User login
Official Newspaper of the American College of Surgeons
Doctors share the wealth with congressional candidates
Doctors and other health professionals are contributing generously to candidates from both parties in this year’s mid-term election, OpenSecrets.org reported.
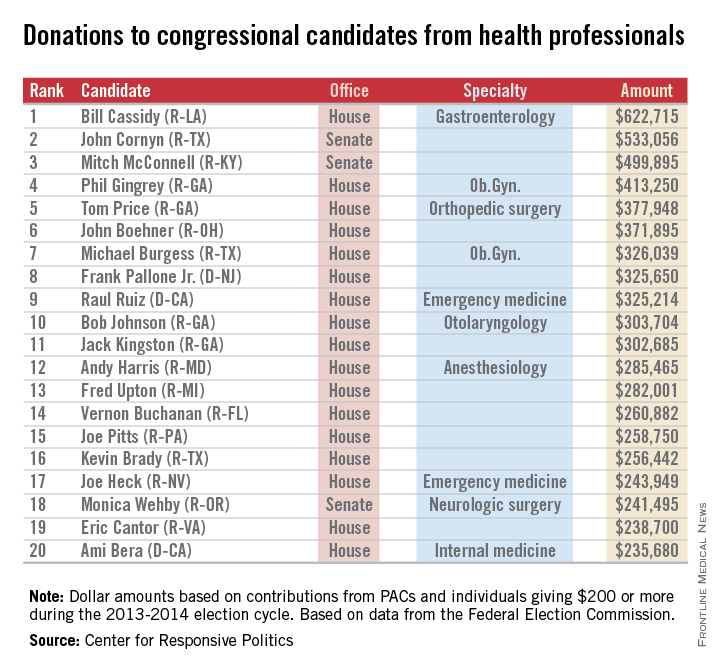
Rep. Bill Cassidy (R-La.), a gastroenterologist, has reaped almost $623,000 from his fellow health professionals – more money than any other candidate in the 2013-2014 election cycle. On the other side of Capitol Hill, Sen. John Cornyn (R-Tex.) has taken in the most in donations, totalling just over $533,000 in this election cycle. Senate Minority Leader Mitch McConnell (R-Ky.) has received just under $500,000, according to the Center for Responsive Politics, which operates OpenSecrets.com.
In the House, Rep. Phil Gingrey (R-Ga.), an ob.gyn., has seen about $413,000 in donations. Ten physician-legislators are included in the top 20 candidates most generously supported by health professionals.
The report from the Center for Responsive Politics is based on data released by the Federal Election Commission on Sept. 8, 2014, and covers contributions of $200 or more from political action committees and individuals.
The health professionals category includes physicians, dentists, nurses, physician assistants, other health professionals, and the political action committees that represent them.

Doctors and other health professionals are contributing generously to candidates from both parties in this year’s mid-term election, OpenSecrets.org reported.
Rep. Bill Cassidy (R-La.), a gastroenterologist, has reaped almost $623,000 from his fellow health professionals – more money than any other candidate in the 2013-2014 election cycle. On the other side of Capitol Hill, Sen. John Cornyn (R-Tex.) has taken in the most in donations, totalling just over $533,000 in this election cycle. Senate Minority Leader Mitch McConnell (R-Ky.) has received just under $500,000, according to the Center for Responsive Politics, which operates OpenSecrets.com.
In the House, Rep. Phil Gingrey (R-Ga.), an ob.gyn., has seen about $413,000 in donations. Ten physician-legislators are included in the top 20 candidates most generously supported by health professionals.
The report from the Center for Responsive Politics is based on data released by the Federal Election Commission on Sept. 8, 2014, and covers contributions of $200 or more from political action committees and individuals.
The health professionals category includes physicians, dentists, nurses, physician assistants, other health professionals, and the political action committees that represent them.

Doctors and other health professionals are contributing generously to candidates from both parties in this year’s mid-term election, OpenSecrets.org reported.
Rep. Bill Cassidy (R-La.), a gastroenterologist, has reaped almost $623,000 from his fellow health professionals – more money than any other candidate in the 2013-2014 election cycle. On the other side of Capitol Hill, Sen. John Cornyn (R-Tex.) has taken in the most in donations, totalling just over $533,000 in this election cycle. Senate Minority Leader Mitch McConnell (R-Ky.) has received just under $500,000, according to the Center for Responsive Politics, which operates OpenSecrets.com.
In the House, Rep. Phil Gingrey (R-Ga.), an ob.gyn., has seen about $413,000 in donations. Ten physician-legislators are included in the top 20 candidates most generously supported by health professionals.
The report from the Center for Responsive Politics is based on data released by the Federal Election Commission on Sept. 8, 2014, and covers contributions of $200 or more from political action committees and individuals.
The health professionals category includes physicians, dentists, nurses, physician assistants, other health professionals, and the political action committees that represent them.

Antimicrobial use varies across hospital units
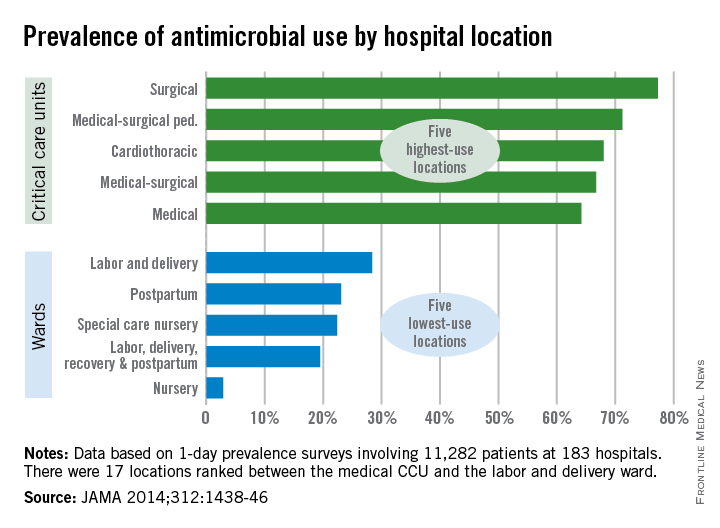
Patients in surgical critical care units were most likely to receive antimicrobial medication, while those in nursery wards were least likely to receive it, according to a recent study.
Nearly 78% of patients who used a surgical critical care unit (CCU) received antimicrobials in 2011, with 71% of patients in medical-surgical pediatric CCUs receiving antimicrobials and patients in cardiothoracic CCUs receiving antimicrobials at a 68% rate, according to Dr. Shelley S. Magill of the Centers for Disease Control and Prevention, Atlanta, and her associates (JAMA 2014;312:1438-46).
With only 3% of patients receiving antimicrobials, nursery wards had the lowest rate of medication, with labor, delivery, recovery, and postpartum wards giving antimicrobials to 20% of patients and 22% of patients receiving antimicrobials in special care nurseries, they reported.
Of the 5,635 patients who received antimicrobials, 4,278 received them for the treatment of infection. Lower respiratory tract infections were the most commonly treated type of infection, with 35% of patients receiving antimicrobials. Urinary tract and skin and soft tissue infections also were commonly treated with antimicrobials at a rate of 22% and 16%, respectively.
Overall (including prophylaxis,noninfection-related reasons, and undocumented rationale), vancomycin was the most commonly received antimicrobial, followed by cefazolin and ceftriaxone, according to Dr. Magill and her associates.
The study used data collected during 1-day prevalence surveys at 183 U.S. acute care hospitals and involving 11,282 patients.

Patients in surgical critical care units were most likely to receive antimicrobial medication, while those in nursery wards were least likely to receive it, according to a recent study.
Nearly 78% of patients who used a surgical critical care unit (CCU) received antimicrobials in 2011, with 71% of patients in medical-surgical pediatric CCUs receiving antimicrobials and patients in cardiothoracic CCUs receiving antimicrobials at a 68% rate, according to Dr. Shelley S. Magill of the Centers for Disease Control and Prevention, Atlanta, and her associates (JAMA 2014;312:1438-46).
With only 3% of patients receiving antimicrobials, nursery wards had the lowest rate of medication, with labor, delivery, recovery, and postpartum wards giving antimicrobials to 20% of patients and 22% of patients receiving antimicrobials in special care nurseries, they reported.
Of the 5,635 patients who received antimicrobials, 4,278 received them for the treatment of infection. Lower respiratory tract infections were the most commonly treated type of infection, with 35% of patients receiving antimicrobials. Urinary tract and skin and soft tissue infections also were commonly treated with antimicrobials at a rate of 22% and 16%, respectively.
Overall (including prophylaxis,noninfection-related reasons, and undocumented rationale), vancomycin was the most commonly received antimicrobial, followed by cefazolin and ceftriaxone, according to Dr. Magill and her associates.
The study used data collected during 1-day prevalence surveys at 183 U.S. acute care hospitals and involving 11,282 patients.

Patients in surgical critical care units were most likely to receive antimicrobial medication, while those in nursery wards were least likely to receive it, according to a recent study.
Nearly 78% of patients who used a surgical critical care unit (CCU) received antimicrobials in 2011, with 71% of patients in medical-surgical pediatric CCUs receiving antimicrobials and patients in cardiothoracic CCUs receiving antimicrobials at a 68% rate, according to Dr. Shelley S. Magill of the Centers for Disease Control and Prevention, Atlanta, and her associates (JAMA 2014;312:1438-46).
With only 3% of patients receiving antimicrobials, nursery wards had the lowest rate of medication, with labor, delivery, recovery, and postpartum wards giving antimicrobials to 20% of patients and 22% of patients receiving antimicrobials in special care nurseries, they reported.
Of the 5,635 patients who received antimicrobials, 4,278 received them for the treatment of infection. Lower respiratory tract infections were the most commonly treated type of infection, with 35% of patients receiving antimicrobials. Urinary tract and skin and soft tissue infections also were commonly treated with antimicrobials at a rate of 22% and 16%, respectively.
Overall (including prophylaxis,noninfection-related reasons, and undocumented rationale), vancomycin was the most commonly received antimicrobial, followed by cefazolin and ceftriaxone, according to Dr. Magill and her associates.
The study used data collected during 1-day prevalence surveys at 183 U.S. acute care hospitals and involving 11,282 patients.

FROM JAMA
Industry payments to docs, hospitals top $4.5 billion
The drug and device industry made about $4.6 billion in payments to physicians and teaching hospitals in the last 5 months of 2013, according to updated data from the government.
The Centers for Medicare & Medicaid Services (CMS) recently released a massive data set detailing payments from industry to health care providers as part of the new Open Payments program, a transparency initiative mandated under the Affordable Care Act. In the initial data release on Sept. 30, the agency said that industry payments totaled $3.5 billion across 4.4 million published payment records. But updated figures, released by the agency Oct. 6, show that the industry spent an additional $1.1 billion for 199,000 payment records that have yet to be published.
The CMS held off on publishing payment data for two reasons: Either there was an unresolved dispute at the end of the 45-day review period, or the manufacturer requested a delay in publication because the payments were related to a drug or device that was still under development.
The bulk of the unpublished payment data – about 190,000 records – falls into the “delay in publication” category. In total, manufacturers made $551 million in payments related to products in development. Under the regulations governing the Open Payments program, the delay cannot exceed 4 years or the date when the product is approved by the Food and Drug Administration.
The rest of the unpublished payment data relate to about 9,000 payments totaling $514 million that are still under active dispute. About 91% of the total payments in question are associated with only 40 disputes, according to the CMS.
The next round of Open Payments data, which will include payments from all of 2014, will be published in June 2015.
On Twitter @maryellenny
The drug and device industry made about $4.6 billion in payments to physicians and teaching hospitals in the last 5 months of 2013, according to updated data from the government.
