User login
Integrating Massage Therapy Into the Health Care of Female Veterans
There are approximately 2 million female veterans in the United States, representing about 10% of the veteran population.1 In 2015, 456,000 female veterans used the US Department of Veterans Affairs (VA) health care services. The VA predicts an increase in utilization over the next 20 years.2
Female veterans are more likely to have musculoskeletal disorder multimorbidity compared with male veterans and have higher rates of depressive and bipolar disorders, anxiety, and posttraumatic stress disorder (PTSD).3,4 Compared with male veterans, female veterans are younger, more likely to be unmarried and to have served during the wars in Iraq and Afghanistan.3 Fifty-five percent of women veterans vs 41% of men veterans have a service-connected disability, and a greater percentage of women veterans have a service connection rating > 50%.5 The top service-connected disabilities for women veterans are PTSD, major depressive disorder, migraines, and lumbosacral or cervical strain.2 In addition, one-third of women veterans using VA health care report experiencing military sexual trauma (MST).6 Military service may impact the health of female veterans both physically and mentally. Providing treatments and programs to improve their health and their health care experience are current VA priorities.
The VA is changing the way health care is conceptualized and delivered by implementing a holistic model of care known as Whole Health, which seeks to empower and equip patients to take charge of their health, blending conventional medicine with self-care and complementary and integrative health (CIH) approaches, such as massage therapy, yoga, acupuncture, and meditation.7 CIH therapies can help improve physical and mental health with little to no adverse effects.8-10
As part of the Whole Health initiative at the VA Ann Arbor Healthcare System (VAAAHS) in Michigan, the massage program was expanded in 2017 to offer relaxation massages to female veterans attending the women’s health clinic, which provides gynecologic care. Patients visiting a gynecology clinic often experience anxiety and pain related to invasive procedures and examinations. This is especially true for female veterans who experienced MST.11
VAAAHS has 1 staff massage therapist (MT). To expand the program to the women’s health clinic, volunteer licensed MTs were recruited and trained in specific procedures by the staff MT.
Several studies have demonstrated the effect of therapeutic massage on pain and anxiety in predominantly male veteran study populations, including veterans needing postsurgical and palliative care as well as those experiencing chronic pain and knee osteoarthritis.12-16 Little is known about the effects of massage therapy on female veterans. The purpose of this pilot study was to examine the effects of massage therapy among female veterans participating in the women’s health massage program.
Methods
The setting for this pre-post intervention study was VAAAHS. Veterans were called in advance by clinic staff and scheduled for 60-minute appointments either before or after their clinic appointment, depending on availability. MTs were instructed to provide relaxation massage using Swedish massage techniques with moderate pressure, avoiding deep pressure techniques.
The volunteer MTs gave the participants a survey to provide comments and to rate baseline pain and other symptoms prior to and following the massage. The MT left the room to provide privacy while completing the survey. The staff included the symptom data in the massage note as clinical outcomes and entered them into the electronic health record. Massages were given from October 1, 2017 to June 30, 2018. Data including symptom scores, demographics, the presence of chronic pain, mental health diagnoses, patient comments, and opioid use were abstracted from the electronic health record by 2 members of the study team and entered into an Excel database. This study was approved by the VAAAHS Institutional Review Board.
Study Measures
Pain intensity, pain unpleasantness (the affective component of pain), anxiety, shortness of breath, relaxation, and inner peace were rated pre- and postmassage on a 0 to 10 scale. Shortness of breath was included due to the relationship between breathing and anxiety. Inner peace was assessed to measure the calming effects of massage therapy. Beck and colleagues found the concept of inner peace was an important outcome of massage therapy.17 The scale anchors for pain intensity were “no pain” and “severe pain”; and “not at all unpleasant” and “as unpleasant as it can be” for pain unpleasantness. For anxiety, the anchors were “no anxiety” and “as anxious as I can be.” Anchors for relaxation and inner peace were reversed so that a 0 indicated low relaxation and inner peace while a 10 indicated the highest state of relaxation and inner peace.
Chronic pain was defined as pain existing for > 3 months. A history of chronic pain was determined from a review and synthesis of primary care and specialty care recorded diagnoses, patient concerns, and service-connected disabilities. The diagnoses included lumbosacral or cervical strain, chronic low back, joint (knee, shoulder, hip, ankle), neck, or pelvic pain, fibromyalgia, headache, migraine, osteoarthritis, and myofascial pain syndrome. The presence of mental health conditions, including depression, anxiety, bipolar disorders, and PTSD, were similarly determined by a review of mental health clinical notes. Sex was determined from the gynecology note.
Statistical Analysis
Means and medians were calculated for short-term changes in symptom scores. Due to skewness in the short-term changes, significance was tested using a nonparametric sign test. Significance was adjusted using the Bonferroni correction to protect the overall type I error level at 5% from multiple testing. We also assessed for differences in symptom changes in 4 subgroups, using an unadjusted general linear model: those with (1) chronic pain vs without; (2) an anxiety diagnosis vs without; (3) depression vs without; and (4) a PTSD diagnosis vs without. Data were analyzed using SPSS 25 and SAS 9.4.
Results
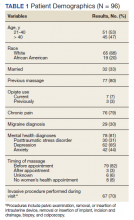
Results are based on the first massage received by 96 unique individuals (Table 1). Fifty-one (53%) patients were aged 21 to 40 years, and 45 (47%) were aged ≥ 41 years. Most participants (80%) had had a previous massage. Seven (7%) participants were currently on prescription opioids; 76 (79%) participants had a history of one or more chronic pain diagnoses (eg, back pain, migraine headaches, fibromyalgia) and 78 (81%) had a history of a mental health diagnosis (eg, depression, anxiety, PTSD). Massage sessions ranged from 30 to 60 minutes; most patients received massage therapy for 50 minutes.
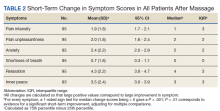
Prior to massage, mean scores were 3.9 pain intensity, 3.7 pain unpleasantness, 3.8 anxiety, 1.0 shortness of breath, 4.0 relaxation, and 4.2 inner peace. Short-term changes in symptom scores are shown in Table 2. The mean score for pain intensity decreased by 1.9 points, pain unpleasantness by 2.0 points, anxiety by 2.4 points. The greatest change occurred for relaxation, which increased by 4.3 points. All changes in symptoms were statistically significant (P < .001). For subgroup comparisons
Verbal feedback and written comments about the massage experience were all favorable: No adverse events were reported.
Discussion
Massage therapy may be a useful treatment for female veterans experiencing chronic pain, anxiety disorders, depression, or situational anxiety related to gynecologic procedures. After receiving a relaxation massage, female veterans reported decreased pain intensity, pain unpleasantness, and anxiety while reporting increased relaxation and feelings of inner peace. The effects of massage were consistent for all the symptoms or characteristics assessed, suggesting that massage may act on the body in multiple ways.
These changes parallel those seen in a palliative care population primarily composed of male veterans.14 However, the female veterans in this cohort experienced greater changes in relaxation and feelings of inner peace, which may be partly due to relief of tension related to an upcoming stressful appointment. The large mean decrease in anxiety level among female veterans with PTSD is notable as well as the larger increase in inner peace in those with chronic pain.
Many patients expressed their gratitude for the massage and interest in having access to more massage therapy. Female patients who have experienced sexual trauma or other trauma may especially benefit from massage prior to painful, invasive gynecologic procedures. Anecdotally, 2 nurse chaperones in the clinic mentioned separately to the massage program supervisor that the massages helped some very anxious women better tolerate an invasive procedure that would have been otherwise extremely difficult.
Female veterans are more likely to have musculoskeletal issues after deployment and have higher rates of anxiety, PTSD, and depression compared with those of male veterans.3,4,18,19 Determining relationships between and causes of chronic pain, depression, and PTSD is very challenging but the increased prevalence of chronic pain and comorbid mental health conditions in female veterans may be partially related to MST or other trauma experiences.20-22 Female veterans are most likely to have more than one source of chronic pain.23-25 Female patients with chronic musculoskeletal pain report more pain-related disability.26 Furthermore, greater disability in the context of depression is reported by women with pain compared with those of men.27 Most (78%) female veterans in a primary care population reported chronic pain.23 Similarly, 79% of the female veterans in this study population had chronic pain and 81% had a history of mental health disorders, including depression, anxiety, and PTSD.
Studies have shown that massage therapy improves pain in populations experiencing chronic low back, neck, and knee pain.28-32 A 2020 Agency for Healthcare Research and Quality review determined there is some evidence that massage therapy is helpful for chronic low back and neck pain and fibromyalgia.33 Research also has demonstrated that massage reduces anxiety and depression in several different population types.13,34,35 Li and colleagues showed that foot massage increased oxytocin levels in healthy males.36 Although further research is needed to determine the mechanisms of massage therapy, there are important physiologic effects. Unlike most medications, massage therapy is unique in that it can impact health and well-being through multiple mechanisms; for example, by reducing pain, improving mood, providing a sense of social connection and/or improving mobility.
Patients using CIH therapies report greater awareness of the need for ongoing engagement in their own care and health behavior changes.37,38
Driscoll and colleagues reported that women veterans are interested in conservative treatment for their chronic musculoskeletal pain and are open to using CIH therapies.39 Research suggests that veterans are interested in and, in some cases, already using massage therapy.23,40-43 Access to massage therapy and other CIH therapies offers patients choice and control over the types and timing of therapy they receive, exemplified by the 80% of patients in our study who previously received a massage and sought another before a potentially stressful situation.
Access to massage therapy or other CIH therapies may reduce the need for more expensive procedures. Although research on the cost-effectiveness of massage therapy is limited, Herman and colleagues did an economic evaluation of CIH therapies in a veteran population, finding that CIH users had lower annual health care costs and lower pain in the year after CIH started. Sensitivity analyses indicated similar results for acupuncture, chiropractic care, and massage but higher costs for those with 8 or more visits.44
The prevalence of comorbid mental health conditions with MSD suggests that female veterans may benefit from multidisciplinary treatment of pain and depression.3,26 Women-centered programs would be both encouraging and validating to women.39 Massage therapy can be combined with physical therapy, yoga, tai chi, and meditation programs to improve pain, anxiety, strength, and flexibility and can be incorporated into a multimodal treatment plan. Likewise, other subpopulations of female veterans with chronic pain, mental health conditions, or cancer could be targeted with multidisciplinary programs that include massage therapy.
Limitations
This study has several limitations including lack of a control group, a self-selected population, the lack of objective biochemical measurements, and possible respondent bias to please the MTs. Eighty percent had previously experienced massage therapy and may have been biased toward the effects of massage before receiving the intervention. The first report of the effects of massage therapy in an exclusively female veteran population is a major strength of this study.
Further research including randomized controlled trials is needed, especially in populations with coexisting chronic pain and mental health disorders, as is exploring the acceptability of massage therapy for female veterans with MST. Finding viable alternatives to medications has become even more important as the nation addresses the challenge of the opioid crisis.45,46
Conclusions
Female veterans are increasingly seeking VA health care.
Acknowledgments
The authors express our gratitude to the Women Veteran Program Manager, Cheryl Allen, RN; Massage Therapists Denise McGee and Kimberly Morro; Dara Ganoczy, MPH, for help with statistical analysis; and Mark Hausman, MD, for leadership support.
1. US Department of Veteran Affairs, National Center for Veterans Analysis and Statistics. Veteran population. Updated April 14, 2021. Accessed January 6, 2022. https://www.va.gov/vetdata/veteran_population.asp
2. US Department of Veteran Affairs. Women veterans report: the past, present, and future of women veterans. Published February 2017. Accessed January 6, 2022. https://www.va.gov/vetdata/docs/specialreports/women_veterans_2015_final.pdf
3. Higgins DM, Fenton BT, Driscoll MA, et al. Gender differences in demographic and clinical correlates among veterans with musculoskeletal disorders. Womens Health Issues. 2017;27(4):463-470. doi:10.1016/j.whi.2017.01.008
4. Lehavot K, Goldberg SB, Chen JA, et al. Do trauma type, stressful life events, and social support explain women veterans’ high prevalence of PTSD?. Soc Psychiatry Psychiatr Epidemiol. 2018;53(9):943-953. doi:10.1007/s00127-018-1550-x
5. Levander XA, Overland MK. Care of women veterans. Med Clin North Am. 2015;99(3):651-662. doi:10.1016/j.mcna.2015.01.013
6. US Department of Veteran Affairs. Facts and statistics about women veterans. Updated May 28. 2020. Accessed January 6, 2022. https://www.womenshealth.va.gov/womenshealth/latestinformation/facts.asp
7. Krejci LP, Carter K, Gaudet T. Whole health: the vision and implementation of personalized, proactive, patient-driven health care for veterans. Med Care. 2014;52(12)(suppl 5):S5-S8. doi:10.1097/MLR.0000000000000226
8. Elwy AR, Taylor SL, Zhao S, et al. Participating in complementary and integrative health approaches is associated with veterans’ patient-reported outcomes over time. Med Care. 2020;58:S125-S132. doi:10.1097/MLR.0000000000001357
9. Smeeding SJ, Bradshaw DH, Kumpfer K, Trevithick S, Stoddard GJ. Outcome evaluation of the Veterans Affairs Salt Lake City Integrative Health Clinic for chronic pain and stress-related depression, anxiety, and post-traumatic stress disorder. J Altern Complement Med. 2010;16(8):823-835. doi:10.1089/acm.2009.0510
10. Hull A, Brooks Holliday S, Eickhoff C, et al. Veteran participation in the integrative health and wellness program: impact on self-reported mental and physical health outcomes. Psychol Serv. 2019;16(3):475-483. doi:10.1037/ser0000192
11. Zephyrin LC. Reproductive health management for the care of women veterans [published correction appears in Obstet Gynecol. 2016 Mar;127(3):605]. Obstet Gynecol. 2016;127(2):383-392. doi:10.1097/AOG.0000000000001252
12. Piotrowski MM, Paterson C, Mitchinson A, Kim HM, Kirsh M, Hinshaw DB. Massage as adjuvant therapy in the management of acute postoperative pain: a preliminary study in men. J Am Coll Surg. 2003;197(6):1037-1046. doi:10.1016/j.jamcollsurg.2003.07.020
13. Mitchinson AR, Kim HM, Rosenberg JM, et al. Acute postoperative pain management using massage as an adjuvant therapy: a randomized trial. Arch Surg. 2007;142(12):1158-1167. doi:10.1001/archsurg.142.12.1158
14. Mitchinson A, Fletcher CE, Kim HM, Montagnini M, Hinshaw DB. Integrating massage therapy within the palliative care of veterans with advanced illnesses: an outcome study. Am J Hosp Palliat Care. 2014;31(1):6-12. doi:10.1177/1049909113476568
15. Fletcher CE, Mitchinson AR, Trumble EL, Hinshaw DB, Dusek JA. Perceptions of other integrative health therapies by veterans with pain who are receiving massage. J Rehabil Res Dev. 2016;53(1):117-126. doi:10.1682/JRRD.2015.01.0015
16. Juberg M, Jerger KK, Allen KD, Dmitrieva NO, Keever T, Perlman AI. Pilot study of massage in veterans with knee osteoarthritis. J Altern Complement Med. 2015;21(6):333-338. doi:10.1089/acm.2014.0254
17. Beck I, Runeson I, Blomqvist K. To find inner peace: soft massage as an established and integrated part of palliative care. Int J Palliate Nurse. 2009;15(11):541-545. doi: 10.12968/ijpn.2009.15.11.45493
18. Haskell SG, Ning Y, Krebs E, et al. Prevalence of painful musculoskeletal conditions in female and male veterans in 7 years after return from deployment in Operation Enduring Freedom/Operation Iraqi Freedom. Clin J Pain. 2012;28(2):163-167. doi:10.1097/AJP.0b013e318223d951
19. Maguen S, Ren L, Bosch JO, Marmar CR, Seal KH. Gender differences in mental health diagnoses among Iraq and Afghanistan veterans enrolled in veterans affairs health care. Am J Public Health. 2010;100(12):2450-2456. doi:10.2105/AJPH.2009.166165
20. Outcalt SD, Kroenke K, Krebs EE, et al. Chronic pain and comorbid mental health conditions: independent associations of posttraumatic stress disorder and depression with pain, disability, and quality of life. J Behav Med. 2015;38(3):535-543. doi:10.1007/s10865-015-9628-3
21. Gibson CJ, Maguen S, Xia F, Barnes DE, Peltz CB, Yaffe K. Military sexual trauma in older women veterans: prevalence and comorbidities. J Gen Intern Med. 2020;35(1):207-213. doi:10.1007/s11606-019-05342-7
22. Tan G, Teo I, Srivastava D, et al. Improving access to care for women veterans suffering from chronic pain and depression associated with trauma. Pain Med. 2013;14(7):1010-1020. doi:10.1111/pme.12131
23. Haskell SG, Heapy A, Reid MC, Papas RK, Kerns RD. The prevalence and age-related characteristics of pain in a sample of women veterans receiving primary care. J Womens Health (Larchmt). 2006;15(7):862-869. doi:10.1089/jwh.2006.15.862
24. Driscoll MA, Higgins D, Shamaskin-Garroway A, et al. Examining gender as a correlate of self-reported pain treatment use among recent service veterans with deployment-related musculoskeletal disorders. Pain Med. 2017;18(9):1767-1777. doi:10.1093/pm/pnx023
25. Weimer MB, Macey TA, Nicolaidis C, Dobscha SK, Duckart JP, Morasco BJ. Sex differences in the medical care of VA patients with chronic non-cancer pain. Pain Med. 2013;14(12):1839-1847. doi:10.1111/pme.12177
26. Stubbs D, Krebs E, Bair M, et al. Sex differences in pain and pain-related disability among primary care patients with chronic musculoskeletal pain. Pain Med. 2010;11(2):232-239. doi:10.1111/j.1526-4637.2009.00760.x
27. Keogh E, McCracken LM, Eccleston C. Gender moderates the association between depression and disability in chronic pain patients. Eur J Pain. 2006;10(5):413-422. doi:10.1016/j.ejpain.2005.05.007
28. Miake-Lye IM, Mak S, Lee J, et al. Massage for pain: an evidence map. J Altern Complement Med. 2019;25(5):475-502. doi:10.1089/acm.2018.0282
29. Cherkin DC, Sherman KJ, Kahn J, et al. A comparison of the effects of 2 types of massage and usual care on chronic low back pain: a randomized, controlled trial. Ann Intern Med. 2011;155(1):1-9. doi:10.7326/0003-4819-155-1-201107050-00002
30. Sherman KJ, Cook AJ, Wellman RD, et al. Five-week outcomes from a dosing trial of therapeutic massage for chronic neck pain. Ann Fam Med. 2014;12(2):112-120. doi:10.1370/afm.1602
31. Perlman AI, Sabina A, Williams AL, Njike VY, Katz DL. Massage therapy for osteoarthritis of the knee: a randomized controlled trial. Arch Intern Med. 2006;166(22):2533-2538. doi:10.1001/archinte.166.22.2533
32. Perlman A, Fogerite SG, Glass O, et al. Efficacy and safety of massage for osteoarthritis of the knee: a randomized clinical trial. J Gen Intern Med. 2019;34(3):379-386. doi:10.1007/s11606-018-4763-5
33. Skelly AC, Chou R, Dettori JR, et al. Noninvasive Nonpharmacological Treatment for Chronic Pain: A Systematic Review Update. Comparative Effectiveness Review. No. 227. Agency for Healthcare Research and Quality; 2020. doi:10.23970/AHRQEPCCER227
34. Moyer CA, Rounds J, Hannum JW. A meta-analysis of massage therapy research. Psychol Bull. 2004;130(1):3-18. doi:10.1037/0033-2909.130.1.3
35. Field T, Hernandez-Reif M, Diego M, Schanberg S, Kuhn C. Cortisol decreases and serotonin and dopamine increase following massage therapy. Int J Neurosci. 2005;115(10):1397-1413. doi:10.1080/ 00207450590956459
36. Li Q, Becker B, Wernicke J, et al. Foot massage evokes oxytocin release and activation of orbitofrontal cortex and superior temporal sulcus. Psychoneuroendocrinology. 2019;101:193-203. doi:10.1016/j.psyneuen.2018.11.016
37. Eaves ER, Sherman KJ, Ritenbaugh C, et al. A qualitative study of changes in expectations over time among patients with chronic low back pain seeking four CAM therapies. BMC Complement Altern Med. 2015;15:12. Published 2015 Feb 5. doi:10.1186/s12906-015-0531-9
38. Bishop FL, Lauche R, Cramer H, et al. Health behavior change and complementary medicine use: National Health Interview Survey 2012. Medicina (Kaunas). 2019;55(10):632. Published 2019 Sep 24. doi:10.3390/medicina55100632
39. Driscoll MA, Knobf MT, Higgins DM, Heapy A, Lee A, Haskell S. Patient experiences navigating chronic pain management in an integrated health care system: a qualitative investigation of women and men. Pain Med. 2018;19(suppl 1):S19-S29. doi:10.1093/pm/pny139
40. Denneson LM, Corson K, Dobscha SK. Complementary and alternative medicine use among veterans with chronic noncancer pain. J Rehabil Res Dev. 2011;48(9):1119-1128. doi:10.1682/jrrd.2010.12.0243
41. Taylor SL, Herman PM, Marshall NJ, et al. Use of complementary and integrated health: a retrospective analysis of U.S. veterans with chronic musculoskeletal pain nationally. J Altern Complement Med. 2019;25(1):32-39. doi:10.1089/acm.2018.0276
42. Evans EA, Herman PM, Washington DL, et al. Gender differences in use of complementary and integrative health by U.S. military veterans with chronic musculoskeletal pain. Womens Health Issues. 2018;28(5):379-386. doi:10.1016/j.whi.2018.07.003
43. Reinhard MJ, Nassif TH, Bloeser K, et al. CAM utilization among OEF/OIF veterans: findings from the National Health Study for a New Generation of US Veterans. Med Care. 2014;52(12)(suppl 5):S45-S49. doi:10.1097/MLR.0000000000000229
44. Herman PM, Yuan AH, Cefalu MS, et al. The use of complementary and integrative health approaches for chronic musculoskeletal pain in younger US Veterans: An economic evaluation. PLoS One. 2019;14(6):e0217831. Published 2019 Jun 5. doi:10.1371/journal.pone.0217831
45. Jonas WB, Schoomaker EB. Pain and opioids in the military: we must do better. JAMA Intern Med. 2014;174(8):1402-1403. doi:10.1001/jamainternmed.2014.2114
46. Han B, Compton WM, Blanco C, Crane E, Lee J, Jones CM. Prescription opioid use, misuse, and use disorders in U.S. adults: 2015 National Survey on Drug Use and Health. Ann Intern Med. 2017;167(5):293-301. doi:10.7326/M17-0865
There are approximately 2 million female veterans in the United States, representing about 10% of the veteran population.1 In 2015, 456,000 female veterans used the US Department of Veterans Affairs (VA) health care services. The VA predicts an increase in utilization over the next 20 years.2
Female veterans are more likely to have musculoskeletal disorder multimorbidity compared with male veterans and have higher rates of depressive and bipolar disorders, anxiety, and posttraumatic stress disorder (PTSD).3,4 Compared with male veterans, female veterans are younger, more likely to be unmarried and to have served during the wars in Iraq and Afghanistan.3 Fifty-five percent of women veterans vs 41% of men veterans have a service-connected disability, and a greater percentage of women veterans have a service connection rating > 50%.5 The top service-connected disabilities for women veterans are PTSD, major depressive disorder, migraines, and lumbosacral or cervical strain.2 In addition, one-third of women veterans using VA health care report experiencing military sexual trauma (MST).6 Military service may impact the health of female veterans both physically and mentally. Providing treatments and programs to improve their health and their health care experience are current VA priorities.
