User login
Emergency Protocol Improves Survival After Severe Head Injury
Preventing low oxygen, low blood pressure, and hyperventilation in people with head injury has been shown to improve survival, according to observational studies. The guidelines for prehospital management of traumatic brain injury (TBI), developed in 2000, were updated in 2007 to reflect those findings. But are they being followed? And if followed, do they help?
The Excellence in Prehospital Injury Care (EPIC) study, the first time the guidelines were assessed in real-world conditions, trained EMS responders in Arizona and compared patient outcomes before and after the guideline implementation.
The study researchers found “a therapeutic sweet spot” in that the guidelines had an “enormous impact” on people with severe TBI. Implementing the guidelines did not affect overall survival of the entire group, which included > 21,000 patients with moderate, severe, and critical injuries. But further analysis showed that they helped double the survival rate of people with severe TBI and tripled the survival rate in severe TBI patients who had to have a breathing tube inserted by EMS personnel.
Daniel Spaite, MD, who led the study, said the patients with moderate injuries would most likely have survived anyway, and those in critical condition may have had injuries too serious to overcome.
The guidelines also were associated with an overall increase in survival to hospital admission.
According to Bentley Bobrow, MD, co-principal investigator, “It was exciting to see such dramatic outcomes resulting from a simple 2-hour training session with EMS personnel.”
The study “demonstrates the significance of conducting studies in real-world settings and brings a strong evidence base to the guidelines,” said Patrick Bellgowan, PhD, program director at the National Institute of Neurological Disorders and Stroke, which supported the study. “It suggests we can systematically increase the chances of saving lives of thousands of people who suffer severe traumatic brain injuries.”
Preventing low oxygen, low blood pressure, and hyperventilation in people with head injury has been shown to improve survival, according to observational studies. The guidelines for prehospital management of traumatic brain injury (TBI), developed in 2000, were updated in 2007 to reflect those findings. But are they being followed? And if followed, do they help?
The Excellence in Prehospital Injury Care (EPIC) study, the first time the guidelines were assessed in real-world conditions, trained EMS responders in Arizona and compared patient outcomes before and after the guideline implementation.
The study researchers found “a therapeutic sweet spot” in that the guidelines had an “enormous impact” on people with severe TBI. Implementing the guidelines did not affect overall survival of the entire group, which included > 21,000 patients with moderate, severe, and critical injuries. But further analysis showed that they helped double the survival rate of people with severe TBI and tripled the survival rate in severe TBI patients who had to have a breathing tube inserted by EMS personnel.
Daniel Spaite, MD, who led the study, said the patients with moderate injuries would most likely have survived anyway, and those in critical condition may have had injuries too serious to overcome.
The guidelines also were associated with an overall increase in survival to hospital admission.
According to Bentley Bobrow, MD, co-principal investigator, “It was exciting to see such dramatic outcomes resulting from a simple 2-hour training session with EMS personnel.”
The study “demonstrates the significance of conducting studies in real-world settings and brings a strong evidence base to the guidelines,” said Patrick Bellgowan, PhD, program director at the National Institute of Neurological Disorders and Stroke, which supported the study. “It suggests we can systematically increase the chances of saving lives of thousands of people who suffer severe traumatic brain injuries.”
Preventing low oxygen, low blood pressure, and hyperventilation in people with head injury has been shown to improve survival, according to observational studies. The guidelines for prehospital management of traumatic brain injury (TBI), developed in 2000, were updated in 2007 to reflect those findings. But are they being followed? And if followed, do they help?
The Excellence in Prehospital Injury Care (EPIC) study, the first time the guidelines were assessed in real-world conditions, trained EMS responders in Arizona and compared patient outcomes before and after the guideline implementation.
The study researchers found “a therapeutic sweet spot” in that the guidelines had an “enormous impact” on people with severe TBI. Implementing the guidelines did not affect overall survival of the entire group, which included > 21,000 patients with moderate, severe, and critical injuries. But further analysis showed that they helped double the survival rate of people with severe TBI and tripled the survival rate in severe TBI patients who had to have a breathing tube inserted by EMS personnel.
Daniel Spaite, MD, who led the study, said the patients with moderate injuries would most likely have survived anyway, and those in critical condition may have had injuries too serious to overcome.
The guidelines also were associated with an overall increase in survival to hospital admission.
According to Bentley Bobrow, MD, co-principal investigator, “It was exciting to see such dramatic outcomes resulting from a simple 2-hour training session with EMS personnel.”
The study “demonstrates the significance of conducting studies in real-world settings and brings a strong evidence base to the guidelines,” said Patrick Bellgowan, PhD, program director at the National Institute of Neurological Disorders and Stroke, which supported the study. “It suggests we can systematically increase the chances of saving lives of thousands of people who suffer severe traumatic brain injuries.”
Biomarkers support impact of concussions on cognitive function
Former athletes with a history of concussion averaged higher levels of total tau in their cerebrospinal fluid than did healthy controls, and those with the highest levels showed signs of reduced cognitive function in a case-control study.
Chronic traumatic encephalopathy (CTE) remains a postmortem diagnosis, but “the potential for treating postconcussion degeneration such as CTE depends on being able to detect the in vivo pathology at an early stage to intervene before the disease progresses to an irreversible stage,” wrote Foad Taghdiri, MD, of the University of Toronto and colleagues.
In a study published in Neurology, the researchers measured concentrations of phosphorylated tau181, total tau (t-tau), and beta-amyloid in the cerebrospinal fluid (CSF) of three groups: 22 former professional athletes who had suffered multiple concussions, 5 healthy controls, and 12 individuals diagnosed with Alzheimer’s disease (AD). The average ages of the groups were 56 years, 57 years, and 60 years, respectively. All the athletes were male, and their sports included snowboarding, hockey, and football.
The average t-tau level in the CSF of the athletes was significantly higher than that of controls (349.3 pg/mL vs. 188.8 pg/mL) and significantly lower than that of AD patients (857.0 pg/mL).
Normal CSF t-tau was defined as 300 pg/mL, and 12 former athletes (45%) had high t-tau levels, with an average of 499.3 pg/mL. In this group of high t-tau former athletes, the average score on the Trail Making Test (TMT) Part B was significantly lower than the average score among the 10 former athletes with normal CSF t-tau levels (t scores 45.6 vs. 62.3; P = .017).
In addition, results from MRI scans showed that fractional anisotropy values across all the tracts were significantly lower for those with high CSF t-tau levels, compared with those who had normal CSF t-tau levels (P = .036).
The findings were limited by several factors, including the small sample size, lack of female athletes, and limited ability to compare white matter integrity between high and normal CSF t-tau groups, the researchers noted.
However, the results suggest that “multiple concussive or subconcussive events may trigger neurodegeneration to a greater degree than expected on the basis of age alone,” they said. Although the study did not allow for diagnosing the participants with CTE, “we are engaged in longitudinal studies to track neurologic and neuropsychological function, CSF biomarkers, and structural brain changes over time to further assess the delayed effects of multiple concussions on the brain,” the researchers wrote.
The study was funded by the Toronto General and Western Hospital Foundation, PSI Foundation, and the Canadian Institute of Health Research. The researchers had no financial conflicts to disclose.
SOURCE: Taghdiri F et al. Neurology. 2019 May 8. doi: 10.1212/WNL.0000000000007608
Former athletes with a history of concussion averaged higher levels of total tau in their cerebrospinal fluid than did healthy controls, and those with the highest levels showed signs of reduced cognitive function in a case-control study.
Chronic traumatic encephalopathy (CTE) remains a postmortem diagnosis, but “the potential for treating postconcussion degeneration such as CTE depends on being able to detect the in vivo pathology at an early stage to intervene before the disease progresses to an irreversible stage,” wrote Foad Taghdiri, MD, of the University of Toronto and colleagues.
In a study published in Neurology, the researchers measured concentrations of phosphorylated tau181, total tau (t-tau), and beta-amyloid in the cerebrospinal fluid (CSF) of three groups: 22 former professional athletes who had suffered multiple concussions, 5 healthy controls, and 12 individuals diagnosed with Alzheimer’s disease (AD). The average ages of the groups were 56 years, 57 years, and 60 years, respectively. All the athletes were male, and their sports included snowboarding, hockey, and football.
The average t-tau level in the CSF of the athletes was significantly higher than that of controls (349.3 pg/mL vs. 188.8 pg/mL) and significantly lower than that of AD patients (857.0 pg/mL).
Normal CSF t-tau was defined as 300 pg/mL, and 12 former athletes (45%) had high t-tau levels, with an average of 499.3 pg/mL. In this group of high t-tau former athletes, the average score on the Trail Making Test (TMT) Part B was significantly lower than the average score among the 10 former athletes with normal CSF t-tau levels (t scores 45.6 vs. 62.3; P = .017).
In addition, results from MRI scans showed that fractional anisotropy values across all the tracts were significantly lower for those with high CSF t-tau levels, compared with those who had normal CSF t-tau levels (P = .036).
The findings were limited by several factors, including the small sample size, lack of female athletes, and limited ability to compare white matter integrity between high and normal CSF t-tau groups, the researchers noted.
However, the results suggest that “multiple concussive or subconcussive events may trigger neurodegeneration to a greater degree than expected on the basis of age alone,” they said. Although the study did not allow for diagnosing the participants with CTE, “we are engaged in longitudinal studies to track neurologic and neuropsychological function, CSF biomarkers, and structural brain changes over time to further assess the delayed effects of multiple concussions on the brain,” the researchers wrote.
The study was funded by the Toronto General and Western Hospital Foundation, PSI Foundation, and the Canadian Institute of Health Research. The researchers had no financial conflicts to disclose.
SOURCE: Taghdiri F et al. Neurology. 2019 May 8. doi: 10.1212/WNL.0000000000007608
Former athletes with a history of concussion averaged higher levels of total tau in their cerebrospinal fluid than did healthy controls, and those with the highest levels showed signs of reduced cognitive function in a case-control study.
Chronic traumatic encephalopathy (CTE) remains a postmortem diagnosis, but “the potential for treating postconcussion degeneration such as CTE depends on being able to detect the in vivo pathology at an early stage to intervene before the disease progresses to an irreversible stage,” wrote Foad Taghdiri, MD, of the University of Toronto and colleagues.
In a study published in Neurology, the researchers measured concentrations of phosphorylated tau181, total tau (t-tau), and beta-amyloid in the cerebrospinal fluid (CSF) of three groups: 22 former professional athletes who had suffered multiple concussions, 5 healthy controls, and 12 individuals diagnosed with Alzheimer’s disease (AD). The average ages of the groups were 56 years, 57 years, and 60 years, respectively. All the athletes were male, and their sports included snowboarding, hockey, and football.
The average t-tau level in the CSF of the athletes was significantly higher than that of controls (349.3 pg/mL vs. 188.8 pg/mL) and significantly lower than that of AD patients (857.0 pg/mL).
Normal CSF t-tau was defined as 300 pg/mL, and 12 former athletes (45%) had high t-tau levels, with an average of 499.3 pg/mL. In this group of high t-tau former athletes, the average score on the Trail Making Test (TMT) Part B was significantly lower than the average score among the 10 former athletes with normal CSF t-tau levels (t scores 45.6 vs. 62.3; P = .017).
In addition, results from MRI scans showed that fractional anisotropy values across all the tracts were significantly lower for those with high CSF t-tau levels, compared with those who had normal CSF t-tau levels (P = .036).
The findings were limited by several factors, including the small sample size, lack of female athletes, and limited ability to compare white matter integrity between high and normal CSF t-tau groups, the researchers noted.
However, the results suggest that “multiple concussive or subconcussive events may trigger neurodegeneration to a greater degree than expected on the basis of age alone,” they said. Although the study did not allow for diagnosing the participants with CTE, “we are engaged in longitudinal studies to track neurologic and neuropsychological function, CSF biomarkers, and structural brain changes over time to further assess the delayed effects of multiple concussions on the brain,” the researchers wrote.