The Centers for Medicare & Medicaid Services (CMS) recently released a massive data set detailing payments from industry to health care providers as part of the new Open Payments program, a transparency initiative mandated under the Affordable Care Act. In the initial data release on Sept. 30, the agency said that industry payments totaled $3.5 billion across 4.4 million published payment records. But updated figures, released by the agency Oct. 6, show that the industry spent an additional $1.1 billion for 199,000 payment records that have yet to be published.
The CMS held off on publishing payment data for two reasons: Either there was an unresolved dispute at the end of the 45-day review period, or the manufacturer requested a delay in publication because the payments were related to a drug or device that was still under development.
The bulk of the unpublished payment data – about 190,000 records – falls into the “delay in publication” category. In total, manufacturers made $551 million in payments related to products in development. Under the regulations governing the Open Payments program, the delay cannot exceed 4 years or the date when the product is approved by the Food and Drug Administration.
The rest of the unpublished payment data relate to about 9,000 payments totaling $514 million that are still under active dispute. About 91% of the total payments in question are associated with only 40 disputes, according to the CMS.
The next round of Open Payments data, which will include payments from all of 2014, will be published in June 2015.
On Twitter @maryellenny
The drug and device industry made about $4.6 billion in payments to physicians and teaching hospitals in the last 5 months of 2013, according to updated data from the government.
The Centers for Medicare & Medicaid Services (CMS) recently released a massive data set detailing payments from industry to health care providers as part of the new Open Payments program, a transparency initiative mandated under the Affordable Care Act. In the initial data release on Sept. 30, the agency said that industry payments totaled $3.5 billion across 4.4 million published payment records. But updated figures, released by the agency Oct. 6, show that the industry spent an additional $1.1 billion for 199,000 payment records that have yet to be published.
The CMS held off on publishing payment data for two reasons: Either there was an unresolved dispute at the end of the 45-day review period, or the manufacturer requested a delay in publication because the payments were related to a drug or device that was still under development.
The bulk of the unpublished payment data – about 190,000 records – falls into the “delay in publication” category. In total, manufacturers made $551 million in payments related to products in development. Under the regulations governing the Open Payments program, the delay cannot exceed 4 years or the date when the product is approved by the Food and Drug Administration.
The rest of the unpublished payment data relate to about 9,000 payments totaling $514 million that are still under active dispute. About 91% of the total payments in question are associated with only 40 disputes, according to the CMS.
The next round of Open Payments data, which will include payments from all of 2014, will be published in June 2015.
On Twitter @maryellenny
Some docs get another chance at 'meaningful use' hardship exemption
Doctors and hospitals are getting another chance to apply for relief from the federal EHR meaningful use program and avoid penalties, officials at the Centers for Medicare & Medicaid Services announced.
The agency is reopening the application period for hardship exemptions, specifically for physicians and hospitals that are attesting to their meaningful use of electronic health records (EHRs) for the first time, and who have been using older technology.
The new deadline for hardship applications is 11:59 p.m. EST on Nov. 30.
The deadline to apply for a hardship exemption originally closed on April 1 for hospitals and July 1 for physicians. But the Centers for Medicare & Medicaid Services (CMS) reopened the process after it became clear that a subset of physicians who were attesting to meaningful use for the first time could see a 1% penalty due to a government website problem.
At the center of the problem is a backlog in the availability of newly certified EHR products. Knowing that many physicians and hospitals would be unable to attest to using newly certified products in time for this year’s meaningful use deadline, the CMS proposed in May to allow them to attest to using older technology during 2014.
The final rule, released in August, gives physicians the flexibility to use either a 2011 certified product, a newer 2014 certified product, or a combination of both, without being penalized. But the website where physicians must apply for the flexibility was not ready in time for the Oct. 1 attestation deadline.
The mismatch in deadlines raised the ire of physician groups and members of Congress, who called on the CMS to find some pathway for physicians to avoid penalties earned through no fault of their own.
On Oct. 7, the CMS announced that it would reopen the hardship application period for physicians and hospitals that had been unable to fully implement 2014 certified EHRs due to delays in their availability, as well as those who were unable to attest to meaningful use by the deadline and were using the flexibility options outlined by the CMS.
The announcement was praised by the American Medical Association. AMA President Robert M. Wah said the change will allow more physicians to avoid an “unfair” penalty in 2015.
“Giving physicians more time to file for a hardship exemption provides necessary relief as many physicians are struggling to meet a number of reporting mandates to avoid multiple penalties,” Dr. Wah said in a statement.
On Twitter @maryellenny
Doctors and hospitals are getting another chance to apply for relief from the federal EHR meaningful use program and avoid penalties, officials at the Centers for Medicare & Medicaid Services announced.
The agency is reopening the application period for hardship exemptions, specifically for physicians and hospitals that are attesting to their meaningful use of electronic health records (EHRs) for the first time, and who have been using older technology.
The new deadline for hardship applications is 11:59 p.m. EST on Nov. 30.
The deadline to apply for a hardship exemption originally closed on April 1 for hospitals and July 1 for physicians. But the Centers for Medicare & Medicaid Services (CMS) reopened the process after it became clear that a subset of physicians who were attesting to meaningful use for the first time could see a 1% penalty due to a government website problem.
At the center of the problem is a backlog in the availability of newly certified EHR products. Knowing that many physicians and hospitals would be unable to attest to using newly certified products in time for this year’s meaningful use deadline, the CMS proposed in May to allow them to attest to using older technology during 2014.
The final rule, released in August, gives physicians the flexibility to use either a 2011 certified product, a newer 2014 certified product, or a combination of both, without being penalized. But the website where physicians must apply for the flexibility was not ready in time for the Oct. 1 attestation deadline.
The mismatch in deadlines raised the ire of physician groups and members of Congress, who called on the CMS to find some pathway for physicians to avoid penalties earned through no fault of their own.
On Oct. 7, the CMS announced that it would reopen the hardship application period for physicians and hospitals that had been unable to fully implement 2014 certified EHRs due to delays in their availability, as well as those who were unable to attest to meaningful use by the deadline and were using the flexibility options outlined by the CMS.
The announcement was praised by the American Medical Association. AMA President Robert M. Wah said the change will allow more physicians to avoid an “unfair” penalty in 2015.
“Giving physicians more time to file for a hardship exemption provides necessary relief as many physicians are struggling to meet a number of reporting mandates to avoid multiple penalties,” Dr. Wah said in a statement.
On Twitter @maryellenny
Doctors and hospitals are getting another chance to apply for relief from the federal EHR meaningful use program and avoid penalties, officials at the Centers for Medicare & Medicaid Services announced.
The agency is reopening the application period for hardship exemptions, specifically for physicians and hospitals that are attesting to their meaningful use of electronic health records (EHRs) for the first time, and who have been using older technology.
The new deadline for hardship applications is 11:59 p.m. EST on Nov. 30.
The deadline to apply for a hardship exemption originally closed on April 1 for hospitals and July 1 for physicians. But the Centers for Medicare & Medicaid Services (CMS) reopened the process after it became clear that a subset of physicians who were attesting to meaningful use for the first time could see a 1% penalty due to a government website problem.
At the center of the problem is a backlog in the availability of newly certified EHR products. Knowing that many physicians and hospitals would be unable to attest to using newly certified products in time for this year’s meaningful use deadline, the CMS proposed in May to allow them to attest to using older technology during 2014.
The final rule, released in August, gives physicians the flexibility to use either a 2011 certified product, a newer 2014 certified product, or a combination of both, without being penalized. But the website where physicians must apply for the flexibility was not ready in time for the Oct. 1 attestation deadline.
The mismatch in deadlines raised the ire of physician groups and members of Congress, who called on the CMS to find some pathway for physicians to avoid penalties earned through no fault of their own.
On Oct. 7, the CMS announced that it would reopen the hardship application period for physicians and hospitals that had been unable to fully implement 2014 certified EHRs due to delays in their availability, as well as those who were unable to attest to meaningful use by the deadline and were using the flexibility options outlined by the CMS.
The announcement was praised by the American Medical Association. AMA President Robert M. Wah said the change will allow more physicians to avoid an “unfair” penalty in 2015.
“Giving physicians more time to file for a hardship exemption provides necessary relief as many physicians are struggling to meet a number of reporting mandates to avoid multiple penalties,” Dr. Wah said in a statement.
On Twitter @maryellenny
Backlog takes toll on physicians who appeal audits
After being audited by Medicare and hit with a $150,000 recoupment demand, California primary care physician Robert E. Feiss was determined to fight the findings.
Auditors had declared that Dr. Feiss was providing care to patients in their homes that did not qualify as being medically necessary. He appealed, and after 3 long years of paperwork, petitions, and hearings, the doctor was successful in overturning the decision.
“It was a scary thing, without a doubt,” said Dr. Feiss, who is in private practice in Ventura, Calif., and treats a large population of seniors in their homes. “But I felt very strongly about what I do and am committed to what I do. It’s a bittersweet victory because, aside from the incredible stress it took, there was no recovery of legal fees. There was no recovery of damages or of the time spent away from my practice.”
Dr. Feiss’s experience is not unique. He is one of thousands of health providers who decide to appeal their audits by the federal government. Win or lose, audit experts say the course of an audit appeal is often long and arduous. Along with financial uncertainty and legal expenses, physicians face substantial delays before their case can be resolved. The wait time for an appeal to be assigned to an administrative law judge is about 2 years, according to a December 2013 letter from the Office of Medicare Hearings and Appeals (OMHA). After assignment, it’s another 6 months before the case is heard.
The wait times reflect OMHA’s enormous backlog of appeals. In fiscal 2013, OMHA received 384,151 new appeals, up from 59,600 in 2011, according to July testimony before Congress by Nancy J. Griswold, OMHA chief administrative law judge. Fiscal 2014 appeals through July 1 totaled 509,124. More than 800,000 OMHA appeals were pending as of July, and the office continues to receive 1 year’s worth of appeals every 4 to 6 weeks, Judge Griswold said. In July a coalition of medical associations, including the American Medical Association, wrote a letter to OMHA urging the agency to immediately remedy the excessive surplus.
“The backlog is a huge problem,” said Jessica L. Gustafson, a Southfield, Mich., health law attorney and vice chair of the American Bar Association Health Law Section’s Physician Issues Interest Group. “One major implication of the backlog is a cash flow interruption to a physician’s practice.”
Ms. Gustafson noted that under federal law, the Centers for Medicare & Medicaid Services is prohibited from recouping an alleged overpayment during the first two stages of an appeal. However, following a reconsideration decision, the CMS is authorized to recoup an alleged overpayment and withhold future payments as an offset to the alleged overpayment. Because of the backlog, the CMS can hold onto a physician’s money for years, until a final determination is made, she said.
In Dr. Feiss’s case, the CMS withheld his Medicare payments during the appeals process, he said. He hired Ms. Gustafson, who assisted him through three rounds of appeals before the case was assigned to an administrative law judge. The process was fraught with unexpected costs, such as hiring an accountant and paying for expert witnesses, he said.
“It was a very stressful period in my life,” Dr. Feiss said in an interview. “It’s almost like having two jobs. I was trying to maintain my practice, but I was trying to compile all this information that was required by Medicare and educate myself in terms of the terminology and definitions of Medicare law to understand their vernacular.”
While the lengthy process of an audit appeal may sound off-putting, experts stress that a large portion of physician appeals are successful. In 2013, 60% of Medicare Part B claims appealed were overturned in providers’ favor, according to a September report released by the CMS. In contrast, only 11% of Medicare Part A claims were overturned on appeal. Of Part B appeals associated with overpayments, about 9% of determinations were overturned in 2013.
For physicians deciding whether to appeal an audit, key considerations include whether their argument against the recoupment is sound and if they have sufficient documentation, said Michael E. Clark, a health law attorney in Houston, speaking in an interview.
