User login
Weight-loss drug lorcaserin’s glycemic effects revealed
BERLIN – Lower rates of incident type 2 diabetes mellitus (T2DM) and improved glycemic control were two of the metabolic effects seen with the appetite-suppressant drug lorcaserin versus placebo on top of existing lifestyle management measures in a large-scale trial of more than 12,000 overweight or obese individuals with established cardiovascular disease or T2DM and other cardiovascular risk factors.
In the CAMELLIA-TIMI 61 trial, treatment with a twice-daily, 10-mg dose of lorcaserin for a median of 3.3 years was associated with a significant 19% reduction in the risk of incident T2DM in participants with prediabetes, compared with placebo (8.5% vs. 10.3%; hazard ratio, 0.81; 95% confidence interval, 0.66-0.99; P = .038). The reduction in the risk of incident T2DM was even greater (23%) in people without diabetes at baseline (6.7% lorcaserin vs. 8.4% placebo; HR, 0.77; 95% CI, 0.63-0.94; P = .012).
Furthermore, in patients with T2DM who had a mean baseline glycated hemoglobin (HbA1c) of 7%, an absolute 0.33% reduction was seen at 1 year between the lorcaserin and placebo groups, with more modest but still significant between-group reductions (–0.09% and –0.08%) in individuals with prediabetes or normoglycemia (all P less than .0001). When baseline HbA1c levels were higher in patients with T2DM (8%), greater net reductions (0.52%) versus placebo were seen (P less than .0001).
These were some of the metabolic findings, published online in the Lancet to coincide with their presentation at the annual meeting of the European Association for the Study of Diabetes, that add to those already released from the CAMELLIA-TIMI 61 trial on cardiovascular safety, lead author and TIMI (Thrombolysis in Myocardial Infarction) group investigator Erin A. Bohula May, MD, observed during a press conference.
The cardiovascular safety data were presented at the 2018 annual congress of the European Society for Cardiology in August and published in the New England Journal of Medicine. These showed no increase with lorcaserin versus placebo in the risk of achieving a major cardiovascular endpoint (MACE) of cardiovascular death, MI, or stroke (HR, 0.99; 95% CI, 0.85-1.14; P less than .001 for noninferiority). There was also no difference between groups in the cumulative incidence of MACE+, which included heart failure, hospitalization for unstable angina, and the need for coronary revascularization (HR, 0.97; 95% CI, 0.87-1.07; P = .55 for superiority).
“We know that weight loss can improve cardiovascular and glycemic risk factors, but it’s difficult to achieve and maintain, and weight-loss agents are guideline-recommended adjuncts to lifestyle modification,” said Dr. Bohula May, who is a cardiovascular medicine and critical care specialist at Brigham and Women’s Hospital in Boston.
“However, prior to this study no agent had convincingly demonstrated cardiovascular safety in a rigorous clinical outcomes study,” she said, noting that several agents, such as the now-withdrawn rimonabant (Acomplia/Zimulti) and sibutramine (Meridia), had been shown to precipitate cardiovascular or psychiatric events, which led the Food and Drug Administration to mandate that all weight-loss drugs be assessed for cardiovascular safety. Lorcaserin (Belviq) is a centrally acting 5-HT2C agonist that works by decreasing appetite and was approved by the FDA in 2012 but is not currently available in Europe.
Long-term data on the effects of weight-loss agents on glycemic parameters were limited, hence the remit of the CAMELLIA-TIMI 61 trial was to assess both the cardiovascular and metabolic safety of lorcaserin. The drug was used on a background of lifestyle modification in 6,000 obese or overweight individuals at high risk of cardiovascular events. A further 6,000 individuals received placebo.
“Lorcaserin induced and maintained weight loss across the glycemic categories,” said coauthor and TIMI group investigator Benjamin Scirica, MD, also of Brigham and Women’s Hospital, who presented the metabolic data during a scientific session at the EASD meeting. Specifically, there was a net weight loss beyond that seen with placebo of 2.6 kg, 2.8 kg, and 3.3 kg in individuals with T2DM, prediabetes, and normoglycemia, respectively.
“Roughly 40% of patients with lorcaserin achieved a 5% weight loss, and about 14%-18% achieved a 10% weight loss across the glycemic categories,” Dr. Scirica reported. The corresponding values for the placebo-treated patients were 17%-18% and 4%-7%.
Naveed Sattar, MD, the independent commentator for the trial, noted the weight-loss reduction seen “was modest in the context of this trial, but I think the important point was that it was sustained. Sustained weight loss is difficult, and it was sustained on top of lifestyle and on top of the other drugs, and that is important.”
However, Dr. Sattar, who is professor and honorary consultant in cardiovascular and medical sciences at the University of Glasgow (Scotland), also observed that “as night follows day, glycemic improvements follow weight loss.” So, did the glycemic parameters improve purely because of the weight loss? While there is some preclinical evidence that lorcaserin may have an effect outside of its weight-lowering effects, Dr. Sattar felt this was unlikely to be clinically significant in itself.
“Obesity is probably the biggest challenge we have in the medical profession. We’ve got excellent cholesterol-lowering, blood pressure–lowering, and diabetes drugs. Yet obesity and complications are rising worldwide” and “safe weight-loss drugs remain sparse,” Dr. Sattar said.
He suggested that lorcaserin may well have an adjunctive place in the current treatment paradigm, but that place is probably “down the line” after other measures with greater weight-reducing effects or proven cardiovascular benefits were used. Not only are lifestyle modification approaches improving, Dr. Sattar said, but there are also over-the-counter options such as orlistat (Xenical), metformin, sodium-glucose cotransporter 2 inhibitors, glucagonlike peptide receptor–1 agonists, and bariatric surgery that are likely to be used first.
“This is a fantastically well done trial, we needed it,” Dr. Sattar said. However, because there was modest weight loss and no real cardiovascular benefit (but also no cardiovascular safety concern) he called the results “a bust” saying that “we have to take them at face value for what they are.”
Dr. Sattar noted that his “gut feeling at the moment is that the clinical role for lorcaserin is probably, at best, a down-the-line adjunct in those who are still obese for additional weight reduction on top of other drugs and lifestyle modifications, particularly in those who are ‘super responders.’ ” This is so long as the safety signals remain strong and there are quality of life benefits, he added.
The study was designed by the TIMI Study Group in conjunction with the executive committee and the trial sponsor, Eisai. Dr. Bohula May and Dr. Scirica reported receiving grants from Eisai, during the conduct of the study. Dr. Sattar reported grant support from Boehringer Ingelheim, and being part of an advisory board or speaker’s bureau for Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen Pharmaceuticals, Novo Nordisk, and Sanofi.
SOURCES: Bohula May EA et al. Lancet. 2018 Oct 4. doi: 10.1016/S0140-6736(18)32328-6; Bohula May EA et al. N Engl J Med. 2018; 379:1107-17; Sattar N. EASD 2018, Session S33.
BERLIN – Lower rates of incident type 2 diabetes mellitus (T2DM) and improved glycemic control were two of the metabolic effects seen with the appetite-suppressant drug lorcaserin versus placebo on top of existing lifestyle management measures in a large-scale trial of more than 12,000 overweight or obese individuals with established cardiovascular disease or T2DM and other cardiovascular risk factors.
In the CAMELLIA-TIMI 61 trial, treatment with a twice-daily, 10-mg dose of lorcaserin for a median of 3.3 years was associated with a significant 19% reduction in the risk of incident T2DM in participants with prediabetes, compared with placebo (8.5% vs. 10.3%; hazard ratio, 0.81; 95% confidence interval, 0.66-0.99; P = .038). The reduction in the risk of incident T2DM was even greater (23%) in people without diabetes at baseline (6.7% lorcaserin vs. 8.4% placebo; HR, 0.77; 95% CI, 0.63-0.94; P = .012).
Furthermore, in patients with T2DM who had a mean baseline glycated hemoglobin (HbA1c) of 7%, an absolute 0.33% reduction was seen at 1 year between the lorcaserin and placebo groups, with more modest but still significant between-group reductions (–0.09% and –0.08%) in individuals with prediabetes or normoglycemia (all P less than .0001). When baseline HbA1c levels were higher in patients with T2DM (8%), greater net reductions (0.52%) versus placebo were seen (P less than .0001).
These were some of the metabolic findings, published online in the Lancet to coincide with their presentation at the annual meeting of the European Association for the Study of Diabetes, that add to those already released from the CAMELLIA-TIMI 61 trial on cardiovascular safety, lead author and TIMI (Thrombolysis in Myocardial Infarction) group investigator Erin A. Bohula May, MD, observed during a press conference.
The cardiovascular safety data were presented at the 2018 annual congress of the European Society for Cardiology in August and published in the New England Journal of Medicine. These showed no increase with lorcaserin versus placebo in the risk of achieving a major cardiovascular endpoint (MACE) of cardiovascular death, MI, or stroke (HR, 0.99; 95% CI, 0.85-1.14; P less than .001 for noninferiority). There was also no difference between groups in the cumulative incidence of MACE+, which included heart failure, hospitalization for unstable angina, and the need for coronary revascularization (HR, 0.97; 95% CI, 0.87-1.07; P = .55 for superiority).
“We know that weight loss can improve cardiovascular and glycemic risk factors, but it’s difficult to achieve and maintain, and weight-loss agents are guideline-recommended adjuncts to lifestyle modification,” said Dr. Bohula May, who is a cardiovascular medicine and critical care specialist at Brigham and Women’s Hospital in Boston.
“However, prior to this study no agent had convincingly demonstrated cardiovascular safety in a rigorous clinical outcomes study,” she said, noting that several agents, such as the now-withdrawn rimonabant (Acomplia/Zimulti) and sibutramine (Meridia), had been shown to precipitate cardiovascular or psychiatric events, which led the Food and Drug Administration to mandate that all weight-loss drugs be assessed for cardiovascular safety. Lorcaserin (Belviq) is a centrally acting 5-HT2C agonist that works by decreasing appetite and was approved by the FDA in 2012 but is not currently available in Europe.
Long-term data on the effects of weight-loss agents on glycemic parameters were limited, hence the remit of the CAMELLIA-TIMI 61 trial was to assess both the cardiovascular and metabolic safety of lorcaserin. The drug was used on a background of lifestyle modification in 6,000 obese or overweight individuals at high risk of cardiovascular events. A further 6,000 individuals received placebo.
“Lorcaserin induced and maintained weight loss across the glycemic categories,” said coauthor and TIMI group investigator Benjamin Scirica, MD, also of Brigham and Women’s Hospital, who presented the metabolic data during a scientific session at the EASD meeting. Specifically, there was a net weight loss beyond that seen with placebo of 2.6 kg, 2.8 kg, and 3.3 kg in individuals with T2DM, prediabetes, and normoglycemia, respectively.
“Roughly 40% of patients with lorcaserin achieved a 5% weight loss, and about 14%-18% achieved a 10% weight loss across the glycemic categories,” Dr. Scirica reported. The corresponding values for the placebo-treated patients were 17%-18% and 4%-7%.
Naveed Sattar, MD, the independent commentator for the trial, noted the weight-loss reduction seen “was modest in the context of this trial, but I think the important point was that it was sustained. Sustained weight loss is difficult, and it was sustained on top of lifestyle and on top of the other drugs, and that is important.”
However, Dr. Sattar, who is professor and honorary consultant in cardiovascular and medical sciences at the University of Glasgow (Scotland), also observed that “as night follows day, glycemic improvements follow weight loss.” So, did the glycemic parameters improve purely because of the weight loss? While there is some preclinical evidence that lorcaserin may have an effect outside of its weight-lowering effects, Dr. Sattar felt this was unlikely to be clinically significant in itself.
“Obesity is probably the biggest challenge we have in the medical profession. We’ve got excellent cholesterol-lowering, blood pressure–lowering, and diabetes drugs. Yet obesity and complications are rising worldwide” and “safe weight-loss drugs remain sparse,” Dr. Sattar said.
He suggested that lorcaserin may well have an adjunctive place in the current treatment paradigm, but that place is probably “down the line” after other measures with greater weight-reducing effects or proven cardiovascular benefits were used. Not only are lifestyle modification approaches improving, Dr. Sattar said, but there are also over-the-counter options such as orlistat (Xenical), metformin, sodium-glucose cotransporter 2 inhibitors, glucagonlike peptide receptor–1 agonists, and bariatric surgery that are likely to be used first.
“This is a fantastically well done trial, we needed it,” Dr. Sattar said. However, because there was modest weight loss and no real cardiovascular benefit (but also no cardiovascular safety concern) he called the results “a bust” saying that “we have to take them at face value for what they are.”
Dr. Sattar noted that his “gut feeling at the moment is that the clinical role for lorcaserin is probably, at best, a down-the-line adjunct in those who are still obese for additional weight reduction on top of other drugs and lifestyle modifications, particularly in those who are ‘super responders.’ ” This is so long as the safety signals remain strong and there are quality of life benefits, he added.
The study was designed by the TIMI Study Group in conjunction with the executive committee and the trial sponsor, Eisai. Dr. Bohula May and Dr. Scirica reported receiving grants from Eisai, during the conduct of the study. Dr. Sattar reported grant support from Boehringer Ingelheim, and being part of an advisory board or speaker’s bureau for Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen Pharmaceuticals, Novo Nordisk, and Sanofi.
SOURCES: Bohula May EA et al. Lancet. 2018 Oct 4. doi: 10.1016/S0140-6736(18)32328-6; Bohula May EA et al. N Engl J Med. 2018; 379:1107-17; Sattar N. EASD 2018, Session S33.
BERLIN – Lower rates of incident type 2 diabetes mellitus (T2DM) and improved glycemic control were two of the metabolic effects seen with the appetite-suppressant drug lorcaserin versus placebo on top of existing lifestyle management measures in a large-scale trial of more than 12,000 overweight or obese individuals with established cardiovascular disease or T2DM and other cardiovascular risk factors.
In the CAMELLIA-TIMI 61 trial, treatment with a twice-daily, 10-mg dose of lorcaserin for a median of 3.3 years was associated with a significant 19% reduction in the risk of incident T2DM in participants with prediabetes, compared with placebo (8.5% vs. 10.3%; hazard ratio, 0.81; 95% confidence interval, 0.66-0.99; P = .038). The reduction in the risk of incident T2DM was even greater (23%) in people without diabetes at baseline (6.7% lorcaserin vs. 8.4% placebo; HR, 0.77; 95% CI, 0.63-0.94; P = .012).
Furthermore, in patients with T2DM who had a mean baseline glycated hemoglobin (HbA1c) of 7%, an absolute 0.33% reduction was seen at 1 year between the lorcaserin and placebo groups, with more modest but still significant between-group reductions (–0.09% and –0.08%) in individuals with prediabetes or normoglycemia (all P less than .0001). When baseline HbA1c levels were higher in patients with T2DM (8%), greater net reductions (0.52%) versus placebo were seen (P less than .0001).
These were some of the metabolic findings, published online in the Lancet to coincide with their presentation at the annual meeting of the European Association for the Study of Diabetes, that add to those already released from the CAMELLIA-TIMI 61 trial on cardiovascular safety, lead author and TIMI (Thrombolysis in Myocardial Infarction) group investigator Erin A. Bohula May, MD, observed during a press conference.
The cardiovascular safety data were presented at the 2018 annual congress of the European Society for Cardiology in August and published in the New England Journal of Medicine. These showed no increase with lorcaserin versus placebo in the risk of achieving a major cardiovascular endpoint (MACE) of cardiovascular death, MI, or stroke (HR, 0.99; 95% CI, 0.85-1.14; P less than .001 for noninferiority). There was also no difference between groups in the cumulative incidence of MACE+, which included heart failure, hospitalization for unstable angina, and the need for coronary revascularization (HR, 0.97; 95% CI, 0.87-1.07; P = .55 for superiority).
“We know that weight loss can improve cardiovascular and glycemic risk factors, but it’s difficult to achieve and maintain, and weight-loss agents are guideline-recommended adjuncts to lifestyle modification,” said Dr. Bohula May, who is a cardiovascular medicine and critical care specialist at Brigham and Women’s Hospital in Boston.
“However, prior to this study no agent had convincingly demonstrated cardiovascular safety in a rigorous clinical outcomes study,” she said, noting that several agents, such as the now-withdrawn rimonabant (Acomplia/Zimulti) and sibutramine (Meridia), had been shown to precipitate cardiovascular or psychiatric events, which led the Food and Drug Administration to mandate that all weight-loss drugs be assessed for cardiovascular safety. Lorcaserin (Belviq) is a centrally acting 5-HT2C agonist that works by decreasing appetite and was approved by the FDA in 2012 but is not currently available in Europe.
Long-term data on the effects of weight-loss agents on glycemic parameters were limited, hence the remit of the CAMELLIA-TIMI 61 trial was to assess both the cardiovascular and metabolic safety of lorcaserin. The drug was used on a background of lifestyle modification in 6,000 obese or overweight individuals at high risk of cardiovascular events. A further 6,000 individuals received placebo.
“Lorcaserin induced and maintained weight loss across the glycemic categories,” said coauthor and TIMI group investigator Benjamin Scirica, MD, also of Brigham and Women’s Hospital, who presented the metabolic data during a scientific session at the EASD meeting. Specifically, there was a net weight loss beyond that seen with placebo of 2.6 kg, 2.8 kg, and 3.3 kg in individuals with T2DM, prediabetes, and normoglycemia, respectively.
“Roughly 40% of patients with lorcaserin achieved a 5% weight loss, and about 14%-18% achieved a 10% weight loss across the glycemic categories,” Dr. Scirica reported. The corresponding values for the placebo-treated patients were 17%-18% and 4%-7%.
Naveed Sattar, MD, the independent commentator for the trial, noted the weight-loss reduction seen “was modest in the context of this trial, but I think the important point was that it was sustained. Sustained weight loss is difficult, and it was sustained on top of lifestyle and on top of the other drugs, and that is important.”
However, Dr. Sattar, who is professor and honorary consultant in cardiovascular and medical sciences at the University of Glasgow (Scotland), also observed that “as night follows day, glycemic improvements follow weight loss.” So, did the glycemic parameters improve purely because of the weight loss? While there is some preclinical evidence that lorcaserin may have an effect outside of its weight-lowering effects, Dr. Sattar felt this was unlikely to be clinically significant in itself.
“Obesity is probably the biggest challenge we have in the medical profession. We’ve got excellent cholesterol-lowering, blood pressure–lowering, and diabetes drugs. Yet obesity and complications are rising worldwide” and “safe weight-loss drugs remain sparse,” Dr. Sattar said.
He suggested that lorcaserin may well have an adjunctive place in the current treatment paradigm, but that place is probably “down the line” after other measures with greater weight-reducing effects or proven cardiovascular benefits were used. Not only are lifestyle modification approaches improving, Dr. Sattar said, but there are also over-the-counter options such as orlistat (Xenical), metformin, sodium-glucose cotransporter 2 inhibitors, glucagonlike peptide receptor–1 agonists, and bariatric surgery that are likely to be used first.
“This is a fantastically well done trial, we needed it,” Dr. Sattar said. However, because there was modest weight loss and no real cardiovascular benefit (but also no cardiovascular safety concern) he called the results “a bust” saying that “we have to take them at face value for what they are.”
Dr. Sattar noted that his “gut feeling at the moment is that the clinical role for lorcaserin is probably, at best, a down-the-line adjunct in those who are still obese for additional weight reduction on top of other drugs and lifestyle modifications, particularly in those who are ‘super responders.’ ” This is so long as the safety signals remain strong and there are quality of life benefits, he added.
The study was designed by the TIMI Study Group in conjunction with the executive committee and the trial sponsor, Eisai. Dr. Bohula May and Dr. Scirica reported receiving grants from Eisai, during the conduct of the study. Dr. Sattar reported grant support from Boehringer Ingelheim, and being part of an advisory board or speaker’s bureau for Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen Pharmaceuticals, Novo Nordisk, and Sanofi.
SOURCES: Bohula May EA et al. Lancet. 2018 Oct 4. doi: 10.1016/S0140-6736(18)32328-6; Bohula May EA et al. N Engl J Med. 2018; 379:1107-17; Sattar N. EASD 2018, Session S33.
REPORTING FROM EASD 2018
Key clinical point: Lorcaserin is an adjunctive treatment to lifestyle modification for chronic weight management that may improve metabolic health.
Major finding: A total of 8.5% of lorcaserin-treated individuals with prediabetes versus 10.3% of placebo-treated individuals developed incident type 2 diabetes mellitus at 1 year (hazard ratio, 0.81; 95% confidence interval, 0.66-0.99; P = .038).
Study details: A randomized, double-blind, placebo-controlled trial of 12,000 overweight or obese individuals with established cardiovascular disease, established or no type 2 diabetes mellitus, and other cardiovascular risk factors.
Disclosures: The study was designed by the Thrombolysis in Myocardial Infarction Study Group in conjunction with the executive committee and the trial sponsor, Eisai. Dr. Bohula May and Dr. Scirica reported receiving grants from Eisai, during the conduct of the study. Dr. Sattar reported grant support from Boehringer Ingelheim and being part of an advisory board or speaker’s bureau for Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen Pharmaceuticals, Novo Nordisk, and Sanofi.
Sources: Bohula May EA et al. Lancet. 2018. doi: 10.1016/S0140-6736(18)32328-6; Bohula May EA et al. N Engl J Med. 2018;379:1107-17; Sattar N. EASD 2018, Session S33.
Implementing the VA/DoD Type 2 Diabetes Mellitus Clinical Practice Guideline (FULL)
Paul Conlin, MD. Thank you all for joining us to talk about the recently released VA/DoD Clinical Practice Guideline for the Management of Type 2 Diabetes Mellitus in Primary Care (CPG). We’ve gathered together a group of experts who were part of the CPG development committee. We’re going to talk about some topics that were highlighted in the CPG that might provide additional detail to those in primary-care practices and help them in their management of patients with diabetes.
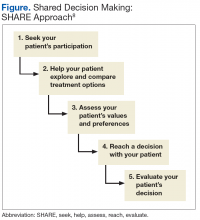
A unique feature of the VA/DoD CPG is that it emphasizes shared decision making as an important tool that clinicians should employ in their patient encounters. Dr. Watts, health care providers may wonder how they can make time for an intervention involving shared decision making using the SHARE approach, (ie, seek, help, assess, reach, and evaluate). Can you give us some advice on this?
Sharon Watts, DNP. Shared decision making is really crucial to success in diabetes. It’s been around for a while. We are trying to make an emphasis on this. The SHARE approach is from the Agency for Healthcare Research and Quality (AHRQ). The AHRQ has a wealth of information on its website. What AHRQ emphasizes is making it brief but conversational when you’re using the SHARE approach with your patient. Most importantly, the patient needs to be in the center of this dialogue, expressing his or her values and preferences of what’s most important to the whole team. This is a team effort. It’s not just with a provider. That’s where providers get overwhelmed. You can ask your nurse to advise the patient to write down 1 or 2 questions that are really important about diabetes before they come to see you, before the encounter. We can refer patients to diabetes classes where a lot of this information is given. The patient can talk to the dietitian or the pharmacist. There’s a whole team out there that will help in SHARE decision making. It’s crucial in the end for the provider to help the patient reach the decision and determine how best to treat the diabetes with them.
Dr. Conlin. Can you give a brief description of the key components of the SHARE approach?
Dr. Watts. Breaking it down simply, providers can start off by asking permission to go over the condition or treatment options because this immediately sets the stage as a signal to the patient that they are important in controlling the dialogue. It’s not the provider giving a discourse. You’re asking for permission. The next step would be to explore the benefits and risks of any course taken. Use decision aids if you have them. Keep in mind your patient’s current health literacy, numeracy, and other limitations.
Next ask about values, preferences, or barriers to whatever treatment you’re talking about. For instance, will this work with your work schedule?
Then the last thing would be ask what the patient wants to do next. Reach a decision on treatment, whatever it is, and make sure that you revisit that decision. Follow up later to see if it’s really working.
Dr. Conlin. If I’m a busy clinician and I have a limited amount of time with a patient, when are the appropriate times to employ the SHARE approach? Can I break it into components, where I address some elements during one visit and other elements in another visit?
Dr. Watts. Absolutely. It can be spread out. Your team is probably already providing information that will help in the SHARE approach. Just chart that you’ve done it. We know the SHARE approach is important because people tend to be adherent if they came up with part of the plan.
Dr. Conlin. Where does diabetes self-management education and diabetes self-management support fall into this framework?
Dr. Watts. Diabetes is a complex disease for providers and for the team and even more so for our patients. Invite them to diabetes classes. There’s so much to understand. The classes go over medications and blood sugar ranges, though you still may have to review it with the patient in your office. It saves the provider time if you have an informed and activated patient. It’s the same with sending a patient to a dietitian. I do all of the above.
Dr. Conlin. Many providers may not be familiar with this type of approach. How can I tell whether or not I’m doing it correctly?
Dr. Watts. The AHRQ website has conversation starters (www.ahrq.gov/professionals/education/curriculum-tools/shareddecisionmaking/tools/index.html). Then make sure when you are with the patient to use Teach-Back. Have that conversation and say, “I want to make sure I understood correctly what we decided would work best for you.” Ask patients to say in their own words what they understand. Then I think you’re off to a great start.
Dr. Conlin. Many patients tend to be deferential to their health care providers. They were brought up in an era where they needed to listen to and respect clinicians rather than participate in discussions about their ongoing care. How do you engage with these patients?
Dr. Watts. That is a tough one. Before the patient leaves the office, I ask them: Are there any barriers? Does this work for your schedule? Is this a preference and value that you have? Is there anything that might get in the way of this working when you go home? I try to pull out a little bit more, making sure to give them some decision aids and revisit it at the next visit to make sure it’s working.
Dr. Conlin. We’ll now turn to a discussion of using hemoglobin A1c (HbA1c) measurements in clinical practice. Dr. Aron, what factors can impact the relationship between HbA1c and blood glucose? How should we use HbA1c in the treatment of patients who come from varied ethnic and racial backgrounds, where the relationship to average blood glucose may be different?
David C. Aron, MD, MS. The identification of HbA1c has been a tremendous advance in our ability to manage patients with diabetes. It represents an average blood glucose over the preceding 3 months but like everything in this world, it has issues. One is the fact that there is a certain degree of inaccuracy in measurement, and that’s true of any measurement that you make of anything. Just as you allow a little bit of wiggle room when you’re driving down the New Jersey Turnpike and watching your speedometer, which is not 100% accurate. It says you are going 65 but it could, for example be 68 or 62. You don’t want to go too fast or you’ll get a speeding ticket. You don’t want to go too slowly or the person behind you will start honking at you. You want to be at the speed limit plus or minus. The first thing to think about in using HbA1c is the issue of accuracy. Rather than choose a specific target number, health care providers should choose a range between this and that. There’ll be more detail on that later.
The second thing is that part of the degree to which HbA1c represents the average blood glucose depends on a lot of factors, and some of these factors are things that we can do absolutely nothing about because we are born with them. African Americans tend to have higher HbA1c levels than do whites for the same glucose. That difference is as much as 0.4. An HbA1c of 6.2 in African Americans gets you a 5.8 in whites for the same average blood glucose. Similarly, Native Americans have somewhat higher HbA1c, although not quite as high as African Americans. Hispanics and Asians do as well, so you have to take your patient’s ethnicity into account.
The second has to do with the way that HbA1c is measured and the fact that there are many things that can affect the measurement. An HbA1c is dependent upon the lifespan of the red blood cell, so if there are alterations in red cell lifespan or if someone has anemia, that can affect HbA1c. Certain hemoglobin variants, for example, hemoglobin F, which is typically elevated in someone with thalassemia, migrates with some assays in the same place as thalassemia, so the assay can’t tell the difference between thalassemia and hemoglobin F. There are drugs and other conditions that can also affect HbA1c. You should think about HbA1c as a guide, but no number should be considered to be written in stone.
Dr. Conlin. I can imagine that this would be particularly important if you were using HbA1c as a criterion for diagnosing diabetes.
Dr. Aron. Quite right. The effects of race and ethnicity on HbA1c account for one of the differences between the VA/DoD guidelines and those of the American Diabetes Association (ADA).
Dr. Conlin. Isn’t < 8% HbA1c a national performance measure that people are asked to adhere to?
Dr. Aron. Not in the VA. In fact, the only performance measure that the VA has with a target is percent of patients with HbA1c > 9%, and we don’t want any of those or very few of them anyway. We have specifically avoided targets like < 8% HbA1c or < 7% HbA1c, which was prevalent some years ago, because the choice of HbA1c is very dependent upon the needs and desires of the individual patient. The VA has had stratified targets based on life expectancy and complications going back more than 15 years.
Dr. Conlin. Another issue that can confuse clinicians is when the HbA1c is in the target range but actually reflects an average of glucose levels that are at times very high and very low. How do we address this problem clinically?
Dr. Aron. In managing patients, you use whatever data you can get. The HbA1c gives you a general indication of average blood glucose, but particularly for those patients who are on insulin, it’s not a complete substitute for measuring blood glucose at appropriate times and taking the degree of glucose variability into account. We don’t want patients getting hypoglycemic, and particularly if they’re elderly, falling, or getting into a car accident. Similarly, we don’t want people to have very high blood sugars, even for limited periods of time, because they can lead to dehydration and other symptoms as well. We use a combination of both HbA1c and individual measures of blood glucose, like finger-stick blood sugar testing, typically.
Dr. Conlin. The VA/DoD CPG differs from other published guidelines in that we proposed patients are treated to HbA1c target ranges, whereas most other guidelines propose upper limits or threshold values, such as the HbA1c should be < 7% or < 8% but without lower bounds. Dr. Colburn, what are the target ranges that are recommended in the CPG? How were they determined?
Maj. Jeffrey A. Colburn, MD. It may be helpful to pull up the Determination of Average Target HbA1c Level Over Time table (page S17), which lays out risk for patients of treatment as well as the benefits of treatment. We first look at the patient’s state of health and whether they have a major comorbidity, a physiologic age that could be high risk, or advanced physiologic age with a diminished life expectancy. In controlling the levels of glucose, we’re often trying to benefit the microvascular health of the patient, realizing also that eventually poor management over time will lead to macrovascular disease as well. The main things that we see in child data is that the benefits of tight glucose control for younger patients with shorter duration of type 2 diabetes mellitus (T2DM) is the prevention of retinopathy, nephropathy, and peripheral neuropathy. Those patients that already have advanced microvascular disease are less likely to benefit from tight control. Trying to push glucose very low can harm the patient. It’s a delicate balance between the possible benefit vs the real harm.
The major trials are the ADVANCE, ACCORD, and the VADT trial, which was done in a VA population. To generalize the results, you are looking at an intensive control, which was trying to keep the HbA1c in general down below the 7% threshold. The patients enrolled in those trials all had microvascular and macrovascular disease and typically longer durations of diabetes at the time of the study. The studies revealed that we were not preventing macrovascular disease, heart attacks, strokes, the types of things that kill patients with diabetes. Individuals at higher HbA1c levels that went down to better HbA1c levels saw some improvement in the microvascular risk. Individuals already at the lower end didn’t see as much improvement. What we saw though that was surprising and concerning was that hypoglycemia, particularly severe hypoglycemia in the VADT trial was a lot more frequent when you try and target the HbA1c on the lower end. Because of these findings, we proposed the table with a set of ranges. As Dr. Aron noted, HbA1c is not a perfect test. It does have some variance in the number it presents. The CPG proposed to give individuals target ranges. They should be individualized based upon physiologic age, comorbidities, and life expectancy.
A criticism of the table that I commonly hear is what’s the magic crystal ball for determining somebody’s life expectancy? We don’t have one. This is a clinician’s judgment. The findings might actually change over time with the patient. A target HbA1c range is something that should be adapted and evolve along with the clinician and patient experience of the diabetes.
There are other important studies. For example, the UKPDS trials that included patients with shorter durations of diabetes and lesser disease to try and get their HbA1c levels on the lower end. We included that in the chart. Another concept we put forward is the idea of relative risk (RR) vs absolute risk. The RR reduction doesn’t speak to what the actual beginning risk is lowered to for a patient. The UKPDS is often cited for RR reduction of microvascular disease as 37% when an HbA1c of 7.9% is targeted down to 7.0%. The absolute risk reduction is actually 5 with the number needed to treat to do so is 20 patients. When we present the data, we give it a fair shake. We want individuals to guide therapy that is going to be both beneficial to preventing outcomes but also not harmful to the patient. I would highly recommend clinicians and patients look at this table together when making their decisions.
Dr. Conlin. In the VA/DoD CPG, the HbA1c target range for individuals with limited life expectancy extends to 9%. That may seem high for some, since most other guidelines propose lower HbA1c levels. How strong are the data that a person with limited life expectancy, say with end-stage renal disease or advanced complications, could be treated to a range of 8% to 9%? Shouldn’t lower levels actually improve life expectancy in such people?
Dr. Colburn. There’s much less data to support this level, which is why it’s cited in CPG as having weaker evidence. The reason it’s proposed is the experience of the workgroup and the evidence that is available of a high risk for patients with low life expectancy when they reduce their HbA1c greatly. One of the concerns about being at that level might be the real issue of renal glycosuria for individuals when their blood glucose is reaching above 180 mg/dL, which correlates to the 8% to 9% HbA1c range. You may have renal loss and risk of dehydration. It is an area where the clinician should be cautious in monitoring a patient in the 8% to 9% HbA1c range. With that being said, a patient who is having a lot of challenges in their health and extremely advanced conditions could be in that range. We would not expect a reversing of a micro- or macrovascular disease with glycemia control. We’re not going to go back from that level of disease they have. The idea about keeping them there is to prevent the risks of overtreatment and harm to the patient.
Dr. Conlin. Since patients with diabetes can progress over their lifetime from no complications to mild-to-moderate complications to advanced complications, how does the HbA1c target range evolve as a patient’s condition changes?
Dr. Colburn. As we check for evidence of microvascular disease or neuropathy signs, that evidence often is good for discussion between the clinician and patient to advise them that better control early on may help stem off or reverse some of that change. As those changes solidify, the patient is challenged by microvascular conditions. I would entertain allowing more relaxed HbA1c ranges to prevent harm to the patient given that we’re not going back. But you have to be careful. We have to consider benefits to the patient and the challenges for controlling glucose.
I hope that this table doesn’t make providers throw up their hands and give up. It’s meant to start a conversation on safety and benefits. With newer agents coming out that can help us control glucose quite well, without as much hypoglycemia risk, clinicians and patients potentially can try and get that HbA1c into a well-managed range.
Dr. Conlin. The CPG discusses various treatment options that might be available for patients who require pharmacologic therapy. The number of agents available is growing quite markedly. Dr. Colburn, can you describe how the CPG put together the pharmacologic therapy preferences.
Dr. Colburn. The CPG expressively stayed away from trying to promote specific regimens of medications. For example, other guidelines promote starting with certain agents followed by a second-line agent by a third-line agents. The concern that we had about that approach is that the medication landscape is rapidly evolving. The options available to clinicians and patients are really diverse at this moment, and the data are not concrete regarding what works best for a single patient.
Rather than trying to go from one agent to the next, we thought it best to discuss with patients using the SHARE decision-making model, the adverse effects (AEs) and relative benefits that are involved with each medication class to determine what might be best for the person. We have many new agents with evidence for possible reductions in cardiovascular outcomes outside of their glycemic control properties. As those evidences promote a potentially better option for a patient, we wanted to allow the room in management to make a decision together. I will say the CPG as well as all of the other applicable diabetes guidelines for T2DM promote metformin as the first therapy to consider for somebody with newly diagnosed T2DM because of safety and availability and the benefit that’s seen with that medication class. We ask clinicians to access the AHRQ website for updates as the medicines evolve.
In a rapidly changing landscape with new drugs coming into the market, each agency has on their individual website information about individual agents and their formulary status, criteria for use, and prior authorization requirements. We refer clinicians to the appropriate website for more information.
Dr. Conlin. There are a series of new medications that have recently come to market that seem to mitigate risk for hypoglycemia. Dr. Lugo, which treatment options carry greater risk? Which treatment options seem to have lesser risk for hypoglycemia?
Amy M. Lugo, PharmD. Insulin and the sulfonylureas have the highest risk of hypoglycemia. The sulfonylureas have fallen out of favor somewhat. One reason is that there are many newer agents that do not cause weight gain or increase the risk of hypoglycemia. Some of the newer insulins may have a lower risk of hypoglycemia and nocturnal hypoglycemia, in particular; however, it is difficult to conclude emphatically that one basal insulin analog is less likely to cause clinically relevant severe or nocturnal hypoglycemia events. This is due to the differences in the definitions of hypoglycemia used in the individual clinical trials, the open label study designs, and the different primary endpoints.
Dr. Conlin. How much affect on HbA1c might I expect to see using SGLT2 inhibitors or GLP-1 agonists? What would be some of the potential AEs I have to be aware of and therefore could counsel patients about?
