User login
‘Father of hematopoietic cytokines’ dies

Photo courtesy of The Walter
and Eliza Hall Institute
of Medical Research
Donald Metcalf, MD, an Australian researcher who has been called “the father of hematopoietic cytokines,” has died at the age of 85.
Dr Metcalf’s studies of blood production led to his speculation that there must be a biological mechanism—one or more hormones—that control white blood cell production.
These substances, which he termed colony-stimulating factors (CSFs), were the focus of more than 50 years of research.
Over this time, Dr Metcalf led researchers to characterize and purify 4 separate CSFs—granulocyte CSF (G-CSF), granulocyte-macrophage CSF (GM-CSF), macrophage CSF (M-CSF), and multi-CSF (now called interleukin-3).
Dr Metcalf recognized that CSFs had a potential role in clinical medicine, and his team was among the first in the world to discover the genes for CSFs.
Dr Metcalf was a central figure in the international clinical trials of CSFs in the 1980s, assessing whether CSFs could boost immune cell numbers in cancer patients whose immune system was weakened as a side effect of chemotherapy. On the basis of these studies, G-CSF (Neupogen) was approved for clinical use in 1991.
Now, an estimated 20 million people have been treated with CSFs. As well as boosting the immune system in patients who receive chemotherapy or have other immune deficiencies, CSFs are thought to have revolutionized hematopoietic stem cell transplantation.
A man with many achievements
Dr Metcalf was born in 1929 and started school at the age of 3, by which time he was already reading. He entered university at the age of 16, ultimately obtaining bachelor’s and medical degrees from the University of Sydney.
After an internship at the Royal Prince Alfred Hospital in Sydney, Dr Metcalf joined the staff of the Walter and Eliza Hall Institute of Medical Research (WEHI) in Melbourne in 1954. He was supported by Cancer Council Victoria’s Carden Fellowship, an award he held until his retirement in 2014. (Dr Metcalf officially retired in 1996 but continued to do research until 2014.)
Dr Metcalf spent his early years at WEHI studying vaccinia virus. In 1965, he began studying blood cell formation and, by association, leukemia. In 1966, he became deputy director of WEHI and the head of its Cancer Research Unit.
Dr Metcalf took several sabbaticals from WEHI, serving as a visiting scientist at Harvard Medical School in Boston, Massachusetts; the Roswell Park Memorial Institute in Buffalo, New York; the Swiss Institute for Experimental Cancer Research in Lausanne, Switzerland; the Radiobiological Institute in Rijswijk, The Netherlands; and the University of Cambridge in the UK.
Among Dr Metcalf’s many honors and awards are the Companion of the Order of Australia (1993), the Albert Lasker Award for Clinical Medical Research (1993), the Gairdner Foundation International Award (1994), the Royal Medal of the Royal Society (1995), the Victoria Prize (2000), and the Prime Minister’s Prize for Science (2001).
Dr Metcalf is survived by his wife Jo; daughters Kate, Johanna, Penelope, and Mary-Ann; grandchildren James, Martin, Patrick, Elizabeth, Rose, and Robert; and their extended families. ![]()

Photo courtesy of The Walter
and Eliza Hall Institute
of Medical Research
Donald Metcalf, MD, an Australian researcher who has been called “the father of hematopoietic cytokines,” has died at the age of 85.
Dr Metcalf’s studies of blood production led to his speculation that there must be a biological mechanism—one or more hormones—that control white blood cell production.
These substances, which he termed colony-stimulating factors (CSFs), were the focus of more than 50 years of research.
Over this time, Dr Metcalf led researchers to characterize and purify 4 separate CSFs—granulocyte CSF (G-CSF), granulocyte-macrophage CSF (GM-CSF), macrophage CSF (M-CSF), and multi-CSF (now called interleukin-3).
Dr Metcalf recognized that CSFs had a potential role in clinical medicine, and his team was among the first in the world to discover the genes for CSFs.
Dr Metcalf was a central figure in the international clinical trials of CSFs in the 1980s, assessing whether CSFs could boost immune cell numbers in cancer patients whose immune system was weakened as a side effect of chemotherapy. On the basis of these studies, G-CSF (Neupogen) was approved for clinical use in 1991.
Now, an estimated 20 million people have been treated with CSFs. As well as boosting the immune system in patients who receive chemotherapy or have other immune deficiencies, CSFs are thought to have revolutionized hematopoietic stem cell transplantation.
A man with many achievements
Dr Metcalf was born in 1929 and started school at the age of 3, by which time he was already reading. He entered university at the age of 16, ultimately obtaining bachelor’s and medical degrees from the University of Sydney.
After an internship at the Royal Prince Alfred Hospital in Sydney, Dr Metcalf joined the staff of the Walter and Eliza Hall Institute of Medical Research (WEHI) in Melbourne in 1954. He was supported by Cancer Council Victoria’s Carden Fellowship, an award he held until his retirement in 2014. (Dr Metcalf officially retired in 1996 but continued to do research until 2014.)
Dr Metcalf spent his early years at WEHI studying vaccinia virus. In 1965, he began studying blood cell formation and, by association, leukemia. In 1966, he became deputy director of WEHI and the head of its Cancer Research Unit.
Dr Metcalf took several sabbaticals from WEHI, serving as a visiting scientist at Harvard Medical School in Boston, Massachusetts; the Roswell Park Memorial Institute in Buffalo, New York; the Swiss Institute for Experimental Cancer Research in Lausanne, Switzerland; the Radiobiological Institute in Rijswijk, The Netherlands; and the University of Cambridge in the UK.
Among Dr Metcalf’s many honors and awards are the Companion of the Order of Australia (1993), the Albert Lasker Award for Clinical Medical Research (1993), the Gairdner Foundation International Award (1994), the Royal Medal of the Royal Society (1995), the Victoria Prize (2000), and the Prime Minister’s Prize for Science (2001).
Dr Metcalf is survived by his wife Jo; daughters Kate, Johanna, Penelope, and Mary-Ann; grandchildren James, Martin, Patrick, Elizabeth, Rose, and Robert; and their extended families. ![]()