The VA is changing the way health care is conceptualized and delivered by implementing a holistic model of care known as Whole Health, which seeks to empower and equip patients to take charge of their health, blending conventional medicine with self-care and complementary and integrative health (CIH) approaches, such as massage therapy, yoga, acupuncture, and meditation.7 CIH therapies can help improve physical and mental health with little to no adverse effects.8-10
As part of the Whole Health initiative at the VA Ann Arbor Healthcare System (VAAAHS) in Michigan, the massage program was expanded in 2017 to offer relaxation massages to female veterans attending the women’s health clinic, which provides gynecologic care. Patients visiting a gynecology clinic often experience anxiety and pain related to invasive procedures and examinations. This is especially true for female veterans who experienced MST.11
VAAAHS has 1 staff massage therapist (MT). To expand the program to the women’s health clinic, volunteer licensed MTs were recruited and trained in specific procedures by the staff MT.
Several studies have demonstrated the effect of therapeutic massage on pain and anxiety in predominantly male veteran study populations, including veterans needing postsurgical and palliative care as well as those experiencing chronic pain and knee osteoarthritis.12-16 Little is known about the effects of massage therapy on female veterans. The purpose of this pilot study was to examine the effects of massage therapy among female veterans participating in the women’s health massage program.
Methods
The setting for this pre-post intervention study was VAAAHS. Veterans were called in advance by clinic staff and scheduled for 60-minute appointments either before or after their clinic appointment, depending on availability. MTs were instructed to provide relaxation massage using Swedish massage techniques with moderate pressure, avoiding deep pressure techniques.
The volunteer MTs gave the participants a survey to provide comments and to rate baseline pain and other symptoms prior to and following the massage. The MT left the room to provide privacy while completing the survey. The staff included the symptom data in the massage note as clinical outcomes and entered them into the electronic health record. Massages were given from October 1, 2017 to June 30, 2018. Data including symptom scores, demographics, the presence of chronic pain, mental health diagnoses, patient comments, and opioid use were abstracted from the electronic health record by 2 members of the study team and entered into an Excel database. This study was approved by the VAAAHS Institutional Review Board.
Study Measures
Pain intensity, pain unpleasantness (the affective component of pain), anxiety, shortness of breath, relaxation, and inner peace were rated pre- and postmassage on a 0 to 10 scale. Shortness of breath was included due to the relationship between breathing and anxiety. Inner peace was assessed to measure the calming effects of massage therapy. Beck and colleagues found the concept of inner peace was an important outcome of massage therapy.17 The scale anchors for pain intensity were “no pain” and “severe pain”; and “not at all unpleasant” and “as unpleasant as it can be” for pain unpleasantness. For anxiety, the anchors were “no anxiety” and “as anxious as I can be.” Anchors for relaxation and inner peace were reversed so that a 0 indicated low relaxation and inner peace while a 10 indicated the highest state of relaxation and inner peace.
Chronic pain was defined as pain existing for > 3 months. A history of chronic pain was determined from a review and synthesis of primary care and specialty care recorded diagnoses, patient concerns, and service-connected disabilities. The diagnoses included lumbosacral or cervical strain, chronic low back, joint (knee, shoulder, hip, ankle), neck, or pelvic pain, fibromyalgia, headache, migraine, osteoarthritis, and myofascial pain syndrome. The presence of mental health conditions, including depression, anxiety, bipolar disorders, and PTSD, were similarly determined by a review of mental health clinical notes. Sex was determined from the gynecology note.
Statistical Analysis
Means and medians were calculated for short-term changes in symptom scores. Due to skewness in the short-term changes, significance was tested using a nonparametric sign test. Significance was adjusted using the Bonferroni correction to protect the overall type I error level at 5% from multiple testing. We also assessed for differences in symptom changes in 4 subgroups, using an unadjusted general linear model: those with (1) chronic pain vs without; (2) an anxiety diagnosis vs without; (3) depression vs without; and (4) a PTSD diagnosis vs without. Data were analyzed using SPSS 25 and SAS 9.4.
Results
Results are based on the first massage received by 96 unique individuals (Table 1). Fifty-one (53%) patients were aged 21 to 40 years, and 45 (47%) were aged ≥ 41 years. Most participants (80%) had had a previous massage. Seven (7%) participants were currently on prescription opioids; 76 (79%) participants had a history of one or more chronic pain diagnoses (eg, back pain, migraine headaches, fibromyalgia) and 78 (81%) had a history of a mental health diagnosis (eg, depression, anxiety, PTSD). Massage sessions ranged from 30 to 60 minutes; most patients received massage therapy for 50 minutes.
Prior to massage, mean scores were 3.9 pain intensity, 3.7 pain unpleasantness, 3.8 anxiety, 1.0 shortness of breath, 4.0 relaxation, and 4.2 inner peace. Short-term changes in symptom scores are shown in Table 2. The mean score for pain intensity decreased by 1.9 points, pain unpleasantness by 2.0 points, anxiety by 2.4 points. The greatest change occurred for relaxation, which increased by 4.3 points. All changes in symptoms were statistically significant (P < .001). For subgroup comparisons
Verbal feedback and written comments about the massage experience were all favorable: No adverse events were reported.
Discussion
Massage therapy may be a useful treatment for female veterans experiencing chronic pain, anxiety disorders, depression, or situational anxiety related to gynecologic procedures. After receiving a relaxation massage, female veterans reported decreased pain intensity, pain unpleasantness, and anxiety while reporting increased relaxation and feelings of inner peace. The effects of massage were consistent for all the symptoms or characteristics assessed, suggesting that massage may act on the body in multiple ways.
These changes parallel those seen in a palliative care population primarily composed of male veterans.14 However, the female veterans in this cohort experienced greater changes in relaxation and feelings of inner peace, which may be partly due to relief of tension related to an upcoming stressful appointment. The large mean decrease in anxiety level among female veterans with PTSD is notable as well as the larger increase in inner peace in those with chronic pain.
Many patients expressed their gratitude for the massage and interest in having access to more massage therapy. Female patients who have experienced sexual trauma or other trauma may especially benefit from massage prior to painful, invasive gynecologic procedures. Anecdotally, 2 nurse chaperones in the clinic mentioned separately to the massage program supervisor that the massages helped some very anxious women better tolerate an invasive procedure that would have been otherwise extremely difficult.
Female veterans are more likely to have musculoskeletal issues after deployment and have higher rates of anxiety, PTSD, and depression compared with those of male veterans.3,4,18,19 Determining relationships between and causes of chronic pain, depression, and PTSD is very challenging but the increased prevalence of chronic pain and comorbid mental health conditions in female veterans may be partially related to MST or other trauma experiences.20-22 Female veterans are most likely to have more than one source of chronic pain.23-25 Female patients with chronic musculoskeletal pain report more pain-related disability.26 Furthermore, greater disability in the context of depression is reported by women with pain compared with those of men.27 Most (78%) female veterans in a primary care population reported chronic pain.23 Similarly, 79% of the female veterans in this study population had chronic pain and 81% had a history of mental health disorders, including depression, anxiety, and PTSD.
Studies have shown that massage therapy improves pain in populations experiencing chronic low back, neck, and knee pain.28-32 A 2020 Agency for Healthcare Research and Quality review determined there is some evidence that massage therapy is helpful for chronic low back and neck pain and fibromyalgia.33 Research also has demonstrated that massage reduces anxiety and depression in several different population types.13,34,35 Li and colleagues showed that foot massage increased oxytocin levels in healthy males.36 Although further research is needed to determine the mechanisms of massage therapy, there are important physiologic effects. Unlike most medications, massage therapy is unique in that it can impact health and well-being through multiple mechanisms; for example, by reducing pain, improving mood, providing a sense of social connection and/or improving mobility.
Patients using CIH therapies report greater awareness of the need for ongoing engagement in their own care and health behavior changes.37,38
Driscoll and colleagues reported that women veterans are interested in conservative treatment for their chronic musculoskeletal pain and are open to using CIH therapies.39 Research suggests that veterans are interested in and, in some cases, already using massage therapy.23,40-43 Access to massage therapy and other CIH therapies offers patients choice and control over the types and timing of therapy they receive, exemplified by the 80% of patients in our study who previously received a massage and sought another before a potentially stressful situation.
Access to massage therapy or other CIH therapies may reduce the need for more expensive procedures. Although research on the cost-effectiveness of massage therapy is limited, Herman and colleagues did an economic evaluation of CIH therapies in a veteran population, finding that CIH users had lower annual health care costs and lower pain in the year after CIH started. Sensitivity analyses indicated similar results for acupuncture, chiropractic care, and massage but higher costs for those with 8 or more visits.44
The prevalence of comorbid mental health conditions with MSD suggests that female veterans may benefit from multidisciplinary treatment of pain and depression.3,26 Women-centered programs would be both encouraging and validating to women.39 Massage therapy can be combined with physical therapy, yoga, tai chi, and meditation programs to improve pain, anxiety, strength, and flexibility and can be incorporated into a multimodal treatment plan. Likewise, other subpopulations of female veterans with chronic pain, mental health conditions, or cancer could be targeted with multidisciplinary programs that include massage therapy.
Limitations
This study has several limitations including lack of a control group, a self-selected population, the lack of objective biochemical measurements, and possible respondent bias to please the MTs. Eighty percent had previously experienced massage therapy and may have been biased toward the effects of massage before receiving the intervention. The first report of the effects of massage therapy in an exclusively female veteran population is a major strength of this study.
Further research including randomized controlled trials is needed, especially in populations with coexisting chronic pain and mental health disorders, as is exploring the acceptability of massage therapy for female veterans with MST. Finding viable alternatives to medications has become even more important as the nation addresses the challenge of the opioid crisis.45,46
Conclusions
Female veterans are increasingly seeking VA health care.
Acknowledgments
The authors express our gratitude to the Women Veteran Program Manager, Cheryl Allen, RN; Massage Therapists Denise McGee and Kimberly Morro; Dara Ganoczy, MPH, for help with statistical analysis; and Mark Hausman, MD, for leadership support.
There are approximately 2 million female veterans in the United States, representing about 10% of the veteran population.1 In 2015, 456,000 female veterans used the US Department of Veterans Affairs (VA) health care services. The VA predicts an increase in utilization over the next 20 years.2
Female veterans are more likely to have musculoskeletal disorder multimorbidity compared with male veterans and have higher rates of depressive and bipolar disorders, anxiety, and posttraumatic stress disorder (PTSD).3,4 Compared with male veterans, female veterans are younger, more likely to be unmarried and to have served during the wars in Iraq and Afghanistan.3 Fifty-five percent of women veterans vs 41% of men veterans have a service-connected disability, and a greater percentage of women veterans have a service connection rating > 50%.5 The top service-connected disabilities for women veterans are PTSD, major depressive disorder, migraines, and lumbosacral or cervical strain.2 In addition, one-third of women veterans using VA health care report experiencing military sexual trauma (MST).6 Military service may impact the health of female veterans both physically and mentally. Providing treatments and programs to improve their health and their health care experience are current VA priorities.
The VA is changing the way health care is conceptualized and delivered by implementing a holistic model of care known as Whole Health, which seeks to empower and equip patients to take charge of their health, blending conventional medicine with self-care and complementary and integrative health (CIH) approaches, such as massage therapy, yoga, acupuncture, and meditation.7 CIH therapies can help improve physical and mental health with little to no adverse effects.8-10
As part of the Whole Health initiative at the VA Ann Arbor Healthcare System (VAAAHS) in Michigan, the massage program was expanded in 2017 to offer relaxation massages to female veterans attending the women’s health clinic, which provides gynecologic care. Patients visiting a gynecology clinic often experience anxiety and pain related to invasive procedures and examinations. This is especially true for female veterans who experienced MST.11
VAAAHS has 1 staff massage therapist (MT). To expand the program to the women’s health clinic, volunteer licensed MTs were recruited and trained in specific procedures by the staff MT.
Several studies have demonstrated the effect of therapeutic massage on pain and anxiety in predominantly male veteran study populations, including veterans needing postsurgical and palliative care as well as those experiencing chronic pain and knee osteoarthritis.12-16 Little is known about the effects of massage therapy on female veterans. The purpose of this pilot study was to examine the effects of massage therapy among female veterans participating in the women’s health massage program.
Methods
The setting for this pre-post intervention study was VAAAHS. Veterans were called in advance by clinic staff and scheduled for 60-minute appointments either before or after their clinic appointment, depending on availability. MTs were instructed to provide relaxation massage using Swedish massage techniques with moderate pressure, avoiding deep pressure techniques.
The volunteer MTs gave the participants a survey to provide comments and to rate baseline pain and other symptoms prior to and following the massage. The MT left the room to provide privacy while completing the survey. The staff included the symptom data in the massage note as clinical outcomes and entered them into the electronic health record. Massages were given from October 1, 2017 to June 30, 2018. Data including symptom scores, demographics, the presence of chronic pain, mental health diagnoses, patient comments, and opioid use were abstracted from the electronic health record by 2 members of the study team and entered into an Excel database. This study was approved by the VAAAHS Institutional Review Board.
Study Measures
Pain intensity, pain unpleasantness (the affective component of pain), anxiety, shortness of breath, relaxation, and inner peace were rated pre- and postmassage on a 0 to 10 scale. Shortness of breath was included due to the relationship between breathing and anxiety. Inner peace was assessed to measure the calming effects of massage therapy. Beck and colleagues found the concept of inner peace was an important outcome of massage therapy.17 The scale anchors for pain intensity were “no pain” and “severe pain”; and “not at all unpleasant” and “as unpleasant as it can be” for pain unpleasantness. For anxiety, the anchors were “no anxiety” and “as anxious as I can be.” Anchors for relaxation and inner peace were reversed so that a 0 indicated low relaxation and inner peace while a 10 indicated the highest state of relaxation and inner peace.
Chronic pain was defined as pain existing for > 3 months. A history of chronic pain was determined from a review and synthesis of primary care and specialty care recorded diagnoses, patient concerns, and service-connected disabilities. The diagnoses included lumbosacral or cervical strain, chronic low back, joint (knee, shoulder, hip, ankle), neck, or pelvic pain, fibromyalgia, headache, migraine, osteoarthritis, and myofascial pain syndrome. The presence of mental health conditions, including depression, anxiety, bipolar disorders, and PTSD, were similarly determined by a review of mental health clinical notes. Sex was determined from the gynecology note.
Statistical Analysis
Means and medians were calculated for short-term changes in symptom scores. Due to skewness in the short-term changes, significance was tested using a nonparametric sign test. Significance was adjusted using the Bonferroni correction to protect the overall type I error level at 5% from multiple testing. We also assessed for differences in symptom changes in 4 subgroups, using an unadjusted general linear model: those with (1) chronic pain vs without; (2) an anxiety diagnosis vs without; (3) depression vs without; and (4) a PTSD diagnosis vs without. Data were analyzed using SPSS 25 and SAS 9.4.
Results
Results are based on the first massage received by 96 unique individuals (Table 1). Fifty-one (53%) patients were aged 21 to 40 years, and 45 (47%) were aged ≥ 41 years. Most participants (80%) had had a previous massage. Seven (7%) participants were currently on prescription opioids; 76 (79%) participants had a history of one or more chronic pain diagnoses (eg, back pain, migraine headaches, fibromyalgia) and 78 (81%) had a history of a mental health diagnosis (eg, depression, anxiety, PTSD). Massage sessions ranged from 30 to 60 minutes; most patients received massage therapy for 50 minutes.
Prior to massage, mean scores were 3.9 pain intensity, 3.7 pain unpleasantness, 3.8 anxiety, 1.0 shortness of breath, 4.0 relaxation, and 4.2 inner peace. Short-term changes in symptom scores are shown in Table 2. The mean score for pain intensity decreased by 1.9 points, pain unpleasantness by 2.0 points, anxiety by 2.4 points. The greatest change occurred for relaxation, which increased by 4.3 points. All changes in symptoms were statistically significant (P < .001). For subgroup comparisons
Verbal feedback and written comments about the massage experience were all favorable: No adverse events were reported.
Discussion
Massage therapy may be a useful treatment for female veterans experiencing chronic pain, anxiety disorders, depression, or situational anxiety related to gynecologic procedures. After receiving a relaxation massage, female veterans reported decreased pain intensity, pain unpleasantness, and anxiety while reporting increased relaxation and feelings of inner peace. The effects of massage were consistent for all the symptoms or characteristics assessed, suggesting that massage may act on the body in multiple ways.
These changes parallel those seen in a palliative care population primarily composed of male veterans.14 However, the female veterans in this cohort experienced greater changes in relaxation and feelings of inner peace, which may be partly due to relief of tension related to an upcoming stressful appointment. The large mean decrease in anxiety level among female veterans with PTSD is notable as well as the larger increase in inner peace in those with chronic pain.
Many patients expressed their gratitude for the massage and interest in having access to more massage therapy. Female patients who have experienced sexual trauma or other trauma may especially benefit from massage prior to painful, invasive gynecologic procedures. Anecdotally, 2 nurse chaperones in the clinic mentioned separately to the massage program supervisor that the massages helped some very anxious women better tolerate an invasive procedure that would have been otherwise extremely difficult.
Female veterans are more likely to have musculoskeletal issues after deployment and have higher rates of anxiety, PTSD, and depression compared with those of male veterans.3,4,18,19 Determining relationships between and causes of chronic pain, depression, and PTSD is very challenging but the increased prevalence of chronic pain and comorbid mental health conditions in female veterans may be partially related to MST or other trauma experiences.20-22 Female veterans are most likely to have more than one source of chronic pain.23-25 Female patients with chronic musculoskeletal pain report more pain-related disability.26 Furthermore, greater disability in the context of depression is reported by women with pain compared with those of men.27 Most (78%) female veterans in a primary care population reported chronic pain.23 Similarly, 79% of the female veterans in this study population had chronic pain and 81% had a history of mental health disorders, including depression, anxiety, and PTSD.
Studies have shown that massage therapy improves pain in populations experiencing chronic low back, neck, and knee pain.28-32 A 2020 Agency for Healthcare Research and Quality review determined there is some evidence that massage therapy is helpful for chronic low back and neck pain and fibromyalgia.33 Research also has demonstrated that massage reduces anxiety and depression in several different population types.13,34,35 Li and colleagues showed that foot massage increased oxytocin levels in healthy males.36 Although further research is needed to determine the mechanisms of massage therapy, there are important physiologic effects. Unlike most medications, massage therapy is unique in that it can impact health and well-being through multiple mechanisms; for example, by reducing pain, improving mood, providing a sense of social connection and/or improving mobility.
Patients using CIH therapies report greater awareness of the need for ongoing engagement in their own care and health behavior changes.37,38
Driscoll and colleagues reported that women veterans are interested in conservative treatment for their chronic musculoskeletal pain and are open to using CIH therapies.39 Research suggests that veterans are interested in and, in some cases, already using massage therapy.23,40-43 Access to massage therapy and other CIH therapies offers patients choice and control over the types and timing of therapy they receive, exemplified by the 80% of patients in our study who previously received a massage and sought another before a potentially stressful situation.
Access to massage therapy or other CIH therapies may reduce the need for more expensive procedures. Although research on the cost-effectiveness of massage therapy is limited, Herman and colleagues did an economic evaluation of CIH therapies in a veteran population, finding that CIH users had lower annual health care costs and lower pain in the year after CIH started. Sensitivity analyses indicated similar results for acupuncture, chiropractic care, and massage but higher costs for those with 8 or more visits.44
The prevalence of comorbid mental health conditions with MSD suggests that female veterans may benefit from multidisciplinary treatment of pain and depression.3,26 Women-centered programs would be both encouraging and validating to women.39 Massage therapy can be combined with physical therapy, yoga, tai chi, and meditation programs to improve pain, anxiety, strength, and flexibility and can be incorporated into a multimodal treatment plan. Likewise, other subpopulations of female veterans with chronic pain, mental health conditions, or cancer could be targeted with multidisciplinary programs that include massage therapy.
Limitations
This study has several limitations including lack of a control group, a self-selected population, the lack of objective biochemical measurements, and possible respondent bias to please the MTs. Eighty percent had previously experienced massage therapy and may have been biased toward the effects of massage before receiving the intervention. The first report of the effects of massage therapy in an exclusively female veteran population is a major strength of this study.
Further research including randomized controlled trials is needed, especially in populations with coexisting chronic pain and mental health disorders, as is exploring the acceptability of massage therapy for female veterans with MST. Finding viable alternatives to medications has become even more important as the nation addresses the challenge of the opioid crisis.45,46
Conclusions
Female veterans are increasingly seeking VA health care.
Acknowledgments
The authors express our gratitude to the Women Veteran Program Manager, Cheryl Allen, RN; Massage Therapists Denise McGee and Kimberly Morro; Dara Ganoczy, MPH, for help with statistical analysis; and Mark Hausman, MD, for leadership support.
