User login
Official Newspaper of the American College of Surgeons
HHS releases tools for patient consent to share data electronically
As more medical practices exchange patients’ health information electronically with hospitals and other providers, physicians have a new problem on their hands: how to explain the process to patients and gain their consent to share the information.
Federal health officials have developed some tools – including customizable patient videos – that aim to simplify the process by creating a standardized explanation of the data exchange process and patient options for sharing their medical information.
The effort, known as meaningful consent, deals specifically with information shared through health information exchange organizations (HIEs). These third-party organizations help health care providers in several ways, including directly exchanging information and orders, allowing providers to request specific patient information, or allowing consumers to aggregate their own data online to share with specific providers.
While state laws and regulations create a patchwork of different requirements for when patient consent is required to release information for treatment, federal officials are encouraging physicians to develop a "meaningful consent" process that includes education about how the information is shared, gives patients time to review the educational materials, and allows them to change or revoke their consent at any time.
The HHS Office of the National Coordinator for Health Information Technology (ONC) recently conducted a pilot project to test the use of tablet computers to educate patients about their options in sharing information through an HIE. The project, which was completed in March 2013, found that patients were most interested in who could access their information, whether sensitive health information would be shared, how the information would be protected from misuse, and why it needed to be shared at all.
"As patients become more engaged in their health care, it’s vitally important that they understand more about various aspects of their choices when it relates to sharing their health in the electronic health information exchange environment," Joy Pritts, ONC’s chief privacy officer, said in a statement.
On Twitter @MaryEllenNY
As more medical practices exchange patients’ health information electronically with hospitals and other providers, physicians have a new problem on their hands: how to explain the process to patients and gain their consent to share the information.
Federal health officials have developed some tools – including customizable patient videos – that aim to simplify the process by creating a standardized explanation of the data exchange process and patient options for sharing their medical information.
The effort, known as meaningful consent, deals specifically with information shared through health information exchange organizations (HIEs). These third-party organizations help health care providers in several ways, including directly exchanging information and orders, allowing providers to request specific patient information, or allowing consumers to aggregate their own data online to share with specific providers.
While state laws and regulations create a patchwork of different requirements for when patient consent is required to release information for treatment, federal officials are encouraging physicians to develop a "meaningful consent" process that includes education about how the information is shared, gives patients time to review the educational materials, and allows them to change or revoke their consent at any time.
The HHS Office of the National Coordinator for Health Information Technology (ONC) recently conducted a pilot project to test the use of tablet computers to educate patients about their options in sharing information through an HIE. The project, which was completed in March 2013, found that patients were most interested in who could access their information, whether sensitive health information would be shared, how the information would be protected from misuse, and why it needed to be shared at all.
"As patients become more engaged in their health care, it’s vitally important that they understand more about various aspects of their choices when it relates to sharing their health in the electronic health information exchange environment," Joy Pritts, ONC’s chief privacy officer, said in a statement.
On Twitter @MaryEllenNY
As more medical practices exchange patients’ health information electronically with hospitals and other providers, physicians have a new problem on their hands: how to explain the process to patients and gain their consent to share the information.
Federal health officials have developed some tools – including customizable patient videos – that aim to simplify the process by creating a standardized explanation of the data exchange process and patient options for sharing their medical information.
The effort, known as meaningful consent, deals specifically with information shared through health information exchange organizations (HIEs). These third-party organizations help health care providers in several ways, including directly exchanging information and orders, allowing providers to request specific patient information, or allowing consumers to aggregate their own data online to share with specific providers.
While state laws and regulations create a patchwork of different requirements for when patient consent is required to release information for treatment, federal officials are encouraging physicians to develop a "meaningful consent" process that includes education about how the information is shared, gives patients time to review the educational materials, and allows them to change or revoke their consent at any time.
The HHS Office of the National Coordinator for Health Information Technology (ONC) recently conducted a pilot project to test the use of tablet computers to educate patients about their options in sharing information through an HIE. The project, which was completed in March 2013, found that patients were most interested in who could access their information, whether sensitive health information would be shared, how the information would be protected from misuse, and why it needed to be shared at all.
"As patients become more engaged in their health care, it’s vitally important that they understand more about various aspects of their choices when it relates to sharing their health in the electronic health information exchange environment," Joy Pritts, ONC’s chief privacy officer, said in a statement.
On Twitter @MaryEllenNY
U.S. physician population grew fastest in South Atlantic region
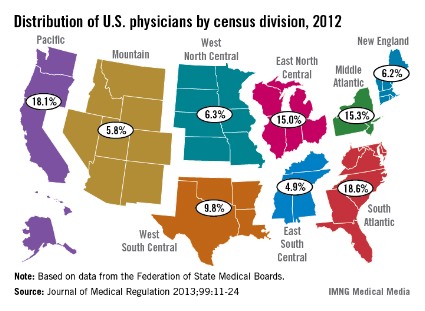
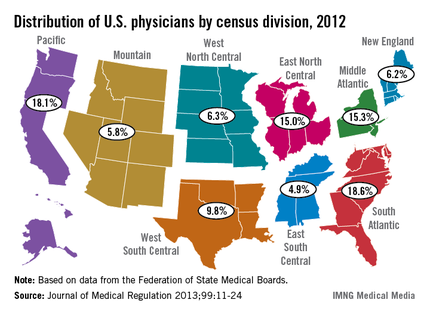
The U.S. South Atlantic states’ growth rate of 10.4% gave that region the fastest-growing population of physicians in the United States between 2010 and 2012, according to data from the Federation of State Medical Boards.
The South Atlantic also had the largest physician population of any census division in 2012 – 163,319 (18.6%) of the 878,194 doctors with an active license, said Aaron Young, Ph.D., of the FSMB in Euless, Tex., and his associates (J. Med. Regul. 2013;99:11-24).
Of the nine geographic divisions defined by the U.S. Census Bureau, five saw their physician population increase, while four experienced decreases from 2010 to 2012. After the South Atlantic, the West South Central region had the second-largest increase, at 9.3%, with the Pacific next at 9.0%. The East North Central (1.9%) and Mountain (0.1%) regions had much smaller increases, the authors reported.
Of the four regions with declines, the West North Central division had the largest decrease, at 5.9%, with New England second, at 5.7%. The Middle Atlantic was down by 2.3%, and the East South Central had a 1.5% decline, Dr. Young and his associates said.
The U.S. South Atlantic states’ growth rate of 10.4% gave that region the fastest-growing population of physicians in the United States between 2010 and 2012, according to data from the Federation of State Medical Boards.
The South Atlantic also had the largest physician population of any census division in 2012 – 163,319 (18.6%) of the 878,194 doctors with an active license, said Aaron Young, Ph.D., of the FSMB in Euless, Tex., and his associates (J. Med. Regul. 2013;99:11-24).
Of the nine geographic divisions defined by the U.S. Census Bureau, five saw their physician population increase, while four experienced decreases from 2010 to 2012. After the South Atlantic, the West South Central region had the second-largest increase, at 9.3%, with the Pacific next at 9.0%. The East North Central (1.9%) and Mountain (0.1%) regions had much smaller increases, the authors reported.
Of the four regions with declines, the West North Central division had the largest decrease, at 5.9%, with New England second, at 5.7%. The Middle Atlantic was down by 2.3%, and the East South Central had a 1.5% decline, Dr. Young and his associates said.

The U.S. South Atlantic states’ growth rate of 10.4% gave that region the fastest-growing population of physicians in the United States between 2010 and 2012, according to data from the Federation of State Medical Boards.
The South Atlantic also had the largest physician population of any census division in 2012 – 163,319 (18.6%) of the 878,194 doctors with an active license, said Aaron Young, Ph.D., of the FSMB in Euless, Tex., and his associates (J. Med. Regul. 2013;99:11-24).
Of the nine geographic divisions defined by the U.S. Census Bureau, five saw their physician population increase, while four experienced decreases from 2010 to 2012. After the South Atlantic, the West South Central region had the second-largest increase, at 9.3%, with the Pacific next at 9.0%. The East North Central (1.9%) and Mountain (0.1%) regions had much smaller increases, the authors reported.
Of the four regions with declines, the West North Central division had the largest decrease, at 5.9%, with New England second, at 5.7%. The Middle Atlantic was down by 2.3%, and the East South Central had a 1.5% decline, Dr. Young and his associates said.

FROM THE JOURNAL OF MEDICAL REGULATION
Apps for physicians
When it comes to health, wellness, and fitness apps, we physicians tend to think of our patients. As in, how can this app help my patient lose weight, exercise more, monitor his diabetes, or help her stay compliant with her medications?
But what about physicians and other health care workers? Can apps help us? Absolutely. According to Dr. Craig Burkhart, a dermatologist and apps expert at the University of North Carolina at Chapel Hill, many physicians aren’t taking advantage of apps that can improve efficiency. "Most physicians I encounter have $1,000-$2,000 smartphones and tablets (when you include the service plans) and only make phone calls, check e-mails, text, and listen to music on them – all things they could do with much less expensive devices."
Dr. Burkhart says that many apps "can turn your devices into tools that help organize your e-mails, organize your schedule, bring clinical information to your fingertips, aid patient-doctor communication, secure important information, speed up and improve note taking, and more."
Apps for physicians are available in categories including patient education, drug reference, medical literature, and general reference.
Check out these handy apps and see what works for you:
• Read by QxMD. Staying up to date on medical journals is a challenge. This app lets you access, organize, and share articles from your favorite medical journals. Its magazinelike interface is popular with users, and makes reading medical journals "as fun as it can be," says Dr. Burkhart.
• PDFpen. Dr. Burkhart says this app is "essential to a paperless workflow." It allows users to sign and send documents without printing actual sheets of paper, making for a greener and more efficient office.
• Visual DX. A reference tool for physicians, this app is a digital medical image library with more than 25,000 images. It allows you to visually confirm a diagnosis and to quickly search on a disease, including symptoms and patient care management. A key feature is its ability to let you create a visual differential of medication-induced diseases for more than 700 different drugs.
• 1Password. We’ve all forgotten passwords from time to time. This app allows you to make and store highly secure and complex passwords. For Dr. Burkhart, it has "replaced memory and random sheets of paper as his password repository."
• Draw MD. We know that visuals often help us to explain complex issues to patients more effectively. This app enables you to draw and modify medical images and surgical procedures in a way that is clear and understandable to patients.
• 3D4Medical. This app can enhance patient education by using 3D technology to allow navigation around the body. You can zoom, rotate, and cut images to create different perspectives.
Dr. Jeffrey Benabio is a partner physician in the department of dermatology of the Southern California Permanente Group in San Diego and a volunteer clinical assistant professor at the University of California, San Diego. He has published numerous scientific articles and is a member and fellow of the American Academy of Dermatology, and a member of the Telemedicine Association and the American Medical Association, among others. He is board certified in dermatology as well as medicine and surgery in the state of California. Dr. Benabio has a special interest in the uses of social media for education and building a dermatology practice. He is the founder of The Derm Blog, an educational website which has had over 2 million unique visitors. Dr. Benabio is also a founding member and the skin care expert for Livestrong.com, a health and wellness website of Lance Armstrong’s the Livestrong Foundation. Dr. Benabio is @Dermdoc on Twitter.
