User login
New AHA/ASA guideline on secondary stroke prevention
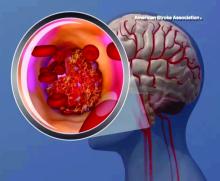
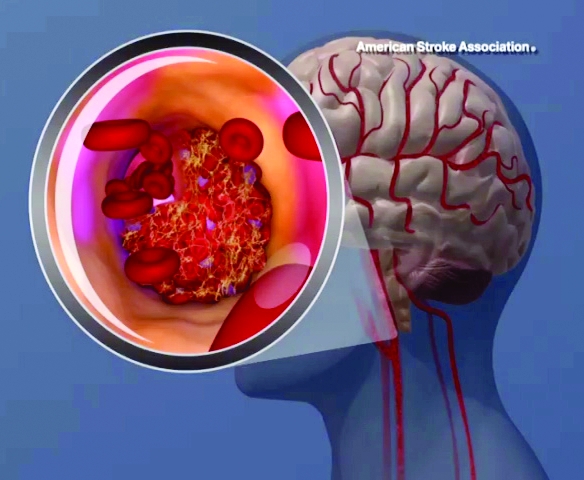
When possible, diagnostic tests to determine the cause of a first stroke or transient ischemic attack (TIA) should be completed within 48 hours after symptom onset, the American Heart Association/American Stroke Association said in an updated clinical practice guideline.
“It is critically important to understand the best ways to prevent another stroke once someone has had a stroke or a TIA,” Dawn O. Kleindorfer, MD, chair of the guideline writing group, said in a news release.
“If we can pinpoint the cause of the first stroke or TIA, we can tailor strategies to prevent a second stroke,” said Dr. Kleindorfer, professor and chair, department of neurology, University of Michigan, Ann Arbor.
The updated guideline was published online May 24, 2021, in Stroke.
“The secondary prevention of stroke guideline is one of the ASA’s ‘flagship’ guidelines, last updated in 2014,” Dr. Kleindorfer said.
The update includes “a number of changes to the writing and formatting of this guideline to make it easier for professionals to understand and locate information more quickly, ultimately greatly improving patient care and preventing more strokes in our patients,” she noted.
Let pathogenic subtype guide prevention
For patients who have survived a stroke or TIA, management of vascular risk factors, particularly hypertension, diabetes, cholesterol/triglyceride levels, and smoking cessation, are key secondary prevention tactics, the guideline said.
Limiting salt intake and/or following a heart-healthy Mediterranean diet is also advised, as is engaging in at least moderate-intensity aerobic activity for at least 10 minutes four times a week or vigorous-intensity aerobic activity for at least 20 minutes twice a week.
“Approximately 80% of strokes can be prevented by controlling blood pressure, eating a healthy diet, engaging in regular physical activity, not smoking and maintaining a healthy weight,” Amytis Towfighi, MD, vice chair of the guideline writing group and director of neurologic services, Los Angeles County Department of Health Services, noted in the release.
For health care professionals, the guideline said specific recommendations for secondary prevention often depend on the ischemic stroke/TIA subtype. “Therefore, new in this guideline is a section describing recommendations for the diagnostic workup after ischemic stroke, to define ischemic stroke pathogenesis (when possible), and to identify targets for treatment to reduce the risk of recurrent ischemic stroke. Recommendations are now segregated by pathogenetic subtype,” the guideline stated.
Among the recommendations:
- Use multidisciplinary care teams to personalize care for patients and employ shared decision-making with the patient to develop care plans that incorporate a patient’s wishes, goals, and concerns.
- Screen for and initiate anticoagulant drug therapy to reduce recurrent events.
- Prescribe antithrombotic therapy, including antiplatelets or anticoagulants, in the absence of contraindications. The guideline noted that the combination of antiplatelets and anticoagulation is typically not recommended for preventing second strokes and that dual antiplatelet therapy (DAPT) – taking along with a second medication to prevent blood clotting – is recommended in the short term and only for specific patients: those with early arriving minor stroke and high-risk TIA or severe symptomatic stenosis.
- Consider or carotid artery stenting for select patients with narrowing of carotid arteries.
- Aggressive medical management of risk factors and short-term DAPT are preferred for patients with severe intracranial stenosis thought to be the cause of first stroke or TIA.
- In some patients, it’s reasonable to consider percutaneous closure of .
The guideline is accompanied by a systematic review and meta-analysis regarding the benefits and risks of dual antiplatelet versus single antiplatelet therapy for secondary stroke prevention. The authors conclude that DAPT may be appropriate for select patients.
“Additional research is needed to determine: the optimal timing of starting treatment relative to the clinical event; the optimal duration of DAPT to maximize the risk-benefit ratio; whether additional populations excluded from POINT and CHANCE [two of the trials examined], such as those with major stroke, may also benefit from early DAPT; and whether certain genetic profiles eliminate the benefit of early DAPT,” concluded the reviewers, led by Devin Brown, MD, University of Michigan.
The guideline was prepared on behalf of and approved by the AHA Stroke Council’s Scientific Statements Oversight Committee on Clinical Practice Guidelines. The writing group included representatives from the AHA/ASA and the American Academy of Neurology. The guideline has been endorsed by the American Association of Neurological Surgeons/Congress of Neurological Surgeons and the Society of Vascular and Interventional Neurology. It has also been affirmed by the AAN as an educational tool for neurologists.
The research had no commercial funding.
A version of this article first appeared on Medscape.com.
When possible, diagnostic tests to determine the cause of a first stroke or transient ischemic attack (TIA) should be completed within 48 hours after symptom onset, the American Heart Association/American Stroke Association said in an updated clinical practice guideline.
“It is critically important to understand the best ways to prevent another stroke once someone has had a stroke or a TIA,” Dawn O. Kleindorfer, MD, chair of the guideline writing group, said in a news release.
“If we can pinpoint the cause of the first stroke or TIA, we can tailor strategies to prevent a second stroke,” said Dr. Kleindorfer, professor and chair, department of neurology, University of Michigan, Ann Arbor.
The updated guideline was published online May 24, 2021, in Stroke.
“The secondary prevention of stroke guideline is one of the ASA’s ‘flagship’ guidelines, last updated in 2014,” Dr. Kleindorfer said.
The update includes “a number of changes to the writing and formatting of this guideline to make it easier for professionals to understand and locate information more quickly, ultimately greatly improving patient care and preventing more strokes in our patients,” she noted.
Let pathogenic subtype guide prevention
For patients who have survived a stroke or TIA, management of vascular risk factors, particularly hypertension, diabetes, cholesterol/triglyceride levels, and smoking cessation, are key secondary prevention tactics, the guideline said.
Limiting salt intake and/or following a heart-healthy Mediterranean diet is also advised, as is engaging in at least moderate-intensity aerobic activity for at least 10 minutes four times a week or vigorous-intensity aerobic activity for at least 20 minutes twice a week.
“Approximately 80% of strokes can be prevented by controlling blood pressure, eating a healthy diet, engaging in regular physical activity, not smoking and maintaining a healthy weight,” Amytis Towfighi, MD, vice chair of the guideline writing group and director of neurologic services, Los Angeles County Department of Health Services, noted in the release.
For health care professionals, the guideline said specific recommendations for secondary prevention often depend on the ischemic stroke/TIA subtype. “Therefore, new in this guideline is a section describing recommendations for the diagnostic workup after ischemic stroke, to define ischemic stroke pathogenesis (when possible), and to identify targets for treatment to reduce the risk of recurrent ischemic stroke. Recommendations are now segregated by pathogenetic subtype,” the guideline stated.
Among the recommendations:
- Use multidisciplinary care teams to personalize care for patients and employ shared decision-making with the patient to develop care plans that incorporate a patient’s wishes, goals, and concerns.
- Screen for and initiate anticoagulant drug therapy to reduce recurrent events.
- Prescribe antithrombotic therapy, including antiplatelets or anticoagulants, in the absence of contraindications. The guideline noted that the combination of antiplatelets and anticoagulation is typically not recommended for preventing second strokes and that dual antiplatelet therapy (DAPT) – taking along with a second medication to prevent blood clotting – is recommended in the short term and only for specific patients: those with early arriving minor stroke and high-risk TIA or severe symptomatic stenosis.
- Consider or carotid artery stenting for select patients with narrowing of carotid arteries.
- Aggressive medical management of risk factors and short-term DAPT are preferred for patients with severe intracranial stenosis thought to be the cause of first stroke or TIA.
- In some patients, it’s reasonable to consider percutaneous closure of .
The guideline is accompanied by a systematic review and meta-analysis regarding the benefits and risks of dual antiplatelet versus single antiplatelet therapy for secondary stroke prevention. The authors conclude that DAPT may be appropriate for select patients.
“Additional research is needed to determine: the optimal timing of starting treatment relative to the clinical event; the optimal duration of DAPT to maximize the risk-benefit ratio; whether additional populations excluded from POINT and CHANCE [two of the trials examined], such as those with major stroke, may also benefit from early DAPT; and whether certain genetic profiles eliminate the benefit of early DAPT,” concluded the reviewers, led by Devin Brown, MD, University of Michigan.
The guideline was prepared on behalf of and approved by the AHA Stroke Council’s Scientific Statements Oversight Committee on Clinical Practice Guidelines. The writing group included representatives from the AHA/ASA and the American Academy of Neurology. The guideline has been endorsed by the American Association of Neurological Surgeons/Congress of Neurological Surgeons and the Society of Vascular and Interventional Neurology. It has also been affirmed by the AAN as an educational tool for neurologists.
The research had no commercial funding.
A version of this article first appeared on Medscape.com.
When possible, diagnostic tests to determine the cause of a first stroke or transient ischemic attack (TIA) should be completed within 48 hours after symptom onset, the American Heart Association/American Stroke Association said in an updated clinical practice guideline.
“It is critically important to understand the best ways to prevent another stroke once someone has had a stroke or a TIA,” Dawn O. Kleindorfer, MD, chair of the guideline writing group, said in a news release.
“If we can pinpoint the cause of the first stroke or TIA, we can tailor strategies to prevent a second stroke,” said Dr. Kleindorfer, professor and chair, department of neurology, University of Michigan, Ann Arbor.
The updated guideline was published online May 24, 2021, in Stroke.
“The secondary prevention of stroke guideline is one of the ASA’s ‘flagship’ guidelines, last updated in 2014,” Dr. Kleindorfer said.
The update includes “a number of changes to the writing and formatting of this guideline to make it easier for professionals to understand and locate information more quickly, ultimately greatly improving patient care and preventing more strokes in our patients,” she noted.
Let pathogenic subtype guide prevention
For patients who have survived a stroke or TIA, management of vascular risk factors, particularly hypertension, diabetes, cholesterol/triglyceride levels, and smoking cessation, are key secondary prevention tactics, the guideline said.
Limiting salt intake and/or following a heart-healthy Mediterranean diet is also advised, as is engaging in at least moderate-intensity aerobic activity for at least 10 minutes four times a week or vigorous-intensity aerobic activity for at least 20 minutes twice a week.
“Approximately 80% of strokes can be prevented by controlling blood pressure, eating a healthy diet, engaging in regular physical activity, not smoking and maintaining a healthy weight,” Amytis Towfighi, MD, vice chair of the guideline writing group and director of neurologic services, Los Angeles County Department of Health Services, noted in the release.
For health care professionals, the guideline said specific recommendations for secondary prevention often depend on the ischemic stroke/TIA subtype. “Therefore, new in this guideline is a section describing recommendations for the diagnostic workup after ischemic stroke, to define ischemic stroke pathogenesis (when possible), and to identify targets for treatment to reduce the risk of recurrent ischemic stroke. Recommendations are now segregated by pathogenetic subtype,” the guideline stated.
Among the recommendations:
- Use multidisciplinary care teams to personalize care for patients and employ shared decision-making with the patient to develop care plans that incorporate a patient’s wishes, goals, and concerns.
- Screen for and initiate anticoagulant drug therapy to reduce recurrent events.
- Prescribe antithrombotic therapy, including antiplatelets or anticoagulants, in the absence of contraindications. The guideline noted that the combination of antiplatelets and anticoagulation is typically not recommended for preventing second strokes and that dual antiplatelet therapy (DAPT) – taking along with a second medication to prevent blood clotting – is recommended in the short term and only for specific patients: those with early arriving minor stroke and high-risk TIA or severe symptomatic stenosis.
- Consider or carotid artery stenting for select patients with narrowing of carotid arteries.
- Aggressive medical management of risk factors and short-term DAPT are preferred for patients with severe intracranial stenosis thought to be the cause of first stroke or TIA.
- In some patients, it’s reasonable to consider percutaneous closure of .
The guideline is accompanied by a systematic review and meta-analysis regarding the benefits and risks of dual antiplatelet versus single antiplatelet therapy for secondary stroke prevention. The authors conclude that DAPT may be appropriate for select patients.
“Additional research is needed to determine: the optimal timing of starting treatment relative to the clinical event; the optimal duration of DAPT to maximize the risk-benefit ratio; whether additional populations excluded from POINT and CHANCE [two of the trials examined], such as those with major stroke, may also benefit from early DAPT; and whether certain genetic profiles eliminate the benefit of early DAPT,” concluded the reviewers, led by Devin Brown, MD, University of Michigan.
The guideline was prepared on behalf of and approved by the AHA Stroke Council’s Scientific Statements Oversight Committee on Clinical Practice Guidelines. The writing group included representatives from the AHA/ASA and the American Academy of Neurology. The guideline has been endorsed by the American Association of Neurological Surgeons/Congress of Neurological Surgeons and the Society of Vascular and Interventional Neurology. It has also been affirmed by the AAN as an educational tool for neurologists.
The research had no commercial funding.
A version of this article first appeared on Medscape.com.
Large vessel stroke linked to AstraZeneca COVID vaccine
The three cases (one of which was fatal) occurred in two women and one man in their 30s or 40s and involved blockages of the carotid and middle cerebral artery. Two of the three patients also had venous thrombosis involving the portal and cerebral venous system. All three also had extremely low platelet counts, confirmed antibodies to platelet factor 4, and raised D-dimer levels, all characteristic of the vaccine-induced immune thrombotic thrombocytopenia (VITT) reaction associated with the AstraZeneca vaccine.
They are described in detail in a letter published online on May 25 in the Journal of Neurology, Neurosurgery & Psychiatry
“These are [the] first detailed reports of arterial stroke believed to be caused by VITT after the AstraZeneca COVID vaccine, although stroke has been mentioned previously in the VITT data,” said senior author David Werring, PhD, FRCP.
“VITT has more commonly presented as CVST [Cerebral venous sinus thrombosis] which is stroke caused by a venous thrombosis; these cases are showing that it can also cause stroke caused by an arterial thrombosis,” explained Dr. Werring, professor of clinical neurology at the Stroke Research Centre, University College London.
“In patients who present with ischemic stroke, especially younger patients, and who have had the AstraZeneca vaccine within the past month, clinicians need to consider VITT as a possible cause, as there is a specific treatment needed for this syndrome,” he said.
Young patients presenting with ischemic stroke after receiving the AstraZeneca vaccine should urgently be evaluated for VITT with laboratory tests, including platelet count, D-dimers, fibrinogen, and anti-PF4 antibodies, the authors wrote, and then managed by a multidisciplinary team, including hematology, neurology, stroke, neurosurgery, and neuroradiology, for rapid access to treatments including intravenous immune globulin, methylprednisolone, plasmapheresis, and nonheparin anticoagulants such as fondaparinux, argatroban, or direct oral anticoagulants.
Dr. Werring noted that these reports do not add anything to the overall risk/benefit of the vaccine, as they are only describing three cases. “While VITT is very serious, the benefit of the vaccine still outweighs its risks,” he said. “Around 40% of patients hospitalized with COVID-19 experience some sort of thrombosis and about 1.5% have an ischemic stroke. Whereas latest figures from the U.K. estimate the incidence of VITT with the AstraZeneca vaccine of 1 in 50,000 to 1 in 100,000.
“Our report doesn’t suggest that VITT is more common than these latest figures estimate, but we are just drawing attention to an alternative presentation,” he added.
Three cases
The first patient in the current case series, a woman in her 30s, experienced an intermittent headache on the right side and around her eyes 6 days after the vaccine. Five days later, she awoke feeling drowsy and with weakness to her left face, arm, and leg.
Imaging revealed a blocked right middle cerebral artery with brain infarction and clots in the right portal vein. She underwent brain surgery to reduce the pressure in her skull, plasma removal and replacement, and received the anticoagulant fondaparinux, but she still unfortunately died.
The second patient, a woman in her late 30s, presented with headache, confusion, weakness in her left arm, and loss of vision on the left side 12 days after having received the vaccine. Imaging showed occlusion of both carotid arteries, as well as pulmonary embolism and a left cerebral venous sinus thrombosis.
Her platelet count increased following plasma removal and replacement and intravenous corticosteroids, and her condition improved after fondaparinux treatment.
The third patient, a man in his early 40s, presented 3 weeks after receiving his vaccination with problems speaking. Imaging showed a clot in the left middle cerebral artery, but there was no evidence of clots in the cerebral venous sinuses. He received a platelet and plasma transfusion, and fondaparinux, and remains stable.
High index of suspicion required
In a linked commentary, Hugh Markus, PhD, FRCP, professor of stroke medicine at the University of Cambridge, United Kingdom, wrote: “This report emphasizes that the immune mediated coagulopathy can also cause arterial thrombosis, including ischemic stroke, although venous thrombosis and especially cerebral venous sinus thrombosis appear more frequent.