1. US Department of Veteran Affairs, National Center for Veterans Analysis and Statistics. Veteran population. Updated April 14, 2021. Accessed January 6, 2022. https://www.va.gov/vetdata/veteran_population.asp
2. US Department of Veteran Affairs. Women veterans report: the past, present, and future of women veterans. Published February 2017. Accessed January 6, 2022. https://www.va.gov/vetdata/docs/specialreports/women_veterans_2015_final.pdf
3. Higgins DM, Fenton BT, Driscoll MA, et al. Gender differences in demographic and clinical correlates among veterans with musculoskeletal disorders. Womens Health Issues. 2017;27(4):463-470. doi:10.1016/j.whi.2017.01.008
4. Lehavot K, Goldberg SB, Chen JA, et al. Do trauma type, stressful life events, and social support explain women veterans’ high prevalence of PTSD?. Soc Psychiatry Psychiatr Epidemiol. 2018;53(9):943-953. doi:10.1007/s00127-018-1550-x
5. Levander XA, Overland MK. Care of women veterans. Med Clin North Am. 2015;99(3):651-662. doi:10.1016/j.mcna.2015.01.013
6. US Department of Veteran Affairs. Facts and statistics about women veterans. Updated May 28. 2020. Accessed January 6, 2022. https://www.womenshealth.va.gov/womenshealth/latestinformation/facts.asp
7. Krejci LP, Carter K, Gaudet T. Whole health: the vision and implementation of personalized, proactive, patient-driven health care for veterans. Med Care. 2014;52(12)(suppl 5):S5-S8. doi:10.1097/MLR.0000000000000226
8. Elwy AR, Taylor SL, Zhao S, et al. Participating in complementary and integrative health approaches is associated with veterans’ patient-reported outcomes over time. Med Care. 2020;58:S125-S132. doi:10.1097/MLR.0000000000001357
9. Smeeding SJ, Bradshaw DH, Kumpfer K, Trevithick S, Stoddard GJ. Outcome evaluation of the Veterans Affairs Salt Lake City Integrative Health Clinic for chronic pain and stress-related depression, anxiety, and post-traumatic stress disorder. J Altern Complement Med. 2010;16(8):823-835. doi:10.1089/acm.2009.0510
10. Hull A, Brooks Holliday S, Eickhoff C, et al. Veteran participation in the integrative health and wellness program: impact on self-reported mental and physical health outcomes. Psychol Serv. 2019;16(3):475-483. doi:10.1037/ser0000192
11. Zephyrin LC. Reproductive health management for the care of women veterans [published correction appears in Obstet Gynecol. 2016 Mar;127(3):605]. Obstet Gynecol. 2016;127(2):383-392. doi:10.1097/AOG.0000000000001252
12. Piotrowski MM, Paterson C, Mitchinson A, Kim HM, Kirsh M, Hinshaw DB. Massage as adjuvant therapy in the management of acute postoperative pain: a preliminary study in men. J Am Coll Surg. 2003;197(6):1037-1046. doi:10.1016/j.jamcollsurg.2003.07.020
13. Mitchinson AR, Kim HM, Rosenberg JM, et al. Acute postoperative pain management using massage as an adjuvant therapy: a randomized trial. Arch Surg. 2007;142(12):1158-1167. doi:10.1001/archsurg.142.12.1158
14. Mitchinson A, Fletcher CE, Kim HM, Montagnini M, Hinshaw DB. Integrating massage therapy within the palliative care of veterans with advanced illnesses: an outcome study. Am J Hosp Palliat Care. 2014;31(1):6-12. doi:10.1177/1049909113476568
15. Fletcher CE, Mitchinson AR, Trumble EL, Hinshaw DB, Dusek JA. Perceptions of other integrative health therapies by veterans with pain who are receiving massage. J Rehabil Res Dev. 2016;53(1):117-126. doi:10.1682/JRRD.2015.01.0015
16. Juberg M, Jerger KK, Allen KD, Dmitrieva NO, Keever T, Perlman AI. Pilot study of massage in veterans with knee osteoarthritis. J Altern Complement Med. 2015;21(6):333-338. doi:10.1089/acm.2014.0254
17. Beck I, Runeson I, Blomqvist K. To find inner peace: soft massage as an established and integrated part of palliative care. Int J Palliate Nurse. 2009;15(11):541-545. doi: 10.12968/ijpn.2009.15.11.45493
18. Haskell SG, Ning Y, Krebs E, et al. Prevalence of painful musculoskeletal conditions in female and male veterans in 7 years after return from deployment in Operation Enduring Freedom/Operation Iraqi Freedom. Clin J Pain. 2012;28(2):163-167. doi:10.1097/AJP.0b013e318223d951
19. Maguen S, Ren L, Bosch JO, Marmar CR, Seal KH. Gender differences in mental health diagnoses among Iraq and Afghanistan veterans enrolled in veterans affairs health care. Am J Public Health. 2010;100(12):2450-2456. doi:10.2105/AJPH.2009.166165
20. Outcalt SD, Kroenke K, Krebs EE, et al. Chronic pain and comorbid mental health conditions: independent associations of posttraumatic stress disorder and depression with pain, disability, and quality of life. J Behav Med. 2015;38(3):535-543. doi:10.1007/s10865-015-9628-3
21. Gibson CJ, Maguen S, Xia F, Barnes DE, Peltz CB, Yaffe K. Military sexual trauma in older women veterans: prevalence and comorbidities. J Gen Intern Med. 2020;35(1):207-213. doi:10.1007/s11606-019-05342-7
22. Tan G, Teo I, Srivastava D, et al. Improving access to care for women veterans suffering from chronic pain and depression associated with trauma. Pain Med. 2013;14(7):1010-1020. doi:10.1111/pme.12131
23. Haskell SG, Heapy A, Reid MC, Papas RK, Kerns RD. The prevalence and age-related characteristics of pain in a sample of women veterans receiving primary care. J Womens Health (Larchmt). 2006;15(7):862-869. doi:10.1089/jwh.2006.15.862
24. Driscoll MA, Higgins D, Shamaskin-Garroway A, et al. Examining gender as a correlate of self-reported pain treatment use among recent service veterans with deployment-related musculoskeletal disorders. Pain Med. 2017;18(9):1767-1777. doi:10.1093/pm/pnx023
25. Weimer MB, Macey TA, Nicolaidis C, Dobscha SK, Duckart JP, Morasco BJ. Sex differences in the medical care of VA patients with chronic non-cancer pain. Pain Med. 2013;14(12):1839-1847. doi:10.1111/pme.12177
26. Stubbs D, Krebs E, Bair M, et al. Sex differences in pain and pain-related disability among primary care patients with chronic musculoskeletal pain. Pain Med. 2010;11(2):232-239. doi:10.1111/j.1526-4637.2009.00760.x
27. Keogh E, McCracken LM, Eccleston C. Gender moderates the association between depression and disability in chronic pain patients. Eur J Pain. 2006;10(5):413-422. doi:10.1016/j.ejpain.2005.05.007
28. Miake-Lye IM, Mak S, Lee J, et al. Massage for pain: an evidence map. J Altern Complement Med. 2019;25(5):475-502. doi:10.1089/acm.2018.0282
29. Cherkin DC, Sherman KJ, Kahn J, et al. A comparison of the effects of 2 types of massage and usual care on chronic low back pain: a randomized, controlled trial. Ann Intern Med. 2011;155(1):1-9. doi:10.7326/0003-4819-155-1-201107050-00002
30. Sherman KJ, Cook AJ, Wellman RD, et al. Five-week outcomes from a dosing trial of therapeutic massage for chronic neck pain. Ann Fam Med. 2014;12(2):112-120. doi:10.1370/afm.1602
31. Perlman AI, Sabina A, Williams AL, Njike VY, Katz DL. Massage therapy for osteoarthritis of the knee: a randomized controlled trial. Arch Intern Med. 2006;166(22):2533-2538. doi:10.1001/archinte.166.22.2533
32. Perlman A, Fogerite SG, Glass O, et al. Efficacy and safety of massage for osteoarthritis of the knee: a randomized clinical trial. J Gen Intern Med. 2019;34(3):379-386. doi:10.1007/s11606-018-4763-5
33. Skelly AC, Chou R, Dettori JR, et al. Noninvasive Nonpharmacological Treatment for Chronic Pain: A Systematic Review Update. Comparative Effectiveness Review. No. 227. Agency for Healthcare Research and Quality; 2020. doi:10.23970/AHRQEPCCER227
34. Moyer CA, Rounds J, Hannum JW. A meta-analysis of massage therapy research. Psychol Bull. 2004;130(1):3-18. doi:10.1037/0033-2909.130.1.3
35. Field T, Hernandez-Reif M, Diego M, Schanberg S, Kuhn C. Cortisol decreases and serotonin and dopamine increase following massage therapy. Int J Neurosci. 2005;115(10):1397-1413. doi:10.1080/ 00207450590956459
36. Li Q, Becker B, Wernicke J, et al. Foot massage evokes oxytocin release and activation of orbitofrontal cortex and superior temporal sulcus. Psychoneuroendocrinology. 2019;101:193-203. doi:10.1016/j.psyneuen.2018.11.016
37. Eaves ER, Sherman KJ, Ritenbaugh C, et al. A qualitative study of changes in expectations over time among patients with chronic low back pain seeking four CAM therapies. BMC Complement Altern Med. 2015;15:12. Published 2015 Feb 5. doi:10.1186/s12906-015-0531-9
38. Bishop FL, Lauche R, Cramer H, et al. Health behavior change and complementary medicine use: National Health Interview Survey 2012. Medicina (Kaunas). 2019;55(10):632. Published 2019 Sep 24. doi:10.3390/medicina55100632
39. Driscoll MA, Knobf MT, Higgins DM, Heapy A, Lee A, Haskell S. Patient experiences navigating chronic pain management in an integrated health care system: a qualitative investigation of women and men. Pain Med. 2018;19(suppl 1):S19-S29. doi:10.1093/pm/pny139
40. Denneson LM, Corson K, Dobscha SK. Complementary and alternative medicine use among veterans with chronic noncancer pain. J Rehabil Res Dev. 2011;48(9):1119-1128. doi:10.1682/jrrd.2010.12.0243
41. Taylor SL, Herman PM, Marshall NJ, et al. Use of complementary and integrated health: a retrospective analysis of U.S. veterans with chronic musculoskeletal pain nationally. J Altern Complement Med. 2019;25(1):32-39. doi:10.1089/acm.2018.0276
42. Evans EA, Herman PM, Washington DL, et al. Gender differences in use of complementary and integrative health by U.S. military veterans with chronic musculoskeletal pain. Womens Health Issues. 2018;28(5):379-386. doi:10.1016/j.whi.2018.07.003
43. Reinhard MJ, Nassif TH, Bloeser K, et al. CAM utilization among OEF/OIF veterans: findings from the National Health Study for a New Generation of US Veterans. Med Care. 2014;52(12)(suppl 5):S45-S49. doi:10.1097/MLR.0000000000000229
44. Herman PM, Yuan AH, Cefalu MS, et al. The use of complementary and integrative health approaches for chronic musculoskeletal pain in younger US Veterans: An economic evaluation. PLoS One. 2019;14(6):e0217831. Published 2019 Jun 5. doi:10.1371/journal.pone.0217831
45. Jonas WB, Schoomaker EB. Pain and opioids in the military: we must do better. JAMA Intern Med. 2014;174(8):1402-1403. doi:10.1001/jamainternmed.2014.2114
46. Han B, Compton WM, Blanco C, Crane E, Lee J, Jones CM. Prescription opioid use, misuse, and use disorders in U.S. adults: 2015 National Survey on Drug Use and Health. Ann Intern Med. 2017;167(5):293-301. doi:10.7326/M17-0865
1. US Department of Veteran Affairs, National Center for Veterans Analysis and Statistics. Veteran population. Updated April 14, 2021. Accessed January 6, 2022. https://www.va.gov/vetdata/veteran_population.asp
2. US Department of Veteran Affairs. Women veterans report: the past, present, and future of women veterans. Published February 2017. Accessed January 6, 2022. https://www.va.gov/vetdata/docs/specialreports/women_veterans_2015_final.pdf
3. Higgins DM, Fenton BT, Driscoll MA, et al. Gender differences in demographic and clinical correlates among veterans with musculoskeletal disorders. Womens Health Issues. 2017;27(4):463-470. doi:10.1016/j.whi.2017.01.008
4. Lehavot K, Goldberg SB, Chen JA, et al. Do trauma type, stressful life events, and social support explain women veterans’ high prevalence of PTSD?. Soc Psychiatry Psychiatr Epidemiol. 2018;53(9):943-953. doi:10.1007/s00127-018-1550-x
5. Levander XA, Overland MK. Care of women veterans. Med Clin North Am. 2015;99(3):651-662. doi:10.1016/j.mcna.2015.01.013
6. US Department of Veteran Affairs. Facts and statistics about women veterans. Updated May 28. 2020. Accessed January 6, 2022. https://www.womenshealth.va.gov/womenshealth/latestinformation/facts.asp
7. Krejci LP, Carter K, Gaudet T. Whole health: the vision and implementation of personalized, proactive, patient-driven health care for veterans. Med Care. 2014;52(12)(suppl 5):S5-S8. doi:10.1097/MLR.0000000000000226
8. Elwy AR, Taylor SL, Zhao S, et al. Participating in complementary and integrative health approaches is associated with veterans’ patient-reported outcomes over time. Med Care. 2020;58:S125-S132. doi:10.1097/MLR.0000000000001357
9. Smeeding SJ, Bradshaw DH, Kumpfer K, Trevithick S, Stoddard GJ. Outcome evaluation of the Veterans Affairs Salt Lake City Integrative Health Clinic for chronic pain and stress-related depression, anxiety, and post-traumatic stress disorder. J Altern Complement Med. 2010;16(8):823-835. doi:10.1089/acm.2009.0510
10. Hull A, Brooks Holliday S, Eickhoff C, et al. Veteran participation in the integrative health and wellness program: impact on self-reported mental and physical health outcomes. Psychol Serv. 2019;16(3):475-483. doi:10.1037/ser0000192
11. Zephyrin LC. Reproductive health management for the care of women veterans [published correction appears in Obstet Gynecol. 2016 Mar;127(3):605]. Obstet Gynecol. 2016;127(2):383-392. doi:10.1097/AOG.0000000000001252
12. Piotrowski MM, Paterson C, Mitchinson A, Kim HM, Kirsh M, Hinshaw DB. Massage as adjuvant therapy in the management of acute postoperative pain: a preliminary study in men. J Am Coll Surg. 2003;197(6):1037-1046. doi:10.1016/j.jamcollsurg.2003.07.020
13. Mitchinson AR, Kim HM, Rosenberg JM, et al. Acute postoperative pain management using massage as an adjuvant therapy: a randomized trial. Arch Surg. 2007;142(12):1158-1167. doi:10.1001/archsurg.142.12.1158
14. Mitchinson A, Fletcher CE, Kim HM, Montagnini M, Hinshaw DB. Integrating massage therapy within the palliative care of veterans with advanced illnesses: an outcome study. Am J Hosp Palliat Care. 2014;31(1):6-12. doi:10.1177/1049909113476568
15. Fletcher CE, Mitchinson AR, Trumble EL, Hinshaw DB, Dusek JA. Perceptions of other integrative health therapies by veterans with pain who are receiving massage. J Rehabil Res Dev. 2016;53(1):117-126. doi:10.1682/JRRD.2015.01.0015
16. Juberg M, Jerger KK, Allen KD, Dmitrieva NO, Keever T, Perlman AI. Pilot study of massage in veterans with knee osteoarthritis. J Altern Complement Med. 2015;21(6):333-338. doi:10.1089/acm.2014.0254
17. Beck I, Runeson I, Blomqvist K. To find inner peace: soft massage as an established and integrated part of palliative care. Int J Palliate Nurse. 2009;15(11):541-545. doi: 10.12968/ijpn.2009.15.11.45493
18. Haskell SG, Ning Y, Krebs E, et al. Prevalence of painful musculoskeletal conditions in female and male veterans in 7 years after return from deployment in Operation Enduring Freedom/Operation Iraqi Freedom. Clin J Pain. 2012;28(2):163-167. doi:10.1097/AJP.0b013e318223d951
19. Maguen S, Ren L, Bosch JO, Marmar CR, Seal KH. Gender differences in mental health diagnoses among Iraq and Afghanistan veterans enrolled in veterans affairs health care. Am J Public Health. 2010;100(12):2450-2456. doi:10.2105/AJPH.2009.166165
20. Outcalt SD, Kroenke K, Krebs EE, et al. Chronic pain and comorbid mental health conditions: independent associations of posttraumatic stress disorder and depression with pain, disability, and quality of life. J Behav Med. 2015;38(3):535-543. doi:10.1007/s10865-015-9628-3
21. Gibson CJ, Maguen S, Xia F, Barnes DE, Peltz CB, Yaffe K. Military sexual trauma in older women veterans: prevalence and comorbidities. J Gen Intern Med. 2020;35(1):207-213. doi:10.1007/s11606-019-05342-7
22. Tan G, Teo I, Srivastava D, et al. Improving access to care for women veterans suffering from chronic pain and depression associated with trauma. Pain Med. 2013;14(7):1010-1020. doi:10.1111/pme.12131
23. Haskell SG, Heapy A, Reid MC, Papas RK, Kerns RD. The prevalence and age-related characteristics of pain in a sample of women veterans receiving primary care. J Womens Health (Larchmt). 2006;15(7):862-869. doi:10.1089/jwh.2006.15.862
24. Driscoll MA, Higgins D, Shamaskin-Garroway A, et al. Examining gender as a correlate of self-reported pain treatment use among recent service veterans with deployment-related musculoskeletal disorders. Pain Med. 2017;18(9):1767-1777. doi:10.1093/pm/pnx023
25. Weimer MB, Macey TA, Nicolaidis C, Dobscha SK, Duckart JP, Morasco BJ. Sex differences in the medical care of VA patients with chronic non-cancer pain. Pain Med. 2013;14(12):1839-1847. doi:10.1111/pme.12177
26. Stubbs D, Krebs E, Bair M, et al. Sex differences in pain and pain-related disability among primary care patients with chronic musculoskeletal pain. Pain Med. 2010;11(2):232-239. doi:10.1111/j.1526-4637.2009.00760.x
27. Keogh E, McCracken LM, Eccleston C. Gender moderates the association between depression and disability in chronic pain patients. Eur J Pain. 2006;10(5):413-422. doi:10.1016/j.ejpain.2005.05.007
28. Miake-Lye IM, Mak S, Lee J, et al. Massage for pain: an evidence map. J Altern Complement Med. 2019;25(5):475-502. doi:10.1089/acm.2018.0282
29. Cherkin DC, Sherman KJ, Kahn J, et al. A comparison of the effects of 2 types of massage and usual care on chronic low back pain: a randomized, controlled trial. Ann Intern Med. 2011;155(1):1-9. doi:10.7326/0003-4819-155-1-201107050-00002
30. Sherman KJ, Cook AJ, Wellman RD, et al. Five-week outcomes from a dosing trial of therapeutic massage for chronic neck pain. Ann Fam Med. 2014;12(2):112-120. doi:10.1370/afm.1602
31. Perlman AI, Sabina A, Williams AL, Njike VY, Katz DL. Massage therapy for osteoarthritis of the knee: a randomized controlled trial. Arch Intern Med. 2006;166(22):2533-2538. doi:10.1001/archinte.166.22.2533
32. Perlman A, Fogerite SG, Glass O, et al. Efficacy and safety of massage for osteoarthritis of the knee: a randomized clinical trial. J Gen Intern Med. 2019;34(3):379-386. doi:10.1007/s11606-018-4763-5
33. Skelly AC, Chou R, Dettori JR, et al. Noninvasive Nonpharmacological Treatment for Chronic Pain: A Systematic Review Update. Comparative Effectiveness Review. No. 227. Agency for Healthcare Research and Quality; 2020. doi:10.23970/AHRQEPCCER227
34. Moyer CA, Rounds J, Hannum JW. A meta-analysis of massage therapy research. Psychol Bull. 2004;130(1):3-18. doi:10.1037/0033-2909.130.1.3
35. Field T, Hernandez-Reif M, Diego M, Schanberg S, Kuhn C. Cortisol decreases and serotonin and dopamine increase following massage therapy. Int J Neurosci. 2005;115(10):1397-1413. doi:10.1080/ 00207450590956459
36. Li Q, Becker B, Wernicke J, et al. Foot massage evokes oxytocin release and activation of orbitofrontal cortex and superior temporal sulcus. Psychoneuroendocrinology. 2019;101:193-203. doi:10.1016/j.psyneuen.2018.11.016
37. Eaves ER, Sherman KJ, Ritenbaugh C, et al. A qualitative study of changes in expectations over time among patients with chronic low back pain seeking four CAM therapies. BMC Complement Altern Med. 2015;15:12. Published 2015 Feb 5. doi:10.1186/s12906-015-0531-9
38. Bishop FL, Lauche R, Cramer H, et al. Health behavior change and complementary medicine use: National Health Interview Survey 2012. Medicina (Kaunas). 2019;55(10):632. Published 2019 Sep 24. doi:10.3390/medicina55100632
39. Driscoll MA, Knobf MT, Higgins DM, Heapy A, Lee A, Haskell S. Patient experiences navigating chronic pain management in an integrated health care system: a qualitative investigation of women and men. Pain Med. 2018;19(suppl 1):S19-S29. doi:10.1093/pm/pny139
40. Denneson LM, Corson K, Dobscha SK. Complementary and alternative medicine use among veterans with chronic noncancer pain. J Rehabil Res Dev. 2011;48(9):1119-1128. doi:10.1682/jrrd.2010.12.0243
41. Taylor SL, Herman PM, Marshall NJ, et al. Use of complementary and integrated health: a retrospective analysis of U.S. veterans with chronic musculoskeletal pain nationally. J Altern Complement Med. 2019;25(1):32-39. doi:10.1089/acm.2018.0276
42. Evans EA, Herman PM, Washington DL, et al. Gender differences in use of complementary and integrative health by U.S. military veterans with chronic musculoskeletal pain. Womens Health Issues. 2018;28(5):379-386. doi:10.1016/j.whi.2018.07.003
43. Reinhard MJ, Nassif TH, Bloeser K, et al. CAM utilization among OEF/OIF veterans: findings from the National Health Study for a New Generation of US Veterans. Med Care. 2014;52(12)(suppl 5):S45-S49. doi:10.1097/MLR.0000000000000229
44. Herman PM, Yuan AH, Cefalu MS, et al. The use of complementary and integrative health approaches for chronic musculoskeletal pain in younger US Veterans: An economic evaluation. PLoS One. 2019;14(6):e0217831. Published 2019 Jun 5. doi:10.1371/journal.pone.0217831
45. Jonas WB, Schoomaker EB. Pain and opioids in the military: we must do better. JAMA Intern Med. 2014;174(8):1402-1403. doi:10.1001/jamainternmed.2014.2114
46. Han B, Compton WM, Blanco C, Crane E, Lee J, Jones CM. Prescription opioid use, misuse, and use disorders in U.S. adults: 2015 National Survey on Drug Use and Health. Ann Intern Med. 2017;167(5):293-301. doi:10.7326/M17-0865
Chewing xylitol gum may modestly reduce preterm birth
In the country with one of the highest rates of preterm birth in the world, these early deliveries dropped by 24% with a simple intervention: chewing gum with xylitol during pregnancy. The decrease in preterm births was linked to improvement in oral health, according to research presented at the meeting sponsored by the Society for Maternal-Fetal Medicine.
