User login
Ask patients, not devices, about impact of PAD
The ankle-brachial index (ABI) is a poor indicator of patient-centered and clinician-based evaluations of functional status in patients with intermittent claudication, according to the results of PORTRAIT, a prospective observational study of patients with newly diagnosed or an exacerbation of nonlimb-threatening peripheral arterial disease (PAD).
PORTRAIT studied 1,251 patients with intermittent claudication enrolled at 16 sites. Researchers studied the correlation of ABI values and Rutherford symptom classification with PAD-specific health status as measured by the Peripheral Artery Questionnaire (PAQ).
ABI values were categorized as mild (greater than 0.80), moderate (0.40-0.79), and severe (less than 0.40). Spearman rank correlation coefficients were calculated between raw ABI values and PAQ scores and between the Rutherford classification and PAQ scores.
ABI explained only 0.1%-2.1% of the variation in PAQ scores and the Rutherford classification had stronger but still modest associations with PAQ scores, according to the researchers.
“This large study of IC patients found that the PAQ offers a unique and complementary
measure of disease burden that is not captured by physiologic or clinician-observed classifications. The findings from this study highlight the clinical complexity of PAD
and the difficulty in using common hemodynamic and symptom measures to classify the impact of this disease on patients’ health status,” the researchers concluded.
Several authors reported serving as consultant for and/or receiving grants from various device and pharmaceutical companies involved with PAD. The senior author. owns the copyright to the Peripheral Artery Questionnaire that formed the basis for the study.
SOURCE: Johnston A et al. J Vasc Surg. 2018;69(3):906-12.
The ankle-brachial index (ABI) is a poor indicator of patient-centered and clinician-based evaluations of functional status in patients with intermittent claudication, according to the results of PORTRAIT, a prospective observational study of patients with newly diagnosed or an exacerbation of nonlimb-threatening peripheral arterial disease (PAD).
PORTRAIT studied 1,251 patients with intermittent claudication enrolled at 16 sites. Researchers studied the correlation of ABI values and Rutherford symptom classification with PAD-specific health status as measured by the Peripheral Artery Questionnaire (PAQ).
ABI values were categorized as mild (greater than 0.80), moderate (0.40-0.79), and severe (less than 0.40). Spearman rank correlation coefficients were calculated between raw ABI values and PAQ scores and between the Rutherford classification and PAQ scores.
ABI explained only 0.1%-2.1% of the variation in PAQ scores and the Rutherford classification had stronger but still modest associations with PAQ scores, according to the researchers.
“This large study of IC patients found that the PAQ offers a unique and complementary
measure of disease burden that is not captured by physiologic or clinician-observed classifications. The findings from this study highlight the clinical complexity of PAD
and the difficulty in using common hemodynamic and symptom measures to classify the impact of this disease on patients’ health status,” the researchers concluded.
Several authors reported serving as consultant for and/or receiving grants from various device and pharmaceutical companies involved with PAD. The senior author. owns the copyright to the Peripheral Artery Questionnaire that formed the basis for the study.
SOURCE: Johnston A et al. J Vasc Surg. 2018;69(3):906-12.
The ankle-brachial index (ABI) is a poor indicator of patient-centered and clinician-based evaluations of functional status in patients with intermittent claudication, according to the results of PORTRAIT, a prospective observational study of patients with newly diagnosed or an exacerbation of nonlimb-threatening peripheral arterial disease (PAD).
PORTRAIT studied 1,251 patients with intermittent claudication enrolled at 16 sites. Researchers studied the correlation of ABI values and Rutherford symptom classification with PAD-specific health status as measured by the Peripheral Artery Questionnaire (PAQ).
ABI values were categorized as mild (greater than 0.80), moderate (0.40-0.79), and severe (less than 0.40). Spearman rank correlation coefficients were calculated between raw ABI values and PAQ scores and between the Rutherford classification and PAQ scores.
ABI explained only 0.1%-2.1% of the variation in PAQ scores and the Rutherford classification had stronger but still modest associations with PAQ scores, according to the researchers.
“This large study of IC patients found that the PAQ offers a unique and complementary
measure of disease burden that is not captured by physiologic or clinician-observed classifications. The findings from this study highlight the clinical complexity of PAD
and the difficulty in using common hemodynamic and symptom measures to classify the impact of this disease on patients’ health status,” the researchers concluded.
Several authors reported serving as consultant for and/or receiving grants from various device and pharmaceutical companies involved with PAD. The senior author. owns the copyright to the Peripheral Artery Questionnaire that formed the basis for the study.
SOURCE: Johnston A et al. J Vasc Surg. 2018;69(3):906-12.
FROM THE JOURNAL OF VASCULAR SURGERY
Spontaneous coronary artery dissection: An often unrecognized cause of acute coronary syndrome
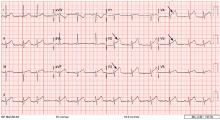
A 12-lead electrocardiogram (Figure 1) showed ST-segment elevation of more than 2 mm in leads V2, V3, V4, and V5, with no reciprocal changes.
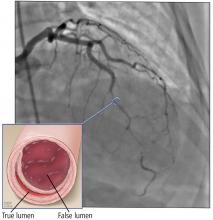
Based on the classic angiographic appearance and the absence of atherosclerotic disease in other coronary arteries, type 2 spontaneous coronary artery dissection (SCAD) was diagnosed.
CORONARY ARTERY WALL SEPARATION
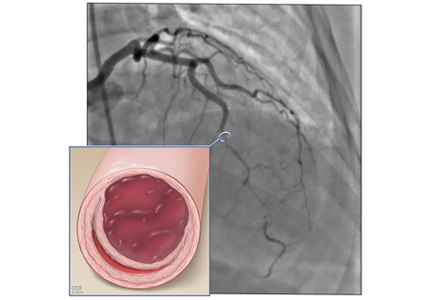
SCAD is defined as a nontraumatic, noniatrogenic intramural hemorrhage leading to separation of the coronary arterial wall and the formation of a false lumen. The separation can occur between any of the coronary artery wall layers and may or may not involve an intimal tear. The bleeding may result in an intramural hematoma and possible narrowing of the arterial lumen. Depending on the severity of narrowing, blood supply to the myocardium could be compromised, resulting in symptoms of ischemia.1
SCAD usually involves a single coronary artery, although multiple coronary artery involvement has been reported.2
CASE CONTINUED: MANAGEMENT
The patient recovered completely and was discharged home with plans to return for outpatient imaging for fibromuscular dysplasia.
SCAD: RARE OR JUST RARELY RECOGNIZED?
SCAD appears to be a rare cause of acute coronary syndrome, but it is likely underdiagnosed and is becoming increasingly recognized worldwide. Typically, it affects women younger than 50, with women in general outnumbering men 9 to 1.3 Overall, SCAD causes up to 4% of acute myocardial infarctions, but in women age 50 or younger, it is responsible for 24% to 35% of acute myocardial infarctions, and the proportion is even higher in pregnant women.4
Not just pregnancy-associated
SCAD was previously thought to be mainly idiopathic and mostly affecting women peripartum. Current understanding paints a different picture: pregnancy-associated SCAD does not account for the majority of cases. That said, SCAD is the most common cause of myocardial infarction peripartum, with the third trimester and early postpartum period being the times of highest risk.5 SCAD development at those times is believed to be related to hormonal changes causing weakening of coronary artery walls.6
Weakening of the coronary artery wall also may occur in the setting of fibromuscular dysplasia, connective tissue disease, recurrent pregnancies, systemic inflammatory disease, hormonal therapy, and other disease states that cause arteriopathy. Exposure to a stressor in a patient with underlying risk factors can lead to either an intimal tear or rupture of the vasa vasorum, with subsequent formation of intramural hemorrhage and eventually SCAD.7 Stressors can be emotional or physical and can include labor and delivery, intense physical exercise, the Valsalva maneuver, and drug abuse.8
Presentation is variable
SCAD presentation depends on the degree of flow limitation and extent of the dissection. Presentation can range from asymptomatic to sudden cardiac death and can include signs and symptoms of acute coronary syndrome caused by ST-segment elevation or non-ST-segment elevation myocardial infarction.
DIAGNOSIS BY ANGIOGRAPHY
SCAD can be diagnosed by coronary angiography. There are 3 angiographic types:
Type 1 (about 25% of SCAD cases) has typical contrast dye staining of the arterial wall and multiple radiolucent luminal abnormalities, with or without dye hang-up.
Type 2 (about 70%) has diffuse, smooth narrowing of the coronary artery, with the left anterior descending artery the most frequently affected.8
Type 3 (about 5%) mimics atherosclerosis, with focal or tubular stenosis.9
Types 1 and 2 are usually easy to recognize. To diagnose type 2, intravenous nitroglycerin should first be administered to rule out coronary spasm.
Type 3 SCAD is more challenging to diagnose because its appearance on angiography is similar to that of atherosclerosis. For equivocal findings in any type, but especially in type 3, intravascular ultrasonography or optical coherence tomography can help.10 Optical coherence tomography is preferred because of superior image resolution, although ultrasonography offers better tissue penetration.11
MANAGE MOST CASES CONSERVATIVELY
Management algorithms for SCAD are available.8,12
The initial and most critical step is to make the correct diagnosis. Although the presentation of acute coronary syndrome caused by SCAD is often identical to that of atherosclerosis, the conditions have different pathophysiologies and thus require different management. Theoretically, systemic anticoagulation may worsen an intramural hemorrhage.
First-line therapy for most patients with SCAD is conservative management and close inpatient monitoring for 3 to 5 days.13 More aggressive management is indicated for any of the following:
- Left main or severe proximal 2-vessel dissection
- Hemodynamic instability
- Ongoing ischemic symptoms.
In a prospective cohort of 168 patients, 134 (80%) were initially treated conservatively; of those, in-hospital myocardial infarction recurred in 4.5%, a major cardiac event occurred within 2 years in 17%, and SCAD recurred in 13%.8
Observational data on patients with SCAD who had repeat angiography weeks to months after the initial event has shown that lesions heal in 70% to 97% of patients.12
WHEN TO CONSIDER AGGRESSIVE MANAGEMENT
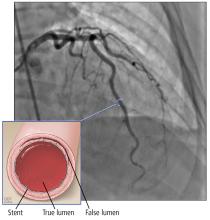
Under the circumstances listed above, revascularization with PCI or coronary artery bypass grafting (CABG) should be considered, with choice of procedure determined by feasibility, technical considerations, and local expertise.
The American Heart Association recommendations are as follows12:
- For left main or severe proximal 2-vessel dissection in clinically stable patients, consider CABG
- For active ischemia or hemodynamic instability, consider PCI if feasible or perform urgent CABG.
A few series have shown that the prognosis with conservative management or CABG is better than with PCI.8,13,14 The success rate for revascularization with PCI is only about 60% because of challenges including risk of inducing iatrogenic dissection, passing the wire into the false lumen and worsening a dissection, and propagating an intramural hematoma with stenting and further compromising coronary blood flow. In addition, dissection tends to extend into distal arteries that are difficult to stent. There is also the risk of stent malapposition after resorption of the intramural hematoma, causing late stent thrombosis.7
SCREEN FOR OTHER VASCULAR PROBLEMS
Imaging of the renal, iliac, and cerebral vasculature is recommended for all patients with SCAD.12 Screening for fibromuscular dysplasia can be done with angiography, computed tomographic angiography (CTA), or magnetic resonance angiography (MRA).12
Multifocal fibromuscular dysplasia in extracoronary arteries occurs with SCAD in 25% to 86% of cases. In a single-center series of 115 patients with confirmed SCAD who underwent CTA from 2010 to 2014, extracoronary vascular abnormalities were found in 66%, with fibromuscular dysplasia being the most common type (45%).15 In another single-center study, 327 patients with SCAD were prospectively followed from 2012 to 2016 with screening for cerebrovascular, renal, and iliac fibromuscular dysplasia using CTA or catheter angiography. Fibromuscular dysplasia was found in 63%, and intracranial aneurysm was found in 14% of patients with fibromuscular dysplasia.9
SCAD can also be associated with connective tissue disorders such as Ehlers-Danlos syndrome type IV and Marfan syndrome.16,17
LONG-TERM MANAGEMENT
Patients with SCAD should start long-term aspirin and 1 year of clopidogrel. Statins are indicated for patients with hyperlipidemia8,18 but otherwise offer no clear benefit for SCAD alone. If there are no contraindications, a beta-adrenergic blocker should be considered, especially if left ventricular dysfunction or arrhythmias are present. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers should also be considered with concomitant left ventricular dysfunction. Antianginal therapy can be used for post-SCAD chest pain syndromes.12
Repeat angiography is recommended only to evaluate recurrent symptoms, to confirm an unclear initial diagnosis, to assess for atherosclerosis-related stenosis, or to evaluate high-risk anatomy, eg, involvement of the left main coronary artery.12
Genetic testing is reserved for patients with a high clinical suspicion of connective tissue disease or systemic arteriopathy.19
- Garcia NA, Khan AN, Boppana RC, Smith HL. Spontaneous coronary artery dissection: a case series and literature review. J Community Hosp Intern Med Perspect 2014; 4(4). doi:10.3402/jchimp.v4.25261
- Lempereur M, Gin K, Saw J. Multivessel spontaneous coronary artery dissection mimicking atherosclerosis. JACC Cardiovasc Interv 2014; 7(7):e87–e88. doi:10.1016/j.jcin.2013.12.207
- Mahmoud AN, Taduru SS, Mentias A, et al. Trends of incidence, clinical presentation, and in-hospital mortality among women with acute myocardial infarction with or without spontaneous coronary artery dissection: a population-based analysis. JACC Cardiovasc Interv 2018; 11(1):80–90. doi:10.1016/j.jcin.2017.08.016
- Saw J. Pregnancy-associated spontaneous coronary artery dissection represents an exceptionally high-risk spontaneous coronary artery dissection cohort. Circ Cardiovasc Interv 2017; 10(3)pii:e005119. doi:10.1161/CIRCINTERVENTIONS.117.005119
- Elkayam U, Jalnapurkar S, Barakkat MN, et al. Pregnancy-associated acute myocardial infarction: a review of contemporary experience in 150 cases between 2006 and 2011. Circulation 2014; 129(16):1695–1702. doi:10.1161/CIRCULATIONAHA.113.002054
- Vijayaraghavan R, Verma S, Gupta N, Saw J. Pregnancy-related spontaneous coronary artery dissection. Circulation 2014; 130(21):1915–1920. doi:10.1161/CIRCULATIONAHA.114.011422
- Saw J, Mancini GBJ, Humphries KH. Contemporary review on spontaneous coronary artery dissection. J Am Coll Cardiol 2016; 68(3):297–312. doi:10.1016/j.jacc.2016.05.034
- Saw J, Aymong E, Sedlak T, et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv 2014; 7(5):645–655. doi:10.1161/CIRCINTERVENTIONS.114.001760
- Saw J, Humphries K ,Aymong E, et al. Spontaneous coronary artery dissection: clinical outcomes and risk of recurrence. J Am Coll Cardiol 2017; 70(9):1148–1158. doi:10.1016/j.jacc.2017.06.053
- Alfonso F, Bastante T, Cuesta J, Rodríguez D, Benedicto A, Rivero F. Spontaneous coronary artery dissection: novel insights on diagnosis and management. Cardiovasc Diagn Ther 2015; 5(2):133–140. doi:10.3978/j.issn.2223-3652.2015.03.05
- Kern MJ, Meier B. Evaluation of the culprit plaque and the physiological significance of coronary atherosclerotic narrowings. Circulation 2001; 103(25):3142–3149. pmid:11425782
- Hayes SN, Kim ESH, Saw J, et al; American Heart Association Council on Peripheral Vascular Disease; Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Genomic and Precision Medicine; and Stroke Council. Spontaneous coronary artery dissection: current state of the science: a scientific statement from the American Heart Association. Circulation 2018; 137(19):e523–e557. doi:10.1161/CIR.0000000000000564
- Tweet MS, Eleid MF, Best PJ, et al. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovasc Interv 2014; 7(6):777–786. doi:10.1161/CIRCINTERVENTIONS.114.001659
- Tweet MS, Hayes SN, Pitta SR, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 2012; 126(5):579–588. doi:10.1161/CIRCULATIONAHA.112.105718
- Prasad M, Tweet MS, Hayes SN, et al. Prevalence of extracoronary vascular abnormalities and fibromuscular dysplasia in patients with spontaneous coronary artery dissection. Am J Cardiol 2015; 115(12):1672–1677. doi:10.1016/j.amjcard.2015.03.011
- Adès LC, Waltham RD, Chiodo AA, Bateman JF. Myocardial infarction resulting from coronary artery dissection in an adolescent with Ehlers-Danlos syndrome type IV due to a type III collagen mutation. Br Heart J 1995; 74(2):112–116. pmid:7546986
- Judge DP, Dietz HC. Marfan’s syndrome. Lancet 2005; 366(9501):1965–1976. doi:10.1016/S0140-6736(05)67789-6
- Saw J. Spontaneous coronary artery dissection. Can J Cardiol 2013; 29(9):1027–1033. doi:10.1016/j.cjca.2012.12.018
- Poloskey SL, Kim ES, Sanghani R, et al. Low yield of genetic testing for known vascular connective tissue disorders in patients with fibromuscular dysplasia. Vasc Med 2012; 17(6):371–378. doi:10.1177/1358863X12459650
A 12-lead electrocardiogram (Figure 1) showed ST-segment elevation of more than 2 mm in leads V2, V3, V4, and V5, with no reciprocal changes.
Based on the classic angiographic appearance and the absence of atherosclerotic disease in other coronary arteries, type 2 spontaneous coronary artery dissection (SCAD) was diagnosed.
CORONARY ARTERY WALL SEPARATION
SCAD is defined as a nontraumatic, noniatrogenic intramural hemorrhage leading to separation of the coronary arterial wall and the formation of a false lumen. The separation can occur between any of the coronary artery wall layers and may or may not involve an intimal tear. The bleeding may result in an intramural hematoma and possible narrowing of the arterial lumen. Depending on the severity of narrowing, blood supply to the myocardium could be compromised, resulting in symptoms of ischemia.1
SCAD usually involves a single coronary artery, although multiple coronary artery involvement has been reported.2
CASE CONTINUED: MANAGEMENT
The patient recovered completely and was discharged home with plans to return for outpatient imaging for fibromuscular dysplasia.
SCAD: RARE OR JUST RARELY RECOGNIZED?
SCAD appears to be a rare cause of acute coronary syndrome, but it is likely underdiagnosed and is becoming increasingly recognized worldwide. Typically, it affects women younger than 50, with women in general outnumbering men 9 to 1.3 Overall, SCAD causes up to 4% of acute myocardial infarctions, but in women age 50 or younger, it is responsible for 24% to 35% of acute myocardial infarctions, and the proportion is even higher in pregnant women.4
Not just pregnancy-associated
SCAD was previously thought to be mainly idiopathic and mostly affecting women peripartum. Current understanding paints a different picture: pregnancy-associated SCAD does not account for the majority of cases. That said, SCAD is the most common cause of myocardial infarction peripartum, with the third trimester and early postpartum period being the times of highest risk.5 SCAD development at those times is believed to be related to hormonal changes causing weakening of coronary artery walls.6
Weakening of the coronary artery wall also may occur in the setting of fibromuscular dysplasia, connective tissue disease, recurrent pregnancies, systemic inflammatory disease, hormonal therapy, and other disease states that cause arteriopathy. Exposure to a stressor in a patient with underlying risk factors can lead to either an intimal tear or rupture of the vasa vasorum, with subsequent formation of intramural hemorrhage and eventually SCAD.7 Stressors can be emotional or physical and can include labor and delivery, intense physical exercise, the Valsalva maneuver, and drug abuse.8
Presentation is variable
SCAD presentation depends on the degree of flow limitation and extent of the dissection. Presentation can range from asymptomatic to sudden cardiac death and can include signs and symptoms of acute coronary syndrome caused by ST-segment elevation or non-ST-segment elevation myocardial infarction.
DIAGNOSIS BY ANGIOGRAPHY
SCAD can be diagnosed by coronary angiography. There are 3 angiographic types:
Type 1 (about 25% of SCAD cases) has typical contrast dye staining of the arterial wall and multiple radiolucent luminal abnormalities, with or without dye hang-up.
Type 2 (about 70%) has diffuse, smooth narrowing of the coronary artery, with the left anterior descending artery the most frequently affected.8
Type 3 (about 5%) mimics atherosclerosis, with focal or tubular stenosis.9
Types 1 and 2 are usually easy to recognize. To diagnose type 2, intravenous nitroglycerin should first be administered to rule out coronary spasm.
Type 3 SCAD is more challenging to diagnose because its appearance on angiography is similar to that of atherosclerosis. For equivocal findings in any type, but especially in type 3, intravascular ultrasonography or optical coherence tomography can help.10 Optical coherence tomography is preferred because of superior image resolution, although ultrasonography offers better tissue penetration.11
MANAGE MOST CASES CONSERVATIVELY
Management algorithms for SCAD are available.8,12
The initial and most critical step is to make the correct diagnosis. Although the presentation of acute coronary syndrome caused by SCAD is often identical to that of atherosclerosis, the conditions have different pathophysiologies and thus require different management. Theoretically, systemic anticoagulation may worsen an intramural hemorrhage.
First-line therapy for most patients with SCAD is conservative management and close inpatient monitoring for 3 to 5 days.13 More aggressive management is indicated for any of the following:
- Left main or severe proximal 2-vessel dissection
- Hemodynamic instability
- Ongoing ischemic symptoms.
In a prospective cohort of 168 patients, 134 (80%) were initially treated conservatively; of those, in-hospital myocardial infarction recurred in 4.5%, a major cardiac event occurred within 2 years in 17%, and SCAD recurred in 13%.8
Observational data on patients with SCAD who had repeat angiography weeks to months after the initial event has shown that lesions heal in 70% to 97% of patients.12
WHEN TO CONSIDER AGGRESSIVE MANAGEMENT
Under the circumstances listed above, revascularization with PCI or coronary artery bypass grafting (CABG) should be considered, with choice of procedure determined by feasibility, technical considerations, and local expertise.
The American Heart Association recommendations are as follows12:
- For left main or severe proximal 2-vessel dissection in clinically stable patients, consider CABG
- For active ischemia or hemodynamic instability, consider PCI if feasible or perform urgent CABG.