“During the current period of COVID vaccination, a high index of suspicion is required to identify thrombotic episodes following vaccination,” he added. “However, it is important to remember that these side effects are rare and much less common than both cerebral venous thrombosis and ischemic stroke associated with COVID-19 infection itself.”
Risk/benefit unaltered
Several experts who commented on these reports for the Science Media Centre all agreed with Dr. Werring and Dr. Markus that these reports do not alter the current risk/benefit estimates with the vaccine.
Ian Douglas, PhD, professor of pharmacoepidemiology, London School of Hygiene & Tropical Medicine, who sits on the U.K.’s Medicines and Healthcare Products Regulatory Agency’s Pharmacovigilance Expert Advisory Group, said: “The picture regarding the rare syndrome of blood clots combined with low platelet counts associated with the AstraZeneca vaccine is becoming clearer. Until now, the cases described have tended to involve clots in veins such as cerebral vein thrombosis. In this series of three case reports, we now have some evidence that the types of blood vessels affected include arteries as well as veins.”
“It’s important to stress that such cases remain very rare, and it’s certainly much rarer in people who have had the AstraZeneca vaccine than it is in people affected by COVID-19 itself,” Dr. Douglas emphasized.
“The description of the cases suggests the patients involved presented with the same kind of symptoms as already described in cases involving cerebral vein thrombosis, and they don’t suggest patients need to be on the alert for anything different,” he added.
“However, the emergence of details like this will help guide health professionals who may be faced with similar cases in future; the sooner such cases are recognized, the more chance they will quickly receive the right kind of treatment, hopefully leading to better outcomes.”
Will Lester, MBChB, PhD, consultant hematologist, University Hospitals Birmingham NHS Foundation Trust, said: “VITT remains a rare complication, and patients with a history of thrombosis, including stroke, should not consider themselves to be at any higher risk of this type of rare thrombosis after vaccination, and COVID infection itself is a significant risk for stroke and other types of thrombosis.”
Many countries have paused use of the AstraZeneca vaccine because of its link to the VITT syndrome or restricted its use to older people as the VITT reaction appears to be slightly more common in younger people. In the United Kingdom, the current recommendation is that individuals under 40 years of age should be offered an alternative to the AstraZeneca vaccine where possible.
A version of this article first appeared on Medscape.com.
The three cases (one of which was fatal) occurred in two women and one man in their 30s or 40s and involved blockages of the carotid and middle cerebral artery. Two of the three patients also had venous thrombosis involving the portal and cerebral venous system. All three also had extremely low platelet counts, confirmed antibodies to platelet factor 4, and raised D-dimer levels, all characteristic of the vaccine-induced immune thrombotic thrombocytopenia (VITT) reaction associated with the AstraZeneca vaccine.
They are described in detail in a letter published online on May 25 in the Journal of Neurology, Neurosurgery & Psychiatry
“These are [the] first detailed reports of arterial stroke believed to be caused by VITT after the AstraZeneca COVID vaccine, although stroke has been mentioned previously in the VITT data,” said senior author David Werring, PhD, FRCP.
“VITT has more commonly presented as CVST [Cerebral venous sinus thrombosis] which is stroke caused by a venous thrombosis; these cases are showing that it can also cause stroke caused by an arterial thrombosis,” explained Dr. Werring, professor of clinical neurology at the Stroke Research Centre, University College London.
“In patients who present with ischemic stroke, especially younger patients, and who have had the AstraZeneca vaccine within the past month, clinicians need to consider VITT as a possible cause, as there is a specific treatment needed for this syndrome,” he said.
Young patients presenting with ischemic stroke after receiving the AstraZeneca vaccine should urgently be evaluated for VITT with laboratory tests, including platelet count, D-dimers, fibrinogen, and anti-PF4 antibodies, the authors wrote, and then managed by a multidisciplinary team, including hematology, neurology, stroke, neurosurgery, and neuroradiology, for rapid access to treatments including intravenous immune globulin, methylprednisolone, plasmapheresis, and nonheparin anticoagulants such as fondaparinux, argatroban, or direct oral anticoagulants.
Dr. Werring noted that these reports do not add anything to the overall risk/benefit of the vaccine, as they are only describing three cases. “While VITT is very serious, the benefit of the vaccine still outweighs its risks,” he said. “Around 40% of patients hospitalized with COVID-19 experience some sort of thrombosis and about 1.5% have an ischemic stroke. Whereas latest figures from the U.K. estimate the incidence of VITT with the AstraZeneca vaccine of 1 in 50,000 to 1 in 100,000.
“Our report doesn’t suggest that VITT is more common than these latest figures estimate, but we are just drawing attention to an alternative presentation,” he added.
Three cases
The first patient in the current case series, a woman in her 30s, experienced an intermittent headache on the right side and around her eyes 6 days after the vaccine. Five days later, she awoke feeling drowsy and with weakness to her left face, arm, and leg.
Imaging revealed a blocked right middle cerebral artery with brain infarction and clots in the right portal vein. She underwent brain surgery to reduce the pressure in her skull, plasma removal and replacement, and received the anticoagulant fondaparinux, but she still unfortunately died.
The second patient, a woman in her late 30s, presented with headache, confusion, weakness in her left arm, and loss of vision on the left side 12 days after having received the vaccine. Imaging showed occlusion of both carotid arteries, as well as pulmonary embolism and a left cerebral venous sinus thrombosis.
Her platelet count increased following plasma removal and replacement and intravenous corticosteroids, and her condition improved after fondaparinux treatment.
The third patient, a man in his early 40s, presented 3 weeks after receiving his vaccination with problems speaking. Imaging showed a clot in the left middle cerebral artery, but there was no evidence of clots in the cerebral venous sinuses. He received a platelet and plasma transfusion, and fondaparinux, and remains stable.
High index of suspicion required
In a linked commentary, Hugh Markus, PhD, FRCP, professor of stroke medicine at the University of Cambridge, United Kingdom, wrote: “This report emphasizes that the immune mediated coagulopathy can also cause arterial thrombosis, including ischemic stroke, although venous thrombosis and especially cerebral venous sinus thrombosis appear more frequent.
“During the current period of COVID vaccination, a high index of suspicion is required to identify thrombotic episodes following vaccination,” he added. “However, it is important to remember that these side effects are rare and much less common than both cerebral venous thrombosis and ischemic stroke associated with COVID-19 infection itself.”
Risk/benefit unaltered
Several experts who commented on these reports for the Science Media Centre all agreed with Dr. Werring and Dr. Markus that these reports do not alter the current risk/benefit estimates with the vaccine.
Ian Douglas, PhD, professor of pharmacoepidemiology, London School of Hygiene & Tropical Medicine, who sits on the U.K.’s Medicines and Healthcare Products Regulatory Agency’s Pharmacovigilance Expert Advisory Group, said: “The picture regarding the rare syndrome of blood clots combined with low platelet counts associated with the AstraZeneca vaccine is becoming clearer. Until now, the cases described have tended to involve clots in veins such as cerebral vein thrombosis. In this series of three case reports, we now have some evidence that the types of blood vessels affected include arteries as well as veins.”
“It’s important to stress that such cases remain very rare, and it’s certainly much rarer in people who have had the AstraZeneca vaccine than it is in people affected by COVID-19 itself,” Dr. Douglas emphasized.
“The description of the cases suggests the patients involved presented with the same kind of symptoms as already described in cases involving cerebral vein thrombosis, and they don’t suggest patients need to be on the alert for anything different,” he added.
“However, the emergence of details like this will help guide health professionals who may be faced with similar cases in future; the sooner such cases are recognized, the more chance they will quickly receive the right kind of treatment, hopefully leading to better outcomes.”
Will Lester, MBChB, PhD, consultant hematologist, University Hospitals Birmingham NHS Foundation Trust, said: “VITT remains a rare complication, and patients with a history of thrombosis, including stroke, should not consider themselves to be at any higher risk of this type of rare thrombosis after vaccination, and COVID infection itself is a significant risk for stroke and other types of thrombosis.”
Many countries have paused use of the AstraZeneca vaccine because of its link to the VITT syndrome or restricted its use to older people as the VITT reaction appears to be slightly more common in younger people. In the United Kingdom, the current recommendation is that individuals under 40 years of age should be offered an alternative to the AstraZeneca vaccine where possible.
A version of this article first appeared on Medscape.com.
The three cases (one of which was fatal) occurred in two women and one man in their 30s or 40s and involved blockages of the carotid and middle cerebral artery. Two of the three patients also had venous thrombosis involving the portal and cerebral venous system. All three also had extremely low platelet counts, confirmed antibodies to platelet factor 4, and raised D-dimer levels, all characteristic of the vaccine-induced immune thrombotic thrombocytopenia (VITT) reaction associated with the AstraZeneca vaccine.
They are described in detail in a letter published online on May 25 in the Journal of Neurology, Neurosurgery & Psychiatry
“These are [the] first detailed reports of arterial stroke believed to be caused by VITT after the AstraZeneca COVID vaccine, although stroke has been mentioned previously in the VITT data,” said senior author David Werring, PhD, FRCP.
“VITT has more commonly presented as CVST [Cerebral venous sinus thrombosis] which is stroke caused by a venous thrombosis; these cases are showing that it can also cause stroke caused by an arterial thrombosis,” explained Dr. Werring, professor of clinical neurology at the Stroke Research Centre, University College London.
“In patients who present with ischemic stroke, especially younger patients, and who have had the AstraZeneca vaccine within the past month, clinicians need to consider VITT as a possible cause, as there is a specific treatment needed for this syndrome,” he said.
Young patients presenting with ischemic stroke after receiving the AstraZeneca vaccine should urgently be evaluated for VITT with laboratory tests, including platelet count, D-dimers, fibrinogen, and anti-PF4 antibodies, the authors wrote, and then managed by a multidisciplinary team, including hematology, neurology, stroke, neurosurgery, and neuroradiology, for rapid access to treatments including intravenous immune globulin, methylprednisolone, plasmapheresis, and nonheparin anticoagulants such as fondaparinux, argatroban, or direct oral anticoagulants.
Dr. Werring noted that these reports do not add anything to the overall risk/benefit of the vaccine, as they are only describing three cases. “While VITT is very serious, the benefit of the vaccine still outweighs its risks,” he said. “Around 40% of patients hospitalized with COVID-19 experience some sort of thrombosis and about 1.5% have an ischemic stroke. Whereas latest figures from the U.K. estimate the incidence of VITT with the AstraZeneca vaccine of 1 in 50,000 to 1 in 100,000.
“Our report doesn’t suggest that VITT is more common than these latest figures estimate, but we are just drawing attention to an alternative presentation,” he added.
Three cases
The first patient in the current case series, a woman in her 30s, experienced an intermittent headache on the right side and around her eyes 6 days after the vaccine. Five days later, she awoke feeling drowsy and with weakness to her left face, arm, and leg.
Imaging revealed a blocked right middle cerebral artery with brain infarction and clots in the right portal vein. She underwent brain surgery to reduce the pressure in her skull, plasma removal and replacement, and received the anticoagulant fondaparinux, but she still unfortunately died.
The second patient, a woman in her late 30s, presented with headache, confusion, weakness in her left arm, and loss of vision on the left side 12 days after having received the vaccine. Imaging showed occlusion of both carotid arteries, as well as pulmonary embolism and a left cerebral venous sinus thrombosis.
Her platelet count increased following plasma removal and replacement and intravenous corticosteroids, and her condition improved after fondaparinux treatment.
The third patient, a man in his early 40s, presented 3 weeks after receiving his vaccination with problems speaking. Imaging showed a clot in the left middle cerebral artery, but there was no evidence of clots in the cerebral venous sinuses. He received a platelet and plasma transfusion, and fondaparinux, and remains stable.
High index of suspicion required
In a linked commentary, Hugh Markus, PhD, FRCP, professor of stroke medicine at the University of Cambridge, United Kingdom, wrote: “This report emphasizes that the immune mediated coagulopathy can also cause arterial thrombosis, including ischemic stroke, although venous thrombosis and especially cerebral venous sinus thrombosis appear more frequent.
“During the current period of COVID vaccination, a high index of suspicion is required to identify thrombotic episodes following vaccination,” he added. “However, it is important to remember that these side effects are rare and much less common than both cerebral venous thrombosis and ischemic stroke associated with COVID-19 infection itself.”
Risk/benefit unaltered
Several experts who commented on these reports for the Science Media Centre all agreed with Dr. Werring and Dr. Markus that these reports do not alter the current risk/benefit estimates with the vaccine.
Ian Douglas, PhD, professor of pharmacoepidemiology, London School of Hygiene & Tropical Medicine, who sits on the U.K.’s Medicines and Healthcare Products Regulatory Agency’s Pharmacovigilance Expert Advisory Group, said: “The picture regarding the rare syndrome of blood clots combined with low platelet counts associated with the AstraZeneca vaccine is becoming clearer. Until now, the cases described have tended to involve clots in veins such as cerebral vein thrombosis. In this series of three case reports, we now have some evidence that the types of blood vessels affected include arteries as well as veins.”
“It’s important to stress that such cases remain very rare, and it’s certainly much rarer in people who have had the AstraZeneca vaccine than it is in people affected by COVID-19 itself,” Dr. Douglas emphasized.
“The description of the cases suggests the patients involved presented with the same kind of symptoms as already described in cases involving cerebral vein thrombosis, and they don’t suggest patients need to be on the alert for anything different,” he added.
“However, the emergence of details like this will help guide health professionals who may be faced with similar cases in future; the sooner such cases are recognized, the more chance they will quickly receive the right kind of treatment, hopefully leading to better outcomes.”
Will Lester, MBChB, PhD, consultant hematologist, University Hospitals Birmingham NHS Foundation Trust, said: “VITT remains a rare complication, and patients with a history of thrombosis, including stroke, should not consider themselves to be at any higher risk of this type of rare thrombosis after vaccination, and COVID infection itself is a significant risk for stroke and other types of thrombosis.”
Many countries have paused use of the AstraZeneca vaccine because of its link to the VITT syndrome or restricted its use to older people as the VITT reaction appears to be slightly more common in younger people. In the United Kingdom, the current recommendation is that individuals under 40 years of age should be offered an alternative to the AstraZeneca vaccine where possible.
A version of this article first appeared on Medscape.com.
Final SPRINT data confirm lower BP is better
The results include data on some outcome events from the trial that had yet to be adjudicated when the primary analysis was released in 2015, as well as posttrial observational follow-up data collected through July 2016.
The data confirm and enhance the earlier findings and show that “lower is better” when it comes to blood pressure, primary investigator Cora E. Lewis, MD, professor and chair, department of epidemiology, University of Alabama at Birmingham, said in an interview.
Final results of the Systolic Blood Pressure Intervention Trial (SPRINT) were published in the May 20 issue of the New England Journal of Medicine.
For the trial, researchers enrolled 9,361 adults 50 years and older with a SBP between 130 and 180 mm Hg who were at increased risk for cardiovascular disease (CVD) but did not have a history of diabetes or stroke. Patients were randomly assigned to an intensive treatment target (SBP < 120 mm Hg) or a standard treatment target (SBP < 140 mm Hg).
In the final analysis, the rate of the primary outcome was 1.77% per year in the intensive-treatment group and 2.40% per year in the standard-treatment group (hazard ratio [HR], 0.73; 95% confidence interval [CR], 0.63-0.86; P < .001), similar to the earlier SPRINT findings.
All-cause mortality was 1.06% per year in the intensive-treatment group and 1.41% per year in the standard-treatment group (HR, 0.75; 95% CI, 0.61-0.92; P = .006), again similar to the previous findings.
“These results were highly statistically significant. It is remarkable in a trial powered for a composite CVD outcome to obtain a significant benefit for total mortality,” Dr. Lewis said.
She noted that one criticism of the initial SPRINT results was that, for the components of the primary outcome, only heart failure and death due to CVD were significantly lower in the intensively treated group.
“Heart failure can be difficult to diagnose from records in a clinical trial, and the critiques were that this was shaky evidence, given that more participants treated to less than 120 were on diuretics, which could decrease swelling, a key symptom of heart failure,” she explained.
“In these final results, SPRINT found that risk of myocardial infarction, heart failure, and death from CVD were significantly lower in the group treated to less than 120, and risk of the primary outcome, excluding heart failure, was still significantly lower in the more intensively treated group,” she noted.
After the trial phase ended, blood pressure treatment was returned to the participants’ usual source of medical care and the trial treatment goals were no longer pursued. SPRINT continued to collect data on the outcomes through July 2016. During this time, SBP rose 6.9 mm Hg in the intensive-treatment group and 2.6 mm Hg in the standard-treatment group.
“Putting all the data together from the trial phase and the phase after randomized interventions had been stopped, there was still a significant benefit for the more intensive treatment on the primary outcome and on death from all causes,” Dr. Lewis said.
In addition, a separate new analysis based on all the data showed significantly fewer first and recurrent primary outcome events with intensive treatment than with standard treatment (435 vs. 552; HR, 0.78; 95% CI, 0.69-0.89; P < .001).
Manageable risk
The pattern of safety events in the final analysis was similar to the 2015 report. In the intervention period, rates of serious adverse events overall did not differ significantly between the groups. However, rates of hypotension, electrolyte abnormalities, syncope (none leading to injurious falls), and acute kidney injury were higher in the intensive-treatment group.