Dr. Lugo. Let’s start with SGLT2 inhibitors. It depends on whether they are used as monotherapy or in combination. We prefer that patients start on metformin unless they have a contraindication. When used as monotherapy, the SGLT2s may decrease HbA1c from 0.4% to 1% from baseline. When combined with additional agents, they can have > 1% improvement in HbA1c from baseline. There are no head-to-head trials between any of the SGLT2 inhibitors. We cannot say that one is more efficacious than another in lowering HbA1c. The most common AEs include genital mycotic infections and urinary tract infections. The SGLT2 inhibitors also should be avoided in renal impairment. There was a recent FDA safety alert for the class for risk of ketoacidosis. Additionally, the FDA warned that patients with a history of bladder cancer should avoid dapagliflozin, and canagliflozin has a warning for increased risk of bone fractures, amputation, and decreased bone density.
Other actions of the SGLT2 inhibitors include a reduction in triglycerides and a modest increase in both low-density lipoprotein cholesterol and high-density lipoprotein cholesterol. The SGLT2 inhibitors also slightly decrease systolic blood pressure (by 4 mm Hg to 6 mm Hg) and body weight (reduction of 1.8 kg)
The GLP-1s are likely to be more efficacious in reducing HbA1c. Typically we see 1% or greater lowering in HbA1c from baseline. As a class, the GLP-1 agonists have a lower risk of hypoglycemia; however, the risk increases when combined with sulfonylureas or insulin. The dose of insulin or sulfonylurea will likely need to be decreased when used concomitantly.
Patients are likely to experience weight loss when on a GLP-1 agonist, which is a great benefit. Gastrointestinal AEs such as nausea are common. Adverse effects may differ somewhat between the agents.
Dr. Conlin. Patients’ experience of care is integral to their engagement with treatment as well as their adherence. Ms. Decesare, what are patients looking for from their health care team?
Elaine M. Decesare. Patients are looking for a knowledgeable and compassionate health care team that has a consistent approach and a consistent message and that the team is updated on the knowledge of appropriate treatments and appropriate lifestyle modifications and targets for the care of diabetes.
Also, I think that the team needs to have some empathy for the challenges of living with diabetes. It’s a 24-hour-a-day disorder, 7 days a week. They can’t take vacation from it. They just can’t take a pill and forget about it. It’s a fairly demanding disorder, and sometimes just acknowledging that with the patient can help you with the dialog.
The second thing I think patients want is an effective treatment plan that’s tailored to their needs and lifestyles. That goes in with the shared decision-making approach, but the plan itself really has to be likely to achieve the targets and the goals that you’ve set up. Sometimes I see patients who are doing all they can with their lifestyle changes, but they can’t get to goal, because there isn’t enough medication in the plan. The plan has to be adequate so that the patient can manage their diabetes. In the shared approach, the patient has to buy in to the plan. With the shared decision making they’re more likely to take the plan on as their plan.
Dr. Conlin. How do you respond to patients who feel treatment burnout from having a new dietary plan, an exercise program, regular monitoring of glucose through finger sticks, and in many cases multiple medications and or injections, while potentially not achieving the goals that you and the patient have arrived at?
Ms. Decesare. First, I want to assess their mood. Sometimes patients are depressed, and they actually need help with that. If they have trouble with just the management, we do have behavioral health psychologists on our team that work with patients to get through some of the barriers and discuss some of the feelings that they have about diabetes and diabetes management.
Sometimes we look at the plan again and see if there’s something we can do to make the plan easier. Occasionally, something has happened in their life. Maybe they’re taking care of an elderly parent or they’ve had other health problems that have come about that we need to reassess the plan and make sure that it’s actually doable for them at this point in time.
Certainly diabetes self-management education can be helpful. Some of those approaches can be helpful for finding something that’s going to work for patients in the long run, because it can be a very difficult disorder to manage as time goes on.
Dr. Colburn. Type 2 DM disproportionately affects individuals who are ≥ 65 years compared with younger individuals. Such older patients also are more likely to have cognitive impairment or visual issues. How do we best manage such patients?
Ms. Decesare. When I’m looking at the care plan, social support is very important. If someone has social support and they have a spouse or a son or daughter or someone else that can help them with their diabetes, we oftentimes will get them involved with the plan, as long as it’s fine with the patient, to offer some help, especially with the patients with cognitive problems, because sometimes the patients just cognitively cannot manage diabetes on their own. Prandial insulin could be a really dangerous product for someone who has cognitive disease.
I think you have to look at all the resources that are available. Sometimes you have to change your HbA1c target range to something that’s going to be manageable for that patient at that time. It might not be perfect, but it would be better to have no hypoglycemia rather than a real aggressive HbA1c target or a target range, if that’s what’s going to keep the patient safe.
Dr. Conlin. We thank our discussants for sharing very practical advice on how to implement the CPG. We hope this information supports clinicians as they develop treatment plans based on each patient's unique characteristics and goals of care.
Paul Conlin, MD. Thank you all for joining us to talk about the recently released VA/DoD Clinical Practice Guideline for the Management of Type 2 Diabetes Mellitus in Primary Care (CPG). We’ve gathered together a group of experts who were part of the CPG development committee. We’re going to talk about some topics that were highlighted in the CPG that might provide additional detail to those in primary-care practices and help them in their management of patients with diabetes.
A unique feature of the VA/DoD CPG is that it emphasizes shared decision making as an important tool that clinicians should employ in their patient encounters. Dr. Watts, health care providers may wonder how they can make time for an intervention involving shared decision making using the SHARE approach, (ie, seek, help, assess, reach, and evaluate). Can you give us some advice on this?
Sharon Watts, DNP. Shared decision making is really crucial to success in diabetes. It’s been around for a while. We are trying to make an emphasis on this. The SHARE approach is from the Agency for Healthcare Research and Quality (AHRQ). The AHRQ has a wealth of information on its website. What AHRQ emphasizes is making it brief but conversational when you’re using the SHARE approach with your patient. Most importantly, the patient needs to be in the center of this dialogue, expressing his or her values and preferences of what’s most important to the whole team. This is a team effort. It’s not just with a provider. That’s where providers get overwhelmed. You can ask your nurse to advise the patient to write down 1 or 2 questions that are really important about diabetes before they come to see you, before the encounter. We can refer patients to diabetes classes where a lot of this information is given. The patient can talk to the dietitian or the pharmacist. There’s a whole team out there that will help in SHARE decision making. It’s crucial in the end for the provider to help the patient reach the decision and determine how best to treat the diabetes with them.
Dr. Conlin. Can you give a brief description of the key components of the SHARE approach?
Dr. Watts. Breaking it down simply, providers can start off by asking permission to go over the condition or treatment options because this immediately sets the stage as a signal to the patient that they are important in controlling the dialogue. It’s not the provider giving a discourse. You’re asking for permission. The next step would be to explore the benefits and risks of any course taken. Use decision aids if you have them. Keep in mind your patient’s current health literacy, numeracy, and other limitations.
Next ask about values, preferences, or barriers to whatever treatment you’re talking about. For instance, will this work with your work schedule?
Then the last thing would be ask what the patient wants to do next. Reach a decision on treatment, whatever it is, and make sure that you revisit that decision. Follow up later to see if it’s really working.
Dr. Conlin. If I’m a busy clinician and I have a limited amount of time with a patient, when are the appropriate times to employ the SHARE approach? Can I break it into components, where I address some elements during one visit and other elements in another visit?
Dr. Watts. Absolutely. It can be spread out. Your team is probably already providing information that will help in the SHARE approach. Just chart that you’ve done it. We know the SHARE approach is important because people tend to be adherent if they came up with part of the plan.
Dr. Conlin. Where does diabetes self-management education and diabetes self-management support fall into this framework?
Dr. Watts. Diabetes is a complex disease for providers and for the team and even more so for our patients. Invite them to diabetes classes. There’s so much to understand. The classes go over medications and blood sugar ranges, though you still may have to review it with the patient in your office. It saves the provider time if you have an informed and activated patient. It’s the same with sending a patient to a dietitian. I do all of the above.
Dr. Conlin. Many providers may not be familiar with this type of approach. How can I tell whether or not I’m doing it correctly?
Dr. Watts. The AHRQ website has conversation starters (www.ahrq.gov/professionals/education/curriculum-tools/shareddecisionmaking/tools/index.html). Then make sure when you are with the patient to use Teach-Back. Have that conversation and say, “I want to make sure I understood correctly what we decided would work best for you.” Ask patients to say in their own words what they understand. Then I think you’re off to a great start.
Dr. Conlin. Many patients tend to be deferential to their health care providers. They were brought up in an era where they needed to listen to and respect clinicians rather than participate in discussions about their ongoing care. How do you engage with these patients?
Dr. Watts. That is a tough one. Before the patient leaves the office, I ask them: Are there any barriers? Does this work for your schedule? Is this a preference and value that you have? Is there anything that might get in the way of this working when you go home? I try to pull out a little bit more, making sure to give them some decision aids and revisit it at the next visit to make sure it’s working.
Dr. Conlin. We’ll now turn to a discussion of using hemoglobin A1c (HbA1c) measurements in clinical practice. Dr. Aron, what factors can impact the relationship between HbA1c and blood glucose? How should we use HbA1c in the treatment of patients who come from varied ethnic and racial backgrounds, where the relationship to average blood glucose may be different?
David C. Aron, MD, MS. The identification of HbA1c has been a tremendous advance in our ability to manage patients with diabetes. It represents an average blood glucose over the preceding 3 months but like everything in this world, it has issues. One is the fact that there is a certain degree of inaccuracy in measurement, and that’s true of any measurement that you make of anything. Just as you allow a little bit of wiggle room when you’re driving down the New Jersey Turnpike and watching your speedometer, which is not 100% accurate. It says you are going 65 but it could, for example be 68 or 62. You don’t want to go too fast or you’ll get a speeding ticket. You don’t want to go too slowly or the person behind you will start honking at you. You want to be at the speed limit plus or minus. The first thing to think about in using HbA1c is the issue of accuracy. Rather than choose a specific target number, health care providers should choose a range between this and that. There’ll be more detail on that later.
The second thing is that part of the degree to which HbA1c represents the average blood glucose depends on a lot of factors, and some of these factors are things that we can do absolutely nothing about because we are born with them. African Americans tend to have higher HbA1c levels than do whites for the same glucose. That difference is as much as 0.4. An HbA1c of 6.2 in African Americans gets you a 5.8 in whites for the same average blood glucose. Similarly, Native Americans have somewhat higher HbA1c, although not quite as high as African Americans. Hispanics and Asians do as well, so you have to take your patient’s ethnicity into account.
The second has to do with the way that HbA1c is measured and the fact that there are many things that can affect the measurement. An HbA1c is dependent upon the lifespan of the red blood cell, so if there are alterations in red cell lifespan or if someone has anemia, that can affect HbA1c. Certain hemoglobin variants, for example, hemoglobin F, which is typically elevated in someone with thalassemia, migrates with some assays in the same place as thalassemia, so the assay can’t tell the difference between thalassemia and hemoglobin F. There are drugs and other conditions that can also affect HbA1c. You should think about HbA1c as a guide, but no number should be considered to be written in stone.
Dr. Conlin. I can imagine that this would be particularly important if you were using HbA1c as a criterion for diagnosing diabetes.
Dr. Aron. Quite right. The effects of race and ethnicity on HbA1c account for one of the differences between the VA/DoD guidelines and those of the American Diabetes Association (ADA).
Dr. Conlin. Isn’t < 8% HbA1c a national performance measure that people are asked to adhere to?
Dr. Aron. Not in the VA. In fact, the only performance measure that the VA has with a target is percent of patients with HbA1c > 9%, and we don’t want any of those or very few of them anyway. We have specifically avoided targets like < 8% HbA1c or < 7% HbA1c, which was prevalent some years ago, because the choice of HbA1c is very dependent upon the needs and desires of the individual patient. The VA has had stratified targets based on life expectancy and complications going back more than 15 years.
Dr. Conlin. Another issue that can confuse clinicians is when the HbA1c is in the target range but actually reflects an average of glucose levels that are at times very high and very low. How do we address this problem clinically?
Dr. Aron. In managing patients, you use whatever data you can get. The HbA1c gives you a general indication of average blood glucose, but particularly for those patients who are on insulin, it’s not a complete substitute for measuring blood glucose at appropriate times and taking the degree of glucose variability into account. We don’t want patients getting hypoglycemic, and particularly if they’re elderly, falling, or getting into a car accident. Similarly, we don’t want people to have very high blood sugars, even for limited periods of time, because they can lead to dehydration and other symptoms as well. We use a combination of both HbA1c and individual measures of blood glucose, like finger-stick blood sugar testing, typically.
Dr. Conlin. The VA/DoD CPG differs from other published guidelines in that we proposed patients are treated to HbA1c target ranges, whereas most other guidelines propose upper limits or threshold values, such as the HbA1c should be < 7% or < 8% but without lower bounds. Dr. Colburn, what are the target ranges that are recommended in the CPG? How were they determined?
Maj. Jeffrey A. Colburn, MD. It may be helpful to pull up the Determination of Average Target HbA1c Level Over Time table (page S17), which lays out risk for patients of treatment as well as the benefits of treatment. We first look at the patient’s state of health and whether they have a major comorbidity, a physiologic age that could be high risk, or advanced physiologic age with a diminished life expectancy. In controlling the levels of glucose, we’re often trying to benefit the microvascular health of the patient, realizing also that eventually poor management over time will lead to macrovascular disease as well. The main things that we see in child data is that the benefits of tight glucose control for younger patients with shorter duration of type 2 diabetes mellitus (T2DM) is the prevention of retinopathy, nephropathy, and peripheral neuropathy. Those patients that already have advanced microvascular disease are less likely to benefit from tight control. Trying to push glucose very low can harm the patient. It’s a delicate balance between the possible benefit vs the real harm.
The major trials are the ADVANCE, ACCORD, and the VADT trial, which was done in a VA population. To generalize the results, you are looking at an intensive control, which was trying to keep the HbA1c in general down below the 7% threshold. The patients enrolled in those trials all had microvascular and macrovascular disease and typically longer durations of diabetes at the time of the study. The studies revealed that we were not preventing macrovascular disease, heart attacks, strokes, the types of things that kill patients with diabetes. Individuals at higher HbA1c levels that went down to better HbA1c levels saw some improvement in the microvascular risk. Individuals already at the lower end didn’t see as much improvement. What we saw though that was surprising and concerning was that hypoglycemia, particularly severe hypoglycemia in the VADT trial was a lot more frequent when you try and target the HbA1c on the lower end. Because of these findings, we proposed the table with a set of ranges. As Dr. Aron noted, HbA1c is not a perfect test. It does have some variance in the number it presents. The CPG proposed to give individuals target ranges. They should be individualized based upon physiologic age, comorbidities, and life expectancy.
A criticism of the table that I commonly hear is what’s the magic crystal ball for determining somebody’s life expectancy? We don’t have one. This is a clinician’s judgment. The findings might actually change over time with the patient. A target HbA1c range is something that should be adapted and evolve along with the clinician and patient experience of the diabetes.
There are other important studies. For example, the UKPDS trials that included patients with shorter durations of diabetes and lesser disease to try and get their HbA1c levels on the lower end. We included that in the chart. Another concept we put forward is the idea of relative risk (RR) vs absolute risk. The RR reduction doesn’t speak to what the actual beginning risk is lowered to for a patient. The UKPDS is often cited for RR reduction of microvascular disease as 37% when an HbA1c of 7.9% is targeted down to 7.0%. The absolute risk reduction is actually 5 with the number needed to treat to do so is 20 patients. When we present the data, we give it a fair shake. We want individuals to guide therapy that is going to be both beneficial to preventing outcomes but also not harmful to the patient. I would highly recommend clinicians and patients look at this table together when making their decisions.
Dr. Conlin. In the VA/DoD CPG, the HbA1c target range for individuals with limited life expectancy extends to 9%. That may seem high for some, since most other guidelines propose lower HbA1c levels. How strong are the data that a person with limited life expectancy, say with end-stage renal disease or advanced complications, could be treated to a range of 8% to 9%? Shouldn’t lower levels actually improve life expectancy in such people?
Dr. Colburn. There’s much less data to support this level, which is why it’s cited in CPG as having weaker evidence. The reason it’s proposed is the experience of the workgroup and the evidence that is available of a high risk for patients with low life expectancy when they reduce their HbA1c greatly. One of the concerns about being at that level might be the real issue of renal glycosuria for individuals when their blood glucose is reaching above 180 mg/dL, which correlates to the 8% to 9% HbA1c range. You may have renal loss and risk of dehydration. It is an area where the clinician should be cautious in monitoring a patient in the 8% to 9% HbA1c range. With that being said, a patient who is having a lot of challenges in their health and extremely advanced conditions could be in that range. We would not expect a reversing of a micro- or macrovascular disease with glycemia control. We’re not going to go back from that level of disease they have. The idea about keeping them there is to prevent the risks of overtreatment and harm to the patient.
Dr. Conlin. Since patients with diabetes can progress over their lifetime from no complications to mild-to-moderate complications to advanced complications, how does the HbA1c target range evolve as a patient’s condition changes?
Dr. Colburn. As we check for evidence of microvascular disease or neuropathy signs, that evidence often is good for discussion between the clinician and patient to advise them that better control early on may help stem off or reverse some of that change. As those changes solidify, the patient is challenged by microvascular conditions. I would entertain allowing more relaxed HbA1c ranges to prevent harm to the patient given that we’re not going back. But you have to be careful. We have to consider benefits to the patient and the challenges for controlling glucose.
I hope that this table doesn’t make providers throw up their hands and give up. It’s meant to start a conversation on safety and benefits. With newer agents coming out that can help us control glucose quite well, without as much hypoglycemia risk, clinicians and patients potentially can try and get that HbA1c into a well-managed range.
Dr. Conlin. The CPG discusses various treatment options that might be available for patients who require pharmacologic therapy. The number of agents available is growing quite markedly. Dr. Colburn, can you describe how the CPG put together the pharmacologic therapy preferences.
Dr. Colburn. The CPG expressively stayed away from trying to promote specific regimens of medications. For example, other guidelines promote starting with certain agents followed by a second-line agent by a third-line agents. The concern that we had about that approach is that the medication landscape is rapidly evolving. The options available to clinicians and patients are really diverse at this moment, and the data are not concrete regarding what works best for a single patient.
Rather than trying to go from one agent to the next, we thought it best to discuss with patients using the SHARE decision-making model, the adverse effects (AEs) and relative benefits that are involved with each medication class to determine what might be best for the person. We have many new agents with evidence for possible reductions in cardiovascular outcomes outside of their glycemic control properties. As those evidences promote a potentially better option for a patient, we wanted to allow the room in management to make a decision together. I will say the CPG as well as all of the other applicable diabetes guidelines for T2DM promote metformin as the first therapy to consider for somebody with newly diagnosed T2DM because of safety and availability and the benefit that’s seen with that medication class. We ask clinicians to access the AHRQ website for updates as the medicines evolve.
In a rapidly changing landscape with new drugs coming into the market, each agency has on their individual website information about individual agents and their formulary status, criteria for use, and prior authorization requirements. We refer clinicians to the appropriate website for more information.
Dr. Conlin. There are a series of new medications that have recently come to market that seem to mitigate risk for hypoglycemia. Dr. Lugo, which treatment options carry greater risk? Which treatment options seem to have lesser risk for hypoglycemia?
Amy M. Lugo, PharmD. Insulin and the sulfonylureas have the highest risk of hypoglycemia. The sulfonylureas have fallen out of favor somewhat. One reason is that there are many newer agents that do not cause weight gain or increase the risk of hypoglycemia. Some of the newer insulins may have a lower risk of hypoglycemia and nocturnal hypoglycemia, in particular; however, it is difficult to conclude emphatically that one basal insulin analog is less likely to cause clinically relevant severe or nocturnal hypoglycemia events. This is due to the differences in the definitions of hypoglycemia used in the individual clinical trials, the open label study designs, and the different primary endpoints.
Dr. Conlin. How much affect on HbA1c might I expect to see using SGLT2 inhibitors or GLP-1 agonists? What would be some of the potential AEs I have to be aware of and therefore could counsel patients about?
Dr. Lugo. Let’s start with SGLT2 inhibitors. It depends on whether they are used as monotherapy or in combination. We prefer that patients start on metformin unless they have a contraindication. When used as monotherapy, the SGLT2s may decrease HbA1c from 0.4% to 1% from baseline. When combined with additional agents, they can have > 1% improvement in HbA1c from baseline. There are no head-to-head trials between any of the SGLT2 inhibitors. We cannot say that one is more efficacious than another in lowering HbA1c. The most common AEs include genital mycotic infections and urinary tract infections. The SGLT2 inhibitors also should be avoided in renal impairment. There was a recent FDA safety alert for the class for risk of ketoacidosis. Additionally, the FDA warned that patients with a history of bladder cancer should avoid dapagliflozin, and canagliflozin has a warning for increased risk of bone fractures, amputation, and decreased bone density.
Other actions of the SGLT2 inhibitors include a reduction in triglycerides and a modest increase in both low-density lipoprotein cholesterol and high-density lipoprotein cholesterol. The SGLT2 inhibitors also slightly decrease systolic blood pressure (by 4 mm Hg to 6 mm Hg) and body weight (reduction of 1.8 kg)
The GLP-1s are likely to be more efficacious in reducing HbA1c. Typically we see 1% or greater lowering in HbA1c from baseline. As a class, the GLP-1 agonists have a lower risk of hypoglycemia; however, the risk increases when combined with sulfonylureas or insulin. The dose of insulin or sulfonylurea will likely need to be decreased when used concomitantly.
Patients are likely to experience weight loss when on a GLP-1 agonist, which is a great benefit. Gastrointestinal AEs such as nausea are common. Adverse effects may differ somewhat between the agents.
Dr. Conlin. Patients’ experience of care is integral to their engagement with treatment as well as their adherence. Ms. Decesare, what are patients looking for from their health care team?
Elaine M. Decesare. Patients are looking for a knowledgeable and compassionate health care team that has a consistent approach and a consistent message and that the team is updated on the knowledge of appropriate treatments and appropriate lifestyle modifications and targets for the care of diabetes.
Also, I think that the team needs to have some empathy for the challenges of living with diabetes. It’s a 24-hour-a-day disorder, 7 days a week. They can’t take vacation from it. They just can’t take a pill and forget about it. It’s a fairly demanding disorder, and sometimes just acknowledging that with the patient can help you with the dialog.
The second thing I think patients want is an effective treatment plan that’s tailored to their needs and lifestyles. That goes in with the shared decision-making approach, but the plan itself really has to be likely to achieve the targets and the goals that you’ve set up. Sometimes I see patients who are doing all they can with their lifestyle changes, but they can’t get to goal, because there isn’t enough medication in the plan. The plan has to be adequate so that the patient can manage their diabetes. In the shared approach, the patient has to buy in to the plan. With the shared decision making they’re more likely to take the plan on as their plan.
Dr. Conlin. How do you respond to patients who feel treatment burnout from having a new dietary plan, an exercise program, regular monitoring of glucose through finger sticks, and in many cases multiple medications and or injections, while potentially not achieving the goals that you and the patient have arrived at?
Ms. Decesare. First, I want to assess their mood. Sometimes patients are depressed, and they actually need help with that. If they have trouble with just the management, we do have behavioral health psychologists on our team that work with patients to get through some of the barriers and discuss some of the feelings that they have about diabetes and diabetes management.
Sometimes we look at the plan again and see if there’s something we can do to make the plan easier. Occasionally, something has happened in their life. Maybe they’re taking care of an elderly parent or they’ve had other health problems that have come about that we need to reassess the plan and make sure that it’s actually doable for them at this point in time.
Certainly diabetes self-management education can be helpful. Some of those approaches can be helpful for finding something that’s going to work for patients in the long run, because it can be a very difficult disorder to manage as time goes on.
Dr. Colburn. Type 2 DM disproportionately affects individuals who are ≥ 65 years compared with younger individuals. Such older patients also are more likely to have cognitive impairment or visual issues. How do we best manage such patients?
Ms. Decesare. When I’m looking at the care plan, social support is very important. If someone has social support and they have a spouse or a son or daughter or someone else that can help them with their diabetes, we oftentimes will get them involved with the plan, as long as it’s fine with the patient, to offer some help, especially with the patients with cognitive problems, because sometimes the patients just cognitively cannot manage diabetes on their own. Prandial insulin could be a really dangerous product for someone who has cognitive disease.
I think you have to look at all the resources that are available. Sometimes you have to change your HbA1c target range to something that’s going to be manageable for that patient at that time. It might not be perfect, but it would be better to have no hypoglycemia rather than a real aggressive HbA1c target or a target range, if that’s what’s going to keep the patient safe.
Dr. Conlin. We thank our discussants for sharing very practical advice on how to implement the CPG. We hope this information supports clinicians as they develop treatment plans based on each patient's unique characteristics and goals of care.
Paul Conlin, MD. Thank you all for joining us to talk about the recently released VA/DoD Clinical Practice Guideline for the Management of Type 2 Diabetes Mellitus in Primary Care (CPG). We’ve gathered together a group of experts who were part of the CPG development committee. We’re going to talk about some topics that were highlighted in the CPG that might provide additional detail to those in primary-care practices and help them in their management of patients with diabetes.
A unique feature of the VA/DoD CPG is that it emphasizes shared decision making as an important tool that clinicians should employ in their patient encounters. Dr. Watts, health care providers may wonder how they can make time for an intervention involving shared decision making using the SHARE approach, (ie, seek, help, assess, reach, and evaluate). Can you give us some advice on this?
Sharon Watts, DNP. Shared decision making is really crucial to success in diabetes. It’s been around for a while. We are trying to make an emphasis on this. The SHARE approach is from the Agency for Healthcare Research and Quality (AHRQ). The AHRQ has a wealth of information on its website. What AHRQ emphasizes is making it brief but conversational when you’re using the SHARE approach with your patient. Most importantly, the patient needs to be in the center of this dialogue, expressing his or her values and preferences of what’s most important to the whole team. This is a team effort. It’s not just with a provider. That’s where providers get overwhelmed. You can ask your nurse to advise the patient to write down 1 or 2 questions that are really important about diabetes before they come to see you, before the encounter. We can refer patients to diabetes classes where a lot of this information is given. The patient can talk to the dietitian or the pharmacist. There’s a whole team out there that will help in SHARE decision making. It’s crucial in the end for the provider to help the patient reach the decision and determine how best to treat the diabetes with them.
Dr. Conlin. Can you give a brief description of the key components of the SHARE approach?
Dr. Watts. Breaking it down simply, providers can start off by asking permission to go over the condition or treatment options because this immediately sets the stage as a signal to the patient that they are important in controlling the dialogue. It’s not the provider giving a discourse. You’re asking for permission. The next step would be to explore the benefits and risks of any course taken. Use decision aids if you have them. Keep in mind your patient’s current health literacy, numeracy, and other limitations.
Next ask about values, preferences, or barriers to whatever treatment you’re talking about. For instance, will this work with your work schedule?
Then the last thing would be ask what the patient wants to do next. Reach a decision on treatment, whatever it is, and make sure that you revisit that decision. Follow up later to see if it’s really working.
Dr. Conlin. If I’m a busy clinician and I have a limited amount of time with a patient, when are the appropriate times to employ the SHARE approach? Can I break it into components, where I address some elements during one visit and other elements in another visit?
Dr. Watts. Absolutely. It can be spread out. Your team is probably already providing information that will help in the SHARE approach. Just chart that you’ve done it. We know the SHARE approach is important because people tend to be adherent if they came up with part of the plan.
Dr. Conlin. Where does diabetes self-management education and diabetes self-management support fall into this framework?
Dr. Watts. Diabetes is a complex disease for providers and for the team and even more so for our patients. Invite them to diabetes classes. There’s so much to understand. The classes go over medications and blood sugar ranges, though you still may have to review it with the patient in your office. It saves the provider time if you have an informed and activated patient. It’s the same with sending a patient to a dietitian. I do all of the above.
Dr. Conlin. Many providers may not be familiar with this type of approach. How can I tell whether or not I’m doing it correctly?
Dr. Watts. The AHRQ website has conversation starters (www.ahrq.gov/professionals/education/curriculum-tools/shareddecisionmaking/tools/index.html). Then make sure when you are with the patient to use Teach-Back. Have that conversation and say, “I want to make sure I understood correctly what we decided would work best for you.” Ask patients to say in their own words what they understand. Then I think you’re off to a great start.
Dr. Conlin. Many patients tend to be deferential to their health care providers. They were brought up in an era where they needed to listen to and respect clinicians rather than participate in discussions about their ongoing care. How do you engage with these patients?
Dr. Watts. That is a tough one. Before the patient leaves the office, I ask them: Are there any barriers? Does this work for your schedule? Is this a preference and value that you have? Is there anything that might get in the way of this working when you go home? I try to pull out a little bit more, making sure to give them some decision aids and revisit it at the next visit to make sure it’s working.
Dr. Conlin. We’ll now turn to a discussion of using hemoglobin A1c (HbA1c) measurements in clinical practice. Dr. Aron, what factors can impact the relationship between HbA1c and blood glucose? How should we use HbA1c in the treatment of patients who come from varied ethnic and racial backgrounds, where the relationship to average blood glucose may be different?
David C. Aron, MD, MS. The identification of HbA1c has been a tremendous advance in our ability to manage patients with diabetes. It represents an average blood glucose over the preceding 3 months but like everything in this world, it has issues. One is the fact that there is a certain degree of inaccuracy in measurement, and that’s true of any measurement that you make of anything. Just as you allow a little bit of wiggle room when you’re driving down the New Jersey Turnpike and watching your speedometer, which is not 100% accurate. It says you are going 65 but it could, for example be 68 or 62. You don’t want to go too fast or you’ll get a speeding ticket. You don’t want to go too slowly or the person behind you will start honking at you. You want to be at the speed limit plus or minus. The first thing to think about in using HbA1c is the issue of accuracy. Rather than choose a specific target number, health care providers should choose a range between this and that. There’ll be more detail on that later.
The second thing is that part of the degree to which HbA1c represents the average blood glucose depends on a lot of factors, and some of these factors are things that we can do absolutely nothing about because we are born with them. African Americans tend to have higher HbA1c levels than do whites for the same glucose. That difference is as much as 0.4. An HbA1c of 6.2 in African Americans gets you a 5.8 in whites for the same average blood glucose. Similarly, Native Americans have somewhat higher HbA1c, although not quite as high as African Americans. Hispanics and Asians do as well, so you have to take your patient’s ethnicity into account.
The second has to do with the way that HbA1c is measured and the fact that there are many things that can affect the measurement. An HbA1c is dependent upon the lifespan of the red blood cell, so if there are alterations in red cell lifespan or if someone has anemia, that can affect HbA1c. Certain hemoglobin variants, for example, hemoglobin F, which is typically elevated in someone with thalassemia, migrates with some assays in the same place as thalassemia, so the assay can’t tell the difference between thalassemia and hemoglobin F. There are drugs and other conditions that can also affect HbA1c. You should think about HbA1c as a guide, but no number should be considered to be written in stone.
Dr. Conlin. I can imagine that this would be particularly important if you were using HbA1c as a criterion for diagnosing diabetes.
Dr. Aron. Quite right. The effects of race and ethnicity on HbA1c account for one of the differences between the VA/DoD guidelines and those of the American Diabetes Association (ADA).
Dr. Conlin. Isn’t < 8% HbA1c a national performance measure that people are asked to adhere to?
Dr. Aron. Not in the VA. In fact, the only performance measure that the VA has with a target is percent of patients with HbA1c > 9%, and we don’t want any of those or very few of them anyway. We have specifically avoided targets like < 8% HbA1c or < 7% HbA1c, which was prevalent some years ago, because the choice of HbA1c is very dependent upon the needs and desires of the individual patient. The VA has had stratified targets based on life expectancy and complications going back more than 15 years.
Dr. Conlin. Another issue that can confuse clinicians is when the HbA1c is in the target range but actually reflects an average of glucose levels that are at times very high and very low. How do we address this problem clinically?
Dr. Aron. In managing patients, you use whatever data you can get. The HbA1c gives you a general indication of average blood glucose, but particularly for those patients who are on insulin, it’s not a complete substitute for measuring blood glucose at appropriate times and taking the degree of glucose variability into account. We don’t want patients getting hypoglycemic, and particularly if they’re elderly, falling, or getting into a car accident. Similarly, we don’t want people to have very high blood sugars, even for limited periods of time, because they can lead to dehydration and other symptoms as well. We use a combination of both HbA1c and individual measures of blood glucose, like finger-stick blood sugar testing, typically.
Dr. Conlin. The VA/DoD CPG differs from other published guidelines in that we proposed patients are treated to HbA1c target ranges, whereas most other guidelines propose upper limits or threshold values, such as the HbA1c should be < 7% or < 8% but without lower bounds. Dr. Colburn, what are the target ranges that are recommended in the CPG? How were they determined?
Maj. Jeffrey A. Colburn, MD. It may be helpful to pull up the Determination of Average Target HbA1c Level Over Time table (page S17), which lays out risk for patients of treatment as well as the benefits of treatment. We first look at the patient’s state of health and whether they have a major comorbidity, a physiologic age that could be high risk, or advanced physiologic age with a diminished life expectancy. In controlling the levels of glucose, we’re often trying to benefit the microvascular health of the patient, realizing also that eventually poor management over time will lead to macrovascular disease as well. The main things that we see in child data is that the benefits of tight glucose control for younger patients with shorter duration of type 2 diabetes mellitus (T2DM) is the prevention of retinopathy, nephropathy, and peripheral neuropathy. Those patients that already have advanced microvascular disease are less likely to benefit from tight control. Trying to push glucose very low can harm the patient. It’s a delicate balance between the possible benefit vs the real harm.
The major trials are the ADVANCE, ACCORD, and the VADT trial, which was done in a VA population. To generalize the results, you are looking at an intensive control, which was trying to keep the HbA1c in general down below the 7% threshold. The patients enrolled in those trials all had microvascular and macrovascular disease and typically longer durations of diabetes at the time of the study. The studies revealed that we were not preventing macrovascular disease, heart attacks, strokes, the types of things that kill patients with diabetes. Individuals at higher HbA1c levels that went down to better HbA1c levels saw some improvement in the microvascular risk. Individuals already at the lower end didn’t see as much improvement. What we saw though that was surprising and concerning was that hypoglycemia, particularly severe hypoglycemia in the VADT trial was a lot more frequent when you try and target the HbA1c on the lower end. Because of these findings, we proposed the table with a set of ranges. As Dr. Aron noted, HbA1c is not a perfect test. It does have some variance in the number it presents. The CPG proposed to give individuals target ranges. They should be individualized based upon physiologic age, comorbidities, and life expectancy.
A criticism of the table that I commonly hear is what’s the magic crystal ball for determining somebody’s life expectancy? We don’t have one. This is a clinician’s judgment. The findings might actually change over time with the patient. A target HbA1c range is something that should be adapted and evolve along with the clinician and patient experience of the diabetes.
There are other important studies. For example, the UKPDS trials that included patients with shorter durations of diabetes and lesser disease to try and get their HbA1c levels on the lower end. We included that in the chart. Another concept we put forward is the idea of relative risk (RR) vs absolute risk. The RR reduction doesn’t speak to what the actual beginning risk is lowered to for a patient. The UKPDS is often cited for RR reduction of microvascular disease as 37% when an HbA1c of 7.9% is targeted down to 7.0%. The absolute risk reduction is actually 5 with the number needed to treat to do so is 20 patients. When we present the data, we give it a fair shake. We want individuals to guide therapy that is going to be both beneficial to preventing outcomes but also not harmful to the patient. I would highly recommend clinicians and patients look at this table together when making their decisions.
Dr. Conlin. In the VA/DoD CPG, the HbA1c target range for individuals with limited life expectancy extends to 9%. That may seem high for some, since most other guidelines propose lower HbA1c levels. How strong are the data that a person with limited life expectancy, say with end-stage renal disease or advanced complications, could be treated to a range of 8% to 9%? Shouldn’t lower levels actually improve life expectancy in such people?
Dr. Colburn. There’s much less data to support this level, which is why it’s cited in CPG as having weaker evidence. The reason it’s proposed is the experience of the workgroup and the evidence that is available of a high risk for patients with low life expectancy when they reduce their HbA1c greatly. One of the concerns about being at that level might be the real issue of renal glycosuria for individuals when their blood glucose is reaching above 180 mg/dL, which correlates to the 8% to 9% HbA1c range. You may have renal loss and risk of dehydration. It is an area where the clinician should be cautious in monitoring a patient in the 8% to 9% HbA1c range. With that being said, a patient who is having a lot of challenges in their health and extremely advanced conditions could be in that range. We would not expect a reversing of a micro- or macrovascular disease with glycemia control. We’re not going to go back from that level of disease they have. The idea about keeping them there is to prevent the risks of overtreatment and harm to the patient.
Dr. Conlin. Since patients with diabetes can progress over their lifetime from no complications to mild-to-moderate complications to advanced complications, how does the HbA1c target range evolve as a patient’s condition changes?
Dr. Colburn. As we check for evidence of microvascular disease or neuropathy signs, that evidence often is good for discussion between the clinician and patient to advise them that better control early on may help stem off or reverse some of that change. As those changes solidify, the patient is challenged by microvascular conditions. I would entertain allowing more relaxed HbA1c ranges to prevent harm to the patient given that we’re not going back. But you have to be careful. We have to consider benefits to the patient and the challenges for controlling glucose.
I hope that this table doesn’t make providers throw up their hands and give up. It’s meant to start a conversation on safety and benefits. With newer agents coming out that can help us control glucose quite well, without as much hypoglycemia risk, clinicians and patients potentially can try and get that HbA1c into a well-managed range.
