User login
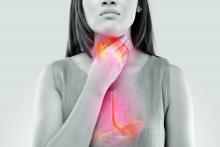
FDA approves first drug for eosinophilic esophagitis
The U.S. Food and Drug Administration has approved dupilumab (Dupixent, Regeneron) to treat eosinophilic esophagitis (EoE) in adults and children aged 12 years and older weighing at least 40 kg.
EoE is a chronic inflammatory disorder driven by type 2 inflammation that damages the esophagus and causes difficulty swallowing and eating.
Dupilumab is a monoclonal antibody that acts to inhibit part of the inflammatory pathway. It’s the first drug to be approved by the FDA for EoE.
In a phase 3 trial, dupilumab 300 mg weekly significantly improved signs and symptoms of eosinophilic esophagitis, compared with placebo, underscoring the role of type 2 inflammation in this disease, Regeneron says in a news release.
According to the company, there are roughly 160,000 patients in the United States living with EoE who are currently using treatments not specifically approved for the disease. Of those patients, about 48,000 continue to experience symptoms despite multiple treatments.
“As researchers and clinicians have gained knowledge about eosinophilic esophagitis in recent years, more cases of the disorder have been recognized and diagnosed in the U.S.,” Jessica Lee, MD, director of the Division of Gastroenterology in the FDA’s Center for Drug Evaluation and Research, said in an FDA news release.
The approval of dupilumab will “fulfill an important unmet need for the increasing number of patients with eosinophilic esophagitis,” Dr. Lee said.
The efficacy and safety of dupilumab in EoE was demonstrated in a randomized, double-blind, parallel-group, multicenter, placebo-controlled trial that included two 24-week treatment periods (parts A and B) that were conducted independently in separate groups of patients.
In both part A and B, patients received dupilumab 300 mg or placebo every week.
In part A of the trial, 60% of the 42 patients who received dupilumab achieved the predetermined level of reduction of eosinophils in the esophagus, compared with 5% of the 39 patients who received placebo, the FDA said.
Patients who received dupilumab also experienced an average improvement of 22 points in the Dysphagia Symptom Questionnaire (DSQ) score, compared with 10 points for patients who received placebo.
In part B, 59% of the 80 patients who received dupilumab achieved the predetermined level of reduction of eosinophils in the esophagus, compared with 6% of the 79 patients who received placebo.
Patients who received dupilumab also experienced an average improvement of 24 points in their DSQ score, compared with 14 points for patients who received placebo.
“Assessments incorporating the perspectives from patients with EoE supported that the DSQ score improvement in patients who received Dupixent in the clinical trial was representative of clinically meaningful improvement in dysphagia,” the FDA noted.
“Treatment for patients with eosinophilic esophagitis can be challenging, particularly with no previously approved medications,” Evan Dellon, MD, principal investigator for the phase 3 trial, said in the company news release.
“Now, patients and their doctors have a treatment option available as part of their management plan that has the potential to control symptoms, improve inflammation, and heal the changes in the esophagus caused by this progressive and burdensome disease,” added Dr. Dellon, who is professor of medicine in the division of gastroenterology and hepatology at the University of North Carolina at Chapel Hill.
The FDA granted dupilumab priority review and breakthrough therapy designations for EoE.
Dupilumab is already approved in the United States for treatment of moderate to severe atopic dermatitis in adults and children aged 6 years and older whose disease is not adequately controlled by topical prescription therapies or for whom those therapies are not advisable.
The drug is also approved as an add-on maintenance treatment for adults and children aged 6 years and older with certain types of moderate to severe asthma and as an add-on maintenance treatment for adults with inadequately controlled chronic rhinosinusitis with nasal polyposis.
A version of this article first appeared on Medscape.com .
Eosinophilic esophagitis (EoE) is a chronic disease requiring long-term treatment for both induction and maintenance of response. For decades, however, Food and Drug Administration–approved therapies for EoE have not been available. Dupilumab is the first drug to receive FDA approval to treat EoE. This human monoclonal antibody directed against the interleukin (IL)4 receptor–alpha component of the type 2 receptor inhibits signaling of IL4 and IL13. Dupilumab has shown efficacy in similar diseases, such as atopic dermatitis and eosinophilic asthma. In 2017 dupilumab was granted Orphan Drug designation for the potential treatment of EoE and in 2020 the FDA granted Breakthrough Therapy designation for EoE. Recent data from the phase 3 trial of dupilumab 300 mg weekly enrolling patients aged 12 years and older demonstrated a significantly greater reduction in disease symptoms, normalization of esophageal eosinophilia, and reduction in endoscopic findings by week 24 compared with placebo.
The highly anticipated approval of dupilumab marks a paradigm shift toward biologic medications for treatment of EoE when historical treatments have relied on proton pump–inhibitor therapy or topical swallowed steroids. As we await updates about availability and access of dupilumab for our patients, we can rest assured that a highly efficacious treatment is now approved and will fill an important treatment gap in EoE, particularly for patients not deriving adequate response with traditionally used strategies. With multiple clinical trials underway, this milestone likely represents the beginning of additional effective therapies (nonbiologic and biologic) that will be available for EoE.
Rena Yadlapati, MD, MSHS, FACG, is associate professor of clinical medicine in the division of gastroenterology at the University of California, San Diego, medical director of the UCSD Center for Esophageal Diseases, and director of the GI Motility Lab. She has no relevant conflicts of interest.
Eosinophilic esophagitis (EoE) is a chronic disease requiring long-term treatment for both induction and maintenance of response. For decades, however, Food and Drug Administration–approved therapies for EoE have not been available. Dupilumab is the first drug to receive FDA approval to treat EoE. This human monoclonal antibody directed against the interleukin (IL)4 receptor–alpha component of the type 2 receptor inhibits signaling of IL4 and IL13. Dupilumab has shown efficacy in similar diseases, such as atopic dermatitis and eosinophilic asthma. In 2017 dupilumab was granted Orphan Drug designation for the potential treatment of EoE and in 2020 the FDA granted Breakthrough Therapy designation for EoE. Recent data from the phase 3 trial of dupilumab 300 mg weekly enrolling patients aged 12 years and older demonstrated a significantly greater reduction in disease symptoms, normalization of esophageal eosinophilia, and reduction in endoscopic findings by week 24 compared with placebo.
The highly anticipated approval of dupilumab marks a paradigm shift toward biologic medications for treatment of EoE when historical treatments have relied on proton pump–inhibitor therapy or topical swallowed steroids. As we await updates about availability and access of dupilumab for our patients, we can rest assured that a highly efficacious treatment is now approved and will fill an important treatment gap in EoE, particularly for patients not deriving adequate response with traditionally used strategies. With multiple clinical trials underway, this milestone likely represents the beginning of additional effective therapies (nonbiologic and biologic) that will be available for EoE.
Rena Yadlapati, MD, MSHS, FACG, is associate professor of clinical medicine in the division of gastroenterology at the University of California, San Diego, medical director of the UCSD Center for Esophageal Diseases, and director of the GI Motility Lab. She has no relevant conflicts of interest.
Eosinophilic esophagitis (EoE) is a chronic disease requiring long-term treatment for both induction and maintenance of response. For decades, however, Food and Drug Administration–approved therapies for EoE have not been available. Dupilumab is the first drug to receive FDA approval to treat EoE. This human monoclonal antibody directed against the interleukin (IL)4 receptor–alpha component of the type 2 receptor inhibits signaling of IL4 and IL13. Dupilumab has shown efficacy in similar diseases, such as atopic dermatitis and eosinophilic asthma. In 2017 dupilumab was granted Orphan Drug designation for the potential treatment of EoE and in 2020 the FDA granted Breakthrough Therapy designation for EoE. Recent data from the phase 3 trial of dupilumab 300 mg weekly enrolling patients aged 12 years and older demonstrated a significantly greater reduction in disease symptoms, normalization of esophageal eosinophilia, and reduction in endoscopic findings by week 24 compared with placebo.
The highly anticipated approval of dupilumab marks a paradigm shift toward biologic medications for treatment of EoE when historical treatments have relied on proton pump–inhibitor therapy or topical swallowed steroids. As we await updates about availability and access of dupilumab for our patients, we can rest assured that a highly efficacious treatment is now approved and will fill an important treatment gap in EoE, particularly for patients not deriving adequate response with traditionally used strategies. With multiple clinical trials underway, this milestone likely represents the beginning of additional effective therapies (nonbiologic and biologic) that will be available for EoE.
Rena Yadlapati, MD, MSHS, FACG, is associate professor of clinical medicine in the division of gastroenterology at the University of California, San Diego, medical director of the UCSD Center for Esophageal Diseases, and director of the GI Motility Lab. She has no relevant conflicts of interest.
The U.S. Food and Drug Administration has approved dupilumab (Dupixent, Regeneron) to treat eosinophilic esophagitis (EoE) in adults and children aged 12 years and older weighing at least 40 kg.
EoE is a chronic inflammatory disorder driven by type 2 inflammation that damages the esophagus and causes difficulty swallowing and eating.
Dupilumab is a monoclonal antibody that acts to inhibit part of the inflammatory pathway. It’s the first drug to be approved by the FDA for EoE.
In a phase 3 trial, dupilumab 300 mg weekly significantly improved signs and symptoms of eosinophilic esophagitis, compared with placebo, underscoring the role of type 2 inflammation in this disease, Regeneron says in a news release.
According to the company, there are roughly 160,000 patients in the United States living with EoE who are currently using treatments not specifically approved for the disease. Of those patients, about 48,000 continue to experience symptoms despite multiple treatments.
“As researchers and clinicians have gained knowledge about eosinophilic esophagitis in recent years, more cases of the disorder have been recognized and diagnosed in the U.S.,” Jessica Lee, MD, director of the Division of Gastroenterology in the FDA’s Center for Drug Evaluation and Research, said in an FDA news release.
The approval of dupilumab will “fulfill an important unmet need for the increasing number of patients with eosinophilic esophagitis,” Dr. Lee said.
The efficacy and safety of dupilumab in EoE was demonstrated in a randomized, double-blind, parallel-group, multicenter, placebo-controlled trial that included two 24-week treatment periods (parts A and B) that were conducted independently in separate groups of patients.
In both part A and B, patients received dupilumab 300 mg or placebo every week.
In part A of the trial, 60% of the 42 patients who received dupilumab achieved the predetermined level of reduction of eosinophils in the esophagus, compared with 5% of the 39 patients who received placebo, the FDA said.
Patients who received dupilumab also experienced an average improvement of 22 points in the Dysphagia Symptom Questionnaire (DSQ) score, compared with 10 points for patients who received placebo.
In part B, 59% of the 80 patients who received dupilumab achieved the predetermined level of reduction of eosinophils in the esophagus, compared with 6% of the 79 patients who received placebo.
Patients who received dupilumab also experienced an average improvement of 24 points in their DSQ score, compared with 14 points for patients who received placebo.
“Assessments incorporating the perspectives from patients with EoE supported that the DSQ score improvement in patients who received Dupixent in the clinical trial was representative of clinically meaningful improvement in dysphagia,” the FDA noted.
“Treatment for patients with eosinophilic esophagitis can be challenging, particularly with no previously approved medications,” Evan Dellon, MD, principal investigator for the phase 3 trial, said in the company news release.
“Now, patients and their doctors have a treatment option available as part of their management plan that has the potential to control symptoms, improve inflammation, and heal the changes in the esophagus caused by this progressive and burdensome disease,” added Dr. Dellon, who is professor of medicine in the division of gastroenterology and hepatology at the University of North Carolina at Chapel Hill.
The FDA granted dupilumab priority review and breakthrough therapy designations for EoE.
Dupilumab is already approved in the United States for treatment of moderate to severe atopic dermatitis in adults and children aged 6 years and older whose disease is not adequately controlled by topical prescription therapies or for whom those therapies are not advisable.
The drug is also approved as an add-on maintenance treatment for adults and children aged 6 years and older with certain types of moderate to severe asthma and as an add-on maintenance treatment for adults with inadequately controlled chronic rhinosinusitis with nasal polyposis.
A version of this article first appeared on Medscape.com .
The U.S. Food and Drug Administration has approved dupilumab (Dupixent, Regeneron) to treat eosinophilic esophagitis (EoE) in adults and children aged 12 years and older weighing at least 40 kg.
EoE is a chronic inflammatory disorder driven by type 2 inflammation that damages the esophagus and causes difficulty swallowing and eating.
Dupilumab is a monoclonal antibody that acts to inhibit part of the inflammatory pathway. It’s the first drug to be approved by the FDA for EoE.
In a phase 3 trial, dupilumab 300 mg weekly significantly improved signs and symptoms of eosinophilic esophagitis, compared with placebo, underscoring the role of type 2 inflammation in this disease, Regeneron says in a news release.
According to the company, there are roughly 160,000 patients in the United States living with EoE who are currently using treatments not specifically approved for the disease. Of those patients, about 48,000 continue to experience symptoms despite multiple treatments.
“As researchers and clinicians have gained knowledge about eosinophilic esophagitis in recent years, more cases of the disorder have been recognized and diagnosed in the U.S.,” Jessica Lee, MD, director of the Division of Gastroenterology in the FDA’s Center for Drug Evaluation and Research, said in an FDA news release.
The approval of dupilumab will “fulfill an important unmet need for the increasing number of patients with eosinophilic esophagitis,” Dr. Lee said.
The efficacy and safety of dupilumab in EoE was demonstrated in a randomized, double-blind, parallel-group, multicenter, placebo-controlled trial that included two 24-week treatment periods (parts A and B) that were conducted independently in separate groups of patients.
In both part A and B, patients received dupilumab 300 mg or placebo every week.
In part A of the trial, 60% of the 42 patients who received dupilumab achieved the predetermined level of reduction of eosinophils in the esophagus, compared with 5% of the 39 patients who received placebo, the FDA said.
Patients who received dupilumab also experienced an average improvement of 22 points in the Dysphagia Symptom Questionnaire (DSQ) score, compared with 10 points for patients who received placebo.
In part B, 59% of the 80 patients who received dupilumab achieved the predetermined level of reduction of eosinophils in the esophagus, compared with 6% of the 79 patients who received placebo.
Patients who received dupilumab also experienced an average improvement of 24 points in their DSQ score, compared with 14 points for patients who received placebo.
“Assessments incorporating the perspectives from patients with EoE supported that the DSQ score improvement in patients who received Dupixent in the clinical trial was representative of clinically meaningful improvement in dysphagia,” the FDA noted.
“Treatment for patients with eosinophilic esophagitis can be challenging, particularly with no previously approved medications,” Evan Dellon, MD, principal investigator for the phase 3 trial, said in the company news release.
“Now, patients and their doctors have a treatment option available as part of their management plan that has the potential to control symptoms, improve inflammation, and heal the changes in the esophagus caused by this progressive and burdensome disease,” added Dr. Dellon, who is professor of medicine in the division of gastroenterology and hepatology at the University of North Carolina at Chapel Hill.
The FDA granted dupilumab priority review and breakthrough therapy designations for EoE.
Dupilumab is already approved in the United States for treatment of moderate to severe atopic dermatitis in adults and children aged 6 years and older whose disease is not adequately controlled by topical prescription therapies or for whom those therapies are not advisable.
The drug is also approved as an add-on maintenance treatment for adults and children aged 6 years and older with certain types of moderate to severe asthma and as an add-on maintenance treatment for adults with inadequately controlled chronic rhinosinusitis with nasal polyposis.
A version of this article first appeared on Medscape.com .
Psychiatric illness associated with eosinophilic esophagitis
People with eosinophilic esophagitis (EoE) may run an increased risk of mood disorders, anxiety, and ADHD and should be screened for those conditions, researchers say.
“It’s important to know that there is an elevated risk of those diagnoses, so you have that in mind when you treat your patients. You can assess their quality of life and the status of their mental state,” said lead author Lovisa Röjler, MD, a pediatrician and doctoral student at Örebro (Sweden) University Hospital.
“Psychiatric disorders are not found with a blood sample or radiology examination,” she said in an interview.
The study was published online in the American Journal of Gastroenterology.
Elevated risk found
Previous studies into the relationship between EoE and anxiety and depression have conflicting conclusions.
In the hope of shedding further light, Dr. Röjler and colleagues analyzed data from Sweden’s ESPRESSO cohort, which consists of more than 6 million biopsy samples from the gastrointestinal tract that were collected from throughout the country during the years 1965-2017.
They identified 1,458 people with EoE who had not experienced psychiatric events before being diagnosed with EoE. Of these, 70% had dysphagia, and 58% had food impaction.
In the study, up to 5 reference persons (6,436 people) without EoE who were identified from the Swedish Total Population Register were matched to the patients with EoE by age, sex, county, and year of diagnosis.
Among the people with EoE, there were 106 events of psychiatric disease, at an incidence of 15.96 per 1,000 person-years versus 10.93 per 1,000 person-years (331 events) among those without EoE. This 50% increased risk for psychiatric illness for people with EoE was statistically significant (hazard ratio, 1.50; 95% confidence interval, 1.20-1.87).
To adjust for genetic and environmental confounding factors, the researchers compared the rate of psychiatric events among 1,055 people with EoE with that of siblings who did not have EoE (1,699 people). There were 74 events of psychiatric disease among the siblings (8.99 per 1000 person-years). From this the researchers calculated a 62% increased risk of psychiatric events for those with EoE (HR, 1.62; 95% CI, 1.14-2.31).
There was no difference in risk for psychiatric disorders by educational attainment, though people for whom there were no data on education were at increased risk.
There was also no difference in psychiatric risk associated with the use of steroids or proton pump inhibitors for EoE, though these medications have sometimes been linked to psychiatric disorders.
After adjusting for inflammatory bowel disease, celiac disease, and asthma, the researchers still found an increased risk of psychiatric events. Also, the people who had EoE were no more likely than the reference persons to have had psychiatric events before their diagnosis, suggesting that EoE caused the psychiatric events rather than the other way around.
Previous researchers have found a similar association with psychiatric illness in people with celiac disease and inflammatory bowel disease. The researchers speculated that people with EoE might develop psychiatric illnesses because their symptoms and treatments, such as restrictive diets, cause stress and chronic pain and thereby cause problems with education, work, and social and economic status.
Dr. Röjler recommended that clinicians use questionnaires to identify mood disorders and ADHD in their patients and then refer them to a mental health professional.
Screen for psychiatric disorders
Tiffany Taft, PsyD, a research associate professor of medicine at Northwestern University, Chicago, who was not involved in the study, agreed that patients with EoE should be screened more often for psychiatric disorders.