The study was funded by the Toronto General and Western Hospital Foundation, PSI Foundation, and the Canadian Institute of Health Research. The researchers had no financial conflicts to disclose.
SOURCE: Taghdiri F et al. Neurology. 2019 May 8. doi: 10.1212/WNL.0000000000007608
FROM NEUROLOGY
Brain volumes after TBI correlate with clinical features
PHILADELPHIA – ” according to a study presented at the annual meeting of the American Academy of Neurology.
Traumatic brain injury (TBI) damages brain tissue and causes subsequent volume loss, which may result in clinical symptoms. It is a prevalent worldwide health problem caused by a mechanical insult to the head, resulting in transient or permanent alteration to brain tissue and/or function. Standard neuroimaging with computerized cranial tomography (CT) and structural magnetic resonance imaging (MRI) is often unrevealing during the evaluation of patients with TBI, particularly those classified as mild TBI. I
In this study, James Rock, MD, of Penn Presbyterian Medical Center and the University of Pennsylvania, and his colleagues sought to examine the value of quantitative analysis of regional brain volumes in the evaluation of TBI. The investigators reviewed the medical records and MRI imaging from 44 patients with TBI evaluated at a Level I trauma center. They also read clinical notes to assess reported symptoms and physical findings.
Regional volumes from TBI subjects were derived using the software package Freesurfer image analysis suite, which utilizes a T1-weighted structural scan to calculate volumetric information. A machine learning algorithm, random forests, was employed across volume measurements from 25 regions of interest to determine the most important regions for classifying subjects based on clinical outcome and symptomology.
Basal ganglia volume showed the highest variable importance with regards to classifying subjects who exhibited symptoms of cognitive dysfunction (Mean Decrease in Gini = 1.067, Mean Decrease in Accuracy = 5.966e-03) in quantitative analysis. Left lateral ventricle volume was important in classifying subjects with motor and vestibular alterations (Mean Decrease in Gini = 2.037, Mean Decrease in Accuracy = 2.92e-02). Left choroid plexus volume was the most important region for classifying subjects with sensation and somatic dysfunction (Mean Decrease in Gini = 0.271, Mean Decrease in Accuracy = 4.82e-03).
The researchers noted that their study is ongoing, in an abstract. “It will be extended to a larger cohort to determine whether volume changes in specific [regions of interest] can act as useful clinical biomarkers for chronic symptoms,” they said.
Dr. Diaz-Arrastia received personal compensation from Neural Analytics, Inc, BrainBox Solutions, Inc, and Bioscience Pharma Partners. Dr. Diaz-Arrastia holds stock and/or stock options in Neural Analytics, Inc. and has received research support from BrainBox Solutions. The other authors reported not having anything to disclose..
SOURCE: Rock J et al. AAN 2019. Abstract S2.006 .
PHILADELPHIA – ” according to a study presented at the annual meeting of the American Academy of Neurology.
Traumatic brain injury (TBI) damages brain tissue and causes subsequent volume loss, which may result in clinical symptoms. It is a prevalent worldwide health problem caused by a mechanical insult to the head, resulting in transient or permanent alteration to brain tissue and/or function. Standard neuroimaging with computerized cranial tomography (CT) and structural magnetic resonance imaging (MRI) is often unrevealing during the evaluation of patients with TBI, particularly those classified as mild TBI. I
In this study, James Rock, MD, of Penn Presbyterian Medical Center and the University of Pennsylvania, and his colleagues sought to examine the value of quantitative analysis of regional brain volumes in the evaluation of TBI. The investigators reviewed the medical records and MRI imaging from 44 patients with TBI evaluated at a Level I trauma center. They also read clinical notes to assess reported symptoms and physical findings.
Regional volumes from TBI subjects were derived using the software package Freesurfer image analysis suite, which utilizes a T1-weighted structural scan to calculate volumetric information. A machine learning algorithm, random forests, was employed across volume measurements from 25 regions of interest to determine the most important regions for classifying subjects based on clinical outcome and symptomology.
Basal ganglia volume showed the highest variable importance with regards to classifying subjects who exhibited symptoms of cognitive dysfunction (Mean Decrease in Gini = 1.067, Mean Decrease in Accuracy = 5.966e-03) in quantitative analysis. Left lateral ventricle volume was important in classifying subjects with motor and vestibular alterations (Mean Decrease in Gini = 2.037, Mean Decrease in Accuracy = 2.92e-02). Left choroid plexus volume was the most important region for classifying subjects with sensation and somatic dysfunction (Mean Decrease in Gini = 0.271, Mean Decrease in Accuracy = 4.82e-03).
The researchers noted that their study is ongoing, in an abstract. “It will be extended to a larger cohort to determine whether volume changes in specific [regions of interest] can act as useful clinical biomarkers for chronic symptoms,” they said.
Dr. Diaz-Arrastia received personal compensation from Neural Analytics, Inc, BrainBox Solutions, Inc, and Bioscience Pharma Partners. Dr. Diaz-Arrastia holds stock and/or stock options in Neural Analytics, Inc. and has received research support from BrainBox Solutions. The other authors reported not having anything to disclose..
SOURCE: Rock J et al. AAN 2019. Abstract S2.006 .
PHILADELPHIA – ” according to a study presented at the annual meeting of the American Academy of Neurology.
Traumatic brain injury (TBI) damages brain tissue and causes subsequent volume loss, which may result in clinical symptoms. It is a prevalent worldwide health problem caused by a mechanical insult to the head, resulting in transient or permanent alteration to brain tissue and/or function. Standard neuroimaging with computerized cranial tomography (CT) and structural magnetic resonance imaging (MRI) is often unrevealing during the evaluation of patients with TBI, particularly those classified as mild TBI. I
In this study, James Rock, MD, of Penn Presbyterian Medical Center and the University of Pennsylvania, and his colleagues sought to examine the value of quantitative analysis of regional brain volumes in the evaluation of TBI. The investigators reviewed the medical records and MRI imaging from 44 patients with TBI evaluated at a Level I trauma center. They also read clinical notes to assess reported symptoms and physical findings.
Regional volumes from TBI subjects were derived using the software package Freesurfer image analysis suite, which utilizes a T1-weighted structural scan to calculate volumetric information. A machine learning algorithm, random forests, was employed across volume measurements from 25 regions of interest to determine the most important regions for classifying subjects based on clinical outcome and symptomology.
Basal ganglia volume showed the highest variable importance with regards to classifying subjects who exhibited symptoms of cognitive dysfunction (Mean Decrease in Gini = 1.067, Mean Decrease in Accuracy = 5.966e-03) in quantitative analysis. Left lateral ventricle volume was important in classifying subjects with motor and vestibular alterations (Mean Decrease in Gini = 2.037, Mean Decrease in Accuracy = 2.92e-02). Left choroid plexus volume was the most important region for classifying subjects with sensation and somatic dysfunction (Mean Decrease in Gini = 0.271, Mean Decrease in Accuracy = 4.82e-03).
The researchers noted that their study is ongoing, in an abstract. “It will be extended to a larger cohort to determine whether volume changes in specific [regions of interest] can act as useful clinical biomarkers for chronic symptoms,” they said.
Dr. Diaz-Arrastia received personal compensation from Neural Analytics, Inc, BrainBox Solutions, Inc, and Bioscience Pharma Partners. Dr. Diaz-Arrastia holds stock and/or stock options in Neural Analytics, Inc. and has received research support from BrainBox Solutions. The other authors reported not having anything to disclose..
SOURCE: Rock J et al. AAN 2019. Abstract S2.006 .
REPORTING FROM AAN 2019
VA Weighs Improvements to Disability Determination Process
The severity of traumatic brain injury (TBI) is typically defined at the time of the initial injury, but a diagnosis may not come for months or even years later. Given the complexities of diagnosing what might be a slowly revealed condition, with signs and symptoms that may manifest over time; the need for self-report of symptoms; and the time that might have elapsed since the original injury, a diagnostician needs not only to have experience with TBI but to stay abreast of the state of the science.
As of now, only health care professionals in 4 specialties—neurologist, neurosurgeon, physiatrist, or psychiatrist—are allowed to diagnose TBI in the VA’s disability compensation process. A new congressionally mandated report by the National Academies of Sciences, Engineering, and Medicine, though, is advising that it’s training and experience that count, not necessarily the specialty.
In Evaluation of the Disability Determination Process for Traumatic Brain Injury in Veterans, a committee of experts in emergency medicine, neurology, neurosurgery, psychiatry, psychology, physical medicine and rehabilitation, and epidemiology and biostatistics review the process and current literature on TBI. The committee advises that any health care professional with “pertinent and ongoing brain injury training and experience” and up-to-date knowledge about TBI should be included in the diagnostic process.
The disability compensation is a tax-free benefit paid to veterans with disabilities resulting from disease or injury incurred or aggravated during active military service. The amount is determined in a 6-step process beginning when the veteran (or a proxy) files a claim. An approved clinician typically must diagnose and evaluate the degree of impairment, functional limitation, and disability.
Between 2000 and 2018, an estimated 384,000 incidents of TBI occurred in the military. That increasing prevalence means more medical specialties now include TBI training in their curriculum. The committee notes that at least 18 brain injury programs are accredited by the Accreditation Council for Graduate Medical Education to train physicians in many specialties to diagnose, treat, and rehabilitate patients with brain injury.
Among other recommendations, the committee advised that the VA take specific actions to increase transparency at both individual and systemwide levels, such as providing veterans full access to the details of their examinations, allowing veterans to rate the quality of their evaluations, and providing public access to detailed systemwide data on the outcomes of evaluations and outcome quality. Those changes will represent a “fundamental enhancement” in the quality of disability evaluations, the committee says, which added that shifting from a focus on the consistency of the process and practitioner qualifications to a focus on the accuracy of the outcome of the evaluation will help identify steps or components in the process that warrant improvement.
It also suggested regularly updating the Veteran Affairs Schedule for Rating Disabilities and the Disability Benefits Questionnaires (DBQs) for residuals of TBI to “better reflect the current state of medical knowledge.” The committee found that 3 important residuals of TBI are not adequately covered by any of the existing DBQs: insomnia, vestibular dysfunction, and near-vision dysfunction. Although 4 DBQs (mental disorder, chronic fatigue syndrome, PTSD, and sleep apnea) contain isolated questions related to insomnia and sleep disruption, no single DBQ, the committee says, combines them all “in a way that captures the full extent of disability associated with post-TBI sleep disruption.” Similarly, no single DBQ captures the full extent of disability associated with post-TBI vestibular dysfunction or the disability associated with near-vision dysfunction.
The committee sums up: “[B]y adopting an explicit learning structure in which the reliability and validity of disability determinations are directly assessed, the VA will be able to devote its resources to those modifications and enhancements … that will have the greatest impact in improving the service provided to injured veterans.”
The severity of traumatic brain injury (TBI) is typically defined at the time of the initial injury, but a diagnosis may not come for months or even years later. Given the complexities of diagnosing what might be a slowly revealed condition, with signs and symptoms that may manifest over time; the need for self-report of symptoms; and the time that might have elapsed since the original injury, a diagnostician needs not only to have experience with TBI but to stay abreast of the state of the science.
As of now, only health care professionals in 4 specialties—neurologist, neurosurgeon, physiatrist, or psychiatrist—are allowed to diagnose TBI in the VA’s disability compensation process. A new congressionally mandated report by the National Academies of Sciences, Engineering, and Medicine, though, is advising that it’s training and experience that count, not necessarily the specialty.
In Evaluation of the Disability Determination Process for Traumatic Brain Injury in Veterans, a committee of experts in emergency medicine, neurology, neurosurgery, psychiatry, psychology, physical medicine and rehabilitation, and epidemiology and biostatistics review the process and current literature on TBI. The committee advises that any health care professional with “pertinent and ongoing brain injury training and experience” and up-to-date knowledge about TBI should be included in the diagnostic process.