Dr. Conlin. The CPG discusses various treatment options that might be available for patients who require pharmacologic therapy. The number of agents available is growing quite markedly. Dr. Colburn, can you describe how the CPG put together the pharmacologic therapy preferences.
Dr. Colburn. The CPG expressively stayed away from trying to promote specific regimens of medications. For example, other guidelines promote starting with certain agents followed by a second-line agent by a third-line agents. The concern that we had about that approach is that the medication landscape is rapidly evolving. The options available to clinicians and patients are really diverse at this moment, and the data are not concrete regarding what works best for a single patient.
Rather than trying to go from one agent to the next, we thought it best to discuss with patients using the SHARE decision-making model, the adverse effects (AEs) and relative benefits that are involved with each medication class to determine what might be best for the person. We have many new agents with evidence for possible reductions in cardiovascular outcomes outside of their glycemic control properties. As those evidences promote a potentially better option for a patient, we wanted to allow the room in management to make a decision together. I will say the CPG as well as all of the other applicable diabetes guidelines for T2DM promote metformin as the first therapy to consider for somebody with newly diagnosed T2DM because of safety and availability and the benefit that’s seen with that medication class. We ask clinicians to access the AHRQ website for updates as the medicines evolve.
In a rapidly changing landscape with new drugs coming into the market, each agency has on their individual website information about individual agents and their formulary status, criteria for use, and prior authorization requirements. We refer clinicians to the appropriate website for more information.
Dr. Conlin. There are a series of new medications that have recently come to market that seem to mitigate risk for hypoglycemia. Dr. Lugo, which treatment options carry greater risk? Which treatment options seem to have lesser risk for hypoglycemia?
Amy M. Lugo, PharmD. Insulin and the sulfonylureas have the highest risk of hypoglycemia. The sulfonylureas have fallen out of favor somewhat. One reason is that there are many newer agents that do not cause weight gain or increase the risk of hypoglycemia. Some of the newer insulins may have a lower risk of hypoglycemia and nocturnal hypoglycemia, in particular; however, it is difficult to conclude emphatically that one basal insulin analog is less likely to cause clinically relevant severe or nocturnal hypoglycemia events. This is due to the differences in the definitions of hypoglycemia used in the individual clinical trials, the open label study designs, and the different primary endpoints.
Dr. Conlin. How much affect on HbA1c might I expect to see using SGLT2 inhibitors or GLP-1 agonists? What would be some of the potential AEs I have to be aware of and therefore could counsel patients about?
Dr. Lugo. Let’s start with SGLT2 inhibitors. It depends on whether they are used as monotherapy or in combination. We prefer that patients start on metformin unless they have a contraindication. When used as monotherapy, the SGLT2s may decrease HbA1c from 0.4% to 1% from baseline. When combined with additional agents, they can have > 1% improvement in HbA1c from baseline. There are no head-to-head trials between any of the SGLT2 inhibitors. We cannot say that one is more efficacious than another in lowering HbA1c. The most common AEs include genital mycotic infections and urinary tract infections. The SGLT2 inhibitors also should be avoided in renal impairment. There was a recent FDA safety alert for the class for risk of ketoacidosis. Additionally, the FDA warned that patients with a history of bladder cancer should avoid dapagliflozin, and canagliflozin has a warning for increased risk of bone fractures, amputation, and decreased bone density.
Other actions of the SGLT2 inhibitors include a reduction in triglycerides and a modest increase in both low-density lipoprotein cholesterol and high-density lipoprotein cholesterol. The SGLT2 inhibitors also slightly decrease systolic blood pressure (by 4 mm Hg to 6 mm Hg) and body weight (reduction of 1.8 kg)
The GLP-1s are likely to be more efficacious in reducing HbA1c. Typically we see 1% or greater lowering in HbA1c from baseline. As a class, the GLP-1 agonists have a lower risk of hypoglycemia; however, the risk increases when combined with sulfonylureas or insulin. The dose of insulin or sulfonylurea will likely need to be decreased when used concomitantly.
Patients are likely to experience weight loss when on a GLP-1 agonist, which is a great benefit. Gastrointestinal AEs such as nausea are common. Adverse effects may differ somewhat between the agents.
Dr. Conlin. Patients’ experience of care is integral to their engagement with treatment as well as their adherence. Ms. Decesare, what are patients looking for from their health care team?
Elaine M. Decesare. Patients are looking for a knowledgeable and compassionate health care team that has a consistent approach and a consistent message and that the team is updated on the knowledge of appropriate treatments and appropriate lifestyle modifications and targets for the care of diabetes.
Also, I think that the team needs to have some empathy for the challenges of living with diabetes. It’s a 24-hour-a-day disorder, 7 days a week. They can’t take vacation from it. They just can’t take a pill and forget about it. It’s a fairly demanding disorder, and sometimes just acknowledging that with the patient can help you with the dialog.
The second thing I think patients want is an effective treatment plan that’s tailored to their needs and lifestyles. That goes in with the shared decision-making approach, but the plan itself really has to be likely to achieve the targets and the goals that you’ve set up. Sometimes I see patients who are doing all they can with their lifestyle changes, but they can’t get to goal, because there isn’t enough medication in the plan. The plan has to be adequate so that the patient can manage their diabetes. In the shared approach, the patient has to buy in to the plan. With the shared decision making they’re more likely to take the plan on as their plan.
Dr. Conlin. How do you respond to patients who feel treatment burnout from having a new dietary plan, an exercise program, regular monitoring of glucose through finger sticks, and in many cases multiple medications and or injections, while potentially not achieving the goals that you and the patient have arrived at?
Ms. Decesare. First, I want to assess their mood. Sometimes patients are depressed, and they actually need help with that. If they have trouble with just the management, we do have behavioral health psychologists on our team that work with patients to get through some of the barriers and discuss some of the feelings that they have about diabetes and diabetes management.
Sometimes we look at the plan again and see if there’s something we can do to make the plan easier. Occasionally, something has happened in their life. Maybe they’re taking care of an elderly parent or they’ve had other health problems that have come about that we need to reassess the plan and make sure that it’s actually doable for them at this point in time.
Certainly diabetes self-management education can be helpful. Some of those approaches can be helpful for finding something that’s going to work for patients in the long run, because it can be a very difficult disorder to manage as time goes on.
Dr. Colburn. Type 2 DM disproportionately affects individuals who are ≥ 65 years compared with younger individuals. Such older patients also are more likely to have cognitive impairment or visual issues. How do we best manage such patients?
Ms. Decesare. When I’m looking at the care plan, social support is very important. If someone has social support and they have a spouse or a son or daughter or someone else that can help them with their diabetes, we oftentimes will get them involved with the plan, as long as it’s fine with the patient, to offer some help, especially with the patients with cognitive problems, because sometimes the patients just cognitively cannot manage diabetes on their own. Prandial insulin could be a really dangerous product for someone who has cognitive disease.
I think you have to look at all the resources that are available. Sometimes you have to change your HbA1c target range to something that’s going to be manageable for that patient at that time. It might not be perfect, but it would be better to have no hypoglycemia rather than a real aggressive HbA1c target or a target range, if that’s what’s going to keep the patient safe.
Dr. Conlin. We thank our discussants for sharing very practical advice on how to implement the CPG. We hope this information supports clinicians as they develop treatment plans based on each patient's unique characteristics and goals of care.
Implementing the 2017 VA/DoD Diabetes Clinical Practice Guideline (FULL)
Paul Conlin, MD. Thank you all for joining us to talk about the recently released VA/DoD Clinical Practice Guideline for the Management of Type 2 Diabetes Mellitus in Primary Care (CPG). We’ve gathered together a group of experts who were part of the CPG development committee. We’re going to talk about some topics that were highlighted in the CPG that might provide additional detail to those in primary-care practices and help them in their management of patients with diabetes.
A unique feature of the VA/DoD CPG is that it emphasizes shared decision making as an important tool that clinicians should employ in their patient encounters. Dr. Watts, health care providers may wonder how they can make time for an intervention involving shared decision making using the SHARE approach, (ie, seek, help, assess, reach, and evaluate). Can you give us some advice on this?
Sharon Watts, DNP. Shared decision making is really crucial to success in diabetes. It’s been around for a while. We are trying to make an emphasis on this. The SHARE approach is from the Agency for Healthcare Research and Quality (AHRQ). The AHRQ has a wealth of information on its website. What AHRQ emphasizes is making it brief but conversational when you’re using the SHARE approach with your patient. Most importantly, the patient needs to be in the center of this dialogue, expressing his or her values and preferences of what’s most important to the whole team. This is a team effort. It’s not just with a provider. That’s where providers get overwhelmed. You can ask your nurse to advise the patient to write down 1 or 2 questions that are really important about diabetes before they come to see you, before the encounter. We can refer patients to diabetes classes where a lot of this information is given. The patient can talk to the dietitian or the pharmacist. There’s a whole team out there that will help in SHARE decision making. It’s crucial in the end for the provider to help the patient reach the decision and determine how best to treat the diabetes with them.
Dr. Conlin. Can you give a brief description of the key components of the SHARE approach?
Dr. Watts. Breaking it down simply, providers can start off by asking permission to go over the condition or treatment options because this immediately sets the stage as a signal to the patient that they are important in controlling the dialogue. It’s not the provider giving a discourse. You’re asking for permission. The next step would be to explore the benefits and risks of any course taken. Use decision aids if you have them. Keep in mind your patient’s current health literacy, numeracy, and other limitations.
Next ask about values, preferences, or barriers to whatever treatment you’re talking about. For instance, will this work with your work schedule?
Then the last thing would be ask what the patient wants to do next. Reach a decision on treatment, whatever it is, and make sure that you revisit that decision. Follow up later to see if it’s really working.
Dr. Conlin. If I’m a busy clinician and I have a limited amount of time with a patient, when are the appropriate times to employ the SHARE approach? Can I break it into components, where I address some elements during one visit and other elements in another visit?
Dr. Watts. Absolutely. It can be spread out. Your team is probably already providing information that will help in the SHARE approach. Just chart that you’ve done it. We know the SHARE approach is important because people
tend to be adherent if they came up with part of the plan.
Dr. Conlin. Where does diabetes self-management education and diabetes self-management support fall into this framework?
Dr. Watts. Diabetes is a complex disease for providers and for the team and even more so for our patients. Invite them to diabetes classes. There’s so much to understand. The classes go over medications and blood sugar ranges, though you still may have to review it with the patient in your office. It saves the provider time if you have an informed and activated patient. It’s the same with sending a patient to a dietitian. I do all of the above.
Dr. Conlin. Many providers may not be familiar with this type of approach. How can I tell whether or not I’m doing it correctly?
Dr. Watts. The AHRQ website has conversation starters (www.ahrq.gov/professionals/education/curriculum-tools/shareddecisionmaking/tools/index.html). Then make sure when you are with the patient to use Teach-Back. Have that conversation and say, “I want to make sure I understood correctly what we decided would work best for you.” Ask patients to say in their own words what they understand. Then I think you’re off to a great start.
Dr. Conlin. Many patients tend to be deferential to their health care providers. They were brought up in an era where they needed to listen to and respect clinicians rather than participate in discussions about their ongoing care. How do you engage with these patients?
Dr. Watts. That is a tough one. Before the patient leaves the office, I ask them: Are there any barriers? Does this work for your schedule? Is this a preference and value that you have? Is there anything that might get in the way of this working when you go home? I try to pull out a little bit more, making sure to give them some decision aids and revisit it at the next visit to make sure it’s working.
Dr. Conlin. We’ll now turn to a discussion of using hemoglobin A1c (HbA1c) measurements in clinical practice. Dr. Aron, what factors can impact the relationship between HbA1c and blood glucose? How should we use HbA1c in the treatment of patients who come from varied ethnic and racial backgrounds, where the relationship to average blood glucose may be different?
David C. Aron, MD, MS. The identification of HbA1c has been a tremendous advance in our ability to manage patients with diabetes. It represents an average blood glucose over the preceding 3 months but like everything in this world, it has issues. One is the fact that there is a certain degree of inaccuracy in measurement, and that’s true of any measurement that you make of anything. Just as you allow a little bit of wiggle room when you’re driving down the New Jersey Turnpike and watching your speedometer, which is not 100% accurate. It says you are going 65 but it could, for example be 68 or 62. You don’t want to go too fast or you’ll get a speeding ticket. You don’t want to go too slowly or the person behind you will start honking at you. You want to be at the speed limit plus or minus. The first thing to think about in using HbA1c is the issue of accuracy. Rather than choose a specific target number, health care providers should choose a range between this and that. There’ll be more detail on that later.
The second thing is that part of the degree to which HbA1c represents the average blood glucose depends on a lot of factors, and some of these factors are things that we can do absolutely nothing about because we are born with them. African Americans tend to have higher HbA1c levels than do whites for the same glucose. That difference is as much as 0.4. An HbA1c of 6.2 in African Americans gets you a 5.8 in whites for the same average blood glucose. Similarly, Native Americans have somewhat higher HbA1c, although not quite as high as African Americans. Hispanics and Asians do as well, so you have to take your patient’s ethnicity into account.
The second has to do with the way that HbA1c is measured and the fact that there are many things that can affect the measurement. An HbA1c is dependent upon the lifespan of the red blood cell, so if there are alterations in red cell lifespan or if someone has anemia, that can affect HbA1c. Certain hemoglobin variants, for example, hemoglobin F, which is typically elevated in someone with thalassemia, migrates with some assays in the same place as thalassemia, so the assay can’t tell the difference between thalassemia and hemoglobin F. There are drugs and other conditions that can also affect HbA1c. You should think about HbA1c as a guide, but no number should be considered to be written in stone.
Dr. Conlin. I can imagine that this would be particularly important if you were using HbA1c as a criterion for diagnosing diabetes.
Dr. Aron. Quite right. The effects of race and ethnicity on HbA1c account for one of the differences between the VA/DoD guidelines and those of the American Diabetes Association (ADA).
Dr. Conlin. Isn’t < 8% HbA1c a national performance measure that people are asked to adhere to?
Dr. Aron. Not in the VA. In fact, the only performance measure that the VA has with a target is percent of patients with HbA1c > 9%, and we don’t want any of those or very few of them anyway. We have specifically avoided targets like < 8% HbA1c or < 7% HbA1c, which was prevalent some years ago, because the choice of HbA1c is very dependent upon the needs and desires of the individual patient. The VA has had stratified targets based on life expectancy and complications going back more than 15 years.
Dr. Conlin. Another issue that can confuse clinicians is when the HbA1c is in the target range but actually reflects an average of glucose levels that are at times very high and very low. How do we address this problem clinically?
Dr. Aron. In managing patients, you use whatever data you can get. The HbA1c gives you a general indication of average blood glucose, but particularly for those patients who are on insulin, it’s not a complete substitute for measuring blood glucose at appropriate times and taking the degree of glucose variability into account. We don’t want patients getting hypoglycemic, and particularly if they’re elderly, falling, or getting into a car accident. Similarly, we don’t want people to have very high blood sugars, even for limited periods of time, because they can lead to dehydration and other symptoms as well. We use a combination of both HbA1c and individual measures of blood glucose, like finger-stick blood sugar testing, typically.
Dr. Conlin. The VA/DoD CPG differs from other published guidelines in that we proposed patients are treated to HbA1c target ranges, whereas most other guidelines propose upper limits or threshold values, such as the HbA1c should be < 7% or < 8% but without lower bounds. Dr. Colburn, what are the target ranges that are recommended in the CPG? How were they determined?
Maj. Jeffrey A. Colburn, MD. It may be helpful to pull up the Determination of Average Target HbA1c Level Over Time table (page S17), which lays out risk for patients of treatment as well as the benefits of treatment. We first look at the patient’s state of health and whether they have a major comorbidity, a physiologic age that could be high risk, or advanced physiologic age with a diminished life expectancy. In controlling the levels of glucose, we’re often trying to benefit the microvascular health of the patient, realizing also that eventually poor management over time will lead to macrovascular disease as well. The main things that we see in child data is that the benefits of tight glucose control for younger patients with shorter duration of type 2 diabetes mellitus (T2DM) is the prevention of retinopathy, nephropathy, and peripheral neuropathy. Those patients that already have advanced microvascular disease are less likely to benefit from tight control. Trying to push glucose very low can harm the patient. It’s a delicate balance between the possible benefit vs the real harm.
The major trials are the ADVANCE, ACCORD, and the VADT trial, which was done in a VA population. To generalize the results, you are looking at an intensive control, which was trying to keep the HbA1c in general down below the 7% threshold. The patients enrolled in those trials all had microvascular and macrovascular disease and typically longer durations of diabetes at the time of the study. The studies revealed that we were not preventing macrovascular disease, heart attacks, strokes, the types of things that kill patients with diabetes. Individuals at higher HbA1c levels that went down to better HbA1c levels saw some improvement in the microvascular risk. Individuals already at the lower end didn’t see as much improvement. What we saw though that was surprising and concerning was that hypoglycemia, particularly severe hypoglycemia in the VADT trial was a lot more frequent when you try and target the HbA1c on the lower end. Because of these findings, we proposed the table with a set of ranges. As Dr. Aron noted, HbA1c is not a perfect test. It does have some variance in the number it presents. The CPG proposed to give individuals target ranges. They should be individualized based upon physiologic age, comorbidities, and life expectancy.
A criticism of the table that I commonly hear is what’s the magic crystal ball for determining somebody’s life expectancy? We don’t have one. This is a clinician’s judgment. The findings might actually change over time with the patient. A target HbA1c range is something that should be adapted and evolve along with the clinician and patient experience of the diabetes.
There are other important studies. For example, the UKPDS trials that included patients with shorter durations of diabetes and lesser disease to try and get their HbA1c levels on the lower end. We included that in the chart. Another concept we put forward is the idea of relative risk (RR) vs absolute risk. The RR reduction doesn’t speak to what the actual beginning risk is lowered to for a patient. The UKPDS is often cited for RR reduction of microvascular disease as 37% when an HbA1c of 7.9% is targeted down to 7.0%. The absolute risk reduction is actually 5 with the number needed to treat to do so is 20 patients. When we present the data, we give it a fair shake. We want individuals to guide therapy that is going to be both beneficial to preventing outcomes but also not harmful to the patient. I would highly recommend clinicians and patients look at this table together when making their decisions.
Dr. Conlin. In the VA/DoD CPG, the HbA1c target range for individuals with limited life expectancy extends to 9%. That may seem high for some, since most other guidelines propose lower HbA1c levels. How strong are the data that a person with limited life expectancy, say with end-stage renal disease or advanced complications, could be treated to a range of 8% to 9%? Shouldn’t lower levels actually improve life expectancy in such people?
Dr. Colburn. There’s much less data to support this level, which is why it’s cited in CPG as having weaker evidence. The reason it’s proposed is the experience of the workgroup and the evidence that is available of a high risk for patients with low life expectancy when they reduce their HbA1c greatly. One of the concerns about being at that level might be the real issue of renal glycosuria for individuals when their blood glucose is reaching above 180 mg/dL, which correlates to the 8% to 9% HbA1c range. You may have renal loss and risk of dehydration. It is an area where the clinician should be cautious in monitoring a patient in the 8% to 9% HbA1c range. With that being said, a patient who is having a lot of challenges in their health and extremely advanced conditions could be in that range. We would not expect a reversing of a micro- or macrovascular disease with glycemia control. We’re not going to go back from that level of disease they have. The idea about keeping them there is to prevent the risks of overtreatment and harm to the patient.
Dr. Conlin. Since patients with diabetes can progress over their lifetime from no complications to mild-to-moderate complications to advanced complications, how does the HbA1c target range evolve as a patient’s condition changes?
Dr. Colburn. As we check for evidence of microvascular disease or neuropathy signs, that evidence often is good for discussion between the clinician and patient to advise them that better control early on may help stem off or reverse some of that change. As those changes solidify, the patient is challenged by microvascular conditions. I would entertain allowing more relaxed HbA1c ranges to prevent harm to the patient given that we’re not going back. But you have to be careful. We have to consider benefits to the patient and the challenges for controlling glucose.
I hope that this table doesn’t make providers throw up their hands and give up. It’s meant to start a conversation on safety and benefits. With newer agents coming out that can help us control glucose quite well, without as much hypoglycemia risk, clinicians and patients potentially can try and get that HbA1c into a well-managed range.
Dr. Conlin. The CPG discusses various treatment options that might be available for patients who require pharmacologic therapy. The number of agents available is growing quite markedly. Dr. Colburn, can you describe how the CPG put together the pharmacologic therapy preferences.
Dr. Colburn. The CPG expressively stayed away from trying to promote specific regimens of medications. For example, other guidelines promote starting with certain agents followed by a second-line agent by a third-line agents. The concern that we had about that approach is that the medication landscape is rapidly evolving. The options available to clinicians and patients are really diverse at this moment, and the data are not concrete regarding what works best for a single patient.
Rather than trying to go from one agent to the next, we thought it best to discuss with patients using the SHARE decision-making model, the adverse effects (AEs) and relative benefits that are involved with each medication class to determine what might be best for the person. We have many new agents with evidence for possible reductions in cardiovascular outcomes outside of their glycemic control properties. As those evidences promote a potentially better option for a patient, we wanted to allow the room in management tomake a decision together. I will say the CPG as well as all of the other applicable diabetes guidelines for T2DM promote metformin as the first therapy to consider for somebody with newly diagnosed T2DM because of safety and availability and the benefit that’s seen with that medication class. We ask clinicians to access the AHRQ website for updates as the medicines evolve.
In a rapidly changing landscape with new drugs coming into the market, each agency has on their individual website information about individual agents and their formulary status, criteria for use, and prior authorization requirements. We refer clinicians to the appropriate website for more information.
Dr. Conlin. There are a series of new medications that have recently come to market that seem to mitigate risk for hypoglycemia. Dr. Lugo, which treatment options carry greater risk? Which treatment options seem to have lesser risk for hypoglycemia?
Amy M. Lugo, PharmD. Insulin and the sulfonylureas have the highest risk of hypoglycemia. The sulfonylureas have fallen out of favor somewhat. One reason is that there are many newer agents that do not cause weight gain or increase the risk of hypoglycemia. Some of the newer insulins may have a lower risk of hypoglycemia and nocturnal hypoglycemia, in particular; however, it is difficult to conclude emphatically that one basal insulin analog is less likely to cause clinically relevant severe or nocturnal hypoglycemia events. This is due to the differences in the definitions of hypoglycemia used in the individual clinical trials, the open label study designs, and the different primary endpoints.
Dr. Conlin. How much affect on HbA1c might I expect to see using SGLT2 inhibitors or GLP-1 agonists? What would be some of the potential AEs I have to be aware of and therefore could counsel patients about?
Dr. Lugo. Let’s start with SGLT2 inhibitors. It depends on whether they are used as monotherapy or in combination. We prefer that patients start on metformin unless they have a contraindication. When used as monotherapy, the SGLT2s may decrease HbA1c from 0.4% to 1% from baseline. When combined with additional agents, they can have > 1% improvement in HbA1c from baseline. There are no head-to-head trials between any of the SGLT2 inhibitors. We cannot say that one is more efficacious than another in lowering HbA1c. The most common AEs include genital mycotic infections and urinary tract infections. The SGLT2 inhibitors also should be avoided in renal impairment. There was a recent FDA safety alert for the class for risk of ketoacidosis. Additionally, the FDA warned that patients
with a history of bladder cancer should avoid dapagliflozin, and canagliflozin has a warning for increased risk of bone fractures, amputation, and decreased bone density.
Other actions of the SGLT2 inhibitors include a reduction in triglycerides and a modest increase in both low-density lipoprotein cholesterol and highdensity lipoprotein cholesterol. The SGLT2 inhibitors also slightly decrease systolic blood pressure (by 4 mm Hg to 6 mm Hg) and body weight (reduction of 1.8 kg)
The GLP-1s are likely to be more efficacious in reducing HbA1c. Typically we see 1% or greater lowering in HbA1c from baseline. As a class, the GLP-1 agonists have a lower risk of hypoglycemia; however, the risk increases when combined with sulfonylureas or insulin. The dose of insulin or sulfonylurea will likely need to be decreased when used concomitantly.
Patients are likely to experience weight loss when on a GLP-1 agonist, which is a great benefit. Gastrointestinal AEs such as nausea are common. Adverse effects may differ somewhat between the agents.
Dr. Conlin. Patients’ experience of care is integral to their engagement with treatment as well as their adherence. Ms. Decesare, what are patients looking for from their health care team?
Elaine M. Decesare. Patients are looking for a knowledgeable and compassionate health care team that has a consistent approach and a consistent message and that the team is updated on the knowledge of appropriate treatments and appropriate lifestyle modifications and targets for the care of diabetes.
Also, I think that the team needs to have some empathy for the challenges of living with diabetes. It’s a 24-hour-a-day disorder, 7 days a week. They can’t take vacation from it. They just can’t take a pill and forget about it. It’s a fairly demanding disorder, and sometimes just acknowledging that with the patient can help you with the dialog.
The second thing I think patients want is an effective treatment plan that’s tailored to their needs and lifestyles. That goes in with the shared decision-making approach, but the plan itself really has to be likely to achieve the targets and the goals that you’ve set up. Sometimes I see patients who are doing all they can with their lifestyle changes, but they can’t get to goal, because there isn’t enough medication in the plan. The plan has to be adequate so that the patient can manage their diabetes. In the shared approach, the patient has to buy in to the plan. With the shared decision making they’re more likely to take the plan on as their plan.
Dr. Conlin. How do you respond to patients who feel treatment burnout from having a new dietary plan, an exercise program, regular monitoring of glucose through finger sticks, and in many cases multiple medications and or injections, while potentially not achieving the goals that you and the patient have arrived at?
Ms. Decesare. First, I want to assess their mood. Sometimes patients are depressed, and they actually need help with that. If they have trouble with just the management, we do have behavioral health psychologists on our team that work with patients to get through some of the barriers and discuss some of the feelings that they have about diabetes and diabetes management.
Sometimes we look at the plan again and see if there’s something we can do to make the plan easier. Occasionally, something has happened in their life. Maybe they’re taking care of an elderly parent or they’ve had other health problems that have come about that we need to reassess the plan and make sure that it’s actually doable for them at this point in time.
Certainly diabetes self-management education can be helpful. Some of those approaches can be helpful for finding something that’s going to work for patients in the long run, because it can be a very difficult disorder to manage as time goes on.
Dr. Colburn. Type 2 DM disproportionately affects individuals who are ≥ 65 years compared with younger individuals. Such older patients also are more likely to have cognitive impairment or visual issues. How do we best manage such patients?
Ms. Decesare. When I’m looking at the care plan, social support is very important. If someone has social support and they have a spouse or a son or daughter or someone else that can help them with their diabetes, we oftentimes will get them involved with the plan, as long as it’s fine with the patient, to offer some help, especially with the patients with cognitive problems, because sometimes the patients just cognitively cannot manage diabetes on their own. Prandial insulin could be a really dangerous product for someone who has cognitive disease.
I think you have to look at all the resources that are available. Sometimes you have to change your HbA1c target range to something that’s going to be manageable for that patient at that time. It might not be perfect, but it would be better to have no hypoglycemia rather than a real aggressive HbA1c target or a target range, if that’s what’s going to keep the patient safe.
Dr. Conlin. We thank our discussants for sharing very practical advice on how to implement the CPG. We hope this information supports clinicians as they develop treatment plans based on each patient's unique characteristics and goals of care.
Click here to read the digital edition.
Paul Conlin, MD. Thank you all for joining us to talk about the recently released VA/DoD Clinical Practice Guideline for the Management of Type 2 Diabetes Mellitus in Primary Care (CPG). We’ve gathered together a group of experts who were part of the CPG development committee. We’re going to talk about some topics that were highlighted in the CPG that might provide additional detail to those in primary-care practices and help them in their management of patients with diabetes.
A unique feature of the VA/DoD CPG is that it emphasizes shared decision making as an important tool that clinicians should employ in their patient encounters. Dr. Watts, health care providers may wonder how they can make time for an intervention involving shared decision making using the SHARE approach, (ie, seek, help, assess, reach, and evaluate). Can you give us some advice on this?
Sharon Watts, DNP. Shared decision making is really crucial to success in diabetes. It’s been around for a while. We are trying to make an emphasis on this. The SHARE approach is from the Agency for Healthcare Research and Quality (AHRQ). The AHRQ has a wealth of information on its website. What AHRQ emphasizes is making it brief but conversational when you’re using the SHARE approach with your patient. Most importantly, the patient needs to be in the center of this dialogue, expressing his or her values and preferences of what’s most important to the whole team. This is a team effort. It’s not just with a provider. That’s where providers get overwhelmed. You can ask your nurse to advise the patient to write down 1 or 2 questions that are really important about diabetes before they come to see you, before the encounter. We can refer patients to diabetes classes where a lot of this information is given. The patient can talk to the dietitian or the pharmacist. There’s a whole team out there that will help in SHARE decision making. It’s crucial in the end for the provider to help the patient reach the decision and determine how best to treat the diabetes with them.
Dr. Conlin. Can you give a brief description of the key components of the SHARE approach?
Dr. Watts. Breaking it down simply, providers can start off by asking permission to go over the condition or treatment options because this immediately sets the stage as a signal to the patient that they are important in controlling the dialogue. It’s not the provider giving a discourse. You’re asking for permission. The next step would be to explore the benefits and risks of any course taken. Use decision aids if you have them. Keep in mind your patient’s current health literacy, numeracy, and other limitations.
Next ask about values, preferences, or barriers to whatever treatment you’re talking about. For instance, will this work with your work schedule?
Then the last thing would be ask what the patient wants to do next. Reach a decision on treatment, whatever it is, and make sure that you revisit that decision. Follow up later to see if it’s really working.
Dr. Conlin. If I’m a busy clinician and I have a limited amount of time with a patient, when are the appropriate times to employ the SHARE approach? Can I break it into components, where I address some elements during one visit and other elements in another visit?
Dr. Watts. Absolutely. It can be spread out. Your team is probably already providing information that will help in the SHARE approach. Just chart that you’ve done it. We know the SHARE approach is important because people
tend to be adherent if they came up with part of the plan.
Dr. Conlin. Where does diabetes self-management education and diabetes self-management support fall into this framework?
Dr. Watts. Diabetes is a complex disease for providers and for the team and even more so for our patients. Invite them to diabetes classes. There’s so much to understand. The classes go over medications and blood sugar ranges, though you still may have to review it with the patient in your office. It saves the provider time if you have an informed and activated patient. It’s the same with sending a patient to a dietitian. I do all of the above.
Dr. Conlin. Many providers may not be familiar with this type of approach. How can I tell whether or not I’m doing it correctly?
Dr. Watts. The AHRQ website has conversation starters (www.ahrq.gov/professionals/education/curriculum-tools/shareddecisionmaking/tools/index.html). Then make sure when you are with the patient to use Teach-Back. Have that conversation and say, “I want to make sure I understood correctly what we decided would work best for you.” Ask patients to say in their own words what they understand. Then I think you’re off to a great start.
Dr. Conlin. Many patients tend to be deferential to their health care providers. They were brought up in an era where they needed to listen to and respect clinicians rather than participate in discussions about their ongoing care. How do you engage with these patients?
Dr. Watts. That is a tough one. Before the patient leaves the office, I ask them: Are there any barriers? Does this work for your schedule? Is this a preference and value that you have? Is there anything that might get in the way of this working when you go home? I try to pull out a little bit more, making sure to give them some decision aids and revisit it at the next visit to make sure it’s working.
Dr. Conlin. We’ll now turn to a discussion of using hemoglobin A1c (HbA1c) measurements in clinical practice. Dr. Aron, what factors can impact the relationship between HbA1c and blood glucose? How should we use HbA1c in the treatment of patients who come from varied ethnic and racial backgrounds, where the relationship to average blood glucose may be different?
David C. Aron, MD, MS. The identification of HbA1c has been a tremendous advance in our ability to manage patients with diabetes. It represents an average blood glucose over the preceding 3 months but like everything in this world, it has issues. One is the fact that there is a certain degree of inaccuracy in measurement, and that’s true of any measurement that you make of anything. Just as you allow a little bit of wiggle room when you’re driving down the New Jersey Turnpike and watching your speedometer, which is not 100% accurate. It says you are going 65 but it could, for example be 68 or 62. You don’t want to go too fast or you’ll get a speeding ticket. You don’t want to go too slowly or the person behind you will start honking at you. You want to be at the speed limit plus or minus. The first thing to think about in using HbA1c is the issue of accuracy. Rather than choose a specific target number, health care providers should choose a range between this and that. There’ll be more detail on that later.
The second thing is that part of the degree to which HbA1c represents the average blood glucose depends on a lot of factors, and some of these factors are things that we can do absolutely nothing about because we are born with them. African Americans tend to have higher HbA1c levels than do whites for the same glucose. That difference is as much as 0.4. An HbA1c of 6.2 in African Americans gets you a 5.8 in whites for the same average blood glucose. Similarly, Native Americans have somewhat higher HbA1c, although not quite as high as African Americans. Hispanics and Asians do as well, so you have to take your patient’s ethnicity into account.
The second has to do with the way that HbA1c is measured and the fact that there are many things that can affect the measurement. An HbA1c is dependent upon the lifespan of the red blood cell, so if there are alterations in red cell lifespan or if someone has anemia, that can affect HbA1c. Certain hemoglobin variants, for example, hemoglobin F, which is typically elevated in someone with thalassemia, migrates with some assays in the same place as thalassemia, so the assay can’t tell the difference between thalassemia and hemoglobin F. There are drugs and other conditions that can also affect HbA1c. You should think about HbA1c as a guide, but no number should be considered to be written in stone.
Dr. Conlin. I can imagine that this would be particularly important if you were using HbA1c as a criterion for diagnosing diabetes.
Dr. Aron. Quite right. The effects of race and ethnicity on HbA1c account for one of the differences between the VA/DoD guidelines and those of the American Diabetes Association (ADA).
Dr. Conlin. Isn’t < 8% HbA1c a national performance measure that people are asked to adhere to?
Dr. Aron. Not in the VA. In fact, the only performance measure that the VA has with a target is percent of patients with HbA1c > 9%, and we don’t want any of those or very few of them anyway. We have specifically avoided targets like < 8% HbA1c or < 7% HbA1c, which was prevalent some years ago, because the choice of HbA1c is very dependent upon the needs and desires of the individual patient. The VA has had stratified targets based on life expectancy and complications going back more than 15 years.
Dr. Conlin. Another issue that can confuse clinicians is when the HbA1c is in the target range but actually reflects an average of glucose levels that are at times very high and very low. How do we address this problem clinically?
Dr. Aron. In managing patients, you use whatever data you can get. The HbA1c gives you a general indication of average blood glucose, but particularly for those patients who are on insulin, it’s not a complete substitute for measuring blood glucose at appropriate times and taking the degree of glucose variability into account. We don’t want patients getting hypoglycemic, and particularly if they’re elderly, falling, or getting into a car accident. Similarly, we don’t want people to have very high blood sugars, even for limited periods of time, because they can lead to dehydration and other symptoms as well. We use a combination of both HbA1c and individual measures of blood glucose, like finger-stick blood sugar testing, typically.
Dr. Conlin. The VA/DoD CPG differs from other published guidelines in that we proposed patients are treated to HbA1c target ranges, whereas most other guidelines propose upper limits or threshold values, such as the HbA1c should be < 7% or < 8% but without lower bounds. Dr. Colburn, what are the target ranges that are recommended in the CPG? How were they determined?
Maj. Jeffrey A. Colburn, MD. It may be helpful to pull up the Determination of Average Target HbA1c Level Over Time table (page S17), which lays out risk for patients of treatment as well as the benefits of treatment. We first look at the patient’s state of health and whether they have a major comorbidity, a physiologic age that could be high risk, or advanced physiologic age with a diminished life expectancy. In controlling the levels of glucose, we’re often trying to benefit the microvascular health of the patient, realizing also that eventually poor management over time will lead to macrovascular disease as well. The main things that we see in child data is that the benefits of tight glucose control for younger patients with shorter duration of type 2 diabetes mellitus (T2DM) is the prevention of retinopathy, nephropathy, and peripheral neuropathy. Those patients that already have advanced microvascular disease are less likely to benefit from tight control. Trying to push glucose very low can harm the patient. It’s a delicate balance between the possible benefit vs the real harm.
The major trials are the ADVANCE, ACCORD, and the VADT trial, which was done in a VA population. To generalize the results, you are looking at an intensive control, which was trying to keep the HbA1c in general down below the 7% threshold. The patients enrolled in those trials all had microvascular and macrovascular disease and typically longer durations of diabetes at the time of the study. The studies revealed that we were not preventing macrovascular disease, heart attacks, strokes, the types of things that kill patients with diabetes. Individuals at higher HbA1c levels that went down to better HbA1c levels saw some improvement in the microvascular risk. Individuals already at the lower end didn’t see as much improvement. What we saw though that was surprising and concerning was that hypoglycemia, particularly severe hypoglycemia in the VADT trial was a lot more frequent when you try and target the HbA1c on the lower end. Because of these findings, we proposed the table with a set of ranges. As Dr. Aron noted, HbA1c is not a perfect test. It does have some variance in the number it presents. The CPG proposed to give individuals target ranges. They should be individualized based upon physiologic age, comorbidities, and life expectancy.