As in other SPRINT reports, “acute kidney injury safety events were generally mild and there was nearly complete recovery of kidney function within 1 year,” Dr. Lewis said. “This and other analyses we have published indicate this is probably a hemodynamic effect.”
“Intensive treatment can be well tolerated and is generally safe with proper patient selection and monitoring. There are advantages to intensive therapy, and some risks, but I don’t think the risks are such that we should just throw the idea of more intensive treatment out the window,” Dr. Lewis said.
Reached for comment, Carlos G. Santos-Gallego, MD, from the Icahn School of Medicine at Mount Sinai in New York, said there has been “controversy” over whether intensive blood pressure control targeting systolic to below 120 mm Hg is beneficial.
“The original SPRINT trial is incredibly important, in that it conclusively demonstrated that among patients with hypertension and increased cardiovascular risk, targeting systolic blood pressure to below 120 mm Hg resulted in lower rates of adverse cardiovascular events and, importantly, all-cause mortality," compared with the conventional target of 140 mm Hg, he said in an interview.
“This final report of the SPRINT trial basically consolidates, confirms, and corroborates the original SPRINT data,” he noted. However, the final data are “more robust, with additional primary outcome events and all events having been adjudicated by a central committee, and there is an additional observation period of 1 extra year in which the treatment has been discontinued,” he said.
“Over time, we are becoming more and more certain that lower is better with blood pressure. We still have a long way to go, but the cardiology community is slowly becoming more intense in our treatment of blood pressure for our patients,” Dr. Santos-Gallego said.
The potential adverse effects of intensive blood pressure control are “very manageable,” he added.
Support for SPRINT was provided by the National Institutes of Health. Full disclosures for authors are available in the original article. Dr. Santos-Gallego has no relevant disclosures.
A version of this article first appeared on Medscape.com.
The results include data on some outcome events from the trial that had yet to be adjudicated when the primary analysis was released in 2015, as well as posttrial observational follow-up data collected through July 2016.
The data confirm and enhance the earlier findings and show that “lower is better” when it comes to blood pressure, primary investigator Cora E. Lewis, MD, professor and chair, department of epidemiology, University of Alabama at Birmingham, said in an interview.
Final results of the Systolic Blood Pressure Intervention Trial (SPRINT) were published in the May 20 issue of the New England Journal of Medicine.
For the trial, researchers enrolled 9,361 adults 50 years and older with a SBP between 130 and 180 mm Hg who were at increased risk for cardiovascular disease (CVD) but did not have a history of diabetes or stroke. Patients were randomly assigned to an intensive treatment target (SBP < 120 mm Hg) or a standard treatment target (SBP < 140 mm Hg).
In the final analysis, the rate of the primary outcome was 1.77% per year in the intensive-treatment group and 2.40% per year in the standard-treatment group (hazard ratio [HR], 0.73; 95% confidence interval [CR], 0.63-0.86; P < .001), similar to the earlier SPRINT findings.
All-cause mortality was 1.06% per year in the intensive-treatment group and 1.41% per year in the standard-treatment group (HR, 0.75; 95% CI, 0.61-0.92; P = .006), again similar to the previous findings.
“These results were highly statistically significant. It is remarkable in a trial powered for a composite CVD outcome to obtain a significant benefit for total mortality,” Dr. Lewis said.
She noted that one criticism of the initial SPRINT results was that, for the components of the primary outcome, only heart failure and death due to CVD were significantly lower in the intensively treated group.
“Heart failure can be difficult to diagnose from records in a clinical trial, and the critiques were that this was shaky evidence, given that more participants treated to less than 120 were on diuretics, which could decrease swelling, a key symptom of heart failure,” she explained.
“In these final results, SPRINT found that risk of myocardial infarction, heart failure, and death from CVD were significantly lower in the group treated to less than 120, and risk of the primary outcome, excluding heart failure, was still significantly lower in the more intensively treated group,” she noted.
After the trial phase ended, blood pressure treatment was returned to the participants’ usual source of medical care and the trial treatment goals were no longer pursued. SPRINT continued to collect data on the outcomes through July 2016. During this time, SBP rose 6.9 mm Hg in the intensive-treatment group and 2.6 mm Hg in the standard-treatment group.
“Putting all the data together from the trial phase and the phase after randomized interventions had been stopped, there was still a significant benefit for the more intensive treatment on the primary outcome and on death from all causes,” Dr. Lewis said.
In addition, a separate new analysis based on all the data showed significantly fewer first and recurrent primary outcome events with intensive treatment than with standard treatment (435 vs. 552; HR, 0.78; 95% CI, 0.69-0.89; P < .001).
Manageable risk
The pattern of safety events in the final analysis was similar to the 2015 report. In the intervention period, rates of serious adverse events overall did not differ significantly between the groups. However, rates of hypotension, electrolyte abnormalities, syncope (none leading to injurious falls), and acute kidney injury were higher in the intensive-treatment group.
As in other SPRINT reports, “acute kidney injury safety events were generally mild and there was nearly complete recovery of kidney function within 1 year,” Dr. Lewis said. “This and other analyses we have published indicate this is probably a hemodynamic effect.”
“Intensive treatment can be well tolerated and is generally safe with proper patient selection and monitoring. There are advantages to intensive therapy, and some risks, but I don’t think the risks are such that we should just throw the idea of more intensive treatment out the window,” Dr. Lewis said.
Reached for comment, Carlos G. Santos-Gallego, MD, from the Icahn School of Medicine at Mount Sinai in New York, said there has been “controversy” over whether intensive blood pressure control targeting systolic to below 120 mm Hg is beneficial.
“The original SPRINT trial is incredibly important, in that it conclusively demonstrated that among patients with hypertension and increased cardiovascular risk, targeting systolic blood pressure to below 120 mm Hg resulted in lower rates of adverse cardiovascular events and, importantly, all-cause mortality," compared with the conventional target of 140 mm Hg, he said in an interview.
“This final report of the SPRINT trial basically consolidates, confirms, and corroborates the original SPRINT data,” he noted. However, the final data are “more robust, with additional primary outcome events and all events having been adjudicated by a central committee, and there is an additional observation period of 1 extra year in which the treatment has been discontinued,” he said.
“Over time, we are becoming more and more certain that lower is better with blood pressure. We still have a long way to go, but the cardiology community is slowly becoming more intense in our treatment of blood pressure for our patients,” Dr. Santos-Gallego said.
The potential adverse effects of intensive blood pressure control are “very manageable,” he added.
Support for SPRINT was provided by the National Institutes of Health. Full disclosures for authors are available in the original article. Dr. Santos-Gallego has no relevant disclosures.
A version of this article first appeared on Medscape.com.
The results include data on some outcome events from the trial that had yet to be adjudicated when the primary analysis was released in 2015, as well as posttrial observational follow-up data collected through July 2016.
The data confirm and enhance the earlier findings and show that “lower is better” when it comes to blood pressure, primary investigator Cora E. Lewis, MD, professor and chair, department of epidemiology, University of Alabama at Birmingham, said in an interview.
Final results of the Systolic Blood Pressure Intervention Trial (SPRINT) were published in the May 20 issue of the New England Journal of Medicine.
For the trial, researchers enrolled 9,361 adults 50 years and older with a SBP between 130 and 180 mm Hg who were at increased risk for cardiovascular disease (CVD) but did not have a history of diabetes or stroke. Patients were randomly assigned to an intensive treatment target (SBP < 120 mm Hg) or a standard treatment target (SBP < 140 mm Hg).
In the final analysis, the rate of the primary outcome was 1.77% per year in the intensive-treatment group and 2.40% per year in the standard-treatment group (hazard ratio [HR], 0.73; 95% confidence interval [CR], 0.63-0.86; P < .001), similar to the earlier SPRINT findings.
All-cause mortality was 1.06% per year in the intensive-treatment group and 1.41% per year in the standard-treatment group (HR, 0.75; 95% CI, 0.61-0.92; P = .006), again similar to the previous findings.
“These results were highly statistically significant. It is remarkable in a trial powered for a composite CVD outcome to obtain a significant benefit for total mortality,” Dr. Lewis said.
She noted that one criticism of the initial SPRINT results was that, for the components of the primary outcome, only heart failure and death due to CVD were significantly lower in the intensively treated group.
“Heart failure can be difficult to diagnose from records in a clinical trial, and the critiques were that this was shaky evidence, given that more participants treated to less than 120 were on diuretics, which could decrease swelling, a key symptom of heart failure,” she explained.
“In these final results, SPRINT found that risk of myocardial infarction, heart failure, and death from CVD were significantly lower in the group treated to less than 120, and risk of the primary outcome, excluding heart failure, was still significantly lower in the more intensively treated group,” she noted.
After the trial phase ended, blood pressure treatment was returned to the participants’ usual source of medical care and the trial treatment goals were no longer pursued. SPRINT continued to collect data on the outcomes through July 2016. During this time, SBP rose 6.9 mm Hg in the intensive-treatment group and 2.6 mm Hg in the standard-treatment group.
“Putting all the data together from the trial phase and the phase after randomized interventions had been stopped, there was still a significant benefit for the more intensive treatment on the primary outcome and on death from all causes,” Dr. Lewis said.
In addition, a separate new analysis based on all the data showed significantly fewer first and recurrent primary outcome events with intensive treatment than with standard treatment (435 vs. 552; HR, 0.78; 95% CI, 0.69-0.89; P < .001).
Manageable risk
The pattern of safety events in the final analysis was similar to the 2015 report. In the intervention period, rates of serious adverse events overall did not differ significantly between the groups. However, rates of hypotension, electrolyte abnormalities, syncope (none leading to injurious falls), and acute kidney injury were higher in the intensive-treatment group.
As in other SPRINT reports, “acute kidney injury safety events were generally mild and there was nearly complete recovery of kidney function within 1 year,” Dr. Lewis said. “This and other analyses we have published indicate this is probably a hemodynamic effect.”
“Intensive treatment can be well tolerated and is generally safe with proper patient selection and monitoring. There are advantages to intensive therapy, and some risks, but I don’t think the risks are such that we should just throw the idea of more intensive treatment out the window,” Dr. Lewis said.
Reached for comment, Carlos G. Santos-Gallego, MD, from the Icahn School of Medicine at Mount Sinai in New York, said there has been “controversy” over whether intensive blood pressure control targeting systolic to below 120 mm Hg is beneficial.
“The original SPRINT trial is incredibly important, in that it conclusively demonstrated that among patients with hypertension and increased cardiovascular risk, targeting systolic blood pressure to below 120 mm Hg resulted in lower rates of adverse cardiovascular events and, importantly, all-cause mortality," compared with the conventional target of 140 mm Hg, he said in an interview.
“This final report of the SPRINT trial basically consolidates, confirms, and corroborates the original SPRINT data,” he noted. However, the final data are “more robust, with additional primary outcome events and all events having been adjudicated by a central committee, and there is an additional observation period of 1 extra year in which the treatment has been discontinued,” he said.
“Over time, we are becoming more and more certain that lower is better with blood pressure. We still have a long way to go, but the cardiology community is slowly becoming more intense in our treatment of blood pressure for our patients,” Dr. Santos-Gallego said.
The potential adverse effects of intensive blood pressure control are “very manageable,” he added.
Support for SPRINT was provided by the National Institutes of Health. Full disclosures for authors are available in the original article. Dr. Santos-Gallego has no relevant disclosures.
A version of this article first appeared on Medscape.com.
LAAOS III: Surgical LAA closure cuts AFib stroke risk by one third
Left atrial appendage occlusion performed at the time of other heart surgery reduces the risk for stroke by about one-third in high-risk patients with atrial fibrillation (AFib), according to results of the Left Atrial Appendage Occlusion Study III (LAAOS III).
At 3.8 years’ follow-up, the primary endpoint of ischemic stroke or systemic embolism occurred in 4.8% of patients randomly assigned to left atrial appendage occlusion (LAAO) and 7.0% of those with no occlusion. This translated into a 33% relative risk reduction (hazard ratio, 0.67; 95% confidence interval, 0.53-0.85; P = .001).
In a landmark analysis, the effect was present early on but was more pronounced after the first 30 days, reducing the relative risk by 42% (HR, 0.58; 95% CI, 0.42-0.80), the researchers report.
The reduction in ongoing stroke risk was on top of oral anticoagulation (OAC) and consistent across all subgroups, Richard Whitlock, MD, PhD, professor of surgery, McMaster University, Hamilton, Ont., reported in a late-breaking trial session at the annual scientific sessions of the American College of Cardiology.
The procedure was safe and added, on average, just 6 minutes to cardiopulmonary bypass time, according to the results, simultaneously published in the New England Journal of Medicine.
“Any patient who comes to the operating room who fits the profile of a LAAOS III patient – so has atrial fibrillation and an elevated stroke risk based on their CHA2DS2-VASc score – the appendage should come off,” he said in an interview.
Commenting during the formal discussion, panelist Michael J. Mack, MD, of Baylor Health Care System in Houston, said, “This is potentially a game-changing, practice-changing study” but asked if there are any patients who shouldn’t undergo LAAO, such as those with heart failure (HF).
Dr. Whitlock said about 10%-15% of patients coming for heart surgery have a history of AFib and “as surgeons, you do need to individualize therapy. If you have a very frail patient, have concerns about tissue quality, you really need to think about how you would occlude the left atrial appendage or if you would occlude.”
Reassuringly, he noted, the data show no increase in HF hospitalizations and a beneficial effect on stroke among patients with HF and those with low ejection fractions, below 50%.
Observational data on surgical occlusion have been inconsistent, and current guidelines offer a weak recommendation in patients with AFib who have a contraindication to long-term anticoagulation. This is the first study to definitively prove that ischemic stroke is reduced by managing the left atrial appendage, he said in an interview.
“The previous percutaneous trials failed to demonstrate that; they demonstrated noninferiority but it was driven primarily by the avoidance of hemorrhagic events or strokes through taking patients off oral anticoagulation,” he said.
The results should translate into a class I guideline recommendation, he added. “This opens up a new paradigm of treatment for atrial fibrillation and stroke prevention in that it is really the first study that has looked at the additive effects of managing the left atrial appendage in addition to oral anticoagulation, and it’s protective on top of oral anticoagulation. That is a paradigm shift.”
In an accompanying editorial, Richard L. Page, MD, University of Vermont in Burlington, said the trial provides no insight on the possible benefit of surgical occlusion in patients unable to receive anticoagulation or with a lower CHA2DS2-VASc score, but he agreed a class I recommendation is likely for the population studied.
“I hope and anticipate that the results of this paper will strengthen the guideline indications for surgical left atrial appendage occlusion and will increase the number of cardiac surgeons who routinely perform this add-on procedure,” he said. “While many already perform this procedure, cardiac surgeons should now feel more comfortable that surgical left atrial appendage occlusion is indicated and supported by high-quality randomized data.”
Unfortunately, LAAOS III does not answer the question of whether patients can come off anticoagulation, but it does show surgical occlusion provides added protection from strokes, which can be huge with atrial fibrillation, Dr. Whitlock said.
“I spoke with a patient today who is an active 66-year-old individual on a [direct oral anticoagulant], and his stroke risk has been further reduced by 30%-40%, so he was ecstatic to hear the results,” Dr. Whitlock said. “I think it’s peace of mind.”
Global, nonindustry effort
LAAOS III investigators at 105 centers in 27 countries enrolled 4,811 patients undergoing cardiac surgery (mean age, 71 years; 68% male) who had a CHA2DS2-VASc score of at least 2.
In all, 4,770 were randomly assigned to no LAAO or occlusion via the preferred technique of amputation with suture closure of the stump as well as stapler occlusion, or epicardial device closure with the AtriClip (AtriCure) or TigerPaw (Maquet Medical). The treating team, researchers, and patients were blinded to assignment.
Patients were followed every 6 months with a validated stroke questionnaire. The trial was stopped early by the data safety monitoring board after the second interim analysis.
The mean CHA2DS2-VASc score was 4.2, one-third of patients had permanent AFib, 9% had a history of stroke, and more than two-thirds underwent a valve procedure, which makes LAAOS III unique, as many previous trials excluded valvular AFib, Dr. Whitlock pointed out.
Operative outcomes in the LAAO and no-LAAO groups were as follows:
- Bypass time: mean, 119 minutes vs. 113 minutes.
- Cross-clamp time: mean, 86 minutes vs. 82 minutes.
- Chest tube output: median, 520 mL vs. 500 mL.
- Reoperation for bleeding: both, 4.0%.
- Prolonged hospitalization due to HF: 5 vs. 14 events.
- 30-day mortality: 3.7% vs 4.0%.
The primary safety outcome of HF hospitalization at 3.8 years occurred in 7.7% of patients with LAAO and 6.8% without occlusion (HR, 1.13; 95% CI, 0.92-1.40), despite concerns that taking off the appendage could worsen HF risk by impairing renal clearance of salt and water.
“There’s observational data on either side of the fence, so it was an important endpoint that people were concerned about,” Dr. Whitlock told this news organization. “We had a data collection firm dedicated to admission for heart failure to really tease that out and, in the end, we saw no adverse effect.”
Although rates of ischemic stroke at 3.8 years were lower with LAAO than without (4.2% vs. 6.6%; HR, 0.62; 95% CI, 0.48-0.80), there was no difference in systemic embolism (0.3% for both) or death (22.6% vs. 22.5%).
In LAAOS III, fewer than 2% of the deaths were attributed to stroke, which is consistent with large stroke registries, Dr. Whitlock said. “Stroke is not what causes people with atrial fibrillation to die; it’s actually the progression on to heart failure.”