The disability compensation is a tax-free benefit paid to veterans with disabilities resulting from disease or injury incurred or aggravated during active military service. The amount is determined in a 6-step process beginning when the veteran (or a proxy) files a claim. An approved clinician typically must diagnose and evaluate the degree of impairment, functional limitation, and disability.
Between 2000 and 2018, an estimated 384,000 incidents of TBI occurred in the military. That increasing prevalence means more medical specialties now include TBI training in their curriculum. The committee notes that at least 18 brain injury programs are accredited by the Accreditation Council for Graduate Medical Education to train physicians in many specialties to diagnose, treat, and rehabilitate patients with brain injury.
Among other recommendations, the committee advised that the VA take specific actions to increase transparency at both individual and systemwide levels, such as providing veterans full access to the details of their examinations, allowing veterans to rate the quality of their evaluations, and providing public access to detailed systemwide data on the outcomes of evaluations and outcome quality. Those changes will represent a “fundamental enhancement” in the quality of disability evaluations, the committee says, which added that shifting from a focus on the consistency of the process and practitioner qualifications to a focus on the accuracy of the outcome of the evaluation will help identify steps or components in the process that warrant improvement.
It also suggested regularly updating the Veteran Affairs Schedule for Rating Disabilities and the Disability Benefits Questionnaires (DBQs) for residuals of TBI to “better reflect the current state of medical knowledge.” The committee found that 3 important residuals of TBI are not adequately covered by any of the existing DBQs: insomnia, vestibular dysfunction, and near-vision dysfunction. Although 4 DBQs (mental disorder, chronic fatigue syndrome, PTSD, and sleep apnea) contain isolated questions related to insomnia and sleep disruption, no single DBQ, the committee says, combines them all “in a way that captures the full extent of disability associated with post-TBI sleep disruption.” Similarly, no single DBQ captures the full extent of disability associated with post-TBI vestibular dysfunction or the disability associated with near-vision dysfunction.
The committee sums up: “[B]y adopting an explicit learning structure in which the reliability and validity of disability determinations are directly assessed, the VA will be able to devote its resources to those modifications and enhancements … that will have the greatest impact in improving the service provided to injured veterans.”
The severity of traumatic brain injury (TBI) is typically defined at the time of the initial injury, but a diagnosis may not come for months or even years later. Given the complexities of diagnosing what might be a slowly revealed condition, with signs and symptoms that may manifest over time; the need for self-report of symptoms; and the time that might have elapsed since the original injury, a diagnostician needs not only to have experience with TBI but to stay abreast of the state of the science.
As of now, only health care professionals in 4 specialties—neurologist, neurosurgeon, physiatrist, or psychiatrist—are allowed to diagnose TBI in the VA’s disability compensation process. A new congressionally mandated report by the National Academies of Sciences, Engineering, and Medicine, though, is advising that it’s training and experience that count, not necessarily the specialty.
In Evaluation of the Disability Determination Process for Traumatic Brain Injury in Veterans, a committee of experts in emergency medicine, neurology, neurosurgery, psychiatry, psychology, physical medicine and rehabilitation, and epidemiology and biostatistics review the process and current literature on TBI. The committee advises that any health care professional with “pertinent and ongoing brain injury training and experience” and up-to-date knowledge about TBI should be included in the diagnostic process.
The disability compensation is a tax-free benefit paid to veterans with disabilities resulting from disease or injury incurred or aggravated during active military service. The amount is determined in a 6-step process beginning when the veteran (or a proxy) files a claim. An approved clinician typically must diagnose and evaluate the degree of impairment, functional limitation, and disability.
Between 2000 and 2018, an estimated 384,000 incidents of TBI occurred in the military. That increasing prevalence means more medical specialties now include TBI training in their curriculum. The committee notes that at least 18 brain injury programs are accredited by the Accreditation Council for Graduate Medical Education to train physicians in many specialties to diagnose, treat, and rehabilitate patients with brain injury.
Among other recommendations, the committee advised that the VA take specific actions to increase transparency at both individual and systemwide levels, such as providing veterans full access to the details of their examinations, allowing veterans to rate the quality of their evaluations, and providing public access to detailed systemwide data on the outcomes of evaluations and outcome quality. Those changes will represent a “fundamental enhancement” in the quality of disability evaluations, the committee says, which added that shifting from a focus on the consistency of the process and practitioner qualifications to a focus on the accuracy of the outcome of the evaluation will help identify steps or components in the process that warrant improvement.
It also suggested regularly updating the Veteran Affairs Schedule for Rating Disabilities and the Disability Benefits Questionnaires (DBQs) for residuals of TBI to “better reflect the current state of medical knowledge.” The committee found that 3 important residuals of TBI are not adequately covered by any of the existing DBQs: insomnia, vestibular dysfunction, and near-vision dysfunction. Although 4 DBQs (mental disorder, chronic fatigue syndrome, PTSD, and sleep apnea) contain isolated questions related to insomnia and sleep disruption, no single DBQ, the committee says, combines them all “in a way that captures the full extent of disability associated with post-TBI sleep disruption.” Similarly, no single DBQ captures the full extent of disability associated with post-TBI vestibular dysfunction or the disability associated with near-vision dysfunction.
The committee sums up: “[B]y adopting an explicit learning structure in which the reliability and validity of disability determinations are directly assessed, the VA will be able to devote its resources to those modifications and enhancements … that will have the greatest impact in improving the service provided to injured veterans.”
Symptomatic former NFL players may have tau deposition consistent with CTE
according to research published online ahead of print April 10 in the New England Journal of Medicine. The distribution of tau in the players’ brains appears to be similar to that in persons with chronic traumatic encephalopathy (CTE).
CTE is a neurodegenerative disease that has been associated with a history of repetitive head impacts, such as those withstood in contact sports. The basis for the neuropathological diagnosis of CTE is a distinct pattern of tau deposition with minimal deposition of amyloid-beta. Paired helical filament tau aggregates are first observed in the frontal, temporal, and parietal cortices. They later spread throughout the cerebral cortex, medial temporal lobe, diencephalon, and brainstem. CTE is diagnosed only through post mortem neuropathological examinations.
To examine whether tau and amyloid deposition can be detected in the brains of living people at risk for CTE, Robert A. Stern, PhD, and his colleagues studied living former NFL players and asymptomatic controls with flortaucipir PET (to detect tau) and 18F-florbetapir PET (to detect amyloid-beta). Dr. Stern is director of clinical research at the CTE Center at Boston University. Eligible former players were male, aged 40-69 years, had played football in the NFL for at least 2 years, had had at least 12 years of total tackle football experience, and reported cognitive, behavioral, and mood symptoms through telephone screening. Eligible controls were male, aged 40-69 years, and had no cognitive symptoms or history of traumatic brain injury.
All subjects underwent flortaucipir PET, florbetapir PET, and T1-weighted volumetric MRI of the head. Dr. Stern and his colleagues used automated image-analysis algorithms to compare the regional tau standardized uptake value ratio (SUVR) between the two patient groups and to evaluate potential associations between that ratio and symptom severity or years of football play.
The investigators included 26 former players and 31 controls in their analysis. The group of former players had a higher percentage of black participants and a lower mean Mini-Mental State Examination score, compared with controls. The mean flortaucipir SUVR was higher among former players than among controls in the bilateral superior frontal (1.09 vs. 0.98), bilateral medial temporal (1.23 vs. 1.12), and left parietal (1.12 vs. 1.01) regions. Dr. Stern and his colleagues found no association between tau deposition in those regions and results on cognitive and neuropsychiatric tests. In a post hoc analysis, they calculated the correlation coefficients in the three brain regions between the SUVRs and years of play to be 0.58 in the bilateral superior frontal region, 0.45 in the bilateral medial temporal region, and 0.50 in the left parietal region. Mean cortical:cerebellar florbetapir SUVRs did not differ significantly between groups.
“These findings suggest that the cognitive difficulties reported by the former players were not related to Alzheimer’s disease amyloid-beta deposition,” said the authors. The study may have been insufficiently powered to detect associations between flortaucipir uptake and the clinical measures, they added. Also, paired helical filament tau pathology alone may not be associated with the former players’ neuropsychiatric symptoms and cognitive impairment. “Although this study showed between-group differences in flortaucipir PET measurements, our analyses do not pertain to detection of tau pathology in individual participants,” the authors concluded.
The study was supported by an investigator-initiated grant from Avid Radiopharmaceuticals. The National Institutes of Health, the state of Arizona, and the U.S. Department of Defense also supported the study.
SOURCE: Stern RA et al. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMoa1900757 (Epub ahead of print).
The study by Stern et al offers valuable information, but the relationships between various features of chronic traumatic encephalopathy (CTE) still are not well understood, said Allan H. Ropper, MD, executive vice chair of neurology at Harvard Medical School in Boston, in an accompanying editorial (N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMe1903746). The risk of CTE associated with a long period of playing football does not correspond with the number, severity, or serial occurrence of concussions, he observed. In addition, “individual factors such as the player’s size, head-to-neck configuration, style of play, and position, as well as biologic attributes, may influence the deposition of tau.” Because of the absence of an association between neuropsychological test results and tau deposition, neurologists can draw few conclusions based on the presence of neuropsychological abnormalities in athletes who are at risk for CTE, said Dr. Ropper.
“As with Alzheimer’s disease, the CTE field is in a phase of fumbling with circumstantial evidence for a connection between tau deposition and a clinical syndrome. ... The report in this issue certainly does strengthen the case that tau is the offender early in CTE, but other links remain to be clarified,” he concluded.
Dr. Ropper reported no relevant conflicts of interest. He is deputy editor of the New England Journal of Medicine.
The study by Stern et al offers valuable information, but the relationships between various features of chronic traumatic encephalopathy (CTE) still are not well understood, said Allan H. Ropper, MD, executive vice chair of neurology at Harvard Medical School in Boston, in an accompanying editorial (N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMe1903746). The risk of CTE associated with a long period of playing football does not correspond with the number, severity, or serial occurrence of concussions, he observed. In addition, “individual factors such as the player’s size, head-to-neck configuration, style of play, and position, as well as biologic attributes, may influence the deposition of tau.” Because of the absence of an association between neuropsychological test results and tau deposition, neurologists can draw few conclusions based on the presence of neuropsychological abnormalities in athletes who are at risk for CTE, said Dr. Ropper.
“As with Alzheimer’s disease, the CTE field is in a phase of fumbling with circumstantial evidence for a connection between tau deposition and a clinical syndrome. ... The report in this issue certainly does strengthen the case that tau is the offender early in CTE, but other links remain to be clarified,” he concluded.
Dr. Ropper reported no relevant conflicts of interest. He is deputy editor of the New England Journal of Medicine.
The study by Stern et al offers valuable information, but the relationships between various features of chronic traumatic encephalopathy (CTE) still are not well understood, said Allan H. Ropper, MD, executive vice chair of neurology at Harvard Medical School in Boston, in an accompanying editorial (N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMe1903746). The risk of CTE associated with a long period of playing football does not correspond with the number, severity, or serial occurrence of concussions, he observed. In addition, “individual factors such as the player’s size, head-to-neck configuration, style of play, and position, as well as biologic attributes, may influence the deposition of tau.” Because of the absence of an association between neuropsychological test results and tau deposition, neurologists can draw few conclusions based on the presence of neuropsychological abnormalities in athletes who are at risk for CTE, said Dr. Ropper.
“As with Alzheimer’s disease, the CTE field is in a phase of fumbling with circumstantial evidence for a connection between tau deposition and a clinical syndrome. ... The report in this issue certainly does strengthen the case that tau is the offender early in CTE, but other links remain to be clarified,” he concluded.