“Physicians should assess whether they have meritorious grounds for an appeal before doing so because if the Qualified Independent Contractor renders an adverse decision then they will be required to pay interest on the overpayments in addition to the amount of the overpayment,” said Mr. Clark, who is chair of the American Bar Association’s Health Law Section. “Some basic issues are whether the underlying documentation is complete and supports the claims for reimbursement that have been made and whether the coding used is appropriate.”
Physicians should also consider the number of claims at issue and the amount of payment before deciding whether to pursue the appeal, Ms. Gustafson adds.
“In many physician cases, the amount at issue may not be worth the time associated with pursuing the appeal,” she said.
On the other hand, appealing is the only way to stop a recoupment demand, said Dr. Brent Moody, a member of the American Academy of Dermatology’s Workgroup on Innovation Payment Delivery and an American Academy of Dermatology Association adviser to the American Medical Association’s Relative Value Scale Update Committee (RUC).
“If a provider thinks they’re in the right, they should appeal,” Dr. Moody said in an interview. “It’s their right to appeal. They should exercise that right if they believe the RAC audit is incorrect.”
The government meanwhile, is taking steps to reduce the appeals backlog. Earlier this year, CMS offered to pay hospitals 68% of inpatient-status claims in exchange for withdrawing their pending appeals. If hospitals accept the offer, the move would free up the system for other pending claims, Ms. Gustafson said.
Mr. Clark does not foresee the appeals bottleneck truly loosening until Congress funds additional administrative law judge positions, he said.
“There’s a well known saying that well describes this problem: ‘Justice delayed is justice denied,’ ” Mr. Clark said. “The specter of having to wait months or years before an appeal can be heard is simply inexcusable given the intrusive nature of the RAC audits, the uncertainties involved, and the impact on the ability of physicians to practice medicine without fear of financial ruin.”
On Twitter @legal_med
After being audited by Medicare and hit with a $150,000 recoupment demand, California primary care physician Robert E. Feiss was determined to fight the findings.
Auditors had declared that Dr. Feiss was providing care to patients in their homes that did not qualify as being medically necessary. He appealed, and after 3 long years of paperwork, petitions, and hearings, the doctor was successful in overturning the decision.
“It was a scary thing, without a doubt,” said Dr. Feiss, who is in private practice in Ventura, Calif., and treats a large population of seniors in their homes. “But I felt very strongly about what I do and am committed to what I do. It’s a bittersweet victory because, aside from the incredible stress it took, there was no recovery of legal fees. There was no recovery of damages or of the time spent away from my practice.”
Dr. Feiss’s experience is not unique. He is one of thousands of health providers who decide to appeal their audits by the federal government. Win or lose, audit experts say the course of an audit appeal is often long and arduous. Along with financial uncertainty and legal expenses, physicians face substantial delays before their case can be resolved. The wait time for an appeal to be assigned to an administrative law judge is about 2 years, according to a December 2013 letter from the Office of Medicare Hearings and Appeals (OMHA). After assignment, it’s another 6 months before the case is heard.
The wait times reflect OMHA’s enormous backlog of appeals. In fiscal 2013, OMHA received 384,151 new appeals, up from 59,600 in 2011, according to July testimony before Congress by Nancy J. Griswold, OMHA chief administrative law judge. Fiscal 2014 appeals through July 1 totaled 509,124. More than 800,000 OMHA appeals were pending as of July, and the office continues to receive 1 year’s worth of appeals every 4 to 6 weeks, Judge Griswold said. In July a coalition of medical associations, including the American Medical Association, wrote a letter to OMHA urging the agency to immediately remedy the excessive surplus.
“The backlog is a huge problem,” said Jessica L. Gustafson, a Southfield, Mich., health law attorney and vice chair of the American Bar Association Health Law Section’s Physician Issues Interest Group. “One major implication of the backlog is a cash flow interruption to a physician’s practice.”
Ms. Gustafson noted that under federal law, the Centers for Medicare & Medicaid Services is prohibited from recouping an alleged overpayment during the first two stages of an appeal. However, following a reconsideration decision, the CMS is authorized to recoup an alleged overpayment and withhold future payments as an offset to the alleged overpayment. Because of the backlog, the CMS can hold onto a physician’s money for years, until a final determination is made, she said.
In Dr. Feiss’s case, the CMS withheld his Medicare payments during the appeals process, he said. He hired Ms. Gustafson, who assisted him through three rounds of appeals before the case was assigned to an administrative law judge. The process was fraught with unexpected costs, such as hiring an accountant and paying for expert witnesses, he said.
“It was a very stressful period in my life,” Dr. Feiss said in an interview. “It’s almost like having two jobs. I was trying to maintain my practice, but I was trying to compile all this information that was required by Medicare and educate myself in terms of the terminology and definitions of Medicare law to understand their vernacular.”
While the lengthy process of an audit appeal may sound off-putting, experts stress that a large portion of physician appeals are successful. In 2013, 60% of Medicare Part B claims appealed were overturned in providers’ favor, according to a September report released by the CMS. In contrast, only 11% of Medicare Part A claims were overturned on appeal. Of Part B appeals associated with overpayments, about 9% of determinations were overturned in 2013.
For physicians deciding whether to appeal an audit, key considerations include whether their argument against the recoupment is sound and if they have sufficient documentation, said Michael E. Clark, a health law attorney in Houston, speaking in an interview.
“Physicians should assess whether they have meritorious grounds for an appeal before doing so because if the Qualified Independent Contractor renders an adverse decision then they will be required to pay interest on the overpayments in addition to the amount of the overpayment,” said Mr. Clark, who is chair of the American Bar Association’s Health Law Section. “Some basic issues are whether the underlying documentation is complete and supports the claims for reimbursement that have been made and whether the coding used is appropriate.”
Physicians should also consider the number of claims at issue and the amount of payment before deciding whether to pursue the appeal, Ms. Gustafson adds.

“In many physician cases, the amount at issue may not be worth the time associated with pursuing the appeal,” she said.
On the other hand, appealing is the only way to stop a recoupment demand, said Dr. Brent Moody, a member of the American Academy of Dermatology’s Workgroup on Innovation Payment Delivery and an American Academy of Dermatology Association adviser to the American Medical Association’s Relative Value Scale Update Committee (RUC).
“If a provider thinks they’re in the right, they should appeal,” Dr. Moody said in an interview. “It’s their right to appeal. They should exercise that right if they believe the RAC audit is incorrect.”
The government meanwhile, is taking steps to reduce the appeals backlog. Earlier this year, CMS offered to pay hospitals 68% of inpatient-status claims in exchange for withdrawing their pending appeals. If hospitals accept the offer, the move would free up the system for other pending claims, Ms. Gustafson said.
Mr. Clark does not foresee the appeals bottleneck truly loosening until Congress funds additional administrative law judge positions, he said.
“There’s a well known saying that well describes this problem: ‘Justice delayed is justice denied,’ ” Mr. Clark said. “The specter of having to wait months or years before an appeal can be heard is simply inexcusable given the intrusive nature of the RAC audits, the uncertainties involved, and the impact on the ability of physicians to practice medicine without fear of financial ruin.”
On Twitter @legal_med
After being audited by Medicare and hit with a $150,000 recoupment demand, California primary care physician Robert E. Feiss was determined to fight the findings.
Auditors had declared that Dr. Feiss was providing care to patients in their homes that did not qualify as being medically necessary. He appealed, and after 3 long years of paperwork, petitions, and hearings, the doctor was successful in overturning the decision.
“It was a scary thing, without a doubt,” said Dr. Feiss, who is in private practice in Ventura, Calif., and treats a large population of seniors in their homes. “But I felt very strongly about what I do and am committed to what I do. It’s a bittersweet victory because, aside from the incredible stress it took, there was no recovery of legal fees. There was no recovery of damages or of the time spent away from my practice.”
Dr. Feiss’s experience is not unique. He is one of thousands of health providers who decide to appeal their audits by the federal government. Win or lose, audit experts say the course of an audit appeal is often long and arduous. Along with financial uncertainty and legal expenses, physicians face substantial delays before their case can be resolved. The wait time for an appeal to be assigned to an administrative law judge is about 2 years, according to a December 2013 letter from the Office of Medicare Hearings and Appeals (OMHA). After assignment, it’s another 6 months before the case is heard.
The wait times reflect OMHA’s enormous backlog of appeals. In fiscal 2013, OMHA received 384,151 new appeals, up from 59,600 in 2011, according to July testimony before Congress by Nancy J. Griswold, OMHA chief administrative law judge. Fiscal 2014 appeals through July 1 totaled 509,124. More than 800,000 OMHA appeals were pending as of July, and the office continues to receive 1 year’s worth of appeals every 4 to 6 weeks, Judge Griswold said. In July a coalition of medical associations, including the American Medical Association, wrote a letter to OMHA urging the agency to immediately remedy the excessive surplus.
“The backlog is a huge problem,” said Jessica L. Gustafson, a Southfield, Mich., health law attorney and vice chair of the American Bar Association Health Law Section’s Physician Issues Interest Group. “One major implication of the backlog is a cash flow interruption to a physician’s practice.”
Ms. Gustafson noted that under federal law, the Centers for Medicare & Medicaid Services is prohibited from recouping an alleged overpayment during the first two stages of an appeal. However, following a reconsideration decision, the CMS is authorized to recoup an alleged overpayment and withhold future payments as an offset to the alleged overpayment. Because of the backlog, the CMS can hold onto a physician’s money for years, until a final determination is made, she said.
In Dr. Feiss’s case, the CMS withheld his Medicare payments during the appeals process, he said. He hired Ms. Gustafson, who assisted him through three rounds of appeals before the case was assigned to an administrative law judge. The process was fraught with unexpected costs, such as hiring an accountant and paying for expert witnesses, he said.
“It was a very stressful period in my life,” Dr. Feiss said in an interview. “It’s almost like having two jobs. I was trying to maintain my practice, but I was trying to compile all this information that was required by Medicare and educate myself in terms of the terminology and definitions of Medicare law to understand their vernacular.”
While the lengthy process of an audit appeal may sound off-putting, experts stress that a large portion of physician appeals are successful. In 2013, 60% of Medicare Part B claims appealed were overturned in providers’ favor, according to a September report released by the CMS. In contrast, only 11% of Medicare Part A claims were overturned on appeal. Of Part B appeals associated with overpayments, about 9% of determinations were overturned in 2013.
For physicians deciding whether to appeal an audit, key considerations include whether their argument against the recoupment is sound and if they have sufficient documentation, said Michael E. Clark, a health law attorney in Houston, speaking in an interview.
“Physicians should assess whether they have meritorious grounds for an appeal before doing so because if the Qualified Independent Contractor renders an adverse decision then they will be required to pay interest on the overpayments in addition to the amount of the overpayment,” said Mr. Clark, who is chair of the American Bar Association’s Health Law Section. “Some basic issues are whether the underlying documentation is complete and supports the claims for reimbursement that have been made and whether the coding used is appropriate.”
Physicians should also consider the number of claims at issue and the amount of payment before deciding whether to pursue the appeal, Ms. Gustafson adds.

“In many physician cases, the amount at issue may not be worth the time associated with pursuing the appeal,” she said.
On the other hand, appealing is the only way to stop a recoupment demand, said Dr. Brent Moody, a member of the American Academy of Dermatology’s Workgroup on Innovation Payment Delivery and an American Academy of Dermatology Association adviser to the American Medical Association’s Relative Value Scale Update Committee (RUC).
“If a provider thinks they’re in the right, they should appeal,” Dr. Moody said in an interview. “It’s their right to appeal. They should exercise that right if they believe the RAC audit is incorrect.”
The government meanwhile, is taking steps to reduce the appeals backlog. Earlier this year, CMS offered to pay hospitals 68% of inpatient-status claims in exchange for withdrawing their pending appeals. If hospitals accept the offer, the move would free up the system for other pending claims, Ms. Gustafson said.