The positive effect on stroke was consistent across all subgroups, including sex, age, rheumatic heart disease, type of OAC at baseline, CHA2DS2-VASc score (≤4 vs. >4), type of surgery, history of heart failure or hypertension, and prior stroke/transient ischemic attack/systemic embolism.
Panelist Anne B. Curtis, MD, State University of New York at Buffalo, expressed surprise that about half of patients at baseline were not receiving anticoagulation and questioned whether event rates varied among those who did and didn’t stay on OAC.
Dr. Whitlock noted that OAC is often underused in AFib and that analyses showed the effects were consistent whether patients were on or off anticoagulants.
The study was sponsored by the Population Health Research Institute, McMaster University. Dr. Whitlock reported no relevant disclosures. Dr. Curtis reported consultant fees/honoraria from Abbott, Janssen, Medtronic, Milestone Pharmaceuticals, and Sanofi Aventis, and data safety monitoring board participation for Medtronic.
A version of this article first appeared on Medscape.com.
Left atrial appendage occlusion performed at the time of other heart surgery reduces the risk for stroke by about one-third in high-risk patients with atrial fibrillation (AFib), according to results of the Left Atrial Appendage Occlusion Study III (LAAOS III).
At 3.8 years’ follow-up, the primary endpoint of ischemic stroke or systemic embolism occurred in 4.8% of patients randomly assigned to left atrial appendage occlusion (LAAO) and 7.0% of those with no occlusion. This translated into a 33% relative risk reduction (hazard ratio, 0.67; 95% confidence interval, 0.53-0.85; P = .001).
In a landmark analysis, the effect was present early on but was more pronounced after the first 30 days, reducing the relative risk by 42% (HR, 0.58; 95% CI, 0.42-0.80), the researchers report.
The reduction in ongoing stroke risk was on top of oral anticoagulation (OAC) and consistent across all subgroups, Richard Whitlock, MD, PhD, professor of surgery, McMaster University, Hamilton, Ont., reported in a late-breaking trial session at the annual scientific sessions of the American College of Cardiology.
The procedure was safe and added, on average, just 6 minutes to cardiopulmonary bypass time, according to the results, simultaneously published in the New England Journal of Medicine.
“Any patient who comes to the operating room who fits the profile of a LAAOS III patient – so has atrial fibrillation and an elevated stroke risk based on their CHA2DS2-VASc score – the appendage should come off,” he said in an interview.
Commenting during the formal discussion, panelist Michael J. Mack, MD, of Baylor Health Care System in Houston, said, “This is potentially a game-changing, practice-changing study” but asked if there are any patients who shouldn’t undergo LAAO, such as those with heart failure (HF).
Dr. Whitlock said about 10%-15% of patients coming for heart surgery have a history of AFib and “as surgeons, you do need to individualize therapy. If you have a very frail patient, have concerns about tissue quality, you really need to think about how you would occlude the left atrial appendage or if you would occlude.”
Reassuringly, he noted, the data show no increase in HF hospitalizations and a beneficial effect on stroke among patients with HF and those with low ejection fractions, below 50%.
Observational data on surgical occlusion have been inconsistent, and current guidelines offer a weak recommendation in patients with AFib who have a contraindication to long-term anticoagulation. This is the first study to definitively prove that ischemic stroke is reduced by managing the left atrial appendage, he said in an interview.
“The previous percutaneous trials failed to demonstrate that; they demonstrated noninferiority but it was driven primarily by the avoidance of hemorrhagic events or strokes through taking patients off oral anticoagulation,” he said.
The results should translate into a class I guideline recommendation, he added. “This opens up a new paradigm of treatment for atrial fibrillation and stroke prevention in that it is really the first study that has looked at the additive effects of managing the left atrial appendage in addition to oral anticoagulation, and it’s protective on top of oral anticoagulation. That is a paradigm shift.”
In an accompanying editorial, Richard L. Page, MD, University of Vermont in Burlington, said the trial provides no insight on the possible benefit of surgical occlusion in patients unable to receive anticoagulation or with a lower CHA2DS2-VASc score, but he agreed a class I recommendation is likely for the population studied.
“I hope and anticipate that the results of this paper will strengthen the guideline indications for surgical left atrial appendage occlusion and will increase the number of cardiac surgeons who routinely perform this add-on procedure,” he said. “While many already perform this procedure, cardiac surgeons should now feel more comfortable that surgical left atrial appendage occlusion is indicated and supported by high-quality randomized data.”
Unfortunately, LAAOS III does not answer the question of whether patients can come off anticoagulation, but it does show surgical occlusion provides added protection from strokes, which can be huge with atrial fibrillation, Dr. Whitlock said.
“I spoke with a patient today who is an active 66-year-old individual on a [direct oral anticoagulant], and his stroke risk has been further reduced by 30%-40%, so he was ecstatic to hear the results,” Dr. Whitlock said. “I think it’s peace of mind.”
Global, nonindustry effort
LAAOS III investigators at 105 centers in 27 countries enrolled 4,811 patients undergoing cardiac surgery (mean age, 71 years; 68% male) who had a CHA2DS2-VASc score of at least 2.
In all, 4,770 were randomly assigned to no LAAO or occlusion via the preferred technique of amputation with suture closure of the stump as well as stapler occlusion, or epicardial device closure with the AtriClip (AtriCure) or TigerPaw (Maquet Medical). The treating team, researchers, and patients were blinded to assignment.
Patients were followed every 6 months with a validated stroke questionnaire. The trial was stopped early by the data safety monitoring board after the second interim analysis.
The mean CHA2DS2-VASc score was 4.2, one-third of patients had permanent AFib, 9% had a history of stroke, and more than two-thirds underwent a valve procedure, which makes LAAOS III unique, as many previous trials excluded valvular AFib, Dr. Whitlock pointed out.
Operative outcomes in the LAAO and no-LAAO groups were as follows:
- Bypass time: mean, 119 minutes vs. 113 minutes.
- Cross-clamp time: mean, 86 minutes vs. 82 minutes.
- Chest tube output: median, 520 mL vs. 500 mL.
- Reoperation for bleeding: both, 4.0%.
- Prolonged hospitalization due to HF: 5 vs. 14 events.
- 30-day mortality: 3.7% vs 4.0%.
The primary safety outcome of HF hospitalization at 3.8 years occurred in 7.7% of patients with LAAO and 6.8% without occlusion (HR, 1.13; 95% CI, 0.92-1.40), despite concerns that taking off the appendage could worsen HF risk by impairing renal clearance of salt and water.
“There’s observational data on either side of the fence, so it was an important endpoint that people were concerned about,” Dr. Whitlock told this news organization. “We had a data collection firm dedicated to admission for heart failure to really tease that out and, in the end, we saw no adverse effect.”
Although rates of ischemic stroke at 3.8 years were lower with LAAO than without (4.2% vs. 6.6%; HR, 0.62; 95% CI, 0.48-0.80), there was no difference in systemic embolism (0.3% for both) or death (22.6% vs. 22.5%).
In LAAOS III, fewer than 2% of the deaths were attributed to stroke, which is consistent with large stroke registries, Dr. Whitlock said. “Stroke is not what causes people with atrial fibrillation to die; it’s actually the progression on to heart failure.”
The positive effect on stroke was consistent across all subgroups, including sex, age, rheumatic heart disease, type of OAC at baseline, CHA2DS2-VASc score (≤4 vs. >4), type of surgery, history of heart failure or hypertension, and prior stroke/transient ischemic attack/systemic embolism.
Panelist Anne B. Curtis, MD, State University of New York at Buffalo, expressed surprise that about half of patients at baseline were not receiving anticoagulation and questioned whether event rates varied among those who did and didn’t stay on OAC.
Dr. Whitlock noted that OAC is often underused in AFib and that analyses showed the effects were consistent whether patients were on or off anticoagulants.
The study was sponsored by the Population Health Research Institute, McMaster University. Dr. Whitlock reported no relevant disclosures. Dr. Curtis reported consultant fees/honoraria from Abbott, Janssen, Medtronic, Milestone Pharmaceuticals, and Sanofi Aventis, and data safety monitoring board participation for Medtronic.
A version of this article first appeared on Medscape.com.
Left atrial appendage occlusion performed at the time of other heart surgery reduces the risk for stroke by about one-third in high-risk patients with atrial fibrillation (AFib), according to results of the Left Atrial Appendage Occlusion Study III (LAAOS III).
At 3.8 years’ follow-up, the primary endpoint of ischemic stroke or systemic embolism occurred in 4.8% of patients randomly assigned to left atrial appendage occlusion (LAAO) and 7.0% of those with no occlusion. This translated into a 33% relative risk reduction (hazard ratio, 0.67; 95% confidence interval, 0.53-0.85; P = .001).
In a landmark analysis, the effect was present early on but was more pronounced after the first 30 days, reducing the relative risk by 42% (HR, 0.58; 95% CI, 0.42-0.80), the researchers report.
The reduction in ongoing stroke risk was on top of oral anticoagulation (OAC) and consistent across all subgroups, Richard Whitlock, MD, PhD, professor of surgery, McMaster University, Hamilton, Ont., reported in a late-breaking trial session at the annual scientific sessions of the American College of Cardiology.
The procedure was safe and added, on average, just 6 minutes to cardiopulmonary bypass time, according to the results, simultaneously published in the New England Journal of Medicine.
“Any patient who comes to the operating room who fits the profile of a LAAOS III patient – so has atrial fibrillation and an elevated stroke risk based on their CHA2DS2-VASc score – the appendage should come off,” he said in an interview.
Commenting during the formal discussion, panelist Michael J. Mack, MD, of Baylor Health Care System in Houston, said, “This is potentially a game-changing, practice-changing study” but asked if there are any patients who shouldn’t undergo LAAO, such as those with heart failure (HF).
Dr. Whitlock said about 10%-15% of patients coming for heart surgery have a history of AFib and “as surgeons, you do need to individualize therapy. If you have a very frail patient, have concerns about tissue quality, you really need to think about how you would occlude the left atrial appendage or if you would occlude.”
Reassuringly, he noted, the data show no increase in HF hospitalizations and a beneficial effect on stroke among patients with HF and those with low ejection fractions, below 50%.
Observational data on surgical occlusion have been inconsistent, and current guidelines offer a weak recommendation in patients with AFib who have a contraindication to long-term anticoagulation. This is the first study to definitively prove that ischemic stroke is reduced by managing the left atrial appendage, he said in an interview.
“The previous percutaneous trials failed to demonstrate that; they demonstrated noninferiority but it was driven primarily by the avoidance of hemorrhagic events or strokes through taking patients off oral anticoagulation,” he said.
The results should translate into a class I guideline recommendation, he added. “This opens up a new paradigm of treatment for atrial fibrillation and stroke prevention in that it is really the first study that has looked at the additive effects of managing the left atrial appendage in addition to oral anticoagulation, and it’s protective on top of oral anticoagulation. That is a paradigm shift.”
In an accompanying editorial, Richard L. Page, MD, University of Vermont in Burlington, said the trial provides no insight on the possible benefit of surgical occlusion in patients unable to receive anticoagulation or with a lower CHA2DS2-VASc score, but he agreed a class I recommendation is likely for the population studied.
“I hope and anticipate that the results of this paper will strengthen the guideline indications for surgical left atrial appendage occlusion and will increase the number of cardiac surgeons who routinely perform this add-on procedure,” he said. “While many already perform this procedure, cardiac surgeons should now feel more comfortable that surgical left atrial appendage occlusion is indicated and supported by high-quality randomized data.”
Unfortunately, LAAOS III does not answer the question of whether patients can come off anticoagulation, but it does show surgical occlusion provides added protection from strokes, which can be huge with atrial fibrillation, Dr. Whitlock said.
“I spoke with a patient today who is an active 66-year-old individual on a [direct oral anticoagulant], and his stroke risk has been further reduced by 30%-40%, so he was ecstatic to hear the results,” Dr. Whitlock said. “I think it’s peace of mind.”
Global, nonindustry effort
LAAOS III investigators at 105 centers in 27 countries enrolled 4,811 patients undergoing cardiac surgery (mean age, 71 years; 68% male) who had a CHA2DS2-VASc score of at least 2.
In all, 4,770 were randomly assigned to no LAAO or occlusion via the preferred technique of amputation with suture closure of the stump as well as stapler occlusion, or epicardial device closure with the AtriClip (AtriCure) or TigerPaw (Maquet Medical). The treating team, researchers, and patients were blinded to assignment.
Patients were followed every 6 months with a validated stroke questionnaire. The trial was stopped early by the data safety monitoring board after the second interim analysis.
The mean CHA2DS2-VASc score was 4.2, one-third of patients had permanent AFib, 9% had a history of stroke, and more than two-thirds underwent a valve procedure, which makes LAAOS III unique, as many previous trials excluded valvular AFib, Dr. Whitlock pointed out.
Operative outcomes in the LAAO and no-LAAO groups were as follows:
- Bypass time: mean, 119 minutes vs. 113 minutes.
- Cross-clamp time: mean, 86 minutes vs. 82 minutes.
- Chest tube output: median, 520 mL vs. 500 mL.
- Reoperation for bleeding: both, 4.0%.
- Prolonged hospitalization due to HF: 5 vs. 14 events.
- 30-day mortality: 3.7% vs 4.0%.
The primary safety outcome of HF hospitalization at 3.8 years occurred in 7.7% of patients with LAAO and 6.8% without occlusion (HR, 1.13; 95% CI, 0.92-1.40), despite concerns that taking off the appendage could worsen HF risk by impairing renal clearance of salt and water.
“There’s observational data on either side of the fence, so it was an important endpoint that people were concerned about,” Dr. Whitlock told this news organization. “We had a data collection firm dedicated to admission for heart failure to really tease that out and, in the end, we saw no adverse effect.”
Although rates of ischemic stroke at 3.8 years were lower with LAAO than without (4.2% vs. 6.6%; HR, 0.62; 95% CI, 0.48-0.80), there was no difference in systemic embolism (0.3% for both) or death (22.6% vs. 22.5%).
In LAAOS III, fewer than 2% of the deaths were attributed to stroke, which is consistent with large stroke registries, Dr. Whitlock said. “Stroke is not what causes people with atrial fibrillation to die; it’s actually the progression on to heart failure.”
The positive effect on stroke was consistent across all subgroups, including sex, age, rheumatic heart disease, type of OAC at baseline, CHA2DS2-VASc score (≤4 vs. >4), type of surgery, history of heart failure or hypertension, and prior stroke/transient ischemic attack/systemic embolism.
Panelist Anne B. Curtis, MD, State University of New York at Buffalo, expressed surprise that about half of patients at baseline were not receiving anticoagulation and questioned whether event rates varied among those who did and didn’t stay on OAC.
Dr. Whitlock noted that OAC is often underused in AFib and that analyses showed the effects were consistent whether patients were on or off anticoagulants.
The study was sponsored by the Population Health Research Institute, McMaster University. Dr. Whitlock reported no relevant disclosures. Dr. Curtis reported consultant fees/honoraria from Abbott, Janssen, Medtronic, Milestone Pharmaceuticals, and Sanofi Aventis, and data safety monitoring board participation for Medtronic.
A version of this article first appeared on Medscape.com.
FROM ACC 2021
ADAPTABLE: Low-dose aspirin as good as high-dose in CHD?
No significant difference in cardiovascular events or major bleeding was shown between patients with established coronary heart disease assigned to a daily aspirin dose of 81 mg and those receiving a dose of 325 mg in the 15,000-patient ADAPTABLE trial.
Although substantial dose switching occurred in the trial, particularly from the higher to the lower dose, lead investigator W. Schuyler Jones, MD, believes the results support the use of the 81-mg dose in most patients.
“While we would have liked to see higher adherence to the assigned doses, we think the results of the trial are reliable,” Dr. Jones said in an interview.
The real-world, open-label, pragmatic trial also involved an innovative low-cost design allowing researchers to identify and communicate with eligible patients directly, opening up a new cost-effective method to conduct clinical research going forward.
Dr. Jones, a cardiologist and associate professor of medicine at Duke University Medical Center, Durham, N.C., presented the ADAPTABLE results at the annual scientific sessions of the American College of Cardiology. They were simultaneously published online in the New England Journal of Medicine.
He noted there were mixed signals in the results. “For example, the main intent-to-treat analysis showed a trend to a lower rate of all-cause death in the 81-mg group, but the subgroup of patients who stayed on the 325-mg dose throughout the study had a lower event rate. But overall, there was no difference.”
Dr. Jones said the investigators had the following take-home messages to patients: “If a patient is already taking 81 mg, staying on this dose is probably right given the similar study results for the primary endpoint and that we didn’t find conclusive evidence that 325 mg is better. But for patients who have tolerated 325 mg long term, then they may want to stay on this dose as it may be associated with moderate benefit.”
Dr. Jones pointed out that, overall, patients who switched doses tended to do worse, but he suggested this may have been more to do with underlying reasons for switching rather than the different dose itself. “For example, switching often happens after bleeding or bruising, which can also often preempt an ischemic event, and other illnesses, such as cancer or atrial fibrillation, can also lead patients to change doses.”
“With the caveat that this trial did not include new patients (the vast majority of patients had been taking aspirin previously) the results support the approach of starting new patients on 81 mg, which is what we have been seeing in evolving clinical practice in recent years,” he added.
Dr. Jones explained that the trial set out to answer the simple but important question about the best dose of aspirin in patients with heart disease.
“Aspirin has been established as an appropriate long-term medication for patients with ischemic heart disease since the 1980s, but we really don’t have any good information on the correct dose.