Dr. Ropper reported no relevant conflicts of interest. He is deputy editor of the New England Journal of Medicine.
according to research published online ahead of print April 10 in the New England Journal of Medicine. The distribution of tau in the players’ brains appears to be similar to that in persons with chronic traumatic encephalopathy (CTE).
CTE is a neurodegenerative disease that has been associated with a history of repetitive head impacts, such as those withstood in contact sports. The basis for the neuropathological diagnosis of CTE is a distinct pattern of tau deposition with minimal deposition of amyloid-beta. Paired helical filament tau aggregates are first observed in the frontal, temporal, and parietal cortices. They later spread throughout the cerebral cortex, medial temporal lobe, diencephalon, and brainstem. CTE is diagnosed only through post mortem neuropathological examinations.
To examine whether tau and amyloid deposition can be detected in the brains of living people at risk for CTE, Robert A. Stern, PhD, and his colleagues studied living former NFL players and asymptomatic controls with flortaucipir PET (to detect tau) and 18F-florbetapir PET (to detect amyloid-beta). Dr. Stern is director of clinical research at the CTE Center at Boston University. Eligible former players were male, aged 40-69 years, had played football in the NFL for at least 2 years, had had at least 12 years of total tackle football experience, and reported cognitive, behavioral, and mood symptoms through telephone screening. Eligible controls were male, aged 40-69 years, and had no cognitive symptoms or history of traumatic brain injury.
All subjects underwent flortaucipir PET, florbetapir PET, and T1-weighted volumetric MRI of the head. Dr. Stern and his colleagues used automated image-analysis algorithms to compare the regional tau standardized uptake value ratio (SUVR) between the two patient groups and to evaluate potential associations between that ratio and symptom severity or years of football play.
The investigators included 26 former players and 31 controls in their analysis. The group of former players had a higher percentage of black participants and a lower mean Mini-Mental State Examination score, compared with controls. The mean flortaucipir SUVR was higher among former players than among controls in the bilateral superior frontal (1.09 vs. 0.98), bilateral medial temporal (1.23 vs. 1.12), and left parietal (1.12 vs. 1.01) regions. Dr. Stern and his colleagues found no association between tau deposition in those regions and results on cognitive and neuropsychiatric tests. In a post hoc analysis, they calculated the correlation coefficients in the three brain regions between the SUVRs and years of play to be 0.58 in the bilateral superior frontal region, 0.45 in the bilateral medial temporal region, and 0.50 in the left parietal region. Mean cortical:cerebellar florbetapir SUVRs did not differ significantly between groups.
“These findings suggest that the cognitive difficulties reported by the former players were not related to Alzheimer’s disease amyloid-beta deposition,” said the authors. The study may have been insufficiently powered to detect associations between flortaucipir uptake and the clinical measures, they added. Also, paired helical filament tau pathology alone may not be associated with the former players’ neuropsychiatric symptoms and cognitive impairment. “Although this study showed between-group differences in flortaucipir PET measurements, our analyses do not pertain to detection of tau pathology in individual participants,” the authors concluded.
The study was supported by an investigator-initiated grant from Avid Radiopharmaceuticals. The National Institutes of Health, the state of Arizona, and the U.S. Department of Defense also supported the study.
SOURCE: Stern RA et al. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMoa1900757 (Epub ahead of print).
according to research published online ahead of print April 10 in the New England Journal of Medicine. The distribution of tau in the players’ brains appears to be similar to that in persons with chronic traumatic encephalopathy (CTE).
CTE is a neurodegenerative disease that has been associated with a history of repetitive head impacts, such as those withstood in contact sports. The basis for the neuropathological diagnosis of CTE is a distinct pattern of tau deposition with minimal deposition of amyloid-beta. Paired helical filament tau aggregates are first observed in the frontal, temporal, and parietal cortices. They later spread throughout the cerebral cortex, medial temporal lobe, diencephalon, and brainstem. CTE is diagnosed only through post mortem neuropathological examinations.
To examine whether tau and amyloid deposition can be detected in the brains of living people at risk for CTE, Robert A. Stern, PhD, and his colleagues studied living former NFL players and asymptomatic controls with flortaucipir PET (to detect tau) and 18F-florbetapir PET (to detect amyloid-beta). Dr. Stern is director of clinical research at the CTE Center at Boston University. Eligible former players were male, aged 40-69 years, had played football in the NFL for at least 2 years, had had at least 12 years of total tackle football experience, and reported cognitive, behavioral, and mood symptoms through telephone screening. Eligible controls were male, aged 40-69 years, and had no cognitive symptoms or history of traumatic brain injury.
All subjects underwent flortaucipir PET, florbetapir PET, and T1-weighted volumetric MRI of the head. Dr. Stern and his colleagues used automated image-analysis algorithms to compare the regional tau standardized uptake value ratio (SUVR) between the two patient groups and to evaluate potential associations between that ratio and symptom severity or years of football play.
The investigators included 26 former players and 31 controls in their analysis. The group of former players had a higher percentage of black participants and a lower mean Mini-Mental State Examination score, compared with controls. The mean flortaucipir SUVR was higher among former players than among controls in the bilateral superior frontal (1.09 vs. 0.98), bilateral medial temporal (1.23 vs. 1.12), and left parietal (1.12 vs. 1.01) regions. Dr. Stern and his colleagues found no association between tau deposition in those regions and results on cognitive and neuropsychiatric tests. In a post hoc analysis, they calculated the correlation coefficients in the three brain regions between the SUVRs and years of play to be 0.58 in the bilateral superior frontal region, 0.45 in the bilateral medial temporal region, and 0.50 in the left parietal region. Mean cortical:cerebellar florbetapir SUVRs did not differ significantly between groups.
“These findings suggest that the cognitive difficulties reported by the former players were not related to Alzheimer’s disease amyloid-beta deposition,” said the authors. The study may have been insufficiently powered to detect associations between flortaucipir uptake and the clinical measures, they added. Also, paired helical filament tau pathology alone may not be associated with the former players’ neuropsychiatric symptoms and cognitive impairment. “Although this study showed between-group differences in flortaucipir PET measurements, our analyses do not pertain to detection of tau pathology in individual participants,” the authors concluded.
The study was supported by an investigator-initiated grant from Avid Radiopharmaceuticals. The National Institutes of Health, the state of Arizona, and the U.S. Department of Defense also supported the study.
SOURCE: Stern RA et al. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMoa1900757 (Epub ahead of print).
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Trial to Test Effectiveness of CBT Phone Sessions for Chronic Pain After TBI
As many as 81.5% of veterans may experience chronic pain, pain that lasts beyond the point of healing and for at least 3 months. It is also particularly prevalent among veterans with traumatic brain injury (TBI) , often accompanied by comorbid conditions. Nearly 90% of veterans with a history of TBI have a psychiatric diagnosis, about 75% have insomnia, and 70% have a pain diagnosis, say researchers from University of Washington and Veterans Administration Puget Sound Health Care System (VAPSHCS).
Cognitive behavioral therapy (CBT) has been shown to help reduce pain, as well as pain-related disability and distress, but no randomized controlled trials (RCT) have examined CBT’s efficacy for pain after TBI in veterans, the researchers say.
In response, the VAPSHCS researchers have designed an RCT to compare telephone-based CBT with telephone-delivered pain education for veterans with TBI and chronic pain. The single-center 2-group trial will enroll up to 160 veterans with TBI to examine the relative efficacy of the interventions on average pain intensity, pain interference, sleep, depression, and life satisfaction.
The participants will be drawn from VAPSHCS, and can be enrolled via clinician referral, electronic health record review, and self-referral. Outcome variables will be collected pre-, mid-, and posttreatment, and 6 months following randomization.
Both interventions will consist of 8 hour-long phone sessions over approximately 8 to 12 weeks, scheduled at times convenient for the participants. Both interventions will also use a participant treatment workbook, with session-specific content to be discussed during the telephone sessions, and audio-recordings to augment material covered. Clinicians will make brief “booster” calls 2, 6, and 10 weeks after the final treatment session.
The trial is innovative, the researchers say, in that it is tailored to veterans, through relatable examples, and to those with TBI, by reducing content and providing multiple methods of engaging with information, as well as using known strategies to help with recall. If effective, the intervention could be disseminated throughout the VHA system, potentially to other personnel who have difficulty accessing specialty pain care.
The trial is registered at ClinicalTrials.gov, protocol NCT01768650.
As many as 81.5% of veterans may experience chronic pain, pain that lasts beyond the point of healing and for at least 3 months. It is also particularly prevalent among veterans with traumatic brain injury (TBI) , often accompanied by comorbid conditions. Nearly 90% of veterans with a history of TBI have a psychiatric diagnosis, about 75% have insomnia, and 70% have a pain diagnosis, say researchers from University of Washington and Veterans Administration Puget Sound Health Care System (VAPSHCS).
Cognitive behavioral therapy (CBT) has been shown to help reduce pain, as well as pain-related disability and distress, but no randomized controlled trials (RCT) have examined CBT’s efficacy for pain after TBI in veterans, the researchers say.
In response, the VAPSHCS researchers have designed an RCT to compare telephone-based CBT with telephone-delivered pain education for veterans with TBI and chronic pain. The single-center 2-group trial will enroll up to 160 veterans with TBI to examine the relative efficacy of the interventions on average pain intensity, pain interference, sleep, depression, and life satisfaction.
The participants will be drawn from VAPSHCS, and can be enrolled via clinician referral, electronic health record review, and self-referral. Outcome variables will be collected pre-, mid-, and posttreatment, and 6 months following randomization.
Both interventions will consist of 8 hour-long phone sessions over approximately 8 to 12 weeks, scheduled at times convenient for the participants. Both interventions will also use a participant treatment workbook, with session-specific content to be discussed during the telephone sessions, and audio-recordings to augment material covered. Clinicians will make brief “booster” calls 2, 6, and 10 weeks after the final treatment session.
The trial is innovative, the researchers say, in that it is tailored to veterans, through relatable examples, and to those with TBI, by reducing content and providing multiple methods of engaging with information, as well as using known strategies to help with recall. If effective, the intervention could be disseminated throughout the VHA system, potentially to other personnel who have difficulty accessing specialty pain care.
The trial is registered at ClinicalTrials.gov, protocol NCT01768650.
As many as 81.5% of veterans may experience chronic pain, pain that lasts beyond the point of healing and for at least 3 months. It is also particularly prevalent among veterans with traumatic brain injury (TBI) , often accompanied by comorbid conditions. Nearly 90% of veterans with a history of TBI have a psychiatric diagnosis, about 75% have insomnia, and 70% have a pain diagnosis, say researchers from University of Washington and Veterans Administration Puget Sound Health Care System (VAPSHCS).
Cognitive behavioral therapy (CBT) has been shown to help reduce pain, as well as pain-related disability and distress, but no randomized controlled trials (RCT) have examined CBT’s efficacy for pain after TBI in veterans, the researchers say.
In response, the VAPSHCS researchers have designed an RCT to compare telephone-based CBT with telephone-delivered pain education for veterans with TBI and chronic pain. The single-center 2-group trial will enroll up to 160 veterans with TBI to examine the relative efficacy of the interventions on average pain intensity, pain interference, sleep, depression, and life satisfaction.
The participants will be drawn from VAPSHCS, and can be enrolled via clinician referral, electronic health record review, and self-referral. Outcome variables will be collected pre-, mid-, and posttreatment, and 6 months following randomization.
Both interventions will consist of 8 hour-long phone sessions over approximately 8 to 12 weeks, scheduled at times convenient for the participants. Both interventions will also use a participant treatment workbook, with session-specific content to be discussed during the telephone sessions, and audio-recordings to augment material covered. Clinicians will make brief “booster” calls 2, 6, and 10 weeks after the final treatment session.