A criticism of the table that I commonly hear is what’s the magic crystal ball for determining somebody’s life expectancy? We don’t have one. This is a clinician’s judgment. The findings might actually change over time with the patient. A target HbA1c range is something that should be adapted and evolve along with the clinician and patient experience of the diabetes.
There are other important studies. For example, the UKPDS trials that included patients with shorter durations of diabetes and lesser disease to try and get their HbA1c levels on the lower end. We included that in the chart. Another concept we put forward is the idea of relative risk (RR) vs absolute risk. The RR reduction doesn’t speak to what the actual beginning risk is lowered to for a patient. The UKPDS is often cited for RR reduction of microvascular disease as 37% when an HbA1c of 7.9% is targeted down to 7.0%. The absolute risk reduction is actually 5 with the number needed to treat to do so is 20 patients. When we present the data, we give it a fair shake. We want individuals to guide therapy that is going to be both beneficial to preventing outcomes but also not harmful to the patient. I would highly recommend clinicians and patients look at this table together when making their decisions.
Dr. Conlin. In the VA/DoD CPG, the HbA1c target range for individuals with limited life expectancy extends to 9%. That may seem high for some, since most other guidelines propose lower HbA1c levels. How strong are the data that a person with limited life expectancy, say with end-stage renal disease or advanced complications, could be treated to a range of 8% to 9%? Shouldn’t lower levels actually improve life expectancy in such people?
Dr. Colburn. There’s much less data to support this level, which is why it’s cited in CPG as having weaker evidence. The reason it’s proposed is the experience of the workgroup and the evidence that is available of a high risk for patients with low life expectancy when they reduce their HbA1c greatly. One of the concerns about being at that level might be the real issue of renal glycosuria for individuals when their blood glucose is reaching above 180 mg/dL, which correlates to the 8% to 9% HbA1c range. You may have renal loss and risk of dehydration. It is an area where the clinician should be cautious in monitoring a patient in the 8% to 9% HbA1c range. With that being said, a patient who is having a lot of challenges in their health and extremely advanced conditions could be in that range. We would not expect a reversing of a micro- or macrovascular disease with glycemia control. We’re not going to go back from that level of disease they have. The idea about keeping them there is to prevent the risks of overtreatment and harm to the patient.
Dr. Conlin. Since patients with diabetes can progress over their lifetime from no complications to mild-to-moderate complications to advanced complications, how does the HbA1c target range evolve as a patient’s condition changes?
Dr. Colburn. As we check for evidence of microvascular disease or neuropathy signs, that evidence often is good for discussion between the clinician and patient to advise them that better control early on may help stem off or reverse some of that change. As those changes solidify, the patient is challenged by microvascular conditions. I would entertain allowing more relaxed HbA1c ranges to prevent harm to the patient given that we’re not going back. But you have to be careful. We have to consider benefits to the patient and the challenges for controlling glucose.
I hope that this table doesn’t make providers throw up their hands and give up. It’s meant to start a conversation on safety and benefits. With newer agents coming out that can help us control glucose quite well, without as much hypoglycemia risk, clinicians and patients potentially can try and get that HbA1c into a well-managed range.
Dr. Conlin. The CPG discusses various treatment options that might be available for patients who require pharmacologic therapy. The number of agents available is growing quite markedly. Dr. Colburn, can you describe how the CPG put together the pharmacologic therapy preferences.
Dr. Colburn. The CPG expressively stayed away from trying to promote specific regimens of medications. For example, other guidelines promote starting with certain agents followed by a second-line agent by a third-line agents. The concern that we had about that approach is that the medication landscape is rapidly evolving. The options available to clinicians and patients are really diverse at this moment, and the data are not concrete regarding what works best for a single patient.
Rather than trying to go from one agent to the next, we thought it best to discuss with patients using the SHARE decision-making model, the adverse effects (AEs) and relative benefits that are involved with each medication class to determine what might be best for the person. We have many new agents with evidence for possible reductions in cardiovascular outcomes outside of their glycemic control properties. As those evidences promote a potentially better option for a patient, we wanted to allow the room in management tomake a decision together. I will say the CPG as well as all of the other applicable diabetes guidelines for T2DM promote metformin as the first therapy to consider for somebody with newly diagnosed T2DM because of safety and availability and the benefit that’s seen with that medication class. We ask clinicians to access the AHRQ website for updates as the medicines evolve.
In a rapidly changing landscape with new drugs coming into the market, each agency has on their individual website information about individual agents and their formulary status, criteria for use, and prior authorization requirements. We refer clinicians to the appropriate website for more information.
Dr. Conlin. There are a series of new medications that have recently come to market that seem to mitigate risk for hypoglycemia. Dr. Lugo, which treatment options carry greater risk? Which treatment options seem to have lesser risk for hypoglycemia?
Amy M. Lugo, PharmD. Insulin and the sulfonylureas have the highest risk of hypoglycemia. The sulfonylureas have fallen out of favor somewhat. One reason is that there are many newer agents that do not cause weight gain or increase the risk of hypoglycemia. Some of the newer insulins may have a lower risk of hypoglycemia and nocturnal hypoglycemia, in particular; however, it is difficult to conclude emphatically that one basal insulin analog is less likely to cause clinically relevant severe or nocturnal hypoglycemia events. This is due to the differences in the definitions of hypoglycemia used in the individual clinical trials, the open label study designs, and the different primary endpoints.
Dr. Conlin. How much affect on HbA1c might I expect to see using SGLT2 inhibitors or GLP-1 agonists? What would be some of the potential AEs I have to be aware of and therefore could counsel patients about?
Dr. Lugo. Let’s start with SGLT2 inhibitors. It depends on whether they are used as monotherapy or in combination. We prefer that patients start on metformin unless they have a contraindication. When used as monotherapy, the SGLT2s may decrease HbA1c from 0.4% to 1% from baseline. When combined with additional agents, they can have > 1% improvement in HbA1c from baseline. There are no head-to-head trials between any of the SGLT2 inhibitors. We cannot say that one is more efficacious than another in lowering HbA1c. The most common AEs include genital mycotic infections and urinary tract infections. The SGLT2 inhibitors also should be avoided in renal impairment. There was a recent FDA safety alert for the class for risk of ketoacidosis. Additionally, the FDA warned that patients
with a history of bladder cancer should avoid dapagliflozin, and canagliflozin has a warning for increased risk of bone fractures, amputation, and decreased bone density.
Other actions of the SGLT2 inhibitors include a reduction in triglycerides and a modest increase in both low-density lipoprotein cholesterol and highdensity lipoprotein cholesterol. The SGLT2 inhibitors also slightly decrease systolic blood pressure (by 4 mm Hg to 6 mm Hg) and body weight (reduction of 1.8 kg)
The GLP-1s are likely to be more efficacious in reducing HbA1c. Typically we see 1% or greater lowering in HbA1c from baseline. As a class, the GLP-1 agonists have a lower risk of hypoglycemia; however, the risk increases when combined with sulfonylureas or insulin. The dose of insulin or sulfonylurea will likely need to be decreased when used concomitantly.
Patients are likely to experience weight loss when on a GLP-1 agonist, which is a great benefit. Gastrointestinal AEs such as nausea are common. Adverse effects may differ somewhat between the agents.
Dr. Conlin. Patients’ experience of care is integral to their engagement with treatment as well as their adherence. Ms. Decesare, what are patients looking for from their health care team?
Elaine M. Decesare. Patients are looking for a knowledgeable and compassionate health care team that has a consistent approach and a consistent message and that the team is updated on the knowledge of appropriate treatments and appropriate lifestyle modifications and targets for the care of diabetes.
Also, I think that the team needs to have some empathy for the challenges of living with diabetes. It’s a 24-hour-a-day disorder, 7 days a week. They can’t take vacation from it. They just can’t take a pill and forget about it. It’s a fairly demanding disorder, and sometimes just acknowledging that with the patient can help you with the dialog.
The second thing I think patients want is an effective treatment plan that’s tailored to their needs and lifestyles. That goes in with the shared decision-making approach, but the plan itself really has to be likely to achieve the targets and the goals that you’ve set up. Sometimes I see patients who are doing all they can with their lifestyle changes, but they can’t get to goal, because there isn’t enough medication in the plan. The plan has to be adequate so that the patient can manage their diabetes. In the shared approach, the patient has to buy in to the plan. With the shared decision making they’re more likely to take the plan on as their plan.
Dr. Conlin. How do you respond to patients who feel treatment burnout from having a new dietary plan, an exercise program, regular monitoring of glucose through finger sticks, and in many cases multiple medications and or injections, while potentially not achieving the goals that you and the patient have arrived at?
Ms. Decesare. First, I want to assess their mood. Sometimes patients are depressed, and they actually need help with that. If they have trouble with just the management, we do have behavioral health psychologists on our team that work with patients to get through some of the barriers and discuss some of the feelings that they have about diabetes and diabetes management.
Sometimes we look at the plan again and see if there’s something we can do to make the plan easier. Occasionally, something has happened in their life. Maybe they’re taking care of an elderly parent or they’ve had other health problems that have come about that we need to reassess the plan and make sure that it’s actually doable for them at this point in time.
Certainly diabetes self-management education can be helpful. Some of those approaches can be helpful for finding something that’s going to work for patients in the long run, because it can be a very difficult disorder to manage as time goes on.
Dr. Colburn. Type 2 DM disproportionately affects individuals who are ≥ 65 years compared with younger individuals. Such older patients also are more likely to have cognitive impairment or visual issues. How do we best manage such patients?
Ms. Decesare. When I’m looking at the care plan, social support is very important. If someone has social support and they have a spouse or a son or daughter or someone else that can help them with their diabetes, we oftentimes will get them involved with the plan, as long as it’s fine with the patient, to offer some help, especially with the patients with cognitive problems, because sometimes the patients just cognitively cannot manage diabetes on their own. Prandial insulin could be a really dangerous product for someone who has cognitive disease.
I think you have to look at all the resources that are available. Sometimes you have to change your HbA1c target range to something that’s going to be manageable for that patient at that time. It might not be perfect, but it would be better to have no hypoglycemia rather than a real aggressive HbA1c target or a target range, if that’s what’s going to keep the patient safe.
Dr. Conlin. We thank our discussants for sharing very practical advice on how to implement the CPG. We hope this information supports clinicians as they develop treatment plans based on each patient's unique characteristics and goals of care.
Click here to read the digital edition.
Paul Conlin, MD. Thank you all for joining us to talk about the recently released VA/DoD Clinical Practice Guideline for the Management of Type 2 Diabetes Mellitus in Primary Care (CPG). We’ve gathered together a group of experts who were part of the CPG development committee. We’re going to talk about some topics that were highlighted in the CPG that might provide additional detail to those in primary-care practices and help them in their management of patients with diabetes.
A unique feature of the VA/DoD CPG is that it emphasizes shared decision making as an important tool that clinicians should employ in their patient encounters. Dr. Watts, health care providers may wonder how they can make time for an intervention involving shared decision making using the SHARE approach, (ie, seek, help, assess, reach, and evaluate). Can you give us some advice on this?
Sharon Watts, DNP. Shared decision making is really crucial to success in diabetes. It’s been around for a while. We are trying to make an emphasis on this. The SHARE approach is from the Agency for Healthcare Research and Quality (AHRQ). The AHRQ has a wealth of information on its website. What AHRQ emphasizes is making it brief but conversational when you’re using the SHARE approach with your patient. Most importantly, the patient needs to be in the center of this dialogue, expressing his or her values and preferences of what’s most important to the whole team. This is a team effort. It’s not just with a provider. That’s where providers get overwhelmed. You can ask your nurse to advise the patient to write down 1 or 2 questions that are really important about diabetes before they come to see you, before the encounter. We can refer patients to diabetes classes where a lot of this information is given. The patient can talk to the dietitian or the pharmacist. There’s a whole team out there that will help in SHARE decision making. It’s crucial in the end for the provider to help the patient reach the decision and determine how best to treat the diabetes with them.
Dr. Conlin. Can you give a brief description of the key components of the SHARE approach?
Dr. Watts. Breaking it down simply, providers can start off by asking permission to go over the condition or treatment options because this immediately sets the stage as a signal to the patient that they are important in controlling the dialogue. It’s not the provider giving a discourse. You’re asking for permission. The next step would be to explore the benefits and risks of any course taken. Use decision aids if you have them. Keep in mind your patient’s current health literacy, numeracy, and other limitations.
Next ask about values, preferences, or barriers to whatever treatment you’re talking about. For instance, will this work with your work schedule?
Then the last thing would be ask what the patient wants to do next. Reach a decision on treatment, whatever it is, and make sure that you revisit that decision. Follow up later to see if it’s really working.
Dr. Conlin. If I’m a busy clinician and I have a limited amount of time with a patient, when are the appropriate times to employ the SHARE approach? Can I break it into components, where I address some elements during one visit and other elements in another visit?
Dr. Watts. Absolutely. It can be spread out. Your team is probably already providing information that will help in the SHARE approach. Just chart that you’ve done it. We know the SHARE approach is important because people
tend to be adherent if they came up with part of the plan.
Dr. Conlin. Where does diabetes self-management education and diabetes self-management support fall into this framework?
Dr. Watts. Diabetes is a complex disease for providers and for the team and even more so for our patients. Invite them to diabetes classes. There’s so much to understand. The classes go over medications and blood sugar ranges, though you still may have to review it with the patient in your office. It saves the provider time if you have an informed and activated patient. It’s the same with sending a patient to a dietitian. I do all of the above.
Dr. Conlin. Many providers may not be familiar with this type of approach. How can I tell whether or not I’m doing it correctly?
Dr. Watts. The AHRQ website has conversation starters (www.ahrq.gov/professionals/education/curriculum-tools/shareddecisionmaking/tools/index.html). Then make sure when you are with the patient to use Teach-Back. Have that conversation and say, “I want to make sure I understood correctly what we decided would work best for you.” Ask patients to say in their own words what they understand. Then I think you’re off to a great start.
Dr. Conlin. Many patients tend to be deferential to their health care providers. They were brought up in an era where they needed to listen to and respect clinicians rather than participate in discussions about their ongoing care. How do you engage with these patients?
Dr. Watts. That is a tough one. Before the patient leaves the office, I ask them: Are there any barriers? Does this work for your schedule? Is this a preference and value that you have? Is there anything that might get in the way of this working when you go home? I try to pull out a little bit more, making sure to give them some decision aids and revisit it at the next visit to make sure it’s working.
Dr. Conlin. We’ll now turn to a discussion of using hemoglobin A1c (HbA1c) measurements in clinical practice. Dr. Aron, what factors can impact the relationship between HbA1c and blood glucose? How should we use HbA1c in the treatment of patients who come from varied ethnic and racial backgrounds, where the relationship to average blood glucose may be different?
David C. Aron, MD, MS. The identification of HbA1c has been a tremendous advance in our ability to manage patients with diabetes. It represents an average blood glucose over the preceding 3 months but like everything in this world, it has issues. One is the fact that there is a certain degree of inaccuracy in measurement, and that’s true of any measurement that you make of anything. Just as you allow a little bit of wiggle room when you’re driving down the New Jersey Turnpike and watching your speedometer, which is not 100% accurate. It says you are going 65 but it could, for example be 68 or 62. You don’t want to go too fast or you’ll get a speeding ticket. You don’t want to go too slowly or the person behind you will start honking at you. You want to be at the speed limit plus or minus. The first thing to think about in using HbA1c is the issue of accuracy. Rather than choose a specific target number, health care providers should choose a range between this and that. There’ll be more detail on that later.
The second thing is that part of the degree to which HbA1c represents the average blood glucose depends on a lot of factors, and some of these factors are things that we can do absolutely nothing about because we are born with them. African Americans tend to have higher HbA1c levels than do whites for the same glucose. That difference is as much as 0.4. An HbA1c of 6.2 in African Americans gets you a 5.8 in whites for the same average blood glucose. Similarly, Native Americans have somewhat higher HbA1c, although not quite as high as African Americans. Hispanics and Asians do as well, so you have to take your patient’s ethnicity into account.
The second has to do with the way that HbA1c is measured and the fact that there are many things that can affect the measurement. An HbA1c is dependent upon the lifespan of the red blood cell, so if there are alterations in red cell lifespan or if someone has anemia, that can affect HbA1c. Certain hemoglobin variants, for example, hemoglobin F, which is typically elevated in someone with thalassemia, migrates with some assays in the same place as thalassemia, so the assay can’t tell the difference between thalassemia and hemoglobin F. There are drugs and other conditions that can also affect HbA1c. You should think about HbA1c as a guide, but no number should be considered to be written in stone.
Dr. Conlin. I can imagine that this would be particularly important if you were using HbA1c as a criterion for diagnosing diabetes.
Dr. Aron. Quite right. The effects of race and ethnicity on HbA1c account for one of the differences between the VA/DoD guidelines and those of the American Diabetes Association (ADA).
Dr. Conlin. Isn’t < 8% HbA1c a national performance measure that people are asked to adhere to?
Dr. Aron. Not in the VA. In fact, the only performance measure that the VA has with a target is percent of patients with HbA1c > 9%, and we don’t want any of those or very few of them anyway. We have specifically avoided targets like < 8% HbA1c or < 7% HbA1c, which was prevalent some years ago, because the choice of HbA1c is very dependent upon the needs and desires of the individual patient. The VA has had stratified targets based on life expectancy and complications going back more than 15 years.
Dr. Conlin. Another issue that can confuse clinicians is when the HbA1c is in the target range but actually reflects an average of glucose levels that are at times very high and very low. How do we address this problem clinically?
Dr. Aron. In managing patients, you use whatever data you can get. The HbA1c gives you a general indication of average blood glucose, but particularly for those patients who are on insulin, it’s not a complete substitute for measuring blood glucose at appropriate times and taking the degree of glucose variability into account. We don’t want patients getting hypoglycemic, and particularly if they’re elderly, falling, or getting into a car accident. Similarly, we don’t want people to have very high blood sugars, even for limited periods of time, because they can lead to dehydration and other symptoms as well. We use a combination of both HbA1c and individual measures of blood glucose, like finger-stick blood sugar testing, typically.
Dr. Conlin. The VA/DoD CPG differs from other published guidelines in that we proposed patients are treated to HbA1c target ranges, whereas most other guidelines propose upper limits or threshold values, such as the HbA1c should be < 7% or < 8% but without lower bounds. Dr. Colburn, what are the target ranges that are recommended in the CPG? How were they determined?
Maj. Jeffrey A. Colburn, MD. It may be helpful to pull up the Determination of Average Target HbA1c Level Over Time table (page S17), which lays out risk for patients of treatment as well as the benefits of treatment. We first look at the patient’s state of health and whether they have a major comorbidity, a physiologic age that could be high risk, or advanced physiologic age with a diminished life expectancy. In controlling the levels of glucose, we’re often trying to benefit the microvascular health of the patient, realizing also that eventually poor management over time will lead to macrovascular disease as well. The main things that we see in child data is that the benefits of tight glucose control for younger patients with shorter duration of type 2 diabetes mellitus (T2DM) is the prevention of retinopathy, nephropathy, and peripheral neuropathy. Those patients that already have advanced microvascular disease are less likely to benefit from tight control. Trying to push glucose very low can harm the patient. It’s a delicate balance between the possible benefit vs the real harm.
The major trials are the ADVANCE, ACCORD, and the VADT trial, which was done in a VA population. To generalize the results, you are looking at an intensive control, which was trying to keep the HbA1c in general down below the 7% threshold. The patients enrolled in those trials all had microvascular and macrovascular disease and typically longer durations of diabetes at the time of the study. The studies revealed that we were not preventing macrovascular disease, heart attacks, strokes, the types of things that kill patients with diabetes. Individuals at higher HbA1c levels that went down to better HbA1c levels saw some improvement in the microvascular risk. Individuals already at the lower end didn’t see as much improvement. What we saw though that was surprising and concerning was that hypoglycemia, particularly severe hypoglycemia in the VADT trial was a lot more frequent when you try and target the HbA1c on the lower end. Because of these findings, we proposed the table with a set of ranges. As Dr. Aron noted, HbA1c is not a perfect test. It does have some variance in the number it presents. The CPG proposed to give individuals target ranges. They should be individualized based upon physiologic age, comorbidities, and life expectancy.
A criticism of the table that I commonly hear is what’s the magic crystal ball for determining somebody’s life expectancy? We don’t have one. This is a clinician’s judgment. The findings might actually change over time with the patient. A target HbA1c range is something that should be adapted and evolve along with the clinician and patient experience of the diabetes.
There are other important studies. For example, the UKPDS trials that included patients with shorter durations of diabetes and lesser disease to try and get their HbA1c levels on the lower end. We included that in the chart. Another concept we put forward is the idea of relative risk (RR) vs absolute risk. The RR reduction doesn’t speak to what the actual beginning risk is lowered to for a patient. The UKPDS is often cited for RR reduction of microvascular disease as 37% when an HbA1c of 7.9% is targeted down to 7.0%. The absolute risk reduction is actually 5 with the number needed to treat to do so is 20 patients. When we present the data, we give it a fair shake. We want individuals to guide therapy that is going to be both beneficial to preventing outcomes but also not harmful to the patient. I would highly recommend clinicians and patients look at this table together when making their decisions.
Dr. Conlin. In the VA/DoD CPG, the HbA1c target range for individuals with limited life expectancy extends to 9%. That may seem high for some, since most other guidelines propose lower HbA1c levels. How strong are the data that a person with limited life expectancy, say with end-stage renal disease or advanced complications, could be treated to a range of 8% to 9%? Shouldn’t lower levels actually improve life expectancy in such people?
Dr. Colburn. There’s much less data to support this level, which is why it’s cited in CPG as having weaker evidence. The reason it’s proposed is the experience of the workgroup and the evidence that is available of a high risk for patients with low life expectancy when they reduce their HbA1c greatly. One of the concerns about being at that level might be the real issue of renal glycosuria for individuals when their blood glucose is reaching above 180 mg/dL, which correlates to the 8% to 9% HbA1c range. You may have renal loss and risk of dehydration. It is an area where the clinician should be cautious in monitoring a patient in the 8% to 9% HbA1c range. With that being said, a patient who is having a lot of challenges in their health and extremely advanced conditions could be in that range. We would not expect a reversing of a micro- or macrovascular disease with glycemia control. We’re not going to go back from that level of disease they have. The idea about keeping them there is to prevent the risks of overtreatment and harm to the patient.
Dr. Conlin. Since patients with diabetes can progress over their lifetime from no complications to mild-to-moderate complications to advanced complications, how does the HbA1c target range evolve as a patient’s condition changes?
Dr. Colburn. As we check for evidence of microvascular disease or neuropathy signs, that evidence often is good for discussion between the clinician and patient to advise them that better control early on may help stem off or reverse some of that change. As those changes solidify, the patient is challenged by microvascular conditions. I would entertain allowing more relaxed HbA1c ranges to prevent harm to the patient given that we’re not going back. But you have to be careful. We have to consider benefits to the patient and the challenges for controlling glucose.
I hope that this table doesn’t make providers throw up their hands and give up. It’s meant to start a conversation on safety and benefits. With newer agents coming out that can help us control glucose quite well, without as much hypoglycemia risk, clinicians and patients potentially can try and get that HbA1c into a well-managed range.
Dr. Conlin. The CPG discusses various treatment options that might be available for patients who require pharmacologic therapy. The number of agents available is growing quite markedly. Dr. Colburn, can you describe how the CPG put together the pharmacologic therapy preferences.
Dr. Colburn. The CPG expressively stayed away from trying to promote specific regimens of medications. For example, other guidelines promote starting with certain agents followed by a second-line agent by a third-line agents. The concern that we had about that approach is that the medication landscape is rapidly evolving. The options available to clinicians and patients are really diverse at this moment, and the data are not concrete regarding what works best for a single patient.
Rather than trying to go from one agent to the next, we thought it best to discuss with patients using the SHARE decision-making model, the adverse effects (AEs) and relative benefits that are involved with each medication class to determine what might be best for the person. We have many new agents with evidence for possible reductions in cardiovascular outcomes outside of their glycemic control properties. As those evidences promote a potentially better option for a patient, we wanted to allow the room in management tomake a decision together. I will say the CPG as well as all of the other applicable diabetes guidelines for T2DM promote metformin as the first therapy to consider for somebody with newly diagnosed T2DM because of safety and availability and the benefit that’s seen with that medication class. We ask clinicians to access the AHRQ website for updates as the medicines evolve.
In a rapidly changing landscape with new drugs coming into the market, each agency has on their individual website information about individual agents and their formulary status, criteria for use, and prior authorization requirements. We refer clinicians to the appropriate website for more information.
Dr. Conlin. There are a series of new medications that have recently come to market that seem to mitigate risk for hypoglycemia. Dr. Lugo, which treatment options carry greater risk? Which treatment options seem to have lesser risk for hypoglycemia?
Amy M. Lugo, PharmD. Insulin and the sulfonylureas have the highest risk of hypoglycemia. The sulfonylureas have fallen out of favor somewhat. One reason is that there are many newer agents that do not cause weight gain or increase the risk of hypoglycemia. Some of the newer insulins may have a lower risk of hypoglycemia and nocturnal hypoglycemia, in particular; however, it is difficult to conclude emphatically that one basal insulin analog is less likely to cause clinically relevant severe or nocturnal hypoglycemia events. This is due to the differences in the definitions of hypoglycemia used in the individual clinical trials, the open label study designs, and the different primary endpoints.
Dr. Conlin. How much affect on HbA1c might I expect to see using SGLT2 inhibitors or GLP-1 agonists? What would be some of the potential AEs I have to be aware of and therefore could counsel patients about?
Dr. Lugo. Let’s start with SGLT2 inhibitors. It depends on whether they are used as monotherapy or in combination. We prefer that patients start on metformin unless they have a contraindication. When used as monotherapy, the SGLT2s may decrease HbA1c from 0.4% to 1% from baseline. When combined with additional agents, they can have > 1% improvement in HbA1c from baseline. There are no head-to-head trials between any of the SGLT2 inhibitors. We cannot say that one is more efficacious than another in lowering HbA1c. The most common AEs include genital mycotic infections and urinary tract infections. The SGLT2 inhibitors also should be avoided in renal impairment. There was a recent FDA safety alert for the class for risk of ketoacidosis. Additionally, the FDA warned that patients
with a history of bladder cancer should avoid dapagliflozin, and canagliflozin has a warning for increased risk of bone fractures, amputation, and decreased bone density.
Other actions of the SGLT2 inhibitors include a reduction in triglycerides and a modest increase in both low-density lipoprotein cholesterol and highdensity lipoprotein cholesterol. The SGLT2 inhibitors also slightly decrease systolic blood pressure (by 4 mm Hg to 6 mm Hg) and body weight (reduction of 1.8 kg)
The GLP-1s are likely to be more efficacious in reducing HbA1c. Typically we see 1% or greater lowering in HbA1c from baseline. As a class, the GLP-1 agonists have a lower risk of hypoglycemia; however, the risk increases when combined with sulfonylureas or insulin. The dose of insulin or sulfonylurea will likely need to be decreased when used concomitantly.
Patients are likely to experience weight loss when on a GLP-1 agonist, which is a great benefit. Gastrointestinal AEs such as nausea are common. Adverse effects may differ somewhat between the agents.
Dr. Conlin. Patients’ experience of care is integral to their engagement with treatment as well as their adherence. Ms. Decesare, what are patients looking for from their health care team?
Elaine M. Decesare. Patients are looking for a knowledgeable and compassionate health care team that has a consistent approach and a consistent message and that the team is updated on the knowledge of appropriate treatments and appropriate lifestyle modifications and targets for the care of diabetes.
Also, I think that the team needs to have some empathy for the challenges of living with diabetes. It’s a 24-hour-a-day disorder, 7 days a week. They can’t take vacation from it. They just can’t take a pill and forget about it. It’s a fairly demanding disorder, and sometimes just acknowledging that with the patient can help you with the dialog.
The second thing I think patients want is an effective treatment plan that’s tailored to their needs and lifestyles. That goes in with the shared decision-making approach, but the plan itself really has to be likely to achieve the targets and the goals that you’ve set up. Sometimes I see patients who are doing all they can with their lifestyle changes, but they can’t get to goal, because there isn’t enough medication in the plan. The plan has to be adequate so that the patient can manage their diabetes. In the shared approach, the patient has to buy in to the plan. With the shared decision making they’re more likely to take the plan on as their plan.
Dr. Conlin. How do you respond to patients who feel treatment burnout from having a new dietary plan, an exercise program, regular monitoring of glucose through finger sticks, and in many cases multiple medications and or injections, while potentially not achieving the goals that you and the patient have arrived at?
Ms. Decesare. First, I want to assess their mood. Sometimes patients are depressed, and they actually need help with that. If they have trouble with just the management, we do have behavioral health psychologists on our team that work with patients to get through some of the barriers and discuss some of the feelings that they have about diabetes and diabetes management.
Sometimes we look at the plan again and see if there’s something we can do to make the plan easier. Occasionally, something has happened in their life. Maybe they’re taking care of an elderly parent or they’ve had other health problems that have come about that we need to reassess the plan and make sure that it’s actually doable for them at this point in time.
Certainly diabetes self-management education can be helpful. Some of those approaches can be helpful for finding something that’s going to work for patients in the long run, because it can be a very difficult disorder to manage as time goes on.
Dr. Colburn. Type 2 DM disproportionately affects individuals who are ≥ 65 years compared with younger individuals. Such older patients also are more likely to have cognitive impairment or visual issues. How do we best manage such patients?
Ms. Decesare. When I’m looking at the care plan, social support is very important. If someone has social support and they have a spouse or a son or daughter or someone else that can help them with their diabetes, we oftentimes will get them involved with the plan, as long as it’s fine with the patient, to offer some help, especially with the patients with cognitive problems, because sometimes the patients just cognitively cannot manage diabetes on their own. Prandial insulin could be a really dangerous product for someone who has cognitive disease.
I think you have to look at all the resources that are available. Sometimes you have to change your HbA1c target range to something that’s going to be manageable for that patient at that time. It might not be perfect, but it would be better to have no hypoglycemia rather than a real aggressive HbA1c target or a target range, if that’s what’s going to keep the patient safe.
Dr. Conlin. We thank our discussants for sharing very practical advice on how to implement the CPG. We hope this information supports clinicians as they develop treatment plans based on each patient's unique characteristics and goals of care.
Click here to read the digital edition.
Guidelines outline patient-centered approach to type 2 diabetes
issued jointly by the American Diabetes Association and the European Association for the Study of Diabetes.
The 2018 ADA/EASD Consensus Report also addresses clinical inertia and notes that medication adherence and persistence should be facilitated. All patients should be offered ongoing self-management education and support, Melanie J. Davies, MD, one of the two cochairs of the report-writing committee, said during a press conference at the annual meeting of the European Association for the Study of Diabetes.
The report also addresses preferred choices for glucose-lowering medications, largely based on recent findings of large-scale cardiovascular outcomes trials. There also is specific guidance on how to manage hyperglycemia in patients with atherosclerotic cardiovascular disease, chronic kidney disease, and heart failure.
“The consensus report focuses on not what an individual’s glycemic target should be or how to individualize goals but really addresses how each patient can achieve their individualized glycemic target,” Dr. Davies said.
Dr. Davies, who is professor of diabetes medicine at the University of Leicester (England) and an honorary consultant diabetologist at the University Hospitals of Leicester NHS Trust also said that the report looked at taking patient factors and preferences into account but also considered “the ever-increasing complexity around the availability of glucose-lowering agents.”
Practical guide to managing patients
The consensus report, which was simultaneously published in the official journals of the ADA (Diabetes Care 2018 Sep; dci180033) and the EASD (Diabetologia. 2018 Sep. doi: 10.1007/s00125-018-4729-5) to coincide with its presentation at the EASD meeting, is much more visual and aims to be more of a practical aid than was the previous position statement from 2015 (Diabetologia. 2015 Mar;58:429-42; Diabetes Care 2015 Jan;38[1]:140-9), on which it was based, Dr. Davies said.
The patient has been placed firmly at the center of the decision cycle, she observed, which starts with assessment of patient characteristics and consideration of their lifestyle, comorbidities, and clinical parameters. Specific factors that may affect the choice of treatment, such as the individualized glycosylated hemoglobin (HbA1c) target or side effect profiles of medications, are included, as is working together with the patient to make, continually monitor, and reevaluate a shared decision plan.
In terms of lifestyle, one of the consensus recommendations is that “an individualized program of medical nutritional therapy should be offered to all patients,” with the more specific recommendation that those who are overweight or obese be advised of the health benefits of weight loss and be encouraged to participate in dietary modifications that may include food substitution. Increasing activity is also highly recommended based on long-established evidence that this can help reduce HbA1c level. Recommendations for when to consider bariatric surgery for weight management also are included.
Clarity on treating comorbidities
Previously discussed in June at the ADA’s annual meeting, the consensus report has undergone fine-tuning and multiple revisions. The report was based on a comprehensive and systematic review of the diabetes literature available from 2014 through February 2018. Overall, more than 6,000 randomized trials, reviews, and meta-analyses were considered and distilled down to a list of around 500 papers that were then thoroughly reviewed by an expert panel.
“I guarantee, there’s never been a paper that’s been more peer reviewed,” said John Buse, MD, PhD, the other cochair of the report’s writing committee. A total of 35 named individuals reviewed and provided more than 800 detailed comments among them, which were considered and reflected in the final version.
Dr. Buse is the Verne S. Caviness Distinguished Professor, chief of the division of endocrinology, and director of the diabetes center at the University of North Carolina at Chapel Hill.
“There’s much more clarity now,” added Dr. Davies, referring to the changes made to how patients with comorbidities are managed. If somebody does have atherosclerotic cardiovascular disease or chronic kidney disease, there is now clear direction on which glucose-lowering therapy should be considered first, and what to do if the HbA1c remains above target.
For example, in patients who have established atherosclerotic cardiovascular disease, the recommendation is, after metformin, to choose either a glucagonlike peptide–1 (GLP-1) receptor agonist or a sodium-glucose cotransporter 2 (SGLT2) inhibitor with proven cardiovascular benefit.
If heart failure or chronic kidney disease coexist, then an SGLT2 inhibitor shown to reduce their progression should be favored, or if contraindicated or not preferred, a GLP-1 receptor agonist with proven cardiovascular benefit should be given.
The main action, pros and cons of interventions, and the various medications are shown in tables to clearly guide clinicians in the decision-making process, Dr. Buse said.
First-line management
The first line recommended glucose-lowering therapy for hyperglycemia in type 2 diabetes remains metformin, together with comprehensive lifestyle advice, Dr. Buse observed.
“A huge controversy in the [diabetes] community asks, ‘Is metformin the first-line therapy because it’s cheap and was the first oral agent studied and has a long history?’ or is it something that really is based on medical evidence?” Dr. Buse acknowledged. Although combinations of glucose-lowering drugs have been proposed upfront, “the evidence for that is largely from small studies, in limited numbers of sites, such that, for now, we generally recommend starting on a single-agent medication if lifestyle management is not enough to control glucose.”
If there is a need to intensify treatment as the patient’s HbA1c remains above their individualized target, then other drugs may be added to step up the treatment. The consensus report then looks at which drugs might be best to add, based on the need to avoid hypoglycemia, promote weight loss, and/or if cost or availability is a major issue.
If patients need the greater glucose-lowering effects of an injectable medication, a GLP-1 receptor agonist – not insulin – is recommended, Dr. Buse observed. However, for patients with extreme and symptomatic hyperglycemia, insulin is then recommended.
There also is guidance on when to consider oral therapies in conjunction with injectable therapies, with the consensus recommendation stating: Patients who are unable to maintain glycemic targets on basal insulin in combination with oral medication can have treatment intensified with GLP-1 receptor agonists, SGLT2 inhibitors, or prandial insulin.
The ADA perspective
William T. Cefalu, MD, chief scientific, medical and mission officer of the ADA observed that the “ADA fully endorses the ADA/EASD Consensus Report” and had already added a statement on the recommendations into its Standards of Medical Care in Diabetes – 2018 as part of the organization’s Living Standards Update. This was a change made last year to allow real-time updates of practice recommendations based on new and evolving evidence released in between the annual process of updating the Society’s Standards.
“Much, if not all, of these recommendations from this paper will be incorporated into our Standards,” said Dr. Cefalu. “We applaud the authors of the consensus paper; we think this was an outstanding group, and we really feel that this is a paradigm change in diabetes management,” he added. “Instead of relying on the [HbA1c] number in an algorithm, this puts the patient at the center; patient-related factors, patient preferences, adherence, compliance, and more importantly, the underlying disease state … this really is a comprehensive approach to management.”
The stratification of patients by cardiovascular disease, kidney disease, or heart failure is a particularly noteworthy, as is the advice on which agent to choose if weight management is an issue. Finally, there are the considerations of costs of therapy, and what to do if there is the risk of hypoglycemia. “The consensus recommendations and approach to glycemic management in adults with type 2 diabetes presented within the report reflects the current view of the ADA,” Dr. Cefalu confirmed.