A few series have shown that the prognosis with conservative management or CABG is better than with PCI.8,13,14 The success rate for revascularization with PCI is only about 60% because of challenges including risk of inducing iatrogenic dissection, passing the wire into the false lumen and worsening a dissection, and propagating an intramural hematoma with stenting and further compromising coronary blood flow. In addition, dissection tends to extend into distal arteries that are difficult to stent. There is also the risk of stent malapposition after resorption of the intramural hematoma, causing late stent thrombosis.7
SCREEN FOR OTHER VASCULAR PROBLEMS
Imaging of the renal, iliac, and cerebral vasculature is recommended for all patients with SCAD.12 Screening for fibromuscular dysplasia can be done with angiography, computed tomographic angiography (CTA), or magnetic resonance angiography (MRA).12
Multifocal fibromuscular dysplasia in extracoronary arteries occurs with SCAD in 25% to 86% of cases. In a single-center series of 115 patients with confirmed SCAD who underwent CTA from 2010 to 2014, extracoronary vascular abnormalities were found in 66%, with fibromuscular dysplasia being the most common type (45%).15 In another single-center study, 327 patients with SCAD were prospectively followed from 2012 to 2016 with screening for cerebrovascular, renal, and iliac fibromuscular dysplasia using CTA or catheter angiography. Fibromuscular dysplasia was found in 63%, and intracranial aneurysm was found in 14% of patients with fibromuscular dysplasia.9
SCAD can also be associated with connective tissue disorders such as Ehlers-Danlos syndrome type IV and Marfan syndrome.16,17
LONG-TERM MANAGEMENT
Patients with SCAD should start long-term aspirin and 1 year of clopidogrel. Statins are indicated for patients with hyperlipidemia8,18 but otherwise offer no clear benefit for SCAD alone. If there are no contraindications, a beta-adrenergic blocker should be considered, especially if left ventricular dysfunction or arrhythmias are present. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers should also be considered with concomitant left ventricular dysfunction. Antianginal therapy can be used for post-SCAD chest pain syndromes.12
Repeat angiography is recommended only to evaluate recurrent symptoms, to confirm an unclear initial diagnosis, to assess for atherosclerosis-related stenosis, or to evaluate high-risk anatomy, eg, involvement of the left main coronary artery.12
Genetic testing is reserved for patients with a high clinical suspicion of connective tissue disease or systemic arteriopathy.19
A 12-lead electrocardiogram (Figure 1) showed ST-segment elevation of more than 2 mm in leads V2, V3, V4, and V5, with no reciprocal changes.
Based on the classic angiographic appearance and the absence of atherosclerotic disease in other coronary arteries, type 2 spontaneous coronary artery dissection (SCAD) was diagnosed.
CORONARY ARTERY WALL SEPARATION
SCAD is defined as a nontraumatic, noniatrogenic intramural hemorrhage leading to separation of the coronary arterial wall and the formation of a false lumen. The separation can occur between any of the coronary artery wall layers and may or may not involve an intimal tear. The bleeding may result in an intramural hematoma and possible narrowing of the arterial lumen. Depending on the severity of narrowing, blood supply to the myocardium could be compromised, resulting in symptoms of ischemia.1
SCAD usually involves a single coronary artery, although multiple coronary artery involvement has been reported.2
CASE CONTINUED: MANAGEMENT
The patient recovered completely and was discharged home with plans to return for outpatient imaging for fibromuscular dysplasia.
SCAD: RARE OR JUST RARELY RECOGNIZED?
SCAD appears to be a rare cause of acute coronary syndrome, but it is likely underdiagnosed and is becoming increasingly recognized worldwide. Typically, it affects women younger than 50, with women in general outnumbering men 9 to 1.3 Overall, SCAD causes up to 4% of acute myocardial infarctions, but in women age 50 or younger, it is responsible for 24% to 35% of acute myocardial infarctions, and the proportion is even higher in pregnant women.4
Not just pregnancy-associated
SCAD was previously thought to be mainly idiopathic and mostly affecting women peripartum. Current understanding paints a different picture: pregnancy-associated SCAD does not account for the majority of cases. That said, SCAD is the most common cause of myocardial infarction peripartum, with the third trimester and early postpartum period being the times of highest risk.5 SCAD development at those times is believed to be related to hormonal changes causing weakening of coronary artery walls.6
Weakening of the coronary artery wall also may occur in the setting of fibromuscular dysplasia, connective tissue disease, recurrent pregnancies, systemic inflammatory disease, hormonal therapy, and other disease states that cause arteriopathy. Exposure to a stressor in a patient with underlying risk factors can lead to either an intimal tear or rupture of the vasa vasorum, with subsequent formation of intramural hemorrhage and eventually SCAD.7 Stressors can be emotional or physical and can include labor and delivery, intense physical exercise, the Valsalva maneuver, and drug abuse.8
Presentation is variable
SCAD presentation depends on the degree of flow limitation and extent of the dissection. Presentation can range from asymptomatic to sudden cardiac death and can include signs and symptoms of acute coronary syndrome caused by ST-segment elevation or non-ST-segment elevation myocardial infarction.
DIAGNOSIS BY ANGIOGRAPHY
SCAD can be diagnosed by coronary angiography. There are 3 angiographic types:
Type 1 (about 25% of SCAD cases) has typical contrast dye staining of the arterial wall and multiple radiolucent luminal abnormalities, with or without dye hang-up.
Type 2 (about 70%) has diffuse, smooth narrowing of the coronary artery, with the left anterior descending artery the most frequently affected.8
Type 3 (about 5%) mimics atherosclerosis, with focal or tubular stenosis.9
Types 1 and 2 are usually easy to recognize. To diagnose type 2, intravenous nitroglycerin should first be administered to rule out coronary spasm.
Type 3 SCAD is more challenging to diagnose because its appearance on angiography is similar to that of atherosclerosis. For equivocal findings in any type, but especially in type 3, intravascular ultrasonography or optical coherence tomography can help.10 Optical coherence tomography is preferred because of superior image resolution, although ultrasonography offers better tissue penetration.11
MANAGE MOST CASES CONSERVATIVELY
Management algorithms for SCAD are available.8,12
The initial and most critical step is to make the correct diagnosis. Although the presentation of acute coronary syndrome caused by SCAD is often identical to that of atherosclerosis, the conditions have different pathophysiologies and thus require different management. Theoretically, systemic anticoagulation may worsen an intramural hemorrhage.
First-line therapy for most patients with SCAD is conservative management and close inpatient monitoring for 3 to 5 days.13 More aggressive management is indicated for any of the following:
- Left main or severe proximal 2-vessel dissection
- Hemodynamic instability
- Ongoing ischemic symptoms.
In a prospective cohort of 168 patients, 134 (80%) were initially treated conservatively; of those, in-hospital myocardial infarction recurred in 4.5%, a major cardiac event occurred within 2 years in 17%, and SCAD recurred in 13%.8
Observational data on patients with SCAD who had repeat angiography weeks to months after the initial event has shown that lesions heal in 70% to 97% of patients.12
WHEN TO CONSIDER AGGRESSIVE MANAGEMENT
Under the circumstances listed above, revascularization with PCI or coronary artery bypass grafting (CABG) should be considered, with choice of procedure determined by feasibility, technical considerations, and local expertise.
The American Heart Association recommendations are as follows12:
- For left main or severe proximal 2-vessel dissection in clinically stable patients, consider CABG
- For active ischemia or hemodynamic instability, consider PCI if feasible or perform urgent CABG.
A few series have shown that the prognosis with conservative management or CABG is better than with PCI.8,13,14 The success rate for revascularization with PCI is only about 60% because of challenges including risk of inducing iatrogenic dissection, passing the wire into the false lumen and worsening a dissection, and propagating an intramural hematoma with stenting and further compromising coronary blood flow. In addition, dissection tends to extend into distal arteries that are difficult to stent. There is also the risk of stent malapposition after resorption of the intramural hematoma, causing late stent thrombosis.7
SCREEN FOR OTHER VASCULAR PROBLEMS
Imaging of the renal, iliac, and cerebral vasculature is recommended for all patients with SCAD.12 Screening for fibromuscular dysplasia can be done with angiography, computed tomographic angiography (CTA), or magnetic resonance angiography (MRA).12
Multifocal fibromuscular dysplasia in extracoronary arteries occurs with SCAD in 25% to 86% of cases. In a single-center series of 115 patients with confirmed SCAD who underwent CTA from 2010 to 2014, extracoronary vascular abnormalities were found in 66%, with fibromuscular dysplasia being the most common type (45%).15 In another single-center study, 327 patients with SCAD were prospectively followed from 2012 to 2016 with screening for cerebrovascular, renal, and iliac fibromuscular dysplasia using CTA or catheter angiography. Fibromuscular dysplasia was found in 63%, and intracranial aneurysm was found in 14% of patients with fibromuscular dysplasia.9
SCAD can also be associated with connective tissue disorders such as Ehlers-Danlos syndrome type IV and Marfan syndrome.16,17
LONG-TERM MANAGEMENT
Patients with SCAD should start long-term aspirin and 1 year of clopidogrel. Statins are indicated for patients with hyperlipidemia8,18 but otherwise offer no clear benefit for SCAD alone. If there are no contraindications, a beta-adrenergic blocker should be considered, especially if left ventricular dysfunction or arrhythmias are present. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers should also be considered with concomitant left ventricular dysfunction. Antianginal therapy can be used for post-SCAD chest pain syndromes.12
Repeat angiography is recommended only to evaluate recurrent symptoms, to confirm an unclear initial diagnosis, to assess for atherosclerosis-related stenosis, or to evaluate high-risk anatomy, eg, involvement of the left main coronary artery.12
Genetic testing is reserved for patients with a high clinical suspicion of connective tissue disease or systemic arteriopathy.19
- Garcia NA, Khan AN, Boppana RC, Smith HL. Spontaneous coronary artery dissection: a case series and literature review. J Community Hosp Intern Med Perspect 2014; 4(4). doi:10.3402/jchimp.v4.25261
- Lempereur M, Gin K, Saw J. Multivessel spontaneous coronary artery dissection mimicking atherosclerosis. JACC Cardiovasc Interv 2014; 7(7):e87–e88. doi:10.1016/j.jcin.2013.12.207
- Mahmoud AN, Taduru SS, Mentias A, et al. Trends of incidence, clinical presentation, and in-hospital mortality among women with acute myocardial infarction with or without spontaneous coronary artery dissection: a population-based analysis. JACC Cardiovasc Interv 2018; 11(1):80–90. doi:10.1016/j.jcin.2017.08.016
- Saw J. Pregnancy-associated spontaneous coronary artery dissection represents an exceptionally high-risk spontaneous coronary artery dissection cohort. Circ Cardiovasc Interv 2017; 10(3)pii:e005119. doi:10.1161/CIRCINTERVENTIONS.117.005119
- Elkayam U, Jalnapurkar S, Barakkat MN, et al. Pregnancy-associated acute myocardial infarction: a review of contemporary experience in 150 cases between 2006 and 2011. Circulation 2014; 129(16):1695–1702. doi:10.1161/CIRCULATIONAHA.113.002054
- Vijayaraghavan R, Verma S, Gupta N, Saw J. Pregnancy-related spontaneous coronary artery dissection. Circulation 2014; 130(21):1915–1920. doi:10.1161/CIRCULATIONAHA.114.011422
- Saw J, Mancini GBJ, Humphries KH. Contemporary review on spontaneous coronary artery dissection. J Am Coll Cardiol 2016; 68(3):297–312. doi:10.1016/j.jacc.2016.05.034
- Saw J, Aymong E, Sedlak T, et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv 2014; 7(5):645–655. doi:10.1161/CIRCINTERVENTIONS.114.001760
- Saw J, Humphries K ,Aymong E, et al. Spontaneous coronary artery dissection: clinical outcomes and risk of recurrence. J Am Coll Cardiol 2017; 70(9):1148–1158. doi:10.1016/j.jacc.2017.06.053
- Alfonso F, Bastante T, Cuesta J, Rodríguez D, Benedicto A, Rivero F. Spontaneous coronary artery dissection: novel insights on diagnosis and management. Cardiovasc Diagn Ther 2015; 5(2):133–140. doi:10.3978/j.issn.2223-3652.2015.03.05
- Kern MJ, Meier B. Evaluation of the culprit plaque and the physiological significance of coronary atherosclerotic narrowings. Circulation 2001; 103(25):3142–3149. pmid:11425782
- Hayes SN, Kim ESH, Saw J, et al; American Heart Association Council on Peripheral Vascular Disease; Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Genomic and Precision Medicine; and Stroke Council. Spontaneous coronary artery dissection: current state of the science: a scientific statement from the American Heart Association. Circulation 2018; 137(19):e523–e557. doi:10.1161/CIR.0000000000000564
- Tweet MS, Eleid MF, Best PJ, et al. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovasc Interv 2014; 7(6):777–786. doi:10.1161/CIRCINTERVENTIONS.114.001659
- Tweet MS, Hayes SN, Pitta SR, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 2012; 126(5):579–588. doi:10.1161/CIRCULATIONAHA.112.105718
- Prasad M, Tweet MS, Hayes SN, et al. Prevalence of extracoronary vascular abnormalities and fibromuscular dysplasia in patients with spontaneous coronary artery dissection. Am J Cardiol 2015; 115(12):1672–1677. doi:10.1016/j.amjcard.2015.03.011
- Adès LC, Waltham RD, Chiodo AA, Bateman JF. Myocardial infarction resulting from coronary artery dissection in an adolescent with Ehlers-Danlos syndrome type IV due to a type III collagen mutation. Br Heart J 1995; 74(2):112–116. pmid:7546986
- Judge DP, Dietz HC. Marfan’s syndrome. Lancet 2005; 366(9501):1965–1976. doi:10.1016/S0140-6736(05)67789-6
- Saw J. Spontaneous coronary artery dissection. Can J Cardiol 2013; 29(9):1027–1033. doi:10.1016/j.cjca.2012.12.018
- Poloskey SL, Kim ES, Sanghani R, et al. Low yield of genetic testing for known vascular connective tissue disorders in patients with fibromuscular dysplasia. Vasc Med 2012; 17(6):371–378. doi:10.1177/1358863X12459650
- Garcia NA, Khan AN, Boppana RC, Smith HL. Spontaneous coronary artery dissection: a case series and literature review. J Community Hosp Intern Med Perspect 2014; 4(4). doi:10.3402/jchimp.v4.25261
- Lempereur M, Gin K, Saw J. Multivessel spontaneous coronary artery dissection mimicking atherosclerosis. JACC Cardiovasc Interv 2014; 7(7):e87–e88. doi:10.1016/j.jcin.2013.12.207
- Mahmoud AN, Taduru SS, Mentias A, et al. Trends of incidence, clinical presentation, and in-hospital mortality among women with acute myocardial infarction with or without spontaneous coronary artery dissection: a population-based analysis. JACC Cardiovasc Interv 2018; 11(1):80–90. doi:10.1016/j.jcin.2017.08.016
- Saw J. Pregnancy-associated spontaneous coronary artery dissection represents an exceptionally high-risk spontaneous coronary artery dissection cohort. Circ Cardiovasc Interv 2017; 10(3)pii:e005119. doi:10.1161/CIRCINTERVENTIONS.117.005119
- Elkayam U, Jalnapurkar S, Barakkat MN, et al. Pregnancy-associated acute myocardial infarction: a review of contemporary experience in 150 cases between 2006 and 2011. Circulation 2014; 129(16):1695–1702. doi:10.1161/CIRCULATIONAHA.113.002054
- Vijayaraghavan R, Verma S, Gupta N, Saw J. Pregnancy-related spontaneous coronary artery dissection. Circulation 2014; 130(21):1915–1920. doi:10.1161/CIRCULATIONAHA.114.011422
- Saw J, Mancini GBJ, Humphries KH. Contemporary review on spontaneous coronary artery dissection. J Am Coll Cardiol 2016; 68(3):297–312. doi:10.1016/j.jacc.2016.05.034
- Saw J, Aymong E, Sedlak T, et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv 2014; 7(5):645–655. doi:10.1161/CIRCINTERVENTIONS.114.001760
- Saw J, Humphries K ,Aymong E, et al. Spontaneous coronary artery dissection: clinical outcomes and risk of recurrence. J Am Coll Cardiol 2017; 70(9):1148–1158. doi:10.1016/j.jacc.2017.06.053
- Alfonso F, Bastante T, Cuesta J, Rodríguez D, Benedicto A, Rivero F. Spontaneous coronary artery dissection: novel insights on diagnosis and management. Cardiovasc Diagn Ther 2015; 5(2):133–140. doi:10.3978/j.issn.2223-3652.2015.03.05
- Kern MJ, Meier B. Evaluation of the culprit plaque and the physiological significance of coronary atherosclerotic narrowings. Circulation 2001; 103(25):3142–3149. pmid:11425782
- Hayes SN, Kim ESH, Saw J, et al; American Heart Association Council on Peripheral Vascular Disease; Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Genomic and Precision Medicine; and Stroke Council. Spontaneous coronary artery dissection: current state of the science: a scientific statement from the American Heart Association. Circulation 2018; 137(19):e523–e557. doi:10.1161/CIR.0000000000000564
- Tweet MS, Eleid MF, Best PJ, et al. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovasc Interv 2014; 7(6):777–786. doi:10.1161/CIRCINTERVENTIONS.114.001659
- Tweet MS, Hayes SN, Pitta SR, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 2012; 126(5):579–588. doi:10.1161/CIRCULATIONAHA.112.105718
- Prasad M, Tweet MS, Hayes SN, et al. Prevalence of extracoronary vascular abnormalities and fibromuscular dysplasia in patients with spontaneous coronary artery dissection. Am J Cardiol 2015; 115(12):1672–1677. doi:10.1016/j.amjcard.2015.03.011
- Adès LC, Waltham RD, Chiodo AA, Bateman JF. Myocardial infarction resulting from coronary artery dissection in an adolescent with Ehlers-Danlos syndrome type IV due to a type III collagen mutation. Br Heart J 1995; 74(2):112–116. pmid:7546986
- Judge DP, Dietz HC. Marfan’s syndrome. Lancet 2005; 366(9501):1965–1976. doi:10.1016/S0140-6736(05)67789-6
- Saw J. Spontaneous coronary artery dissection. Can J Cardiol 2013; 29(9):1027–1033. doi:10.1016/j.cjca.2012.12.018
- Poloskey SL, Kim ES, Sanghani R, et al. Low yield of genetic testing for known vascular connective tissue disorders in patients with fibromuscular dysplasia. Vasc Med 2012; 17(6):371–378. doi:10.1177/1358863X12459650
KEY POINTS
- SCAD often presents with symptoms of acute coronary syndrome but can be asymptomatic or cause sudden death.
- Management is generally conservative, but a left main or severe proximal 2-vessel dissection, hemodynamic instability, or ongoing ischemic symptoms may warrant revascularization.
- All patients with SCAD should be screened for other vascular problems, especially fibromuscular dysplasia.
- Long-term aspirin therapy and 1 year of clopidogrel are recommended after an episode of SCAD.
EVAR insights from the GREAT registry
CHICAGO – The Global Registry for Endovascular Aortic Treatment is a unique resource that, although still early in its planned 10-year follow-up period, has already yielded important insights into one of the hottest topics in endovascular repair of abdominal aortic aneurysms: that is, the impact of the proximal aortic neck, Clayton J. Brinster, MD, said at a symposium on vascular surgery sponsored by Northwestern University.
The Global Registry for Endovascular Aortic Treatment (GREAT) is a prospective, observational, real-world registry that enrolled more than 5,000 consecutive patients undergoing endovascular aortic repair (EVAR) in the United States, Europe, Australia, New Zealand, and Brazil before enrollment closed in October 2016.
GREAT is the largest stent graft registry in the world. One of its special features is that it has essentially no exclusion criteria. This enables researchers to compare outcomes in patients undergoing on-label EVAR using devices deployed within the official instructions for use (IFU) to results in real-world practice, which not infrequently entails treatment for nonstandard indications using devices outside the narrowly defined IFU generated via pivotal clinical trials, explained Dr. Brinster, a vascular surgeon at the Ochsner Clinic Foundation in New Orleans.
The biggest limitation of GREAT is that it’s sponsored by Gore and restricted to recipients of GORE thoracic and abdominal stent grafts. However, the registry has an oversight and safety monitoring board that is independent of the company, Dr. Brinster continued.
He highlighted three recently published studies that have utilized early GREAT data to examine the impact on EVAR outcomes of various features of the proximal aortic neck.
Noncylindrical neck anatomy
An international team of investigators analyzed the incidence and impact of noncylindrical neck anatomy, defined as a 2-mm or greater change in diameter over the first 15 mm of proximal aortic neck length. Of 3,077 GREAT participants treated with the Gore Excluder endograft, 1,312, or 43%, had an hourglass, tapered, or conical neck shape that qualified as noncylindrical. Noncylindrical necks were more common in women. Fifteen percent of patients with a noncylindrical neck received the device outside the Excluder IFU, as did 11% with a cylindrical neck.
After an average follow-up of about 20 months, the noncylindrical neck group had a 3.1% rate of device-related intervention, significantly better than the 4.9% rate in patients with a cylindrical neck. In a multivariate regression analysis, female gender and maximum abdominal aortic aneurysm diameter were significant risk factors for device-related or endoleak-specific reintervention; noncylindrical neck morphology was not. Indeed, women were 2.2-fold more likely to require device-related reintervention than men (J Vasc Surg. 2018; 68[6]:1714-24).
Large proximal aortic neck
Of 3,166 consecutive patients in GREAT, 37.6% had a large aortic neck diameter, defined as 25 mm or wider. The rate of 5-year freedom from type Ia endoleak was 96.8% in the large-neck group, significantly less than the 98.6% rate in patients with a normal aortic neck diameter. Of note, rates didn’t diverge until after 2 years of follow-up, emphasizing the need for careful long-term surveillance despite initial technical success.
The 5-year rate of freedom from the primary composite endpoint of type Ia endoleak, reintervention, aortic rupture, or isolated aortic-related mortality was also significantly worse in the large-neck group: 81.3% versus 87%. Moreover, the 5-year survival rate was only 64.6% in the large–aortic neck group, compared with 76.5% in the comparator arm, even though aortic-related mortality didn’t differ between the two groups. “The findings raise the question of whether young patients, with predicted life expectancies exceeding 10 years, should receive standard endovascular intervention if they have large aortic neck diameters at baseline (Eur J Vasc Endovasc Surg. 2018;56[2]:189-99).