When it comes to health, wellness, and fitness apps, we physicians tend to think of our patients. As in, how can this app help my patient lose weight, exercise more, monitor his diabetes, or help her stay compliant with her medications?
But what about physicians and other health care workers? Can apps help us? Absolutely. According to Dr. Craig Burkhart, a dermatologist and apps expert at the University of North Carolina at Chapel Hill, many physicians aren’t taking advantage of apps that can improve efficiency. "Most physicians I encounter have $1,000-$2,000 smartphones and tablets (when you include the service plans) and only make phone calls, check e-mails, text, and listen to music on them – all things they could do with much less expensive devices."
Dr. Burkhart says that many apps "can turn your devices into tools that help organize your e-mails, organize your schedule, bring clinical information to your fingertips, aid patient-doctor communication, secure important information, speed up and improve note taking, and more."
Apps for physicians are available in categories including patient education, drug reference, medical literature, and general reference.
Check out these handy apps and see what works for you:
• Read by QxMD. Staying up to date on medical journals is a challenge. This app lets you access, organize, and share articles from your favorite medical journals. Its magazinelike interface is popular with users, and makes reading medical journals "as fun as it can be," says Dr. Burkhart.
• PDFpen. Dr. Burkhart says this app is "essential to a paperless workflow." It allows users to sign and send documents without printing actual sheets of paper, making for a greener and more efficient office.
• Visual DX. A reference tool for physicians, this app is a digital medical image library with more than 25,000 images. It allows you to visually confirm a diagnosis and to quickly search on a disease, including symptoms and patient care management. A key feature is its ability to let you create a visual differential of medication-induced diseases for more than 700 different drugs.
• 1Password. We’ve all forgotten passwords from time to time. This app allows you to make and store highly secure and complex passwords. For Dr. Burkhart, it has "replaced memory and random sheets of paper as his password repository."
• Draw MD. We know that visuals often help us to explain complex issues to patients more effectively. This app enables you to draw and modify medical images and surgical procedures in a way that is clear and understandable to patients.
• 3D4Medical. This app can enhance patient education by using 3D technology to allow navigation around the body. You can zoom, rotate, and cut images to create different perspectives.
Dr. Jeffrey Benabio is a partner physician in the department of dermatology of the Southern California Permanente Group in San Diego and a volunteer clinical assistant professor at the University of California, San Diego. He has published numerous scientific articles and is a member and fellow of the American Academy of Dermatology, and a member of the Telemedicine Association and the American Medical Association, among others. He is board certified in dermatology as well as medicine and surgery in the state of California. Dr. Benabio has a special interest in the uses of social media for education and building a dermatology practice. He is the founder of The Derm Blog, an educational website which has had over 2 million unique visitors. Dr. Benabio is also a founding member and the skin care expert for Livestrong.com, a health and wellness website of Lance Armstrong’s the Livestrong Foundation. Dr. Benabio is @Dermdoc on Twitter.
When it comes to health, wellness, and fitness apps, we physicians tend to think of our patients. As in, how can this app help my patient lose weight, exercise more, monitor his diabetes, or help her stay compliant with her medications?
But what about physicians and other health care workers? Can apps help us? Absolutely. According to Dr. Craig Burkhart, a dermatologist and apps expert at the University of North Carolina at Chapel Hill, many physicians aren’t taking advantage of apps that can improve efficiency. "Most physicians I encounter have $1,000-$2,000 smartphones and tablets (when you include the service plans) and only make phone calls, check e-mails, text, and listen to music on them – all things they could do with much less expensive devices."
Dr. Burkhart says that many apps "can turn your devices into tools that help organize your e-mails, organize your schedule, bring clinical information to your fingertips, aid patient-doctor communication, secure important information, speed up and improve note taking, and more."
Apps for physicians are available in categories including patient education, drug reference, medical literature, and general reference.
Check out these handy apps and see what works for you:
• Read by QxMD. Staying up to date on medical journals is a challenge. This app lets you access, organize, and share articles from your favorite medical journals. Its magazinelike interface is popular with users, and makes reading medical journals "as fun as it can be," says Dr. Burkhart.
• PDFpen. Dr. Burkhart says this app is "essential to a paperless workflow." It allows users to sign and send documents without printing actual sheets of paper, making for a greener and more efficient office.
• Visual DX. A reference tool for physicians, this app is a digital medical image library with more than 25,000 images. It allows you to visually confirm a diagnosis and to quickly search on a disease, including symptoms and patient care management. A key feature is its ability to let you create a visual differential of medication-induced diseases for more than 700 different drugs.
• 1Password. We’ve all forgotten passwords from time to time. This app allows you to make and store highly secure and complex passwords. For Dr. Burkhart, it has "replaced memory and random sheets of paper as his password repository."
• Draw MD. We know that visuals often help us to explain complex issues to patients more effectively. This app enables you to draw and modify medical images and surgical procedures in a way that is clear and understandable to patients.
• 3D4Medical. This app can enhance patient education by using 3D technology to allow navigation around the body. You can zoom, rotate, and cut images to create different perspectives.
Dr. Jeffrey Benabio is a partner physician in the department of dermatology of the Southern California Permanente Group in San Diego and a volunteer clinical assistant professor at the University of California, San Diego. He has published numerous scientific articles and is a member and fellow of the American Academy of Dermatology, and a member of the Telemedicine Association and the American Medical Association, among others. He is board certified in dermatology as well as medicine and surgery in the state of California. Dr. Benabio has a special interest in the uses of social media for education and building a dermatology practice. He is the founder of The Derm Blog, an educational website which has had over 2 million unique visitors. Dr. Benabio is also a founding member and the skin care expert for Livestrong.com, a health and wellness website of Lance Armstrong’s the Livestrong Foundation. Dr. Benabio is @Dermdoc on Twitter.
Fear, anxiety drive contralateral mastectomy, survey finds
Young women who elect contralateral prophylactic mastectomy do so, in part, because they want to improve their chances for survival, even though they realize there is no convincing evidence of a survival benefit, based on results from a survey.
Moreover, many women, particularly those without a known BRCA mutation, substantially overestimate their risk of developing cancer in the unaffected breast, Shoshana M. Rosenberg, Sc.D., of the Dana-Farber Cancer Institute and the Harvard School of Public Health, both in Boston, and her colleagues reported in the Sept. 17 issue of Annals of Internal Medicine.
The findings suggest "some degree of cognitive dissonance," since almost all respondents cited both the desire to improve survival or extend life and a desire to prevent metastatic disease as extremely or very important reasons for choosing contralateral prophylactic mastectomy, while also demonstrating awareness that bilateral mastectomy would not extend survival, the researchers said. Anxiety and fear of recurrence likely influenced the decision-making process and led women to identify their desire to extend life and prevent metastatic disease as among the most important reasons for having contralateral prophylactic mastectomy.
Of 550 women diagnosed at age 40 years or younger with breast cancer in one breast, 123 (22%) who underwent bilateral mastectomy were included in the analysis. Of those women, 94% said the desire to improve survival was an extremely or very important factor in their decision to undergo contralateral prophylactic mastectomy. Yet 74% of mutation carriers and 84% of noncarriers acknowledged that most women who are diagnosed with early-stage breast cancer and undergo treatment will ultimately die of something other than breast cancer.
Also, 98% cited a desire to decrease their risk of contralateral breast cancer and 95% cited a desire for peace of mind as extremely or very important factors in their decision (Ann. Intern. Med. 2013;17:373-81).
Mutation carriers estimated their risk of developing cancer in the contralateral breast in the 5 years after unilateral treatment at 20%, noncarriers estimated that risk at 10%. The risk for women with BRCA mutations is estimated to be 24%-31%. The group without mutations overestimated their risk, which is actually about 2%-4% over 5 years. Women in both groups estimated the risk of chest wall recurrence after bilateral mastectomy at 5%, which is actually estimated at less than 1%, the researchers noted.
Survey participants were women whose median age was 37 years at cancer diagnosis and who were recruited from four academic and five community hospitals in Massachusetts, and from one academic site in Toronto between November 2006 and November 2010. The participants were recruited as part of the Helping Ourselves, Helping Others: Young Women’s Breast Cancer Study. Most had stage I or stage II breast cancer, and all were believed to have breast cancer only in one breast. About 60% of tumors were estrogen receptor–positive, and about 25% of the women were BRCAmutation carriers.
The survey was a one-time supplement to the ongoing prospective cohort study. It is one of the largest surveys to date to examine decision-making, risk perceptions, and psychosocial aspects of contralateral prophylactic mastectomy among young women with breast cancer, the investigators said. The survey findings are important, given that the rates of contralateral prophylactic mastectomy have increased dramatically in recent years, from 4%-6% in the late 1990s to 11%-25% in more recent reports, even though "the value of the procedure for most women with unilateral early-stage breast cancer is unclear," the researchers said.
The study was limited by the lack of validation of the survey, the possibility of recall bias (since the women were surveyed an average of 2 years following surgery), and the possibility of limited generalizability of the findings because the study population was primarily white, non-Hispanic, and college educated. Yet, the findings highlight a need for improved communication with patients, they said.
Although 96%-97% of participants believed they were clear about benefits and risks, and which mattered most, many women reported that several outcomes associated with surgery were worse than they had expected. For example, 33% reported needing a higher-than-expected number of operations or procedures, and 28% said that numbness or tingling in the chest was worse than expected.
"With respect to QOL outcomes, 42% reported that their sense of sexuality was worse than they expected after surgery, and nearly one-third indicated that self-consciousness about appearance was also worse than expected," the researchers reported.
Only about half of the participants indicated that their physicians had talked at least to some degree about reasons not to have contralateral prophylactic mastectomy, suggesting a potential role for "interventions that ensure women are sufficiently informed and the actual risk for contralateral disease is effectively communicated," the investigators said.
Additional clarification of these conflicting responses would be helpful, they said, suggesting that future investigation "might include focus groups or collection of qualitative data with the goal of elucidating the role of cognitive biases in making treatment decisions."
This study was primarily funded by Susan G. Komen for the Cure. Dr. Rosenberg reported receiving support from the National Cancer Institute.
Young women who elect contralateral prophylactic mastectomy do so, in part, because they want to improve their chances for survival, even though they realize there is no convincing evidence of a survival benefit, based on results from a survey.
Moreover, many women, particularly those without a known BRCA mutation, substantially overestimate their risk of developing cancer in the unaffected breast, Shoshana M. Rosenberg, Sc.D., of the Dana-Farber Cancer Institute and the Harvard School of Public Health, both in Boston, and her colleagues reported in the Sept. 17 issue of Annals of Internal Medicine.