“We’ve found that symptom-specific anxiety is prevalent and associated with other outcomes, like quality of life, so it may not be the typical anxiety that you would diagnose from the Diagnostic and Statistical Manual of Mental Disorders,” Dr. Taft said in an interview.
While anxiety is not likely to trigger EoE, it can worsen the symptoms, she said. Sometimes helping patients make the connection between their mental health and EoE can address the anxiety itself.
“Education is good enough for a certain chunk of patients,” Dr. Taft said.
Other patients benefit from cognitive-behavioral therapy, which gives them a more realistic understanding of their situation.
“We also add in relaxation, deep breathing, and guided imagery to calm down the stress response in the body, which is part of that brain-gut connection that enhances symptom severity,” she said.
Some patients prefer medications, or they rely on medication because that is what their insurance provides, she said, adding that most patients do best with a combination of medication and talk therapy.
Ideally, people with these disorders would be referred to someone such as herself, a psychotherapist with a specialty in gastroenterology, Dr. Taft said. But there are not many people in that subspecialty, so if a gastroenterology psychologist is not available, a psychologist who specializes in treating mental illness associated with chronic diseases is a good second choice.
The study was funded by Örebro County Council and Karolinska Institutet. Dr. Röjler and Dr. Taft reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
People with eosinophilic esophagitis (EoE) may run an increased risk of mood disorders, anxiety, and ADHD and should be screened for those conditions, researchers say.
“It’s important to know that there is an elevated risk of those diagnoses, so you have that in mind when you treat your patients. You can assess their quality of life and the status of their mental state,” said lead author Lovisa Röjler, MD, a pediatrician and doctoral student at Örebro (Sweden) University Hospital.
“Psychiatric disorders are not found with a blood sample or radiology examination,” she said in an interview.
The study was published online in the American Journal of Gastroenterology.
Elevated risk found
Previous studies into the relationship between EoE and anxiety and depression have conflicting conclusions.
In the hope of shedding further light, Dr. Röjler and colleagues analyzed data from Sweden’s ESPRESSO cohort, which consists of more than 6 million biopsy samples from the gastrointestinal tract that were collected from throughout the country during the years 1965-2017.
They identified 1,458 people with EoE who had not experienced psychiatric events before being diagnosed with EoE. Of these, 70% had dysphagia, and 58% had food impaction.
In the study, up to 5 reference persons (6,436 people) without EoE who were identified from the Swedish Total Population Register were matched to the patients with EoE by age, sex, county, and year of diagnosis.
Among the people with EoE, there were 106 events of psychiatric disease, at an incidence of 15.96 per 1,000 person-years versus 10.93 per 1,000 person-years (331 events) among those without EoE. This 50% increased risk for psychiatric illness for people with EoE was statistically significant (hazard ratio, 1.50; 95% confidence interval, 1.20-1.87).
To adjust for genetic and environmental confounding factors, the researchers compared the rate of psychiatric events among 1,055 people with EoE with that of siblings who did not have EoE (1,699 people). There were 74 events of psychiatric disease among the siblings (8.99 per 1000 person-years). From this the researchers calculated a 62% increased risk of psychiatric events for those with EoE (HR, 1.62; 95% CI, 1.14-2.31).
There was no difference in risk for psychiatric disorders by educational attainment, though people for whom there were no data on education were at increased risk.
There was also no difference in psychiatric risk associated with the use of steroids or proton pump inhibitors for EoE, though these medications have sometimes been linked to psychiatric disorders.
After adjusting for inflammatory bowel disease, celiac disease, and asthma, the researchers still found an increased risk of psychiatric events. Also, the people who had EoE were no more likely than the reference persons to have had psychiatric events before their diagnosis, suggesting that EoE caused the psychiatric events rather than the other way around.
Previous researchers have found a similar association with psychiatric illness in people with celiac disease and inflammatory bowel disease. The researchers speculated that people with EoE might develop psychiatric illnesses because their symptoms and treatments, such as restrictive diets, cause stress and chronic pain and thereby cause problems with education, work, and social and economic status.
Dr. Röjler recommended that clinicians use questionnaires to identify mood disorders and ADHD in their patients and then refer them to a mental health professional.
Screen for psychiatric disorders
Tiffany Taft, PsyD, a research associate professor of medicine at Northwestern University, Chicago, who was not involved in the study, agreed that patients with EoE should be screened more often for psychiatric disorders.
“We’ve found that symptom-specific anxiety is prevalent and associated with other outcomes, like quality of life, so it may not be the typical anxiety that you would diagnose from the Diagnostic and Statistical Manual of Mental Disorders,” Dr. Taft said in an interview.
While anxiety is not likely to trigger EoE, it can worsen the symptoms, she said. Sometimes helping patients make the connection between their mental health and EoE can address the anxiety itself.
“Education is good enough for a certain chunk of patients,” Dr. Taft said.
Other patients benefit from cognitive-behavioral therapy, which gives them a more realistic understanding of their situation.
“We also add in relaxation, deep breathing, and guided imagery to calm down the stress response in the body, which is part of that brain-gut connection that enhances symptom severity,” she said.
Some patients prefer medications, or they rely on medication because that is what their insurance provides, she said, adding that most patients do best with a combination of medication and talk therapy.
Ideally, people with these disorders would be referred to someone such as herself, a psychotherapist with a specialty in gastroenterology, Dr. Taft said. But there are not many people in that subspecialty, so if a gastroenterology psychologist is not available, a psychologist who specializes in treating mental illness associated with chronic diseases is a good second choice.
The study was funded by Örebro County Council and Karolinska Institutet. Dr. Röjler and Dr. Taft reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
People with eosinophilic esophagitis (EoE) may run an increased risk of mood disorders, anxiety, and ADHD and should be screened for those conditions, researchers say.
“It’s important to know that there is an elevated risk of those diagnoses, so you have that in mind when you treat your patients. You can assess their quality of life and the status of their mental state,” said lead author Lovisa Röjler, MD, a pediatrician and doctoral student at Örebro (Sweden) University Hospital.
“Psychiatric disorders are not found with a blood sample or radiology examination,” she said in an interview.
The study was published online in the American Journal of Gastroenterology.
Elevated risk found
Previous studies into the relationship between EoE and anxiety and depression have conflicting conclusions.
In the hope of shedding further light, Dr. Röjler and colleagues analyzed data from Sweden’s ESPRESSO cohort, which consists of more than 6 million biopsy samples from the gastrointestinal tract that were collected from throughout the country during the years 1965-2017.
They identified 1,458 people with EoE who had not experienced psychiatric events before being diagnosed with EoE. Of these, 70% had dysphagia, and 58% had food impaction.
In the study, up to 5 reference persons (6,436 people) without EoE who were identified from the Swedish Total Population Register were matched to the patients with EoE by age, sex, county, and year of diagnosis.
Among the people with EoE, there were 106 events of psychiatric disease, at an incidence of 15.96 per 1,000 person-years versus 10.93 per 1,000 person-years (331 events) among those without EoE. This 50% increased risk for psychiatric illness for people with EoE was statistically significant (hazard ratio, 1.50; 95% confidence interval, 1.20-1.87).
To adjust for genetic and environmental confounding factors, the researchers compared the rate of psychiatric events among 1,055 people with EoE with that of siblings who did not have EoE (1,699 people). There were 74 events of psychiatric disease among the siblings (8.99 per 1000 person-years). From this the researchers calculated a 62% increased risk of psychiatric events for those with EoE (HR, 1.62; 95% CI, 1.14-2.31).
There was no difference in risk for psychiatric disorders by educational attainment, though people for whom there were no data on education were at increased risk.
There was also no difference in psychiatric risk associated with the use of steroids or proton pump inhibitors for EoE, though these medications have sometimes been linked to psychiatric disorders.
After adjusting for inflammatory bowel disease, celiac disease, and asthma, the researchers still found an increased risk of psychiatric events. Also, the people who had EoE were no more likely than the reference persons to have had psychiatric events before their diagnosis, suggesting that EoE caused the psychiatric events rather than the other way around.
Previous researchers have found a similar association with psychiatric illness in people with celiac disease and inflammatory bowel disease. The researchers speculated that people with EoE might develop psychiatric illnesses because their symptoms and treatments, such as restrictive diets, cause stress and chronic pain and thereby cause problems with education, work, and social and economic status.
Dr. Röjler recommended that clinicians use questionnaires to identify mood disorders and ADHD in their patients and then refer them to a mental health professional.
Screen for psychiatric disorders
Tiffany Taft, PsyD, a research associate professor of medicine at Northwestern University, Chicago, who was not involved in the study, agreed that patients with EoE should be screened more often for psychiatric disorders.
“We’ve found that symptom-specific anxiety is prevalent and associated with other outcomes, like quality of life, so it may not be the typical anxiety that you would diagnose from the Diagnostic and Statistical Manual of Mental Disorders,” Dr. Taft said in an interview.
While anxiety is not likely to trigger EoE, it can worsen the symptoms, she said. Sometimes helping patients make the connection between their mental health and EoE can address the anxiety itself.
“Education is good enough for a certain chunk of patients,” Dr. Taft said.
Other patients benefit from cognitive-behavioral therapy, which gives them a more realistic understanding of their situation.
“We also add in relaxation, deep breathing, and guided imagery to calm down the stress response in the body, which is part of that brain-gut connection that enhances symptom severity,” she said.
Some patients prefer medications, or they rely on medication because that is what their insurance provides, she said, adding that most patients do best with a combination of medication and talk therapy.
Ideally, people with these disorders would be referred to someone such as herself, a psychotherapist with a specialty in gastroenterology, Dr. Taft said. But there are not many people in that subspecialty, so if a gastroenterology psychologist is not available, a psychologist who specializes in treating mental illness associated with chronic diseases is a good second choice.
The study was funded by Örebro County Council and Karolinska Institutet. Dr. Röjler and Dr. Taft reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF GASTROENTEROLOGY
Vibrating wearable device may help night time GERD
A vibrating wearable device helped people with gastroesophageal reflux disease (GERD) stay positioned on their left side while sleeping, alleviating night time reflux symptoms, compared with sham treatment, a small, randomized study suggests.
People often report having more reflux symptoms when sleeping on their right side, and experimental studies suggest that sleeping on the right side is associated with higher esophageal acid exposure time and slower esophageal acid clearance, compared with sleeping on the left side, the authors wrote.
They cite a possible cause as the stomach being above the esophagus when a person is sleeping on their right side, resulting in more reflux.
“There are two very exciting new things that can be learned,” Arjan Bredenoord, MD, PhD, a principal investigator of the study and professor of neurogastroenterology and motility at the Academic Medical Center in Amsterdam, told this news organization. “First, we show that a device that trains people to sleep on the left side really helps to relieve nocturnal reflux symptoms. Second, the study was performed completely remotely, with the patients being at home.”
“The devices were shipped to the patients. All contact was via video calling, and questionnaires were done via links in emails that were linked to secure databases to store the patients’ symptom responses,” he added.
The findings were published online in Clinical Gastroenterology and Hepatology.
A study in sleep positional therapy
Researchers performed a double-blind, randomized, sham-controlled trial in 100 patients with night time GERD symptoms who wore a programmed device (about 1.5 inches square) on their chest, midsternum.
Patients were advised to sleep on their left side and randomly assigned (1:1) either to a group whose device produced a gentle vibration when they flipped onto their right side throughout sleep or to the group whose device vibrated when they flipped to the right side but only for the first 20 minutes of use (the sham intervention).
The primary outcome for success was at least a 50% reduction in the Nocturnal Gastroesophageal Reflux Disease Symptom Severity and Impact Questionnaire (N-GSSIQ) score. Secondary outcomes included change in sleep position and reflux symptoms.
In the intention-to-treat analysis, the rate of treatment success was 44% in the intervention group versus 24% in the sham group. The risk difference was 20% (95% confidence interval, 1.8% to 38.2%; P = .03).
Treatment led to a significant avoidance of sleeping on the right side (intervention 2.2% vs. sham 23.5%; P ≤ .0001) and an increased time of sleeping on the left side (intervention 60.9% vs. sham 38.5%; P ≤ .0001).
Patients in the intervention group also had more reflux-free nights (9 nights vs. 6 nights for the sham group).
After 2 weeks of treatment, the average total N-GSSIQ scores were lower in the active device group (18.8 vs. 23.7 in the sham group; P = .04).
Most with GERD have night time symptoms
The authors pointed out that up to 80% of patients with GERD experience symptoms during the night, such as heartburn and regurgitation, which can significantly impair sleep quality and daytime functioning.
Solutions are of high interest because current measures have shortcomings.
Raising the head-end of the bed and lengthening the time between dinner and bedtime have limited effect, the authors explained. And while proton pump inhibitors are very effective for daytime symptoms, they have limited efficacy for night time reflux symptoms.
Antireflux pillows, which are designed to keep patients on their left side through the night, have been found to result in less recumbent acid exposure and less self-reported night time reflux symptoms, but they do not allow for spontaneous body movements and can be uncomfortable, they explained.
The lightweight vibration device, made by Side Sleep Technologies BV, registers the sleep position of a subject at 30-second intervals. It categorizes sleep position as supine, right, left, prone, or upright.
Michiel Allessie, CEO of Side Sleep Technologies, told this news organization that the wearable V1.0 is sold as a consumer electronic device rather than a medical device in the United Kingdom for £99. He said the company expects to sell the V1.0 in the United States starting in June, with a target price of $99.
Promising but device still needs real-world testing
When asked to comment, Philip Katz, MD, a gastroenterologist at Weill Cornell Medicine, New York, said it was a fantastic study scientifically and academically incredibly interesting, but the device is not a panacea.
Dr. Katz said he will remain skeptical until the device is tested in real life and added that it’s important to remember this is one study with 100 people.
He also wondered whether there might be an even better solution in a well-designed wedge, for example, and whether the buzzing of this product might affect sleep quality. If so, would that be worth the tradeoff?
Dr. Katz noted that busy physicians may not have the time to determine whether patients truly have nocturnal GERD or just similar symptoms. This study included people who were carefully screened by the researchers for nocturnal reflux symptoms, he pointed out.
Based on this study, Dr. Katz said he would tell patients, “You have a 50% chance to be helped because their primary outcome was met by 44%.”
He said the decision is up to the patients and comes down to this: “It’s better than nothing for sure. Is it worth $100? You tell me.”
Dr. Bredenoord said the next step is a study using pH-impedance monitoring of the esophagus to show that there is also an effect on reflux episodes.
The investigational medical devices were provided free of charge and without restrictions by Side Sleep Technologies BV. Dr. Bredenoord disclosed research funding from Nutricia, Norgine, SST, Thelial, and Bayer; speaker and/or consulting fees from Laborie, EsoCap, Medtronic, Dr. Falk Pharma, Calypso Biotech, Alimentiv, Reckett Benkiser, Regeneron, and AstraZeneca; and previously owned shares in Side Sleep Technologies BV. Another coauthor received research funding from Boston Scientific and speaker and/or consulting fees from Cook and Olympus. The remaining authors have disclosed no relevant financial relationships. Dr. Katz reported being a consultant for Phathom Pharmaceuticals and Sebela.
A version of this article first appeared on Medscape.com.
A vibrating wearable device helped people with gastroesophageal reflux disease (GERD) stay positioned on their left side while sleeping, alleviating night time reflux symptoms, compared with sham treatment, a small, randomized study suggests.
People often report having more reflux symptoms when sleeping on their right side, and experimental studies suggest that sleeping on the right side is associated with higher esophageal acid exposure time and slower esophageal acid clearance, compared with sleeping on the left side, the authors wrote.
They cite a possible cause as the stomach being above the esophagus when a person is sleeping on their right side, resulting in more reflux.
“There are two very exciting new things that can be learned,” Arjan Bredenoord, MD, PhD, a principal investigator of the study and professor of neurogastroenterology and motility at the Academic Medical Center in Amsterdam, told this news organization. “First, we show that a device that trains people to sleep on the left side really helps to relieve nocturnal reflux symptoms. Second, the study was performed completely remotely, with the patients being at home.”
“The devices were shipped to the patients. All contact was via video calling, and questionnaires were done via links in emails that were linked to secure databases to store the patients’ symptom responses,” he added.
The findings were published online in Clinical Gastroenterology and Hepatology.
A study in sleep positional therapy
Researchers performed a double-blind, randomized, sham-controlled trial in 100 patients with night time GERD symptoms who wore a programmed device (about 1.5 inches square) on their chest, midsternum.
Patients were advised to sleep on their left side and randomly assigned (1:1) either to a group whose device produced a gentle vibration when they flipped onto their right side throughout sleep or to the group whose device vibrated when they flipped to the right side but only for the first 20 minutes of use (the sham intervention).
The primary outcome for success was at least a 50% reduction in the Nocturnal Gastroesophageal Reflux Disease Symptom Severity and Impact Questionnaire (N-GSSIQ) score. Secondary outcomes included change in sleep position and reflux symptoms.
In the intention-to-treat analysis, the rate of treatment success was 44% in the intervention group versus 24% in the sham group. The risk difference was 20% (95% confidence interval, 1.8% to 38.2%; P = .03).
Treatment led to a significant avoidance of sleeping on the right side (intervention 2.2% vs. sham 23.5%; P ≤ .0001) and an increased time of sleeping on the left side (intervention 60.9% vs. sham 38.5%; P ≤ .0001).
Patients in the intervention group also had more reflux-free nights (9 nights vs. 6 nights for the sham group).
After 2 weeks of treatment, the average total N-GSSIQ scores were lower in the active device group (18.8 vs. 23.7 in the sham group; P = .04).
Most with GERD have night time symptoms
The authors pointed out that up to 80% of patients with GERD experience symptoms during the night, such as heartburn and regurgitation, which can significantly impair sleep quality and daytime functioning.
Solutions are of high interest because current measures have shortcomings.
Raising the head-end of the bed and lengthening the time between dinner and bedtime have limited effect, the authors explained. And while proton pump inhibitors are very effective for daytime symptoms, they have limited efficacy for night time reflux symptoms.
Antireflux pillows, which are designed to keep patients on their left side through the night, have been found to result in less recumbent acid exposure and less self-reported night time reflux symptoms, but they do not allow for spontaneous body movements and can be uncomfortable, they explained.
The lightweight vibration device, made by Side Sleep Technologies BV, registers the sleep position of a subject at 30-second intervals. It categorizes sleep position as supine, right, left, prone, or upright.
