User login
Aortic stenosis: Who should undergo surgery, transcatheter valve replacement?
For some patients with aortic stenosis, the choice of management is simple; for others it is less so. Patients who have severe, symptomatic stenosis and who have low surgical risk should undergo aortic valve replacement. But if the stenosis is severe but asymptomatic, or if the patient is at higher surgical risk, or if there seems to be a mismatch in the hemodynamic variables, the situation is more complicated.
Fortunately, we have evidence and guidelines to go on. In this paper we review the indications for surgical and transcatheter aortic valve replacement, focusing on the areas of less certainty.
AN INDOLENT DISEASE, UNTIL IT ISN’T
Aortic stenosis is the most common valvular disease and the third most prevalent form of cardiovascular disease in the Western world, after hypertension and coronary artery disease. It is largely a disease of the elderly; its prevalence increases with age, and it is present in 2% to 7% of patients over age 65.1,2
At first, its course is indolent, as it progresses slowly over years to decades. However, this is followed by rapid clinical deterioration and a high death rate after symptoms develop.
SURGICAL AORTIC VALVE REPLACEMENT FOR SEVERE SYMPTOMATIC STENOSIS
Classic symptoms of aortic stenosis include angina, heart failure, and syncope. Once symptoms appear, patients with severe aortic stenosis should be promptly referred for surgical aortic valve replacement, as survival is poor unless outflow obstruction is relieved (Figure 1). The onset of symptoms confers a poor prognosis: patients die within an average of 5 years after the onset of angina, 3 years after the onset of syncope, and 2 years after the onset of heart failure symptoms. The overall mortality rate is 75% at 3 years without surgery.3,4 Furthermore, 8% to 34% of patients with symptoms die suddenly.
Advances in prosthetic-valve design, cardiopulmonary bypass, surgical technique, and anesthesia have steadily improved the outcomes of aortic valve surgery. An analysis of the Society of Thoracic Surgeons (STS) database in 2006 showed that during the previous decade the death rate during isolated aortic valve replacement decreased from 3.4% to 2.6%. For patients under age 70 at the time of surgery, the rate of death was 1.3%, and in those ages 80 to 85, the 30-day mortality rate was less than 5%.5
Patients who survive surgery enjoy a near-normal life expectancy: 99% survive at least 5 years, 85% at least 10 years, and 82% at least 15 years.6,7 Nearly all have improvement in their ejection fraction and heart failure symptoms, and those who had more advanced symptoms before surgery enjoy the most benefit afterward.8,9
Recommendation. Surgical valve replacement for symptomatic severe aortic stenosis receives a class I recommendation, level of evidence B, in the current guidelines from the American College of Cardiology (ACC) and the American Heart Association (AHA).10,11 (See Table 1 for an explanation of the classes of recommendations and levels of evidence.)
TWO RISK-ASSESSMENT SCORES
There are two widely used scores for assessing the risk of aortic valve replacement: the European System for Cardiac Operative Risk Evaluation (EuroSCORE) and the STS score. Each has limitations.
The EuroSCORE was developed to predict the risk of dying in the hospital after adult cardiac surgery. It has been shown to predict the short-term and the long-term risk of death after heart valve surgery.12 Unfortunately, it overestimates the dangers of isolated aortic valve replacement in the patients at highest risk.13,14
The STS score, a logistic model, reflects more closely the operative and 30-day mortality rates for the patients at highest risk undergoing surgical aortic valve replacement.15,16 It was used to assess patients for surgical or transcatheter aortic valve replacement in the Placement of Aortic Transcatheter Valves (PARTNER) trial.17
These risk scores, though not perfect, are helpful as part of an overall estimation of risk that includes functional status, cardiac function, and comorbidities.
OTHER INDICATIONS FOR SURGICAL AORTIC VALVE REPLACEMENT
For patients with severe but asymptomatic aortic stenosis, surgical referral is standard practice in several circumstances.
Asymptomatic severe aortic stenosis with a low ejection fraction
Early studies found significant differences in survival beginning as early as 3 years after valve replacement between those whose preoperative ejection fraction was greater than 50% and those with a lower ejection fraction.4 Delaying surgery in these patients may lead to irreversible left ventricular dysfunction and worse survival.
Recommendation. The AHA and the ACC recommend surgical aortic valve replacement for patients who have no symptoms and whose left ventricular ejection fraction is less than 50% (class I indication, level of evidence C).10,11
Asymptomatic severe aortic stenosis in patients undergoing other cardiac surgery
Recommendation. Even if it is causing no symptoms, a severely stenotic aortic valve ought to be replaced if the ejection fraction is greater than 50% and the patient is undergoing another type of heart surgery, such as coronary artery bypass grafting, aortic surgery, or surgery on other heart valves (class I indication, level of evidence B).10,11
Asymptomatic moderate aortic stenosis in patients undergoing other cardiac surgery
When patients with a mildly or moderately stenotic aortic valve undergo other types of cardiac surgery, the decision to replace the valve is more difficult. Clinicians have to consider the increase in risk caused by adding aortic valve replacement to the planned surgery compared with the future likelihood of aortic stenosis progressing to a severe symptomatic state and eventually requiring a second cardiac surgery.
We have no evidence from a large prospective randomized controlled trial regarding prophylactic valve replacement at the time of coronary bypass surgery. However, a review of outcomes from the STS database between 1995 and 2000 found that patients under age 70 with a peak aortic gradient greater than “about 28 mm Hg” (correlating with a moderate degree of stenosis) benefited from prophylactic valve replacement at the time of coronary artery bypass surgery.18
These conclusions were supported by a subsequent retrospective analysis that found a significant survival advantage at 8 years in favor of prophylactic valve replacement at the time of bypass surgery for those with moderate (but not mild) aortic stenosis.19
Recommendation. The AHA and ACC give a class IIb endorsement, level of evidence B, for aortic valve replacement in patients with asymptomatic moderate aortic stenosis undergoing coronary bypass, valve, or aortic surgery.10,11
SEVERE ASYMPTOMATIC STENOSIS: WHICH TESTS HELP IN DECIDING?
A patient without symptoms presents a greater challenge than one with symptoms.
If surgery is deferred, the prognosis is usually excellent in such patients. Pellikka et al20 found that patients with severe asymptomatic aortic stenosis who did not undergo surgery had a rate of sudden cardiac death of about 1% per year of follow-up. However, physicians worry about missing the rapid development of symptoms of aortic stenosis in patients who previously had none. Pallikka et al also found that, at 5 years, only 20% of patients had not undergone aortic valve replacement or had not died of cardiovascular causes.20
Many researchers advocate surgical aortic valve replacement for severe asymptomatic aortic stenosis. However, the operative risk is 3% overall and has to be weighed against the 1%-per-year risk of death in patients who do not undergo surgery. Therefore, we need a way to identify a subgroup of patients without symptoms who are at higher risk.
Exercise stress testing
Some patients might subconsciously adapt to aortic stenosis by reducing their physical activity. In these “asymptomatic” patients, exercise stress testing can uncover symptoms in around 40%.21
In a group of people with severe asymptomatic aortic stenosis, a positive treadmill test (defined as an abnormal blood pressure response, ST segment changes, symptoms such as limiting dyspnea, chest discomfort, or dizziness on a modified Bruce protocol, or complex ventricular arrhythmias) strongly predicted the onset of symptoms or the need for surgery. At 24 months, only 19% of those who had had a positive exercise test result remained alive, symptom-free, and without valve replacement, compared with 85% of those who had had a negative test result.22
Subsequent study found that symptoms with exercise were the strongest predictor of the onset of symptoms of aortic stenosis, especially among patients under age 70, in whom the symptoms of fatigue and breathlessness are more specific than in the elderly.23
Recommendation. Exercise testing is recommended in patients with severe asymptomatic aortic stenosis (class IIa indication, level of evidence B) as a means of identifying those who are likely to develop symptoms or who might benefit from surgery. Surgery for those who have an abnormal exercise stress response receives a class IIb, level of evidence C recommendation from the ACC/AHA and a class IC from the European Society of Cardiology.24,25
Exercise stress echocardiography to measure change in transvalvular gradient
Emerging data suggest that exercise stress echocardiography may provide incremental prognostic information in patients with severe asymptomatic aortic stenosis. In fact, two studies showed that an exercise-induced increase in the transvalvular gradient of more than 20 mm Hg26 or 18 mm Hg27 predicts future cardiac events. This increase reflects fixed valve stenosis with limited valve compliance.
Other echocardiographic variables
Additional data have shown that severe aortic stenosis (valve area < 0.6 cm2), aortic velocity greater than 4.0 m/s, and severe calcification confer a higher risk of developing symptoms.28,29
Recommendation. The ACC and AHA say that surgical aortic valve replacement may be considered in patients without symptoms who have a high likelihood of rapid progression of aortic stenosis (ie, who are older or have severe calcification or coronary artery disease) or if surgery might be delayed at the time of symptom onset (class IIb, level of evidence C).
Aortic valve replacement can also be considered for extremely severe aortic stenosis (valve area < 0.6 cm2), mean gradient > 60 mm Hg, and velocity > 5.0 m/s if the operative mortality rate is 1.0% or less (class IIb, level of evidence C).
Brain natriuretic peptide levels
Measuring the brain natriuretic peptide (BNP) level may help if symptoms are unclear; higher levels suggest cardiac decompensation.28
One study showed that BNP levels are higher in patients with symptomatic aortic stenosis than in those with asymptomatic severe disease, and correlate with symptom severity.30 In addition, in two other studies, higher BNP and N-terminal BNP levels were shown to predict disease progression, symptom onset, and poorer event-free survival.31,32
In severe asymptomatic aortic stenosis, natriuretic peptides may provide important prognostic information beyond clinical and echocardiographic evaluation. Furthermore, in a recent study, Monin et al33 proposed a risk score that integrates peak aortic jet velocity, BNP level, and sex (women being at higher risk) in predicting who would benefit from early surgery in patients with severe asymptomatic aortic stenosis.33
SPECIAL CONSIDERATIONS
Low-output, low-gradient aortic stenosis: True severe stenosis vs pseudostenosis
Patients with a low ejection fraction (< 50%) and a high mean transvalvular gradient (> 30 or 40 mm Hg) pose no therapeutic dilemma. They have true afterload mismatch and improve markedly with surgery.34 However, patients with an even lower ejection fraction (< 35% or 40%) and a low mean transvalvular gradient (< 30 or 40 mm Hg) pose more of a problem.
It is hard to tell if these patients have true severe aortic stenosis or pseudostenosis due to primary myocardial dysfunction. In pseudostenosis, the aortic valves are moderately diseased, and leaflet opening is reduced by a failing ventricle. When cardiac output is low, the formulae used to calculate the aortic valve area become less accurate, so that patients with cardiomyopathy who have only mild or moderate aortic stenosis may appear to have severe stenosis.
Patients with pseudostenosis have a high risk of dying during surgical aortic valve replacement, approaching 50%, and benefit more from evidence-based heart failure management.35,36 In patients with true stenosis, ventricular dysfunction is mainly a result of severe stenosis and should improve after aortic valve replacement.
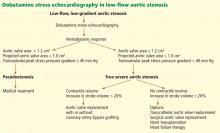
Dobutamine stress echocardiography can be used in patients with low-flow, low-gradient aortic stenosis to distinguish true severe stenosis from pseudostenosis. Dobutamine, an inotropic drug, increases the stroke volume so that patients with true severe aortic stenosis increase their transvalvular gradient and velocity with no or minimal change in the valve area. Conversely, in patients with pseudostenosis, the increase in stroke volume will open the aortic valve further and cause no or minimal increase in transvalvular gradient and velocity, but will increase the calculated valve area, confirming that aortic stenosis only is mild to moderate.37
Patients with low-flow, low-gradient aortic stenosis are at higher risk during surgical aortic valve replacement. Many studies have reported a 30-day mortality rate between 9% and 18%, although risks vary considerably within this population.38,39
Contractile reserve. Dobutamine stress echocardiography has also been used to identify patients with severe aortic stenosis who can increase their ejection fraction and stroke volume (Figure 2).40,41 These patients are said to have “contractile reserve” and do better with surgery than those who lack adequate contractile reserve. Contractile reserve is defined as an increase of more than 20% in stroke volume during low-dose dobutamine infusion.42,43 In one small nonrandomized study, patients with contractile reserve had a 5% mortality rate at 30 days, compared with 32% in patients with no contractile reserve.44,45
In fact, patients with no contractile reserve have a high operative mortality rate during aortic valve replacement, but those who survive the operation have improvements in symptoms, functional class, and ejection fraction similar to those in patients who do have contractile reserve.46
On the other hand, if patients with no contractile reserve are treated conservatively, they have a much worse prognosis than those managed surgically.47 While it is true that patients without contractile reserve did not have a statistically significant difference in mortality rates with aortic valve replacement (P = .07) in a study by Monin et al,44 the difference was staggering between the group who underwent aortic valve replacement and the group who received medical treatment alone (hazard ratio = 0.47, 95% confidence interval 0.31–1.05, P = .07). The difference in the mortality rates may not have reached statistical significance because of the study’s small sample size.
A few years later, the same group published a similar paper with a larger study sample, focusing on patients with no contractile reserve. Using 42 propensity-matched patients, they found a statistically significantly higher 5-year survival rate in patients with no contractile reserve who underwent aortic valve replacement than in similar patients who received medical management (65% ± 11% vs 11 ± 7%, P = .019).47
Hence, surgery may be a better option than medical treatment for this select high-risk group despite the higher operative mortality risk. Transcatheter aortic valve implantation may also offer an interesting alternative to surgical aortic valve replacement in this particular subset of patients.48
Low-gradient ‘severe’ aortic stenosis with preserved ejection fraction or ‘paradoxically low-flow aortic stenosis’
Low-gradient “severe” aortic stenosis with a preserved left ventricular ejection fraction is a recently recognized clinical entity in patients with severe aortic stenosis who present with a lower-than-expected transvalvular gradient on the basis of generally accepted values.49 (A patient with severe aortic stenosis and preserved ejection fraction is expected to generate a mean transaortic gradient greater than 40 mm Hg.24) This situation remains incompletely understood but has been shown in retrospective studies to foretell a poor prognosis.50–52
This subgroup of patients has pronounced left ventricular concentric remodeling with a small left ventricular cavity, impaired left ventricular filling, and reduced systolic longitudinal myocardial shortening.44
Herrmann et al53 provided more insight into the pathophysiology by showing that patients with this condition exhibit more pronounced myocardial fibrosis on myocardial biopsy and more pronounced late subendocardial enhancement on magnetic resonance imaging. These patients also displayed a significant decrease in mitral ring displacement and systolic strain. These abnormalities result in a low stroke volume despite a preserved ejection fraction and consequently a lower transvalvular gradient (< 40 mm Hg).
This disease pattern, in which the low gradient is interpreted as mild to moderate aortic stenosis, may lead to underestimation of stenosis severity and, thus, to inappropriate delay of aortic valve replacement.
However, other conditions can cause this hemodynamic situation with a lower-than-expected gradient. It can arise from a small left ventricle that correlates with a small body size, yielding a lower-than-normal stroke volume, measurement errors in determining stroke volume and valve area by Doppler echocardiography, systemic hypertension (which can influence estimation of the gradient by Doppler echocardiography), and inconsistency in the definition of severe aortic stenosis in the current guidelines relating to cutoffs of valve area in relation to those of jet velocity and gradient.54
This subgroup of patients seems to be at a more advanced stage and has a poorer prognosis if treated medically rather than surgically. When symptomatic, low-gradient severe aortic stenosis should be treated surgically, with one study showing excellent outcomes with aortic valve replacement.50
However, a recent study by Jander et al55 showed that patients with low-gradient severe aortic stenosis and normal ejection fraction have outcomes similar to those in patients with moderate aortic stenosis, suggesting a strategy of medical therapy and close monitoring.55 Of note, the subset of patients reported in this substudy of the Simvastatin and Ezetimibe in Aortic Stenosis (SEAS) trial did not really fit the pattern of low-gradient severe aortic stenosis described by Hachicha et al50 and other groups.51,56 These patients had aortic valve areas in the severe range but mean transaortic gradients in the moderate range, and in light of the other echocardiographic findings in these patients, the area-gradient discordances were predominantly due to small body surface area and measurement errors. These patients indeed had near-normal left ventricular size, no left ventricular hypertrophy, and no evidence of concentric remodeling.
Finally, the findings of the study by Jander et al55 are discordant with those of another substudy of the SEAS trial,57 which reported that paradoxical low-flow aortic stenosis occurred in about 7% of the cohort (compared with 52% in the study by Jander et al55) and was associated with more pronounced concentric remodeling and more severe impairment of myocardial function.
Whether intervention in patients with low-gradient severe aortic stenosis and valve area less than 1.0 cm2 improves outcomes remains to be confirmed and reproduced in future prospective studies.
Elderly patients
The risks of cardiac surgery increase with age. Older patients may be more deconditioned and have more comorbidities than younger patients, placing them at greater risk of a poor outcome.
Several retrospective studies of valve replacement in octogenarians have found that operative mortality rates range from 5.7% to 9% during isolated aortic valve replacement.58–60 Note that, using the STS score, the operative mortality risk increases only from 1.2% in a 70-year-old man with no comorbidities to 1.8% in an 80-year-old man undergoing aortic valve replacement plus coronary artery bypass grafting.61
As in younger patients, valve replacement results in a significant survival benefit and symptomatic improvement. Yet up to 30% of patients with severe aortic stenosis are not referred for surgery because surgery is believed to be too risky.62 The conditions most frequently cited by physicians when declining to refer patients for surgery include a low ejection fraction, advanced age, and advanced comorbidities. None of these is an absolute contraindication to surgery.
A recent retrospective study of 443 elderly patients (mean age 79.5) showed that those with left ventricular concentric remodeling, lower stroke volume, elevated left ventricular filling pressures, and mildly elevated pulmonary artery pressures have a very bad prognosis, with a mortality rate of 50.5% at 3.3 ± 2.7 years.63
Despite the higher operative mortality risk, these patients face a dismal prognosis when treated medically and should be referred to a cardiologist or cardiothoracic surgeon for an assessment of their operative risk and, potentially, for referral for catheter-based valve replacement.
Acutely ill patients
In critically ill patients with aortic stenosis and cardiogenic shock, the use of intravenous sodium nitroprusside increases cardiac output and decreases pulmonary artery wedge pressure, allowing patients to transition to surgery or vasodilator therapy. The mechanism seems to be an increase in myocardial contractility rather than a decrease in peripheral resistance. The reduction in filling pressure and concurrent increase in coronary blood flow relieves ischemia and subsequently enhances contractility.64
TRANSCATHETER AORTIC VALVE REPLACEMENT
Until recently, patients with severe aortic stenosis who were deemed to be at high surgical risk were referred for balloon valvuloplasty as a palliative option. The procedure consists of balloon inflation across the aortic valve to relieve the stenosis.
Most patients have improved symptoms and a decrease in pressure gradient immediately after the procedure, but the results are not durable, with a high restenosis rate within 6 to 12 months and no decrease in the mortality rate.65 (There is some evidence that serial balloon dilation improves survival.66)
The procedure has several limitations, including a risk of embolic stroke, myocardial infarction, and, sometimes, perforation of the left ventricle. It is only used in people who do not wish to have surgery or as a bridge to surgical aortic valve replacement in hemodynamically unstable patients.
Advances in transcatheter technologies have made nonsurgical valve replacement a reality that is increasingly available to a broader population of patients. The first percutaneous valve replacement in a human was performed in 2002.67 Since then, multiple registries from centers around the world, especially in Europe, have shown that it can be performed in high-risk patients with outcomes very comparable to those of surgical aortic valve replacement as predicted by the STS score and EuroSCORE.68,69 Procedural success rates have increased from around 80% in the initial experience to over 95% in the most current series.70
Results from randomized trials
The long-awaited PARTNER A and B trials have been published.
The PARTNER B trial17 randomized patients with severe aortic stenosis who were not considered by the STS score to be suitable candidates for surgery to standard therapy (which included balloon valvoplasty in 84%) or transcatheter aortic valve replacement. There was a dramatic 20% absolute improvement in survival at 1 year with transcatheter replacement, with the survival curve continuing to diverge at 1 year. The rate of death from any cause was 30.7% with transcatheter aortic valve replacement vs 50.7% with standard therapy (hazard ratio with transcatheter replacement 0.55; P < .001).
The major concerns about transcatheter aortic valve replacement borne out in the study are procedural complications, namely stroke and vascular events. At 30 days, transcatheter replacement, as compared with standard therapy, was associated with a higher incidence of major stroke (5.0% vs 1.1%, P = .06) and major vascular complications (16.2% vs 1.1%, P < .001).17
On the other hand, the PARTNER A trial randomized high-risk patients deemed operable by the STS score to either transcatheter or surgical aortic valve replacement. The rate of death at 1 year from any cause was similar in both groups (24.2% vs 26.8%; P = .44), but again at the expense of higher rates of vascular complications (11.0% vs 3.2%, P < .001 at 30 days) and stroke (5.1% vs 2.4%; P = .07 at 1 year) in the transcatheter group. However, the surgical group had higher rates of major bleeding (19.5% vs 9.3%; P < .001) and new-onset atrial fibrillation (16.0% vs 8.6%, P = .06).71
Transcatheter aortic valve replacement has modernized the way we treat aortic stenosis and without a shred of doubt will become the standard of therapy for severe symptomatic aortic stenosis in patients who are not candidates for surgery. For the high-risk operable patient, the benefit of avoiding a sternotomy should be weighed against the higher risk of stroke and vascular complications with the transcatheter procedure. The availability of smaller delivery systems, better expertise, and better vascular access selection should decrease the rate of complications in the future.
- Stewart BF, Siscovick D, Lind BK, et al. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol 1997; 29:630–634.
- Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J 2003; 24:1231–1243.
- Ross J, Braunwald E. Aortic stenosis. Circulation 1968; 38(suppl 1):61–67.
- Schwarz F, Baumann P, Manthey J, et al. The effect of aortic valve replacement on survival. Circulation 1982; 66:1105–1110.
- Brown JM, O’Brien SM, Wu C, Sikora JA, Griffith BP, Gammie JS. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J Thorac Cardiovasc Surg 2009; 137:82–90.
- Kvidal P, Bergström R, Malm T, Ståhle E. Long-term follow-up of morbidity and mortality after aortic valve replacement with a mechanical valve prosthesis. Eur Heart J 2000; 21:1099–1111.
- Ståhle E, Kvidal P, Nyström SO, Bergström R. Long-term relative survival after primary heart valve replacement. Eur J Cardiothorac Surg 1997; 11:81–91.
- Sharma UC, Barenbrug P, Pokharel S, Dassen WR, Pinto YM, Maessen JG. Systematic review of the outcome of aortic valve replacement in patients with aortic stenosis. Ann Thorac Surg 2004; 78:90–95.
- Vaquette B, Corbineau H, Laurent M, et al. Valve replacement in patients with critical aortic stenosis and depressed left ventricular function: predictors of operative risk, left ventricular function recovery, and long term outcome. Heart 2005; 91:1324–1329.
- American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons, Bonow RO, Carabello BA, Kanu C, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation 2006; 114:e84–e231.
- Bonow RO, Carabello BA, Chatterjee K, et al; 2006 Writing Committee Members; American College of Cardiology/American Heart Association Task Force. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2008; 118:e523–e661.
- Nashef SA, Roques F, Hammill BG, et al; EuroSCORE Project Group. Validation of European System for Cardiac Operative Risk Evaluation (EuroSCORE) in North American cardiac surgery. Eur J Cardiothorac Surg 2002; 22:101–105.
- Grossi EA, Schwartz CF, Yu PJ, et al. High-risk aortic valve replacement: are the outcomes as bad as predicted? Ann Thorac Surg 2008; 85:102–106.
- Kalavrouziotis D, Li D, Buth KJ, Légaré JF. The European System for Cardiac Operative Risk Evaluation (EuroSCORE) is not appropriate for withholding surgery in high-risk patients with aortic stenosis: a retrospective cohort study. J Cardiothorac Surg 2009; 4:32.
- Dewey TM, Brown D, Ryan WH, Herbert MA, Prince SL, Mack MJ. Reliability of risk algorithms in predicting early and late operative outcomes in high-risk patients undergoing aortic valve replacement. J Thorac Cardiovasc Surg 2008; 135:180–187.
- Wendt D, Osswald BR, Kayser K, et al. Society of Thoracic Surgeons score is superior to the EuroSCORE determining mortality in high risk patients undergoing isolated aortic valve replacement. Ann Thorac Surg 2009; 88:468–474.
- Leon MB, Smith CR, Mack M, et al; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010; 363:1597–1607.
- Smith WT, Ferguson TB, Ryan T, Landolfo CK, Peterson ED. Should coronary artery bypass graft surgery patients with mild or moderate aortic stenosis undergo concomitant aortic valve replacement? A decision analysis approach to the surgical dilemma. J Am Coll Cardiol 2004; 44:1241–1247.
- Pereira JJ, Balaban K, Lauer MS, Lytle B, Thomas JD, Garcia MJ. Aortic valve replacement in patients with mild or moderate aortic stenosis and coronary bypass surgery. Am J Med 2005; 118:735–742.
- Pellikka PA, Sarano ME, Nishimura RA, et al. Outcome of 622 adults with asymptomatic, hemodynamically significant aortic stenosis during prolonged follow-up. Circulation 2005; 111:3290–3295.
- Ennezat PV, Maréchaux S, Iung B, Chauvel C, LeJemtel TH, Pibarot P. Exercise testing and exercise stress echocardiography in asymptomatic aortic valve stenosis. Heart 2009; 95:877–884.
- Amato MC, Moffa PJ, Werner KE, Ramires JA. Treatment decision in asymptomatic aortic valve stenosis: role of exercise testing. Heart 2001; 86:381–386.
- Das P, Rimington H, Chambers J. Exercise testing to stratify risk in aortic stenosis. Eur Heart J 2005; 26:1309–1313.
- American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease); Society of Cardiovascular Anesthesiologists, Bonow RO, Carabello BA, Chatterjee K, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing Committee to Revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the Society of Cardiovascular Anesthesiologists endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol 2006; 48:e1–e148.
- Vahanian A, Baumgartner H, Bax J, et al; Task Force on the Management of Valvular Hearth Disease of the European Society of Cardiology; ESC Committee for Practice Guidelines. Guidelines on the management of valvular heart disease: The Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur Heart J 2007; 28:230–268.
- Maréchaux S, Hachicha Z, Bellouin A, et al. Usefulness of exercise-stress echocardiography for risk stratification of true asymptomatic patients with aortic valve stenosis. Eur Heart J 2010; 31:1390–1397.
- Lancellotti P, Lebois F, Simon M, Tombeux C, Chauvel C, Pierard LA. Prognostic importance of quantitative exercise Doppler echocardiography in asymptomatic valvular aortic stenosis. Circulation 2005; 112(suppl 9):I377–1382.
- Otto CM. Valvular aortic stenosis: disease severity and timing of intervention. J Am Coll Cardiol 2006; 47:2141–2151.
- Rosenhek R, Binder T, Porenta G, et al. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med 2000; 343:611–617.
- Lim P, Monin JL, Monchi M, et al. Predictors of outcome in patients with severe aortic stenosis and normal left ventricular function: role of B-type natriuretic peptide. Eur Heart J 2004; 25:2048–2053.
- Gerber IL, Legget ME, West TM, Richards AM, Stewart RA. Usefulness of serial measurement of N-terminal pro-brain natriuretic peptide plasma levels in asymptomatic patients with aortic stenosis to predict symptomatic deterioration. Am J Cardiol 2005; 95:898–901.
- Bergler-Klein J, Klaar U, Heger M, et al. Natriuretic peptides predict symptom-free survival and postoperative outcome in severe aortic stenosis. Circulation 2004; 109:2302–2308.