Photo courtesy of The Walter
and Eliza Hall Institute
of Medical Research
Donald Metcalf, MD, an Australian researcher who has been called “the father of hematopoietic cytokines,” has died at the age of 85.
Dr Metcalf’s studies of blood production led to his speculation that there must be a biological mechanism—one or more hormones—that control white blood cell production.
These substances, which he termed colony-stimulating factors (CSFs), were the focus of more than 50 years of research.
Over this time, Dr Metcalf led researchers to characterize and purify 4 separate CSFs—granulocyte CSF (G-CSF), granulocyte-macrophage CSF (GM-CSF), macrophage CSF (M-CSF), and multi-CSF (now called interleukin-3).
Dr Metcalf recognized that CSFs had a potential role in clinical medicine, and his team was among the first in the world to discover the genes for CSFs.
Dr Metcalf was a central figure in the international clinical trials of CSFs in the 1980s, assessing whether CSFs could boost immune cell numbers in cancer patients whose immune system was weakened as a side effect of chemotherapy. On the basis of these studies, G-CSF (Neupogen) was approved for clinical use in 1991.
Now, an estimated 20 million people have been treated with CSFs. As well as boosting the immune system in patients who receive chemotherapy or have other immune deficiencies, CSFs are thought to have revolutionized hematopoietic stem cell transplantation.
A man with many achievements
Dr Metcalf was born in 1929 and started school at the age of 3, by which time he was already reading. He entered university at the age of 16, ultimately obtaining bachelor’s and medical degrees from the University of Sydney.
After an internship at the Royal Prince Alfred Hospital in Sydney, Dr Metcalf joined the staff of the Walter and Eliza Hall Institute of Medical Research (WEHI) in Melbourne in 1954. He was supported by Cancer Council Victoria’s Carden Fellowship, an award he held until his retirement in 2014. (Dr Metcalf officially retired in 1996 but continued to do research until 2014.)
Dr Metcalf spent his early years at WEHI studying vaccinia virus. In 1965, he began studying blood cell formation and, by association, leukemia. In 1966, he became deputy director of WEHI and the head of its Cancer Research Unit.
Dr Metcalf took several sabbaticals from WEHI, serving as a visiting scientist at Harvard Medical School in Boston, Massachusetts; the Roswell Park Memorial Institute in Buffalo, New York; the Swiss Institute for Experimental Cancer Research in Lausanne, Switzerland; the Radiobiological Institute in Rijswijk, The Netherlands; and the University of Cambridge in the UK.
Among Dr Metcalf’s many honors and awards are the Companion of the Order of Australia (1993), the Albert Lasker Award for Clinical Medical Research (1993), the Gairdner Foundation International Award (1994), the Royal Medal of the Royal Society (1995), the Victoria Prize (2000), and the Prime Minister’s Prize for Science (2001).
Dr Metcalf is survived by his wife Jo; daughters Kate, Johanna, Penelope, and Mary-Ann; grandchildren James, Martin, Patrick, Elizabeth, Rose, and Robert; and their extended families. ![]()
PFS improvement will translate to OS, speaker says
SAN FRANCISCO—Administering brentuximab vedotin immediately after autologous stem cell transplant can improve progression-free survival (PFS) in patients with Hodgkin lymphoma (HL), results of the phase 3 AETHERA trial suggest.
The overall survival (OS) data for this study are not yet mature, but the significant improvement in PFS will likely translate to improved OS in a few years’ time, according to Craig Moskowitz, MD, of Memorial Sloan Kettering Cancer Center in New York.
Dr Moskowitz presented results from the AETHERA trial at the 2014 ASH Annual Meeting as abstract 673. The trial was funded by Seattle Genetics, Inc., and Takeda Pharmaceutical Company Limited, the companies developing brentuximab.
The trial included HL patients with at least one risk factor for progression. Eligible patients must have had a history of refractory HL, relapsed within a year of receiving frontline chemotherapy, and/or had disease outside of the lymph nodes at the time of pre-transplant relapse.
Researchers enrolled 329 patients, and they were randomized to receive brentuximab or placebo every 3 weeks for up to about a year. Baseline characteristics were similar between the 2 arms.
Dr Moskowitz pointed out that 43% of patients in the brentuximab arm and 48% in the placebo arm had required 2 or more prior salvage therapies, and 60% and 59%, respectively, had primary refractory HL.
Patients in both arms received a median of 15 treatment cycles, with an average of 12 cycles on the brentuximab arm and 11 cycles on the placebo arm.
“Patients who progressed in the placebo arm could be unblinded and subsequently receive brentuximab on a companion study,” Dr Moskowitz noted. “So technically, this was a cross-over design, making overall survival at 24 months quite unlikely.”
Efficacy/survival results
About half of patients in each arm completed treatment—47% in the brentuximab arm and 49% in the placebo arm. The reasons for discontinuation included disease progression (15% and 42%, respectively), adverse events (33% and 6%, respectively), and patient decision (5% and 2%, respectively).
Still, the trial achieved its primary endpoint, demonstrating a significant increase in PFS, according to an independent review facility (IRF).
The median PFS per the IRF was 43 months for patients in the brentuximab arm and 24 months in the placebo arm (hazard ratio=0.57, P=0.001). The 2-year PFS rates per the IRF were 63% and 51%, respectively.
The 2-year PFS rate according to investigators was 65% in the brentuximab arm and 45% in the placebo arm. The median PFS per investigators has not yet been reached for brentuximab but was 16 months for placebo.
The PFS benefit was consistent across all pre-specified subgroups, Dr Moskowitz noted, including primary refractory patients, patients who relapsed within 12 months of frontline therapy, and patients who relapsed after 12 months with extranodal disease.
Patients who experienced disease progression received a variety of subsequent therapies.
In the brentuximab arm, 16% of patients receiving subsequent therapy were treated with brentuximab after relapse. In the placebo arm, 85% of patients receiving subsequent therapy were treated with single-agent brentuximab.
Twenty-eight percent of patients in the placebo arm and 25% in the brentuximab arm received stem cell transplant as subsequent therapy, the majority of which were allogeneic transplants. Dr Moskowitz said a second transplant could have improved survival in these patients, but whether it actually did is unclear.
He noted that the OS data are immature, but there is currently no significant difference in OS between the treatment arms (hazard ratio=1.15; P=0.62).
“The median follow-up right now is 24 months,” he said. “So one will have to wait for a survival advantage or disadvantage, but from my point of view, a PFS of 65% at 2 years will translate to an overall survival difference. We’re just going to have to wait a few more years.”
Dr Moskowitz said another analysis of OS is planned in 2016.
Safety data
The most common adverse events in the brentuximab arm were peripheral sensory neuropathy (56%), neutropenia (35%), upper respiratory tract infection (26%), fatigue (24%), and peripheral motor neuropathy (23%).
The most common adverse events in the placebo arm were upper respiratory tract infection (23%), fatigue (18%), peripheral sensory neuropathy (16%), cough (16%), and neutropenia (12%).
Eighty-five percent of patients with peripheral neuropathy in the brentuximab arm had a resolution or improvement in symptoms, with a median time to improvement of 23.4 weeks.
Grade 3 or higher adverse events in the brentuximab arm included neutropenia, peripheral sensory neuropathy, peripheral motor neuropathy, nausea, fatigue, and diarrhea.
Grade 3 or higher adverse events in the placebo arm included neutropenia, fatigue, peripheral motor neuropathy, diarrhea, and peripheral sensory neuropathy. No Grade 4 peripheral neuropathy events occurred.
One death occurred within 30 days of brentuximab treatment. The patient died from treatment-related acute respiratory distress syndrome (ARDS) associated with pneumonitis.
Another death occurred on the brentuximab arm at day 40 from ARDS following an episode of treatment-related acute pancreatitis, which had resolved at the time of death.
Nevertheless, Dr Moskowitz characterized brentuximab consolidation as “very well-tolerated” in this patient population.
He concluded, “For patients with a remission duration of less than a year, patients with primary refractory Hodgkin lymphoma, and patients with Hodgkin lymphoma with extranodal involvement, I do believe this will become standard treatment.” ![]()
SAN FRANCISCO—Administering brentuximab vedotin immediately after autologous stem cell transplant can improve progression-free survival (PFS) in patients with Hodgkin lymphoma (HL), results of the phase 3 AETHERA trial suggest.
The overall survival (OS) data for this study are not yet mature, but the significant improvement in PFS will likely translate to improved OS in a few years’ time, according to Craig Moskowitz, MD, of Memorial Sloan Kettering Cancer Center in New York.
Dr Moskowitz presented results from the AETHERA trial at the 2014 ASH Annual Meeting as abstract 673. The trial was funded by Seattle Genetics, Inc., and Takeda Pharmaceutical Company Limited, the companies developing brentuximab.
The trial included HL patients with at least one risk factor for progression. Eligible patients must have had a history of refractory HL, relapsed within a year of receiving frontline chemotherapy, and/or had disease outside of the lymph nodes at the time of pre-transplant relapse.
Researchers enrolled 329 patients, and they were randomized to receive brentuximab or placebo every 3 weeks for up to about a year. Baseline characteristics were similar between the 2 arms.
Dr Moskowitz pointed out that 43% of patients in the brentuximab arm and 48% in the placebo arm had required 2 or more prior salvage therapies, and 60% and 59%, respectively, had primary refractory HL.
Patients in both arms received a median of 15 treatment cycles, with an average of 12 cycles on the brentuximab arm and 11 cycles on the placebo arm.
“Patients who progressed in the placebo arm could be unblinded and subsequently receive brentuximab on a companion study,” Dr Moskowitz noted. “So technically, this was a cross-over design, making overall survival at 24 months quite unlikely.”
Efficacy/survival results
About half of patients in each arm completed treatment—47% in the brentuximab arm and 49% in the placebo arm. The reasons for discontinuation included disease progression (15% and 42%, respectively), adverse events (33% and 6%, respectively), and patient decision (5% and 2%, respectively).
Still, the trial achieved its primary endpoint, demonstrating a significant increase in PFS, according to an independent review facility (IRF).
The median PFS per the IRF was 43 months for patients in the brentuximab arm and 24 months in the placebo arm (hazard ratio=0.57, P=0.001). The 2-year PFS rates per the IRF were 63% and 51%, respectively.
The 2-year PFS rate according to investigators was 65% in the brentuximab arm and 45% in the placebo arm. The median PFS per investigators has not yet been reached for brentuximab but was 16 months for placebo.
The PFS benefit was consistent across all pre-specified subgroups, Dr Moskowitz noted, including primary refractory patients, patients who relapsed within 12 months of frontline therapy, and patients who relapsed after 12 months with extranodal disease.
Patients who experienced disease progression received a variety of subsequent therapies.
In the brentuximab arm, 16% of patients receiving subsequent therapy were treated with brentuximab after relapse. In the placebo arm, 85% of patients receiving subsequent therapy were treated with single-agent brentuximab.
Twenty-eight percent of patients in the placebo arm and 25% in the brentuximab arm received stem cell transplant as subsequent therapy, the majority of which were allogeneic transplants. Dr Moskowitz said a second transplant could have improved survival in these patients, but whether it actually did is unclear.
He noted that the OS data are immature, but there is currently no significant difference in OS between the treatment arms (hazard ratio=1.15; P=0.62).
“The median follow-up right now is 24 months,” he said. “So one will have to wait for a survival advantage or disadvantage, but from my point of view, a PFS of 65% at 2 years will translate to an overall survival difference. We’re just going to have to wait a few more years.”
Dr Moskowitz said another analysis of OS is planned in 2016.
Safety data
The most common adverse events in the brentuximab arm were peripheral sensory neuropathy (56%), neutropenia (35%), upper respiratory tract infection (26%), fatigue (24%), and peripheral motor neuropathy (23%).
The most common adverse events in the placebo arm were upper respiratory tract infection (23%), fatigue (18%), peripheral sensory neuropathy (16%), cough (16%), and neutropenia (12%).
Eighty-five percent of patients with peripheral neuropathy in the brentuximab arm had a resolution or improvement in symptoms, with a median time to improvement of 23.4 weeks.
Grade 3 or higher adverse events in the brentuximab arm included neutropenia, peripheral sensory neuropathy, peripheral motor neuropathy, nausea, fatigue, and diarrhea.
Grade 3 or higher adverse events in the placebo arm included neutropenia, fatigue, peripheral motor neuropathy, diarrhea, and peripheral sensory neuropathy. No Grade 4 peripheral neuropathy events occurred.
One death occurred within 30 days of brentuximab treatment. The patient died from treatment-related acute respiratory distress syndrome (ARDS) associated with pneumonitis.
Another death occurred on the brentuximab arm at day 40 from ARDS following an episode of treatment-related acute pancreatitis, which had resolved at the time of death.
Nevertheless, Dr Moskowitz characterized brentuximab consolidation as “very well-tolerated” in this patient population.
He concluded, “For patients with a remission duration of less than a year, patients with primary refractory Hodgkin lymphoma, and patients with Hodgkin lymphoma with extranodal involvement, I do believe this will become standard treatment.” ![]()
SAN FRANCISCO—Administering brentuximab vedotin immediately after autologous stem cell transplant can improve progression-free survival (PFS) in patients with Hodgkin lymphoma (HL), results of the phase 3 AETHERA trial suggest.
The overall survival (OS) data for this study are not yet mature, but the significant improvement in PFS will likely translate to improved OS in a few years’ time, according to Craig Moskowitz, MD, of Memorial Sloan Kettering Cancer Center in New York.
Dr Moskowitz presented results from the AETHERA trial at the 2014 ASH Annual Meeting as abstract 673. The trial was funded by Seattle Genetics, Inc., and Takeda Pharmaceutical Company Limited, the companies developing brentuximab.
The trial included HL patients with at least one risk factor for progression. Eligible patients must have had a history of refractory HL, relapsed within a year of receiving frontline chemotherapy, and/or had disease outside of the lymph nodes at the time of pre-transplant relapse.
Researchers enrolled 329 patients, and they were randomized to receive brentuximab or placebo every 3 weeks for up to about a year. Baseline characteristics were similar between the 2 arms.
Dr Moskowitz pointed out that 43% of patients in the brentuximab arm and 48% in the placebo arm had required 2 or more prior salvage therapies, and 60% and 59%, respectively, had primary refractory HL.
Patients in both arms received a median of 15 treatment cycles, with an average of 12 cycles on the brentuximab arm and 11 cycles on the placebo arm.
“Patients who progressed in the placebo arm could be unblinded and subsequently receive brentuximab on a companion study,” Dr Moskowitz noted. “So technically, this was a cross-over design, making overall survival at 24 months quite unlikely.”
Efficacy/survival results
About half of patients in each arm completed treatment—47% in the brentuximab arm and 49% in the placebo arm. The reasons for discontinuation included disease progression (15% and 42%, respectively), adverse events (33% and 6%, respectively), and patient decision (5% and 2%, respectively).
Still, the trial achieved its primary endpoint, demonstrating a significant increase in PFS, according to an independent review facility (IRF).
The median PFS per the IRF was 43 months for patients in the brentuximab arm and 24 months in the placebo arm (hazard ratio=0.57, P=0.001). The 2-year PFS rates per the IRF were 63% and 51%, respectively.
The 2-year PFS rate according to investigators was 65% in the brentuximab arm and 45% in the placebo arm. The median PFS per investigators has not yet been reached for brentuximab but was 16 months for placebo.
The PFS benefit was consistent across all pre-specified subgroups, Dr Moskowitz noted, including primary refractory patients, patients who relapsed within 12 months of frontline therapy, and patients who relapsed after 12 months with extranodal disease.
Patients who experienced disease progression received a variety of subsequent therapies.
In the brentuximab arm, 16% of patients receiving subsequent therapy were treated with brentuximab after relapse. In the placebo arm, 85% of patients receiving subsequent therapy were treated with single-agent brentuximab.
Twenty-eight percent of patients in the placebo arm and 25% in the brentuximab arm received stem cell transplant as subsequent therapy, the majority of which were allogeneic transplants. Dr Moskowitz said a second transplant could have improved survival in these patients, but whether it actually did is unclear.
He noted that the OS data are immature, but there is currently no significant difference in OS between the treatment arms (hazard ratio=1.15; P=0.62).
“The median follow-up right now is 24 months,” he said. “So one will have to wait for a survival advantage or disadvantage, but from my point of view, a PFS of 65% at 2 years will translate to an overall survival difference. We’re just going to have to wait a few more years.”
Dr Moskowitz said another analysis of OS is planned in 2016.
Safety data
The most common adverse events in the brentuximab arm were peripheral sensory neuropathy (56%), neutropenia (35%), upper respiratory tract infection (26%), fatigue (24%), and peripheral motor neuropathy (23%).
The most common adverse events in the placebo arm were upper respiratory tract infection (23%), fatigue (18%), peripheral sensory neuropathy (16%), cough (16%), and neutropenia (12%).
Eighty-five percent of patients with peripheral neuropathy in the brentuximab arm had a resolution or improvement in symptoms, with a median time to improvement of 23.4 weeks.
Grade 3 or higher adverse events in the brentuximab arm included neutropenia, peripheral sensory neuropathy, peripheral motor neuropathy, nausea, fatigue, and diarrhea.
Grade 3 or higher adverse events in the placebo arm included neutropenia, fatigue, peripheral motor neuropathy, diarrhea, and peripheral sensory neuropathy. No Grade 4 peripheral neuropathy events occurred.
One death occurred within 30 days of brentuximab treatment. The patient died from treatment-related acute respiratory distress syndrome (ARDS) associated with pneumonitis.
Another death occurred on the brentuximab arm at day 40 from ARDS following an episode of treatment-related acute pancreatitis, which had resolved at the time of death.
Nevertheless, Dr Moskowitz characterized brentuximab consolidation as “very well-tolerated” in this patient population.
He concluded, “For patients with a remission duration of less than a year, patients with primary refractory Hodgkin lymphoma, and patients with Hodgkin lymphoma with extranodal involvement, I do believe this will become standard treatment.” ![]()
Method may predict likelihood of GVHD