Michiel Allessie, CEO of Side Sleep Technologies, told this news organization that the wearable V1.0 is sold as a consumer electronic device rather than a medical device in the United Kingdom for £99. He said the company expects to sell the V1.0 in the United States starting in June, with a target price of $99.
Promising but device still needs real-world testing
When asked to comment, Philip Katz, MD, a gastroenterologist at Weill Cornell Medicine, New York, said it was a fantastic study scientifically and academically incredibly interesting, but the device is not a panacea.
Dr. Katz said he will remain skeptical until the device is tested in real life and added that it’s important to remember this is one study with 100 people.
He also wondered whether there might be an even better solution in a well-designed wedge, for example, and whether the buzzing of this product might affect sleep quality. If so, would that be worth the tradeoff?
Dr. Katz noted that busy physicians may not have the time to determine whether patients truly have nocturnal GERD or just similar symptoms. This study included people who were carefully screened by the researchers for nocturnal reflux symptoms, he pointed out.
Based on this study, Dr. Katz said he would tell patients, “You have a 50% chance to be helped because their primary outcome was met by 44%.”
He said the decision is up to the patients and comes down to this: “It’s better than nothing for sure. Is it worth $100? You tell me.”
Dr. Bredenoord said the next step is a study using pH-impedance monitoring of the esophagus to show that there is also an effect on reflux episodes.
The investigational medical devices were provided free of charge and without restrictions by Side Sleep Technologies BV. Dr. Bredenoord disclosed research funding from Nutricia, Norgine, SST, Thelial, and Bayer; speaker and/or consulting fees from Laborie, EsoCap, Medtronic, Dr. Falk Pharma, Calypso Biotech, Alimentiv, Reckett Benkiser, Regeneron, and AstraZeneca; and previously owned shares in Side Sleep Technologies BV. Another coauthor received research funding from Boston Scientific and speaker and/or consulting fees from Cook and Olympus. The remaining authors have disclosed no relevant financial relationships. Dr. Katz reported being a consultant for Phathom Pharmaceuticals and Sebela.
A version of this article first appeared on Medscape.com.
A vibrating wearable device helped people with gastroesophageal reflux disease (GERD) stay positioned on their left side while sleeping, alleviating night time reflux symptoms, compared with sham treatment, a small, randomized study suggests.
People often report having more reflux symptoms when sleeping on their right side, and experimental studies suggest that sleeping on the right side is associated with higher esophageal acid exposure time and slower esophageal acid clearance, compared with sleeping on the left side, the authors wrote.
They cite a possible cause as the stomach being above the esophagus when a person is sleeping on their right side, resulting in more reflux.
“There are two very exciting new things that can be learned,” Arjan Bredenoord, MD, PhD, a principal investigator of the study and professor of neurogastroenterology and motility at the Academic Medical Center in Amsterdam, told this news organization. “First, we show that a device that trains people to sleep on the left side really helps to relieve nocturnal reflux symptoms. Second, the study was performed completely remotely, with the patients being at home.”
“The devices were shipped to the patients. All contact was via video calling, and questionnaires were done via links in emails that were linked to secure databases to store the patients’ symptom responses,” he added.
The findings were published online in Clinical Gastroenterology and Hepatology.
A study in sleep positional therapy
Researchers performed a double-blind, randomized, sham-controlled trial in 100 patients with night time GERD symptoms who wore a programmed device (about 1.5 inches square) on their chest, midsternum.
Patients were advised to sleep on their left side and randomly assigned (1:1) either to a group whose device produced a gentle vibration when they flipped onto their right side throughout sleep or to the group whose device vibrated when they flipped to the right side but only for the first 20 minutes of use (the sham intervention).
The primary outcome for success was at least a 50% reduction in the Nocturnal Gastroesophageal Reflux Disease Symptom Severity and Impact Questionnaire (N-GSSIQ) score. Secondary outcomes included change in sleep position and reflux symptoms.
In the intention-to-treat analysis, the rate of treatment success was 44% in the intervention group versus 24% in the sham group. The risk difference was 20% (95% confidence interval, 1.8% to 38.2%; P = .03).
Treatment led to a significant avoidance of sleeping on the right side (intervention 2.2% vs. sham 23.5%; P ≤ .0001) and an increased time of sleeping on the left side (intervention 60.9% vs. sham 38.5%; P ≤ .0001).
Patients in the intervention group also had more reflux-free nights (9 nights vs. 6 nights for the sham group).
After 2 weeks of treatment, the average total N-GSSIQ scores were lower in the active device group (18.8 vs. 23.7 in the sham group; P = .04).
Most with GERD have night time symptoms
The authors pointed out that up to 80% of patients with GERD experience symptoms during the night, such as heartburn and regurgitation, which can significantly impair sleep quality and daytime functioning.
Solutions are of high interest because current measures have shortcomings.
Raising the head-end of the bed and lengthening the time between dinner and bedtime have limited effect, the authors explained. And while proton pump inhibitors are very effective for daytime symptoms, they have limited efficacy for night time reflux symptoms.
Antireflux pillows, which are designed to keep patients on their left side through the night, have been found to result in less recumbent acid exposure and less self-reported night time reflux symptoms, but they do not allow for spontaneous body movements and can be uncomfortable, they explained.
The lightweight vibration device, made by Side Sleep Technologies BV, registers the sleep position of a subject at 30-second intervals. It categorizes sleep position as supine, right, left, prone, or upright.
Michiel Allessie, CEO of Side Sleep Technologies, told this news organization that the wearable V1.0 is sold as a consumer electronic device rather than a medical device in the United Kingdom for £99. He said the company expects to sell the V1.0 in the United States starting in June, with a target price of $99.
Promising but device still needs real-world testing
When asked to comment, Philip Katz, MD, a gastroenterologist at Weill Cornell Medicine, New York, said it was a fantastic study scientifically and academically incredibly interesting, but the device is not a panacea.
Dr. Katz said he will remain skeptical until the device is tested in real life and added that it’s important to remember this is one study with 100 people.
He also wondered whether there might be an even better solution in a well-designed wedge, for example, and whether the buzzing of this product might affect sleep quality. If so, would that be worth the tradeoff?
Dr. Katz noted that busy physicians may not have the time to determine whether patients truly have nocturnal GERD or just similar symptoms. This study included people who were carefully screened by the researchers for nocturnal reflux symptoms, he pointed out.
Based on this study, Dr. Katz said he would tell patients, “You have a 50% chance to be helped because their primary outcome was met by 44%.”
He said the decision is up to the patients and comes down to this: “It’s better than nothing for sure. Is it worth $100? You tell me.”
Dr. Bredenoord said the next step is a study using pH-impedance monitoring of the esophagus to show that there is also an effect on reflux episodes.
The investigational medical devices were provided free of charge and without restrictions by Side Sleep Technologies BV. Dr. Bredenoord disclosed research funding from Nutricia, Norgine, SST, Thelial, and Bayer; speaker and/or consulting fees from Laborie, EsoCap, Medtronic, Dr. Falk Pharma, Calypso Biotech, Alimentiv, Reckett Benkiser, Regeneron, and AstraZeneca; and previously owned shares in Side Sleep Technologies BV. Another coauthor received research funding from Boston Scientific and speaker and/or consulting fees from Cook and Olympus. The remaining authors have disclosed no relevant financial relationships. Dr. Katz reported being a consultant for Phathom Pharmaceuticals and Sebela.
A version of this article first appeared on Medscape.com.
AGA Clinical Practice Update: Expert review on personalizing GERD management
A recent American Gastroenterological Association Clinical Practice Update for evaluation and management of gastroesophageal reflux disease (GERD) focuses on delivering personalized diagnostic and therapeutic strategies.
The document includes new advice on use of upfront objective testing for isolated extraesophageal symptoms, confirmation of GERD diagnosis prior to long-term GERD therapy even in PPI responders, as well as important elements focused on personalization of therapy.
Although GERD is common, with an estimated 30% of people in the United States experiencing symptoms, up to half of all individuals on proton pump inhibitor (PPI) therapy report incomplete symptom improvement. That could be due to the heterogeneous nature of symptoms, which may include heartburn and regurgitation, chest pain, and cough or sore throat, among others. Other conditions may produce similar symptoms or could be exacerbated by the presence of GERD.
The authors of the expert review, published in Clinical Gastroenterology and Hepatology, note that these considerations have driven increased interest in personalized approaches to the management of GERD. The practice update includes sections on how to approach GERD symptoms in the clinic, personalized diagnosis related to GERD symptoms, and precision management.
In the initial management, the authors offer advice on involving the patient in creating a care plan, patient education, and conducting a 4- to 8-week PPI trial in patients with heartburn, regurgitation, or noncardiac chest pains without accompanying alarm signals. If symptoms don’t improve to the patient’s satisfaction, dosing can be boosted to twice per day, or a more effective acid suppressor can be substituted and continued at a once-daily dose. When the response to PPIs is adequate, the dose should be reduced until the lowest effective dose is reached, or the patient could potentially be moved to H2 receptor antagonists or other antacids. However, patients with erosive esophagitis, biopsy-confirmed Barrett’s esophagus, or peptic stricture must stay on long-term PPI therapy.
The authors also gave advice on when to conduct objective testing. When a PPI trial doesn’t adequately address troublesome heartburn, regurgitation, and/or noncardiac chest pain, or if alarm systems are present, endoscopy should be employed to look for erosive reflux disease or long-segment Barrett’s esophagus as conclusive evidence for GERD. If these are absent, prolonged wireless pH monitoring while a patient is off medication is suggested. In addition, patients with extraesophageal symptoms suspected to be caused by reflux should undergo upfront objective reflux testing while off PPI therapy rather than doing an empiric PPI trial.
The authors advise that, if patients don’t have proven GERD and are continued on PPI therapy, they should be evaluated within 12 months to ensure that the therapy and dose are appropriate. Physicians should offer endoscopy with prolonged wireless reflux monitoring in the absence of PPI therapy (ideally after 2-4 weeks of withdrawal) to confirm that long-term PPI therapy is needed.
In the section on personalization of disease management, the authors note that ambulatory reflux monitoring and upper gastrointestinal endoscopy can be used to guide management of GERD. When upper GI endoscopy reveals no erosive findings and esophageal acid exposure time (AET) is less than 4% throughout all days of prolonged wireless pH monitoring, the physician can conclude that the patient has no pathologic gastroesophageal reflux and is likely to have a functional esophageal disorder. In contrast, erosive findings during upper GI endoscopy and/or AET more than 4% across at least 1 day of wireless pH monitoring suggests a GERD diagnosis.
Optimization of PPI is important among patients with GERD, and the authors stress that patients should be educated about the safety of PPI use.
Adjunctive pharmacotherapy is useful and can include alginate antacids for breakthrough symptoms, H2RAs for nocturnal symptoms, baclofen to counter regurgitation or belching, and prokinetics for accompanying gastroparesis. The choice of medications depends on the phenotype, and they should not be used empirically.
For patients with functional heartburn or reflux disease linked to esophageal hypervigilance, reflux sensitivity, or behavioral disorders, options include pharmacologic neuromodulation, hypnotherapy provided by a behavioral therapist, cognitive behavioral therapy, and diaphragmatic breathing and relaxation.
If symptoms persist despite efforts at optimization of treatments and lifestyle factors, ambulatory 24-hour pH-impedance monitoring on PPI can be used to investigate mechanistic causes, especially when there is no known antireflux barrier abnormality, but the technique requires expertise to correctly interpret. This can ensure that the symptoms are not due to reflux hypersensitivity, rumination syndrome, or a belching disorder. When symptoms are confirmed to be treatment resistant, therapy should be escalated, using a strategy that incorporates a pattern of reflux, integrity of the antireflux barrier, obesity if present, and psychological factors.
Surgical options for confirmed GERD include laparoscopic fundoplication and magnetic sphincter augmentation. Transoral incisionless fundoplication can be performed endoscopically in selected patients. For obese patients with confirmed GERD, Roux-en-Y gastric bypass is effective at reducing reflux and can be used as a salvage treatment for nonobese patients. Sleeve gastrectomy may exacerbate GERD.
The authors reported relationships with Medtronic, Diversatek, Ironwood, and Takeda. The authors also reported funding from National Institutes of Health grants.
A recent American Gastroenterological Association Clinical Practice Update for evaluation and management of gastroesophageal reflux disease (GERD) focuses on delivering personalized diagnostic and therapeutic strategies.
The document includes new advice on use of upfront objective testing for isolated extraesophageal symptoms, confirmation of GERD diagnosis prior to long-term GERD therapy even in PPI responders, as well as important elements focused on personalization of therapy.
Although GERD is common, with an estimated 30% of people in the United States experiencing symptoms, up to half of all individuals on proton pump inhibitor (PPI) therapy report incomplete symptom improvement. That could be due to the heterogeneous nature of symptoms, which may include heartburn and regurgitation, chest pain, and cough or sore throat, among others. Other conditions may produce similar symptoms or could be exacerbated by the presence of GERD.
The authors of the expert review, published in Clinical Gastroenterology and Hepatology, note that these considerations have driven increased interest in personalized approaches to the management of GERD. The practice update includes sections on how to approach GERD symptoms in the clinic, personalized diagnosis related to GERD symptoms, and precision management.
In the initial management, the authors offer advice on involving the patient in creating a care plan, patient education, and conducting a 4- to 8-week PPI trial in patients with heartburn, regurgitation, or noncardiac chest pains without accompanying alarm signals. If symptoms don’t improve to the patient’s satisfaction, dosing can be boosted to twice per day, or a more effective acid suppressor can be substituted and continued at a once-daily dose. When the response to PPIs is adequate, the dose should be reduced until the lowest effective dose is reached, or the patient could potentially be moved to H2 receptor antagonists or other antacids. However, patients with erosive esophagitis, biopsy-confirmed Barrett’s esophagus, or peptic stricture must stay on long-term PPI therapy.
The authors also gave advice on when to conduct objective testing. When a PPI trial doesn’t adequately address troublesome heartburn, regurgitation, and/or noncardiac chest pain, or if alarm systems are present, endoscopy should be employed to look for erosive reflux disease or long-segment Barrett’s esophagus as conclusive evidence for GERD. If these are absent, prolonged wireless pH monitoring while a patient is off medication is suggested. In addition, patients with extraesophageal symptoms suspected to be caused by reflux should undergo upfront objective reflux testing while off PPI therapy rather than doing an empiric PPI trial.
The authors advise that, if patients don’t have proven GERD and are continued on PPI therapy, they should be evaluated within 12 months to ensure that the therapy and dose are appropriate. Physicians should offer endoscopy with prolonged wireless reflux monitoring in the absence of PPI therapy (ideally after 2-4 weeks of withdrawal) to confirm that long-term PPI therapy is needed.
In the section on personalization of disease management, the authors note that ambulatory reflux monitoring and upper gastrointestinal endoscopy can be used to guide management of GERD. When upper GI endoscopy reveals no erosive findings and esophageal acid exposure time (AET) is less than 4% throughout all days of prolonged wireless pH monitoring, the physician can conclude that the patient has no pathologic gastroesophageal reflux and is likely to have a functional esophageal disorder. In contrast, erosive findings during upper GI endoscopy and/or AET more than 4% across at least 1 day of wireless pH monitoring suggests a GERD diagnosis.
Optimization of PPI is important among patients with GERD, and the authors stress that patients should be educated about the safety of PPI use.
Adjunctive pharmacotherapy is useful and can include alginate antacids for breakthrough symptoms, H2RAs for nocturnal symptoms, baclofen to counter regurgitation or belching, and prokinetics for accompanying gastroparesis. The choice of medications depends on the phenotype, and they should not be used empirically.
For patients with functional heartburn or reflux disease linked to esophageal hypervigilance, reflux sensitivity, or behavioral disorders, options include pharmacologic neuromodulation, hypnotherapy provided by a behavioral therapist, cognitive behavioral therapy, and diaphragmatic breathing and relaxation.
If symptoms persist despite efforts at optimization of treatments and lifestyle factors, ambulatory 24-hour pH-impedance monitoring on PPI can be used to investigate mechanistic causes, especially when there is no known antireflux barrier abnormality, but the technique requires expertise to correctly interpret. This can ensure that the symptoms are not due to reflux hypersensitivity, rumination syndrome, or a belching disorder. When symptoms are confirmed to be treatment resistant, therapy should be escalated, using a strategy that incorporates a pattern of reflux, integrity of the antireflux barrier, obesity if present, and psychological factors.
Surgical options for confirmed GERD include laparoscopic fundoplication and magnetic sphincter augmentation. Transoral incisionless fundoplication can be performed endoscopically in selected patients. For obese patients with confirmed GERD, Roux-en-Y gastric bypass is effective at reducing reflux and can be used as a salvage treatment for nonobese patients. Sleeve gastrectomy may exacerbate GERD.
The authors reported relationships with Medtronic, Diversatek, Ironwood, and Takeda. The authors also reported funding from National Institutes of Health grants.
A recent American Gastroenterological Association Clinical Practice Update for evaluation and management of gastroesophageal reflux disease (GERD) focuses on delivering personalized diagnostic and therapeutic strategies.
The document includes new advice on use of upfront objective testing for isolated extraesophageal symptoms, confirmation of GERD diagnosis prior to long-term GERD therapy even in PPI responders, as well as important elements focused on personalization of therapy.
Although GERD is common, with an estimated 30% of people in the United States experiencing symptoms, up to half of all individuals on proton pump inhibitor (PPI) therapy report incomplete symptom improvement. That could be due to the heterogeneous nature of symptoms, which may include heartburn and regurgitation, chest pain, and cough or sore throat, among others. Other conditions may produce similar symptoms or could be exacerbated by the presence of GERD.
The authors of the expert review, published in Clinical Gastroenterology and Hepatology, note that these considerations have driven increased interest in personalized approaches to the management of GERD. The practice update includes sections on how to approach GERD symptoms in the clinic, personalized diagnosis related to GERD symptoms, and precision management.
In the initial management, the authors offer advice on involving the patient in creating a care plan, patient education, and conducting a 4- to 8-week PPI trial in patients with heartburn, regurgitation, or noncardiac chest pains without accompanying alarm signals. If symptoms don’t improve to the patient’s satisfaction, dosing can be boosted to twice per day, or a more effective acid suppressor can be substituted and continued at a once-daily dose. When the response to PPIs is adequate, the dose should be reduced until the lowest effective dose is reached, or the patient could potentially be moved to H2 receptor antagonists or other antacids. However, patients with erosive esophagitis, biopsy-confirmed Barrett’s esophagus, or peptic stricture must stay on long-term PPI therapy.