Mr. Clark does not foresee the appeals bottleneck truly loosening until Congress funds additional administrative law judge positions, he said.
“There’s a well known saying that well describes this problem: ‘Justice delayed is justice denied,’ ” Mr. Clark said. “The specter of having to wait months or years before an appeal can be heard is simply inexcusable given the intrusive nature of the RAC audits, the uncertainties involved, and the impact on the ability of physicians to practice medicine without fear of financial ruin.”
On Twitter @legal_med
When to use mesh in laparoscopic hiatal hernia repair
LAS VEGAS – Routine use of mesh reinforcement when performing laparoscopic repair of hiatal hernia defects 5 cm or larger in diameter is associated with a low recurrence rate, Dr. Chetan V. Aher reported at the annual Minimally Invasive Surgery Week.
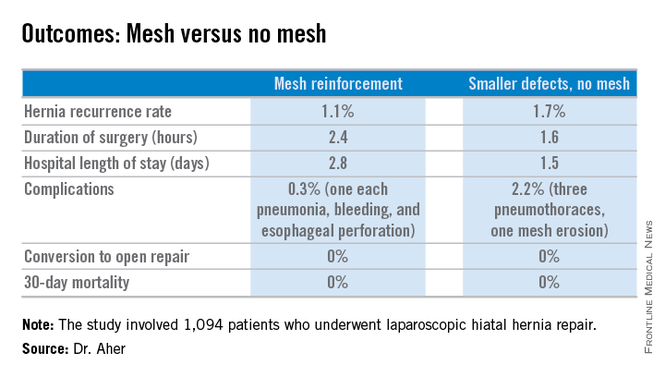
His coinvestigators had shown in an earlier randomized controlled trial that mesh reinforcement of primary cruroplasty in patients with a hernia of 8 cm or greater was associated with no recurrences. Repair with simple cruroplasty was associated with a 22% recurrence rate (Arch. Surg. 2002;137:649-52).
However, Dr. Aher and his coinvestigators subsequently observed a high recurrence rate following mesh-free simple cruroplasty for defects in the 5- to 8-cm range. He presented a case series involving 1,094 laparoscopic hiatal hernia repairs performed since he and his colleagues changed their practice by lowering their threshold for polytetrafluoroethylene mesh reinforcement to defects of at least 5 cm from their prior standard of 8 cm or more.
Hernias were less than 5 cm in diameter in 84% of the patients, so mesh wasn’t used for those repairs. In the remaining 178 patients – those with hernias of at least 5 cm – PTFE mesh was utilized to circumferentially reinforce the cruroplasty.
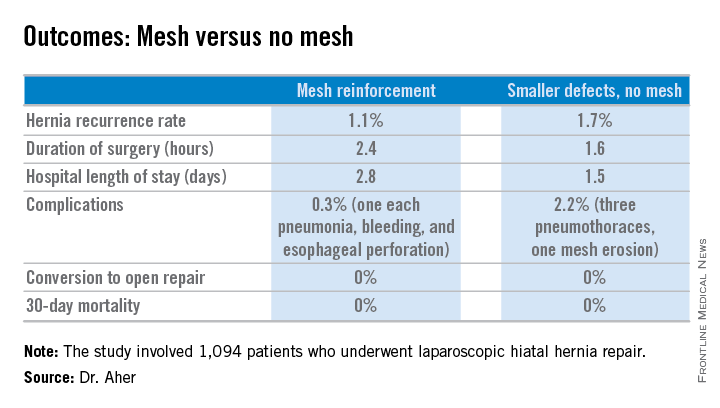
During a mean follow-up of 3.1 years, the hernia recurrence rate was 1.7% in the group with hernia defects of less than 5 cm and similar at 1.1% in those who received mesh reinforcement because their hernias were larger, reported Dr. Aher of Rush University Medical Center in Chicago.
Operative time and length of stay were longer in the mesh reinforcement group (see chart).
“There’s more dissection when using mesh, and obviously the placement of the mesh takes a little longer,” he noted at the meeting presented by the Society of Laparoscopic Surgeons and affiliated societies.
All repairs were performed using cruroplasty with interrupted nonabsorbable sutures approximating the right and left bundles of the right crura.
Laparoscopic repair has become the standard approach in the primary repair of hiatal hernias. In a 2010 survey of members of the Society of Gastrointestinal and Endoscopic Surgeons conducted by Dr. Aher’s colleagues, respondents indicated they laparoscopically performed 77% of their mesh-reinforced repairs. However, the survey results underscored a lack of consensus within the surgical community regarding mesh usage. Biologic mesh was used by 28% of surgeons; 25% used PTFE (polytetrafluoroethylene), and 21% polypropylene. Mesh placement practices also varied widely: 14% of surgeons utilized anterior placement, 34% posterior, and only 10% circumferential (Surg. Endosc. 2010;24:1017-24).
Asked how he counsels patients about the competing risks of mesh erosion and hernia recurrence in the absence of mesh reinforcement, Dr. Aher pointed to the 22% recurrence risk with large hernias in the earlier randomized trial.
“I would counsel my own family that if you have a large hernia, the risk of mesh erosion is very low and the risk of undergoing a recurrent operation if there is no mesh reinforcement is, I think, overall higher. So I would say they should get the mesh reinforcement,” he concluded.
Dr. Aher reported having no financial conflicts regarding this study.
LAS VEGAS – Routine use of mesh reinforcement when performing laparoscopic repair of hiatal hernia defects 5 cm or larger in diameter is associated with a low recurrence rate, Dr. Chetan V. Aher reported at the annual Minimally Invasive Surgery Week.
His coinvestigators had shown in an earlier randomized controlled trial that mesh reinforcement of primary cruroplasty in patients with a hernia of 8 cm or greater was associated with no recurrences. Repair with simple cruroplasty was associated with a 22% recurrence rate (Arch. Surg. 2002;137:649-52).

However, Dr. Aher and his coinvestigators subsequently observed a high recurrence rate following mesh-free simple cruroplasty for defects in the 5- to 8-cm range. He presented a case series involving 1,094 laparoscopic hiatal hernia repairs performed since he and his colleagues changed their practice by lowering their threshold for polytetrafluoroethylene mesh reinforcement to defects of at least 5 cm from their prior standard of 8 cm or more.
Hernias were less than 5 cm in diameter in 84% of the patients, so mesh wasn’t used for those repairs. In the remaining 178 patients – those with hernias of at least 5 cm – PTFE mesh was utilized to circumferentially reinforce the cruroplasty.
During a mean follow-up of 3.1 years, the hernia recurrence rate was 1.7% in the group with hernia defects of less than 5 cm and similar at 1.1% in those who received mesh reinforcement because their hernias were larger, reported Dr. Aher of Rush University Medical Center in Chicago.
Operative time and length of stay were longer in the mesh reinforcement group (see chart).
“There’s more dissection when using mesh, and obviously the placement of the mesh takes a little longer,” he noted at the meeting presented by the Society of Laparoscopic Surgeons and affiliated societies.
All repairs were performed using cruroplasty with interrupted nonabsorbable sutures approximating the right and left bundles of the right crura.
Laparoscopic repair has become the standard approach in the primary repair of hiatal hernias. In a 2010 survey of members of the Society of Gastrointestinal and Endoscopic Surgeons conducted by Dr. Aher’s colleagues, respondents indicated they laparoscopically performed 77% of their mesh-reinforced repairs. However, the survey results underscored a lack of consensus within the surgical community regarding mesh usage. Biologic mesh was used by 28% of surgeons; 25% used PTFE (polytetrafluoroethylene), and 21% polypropylene. Mesh placement practices also varied widely: 14% of surgeons utilized anterior placement, 34% posterior, and only 10% circumferential (Surg. Endosc. 2010;24:1017-24).
Asked how he counsels patients about the competing risks of mesh erosion and hernia recurrence in the absence of mesh reinforcement, Dr. Aher pointed to the 22% recurrence risk with large hernias in the earlier randomized trial.
“I would counsel my own family that if you have a large hernia, the risk of mesh erosion is very low and the risk of undergoing a recurrent operation if there is no mesh reinforcement is, I think, overall higher. So I would say they should get the mesh reinforcement,” he concluded.
Dr. Aher reported having no financial conflicts regarding this study.
LAS VEGAS – Routine use of mesh reinforcement when performing laparoscopic repair of hiatal hernia defects 5 cm or larger in diameter is associated with a low recurrence rate, Dr. Chetan V. Aher reported at the annual Minimally Invasive Surgery Week.
His coinvestigators had shown in an earlier randomized controlled trial that mesh reinforcement of primary cruroplasty in patients with a hernia of 8 cm or greater was associated with no recurrences. Repair with simple cruroplasty was associated with a 22% recurrence rate (Arch. Surg. 2002;137:649-52).

However, Dr. Aher and his coinvestigators subsequently observed a high recurrence rate following mesh-free simple cruroplasty for defects in the 5- to 8-cm range. He presented a case series involving 1,094 laparoscopic hiatal hernia repairs performed since he and his colleagues changed their practice by lowering their threshold for polytetrafluoroethylene mesh reinforcement to defects of at least 5 cm from their prior standard of 8 cm or more.
Hernias were less than 5 cm in diameter in 84% of the patients, so mesh wasn’t used for those repairs. In the remaining 178 patients – those with hernias of at least 5 cm – PTFE mesh was utilized to circumferentially reinforce the cruroplasty.
During a mean follow-up of 3.1 years, the hernia recurrence rate was 1.7% in the group with hernia defects of less than 5 cm and similar at 1.1% in those who received mesh reinforcement because their hernias were larger, reported Dr. Aher of Rush University Medical Center in Chicago.
Operative time and length of stay were longer in the mesh reinforcement group (see chart).
“There’s more dissection when using mesh, and obviously the placement of the mesh takes a little longer,” he noted at the meeting presented by the Society of Laparoscopic Surgeons and affiliated societies.
All repairs were performed using cruroplasty with interrupted nonabsorbable sutures approximating the right and left bundles of the right crura.
Laparoscopic repair has become the standard approach in the primary repair of hiatal hernias. In a 2010 survey of members of the Society of Gastrointestinal and Endoscopic Surgeons conducted by Dr. Aher’s colleagues, respondents indicated they laparoscopically performed 77% of their mesh-reinforced repairs. However, the survey results underscored a lack of consensus within the surgical community regarding mesh usage. Biologic mesh was used by 28% of surgeons; 25% used PTFE (polytetrafluoroethylene), and 21% polypropylene. Mesh placement practices also varied widely: 14% of surgeons utilized anterior placement, 34% posterior, and only 10% circumferential (Surg. Endosc. 2010;24:1017-24).
Asked how he counsels patients about the competing risks of mesh erosion and hernia recurrence in the absence of mesh reinforcement, Dr. Aher pointed to the 22% recurrence risk with large hernias in the earlier randomized trial.
“I would counsel my own family that if you have a large hernia, the risk of mesh erosion is very low and the risk of undergoing a recurrent operation if there is no mesh reinforcement is, I think, overall higher. So I would say they should get the mesh reinforcement,” he concluded.
Dr. Aher reported having no financial conflicts regarding this study.
AT MINIMALLY INVASIVE SURGERY WEEK
Key clinical point: Using hernia defect size to guide selective use of mesh reinforcement in laparoscopic hiatal hernia repair results in a low recurrence rate and excellent safety.
Major finding: The hernia recurrence rate was 1.7% in patients who underwent primary cruroplasty for hernias less than 5 cm in diameter and 1.1% in those who received mesh reinforcement because their hernias exceeded that size.
Data source: This was a retrospective study of 1,094 patients who underwent laparoscopic hiatal hernia repair since the investigators changed their threshold for utilizing mesh reinforcement from 8- to 5-cm hernia defects.