The trial is innovative, the researchers say, in that it is tailored to veterans, through relatable examples, and to those with TBI, by reducing content and providing multiple methods of engaging with information, as well as using known strategies to help with recall. If effective, the intervention could be disseminated throughout the VHA system, potentially to other personnel who have difficulty accessing specialty pain care.
The trial is registered at ClinicalTrials.gov, protocol NCT01768650.
Functional MRI detects consciousness after brain damage
Functional MRI can measure patterns of connectivity to determine levels of consciousness in nonresponsive patients with brain injury, according to results from a multicenter, cross-sectional, observational study.
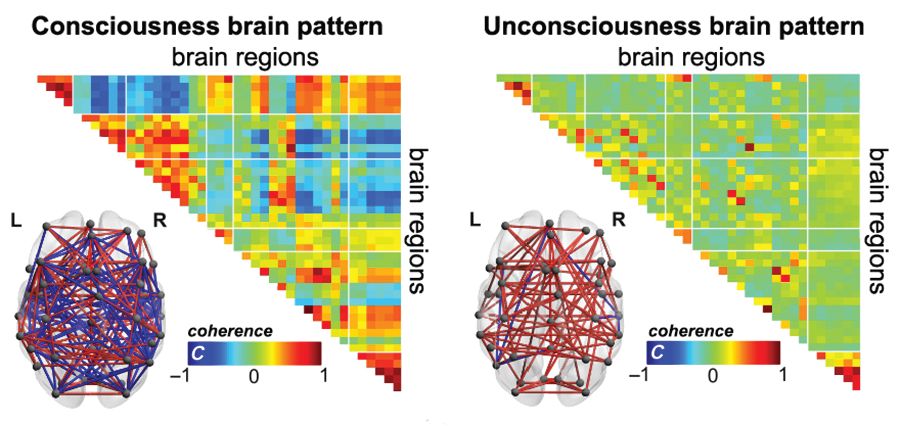
Blood oxygen level–dependent (BOLD) fMRI showed that brain-wide coordination patterns of high complexity became increasingly common moving from unresponsive patients to those with minimal consciousness to healthy individuals, reported lead author Athena Demertzi, PhD, of GIGA Research Institute at the University of Liège in Belgium, and her colleagues.
“Finding reliable markers indicating the presence or absence of consciousness represents an outstanding open problem for science,” the investigators wrote in Science Advances.
In medicine, an fMRI-based measure of consciousness could supplement behavioral assessments of awareness and guide therapeutic strategies; more broadly, image-based markers could help elucidate the nature of consciousness itself.
“We postulate that consciousness has specific characteristics that are based on the temporal dynamics of ongoing brain activity and its coordination over distant cortical regions,” the investigators wrote. “Our hypothesis stems from the common stance of various contemporary theories which propose that consciousness relates to a dynamic process of self-sustained, coordinated brain-scale activity assisting the tuning to a constantly evolving environment, rather than in static descriptions of brain function.”
There is a need for a reliable way of distinguishing consciousness from unconscious states, the investigators said. “Given that nonresponsiveness can be associated with a variety of brain lesions, varying levels of vigilance, and covert cognition, we highlight the need to determine a common set of features capable of accounting for the capacity to sustain conscious experience.”
To search for patterns of brain signal coordination that correlate with consciousness, four independent research centers performed BOLD fMRI scans of participants at rest or under anesthesia with propofol. Of 159 total participants, 47 were healthy individuals and 112 were patients in a vegetative state/with unresponsive wakefulness syndrome (UWS) or in a minimally conscious state (MCS), based on standardized behavioral assessments. The main data analysis, which included 125 participants, assessed BOLD fMRI signal coordination between six brain networks known to have roles in cognitive and functional processes.
The researchers’ analysis revealed four distinct and recurring brain-wide coordination patterns ranging on a scale from highest activity (pattern 1) to lowest activity (pattern 4). Pattern 1, which exhibited most long-distance edges, spatial complexity, efficiency, and community structure, became increasingly common when moving from UWS patients to MCS patients to healthy control individuals (UWS < MCS < HC, rho = 0.7, Spearman rank correlation between rate and group, P less than 1 x 10-16).
In contrast, pattern 4, characterized by low interareal coordination, showed an inverse trend; it became less common when moving from vegetative patients to healthy individuals (UWS > MCS > HC, Spearman rank correlation between rate and group, rho = –0.6, P less than 1 x 10-11). Although patterns 2 and 3 occurred with equal frequency across all groups, the investigators noted that switching between patterns was most common and predictably sequential in healthy individuals, versus patients with UWS, who were least likely to switch patterns. A total of 23 patients who were scanned under propofol anesthesia were equally likely to exhibit pattern 4, regardless of health status, suggesting that pattern 4 depends upon fixed anatomical pathways. Results were not affected by scanning site or other patient characteristics, such as age, gender, etiology, or chronicity.
“We conclude that these patterns of transient brain signal coordination are characteristic of conscious and unconscious brain states,” the investigators wrote, “warranting future research concerning their relationship to ongoing conscious content, and the possibility of modifying their prevalence by external perturbations, both in healthy and pathological individuals, as well as across species.”
The study was funded by a James S. McDonnell Foundation Collaborative Activity Award, INSERM, the Belgian National Funds for Scientific Research, the Canada Excellence Research Chairs program, and others. The authors declared having no conflicts of interest.
SOURCE: Demertzi A et al. Sci Adv. 2019 Feb 6. doi: 10.1126/sciadv.aat7603.
Functional MRI can measure patterns of connectivity to determine levels of consciousness in nonresponsive patients with brain injury, according to results from a multicenter, cross-sectional, observational study.

Blood oxygen level–dependent (BOLD) fMRI showed that brain-wide coordination patterns of high complexity became increasingly common moving from unresponsive patients to those with minimal consciousness to healthy individuals, reported lead author Athena Demertzi, PhD, of GIGA Research Institute at the University of Liège in Belgium, and her colleagues.
“Finding reliable markers indicating the presence or absence of consciousness represents an outstanding open problem for science,” the investigators wrote in Science Advances.
In medicine, an fMRI-based measure of consciousness could supplement behavioral assessments of awareness and guide therapeutic strategies; more broadly, image-based markers could help elucidate the nature of consciousness itself.
“We postulate that consciousness has specific characteristics that are based on the temporal dynamics of ongoing brain activity and its coordination over distant cortical regions,” the investigators wrote. “Our hypothesis stems from the common stance of various contemporary theories which propose that consciousness relates to a dynamic process of self-sustained, coordinated brain-scale activity assisting the tuning to a constantly evolving environment, rather than in static descriptions of brain function.”
There is a need for a reliable way of distinguishing consciousness from unconscious states, the investigators said. “Given that nonresponsiveness can be associated with a variety of brain lesions, varying levels of vigilance, and covert cognition, we highlight the need to determine a common set of features capable of accounting for the capacity to sustain conscious experience.”
To search for patterns of brain signal coordination that correlate with consciousness, four independent research centers performed BOLD fMRI scans of participants at rest or under anesthesia with propofol. Of 159 total participants, 47 were healthy individuals and 112 were patients in a vegetative state/with unresponsive wakefulness syndrome (UWS) or in a minimally conscious state (MCS), based on standardized behavioral assessments. The main data analysis, which included 125 participants, assessed BOLD fMRI signal coordination between six brain networks known to have roles in cognitive and functional processes.
The researchers’ analysis revealed four distinct and recurring brain-wide coordination patterns ranging on a scale from highest activity (pattern 1) to lowest activity (pattern 4). Pattern 1, which exhibited most long-distance edges, spatial complexity, efficiency, and community structure, became increasingly common when moving from UWS patients to MCS patients to healthy control individuals (UWS < MCS < HC, rho = 0.7, Spearman rank correlation between rate and group, P less than 1 x 10-16).
In contrast, pattern 4, characterized by low interareal coordination, showed an inverse trend; it became less common when moving from vegetative patients to healthy individuals (UWS > MCS > HC, Spearman rank correlation between rate and group, rho = –0.6, P less than 1 x 10-11). Although patterns 2 and 3 occurred with equal frequency across all groups, the investigators noted that switching between patterns was most common and predictably sequential in healthy individuals, versus patients with UWS, who were least likely to switch patterns. A total of 23 patients who were scanned under propofol anesthesia were equally likely to exhibit pattern 4, regardless of health status, suggesting that pattern 4 depends upon fixed anatomical pathways. Results were not affected by scanning site or other patient characteristics, such as age, gender, etiology, or chronicity.
“We conclude that these patterns of transient brain signal coordination are characteristic of conscious and unconscious brain states,” the investigators wrote, “warranting future research concerning their relationship to ongoing conscious content, and the possibility of modifying their prevalence by external perturbations, both in healthy and pathological individuals, as well as across species.”
The study was funded by a James S. McDonnell Foundation Collaborative Activity Award, INSERM, the Belgian National Funds for Scientific Research, the Canada Excellence Research Chairs program, and others. The authors declared having no conflicts of interest.
SOURCE: Demertzi A et al. Sci Adv. 2019 Feb 6. doi: 10.1126/sciadv.aat7603.
Functional MRI can measure patterns of connectivity to determine levels of consciousness in nonresponsive patients with brain injury, according to results from a multicenter, cross-sectional, observational study.

Blood oxygen level–dependent (BOLD) fMRI showed that brain-wide coordination patterns of high complexity became increasingly common moving from unresponsive patients to those with minimal consciousness to healthy individuals, reported lead author Athena Demertzi, PhD, of GIGA Research Institute at the University of Liège in Belgium, and her colleagues.
“Finding reliable markers indicating the presence or absence of consciousness represents an outstanding open problem for science,” the investigators wrote in Science Advances.
In medicine, an fMRI-based measure of consciousness could supplement behavioral assessments of awareness and guide therapeutic strategies; more broadly, image-based markers could help elucidate the nature of consciousness itself.
“We postulate that consciousness has specific characteristics that are based on the temporal dynamics of ongoing brain activity and its coordination over distant cortical regions,” the investigators wrote. “Our hypothesis stems from the common stance of various contemporary theories which propose that consciousness relates to a dynamic process of self-sustained, coordinated brain-scale activity assisting the tuning to a constantly evolving environment, rather than in static descriptions of brain function.”
There is a need for a reliable way of distinguishing consciousness from unconscious states, the investigators said. “Given that nonresponsiveness can be associated with a variety of brain lesions, varying levels of vigilance, and covert cognition, we highlight the need to determine a common set of features capable of accounting for the capacity to sustain conscious experience.”
To search for patterns of brain signal coordination that correlate with consciousness, four independent research centers performed BOLD fMRI scans of participants at rest or under anesthesia with propofol. Of 159 total participants, 47 were healthy individuals and 112 were patients in a vegetative state/with unresponsive wakefulness syndrome (UWS) or in a minimally conscious state (MCS), based on standardized behavioral assessments. The main data analysis, which included 125 participants, assessed BOLD fMRI signal coordination between six brain networks known to have roles in cognitive and functional processes.
The researchers’ analysis revealed four distinct and recurring brain-wide coordination patterns ranging on a scale from highest activity (pattern 1) to lowest activity (pattern 4). Pattern 1, which exhibited most long-distance edges, spatial complexity, efficiency, and community structure, became increasingly common when moving from UWS patients to MCS patients to healthy control individuals (UWS < MCS < HC, rho = 0.7, Spearman rank correlation between rate and group, P less than 1 x 10-16).
In contrast, pattern 4, characterized by low interareal coordination, showed an inverse trend; it became less common when moving from vegetative patients to healthy individuals (UWS > MCS > HC, Spearman rank correlation between rate and group, rho = –0.6, P less than 1 x 10-11). Although patterns 2 and 3 occurred with equal frequency across all groups, the investigators noted that switching between patterns was most common and predictably sequential in healthy individuals, versus patients with UWS, who were least likely to switch patterns. A total of 23 patients who were scanned under propofol anesthesia were equally likely to exhibit pattern 4, regardless of health status, suggesting that pattern 4 depends upon fixed anatomical pathways. Results were not affected by scanning site or other patient characteristics, such as age, gender, etiology, or chronicity.