Although the findings, from a randomized controlled trial of women in Malawi, barely reached statistical significance, the researchers also documented a reduction in periodontitis (gum disease) that appears to correlate with the reduction in early deliveries, according to Kjersti Aagaard, MD, PhD, of Baylor College of Medicine and Texas Children’s Hospital, both in Houston.
“For a while, we have known about the association with poor oral health and preterm birth but I am not aware of a study of this magnitude suggesting a simple and effective treatment option,” said Ilina Pluym, MD, an assistant professor in maternal fetal medicine at the University of California, Los Angeles, who attended the presentation. Dr. Pluym called the new data “compelling” and said the study “adds to our possible strategies to treat a condition that causes a significant burden of disease worldwide.” The findings must be replicated, ideally in countries with lower rates of preterm birth and periodontal disease to see if the effect is similar, before broadly implementing this cheap and simple intervention.
Preterm birth is a leading cause of infant mortality and a major underlying cause of health problems in children under 5 worldwide. As many as 42% of children born preterm have a health condition related to their prematurity or do not survive childhood.
About one in five babies in Malawi are born between 26 and 37 weeks, about double the U.S. rate of 10.8% preterm births, which in this country is considered births that occur between 23 and 37 weeks’ gestation. Researchers also chose Malawi for the trial because residents there see preterm birth as a widespread problem that must be addressed, Dr. Aagaard said.
Multiple previous studies have found a link between periodontal disease and deliveries that are preterm or low birth weight, Dr. Aagaard told attendees. However, 11 randomized controlled trials that involved treating periodontal disease did not reduce preterm birth despite improving periodontitis and oral health.
Dr. Aagaard’s team decided to test the effectiveness of xylitol – a natural prebiotic found in fruits, vegetables, and bran – because harmful oral bacteria cannot metabolize the substance, and regular use of xylitol reduces the number of harmful mouth bacteria while increasing the number of good microbes in the mouth. In addition, a study in 2006 found that children up to 4 years old had fewer cavities and ear infections when their mothers chewed gum containing xylitol and other compounds. Dr. Aagaard noted that gums without xylitol do not appear to produce the same improvements in oral health.
Before beginning the trial, Dr. Aagaard’s group spent 3 years doing a “run-in” study to ensure a larger, longer-term trial in Malawi was feasible. That initial study found a reduction in tooth decay and periodontal inflammation with use of xylitol. The researchers also learned that participants preferred gum over lozenges or lollipops. Nearly all the participants (92%) chewed the gum twice daily.
Among 10,069 women who enrolled in the trial, 96% remained in it until the end. Of the initial total, 4,029 participants underwent an oral health assessment at the start of the study, and 920 had a follow-up oral health assessment.
Of the 4,349 women who chewed xylitol gum, 12.6% gave birth before 37 weeks, compared with 16.5% preterm births among the 5,321 women in the control group – a 24% reduction (P = .045). The 16.5% rate among women not chewing gum was still lower than the national rate of 19.6%, possibly related to the education the participants received, according to the researchers.
No statistically significant reduction occurred for births at less than 34 weeks, but the reduction in late preterm births – babies born between 34 and 37 weeks – was also borderline in statistical significance (P = .049). Only 9.9% of women chewing xylitol gum had a late preterm birth compared to 13.5% of women who only received health education.
The researchers estimated it would take 26 pregnant women chewing xylitol gum to prevent one preterm birth. At a cost of $24-$29 per pregnancy for the gum, preventing each preterm birth in a community would cost $623-$754.
The researchers also observed a 30% reduction in newborns weighing less than 2,500 g (5.5 pounds), with 8.9% of low-birth-weight babies born to moms chewing gum and 12.9% of low-birth-weight babies born to those not provided gum (P = .046). They attributed this reduction in low birth weight to the lower proportion of late preterm births. The groups showed no significant differences in stillbirths or newborn deaths.
The researchers did, however, find a significant reduction in periodontitis among the women who chewed xylitol gum who came for follow-up dental visits. The prevalence of periodontal disease dropped from 31% to 27% in those not chewing gum but from 31% to 21% in gum chewers (P = .04).
“This cannot be attributed to overall oral health, as dental caries composite scores did not significantly differ while periodontitis measures did,” Dr. Aagaard said.
One limitation of the trial is that it was randomized by health centers instead by individual women, although the researchers tried to account for differences that might exist between the populations going to different facilities. Nor did the researchers assess how frequently the participants chewed gum – although the fact that the gum-chewing group had better oral health suggests they appear to have done so regularly.
Whether recommending xylitol chewing gum to pregnant women in other countries would affect rates of preterm birth is unclear. The ideal population for an intervention like this is one where the population has a high rate of periodontal disease or other preterm birth risk factors, Dr. Pluym said.
”Preterm birth is multifactorial,” she said. “There are often multiple risk factors and causes to the complex pathophysiological process and a quick fix is not the solution for everyone.”
The study was funded by the Thrasher Research Fund. Dr. Aagaard and Dr. Pluym reported no disclosures.
This story was updated on Feb. 4, 2022.
In the country with one of the highest rates of preterm birth in the world, these early deliveries dropped by 24% with a simple intervention: chewing gum with xylitol during pregnancy. The decrease in preterm births was linked to improvement in oral health, according to research presented at the meeting sponsored by the Society for Maternal-Fetal Medicine.
Although the findings, from a randomized controlled trial of women in Malawi, barely reached statistical significance, the researchers also documented a reduction in periodontitis (gum disease) that appears to correlate with the reduction in early deliveries, according to Kjersti Aagaard, MD, PhD, of Baylor College of Medicine and Texas Children’s Hospital, both in Houston.
“For a while, we have known about the association with poor oral health and preterm birth but I am not aware of a study of this magnitude suggesting a simple and effective treatment option,” said Ilina Pluym, MD, an assistant professor in maternal fetal medicine at the University of California, Los Angeles, who attended the presentation. Dr. Pluym called the new data “compelling” and said the study “adds to our possible strategies to treat a condition that causes a significant burden of disease worldwide.” The findings must be replicated, ideally in countries with lower rates of preterm birth and periodontal disease to see if the effect is similar, before broadly implementing this cheap and simple intervention.
Preterm birth is a leading cause of infant mortality and a major underlying cause of health problems in children under 5 worldwide. As many as 42% of children born preterm have a health condition related to their prematurity or do not survive childhood.
About one in five babies in Malawi are born between 26 and 37 weeks, about double the U.S. rate of 10.8% preterm births, which in this country is considered births that occur between 23 and 37 weeks’ gestation. Researchers also chose Malawi for the trial because residents there see preterm birth as a widespread problem that must be addressed, Dr. Aagaard said.
Multiple previous studies have found a link between periodontal disease and deliveries that are preterm or low birth weight, Dr. Aagaard told attendees. However, 11 randomized controlled trials that involved treating periodontal disease did not reduce preterm birth despite improving periodontitis and oral health.
Dr. Aagaard’s team decided to test the effectiveness of xylitol – a natural prebiotic found in fruits, vegetables, and bran – because harmful oral bacteria cannot metabolize the substance, and regular use of xylitol reduces the number of harmful mouth bacteria while increasing the number of good microbes in the mouth. In addition, a study in 2006 found that children up to 4 years old had fewer cavities and ear infections when their mothers chewed gum containing xylitol and other compounds. Dr. Aagaard noted that gums without xylitol do not appear to produce the same improvements in oral health.
Before beginning the trial, Dr. Aagaard’s group spent 3 years doing a “run-in” study to ensure a larger, longer-term trial in Malawi was feasible. That initial study found a reduction in tooth decay and periodontal inflammation with use of xylitol. The researchers also learned that participants preferred gum over lozenges or lollipops. Nearly all the participants (92%) chewed the gum twice daily.
Among 10,069 women who enrolled in the trial, 96% remained in it until the end. Of the initial total, 4,029 participants underwent an oral health assessment at the start of the study, and 920 had a follow-up oral health assessment.
Of the 4,349 women who chewed xylitol gum, 12.6% gave birth before 37 weeks, compared with 16.5% preterm births among the 5,321 women in the control group – a 24% reduction (P = .045). The 16.5% rate among women not chewing gum was still lower than the national rate of 19.6%, possibly related to the education the participants received, according to the researchers.
No statistically significant reduction occurred for births at less than 34 weeks, but the reduction in late preterm births – babies born between 34 and 37 weeks – was also borderline in statistical significance (P = .049). Only 9.9% of women chewing xylitol gum had a late preterm birth compared to 13.5% of women who only received health education.
The researchers estimated it would take 26 pregnant women chewing xylitol gum to prevent one preterm birth. At a cost of $24-$29 per pregnancy for the gum, preventing each preterm birth in a community would cost $623-$754.
The researchers also observed a 30% reduction in newborns weighing less than 2,500 g (5.5 pounds), with 8.9% of low-birth-weight babies born to moms chewing gum and 12.9% of low-birth-weight babies born to those not provided gum (P = .046). They attributed this reduction in low birth weight to the lower proportion of late preterm births. The groups showed no significant differences in stillbirths or newborn deaths.
The researchers did, however, find a significant reduction in periodontitis among the women who chewed xylitol gum who came for follow-up dental visits. The prevalence of periodontal disease dropped from 31% to 27% in those not chewing gum but from 31% to 21% in gum chewers (P = .04).
“This cannot be attributed to overall oral health, as dental caries composite scores did not significantly differ while periodontitis measures did,” Dr. Aagaard said.
One limitation of the trial is that it was randomized by health centers instead by individual women, although the researchers tried to account for differences that might exist between the populations going to different facilities. Nor did the researchers assess how frequently the participants chewed gum – although the fact that the gum-chewing group had better oral health suggests they appear to have done so regularly.
Whether recommending xylitol chewing gum to pregnant women in other countries would affect rates of preterm birth is unclear. The ideal population for an intervention like this is one where the population has a high rate of periodontal disease or other preterm birth risk factors, Dr. Pluym said.
”Preterm birth is multifactorial,” she said. “There are often multiple risk factors and causes to the complex pathophysiological process and a quick fix is not the solution for everyone.”
The study was funded by the Thrasher Research Fund. Dr. Aagaard and Dr. Pluym reported no disclosures.
This story was updated on Feb. 4, 2022.
In the country with one of the highest rates of preterm birth in the world, these early deliveries dropped by 24% with a simple intervention: chewing gum with xylitol during pregnancy. The decrease in preterm births was linked to improvement in oral health, according to research presented at the meeting sponsored by the Society for Maternal-Fetal Medicine.
Although the findings, from a randomized controlled trial of women in Malawi, barely reached statistical significance, the researchers also documented a reduction in periodontitis (gum disease) that appears to correlate with the reduction in early deliveries, according to Kjersti Aagaard, MD, PhD, of Baylor College of Medicine and Texas Children’s Hospital, both in Houston.
“For a while, we have known about the association with poor oral health and preterm birth but I am not aware of a study of this magnitude suggesting a simple and effective treatment option,” said Ilina Pluym, MD, an assistant professor in maternal fetal medicine at the University of California, Los Angeles, who attended the presentation. Dr. Pluym called the new data “compelling” and said the study “adds to our possible strategies to treat a condition that causes a significant burden of disease worldwide.” The findings must be replicated, ideally in countries with lower rates of preterm birth and periodontal disease to see if the effect is similar, before broadly implementing this cheap and simple intervention.
Preterm birth is a leading cause of infant mortality and a major underlying cause of health problems in children under 5 worldwide. As many as 42% of children born preterm have a health condition related to their prematurity or do not survive childhood.
About one in five babies in Malawi are born between 26 and 37 weeks, about double the U.S. rate of 10.8% preterm births, which in this country is considered births that occur between 23 and 37 weeks’ gestation. Researchers also chose Malawi for the trial because residents there see preterm birth as a widespread problem that must be addressed, Dr. Aagaard said.
Multiple previous studies have found a link between periodontal disease and deliveries that are preterm or low birth weight, Dr. Aagaard told attendees. However, 11 randomized controlled trials that involved treating periodontal disease did not reduce preterm birth despite improving periodontitis and oral health.
Dr. Aagaard’s team decided to test the effectiveness of xylitol – a natural prebiotic found in fruits, vegetables, and bran – because harmful oral bacteria cannot metabolize the substance, and regular use of xylitol reduces the number of harmful mouth bacteria while increasing the number of good microbes in the mouth. In addition, a study in 2006 found that children up to 4 years old had fewer cavities and ear infections when their mothers chewed gum containing xylitol and other compounds. Dr. Aagaard noted that gums without xylitol do not appear to produce the same improvements in oral health.
Before beginning the trial, Dr. Aagaard’s group spent 3 years doing a “run-in” study to ensure a larger, longer-term trial in Malawi was feasible. That initial study found a reduction in tooth decay and periodontal inflammation with use of xylitol. The researchers also learned that participants preferred gum over lozenges or lollipops. Nearly all the participants (92%) chewed the gum twice daily.
Among 10,069 women who enrolled in the trial, 96% remained in it until the end. Of the initial total, 4,029 participants underwent an oral health assessment at the start of the study, and 920 had a follow-up oral health assessment.
Of the 4,349 women who chewed xylitol gum, 12.6% gave birth before 37 weeks, compared with 16.5% preterm births among the 5,321 women in the control group – a 24% reduction (P = .045). The 16.5% rate among women not chewing gum was still lower than the national rate of 19.6%, possibly related to the education the participants received, according to the researchers.
No statistically significant reduction occurred for births at less than 34 weeks, but the reduction in late preterm births – babies born between 34 and 37 weeks – was also borderline in statistical significance (P = .049). Only 9.9% of women chewing xylitol gum had a late preterm birth compared to 13.5% of women who only received health education.
The researchers estimated it would take 26 pregnant women chewing xylitol gum to prevent one preterm birth. At a cost of $24-$29 per pregnancy for the gum, preventing each preterm birth in a community would cost $623-$754.
The researchers also observed a 30% reduction in newborns weighing less than 2,500 g (5.5 pounds), with 8.9% of low-birth-weight babies born to moms chewing gum and 12.9% of low-birth-weight babies born to those not provided gum (P = .046). They attributed this reduction in low birth weight to the lower proportion of late preterm births. The groups showed no significant differences in stillbirths or newborn deaths.
The researchers did, however, find a significant reduction in periodontitis among the women who chewed xylitol gum who came for follow-up dental visits. The prevalence of periodontal disease dropped from 31% to 27% in those not chewing gum but from 31% to 21% in gum chewers (P = .04).
“This cannot be attributed to overall oral health, as dental caries composite scores did not significantly differ while periodontitis measures did,” Dr. Aagaard said.
One limitation of the trial is that it was randomized by health centers instead by individual women, although the researchers tried to account for differences that might exist between the populations going to different facilities. Nor did the researchers assess how frequently the participants chewed gum – although the fact that the gum-chewing group had better oral health suggests they appear to have done so regularly.
Whether recommending xylitol chewing gum to pregnant women in other countries would affect rates of preterm birth is unclear. The ideal population for an intervention like this is one where the population has a high rate of periodontal disease or other preterm birth risk factors, Dr. Pluym said.
”Preterm birth is multifactorial,” she said. “There are often multiple risk factors and causes to the complex pathophysiological process and a quick fix is not the solution for everyone.”
The study was funded by the Thrasher Research Fund. Dr. Aagaard and Dr. Pluym reported no disclosures.
This story was updated on Feb. 4, 2022.
FROM THE PREGNANCY MEETING
Lifestyle likely responsible for obesity in children, not mother’s BMI
Lifestyle is more likely to affect a child’s body mass index than the weight of their mother before and during pregnancy say researchers who have found that a mother’s high BMI before and during pregnancy is not a major cause of high BMI in their offspring – indicating that childhood and teen obesity is more likely to be a result of lifestyle factors.
According to UK Government figures 9.9% of reception age children (age 4-5) are obese, with a further 13.1% overweight. At age 10-11 (year 6), 21.0% are obese and 14.1% overweight.
Research from the Centre for Longitudinal Studies (CLS) at the UCL Social Research Institute, published in December 2020, showed that one in five (21%) young people were obese at age 17, and a further one in seven (14%) were overweight.
Nature or nurture
Greater maternal BMI before or during pregnancy is known to be associated with higher BMI throughout childhood, but exactly how much a mother’s weight before or during pregnancy contributes to childhood obesity, or whether it is lifestyle and environmental factors that are responsible, remains unclear.
To investigate this question researchers from the University of Bristol (England) and Imperial College London used data from the “Children of the 90s” (also known as the Avon Longitudinal Study of Parents and Children), and data from the “Born in Bradford” longitudinal study.
For their study, published in BMC Medicine, researchers used Mendelian randomisation, measuring variation in genes to determine the effect of an exposure on an outcome, along with polygenic risk scores, to investigate if associations between before and during early pregnancy BMI, and a child’s BMI from birth to adolescence, are causal.
They looked at birth weight and BMI at age 1 and 4 years in both “Children of the 90s” and “Born in Bradford” participants, and then also BMI at age 10 and 15 years in just the Children of the 90s participants.
Since the effects being explored may differ by ethnicity the authors reported that they limited analyses to two ethnic groups – White European and South Asian.
Interventions targeting everyone needed
The researchers found that there was a moderate causal effect between maternal BMI and the birth weight of children, however they said they “found no strong evidence for a causal effect of maternal BMI on offspring adiposity beyond birth”.
Tom Bond, MSc, senior research associate at the University of Bristol, explained: “We found that if women are heavier at the start of pregnancy this isn’t a strong cause of their children being heavier as teenagers.”
The authors wrote that their results suggested that “higher maternal pre-/early-pregnancy BMI is not a key driver of higher adiposity in the next generation,” something that Mr. Bond said was “important to know”.
The authors concluded that their findings “support interventions that target the whole population for reducing overweight and obesity, rather than a specific focus on women of reproductive age”.
Mr. Bond pointed out that “it isn’t enough to just focus on women entering pregnancy.” However, “there is good evidence that maternal obesity causes other health problems for mothers and babies, so prospective mothers should still be encouraged and supported to maintain a healthy weight.”
A version of this article first appeared on Medscape UK.
Lifestyle is more likely to affect a child’s body mass index than the weight of their mother before and during pregnancy say researchers who have found that a mother’s high BMI before and during pregnancy is not a major cause of high BMI in their offspring – indicating that childhood and teen obesity is more likely to be a result of lifestyle factors.
According to UK Government figures 9.9% of reception age children (age 4-5) are obese, with a further 13.1% overweight. At age 10-11 (year 6), 21.0% are obese and 14.1% overweight.
Research from the Centre for Longitudinal Studies (CLS) at the UCL Social Research Institute, published in December 2020, showed that one in five (21%) young people were obese at age 17, and a further one in seven (14%) were overweight.
Nature or nurture
Greater maternal BMI before or during pregnancy is known to be associated with higher BMI throughout childhood, but exactly how much a mother’s weight before or during pregnancy contributes to childhood obesity, or whether it is lifestyle and environmental factors that are responsible, remains unclear.
To investigate this question researchers from the University of Bristol (England) and Imperial College London used data from the “Children of the 90s” (also known as the Avon Longitudinal Study of Parents and Children), and data from the “Born in Bradford” longitudinal study.
For their study, published in BMC Medicine, researchers used Mendelian randomisation, measuring variation in genes to determine the effect of an exposure on an outcome, along with polygenic risk scores, to investigate if associations between before and during early pregnancy BMI, and a child’s BMI from birth to adolescence, are causal.
They looked at birth weight and BMI at age 1 and 4 years in both “Children of the 90s” and “Born in Bradford” participants, and then also BMI at age 10 and 15 years in just the Children of the 90s participants.
Since the effects being explored may differ by ethnicity the authors reported that they limited analyses to two ethnic groups – White European and South Asian.
Interventions targeting everyone needed
The researchers found that there was a moderate causal effect between maternal BMI and the birth weight of children, however they said they “found no strong evidence for a causal effect of maternal BMI on offspring adiposity beyond birth”.
Tom Bond, MSc, senior research associate at the University of Bristol, explained: “We found that if women are heavier at the start of pregnancy this isn’t a strong cause of their children being heavier as teenagers.”
The authors wrote that their results suggested that “higher maternal pre-/early-pregnancy BMI is not a key driver of higher adiposity in the next generation,” something that Mr. Bond said was “important to know”.
The authors concluded that their findings “support interventions that target the whole population for reducing overweight and obesity, rather than a specific focus on women of reproductive age”.
Mr. Bond pointed out that “it isn’t enough to just focus on women entering pregnancy.” However, “there is good evidence that maternal obesity causes other health problems for mothers and babies, so prospective mothers should still be encouraged and supported to maintain a healthy weight.”
A version of this article first appeared on Medscape UK.
Lifestyle is more likely to affect a child’s body mass index than the weight of their mother before and during pregnancy say researchers who have found that a mother’s high BMI before and during pregnancy is not a major cause of high BMI in their offspring – indicating that childhood and teen obesity is more likely to be a result of lifestyle factors.
According to UK Government figures 9.9% of reception age children (age 4-5) are obese, with a further 13.1% overweight. At age 10-11 (year 6), 21.0% are obese and 14.1% overweight.
Research from the Centre for Longitudinal Studies (CLS) at the UCL Social Research Institute, published in December 2020, showed that one in five (21%) young people were obese at age 17, and a further one in seven (14%) were overweight.
Nature or nurture
Greater maternal BMI before or during pregnancy is known to be associated with higher BMI throughout childhood, but exactly how much a mother’s weight before or during pregnancy contributes to childhood obesity, or whether it is lifestyle and environmental factors that are responsible, remains unclear.
To investigate this question researchers from the University of Bristol (England) and Imperial College London used data from the “Children of the 90s” (also known as the Avon Longitudinal Study of Parents and Children), and data from the “Born in Bradford” longitudinal study.
For their study, published in BMC Medicine, researchers used Mendelian randomisation, measuring variation in genes to determine the effect of an exposure on an outcome, along with polygenic risk scores, to investigate if associations between before and during early pregnancy BMI, and a child’s BMI from birth to adolescence, are causal.
They looked at birth weight and BMI at age 1 and 4 years in both “Children of the 90s” and “Born in Bradford” participants, and then also BMI at age 10 and 15 years in just the Children of the 90s participants.
Since the effects being explored may differ by ethnicity the authors reported that they limited analyses to two ethnic groups – White European and South Asian.
Interventions targeting everyone needed
The researchers found that there was a moderate causal effect between maternal BMI and the birth weight of children, however they said they “found no strong evidence for a causal effect of maternal BMI on offspring adiposity beyond birth”.
Tom Bond, MSc, senior research associate at the University of Bristol, explained: “We found that if women are heavier at the start of pregnancy this isn’t a strong cause of their children being heavier as teenagers.”
The authors wrote that their results suggested that “higher maternal pre-/early-pregnancy BMI is not a key driver of higher adiposity in the next generation,” something that Mr. Bond said was “important to know”.