The EASD perspective
“The EASD was again delighted to go into cooperation with our colleagues and friends at the ADA because is it is so important to bring out a consensus on where we need to go in this forest of glucose-lowering therapies,” said Chantal Mathieu, MD, PhD, vice-president of the EASD.
“The fact that this consensus paper puts the patient front and center, and makes that an integral part of glucose-lowering therapy, and also that lifestyle is being accentuated again, together with education in every patient is crucial,” Dr. Mathieu, who is professor of medicine at the Katholieke Universiteit Leuven (Belgium), and a coauthor of the report, added.
“At EASD, we also believe that it is very important to bring this consensus paper to life,” she added, which is part of her role as the chair of postgraduate education at the EASD. Two of the EASD’s main remits is to ensure that the results of research and education are brought to the diabetes community at large, she said.
In every figure in the paper there is a highlight to say, “please avoid clinical inertia, reassess and modify treatment if necessary, at least every 3-6 months,” Dr. Mathieu noted during the EASD congress.
David Matthews, MD, professor of diabetic medicine at the University of Oxford (England) and president-elect of the EASD, commented on the 2018 ADA/EASD Consensus Report after its presentation at the EASD meeting. “The reality is that you’ve got to think extremely hard with your patients about what the balances between risks and benefits are,” Dr. Matthews said. “We encourage you to do this, what you have here is a wonderful handbook to guide you in your decision making.”
Dr. Davies reported receiving personal fees and/or grants from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Gilead, Intarcia Therapeutics/Servier, Janssen, Merck Sharp & Dohme, Mitsubishi Tanabi, Novo Nordisk, Sanofi, and Takeda.
Dr. Buse disclosed acting as a consultant to and/or receiving research support from Adocia, AstraZeneca, Eli Lilly, GI Dynamics, Intarcia Therapeutics, MannKind, NovaTarg, Neurimmune, Novo Nordisk, Senseonics, and vTv Therapeutics with fees paid to the University of North Carolina. He holds stock options in Mellitus Health, PhaseBio and Stability Health.
Dr. Mathieu disclosed relationships with (advisory boards, speakers bureaus, and/or research support) from Abbott, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Hanmi Pharmaceuticals, Intrexon, Janssen Pharmaceuticals, MannKind, Medtronic, MSD, Novartis, Novo Nordisk, Pfizer, Roche Diagnostics, Sanofi, and UCB.
Dr. Cefalu had no disclosures and Dr. Matthews had no relevant conflicts of interest other than becoming the EASD president-elect during the meeting.
issued jointly by the American Diabetes Association and the European Association for the Study of Diabetes.
The 2018 ADA/EASD Consensus Report also addresses clinical inertia and notes that medication adherence and persistence should be facilitated. All patients should be offered ongoing self-management education and support, Melanie J. Davies, MD, one of the two cochairs of the report-writing committee, said during a press conference at the annual meeting of the European Association for the Study of Diabetes.
The report also addresses preferred choices for glucose-lowering medications, largely based on recent findings of large-scale cardiovascular outcomes trials. There also is specific guidance on how to manage hyperglycemia in patients with atherosclerotic cardiovascular disease, chronic kidney disease, and heart failure.
“The consensus report focuses on not what an individual’s glycemic target should be or how to individualize goals but really addresses how each patient can achieve their individualized glycemic target,” Dr. Davies said.
Dr. Davies, who is professor of diabetes medicine at the University of Leicester (England) and an honorary consultant diabetologist at the University Hospitals of Leicester NHS Trust also said that the report looked at taking patient factors and preferences into account but also considered “the ever-increasing complexity around the availability of glucose-lowering agents.”
Practical guide to managing patients
The consensus report, which was simultaneously published in the official journals of the ADA (Diabetes Care 2018 Sep; dci180033) and the EASD (Diabetologia. 2018 Sep. doi: 10.1007/s00125-018-4729-5) to coincide with its presentation at the EASD meeting, is much more visual and aims to be more of a practical aid than was the previous position statement from 2015 (Diabetologia. 2015 Mar;58:429-42; Diabetes Care 2015 Jan;38[1]:140-9), on which it was based, Dr. Davies said.
The patient has been placed firmly at the center of the decision cycle, she observed, which starts with assessment of patient characteristics and consideration of their lifestyle, comorbidities, and clinical parameters. Specific factors that may affect the choice of treatment, such as the individualized glycosylated hemoglobin (HbA1c) target or side effect profiles of medications, are included, as is working together with the patient to make, continually monitor, and reevaluate a shared decision plan.
In terms of lifestyle, one of the consensus recommendations is that “an individualized program of medical nutritional therapy should be offered to all patients,” with the more specific recommendation that those who are overweight or obese be advised of the health benefits of weight loss and be encouraged to participate in dietary modifications that may include food substitution. Increasing activity is also highly recommended based on long-established evidence that this can help reduce HbA1c level. Recommendations for when to consider bariatric surgery for weight management also are included.
Clarity on treating comorbidities
Previously discussed in June at the ADA’s annual meeting, the consensus report has undergone fine-tuning and multiple revisions. The report was based on a comprehensive and systematic review of the diabetes literature available from 2014 through February 2018. Overall, more than 6,000 randomized trials, reviews, and meta-analyses were considered and distilled down to a list of around 500 papers that were then thoroughly reviewed by an expert panel.
“I guarantee, there’s never been a paper that’s been more peer reviewed,” said John Buse, MD, PhD, the other cochair of the report’s writing committee. A total of 35 named individuals reviewed and provided more than 800 detailed comments among them, which were considered and reflected in the final version.
Dr. Buse is the Verne S. Caviness Distinguished Professor, chief of the division of endocrinology, and director of the diabetes center at the University of North Carolina at Chapel Hill.
“There’s much more clarity now,” added Dr. Davies, referring to the changes made to how patients with comorbidities are managed. If somebody does have atherosclerotic cardiovascular disease or chronic kidney disease, there is now clear direction on which glucose-lowering therapy should be considered first, and what to do if the HbA1c remains above target.
For example, in patients who have established atherosclerotic cardiovascular disease, the recommendation is, after metformin, to choose either a glucagonlike peptide–1 (GLP-1) receptor agonist or a sodium-glucose cotransporter 2 (SGLT2) inhibitor with proven cardiovascular benefit.
If heart failure or chronic kidney disease coexist, then an SGLT2 inhibitor shown to reduce their progression should be favored, or if contraindicated or not preferred, a GLP-1 receptor agonist with proven cardiovascular benefit should be given.
The main action, pros and cons of interventions, and the various medications are shown in tables to clearly guide clinicians in the decision-making process, Dr. Buse said.
First-line management
The first line recommended glucose-lowering therapy for hyperglycemia in type 2 diabetes remains metformin, together with comprehensive lifestyle advice, Dr. Buse observed.
“A huge controversy in the [diabetes] community asks, ‘Is metformin the first-line therapy because it’s cheap and was the first oral agent studied and has a long history?’ or is it something that really is based on medical evidence?” Dr. Buse acknowledged. Although combinations of glucose-lowering drugs have been proposed upfront, “the evidence for that is largely from small studies, in limited numbers of sites, such that, for now, we generally recommend starting on a single-agent medication if lifestyle management is not enough to control glucose.”
If there is a need to intensify treatment as the patient’s HbA1c remains above their individualized target, then other drugs may be added to step up the treatment. The consensus report then looks at which drugs might be best to add, based on the need to avoid hypoglycemia, promote weight loss, and/or if cost or availability is a major issue.
If patients need the greater glucose-lowering effects of an injectable medication, a GLP-1 receptor agonist – not insulin – is recommended, Dr. Buse observed. However, for patients with extreme and symptomatic hyperglycemia, insulin is then recommended.
There also is guidance on when to consider oral therapies in conjunction with injectable therapies, with the consensus recommendation stating: Patients who are unable to maintain glycemic targets on basal insulin in combination with oral medication can have treatment intensified with GLP-1 receptor agonists, SGLT2 inhibitors, or prandial insulin.
The ADA perspective
William T. Cefalu, MD, chief scientific, medical and mission officer of the ADA observed that the “ADA fully endorses the ADA/EASD Consensus Report” and had already added a statement on the recommendations into its Standards of Medical Care in Diabetes – 2018 as part of the organization’s Living Standards Update. This was a change made last year to allow real-time updates of practice recommendations based on new and evolving evidence released in between the annual process of updating the Society’s Standards.
“Much, if not all, of these recommendations from this paper will be incorporated into our Standards,” said Dr. Cefalu. “We applaud the authors of the consensus paper; we think this was an outstanding group, and we really feel that this is a paradigm change in diabetes management,” he added. “Instead of relying on the [HbA1c] number in an algorithm, this puts the patient at the center; patient-related factors, patient preferences, adherence, compliance, and more importantly, the underlying disease state … this really is a comprehensive approach to management.”
The stratification of patients by cardiovascular disease, kidney disease, or heart failure is a particularly noteworthy, as is the advice on which agent to choose if weight management is an issue. Finally, there are the considerations of costs of therapy, and what to do if there is the risk of hypoglycemia. “The consensus recommendations and approach to glycemic management in adults with type 2 diabetes presented within the report reflects the current view of the ADA,” Dr. Cefalu confirmed.
The EASD perspective
“The EASD was again delighted to go into cooperation with our colleagues and friends at the ADA because is it is so important to bring out a consensus on where we need to go in this forest of glucose-lowering therapies,” said Chantal Mathieu, MD, PhD, vice-president of the EASD.
“The fact that this consensus paper puts the patient front and center, and makes that an integral part of glucose-lowering therapy, and also that lifestyle is being accentuated again, together with education in every patient is crucial,” Dr. Mathieu, who is professor of medicine at the Katholieke Universiteit Leuven (Belgium), and a coauthor of the report, added.
“At EASD, we also believe that it is very important to bring this consensus paper to life,” she added, which is part of her role as the chair of postgraduate education at the EASD. Two of the EASD’s main remits is to ensure that the results of research and education are brought to the diabetes community at large, she said.
In every figure in the paper there is a highlight to say, “please avoid clinical inertia, reassess and modify treatment if necessary, at least every 3-6 months,” Dr. Mathieu noted during the EASD congress.
David Matthews, MD, professor of diabetic medicine at the University of Oxford (England) and president-elect of the EASD, commented on the 2018 ADA/EASD Consensus Report after its presentation at the EASD meeting. “The reality is that you’ve got to think extremely hard with your patients about what the balances between risks and benefits are,” Dr. Matthews said. “We encourage you to do this, what you have here is a wonderful handbook to guide you in your decision making.”
Dr. Davies reported receiving personal fees and/or grants from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Gilead, Intarcia Therapeutics/Servier, Janssen, Merck Sharp & Dohme, Mitsubishi Tanabi, Novo Nordisk, Sanofi, and Takeda.
Dr. Buse disclosed acting as a consultant to and/or receiving research support from Adocia, AstraZeneca, Eli Lilly, GI Dynamics, Intarcia Therapeutics, MannKind, NovaTarg, Neurimmune, Novo Nordisk, Senseonics, and vTv Therapeutics with fees paid to the University of North Carolina. He holds stock options in Mellitus Health, PhaseBio and Stability Health.
Dr. Mathieu disclosed relationships with (advisory boards, speakers bureaus, and/or research support) from Abbott, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Hanmi Pharmaceuticals, Intrexon, Janssen Pharmaceuticals, MannKind, Medtronic, MSD, Novartis, Novo Nordisk, Pfizer, Roche Diagnostics, Sanofi, and UCB.
Dr. Cefalu had no disclosures and Dr. Matthews had no relevant conflicts of interest other than becoming the EASD president-elect during the meeting.
issued jointly by the American Diabetes Association and the European Association for the Study of Diabetes.
The 2018 ADA/EASD Consensus Report also addresses clinical inertia and notes that medication adherence and persistence should be facilitated. All patients should be offered ongoing self-management education and support, Melanie J. Davies, MD, one of the two cochairs of the report-writing committee, said during a press conference at the annual meeting of the European Association for the Study of Diabetes.
The report also addresses preferred choices for glucose-lowering medications, largely based on recent findings of large-scale cardiovascular outcomes trials. There also is specific guidance on how to manage hyperglycemia in patients with atherosclerotic cardiovascular disease, chronic kidney disease, and heart failure.
“The consensus report focuses on not what an individual’s glycemic target should be or how to individualize goals but really addresses how each patient can achieve their individualized glycemic target,” Dr. Davies said.
Dr. Davies, who is professor of diabetes medicine at the University of Leicester (England) and an honorary consultant diabetologist at the University Hospitals of Leicester NHS Trust also said that the report looked at taking patient factors and preferences into account but also considered “the ever-increasing complexity around the availability of glucose-lowering agents.”
Practical guide to managing patients
The consensus report, which was simultaneously published in the official journals of the ADA (Diabetes Care 2018 Sep; dci180033) and the EASD (Diabetologia. 2018 Sep. doi: 10.1007/s00125-018-4729-5) to coincide with its presentation at the EASD meeting, is much more visual and aims to be more of a practical aid than was the previous position statement from 2015 (Diabetologia. 2015 Mar;58:429-42; Diabetes Care 2015 Jan;38[1]:140-9), on which it was based, Dr. Davies said.
The patient has been placed firmly at the center of the decision cycle, she observed, which starts with assessment of patient characteristics and consideration of their lifestyle, comorbidities, and clinical parameters. Specific factors that may affect the choice of treatment, such as the individualized glycosylated hemoglobin (HbA1c) target or side effect profiles of medications, are included, as is working together with the patient to make, continually monitor, and reevaluate a shared decision plan.
In terms of lifestyle, one of the consensus recommendations is that “an individualized program of medical nutritional therapy should be offered to all patients,” with the more specific recommendation that those who are overweight or obese be advised of the health benefits of weight loss and be encouraged to participate in dietary modifications that may include food substitution. Increasing activity is also highly recommended based on long-established evidence that this can help reduce HbA1c level. Recommendations for when to consider bariatric surgery for weight management also are included.
Clarity on treating comorbidities
Previously discussed in June at the ADA’s annual meeting, the consensus report has undergone fine-tuning and multiple revisions. The report was based on a comprehensive and systematic review of the diabetes literature available from 2014 through February 2018. Overall, more than 6,000 randomized trials, reviews, and meta-analyses were considered and distilled down to a list of around 500 papers that were then thoroughly reviewed by an expert panel.
“I guarantee, there’s never been a paper that’s been more peer reviewed,” said John Buse, MD, PhD, the other cochair of the report’s writing committee. A total of 35 named individuals reviewed and provided more than 800 detailed comments among them, which were considered and reflected in the final version.
Dr. Buse is the Verne S. Caviness Distinguished Professor, chief of the division of endocrinology, and director of the diabetes center at the University of North Carolina at Chapel Hill.
“There’s much more clarity now,” added Dr. Davies, referring to the changes made to how patients with comorbidities are managed. If somebody does have atherosclerotic cardiovascular disease or chronic kidney disease, there is now clear direction on which glucose-lowering therapy should be considered first, and what to do if the HbA1c remains above target.
For example, in patients who have established atherosclerotic cardiovascular disease, the recommendation is, after metformin, to choose either a glucagonlike peptide–1 (GLP-1) receptor agonist or a sodium-glucose cotransporter 2 (SGLT2) inhibitor with proven cardiovascular benefit.
If heart failure or chronic kidney disease coexist, then an SGLT2 inhibitor shown to reduce their progression should be favored, or if contraindicated or not preferred, a GLP-1 receptor agonist with proven cardiovascular benefit should be given.
The main action, pros and cons of interventions, and the various medications are shown in tables to clearly guide clinicians in the decision-making process, Dr. Buse said.
First-line management
The first line recommended glucose-lowering therapy for hyperglycemia in type 2 diabetes remains metformin, together with comprehensive lifestyle advice, Dr. Buse observed.
“A huge controversy in the [diabetes] community asks, ‘Is metformin the first-line therapy because it’s cheap and was the first oral agent studied and has a long history?’ or is it something that really is based on medical evidence?” Dr. Buse acknowledged. Although combinations of glucose-lowering drugs have been proposed upfront, “the evidence for that is largely from small studies, in limited numbers of sites, such that, for now, we generally recommend starting on a single-agent medication if lifestyle management is not enough to control glucose.”
If there is a need to intensify treatment as the patient’s HbA1c remains above their individualized target, then other drugs may be added to step up the treatment. The consensus report then looks at which drugs might be best to add, based on the need to avoid hypoglycemia, promote weight loss, and/or if cost or availability is a major issue.
If patients need the greater glucose-lowering effects of an injectable medication, a GLP-1 receptor agonist – not insulin – is recommended, Dr. Buse observed. However, for patients with extreme and symptomatic hyperglycemia, insulin is then recommended.
There also is guidance on when to consider oral therapies in conjunction with injectable therapies, with the consensus recommendation stating: Patients who are unable to maintain glycemic targets on basal insulin in combination with oral medication can have treatment intensified with GLP-1 receptor agonists, SGLT2 inhibitors, or prandial insulin.
The ADA perspective
William T. Cefalu, MD, chief scientific, medical and mission officer of the ADA observed that the “ADA fully endorses the ADA/EASD Consensus Report” and had already added a statement on the recommendations into its Standards of Medical Care in Diabetes – 2018 as part of the organization’s Living Standards Update. This was a change made last year to allow real-time updates of practice recommendations based on new and evolving evidence released in between the annual process of updating the Society’s Standards.
“Much, if not all, of these recommendations from this paper will be incorporated into our Standards,” said Dr. Cefalu. “We applaud the authors of the consensus paper; we think this was an outstanding group, and we really feel that this is a paradigm change in diabetes management,” he added. “Instead of relying on the [HbA1c] number in an algorithm, this puts the patient at the center; patient-related factors, patient preferences, adherence, compliance, and more importantly, the underlying disease state … this really is a comprehensive approach to management.”
The stratification of patients by cardiovascular disease, kidney disease, or heart failure is a particularly noteworthy, as is the advice on which agent to choose if weight management is an issue. Finally, there are the considerations of costs of therapy, and what to do if there is the risk of hypoglycemia. “The consensus recommendations and approach to glycemic management in adults with type 2 diabetes presented within the report reflects the current view of the ADA,” Dr. Cefalu confirmed.
The EASD perspective
“The EASD was again delighted to go into cooperation with our colleagues and friends at the ADA because is it is so important to bring out a consensus on where we need to go in this forest of glucose-lowering therapies,” said Chantal Mathieu, MD, PhD, vice-president of the EASD.
“The fact that this consensus paper puts the patient front and center, and makes that an integral part of glucose-lowering therapy, and also that lifestyle is being accentuated again, together with education in every patient is crucial,” Dr. Mathieu, who is professor of medicine at the Katholieke Universiteit Leuven (Belgium), and a coauthor of the report, added.
“At EASD, we also believe that it is very important to bring this consensus paper to life,” she added, which is part of her role as the chair of postgraduate education at the EASD. Two of the EASD’s main remits is to ensure that the results of research and education are brought to the diabetes community at large, she said.
In every figure in the paper there is a highlight to say, “please avoid clinical inertia, reassess and modify treatment if necessary, at least every 3-6 months,” Dr. Mathieu noted during the EASD congress.
David Matthews, MD, professor of diabetic medicine at the University of Oxford (England) and president-elect of the EASD, commented on the 2018 ADA/EASD Consensus Report after its presentation at the EASD meeting. “The reality is that you’ve got to think extremely hard with your patients about what the balances between risks and benefits are,” Dr. Matthews said. “We encourage you to do this, what you have here is a wonderful handbook to guide you in your decision making.”
Dr. Davies reported receiving personal fees and/or grants from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Gilead, Intarcia Therapeutics/Servier, Janssen, Merck Sharp & Dohme, Mitsubishi Tanabi, Novo Nordisk, Sanofi, and Takeda.
Dr. Buse disclosed acting as a consultant to and/or receiving research support from Adocia, AstraZeneca, Eli Lilly, GI Dynamics, Intarcia Therapeutics, MannKind, NovaTarg, Neurimmune, Novo Nordisk, Senseonics, and vTv Therapeutics with fees paid to the University of North Carolina. He holds stock options in Mellitus Health, PhaseBio and Stability Health.
Dr. Mathieu disclosed relationships with (advisory boards, speakers bureaus, and/or research support) from Abbott, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Hanmi Pharmaceuticals, Intrexon, Janssen Pharmaceuticals, MannKind, Medtronic, MSD, Novartis, Novo Nordisk, Pfizer, Roche Diagnostics, Sanofi, and UCB.
Dr. Cefalu had no disclosures and Dr. Matthews had no relevant conflicts of interest other than becoming the EASD president-elect during the meeting.
REPORTING FROM EASD 2018
‘Twincreatin’ produces ‘impressive’ HbA1c, weight control in T2DM
BERLIN – Patients with type 2 diabetes mellitus (T2DM) achieved “impressive” drops in glycated hemoglobin, or HbA1c, of up to 2.4% and in body weight of up to 11.3 kg with the investigational drug LY3298176 in a phase 2b trial.
The primary endpoint was the change in HbA1c at 26 weeks, which showed significant reductions from baseline of 1.6%, 2%, and 2.4% for the 5-mg, 10-mg, and 15-mg doses, respectively, of the drug tested. By comparison, a drop of just 1.1% was seen for dulaglutide, and 0.1% for placebo.
The higher doses of LY3298176, which is a dual agonist of glucose-dependent insulinotropic polypeptide (GIP) and the glucagonlike peptide–1 (GLP-1) receptor, used in the trial helped up to 90% of patients achieve an HbA1c of 7% or less, 82% of patients to achieve an HbA1c of 6.5% or less, and up to 30% of patients to achieve an HbA1c of less than 5.7%, a range which is considered normal for people without diabetes.
As for weight loss, the 5-mg, 10-mg, and 15-mg doses of LY3298176 helped patients achieve significant –4.8-kg, –8.7-kg, and –11.3-kg changes from baseline, respectively. These were greater than those seen with dulaglutide (–2.7 kg) and placebo (–0.4 kg).
More than one third of patients treated with the novel drug achieved at least a 10% or more change in their starting body weight, with a quarter of patients on the 15-mg dose achieving a 15% or more change in bodyweight.
“These are very, very impressive data both from an A1c-lowering perspective and also from a weight reduction perspective,” said the study’s principal investigator Juan Frias, MD, at a press briefing held at the annual meeting of the European Association for the Study of Diabetes (EASD).
GLP-1 receptor agonist therapy is a recommended treatment for T2DM because it addresses many of the important pathophysiologic problems, from blood glucose control and weight loss to reducing cardiovascular risk, Dr. Frias observed.
He added that the first GLP-1 receptor agonists became available in the United States in 2005. Their use in T2DM has recently been further endorsed in a consensus report (Diabetes Care. 2018 Sep. doi: rg/10.2337/dci18-0033; Diabetologia. 2018. doi: 10.1007/s00125-018-4729-5) issued jointly by the American Diabetes Association and the EASD.
“Despite the potency of these agents, many of our patients are not achieving adequate glycemic or weight control, so we could always use new agents that are more potent as long as they’re safe for the patient,” said Dr. Frias, who is a clinical endocrinologist and the chief executive officer at the National Research Institute in Los Angeles
LY3298176 is a novel unimolecular, multifunctional peptide that is being developed by Eli Lilly as a dual GIP/GLP-1 receptor agonist for the treatment of T2DM. It consists of 39 amino acids linked to a C20 fatty–diacid moiety and has a mean half-life of around 5 days, which means it can be dosed weekly, Dr. Frias observed.
The effect of targeting both GIP and the GLP-1 receptor simultaneously is possibly both additive and complementary, Dr. Frias suggested. Additive effects may include a decrease in food intake and an increase in insulin secretion, with opposing effects on glucagon secretion, with the additional effects of increased glucose uptake with GIP and delayed gastric emptying with GLP-1 receptor agonism.
“When you think back on what the history, biology, and discovery of these compounds was, it isn’t at all obvious you’d pair these two [to make] a so-called ‘twincreatin’ – there is so much overlap,” said Matthias Tschöp, MD, who was invited to discuss the findings after the presentation at the EASD meeting.
Dr. Tschöp, who is the head of the division of neuroendocrinology at the Institute for Diabetes and Obesity at Helmholtz Zentrum München in Neuherberg , Germany, noted that it’s an interesting combination, but it’s not the only approach being tested – there are other twincretin combinations. GIP on its own doesn’t seem to do much, but it must be the combination that is important, it was suggested during discussion.
A total of 318 patients with T2DM and a starting HbA1c of 7%-10.5% were studied and randomized equally to one of six weekly treatment groups: LY3298176 at doses of either 1 mg, 5 mg, 10 mg, or 15 mg, given subcutaneously; 1.5 mg of dulaglutide; or a placebo injection.
The two highest doses of LY3298176 were titrated rather quickly, Dr. Frias said, and this is important when it comes to the side effect profile. In a subsequent study, the dose titration schedule was amended to prolong the titration period to try to avoid some of the side effects seen, according to Dr. Frias.
Adverse events predominantly affected the gastrointestinal system, with any grade nausea, vomiting, and diarrhea seen in a respective 20%-40%, 7%-26%, and 24%-32% of patients taking the 5-mg, 10-mg, and 15-mg doses. Corresponding rates in dulaglutide-treated patients were, approximately, 30%, 9%, and 17%.
“The safety profile of the dual agonist is really similar to that which you see with selective GLP-1 receptor agonists,” Dr. Frias observed. “The most common adverse events were seen at the higher doses and pertained to GI tolerability, but the majority of these events were mild to moderate and dissipated with time and [could] be reduced significantly by appropriate titration.”
Dr. Frias said: “This novel dual GIP/GLP-1 receptor agonist certainly has the potential to be become a very promising treatment option for patients with type 2 diabetes.”
He added: “In my experience as an investigator, as a clinician, I’ve never really seen this magnitude of A1c reduction in this percentage of patients and also with this level of weight loss as well, certainly greater than with a selective GLP-1 receptor agonist.”
Further long-term studies are needed, and the phase 3 program in T2DM will be called SURPASS, according to a press statement issued by Eli Lilly. The phase 3 studies are expected to begin “no later than early 2019,” the statement said, and be completed in late 2021. The drug company also noted that it is “evaluating next steps in the study of GIP/GLP-1 RA for obesity and other conditions.”
SOURCE: Frias JP et al. EASD 2018, Session S31; Frias JP et al. Lancet. 2018 Oct 4. doi: 10.1016/S0140-6736(18)32260-8.
BERLIN – Patients with type 2 diabetes mellitus (T2DM) achieved “impressive” drops in glycated hemoglobin, or HbA1c, of up to 2.4% and in body weight of up to 11.3 kg with the investigational drug LY3298176 in a phase 2b trial.
The primary endpoint was the change in HbA1c at 26 weeks, which showed significant reductions from baseline of 1.6%, 2%, and 2.4% for the 5-mg, 10-mg, and 15-mg doses, respectively, of the drug tested. By comparison, a drop of just 1.1% was seen for dulaglutide, and 0.1% for placebo.
The higher doses of LY3298176, which is a dual agonist of glucose-dependent insulinotropic polypeptide (GIP) and the glucagonlike peptide–1 (GLP-1) receptor, used in the trial helped up to 90% of patients achieve an HbA1c of 7% or less, 82% of patients to achieve an HbA1c of 6.5% or less, and up to 30% of patients to achieve an HbA1c of less than 5.7%, a range which is considered normal for people without diabetes.
As for weight loss, the 5-mg, 10-mg, and 15-mg doses of LY3298176 helped patients achieve significant –4.8-kg, –8.7-kg, and –11.3-kg changes from baseline, respectively. These were greater than those seen with dulaglutide (–2.7 kg) and placebo (–0.4 kg).
More than one third of patients treated with the novel drug achieved at least a 10% or more change in their starting body weight, with a quarter of patients on the 15-mg dose achieving a 15% or more change in bodyweight.
“These are very, very impressive data both from an A1c-lowering perspective and also from a weight reduction perspective,” said the study’s principal investigator Juan Frias, MD, at a press briefing held at the annual meeting of the European Association for the Study of Diabetes (EASD).
GLP-1 receptor agonist therapy is a recommended treatment for T2DM because it addresses many of the important pathophysiologic problems, from blood glucose control and weight loss to reducing cardiovascular risk, Dr. Frias observed.
He added that the first GLP-1 receptor agonists became available in the United States in 2005. Their use in T2DM has recently been further endorsed in a consensus report (Diabetes Care. 2018 Sep. doi: rg/10.2337/dci18-0033; Diabetologia. 2018. doi: 10.1007/s00125-018-4729-5) issued jointly by the American Diabetes Association and the EASD.
“Despite the potency of these agents, many of our patients are not achieving adequate glycemic or weight control, so we could always use new agents that are more potent as long as they’re safe for the patient,” said Dr. Frias, who is a clinical endocrinologist and the chief executive officer at the National Research Institute in Los Angeles
LY3298176 is a novel unimolecular, multifunctional peptide that is being developed by Eli Lilly as a dual GIP/GLP-1 receptor agonist for the treatment of T2DM. It consists of 39 amino acids linked to a C20 fatty–diacid moiety and has a mean half-life of around 5 days, which means it can be dosed weekly, Dr. Frias observed.
The effect of targeting both GIP and the GLP-1 receptor simultaneously is possibly both additive and complementary, Dr. Frias suggested. Additive effects may include a decrease in food intake and an increase in insulin secretion, with opposing effects on glucagon secretion, with the additional effects of increased glucose uptake with GIP and delayed gastric emptying with GLP-1 receptor agonism.
“When you think back on what the history, biology, and discovery of these compounds was, it isn’t at all obvious you’d pair these two [to make] a so-called ‘twincreatin’ – there is so much overlap,” said Matthias Tschöp, MD, who was invited to discuss the findings after the presentation at the EASD meeting.
Dr. Tschöp, who is the head of the division of neuroendocrinology at the Institute for Diabetes and Obesity at Helmholtz Zentrum München in Neuherberg , Germany, noted that it’s an interesting combination, but it’s not the only approach being tested – there are other twincretin combinations. GIP on its own doesn’t seem to do much, but it must be the combination that is important, it was suggested during discussion.
A total of 318 patients with T2DM and a starting HbA1c of 7%-10.5% were studied and randomized equally to one of six weekly treatment groups: LY3298176 at doses of either 1 mg, 5 mg, 10 mg, or 15 mg, given subcutaneously; 1.5 mg of dulaglutide; or a placebo injection.
The two highest doses of LY3298176 were titrated rather quickly, Dr. Frias said, and this is important when it comes to the side effect profile. In a subsequent study, the dose titration schedule was amended to prolong the titration period to try to avoid some of the side effects seen, according to Dr. Frias.
Adverse events predominantly affected the gastrointestinal system, with any grade nausea, vomiting, and diarrhea seen in a respective 20%-40%, 7%-26%, and 24%-32% of patients taking the 5-mg, 10-mg, and 15-mg doses. Corresponding rates in dulaglutide-treated patients were, approximately, 30%, 9%, and 17%.
“The safety profile of the dual agonist is really similar to that which you see with selective GLP-1 receptor agonists,” Dr. Frias observed. “The most common adverse events were seen at the higher doses and pertained to GI tolerability, but the majority of these events were mild to moderate and dissipated with time and [could] be reduced significantly by appropriate titration.”
Dr. Frias said: “This novel dual GIP/GLP-1 receptor agonist certainly has the potential to be become a very promising treatment option for patients with type 2 diabetes.”
He added: “In my experience as an investigator, as a clinician, I’ve never really seen this magnitude of A1c reduction in this percentage of patients and also with this level of weight loss as well, certainly greater than with a selective GLP-1 receptor agonist.”
Further long-term studies are needed, and the phase 3 program in T2DM will be called SURPASS, according to a press statement issued by Eli Lilly. The phase 3 studies are expected to begin “no later than early 2019,” the statement said, and be completed in late 2021. The drug company also noted that it is “evaluating next steps in the study of GIP/GLP-1 RA for obesity and other conditions.”
SOURCE: Frias JP et al. EASD 2018, Session S31; Frias JP et al. Lancet. 2018 Oct 4. doi: 10.1016/S0140-6736(18)32260-8.
BERLIN – Patients with type 2 diabetes mellitus (T2DM) achieved “impressive” drops in glycated hemoglobin, or HbA1c, of up to 2.4% and in body weight of up to 11.3 kg with the investigational drug LY3298176 in a phase 2b trial.
The primary endpoint was the change in HbA1c at 26 weeks, which showed significant reductions from baseline of 1.6%, 2%, and 2.4% for the 5-mg, 10-mg, and 15-mg doses, respectively, of the drug tested. By comparison, a drop of just 1.1% was seen for dulaglutide, and 0.1% for placebo.
The higher doses of LY3298176, which is a dual agonist of glucose-dependent insulinotropic polypeptide (GIP) and the glucagonlike peptide–1 (GLP-1) receptor, used in the trial helped up to 90% of patients achieve an HbA1c of 7% or less, 82% of patients to achieve an HbA1c of 6.5% or less, and up to 30% of patients to achieve an HbA1c of less than 5.7%, a range which is considered normal for people without diabetes.
As for weight loss, the 5-mg, 10-mg, and 15-mg doses of LY3298176 helped patients achieve significant –4.8-kg, –8.7-kg, and –11.3-kg changes from baseline, respectively. These were greater than those seen with dulaglutide (–2.7 kg) and placebo (–0.4 kg).
More than one third of patients treated with the novel drug achieved at least a 10% or more change in their starting body weight, with a quarter of patients on the 15-mg dose achieving a 15% or more change in bodyweight.
“These are very, very impressive data both from an A1c-lowering perspective and also from a weight reduction perspective,” said the study’s principal investigator Juan Frias, MD, at a press briefing held at the annual meeting of the European Association for the Study of Diabetes (EASD).
GLP-1 receptor agonist therapy is a recommended treatment for T2DM because it addresses many of the important pathophysiologic problems, from blood glucose control and weight loss to reducing cardiovascular risk, Dr. Frias observed.
He added that the first GLP-1 receptor agonists became available in the United States in 2005. Their use in T2DM has recently been further endorsed in a consensus report (Diabetes Care. 2018 Sep. doi: rg/10.2337/dci18-0033; Diabetologia. 2018. doi: 10.1007/s00125-018-4729-5) issued jointly by the American Diabetes Association and the EASD.
“Despite the potency of these agents, many of our patients are not achieving adequate glycemic or weight control, so we could always use new agents that are more potent as long as they’re safe for the patient,” said Dr. Frias, who is a clinical endocrinologist and the chief executive officer at the National Research Institute in Los Angeles
LY3298176 is a novel unimolecular, multifunctional peptide that is being developed by Eli Lilly as a dual GIP/GLP-1 receptor agonist for the treatment of T2DM. It consists of 39 amino acids linked to a C20 fatty–diacid moiety and has a mean half-life of around 5 days, which means it can be dosed weekly, Dr. Frias observed.
The effect of targeting both GIP and the GLP-1 receptor simultaneously is possibly both additive and complementary, Dr. Frias suggested. Additive effects may include a decrease in food intake and an increase in insulin secretion, with opposing effects on glucagon secretion, with the additional effects of increased glucose uptake with GIP and delayed gastric emptying with GLP-1 receptor agonism.
“When you think back on what the history, biology, and discovery of these compounds was, it isn’t at all obvious you’d pair these two [to make] a so-called ‘twincreatin’ – there is so much overlap,” said Matthias Tschöp, MD, who was invited to discuss the findings after the presentation at the EASD meeting.
Dr. Tschöp, who is the head of the division of neuroendocrinology at the Institute for Diabetes and Obesity at Helmholtz Zentrum München in Neuherberg , Germany, noted that it’s an interesting combination, but it’s not the only approach being tested – there are other twincretin combinations. GIP on its own doesn’t seem to do much, but it must be the combination that is important, it was suggested during discussion.
A total of 318 patients with T2DM and a starting HbA1c of 7%-10.5% were studied and randomized equally to one of six weekly treatment groups: LY3298176 at doses of either 1 mg, 5 mg, 10 mg, or 15 mg, given subcutaneously; 1.5 mg of dulaglutide; or a placebo injection.
The two highest doses of LY3298176 were titrated rather quickly, Dr. Frias said, and this is important when it comes to the side effect profile. In a subsequent study, the dose titration schedule was amended to prolong the titration period to try to avoid some of the side effects seen, according to Dr. Frias.
Adverse events predominantly affected the gastrointestinal system, with any grade nausea, vomiting, and diarrhea seen in a respective 20%-40%, 7%-26%, and 24%-32% of patients taking the 5-mg, 10-mg, and 15-mg doses. Corresponding rates in dulaglutide-treated patients were, approximately, 30%, 9%, and 17%.
“The safety profile of the dual agonist is really similar to that which you see with selective GLP-1 receptor agonists,” Dr. Frias observed. “The most common adverse events were seen at the higher doses and pertained to GI tolerability, but the majority of these events were mild to moderate and dissipated with time and [could] be reduced significantly by appropriate titration.”
Dr. Frias said: “This novel dual GIP/GLP-1 receptor agonist certainly has the potential to be become a very promising treatment option for patients with type 2 diabetes.”
He added: “In my experience as an investigator, as a clinician, I’ve never really seen this magnitude of A1c reduction in this percentage of patients and also with this level of weight loss as well, certainly greater than with a selective GLP-1 receptor agonist.”