Disclosures: The presenter reported having no financial conflicts.
Lungs donated after asphyxiation, drowning found suitable for transplant
Patients who received lung transplants from donors who died of asphyxiation or drowning had similar survival rates and clinical outcomes as those whose donors died of other causes, according to a large registry analysis in the October issue of The Annals of Thoracic Surgery.
“Asphyxiation or drowning as a donor cause of death should not automatically exclude the organ from transplant consideration,” said Dr. Bryan A. Whitson of Ohio State University, Columbus, and his associates. Donor death from asphyxiation or drowning did not significantly affect rates of airway dehiscence, transplant rejection, posttransplant stroke or dialysis, or long-term survival.
Lungs donated after asphyxiation or drowning should be carefully evaluated for parenchymal injury, microbial contamination, and the possibility of primary graft dysfunction, the researchers cautioned. For example, asphyxiation and drowning can alter lung surfactant levels (Ann. Thorac. Surg. 2014;98:1145-51).
The analysis included 18,205 U.S. adults who underwent lung transplantation between 1987 and 2010, including 309 patients whose donors had reportedly died from drowning or asphyxiation. Patients were identified from the UNOS/OPTN STAR (United Network for Organ Sharing/Organ Procurement and Transplantation Network Standard Transplant Analysis and Research) database, which is overseen by the U.S. Department of Health & Human Services.
Ten-year survival curves did not vary based on donor cause of death, either when analyzed individually or when asphyxiation or drowning was compared with all other causes (P = .52), the researchers said. In fact, pulmonary deaths were significantly less common (5.8%) among recipients whose donors had died of asphyxiation or drowning compared with other causes (9.5%; P = .02).
Donor death from drowning and asphyxiation also did not significantly affect rates of treatment for transplant rejection within the first year after surgery (50.8% vs. 47.4% for all other causes of donor death), or posttransplant rates of stroke (0.7% vs. 2.1%) or dialysis (5.4% vs. 5.2%), the investigators said. However, hospital length of stay averaged 0.8 days longer when donors had died of asphyxiation or drowning compared with other causes (27.3 vs. 26.5 days; P < 0.001).
Dr. Jacques-Pierre Fontaine comments: The shortage of suitable donor lungs remains an important problem. Less than 20% of lungs being offered for donation are being used. The notion that asphyxiation or drowning excludes a patient from being a potential donor is widespread among some clinicians.
This extensive retrospective review of the robust UNOS Database demonstrates that recipients of lungs from donors who died from asphyxiation or drowning have similar 10-year survival and post-transplant complication rates. In carefully selected donors, these lungs may be successfully used. Furthermore, "optimization" of marginal donor lungs may become more prevalent as ex-vivo lung perfusion technology evolves.
Dr. Fontaine specializes in thoracic surgery at the Moffitt Cancer Center in Tampa, Florida.
Dr. Jacques-Pierre Fontaine comments: The shortage of suitable donor lungs remains an important problem. Less than 20% of lungs being offered for donation are being used. The notion that asphyxiation or drowning excludes a patient from being a potential donor is widespread among some clinicians.
This extensive retrospective review of the robust UNOS Database demonstrates that recipients of lungs from donors who died from asphyxiation or drowning have similar 10-year survival and post-transplant complication rates. In carefully selected donors, these lungs may be successfully used. Furthermore, "optimization" of marginal donor lungs may become more prevalent as ex-vivo lung perfusion technology evolves.
Dr. Fontaine specializes in thoracic surgery at the Moffitt Cancer Center in Tampa, Florida.
Dr. Jacques-Pierre Fontaine comments: The shortage of suitable donor lungs remains an important problem. Less than 20% of lungs being offered for donation are being used. The notion that asphyxiation or drowning excludes a patient from being a potential donor is widespread among some clinicians.
This extensive retrospective review of the robust UNOS Database demonstrates that recipients of lungs from donors who died from asphyxiation or drowning have similar 10-year survival and post-transplant complication rates. In carefully selected donors, these lungs may be successfully used. Furthermore, "optimization" of marginal donor lungs may become more prevalent as ex-vivo lung perfusion technology evolves.
Dr. Fontaine specializes in thoracic surgery at the Moffitt Cancer Center in Tampa, Florida.
Patients who received lung transplants from donors who died of asphyxiation or drowning had similar survival rates and clinical outcomes as those whose donors died of other causes, according to a large registry analysis in the October issue of The Annals of Thoracic Surgery.
“Asphyxiation or drowning as a donor cause of death should not automatically exclude the organ from transplant consideration,” said Dr. Bryan A. Whitson of Ohio State University, Columbus, and his associates. Donor death from asphyxiation or drowning did not significantly affect rates of airway dehiscence, transplant rejection, posttransplant stroke or dialysis, or long-term survival.
Lungs donated after asphyxiation or drowning should be carefully evaluated for parenchymal injury, microbial contamination, and the possibility of primary graft dysfunction, the researchers cautioned. For example, asphyxiation and drowning can alter lung surfactant levels (Ann. Thorac. Surg. 2014;98:1145-51).
The analysis included 18,205 U.S. adults who underwent lung transplantation between 1987 and 2010, including 309 patients whose donors had reportedly died from drowning or asphyxiation. Patients were identified from the UNOS/OPTN STAR (United Network for Organ Sharing/Organ Procurement and Transplantation Network Standard Transplant Analysis and Research) database, which is overseen by the U.S. Department of Health & Human Services.
Ten-year survival curves did not vary based on donor cause of death, either when analyzed individually or when asphyxiation or drowning was compared with all other causes (P = .52), the researchers said. In fact, pulmonary deaths were significantly less common (5.8%) among recipients whose donors had died of asphyxiation or drowning compared with other causes (9.5%; P = .02).
Donor death from drowning and asphyxiation also did not significantly affect rates of treatment for transplant rejection within the first year after surgery (50.8% vs. 47.4% for all other causes of donor death), or posttransplant rates of stroke (0.7% vs. 2.1%) or dialysis (5.4% vs. 5.2%), the investigators said. However, hospital length of stay averaged 0.8 days longer when donors had died of asphyxiation or drowning compared with other causes (27.3 vs. 26.5 days; P < 0.001).
Patients who received lung transplants from donors who died of asphyxiation or drowning had similar survival rates and clinical outcomes as those whose donors died of other causes, according to a large registry analysis in the October issue of The Annals of Thoracic Surgery.
“Asphyxiation or drowning as a donor cause of death should not automatically exclude the organ from transplant consideration,” said Dr. Bryan A. Whitson of Ohio State University, Columbus, and his associates. Donor death from asphyxiation or drowning did not significantly affect rates of airway dehiscence, transplant rejection, posttransplant stroke or dialysis, or long-term survival.
Lungs donated after asphyxiation or drowning should be carefully evaluated for parenchymal injury, microbial contamination, and the possibility of primary graft dysfunction, the researchers cautioned. For example, asphyxiation and drowning can alter lung surfactant levels (Ann. Thorac. Surg. 2014;98:1145-51).
The analysis included 18,205 U.S. adults who underwent lung transplantation between 1987 and 2010, including 309 patients whose donors had reportedly died from drowning or asphyxiation. Patients were identified from the UNOS/OPTN STAR (United Network for Organ Sharing/Organ Procurement and Transplantation Network Standard Transplant Analysis and Research) database, which is overseen by the U.S. Department of Health & Human Services.
Ten-year survival curves did not vary based on donor cause of death, either when analyzed individually or when asphyxiation or drowning was compared with all other causes (P = .52), the researchers said. In fact, pulmonary deaths were significantly less common (5.8%) among recipients whose donors had died of asphyxiation or drowning compared with other causes (9.5%; P = .02).
Donor death from drowning and asphyxiation also did not significantly affect rates of treatment for transplant rejection within the first year after surgery (50.8% vs. 47.4% for all other causes of donor death), or posttransplant rates of stroke (0.7% vs. 2.1%) or dialysis (5.4% vs. 5.2%), the investigators said. However, hospital length of stay averaged 0.8 days longer when donors had died of asphyxiation or drowning compared with other causes (27.3 vs. 26.5 days; P < 0.001).
Key clinical point: Lung transplant recipients had good outcomes and long-term survival in cases involving donors who died of asphyxiation or drowning.
Major finding: Pulmonary deaths were significantly less common (5.8%) among recipients whose donors had died of asphyxiation or drowning compared with other causes (9.5%; P = .02).
Data source: Retrospective registry analysis of 18,250 lung transplant recipients.
Disclosures: The authors did not report funding sources or conflicts of interest.
Ovarian cancer often arises from precursor endometriosis
LAS VEGAS – Gynecologists, general surgeons, and primary care physicians now share an unprecedented opportunity to put a major dent in the incidence of ovarian cancer, according to Dr. Farr R. Nezhat.
Mounting evidence suggests that identification and complete surgical removal of endometriosis reduce the risk of several histologic types of ovarian cancer. So when a woman visits her primary care physician for pelvic pain or vaginal bleeding that might be due to endometrial pathology, or a general surgeon finds asymptomatic endometriosis during pelvic surgery, these encounters provide an opportunity for preventive intervention, explained Dr. Nezhat, professor of ob.gyn. and director of minimally invasive surgery and gynecologic robotics at Mount Sinai Medical Center, New York.
The latest thinking about the pathophysiology of ovarian cancer, he noted, is that there are two different types of the malignancy. One type, which likely arises from endometriosis as the precursor lesion, is characterized by low-grade serous, clear cell, and endometrioid carcinomas, which tend to present at an earlier stage and are more indolent. They are associated with mutations in the PTEN, BCL2, and ARID1A genes.
A pooled analysis of 13 ovarian cancer case-control studies conducted by investigators in the Ovarian Cancer Association Consortium made the point that women with endometriosis are at increased risk of specific subtypes of the malignancy. The analysis, which included 7,911 women with invasive ovarian cancer, 1,907 others with borderline ovarian cancer, and more than 13,000 controls, concluded that women with a self-reported history of endometriosis had a 3.05-fold increased risk of clear cell invasive ovarian cancer, compared with controls, a 2.04-fold increased risk of endometrioid ovarian cancer, and a 2.11-fold greater likelihood of low-grade serous ovarian cancer.
In contrast, no association was apparent between endometriosis and the risk of high-grade serous or mucinous invasive ovarian cancer or borderline tumors. Thus, the pathogenesis of low- and high-grade serous ovarian cancers may differ (Lancet Oncol. 2012;13:385-94).
Dr. Nezhat cited as another influential study a Swedish national registry case-control study involving all Swedes with a first-time hospital discharge diagnosis of endometriosis during 1969-2007. The cases in this study were all 220 Swedish women diagnosed with epithelial ovarian cancer at least 1 year after their endometriosis was diagnosed. Each was matched with two controls with no ovarian cancer diagnosis before the date of the case’s cancer diagnosis.
This was the first published study to demonstrate that treatment of endometriosis has a salutary impact on subsequent risk of ovarian cancer. Complete surgical removal of all visible endometriotic tissue was associated with a 63% reduction in the risk of ovarian cancer in a univariate analysis and a 70% relative risk reduction in a multivariate analysis. One-sided oophorectomy involving the endometriosis-involved ovary was similarly associated with a 58% risk reduction for ovarian cancer in a univariate analysis and an 81% reduction in risk in a multivariate analysis (Acta Obstet. Gynecol. Scand. 2013:92:546-54).
An earlier study in which Dr. Nezhat was senior author highlighted that different histologic types of early-stage ovarian carcinoma feature distinctive patterns of clinical symptoms. The study included 76 consecutive patients with FIGO stage I ovarian carcinoma, of which 54 – that is, more than two-thirds – were nonserous, which is a much higher proportion than is seen in women diagnosed with stage III and IV disease.