Severe neck angulation
Australian investigators wondered if the IFU for the Gore C3 Excluder was overly restrictive in defining abdominal aortic aneurysms with necks greater than 60 degrees as off-label for the device. In the first 1,394 patients enrolled in GREAT, the researchers identified 127 (9.2%) who exhibited more than 60 and less than 140 degrees of neck angulation and didn’t require endoanchors for proximal fixation. Their mean neck angle was 78 degrees, with a mean neck length of 29 mm. Mean graft oversizing was 23.5%, which was also outside the Excluder IFU.
During a median follow-up of 236 days there were 7 type Ia endoleaks, for an incidence of 5.6%. The degree of neck angulation, neck length, and the amount of oversizing were not associated with endoleak (Ann Vasc Surg. 2018;49:152-57). However, Dr. Brinster wants to see longer follow-up data before he is prepared to accept that a mean 23.5% graft oversizing is a benign intervention.
“One must remember that, with that percentage of oversizing in an already abnormal neck, aortic neck dilation could be a significant problem longer term,” the vascular surgeon said.
Dr. Brinster reported having no conflicts regarding his presentation.
[email protected]
CHICAGO – The Global Registry for Endovascular Aortic Treatment is a unique resource that, although still early in its planned 10-year follow-up period, has already yielded important insights into one of the hottest topics in endovascular repair of abdominal aortic aneurysms: that is, the impact of the proximal aortic neck, Clayton J. Brinster, MD, said at a symposium on vascular surgery sponsored by Northwestern University.
The Global Registry for Endovascular Aortic Treatment (GREAT) is a prospective, observational, real-world registry that enrolled more than 5,000 consecutive patients undergoing endovascular aortic repair (EVAR) in the United States, Europe, Australia, New Zealand, and Brazil before enrollment closed in October 2016.
GREAT is the largest stent graft registry in the world. One of its special features is that it has essentially no exclusion criteria. This enables researchers to compare outcomes in patients undergoing on-label EVAR using devices deployed within the official instructions for use (IFU) to results in real-world practice, which not infrequently entails treatment for nonstandard indications using devices outside the narrowly defined IFU generated via pivotal clinical trials, explained Dr. Brinster, a vascular surgeon at the Ochsner Clinic Foundation in New Orleans.
The biggest limitation of GREAT is that it’s sponsored by Gore and restricted to recipients of GORE thoracic and abdominal stent grafts. However, the registry has an oversight and safety monitoring board that is independent of the company, Dr. Brinster continued.
He highlighted three recently published studies that have utilized early GREAT data to examine the impact on EVAR outcomes of various features of the proximal aortic neck.
Noncylindrical neck anatomy
An international team of investigators analyzed the incidence and impact of noncylindrical neck anatomy, defined as a 2-mm or greater change in diameter over the first 15 mm of proximal aortic neck length. Of 3,077 GREAT participants treated with the Gore Excluder endograft, 1,312, or 43%, had an hourglass, tapered, or conical neck shape that qualified as noncylindrical. Noncylindrical necks were more common in women. Fifteen percent of patients with a noncylindrical neck received the device outside the Excluder IFU, as did 11% with a cylindrical neck.
After an average follow-up of about 20 months, the noncylindrical neck group had a 3.1% rate of device-related intervention, significantly better than the 4.9% rate in patients with a cylindrical neck. In a multivariate regression analysis, female gender and maximum abdominal aortic aneurysm diameter were significant risk factors for device-related or endoleak-specific reintervention; noncylindrical neck morphology was not. Indeed, women were 2.2-fold more likely to require device-related reintervention than men (J Vasc Surg. 2018; 68[6]:1714-24).
Large proximal aortic neck
Of 3,166 consecutive patients in GREAT, 37.6% had a large aortic neck diameter, defined as 25 mm or wider. The rate of 5-year freedom from type Ia endoleak was 96.8% in the large-neck group, significantly less than the 98.6% rate in patients with a normal aortic neck diameter. Of note, rates didn’t diverge until after 2 years of follow-up, emphasizing the need for careful long-term surveillance despite initial technical success.
The 5-year rate of freedom from the primary composite endpoint of type Ia endoleak, reintervention, aortic rupture, or isolated aortic-related mortality was also significantly worse in the large-neck group: 81.3% versus 87%. Moreover, the 5-year survival rate was only 64.6% in the large–aortic neck group, compared with 76.5% in the comparator arm, even though aortic-related mortality didn’t differ between the two groups. “The findings raise the question of whether young patients, with predicted life expectancies exceeding 10 years, should receive standard endovascular intervention if they have large aortic neck diameters at baseline (Eur J Vasc Endovasc Surg. 2018;56[2]:189-99).
Severe neck angulation
Australian investigators wondered if the IFU for the Gore C3 Excluder was overly restrictive in defining abdominal aortic aneurysms with necks greater than 60 degrees as off-label for the device. In the first 1,394 patients enrolled in GREAT, the researchers identified 127 (9.2%) who exhibited more than 60 and less than 140 degrees of neck angulation and didn’t require endoanchors for proximal fixation. Their mean neck angle was 78 degrees, with a mean neck length of 29 mm. Mean graft oversizing was 23.5%, which was also outside the Excluder IFU.
During a median follow-up of 236 days there were 7 type Ia endoleaks, for an incidence of 5.6%. The degree of neck angulation, neck length, and the amount of oversizing were not associated with endoleak (Ann Vasc Surg. 2018;49:152-57). However, Dr. Brinster wants to see longer follow-up data before he is prepared to accept that a mean 23.5% graft oversizing is a benign intervention.
“One must remember that, with that percentage of oversizing in an already abnormal neck, aortic neck dilation could be a significant problem longer term,” the vascular surgeon said.
Dr. Brinster reported having no conflicts regarding his presentation.
[email protected]
CHICAGO – The Global Registry for Endovascular Aortic Treatment is a unique resource that, although still early in its planned 10-year follow-up period, has already yielded important insights into one of the hottest topics in endovascular repair of abdominal aortic aneurysms: that is, the impact of the proximal aortic neck, Clayton J. Brinster, MD, said at a symposium on vascular surgery sponsored by Northwestern University.
The Global Registry for Endovascular Aortic Treatment (GREAT) is a prospective, observational, real-world registry that enrolled more than 5,000 consecutive patients undergoing endovascular aortic repair (EVAR) in the United States, Europe, Australia, New Zealand, and Brazil before enrollment closed in October 2016.
GREAT is the largest stent graft registry in the world. One of its special features is that it has essentially no exclusion criteria. This enables researchers to compare outcomes in patients undergoing on-label EVAR using devices deployed within the official instructions for use (IFU) to results in real-world practice, which not infrequently entails treatment for nonstandard indications using devices outside the narrowly defined IFU generated via pivotal clinical trials, explained Dr. Brinster, a vascular surgeon at the Ochsner Clinic Foundation in New Orleans.
The biggest limitation of GREAT is that it’s sponsored by Gore and restricted to recipients of GORE thoracic and abdominal stent grafts. However, the registry has an oversight and safety monitoring board that is independent of the company, Dr. Brinster continued.
He highlighted three recently published studies that have utilized early GREAT data to examine the impact on EVAR outcomes of various features of the proximal aortic neck.
Noncylindrical neck anatomy
An international team of investigators analyzed the incidence and impact of noncylindrical neck anatomy, defined as a 2-mm or greater change in diameter over the first 15 mm of proximal aortic neck length. Of 3,077 GREAT participants treated with the Gore Excluder endograft, 1,312, or 43%, had an hourglass, tapered, or conical neck shape that qualified as noncylindrical. Noncylindrical necks were more common in women. Fifteen percent of patients with a noncylindrical neck received the device outside the Excluder IFU, as did 11% with a cylindrical neck.
After an average follow-up of about 20 months, the noncylindrical neck group had a 3.1% rate of device-related intervention, significantly better than the 4.9% rate in patients with a cylindrical neck. In a multivariate regression analysis, female gender and maximum abdominal aortic aneurysm diameter were significant risk factors for device-related or endoleak-specific reintervention; noncylindrical neck morphology was not. Indeed, women were 2.2-fold more likely to require device-related reintervention than men (J Vasc Surg. 2018; 68[6]:1714-24).
Large proximal aortic neck
Of 3,166 consecutive patients in GREAT, 37.6% had a large aortic neck diameter, defined as 25 mm or wider. The rate of 5-year freedom from type Ia endoleak was 96.8% in the large-neck group, significantly less than the 98.6% rate in patients with a normal aortic neck diameter. Of note, rates didn’t diverge until after 2 years of follow-up, emphasizing the need for careful long-term surveillance despite initial technical success.
The 5-year rate of freedom from the primary composite endpoint of type Ia endoleak, reintervention, aortic rupture, or isolated aortic-related mortality was also significantly worse in the large-neck group: 81.3% versus 87%. Moreover, the 5-year survival rate was only 64.6% in the large–aortic neck group, compared with 76.5% in the comparator arm, even though aortic-related mortality didn’t differ between the two groups. “The findings raise the question of whether young patients, with predicted life expectancies exceeding 10 years, should receive standard endovascular intervention if they have large aortic neck diameters at baseline (Eur J Vasc Endovasc Surg. 2018;56[2]:189-99).
Severe neck angulation
Australian investigators wondered if the IFU for the Gore C3 Excluder was overly restrictive in defining abdominal aortic aneurysms with necks greater than 60 degrees as off-label for the device. In the first 1,394 patients enrolled in GREAT, the researchers identified 127 (9.2%) who exhibited more than 60 and less than 140 degrees of neck angulation and didn’t require endoanchors for proximal fixation. Their mean neck angle was 78 degrees, with a mean neck length of 29 mm. Mean graft oversizing was 23.5%, which was also outside the Excluder IFU.
During a median follow-up of 236 days there were 7 type Ia endoleaks, for an incidence of 5.6%. The degree of neck angulation, neck length, and the amount of oversizing were not associated with endoleak (Ann Vasc Surg. 2018;49:152-57). However, Dr. Brinster wants to see longer follow-up data before he is prepared to accept that a mean 23.5% graft oversizing is a benign intervention.
“One must remember that, with that percentage of oversizing in an already abnormal neck, aortic neck dilation could be a significant problem longer term,” the vascular surgeon said.
Dr. Brinster reported having no conflicts regarding his presentation.
[email protected]
EXPERT ANALYSIS FROM THE NORTHWESTERN VASCULAR SYMPOSIUM
Repeating blood cultures after initial bacteremia: When and how often?
Repeat cultures are indicated in specific scenarios, but for most patients, frequent and indiscriminate repetition after an initial positive culture is unnecessary and may be associated with excessive use of resources. Prospective studies and practice guidelines are needed to help further define the indications.
THE TENDENCY TO REPEAT CULTURES
Current literature lacks strong evidence for repeating previously positive blood cultures collected appropriately—ie, 10 mL of blood for aerobic culture and 10 mL for anaerobic culture from 2 different sites, and a positive result from both sets. However, because of the risk of serious complications of bacteremia, particularly in critically ill patients, many clinicians order multiple, repeated sets of blood cultures.
Tabriz et al1 found that one-third of hospitalized patients got repeat cultures after an initial set, regardless of the result of the first set. Most (83.4%) of those cultures yielded no growth, 9.1% grew the same pathogen, and 5.0% were contaminated. Finding a new pathogen was rare, occurring in only 2.5% of repeated cultures.
Wiggers et al2 reported an even higher number of repeat cultures ordered for patients who had an initially positive culture: 38.9%.2 And in another study,3 half of the patients received more than 2 consecutive cultures.
Drawbacks
Unrestrained ordering of repeat blood cultures can increase the risk of a false-positive result, leading to more cultures, echocardiography, other imaging tests, and unnecessary antimicrobial therapy, all of which puts patients at risk of adverse effects of treatment and missed alternative diagnoses and increases the length and cost of hospitalization.4
Advantages
On the other hand, repeat blood cultures may increase the diagnostic yield for conditions such as infective endocarditis and may have implications for the duration of antibiotic therapy.1 The duration of therapy for bacteremia is usually determined from the last negative culture; hence, documenting clearance of bacteremia can determine a precise end-date for antibiotic therapy.
Bacteremia due to Staphylococcus aureus and to endovascular and epidural sources has been found to be independently associated with persistent bacteremia, detected in 6.6% of 1,801 index cases of bacteremia in a retrospective cohort study.2 An endovascular source (adjusted odds ratio [OR] 7.66, 95% confidence interval [CI] 2.30–25.48), an epidural source (adjusted OR 26.99, 95% CI, 1.91–391.08), and S aureus bacteremia (adjusted OR 4.49, 95% CI 1.88–10.73) were independently associated with persistent bacteremia. Escherichia coli (5.1%, P = .006), viridans group streptococci (1.7%, P = .035), and beta-hemolytic streptococci (0%, P = .028) were associated with a lower likelihood of persistent bacteremia. Patients with persistent bacteremia were less likely to have achieved source control within 48 hours of the index event (29.7% vs 52.5%, P < .001).2
WHEN REPEATING CULTURES IS APPROPRIATE
Repeating blood cultures after an initial positive result is superfluous, except in certain situations.
Suspected endovascular infection
Patients with endocarditis, thrombophlebitis, an indwelling device for epidural access, or a cardiovascular implantable electronic device should have repeat cultures after an initial positive culture. Implantable electronic device infection is suspected in the following cases: sustained positive blood culture (> 24 hours); relapsing bacteremia despite a course of appropriate antibiotic therapy; presence of an implantable cardioverter defibrillator; presence of a prosthetic cardiac valve; and an episode of bacteremia within 3 months of device placement.5
S aureus bacteremia
Repeat blood culture is warranted for S aureus bacteremia regardless of methicillin susceptibility.1 But persistent methicillin-resistant S aureus (MRSA) bacteremia changes the management of these patients.6 For example, the source of infection should be identified, followed by debridement or drainage, and then either high-dose or combination antimicrobial therapy.6 Infective endocarditis from persistent MRSA bacteremia is an indication for surgery.6
Persistent S aureus bacteremia may change the duration of therapy, as the common practice is to continue treating uncomplicated gram-positive bacteremia for 14 days from the date of the first negative culture. Infection leading to infective endocarditis increases the duration of antibiotic therapy to at least 4 weeks.
Candidemia
Candidemia is an absolute indication for repeat blood culture.7 Patients with persistent candidemia should undergo imaging of the genitourinary tract, liver, and spleen as part of the evaluation for a deep-tissue source of infection.7 Also, if the patient is initially treated with an echinocandin, therapy can be transitioned to fluconazole if the isolate is azole-susceptible, the patient’s condition is clinically stable, and repeat cultures are negative.7 Therefore, repeating cultures has therapeutic implications.
Confirming response to therapy
In patients with infective endocarditis or other endovascular infection caused by S aureus, Enterococcus species, or gram-negative bacilli,1 repeat blood culture should be done to confirm therapeutic response. Patients with infective endocarditis whose condition is stable can be discharged to receive outpatient parenteral antibiotic therapy. However, patients with uncontrolled heart failure, systemic emboli, abscess, persistent fever, or persistently positive cultures are not candidates for outpatient therapy and require repeat cultures.8
Multidrug-resistant gram-negative bacilli
Bacteremia due to multidrug-resistant gram-negative bacilli requires repeat blood cultures to document clearance of bacteremia and to ensure the efficacy of antibiotics, as these organisms pose a higher risk of treatment failure, and combination synergistic regimens may be needed if bacteremia does not clear.
Febrile neutropenia
Blood cultures are important in the management of febrile neutropenia. In a study by Rosenblum et al,9 repeat cultures were positive in 10.9% of patients with febrile neutropenia after an initial negative culture, but many of those organisms were of low pathogenicity, and a significant proportion were coagulase-negative staphylococci.10 Another study showed that the frequency of detecting new pathogens by repeat culture in recurrent febrile neutropenia was higher than that in persistent febrile neutropenia (8% vs 2%) (P = .0491); a history of recent bacteremia was identified as a significant predictor of positive culture in recurrent febrile neutropenia.11
Persistent or new infection
Persistence of fever, leukocytosis, or other signs of infection 72 hours after appropriate antibiotic therapy is started requires follow-up blood cultures.
New episode of sepsis. A new episode of sepsis should be confirmed12 using the systemic inflammatory response syndrome criteria, the newer definition of Sepsis-related Organ Failure Assessment (SOFA) in the intensive-care unit, or the quick SOFA in general units. If the patient develops new signs of sepsis after response to treatment for initial bacteremia, repeat blood cultures should be considered.
Central line-associated bloodstream infection requires repeat cultures.13 Persistence of bacteremia in this type of infection extends the duration of therapy, as most clinicians determine treatment duration from the last negative culture. Persistent bacteremia also influences the decision to salvage or remove the catheter. Microbiologic clearance of bacteremia on blood culture can also guide the time of reinsertion if the catheter was removed.
Concern for an unresolved focus of infection such as abscess, joint infection, or retained catheter is an indication for repeat blood cultures.
Bacteremia of unknown source. In clinical practice, we encounter scenarios in which blood cultures are positive but no source can be identified. In those situations, it is important to repeat blood cultures to document clearance. If bacteremia persists, we need to continue searching for the source.
WHEN ROUTINELY REPEATING CULTURES IS NOT INDICATED
Repeat blood cultures are not routinely indicated in patients with streptococcal bacteremia, uncomplicated gram-negative bacteremia, and bacteremia associated with localized infection such as cellulitis, community-acquired pneumonia, or pyelonephritis.2,4 A study of patients with gram-negative bacteremia found that 17 repeated cultures needed to be drawn to yield 1 positive culture.14
Isolated fever or leukocytosis does not accurately predict bacteremia.4 A study that excluded neutropenic and intensive-care patients reported none of the initially negative cultures to be positive when repeated.15
Ordering repeat cultures in response to persistent fever is a common practice, even though fever is typical in the first 72 hours of antibiotic therapy. Such cultures rarely if ever reveal new pathogens, and results can be predicted based on cultures before the start of antibiotics.15 For patients on antibiotics, physicians should therefore wait for results of the preantibiotic cultures rather than order new cultures in response to persistent fever.15
WOULD WE MISS PERSISTENT BACTEREMIA?
In theory, not repeating blood cultures could miss persistent bacteremia, but this is unlikely if the concerns discussed above are considered. Further, persistent bacteremia would result in clinical signs and symptoms that should prompt repeat cultures.
FREQUENCY OF REPEAT BLOOD CULTURES
There are no evidence-based guidelines for the frequency of repeating cultures. The Infectious Diseases Society of America recommends repeating blood cultures 2 to 4 days after the index positive culture in the case of multidrug-resistant S aureus bacteremia, and every day or every other day for candidemia.6,7,9
A study evaluating the practice patterns of repeating cultures after an initial bacteremia showed that 34.7% were done within 24 hours and 44.7% were done in 2 to 4 days.1 There is no evidence that repeating blood cultures daily is necessary in these patients. As a general rule, it should be done 48 to 72 hours after a positive culture.
- Tabriz MS, Riederer K, Baran J Jr, Khatib R. Repeating blood cultures during hospital stay: practice pattern at a teaching hospital and a proposal for guidelines. Clin Microbiol Infect 2004; 10(7):624–627. doi:10.1111/j.1469-0691.2004.00893.x
- Wiggers JB, Xiong W, Daneman N. Sending repeat cultures: is there a role in the management of bacteremic episodes? (SCRIBE study). BMC Infect Dis 2016; 16:286. doi:10.1186/s12879-016-1622-z
- Kang CK, Kim ES, Song KH, et al. Can a routine follow-up blood culture be justified in Klebsiella pneumoniae bacteremia? A retrospective case–control study. BMC Infect Dis 2013; 13:365. doi:10.1186/1471-2334-13-365
- Coburn B, Morris AM, Tomlinson G, Detsky AS. Does this adult patient with suspected bacteremia require blood cultures? JAMA 2012; 308(5):502–511. doi:10.1001/jama.2012.8262
- Baddour LM, Epstein AE, Erickson CC, et al; American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee; Council on Cardiovascular Disease in Young; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Nursing; Council on Clinical Cardiology; Interdisciplinary Council on Quality of Care; American Heart Association. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation 2010; 121(3):458–477. doi:10.1161/CIRCULATIONAHA.109.192665
- Liu C, Bayer A, Cosgrove SE, et al; Infectious Diseases Society of America. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011; 52(3):e18–e55. doi:10.1093/cid/ciq146
- Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 62(4):e1–e50. doi:10.1093/cid/civ933
- Baddour LM, Wilson WR, Bayer AS, et al; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi:10.1161/CIR.0000000000000296
- Rosenblum J, Lin J, Kim M, Levy AS. Repeating blood cultures in neutropenic children with persistent fevers when the initial blood culture is negative. Pediatr Blood Cancer 2013; 60(6):923–927. doi:10.1002/pbc.24358
- Thomas MW, Chauvenet AR, O'Suoji C. Repeating blood cultures in neutropenic children with persistent fevers when the initial blood culture is negative. Pediatr Blood Cancer 2014; 61(2):194. doi:10.1002/pbc.24834
- Kimura SI, Gomyo A, Hayakawa J, et al. Clinical significance of repeat blood cultures during febrile neutropenia in adult acute myeloid leukaemia patients undergoing intensive chemotherapy. Infect Dis (Lond) 2017; 49(10):748–757. doi:10.1080/23744235.2017.1340665
- Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315(8):801–810. doi:10.1001/jama.2016.0287
- Shah H, Bosch W, Thompson KM, Hellinger WC. Intravascular catheter-related bloodstream infection. Neurohospitalist 2013; 3(3):144–151. doi:10.1177/1941874413476043
- Canzoneri CN, Akhavan BJ, Tosur Z, Andrade PEA, Aisenberg GM. Follow-up blood cultures in gram-negative bacteremia: are they needed? Clin Infect Dis 2017; 65(11):1776–1779. doi:10.1093/cid/cix648
- Grace CJ, Lieberman J, Pierce K, Littenberg B. Usefulness of blood culture for hospitalized patients who are receiving antibiotic therapy. Clin Infect Dis 2001; 32(11):1651–1655. doi:10.1086/320527
Repeat cultures are indicated in specific scenarios, but for most patients, frequent and indiscriminate repetition after an initial positive culture is unnecessary and may be associated with excessive use of resources. Prospective studies and practice guidelines are needed to help further define the indications.
THE TENDENCY TO REPEAT CULTURES
Current literature lacks strong evidence for repeating previously positive blood cultures collected appropriately—ie, 10 mL of blood for aerobic culture and 10 mL for anaerobic culture from 2 different sites, and a positive result from both sets. However, because of the risk of serious complications of bacteremia, particularly in critically ill patients, many clinicians order multiple, repeated sets of blood cultures.
