User login
Bert Vargas, MD
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Bert B. Vargas, MD
Limiting Full-Contact Practice Reduces Football Concussions
WASHINGTON, DC—Limiting the amount of full-contact tackling that occurs in high school football practice reduces the rate of sports-related concussions among the athletes, according to a prospective study.
“Something as simple as saying they can’t tackle all the time, limiting the amount of minutes each month, reduced the incidence,” said Timothy A. McGuine, PhD, Senior Scientist at the University of Wisconsin, Madison, at the American Academy of Pediatrics Annual Meeting.
“The majority of sports-related concussions sustained in high school football practice occurred during full-contact activities,” he said. “The rate of sports-related concussions sustained in high school football practice was more than twice as high in the two seasons prior to a rule change limiting the amount and duration of full-contact activities.”
Testing a Tackle-Limiting Rule
In their study, Dr. McGuine and his associates tested the effects of a tackle-limiting rule implemented in 2014 in a state interscholastic athletic association for all players in grades 9 through 12. The rule prohibited full-contact play during the first practice week, and full contact was defined as “drills or game situations that occur at game speed when full tackles are made at a competitive pace and players are taken to the ground.” The players engaged in full-contact play for as long as 75 minutes total during the second week of practice and for a maximum of 60 min/week for all subsequent weeks in the practice season. The rule did not apply to games.
For data on the two years before the rule change, 2,081 athletes with a mean age of 16 reported their concussion history in the 2012 season, which included 36 schools, and the 2013 season, which included 18 schools. In 2014, licensed athletic trainers recorded the incidence and severity of each sports-related concussion for the 945 players at 26 schools. During all three seasons, almost half the concussions (46%) occurred during tackling. Although the overall rate of concussions dropped from 1.57 per 1,000 athletic exposures in the combined 2012 and 2013 seasons to 1.28 per 1,000 athletic exposures in the 2014 season, the difference was not significant. During the 2012 and 2013 seasons combined, 206 players (9%) sustained 211 concussions, compared with 67 players (7%) with 70 concussions in 2014.
The difference in concussions occurring during practice, however, did differ significantly before and after the rule change. The rate of concussions during practice in 2014 was 0.33 concussions per 1,000 athletic exposures, compared with 0.76 concussions per 1,000 exposures in the 2012 and 2013 seasons. Twelve of 15 concussions in 2014 practices occurred during full-contact practices, a rate of 0.57 per 1,000 exposures, and 82 of 86 concussions in the 2012 and 2013 seasons occurred during full contact practices, a rate of 0.87 per 1,000 exposures.
The investigators observed no difference in concussion rate during the games following the rule change. The 2014 rate of concussions during games was 5.74 per 1,000 exposures, compared with 5.81 per 1,000 exposures in the combined 2012 and 2013 seasons. The severity of concussions sustained before and after the rule change also did not differ, and athletes’ years of football-playing experience had no effect on the concussion incidence in 2014.
To Tackle or Not to Tackle?
Despite the relationship between full-contact play and concussions, Dr. McGuine said that he would not support banning tackling from football.
“I think the benefits of the sport far outweigh the risks,” Dr. McGuine said. “Concussions particularly have transcended a sports issue and become a public health issue and have become political, and I’m very much against legislators, policy makers, [and] associations making blanket rules without the evidence to back those,” he said. “There are lingering long-term effects from all orthopedic injuries, but we’re focusing on concussions.”
Equipment modification is unlikely to make much difference in concussion rates either, said Dr. McGuine, whose previous study on football helmets found that the brand and model did not influence concussion risk. “Concussions are multifactorial,” he said. “We can’t just limit the amount of force transmitted to the brain and say we’re going to stop these injuries from occurring.”
One important strategy for reducing concussions is increasing parents’ and athletes’ awareness about multiple injuries and about ways to reduce the risk, Dr. McGuine said.
“Concussions are like any other injury [such as] ankle sprains, knee injuries and surgeries, [and] shoulder dislocations,” he said. “If you have one, you’re more susceptible to having another one, as opposed to somebody who never had that injury, so the problems are repeat injuries and lingering injuries.” Any of these injuries can have a lasting effect on a young athlete’s quality of life, Dr. McGuine added.
Another way to decrease the incidence of concussions is to enforce rules against leading, or lowering, athletes’ heads during tackling.
“A big issue now is penalizing players for leading with their head and face, but I think we need to be consistent there, too,” Dr. McGuine said. “We can’t penalize defensive players for lowering their helmet if we’re not going to penalize running backs and wide receivers.”
—Tara Haelle
WASHINGTON, DC—Limiting the amount of full-contact tackling that occurs in high school football practice reduces the rate of sports-related concussions among the athletes, according to a prospective study.
“Something as simple as saying they can’t tackle all the time, limiting the amount of minutes each month, reduced the incidence,” said Timothy A. McGuine, PhD, Senior Scientist at the University of Wisconsin, Madison, at the American Academy of Pediatrics Annual Meeting.
“The majority of sports-related concussions sustained in high school football practice occurred during full-contact activities,” he said. “The rate of sports-related concussions sustained in high school football practice was more than twice as high in the two seasons prior to a rule change limiting the amount and duration of full-contact activities.”
Testing a Tackle-Limiting Rule
In their study, Dr. McGuine and his associates tested the effects of a tackle-limiting rule implemented in 2014 in a state interscholastic athletic association for all players in grades 9 through 12. The rule prohibited full-contact play during the first practice week, and full contact was defined as “drills or game situations that occur at game speed when full tackles are made at a competitive pace and players are taken to the ground.” The players engaged in full-contact play for as long as 75 minutes total during the second week of practice and for a maximum of 60 min/week for all subsequent weeks in the practice season. The rule did not apply to games.
For data on the two years before the rule change, 2,081 athletes with a mean age of 16 reported their concussion history in the 2012 season, which included 36 schools, and the 2013 season, which included 18 schools. In 2014, licensed athletic trainers recorded the incidence and severity of each sports-related concussion for the 945 players at 26 schools. During all three seasons, almost half the concussions (46%) occurred during tackling. Although the overall rate of concussions dropped from 1.57 per 1,000 athletic exposures in the combined 2012 and 2013 seasons to 1.28 per 1,000 athletic exposures in the 2014 season, the difference was not significant. During the 2012 and 2013 seasons combined, 206 players (9%) sustained 211 concussions, compared with 67 players (7%) with 70 concussions in 2014.
The difference in concussions occurring during practice, however, did differ significantly before and after the rule change. The rate of concussions during practice in 2014 was 0.33 concussions per 1,000 athletic exposures, compared with 0.76 concussions per 1,000 exposures in the 2012 and 2013 seasons. Twelve of 15 concussions in 2014 practices occurred during full-contact practices, a rate of 0.57 per 1,000 exposures, and 82 of 86 concussions in the 2012 and 2013 seasons occurred during full contact practices, a rate of 0.87 per 1,000 exposures.
The investigators observed no difference in concussion rate during the games following the rule change. The 2014 rate of concussions during games was 5.74 per 1,000 exposures, compared with 5.81 per 1,000 exposures in the combined 2012 and 2013 seasons. The severity of concussions sustained before and after the rule change also did not differ, and athletes’ years of football-playing experience had no effect on the concussion incidence in 2014.
To Tackle or Not to Tackle?
Despite the relationship between full-contact play and concussions, Dr. McGuine said that he would not support banning tackling from football.
“I think the benefits of the sport far outweigh the risks,” Dr. McGuine said. “Concussions particularly have transcended a sports issue and become a public health issue and have become political, and I’m very much against legislators, policy makers, [and] associations making blanket rules without the evidence to back those,” he said. “There are lingering long-term effects from all orthopedic injuries, but we’re focusing on concussions.”
Equipment modification is unlikely to make much difference in concussion rates either, said Dr. McGuine, whose previous study on football helmets found that the brand and model did not influence concussion risk. “Concussions are multifactorial,” he said. “We can’t just limit the amount of force transmitted to the brain and say we’re going to stop these injuries from occurring.”
One important strategy for reducing concussions is increasing parents’ and athletes’ awareness about multiple injuries and about ways to reduce the risk, Dr. McGuine said.
“Concussions are like any other injury [such as] ankle sprains, knee injuries and surgeries, [and] shoulder dislocations,” he said. “If you have one, you’re more susceptible to having another one, as opposed to somebody who never had that injury, so the problems are repeat injuries and lingering injuries.” Any of these injuries can have a lasting effect on a young athlete’s quality of life, Dr. McGuine added.
Another way to decrease the incidence of concussions is to enforce rules against leading, or lowering, athletes’ heads during tackling.
“A big issue now is penalizing players for leading with their head and face, but I think we need to be consistent there, too,” Dr. McGuine said. “We can’t penalize defensive players for lowering their helmet if we’re not going to penalize running backs and wide receivers.”
—Tara Haelle
WASHINGTON, DC—Limiting the amount of full-contact tackling that occurs in high school football practice reduces the rate of sports-related concussions among the athletes, according to a prospective study.
“Something as simple as saying they can’t tackle all the time, limiting the amount of minutes each month, reduced the incidence,” said Timothy A. McGuine, PhD, Senior Scientist at the University of Wisconsin, Madison, at the American Academy of Pediatrics Annual Meeting.
“The majority of sports-related concussions sustained in high school football practice occurred during full-contact activities,” he said. “The rate of sports-related concussions sustained in high school football practice was more than twice as high in the two seasons prior to a rule change limiting the amount and duration of full-contact activities.”
Testing a Tackle-Limiting Rule
In their study, Dr. McGuine and his associates tested the effects of a tackle-limiting rule implemented in 2014 in a state interscholastic athletic association for all players in grades 9 through 12. The rule prohibited full-contact play during the first practice week, and full contact was defined as “drills or game situations that occur at game speed when full tackles are made at a competitive pace and players are taken to the ground.” The players engaged in full-contact play for as long as 75 minutes total during the second week of practice and for a maximum of 60 min/week for all subsequent weeks in the practice season. The rule did not apply to games.
For data on the two years before the rule change, 2,081 athletes with a mean age of 16 reported their concussion history in the 2012 season, which included 36 schools, and the 2013 season, which included 18 schools. In 2014, licensed athletic trainers recorded the incidence and severity of each sports-related concussion for the 945 players at 26 schools. During all three seasons, almost half the concussions (46%) occurred during tackling. Although the overall rate of concussions dropped from 1.57 per 1,000 athletic exposures in the combined 2012 and 2013 seasons to 1.28 per 1,000 athletic exposures in the 2014 season, the difference was not significant. During the 2012 and 2013 seasons combined, 206 players (9%) sustained 211 concussions, compared with 67 players (7%) with 70 concussions in 2014.
The difference in concussions occurring during practice, however, did differ significantly before and after the rule change. The rate of concussions during practice in 2014 was 0.33 concussions per 1,000 athletic exposures, compared with 0.76 concussions per 1,000 exposures in the 2012 and 2013 seasons. Twelve of 15 concussions in 2014 practices occurred during full-contact practices, a rate of 0.57 per 1,000 exposures, and 82 of 86 concussions in the 2012 and 2013 seasons occurred during full contact practices, a rate of 0.87 per 1,000 exposures.
The investigators observed no difference in concussion rate during the games following the rule change. The 2014 rate of concussions during games was 5.74 per 1,000 exposures, compared with 5.81 per 1,000 exposures in the combined 2012 and 2013 seasons. The severity of concussions sustained before and after the rule change also did not differ, and athletes’ years of football-playing experience had no effect on the concussion incidence in 2014.
To Tackle or Not to Tackle?
Despite the relationship between full-contact play and concussions, Dr. McGuine said that he would not support banning tackling from football.
“I think the benefits of the sport far outweigh the risks,” Dr. McGuine said. “Concussions particularly have transcended a sports issue and become a public health issue and have become political, and I’m very much against legislators, policy makers, [and] associations making blanket rules without the evidence to back those,” he said. “There are lingering long-term effects from all orthopedic injuries, but we’re focusing on concussions.”
Equipment modification is unlikely to make much difference in concussion rates either, said Dr. McGuine, whose previous study on football helmets found that the brand and model did not influence concussion risk. “Concussions are multifactorial,” he said. “We can’t just limit the amount of force transmitted to the brain and say we’re going to stop these injuries from occurring.”
One important strategy for reducing concussions is increasing parents’ and athletes’ awareness about multiple injuries and about ways to reduce the risk, Dr. McGuine said.
“Concussions are like any other injury [such as] ankle sprains, knee injuries and surgeries, [and] shoulder dislocations,” he said. “If you have one, you’re more susceptible to having another one, as opposed to somebody who never had that injury, so the problems are repeat injuries and lingering injuries.” Any of these injuries can have a lasting effect on a young athlete’s quality of life, Dr. McGuine added.
Another way to decrease the incidence of concussions is to enforce rules against leading, or lowering, athletes’ heads during tackling.
“A big issue now is penalizing players for leading with their head and face, but I think we need to be consistent there, too,” Dr. McGuine said. “We can’t penalize defensive players for lowering their helmet if we’re not going to penalize running backs and wide receivers.”
—Tara Haelle
Idiopathic Intracranial Hypertension in Pregnancy
A 27-year-old white woman presented to the clinic with headaches and decreased vision through her reading glasses while performing near tasks. Her medical history was significant for herpes simplex, hyperlipidemia, and migraine headaches with aura. Her migraines began following an earlier motor vehicle accident, and her most recent magnetic resonance imaging (MRI) showed no abnormalities. Her current medications included prophylactic acyclovir for herpes and acetaminophen and caffeine tablets as needed for headache. She reported no other trauma or surgery and no known allergies. The patient’s best-corrected Snellen visual acuities in both eyes were 20/20 (distance) and 20/30 (near).
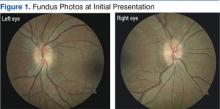
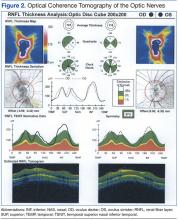
Preliminary testing, including pupils, extraocular motilities, confrontation fields, and color vision, were all within normal limits. Her slit-lamp examination was unremarkable. A dilated fundus examination revealed crowded, elevated discs without vessel obscuration, hemorrhage, hyperemia, or drusen (Figure 1). The fundus examination was otherwise unremarkable. Optical coherence tomography of the optic nerves showed increased nerve fiber layer thickness in both eyes (Figure 2). Her blood pressure (BP) at this visit was 106/77 mm/Hg.
The diagnosis based on these findings was bilateral optic nerve elevation with long-standing migraine headaches. The plan was for the patient to return to the clinic for repeat visual field testing and B-scan ultrasonography to rule out buried optic nerve head drusen.
Two months later, the patient presented to the clinic 19 weeks pregnant and reported that her headaches had increased in frequency, but she had no diplopia. All preliminary testing, including visual acuities, pupil reaction, color vision, and slit-lamp examination remained normal. Fundus examination showed the patient’s nerves were unchanged in appearance from the initial presentation. Visual fields revealed an enlarged blind spot in the right eye and paracentral defects in the left eye. The B-scan testing was negative for optic nerve drusen. Due to the increased frequency of headaches, pregnancy, and suspicious optic nerves, an urgent consult was placed to neurology.
At the neurology appointment 1 month later, the patient was diagnosed with migraine headache syndrome and idiopathic intracranial hypertension (IIH). The neurologist believed her headaches might have been resulting from analgesic rebound. He suggested that the patient discontinue or decrease use of oral butalbital, acetaminophen and caffeine tablets, and other forms of caffeine. It was decided that divalproxen sodium and verapamil were not feasible due to pregnancy. The neurologist started her on oral acetazolamide 500 mg twice daily.
The patient returned to her obstetrician 1 month later for a routine follow-up; the headaches had worsened and were now accompanied by nausea and vomiting twice daily on average. Her medications still included acetaminophen and caffeine tablets, although it had been recommended she discontinue them, prochlorperazine, and acetazolamide. Due to the worsening of her symptoms and visual fields (eFigure 1), the obstetrician recommended that the patient deliver by cesarean section at 38 to 39 weeks.