“We conclude that these patterns of transient brain signal coordination are characteristic of conscious and unconscious brain states,” the investigators wrote, “warranting future research concerning their relationship to ongoing conscious content, and the possibility of modifying their prevalence by external perturbations, both in healthy and pathological individuals, as well as across species.”
The study was funded by a James S. McDonnell Foundation Collaborative Activity Award, INSERM, the Belgian National Funds for Scientific Research, the Canada Excellence Research Chairs program, and others. The authors declared having no conflicts of interest.
SOURCE: Demertzi A et al. Sci Adv. 2019 Feb 6. doi: 10.1126/sciadv.aat7603.
FROM SCIENCE ADVANCES
Key clinical point:
Major finding: A brain-wide coordination pattern of high complexity became increasingly common when moving from patients with unresponsive wakefulness syndrome (UWS) to patients in a minimally conscious state (MCS) to healthy control individuals.
Study details: A study involving blood oxygen level–dependent (BOLD) fMRI scans at rest or under anesthesia in 159 participants at four independent research facilities.
Disclosures: The study was funded by a James S. McDonnell Foundation Collaborative Activity Award, INSERM, the Belgian National Funds for Scientific Research, the Canada Excellence Research Chairs program, and others. The authors declared having no conflicts of interest.
Source: Demertzi A et al. Sci Adv. 2019 Feb 6. doi: 10.1126/sciadv.aat7603.
Mild aerobic exercise speeds sports concussion recovery
Mild aerobic exercise significantly shortened recovery time from sports-related concussion in adolescent athletes, compared with a stretching program in a randomized trial of 103 participants.
Sports-related concussion (SRC) remains a major public health problem with no effective treatment, wrote John J. Leddy, MD, of the State University of New York at Buffalo, and his colleagues.
Exercise tolerance after SRC has not been well studied. However, given the demonstrated benefits of aerobic exercise training on autonomic nervous system regulation, cerebral blood flow regulation, cardiovascular physiology, and brain neuroplasticity, the researchers hypothesized that exercise at a level that does not exacerbate symptoms might facilitate recovery in concussion patients.
In a study published in JAMA Pediatrics, the researchers randomized 103 adolescent athletes aged 13-18 years to a program of subsymptom aerobic exercise or a placebo stretching program. The participants were enrolled in the study within 10 days of an SRC, and were followed for 30 days or until recovery.
Athletes in the aerobic exercise group recovered in a median of 13 days, compared with 17 days for those in the stretching group (P = .009). Recovery was defined as “symptom resolution to normal,” based on normal physical and neurological examinations, “further confirmed by demonstration of the ability to exercise to exhaustion without exacerbation of symptoms” according to the Buffalo Concussion Treadmill Test, the researchers wrote.
No demographic differences or difference in previous concussions, time from injury until treatment, initial symptom severity score, initial exercise treadmill test, or physical exam were noted between the groups.
The average age of the participants was 15 years, 47% were female. The athletes performed the aerobic exercise or stretching programs approximately 20 minutes per day, and reported their daily symptoms and compliance via a website. The aerobic exercise consisted of walking or jogging on a treadmill or outdoors, or riding a stationary bike while wearing a heart rate monitor to maintain a target heart rate. The target heart rate was calculated as 80% of the heart rate at symptom exacerbation during the Buffalo Concussion Treadmill Test at each participant’s initial visit.
No adverse events related to the exercise intervention were reported, which supports the safety of subsymptom threshhold exercise, in the study population, Dr. Leddy and his associates noted.
The researchers also found lower rates of persistent symptoms at 1 month in the exercise group, compared with the stretching group (two participants vs. seven participants), but this difference was not statistically significant.
The study findings were limited by several factors, including the unblinded design and failure to address the mechanism of action for the effects of exercise. In addition, the results are not generalizable to younger children or other demographic groups, including those with concussions from causes other than sports and adults with heart conditions, the researchers noted.
However, “the results of this study should give clinicians confidence that moderate levels of physical activity, including prescribed subsymptom threshold aerobic exercise, after the first 48 hours following SRC can safely and significantly speed recovery,” Dr. Leddy and his associates concluded.
The study was supported by grants from the National Institutes of Health. The researchers had no financial conflicts to disclose.
SOURCE: Leddy JJ et al. JAMA Pediatr. 2019 Feb 4. doi: 10.1001/jamapediatrics.2018.4397.
In 2009 and 2010, the culture of sports concussion care began to shift with the publication of an initial study by Leddy et al. on the use of exercise at subsymptom levels as part of concussion rehabilitation, Sara P. D. Chrisman, MD, MPH, wrote in an accompanying editorial. Previous guidelines had emphasized total avoidance of physical activity, as well as avoidance of screen time and social activity, until patients were asymptomatic; however, “no definition was provided for the term asymptomatic, and no time limits were placed on rest, and as a result, rest often continued for weeks or months,” Dr. Chrisman said. Additional research over the past decade supported the potential value of moderate exercise, and the 2016 meeting of the Concussion in Sport Group resulted in recommendations limiting rest to 24-48 hours, which prompted further studies of exercise intervention.
The current study by Leddy et al. is a clinical trial using exercise “to treat acute concussion with a goal of reducing symptom duration,” she said. Despite the study’s limitations, including the inability to estimate how much exercise was needed to achieve the treatment outcome, “this is a landmark study that may shift the standard of care toward the use of rehabilitative exercise to decrease the duration of concussion symptoms.
“Future studies will need to explore the limits of exercise treatment for concussion,” and should address questions including the timing, intensity, and duration of exercise and whether the strategy is appropriate for other populations, such as those with mental health comorbidities, Dr. Chrisman concluded.
Dr. Chrisman is at the Center for Child Health, Behavior, and Development, Seattle Children’s Research Institute. These comments are from her editorial accompanying the article by Leddy et al. (JAMA Pedatr. 2019 Feb 4. doi: 10.1001/jamapediatrics.2018.5281). She had no financial conflicts to disclose.
In 2009 and 2010, the culture of sports concussion care began to shift with the publication of an initial study by Leddy et al. on the use of exercise at subsymptom levels as part of concussion rehabilitation, Sara P. D. Chrisman, MD, MPH, wrote in an accompanying editorial. Previous guidelines had emphasized total avoidance of physical activity, as well as avoidance of screen time and social activity, until patients were asymptomatic; however, “no definition was provided for the term asymptomatic, and no time limits were placed on rest, and as a result, rest often continued for weeks or months,” Dr. Chrisman said. Additional research over the past decade supported the potential value of moderate exercise, and the 2016 meeting of the Concussion in Sport Group resulted in recommendations limiting rest to 24-48 hours, which prompted further studies of exercise intervention.
The current study by Leddy et al. is a clinical trial using exercise “to treat acute concussion with a goal of reducing symptom duration,” she said. Despite the study’s limitations, including the inability to estimate how much exercise was needed to achieve the treatment outcome, “this is a landmark study that may shift the standard of care toward the use of rehabilitative exercise to decrease the duration of concussion symptoms.
“Future studies will need to explore the limits of exercise treatment for concussion,” and should address questions including the timing, intensity, and duration of exercise and whether the strategy is appropriate for other populations, such as those with mental health comorbidities, Dr. Chrisman concluded.
Dr. Chrisman is at the Center for Child Health, Behavior, and Development, Seattle Children’s Research Institute. These comments are from her editorial accompanying the article by Leddy et al. (JAMA Pedatr. 2019 Feb 4. doi: 10.1001/jamapediatrics.2018.5281). She had no financial conflicts to disclose.
In 2009 and 2010, the culture of sports concussion care began to shift with the publication of an initial study by Leddy et al. on the use of exercise at subsymptom levels as part of concussion rehabilitation, Sara P. D. Chrisman, MD, MPH, wrote in an accompanying editorial. Previous guidelines had emphasized total avoidance of physical activity, as well as avoidance of screen time and social activity, until patients were asymptomatic; however, “no definition was provided for the term asymptomatic, and no time limits were placed on rest, and as a result, rest often continued for weeks or months,” Dr. Chrisman said. Additional research over the past decade supported the potential value of moderate exercise, and the 2016 meeting of the Concussion in Sport Group resulted in recommendations limiting rest to 24-48 hours, which prompted further studies of exercise intervention.
The current study by Leddy et al. is a clinical trial using exercise “to treat acute concussion with a goal of reducing symptom duration,” she said. Despite the study’s limitations, including the inability to estimate how much exercise was needed to achieve the treatment outcome, “this is a landmark study that may shift the standard of care toward the use of rehabilitative exercise to decrease the duration of concussion symptoms.
“Future studies will need to explore the limits of exercise treatment for concussion,” and should address questions including the timing, intensity, and duration of exercise and whether the strategy is appropriate for other populations, such as those with mental health comorbidities, Dr. Chrisman concluded.
Dr. Chrisman is at the Center for Child Health, Behavior, and Development, Seattle Children’s Research Institute. These comments are from her editorial accompanying the article by Leddy et al. (JAMA Pedatr. 2019 Feb 4. doi: 10.1001/jamapediatrics.2018.5281). She had no financial conflicts to disclose.
Mild aerobic exercise significantly shortened recovery time from sports-related concussion in adolescent athletes, compared with a stretching program in a randomized trial of 103 participants.
Sports-related concussion (SRC) remains a major public health problem with no effective treatment, wrote John J. Leddy, MD, of the State University of New York at Buffalo, and his colleagues.
Exercise tolerance after SRC has not been well studied. However, given the demonstrated benefits of aerobic exercise training on autonomic nervous system regulation, cerebral blood flow regulation, cardiovascular physiology, and brain neuroplasticity, the researchers hypothesized that exercise at a level that does not exacerbate symptoms might facilitate recovery in concussion patients.
In a study published in JAMA Pediatrics, the researchers randomized 103 adolescent athletes aged 13-18 years to a program of subsymptom aerobic exercise or a placebo stretching program. The participants were enrolled in the study within 10 days of an SRC, and were followed for 30 days or until recovery.
Athletes in the aerobic exercise group recovered in a median of 13 days, compared with 17 days for those in the stretching group (P = .009). Recovery was defined as “symptom resolution to normal,” based on normal physical and neurological examinations, “further confirmed by demonstration of the ability to exercise to exhaustion without exacerbation of symptoms” according to the Buffalo Concussion Treadmill Test, the researchers wrote.
No demographic differences or difference in previous concussions, time from injury until treatment, initial symptom severity score, initial exercise treadmill test, or physical exam were noted between the groups.
The average age of the participants was 15 years, 47% were female. The athletes performed the aerobic exercise or stretching programs approximately 20 minutes per day, and reported their daily symptoms and compliance via a website. The aerobic exercise consisted of walking or jogging on a treadmill or outdoors, or riding a stationary bike while wearing a heart rate monitor to maintain a target heart rate. The target heart rate was calculated as 80% of the heart rate at symptom exacerbation during the Buffalo Concussion Treadmill Test at each participant’s initial visit.
No adverse events related to the exercise intervention were reported, which supports the safety of subsymptom threshhold exercise, in the study population, Dr. Leddy and his associates noted.
The researchers also found lower rates of persistent symptoms at 1 month in the exercise group, compared with the stretching group (two participants vs. seven participants), but this difference was not statistically significant.
The study findings were limited by several factors, including the unblinded design and failure to address the mechanism of action for the effects of exercise. In addition, the results are not generalizable to younger children or other demographic groups, including those with concussions from causes other than sports and adults with heart conditions, the researchers noted.