The findings suggest "some degree of cognitive dissonance," since almost all respondents cited both the desire to improve survival or extend life and a desire to prevent metastatic disease as extremely or very important reasons for choosing contralateral prophylactic mastectomy, while also demonstrating awareness that bilateral mastectomy would not extend survival, the researchers said. Anxiety and fear of recurrence likely influenced the decision-making process and led women to identify their desire to extend life and prevent metastatic disease as among the most important reasons for having contralateral prophylactic mastectomy.
Of 550 women diagnosed at age 40 years or younger with breast cancer in one breast, 123 (22%) who underwent bilateral mastectomy were included in the analysis. Of those women, 94% said the desire to improve survival was an extremely or very important factor in their decision to undergo contralateral prophylactic mastectomy. Yet 74% of mutation carriers and 84% of noncarriers acknowledged that most women who are diagnosed with early-stage breast cancer and undergo treatment will ultimately die of something other than breast cancer.
Also, 98% cited a desire to decrease their risk of contralateral breast cancer and 95% cited a desire for peace of mind as extremely or very important factors in their decision (Ann. Intern. Med. 2013;17:373-81).
Mutation carriers estimated their risk of developing cancer in the contralateral breast in the 5 years after unilateral treatment at 20%, noncarriers estimated that risk at 10%. The risk for women with BRCA mutations is estimated to be 24%-31%. The group without mutations overestimated their risk, which is actually about 2%-4% over 5 years. Women in both groups estimated the risk of chest wall recurrence after bilateral mastectomy at 5%, which is actually estimated at less than 1%, the researchers noted.
Survey participants were women whose median age was 37 years at cancer diagnosis and who were recruited from four academic and five community hospitals in Massachusetts, and from one academic site in Toronto between November 2006 and November 2010. The participants were recruited as part of the Helping Ourselves, Helping Others: Young Women’s Breast Cancer Study. Most had stage I or stage II breast cancer, and all were believed to have breast cancer only in one breast. About 60% of tumors were estrogen receptor–positive, and about 25% of the women were BRCAmutation carriers.
The survey was a one-time supplement to the ongoing prospective cohort study. It is one of the largest surveys to date to examine decision-making, risk perceptions, and psychosocial aspects of contralateral prophylactic mastectomy among young women with breast cancer, the investigators said. The survey findings are important, given that the rates of contralateral prophylactic mastectomy have increased dramatically in recent years, from 4%-6% in the late 1990s to 11%-25% in more recent reports, even though "the value of the procedure for most women with unilateral early-stage breast cancer is unclear," the researchers said.
The study was limited by the lack of validation of the survey, the possibility of recall bias (since the women were surveyed an average of 2 years following surgery), and the possibility of limited generalizability of the findings because the study population was primarily white, non-Hispanic, and college educated. Yet, the findings highlight a need for improved communication with patients, they said.
Although 96%-97% of participants believed they were clear about benefits and risks, and which mattered most, many women reported that several outcomes associated with surgery were worse than they had expected. For example, 33% reported needing a higher-than-expected number of operations or procedures, and 28% said that numbness or tingling in the chest was worse than expected.
"With respect to QOL outcomes, 42% reported that their sense of sexuality was worse than they expected after surgery, and nearly one-third indicated that self-consciousness about appearance was also worse than expected," the researchers reported.
Only about half of the participants indicated that their physicians had talked at least to some degree about reasons not to have contralateral prophylactic mastectomy, suggesting a potential role for "interventions that ensure women are sufficiently informed and the actual risk for contralateral disease is effectively communicated," the investigators said.
Additional clarification of these conflicting responses would be helpful, they said, suggesting that future investigation "might include focus groups or collection of qualitative data with the goal of elucidating the role of cognitive biases in making treatment decisions."
This study was primarily funded by Susan G. Komen for the Cure. Dr. Rosenberg reported receiving support from the National Cancer Institute.
Young women who elect contralateral prophylactic mastectomy do so, in part, because they want to improve their chances for survival, even though they realize there is no convincing evidence of a survival benefit, based on results from a survey.
Moreover, many women, particularly those without a known BRCA mutation, substantially overestimate their risk of developing cancer in the unaffected breast, Shoshana M. Rosenberg, Sc.D., of the Dana-Farber Cancer Institute and the Harvard School of Public Health, both in Boston, and her colleagues reported in the Sept. 17 issue of Annals of Internal Medicine.
The findings suggest "some degree of cognitive dissonance," since almost all respondents cited both the desire to improve survival or extend life and a desire to prevent metastatic disease as extremely or very important reasons for choosing contralateral prophylactic mastectomy, while also demonstrating awareness that bilateral mastectomy would not extend survival, the researchers said. Anxiety and fear of recurrence likely influenced the decision-making process and led women to identify their desire to extend life and prevent metastatic disease as among the most important reasons for having contralateral prophylactic mastectomy.
Of 550 women diagnosed at age 40 years or younger with breast cancer in one breast, 123 (22%) who underwent bilateral mastectomy were included in the analysis. Of those women, 94% said the desire to improve survival was an extremely or very important factor in their decision to undergo contralateral prophylactic mastectomy. Yet 74% of mutation carriers and 84% of noncarriers acknowledged that most women who are diagnosed with early-stage breast cancer and undergo treatment will ultimately die of something other than breast cancer.
Also, 98% cited a desire to decrease their risk of contralateral breast cancer and 95% cited a desire for peace of mind as extremely or very important factors in their decision (Ann. Intern. Med. 2013;17:373-81).
Mutation carriers estimated their risk of developing cancer in the contralateral breast in the 5 years after unilateral treatment at 20%, noncarriers estimated that risk at 10%. The risk for women with BRCA mutations is estimated to be 24%-31%. The group without mutations overestimated their risk, which is actually about 2%-4% over 5 years. Women in both groups estimated the risk of chest wall recurrence after bilateral mastectomy at 5%, which is actually estimated at less than 1%, the researchers noted.
Survey participants were women whose median age was 37 years at cancer diagnosis and who were recruited from four academic and five community hospitals in Massachusetts, and from one academic site in Toronto between November 2006 and November 2010. The participants were recruited as part of the Helping Ourselves, Helping Others: Young Women’s Breast Cancer Study. Most had stage I or stage II breast cancer, and all were believed to have breast cancer only in one breast. About 60% of tumors were estrogen receptor–positive, and about 25% of the women were BRCAmutation carriers.
The survey was a one-time supplement to the ongoing prospective cohort study. It is one of the largest surveys to date to examine decision-making, risk perceptions, and psychosocial aspects of contralateral prophylactic mastectomy among young women with breast cancer, the investigators said. The survey findings are important, given that the rates of contralateral prophylactic mastectomy have increased dramatically in recent years, from 4%-6% in the late 1990s to 11%-25% in more recent reports, even though "the value of the procedure for most women with unilateral early-stage breast cancer is unclear," the researchers said.
The study was limited by the lack of validation of the survey, the possibility of recall bias (since the women were surveyed an average of 2 years following surgery), and the possibility of limited generalizability of the findings because the study population was primarily white, non-Hispanic, and college educated. Yet, the findings highlight a need for improved communication with patients, they said.
Although 96%-97% of participants believed they were clear about benefits and risks, and which mattered most, many women reported that several outcomes associated with surgery were worse than they had expected. For example, 33% reported needing a higher-than-expected number of operations or procedures, and 28% said that numbness or tingling in the chest was worse than expected.
"With respect to QOL outcomes, 42% reported that their sense of sexuality was worse than they expected after surgery, and nearly one-third indicated that self-consciousness about appearance was also worse than expected," the researchers reported.
Only about half of the participants indicated that their physicians had talked at least to some degree about reasons not to have contralateral prophylactic mastectomy, suggesting a potential role for "interventions that ensure women are sufficiently informed and the actual risk for contralateral disease is effectively communicated," the investigators said.
Additional clarification of these conflicting responses would be helpful, they said, suggesting that future investigation "might include focus groups or collection of qualitative data with the goal of elucidating the role of cognitive biases in making treatment decisions."
This study was primarily funded by Susan G. Komen for the Cure. Dr. Rosenberg reported receiving support from the National Cancer Institute.
FROM ANNALS OF INTERNAL MEDICINE
Major finding: A total of 94% of respondents cited a desire to improve survival as an extremely or very important factor in their decision to undergo contralateral prophylactic mastectomy, yet 74%-84% acknowledged that most women diagnosed with early-stage breast cancer who undergo treatment will ultimately die of something else.
Data source: A survey conducted as part of a prospective cohort study.
Disclosures: This study was primarily funded by Susan G. Komen for the Cure. Dr. Rosenberg reported receiving support from the National Cancer Institute.
SGR replacement cost now up to $176 billion
The price tag to replace the Medicare Sustainable Growth Rate formula has grown to an estimated $176 billion over 10 years, up $40 billion from the most recent estimates, according to a Sept. 13 Congressional Budget Office analysis.
This time, the nonpartisan CBO analyzed the Medicare Patient Access and Quality Improvement Act of 2013 (H.R. 2810), which was approved unanimously by the House Energy and Commerce Committee in July. The bill has not seen any further movement since that time, but it is expected that Congress will take up SGR reform again this fall.
In its analysis, the CBO said that it thought that H.R. 2810, in part because it continues to rely largely on fee-for-service, would continue to drive up Medicare spending. Between 2014 and 2018, the bill proposes to increase physician fees by a flat 0.5%.
Starting in 2019, physicians will be paid based on performance in a program that gives rewards for quality, or on so-called alternative payment models.
The CBO said it believed that physicians would choose payment models that would increase their fees, which would, in turn, create a rise in Medicare spending. The pay increases for 2014-2018 will likely cost $64 billion, according to the agency. From 2019 to 2023, spending on physician fees is expected to hit $112 billion.
In addition, the agency said that Medicare would end up spending about $0.3 billion on other payment reforms proposed in the legislation, including modifying payment rates in certain California counties, adjusting relative value units for certain physicians’ services, and requiring the development of payment codes to encourage care coordination and the use of medical homes.
On Twitter @aliciaault
The price tag to replace the Medicare Sustainable Growth Rate formula has grown to an estimated $176 billion over 10 years, up $40 billion from the most recent estimates, according to a Sept. 13 Congressional Budget Office analysis.
This time, the nonpartisan CBO analyzed the Medicare Patient Access and Quality Improvement Act of 2013 (H.R. 2810), which was approved unanimously by the House Energy and Commerce Committee in July. The bill has not seen any further movement since that time, but it is expected that Congress will take up SGR reform again this fall.
In its analysis, the CBO said that it thought that H.R. 2810, in part because it continues to rely largely on fee-for-service, would continue to drive up Medicare spending. Between 2014 and 2018, the bill proposes to increase physician fees by a flat 0.5%.
Starting in 2019, physicians will be paid based on performance in a program that gives rewards for quality, or on so-called alternative payment models.
The CBO said it believed that physicians would choose payment models that would increase their fees, which would, in turn, create a rise in Medicare spending. The pay increases for 2014-2018 will likely cost $64 billion, according to the agency. From 2019 to 2023, spending on physician fees is expected to hit $112 billion.