He noted that the U.S. guidelines suggest any dose in the range of 81 mg to 325 mg daily can be used, whereas the European guidelines recommend 81 mg daily, although this is mainly based on observational data and expert opinion; there is little hard, randomized-trial evidence.
The ADAPTABLE trial randomly assigned 15,076 patients with established heart disease to receive 81 mg or 325 mg of aspirin. Before randomization, 96% of those with available information were already taking aspirin, 85% of whom were taking 81 mg.
After a mean follow-up of 26 months, the primary efficacy endpoint – a composite of all-cause death, myocardial infarction, or stroke – had occurred in 7.28% of the 81-mg group and 7.51% of the 325-mg group (hazard ratio, 1.02; 95% confidence interval, 0.91-1.14).
The main safety endpoint, hospitalization for major bleeding with an associated blood transfusion, occurred in 0.63% of the 81-mg group and 0.60% of the 325-mg group (HR, 1.18; 95% CI, 0.79-1.77).
“The bleeding safety endpoint looked similar, which may be counterintuitive to what may have been expected,” Dr. Jones commented. “However, the safety endpoint was very stringent. We still haven’t analyzed all the less serious ADR [adverse drug event]/bleeding data, but overall, it does appear to be balanced.”
He added: “Most cardiologists probably may not have expected to see much difference in efficacy between these two doses but would maybe have anticipated a lower bleeding rate with the low dose. I was a little surprised to see such a low bleeding rate in the 325-mg group.”
Patients assigned to 325 mg had a higher incidence of dose switching (41.6%) than those assigned to 81 mg (7.1%) and were more likely to discontinue treatment (11.1% vs. 7.0%). This resulted in fewer median days of exposure to the assigned dose in the 325-mg group (434 vs. 650 days).
“This was an open-label study, and such studies always suffer from a degree of infidelity to the assigned treatment group,” Dr. Jones said. “In ADAPTABLE, this was unbalanced in that a much greater number of patients switched from 325 mg to 81 mg than the other way round.”
“But our results do reflect what happens in normal life,” he added. “People behaved in the study like they do in the real world. They sometimes changed their dose and sometimes stopped taking aspirin altogether. So, I think the results are an accurate representation of the real world.”
A sensitivity analysis based on which dose the patient actually reported taking showed a higher risk for death, MI, or stroke in patients who took 81 mg than those who took 325 mg (HR, 1.25; 95% CI, 1.10-1.43). But as with any postrandomization analysis, this approach has many inherent biases, Dr. Jones cautioned.
Innovative study design
The ADAPTABLE study used an innovative low-cost design, which involved direct communication with the patients themselves.
Using the National Patient-Centered Clinical Research Network (PCORnet), a group of 40 U.S. centers committed to compiling data in a common format, invitations to enroll in the study were sent to eligible patients identified from medical records. Consent and randomization took place on the patient web portal.
Participants then purchased aspirin at the assigned dose themselves, and all follow-up was done virtually or on the phone, with outcomes ascertained remotely (from patient reports, electronic medical records, and insurance claims) without adjudication.
“This is a pretty neat way to do clinical research, enabling us to conduct a 15,000-patient trial on a very tight budget,” Dr. Jones commented.
He estimated that the trial cost around $18 to $19 million. “No industry funder would have sponsored such a study of aspirin, and a typical trial with this many patients conducted in the traditional way would have cost at least 5 or 10 times more,” he said.
“This is the first time this type of study has been done in the U.S. on such a large scale, and it opens up this method for future research.”
He explained that this design, communicating directly with patients, somewhat limits the questions that can be addressed. “As aspirin is purchased over the counter by patients themselves, this is a question that lent itself to be answered in this way.”
Another innovative design feature was the inclusion of “patient partners,” with one patient nominated by each center to be part of the organization of the trial. “This helped keep the research relevant to what patients care about.
They also helped with the recruitment strategy and communication with participants. I think this is something we need to continue and prioritize in clinical research going forward,” Dr. Jones noted.
‘Pioneering’ trial
Discussants of the study at the ACC presentation congratulated the investigators on conducting such an innovative trial.
Donald Lloyd-Jones, MD, chair of preventive medicine at Northwestern University, Chicago, said, “This is really a pioneering large pragmatic trial, and we’re going to need to see more of these over the next few years. The most important legacy from this trial for me is that you did it, and that you showed us many of the promises and some of the pitfalls of these large pragmatic designs.”
Akshay Desai, MD, associate professor of medicine, Harvard Medical School, Boston, added: “This was an innovative approach to answering an important question for daily clinical practice.”
On the results of the study, Dr. Lloyd-Jones said, “Maybe the outcomes were not too surprising, and I certainly endorse your cautious status quo statement about patients staying on the dose that they are on.”
But he suggested that the bleeding safety outcomes were perhaps a little unexpected, being a little lower in the lower-dose group, and he asked whether there was a sensitivity analysis looking at bleeding on a per protocol basis. Dr. Jones answered that this was planned.
Dr. Desai also raised questions about the “unusual bleeding endpoint,” noting that the rates of bleeding were far lower than would be expected, compared with other clinical trials.
Dr. Jones replied that the bleeding endpoint with blood product transfusion was chosen to allow the researchers to accurately identify these events in claims codes. He said the endpoint probably mirrored the GUSTO (Global Use of Strategies to Open Occluded Coronary Arteries) severe bleeding classification.
In an editorial accompanying the publication of ADAPTABLE, Colin Baigent, FMedSci, says the study provides proof of principle that large pragmatic randomized trials can be conducted in the United States.
But Dr. Baigent, who is professor of epidemiology and director of the Medical Research Council Population Health Research Unit at the University of Oxford (England), says that the high degree of switching between dosages that occurred during the trial gives rise to some uncertainty about the results.
“Because switching was not likely to have been at random, bias arising from this degree of crossover could have obscured a true difference in efficacy or safety (or both), and moreover it is also not possible to conclude that the lack of any significant difference between the two dose groups implies equivalence of the effects of the doses,” he writes.
He suggests that a pilot study may have identified a preference for the 81-mg dose and allowed methods to facilitate equipoise, such as a run-in period with both doses, and only patients adhering being considered for randomization.
But Dr. Baigent concludes that the ADAPTABLE trial is a “major achievement” in that it paves the way for low-cost randomized trials in the United States, which should allow many more clinical questions to be answered.
The trial was supported by an award from the Patient-Centred Outcomes Research Institute. Dr. Schuyler Jones reports consultant fees/honoraria from Bayer Healthcare and Janssen and research grants from Boehringer Ingelheim, Bristol Myers Squibb, and the Patient-Centered Outcomes Research Institute. Dr. Baigent reports grants from Boehringer Ingelheim, Medical Research Council, British Heart Foundation, and National Institute of Health Research, outside the submitted work.
A version of this article first appeared on Medscape.com.
No significant difference in cardiovascular events or major bleeding was shown between patients with established coronary heart disease assigned to a daily aspirin dose of 81 mg and those receiving a dose of 325 mg in the 15,000-patient ADAPTABLE trial.
Although substantial dose switching occurred in the trial, particularly from the higher to the lower dose, lead investigator W. Schuyler Jones, MD, believes the results support the use of the 81-mg dose in most patients.
“While we would have liked to see higher adherence to the assigned doses, we think the results of the trial are reliable,” Dr. Jones said in an interview.
The real-world, open-label, pragmatic trial also involved an innovative low-cost design allowing researchers to identify and communicate with eligible patients directly, opening up a new cost-effective method to conduct clinical research going forward.
Dr. Jones, a cardiologist and associate professor of medicine at Duke University Medical Center, Durham, N.C., presented the ADAPTABLE results at the annual scientific sessions of the American College of Cardiology. They were simultaneously published online in the New England Journal of Medicine.
He noted there were mixed signals in the results. “For example, the main intent-to-treat analysis showed a trend to a lower rate of all-cause death in the 81-mg group, but the subgroup of patients who stayed on the 325-mg dose throughout the study had a lower event rate. But overall, there was no difference.”
Dr. Jones said the investigators had the following take-home messages to patients: “If a patient is already taking 81 mg, staying on this dose is probably right given the similar study results for the primary endpoint and that we didn’t find conclusive evidence that 325 mg is better. But for patients who have tolerated 325 mg long term, then they may want to stay on this dose as it may be associated with moderate benefit.”
Dr. Jones pointed out that, overall, patients who switched doses tended to do worse, but he suggested this may have been more to do with underlying reasons for switching rather than the different dose itself. “For example, switching often happens after bleeding or bruising, which can also often preempt an ischemic event, and other illnesses, such as cancer or atrial fibrillation, can also lead patients to change doses.”
“With the caveat that this trial did not include new patients (the vast majority of patients had been taking aspirin previously) the results support the approach of starting new patients on 81 mg, which is what we have been seeing in evolving clinical practice in recent years,” he added.
Dr. Jones explained that the trial set out to answer the simple but important question about the best dose of aspirin in patients with heart disease.
“Aspirin has been established as an appropriate long-term medication for patients with ischemic heart disease since the 1980s, but we really don’t have any good information on the correct dose.
He noted that the U.S. guidelines suggest any dose in the range of 81 mg to 325 mg daily can be used, whereas the European guidelines recommend 81 mg daily, although this is mainly based on observational data and expert opinion; there is little hard, randomized-trial evidence.
The ADAPTABLE trial randomly assigned 15,076 patients with established heart disease to receive 81 mg or 325 mg of aspirin. Before randomization, 96% of those with available information were already taking aspirin, 85% of whom were taking 81 mg.
After a mean follow-up of 26 months, the primary efficacy endpoint – a composite of all-cause death, myocardial infarction, or stroke – had occurred in 7.28% of the 81-mg group and 7.51% of the 325-mg group (hazard ratio, 1.02; 95% confidence interval, 0.91-1.14).
The main safety endpoint, hospitalization for major bleeding with an associated blood transfusion, occurred in 0.63% of the 81-mg group and 0.60% of the 325-mg group (HR, 1.18; 95% CI, 0.79-1.77).
“The bleeding safety endpoint looked similar, which may be counterintuitive to what may have been expected,” Dr. Jones commented. “However, the safety endpoint was very stringent. We still haven’t analyzed all the less serious ADR [adverse drug event]/bleeding data, but overall, it does appear to be balanced.”
He added: “Most cardiologists probably may not have expected to see much difference in efficacy between these two doses but would maybe have anticipated a lower bleeding rate with the low dose. I was a little surprised to see such a low bleeding rate in the 325-mg group.”
Patients assigned to 325 mg had a higher incidence of dose switching (41.6%) than those assigned to 81 mg (7.1%) and were more likely to discontinue treatment (11.1% vs. 7.0%). This resulted in fewer median days of exposure to the assigned dose in the 325-mg group (434 vs. 650 days).
“This was an open-label study, and such studies always suffer from a degree of infidelity to the assigned treatment group,” Dr. Jones said. “In ADAPTABLE, this was unbalanced in that a much greater number of patients switched from 325 mg to 81 mg than the other way round.”
“But our results do reflect what happens in normal life,” he added. “People behaved in the study like they do in the real world. They sometimes changed their dose and sometimes stopped taking aspirin altogether. So, I think the results are an accurate representation of the real world.”
A sensitivity analysis based on which dose the patient actually reported taking showed a higher risk for death, MI, or stroke in patients who took 81 mg than those who took 325 mg (HR, 1.25; 95% CI, 1.10-1.43). But as with any postrandomization analysis, this approach has many inherent biases, Dr. Jones cautioned.
Innovative study design
The ADAPTABLE study used an innovative low-cost design, which involved direct communication with the patients themselves.
Using the National Patient-Centered Clinical Research Network (PCORnet), a group of 40 U.S. centers committed to compiling data in a common format, invitations to enroll in the study were sent to eligible patients identified from medical records. Consent and randomization took place on the patient web portal.
Participants then purchased aspirin at the assigned dose themselves, and all follow-up was done virtually or on the phone, with outcomes ascertained remotely (from patient reports, electronic medical records, and insurance claims) without adjudication.
“This is a pretty neat way to do clinical research, enabling us to conduct a 15,000-patient trial on a very tight budget,” Dr. Jones commented.
He estimated that the trial cost around $18 to $19 million. “No industry funder would have sponsored such a study of aspirin, and a typical trial with this many patients conducted in the traditional way would have cost at least 5 or 10 times more,” he said.
“This is the first time this type of study has been done in the U.S. on such a large scale, and it opens up this method for future research.”
He explained that this design, communicating directly with patients, somewhat limits the questions that can be addressed. “As aspirin is purchased over the counter by patients themselves, this is a question that lent itself to be answered in this way.”
Another innovative design feature was the inclusion of “patient partners,” with one patient nominated by each center to be part of the organization of the trial. “This helped keep the research relevant to what patients care about.
They also helped with the recruitment strategy and communication with participants. I think this is something we need to continue and prioritize in clinical research going forward,” Dr. Jones noted.
‘Pioneering’ trial
Discussants of the study at the ACC presentation congratulated the investigators on conducting such an innovative trial.
Donald Lloyd-Jones, MD, chair of preventive medicine at Northwestern University, Chicago, said, “This is really a pioneering large pragmatic trial, and we’re going to need to see more of these over the next few years. The most important legacy from this trial for me is that you did it, and that you showed us many of the promises and some of the pitfalls of these large pragmatic designs.”
Akshay Desai, MD, associate professor of medicine, Harvard Medical School, Boston, added: “This was an innovative approach to answering an important question for daily clinical practice.”
On the results of the study, Dr. Lloyd-Jones said, “Maybe the outcomes were not too surprising, and I certainly endorse your cautious status quo statement about patients staying on the dose that they are on.”
But he suggested that the bleeding safety outcomes were perhaps a little unexpected, being a little lower in the lower-dose group, and he asked whether there was a sensitivity analysis looking at bleeding on a per protocol basis. Dr. Jones answered that this was planned.
Dr. Desai also raised questions about the “unusual bleeding endpoint,” noting that the rates of bleeding were far lower than would be expected, compared with other clinical trials.
Dr. Jones replied that the bleeding endpoint with blood product transfusion was chosen to allow the researchers to accurately identify these events in claims codes. He said the endpoint probably mirrored the GUSTO (Global Use of Strategies to Open Occluded Coronary Arteries) severe bleeding classification.
In an editorial accompanying the publication of ADAPTABLE, Colin Baigent, FMedSci, says the study provides proof of principle that large pragmatic randomized trials can be conducted in the United States.
But Dr. Baigent, who is professor of epidemiology and director of the Medical Research Council Population Health Research Unit at the University of Oxford (England), says that the high degree of switching between dosages that occurred during the trial gives rise to some uncertainty about the results.
“Because switching was not likely to have been at random, bias arising from this degree of crossover could have obscured a true difference in efficacy or safety (or both), and moreover it is also not possible to conclude that the lack of any significant difference between the two dose groups implies equivalence of the effects of the doses,” he writes.
He suggests that a pilot study may have identified a preference for the 81-mg dose and allowed methods to facilitate equipoise, such as a run-in period with both doses, and only patients adhering being considered for randomization.
But Dr. Baigent concludes that the ADAPTABLE trial is a “major achievement” in that it paves the way for low-cost randomized trials in the United States, which should allow many more clinical questions to be answered.
The trial was supported by an award from the Patient-Centred Outcomes Research Institute. Dr. Schuyler Jones reports consultant fees/honoraria from Bayer Healthcare and Janssen and research grants from Boehringer Ingelheim, Bristol Myers Squibb, and the Patient-Centered Outcomes Research Institute. Dr. Baigent reports grants from Boehringer Ingelheim, Medical Research Council, British Heart Foundation, and National Institute of Health Research, outside the submitted work.
A version of this article first appeared on Medscape.com.
No significant difference in cardiovascular events or major bleeding was shown between patients with established coronary heart disease assigned to a daily aspirin dose of 81 mg and those receiving a dose of 325 mg in the 15,000-patient ADAPTABLE trial.
Although substantial dose switching occurred in the trial, particularly from the higher to the lower dose, lead investigator W. Schuyler Jones, MD, believes the results support the use of the 81-mg dose in most patients.
“While we would have liked to see higher adherence to the assigned doses, we think the results of the trial are reliable,” Dr. Jones said in an interview.
The real-world, open-label, pragmatic trial also involved an innovative low-cost design allowing researchers to identify and communicate with eligible patients directly, opening up a new cost-effective method to conduct clinical research going forward.
Dr. Jones, a cardiologist and associate professor of medicine at Duke University Medical Center, Durham, N.C., presented the ADAPTABLE results at the annual scientific sessions of the American College of Cardiology. They were simultaneously published online in the New England Journal of Medicine.
He noted there were mixed signals in the results. “For example, the main intent-to-treat analysis showed a trend to a lower rate of all-cause death in the 81-mg group, but the subgroup of patients who stayed on the 325-mg dose throughout the study had a lower event rate. But overall, there was no difference.”
Dr. Jones said the investigators had the following take-home messages to patients: “If a patient is already taking 81 mg, staying on this dose is probably right given the similar study results for the primary endpoint and that we didn’t find conclusive evidence that 325 mg is better. But for patients who have tolerated 325 mg long term, then they may want to stay on this dose as it may be associated with moderate benefit.”
Dr. Jones pointed out that, overall, patients who switched doses tended to do worse, but he suggested this may have been more to do with underlying reasons for switching rather than the different dose itself. “For example, switching often happens after bleeding or bruising, which can also often preempt an ischemic event, and other illnesses, such as cancer or atrial fibrillation, can also lead patients to change doses.”