The authors also gave advice on when to conduct objective testing. When a PPI trial doesn’t adequately address troublesome heartburn, regurgitation, and/or noncardiac chest pain, or if alarm systems are present, endoscopy should be employed to look for erosive reflux disease or long-segment Barrett’s esophagus as conclusive evidence for GERD. If these are absent, prolonged wireless pH monitoring while a patient is off medication is suggested. In addition, patients with extraesophageal symptoms suspected to be caused by reflux should undergo upfront objective reflux testing while off PPI therapy rather than doing an empiric PPI trial.
The authors advise that, if patients don’t have proven GERD and are continued on PPI therapy, they should be evaluated within 12 months to ensure that the therapy and dose are appropriate. Physicians should offer endoscopy with prolonged wireless reflux monitoring in the absence of PPI therapy (ideally after 2-4 weeks of withdrawal) to confirm that long-term PPI therapy is needed.
In the section on personalization of disease management, the authors note that ambulatory reflux monitoring and upper gastrointestinal endoscopy can be used to guide management of GERD. When upper GI endoscopy reveals no erosive findings and esophageal acid exposure time (AET) is less than 4% throughout all days of prolonged wireless pH monitoring, the physician can conclude that the patient has no pathologic gastroesophageal reflux and is likely to have a functional esophageal disorder. In contrast, erosive findings during upper GI endoscopy and/or AET more than 4% across at least 1 day of wireless pH monitoring suggests a GERD diagnosis.
Optimization of PPI is important among patients with GERD, and the authors stress that patients should be educated about the safety of PPI use.
Adjunctive pharmacotherapy is useful and can include alginate antacids for breakthrough symptoms, H2RAs for nocturnal symptoms, baclofen to counter regurgitation or belching, and prokinetics for accompanying gastroparesis. The choice of medications depends on the phenotype, and they should not be used empirically.
For patients with functional heartburn or reflux disease linked to esophageal hypervigilance, reflux sensitivity, or behavioral disorders, options include pharmacologic neuromodulation, hypnotherapy provided by a behavioral therapist, cognitive behavioral therapy, and diaphragmatic breathing and relaxation.
If symptoms persist despite efforts at optimization of treatments and lifestyle factors, ambulatory 24-hour pH-impedance monitoring on PPI can be used to investigate mechanistic causes, especially when there is no known antireflux barrier abnormality, but the technique requires expertise to correctly interpret. This can ensure that the symptoms are not due to reflux hypersensitivity, rumination syndrome, or a belching disorder. When symptoms are confirmed to be treatment resistant, therapy should be escalated, using a strategy that incorporates a pattern of reflux, integrity of the antireflux barrier, obesity if present, and psychological factors.
Surgical options for confirmed GERD include laparoscopic fundoplication and magnetic sphincter augmentation. Transoral incisionless fundoplication can be performed endoscopically in selected patients. For obese patients with confirmed GERD, Roux-en-Y gastric bypass is effective at reducing reflux and can be used as a salvage treatment for nonobese patients. Sleeve gastrectomy may exacerbate GERD.
The authors reported relationships with Medtronic, Diversatek, Ironwood, and Takeda. The authors also reported funding from National Institutes of Health grants.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
AGA Clinical Practice Update: Expert review on deprescribing PPIs
An American Gastroenterological Association practice update on deprescribing proton-pump inhibitors (PPIs) delineates conditions under which drug withdrawal should be considered, and acknowledges that conversations between physicians and patients can be complicated. An inappropriate decision to discontinue PPI therapy can have significant consequences for the patient, while continued inappropriate use raises health care costs and may rarely lead to adverse effects.
One purpose of the update is to provide guidance when patients and providers don’t have the resources to systematically examine the issue, especially when other medical concerns may be in play. The authors also suggested that physicians include pharmacists in the employment of the best practices advice.
“None of these statements represents a radical departure from previously published guidance on PPI appropriateness and deprescribing: Our [recommendations] simply seek to summarize the evidence and to provide the clinician with a single document which distills the evidence down into clinically applicable guidance statements,” Laura Targownik, MD, associate professor of medicine at the University of Toronto and corresponding author of the practice update published in Gastroenterology said in an interview.
“PPIs are highly effective medications for specific gastrointestinal conditions, and are largely safe. However, PPIs are often used in situations where they have minimal and no proven benefit, leading to unnecessary health care spending and unnecessary exposure to drugs. Our paper helps clinicians identify which patients require long-term PPI use as well as those who may be using them unnecessarily, and provides actionable advice on how to deprescribe PPIs from those deemed to be using them without clear benefit,” said Dr. Targownik.
An estimated 7%-15% of health care patients in general and 40% of those over 70 use PPIs at any given time, making them among the most commonly used drugs. About one in four patients who start PPIs will use them for a year or more. Aside from their use for acid-mediated upper gastrointestinal conditions, PPIs often find use for less well-defined complaints. Since PPIs are available over the counter, physicians may not even be involved in a patient’s decision to use them.
Although PPI use has been associated with adverse events, including chronic kidney disease, fractures, dementia, and greater risk of COVID-19 infection, there is not high-quality evidence to suggest that PPIs are directly responsible for any of these adverse events.
The authors suggested the primary care provider should periodically review and document the complaints or indications that prompt PPI use. When a patient is found to have no chronic condition that PPIs could reasonably address, the physician should consider a trial withdrawal. Patients who take PPIs twice daily for a known chronic condition should be considered for a reduction to a once-daily dose.
In general, PPI discontinuation is not a good option for most patients with complicated gastroesophageal reflux disease, such as those with a history of severe erosive esophagitis, esophageal ulcer, or peptic stricture. The same is true for patients with Barrett’s esophagus, eosinophilic esophagitis, or idiopathic pulmonary fibrosis.
Before any deprescribing is considered, the patient should be evaluated for risk of upper gastrointestinal bleeding, and those at high risk are not candidates for PPI deprescribing.
When the decision is made to withdraw PPIs, the patient should be advised of an increased risk of transient upper gastrointestinal symptoms caused by rebound acid hypersecretion.
The withdrawal of PPIs can be done abruptly, or the dose can be tapered gradually.
PPI-associated adverse events should not be a consideration when discussing the option of withdrawing from PPIs. Instead, the decision should be based on the absence of a specific reason for their use. A history of such adverse events, or a current adverse event, should not be a sole reason for discontinuation, nor should risk factors associated with risk of adverse events. Concerns about adverse events have driven recent interest in reducing use of PPIs, but those adverse events were identified through retrospective studies and may be only associated with PPI use rather than caused by it. In many cases there is no plausible mechanistic cause, and no clinical trials have demonstrated increased adverse events in PPI users.
Three-quarters of physicians say they have altered treatment plans for patients because of concerns about PPI adverse events, and 80% say they would advise patients to withdraw PPIs if they learned the patient was at increased risk of upper gastrointestinal bleeding. Unnecessary withdrawal can lead to recurrent symptoms and complications when PPIs are effective treatments. “Therefore, physicians should not use concern about unproven complications of PPI use as a justification for PPI deprescribing if there remain ongoing valid indications for PPI use,” the authors wrote.
Dr. Targownik has received investigator-initiated funding from Janssen Canada and served on advisory boards for AbbVie Canada, Takeda Canada, Merck Canada, Pfizer Canada, Janssen Canada, Roche Canada, and Sandoz Canada. She is the lead on an IBD registry supported by AbbVie Canada, Takeda Canada, Merck Canada, Pfizer Canada, Amgen Canada, Roche Canada, and Sandoz Canada. None of the companies with whom Dr. Targownik has a relation are involved in the manufacturing, distribution, or sales of PPIs or any other agents mentioned in the manuscript.
An American Gastroenterological Association practice update on deprescribing proton-pump inhibitors (PPIs) delineates conditions under which drug withdrawal should be considered, and acknowledges that conversations between physicians and patients can be complicated. An inappropriate decision to discontinue PPI therapy can have significant consequences for the patient, while continued inappropriate use raises health care costs and may rarely lead to adverse effects.
One purpose of the update is to provide guidance when patients and providers don’t have the resources to systematically examine the issue, especially when other medical concerns may be in play. The authors also suggested that physicians include pharmacists in the employment of the best practices advice.
“None of these statements represents a radical departure from previously published guidance on PPI appropriateness and deprescribing: Our [recommendations] simply seek to summarize the evidence and to provide the clinician with a single document which distills the evidence down into clinically applicable guidance statements,” Laura Targownik, MD, associate professor of medicine at the University of Toronto and corresponding author of the practice update published in Gastroenterology said in an interview.
“PPIs are highly effective medications for specific gastrointestinal conditions, and are largely safe. However, PPIs are often used in situations where they have minimal and no proven benefit, leading to unnecessary health care spending and unnecessary exposure to drugs. Our paper helps clinicians identify which patients require long-term PPI use as well as those who may be using them unnecessarily, and provides actionable advice on how to deprescribe PPIs from those deemed to be using them without clear benefit,” said Dr. Targownik.
An estimated 7%-15% of health care patients in general and 40% of those over 70 use PPIs at any given time, making them among the most commonly used drugs. About one in four patients who start PPIs will use them for a year or more. Aside from their use for acid-mediated upper gastrointestinal conditions, PPIs often find use for less well-defined complaints. Since PPIs are available over the counter, physicians may not even be involved in a patient’s decision to use them.
Although PPI use has been associated with adverse events, including chronic kidney disease, fractures, dementia, and greater risk of COVID-19 infection, there is not high-quality evidence to suggest that PPIs are directly responsible for any of these adverse events.
The authors suggested the primary care provider should periodically review and document the complaints or indications that prompt PPI use. When a patient is found to have no chronic condition that PPIs could reasonably address, the physician should consider a trial withdrawal. Patients who take PPIs twice daily for a known chronic condition should be considered for a reduction to a once-daily dose.
In general, PPI discontinuation is not a good option for most patients with complicated gastroesophageal reflux disease, such as those with a history of severe erosive esophagitis, esophageal ulcer, or peptic stricture. The same is true for patients with Barrett’s esophagus, eosinophilic esophagitis, or idiopathic pulmonary fibrosis.
Before any deprescribing is considered, the patient should be evaluated for risk of upper gastrointestinal bleeding, and those at high risk are not candidates for PPI deprescribing.
When the decision is made to withdraw PPIs, the patient should be advised of an increased risk of transient upper gastrointestinal symptoms caused by rebound acid hypersecretion.
The withdrawal of PPIs can be done abruptly, or the dose can be tapered gradually.
PPI-associated adverse events should not be a consideration when discussing the option of withdrawing from PPIs. Instead, the decision should be based on the absence of a specific reason for their use. A history of such adverse events, or a current adverse event, should not be a sole reason for discontinuation, nor should risk factors associated with risk of adverse events. Concerns about adverse events have driven recent interest in reducing use of PPIs, but those adverse events were identified through retrospective studies and may be only associated with PPI use rather than caused by it. In many cases there is no plausible mechanistic cause, and no clinical trials have demonstrated increased adverse events in PPI users.
Three-quarters of physicians say they have altered treatment plans for patients because of concerns about PPI adverse events, and 80% say they would advise patients to withdraw PPIs if they learned the patient was at increased risk of upper gastrointestinal bleeding. Unnecessary withdrawal can lead to recurrent symptoms and complications when PPIs are effective treatments. “Therefore, physicians should not use concern about unproven complications of PPI use as a justification for PPI deprescribing if there remain ongoing valid indications for PPI use,” the authors wrote.
Dr. Targownik has received investigator-initiated funding from Janssen Canada and served on advisory boards for AbbVie Canada, Takeda Canada, Merck Canada, Pfizer Canada, Janssen Canada, Roche Canada, and Sandoz Canada. She is the lead on an IBD registry supported by AbbVie Canada, Takeda Canada, Merck Canada, Pfizer Canada, Amgen Canada, Roche Canada, and Sandoz Canada. None of the companies with whom Dr. Targownik has a relation are involved in the manufacturing, distribution, or sales of PPIs or any other agents mentioned in the manuscript.
An American Gastroenterological Association practice update on deprescribing proton-pump inhibitors (PPIs) delineates conditions under which drug withdrawal should be considered, and acknowledges that conversations between physicians and patients can be complicated. An inappropriate decision to discontinue PPI therapy can have significant consequences for the patient, while continued inappropriate use raises health care costs and may rarely lead to adverse effects.
One purpose of the update is to provide guidance when patients and providers don’t have the resources to systematically examine the issue, especially when other medical concerns may be in play. The authors also suggested that physicians include pharmacists in the employment of the best practices advice.
“None of these statements represents a radical departure from previously published guidance on PPI appropriateness and deprescribing: Our [recommendations] simply seek to summarize the evidence and to provide the clinician with a single document which distills the evidence down into clinically applicable guidance statements,” Laura Targownik, MD, associate professor of medicine at the University of Toronto and corresponding author of the practice update published in Gastroenterology said in an interview.
“PPIs are highly effective medications for specific gastrointestinal conditions, and are largely safe. However, PPIs are often used in situations where they have minimal and no proven benefit, leading to unnecessary health care spending and unnecessary exposure to drugs. Our paper helps clinicians identify which patients require long-term PPI use as well as those who may be using them unnecessarily, and provides actionable advice on how to deprescribe PPIs from those deemed to be using them without clear benefit,” said Dr. Targownik.
An estimated 7%-15% of health care patients in general and 40% of those over 70 use PPIs at any given time, making them among the most commonly used drugs. About one in four patients who start PPIs will use them for a year or more. Aside from their use for acid-mediated upper gastrointestinal conditions, PPIs often find use for less well-defined complaints. Since PPIs are available over the counter, physicians may not even be involved in a patient’s decision to use them.
Although PPI use has been associated with adverse events, including chronic kidney disease, fractures, dementia, and greater risk of COVID-19 infection, there is not high-quality evidence to suggest that PPIs are directly responsible for any of these adverse events.
The authors suggested the primary care provider should periodically review and document the complaints or indications that prompt PPI use. When a patient is found to have no chronic condition that PPIs could reasonably address, the physician should consider a trial withdrawal. Patients who take PPIs twice daily for a known chronic condition should be considered for a reduction to a once-daily dose.
In general, PPI discontinuation is not a good option for most patients with complicated gastroesophageal reflux disease, such as those with a history of severe erosive esophagitis, esophageal ulcer, or peptic stricture. The same is true for patients with Barrett’s esophagus, eosinophilic esophagitis, or idiopathic pulmonary fibrosis.
Before any deprescribing is considered, the patient should be evaluated for risk of upper gastrointestinal bleeding, and those at high risk are not candidates for PPI deprescribing.
When the decision is made to withdraw PPIs, the patient should be advised of an increased risk of transient upper gastrointestinal symptoms caused by rebound acid hypersecretion.
The withdrawal of PPIs can be done abruptly, or the dose can be tapered gradually.
PPI-associated adverse events should not be a consideration when discussing the option of withdrawing from PPIs. Instead, the decision should be based on the absence of a specific reason for their use. A history of such adverse events, or a current adverse event, should not be a sole reason for discontinuation, nor should risk factors associated with risk of adverse events. Concerns about adverse events have driven recent interest in reducing use of PPIs, but those adverse events were identified through retrospective studies and may be only associated with PPI use rather than caused by it. In many cases there is no plausible mechanistic cause, and no clinical trials have demonstrated increased adverse events in PPI users.
Three-quarters of physicians say they have altered treatment plans for patients because of concerns about PPI adverse events, and 80% say they would advise patients to withdraw PPIs if they learned the patient was at increased risk of upper gastrointestinal bleeding. Unnecessary withdrawal can lead to recurrent symptoms and complications when PPIs are effective treatments. “Therefore, physicians should not use concern about unproven complications of PPI use as a justification for PPI deprescribing if there remain ongoing valid indications for PPI use,” the authors wrote.
Dr. Targownik has received investigator-initiated funding from Janssen Canada and served on advisory boards for AbbVie Canada, Takeda Canada, Merck Canada, Pfizer Canada, Janssen Canada, Roche Canada, and Sandoz Canada. She is the lead on an IBD registry supported by AbbVie Canada, Takeda Canada, Merck Canada, Pfizer Canada, Amgen Canada, Roche Canada, and Sandoz Canada. None of the companies with whom Dr. Targownik has a relation are involved in the manufacturing, distribution, or sales of PPIs or any other agents mentioned in the manuscript.
FROM GASTROENTEROLOGY
Topical steroid shows promise for EOE
A topical formulation of fluticasone designed to dissolve and coat the esophagus appears safe and effective for the treatment of eosinophilic esophagitis (EOE), according to new results from a phase 2b study. The results pave the way for phase 3 clinical trials.
Topical steroids are frequently used off-label for EOE. They may be repurposed from nebulizers used for asthma, with patients mixing the drugs themselves or sending them to a pharmacy to be compounded. Patients remove the spacer from a nebulizer in order to swallow the active compound or mix the liquid that would be nebulized with honey or Splenda to thicken it to maximize its contact with the esophagus. “Both of these things are very cumbersome and difficult. I get a lot of complaints from patients [that] it doesn’t taste good. So, the fact that we have a drug that we are already using, but it’s designed for the esophagus, is really exciting,” said Nielsen Fernandez-Becker, MD, PhD, of the department of gastroenterology and hepatology at Stanford (Calif.) University. Dr. Fernandez-Becker referred some patients to the trial and performed some procedures.
“I don’t think the findings are unexpected, given what we’ve seen with swallowed inhalers with fluticasone, but I think the real importance of this is that it does look like a dedicated swallow form works. And if this leads to [Food and Drug Administration] approval, then I think that that really becomes a game-changer for this EOE population. Getting something that’s FDA approved to treat this disorder is a key unmet need,” said John Clarke, MD, who was not involved in the study.
He also pointed out that the safety profile of the drug appears good with respect to both candidiasis and adrenal suppression. “It at least seems comparable, if not better than what we’re currently doing with the inhaler,” said Dr. Clarke, a clinical professor of medicine and director of the esophagus program at Stanford University.
Current options for EOE are limited primarily to the use of proton pump inhibitors and food-elimination diets. Oral budesonide is available to patients in Europe and under investigation in the United States.
The new formulation (APT-1011, Ellodi Pharmaceuticals) is meant to be taken without water and dissolves on the tongue and then coats the esophagus.