In addition, the agency said that Medicare would end up spending about $0.3 billion on other payment reforms proposed in the legislation, including modifying payment rates in certain California counties, adjusting relative value units for certain physicians’ services, and requiring the development of payment codes to encourage care coordination and the use of medical homes.
On Twitter @aliciaault
The price tag to replace the Medicare Sustainable Growth Rate formula has grown to an estimated $176 billion over 10 years, up $40 billion from the most recent estimates, according to a Sept. 13 Congressional Budget Office analysis.
This time, the nonpartisan CBO analyzed the Medicare Patient Access and Quality Improvement Act of 2013 (H.R. 2810), which was approved unanimously by the House Energy and Commerce Committee in July. The bill has not seen any further movement since that time, but it is expected that Congress will take up SGR reform again this fall.
In its analysis, the CBO said that it thought that H.R. 2810, in part because it continues to rely largely on fee-for-service, would continue to drive up Medicare spending. Between 2014 and 2018, the bill proposes to increase physician fees by a flat 0.5%.
Starting in 2019, physicians will be paid based on performance in a program that gives rewards for quality, or on so-called alternative payment models.
The CBO said it believed that physicians would choose payment models that would increase their fees, which would, in turn, create a rise in Medicare spending. The pay increases for 2014-2018 will likely cost $64 billion, according to the agency. From 2019 to 2023, spending on physician fees is expected to hit $112 billion.
In addition, the agency said that Medicare would end up spending about $0.3 billion on other payment reforms proposed in the legislation, including modifying payment rates in certain California counties, adjusting relative value units for certain physicians’ services, and requiring the development of payment codes to encourage care coordination and the use of medical homes.
On Twitter @aliciaault
Stats show MRSA declining, especially in hospitals
The estimated total of invasive MRSA infections across the United States fell 31% between 2005 and 2011, according to a report published online Sept. 16 in JAMA Internal Medicine.
And for the first time, the estimated number of hospital-onset invasive MRSA infections was lower than that of community-associated infections, said Dr. Raymund Dantes of the Epidemic Intelligence Service of the Centers for Disease Control and Prevention, Atlanta.
The greatest burden of disease continues to fall upon patients in the community who have recent or ongoing exposure to health care services. That includes those recently discharged from acute medical care, long-term–care residents, and patients who require repeated medical visits, such as for diabetes care or dialysis, according to Dr. Dantes and his colleagues.
Those findings, together with other results from their analysis of data in the CDC’s emerging infections surveillance system, indicate that "the U.S. is on track to meet the Department of Health and Human Services 2013 target of reducing health care–associated MRSA invasive infections by 50%," they noted (JAMA Intern. Med. 2013 Sept. 16 [doi:10.1001/jamainternmed.2013.10423]).
To better understand the national burden of invasive MRSA infections, Dr. Dantes and his colleagues analyzed data from nine states participating in the surveillance program: California, Colorado, Connecticut, Georgia, Maryland, Minnesota, New York, Oregon, and Tennessee. They focused on 2011, the most recent year for which complete data are available, and compared the information with that gathered in 2005.
Those nine sites, which represented 16,489,254 people in 2005 and 19,393,677 people in 2011, reported 4,872 cases of invasive MRSA among 4,445 patients in 2011. A total of 18% were classified as hospital-onset infections, 60% as "health-care–associated community onset" infections, and 20% as community-associated infections.
Extrapolating those results to the entire U.S. population, the investigators estimated that 80,461 invasive MRSA infections occurred nationally in 2011, of which 14,156 were hospital-onset, 48,353 were health-care–associated community onset, and 16,560 were community-associated.
"Compared with 6 years earlier, the estimated national rate of invasive MRSA has decreased by 31.2%," Dr. Dantes and his associates said. "Although this rate decrease was most precipitous among hospital-onset infections, at 54.2%, rate decreases were evident among other categories as well: health-care–associated by 27.7%, and community-associated by 5.0%," they wrote.
The 54% decline in hospital-onset cases was particularly encouraging. The exact reason for that reduction is not yet known, but it is likely that implementation of infection prevention measures contributed, especially those targeting intravascular catheter-related infections and health care transmission of multidrug-resistant organisms, they added.
"It is notable that the incidence of community-associated invasive MRSA infections, although relatively stable, has not increased over this time, despite increases in hospitalizations related to MRSA skin and soft-tissue infections documented in discharge data," the investigators said. "Progress in reducing infections among this population is likely to be most challenging due to a lack of clearly effective strategies to control endemic MRSA transmission in the community setting.
Although there is guidance on preventing community-associated MRSA transmission in institutions such as athletic facilities, correctional facilities, and schools, guidance on prevention in other community settings isn’t as well established. Changes in community settings "may be related to transmission in households, prevention of invasive disease from improved early treatment of noninvasive infections, or the natural evolution of this pathogen," they noted.
To substantially reduce the overall burden of invasive MRSA infections, significant progress must be made to prevent postdischarge and dialysis-related cases, the researchers added. In 2011, most cases of health care–associated community-onset invasive MRSA occurred among dialysis patients and patients who had been hospitalized recently.
"Invasive devices that remain placed during the postdischarge period, progression from colonization to clinical infection, and breakdowns in host defense and skin integrity during hospitalization may account for this increased risk," Dr. Dantes and his associates said.
An estimated 11,285 patients with invasive MRSA infections died of all causes during hospitalization in 2011, they added.
The Emerging Infections Program and the National Center for Emerging Zoonotic Infectious Diseases at the Centers for Disease Control and Prevention supported the study. No financial conflicts of interest were reported.
The estimated total of invasive MRSA infections across the United States fell 31% between 2005 and 2011, according to a report published online Sept. 16 in JAMA Internal Medicine.
And for the first time, the estimated number of hospital-onset invasive MRSA infections was lower than that of community-associated infections, said Dr. Raymund Dantes of the Epidemic Intelligence Service of the Centers for Disease Control and Prevention, Atlanta.
The greatest burden of disease continues to fall upon patients in the community who have recent or ongoing exposure to health care services. That includes those recently discharged from acute medical care, long-term–care residents, and patients who require repeated medical visits, such as for diabetes care or dialysis, according to Dr. Dantes and his colleagues.
Those findings, together with other results from their analysis of data in the CDC’s emerging infections surveillance system, indicate that "the U.S. is on track to meet the Department of Health and Human Services 2013 target of reducing health care–associated MRSA invasive infections by 50%," they noted (JAMA Intern. Med. 2013 Sept. 16 [doi:10.1001/jamainternmed.2013.10423]).
To better understand the national burden of invasive MRSA infections, Dr. Dantes and his colleagues analyzed data from nine states participating in the surveillance program: California, Colorado, Connecticut, Georgia, Maryland, Minnesota, New York, Oregon, and Tennessee. They focused on 2011, the most recent year for which complete data are available, and compared the information with that gathered in 2005.
Those nine sites, which represented 16,489,254 people in 2005 and 19,393,677 people in 2011, reported 4,872 cases of invasive MRSA among 4,445 patients in 2011. A total of 18% were classified as hospital-onset infections, 60% as "health-care–associated community onset" infections, and 20% as community-associated infections.
Extrapolating those results to the entire U.S. population, the investigators estimated that 80,461 invasive MRSA infections occurred nationally in 2011, of which 14,156 were hospital-onset, 48,353 were health-care–associated community onset, and 16,560 were community-associated.
"Compared with 6 years earlier, the estimated national rate of invasive MRSA has decreased by 31.2%," Dr. Dantes and his associates said. "Although this rate decrease was most precipitous among hospital-onset infections, at 54.2%, rate decreases were evident among other categories as well: health-care–associated by 27.7%, and community-associated by 5.0%," they wrote.
The 54% decline in hospital-onset cases was particularly encouraging. The exact reason for that reduction is not yet known, but it is likely that implementation of infection prevention measures contributed, especially those targeting intravascular catheter-related infections and health care transmission of multidrug-resistant organisms, they added.
"It is notable that the incidence of community-associated invasive MRSA infections, although relatively stable, has not increased over this time, despite increases in hospitalizations related to MRSA skin and soft-tissue infections documented in discharge data," the investigators said. "Progress in reducing infections among this population is likely to be most challenging due to a lack of clearly effective strategies to control endemic MRSA transmission in the community setting.
Although there is guidance on preventing community-associated MRSA transmission in institutions such as athletic facilities, correctional facilities, and schools, guidance on prevention in other community settings isn’t as well established. Changes in community settings "may be related to transmission in households, prevention of invasive disease from improved early treatment of noninvasive infections, or the natural evolution of this pathogen," they noted.
To substantially reduce the overall burden of invasive MRSA infections, significant progress must be made to prevent postdischarge and dialysis-related cases, the researchers added. In 2011, most cases of health care–associated community-onset invasive MRSA occurred among dialysis patients and patients who had been hospitalized recently.
"Invasive devices that remain placed during the postdischarge period, progression from colonization to clinical infection, and breakdowns in host defense and skin integrity during hospitalization may account for this increased risk," Dr. Dantes and his associates said.
An estimated 11,285 patients with invasive MRSA infections died of all causes during hospitalization in 2011, they added.
The Emerging Infections Program and the National Center for Emerging Zoonotic Infectious Diseases at the Centers for Disease Control and Prevention supported the study. No financial conflicts of interest were reported.
The estimated total of invasive MRSA infections across the United States fell 31% between 2005 and 2011, according to a report published online Sept. 16 in JAMA Internal Medicine.
And for the first time, the estimated number of hospital-onset invasive MRSA infections was lower than that of community-associated infections, said Dr. Raymund Dantes of the Epidemic Intelligence Service of the Centers for Disease Control and Prevention, Atlanta.
The greatest burden of disease continues to fall upon patients in the community who have recent or ongoing exposure to health care services. That includes those recently discharged from acute medical care, long-term–care residents, and patients who require repeated medical visits, such as for diabetes care or dialysis, according to Dr. Dantes and his colleagues.
Those findings, together with other results from their analysis of data in the CDC’s emerging infections surveillance system, indicate that "the U.S. is on track to meet the Department of Health and Human Services 2013 target of reducing health care–associated MRSA invasive infections by 50%," they noted (JAMA Intern. Med. 2013 Sept. 16 [doi:10.1001/jamainternmed.2013.10423]).
To better understand the national burden of invasive MRSA infections, Dr. Dantes and his colleagues analyzed data from nine states participating in the surveillance program: California, Colorado, Connecticut, Georgia, Maryland, Minnesota, New York, Oregon, and Tennessee. They focused on 2011, the most recent year for which complete data are available, and compared the information with that gathered in 2005.
Those nine sites, which represented 16,489,254 people in 2005 and 19,393,677 people in 2011, reported 4,872 cases of invasive MRSA among 4,445 patients in 2011. A total of 18% were classified as hospital-onset infections, 60% as "health-care–associated community onset" infections, and 20% as community-associated infections.