Tabriz et al1 found that one-third of hospitalized patients got repeat cultures after an initial set, regardless of the result of the first set. Most (83.4%) of those cultures yielded no growth, 9.1% grew the same pathogen, and 5.0% were contaminated. Finding a new pathogen was rare, occurring in only 2.5% of repeated cultures.
Wiggers et al2 reported an even higher number of repeat cultures ordered for patients who had an initially positive culture: 38.9%.2 And in another study,3 half of the patients received more than 2 consecutive cultures.
Drawbacks
Unrestrained ordering of repeat blood cultures can increase the risk of a false-positive result, leading to more cultures, echocardiography, other imaging tests, and unnecessary antimicrobial therapy, all of which puts patients at risk of adverse effects of treatment and missed alternative diagnoses and increases the length and cost of hospitalization.4
Advantages
On the other hand, repeat blood cultures may increase the diagnostic yield for conditions such as infective endocarditis and may have implications for the duration of antibiotic therapy.1 The duration of therapy for bacteremia is usually determined from the last negative culture; hence, documenting clearance of bacteremia can determine a precise end-date for antibiotic therapy.
Bacteremia due to Staphylococcus aureus and to endovascular and epidural sources has been found to be independently associated with persistent bacteremia, detected in 6.6% of 1,801 index cases of bacteremia in a retrospective cohort study.2 An endovascular source (adjusted odds ratio [OR] 7.66, 95% confidence interval [CI] 2.30–25.48), an epidural source (adjusted OR 26.99, 95% CI, 1.91–391.08), and S aureus bacteremia (adjusted OR 4.49, 95% CI 1.88–10.73) were independently associated with persistent bacteremia. Escherichia coli (5.1%, P = .006), viridans group streptococci (1.7%, P = .035), and beta-hemolytic streptococci (0%, P = .028) were associated with a lower likelihood of persistent bacteremia. Patients with persistent bacteremia were less likely to have achieved source control within 48 hours of the index event (29.7% vs 52.5%, P < .001).2
WHEN REPEATING CULTURES IS APPROPRIATE
Repeating blood cultures after an initial positive result is superfluous, except in certain situations.
Suspected endovascular infection
Patients with endocarditis, thrombophlebitis, an indwelling device for epidural access, or a cardiovascular implantable electronic device should have repeat cultures after an initial positive culture. Implantable electronic device infection is suspected in the following cases: sustained positive blood culture (> 24 hours); relapsing bacteremia despite a course of appropriate antibiotic therapy; presence of an implantable cardioverter defibrillator; presence of a prosthetic cardiac valve; and an episode of bacteremia within 3 months of device placement.5
S aureus bacteremia
Repeat blood culture is warranted for S aureus bacteremia regardless of methicillin susceptibility.1 But persistent methicillin-resistant S aureus (MRSA) bacteremia changes the management of these patients.6 For example, the source of infection should be identified, followed by debridement or drainage, and then either high-dose or combination antimicrobial therapy.6 Infective endocarditis from persistent MRSA bacteremia is an indication for surgery.6
Persistent S aureus bacteremia may change the duration of therapy, as the common practice is to continue treating uncomplicated gram-positive bacteremia for 14 days from the date of the first negative culture. Infection leading to infective endocarditis increases the duration of antibiotic therapy to at least 4 weeks.
Candidemia
Candidemia is an absolute indication for repeat blood culture.7 Patients with persistent candidemia should undergo imaging of the genitourinary tract, liver, and spleen as part of the evaluation for a deep-tissue source of infection.7 Also, if the patient is initially treated with an echinocandin, therapy can be transitioned to fluconazole if the isolate is azole-susceptible, the patient’s condition is clinically stable, and repeat cultures are negative.7 Therefore, repeating cultures has therapeutic implications.
Confirming response to therapy
In patients with infective endocarditis or other endovascular infection caused by S aureus, Enterococcus species, or gram-negative bacilli,1 repeat blood culture should be done to confirm therapeutic response. Patients with infective endocarditis whose condition is stable can be discharged to receive outpatient parenteral antibiotic therapy. However, patients with uncontrolled heart failure, systemic emboli, abscess, persistent fever, or persistently positive cultures are not candidates for outpatient therapy and require repeat cultures.8
Multidrug-resistant gram-negative bacilli
Bacteremia due to multidrug-resistant gram-negative bacilli requires repeat blood cultures to document clearance of bacteremia and to ensure the efficacy of antibiotics, as these organisms pose a higher risk of treatment failure, and combination synergistic regimens may be needed if bacteremia does not clear.
Febrile neutropenia
Blood cultures are important in the management of febrile neutropenia. In a study by Rosenblum et al,9 repeat cultures were positive in 10.9% of patients with febrile neutropenia after an initial negative culture, but many of those organisms were of low pathogenicity, and a significant proportion were coagulase-negative staphylococci.10 Another study showed that the frequency of detecting new pathogens by repeat culture in recurrent febrile neutropenia was higher than that in persistent febrile neutropenia (8% vs 2%) (P = .0491); a history of recent bacteremia was identified as a significant predictor of positive culture in recurrent febrile neutropenia.11
Persistent or new infection
Persistence of fever, leukocytosis, or other signs of infection 72 hours after appropriate antibiotic therapy is started requires follow-up blood cultures.
New episode of sepsis. A new episode of sepsis should be confirmed12 using the systemic inflammatory response syndrome criteria, the newer definition of Sepsis-related Organ Failure Assessment (SOFA) in the intensive-care unit, or the quick SOFA in general units. If the patient develops new signs of sepsis after response to treatment for initial bacteremia, repeat blood cultures should be considered.
Central line-associated bloodstream infection requires repeat cultures.13 Persistence of bacteremia in this type of infection extends the duration of therapy, as most clinicians determine treatment duration from the last negative culture. Persistent bacteremia also influences the decision to salvage or remove the catheter. Microbiologic clearance of bacteremia on blood culture can also guide the time of reinsertion if the catheter was removed.
Concern for an unresolved focus of infection such as abscess, joint infection, or retained catheter is an indication for repeat blood cultures.
Bacteremia of unknown source. In clinical practice, we encounter scenarios in which blood cultures are positive but no source can be identified. In those situations, it is important to repeat blood cultures to document clearance. If bacteremia persists, we need to continue searching for the source.
WHEN ROUTINELY REPEATING CULTURES IS NOT INDICATED
Repeat blood cultures are not routinely indicated in patients with streptococcal bacteremia, uncomplicated gram-negative bacteremia, and bacteremia associated with localized infection such as cellulitis, community-acquired pneumonia, or pyelonephritis.2,4 A study of patients with gram-negative bacteremia found that 17 repeated cultures needed to be drawn to yield 1 positive culture.14
Isolated fever or leukocytosis does not accurately predict bacteremia.4 A study that excluded neutropenic and intensive-care patients reported none of the initially negative cultures to be positive when repeated.15
Ordering repeat cultures in response to persistent fever is a common practice, even though fever is typical in the first 72 hours of antibiotic therapy. Such cultures rarely if ever reveal new pathogens, and results can be predicted based on cultures before the start of antibiotics.15 For patients on antibiotics, physicians should therefore wait for results of the preantibiotic cultures rather than order new cultures in response to persistent fever.15
WOULD WE MISS PERSISTENT BACTEREMIA?
In theory, not repeating blood cultures could miss persistent bacteremia, but this is unlikely if the concerns discussed above are considered. Further, persistent bacteremia would result in clinical signs and symptoms that should prompt repeat cultures.
FREQUENCY OF REPEAT BLOOD CULTURES
There are no evidence-based guidelines for the frequency of repeating cultures. The Infectious Diseases Society of America recommends repeating blood cultures 2 to 4 days after the index positive culture in the case of multidrug-resistant S aureus bacteremia, and every day or every other day for candidemia.6,7,9
A study evaluating the practice patterns of repeating cultures after an initial bacteremia showed that 34.7% were done within 24 hours and 44.7% were done in 2 to 4 days.1 There is no evidence that repeating blood cultures daily is necessary in these patients. As a general rule, it should be done 48 to 72 hours after a positive culture.
Repeat cultures are indicated in specific scenarios, but for most patients, frequent and indiscriminate repetition after an initial positive culture is unnecessary and may be associated with excessive use of resources. Prospective studies and practice guidelines are needed to help further define the indications.
THE TENDENCY TO REPEAT CULTURES
Current literature lacks strong evidence for repeating previously positive blood cultures collected appropriately—ie, 10 mL of blood for aerobic culture and 10 mL for anaerobic culture from 2 different sites, and a positive result from both sets. However, because of the risk of serious complications of bacteremia, particularly in critically ill patients, many clinicians order multiple, repeated sets of blood cultures.
Tabriz et al1 found that one-third of hospitalized patients got repeat cultures after an initial set, regardless of the result of the first set. Most (83.4%) of those cultures yielded no growth, 9.1% grew the same pathogen, and 5.0% were contaminated. Finding a new pathogen was rare, occurring in only 2.5% of repeated cultures.
Wiggers et al2 reported an even higher number of repeat cultures ordered for patients who had an initially positive culture: 38.9%.2 And in another study,3 half of the patients received more than 2 consecutive cultures.
Drawbacks
Unrestrained ordering of repeat blood cultures can increase the risk of a false-positive result, leading to more cultures, echocardiography, other imaging tests, and unnecessary antimicrobial therapy, all of which puts patients at risk of adverse effects of treatment and missed alternative diagnoses and increases the length and cost of hospitalization.4
Advantages
On the other hand, repeat blood cultures may increase the diagnostic yield for conditions such as infective endocarditis and may have implications for the duration of antibiotic therapy.1 The duration of therapy for bacteremia is usually determined from the last negative culture; hence, documenting clearance of bacteremia can determine a precise end-date for antibiotic therapy.
Bacteremia due to Staphylococcus aureus and to endovascular and epidural sources has been found to be independently associated with persistent bacteremia, detected in 6.6% of 1,801 index cases of bacteremia in a retrospective cohort study.2 An endovascular source (adjusted odds ratio [OR] 7.66, 95% confidence interval [CI] 2.30–25.48), an epidural source (adjusted OR 26.99, 95% CI, 1.91–391.08), and S aureus bacteremia (adjusted OR 4.49, 95% CI 1.88–10.73) were independently associated with persistent bacteremia. Escherichia coli (5.1%, P = .006), viridans group streptococci (1.7%, P = .035), and beta-hemolytic streptococci (0%, P = .028) were associated with a lower likelihood of persistent bacteremia. Patients with persistent bacteremia were less likely to have achieved source control within 48 hours of the index event (29.7% vs 52.5%, P < .001).2
WHEN REPEATING CULTURES IS APPROPRIATE
Repeating blood cultures after an initial positive result is superfluous, except in certain situations.
Suspected endovascular infection
Patients with endocarditis, thrombophlebitis, an indwelling device for epidural access, or a cardiovascular implantable electronic device should have repeat cultures after an initial positive culture. Implantable electronic device infection is suspected in the following cases: sustained positive blood culture (> 24 hours); relapsing bacteremia despite a course of appropriate antibiotic therapy; presence of an implantable cardioverter defibrillator; presence of a prosthetic cardiac valve; and an episode of bacteremia within 3 months of device placement.5
S aureus bacteremia
Repeat blood culture is warranted for S aureus bacteremia regardless of methicillin susceptibility.1 But persistent methicillin-resistant S aureus (MRSA) bacteremia changes the management of these patients.6 For example, the source of infection should be identified, followed by debridement or drainage, and then either high-dose or combination antimicrobial therapy.6 Infective endocarditis from persistent MRSA bacteremia is an indication for surgery.6
Persistent S aureus bacteremia may change the duration of therapy, as the common practice is to continue treating uncomplicated gram-positive bacteremia for 14 days from the date of the first negative culture. Infection leading to infective endocarditis increases the duration of antibiotic therapy to at least 4 weeks.
Candidemia
Candidemia is an absolute indication for repeat blood culture.7 Patients with persistent candidemia should undergo imaging of the genitourinary tract, liver, and spleen as part of the evaluation for a deep-tissue source of infection.7 Also, if the patient is initially treated with an echinocandin, therapy can be transitioned to fluconazole if the isolate is azole-susceptible, the patient’s condition is clinically stable, and repeat cultures are negative.7 Therefore, repeating cultures has therapeutic implications.
Confirming response to therapy
In patients with infective endocarditis or other endovascular infection caused by S aureus, Enterococcus species, or gram-negative bacilli,1 repeat blood culture should be done to confirm therapeutic response. Patients with infective endocarditis whose condition is stable can be discharged to receive outpatient parenteral antibiotic therapy. However, patients with uncontrolled heart failure, systemic emboli, abscess, persistent fever, or persistently positive cultures are not candidates for outpatient therapy and require repeat cultures.8
Multidrug-resistant gram-negative bacilli
Bacteremia due to multidrug-resistant gram-negative bacilli requires repeat blood cultures to document clearance of bacteremia and to ensure the efficacy of antibiotics, as these organisms pose a higher risk of treatment failure, and combination synergistic regimens may be needed if bacteremia does not clear.
Febrile neutropenia
Blood cultures are important in the management of febrile neutropenia. In a study by Rosenblum et al,9 repeat cultures were positive in 10.9% of patients with febrile neutropenia after an initial negative culture, but many of those organisms were of low pathogenicity, and a significant proportion were coagulase-negative staphylococci.10 Another study showed that the frequency of detecting new pathogens by repeat culture in recurrent febrile neutropenia was higher than that in persistent febrile neutropenia (8% vs 2%) (P = .0491); a history of recent bacteremia was identified as a significant predictor of positive culture in recurrent febrile neutropenia.11
Persistent or new infection
Persistence of fever, leukocytosis, or other signs of infection 72 hours after appropriate antibiotic therapy is started requires follow-up blood cultures.
New episode of sepsis. A new episode of sepsis should be confirmed12 using the systemic inflammatory response syndrome criteria, the newer definition of Sepsis-related Organ Failure Assessment (SOFA) in the intensive-care unit, or the quick SOFA in general units. If the patient develops new signs of sepsis after response to treatment for initial bacteremia, repeat blood cultures should be considered.
Central line-associated bloodstream infection requires repeat cultures.13 Persistence of bacteremia in this type of infection extends the duration of therapy, as most clinicians determine treatment duration from the last negative culture. Persistent bacteremia also influences the decision to salvage or remove the catheter. Microbiologic clearance of bacteremia on blood culture can also guide the time of reinsertion if the catheter was removed.
Concern for an unresolved focus of infection such as abscess, joint infection, or retained catheter is an indication for repeat blood cultures.
Bacteremia of unknown source. In clinical practice, we encounter scenarios in which blood cultures are positive but no source can be identified. In those situations, it is important to repeat blood cultures to document clearance. If bacteremia persists, we need to continue searching for the source.
WHEN ROUTINELY REPEATING CULTURES IS NOT INDICATED
Repeat blood cultures are not routinely indicated in patients with streptococcal bacteremia, uncomplicated gram-negative bacteremia, and bacteremia associated with localized infection such as cellulitis, community-acquired pneumonia, or pyelonephritis.2,4 A study of patients with gram-negative bacteremia found that 17 repeated cultures needed to be drawn to yield 1 positive culture.14
Isolated fever or leukocytosis does not accurately predict bacteremia.4 A study that excluded neutropenic and intensive-care patients reported none of the initially negative cultures to be positive when repeated.15
Ordering repeat cultures in response to persistent fever is a common practice, even though fever is typical in the first 72 hours of antibiotic therapy. Such cultures rarely if ever reveal new pathogens, and results can be predicted based on cultures before the start of antibiotics.15 For patients on antibiotics, physicians should therefore wait for results of the preantibiotic cultures rather than order new cultures in response to persistent fever.15
WOULD WE MISS PERSISTENT BACTEREMIA?
In theory, not repeating blood cultures could miss persistent bacteremia, but this is unlikely if the concerns discussed above are considered. Further, persistent bacteremia would result in clinical signs and symptoms that should prompt repeat cultures.
FREQUENCY OF REPEAT BLOOD CULTURES
There are no evidence-based guidelines for the frequency of repeating cultures. The Infectious Diseases Society of America recommends repeating blood cultures 2 to 4 days after the index positive culture in the case of multidrug-resistant S aureus bacteremia, and every day or every other day for candidemia.6,7,9
A study evaluating the practice patterns of repeating cultures after an initial bacteremia showed that 34.7% were done within 24 hours and 44.7% were done in 2 to 4 days.1 There is no evidence that repeating blood cultures daily is necessary in these patients. As a general rule, it should be done 48 to 72 hours after a positive culture.
- Tabriz MS, Riederer K, Baran J Jr, Khatib R. Repeating blood cultures during hospital stay: practice pattern at a teaching hospital and a proposal for guidelines. Clin Microbiol Infect 2004; 10(7):624–627. doi:10.1111/j.1469-0691.2004.00893.x
- Wiggers JB, Xiong W, Daneman N. Sending repeat cultures: is there a role in the management of bacteremic episodes? (SCRIBE study). BMC Infect Dis 2016; 16:286. doi:10.1186/s12879-016-1622-z
- Kang CK, Kim ES, Song KH, et al. Can a routine follow-up blood culture be justified in Klebsiella pneumoniae bacteremia? A retrospective case–control study. BMC Infect Dis 2013; 13:365. doi:10.1186/1471-2334-13-365
- Coburn B, Morris AM, Tomlinson G, Detsky AS. Does this adult patient with suspected bacteremia require blood cultures? JAMA 2012; 308(5):502–511. doi:10.1001/jama.2012.8262
- Baddour LM, Epstein AE, Erickson CC, et al; American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee; Council on Cardiovascular Disease in Young; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Nursing; Council on Clinical Cardiology; Interdisciplinary Council on Quality of Care; American Heart Association. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation 2010; 121(3):458–477. doi:10.1161/CIRCULATIONAHA.109.192665
- Liu C, Bayer A, Cosgrove SE, et al; Infectious Diseases Society of America. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011; 52(3):e18–e55. doi:10.1093/cid/ciq146
- Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 62(4):e1–e50. doi:10.1093/cid/civ933
- Baddour LM, Wilson WR, Bayer AS, et al; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi:10.1161/CIR.0000000000000296
- Rosenblum J, Lin J, Kim M, Levy AS. Repeating blood cultures in neutropenic children with persistent fevers when the initial blood culture is negative. Pediatr Blood Cancer 2013; 60(6):923–927. doi:10.1002/pbc.24358
- Thomas MW, Chauvenet AR, O'Suoji C. Repeating blood cultures in neutropenic children with persistent fevers when the initial blood culture is negative. Pediatr Blood Cancer 2014; 61(2):194. doi:10.1002/pbc.24834
- Kimura SI, Gomyo A, Hayakawa J, et al. Clinical significance of repeat blood cultures during febrile neutropenia in adult acute myeloid leukaemia patients undergoing intensive chemotherapy. Infect Dis (Lond) 2017; 49(10):748–757. doi:10.1080/23744235.2017.1340665
- Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315(8):801–810. doi:10.1001/jama.2016.0287
- Shah H, Bosch W, Thompson KM, Hellinger WC. Intravascular catheter-related bloodstream infection. Neurohospitalist 2013; 3(3):144–151. doi:10.1177/1941874413476043
- Canzoneri CN, Akhavan BJ, Tosur Z, Andrade PEA, Aisenberg GM. Follow-up blood cultures in gram-negative bacteremia: are they needed? Clin Infect Dis 2017; 65(11):1776–1779. doi:10.1093/cid/cix648
- Grace CJ, Lieberman J, Pierce K, Littenberg B. Usefulness of blood culture for hospitalized patients who are receiving antibiotic therapy. Clin Infect Dis 2001; 32(11):1651–1655. doi:10.1086/320527
- Tabriz MS, Riederer K, Baran J Jr, Khatib R. Repeating blood cultures during hospital stay: practice pattern at a teaching hospital and a proposal for guidelines. Clin Microbiol Infect 2004; 10(7):624–627. doi:10.1111/j.1469-0691.2004.00893.x
- Wiggers JB, Xiong W, Daneman N. Sending repeat cultures: is there a role in the management of bacteremic episodes? (SCRIBE study). BMC Infect Dis 2016; 16:286. doi:10.1186/s12879-016-1622-z
- Kang CK, Kim ES, Song KH, et al. Can a routine follow-up blood culture be justified in Klebsiella pneumoniae bacteremia? A retrospective case–control study. BMC Infect Dis 2013; 13:365. doi:10.1186/1471-2334-13-365
- Coburn B, Morris AM, Tomlinson G, Detsky AS. Does this adult patient with suspected bacteremia require blood cultures? JAMA 2012; 308(5):502–511. doi:10.1001/jama.2012.8262
- Baddour LM, Epstein AE, Erickson CC, et al; American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee; Council on Cardiovascular Disease in Young; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Nursing; Council on Clinical Cardiology; Interdisciplinary Council on Quality of Care; American Heart Association. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation 2010; 121(3):458–477. doi:10.1161/CIRCULATIONAHA.109.192665
- Liu C, Bayer A, Cosgrove SE, et al; Infectious Diseases Society of America. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011; 52(3):e18–e55. doi:10.1093/cid/ciq146
- Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 62(4):e1–e50. doi:10.1093/cid/civ933
- Baddour LM, Wilson WR, Bayer AS, et al; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi:10.1161/CIR.0000000000000296
- Rosenblum J, Lin J, Kim M, Levy AS. Repeating blood cultures in neutropenic children with persistent fevers when the initial blood culture is negative. Pediatr Blood Cancer 2013; 60(6):923–927. doi:10.1002/pbc.24358
- Thomas MW, Chauvenet AR, O'Suoji C. Repeating blood cultures in neutropenic children with persistent fevers when the initial blood culture is negative. Pediatr Blood Cancer 2014; 61(2):194. doi:10.1002/pbc.24834
- Kimura SI, Gomyo A, Hayakawa J, et al. Clinical significance of repeat blood cultures during febrile neutropenia in adult acute myeloid leukaemia patients undergoing intensive chemotherapy. Infect Dis (Lond) 2017; 49(10):748–757. doi:10.1080/23744235.2017.1340665
- Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315(8):801–810. doi:10.1001/jama.2016.0287
- Shah H, Bosch W, Thompson KM, Hellinger WC. Intravascular catheter-related bloodstream infection. Neurohospitalist 2013; 3(3):144–151. doi:10.1177/1941874413476043
- Canzoneri CN, Akhavan BJ, Tosur Z, Andrade PEA, Aisenberg GM. Follow-up blood cultures in gram-negative bacteremia: are they needed? Clin Infect Dis 2017; 65(11):1776–1779. doi:10.1093/cid/cix648
- Grace CJ, Lieberman J, Pierce K, Littenberg B. Usefulness of blood culture for hospitalized patients who are receiving antibiotic therapy. Clin Infect Dis 2001; 32(11):1651–1655. doi:10.1086/320527
Follow-up blood cultures are often needed after bacteremia
Bacteremia is common and associated with significant morbidity and mortality. Bloodstream infections rank among the leading causes of death in North America and Europe.1
In this issue, Mushtaq et al2 contend that follow-up blood cultures after initial bacteremia are not needed for most hospitalized patients. Not repeating blood cultures after initial bacteremia has been proposed to decrease hospitalization length, consultations, and healthcare costs in some clinical settings. However, without follow-up cultures, it can be difficult to assess the adequacy of treatment of bacteremia and associated underlying infections.