“With the caveat that this trial did not include new patients (the vast majority of patients had been taking aspirin previously) the results support the approach of starting new patients on 81 mg, which is what we have been seeing in evolving clinical practice in recent years,” he added.
Dr. Jones explained that the trial set out to answer the simple but important question about the best dose of aspirin in patients with heart disease.
“Aspirin has been established as an appropriate long-term medication for patients with ischemic heart disease since the 1980s, but we really don’t have any good information on the correct dose.
He noted that the U.S. guidelines suggest any dose in the range of 81 mg to 325 mg daily can be used, whereas the European guidelines recommend 81 mg daily, although this is mainly based on observational data and expert opinion; there is little hard, randomized-trial evidence.
The ADAPTABLE trial randomly assigned 15,076 patients with established heart disease to receive 81 mg or 325 mg of aspirin. Before randomization, 96% of those with available information were already taking aspirin, 85% of whom were taking 81 mg.
After a mean follow-up of 26 months, the primary efficacy endpoint – a composite of all-cause death, myocardial infarction, or stroke – had occurred in 7.28% of the 81-mg group and 7.51% of the 325-mg group (hazard ratio, 1.02; 95% confidence interval, 0.91-1.14).
The main safety endpoint, hospitalization for major bleeding with an associated blood transfusion, occurred in 0.63% of the 81-mg group and 0.60% of the 325-mg group (HR, 1.18; 95% CI, 0.79-1.77).
“The bleeding safety endpoint looked similar, which may be counterintuitive to what may have been expected,” Dr. Jones commented. “However, the safety endpoint was very stringent. We still haven’t analyzed all the less serious ADR [adverse drug event]/bleeding data, but overall, it does appear to be balanced.”
He added: “Most cardiologists probably may not have expected to see much difference in efficacy between these two doses but would maybe have anticipated a lower bleeding rate with the low dose. I was a little surprised to see such a low bleeding rate in the 325-mg group.”
Patients assigned to 325 mg had a higher incidence of dose switching (41.6%) than those assigned to 81 mg (7.1%) and were more likely to discontinue treatment (11.1% vs. 7.0%). This resulted in fewer median days of exposure to the assigned dose in the 325-mg group (434 vs. 650 days).
“This was an open-label study, and such studies always suffer from a degree of infidelity to the assigned treatment group,” Dr. Jones said. “In ADAPTABLE, this was unbalanced in that a much greater number of patients switched from 325 mg to 81 mg than the other way round.”
“But our results do reflect what happens in normal life,” he added. “People behaved in the study like they do in the real world. They sometimes changed their dose and sometimes stopped taking aspirin altogether. So, I think the results are an accurate representation of the real world.”
A sensitivity analysis based on which dose the patient actually reported taking showed a higher risk for death, MI, or stroke in patients who took 81 mg than those who took 325 mg (HR, 1.25; 95% CI, 1.10-1.43). But as with any postrandomization analysis, this approach has many inherent biases, Dr. Jones cautioned.
Innovative study design
The ADAPTABLE study used an innovative low-cost design, which involved direct communication with the patients themselves.
Using the National Patient-Centered Clinical Research Network (PCORnet), a group of 40 U.S. centers committed to compiling data in a common format, invitations to enroll in the study were sent to eligible patients identified from medical records. Consent and randomization took place on the patient web portal.
Participants then purchased aspirin at the assigned dose themselves, and all follow-up was done virtually or on the phone, with outcomes ascertained remotely (from patient reports, electronic medical records, and insurance claims) without adjudication.
“This is a pretty neat way to do clinical research, enabling us to conduct a 15,000-patient trial on a very tight budget,” Dr. Jones commented.
He estimated that the trial cost around $18 to $19 million. “No industry funder would have sponsored such a study of aspirin, and a typical trial with this many patients conducted in the traditional way would have cost at least 5 or 10 times more,” he said.
“This is the first time this type of study has been done in the U.S. on such a large scale, and it opens up this method for future research.”
He explained that this design, communicating directly with patients, somewhat limits the questions that can be addressed. “As aspirin is purchased over the counter by patients themselves, this is a question that lent itself to be answered in this way.”
Another innovative design feature was the inclusion of “patient partners,” with one patient nominated by each center to be part of the organization of the trial. “This helped keep the research relevant to what patients care about.
They also helped with the recruitment strategy and communication with participants. I think this is something we need to continue and prioritize in clinical research going forward,” Dr. Jones noted.
‘Pioneering’ trial
Discussants of the study at the ACC presentation congratulated the investigators on conducting such an innovative trial.
Donald Lloyd-Jones, MD, chair of preventive medicine at Northwestern University, Chicago, said, “This is really a pioneering large pragmatic trial, and we’re going to need to see more of these over the next few years. The most important legacy from this trial for me is that you did it, and that you showed us many of the promises and some of the pitfalls of these large pragmatic designs.”
Akshay Desai, MD, associate professor of medicine, Harvard Medical School, Boston, added: “This was an innovative approach to answering an important question for daily clinical practice.”
On the results of the study, Dr. Lloyd-Jones said, “Maybe the outcomes were not too surprising, and I certainly endorse your cautious status quo statement about patients staying on the dose that they are on.”
But he suggested that the bleeding safety outcomes were perhaps a little unexpected, being a little lower in the lower-dose group, and he asked whether there was a sensitivity analysis looking at bleeding on a per protocol basis. Dr. Jones answered that this was planned.
Dr. Desai also raised questions about the “unusual bleeding endpoint,” noting that the rates of bleeding were far lower than would be expected, compared with other clinical trials.
Dr. Jones replied that the bleeding endpoint with blood product transfusion was chosen to allow the researchers to accurately identify these events in claims codes. He said the endpoint probably mirrored the GUSTO (Global Use of Strategies to Open Occluded Coronary Arteries) severe bleeding classification.
In an editorial accompanying the publication of ADAPTABLE, Colin Baigent, FMedSci, says the study provides proof of principle that large pragmatic randomized trials can be conducted in the United States.
But Dr. Baigent, who is professor of epidemiology and director of the Medical Research Council Population Health Research Unit at the University of Oxford (England), says that the high degree of switching between dosages that occurred during the trial gives rise to some uncertainty about the results.
“Because switching was not likely to have been at random, bias arising from this degree of crossover could have obscured a true difference in efficacy or safety (or both), and moreover it is also not possible to conclude that the lack of any significant difference between the two dose groups implies equivalence of the effects of the doses,” he writes.
He suggests that a pilot study may have identified a preference for the 81-mg dose and allowed methods to facilitate equipoise, such as a run-in period with both doses, and only patients adhering being considered for randomization.
But Dr. Baigent concludes that the ADAPTABLE trial is a “major achievement” in that it paves the way for low-cost randomized trials in the United States, which should allow many more clinical questions to be answered.
The trial was supported by an award from the Patient-Centred Outcomes Research Institute. Dr. Schuyler Jones reports consultant fees/honoraria from Bayer Healthcare and Janssen and research grants from Boehringer Ingelheim, Bristol Myers Squibb, and the Patient-Centered Outcomes Research Institute. Dr. Baigent reports grants from Boehringer Ingelheim, Medical Research Council, British Heart Foundation, and National Institute of Health Research, outside the submitted work.
A version of this article first appeared on Medscape.com.
FROM ACC 2021
High teen BMI linked to stroke risk in young adulthood
High and even high-normal body mass index (BMI) were linked to increased ischemic stroke risk, regardless of whether or not individuals had diabetes.
Overweight and obese adolescent groups in the study had a roughly two- to threefold increased risk of ischemic stroke, which was apparent even before age 30 years in the study that was based on records of Israeli adolescents evaluated prior to mandatory military service.
These findings highlight the importance of treating and preventing high BMI among adolescence, study coauthor Gilad Twig, MD, MPH, PhD, said in a press release.
“Adults who survive stroke earlier in life face poor functional outcomes, which can lead to unemployment, depression and anxiety,” said Dr. Twig, associate professor in the department of military medicine in The Hebrew University in Jerusalem.
The costs of stroke prevention and care, already high, are expected to become even higher as the adolescent obesity prevalence goes up, fueling further increases in stroke rate, Dr. Twig added.
This is believed to be the first study showing that stroke risk is associated with higher BMI values in both men and women, not just men, Dr. Twig and coauthors said in their article, published May 13, 2021 in the journal Stroke. Previous studies assessing the stroke-BMI relationship in adolescents were based on records of Swedish men evaluated during military conscription at age 18.
In the present study, Dr. Twig and coauthors assessed the linkage between adolescent BMI and first stroke event in 1.9 million male and female adolescents in Israel who were evaluated 1 year prior to mandatory military service, between the years of 1985 and 2013.
They cross-referenced that information with stroke events in a national registry to which all hospitals in Israel are required to report.
The adolescents were about 17 years of age on average at the time of evaluation, 58% were male, and 84% were born in Israel. The mean age at the beginning of follow-up for stroke was about 31 years.
Over the follow-up period, investigators identified 1,088 first stroke events, including 921 ischemic and 167 hemorrhagic strokes.
A gradual increase in stroke rate was seen across BMI categories for ischemic strokes, but not so much for hemorrhagic strokes, investigators found.
Hazard ratios for first ischemic stroke event were 1.4 (95% confidence interval, 1.2-1.6) for the high-normal BMI group, 2.0 (95% CI, 1.6-2.4) for the overweight group, and 3.5 (95% CI, 2.8-4.5) for the obese group after adjusting for age and sex at beginning of follow-up, investigators reported.
When the adjusted results were stratified by presence or absence of diabetes, estimates were similar to what was seen in the overall risk model, they added.
Among those young adults who developed ischemic stroke, 43% smoked, 29% had high blood pressure, 17% had diabetes, and 32% had abnormal lipids at the time of diagnosis, the reported data showed.
The clinical and public health implications of these findings could be substantial, since strokes are associated with worse medical and socioeconomic outcomes in younger as compared with older individuals, according to Dr. Twig and coauthors.
Younger individuals with stroke have a higher risk of recurrent stroke, heart attack, long-term care, or death, they said. Moreover, about half of young-adult stroke survivors have poor functional outcomes, and their risk of unemployment and depression/anxiety is higher than in young individuals without stroke.
One limitation of the study is that follow-up BMI data were not available for all participants. As a result, the contribution of obesity to stroke risk over time could not be assessed, and the independent risk of BMI during adolescence could not be determined. In addition, the authors said the study underrepresents orthodox and ultraorthodox Jewish women, as they are not obligated to serve in the Israeli military.
The study authors had no disclosures related to the study, which was supported by a medical corps Israel Defense Forces research grant.
High and even high-normal body mass index (BMI) were linked to increased ischemic stroke risk, regardless of whether or not individuals had diabetes.
Overweight and obese adolescent groups in the study had a roughly two- to threefold increased risk of ischemic stroke, which was apparent even before age 30 years in the study that was based on records of Israeli adolescents evaluated prior to mandatory military service.
These findings highlight the importance of treating and preventing high BMI among adolescence, study coauthor Gilad Twig, MD, MPH, PhD, said in a press release.
“Adults who survive stroke earlier in life face poor functional outcomes, which can lead to unemployment, depression and anxiety,” said Dr. Twig, associate professor in the department of military medicine in The Hebrew University in Jerusalem.
The costs of stroke prevention and care, already high, are expected to become even higher as the adolescent obesity prevalence goes up, fueling further increases in stroke rate, Dr. Twig added.
This is believed to be the first study showing that stroke risk is associated with higher BMI values in both men and women, not just men, Dr. Twig and coauthors said in their article, published May 13, 2021 in the journal Stroke. Previous studies assessing the stroke-BMI relationship in adolescents were based on records of Swedish men evaluated during military conscription at age 18.
In the present study, Dr. Twig and coauthors assessed the linkage between adolescent BMI and first stroke event in 1.9 million male and female adolescents in Israel who were evaluated 1 year prior to mandatory military service, between the years of 1985 and 2013.
They cross-referenced that information with stroke events in a national registry to which all hospitals in Israel are required to report.
The adolescents were about 17 years of age on average at the time of evaluation, 58% were male, and 84% were born in Israel. The mean age at the beginning of follow-up for stroke was about 31 years.
Over the follow-up period, investigators identified 1,088 first stroke events, including 921 ischemic and 167 hemorrhagic strokes.
A gradual increase in stroke rate was seen across BMI categories for ischemic strokes, but not so much for hemorrhagic strokes, investigators found.
Hazard ratios for first ischemic stroke event were 1.4 (95% confidence interval, 1.2-1.6) for the high-normal BMI group, 2.0 (95% CI, 1.6-2.4) for the overweight group, and 3.5 (95% CI, 2.8-4.5) for the obese group after adjusting for age and sex at beginning of follow-up, investigators reported.
When the adjusted results were stratified by presence or absence of diabetes, estimates were similar to what was seen in the overall risk model, they added.
Among those young adults who developed ischemic stroke, 43% smoked, 29% had high blood pressure, 17% had diabetes, and 32% had abnormal lipids at the time of diagnosis, the reported data showed.
The clinical and public health implications of these findings could be substantial, since strokes are associated with worse medical and socioeconomic outcomes in younger as compared with older individuals, according to Dr. Twig and coauthors.
Younger individuals with stroke have a higher risk of recurrent stroke, heart attack, long-term care, or death, they said. Moreover, about half of young-adult stroke survivors have poor functional outcomes, and their risk of unemployment and depression/anxiety is higher than in young individuals without stroke.
One limitation of the study is that follow-up BMI data were not available for all participants. As a result, the contribution of obesity to stroke risk over time could not be assessed, and the independent risk of BMI during adolescence could not be determined. In addition, the authors said the study underrepresents orthodox and ultraorthodox Jewish women, as they are not obligated to serve in the Israeli military.
The study authors had no disclosures related to the study, which was supported by a medical corps Israel Defense Forces research grant.
High and even high-normal body mass index (BMI) were linked to increased ischemic stroke risk, regardless of whether or not individuals had diabetes.
Overweight and obese adolescent groups in the study had a roughly two- to threefold increased risk of ischemic stroke, which was apparent even before age 30 years in the study that was based on records of Israeli adolescents evaluated prior to mandatory military service.
These findings highlight the importance of treating and preventing high BMI among adolescence, study coauthor Gilad Twig, MD, MPH, PhD, said in a press release.
“Adults who survive stroke earlier in life face poor functional outcomes, which can lead to unemployment, depression and anxiety,” said Dr. Twig, associate professor in the department of military medicine in The Hebrew University in Jerusalem.
The costs of stroke prevention and care, already high, are expected to become even higher as the adolescent obesity prevalence goes up, fueling further increases in stroke rate, Dr. Twig added.
This is believed to be the first study showing that stroke risk is associated with higher BMI values in both men and women, not just men, Dr. Twig and coauthors said in their article, published May 13, 2021 in the journal Stroke. Previous studies assessing the stroke-BMI relationship in adolescents were based on records of Swedish men evaluated during military conscription at age 18.
In the present study, Dr. Twig and coauthors assessed the linkage between adolescent BMI and first stroke event in 1.9 million male and female adolescents in Israel who were evaluated 1 year prior to mandatory military service, between the years of 1985 and 2013.
They cross-referenced that information with stroke events in a national registry to which all hospitals in Israel are required to report.
The adolescents were about 17 years of age on average at the time of evaluation, 58% were male, and 84% were born in Israel. The mean age at the beginning of follow-up for stroke was about 31 years.
Over the follow-up period, investigators identified 1,088 first stroke events, including 921 ischemic and 167 hemorrhagic strokes.
A gradual increase in stroke rate was seen across BMI categories for ischemic strokes, but not so much for hemorrhagic strokes, investigators found.
Hazard ratios for first ischemic stroke event were 1.4 (95% confidence interval, 1.2-1.6) for the high-normal BMI group, 2.0 (95% CI, 1.6-2.4) for the overweight group, and 3.5 (95% CI, 2.8-4.5) for the obese group after adjusting for age and sex at beginning of follow-up, investigators reported.
When the adjusted results were stratified by presence or absence of diabetes, estimates were similar to what was seen in the overall risk model, they added.
Among those young adults who developed ischemic stroke, 43% smoked, 29% had high blood pressure, 17% had diabetes, and 32% had abnormal lipids at the time of diagnosis, the reported data showed.
The clinical and public health implications of these findings could be substantial, since strokes are associated with worse medical and socioeconomic outcomes in younger as compared with older individuals, according to Dr. Twig and coauthors.
Younger individuals with stroke have a higher risk of recurrent stroke, heart attack, long-term care, or death, they said. Moreover, about half of young-adult stroke survivors have poor functional outcomes, and their risk of unemployment and depression/anxiety is higher than in young individuals without stroke.
One limitation of the study is that follow-up BMI data were not available for all participants. As a result, the contribution of obesity to stroke risk over time could not be assessed, and the independent risk of BMI during adolescence could not be determined. In addition, the authors said the study underrepresents orthodox and ultraorthodox Jewish women, as they are not obligated to serve in the Israeli military.
The study authors had no disclosures related to the study, which was supported by a medical corps Israel Defense Forces research grant.
FROM STROKE
FDA class I recall for some Cordis carotid stent systems
Cordis, part of Cardinal Health, has recalled certain lots of its Precise PRO Rx carotid stent system because of a risk of separation of the distal tip of the sheathed delivery system during use.