Credit: Darren Baker
Researchers say that computer modeling of next-generation DNA sequencing data can help us understand the variable outcomes of stem cell transplant and provide a theoretical framework to make transplant a possibility for more patients who don’t have a related donor.
The team analyzed data obtained from whole-exome sequencing of 9 donor-recipient pairs (DRPs) and found it’s possible to predict the risk of graft-vs-host disease (GVHD).
This finding could one day help physicians tailor immunosuppressive therapies to possibly improve transplant outcomes.
The investigators say their data provide evidence that the way a patient’s immune system rebuilds itself following transplant is representative of a dynamical system, a system in which the current state determines what future state will follow.
“The immune system seems chaotic, but that is because there are so many variables involved,” said Amir Toor, MD, of the Virginia Commonwealth University in Richmond.
“We have found evidence of an underlying order. Using next-generation DNA sequencing technology, it may be possible to account for many of the molecular variables that eventually determine how well a donor’s immune system will graft to a patient.”
Dr Toor and his colleagues describe this work in two articles in Frontiers in Immunology.
In the first paper, the researchers recount how they used whole-exome sequencing to examine variation in minor histocompatibility antigens (mHAs) of transplant DRPs.
Using advanced computer-based analysis, the investigators examined potential interactions between mHAs and HLAs and discovered a high level of mHA variation in HLA-matched DRPs that could potentially contribute to GVHD.
These findings may help explain why many HLA-matched recipients experience GVHD, but why some HLA-mismatched recipients do not develop GVHD remains a mystery.
The researchers offer an explanation for this seeming paradox in a companion article. In this paper, they suggest that by inhibiting peptide generation through immunosuppressive therapies in the earliest weeks following stem cell transplant, antigen presentation to donor T cells could be diminished, which reduces the risk of GVHD as the recipients reconstitute their T-cell repertoire.
In previous research, Dr Toor and his colleagues discovered a fractal pattern in the DNA of recipients’ T-cell repertoires. (Fractals are self-similar patterns that repeat themselves at every scale.)
Based on their data, the researchers believe that the presentation of mHAs following transplant helps shape the development of T-cell clonal families.
Thus, inhibiting this antigen presentation through immunosuppressive therapies in patients who have high mHA variation can potentially reduce the risk of GVHD by influencing the development of their T-cell repertoire. This is supported by data from clinical studies showing immune suppression soon after transplant improves outcomes in unrelated DRPs.
The investigators suggest that an equation such as the logistic model of growth, a mathematical formula used to explain population growth, could be employed to predict the evolution of T-cell clones and determine a patient’s future risk of GVHD.
“Currently, we rely on population-based outcomes derived from probabilistic studies to determine the best way to perform stem cell transplants,” Dr Toor said. “The development of accurate mathematical models that account for the key variables influencing transplant outcomes may allow us to treat patients using a systematic and personalized approach.”
“We plan to keep exploring this concept in hopes that we can tailor the transplantation process to each individual in order to improve outcomes and make transplantation an option for more patients.” ![]()