- Monin JL, Lancellotti P, Monchi M, et al. Risk score for predicting outcome in patients with asymptomatic aortic stenosis. Circulation 2009; 120:69–75.
- Carabello BA, Green LH, Grossman W, Cohn LH, Koster JK, Collins JJ. Hemodynamic determinants of prognosis of aortic valve replacement in critical aortic stenosis and advanced congestive heart failure. Circulation 1980; 62:42–48.
- Connolly HM, Oh JK, Schaff HV, et al. Severe aortic stenosis with low transvalvular gradient and severe left ventricular dysfunction: result of aortic valve replacement in 52 patients. Circulation 2000; 101:1940–1946.
- Brogan WC, Grayburn PA, Lange RA, Hillis LD. Prognosis after valve replacement in patients with severe aortic stenosis and a low transvalvular pressure gradient. J Am Coll Cardiol 1993; 21:1657–1660.
- Burwash IG. Low-flow, low-gradient aortic stenosis: from evaluation to treatment. Curr Opin Cardiol 2007; 22:84–91.
- Connolly HM, Oh JK, Orszulak TA, et al. Aortic valve replacement for aortic stenosis with severe left ventricular dysfunction. Prognostic indicators. Circulation 1997; 95:2395–2400.
- Pai RG, Varadarajan P, Razzouk A. Survival benefit of aortic valve replacement in patients with severe aortic stenosis with low ejection fraction and low gradient with normal ejection fraction. Ann Thorac Surg 2008; 86:1781–1789.
- Blais C, Burwash IG, Mundigler G, et al. Projected valve area at normal flow rate improves the assessment of stenosis severity in patients with low-flow, low-gradient aortic stenosis: the multicenter TOPAS (Truly or Pseudo-Severe Aortic Stenosis) study. Circulation 2006; 113:711–721.
- Clavel MA, Burwash IG, Mundigler G, et al. Validation of conventional and simplified methods to calculate projected valve area at normal flow rate in patients with low flow, low gradient aortic stenosis: the multicenter TOPAS (True or Pseudo Severe Aortic Stenosis) study. J Am Soc Echocardiogr 2010; 23:380–386.
- Monin JL, Monchi M, Gest V, Duval-Moulin AM, Dubois-Rande JL, Gueret P. Aortic stenosis with severe left ventricular dysfunction and low transvalvular pressure gradients: risk stratification by low-dose dobutamine echocardiography. J Am Coll Cardiol 2001; 37:2101–2107.
- Nishimura RA, Grantham JA, Connolly HM, Schaff HV, Higano ST, Holmes DR. Low-output, low-gradient aortic stenosis in patients with depressed left ventricular systolic function: the clinical utility of the dobutamine challenge in the catheterization laboratory. Circulation 2002; 106:809–813.
- Monin JL, Quéré JP, Monchi M, et al. Low-gradient aortic stenosis: operative risk stratification and predictors for long-term outcome: a multicenter study using dobutamine stress hemodynamics. Circulation 2003; 108:319–324.
- Monin JL, Guéret P. Calcified aortic stenosis with left ventricular dysfunction and low transvalvular gradients. Must one reject surgery in certain cases?. (In French.) Arch Mal Coeur Vaiss 2003; 96:864–870.
- Quere JP, Monin JL, Levy F, et al. Influence of preoperative left ventricular contractile reserve on postoperative ejection fraction in low-gradient aortic stenosis. Circulation 2006; 113:1738–1744.
- Tribouilloy C, Lévy F, Rusinaru D, et al. Outcome after aortic valve replacement for low-flow/low-gradient aortic stenosis without contractile reserve on dobutamine stress echocardiography. J Am Coll Cardiol 2009; 53:1865–1873.
- Clavel MA, Webb JG, Rodés-Cabau J, et al. Comparison between transcatheter and surgical prosthetic valve implantation in patients with severe aortic stenosis and reduced left ventricular ejection fraction. Circulation 2010; 122:1928–1936.
- Dumesnil JG, Pibarot P, Carabello B. Paradoxical low flow and/or low gradient severe aortic stenosis despite preserved left ventricular ejection fraction: implications for diagnosis and treatment. Eur Heart J 2010; 31:281–289.
- Hachicha Z, Dumesnil JG, Bogaty P, Pibarot P. Paradoxical low-flow, low-gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation 2007; 115:2856–2864.
- Barasch E, Fan D, Chukwu EO, et al. Severe isolated aortic stenosis with normal left ventricular systolic function and low transvalvular gradients: pathophysiologic and prognostic insights. J Heart Valve Dis 2008; 17:81–88.
- Dumesnil JG, Pibarot P, Carabello B. Paradoxical low flow and/or low gradient severe aortic stenosis despite preserved left ventricular ejection fraction: implications for diagnosis and treatment. Eur Heart J 2010; 31:281–289.
- Herrmann S, Störk S, Niemann M, et al. Low-gradient aortic valve stenosis myocardial fibrosis and its influence on function and outcome. J Am Coll Cardiol 2011; 58:402–412.
- Minners J, Allgeier M, Gohlke-Baerwolf C, Kienzle RP, Neumann FJ, Jander N. Inconsistent grading of aortic valve stenosis by current guidelines: haemodynamic studies in patients with apparently normal left ventricular function. Heart 2010; 96:1463–1468.
- Jander N, Minners J, Holme I, et al. Outcome of patients with low-gradient “severe” aortic stenosis and preserved ejection fraction. Circulation 2011; 123:887–895.
- Lancellotti P, Donal E, Magne J, et al. Impact of global left ventricular afterload on left ventricular function in asymptomatic severe aortic stenosis: a two-dimensional speckle-tracking study. Eur J Echocardiogr 2010; 11:537–543.
- Cramariuc D, Cioffi G, Rieck AE, et al. Low-flow aortic stenosis in asymptomatic patients: valvular-arterial impedance and systolic function from the SEAS Substudy. JACC Cardiovasc Imaging 2009; 2:390–399.
- Craver JM, Puskas JD, Weintraub WW, et al. 601 octogenarians undergoing cardiac surgery: outcome and comparison with younger age groups. Ann Thorac Surg 1999; 67:1104–1110.
- Alexander KP, Anstrom KJ, Muhlbaier LH, et al. Outcomes of cardiac surgery in patients > or = 80 years: results from the National Cardiovascular Network. J Am Coll Cardiol 2000; 35:731–738.
- Collart F, Feier H, Kerbaul F, et al. Valvular surgery in octogenarians: operative risks factors, evaluation of Euroscore and long term results. Eur J Cardiothorac Surg 2005; 27:276–280.
- Kurtz CE, Otto CM. Aortic stenosis: clinical aspects of diagnosis and management, with 10 illustrative case reports from a 25-year experience. Medicine (Baltimore) 2010; 89:349–379.
- Iung B, Cachier A, Baron G, et al. Decision-making in elderly patients with severe aortic stenosis: why are so many denied surgery? Eur Heart J 2005; 26:2714–2720.
- Kahn J, Petillo F, Rhee PDY, et al. Echocardiographic predictors of mortality in patients with severe isolated aortic stenosis and normal left ventricular ejection fraction who do not undergo aortic valve replacement. American Society of Echocardiography 2011 Scientific Sessions; June 13, 2011; Montreal, QC. http://www.abstractsonline.com/Plan/ViewAbstract.aspx?sKey=845e6287-66e1-4df5-8aef-8f5da16ef94a&cKey=5e5438dd-20df-48bfbee7-5f867fce66e6&mKey=%7bAE58A7EE-7140-41D6-9C7ED375E33DDABD%7d. Accessed May 27, 2012.
- Popovic ZB, Khot UN, Novaro GM, et al. Effects of sodium nitroprusside in aortic stenosis associated with severe heart failure: pressure-volume loop analysis using a numerical model. Am J Physiol Heart Circ Physiol 2005; 288:H416–H423.
- Otto CM, Mickel MC, Kennedy JW, et al. Three-year outcome after balloon aortic valvuloplasty. Insights into prognosis of valvular aortic stenosis. Circulation 1994; 89:642–650.
- Letac B, Cribier A, Eltchaninoff H, Koning R, Derumeaux G. Evaluation of restenosis after balloon dilatation in adult aortic stenosis by repeat catheterization. Am Heart J 1991; 122:55–60.
- Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation 2002; 106:3006–3008.
- Grube E, Schuler G, Buellesfeld L, et al. Percutaneous aortic valve replacement for severe aortic stenosis in high-risk patients using the second- and current third-generation self-expanding CoreValve prosthesis: device success and 30-day clinical outcome. J Am Coll Cardiol 2007; 50:69–76.
- Webb JG, Altwegg L, Masson JB, Al Bugami S, Al Ali A, Boone RA. A new transcatheter aortic valve and percutaneous valve delivery system. J Am Coll Cardiol 2009; 53:1855–1858.
- Clavel MA, Webb JG, Pibarot P, et al. Comparison of the hemodynamic performance of percutaneous and surgical bioprostheses for the treatment of severe aortic stenosis. J Am Coll Cardiol 2009; 53:1883–1891.
- Smith CR, Leon MB, Mack MJ, et al; PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011; 364:2187–2198.
For some patients with aortic stenosis, the choice of management is simple; for others it is less so. Patients who have severe, symptomatic stenosis and who have low surgical risk should undergo aortic valve replacement. But if the stenosis is severe but asymptomatic, or if the patient is at higher surgical risk, or if there seems to be a mismatch in the hemodynamic variables, the situation is more complicated.
Fortunately, we have evidence and guidelines to go on. In this paper we review the indications for surgical and transcatheter aortic valve replacement, focusing on the areas of less certainty.
AN INDOLENT DISEASE, UNTIL IT ISN’T
Aortic stenosis is the most common valvular disease and the third most prevalent form of cardiovascular disease in the Western world, after hypertension and coronary artery disease. It is largely a disease of the elderly; its prevalence increases with age, and it is present in 2% to 7% of patients over age 65.1,2
At first, its course is indolent, as it progresses slowly over years to decades. However, this is followed by rapid clinical deterioration and a high death rate after symptoms develop.
SURGICAL AORTIC VALVE REPLACEMENT FOR SEVERE SYMPTOMATIC STENOSIS
Classic symptoms of aortic stenosis include angina, heart failure, and syncope. Once symptoms appear, patients with severe aortic stenosis should be promptly referred for surgical aortic valve replacement, as survival is poor unless outflow obstruction is relieved (Figure 1). The onset of symptoms confers a poor prognosis: patients die within an average of 5 years after the onset of angina, 3 years after the onset of syncope, and 2 years after the onset of heart failure symptoms. The overall mortality rate is 75% at 3 years without surgery.3,4 Furthermore, 8% to 34% of patients with symptoms die suddenly.
Advances in prosthetic-valve design, cardiopulmonary bypass, surgical technique, and anesthesia have steadily improved the outcomes of aortic valve surgery. An analysis of the Society of Thoracic Surgeons (STS) database in 2006 showed that during the previous decade the death rate during isolated aortic valve replacement decreased from 3.4% to 2.6%. For patients under age 70 at the time of surgery, the rate of death was 1.3%, and in those ages 80 to 85, the 30-day mortality rate was less than 5%.5
Patients who survive surgery enjoy a near-normal life expectancy: 99% survive at least 5 years, 85% at least 10 years, and 82% at least 15 years.6,7 Nearly all have improvement in their ejection fraction and heart failure symptoms, and those who had more advanced symptoms before surgery enjoy the most benefit afterward.8,9
Recommendation. Surgical valve replacement for symptomatic severe aortic stenosis receives a class I recommendation, level of evidence B, in the current guidelines from the American College of Cardiology (ACC) and the American Heart Association (AHA).10,11 (See Table 1 for an explanation of the classes of recommendations and levels of evidence.)
TWO RISK-ASSESSMENT SCORES
There are two widely used scores for assessing the risk of aortic valve replacement: the European System for Cardiac Operative Risk Evaluation (EuroSCORE) and the STS score. Each has limitations.
The EuroSCORE was developed to predict the risk of dying in the hospital after adult cardiac surgery. It has been shown to predict the short-term and the long-term risk of death after heart valve surgery.12 Unfortunately, it overestimates the dangers of isolated aortic valve replacement in the patients at highest risk.13,14
The STS score, a logistic model, reflects more closely the operative and 30-day mortality rates for the patients at highest risk undergoing surgical aortic valve replacement.15,16 It was used to assess patients for surgical or transcatheter aortic valve replacement in the Placement of Aortic Transcatheter Valves (PARTNER) trial.17
These risk scores, though not perfect, are helpful as part of an overall estimation of risk that includes functional status, cardiac function, and comorbidities.
OTHER INDICATIONS FOR SURGICAL AORTIC VALVE REPLACEMENT
For patients with severe but asymptomatic aortic stenosis, surgical referral is standard practice in several circumstances.
Asymptomatic severe aortic stenosis with a low ejection fraction
Early studies found significant differences in survival beginning as early as 3 years after valve replacement between those whose preoperative ejection fraction was greater than 50% and those with a lower ejection fraction.4 Delaying surgery in these patients may lead to irreversible left ventricular dysfunction and worse survival.
Recommendation. The AHA and the ACC recommend surgical aortic valve replacement for patients who have no symptoms and whose left ventricular ejection fraction is less than 50% (class I indication, level of evidence C).10,11
Asymptomatic severe aortic stenosis in patients undergoing other cardiac surgery
Recommendation. Even if it is causing no symptoms, a severely stenotic aortic valve ought to be replaced if the ejection fraction is greater than 50% and the patient is undergoing another type of heart surgery, such as coronary artery bypass grafting, aortic surgery, or surgery on other heart valves (class I indication, level of evidence B).10,11
Asymptomatic moderate aortic stenosis in patients undergoing other cardiac surgery
When patients with a mildly or moderately stenotic aortic valve undergo other types of cardiac surgery, the decision to replace the valve is more difficult. Clinicians have to consider the increase in risk caused by adding aortic valve replacement to the planned surgery compared with the future likelihood of aortic stenosis progressing to a severe symptomatic state and eventually requiring a second cardiac surgery.
We have no evidence from a large prospective randomized controlled trial regarding prophylactic valve replacement at the time of coronary bypass surgery. However, a review of outcomes from the STS database between 1995 and 2000 found that patients under age 70 with a peak aortic gradient greater than “about 28 mm Hg” (correlating with a moderate degree of stenosis) benefited from prophylactic valve replacement at the time of coronary artery bypass surgery.18
These conclusions were supported by a subsequent retrospective analysis that found a significant survival advantage at 8 years in favor of prophylactic valve replacement at the time of bypass surgery for those with moderate (but not mild) aortic stenosis.19
Recommendation. The AHA and ACC give a class IIb endorsement, level of evidence B, for aortic valve replacement in patients with asymptomatic moderate aortic stenosis undergoing coronary bypass, valve, or aortic surgery.10,11
SEVERE ASYMPTOMATIC STENOSIS: WHICH TESTS HELP IN DECIDING?
A patient without symptoms presents a greater challenge than one with symptoms.
If surgery is deferred, the prognosis is usually excellent in such patients. Pellikka et al20 found that patients with severe asymptomatic aortic stenosis who did not undergo surgery had a rate of sudden cardiac death of about 1% per year of follow-up. However, physicians worry about missing the rapid development of symptoms of aortic stenosis in patients who previously had none. Pallikka et al also found that, at 5 years, only 20% of patients had not undergone aortic valve replacement or had not died of cardiovascular causes.20
Many researchers advocate surgical aortic valve replacement for severe asymptomatic aortic stenosis. However, the operative risk is 3% overall and has to be weighed against the 1%-per-year risk of death in patients who do not undergo surgery. Therefore, we need a way to identify a subgroup of patients without symptoms who are at higher risk.
Exercise stress testing
Some patients might subconsciously adapt to aortic stenosis by reducing their physical activity. In these “asymptomatic” patients, exercise stress testing can uncover symptoms in around 40%.21
In a group of people with severe asymptomatic aortic stenosis, a positive treadmill test (defined as an abnormal blood pressure response, ST segment changes, symptoms such as limiting dyspnea, chest discomfort, or dizziness on a modified Bruce protocol, or complex ventricular arrhythmias) strongly predicted the onset of symptoms or the need for surgery. At 24 months, only 19% of those who had had a positive exercise test result remained alive, symptom-free, and without valve replacement, compared with 85% of those who had had a negative test result.22
Subsequent study found that symptoms with exercise were the strongest predictor of the onset of symptoms of aortic stenosis, especially among patients under age 70, in whom the symptoms of fatigue and breathlessness are more specific than in the elderly.23
Recommendation. Exercise testing is recommended in patients with severe asymptomatic aortic stenosis (class IIa indication, level of evidence B) as a means of identifying those who are likely to develop symptoms or who might benefit from surgery. Surgery for those who have an abnormal exercise stress response receives a class IIb, level of evidence C recommendation from the ACC/AHA and a class IC from the European Society of Cardiology.24,25
Exercise stress echocardiography to measure change in transvalvular gradient
Emerging data suggest that exercise stress echocardiography may provide incremental prognostic information in patients with severe asymptomatic aortic stenosis. In fact, two studies showed that an exercise-induced increase in the transvalvular gradient of more than 20 mm Hg26 or 18 mm Hg27 predicts future cardiac events. This increase reflects fixed valve stenosis with limited valve compliance.
Other echocardiographic variables
Additional data have shown that severe aortic stenosis (valve area < 0.6 cm2), aortic velocity greater than 4.0 m/s, and severe calcification confer a higher risk of developing symptoms.28,29
Recommendation. The ACC and AHA say that surgical aortic valve replacement may be considered in patients without symptoms who have a high likelihood of rapid progression of aortic stenosis (ie, who are older or have severe calcification or coronary artery disease) or if surgery might be delayed at the time of symptom onset (class IIb, level of evidence C).
Aortic valve replacement can also be considered for extremely severe aortic stenosis (valve area < 0.6 cm2), mean gradient > 60 mm Hg, and velocity > 5.0 m/s if the operative mortality rate is 1.0% or less (class IIb, level of evidence C).
Brain natriuretic peptide levels
Measuring the brain natriuretic peptide (BNP) level may help if symptoms are unclear; higher levels suggest cardiac decompensation.28
One study showed that BNP levels are higher in patients with symptomatic aortic stenosis than in those with asymptomatic severe disease, and correlate with symptom severity.30 In addition, in two other studies, higher BNP and N-terminal BNP levels were shown to predict disease progression, symptom onset, and poorer event-free survival.31,32
In severe asymptomatic aortic stenosis, natriuretic peptides may provide important prognostic information beyond clinical and echocardiographic evaluation. Furthermore, in a recent study, Monin et al33 proposed a risk score that integrates peak aortic jet velocity, BNP level, and sex (women being at higher risk) in predicting who would benefit from early surgery in patients with severe asymptomatic aortic stenosis.33
SPECIAL CONSIDERATIONS
Low-output, low-gradient aortic stenosis: True severe stenosis vs pseudostenosis
Patients with a low ejection fraction (< 50%) and a high mean transvalvular gradient (> 30 or 40 mm Hg) pose no therapeutic dilemma. They have true afterload mismatch and improve markedly with surgery.34 However, patients with an even lower ejection fraction (< 35% or 40%) and a low mean transvalvular gradient (< 30 or 40 mm Hg) pose more of a problem.
It is hard to tell if these patients have true severe aortic stenosis or pseudostenosis due to primary myocardial dysfunction. In pseudostenosis, the aortic valves are moderately diseased, and leaflet opening is reduced by a failing ventricle. When cardiac output is low, the formulae used to calculate the aortic valve area become less accurate, so that patients with cardiomyopathy who have only mild or moderate aortic stenosis may appear to have severe stenosis.
Patients with pseudostenosis have a high risk of dying during surgical aortic valve replacement, approaching 50%, and benefit more from evidence-based heart failure management.35,36 In patients with true stenosis, ventricular dysfunction is mainly a result of severe stenosis and should improve after aortic valve replacement.
Dobutamine stress echocardiography can be used in patients with low-flow, low-gradient aortic stenosis to distinguish true severe stenosis from pseudostenosis. Dobutamine, an inotropic drug, increases the stroke volume so that patients with true severe aortic stenosis increase their transvalvular gradient and velocity with no or minimal change in the valve area. Conversely, in patients with pseudostenosis, the increase in stroke volume will open the aortic valve further and cause no or minimal increase in transvalvular gradient and velocity, but will increase the calculated valve area, confirming that aortic stenosis only is mild to moderate.37
Patients with low-flow, low-gradient aortic stenosis are at higher risk during surgical aortic valve replacement. Many studies have reported a 30-day mortality rate between 9% and 18%, although risks vary considerably within this population.38,39
Contractile reserve. Dobutamine stress echocardiography has also been used to identify patients with severe aortic stenosis who can increase their ejection fraction and stroke volume (Figure 2).40,41 These patients are said to have “contractile reserve” and do better with surgery than those who lack adequate contractile reserve. Contractile reserve is defined as an increase of more than 20% in stroke volume during low-dose dobutamine infusion.42,43 In one small nonrandomized study, patients with contractile reserve had a 5% mortality rate at 30 days, compared with 32% in patients with no contractile reserve.44,45
In fact, patients with no contractile reserve have a high operative mortality rate during aortic valve replacement, but those who survive the operation have improvements in symptoms, functional class, and ejection fraction similar to those in patients who do have contractile reserve.46
On the other hand, if patients with no contractile reserve are treated conservatively, they have a much worse prognosis than those managed surgically.47 While it is true that patients without contractile reserve did not have a statistically significant difference in mortality rates with aortic valve replacement (P = .07) in a study by Monin et al,44 the difference was staggering between the group who underwent aortic valve replacement and the group who received medical treatment alone (hazard ratio = 0.47, 95% confidence interval 0.31–1.05, P = .07). The difference in the mortality rates may not have reached statistical significance because of the study’s small sample size.
A few years later, the same group published a similar paper with a larger study sample, focusing on patients with no contractile reserve. Using 42 propensity-matched patients, they found a statistically significantly higher 5-year survival rate in patients with no contractile reserve who underwent aortic valve replacement than in similar patients who received medical management (65% ± 11% vs 11 ± 7%, P = .019).47
Hence, surgery may be a better option than medical treatment for this select high-risk group despite the higher operative mortality risk. Transcatheter aortic valve implantation may also offer an interesting alternative to surgical aortic valve replacement in this particular subset of patients.48
Low-gradient ‘severe’ aortic stenosis with preserved ejection fraction or ‘paradoxically low-flow aortic stenosis’
Low-gradient “severe” aortic stenosis with a preserved left ventricular ejection fraction is a recently recognized clinical entity in patients with severe aortic stenosis who present with a lower-than-expected transvalvular gradient on the basis of generally accepted values.49 (A patient with severe aortic stenosis and preserved ejection fraction is expected to generate a mean transaortic gradient greater than 40 mm Hg.24) This situation remains incompletely understood but has been shown in retrospective studies to foretell a poor prognosis.50–52
This subgroup of patients has pronounced left ventricular concentric remodeling with a small left ventricular cavity, impaired left ventricular filling, and reduced systolic longitudinal myocardial shortening.44
Herrmann et al53 provided more insight into the pathophysiology by showing that patients with this condition exhibit more pronounced myocardial fibrosis on myocardial biopsy and more pronounced late subendocardial enhancement on magnetic resonance imaging. These patients also displayed a significant decrease in mitral ring displacement and systolic strain. These abnormalities result in a low stroke volume despite a preserved ejection fraction and consequently a lower transvalvular gradient (< 40 mm Hg).
This disease pattern, in which the low gradient is interpreted as mild to moderate aortic stenosis, may lead to underestimation of stenosis severity and, thus, to inappropriate delay of aortic valve replacement.
However, other conditions can cause this hemodynamic situation with a lower-than-expected gradient. It can arise from a small left ventricle that correlates with a small body size, yielding a lower-than-normal stroke volume, measurement errors in determining stroke volume and valve area by Doppler echocardiography, systemic hypertension (which can influence estimation of the gradient by Doppler echocardiography), and inconsistency in the definition of severe aortic stenosis in the current guidelines relating to cutoffs of valve area in relation to those of jet velocity and gradient.54
This subgroup of patients seems to be at a more advanced stage and has a poorer prognosis if treated medically rather than surgically. When symptomatic, low-gradient severe aortic stenosis should be treated surgically, with one study showing excellent outcomes with aortic valve replacement.50
However, a recent study by Jander et al55 showed that patients with low-gradient severe aortic stenosis and normal ejection fraction have outcomes similar to those in patients with moderate aortic stenosis, suggesting a strategy of medical therapy and close monitoring.55 Of note, the subset of patients reported in this substudy of the Simvastatin and Ezetimibe in Aortic Stenosis (SEAS) trial did not really fit the pattern of low-gradient severe aortic stenosis described by Hachicha et al50 and other groups.51,56 These patients had aortic valve areas in the severe range but mean transaortic gradients in the moderate range, and in light of the other echocardiographic findings in these patients, the area-gradient discordances were predominantly due to small body surface area and measurement errors. These patients indeed had near-normal left ventricular size, no left ventricular hypertrophy, and no evidence of concentric remodeling.
Finally, the findings of the study by Jander et al55 are discordant with those of another substudy of the SEAS trial,57 which reported that paradoxical low-flow aortic stenosis occurred in about 7% of the cohort (compared with 52% in the study by Jander et al55) and was associated with more pronounced concentric remodeling and more severe impairment of myocardial function.
Whether intervention in patients with low-gradient severe aortic stenosis and valve area less than 1.0 cm2 improves outcomes remains to be confirmed and reproduced in future prospective studies.
Elderly patients
The risks of cardiac surgery increase with age. Older patients may be more deconditioned and have more comorbidities than younger patients, placing them at greater risk of a poor outcome.
Several retrospective studies of valve replacement in octogenarians have found that operative mortality rates range from 5.7% to 9% during isolated aortic valve replacement.58–60 Note that, using the STS score, the operative mortality risk increases only from 1.2% in a 70-year-old man with no comorbidities to 1.8% in an 80-year-old man undergoing aortic valve replacement plus coronary artery bypass grafting.61
As in younger patients, valve replacement results in a significant survival benefit and symptomatic improvement. Yet up to 30% of patients with severe aortic stenosis are not referred for surgery because surgery is believed to be too risky.62 The conditions most frequently cited by physicians when declining to refer patients for surgery include a low ejection fraction, advanced age, and advanced comorbidities. None of these is an absolute contraindication to surgery.
A recent retrospective study of 443 elderly patients (mean age 79.5) showed that those with left ventricular concentric remodeling, lower stroke volume, elevated left ventricular filling pressures, and mildly elevated pulmonary artery pressures have a very bad prognosis, with a mortality rate of 50.5% at 3.3 ± 2.7 years.63
Despite the higher operative mortality risk, these patients face a dismal prognosis when treated medically and should be referred to a cardiologist or cardiothoracic surgeon for an assessment of their operative risk and, potentially, for referral for catheter-based valve replacement.
Acutely ill patients
In critically ill patients with aortic stenosis and cardiogenic shock, the use of intravenous sodium nitroprusside increases cardiac output and decreases pulmonary artery wedge pressure, allowing patients to transition to surgery or vasodilator therapy. The mechanism seems to be an increase in myocardial contractility rather than a decrease in peripheral resistance. The reduction in filling pressure and concurrent increase in coronary blood flow relieves ischemia and subsequently enhances contractility.64
TRANSCATHETER AORTIC VALVE REPLACEMENT
Until recently, patients with severe aortic stenosis who were deemed to be at high surgical risk were referred for balloon valvuloplasty as a palliative option. The procedure consists of balloon inflation across the aortic valve to relieve the stenosis.