GRAM-NEGATIVE ORGANISMS
Results of retrospective studies indicate that follow-up cultures may not be routinely needed for gram-negative bacteremia. In a review by Canzoneri et al of 383 cases with subsequent follow-up cultures,3 55 (14%) were positive. The mean duration of bacteremia was 2.8 days (range 1 to 15 days). Of the 55 persistently positive blood cultures, only 8 (15%) were caused by gram-negative organisms. Limitations to this study included the lack of patient outcome data, a low event rate, and the retrospective design.4
In a retrospective case-control study of follow-up cultures for 862 episodes of Klebsiella pneumoniae bacteremia,5 independent risk factors for persistent bacteremia were intra-abdominal infection, higher Charlson comorbidity index score, solid-organ transplant, and unfavorable treatment response.
These studies confirm that persistent bacteremia is uncommon with gram-negative organisms. They also support using comorbidities and treatment response to guide the ordering of follow-up blood cultures.
WHEN IS FOLLOW-UP CULTURE USEFUL?
Although follow-up blood cultures may not be needed routinely in patients with gram- negative bacteremia, it would be difficult to extrapolate this to gram-positive organisms, especially Staphylococcus aureus.
In Canzoneri et al,3 43 (78%) of the 55 positive follow-up cultures were due to gram-positive organisms. Factors associated with positive follow-up cultures were concurrent fever, presence of a central intravenous line, end-stage renal disease on hemodialysis, and diabetes mellitus. In addition, infectious disease consultation to decide the need for follow-up cultures for S aureus bacteremia has been associated with fewer deaths, fewer relapses, and lower readmission rates.6,7
In certain clinical scenarios, follow-up blood cultures can provide useful information, such as when the source of bacteremia is endocarditis or cardiac device infection, a vascular graft, or an intravascular line. In the Infectious Diseases Society of America guidelines for diagnosis and management of catheter-related bloodstream infections, persistent or relapsing bacteremia for some organisms is a criterion for removal of a long-term central venous catheter.8
Follow-up cultures are especially useful when the focus of infection is protected from antibiotic penetration, such as in the central nervous system, joints, and abdominal or other abscess. These foci may require drainage for cure. In these cases or in the setting of unfavorable clinical treatment response, follow-up blood cultures showing persistent bacteremia can prompt a search for unaddressed or incompletely addressed foci of infection and allow for source control.
The timing of follow-up cultures is generally 1 to 2 days after the initial culture. Although Mushtaq et al propose a different approach, traditional teaching has been that the last blood culture should not be positive, and this leads to ordering follow-up blood cultures until clearance of bacteremia is documented.
- Goto M, Al-Hasan MN. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect 2013; 19(6):501–509. doi:10.1111/1469-0691.12195
- Mushtaq A, Bredell B, Soubani A. Repeating blood cultures after an initial bacteremia: when and how often? Cleve Clin J Med 2019; 86(2):89–92. doi:10.3949/ccjm.86a.18001
- Canzoneri CN, Akhavan BJ, Tosur Z, Andrade PEA, Aisenberg GM. Follow-up blood cultures in gram-negative bacteremia: are they needed? Clin Infect Dis 2017; 65(11):1776–1779. doi:10.1093/cid/cix648
- Jones RB, Paruchuri A, Shah SS. Prospective trials are required to alter practice for follow-up blood cultures for gram-negative bacilli bacteremia. Clin Infect Dis 2018; 67(2):315–316. doi:10.1093/cid/ciy070
- Kang CK, Kim ES, Song KH, et al. Can a routine follow-up blood culture be justified in Klebsiella pneumoniae bacteremia? A retrospective case-control study. BMC Infect Dis 2013; 13:365. doi:10.1186/1471-2334-13-365
- Honda H, Krauss MJ, Jones JC, Olsen MA, Warren DK. The value of infectious diseases consultation in Staphylococcus aureus bacteremia. Am J Med 2010; 123(7):631–637. doi:10.1016/j.amjmed.2010.01.015
- Fowler VG Jr, Sanders LL, Sexton DJ, et al. Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: experience with 244 patients. Clin Infect Dis 1998; 27(3):478–486. pmid:9770144
- Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 49(1):1–45. doi:10.1086/599376
Bacteremia is common and associated with significant morbidity and mortality. Bloodstream infections rank among the leading causes of death in North America and Europe.1
In this issue, Mushtaq et al2 contend that follow-up blood cultures after initial bacteremia are not needed for most hospitalized patients. Not repeating blood cultures after initial bacteremia has been proposed to decrease hospitalization length, consultations, and healthcare costs in some clinical settings. However, without follow-up cultures, it can be difficult to assess the adequacy of treatment of bacteremia and associated underlying infections.
GRAM-NEGATIVE ORGANISMS
Results of retrospective studies indicate that follow-up cultures may not be routinely needed for gram-negative bacteremia. In a review by Canzoneri et al of 383 cases with subsequent follow-up cultures,3 55 (14%) were positive. The mean duration of bacteremia was 2.8 days (range 1 to 15 days). Of the 55 persistently positive blood cultures, only 8 (15%) were caused by gram-negative organisms. Limitations to this study included the lack of patient outcome data, a low event rate, and the retrospective design.4
In a retrospective case-control study of follow-up cultures for 862 episodes of Klebsiella pneumoniae bacteremia,5 independent risk factors for persistent bacteremia were intra-abdominal infection, higher Charlson comorbidity index score, solid-organ transplant, and unfavorable treatment response.
These studies confirm that persistent bacteremia is uncommon with gram-negative organisms. They also support using comorbidities and treatment response to guide the ordering of follow-up blood cultures.
WHEN IS FOLLOW-UP CULTURE USEFUL?
Although follow-up blood cultures may not be needed routinely in patients with gram- negative bacteremia, it would be difficult to extrapolate this to gram-positive organisms, especially Staphylococcus aureus.
In Canzoneri et al,3 43 (78%) of the 55 positive follow-up cultures were due to gram-positive organisms. Factors associated with positive follow-up cultures were concurrent fever, presence of a central intravenous line, end-stage renal disease on hemodialysis, and diabetes mellitus. In addition, infectious disease consultation to decide the need for follow-up cultures for S aureus bacteremia has been associated with fewer deaths, fewer relapses, and lower readmission rates.6,7
In certain clinical scenarios, follow-up blood cultures can provide useful information, such as when the source of bacteremia is endocarditis or cardiac device infection, a vascular graft, or an intravascular line. In the Infectious Diseases Society of America guidelines for diagnosis and management of catheter-related bloodstream infections, persistent or relapsing bacteremia for some organisms is a criterion for removal of a long-term central venous catheter.8
Follow-up cultures are especially useful when the focus of infection is protected from antibiotic penetration, such as in the central nervous system, joints, and abdominal or other abscess. These foci may require drainage for cure. In these cases or in the setting of unfavorable clinical treatment response, follow-up blood cultures showing persistent bacteremia can prompt a search for unaddressed or incompletely addressed foci of infection and allow for source control.
The timing of follow-up cultures is generally 1 to 2 days after the initial culture. Although Mushtaq et al propose a different approach, traditional teaching has been that the last blood culture should not be positive, and this leads to ordering follow-up blood cultures until clearance of bacteremia is documented.
Bacteremia is common and associated with significant morbidity and mortality. Bloodstream infections rank among the leading causes of death in North America and Europe.1
In this issue, Mushtaq et al2 contend that follow-up blood cultures after initial bacteremia are not needed for most hospitalized patients. Not repeating blood cultures after initial bacteremia has been proposed to decrease hospitalization length, consultations, and healthcare costs in some clinical settings. However, without follow-up cultures, it can be difficult to assess the adequacy of treatment of bacteremia and associated underlying infections.
GRAM-NEGATIVE ORGANISMS
Results of retrospective studies indicate that follow-up cultures may not be routinely needed for gram-negative bacteremia. In a review by Canzoneri et al of 383 cases with subsequent follow-up cultures,3 55 (14%) were positive. The mean duration of bacteremia was 2.8 days (range 1 to 15 days). Of the 55 persistently positive blood cultures, only 8 (15%) were caused by gram-negative organisms. Limitations to this study included the lack of patient outcome data, a low event rate, and the retrospective design.4
In a retrospective case-control study of follow-up cultures for 862 episodes of Klebsiella pneumoniae bacteremia,5 independent risk factors for persistent bacteremia were intra-abdominal infection, higher Charlson comorbidity index score, solid-organ transplant, and unfavorable treatment response.
These studies confirm that persistent bacteremia is uncommon with gram-negative organisms. They also support using comorbidities and treatment response to guide the ordering of follow-up blood cultures.
WHEN IS FOLLOW-UP CULTURE USEFUL?
Although follow-up blood cultures may not be needed routinely in patients with gram- negative bacteremia, it would be difficult to extrapolate this to gram-positive organisms, especially Staphylococcus aureus.
In Canzoneri et al,3 43 (78%) of the 55 positive follow-up cultures were due to gram-positive organisms. Factors associated with positive follow-up cultures were concurrent fever, presence of a central intravenous line, end-stage renal disease on hemodialysis, and diabetes mellitus. In addition, infectious disease consultation to decide the need for follow-up cultures for S aureus bacteremia has been associated with fewer deaths, fewer relapses, and lower readmission rates.6,7
In certain clinical scenarios, follow-up blood cultures can provide useful information, such as when the source of bacteremia is endocarditis or cardiac device infection, a vascular graft, or an intravascular line. In the Infectious Diseases Society of America guidelines for diagnosis and management of catheter-related bloodstream infections, persistent or relapsing bacteremia for some organisms is a criterion for removal of a long-term central venous catheter.8
Follow-up cultures are especially useful when the focus of infection is protected from antibiotic penetration, such as in the central nervous system, joints, and abdominal or other abscess. These foci may require drainage for cure. In these cases or in the setting of unfavorable clinical treatment response, follow-up blood cultures showing persistent bacteremia can prompt a search for unaddressed or incompletely addressed foci of infection and allow for source control.
The timing of follow-up cultures is generally 1 to 2 days after the initial culture. Although Mushtaq et al propose a different approach, traditional teaching has been that the last blood culture should not be positive, and this leads to ordering follow-up blood cultures until clearance of bacteremia is documented.
- Goto M, Al-Hasan MN. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect 2013; 19(6):501–509. doi:10.1111/1469-0691.12195
- Mushtaq A, Bredell B, Soubani A. Repeating blood cultures after an initial bacteremia: when and how often? Cleve Clin J Med 2019; 86(2):89–92. doi:10.3949/ccjm.86a.18001
- Canzoneri CN, Akhavan BJ, Tosur Z, Andrade PEA, Aisenberg GM. Follow-up blood cultures in gram-negative bacteremia: are they needed? Clin Infect Dis 2017; 65(11):1776–1779. doi:10.1093/cid/cix648
- Jones RB, Paruchuri A, Shah SS. Prospective trials are required to alter practice for follow-up blood cultures for gram-negative bacilli bacteremia. Clin Infect Dis 2018; 67(2):315–316. doi:10.1093/cid/ciy070
- Kang CK, Kim ES, Song KH, et al. Can a routine follow-up blood culture be justified in Klebsiella pneumoniae bacteremia? A retrospective case-control study. BMC Infect Dis 2013; 13:365. doi:10.1186/1471-2334-13-365
- Honda H, Krauss MJ, Jones JC, Olsen MA, Warren DK. The value of infectious diseases consultation in Staphylococcus aureus bacteremia. Am J Med 2010; 123(7):631–637. doi:10.1016/j.amjmed.2010.01.015
- Fowler VG Jr, Sanders LL, Sexton DJ, et al. Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: experience with 244 patients. Clin Infect Dis 1998; 27(3):478–486. pmid:9770144
- Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 49(1):1–45. doi:10.1086/599376
- Goto M, Al-Hasan MN. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect 2013; 19(6):501–509. doi:10.1111/1469-0691.12195
- Mushtaq A, Bredell B, Soubani A. Repeating blood cultures after an initial bacteremia: when and how often? Cleve Clin J Med 2019; 86(2):89–92. doi:10.3949/ccjm.86a.18001
- Canzoneri CN, Akhavan BJ, Tosur Z, Andrade PEA, Aisenberg GM. Follow-up blood cultures in gram-negative bacteremia: are they needed? Clin Infect Dis 2017; 65(11):1776–1779. doi:10.1093/cid/cix648
- Jones RB, Paruchuri A, Shah SS. Prospective trials are required to alter practice for follow-up blood cultures for gram-negative bacilli bacteremia. Clin Infect Dis 2018; 67(2):315–316. doi:10.1093/cid/ciy070
- Kang CK, Kim ES, Song KH, et al. Can a routine follow-up blood culture be justified in Klebsiella pneumoniae bacteremia? A retrospective case-control study. BMC Infect Dis 2013; 13:365. doi:10.1186/1471-2334-13-365
- Honda H, Krauss MJ, Jones JC, Olsen MA, Warren DK. The value of infectious diseases consultation in Staphylococcus aureus bacteremia. Am J Med 2010; 123(7):631–637. doi:10.1016/j.amjmed.2010.01.015
- Fowler VG Jr, Sanders LL, Sexton DJ, et al. Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: experience with 244 patients. Clin Infect Dis 1998; 27(3):478–486. pmid:9770144
- Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 49(1):1–45. doi:10.1086/599376
FDA: Benefits still outweigh risks from paclitaxel-coated devices for PAD
The Food and Drug Administration has issued a letter alerting health care providers that it is aware of and examining recent data on an increase in long-term mortality rates for patients receiving paclitaxel-coated balloons and paclitaxel-eluting stents for treatment of peripheral artery disease.
“Currently, the FDA believes that the benefits continue to outweigh the risks for approved paclitaxel-coated balloons and paclitaxel-eluting stents when used in accordance with their indications for use,” William Maisel, MD, MPH, chief medical officer of the Center for Devices and Radiological Health at the FDA, wrote in a letter to health care providers.
The FDA letter was in response to a recent systematic review of paclitaxel-coated balloons and stents recently published in the Journal of the American Heart Association. Konstantinos Katsanos, MD, PhD, from Patras University Hospital in Rion, Greece, and colleagues performed the systematic review and meta-analysis of 28 randomized controlled trials with 4,663 patients who received paclitaxel-coated devices in the femoral and/or popliteal arteries and found similar 1-year risk of all-cause patient mortality (2.3%; risk ratio, 1.08; 95% confidence interval, 0.72-1.61). However, there was an increased risk of all-cause mortality for patients with paclitaxel-coated devices at 2 years (7.2% vs. 3.8%; RR, 1.68; 95% CI, 1.15-2.47) and at 5 years (14.7% vs. 8.1%; RR, 1.93; 95% CI, 1.27-2.93), compared with control groups. The number needed to harm at 2 years was 29 patients (95% CI, 19-59) and 14 patients (95% CI, 9-32) at 5 years. Their meta regression analysis found a significant link between paclitaxel exposure and absolute risk of death.
“Actual causes for this serious late side effect remain unknown, and further investigations with longer-term follow-up are urgently warranted,” Dr. Katsanos and colleagues wrote in their review.
The FDA told health care providers of patients with paclitaxel-coated balloons and paclitaxel-eluting stents to continue surveillance of these patients per standard of care, to discuss the risks and benefits of PAD treatment options with patients, and to report any adverse or suspected adverse events to MedWatch.
The FDA said they are currently evaluating long-term data on paclitaxel-coated products to determine whether the devices carry an increased risk of death or other long-term risks, and noted there were several paclitaxel-coated balloons or paclitaxel-eluting stents that have either been approved or are under study in the United States.
SOURCE: Katsanos K et al. J Am Heart Assoc. 2018. doi: 10.1161/JAHA.118.011245.
The Food and Drug Administration has issued a letter alerting health care providers that it is aware of and examining recent data on an increase in long-term mortality rates for patients receiving paclitaxel-coated balloons and paclitaxel-eluting stents for treatment of peripheral artery disease.
“Currently, the FDA believes that the benefits continue to outweigh the risks for approved paclitaxel-coated balloons and paclitaxel-eluting stents when used in accordance with their indications for use,” William Maisel, MD, MPH, chief medical officer of the Center for Devices and Radiological Health at the FDA, wrote in a letter to health care providers.
The FDA letter was in response to a recent systematic review of paclitaxel-coated balloons and stents recently published in the Journal of the American Heart Association. Konstantinos Katsanos, MD, PhD, from Patras University Hospital in Rion, Greece, and colleagues performed the systematic review and meta-analysis of 28 randomized controlled trials with 4,663 patients who received paclitaxel-coated devices in the femoral and/or popliteal arteries and found similar 1-year risk of all-cause patient mortality (2.3%; risk ratio, 1.08; 95% confidence interval, 0.72-1.61). However, there was an increased risk of all-cause mortality for patients with paclitaxel-coated devices at 2 years (7.2% vs. 3.8%; RR, 1.68; 95% CI, 1.15-2.47) and at 5 years (14.7% vs. 8.1%; RR, 1.93; 95% CI, 1.27-2.93), compared with control groups. The number needed to harm at 2 years was 29 patients (95% CI, 19-59) and 14 patients (95% CI, 9-32) at 5 years. Their meta regression analysis found a significant link between paclitaxel exposure and absolute risk of death.
“Actual causes for this serious late side effect remain unknown, and further investigations with longer-term follow-up are urgently warranted,” Dr. Katsanos and colleagues wrote in their review.
The FDA told health care providers of patients with paclitaxel-coated balloons and paclitaxel-eluting stents to continue surveillance of these patients per standard of care, to discuss the risks and benefits of PAD treatment options with patients, and to report any adverse or suspected adverse events to MedWatch.
The FDA said they are currently evaluating long-term data on paclitaxel-coated products to determine whether the devices carry an increased risk of death or other long-term risks, and noted there were several paclitaxel-coated balloons or paclitaxel-eluting stents that have either been approved or are under study in the United States.
SOURCE: Katsanos K et al. J Am Heart Assoc. 2018. doi: 10.1161/JAHA.118.011245.
The Food and Drug Administration has issued a letter alerting health care providers that it is aware of and examining recent data on an increase in long-term mortality rates for patients receiving paclitaxel-coated balloons and paclitaxel-eluting stents for treatment of peripheral artery disease.
“Currently, the FDA believes that the benefits continue to outweigh the risks for approved paclitaxel-coated balloons and paclitaxel-eluting stents when used in accordance with their indications for use,” William Maisel, MD, MPH, chief medical officer of the Center for Devices and Radiological Health at the FDA, wrote in a letter to health care providers.
The FDA letter was in response to a recent systematic review of paclitaxel-coated balloons and stents recently published in the Journal of the American Heart Association. Konstantinos Katsanos, MD, PhD, from Patras University Hospital in Rion, Greece, and colleagues performed the systematic review and meta-analysis of 28 randomized controlled trials with 4,663 patients who received paclitaxel-coated devices in the femoral and/or popliteal arteries and found similar 1-year risk of all-cause patient mortality (2.3%; risk ratio, 1.08; 95% confidence interval, 0.72-1.61). However, there was an increased risk of all-cause mortality for patients with paclitaxel-coated devices at 2 years (7.2% vs. 3.8%; RR, 1.68; 95% CI, 1.15-2.47) and at 5 years (14.7% vs. 8.1%; RR, 1.93; 95% CI, 1.27-2.93), compared with control groups. The number needed to harm at 2 years was 29 patients (95% CI, 19-59) and 14 patients (95% CI, 9-32) at 5 years. Their meta regression analysis found a significant link between paclitaxel exposure and absolute risk of death.
“Actual causes for this serious late side effect remain unknown, and further investigations with longer-term follow-up are urgently warranted,” Dr. Katsanos and colleagues wrote in their review.
The FDA told health care providers of patients with paclitaxel-coated balloons and paclitaxel-eluting stents to continue surveillance of these patients per standard of care, to discuss the risks and benefits of PAD treatment options with patients, and to report any adverse or suspected adverse events to MedWatch.
The FDA said they are currently evaluating long-term data on paclitaxel-coated products to determine whether the devices carry an increased risk of death or other long-term risks, and noted there were several paclitaxel-coated balloons or paclitaxel-eluting stents that have either been approved or are under study in the United States.
SOURCE: Katsanos K et al. J Am Heart Assoc. 2018. doi: 10.1161/JAHA.118.011245.
Key clinical point: In a letter to health care providers, FDA said it was investigating data from a recent meta-analysis of increased long-term mortality risk from paclitaxel-coated balloons and paclitaxel-eluting stents for treatment of peripheral artery disease.
Major finding: All-cause mortality increased significantly after 2 years (7.2% vs. 3.8%) and 5 years (14.7% vs. 8.1%) compared with control groups.
Study details: A systematic review and meta-analysis of 28 randomized controlled trials with 4,663 patients.
Source: Katsanos K et al. J Am Heart Assoc. 2018. doi: 10.1161/JAHA.118.011245.
ATTRACT trial shouldn’t detract from pharmacomechanical thrombolysis
CHICAGO – A closer look at the landmark ATTRACT trial of pharmacomechanical catheter-directed thrombolysis for acute deep vein thrombosis (DVT) shows multiple benefits for the intervention versus standard anticoagulation alone in the subset of participants with iliofemoral DVT, Kush R. Desai, MD, said at a symposium on vascular surgery sponsored by Northwestern University.
ATTRACT, a National Institutes of Health–sponsored, phase 3, multicenter, open-label, assessor-blinded study, was the first-ever randomized trial of pharmacomechanical catheter-directed thrombolysis (PCDT) for acute DVT.
The results caused a major stir because, despite a sound therapeutic rationale for the procedure, the incidence of chronic postthrombotic syndrome (PTS) at 24 months of follow-up was 47% in the PCDT plus anticoagulation group and 48% in controls on anticoagulation alone (N Engl J Med. 2017 Dec 7;377[23]:2240-52). Since then, that overall negative trial has been one of the hottest topics in DVT.