Further long-term studies are needed, and the phase 3 program in T2DM will be called SURPASS, according to a press statement issued by Eli Lilly. The phase 3 studies are expected to begin “no later than early 2019,” the statement said, and be completed in late 2021. The drug company also noted that it is “evaluating next steps in the study of GIP/GLP-1 RA for obesity and other conditions.”
SOURCE: Frias JP et al. EASD 2018, Session S31; Frias JP et al. Lancet. 2018 Oct 4. doi: 10.1016/S0140-6736(18)32260-8.
REPORTING FROM EASD 2018
Key clinical point: The investigational dual GIP/GLP-1 receptor agonist LY3298176 could prove to be a promising treatment for T2DM.
Major finding: HbA1c fell by up to 2.4%, and body weight reduced by up to 11.3 kg depending on the dose tested.
Study details: A 26-week, phase 2b, double-blind, placebo-controlled study of 318 subjects with T2DM and a starting HbA1c of 7%-10.5%.
Disclosures: The study was funded by Eli Lilly. The presenting author Dr. Frias declared receiving research support and consulting honorarium from the company, as well as from multiple other pharmaceutical companies. The commentator Dr. Tschöp disclosed being a scientific advisory board member of ERX Pharmaceuticals.
Sources: Frias JP et al. EASD 2018, Session S31; Frias JP et al. Lancet. 2018 Oct 4. doi: 10.1016/S0140-6736(18)32260-8.
Overview and Discussion of the 2017 VA/DoD Clinical Practice Guideline for the Management of Type 2 Diabetes Mellitus in Primary Care (FULL)
Diabetes mellitus (DM) is an epidemic in the U.S. More than 30 million people (9.4% of the total population) have DM; type 2 DM (T2SM) accounts for 95% of these cases.1 The estimated prevalence of DM among individuals aged > 65 years is about 3 times higher at 26%. The prevalence among veterans enrolled in the VA is higher than in the general population; about 25% of VA users have been diagnosed with DM.2 As a result, DM is the leading cause of blindness, end stage renal disease, amputations, and a significant contributor to myocardial infarction and stroke. Older adults with DM have an increased risk of mortality compared with individuals without DM.3 In 2012, DM was estimated to cost $176 billion in direct and indirect medical costs.4 These health and cost implications make effective management of DM a priority for health care providers (HCPs), policy makers, and patients nationwide.
The 2017 VA/DoD Clinical Practice Guideline (CPG) for the Management of T2DM in Primary Care provides the primary care team an evidence-based and individualized approach to holistic care of the patient with T2DM.5 Key recommendations were developed based on methods established by the VA/DoD Evidence-Based Practice Working Group (EBPWG) and are aligned with standards for trustworthy guidelines by using the Grading of Recommendations Assessment, Development and Evaluation system to assess the quality of the evidence base and assign a grade for the strength for each recommendation.6,7 The EBPWG included a multidisciplinary panel of practicing clinician stakeholders, including primary care physicians, endocrinologists, medical nutritionists, pharmacists, diabetes educators, and nurse practitioners. The CPG development process also included a patient focus group. Important themes from the focus group were shared with the EBPWG to help address the needs and perspectives of patients receiving treatment for DM in the VA and DoD.
In this article, the authors briefly review several of the most pertinent CPG updates for the busy clinician.
Shared Decision Making
Shared decision making (SDM) is a central component of the approach to patients with DM. Shared decision making involves the patient and care providers together making important decisions about the treatment plan and goals of care, using communication tools and exploring patient preferences.8
Using an empathetic and nonjudgmental approach facilitates discussions about a patient’s specific health care needs and goals for care. Shared decision making also can provide culturally appropriate treatment and care information to meet the needs of those with limited literacy or numeracy skills, or other learning barriers, such as physical, sensory, or learning disabilities. Family involvement is an important component of SDM when desired by the patient.9
The goals of successful SDM include a decrease in patient anxiety and an increase in trust in the health care team, ideally leading to improvement in adherence and patient outcomes.8,10-12 Improved patient-clinician communication conveys openness to discuss any future concerns. Furthermore, SDM does not need to take a significant amount of a clinician’s time to create an environment of consideration and goal formation. Training in communication skills may be helpful for those unfamiliar with SDM techniques. Patients are most likely to participate in the SDM process when they are comfortable speaking with clinicians and have some knowledge about their specific disease process.13
The clinical team can review all prior treatment attempts with the patient to understand the patient's perspective on these interventions. Lastly, patients are involved in prioritizing problems to be addressed and in setting specific goals. A 5-step SDM process prompted by the SHARE acronym can be used:
- Seek your patient’s participation;
- Help your patient explore and compare treatment options;
- Assess your patient’s values and preferences;
- Reach a decision with your patient; and
- Evaluate your patient’s decision (Figure).8
The VA/DoD CPG noted that there is high-quality evidence supporting SDM for improving patients’ knowledge, satisfaction, and engagement with their treatment plan.14-16 Specific methodologic approaches to SDM are not well defined for individual patient groups, which represents a significant research gap. Patients diagnosed with T2DM might respond differently to SDM depending on personal goals, life experiences, and coping strategies.14-16 Shared decision making should be used at every decision point in the treatment process, from the diagnosis of pre-diabetes to the patient with advanced complications. This includes— at a minimum—at initial diagnosis, when experiencing difficulties in management, and at times of transition or development of complications.16
A shared understanding is critical to the SDM process. Diabetes self-management education and diabetes self-management support provide a framework that involves a collaborative, ongoing, interactive process to help patients gain knowledge, modify behavior, and successfully manage the disease. The goal of DM education in SDM is to ensure that the patient has sufficient knowledge and skills to achieve the treatment goals they set with their health care team. Assessment of patient understanding in the clinic could include use of the “teach-back method.”17 Health coaching and motivational interviewing strategies also may help clinicians understand patients’ perceptions, values, and beliefs regarding their condition, treatment, and self-management options, particularly when patients seem to be reluctant to fully participate in decisions and care.
A challenge for HCPs is to help patients understand how they can successfully manage DM and partner with health care teams to express their goals and preferences to aid in individualized health care decisions. Using SDM tools and ensuring that clinicians can use patient-centered communication skills increase patients’ willingness to share in decision making and engage in the treatment plan.
Nutrition Recommendations
Nutrition therapy is a key component of any successful DM management plan. The EBPWG added 2 strong recommendations for DM nutrition strategies. The first recommendation is to follow a Mediterranean diet, if this resonates with the patient’s values and preferences (Table 1).
- High intake of vegetables, fruits, nuts, unrefined grains, and olive oil;
- Moderate intake of fish and poultry;
- Low or moderate intake of wine; and
- Low intake of red meat, processed meat, dairy, and sweets.
The Mediterranean diet effectively improves glycemic control, delays the time to first pharmacologic intervention, and reduces cardiovascular risk factors in individuals with diabetes.18 An additional benefits of this dietary pattern includes significant hemoglobin A1c (HbA1c) reduction.19,20 A Mediterranean diet also has been linked to improved cardiovascular outcomes and weight loss. In general, the evidence supporting a Mediterranean diet are robust, but securing and adapting to these types of foods can be challenging for some patients.
The second nutrition recommendation is to reduce the percentage of energy from carbohydrates to between 14% and 45% per day and/or eat foods with lower glycemic index. Patients who do not choose a Mediterranean diet can employ this dietary pattern. A systematic review compared dietary interventions, including lower carbohydrate and low-glycemic index diets, and showed both dietary interventions improved glycemic control.18 Unfortunately, many studies compare different intervention diets rather than comparing an intervention against a control diet. However, based on the available evidence, the Working Group endorses a Mediterranean diet and carbohydrate reduction and low glycemic index foods as dietary options in which the benefits seem to outweigh harms.
Target Hemoglobin A1c
The EBPWG reviewed several large, intensive glucose control trials to apply recent evidence to ongoing HbA1c treatment targets. The CPG strongly reaffirms that rather than assigning a single glycemic goal for all patients, clinicians should use SDM to develop an HbA1c target range that is risk-stratified (Table 2).
The ARR of complications must be balanced against the risk of therapy. Several major trials tested the hypothesis that intensive glycemic control (target HbA1c at least < 7%) improved cardiovascular outcomes in patients with T2DM.23-25 These trials did not demonstrate cardiovascular benefit from intensive control to reach HbA1c < 7%, and the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study revealed possible cardiovascular harm.24
In addition, because these studies enrolled patients with established T2DM, they demonstrated less reduction in microvascular complications than was seen in newly diagnosed patients in UKPDS.22 Systematic reviews comparing intensive and conventional glucose control showed no significant differences in all-cause mortality or death from cardiovascular disease.26,27 Therefore, intensive control of T2DM has the greatest impact on microvascular complications and is most successful when initiated early in the disease process.
A target HbA1c range is recommended rather than a threshold value (eg, HbA1c < 8.0%) for several reasons. Most important the clinical trials that provide evidence for improved glycemic control used an HbA1c value recorded over time, not a single value measured at one point in time. Many factors influence HbA1c measurements other than just glycemic control.28 These include anemia, chronic kidney disease, race/ethnicity, and hemoglobinapathies.29-32 Patients can have clinically significant variation in HbA1c results between test samples, even when obtained from the same laboratory.33 For these reasons, the CPG continues to recommend use of fasting glucose ≥ 126 mg/dL to establish a DM diagnosis when the HbA1c is < 7.0%. This limits the likelihood that patients will be incorrectly diagnosed with DM, which can affect insurability, disability, or the trajectory of a military career. For patients with diagnosed T2DM, glycemic control over time remains important, but overreliance on a single HbA1c test could lead to overtreatment and potential adverse outcomes.
The EBPWG considered the target HbA1c and outcomes in UKPDS, ACCORD, Veterans Affairs Diabetes Trial (VADT), and the Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) when considering HbA1c target ranges.23,25,34,35 Indeed, target HbA1c ranges, with both lower and upper bounds, were considered a better way to balance the potential risks and benefits of therapy. For example, a target HbA1c range of 6% to 7% might be appropriate in patients with a life expectancy more than 10 to 15 years with no significant microvascular disease and no other socioeconomic limitations to therapy. For patients with established microvascular disease or a life expectancy < 10 years, target ranges from 7% to 9% might be appropriate depending on patient-specific factors. A patient with advanced disease or limited life expectancy is less likely to derive benefit from intensive control, yet they would be exposed to the adverse effects from intensive therapy. For these patients, consider a less-intensive HbA1c target range. Although life expectancy can be difficult to estimate, this framework can be helpful to reach a target range using SDM with the patient.
An important issue in current DM management is potential overtreatment, which sits at the intersection of overuse of low value practices and medication safety. Up to 65% of older veterans with DM taking hypoglycemic agents might be overtreated based on the presence of DM complications, medical comorbidities, and decreased life expectancy that confer more risk than benefit from lower HbA1c levels.36 Harms from intensive glycemic control, such as increased risk of death from cardiovascular events and severe hypoglycemia must be considered.24 Patient-specific factors that could increase risk of hypoglycemia include the use of specific drugs (insulin and sulfonylureas), advanced age (> 75 years), cognitive impairment, chronic renal insufficiency, and food insufficiency.37-39
The CPG did not address specific pharmacologic treatment options because these can change rapidly as the literature evolves. Instead, the CPG refers clinicians to current criteria issued by the VA and DoD, which are updated frequently. In line with recent reviews, the CPG continues to recommend metformin as a first-line therapy for most patients with T2DM.40 An important consideration in the future will be the potential for cardiovascular risk reduction from specific medications or classes of medication independent of HbA1c reduction. As ongoing clinical trials are completed, SDM, ARR, and potential harm from therapy will remain important considerations.
Conclusion
The VA/DoD Diabetes Clinical Practice Guideline for the Management of Type 2 Diabetes Mellitus in Primary Care strongly recommend SDM in setting management and treatment goals, lifestyle changes that favor a Mediterranean or reduced carbohydrate diet, and targeting HbA1c levels to a range that balances benefits and harms for an individual patient.
This CPG represents a significant step foward in improving the treatment and management of patients with DM in the VA and DoD. This document represents a synthesis of the best available evidence regarding DM care as of March 2016. It is the authors' hope that such recommendations are implemented at the individual practice level. The CPG can help HCPs, but use of such recommendations should be placed in the context of clinical judgment, the patient’s values and preferences, and other available evidence as scientific knowledge and technology advance and treatment patterns evolve.
Application of these CPG recommendations will help VA and DoD clinicians deliver high-quality DM care in a personalized, proactive, and patient-driven manner, that inspires patients to achieve a state of health and well-being that is tailored to their unique characteristics and goals of care.
Click here to read the digital edition.
1. Centers for Disease Control and Prevention. 2017 National Diabetes Statistics Report. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics -report.pdf. Accessed 9/7/2017.
2. Miller DR, Safford MM, Pogach LM. Who has diabetes? Best estimates of diabetes prevalence in the Department of Veterans Affairs based on computerized patient data. Diabetes Care. 2004;27(suppl 2):B10-B21.
3. Bethel MA, Sloan FA, Belsky D, Feinglos MN. Longitudinal incidence and prevalence of adverse outcomes of diabetes mellitus in elderly patients. Arch Intern Med. 2007;167(9):921-927.
4. American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36(4):1033-1046.
5. U.S. Department of Veteran Affairs, U.S. Department of Defense.VA/DoD clinical practice guidelines: management of diabetes mellitus in primary care. https://www.healthquality.va.gov/guidelines/CD/diabetes/. Updated April 18, 2017. Accessed August 28, 2017.
6. U.S. Department of Veteran Affairs, U.S. Department of Defense. VA/DoD clinical practice guidelines: CPG policy guidance: guidelines for guidelines. https://www.healthquality.va.gov/documents/cpgGuidelinesForGuidelinesFinalRevisions051214.docx . Updated February 8, 2017. Accessed August 28,2017.
7. Andrews J, Guyatt G, Oxman AD, et al. GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. J Clin Epidemiol. 2013;66(7):719-725.
8. Agency for Healthcare Research and Quality. The SHARE approach. https://www.ahrq.gov/professionals/education/curriculum-tools/shareddecisionmaking/index.html. Updated February 2017. Accessed August 28, 2017.
9. Kirkman MS, Briscoe VJ, Clark N, et al; Consensus Development Conference on Diabetes and Older Adults. Diabetes in older adults: a consensus report. J Am Geriatr Soc. 2012;60(12):2342-2356.
10. VA/DoD Evidence-based Practice. Shared Decision Making, A Guide for Busy Clinicians. https://www.qmo.amedd.army.mil/asthma/SDM-POCKETGuide.pdf. Accessed 3/17/2017.
11. Bertakis KD, Azari R. Patient-centered care is associated with decreased health care utilization. J Am Board Fam Med. 2011;24(3):229-239.
12. Robinson JH, Callister LC, Berry JA, Dearing KA. Patient-centered care and adherence: definitions and applications to improve outcomes. J Am Acad Nurse Pract. 2008;20(12):600-607.
13. Mullan RJ, Montori VM, Shah ND, et al. The diabetes mellitus medication choice decision aid: a randomized trial. Arch Intern Med. 2009;169(17):1560-1568.
14. Branda ME, LeBlanc A, Shah ND, et al. Shared decision making for patients with type 2 diabetes: a randomized trial in primary care. BMC Health Serv Res. 2013;13:301.
15. Hsu WC, Lau KH, Huang R, et al. Utilization of a cloud-based diabetes management program for insulin initiation and titration enables collaborative decision making between healthcare providers and patients. Diabetes Technol Ther. 2016;18(2):59-67.
16. Buhse S, Mühlhauser I, Heller T, et al. Informed shared decision-making programme on the prevention of myocardial infarction in type 2 diabetes: a randomised controlled trial. BMJ Open. 2015;5(11):e009116.
17. Agency for Healthcare Research and Quality. Health literacy universal precautions tool kit, 2nd edition. Use the teach-back method: tool #5. http://www.ahrq .gov/professionals/quality-patient-safety/quality-resources/tools/literacy-toolkit/healthlittoolkit2-tool5.html. Updated February 2015. Accessed August 28, 2017.
18. Ajala O, English P, Pinkney J. Systematic review and meta-analysis of different dietary approaches to the management of type 2 diabetes. Am J Clin Nutr. 2013;97(3):505-516.
19. Huo R, Du T, Xu Y, et al. Effects of Mediterranean-style diet on glycemic control, weight loss and cardiovascular risk factors among type 2 diabetes individuals: a meta-analysis. Eur J Clin Nutr. 2015;69(11):1200-1208.
20. Esposito K, Maiorino MI, Bellastella G, Chiodini P, Panagiotakos D, Giugliano D. A journey into a Mediterranean diet and type 2 diabetes: a systematic review with meta-analyses. BMJ Open. 2015;5(8):e008222.
21. Laine C, Taichman DB, Mulrow C. Trustworthy clinical guidelines. Ann Intern Med. 2011;154(11):774-775.
22. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837-853.
23. Beulens JW, Patel A, Vingerling JR, et al; AdRem project team; ADVANCE management committee. Effects of blood pressure lowering and intensive glucose control on the incidence and progression of retinopathy in patients with type 2 diabetes mellitus: a randomised controlled trial. Diabetologia. 2009;52(10):2027-2036.
24. ACCORD Study Group, Gerstein HC, Miller ME, et al. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med. 2011;364(9):818-828.
25. Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129-139.
26. Hemmingsen B, Lund SS, Gluud C, et al. Targeting intensive glycaemic control versus targeting conventional glycaemic control for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2011;(6):CD008143.
27. Hasan R, Firwana B, Elraiyah T, et al. A systematic review and meta-analysis of glycemic control for the prevention of diabetic foot syndrome. J Vasc Surg. 2016;63(suppl 2):22S-28S.e1-2.
28. Radin MS. Pitfalls in hemoglobin A1c measurement: when results may be misleading. J Gen Intern Med. 2014;29(2):388-394.
29. English E, Idris I, Smith G, Dhatariya K, Kilpatrick ES, John WG. The effect of anaemia and abnormalities of erythrocyte indices on HbA1c analysis: a systematic review. Diabetologia. 2015;58(7):1409-1421.
30. Goldstein DE, Little RR, Lorenz RA, et al. Tests of glycemia in diabetes. Diabetes Care. 2004;27(7):1761-1773.
31. Herman WH, Ma Y, Uwaifo G, et al; Diabetes Prevention Program Research Group. Differences in A1c by race and ethnicity among patients with impaired glucose tolerance in the Diabetes Prevention Program. Diabetes Care. 2007;30(10):2453-2457.
32. Little RR, Rohlfing CL, Hanson S, et al. Effects of hemoglobin (Hb) E and HbD traits on measurements of glycated Hb (HbA1c) by 23 methods. Clin Chem. 2008;54(8):1277-1282.
33. Sacks DB, Arnold M, Bakris GL, et al; Evidence-Based Laboratory Medicine Committee of the American Association for Clinical Chemistry. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Diabetes Care. 2011;34(6):e61-e99.
34. Hayward RA, Reaven PD, Wiitala WL, et al; VADT Investigators. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372(23):2197-2206.
35. Zoungas S, Chalmers J, Neal B, et al; ADVANCE-ON Collaborative Group. Follow-up of blood-pressure lowering and glucose control in type 2 diabetes. N Engl J Med. 2014;371(15):1392-1406.
36. Tseng CL, Soroka O, Maney M, Aron DC, Pogach LM. Assessing potential glycemic overtreatment in persons at hypoglycemic risk. JAMA Intern Med. 2014;174(2):259-268.
37. Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36(5):1384-1395.
38. ORIGIN Trial Investigators. Predictors of nonsevere and severe hypoglycemia during glucose-lowering treatment with insulin glargine or standard drugs in the ORIGIN trial. Diabetes Care. 2015;38(1):22-28.
39. Bruderer SG, Bodmer M, Jick SS, Bader G, Schlienger RG, Meier CR. Incidence of and risk factors for severe hypoglycaemia in treated type 2 diabetes mellitus patients in the UK—a nested case-control analysis. Diabetes Obes Metab. 2014;16(9):801-811.
40. Maruthur NM, Tseng E, Hutfless S, et al. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2016;164(11):740-751.
Diabetes mellitus (DM) is an epidemic in the U.S. More than 30 million people (9.4% of the total population) have DM; type 2 DM (T2SM) accounts for 95% of these cases.1 The estimated prevalence of DM among individuals aged > 65 years is about 3 times higher at 26%. The prevalence among veterans enrolled in the VA is higher than in the general population; about 25% of VA users have been diagnosed with DM.2 As a result, DM is the leading cause of blindness, end stage renal disease, amputations, and a significant contributor to myocardial infarction and stroke. Older adults with DM have an increased risk of mortality compared with individuals without DM.3 In 2012, DM was estimated to cost $176 billion in direct and indirect medical costs.4 These health and cost implications make effective management of DM a priority for health care providers (HCPs), policy makers, and patients nationwide.
The 2017 VA/DoD Clinical Practice Guideline (CPG) for the Management of T2DM in Primary Care provides the primary care team an evidence-based and individualized approach to holistic care of the patient with T2DM.5 Key recommendations were developed based on methods established by the VA/DoD Evidence-Based Practice Working Group (EBPWG) and are aligned with standards for trustworthy guidelines by using the Grading of Recommendations Assessment, Development and Evaluation system to assess the quality of the evidence base and assign a grade for the strength for each recommendation.6,7 The EBPWG included a multidisciplinary panel of practicing clinician stakeholders, including primary care physicians, endocrinologists, medical nutritionists, pharmacists, diabetes educators, and nurse practitioners. The CPG development process also included a patient focus group. Important themes from the focus group were shared with the EBPWG to help address the needs and perspectives of patients receiving treatment for DM in the VA and DoD.
In this article, the authors briefly review several of the most pertinent CPG updates for the busy clinician.
Shared Decision Making
Shared decision making (SDM) is a central component of the approach to patients with DM. Shared decision making involves the patient and care providers together making important decisions about the treatment plan and goals of care, using communication tools and exploring patient preferences.8
Using an empathetic and nonjudgmental approach facilitates discussions about a patient’s specific health care needs and goals for care. Shared decision making also can provide culturally appropriate treatment and care information to meet the needs of those with limited literacy or numeracy skills, or other learning barriers, such as physical, sensory, or learning disabilities. Family involvement is an important component of SDM when desired by the patient.9
The goals of successful SDM include a decrease in patient anxiety and an increase in trust in the health care team, ideally leading to improvement in adherence and patient outcomes.8,10-12 Improved patient-clinician communication conveys openness to discuss any future concerns. Furthermore, SDM does not need to take a significant amount of a clinician’s time to create an environment of consideration and goal formation. Training in communication skills may be helpful for those unfamiliar with SDM techniques. Patients are most likely to participate in the SDM process when they are comfortable speaking with clinicians and have some knowledge about their specific disease process.13
The clinical team can review all prior treatment attempts with the patient to understand the patient's perspective on these interventions. Lastly, patients are involved in prioritizing problems to be addressed and in setting specific goals. A 5-step SDM process prompted by the SHARE acronym can be used:
- Seek your patient’s participation;
- Help your patient explore and compare treatment options;
- Assess your patient’s values and preferences;
- Reach a decision with your patient; and
- Evaluate your patient’s decision (Figure).8
The VA/DoD CPG noted that there is high-quality evidence supporting SDM for improving patients’ knowledge, satisfaction, and engagement with their treatment plan.14-16 Specific methodologic approaches to SDM are not well defined for individual patient groups, which represents a significant research gap. Patients diagnosed with T2DM might respond differently to SDM depending on personal goals, life experiences, and coping strategies.14-16 Shared decision making should be used at every decision point in the treatment process, from the diagnosis of pre-diabetes to the patient with advanced complications. This includes— at a minimum—at initial diagnosis, when experiencing difficulties in management, and at times of transition or development of complications.16
A shared understanding is critical to the SDM process. Diabetes self-management education and diabetes self-management support provide a framework that involves a collaborative, ongoing, interactive process to help patients gain knowledge, modify behavior, and successfully manage the disease. The goal of DM education in SDM is to ensure that the patient has sufficient knowledge and skills to achieve the treatment goals they set with their health care team. Assessment of patient understanding in the clinic could include use of the “teach-back method.”17 Health coaching and motivational interviewing strategies also may help clinicians understand patients’ perceptions, values, and beliefs regarding their condition, treatment, and self-management options, particularly when patients seem to be reluctant to fully participate in decisions and care.
A challenge for HCPs is to help patients understand how they can successfully manage DM and partner with health care teams to express their goals and preferences to aid in individualized health care decisions. Using SDM tools and ensuring that clinicians can use patient-centered communication skills increase patients’ willingness to share in decision making and engage in the treatment plan.
Nutrition Recommendations
Nutrition therapy is a key component of any successful DM management plan. The EBPWG added 2 strong recommendations for DM nutrition strategies. The first recommendation is to follow a Mediterranean diet, if this resonates with the patient’s values and preferences (Table 1).
- High intake of vegetables, fruits, nuts, unrefined grains, and olive oil;
- Moderate intake of fish and poultry;
- Low or moderate intake of wine; and
- Low intake of red meat, processed meat, dairy, and sweets.
The Mediterranean diet effectively improves glycemic control, delays the time to first pharmacologic intervention, and reduces cardiovascular risk factors in individuals with diabetes.18 An additional benefits of this dietary pattern includes significant hemoglobin A1c (HbA1c) reduction.19,20 A Mediterranean diet also has been linked to improved cardiovascular outcomes and weight loss. In general, the evidence supporting a Mediterranean diet are robust, but securing and adapting to these types of foods can be challenging for some patients.
The second nutrition recommendation is to reduce the percentage of energy from carbohydrates to between 14% and 45% per day and/or eat foods with lower glycemic index. Patients who do not choose a Mediterranean diet can employ this dietary pattern. A systematic review compared dietary interventions, including lower carbohydrate and low-glycemic index diets, and showed both dietary interventions improved glycemic control.18 Unfortunately, many studies compare different intervention diets rather than comparing an intervention against a control diet. However, based on the available evidence, the Working Group endorses a Mediterranean diet and carbohydrate reduction and low glycemic index foods as dietary options in which the benefits seem to outweigh harms.
Target Hemoglobin A1c
The EBPWG reviewed several large, intensive glucose control trials to apply recent evidence to ongoing HbA1c treatment targets. The CPG strongly reaffirms that rather than assigning a single glycemic goal for all patients, clinicians should use SDM to develop an HbA1c target range that is risk-stratified (Table 2).
The ARR of complications must be balanced against the risk of therapy. Several major trials tested the hypothesis that intensive glycemic control (target HbA1c at least < 7%) improved cardiovascular outcomes in patients with T2DM.23-25 These trials did not demonstrate cardiovascular benefit from intensive control to reach HbA1c < 7%, and the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study revealed possible cardiovascular harm.24
In addition, because these studies enrolled patients with established T2DM, they demonstrated less reduction in microvascular complications than was seen in newly diagnosed patients in UKPDS.22 Systematic reviews comparing intensive and conventional glucose control showed no significant differences in all-cause mortality or death from cardiovascular disease.26,27 Therefore, intensive control of T2DM has the greatest impact on microvascular complications and is most successful when initiated early in the disease process.
A target HbA1c range is recommended rather than a threshold value (eg, HbA1c < 8.0%) for several reasons. Most important the clinical trials that provide evidence for improved glycemic control used an HbA1c value recorded over time, not a single value measured at one point in time. Many factors influence HbA1c measurements other than just glycemic control.28 These include anemia, chronic kidney disease, race/ethnicity, and hemoglobinapathies.29-32 Patients can have clinically significant variation in HbA1c results between test samples, even when obtained from the same laboratory.33 For these reasons, the CPG continues to recommend use of fasting glucose ≥ 126 mg/dL to establish a DM diagnosis when the HbA1c is < 7.0%. This limits the likelihood that patients will be incorrectly diagnosed with DM, which can affect insurability, disability, or the trajectory of a military career. For patients with diagnosed T2DM, glycemic control over time remains important, but overreliance on a single HbA1c test could lead to overtreatment and potential adverse outcomes.
The EBPWG considered the target HbA1c and outcomes in UKPDS, ACCORD, Veterans Affairs Diabetes Trial (VADT), and the Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) when considering HbA1c target ranges.23,25,34,35 Indeed, target HbA1c ranges, with both lower and upper bounds, were considered a better way to balance the potential risks and benefits of therapy. For example, a target HbA1c range of 6% to 7% might be appropriate in patients with a life expectancy more than 10 to 15 years with no significant microvascular disease and no other socioeconomic limitations to therapy. For patients with established microvascular disease or a life expectancy < 10 years, target ranges from 7% to 9% might be appropriate depending on patient-specific factors. A patient with advanced disease or limited life expectancy is less likely to derive benefit from intensive control, yet they would be exposed to the adverse effects from intensive therapy. For these patients, consider a less-intensive HbA1c target range. Although life expectancy can be difficult to estimate, this framework can be helpful to reach a target range using SDM with the patient.
An important issue in current DM management is potential overtreatment, which sits at the intersection of overuse of low value practices and medication safety. Up to 65% of older veterans with DM taking hypoglycemic agents might be overtreated based on the presence of DM complications, medical comorbidities, and decreased life expectancy that confer more risk than benefit from lower HbA1c levels.36 Harms from intensive glycemic control, such as increased risk of death from cardiovascular events and severe hypoglycemia must be considered.24 Patient-specific factors that could increase risk of hypoglycemia include the use of specific drugs (insulin and sulfonylureas), advanced age (> 75 years), cognitive impairment, chronic renal insufficiency, and food insufficiency.37-39
The CPG did not address specific pharmacologic treatment options because these can change rapidly as the literature evolves. Instead, the CPG refers clinicians to current criteria issued by the VA and DoD, which are updated frequently. In line with recent reviews, the CPG continues to recommend metformin as a first-line therapy for most patients with T2DM.40 An important consideration in the future will be the potential for cardiovascular risk reduction from specific medications or classes of medication independent of HbA1c reduction. As ongoing clinical trials are completed, SDM, ARR, and potential harm from therapy will remain important considerations.
Conclusion
The VA/DoD Diabetes Clinical Practice Guideline for the Management of Type 2 Diabetes Mellitus in Primary Care strongly recommend SDM in setting management and treatment goals, lifestyle changes that favor a Mediterranean or reduced carbohydrate diet, and targeting HbA1c levels to a range that balances benefits and harms for an individual patient.
This CPG represents a significant step foward in improving the treatment and management of patients with DM in the VA and DoD. This document represents a synthesis of the best available evidence regarding DM care as of March 2016. It is the authors' hope that such recommendations are implemented at the individual practice level. The CPG can help HCPs, but use of such recommendations should be placed in the context of clinical judgment, the patient’s values and preferences, and other available evidence as scientific knowledge and technology advance and treatment patterns evolve.
Application of these CPG recommendations will help VA and DoD clinicians deliver high-quality DM care in a personalized, proactive, and patient-driven manner, that inspires patients to achieve a state of health and well-being that is tailored to their unique characteristics and goals of care.
Click here to read the digital edition.
Diabetes mellitus (DM) is an epidemic in the U.S. More than 30 million people (9.4% of the total population) have DM; type 2 DM (T2SM) accounts for 95% of these cases.1 The estimated prevalence of DM among individuals aged > 65 years is about 3 times higher at 26%. The prevalence among veterans enrolled in the VA is higher than in the general population; about 25% of VA users have been diagnosed with DM.2 As a result, DM is the leading cause of blindness, end stage renal disease, amputations, and a significant contributor to myocardial infarction and stroke. Older adults with DM have an increased risk of mortality compared with individuals without DM.3 In 2012, DM was estimated to cost $176 billion in direct and indirect medical costs.4 These health and cost implications make effective management of DM a priority for health care providers (HCPs), policy makers, and patients nationwide.
The 2017 VA/DoD Clinical Practice Guideline (CPG) for the Management of T2DM in Primary Care provides the primary care team an evidence-based and individualized approach to holistic care of the patient with T2DM.5 Key recommendations were developed based on methods established by the VA/DoD Evidence-Based Practice Working Group (EBPWG) and are aligned with standards for trustworthy guidelines by using the Grading of Recommendations Assessment, Development and Evaluation system to assess the quality of the evidence base and assign a grade for the strength for each recommendation.6,7 The EBPWG included a multidisciplinary panel of practicing clinician stakeholders, including primary care physicians, endocrinologists, medical nutritionists, pharmacists, diabetes educators, and nurse practitioners. The CPG development process also included a patient focus group. Important themes from the focus group were shared with the EBPWG to help address the needs and perspectives of patients receiving treatment for DM in the VA and DoD.
In this article, the authors briefly review several of the most pertinent CPG updates for the busy clinician.
Shared Decision Making
Shared decision making (SDM) is a central component of the approach to patients with DM. Shared decision making involves the patient and care providers together making important decisions about the treatment plan and goals of care, using communication tools and exploring patient preferences.8
Using an empathetic and nonjudgmental approach facilitates discussions about a patient’s specific health care needs and goals for care. Shared decision making also can provide culturally appropriate treatment and care information to meet the needs of those with limited literacy or numeracy skills, or other learning barriers, such as physical, sensory, or learning disabilities. Family involvement is an important component of SDM when desired by the patient.9
The goals of successful SDM include a decrease in patient anxiety and an increase in trust in the health care team, ideally leading to improvement in adherence and patient outcomes.8,10-12 Improved patient-clinician communication conveys openness to discuss any future concerns. Furthermore, SDM does not need to take a significant amount of a clinician’s time to create an environment of consideration and goal formation. Training in communication skills may be helpful for those unfamiliar with SDM techniques. Patients are most likely to participate in the SDM process when they are comfortable speaking with clinicians and have some knowledge about their specific disease process.13
The clinical team can review all prior treatment attempts with the patient to understand the patient's perspective on these interventions. Lastly, patients are involved in prioritizing problems to be addressed and in setting specific goals. A 5-step SDM process prompted by the SHARE acronym can be used:
- Seek your patient’s participation;
- Help your patient explore and compare treatment options;
- Assess your patient’s values and preferences;
- Reach a decision with your patient; and
- Evaluate your patient’s decision (Figure).8
The VA/DoD CPG noted that there is high-quality evidence supporting SDM for improving patients’ knowledge, satisfaction, and engagement with their treatment plan.14-16 Specific methodologic approaches to SDM are not well defined for individual patient groups, which represents a significant research gap. Patients diagnosed with T2DM might respond differently to SDM depending on personal goals, life experiences, and coping strategies.14-16 Shared decision making should be used at every decision point in the treatment process, from the diagnosis of pre-diabetes to the patient with advanced complications. This includes— at a minimum—at initial diagnosis, when experiencing difficulties in management, and at times of transition or development of complications.16
A shared understanding is critical to the SDM process. Diabetes self-management education and diabetes self-management support provide a framework that involves a collaborative, ongoing, interactive process to help patients gain knowledge, modify behavior, and successfully manage the disease. The goal of DM education in SDM is to ensure that the patient has sufficient knowledge and skills to achieve the treatment goals they set with their health care team. Assessment of patient understanding in the clinic could include use of the “teach-back method.”17 Health coaching and motivational interviewing strategies also may help clinicians understand patients’ perceptions, values, and beliefs regarding their condition, treatment, and self-management options, particularly when patients seem to be reluctant to fully participate in decisions and care.
A challenge for HCPs is to help patients understand how they can successfully manage DM and partner with health care teams to express their goals and preferences to aid in individualized health care decisions. Using SDM tools and ensuring that clinicians can use patient-centered communication skills increase patients’ willingness to share in decision making and engage in the treatment plan.
Nutrition Recommendations
Nutrition therapy is a key component of any successful DM management plan. The EBPWG added 2 strong recommendations for DM nutrition strategies. The first recommendation is to follow a Mediterranean diet, if this resonates with the patient’s values and preferences (Table 1).
- High intake of vegetables, fruits, nuts, unrefined grains, and olive oil;
- Moderate intake of fish and poultry;
- Low or moderate intake of wine; and
- Low intake of red meat, processed meat, dairy, and sweets.
The Mediterranean diet effectively improves glycemic control, delays the time to first pharmacologic intervention, and reduces cardiovascular risk factors in individuals with diabetes.18 An additional benefits of this dietary pattern includes significant hemoglobin A1c (HbA1c) reduction.19,20 A Mediterranean diet also has been linked to improved cardiovascular outcomes and weight loss. In general, the evidence supporting a Mediterranean diet are robust, but securing and adapting to these types of foods can be challenging for some patients.
The second nutrition recommendation is to reduce the percentage of energy from carbohydrates to between 14% and 45% per day and/or eat foods with lower glycemic index. Patients who do not choose a Mediterranean diet can employ this dietary pattern. A systematic review compared dietary interventions, including lower carbohydrate and low-glycemic index diets, and showed both dietary interventions improved glycemic control.18 Unfortunately, many studies compare different intervention diets rather than comparing an intervention against a control diet. However, based on the available evidence, the Working Group endorses a Mediterranean diet and carbohydrate reduction and low glycemic index foods as dietary options in which the benefits seem to outweigh harms.
Target Hemoglobin A1c
The EBPWG reviewed several large, intensive glucose control trials to apply recent evidence to ongoing HbA1c treatment targets. The CPG strongly reaffirms that rather than assigning a single glycemic goal for all patients, clinicians should use SDM to develop an HbA1c target range that is risk-stratified (Table 2).