Most patients have improved symptoms and a decrease in pressure gradient immediately after the procedure, but the results are not durable, with a high restenosis rate within 6 to 12 months and no decrease in the mortality rate.65 (There is some evidence that serial balloon dilation improves survival.66)
The procedure has several limitations, including a risk of embolic stroke, myocardial infarction, and, sometimes, perforation of the left ventricle. It is only used in people who do not wish to have surgery or as a bridge to surgical aortic valve replacement in hemodynamically unstable patients.
Advances in transcatheter technologies have made nonsurgical valve replacement a reality that is increasingly available to a broader population of patients. The first percutaneous valve replacement in a human was performed in 2002.67 Since then, multiple registries from centers around the world, especially in Europe, have shown that it can be performed in high-risk patients with outcomes very comparable to those of surgical aortic valve replacement as predicted by the STS score and EuroSCORE.68,69 Procedural success rates have increased from around 80% in the initial experience to over 95% in the most current series.70
Results from randomized trials
The long-awaited PARTNER A and B trials have been published.
The PARTNER B trial17 randomized patients with severe aortic stenosis who were not considered by the STS score to be suitable candidates for surgery to standard therapy (which included balloon valvoplasty in 84%) or transcatheter aortic valve replacement. There was a dramatic 20% absolute improvement in survival at 1 year with transcatheter replacement, with the survival curve continuing to diverge at 1 year. The rate of death from any cause was 30.7% with transcatheter aortic valve replacement vs 50.7% with standard therapy (hazard ratio with transcatheter replacement 0.55; P < .001).
The major concerns about transcatheter aortic valve replacement borne out in the study are procedural complications, namely stroke and vascular events. At 30 days, transcatheter replacement, as compared with standard therapy, was associated with a higher incidence of major stroke (5.0% vs 1.1%, P = .06) and major vascular complications (16.2% vs 1.1%, P < .001).17
On the other hand, the PARTNER A trial randomized high-risk patients deemed operable by the STS score to either transcatheter or surgical aortic valve replacement. The rate of death at 1 year from any cause was similar in both groups (24.2% vs 26.8%; P = .44), but again at the expense of higher rates of vascular complications (11.0% vs 3.2%, P < .001 at 30 days) and stroke (5.1% vs 2.4%; P = .07 at 1 year) in the transcatheter group. However, the surgical group had higher rates of major bleeding (19.5% vs 9.3%; P < .001) and new-onset atrial fibrillation (16.0% vs 8.6%, P = .06).71
Transcatheter aortic valve replacement has modernized the way we treat aortic stenosis and without a shred of doubt will become the standard of therapy for severe symptomatic aortic stenosis in patients who are not candidates for surgery. For the high-risk operable patient, the benefit of avoiding a sternotomy should be weighed against the higher risk of stroke and vascular complications with the transcatheter procedure. The availability of smaller delivery systems, better expertise, and better vascular access selection should decrease the rate of complications in the future.
For some patients with aortic stenosis, the choice of management is simple; for others it is less so. Patients who have severe, symptomatic stenosis and who have low surgical risk should undergo aortic valve replacement. But if the stenosis is severe but asymptomatic, or if the patient is at higher surgical risk, or if there seems to be a mismatch in the hemodynamic variables, the situation is more complicated.
Fortunately, we have evidence and guidelines to go on. In this paper we review the indications for surgical and transcatheter aortic valve replacement, focusing on the areas of less certainty.
AN INDOLENT DISEASE, UNTIL IT ISN’T
Aortic stenosis is the most common valvular disease and the third most prevalent form of cardiovascular disease in the Western world, after hypertension and coronary artery disease. It is largely a disease of the elderly; its prevalence increases with age, and it is present in 2% to 7% of patients over age 65.1,2
At first, its course is indolent, as it progresses slowly over years to decades. However, this is followed by rapid clinical deterioration and a high death rate after symptoms develop.
SURGICAL AORTIC VALVE REPLACEMENT FOR SEVERE SYMPTOMATIC STENOSIS
Classic symptoms of aortic stenosis include angina, heart failure, and syncope. Once symptoms appear, patients with severe aortic stenosis should be promptly referred for surgical aortic valve replacement, as survival is poor unless outflow obstruction is relieved (Figure 1). The onset of symptoms confers a poor prognosis: patients die within an average of 5 years after the onset of angina, 3 years after the onset of syncope, and 2 years after the onset of heart failure symptoms. The overall mortality rate is 75% at 3 years without surgery.3,4 Furthermore, 8% to 34% of patients with symptoms die suddenly.
Advances in prosthetic-valve design, cardiopulmonary bypass, surgical technique, and anesthesia have steadily improved the outcomes of aortic valve surgery. An analysis of the Society of Thoracic Surgeons (STS) database in 2006 showed that during the previous decade the death rate during isolated aortic valve replacement decreased from 3.4% to 2.6%. For patients under age 70 at the time of surgery, the rate of death was 1.3%, and in those ages 80 to 85, the 30-day mortality rate was less than 5%.5
Patients who survive surgery enjoy a near-normal life expectancy: 99% survive at least 5 years, 85% at least 10 years, and 82% at least 15 years.6,7 Nearly all have improvement in their ejection fraction and heart failure symptoms, and those who had more advanced symptoms before surgery enjoy the most benefit afterward.8,9
Recommendation. Surgical valve replacement for symptomatic severe aortic stenosis receives a class I recommendation, level of evidence B, in the current guidelines from the American College of Cardiology (ACC) and the American Heart Association (AHA).10,11 (See Table 1 for an explanation of the classes of recommendations and levels of evidence.)
TWO RISK-ASSESSMENT SCORES
There are two widely used scores for assessing the risk of aortic valve replacement: the European System for Cardiac Operative Risk Evaluation (EuroSCORE) and the STS score. Each has limitations.
The EuroSCORE was developed to predict the risk of dying in the hospital after adult cardiac surgery. It has been shown to predict the short-term and the long-term risk of death after heart valve surgery.12 Unfortunately, it overestimates the dangers of isolated aortic valve replacement in the patients at highest risk.13,14
The STS score, a logistic model, reflects more closely the operative and 30-day mortality rates for the patients at highest risk undergoing surgical aortic valve replacement.15,16 It was used to assess patients for surgical or transcatheter aortic valve replacement in the Placement of Aortic Transcatheter Valves (PARTNER) trial.17
These risk scores, though not perfect, are helpful as part of an overall estimation of risk that includes functional status, cardiac function, and comorbidities.
OTHER INDICATIONS FOR SURGICAL AORTIC VALVE REPLACEMENT
For patients with severe but asymptomatic aortic stenosis, surgical referral is standard practice in several circumstances.
Asymptomatic severe aortic stenosis with a low ejection fraction
Early studies found significant differences in survival beginning as early as 3 years after valve replacement between those whose preoperative ejection fraction was greater than 50% and those with a lower ejection fraction.4 Delaying surgery in these patients may lead to irreversible left ventricular dysfunction and worse survival.
Recommendation. The AHA and the ACC recommend surgical aortic valve replacement for patients who have no symptoms and whose left ventricular ejection fraction is less than 50% (class I indication, level of evidence C).10,11
Asymptomatic severe aortic stenosis in patients undergoing other cardiac surgery
Recommendation. Even if it is causing no symptoms, a severely stenotic aortic valve ought to be replaced if the ejection fraction is greater than 50% and the patient is undergoing another type of heart surgery, such as coronary artery bypass grafting, aortic surgery, or surgery on other heart valves (class I indication, level of evidence B).10,11
Asymptomatic moderate aortic stenosis in patients undergoing other cardiac surgery
When patients with a mildly or moderately stenotic aortic valve undergo other types of cardiac surgery, the decision to replace the valve is more difficult. Clinicians have to consider the increase in risk caused by adding aortic valve replacement to the planned surgery compared with the future likelihood of aortic stenosis progressing to a severe symptomatic state and eventually requiring a second cardiac surgery.
We have no evidence from a large prospective randomized controlled trial regarding prophylactic valve replacement at the time of coronary bypass surgery. However, a review of outcomes from the STS database between 1995 and 2000 found that patients under age 70 with a peak aortic gradient greater than “about 28 mm Hg” (correlating with a moderate degree of stenosis) benefited from prophylactic valve replacement at the time of coronary artery bypass surgery.18
These conclusions were supported by a subsequent retrospective analysis that found a significant survival advantage at 8 years in favor of prophylactic valve replacement at the time of bypass surgery for those with moderate (but not mild) aortic stenosis.19
Recommendation. The AHA and ACC give a class IIb endorsement, level of evidence B, for aortic valve replacement in patients with asymptomatic moderate aortic stenosis undergoing coronary bypass, valve, or aortic surgery.10,11
SEVERE ASYMPTOMATIC STENOSIS: WHICH TESTS HELP IN DECIDING?
A patient without symptoms presents a greater challenge than one with symptoms.
If surgery is deferred, the prognosis is usually excellent in such patients. Pellikka et al20 found that patients with severe asymptomatic aortic stenosis who did not undergo surgery had a rate of sudden cardiac death of about 1% per year of follow-up. However, physicians worry about missing the rapid development of symptoms of aortic stenosis in patients who previously had none. Pallikka et al also found that, at 5 years, only 20% of patients had not undergone aortic valve replacement or had not died of cardiovascular causes.20
Many researchers advocate surgical aortic valve replacement for severe asymptomatic aortic stenosis. However, the operative risk is 3% overall and has to be weighed against the 1%-per-year risk of death in patients who do not undergo surgery. Therefore, we need a way to identify a subgroup of patients without symptoms who are at higher risk.
Exercise stress testing
Some patients might subconsciously adapt to aortic stenosis by reducing their physical activity. In these “asymptomatic” patients, exercise stress testing can uncover symptoms in around 40%.21
In a group of people with severe asymptomatic aortic stenosis, a positive treadmill test (defined as an abnormal blood pressure response, ST segment changes, symptoms such as limiting dyspnea, chest discomfort, or dizziness on a modified Bruce protocol, or complex ventricular arrhythmias) strongly predicted the onset of symptoms or the need for surgery. At 24 months, only 19% of those who had had a positive exercise test result remained alive, symptom-free, and without valve replacement, compared with 85% of those who had had a negative test result.22
Subsequent study found that symptoms with exercise were the strongest predictor of the onset of symptoms of aortic stenosis, especially among patients under age 70, in whom the symptoms of fatigue and breathlessness are more specific than in the elderly.23
Recommendation. Exercise testing is recommended in patients with severe asymptomatic aortic stenosis (class IIa indication, level of evidence B) as a means of identifying those who are likely to develop symptoms or who might benefit from surgery. Surgery for those who have an abnormal exercise stress response receives a class IIb, level of evidence C recommendation from the ACC/AHA and a class IC from the European Society of Cardiology.24,25
Exercise stress echocardiography to measure change in transvalvular gradient
Emerging data suggest that exercise stress echocardiography may provide incremental prognostic information in patients with severe asymptomatic aortic stenosis. In fact, two studies showed that an exercise-induced increase in the transvalvular gradient of more than 20 mm Hg26 or 18 mm Hg27 predicts future cardiac events. This increase reflects fixed valve stenosis with limited valve compliance.
Other echocardiographic variables
Additional data have shown that severe aortic stenosis (valve area < 0.6 cm2), aortic velocity greater than 4.0 m/s, and severe calcification confer a higher risk of developing symptoms.28,29
Recommendation. The ACC and AHA say that surgical aortic valve replacement may be considered in patients without symptoms who have a high likelihood of rapid progression of aortic stenosis (ie, who are older or have severe calcification or coronary artery disease) or if surgery might be delayed at the time of symptom onset (class IIb, level of evidence C).
Aortic valve replacement can also be considered for extremely severe aortic stenosis (valve area < 0.6 cm2), mean gradient > 60 mm Hg, and velocity > 5.0 m/s if the operative mortality rate is 1.0% or less (class IIb, level of evidence C).
Brain natriuretic peptide levels
Measuring the brain natriuretic peptide (BNP) level may help if symptoms are unclear; higher levels suggest cardiac decompensation.28
One study showed that BNP levels are higher in patients with symptomatic aortic stenosis than in those with asymptomatic severe disease, and correlate with symptom severity.30 In addition, in two other studies, higher BNP and N-terminal BNP levels were shown to predict disease progression, symptom onset, and poorer event-free survival.31,32
In severe asymptomatic aortic stenosis, natriuretic peptides may provide important prognostic information beyond clinical and echocardiographic evaluation. Furthermore, in a recent study, Monin et al33 proposed a risk score that integrates peak aortic jet velocity, BNP level, and sex (women being at higher risk) in predicting who would benefit from early surgery in patients with severe asymptomatic aortic stenosis.33
SPECIAL CONSIDERATIONS
Low-output, low-gradient aortic stenosis: True severe stenosis vs pseudostenosis
Patients with a low ejection fraction (< 50%) and a high mean transvalvular gradient (> 30 or 40 mm Hg) pose no therapeutic dilemma. They have true afterload mismatch and improve markedly with surgery.34 However, patients with an even lower ejection fraction (< 35% or 40%) and a low mean transvalvular gradient (< 30 or 40 mm Hg) pose more of a problem.
It is hard to tell if these patients have true severe aortic stenosis or pseudostenosis due to primary myocardial dysfunction. In pseudostenosis, the aortic valves are moderately diseased, and leaflet opening is reduced by a failing ventricle. When cardiac output is low, the formulae used to calculate the aortic valve area become less accurate, so that patients with cardiomyopathy who have only mild or moderate aortic stenosis may appear to have severe stenosis.
Patients with pseudostenosis have a high risk of dying during surgical aortic valve replacement, approaching 50%, and benefit more from evidence-based heart failure management.35,36 In patients with true stenosis, ventricular dysfunction is mainly a result of severe stenosis and should improve after aortic valve replacement.
Dobutamine stress echocardiography can be used in patients with low-flow, low-gradient aortic stenosis to distinguish true severe stenosis from pseudostenosis. Dobutamine, an inotropic drug, increases the stroke volume so that patients with true severe aortic stenosis increase their transvalvular gradient and velocity with no or minimal change in the valve area. Conversely, in patients with pseudostenosis, the increase in stroke volume will open the aortic valve further and cause no or minimal increase in transvalvular gradient and velocity, but will increase the calculated valve area, confirming that aortic stenosis only is mild to moderate.37
Patients with low-flow, low-gradient aortic stenosis are at higher risk during surgical aortic valve replacement. Many studies have reported a 30-day mortality rate between 9% and 18%, although risks vary considerably within this population.38,39
Contractile reserve. Dobutamine stress echocardiography has also been used to identify patients with severe aortic stenosis who can increase their ejection fraction and stroke volume (Figure 2).40,41 These patients are said to have “contractile reserve” and do better with surgery than those who lack adequate contractile reserve. Contractile reserve is defined as an increase of more than 20% in stroke volume during low-dose dobutamine infusion.42,43 In one small nonrandomized study, patients with contractile reserve had a 5% mortality rate at 30 days, compared with 32% in patients with no contractile reserve.44,45
In fact, patients with no contractile reserve have a high operative mortality rate during aortic valve replacement, but those who survive the operation have improvements in symptoms, functional class, and ejection fraction similar to those in patients who do have contractile reserve.46
On the other hand, if patients with no contractile reserve are treated conservatively, they have a much worse prognosis than those managed surgically.47 While it is true that patients without contractile reserve did not have a statistically significant difference in mortality rates with aortic valve replacement (P = .07) in a study by Monin et al,44 the difference was staggering between the group who underwent aortic valve replacement and the group who received medical treatment alone (hazard ratio = 0.47, 95% confidence interval 0.31–1.05, P = .07). The difference in the mortality rates may not have reached statistical significance because of the study’s small sample size.
A few years later, the same group published a similar paper with a larger study sample, focusing on patients with no contractile reserve. Using 42 propensity-matched patients, they found a statistically significantly higher 5-year survival rate in patients with no contractile reserve who underwent aortic valve replacement than in similar patients who received medical management (65% ± 11% vs 11 ± 7%, P = .019).47
Hence, surgery may be a better option than medical treatment for this select high-risk group despite the higher operative mortality risk. Transcatheter aortic valve implantation may also offer an interesting alternative to surgical aortic valve replacement in this particular subset of patients.48
Low-gradient ‘severe’ aortic stenosis with preserved ejection fraction or ‘paradoxically low-flow aortic stenosis’
Low-gradient “severe” aortic stenosis with a preserved left ventricular ejection fraction is a recently recognized clinical entity in patients with severe aortic stenosis who present with a lower-than-expected transvalvular gradient on the basis of generally accepted values.49 (A patient with severe aortic stenosis and preserved ejection fraction is expected to generate a mean transaortic gradient greater than 40 mm Hg.24) This situation remains incompletely understood but has been shown in retrospective studies to foretell a poor prognosis.50–52
This subgroup of patients has pronounced left ventricular concentric remodeling with a small left ventricular cavity, impaired left ventricular filling, and reduced systolic longitudinal myocardial shortening.44
Herrmann et al53 provided more insight into the pathophysiology by showing that patients with this condition exhibit more pronounced myocardial fibrosis on myocardial biopsy and more pronounced late subendocardial enhancement on magnetic resonance imaging. These patients also displayed a significant decrease in mitral ring displacement and systolic strain. These abnormalities result in a low stroke volume despite a preserved ejection fraction and consequently a lower transvalvular gradient (< 40 mm Hg).
This disease pattern, in which the low gradient is interpreted as mild to moderate aortic stenosis, may lead to underestimation of stenosis severity and, thus, to inappropriate delay of aortic valve replacement.
However, other conditions can cause this hemodynamic situation with a lower-than-expected gradient. It can arise from a small left ventricle that correlates with a small body size, yielding a lower-than-normal stroke volume, measurement errors in determining stroke volume and valve area by Doppler echocardiography, systemic hypertension (which can influence estimation of the gradient by Doppler echocardiography), and inconsistency in the definition of severe aortic stenosis in the current guidelines relating to cutoffs of valve area in relation to those of jet velocity and gradient.54
This subgroup of patients seems to be at a more advanced stage and has a poorer prognosis if treated medically rather than surgically. When symptomatic, low-gradient severe aortic stenosis should be treated surgically, with one study showing excellent outcomes with aortic valve replacement.50
However, a recent study by Jander et al55 showed that patients with low-gradient severe aortic stenosis and normal ejection fraction have outcomes similar to those in patients with moderate aortic stenosis, suggesting a strategy of medical therapy and close monitoring.55 Of note, the subset of patients reported in this substudy of the Simvastatin and Ezetimibe in Aortic Stenosis (SEAS) trial did not really fit the pattern of low-gradient severe aortic stenosis described by Hachicha et al50 and other groups.51,56 These patients had aortic valve areas in the severe range but mean transaortic gradients in the moderate range, and in light of the other echocardiographic findings in these patients, the area-gradient discordances were predominantly due to small body surface area and measurement errors. These patients indeed had near-normal left ventricular size, no left ventricular hypertrophy, and no evidence of concentric remodeling.
Finally, the findings of the study by Jander et al55 are discordant with those of another substudy of the SEAS trial,57 which reported that paradoxical low-flow aortic stenosis occurred in about 7% of the cohort (compared with 52% in the study by Jander et al55) and was associated with more pronounced concentric remodeling and more severe impairment of myocardial function.
Whether intervention in patients with low-gradient severe aortic stenosis and valve area less than 1.0 cm2 improves outcomes remains to be confirmed and reproduced in future prospective studies.
Elderly patients
The risks of cardiac surgery increase with age. Older patients may be more deconditioned and have more comorbidities than younger patients, placing them at greater risk of a poor outcome.
Several retrospective studies of valve replacement in octogenarians have found that operative mortality rates range from 5.7% to 9% during isolated aortic valve replacement.58–60 Note that, using the STS score, the operative mortality risk increases only from 1.2% in a 70-year-old man with no comorbidities to 1.8% in an 80-year-old man undergoing aortic valve replacement plus coronary artery bypass grafting.61
As in younger patients, valve replacement results in a significant survival benefit and symptomatic improvement. Yet up to 30% of patients with severe aortic stenosis are not referred for surgery because surgery is believed to be too risky.62 The conditions most frequently cited by physicians when declining to refer patients for surgery include a low ejection fraction, advanced age, and advanced comorbidities. None of these is an absolute contraindication to surgery.
A recent retrospective study of 443 elderly patients (mean age 79.5) showed that those with left ventricular concentric remodeling, lower stroke volume, elevated left ventricular filling pressures, and mildly elevated pulmonary artery pressures have a very bad prognosis, with a mortality rate of 50.5% at 3.3 ± 2.7 years.63
Despite the higher operative mortality risk, these patients face a dismal prognosis when treated medically and should be referred to a cardiologist or cardiothoracic surgeon for an assessment of their operative risk and, potentially, for referral for catheter-based valve replacement.
Acutely ill patients
In critically ill patients with aortic stenosis and cardiogenic shock, the use of intravenous sodium nitroprusside increases cardiac output and decreases pulmonary artery wedge pressure, allowing patients to transition to surgery or vasodilator therapy. The mechanism seems to be an increase in myocardial contractility rather than a decrease in peripheral resistance. The reduction in filling pressure and concurrent increase in coronary blood flow relieves ischemia and subsequently enhances contractility.64
TRANSCATHETER AORTIC VALVE REPLACEMENT
Until recently, patients with severe aortic stenosis who were deemed to be at high surgical risk were referred for balloon valvuloplasty as a palliative option. The procedure consists of balloon inflation across the aortic valve to relieve the stenosis.
Most patients have improved symptoms and a decrease in pressure gradient immediately after the procedure, but the results are not durable, with a high restenosis rate within 6 to 12 months and no decrease in the mortality rate.65 (There is some evidence that serial balloon dilation improves survival.66)
The procedure has several limitations, including a risk of embolic stroke, myocardial infarction, and, sometimes, perforation of the left ventricle. It is only used in people who do not wish to have surgery or as a bridge to surgical aortic valve replacement in hemodynamically unstable patients.
Advances in transcatheter technologies have made nonsurgical valve replacement a reality that is increasingly available to a broader population of patients. The first percutaneous valve replacement in a human was performed in 2002.67 Since then, multiple registries from centers around the world, especially in Europe, have shown that it can be performed in high-risk patients with outcomes very comparable to those of surgical aortic valve replacement as predicted by the STS score and EuroSCORE.68,69 Procedural success rates have increased from around 80% in the initial experience to over 95% in the most current series.70
Results from randomized trials
The long-awaited PARTNER A and B trials have been published.
The PARTNER B trial17 randomized patients with severe aortic stenosis who were not considered by the STS score to be suitable candidates for surgery to standard therapy (which included balloon valvoplasty in 84%) or transcatheter aortic valve replacement. There was a dramatic 20% absolute improvement in survival at 1 year with transcatheter replacement, with the survival curve continuing to diverge at 1 year. The rate of death from any cause was 30.7% with transcatheter aortic valve replacement vs 50.7% with standard therapy (hazard ratio with transcatheter replacement 0.55; P < .001).
The major concerns about transcatheter aortic valve replacement borne out in the study are procedural complications, namely stroke and vascular events. At 30 days, transcatheter replacement, as compared with standard therapy, was associated with a higher incidence of major stroke (5.0% vs 1.1%, P = .06) and major vascular complications (16.2% vs 1.1%, P < .001).17
On the other hand, the PARTNER A trial randomized high-risk patients deemed operable by the STS score to either transcatheter or surgical aortic valve replacement. The rate of death at 1 year from any cause was similar in both groups (24.2% vs 26.8%; P = .44), but again at the expense of higher rates of vascular complications (11.0% vs 3.2%, P < .001 at 30 days) and stroke (5.1% vs 2.4%; P = .07 at 1 year) in the transcatheter group. However, the surgical group had higher rates of major bleeding (19.5% vs 9.3%; P < .001) and new-onset atrial fibrillation (16.0% vs 8.6%, P = .06).71
Transcatheter aortic valve replacement has modernized the way we treat aortic stenosis and without a shred of doubt will become the standard of therapy for severe symptomatic aortic stenosis in patients who are not candidates for surgery. For the high-risk operable patient, the benefit of avoiding a sternotomy should be weighed against the higher risk of stroke and vascular complications with the transcatheter procedure. The availability of smaller delivery systems, better expertise, and better vascular access selection should decrease the rate of complications in the future.
- Stewart BF, Siscovick D, Lind BK, et al. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol 1997; 29:630–634.
- Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J 2003; 24:1231–1243.
- Ross J, Braunwald E. Aortic stenosis. Circulation 1968; 38(suppl 1):61–67.
- Schwarz F, Baumann P, Manthey J, et al. The effect of aortic valve replacement on survival. Circulation 1982; 66:1105–1110.
- Brown JM, O’Brien SM, Wu C, Sikora JA, Griffith BP, Gammie JS. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J Thorac Cardiovasc Surg 2009; 137:82–90.
- Kvidal P, Bergström R, Malm T, Ståhle E. Long-term follow-up of morbidity and mortality after aortic valve replacement with a mechanical valve prosthesis. Eur Heart J 2000; 21:1099–1111.
- Ståhle E, Kvidal P, Nyström SO, Bergström R. Long-term relative survival after primary heart valve replacement. Eur J Cardiothorac Surg 1997; 11:81–91.
- Sharma UC, Barenbrug P, Pokharel S, Dassen WR, Pinto YM, Maessen JG. Systematic review of the outcome of aortic valve replacement in patients with aortic stenosis. Ann Thorac Surg 2004; 78:90–95.
- Vaquette B, Corbineau H, Laurent M, et al. Valve replacement in patients with critical aortic stenosis and depressed left ventricular function: predictors of operative risk, left ventricular function recovery, and long term outcome. Heart 2005; 91:1324–1329.
- American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons, Bonow RO, Carabello BA, Kanu C, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation 2006; 114:e84–e231.
- Bonow RO, Carabello BA, Chatterjee K, et al; 2006 Writing Committee Members; American College of Cardiology/American Heart Association Task Force. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2008; 118:e523–e661.
- Nashef SA, Roques F, Hammill BG, et al; EuroSCORE Project Group. Validation of European System for Cardiac Operative Risk Evaluation (EuroSCORE) in North American cardiac surgery. Eur J Cardiothorac Surg 2002; 22:101–105.
- Grossi EA, Schwartz CF, Yu PJ, et al. High-risk aortic valve replacement: are the outcomes as bad as predicted? Ann Thorac Surg 2008; 85:102–106.
- Kalavrouziotis D, Li D, Buth KJ, Légaré JF. The European System for Cardiac Operative Risk Evaluation (EuroSCORE) is not appropriate for withholding surgery in high-risk patients with aortic stenosis: a retrospective cohort study. J Cardiothorac Surg 2009; 4:32.
- Dewey TM, Brown D, Ryan WH, Herbert MA, Prince SL, Mack MJ. Reliability of risk algorithms in predicting early and late operative outcomes in high-risk patients undergoing aortic valve replacement. J Thorac Cardiovasc Surg 2008; 135:180–187.
- Wendt D, Osswald BR, Kayser K, et al. Society of Thoracic Surgeons score is superior to the EuroSCORE determining mortality in high risk patients undergoing isolated aortic valve replacement. Ann Thorac Surg 2009; 88:468–474.
- Leon MB, Smith CR, Mack M, et al; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010; 363:1597–1607.
- Smith WT, Ferguson TB, Ryan T, Landolfo CK, Peterson ED. Should coronary artery bypass graft surgery patients with mild or moderate aortic stenosis undergo concomitant aortic valve replacement? A decision analysis approach to the surgical dilemma. J Am Coll Cardiol 2004; 44:1241–1247.
- Pereira JJ, Balaban K, Lauer MS, Lytle B, Thomas JD, Garcia MJ. Aortic valve replacement in patients with mild or moderate aortic stenosis and coronary bypass surgery. Am J Med 2005; 118:735–742.
- Pellikka PA, Sarano ME, Nishimura RA, et al. Outcome of 622 adults with asymptomatic, hemodynamically significant aortic stenosis during prolonged follow-up. Circulation 2005; 111:3290–3295.