Most patients with serous papillary carcinoma in this series presented with an asymptomatic pelvic mass. In contrast, most of those with endometrioid or clear cell carcinoma presented with pelvic pain or abnormal vaginal bleeding with or without a pelvic mass (Fertil. Steril. 2007;88:906-10).
Endometrioisis is a pervasive condition. Dr. Nezhat said the endometriosis patients he considers to be at possible increased risk for ovarian cancer include those with longstanding endometriosis, a history of infertility, endometriosis diagnosed at an early age, as well as those with ovarian endometriomas. Eventually it will be possible to pin down more precisely the ovarian cancer risk of an individual with endometriosis through screening for genetic mutations, but the evidence base isn’t yet sufficient to introduce this into everyday practice, he said.
One audience member said it’s her practice and that of many of her gynecologic colleagues that when they incidentally find a patient has asymptomatic endometriosis, for example, during surgery for ectopic pregnancy, they will often leave it in place, even if it is quite severe. Is it time to rethink that practice and instead remove all visible endometriosis, even if the patient is asymptomatic? she asked.
“The short answer is, Yes,” Dr. Nezhat replied. “The most important thing is that when you do surgery, remove it all or else do biopsies to make sure you’re not leaving early ovarian cancer behind. Draining endometriomas is not adequate.”
He reported having no relevant financial conflicts.
LAS VEGAS – Gynecologists, general surgeons, and primary care physicians now share an unprecedented opportunity to put a major dent in the incidence of ovarian cancer, according to Dr. Farr R. Nezhat.
Mounting evidence suggests that identification and complete surgical removal of endometriosis reduce the risk of several histologic types of ovarian cancer. So when a woman visits her primary care physician for pelvic pain or vaginal bleeding that might be due to endometrial pathology, or a general surgeon finds asymptomatic endometriosis during pelvic surgery, these encounters provide an opportunity for preventive intervention, explained Dr. Nezhat, professor of ob.gyn. and director of minimally invasive surgery and gynecologic robotics at Mount Sinai Medical Center, New York.
The latest thinking about the pathophysiology of ovarian cancer, he noted, is that there are two different types of the malignancy. One type, which likely arises from endometriosis as the precursor lesion, is characterized by low-grade serous, clear cell, and endometrioid carcinomas, which tend to present at an earlier stage and are more indolent. They are associated with mutations in the PTEN, BCL2, and ARID1A genes.
A pooled analysis of 13 ovarian cancer case-control studies conducted by investigators in the Ovarian Cancer Association Consortium made the point that women with endometriosis are at increased risk of specific subtypes of the malignancy. The analysis, which included 7,911 women with invasive ovarian cancer, 1,907 others with borderline ovarian cancer, and more than 13,000 controls, concluded that women with a self-reported history of endometriosis had a 3.05-fold increased risk of clear cell invasive ovarian cancer, compared with controls, a 2.04-fold increased risk of endometrioid ovarian cancer, and a 2.11-fold greater likelihood of low-grade serous ovarian cancer.
In contrast, no association was apparent between endometriosis and the risk of high-grade serous or mucinous invasive ovarian cancer or borderline tumors. Thus, the pathogenesis of low- and high-grade serous ovarian cancers may differ (Lancet Oncol. 2012;13:385-94).
Dr. Nezhat cited as another influential study a Swedish national registry case-control study involving all Swedes with a first-time hospital discharge diagnosis of endometriosis during 1969-2007. The cases in this study were all 220 Swedish women diagnosed with epithelial ovarian cancer at least 1 year after their endometriosis was diagnosed. Each was matched with two controls with no ovarian cancer diagnosis before the date of the case’s cancer diagnosis.
This was the first published study to demonstrate that treatment of endometriosis has a salutary impact on subsequent risk of ovarian cancer. Complete surgical removal of all visible endometriotic tissue was associated with a 63% reduction in the risk of ovarian cancer in a univariate analysis and a 70% relative risk reduction in a multivariate analysis. One-sided oophorectomy involving the endometriosis-involved ovary was similarly associated with a 58% risk reduction for ovarian cancer in a univariate analysis and an 81% reduction in risk in a multivariate analysis (Acta Obstet. Gynecol. Scand. 2013:92:546-54).
An earlier study in which Dr. Nezhat was senior author highlighted that different histologic types of early-stage ovarian carcinoma feature distinctive patterns of clinical symptoms. The study included 76 consecutive patients with FIGO stage I ovarian carcinoma, of which 54 – that is, more than two-thirds – were nonserous, which is a much higher proportion than is seen in women diagnosed with stage III and IV disease.
Most patients with serous papillary carcinoma in this series presented with an asymptomatic pelvic mass. In contrast, most of those with endometrioid or clear cell carcinoma presented with pelvic pain or abnormal vaginal bleeding with or without a pelvic mass (Fertil. Steril. 2007;88:906-10).
Endometrioisis is a pervasive condition. Dr. Nezhat said the endometriosis patients he considers to be at possible increased risk for ovarian cancer include those with longstanding endometriosis, a history of infertility, endometriosis diagnosed at an early age, as well as those with ovarian endometriomas. Eventually it will be possible to pin down more precisely the ovarian cancer risk of an individual with endometriosis through screening for genetic mutations, but the evidence base isn’t yet sufficient to introduce this into everyday practice, he said.
One audience member said it’s her practice and that of many of her gynecologic colleagues that when they incidentally find a patient has asymptomatic endometriosis, for example, during surgery for ectopic pregnancy, they will often leave it in place, even if it is quite severe. Is it time to rethink that practice and instead remove all visible endometriosis, even if the patient is asymptomatic? she asked.
“The short answer is, Yes,” Dr. Nezhat replied. “The most important thing is that when you do surgery, remove it all or else do biopsies to make sure you’re not leaving early ovarian cancer behind. Draining endometriomas is not adequate.”
He reported having no relevant financial conflicts.
LAS VEGAS – Gynecologists, general surgeons, and primary care physicians now share an unprecedented opportunity to put a major dent in the incidence of ovarian cancer, according to Dr. Farr R. Nezhat.
Mounting evidence suggests that identification and complete surgical removal of endometriosis reduce the risk of several histologic types of ovarian cancer. So when a woman visits her primary care physician for pelvic pain or vaginal bleeding that might be due to endometrial pathology, or a general surgeon finds asymptomatic endometriosis during pelvic surgery, these encounters provide an opportunity for preventive intervention, explained Dr. Nezhat, professor of ob.gyn. and director of minimally invasive surgery and gynecologic robotics at Mount Sinai Medical Center, New York.
The latest thinking about the pathophysiology of ovarian cancer, he noted, is that there are two different types of the malignancy. One type, which likely arises from endometriosis as the precursor lesion, is characterized by low-grade serous, clear cell, and endometrioid carcinomas, which tend to present at an earlier stage and are more indolent. They are associated with mutations in the PTEN, BCL2, and ARID1A genes.
A pooled analysis of 13 ovarian cancer case-control studies conducted by investigators in the Ovarian Cancer Association Consortium made the point that women with endometriosis are at increased risk of specific subtypes of the malignancy. The analysis, which included 7,911 women with invasive ovarian cancer, 1,907 others with borderline ovarian cancer, and more than 13,000 controls, concluded that women with a self-reported history of endometriosis had a 3.05-fold increased risk of clear cell invasive ovarian cancer, compared with controls, a 2.04-fold increased risk of endometrioid ovarian cancer, and a 2.11-fold greater likelihood of low-grade serous ovarian cancer.
In contrast, no association was apparent between endometriosis and the risk of high-grade serous or mucinous invasive ovarian cancer or borderline tumors. Thus, the pathogenesis of low- and high-grade serous ovarian cancers may differ (Lancet Oncol. 2012;13:385-94).
Dr. Nezhat cited as another influential study a Swedish national registry case-control study involving all Swedes with a first-time hospital discharge diagnosis of endometriosis during 1969-2007. The cases in this study were all 220 Swedish women diagnosed with epithelial ovarian cancer at least 1 year after their endometriosis was diagnosed. Each was matched with two controls with no ovarian cancer diagnosis before the date of the case’s cancer diagnosis.
This was the first published study to demonstrate that treatment of endometriosis has a salutary impact on subsequent risk of ovarian cancer. Complete surgical removal of all visible endometriotic tissue was associated with a 63% reduction in the risk of ovarian cancer in a univariate analysis and a 70% relative risk reduction in a multivariate analysis. One-sided oophorectomy involving the endometriosis-involved ovary was similarly associated with a 58% risk reduction for ovarian cancer in a univariate analysis and an 81% reduction in risk in a multivariate analysis (Acta Obstet. Gynecol. Scand. 2013:92:546-54).
An earlier study in which Dr. Nezhat was senior author highlighted that different histologic types of early-stage ovarian carcinoma feature distinctive patterns of clinical symptoms. The study included 76 consecutive patients with FIGO stage I ovarian carcinoma, of which 54 – that is, more than two-thirds – were nonserous, which is a much higher proportion than is seen in women diagnosed with stage III and IV disease.
Most patients with serous papillary carcinoma in this series presented with an asymptomatic pelvic mass. In contrast, most of those with endometrioid or clear cell carcinoma presented with pelvic pain or abnormal vaginal bleeding with or without a pelvic mass (Fertil. Steril. 2007;88:906-10).
Endometrioisis is a pervasive condition. Dr. Nezhat said the endometriosis patients he considers to be at possible increased risk for ovarian cancer include those with longstanding endometriosis, a history of infertility, endometriosis diagnosed at an early age, as well as those with ovarian endometriomas. Eventually it will be possible to pin down more precisely the ovarian cancer risk of an individual with endometriosis through screening for genetic mutations, but the evidence base isn’t yet sufficient to introduce this into everyday practice, he said.
One audience member said it’s her practice and that of many of her gynecologic colleagues that when they incidentally find a patient has asymptomatic endometriosis, for example, during surgery for ectopic pregnancy, they will often leave it in place, even if it is quite severe. Is it time to rethink that practice and instead remove all visible endometriosis, even if the patient is asymptomatic? she asked.
“The short answer is, Yes,” Dr. Nezhat replied. “The most important thing is that when you do surgery, remove it all or else do biopsies to make sure you’re not leaving early ovarian cancer behind. Draining endometriomas is not adequate.”
He reported having no relevant financial conflicts.
EXPERT ANALYSIS FROM MINIMALLY INVASIVE SURGERY WEEK
S.D. voters to decide ‘any willing provider’ question; other state initiatives highlighted
South Dakota voters’ decision on an “any willing provider” ballot initiative is among a number of health care–related ballot initiatives that will be voted on in the upcoming midterm elections.
Others include a measure in Illinois that would require insurance providers that have prescription drug coverage to provide prescription contraceptives, and an Arizona ballot question regarding terminally ill patients’ access to experimental drugs.
If approved, South Dakota’s Initiative Measure 17 would allow providers who are willing to meet a health insurer’s coverage terms to provide health care services to insured patients without those patients incurring any out-of-network fees.
In recent years, the state has seen a significant increase in the number of closed and narrow provider networks, noted Dr. Mary J. Milroy, president of the South Dakota State Medical Association. That has disrupted the patient-physician relationship as patients find their long-time physicians are no longer in their health plan’s networks, said Dr. Milroy, a general surgeon from Yankton, S.D.
“Most patients don’t automatically choose their insurance company,” Dr. Milroy said in an interview. Employers can change employees’ insurance coverage, or coverage could shift with an employment change.
“If your personal physician, who maybe has been taking care of you for years, is not included in the panel for the new plan, then you have to switch or you have to incur out-of-pocket costs,” she explained. “We do not think that is good for continued and comprehensive medical care.”
Under the ballot question, physicians not in a patient’s insurance network but who want to continue a relationship with that patient would simply have to agree to the terms that the insurance provider has for physicians in its network. There would be no special treatment and physicians would have to uphold any terms imposed on in-network providers, including agreeing to reimbursement rates, quality standards, and other terms. If they agree, the patient’s visit would be treated like an in-network visit.