(eFigure 1.Visual Fields at Follow-up 1 and 2)
Right eye
Left Eye
Following an uncomplicated cesarean delivery at 38 weeks, the patient returned to the clinic for visual field testing. Humphrey visual fields were full in the right eye and showed some scattered central depressions in the left. Both eyes were significantly improved from previous fields (eFigure 2) . The patient had discontinued acetazolamide and reported minor tension headaches she believed were due to lack of sleep but stated that she was no longer having migraines. There was no papilledema noted on fundus examination, and Snellen distance visual acuity measured 20/20 in both eyes. An MRI had been performed after delivery and was negative for intracranial hemorrhage, mass, or hydrocephalus).
(eFigure 2. Visual Fields Postpartum)
Right eye
Left eye
Three months later, the patient returned for her yearly comprehensive examination. At that visit, she reported a decrease in frequency of the migraine headaches. Optical coherence tomography was performed and showed a significant decrease in optic nerve head swelling.
Related: Diabetes on the Rise Among Other Pregnancy Problems
Clinical Picture
Idiopathic intracranial hypertension presents clinically with signs and symptoms of increased intracranial pressure (ICP). Headache is the most common symptom, usually presenting as daily and pulsatile.1 Nausea may be associated with the headache, although vomiting is rare, and the headache may awaken the patient. The headache may remain after resolution of elevated ICP (Table).2
Papilledema is the most common sign of IIH.1,2 Visual loss associated with papilledema is generally mild at first but progressive. Transient blur lasts usually 30 seconds and may be monocular or binocular.1 The cause is thought to be related to transient ischemia of the optic nerve.1 Vision loss is typically reversible with resolution of optic nerve swelling, but 25% of patients may develop optic atrophy, which results in permanent vision loss.2 Common patterns of visual abnormalities include enlargement of the physiologic blind spot, inferonasal and arcuate defects, and eventually severe peripheral constriction.1,2 It is imperative that all patients with IIH have visual field testing performed.
About one-third of patients with IIH experience diplopia. This binocular, horizontal diplopia is caused by a sixth nerve palsy in 10% to 20% of patients.1 Cranial nerves II, VI, and VII make a 90-degree bend and seem to be prone to damage at the site of the bend.1
Pulse-synchronous tinnitus is common in IIH as well.2,3 This generally occurs unilaterally and may be eliminated by jugular compression or the head turning to the ipsilateral side.1,3 The sound is caused by the transmission of an increase in the vascular pulse due to high pressure on the cerebrospinal fluid (CSF).1,3
Idiopathic intracranial hypertension most typically presents in obese women of childbearing age.1-3 An increasing degree of obesity is generally associated with an increased risk of vision loss.1,2 Men seem to have worse acuity and visual fields at presentation than do women.2 Men are less likely to report headaches than are women and, therefore, have double the likelihood of severe vision loss.2 Hence, closer monitoring and more aggressive intervention is recommended for men due to their lesser tendency for headaches.2 Black patients also demonstrate more aggressive disease and, therefore, require closer monitoring and early aggressive intervention.1,2
Papilledema is the most common sign of IIH and may be caused by several processes. In this case, most were ruled out given the patient’s normal visual acuities, pupillary reaction, color vision testing, BP measurement, and B-scan imaging. The patient’s systemic history was negative for thyroid-related disease, diabetes, hypertension, autoimmune disease, or infection. She had no family history of vision loss or hereditary ocular conditions. The most recent MRI was negative for any long-standing space-occupying lesion or hydrocephalus.
Pathophysiology
Several mechanisms leading to increased ICP have been proposed. These include increased brain water content, excess CSF production, reduced CSF absorption, and increased cerebral venous pressure.2,3 There is also a suspicion of the role of sex hormones in IIH due to its high predilection for females.2
The role of vitamin A metabolism has also been studied in IIH.1 Retinol levels are elevated in the CSF of patients with IIH. Patients may ingest an abnormally large amount of vitamin A, metabolize it abnormally, or be sensitive to its effects.2,4 The function of adipose tissue as an actively secreting endocrine tissue may play a role in IIH due to its release of adipose tissue-derived retinol binding protein.2 Other adipose-produced cytokines include leptin, which has been implicated in IIH due to its elevated levels found in the CSF of patients with IIH.2
Stenosis of the cerebral sinuses is another proposed mechanism of IIH.1-3 Cerebrospinal fluid exits the cranium into the venous sinuses via the arachnoid villi.2 An obstruction in these sinuses may impair CSF outflow and result in intracranial hypertension. Microthrombosis caused by hypercoaguable disorders may result in increased cerebral venous pressure and impaired CSF absorption as well.2,4
Some medications have been found in association with IIH. These include tetracycline, cyclosporine, lithium, nalidixic acid, nitrofurantoin, oral contraceptives, levonorgestrel, danaxol, and tamoxifen.1-4 Tetracycline seems to have the strongest association with IIH and should be discontinued in those patients where the association is very likely to be the causative factor.2 The link to oral contraceptives may occur simply due to their association with young women most at risk for IIH.1-3
Related:Young Man With Headache, Confusion, and Hearing Loss
Management
The goals of treatment with IIH are to preserve vision and relieve symptoms, particularly headache. The general recommendation is that pregnant women with IIH should be managed and treated the same as any other patient with IIH. However, imaging and some drug contraindications exist between these 2 groups.
The diagnostic test for IIH is a lumbar puncture, which is also the most effective treatment.1-3,5 Lumbar puncture should be performed in the relaxed lateral decubitus position without sedation.1-3 The opening pressure should be measured and is the most clinically significant diagnostic tool for diagnosis of IIH. Opening pressures of > 250 mm H2O are diagnostic of IIH.1-3,5
Weight loss is an essential part of treatment in obese patients with IIH.1-3 A low-calorie, low-salt diet with mild fluid restriction seems to reverse the symptoms of IIH. A 5% to 10% reduction in body weight may reduce symptoms and signs of IIH.2
Carbonic anhydrase inhibitors (CAIs), such as acetalzolamide, have a multifactorial role in IIH.4 They are usually prescribed in 1 to 2 grams over several doses and function by decreasing CSF production.1 Carbonic anhydrase inhibitors also are known to change the taste of foods and may, therefore, aid in weight loss.1,2 Patients prescribed CAIs commonly experience a tingling in their fingers, toes, and perioral region, an indication that the medication is working.1,2 A rare but serious adverse effect (AE) is aplastic anemia, which generally occurs in the first 6 months of treatment in elderly patients.1 The use of CAIs in pregnancy is controversial, and although rare complications are reported, it is considered a class C drug.5
In patients with rapidly progressive vision loss but with minimal headache, optic nerve sheath fenestration (ONSF) is the surgical treatment of choice.2,3,6 In this procedure, a window or series of slits are created behind the globe in the optic nerve sheath.1 About 50% of patients achieve adequate headache control with ONSF, especially for frontal headaches.1,2
For patients with vision loss, papilledema, and headache that do not respond to medical therapy, a CSF diversion procedure is the preferred treatment. Cerebrospinal fluid diversion with ventriculoperitoneal or lumboperitoneal shunts may prevent progressive loss of vision.1,4,6 However, variable response rates and shunt failure requiring subsequent revisions are common and may occur in as many as half of patients undergoing these procedures.1
Increased intracranial venous pressure due to stenosis of the venous sinuses has been thought to be a possible cause of IIH. Stenting of the transverse venous sinus stenosis has been shown to reduce cerebral venous pressure, reduce ICP, and improve symptoms in patients with IIH.1-3 It is unclear whether elevations in ICP cause transverse sinus stenosis or whether transverse sinus stenosis causes increased ICP.2 Regardless, stents have a high rate of complications, including subdural hemorrhage, venous sinus perforation, in-stent thrombosis, and recurrent stenosis proximal to the stent.2
Steroids have been used to treat IIH in the past, although their mechanism of action remains unclear.2 There may be recurrence of papilledema if they are tapered too quickly. Due to their association with long-term AEs, including weight gain, they should be avoided.2
Management in Pregnancy
Several studies agree that vision loss occurs in the same frequency in pregnant and nonpregnant patients with IIH.4,7 Idiopathic intracranial hypertension can occur in any trimester in pregnancy. It has been found that patients have the same spontaneous abortion rate and visual outcomes as the general population.6-8 It has also been concluded that treatment should be the same in both patient populations with slight variability in the use of acetazolamide.4,6,7
The use of dilating drops during pregnancy is controversial. Although there have been no teratogenic effects reported with use of topical anesthetics and dilating drops, all drugs should be avoided during the first trimester.7-10 Guidelines have been established by the American Congress of Obstetricians and Gynecologists for X-ray examination and exposure during pregnancy. It has been determined that exposure from a single diagnostic X-ray procedure does not result in harmful fetal effects.11 Magnetic resonance imaging is not associated with any known adverse fetal effects and is a better imaging option during pregnancy, because it is not associated with the use of ionizing radiation.11
The use of CAIs in the first trimester is controversial.4,7 Some believe it should be avoided because it is a Pregnancy Category C drug. However, a single case of sacrococcygeal teratoma has been reported in humans; therefore, some believe this is not a strong basis for withholding the medication in patients with the potential risk for severe vision loss.4,7 In this case, a consult to the patient’s obstetrician was made, and the use of acetazolamide had no effect on the health of the baby.
In pregnant women with IIH with progressive vision loss, failed treatment, or nonadherence, surgery may be necessary. Optic nerve sheath fenestration is preferred due to lower morbidity and mortality compared with shunting procedures.1,2,4,6 The growing fetus may be affected by the peritoneal end of the shunt.4
Related: 49-Year-Old Woman With a Broken Heart
Conclusions
Vision loss associated with IIH can be severe and permanent if left untreated. The best treatments and often the most effective involve weight loss and lumbar puncture. Acetazolamide has been a proven effective treatment in some patients, but some debate exists over the safety of its use during pregnancy. This patient did not have any AEs from its use; however, it did not prove valuable in her treatment. Studies often disagree on the use of acetazolamide in pregnancy; however, all agree that proper patient counseling on potential AEs and management by an obstetrician are important. With proper management, pregnant women with IIH have had outcomes similar to those of the general population.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Wall M. Idiopathic intracranial hypertension. Neurol Clin. 2010;28(3):593-617.
2. Bruce BB, Biousee V, Newman NJ. Update on idiopathic intracranial hypertension. Am J Ophthalmol. 2011;152(2):163-169.
3. Fields JD, Javendani PP, Falardeau J, et al. Dural venous sinus angioplasty and stenting for the treatment of idiopathic intracranial hypertension. J Neurointerv Surg. 2013;5(1):62-68.
4. Evans RW, Lee AG. Idiopathic intracranial hypertension in pregnancy. Headache. 2010;50(9):1513-1515.
5. Friedman DI, Jacobson DM. Diagnostic criteria for idiopathic intracranial hypertension. Neurology. 2002;59(10):1492-1495.
6. Martínez-Varea A, Diago-Almela VJ, Abad-Carrascosa A, Perales-Marín A. Progressive visual loss in a pregnant woman with idiopathic intracranial hypertension. Eur J Obstet Gynecol Reprod Biol. 2012;163(1):117-122.
7. Falardeau J, Lobb B, Golden S, Maxfield SD, Tanne E. The use of acetazolamide during pregnancy in intracranial hypertension patients. J Neuroophthalmol. 2013;33(1):9-12.
8. Dinn RB, Harris A, Marcus PS. Ocular changes in pregnancy. Obstet Gynecol Surg. 2003;58(2):137-144.
9. Shultz KL, Birnbaum AD, Goldstein DA. Ocular disease in pregnancy. Curr Opin Ophthalmol. 2005;16(5):308-314.
10. Chung CY, Kwok AKH, Chung KL. Use of ophthalmic medications during pregnancy. Hong Kong Med J. 2004;10(3):191-195.
11. American Congress of Obstetricians and Gynecologists. Committee Opinion. Guidelines for diagnostic imaging during pregnancy. American Congress of Obstetricians and Gynecologists Website. http://www.acog.org/-/media/Committee-Opinions/Committee-on-Obstetric-Practice/co299.pdf. Published 2004. Accessed October 9, 2015.
A 27-year-old white woman presented to the clinic with headaches and decreased vision through her reading glasses while performing near tasks. Her medical history was significant for herpes simplex, hyperlipidemia, and migraine headaches with aura. Her migraines began following an earlier motor vehicle accident, and her most recent magnetic resonance imaging (MRI) showed no abnormalities. Her current medications included prophylactic acyclovir for herpes and acetaminophen and caffeine tablets as needed for headache. She reported no other trauma or surgery and no known allergies. The patient’s best-corrected Snellen visual acuities in both eyes were 20/20 (distance) and 20/30 (near).
Preliminary testing, including pupils, extraocular motilities, confrontation fields, and color vision, were all within normal limits. Her slit-lamp examination was unremarkable. A dilated fundus examination revealed crowded, elevated discs without vessel obscuration, hemorrhage, hyperemia, or drusen (Figure 1). The fundus examination was otherwise unremarkable. Optical coherence tomography of the optic nerves showed increased nerve fiber layer thickness in both eyes (Figure 2). Her blood pressure (BP) at this visit was 106/77 mm/Hg.
The diagnosis based on these findings was bilateral optic nerve elevation with long-standing migraine headaches. The plan was for the patient to return to the clinic for repeat visual field testing and B-scan ultrasonography to rule out buried optic nerve head drusen.
Two months later, the patient presented to the clinic 19 weeks pregnant and reported that her headaches had increased in frequency, but she had no diplopia. All preliminary testing, including visual acuities, pupil reaction, color vision, and slit-lamp examination remained normal. Fundus examination showed the patient’s nerves were unchanged in appearance from the initial presentation. Visual fields revealed an enlarged blind spot in the right eye and paracentral defects in the left eye. The B-scan testing was negative for optic nerve drusen. Due to the increased frequency of headaches, pregnancy, and suspicious optic nerves, an urgent consult was placed to neurology.
At the neurology appointment 1 month later, the patient was diagnosed with migraine headache syndrome and idiopathic intracranial hypertension (IIH). The neurologist believed her headaches might have been resulting from analgesic rebound. He suggested that the patient discontinue or decrease use of oral butalbital, acetaminophen and caffeine tablets, and other forms of caffeine. It was decided that divalproxen sodium and verapamil were not feasible due to pregnancy. The neurologist started her on oral acetazolamide 500 mg twice daily.
The patient returned to her obstetrician 1 month later for a routine follow-up; the headaches had worsened and were now accompanied by nausea and vomiting twice daily on average. Her medications still included acetaminophen and caffeine tablets, although it had been recommended she discontinue them, prochlorperazine, and acetazolamide. Due to the worsening of her symptoms and visual fields (eFigure 1), the obstetrician recommended that the patient deliver by cesarean section at 38 to 39 weeks.
(eFigure 1.Visual Fields at Follow-up 1 and 2)
Right eye
Left Eye
Following an uncomplicated cesarean delivery at 38 weeks, the patient returned to the clinic for visual field testing. Humphrey visual fields were full in the right eye and showed some scattered central depressions in the left. Both eyes were significantly improved from previous fields (eFigure 2) . The patient had discontinued acetazolamide and reported minor tension headaches she believed were due to lack of sleep but stated that she was no longer having migraines. There was no papilledema noted on fundus examination, and Snellen distance visual acuity measured 20/20 in both eyes. An MRI had been performed after delivery and was negative for intracranial hemorrhage, mass, or hydrocephalus).
(eFigure 2. Visual Fields Postpartum)
Right eye
Left eye
Three months later, the patient returned for her yearly comprehensive examination. At that visit, she reported a decrease in frequency of the migraine headaches. Optical coherence tomography was performed and showed a significant decrease in optic nerve head swelling.
Related: Diabetes on the Rise Among Other Pregnancy Problems
Clinical Picture
Idiopathic intracranial hypertension presents clinically with signs and symptoms of increased intracranial pressure (ICP). Headache is the most common symptom, usually presenting as daily and pulsatile.1 Nausea may be associated with the headache, although vomiting is rare, and the headache may awaken the patient. The headache may remain after resolution of elevated ICP (Table).2
Papilledema is the most common sign of IIH.1,2 Visual loss associated with papilledema is generally mild at first but progressive. Transient blur lasts usually 30 seconds and may be monocular or binocular.1 The cause is thought to be related to transient ischemia of the optic nerve.1 Vision loss is typically reversible with resolution of optic nerve swelling, but 25% of patients may develop optic atrophy, which results in permanent vision loss.2 Common patterns of visual abnormalities include enlargement of the physiologic blind spot, inferonasal and arcuate defects, and eventually severe peripheral constriction.1,2 It is imperative that all patients with IIH have visual field testing performed.