- Ennezat PV, Maréchaux S, Iung B, Chauvel C, LeJemtel TH, Pibarot P. Exercise testing and exercise stress echocardiography in asymptomatic aortic valve stenosis. Heart 2009; 95:877–884.
- Amato MC, Moffa PJ, Werner KE, Ramires JA. Treatment decision in asymptomatic aortic valve stenosis: role of exercise testing. Heart 2001; 86:381–386.
- Das P, Rimington H, Chambers J. Exercise testing to stratify risk in aortic stenosis. Eur Heart J 2005; 26:1309–1313.
- American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease); Society of Cardiovascular Anesthesiologists, Bonow RO, Carabello BA, Chatterjee K, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing Committee to Revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the Society of Cardiovascular Anesthesiologists endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol 2006; 48:e1–e148.
- Vahanian A, Baumgartner H, Bax J, et al; Task Force on the Management of Valvular Hearth Disease of the European Society of Cardiology; ESC Committee for Practice Guidelines. Guidelines on the management of valvular heart disease: The Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur Heart J 2007; 28:230–268.
- Maréchaux S, Hachicha Z, Bellouin A, et al. Usefulness of exercise-stress echocardiography for risk stratification of true asymptomatic patients with aortic valve stenosis. Eur Heart J 2010; 31:1390–1397.
- Lancellotti P, Lebois F, Simon M, Tombeux C, Chauvel C, Pierard LA. Prognostic importance of quantitative exercise Doppler echocardiography in asymptomatic valvular aortic stenosis. Circulation 2005; 112(suppl 9):I377–1382.
- Otto CM. Valvular aortic stenosis: disease severity and timing of intervention. J Am Coll Cardiol 2006; 47:2141–2151.
- Rosenhek R, Binder T, Porenta G, et al. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med 2000; 343:611–617.
- Lim P, Monin JL, Monchi M, et al. Predictors of outcome in patients with severe aortic stenosis and normal left ventricular function: role of B-type natriuretic peptide. Eur Heart J 2004; 25:2048–2053.
- Gerber IL, Legget ME, West TM, Richards AM, Stewart RA. Usefulness of serial measurement of N-terminal pro-brain natriuretic peptide plasma levels in asymptomatic patients with aortic stenosis to predict symptomatic deterioration. Am J Cardiol 2005; 95:898–901.
- Bergler-Klein J, Klaar U, Heger M, et al. Natriuretic peptides predict symptom-free survival and postoperative outcome in severe aortic stenosis. Circulation 2004; 109:2302–2308.
- Monin JL, Lancellotti P, Monchi M, et al. Risk score for predicting outcome in patients with asymptomatic aortic stenosis. Circulation 2009; 120:69–75.
- Carabello BA, Green LH, Grossman W, Cohn LH, Koster JK, Collins JJ. Hemodynamic determinants of prognosis of aortic valve replacement in critical aortic stenosis and advanced congestive heart failure. Circulation 1980; 62:42–48.
- Connolly HM, Oh JK, Schaff HV, et al. Severe aortic stenosis with low transvalvular gradient and severe left ventricular dysfunction: result of aortic valve replacement in 52 patients. Circulation 2000; 101:1940–1946.
- Brogan WC, Grayburn PA, Lange RA, Hillis LD. Prognosis after valve replacement in patients with severe aortic stenosis and a low transvalvular pressure gradient. J Am Coll Cardiol 1993; 21:1657–1660.
- Burwash IG. Low-flow, low-gradient aortic stenosis: from evaluation to treatment. Curr Opin Cardiol 2007; 22:84–91.
- Connolly HM, Oh JK, Orszulak TA, et al. Aortic valve replacement for aortic stenosis with severe left ventricular dysfunction. Prognostic indicators. Circulation 1997; 95:2395–2400.
- Pai RG, Varadarajan P, Razzouk A. Survival benefit of aortic valve replacement in patients with severe aortic stenosis with low ejection fraction and low gradient with normal ejection fraction. Ann Thorac Surg 2008; 86:1781–1789.
- Blais C, Burwash IG, Mundigler G, et al. Projected valve area at normal flow rate improves the assessment of stenosis severity in patients with low-flow, low-gradient aortic stenosis: the multicenter TOPAS (Truly or Pseudo-Severe Aortic Stenosis) study. Circulation 2006; 113:711–721.
- Clavel MA, Burwash IG, Mundigler G, et al. Validation of conventional and simplified methods to calculate projected valve area at normal flow rate in patients with low flow, low gradient aortic stenosis: the multicenter TOPAS (True or Pseudo Severe Aortic Stenosis) study. J Am Soc Echocardiogr 2010; 23:380–386.
- Monin JL, Monchi M, Gest V, Duval-Moulin AM, Dubois-Rande JL, Gueret P. Aortic stenosis with severe left ventricular dysfunction and low transvalvular pressure gradients: risk stratification by low-dose dobutamine echocardiography. J Am Coll Cardiol 2001; 37:2101–2107.
- Nishimura RA, Grantham JA, Connolly HM, Schaff HV, Higano ST, Holmes DR. Low-output, low-gradient aortic stenosis in patients with depressed left ventricular systolic function: the clinical utility of the dobutamine challenge in the catheterization laboratory. Circulation 2002; 106:809–813.
- Monin JL, Quéré JP, Monchi M, et al. Low-gradient aortic stenosis: operative risk stratification and predictors for long-term outcome: a multicenter study using dobutamine stress hemodynamics. Circulation 2003; 108:319–324.
- Monin JL, Guéret P. Calcified aortic stenosis with left ventricular dysfunction and low transvalvular gradients. Must one reject surgery in certain cases?. (In French.) Arch Mal Coeur Vaiss 2003; 96:864–870.
- Quere JP, Monin JL, Levy F, et al. Influence of preoperative left ventricular contractile reserve on postoperative ejection fraction in low-gradient aortic stenosis. Circulation 2006; 113:1738–1744.
- Tribouilloy C, Lévy F, Rusinaru D, et al. Outcome after aortic valve replacement for low-flow/low-gradient aortic stenosis without contractile reserve on dobutamine stress echocardiography. J Am Coll Cardiol 2009; 53:1865–1873.
- Clavel MA, Webb JG, Rodés-Cabau J, et al. Comparison between transcatheter and surgical prosthetic valve implantation in patients with severe aortic stenosis and reduced left ventricular ejection fraction. Circulation 2010; 122:1928–1936.
- Dumesnil JG, Pibarot P, Carabello B. Paradoxical low flow and/or low gradient severe aortic stenosis despite preserved left ventricular ejection fraction: implications for diagnosis and treatment. Eur Heart J 2010; 31:281–289.
- Hachicha Z, Dumesnil JG, Bogaty P, Pibarot P. Paradoxical low-flow, low-gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation 2007; 115:2856–2864.
- Barasch E, Fan D, Chukwu EO, et al. Severe isolated aortic stenosis with normal left ventricular systolic function and low transvalvular gradients: pathophysiologic and prognostic insights. J Heart Valve Dis 2008; 17:81–88.
- Dumesnil JG, Pibarot P, Carabello B. Paradoxical low flow and/or low gradient severe aortic stenosis despite preserved left ventricular ejection fraction: implications for diagnosis and treatment. Eur Heart J 2010; 31:281–289.
- Herrmann S, Störk S, Niemann M, et al. Low-gradient aortic valve stenosis myocardial fibrosis and its influence on function and outcome. J Am Coll Cardiol 2011; 58:402–412.
- Minners J, Allgeier M, Gohlke-Baerwolf C, Kienzle RP, Neumann FJ, Jander N. Inconsistent grading of aortic valve stenosis by current guidelines: haemodynamic studies in patients with apparently normal left ventricular function. Heart 2010; 96:1463–1468.
- Jander N, Minners J, Holme I, et al. Outcome of patients with low-gradient “severe” aortic stenosis and preserved ejection fraction. Circulation 2011; 123:887–895.
- Lancellotti P, Donal E, Magne J, et al. Impact of global left ventricular afterload on left ventricular function in asymptomatic severe aortic stenosis: a two-dimensional speckle-tracking study. Eur J Echocardiogr 2010; 11:537–543.
- Cramariuc D, Cioffi G, Rieck AE, et al. Low-flow aortic stenosis in asymptomatic patients: valvular-arterial impedance and systolic function from the SEAS Substudy. JACC Cardiovasc Imaging 2009; 2:390–399.
- Craver JM, Puskas JD, Weintraub WW, et al. 601 octogenarians undergoing cardiac surgery: outcome and comparison with younger age groups. Ann Thorac Surg 1999; 67:1104–1110.
- Alexander KP, Anstrom KJ, Muhlbaier LH, et al. Outcomes of cardiac surgery in patients > or = 80 years: results from the National Cardiovascular Network. J Am Coll Cardiol 2000; 35:731–738.
- Collart F, Feier H, Kerbaul F, et al. Valvular surgery in octogenarians: operative risks factors, evaluation of Euroscore and long term results. Eur J Cardiothorac Surg 2005; 27:276–280.
- Kurtz CE, Otto CM. Aortic stenosis: clinical aspects of diagnosis and management, with 10 illustrative case reports from a 25-year experience. Medicine (Baltimore) 2010; 89:349–379.
- Iung B, Cachier A, Baron G, et al. Decision-making in elderly patients with severe aortic stenosis: why are so many denied surgery? Eur Heart J 2005; 26:2714–2720.
- Kahn J, Petillo F, Rhee PDY, et al. Echocardiographic predictors of mortality in patients with severe isolated aortic stenosis and normal left ventricular ejection fraction who do not undergo aortic valve replacement. American Society of Echocardiography 2011 Scientific Sessions; June 13, 2011; Montreal, QC. http://www.abstractsonline.com/Plan/ViewAbstract.aspx?sKey=845e6287-66e1-4df5-8aef-8f5da16ef94a&cKey=5e5438dd-20df-48bfbee7-5f867fce66e6&mKey=%7bAE58A7EE-7140-41D6-9C7ED375E33DDABD%7d. Accessed May 27, 2012.
- Popovic ZB, Khot UN, Novaro GM, et al. Effects of sodium nitroprusside in aortic stenosis associated with severe heart failure: pressure-volume loop analysis using a numerical model. Am J Physiol Heart Circ Physiol 2005; 288:H416–H423.
- Otto CM, Mickel MC, Kennedy JW, et al. Three-year outcome after balloon aortic valvuloplasty. Insights into prognosis of valvular aortic stenosis. Circulation 1994; 89:642–650.
- Letac B, Cribier A, Eltchaninoff H, Koning R, Derumeaux G. Evaluation of restenosis after balloon dilatation in adult aortic stenosis by repeat catheterization. Am Heart J 1991; 122:55–60.
- Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation 2002; 106:3006–3008.
- Grube E, Schuler G, Buellesfeld L, et al. Percutaneous aortic valve replacement for severe aortic stenosis in high-risk patients using the second- and current third-generation self-expanding CoreValve prosthesis: device success and 30-day clinical outcome. J Am Coll Cardiol 2007; 50:69–76.
- Webb JG, Altwegg L, Masson JB, Al Bugami S, Al Ali A, Boone RA. A new transcatheter aortic valve and percutaneous valve delivery system. J Am Coll Cardiol 2009; 53:1855–1858.
- Clavel MA, Webb JG, Pibarot P, et al. Comparison of the hemodynamic performance of percutaneous and surgical bioprostheses for the treatment of severe aortic stenosis. J Am Coll Cardiol 2009; 53:1883–1891.
- Smith CR, Leon MB, Mack MJ, et al; PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011; 364:2187–2198.
- Stewart BF, Siscovick D, Lind BK, et al. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol 1997; 29:630–634.
- Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J 2003; 24:1231–1243.
- Ross J, Braunwald E. Aortic stenosis. Circulation 1968; 38(suppl 1):61–67.
- Schwarz F, Baumann P, Manthey J, et al. The effect of aortic valve replacement on survival. Circulation 1982; 66:1105–1110.
- Brown JM, O’Brien SM, Wu C, Sikora JA, Griffith BP, Gammie JS. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J Thorac Cardiovasc Surg 2009; 137:82–90.
- Kvidal P, Bergström R, Malm T, Ståhle E. Long-term follow-up of morbidity and mortality after aortic valve replacement with a mechanical valve prosthesis. Eur Heart J 2000; 21:1099–1111.
- Ståhle E, Kvidal P, Nyström SO, Bergström R. Long-term relative survival after primary heart valve replacement. Eur J Cardiothorac Surg 1997; 11:81–91.
- Sharma UC, Barenbrug P, Pokharel S, Dassen WR, Pinto YM, Maessen JG. Systematic review of the outcome of aortic valve replacement in patients with aortic stenosis. Ann Thorac Surg 2004; 78:90–95.
- Vaquette B, Corbineau H, Laurent M, et al. Valve replacement in patients with critical aortic stenosis and depressed left ventricular function: predictors of operative risk, left ventricular function recovery, and long term outcome. Heart 2005; 91:1324–1329.
- American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons, Bonow RO, Carabello BA, Kanu C, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation 2006; 114:e84–e231.
- Bonow RO, Carabello BA, Chatterjee K, et al; 2006 Writing Committee Members; American College of Cardiology/American Heart Association Task Force. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2008; 118:e523–e661.
- Nashef SA, Roques F, Hammill BG, et al; EuroSCORE Project Group. Validation of European System for Cardiac Operative Risk Evaluation (EuroSCORE) in North American cardiac surgery. Eur J Cardiothorac Surg 2002; 22:101–105.
- Grossi EA, Schwartz CF, Yu PJ, et al. High-risk aortic valve replacement: are the outcomes as bad as predicted? Ann Thorac Surg 2008; 85:102–106.
- Kalavrouziotis D, Li D, Buth KJ, Légaré JF. The European System for Cardiac Operative Risk Evaluation (EuroSCORE) is not appropriate for withholding surgery in high-risk patients with aortic stenosis: a retrospective cohort study. J Cardiothorac Surg 2009; 4:32.
- Dewey TM, Brown D, Ryan WH, Herbert MA, Prince SL, Mack MJ. Reliability of risk algorithms in predicting early and late operative outcomes in high-risk patients undergoing aortic valve replacement. J Thorac Cardiovasc Surg 2008; 135:180–187.
- Wendt D, Osswald BR, Kayser K, et al. Society of Thoracic Surgeons score is superior to the EuroSCORE determining mortality in high risk patients undergoing isolated aortic valve replacement. Ann Thorac Surg 2009; 88:468–474.
- Leon MB, Smith CR, Mack M, et al; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010; 363:1597–1607.
- Smith WT, Ferguson TB, Ryan T, Landolfo CK, Peterson ED. Should coronary artery bypass graft surgery patients with mild or moderate aortic stenosis undergo concomitant aortic valve replacement? A decision analysis approach to the surgical dilemma. J Am Coll Cardiol 2004; 44:1241–1247.
- Pereira JJ, Balaban K, Lauer MS, Lytle B, Thomas JD, Garcia MJ. Aortic valve replacement in patients with mild or moderate aortic stenosis and coronary bypass surgery. Am J Med 2005; 118:735–742.
- Pellikka PA, Sarano ME, Nishimura RA, et al. Outcome of 622 adults with asymptomatic, hemodynamically significant aortic stenosis during prolonged follow-up. Circulation 2005; 111:3290–3295.
- Ennezat PV, Maréchaux S, Iung B, Chauvel C, LeJemtel TH, Pibarot P. Exercise testing and exercise stress echocardiography in asymptomatic aortic valve stenosis. Heart 2009; 95:877–884.
- Amato MC, Moffa PJ, Werner KE, Ramires JA. Treatment decision in asymptomatic aortic valve stenosis: role of exercise testing. Heart 2001; 86:381–386.
- Das P, Rimington H, Chambers J. Exercise testing to stratify risk in aortic stenosis. Eur Heart J 2005; 26:1309–1313.
- American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease); Society of Cardiovascular Anesthesiologists, Bonow RO, Carabello BA, Chatterjee K, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing Committee to Revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the Society of Cardiovascular Anesthesiologists endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol 2006; 48:e1–e148.
- Vahanian A, Baumgartner H, Bax J, et al; Task Force on the Management of Valvular Hearth Disease of the European Society of Cardiology; ESC Committee for Practice Guidelines. Guidelines on the management of valvular heart disease: The Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur Heart J 2007; 28:230–268.
- Maréchaux S, Hachicha Z, Bellouin A, et al. Usefulness of exercise-stress echocardiography for risk stratification of true asymptomatic patients with aortic valve stenosis. Eur Heart J 2010; 31:1390–1397.
- Lancellotti P, Lebois F, Simon M, Tombeux C, Chauvel C, Pierard LA. Prognostic importance of quantitative exercise Doppler echocardiography in asymptomatic valvular aortic stenosis. Circulation 2005; 112(suppl 9):I377–1382.
- Otto CM. Valvular aortic stenosis: disease severity and timing of intervention. J Am Coll Cardiol 2006; 47:2141–2151.
- Rosenhek R, Binder T, Porenta G, et al. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med 2000; 343:611–617.
- Lim P, Monin JL, Monchi M, et al. Predictors of outcome in patients with severe aortic stenosis and normal left ventricular function: role of B-type natriuretic peptide. Eur Heart J 2004; 25:2048–2053.
- Gerber IL, Legget ME, West TM, Richards AM, Stewart RA. Usefulness of serial measurement of N-terminal pro-brain natriuretic peptide plasma levels in asymptomatic patients with aortic stenosis to predict symptomatic deterioration. Am J Cardiol 2005; 95:898–901.
- Bergler-Klein J, Klaar U, Heger M, et al. Natriuretic peptides predict symptom-free survival and postoperative outcome in severe aortic stenosis. Circulation 2004; 109:2302–2308.
- Monin JL, Lancellotti P, Monchi M, et al. Risk score for predicting outcome in patients with asymptomatic aortic stenosis. Circulation 2009; 120:69–75.
- Carabello BA, Green LH, Grossman W, Cohn LH, Koster JK, Collins JJ. Hemodynamic determinants of prognosis of aortic valve replacement in critical aortic stenosis and advanced congestive heart failure. Circulation 1980; 62:42–48.
- Connolly HM, Oh JK, Schaff HV, et al. Severe aortic stenosis with low transvalvular gradient and severe left ventricular dysfunction: result of aortic valve replacement in 52 patients. Circulation 2000; 101:1940–1946.
- Brogan WC, Grayburn PA, Lange RA, Hillis LD. Prognosis after valve replacement in patients with severe aortic stenosis and a low transvalvular pressure gradient. J Am Coll Cardiol 1993; 21:1657–1660.
- Burwash IG. Low-flow, low-gradient aortic stenosis: from evaluation to treatment. Curr Opin Cardiol 2007; 22:84–91.
- Connolly HM, Oh JK, Orszulak TA, et al. Aortic valve replacement for aortic stenosis with severe left ventricular dysfunction. Prognostic indicators. Circulation 1997; 95:2395–2400.
- Pai RG, Varadarajan P, Razzouk A. Survival benefit of aortic valve replacement in patients with severe aortic stenosis with low ejection fraction and low gradient with normal ejection fraction. Ann Thorac Surg 2008; 86:1781–1789.
- Blais C, Burwash IG, Mundigler G, et al. Projected valve area at normal flow rate improves the assessment of stenosis severity in patients with low-flow, low-gradient aortic stenosis: the multicenter TOPAS (Truly or Pseudo-Severe Aortic Stenosis) study. Circulation 2006; 113:711–721.
- Clavel MA, Burwash IG, Mundigler G, et al. Validation of conventional and simplified methods to calculate projected valve area at normal flow rate in patients with low flow, low gradient aortic stenosis: the multicenter TOPAS (True or Pseudo Severe Aortic Stenosis) study. J Am Soc Echocardiogr 2010; 23:380–386.
- Monin JL, Monchi M, Gest V, Duval-Moulin AM, Dubois-Rande JL, Gueret P. Aortic stenosis with severe left ventricular dysfunction and low transvalvular pressure gradients: risk stratification by low-dose dobutamine echocardiography. J Am Coll Cardiol 2001; 37:2101–2107.
- Nishimura RA, Grantham JA, Connolly HM, Schaff HV, Higano ST, Holmes DR. Low-output, low-gradient aortic stenosis in patients with depressed left ventricular systolic function: the clinical utility of the dobutamine challenge in the catheterization laboratory. Circulation 2002; 106:809–813.
- Monin JL, Quéré JP, Monchi M, et al. Low-gradient aortic stenosis: operative risk stratification and predictors for long-term outcome: a multicenter study using dobutamine stress hemodynamics. Circulation 2003; 108:319–324.
- Monin JL, Guéret P. Calcified aortic stenosis with left ventricular dysfunction and low transvalvular gradients. Must one reject surgery in certain cases?. (In French.) Arch Mal Coeur Vaiss 2003; 96:864–870.
- Quere JP, Monin JL, Levy F, et al. Influence of preoperative left ventricular contractile reserve on postoperative ejection fraction in low-gradient aortic stenosis. Circulation 2006; 113:1738–1744.
- Tribouilloy C, Lévy F, Rusinaru D, et al. Outcome after aortic valve replacement for low-flow/low-gradient aortic stenosis without contractile reserve on dobutamine stress echocardiography. J Am Coll Cardiol 2009; 53:1865–1873.
- Clavel MA, Webb JG, Rodés-Cabau J, et al. Comparison between transcatheter and surgical prosthetic valve implantation in patients with severe aortic stenosis and reduced left ventricular ejection fraction. Circulation 2010; 122:1928–1936.
- Dumesnil JG, Pibarot P, Carabello B. Paradoxical low flow and/or low gradient severe aortic stenosis despite preserved left ventricular ejection fraction: implications for diagnosis and treatment. Eur Heart J 2010; 31:281–289.
- Hachicha Z, Dumesnil JG, Bogaty P, Pibarot P. Paradoxical low-flow, low-gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation 2007; 115:2856–2864.
- Barasch E, Fan D, Chukwu EO, et al. Severe isolated aortic stenosis with normal left ventricular systolic function and low transvalvular gradients: pathophysiologic and prognostic insights. J Heart Valve Dis 2008; 17:81–88.
- Dumesnil JG, Pibarot P, Carabello B. Paradoxical low flow and/or low gradient severe aortic stenosis despite preserved left ventricular ejection fraction: implications for diagnosis and treatment. Eur Heart J 2010; 31:281–289.
- Herrmann S, Störk S, Niemann M, et al. Low-gradient aortic valve stenosis myocardial fibrosis and its influence on function and outcome. J Am Coll Cardiol 2011; 58:402–412.
- Minners J, Allgeier M, Gohlke-Baerwolf C, Kienzle RP, Neumann FJ, Jander N. Inconsistent grading of aortic valve stenosis by current guidelines: haemodynamic studies in patients with apparently normal left ventricular function. Heart 2010; 96:1463–1468.
- Jander N, Minners J, Holme I, et al. Outcome of patients with low-gradient “severe” aortic stenosis and preserved ejection fraction. Circulation 2011; 123:887–895.
- Lancellotti P, Donal E, Magne J, et al. Impact of global left ventricular afterload on left ventricular function in asymptomatic severe aortic stenosis: a two-dimensional speckle-tracking study. Eur J Echocardiogr 2010; 11:537–543.
- Cramariuc D, Cioffi G, Rieck AE, et al. Low-flow aortic stenosis in asymptomatic patients: valvular-arterial impedance and systolic function from the SEAS Substudy. JACC Cardiovasc Imaging 2009; 2:390–399.
- Craver JM, Puskas JD, Weintraub WW, et al. 601 octogenarians undergoing cardiac surgery: outcome and comparison with younger age groups. Ann Thorac Surg 1999; 67:1104–1110.
- Alexander KP, Anstrom KJ, Muhlbaier LH, et al. Outcomes of cardiac surgery in patients > or = 80 years: results from the National Cardiovascular Network. J Am Coll Cardiol 2000; 35:731–738.
- Collart F, Feier H, Kerbaul F, et al. Valvular surgery in octogenarians: operative risks factors, evaluation of Euroscore and long term results. Eur J Cardiothorac Surg 2005; 27:276–280.
- Kurtz CE, Otto CM. Aortic stenosis: clinical aspects of diagnosis and management, with 10 illustrative case reports from a 25-year experience. Medicine (Baltimore) 2010; 89:349–379.
- Iung B, Cachier A, Baron G, et al. Decision-making in elderly patients with severe aortic stenosis: why are so many denied surgery? Eur Heart J 2005; 26:2714–2720.
- Kahn J, Petillo F, Rhee PDY, et al. Echocardiographic predictors of mortality in patients with severe isolated aortic stenosis and normal left ventricular ejection fraction who do not undergo aortic valve replacement. American Society of Echocardiography 2011 Scientific Sessions; June 13, 2011; Montreal, QC. http://www.abstractsonline.com/Plan/ViewAbstract.aspx?sKey=845e6287-66e1-4df5-8aef-8f5da16ef94a&cKey=5e5438dd-20df-48bfbee7-5f867fce66e6&mKey=%7bAE58A7EE-7140-41D6-9C7ED375E33DDABD%7d. Accessed May 27, 2012.
- Popovic ZB, Khot UN, Novaro GM, et al. Effects of sodium nitroprusside in aortic stenosis associated with severe heart failure: pressure-volume loop analysis using a numerical model. Am J Physiol Heart Circ Physiol 2005; 288:H416–H423.
- Otto CM, Mickel MC, Kennedy JW, et al. Three-year outcome after balloon aortic valvuloplasty. Insights into prognosis of valvular aortic stenosis. Circulation 1994; 89:642–650.
- Letac B, Cribier A, Eltchaninoff H, Koning R, Derumeaux G. Evaluation of restenosis after balloon dilatation in adult aortic stenosis by repeat catheterization. Am Heart J 1991; 122:55–60.
- Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation 2002; 106:3006–3008.
- Grube E, Schuler G, Buellesfeld L, et al. Percutaneous aortic valve replacement for severe aortic stenosis in high-risk patients using the second- and current third-generation self-expanding CoreValve prosthesis: device success and 30-day clinical outcome. J Am Coll Cardiol 2007; 50:69–76.
- Webb JG, Altwegg L, Masson JB, Al Bugami S, Al Ali A, Boone RA. A new transcatheter aortic valve and percutaneous valve delivery system. J Am Coll Cardiol 2009; 53:1855–1858.
- Clavel MA, Webb JG, Pibarot P, et al. Comparison of the hemodynamic performance of percutaneous and surgical bioprostheses for the treatment of severe aortic stenosis. J Am Coll Cardiol 2009; 53:1883–1891.
- Smith CR, Leon MB, Mack MJ, et al; PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011; 364:2187–2198.
KEY POINTS
- The management of severe but asymptomatic aortic stenosis is challenging. An abnormal response to exercise stress testing and elevated biomarkers may identify a higher-risk group that might benefit from closer followup and earlier surgery.
- Even patients with impaired left ventricular function and advanced disease can have a good outcome from surgery.
- Dobutamine infusion can help ascertain which patients with low-flow, low-gradient aortic valve stenosis have true severe stenosis (as opposed to pseudostenosis) and are most likely to benefit from aortic valve replacement.
- Transcatheter aortic valve implantation will soon become the procedure of choice for patients at high risk for whom surgery is not feasible, and it may be an alternative to surgery in other patients at high risk even if they can undergo surgery.
Older Patients Benefited Most From EVAR
SAN FRANCISCO – The introduction of endovascular aneurysm repair, or EVAR, has improved outcomes for the repair of intact and ruptured aortic abdominal aneurysms, particularly among those aged 80 years and older, based on a review of more than 400,000 Medicare beneficiaries.