The Food and Drug Administration has classified this recall as class I, the most serious type, because of the potential for serious injury or death.
“If the device separates during use this may cause serious adverse events such as removal of the separated tip from the carotid artery, embolization distally, or stroke,” noted the recall notice posted on the FDA website.
To date, there have been seven complaints, including five reported injuries, related to this device issue. No deaths have been reported.
The Precise PRO Rx stent system is used in patients with stenotic lesions of the carotid arteries. The system includes a metal (nitinol) self-expanding stent preloaded on a delivery catheter used to place the stent.
The recall covers 7,300 devices made between October 2019 and August 2020 and distributed between Dec. 6, 2019, to Feb. 8, 2021.
The FDA has a complete list of product and lot numbers for the recalled devices on their website.
The company sent an urgent medical device recall letter to all affected customers asking them to check inventories and providing instructions on how to return any recalled product they have on hand.
Health care providers with questions about this recall can contact the company by email at [email protected] or by phone at 786-313-2087.
Health care providers can report adverse reactions or quality problems they experience using these devices to the FDA’s MedWatch program.
A version of this article first appeared on Medscape.com.
Cordis, part of Cardinal Health, has recalled certain lots of its Precise PRO Rx carotid stent system because of a risk of separation of the distal tip of the sheathed delivery system during use.
The Food and Drug Administration has classified this recall as class I, the most serious type, because of the potential for serious injury or death.
“If the device separates during use this may cause serious adverse events such as removal of the separated tip from the carotid artery, embolization distally, or stroke,” noted the recall notice posted on the FDA website.
To date, there have been seven complaints, including five reported injuries, related to this device issue. No deaths have been reported.
The Precise PRO Rx stent system is used in patients with stenotic lesions of the carotid arteries. The system includes a metal (nitinol) self-expanding stent preloaded on a delivery catheter used to place the stent.
The recall covers 7,300 devices made between October 2019 and August 2020 and distributed between Dec. 6, 2019, to Feb. 8, 2021.
The FDA has a complete list of product and lot numbers for the recalled devices on their website.
The company sent an urgent medical device recall letter to all affected customers asking them to check inventories and providing instructions on how to return any recalled product they have on hand.
Health care providers with questions about this recall can contact the company by email at [email protected] or by phone at 786-313-2087.
Health care providers can report adverse reactions or quality problems they experience using these devices to the FDA’s MedWatch program.
A version of this article first appeared on Medscape.com.
Cordis, part of Cardinal Health, has recalled certain lots of its Precise PRO Rx carotid stent system because of a risk of separation of the distal tip of the sheathed delivery system during use.
The Food and Drug Administration has classified this recall as class I, the most serious type, because of the potential for serious injury or death.
“If the device separates during use this may cause serious adverse events such as removal of the separated tip from the carotid artery, embolization distally, or stroke,” noted the recall notice posted on the FDA website.
To date, there have been seven complaints, including five reported injuries, related to this device issue. No deaths have been reported.
The Precise PRO Rx stent system is used in patients with stenotic lesions of the carotid arteries. The system includes a metal (nitinol) self-expanding stent preloaded on a delivery catheter used to place the stent.
The recall covers 7,300 devices made between October 2019 and August 2020 and distributed between Dec. 6, 2019, to Feb. 8, 2021.
The FDA has a complete list of product and lot numbers for the recalled devices on their website.
The company sent an urgent medical device recall letter to all affected customers asking them to check inventories and providing instructions on how to return any recalled product they have on hand.
Health care providers with questions about this recall can contact the company by email at [email protected] or by phone at 786-313-2087.
Health care providers can report adverse reactions or quality problems they experience using these devices to the FDA’s MedWatch program.
A version of this article first appeared on Medscape.com.
Modest clinical gain for AF screening of asymptomatic elderly: STROKESTOP
Some, perhaps many, previously unrecognized cases of atrial fibrillation (AF) will come to light in a screening program aimed at older asymptomatic adults. The key question is whether the challenges of such systematic but age-restricted AF screening in the community, with oral anticoagulation (OAC) offered to those found to have the arrhythmia, is worthwhile in preventing events such as death or stroke.
Now there is evidence supporting such a clinical benefit from a large, prospective, randomized trial. A screening program restricted to people 75 or 76 years of age in two Swedish communities, which called on them to use a handheld single-lead ECG system at home intermittently for 2 weeks, was followed by a slight drop in clinical events over about 7 years.
The 4% decline in risk (P = .045) in the STROKESTOP trial’s “intention-to-treat” (ITT) analysis yielded a number needed to treat of 91; that is, that many people had to be targeted by the screening program to prevent one primary-endpoint clinical event.
Those included ischemic stroke, systemic thromboembolism, hospitalization for severe bleeding, and death from any cause, investigators reported April 23 during the virtual European Heart Rhythm Association (EHRA) 2021 Congress.
If that benefit and its significance seem marginal, some secondary findings might be reassuring. Half the population of the target age in the two communities – 13,979 randomly selected people – were invited to join the trial and follow the screening protocol, comprising the ITT cohort. The other half, numbering 13,996, was not invited and served as control subjects.
However, only 51% of the ITT cohort accepted the invitation and participated in the trial; they represented the “as-treated” cohort, observed Emma Svennberg, MD, PhD, Karolinska Institute, Danderyd Hospital, Stockholm, who presented the analysis at the EHRA sessions.
The screening protocol identified untreated AF, whether previously known or unknown, in about 5% of the 7,165 as-treated screening participants; OAC was initiated in about three-fourths of those cases.
The as-treated group, on their own, benefited with a 24% drop in the prospectively defined secondary endpoint of ischemic stroke, compared with the entire control group.
The clinical benefit in the ITT population was “small but significant,” but over the same period in the as-treated cohort, there was a highly significant drop in risk for ischemic stroke, Dr. Svennberg said in an interview.
The trial’s lead message, she said, is that “screening for atrial fibrillation in an elderly population reduces the risk of death and ischemic stroke without increasing the risk of bleeding.”
Caveats: As-treated vs. ITT
But there are caveats that complicate interpretation of the trial and, Dr. Svennberg proposed, point to the importance of that interpretation of both the ITT and as-treated analyses.
“We detected significantly more atrial fibrillation in the group that was randomized to screening. A major strength of our study was that we referred all of those individuals for a structured follow-up within the study,” she said. “Although the focus of the follow-up was oral anticoagulant therapy, other risk factors were also assessed and managed, such as hypertension and diabetes.”
It’s possible that increased detection of AF followed by such structured management contributed to the observed benefit, Dr. Svennberg proposed.
However, the exclusion of those in the prespecified ITT population who declined to be screened or otherwise didn’t participate left an as-treated cohort that was healthier than the ITT population or the control group.
Indeed, the nonparticipating invitees were sicker, with significantly more diabetes, vascular disease, hypertension, and heart failure, and higher CHA2DS2VASc stroke risk scores than those who agreed to participate.
“We took a more difficult route in setting up this study, in that we identified all individuals aged 75 to 76 residing in our two regions and excluded no one,” Dr. Svennberg said in an interview. “That means even individuals with end-stage disease, severe dementia, bedridden in nursing homes, et cetera, were also randomized but perhaps not likely or eligible to participate.”
Therefore, some invitees were unable to join the study even as others might have declined “out of low interest” or other personal reasons, she said. “We believe that this mimics how a population-based screening program would be performed if done in our country.”
In the ITT analysis, screening successfully identified previously unknown or untreated cases of AF, which led to expanded OAC use and intensified risk-factor management, “which was key to a successful outcome.”
In the as-treated analysis, Dr. Svennberg said, “I think a combination of the intervention and the population being overall more healthy was driving the secondary endpoint.”
Systematic vs. opportunistic screening
Although “opportunistic screening in individuals aged 65 and older” is recommended by current European Society of Cardiology guidelines, systematic screening, such as that used in STROKESTOP, has a much weaker evidence base, observed Renate B. Schnabel, MD, PhD, University Heart & Vascular Center, Hamburg, Germany, as the invited discussant after the STROKESTOP presentation.
STROKESTOP “is one of the first studies, if not the first study,” to show a clinical benefit from screening for AF, Dr. Schnabel said.
Fewer-than-projected primary outcome events were seen during the trial, and event curves for screened and control participants didn’t start to separate until about 4 years into the study, she said. It therefore might take a long time for the screened elderly to realize the clinical benefits of screening.
Studies such as the recent SCREEN-AF and mSTOPS have amply shown that AF screening in the asymptomatic elderly can reveal previously unrecognized AF far more often than would be detected in routine practice, allowing them the opportunity to go on OAC. But the trials weren’t able to show whether the benefits of such management outweigh the risks or costs.
Indeed, on April 20, the U.S. Preventive Services Task Force (USPSTF) released a draft recommendation statement concluding that “the current evidence is insufficient to assess the balance of benefits and harms” associated with AF screening in asymptomatic people at least 50 years of age.
In STROKESTOP, however, benefit for the primary outcome reached significance in the prespecified ITT analysis and “appeared to be driven by the reduction in ischemic stroke incidence,” Dr. Schnabel said.
“The future guidelines have gained strong evidence to judge on systematic atrial fibrillation screening” as it was performed in the trial, she said. “How to implement atrial fibrillation screening, including systematic screening in health care systems across Europe and beyond, remains an open question.”
A randomized population
STROKESTOP considered all 75- and 76-year-olds living in Sweden’s Stockholm County (n = 23,888) and the Halland region (n = 4,880) and randomly assigned them to the ITT group or a control group, with stratification by sex, birth year, and geographic region. In both groups, 54.6% were female and the mean CHA2DS2VASc score was 3.5.
People assigned to the ITT cohort were invited to be screened and followed. Those who agreed to participate underwent a baseline ECG assessment to detect or rule out permanent AF. Guideline-based OAC and follow-up was offered to those found with the arrhythmia. Those in sinus rhythm with no history of AF used a handheld single-lead ECG recorder (Zenicor) for 30 seconds twice daily for 14 days.
Structured management, including OAC, was offered to anyone demonstrating sufficient AF, that is, at least one bout without p waves in one 30-second recording or at least two such episodes lasting 10-29 seconds during the 2-week screening period.
In the ITT analysis, the hazard ratio (HR) for the composite clinical primary endpoint was 0.96 (95% confidence interval, 0.920-0.999; P = .045), but in the as-treated analysis, the HR for ischemic stroke was 0.76 (95% CI, 0.68-0.87; P < .001).
“I believe that this will likely be generalizable to most countries’ elderly residents,” Dr. Svennberg said. “I think if we can find a significant difference in our elderly population in Sweden, most countries will be able to do so, or find even more significant results.”
That’s because “baseline detection of AF in Sweden is high,” she said, “so new detection is likely more difficult.” Also, in Sweden, “care can be sought without monetary concern, and prescriptions are provided at low costs to the patients.” Therefore, patients newly identified with AF, whether in studies or not, “would likely be started on therapy.”
It will be important to know whether the screening strategy is cost-effective, Dr. Schnabel said, because “the overall effect, with a hazard ratio of 0.96, is not too big, and costs incurred by systematic screening are comparatively high.”
STROKESTOP “now provides sound information for cost-effectiveness analyses, which to date have largely relied on assumptions.”
STROKESTOP was partially supported by Carl Bennet AB, Boehringer-Ingelheim, Bayer, Bristol-Meyers Squibb, and Pfizer. Dr. Svennberg disclosed receiving fees for lectures or consulting from Bayer, Bristol-Meyers Squibb, Pfizer, Boehringer-Ingelheim, Merck Sharp & Dohme, and Sanofi; and institutional grants from Roche Diagnostics and Carl Bennett Ltd.
A version of this article first appeared on Medscape.com.
Some, perhaps many, previously unrecognized cases of atrial fibrillation (AF) will come to light in a screening program aimed at older asymptomatic adults. The key question is whether the challenges of such systematic but age-restricted AF screening in the community, with oral anticoagulation (OAC) offered to those found to have the arrhythmia, is worthwhile in preventing events such as death or stroke.
Now there is evidence supporting such a clinical benefit from a large, prospective, randomized trial. A screening program restricted to people 75 or 76 years of age in two Swedish communities, which called on them to use a handheld single-lead ECG system at home intermittently for 2 weeks, was followed by a slight drop in clinical events over about 7 years.
The 4% decline in risk (P = .045) in the STROKESTOP trial’s “intention-to-treat” (ITT) analysis yielded a number needed to treat of 91; that is, that many people had to be targeted by the screening program to prevent one primary-endpoint clinical event.
Those included ischemic stroke, systemic thromboembolism, hospitalization for severe bleeding, and death from any cause, investigators reported April 23 during the virtual European Heart Rhythm Association (EHRA) 2021 Congress.
If that benefit and its significance seem marginal, some secondary findings might be reassuring. Half the population of the target age in the two communities – 13,979 randomly selected people – were invited to join the trial and follow the screening protocol, comprising the ITT cohort. The other half, numbering 13,996, was not invited and served as control subjects.
However, only 51% of the ITT cohort accepted the invitation and participated in the trial; they represented the “as-treated” cohort, observed Emma Svennberg, MD, PhD, Karolinska Institute, Danderyd Hospital, Stockholm, who presented the analysis at the EHRA sessions.
The screening protocol identified untreated AF, whether previously known or unknown, in about 5% of the 7,165 as-treated screening participants; OAC was initiated in about three-fourths of those cases.
The as-treated group, on their own, benefited with a 24% drop in the prospectively defined secondary endpoint of ischemic stroke, compared with the entire control group.
The clinical benefit in the ITT population was “small but significant,” but over the same period in the as-treated cohort, there was a highly significant drop in risk for ischemic stroke, Dr. Svennberg said in an interview.
The trial’s lead message, she said, is that “screening for atrial fibrillation in an elderly population reduces the risk of death and ischemic stroke without increasing the risk of bleeding.”
Caveats: As-treated vs. ITT
But there are caveats that complicate interpretation of the trial and, Dr. Svennberg proposed, point to the importance of that interpretation of both the ITT and as-treated analyses.
“We detected significantly more atrial fibrillation in the group that was randomized to screening. A major strength of our study was that we referred all of those individuals for a structured follow-up within the study,” she said. “Although the focus of the follow-up was oral anticoagulant therapy, other risk factors were also assessed and managed, such as hypertension and diabetes.”
It’s possible that increased detection of AF followed by such structured management contributed to the observed benefit, Dr. Svennberg proposed.
However, the exclusion of those in the prespecified ITT population who declined to be screened or otherwise didn’t participate left an as-treated cohort that was healthier than the ITT population or the control group.
Indeed, the nonparticipating invitees were sicker, with significantly more diabetes, vascular disease, hypertension, and heart failure, and higher CHA2DS2VASc stroke risk scores than those who agreed to participate.
“We took a more difficult route in setting up this study, in that we identified all individuals aged 75 to 76 residing in our two regions and excluded no one,” Dr. Svennberg said in an interview. “That means even individuals with end-stage disease, severe dementia, bedridden in nursing homes, et cetera, were also randomized but perhaps not likely or eligible to participate.”
Therefore, some invitees were unable to join the study even as others might have declined “out of low interest” or other personal reasons, she said. “We believe that this mimics how a population-based screening program would be performed if done in our country.”
In the ITT analysis, screening successfully identified previously unknown or untreated cases of AF, which led to expanded OAC use and intensified risk-factor management, “which was key to a successful outcome.”
In the as-treated analysis, Dr. Svennberg said, “I think a combination of the intervention and the population being overall more healthy was driving the secondary endpoint.”
Systematic vs. opportunistic screening
Although “opportunistic screening in individuals aged 65 and older” is recommended by current European Society of Cardiology guidelines, systematic screening, such as that used in STROKESTOP, has a much weaker evidence base, observed Renate B. Schnabel, MD, PhD, University Heart & Vascular Center, Hamburg, Germany, as the invited discussant after the STROKESTOP presentation.
STROKESTOP “is one of the first studies, if not the first study,” to show a clinical benefit from screening for AF, Dr. Schnabel said.
Fewer-than-projected primary outcome events were seen during the trial, and event curves for screened and control participants didn’t start to separate until about 4 years into the study, she said. It therefore might take a long time for the screened elderly to realize the clinical benefits of screening.
Studies such as the recent SCREEN-AF and mSTOPS have amply shown that AF screening in the asymptomatic elderly can reveal previously unrecognized AF far more often than would be detected in routine practice, allowing them the opportunity to go on OAC. But the trials weren’t able to show whether the benefits of such management outweigh the risks or costs.
Indeed, on April 20, the U.S. Preventive Services Task Force (USPSTF) released a draft recommendation statement concluding that “the current evidence is insufficient to assess the balance of benefits and harms” associated with AF screening in asymptomatic people at least 50 years of age.
In STROKESTOP, however, benefit for the primary outcome reached significance in the prespecified ITT analysis and “appeared to be driven by the reduction in ischemic stroke incidence,” Dr. Schnabel said.
“The future guidelines have gained strong evidence to judge on systematic atrial fibrillation screening” as it was performed in the trial, she said. “How to implement atrial fibrillation screening, including systematic screening in health care systems across Europe and beyond, remains an open question.”
A randomized population
STROKESTOP considered all 75- and 76-year-olds living in Sweden’s Stockholm County (n = 23,888) and the Halland region (n = 4,880) and randomly assigned them to the ITT group or a control group, with stratification by sex, birth year, and geographic region. In both groups, 54.6% were female and the mean CHA2DS2VASc score was 3.5.