About one-third of patients with IIH experience diplopia. This binocular, horizontal diplopia is caused by a sixth nerve palsy in 10% to 20% of patients.1 Cranial nerves II, VI, and VII make a 90-degree bend and seem to be prone to damage at the site of the bend.1
Pulse-synchronous tinnitus is common in IIH as well.2,3 This generally occurs unilaterally and may be eliminated by jugular compression or the head turning to the ipsilateral side.1,3 The sound is caused by the transmission of an increase in the vascular pulse due to high pressure on the cerebrospinal fluid (CSF).1,3
Idiopathic intracranial hypertension most typically presents in obese women of childbearing age.1-3 An increasing degree of obesity is generally associated with an increased risk of vision loss.1,2 Men seem to have worse acuity and visual fields at presentation than do women.2 Men are less likely to report headaches than are women and, therefore, have double the likelihood of severe vision loss.2 Hence, closer monitoring and more aggressive intervention is recommended for men due to their lesser tendency for headaches.2 Black patients also demonstrate more aggressive disease and, therefore, require closer monitoring and early aggressive intervention.1,2
Papilledema is the most common sign of IIH and may be caused by several processes. In this case, most were ruled out given the patient’s normal visual acuities, pupillary reaction, color vision testing, BP measurement, and B-scan imaging. The patient’s systemic history was negative for thyroid-related disease, diabetes, hypertension, autoimmune disease, or infection. She had no family history of vision loss or hereditary ocular conditions. The most recent MRI was negative for any long-standing space-occupying lesion or hydrocephalus.
Pathophysiology
Several mechanisms leading to increased ICP have been proposed. These include increased brain water content, excess CSF production, reduced CSF absorption, and increased cerebral venous pressure.2,3 There is also a suspicion of the role of sex hormones in IIH due to its high predilection for females.2
The role of vitamin A metabolism has also been studied in IIH.1 Retinol levels are elevated in the CSF of patients with IIH. Patients may ingest an abnormally large amount of vitamin A, metabolize it abnormally, or be sensitive to its effects.2,4 The function of adipose tissue as an actively secreting endocrine tissue may play a role in IIH due to its release of adipose tissue-derived retinol binding protein.2 Other adipose-produced cytokines include leptin, which has been implicated in IIH due to its elevated levels found in the CSF of patients with IIH.2
Stenosis of the cerebral sinuses is another proposed mechanism of IIH.1-3 Cerebrospinal fluid exits the cranium into the venous sinuses via the arachnoid villi.2 An obstruction in these sinuses may impair CSF outflow and result in intracranial hypertension. Microthrombosis caused by hypercoaguable disorders may result in increased cerebral venous pressure and impaired CSF absorption as well.2,4
Some medications have been found in association with IIH. These include tetracycline, cyclosporine, lithium, nalidixic acid, nitrofurantoin, oral contraceptives, levonorgestrel, danaxol, and tamoxifen.1-4 Tetracycline seems to have the strongest association with IIH and should be discontinued in those patients where the association is very likely to be the causative factor.2 The link to oral contraceptives may occur simply due to their association with young women most at risk for IIH.1-3
Related:Young Man With Headache, Confusion, and Hearing Loss
Management
The goals of treatment with IIH are to preserve vision and relieve symptoms, particularly headache. The general recommendation is that pregnant women with IIH should be managed and treated the same as any other patient with IIH. However, imaging and some drug contraindications exist between these 2 groups.
The diagnostic test for IIH is a lumbar puncture, which is also the most effective treatment.1-3,5 Lumbar puncture should be performed in the relaxed lateral decubitus position without sedation.1-3 The opening pressure should be measured and is the most clinically significant diagnostic tool for diagnosis of IIH. Opening pressures of > 250 mm H2O are diagnostic of IIH.1-3,5
Weight loss is an essential part of treatment in obese patients with IIH.1-3 A low-calorie, low-salt diet with mild fluid restriction seems to reverse the symptoms of IIH. A 5% to 10% reduction in body weight may reduce symptoms and signs of IIH.2
Carbonic anhydrase inhibitors (CAIs), such as acetalzolamide, have a multifactorial role in IIH.4 They are usually prescribed in 1 to 2 grams over several doses and function by decreasing CSF production.1 Carbonic anhydrase inhibitors also are known to change the taste of foods and may, therefore, aid in weight loss.1,2 Patients prescribed CAIs commonly experience a tingling in their fingers, toes, and perioral region, an indication that the medication is working.1,2 A rare but serious adverse effect (AE) is aplastic anemia, which generally occurs in the first 6 months of treatment in elderly patients.1 The use of CAIs in pregnancy is controversial, and although rare complications are reported, it is considered a class C drug.5
In patients with rapidly progressive vision loss but with minimal headache, optic nerve sheath fenestration (ONSF) is the surgical treatment of choice.2,3,6 In this procedure, a window or series of slits are created behind the globe in the optic nerve sheath.1 About 50% of patients achieve adequate headache control with ONSF, especially for frontal headaches.1,2
For patients with vision loss, papilledema, and headache that do not respond to medical therapy, a CSF diversion procedure is the preferred treatment. Cerebrospinal fluid diversion with ventriculoperitoneal or lumboperitoneal shunts may prevent progressive loss of vision.1,4,6 However, variable response rates and shunt failure requiring subsequent revisions are common and may occur in as many as half of patients undergoing these procedures.1
Increased intracranial venous pressure due to stenosis of the venous sinuses has been thought to be a possible cause of IIH. Stenting of the transverse venous sinus stenosis has been shown to reduce cerebral venous pressure, reduce ICP, and improve symptoms in patients with IIH.1-3 It is unclear whether elevations in ICP cause transverse sinus stenosis or whether transverse sinus stenosis causes increased ICP.2 Regardless, stents have a high rate of complications, including subdural hemorrhage, venous sinus perforation, in-stent thrombosis, and recurrent stenosis proximal to the stent.2
Steroids have been used to treat IIH in the past, although their mechanism of action remains unclear.2 There may be recurrence of papilledema if they are tapered too quickly. Due to their association with long-term AEs, including weight gain, they should be avoided.2
Management in Pregnancy
Several studies agree that vision loss occurs in the same frequency in pregnant and nonpregnant patients with IIH.4,7 Idiopathic intracranial hypertension can occur in any trimester in pregnancy. It has been found that patients have the same spontaneous abortion rate and visual outcomes as the general population.6-8 It has also been concluded that treatment should be the same in both patient populations with slight variability in the use of acetazolamide.4,6,7
The use of dilating drops during pregnancy is controversial. Although there have been no teratogenic effects reported with use of topical anesthetics and dilating drops, all drugs should be avoided during the first trimester.7-10 Guidelines have been established by the American Congress of Obstetricians and Gynecologists for X-ray examination and exposure during pregnancy. It has been determined that exposure from a single diagnostic X-ray procedure does not result in harmful fetal effects.11 Magnetic resonance imaging is not associated with any known adverse fetal effects and is a better imaging option during pregnancy, because it is not associated with the use of ionizing radiation.11
The use of CAIs in the first trimester is controversial.4,7 Some believe it should be avoided because it is a Pregnancy Category C drug. However, a single case of sacrococcygeal teratoma has been reported in humans; therefore, some believe this is not a strong basis for withholding the medication in patients with the potential risk for severe vision loss.4,7 In this case, a consult to the patient’s obstetrician was made, and the use of acetazolamide had no effect on the health of the baby.
In pregnant women with IIH with progressive vision loss, failed treatment, or nonadherence, surgery may be necessary. Optic nerve sheath fenestration is preferred due to lower morbidity and mortality compared with shunting procedures.1,2,4,6 The growing fetus may be affected by the peritoneal end of the shunt.4
Related: 49-Year-Old Woman With a Broken Heart
Conclusions
Vision loss associated with IIH can be severe and permanent if left untreated. The best treatments and often the most effective involve weight loss and lumbar puncture. Acetazolamide has been a proven effective treatment in some patients, but some debate exists over the safety of its use during pregnancy. This patient did not have any AEs from its use; however, it did not prove valuable in her treatment. Studies often disagree on the use of acetazolamide in pregnancy; however, all agree that proper patient counseling on potential AEs and management by an obstetrician are important. With proper management, pregnant women with IIH have had outcomes similar to those of the general population.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
A 27-year-old white woman presented to the clinic with headaches and decreased vision through her reading glasses while performing near tasks. Her medical history was significant for herpes simplex, hyperlipidemia, and migraine headaches with aura. Her migraines began following an earlier motor vehicle accident, and her most recent magnetic resonance imaging (MRI) showed no abnormalities. Her current medications included prophylactic acyclovir for herpes and acetaminophen and caffeine tablets as needed for headache. She reported no other trauma or surgery and no known allergies. The patient’s best-corrected Snellen visual acuities in both eyes were 20/20 (distance) and 20/30 (near).
Preliminary testing, including pupils, extraocular motilities, confrontation fields, and color vision, were all within normal limits. Her slit-lamp examination was unremarkable. A dilated fundus examination revealed crowded, elevated discs without vessel obscuration, hemorrhage, hyperemia, or drusen (Figure 1). The fundus examination was otherwise unremarkable. Optical coherence tomography of the optic nerves showed increased nerve fiber layer thickness in both eyes (Figure 2). Her blood pressure (BP) at this visit was 106/77 mm/Hg.
The diagnosis based on these findings was bilateral optic nerve elevation with long-standing migraine headaches. The plan was for the patient to return to the clinic for repeat visual field testing and B-scan ultrasonography to rule out buried optic nerve head drusen.
Two months later, the patient presented to the clinic 19 weeks pregnant and reported that her headaches had increased in frequency, but she had no diplopia. All preliminary testing, including visual acuities, pupil reaction, color vision, and slit-lamp examination remained normal. Fundus examination showed the patient’s nerves were unchanged in appearance from the initial presentation. Visual fields revealed an enlarged blind spot in the right eye and paracentral defects in the left eye. The B-scan testing was negative for optic nerve drusen. Due to the increased frequency of headaches, pregnancy, and suspicious optic nerves, an urgent consult was placed to neurology.
At the neurology appointment 1 month later, the patient was diagnosed with migraine headache syndrome and idiopathic intracranial hypertension (IIH). The neurologist believed her headaches might have been resulting from analgesic rebound. He suggested that the patient discontinue or decrease use of oral butalbital, acetaminophen and caffeine tablets, and other forms of caffeine. It was decided that divalproxen sodium and verapamil were not feasible due to pregnancy. The neurologist started her on oral acetazolamide 500 mg twice daily.
The patient returned to her obstetrician 1 month later for a routine follow-up; the headaches had worsened and were now accompanied by nausea and vomiting twice daily on average. Her medications still included acetaminophen and caffeine tablets, although it had been recommended she discontinue them, prochlorperazine, and acetazolamide. Due to the worsening of her symptoms and visual fields (eFigure 1), the obstetrician recommended that the patient deliver by cesarean section at 38 to 39 weeks.
(eFigure 1.Visual Fields at Follow-up 1 and 2)
Right eye
Left Eye
Following an uncomplicated cesarean delivery at 38 weeks, the patient returned to the clinic for visual field testing. Humphrey visual fields were full in the right eye and showed some scattered central depressions in the left. Both eyes were significantly improved from previous fields (eFigure 2) . The patient had discontinued acetazolamide and reported minor tension headaches she believed were due to lack of sleep but stated that she was no longer having migraines. There was no papilledema noted on fundus examination, and Snellen distance visual acuity measured 20/20 in both eyes. An MRI had been performed after delivery and was negative for intracranial hemorrhage, mass, or hydrocephalus).
(eFigure 2. Visual Fields Postpartum)
Right eye
Left eye
Three months later, the patient returned for her yearly comprehensive examination. At that visit, she reported a decrease in frequency of the migraine headaches. Optical coherence tomography was performed and showed a significant decrease in optic nerve head swelling.
Related: Diabetes on the Rise Among Other Pregnancy Problems
Clinical Picture
Idiopathic intracranial hypertension presents clinically with signs and symptoms of increased intracranial pressure (ICP). Headache is the most common symptom, usually presenting as daily and pulsatile.1 Nausea may be associated with the headache, although vomiting is rare, and the headache may awaken the patient. The headache may remain after resolution of elevated ICP (Table).2
Papilledema is the most common sign of IIH.1,2 Visual loss associated with papilledema is generally mild at first but progressive. Transient blur lasts usually 30 seconds and may be monocular or binocular.1 The cause is thought to be related to transient ischemia of the optic nerve.1 Vision loss is typically reversible with resolution of optic nerve swelling, but 25% of patients may develop optic atrophy, which results in permanent vision loss.2 Common patterns of visual abnormalities include enlargement of the physiologic blind spot, inferonasal and arcuate defects, and eventually severe peripheral constriction.1,2 It is imperative that all patients with IIH have visual field testing performed.
About one-third of patients with IIH experience diplopia. This binocular, horizontal diplopia is caused by a sixth nerve palsy in 10% to 20% of patients.1 Cranial nerves II, VI, and VII make a 90-degree bend and seem to be prone to damage at the site of the bend.1
Pulse-synchronous tinnitus is common in IIH as well.2,3 This generally occurs unilaterally and may be eliminated by jugular compression or the head turning to the ipsilateral side.1,3 The sound is caused by the transmission of an increase in the vascular pulse due to high pressure on the cerebrospinal fluid (CSF).1,3
Idiopathic intracranial hypertension most typically presents in obese women of childbearing age.1-3 An increasing degree of obesity is generally associated with an increased risk of vision loss.1,2 Men seem to have worse acuity and visual fields at presentation than do women.2 Men are less likely to report headaches than are women and, therefore, have double the likelihood of severe vision loss.2 Hence, closer monitoring and more aggressive intervention is recommended for men due to their lesser tendency for headaches.2 Black patients also demonstrate more aggressive disease and, therefore, require closer monitoring and early aggressive intervention.1,2
Papilledema is the most common sign of IIH and may be caused by several processes. In this case, most were ruled out given the patient’s normal visual acuities, pupillary reaction, color vision testing, BP measurement, and B-scan imaging. The patient’s systemic history was negative for thyroid-related disease, diabetes, hypertension, autoimmune disease, or infection. She had no family history of vision loss or hereditary ocular conditions. The most recent MRI was negative for any long-standing space-occupying lesion or hydrocephalus.
Pathophysiology
Several mechanisms leading to increased ICP have been proposed. These include increased brain water content, excess CSF production, reduced CSF absorption, and increased cerebral venous pressure.2,3 There is also a suspicion of the role of sex hormones in IIH due to its high predilection for females.2
The role of vitamin A metabolism has also been studied in IIH.1 Retinol levels are elevated in the CSF of patients with IIH. Patients may ingest an abnormally large amount of vitamin A, metabolize it abnormally, or be sensitive to its effects.2,4 The function of adipose tissue as an actively secreting endocrine tissue may play a role in IIH due to its release of adipose tissue-derived retinol binding protein.2 Other adipose-produced cytokines include leptin, which has been implicated in IIH due to its elevated levels found in the CSF of patients with IIH.2
Stenosis of the cerebral sinuses is another proposed mechanism of IIH.1-3 Cerebrospinal fluid exits the cranium into the venous sinuses via the arachnoid villi.2 An obstruction in these sinuses may impair CSF outflow and result in intracranial hypertension. Microthrombosis caused by hypercoaguable disorders may result in increased cerebral venous pressure and impaired CSF absorption as well.2,4
Some medications have been found in association with IIH. These include tetracycline, cyclosporine, lithium, nalidixic acid, nitrofurantoin, oral contraceptives, levonorgestrel, danaxol, and tamoxifen.1-4 Tetracycline seems to have the strongest association with IIH and should be discontinued in those patients where the association is very likely to be the causative factor.2 The link to oral contraceptives may occur simply due to their association with young women most at risk for IIH.1-3
Related:Young Man With Headache, Confusion, and Hearing Loss
Management
The goals of treatment with IIH are to preserve vision and relieve symptoms, particularly headache. The general recommendation is that pregnant women with IIH should be managed and treated the same as any other patient with IIH. However, imaging and some drug contraindications exist between these 2 groups.