“We don’t see that such a change would negatively affect cost of health care,” Dr. Milroy said. “In fact, we think it would actually increase competition.”
If voters agree and vote positively on the initiative, it will have the force of law, according to Dr. Milroy.
Illinois voters will be asked to vote yes or no on whether any insurance plan in the state that offers prescription drug coverage “be required to include prescription birth control as part of that coverage.”
How that is to be implemented and what exactly would be covered as part of prescription birth control would be determined when the state legislature crafted a bill following voters’ approval of the question, which would leave the question of whether emergency contraception would be covered.
Dr. Maura P. Quinlan, chair of the Illinois Section of the American Congress of Obstetricians and Gynecologists, said in an interview that the ballot initiative is a way for voters to weigh in on the debate, noting cases like the recent Hobby Lobby Supreme Court decision had “no role for voters in Illinois to say how they feel about this.”
In general, the Illinois section supports the ballot initiative, commenting that while there might be costs associated with coverage, “it is so much cheaper to cover the cost to prevent an unplanned pregnancy than to pay for the pregnancy, the care of the child, etc. Always, preventive measures are much cheaper when insurance is trying to decide what they cover,” said Dr. Quinlan, who is in private practice in La Grange, Ill.
In Illinois, the ballot question does not have the force of law but would require the state legislature to enact a law to implement the initiative.
In another ballot initiative, Arizona voters will be asked to decide whether terminal patients in the state should have access to experimental drugs that have not yet received FDA approval.
According to the text of the “Terminal Patients’ Right to Try” act, an eligible patient must have a terminal illness as determined by the person’s physician, and the physician has determined there is “no comparable or satisfactory” FDA-approved treatment option “available to diagnose, monitor, or treat the disease or condition involved and that the probable risk to the person from the investigational drug, biological product, or device is not greater than the probable risk from the disease or condition.”
Products in question need to have successfully undergone phase I study and remain under clinical investigation. Manufacturers would not be required to provide access to the investigational drug, and it would be up to the company as to whether it collects compensation for the product or requires data collection on the drug’s effects as part of providing access to the product.
Similarly, insurance companies would not be required to cover access to the investigational product.
The measure also provides protections for the physician, stating that a state regulatory authority cannot take action against a physician’s license “based solely on a physician’s recommendation to an eligible patient regarding or prescribing for or treatment with an investigational drug, biological product, or device.”
The language of the ballot measure passed the state House on March 4 and state Senate on April 15.
South Dakota voters’ decision on an “any willing provider” ballot initiative is among a number of health care–related ballot initiatives that will be voted on in the upcoming midterm elections.
Others include a measure in Illinois that would require insurance providers that have prescription drug coverage to provide prescription contraceptives, and an Arizona ballot question regarding terminally ill patients’ access to experimental drugs.
If approved, South Dakota’s Initiative Measure 17 would allow providers who are willing to meet a health insurer’s coverage terms to provide health care services to insured patients without those patients incurring any out-of-network fees.
In recent years, the state has seen a significant increase in the number of closed and narrow provider networks, noted Dr. Mary J. Milroy, president of the South Dakota State Medical Association. That has disrupted the patient-physician relationship as patients find their long-time physicians are no longer in their health plan’s networks, said Dr. Milroy, a general surgeon from Yankton, S.D.
“Most patients don’t automatically choose their insurance company,” Dr. Milroy said in an interview. Employers can change employees’ insurance coverage, or coverage could shift with an employment change.
“If your personal physician, who maybe has been taking care of you for years, is not included in the panel for the new plan, then you have to switch or you have to incur out-of-pocket costs,” she explained. “We do not think that is good for continued and comprehensive medical care.”
Under the ballot question, physicians not in a patient’s insurance network but who want to continue a relationship with that patient would simply have to agree to the terms that the insurance provider has for physicians in its network. There would be no special treatment and physicians would have to uphold any terms imposed on in-network providers, including agreeing to reimbursement rates, quality standards, and other terms. If they agree, the patient’s visit would be treated like an in-network visit.
“We don’t see that such a change would negatively affect cost of health care,” Dr. Milroy said. “In fact, we think it would actually increase competition.”
If voters agree and vote positively on the initiative, it will have the force of law, according to Dr. Milroy.
Illinois voters will be asked to vote yes or no on whether any insurance plan in the state that offers prescription drug coverage “be required to include prescription birth control as part of that coverage.”
How that is to be implemented and what exactly would be covered as part of prescription birth control would be determined when the state legislature crafted a bill following voters’ approval of the question, which would leave the question of whether emergency contraception would be covered.
Dr. Maura P. Quinlan, chair of the Illinois Section of the American Congress of Obstetricians and Gynecologists, said in an interview that the ballot initiative is a way for voters to weigh in on the debate, noting cases like the recent Hobby Lobby Supreme Court decision had “no role for voters in Illinois to say how they feel about this.”
In general, the Illinois section supports the ballot initiative, commenting that while there might be costs associated with coverage, “it is so much cheaper to cover the cost to prevent an unplanned pregnancy than to pay for the pregnancy, the care of the child, etc. Always, preventive measures are much cheaper when insurance is trying to decide what they cover,” said Dr. Quinlan, who is in private practice in La Grange, Ill.
In Illinois, the ballot question does not have the force of law but would require the state legislature to enact a law to implement the initiative.
In another ballot initiative, Arizona voters will be asked to decide whether terminal patients in the state should have access to experimental drugs that have not yet received FDA approval.
According to the text of the “Terminal Patients’ Right to Try” act, an eligible patient must have a terminal illness as determined by the person’s physician, and the physician has determined there is “no comparable or satisfactory” FDA-approved treatment option “available to diagnose, monitor, or treat the disease or condition involved and that the probable risk to the person from the investigational drug, biological product, or device is not greater than the probable risk from the disease or condition.”
Products in question need to have successfully undergone phase I study and remain under clinical investigation. Manufacturers would not be required to provide access to the investigational drug, and it would be up to the company as to whether it collects compensation for the product or requires data collection on the drug’s effects as part of providing access to the product.
Similarly, insurance companies would not be required to cover access to the investigational product.
The measure also provides protections for the physician, stating that a state regulatory authority cannot take action against a physician’s license “based solely on a physician’s recommendation to an eligible patient regarding or prescribing for or treatment with an investigational drug, biological product, or device.”
The language of the ballot measure passed the state House on March 4 and state Senate on April 15.
South Dakota voters’ decision on an “any willing provider” ballot initiative is among a number of health care–related ballot initiatives that will be voted on in the upcoming midterm elections.
Others include a measure in Illinois that would require insurance providers that have prescription drug coverage to provide prescription contraceptives, and an Arizona ballot question regarding terminally ill patients’ access to experimental drugs.
If approved, South Dakota’s Initiative Measure 17 would allow providers who are willing to meet a health insurer’s coverage terms to provide health care services to insured patients without those patients incurring any out-of-network fees.
In recent years, the state has seen a significant increase in the number of closed and narrow provider networks, noted Dr. Mary J. Milroy, president of the South Dakota State Medical Association. That has disrupted the patient-physician relationship as patients find their long-time physicians are no longer in their health plan’s networks, said Dr. Milroy, a general surgeon from Yankton, S.D.
“Most patients don’t automatically choose their insurance company,” Dr. Milroy said in an interview. Employers can change employees’ insurance coverage, or coverage could shift with an employment change.
“If your personal physician, who maybe has been taking care of you for years, is not included in the panel for the new plan, then you have to switch or you have to incur out-of-pocket costs,” she explained. “We do not think that is good for continued and comprehensive medical care.”
Under the ballot question, physicians not in a patient’s insurance network but who want to continue a relationship with that patient would simply have to agree to the terms that the insurance provider has for physicians in its network. There would be no special treatment and physicians would have to uphold any terms imposed on in-network providers, including agreeing to reimbursement rates, quality standards, and other terms. If they agree, the patient’s visit would be treated like an in-network visit.
“We don’t see that such a change would negatively affect cost of health care,” Dr. Milroy said. “In fact, we think it would actually increase competition.”
If voters agree and vote positively on the initiative, it will have the force of law, according to Dr. Milroy.
Illinois voters will be asked to vote yes or no on whether any insurance plan in the state that offers prescription drug coverage “be required to include prescription birth control as part of that coverage.”
How that is to be implemented and what exactly would be covered as part of prescription birth control would be determined when the state legislature crafted a bill following voters’ approval of the question, which would leave the question of whether emergency contraception would be covered.
Dr. Maura P. Quinlan, chair of the Illinois Section of the American Congress of Obstetricians and Gynecologists, said in an interview that the ballot initiative is a way for voters to weigh in on the debate, noting cases like the recent Hobby Lobby Supreme Court decision had “no role for voters in Illinois to say how they feel about this.”
In general, the Illinois section supports the ballot initiative, commenting that while there might be costs associated with coverage, “it is so much cheaper to cover the cost to prevent an unplanned pregnancy than to pay for the pregnancy, the care of the child, etc. Always, preventive measures are much cheaper when insurance is trying to decide what they cover,” said Dr. Quinlan, who is in private practice in La Grange, Ill.
In Illinois, the ballot question does not have the force of law but would require the state legislature to enact a law to implement the initiative.
In another ballot initiative, Arizona voters will be asked to decide whether terminal patients in the state should have access to experimental drugs that have not yet received FDA approval.
According to the text of the “Terminal Patients’ Right to Try” act, an eligible patient must have a terminal illness as determined by the person’s physician, and the physician has determined there is “no comparable or satisfactory” FDA-approved treatment option “available to diagnose, monitor, or treat the disease or condition involved and that the probable risk to the person from the investigational drug, biological product, or device is not greater than the probable risk from the disease or condition.”
Products in question need to have successfully undergone phase I study and remain under clinical investigation. Manufacturers would not be required to provide access to the investigational drug, and it would be up to the company as to whether it collects compensation for the product or requires data collection on the drug’s effects as part of providing access to the product.
Similarly, insurance companies would not be required to cover access to the investigational product.
The measure also provides protections for the physician, stating that a state regulatory authority cannot take action against a physician’s license “based solely on a physician’s recommendation to an eligible patient regarding or prescribing for or treatment with an investigational drug, biological product, or device.”
The language of the ballot measure passed the state House on March 4 and state Senate on April 15.
More docs need to use available Rx-monitoring programs to help curb opioid abuse
WASHINGTON – The prescription drug–monitoring programs that exist in every state are not going to make a dent in opioid abuse unless physicians use them.
Because each state governs its own prescription drug–monitoring program (PDMP), the efficiency and effectiveness of each varies widely from the others. In the ideal, a PDMP collects information on which prescriptions are being filled by what patients as well as which physicians are prescribing the drugs. Information from the databases is used to help determine which patients might be doctor shopping for opioids as well as identify physicians who might be operating “pill mills” and contributing to the availability of narcotic pain medication that is used for other than clinical purposes.
Data from several studies have shown that in many instances, doctors do not realize the detail and usefulness of data from PDMPs, Allan Coukell, senior director for drugs and medical devices at Pew Charitable Trusts, Washington, said at a panel discussion on prescription drug abuse hosted by the Alliance for Health Reform and the Pharmaceutical Care Management Association, the industry lobby for pharmacy benefit managers.
When shown data on a specific patient, a physician was often “surprised to know how many other physicians that patient was seeing, and having that information changed their prescribing,” Mr. Coukell said, noting that in some cases, the information led to physicians refusing to prescribe pain medication and in others, it eased fears that a patient might be doctor shopping, giving the physician some peace of mind about writing a prescription for a narcotic.