In the phase 2b study published in Clinical Gastroenterology and Hepatology, researchers randomized 106 adults from six countries with EOE to receive one of four doses of APT-1011, or placebo. Participants had to have current symptoms of dysphagia and active disease after no histologic response from at least 8 weeks of high-dose (20-40 mg/day) proton pump inhibitors. The study included a placebo-controlled, 12-week induction period followed by 40 weeks of maintenance therapy with no placebo arm. The researchers considered a count of fewer than six eosinophils per high-powered field, as measured during an esophageal biopsy, to be a histologic response.
No patients in the placebo group had a response. The response rate was 80% among patients taking a 3-mg dose twice per day; 67% among those taking a 3-mg dose only at bedtime; 86% for those taking 1.5 mg twice per day; and 48% for 1.5 mg only at bedtime (P < .001 for all comparisons to placebo).
After 12 weeks, EOE Endoscopic Reference Score (EREFS) improved from 4.5 to 2.3 in the 3-mg b.i.d. group (5.3-2.1 for bedtime only), and from 4.6 to 1.7 for the 1.5-mg b.i.d. group (5.3-2.9 for 1.5 bedtime only). In the placebo group, the change was from 5.2 to 4.5.
Among those who responded during the induction period, the majority continued to be responders at weeks 26 and 52, including the 3-mg b.i.d. group (88% and 69%, respectively), the 3-mg bedtime-only group (79% and 64%), the 1.5-mg b.i.d. group (89% and 84%), and the 1.5-mg bedtime-only group (70% and 30%).
If approved, the new formulation will likely have a big impact on EOE patients, according to Dr. Fernandez-Becker. “The treatment that we decide on ultimately is through shared decision-making with the physician and the patient. I have many patients who want to go with diet, but it’s very difficult and it takes a long time to tailor the therapy, and many patients are not interested in proton pump inhibitors. So topical steroids are something that I prescribe a lot for patients,” she said.
The fact that the formulation is based on a drug with a known safety record is encouraging, but more research needs to be done. “I don’t expect that this would be any different, but that’s something that’s going to be studied,” said Dr. Fernandez-Becker.
The study was funded by Ellodi Pharmaceuticals. Dr. Clarke has no relevant financial disclosures. Dr. Fernandez-Becker has no relevant financial disclosures but was a participant in the study.
A topical formulation of fluticasone designed to dissolve and coat the esophagus appears safe and effective for the treatment of eosinophilic esophagitis (EOE), according to new results from a phase 2b study. The results pave the way for phase 3 clinical trials.
Topical steroids are frequently used off-label for EOE. They may be repurposed from nebulizers used for asthma, with patients mixing the drugs themselves or sending them to a pharmacy to be compounded. Patients remove the spacer from a nebulizer in order to swallow the active compound or mix the liquid that would be nebulized with honey or Splenda to thicken it to maximize its contact with the esophagus. “Both of these things are very cumbersome and difficult. I get a lot of complaints from patients [that] it doesn’t taste good. So, the fact that we have a drug that we are already using, but it’s designed for the esophagus, is really exciting,” said Nielsen Fernandez-Becker, MD, PhD, of the department of gastroenterology and hepatology at Stanford (Calif.) University. Dr. Fernandez-Becker referred some patients to the trial and performed some procedures.
“I don’t think the findings are unexpected, given what we’ve seen with swallowed inhalers with fluticasone, but I think the real importance of this is that it does look like a dedicated swallow form works. And if this leads to [Food and Drug Administration] approval, then I think that that really becomes a game-changer for this EOE population. Getting something that’s FDA approved to treat this disorder is a key unmet need,” said John Clarke, MD, who was not involved in the study.
He also pointed out that the safety profile of the drug appears good with respect to both candidiasis and adrenal suppression. “It at least seems comparable, if not better than what we’re currently doing with the inhaler,” said Dr. Clarke, a clinical professor of medicine and director of the esophagus program at Stanford University.
Current options for EOE are limited primarily to the use of proton pump inhibitors and food-elimination diets. Oral budesonide is available to patients in Europe and under investigation in the United States.
The new formulation (APT-1011, Ellodi Pharmaceuticals) is meant to be taken without water and dissolves on the tongue and then coats the esophagus.
In the phase 2b study published in Clinical Gastroenterology and Hepatology, researchers randomized 106 adults from six countries with EOE to receive one of four doses of APT-1011, or placebo. Participants had to have current symptoms of dysphagia and active disease after no histologic response from at least 8 weeks of high-dose (20-40 mg/day) proton pump inhibitors. The study included a placebo-controlled, 12-week induction period followed by 40 weeks of maintenance therapy with no placebo arm. The researchers considered a count of fewer than six eosinophils per high-powered field, as measured during an esophageal biopsy, to be a histologic response.
No patients in the placebo group had a response. The response rate was 80% among patients taking a 3-mg dose twice per day; 67% among those taking a 3-mg dose only at bedtime; 86% for those taking 1.5 mg twice per day; and 48% for 1.5 mg only at bedtime (P < .001 for all comparisons to placebo).
After 12 weeks, EOE Endoscopic Reference Score (EREFS) improved from 4.5 to 2.3 in the 3-mg b.i.d. group (5.3-2.1 for bedtime only), and from 4.6 to 1.7 for the 1.5-mg b.i.d. group (5.3-2.9 for 1.5 bedtime only). In the placebo group, the change was from 5.2 to 4.5.
Among those who responded during the induction period, the majority continued to be responders at weeks 26 and 52, including the 3-mg b.i.d. group (88% and 69%, respectively), the 3-mg bedtime-only group (79% and 64%), the 1.5-mg b.i.d. group (89% and 84%), and the 1.5-mg bedtime-only group (70% and 30%).
If approved, the new formulation will likely have a big impact on EOE patients, according to Dr. Fernandez-Becker. “The treatment that we decide on ultimately is through shared decision-making with the physician and the patient. I have many patients who want to go with diet, but it’s very difficult and it takes a long time to tailor the therapy, and many patients are not interested in proton pump inhibitors. So topical steroids are something that I prescribe a lot for patients,” she said.
The fact that the formulation is based on a drug with a known safety record is encouraging, but more research needs to be done. “I don’t expect that this would be any different, but that’s something that’s going to be studied,” said Dr. Fernandez-Becker.
The study was funded by Ellodi Pharmaceuticals. Dr. Clarke has no relevant financial disclosures. Dr. Fernandez-Becker has no relevant financial disclosures but was a participant in the study.
A topical formulation of fluticasone designed to dissolve and coat the esophagus appears safe and effective for the treatment of eosinophilic esophagitis (EOE), according to new results from a phase 2b study. The results pave the way for phase 3 clinical trials.
Topical steroids are frequently used off-label for EOE. They may be repurposed from nebulizers used for asthma, with patients mixing the drugs themselves or sending them to a pharmacy to be compounded. Patients remove the spacer from a nebulizer in order to swallow the active compound or mix the liquid that would be nebulized with honey or Splenda to thicken it to maximize its contact with the esophagus. “Both of these things are very cumbersome and difficult. I get a lot of complaints from patients [that] it doesn’t taste good. So, the fact that we have a drug that we are already using, but it’s designed for the esophagus, is really exciting,” said Nielsen Fernandez-Becker, MD, PhD, of the department of gastroenterology and hepatology at Stanford (Calif.) University. Dr. Fernandez-Becker referred some patients to the trial and performed some procedures.
“I don’t think the findings are unexpected, given what we’ve seen with swallowed inhalers with fluticasone, but I think the real importance of this is that it does look like a dedicated swallow form works. And if this leads to [Food and Drug Administration] approval, then I think that that really becomes a game-changer for this EOE population. Getting something that’s FDA approved to treat this disorder is a key unmet need,” said John Clarke, MD, who was not involved in the study.
He also pointed out that the safety profile of the drug appears good with respect to both candidiasis and adrenal suppression. “It at least seems comparable, if not better than what we’re currently doing with the inhaler,” said Dr. Clarke, a clinical professor of medicine and director of the esophagus program at Stanford University.
Current options for EOE are limited primarily to the use of proton pump inhibitors and food-elimination diets. Oral budesonide is available to patients in Europe and under investigation in the United States.
The new formulation (APT-1011, Ellodi Pharmaceuticals) is meant to be taken without water and dissolves on the tongue and then coats the esophagus.
In the phase 2b study published in Clinical Gastroenterology and Hepatology, researchers randomized 106 adults from six countries with EOE to receive one of four doses of APT-1011, or placebo. Participants had to have current symptoms of dysphagia and active disease after no histologic response from at least 8 weeks of high-dose (20-40 mg/day) proton pump inhibitors. The study included a placebo-controlled, 12-week induction period followed by 40 weeks of maintenance therapy with no placebo arm. The researchers considered a count of fewer than six eosinophils per high-powered field, as measured during an esophageal biopsy, to be a histologic response.
No patients in the placebo group had a response. The response rate was 80% among patients taking a 3-mg dose twice per day; 67% among those taking a 3-mg dose only at bedtime; 86% for those taking 1.5 mg twice per day; and 48% for 1.5 mg only at bedtime (P < .001 for all comparisons to placebo).
After 12 weeks, EOE Endoscopic Reference Score (EREFS) improved from 4.5 to 2.3 in the 3-mg b.i.d. group (5.3-2.1 for bedtime only), and from 4.6 to 1.7 for the 1.5-mg b.i.d. group (5.3-2.9 for 1.5 bedtime only). In the placebo group, the change was from 5.2 to 4.5.
Among those who responded during the induction period, the majority continued to be responders at weeks 26 and 52, including the 3-mg b.i.d. group (88% and 69%, respectively), the 3-mg bedtime-only group (79% and 64%), the 1.5-mg b.i.d. group (89% and 84%), and the 1.5-mg bedtime-only group (70% and 30%).
If approved, the new formulation will likely have a big impact on EOE patients, according to Dr. Fernandez-Becker. “The treatment that we decide on ultimately is through shared decision-making with the physician and the patient. I have many patients who want to go with diet, but it’s very difficult and it takes a long time to tailor the therapy, and many patients are not interested in proton pump inhibitors. So topical steroids are something that I prescribe a lot for patients,” she said.
The fact that the formulation is based on a drug with a known safety record is encouraging, but more research needs to be done. “I don’t expect that this would be any different, but that’s something that’s going to be studied,” said Dr. Fernandez-Becker.
The study was funded by Ellodi Pharmaceuticals. Dr. Clarke has no relevant financial disclosures. Dr. Fernandez-Becker has no relevant financial disclosures but was a participant in the study.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
AGA clinical practice update: Expert review on managing refractory gastroparesis
Gastroparesis can be tricky to diagnose and treat, in part because its symptoms can be difficult to distinguish from functional dyspepsia. A new clinical practice update from the American Gastroenterological Association aims to help physicians treat medically refractory gastroparesis with practical advice stemming from expert opinion and a literature review.
Although gastroparesis can be caused by known factors such as diabetes and medications, the largest group is idiopathic. The authors define medically refractory gastroparesis as symptoms that are not due to medication use, that continue despite dietary changes and first-line treatment with metoclopramide.
Although the authors outline several best practice advice statements on symptom identification and management, they acknowledge that much uncertainty still exists. “Our knowledge gap remains vast, and areas for future research include study of pathophysiology and etiology, as well as identification of clinical and investigation-based predictors of response to each management approach,” the authors wrote. Their report is in Clinical Gastroenterology and Hepatology.
They also call for research to identify gastroparesis phenotypes that are most likely to respond to individual management approaches.
Common gastroparesis symptoms include nausea, vomiting, early satiety, bloating, postprandial fullness, abdominal pain, and weight loss. Many of these overlap with functional dyspepsia (FD). In fact, one study found that 42% of gastroparesis could be reclassified as having functional dyspepsia, and 37% of FD patients as having gastroparesis.
About 5 million adults in the United States, and 7.2% of the world population, report gastroparesis-like symptoms. The similarities between the two groups poses a significant diagnostic challenge. However, a careful history, physical exam, and appropriate diagnostic tests should allow the physician to rule out other conditions that may mimic gastroparesis. Repeating scintigraphy may change diagnosis from gastroparesis to FD or vice versa, but the authors note that this technique is often performed incorrectly and so should be conducted at centers that closely follow guidelines. They suggest a 4 hour meal-based test of gastric emptying over the wireless motility capsule because it provides a better physiological assessment.
They also suggest that treatment should focus on the most bothersome symptom, along with reducing the potential for complications such as esophagitis, malnutrition, and weight loss, as well as improving quality of life.
There are medications available for nausea and vomiting, although most have not been studied in large randomized controlled trials. These agents include domperidone, 5-hydroxytryptamine3 receptor antagonists, neurokinin receptor antagonists, and phenothiazine antipsychotics.
There are also medications available to increase the rate of gastric emptying. Erythromycin can be used intravenously or orally ahead of meals, while the 5-HT4 receptor agonist velusetrag improved gastric emptying in healthy volunteers with no sign of cardiac side effects. The commonly available 5-HT4 agonist prucalopride has also shown promise in improving gastric emptying.
For visceral pain, the authors suggest not using opioids because they may slow gastric emptying and increase pain perception. It is believed that neuromodulators such as tricyclic antidepressants (TCAs) and serotonin norepinephrine reuptake inhibitors (SNRIs) may reduce perception of pain, but there is limited high-quality evidence available for these therapies. The authors suggest that higher potency tertiary tricyclic amines such as amitriptyline or imipramine may be effective, particularly in diabetic gastroparesis since they provide relief in FD.
Nonpharmaceutical options include gastric electrical stimulation (GES), which improves refractory nausea and vomiting in some patients with gastroparesis, but does not accelerate gastric emptying. It may also improve glycemic control, nutritional status, and quality of life. The treatment may be well suited to opioid-free patients with refractory or intractable nausea and vomiting whose predominant symptom is not abdominal pain.
Other therapies focus on the pylorus and its role in gastric emptying, which can be impaired as a result of abnormalities of pyloric tone and pressure. Functional lumen imaging probe (FLIP) can be used to probe pyloric tone and pressure, but it is expensive, invasive, and not widely available.
Outside of clinical trial settings, the authors advise against the use of intrapyloric botulinum toxic injection and transpyloric stent placement. Per oral endoscopic myotomy (POEM) has shown some efficacy at improving symptoms and reducing gastric emptying times, but it has not been studied in sham-controlled trials. The authors call the technique intriguing, but say it should not be considered a first-line therapy, and should be performed only at tertiary centers with expert motility specialists and endoscopists.
In extreme cases, enteral nutrition may be necessary, and a transjejunal tube or combined gastrojejunostomy tube should be emplaced beyond the pylorus. In a retrospective case series, patients experienced weight recovery with acceptable morbidity and mortality, and the implant was removed at an average of 20 months.
The authors have consulted or been on scientific advisory boards for Salix, Ironwood, Allergan, Arena, Allakos, Medtronic, Diversatek, Takeda, Quintiles, and IsoThrive.
This article was updated Feb. 17, 2022.
Gastroparesis can be tricky to diagnose and treat, in part because its symptoms can be difficult to distinguish from functional dyspepsia. A new clinical practice update from the American Gastroenterological Association aims to help physicians treat medically refractory gastroparesis with practical advice stemming from expert opinion and a literature review.
Although gastroparesis can be caused by known factors such as diabetes and medications, the largest group is idiopathic. The authors define medically refractory gastroparesis as symptoms that are not due to medication use, that continue despite dietary changes and first-line treatment with metoclopramide.
Although the authors outline several best practice advice statements on symptom identification and management, they acknowledge that much uncertainty still exists. “Our knowledge gap remains vast, and areas for future research include study of pathophysiology and etiology, as well as identification of clinical and investigation-based predictors of response to each management approach,” the authors wrote. Their report is in Clinical Gastroenterology and Hepatology.
They also call for research to identify gastroparesis phenotypes that are most likely to respond to individual management approaches.
Common gastroparesis symptoms include nausea, vomiting, early satiety, bloating, postprandial fullness, abdominal pain, and weight loss. Many of these overlap with functional dyspepsia (FD). In fact, one study found that 42% of gastroparesis could be reclassified as having functional dyspepsia, and 37% of FD patients as having gastroparesis.
About 5 million adults in the United States, and 7.2% of the world population, report gastroparesis-like symptoms. The similarities between the two groups poses a significant diagnostic challenge. However, a careful history, physical exam, and appropriate diagnostic tests should allow the physician to rule out other conditions that may mimic gastroparesis. Repeating scintigraphy may change diagnosis from gastroparesis to FD or vice versa, but the authors note that this technique is often performed incorrectly and so should be conducted at centers that closely follow guidelines. They suggest a 4 hour meal-based test of gastric emptying over the wireless motility capsule because it provides a better physiological assessment.
They also suggest that treatment should focus on the most bothersome symptom, along with reducing the potential for complications such as esophagitis, malnutrition, and weight loss, as well as improving quality of life.
There are medications available for nausea and vomiting, although most have not been studied in large randomized controlled trials. These agents include domperidone, 5-hydroxytryptamine3 receptor antagonists, neurokinin receptor antagonists, and phenothiazine antipsychotics.
There are also medications available to increase the rate of gastric emptying. Erythromycin can be used intravenously or orally ahead of meals, while the 5-HT4 receptor agonist velusetrag improved gastric emptying in healthy volunteers with no sign of cardiac side effects. The commonly available 5-HT4 agonist prucalopride has also shown promise in improving gastric emptying.
For visceral pain, the authors suggest not using opioids because they may slow gastric emptying and increase pain perception. It is believed that neuromodulators such as tricyclic antidepressants (TCAs) and serotonin norepinephrine reuptake inhibitors (SNRIs) may reduce perception of pain, but there is limited high-quality evidence available for these therapies. The authors suggest that higher potency tertiary tricyclic amines such as amitriptyline or imipramine may be effective, particularly in diabetic gastroparesis since they provide relief in FD.
Nonpharmaceutical options include gastric electrical stimulation (GES), which improves refractory nausea and vomiting in some patients with gastroparesis, but does not accelerate gastric emptying. It may also improve glycemic control, nutritional status, and quality of life. The treatment may be well suited to opioid-free patients with refractory or intractable nausea and vomiting whose predominant symptom is not abdominal pain.
Other therapies focus on the pylorus and its role in gastric emptying, which can be impaired as a result of abnormalities of pyloric tone and pressure. Functional lumen imaging probe (FLIP) can be used to probe pyloric tone and pressure, but it is expensive, invasive, and not widely available.