The diagnostic test for IIH is a lumbar puncture, which is also the most effective treatment.1-3,5 Lumbar puncture should be performed in the relaxed lateral decubitus position without sedation.1-3 The opening pressure should be measured and is the most clinically significant diagnostic tool for diagnosis of IIH. Opening pressures of > 250 mm H2O are diagnostic of IIH.1-3,5
Weight loss is an essential part of treatment in obese patients with IIH.1-3 A low-calorie, low-salt diet with mild fluid restriction seems to reverse the symptoms of IIH. A 5% to 10% reduction in body weight may reduce symptoms and signs of IIH.2
Carbonic anhydrase inhibitors (CAIs), such as acetalzolamide, have a multifactorial role in IIH.4 They are usually prescribed in 1 to 2 grams over several doses and function by decreasing CSF production.1 Carbonic anhydrase inhibitors also are known to change the taste of foods and may, therefore, aid in weight loss.1,2 Patients prescribed CAIs commonly experience a tingling in their fingers, toes, and perioral region, an indication that the medication is working.1,2 A rare but serious adverse effect (AE) is aplastic anemia, which generally occurs in the first 6 months of treatment in elderly patients.1 The use of CAIs in pregnancy is controversial, and although rare complications are reported, it is considered a class C drug.5
In patients with rapidly progressive vision loss but with minimal headache, optic nerve sheath fenestration (ONSF) is the surgical treatment of choice.2,3,6 In this procedure, a window or series of slits are created behind the globe in the optic nerve sheath.1 About 50% of patients achieve adequate headache control with ONSF, especially for frontal headaches.1,2
For patients with vision loss, papilledema, and headache that do not respond to medical therapy, a CSF diversion procedure is the preferred treatment. Cerebrospinal fluid diversion with ventriculoperitoneal or lumboperitoneal shunts may prevent progressive loss of vision.1,4,6 However, variable response rates and shunt failure requiring subsequent revisions are common and may occur in as many as half of patients undergoing these procedures.1
Increased intracranial venous pressure due to stenosis of the venous sinuses has been thought to be a possible cause of IIH. Stenting of the transverse venous sinus stenosis has been shown to reduce cerebral venous pressure, reduce ICP, and improve symptoms in patients with IIH.1-3 It is unclear whether elevations in ICP cause transverse sinus stenosis or whether transverse sinus stenosis causes increased ICP.2 Regardless, stents have a high rate of complications, including subdural hemorrhage, venous sinus perforation, in-stent thrombosis, and recurrent stenosis proximal to the stent.2
Steroids have been used to treat IIH in the past, although their mechanism of action remains unclear.2 There may be recurrence of papilledema if they are tapered too quickly. Due to their association with long-term AEs, including weight gain, they should be avoided.2
Management in Pregnancy
Several studies agree that vision loss occurs in the same frequency in pregnant and nonpregnant patients with IIH.4,7 Idiopathic intracranial hypertension can occur in any trimester in pregnancy. It has been found that patients have the same spontaneous abortion rate and visual outcomes as the general population.6-8 It has also been concluded that treatment should be the same in both patient populations with slight variability in the use of acetazolamide.4,6,7
The use of dilating drops during pregnancy is controversial. Although there have been no teratogenic effects reported with use of topical anesthetics and dilating drops, all drugs should be avoided during the first trimester.7-10 Guidelines have been established by the American Congress of Obstetricians and Gynecologists for X-ray examination and exposure during pregnancy. It has been determined that exposure from a single diagnostic X-ray procedure does not result in harmful fetal effects.11 Magnetic resonance imaging is not associated with any known adverse fetal effects and is a better imaging option during pregnancy, because it is not associated with the use of ionizing radiation.11
The use of CAIs in the first trimester is controversial.4,7 Some believe it should be avoided because it is a Pregnancy Category C drug. However, a single case of sacrococcygeal teratoma has been reported in humans; therefore, some believe this is not a strong basis for withholding the medication in patients with the potential risk for severe vision loss.4,7 In this case, a consult to the patient’s obstetrician was made, and the use of acetazolamide had no effect on the health of the baby.
In pregnant women with IIH with progressive vision loss, failed treatment, or nonadherence, surgery may be necessary. Optic nerve sheath fenestration is preferred due to lower morbidity and mortality compared with shunting procedures.1,2,4,6 The growing fetus may be affected by the peritoneal end of the shunt.4
Related: 49-Year-Old Woman With a Broken Heart
Conclusions
Vision loss associated with IIH can be severe and permanent if left untreated. The best treatments and often the most effective involve weight loss and lumbar puncture. Acetazolamide has been a proven effective treatment in some patients, but some debate exists over the safety of its use during pregnancy. This patient did not have any AEs from its use; however, it did not prove valuable in her treatment. Studies often disagree on the use of acetazolamide in pregnancy; however, all agree that proper patient counseling on potential AEs and management by an obstetrician are important. With proper management, pregnant women with IIH have had outcomes similar to those of the general population.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Wall M. Idiopathic intracranial hypertension. Neurol Clin. 2010;28(3):593-617.
2. Bruce BB, Biousee V, Newman NJ. Update on idiopathic intracranial hypertension. Am J Ophthalmol. 2011;152(2):163-169.
3. Fields JD, Javendani PP, Falardeau J, et al. Dural venous sinus angioplasty and stenting for the treatment of idiopathic intracranial hypertension. J Neurointerv Surg. 2013;5(1):62-68.
4. Evans RW, Lee AG. Idiopathic intracranial hypertension in pregnancy. Headache. 2010;50(9):1513-1515.
5. Friedman DI, Jacobson DM. Diagnostic criteria for idiopathic intracranial hypertension. Neurology. 2002;59(10):1492-1495.
6. Martínez-Varea A, Diago-Almela VJ, Abad-Carrascosa A, Perales-Marín A. Progressive visual loss in a pregnant woman with idiopathic intracranial hypertension. Eur J Obstet Gynecol Reprod Biol. 2012;163(1):117-122.
7. Falardeau J, Lobb B, Golden S, Maxfield SD, Tanne E. The use of acetazolamide during pregnancy in intracranial hypertension patients. J Neuroophthalmol. 2013;33(1):9-12.
8. Dinn RB, Harris A, Marcus PS. Ocular changes in pregnancy. Obstet Gynecol Surg. 2003;58(2):137-144.
9. Shultz KL, Birnbaum AD, Goldstein DA. Ocular disease in pregnancy. Curr Opin Ophthalmol. 2005;16(5):308-314.
10. Chung CY, Kwok AKH, Chung KL. Use of ophthalmic medications during pregnancy. Hong Kong Med J. 2004;10(3):191-195.
11. American Congress of Obstetricians and Gynecologists. Committee Opinion. Guidelines for diagnostic imaging during pregnancy. American Congress of Obstetricians and Gynecologists Website. http://www.acog.org/-/media/Committee-Opinions/Committee-on-Obstetric-Practice/co299.pdf. Published 2004. Accessed October 9, 2015.
1. Wall M. Idiopathic intracranial hypertension. Neurol Clin. 2010;28(3):593-617.
2. Bruce BB, Biousee V, Newman NJ. Update on idiopathic intracranial hypertension. Am J Ophthalmol. 2011;152(2):163-169.
3. Fields JD, Javendani PP, Falardeau J, et al. Dural venous sinus angioplasty and stenting for the treatment of idiopathic intracranial hypertension. J Neurointerv Surg. 2013;5(1):62-68.
4. Evans RW, Lee AG. Idiopathic intracranial hypertension in pregnancy. Headache. 2010;50(9):1513-1515.
5. Friedman DI, Jacobson DM. Diagnostic criteria for idiopathic intracranial hypertension. Neurology. 2002;59(10):1492-1495.
6. Martínez-Varea A, Diago-Almela VJ, Abad-Carrascosa A, Perales-Marín A. Progressive visual loss in a pregnant woman with idiopathic intracranial hypertension. Eur J Obstet Gynecol Reprod Biol. 2012;163(1):117-122.
7. Falardeau J, Lobb B, Golden S, Maxfield SD, Tanne E. The use of acetazolamide during pregnancy in intracranial hypertension patients. J Neuroophthalmol. 2013;33(1):9-12.
8. Dinn RB, Harris A, Marcus PS. Ocular changes in pregnancy. Obstet Gynecol Surg. 2003;58(2):137-144.
9. Shultz KL, Birnbaum AD, Goldstein DA. Ocular disease in pregnancy. Curr Opin Ophthalmol. 2005;16(5):308-314.
10. Chung CY, Kwok AKH, Chung KL. Use of ophthalmic medications during pregnancy. Hong Kong Med J. 2004;10(3):191-195.
11. American Congress of Obstetricians and Gynecologists. Committee Opinion. Guidelines for diagnostic imaging during pregnancy. American Congress of Obstetricians and Gynecologists Website. http://www.acog.org/-/media/Committee-Opinions/Committee-on-Obstetric-Practice/co299.pdf. Published 2004. Accessed October 9, 2015.
Hypothermia Therapy in Traumatic Brain Injury
In patients with an intracranial pressure of more than 20 mm Hg after traumatic brain injury (TBI), therapeutic hypothermia plus standard care to reduce intracranial pressure did not result in outcomes better than those with standard care alone, according to a study of 387 patients with TBI. Researchers found:
• Stage 3 treatments were needed to control intracranial pressure in 54% of controls and 44% of the hypothermia group.
• Adjusted common odds ratio for the Extended Glasgow Outcome Scale (GOS-E) was 1.53, indicating a worse outcome in the hypothermia group vs controls.
• A favorable outcome occurred in 26% of the hypothermia group vs 37% in controls.
Citation: Andrews PJ, Sinclair HL, Rodriguez A, et al. Hypothermia for intracranial hypertension after traumatic brain injury. [Published online ahead of print October 7, 2015]. N Engl J Med. doi: 10.1056/NEJMoa1507581.
In patients with an intracranial pressure of more than 20 mm Hg after traumatic brain injury (TBI), therapeutic hypothermia plus standard care to reduce intracranial pressure did not result in outcomes better than those with standard care alone, according to a study of 387 patients with TBI. Researchers found:
• Stage 3 treatments were needed to control intracranial pressure in 54% of controls and 44% of the hypothermia group.
• Adjusted common odds ratio for the Extended Glasgow Outcome Scale (GOS-E) was 1.53, indicating a worse outcome in the hypothermia group vs controls.
• A favorable outcome occurred in 26% of the hypothermia group vs 37% in controls.
Citation: Andrews PJ, Sinclair HL, Rodriguez A, et al. Hypothermia for intracranial hypertension after traumatic brain injury. [Published online ahead of print October 7, 2015]. N Engl J Med. doi: 10.1056/NEJMoa1507581.
In patients with an intracranial pressure of more than 20 mm Hg after traumatic brain injury (TBI), therapeutic hypothermia plus standard care to reduce intracranial pressure did not result in outcomes better than those with standard care alone, according to a study of 387 patients with TBI. Researchers found:
• Stage 3 treatments were needed to control intracranial pressure in 54% of controls and 44% of the hypothermia group.
• Adjusted common odds ratio for the Extended Glasgow Outcome Scale (GOS-E) was 1.53, indicating a worse outcome in the hypothermia group vs controls.
• A favorable outcome occurred in 26% of the hypothermia group vs 37% in controls.
Citation: Andrews PJ, Sinclair HL, Rodriguez A, et al. Hypothermia for intracranial hypertension after traumatic brain injury. [Published online ahead of print October 7, 2015]. N Engl J Med. doi: 10.1056/NEJMoa1507581.
Helping Veterans Manage Pain
Patients with a history of traumatic brain injury (TBI) may be coping with lingering pain in safe and healthy ways, but there are still many areas where their health care practitioners can help, according to a pilot study of how veterans with TBI are managing their pain.
Researchers from VA Western New York Healthcare System in Buffalo, State University of New York in Buffalo, and the Syracuse VAMC in New York surveyed 24 outpatients with a history of mild-to-moderate TBI. Participants completed a series of self-reports, including the Pain Outcomes Questionnaire and Pain Symptom Survey pain scale.
Related: Veterans' Health and Opioid Safety—Contexts, Risks, and Outreach Implications
Most rated their health as average or better, although more than half reported having pain at least 3 days per week, and more than one-third reported having pain daily. About two-thirds reported headache and/or lower back pain; 54% had joint pain, and 42% had neck pain. The researchers note that the pain was not necessarily attributable to TBI, although factors such as the number and severity of injuries might have played a role.
Related: TBI Biomarker Development on the Horizon
The veterans were mostly using exercise, nonopioid analgesics, and rest to manage their pain. They also used, to a lesser degree, heat therapy, antidepressants, and opioids. Only regular use of antidepressants was associated with greater benefit.
The takeaway for practitioners, the researchers suggest, is multifaceted. First, they need to be prepared to manage pain of various etiologies in the context of other comorbid conditions. Second, the survey indicates that patients may be underusing some effective pain management tools: 10 of 14 possible strategies in the survey were endorsed by fewer than half the veterans.
Related: Complementary and Alternative Medicine for Chronic Musculoskeletal Pain
Third, the researchers suggest that because some of the participants were concerned about exercise leading to reinjury, it might be helpful to provide additional education and support about the value of pacing (ie, moderate exercise and rest vs overexertion followed by extended bed rest). VA mental health providers, they add, could also address common anxieties about reinjury and offer nonpharmacologic interventions. Studies of veterans with pain and posttraumatic stress disorder, for instance, have shown that cognitive behavioral approaches such as progressive muscle relaxation, biofeedback, and diaphragmatic breathing, which were underused in the survey group, are effective and well received.
Source
King PR, Beehler GP, Wade MJ. Mil Med. 2015;180(8):863-868.
doi: 10.7205/MILMED-D-14-00472.
Patients with a history of traumatic brain injury (TBI) may be coping with lingering pain in safe and healthy ways, but there are still many areas where their health care practitioners can help, according to a pilot study of how veterans with TBI are managing their pain.
Researchers from VA Western New York Healthcare System in Buffalo, State University of New York in Buffalo, and the Syracuse VAMC in New York surveyed 24 outpatients with a history of mild-to-moderate TBI. Participants completed a series of self-reports, including the Pain Outcomes Questionnaire and Pain Symptom Survey pain scale.
Related: Veterans' Health and Opioid Safety—Contexts, Risks, and Outreach Implications
Most rated their health as average or better, although more than half reported having pain at least 3 days per week, and more than one-third reported having pain daily. About two-thirds reported headache and/or lower back pain; 54% had joint pain, and 42% had neck pain. The researchers note that the pain was not necessarily attributable to TBI, although factors such as the number and severity of injuries might have played a role.
Related: TBI Biomarker Development on the Horizon
The veterans were mostly using exercise, nonopioid analgesics, and rest to manage their pain. They also used, to a lesser degree, heat therapy, antidepressants, and opioids. Only regular use of antidepressants was associated with greater benefit.
The takeaway for practitioners, the researchers suggest, is multifaceted. First, they need to be prepared to manage pain of various etiologies in the context of other comorbid conditions. Second, the survey indicates that patients may be underusing some effective pain management tools: 10 of 14 possible strategies in the survey were endorsed by fewer than half the veterans.
Related: Complementary and Alternative Medicine for Chronic Musculoskeletal Pain
Third, the researchers suggest that because some of the participants were concerned about exercise leading to reinjury, it might be helpful to provide additional education and support about the value of pacing (ie, moderate exercise and rest vs overexertion followed by extended bed rest). VA mental health providers, they add, could also address common anxieties about reinjury and offer nonpharmacologic interventions. Studies of veterans with pain and posttraumatic stress disorder, for instance, have shown that cognitive behavioral approaches such as progressive muscle relaxation, biofeedback, and diaphragmatic breathing, which were underused in the survey group, are effective and well received.
Source
King PR, Beehler GP, Wade MJ. Mil Med. 2015;180(8):863-868.
doi: 10.7205/MILMED-D-14-00472.
Patients with a history of traumatic brain injury (TBI) may be coping with lingering pain in safe and healthy ways, but there are still many areas where their health care practitioners can help, according to a pilot study of how veterans with TBI are managing their pain.
Researchers from VA Western New York Healthcare System in Buffalo, State University of New York in Buffalo, and the Syracuse VAMC in New York surveyed 24 outpatients with a history of mild-to-moderate TBI. Participants completed a series of self-reports, including the Pain Outcomes Questionnaire and Pain Symptom Survey pain scale.