Credit: Darren Baker
Researchers say that computer modeling of next-generation DNA sequencing data can help us understand the variable outcomes of stem cell transplant and provide a theoretical framework to make transplant a possibility for more patients who don’t have a related donor.
The team analyzed data obtained from whole-exome sequencing of 9 donor-recipient pairs (DRPs) and found it’s possible to predict the risk of graft-vs-host disease (GVHD).
This finding could one day help physicians tailor immunosuppressive therapies to possibly improve transplant outcomes.
The investigators say their data provide evidence that the way a patient’s immune system rebuilds itself following transplant is representative of a dynamical system, a system in which the current state determines what future state will follow.
“The immune system seems chaotic, but that is because there are so many variables involved,” said Amir Toor, MD, of the Virginia Commonwealth University in Richmond.
“We have found evidence of an underlying order. Using next-generation DNA sequencing technology, it may be possible to account for many of the molecular variables that eventually determine how well a donor’s immune system will graft to a patient.”
Dr Toor and his colleagues describe this work in two articles in Frontiers in Immunology.
In the first paper, the researchers recount how they used whole-exome sequencing to examine variation in minor histocompatibility antigens (mHAs) of transplant DRPs.
Using advanced computer-based analysis, the investigators examined potential interactions between mHAs and HLAs and discovered a high level of mHA variation in HLA-matched DRPs that could potentially contribute to GVHD.
These findings may help explain why many HLA-matched recipients experience GVHD, but why some HLA-mismatched recipients do not develop GVHD remains a mystery.
The researchers offer an explanation for this seeming paradox in a companion article. In this paper, they suggest that by inhibiting peptide generation through immunosuppressive therapies in the earliest weeks following stem cell transplant, antigen presentation to donor T cells could be diminished, which reduces the risk of GVHD as the recipients reconstitute their T-cell repertoire.
In previous research, Dr Toor and his colleagues discovered a fractal pattern in the DNA of recipients’ T-cell repertoires. (Fractals are self-similar patterns that repeat themselves at every scale.)
Based on their data, the researchers believe that the presentation of mHAs following transplant helps shape the development of T-cell clonal families.
Thus, inhibiting this antigen presentation through immunosuppressive therapies in patients who have high mHA variation can potentially reduce the risk of GVHD by influencing the development of their T-cell repertoire. This is supported by data from clinical studies showing immune suppression soon after transplant improves outcomes in unrelated DRPs.
The investigators suggest that an equation such as the logistic model of growth, a mathematical formula used to explain population growth, could be employed to predict the evolution of T-cell clones and determine a patient’s future risk of GVHD.
“Currently, we rely on population-based outcomes derived from probabilistic studies to determine the best way to perform stem cell transplants,” Dr Toor said. “The development of accurate mathematical models that account for the key variables influencing transplant outcomes may allow us to treat patients using a systematic and personalized approach.”
“We plan to keep exploring this concept in hopes that we can tailor the transplantation process to each individual in order to improve outcomes and make transplantation an option for more patients.” ![]()

Credit: Darren Baker
Researchers say that computer modeling of next-generation DNA sequencing data can help us understand the variable outcomes of stem cell transplant and provide a theoretical framework to make transplant a possibility for more patients who don’t have a related donor.
The team analyzed data obtained from whole-exome sequencing of 9 donor-recipient pairs (DRPs) and found it’s possible to predict the risk of graft-vs-host disease (GVHD).
This finding could one day help physicians tailor immunosuppressive therapies to possibly improve transplant outcomes.
The investigators say their data provide evidence that the way a patient’s immune system rebuilds itself following transplant is representative of a dynamical system, a system in which the current state determines what future state will follow.
“The immune system seems chaotic, but that is because there are so many variables involved,” said Amir Toor, MD, of the Virginia Commonwealth University in Richmond.
“We have found evidence of an underlying order. Using next-generation DNA sequencing technology, it may be possible to account for many of the molecular variables that eventually determine how well a donor’s immune system will graft to a patient.”
Dr Toor and his colleagues describe this work in two articles in Frontiers in Immunology.
In the first paper, the researchers recount how they used whole-exome sequencing to examine variation in minor histocompatibility antigens (mHAs) of transplant DRPs.
Using advanced computer-based analysis, the investigators examined potential interactions between mHAs and HLAs and discovered a high level of mHA variation in HLA-matched DRPs that could potentially contribute to GVHD.
These findings may help explain why many HLA-matched recipients experience GVHD, but why some HLA-mismatched recipients do not develop GVHD remains a mystery.
The researchers offer an explanation for this seeming paradox in a companion article. In this paper, they suggest that by inhibiting peptide generation through immunosuppressive therapies in the earliest weeks following stem cell transplant, antigen presentation to donor T cells could be diminished, which reduces the risk of GVHD as the recipients reconstitute their T-cell repertoire.
In previous research, Dr Toor and his colleagues discovered a fractal pattern in the DNA of recipients’ T-cell repertoires. (Fractals are self-similar patterns that repeat themselves at every scale.)
Based on their data, the researchers believe that the presentation of mHAs following transplant helps shape the development of T-cell clonal families.
Thus, inhibiting this antigen presentation through immunosuppressive therapies in patients who have high mHA variation can potentially reduce the risk of GVHD by influencing the development of their T-cell repertoire. This is supported by data from clinical studies showing immune suppression soon after transplant improves outcomes in unrelated DRPs.
The investigators suggest that an equation such as the logistic model of growth, a mathematical formula used to explain population growth, could be employed to predict the evolution of T-cell clones and determine a patient’s future risk of GVHD.
“Currently, we rely on population-based outcomes derived from probabilistic studies to determine the best way to perform stem cell transplants,” Dr Toor said. “The development of accurate mathematical models that account for the key variables influencing transplant outcomes may allow us to treat patients using a systematic and personalized approach.”
“We plan to keep exploring this concept in hopes that we can tailor the transplantation process to each individual in order to improve outcomes and make transplantation an option for more patients.” ![]()
Protein inhibits HSC engraftment

in the bone marrow
Researchers say they have identified a protein that plays a key role in regulating hematopoietic stem cell (HSC) engraftment.
Experiments revealed that protein tyrosine phosphatase-sigma (PTP-sigma) suppresses normal HSC engraftment capacity, but targeted inhibition of
PTP-sigma can substantially improve mouse and human HSC engraftment.
The researchers described these findings in The Journal of Clinical Investigation.
Mamle Quarmyne, a graduate student at the University of California, Los Angeles, and her colleagues first found that PTP-sigma is expressed on a high percentage of mouse and human HSCs.
When the team deleted PTP-sigma in mice, they observed a marked increase in HSCs’ ability to engraft.
When they selected human HSCs that did not express PTP-sigma and transplanted these cells into immune-deficient mice, the researchers observed a 15-fold increase in HSC engraftment.
The team also discovered that PTP-sigma regulates HSC function by suppressing a protein called RAC1, which is known to promote HSC engraftment.
“These findings have tremendous therapeutic potential, since we have identified a new receptor on HSCs, PTP-sigma, which can be specifically targeted as a means to potently increase the engraftment of transplanted HSCs in patients,” said John P. Chute, MD, of the University of California, Los Angeles.
“This approach can also potentially accelerate hematologic recovery in cancer patients receiving chemotherapy and/or radiation, which also suppress the blood and immune systems.”
Now, the researchers are testing small molecules for their ability to inhibit PTP-sigma on HSCs. If these studies are successful, the team aims to translate these findings into clinical trials in the near future. ![]()

in the bone marrow
Researchers say they have identified a protein that plays a key role in regulating hematopoietic stem cell (HSC) engraftment.
Experiments revealed that protein tyrosine phosphatase-sigma (PTP-sigma) suppresses normal HSC engraftment capacity, but targeted inhibition of
PTP-sigma can substantially improve mouse and human HSC engraftment.
The researchers described these findings in The Journal of Clinical Investigation.
Mamle Quarmyne, a graduate student at the University of California, Los Angeles, and her colleagues first found that PTP-sigma is expressed on a high percentage of mouse and human HSCs.
When the team deleted PTP-sigma in mice, they observed a marked increase in HSCs’ ability to engraft.
When they selected human HSCs that did not express PTP-sigma and transplanted these cells into immune-deficient mice, the researchers observed a 15-fold increase in HSC engraftment.
The team also discovered that PTP-sigma regulates HSC function by suppressing a protein called RAC1, which is known to promote HSC engraftment.
“These findings have tremendous therapeutic potential, since we have identified a new receptor on HSCs, PTP-sigma, which can be specifically targeted as a means to potently increase the engraftment of transplanted HSCs in patients,” said John P. Chute, MD, of the University of California, Los Angeles.
“This approach can also potentially accelerate hematologic recovery in cancer patients receiving chemotherapy and/or radiation, which also suppress the blood and immune systems.”
Now, the researchers are testing small molecules for their ability to inhibit PTP-sigma on HSCs. If these studies are successful, the team aims to translate these findings into clinical trials in the near future. ![]()

in the bone marrow
Researchers say they have identified a protein that plays a key role in regulating hematopoietic stem cell (HSC) engraftment.
Experiments revealed that protein tyrosine phosphatase-sigma (PTP-sigma) suppresses normal HSC engraftment capacity, but targeted inhibition of
PTP-sigma can substantially improve mouse and human HSC engraftment.
The researchers described these findings in The Journal of Clinical Investigation.
Mamle Quarmyne, a graduate student at the University of California, Los Angeles, and her colleagues first found that PTP-sigma is expressed on a high percentage of mouse and human HSCs.
When the team deleted PTP-sigma in mice, they observed a marked increase in HSCs’ ability to engraft.
When they selected human HSCs that did not express PTP-sigma and transplanted these cells into immune-deficient mice, the researchers observed a 15-fold increase in HSC engraftment.
The team also discovered that PTP-sigma regulates HSC function by suppressing a protein called RAC1, which is known to promote HSC engraftment.
“These findings have tremendous therapeutic potential, since we have identified a new receptor on HSCs, PTP-sigma, which can be specifically targeted as a means to potently increase the engraftment of transplanted HSCs in patients,” said John P. Chute, MD, of the University of California, Los Angeles.
“This approach can also potentially accelerate hematologic recovery in cancer patients receiving chemotherapy and/or radiation, which also suppress the blood and immune systems.”
Now, the researchers are testing small molecules for their ability to inhibit PTP-sigma on HSCs. If these studies are successful, the team aims to translate these findings into clinical trials in the near future. ![]()
IL-6 inhibitor helps prevent GVHD