“This is the first thing your educated patients who come to the emergency department with DVT will ask about. It’s the first thing they’ll see when they go online and type in ‘thrombolysis DVT,’ ” noted Dr. Desai, an interventional radiologist at Northwestern University, Chicago.
But the trial has several major flaws, he cautioned. And contrary to popular opinion, ATTRACT is not the death knell for PCDT. Far from it.
“I don’t think the story stops with ATTRACT. This isn’t the end for PCDT in patients with iliofemoral DVT,” he asserted.
That’s in part because 301 of the 692 participants in ATTRACT had DVT of the femoropopliteal segment. That’s a population in which Dr. Desai and other interventionalists wouldn’t have anticipated seeing a benefit for PCDT, because their risk of PTS is so low.
“We know through historical data that patients with iliofemoral DVT are much more likely to develop PTS and to have recurrent DVT, so this is probably one of the major shortcomings of the trial,” he explained. “It’s through no fault of the trial investigators, because the study was planned years ago when we just didn’t know as much about PTS as we do now.
“The way I look at it is, I don’t practice in the way that ATTRACT was designed,” Dr. Desai said. “I don’t typically lyse or get referrals for lysis or thrombectomy in patients who have isolated femoropopliteal DVT. It has to involve at least the common femoral vein and frequently goes up to the iliac vein.”
The ATTRACT investigators’ recent subanalysis of the 391 participants with iliofemoral DVT showed that, although there was no difference between the two study arms in the occurrence of PTS through the first 24 months of follow-up, PCDT led to a 35% reduction in the incidence of moderate or severe PTS – by a margin of 18% versus 28% in controls.
Patients in the PCDT arm also experienced significantly greater improvement in venous disease-specific quality of life through 24 months, and a greater reduction in leg pain and swelling at 10 and 30 days (Circulation. 2018 Dec 4. doi: 10.1161/CIRCULATIONAHA.118.037425).
And moderate to severe PTS is a key outcome, Dr. Desai continued. Multiple studies have shown that patients with PTS have a worse quality of life than those with chronic lung disease, arthritis, or diabetes. Moreover, the 5%-10% of patients with symptomatic DVT who develop the most-severe form of PTS – characterized by severe pain, chronic ulcerations, stasis dermatitis, venous claudication, and intractable edema – have a quality of life comparable with patients with cancer or heart failure.
The 1.5% incidence of major bleeding within 10 days in the PCDT group was 200% higher than in controls, but none of it was life threatening.
“This is reassuring: Nobody had intracranial hemorrhage; nobody had a GUSTO 5 bleed,” Dr. Desai said.
Another limitation of the ATTRACT trial is that all but one of the devices utilized for PCDT were used off label. They weren’t designed for venous application. Several on-label rheolytic, rotational thrombectomy, or clot aspiration devices have been approved since enrollment in ATTRACT was closed. Future randomized trials will utilize on-label devices in patients with acute iliofemoral DVT to clarify the role of PCDT.
It’s noteworthy that nearly half of ATTRACT participants developed PTS within 24 months of their DVT despite being on optimal anticoagulation. It’s a finding that underscores the need for improved therapies. That was the impetus for development of first-generation catheter-directed thrombolysis utilizing a percutaneously inserted catheter to infuse a fibrinolytic drug directly to the thrombus to dissolve it rapidly.
But that form of catheter-directed thrombolysis has major disadvantages, Dr. Desai explained: It’s a multiday procedure requiring ICU-level care and prolonged exposure to powerful lytic agents.
“This is where things have changed with PCDT,” he said. “We can now, with on-label devices, accelerate the thrombolysis time, reduce lytic exposure, and I think also reduce the bleeding risk, although that hasn’t been shown in a trial yet. PCDT also reduces the necessity for ICU-level care and prolonged hospitalization.”
Dr. Desai no longer performs multiday lytic procedures. “In fact, with the introduction of the newer on-label devices, I haven’t done a multiday unilateral limb lytic procedure in a couple years. I think we’ve gotten to the point where we don’t need to do that anymore.”
Indeed, PCDT makes recanalization possible as a single-day, single-session procedure.
Dr. Desai views the recent ATTRACT subanalysis as hypothesis generating.
“Should PCDT be the first-line treatment in all proximal DVT patients? No it should not – and that’s not what I would have advocated even before ATTRACT came out,” he explained. “It’s sort of a salvage procedure for patients with iliofemoral DVT and moderate to severe symptoms. And there are a significant number of such patients.”
Current understanding of the pathophysiology of PTS is that a nondissolved thrombus at the valve leaflets becomes inflammatory, with resultant valvular dysfunction leading to venous reflux and venous hypertension. PCDT is consistent with the open-vein hypothesis, which posits that, by eliminating thrombus much faster than achievable via anticoagulation, valve integrity is maintained and PTS is prevented.
Dr. Desai reported receiving consulting fees from AngioDynamics, Boston Scientific, Cook Medical, and Spectranetics.
CHICAGO – A closer look at the landmark ATTRACT trial of pharmacomechanical catheter-directed thrombolysis for acute deep vein thrombosis (DVT) shows multiple benefits for the intervention versus standard anticoagulation alone in the subset of participants with iliofemoral DVT, Kush R. Desai, MD, said at a symposium on vascular surgery sponsored by Northwestern University.
ATTRACT, a National Institutes of Health–sponsored, phase 3, multicenter, open-label, assessor-blinded study, was the first-ever randomized trial of pharmacomechanical catheter-directed thrombolysis (PCDT) for acute DVT.
The results caused a major stir because, despite a sound therapeutic rationale for the procedure, the incidence of chronic postthrombotic syndrome (PTS) at 24 months of follow-up was 47% in the PCDT plus anticoagulation group and 48% in controls on anticoagulation alone (N Engl J Med. 2017 Dec 7;377[23]:2240-52). Since then, that overall negative trial has been one of the hottest topics in DVT.
“This is the first thing your educated patients who come to the emergency department with DVT will ask about. It’s the first thing they’ll see when they go online and type in ‘thrombolysis DVT,’ ” noted Dr. Desai, an interventional radiologist at Northwestern University, Chicago.
But the trial has several major flaws, he cautioned. And contrary to popular opinion, ATTRACT is not the death knell for PCDT. Far from it.
“I don’t think the story stops with ATTRACT. This isn’t the end for PCDT in patients with iliofemoral DVT,” he asserted.
That’s in part because 301 of the 692 participants in ATTRACT had DVT of the femoropopliteal segment. That’s a population in which Dr. Desai and other interventionalists wouldn’t have anticipated seeing a benefit for PCDT, because their risk of PTS is so low.
“We know through historical data that patients with iliofemoral DVT are much more likely to develop PTS and to have recurrent DVT, so this is probably one of the major shortcomings of the trial,” he explained. “It’s through no fault of the trial investigators, because the study was planned years ago when we just didn’t know as much about PTS as we do now.
“The way I look at it is, I don’t practice in the way that ATTRACT was designed,” Dr. Desai said. “I don’t typically lyse or get referrals for lysis or thrombectomy in patients who have isolated femoropopliteal DVT. It has to involve at least the common femoral vein and frequently goes up to the iliac vein.”
The ATTRACT investigators’ recent subanalysis of the 391 participants with iliofemoral DVT showed that, although there was no difference between the two study arms in the occurrence of PTS through the first 24 months of follow-up, PCDT led to a 35% reduction in the incidence of moderate or severe PTS – by a margin of 18% versus 28% in controls.
Patients in the PCDT arm also experienced significantly greater improvement in venous disease-specific quality of life through 24 months, and a greater reduction in leg pain and swelling at 10 and 30 days (Circulation. 2018 Dec 4. doi: 10.1161/CIRCULATIONAHA.118.037425).
And moderate to severe PTS is a key outcome, Dr. Desai continued. Multiple studies have shown that patients with PTS have a worse quality of life than those with chronic lung disease, arthritis, or diabetes. Moreover, the 5%-10% of patients with symptomatic DVT who develop the most-severe form of PTS – characterized by severe pain, chronic ulcerations, stasis dermatitis, venous claudication, and intractable edema – have a quality of life comparable with patients with cancer or heart failure.
The 1.5% incidence of major bleeding within 10 days in the PCDT group was 200% higher than in controls, but none of it was life threatening.
“This is reassuring: Nobody had intracranial hemorrhage; nobody had a GUSTO 5 bleed,” Dr. Desai said.
Another limitation of the ATTRACT trial is that all but one of the devices utilized for PCDT were used off label. They weren’t designed for venous application. Several on-label rheolytic, rotational thrombectomy, or clot aspiration devices have been approved since enrollment in ATTRACT was closed. Future randomized trials will utilize on-label devices in patients with acute iliofemoral DVT to clarify the role of PCDT.
It’s noteworthy that nearly half of ATTRACT participants developed PTS within 24 months of their DVT despite being on optimal anticoagulation. It’s a finding that underscores the need for improved therapies. That was the impetus for development of first-generation catheter-directed thrombolysis utilizing a percutaneously inserted catheter to infuse a fibrinolytic drug directly to the thrombus to dissolve it rapidly.
But that form of catheter-directed thrombolysis has major disadvantages, Dr. Desai explained: It’s a multiday procedure requiring ICU-level care and prolonged exposure to powerful lytic agents.
“This is where things have changed with PCDT,” he said. “We can now, with on-label devices, accelerate the thrombolysis time, reduce lytic exposure, and I think also reduce the bleeding risk, although that hasn’t been shown in a trial yet. PCDT also reduces the necessity for ICU-level care and prolonged hospitalization.”
Dr. Desai no longer performs multiday lytic procedures. “In fact, with the introduction of the newer on-label devices, I haven’t done a multiday unilateral limb lytic procedure in a couple years. I think we’ve gotten to the point where we don’t need to do that anymore.”
Indeed, PCDT makes recanalization possible as a single-day, single-session procedure.
Dr. Desai views the recent ATTRACT subanalysis as hypothesis generating.
“Should PCDT be the first-line treatment in all proximal DVT patients? No it should not – and that’s not what I would have advocated even before ATTRACT came out,” he explained. “It’s sort of a salvage procedure for patients with iliofemoral DVT and moderate to severe symptoms. And there are a significant number of such patients.”
Current understanding of the pathophysiology of PTS is that a nondissolved thrombus at the valve leaflets becomes inflammatory, with resultant valvular dysfunction leading to venous reflux and venous hypertension. PCDT is consistent with the open-vein hypothesis, which posits that, by eliminating thrombus much faster than achievable via anticoagulation, valve integrity is maintained and PTS is prevented.
Dr. Desai reported receiving consulting fees from AngioDynamics, Boston Scientific, Cook Medical, and Spectranetics.
CHICAGO – A closer look at the landmark ATTRACT trial of pharmacomechanical catheter-directed thrombolysis for acute deep vein thrombosis (DVT) shows multiple benefits for the intervention versus standard anticoagulation alone in the subset of participants with iliofemoral DVT, Kush R. Desai, MD, said at a symposium on vascular surgery sponsored by Northwestern University.
ATTRACT, a National Institutes of Health–sponsored, phase 3, multicenter, open-label, assessor-blinded study, was the first-ever randomized trial of pharmacomechanical catheter-directed thrombolysis (PCDT) for acute DVT.
The results caused a major stir because, despite a sound therapeutic rationale for the procedure, the incidence of chronic postthrombotic syndrome (PTS) at 24 months of follow-up was 47% in the PCDT plus anticoagulation group and 48% in controls on anticoagulation alone (N Engl J Med. 2017 Dec 7;377[23]:2240-52). Since then, that overall negative trial has been one of the hottest topics in DVT.
“This is the first thing your educated patients who come to the emergency department with DVT will ask about. It’s the first thing they’ll see when they go online and type in ‘thrombolysis DVT,’ ” noted Dr. Desai, an interventional radiologist at Northwestern University, Chicago.
But the trial has several major flaws, he cautioned. And contrary to popular opinion, ATTRACT is not the death knell for PCDT. Far from it.
“I don’t think the story stops with ATTRACT. This isn’t the end for PCDT in patients with iliofemoral DVT,” he asserted.
That’s in part because 301 of the 692 participants in ATTRACT had DVT of the femoropopliteal segment. That’s a population in which Dr. Desai and other interventionalists wouldn’t have anticipated seeing a benefit for PCDT, because their risk of PTS is so low.
“We know through historical data that patients with iliofemoral DVT are much more likely to develop PTS and to have recurrent DVT, so this is probably one of the major shortcomings of the trial,” he explained. “It’s through no fault of the trial investigators, because the study was planned years ago when we just didn’t know as much about PTS as we do now.
“The way I look at it is, I don’t practice in the way that ATTRACT was designed,” Dr. Desai said. “I don’t typically lyse or get referrals for lysis or thrombectomy in patients who have isolated femoropopliteal DVT. It has to involve at least the common femoral vein and frequently goes up to the iliac vein.”
The ATTRACT investigators’ recent subanalysis of the 391 participants with iliofemoral DVT showed that, although there was no difference between the two study arms in the occurrence of PTS through the first 24 months of follow-up, PCDT led to a 35% reduction in the incidence of moderate or severe PTS – by a margin of 18% versus 28% in controls.
Patients in the PCDT arm also experienced significantly greater improvement in venous disease-specific quality of life through 24 months, and a greater reduction in leg pain and swelling at 10 and 30 days (Circulation. 2018 Dec 4. doi: 10.1161/CIRCULATIONAHA.118.037425).
And moderate to severe PTS is a key outcome, Dr. Desai continued. Multiple studies have shown that patients with PTS have a worse quality of life than those with chronic lung disease, arthritis, or diabetes. Moreover, the 5%-10% of patients with symptomatic DVT who develop the most-severe form of PTS – characterized by severe pain, chronic ulcerations, stasis dermatitis, venous claudication, and intractable edema – have a quality of life comparable with patients with cancer or heart failure.
The 1.5% incidence of major bleeding within 10 days in the PCDT group was 200% higher than in controls, but none of it was life threatening.
“This is reassuring: Nobody had intracranial hemorrhage; nobody had a GUSTO 5 bleed,” Dr. Desai said.
Another limitation of the ATTRACT trial is that all but one of the devices utilized for PCDT were used off label. They weren’t designed for venous application. Several on-label rheolytic, rotational thrombectomy, or clot aspiration devices have been approved since enrollment in ATTRACT was closed. Future randomized trials will utilize on-label devices in patients with acute iliofemoral DVT to clarify the role of PCDT.
It’s noteworthy that nearly half of ATTRACT participants developed PTS within 24 months of their DVT despite being on optimal anticoagulation. It’s a finding that underscores the need for improved therapies. That was the impetus for development of first-generation catheter-directed thrombolysis utilizing a percutaneously inserted catheter to infuse a fibrinolytic drug directly to the thrombus to dissolve it rapidly.
But that form of catheter-directed thrombolysis has major disadvantages, Dr. Desai explained: It’s a multiday procedure requiring ICU-level care and prolonged exposure to powerful lytic agents.
“This is where things have changed with PCDT,” he said. “We can now, with on-label devices, accelerate the thrombolysis time, reduce lytic exposure, and I think also reduce the bleeding risk, although that hasn’t been shown in a trial yet. PCDT also reduces the necessity for ICU-level care and prolonged hospitalization.”
Dr. Desai no longer performs multiday lytic procedures. “In fact, with the introduction of the newer on-label devices, I haven’t done a multiday unilateral limb lytic procedure in a couple years. I think we’ve gotten to the point where we don’t need to do that anymore.”
Indeed, PCDT makes recanalization possible as a single-day, single-session procedure.
Dr. Desai views the recent ATTRACT subanalysis as hypothesis generating.
“Should PCDT be the first-line treatment in all proximal DVT patients? No it should not – and that’s not what I would have advocated even before ATTRACT came out,” he explained. “It’s sort of a salvage procedure for patients with iliofemoral DVT and moderate to severe symptoms. And there are a significant number of such patients.”
Current understanding of the pathophysiology of PTS is that a nondissolved thrombus at the valve leaflets becomes inflammatory, with resultant valvular dysfunction leading to venous reflux and venous hypertension. PCDT is consistent with the open-vein hypothesis, which posits that, by eliminating thrombus much faster than achievable via anticoagulation, valve integrity is maintained and PTS is prevented.
Dr. Desai reported receiving consulting fees from AngioDynamics, Boston Scientific, Cook Medical, and Spectranetics.
EXPERT ANALYSIS FROM THE NORTHWESTERN VASCULAR SYMPOSIUM
In Medicare population, carotid revascularization has declined
NEW YORK – The rates of carotid artery revascularization with either endarterectomy or stenting declined precipitously over a recent 15-year period, at least among Medicare fee-for-service beneficiaries, according to data presented at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation.
A reduction in carotid endarterectomies (CEA) largely accounted for the decline during 1999-2014 although there was a cumulative decline in all carotid revascularization procedures when rates of CEA and stenting were combined, according to Brajesh K. Lal, MD, professor of surgery, University of Maryland Medical System, Baltimore.
In 1999, when enthusiasm for CEA appears to have peaked, 81,306 patients received this procedure, but a steady decline was observed until 2014, when 36,325 patients were being treated annually in the Medicare database. When calculated as endarterectomies per 100,000 beneficiaries, the rate declined from 298 to 128 (57%; P less than .001) over this 15-year period.
The number of stenting procedures had not reached its peak in 1999, when 10,416 were performed. Rather, the number performed annually nearly doubled to, 22.865 by 2006. However, it then began to decline and reached 10,208 by 2014, which was slightly fewer than in 1999, according to Dr. Lal.
These trends have been observed even though outcomes are getting better, at least for CEA, according to Dr. Lal. From the same pool of data, there was a 31% (1.1% vs. 1.6%) reduction from 1999 to 2014 in mortality at 30 days following CEA. For a composite of ischemic stroke and all-cause mortality, the rate fell 29.5% (3.1% vs. 4.4%). Both reductions were called statistically significant by Dr. Lal.
The improvements in CEA outcomes were observed even though “the treated patients got sicker when looking at comorbidities and risk factors, particularly hypertension, renal insufficiency, and diabetes,” Dr. Lal said.
Outcomes also improved among patients undergoing carotid stenting in general, although the patterns were described as “more complex.” In general, there was steady improvement on outcomes during 1999-2006, but there was no further gain and some lost ground during 2006-2014. For example, ischemic stroke or death fell from 7.0% in 1999 to 4.8% in 2006, but it had climbed back to 7.0% by 2014 with no net change when the first and last year were compared.
However, with risk adjustment, there was a reduction in in-hospital mortality (1.13% vs. 2.78%) over the study period for patients undergoing carotid stenting, according to Dr. Lal, who said this reached statistical significance. Like the CEA group, there was more comorbidity among those treated with stenting at the end, relative to the early part of the study period.
In the stenting group, patients with symptomatic carotid disease rose from 14.4% in 1999 to 25.9% in 2014. This tracks with Medicare policy, which required patients after 2005 to have symptomatic disease for reimbursement, according to Dr. Lal. Prior to 2005, reimbursement was granted for patients participating in clinical trials only.
The rates of carotid revascularization are not evenly distributed geographically in the United States, according to the Medicare data. Endarterectomy in particular has been more common in the south and Midwest than on either coast. This was true in 1999 and remained so in 2014. The distribution was similar for stenting, although it was also relatively common in the southwest in the early part of the study period.
In the beginning of the study, the increased rate of stenting might have contributed to the decline in endarterectomy, but there are several other factors that are implicated in the observed trends, according to Dr. Lal. He suggested that decreasing reimbursement for the performance of these procedures, better clinical management of risk factors, and advances in medical therapy. He cited a physician survey that showed a growing preference for medical management over invasive procedures in patients with high-grade stenosis and indicated that this last factor might be a particularly important driver of the decline in revascularization referrals for asymptomatic carotid disease.
The degree to which these Medicare data are representative of overall trends in the United States is unclear, but Dr. Lal called for further work to understand the forces that these data suggest are driving the changing patterns of carotid revascularization.
NEW YORK – The rates of carotid artery revascularization with either endarterectomy or stenting declined precipitously over a recent 15-year period, at least among Medicare fee-for-service beneficiaries, according to data presented at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation.
A reduction in carotid endarterectomies (CEA) largely accounted for the decline during 1999-2014 although there was a cumulative decline in all carotid revascularization procedures when rates of CEA and stenting were combined, according to Brajesh K. Lal, MD, professor of surgery, University of Maryland Medical System, Baltimore.
In 1999, when enthusiasm for CEA appears to have peaked, 81,306 patients received this procedure, but a steady decline was observed until 2014, when 36,325 patients were being treated annually in the Medicare database. When calculated as endarterectomies per 100,000 beneficiaries, the rate declined from 298 to 128 (57%; P less than .001) over this 15-year period.
The number of stenting procedures had not reached its peak in 1999, when 10,416 were performed. Rather, the number performed annually nearly doubled to, 22.865 by 2006. However, it then began to decline and reached 10,208 by 2014, which was slightly fewer than in 1999, according to Dr. Lal.
These trends have been observed even though outcomes are getting better, at least for CEA, according to Dr. Lal. From the same pool of data, there was a 31% (1.1% vs. 1.6%) reduction from 1999 to 2014 in mortality at 30 days following CEA. For a composite of ischemic stroke and all-cause mortality, the rate fell 29.5% (3.1% vs. 4.4%). Both reductions were called statistically significant by Dr. Lal.
The improvements in CEA outcomes were observed even though “the treated patients got sicker when looking at comorbidities and risk factors, particularly hypertension, renal insufficiency, and diabetes,” Dr. Lal said.
Outcomes also improved among patients undergoing carotid stenting in general, although the patterns were described as “more complex.” In general, there was steady improvement on outcomes during 1999-2006, but there was no further gain and some lost ground during 2006-2014. For example, ischemic stroke or death fell from 7.0% in 1999 to 4.8% in 2006, but it had climbed back to 7.0% by 2014 with no net change when the first and last year were compared.
However, with risk adjustment, there was a reduction in in-hospital mortality (1.13% vs. 2.78%) over the study period for patients undergoing carotid stenting, according to Dr. Lal, who said this reached statistical significance. Like the CEA group, there was more comorbidity among those treated with stenting at the end, relative to the early part of the study period.
In the stenting group, patients with symptomatic carotid disease rose from 14.4% in 1999 to 25.9% in 2014. This tracks with Medicare policy, which required patients after 2005 to have symptomatic disease for reimbursement, according to Dr. Lal. Prior to 2005, reimbursement was granted for patients participating in clinical trials only.