Extrapolating those results to the entire U.S. population, the investigators estimated that 80,461 invasive MRSA infections occurred nationally in 2011, of which 14,156 were hospital-onset, 48,353 were health-care–associated community onset, and 16,560 were community-associated.
"Compared with 6 years earlier, the estimated national rate of invasive MRSA has decreased by 31.2%," Dr. Dantes and his associates said. "Although this rate decrease was most precipitous among hospital-onset infections, at 54.2%, rate decreases were evident among other categories as well: health-care–associated by 27.7%, and community-associated by 5.0%," they wrote.
The 54% decline in hospital-onset cases was particularly encouraging. The exact reason for that reduction is not yet known, but it is likely that implementation of infection prevention measures contributed, especially those targeting intravascular catheter-related infections and health care transmission of multidrug-resistant organisms, they added.
"It is notable that the incidence of community-associated invasive MRSA infections, although relatively stable, has not increased over this time, despite increases in hospitalizations related to MRSA skin and soft-tissue infections documented in discharge data," the investigators said. "Progress in reducing infections among this population is likely to be most challenging due to a lack of clearly effective strategies to control endemic MRSA transmission in the community setting.
Although there is guidance on preventing community-associated MRSA transmission in institutions such as athletic facilities, correctional facilities, and schools, guidance on prevention in other community settings isn’t as well established. Changes in community settings "may be related to transmission in households, prevention of invasive disease from improved early treatment of noninvasive infections, or the natural evolution of this pathogen," they noted.
To substantially reduce the overall burden of invasive MRSA infections, significant progress must be made to prevent postdischarge and dialysis-related cases, the researchers added. In 2011, most cases of health care–associated community-onset invasive MRSA occurred among dialysis patients and patients who had been hospitalized recently.
"Invasive devices that remain placed during the postdischarge period, progression from colonization to clinical infection, and breakdowns in host defense and skin integrity during hospitalization may account for this increased risk," Dr. Dantes and his associates said.
An estimated 11,285 patients with invasive MRSA infections died of all causes during hospitalization in 2011, they added.
The Emerging Infections Program and the National Center for Emerging Zoonotic Infectious Diseases at the Centers for Disease Control and Prevention supported the study. No financial conflicts of interest were reported.
FROM JAMA INTERNAL MEDICINE
Major Finding: The estimated national rate of invasive MRSA infections declined 31% since 2005, with hospital-onset cases dropping 54%, health care–associated cases decreasing 28%, and community-associated cases declining 5%.
Data Source: An analysis of data from a MRSA surveillance program covering more than 16 million residents of nine states in 2005 and more than 19 million residents in those states in 2011.
Disclosures: The Emerging Infections Program and the National Center for Emerging Zoonotic Infectious Diseases at the Centers for Disease Control and Prevention supported the study. No financial conflicts of interest were reported.
FDA advisory panel backs approval of neoadjuvant pertuzumab for breast cancer
SILVER SPRING, MD. – Pertuzumab is likely to be approved for the neoadjuvant treatment of breast cancer in the preoperative setting, based on the recommendation of a Food and Drug Administration advisory panel.
At a meeting on Sept. 12, the FDA’s Oncologic Drugs Advisory Committee panel voted 13-0, with 1 abstention, that treatment with pertuzumab, a human epidermal growth factor receptor 2 (HER2)-targeted monoclonal antibody, had a favorable benefit-to-risk profile as a neoadjuvant treatment in combination with trastuzumab and docetaxel before surgery in patients with locally-advanced, inflammatory, or early-stage breast cancers greater than 2 cm in diameter. The neoadjuvant approach would be part of a complete early breast cancer treatment regimen containing fluorouracil, epirubicin, and cyclophosphamide or carboplatin.
The drug is being reviewed under the accelerated approval process, a mechanism that makes drugs available to fill an unmet medical need in patients with serious diseases. Historically, the usual sequence of the FDA approvals for breast cancer agents starts with approval for metastatic disease, followed by approval for early-stage disease years later after the results of large studies with long follow-up periods are available. Pertuzumab, marketed as Perjeta by Genentech, was just approved in 2012 as a first-line treatment for metastatic HER2-positive breast cancer in women who have not received prior anti-HER2 therapy or chemotherapy for metastatic disease.
Accelerated approvals are based on a surrogate endpoint that is considered “reasonably likely” to predict clinical benefit. In this instance, pathologic complete response rate (pCR) was used in the phase II NeoSphere study. For this trial, pertuzumab was added to trastuzumab (Herceptin) – another HER2 receptor antagonist – and docetaxel, and was given before surgery to women with HER-2-positive, locally advanced, inflammatory or early-stage breast cancer. The comparator group was patients treated preoperatively with trastuzumab and docetaxel.
The women who received the three-drug neoadjuvant regimen had an 18% improvement in pCR as compared to women given trastuzumab and docetaxel only.
In addition to the NeoSphere study, the panel also considered the body of data on pertuzumab in metastatic breast cancer, the known biology of the pertuzumab, and the activity of HER-2 targeted therapies in breast cancer.
Should pertuzumab receive the accelerated approval, its full approval will be contingent on the final results of APHINITY, a confirmatory study. If the APHINITY results do not confirm the NeoSphere results, the FDA can withdraw the approval for this indication.
The vote to support the first approval of a drug for the neoadjuvant treatment of breast cancer is “a historic moment,” said the panel chair, Dr. Mikkael A. Sekeres of the Cleveland Clinic. “In so doing, we are supporting the rapid movement of a highly active drug for metastatic breast cancer to the first-line setting with the hope that women with earlier stages of breast cancer will live longer and better.”
However, he added, “all eyes will be on the confirmatory APHINITY trial” and on Genentech, to “verify this initial signal of efficacy and to confirm the bandwidth of safety we have seen so far.” If the results are negative, he and other panelists urged the company to voluntarily remove the drug for this indication, and avoid what happened with another Genentech drug, bevacizumab (Avastin), which was granted an accelerated approval as a first-line treatment in combination with paclitaxel for metastatic breast cancer in 2008. The FDA decided to withdraw approval of this indication after studies failed to confirm the benefit. The company appealed the decision, however, delaying the FDA’s withdrawal of the approval until 2011.
NeoSphere, a randomized study conducted outside of the United States, compared four treatment regimens in 417 women newly diagnosed with locally advanced, inflammatory or operable HER2-positive early breast cancer, with tumors greater than 2 cm, treated for four cycles before surgery. The median tumor size was about 5 cm, and two-thirds were node positive.
Based on the pCR definition used by Genentech (the absence of invasive cancer in the breast), almost 46% of those on the combination of pertuzumab, trastuzumab, and docetaxel reached the primary endpoint, compared with 29% of those on trastuzumab and docetaxel – a statistically significant difference of nearly 17%. Based on the FDA-preferred definition of pCR definition (the absence of invasive cancer in the breast and lymph nodes), the pCR rate was almost 18% higher with the three-drug regimen (39.3% vs. 21.5%).
The FDA considers pCR as “reasonably likely” to predict outcomes in HER2-positive breast cancer.
The treatment has the potential to cure more patients in this high risk population, said Dr. Suparna Wedam, a medical officer in FDA’s Office of Hematology and Oncology Products. Yet, still to be determined are whether the regimen has long-term safety and results in improvements in overall survival, progression-free survival, and other improvements in long-term outcomes.
Genentech also provided results from the TRYPHAENA phase II study that compared three neoadjuvant treatment regimens before surgery; as well as the CLEOPATRA phase III study, the basis of the 2012 approval of trastuzumab. In TRYPHAENA, 225 women with HER2-positive, locally advanced, operable or inflammatory breast cancer, received one of three neoadjuvant treatment regimens. The pCR rates, a secondary endpoint, ranged from about 55% to 64% when pertuzumab was added to trastuzumab and chemotherapy. CLEOPATRA enrolled 808 women with HER2-positive, locally recurrent, unresectable or metastatic breast cancer previously untreated with a biologic or chemotherapy for metastatic disease. In that trial, pertuzumab in combination with trastuzumab and docetaxel resulted in significant improvements in progression-free survival and overall survival.
The most common adverse events with the three-drug regimen in the NeoSphere study were neutropenia, diarrhea, nausea, fatigue, mucosal inflammation, and rash, according to the company. No unexpected safety signals were observed with the addition of pertuzumab. The addition of pertuzumab did not appear to increase symptomatic cardiac toxicity when added to trastuzumab-based neoadjuvant or metastatic treatment regimens.
The FDA reviewers noted, however, that the rate of left ventricular dysfunction (mostly asymptomatic) was higher with neoadjuvant pertuzumab treatment. Cardiac toxicity appeared to be reversible, however.
Panel member Deborah Armstrong of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, said that there were “some hints of increased cardiac toxicity.” She encouraged the company to closely evaluate patients on longer-term pertuzumab in trials for cardiac toxicity. Dr. Armstrong voted in favor of the benefit risk profile, but like other panelists, was concerned about the potential for approval “opening the floodgates” to the use of this drug in treating patients for whom it may not yet be appropriate.
Citing the clear potential benefit in the intended population, Dr. Michael Menefee of the division of hematology and oncology at the Mayo Clinic, Jacksonville, Fla., also shared his concerns about the potential for toxicity and overuse of the drug. “My hope is that the FDA is very clear in the ultimate labeling so practitioners have clear guidance as to how to use this drug best and most safely.”
Genentech has completed enrollment in the confirmatory, phase III APHINITY study, which will compare chemotherapy plus trastuzumab with or without pertuzumab before surgery in about 4,800 patients with HER-2 positive early breast cancer. The patients will be followed for 10 years, and the study will evaluate invasive disease-free survival. Results are expected to be first available in 2016.
Panel members have been cleared of potential conflicts of interest related to the topic of the meeting. Occasionally, a panelist may be given a waiver, but not at this meeting.
Genentech also markets trastuzumab.
SILVER SPRING, MD. – Pertuzumab is likely to be approved for the neoadjuvant treatment of breast cancer in the preoperative setting, based on the recommendation of a Food and Drug Administration advisory panel.
At a meeting on Sept. 12, the FDA’s Oncologic Drugs Advisory Committee panel voted 13-0, with 1 abstention, that treatment with pertuzumab, a human epidermal growth factor receptor 2 (HER2)-targeted monoclonal antibody, had a favorable benefit-to-risk profile as a neoadjuvant treatment in combination with trastuzumab and docetaxel before surgery in patients with locally-advanced, inflammatory, or early-stage breast cancers greater than 2 cm in diameter. The neoadjuvant approach would be part of a complete early breast cancer treatment regimen containing fluorouracil, epirubicin, and cyclophosphamide or carboplatin.
The drug is being reviewed under the accelerated approval process, a mechanism that makes drugs available to fill an unmet medical need in patients with serious diseases. Historically, the usual sequence of the FDA approvals for breast cancer agents starts with approval for metastatic disease, followed by approval for early-stage disease years later after the results of large studies with long follow-up periods are available. Pertuzumab, marketed as Perjeta by Genentech, was just approved in 2012 as a first-line treatment for metastatic HER2-positive breast cancer in women who have not received prior anti-HER2 therapy or chemotherapy for metastatic disease.