Among patients in this age group, there was an increase in the number of intact abdominal aortic aneurysm (AAA) repairs, coupled with declines in rupture deaths. In those younger than 75 years, intact repair rates decreased about 10%. Those aged 75-79 years had an increase of about 10%. However, those aged 80 years and older had a more dramatic increase of more than 50%. In addition, operative mortality for intact repair has decreased over time for all age groups, although the decline becomes greater with increasing age.
"The greatest benefit [of EVAR] has been seen in those over 80, who had the largest increase in intact repair rates, the largest decline in intact repair mortality, and the largest decline in rupture deaths," Dr. Marc L. Schermerhorn said at the annual meeting of the American Surgical Association.
The researchers used a 100% sample of Medicare data between 1995 and 2008. They identified patients with intact AAAs undergoing repair – by either EVAR or open surgical repair – as well as patients with ruptured AAAs (with and without repair). They determined the 30-day or in-hospital mortality, then standardized the rates (per 100,000 beneficiaries), adjusting for changes in age, sex, and population size over time.
They identified 338,278 intact repairs and 69,653 ruptures (with 47,524 repairs). EVAR use rapidly increased after Food and Drug Administration approval of the procedure in 1999; as of 2008, EVAR was used for 77% of all intact aneurysm repairs.
"There was a more delayed response but an increase nonetheless in the use of EVAR for ruptured aneurysms, to the point where [in 2008] 31% of all ruptured aneurysms are treated with EVAR," said Dr. Schermerhorn. During this time period, the average age of those undergoing repair increased from 73.7 to 75.5 years.
Operative mortality during open intact repair remained steady, at roughly 5%. EVAR mortality dropped from about 2% in 1995 to 1.4% in 2008. The total operative mortality for intact repair (open and EVAR) fell from roughly 5% to 2.4%, despite the fact that there were more repairs in 2008 and the average age of these patients increased.
"As we increase our utilization of EVAR, this is driving down the total mortality of intact aneurysm repair, so that it’s half of what it was at the beginning of that time period," said Dr. Schermerhorn, chief of vascular and endovascular surgery at Beth Israel Deaconess Medical Center in Boston.
The researchers also found that the annual number of ruptures decreased from 6,535 in 1995 to 3,298 in 2008. The greatest decrease in ruptures (repaired or not) was seen in older patients – those 75 years and older – but there was also a decline among patients younger than 75 years. The mortality with open rupture repair remained largely unchanged during the time period (approximately 45%). However, EVAR rupture repair declined from about 45% to 28%. The rate of all rupture repairs also has decreased from about 45% to 36%.
"Rather than suggesting that we’re simply taking the hemodynamically stable patients for EVAR, the best evidence to say that there’s actually a true reduction in mortality is the fact that for the first time in 3 decades, the overall mortality for ruptured aneurysm repair is now well below 40%," Dr. Schermerhorn said. The mortality for all ruptures (repaired or not) has gone down the most dramatically in those aged 80 years and older, although there were declines in the other age groups as well.
Invited discussant Dr. Philip B. Paty noted that reduced rates of intact aneurysm repair and rupture in patients aged 65-74 years may be attributed to a change in the natural history or a decline in the incidence of aneurysms.
"Did you evaluate the age-specific incidence of comorbidities or medical risk over the period of study to see if we are, in fact, dealing with different patient populations? Alternatively, is it possible that repair in recent years was deferred until a larger sac size was present?" he asked.
"We did look at comorbidities over time, and there have been increases in all of the various comorbidities that you would typically associate with aortic aneurysm patients. Coronary artery disease, peripheral arterial disease, hypertension, and [heart failure] all increased. We did not have access to data about rates of cigarette smoking or medication," Dr. Schermerhorn said.
Other studies have shown that the rate of smoking has gone down with time and the use of statins has gone up. There may be better control of hypertension as well. "We think that it’s possible that for those reasons, there may be a decreasing incidence of aortic aneurysm in the United States." Data from the United Kingdom, Sweden, and Australia suggest that there may be a decline in those countries, he added.
Dr. Paty, vice chair of clinical research in the department of surgery at Memorial Sloan-Kettering Cancer Center in New York, also questioned whether the data reflect a change in practice to observing smaller aneurysms, delaying repair.
This may be the case, according to Dr. Schermerhorn. "Are we deferring aneurysms? I would agree completely." Studies have shown "that it’s safe for us to wait until the aneurysms are up in the 5.5-cm range. So I think that a lot of that redistribution of those [younger than 75 years to those just older than 75] may represent that. That should, however, decrease the rate of aneurysm repair that we do, and some patients will die of competitive causes during that observation period. So that should not be reflected in the increased rate of repair that we detected." In addition, the increased use of advanced abdominal imaging has led to the identification of more aneurysms.
The complete manuscript of this presentation is anticipated to be published in the Annals of Surgery pending editorial review.
The authors reported that they have no relevant conflicts of interest.
SAN FRANCISCO – The introduction of endovascular aneurysm repair, or EVAR, has improved outcomes for the repair of intact and ruptured aortic abdominal aneurysms, particularly among those aged 80 years and older, based on a review of more than 400,000 Medicare beneficiaries.
Among patients in this age group, there was an increase in the number of intact abdominal aortic aneurysm (AAA) repairs, coupled with declines in rupture deaths. In those younger than 75 years, intact repair rates decreased about 10%. Those aged 75-79 years had an increase of about 10%. However, those aged 80 years and older had a more dramatic increase of more than 50%. In addition, operative mortality for intact repair has decreased over time for all age groups, although the decline becomes greater with increasing age.
"The greatest benefit [of EVAR] has been seen in those over 80, who had the largest increase in intact repair rates, the largest decline in intact repair mortality, and the largest decline in rupture deaths," Dr. Marc L. Schermerhorn said at the annual meeting of the American Surgical Association.
The researchers used a 100% sample of Medicare data between 1995 and 2008. They identified patients with intact AAAs undergoing repair – by either EVAR or open surgical repair – as well as patients with ruptured AAAs (with and without repair). They determined the 30-day or in-hospital mortality, then standardized the rates (per 100,000 beneficiaries), adjusting for changes in age, sex, and population size over time.
They identified 338,278 intact repairs and 69,653 ruptures (with 47,524 repairs). EVAR use rapidly increased after Food and Drug Administration approval of the procedure in 1999; as of 2008, EVAR was used for 77% of all intact aneurysm repairs.
"There was a more delayed response but an increase nonetheless in the use of EVAR for ruptured aneurysms, to the point where [in 2008] 31% of all ruptured aneurysms are treated with EVAR," said Dr. Schermerhorn. During this time period, the average age of those undergoing repair increased from 73.7 to 75.5 years.
Operative mortality during open intact repair remained steady, at roughly 5%. EVAR mortality dropped from about 2% in 1995 to 1.4% in 2008. The total operative mortality for intact repair (open and EVAR) fell from roughly 5% to 2.4%, despite the fact that there were more repairs in 2008 and the average age of these patients increased.
"As we increase our utilization of EVAR, this is driving down the total mortality of intact aneurysm repair, so that it’s half of what it was at the beginning of that time period," said Dr. Schermerhorn, chief of vascular and endovascular surgery at Beth Israel Deaconess Medical Center in Boston.
The researchers also found that the annual number of ruptures decreased from 6,535 in 1995 to 3,298 in 2008. The greatest decrease in ruptures (repaired or not) was seen in older patients – those 75 years and older – but there was also a decline among patients younger than 75 years. The mortality with open rupture repair remained largely unchanged during the time period (approximately 45%). However, EVAR rupture repair declined from about 45% to 28%. The rate of all rupture repairs also has decreased from about 45% to 36%.
"Rather than suggesting that we’re simply taking the hemodynamically stable patients for EVAR, the best evidence to say that there’s actually a true reduction in mortality is the fact that for the first time in 3 decades, the overall mortality for ruptured aneurysm repair is now well below 40%," Dr. Schermerhorn said. The mortality for all ruptures (repaired or not) has gone down the most dramatically in those aged 80 years and older, although there were declines in the other age groups as well.
Invited discussant Dr. Philip B. Paty noted that reduced rates of intact aneurysm repair and rupture in patients aged 65-74 years may be attributed to a change in the natural history or a decline in the incidence of aneurysms.
"Did you evaluate the age-specific incidence of comorbidities or medical risk over the period of study to see if we are, in fact, dealing with different patient populations? Alternatively, is it possible that repair in recent years was deferred until a larger sac size was present?" he asked.
"We did look at comorbidities over time, and there have been increases in all of the various comorbidities that you would typically associate with aortic aneurysm patients. Coronary artery disease, peripheral arterial disease, hypertension, and [heart failure] all increased. We did not have access to data about rates of cigarette smoking or medication," Dr. Schermerhorn said.
Other studies have shown that the rate of smoking has gone down with time and the use of statins has gone up. There may be better control of hypertension as well. "We think that it’s possible that for those reasons, there may be a decreasing incidence of aortic aneurysm in the United States." Data from the United Kingdom, Sweden, and Australia suggest that there may be a decline in those countries, he added.
Dr. Paty, vice chair of clinical research in the department of surgery at Memorial Sloan-Kettering Cancer Center in New York, also questioned whether the data reflect a change in practice to observing smaller aneurysms, delaying repair.
This may be the case, according to Dr. Schermerhorn. "Are we deferring aneurysms? I would agree completely." Studies have shown "that it’s safe for us to wait until the aneurysms are up in the 5.5-cm range. So I think that a lot of that redistribution of those [younger than 75 years to those just older than 75] may represent that. That should, however, decrease the rate of aneurysm repair that we do, and some patients will die of competitive causes during that observation period. So that should not be reflected in the increased rate of repair that we detected." In addition, the increased use of advanced abdominal imaging has led to the identification of more aneurysms.
The complete manuscript of this presentation is anticipated to be published in the Annals of Surgery pending editorial review.
The authors reported that they have no relevant conflicts of interest.
SAN FRANCISCO – The introduction of endovascular aneurysm repair, or EVAR, has improved outcomes for the repair of intact and ruptured aortic abdominal aneurysms, particularly among those aged 80 years and older, based on a review of more than 400,000 Medicare beneficiaries.
Among patients in this age group, there was an increase in the number of intact abdominal aortic aneurysm (AAA) repairs, coupled with declines in rupture deaths. In those younger than 75 years, intact repair rates decreased about 10%. Those aged 75-79 years had an increase of about 10%. However, those aged 80 years and older had a more dramatic increase of more than 50%. In addition, operative mortality for intact repair has decreased over time for all age groups, although the decline becomes greater with increasing age.
"The greatest benefit [of EVAR] has been seen in those over 80, who had the largest increase in intact repair rates, the largest decline in intact repair mortality, and the largest decline in rupture deaths," Dr. Marc L. Schermerhorn said at the annual meeting of the American Surgical Association.
The researchers used a 100% sample of Medicare data between 1995 and 2008. They identified patients with intact AAAs undergoing repair – by either EVAR or open surgical repair – as well as patients with ruptured AAAs (with and without repair). They determined the 30-day or in-hospital mortality, then standardized the rates (per 100,000 beneficiaries), adjusting for changes in age, sex, and population size over time.
They identified 338,278 intact repairs and 69,653 ruptures (with 47,524 repairs). EVAR use rapidly increased after Food and Drug Administration approval of the procedure in 1999; as of 2008, EVAR was used for 77% of all intact aneurysm repairs.
"There was a more delayed response but an increase nonetheless in the use of EVAR for ruptured aneurysms, to the point where [in 2008] 31% of all ruptured aneurysms are treated with EVAR," said Dr. Schermerhorn. During this time period, the average age of those undergoing repair increased from 73.7 to 75.5 years.
Operative mortality during open intact repair remained steady, at roughly 5%. EVAR mortality dropped from about 2% in 1995 to 1.4% in 2008. The total operative mortality for intact repair (open and EVAR) fell from roughly 5% to 2.4%, despite the fact that there were more repairs in 2008 and the average age of these patients increased.
"As we increase our utilization of EVAR, this is driving down the total mortality of intact aneurysm repair, so that it’s half of what it was at the beginning of that time period," said Dr. Schermerhorn, chief of vascular and endovascular surgery at Beth Israel Deaconess Medical Center in Boston.
The researchers also found that the annual number of ruptures decreased from 6,535 in 1995 to 3,298 in 2008. The greatest decrease in ruptures (repaired or not) was seen in older patients – those 75 years and older – but there was also a decline among patients younger than 75 years. The mortality with open rupture repair remained largely unchanged during the time period (approximately 45%). However, EVAR rupture repair declined from about 45% to 28%. The rate of all rupture repairs also has decreased from about 45% to 36%.
"Rather than suggesting that we’re simply taking the hemodynamically stable patients for EVAR, the best evidence to say that there’s actually a true reduction in mortality is the fact that for the first time in 3 decades, the overall mortality for ruptured aneurysm repair is now well below 40%," Dr. Schermerhorn said. The mortality for all ruptures (repaired or not) has gone down the most dramatically in those aged 80 years and older, although there were declines in the other age groups as well.
Invited discussant Dr. Philip B. Paty noted that reduced rates of intact aneurysm repair and rupture in patients aged 65-74 years may be attributed to a change in the natural history or a decline in the incidence of aneurysms.
"Did you evaluate the age-specific incidence of comorbidities or medical risk over the period of study to see if we are, in fact, dealing with different patient populations? Alternatively, is it possible that repair in recent years was deferred until a larger sac size was present?" he asked.
"We did look at comorbidities over time, and there have been increases in all of the various comorbidities that you would typically associate with aortic aneurysm patients. Coronary artery disease, peripheral arterial disease, hypertension, and [heart failure] all increased. We did not have access to data about rates of cigarette smoking or medication," Dr. Schermerhorn said.
Other studies have shown that the rate of smoking has gone down with time and the use of statins has gone up. There may be better control of hypertension as well. "We think that it’s possible that for those reasons, there may be a decreasing incidence of aortic aneurysm in the United States." Data from the United Kingdom, Sweden, and Australia suggest that there may be a decline in those countries, he added.
Dr. Paty, vice chair of clinical research in the department of surgery at Memorial Sloan-Kettering Cancer Center in New York, also questioned whether the data reflect a change in practice to observing smaller aneurysms, delaying repair.
This may be the case, according to Dr. Schermerhorn. "Are we deferring aneurysms? I would agree completely." Studies have shown "that it’s safe for us to wait until the aneurysms are up in the 5.5-cm range. So I think that a lot of that redistribution of those [younger than 75 years to those just older than 75] may represent that. That should, however, decrease the rate of aneurysm repair that we do, and some patients will die of competitive causes during that observation period. So that should not be reflected in the increased rate of repair that we detected." In addition, the increased use of advanced abdominal imaging has led to the identification of more aneurysms.
The complete manuscript of this presentation is anticipated to be published in the Annals of Surgery pending editorial review.
The authors reported that they have no relevant conflicts of interest.
FROM THE ANNUAL MEETING OF THE AMERICAN SURGICAL ASSOCIATION
Major Finding: The number of intact abdominal aortic aneurysm repairs increased more than 50% in patients aged 80 years and older. Likewise, the largest improvements in mortality with intact repair were seen in the older age groups; the greatest decrease in ruptures (repaired or not) was seen in older patients.
Data Source: The researchers identified Medicare patients with intact (338,278) or ruptured (69,653) AAAs undergoing repair between 1995 and 2008.
Disclosures: The authors reported that they have no relevant conflicts of interest.
Less Vascular Care Tied to More Amputations
NATIONAL HARBOR, MD. – Lower-extremity revascularization can be effective in preventing amputation in peripheral arterial disease, but in some regions of the United States, the amount and intensity of vascular care is inversely related to the amputation rate, a large study of Medicare patients indicates.
To examine the relationship between intensity of vascular care and the risk of amputation, Dr. Philip P. Goodney and his colleagues at Dartmouth-Hitchcock Medical Center and the Dartmouth Institute for Health Policy in Lebanon, N.H., studied all open and endovascular revascularizations provided to 20,464 Medicare patients in the year prior to vascular amputation. They examined associations among patient characteristics, the regional rates of revascularization, and the regional amputation rate among the 307 hospital referral regions, as described in the Dartmouth Atlas of Health Care.
Population-based amputation rates varied across regions, from fewer than 1 to more than 44 amputations per 10,000 Medicare patients. Amputation rates were highest in rural regions of the southern and Appalachian United States, Dr. Goodney said at the Vascular Annual Meeting.
Patients in regions with high amputation rates were more commonly African American than were patients in regions with low amputation rates (50% vs. 12%). Furthermore, those in regions with high amputation rates had lower per-capita income, compared with those in regions with low amputation rates ($17,980 vs. $19,545).
Less vascular care was provided to patients who lived where amputation rates were highest. Those patients had 57% fewer therapeutic revascularization procedures (such as bypass surgery or stent placement) than did patients in regions with low amputation rates (2.2 vs. 4.8 revascularizations per amputation). Even the number of diagnostic angiograms was significantly lower in high amputation regions than in low amputation regions (2.4 vs. 5.0 angiograms per amputation).
"Medicare patients living in regions with the highest amputation rate are commonly poor and African American, and they receive less than half as much vascular care as those in regions with lower burdens of vascular disease," said Dr. Goodney. "In these regions, we believe patients commonly present late in their disease process, with ‘unsalvageable’ limbs. Because of wet gangrene or massive tissue loss, it is often too late for vascular care to matter, and surgeons are forced to simply perform an amputation rather than have the opportunity to revascularize and try to save a patient’s leg. However, we hope that our study will help to limit amputations in the future," Dr. Goodney said.
"Our work provides a ‘blueprint’ for improvement, by targeting the regions of the [United States] where early, integrated efforts to prevent amputation – medical, podiatric, and vascular – have the biggest potential to make a difference," he added.
Dr. Goodney reported no relevant disclosures.
NATIONAL HARBOR, MD. – Lower-extremity revascularization can be effective in preventing amputation in peripheral arterial disease, but in some regions of the United States, the amount and intensity of vascular care is inversely related to the amputation rate, a large study of Medicare patients indicates.
To examine the relationship between intensity of vascular care and the risk of amputation, Dr. Philip P. Goodney and his colleagues at Dartmouth-Hitchcock Medical Center and the Dartmouth Institute for Health Policy in Lebanon, N.H., studied all open and endovascular revascularizations provided to 20,464 Medicare patients in the year prior to vascular amputation. They examined associations among patient characteristics, the regional rates of revascularization, and the regional amputation rate among the 307 hospital referral regions, as described in the Dartmouth Atlas of Health Care.
Population-based amputation rates varied across regions, from fewer than 1 to more than 44 amputations per 10,000 Medicare patients. Amputation rates were highest in rural regions of the southern and Appalachian United States, Dr. Goodney said at the Vascular Annual Meeting.
Patients in regions with high amputation rates were more commonly African American than were patients in regions with low amputation rates (50% vs. 12%). Furthermore, those in regions with high amputation rates had lower per-capita income, compared with those in regions with low amputation rates ($17,980 vs. $19,545).
Less vascular care was provided to patients who lived where amputation rates were highest. Those patients had 57% fewer therapeutic revascularization procedures (such as bypass surgery or stent placement) than did patients in regions with low amputation rates (2.2 vs. 4.8 revascularizations per amputation). Even the number of diagnostic angiograms was significantly lower in high amputation regions than in low amputation regions (2.4 vs. 5.0 angiograms per amputation).
"Medicare patients living in regions with the highest amputation rate are commonly poor and African American, and they receive less than half as much vascular care as those in regions with lower burdens of vascular disease," said Dr. Goodney. "In these regions, we believe patients commonly present late in their disease process, with ‘unsalvageable’ limbs. Because of wet gangrene or massive tissue loss, it is often too late for vascular care to matter, and surgeons are forced to simply perform an amputation rather than have the opportunity to revascularize and try to save a patient’s leg. However, we hope that our study will help to limit amputations in the future," Dr. Goodney said.
"Our work provides a ‘blueprint’ for improvement, by targeting the regions of the [United States] where early, integrated efforts to prevent amputation – medical, podiatric, and vascular – have the biggest potential to make a difference," he added.
Dr. Goodney reported no relevant disclosures.
NATIONAL HARBOR, MD. – Lower-extremity revascularization can be effective in preventing amputation in peripheral arterial disease, but in some regions of the United States, the amount and intensity of vascular care is inversely related to the amputation rate, a large study of Medicare patients indicates.
To examine the relationship between intensity of vascular care and the risk of amputation, Dr. Philip P. Goodney and his colleagues at Dartmouth-Hitchcock Medical Center and the Dartmouth Institute for Health Policy in Lebanon, N.H., studied all open and endovascular revascularizations provided to 20,464 Medicare patients in the year prior to vascular amputation. They examined associations among patient characteristics, the regional rates of revascularization, and the regional amputation rate among the 307 hospital referral regions, as described in the Dartmouth Atlas of Health Care.
Population-based amputation rates varied across regions, from fewer than 1 to more than 44 amputations per 10,000 Medicare patients. Amputation rates were highest in rural regions of the southern and Appalachian United States, Dr. Goodney said at the Vascular Annual Meeting.
Patients in regions with high amputation rates were more commonly African American than were patients in regions with low amputation rates (50% vs. 12%). Furthermore, those in regions with high amputation rates had lower per-capita income, compared with those in regions with low amputation rates ($17,980 vs. $19,545).
Less vascular care was provided to patients who lived where amputation rates were highest. Those patients had 57% fewer therapeutic revascularization procedures (such as bypass surgery or stent placement) than did patients in regions with low amputation rates (2.2 vs. 4.8 revascularizations per amputation). Even the number of diagnostic angiograms was significantly lower in high amputation regions than in low amputation regions (2.4 vs. 5.0 angiograms per amputation).
"Medicare patients living in regions with the highest amputation rate are commonly poor and African American, and they receive less than half as much vascular care as those in regions with lower burdens of vascular disease," said Dr. Goodney. "In these regions, we believe patients commonly present late in their disease process, with ‘unsalvageable’ limbs. Because of wet gangrene or massive tissue loss, it is often too late for vascular care to matter, and surgeons are forced to simply perform an amputation rather than have the opportunity to revascularize and try to save a patient’s leg. However, we hope that our study will help to limit amputations in the future," Dr. Goodney said.
"Our work provides a ‘blueprint’ for improvement, by targeting the regions of the [United States] where early, integrated efforts to prevent amputation – medical, podiatric, and vascular – have the biggest potential to make a difference," he added.
Dr. Goodney reported no relevant disclosures.
FROM THE VASCULAR ANNUAL MEETING
Major Finding: Patients in the highest amputation regions had 57% fewer therapeutic revascularization procedures (such as bypass surgery or stent placement) than did patients in regions with low amputation rates (2.2 vs. 4.8 revascularizations per amputation).
Data Source: The researchers reviewed the database of all open and endovascular revascularizations provided to 20,464 Medicare patients in the year prior to vascular amputation.
Disclosures: Dr. Goodney reported no relevant disclosures.
Research Reveals Predictors of Ischemic Colitis
The high mortality rate among elderly patients who develop ischemic colitis after hybrid endovascular repair of complex aortic aneurysms suggests that a different approach may be warranted, judging by operative outcomes in more than 200 patients.
Patient survival at 1 year was significantly decreased among patients who had ischemic colitis, compared with those who did not have this complication (51% vs. 79%), Dr. Carlos H. Timaran reported at the Vascular Annual Meeting in National Harbor, Md.
Dr. Timaran and his colleagues at the University of Texas Southwestern Medical Center, Dallas, studied the frequency, predictors, and outcomes of ischemic colitis after abdominal debranching combined with aortic stent grafts (ADSG), which was the approach used to treat pararenal and thoracoabdominal aortic aneurysms (TAAs).
They reviewed clinical data in the North American Complex Abdominal Aortic Debranching (NACAAD) Registry of 208 patients treated by ADSG in 13 North American academic centers between 1999 and 2010. Ischemic colitis was identified by colonoscopy and/or operative findings. End points included the need for colon resection, morbidity, and mortality.
The researchers used univariate and multivariate logistic regression analysis to identify predictive factors for ischemic colitis.
Of the 208 patients, 118 men and 90 women (mean age, 72 ± 10 years) were treated for 45 pararenal aneurysms and 163 TAAs.
ADSG required reconstruction of 468 vessels (2.8 per patient), done in a single stage in 92 patients (44%). Ischemic colitis occurred in 13 patients (6%), and four patients (2%) developed transmural necrosis that required colon resection.
The 30-day mortality was 14% for the entire cohort. According to the investigators, this rate was significantly higher among patients who had ischemic colitis (46% vs. 12%; P less than .05), including those who required colon resection (50%).
Univariate analysis found that significantly higher rates of ischemic colitis were associated with age, Society for Vascular Surgery comorbidity score, chronic kidney disease, ruptured or symptomatic aneurysm, and whether patients had undergone a single-stage operation.
Independent predictors for ischemic colitis included age (odds ratio, 1.12), Society for Vascular Surgery comorbidity score (OR, 1.02), and single-stage operation (OR, 1.3).
"In elderly sicker patients, it appears that a staged approach or another treatment strategy, such as fenestrated or branched endovascular repair, may be better alternatives," Dr. Timaran concluded.
Dr. Timaran disclosed that he has received consulting fees or other remuneration from W.L. Gore & Associates.
The high mortality rate among elderly patients who develop ischemic colitis after hybrid endovascular repair of complex aortic aneurysms suggests that a different approach may be warranted, judging by operative outcomes in more than 200 patients.
Patient survival at 1 year was significantly decreased among patients who had ischemic colitis, compared with those who did not have this complication (51% vs. 79%), Dr. Carlos H. Timaran reported at the Vascular Annual Meeting in National Harbor, Md.
Dr. Timaran and his colleagues at the University of Texas Southwestern Medical Center, Dallas, studied the frequency, predictors, and outcomes of ischemic colitis after abdominal debranching combined with aortic stent grafts (ADSG), which was the approach used to treat pararenal and thoracoabdominal aortic aneurysms (TAAs).
They reviewed clinical data in the North American Complex Abdominal Aortic Debranching (NACAAD) Registry of 208 patients treated by ADSG in 13 North American academic centers between 1999 and 2010. Ischemic colitis was identified by colonoscopy and/or operative findings. End points included the need for colon resection, morbidity, and mortality.
The researchers used univariate and multivariate logistic regression analysis to identify predictive factors for ischemic colitis.
Of the 208 patients, 118 men and 90 women (mean age, 72 ± 10 years) were treated for 45 pararenal aneurysms and 163 TAAs.
ADSG required reconstruction of 468 vessels (2.8 per patient), done in a single stage in 92 patients (44%). Ischemic colitis occurred in 13 patients (6%), and four patients (2%) developed transmural necrosis that required colon resection.
The 30-day mortality was 14% for the entire cohort. According to the investigators, this rate was significantly higher among patients who had ischemic colitis (46% vs. 12%; P less than .05), including those who required colon resection (50%).
Univariate analysis found that significantly higher rates of ischemic colitis were associated with age, Society for Vascular Surgery comorbidity score, chronic kidney disease, ruptured or symptomatic aneurysm, and whether patients had undergone a single-stage operation.
Independent predictors for ischemic colitis included age (odds ratio, 1.12), Society for Vascular Surgery comorbidity score (OR, 1.02), and single-stage operation (OR, 1.3).
"In elderly sicker patients, it appears that a staged approach or another treatment strategy, such as fenestrated or branched endovascular repair, may be better alternatives," Dr. Timaran concluded.
Dr. Timaran disclosed that he has received consulting fees or other remuneration from W.L. Gore & Associates.
The high mortality rate among elderly patients who develop ischemic colitis after hybrid endovascular repair of complex aortic aneurysms suggests that a different approach may be warranted, judging by operative outcomes in more than 200 patients.
Patient survival at 1 year was significantly decreased among patients who had ischemic colitis, compared with those who did not have this complication (51% vs. 79%), Dr. Carlos H. Timaran reported at the Vascular Annual Meeting in National Harbor, Md.