The rates of carotid revascularization are not evenly distributed geographically in the United States, according to the Medicare data. Endarterectomy in particular has been more common in the south and Midwest than on either coast. This was true in 1999 and remained so in 2014. The distribution was similar for stenting, although it was also relatively common in the southwest in the early part of the study period.
In the beginning of the study, the increased rate of stenting might have contributed to the decline in endarterectomy, but there are several other factors that are implicated in the observed trends, according to Dr. Lal. He suggested that decreasing reimbursement for the performance of these procedures, better clinical management of risk factors, and advances in medical therapy. He cited a physician survey that showed a growing preference for medical management over invasive procedures in patients with high-grade stenosis and indicated that this last factor might be a particularly important driver of the decline in revascularization referrals for asymptomatic carotid disease.
The degree to which these Medicare data are representative of overall trends in the United States is unclear, but Dr. Lal called for further work to understand the forces that these data suggest are driving the changing patterns of carotid revascularization.
NEW YORK – The rates of carotid artery revascularization with either endarterectomy or stenting declined precipitously over a recent 15-year period, at least among Medicare fee-for-service beneficiaries, according to data presented at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation.
A reduction in carotid endarterectomies (CEA) largely accounted for the decline during 1999-2014 although there was a cumulative decline in all carotid revascularization procedures when rates of CEA and stenting were combined, according to Brajesh K. Lal, MD, professor of surgery, University of Maryland Medical System, Baltimore.
In 1999, when enthusiasm for CEA appears to have peaked, 81,306 patients received this procedure, but a steady decline was observed until 2014, when 36,325 patients were being treated annually in the Medicare database. When calculated as endarterectomies per 100,000 beneficiaries, the rate declined from 298 to 128 (57%; P less than .001) over this 15-year period.
The number of stenting procedures had not reached its peak in 1999, when 10,416 were performed. Rather, the number performed annually nearly doubled to, 22.865 by 2006. However, it then began to decline and reached 10,208 by 2014, which was slightly fewer than in 1999, according to Dr. Lal.
These trends have been observed even though outcomes are getting better, at least for CEA, according to Dr. Lal. From the same pool of data, there was a 31% (1.1% vs. 1.6%) reduction from 1999 to 2014 in mortality at 30 days following CEA. For a composite of ischemic stroke and all-cause mortality, the rate fell 29.5% (3.1% vs. 4.4%). Both reductions were called statistically significant by Dr. Lal.
The improvements in CEA outcomes were observed even though “the treated patients got sicker when looking at comorbidities and risk factors, particularly hypertension, renal insufficiency, and diabetes,” Dr. Lal said.
Outcomes also improved among patients undergoing carotid stenting in general, although the patterns were described as “more complex.” In general, there was steady improvement on outcomes during 1999-2006, but there was no further gain and some lost ground during 2006-2014. For example, ischemic stroke or death fell from 7.0% in 1999 to 4.8% in 2006, but it had climbed back to 7.0% by 2014 with no net change when the first and last year were compared.
However, with risk adjustment, there was a reduction in in-hospital mortality (1.13% vs. 2.78%) over the study period for patients undergoing carotid stenting, according to Dr. Lal, who said this reached statistical significance. Like the CEA group, there was more comorbidity among those treated with stenting at the end, relative to the early part of the study period.
In the stenting group, patients with symptomatic carotid disease rose from 14.4% in 1999 to 25.9% in 2014. This tracks with Medicare policy, which required patients after 2005 to have symptomatic disease for reimbursement, according to Dr. Lal. Prior to 2005, reimbursement was granted for patients participating in clinical trials only.
The rates of carotid revascularization are not evenly distributed geographically in the United States, according to the Medicare data. Endarterectomy in particular has been more common in the south and Midwest than on either coast. This was true in 1999 and remained so in 2014. The distribution was similar for stenting, although it was also relatively common in the southwest in the early part of the study period.
In the beginning of the study, the increased rate of stenting might have contributed to the decline in endarterectomy, but there are several other factors that are implicated in the observed trends, according to Dr. Lal. He suggested that decreasing reimbursement for the performance of these procedures, better clinical management of risk factors, and advances in medical therapy. He cited a physician survey that showed a growing preference for medical management over invasive procedures in patients with high-grade stenosis and indicated that this last factor might be a particularly important driver of the decline in revascularization referrals for asymptomatic carotid disease.
The degree to which these Medicare data are representative of overall trends in the United States is unclear, but Dr. Lal called for further work to understand the forces that these data suggest are driving the changing patterns of carotid revascularization.
REPORTING FROM VEITHSYMPOSIUM
Key clinical point:
Major finding: During 1999-2014, the rate of carotid endarterectomy per 100,000 beneficiaries fell from 291 to 128 (57%; P less than .001).
Study details: Retrospective database review.
Disclosures: Dr. Lal reported having no financial conflicts relevant to the study.
Redo carotid endarterectomy is more risky than previously estimated
NEW YORK – It is well known that reoperative carotid endarterectomy can be technically challenging because of the scarring left from the initial procedure, but an analysis of a large database presented at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation also revealed that the risk of complications, particularly stroke, is greater.
When “redo” carotid endarterectomies were compared with the index primary procedure collected in the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database, the odds ratio for stroke was several times greater (odds ratio, 3.71; P = .002) on univariate analysis, reported Jeffrey J. Siracuse, MD, associate professor of surgery and radiology at Boston University.
Previous single-center reports of redo endarterectomies “showed terrific results, really no perioperative stroke or morbidity, but this is older data from a different era,” said Dr. Siracuse, who undertook this study to determine whether “real-world” data would tell a different story.
In this study, 75,943 primary carotid endarterectomies and 140 redo procedures were identified in the ACS NSQIP database and compared. The redo population had a significantly higher incidence of end-stage renal disease (3.6% vs. 1.1%; P = .004), but history of stroke, whether with deficit (20.8% vs. 15.4%) or without (11.5% vs. 9.1%), was numerically higher among those undergoing a primary procedure even though these differences did not reach statistical significance. Baseline demographics and comorbidities were otherwise similar.
Presumably because of the difficulty of recanalizing scarred tissue, the mean procedure time for redos was longer than that for the primary procedures (137 vs. 49 minutes; P less than .001), but there were no significant differences in the rate of surgical site infections (0.7% vs. 0.3%; P = .482), return to the operating room (3.6% vs. 4%; P = .853), or 30-day readmissions (2.1% vs. 6.9%; P = .810) for the redo and index procedures, respectively.
Although perioperative MI rates were higher in the redo group (2.1%) than in the primary endarterectomy group (0.9%), this difference did not reach statistical significance (P = .125). However, a multivariate analysis associated redo carotid endarterectomy procedures with a nearly threefold increase in risk of a composite of major adverse cardiovascular events when compared on a multivariate analysis (OR, 2.76; P = .007), Dr. Siracuse reported.
For the surgeons considering a redo carotid endarterectomy, these data “inform a risk-benefit analysis,” according to Dr. Siracuse, but he also said that redo procedures still should be considered a viable strategy when considered in the context of other options.
Presenting a case he performed just prior to the VEITHsymposium, Dr. Siracuse displayed CT images that showed internal and common carotids with more than 75% stenosis in an 80-year-old women 7 years after a primary carotid endarterectomy. The tight stenoses and the evidence of substantial intra-arterial debris were concerns, but a decision to perform a redo endarterectomy was reached after other options, including stenting, were considered.
“She did great. She went home and has had no more symptoms,” Dr. Siracuse reported. “The point is you still have to take these [potential redo endarterectomies] on a case-by case basis.”
Dr. Siracuse reported he had no financial relationships relevant to this study.
NEW YORK – It is well known that reoperative carotid endarterectomy can be technically challenging because of the scarring left from the initial procedure, but an analysis of a large database presented at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation also revealed that the risk of complications, particularly stroke, is greater.
When “redo” carotid endarterectomies were compared with the index primary procedure collected in the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database, the odds ratio for stroke was several times greater (odds ratio, 3.71; P = .002) on univariate analysis, reported Jeffrey J. Siracuse, MD, associate professor of surgery and radiology at Boston University.
Previous single-center reports of redo endarterectomies “showed terrific results, really no perioperative stroke or morbidity, but this is older data from a different era,” said Dr. Siracuse, who undertook this study to determine whether “real-world” data would tell a different story.
In this study, 75,943 primary carotid endarterectomies and 140 redo procedures were identified in the ACS NSQIP database and compared. The redo population had a significantly higher incidence of end-stage renal disease (3.6% vs. 1.1%; P = .004), but history of stroke, whether with deficit (20.8% vs. 15.4%) or without (11.5% vs. 9.1%), was numerically higher among those undergoing a primary procedure even though these differences did not reach statistical significance. Baseline demographics and comorbidities were otherwise similar.
Presumably because of the difficulty of recanalizing scarred tissue, the mean procedure time for redos was longer than that for the primary procedures (137 vs. 49 minutes; P less than .001), but there were no significant differences in the rate of surgical site infections (0.7% vs. 0.3%; P = .482), return to the operating room (3.6% vs. 4%; P = .853), or 30-day readmissions (2.1% vs. 6.9%; P = .810) for the redo and index procedures, respectively.
Although perioperative MI rates were higher in the redo group (2.1%) than in the primary endarterectomy group (0.9%), this difference did not reach statistical significance (P = .125). However, a multivariate analysis associated redo carotid endarterectomy procedures with a nearly threefold increase in risk of a composite of major adverse cardiovascular events when compared on a multivariate analysis (OR, 2.76; P = .007), Dr. Siracuse reported.
For the surgeons considering a redo carotid endarterectomy, these data “inform a risk-benefit analysis,” according to Dr. Siracuse, but he also said that redo procedures still should be considered a viable strategy when considered in the context of other options.
Presenting a case he performed just prior to the VEITHsymposium, Dr. Siracuse displayed CT images that showed internal and common carotids with more than 75% stenosis in an 80-year-old women 7 years after a primary carotid endarterectomy. The tight stenoses and the evidence of substantial intra-arterial debris were concerns, but a decision to perform a redo endarterectomy was reached after other options, including stenting, were considered.
“She did great. She went home and has had no more symptoms,” Dr. Siracuse reported. “The point is you still have to take these [potential redo endarterectomies] on a case-by case basis.”
Dr. Siracuse reported he had no financial relationships relevant to this study.
NEW YORK – It is well known that reoperative carotid endarterectomy can be technically challenging because of the scarring left from the initial procedure, but an analysis of a large database presented at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation also revealed that the risk of complications, particularly stroke, is greater.
When “redo” carotid endarterectomies were compared with the index primary procedure collected in the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database, the odds ratio for stroke was several times greater (odds ratio, 3.71; P = .002) on univariate analysis, reported Jeffrey J. Siracuse, MD, associate professor of surgery and radiology at Boston University.
Previous single-center reports of redo endarterectomies “showed terrific results, really no perioperative stroke or morbidity, but this is older data from a different era,” said Dr. Siracuse, who undertook this study to determine whether “real-world” data would tell a different story.
In this study, 75,943 primary carotid endarterectomies and 140 redo procedures were identified in the ACS NSQIP database and compared. The redo population had a significantly higher incidence of end-stage renal disease (3.6% vs. 1.1%; P = .004), but history of stroke, whether with deficit (20.8% vs. 15.4%) or without (11.5% vs. 9.1%), was numerically higher among those undergoing a primary procedure even though these differences did not reach statistical significance. Baseline demographics and comorbidities were otherwise similar.
Presumably because of the difficulty of recanalizing scarred tissue, the mean procedure time for redos was longer than that for the primary procedures (137 vs. 49 minutes; P less than .001), but there were no significant differences in the rate of surgical site infections (0.7% vs. 0.3%; P = .482), return to the operating room (3.6% vs. 4%; P = .853), or 30-day readmissions (2.1% vs. 6.9%; P = .810) for the redo and index procedures, respectively.
Although perioperative MI rates were higher in the redo group (2.1%) than in the primary endarterectomy group (0.9%), this difference did not reach statistical significance (P = .125). However, a multivariate analysis associated redo carotid endarterectomy procedures with a nearly threefold increase in risk of a composite of major adverse cardiovascular events when compared on a multivariate analysis (OR, 2.76; P = .007), Dr. Siracuse reported.
For the surgeons considering a redo carotid endarterectomy, these data “inform a risk-benefit analysis,” according to Dr. Siracuse, but he also said that redo procedures still should be considered a viable strategy when considered in the context of other options.
Presenting a case he performed just prior to the VEITHsymposium, Dr. Siracuse displayed CT images that showed internal and common carotids with more than 75% stenosis in an 80-year-old women 7 years after a primary carotid endarterectomy. The tight stenoses and the evidence of substantial intra-arterial debris were concerns, but a decision to perform a redo endarterectomy was reached after other options, including stenting, were considered.
“She did great. She went home and has had no more symptoms,” Dr. Siracuse reported. “The point is you still have to take these [potential redo endarterectomies] on a case-by case basis.”
Dr. Siracuse reported he had no financial relationships relevant to this study.
REPORTING FROM VEITHSYMPOSIUM
Key clinical point:
Major finding: The odds ratio for stroke is 3.71 times higher (P = .002) with redo than with primary carotid endarterectomy.
Study details: Multivariate retrospective database analysis.
Disclosures: Dr. Siracuse reported he had no financial relationships relevant to this study.
Should toe amputation be delayed in diabetic patients with osteomyelitis?
Amputation: Resistance is not futile!
What’s in a toe you may ask? Why worry about saving it? Just amputate and move on ...
Not so! I implore you to resist the desire. We vascular surgeons are accustomed to cutting off toes, even feet and legs. But when it comes to diabetic feet please reconsider. Just because there is osteomyelitis, I argue that does not necessitate amputation.
We all agree that ischemic gangrene and black mummified digits are beyond salvage. That’s not what my concern is. My focus is nonhealing ulcers with underlying osteomyelitis. Whether ischemic in etiology or neuropathic (or both), give salvage a try.
Why is this so important? My opponent will try to convince you that it’s not. He’ll try to sell you on how well people walk after amputation and that functional outcomes are great. But think beyond that for a second.
Amputation changes the foot architecture and weight distribution. In a person with neuropathy, this only predisposes them to more ulcers. More ulcers will mean more infection, which will lead to more amputations. This finally culminates in a major amputation.
In one reported study,1 researchers followed more than 200,000 diabetics from 2010 until 2013. While the risk of amputation overall was relatively small (0.36% for major and 0.56% for minor amputations), prior minor amputation increased the risk of major amputation 10-fold and increased the risk of another minor (below-ankle) amputation 20-fold. Of those who had a major amputation, 57% died over the 3 years. This is not insignificant.
This does not also consider the morbidity and impact on lifestyle and quality of life for these patients. Many may not walk. Some will be relegated to nursing homes. Some will suffer from phantom limb pain. Many may never return to work. Even more will have difficulty with their daily lives, not to mention the psychological recovery also required.
The foot seems to be the only place where amputation as first-line therapy for osteomyelitis is accepted. We don’t do a hip disarticulation for ischial pressure sores with osteomyelitis. Calvarial osteomyelitis is also treated with antibiotics. I implore you: Don’t treat toes like vestigial organs.
Granted, there are subsets of patients who would benefit from amputations. A patient with painful Charcot foot may elect to have a below-knee amputation and move on with life. Another who has lost jobs or significant time due to recurrence of osteomyelitis may progress. A patient with severe sepsis and infection into a joint may need amputation.
But what other treatment options are there? I’m glad you inquired.
I primarily treat diabetic feet by treating the soft tissue envelope. Even if a patient presents with midfoot infection or necrotizing soft tissue infection, I treat it like a good old-fashioned abscess or necrotizing fasciitis:
1) Drain pus
2) Resect the dead stuff
3) Supportive care (antibiotics, fluids, aggressive wound care, etc.)
I try to leave the bones intact. When bone is exposed I take biopsies for culture and pathology. Any bone destroyed by the infection is focally debrided. I also take a specimen of the “bone margin” that I’m leaving behind and I send this to pathology looking for residual acute osteomyelitis. These steps are important as they dictate duration and choice of antibiotic therapy. This is in keeping with the consensus recommendations published in 2016.2
Even chronic wounds get a similar approach. If there is granulation, let it granulate and see if it will fill the wound. “Just because osteomyelitis is there, it doesn’t mean that for the toe we won’t care!”
There are exceptions of course. If the soft tissue is severely affected so the phalynx protrudes like something from the movie “Coco,” probably that should be amputated. Repeat offenders also may progress to amputation. But otherwise, hold off and give it a chance.
For the inpatient, aggressive irrigation of the wounds using the Veraflo system promotes granulation, even for short hospital stays of 1 week or less. Any ischemic component is worked up and addressed with percutaneous or open revascularization. We treat with prolonged antibiotics, and in questionable cases err on the side of giving long-term courses. These wounds need to be offloaded for tasks of daily living (going to the bathroom, making a sandwich, etc.) but otherwise we instruct patients to be effectively non–weight-bearing on that limb.
We also refer patients for hyperbaric therapy frequently. Now if you’re done groaning, I assure you this is not phony medicine. There is growing evidence to support not only improved rates of healing, but also significant cost savings and improved quality of life.3
In young patients or those with large defects, we also involve plastic and reconstructive surgery for secondary closure approaches (free flaps, adjacent tissue transfers, local autogenous or prosthetic grafting [Integra, Stravix, Dermacell, etc.] or other advanced techniques). This is particularly important in plantar wounds that will need to bear weight in the future, or in young patients for improved functional and cosmetic outcomes. For smaller wounds, we often use dermal/subdermal graft substitutes ourselves.
Even still, in nonambulatory or chronically debilitated and medically high-risk patients, maybe a different option is palliative wound care with or without antibiotics. A nonoperative approach to allow individuals to live the rest of their remaining days without undergoing a morbid and disfiguring amputation is not unreasonable. Many families are thankful for this option when given it. In the absence of refractory pain or overwhelming sepsis, we just let the wound do what it will do, understanding that someday the plan may change. This allows patients to continue to treat the wound without escalation to surgery or resorting to amputation.
In the end, just like we vascular surgeons tailor our “holistic” approach to the needs and desires of a single particular patient, we should approach wounds with a similar attitude. The presence of osteomyelitis in and of itself should not prompt one to bypass an entire algorithm, go straight to amputation, do not pass “Go” or “collect $200” (although the professional fee for a toe amputation is probably around $200). With a multidisciplinary and multimodal approach, and vested patients, salvage is possible in the majority of cases.
References
1. Diabetologia. 2018 Mar;61(3):626-35.
2. Diabet Foot Ankle. 2016 Jul 12. doi: 10.3402/dfa.v7.30079.
3. Int J Technol Assess Health Care. 2008 Spring;24(2):178-83.
Dr. Issam Koleilat is assistant professor and associate program director, Vascular Surgery Residency and Fellowship, Division of Vascular Surgery, Albert Einstein College of Medicine/Montefiore Medical Center, New York. He had no relevant disclosures.
Amputation: Often the best option
For many years there has been debate about the best management strategy for diabetic foot infection including osteomyelitis. The principles of appropriate antibiotics, surgical debridement, good wound care, and proper offloading will always remain. There are no randomized controlled trials of medical vs. surgical management of diabetic foot ulceration with osteomyelitis.
We now have a number of widely accepted ways to define wounds including Wagner and the SVS-adopted WIFI score. Historical papers are somewhat plagued by heterogeneity in the wounds included. This is even more apparent with any attempted meta-analyses. I think everyone would agree that the superficial toe wound with minimal cellulitis is best managed medically. The issue at hand is the profoundly neuropathic diabetic often with underlying anatomic abnormality and osteomyelitis. My esteemed colleague would suggest that we are too quick to pull a trigger and amputate a toe with underlying osteomyelitis.
I think the initial item for debate is the technique of diagnosis of osteomyelitis. We have multiple ways this is reported. Plain x-ray, bone scan, MRI, and “clinical osteomyelitis” are among the alternative ways osteomyelitis is diagnosed. The reliability of the last is the most variable because clinical osteomyelitis ranges from “probes close to bone” to exposed bone visible protruding from the wound bed. Given the variability of diagnostic techniques, the literature is an amalgam of clinical scenarios and difficult to navigate in a way to affect treatment decisions.
In addition, the medical treatment for osteomyelitis is highly variable. This commonly involves tunneled catheter insertion and 6 weeks to 3 months of IV antibiotics. In some institutions antibiotics are tailored to “wound culture.” Several of our infectious disease specialists prefer bone culture and pathology of bone demonstrating an acute destructive process. Obviously, this often requires surgical debridement to obtain a specimen. Antibiotic duration recommendations may vary from 1 week (if all infected bone is resected) to 90 days if a standalone antibiotic management is selected. Chronic osteomyelitis has a reinfection rate of up to 30%.1
Medical management is not without risk. These risks include recurring infection with resistant organisms, wound deterioration, gastrointestinal complications (Clostridium difficile), catheter-related complications, and acute kidney injury. A recent paper found over 30% of patients treated medically for osteomyelitis developed acute kidney injury. These patients had more frequent hospitalization, recurring ulceration, and infection.2 We have all experienced the patient with multiple hospitalizations and episodic AKI that culminates in ESRD requiring hemodialysis.
If the argument is with good follow-up these patients will ultimately experience preservation of the toe, I would take the stance that in our patient population of diabetics presenting with foot ulcer and osteomyelitis the average hemoglobin A1c is over 9. Although this is not only related to patient compliance, in many instances this is a large piece of the puzzle. It is hard to infer that suddenly with biopsy-proven osteomyelitis the patient will become compliant with medical management of the disease process. Certainly, in some circumstances, this is the case. There are a number of studies with a wide range of findings on HbA1c as it relates to predictive value of wound healing.
There are various studies comparing surgical to medical management for osteomyelitis. Limb salvage is contingent upon location (forefoot, midfoot, hindfoot), the extent of infection, and patient comorbidities. The conclusion of the majority of these studies is that a standalone antibiotic treatment algorithm results in greater limb loss. Patients with peripheral occlusive disease and preadmission antibiotic use have been shown to have decreased wound healing. Minor amputation has been shown to be protective from mortality, risk of major amputation, and unfavorable discharge in patients admitted with a diagnosis of osteomyelitis.3 The major limb amputation rate for antibiotics alone is 20%-30% according to two trials with duration of antibiotics of 3 months.4,5 The available randomized trials tend to exclude patients with severe infection (poorly defined), those with PAD, or those with severe comorbid conditions.