The authors concluded that their findings “support interventions that target the whole population for reducing overweight and obesity, rather than a specific focus on women of reproductive age”.
Mr. Bond pointed out that “it isn’t enough to just focus on women entering pregnancy.” However, “there is good evidence that maternal obesity causes other health problems for mothers and babies, so prospective mothers should still be encouraged and supported to maintain a healthy weight.”
A version of this article first appeared on Medscape UK.
FROM BMC MEDICINE
Motherhood and mortality: Navigating miscarriages as a physician
One clinic morning in an office visit, I stood next to the door talking, hand on the doorknob ready to exit. My elderly patient was sitting in the chair next to the door, family member in another, as I attempted my exit. Suddenly, as if looking for something, my patient locked her gaze to my abdomen and began to slowly advance herself forward, eyes squinting for a better view. She had found something. Poke, poke, poke. Three pokes in quick succession into my apparently protruding abdomen stoked an internal horror that I dared not release onto my face. How in the hell could she know? My heart sank – the signs were still there.
“There’s something in there,” she said with a seasoned certainty.
“No there’s not,” I said trying hard to hide any emotion.
“Yes, there is,” she said flatly.
“Grannie, no there isn’t,” her family member interrupted, unknowingly saving me. I thanked them again and quickly left the room.
My patient had the ongoings of slowly progressing dementia. Little did she know she was right. Maybe she had known something in another time and space. Either way, I wasn’t prepared to tell the story. She wasn’t prepared to fully understand.
I tried to forge on to see the next patient. Tears began welling in both eyes. I tilted my head back slightly to prevent the water from falling. I wanted to feel offended, but she couldn’t have known the war my body was fighting at the time. I had not yet shared the pregnancy news with this particular patient, and yet her knowing was telling in a sense. I’m learning that the old folks always know.
I was at work, actively having yet another miscarriage. This was the second of two. This most recent time, we found out at 9 weeks that our baby had stopped growing about a week or so earlier. Cue the denial. Cue the rage. Cue the devastation.
Thinking back, with each pregnancy discovery, we did not wait the customary 3 months before telling anyone. Just about everyone knew. We were immediately excited to start sharing with friends, family, coworkers, and even patients early on. We knew the risks in my 40-something age group but were quintessentially optimistic.
I am a family medicine physician with expert-level knowledge and clinical experiences in women’s health counseling, contraception, conception, and pregnancy. In my training, I’ve delivered babies, been elbow-deep searching for wayward tissue from bleeding uteri, and sutured gaping vaginal lacerations. I’ve cried with new mothers at the end of long labors. I’ve been bear-hugged by doting new fathers. I have an abundance of medical knowledge, and yet the pain and struggle of miscarriage over the past 2.5 years has twice reduced me to absolute pieces. There was no course to teach me how to navigate loss within my own body, no textbook to study so that I could test out of the experience. Life hit us dead-on, and I was broken.
I can say that the experience of a miscarriage does not get easier with each subsequent loss. At least for me, the emotions were always raw and tender. Each one was a new gash to my emotional and physical health. My sanity bled out. I was physically exhausted. The struggles of being a health care worker in the midst of a global pandemic I’m sure did not help the situation. My first miscarriage was just before the start of the pandemic. I was in New York visiting family and after dinner at Tavern on the Green, of all places, when I began showing signs. Two days later, I was at the coffee station in our clinic cafeteria adding my cream and sugar when my ob.gyn.’s office called. The hCG levels were probably too low; a miscarriage was likely. I kept my composure, walked out of the cafeteria, got my car keys, went to my car, and proceeded to scream at the top of my lungs for a few minutes. Afterward, I went back to finish up my work and canceled my clinic for the rest of the day.
For my second miscarriage, I was laying in my doctor’s office getting an ultrasound. I had started bleeding the previous day but thought that the subchorionic hemorrhage noted on the last ultrasound might be the culprit. The bleeding was light. That’s the thing about being a pregnant physician: We know too much. The image on the screen looked abnormal, the remnants a ghost of its former self. I knew something was wrong but held out some hope. She searched and turned and pressed the transducer into my belly for a seemingly better view. She apologized for not finding the heartbeat. How is this happening again?
So how does one get through the loss of multiple pregnancies? I know my husband and I worked hard to get through each loss. We did all the right things a good therapist would recommend: Be present in the moment, go with your feelings, allow yourself to feel everything. There were no wrong emotions. Little by little we grieved and healed, grieved and healed. Having a successful pregnancy did help. Miracles are not promised but I believe we were sent one, and her name is Giavonna Barbara. Bookended by miscarriages, she has made me realize just how precious and delicate life really is. She is our absolute world and joy.
I’ve learned twice now that men mourn differently than women. Not any less, just in a different way. There is a pain in the silence that often goes unvocalized, but it is of no less value. My husband and I allowed each other to heal in our own unique ways, and that has made all of the difference. I think I knew I was doing okay when one day I found something funny and I let out the heartiest laugh my belly could muster. A different purpose was renewed. Tears were harder to come by. Hope for the future again sprung eternal. Life went on and so did we.
Looking back, I realize that having a miscarriage and working as a physician in the middle of a global pandemic pushed me to my emotional and physical limits. There is a second-guessing of sorts that occurs. Did the miscarriage happen because I was under so much stress at work? It had happened in the past, was this going to continue to happen?
I can say that I was great at compartmentalizing emotions. I’d try and box them away until I got off of work and then turn them on like a switch once I hit the driver’s seat. It’s easy as a busy physician with so many patients to see, messages to return, notes to write, students and residents to teach, and programs to run to completely tune out the thought of mourning. Temporarily anyway. Work was actually a welcome distraction at times. A purpose. The journey to healing is individualized and can’t be rushed. I like to think that I heal a little bit more every day thinking about the losses and gains that I’ve had. I’m grateful for the experience and growth.
In 2022, I’m looking forward to continuing my healing journey among the twists and turns of the pandemic. I now bring a different level of understanding and empathy to my patients who are undergoing or who have undergone a miscarriage. There will always be a piece of me that viscerally mourns with them. We have a hidden shared experience. I believe I am a better physician because of those lessons learned from my own personal tragedy. Now, I look forward to sharing big belly laughs with my family and friends and savoring the small, quiet moments with my husband and daughter.
A version of this article first appeared on Medscape.com.
One clinic morning in an office visit, I stood next to the door talking, hand on the doorknob ready to exit. My elderly patient was sitting in the chair next to the door, family member in another, as I attempted my exit. Suddenly, as if looking for something, my patient locked her gaze to my abdomen and began to slowly advance herself forward, eyes squinting for a better view. She had found something. Poke, poke, poke. Three pokes in quick succession into my apparently protruding abdomen stoked an internal horror that I dared not release onto my face. How in the hell could she know? My heart sank – the signs were still there.
“There’s something in there,” she said with a seasoned certainty.
“No there’s not,” I said trying hard to hide any emotion.
“Yes, there is,” she said flatly.
“Grannie, no there isn’t,” her family member interrupted, unknowingly saving me. I thanked them again and quickly left the room.
My patient had the ongoings of slowly progressing dementia. Little did she know she was right. Maybe she had known something in another time and space. Either way, I wasn’t prepared to tell the story. She wasn’t prepared to fully understand.
I tried to forge on to see the next patient. Tears began welling in both eyes. I tilted my head back slightly to prevent the water from falling. I wanted to feel offended, but she couldn’t have known the war my body was fighting at the time. I had not yet shared the pregnancy news with this particular patient, and yet her knowing was telling in a sense. I’m learning that the old folks always know.
I was at work, actively having yet another miscarriage. This was the second of two. This most recent time, we found out at 9 weeks that our baby had stopped growing about a week or so earlier. Cue the denial. Cue the rage. Cue the devastation.
Thinking back, with each pregnancy discovery, we did not wait the customary 3 months before telling anyone. Just about everyone knew. We were immediately excited to start sharing with friends, family, coworkers, and even patients early on. We knew the risks in my 40-something age group but were quintessentially optimistic.
I am a family medicine physician with expert-level knowledge and clinical experiences in women’s health counseling, contraception, conception, and pregnancy. In my training, I’ve delivered babies, been elbow-deep searching for wayward tissue from bleeding uteri, and sutured gaping vaginal lacerations. I’ve cried with new mothers at the end of long labors. I’ve been bear-hugged by doting new fathers. I have an abundance of medical knowledge, and yet the pain and struggle of miscarriage over the past 2.5 years has twice reduced me to absolute pieces. There was no course to teach me how to navigate loss within my own body, no textbook to study so that I could test out of the experience. Life hit us dead-on, and I was broken.
I can say that the experience of a miscarriage does not get easier with each subsequent loss. At least for me, the emotions were always raw and tender. Each one was a new gash to my emotional and physical health. My sanity bled out. I was physically exhausted. The struggles of being a health care worker in the midst of a global pandemic I’m sure did not help the situation. My first miscarriage was just before the start of the pandemic. I was in New York visiting family and after dinner at Tavern on the Green, of all places, when I began showing signs. Two days later, I was at the coffee station in our clinic cafeteria adding my cream and sugar when my ob.gyn.’s office called. The hCG levels were probably too low; a miscarriage was likely. I kept my composure, walked out of the cafeteria, got my car keys, went to my car, and proceeded to scream at the top of my lungs for a few minutes. Afterward, I went back to finish up my work and canceled my clinic for the rest of the day.
For my second miscarriage, I was laying in my doctor’s office getting an ultrasound. I had started bleeding the previous day but thought that the subchorionic hemorrhage noted on the last ultrasound might be the culprit. The bleeding was light. That’s the thing about being a pregnant physician: We know too much. The image on the screen looked abnormal, the remnants a ghost of its former self. I knew something was wrong but held out some hope. She searched and turned and pressed the transducer into my belly for a seemingly better view. She apologized for not finding the heartbeat. How is this happening again?
So how does one get through the loss of multiple pregnancies? I know my husband and I worked hard to get through each loss. We did all the right things a good therapist would recommend: Be present in the moment, go with your feelings, allow yourself to feel everything. There were no wrong emotions. Little by little we grieved and healed, grieved and healed. Having a successful pregnancy did help. Miracles are not promised but I believe we were sent one, and her name is Giavonna Barbara. Bookended by miscarriages, she has made me realize just how precious and delicate life really is. She is our absolute world and joy.
I’ve learned twice now that men mourn differently than women. Not any less, just in a different way. There is a pain in the silence that often goes unvocalized, but it is of no less value. My husband and I allowed each other to heal in our own unique ways, and that has made all of the difference. I think I knew I was doing okay when one day I found something funny and I let out the heartiest laugh my belly could muster. A different purpose was renewed. Tears were harder to come by. Hope for the future again sprung eternal. Life went on and so did we.
Looking back, I realize that having a miscarriage and working as a physician in the middle of a global pandemic pushed me to my emotional and physical limits. There is a second-guessing of sorts that occurs. Did the miscarriage happen because I was under so much stress at work? It had happened in the past, was this going to continue to happen?
I can say that I was great at compartmentalizing emotions. I’d try and box them away until I got off of work and then turn them on like a switch once I hit the driver’s seat. It’s easy as a busy physician with so many patients to see, messages to return, notes to write, students and residents to teach, and programs to run to completely tune out the thought of mourning. Temporarily anyway. Work was actually a welcome distraction at times. A purpose. The journey to healing is individualized and can’t be rushed. I like to think that I heal a little bit more every day thinking about the losses and gains that I’ve had. I’m grateful for the experience and growth.
In 2022, I’m looking forward to continuing my healing journey among the twists and turns of the pandemic. I now bring a different level of understanding and empathy to my patients who are undergoing or who have undergone a miscarriage. There will always be a piece of me that viscerally mourns with them. We have a hidden shared experience. I believe I am a better physician because of those lessons learned from my own personal tragedy. Now, I look forward to sharing big belly laughs with my family and friends and savoring the small, quiet moments with my husband and daughter.
A version of this article first appeared on Medscape.com.
One clinic morning in an office visit, I stood next to the door talking, hand on the doorknob ready to exit. My elderly patient was sitting in the chair next to the door, family member in another, as I attempted my exit. Suddenly, as if looking for something, my patient locked her gaze to my abdomen and began to slowly advance herself forward, eyes squinting for a better view. She had found something. Poke, poke, poke. Three pokes in quick succession into my apparently protruding abdomen stoked an internal horror that I dared not release onto my face. How in the hell could she know? My heart sank – the signs were still there.
“There’s something in there,” she said with a seasoned certainty.
“No there’s not,” I said trying hard to hide any emotion.
“Yes, there is,” she said flatly.
“Grannie, no there isn’t,” her family member interrupted, unknowingly saving me. I thanked them again and quickly left the room.
My patient had the ongoings of slowly progressing dementia. Little did she know she was right. Maybe she had known something in another time and space. Either way, I wasn’t prepared to tell the story. She wasn’t prepared to fully understand.
I tried to forge on to see the next patient. Tears began welling in both eyes. I tilted my head back slightly to prevent the water from falling. I wanted to feel offended, but she couldn’t have known the war my body was fighting at the time. I had not yet shared the pregnancy news with this particular patient, and yet her knowing was telling in a sense. I’m learning that the old folks always know.
I was at work, actively having yet another miscarriage. This was the second of two. This most recent time, we found out at 9 weeks that our baby had stopped growing about a week or so earlier. Cue the denial. Cue the rage. Cue the devastation.
Thinking back, with each pregnancy discovery, we did not wait the customary 3 months before telling anyone. Just about everyone knew. We were immediately excited to start sharing with friends, family, coworkers, and even patients early on. We knew the risks in my 40-something age group but were quintessentially optimistic.
I am a family medicine physician with expert-level knowledge and clinical experiences in women’s health counseling, contraception, conception, and pregnancy. In my training, I’ve delivered babies, been elbow-deep searching for wayward tissue from bleeding uteri, and sutured gaping vaginal lacerations. I’ve cried with new mothers at the end of long labors. I’ve been bear-hugged by doting new fathers. I have an abundance of medical knowledge, and yet the pain and struggle of miscarriage over the past 2.5 years has twice reduced me to absolute pieces. There was no course to teach me how to navigate loss within my own body, no textbook to study so that I could test out of the experience. Life hit us dead-on, and I was broken.
I can say that the experience of a miscarriage does not get easier with each subsequent loss. At least for me, the emotions were always raw and tender. Each one was a new gash to my emotional and physical health. My sanity bled out. I was physically exhausted. The struggles of being a health care worker in the midst of a global pandemic I’m sure did not help the situation. My first miscarriage was just before the start of the pandemic. I was in New York visiting family and after dinner at Tavern on the Green, of all places, when I began showing signs. Two days later, I was at the coffee station in our clinic cafeteria adding my cream and sugar when my ob.gyn.’s office called. The hCG levels were probably too low; a miscarriage was likely. I kept my composure, walked out of the cafeteria, got my car keys, went to my car, and proceeded to scream at the top of my lungs for a few minutes. Afterward, I went back to finish up my work and canceled my clinic for the rest of the day.
For my second miscarriage, I was laying in my doctor’s office getting an ultrasound. I had started bleeding the previous day but thought that the subchorionic hemorrhage noted on the last ultrasound might be the culprit. The bleeding was light. That’s the thing about being a pregnant physician: We know too much. The image on the screen looked abnormal, the remnants a ghost of its former self. I knew something was wrong but held out some hope. She searched and turned and pressed the transducer into my belly for a seemingly better view. She apologized for not finding the heartbeat. How is this happening again?
So how does one get through the loss of multiple pregnancies? I know my husband and I worked hard to get through each loss. We did all the right things a good therapist would recommend: Be present in the moment, go with your feelings, allow yourself to feel everything. There were no wrong emotions. Little by little we grieved and healed, grieved and healed. Having a successful pregnancy did help. Miracles are not promised but I believe we were sent one, and her name is Giavonna Barbara. Bookended by miscarriages, she has made me realize just how precious and delicate life really is. She is our absolute world and joy.
I’ve learned twice now that men mourn differently than women. Not any less, just in a different way. There is a pain in the silence that often goes unvocalized, but it is of no less value. My husband and I allowed each other to heal in our own unique ways, and that has made all of the difference. I think I knew I was doing okay when one day I found something funny and I let out the heartiest laugh my belly could muster. A different purpose was renewed. Tears were harder to come by. Hope for the future again sprung eternal. Life went on and so did we.
Looking back, I realize that having a miscarriage and working as a physician in the middle of a global pandemic pushed me to my emotional and physical limits. There is a second-guessing of sorts that occurs. Did the miscarriage happen because I was under so much stress at work? It had happened in the past, was this going to continue to happen?
I can say that I was great at compartmentalizing emotions. I’d try and box them away until I got off of work and then turn them on like a switch once I hit the driver’s seat. It’s easy as a busy physician with so many patients to see, messages to return, notes to write, students and residents to teach, and programs to run to completely tune out the thought of mourning. Temporarily anyway. Work was actually a welcome distraction at times. A purpose. The journey to healing is individualized and can’t be rushed. I like to think that I heal a little bit more every day thinking about the losses and gains that I’ve had. I’m grateful for the experience and growth.
In 2022, I’m looking forward to continuing my healing journey among the twists and turns of the pandemic. I now bring a different level of understanding and empathy to my patients who are undergoing or who have undergone a miscarriage. There will always be a piece of me that viscerally mourns with them. We have a hidden shared experience. I believe I am a better physician because of those lessons learned from my own personal tragedy. Now, I look forward to sharing big belly laughs with my family and friends and savoring the small, quiet moments with my husband and daughter.
A version of this article first appeared on Medscape.com.
Some U.S. women not getting ET for curable breast cancer
A standard treatment for early breast cancer is endocrine therapy (ET), with drugs such a tamoxifen and aromatase inhibitors.
But the study found that ET was not being used in about half of the eligible patients.
For example, only 13,115 of 26,255 eligible patients (48.8%) initiated ET within 1 year of diagnosis, and only 13,944 (52.1%) continued with ET.
“This is remarkable, considering that ET confers an impressive one-third reduction in the risk of death from breast cancer in the first 15 years after diagnosis,” comment authors Michael J. Hassett, MD, of the Dana-Farber Cancer Institute, Boston, and colleagues.
The findings were published online on Jan. 27 in JAMA Oncology.
This study provides an “important and disturbing” glimpse of the hidden barriers patients face when seeking quality, guideline-concordant care, says Kathy Miller, MD, the Ballve Lantero professor of oncology at Indiana University School of Medicine and associate director of clinical research at the IU Simon Comprehensive Cancer Center, Indianapolis, who was approached for comment.
Geographical variations
In their study, Dr. Hasset and colleagues set out determine the extent to which geospatial variations in early breast cancer care are attributable to health service area versus patient factors. They analyzed Surveillance, Epidemiology, and End Results (SEER) Medicare data for 31,571 patients with newly diagnosed with stage I-II nonmetastatic breast cancer between 2007 and 2013 who were followed for at least 3 years.
The patients had a median age of 71 years, and 61.4% had stage I disease at diagnosis.
Geospatial density maps (heat maps) in the paper highlight regional performance patterns. For initiation of ET within 1 year of diagnosis, the regions that appeared the worst (with less than 50% of patients getting this treatment) were parts of California, Utah, New Mexico, Louisiana, Georgia, Kentucky, Washington, and an isolated patch in Michigan.
In addition to the striking finding that nearly half of all women who are eligible for ET did not receive that therapy, the investigators found that 81.6% of 21,190 eligible patients received radiation therapy and 72.8% of 9,903 eligible patients received chemotherapy.
This also varied across the graphical regions, with the heat maps showing that the areas that were delivering radiation and chemotherapy to 70% to 80% of women were similar to the areas that were not initiating ET in about half of these women.
The authors found that the geographical region and health service area (HSA) explained more observed variation (24% to 48%) than patient factors (1% to 4%).
“While patient characteristics, such as race and ethnicity, were significantly associated with variation in breast cancer care, they explained a relatively small proportion of the total observed geospatial variance,” the authors comment.
“In fact, most of the total observed variance was owing to randomness or unexplained factors,” they add. The largest share of variation – 35% to 45% – was unexplained.
“The ET metrics demonstrated the largest total observed variance, the lowest absolute performance (only 49% of patients had an ET prescription within 1 year of diagnosis), and the strongest association with region/HSA,” they conclude.
Though limited by factors inherent in a retrospective review of SEER-Medicare data, the “unexplained nature of most geospatial variation in initial breast cancer care is not likely to change,” they comment.
Future quality improvement efforts should focus on reducing this unwarranted geospatial variation, particularly through the use of ET in eligible patients and with strategies that work across health care delivery systems, they suggest.
Approached for comment on the new findings, Dr. Miller posits that “many factors may be at play.”
“Unfortunately, the SEER database doesn’t allow us to sort out the impact of poverty/cost of care, distance to medical care, availability of specialty and subspecialty care, and payer/provider networks that may limit choices and options for second opinions,” Dr. Miller told this news organization.
She said that patients should be encouraged to consult reliable patient-focused information, such as that provided by the American Society of Clinical Oncology through its disease-specific sites, and to seek a second opinion from a university center. In many cases, major centers have become more accessible through virtual visits made available in the wake of the COVID-19 pandemic, she noted.
This study was supported by Dana-Farber Cancer Institute and the American Cancer Society. The authors and Dr. Miller have disclosed no relevant financial relationships. Dr. Miller is a regular contributor to Medscape with her Miller on Oncology column.
A version of this article first appeared on Medscape.com.
A standard treatment for early breast cancer is endocrine therapy (ET), with drugs such a tamoxifen and aromatase inhibitors.
But the study found that ET was not being used in about half of the eligible patients.
For example, only 13,115 of 26,255 eligible patients (48.8%) initiated ET within 1 year of diagnosis, and only 13,944 (52.1%) continued with ET.
“This is remarkable, considering that ET confers an impressive one-third reduction in the risk of death from breast cancer in the first 15 years after diagnosis,” comment authors Michael J. Hassett, MD, of the Dana-Farber Cancer Institute, Boston, and colleagues.
The findings were published online on Jan. 27 in JAMA Oncology.
This study provides an “important and disturbing” glimpse of the hidden barriers patients face when seeking quality, guideline-concordant care, says Kathy Miller, MD, the Ballve Lantero professor of oncology at Indiana University School of Medicine and associate director of clinical research at the IU Simon Comprehensive Cancer Center, Indianapolis, who was approached for comment.
Geographical variations
In their study, Dr. Hasset and colleagues set out determine the extent to which geospatial variations in early breast cancer care are attributable to health service area versus patient factors. They analyzed Surveillance, Epidemiology, and End Results (SEER) Medicare data for 31,571 patients with newly diagnosed with stage I-II nonmetastatic breast cancer between 2007 and 2013 who were followed for at least 3 years.