However, “the results of this study should give clinicians confidence that moderate levels of physical activity, including prescribed subsymptom threshold aerobic exercise, after the first 48 hours following SRC can safely and significantly speed recovery,” Dr. Leddy and his associates concluded.
The study was supported by grants from the National Institutes of Health. The researchers had no financial conflicts to disclose.
SOURCE: Leddy JJ et al. JAMA Pediatr. 2019 Feb 4. doi: 10.1001/jamapediatrics.2018.4397.
Mild aerobic exercise significantly shortened recovery time from sports-related concussion in adolescent athletes, compared with a stretching program in a randomized trial of 103 participants.
Sports-related concussion (SRC) remains a major public health problem with no effective treatment, wrote John J. Leddy, MD, of the State University of New York at Buffalo, and his colleagues.
Exercise tolerance after SRC has not been well studied. However, given the demonstrated benefits of aerobic exercise training on autonomic nervous system regulation, cerebral blood flow regulation, cardiovascular physiology, and brain neuroplasticity, the researchers hypothesized that exercise at a level that does not exacerbate symptoms might facilitate recovery in concussion patients.
In a study published in JAMA Pediatrics, the researchers randomized 103 adolescent athletes aged 13-18 years to a program of subsymptom aerobic exercise or a placebo stretching program. The participants were enrolled in the study within 10 days of an SRC, and were followed for 30 days or until recovery.
Athletes in the aerobic exercise group recovered in a median of 13 days, compared with 17 days for those in the stretching group (P = .009). Recovery was defined as “symptom resolution to normal,” based on normal physical and neurological examinations, “further confirmed by demonstration of the ability to exercise to exhaustion without exacerbation of symptoms” according to the Buffalo Concussion Treadmill Test, the researchers wrote.
No demographic differences or difference in previous concussions, time from injury until treatment, initial symptom severity score, initial exercise treadmill test, or physical exam were noted between the groups.
The average age of the participants was 15 years, 47% were female. The athletes performed the aerobic exercise or stretching programs approximately 20 minutes per day, and reported their daily symptoms and compliance via a website. The aerobic exercise consisted of walking or jogging on a treadmill or outdoors, or riding a stationary bike while wearing a heart rate monitor to maintain a target heart rate. The target heart rate was calculated as 80% of the heart rate at symptom exacerbation during the Buffalo Concussion Treadmill Test at each participant’s initial visit.
No adverse events related to the exercise intervention were reported, which supports the safety of subsymptom threshhold exercise, in the study population, Dr. Leddy and his associates noted.
The researchers also found lower rates of persistent symptoms at 1 month in the exercise group, compared with the stretching group (two participants vs. seven participants), but this difference was not statistically significant.
The study findings were limited by several factors, including the unblinded design and failure to address the mechanism of action for the effects of exercise. In addition, the results are not generalizable to younger children or other demographic groups, including those with concussions from causes other than sports and adults with heart conditions, the researchers noted.
However, “the results of this study should give clinicians confidence that moderate levels of physical activity, including prescribed subsymptom threshold aerobic exercise, after the first 48 hours following SRC can safely and significantly speed recovery,” Dr. Leddy and his associates concluded.
The study was supported by grants from the National Institutes of Health. The researchers had no financial conflicts to disclose.
SOURCE: Leddy JJ et al. JAMA Pediatr. 2019 Feb 4. doi: 10.1001/jamapediatrics.2018.4397.
FROM JAMA PEDIATRICS
Key clinical point:
Major finding: Teen athletes who performed aerobic exercise recovered from sports-related concussions in 13 days, compared with 17 days for those in a placebo-stretching group.
Study details: The data come from a randomized trial of 103 athletes aged 13-18 years.
Disclosures: The study was supported by grants from the National Institutes of Health. The researchers had no financial conflicts to disclose.
Source: Leddy JJ et al. JAMA Pediatr. 2019 Feb 4. doi: 10.1001/jamapediatrics.2018.4397.
Race/ethnicity, other factors predict PTSD and depression after mild TBI
Civilian patients with mild traumatic brain injury (TBI) who are black, have psychiatric history or lower education, or whose injury was caused by assault might be at greater risk of developing posttraumatic stress disorder or major depression, a longitudinal study suggests.
“Our findings may have implications for surveillance and treatment of mental disorders after TBI,” wrote Murray B. Stein, MD, MPH, and his associates. The study was published Jan. 30 in JAMA Psychiatry.
The researchers looked at the risk factors for and prevalence of posttraumatic stress disorder (PTSD) and major depressive disorder among 1,155 patients. The patients were enrolled at 11 level 1 trauma centers across the United States after they were evaluated for mild TBI in emergency departments as part of a prospective study called Transforming Research and Clinical Knowledge in Traumatic Brain Injury, or TRACK-TBI. The comparison group was 230 patients with nonhead orthopedic trauma injuries, wrote Dr. Stein, distinguished professor of psychiatry and family medicine and public health at the University of California, San Diego, and his associates.
They found that each additional year of education was associated with a significant 11% reduction in the risk of developing PTSD after mild TBI (P = .005). Also, black patients had a greater than fivefold higher risk of PTSD (P less than.001) than that of individuals who were not black.
Among patients with a history of mental illness and those who had experienced their injury as a result of assault or violence – as opposed to a motor vehicle accident or fall, for example – both had a greater than threefold higher risk of developing PTSD (odds ratio, 3.57 and 3.43 respectively). A prior TBI was nonsignificantly associated with an increased risk of developing PTSD.
Lower education duration, being black, or a history of mental illness also were all significantly associated with an increased risk of developing major depressive disorder after mild TBI.
However, duration of lost consciousness or posttraumatic amnesia, evidence of brain injury on CT, or hospitalization did not predict an increased risk of PTSD or major depression.
“Although MDD and PTSD are prevalent after TBI, little is known about which patients are at risk for developing them,” Dr. Stein and his associates wrote.
Noting that having a prior mental health problem was an “exceptionally strong” risk factor for PTSD and MDD after TBI, the authors said this could represent continuation or exacerbation of the prior mental health issue, or the triggering of a new episode in a person with a past history who had recovered.
“However, in either case this finding underscores the importance of clinicians being aware of the mental health history of their patients with [mild TBI], as this information is central to expectations regarding both short-term and long-term outcome,” they wrote.
Dr. Stein and his associates cited as a limitation their reliance on patient or family report. In addition, they said, the elevated risk for mental disorders among black individuals after mild TBI, which was independent of socioeconomic status or cause of injury, was not understood. “Unmeasured covariates may be part of the explanation; this is a topic needing further study,” they wrote.
The study was supported by the National Institutes of Health, the U.S. Department of Defense, Abbott Laboratories, and One Mind. Four authors declared consultancies, advisory board positions, speaking fees, and shares or stock options with the pharmaceutical and private industry. Two authors declared grants from the study sponsors.
SOURCE: Stein MB et al. JAMA Psychiatry. 2019. Jan 30. doi: 10.1001/jamapsychiatry.2018.4288.
Civilian patients with mild traumatic brain injury (TBI) who are black, have psychiatric history or lower education, or whose injury was caused by assault might be at greater risk of developing posttraumatic stress disorder or major depression, a longitudinal study suggests.
“Our findings may have implications for surveillance and treatment of mental disorders after TBI,” wrote Murray B. Stein, MD, MPH, and his associates. The study was published Jan. 30 in JAMA Psychiatry.
The researchers looked at the risk factors for and prevalence of posttraumatic stress disorder (PTSD) and major depressive disorder among 1,155 patients. The patients were enrolled at 11 level 1 trauma centers across the United States after they were evaluated for mild TBI in emergency departments as part of a prospective study called Transforming Research and Clinical Knowledge in Traumatic Brain Injury, or TRACK-TBI. The comparison group was 230 patients with nonhead orthopedic trauma injuries, wrote Dr. Stein, distinguished professor of psychiatry and family medicine and public health at the University of California, San Diego, and his associates.
They found that each additional year of education was associated with a significant 11% reduction in the risk of developing PTSD after mild TBI (P = .005). Also, black patients had a greater than fivefold higher risk of PTSD (P less than.001) than that of individuals who were not black.
Among patients with a history of mental illness and those who had experienced their injury as a result of assault or violence – as opposed to a motor vehicle accident or fall, for example – both had a greater than threefold higher risk of developing PTSD (odds ratio, 3.57 and 3.43 respectively). A prior TBI was nonsignificantly associated with an increased risk of developing PTSD.
Lower education duration, being black, or a history of mental illness also were all significantly associated with an increased risk of developing major depressive disorder after mild TBI.
However, duration of lost consciousness or posttraumatic amnesia, evidence of brain injury on CT, or hospitalization did not predict an increased risk of PTSD or major depression.
“Although MDD and PTSD are prevalent after TBI, little is known about which patients are at risk for developing them,” Dr. Stein and his associates wrote.
Noting that having a prior mental health problem was an “exceptionally strong” risk factor for PTSD and MDD after TBI, the authors said this could represent continuation or exacerbation of the prior mental health issue, or the triggering of a new episode in a person with a past history who had recovered.
“However, in either case this finding underscores the importance of clinicians being aware of the mental health history of their patients with [mild TBI], as this information is central to expectations regarding both short-term and long-term outcome,” they wrote.
Dr. Stein and his associates cited as a limitation their reliance on patient or family report. In addition, they said, the elevated risk for mental disorders among black individuals after mild TBI, which was independent of socioeconomic status or cause of injury, was not understood. “Unmeasured covariates may be part of the explanation; this is a topic needing further study,” they wrote.
The study was supported by the National Institutes of Health, the U.S. Department of Defense, Abbott Laboratories, and One Mind. Four authors declared consultancies, advisory board positions, speaking fees, and shares or stock options with the pharmaceutical and private industry. Two authors declared grants from the study sponsors.
SOURCE: Stein MB et al. JAMA Psychiatry. 2019. Jan 30. doi: 10.1001/jamapsychiatry.2018.4288.
Civilian patients with mild traumatic brain injury (TBI) who are black, have psychiatric history or lower education, or whose injury was caused by assault might be at greater risk of developing posttraumatic stress disorder or major depression, a longitudinal study suggests.
“Our findings may have implications for surveillance and treatment of mental disorders after TBI,” wrote Murray B. Stein, MD, MPH, and his associates. The study was published Jan. 30 in JAMA Psychiatry.
The researchers looked at the risk factors for and prevalence of posttraumatic stress disorder (PTSD) and major depressive disorder among 1,155 patients. The patients were enrolled at 11 level 1 trauma centers across the United States after they were evaluated for mild TBI in emergency departments as part of a prospective study called Transforming Research and Clinical Knowledge in Traumatic Brain Injury, or TRACK-TBI. The comparison group was 230 patients with nonhead orthopedic trauma injuries, wrote Dr. Stein, distinguished professor of psychiatry and family medicine and public health at the University of California, San Diego, and his associates.
They found that each additional year of education was associated with a significant 11% reduction in the risk of developing PTSD after mild TBI (P = .005). Also, black patients had a greater than fivefold higher risk of PTSD (P less than.001) than that of individuals who were not black.
Among patients with a history of mental illness and those who had experienced their injury as a result of assault or violence – as opposed to a motor vehicle accident or fall, for example – both had a greater than threefold higher risk of developing PTSD (odds ratio, 3.57 and 3.43 respectively). A prior TBI was nonsignificantly associated with an increased risk of developing PTSD.
Lower education duration, being black, or a history of mental illness also were all significantly associated with an increased risk of developing major depressive disorder after mild TBI.
However, duration of lost consciousness or posttraumatic amnesia, evidence of brain injury on CT, or hospitalization did not predict an increased risk of PTSD or major depression.
“Although MDD and PTSD are prevalent after TBI, little is known about which patients are at risk for developing them,” Dr. Stein and his associates wrote.