Cost of treatment is even more poorly delineated. Obviously, surgical treatment is not without cost to the health care system. Toe amputation especially when including the metatarsal head shifts pressure points and in the neuropathic patient may lead to recurrent ulceration. The average outpatient cost per patient per ulcer is often over $30,000. The goal of surgical treatment can be defined as trying to maintain the greatest degree of function with the least risk. Removing infected bone (i.e., minor amputation) limits exposure to prolonged antibiotic treatment and hopefully lessens recurring ulceration and hospitalization. This is only one piece of the puzzle, however. A multidisciplinary approach with endocrinology, infectious disease, and orthotics for offloading are keys to decrease future ulceration.
Although I do not advocate for widespread toe carnage as suggested by Dr. Koleilat, I do think liberal application of minor amputation to limit hospital stay, limit antibiotic duration and its inherent risk, and possibly affect readmission is often in the best interest of the patient and the system as a whole. Obviously, based on the variable reports in the literature there cannot be a single approach to these patients and the treatment must be individualized based on extent of infection, compliance of the patient, access to multidisciplinary care, and comorbid conditions.
References
1. World J Diabetes. 2017 Apr 15;8(4):135-42.
2. Diabetes Res Clin Pract. 2018 Jan;135:58-64.
3. Ann Surg. 2005;241(6):885-94.
4. Am J Med. 1987 Oct;83(4):653-60.
5. Am J Med.1989 Jun;86(6 Pt 2):801-8.
Dr. Mark P. Androes is division chief, vascular surgery, Greenville (S.C.) Health System. He had no relevant disclosures.
Amputation: Resistance is not futile!
What’s in a toe you may ask? Why worry about saving it? Just amputate and move on ...
Not so! I implore you to resist the desire. We vascular surgeons are accustomed to cutting off toes, even feet and legs. But when it comes to diabetic feet please reconsider. Just because there is osteomyelitis, I argue that does not necessitate amputation.
We all agree that ischemic gangrene and black mummified digits are beyond salvage. That’s not what my concern is. My focus is nonhealing ulcers with underlying osteomyelitis. Whether ischemic in etiology or neuropathic (or both), give salvage a try.
Why is this so important? My opponent will try to convince you that it’s not. He’ll try to sell you on how well people walk after amputation and that functional outcomes are great. But think beyond that for a second.
Amputation changes the foot architecture and weight distribution. In a person with neuropathy, this only predisposes them to more ulcers. More ulcers will mean more infection, which will lead to more amputations. This finally culminates in a major amputation.
In one reported study,1 researchers followed more than 200,000 diabetics from 2010 until 2013. While the risk of amputation overall was relatively small (0.36% for major and 0.56% for minor amputations), prior minor amputation increased the risk of major amputation 10-fold and increased the risk of another minor (below-ankle) amputation 20-fold. Of those who had a major amputation, 57% died over the 3 years. This is not insignificant.
This does not also consider the morbidity and impact on lifestyle and quality of life for these patients. Many may not walk. Some will be relegated to nursing homes. Some will suffer from phantom limb pain. Many may never return to work. Even more will have difficulty with their daily lives, not to mention the psychological recovery also required.
The foot seems to be the only place where amputation as first-line therapy for osteomyelitis is accepted. We don’t do a hip disarticulation for ischial pressure sores with osteomyelitis. Calvarial osteomyelitis is also treated with antibiotics. I implore you: Don’t treat toes like vestigial organs.
Granted, there are subsets of patients who would benefit from amputations. A patient with painful Charcot foot may elect to have a below-knee amputation and move on with life. Another who has lost jobs or significant time due to recurrence of osteomyelitis may progress. A patient with severe sepsis and infection into a joint may need amputation.
But what other treatment options are there? I’m glad you inquired.
I primarily treat diabetic feet by treating the soft tissue envelope. Even if a patient presents with midfoot infection or necrotizing soft tissue infection, I treat it like a good old-fashioned abscess or necrotizing fasciitis:
1) Drain pus
2) Resect the dead stuff
3) Supportive care (antibiotics, fluids, aggressive wound care, etc.)
I try to leave the bones intact. When bone is exposed I take biopsies for culture and pathology. Any bone destroyed by the infection is focally debrided. I also take a specimen of the “bone margin” that I’m leaving behind and I send this to pathology looking for residual acute osteomyelitis. These steps are important as they dictate duration and choice of antibiotic therapy. This is in keeping with the consensus recommendations published in 2016.2
Even chronic wounds get a similar approach. If there is granulation, let it granulate and see if it will fill the wound. “Just because osteomyelitis is there, it doesn’t mean that for the toe we won’t care!”
There are exceptions of course. If the soft tissue is severely affected so the phalynx protrudes like something from the movie “Coco,” probably that should be amputated. Repeat offenders also may progress to amputation. But otherwise, hold off and give it a chance.
For the inpatient, aggressive irrigation of the wounds using the Veraflo system promotes granulation, even for short hospital stays of 1 week or less. Any ischemic component is worked up and addressed with percutaneous or open revascularization. We treat with prolonged antibiotics, and in questionable cases err on the side of giving long-term courses. These wounds need to be offloaded for tasks of daily living (going to the bathroom, making a sandwich, etc.) but otherwise we instruct patients to be effectively non–weight-bearing on that limb.
We also refer patients for hyperbaric therapy frequently. Now if you’re done groaning, I assure you this is not phony medicine. There is growing evidence to support not only improved rates of healing, but also significant cost savings and improved quality of life.3
In young patients or those with large defects, we also involve plastic and reconstructive surgery for secondary closure approaches (free flaps, adjacent tissue transfers, local autogenous or prosthetic grafting [Integra, Stravix, Dermacell, etc.] or other advanced techniques). This is particularly important in plantar wounds that will need to bear weight in the future, or in young patients for improved functional and cosmetic outcomes. For smaller wounds, we often use dermal/subdermal graft substitutes ourselves.
Even still, in nonambulatory or chronically debilitated and medically high-risk patients, maybe a different option is palliative wound care with or without antibiotics. A nonoperative approach to allow individuals to live the rest of their remaining days without undergoing a morbid and disfiguring amputation is not unreasonable. Many families are thankful for this option when given it. In the absence of refractory pain or overwhelming sepsis, we just let the wound do what it will do, understanding that someday the plan may change. This allows patients to continue to treat the wound without escalation to surgery or resorting to amputation.
In the end, just like we vascular surgeons tailor our “holistic” approach to the needs and desires of a single particular patient, we should approach wounds with a similar attitude. The presence of osteomyelitis in and of itself should not prompt one to bypass an entire algorithm, go straight to amputation, do not pass “Go” or “collect $200” (although the professional fee for a toe amputation is probably around $200). With a multidisciplinary and multimodal approach, and vested patients, salvage is possible in the majority of cases.
References
1. Diabetologia. 2018 Mar;61(3):626-35.
2. Diabet Foot Ankle. 2016 Jul 12. doi: 10.3402/dfa.v7.30079.
3. Int J Technol Assess Health Care. 2008 Spring;24(2):178-83.
Dr. Issam Koleilat is assistant professor and associate program director, Vascular Surgery Residency and Fellowship, Division of Vascular Surgery, Albert Einstein College of Medicine/Montefiore Medical Center, New York. He had no relevant disclosures.
Amputation: Often the best option
For many years there has been debate about the best management strategy for diabetic foot infection including osteomyelitis. The principles of appropriate antibiotics, surgical debridement, good wound care, and proper offloading will always remain. There are no randomized controlled trials of medical vs. surgical management of diabetic foot ulceration with osteomyelitis.
We now have a number of widely accepted ways to define wounds including Wagner and the SVS-adopted WIFI score. Historical papers are somewhat plagued by heterogeneity in the wounds included. This is even more apparent with any attempted meta-analyses. I think everyone would agree that the superficial toe wound with minimal cellulitis is best managed medically. The issue at hand is the profoundly neuropathic diabetic often with underlying anatomic abnormality and osteomyelitis. My esteemed colleague would suggest that we are too quick to pull a trigger and amputate a toe with underlying osteomyelitis.
I think the initial item for debate is the technique of diagnosis of osteomyelitis. We have multiple ways this is reported. Plain x-ray, bone scan, MRI, and “clinical osteomyelitis” are among the alternative ways osteomyelitis is diagnosed. The reliability of the last is the most variable because clinical osteomyelitis ranges from “probes close to bone” to exposed bone visible protruding from the wound bed. Given the variability of diagnostic techniques, the literature is an amalgam of clinical scenarios and difficult to navigate in a way to affect treatment decisions.
In addition, the medical treatment for osteomyelitis is highly variable. This commonly involves tunneled catheter insertion and 6 weeks to 3 months of IV antibiotics. In some institutions antibiotics are tailored to “wound culture.” Several of our infectious disease specialists prefer bone culture and pathology of bone demonstrating an acute destructive process. Obviously, this often requires surgical debridement to obtain a specimen. Antibiotic duration recommendations may vary from 1 week (if all infected bone is resected) to 90 days if a standalone antibiotic management is selected. Chronic osteomyelitis has a reinfection rate of up to 30%.1
Medical management is not without risk. These risks include recurring infection with resistant organisms, wound deterioration, gastrointestinal complications (Clostridium difficile), catheter-related complications, and acute kidney injury. A recent paper found over 30% of patients treated medically for osteomyelitis developed acute kidney injury. These patients had more frequent hospitalization, recurring ulceration, and infection.2 We have all experienced the patient with multiple hospitalizations and episodic AKI that culminates in ESRD requiring hemodialysis.
If the argument is with good follow-up these patients will ultimately experience preservation of the toe, I would take the stance that in our patient population of diabetics presenting with foot ulcer and osteomyelitis the average hemoglobin A1c is over 9. Although this is not only related to patient compliance, in many instances this is a large piece of the puzzle. It is hard to infer that suddenly with biopsy-proven osteomyelitis the patient will become compliant with medical management of the disease process. Certainly, in some circumstances, this is the case. There are a number of studies with a wide range of findings on HbA1c as it relates to predictive value of wound healing.
There are various studies comparing surgical to medical management for osteomyelitis. Limb salvage is contingent upon location (forefoot, midfoot, hindfoot), the extent of infection, and patient comorbidities. The conclusion of the majority of these studies is that a standalone antibiotic treatment algorithm results in greater limb loss. Patients with peripheral occlusive disease and preadmission antibiotic use have been shown to have decreased wound healing. Minor amputation has been shown to be protective from mortality, risk of major amputation, and unfavorable discharge in patients admitted with a diagnosis of osteomyelitis.3 The major limb amputation rate for antibiotics alone is 20%-30% according to two trials with duration of antibiotics of 3 months.4,5 The available randomized trials tend to exclude patients with severe infection (poorly defined), those with PAD, or those with severe comorbid conditions.
Cost of treatment is even more poorly delineated. Obviously, surgical treatment is not without cost to the health care system. Toe amputation especially when including the metatarsal head shifts pressure points and in the neuropathic patient may lead to recurrent ulceration. The average outpatient cost per patient per ulcer is often over $30,000. The goal of surgical treatment can be defined as trying to maintain the greatest degree of function with the least risk. Removing infected bone (i.e., minor amputation) limits exposure to prolonged antibiotic treatment and hopefully lessens recurring ulceration and hospitalization. This is only one piece of the puzzle, however. A multidisciplinary approach with endocrinology, infectious disease, and orthotics for offloading are keys to decrease future ulceration.
Although I do not advocate for widespread toe carnage as suggested by Dr. Koleilat, I do think liberal application of minor amputation to limit hospital stay, limit antibiotic duration and its inherent risk, and possibly affect readmission is often in the best interest of the patient and the system as a whole. Obviously, based on the variable reports in the literature there cannot be a single approach to these patients and the treatment must be individualized based on extent of infection, compliance of the patient, access to multidisciplinary care, and comorbid conditions.
References
1. World J Diabetes. 2017 Apr 15;8(4):135-42.
2. Diabetes Res Clin Pract. 2018 Jan;135:58-64.
3. Ann Surg. 2005;241(6):885-94.
4. Am J Med. 1987 Oct;83(4):653-60.
5. Am J Med.1989 Jun;86(6 Pt 2):801-8.
Dr. Mark P. Androes is division chief, vascular surgery, Greenville (S.C.) Health System. He had no relevant disclosures.
Amputation: Resistance is not futile!
What’s in a toe you may ask? Why worry about saving it? Just amputate and move on ...
Not so! I implore you to resist the desire. We vascular surgeons are accustomed to cutting off toes, even feet and legs. But when it comes to diabetic feet please reconsider. Just because there is osteomyelitis, I argue that does not necessitate amputation.
We all agree that ischemic gangrene and black mummified digits are beyond salvage. That’s not what my concern is. My focus is nonhealing ulcers with underlying osteomyelitis. Whether ischemic in etiology or neuropathic (or both), give salvage a try.
Why is this so important? My opponent will try to convince you that it’s not. He’ll try to sell you on how well people walk after amputation and that functional outcomes are great. But think beyond that for a second.
Amputation changes the foot architecture and weight distribution. In a person with neuropathy, this only predisposes them to more ulcers. More ulcers will mean more infection, which will lead to more amputations. This finally culminates in a major amputation.
In one reported study,1 researchers followed more than 200,000 diabetics from 2010 until 2013. While the risk of amputation overall was relatively small (0.36% for major and 0.56% for minor amputations), prior minor amputation increased the risk of major amputation 10-fold and increased the risk of another minor (below-ankle) amputation 20-fold. Of those who had a major amputation, 57% died over the 3 years. This is not insignificant.
This does not also consider the morbidity and impact on lifestyle and quality of life for these patients. Many may not walk. Some will be relegated to nursing homes. Some will suffer from phantom limb pain. Many may never return to work. Even more will have difficulty with their daily lives, not to mention the psychological recovery also required.
The foot seems to be the only place where amputation as first-line therapy for osteomyelitis is accepted. We don’t do a hip disarticulation for ischial pressure sores with osteomyelitis. Calvarial osteomyelitis is also treated with antibiotics. I implore you: Don’t treat toes like vestigial organs.
Granted, there are subsets of patients who would benefit from amputations. A patient with painful Charcot foot may elect to have a below-knee amputation and move on with life. Another who has lost jobs or significant time due to recurrence of osteomyelitis may progress. A patient with severe sepsis and infection into a joint may need amputation.
But what other treatment options are there? I’m glad you inquired.
I primarily treat diabetic feet by treating the soft tissue envelope. Even if a patient presents with midfoot infection or necrotizing soft tissue infection, I treat it like a good old-fashioned abscess or necrotizing fasciitis:
1) Drain pus
2) Resect the dead stuff
3) Supportive care (antibiotics, fluids, aggressive wound care, etc.)
I try to leave the bones intact. When bone is exposed I take biopsies for culture and pathology. Any bone destroyed by the infection is focally debrided. I also take a specimen of the “bone margin” that I’m leaving behind and I send this to pathology looking for residual acute osteomyelitis. These steps are important as they dictate duration and choice of antibiotic therapy. This is in keeping with the consensus recommendations published in 2016.2
Even chronic wounds get a similar approach. If there is granulation, let it granulate and see if it will fill the wound. “Just because osteomyelitis is there, it doesn’t mean that for the toe we won’t care!”
There are exceptions of course. If the soft tissue is severely affected so the phalynx protrudes like something from the movie “Coco,” probably that should be amputated. Repeat offenders also may progress to amputation. But otherwise, hold off and give it a chance.
For the inpatient, aggressive irrigation of the wounds using the Veraflo system promotes granulation, even for short hospital stays of 1 week or less. Any ischemic component is worked up and addressed with percutaneous or open revascularization. We treat with prolonged antibiotics, and in questionable cases err on the side of giving long-term courses. These wounds need to be offloaded for tasks of daily living (going to the bathroom, making a sandwich, etc.) but otherwise we instruct patients to be effectively non–weight-bearing on that limb.
We also refer patients for hyperbaric therapy frequently. Now if you’re done groaning, I assure you this is not phony medicine. There is growing evidence to support not only improved rates of healing, but also significant cost savings and improved quality of life.3
In young patients or those with large defects, we also involve plastic and reconstructive surgery for secondary closure approaches (free flaps, adjacent tissue transfers, local autogenous or prosthetic grafting [Integra, Stravix, Dermacell, etc.] or other advanced techniques). This is particularly important in plantar wounds that will need to bear weight in the future, or in young patients for improved functional and cosmetic outcomes. For smaller wounds, we often use dermal/subdermal graft substitutes ourselves.
Even still, in nonambulatory or chronically debilitated and medically high-risk patients, maybe a different option is palliative wound care with or without antibiotics. A nonoperative approach to allow individuals to live the rest of their remaining days without undergoing a morbid and disfiguring amputation is not unreasonable. Many families are thankful for this option when given it. In the absence of refractory pain or overwhelming sepsis, we just let the wound do what it will do, understanding that someday the plan may change. This allows patients to continue to treat the wound without escalation to surgery or resorting to amputation.
In the end, just like we vascular surgeons tailor our “holistic” approach to the needs and desires of a single particular patient, we should approach wounds with a similar attitude. The presence of osteomyelitis in and of itself should not prompt one to bypass an entire algorithm, go straight to amputation, do not pass “Go” or “collect $200” (although the professional fee for a toe amputation is probably around $200). With a multidisciplinary and multimodal approach, and vested patients, salvage is possible in the majority of cases.
References
1. Diabetologia. 2018 Mar;61(3):626-35.
2. Diabet Foot Ankle. 2016 Jul 12. doi: 10.3402/dfa.v7.30079.
3. Int J Technol Assess Health Care. 2008 Spring;24(2):178-83.
Dr. Issam Koleilat is assistant professor and associate program director, Vascular Surgery Residency and Fellowship, Division of Vascular Surgery, Albert Einstein College of Medicine/Montefiore Medical Center, New York. He had no relevant disclosures.
Amputation: Often the best option
For many years there has been debate about the best management strategy for diabetic foot infection including osteomyelitis. The principles of appropriate antibiotics, surgical debridement, good wound care, and proper offloading will always remain. There are no randomized controlled trials of medical vs. surgical management of diabetic foot ulceration with osteomyelitis.
We now have a number of widely accepted ways to define wounds including Wagner and the SVS-adopted WIFI score. Historical papers are somewhat plagued by heterogeneity in the wounds included. This is even more apparent with any attempted meta-analyses. I think everyone would agree that the superficial toe wound with minimal cellulitis is best managed medically. The issue at hand is the profoundly neuropathic diabetic often with underlying anatomic abnormality and osteomyelitis. My esteemed colleague would suggest that we are too quick to pull a trigger and amputate a toe with underlying osteomyelitis.
I think the initial item for debate is the technique of diagnosis of osteomyelitis. We have multiple ways this is reported. Plain x-ray, bone scan, MRI, and “clinical osteomyelitis” are among the alternative ways osteomyelitis is diagnosed. The reliability of the last is the most variable because clinical osteomyelitis ranges from “probes close to bone” to exposed bone visible protruding from the wound bed. Given the variability of diagnostic techniques, the literature is an amalgam of clinical scenarios and difficult to navigate in a way to affect treatment decisions.
In addition, the medical treatment for osteomyelitis is highly variable. This commonly involves tunneled catheter insertion and 6 weeks to 3 months of IV antibiotics. In some institutions antibiotics are tailored to “wound culture.” Several of our infectious disease specialists prefer bone culture and pathology of bone demonstrating an acute destructive process. Obviously, this often requires surgical debridement to obtain a specimen. Antibiotic duration recommendations may vary from 1 week (if all infected bone is resected) to 90 days if a standalone antibiotic management is selected. Chronic osteomyelitis has a reinfection rate of up to 30%.1
Medical management is not without risk. These risks include recurring infection with resistant organisms, wound deterioration, gastrointestinal complications (Clostridium difficile), catheter-related complications, and acute kidney injury. A recent paper found over 30% of patients treated medically for osteomyelitis developed acute kidney injury. These patients had more frequent hospitalization, recurring ulceration, and infection.2 We have all experienced the patient with multiple hospitalizations and episodic AKI that culminates in ESRD requiring hemodialysis.
If the argument is with good follow-up these patients will ultimately experience preservation of the toe, I would take the stance that in our patient population of diabetics presenting with foot ulcer and osteomyelitis the average hemoglobin A1c is over 9. Although this is not only related to patient compliance, in many instances this is a large piece of the puzzle. It is hard to infer that suddenly with biopsy-proven osteomyelitis the patient will become compliant with medical management of the disease process. Certainly, in some circumstances, this is the case. There are a number of studies with a wide range of findings on HbA1c as it relates to predictive value of wound healing.
There are various studies comparing surgical to medical management for osteomyelitis. Limb salvage is contingent upon location (forefoot, midfoot, hindfoot), the extent of infection, and patient comorbidities. The conclusion of the majority of these studies is that a standalone antibiotic treatment algorithm results in greater limb loss. Patients with peripheral occlusive disease and preadmission antibiotic use have been shown to have decreased wound healing. Minor amputation has been shown to be protective from mortality, risk of major amputation, and unfavorable discharge in patients admitted with a diagnosis of osteomyelitis.3 The major limb amputation rate for antibiotics alone is 20%-30% according to two trials with duration of antibiotics of 3 months.4,5 The available randomized trials tend to exclude patients with severe infection (poorly defined), those with PAD, or those with severe comorbid conditions.
Cost of treatment is even more poorly delineated. Obviously, surgical treatment is not without cost to the health care system. Toe amputation especially when including the metatarsal head shifts pressure points and in the neuropathic patient may lead to recurrent ulceration. The average outpatient cost per patient per ulcer is often over $30,000. The goal of surgical treatment can be defined as trying to maintain the greatest degree of function with the least risk. Removing infected bone (i.e., minor amputation) limits exposure to prolonged antibiotic treatment and hopefully lessens recurring ulceration and hospitalization. This is only one piece of the puzzle, however. A multidisciplinary approach with endocrinology, infectious disease, and orthotics for offloading are keys to decrease future ulceration.
Although I do not advocate for widespread toe carnage as suggested by Dr. Koleilat, I do think liberal application of minor amputation to limit hospital stay, limit antibiotic duration and its inherent risk, and possibly affect readmission is often in the best interest of the patient and the system as a whole. Obviously, based on the variable reports in the literature there cannot be a single approach to these patients and the treatment must be individualized based on extent of infection, compliance of the patient, access to multidisciplinary care, and comorbid conditions.
References
1. World J Diabetes. 2017 Apr 15;8(4):135-42.
2. Diabetes Res Clin Pract. 2018 Jan;135:58-64.
3. Ann Surg. 2005;241(6):885-94.
4. Am J Med. 1987 Oct;83(4):653-60.
5. Am J Med.1989 Jun;86(6 Pt 2):801-8.
Dr. Mark P. Androes is division chief, vascular surgery, Greenville (S.C.) Health System. He had no relevant disclosures.