Related: Veterans' Health and Opioid Safety—Contexts, Risks, and Outreach Implications
Most rated their health as average or better, although more than half reported having pain at least 3 days per week, and more than one-third reported having pain daily. About two-thirds reported headache and/or lower back pain; 54% had joint pain, and 42% had neck pain. The researchers note that the pain was not necessarily attributable to TBI, although factors such as the number and severity of injuries might have played a role.
Related: TBI Biomarker Development on the Horizon
The veterans were mostly using exercise, nonopioid analgesics, and rest to manage their pain. They also used, to a lesser degree, heat therapy, antidepressants, and opioids. Only regular use of antidepressants was associated with greater benefit.
The takeaway for practitioners, the researchers suggest, is multifaceted. First, they need to be prepared to manage pain of various etiologies in the context of other comorbid conditions. Second, the survey indicates that patients may be underusing some effective pain management tools: 10 of 14 possible strategies in the survey were endorsed by fewer than half the veterans.
Related: Complementary and Alternative Medicine for Chronic Musculoskeletal Pain
Third, the researchers suggest that because some of the participants were concerned about exercise leading to reinjury, it might be helpful to provide additional education and support about the value of pacing (ie, moderate exercise and rest vs overexertion followed by extended bed rest). VA mental health providers, they add, could also address common anxieties about reinjury and offer nonpharmacologic interventions. Studies of veterans with pain and posttraumatic stress disorder, for instance, have shown that cognitive behavioral approaches such as progressive muscle relaxation, biofeedback, and diaphragmatic breathing, which were underused in the survey group, are effective and well received.
Source
King PR, Beehler GP, Wade MJ. Mil Med. 2015;180(8):863-868.
doi: 10.7205/MILMED-D-14-00472.
TBI Biomarker Development on the Horizon
A number of advanced efforts in traumatic brain injury (TBI) research are on the verge of reporting new data, according to a roundtable discussion that took place during the Military Health System Research Symposium on August 19. Among the most notable is in the area of biomarker development.
A 2,000-patient pivotal trial recently closed, and analysis should be completed by the end of the year. By March 2016, the research team expects to submit for FDA clearance a first-ever blood test for TBI. In addition, TBI research is currently being conducted in the areas of eye movement and balance.
Related: Brain Training for TBI Patients
A problem with assessing and treating the complexities of TBI up to this point is that although there are hundreds of measures of brain function, the evidence isn’t strong enough to provide a gold standard. “The whole area of drugs in neuroscience has been very difficult,” said Col. Dallas Hack, MD, senior medical advisor to the principal assistant for research and technology. “We have a couple of major efforts that are aimed at solving the problems of achieving results that can be measured according to the standards required to have them approved.”
Related: Stopping TBI-Related Brain Degeneration
To address the lack of a gold standard, the TBI Endpoints Development multiyear effort is making progress to give validity to the many existing measures of brain injury. Another effort is the VA/DoD Chronic Effects of Neurotrauma Consortium (CENC), a federally funded program that identifies gaps in research and provides support services for scientific, clinical, and translational research projects focused on the long-term effects of mild TBI in veterans and active-duty service members.
For information about the CENC, click here.
A number of advanced efforts in traumatic brain injury (TBI) research are on the verge of reporting new data, according to a roundtable discussion that took place during the Military Health System Research Symposium on August 19. Among the most notable is in the area of biomarker development.
A 2,000-patient pivotal trial recently closed, and analysis should be completed by the end of the year. By March 2016, the research team expects to submit for FDA clearance a first-ever blood test for TBI. In addition, TBI research is currently being conducted in the areas of eye movement and balance.
Related: Brain Training for TBI Patients
A problem with assessing and treating the complexities of TBI up to this point is that although there are hundreds of measures of brain function, the evidence isn’t strong enough to provide a gold standard. “The whole area of drugs in neuroscience has been very difficult,” said Col. Dallas Hack, MD, senior medical advisor to the principal assistant for research and technology. “We have a couple of major efforts that are aimed at solving the problems of achieving results that can be measured according to the standards required to have them approved.”
Related: Stopping TBI-Related Brain Degeneration
To address the lack of a gold standard, the TBI Endpoints Development multiyear effort is making progress to give validity to the many existing measures of brain injury. Another effort is the VA/DoD Chronic Effects of Neurotrauma Consortium (CENC), a federally funded program that identifies gaps in research and provides support services for scientific, clinical, and translational research projects focused on the long-term effects of mild TBI in veterans and active-duty service members.
For information about the CENC, click here.
A number of advanced efforts in traumatic brain injury (TBI) research are on the verge of reporting new data, according to a roundtable discussion that took place during the Military Health System Research Symposium on August 19. Among the most notable is in the area of biomarker development.
A 2,000-patient pivotal trial recently closed, and analysis should be completed by the end of the year. By March 2016, the research team expects to submit for FDA clearance a first-ever blood test for TBI. In addition, TBI research is currently being conducted in the areas of eye movement and balance.
Related: Brain Training for TBI Patients
A problem with assessing and treating the complexities of TBI up to this point is that although there are hundreds of measures of brain function, the evidence isn’t strong enough to provide a gold standard. “The whole area of drugs in neuroscience has been very difficult,” said Col. Dallas Hack, MD, senior medical advisor to the principal assistant for research and technology. “We have a couple of major efforts that are aimed at solving the problems of achieving results that can be measured according to the standards required to have them approved.”
Related: Stopping TBI-Related Brain Degeneration
To address the lack of a gold standard, the TBI Endpoints Development multiyear effort is making progress to give validity to the many existing measures of brain injury. Another effort is the VA/DoD Chronic Effects of Neurotrauma Consortium (CENC), a federally funded program that identifies gaps in research and provides support services for scientific, clinical, and translational research projects focused on the long-term effects of mild TBI in veterans and active-duty service members.
For information about the CENC, click here.
Plasma Tau Level Is Chronically Elevated in TBI
Peripheral plasma levels of the CNS protein tau are chronically elevated after traumatic brain injury (TBI) and appear to correlate with the severity of postconcussive symptoms, according to a report published online ahead of print August 3 in JAMA Neurology.
If these findings are confirmed, tau may be the first biomarker that is sensitive and specific to persistent TBI-related symptoms. The results also suggest that “months to years after the primary brain injury, there may be a continuation of secondary injuries with residual axonal degeneration and blood–brain barrier disruptions in this population that may contribute to the maintenance of postconcussive disorder symptoms and affect symptom severity,” said Anlys Olivera, PhD, of the National Institute of Nursing Research in Bethesda, Maryland, and her associates.
Tau stabilizes the structure of the axonal cytoskeleton. It is elevated in the CSF and the peripheral blood (albeit in extremely low concentrations) of patients with severe TBI, professional boxers, and athletes who sustain concussions. The extremely low levels of tau in the peripheral blood have been difficult to measure until the recent development of an ultrahigh-sensitivity immunoassay technology. Using this innovation, the researchers were able to examine for the first time the associations between plasma tau levels and the frequency and severity of deployment-related TBIs.
Over a two-year period, Dr. Olivera and her associates assessed tau levels in 70 members of the military who self-reported one or more TBIs and 28 military control subjects without TBI who were matched for age, sex, race, time since deployment, and number of deployments. Almost all participants in the TBI group had been injured at least 18 months previously. The most common causes of TBI were blows to the head, exposure to blasts, vehicular crashes, and sports-related concussions.
Total tau was significantly increased in the TBI group (mean level, 1.13 pg/mL), compared with the control group (0.63 pg/mL). Total tau also increased with increasing severity of the initial brain injury, with increasing number of TBIs, and with increasing severity of present-day postconcussive symptoms. These associations, moreover, were independent of symptoms of post-traumatic stress disorder and depression, which were prevalent in the TBI group, the investigators said.
Tau is not only a marker of brain injury, it also can contribute to secondary injury processes such as inflammation, which makes it a potential target for therapy. If the findings of this study are confirmed and extended to demonstrate a direct mechanistic relationship between TBI and tau aggregation, treatments such as the direct delivery of proteasomes “would be invaluable, considering the dearth of treatments for TBIs and chronic [postconcussive disorder] symptoms,” Dr. Olivera and her associates said.
Among the study limitations cited by the investigators are lack of neuroimaging and neuropsychologic data.
—Mary Ann Moon
Suggested Reading
Olivera A, Lejbman N, Jeromin A, et al. Peripheral total tau in military personnel who sustain traumatic brain injuries during deployment. JAMA Neurol. 2015 Aug 3 [Epub ahead of print].
Peripheral plasma levels of the CNS protein tau are chronically elevated after traumatic brain injury (TBI) and appear to correlate with the severity of postconcussive symptoms, according to a report published online ahead of print August 3 in JAMA Neurology.
If these findings are confirmed, tau may be the first biomarker that is sensitive and specific to persistent TBI-related symptoms. The results also suggest that “months to years after the primary brain injury, there may be a continuation of secondary injuries with residual axonal degeneration and blood–brain barrier disruptions in this population that may contribute to the maintenance of postconcussive disorder symptoms and affect symptom severity,” said Anlys Olivera, PhD, of the National Institute of Nursing Research in Bethesda, Maryland, and her associates.
Tau stabilizes the structure of the axonal cytoskeleton. It is elevated in the CSF and the peripheral blood (albeit in extremely low concentrations) of patients with severe TBI, professional boxers, and athletes who sustain concussions. The extremely low levels of tau in the peripheral blood have been difficult to measure until the recent development of an ultrahigh-sensitivity immunoassay technology. Using this innovation, the researchers were able to examine for the first time the associations between plasma tau levels and the frequency and severity of deployment-related TBIs.
Over a two-year period, Dr. Olivera and her associates assessed tau levels in 70 members of the military who self-reported one or more TBIs and 28 military control subjects without TBI who were matched for age, sex, race, time since deployment, and number of deployments. Almost all participants in the TBI group had been injured at least 18 months previously. The most common causes of TBI were blows to the head, exposure to blasts, vehicular crashes, and sports-related concussions.
Total tau was significantly increased in the TBI group (mean level, 1.13 pg/mL), compared with the control group (0.63 pg/mL). Total tau also increased with increasing severity of the initial brain injury, with increasing number of TBIs, and with increasing severity of present-day postconcussive symptoms. These associations, moreover, were independent of symptoms of post-traumatic stress disorder and depression, which were prevalent in the TBI group, the investigators said.
Tau is not only a marker of brain injury, it also can contribute to secondary injury processes such as inflammation, which makes it a potential target for therapy. If the findings of this study are confirmed and extended to demonstrate a direct mechanistic relationship between TBI and tau aggregation, treatments such as the direct delivery of proteasomes “would be invaluable, considering the dearth of treatments for TBIs and chronic [postconcussive disorder] symptoms,” Dr. Olivera and her associates said.
Among the study limitations cited by the investigators are lack of neuroimaging and neuropsychologic data.
—Mary Ann Moon
Peripheral plasma levels of the CNS protein tau are chronically elevated after traumatic brain injury (TBI) and appear to correlate with the severity of postconcussive symptoms, according to a report published online ahead of print August 3 in JAMA Neurology.
If these findings are confirmed, tau may be the first biomarker that is sensitive and specific to persistent TBI-related symptoms. The results also suggest that “months to years after the primary brain injury, there may be a continuation of secondary injuries with residual axonal degeneration and blood–brain barrier disruptions in this population that may contribute to the maintenance of postconcussive disorder symptoms and affect symptom severity,” said Anlys Olivera, PhD, of the National Institute of Nursing Research in Bethesda, Maryland, and her associates.
Tau stabilizes the structure of the axonal cytoskeleton. It is elevated in the CSF and the peripheral blood (albeit in extremely low concentrations) of patients with severe TBI, professional boxers, and athletes who sustain concussions. The extremely low levels of tau in the peripheral blood have been difficult to measure until the recent development of an ultrahigh-sensitivity immunoassay technology. Using this innovation, the researchers were able to examine for the first time the associations between plasma tau levels and the frequency and severity of deployment-related TBIs.
Over a two-year period, Dr. Olivera and her associates assessed tau levels in 70 members of the military who self-reported one or more TBIs and 28 military control subjects without TBI who were matched for age, sex, race, time since deployment, and number of deployments. Almost all participants in the TBI group had been injured at least 18 months previously. The most common causes of TBI were blows to the head, exposure to blasts, vehicular crashes, and sports-related concussions.
Total tau was significantly increased in the TBI group (mean level, 1.13 pg/mL), compared with the control group (0.63 pg/mL). Total tau also increased with increasing severity of the initial brain injury, with increasing number of TBIs, and with increasing severity of present-day postconcussive symptoms. These associations, moreover, were independent of symptoms of post-traumatic stress disorder and depression, which were prevalent in the TBI group, the investigators said.
Tau is not only a marker of brain injury, it also can contribute to secondary injury processes such as inflammation, which makes it a potential target for therapy. If the findings of this study are confirmed and extended to demonstrate a direct mechanistic relationship between TBI and tau aggregation, treatments such as the direct delivery of proteasomes “would be invaluable, considering the dearth of treatments for TBIs and chronic [postconcussive disorder] symptoms,” Dr. Olivera and her associates said.
Among the study limitations cited by the investigators are lack of neuroimaging and neuropsychologic data.
—Mary Ann Moon
Suggested Reading
Olivera A, Lejbman N, Jeromin A, et al. Peripheral total tau in military personnel who sustain traumatic brain injuries during deployment. JAMA Neurol. 2015 Aug 3 [Epub ahead of print].
Suggested Reading
Olivera A, Lejbman N, Jeromin A, et al. Peripheral total tau in military personnel who sustain traumatic brain injuries during deployment. JAMA Neurol. 2015 Aug 3 [Epub ahead of print].
The VA/DoD Chronic Effects of Neurotrauma Consortium: An Overview at Year 1
The Chronic Effects of Neuro-trauma Consortium (CENC) is a federally funded research project devised to address the long-term effects of mild traumatic brain injury (mTBI) in military service members (SMs) and veterans. Announced by President Barack Obama on August 20, 2013, the CENC is one of 2 major initiatives developed in response to injuries incurred by U.S. service personnel during Operation Enduring Freedom (OEF) and Operation Iraqi Freedom (OIF) as part of the National Research Action Plan. The CENC is jointly funded by the DoD and the VA, with a budget of $62.175 million over 5 years.
The consortium funds basic science, clinical, and translational research efforts with a closely integrated supportive infrastructure, including administrative services, regulatory guidance, study design, biostatistical consultation, data management, common data element application, and interdisciplinary communication. In addition, the consortium facilitates and integrates the activities of a diverse group of skilled specialty research teams, allowing them to fully focus their efforts on understanding and clarifying the relationship between combat-related mTBI and chronic neurotrauma effects, including neurodegeneration.
Background
Nearly 20% of the more than 2.6 million U.S. SMs deployed since 2003 to OEF and OIF have sustained at least 1 TBI, predominantly mTBI. Almost 8% of all OEF/OIF veterans demonstrate persistent post-TBI symptoms more than 6 months postinjury. Acute mTBI effects are typically transient, with headache, cognitive, behavioral, balance, and sleep symptoms most often seen, but symptoms may persist and even lead to lifelong disability. In these individuals, additional chronic effects, such as neuroendocrinologic abnormalities, seizures and seizurelike disorders, fatigue, vision and hearing abnormalities, and numerous other somatic symptoms are more common over time. The long-term effects from single or repeated mTBIs on the persistence of these symptoms, on combat and trauma-related comorbidities, and on long-term brain functioning are unknown.
Increasing evidence supports the link between both concussions and combat-related trauma with chronic traumatic encephalopathy (CTE), which results in progressive cognitive and behavioral decline in subpopulations 5 to 50 years out from repeated or cumulative mTBI exposures. The possibility of a link between mTBI, persistent symptoms, and early dementia has widespread implications for SMs and veterans; however, these chronic and late-life effects of mTBI are poorly understood.
Traumatic brain injuries of mixed severity have been linked to a higher incidence of Alzheimer disease (AD) and other dementias and an earlier onset of AD, although negative findings have also been reported. Chronic traumatic encephalopathy has been reported to occur in retired boxers at higher rates and at younger ages compared with dementia in the general population. More recently, brain autopsies of athletes from a variety of sports with confirmed CTE have demonstrated elevated tau proteins, tau-immunoreactive neurofibrillary tangles, and neuropil threads, suggesting that pathologic processes similar to those occurring in AD may be involved. Longitudinal research bridging SMs, veterans, and athletes with neurotrauma has been fragmented and incompletely focused on the strategic needs (eg, troop readiness) and vision of the DoD and VA.