Credit: Chad McNeeley
Adding the interleukin 6 (IL-6) inhibitor tocilizumab can improve standard prophylaxis for graft-vs-host disease (GVHD), researchers have reported in The Lancet Oncology.
IL-6 is the main detectable and dysregulated cytokine secreted after allogeneic stem cell transplant (allo-SCT).
So the researchers theorized that inhibiting IL-6 with tocilizumab might protect patients from acute GVHD despite robust immune reconstitution.
In their phase 1/2 study, the team saw acute GVHD drop from the usual 50% observed in patients receiving standard GVHD prophylaxis to 12% in patients receiving tocilizumab.
“Severe cases—which often result in death—were reduced from 21% to 4%,” said Geoff Hill, MD, of QIMR Berghofer Medical Research Institute in Brisbane, Queensland, Australia.
He and his colleagues enrolled 48 patients in this study. They ranged in age from 18 to 65 and underwent T-replete, HLA-matched, allo-SCT from unrelated or sibling donors. Patients received either total-body-irradiation-based myeloablative conditioning or reduced-intensity conditioning.
As GVHD prophylaxis, patients received a single intravenous dose of tocilizumab (8 mg/kg, capped at 800 mg, over a 60-minute infusion) the day before transplant.
They also received standard GVHD prophylaxis—cyclosporin (5 mg/kg per day on days −1 to +1, then 3 mg/kg per day to maintain therapeutic levels [trough levels of 140-300 ng/mL] for 100 days) and methotrexate (15 mg/m2 on day 1, then 10 mg/m2 on days 3, 6, and 11).
The primary endpoint was the incidence of grade 2-4 acute GVHD at day 100, which was 12%. The incidence of grade 3-4 acute GVHD was 4%.
Five patients (10%) had grade 2-4 acute GVHD involving the skin, and 4 (8%) had grade 2-4 acute GVHD involving the gastrointestinal tract. None of the patients had GVHD involving the liver.
The researchers noted that the rate of grade 2-4 acute GVHD was low regardless of the conditioning regimen a patient received. The rate was 12% for both myeloablative and reduced-intensity conditioning.
In addition, patients’ immune reconstitution was preserved after receiving tocilizumab, but the researchers did observe suppression of known pathogenic STAT3-dependent pathways.
These results represent a significant advance in allo-SCT, according to study author Glen Kennedy, MBBS, also of QIMR Berghofer Medical Research Institute.
“The new therapy has the potential to make transplant safer,” he said, “and applicable to a larger group of patients.”
A phase 3 study of tocilizumab as GVHD prophylaxis is now underway. The drug is currently approved to treat rheumatoid arthritis. ![]()

Credit: Chad McNeeley
Adding the interleukin 6 (IL-6) inhibitor tocilizumab can improve standard prophylaxis for graft-vs-host disease (GVHD), researchers have reported in The Lancet Oncology.
IL-6 is the main detectable and dysregulated cytokine secreted after allogeneic stem cell transplant (allo-SCT).
So the researchers theorized that inhibiting IL-6 with tocilizumab might protect patients from acute GVHD despite robust immune reconstitution.
In their phase 1/2 study, the team saw acute GVHD drop from the usual 50% observed in patients receiving standard GVHD prophylaxis to 12% in patients receiving tocilizumab.
“Severe cases—which often result in death—were reduced from 21% to 4%,” said Geoff Hill, MD, of QIMR Berghofer Medical Research Institute in Brisbane, Queensland, Australia.
He and his colleagues enrolled 48 patients in this study. They ranged in age from 18 to 65 and underwent T-replete, HLA-matched, allo-SCT from unrelated or sibling donors. Patients received either total-body-irradiation-based myeloablative conditioning or reduced-intensity conditioning.
As GVHD prophylaxis, patients received a single intravenous dose of tocilizumab (8 mg/kg, capped at 800 mg, over a 60-minute infusion) the day before transplant.
They also received standard GVHD prophylaxis—cyclosporin (5 mg/kg per day on days −1 to +1, then 3 mg/kg per day to maintain therapeutic levels [trough levels of 140-300 ng/mL] for 100 days) and methotrexate (15 mg/m2 on day 1, then 10 mg/m2 on days 3, 6, and 11).
The primary endpoint was the incidence of grade 2-4 acute GVHD at day 100, which was 12%. The incidence of grade 3-4 acute GVHD was 4%.
Five patients (10%) had grade 2-4 acute GVHD involving the skin, and 4 (8%) had grade 2-4 acute GVHD involving the gastrointestinal tract. None of the patients had GVHD involving the liver.
The researchers noted that the rate of grade 2-4 acute GVHD was low regardless of the conditioning regimen a patient received. The rate was 12% for both myeloablative and reduced-intensity conditioning.
In addition, patients’ immune reconstitution was preserved after receiving tocilizumab, but the researchers did observe suppression of known pathogenic STAT3-dependent pathways.
These results represent a significant advance in allo-SCT, according to study author Glen Kennedy, MBBS, also of QIMR Berghofer Medical Research Institute.
“The new therapy has the potential to make transplant safer,” he said, “and applicable to a larger group of patients.”
A phase 3 study of tocilizumab as GVHD prophylaxis is now underway. The drug is currently approved to treat rheumatoid arthritis. ![]()

Credit: Chad McNeeley
Adding the interleukin 6 (IL-6) inhibitor tocilizumab can improve standard prophylaxis for graft-vs-host disease (GVHD), researchers have reported in The Lancet Oncology.
IL-6 is the main detectable and dysregulated cytokine secreted after allogeneic stem cell transplant (allo-SCT).
So the researchers theorized that inhibiting IL-6 with tocilizumab might protect patients from acute GVHD despite robust immune reconstitution.
In their phase 1/2 study, the team saw acute GVHD drop from the usual 50% observed in patients receiving standard GVHD prophylaxis to 12% in patients receiving tocilizumab.
“Severe cases—which often result in death—were reduced from 21% to 4%,” said Geoff Hill, MD, of QIMR Berghofer Medical Research Institute in Brisbane, Queensland, Australia.
He and his colleagues enrolled 48 patients in this study. They ranged in age from 18 to 65 and underwent T-replete, HLA-matched, allo-SCT from unrelated or sibling donors. Patients received either total-body-irradiation-based myeloablative conditioning or reduced-intensity conditioning.
As GVHD prophylaxis, patients received a single intravenous dose of tocilizumab (8 mg/kg, capped at 800 mg, over a 60-minute infusion) the day before transplant.
They also received standard GVHD prophylaxis—cyclosporin (5 mg/kg per day on days −1 to +1, then 3 mg/kg per day to maintain therapeutic levels [trough levels of 140-300 ng/mL] for 100 days) and methotrexate (15 mg/m2 on day 1, then 10 mg/m2 on days 3, 6, and 11).
The primary endpoint was the incidence of grade 2-4 acute GVHD at day 100, which was 12%. The incidence of grade 3-4 acute GVHD was 4%.
Five patients (10%) had grade 2-4 acute GVHD involving the skin, and 4 (8%) had grade 2-4 acute GVHD involving the gastrointestinal tract. None of the patients had GVHD involving the liver.
The researchers noted that the rate of grade 2-4 acute GVHD was low regardless of the conditioning regimen a patient received. The rate was 12% for both myeloablative and reduced-intensity conditioning.
In addition, patients’ immune reconstitution was preserved after receiving tocilizumab, but the researchers did observe suppression of known pathogenic STAT3-dependent pathways.
These results represent a significant advance in allo-SCT, according to study author Glen Kennedy, MBBS, also of QIMR Berghofer Medical Research Institute.
“The new therapy has the potential to make transplant safer,” he said, “and applicable to a larger group of patients.”
A phase 3 study of tocilizumab as GVHD prophylaxis is now underway. The drug is currently approved to treat rheumatoid arthritis. ![]()
Protein discovery paves way for patient-specific HSCs

Credit: John Perry
A protein known as GPI-80 is integral to the self-renewal of hematopoietic stem cells (HSCs) during human development, investigators have reported in Cell Stem Cell.
The team says this discovery lays the groundwork for researchers to generate HSCs in the lab that better mirror HSCs in their natural environment.
This could lead to improved therapies for hematologic disorders by enabling the creation of patient-specific HSCs for transplantation.
In a 5-year study, Hanna Katri Annikki Mikkola, MD, PhD, of the University of California, Los Angeles, and her colleagues investigated a unique HSC surface protein called GPI-80.
They found that GPI-80 is produced by a subpopulation of human fetal hematopoietic stem/progenitor cells (HSPCs)—the only group of cells that could self-renew and differentiate into various blood cell types.
The investigators also found that this subpopulation—CD34+CD38lo/-CD90+GPI-80+ HSPCs—was the sole population able to permanently integrate into and thrive within the blood system of a recipient mouse.
Dr Mikkola and her colleagues further discovered that GPI-80 identifies human HSPCs during multiple phases of development and migration.
These include the early first trimester of fetal development, when newly generated HSCs can be found in the placenta, and the second trimester, when HSCs are actively replicating in the fetal liver and the fetal bone marrow.
“We found that whatever HSC niche we investigated, we could use GPI-80 as the best determinant to find the stem cell as it was being generated or colonized different hematopoietic tissues,” Dr Mikkola said.
“Moreover, loss of GPI-80 caused the stem cells to differentiate. This essentially tells us that GPI-80 must be present to make HSCs. We now have a very unique marker for investigating how human hematopoietic cells develop, migrate, and function.”
Dr Mikkola’s team is exploring different stages of human HSC development and pluripotent stem cell differentiation based on the GPI-80 marker and comparing how HSCs are being generated in vitro and in vivo.
The group says this paves the way for scientists to redirect pluripotent stem cells into patient-specific HSCs for transplantation into a patient without the need to find a suitable donor.
“Now that we can use GPI-80 as a marker to isolate the human hematopoietic stem cell at different stages of development, this can serve as a guide for identifying and overcoming the barriers to making human HSCs in vitro, which has never been done successfully,” Dr Mikkola said.
“We can now better understand the missing molecular elements that in vitro-derived cells don’t have, which is critical to fulfilling the functional and safety criteria for transplantation to patients.” ![]()