Dr. Timaran and his colleagues at the University of Texas Southwestern Medical Center, Dallas, studied the frequency, predictors, and outcomes of ischemic colitis after abdominal debranching combined with aortic stent grafts (ADSG), which was the approach used to treat pararenal and thoracoabdominal aortic aneurysms (TAAs).
They reviewed clinical data in the North American Complex Abdominal Aortic Debranching (NACAAD) Registry of 208 patients treated by ADSG in 13 North American academic centers between 1999 and 2010. Ischemic colitis was identified by colonoscopy and/or operative findings. End points included the need for colon resection, morbidity, and mortality.
The researchers used univariate and multivariate logistic regression analysis to identify predictive factors for ischemic colitis.
Of the 208 patients, 118 men and 90 women (mean age, 72 ± 10 years) were treated for 45 pararenal aneurysms and 163 TAAs.
ADSG required reconstruction of 468 vessels (2.8 per patient), done in a single stage in 92 patients (44%). Ischemic colitis occurred in 13 patients (6%), and four patients (2%) developed transmural necrosis that required colon resection.
The 30-day mortality was 14% for the entire cohort. According to the investigators, this rate was significantly higher among patients who had ischemic colitis (46% vs. 12%; P less than .05), including those who required colon resection (50%).
Univariate analysis found that significantly higher rates of ischemic colitis were associated with age, Society for Vascular Surgery comorbidity score, chronic kidney disease, ruptured or symptomatic aneurysm, and whether patients had undergone a single-stage operation.
Independent predictors for ischemic colitis included age (odds ratio, 1.12), Society for Vascular Surgery comorbidity score (OR, 1.02), and single-stage operation (OR, 1.3).
"In elderly sicker patients, it appears that a staged approach or another treatment strategy, such as fenestrated or branched endovascular repair, may be better alternatives," Dr. Timaran concluded.
Dr. Timaran disclosed that he has received consulting fees or other remuneration from W.L. Gore & Associates.
FROM THE VASCULAR ANNUAL MEETING
Major Finding: Patient survival at 1 year was significantly lower among patients who had ischemic colitis, compared with those who did not have this complication (51% vs. 79%).
Data Source: The researchers reviewed clinical data in the North American Complex Abdominal Aortic Debranching Registry of 208 patients treated in 13 North American academic centers between 1999 and 2010.
Disclosures: Dr. Timaran disclosed that he has received consulting fees or other remuneration from W.L. Gore & Associates.
U.S. Medicare Leg Amputations Down From 2000 to 2008
CHICAGO – Leg and foot amputations for elderly U.S. patients with peripheral artery disease dropped by more than half during the period 2000-2008 for unknown reasons, based on a review of Medicare data.
During the 9-year period studied, the rate of lower-extremity amputations among U.S. Medicare beneficiaries fell from 9,650/100,000 beneficiaries with peripheral artery disease (PAD) to 4,274/100,000, Dr. W. Schuyler Jones and his associates reported in a poster at the meeting.
Their analysis of the 2000-2008 Medicare data also showed significant regional variations in lower-extremity amputation rates in PAD patients, with the highest rate in New England and the lowest rate in the East North Central region (Illinois, Indiana, Michigan, Ohio, and Wisconsin). Again, no clear explanation exists for this pattern, said Dr. Jones, an interventional cardiologist at Duke University in Durham, N.C., and his associates.
Their analysis of data from the Centers for Medicare & Medicaid Services included 3,354,264 diagnosed with PAD during 2000-2008, of whom 249,310 (7%) underwent amputation of a foot or leg, either below or above the knee. The average age of all PAD patients was 77 years old among both those who underwent amputations and those who did not have this surgery. The analysis identified four factors that were significant, independent predictors of having an amputation: male sex, African American race, and the presence of renal disease or diabetes.
During the 9-year period, the annual incidence of patients newly diagnosed with PAD remained relatively steady, with 398,000 diagnosed in 2000 and 397,000 diagnosed in 2008. The rates showed some year-to-year fluctuation, ranging from a low of 304,000 in 2003 to a high of 517,000 in 2006.
In contrast, the annual incidence of lower-extremity amputations showed a clear downward trajectory, with a sharp drop starting in 2005. During 2000-2004, the annual amputation rate hovered at about 10,000 cases/100,000 patients with PAD, but then fell to 7,455/100,000 in 2005, and fell further to 4,261/100,000 in 2006. During the subsequent 2 years the rate remained at about the same low level first reached in 2006. Roughly similar patterns existed for the subgroups of patients who underwent above-the-knee amputations, below-the-knee amputations, or foot amputations.
The authors of the report performed an analysis of amputation rates by U.S. Census geographic regions for the entire period of 2000-2008 that they adjusted by regional differences in patients’ age, sex, race, comorbidities, and year of amputation. They set the amputation rate in the South Atlantic region as their reference level, and found four regions with significantly higher rates of amputations: New England ran 16% higher, the West South Central region (Arkansas, Louisiana, Oklahoma, and Texas) ran 12% higher, Mid Atlantic (Connecticut, New Jersey, New York, and Pennsylvania) was 8% higher, and the Pacific region (Alaska, California, Hawaii, Oregon, and Washington) was 3% above the reference level. Three other U.S. regions had amputations rates below the reference level, headed by the East North Central which ran 11% below the South Atlantic region, followed by the Mountain region at 10% lower, and the West North Central region (Iowa, Kansas, Minnesota, Missouri, Nebraska, North Dakota, and South Dakota) which was 5% below the reference level.
Dr. Jones said that he had no disclosures.
CHICAGO – Leg and foot amputations for elderly U.S. patients with peripheral artery disease dropped by more than half during the period 2000-2008 for unknown reasons, based on a review of Medicare data.
During the 9-year period studied, the rate of lower-extremity amputations among U.S. Medicare beneficiaries fell from 9,650/100,000 beneficiaries with peripheral artery disease (PAD) to 4,274/100,000, Dr. W. Schuyler Jones and his associates reported in a poster at the meeting.
Their analysis of the 2000-2008 Medicare data also showed significant regional variations in lower-extremity amputation rates in PAD patients, with the highest rate in New England and the lowest rate in the East North Central region (Illinois, Indiana, Michigan, Ohio, and Wisconsin). Again, no clear explanation exists for this pattern, said Dr. Jones, an interventional cardiologist at Duke University in Durham, N.C., and his associates.
Their analysis of data from the Centers for Medicare & Medicaid Services included 3,354,264 diagnosed with PAD during 2000-2008, of whom 249,310 (7%) underwent amputation of a foot or leg, either below or above the knee. The average age of all PAD patients was 77 years old among both those who underwent amputations and those who did not have this surgery. The analysis identified four factors that were significant, independent predictors of having an amputation: male sex, African American race, and the presence of renal disease or diabetes.
During the 9-year period, the annual incidence of patients newly diagnosed with PAD remained relatively steady, with 398,000 diagnosed in 2000 and 397,000 diagnosed in 2008. The rates showed some year-to-year fluctuation, ranging from a low of 304,000 in 2003 to a high of 517,000 in 2006.
In contrast, the annual incidence of lower-extremity amputations showed a clear downward trajectory, with a sharp drop starting in 2005. During 2000-2004, the annual amputation rate hovered at about 10,000 cases/100,000 patients with PAD, but then fell to 7,455/100,000 in 2005, and fell further to 4,261/100,000 in 2006. During the subsequent 2 years the rate remained at about the same low level first reached in 2006. Roughly similar patterns existed for the subgroups of patients who underwent above-the-knee amputations, below-the-knee amputations, or foot amputations.
The authors of the report performed an analysis of amputation rates by U.S. Census geographic regions for the entire period of 2000-2008 that they adjusted by regional differences in patients’ age, sex, race, comorbidities, and year of amputation. They set the amputation rate in the South Atlantic region as their reference level, and found four regions with significantly higher rates of amputations: New England ran 16% higher, the West South Central region (Arkansas, Louisiana, Oklahoma, and Texas) ran 12% higher, Mid Atlantic (Connecticut, New Jersey, New York, and Pennsylvania) was 8% higher, and the Pacific region (Alaska, California, Hawaii, Oregon, and Washington) was 3% above the reference level. Three other U.S. regions had amputations rates below the reference level, headed by the East North Central which ran 11% below the South Atlantic region, followed by the Mountain region at 10% lower, and the West North Central region (Iowa, Kansas, Minnesota, Missouri, Nebraska, North Dakota, and South Dakota) which was 5% below the reference level.
Dr. Jones said that he had no disclosures.
CHICAGO – Leg and foot amputations for elderly U.S. patients with peripheral artery disease dropped by more than half during the period 2000-2008 for unknown reasons, based on a review of Medicare data.
During the 9-year period studied, the rate of lower-extremity amputations among U.S. Medicare beneficiaries fell from 9,650/100,000 beneficiaries with peripheral artery disease (PAD) to 4,274/100,000, Dr. W. Schuyler Jones and his associates reported in a poster at the meeting.
Their analysis of the 2000-2008 Medicare data also showed significant regional variations in lower-extremity amputation rates in PAD patients, with the highest rate in New England and the lowest rate in the East North Central region (Illinois, Indiana, Michigan, Ohio, and Wisconsin). Again, no clear explanation exists for this pattern, said Dr. Jones, an interventional cardiologist at Duke University in Durham, N.C., and his associates.
Their analysis of data from the Centers for Medicare & Medicaid Services included 3,354,264 diagnosed with PAD during 2000-2008, of whom 249,310 (7%) underwent amputation of a foot or leg, either below or above the knee. The average age of all PAD patients was 77 years old among both those who underwent amputations and those who did not have this surgery. The analysis identified four factors that were significant, independent predictors of having an amputation: male sex, African American race, and the presence of renal disease or diabetes.
During the 9-year period, the annual incidence of patients newly diagnosed with PAD remained relatively steady, with 398,000 diagnosed in 2000 and 397,000 diagnosed in 2008. The rates showed some year-to-year fluctuation, ranging from a low of 304,000 in 2003 to a high of 517,000 in 2006.
In contrast, the annual incidence of lower-extremity amputations showed a clear downward trajectory, with a sharp drop starting in 2005. During 2000-2004, the annual amputation rate hovered at about 10,000 cases/100,000 patients with PAD, but then fell to 7,455/100,000 in 2005, and fell further to 4,261/100,000 in 2006. During the subsequent 2 years the rate remained at about the same low level first reached in 2006. Roughly similar patterns existed for the subgroups of patients who underwent above-the-knee amputations, below-the-knee amputations, or foot amputations.
The authors of the report performed an analysis of amputation rates by U.S. Census geographic regions for the entire period of 2000-2008 that they adjusted by regional differences in patients’ age, sex, race, comorbidities, and year of amputation. They set the amputation rate in the South Atlantic region as their reference level, and found four regions with significantly higher rates of amputations: New England ran 16% higher, the West South Central region (Arkansas, Louisiana, Oklahoma, and Texas) ran 12% higher, Mid Atlantic (Connecticut, New Jersey, New York, and Pennsylvania) was 8% higher, and the Pacific region (Alaska, California, Hawaii, Oregon, and Washington) was 3% above the reference level. Three other U.S. regions had amputations rates below the reference level, headed by the East North Central which ran 11% below the South Atlantic region, followed by the Mountain region at 10% lower, and the West North Central region (Iowa, Kansas, Minnesota, Missouri, Nebraska, North Dakota, and South Dakota) which was 5% below the reference level.
Dr. Jones said that he had no disclosures.
FROM THE ANNUAL MEETING OF THE AMERICAN COLLEGE OF CARDIOLOGY
Major Finding: Lower-extremity amputations among Medicare beneficiaries with PAD dropped from 9,650/100,000 in 2000 to 4,274/100,000 in 2008.
Data Source: Data came from an analysis of U.S. Medicare beneficiaries during 2000-2008 using information compiled by the Centers for Medicare & Medicaid Services.
Disclosures: Dr. Jones said that he had no disclosures.
Community Hospital Offers Catheter-Directed Thrombolysis
With the advantage of around-the-clock hospitalist services, one community hospital has been able to offer catheter-directed thrombolytic therapy – a procedure typically available only at tertiary-care hospitals.
Dr. Brian A. Carpenter, medical director of the adult hospitalist service at Shady Grove Adventist Hospital in Rockville, Md., said that at least 12 patients with massive or submassive pulmonary embolisms have undergone catheter-directed thrombolysis at the hospital. Many others have undergone the procedure for deep vein thrombosis in a lower extremity with vascular extension into the larger vessels of the pelvis.
Before the service was introduced, patients with massive or submassive pulmonary embolism were sent to a tertiary-care hospital if they were stable enough for transfer. Otherwise, they were monitored in the ICU, managed medically with systemic anticoagulation, and transferred later if they still needed further treatment.
Systemic anticoagulation is the mainstay of treatment for pulmonary embolism, but the American Heart Association and the American College of Chest Physicians recommend a more aggressive approach for massive and submassive PE. Up to 60% of patients with massive PE die, data suggest, with two-thirds of the deaths occurring in the first hour after the embolus forms. Within 30 days after submassive PE, 15%-20% of patients die secondary to pulmonary hypertension and subsequent cor pulmonale, according to a data presented at the annual meeting of the Southern Association for Vascular Surgery.
The catheter-directed thrombolytic therapy at Shady Grove Adventist largely owes its success to a vascular surgeon who took the lead in establishing protocols and training support staff, with hospitalists as a key part of the team.
The vascular surgeon, Dr. Jeffrey Y. Wang, reported on the outcomes of the first 12 patients, in whom the procedures were all technically successful. One patient developed hemodynamically significant bradycardia, but all patients were off supplemental oxygen within 24 hours of the procedure. There were no bleeding complications.
One patient died 14 hours after the procedure, most likely because of a paradoxical embolus to the intestine. The 11 surviving patients were discharged to home within 48 hours of the intervention, according to Dr. Wang of Horizon Vascular Specialists. The group contracts to provide vascular surgical care at Shady Grove Adventist and two other hospitals in Maryland.
Catheter-directed thrombolytic therapy can shorten stays in the ICU and the hospital, reduce or eliminate the need for home oxygen therapy, and help restore right heart function in patients with massive or submassive pulmonary embolism, Dr. Wang said. Patients with massive or submassive PE are offered catheter-directed thrombolytic therapy if they are hemodynamically unstable; if they have right heart dysfunction, elevated troponin, or pulmonary artery pressures greater than 70 mmHg; or if they are not weaning off intubation for oxygen within 5 days, Dr. Wang said. He excludes patients who are actively bleeding or who are not able to tolerate any systemic anticoagulation.
Recent surgery was not a disqualifying factor in his case series. "Typically, those patients were orthopedic in nature, with a hip or knee replacement," Dr. Wang said. The patient would develop a big pulmonary embolus, and the orthopedist would give a green light for aggressive treatment.
But most of the patients who have received catheter-directed thrombolytic procedures presented to the emergency department with lower extremity swelling, and were found on sonography to have a thrombus extending into the large pelvic vessels. Dr. Carpenter’s service admits approximately 90% of adult inpatients, so the hospitalists usually are the ones to determine which patients should be considered for the interventional approach and which ones get medical therapy.
Dr. Wang emphasized that the protocols are as important as technical expertise in catheter-directed thrombolysis. Protocols are in place for the ED, the ICU, and the hospitalist team for the early detection of DVT and pulmonary emboli, notification of the appropriate staff, and posttreatment care of patients. Dr. Wang also took the lead on the anticoagulation aspect of computerized physician order entry.
"In our institution, we use the same protocols for call-in and transport to the cath lab as for ST-elevation myocardial infarction, which allows us to get the patient up and into the fluoroscopy suite within 30 minutes," Dr. Wang said. The fluoroscopy suite must be available on an emergency basis.
"It’s amazing to see such a dramatic improvement in patient symptoms almost immediately post procedure," said Dr. Carpenter.
Patients who undergo catheter-directed thrombolysis require close postoperative monitoring, said Dr. Carpenter, who is with Inpatient Specialists, a group that contracts with hospitals to provide hospitalist services. The main risks are bleeding, low blood pressure, or respiratory distress. "The bleeding might not necessarily be obvious," as the antithrombotic agent is given locally and bleeding can be local as well.
Community hospitals that offer catheter-directed thrombolysis need sufficient commitment from vascular surgeons and robust postprocedure support, Dr. Carpenter said. The vascular surgery group should be able to offer the procedure to all patients who need it.
Around-the-clock hospitalist availability is a good idea, especially if the surgeon is not on the in-house staff. "Don’t do [this procedure] if the hospitalist is providing triage services at night," he advised.
With 23 full-time positions (translating into 40 full-time or part-time physicians), the adult hospitalist service at Shady Grove Adventist, a 339-bed hospital, typically provides 10 hospitalists during weekdays (including one medical-psychiatric physician) and 8 on weekend days.
Besides the adult hospitalist group, the hospital has a pediatric hospitalist service, 24-hour in-house ICU hospitalist coverage, and a surgical hospitalist group. "Not many hospitals have surgical hospitalists," Dr. Carpenter noted. Laborists also are available 24 hours a day for in-house ob.gyn. consultations.
Dr. Carpenter, who has been a hospitalist since 2006, said that this "is how hospital-based medicine is progressing. "Shady Grove has been an early adopter" of expanded hospitalist services.
Dr. Carpenter and Dr. Wang reported having no financial disclosures.
With the advantage of around-the-clock hospitalist services, one community hospital has been able to offer catheter-directed thrombolytic therapy – a procedure typically available only at tertiary-care hospitals.
Dr. Brian A. Carpenter, medical director of the adult hospitalist service at Shady Grove Adventist Hospital in Rockville, Md., said that at least 12 patients with massive or submassive pulmonary embolisms have undergone catheter-directed thrombolysis at the hospital. Many others have undergone the procedure for deep vein thrombosis in a lower extremity with vascular extension into the larger vessels of the pelvis.
Before the service was introduced, patients with massive or submassive pulmonary embolism were sent to a tertiary-care hospital if they were stable enough for transfer. Otherwise, they were monitored in the ICU, managed medically with systemic anticoagulation, and transferred later if they still needed further treatment.
Systemic anticoagulation is the mainstay of treatment for pulmonary embolism, but the American Heart Association and the American College of Chest Physicians recommend a more aggressive approach for massive and submassive PE. Up to 60% of patients with massive PE die, data suggest, with two-thirds of the deaths occurring in the first hour after the embolus forms. Within 30 days after submassive PE, 15%-20% of patients die secondary to pulmonary hypertension and subsequent cor pulmonale, according to a data presented at the annual meeting of the Southern Association for Vascular Surgery.
The catheter-directed thrombolytic therapy at Shady Grove Adventist largely owes its success to a vascular surgeon who took the lead in establishing protocols and training support staff, with hospitalists as a key part of the team.
The vascular surgeon, Dr. Jeffrey Y. Wang, reported on the outcomes of the first 12 patients, in whom the procedures were all technically successful. One patient developed hemodynamically significant bradycardia, but all patients were off supplemental oxygen within 24 hours of the procedure. There were no bleeding complications.
One patient died 14 hours after the procedure, most likely because of a paradoxical embolus to the intestine. The 11 surviving patients were discharged to home within 48 hours of the intervention, according to Dr. Wang of Horizon Vascular Specialists. The group contracts to provide vascular surgical care at Shady Grove Adventist and two other hospitals in Maryland.
Catheter-directed thrombolytic therapy can shorten stays in the ICU and the hospital, reduce or eliminate the need for home oxygen therapy, and help restore right heart function in patients with massive or submassive pulmonary embolism, Dr. Wang said. Patients with massive or submassive PE are offered catheter-directed thrombolytic therapy if they are hemodynamically unstable; if they have right heart dysfunction, elevated troponin, or pulmonary artery pressures greater than 70 mmHg; or if they are not weaning off intubation for oxygen within 5 days, Dr. Wang said. He excludes patients who are actively bleeding or who are not able to tolerate any systemic anticoagulation.
Recent surgery was not a disqualifying factor in his case series. "Typically, those patients were orthopedic in nature, with a hip or knee replacement," Dr. Wang said. The patient would develop a big pulmonary embolus, and the orthopedist would give a green light for aggressive treatment.
But most of the patients who have received catheter-directed thrombolytic procedures presented to the emergency department with lower extremity swelling, and were found on sonography to have a thrombus extending into the large pelvic vessels. Dr. Carpenter’s service admits approximately 90% of adult inpatients, so the hospitalists usually are the ones to determine which patients should be considered for the interventional approach and which ones get medical therapy.
Dr. Wang emphasized that the protocols are as important as technical expertise in catheter-directed thrombolysis. Protocols are in place for the ED, the ICU, and the hospitalist team for the early detection of DVT and pulmonary emboli, notification of the appropriate staff, and posttreatment care of patients. Dr. Wang also took the lead on the anticoagulation aspect of computerized physician order entry.
"In our institution, we use the same protocols for call-in and transport to the cath lab as for ST-elevation myocardial infarction, which allows us to get the patient up and into the fluoroscopy suite within 30 minutes," Dr. Wang said. The fluoroscopy suite must be available on an emergency basis.
"It’s amazing to see such a dramatic improvement in patient symptoms almost immediately post procedure," said Dr. Carpenter.
Patients who undergo catheter-directed thrombolysis require close postoperative monitoring, said Dr. Carpenter, who is with Inpatient Specialists, a group that contracts with hospitals to provide hospitalist services. The main risks are bleeding, low blood pressure, or respiratory distress. "The bleeding might not necessarily be obvious," as the antithrombotic agent is given locally and bleeding can be local as well.
Community hospitals that offer catheter-directed thrombolysis need sufficient commitment from vascular surgeons and robust postprocedure support, Dr. Carpenter said. The vascular surgery group should be able to offer the procedure to all patients who need it.
Around-the-clock hospitalist availability is a good idea, especially if the surgeon is not on the in-house staff. "Don’t do [this procedure] if the hospitalist is providing triage services at night," he advised.
With 23 full-time positions (translating into 40 full-time or part-time physicians), the adult hospitalist service at Shady Grove Adventist, a 339-bed hospital, typically provides 10 hospitalists during weekdays (including one medical-psychiatric physician) and 8 on weekend days.
Besides the adult hospitalist group, the hospital has a pediatric hospitalist service, 24-hour in-house ICU hospitalist coverage, and a surgical hospitalist group. "Not many hospitals have surgical hospitalists," Dr. Carpenter noted. Laborists also are available 24 hours a day for in-house ob.gyn. consultations.
Dr. Carpenter, who has been a hospitalist since 2006, said that this "is how hospital-based medicine is progressing. "Shady Grove has been an early adopter" of expanded hospitalist services.
Dr. Carpenter and Dr. Wang reported having no financial disclosures.
With the advantage of around-the-clock hospitalist services, one community hospital has been able to offer catheter-directed thrombolytic therapy – a procedure typically available only at tertiary-care hospitals.
Dr. Brian A. Carpenter, medical director of the adult hospitalist service at Shady Grove Adventist Hospital in Rockville, Md., said that at least 12 patients with massive or submassive pulmonary embolisms have undergone catheter-directed thrombolysis at the hospital. Many others have undergone the procedure for deep vein thrombosis in a lower extremity with vascular extension into the larger vessels of the pelvis.
Before the service was introduced, patients with massive or submassive pulmonary embolism were sent to a tertiary-care hospital if they were stable enough for transfer. Otherwise, they were monitored in the ICU, managed medically with systemic anticoagulation, and transferred later if they still needed further treatment.
Systemic anticoagulation is the mainstay of treatment for pulmonary embolism, but the American Heart Association and the American College of Chest Physicians recommend a more aggressive approach for massive and submassive PE. Up to 60% of patients with massive PE die, data suggest, with two-thirds of the deaths occurring in the first hour after the embolus forms. Within 30 days after submassive PE, 15%-20% of patients die secondary to pulmonary hypertension and subsequent cor pulmonale, according to a data presented at the annual meeting of the Southern Association for Vascular Surgery.
The catheter-directed thrombolytic therapy at Shady Grove Adventist largely owes its success to a vascular surgeon who took the lead in establishing protocols and training support staff, with hospitalists as a key part of the team.
The vascular surgeon, Dr. Jeffrey Y. Wang, reported on the outcomes of the first 12 patients, in whom the procedures were all technically successful. One patient developed hemodynamically significant bradycardia, but all patients were off supplemental oxygen within 24 hours of the procedure. There were no bleeding complications.
One patient died 14 hours after the procedure, most likely because of a paradoxical embolus to the intestine. The 11 surviving patients were discharged to home within 48 hours of the intervention, according to Dr. Wang of Horizon Vascular Specialists. The group contracts to provide vascular surgical care at Shady Grove Adventist and two other hospitals in Maryland.
Catheter-directed thrombolytic therapy can shorten stays in the ICU and the hospital, reduce or eliminate the need for home oxygen therapy, and help restore right heart function in patients with massive or submassive pulmonary embolism, Dr. Wang said. Patients with massive or submassive PE are offered catheter-directed thrombolytic therapy if they are hemodynamically unstable; if they have right heart dysfunction, elevated troponin, or pulmonary artery pressures greater than 70 mmHg; or if they are not weaning off intubation for oxygen within 5 days, Dr. Wang said. He excludes patients who are actively bleeding or who are not able to tolerate any systemic anticoagulation.
Recent surgery was not a disqualifying factor in his case series. "Typically, those patients were orthopedic in nature, with a hip or knee replacement," Dr. Wang said. The patient would develop a big pulmonary embolus, and the orthopedist would give a green light for aggressive treatment.
But most of the patients who have received catheter-directed thrombolytic procedures presented to the emergency department with lower extremity swelling, and were found on sonography to have a thrombus extending into the large pelvic vessels. Dr. Carpenter’s service admits approximately 90% of adult inpatients, so the hospitalists usually are the ones to determine which patients should be considered for the interventional approach and which ones get medical therapy.
Dr. Wang emphasized that the protocols are as important as technical expertise in catheter-directed thrombolysis. Protocols are in place for the ED, the ICU, and the hospitalist team for the early detection of DVT and pulmonary emboli, notification of the appropriate staff, and posttreatment care of patients. Dr. Wang also took the lead on the anticoagulation aspect of computerized physician order entry.
"In our institution, we use the same protocols for call-in and transport to the cath lab as for ST-elevation myocardial infarction, which allows us to get the patient up and into the fluoroscopy suite within 30 minutes," Dr. Wang said. The fluoroscopy suite must be available on an emergency basis.
"It’s amazing to see such a dramatic improvement in patient symptoms almost immediately post procedure," said Dr. Carpenter.
Patients who undergo catheter-directed thrombolysis require close postoperative monitoring, said Dr. Carpenter, who is with Inpatient Specialists, a group that contracts with hospitals to provide hospitalist services. The main risks are bleeding, low blood pressure, or respiratory distress. "The bleeding might not necessarily be obvious," as the antithrombotic agent is given locally and bleeding can be local as well.
Community hospitals that offer catheter-directed thrombolysis need sufficient commitment from vascular surgeons and robust postprocedure support, Dr. Carpenter said. The vascular surgery group should be able to offer the procedure to all patients who need it.
Around-the-clock hospitalist availability is a good idea, especially if the surgeon is not on the in-house staff. "Don’t do [this procedure] if the hospitalist is providing triage services at night," he advised.
With 23 full-time positions (translating into 40 full-time or part-time physicians), the adult hospitalist service at Shady Grove Adventist, a 339-bed hospital, typically provides 10 hospitalists during weekdays (including one medical-psychiatric physician) and 8 on weekend days.
Besides the adult hospitalist group, the hospital has a pediatric hospitalist service, 24-hour in-house ICU hospitalist coverage, and a surgical hospitalist group. "Not many hospitals have surgical hospitalists," Dr. Carpenter noted. Laborists also are available 24 hours a day for in-house ob.gyn. consultations.