Noting that having a prior mental health problem was an “exceptionally strong” risk factor for PTSD and MDD after TBI, the authors said this could represent continuation or exacerbation of the prior mental health issue, or the triggering of a new episode in a person with a past history who had recovered.
“However, in either case this finding underscores the importance of clinicians being aware of the mental health history of their patients with [mild TBI], as this information is central to expectations regarding both short-term and long-term outcome,” they wrote.
Dr. Stein and his associates cited as a limitation their reliance on patient or family report. In addition, they said, the elevated risk for mental disorders among black individuals after mild TBI, which was independent of socioeconomic status or cause of injury, was not understood. “Unmeasured covariates may be part of the explanation; this is a topic needing further study,” they wrote.
The study was supported by the National Institutes of Health, the U.S. Department of Defense, Abbott Laboratories, and One Mind. Four authors declared consultancies, advisory board positions, speaking fees, and shares or stock options with the pharmaceutical and private industry. Two authors declared grants from the study sponsors.
SOURCE: Stein MB et al. JAMA Psychiatry. 2019. Jan 30. doi: 10.1001/jamapsychiatry.2018.4288.
FROM JAMA PSYCHIATRY
Key clinical point: The findings underscore “the importance of clinicians being aware of the mental health history of their patients with [mild TBI], as this information is central to expectations regarding both short-term and long-term outcome.”
Major finding: Black patients have fivefold higher risk of PTSD after brain injury.
Study details: Longitudinal cohort study of 1,155 patients with mild traumatic brain injury.
Disclosures: The study was supported by the National Institutes of Health, the U.S. Department of Defense, Abbott Laboratories, and One Mind. Four authors declared consultancies, advisory board positions, and speaking fees, shares, or stock options with the pharmaceutical and private industry. Two authors declared grants from the study sponsors.
Source: Stein MB et al. JAMA Psychiatry 2019. Jan 30. doi: 10.1001/jamapsychiatry.2018.4288.
Survey Identifies Variations in Management of Pediatric Posttraumatic Headache
Findings highlight a need to establish best evidence-based practices, researchers say.
CHICAGO—Child neurologists differ in their approach to diagnosing and managing posttraumatic headache, according to survey results presented at the 47th Annual Meeting of the Child Neurology Society.
For example, practice differs as to when posttraumatic headaches are considered persistent and when to recommend preventive therapy.
“As there are no established guidelines on management of posttraumatic headache, it is not surprising that diagnosis and management vary considerably,” said Rachel Pearson, MD, a child neurology resident at Children’s Hospital of Orange County in Orange, California, and colleagues. “Further studies are needed to define the best evidence-based practices for pediatric posttraumatic headache.”
Research indicates that about 7% of children ages 3 to 17 experience a significant head injury, and headache is the most common postconcussive symptom. Headache persists at three months in as much as 43% of cases, according to current studies. To better understand the current clinical practices of child neurologists in the diagnosis and treatment of posttraumatic headache, Dr. Pearson and colleagues sent all active, nonresident members of the Child Neurology Society a link to an online survey. The survey covered diagnosis, management, and return-to-play guidelines. Ninety-five members responded to the survey.
Persistence Threshold: Four Weeks or Three Months?
Although 39% of respondents reported that they always use ICHD diagnostic criteria to diagnose posttraumatic headache, and 31% sometimes use ICHD criteria, “only 19% of respondents correctly defined persistent posttraumatic headache per ICHD diagnostic criteria” as lasting more than three months, the researchers said. “The largest number of participants considered posttraumatic headache to be persistent at four weeks,” they said. “This may have implications for when prophylactic headache medications are considered.”
More than 90% recommend NSAIDs as abortive therapy. One-third consider starting preventive headache therapy within one month, and one-third between one and two months.
The most commonly used preventive medications are amitriptyline and nortriptyline (93.7%) and topiramate (71.6%). Amitriptyline and nortriptyline may be widely used because they “can also address other postconcussive symptoms, such as sleep or mood disturbance,” the investigators noted.
Treatment Options
In addition, 59% of providers use vitamins and supplements (eg, magnesium, riboflavin, melatonin, and CoQ10) as preventive treatments. “These are considered generally safe and have few adverse effects,” and “families may prefer these treatment options as they are perceived as ‘natural,’” Dr. Pearson and colleagues said. More than half of respondents use nonmedicinal therapies such as physical therapy, pain-focused cognitive behavioral therapy, and biofeedback.
Thirty-eight percent use injection-based therapies (eg, nerve blocks, botulinum toxin, and trigger point injections), and 14% of providers administer injections themselves.
One-third of respondents recommend cognitive and physical rest for one to three days, followed by a progressive return to activities, consistent with evidence-based recommendations. Approximately one-third advise patients to rest for seven to 14 days before returning to play.
“As a whole, these findings can guide additional research in this area and serve as a platform on which to base future randomized controlled trials,” said Dr. Pearson and colleagues.
—Jake Remaly
Suggested Reading
Blume HK, Vavilala MS, Jaffe KM, et al. Headache after pediatric traumatic brain injury: a cohort study. Pediatrics. 2012;129(1):e31-e39.
Blume HK. Headaches after concussion in pediatrics: a review. Curr Pain Headache Rep. 2015;19(9):42.
Choe MC, Blume HK. Pediatric posttraumatic headache: a review. J Child Neurol. 2016;31(1): 76-85.
Findings highlight a need to establish best evidence-based practices, researchers say.
Findings highlight a need to establish best evidence-based practices, researchers say.
CHICAGO—Child neurologists differ in their approach to diagnosing and managing posttraumatic headache, according to survey results presented at the 47th Annual Meeting of the Child Neurology Society.
For example, practice differs as to when posttraumatic headaches are considered persistent and when to recommend preventive therapy.
“As there are no established guidelines on management of posttraumatic headache, it is not surprising that diagnosis and management vary considerably,” said Rachel Pearson, MD, a child neurology resident at Children’s Hospital of Orange County in Orange, California, and colleagues. “Further studies are needed to define the best evidence-based practices for pediatric posttraumatic headache.”
Research indicates that about 7% of children ages 3 to 17 experience a significant head injury, and headache is the most common postconcussive symptom. Headache persists at three months in as much as 43% of cases, according to current studies. To better understand the current clinical practices of child neurologists in the diagnosis and treatment of posttraumatic headache, Dr. Pearson and colleagues sent all active, nonresident members of the Child Neurology Society a link to an online survey. The survey covered diagnosis, management, and return-to-play guidelines. Ninety-five members responded to the survey.
Persistence Threshold: Four Weeks or Three Months?
Although 39% of respondents reported that they always use ICHD diagnostic criteria to diagnose posttraumatic headache, and 31% sometimes use ICHD criteria, “only 19% of respondents correctly defined persistent posttraumatic headache per ICHD diagnostic criteria” as lasting more than three months, the researchers said. “The largest number of participants considered posttraumatic headache to be persistent at four weeks,” they said. “This may have implications for when prophylactic headache medications are considered.”
More than 90% recommend NSAIDs as abortive therapy. One-third consider starting preventive headache therapy within one month, and one-third between one and two months.
The most commonly used preventive medications are amitriptyline and nortriptyline (93.7%) and topiramate (71.6%). Amitriptyline and nortriptyline may be widely used because they “can also address other postconcussive symptoms, such as sleep or mood disturbance,” the investigators noted.
Treatment Options
In addition, 59% of providers use vitamins and supplements (eg, magnesium, riboflavin, melatonin, and CoQ10) as preventive treatments. “These are considered generally safe and have few adverse effects,” and “families may prefer these treatment options as they are perceived as ‘natural,’” Dr. Pearson and colleagues said. More than half of respondents use nonmedicinal therapies such as physical therapy, pain-focused cognitive behavioral therapy, and biofeedback.
Thirty-eight percent use injection-based therapies (eg, nerve blocks, botulinum toxin, and trigger point injections), and 14% of providers administer injections themselves.
One-third of respondents recommend cognitive and physical rest for one to three days, followed by a progressive return to activities, consistent with evidence-based recommendations. Approximately one-third advise patients to rest for seven to 14 days before returning to play.
“As a whole, these findings can guide additional research in this area and serve as a platform on which to base future randomized controlled trials,” said Dr. Pearson and colleagues.
—Jake Remaly
Suggested Reading
Blume HK, Vavilala MS, Jaffe KM, et al. Headache after pediatric traumatic brain injury: a cohort study. Pediatrics. 2012;129(1):e31-e39.
Blume HK. Headaches after concussion in pediatrics: a review. Curr Pain Headache Rep. 2015;19(9):42.
Choe MC, Blume HK. Pediatric posttraumatic headache: a review. J Child Neurol. 2016;31(1): 76-85.
CHICAGO—Child neurologists differ in their approach to diagnosing and managing posttraumatic headache, according to survey results presented at the 47th Annual Meeting of the Child Neurology Society.
For example, practice differs as to when posttraumatic headaches are considered persistent and when to recommend preventive therapy.
“As there are no established guidelines on management of posttraumatic headache, it is not surprising that diagnosis and management vary considerably,” said Rachel Pearson, MD, a child neurology resident at Children’s Hospital of Orange County in Orange, California, and colleagues. “Further studies are needed to define the best evidence-based practices for pediatric posttraumatic headache.”
Research indicates that about 7% of children ages 3 to 17 experience a significant head injury, and headache is the most common postconcussive symptom. Headache persists at three months in as much as 43% of cases, according to current studies. To better understand the current clinical practices of child neurologists in the diagnosis and treatment of posttraumatic headache, Dr. Pearson and colleagues sent all active, nonresident members of the Child Neurology Society a link to an online survey. The survey covered diagnosis, management, and return-to-play guidelines. Ninety-five members responded to the survey.
Persistence Threshold: Four Weeks or Three Months?
Although 39% of respondents reported that they always use ICHD diagnostic criteria to diagnose posttraumatic headache, and 31% sometimes use ICHD criteria, “only 19% of respondents correctly defined persistent posttraumatic headache per ICHD diagnostic criteria” as lasting more than three months, the researchers said. “The largest number of participants considered posttraumatic headache to be persistent at four weeks,” they said. “This may have implications for when prophylactic headache medications are considered.”
More than 90% recommend NSAIDs as abortive therapy. One-third consider starting preventive headache therapy within one month, and one-third between one and two months.
The most commonly used preventive medications are amitriptyline and nortriptyline (93.7%) and topiramate (71.6%). Amitriptyline and nortriptyline may be widely used because they “can also address other postconcussive symptoms, such as sleep or mood disturbance,” the investigators noted.
Treatment Options
In addition, 59% of providers use vitamins and supplements (eg, magnesium, riboflavin, melatonin, and CoQ10) as preventive treatments. “These are considered generally safe and have few adverse effects,” and “families may prefer these treatment options as they are perceived as ‘natural,’” Dr. Pearson and colleagues said. More than half of respondents use nonmedicinal therapies such as physical therapy, pain-focused cognitive behavioral therapy, and biofeedback.
Thirty-eight percent use injection-based therapies (eg, nerve blocks, botulinum toxin, and trigger point injections), and 14% of providers administer injections themselves.
One-third of respondents recommend cognitive and physical rest for one to three days, followed by a progressive return to activities, consistent with evidence-based recommendations. Approximately one-third advise patients to rest for seven to 14 days before returning to play.
“As a whole, these findings can guide additional research in this area and serve as a platform on which to base future randomized controlled trials,” said Dr. Pearson and colleagues.
—Jake Remaly
Suggested Reading
Blume HK, Vavilala MS, Jaffe KM, et al. Headache after pediatric traumatic brain injury: a cohort study. Pediatrics. 2012;129(1):e31-e39.
Blume HK. Headaches after concussion in pediatrics: a review. Curr Pain Headache Rep. 2015;19(9):42.
Choe MC, Blume HK. Pediatric posttraumatic headache: a review. J Child Neurol. 2016;31(1): 76-85.