Credit: John Perry
A protein known as GPI-80 is integral to the self-renewal of hematopoietic stem cells (HSCs) during human development, investigators have reported in Cell Stem Cell.
The team says this discovery lays the groundwork for researchers to generate HSCs in the lab that better mirror HSCs in their natural environment.
This could lead to improved therapies for hematologic disorders by enabling the creation of patient-specific HSCs for transplantation.
In a 5-year study, Hanna Katri Annikki Mikkola, MD, PhD, of the University of California, Los Angeles, and her colleagues investigated a unique HSC surface protein called GPI-80.
They found that GPI-80 is produced by a subpopulation of human fetal hematopoietic stem/progenitor cells (HSPCs)—the only group of cells that could self-renew and differentiate into various blood cell types.
The investigators also found that this subpopulation—CD34+CD38lo/-CD90+GPI-80+ HSPCs—was the sole population able to permanently integrate into and thrive within the blood system of a recipient mouse.
Dr Mikkola and her colleagues further discovered that GPI-80 identifies human HSPCs during multiple phases of development and migration.
These include the early first trimester of fetal development, when newly generated HSCs can be found in the placenta, and the second trimester, when HSCs are actively replicating in the fetal liver and the fetal bone marrow.
“We found that whatever HSC niche we investigated, we could use GPI-80 as the best determinant to find the stem cell as it was being generated or colonized different hematopoietic tissues,” Dr Mikkola said.
“Moreover, loss of GPI-80 caused the stem cells to differentiate. This essentially tells us that GPI-80 must be present to make HSCs. We now have a very unique marker for investigating how human hematopoietic cells develop, migrate, and function.”
Dr Mikkola’s team is exploring different stages of human HSC development and pluripotent stem cell differentiation based on the GPI-80 marker and comparing how HSCs are being generated in vitro and in vivo.
The group says this paves the way for scientists to redirect pluripotent stem cells into patient-specific HSCs for transplantation into a patient without the need to find a suitable donor.
“Now that we can use GPI-80 as a marker to isolate the human hematopoietic stem cell at different stages of development, this can serve as a guide for identifying and overcoming the barriers to making human HSCs in vitro, which has never been done successfully,” Dr Mikkola said.
“We can now better understand the missing molecular elements that in vitro-derived cells don’t have, which is critical to fulfilling the functional and safety criteria for transplantation to patients.” ![]()

Credit: John Perry
A protein known as GPI-80 is integral to the self-renewal of hematopoietic stem cells (HSCs) during human development, investigators have reported in Cell Stem Cell.
The team says this discovery lays the groundwork for researchers to generate HSCs in the lab that better mirror HSCs in their natural environment.
This could lead to improved therapies for hematologic disorders by enabling the creation of patient-specific HSCs for transplantation.
In a 5-year study, Hanna Katri Annikki Mikkola, MD, PhD, of the University of California, Los Angeles, and her colleagues investigated a unique HSC surface protein called GPI-80.
They found that GPI-80 is produced by a subpopulation of human fetal hematopoietic stem/progenitor cells (HSPCs)—the only group of cells that could self-renew and differentiate into various blood cell types.
The investigators also found that this subpopulation—CD34+CD38lo/-CD90+GPI-80+ HSPCs—was the sole population able to permanently integrate into and thrive within the blood system of a recipient mouse.
Dr Mikkola and her colleagues further discovered that GPI-80 identifies human HSPCs during multiple phases of development and migration.
These include the early first trimester of fetal development, when newly generated HSCs can be found in the placenta, and the second trimester, when HSCs are actively replicating in the fetal liver and the fetal bone marrow.
“We found that whatever HSC niche we investigated, we could use GPI-80 as the best determinant to find the stem cell as it was being generated or colonized different hematopoietic tissues,” Dr Mikkola said.
“Moreover, loss of GPI-80 caused the stem cells to differentiate. This essentially tells us that GPI-80 must be present to make HSCs. We now have a very unique marker for investigating how human hematopoietic cells develop, migrate, and function.”
Dr Mikkola’s team is exploring different stages of human HSC development and pluripotent stem cell differentiation based on the GPI-80 marker and comparing how HSCs are being generated in vitro and in vivo.
The group says this paves the way for scientists to redirect pluripotent stem cells into patient-specific HSCs for transplantation into a patient without the need to find a suitable donor.
“Now that we can use GPI-80 as a marker to isolate the human hematopoietic stem cell at different stages of development, this can serve as a guide for identifying and overcoming the barriers to making human HSCs in vitro, which has never been done successfully,” Dr Mikkola said.
“We can now better understand the missing molecular elements that in vitro-derived cells don’t have, which is critical to fulfilling the functional and safety criteria for transplantation to patients.” ![]()
Team discovers key aspects of HSC development

New research suggests proinflammatory signaling is crucial to the creation of hematopoietic stem cells (HSCs) during embryonic development, a finding that could help scientists reproduce HSCs for therapeutic use.
Researchers discovered that TNFR2, via TNFα, activates the Notch and NF-kB signaling pathways to establish HSC fate, which suggests inflammatory signaling is required for HSC generation.
The group also found that primitive neutrophils are the major source of TNFa. So it seems these cells are crucial to HSC development as well.
The researchers described these findings in Cell.
“The development of some mature cell lineages from iPSCs [induced pluripotent stem cells], such as cardiac and neural, has been reasonably straightforward, but not with HSCs,” said principal investigator David Traver, PhD, of the University of California, San Diego School of Medicine.
“This is likely due, at least in part, to not fully understanding all of the factors used by the embryo to generate HSCs. We believe the discovery that proinflammatory cues are important in vivo will help us recapitulate instruction of HSC fate in vitro from iPSCs.”
For this study, Dr Traver and his colleagues decided to examine the role of TNFα in HSC development, extending previous research by Victoriano Mulero, PhD, of the University of Murcia in Spain.
Dr Mulero reported that TNFα is important in the function of the embryonic vascular system. And, in animal models where TNF function was absent, blood defects resulted.
Raquel Espin-Palazon, PhD, a researcher in Dr Traver’s lab and a former colleague of Dr Mulero’s, determined that TNFα is required for the emergence of HSCs during embryogenesis in zebrafish.
Dr Traver said the finding was completely unexpected because HSCs emerge relatively early in embryonic formation, when the developing organism is considered to be largely sterile and devoid of infection.
“Thus, there was no expectation that proinflammatory signaling would be active at this time or in the blood-forming regions,” Dr Traver said. “Equally surprising, we found that a population of embryonic myeloid cells, which are transient cells produced before HSCs arise, are the producers of the TNFα needed to establish HSC fate.”
“So it turns out that a small subset of myeloid cells that persist for only a few days in development are necessary to help generate the lineal precursors of the entire adult blood-forming system.” ![]()

New research suggests proinflammatory signaling is crucial to the creation of hematopoietic stem cells (HSCs) during embryonic development, a finding that could help scientists reproduce HSCs for therapeutic use.
Researchers discovered that TNFR2, via TNFα, activates the Notch and NF-kB signaling pathways to establish HSC fate, which suggests inflammatory signaling is required for HSC generation.
The group also found that primitive neutrophils are the major source of TNFa. So it seems these cells are crucial to HSC development as well.
The researchers described these findings in Cell.
“The development of some mature cell lineages from iPSCs [induced pluripotent stem cells], such as cardiac and neural, has been reasonably straightforward, but not with HSCs,” said principal investigator David Traver, PhD, of the University of California, San Diego School of Medicine.
“This is likely due, at least in part, to not fully understanding all of the factors used by the embryo to generate HSCs. We believe the discovery that proinflammatory cues are important in vivo will help us recapitulate instruction of HSC fate in vitro from iPSCs.”
For this study, Dr Traver and his colleagues decided to examine the role of TNFα in HSC development, extending previous research by Victoriano Mulero, PhD, of the University of Murcia in Spain.
Dr Mulero reported that TNFα is important in the function of the embryonic vascular system. And, in animal models where TNF function was absent, blood defects resulted.
Raquel Espin-Palazon, PhD, a researcher in Dr Traver’s lab and a former colleague of Dr Mulero’s, determined that TNFα is required for the emergence of HSCs during embryogenesis in zebrafish.
Dr Traver said the finding was completely unexpected because HSCs emerge relatively early in embryonic formation, when the developing organism is considered to be largely sterile and devoid of infection.
“Thus, there was no expectation that proinflammatory signaling would be active at this time or in the blood-forming regions,” Dr Traver said. “Equally surprising, we found that a population of embryonic myeloid cells, which are transient cells produced before HSCs arise, are the producers of the TNFα needed to establish HSC fate.”
“So it turns out that a small subset of myeloid cells that persist for only a few days in development are necessary to help generate the lineal precursors of the entire adult blood-forming system.” ![]()