The ARR of complications must be balanced against the risk of therapy. Several major trials tested the hypothesis that intensive glycemic control (target HbA1c at least < 7%) improved cardiovascular outcomes in patients with T2DM.23-25 These trials did not demonstrate cardiovascular benefit from intensive control to reach HbA1c < 7%, and the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study revealed possible cardiovascular harm.24
In addition, because these studies enrolled patients with established T2DM, they demonstrated less reduction in microvascular complications than was seen in newly diagnosed patients in UKPDS.22 Systematic reviews comparing intensive and conventional glucose control showed no significant differences in all-cause mortality or death from cardiovascular disease.26,27 Therefore, intensive control of T2DM has the greatest impact on microvascular complications and is most successful when initiated early in the disease process.
A target HbA1c range is recommended rather than a threshold value (eg, HbA1c < 8.0%) for several reasons. Most important the clinical trials that provide evidence for improved glycemic control used an HbA1c value recorded over time, not a single value measured at one point in time. Many factors influence HbA1c measurements other than just glycemic control.28 These include anemia, chronic kidney disease, race/ethnicity, and hemoglobinapathies.29-32 Patients can have clinically significant variation in HbA1c results between test samples, even when obtained from the same laboratory.33 For these reasons, the CPG continues to recommend use of fasting glucose ≥ 126 mg/dL to establish a DM diagnosis when the HbA1c is < 7.0%. This limits the likelihood that patients will be incorrectly diagnosed with DM, which can affect insurability, disability, or the trajectory of a military career. For patients with diagnosed T2DM, glycemic control over time remains important, but overreliance on a single HbA1c test could lead to overtreatment and potential adverse outcomes.
The EBPWG considered the target HbA1c and outcomes in UKPDS, ACCORD, Veterans Affairs Diabetes Trial (VADT), and the Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) when considering HbA1c target ranges.23,25,34,35 Indeed, target HbA1c ranges, with both lower and upper bounds, were considered a better way to balance the potential risks and benefits of therapy. For example, a target HbA1c range of 6% to 7% might be appropriate in patients with a life expectancy more than 10 to 15 years with no significant microvascular disease and no other socioeconomic limitations to therapy. For patients with established microvascular disease or a life expectancy < 10 years, target ranges from 7% to 9% might be appropriate depending on patient-specific factors. A patient with advanced disease or limited life expectancy is less likely to derive benefit from intensive control, yet they would be exposed to the adverse effects from intensive therapy. For these patients, consider a less-intensive HbA1c target range. Although life expectancy can be difficult to estimate, this framework can be helpful to reach a target range using SDM with the patient.
An important issue in current DM management is potential overtreatment, which sits at the intersection of overuse of low value practices and medication safety. Up to 65% of older veterans with DM taking hypoglycemic agents might be overtreated based on the presence of DM complications, medical comorbidities, and decreased life expectancy that confer more risk than benefit from lower HbA1c levels.36 Harms from intensive glycemic control, such as increased risk of death from cardiovascular events and severe hypoglycemia must be considered.24 Patient-specific factors that could increase risk of hypoglycemia include the use of specific drugs (insulin and sulfonylureas), advanced age (> 75 years), cognitive impairment, chronic renal insufficiency, and food insufficiency.37-39
The CPG did not address specific pharmacologic treatment options because these can change rapidly as the literature evolves. Instead, the CPG refers clinicians to current criteria issued by the VA and DoD, which are updated frequently. In line with recent reviews, the CPG continues to recommend metformin as a first-line therapy for most patients with T2DM.40 An important consideration in the future will be the potential for cardiovascular risk reduction from specific medications or classes of medication independent of HbA1c reduction. As ongoing clinical trials are completed, SDM, ARR, and potential harm from therapy will remain important considerations.
Conclusion
The VA/DoD Diabetes Clinical Practice Guideline for the Management of Type 2 Diabetes Mellitus in Primary Care strongly recommend SDM in setting management and treatment goals, lifestyle changes that favor a Mediterranean or reduced carbohydrate diet, and targeting HbA1c levels to a range that balances benefits and harms for an individual patient.
This CPG represents a significant step foward in improving the treatment and management of patients with DM in the VA and DoD. This document represents a synthesis of the best available evidence regarding DM care as of March 2016. It is the authors' hope that such recommendations are implemented at the individual practice level. The CPG can help HCPs, but use of such recommendations should be placed in the context of clinical judgment, the patient’s values and preferences, and other available evidence as scientific knowledge and technology advance and treatment patterns evolve.
Application of these CPG recommendations will help VA and DoD clinicians deliver high-quality DM care in a personalized, proactive, and patient-driven manner, that inspires patients to achieve a state of health and well-being that is tailored to their unique characteristics and goals of care.
Click here to read the digital edition.
1. Centers for Disease Control and Prevention. 2017 National Diabetes Statistics Report. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics -report.pdf. Accessed 9/7/2017.
2. Miller DR, Safford MM, Pogach LM. Who has diabetes? Best estimates of diabetes prevalence in the Department of Veterans Affairs based on computerized patient data. Diabetes Care. 2004;27(suppl 2):B10-B21.
3. Bethel MA, Sloan FA, Belsky D, Feinglos MN. Longitudinal incidence and prevalence of adverse outcomes of diabetes mellitus in elderly patients. Arch Intern Med. 2007;167(9):921-927.
4. American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36(4):1033-1046.
5. U.S. Department of Veteran Affairs, U.S. Department of Defense.VA/DoD clinical practice guidelines: management of diabetes mellitus in primary care. https://www.healthquality.va.gov/guidelines/CD/diabetes/. Updated April 18, 2017. Accessed August 28, 2017.
6. U.S. Department of Veteran Affairs, U.S. Department of Defense. VA/DoD clinical practice guidelines: CPG policy guidance: guidelines for guidelines. https://www.healthquality.va.gov/documents/cpgGuidelinesForGuidelinesFinalRevisions051214.docx . Updated February 8, 2017. Accessed August 28,2017.
7. Andrews J, Guyatt G, Oxman AD, et al. GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. J Clin Epidemiol. 2013;66(7):719-725.
8. Agency for Healthcare Research and Quality. The SHARE approach. https://www.ahrq.gov/professionals/education/curriculum-tools/shareddecisionmaking/index.html. Updated February 2017. Accessed August 28, 2017.
9. Kirkman MS, Briscoe VJ, Clark N, et al; Consensus Development Conference on Diabetes and Older Adults. Diabetes in older adults: a consensus report. J Am Geriatr Soc. 2012;60(12):2342-2356.
10. VA/DoD Evidence-based Practice. Shared Decision Making, A Guide for Busy Clinicians. https://www.qmo.amedd.army.mil/asthma/SDM-POCKETGuide.pdf. Accessed 3/17/2017.
11. Bertakis KD, Azari R. Patient-centered care is associated with decreased health care utilization. J Am Board Fam Med. 2011;24(3):229-239.
12. Robinson JH, Callister LC, Berry JA, Dearing KA. Patient-centered care and adherence: definitions and applications to improve outcomes. J Am Acad Nurse Pract. 2008;20(12):600-607.
13. Mullan RJ, Montori VM, Shah ND, et al. The diabetes mellitus medication choice decision aid: a randomized trial. Arch Intern Med. 2009;169(17):1560-1568.
14. Branda ME, LeBlanc A, Shah ND, et al. Shared decision making for patients with type 2 diabetes: a randomized trial in primary care. BMC Health Serv Res. 2013;13:301.
15. Hsu WC, Lau KH, Huang R, et al. Utilization of a cloud-based diabetes management program for insulin initiation and titration enables collaborative decision making between healthcare providers and patients. Diabetes Technol Ther. 2016;18(2):59-67.
16. Buhse S, Mühlhauser I, Heller T, et al. Informed shared decision-making programme on the prevention of myocardial infarction in type 2 diabetes: a randomised controlled trial. BMJ Open. 2015;5(11):e009116.
17. Agency for Healthcare Research and Quality. Health literacy universal precautions tool kit, 2nd edition. Use the teach-back method: tool #5. http://www.ahrq .gov/professionals/quality-patient-safety/quality-resources/tools/literacy-toolkit/healthlittoolkit2-tool5.html. Updated February 2015. Accessed August 28, 2017.
18. Ajala O, English P, Pinkney J. Systematic review and meta-analysis of different dietary approaches to the management of type 2 diabetes. Am J Clin Nutr. 2013;97(3):505-516.
19. Huo R, Du T, Xu Y, et al. Effects of Mediterranean-style diet on glycemic control, weight loss and cardiovascular risk factors among type 2 diabetes individuals: a meta-analysis. Eur J Clin Nutr. 2015;69(11):1200-1208.
20. Esposito K, Maiorino MI, Bellastella G, Chiodini P, Panagiotakos D, Giugliano D. A journey into a Mediterranean diet and type 2 diabetes: a systematic review with meta-analyses. BMJ Open. 2015;5(8):e008222.
21. Laine C, Taichman DB, Mulrow C. Trustworthy clinical guidelines. Ann Intern Med. 2011;154(11):774-775.
22. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837-853.
23. Beulens JW, Patel A, Vingerling JR, et al; AdRem project team; ADVANCE management committee. Effects of blood pressure lowering and intensive glucose control on the incidence and progression of retinopathy in patients with type 2 diabetes mellitus: a randomised controlled trial. Diabetologia. 2009;52(10):2027-2036.
24. ACCORD Study Group, Gerstein HC, Miller ME, et al. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med. 2011;364(9):818-828.
25. Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129-139.
26. Hemmingsen B, Lund SS, Gluud C, et al. Targeting intensive glycaemic control versus targeting conventional glycaemic control for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2011;(6):CD008143.
27. Hasan R, Firwana B, Elraiyah T, et al. A systematic review and meta-analysis of glycemic control for the prevention of diabetic foot syndrome. J Vasc Surg. 2016;63(suppl 2):22S-28S.e1-2.
28. Radin MS. Pitfalls in hemoglobin A1c measurement: when results may be misleading. J Gen Intern Med. 2014;29(2):388-394.
29. English E, Idris I, Smith G, Dhatariya K, Kilpatrick ES, John WG. The effect of anaemia and abnormalities of erythrocyte indices on HbA1c analysis: a systematic review. Diabetologia. 2015;58(7):1409-1421.
30. Goldstein DE, Little RR, Lorenz RA, et al. Tests of glycemia in diabetes. Diabetes Care. 2004;27(7):1761-1773.
31. Herman WH, Ma Y, Uwaifo G, et al; Diabetes Prevention Program Research Group. Differences in A1c by race and ethnicity among patients with impaired glucose tolerance in the Diabetes Prevention Program. Diabetes Care. 2007;30(10):2453-2457.
32. Little RR, Rohlfing CL, Hanson S, et al. Effects of hemoglobin (Hb) E and HbD traits on measurements of glycated Hb (HbA1c) by 23 methods. Clin Chem. 2008;54(8):1277-1282.
33. Sacks DB, Arnold M, Bakris GL, et al; Evidence-Based Laboratory Medicine Committee of the American Association for Clinical Chemistry. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Diabetes Care. 2011;34(6):e61-e99.
34. Hayward RA, Reaven PD, Wiitala WL, et al; VADT Investigators. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372(23):2197-2206.
35. Zoungas S, Chalmers J, Neal B, et al; ADVANCE-ON Collaborative Group. Follow-up of blood-pressure lowering and glucose control in type 2 diabetes. N Engl J Med. 2014;371(15):1392-1406.
36. Tseng CL, Soroka O, Maney M, Aron DC, Pogach LM. Assessing potential glycemic overtreatment in persons at hypoglycemic risk. JAMA Intern Med. 2014;174(2):259-268.
37. Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36(5):1384-1395.
38. ORIGIN Trial Investigators. Predictors of nonsevere and severe hypoglycemia during glucose-lowering treatment with insulin glargine or standard drugs in the ORIGIN trial. Diabetes Care. 2015;38(1):22-28.
39. Bruderer SG, Bodmer M, Jick SS, Bader G, Schlienger RG, Meier CR. Incidence of and risk factors for severe hypoglycaemia in treated type 2 diabetes mellitus patients in the UK—a nested case-control analysis. Diabetes Obes Metab. 2014;16(9):801-811.
40. Maruthur NM, Tseng E, Hutfless S, et al. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2016;164(11):740-751.
1. Centers for Disease Control and Prevention. 2017 National Diabetes Statistics Report. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics -report.pdf. Accessed 9/7/2017.
2. Miller DR, Safford MM, Pogach LM. Who has diabetes? Best estimates of diabetes prevalence in the Department of Veterans Affairs based on computerized patient data. Diabetes Care. 2004;27(suppl 2):B10-B21.
3. Bethel MA, Sloan FA, Belsky D, Feinglos MN. Longitudinal incidence and prevalence of adverse outcomes of diabetes mellitus in elderly patients. Arch Intern Med. 2007;167(9):921-927.
4. American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36(4):1033-1046.
5. U.S. Department of Veteran Affairs, U.S. Department of Defense.VA/DoD clinical practice guidelines: management of diabetes mellitus in primary care. https://www.healthquality.va.gov/guidelines/CD/diabetes/. Updated April 18, 2017. Accessed August 28, 2017.
6. U.S. Department of Veteran Affairs, U.S. Department of Defense. VA/DoD clinical practice guidelines: CPG policy guidance: guidelines for guidelines. https://www.healthquality.va.gov/documents/cpgGuidelinesForGuidelinesFinalRevisions051214.docx . Updated February 8, 2017. Accessed August 28,2017.
7. Andrews J, Guyatt G, Oxman AD, et al. GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. J Clin Epidemiol. 2013;66(7):719-725.
8. Agency for Healthcare Research and Quality. The SHARE approach. https://www.ahrq.gov/professionals/education/curriculum-tools/shareddecisionmaking/index.html. Updated February 2017. Accessed August 28, 2017.
9. Kirkman MS, Briscoe VJ, Clark N, et al; Consensus Development Conference on Diabetes and Older Adults. Diabetes in older adults: a consensus report. J Am Geriatr Soc. 2012;60(12):2342-2356.
10. VA/DoD Evidence-based Practice. Shared Decision Making, A Guide for Busy Clinicians. https://www.qmo.amedd.army.mil/asthma/SDM-POCKETGuide.pdf. Accessed 3/17/2017.
11. Bertakis KD, Azari R. Patient-centered care is associated with decreased health care utilization. J Am Board Fam Med. 2011;24(3):229-239.
12. Robinson JH, Callister LC, Berry JA, Dearing KA. Patient-centered care and adherence: definitions and applications to improve outcomes. J Am Acad Nurse Pract. 2008;20(12):600-607.
13. Mullan RJ, Montori VM, Shah ND, et al. The diabetes mellitus medication choice decision aid: a randomized trial. Arch Intern Med. 2009;169(17):1560-1568.
14. Branda ME, LeBlanc A, Shah ND, et al. Shared decision making for patients with type 2 diabetes: a randomized trial in primary care. BMC Health Serv Res. 2013;13:301.
15. Hsu WC, Lau KH, Huang R, et al. Utilization of a cloud-based diabetes management program for insulin initiation and titration enables collaborative decision making between healthcare providers and patients. Diabetes Technol Ther. 2016;18(2):59-67.
16. Buhse S, Mühlhauser I, Heller T, et al. Informed shared decision-making programme on the prevention of myocardial infarction in type 2 diabetes: a randomised controlled trial. BMJ Open. 2015;5(11):e009116.
17. Agency for Healthcare Research and Quality. Health literacy universal precautions tool kit, 2nd edition. Use the teach-back method: tool #5. http://www.ahrq .gov/professionals/quality-patient-safety/quality-resources/tools/literacy-toolkit/healthlittoolkit2-tool5.html. Updated February 2015. Accessed August 28, 2017.
18. Ajala O, English P, Pinkney J. Systematic review and meta-analysis of different dietary approaches to the management of type 2 diabetes. Am J Clin Nutr. 2013;97(3):505-516.
19. Huo R, Du T, Xu Y, et al. Effects of Mediterranean-style diet on glycemic control, weight loss and cardiovascular risk factors among type 2 diabetes individuals: a meta-analysis. Eur J Clin Nutr. 2015;69(11):1200-1208.
20. Esposito K, Maiorino MI, Bellastella G, Chiodini P, Panagiotakos D, Giugliano D. A journey into a Mediterranean diet and type 2 diabetes: a systematic review with meta-analyses. BMJ Open. 2015;5(8):e008222.
21. Laine C, Taichman DB, Mulrow C. Trustworthy clinical guidelines. Ann Intern Med. 2011;154(11):774-775.
22. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837-853.
23. Beulens JW, Patel A, Vingerling JR, et al; AdRem project team; ADVANCE management committee. Effects of blood pressure lowering and intensive glucose control on the incidence and progression of retinopathy in patients with type 2 diabetes mellitus: a randomised controlled trial. Diabetologia. 2009;52(10):2027-2036.
24. ACCORD Study Group, Gerstein HC, Miller ME, et al. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med. 2011;364(9):818-828.
25. Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129-139.
26. Hemmingsen B, Lund SS, Gluud C, et al. Targeting intensive glycaemic control versus targeting conventional glycaemic control for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2011;(6):CD008143.
27. Hasan R, Firwana B, Elraiyah T, et al. A systematic review and meta-analysis of glycemic control for the prevention of diabetic foot syndrome. J Vasc Surg. 2016;63(suppl 2):22S-28S.e1-2.
28. Radin MS. Pitfalls in hemoglobin A1c measurement: when results may be misleading. J Gen Intern Med. 2014;29(2):388-394.
29. English E, Idris I, Smith G, Dhatariya K, Kilpatrick ES, John WG. The effect of anaemia and abnormalities of erythrocyte indices on HbA1c analysis: a systematic review. Diabetologia. 2015;58(7):1409-1421.
30. Goldstein DE, Little RR, Lorenz RA, et al. Tests of glycemia in diabetes. Diabetes Care. 2004;27(7):1761-1773.
31. Herman WH, Ma Y, Uwaifo G, et al; Diabetes Prevention Program Research Group. Differences in A1c by race and ethnicity among patients with impaired glucose tolerance in the Diabetes Prevention Program. Diabetes Care. 2007;30(10):2453-2457.
32. Little RR, Rohlfing CL, Hanson S, et al. Effects of hemoglobin (Hb) E and HbD traits on measurements of glycated Hb (HbA1c) by 23 methods. Clin Chem. 2008;54(8):1277-1282.
33. Sacks DB, Arnold M, Bakris GL, et al; Evidence-Based Laboratory Medicine Committee of the American Association for Clinical Chemistry. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Diabetes Care. 2011;34(6):e61-e99.
34. Hayward RA, Reaven PD, Wiitala WL, et al; VADT Investigators. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372(23):2197-2206.
35. Zoungas S, Chalmers J, Neal B, et al; ADVANCE-ON Collaborative Group. Follow-up of blood-pressure lowering and glucose control in type 2 diabetes. N Engl J Med. 2014;371(15):1392-1406.
36. Tseng CL, Soroka O, Maney M, Aron DC, Pogach LM. Assessing potential glycemic overtreatment in persons at hypoglycemic risk. JAMA Intern Med. 2014;174(2):259-268.
37. Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36(5):1384-1395.
38. ORIGIN Trial Investigators. Predictors of nonsevere and severe hypoglycemia during glucose-lowering treatment with insulin glargine or standard drugs in the ORIGIN trial. Diabetes Care. 2015;38(1):22-28.
39. Bruderer SG, Bodmer M, Jick SS, Bader G, Schlienger RG, Meier CR. Incidence of and risk factors for severe hypoglycaemia in treated type 2 diabetes mellitus patients in the UK—a nested case-control analysis. Diabetes Obes Metab. 2014;16(9):801-811.
40. Maruthur NM, Tseng E, Hutfless S, et al. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2016;164(11):740-751.
Gastric banding, metformin “equal” for slowing early T2DM progression
BERLIN – Gastric banding surgery and metformin produce similar improvements in insulin sensitivity and parameters indicative of preserved beta-cell function in patients with impaired glucose tolerance (IGT) or newly diagnosed type 2 diabetes mellitus (T2DM), according to the results of a study conducted by the Restoring Insulin Secretion (RISE) Consortium.
“Both interventions resulted in about 50% improvements in insulin sensitivity at 1 year, which was attenuated at 2 years,” reported study investigator Thomas Buchanan, MD, of the University of Southern California, Los Angeles, at the annual meeting of the European Association for the Study of Diabetes.
“The beta-cell responses fell in a pattern that maintained relatively, but not perfectly, stable compensation for insulin resistance,” he added.
Although glucose levels improved “only slightly,” he said, “acute compensation to glucose improved significantly with gastric banding and beta-cell compensation at maximal stimulation fell significantly with metformin.”
Results of the BetaFat (Beta Cell Restoration through Fat Mitigation) study, which are now published online in Diabetes Care, also showed that greater weight loss could be achieved with surgery versus metformin, with a 8.9 kg difference between the groups at 2 years (10.6 vs. 1.7 kg, respectively, P less than .01).
HDL cholesterol levels also rose with both interventions, and gastric banding resulted in a greater effect on very low–density lipoprotein cholesterol and triglycerides, as well as serum ALT, Dr. Buchanan said.
The BetaFat study is one of three “proof-of principle” studies currently being conducted by the RISE Consortium in patients with IGT, sometimes called prediabetes, and T2DM, explained Steven E. Kahn, MB, ChB, the chair for the RISE studies.
The other two multicenter, randomized trials being conducted by the RISE Consortium are looking at the effects of medications on preserving beta-cell function in pediatric/adolescent (10-19 years) and adult (21-65 years) populations with IGT or mild, recently diagnosed T2DM. The design, and some results, of these trials can be viewed on a dedicated section of the Diabetes Care website.
Beta-cell function is being assessed using “state-of-the-art” methods; the coprimary endpoint of the surgery versus metformin study was the steady state C-peptide level and acute C-peptide response at maximal glycemia measured using a hyperglycemic “clamp.”
The goal of the RISE studies is to test different approaches to preserve beta-cell function. It is designed to answer the question of which is more effective in this setting: sustained weight loss through gastric banding such as in the BetaFat study or medication.
Patients were eligible for inclusion in the study if they were aged 21-65 years, had a body mass index of 30-40 kg/m2, and had IGT or a diagnosis of T2DM within the past year for which they had received no diabetes medication at recruitment.
A total of 88 individuals were randomized with exactly half undergoing gastric banding. This consisted of a gastric band placed laparoscopically and adjusted every 2 months for the first year, and then every 3 months for the following year depending on symptoms and weight change.
Normoglycemia was observed in none of the study subjects at baseline but in 22% and 15% of those who had gastric banding or metformin, respectively, at 2 years (P = .66).
As for tolerability, five patients who underwent gastric banding experienced serious adverse events, of which two were caused by band slippage and three were caused other reasons. In the metformin arm, there were two serious adverse events, both unrelated to the medication.
“Gastric banding and metformin offered approximately equal approaches for improving insulin sensitivity in adults with mild to moderate obesity and impaired glucose tolerance or early, mild type 2 diabetes,” Dr. Buchanan concluded. “The predominant beta-cell response was a reduction in secretion to maintain a relatively constant compensation for insulin resistance, with only a small improvement in glucose. Whether these interventions will have different effects on beta-cell function over the long-term remains to be determined.”
Commenting on the study, Roy Taylor, MD, professor of medicine and metabolism at Newcastle University (England), noted that the changes in the lipid and liver parameters were important. Fasting plasma triglyceride levels fell from 1.3 mmol/L at baseline to 1.1 mmol/L at 2 years with surgery but stayed more or less the same with metformin (1.23 mmol/L and 1.28 mmol/L; P less than .009 comparing surgery and metformin groups at 2 years). Change in ALT levels were also significant comparing baseline values with results at 2 years, decreasing in the surgical group to a greater extent than in the metformin groups.
“There’s a really important message here, the predictors of a better response to the weight loss [i.e. changes in triglycerides and liver enzymes] are all there,” Dr. Taylor observed. “RISE has looked at 2 years of this effect, but the conversion to type 2 diabetes is probably going to happen over a longer time course.”
He added that “although the primary outcome measure of change in insulin secretion was not achieved, the writing is on the wall. These people, provided they maintain their weight loss, are likely to succeed. We see all the hallmarks of a successful outcome for the weight loss group – remove the primary driver for type 2 diabetes, and that group is on track.”
The RISE Consortium conducted the BetaFat study. The RISE Consortium is supported by grants from the National Institutes for Health. Further support came from the Department of Veterans Affairs, Kaiser Permanente Southern California, the American Diabetes Association, and Allergan. Additional donations of supplies were provided by Allergan, Apollo Endosurgery, Abbott, and Novo Nordisk. Dr. Buchanan reported receiving research funding from Allergan and Apollo Endosurgery. Dr. Taylor had no conflicts of interest.
SOURCES: Buchanan T et al. EASD 2018, Session S09; Xiang AH et al. Diabetes Care. 2018 Oct; dc181662.
BERLIN – Gastric banding surgery and metformin produce similar improvements in insulin sensitivity and parameters indicative of preserved beta-cell function in patients with impaired glucose tolerance (IGT) or newly diagnosed type 2 diabetes mellitus (T2DM), according to the results of a study conducted by the Restoring Insulin Secretion (RISE) Consortium.
“Both interventions resulted in about 50% improvements in insulin sensitivity at 1 year, which was attenuated at 2 years,” reported study investigator Thomas Buchanan, MD, of the University of Southern California, Los Angeles, at the annual meeting of the European Association for the Study of Diabetes.
“The beta-cell responses fell in a pattern that maintained relatively, but not perfectly, stable compensation for insulin resistance,” he added.
Although glucose levels improved “only slightly,” he said, “acute compensation to glucose improved significantly with gastric banding and beta-cell compensation at maximal stimulation fell significantly with metformin.”
Results of the BetaFat (Beta Cell Restoration through Fat Mitigation) study, which are now published online in Diabetes Care, also showed that greater weight loss could be achieved with surgery versus metformin, with a 8.9 kg difference between the groups at 2 years (10.6 vs. 1.7 kg, respectively, P less than .01).
HDL cholesterol levels also rose with both interventions, and gastric banding resulted in a greater effect on very low–density lipoprotein cholesterol and triglycerides, as well as serum ALT, Dr. Buchanan said.
The BetaFat study is one of three “proof-of principle” studies currently being conducted by the RISE Consortium in patients with IGT, sometimes called prediabetes, and T2DM, explained Steven E. Kahn, MB, ChB, the chair for the RISE studies.
The other two multicenter, randomized trials being conducted by the RISE Consortium are looking at the effects of medications on preserving beta-cell function in pediatric/adolescent (10-19 years) and adult (21-65 years) populations with IGT or mild, recently diagnosed T2DM. The design, and some results, of these trials can be viewed on a dedicated section of the Diabetes Care website.
Beta-cell function is being assessed using “state-of-the-art” methods; the coprimary endpoint of the surgery versus metformin study was the steady state C-peptide level and acute C-peptide response at maximal glycemia measured using a hyperglycemic “clamp.”
The goal of the RISE studies is to test different approaches to preserve beta-cell function. It is designed to answer the question of which is more effective in this setting: sustained weight loss through gastric banding such as in the BetaFat study or medication.
Patients were eligible for inclusion in the study if they were aged 21-65 years, had a body mass index of 30-40 kg/m2, and had IGT or a diagnosis of T2DM within the past year for which they had received no diabetes medication at recruitment.
A total of 88 individuals were randomized with exactly half undergoing gastric banding. This consisted of a gastric band placed laparoscopically and adjusted every 2 months for the first year, and then every 3 months for the following year depending on symptoms and weight change.
Normoglycemia was observed in none of the study subjects at baseline but in 22% and 15% of those who had gastric banding or metformin, respectively, at 2 years (P = .66).
As for tolerability, five patients who underwent gastric banding experienced serious adverse events, of which two were caused by band slippage and three were caused other reasons. In the metformin arm, there were two serious adverse events, both unrelated to the medication.
“Gastric banding and metformin offered approximately equal approaches for improving insulin sensitivity in adults with mild to moderate obesity and impaired glucose tolerance or early, mild type 2 diabetes,” Dr. Buchanan concluded. “The predominant beta-cell response was a reduction in secretion to maintain a relatively constant compensation for insulin resistance, with only a small improvement in glucose. Whether these interventions will have different effects on beta-cell function over the long-term remains to be determined.”
Commenting on the study, Roy Taylor, MD, professor of medicine and metabolism at Newcastle University (England), noted that the changes in the lipid and liver parameters were important. Fasting plasma triglyceride levels fell from 1.3 mmol/L at baseline to 1.1 mmol/L at 2 years with surgery but stayed more or less the same with metformin (1.23 mmol/L and 1.28 mmol/L; P less than .009 comparing surgery and metformin groups at 2 years). Change in ALT levels were also significant comparing baseline values with results at 2 years, decreasing in the surgical group to a greater extent than in the metformin groups.
“There’s a really important message here, the predictors of a better response to the weight loss [i.e. changes in triglycerides and liver enzymes] are all there,” Dr. Taylor observed. “RISE has looked at 2 years of this effect, but the conversion to type 2 diabetes is probably going to happen over a longer time course.”
He added that “although the primary outcome measure of change in insulin secretion was not achieved, the writing is on the wall. These people, provided they maintain their weight loss, are likely to succeed. We see all the hallmarks of a successful outcome for the weight loss group – remove the primary driver for type 2 diabetes, and that group is on track.”
The RISE Consortium conducted the BetaFat study. The RISE Consortium is supported by grants from the National Institutes for Health. Further support came from the Department of Veterans Affairs, Kaiser Permanente Southern California, the American Diabetes Association, and Allergan. Additional donations of supplies were provided by Allergan, Apollo Endosurgery, Abbott, and Novo Nordisk. Dr. Buchanan reported receiving research funding from Allergan and Apollo Endosurgery. Dr. Taylor had no conflicts of interest.
SOURCES: Buchanan T et al. EASD 2018, Session S09; Xiang AH et al. Diabetes Care. 2018 Oct; dc181662.
BERLIN – Gastric banding surgery and metformin produce similar improvements in insulin sensitivity and parameters indicative of preserved beta-cell function in patients with impaired glucose tolerance (IGT) or newly diagnosed type 2 diabetes mellitus (T2DM), according to the results of a study conducted by the Restoring Insulin Secretion (RISE) Consortium.
“Both interventions resulted in about 50% improvements in insulin sensitivity at 1 year, which was attenuated at 2 years,” reported study investigator Thomas Buchanan, MD, of the University of Southern California, Los Angeles, at the annual meeting of the European Association for the Study of Diabetes.
“The beta-cell responses fell in a pattern that maintained relatively, but not perfectly, stable compensation for insulin resistance,” he added.
Although glucose levels improved “only slightly,” he said, “acute compensation to glucose improved significantly with gastric banding and beta-cell compensation at maximal stimulation fell significantly with metformin.”
Results of the BetaFat (Beta Cell Restoration through Fat Mitigation) study, which are now published online in Diabetes Care, also showed that greater weight loss could be achieved with surgery versus metformin, with a 8.9 kg difference between the groups at 2 years (10.6 vs. 1.7 kg, respectively, P less than .01).
HDL cholesterol levels also rose with both interventions, and gastric banding resulted in a greater effect on very low–density lipoprotein cholesterol and triglycerides, as well as serum ALT, Dr. Buchanan said.
The BetaFat study is one of three “proof-of principle” studies currently being conducted by the RISE Consortium in patients with IGT, sometimes called prediabetes, and T2DM, explained Steven E. Kahn, MB, ChB, the chair for the RISE studies.
The other two multicenter, randomized trials being conducted by the RISE Consortium are looking at the effects of medications on preserving beta-cell function in pediatric/adolescent (10-19 years) and adult (21-65 years) populations with IGT or mild, recently diagnosed T2DM. The design, and some results, of these trials can be viewed on a dedicated section of the Diabetes Care website.
Beta-cell function is being assessed using “state-of-the-art” methods; the coprimary endpoint of the surgery versus metformin study was the steady state C-peptide level and acute C-peptide response at maximal glycemia measured using a hyperglycemic “clamp.”
The goal of the RISE studies is to test different approaches to preserve beta-cell function. It is designed to answer the question of which is more effective in this setting: sustained weight loss through gastric banding such as in the BetaFat study or medication.
Patients were eligible for inclusion in the study if they were aged 21-65 years, had a body mass index of 30-40 kg/m2, and had IGT or a diagnosis of T2DM within the past year for which they had received no diabetes medication at recruitment.
A total of 88 individuals were randomized with exactly half undergoing gastric banding. This consisted of a gastric band placed laparoscopically and adjusted every 2 months for the first year, and then every 3 months for the following year depending on symptoms and weight change.
Normoglycemia was observed in none of the study subjects at baseline but in 22% and 15% of those who had gastric banding or metformin, respectively, at 2 years (P = .66).
As for tolerability, five patients who underwent gastric banding experienced serious adverse events, of which two were caused by band slippage and three were caused other reasons. In the metformin arm, there were two serious adverse events, both unrelated to the medication.
“Gastric banding and metformin offered approximately equal approaches for improving insulin sensitivity in adults with mild to moderate obesity and impaired glucose tolerance or early, mild type 2 diabetes,” Dr. Buchanan concluded. “The predominant beta-cell response was a reduction in secretion to maintain a relatively constant compensation for insulin resistance, with only a small improvement in glucose. Whether these interventions will have different effects on beta-cell function over the long-term remains to be determined.”
Commenting on the study, Roy Taylor, MD, professor of medicine and metabolism at Newcastle University (England), noted that the changes in the lipid and liver parameters were important. Fasting plasma triglyceride levels fell from 1.3 mmol/L at baseline to 1.1 mmol/L at 2 years with surgery but stayed more or less the same with metformin (1.23 mmol/L and 1.28 mmol/L; P less than .009 comparing surgery and metformin groups at 2 years). Change in ALT levels were also significant comparing baseline values with results at 2 years, decreasing in the surgical group to a greater extent than in the metformin groups.
“There’s a really important message here, the predictors of a better response to the weight loss [i.e. changes in triglycerides and liver enzymes] are all there,” Dr. Taylor observed. “RISE has looked at 2 years of this effect, but the conversion to type 2 diabetes is probably going to happen over a longer time course.”
He added that “although the primary outcome measure of change in insulin secretion was not achieved, the writing is on the wall. These people, provided they maintain their weight loss, are likely to succeed. We see all the hallmarks of a successful outcome for the weight loss group – remove the primary driver for type 2 diabetes, and that group is on track.”
The RISE Consortium conducted the BetaFat study. The RISE Consortium is supported by grants from the National Institutes for Health. Further support came from the Department of Veterans Affairs, Kaiser Permanente Southern California, the American Diabetes Association, and Allergan. Additional donations of supplies were provided by Allergan, Apollo Endosurgery, Abbott, and Novo Nordisk. Dr. Buchanan reported receiving research funding from Allergan and Apollo Endosurgery. Dr. Taylor had no conflicts of interest.
SOURCES: Buchanan T et al. EASD 2018, Session S09; Xiang AH et al. Diabetes Care. 2018 Oct; dc181662.
REPORTING FROM EASD 2018
Key clinical point: Over 2 years, gastric banding surgery and metformin produced similar improvements in insulin sensitivity and parameters suggestive of preserved beta-cell function in patients with prediabetes or early type 2 diabetes.
Major finding: Around a 50% improvement in insulin sensitivity was seen in both study groups at 1 year with attenuation of the effect at 2 years.
Study details: The BetaFat study included 88 obese adults with impaired glucose tolerance or newly diagnosed early type 2 diabetes.
Disclosures: The study was part of the RISE studies, which are supported by grants from the National Institutes for Health. Further support comes from the Department of Veterans Affairs, Kaiser Permanente Southern California, the American Diabetes Association, and Allergan. Additional donations of supplies are provided by Allergan, Apollo Endosurgery, Abbott Laboratories, and Novo Nordisk. Dr. Buchanan reported research funding from Allergan and Apollo Endosurgery. Dr. Taylor had no conflicts of interest.
Sources: Buchanan T et al. EASD 2018, Session S09; Xiang AH et al. Diabetes Care. 2018 Oct; dc181662.
Shelved GLP-1 agonist reduced cardiovascular risk in type 2 diabetes mellitus
BERLIN – Albiglutide, a glucagonlike peptide–1 (GLP-1) agonist, added on top of the standard of care reduced the incidence of major cardiovascular events (MACE) in patients with type 2 diabetes mellitus (T2DM) with established cardiovascular disease by a significant 22% versus placebo in the HARMONY Outcomes trial, according to results reported at the annual meeting of the European Association for the Study of Diabetes.
The trial’s findings, which were published simultaneously in the Lancet, have added “further support that evidence-based GLP-1 receptor agonists should be part of a comprehensive strategy to reduce the risk for cardiovascular events in patients with type 2 diabetes as recommended by recent cardiology and diabetes guidelines,” said study investigator Lawrence Leiter, MD.
Albiglutide was approved for the treatment of T2DM by the European Medicines Agency as Eperzan and by the Food and Drug Administration in the United States as Tanzeum in 2014. Last year, however, its manufacturer, GlaxoSmithKline, announced that it would cease further research and development, manufacturing, and sales activity for albiglutide. Nevertheless, the company remained committed to completing the HARMONY Outcomes trial, begun in 2015.