Critical gaps exist in the literature with few prospective, well-controlled, longitudinal studies on late-life outcomes and neurodegeneration after mTBI, as well as in related basic science research. These research gaps are particularly prominent in the potentially unique injuries and difficulties seen in combat-exposed populations. The existing research, although suggestive, is not rigorous or robust enough to allow for a clear understanding of the relationships, risks, and potential effective interventions for mTBI, chronic symptoms, and neurodegeneration.
The CENC was developed to create a road map of existing knowledge gaps, to recruit the top relevant subject matter experts in the country, to develop and establish a cohesive set of rigorously designed studies to address these knowledge voids, and to leverage core consortium resources both efficiently and effectively.
Related: The Right Care at the Right Time and in the Right Place: The Role of Technology in the VHA
Given these gaps in scientific research and knowledge, the DoD and VA jointly issued a request for proposals to fund a project to address these concerns. After a competitive application process, an integrated proposal, led by researchers at Virginia Commonwealth University (VCU) was announced as the recipient of the Presidential award.
Consortium Structure
The CENC, serving as the comprehensive research network for DoD and VA, focuses on (1) identifying and characterizing the anatomic, molecular, and physiologic mechanisms of chronic injury from mTBI and potential neurodegeneration; (2) investigating the relationship of comorbidities (psychological, neurologic, sensory, motor, pain, cognitive, and neuroendocrine) of trauma and combat exposure to TBI with neurodegeneration; and (3) assessing the efficacy of existing and novel treatment and rehabilitation strategies for chronic effects and neurodegeneration following TBI.
The consortium is a collaboration among more than 30 universities, nonprofit research organizations, VAMCs, and military medical centers made up of a leadership core, 5 research infrastructure cores, 8 active studies, a data safety monitoring committee, a consumer advisory board, a scientific advisory board, and an independent granting mechanism to foster additional research in chronic effects after mTBI.
Leadership Core
The principal investigator for CENC is David X. Cifu, MD, chairman and professor of the VCU Department of Physical Medicine and Rehabilitation in Richmond, Virginia. The consortium co-principal investigators are Ramon Diaz-Arrastia, MD, PhD, professor of neurology, Uniformed Services University of the Health Sciences (USUHS) and director of the clinical research at the Center for Neuroscience and Regenerative Medicine in Bethesda, Maryland, and Rick L. Williams, PhD, co-principal investigator for CENC and senior statistician at RTI International in Raleigh, North Carolina.
Research Cores
The CENC operates 5 research infrastructure cores. The Biorepository Core, led by Dr. Diaz-Arrastia at USUHS, manages the storage and processing of biologic (blood and saliva) samples collected through all CENC protocols. The Biostatistics Core, led by Dr. Williams; Nancy Temkin, PhD; and Heather Belanger, PhD at RTI, provides study design guidance and biostatistical analysis to facilitate knowledge translation and dissemination.
The Data and Study Management Core is led by Dr. Williams at RTI. It centrally and securely maintains all collected data; oversees the clinical monitoring of research sites; provides a consortium research manager for each study who interacts with the study leadership, study site leaders, and staff; expedites and guides clinical protocols through regulatory approval processes; coordinates patient accrual and study activities across sites; develops and monitors data acquisition compliance; and facilitates exportation of all data collection to the Federal Interagency Traumatic Brain Injury Research informatics system.
The Neuroimaging Core is led by Elisabeth Wilde, PhD, at Baylor College of Medicine and the Michael E. DeBakey VAMC in Houston, Texas. This core facilitates sequence development and pulse programming; provides training and supervision of technologists and support personnel; ensures acquisition, transfer, and storage of imaging data; oversees quality assurance; performs conventional and advanced imaging analysis; and interprets neuroimaging data.
The Neuropathology Core is led by Dr. Dan Perl and colocated at USUHS and Edith Norse Rogers Memorial Veterans Hospital/VA Boston Healthcare System. Dr. Perl manages the collection of brain specimens from the participants, using an existing national network of dieners and neuropathologists, catalogs and stores tissues, and administers requests for use of these tissues.
Active Research Studies
The Longitudinal Cohort Study addresses a critical research gap by identifying and characterizing the late effects of mTBI and assessing the influence and interaction of the many potential risk factors for early dementia. The study uses a wide array of self-report, laboratory, biophysical, neuropsychologic, and imaging assessment tools to evaluate a cohort (n = 880) of U.S. OEF/OIF combatants who have had at least 1 mTBI and a control group of participants (n = 220) who have experienced combat but have not had a mTBI, and then re-assesses them annually (in person or via telephone), with the goal of following the cohort for as long as resources are available.
Collaborating sites for this study include Hunter Holmes McGuire VAMC in Richmond, Virginia; James A. Haley Veterans’ Hospital in Tampa, Florida; Michael E. DeBakey VAMC in Houston, Texas; Audie L. Murphy Memorial Veterans Hospital in San Antonio, Texas; VA Boston Healthcare System; Minneapolis VA Health Care System in Minnesota; and Fort Belvoir in Virginia. Dr. Cifu and Dr. William Walker lead this study.
Epidemiology of mTBI and Neurosensory Outcomes
This project integrates and analyzes several VA, DoD, and Centers for Medicare and Medicaid Services health care system data sets to study the chronic effects of mTBI on neurodegenerative disease and other comorbidities. The primary aims of the project include evaluating the association between mTBI and short-term clinical outcomes, including factors associated with resilience and effects of treatment; investigating long-term clinical outcomes, including neurosensory disorders and mortality; and identifying factors associated with low- and high-distress trajectories of comorbid burden after mTBI. Dr. Kristine Yaffe, Dr. Mary Jo Pugh, and Dr. Michael McCrea, are the leads of this study.
Tau Modification and Aggregation in TBI
This study aims to develop an animal model of repetitive-mTBI, which will allow the tracking of progressive intraneuronal tau alterations that can be correlated with behavioral dysfunction, neuronal protein, and gene expression signatures that can be used to assess the effects of interventions. The observations made in the animal model will be compared with findings generated from tissue obtained at autopsy from deceased SMs and veterans who sustained repetitive-mTBI. Dr. Fiona Crawford and Dr. Elliott Mufson lead this study.
Otolith Dysfunction
This study is examining the effect of inner ear dysfunction on balance, gait, and quality of life (QOL). Recent evidence suggests that otolith organ dysfunction can occur in patients with mTBI or blast exposure. If the dizziness and imbalance symptoms that occur following head injury or blast exposure are related to injury to the otolith organs rather than to the horizontal semicircular canal, then new treatment approaches may be necessary to focus on otolith organ pathway recovery. Performance on balance tasks while standing and walking and questionnaires on the impact on QOL will be compared in 4 groups of individuals (n = 120) with and without head injury/blast exposure (otolith organ dysfunction, horizontal canal dysfunction, both otolith and horizontal canal dysfunction, and healthy individuals). Dr. Faith Akin leads this study.
ADAPT
The ADAPT study (Assessment and Long-term Outcome and Disability in Active Duty Military Prospectively Examined following Concussive TBI) is investigating the association of early clinical and imaging measures with late (5 year) clinical outcome after blast-related mTBI from combat. The study (n = 100) will use 5-year follow-up advanced magnetic resonance imaging (MRI) and clinical outcome measures of combat mTBI, as a continuation of previous longitudinal research efforts (n = 575). Two groups of subjects will be studied: subjects who sustained a mTBI from blast during deployment and subjects without history of blast exposure and no diagnosis of deployment mTBI. Dr. Christine MacDonald leads this study.
Diffusion Tensor Imaging Phantom Study
This study involves the development and testing of a novel phantom that would be used to enhance accuracy, consistency, and reliability in both isotropic and anisotropic measurements derived from diffusion imaging, as well as other MRI-based measurements, using universal fluid disk chambers in a single phantom. Currently, the acquisition of diffusion data in large studies and clinical trials lacks standardization, and important differences exist in how data are acquired on scanners of different manufacturers, using different hardware or software, or when different acquisition parameters are used. As a result, development of large pools of data and the creation of normative data are hampered by inhomogeneity in the data set, which is difficult to analyze. The study team will perform detailed testing of the phantom materials and phantoms themselves, as well as examine diffusion imaging on 1 to 2 human volunteers at each of the 4 sites. Intra- and interscanner differences will be measured, and based on these findings, a more standardized imaging protocol that will provide optimal uniformity of diffusion imaging will be designed. Dr. Elisabeth Wilde leads this study.
Novel White Matter Imaging to Improve mTBI Diagnosis
This study will use myelin-sensitive novel imaging techniques (McDespot [multi-component driven equilibrium single pulse observation of T1/T2]) to improve correspondence with diagnostic groups after trauma exposure and correlation with cognitive deficits in mTBI. The study will recruit individuals (n = 82) from 4 groups, comorbid mTBI and posttraumatic stress disorder (PTSD), only mTBI, only PTSD, and controls who will be prospectively comprehensively assessed clinically (clinical interview, physical exam, neuropsychological assessment) and with advanced imaging (including McDespot, diffusion tensor imaging, and other forms of imaging). Dr. Amy Jak leads this study.
Peer Review Program
The CENC has an integrated grant program to identify scientifically valid and strategically important research projects. To date, 2 rounds of proposal requests and project support have been completed. Scientific review is conducted under the CENC Peer Review Program. Scientifically meritorious studies are identified by independent peer review and then undergo a Programmatic Review by CENC leadership before being recommended for funding to the Government Steering Committee (GSC). Studies that are recommended must address road map gaps, develop innovative approaches, or provide an avenue for new researchers and novel research approaches to contribute to the consortium mission to advance the science of brain injury treatment and prevention. The CENC grant program is administered by Dr. Steven L. West.
Consumer Advisory Board
The Consumer Advisory Board (CAB) advises and makes nonbinding recommendations to CENC. The responsibilities of the committee members include (1) providing information that helps CENC leadership better appreciate and understand the issues and needs of TBI survivors and their support networks so appropriate research can be designed and implemented; (2) evaluating existing research and making recommendations for additions and/or modifications to project procedures; (3) providing input for the road map for future research based on members’ personal experiences and knowledge; and (4) providing linkages to targeted communities for direct feedback and to assist in forming collaborative partnerships.
The CAB is composed of survivors of TBI, family members of survivors of TBI, providers of TBI services, service organizations with specific ties to SMs and veterans, and clinical and corporate representatives of transportation services for the disabled, the independent living movement, and assistive technology. Persons who are heavily engaged in political activity or who actively endorse a specific device or product are not eligible for membership on the CAB. Membership is composed of persons nominated by CENC leadership and approved by the GSC. The CAB is co-chaired by Charles Gatlin, MS, and General (Ret.) Peter Chiarelli.
Scientific Advisory Board
The members of the Scientific Advisory Board (SAB) advise and make nonbinding recommendations to CENC. Responsibilities of the committee members include (1) providing information that may help the consortium leadership better understand the issues related to TBI; (2) evaluating existing research; (3) recommending additions and/or modifications to project procedures; and (4) assisting CENC by helping leverage relationships with other researchers. The SAB is composed of members of the research community on TBI who are not part of CENC. Persons who may be considered to have positions of authority, such as active or retired flag officers or chief executive officers, may be eligible for general SAB membership but are not be eligible for chair positions. Membership is composed of persons nominated by CENC leadership and approved by the GSC. Col. Jamie Grimes, MD, and Henry Lew, MD, PhD, co-chair the SAB.
Federal Oversight
The GSC oversees CENC. Members of the GSC are DoD and VA appointed and represent both government agencies and nongovernment subject matter experts. The GSC approves all studies to be conducted, recommends new studies, and identifies existing and new requirements. The GSC is the overall main governing and management committee for the project and the committee through which the DoD and VA interact and collaborate with the CENC. The GSC determines all major scientific decisions, and clinical studies proposed by the CENC committee proceed to the implementation stage only with the approval of the GSC.
Acknowledgements
This research is supported by grants 1-I01-RX-001135-01-A2 (PI: F. Aiken), 1-I01-RX-001774-01 (PI: F. Crawford), 1-I01-RX-001880-01 (PI: E. Wilde), 1-I01-CX-001135-01 (PI: S. Cifu), and 1-I01-CX-001246-01 (PI: K. Yaffe) from the U.S. Department of Veterans Affairs and by grant W81XWH-13-2-0095 (PI: D. Cifu) from the U.S. Department of Defense, Congressionally Directed Medical Research Programs. The ideas and opinions expressed in this paper do not necessarily represent the views of the Department of Veterans Affairs, the Department of Defense, or the U.S. Government.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
The Chronic Effects of Neuro-trauma Consortium (CENC) is a federally funded research project devised to address the long-term effects of mild traumatic brain injury (mTBI) in military service members (SMs) and veterans. Announced by President Barack Obama on August 20, 2013, the CENC is one of 2 major initiatives developed in response to injuries incurred by U.S. service personnel during Operation Enduring Freedom (OEF) and Operation Iraqi Freedom (OIF) as part of the National Research Action Plan. The CENC is jointly funded by the DoD and the VA, with a budget of $62.175 million over 5 years.
The consortium funds basic science, clinical, and translational research efforts with a closely integrated supportive infrastructure, including administrative services, regulatory guidance, study design, biostatistical consultation, data management, common data element application, and interdisciplinary communication. In addition, the consortium facilitates and integrates the activities of a diverse group of skilled specialty research teams, allowing them to fully focus their efforts on understanding and clarifying the relationship between combat-related mTBI and chronic neurotrauma effects, including neurodegeneration.
Background
Nearly 20% of the more than 2.6 million U.S. SMs deployed since 2003 to OEF and OIF have sustained at least 1 TBI, predominantly mTBI. Almost 8% of all OEF/OIF veterans demonstrate persistent post-TBI symptoms more than 6 months postinjury. Acute mTBI effects are typically transient, with headache, cognitive, behavioral, balance, and sleep symptoms most often seen, but symptoms may persist and even lead to lifelong disability. In these individuals, additional chronic effects, such as neuroendocrinologic abnormalities, seizures and seizurelike disorders, fatigue, vision and hearing abnormalities, and numerous other somatic symptoms are more common over time. The long-term effects from single or repeated mTBIs on the persistence of these symptoms, on combat and trauma-related comorbidities, and on long-term brain functioning are unknown.
Increasing evidence supports the link between both concussions and combat-related trauma with chronic traumatic encephalopathy (CTE), which results in progressive cognitive and behavioral decline in subpopulations 5 to 50 years out from repeated or cumulative mTBI exposures. The possibility of a link between mTBI, persistent symptoms, and early dementia has widespread implications for SMs and veterans; however, these chronic and late-life effects of mTBI are poorly understood.
Traumatic brain injuries of mixed severity have been linked to a higher incidence of Alzheimer disease (AD) and other dementias and an earlier onset of AD, although negative findings have also been reported. Chronic traumatic encephalopathy has been reported to occur in retired boxers at higher rates and at younger ages compared with dementia in the general population. More recently, brain autopsies of athletes from a variety of sports with confirmed CTE have demonstrated elevated tau proteins, tau-immunoreactive neurofibrillary tangles, and neuropil threads, suggesting that pathologic processes similar to those occurring in AD may be involved. Longitudinal research bridging SMs, veterans, and athletes with neurotrauma has been fragmented and incompletely focused on the strategic needs (eg, troop readiness) and vision of the DoD and VA.
Critical gaps exist in the literature with few prospective, well-controlled, longitudinal studies on late-life outcomes and neurodegeneration after mTBI, as well as in related basic science research. These research gaps are particularly prominent in the potentially unique injuries and difficulties seen in combat-exposed populations. The existing research, although suggestive, is not rigorous or robust enough to allow for a clear understanding of the relationships, risks, and potential effective interventions for mTBI, chronic symptoms, and neurodegeneration.
The CENC was developed to create a road map of existing knowledge gaps, to recruit the top relevant subject matter experts in the country, to develop and establish a cohesive set of rigorously designed studies to address these knowledge voids, and to leverage core consortium resources both efficiently and effectively.