The patients had a median age of 71 years, and 61.4% had stage I disease at diagnosis.
Geospatial density maps (heat maps) in the paper highlight regional performance patterns. For initiation of ET within 1 year of diagnosis, the regions that appeared the worst (with less than 50% of patients getting this treatment) were parts of California, Utah, New Mexico, Louisiana, Georgia, Kentucky, Washington, and an isolated patch in Michigan.
In addition to the striking finding that nearly half of all women who are eligible for ET did not receive that therapy, the investigators found that 81.6% of 21,190 eligible patients received radiation therapy and 72.8% of 9,903 eligible patients received chemotherapy.
This also varied across the graphical regions, with the heat maps showing that the areas that were delivering radiation and chemotherapy to 70% to 80% of women were similar to the areas that were not initiating ET in about half of these women.
The authors found that the geographical region and health service area (HSA) explained more observed variation (24% to 48%) than patient factors (1% to 4%).
“While patient characteristics, such as race and ethnicity, were significantly associated with variation in breast cancer care, they explained a relatively small proportion of the total observed geospatial variance,” the authors comment.
“In fact, most of the total observed variance was owing to randomness or unexplained factors,” they add. The largest share of variation – 35% to 45% – was unexplained.
“The ET metrics demonstrated the largest total observed variance, the lowest absolute performance (only 49% of patients had an ET prescription within 1 year of diagnosis), and the strongest association with region/HSA,” they conclude.
Though limited by factors inherent in a retrospective review of SEER-Medicare data, the “unexplained nature of most geospatial variation in initial breast cancer care is not likely to change,” they comment.
Future quality improvement efforts should focus on reducing this unwarranted geospatial variation, particularly through the use of ET in eligible patients and with strategies that work across health care delivery systems, they suggest.
Approached for comment on the new findings, Dr. Miller posits that “many factors may be at play.”
“Unfortunately, the SEER database doesn’t allow us to sort out the impact of poverty/cost of care, distance to medical care, availability of specialty and subspecialty care, and payer/provider networks that may limit choices and options for second opinions,” Dr. Miller told this news organization.
She said that patients should be encouraged to consult reliable patient-focused information, such as that provided by the American Society of Clinical Oncology through its disease-specific sites, and to seek a second opinion from a university center. In many cases, major centers have become more accessible through virtual visits made available in the wake of the COVID-19 pandemic, she noted.
This study was supported by Dana-Farber Cancer Institute and the American Cancer Society. The authors and Dr. Miller have disclosed no relevant financial relationships. Dr. Miller is a regular contributor to Medscape with her Miller on Oncology column.
A version of this article first appeared on Medscape.com.
A standard treatment for early breast cancer is endocrine therapy (ET), with drugs such a tamoxifen and aromatase inhibitors.
But the study found that ET was not being used in about half of the eligible patients.
For example, only 13,115 of 26,255 eligible patients (48.8%) initiated ET within 1 year of diagnosis, and only 13,944 (52.1%) continued with ET.
“This is remarkable, considering that ET confers an impressive one-third reduction in the risk of death from breast cancer in the first 15 years after diagnosis,” comment authors Michael J. Hassett, MD, of the Dana-Farber Cancer Institute, Boston, and colleagues.
The findings were published online on Jan. 27 in JAMA Oncology.
This study provides an “important and disturbing” glimpse of the hidden barriers patients face when seeking quality, guideline-concordant care, says Kathy Miller, MD, the Ballve Lantero professor of oncology at Indiana University School of Medicine and associate director of clinical research at the IU Simon Comprehensive Cancer Center, Indianapolis, who was approached for comment.
Geographical variations
In their study, Dr. Hasset and colleagues set out determine the extent to which geospatial variations in early breast cancer care are attributable to health service area versus patient factors. They analyzed Surveillance, Epidemiology, and End Results (SEER) Medicare data for 31,571 patients with newly diagnosed with stage I-II nonmetastatic breast cancer between 2007 and 2013 who were followed for at least 3 years.
The patients had a median age of 71 years, and 61.4% had stage I disease at diagnosis.
Geospatial density maps (heat maps) in the paper highlight regional performance patterns. For initiation of ET within 1 year of diagnosis, the regions that appeared the worst (with less than 50% of patients getting this treatment) were parts of California, Utah, New Mexico, Louisiana, Georgia, Kentucky, Washington, and an isolated patch in Michigan.
In addition to the striking finding that nearly half of all women who are eligible for ET did not receive that therapy, the investigators found that 81.6% of 21,190 eligible patients received radiation therapy and 72.8% of 9,903 eligible patients received chemotherapy.
This also varied across the graphical regions, with the heat maps showing that the areas that were delivering radiation and chemotherapy to 70% to 80% of women were similar to the areas that were not initiating ET in about half of these women.
The authors found that the geographical region and health service area (HSA) explained more observed variation (24% to 48%) than patient factors (1% to 4%).
“While patient characteristics, such as race and ethnicity, were significantly associated with variation in breast cancer care, they explained a relatively small proportion of the total observed geospatial variance,” the authors comment.
“In fact, most of the total observed variance was owing to randomness or unexplained factors,” they add. The largest share of variation – 35% to 45% – was unexplained.
“The ET metrics demonstrated the largest total observed variance, the lowest absolute performance (only 49% of patients had an ET prescription within 1 year of diagnosis), and the strongest association with region/HSA,” they conclude.
Though limited by factors inherent in a retrospective review of SEER-Medicare data, the “unexplained nature of most geospatial variation in initial breast cancer care is not likely to change,” they comment.
Future quality improvement efforts should focus on reducing this unwarranted geospatial variation, particularly through the use of ET in eligible patients and with strategies that work across health care delivery systems, they suggest.
Approached for comment on the new findings, Dr. Miller posits that “many factors may be at play.”
“Unfortunately, the SEER database doesn’t allow us to sort out the impact of poverty/cost of care, distance to medical care, availability of specialty and subspecialty care, and payer/provider networks that may limit choices and options for second opinions,” Dr. Miller told this news organization.
She said that patients should be encouraged to consult reliable patient-focused information, such as that provided by the American Society of Clinical Oncology through its disease-specific sites, and to seek a second opinion from a university center. In many cases, major centers have become more accessible through virtual visits made available in the wake of the COVID-19 pandemic, she noted.
This study was supported by Dana-Farber Cancer Institute and the American Cancer Society. The authors and Dr. Miller have disclosed no relevant financial relationships. Dr. Miller is a regular contributor to Medscape with her Miller on Oncology column.
A version of this article first appeared on Medscape.com.
FROM JAMA ONCOLOGY
Researchers eye cannabis for gynecologic pain
Many women use cannabis to help manage gynecologic pain conditions.
When patients ask or tell clinicians about this treatment approach, however, few if any controlled trials exist to inform medical guidance.
A recent review of studies in this area presents a “thorough analysis of this very relevant topic,” said Erin A. Blake, MD, of Presbyterian Cancer Care, Rio Rancho, N.M..
The findings “are consistent with my anecdotal clinical findings as well as the results of my own research,” Dr. Blake said. “Cannabis products represent an underutilized but likely effective modality to relieve pain and other symptoms experienced by our patients.”
Mostly in the dark
Cannabis products “are unregulated and the data we have surrounding them is extremely limited due to outdated federal laws,” said Dr. Blake, who in 2019 described nonprescription cannabis use for symptom management by women with gynecologic malignancies. “Our ability to practice evidence-based medicine related to cannabis products will be limited until we are legally and financially able to design trials to evaluate them in a controlled fashion.”
For the new review, Jenell S. Coleman, MD, MPH, with Johns Hopkins University, Baltimore, and colleagues, identified 16 studies since 1990, including Dr. Blake’s, that examined the use of cannabinoids for managing pain from gynecologic conditions.
Dr. Coleman and her coauthors, Angela L. Liang and Erin L. Gingher, analyzed eight cross-sectional studies, six prospective studies, and two randomized controlled trials.
Patients who used cannabis tended to do so “multiple times per week, and they used a variety of delivery methods and a wide range of doses,” the authors said. “One of the most common reasons for cannabis use was pain management, and all the cross-sectional studies found that most women reported pain relief with cannabis use, especially among women who used a combination of CBD plus THC compared with either cannabinoid alone.”
Cross-sectional studies included patients with chronic pelvic pain (in two of the studies), vulvodynia (one), endometriosis (four), and gynecologic malignancy (two). These studies included between 36 and 3,426 participants and were conducted in the United States, Canada, Australia, and New Zealand.
In one Australian study, for example, Armour and colleagues asked 484 patients with endometriosis to rate the effectiveness of self-management strategies, including cannabis, heat, diet, and exercise, for reducing pelvic pain. Cannabis was used by 13% of the participants and had the highest average effectiveness rating: 7.6 on a 10-point scale.
In some cases, patients who use cannabis may decrease their use of other pain medications, the review found.
Cannabis side effects may include dry mouth, sleepiness, increased appetite, palpitations, and a “high” associated with THC.
Enhancing endogenous cannabinoids
The six prospective cohort studies and two randomized controlled trials examined the effectiveness of compounds – including palmitoylethanolamide (PEA) and a fatty acid amide hydrolase inhibitor – that can enhance endogenous cannabinoids.
Studies of PEA combined with antioxidants showed that these treatments significantly decreased pain from primary dysmenorrhea, pelvic pain, and interstitial cystitis. PEA-combination medications were well tolerated, with nausea and spotting as potential side effects.
On the other hand, a study that assessed a fatty acid amide hydrolase inhibitor found that it did not decrease pain from interstitial cystitis.
Dr. Coleman began reviewing the endocannabinoid system and cannabis research after hearing from patients who were using cannabis for pelvic pain.
Seeing various preclinical data that suggest cannabis could be useful for pain conditions came as a surprise.
Still, the existing evidence base for clinical effectiveness is poor quality, Dr. Coleman said in an interview. Rigorous trials are needed.
“It is a whole field that is just waiting for the U.S. to do something in terms of legalization so that we can actually study to see, does this make sense?” Dr. Coleman said.
Cannabis should not be used while pregnant
In a recent meta-analysis based on data from nearly 60,000 individuals, women who used marijuana during pregnancy were at increased risk for adverse neonatal outcomes such as low birth weight and preterm birth. Study author Greg J. Marchand, MD, of the Marchand Institute for Minimally Invasive Surgery, Mesa, Ariz., noted that the results will force some difficult decisions for mothers who use marijuana to treat medical problems, and that there may not be good substitute treatments for some of these conditions, especially chronic pain and anxiety.
Dr. Coleman disclosed investments in a cannabis exchange-traded fund. Dr. Blake and Dr. Marchand had no relevant financial disclosures.
Many women use cannabis to help manage gynecologic pain conditions.
When patients ask or tell clinicians about this treatment approach, however, few if any controlled trials exist to inform medical guidance.
A recent review of studies in this area presents a “thorough analysis of this very relevant topic,” said Erin A. Blake, MD, of Presbyterian Cancer Care, Rio Rancho, N.M..
The findings “are consistent with my anecdotal clinical findings as well as the results of my own research,” Dr. Blake said. “Cannabis products represent an underutilized but likely effective modality to relieve pain and other symptoms experienced by our patients.”
Mostly in the dark
Cannabis products “are unregulated and the data we have surrounding them is extremely limited due to outdated federal laws,” said Dr. Blake, who in 2019 described nonprescription cannabis use for symptom management by women with gynecologic malignancies. “Our ability to practice evidence-based medicine related to cannabis products will be limited until we are legally and financially able to design trials to evaluate them in a controlled fashion.”
For the new review, Jenell S. Coleman, MD, MPH, with Johns Hopkins University, Baltimore, and colleagues, identified 16 studies since 1990, including Dr. Blake’s, that examined the use of cannabinoids for managing pain from gynecologic conditions.
Dr. Coleman and her coauthors, Angela L. Liang and Erin L. Gingher, analyzed eight cross-sectional studies, six prospective studies, and two randomized controlled trials.
Patients who used cannabis tended to do so “multiple times per week, and they used a variety of delivery methods and a wide range of doses,” the authors said. “One of the most common reasons for cannabis use was pain management, and all the cross-sectional studies found that most women reported pain relief with cannabis use, especially among women who used a combination of CBD plus THC compared with either cannabinoid alone.”
Cross-sectional studies included patients with chronic pelvic pain (in two of the studies), vulvodynia (one), endometriosis (four), and gynecologic malignancy (two). These studies included between 36 and 3,426 participants and were conducted in the United States, Canada, Australia, and New Zealand.
In one Australian study, for example, Armour and colleagues asked 484 patients with endometriosis to rate the effectiveness of self-management strategies, including cannabis, heat, diet, and exercise, for reducing pelvic pain. Cannabis was used by 13% of the participants and had the highest average effectiveness rating: 7.6 on a 10-point scale.
In some cases, patients who use cannabis may decrease their use of other pain medications, the review found.
Cannabis side effects may include dry mouth, sleepiness, increased appetite, palpitations, and a “high” associated with THC.
Enhancing endogenous cannabinoids
The six prospective cohort studies and two randomized controlled trials examined the effectiveness of compounds – including palmitoylethanolamide (PEA) and a fatty acid amide hydrolase inhibitor – that can enhance endogenous cannabinoids.
Studies of PEA combined with antioxidants showed that these treatments significantly decreased pain from primary dysmenorrhea, pelvic pain, and interstitial cystitis. PEA-combination medications were well tolerated, with nausea and spotting as potential side effects.
On the other hand, a study that assessed a fatty acid amide hydrolase inhibitor found that it did not decrease pain from interstitial cystitis.
Dr. Coleman began reviewing the endocannabinoid system and cannabis research after hearing from patients who were using cannabis for pelvic pain.
Seeing various preclinical data that suggest cannabis could be useful for pain conditions came as a surprise.
Still, the existing evidence base for clinical effectiveness is poor quality, Dr. Coleman said in an interview. Rigorous trials are needed.
“It is a whole field that is just waiting for the U.S. to do something in terms of legalization so that we can actually study to see, does this make sense?” Dr. Coleman said.
Cannabis should not be used while pregnant
In a recent meta-analysis based on data from nearly 60,000 individuals, women who used marijuana during pregnancy were at increased risk for adverse neonatal outcomes such as low birth weight and preterm birth. Study author Greg J. Marchand, MD, of the Marchand Institute for Minimally Invasive Surgery, Mesa, Ariz., noted that the results will force some difficult decisions for mothers who use marijuana to treat medical problems, and that there may not be good substitute treatments for some of these conditions, especially chronic pain and anxiety.
Dr. Coleman disclosed investments in a cannabis exchange-traded fund. Dr. Blake and Dr. Marchand had no relevant financial disclosures.
Many women use cannabis to help manage gynecologic pain conditions.
When patients ask or tell clinicians about this treatment approach, however, few if any controlled trials exist to inform medical guidance.
A recent review of studies in this area presents a “thorough analysis of this very relevant topic,” said Erin A. Blake, MD, of Presbyterian Cancer Care, Rio Rancho, N.M..
The findings “are consistent with my anecdotal clinical findings as well as the results of my own research,” Dr. Blake said. “Cannabis products represent an underutilized but likely effective modality to relieve pain and other symptoms experienced by our patients.”
Mostly in the dark
Cannabis products “are unregulated and the data we have surrounding them is extremely limited due to outdated federal laws,” said Dr. Blake, who in 2019 described nonprescription cannabis use for symptom management by women with gynecologic malignancies. “Our ability to practice evidence-based medicine related to cannabis products will be limited until we are legally and financially able to design trials to evaluate them in a controlled fashion.”
For the new review, Jenell S. Coleman, MD, MPH, with Johns Hopkins University, Baltimore, and colleagues, identified 16 studies since 1990, including Dr. Blake’s, that examined the use of cannabinoids for managing pain from gynecologic conditions.
Dr. Coleman and her coauthors, Angela L. Liang and Erin L. Gingher, analyzed eight cross-sectional studies, six prospective studies, and two randomized controlled trials.
Patients who used cannabis tended to do so “multiple times per week, and they used a variety of delivery methods and a wide range of doses,” the authors said. “One of the most common reasons for cannabis use was pain management, and all the cross-sectional studies found that most women reported pain relief with cannabis use, especially among women who used a combination of CBD plus THC compared with either cannabinoid alone.”
Cross-sectional studies included patients with chronic pelvic pain (in two of the studies), vulvodynia (one), endometriosis (four), and gynecologic malignancy (two). These studies included between 36 and 3,426 participants and were conducted in the United States, Canada, Australia, and New Zealand.
In one Australian study, for example, Armour and colleagues asked 484 patients with endometriosis to rate the effectiveness of self-management strategies, including cannabis, heat, diet, and exercise, for reducing pelvic pain. Cannabis was used by 13% of the participants and had the highest average effectiveness rating: 7.6 on a 10-point scale.
In some cases, patients who use cannabis may decrease their use of other pain medications, the review found.
Cannabis side effects may include dry mouth, sleepiness, increased appetite, palpitations, and a “high” associated with THC.
Enhancing endogenous cannabinoids
The six prospective cohort studies and two randomized controlled trials examined the effectiveness of compounds – including palmitoylethanolamide (PEA) and a fatty acid amide hydrolase inhibitor – that can enhance endogenous cannabinoids.
Studies of PEA combined with antioxidants showed that these treatments significantly decreased pain from primary dysmenorrhea, pelvic pain, and interstitial cystitis. PEA-combination medications were well tolerated, with nausea and spotting as potential side effects.
On the other hand, a study that assessed a fatty acid amide hydrolase inhibitor found that it did not decrease pain from interstitial cystitis.
Dr. Coleman began reviewing the endocannabinoid system and cannabis research after hearing from patients who were using cannabis for pelvic pain.
Seeing various preclinical data that suggest cannabis could be useful for pain conditions came as a surprise.
Still, the existing evidence base for clinical effectiveness is poor quality, Dr. Coleman said in an interview. Rigorous trials are needed.
“It is a whole field that is just waiting for the U.S. to do something in terms of legalization so that we can actually study to see, does this make sense?” Dr. Coleman said.
Cannabis should not be used while pregnant
In a recent meta-analysis based on data from nearly 60,000 individuals, women who used marijuana during pregnancy were at increased risk for adverse neonatal outcomes such as low birth weight and preterm birth. Study author Greg J. Marchand, MD, of the Marchand Institute for Minimally Invasive Surgery, Mesa, Ariz., noted that the results will force some difficult decisions for mothers who use marijuana to treat medical problems, and that there may not be good substitute treatments for some of these conditions, especially chronic pain and anxiety.
Dr. Coleman disclosed investments in a cannabis exchange-traded fund. Dr. Blake and Dr. Marchand had no relevant financial disclosures.
FROM OBSTETRICS & GYNECOLOGY
A range of healthy dietary patterns can reduce risk of gout in women
A new study of thousands of women has found that sticking to recommended healthy dietary patterns can lessen the risk of new-onset gout.
“The identification of multiple patterns of eating that can similarly reduce a woman’s risk of incident gout in our study allows more choice for potential personalization of dietary recommendations according to culinary traditions and personal preferences to enhance adherence,” Chio Yokose, MD, of Harvard Medical School, Boston, and coauthors wrote. The study was published Jan. 31, 2022, in JAMA Internal Medicine.
To determine whether consistent healthy eating plays a role in preventing gout in women, the authors launched a prospective cohort study tied to the Nurses’ Health Study, an ongoing endeavor that has been questioning its participants’ food and beverage intake since 1984. Based on the 2020 to 2025 Dietary Guidelines for Americans, four healthy eating patterns were identified for assessment: the Dietary Approaches to Stop Hypertension (DASH), the Mediterranean diet, the Alternative Healthy Eating Index, and the Prudent diet, as well as the unhealthy Western dietary pattern for comparison.
Over 34 years of follow-up, the researchers identified 3,890 cases of gout among 80,039 women with an average age of 50.5 and an average body mass index (BMI) of 25.0 kg/m2. Women who strongly adhered to either of the four healthy dietary patterns had a significantly lower risk of gout, especially those who stuck to DASH (multivariable hazard ratio, 0.68; 95% confidence interval, 0.61-0.76) and Prudent (HR, 0.75; 95% CI, 0.73-0.90). In contrast, women with high Western diet scores had a 49% increased risk of gout (HR, 1.49; 95% CI, 1.33-1.68), compared with those who had low scores.
After additional analysis that factored in variables like diuretic use, alcohol use, and obesity, the associations between each diet and their risk of gout persisted in almost every instance. In particular, the most DASH-adherent women with normal BMI had a 68% lower risk of gout (HR, 0.32; 95% CI, 0.26-0.38), compared with the least-adherent women who were overweight or obese. Strong DASH adherence and no diuretic use also led to a 65% gout risk reduction (HR, 0.35; 95% CI, 0.30-0.41).
Healthy eating offers broad benefits for gout patients
“These results are consistent with a lot of the conversations we have on a day-to-day basis with patients,” Ted Mikuls, MD, of the University of Nebraska Medical Center, Omaha, said in an interview. “But I will say, I don’t get a lot of patients coming in saying: ‘Hey, what can I do to prevent gout?’ You’re usually seeing them after the fact.”
“These results shouldn’t be confused with that,” he said. “In other words, I wouldn’t want people interpreting this study to mean diet is always a satisfactory treatment for someone with established gout. The fact of the matter is, often it’s not. We need medication to effectively treat gout. I think this and other studies like it call for future research that can look at these dietary interventions as either standalone or probably adjuvant therapies in gout treatment.”
But, he added, that doesn’t mean conversations about diet aren’t of the utmost importance for gout patients.
“That shouldn’t stop clinicians from talking to patients about dietary changes that holistically are going to have positive benefits,” he said. “By the time you meet them, gout patients often already have other health conditions: high blood pressure, diabetes, obesity. The dietary changes that these authors studied are going to have a holistic benefit that goes well beyond gout risk, and that’s important. That’s a conversation that physicians and health care providers can and should be having right now with their patients.”
The authors acknowledged their study’s limitations, including the unmeasured or residual confounding that could come with any observational study as well as these rates of gout and these dietary patterns not necessarily being representative of a random sample of American women. “Future research could examine the population contributions of diets and other risk factors for incident female gout, as done in men.”
The study was funded by the National Institutes of Health. The authors reported several potential conflicts of interest, including receiving grants from the NIH and grants and personal fees from other organizations and pharmaceutical companies. Dr. Mikuls reported receiving past funding from Horizon Therapeutics and serving for them in a consulting capacity.
A new study of thousands of women has found that sticking to recommended healthy dietary patterns can lessen the risk of new-onset gout.
“The identification of multiple patterns of eating that can similarly reduce a woman’s risk of incident gout in our study allows more choice for potential personalization of dietary recommendations according to culinary traditions and personal preferences to enhance adherence,” Chio Yokose, MD, of Harvard Medical School, Boston, and coauthors wrote. The study was published Jan. 31, 2022, in JAMA Internal Medicine.