Outside of clinical trial settings, the authors advise against the use of intrapyloric botulinum toxic injection and transpyloric stent placement. Per oral endoscopic myotomy (POEM) has shown some efficacy at improving symptoms and reducing gastric emptying times, but it has not been studied in sham-controlled trials. The authors call the technique intriguing, but say it should not be considered a first-line therapy, and should be performed only at tertiary centers with expert motility specialists and endoscopists.
In extreme cases, enteral nutrition may be necessary, and a transjejunal tube or combined gastrojejunostomy tube should be emplaced beyond the pylorus. In a retrospective case series, patients experienced weight recovery with acceptable morbidity and mortality, and the implant was removed at an average of 20 months.
The authors have consulted or been on scientific advisory boards for Salix, Ironwood, Allergan, Arena, Allakos, Medtronic, Diversatek, Takeda, Quintiles, and IsoThrive.
This article was updated Feb. 17, 2022.
Gastroparesis can be tricky to diagnose and treat, in part because its symptoms can be difficult to distinguish from functional dyspepsia. A new clinical practice update from the American Gastroenterological Association aims to help physicians treat medically refractory gastroparesis with practical advice stemming from expert opinion and a literature review.
Although gastroparesis can be caused by known factors such as diabetes and medications, the largest group is idiopathic. The authors define medically refractory gastroparesis as symptoms that are not due to medication use, that continue despite dietary changes and first-line treatment with metoclopramide.
Although the authors outline several best practice advice statements on symptom identification and management, they acknowledge that much uncertainty still exists. “Our knowledge gap remains vast, and areas for future research include study of pathophysiology and etiology, as well as identification of clinical and investigation-based predictors of response to each management approach,” the authors wrote. Their report is in Clinical Gastroenterology and Hepatology.
They also call for research to identify gastroparesis phenotypes that are most likely to respond to individual management approaches.
Common gastroparesis symptoms include nausea, vomiting, early satiety, bloating, postprandial fullness, abdominal pain, and weight loss. Many of these overlap with functional dyspepsia (FD). In fact, one study found that 42% of gastroparesis could be reclassified as having functional dyspepsia, and 37% of FD patients as having gastroparesis.
About 5 million adults in the United States, and 7.2% of the world population, report gastroparesis-like symptoms. The similarities between the two groups poses a significant diagnostic challenge. However, a careful history, physical exam, and appropriate diagnostic tests should allow the physician to rule out other conditions that may mimic gastroparesis. Repeating scintigraphy may change diagnosis from gastroparesis to FD or vice versa, but the authors note that this technique is often performed incorrectly and so should be conducted at centers that closely follow guidelines. They suggest a 4 hour meal-based test of gastric emptying over the wireless motility capsule because it provides a better physiological assessment.
They also suggest that treatment should focus on the most bothersome symptom, along with reducing the potential for complications such as esophagitis, malnutrition, and weight loss, as well as improving quality of life.
There are medications available for nausea and vomiting, although most have not been studied in large randomized controlled trials. These agents include domperidone, 5-hydroxytryptamine3 receptor antagonists, neurokinin receptor antagonists, and phenothiazine antipsychotics.
There are also medications available to increase the rate of gastric emptying. Erythromycin can be used intravenously or orally ahead of meals, while the 5-HT4 receptor agonist velusetrag improved gastric emptying in healthy volunteers with no sign of cardiac side effects. The commonly available 5-HT4 agonist prucalopride has also shown promise in improving gastric emptying.
For visceral pain, the authors suggest not using opioids because they may slow gastric emptying and increase pain perception. It is believed that neuromodulators such as tricyclic antidepressants (TCAs) and serotonin norepinephrine reuptake inhibitors (SNRIs) may reduce perception of pain, but there is limited high-quality evidence available for these therapies. The authors suggest that higher potency tertiary tricyclic amines such as amitriptyline or imipramine may be effective, particularly in diabetic gastroparesis since they provide relief in FD.
Nonpharmaceutical options include gastric electrical stimulation (GES), which improves refractory nausea and vomiting in some patients with gastroparesis, but does not accelerate gastric emptying. It may also improve glycemic control, nutritional status, and quality of life. The treatment may be well suited to opioid-free patients with refractory or intractable nausea and vomiting whose predominant symptom is not abdominal pain.
Other therapies focus on the pylorus and its role in gastric emptying, which can be impaired as a result of abnormalities of pyloric tone and pressure. Functional lumen imaging probe (FLIP) can be used to probe pyloric tone and pressure, but it is expensive, invasive, and not widely available.
Outside of clinical trial settings, the authors advise against the use of intrapyloric botulinum toxic injection and transpyloric stent placement. Per oral endoscopic myotomy (POEM) has shown some efficacy at improving symptoms and reducing gastric emptying times, but it has not been studied in sham-controlled trials. The authors call the technique intriguing, but say it should not be considered a first-line therapy, and should be performed only at tertiary centers with expert motility specialists and endoscopists.
In extreme cases, enteral nutrition may be necessary, and a transjejunal tube or combined gastrojejunostomy tube should be emplaced beyond the pylorus. In a retrospective case series, patients experienced weight recovery with acceptable morbidity and mortality, and the implant was removed at an average of 20 months.
The authors have consulted or been on scientific advisory boards for Salix, Ironwood, Allergan, Arena, Allakos, Medtronic, Diversatek, Takeda, Quintiles, and IsoThrive.
This article was updated Feb. 17, 2022.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
GERD: Upper endoscopy may reduce GI cancer mortality
Among individuals with gastroesophageal reflux disease (GERD), a negative upper endoscopy is associated with decreased risk in incidence and mortality from gastrointestinal cancer. The benefit persisted through 5-10 years following the procedure.
The finding is similar to the survival benefit seen with colonoscopies and colorectal cancer, and may be attributable to endoscopic treatment of premalignant lesions.
“The relatively high incidence rate of upper gastrointestinal cancer in patients with GERD indicates that a one-time upper endoscopy may be beneficial,” wrote the authors, who were led by Dag Holmberg, MD, PhD, of the department of molecular medicine and surgery at the Karolinska Institutet and Karolinska University Hospital, both in Stockholm. The study was published in Gastroenterology.
GERD is the most frequent reason patients undergo an upper endoscopy, but the results are often negative. It is generally a benign condition, but can lead to Barrett’s esophagus, as well as esophageal and gastric cardia adenocarcinoma. Upper endoscopy can identify other esophageal cancers like gastric noncardia cancer and duodenal cancer, which may cause dyspepsia or GERD-like symptoms.
To determine the potential benefit of upper endoscopy, the researchers conducted a population-based, four-nation cohort study that included 1,062,740 individuals with GERD in Denmark, Finland, Norway, and Sweden. The data were gathered from national patient registries, cancer registries, and cause of death registries. The study encompassed data from 1979 through the end of 2018.
The median age was 58 years, and 52% of participants were women.
The researchers defined a negative endoscopy as no diagnosis of gastrointestinal cancer within 6 months of the procedure; 69.3% of procedures were negative.
During the follow-up period, 0.34% of participants developed and 0.27% died of upper gastrointestinal cancer. Among those with negative endoscopies, 0.23% developed and 0.22% died from upper gastrointestinal cancer.
Participants with a negative endoscopy had a lower risk of being diagnosed with upper gastrointestinal cancer during the follow-up period (adjusted hazard ratio, 0.45; 95% confidence interval, 0.43-0.48). The reduction in risk was similar across age sexes and age groups, but among procedures performed after 2008, the risk reduction was even higher (aHR, 0.34; P < .001).
The effect was strongest in the first year after the procedure, but it persisted out to 5 years before returning to baseline risk levels.
A negative endoscopy was also associated with decreased mortality risk from upper gastrointestinal cancer versus those who hadn’t had an endoscopy (aHR, 0.39; 95% CI, 0.37-0.42). The protective value continued for at least 10 years.
Esophageal adenocarcinoma developed in 0.12% of participants, and 0.10% died of the disease. Among those with a negative endoscopy, 0.09% developed adenocarcinoma, and 0.07% died (aHR vs. no upper endoscopy, 0.33; 95% CI, 0.30-0.37).
The rapid return to baseline risk was notable, and different from what occurs after negative colonoscopies. However, new tumors can readily form within one year, and the risk may reflect early malignant or premalignant lesions that were missed during the procedure.
In fact, a meta-analysis found that 11.3% of upper gastrointestinal cancers had escaped detection during an endoscopy in the previous 3 years before diagnosis, and case reviews of patients diagnosed with gastrointestinal cancer soon after an upper endoscopy usually reveal suspicious or indeterminate results that the endoscopist or pathologist missed.
Quality indicators for upper endoscopy include procedure time, rate of targeted biopsies, and computer-aided detection, but it isn’t clear what impact these measures have on outcomes. However, the greater risk reduction found with endoscopies performed more recently suggests that newer quality indicators and technological improvements may be improving outcomes.
The relatively low incidence of esophageal and gastric cancer in Western countries has discouraged widespread adoption of endoscopic screening, but the researchers point out that the risk of gastrointestinal cancer among individuals with GERD is similar to the risk of colorectal cancer in the 60-69 age group in the United States, for whom colonoscopy is recommended.
“The present study indicates that upper endoscopy may be beneficial for patients with GERD, but to make upper endoscopy screening more cost beneficial at its initiation, the target group may be limited to include patients at highest risk of cancer. Such previous cost-effectiveness studies have indicated that endoscopy is cost effective in men at aged 50 years or older with chronic GERD,” the authors wrote.
The study was funded by Swedish Research Council and Swedish Cancer Society. The authors disclosed no relevant conflicts of interest.
This study from Holmberg and colleagues has the potential to revolutionize future clinical guidelines determining endoscopic investigations for GERD patients.
The cohort for analysis is staggering in magnitude: The authors analyzed real-world data from over 1 million participants with GERD in four Scandinavian databases. The results show strong and precise reductions in both risk and mortality from upper gastrointestinal cancer in the whole cohort. This reduction was consistent across all subgroup and sensitivity analyses.
These findings are important as GERD alone does not necessarily warrant an upper endoscopy investigation in current practice. This study provides strong evidence that a one-off endoscopic investigation in patients with GERD could bring meaningful opportunities for early detection of esophageal and gastric cancers – and in turn lead to fewer patients dying from these tumors. The immediacy of the return for investment is also impressive; with the risk reduction being strongest in the first few years of follow-up.
The elusive next step, as highlighted by the authors, is to ensure implementation of endoscopic screening can be done in a cost-effective manner. This is even more important because many health care systems across the world struggle with endoscopy capacity during the COVID-19 pandemic.
Helen Coleman, PhD, BSc(Hons), is a professor of cancer epidemiology at Queen’s University Belfast (Northern Ireland); joint deputy director of the Northern Ireland Cancer Registry; a Cancer Research UK Fellow; and a visiting scientist with the Fitzgerald Lab at the University of Cambridge (England). She has no conflicts.
This study from Holmberg and colleagues has the potential to revolutionize future clinical guidelines determining endoscopic investigations for GERD patients.
The cohort for analysis is staggering in magnitude: The authors analyzed real-world data from over 1 million participants with GERD in four Scandinavian databases. The results show strong and precise reductions in both risk and mortality from upper gastrointestinal cancer in the whole cohort. This reduction was consistent across all subgroup and sensitivity analyses.
These findings are important as GERD alone does not necessarily warrant an upper endoscopy investigation in current practice. This study provides strong evidence that a one-off endoscopic investigation in patients with GERD could bring meaningful opportunities for early detection of esophageal and gastric cancers – and in turn lead to fewer patients dying from these tumors. The immediacy of the return for investment is also impressive; with the risk reduction being strongest in the first few years of follow-up.
The elusive next step, as highlighted by the authors, is to ensure implementation of endoscopic screening can be done in a cost-effective manner. This is even more important because many health care systems across the world struggle with endoscopy capacity during the COVID-19 pandemic.
Helen Coleman, PhD, BSc(Hons), is a professor of cancer epidemiology at Queen’s University Belfast (Northern Ireland); joint deputy director of the Northern Ireland Cancer Registry; a Cancer Research UK Fellow; and a visiting scientist with the Fitzgerald Lab at the University of Cambridge (England). She has no conflicts.
This study from Holmberg and colleagues has the potential to revolutionize future clinical guidelines determining endoscopic investigations for GERD patients.
The cohort for analysis is staggering in magnitude: The authors analyzed real-world data from over 1 million participants with GERD in four Scandinavian databases. The results show strong and precise reductions in both risk and mortality from upper gastrointestinal cancer in the whole cohort. This reduction was consistent across all subgroup and sensitivity analyses.
These findings are important as GERD alone does not necessarily warrant an upper endoscopy investigation in current practice. This study provides strong evidence that a one-off endoscopic investigation in patients with GERD could bring meaningful opportunities for early detection of esophageal and gastric cancers – and in turn lead to fewer patients dying from these tumors. The immediacy of the return for investment is also impressive; with the risk reduction being strongest in the first few years of follow-up.
The elusive next step, as highlighted by the authors, is to ensure implementation of endoscopic screening can be done in a cost-effective manner. This is even more important because many health care systems across the world struggle with endoscopy capacity during the COVID-19 pandemic.
Helen Coleman, PhD, BSc(Hons), is a professor of cancer epidemiology at Queen’s University Belfast (Northern Ireland); joint deputy director of the Northern Ireland Cancer Registry; a Cancer Research UK Fellow; and a visiting scientist with the Fitzgerald Lab at the University of Cambridge (England). She has no conflicts.
Among individuals with gastroesophageal reflux disease (GERD), a negative upper endoscopy is associated with decreased risk in incidence and mortality from gastrointestinal cancer. The benefit persisted through 5-10 years following the procedure.
The finding is similar to the survival benefit seen with colonoscopies and colorectal cancer, and may be attributable to endoscopic treatment of premalignant lesions.
“The relatively high incidence rate of upper gastrointestinal cancer in patients with GERD indicates that a one-time upper endoscopy may be beneficial,” wrote the authors, who were led by Dag Holmberg, MD, PhD, of the department of molecular medicine and surgery at the Karolinska Institutet and Karolinska University Hospital, both in Stockholm. The study was published in Gastroenterology.
GERD is the most frequent reason patients undergo an upper endoscopy, but the results are often negative. It is generally a benign condition, but can lead to Barrett’s esophagus, as well as esophageal and gastric cardia adenocarcinoma. Upper endoscopy can identify other esophageal cancers like gastric noncardia cancer and duodenal cancer, which may cause dyspepsia or GERD-like symptoms.
To determine the potential benefit of upper endoscopy, the researchers conducted a population-based, four-nation cohort study that included 1,062,740 individuals with GERD in Denmark, Finland, Norway, and Sweden. The data were gathered from national patient registries, cancer registries, and cause of death registries. The study encompassed data from 1979 through the end of 2018.
The median age was 58 years, and 52% of participants were women.
The researchers defined a negative endoscopy as no diagnosis of gastrointestinal cancer within 6 months of the procedure; 69.3% of procedures were negative.
During the follow-up period, 0.34% of participants developed and 0.27% died of upper gastrointestinal cancer. Among those with negative endoscopies, 0.23% developed and 0.22% died from upper gastrointestinal cancer.
Participants with a negative endoscopy had a lower risk of being diagnosed with upper gastrointestinal cancer during the follow-up period (adjusted hazard ratio, 0.45; 95% confidence interval, 0.43-0.48). The reduction in risk was similar across age sexes and age groups, but among procedures performed after 2008, the risk reduction was even higher (aHR, 0.34; P < .001).
The effect was strongest in the first year after the procedure, but it persisted out to 5 years before returning to baseline risk levels.
A negative endoscopy was also associated with decreased mortality risk from upper gastrointestinal cancer versus those who hadn’t had an endoscopy (aHR, 0.39; 95% CI, 0.37-0.42). The protective value continued for at least 10 years.
Esophageal adenocarcinoma developed in 0.12% of participants, and 0.10% died of the disease. Among those with a negative endoscopy, 0.09% developed adenocarcinoma, and 0.07% died (aHR vs. no upper endoscopy, 0.33; 95% CI, 0.30-0.37).
The rapid return to baseline risk was notable, and different from what occurs after negative colonoscopies. However, new tumors can readily form within one year, and the risk may reflect early malignant or premalignant lesions that were missed during the procedure.
In fact, a meta-analysis found that 11.3% of upper gastrointestinal cancers had escaped detection during an endoscopy in the previous 3 years before diagnosis, and case reviews of patients diagnosed with gastrointestinal cancer soon after an upper endoscopy usually reveal suspicious or indeterminate results that the endoscopist or pathologist missed.
Quality indicators for upper endoscopy include procedure time, rate of targeted biopsies, and computer-aided detection, but it isn’t clear what impact these measures have on outcomes. However, the greater risk reduction found with endoscopies performed more recently suggests that newer quality indicators and technological improvements may be improving outcomes.
The relatively low incidence of esophageal and gastric cancer in Western countries has discouraged widespread adoption of endoscopic screening, but the researchers point out that the risk of gastrointestinal cancer among individuals with GERD is similar to the risk of colorectal cancer in the 60-69 age group in the United States, for whom colonoscopy is recommended.
“The present study indicates that upper endoscopy may be beneficial for patients with GERD, but to make upper endoscopy screening more cost beneficial at its initiation, the target group may be limited to include patients at highest risk of cancer. Such previous cost-effectiveness studies have indicated that endoscopy is cost effective in men at aged 50 years or older with chronic GERD,” the authors wrote.
The study was funded by Swedish Research Council and Swedish Cancer Society. The authors disclosed no relevant conflicts of interest.
Among individuals with gastroesophageal reflux disease (GERD), a negative upper endoscopy is associated with decreased risk in incidence and mortality from gastrointestinal cancer. The benefit persisted through 5-10 years following the procedure.
The finding is similar to the survival benefit seen with colonoscopies and colorectal cancer, and may be attributable to endoscopic treatment of premalignant lesions.
“The relatively high incidence rate of upper gastrointestinal cancer in patients with GERD indicates that a one-time upper endoscopy may be beneficial,” wrote the authors, who were led by Dag Holmberg, MD, PhD, of the department of molecular medicine and surgery at the Karolinska Institutet and Karolinska University Hospital, both in Stockholm. The study was published in Gastroenterology.
GERD is the most frequent reason patients undergo an upper endoscopy, but the results are often negative. It is generally a benign condition, but can lead to Barrett’s esophagus, as well as esophageal and gastric cardia adenocarcinoma. Upper endoscopy can identify other esophageal cancers like gastric noncardia cancer and duodenal cancer, which may cause dyspepsia or GERD-like symptoms.