Related: The Right Care at the Right Time and in the Right Place: The Role of Technology in the VHA
Given these gaps in scientific research and knowledge, the DoD and VA jointly issued a request for proposals to fund a project to address these concerns. After a competitive application process, an integrated proposal, led by researchers at Virginia Commonwealth University (VCU) was announced as the recipient of the Presidential award.
Consortium Structure
The CENC, serving as the comprehensive research network for DoD and VA, focuses on (1) identifying and characterizing the anatomic, molecular, and physiologic mechanisms of chronic injury from mTBI and potential neurodegeneration; (2) investigating the relationship of comorbidities (psychological, neurologic, sensory, motor, pain, cognitive, and neuroendocrine) of trauma and combat exposure to TBI with neurodegeneration; and (3) assessing the efficacy of existing and novel treatment and rehabilitation strategies for chronic effects and neurodegeneration following TBI.
The consortium is a collaboration among more than 30 universities, nonprofit research organizations, VAMCs, and military medical centers made up of a leadership core, 5 research infrastructure cores, 8 active studies, a data safety monitoring committee, a consumer advisory board, a scientific advisory board, and an independent granting mechanism to foster additional research in chronic effects after mTBI.
Leadership Core
The principal investigator for CENC is David X. Cifu, MD, chairman and professor of the VCU Department of Physical Medicine and Rehabilitation in Richmond, Virginia. The consortium co-principal investigators are Ramon Diaz-Arrastia, MD, PhD, professor of neurology, Uniformed Services University of the Health Sciences (USUHS) and director of the clinical research at the Center for Neuroscience and Regenerative Medicine in Bethesda, Maryland, and Rick L. Williams, PhD, co-principal investigator for CENC and senior statistician at RTI International in Raleigh, North Carolina.
Research Cores
The CENC operates 5 research infrastructure cores. The Biorepository Core, led by Dr. Diaz-Arrastia at USUHS, manages the storage and processing of biologic (blood and saliva) samples collected through all CENC protocols. The Biostatistics Core, led by Dr. Williams; Nancy Temkin, PhD; and Heather Belanger, PhD at RTI, provides study design guidance and biostatistical analysis to facilitate knowledge translation and dissemination.
The Data and Study Management Core is led by Dr. Williams at RTI. It centrally and securely maintains all collected data; oversees the clinical monitoring of research sites; provides a consortium research manager for each study who interacts with the study leadership, study site leaders, and staff; expedites and guides clinical protocols through regulatory approval processes; coordinates patient accrual and study activities across sites; develops and monitors data acquisition compliance; and facilitates exportation of all data collection to the Federal Interagency Traumatic Brain Injury Research informatics system.
The Neuroimaging Core is led by Elisabeth Wilde, PhD, at Baylor College of Medicine and the Michael E. DeBakey VAMC in Houston, Texas. This core facilitates sequence development and pulse programming; provides training and supervision of technologists and support personnel; ensures acquisition, transfer, and storage of imaging data; oversees quality assurance; performs conventional and advanced imaging analysis; and interprets neuroimaging data.
The Neuropathology Core is led by Dr. Dan Perl and colocated at USUHS and Edith Norse Rogers Memorial Veterans Hospital/VA Boston Healthcare System. Dr. Perl manages the collection of brain specimens from the participants, using an existing national network of dieners and neuropathologists, catalogs and stores tissues, and administers requests for use of these tissues.
Active Research Studies
The Longitudinal Cohort Study addresses a critical research gap by identifying and characterizing the late effects of mTBI and assessing the influence and interaction of the many potential risk factors for early dementia. The study uses a wide array of self-report, laboratory, biophysical, neuropsychologic, and imaging assessment tools to evaluate a cohort (n = 880) of U.S. OEF/OIF combatants who have had at least 1 mTBI and a control group of participants (n = 220) who have experienced combat but have not had a mTBI, and then re-assesses them annually (in person or via telephone), with the goal of following the cohort for as long as resources are available.
Collaborating sites for this study include Hunter Holmes McGuire VAMC in Richmond, Virginia; James A. Haley Veterans’ Hospital in Tampa, Florida; Michael E. DeBakey VAMC in Houston, Texas; Audie L. Murphy Memorial Veterans Hospital in San Antonio, Texas; VA Boston Healthcare System; Minneapolis VA Health Care System in Minnesota; and Fort Belvoir in Virginia. Dr. Cifu and Dr. William Walker lead this study.
Epidemiology of mTBI and Neurosensory Outcomes
This project integrates and analyzes several VA, DoD, and Centers for Medicare and Medicaid Services health care system data sets to study the chronic effects of mTBI on neurodegenerative disease and other comorbidities. The primary aims of the project include evaluating the association between mTBI and short-term clinical outcomes, including factors associated with resilience and effects of treatment; investigating long-term clinical outcomes, including neurosensory disorders and mortality; and identifying factors associated with low- and high-distress trajectories of comorbid burden after mTBI. Dr. Kristine Yaffe, Dr. Mary Jo Pugh, and Dr. Michael McCrea, are the leads of this study.
Tau Modification and Aggregation in TBI
This study aims to develop an animal model of repetitive-mTBI, which will allow the tracking of progressive intraneuronal tau alterations that can be correlated with behavioral dysfunction, neuronal protein, and gene expression signatures that can be used to assess the effects of interventions. The observations made in the animal model will be compared with findings generated from tissue obtained at autopsy from deceased SMs and veterans who sustained repetitive-mTBI. Dr. Fiona Crawford and Dr. Elliott Mufson lead this study.
Otolith Dysfunction
This study is examining the effect of inner ear dysfunction on balance, gait, and quality of life (QOL). Recent evidence suggests that otolith organ dysfunction can occur in patients with mTBI or blast exposure. If the dizziness and imbalance symptoms that occur following head injury or blast exposure are related to injury to the otolith organs rather than to the horizontal semicircular canal, then new treatment approaches may be necessary to focus on otolith organ pathway recovery. Performance on balance tasks while standing and walking and questionnaires on the impact on QOL will be compared in 4 groups of individuals (n = 120) with and without head injury/blast exposure (otolith organ dysfunction, horizontal canal dysfunction, both otolith and horizontal canal dysfunction, and healthy individuals). Dr. Faith Akin leads this study.
ADAPT
The ADAPT study (Assessment and Long-term Outcome and Disability in Active Duty Military Prospectively Examined following Concussive TBI) is investigating the association of early clinical and imaging measures with late (5 year) clinical outcome after blast-related mTBI from combat. The study (n = 100) will use 5-year follow-up advanced magnetic resonance imaging (MRI) and clinical outcome measures of combat mTBI, as a continuation of previous longitudinal research efforts (n = 575). Two groups of subjects will be studied: subjects who sustained a mTBI from blast during deployment and subjects without history of blast exposure and no diagnosis of deployment mTBI. Dr. Christine MacDonald leads this study.
Diffusion Tensor Imaging Phantom Study
This study involves the development and testing of a novel phantom that would be used to enhance accuracy, consistency, and reliability in both isotropic and anisotropic measurements derived from diffusion imaging, as well as other MRI-based measurements, using universal fluid disk chambers in a single phantom. Currently, the acquisition of diffusion data in large studies and clinical trials lacks standardization, and important differences exist in how data are acquired on scanners of different manufacturers, using different hardware or software, or when different acquisition parameters are used. As a result, development of large pools of data and the creation of normative data are hampered by inhomogeneity in the data set, which is difficult to analyze. The study team will perform detailed testing of the phantom materials and phantoms themselves, as well as examine diffusion imaging on 1 to 2 human volunteers at each of the 4 sites. Intra- and interscanner differences will be measured, and based on these findings, a more standardized imaging protocol that will provide optimal uniformity of diffusion imaging will be designed. Dr. Elisabeth Wilde leads this study.
Novel White Matter Imaging to Improve mTBI Diagnosis
This study will use myelin-sensitive novel imaging techniques (McDespot [multi-component driven equilibrium single pulse observation of T1/T2]) to improve correspondence with diagnostic groups after trauma exposure and correlation with cognitive deficits in mTBI. The study will recruit individuals (n = 82) from 4 groups, comorbid mTBI and posttraumatic stress disorder (PTSD), only mTBI, only PTSD, and controls who will be prospectively comprehensively assessed clinically (clinical interview, physical exam, neuropsychological assessment) and with advanced imaging (including McDespot, diffusion tensor imaging, and other forms of imaging). Dr. Amy Jak leads this study.
Peer Review Program
The CENC has an integrated grant program to identify scientifically valid and strategically important research projects. To date, 2 rounds of proposal requests and project support have been completed. Scientific review is conducted under the CENC Peer Review Program. Scientifically meritorious studies are identified by independent peer review and then undergo a Programmatic Review by CENC leadership before being recommended for funding to the Government Steering Committee (GSC). Studies that are recommended must address road map gaps, develop innovative approaches, or provide an avenue for new researchers and novel research approaches to contribute to the consortium mission to advance the science of brain injury treatment and prevention. The CENC grant program is administered by Dr. Steven L. West.
Consumer Advisory Board
The Consumer Advisory Board (CAB) advises and makes nonbinding recommendations to CENC. The responsibilities of the committee members include (1) providing information that helps CENC leadership better appreciate and understand the issues and needs of TBI survivors and their support networks so appropriate research can be designed and implemented; (2) evaluating existing research and making recommendations for additions and/or modifications to project procedures; (3) providing input for the road map for future research based on members’ personal experiences and knowledge; and (4) providing linkages to targeted communities for direct feedback and to assist in forming collaborative partnerships.
The CAB is composed of survivors of TBI, family members of survivors of TBI, providers of TBI services, service organizations with specific ties to SMs and veterans, and clinical and corporate representatives of transportation services for the disabled, the independent living movement, and assistive technology. Persons who are heavily engaged in political activity or who actively endorse a specific device or product are not eligible for membership on the CAB. Membership is composed of persons nominated by CENC leadership and approved by the GSC. The CAB is co-chaired by Charles Gatlin, MS, and General (Ret.) Peter Chiarelli.
Scientific Advisory Board
The members of the Scientific Advisory Board (SAB) advise and make nonbinding recommendations to CENC. Responsibilities of the committee members include (1) providing information that may help the consortium leadership better understand the issues related to TBI; (2) evaluating existing research; (3) recommending additions and/or modifications to project procedures; and (4) assisting CENC by helping leverage relationships with other researchers. The SAB is composed of members of the research community on TBI who are not part of CENC. Persons who may be considered to have positions of authority, such as active or retired flag officers or chief executive officers, may be eligible for general SAB membership but are not be eligible for chair positions. Membership is composed of persons nominated by CENC leadership and approved by the GSC. Col. Jamie Grimes, MD, and Henry Lew, MD, PhD, co-chair the SAB.
Federal Oversight
The GSC oversees CENC. Members of the GSC are DoD and VA appointed and represent both government agencies and nongovernment subject matter experts. The GSC approves all studies to be conducted, recommends new studies, and identifies existing and new requirements. The GSC is the overall main governing and management committee for the project and the committee through which the DoD and VA interact and collaborate with the CENC. The GSC determines all major scientific decisions, and clinical studies proposed by the CENC committee proceed to the implementation stage only with the approval of the GSC.
Acknowledgements
This research is supported by grants 1-I01-RX-001135-01-A2 (PI: F. Aiken), 1-I01-RX-001774-01 (PI: F. Crawford), 1-I01-RX-001880-01 (PI: E. Wilde), 1-I01-CX-001135-01 (PI: S. Cifu), and 1-I01-CX-001246-01 (PI: K. Yaffe) from the U.S. Department of Veterans Affairs and by grant W81XWH-13-2-0095 (PI: D. Cifu) from the U.S. Department of Defense, Congressionally Directed Medical Research Programs. The ideas and opinions expressed in this paper do not necessarily represent the views of the Department of Veterans Affairs, the Department of Defense, or the U.S. Government.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
The Chronic Effects of Neuro-trauma Consortium (CENC) is a federally funded research project devised to address the long-term effects of mild traumatic brain injury (mTBI) in military service members (SMs) and veterans. Announced by President Barack Obama on August 20, 2013, the CENC is one of 2 major initiatives developed in response to injuries incurred by U.S. service personnel during Operation Enduring Freedom (OEF) and Operation Iraqi Freedom (OIF) as part of the National Research Action Plan. The CENC is jointly funded by the DoD and the VA, with a budget of $62.175 million over 5 years.
The consortium funds basic science, clinical, and translational research efforts with a closely integrated supportive infrastructure, including administrative services, regulatory guidance, study design, biostatistical consultation, data management, common data element application, and interdisciplinary communication. In addition, the consortium facilitates and integrates the activities of a diverse group of skilled specialty research teams, allowing them to fully focus their efforts on understanding and clarifying the relationship between combat-related mTBI and chronic neurotrauma effects, including neurodegeneration.
Background
Nearly 20% of the more than 2.6 million U.S. SMs deployed since 2003 to OEF and OIF have sustained at least 1 TBI, predominantly mTBI. Almost 8% of all OEF/OIF veterans demonstrate persistent post-TBI symptoms more than 6 months postinjury. Acute mTBI effects are typically transient, with headache, cognitive, behavioral, balance, and sleep symptoms most often seen, but symptoms may persist and even lead to lifelong disability. In these individuals, additional chronic effects, such as neuroendocrinologic abnormalities, seizures and seizurelike disorders, fatigue, vision and hearing abnormalities, and numerous other somatic symptoms are more common over time. The long-term effects from single or repeated mTBIs on the persistence of these symptoms, on combat and trauma-related comorbidities, and on long-term brain functioning are unknown.
Increasing evidence supports the link between both concussions and combat-related trauma with chronic traumatic encephalopathy (CTE), which results in progressive cognitive and behavioral decline in subpopulations 5 to 50 years out from repeated or cumulative mTBI exposures. The possibility of a link between mTBI, persistent symptoms, and early dementia has widespread implications for SMs and veterans; however, these chronic and late-life effects of mTBI are poorly understood.
Traumatic brain injuries of mixed severity have been linked to a higher incidence of Alzheimer disease (AD) and other dementias and an earlier onset of AD, although negative findings have also been reported. Chronic traumatic encephalopathy has been reported to occur in retired boxers at higher rates and at younger ages compared with dementia in the general population. More recently, brain autopsies of athletes from a variety of sports with confirmed CTE have demonstrated elevated tau proteins, tau-immunoreactive neurofibrillary tangles, and neuropil threads, suggesting that pathologic processes similar to those occurring in AD may be involved. Longitudinal research bridging SMs, veterans, and athletes with neurotrauma has been fragmented and incompletely focused on the strategic needs (eg, troop readiness) and vision of the DoD and VA.
Critical gaps exist in the literature with few prospective, well-controlled, longitudinal studies on late-life outcomes and neurodegeneration after mTBI, as well as in related basic science research. These research gaps are particularly prominent in the potentially unique injuries and difficulties seen in combat-exposed populations. The existing research, although suggestive, is not rigorous or robust enough to allow for a clear understanding of the relationships, risks, and potential effective interventions for mTBI, chronic symptoms, and neurodegeneration.
The CENC was developed to create a road map of existing knowledge gaps, to recruit the top relevant subject matter experts in the country, to develop and establish a cohesive set of rigorously designed studies to address these knowledge voids, and to leverage core consortium resources both efficiently and effectively.
Related: The Right Care at the Right Time and in the Right Place: The Role of Technology in the VHA
Given these gaps in scientific research and knowledge, the DoD and VA jointly issued a request for proposals to fund a project to address these concerns. After a competitive application process, an integrated proposal, led by researchers at Virginia Commonwealth University (VCU) was announced as the recipient of the Presidential award.
Consortium Structure
The CENC, serving as the comprehensive research network for DoD and VA, focuses on (1) identifying and characterizing the anatomic, molecular, and physiologic mechanisms of chronic injury from mTBI and potential neurodegeneration; (2) investigating the relationship of comorbidities (psychological, neurologic, sensory, motor, pain, cognitive, and neuroendocrine) of trauma and combat exposure to TBI with neurodegeneration; and (3) assessing the efficacy of existing and novel treatment and rehabilitation strategies for chronic effects and neurodegeneration following TBI.
The consortium is a collaboration among more than 30 universities, nonprofit research organizations, VAMCs, and military medical centers made up of a leadership core, 5 research infrastructure cores, 8 active studies, a data safety monitoring committee, a consumer advisory board, a scientific advisory board, and an independent granting mechanism to foster additional research in chronic effects after mTBI.