Accelerated approvals are based on a surrogate endpoint that is considered “reasonably likely” to predict clinical benefit. In this instance, pathologic complete response rate (pCR) was used in the phase II NeoSphere study. For this trial, pertuzumab was added to trastuzumab (Herceptin) – another HER2 receptor antagonist – and docetaxel, and was given before surgery to women with HER-2-positive, locally advanced, inflammatory or early-stage breast cancer. The comparator group was patients treated preoperatively with trastuzumab and docetaxel.
The women who received the three-drug neoadjuvant regimen had an 18% improvement in pCR as compared to women given trastuzumab and docetaxel only.
In addition to the NeoSphere study, the panel also considered the body of data on pertuzumab in metastatic breast cancer, the known biology of the pertuzumab, and the activity of HER-2 targeted therapies in breast cancer.
Should pertuzumab receive the accelerated approval, its full approval will be contingent on the final results of APHINITY, a confirmatory study. If the APHINITY results do not confirm the NeoSphere results, the FDA can withdraw the approval for this indication.
The vote to support the first approval of a drug for the neoadjuvant treatment of breast cancer is “a historic moment,” said the panel chair, Dr. Mikkael A. Sekeres of the Cleveland Clinic. “In so doing, we are supporting the rapid movement of a highly active drug for metastatic breast cancer to the first-line setting with the hope that women with earlier stages of breast cancer will live longer and better.”
However, he added, “all eyes will be on the confirmatory APHINITY trial” and on Genentech, to “verify this initial signal of efficacy and to confirm the bandwidth of safety we have seen so far.” If the results are negative, he and other panelists urged the company to voluntarily remove the drug for this indication, and avoid what happened with another Genentech drug, bevacizumab (Avastin), which was granted an accelerated approval as a first-line treatment in combination with paclitaxel for metastatic breast cancer in 2008. The FDA decided to withdraw approval of this indication after studies failed to confirm the benefit. The company appealed the decision, however, delaying the FDA’s withdrawal of the approval until 2011.
NeoSphere, a randomized study conducted outside of the United States, compared four treatment regimens in 417 women newly diagnosed with locally advanced, inflammatory or operable HER2-positive early breast cancer, with tumors greater than 2 cm, treated for four cycles before surgery. The median tumor size was about 5 cm, and two-thirds were node positive.
Based on the pCR definition used by Genentech (the absence of invasive cancer in the breast), almost 46% of those on the combination of pertuzumab, trastuzumab, and docetaxel reached the primary endpoint, compared with 29% of those on trastuzumab and docetaxel – a statistically significant difference of nearly 17%. Based on the FDA-preferred definition of pCR definition (the absence of invasive cancer in the breast and lymph nodes), the pCR rate was almost 18% higher with the three-drug regimen (39.3% vs. 21.5%).
The FDA considers pCR as “reasonably likely” to predict outcomes in HER2-positive breast cancer.
The treatment has the potential to cure more patients in this high risk population, said Dr. Suparna Wedam, a medical officer in FDA’s Office of Hematology and Oncology Products. Yet, still to be determined are whether the regimen has long-term safety and results in improvements in overall survival, progression-free survival, and other improvements in long-term outcomes.
Genentech also provided results from the TRYPHAENA phase II study that compared three neoadjuvant treatment regimens before surgery; as well as the CLEOPATRA phase III study, the basis of the 2012 approval of trastuzumab. In TRYPHAENA, 225 women with HER2-positive, locally advanced, operable or inflammatory breast cancer, received one of three neoadjuvant treatment regimens. The pCR rates, a secondary endpoint, ranged from about 55% to 64% when pertuzumab was added to trastuzumab and chemotherapy. CLEOPATRA enrolled 808 women with HER2-positive, locally recurrent, unresectable or metastatic breast cancer previously untreated with a biologic or chemotherapy for metastatic disease. In that trial, pertuzumab in combination with trastuzumab and docetaxel resulted in significant improvements in progression-free survival and overall survival.
The most common adverse events with the three-drug regimen in the NeoSphere study were neutropenia, diarrhea, nausea, fatigue, mucosal inflammation, and rash, according to the company. No unexpected safety signals were observed with the addition of pertuzumab. The addition of pertuzumab did not appear to increase symptomatic cardiac toxicity when added to trastuzumab-based neoadjuvant or metastatic treatment regimens.
The FDA reviewers noted, however, that the rate of left ventricular dysfunction (mostly asymptomatic) was higher with neoadjuvant pertuzumab treatment. Cardiac toxicity appeared to be reversible, however.
Panel member Deborah Armstrong of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, said that there were “some hints of increased cardiac toxicity.” She encouraged the company to closely evaluate patients on longer-term pertuzumab in trials for cardiac toxicity. Dr. Armstrong voted in favor of the benefit risk profile, but like other panelists, was concerned about the potential for approval “opening the floodgates” to the use of this drug in treating patients for whom it may not yet be appropriate.
Citing the clear potential benefit in the intended population, Dr. Michael Menefee of the division of hematology and oncology at the Mayo Clinic, Jacksonville, Fla., also shared his concerns about the potential for toxicity and overuse of the drug. “My hope is that the FDA is very clear in the ultimate labeling so practitioners have clear guidance as to how to use this drug best and most safely.”
Genentech has completed enrollment in the confirmatory, phase III APHINITY study, which will compare chemotherapy plus trastuzumab with or without pertuzumab before surgery in about 4,800 patients with HER-2 positive early breast cancer. The patients will be followed for 10 years, and the study will evaluate invasive disease-free survival. Results are expected to be first available in 2016.
Panel members have been cleared of potential conflicts of interest related to the topic of the meeting. Occasionally, a panelist may be given a waiver, but not at this meeting.
Genentech also markets trastuzumab.
SILVER SPRING, MD. – Pertuzumab is likely to be approved for the neoadjuvant treatment of breast cancer in the preoperative setting, based on the recommendation of a Food and Drug Administration advisory panel.
At a meeting on Sept. 12, the FDA’s Oncologic Drugs Advisory Committee panel voted 13-0, with 1 abstention, that treatment with pertuzumab, a human epidermal growth factor receptor 2 (HER2)-targeted monoclonal antibody, had a favorable benefit-to-risk profile as a neoadjuvant treatment in combination with trastuzumab and docetaxel before surgery in patients with locally-advanced, inflammatory, or early-stage breast cancers greater than 2 cm in diameter. The neoadjuvant approach would be part of a complete early breast cancer treatment regimen containing fluorouracil, epirubicin, and cyclophosphamide or carboplatin.
The drug is being reviewed under the accelerated approval process, a mechanism that makes drugs available to fill an unmet medical need in patients with serious diseases. Historically, the usual sequence of the FDA approvals for breast cancer agents starts with approval for metastatic disease, followed by approval for early-stage disease years later after the results of large studies with long follow-up periods are available. Pertuzumab, marketed as Perjeta by Genentech, was just approved in 2012 as a first-line treatment for metastatic HER2-positive breast cancer in women who have not received prior anti-HER2 therapy or chemotherapy for metastatic disease.
Accelerated approvals are based on a surrogate endpoint that is considered “reasonably likely” to predict clinical benefit. In this instance, pathologic complete response rate (pCR) was used in the phase II NeoSphere study. For this trial, pertuzumab was added to trastuzumab (Herceptin) – another HER2 receptor antagonist – and docetaxel, and was given before surgery to women with HER-2-positive, locally advanced, inflammatory or early-stage breast cancer. The comparator group was patients treated preoperatively with trastuzumab and docetaxel.
The women who received the three-drug neoadjuvant regimen had an 18% improvement in pCR as compared to women given trastuzumab and docetaxel only.
In addition to the NeoSphere study, the panel also considered the body of data on pertuzumab in metastatic breast cancer, the known biology of the pertuzumab, and the activity of HER-2 targeted therapies in breast cancer.
Should pertuzumab receive the accelerated approval, its full approval will be contingent on the final results of APHINITY, a confirmatory study. If the APHINITY results do not confirm the NeoSphere results, the FDA can withdraw the approval for this indication.
The vote to support the first approval of a drug for the neoadjuvant treatment of breast cancer is “a historic moment,” said the panel chair, Dr. Mikkael A. Sekeres of the Cleveland Clinic. “In so doing, we are supporting the rapid movement of a highly active drug for metastatic breast cancer to the first-line setting with the hope that women with earlier stages of breast cancer will live longer and better.”
However, he added, “all eyes will be on the confirmatory APHINITY trial” and on Genentech, to “verify this initial signal of efficacy and to confirm the bandwidth of safety we have seen so far.” If the results are negative, he and other panelists urged the company to voluntarily remove the drug for this indication, and avoid what happened with another Genentech drug, bevacizumab (Avastin), which was granted an accelerated approval as a first-line treatment in combination with paclitaxel for metastatic breast cancer in 2008. The FDA decided to withdraw approval of this indication after studies failed to confirm the benefit. The company appealed the decision, however, delaying the FDA’s withdrawal of the approval until 2011.
NeoSphere, a randomized study conducted outside of the United States, compared four treatment regimens in 417 women newly diagnosed with locally advanced, inflammatory or operable HER2-positive early breast cancer, with tumors greater than 2 cm, treated for four cycles before surgery. The median tumor size was about 5 cm, and two-thirds were node positive.
Based on the pCR definition used by Genentech (the absence of invasive cancer in the breast), almost 46% of those on the combination of pertuzumab, trastuzumab, and docetaxel reached the primary endpoint, compared with 29% of those on trastuzumab and docetaxel – a statistically significant difference of nearly 17%. Based on the FDA-preferred definition of pCR definition (the absence of invasive cancer in the breast and lymph nodes), the pCR rate was almost 18% higher with the three-drug regimen (39.3% vs. 21.5%).
The FDA considers pCR as “reasonably likely” to predict outcomes in HER2-positive breast cancer.
The treatment has the potential to cure more patients in this high risk population, said Dr. Suparna Wedam, a medical officer in FDA’s Office of Hematology and Oncology Products. Yet, still to be determined are whether the regimen has long-term safety and results in improvements in overall survival, progression-free survival, and other improvements in long-term outcomes.
Genentech also provided results from the TRYPHAENA phase II study that compared three neoadjuvant treatment regimens before surgery; as well as the CLEOPATRA phase III study, the basis of the 2012 approval of trastuzumab. In TRYPHAENA, 225 women with HER2-positive, locally advanced, operable or inflammatory breast cancer, received one of three neoadjuvant treatment regimens. The pCR rates, a secondary endpoint, ranged from about 55% to 64% when pertuzumab was added to trastuzumab and chemotherapy. CLEOPATRA enrolled 808 women with HER2-positive, locally recurrent, unresectable or metastatic breast cancer previously untreated with a biologic or chemotherapy for metastatic disease. In that trial, pertuzumab in combination with trastuzumab and docetaxel resulted in significant improvements in progression-free survival and overall survival.