Dr. Carpenter, who has been a hospitalist since 2006, said that this "is how hospital-based medicine is progressing. "Shady Grove has been an early adopter" of expanded hospitalist services.
Dr. Carpenter and Dr. Wang reported having no financial disclosures.
Endovascular Repair Effective for Inflammatory AAA
SCOTTSDALE, ARIZ. – Inflammatory abdominal aortic aneurysms can be treated safely and effectively by endovascular aneurysm repair, though patients with significant hydronephrosis may have worse outcomes, a retrospective study suggests.
The study included 69 patients who underwent either endovascular repair (10 patients) or open surgical repair (59 patients) at the surgeon’s discretion at Mayo Clinics in Minnesota, Florida, or Arizona between 1999 and 2011.
Previous case series and meta-analyses have reported mixed results with endovascular aneurysm repair (EVAR), but the current study suggests that EVAR is appropriate management for inflammatory abdominal aortic aneurysms (AAAs), Dr. William M. Stone said at the annual meeting of the Southern Association for Vascular Surgery.
In the study’s EVAR group, eight patients presented with symptoms (80%), including one with rupture. In the open-repair group, 31 patients (52%) presented with symptoms, including 3 with rupture (5%).
Ureteral involvement was seen in 23 patients: 2 patients in the EVAR group (20%) and 21 in the open group (34%). Of these 23 patients, 21 underwent preoperative ureteral stent placement (91%). Thirty-six patients in the open-repair group had suprarenal aortic cross clamps (61%).
All of the EVAR procedures were technically successful, and patients were followed for an average of 34 months. The mean aneurysm size in the EVAR group decreased from 5.94 cm preoperatively to 4.73 cm after EVAR, an average change of nearly 18%, reported Dr. Stone of the Mayo Clinic in Phoenix.
Aneurysm size decreased in seven patients (70%), and did not change in two patients (20%) after EVAR. One EVAR patient was lost to follow-up. Preoperative hydronephrosis in one patient (10%) before EVAR did not change after EVAR, and one patient developed new hydronephrosis after EVAR. No patients in the EVAR group died, developed endoleaks, or needed steroids. Two patients developed atrial fibrillation after EVAR and were treated for it.
The inflammatory rind measured 5.4 cm before EVAR and 2.7 cm afterward on average, a mean change of 50%, he said.
In the open-repair group, surgeons used midline incisions in 52 patients (88%), a left flank approach in 6 patients (10%), and a bilateral subcostal approach in 1 (2%) to repair aneurysms with a mean size of 6.3 cm.
One patient in the open-repair group died postoperatively of a ruptured suprarenal inflammatory aneurysm. Major complications were seen in 22 patients (38%), including renal complications in 5 (8%), ischemic colitis in 2 (3%), and ureteral obstruction that required intervention in 3 (5%). A late anastomotic rupture in one patient in the open-surgery group was repaired by EVAR.
During a mean follow-up of 43 months, 17 patients in the open-repair group were lost to follow-up (28%). All of the remaining patients had resolution of their aneurysm sac. Four patients (7%) received steroids, not to treat the aneurysm but for comorbidities.
Among 12 patients in the open-repair group with preoperative hydronephrosis (20%), the hydronephrosis resolved in 7 of the 12 (58%), remained stable in 4 patients (33%), and worsened in 1 patient (8%). The patient with worsening hydronephrosis underwent ureterolysis. Two patients developed renal atrophy.
Although the retrospective study was small, it appeared to show a trend toward less severe complications in the EVAR group, "which is what we intuitively would have guessed," Dr. Stone said.
Previous data from the EUROSTAR (European Collaborators on Stent-Graft Techniques for Abdominal Aortic Aneurysm Repair) registry suggested that inflammatory AAA size decreased by 87% after EVAR, he noted (Semin. Interv. Cardiol. 2000;5:29-33). Aneurysm wall thickness averaged 21 mm preoperatively, 17 mm at intermediate follow-up, and 13 mm with long-term follow-up, which is "consistent with our review," he said.
Patients in the EUROSTAR database had problems with graft limb stenosis that were not seen in the current series, Dr. Stone noted. A ureteral stent and/or ureterolysis were required in 18% of the EUROSTAR patients who underwent EVAR. The rate of ureteral "entrapment" (inflammation around the ureters) improved from 45% to 27% after EVAR.
In a previous meta-analysis of data on 46 patients in 14 studies, EVAR decreased aneurysm size in most patients, with a median decrease of 11 mm. Periaortic fibrosis decreased in 52%, was unchanged in 42%, and progressed in 7% (J. Endovasc. Ther. 2005;12:560-7).
A separate meta-analysis of 56 studies with 1,120 patients compared EVAR with open repair of AAA, and found 1-year all-cause mortality rates of 2% with EVAR and 14% with open repair (Eur. J. Vasc. Endovasc. Surg. 2009;38:291-7). Periaortic fibrosis decreased in 65% of the EVAR group and in 73% of the open-repair group. Hydronephrosis regressed in 38% of the EVAR group and in 69% of the open-repair group.
In the current study, the two groups did not differ significantly in mean age, preoperative aneurysm size, or length of follow-up. In the EVAR group, patients averaged 72 years of age, and eight patients (80%) were male. In the open-repair group, patients averaged 66 years of age, and 45 (77%) were male.
Patients were excluded from endovascular treatment if they had infected aneurysms or aneurysms with inflammation that were not deemed to be true inflammatory aneurysms. The diagnosis of inflammatory aortic aneurysm was confirmed preoperatively by CT scan in the EVAR group or at the time of surgery in the open-repair group.
Dr. Stone reported having no financial disclosures.
SCOTTSDALE, ARIZ. – Inflammatory abdominal aortic aneurysms can be treated safely and effectively by endovascular aneurysm repair, though patients with significant hydronephrosis may have worse outcomes, a retrospective study suggests.
The study included 69 patients who underwent either endovascular repair (10 patients) or open surgical repair (59 patients) at the surgeon’s discretion at Mayo Clinics in Minnesota, Florida, or Arizona between 1999 and 2011.
Previous case series and meta-analyses have reported mixed results with endovascular aneurysm repair (EVAR), but the current study suggests that EVAR is appropriate management for inflammatory abdominal aortic aneurysms (AAAs), Dr. William M. Stone said at the annual meeting of the Southern Association for Vascular Surgery.
In the study’s EVAR group, eight patients presented with symptoms (80%), including one with rupture. In the open-repair group, 31 patients (52%) presented with symptoms, including 3 with rupture (5%).
Ureteral involvement was seen in 23 patients: 2 patients in the EVAR group (20%) and 21 in the open group (34%). Of these 23 patients, 21 underwent preoperative ureteral stent placement (91%). Thirty-six patients in the open-repair group had suprarenal aortic cross clamps (61%).
All of the EVAR procedures were technically successful, and patients were followed for an average of 34 months. The mean aneurysm size in the EVAR group decreased from 5.94 cm preoperatively to 4.73 cm after EVAR, an average change of nearly 18%, reported Dr. Stone of the Mayo Clinic in Phoenix.
Aneurysm size decreased in seven patients (70%), and did not change in two patients (20%) after EVAR. One EVAR patient was lost to follow-up. Preoperative hydronephrosis in one patient (10%) before EVAR did not change after EVAR, and one patient developed new hydronephrosis after EVAR. No patients in the EVAR group died, developed endoleaks, or needed steroids. Two patients developed atrial fibrillation after EVAR and were treated for it.
The inflammatory rind measured 5.4 cm before EVAR and 2.7 cm afterward on average, a mean change of 50%, he said.
In the open-repair group, surgeons used midline incisions in 52 patients (88%), a left flank approach in 6 patients (10%), and a bilateral subcostal approach in 1 (2%) to repair aneurysms with a mean size of 6.3 cm.
One patient in the open-repair group died postoperatively of a ruptured suprarenal inflammatory aneurysm. Major complications were seen in 22 patients (38%), including renal complications in 5 (8%), ischemic colitis in 2 (3%), and ureteral obstruction that required intervention in 3 (5%). A late anastomotic rupture in one patient in the open-surgery group was repaired by EVAR.
During a mean follow-up of 43 months, 17 patients in the open-repair group were lost to follow-up (28%). All of the remaining patients had resolution of their aneurysm sac. Four patients (7%) received steroids, not to treat the aneurysm but for comorbidities.
Among 12 patients in the open-repair group with preoperative hydronephrosis (20%), the hydronephrosis resolved in 7 of the 12 (58%), remained stable in 4 patients (33%), and worsened in 1 patient (8%). The patient with worsening hydronephrosis underwent ureterolysis. Two patients developed renal atrophy.
Although the retrospective study was small, it appeared to show a trend toward less severe complications in the EVAR group, "which is what we intuitively would have guessed," Dr. Stone said.
Previous data from the EUROSTAR (European Collaborators on Stent-Graft Techniques for Abdominal Aortic Aneurysm Repair) registry suggested that inflammatory AAA size decreased by 87% after EVAR, he noted (Semin. Interv. Cardiol. 2000;5:29-33). Aneurysm wall thickness averaged 21 mm preoperatively, 17 mm at intermediate follow-up, and 13 mm with long-term follow-up, which is "consistent with our review," he said.
Patients in the EUROSTAR database had problems with graft limb stenosis that were not seen in the current series, Dr. Stone noted. A ureteral stent and/or ureterolysis were required in 18% of the EUROSTAR patients who underwent EVAR. The rate of ureteral "entrapment" (inflammation around the ureters) improved from 45% to 27% after EVAR.
In a previous meta-analysis of data on 46 patients in 14 studies, EVAR decreased aneurysm size in most patients, with a median decrease of 11 mm. Periaortic fibrosis decreased in 52%, was unchanged in 42%, and progressed in 7% (J. Endovasc. Ther. 2005;12:560-7).
A separate meta-analysis of 56 studies with 1,120 patients compared EVAR with open repair of AAA, and found 1-year all-cause mortality rates of 2% with EVAR and 14% with open repair (Eur. J. Vasc. Endovasc. Surg. 2009;38:291-7). Periaortic fibrosis decreased in 65% of the EVAR group and in 73% of the open-repair group. Hydronephrosis regressed in 38% of the EVAR group and in 69% of the open-repair group.
In the current study, the two groups did not differ significantly in mean age, preoperative aneurysm size, or length of follow-up. In the EVAR group, patients averaged 72 years of age, and eight patients (80%) were male. In the open-repair group, patients averaged 66 years of age, and 45 (77%) were male.
Patients were excluded from endovascular treatment if they had infected aneurysms or aneurysms with inflammation that were not deemed to be true inflammatory aneurysms. The diagnosis of inflammatory aortic aneurysm was confirmed preoperatively by CT scan in the EVAR group or at the time of surgery in the open-repair group.
Dr. Stone reported having no financial disclosures.
SCOTTSDALE, ARIZ. – Inflammatory abdominal aortic aneurysms can be treated safely and effectively by endovascular aneurysm repair, though patients with significant hydronephrosis may have worse outcomes, a retrospective study suggests.
The study included 69 patients who underwent either endovascular repair (10 patients) or open surgical repair (59 patients) at the surgeon’s discretion at Mayo Clinics in Minnesota, Florida, or Arizona between 1999 and 2011.
Previous case series and meta-analyses have reported mixed results with endovascular aneurysm repair (EVAR), but the current study suggests that EVAR is appropriate management for inflammatory abdominal aortic aneurysms (AAAs), Dr. William M. Stone said at the annual meeting of the Southern Association for Vascular Surgery.
In the study’s EVAR group, eight patients presented with symptoms (80%), including one with rupture. In the open-repair group, 31 patients (52%) presented with symptoms, including 3 with rupture (5%).
Ureteral involvement was seen in 23 patients: 2 patients in the EVAR group (20%) and 21 in the open group (34%). Of these 23 patients, 21 underwent preoperative ureteral stent placement (91%). Thirty-six patients in the open-repair group had suprarenal aortic cross clamps (61%).
All of the EVAR procedures were technically successful, and patients were followed for an average of 34 months. The mean aneurysm size in the EVAR group decreased from 5.94 cm preoperatively to 4.73 cm after EVAR, an average change of nearly 18%, reported Dr. Stone of the Mayo Clinic in Phoenix.
Aneurysm size decreased in seven patients (70%), and did not change in two patients (20%) after EVAR. One EVAR patient was lost to follow-up. Preoperative hydronephrosis in one patient (10%) before EVAR did not change after EVAR, and one patient developed new hydronephrosis after EVAR. No patients in the EVAR group died, developed endoleaks, or needed steroids. Two patients developed atrial fibrillation after EVAR and were treated for it.
The inflammatory rind measured 5.4 cm before EVAR and 2.7 cm afterward on average, a mean change of 50%, he said.
In the open-repair group, surgeons used midline incisions in 52 patients (88%), a left flank approach in 6 patients (10%), and a bilateral subcostal approach in 1 (2%) to repair aneurysms with a mean size of 6.3 cm.
One patient in the open-repair group died postoperatively of a ruptured suprarenal inflammatory aneurysm. Major complications were seen in 22 patients (38%), including renal complications in 5 (8%), ischemic colitis in 2 (3%), and ureteral obstruction that required intervention in 3 (5%). A late anastomotic rupture in one patient in the open-surgery group was repaired by EVAR.
During a mean follow-up of 43 months, 17 patients in the open-repair group were lost to follow-up (28%). All of the remaining patients had resolution of their aneurysm sac. Four patients (7%) received steroids, not to treat the aneurysm but for comorbidities.
Among 12 patients in the open-repair group with preoperative hydronephrosis (20%), the hydronephrosis resolved in 7 of the 12 (58%), remained stable in 4 patients (33%), and worsened in 1 patient (8%). The patient with worsening hydronephrosis underwent ureterolysis. Two patients developed renal atrophy.
Although the retrospective study was small, it appeared to show a trend toward less severe complications in the EVAR group, "which is what we intuitively would have guessed," Dr. Stone said.
Previous data from the EUROSTAR (European Collaborators on Stent-Graft Techniques for Abdominal Aortic Aneurysm Repair) registry suggested that inflammatory AAA size decreased by 87% after EVAR, he noted (Semin. Interv. Cardiol. 2000;5:29-33). Aneurysm wall thickness averaged 21 mm preoperatively, 17 mm at intermediate follow-up, and 13 mm with long-term follow-up, which is "consistent with our review," he said.
Patients in the EUROSTAR database had problems with graft limb stenosis that were not seen in the current series, Dr. Stone noted. A ureteral stent and/or ureterolysis were required in 18% of the EUROSTAR patients who underwent EVAR. The rate of ureteral "entrapment" (inflammation around the ureters) improved from 45% to 27% after EVAR.
In a previous meta-analysis of data on 46 patients in 14 studies, EVAR decreased aneurysm size in most patients, with a median decrease of 11 mm. Periaortic fibrosis decreased in 52%, was unchanged in 42%, and progressed in 7% (J. Endovasc. Ther. 2005;12:560-7).
A separate meta-analysis of 56 studies with 1,120 patients compared EVAR with open repair of AAA, and found 1-year all-cause mortality rates of 2% with EVAR and 14% with open repair (Eur. J. Vasc. Endovasc. Surg. 2009;38:291-7). Periaortic fibrosis decreased in 65% of the EVAR group and in 73% of the open-repair group. Hydronephrosis regressed in 38% of the EVAR group and in 69% of the open-repair group.
In the current study, the two groups did not differ significantly in mean age, preoperative aneurysm size, or length of follow-up. In the EVAR group, patients averaged 72 years of age, and eight patients (80%) were male. In the open-repair group, patients averaged 66 years of age, and 45 (77%) were male.
Patients were excluded from endovascular treatment if they had infected aneurysms or aneurysms with inflammation that were not deemed to be true inflammatory aneurysms. The diagnosis of inflammatory aortic aneurysm was confirmed preoperatively by CT scan in the EVAR group or at the time of surgery in the open-repair group.
Dr. Stone reported having no financial disclosures.
FROM THE ANNUAL MEETING OF THE SOUTHERN ASSOCIATION FOR VASCULAR SURGERY
Virtual Reality Allows Patient-Specific Surgery Simulation
Physicians and patients heading into the operating room have to hope that the surgeon or proceduralist does the job right the first time. Generally, needing a do-over is not a good thing.
Soon, however, vascular and cardiac surgeons at Cleveland Clinic will be able to take several consequences-free do-overs that should lower risk for the patient. Some of the latest advances in imaging and technology are being incorporated into new simulation rooms built adjacent to a few new ORs. In these rooms physicians can rehearse the procedure they’re about to do on a three-dimensional simulation of the patient, who is being prepared for surgery next door.
For example, “fusion” imaging can help in the repair of aortic aneurysms. The results of an angiogram taken at the time of the procedure are superimposed on a preoperative CT scan of the patient to create three-dimensional imaging studies “telling us where all the arteries are without using any contrast,” Dr. Roy K. Greenberg said at the annual meeting of the Southern Association for Vascular Surgery. Not having to use catheters and wires to figure that out makes the procedure much easier, said Dr. Greenberg of the Cleveland Clinic Foundation.
His talk on “Aortic Care for the 21st Century” at the meeting was the first Jesse E. Thompson, M.D. Distinguished Guest Lecture.
“The imaging can be loaded in patient-specific simulators, providing a means to ‘test’ the procedures in virtual reality, train the team, and problem-solve `off-line’ ” with less risk to the patient, he said.
The Cleveland Clinic is no slouch when it comes to simulation. Their doctors-in-training practice on mannequins, robots, and other virtual stand-ins for patients. There is an Anesthesia Simulation Lab. The Clinic is even building an entire Center for Multidisciplinary Simulation.
The two new ORs that Dr. Greenberg described take simulation to the next level. The simulators are not in a remote room, they’re not in a separate building, and they’re not at a course that physicians go to for two or three days before the surgery. “I think they have to be integrated into the actual work flow and case,” he said.
The ORs should be finished in the fourth quarter of 2012, and will feature a control room behind each OR. Behind each control room will be a simulator room.
Dr. Greenberg described the work flow for a fenestrated endovascular aneurysm repair as something like this: The preoperative CT scan is used for patient selection and device design, and is imported into the OR and the simulator before the patient arrives. The CT scan and fluoroscopic imaging studies are used in fusion imaging in the OR, minimizing the use of contrast agents and radiation.
Standard clinic procedures required team sign-ins when the patient is on the table. Each team members signs in, introduces themselves, and makes sure they're operating on the correct anatomic part. Then “we have that obligatory 2.5 hours while anesthesia gets everything else ready,” during which the operative team goes to the simulator room. Residents, fellows, and the rest of the team get to practice the procedure using the repair device on a simulation of that particular patient.
“So there’s no question about what steps are next” during the live surgery, he said. If something doesn’t seem right during rehearsal, “you can say, `Why not?’ Is that a problem with the simulator, or is it going to be a problem with the procedure?”
Dr. Greenberg said he doesn’t know of any other institutions doing patient-specific simulations like this, but “I hope they will.”
Dr. Greenberg disclosed receiving royalties for intellectual property licensed to Cook, Inc. and funds for research and travel from Cook.
Physicians and patients heading into the operating room have to hope that the surgeon or proceduralist does the job right the first time. Generally, needing a do-over is not a good thing.
Soon, however, vascular and cardiac surgeons at Cleveland Clinic will be able to take several consequences-free do-overs that should lower risk for the patient. Some of the latest advances in imaging and technology are being incorporated into new simulation rooms built adjacent to a few new ORs. In these rooms physicians can rehearse the procedure they’re about to do on a three-dimensional simulation of the patient, who is being prepared for surgery next door.
For example, “fusion” imaging can help in the repair of aortic aneurysms. The results of an angiogram taken at the time of the procedure are superimposed on a preoperative CT scan of the patient to create three-dimensional imaging studies “telling us where all the arteries are without using any contrast,” Dr. Roy K. Greenberg said at the annual meeting of the Southern Association for Vascular Surgery. Not having to use catheters and wires to figure that out makes the procedure much easier, said Dr. Greenberg of the Cleveland Clinic Foundation.
His talk on “Aortic Care for the 21st Century” at the meeting was the first Jesse E. Thompson, M.D. Distinguished Guest Lecture.
“The imaging can be loaded in patient-specific simulators, providing a means to ‘test’ the procedures in virtual reality, train the team, and problem-solve `off-line’ ” with less risk to the patient, he said.
The Cleveland Clinic is no slouch when it comes to simulation. Their doctors-in-training practice on mannequins, robots, and other virtual stand-ins for patients. There is an Anesthesia Simulation Lab. The Clinic is even building an entire Center for Multidisciplinary Simulation.
The two new ORs that Dr. Greenberg described take simulation to the next level. The simulators are not in a remote room, they’re not in a separate building, and they’re not at a course that physicians go to for two or three days before the surgery. “I think they have to be integrated into the actual work flow and case,” he said.
The ORs should be finished in the fourth quarter of 2012, and will feature a control room behind each OR. Behind each control room will be a simulator room.
Dr. Greenberg described the work flow for a fenestrated endovascular aneurysm repair as something like this: The preoperative CT scan is used for patient selection and device design, and is imported into the OR and the simulator before the patient arrives. The CT scan and fluoroscopic imaging studies are used in fusion imaging in the OR, minimizing the use of contrast agents and radiation.
Standard clinic procedures required team sign-ins when the patient is on the table. Each team members signs in, introduces themselves, and makes sure they're operating on the correct anatomic part. Then “we have that obligatory 2.5 hours while anesthesia gets everything else ready,” during which the operative team goes to the simulator room. Residents, fellows, and the rest of the team get to practice the procedure using the repair device on a simulation of that particular patient.
“So there’s no question about what steps are next” during the live surgery, he said. If something doesn’t seem right during rehearsal, “you can say, `Why not?’ Is that a problem with the simulator, or is it going to be a problem with the procedure?”
Dr. Greenberg said he doesn’t know of any other institutions doing patient-specific simulations like this, but “I hope they will.”
Dr. Greenberg disclosed receiving royalties for intellectual property licensed to Cook, Inc. and funds for research and travel from Cook.
Physicians and patients heading into the operating room have to hope that the surgeon or proceduralist does the job right the first time. Generally, needing a do-over is not a good thing.
Soon, however, vascular and cardiac surgeons at Cleveland Clinic will be able to take several consequences-free do-overs that should lower risk for the patient. Some of the latest advances in imaging and technology are being incorporated into new simulation rooms built adjacent to a few new ORs. In these rooms physicians can rehearse the procedure they’re about to do on a three-dimensional simulation of the patient, who is being prepared for surgery next door.
For example, “fusion” imaging can help in the repair of aortic aneurysms. The results of an angiogram taken at the time of the procedure are superimposed on a preoperative CT scan of the patient to create three-dimensional imaging studies “telling us where all the arteries are without using any contrast,” Dr. Roy K. Greenberg said at the annual meeting of the Southern Association for Vascular Surgery. Not having to use catheters and wires to figure that out makes the procedure much easier, said Dr. Greenberg of the Cleveland Clinic Foundation.
His talk on “Aortic Care for the 21st Century” at the meeting was the first Jesse E. Thompson, M.D. Distinguished Guest Lecture.
“The imaging can be loaded in patient-specific simulators, providing a means to ‘test’ the procedures in virtual reality, train the team, and problem-solve `off-line’ ” with less risk to the patient, he said.
The Cleveland Clinic is no slouch when it comes to simulation. Their doctors-in-training practice on mannequins, robots, and other virtual stand-ins for patients. There is an Anesthesia Simulation Lab. The Clinic is even building an entire Center for Multidisciplinary Simulation.
The two new ORs that Dr. Greenberg described take simulation to the next level. The simulators are not in a remote room, they’re not in a separate building, and they’re not at a course that physicians go to for two or three days before the surgery. “I think they have to be integrated into the actual work flow and case,” he said.
The ORs should be finished in the fourth quarter of 2012, and will feature a control room behind each OR. Behind each control room will be a simulator room.
Dr. Greenberg described the work flow for a fenestrated endovascular aneurysm repair as something like this: The preoperative CT scan is used for patient selection and device design, and is imported into the OR and the simulator before the patient arrives. The CT scan and fluoroscopic imaging studies are used in fusion imaging in the OR, minimizing the use of contrast agents and radiation.
Standard clinic procedures required team sign-ins when the patient is on the table. Each team members signs in, introduces themselves, and makes sure they're operating on the correct anatomic part. Then “we have that obligatory 2.5 hours while anesthesia gets everything else ready,” during which the operative team goes to the simulator room. Residents, fellows, and the rest of the team get to practice the procedure using the repair device on a simulation of that particular patient.
“So there’s no question about what steps are next” during the live surgery, he said. If something doesn’t seem right during rehearsal, “you can say, `Why not?’ Is that a problem with the simulator, or is it going to be a problem with the procedure?”
Dr. Greenberg said he doesn’t know of any other institutions doing patient-specific simulations like this, but “I hope they will.”
Dr. Greenberg disclosed receiving royalties for intellectual property licensed to Cook, Inc. and funds for research and travel from Cook.
Local, Regional Anesthesia Surpass General for AAA EVAR
MIAMI BEACH – Local or regional anesthesia is a better option than is general anesthesia for patients undergoing endovascular repair of an abdominal aortic aneurysm, based on findings from a registry with nearly 1,200 patients.
Both local anesthesia and regional anesthesia each surpassed general anesthesia in two periprocedural measures: significantly reducing procedure time, and significantly reducing postoperative hospitalization, Dr. Rutger A. Stokmans said at ISET 2012, an international symposium on endovascular therapy.
In addition, both local and regional anesthesia led to trends in reduced rates of major adverse events during the 30 days following surgery, although these differences did not reach statistical significance. All three anesthesia types linked with similar rates of both technical and clinical success of the aneurysm repairs. Regional anesthesia also led to a significantly lower rate of ICU admission, compared with both general and local anesthesia; local anesthesia showed no significant difference for this measure, compared with general anesthesia.
Based on these findings, local or regional anesthesia should be preferred when performing endovascular aneurysm repair (EVAR), whereas general anesthesia should usually be avoided, said Dr. Stokmans, a vascular surgeon at Catharina Hospital in Eindhoven, the Netherlands.
These findings support the most recent anesthesia recommendations of the European Society for Vascular Surgery, which in 2011 guidelines for managing abdominal aortic aneurysms (AAA) cited local anesthesia as preferred for EVAR, with regional or general anesthesia reserved for patients with contraindications for local anesthesia, he said (Euro. J. Vasc. Endovasc. Surg .2011;41[suppl. 1]:S1-S58). The most recent guidelines for AAA management from the Society for Vascular Surgery suggested using local or regional anesthesia over general anesthesia, he added (J. Vasc. Surg. 2009[suppl.]:50:S2-S49).
But despite these recommendations, the most commonly used anesthesia type worldwide for EVAR repair of AAA has been general anesthesia, followed by regional anesthesia, with local treatment used least often, according to the registry data reported by Dr. Stokmans. Among the 1,199 patients enrolled in ENGAGE (Endurant Stent Graft Natural Selection Global Postmarketing Registry) during March 2009 to December 2010 in 30 countries on five continents, 749 (62%) underwent their EVAR with general anesthesia, 325 (27%) with regional, and 125 (10%) with local anesthesia. (Percentages do not add up to 100% because of rounding.)
The registry data also showed striking regional variations in anesthesia use, with general anesthesia used on about 90% of patients in Canada, Australia, and New Zealand, and on about 70% of patients in Scandinavian countries and the United Kingdom. But in Central Europe, regional anesthesia – used on nearly 70% of EVAR patients – dominated. The only region favoring local anesthesia was South America (Argentina, Columbia, and Uruguay), where about 50% of patients received local, but more than 40% received general anesthesia, he said. The registry contained no U.S. patients, although the Endurant AAA stent graft system is marketed in the United States.