Leadership Core
The principal investigator for CENC is David X. Cifu, MD, chairman and professor of the VCU Department of Physical Medicine and Rehabilitation in Richmond, Virginia. The consortium co-principal investigators are Ramon Diaz-Arrastia, MD, PhD, professor of neurology, Uniformed Services University of the Health Sciences (USUHS) and director of the clinical research at the Center for Neuroscience and Regenerative Medicine in Bethesda, Maryland, and Rick L. Williams, PhD, co-principal investigator for CENC and senior statistician at RTI International in Raleigh, North Carolina.
Research Cores
The CENC operates 5 research infrastructure cores. The Biorepository Core, led by Dr. Diaz-Arrastia at USUHS, manages the storage and processing of biologic (blood and saliva) samples collected through all CENC protocols. The Biostatistics Core, led by Dr. Williams; Nancy Temkin, PhD; and Heather Belanger, PhD at RTI, provides study design guidance and biostatistical analysis to facilitate knowledge translation and dissemination.
The Data and Study Management Core is led by Dr. Williams at RTI. It centrally and securely maintains all collected data; oversees the clinical monitoring of research sites; provides a consortium research manager for each study who interacts with the study leadership, study site leaders, and staff; expedites and guides clinical protocols through regulatory approval processes; coordinates patient accrual and study activities across sites; develops and monitors data acquisition compliance; and facilitates exportation of all data collection to the Federal Interagency Traumatic Brain Injury Research informatics system.
The Neuroimaging Core is led by Elisabeth Wilde, PhD, at Baylor College of Medicine and the Michael E. DeBakey VAMC in Houston, Texas. This core facilitates sequence development and pulse programming; provides training and supervision of technologists and support personnel; ensures acquisition, transfer, and storage of imaging data; oversees quality assurance; performs conventional and advanced imaging analysis; and interprets neuroimaging data.
The Neuropathology Core is led by Dr. Dan Perl and colocated at USUHS and Edith Norse Rogers Memorial Veterans Hospital/VA Boston Healthcare System. Dr. Perl manages the collection of brain specimens from the participants, using an existing national network of dieners and neuropathologists, catalogs and stores tissues, and administers requests for use of these tissues.
Active Research Studies
The Longitudinal Cohort Study addresses a critical research gap by identifying and characterizing the late effects of mTBI and assessing the influence and interaction of the many potential risk factors for early dementia. The study uses a wide array of self-report, laboratory, biophysical, neuropsychologic, and imaging assessment tools to evaluate a cohort (n = 880) of U.S. OEF/OIF combatants who have had at least 1 mTBI and a control group of participants (n = 220) who have experienced combat but have not had a mTBI, and then re-assesses them annually (in person or via telephone), with the goal of following the cohort for as long as resources are available.
Collaborating sites for this study include Hunter Holmes McGuire VAMC in Richmond, Virginia; James A. Haley Veterans’ Hospital in Tampa, Florida; Michael E. DeBakey VAMC in Houston, Texas; Audie L. Murphy Memorial Veterans Hospital in San Antonio, Texas; VA Boston Healthcare System; Minneapolis VA Health Care System in Minnesota; and Fort Belvoir in Virginia. Dr. Cifu and Dr. William Walker lead this study.
Epidemiology of mTBI and Neurosensory Outcomes
This project integrates and analyzes several VA, DoD, and Centers for Medicare and Medicaid Services health care system data sets to study the chronic effects of mTBI on neurodegenerative disease and other comorbidities. The primary aims of the project include evaluating the association between mTBI and short-term clinical outcomes, including factors associated with resilience and effects of treatment; investigating long-term clinical outcomes, including neurosensory disorders and mortality; and identifying factors associated with low- and high-distress trajectories of comorbid burden after mTBI. Dr. Kristine Yaffe, Dr. Mary Jo Pugh, and Dr. Michael McCrea, are the leads of this study.
Tau Modification and Aggregation in TBI
This study aims to develop an animal model of repetitive-mTBI, which will allow the tracking of progressive intraneuronal tau alterations that can be correlated with behavioral dysfunction, neuronal protein, and gene expression signatures that can be used to assess the effects of interventions. The observations made in the animal model will be compared with findings generated from tissue obtained at autopsy from deceased SMs and veterans who sustained repetitive-mTBI. Dr. Fiona Crawford and Dr. Elliott Mufson lead this study.
Otolith Dysfunction
This study is examining the effect of inner ear dysfunction on balance, gait, and quality of life (QOL). Recent evidence suggests that otolith organ dysfunction can occur in patients with mTBI or blast exposure. If the dizziness and imbalance symptoms that occur following head injury or blast exposure are related to injury to the otolith organs rather than to the horizontal semicircular canal, then new treatment approaches may be necessary to focus on otolith organ pathway recovery. Performance on balance tasks while standing and walking and questionnaires on the impact on QOL will be compared in 4 groups of individuals (n = 120) with and without head injury/blast exposure (otolith organ dysfunction, horizontal canal dysfunction, both otolith and horizontal canal dysfunction, and healthy individuals). Dr. Faith Akin leads this study.
ADAPT
The ADAPT study (Assessment and Long-term Outcome and Disability in Active Duty Military Prospectively Examined following Concussive TBI) is investigating the association of early clinical and imaging measures with late (5 year) clinical outcome after blast-related mTBI from combat. The study (n = 100) will use 5-year follow-up advanced magnetic resonance imaging (MRI) and clinical outcome measures of combat mTBI, as a continuation of previous longitudinal research efforts (n = 575). Two groups of subjects will be studied: subjects who sustained a mTBI from blast during deployment and subjects without history of blast exposure and no diagnosis of deployment mTBI. Dr. Christine MacDonald leads this study.
Diffusion Tensor Imaging Phantom Study
This study involves the development and testing of a novel phantom that would be used to enhance accuracy, consistency, and reliability in both isotropic and anisotropic measurements derived from diffusion imaging, as well as other MRI-based measurements, using universal fluid disk chambers in a single phantom. Currently, the acquisition of diffusion data in large studies and clinical trials lacks standardization, and important differences exist in how data are acquired on scanners of different manufacturers, using different hardware or software, or when different acquisition parameters are used. As a result, development of large pools of data and the creation of normative data are hampered by inhomogeneity in the data set, which is difficult to analyze. The study team will perform detailed testing of the phantom materials and phantoms themselves, as well as examine diffusion imaging on 1 to 2 human volunteers at each of the 4 sites. Intra- and interscanner differences will be measured, and based on these findings, a more standardized imaging protocol that will provide optimal uniformity of diffusion imaging will be designed. Dr. Elisabeth Wilde leads this study.
Novel White Matter Imaging to Improve mTBI Diagnosis
This study will use myelin-sensitive novel imaging techniques (McDespot [multi-component driven equilibrium single pulse observation of T1/T2]) to improve correspondence with diagnostic groups after trauma exposure and correlation with cognitive deficits in mTBI. The study will recruit individuals (n = 82) from 4 groups, comorbid mTBI and posttraumatic stress disorder (PTSD), only mTBI, only PTSD, and controls who will be prospectively comprehensively assessed clinically (clinical interview, physical exam, neuropsychological assessment) and with advanced imaging (including McDespot, diffusion tensor imaging, and other forms of imaging). Dr. Amy Jak leads this study.
Peer Review Program
The CENC has an integrated grant program to identify scientifically valid and strategically important research projects. To date, 2 rounds of proposal requests and project support have been completed. Scientific review is conducted under the CENC Peer Review Program. Scientifically meritorious studies are identified by independent peer review and then undergo a Programmatic Review by CENC leadership before being recommended for funding to the Government Steering Committee (GSC). Studies that are recommended must address road map gaps, develop innovative approaches, or provide an avenue for new researchers and novel research approaches to contribute to the consortium mission to advance the science of brain injury treatment and prevention. The CENC grant program is administered by Dr. Steven L. West.
Consumer Advisory Board
The Consumer Advisory Board (CAB) advises and makes nonbinding recommendations to CENC. The responsibilities of the committee members include (1) providing information that helps CENC leadership better appreciate and understand the issues and needs of TBI survivors and their support networks so appropriate research can be designed and implemented; (2) evaluating existing research and making recommendations for additions and/or modifications to project procedures; (3) providing input for the road map for future research based on members’ personal experiences and knowledge; and (4) providing linkages to targeted communities for direct feedback and to assist in forming collaborative partnerships.
The CAB is composed of survivors of TBI, family members of survivors of TBI, providers of TBI services, service organizations with specific ties to SMs and veterans, and clinical and corporate representatives of transportation services for the disabled, the independent living movement, and assistive technology. Persons who are heavily engaged in political activity or who actively endorse a specific device or product are not eligible for membership on the CAB. Membership is composed of persons nominated by CENC leadership and approved by the GSC. The CAB is co-chaired by Charles Gatlin, MS, and General (Ret.) Peter Chiarelli.
Scientific Advisory Board
The members of the Scientific Advisory Board (SAB) advise and make nonbinding recommendations to CENC. Responsibilities of the committee members include (1) providing information that may help the consortium leadership better understand the issues related to TBI; (2) evaluating existing research; (3) recommending additions and/or modifications to project procedures; and (4) assisting CENC by helping leverage relationships with other researchers. The SAB is composed of members of the research community on TBI who are not part of CENC. Persons who may be considered to have positions of authority, such as active or retired flag officers or chief executive officers, may be eligible for general SAB membership but are not be eligible for chair positions. Membership is composed of persons nominated by CENC leadership and approved by the GSC. Col. Jamie Grimes, MD, and Henry Lew, MD, PhD, co-chair the SAB.
Federal Oversight
The GSC oversees CENC. Members of the GSC are DoD and VA appointed and represent both government agencies and nongovernment subject matter experts. The GSC approves all studies to be conducted, recommends new studies, and identifies existing and new requirements. The GSC is the overall main governing and management committee for the project and the committee through which the DoD and VA interact and collaborate with the CENC. The GSC determines all major scientific decisions, and clinical studies proposed by the CENC committee proceed to the implementation stage only with the approval of the GSC.
Acknowledgements
This research is supported by grants 1-I01-RX-001135-01-A2 (PI: F. Aiken), 1-I01-RX-001774-01 (PI: F. Crawford), 1-I01-RX-001880-01 (PI: E. Wilde), 1-I01-CX-001135-01 (PI: S. Cifu), and 1-I01-CX-001246-01 (PI: K. Yaffe) from the U.S. Department of Veterans Affairs and by grant W81XWH-13-2-0095 (PI: D. Cifu) from the U.S. Department of Defense, Congressionally Directed Medical Research Programs. The ideas and opinions expressed in this paper do not necessarily represent the views of the Department of Veterans Affairs, the Department of Defense, or the U.S. Government.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
Player-to-Player Contact Is the Main Source of High School Soccer Concussions
Head contact with other players, not with the ball, is the main source of concussions among high school soccer players, according to research published online ahead of print July 13 in JAMA Pediatrics.
Several studies have shown that heading the ball is responsible for many soccer-related concussions. Some people have called for banning heading, especially among children and adolescents, to make the sport safer. No large study, however, had examined the exact mechanism of head injuries among school-aged soccer players, so such prevention efforts could not be considered evidence-based, said R. Dawn Comstock, PhD, an epidemiologist at the University of Colorado Denver in Aurora.
Dr. Comstock and colleagues performed a retrospective analysis of data from a large, Internet-based sports injury surveillance study, focusing on concussions sustained during soccer practices or games that required medical attention and restricted the athlete’s participation for one or more days. The investigators assessed nationally representative samples of 100 high schools every year for nine years. There were 627 concussions during 1,393,753 athlete exposures among girls (4.50 per 10,000 exposures) and 442 concussions during 1,592,238 athlete exposures among boys (2.78 per 10,000 exposures).
The most common mechanism of concussion was player-to-player contact among boys (68.8%) and girls (51.3%). Contact with the ball accounted for 17% of concussions among boys and 29% among girls.
The number and types of concussion symptoms were the same, regardless of whether the concussion was caused by player-to-player contact or player-to-ball contact. However, symptom resolution time was slightly but significantly longer for both boys and girls when the concussion was caused by collision with a ball or goal post.
“We postulate that banning heading from soccer will have limited effectiveness as a primary prevention mechanism unless such a ban is combined with concurrent efforts to reduce athlete–athlete contact throughout the game,” Dr. Comstock and her associates said.
“It may be culturally more tolerable to the soccer community to attempt to reduce athlete–athlete contact across all phases of play through better enforcement of existing rules, enhanced education of athletes on the rules of the game, and improved coaching of activities such as heading,” rather than simply banning heading, said the researchers.
—Mary Ann Moon
Head contact with other players, not with the ball, is the main source of concussions among high school soccer players, according to research published online ahead of print July 13 in JAMA Pediatrics.
Several studies have shown that heading the ball is responsible for many soccer-related concussions. Some people have called for banning heading, especially among children and adolescents, to make the sport safer. No large study, however, had examined the exact mechanism of head injuries among school-aged soccer players, so such prevention efforts could not be considered evidence-based, said R. Dawn Comstock, PhD, an epidemiologist at the University of Colorado Denver in Aurora.
Dr. Comstock and colleagues performed a retrospective analysis of data from a large, Internet-based sports injury surveillance study, focusing on concussions sustained during soccer practices or games that required medical attention and restricted the athlete’s participation for one or more days. The investigators assessed nationally representative samples of 100 high schools every year for nine years. There were 627 concussions during 1,393,753 athlete exposures among girls (4.50 per 10,000 exposures) and 442 concussions during 1,592,238 athlete exposures among boys (2.78 per 10,000 exposures).
The most common mechanism of concussion was player-to-player contact among boys (68.8%) and girls (51.3%). Contact with the ball accounted for 17% of concussions among boys and 29% among girls.
The number and types of concussion symptoms were the same, regardless of whether the concussion was caused by player-to-player contact or player-to-ball contact. However, symptom resolution time was slightly but significantly longer for both boys and girls when the concussion was caused by collision with a ball or goal post.
“We postulate that banning heading from soccer will have limited effectiveness as a primary prevention mechanism unless such a ban is combined with concurrent efforts to reduce athlete–athlete contact throughout the game,” Dr. Comstock and her associates said.
“It may be culturally more tolerable to the soccer community to attempt to reduce athlete–athlete contact across all phases of play through better enforcement of existing rules, enhanced education of athletes on the rules of the game, and improved coaching of activities such as heading,” rather than simply banning heading, said the researchers.
—Mary Ann Moon
Head contact with other players, not with the ball, is the main source of concussions among high school soccer players, according to research published online ahead of print July 13 in JAMA Pediatrics.
Several studies have shown that heading the ball is responsible for many soccer-related concussions. Some people have called for banning heading, especially among children and adolescents, to make the sport safer. No large study, however, had examined the exact mechanism of head injuries among school-aged soccer players, so such prevention efforts could not be considered evidence-based, said R. Dawn Comstock, PhD, an epidemiologist at the University of Colorado Denver in Aurora.
Dr. Comstock and colleagues performed a retrospective analysis of data from a large, Internet-based sports injury surveillance study, focusing on concussions sustained during soccer practices or games that required medical attention and restricted the athlete’s participation for one or more days. The investigators assessed nationally representative samples of 100 high schools every year for nine years. There were 627 concussions during 1,393,753 athlete exposures among girls (4.50 per 10,000 exposures) and 442 concussions during 1,592,238 athlete exposures among boys (2.78 per 10,000 exposures).
The most common mechanism of concussion was player-to-player contact among boys (68.8%) and girls (51.3%). Contact with the ball accounted for 17% of concussions among boys and 29% among girls.
The number and types of concussion symptoms were the same, regardless of whether the concussion was caused by player-to-player contact or player-to-ball contact. However, symptom resolution time was slightly but significantly longer for both boys and girls when the concussion was caused by collision with a ball or goal post.
“We postulate that banning heading from soccer will have limited effectiveness as a primary prevention mechanism unless such a ban is combined with concurrent efforts to reduce athlete–athlete contact throughout the game,” Dr. Comstock and her associates said.
“It may be culturally more tolerable to the soccer community to attempt to reduce athlete–athlete contact across all phases of play through better enforcement of existing rules, enhanced education of athletes on the rules of the game, and improved coaching of activities such as heading,” rather than simply banning heading, said the researchers.
—Mary Ann Moon