The most common adverse events with the three-drug regimen in the NeoSphere study were neutropenia, diarrhea, nausea, fatigue, mucosal inflammation, and rash, according to the company. No unexpected safety signals were observed with the addition of pertuzumab. The addition of pertuzumab did not appear to increase symptomatic cardiac toxicity when added to trastuzumab-based neoadjuvant or metastatic treatment regimens.
The FDA reviewers noted, however, that the rate of left ventricular dysfunction (mostly asymptomatic) was higher with neoadjuvant pertuzumab treatment. Cardiac toxicity appeared to be reversible, however.
Panel member Deborah Armstrong of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, said that there were “some hints of increased cardiac toxicity.” She encouraged the company to closely evaluate patients on longer-term pertuzumab in trials for cardiac toxicity. Dr. Armstrong voted in favor of the benefit risk profile, but like other panelists, was concerned about the potential for approval “opening the floodgates” to the use of this drug in treating patients for whom it may not yet be appropriate.
Citing the clear potential benefit in the intended population, Dr. Michael Menefee of the division of hematology and oncology at the Mayo Clinic, Jacksonville, Fla., also shared his concerns about the potential for toxicity and overuse of the drug. “My hope is that the FDA is very clear in the ultimate labeling so practitioners have clear guidance as to how to use this drug best and most safely.”
Genentech has completed enrollment in the confirmatory, phase III APHINITY study, which will compare chemotherapy plus trastuzumab with or without pertuzumab before surgery in about 4,800 patients with HER-2 positive early breast cancer. The patients will be followed for 10 years, and the study will evaluate invasive disease-free survival. Results are expected to be first available in 2016.
Panel members have been cleared of potential conflicts of interest related to the topic of the meeting. Occasionally, a panelist may be given a waiver, but not at this meeting.
Genentech also markets trastuzumab.
Sen. Cardin hears doctors’ concerns on ACA
On Oct. 1, the Affordable Care Act’s health insurance exchanges will be open for business in all 50 states and the District of Columbia. Some members of Congress have been meeting with constituents to talk about this milestone and what it means to them. Sen. Ben Cardin (D-Md.) recently held an open forum on health reform with physicians and administrators at the Prince George’s Hospital Center in Cheverly, Md.
The hospital is a safety net facility, treating many underinsured and uninsured patients. Sen. Cardin listened to doctors’ concerns about being able to meet their patients’ needs and also let them know that Democrats were aiming to make sure that the law would continue to be implemented.
On Twitter @aliciaault
On Oct. 1, the Affordable Care Act’s health insurance exchanges will be open for business in all 50 states and the District of Columbia. Some members of Congress have been meeting with constituents to talk about this milestone and what it means to them. Sen. Ben Cardin (D-Md.) recently held an open forum on health reform with physicians and administrators at the Prince George’s Hospital Center in Cheverly, Md.
The hospital is a safety net facility, treating many underinsured and uninsured patients. Sen. Cardin listened to doctors’ concerns about being able to meet their patients’ needs and also let them know that Democrats were aiming to make sure that the law would continue to be implemented.
On Twitter @aliciaault
On Oct. 1, the Affordable Care Act’s health insurance exchanges will be open for business in all 50 states and the District of Columbia. Some members of Congress have been meeting with constituents to talk about this milestone and what it means to them. Sen. Ben Cardin (D-Md.) recently held an open forum on health reform with physicians and administrators at the Prince George’s Hospital Center in Cheverly, Md.
The hospital is a safety net facility, treating many underinsured and uninsured patients. Sen. Cardin listened to doctors’ concerns about being able to meet their patients’ needs and also let them know that Democrats were aiming to make sure that the law would continue to be implemented.
On Twitter @aliciaault
Patients with cirrhosis did well with laparoscopic cholecystectomy
Laparoscopic cholecystectomy is a good choice for many patients with liver cirrhosis who need the procedure.
In addition to quickly and effectively addressing the acute illness, laparoscopic cholecystectomy may offer a future advantage, Dr. Vincenzo Neri said at the minimally invasive surgery week annual meeting and endo expo.
"Some cirrhotic patients may be candidates for liver transplantation in the future," said Dr. Neri of the University of Foggia, Italy. "Laparoscopic cholecystectomy offers the chance of fewer right upper quadrant postoperative adhesions" that might complicate later transplant surgery.
He presented a retrospective analysis designed to evaluate the safety and usefulness of a laparoscopic approach in cirrhotic patients undergoing a cholecystectomy. The series comprised 65 patients with hepatic cirrhosis and symptomatic gallstone disease. Of these, six had planned open procedures and the rest laparoscopic procedures. There were 12 conversions to open surgery.
The patients were a mean of 58 years old. More than half had at least two comorbid conditions, including hypertension (14%), cardiac disease (9%), diabetes (12%), respiratory conditions (8%), cerebrovascular disease (4%), and other problems (11%).
Total bilirubin was more than 1 mg/dL in 51% of the group. Albumin was elevated in 61%, and platelets were below 160,000/mcL in 31%. More than a quarter (27%) had a prolonged prothrombin time. About 45% were a Child-Pugh class A, 20% were class B, and the rest were class C.
Cirrhosis was known preoperatively in only 24 patients. The diagnosis was made during the hospital stay in the rest of the patients.
The most common indication for admission and surgery was biliary colic (37%). Other indications included acute cholecystitis (17%), acute biliary pancreatitis (5%), gallbladder and common bile duct stones (5%), and acute cholecystitis with cholangitis (1%). Other indications were not specified.
Of the 12 conversions, 4 were due to acute cholecystitis. Other reasons for conversion were previous laparoscopy (3), acute pancreatitis (2), hypertrophic left hepatic lobe (2), and intraoperative cholangiography (1).
The investigators compared surgical outcomes to those in an unselected control group of 81 patients without cirrhosis who had undergone laparoscopic cholecystectomy.
The mean operative time in the laparoscopic cirrhotic group was 89 minutes – similar to that in the control group (85 minutes). Among the cirrhotic patients, both planned open and converted procedures lasted about the same time (141 and 149 minutes, respectively).
Length of stay was 5 days in the cirrhotic laparoscopy group and 3 in the noncirrhotic control group. Patients with open or converted surgery stayed a mean of 9 and 8 days, respectively.
The blood transfusion rate was 4% in the laparoscopic group, and 17% in both the open and converted groups. Fourteen percent of the laparoscopic group needed transfusion of blood products, compared with 17% of the open group and 33% of the converted group. Transfusions were significantly more common among patients with a Child-Pugh B score, with 26% needing plasma, 21% blood, and 21% platelets. Among Child-Pugh class A patients, 4% needed plasma, 3% blood, and 3% platelets. There were no transfusions in the Child-Pugh class C patients.
Postoperative complications were significantly more common among patients with planned open and converted procedures than total laparoscopies (27% vs. 5%). These included transient ascites (16% vs. 8%) and wound hematoma (8% vs. 4%).
The meeting was presented by the Society of Laparoendoscopic Surgeons and affiliated societies. Dr. Neri had no financial disclosures.
Laparoscopic cholecystectomy is a good choice for many patients with liver cirrhosis who need the procedure.
In addition to quickly and effectively addressing the acute illness, laparoscopic cholecystectomy may offer a future advantage, Dr. Vincenzo Neri said at the minimally invasive surgery week annual meeting and endo expo.
"Some cirrhotic patients may be candidates for liver transplantation in the future," said Dr. Neri of the University of Foggia, Italy. "Laparoscopic cholecystectomy offers the chance of fewer right upper quadrant postoperative adhesions" that might complicate later transplant surgery.
He presented a retrospective analysis designed to evaluate the safety and usefulness of a laparoscopic approach in cirrhotic patients undergoing a cholecystectomy. The series comprised 65 patients with hepatic cirrhosis and symptomatic gallstone disease. Of these, six had planned open procedures and the rest laparoscopic procedures. There were 12 conversions to open surgery.
The patients were a mean of 58 years old. More than half had at least two comorbid conditions, including hypertension (14%), cardiac disease (9%), diabetes (12%), respiratory conditions (8%), cerebrovascular disease (4%), and other problems (11%).
Total bilirubin was more than 1 mg/dL in 51% of the group. Albumin was elevated in 61%, and platelets were below 160,000/mcL in 31%. More than a quarter (27%) had a prolonged prothrombin time. About 45% were a Child-Pugh class A, 20% were class B, and the rest were class C.
Cirrhosis was known preoperatively in only 24 patients. The diagnosis was made during the hospital stay in the rest of the patients.
The most common indication for admission and surgery was biliary colic (37%). Other indications included acute cholecystitis (17%), acute biliary pancreatitis (5%), gallbladder and common bile duct stones (5%), and acute cholecystitis with cholangitis (1%). Other indications were not specified.
Of the 12 conversions, 4 were due to acute cholecystitis. Other reasons for conversion were previous laparoscopy (3), acute pancreatitis (2), hypertrophic left hepatic lobe (2), and intraoperative cholangiography (1).
The investigators compared surgical outcomes to those in an unselected control group of 81 patients without cirrhosis who had undergone laparoscopic cholecystectomy.
The mean operative time in the laparoscopic cirrhotic group was 89 minutes – similar to that in the control group (85 minutes). Among the cirrhotic patients, both planned open and converted procedures lasted about the same time (141 and 149 minutes, respectively).
Length of stay was 5 days in the cirrhotic laparoscopy group and 3 in the noncirrhotic control group. Patients with open or converted surgery stayed a mean of 9 and 8 days, respectively.
The blood transfusion rate was 4% in the laparoscopic group, and 17% in both the open and converted groups. Fourteen percent of the laparoscopic group needed transfusion of blood products, compared with 17% of the open group and 33% of the converted group. Transfusions were significantly more common among patients with a Child-Pugh B score, with 26% needing plasma, 21% blood, and 21% platelets. Among Child-Pugh class A patients, 4% needed plasma, 3% blood, and 3% platelets. There were no transfusions in the Child-Pugh class C patients.
Postoperative complications were significantly more common among patients with planned open and converted procedures than total laparoscopies (27% vs. 5%). These included transient ascites (16% vs. 8%) and wound hematoma (8% vs. 4%).
The meeting was presented by the Society of Laparoendoscopic Surgeons and affiliated societies. Dr. Neri had no financial disclosures.
Laparoscopic cholecystectomy is a good choice for many patients with liver cirrhosis who need the procedure.
In addition to quickly and effectively addressing the acute illness, laparoscopic cholecystectomy may offer a future advantage, Dr. Vincenzo Neri said at the minimally invasive surgery week annual meeting and endo expo.
"Some cirrhotic patients may be candidates for liver transplantation in the future," said Dr. Neri of the University of Foggia, Italy. "Laparoscopic cholecystectomy offers the chance of fewer right upper quadrant postoperative adhesions" that might complicate later transplant surgery.