Panelist Dr. Sarah Chouinard, medical director of Community Care of West Virginia, noted that physicians often are surprised when presented with data collected by PDMPs about their own individual prescribing habits.
“Eight out of 10 people were very surprised at the number of hydrocodone prescriptions they had written in the last 3 months,” Dr. Chouinard said.
In addition, the culture of administering pain medicine has gone through a shift in the last 10 years that is contributing to the problem of how many pills are available.
“About 10 years ago, there were doctors wandering around with buttons that said ‘No Pain’ with a red circle and a line through it because we were accused of under treating pain,” she observed. “Now, the pendulum has swung in the opposite direction. They used to say, ‘Hey, we’ll get sued if we don’t write pain medicine for these people,’ and now it’s, ‘Gosh, don’t write anything, or we will be a pill mill.’ I think the real answer is getting back to the middle and allowing the word to be out there that you don’t have to use a narcotic for every bump.”
To that end, Dr. Chouinard shared a solution her community organization has implemented. She said that “none of the [family physicians] are equipped, myself included, to treat chronic pain. Chronic pain is a specialty. ... It requires special training. The training that we as family doctors get now, after residency, is a mandatory 3 hours every 2 years.”
So Community Health of West Virgina hired an anesthetist who is not interested in family medicine but rather has a special interest in addictions medicine and outpatient pain medicine.
“Every one of our patients who has chronic pain [is sent] to this physician first,” Dr. Chouinard explained. “He does the work-up. He looks to see if the patients are amenable to any kind of alternatives and then sends those patients back to us as primary care doctors.”
After that, every patient gets a pain contract that essentially provides consent to have urine tested on a regular basis to track adherence, as well as provide pill counts and office visits as required by the doctors. The physicians also are required to regularly check the PDMP database.
Patients who need this kind of treatment do not complain that the requirements are burdensome, said Dr. Chouinard, who described the program as successful overall. She said 30% have stayed properly adherent to their prescription narcotics and are living within the agreements of their contracts, while another 20% were taken off their medication because they were able to be placed on alternate, nonopioid therapies. About 30% ended up recommended for or enrolled in an addiction program, while 20% failed to maintain their contracts, and thus were unable to get their prescriptions.
Dr. Chouinard said a program like this could easily be rolled out across the country to help address the opioid abuse problem, as well as help remove primary care doctors from the prescribing loop.
“None of these people went into medicine because they had an interest in treating chronic pain,” she said.
In keeping with the overall message of getting doctors to use PDMPs more, Mr. Coukell said that getting this information integrated into electronic health records is “the Holy Grail,” though he added that getting practice alerts are a good short-term fix. He also said that getting consistency of reporting practice uniform across states and getting more states to trade information also was key, as is getting rules in place to allow someone in the office other than the doctor to have access to the PDMP databases (which some states do allow).
Centers for Disease Control and Prevention Director of the Division of Unintentional Injury Prevention Grant Baldwin, Ph.D., concurred.
“Providers have a limited amount of time to see patients,” Dr. Baldwin said. “Integrating all of these fixes will not necessarily extend the amount of time, but it actually makes it easier for the health care team to do their job in an efficient manner in that same 10-15 minute time."
WASHINGTON – The prescription drug–monitoring programs that exist in every state are not going to make a dent in opioid abuse unless physicians use them.
Because each state governs its own prescription drug–monitoring program (PDMP), the efficiency and effectiveness of each varies widely from the others. In the ideal, a PDMP collects information on which prescriptions are being filled by what patients as well as which physicians are prescribing the drugs. Information from the databases is used to help determine which patients might be doctor shopping for opioids as well as identify physicians who might be operating “pill mills” and contributing to the availability of narcotic pain medication that is used for other than clinical purposes.
Data from several studies have shown that in many instances, doctors do not realize the detail and usefulness of data from PDMPs, Allan Coukell, senior director for drugs and medical devices at Pew Charitable Trusts, Washington, said at a panel discussion on prescription drug abuse hosted by the Alliance for Health Reform and the Pharmaceutical Care Management Association, the industry lobby for pharmacy benefit managers.
When shown data on a specific patient, a physician was often “surprised to know how many other physicians that patient was seeing, and having that information changed their prescribing,” Mr. Coukell said, noting that in some cases, the information led to physicians refusing to prescribe pain medication and in others, it eased fears that a patient might be doctor shopping, giving the physician some peace of mind about writing a prescription for a narcotic.
Panelist Dr. Sarah Chouinard, medical director of Community Care of West Virginia, noted that physicians often are surprised when presented with data collected by PDMPs about their own individual prescribing habits.
“Eight out of 10 people were very surprised at the number of hydrocodone prescriptions they had written in the last 3 months,” Dr. Chouinard said.
In addition, the culture of administering pain medicine has gone through a shift in the last 10 years that is contributing to the problem of how many pills are available.
“About 10 years ago, there were doctors wandering around with buttons that said ‘No Pain’ with a red circle and a line through it because we were accused of under treating pain,” she observed. “Now, the pendulum has swung in the opposite direction. They used to say, ‘Hey, we’ll get sued if we don’t write pain medicine for these people,’ and now it’s, ‘Gosh, don’t write anything, or we will be a pill mill.’ I think the real answer is getting back to the middle and allowing the word to be out there that you don’t have to use a narcotic for every bump.”
To that end, Dr. Chouinard shared a solution her community organization has implemented. She said that “none of the [family physicians] are equipped, myself included, to treat chronic pain. Chronic pain is a specialty. ... It requires special training. The training that we as family doctors get now, after residency, is a mandatory 3 hours every 2 years.”
So Community Health of West Virgina hired an anesthetist who is not interested in family medicine but rather has a special interest in addictions medicine and outpatient pain medicine.
“Every one of our patients who has chronic pain [is sent] to this physician first,” Dr. Chouinard explained. “He does the work-up. He looks to see if the patients are amenable to any kind of alternatives and then sends those patients back to us as primary care doctors.”
After that, every patient gets a pain contract that essentially provides consent to have urine tested on a regular basis to track adherence, as well as provide pill counts and office visits as required by the doctors. The physicians also are required to regularly check the PDMP database.
Patients who need this kind of treatment do not complain that the requirements are burdensome, said Dr. Chouinard, who described the program as successful overall. She said 30% have stayed properly adherent to their prescription narcotics and are living within the agreements of their contracts, while another 20% were taken off their medication because they were able to be placed on alternate, nonopioid therapies. About 30% ended up recommended for or enrolled in an addiction program, while 20% failed to maintain their contracts, and thus were unable to get their prescriptions.
Dr. Chouinard said a program like this could easily be rolled out across the country to help address the opioid abuse problem, as well as help remove primary care doctors from the prescribing loop.
“None of these people went into medicine because they had an interest in treating chronic pain,” she said.
In keeping with the overall message of getting doctors to use PDMPs more, Mr. Coukell said that getting this information integrated into electronic health records is “the Holy Grail,” though he added that getting practice alerts are a good short-term fix. He also said that getting consistency of reporting practice uniform across states and getting more states to trade information also was key, as is getting rules in place to allow someone in the office other than the doctor to have access to the PDMP databases (which some states do allow).
Centers for Disease Control and Prevention Director of the Division of Unintentional Injury Prevention Grant Baldwin, Ph.D., concurred.
“Providers have a limited amount of time to see patients,” Dr. Baldwin said. “Integrating all of these fixes will not necessarily extend the amount of time, but it actually makes it easier for the health care team to do their job in an efficient manner in that same 10-15 minute time."
WASHINGTON – The prescription drug–monitoring programs that exist in every state are not going to make a dent in opioid abuse unless physicians use them.
Because each state governs its own prescription drug–monitoring program (PDMP), the efficiency and effectiveness of each varies widely from the others. In the ideal, a PDMP collects information on which prescriptions are being filled by what patients as well as which physicians are prescribing the drugs. Information from the databases is used to help determine which patients might be doctor shopping for opioids as well as identify physicians who might be operating “pill mills” and contributing to the availability of narcotic pain medication that is used for other than clinical purposes.
Data from several studies have shown that in many instances, doctors do not realize the detail and usefulness of data from PDMPs, Allan Coukell, senior director for drugs and medical devices at Pew Charitable Trusts, Washington, said at a panel discussion on prescription drug abuse hosted by the Alliance for Health Reform and the Pharmaceutical Care Management Association, the industry lobby for pharmacy benefit managers.
When shown data on a specific patient, a physician was often “surprised to know how many other physicians that patient was seeing, and having that information changed their prescribing,” Mr. Coukell said, noting that in some cases, the information led to physicians refusing to prescribe pain medication and in others, it eased fears that a patient might be doctor shopping, giving the physician some peace of mind about writing a prescription for a narcotic.
Panelist Dr. Sarah Chouinard, medical director of Community Care of West Virginia, noted that physicians often are surprised when presented with data collected by PDMPs about their own individual prescribing habits.
“Eight out of 10 people were very surprised at the number of hydrocodone prescriptions they had written in the last 3 months,” Dr. Chouinard said.
In addition, the culture of administering pain medicine has gone through a shift in the last 10 years that is contributing to the problem of how many pills are available.
“About 10 years ago, there were doctors wandering around with buttons that said ‘No Pain’ with a red circle and a line through it because we were accused of under treating pain,” she observed. “Now, the pendulum has swung in the opposite direction. They used to say, ‘Hey, we’ll get sued if we don’t write pain medicine for these people,’ and now it’s, ‘Gosh, don’t write anything, or we will be a pill mill.’ I think the real answer is getting back to the middle and allowing the word to be out there that you don’t have to use a narcotic for every bump.”
To that end, Dr. Chouinard shared a solution her community organization has implemented. She said that “none of the [family physicians] are equipped, myself included, to treat chronic pain. Chronic pain is a specialty. ... It requires special training. The training that we as family doctors get now, after residency, is a mandatory 3 hours every 2 years.”
So Community Health of West Virgina hired an anesthetist who is not interested in family medicine but rather has a special interest in addictions medicine and outpatient pain medicine.
“Every one of our patients who has chronic pain [is sent] to this physician first,” Dr. Chouinard explained. “He does the work-up. He looks to see if the patients are amenable to any kind of alternatives and then sends those patients back to us as primary care doctors.”
After that, every patient gets a pain contract that essentially provides consent to have urine tested on a regular basis to track adherence, as well as provide pill counts and office visits as required by the doctors. The physicians also are required to regularly check the PDMP database.
Patients who need this kind of treatment do not complain that the requirements are burdensome, said Dr. Chouinard, who described the program as successful overall. She said 30% have stayed properly adherent to their prescription narcotics and are living within the agreements of their contracts, while another 20% were taken off their medication because they were able to be placed on alternate, nonopioid therapies. About 30% ended up recommended for or enrolled in an addiction program, while 20% failed to maintain their contracts, and thus were unable to get their prescriptions.
Dr. Chouinard said a program like this could easily be rolled out across the country to help address the opioid abuse problem, as well as help remove primary care doctors from the prescribing loop.
“None of these people went into medicine because they had an interest in treating chronic pain,” she said.
In keeping with the overall message of getting doctors to use PDMPs more, Mr. Coukell said that getting this information integrated into electronic health records is “the Holy Grail,” though he added that getting practice alerts are a good short-term fix. He also said that getting consistency of reporting practice uniform across states and getting more states to trade information also was key, as is getting rules in place to allow someone in the office other than the doctor to have access to the PDMP databases (which some states do allow).
Centers for Disease Control and Prevention Director of the Division of Unintentional Injury Prevention Grant Baldwin, Ph.D., concurred.
“Providers have a limited amount of time to see patients,” Dr. Baldwin said. “Integrating all of these fixes will not necessarily extend the amount of time, but it actually makes it easier for the health care team to do their job in an efficient manner in that same 10-15 minute time."
AT A PEW CHARITABLE TRUSTS–HOSTED PANEL