The average age of the EVAR patients in the registry was about 73 years. Those patients who underwent general anesthesia were significantly older, by an average of about 18 months, compared with those who received local or regional anesthesia.
The proportions of patients undergoing general, regional, or local anesthesia were similar in the subgroups of patients with American Society of Anesthesiologists (ASA) physical status scores of 1, 2, or 3. However, among the highest-risk patients included in the study – those with an ASA score of 4 – a significantly greater proportion of patients received general anesthesia. The multivariate models used in the analysis, therefore, were adjusted for age and for ASA score. About 42% of patients were in ASA class 2, and another 42% in class 3.
All patients were hemodynamically stable at the time of their enrollment. The maximum AAA diameter of registry patients was 6 cm, and about 88% of patients had an AAA diameter greater than 5 cm.
Average procedure times were 106 minutes in the general anesthesia patients, 95 minutes in the regional patients, and 81 minutes in the local anesthesia patients – statistically significant differences among the three groups.
The average postoperative hospitalization was 5.2 days in the general anesthesia patients, 4.3 days in the regional patients, and 3.6 days in the local anesthesia patients, differences that were statistically significant among each of the three groups.
The rate of technical surgical success was 98% in all three subgroups, and the rate of clinical success reached 97%-98% in all three groups.
The rates of major adverse events during the 30 days following surgery were 5.1% in the general anesthesia patients, 3.2% in the local patients, and 2.2% in the regional anesthesia patients. None of these differences reached statistical significance. Major adverse events included death, MI, stroke, renal failure, blood loss greater than 1 L, and bowel ischemia. No patients developed paraplegia or respiratory failure.
Postoperative ICU admission occurred for 27% of the regional anesthesia patients, 35% of the general patients, and 42% of the local anesthesia patients. The rate among the regional patients was significantly less than in the other two groups, but the difference in rates between the general and local anesthesia patients did not reach statistical significance.
The ENGAGE registry was organized and sponsored by Medtronic, which markets the Endurant stent. Dr. Stokmans and his associates received an unrestricted research grant from Medtronic. Dr. Stokmans said that he had no other disclosures.
MIAMI BEACH – Local or regional anesthesia is a better option than is general anesthesia for patients undergoing endovascular repair of an abdominal aortic aneurysm, based on findings from a registry with nearly 1,200 patients.
Both local anesthesia and regional anesthesia each surpassed general anesthesia in two periprocedural measures: significantly reducing procedure time, and significantly reducing postoperative hospitalization, Dr. Rutger A. Stokmans said at ISET 2012, an international symposium on endovascular therapy.
In addition, both local and regional anesthesia led to trends in reduced rates of major adverse events during the 30 days following surgery, although these differences did not reach statistical significance. All three anesthesia types linked with similar rates of both technical and clinical success of the aneurysm repairs. Regional anesthesia also led to a significantly lower rate of ICU admission, compared with both general and local anesthesia; local anesthesia showed no significant difference for this measure, compared with general anesthesia.
Based on these findings, local or regional anesthesia should be preferred when performing endovascular aneurysm repair (EVAR), whereas general anesthesia should usually be avoided, said Dr. Stokmans, a vascular surgeon at Catharina Hospital in Eindhoven, the Netherlands.
These findings support the most recent anesthesia recommendations of the European Society for Vascular Surgery, which in 2011 guidelines for managing abdominal aortic aneurysms (AAA) cited local anesthesia as preferred for EVAR, with regional or general anesthesia reserved for patients with contraindications for local anesthesia, he said (Euro. J. Vasc. Endovasc. Surg .2011;41[suppl. 1]:S1-S58). The most recent guidelines for AAA management from the Society for Vascular Surgery suggested using local or regional anesthesia over general anesthesia, he added (J. Vasc. Surg. 2009[suppl.]:50:S2-S49).
But despite these recommendations, the most commonly used anesthesia type worldwide for EVAR repair of AAA has been general anesthesia, followed by regional anesthesia, with local treatment used least often, according to the registry data reported by Dr. Stokmans. Among the 1,199 patients enrolled in ENGAGE (Endurant Stent Graft Natural Selection Global Postmarketing Registry) during March 2009 to December 2010 in 30 countries on five continents, 749 (62%) underwent their EVAR with general anesthesia, 325 (27%) with regional, and 125 (10%) with local anesthesia. (Percentages do not add up to 100% because of rounding.)
The registry data also showed striking regional variations in anesthesia use, with general anesthesia used on about 90% of patients in Canada, Australia, and New Zealand, and on about 70% of patients in Scandinavian countries and the United Kingdom. But in Central Europe, regional anesthesia – used on nearly 70% of EVAR patients – dominated. The only region favoring local anesthesia was South America (Argentina, Columbia, and Uruguay), where about 50% of patients received local, but more than 40% received general anesthesia, he said. The registry contained no U.S. patients, although the Endurant AAA stent graft system is marketed in the United States.
The average age of the EVAR patients in the registry was about 73 years. Those patients who underwent general anesthesia were significantly older, by an average of about 18 months, compared with those who received local or regional anesthesia.
The proportions of patients undergoing general, regional, or local anesthesia were similar in the subgroups of patients with American Society of Anesthesiologists (ASA) physical status scores of 1, 2, or 3. However, among the highest-risk patients included in the study – those with an ASA score of 4 – a significantly greater proportion of patients received general anesthesia. The multivariate models used in the analysis, therefore, were adjusted for age and for ASA score. About 42% of patients were in ASA class 2, and another 42% in class 3.
All patients were hemodynamically stable at the time of their enrollment. The maximum AAA diameter of registry patients was 6 cm, and about 88% of patients had an AAA diameter greater than 5 cm.
Average procedure times were 106 minutes in the general anesthesia patients, 95 minutes in the regional patients, and 81 minutes in the local anesthesia patients – statistically significant differences among the three groups.
The average postoperative hospitalization was 5.2 days in the general anesthesia patients, 4.3 days in the regional patients, and 3.6 days in the local anesthesia patients, differences that were statistically significant among each of the three groups.
The rate of technical surgical success was 98% in all three subgroups, and the rate of clinical success reached 97%-98% in all three groups.
The rates of major adverse events during the 30 days following surgery were 5.1% in the general anesthesia patients, 3.2% in the local patients, and 2.2% in the regional anesthesia patients. None of these differences reached statistical significance. Major adverse events included death, MI, stroke, renal failure, blood loss greater than 1 L, and bowel ischemia. No patients developed paraplegia or respiratory failure.
Postoperative ICU admission occurred for 27% of the regional anesthesia patients, 35% of the general patients, and 42% of the local anesthesia patients. The rate among the regional patients was significantly less than in the other two groups, but the difference in rates between the general and local anesthesia patients did not reach statistical significance.
The ENGAGE registry was organized and sponsored by Medtronic, which markets the Endurant stent. Dr. Stokmans and his associates received an unrestricted research grant from Medtronic. Dr. Stokmans said that he had no other disclosures.
MIAMI BEACH – Local or regional anesthesia is a better option than is general anesthesia for patients undergoing endovascular repair of an abdominal aortic aneurysm, based on findings from a registry with nearly 1,200 patients.
Both local anesthesia and regional anesthesia each surpassed general anesthesia in two periprocedural measures: significantly reducing procedure time, and significantly reducing postoperative hospitalization, Dr. Rutger A. Stokmans said at ISET 2012, an international symposium on endovascular therapy.
In addition, both local and regional anesthesia led to trends in reduced rates of major adverse events during the 30 days following surgery, although these differences did not reach statistical significance. All three anesthesia types linked with similar rates of both technical and clinical success of the aneurysm repairs. Regional anesthesia also led to a significantly lower rate of ICU admission, compared with both general and local anesthesia; local anesthesia showed no significant difference for this measure, compared with general anesthesia.
Based on these findings, local or regional anesthesia should be preferred when performing endovascular aneurysm repair (EVAR), whereas general anesthesia should usually be avoided, said Dr. Stokmans, a vascular surgeon at Catharina Hospital in Eindhoven, the Netherlands.
These findings support the most recent anesthesia recommendations of the European Society for Vascular Surgery, which in 2011 guidelines for managing abdominal aortic aneurysms (AAA) cited local anesthesia as preferred for EVAR, with regional or general anesthesia reserved for patients with contraindications for local anesthesia, he said (Euro. J. Vasc. Endovasc. Surg .2011;41[suppl. 1]:S1-S58). The most recent guidelines for AAA management from the Society for Vascular Surgery suggested using local or regional anesthesia over general anesthesia, he added (J. Vasc. Surg. 2009[suppl.]:50:S2-S49).
But despite these recommendations, the most commonly used anesthesia type worldwide for EVAR repair of AAA has been general anesthesia, followed by regional anesthesia, with local treatment used least often, according to the registry data reported by Dr. Stokmans. Among the 1,199 patients enrolled in ENGAGE (Endurant Stent Graft Natural Selection Global Postmarketing Registry) during March 2009 to December 2010 in 30 countries on five continents, 749 (62%) underwent their EVAR with general anesthesia, 325 (27%) with regional, and 125 (10%) with local anesthesia. (Percentages do not add up to 100% because of rounding.)
The registry data also showed striking regional variations in anesthesia use, with general anesthesia used on about 90% of patients in Canada, Australia, and New Zealand, and on about 70% of patients in Scandinavian countries and the United Kingdom. But in Central Europe, regional anesthesia – used on nearly 70% of EVAR patients – dominated. The only region favoring local anesthesia was South America (Argentina, Columbia, and Uruguay), where about 50% of patients received local, but more than 40% received general anesthesia, he said. The registry contained no U.S. patients, although the Endurant AAA stent graft system is marketed in the United States.
The average age of the EVAR patients in the registry was about 73 years. Those patients who underwent general anesthesia were significantly older, by an average of about 18 months, compared with those who received local or regional anesthesia.
The proportions of patients undergoing general, regional, or local anesthesia were similar in the subgroups of patients with American Society of Anesthesiologists (ASA) physical status scores of 1, 2, or 3. However, among the highest-risk patients included in the study – those with an ASA score of 4 – a significantly greater proportion of patients received general anesthesia. The multivariate models used in the analysis, therefore, were adjusted for age and for ASA score. About 42% of patients were in ASA class 2, and another 42% in class 3.
All patients were hemodynamically stable at the time of their enrollment. The maximum AAA diameter of registry patients was 6 cm, and about 88% of patients had an AAA diameter greater than 5 cm.
Average procedure times were 106 minutes in the general anesthesia patients, 95 minutes in the regional patients, and 81 minutes in the local anesthesia patients – statistically significant differences among the three groups.
The average postoperative hospitalization was 5.2 days in the general anesthesia patients, 4.3 days in the regional patients, and 3.6 days in the local anesthesia patients, differences that were statistically significant among each of the three groups.
The rate of technical surgical success was 98% in all three subgroups, and the rate of clinical success reached 97%-98% in all three groups.
The rates of major adverse events during the 30 days following surgery were 5.1% in the general anesthesia patients, 3.2% in the local patients, and 2.2% in the regional anesthesia patients. None of these differences reached statistical significance. Major adverse events included death, MI, stroke, renal failure, blood loss greater than 1 L, and bowel ischemia. No patients developed paraplegia or respiratory failure.
Postoperative ICU admission occurred for 27% of the regional anesthesia patients, 35% of the general patients, and 42% of the local anesthesia patients. The rate among the regional patients was significantly less than in the other two groups, but the difference in rates between the general and local anesthesia patients did not reach statistical significance.
The ENGAGE registry was organized and sponsored by Medtronic, which markets the Endurant stent. Dr. Stokmans and his associates received an unrestricted research grant from Medtronic. Dr. Stokmans said that he had no other disclosures.
FROM ISET 2012, AN INTERNATIONAL SYMPOSIUM ON ENDOVASCULAR THERAPY
Major Finding: Postoperative hospitalization averaged 5.2 days in EVAR patients treated with general anesthesia, 4.3 days with regional anesthesia, and 3.6 days with local anesthesia.
Data Source: ENGAGE, an international registry of 1,199 patients who underwent EVAR for an abdominal aortic aneurysm during 2009 or 2010.
Disclosures: The ENGAGE registry was organized and sponsored by Medtronic, which markets the Endurant EVAR stent. Dr. Stokmans and his associates received an unrestricted research grant from Medtronic. Dr. Stokmans said that he had no other disclosures.
Postsurgery Complications and Readmissions Common, Costly
SAN FRANCISCO – A majority of patients who are rehospitalized after surgery have a postoperative complication, most commonly after colectomy, lower extremity bypass, or carotid endarterectomy.
Reducing postoperative complications could reduce costs associated with readmissions by millions of dollars per year, a retrospective study of data on 90,932 patients from 214 hospitals suggests.
Investigators linked records from the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) and the Medicare Provider Analysis and Review files for patients aged 65 years or older who underwent surgery in 2005-2008.
Within 30 days of surgery, 13% of patients were readmitted. A postoperative complication listed in the ACS-NSQIP registry was seen in 53% of readmitted patients compared with 16% of patients who did not need readmission, Dr. Elise H. Lawson and her associates reported at the annual clinical congress of the American College of Surgeons.
The study looked at 20 postoperative complications, including surgical site infections, wound disruption, pneumonia, unplanned intubation, pulmonary embolism, progressive renal insufficiency, acute renal failure, urinary tract infection, stroke, coma, cardiac arrest requiring CPR, myocardial infarction, bleeding requiring transfusion, deep venous thrombosis, sepsis or septic shock, being on a ventilator for more than 48 hours, and an unplanned return to the OR, among others.
Colectomy was associated with the greatest number of readmissions, followed by lower extremity bypass and carotid endarterectomy. After colectomy, 27% of patients developed a complication, and 13.4% of all colectomy patients were readmitted within 30 days.
Readmission rates after colectomy were 28% for patients who developed postoperative complications and 8% for patients without complications, said Dr. Lawson of the University of California, Los Angeles. She won the College’s 2011 Excellence in Research Award for her study.
Hypothetically, if postoperative complications could be prevented after colectomy, the risk-adjusted probability of readmission within 30 days would be 8%, she said. The study adjusted for the effects of many other factors that influenced the risk of having a postoperative complication, including age, sex, body mass index, functional status, emergency procedures, smoking, renal failure, and diabetes.
Not only did patients with complications have more readmissions, but those readmissions were more expensive. The cost for readmission after colectomy was $13,400 for patients with a complication and $7,500 for those without complications.
It’s unrealistic to think that a hospital could prevent all postoperative complications, Dr. Lawson said. Reducing complications after colectomy by even 10% (to 24%) would lower the overall postcolectomy readmission rate from 13.4% to 12.8%, the investigators estimated. For the 108,820 colectomies performed each year in Medicare beneficiaries aged 65 years or older, a 10% reduction in postoperative complications would reduce costs from readmissions alone by $9.3 million per year, she said.
Reducing complications after colectomy by 30% (to 19%) would lower the postcolectomy readmission rate to 11.7% and save an estimated $28 million per year in readmission costs. Halving the postcolectomy complication rate (to 13.5%) would reduce the readmission rate to 10.6% and save an estimated $46 million per year in readmission costs.
Previous data suggest that 13% of surgical patients and 16% of medical patients are readmitted after discharge from hospitalization, accounting for an estimated $17 billion in Medicare costs. Medicare plans to reduce payments for readmissions starting in 2013.
The reasons that patients are readmitted are not well understood, which was one motivation for the study, Dr. Lawson said. Unplanned readmissions that are related to the initial surgery may be due to postoperative complications or exacerbations of a preoperative comorbidity. Unplanned readmissions also may be for reasons unrelated to the initial surgery, such as for trauma or falls. In other cases, readmission may be planned for chemotherapy or elective procedures. The study excluded patients who died before discharge or who were not discharged from the primary hospitalization.
Dr. Lawson said she has no relevant conflicts of interest.
I’d like to congratulate Dr. Lawson on an excellent presentation and a well-deserved award. Clearly, reducing postoperative morbidity will decrease costs by decreasing lengths of stay and decreasing resource utilization.
When I was in training it was thought that central line–associated bloodstream infections and complications of central lines couldn’t be prevented in some cases. We’ve clearly shown that that is not the case, and with very simple measures we’ve been able to almost eliminate central line infections.
But colon surgery involves complex procedures. How often can we identify individual- or system-level error and correct it in systematic fashion to improve outcomes?

|
|
I suspect that all complications are not equally associated with readmission. Identifying those that do increase readmission risk will help us increase our observation of those patients postoperatively and our perceived risk for those patients.
I was surprised that carotid endarterectomy was one of the top three procedures on the list. It makes me think that there’s an interaction between the procedure type and complications in terms of readmission. For example, I think complications would be far more predictive of readmission for something like colectomy than something like carotid endarterectomy. My suspicion is that the majority of readmissions after carotid endarterectomy were related to patients’ preoperative comorbidities. If so, the approach to reducing readmissions might vary significantly depending on the procedure in question.
Finally, 47% of readmissions were not associated with postoperative complications. If we understood what was driving these readmissions, we might be able to prevent them and further decrease costs. For instance, does improved continuity of care decrease readmissions? If patients had primary care physicians, were they less likely to be readmitted? Or if they saw their primary care physicians within 2 weeks of discharge, were they less likely to be readmitted?
Dr. Taylor S. Riall, an ACS Fellow at the University of Texas Medical Branch, Galveston, made these remarks as the discussant after Dr. Lawson’s presentation.
I’d like to congratulate Dr. Lawson on an excellent presentation and a well-deserved award. Clearly, reducing postoperative morbidity will decrease costs by decreasing lengths of stay and decreasing resource utilization.
When I was in training it was thought that central line–associated bloodstream infections and complications of central lines couldn’t be prevented in some cases. We’ve clearly shown that that is not the case, and with very simple measures we’ve been able to almost eliminate central line infections.
But colon surgery involves complex procedures. How often can we identify individual- or system-level error and correct it in systematic fashion to improve outcomes?

|
|
I suspect that all complications are not equally associated with readmission. Identifying those that do increase readmission risk will help us increase our observation of those patients postoperatively and our perceived risk for those patients.
I was surprised that carotid endarterectomy was one of the top three procedures on the list. It makes me think that there’s an interaction between the procedure type and complications in terms of readmission. For example, I think complications would be far more predictive of readmission for something like colectomy than something like carotid endarterectomy. My suspicion is that the majority of readmissions after carotid endarterectomy were related to patients’ preoperative comorbidities. If so, the approach to reducing readmissions might vary significantly depending on the procedure in question.
Finally, 47% of readmissions were not associated with postoperative complications. If we understood what was driving these readmissions, we might be able to prevent them and further decrease costs. For instance, does improved continuity of care decrease readmissions? If patients had primary care physicians, were they less likely to be readmitted? Or if they saw their primary care physicians within 2 weeks of discharge, were they less likely to be readmitted?
Dr. Taylor S. Riall, an ACS Fellow at the University of Texas Medical Branch, Galveston, made these remarks as the discussant after Dr. Lawson’s presentation.
I’d like to congratulate Dr. Lawson on an excellent presentation and a well-deserved award. Clearly, reducing postoperative morbidity will decrease costs by decreasing lengths of stay and decreasing resource utilization.
When I was in training it was thought that central line–associated bloodstream infections and complications of central lines couldn’t be prevented in some cases. We’ve clearly shown that that is not the case, and with very simple measures we’ve been able to almost eliminate central line infections.
But colon surgery involves complex procedures. How often can we identify individual- or system-level error and correct it in systematic fashion to improve outcomes?

|
|
I suspect that all complications are not equally associated with readmission. Identifying those that do increase readmission risk will help us increase our observation of those patients postoperatively and our perceived risk for those patients.
I was surprised that carotid endarterectomy was one of the top three procedures on the list. It makes me think that there’s an interaction between the procedure type and complications in terms of readmission. For example, I think complications would be far more predictive of readmission for something like colectomy than something like carotid endarterectomy. My suspicion is that the majority of readmissions after carotid endarterectomy were related to patients’ preoperative comorbidities. If so, the approach to reducing readmissions might vary significantly depending on the procedure in question.
Finally, 47% of readmissions were not associated with postoperative complications. If we understood what was driving these readmissions, we might be able to prevent them and further decrease costs. For instance, does improved continuity of care decrease readmissions? If patients had primary care physicians, were they less likely to be readmitted? Or if they saw their primary care physicians within 2 weeks of discharge, were they less likely to be readmitted?
Dr. Taylor S. Riall, an ACS Fellow at the University of Texas Medical Branch, Galveston, made these remarks as the discussant after Dr. Lawson’s presentation.
SAN FRANCISCO – A majority of patients who are rehospitalized after surgery have a postoperative complication, most commonly after colectomy, lower extremity bypass, or carotid endarterectomy.
Reducing postoperative complications could reduce costs associated with readmissions by millions of dollars per year, a retrospective study of data on 90,932 patients from 214 hospitals suggests.
Investigators linked records from the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) and the Medicare Provider Analysis and Review files for patients aged 65 years or older who underwent surgery in 2005-2008.
Within 30 days of surgery, 13% of patients were readmitted. A postoperative complication listed in the ACS-NSQIP registry was seen in 53% of readmitted patients compared with 16% of patients who did not need readmission, Dr. Elise H. Lawson and her associates reported at the annual clinical congress of the American College of Surgeons.
The study looked at 20 postoperative complications, including surgical site infections, wound disruption, pneumonia, unplanned intubation, pulmonary embolism, progressive renal insufficiency, acute renal failure, urinary tract infection, stroke, coma, cardiac arrest requiring CPR, myocardial infarction, bleeding requiring transfusion, deep venous thrombosis, sepsis or septic shock, being on a ventilator for more than 48 hours, and an unplanned return to the OR, among others.
Colectomy was associated with the greatest number of readmissions, followed by lower extremity bypass and carotid endarterectomy. After colectomy, 27% of patients developed a complication, and 13.4% of all colectomy patients were readmitted within 30 days.
Readmission rates after colectomy were 28% for patients who developed postoperative complications and 8% for patients without complications, said Dr. Lawson of the University of California, Los Angeles. She won the College’s 2011 Excellence in Research Award for her study.
Hypothetically, if postoperative complications could be prevented after colectomy, the risk-adjusted probability of readmission within 30 days would be 8%, she said. The study adjusted for the effects of many other factors that influenced the risk of having a postoperative complication, including age, sex, body mass index, functional status, emergency procedures, smoking, renal failure, and diabetes.
Not only did patients with complications have more readmissions, but those readmissions were more expensive. The cost for readmission after colectomy was $13,400 for patients with a complication and $7,500 for those without complications.
It’s unrealistic to think that a hospital could prevent all postoperative complications, Dr. Lawson said. Reducing complications after colectomy by even 10% (to 24%) would lower the overall postcolectomy readmission rate from 13.4% to 12.8%, the investigators estimated. For the 108,820 colectomies performed each year in Medicare beneficiaries aged 65 years or older, a 10% reduction in postoperative complications would reduce costs from readmissions alone by $9.3 million per year, she said.
Reducing complications after colectomy by 30% (to 19%) would lower the postcolectomy readmission rate to 11.7% and save an estimated $28 million per year in readmission costs. Halving the postcolectomy complication rate (to 13.5%) would reduce the readmission rate to 10.6% and save an estimated $46 million per year in readmission costs.
Previous data suggest that 13% of surgical patients and 16% of medical patients are readmitted after discharge from hospitalization, accounting for an estimated $17 billion in Medicare costs. Medicare plans to reduce payments for readmissions starting in 2013.
The reasons that patients are readmitted are not well understood, which was one motivation for the study, Dr. Lawson said. Unplanned readmissions that are related to the initial surgery may be due to postoperative complications or exacerbations of a preoperative comorbidity. Unplanned readmissions also may be for reasons unrelated to the initial surgery, such as for trauma or falls. In other cases, readmission may be planned for chemotherapy or elective procedures. The study excluded patients who died before discharge or who were not discharged from the primary hospitalization.
Dr. Lawson said she has no relevant conflicts of interest.
SAN FRANCISCO – A majority of patients who are rehospitalized after surgery have a postoperative complication, most commonly after colectomy, lower extremity bypass, or carotid endarterectomy.
Reducing postoperative complications could reduce costs associated with readmissions by millions of dollars per year, a retrospective study of data on 90,932 patients from 214 hospitals suggests.
Investigators linked records from the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) and the Medicare Provider Analysis and Review files for patients aged 65 years or older who underwent surgery in 2005-2008.
Within 30 days of surgery, 13% of patients were readmitted. A postoperative complication listed in the ACS-NSQIP registry was seen in 53% of readmitted patients compared with 16% of patients who did not need readmission, Dr. Elise H. Lawson and her associates reported at the annual clinical congress of the American College of Surgeons.
The study looked at 20 postoperative complications, including surgical site infections, wound disruption, pneumonia, unplanned intubation, pulmonary embolism, progressive renal insufficiency, acute renal failure, urinary tract infection, stroke, coma, cardiac arrest requiring CPR, myocardial infarction, bleeding requiring transfusion, deep venous thrombosis, sepsis or septic shock, being on a ventilator for more than 48 hours, and an unplanned return to the OR, among others.
Colectomy was associated with the greatest number of readmissions, followed by lower extremity bypass and carotid endarterectomy. After colectomy, 27% of patients developed a complication, and 13.4% of all colectomy patients were readmitted within 30 days.
Readmission rates after colectomy were 28% for patients who developed postoperative complications and 8% for patients without complications, said Dr. Lawson of the University of California, Los Angeles. She won the College’s 2011 Excellence in Research Award for her study.
Hypothetically, if postoperative complications could be prevented after colectomy, the risk-adjusted probability of readmission within 30 days would be 8%, she said. The study adjusted for the effects of many other factors that influenced the risk of having a postoperative complication, including age, sex, body mass index, functional status, emergency procedures, smoking, renal failure, and diabetes.
Not only did patients with complications have more readmissions, but those readmissions were more expensive. The cost for readmission after colectomy was $13,400 for patients with a complication and $7,500 for those without complications.
It’s unrealistic to think that a hospital could prevent all postoperative complications, Dr. Lawson said. Reducing complications after colectomy by even 10% (to 24%) would lower the overall postcolectomy readmission rate from 13.4% to 12.8%, the investigators estimated. For the 108,820 colectomies performed each year in Medicare beneficiaries aged 65 years or older, a 10% reduction in postoperative complications would reduce costs from readmissions alone by $9.3 million per year, she said.
Reducing complications after colectomy by 30% (to 19%) would lower the postcolectomy readmission rate to 11.7% and save an estimated $28 million per year in readmission costs. Halving the postcolectomy complication rate (to 13.5%) would reduce the readmission rate to 10.6% and save an estimated $46 million per year in readmission costs.
Previous data suggest that 13% of surgical patients and 16% of medical patients are readmitted after discharge from hospitalization, accounting for an estimated $17 billion in Medicare costs. Medicare plans to reduce payments for readmissions starting in 2013.
The reasons that patients are readmitted are not well understood, which was one motivation for the study, Dr. Lawson said. Unplanned readmissions that are related to the initial surgery may be due to postoperative complications or exacerbations of a preoperative comorbidity. Unplanned readmissions also may be for reasons unrelated to the initial surgery, such as for trauma or falls. In other cases, readmission may be planned for chemotherapy or elective procedures. The study excluded patients who died before discharge or who were not discharged from the primary hospitalization.
Dr. Lawson said she has no relevant conflicts of interest.
FROM THE ANNUAL CLINICAL CONGRESS OF THE AMERICAN COLLEGE OF SURGEONS
Major Finding: Postoperative complications developed in 53% of patients who needed readmission within 30 days compared with 16% of patients who did not require readmission. A 10% reduction in complications after colectomy alone could avoid $9.3 million/year in costs for readmissions.
Data Source: A retrospective study of data on 90,932 patients aged 65 years or older who underwent surgery in 2005-2008.
Disclosures: Dr. Lawson said she has no relevant conflicts of interest.