He presented a retrospective analysis designed to evaluate the safety and usefulness of a laparoscopic approach in cirrhotic patients undergoing a cholecystectomy. The series comprised 65 patients with hepatic cirrhosis and symptomatic gallstone disease. Of these, six had planned open procedures and the rest laparoscopic procedures. There were 12 conversions to open surgery.
The patients were a mean of 58 years old. More than half had at least two comorbid conditions, including hypertension (14%), cardiac disease (9%), diabetes (12%), respiratory conditions (8%), cerebrovascular disease (4%), and other problems (11%).
Total bilirubin was more than 1 mg/dL in 51% of the group. Albumin was elevated in 61%, and platelets were below 160,000/mcL in 31%. More than a quarter (27%) had a prolonged prothrombin time. About 45% were a Child-Pugh class A, 20% were class B, and the rest were class C.
Cirrhosis was known preoperatively in only 24 patients. The diagnosis was made during the hospital stay in the rest of the patients.
The most common indication for admission and surgery was biliary colic (37%). Other indications included acute cholecystitis (17%), acute biliary pancreatitis (5%), gallbladder and common bile duct stones (5%), and acute cholecystitis with cholangitis (1%). Other indications were not specified.
Of the 12 conversions, 4 were due to acute cholecystitis. Other reasons for conversion were previous laparoscopy (3), acute pancreatitis (2), hypertrophic left hepatic lobe (2), and intraoperative cholangiography (1).
The investigators compared surgical outcomes to those in an unselected control group of 81 patients without cirrhosis who had undergone laparoscopic cholecystectomy.
The mean operative time in the laparoscopic cirrhotic group was 89 minutes – similar to that in the control group (85 minutes). Among the cirrhotic patients, both planned open and converted procedures lasted about the same time (141 and 149 minutes, respectively).
Length of stay was 5 days in the cirrhotic laparoscopy group and 3 in the noncirrhotic control group. Patients with open or converted surgery stayed a mean of 9 and 8 days, respectively.
The blood transfusion rate was 4% in the laparoscopic group, and 17% in both the open and converted groups. Fourteen percent of the laparoscopic group needed transfusion of blood products, compared with 17% of the open group and 33% of the converted group. Transfusions were significantly more common among patients with a Child-Pugh B score, with 26% needing plasma, 21% blood, and 21% platelets. Among Child-Pugh class A patients, 4% needed plasma, 3% blood, and 3% platelets. There were no transfusions in the Child-Pugh class C patients.
Postoperative complications were significantly more common among patients with planned open and converted procedures than total laparoscopies (27% vs. 5%). These included transient ascites (16% vs. 8%) and wound hematoma (8% vs. 4%).
The meeting was presented by the Society of Laparoendoscopic Surgeons and affiliated societies. Dr. Neri had no financial disclosures.
FROM MINIMALLY INVASIVE SURGERY WEEK
Major finding: Hepatic cirrhosis patients who underwent a laparoscopic cholecystectomy had surgical outcomes similar to those of patients with normal livers, with mean operative times of 89 and 85 minutes, respectively.
Data source: Retrospective study of 65 patients with hepatic cirrhosis and 81 with normal livers.
Disclosures: The meeting was presented by the Society of Laparoendoscopic Surgeons and affiliated societies. Dr. Neri had no financial disclosures.
MammoSite has comparatively high long-term complication rate
The rate of long-term complications such as palpable masses and telangiectasias was nearly five times higher in women who had MammoSite therapy, compared with those who underwent whole breast radiation therapy, results from a retrospective study showed.
Dr. Kari Rosenkranz and her associates at Dartmouth Hitchcock Medical Center, Hanover, N.H., analyzed the data charts of all women who met criteria for brachytherapy and underwent MammoSite (n = 71) or whole breast radiation therapy (WBRT) (n = 245) at the center between 2003 and 2008.
The incidence of palpable masses at the site of the lumpectomy, telangiectasias, and local recurrence were the studied endpoints. No significant differences existed between the study groups regarding age (average was 63.5 years), mean size of tumor (average was 1.1 cm), the percentage of patients with estrogen receptor–positive tumors (92% in total), or the length of follow-up (median was 4 years).
In the MammoSite cohort with hormone receptor–positive tumors, 83% received adjuvant endocrine therapy; 94% of the WBRT group with hormone receptor–positive tumors had endocrine therapy. No significant difference was found in systemic chemotherapy rates.
The rate of long-term complications such as palpable masses, telangiectasias, or both were found to have occurred in 42% of MammoSite patients, compared with 9% in the WBRT group (J. Am. Coll. Surg. 2013;217:497-502).
During follow-up in the MammoSite group, the incidence rate of palpable mass detection at the lumpectomy site was nearly 27%; for the WBRT group, the rate was approximately 7%. MammoSite patients were three times more likely to require a core biopsy of the mass to rule out malignancy than were WBRT patients (16.9% vs. 4.9%, respectively). Telangiectasia was six times more likely to develop in MammoSite patients than in WBRT patients (24% vs. 4%).
Dr. Rosenkranz and her colleagues reported that a prospective, randomized clinical trial which began in 2005, sponsored by the National Surgical Adjuvant Breast and Bowel Project (NSABP)/Radiation Therapy Oncology Group (RTOG), is currently underway to compare WBRT with partial radiation therapies such as MammoSite. The primary endpoint of this study in women who have had surgery for ductal carcinoma in situ or stage I or stage II breast cancer, is breast tumor recurrence; secondary endpoints include toxicity. The study is expected to end in 2015, according to the researchers.
"Until this prospective, randomized trial reports, the increased rate of long-term local toxicity found in our institution’s experience with MammoSite brachytherapy should be considered when counseling women on options for adjuvant radiation therapy after breast-conserving surgery," concluded Dr. Rosenkranz and her associates.
The researchers reported no relevant disclosures.
The rate of long-term complications such as palpable masses and telangiectasias was nearly five times higher in women who had MammoSite therapy, compared with those who underwent whole breast radiation therapy, results from a retrospective study showed.
Dr. Kari Rosenkranz and her associates at Dartmouth Hitchcock Medical Center, Hanover, N.H., analyzed the data charts of all women who met criteria for brachytherapy and underwent MammoSite (n = 71) or whole breast radiation therapy (WBRT) (n = 245) at the center between 2003 and 2008.
The incidence of palpable masses at the site of the lumpectomy, telangiectasias, and local recurrence were the studied endpoints. No significant differences existed between the study groups regarding age (average was 63.5 years), mean size of tumor (average was 1.1 cm), the percentage of patients with estrogen receptor–positive tumors (92% in total), or the length of follow-up (median was 4 years).
In the MammoSite cohort with hormone receptor–positive tumors, 83% received adjuvant endocrine therapy; 94% of the WBRT group with hormone receptor–positive tumors had endocrine therapy. No significant difference was found in systemic chemotherapy rates.
The rate of long-term complications such as palpable masses, telangiectasias, or both were found to have occurred in 42% of MammoSite patients, compared with 9% in the WBRT group (J. Am. Coll. Surg. 2013;217:497-502).
During follow-up in the MammoSite group, the incidence rate of palpable mass detection at the lumpectomy site was nearly 27%; for the WBRT group, the rate was approximately 7%. MammoSite patients were three times more likely to require a core biopsy of the mass to rule out malignancy than were WBRT patients (16.9% vs. 4.9%, respectively). Telangiectasia was six times more likely to develop in MammoSite patients than in WBRT patients (24% vs. 4%).
Dr. Rosenkranz and her colleagues reported that a prospective, randomized clinical trial which began in 2005, sponsored by the National Surgical Adjuvant Breast and Bowel Project (NSABP)/Radiation Therapy Oncology Group (RTOG), is currently underway to compare WBRT with partial radiation therapies such as MammoSite. The primary endpoint of this study in women who have had surgery for ductal carcinoma in situ or stage I or stage II breast cancer, is breast tumor recurrence; secondary endpoints include toxicity. The study is expected to end in 2015, according to the researchers.
"Until this prospective, randomized trial reports, the increased rate of long-term local toxicity found in our institution’s experience with MammoSite brachytherapy should be considered when counseling women on options for adjuvant radiation therapy after breast-conserving surgery," concluded Dr. Rosenkranz and her associates.
The researchers reported no relevant disclosures.
The rate of long-term complications such as palpable masses and telangiectasias was nearly five times higher in women who had MammoSite therapy, compared with those who underwent whole breast radiation therapy, results from a retrospective study showed.
Dr. Kari Rosenkranz and her associates at Dartmouth Hitchcock Medical Center, Hanover, N.H., analyzed the data charts of all women who met criteria for brachytherapy and underwent MammoSite (n = 71) or whole breast radiation therapy (WBRT) (n = 245) at the center between 2003 and 2008.
The incidence of palpable masses at the site of the lumpectomy, telangiectasias, and local recurrence were the studied endpoints. No significant differences existed between the study groups regarding age (average was 63.5 years), mean size of tumor (average was 1.1 cm), the percentage of patients with estrogen receptor–positive tumors (92% in total), or the length of follow-up (median was 4 years).
In the MammoSite cohort with hormone receptor–positive tumors, 83% received adjuvant endocrine therapy; 94% of the WBRT group with hormone receptor–positive tumors had endocrine therapy. No significant difference was found in systemic chemotherapy rates.
The rate of long-term complications such as palpable masses, telangiectasias, or both were found to have occurred in 42% of MammoSite patients, compared with 9% in the WBRT group (J. Am. Coll. Surg. 2013;217:497-502).
During follow-up in the MammoSite group, the incidence rate of palpable mass detection at the lumpectomy site was nearly 27%; for the WBRT group, the rate was approximately 7%. MammoSite patients were three times more likely to require a core biopsy of the mass to rule out malignancy than were WBRT patients (16.9% vs. 4.9%, respectively). Telangiectasia was six times more likely to develop in MammoSite patients than in WBRT patients (24% vs. 4%).
Dr. Rosenkranz and her colleagues reported that a prospective, randomized clinical trial which began in 2005, sponsored by the National Surgical Adjuvant Breast and Bowel Project (NSABP)/Radiation Therapy Oncology Group (RTOG), is currently underway to compare WBRT with partial radiation therapies such as MammoSite. The primary endpoint of this study in women who have had surgery for ductal carcinoma in situ or stage I or stage II breast cancer, is breast tumor recurrence; secondary endpoints include toxicity. The study is expected to end in 2015, according to the researchers.
"Until this prospective, randomized trial reports, the increased rate of long-term local toxicity found in our institution’s experience with MammoSite brachytherapy should be considered when counseling women on options for adjuvant radiation therapy after breast-conserving surgery," concluded Dr. Rosenkranz and her associates.
The researchers reported no relevant disclosures.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF SURGEONS
Major finding: Forty-two percent of MammoSite patients had long-term complications, vs. 9% of those who had whole breast radiation therapy.
Data source: Retrospective study of 71 women who underwent MammoSite brachytherapy and 245 who had whole breast radiation therapy at a single academic medical center between 2003 and 2008.
Disclosures: Dr. Rosenkranz and her associates reported no relevant disclosures.