To determine the potential benefit of upper endoscopy, the researchers conducted a population-based, four-nation cohort study that included 1,062,740 individuals with GERD in Denmark, Finland, Norway, and Sweden. The data were gathered from national patient registries, cancer registries, and cause of death registries. The study encompassed data from 1979 through the end of 2018.
The median age was 58 years, and 52% of participants were women.
The researchers defined a negative endoscopy as no diagnosis of gastrointestinal cancer within 6 months of the procedure; 69.3% of procedures were negative.
During the follow-up period, 0.34% of participants developed and 0.27% died of upper gastrointestinal cancer. Among those with negative endoscopies, 0.23% developed and 0.22% died from upper gastrointestinal cancer.
Participants with a negative endoscopy had a lower risk of being diagnosed with upper gastrointestinal cancer during the follow-up period (adjusted hazard ratio, 0.45; 95% confidence interval, 0.43-0.48). The reduction in risk was similar across age sexes and age groups, but among procedures performed after 2008, the risk reduction was even higher (aHR, 0.34; P < .001).
The effect was strongest in the first year after the procedure, but it persisted out to 5 years before returning to baseline risk levels.
A negative endoscopy was also associated with decreased mortality risk from upper gastrointestinal cancer versus those who hadn’t had an endoscopy (aHR, 0.39; 95% CI, 0.37-0.42). The protective value continued for at least 10 years.
Esophageal adenocarcinoma developed in 0.12% of participants, and 0.10% died of the disease. Among those with a negative endoscopy, 0.09% developed adenocarcinoma, and 0.07% died (aHR vs. no upper endoscopy, 0.33; 95% CI, 0.30-0.37).
The rapid return to baseline risk was notable, and different from what occurs after negative colonoscopies. However, new tumors can readily form within one year, and the risk may reflect early malignant or premalignant lesions that were missed during the procedure.
In fact, a meta-analysis found that 11.3% of upper gastrointestinal cancers had escaped detection during an endoscopy in the previous 3 years before diagnosis, and case reviews of patients diagnosed with gastrointestinal cancer soon after an upper endoscopy usually reveal suspicious or indeterminate results that the endoscopist or pathologist missed.
Quality indicators for upper endoscopy include procedure time, rate of targeted biopsies, and computer-aided detection, but it isn’t clear what impact these measures have on outcomes. However, the greater risk reduction found with endoscopies performed more recently suggests that newer quality indicators and technological improvements may be improving outcomes.
The relatively low incidence of esophageal and gastric cancer in Western countries has discouraged widespread adoption of endoscopic screening, but the researchers point out that the risk of gastrointestinal cancer among individuals with GERD is similar to the risk of colorectal cancer in the 60-69 age group in the United States, for whom colonoscopy is recommended.
“The present study indicates that upper endoscopy may be beneficial for patients with GERD, but to make upper endoscopy screening more cost beneficial at its initiation, the target group may be limited to include patients at highest risk of cancer. Such previous cost-effectiveness studies have indicated that endoscopy is cost effective in men at aged 50 years or older with chronic GERD,” the authors wrote.
The study was funded by Swedish Research Council and Swedish Cancer Society. The authors disclosed no relevant conflicts of interest.
FROM GASTROENTEROLOGY
Hemostatic powder noninferior in nonvariceal upper GI bleeds
TC-325, a bentonite-derived hemostatic powder, was not inferior to standard therapy for the endoscopic management of acute nonvariceal upper GI bleeding, according to a new study.
The findings from the study, lead investigator and study author James Y.W. Lau, MD, of the Prince of Wales Hospital in Hong Kong, said in an interview, suggest TC-325 could “be considered one of the primary endoscopic treatments to actively stop nonvariceal bleeding,” particularly in cases when other therapies prove unsuccessful. The study findings were published in Annals of Internal Medicine.
The study team noted that, after they first reported the use of TC-325 in active bleeding from gastroduodenal ulcers in 2011, there have been other studies of its use with acute nonvariceal upper GI bleeding, but to date there has been only two randomized controlled trials of it as a sole endoscopic treatment option for acute nonvariceal upper GI bleeding. To close this research gap, Dr. Lau and researchers enrolled 224 adult patients with acute bleeding from a nonvariceal source on upper GI endoscopy and randomly assigned these patients to receive either TC-325 (n = 111) or standard hemostatic treatment (n = 113). Standard endoscopic bleeding management consisted of contact thermocoagulation using a heater probe or bipolar probe, or hemoclipping with or without previously injected diluted epinephrine.
Success of assigned treatment was defined by the cessation of active bleeding as well as flattening of the protuberance or vessel with a heater or bipolar probe. For the primary outcome of the study, the investigators assessed the rate of bleeding control within 30 days following randomization. Additionally, the researchers compared the treatment groups to identify differences in the failure to control bleeding during the initial endoscopy and recurrent bleeding following hemostasis.
Treatment groups were even in regard to the proportions of patients with bleeding gastroduodenal ulcers (61.3% vs. 60.2%). A smaller proportion of patients in the TC-325 arm had a history of alcohol use (3.0% vs. 9.8%) and current use of NSAIDs (8.1% vs. 20.4%). The group assigned to TC-325 had more bleeding tumors (20.7% vs. 8.8%) and fewer Dieulafoy lesions (5.4% vs. 14.2%), compared with the standard treatment arm. Additionally, patients in the TC-325 group had a higher median Glasgow-Blatchford Score at hospital admission than the standard endoscopy management group (12 vs. 11, respectively; P < .05).
Although a greater proportion of patients assigned TC-325 had bleeding controlled within 30 days of randomization (90.1% vs. 81.4%; risk difference, 8.7 percentage points; 1-sided 95% CI, 0.95 percentage points), the researchers noted that the lower limit of the confidence interval for treatment difference “did not extend beyond the prespecified noninferiority margin of 10 percentage points, indicating that TC-325 is not inferior to standard treatment in the control of bleeding.”
Fewer failures of hemostasis were observed with TC-325 during index endoscopy (2.7% vs. 9.7%; odds ratio, 0.26; 95% CI, 0.07-0.95). After initial endoscopic control, recurrent bleeding was observed in 9 patients in the TC-325 arm and 10 patients in the standard treatment group.
The authors suggested that the low recurrent bleeding rate in the TC-325 arm may reflect enhanced responsiveness in the predominantly Asian study population, a group with lower parietal cell masses and higher rates of Helicobacter pylori infections. In an accompanying editorial published online in Annals of Internal Medicine, Alan N. Barkun, MD, McGill University and McGill University Health Centre in Montreal, and Ali Alali, MB BCh BAO, in the department of medicine at Kuwait University, Kuwait City, noted that “possible additional reasons for the enhanced effectiveness of TC-325 observed in the current trial may be its varied performance in the patients with nonulcer bleeding.”
No difference was found between the treatment strategies in terms of the need for additional interventions within 30 days. The need for further endoscopic treatment was reported in 7.2% of patients in the TC-325 groups versus 8.8% of patients assigned to standard treatment. In addition, further angiography was required in 1.8% and 3.5% of patients, while further surgery was required in 0.9% of patients treated with TC-325 versus none in the standard treatment group. Each group reported 14 deaths.
Dr. Lau noted that the study enrolled Asian patients who were more responsive to proton pump inhibitor therapy, which may limit the generalizability of the findings. “We also included patients with mixed etiologies,” he added. “Studies that focus on specific lesions would further inform our practice, and larger observational studies are required to understand failures with TC-325.”
Based on the study findings, corresponding editorial author Dr. Barkun wrote that “TC-325 can be used alone in nonvariceal upper gastrointestinal bleeding or as rescue therapy but should be reserved for patients with actively bleeding lesions” and suggests the treatment option “is likely one of the most effective modalities in achieving immediate hemostasis.”
The researchers reported no conflicts of interest with the pharmaceutical industry. The editorialists also reported no disclosures of interest.
TC-325, a bentonite-derived hemostatic powder, was not inferior to standard therapy for the endoscopic management of acute nonvariceal upper GI bleeding, according to a new study.
The findings from the study, lead investigator and study author James Y.W. Lau, MD, of the Prince of Wales Hospital in Hong Kong, said in an interview, suggest TC-325 could “be considered one of the primary endoscopic treatments to actively stop nonvariceal bleeding,” particularly in cases when other therapies prove unsuccessful. The study findings were published in Annals of Internal Medicine.
The study team noted that, after they first reported the use of TC-325 in active bleeding from gastroduodenal ulcers in 2011, there have been other studies of its use with acute nonvariceal upper GI bleeding, but to date there has been only two randomized controlled trials of it as a sole endoscopic treatment option for acute nonvariceal upper GI bleeding. To close this research gap, Dr. Lau and researchers enrolled 224 adult patients with acute bleeding from a nonvariceal source on upper GI endoscopy and randomly assigned these patients to receive either TC-325 (n = 111) or standard hemostatic treatment (n = 113). Standard endoscopic bleeding management consisted of contact thermocoagulation using a heater probe or bipolar probe, or hemoclipping with or without previously injected diluted epinephrine.
Success of assigned treatment was defined by the cessation of active bleeding as well as flattening of the protuberance or vessel with a heater or bipolar probe. For the primary outcome of the study, the investigators assessed the rate of bleeding control within 30 days following randomization. Additionally, the researchers compared the treatment groups to identify differences in the failure to control bleeding during the initial endoscopy and recurrent bleeding following hemostasis.
Treatment groups were even in regard to the proportions of patients with bleeding gastroduodenal ulcers (61.3% vs. 60.2%). A smaller proportion of patients in the TC-325 arm had a history of alcohol use (3.0% vs. 9.8%) and current use of NSAIDs (8.1% vs. 20.4%). The group assigned to TC-325 had more bleeding tumors (20.7% vs. 8.8%) and fewer Dieulafoy lesions (5.4% vs. 14.2%), compared with the standard treatment arm. Additionally, patients in the TC-325 group had a higher median Glasgow-Blatchford Score at hospital admission than the standard endoscopy management group (12 vs. 11, respectively; P < .05).
Although a greater proportion of patients assigned TC-325 had bleeding controlled within 30 days of randomization (90.1% vs. 81.4%; risk difference, 8.7 percentage points; 1-sided 95% CI, 0.95 percentage points), the researchers noted that the lower limit of the confidence interval for treatment difference “did not extend beyond the prespecified noninferiority margin of 10 percentage points, indicating that TC-325 is not inferior to standard treatment in the control of bleeding.”
Fewer failures of hemostasis were observed with TC-325 during index endoscopy (2.7% vs. 9.7%; odds ratio, 0.26; 95% CI, 0.07-0.95). After initial endoscopic control, recurrent bleeding was observed in 9 patients in the TC-325 arm and 10 patients in the standard treatment group.
The authors suggested that the low recurrent bleeding rate in the TC-325 arm may reflect enhanced responsiveness in the predominantly Asian study population, a group with lower parietal cell masses and higher rates of Helicobacter pylori infections. In an accompanying editorial published online in Annals of Internal Medicine, Alan N. Barkun, MD, McGill University and McGill University Health Centre in Montreal, and Ali Alali, MB BCh BAO, in the department of medicine at Kuwait University, Kuwait City, noted that “possible additional reasons for the enhanced effectiveness of TC-325 observed in the current trial may be its varied performance in the patients with nonulcer bleeding.”
No difference was found between the treatment strategies in terms of the need for additional interventions within 30 days. The need for further endoscopic treatment was reported in 7.2% of patients in the TC-325 groups versus 8.8% of patients assigned to standard treatment. In addition, further angiography was required in 1.8% and 3.5% of patients, while further surgery was required in 0.9% of patients treated with TC-325 versus none in the standard treatment group. Each group reported 14 deaths.
Dr. Lau noted that the study enrolled Asian patients who were more responsive to proton pump inhibitor therapy, which may limit the generalizability of the findings. “We also included patients with mixed etiologies,” he added. “Studies that focus on specific lesions would further inform our practice, and larger observational studies are required to understand failures with TC-325.”
Based on the study findings, corresponding editorial author Dr. Barkun wrote that “TC-325 can be used alone in nonvariceal upper gastrointestinal bleeding or as rescue therapy but should be reserved for patients with actively bleeding lesions” and suggests the treatment option “is likely one of the most effective modalities in achieving immediate hemostasis.”
The researchers reported no conflicts of interest with the pharmaceutical industry. The editorialists also reported no disclosures of interest.
TC-325, a bentonite-derived hemostatic powder, was not inferior to standard therapy for the endoscopic management of acute nonvariceal upper GI bleeding, according to a new study.
The findings from the study, lead investigator and study author James Y.W. Lau, MD, of the Prince of Wales Hospital in Hong Kong, said in an interview, suggest TC-325 could “be considered one of the primary endoscopic treatments to actively stop nonvariceal bleeding,” particularly in cases when other therapies prove unsuccessful. The study findings were published in Annals of Internal Medicine.
The study team noted that, after they first reported the use of TC-325 in active bleeding from gastroduodenal ulcers in 2011, there have been other studies of its use with acute nonvariceal upper GI bleeding, but to date there has been only two randomized controlled trials of it as a sole endoscopic treatment option for acute nonvariceal upper GI bleeding. To close this research gap, Dr. Lau and researchers enrolled 224 adult patients with acute bleeding from a nonvariceal source on upper GI endoscopy and randomly assigned these patients to receive either TC-325 (n = 111) or standard hemostatic treatment (n = 113). Standard endoscopic bleeding management consisted of contact thermocoagulation using a heater probe or bipolar probe, or hemoclipping with or without previously injected diluted epinephrine.
Success of assigned treatment was defined by the cessation of active bleeding as well as flattening of the protuberance or vessel with a heater or bipolar probe. For the primary outcome of the study, the investigators assessed the rate of bleeding control within 30 days following randomization. Additionally, the researchers compared the treatment groups to identify differences in the failure to control bleeding during the initial endoscopy and recurrent bleeding following hemostasis.
Treatment groups were even in regard to the proportions of patients with bleeding gastroduodenal ulcers (61.3% vs. 60.2%). A smaller proportion of patients in the TC-325 arm had a history of alcohol use (3.0% vs. 9.8%) and current use of NSAIDs (8.1% vs. 20.4%). The group assigned to TC-325 had more bleeding tumors (20.7% vs. 8.8%) and fewer Dieulafoy lesions (5.4% vs. 14.2%), compared with the standard treatment arm. Additionally, patients in the TC-325 group had a higher median Glasgow-Blatchford Score at hospital admission than the standard endoscopy management group (12 vs. 11, respectively; P < .05).
Although a greater proportion of patients assigned TC-325 had bleeding controlled within 30 days of randomization (90.1% vs. 81.4%; risk difference, 8.7 percentage points; 1-sided 95% CI, 0.95 percentage points), the researchers noted that the lower limit of the confidence interval for treatment difference “did not extend beyond the prespecified noninferiority margin of 10 percentage points, indicating that TC-325 is not inferior to standard treatment in the control of bleeding.”
Fewer failures of hemostasis were observed with TC-325 during index endoscopy (2.7% vs. 9.7%; odds ratio, 0.26; 95% CI, 0.07-0.95). After initial endoscopic control, recurrent bleeding was observed in 9 patients in the TC-325 arm and 10 patients in the standard treatment group.
The authors suggested that the low recurrent bleeding rate in the TC-325 arm may reflect enhanced responsiveness in the predominantly Asian study population, a group with lower parietal cell masses and higher rates of Helicobacter pylori infections. In an accompanying editorial published online in Annals of Internal Medicine, Alan N. Barkun, MD, McGill University and McGill University Health Centre in Montreal, and Ali Alali, MB BCh BAO, in the department of medicine at Kuwait University, Kuwait City, noted that “possible additional reasons for the enhanced effectiveness of TC-325 observed in the current trial may be its varied performance in the patients with nonulcer bleeding.”
No difference was found between the treatment strategies in terms of the need for additional interventions within 30 days. The need for further endoscopic treatment was reported in 7.2% of patients in the TC-325 groups versus 8.8% of patients assigned to standard treatment. In addition, further angiography was required in 1.8% and 3.5% of patients, while further surgery was required in 0.9% of patients treated with TC-325 versus none in the standard treatment group. Each group reported 14 deaths.
Dr. Lau noted that the study enrolled Asian patients who were more responsive to proton pump inhibitor therapy, which may limit the generalizability of the findings. “We also included patients with mixed etiologies,” he added. “Studies that focus on specific lesions would further inform our practice, and larger observational studies are required to understand failures with TC-325.”
Based on the study findings, corresponding editorial author Dr. Barkun wrote that “TC-325 can be used alone in nonvariceal upper gastrointestinal bleeding or as rescue therapy but should be reserved for patients with actively bleeding lesions” and suggests the treatment option “is likely one of the most effective modalities in achieving immediate hemostasis.”
The researchers reported no conflicts of interest with the pharmaceutical industry. The editorialists also reported no disclosures of interest.
FROM ANNALS OF INTERNAL MEDICINE
Vonoprazan beats PPIs in H. pylori eradication
LAS VEGAS – In the treatment of Helicobacter pylori infection, combination therapies using the oral potassium-competitive acid blocker vonoprazan were superior to standard proton pump inhibitor (PPI)–based triple therapy, producing higher eradication rates, according to combined data from a U.S. and a European phase 3 randomized, controlled trial.
Vonoprazan has been submitted to the Food and Drug Administration for approval with a Fast Track designation in combination with amoxicillin and clarithromycin (triple therapy) or amoxicillin alone (dual therapy) for treating H. pylori infection. It has already been approved in Japan for the treatment of gastric and duodenal ulcers, reflux esophagitis, secondary prevention of low-dose aspirin– or nonsteroidal anti-inflammatory drug–induced gastric mucosal damage, and for first and second-line H. pylori eradication therapy.
Study details
The study included 1,046 treatment-naive patients who had dyspepsia, a recent or new diagnosis of a nonbleeding peptic ulcer, a history of a peptic ulcer, or long-term stable use of an NSAID. Patients were randomized to PPI-based triple therapy (lansoprazole, amoxicillin, clarithromycin), vonoprazan triple therapy (plus amoxicillin, clarithromycin), or vonoprazan dual therapy (amoxicillin). The treatment period was 14 days, followed by 13C urea breath test (UBT) 4 weeks after treatment.
The researchers conducted several analyses, including: Modified intention-to-treat analyses, which included all enrollees; per protocol analyses, which included patients who took at least 75% of each study medication and underwent 13C UBT in the expected time frame; and a safety population of all patients who took at least one study drug.