To determine whether consistent healthy eating plays a role in preventing gout in women, the authors launched a prospective cohort study tied to the Nurses’ Health Study, an ongoing endeavor that has been questioning its participants’ food and beverage intake since 1984. Based on the 2020 to 2025 Dietary Guidelines for Americans, four healthy eating patterns were identified for assessment: the Dietary Approaches to Stop Hypertension (DASH), the Mediterranean diet, the Alternative Healthy Eating Index, and the Prudent diet, as well as the unhealthy Western dietary pattern for comparison.
Over 34 years of follow-up, the researchers identified 3,890 cases of gout among 80,039 women with an average age of 50.5 and an average body mass index (BMI) of 25.0 kg/m2. Women who strongly adhered to either of the four healthy dietary patterns had a significantly lower risk of gout, especially those who stuck to DASH (multivariable hazard ratio, 0.68; 95% confidence interval, 0.61-0.76) and Prudent (HR, 0.75; 95% CI, 0.73-0.90). In contrast, women with high Western diet scores had a 49% increased risk of gout (HR, 1.49; 95% CI, 1.33-1.68), compared with those who had low scores.
After additional analysis that factored in variables like diuretic use, alcohol use, and obesity, the associations between each diet and their risk of gout persisted in almost every instance. In particular, the most DASH-adherent women with normal BMI had a 68% lower risk of gout (HR, 0.32; 95% CI, 0.26-0.38), compared with the least-adherent women who were overweight or obese. Strong DASH adherence and no diuretic use also led to a 65% gout risk reduction (HR, 0.35; 95% CI, 0.30-0.41).
Healthy eating offers broad benefits for gout patients
“These results are consistent with a lot of the conversations we have on a day-to-day basis with patients,” Ted Mikuls, MD, of the University of Nebraska Medical Center, Omaha, said in an interview. “But I will say, I don’t get a lot of patients coming in saying: ‘Hey, what can I do to prevent gout?’ You’re usually seeing them after the fact.”
“These results shouldn’t be confused with that,” he said. “In other words, I wouldn’t want people interpreting this study to mean diet is always a satisfactory treatment for someone with established gout. The fact of the matter is, often it’s not. We need medication to effectively treat gout. I think this and other studies like it call for future research that can look at these dietary interventions as either standalone or probably adjuvant therapies in gout treatment.”
But, he added, that doesn’t mean conversations about diet aren’t of the utmost importance for gout patients.
“That shouldn’t stop clinicians from talking to patients about dietary changes that holistically are going to have positive benefits,” he said. “By the time you meet them, gout patients often already have other health conditions: high blood pressure, diabetes, obesity. The dietary changes that these authors studied are going to have a holistic benefit that goes well beyond gout risk, and that’s important. That’s a conversation that physicians and health care providers can and should be having right now with their patients.”
The authors acknowledged their study’s limitations, including the unmeasured or residual confounding that could come with any observational study as well as these rates of gout and these dietary patterns not necessarily being representative of a random sample of American women. “Future research could examine the population contributions of diets and other risk factors for incident female gout, as done in men.”
The study was funded by the National Institutes of Health. The authors reported several potential conflicts of interest, including receiving grants from the NIH and grants and personal fees from other organizations and pharmaceutical companies. Dr. Mikuls reported receiving past funding from Horizon Therapeutics and serving for them in a consulting capacity.
A new study of thousands of women has found that sticking to recommended healthy dietary patterns can lessen the risk of new-onset gout.
“The identification of multiple patterns of eating that can similarly reduce a woman’s risk of incident gout in our study allows more choice for potential personalization of dietary recommendations according to culinary traditions and personal preferences to enhance adherence,” Chio Yokose, MD, of Harvard Medical School, Boston, and coauthors wrote. The study was published Jan. 31, 2022, in JAMA Internal Medicine.
To determine whether consistent healthy eating plays a role in preventing gout in women, the authors launched a prospective cohort study tied to the Nurses’ Health Study, an ongoing endeavor that has been questioning its participants’ food and beverage intake since 1984. Based on the 2020 to 2025 Dietary Guidelines for Americans, four healthy eating patterns were identified for assessment: the Dietary Approaches to Stop Hypertension (DASH), the Mediterranean diet, the Alternative Healthy Eating Index, and the Prudent diet, as well as the unhealthy Western dietary pattern for comparison.
Over 34 years of follow-up, the researchers identified 3,890 cases of gout among 80,039 women with an average age of 50.5 and an average body mass index (BMI) of 25.0 kg/m2. Women who strongly adhered to either of the four healthy dietary patterns had a significantly lower risk of gout, especially those who stuck to DASH (multivariable hazard ratio, 0.68; 95% confidence interval, 0.61-0.76) and Prudent (HR, 0.75; 95% CI, 0.73-0.90). In contrast, women with high Western diet scores had a 49% increased risk of gout (HR, 1.49; 95% CI, 1.33-1.68), compared with those who had low scores.
After additional analysis that factored in variables like diuretic use, alcohol use, and obesity, the associations between each diet and their risk of gout persisted in almost every instance. In particular, the most DASH-adherent women with normal BMI had a 68% lower risk of gout (HR, 0.32; 95% CI, 0.26-0.38), compared with the least-adherent women who were overweight or obese. Strong DASH adherence and no diuretic use also led to a 65% gout risk reduction (HR, 0.35; 95% CI, 0.30-0.41).
Healthy eating offers broad benefits for gout patients
“These results are consistent with a lot of the conversations we have on a day-to-day basis with patients,” Ted Mikuls, MD, of the University of Nebraska Medical Center, Omaha, said in an interview. “But I will say, I don’t get a lot of patients coming in saying: ‘Hey, what can I do to prevent gout?’ You’re usually seeing them after the fact.”
“These results shouldn’t be confused with that,” he said. “In other words, I wouldn’t want people interpreting this study to mean diet is always a satisfactory treatment for someone with established gout. The fact of the matter is, often it’s not. We need medication to effectively treat gout. I think this and other studies like it call for future research that can look at these dietary interventions as either standalone or probably adjuvant therapies in gout treatment.”
But, he added, that doesn’t mean conversations about diet aren’t of the utmost importance for gout patients.
“That shouldn’t stop clinicians from talking to patients about dietary changes that holistically are going to have positive benefits,” he said. “By the time you meet them, gout patients often already have other health conditions: high blood pressure, diabetes, obesity. The dietary changes that these authors studied are going to have a holistic benefit that goes well beyond gout risk, and that’s important. That’s a conversation that physicians and health care providers can and should be having right now with their patients.”
The authors acknowledged their study’s limitations, including the unmeasured or residual confounding that could come with any observational study as well as these rates of gout and these dietary patterns not necessarily being representative of a random sample of American women. “Future research could examine the population contributions of diets and other risk factors for incident female gout, as done in men.”
The study was funded by the National Institutes of Health. The authors reported several potential conflicts of interest, including receiving grants from the NIH and grants and personal fees from other organizations and pharmaceutical companies. Dr. Mikuls reported receiving past funding from Horizon Therapeutics and serving for them in a consulting capacity.
FROM JAMA INTERNAL MEDICINE
Marijuana use during pregnancy raised risk of adverse neonatal outcomes
Women who used marijuana during pregnancy were at increased risk for adverse neonatal outcomes, based on data from a meta-analysis of nearly 60,000 individuals.
Marijuana misuse remains a top substance use disorder and studies of prenatal use show a prevalence as high as 22% worldwide, wrote Greg J. Marchand, MD, of the Marchand Institute for Minimally Invasive Surgery, Mesa, Ariz., and colleagues.
“The prevalence of marijuana use during pregnancy may continue to increase, given that there is a suggested association between legalized recreational marijuana and increased use in prenatal and postpartum periods,” they wrote. “Remarkably, 34%-60% of individuals who use marijuana keep using it during pregnancy,” and many women cite a belief that marijuana is safe to use while pregnant, they noted.
Cannabinoid receptors are present in the developing fetus by the start of the second trimester, and exposure to exogenous cannabinoids may be associated with changes in the prefrontal cortex, including development and function, the researchers said. However, previous studies of an association between maternal marijuana use and poor neonatal outcomes have been inconsistent, they added.
In a study published in JAMA Network Open, the researchers identified 16 interventional and observational studies including 59,138 patients; each study included pregnant women who were exposed to marijuana, compared with those not exposed to marijuana, along with neonatal outcomes. The data selection included studies published until Aug. 16, 2021, and 10 studies were published in 2015 or later.
Overall, the risk for seven adverse neonatal outcomes was significantly increased among women who were exposed to marijuana during pregnancy, compared with those not exposed. The researchers identified increased risk for birth weight less than 2,500 g (relative risk, 2.06; P = .005), small for gestational age (RR, 1.61; P < .001), preterm delivery (RR, 1.28; P < .001), and NICU admission (RR, 1.38; P < .001). In addition, they found significant differences in mean birth weight (mean difference, −112.30 g; P < .001), Apgar score at 1 minute (mean difference, −0.26; P = .002), and infant head circumference (mean difference, −0.34cm; P = .02) between women who used marijuana during pregnancy and those who did not.
The study findings were limited by several factors, including the assessment of only cohort studies, which might suffer from bias given their retrospective designs, the researchers noted. Other limitations included the reliance on self-reports, the inability to adjust for tobacco/marijuana coexposure, and the lack of differentiation between levels of use and between different types of marijuana ingestion, they added.
However, the results support an association between marijuana use and adverse neonatal outcomes, and the researchers recommended additional studies of both maternal and neonatal outcomes associated with marijuana exposure. “Given increasing marijuana legalization and use worldwide, raising awareness and educating patients about these adverse outcomes may help to improve neonatal health,” they concluded.
New research prompted new review
The motivation to conduct this analysis at this time was prompted by the publication of several new, high-quality studies on the use of marijuana in pregnancy, according to Dr. Marchand. “It’s been a few years since a full analysis of all of the available data had been done, so we decided it was time to see if the old conclusions still held,” he said in an interview.
Dr. Marchand said he was surprised to see such a clear connection to preterm deliveries and lower birth weights. “When we perform a meta-analysis, we use all of the available data, and some important studies performed as recently as the past few years provided the depth of evidence behind these connections,” he said. “We didn’t have that level of evidence the last time this topic was studied only a few years ago,” he added.
The study is the largest meta-analysis on this topic to date, so the message to clinicians is highly significant, Dr. Marchand said. That message is “that we now have a very high level of evidence to say that smoking marijuana during pregnancy is harmful, and we (physicians especially) can no longer state that we just don’t know,” he said. “This is going to mean that deciding to smoke marijuana during your pregnancy is also deciding to do something that can harm your baby,” he emphasized. “This paper also will force some difficult decisions for mothers who use marijuana to treat medical problems, and there may not be good substitute treatments for some of these conditions, especially chronic pain and anxiety,” Dr. Marchand noted. “This will set up a difficult risk-versus-benefits situation, where these mothers, ideally with the help of their physicians, will have to decide if the risks of stopping marijuana outweigh the possible harm to the unborn baby,” he said.
As for additional research, long-term studies to assess behavioral changes as exposed children grow up would be beneficial, Dr. Marchand said. Such studies “could really help us balance the risk of marijuana exposure in pregnancy, especially if it is being used to treat serious medical conditions,” he noted.
Findings are a call to action
The view among many women that prenatal cannabis use is safe and without consequence “is a false narrative perpetuated by a combination of outdated evidence and recent changes to state-level cannabis policies,” wrote Kara R. Skelton, PhD, of Towson (Md.) University, and Sara E. Benjamin-Neelon, PhD, of Johns Hopkins University, Baltimore, in an accompanying editorial.
The findings from the current study add to the growing evidence that prenatal cannabis use is associated with adverse birth outcomes, they wrote. “Clinician-directed communication about cannabis has been criticized by pregnant women, with recent findings supporting a need for increased cannabis communication by clinicians,” and not only clinicians, but all health professionals who encounter women who are pregnant or attempting pregnancy should not miss the opportunity to communicate the risks of prenatal cannabis use, they emphasized.
The authors highlighted some of the current study’s limitations, including the inability to determine a dose-response association, the reliance on self-reports, and the lack of adjustment for tobacco/marijuana coexposure. However, they noted that the inclusion of recent studies (10 published in 2015 or later) strengthens the results because of the significant increase in the potency of Δ-9-tetrahydrocannabinol (THC), the main psychoactive ingredient in cannabis, in recent decades.
“We urge clinicians, public health professionals, and policy makers to carefully consider the consequences of in utero cannabis exposure identified by Marchand et al. and partner to ensure prioritization of infant and child health during this time of precipitous cannabis legalization and commercialization,” the authors emphasized. “Without necessary safeguards to protect neonatal health, prenatal cannabis use poses a substantial threat to current and future generations of children,” they wrote.
The study received no outside funding. The researchers had no financial conflicts to disclose. The editorialists had no financial conflicts to disclose.
Women who used marijuana during pregnancy were at increased risk for adverse neonatal outcomes, based on data from a meta-analysis of nearly 60,000 individuals.
Marijuana misuse remains a top substance use disorder and studies of prenatal use show a prevalence as high as 22% worldwide, wrote Greg J. Marchand, MD, of the Marchand Institute for Minimally Invasive Surgery, Mesa, Ariz., and colleagues.
“The prevalence of marijuana use during pregnancy may continue to increase, given that there is a suggested association between legalized recreational marijuana and increased use in prenatal and postpartum periods,” they wrote. “Remarkably, 34%-60% of individuals who use marijuana keep using it during pregnancy,” and many women cite a belief that marijuana is safe to use while pregnant, they noted.
Cannabinoid receptors are present in the developing fetus by the start of the second trimester, and exposure to exogenous cannabinoids may be associated with changes in the prefrontal cortex, including development and function, the researchers said. However, previous studies of an association between maternal marijuana use and poor neonatal outcomes have been inconsistent, they added.
In a study published in JAMA Network Open, the researchers identified 16 interventional and observational studies including 59,138 patients; each study included pregnant women who were exposed to marijuana, compared with those not exposed to marijuana, along with neonatal outcomes. The data selection included studies published until Aug. 16, 2021, and 10 studies were published in 2015 or later.
Overall, the risk for seven adverse neonatal outcomes was significantly increased among women who were exposed to marijuana during pregnancy, compared with those not exposed. The researchers identified increased risk for birth weight less than 2,500 g (relative risk, 2.06; P = .005), small for gestational age (RR, 1.61; P < .001), preterm delivery (RR, 1.28; P < .001), and NICU admission (RR, 1.38; P < .001). In addition, they found significant differences in mean birth weight (mean difference, −112.30 g; P < .001), Apgar score at 1 minute (mean difference, −0.26; P = .002), and infant head circumference (mean difference, −0.34cm; P = .02) between women who used marijuana during pregnancy and those who did not.
The study findings were limited by several factors, including the assessment of only cohort studies, which might suffer from bias given their retrospective designs, the researchers noted. Other limitations included the reliance on self-reports, the inability to adjust for tobacco/marijuana coexposure, and the lack of differentiation between levels of use and between different types of marijuana ingestion, they added.
However, the results support an association between marijuana use and adverse neonatal outcomes, and the researchers recommended additional studies of both maternal and neonatal outcomes associated with marijuana exposure. “Given increasing marijuana legalization and use worldwide, raising awareness and educating patients about these adverse outcomes may help to improve neonatal health,” they concluded.
New research prompted new review
The motivation to conduct this analysis at this time was prompted by the publication of several new, high-quality studies on the use of marijuana in pregnancy, according to Dr. Marchand. “It’s been a few years since a full analysis of all of the available data had been done, so we decided it was time to see if the old conclusions still held,” he said in an interview.
Dr. Marchand said he was surprised to see such a clear connection to preterm deliveries and lower birth weights. “When we perform a meta-analysis, we use all of the available data, and some important studies performed as recently as the past few years provided the depth of evidence behind these connections,” he said. “We didn’t have that level of evidence the last time this topic was studied only a few years ago,” he added.
The study is the largest meta-analysis on this topic to date, so the message to clinicians is highly significant, Dr. Marchand said. That message is “that we now have a very high level of evidence to say that smoking marijuana during pregnancy is harmful, and we (physicians especially) can no longer state that we just don’t know,” he said. “This is going to mean that deciding to smoke marijuana during your pregnancy is also deciding to do something that can harm your baby,” he emphasized. “This paper also will force some difficult decisions for mothers who use marijuana to treat medical problems, and there may not be good substitute treatments for some of these conditions, especially chronic pain and anxiety,” Dr. Marchand noted. “This will set up a difficult risk-versus-benefits situation, where these mothers, ideally with the help of their physicians, will have to decide if the risks of stopping marijuana outweigh the possible harm to the unborn baby,” he said.
As for additional research, long-term studies to assess behavioral changes as exposed children grow up would be beneficial, Dr. Marchand said. Such studies “could really help us balance the risk of marijuana exposure in pregnancy, especially if it is being used to treat serious medical conditions,” he noted.
Findings are a call to action
The view among many women that prenatal cannabis use is safe and without consequence “is a false narrative perpetuated by a combination of outdated evidence and recent changes to state-level cannabis policies,” wrote Kara R. Skelton, PhD, of Towson (Md.) University, and Sara E. Benjamin-Neelon, PhD, of Johns Hopkins University, Baltimore, in an accompanying editorial.
The findings from the current study add to the growing evidence that prenatal cannabis use is associated with adverse birth outcomes, they wrote. “Clinician-directed communication about cannabis has been criticized by pregnant women, with recent findings supporting a need for increased cannabis communication by clinicians,” and not only clinicians, but all health professionals who encounter women who are pregnant or attempting pregnancy should not miss the opportunity to communicate the risks of prenatal cannabis use, they emphasized.
The authors highlighted some of the current study’s limitations, including the inability to determine a dose-response association, the reliance on self-reports, and the lack of adjustment for tobacco/marijuana coexposure. However, they noted that the inclusion of recent studies (10 published in 2015 or later) strengthens the results because of the significant increase in the potency of Δ-9-tetrahydrocannabinol (THC), the main psychoactive ingredient in cannabis, in recent decades.
“We urge clinicians, public health professionals, and policy makers to carefully consider the consequences of in utero cannabis exposure identified by Marchand et al. and partner to ensure prioritization of infant and child health during this time of precipitous cannabis legalization and commercialization,” the authors emphasized. “Without necessary safeguards to protect neonatal health, prenatal cannabis use poses a substantial threat to current and future generations of children,” they wrote.
The study received no outside funding. The researchers had no financial conflicts to disclose. The editorialists had no financial conflicts to disclose.
Women who used marijuana during pregnancy were at increased risk for adverse neonatal outcomes, based on data from a meta-analysis of nearly 60,000 individuals.
Marijuana misuse remains a top substance use disorder and studies of prenatal use show a prevalence as high as 22% worldwide, wrote Greg J. Marchand, MD, of the Marchand Institute for Minimally Invasive Surgery, Mesa, Ariz., and colleagues.
“The prevalence of marijuana use during pregnancy may continue to increase, given that there is a suggested association between legalized recreational marijuana and increased use in prenatal and postpartum periods,” they wrote. “Remarkably, 34%-60% of individuals who use marijuana keep using it during pregnancy,” and many women cite a belief that marijuana is safe to use while pregnant, they noted.
Cannabinoid receptors are present in the developing fetus by the start of the second trimester, and exposure to exogenous cannabinoids may be associated with changes in the prefrontal cortex, including development and function, the researchers said. However, previous studies of an association between maternal marijuana use and poor neonatal outcomes have been inconsistent, they added.
In a study published in JAMA Network Open, the researchers identified 16 interventional and observational studies including 59,138 patients; each study included pregnant women who were exposed to marijuana, compared with those not exposed to marijuana, along with neonatal outcomes. The data selection included studies published until Aug. 16, 2021, and 10 studies were published in 2015 or later.
Overall, the risk for seven adverse neonatal outcomes was significantly increased among women who were exposed to marijuana during pregnancy, compared with those not exposed. The researchers identified increased risk for birth weight less than 2,500 g (relative risk, 2.06; P = .005), small for gestational age (RR, 1.61; P < .001), preterm delivery (RR, 1.28; P < .001), and NICU admission (RR, 1.38; P < .001). In addition, they found significant differences in mean birth weight (mean difference, −112.30 g; P < .001), Apgar score at 1 minute (mean difference, −0.26; P = .002), and infant head circumference (mean difference, −0.34cm; P = .02) between women who used marijuana during pregnancy and those who did not.
The study findings were limited by several factors, including the assessment of only cohort studies, which might suffer from bias given their retrospective designs, the researchers noted. Other limitations included the reliance on self-reports, the inability to adjust for tobacco/marijuana coexposure, and the lack of differentiation between levels of use and between different types of marijuana ingestion, they added.
However, the results support an association between marijuana use and adverse neonatal outcomes, and the researchers recommended additional studies of both maternal and neonatal outcomes associated with marijuana exposure. “Given increasing marijuana legalization and use worldwide, raising awareness and educating patients about these adverse outcomes may help to improve neonatal health,” they concluded.
New research prompted new review
The motivation to conduct this analysis at this time was prompted by the publication of several new, high-quality studies on the use of marijuana in pregnancy, according to Dr. Marchand. “It’s been a few years since a full analysis of all of the available data had been done, so we decided it was time to see if the old conclusions still held,” he said in an interview.
Dr. Marchand said he was surprised to see such a clear connection to preterm deliveries and lower birth weights. “When we perform a meta-analysis, we use all of the available data, and some important studies performed as recently as the past few years provided the depth of evidence behind these connections,” he said. “We didn’t have that level of evidence the last time this topic was studied only a few years ago,” he added.
The study is the largest meta-analysis on this topic to date, so the message to clinicians is highly significant, Dr. Marchand said. That message is “that we now have a very high level of evidence to say that smoking marijuana during pregnancy is harmful, and we (physicians especially) can no longer state that we just don’t know,” he said. “This is going to mean that deciding to smoke marijuana during your pregnancy is also deciding to do something that can harm your baby,” he emphasized. “This paper also will force some difficult decisions for mothers who use marijuana to treat medical problems, and there may not be good substitute treatments for some of these conditions, especially chronic pain and anxiety,” Dr. Marchand noted. “This will set up a difficult risk-versus-benefits situation, where these mothers, ideally with the help of their physicians, will have to decide if the risks of stopping marijuana outweigh the possible harm to the unborn baby,” he said.
As for additional research, long-term studies to assess behavioral changes as exposed children grow up would be beneficial, Dr. Marchand said. Such studies “could really help us balance the risk of marijuana exposure in pregnancy, especially if it is being used to treat serious medical conditions,” he noted.