In a press release issued by GSK on Oct. 2, 2018, the same day as the trial’s findings were revealed, John Lepore, MD, the senior vice president of GSK’s R&D pipeline said, “HARMONY Outcomes was an important study for us to complete to generate new data and insights about the role of the GLP-1 receptor agonist class in the management of patients with diabetes and cardiovascular disease.”
Dr. Lepore added, “GSK continued to invest in this study… and we continue to explore opportunities to divest this medicine to a company with the right expertise and resources to realize its full potential for patients.”
During his summing up of the HARMONY Outcomes data, Dr. Leiter of the University of Toronto observed that all components of the composite primary endpoint – which included MI, cardiovascular death, and stroke – were “directionally consistent with overall benefit.” However, it was the 25% reduction in MI that drove the overall benefit seen.
With an average duration of follow-up of just 1.6 years, it was no wonder perhaps that no effect on a long-term outcome such as cardiovascular death was seen, Dr. Leiter suggested. Insufficient trial length was a fact picked up by the independent commentator for the trial David Matthews, MD, professor of diabetic medicine at the University of Oxford (England).
“HARMONY recruited patients who were extremely near the edge of a cliff,” Dr. Matthews observed, noting that, if a trial was to be completed in such a short span of time, a very-high-risk population needed to be recruited.
Indeed, 100% of the study population in the trial had cardiovascular disease; specifically, 70% had coronary artery disease, 47% had a prior MI, 43% had undergone percutaneous coronary intervention, and 25% had peripheral arterial disease. In addition, 86% had hypertension, 20% had heart failure, and 18% had experienced a stroke. Furthermore, the average hemoglobin A1c (HbA1c) at baseline was 8.7%.
When you are thinking about trial design, you want to recruit patients who are near the edge so that you see lots of events, but not too near such that treatment makes no difference and not too far from the edge or the trial will go on and on, Dr. Matthews observed.
With regards to the primary composite endpoint, he noted that no adjustment of the significance level was needed to test the superiority of albiglutide over placebo. The hazard ratio was 0.78, with a P value of less than .0001 for noninferiority and P = .0006 for superiority, and event rates per 100 patient-years were 4.57 for albiglutide and 5.87 for placebo.
The mean change in HbA1c over time was greater with albiglutide than with placebo, with a between-group difference of –0.63% at 8 months and –0.52% at 16 months. These data suggest that albiglutide seems to have weaker effects than semaglutide, Dr. Matthews noted.
“The odd thing about albiglutide was the weight didn’t change,” Dr. Matthews observed when discussing some of the secondary endpoints. The difference in body weight between albiglutide and placebo was –0.66 kg at 8 months and –0.83 kg at 16 months.
If the results on body mass index with another GLP-1 agonist, semaglutide, were considered, effects on body weight in the HARMONY Outcomes trial were negligible, Dr. Matthews added. This point was something Twitter users also commented on.
“The weight loss is really modest with albiglutide in HARMONY”, said Abd Tahrani, MD, an National Institute for Health Research clinician scientist at the University of Birmingham (U.K.) and an honorary consultant endocrinologist the Heart of England National Health Service Foundation Trust in Birmingham.
Syed Gilani, MD, a general practitioner and champion for Diabetes UK, as well as being a clinical research fellow in diabetes and senior lecturer at the University of Wolverhampton (England), agreed and tweeted: “Is there a hint of GLP-1 class effect?”
While another U.K. diabetes consultant, Partha Kar, MD, a diabetes consultant and endocrinologist at Queen Alexandria Hospital, Portsmouth, England, tweeted: “Game-changer or confirmatory of class effect with better options available?”
The lack of a weight effect could be an advantage of course, Dr. Matthews observed; differences in the GLP-1 agonists could be matched to patients’ needs, with those you do not want to lose weight being given albiglutide.
In an editorial also published in the Lancet (2018 Oct 2. doi: 10.1016/S0140-6736[18]32348-1), Marion Mafham and David Preiss, PhD, who are both from the University of Oxford, observed that “given the clear cardiovascular benefit observed with albiglutide … GlaxoSmithKline should reconsider making it available to patients.”
Ms. Mafham and Dr. Preiss also noted in their comments that, while there has been inconsistency among GLP-1 trials, the HARMONY Outcomes data now add to the evidence of a cardiovascular benefit as seen in the SUSTAIN-6 trial with semaglutide and in the LEADER trial with liraglutide.
“International guidelines should reflect the increasing weight of evidence that supports the use of GLP-1 receptor agonists in patients with type 2 diabetes and cardiovascular disease,” the editorialists wrote.
The study was sponsored by GlaxoSmithKline. Dr. Leiter was an investigator in the study and disclosed receiving research funding and honoraria from GlaxoSmithKline, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Novo Nordisk, and Sanofi Aventis, as well as honoraria from Servier. Dr. Lepore is an employee of GlaxoSmithKline. Dr. Matthews disclosed acting as an advisory board member for and receiving consulting fees or honoraria from GlaxoSmithKline, Novo Nordisk, Novartis, Eli Lilly, Sanofi Aventis, Janssen, and Servier. Ms. Mafham has no competing interests. Dr. Preiss is an investigator in a trial funded by Boehringer Ingelheim.
SOURCE: Hernandez AF et al. Lancet. 2018 Oct 2. doi: 10.1016/S0140-6736(18)32261-X.
BERLIN – Albiglutide, a glucagonlike peptide–1 (GLP-1) agonist, added on top of the standard of care reduced the incidence of major cardiovascular events (MACE) in patients with type 2 diabetes mellitus (T2DM) with established cardiovascular disease by a significant 22% versus placebo in the HARMONY Outcomes trial, according to results reported at the annual meeting of the European Association for the Study of Diabetes.
The trial’s findings, which were published simultaneously in the Lancet, have added “further support that evidence-based GLP-1 receptor agonists should be part of a comprehensive strategy to reduce the risk for cardiovascular events in patients with type 2 diabetes as recommended by recent cardiology and diabetes guidelines,” said study investigator Lawrence Leiter, MD.
Albiglutide was approved for the treatment of T2DM by the European Medicines Agency as Eperzan and by the Food and Drug Administration in the United States as Tanzeum in 2014. Last year, however, its manufacturer, GlaxoSmithKline, announced that it would cease further research and development, manufacturing, and sales activity for albiglutide. Nevertheless, the company remained committed to completing the HARMONY Outcomes trial, begun in 2015.
In a press release issued by GSK on Oct. 2, 2018, the same day as the trial’s findings were revealed, John Lepore, MD, the senior vice president of GSK’s R&D pipeline said, “HARMONY Outcomes was an important study for us to complete to generate new data and insights about the role of the GLP-1 receptor agonist class in the management of patients with diabetes and cardiovascular disease.”
Dr. Lepore added, “GSK continued to invest in this study… and we continue to explore opportunities to divest this medicine to a company with the right expertise and resources to realize its full potential for patients.”
During his summing up of the HARMONY Outcomes data, Dr. Leiter of the University of Toronto observed that all components of the composite primary endpoint – which included MI, cardiovascular death, and stroke – were “directionally consistent with overall benefit.” However, it was the 25% reduction in MI that drove the overall benefit seen.
With an average duration of follow-up of just 1.6 years, it was no wonder perhaps that no effect on a long-term outcome such as cardiovascular death was seen, Dr. Leiter suggested. Insufficient trial length was a fact picked up by the independent commentator for the trial David Matthews, MD, professor of diabetic medicine at the University of Oxford (England).
“HARMONY recruited patients who were extremely near the edge of a cliff,” Dr. Matthews observed, noting that, if a trial was to be completed in such a short span of time, a very-high-risk population needed to be recruited.
Indeed, 100% of the study population in the trial had cardiovascular disease; specifically, 70% had coronary artery disease, 47% had a prior MI, 43% had undergone percutaneous coronary intervention, and 25% had peripheral arterial disease. In addition, 86% had hypertension, 20% had heart failure, and 18% had experienced a stroke. Furthermore, the average hemoglobin A1c (HbA1c) at baseline was 8.7%.
When you are thinking about trial design, you want to recruit patients who are near the edge so that you see lots of events, but not too near such that treatment makes no difference and not too far from the edge or the trial will go on and on, Dr. Matthews observed.
With regards to the primary composite endpoint, he noted that no adjustment of the significance level was needed to test the superiority of albiglutide over placebo. The hazard ratio was 0.78, with a P value of less than .0001 for noninferiority and P = .0006 for superiority, and event rates per 100 patient-years were 4.57 for albiglutide and 5.87 for placebo.
The mean change in HbA1c over time was greater with albiglutide than with placebo, with a between-group difference of –0.63% at 8 months and –0.52% at 16 months. These data suggest that albiglutide seems to have weaker effects than semaglutide, Dr. Matthews noted.
“The odd thing about albiglutide was the weight didn’t change,” Dr. Matthews observed when discussing some of the secondary endpoints. The difference in body weight between albiglutide and placebo was –0.66 kg at 8 months and –0.83 kg at 16 months.
If the results on body mass index with another GLP-1 agonist, semaglutide, were considered, effects on body weight in the HARMONY Outcomes trial were negligible, Dr. Matthews added. This point was something Twitter users also commented on.
“The weight loss is really modest with albiglutide in HARMONY”, said Abd Tahrani, MD, an National Institute for Health Research clinician scientist at the University of Birmingham (U.K.) and an honorary consultant endocrinologist the Heart of England National Health Service Foundation Trust in Birmingham.
Syed Gilani, MD, a general practitioner and champion for Diabetes UK, as well as being a clinical research fellow in diabetes and senior lecturer at the University of Wolverhampton (England), agreed and tweeted: “Is there a hint of GLP-1 class effect?”
While another U.K. diabetes consultant, Partha Kar, MD, a diabetes consultant and endocrinologist at Queen Alexandria Hospital, Portsmouth, England, tweeted: “Game-changer or confirmatory of class effect with better options available?”
The lack of a weight effect could be an advantage of course, Dr. Matthews observed; differences in the GLP-1 agonists could be matched to patients’ needs, with those you do not want to lose weight being given albiglutide.
In an editorial also published in the Lancet (2018 Oct 2. doi: 10.1016/S0140-6736[18]32348-1), Marion Mafham and David Preiss, PhD, who are both from the University of Oxford, observed that “given the clear cardiovascular benefit observed with albiglutide … GlaxoSmithKline should reconsider making it available to patients.”
Ms. Mafham and Dr. Preiss also noted in their comments that, while there has been inconsistency among GLP-1 trials, the HARMONY Outcomes data now add to the evidence of a cardiovascular benefit as seen in the SUSTAIN-6 trial with semaglutide and in the LEADER trial with liraglutide.
“International guidelines should reflect the increasing weight of evidence that supports the use of GLP-1 receptor agonists in patients with type 2 diabetes and cardiovascular disease,” the editorialists wrote.
The study was sponsored by GlaxoSmithKline. Dr. Leiter was an investigator in the study and disclosed receiving research funding and honoraria from GlaxoSmithKline, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Novo Nordisk, and Sanofi Aventis, as well as honoraria from Servier. Dr. Lepore is an employee of GlaxoSmithKline. Dr. Matthews disclosed acting as an advisory board member for and receiving consulting fees or honoraria from GlaxoSmithKline, Novo Nordisk, Novartis, Eli Lilly, Sanofi Aventis, Janssen, and Servier. Ms. Mafham has no competing interests. Dr. Preiss is an investigator in a trial funded by Boehringer Ingelheim.
SOURCE: Hernandez AF et al. Lancet. 2018 Oct 2. doi: 10.1016/S0140-6736(18)32261-X.
BERLIN – Albiglutide, a glucagonlike peptide–1 (GLP-1) agonist, added on top of the standard of care reduced the incidence of major cardiovascular events (MACE) in patients with type 2 diabetes mellitus (T2DM) with established cardiovascular disease by a significant 22% versus placebo in the HARMONY Outcomes trial, according to results reported at the annual meeting of the European Association for the Study of Diabetes.
The trial’s findings, which were published simultaneously in the Lancet, have added “further support that evidence-based GLP-1 receptor agonists should be part of a comprehensive strategy to reduce the risk for cardiovascular events in patients with type 2 diabetes as recommended by recent cardiology and diabetes guidelines,” said study investigator Lawrence Leiter, MD.
Albiglutide was approved for the treatment of T2DM by the European Medicines Agency as Eperzan and by the Food and Drug Administration in the United States as Tanzeum in 2014. Last year, however, its manufacturer, GlaxoSmithKline, announced that it would cease further research and development, manufacturing, and sales activity for albiglutide. Nevertheless, the company remained committed to completing the HARMONY Outcomes trial, begun in 2015.
In a press release issued by GSK on Oct. 2, 2018, the same day as the trial’s findings were revealed, John Lepore, MD, the senior vice president of GSK’s R&D pipeline said, “HARMONY Outcomes was an important study for us to complete to generate new data and insights about the role of the GLP-1 receptor agonist class in the management of patients with diabetes and cardiovascular disease.”
Dr. Lepore added, “GSK continued to invest in this study… and we continue to explore opportunities to divest this medicine to a company with the right expertise and resources to realize its full potential for patients.”
During his summing up of the HARMONY Outcomes data, Dr. Leiter of the University of Toronto observed that all components of the composite primary endpoint – which included MI, cardiovascular death, and stroke – were “directionally consistent with overall benefit.” However, it was the 25% reduction in MI that drove the overall benefit seen.
With an average duration of follow-up of just 1.6 years, it was no wonder perhaps that no effect on a long-term outcome such as cardiovascular death was seen, Dr. Leiter suggested. Insufficient trial length was a fact picked up by the independent commentator for the trial David Matthews, MD, professor of diabetic medicine at the University of Oxford (England).
“HARMONY recruited patients who were extremely near the edge of a cliff,” Dr. Matthews observed, noting that, if a trial was to be completed in such a short span of time, a very-high-risk population needed to be recruited.
Indeed, 100% of the study population in the trial had cardiovascular disease; specifically, 70% had coronary artery disease, 47% had a prior MI, 43% had undergone percutaneous coronary intervention, and 25% had peripheral arterial disease. In addition, 86% had hypertension, 20% had heart failure, and 18% had experienced a stroke. Furthermore, the average hemoglobin A1c (HbA1c) at baseline was 8.7%.
When you are thinking about trial design, you want to recruit patients who are near the edge so that you see lots of events, but not too near such that treatment makes no difference and not too far from the edge or the trial will go on and on, Dr. Matthews observed.
With regards to the primary composite endpoint, he noted that no adjustment of the significance level was needed to test the superiority of albiglutide over placebo. The hazard ratio was 0.78, with a P value of less than .0001 for noninferiority and P = .0006 for superiority, and event rates per 100 patient-years were 4.57 for albiglutide and 5.87 for placebo.
The mean change in HbA1c over time was greater with albiglutide than with placebo, with a between-group difference of –0.63% at 8 months and –0.52% at 16 months. These data suggest that albiglutide seems to have weaker effects than semaglutide, Dr. Matthews noted.
“The odd thing about albiglutide was the weight didn’t change,” Dr. Matthews observed when discussing some of the secondary endpoints. The difference in body weight between albiglutide and placebo was –0.66 kg at 8 months and –0.83 kg at 16 months.
If the results on body mass index with another GLP-1 agonist, semaglutide, were considered, effects on body weight in the HARMONY Outcomes trial were negligible, Dr. Matthews added. This point was something Twitter users also commented on.
“The weight loss is really modest with albiglutide in HARMONY”, said Abd Tahrani, MD, an National Institute for Health Research clinician scientist at the University of Birmingham (U.K.) and an honorary consultant endocrinologist the Heart of England National Health Service Foundation Trust in Birmingham.
Syed Gilani, MD, a general practitioner and champion for Diabetes UK, as well as being a clinical research fellow in diabetes and senior lecturer at the University of Wolverhampton (England), agreed and tweeted: “Is there a hint of GLP-1 class effect?”
While another U.K. diabetes consultant, Partha Kar, MD, a diabetes consultant and endocrinologist at Queen Alexandria Hospital, Portsmouth, England, tweeted: “Game-changer or confirmatory of class effect with better options available?”
The lack of a weight effect could be an advantage of course, Dr. Matthews observed; differences in the GLP-1 agonists could be matched to patients’ needs, with those you do not want to lose weight being given albiglutide.
In an editorial also published in the Lancet (2018 Oct 2. doi: 10.1016/S0140-6736[18]32348-1), Marion Mafham and David Preiss, PhD, who are both from the University of Oxford, observed that “given the clear cardiovascular benefit observed with albiglutide … GlaxoSmithKline should reconsider making it available to patients.”
Ms. Mafham and Dr. Preiss also noted in their comments that, while there has been inconsistency among GLP-1 trials, the HARMONY Outcomes data now add to the evidence of a cardiovascular benefit as seen in the SUSTAIN-6 trial with semaglutide and in the LEADER trial with liraglutide.
“International guidelines should reflect the increasing weight of evidence that supports the use of GLP-1 receptor agonists in patients with type 2 diabetes and cardiovascular disease,” the editorialists wrote.
The study was sponsored by GlaxoSmithKline. Dr. Leiter was an investigator in the study and disclosed receiving research funding and honoraria from GlaxoSmithKline, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Novo Nordisk, and Sanofi Aventis, as well as honoraria from Servier. Dr. Lepore is an employee of GlaxoSmithKline. Dr. Matthews disclosed acting as an advisory board member for and receiving consulting fees or honoraria from GlaxoSmithKline, Novo Nordisk, Novartis, Eli Lilly, Sanofi Aventis, Janssen, and Servier. Ms. Mafham has no competing interests. Dr. Preiss is an investigator in a trial funded by Boehringer Ingelheim.
SOURCE: Hernandez AF et al. Lancet. 2018 Oct 2. doi: 10.1016/S0140-6736(18)32261-X.
REPORTING FROM EASD 2018
Key clinical point: The overall reduction was led by a reduction in the rate of MI.
Major finding: Albiglutide reduced the risk of major cardiovascular events by 22%, compared with placebo, in patients with T2DM and cardiovascular disease.
Study details: HARMONY Outcomes, a postapproval, double-blind, placebo-controlled trial of once-weekly, subcutaneous albiglutide (30-50 mg) versus matched placebo in 9,463 randomized patients.
Disclosures: The study was sponsored by GlaxoSmithKline. Dr. Leiter was an investigator in the study and disclosed receiving research funding and honoraria from GlaxoSmithKline, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Novo Nordisk, and Sanofi Aventis, as well as honoraria from Servier. Dr. Lepore is an employee of GlaxoSmithKline. Dr. Matthews disclosed acting as an advisory board member for and receiving consulting fees or honoraria from GlaxoSmithKline, Novo Nordisk, Novartis, Eli Lilly, Sanofi Aventis, Janssen, and Servier. Dr. Mafham has no competing interests. Dr. Preiss is an investigator in a trial funded by Boehringer Ingelheim.
Source: Hernandez AF et al. Lancet. 2018 Oct 2. doi: 10.1016/S0140-6736(18)32261-X.
Perioperative diabetes and HbA1c in mortality
Clinical question: Do preoperative hemoglobin A1c (HbA1c) and perioperative glucose predict outcomes in patients undergoing noncardiac and cardiac surgeries?
Background: Hyperglycemia in the perioperative period has been associated with infection, delayed wound healing, and postoperative mortality. Studies have investigated the effects of HbA1c or hyperglycemia on postoperative outcomes, but none have been performed to assess the effect of one while controlling for the other.
Study design: Retrospective analysis.
Setting: Single-center, Duke University Health System.
Synopsis: Using a database of electronic health records at Duke University Health System, Durham, N.C., investigators reviewed 13,077 surgeries (6,684 noncardiac and 6,393 cardiac) to determine the association of preoperative HbA1c with perioperative glucose and 30-day mortality. For noncardiac surgery, increased average perioperative glucose was associated with increased mortality (P = .04). In cardiac surgery both low and high average glucose was associated with increased mortality (P = .001). By contrast, HbA1c was not a significant predictor of postoperative mortality in cardiac surgery (P = .08), and in noncardiac surgery, HbA1C was negatively associated with 30-day mortality (P = .01). Overall, perioperative glucose was predictive of 30-day mortality, but HbA1c was not associated with 30-day mortality after researchers controlled for glucose.
Because the study is retrospective, no causal relationship can be established. Hospitalists involved in perioperative care should aim for optimization of glucose control regardless of preoperative HbA1c.
Bottom line: Perioperative glucose is related to surgical outcomes, but HbA1c is a less useful indicator of 30-day postoperative mortality.
Citation: Van den Boom W et al. Effect of A1C and glucose on postoperative mortality in noncardiac and cardiac surgeries. Diabetes Care. 2018 Feb;41:782-8.
Clinical question: Do preoperative hemoglobin A1c (HbA1c) and perioperative glucose predict outcomes in patients undergoing noncardiac and cardiac surgeries?
Background: Hyperglycemia in the perioperative period has been associated with infection, delayed wound healing, and postoperative mortality. Studies have investigated the effects of HbA1c or hyperglycemia on postoperative outcomes, but none have been performed to assess the effect of one while controlling for the other.
Study design: Retrospective analysis.
Setting: Single-center, Duke University Health System.
Synopsis: Using a database of electronic health records at Duke University Health System, Durham, N.C., investigators reviewed 13,077 surgeries (6,684 noncardiac and 6,393 cardiac) to determine the association of preoperative HbA1c with perioperative glucose and 30-day mortality. For noncardiac surgery, increased average perioperative glucose was associated with increased mortality (P = .04). In cardiac surgery both low and high average glucose was associated with increased mortality (P = .001). By contrast, HbA1c was not a significant predictor of postoperative mortality in cardiac surgery (P = .08), and in noncardiac surgery, HbA1C was negatively associated with 30-day mortality (P = .01). Overall, perioperative glucose was predictive of 30-day mortality, but HbA1c was not associated with 30-day mortality after researchers controlled for glucose.
Because the study is retrospective, no causal relationship can be established. Hospitalists involved in perioperative care should aim for optimization of glucose control regardless of preoperative HbA1c.
Bottom line: Perioperative glucose is related to surgical outcomes, but HbA1c is a less useful indicator of 30-day postoperative mortality.
Citation: Van den Boom W et al. Effect of A1C and glucose on postoperative mortality in noncardiac and cardiac surgeries. Diabetes Care. 2018 Feb;41:782-8.
Clinical question: Do preoperative hemoglobin A1c (HbA1c) and perioperative glucose predict outcomes in patients undergoing noncardiac and cardiac surgeries?
Background: Hyperglycemia in the perioperative period has been associated with infection, delayed wound healing, and postoperative mortality. Studies have investigated the effects of HbA1c or hyperglycemia on postoperative outcomes, but none have been performed to assess the effect of one while controlling for the other.
Study design: Retrospective analysis.
Setting: Single-center, Duke University Health System.
Synopsis: Using a database of electronic health records at Duke University Health System, Durham, N.C., investigators reviewed 13,077 surgeries (6,684 noncardiac and 6,393 cardiac) to determine the association of preoperative HbA1c with perioperative glucose and 30-day mortality. For noncardiac surgery, increased average perioperative glucose was associated with increased mortality (P = .04). In cardiac surgery both low and high average glucose was associated with increased mortality (P = .001). By contrast, HbA1c was not a significant predictor of postoperative mortality in cardiac surgery (P = .08), and in noncardiac surgery, HbA1C was negatively associated with 30-day mortality (P = .01). Overall, perioperative glucose was predictive of 30-day mortality, but HbA1c was not associated with 30-day mortality after researchers controlled for glucose.
Because the study is retrospective, no causal relationship can be established. Hospitalists involved in perioperative care should aim for optimization of glucose control regardless of preoperative HbA1c.
Bottom line: Perioperative glucose is related to surgical outcomes, but HbA1c is a less useful indicator of 30-day postoperative mortality.
Citation: Van den Boom W et al. Effect of A1C and glucose on postoperative mortality in noncardiac and cardiac surgeries. Diabetes Care. 2018 Feb;41:782-8.
Breast cancer risk in type 2 diabetes related to adiposity
ORLANDO – findings from meta-analyses suggest.
In one meta-analysis of data from 21 prospective studies with a total of nearly 15.2 million women, 325,117 breast cancer cases, and a mean follow-up time of 8 years (nearly 33 million person-years), the risk of breast cancer was significantly greater among patients with diabetes than it was among patients without diabetes (summary relative risk, 1.11), Maria Bota reported at the annual scientific sessions of the American Diabetes Association.
However, there was substantial unexplained heterogeneity of results across the individual studies (I2 = 82%), said Ms. Bota, a faculty member at the International Prevention Research Institute, Lyon, France.
“When the analysis was restricted to the 12 studies that adjusted for [body mass index], the summary relative risk decreased to 1.09 and the heterogeneity also decreased to a moderate value of 32%; when the analysis was restricted to the 9 studies that did not adjust for BMI, the summary relative risk increased to 1.14 again, and the heterogeneity increased even more to 91%,” she said.
In an analysis that combined the results of the nine studies that did not adjust for BMI along with crude relative risks from studies that reported both crude and BMI-adjusted relative risks (17 studies in all), the SRR was 1.12, and heterogeneity among the studies was high at 84%.
Additionally, an analysis by menopausal status based on four studies that reported breast cancer in both pre- and postmenopausal women showed SRRs for breast cancer of 0.97 (a 3% decrease in risk) and 1.14 among diabetic vs. nondiabetic premenopausal women and postmenopausal women, respectively, she said, noting that heterogeneity was low (I2 = 0%) among the premenopausal breast cancer study groups and high (I2 = 70%) among the postmenopausal study groups.
The findings provide evidence for a moderately increased risk of breast cancer in women with T2DM, Ms. Bota said.
“However, the effect of the adjustment or lack of adjustment on the heterogeneity suggests that the higher risk of breast cancer in women with diabetes may not be due to diabetes itself, but to adiposity,” she said, adding that “this hypothesis is equally supported by our subgroup analysis according to menopausal status because we saw that the risk of breast cancer was only associated with diabetes in postmenopausal women and this pattern resembles the pattern of the risk of breast cancer associated with adiposity, which is also only increased in postmenopausal women.”
This study was limited by insufficient data for investigating the sources of heterogeneity, she said.
“Therefore we propose ... future pooled analyses based on individual data from good quality prospective studies in order to increase the study power and to do some detailed analysis of the links between adiposity, diabetes, and breast cancer,” she concluded, adding that new studies to examine those relationships only in premenopausal women are also needed
In a separate meta-analysis, she and her colleagues, including Peter Boyle, PhD, president of the International Prevention Research Institute, assessed the association between insulin treatment and breast cancer risk in patients with diabetes.
“The long-acting insulin analogues glargine and detemir have been shown in some studies to be associated with increased risk of breast cancer, and other studies have shown no association between the use of these two compounds and the risk of breast cancer,” Dr. Boyle said in a separate presentation at the ADA meeting. “It was important to sort out a little bit what was going on in the literature.”
Overall, the meta-analysis of data from 12 longitudinal cohort studies – including more than 6,000 cases of breast cancer – showed a slight increase in breast cancer risk in patients taking long-acting insulin (SRR, 1.13) with “a relatively reasonable” level of heterogeneity (I2 = 23%).
“But we see that the story is not that simple,” he said.
For example, some studies included only patients who were prescribed insulin for the first time after the study began (new users), some included only patients who were prescribed insulin before the study began (prevalent users), and some included both (ever users), which may have introduced bias in the results, he explained.
Studies of glargine included 4,168 breast cancer cases over a total of 1,418,743 person-years of observation, and studies of detemir included fewer than 2,047 breast cancer cases (not all studies reported case numbers). Among both new users of glargine and detemir, the SRR was 1.12, suggesting no real association between either glargine or detemir use and breast cancer, he said.
“One important take-home message is that you have to be careful that these pharmaco-epidemiological studies, even when working with the same database, may have conflicting results ... so we still need more robust standards in methods for [such] studies,” he concluded.
Ms. Bota reported having no disclosures. The study presented by Dr. Boyle was funded by Sanofi. Dr. Boyle is president of a charity that has received donations from Pfizer, Roche, Novartis, and Lilly.
SOURCE: Bota M. ADA 2018, Abstract 180-OR; Boyle P. ADA 2018, Abstract 133-OR.
ORLANDO – findings from meta-analyses suggest.
In one meta-analysis of data from 21 prospective studies with a total of nearly 15.2 million women, 325,117 breast cancer cases, and a mean follow-up time of 8 years (nearly 33 million person-years), the risk of breast cancer was significantly greater among patients with diabetes than it was among patients without diabetes (summary relative risk, 1.11), Maria Bota reported at the annual scientific sessions of the American Diabetes Association.
However, there was substantial unexplained heterogeneity of results across the individual studies (I2 = 82%), said Ms. Bota, a faculty member at the International Prevention Research Institute, Lyon, France.
“When the analysis was restricted to the 12 studies that adjusted for [body mass index], the summary relative risk decreased to 1.09 and the heterogeneity also decreased to a moderate value of 32%; when the analysis was restricted to the 9 studies that did not adjust for BMI, the summary relative risk increased to 1.14 again, and the heterogeneity increased even more to 91%,” she said.
In an analysis that combined the results of the nine studies that did not adjust for BMI along with crude relative risks from studies that reported both crude and BMI-adjusted relative risks (17 studies in all), the SRR was 1.12, and heterogeneity among the studies was high at 84%.
Additionally, an analysis by menopausal status based on four studies that reported breast cancer in both pre- and postmenopausal women showed SRRs for breast cancer of 0.97 (a 3% decrease in risk) and 1.14 among diabetic vs. nondiabetic premenopausal women and postmenopausal women, respectively, she said, noting that heterogeneity was low (I2 = 0%) among the premenopausal breast cancer study groups and high (I2 = 70%) among the postmenopausal study groups.
The findings provide evidence for a moderately increased risk of breast cancer in women with T2DM, Ms. Bota said.
“However, the effect of the adjustment or lack of adjustment on the heterogeneity suggests that the higher risk of breast cancer in women with diabetes may not be due to diabetes itself, but to adiposity,” she said, adding that “this hypothesis is equally supported by our subgroup analysis according to menopausal status because we saw that the risk of breast cancer was only associated with diabetes in postmenopausal women and this pattern resembles the pattern of the risk of breast cancer associated with adiposity, which is also only increased in postmenopausal women.”
This study was limited by insufficient data for investigating the sources of heterogeneity, she said.
“Therefore we propose ... future pooled analyses based on individual data from good quality prospective studies in order to increase the study power and to do some detailed analysis of the links between adiposity, diabetes, and breast cancer,” she concluded, adding that new studies to examine those relationships only in premenopausal women are also needed
In a separate meta-analysis, she and her colleagues, including Peter Boyle, PhD, president of the International Prevention Research Institute, assessed the association between insulin treatment and breast cancer risk in patients with diabetes.
“The long-acting insulin analogues glargine and detemir have been shown in some studies to be associated with increased risk of breast cancer, and other studies have shown no association between the use of these two compounds and the risk of breast cancer,” Dr. Boyle said in a separate presentation at the ADA meeting. “It was important to sort out a little bit what was going on in the literature.”
Overall, the meta-analysis of data from 12 longitudinal cohort studies – including more than 6,000 cases of breast cancer – showed a slight increase in breast cancer risk in patients taking long-acting insulin (SRR, 1.13) with “a relatively reasonable” level of heterogeneity (I2 = 23%).
“But we see that the story is not that simple,” he said.
For example, some studies included only patients who were prescribed insulin for the first time after the study began (new users), some included only patients who were prescribed insulin before the study began (prevalent users), and some included both (ever users), which may have introduced bias in the results, he explained.
Studies of glargine included 4,168 breast cancer cases over a total of 1,418,743 person-years of observation, and studies of detemir included fewer than 2,047 breast cancer cases (not all studies reported case numbers). Among both new users of glargine and detemir, the SRR was 1.12, suggesting no real association between either glargine or detemir use and breast cancer, he said.
“One important take-home message is that you have to be careful that these pharmaco-epidemiological studies, even when working with the same database, may have conflicting results ... so we still need more robust standards in methods for [such] studies,” he concluded.
Ms. Bota reported having no disclosures. The study presented by Dr. Boyle was funded by Sanofi. Dr. Boyle is president of a charity that has received donations from Pfizer, Roche, Novartis, and Lilly.
SOURCE: Bota M. ADA 2018, Abstract 180-OR; Boyle P. ADA 2018, Abstract 133-OR.
ORLANDO – findings from meta-analyses suggest.
In one meta-analysis of data from 21 prospective studies with a total of nearly 15.2 million women, 325,117 breast cancer cases, and a mean follow-up time of 8 years (nearly 33 million person-years), the risk of breast cancer was significantly greater among patients with diabetes than it was among patients without diabetes (summary relative risk, 1.11), Maria Bota reported at the annual scientific sessions of the American Diabetes Association.
However, there was substantial unexplained heterogeneity of results across the individual studies (I2 = 82%), said Ms. Bota, a faculty member at the International Prevention Research Institute, Lyon, France.
“When the analysis was restricted to the 12 studies that adjusted for [body mass index], the summary relative risk decreased to 1.09 and the heterogeneity also decreased to a moderate value of 32%; when the analysis was restricted to the 9 studies that did not adjust for BMI, the summary relative risk increased to 1.14 again, and the heterogeneity increased even more to 91%,” she said.
In an analysis that combined the results of the nine studies that did not adjust for BMI along with crude relative risks from studies that reported both crude and BMI-adjusted relative risks (17 studies in all), the SRR was 1.12, and heterogeneity among the studies was high at 84%.
Additionally, an analysis by menopausal status based on four studies that reported breast cancer in both pre- and postmenopausal women showed SRRs for breast cancer of 0.97 (a 3% decrease in risk) and 1.14 among diabetic vs. nondiabetic premenopausal women and postmenopausal women, respectively, she said, noting that heterogeneity was low (I2 = 0%) among the premenopausal breast cancer study groups and high (I2 = 70%) among the postmenopausal study groups.
The findings provide evidence for a moderately increased risk of breast cancer in women with T2DM, Ms. Bota said.
“However, the effect of the adjustment or lack of adjustment on the heterogeneity suggests that the higher risk of breast cancer in women with diabetes may not be due to diabetes itself, but to adiposity,” she said, adding that “this hypothesis is equally supported by our subgroup analysis according to menopausal status because we saw that the risk of breast cancer was only associated with diabetes in postmenopausal women and this pattern resembles the pattern of the risk of breast cancer associated with adiposity, which is also only increased in postmenopausal women.”
This study was limited by insufficient data for investigating the sources of heterogeneity, she said.
“Therefore we propose ... future pooled analyses based on individual data from good quality prospective studies in order to increase the study power and to do some detailed analysis of the links between adiposity, diabetes, and breast cancer,” she concluded, adding that new studies to examine those relationships only in premenopausal women are also needed
In a separate meta-analysis, she and her colleagues, including Peter Boyle, PhD, president of the International Prevention Research Institute, assessed the association between insulin treatment and breast cancer risk in patients with diabetes.
“The long-acting insulin analogues glargine and detemir have been shown in some studies to be associated with increased risk of breast cancer, and other studies have shown no association between the use of these two compounds and the risk of breast cancer,” Dr. Boyle said in a separate presentation at the ADA meeting. “It was important to sort out a little bit what was going on in the literature.”
Overall, the meta-analysis of data from 12 longitudinal cohort studies – including more than 6,000 cases of breast cancer – showed a slight increase in breast cancer risk in patients taking long-acting insulin (SRR, 1.13) with “a relatively reasonable” level of heterogeneity (I2 = 23%).
“But we see that the story is not that simple,” he said.
For example, some studies included only patients who were prescribed insulin for the first time after the study began (new users), some included only patients who were prescribed insulin before the study began (prevalent users), and some included both (ever users), which may have introduced bias in the results, he explained.
Studies of glargine included 4,168 breast cancer cases over a total of 1,418,743 person-years of observation, and studies of detemir included fewer than 2,047 breast cancer cases (not all studies reported case numbers). Among both new users of glargine and detemir, the SRR was 1.12, suggesting no real association between either glargine or detemir use and breast cancer, he said.
“One important take-home message is that you have to be careful that these pharmaco-epidemiological studies, even when working with the same database, may have conflicting results ... so we still need more robust standards in methods for [such] studies,” he concluded.
Ms. Bota reported having no disclosures. The study presented by Dr. Boyle was funded by Sanofi. Dr. Boyle is president of a charity that has received donations from Pfizer, Roche, Novartis, and Lilly.
SOURCE: Bota M. ADA 2018, Abstract 180-OR; Boyle P. ADA 2018, Abstract 133-OR.
REPORTING FROM ADA 2018
Key clinical point: Adiposity accounts for the increased risk of breast cancer among women with diabetes.
Major finding: An analysis of 12 studies that adjusted for BMI showed a summary relative risk for breast cancer of 1.09 in diabetic versus nondiabetic women, with moderate study heterogeneity.
Study details: Meta-analyses including 21 and 12 studies, respectively.
Disclosures: Ms. Bota reported having no disclosures. The study presented by Dr. Boyle was funded by Sanofi. Dr. Boyle is president of a charity that has received donations from Pfizer, Roche, Novartis, and Lilly.
Source: Bota M. ADA 2018, Abstract 180-OR; Boyle P. ADA 2018, Abstract 133-OR.