New research suggests proinflammatory signaling is crucial to the creation of hematopoietic stem cells (HSCs) during embryonic development, a finding that could help scientists reproduce HSCs for therapeutic use.
Researchers discovered that TNFR2, via TNFα, activates the Notch and NF-kB signaling pathways to establish HSC fate, which suggests inflammatory signaling is required for HSC generation.
The group also found that primitive neutrophils are the major source of TNFa. So it seems these cells are crucial to HSC development as well.
The researchers described these findings in Cell.
“The development of some mature cell lineages from iPSCs [induced pluripotent stem cells], such as cardiac and neural, has been reasonably straightforward, but not with HSCs,” said principal investigator David Traver, PhD, of the University of California, San Diego School of Medicine.
“This is likely due, at least in part, to not fully understanding all of the factors used by the embryo to generate HSCs. We believe the discovery that proinflammatory cues are important in vivo will help us recapitulate instruction of HSC fate in vitro from iPSCs.”
For this study, Dr Traver and his colleagues decided to examine the role of TNFα in HSC development, extending previous research by Victoriano Mulero, PhD, of the University of Murcia in Spain.
Dr Mulero reported that TNFα is important in the function of the embryonic vascular system. And, in animal models where TNF function was absent, blood defects resulted.
Raquel Espin-Palazon, PhD, a researcher in Dr Traver’s lab and a former colleague of Dr Mulero’s, determined that TNFα is required for the emergence of HSCs during embryogenesis in zebrafish.
Dr Traver said the finding was completely unexpected because HSCs emerge relatively early in embryonic formation, when the developing organism is considered to be largely sterile and devoid of infection.
“Thus, there was no expectation that proinflammatory signaling would be active at this time or in the blood-forming regions,” Dr Traver said. “Equally surprising, we found that a population of embryonic myeloid cells, which are transient cells produced before HSCs arise, are the producers of the TNFα needed to establish HSC fate.”
“So it turns out that a small subset of myeloid cells that persist for only a few days in development are necessary to help generate the lineal precursors of the entire adult blood-forming system.”
FDA grants drug orphan designation for GVHD

Credit: Bill Branson
The US Food and Drug Administration (FDA) has granted orphan designation for a human alpha-1 antitrypsin (AAT) product known as Glassia to treat graft-vs-host disease (GVHD).
Orphan drug designation carries multiple benefits, including the availability of grant money, certain tax credits, and 7 years of market exclusivity, as well as the possibility of an expedited regulatory process.
Glassia is the first available ready-to-infuse liquid alpha1-proteinase inhibitor.
The product is already approved by the FDA to treat adults with clinically evident emphysema due to severe congenital AAT deficiency. Glassia is given intravenously once a week to augment the levels of AAT, a protein derived from human plasma, in the blood.
In recent years, researchers have discovered that AAT has anti-inflammatory, tissue protective, immunomodulatory, and anti-apoptotic properties in direct or indirect consequence of its underlying antiprotease capabilities.
These properties may attenuate inflammation by lowering levels of proinflammatory mediators such as cytokines, chemokines, and proteases that are associated with GVHD.
Preliminary human and animal studies indicate that Glassia may be able to treat and reduce the severity of GVHD occurring after allogeneic stem cell transplant.
Researchers are now evaluating Glassia in a phase 1/2 study of 24 GVHD patients with inadequate responses to steroid treatment following allogeneic stem cell transplant. The patients are enrolled in 4 dose cohorts, in which they receive up to 8 doses of Glassia. Interim data from this study is expected by the end of this year.
“Results from this phase 1/2 study in GVHD may support global clinical development activities and may serve as a platform to apply for an expansion of the AAT indications to include general organ transplantation, based on a similar mechanism of action,“ said David Tsur, Co-founder and Chief Executive Officer of Kamada, makers of Glassia.
“GVHD is a disease of significant unmet medical need, and both the disease and current therapy options carry considerable side effects. Given the favorable safety profile of Glassia, there is a strong rationale to support the development of this new indication and an increased likelihood of it becoming an effective therapy for this potentially life-threatening disease.”
“We will pursue discussion with the US and European regulators with regard to our development pathway and with an aim to move forward with a more advanced study of Glassia to treat GVHD.”
For more information on Glassia, see the full prescribing information.

Credit: Bill Branson
The US Food and Drug Administration (FDA) has granted orphan designation for a human alpha-1 antitrypsin (AAT) product known as Glassia to treat graft-vs-host disease (GVHD).
Orphan drug designation carries multiple benefits, including the availability of grant money, certain tax credits, and 7 years of market exclusivity, as well as the possibility of an expedited regulatory process.
Glassia is the first available ready-to-infuse liquid alpha1-proteinase inhibitor.
The product is already approved by the FDA to treat adults with clinically evident emphysema due to severe congenital AAT deficiency. Glassia is given intravenously once a week to augment the levels of AAT, a protein derived from human plasma, in the blood.
In recent years, researchers have discovered that AAT has anti-inflammatory, tissue protective, immunomodulatory, and anti-apoptotic properties in direct or indirect consequence of its underlying antiprotease capabilities.
These properties may attenuate inflammation by lowering levels of proinflammatory mediators such as cytokines, chemokines, and proteases that are associated with GVHD.
Preliminary human and animal studies indicate that Glassia may be able to treat and reduce the severity of GVHD occurring after allogeneic stem cell transplant.
Researchers are now evaluating Glassia in a phase 1/2 study of 24 GVHD patients with inadequate responses to steroid treatment following allogeneic stem cell transplant. The patients are enrolled in 4 dose cohorts, in which they receive up to 8 doses of Glassia. Interim data from this study is expected by the end of this year.
“Results from this phase 1/2 study in GVHD may support global clinical development activities and may serve as a platform to apply for an expansion of the AAT indications to include general organ transplantation, based on a similar mechanism of action,“ said David Tsur, Co-founder and Chief Executive Officer of Kamada, makers of Glassia.
“GVHD is a disease of significant unmet medical need, and both the disease and current therapy options carry considerable side effects. Given the favorable safety profile of Glassia, there is a strong rationale to support the development of this new indication and an increased likelihood of it becoming an effective therapy for this potentially life-threatening disease.”
“We will pursue discussion with the US and European regulators with regard to our development pathway and with an aim to move forward with a more advanced study of Glassia to treat GVHD.”
For more information on Glassia, see the full prescribing information.

Credit: Bill Branson
The US Food and Drug Administration (FDA) has granted orphan designation for a human alpha-1 antitrypsin (AAT) product known as Glassia to treat graft-vs-host disease (GVHD).
Orphan drug designation carries multiple benefits, including the availability of grant money, certain tax credits, and 7 years of market exclusivity, as well as the possibility of an expedited regulatory process.
Glassia is the first available ready-to-infuse liquid alpha1-proteinase inhibitor.
The product is already approved by the FDA to treat adults with clinically evident emphysema due to severe congenital AAT deficiency. Glassia is given intravenously once a week to augment the levels of AAT, a protein derived from human plasma, in the blood.
In recent years, researchers have discovered that AAT has anti-inflammatory, tissue protective, immunomodulatory, and anti-apoptotic properties in direct or indirect consequence of its underlying antiprotease capabilities.
These properties may attenuate inflammation by lowering levels of proinflammatory mediators such as cytokines, chemokines, and proteases that are associated with GVHD.
Preliminary human and animal studies indicate that Glassia may be able to treat and reduce the severity of GVHD occurring after allogeneic stem cell transplant.
Researchers are now evaluating Glassia in a phase 1/2 study of 24 GVHD patients with inadequate responses to steroid treatment following allogeneic stem cell transplant. The patients are enrolled in 4 dose cohorts, in which they receive up to 8 doses of Glassia. Interim data from this study is expected by the end of this year.
“Results from this phase 1/2 study in GVHD may support global clinical development activities and may serve as a platform to apply for an expansion of the AAT indications to include general organ transplantation, based on a similar mechanism of action,“ said David Tsur, Co-founder and Chief Executive Officer of Kamada, makers of Glassia.
“GVHD is a disease of significant unmet medical need, and both the disease and current therapy options carry considerable side effects. Given the favorable safety profile of Glassia, there is a strong rationale to support the development of this new indication and an increased likelihood of it becoming an effective therapy for this potentially life-threatening disease.”
“We will pursue discussion with the US and European regulators with regard to our development pathway and with an aim to move forward with a more advanced study of Glassia to treat GVHD.”
For more information on Glassia, see the full prescribing information.
Bortezomib can treat chronic GVHD, study shows
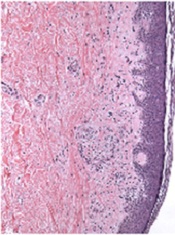
Credit: PLOS ONE
The proteasome inhibitor bortezomib can treat chronic graft-vs-host disease (GVHD), according to research published in Blood.
The study showed that bortezomib provides better outcomes than existing treatments and does not impair the graft-vs-tumor effect.
“Bortezomib helped a group of patients who desperately needed a treatment, having failed multiple different therapies,” said study author Mehrdad Abedi, MD, of the University of California, Davis.
“The drug fights chronic graft-vs-host disease, and, unlike other GVHD therapies such as steroid, cyclosporine, or mycophenolate, it treats chronic GVHD without dampening the graft-vs-tumor effect, which can be critically important to help patients avoid relapse. In fact, because bortezomib is an anticancer drug, it potentially attacks cancer cells in its own right.”
The researchers first studied bortezomib in mice and found the drug suppresses the donor immune cells that cause GVHD.
“We then tested this concept in patients with chronic GVHD . . . ,” Dr Abedi said. “Almost all the patients who tolerated and remained on the treatment responded. In some cases, individual responses were quite dramatic. We were able to stop their other immunosuppressive medications and keep the patients under control with just weekly injections of bortezomib.”
Dr Abedi added that one patient had severe hemolytic anemia that did not respond to several lines of therapy.
“After receiving bortezomib, the patient’s symptoms improved, and we were able to take her completely off steroid and other immunosuppressive medications,” he said. “Another person had multiple ulcers, which completely healed. These were patients who had been on all different kinds of medications and had no response.”
This research is ongoing. Dr Abedi and his colleagues are now looking at a potential oral version of the drug and a similar agent that would alleviate the need for weekly injections and could have fewer side effects.
This research was funded by the National Cancer Institute, the National Institute of Allergy and Infectious Diseases, and Millennium Pharmaceuticals, makers of bortezomib.