Among patients with H. pylori strains that were not resistant to clarithromycin, the PPI-based triple-therapy group had an eradication rate of 78.8%, compared with 84.7% in the vonoprazan triple-therapy group (P < .0001), and 78.5% in the vonoprazan dual-therapy group (P = .0037). In the per protocol analysis, PPI-based triple therapy eradicated H. pylori 82.1% of the time, compared with 90.4% in the vonoprazan triple-therapy group (P < .0001) and 81.2% in the vonoprazan dual-therapy group (P = .0077). Both vonoprazan treatment groups were noninferior to PPI-based triple therapy.
A prespecified exploratory analysis found that vonoprazan triple therapy outperformed PPI-based triple therapy in the modified intention-to-treat population (P = .0408) and the per protocol population (P = .0059).
Among patients with clarithromycin-resistant strains of H. pylori, in the modified intention-to-treat population, 31.9% achieved eradication with PPI triple therapy, compared with 65.8% in the vonoprazan triple-therapy group, and 69.6% in the vonoprazan dual-therapy group. In the per protocol population, the numbers were 29.0% versus 67.2% and 79.5%, respectively (P < .0001 for both versus PPI triple therapy).
Among all patients, in the modified intention-to-treat population, 68.5% achieved eradication with PPI triple therapy, 80.8% with vonoprazan triple therapy (P =. 0001), and 77.2% with vonoprazan dual therapy (P = .0063)*. In the per protocol population, the numbers were 70.0%, 85.7% (P < .0001), and 81.1% (P = .0013), respectively.
Safety outcomes were similar among the three groups, with treatment-emergent adverse events occurring in 34.5% of the PPI triple-therapy group (1.2% discontinued), 34.1% of the vonoprazan triple-therapy group (2.3% discontinued), and 29.9% in the vonoprazan dual-therapy group (0.9% discontinued).
Fighting against resistance
The efficacy of PPI-based clarithromycin-based triple therapy has fallen below 80% in the United States and Europe over the past few decades, largely because of antibiotic resistance, said William Chey, MD, during a presentation of the results at the annual meeting of the American College of Gastroenterology. Dr. Chey is a professor of medicine and director of the GI physiology laboratory at Michigan Medicine.
Vonoprazan is more stable in acid than are PPIs, and produces greater and more durable acid reduction, according to Dr. Chey. That’s important for two reasons: One is that some antibiotics are acid-labile, and so may have their efficacy directly impacted in a more acidic environment. The other factor is that most antibiotics work better on bacteria that are actively replicating, and H. pylori reproduces better in a more neutral environment. “So, you increase the replication, you increase the bioavailability of the antibiotics. And therefore, hopefully, that underlies why we see it working better in the patients with [antibiotic] resistance,” Dr. Chey said in an interview.
It remains to be seen whether or not the drug will receive FDA approval, but he pointed to other regimens like bismuth quadruple therapy and rifabutin-based triple therapy that are already available. “If I had the choice, I would never use a PPI-based triple therapy again. People should not be doing that,” said Dr. Chey.
“More successful H. pylori eradication regimens are certainly needed, and these results are particularly relevant and interesting given the increasing failure of initial treatment regimens,” said Kimberly Harer, MD, who moderated the session. She noted that the secondary analysis of patients with clarithromycin-resistant infections was particularly relevant. “The superiority analysis indicating vonoprazan triple therapy resulted in increased H. pylori eradication compared to lanzoprazole triple therapy was especially interesting,” said Dr. Harer, who is a clinical lecturer at University of Michigan Health, Ann Arbor.
One downside to the study is that it didn’t compare vonoprazan combinations to quadruple therapy of a PPI, bismuth, tetracycline, and a nitroimidazole, said Joseph Jennings, MD, who was asked to comment on the study. Other treatment approaches include sequential antibiotics and other combinations. Dr. Jennings also highlighted the findings that the vonoprazan regimens were superior against clarithromycin-resistant strains. “The more different regimens we can add to the armamentarium, the better chance we have because the resistant patterns fluctuate all throughout the world,” said Dr. Jennings, who is an assistant professor of medicine at Georgetown University and director of the center for GI bleeding at MedStar Georgetown University Hospital, both in Washington.
He also pointed out that physicians can face a conundrum when patients fail multiple lines of therapy and have testing done that shows high levels of resistance. Some have allergies that prevent them from turning to other antibiotics. “That’s a market where lots of doctors struggle. Something like this would be a nice add-on,” said Dr. Jennings.
The study was funded by Phathom Pharmaceuticals.** Dr. Chey has consulted and/or received research support from Abbvie, Alfasigma, Allakos, Alnylam, Bayer, Bioamerica, Cosmo, Intrinsic Medicine, Ironwood, Modify Health, My GI Health, My Nutrition Health, Nestle, Phathom Pharmaceuticals, QOL Medical, Redhill, Salix/Valeant, Takeda, Urovant, and Vibrant. Dr. Harer and Dr. Jennings have no relevant financial disclosures.
*Correction, 10/29/21: An earlier version of this article misstated the percentage of patients in the modified intention-to-treat population who achieved eradication with vonoprazan triple therapy.
**Correction, 10/29/21: An earlier version of this article misstated the name of Phathom Pharmaceuticals.
LAS VEGAS – In the treatment of Helicobacter pylori infection, combination therapies using the oral potassium-competitive acid blocker vonoprazan were superior to standard proton pump inhibitor (PPI)–based triple therapy, producing higher eradication rates, according to combined data from a U.S. and a European phase 3 randomized, controlled trial.
Vonoprazan has been submitted to the Food and Drug Administration for approval with a Fast Track designation in combination with amoxicillin and clarithromycin (triple therapy) or amoxicillin alone (dual therapy) for treating H. pylori infection. It has already been approved in Japan for the treatment of gastric and duodenal ulcers, reflux esophagitis, secondary prevention of low-dose aspirin– or nonsteroidal anti-inflammatory drug–induced gastric mucosal damage, and for first and second-line H. pylori eradication therapy.
Study details
The study included 1,046 treatment-naive patients who had dyspepsia, a recent or new diagnosis of a nonbleeding peptic ulcer, a history of a peptic ulcer, or long-term stable use of an NSAID. Patients were randomized to PPI-based triple therapy (lansoprazole, amoxicillin, clarithromycin), vonoprazan triple therapy (plus amoxicillin, clarithromycin), or vonoprazan dual therapy (amoxicillin). The treatment period was 14 days, followed by 13C urea breath test (UBT) 4 weeks after treatment.
The researchers conducted several analyses, including: Modified intention-to-treat analyses, which included all enrollees; per protocol analyses, which included patients who took at least 75% of each study medication and underwent 13C UBT in the expected time frame; and a safety population of all patients who took at least one study drug.
Among patients with H. pylori strains that were not resistant to clarithromycin, the PPI-based triple-therapy group had an eradication rate of 78.8%, compared with 84.7% in the vonoprazan triple-therapy group (P < .0001), and 78.5% in the vonoprazan dual-therapy group (P = .0037). In the per protocol analysis, PPI-based triple therapy eradicated H. pylori 82.1% of the time, compared with 90.4% in the vonoprazan triple-therapy group (P < .0001) and 81.2% in the vonoprazan dual-therapy group (P = .0077). Both vonoprazan treatment groups were noninferior to PPI-based triple therapy.
A prespecified exploratory analysis found that vonoprazan triple therapy outperformed PPI-based triple therapy in the modified intention-to-treat population (P = .0408) and the per protocol population (P = .0059).
Among patients with clarithromycin-resistant strains of H. pylori, in the modified intention-to-treat population, 31.9% achieved eradication with PPI triple therapy, compared with 65.8% in the vonoprazan triple-therapy group, and 69.6% in the vonoprazan dual-therapy group. In the per protocol population, the numbers were 29.0% versus 67.2% and 79.5%, respectively (P < .0001 for both versus PPI triple therapy).
Among all patients, in the modified intention-to-treat population, 68.5% achieved eradication with PPI triple therapy, 80.8% with vonoprazan triple therapy (P =. 0001), and 77.2% with vonoprazan dual therapy (P = .0063)*. In the per protocol population, the numbers were 70.0%, 85.7% (P < .0001), and 81.1% (P = .0013), respectively.
Safety outcomes were similar among the three groups, with treatment-emergent adverse events occurring in 34.5% of the PPI triple-therapy group (1.2% discontinued), 34.1% of the vonoprazan triple-therapy group (2.3% discontinued), and 29.9% in the vonoprazan dual-therapy group (0.9% discontinued).
Fighting against resistance
The efficacy of PPI-based clarithromycin-based triple therapy has fallen below 80% in the United States and Europe over the past few decades, largely because of antibiotic resistance, said William Chey, MD, during a presentation of the results at the annual meeting of the American College of Gastroenterology. Dr. Chey is a professor of medicine and director of the GI physiology laboratory at Michigan Medicine.
Vonoprazan is more stable in acid than are PPIs, and produces greater and more durable acid reduction, according to Dr. Chey. That’s important for two reasons: One is that some antibiotics are acid-labile, and so may have their efficacy directly impacted in a more acidic environment. The other factor is that most antibiotics work better on bacteria that are actively replicating, and H. pylori reproduces better in a more neutral environment. “So, you increase the replication, you increase the bioavailability of the antibiotics. And therefore, hopefully, that underlies why we see it working better in the patients with [antibiotic] resistance,” Dr. Chey said in an interview.
It remains to be seen whether or not the drug will receive FDA approval, but he pointed to other regimens like bismuth quadruple therapy and rifabutin-based triple therapy that are already available. “If I had the choice, I would never use a PPI-based triple therapy again. People should not be doing that,” said Dr. Chey.
“More successful H. pylori eradication regimens are certainly needed, and these results are particularly relevant and interesting given the increasing failure of initial treatment regimens,” said Kimberly Harer, MD, who moderated the session. She noted that the secondary analysis of patients with clarithromycin-resistant infections was particularly relevant. “The superiority analysis indicating vonoprazan triple therapy resulted in increased H. pylori eradication compared to lanzoprazole triple therapy was especially interesting,” said Dr. Harer, who is a clinical lecturer at University of Michigan Health, Ann Arbor.
One downside to the study is that it didn’t compare vonoprazan combinations to quadruple therapy of a PPI, bismuth, tetracycline, and a nitroimidazole, said Joseph Jennings, MD, who was asked to comment on the study. Other treatment approaches include sequential antibiotics and other combinations. Dr. Jennings also highlighted the findings that the vonoprazan regimens were superior against clarithromycin-resistant strains. “The more different regimens we can add to the armamentarium, the better chance we have because the resistant patterns fluctuate all throughout the world,” said Dr. Jennings, who is an assistant professor of medicine at Georgetown University and director of the center for GI bleeding at MedStar Georgetown University Hospital, both in Washington.
He also pointed out that physicians can face a conundrum when patients fail multiple lines of therapy and have testing done that shows high levels of resistance. Some have allergies that prevent them from turning to other antibiotics. “That’s a market where lots of doctors struggle. Something like this would be a nice add-on,” said Dr. Jennings.
The study was funded by Phathom Pharmaceuticals.** Dr. Chey has consulted and/or received research support from Abbvie, Alfasigma, Allakos, Alnylam, Bayer, Bioamerica, Cosmo, Intrinsic Medicine, Ironwood, Modify Health, My GI Health, My Nutrition Health, Nestle, Phathom Pharmaceuticals, QOL Medical, Redhill, Salix/Valeant, Takeda, Urovant, and Vibrant. Dr. Harer and Dr. Jennings have no relevant financial disclosures.
*Correction, 10/29/21: An earlier version of this article misstated the percentage of patients in the modified intention-to-treat population who achieved eradication with vonoprazan triple therapy.
**Correction, 10/29/21: An earlier version of this article misstated the name of Phathom Pharmaceuticals.
LAS VEGAS – In the treatment of Helicobacter pylori infection, combination therapies using the oral potassium-competitive acid blocker vonoprazan were superior to standard proton pump inhibitor (PPI)–based triple therapy, producing higher eradication rates, according to combined data from a U.S. and a European phase 3 randomized, controlled trial.
Vonoprazan has been submitted to the Food and Drug Administration for approval with a Fast Track designation in combination with amoxicillin and clarithromycin (triple therapy) or amoxicillin alone (dual therapy) for treating H. pylori infection. It has already been approved in Japan for the treatment of gastric and duodenal ulcers, reflux esophagitis, secondary prevention of low-dose aspirin– or nonsteroidal anti-inflammatory drug–induced gastric mucosal damage, and for first and second-line H. pylori eradication therapy.
Study details
The study included 1,046 treatment-naive patients who had dyspepsia, a recent or new diagnosis of a nonbleeding peptic ulcer, a history of a peptic ulcer, or long-term stable use of an NSAID. Patients were randomized to PPI-based triple therapy (lansoprazole, amoxicillin, clarithromycin), vonoprazan triple therapy (plus amoxicillin, clarithromycin), or vonoprazan dual therapy (amoxicillin). The treatment period was 14 days, followed by 13C urea breath test (UBT) 4 weeks after treatment.
The researchers conducted several analyses, including: Modified intention-to-treat analyses, which included all enrollees; per protocol analyses, which included patients who took at least 75% of each study medication and underwent 13C UBT in the expected time frame; and a safety population of all patients who took at least one study drug.
Among patients with H. pylori strains that were not resistant to clarithromycin, the PPI-based triple-therapy group had an eradication rate of 78.8%, compared with 84.7% in the vonoprazan triple-therapy group (P < .0001), and 78.5% in the vonoprazan dual-therapy group (P = .0037). In the per protocol analysis, PPI-based triple therapy eradicated H. pylori 82.1% of the time, compared with 90.4% in the vonoprazan triple-therapy group (P < .0001) and 81.2% in the vonoprazan dual-therapy group (P = .0077). Both vonoprazan treatment groups were noninferior to PPI-based triple therapy.
A prespecified exploratory analysis found that vonoprazan triple therapy outperformed PPI-based triple therapy in the modified intention-to-treat population (P = .0408) and the per protocol population (P = .0059).
Among patients with clarithromycin-resistant strains of H. pylori, in the modified intention-to-treat population, 31.9% achieved eradication with PPI triple therapy, compared with 65.8% in the vonoprazan triple-therapy group, and 69.6% in the vonoprazan dual-therapy group. In the per protocol population, the numbers were 29.0% versus 67.2% and 79.5%, respectively (P < .0001 for both versus PPI triple therapy).
Among all patients, in the modified intention-to-treat population, 68.5% achieved eradication with PPI triple therapy, 80.8% with vonoprazan triple therapy (P =. 0001), and 77.2% with vonoprazan dual therapy (P = .0063)*. In the per protocol population, the numbers were 70.0%, 85.7% (P < .0001), and 81.1% (P = .0013), respectively.
Safety outcomes were similar among the three groups, with treatment-emergent adverse events occurring in 34.5% of the PPI triple-therapy group (1.2% discontinued), 34.1% of the vonoprazan triple-therapy group (2.3% discontinued), and 29.9% in the vonoprazan dual-therapy group (0.9% discontinued).
Fighting against resistance
The efficacy of PPI-based clarithromycin-based triple therapy has fallen below 80% in the United States and Europe over the past few decades, largely because of antibiotic resistance, said William Chey, MD, during a presentation of the results at the annual meeting of the American College of Gastroenterology. Dr. Chey is a professor of medicine and director of the GI physiology laboratory at Michigan Medicine.
Vonoprazan is more stable in acid than are PPIs, and produces greater and more durable acid reduction, according to Dr. Chey. That’s important for two reasons: One is that some antibiotics are acid-labile, and so may have their efficacy directly impacted in a more acidic environment. The other factor is that most antibiotics work better on bacteria that are actively replicating, and H. pylori reproduces better in a more neutral environment. “So, you increase the replication, you increase the bioavailability of the antibiotics. And therefore, hopefully, that underlies why we see it working better in the patients with [antibiotic] resistance,” Dr. Chey said in an interview.
It remains to be seen whether or not the drug will receive FDA approval, but he pointed to other regimens like bismuth quadruple therapy and rifabutin-based triple therapy that are already available. “If I had the choice, I would never use a PPI-based triple therapy again. People should not be doing that,” said Dr. Chey.
“More successful H. pylori eradication regimens are certainly needed, and these results are particularly relevant and interesting given the increasing failure of initial treatment regimens,” said Kimberly Harer, MD, who moderated the session. She noted that the secondary analysis of patients with clarithromycin-resistant infections was particularly relevant. “The superiority analysis indicating vonoprazan triple therapy resulted in increased H. pylori eradication compared to lanzoprazole triple therapy was especially interesting,” said Dr. Harer, who is a clinical lecturer at University of Michigan Health, Ann Arbor.
One downside to the study is that it didn’t compare vonoprazan combinations to quadruple therapy of a PPI, bismuth, tetracycline, and a nitroimidazole, said Joseph Jennings, MD, who was asked to comment on the study. Other treatment approaches include sequential antibiotics and other combinations. Dr. Jennings also highlighted the findings that the vonoprazan regimens were superior against clarithromycin-resistant strains. “The more different regimens we can add to the armamentarium, the better chance we have because the resistant patterns fluctuate all throughout the world,” said Dr. Jennings, who is an assistant professor of medicine at Georgetown University and director of the center for GI bleeding at MedStar Georgetown University Hospital, both in Washington.
He also pointed out that physicians can face a conundrum when patients fail multiple lines of therapy and have testing done that shows high levels of resistance. Some have allergies that prevent them from turning to other antibiotics. “That’s a market where lots of doctors struggle. Something like this would be a nice add-on,” said Dr. Jennings.
The study was funded by Phathom Pharmaceuticals.** Dr. Chey has consulted and/or received research support from Abbvie, Alfasigma, Allakos, Alnylam, Bayer, Bioamerica, Cosmo, Intrinsic Medicine, Ironwood, Modify Health, My GI Health, My Nutrition Health, Nestle, Phathom Pharmaceuticals, QOL Medical, Redhill, Salix/Valeant, Takeda, Urovant, and Vibrant. Dr. Harer and Dr. Jennings have no relevant financial disclosures.
*Correction, 10/29/21: An earlier version of this article misstated the percentage of patients in the modified intention-to-treat population who achieved eradication with vonoprazan triple therapy.
**Correction, 10/29/21: An earlier version of this article misstated the name of Phathom Pharmaceuticals.
AT ACG 2021