Findings are a call to action
The view among many women that prenatal cannabis use is safe and without consequence “is a false narrative perpetuated by a combination of outdated evidence and recent changes to state-level cannabis policies,” wrote Kara R. Skelton, PhD, of Towson (Md.) University, and Sara E. Benjamin-Neelon, PhD, of Johns Hopkins University, Baltimore, in an accompanying editorial.
The findings from the current study add to the growing evidence that prenatal cannabis use is associated with adverse birth outcomes, they wrote. “Clinician-directed communication about cannabis has been criticized by pregnant women, with recent findings supporting a need for increased cannabis communication by clinicians,” and not only clinicians, but all health professionals who encounter women who are pregnant or attempting pregnancy should not miss the opportunity to communicate the risks of prenatal cannabis use, they emphasized.
The authors highlighted some of the current study’s limitations, including the inability to determine a dose-response association, the reliance on self-reports, and the lack of adjustment for tobacco/marijuana coexposure. However, they noted that the inclusion of recent studies (10 published in 2015 or later) strengthens the results because of the significant increase in the potency of Δ-9-tetrahydrocannabinol (THC), the main psychoactive ingredient in cannabis, in recent decades.
“We urge clinicians, public health professionals, and policy makers to carefully consider the consequences of in utero cannabis exposure identified by Marchand et al. and partner to ensure prioritization of infant and child health during this time of precipitous cannabis legalization and commercialization,” the authors emphasized. “Without necessary safeguards to protect neonatal health, prenatal cannabis use poses a substantial threat to current and future generations of children,” they wrote.
The study received no outside funding. The researchers had no financial conflicts to disclose. The editorialists had no financial conflicts to disclose.
FROM JAMA NETWORK OPEN
Primary care docs have role to play in hypertension prevention and treatment for women of reproductive age
The American Heart Association recently released a scientific statement concerning hypertension in pregnancy, which laid out the variety of disorders, the epidemiology, the future impact of pregnant persons, and the current debates regarding treatment and diagnosis.
This statement addresses all stages from preconception through post pregnancy and outlines the many prevention and treatment options available. Although family physicians were not specifically called out to be partners in the statement, we have a large role to play for both our pregnant patients and those of reproductive age who are not pregnant.
Preconception health
One of the first things pointed out was preconception health. Regardless of whether each individual family physician provides prenatal care, we can all focus on preconception health for those of reproductive age.
The statement from the AHA points out that “lifestyle changes before and during pregnancy may ameliorate both maternal and fetal risks.”
As many already do, family physicians should focus on encouraging their patients to practice healthy eating and exercise prior to pregnancy to help establish routines that will decrease the risk of hypertensive disorders in pregnancy.
Focusing on care prior to pregnancy also allows the primary care provider to be involved in quickly linking patients to prenatal care, as it is well established that early and complete prenatal care is important for improving outcomes.
Later-in-life pregnancy
The AHA also highlights that many are choosing to have pregnancies at older ages and with greater comorbidities than in past years. This is another area in which family physicians can provide important care.
We can help by first identifying the chronic conditions, such as hypertension and diabetes, that make the hypertensive disorders of pregnancy more likely. We should then focus on the treatment of these conditions during the preconception time so that they are well controlled prior to pregnancy.
We should also preferentially choose medications that our patients will be able to continue in pregnancy, so that control may be maintained throughout pregnancy.
The statement particularly highlights the avoidance of antihypertensives that are renin-angiotensin system blockers.
We can also help prepare our patients for the additional medications, testing, and precautions they will likely require during their pregnancy so that they know what to expect.
Family physicians are also already starting to utilize home blood pressure monitoring and can introduce this method so that patients may continue to monitor their blood pressures during pregnancy.
Throughout pregnancy, the new statement calls in the current debates of when prenatal care providers should be diagnosing hypertensive disorders and the goals of treatment.
Prenatal care providers can use shared decision-making for medication choices and blood pressure goals. They can also continue to encourage the healthy lifestyle choices such as diet and exercise to reduce the risk of poor outcomes.
This AHA also indicates that prenatal care providers can integrate the use of home blood pressure monitoring as they monitor the blood pressure for patients with hypertensive disorders of pregnancy.
Postpartum care
The postpartum period is another crucial time for family physicians and other primary care providers to greatly impact their patients with hypertensive diseases of pregnancy.
They can work to ensure that blood pressure is closely monitored and controlled, including by prescribing diuretics, which are typically not used during pregnancy.
If a patient’s blood pressure does not go down on its own, the primary care provider can begin treatment for hypertension outside of pregnancy. This can decrease their long-term cardiac risk factors and provide control prior to any future potential pregnancies.
Providing care during this postpartum time also offers a great opportunity to again encourage lifestyle options that may decrease risk.
Family physicians and other primary care providers can also encourage their patient to be involved in registries that gather data on hypertensive disorders in pregnancy.
In the new statement, the AHA acknowledges the great number of things that are not yet known or fully understood and the health inequities that many face.
Family physicians are positioned to help advocate for their patients and utilize a team-based approach to help provide resources to patients. We must continue to be there for our patients at every stage of their lives to help them live their healthiest lives possible.
The statement also indicates that there may be genetic factors at play more than social determinants of health. It is important to identify what those are for the best care of our patients while ensuring we are doing our best to provide our patients with the resources they need.
Dr. Wheat is a family physician at Erie Family Health Center and program director of Northwestern University’s McGaw Family Medicine residency program, both in Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
The American Heart Association recently released a scientific statement concerning hypertension in pregnancy, which laid out the variety of disorders, the epidemiology, the future impact of pregnant persons, and the current debates regarding treatment and diagnosis.
This statement addresses all stages from preconception through post pregnancy and outlines the many prevention and treatment options available. Although family physicians were not specifically called out to be partners in the statement, we have a large role to play for both our pregnant patients and those of reproductive age who are not pregnant.
Preconception health
One of the first things pointed out was preconception health. Regardless of whether each individual family physician provides prenatal care, we can all focus on preconception health for those of reproductive age.
The statement from the AHA points out that “lifestyle changes before and during pregnancy may ameliorate both maternal and fetal risks.”
As many already do, family physicians should focus on encouraging their patients to practice healthy eating and exercise prior to pregnancy to help establish routines that will decrease the risk of hypertensive disorders in pregnancy.
Focusing on care prior to pregnancy also allows the primary care provider to be involved in quickly linking patients to prenatal care, as it is well established that early and complete prenatal care is important for improving outcomes.
Later-in-life pregnancy
The AHA also highlights that many are choosing to have pregnancies at older ages and with greater comorbidities than in past years. This is another area in which family physicians can provide important care.
We can help by first identifying the chronic conditions, such as hypertension and diabetes, that make the hypertensive disorders of pregnancy more likely. We should then focus on the treatment of these conditions during the preconception time so that they are well controlled prior to pregnancy.
We should also preferentially choose medications that our patients will be able to continue in pregnancy, so that control may be maintained throughout pregnancy.
The statement particularly highlights the avoidance of antihypertensives that are renin-angiotensin system blockers.
We can also help prepare our patients for the additional medications, testing, and precautions they will likely require during their pregnancy so that they know what to expect.
Family physicians are also already starting to utilize home blood pressure monitoring and can introduce this method so that patients may continue to monitor their blood pressures during pregnancy.
Throughout pregnancy, the new statement calls in the current debates of when prenatal care providers should be diagnosing hypertensive disorders and the goals of treatment.
Prenatal care providers can use shared decision-making for medication choices and blood pressure goals. They can also continue to encourage the healthy lifestyle choices such as diet and exercise to reduce the risk of poor outcomes.
This AHA also indicates that prenatal care providers can integrate the use of home blood pressure monitoring as they monitor the blood pressure for patients with hypertensive disorders of pregnancy.
Postpartum care
The postpartum period is another crucial time for family physicians and other primary care providers to greatly impact their patients with hypertensive diseases of pregnancy.
They can work to ensure that blood pressure is closely monitored and controlled, including by prescribing diuretics, which are typically not used during pregnancy.
If a patient’s blood pressure does not go down on its own, the primary care provider can begin treatment for hypertension outside of pregnancy. This can decrease their long-term cardiac risk factors and provide control prior to any future potential pregnancies.
Providing care during this postpartum time also offers a great opportunity to again encourage lifestyle options that may decrease risk.
Family physicians and other primary care providers can also encourage their patient to be involved in registries that gather data on hypertensive disorders in pregnancy.
In the new statement, the AHA acknowledges the great number of things that are not yet known or fully understood and the health inequities that many face.
Family physicians are positioned to help advocate for their patients and utilize a team-based approach to help provide resources to patients. We must continue to be there for our patients at every stage of their lives to help them live their healthiest lives possible.
The statement also indicates that there may be genetic factors at play more than social determinants of health. It is important to identify what those are for the best care of our patients while ensuring we are doing our best to provide our patients with the resources they need.
Dr. Wheat is a family physician at Erie Family Health Center and program director of Northwestern University’s McGaw Family Medicine residency program, both in Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
The American Heart Association recently released a scientific statement concerning hypertension in pregnancy, which laid out the variety of disorders, the epidemiology, the future impact of pregnant persons, and the current debates regarding treatment and diagnosis.
This statement addresses all stages from preconception through post pregnancy and outlines the many prevention and treatment options available. Although family physicians were not specifically called out to be partners in the statement, we have a large role to play for both our pregnant patients and those of reproductive age who are not pregnant.
Preconception health
One of the first things pointed out was preconception health. Regardless of whether each individual family physician provides prenatal care, we can all focus on preconception health for those of reproductive age.
The statement from the AHA points out that “lifestyle changes before and during pregnancy may ameliorate both maternal and fetal risks.”
As many already do, family physicians should focus on encouraging their patients to practice healthy eating and exercise prior to pregnancy to help establish routines that will decrease the risk of hypertensive disorders in pregnancy.
Focusing on care prior to pregnancy also allows the primary care provider to be involved in quickly linking patients to prenatal care, as it is well established that early and complete prenatal care is important for improving outcomes.
Later-in-life pregnancy
The AHA also highlights that many are choosing to have pregnancies at older ages and with greater comorbidities than in past years. This is another area in which family physicians can provide important care.
We can help by first identifying the chronic conditions, such as hypertension and diabetes, that make the hypertensive disorders of pregnancy more likely. We should then focus on the treatment of these conditions during the preconception time so that they are well controlled prior to pregnancy.
We should also preferentially choose medications that our patients will be able to continue in pregnancy, so that control may be maintained throughout pregnancy.
The statement particularly highlights the avoidance of antihypertensives that are renin-angiotensin system blockers.
We can also help prepare our patients for the additional medications, testing, and precautions they will likely require during their pregnancy so that they know what to expect.
Family physicians are also already starting to utilize home blood pressure monitoring and can introduce this method so that patients may continue to monitor their blood pressures during pregnancy.
Throughout pregnancy, the new statement calls in the current debates of when prenatal care providers should be diagnosing hypertensive disorders and the goals of treatment.
Prenatal care providers can use shared decision-making for medication choices and blood pressure goals. They can also continue to encourage the healthy lifestyle choices such as diet and exercise to reduce the risk of poor outcomes.
This AHA also indicates that prenatal care providers can integrate the use of home blood pressure monitoring as they monitor the blood pressure for patients with hypertensive disorders of pregnancy.
Postpartum care
The postpartum period is another crucial time for family physicians and other primary care providers to greatly impact their patients with hypertensive diseases of pregnancy.
They can work to ensure that blood pressure is closely monitored and controlled, including by prescribing diuretics, which are typically not used during pregnancy.
If a patient’s blood pressure does not go down on its own, the primary care provider can begin treatment for hypertension outside of pregnancy. This can decrease their long-term cardiac risk factors and provide control prior to any future potential pregnancies.
Providing care during this postpartum time also offers a great opportunity to again encourage lifestyle options that may decrease risk.
Family physicians and other primary care providers can also encourage their patient to be involved in registries that gather data on hypertensive disorders in pregnancy.
In the new statement, the AHA acknowledges the great number of things that are not yet known or fully understood and the health inequities that many face.
Family physicians are positioned to help advocate for their patients and utilize a team-based approach to help provide resources to patients. We must continue to be there for our patients at every stage of their lives to help them live their healthiest lives possible.
The statement also indicates that there may be genetic factors at play more than social determinants of health. It is important to identify what those are for the best care of our patients while ensuring we are doing our best to provide our patients with the resources they need.
Dr. Wheat is a family physician at Erie Family Health Center and program director of Northwestern University’s McGaw Family Medicine residency program, both in Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
Moderate-vigorous stepping seen to lower diabetes risk in older women
More steps per day, particularly at a higher intensity, may reduce the risk of type 2 diabetes in older women, based on a prospective cohort study.
The link between daily stepping and diabetes was not significantly modified by body mass index (BMI) or other common diabetes risk factors, suggesting that the relationship is highly generalizable, lead author Alexis C. Garduno, MPH, a PhD student at the University of California, San Diego, and colleagues reported.
“Physical activity is a key modifiable behavior for diabetes prevention and management,” the investigators wrote in Diabetes Care. “Many prevention studies have demonstrated that regular physical activity, along with improved diet, reduces the risk of diabetes in adults. ... To the best of our knowledge, there are few studies examining the association between objectively measured steps per day and incident diabetes in a community-based setting.”
To this end, the investigators analyzed data from 4,838 older, community-living women in the Objective Physical Activity and Cardiovascular Health Study. Upon enrollment, women were without physician-diagnosed diabetes and had a mean age of 78.9 years. For 1 week, participants wore ActiGraph GT3X+ accelerometers to measure steps per day, as well as step intensity, graded as light or moderate to vigorous.
The relationship between daily activity and diabetes was analyzed using three multivariate models: The first included race/ethnicity and age; the second also included family history of diabetes, education, physical functioning, self-rated health, smoking status, and alcohol consumption; and the third added BMI, “a potential mediator in the causal pathway between steps per day and diabetes,” the investigators wrote.
Participants took an average of 3,729 steps per day, divided roughly evenly between light and moderate to vigorous intensity.
After a median follow-up of 5.7 years, 8.1% of women developed diabetes. The least-adjusted model showed a 14% reduction in diabetes risk per 2,000 steps (hazard ratio, 0.86; 95% confidence interval, 0.80-0.92; P = .007), whereas the second model, adjusting for more confounding variables, showed a 12% reduction in diabetes risk per 2,000 steps (HR, 0.88; 95% CI, 0.78-1.00; P = .045).
The final model, which added BMI, showed a 10% reduction in risk, although it didn’t reach statistical significance (HR, 0.90; 95% CI, 0.80-1.02; P = .11). Furthermore, accelerated failure time models suggested that BMI did not significantly impact the link between steps and diabetes (proportion mediated, 17.7%;95% CI, –55.0 to 142.0; P = .09). Further analyses also found no significant interactions between BMI or other possible confounders.
“The steps per day–diabetes association was not modified by age, race/ethnicity, BMI, physical functioning, or family history of diabetes, which supports the generalizability of these findings to community-living older women,” the investigators wrote.
Increased stepping intensity also appeared to lower risk of diabetes. After adjusting for confounding variables, light stepping was not linked to reduced risk (HR, 0.97; 95% CI, 0.73-1.29; P = .83), whereas moderate to vigorous stepping reduced risk by 14% per 2,000 steps (HR, 0.86; 95% CI, 0.74-1.00; P = .04).
“This study provides evidence supporting an association between steps per day and lower incident diabetes,” the investigators concluded. “While further work is needed to identify whether there is a minimum number of steps per day that results in a clinically significant reduction of diabetes and to evaluate the role that step intensity plays in diabetes etiology for older adults, findings from this study suggest that moderate-vigorous–intensity steps may be more important than lower-intensity steps with respect to incident diabetes. Steps per day–based interventions are needed to advance diabetes prevention science in older adults.”
The study was supported by the National Institute on Aging, the National Institute of Diabetes and Digestive and Kidney Diseases, the Tobacco-Related Disease Research Program, and others. The investigators had no potential conflicts of interest.
More steps per day, particularly at a higher intensity, may reduce the risk of type 2 diabetes in older women, based on a prospective cohort study.
The link between daily stepping and diabetes was not significantly modified by body mass index (BMI) or other common diabetes risk factors, suggesting that the relationship is highly generalizable, lead author Alexis C. Garduno, MPH, a PhD student at the University of California, San Diego, and colleagues reported.
“Physical activity is a key modifiable behavior for diabetes prevention and management,” the investigators wrote in Diabetes Care. “Many prevention studies have demonstrated that regular physical activity, along with improved diet, reduces the risk of diabetes in adults. ... To the best of our knowledge, there are few studies examining the association between objectively measured steps per day and incident diabetes in a community-based setting.”
To this end, the investigators analyzed data from 4,838 older, community-living women in the Objective Physical Activity and Cardiovascular Health Study. Upon enrollment, women were without physician-diagnosed diabetes and had a mean age of 78.9 years. For 1 week, participants wore ActiGraph GT3X+ accelerometers to measure steps per day, as well as step intensity, graded as light or moderate to vigorous.
The relationship between daily activity and diabetes was analyzed using three multivariate models: The first included race/ethnicity and age; the second also included family history of diabetes, education, physical functioning, self-rated health, smoking status, and alcohol consumption; and the third added BMI, “a potential mediator in the causal pathway between steps per day and diabetes,” the investigators wrote.
Participants took an average of 3,729 steps per day, divided roughly evenly between light and moderate to vigorous intensity.
After a median follow-up of 5.7 years, 8.1% of women developed diabetes. The least-adjusted model showed a 14% reduction in diabetes risk per 2,000 steps (hazard ratio, 0.86; 95% confidence interval, 0.80-0.92; P = .007), whereas the second model, adjusting for more confounding variables, showed a 12% reduction in diabetes risk per 2,000 steps (HR, 0.88; 95% CI, 0.78-1.00; P = .045).
The final model, which added BMI, showed a 10% reduction in risk, although it didn’t reach statistical significance (HR, 0.90; 95% CI, 0.80-1.02; P = .11). Furthermore, accelerated failure time models suggested that BMI did not significantly impact the link between steps and diabetes (proportion mediated, 17.7%;95% CI, –55.0 to 142.0; P = .09). Further analyses also found no significant interactions between BMI or other possible confounders.
“The steps per day–diabetes association was not modified by age, race/ethnicity, BMI, physical functioning, or family history of diabetes, which supports the generalizability of these findings to community-living older women,” the investigators wrote.
Increased stepping intensity also appeared to lower risk of diabetes. After adjusting for confounding variables, light stepping was not linked to reduced risk (HR, 0.97; 95% CI, 0.73-1.29; P = .83), whereas moderate to vigorous stepping reduced risk by 14% per 2,000 steps (HR, 0.86; 95% CI, 0.74-1.00; P = .04).
“This study provides evidence supporting an association between steps per day and lower incident diabetes,” the investigators concluded. “While further work is needed to identify whether there is a minimum number of steps per day that results in a clinically significant reduction of diabetes and to evaluate the role that step intensity plays in diabetes etiology for older adults, findings from this study suggest that moderate-vigorous–intensity steps may be more important than lower-intensity steps with respect to incident diabetes. Steps per day–based interventions are needed to advance diabetes prevention science in older adults.”
The study was supported by the National Institute on Aging, the National Institute of Diabetes and Digestive and Kidney Diseases, the Tobacco-Related Disease Research Program, and others. The investigators had no potential conflicts of interest.
More steps per day, particularly at a higher intensity, may reduce the risk of type 2 diabetes in older women, based on a prospective cohort study.
The link between daily stepping and diabetes was not significantly modified by body mass index (BMI) or other common diabetes risk factors, suggesting that the relationship is highly generalizable, lead author Alexis C. Garduno, MPH, a PhD student at the University of California, San Diego, and colleagues reported.
“Physical activity is a key modifiable behavior for diabetes prevention and management,” the investigators wrote in Diabetes Care. “Many prevention studies have demonstrated that regular physical activity, along with improved diet, reduces the risk of diabetes in adults. ... To the best of our knowledge, there are few studies examining the association between objectively measured steps per day and incident diabetes in a community-based setting.”
To this end, the investigators analyzed data from 4,838 older, community-living women in the Objective Physical Activity and Cardiovascular Health Study. Upon enrollment, women were without physician-diagnosed diabetes and had a mean age of 78.9 years. For 1 week, participants wore ActiGraph GT3X+ accelerometers to measure steps per day, as well as step intensity, graded as light or moderate to vigorous.
The relationship between daily activity and diabetes was analyzed using three multivariate models: The first included race/ethnicity and age; the second also included family history of diabetes, education, physical functioning, self-rated health, smoking status, and alcohol consumption; and the third added BMI, “a potential mediator in the causal pathway between steps per day and diabetes,” the investigators wrote.
Participants took an average of 3,729 steps per day, divided roughly evenly between light and moderate to vigorous intensity.
After a median follow-up of 5.7 years, 8.1% of women developed diabetes. The least-adjusted model showed a 14% reduction in diabetes risk per 2,000 steps (hazard ratio, 0.86; 95% confidence interval, 0.80-0.92; P = .007), whereas the second model, adjusting for more confounding variables, showed a 12% reduction in diabetes risk per 2,000 steps (HR, 0.88; 95% CI, 0.78-1.00; P = .045).
The final model, which added BMI, showed a 10% reduction in risk, although it didn’t reach statistical significance (HR, 0.90; 95% CI, 0.80-1.02; P = .11). Furthermore, accelerated failure time models suggested that BMI did not significantly impact the link between steps and diabetes (proportion mediated, 17.7%;95% CI, –55.0 to 142.0; P = .09). Further analyses also found no significant interactions between BMI or other possible confounders.
“The steps per day–diabetes association was not modified by age, race/ethnicity, BMI, physical functioning, or family history of diabetes, which supports the generalizability of these findings to community-living older women,” the investigators wrote.
Increased stepping intensity also appeared to lower risk of diabetes. After adjusting for confounding variables, light stepping was not linked to reduced risk (HR, 0.97; 95% CI, 0.73-1.29; P = .83), whereas moderate to vigorous stepping reduced risk by 14% per 2,000 steps (HR, 0.86; 95% CI, 0.74-1.00; P = .04).
“This study provides evidence supporting an association between steps per day and lower incident diabetes,” the investigators concluded. “While further work is needed to identify whether there is a minimum number of steps per day that results in a clinically significant reduction of diabetes and to evaluate the role that step intensity plays in diabetes etiology for older adults, findings from this study suggest that moderate-vigorous–intensity steps may be more important than lower-intensity steps with respect to incident diabetes. Steps per day–based interventions are needed to advance diabetes prevention science in older adults.”
The study was supported by the National Institute on Aging, the National Institute of Diabetes and Digestive and Kidney Diseases, the Tobacco-Related Disease Research Program, and others. The investigators had no potential conflicts of interest.
FROM DIABETES CARE