Credit: PLOS ONE
The proteasome inhibitor bortezomib can treat chronic graft-vs-host disease (GVHD), according to research published in Blood.
The study showed that bortezomib provides better outcomes than existing treatments and does not impair the graft-vs-tumor effect.
“Bortezomib helped a group of patients who desperately needed a treatment, having failed multiple different therapies,” said study author Mehrdad Abedi, MD, of the University of California, Davis.
“The drug fights chronic graft-vs-host disease, and, unlike other GVHD therapies such as steroid, cyclosporine, or mycophenolate, it treats chronic GVHD without dampening the graft-vs-tumor effect, which can be critically important to help patients avoid relapse. In fact, because bortezomib is an anticancer drug, it potentially attacks cancer cells in its own right.”
The researchers first studied bortezomib in mice and found the drug suppresses the donor immune cells that cause GVHD.
“We then tested this concept in patients with chronic GVHD . . . ,” Dr Abedi said. “Almost all the patients who tolerated and remained on the treatment responded. In some cases, individual responses were quite dramatic. We were able to stop their other immunosuppressive medications and keep the patients under control with just weekly injections of bortezomib.”
Dr Abedi added that one patient had severe hemolytic anemia that did not respond to several lines of therapy.
“After receiving bortezomib, the patient’s symptoms improved, and we were able to take her completely off steroid and other immunosuppressive medications,” he said. “Another person had multiple ulcers, which completely healed. These were patients who had been on all different kinds of medications and had no response.”
This research is ongoing. Dr Abedi and his colleagues are now looking at a potential oral version of the drug and a similar agent that would alleviate the need for weekly injections and could have fewer side effects.
This research was funded by the National Cancer Institute, the National Institute of Allergy and Infectious Diseases, and Millennium Pharmaceuticals, makers of bortezomib.

Credit: PLOS ONE
The proteasome inhibitor bortezomib can treat chronic graft-vs-host disease (GVHD), according to research published in Blood.
The study showed that bortezomib provides better outcomes than existing treatments and does not impair the graft-vs-tumor effect.
“Bortezomib helped a group of patients who desperately needed a treatment, having failed multiple different therapies,” said study author Mehrdad Abedi, MD, of the University of California, Davis.
“The drug fights chronic graft-vs-host disease, and, unlike other GVHD therapies such as steroid, cyclosporine, or mycophenolate, it treats chronic GVHD without dampening the graft-vs-tumor effect, which can be critically important to help patients avoid relapse. In fact, because bortezomib is an anticancer drug, it potentially attacks cancer cells in its own right.”
The researchers first studied bortezomib in mice and found the drug suppresses the donor immune cells that cause GVHD.
“We then tested this concept in patients with chronic GVHD . . . ,” Dr Abedi said. “Almost all the patients who tolerated and remained on the treatment responded. In some cases, individual responses were quite dramatic. We were able to stop their other immunosuppressive medications and keep the patients under control with just weekly injections of bortezomib.”
Dr Abedi added that one patient had severe hemolytic anemia that did not respond to several lines of therapy.
“After receiving bortezomib, the patient’s symptoms improved, and we were able to take her completely off steroid and other immunosuppressive medications,” he said. “Another person had multiple ulcers, which completely healed. These were patients who had been on all different kinds of medications and had no response.”
This research is ongoing. Dr Abedi and his colleagues are now looking at a potential oral version of the drug and a similar agent that would alleviate the need for weekly injections and could have fewer side effects.
This research was funded by the National Cancer Institute, the National Institute of Allergy and Infectious Diseases, and Millennium Pharmaceuticals, makers of bortezomib.
Team creates functional vascular grafts in a week
Credit: Robert Emilsson
Researchers have found they can engineer vascular grafts using autologous peripheral blood and successfully transplant them into patients with portal vein thrombosis.
The team said this research provides early evidence for generating personalized blood vessels using stem cells from a blood sample rather than the bone marrow.
Suchitra Sumitran-Holgersson, PhD, of Sahlgrenska University Hospital in Sweden, and her colleagues described the work in EBioMedicine.
The researchers tested their new method on two young children with portal vein thrombosis.
The team used the patients’ own stem cells to grow new blood vessels, a procedure that had been performed at Sahlgrenska University Hospital once before. The novel finding was that they could do this without extracting the cells from the bone marrow.
The researchers took decellularized allogeneic vascular scaffolds and repopulated them with peripheral whole blood in a bioreactor.
The team found they could use 25 mL of blood, the minimum quantity needed to obtain enough stem cells. And the extraction procedure worked the first time.
“Not only that, but the blood itself accelerated growth of the new vein,” Dr Sumitran-Holgersson said. “The entire process took only a week, as opposed to a month in the first case. The blood contains substances that naturally promote growth.”
Dr Sumitran-Holgersson and her colleagues have treated three patients thus far. Two of those patients are still doing well and have veins that are functioning as they should. In the third case, the child is under medical surveillance, and the outcome is more uncertain.
“We believe that this technological progress can lead to dissemination of the method for the benefit of additional groups of patients, such as those with varicose veins or myocardial infarction, who need new blood vessels,” Dr Sumitran-Holgersson said. “Our dream is to be able to grow complete organs as a way of overcoming the current shortage from donors.”

Credit: Robert Emilsson
Researchers have found they can engineer vascular grafts using autologous peripheral blood and successfully transplant them into patients with portal vein thrombosis.
The team said this research provides early evidence for generating personalized blood vessels using stem cells from a blood sample rather than the bone marrow.
Suchitra Sumitran-Holgersson, PhD, of Sahlgrenska University Hospital in Sweden, and her colleagues described the work in EBioMedicine.
The researchers tested their new method on two young children with portal vein thrombosis.
The team used the patients’ own stem cells to grow new blood vessels, a procedure that had been performed at Sahlgrenska University Hospital once before. The novel finding was that they could do this without extracting the cells from the bone marrow.
The researchers took decellularized allogeneic vascular scaffolds and repopulated them with peripheral whole blood in a bioreactor.
The team found they could use 25 mL of blood, the minimum quantity needed to obtain enough stem cells. And the extraction procedure worked the first time.
“Not only that, but the blood itself accelerated growth of the new vein,” Dr Sumitran-Holgersson said. “The entire process took only a week, as opposed to a month in the first case. The blood contains substances that naturally promote growth.”
Dr Sumitran-Holgersson and her colleagues have treated three patients thus far. Two of those patients are still doing well and have veins that are functioning as they should. In the third case, the child is under medical surveillance, and the outcome is more uncertain.
“We believe that this technological progress can lead to dissemination of the method for the benefit of additional groups of patients, such as those with varicose veins or myocardial infarction, who need new blood vessels,” Dr Sumitran-Holgersson said. “Our dream is to be able to grow complete organs as a way of overcoming the current shortage from donors.”

Credit: Robert Emilsson
Researchers have found they can engineer vascular grafts using autologous peripheral blood and successfully transplant them into patients with portal vein thrombosis.
The team said this research provides early evidence for generating personalized blood vessels using stem cells from a blood sample rather than the bone marrow.
Suchitra Sumitran-Holgersson, PhD, of Sahlgrenska University Hospital in Sweden, and her colleagues described the work in EBioMedicine.
The researchers tested their new method on two young children with portal vein thrombosis.
The team used the patients’ own stem cells to grow new blood vessels, a procedure that had been performed at Sahlgrenska University Hospital once before. The novel finding was that they could do this without extracting the cells from the bone marrow.
The researchers took decellularized allogeneic vascular scaffolds and repopulated them with peripheral whole blood in a bioreactor.
The team found they could use 25 mL of blood, the minimum quantity needed to obtain enough stem cells. And the extraction procedure worked the first time.
“Not only that, but the blood itself accelerated growth of the new vein,” Dr Sumitran-Holgersson said. “The entire process took only a week, as opposed to a month in the first case. The blood contains substances that naturally promote growth.”
Dr Sumitran-Holgersson and her colleagues have treated three patients thus far. Two of those patients are still doing well and have veins that are functioning as they should. In the third case, the child is under medical surveillance, and the outcome is more uncertain.
“We believe that this technological progress can lead to dissemination of the method for the benefit of additional groups of patients, such as those with varicose veins or myocardial infarction, who need new blood vessels,” Dr Sumitran-Holgersson said. “Our dream is to be able to grow complete organs as a way of overcoming the current shortage from donors.”