People assigned to the ITT cohort were invited to be screened and followed. Those who agreed to participate underwent a baseline ECG assessment to detect or rule out permanent AF. Guideline-based OAC and follow-up was offered to those found with the arrhythmia. Those in sinus rhythm with no history of AF used a handheld single-lead ECG recorder (Zenicor) for 30 seconds twice daily for 14 days.
Structured management, including OAC, was offered to anyone demonstrating sufficient AF, that is, at least one bout without p waves in one 30-second recording or at least two such episodes lasting 10-29 seconds during the 2-week screening period.
In the ITT analysis, the hazard ratio (HR) for the composite clinical primary endpoint was 0.96 (95% confidence interval, 0.920-0.999; P = .045), but in the as-treated analysis, the HR for ischemic stroke was 0.76 (95% CI, 0.68-0.87; P < .001).
“I believe that this will likely be generalizable to most countries’ elderly residents,” Dr. Svennberg said. “I think if we can find a significant difference in our elderly population in Sweden, most countries will be able to do so, or find even more significant results.”
That’s because “baseline detection of AF in Sweden is high,” she said, “so new detection is likely more difficult.” Also, in Sweden, “care can be sought without monetary concern, and prescriptions are provided at low costs to the patients.” Therefore, patients newly identified with AF, whether in studies or not, “would likely be started on therapy.”
It will be important to know whether the screening strategy is cost-effective, Dr. Schnabel said, because “the overall effect, with a hazard ratio of 0.96, is not too big, and costs incurred by systematic screening are comparatively high.”
STROKESTOP “now provides sound information for cost-effectiveness analyses, which to date have largely relied on assumptions.”
STROKESTOP was partially supported by Carl Bennet AB, Boehringer-Ingelheim, Bayer, Bristol-Meyers Squibb, and Pfizer. Dr. Svennberg disclosed receiving fees for lectures or consulting from Bayer, Bristol-Meyers Squibb, Pfizer, Boehringer-Ingelheim, Merck Sharp & Dohme, and Sanofi; and institutional grants from Roche Diagnostics and Carl Bennett Ltd.
A version of this article first appeared on Medscape.com.
Some, perhaps many, previously unrecognized cases of atrial fibrillation (AF) will come to light in a screening program aimed at older asymptomatic adults. The key question is whether the challenges of such systematic but age-restricted AF screening in the community, with oral anticoagulation (OAC) offered to those found to have the arrhythmia, is worthwhile in preventing events such as death or stroke.
Now there is evidence supporting such a clinical benefit from a large, prospective, randomized trial. A screening program restricted to people 75 or 76 years of age in two Swedish communities, which called on them to use a handheld single-lead ECG system at home intermittently for 2 weeks, was followed by a slight drop in clinical events over about 7 years.
The 4% decline in risk (P = .045) in the STROKESTOP trial’s “intention-to-treat” (ITT) analysis yielded a number needed to treat of 91; that is, that many people had to be targeted by the screening program to prevent one primary-endpoint clinical event.
Those included ischemic stroke, systemic thromboembolism, hospitalization for severe bleeding, and death from any cause, investigators reported April 23 during the virtual European Heart Rhythm Association (EHRA) 2021 Congress.
If that benefit and its significance seem marginal, some secondary findings might be reassuring. Half the population of the target age in the two communities – 13,979 randomly selected people – were invited to join the trial and follow the screening protocol, comprising the ITT cohort. The other half, numbering 13,996, was not invited and served as control subjects.
However, only 51% of the ITT cohort accepted the invitation and participated in the trial; they represented the “as-treated” cohort, observed Emma Svennberg, MD, PhD, Karolinska Institute, Danderyd Hospital, Stockholm, who presented the analysis at the EHRA sessions.
The screening protocol identified untreated AF, whether previously known or unknown, in about 5% of the 7,165 as-treated screening participants; OAC was initiated in about three-fourths of those cases.
The as-treated group, on their own, benefited with a 24% drop in the prospectively defined secondary endpoint of ischemic stroke, compared with the entire control group.
The clinical benefit in the ITT population was “small but significant,” but over the same period in the as-treated cohort, there was a highly significant drop in risk for ischemic stroke, Dr. Svennberg said in an interview.
The trial’s lead message, she said, is that “screening for atrial fibrillation in an elderly population reduces the risk of death and ischemic stroke without increasing the risk of bleeding.”
Caveats: As-treated vs. ITT
But there are caveats that complicate interpretation of the trial and, Dr. Svennberg proposed, point to the importance of that interpretation of both the ITT and as-treated analyses.
“We detected significantly more atrial fibrillation in the group that was randomized to screening. A major strength of our study was that we referred all of those individuals for a structured follow-up within the study,” she said. “Although the focus of the follow-up was oral anticoagulant therapy, other risk factors were also assessed and managed, such as hypertension and diabetes.”
It’s possible that increased detection of AF followed by such structured management contributed to the observed benefit, Dr. Svennberg proposed.
However, the exclusion of those in the prespecified ITT population who declined to be screened or otherwise didn’t participate left an as-treated cohort that was healthier than the ITT population or the control group.
Indeed, the nonparticipating invitees were sicker, with significantly more diabetes, vascular disease, hypertension, and heart failure, and higher CHA2DS2VASc stroke risk scores than those who agreed to participate.
“We took a more difficult route in setting up this study, in that we identified all individuals aged 75 to 76 residing in our two regions and excluded no one,” Dr. Svennberg said in an interview. “That means even individuals with end-stage disease, severe dementia, bedridden in nursing homes, et cetera, were also randomized but perhaps not likely or eligible to participate.”
Therefore, some invitees were unable to join the study even as others might have declined “out of low interest” or other personal reasons, she said. “We believe that this mimics how a population-based screening program would be performed if done in our country.”
In the ITT analysis, screening successfully identified previously unknown or untreated cases of AF, which led to expanded OAC use and intensified risk-factor management, “which was key to a successful outcome.”
In the as-treated analysis, Dr. Svennberg said, “I think a combination of the intervention and the population being overall more healthy was driving the secondary endpoint.”
Systematic vs. opportunistic screening
Although “opportunistic screening in individuals aged 65 and older” is recommended by current European Society of Cardiology guidelines, systematic screening, such as that used in STROKESTOP, has a much weaker evidence base, observed Renate B. Schnabel, MD, PhD, University Heart & Vascular Center, Hamburg, Germany, as the invited discussant after the STROKESTOP presentation.
STROKESTOP “is one of the first studies, if not the first study,” to show a clinical benefit from screening for AF, Dr. Schnabel said.
Fewer-than-projected primary outcome events were seen during the trial, and event curves for screened and control participants didn’t start to separate until about 4 years into the study, she said. It therefore might take a long time for the screened elderly to realize the clinical benefits of screening.
Studies such as the recent SCREEN-AF and mSTOPS have amply shown that AF screening in the asymptomatic elderly can reveal previously unrecognized AF far more often than would be detected in routine practice, allowing them the opportunity to go on OAC. But the trials weren’t able to show whether the benefits of such management outweigh the risks or costs.
Indeed, on April 20, the U.S. Preventive Services Task Force (USPSTF) released a draft recommendation statement concluding that “the current evidence is insufficient to assess the balance of benefits and harms” associated with AF screening in asymptomatic people at least 50 years of age.
In STROKESTOP, however, benefit for the primary outcome reached significance in the prespecified ITT analysis and “appeared to be driven by the reduction in ischemic stroke incidence,” Dr. Schnabel said.
“The future guidelines have gained strong evidence to judge on systematic atrial fibrillation screening” as it was performed in the trial, she said. “How to implement atrial fibrillation screening, including systematic screening in health care systems across Europe and beyond, remains an open question.”
A randomized population
STROKESTOP considered all 75- and 76-year-olds living in Sweden’s Stockholm County (n = 23,888) and the Halland region (n = 4,880) and randomly assigned them to the ITT group or a control group, with stratification by sex, birth year, and geographic region. In both groups, 54.6% were female and the mean CHA2DS2VASc score was 3.5.
People assigned to the ITT cohort were invited to be screened and followed. Those who agreed to participate underwent a baseline ECG assessment to detect or rule out permanent AF. Guideline-based OAC and follow-up was offered to those found with the arrhythmia. Those in sinus rhythm with no history of AF used a handheld single-lead ECG recorder (Zenicor) for 30 seconds twice daily for 14 days.
Structured management, including OAC, was offered to anyone demonstrating sufficient AF, that is, at least one bout without p waves in one 30-second recording or at least two such episodes lasting 10-29 seconds during the 2-week screening period.
In the ITT analysis, the hazard ratio (HR) for the composite clinical primary endpoint was 0.96 (95% confidence interval, 0.920-0.999; P = .045), but in the as-treated analysis, the HR for ischemic stroke was 0.76 (95% CI, 0.68-0.87; P < .001).
“I believe that this will likely be generalizable to most countries’ elderly residents,” Dr. Svennberg said. “I think if we can find a significant difference in our elderly population in Sweden, most countries will be able to do so, or find even more significant results.”
That’s because “baseline detection of AF in Sweden is high,” she said, “so new detection is likely more difficult.” Also, in Sweden, “care can be sought without monetary concern, and prescriptions are provided at low costs to the patients.” Therefore, patients newly identified with AF, whether in studies or not, “would likely be started on therapy.”
It will be important to know whether the screening strategy is cost-effective, Dr. Schnabel said, because “the overall effect, with a hazard ratio of 0.96, is not too big, and costs incurred by systematic screening are comparatively high.”
STROKESTOP “now provides sound information for cost-effectiveness analyses, which to date have largely relied on assumptions.”
STROKESTOP was partially supported by Carl Bennet AB, Boehringer-Ingelheim, Bayer, Bristol-Meyers Squibb, and Pfizer. Dr. Svennberg disclosed receiving fees for lectures or consulting from Bayer, Bristol-Meyers Squibb, Pfizer, Boehringer-Ingelheim, Merck Sharp & Dohme, and Sanofi; and institutional grants from Roche Diagnostics and Carl Bennett Ltd.
A version of this article first appeared on Medscape.com.
AHA statement flags CV risk of hormonal cancer therapies
Hormonal therapies for the treatment of hormone-dependent breast and prostate cancer could raise the risk for myocardial infarction and stroke, and patients need to be closely monitored to allow early detection and treatment of cardiovascular disease (CVD), the American Heart Association says in a new scientific statement.
“The statement provides data on the risks of each type of hormonal therapy so clinicians can use it as a guide to help manage cardiovascular risks during cancer treatment,” Tochi Okwuosa, DO, chair of the writing group, said in a news release.
“A team-based approach to patient care that includes the oncology team, cardiologist, primary care clinician, dietitian, endocrinologist, and other health care professionals as appropriate is needed to work with each patient to manage and reduce the increased risk of heart disease and strokes associated with hormonal therapy in breast and prostate cancer treatment,” said Dr. Okwuosa, director of cardio-oncology services, Rush University Medical Center, Chicago.
The scientific statement was published online April 26 in Circulation: Genomic and Precision Medicine.
Hormone-dependent cancers, such as prostate and breast cancer, are the most common noncutaneous cancers in the United States and around the world. As hormonal therapies have markedly improved survival in these patients, CVD has emerged as a leading cause illness and death.
The increased CVD burden might be explained by the increasing average age of cancer survivors, leading to higher rates of age-related CV risk factors and coronary artery disease.
The writing group reviewed existing evidence from observational studies and randomized controlled trials on the cardiovascular impact of anticancer hormonal therapies.
Among the key findings:
- In patients with breast cancer, has been shown to increase the risk for venous thromboembolic events, but to have somewhat protective to neutral effects on CVD risk burden and CVD events. Conversely, aromatase inhibitors have been shown to increase the risk for CVD risk factors and events, including MI and stroke.
- Androgen-deprivation therapy for prostate cancer appears to increase the risk for CV events, although gonadotrophin-releasing hormone (GnRH) antagonists are associated with a lower risk for CV events than are GnRH agonists. The oral antiandrogens appear to be associated with increased CVD risk as well, particularly when used for complete androgen blockade as combination GnRH/anti-androgen therapy.
- The duration of hormonal therapies has a significant impact on CVD risk; the longer patients receive hormonal therapy, the greater the risk. More research is needed to better define the risks associated with duration of treatment.
- The data are mixed on the impact of preexisting CV risk factors and CVD on CV events associated with hormonal therapy. Although the presence of baseline CV risk factors and CVD can increase CV events associated with aromatase inhibitors, it is not clear that tamoxifen does.
- Studies suggest that patients with prostate cancer and baseline CVD and CV risk factors have increased rates of CV events when treated with androgen-deprivation therapy.
- Although the prolonged use of some hormonal therapies worsens CV risk factors and , the effects of the duration of therapy on CV events are less clear.
The writing group noted that there are no definitive guidelines for the monitoring and management of hormonal therapy-related CVD risks.
The authors encourage clinicians to be alert for worsening CV problems in those with preexisting heart disease or risk factors, and to recognize that even patients without preexisting CV problems are at higher risk because of their exposure to hormonal therapies.
“For patients who have two or more cardiovascular risk factors, it is likely that referral to a cardiologist would be appropriate prior to beginning hormone treatment. For patients already receiving hormonal therapies, a discussion with the oncology team can help to determine if a cardiology referral is recommended,” Dr. Okwuosa said in the news release.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Cardio-Oncology Subcommittee of the Council on Clinical Cardiology and the Council on Genomic and Precision Medicine; the Council on Arteriosclerosis, Thrombosis, and Vascular Biology; and the Council on Cardiovascular Radiology and Intervention.
The research had no commercial funding. Dr. Okwuosa has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Hormonal therapies for the treatment of hormone-dependent breast and prostate cancer could raise the risk for myocardial infarction and stroke, and patients need to be closely monitored to allow early detection and treatment of cardiovascular disease (CVD), the American Heart Association says in a new scientific statement.
“The statement provides data on the risks of each type of hormonal therapy so clinicians can use it as a guide to help manage cardiovascular risks during cancer treatment,” Tochi Okwuosa, DO, chair of the writing group, said in a news release.
“A team-based approach to patient care that includes the oncology team, cardiologist, primary care clinician, dietitian, endocrinologist, and other health care professionals as appropriate is needed to work with each patient to manage and reduce the increased risk of heart disease and strokes associated with hormonal therapy in breast and prostate cancer treatment,” said Dr. Okwuosa, director of cardio-oncology services, Rush University Medical Center, Chicago.
The scientific statement was published online April 26 in Circulation: Genomic and Precision Medicine.
Hormone-dependent cancers, such as prostate and breast cancer, are the most common noncutaneous cancers in the United States and around the world. As hormonal therapies have markedly improved survival in these patients, CVD has emerged as a leading cause illness and death.
The increased CVD burden might be explained by the increasing average age of cancer survivors, leading to higher rates of age-related CV risk factors and coronary artery disease.
The writing group reviewed existing evidence from observational studies and randomized controlled trials on the cardiovascular impact of anticancer hormonal therapies.
Among the key findings:
- In patients with breast cancer, has been shown to increase the risk for venous thromboembolic events, but to have somewhat protective to neutral effects on CVD risk burden and CVD events. Conversely, aromatase inhibitors have been shown to increase the risk for CVD risk factors and events, including MI and stroke.
- Androgen-deprivation therapy for prostate cancer appears to increase the risk for CV events, although gonadotrophin-releasing hormone (GnRH) antagonists are associated with a lower risk for CV events than are GnRH agonists. The oral antiandrogens appear to be associated with increased CVD risk as well, particularly when used for complete androgen blockade as combination GnRH/anti-androgen therapy.
- The duration of hormonal therapies has a significant impact on CVD risk; the longer patients receive hormonal therapy, the greater the risk. More research is needed to better define the risks associated with duration of treatment.
- The data are mixed on the impact of preexisting CV risk factors and CVD on CV events associated with hormonal therapy. Although the presence of baseline CV risk factors and CVD can increase CV events associated with aromatase inhibitors, it is not clear that tamoxifen does.
- Studies suggest that patients with prostate cancer and baseline CVD and CV risk factors have increased rates of CV events when treated with androgen-deprivation therapy.
- Although the prolonged use of some hormonal therapies worsens CV risk factors and , the effects of the duration of therapy on CV events are less clear.
The writing group noted that there are no definitive guidelines for the monitoring and management of hormonal therapy-related CVD risks.
The authors encourage clinicians to be alert for worsening CV problems in those with preexisting heart disease or risk factors, and to recognize that even patients without preexisting CV problems are at higher risk because of their exposure to hormonal therapies.
“For patients who have two or more cardiovascular risk factors, it is likely that referral to a cardiologist would be appropriate prior to beginning hormone treatment. For patients already receiving hormonal therapies, a discussion with the oncology team can help to determine if a cardiology referral is recommended,” Dr. Okwuosa said in the news release.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Cardio-Oncology Subcommittee of the Council on Clinical Cardiology and the Council on Genomic and Precision Medicine; the Council on Arteriosclerosis, Thrombosis, and Vascular Biology; and the Council on Cardiovascular Radiology and Intervention.
The research had no commercial funding. Dr. Okwuosa has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Hormonal therapies for the treatment of hormone-dependent breast and prostate cancer could raise the risk for myocardial infarction and stroke, and patients need to be closely monitored to allow early detection and treatment of cardiovascular disease (CVD), the American Heart Association says in a new scientific statement.
“The statement provides data on the risks of each type of hormonal therapy so clinicians can use it as a guide to help manage cardiovascular risks during cancer treatment,” Tochi Okwuosa, DO, chair of the writing group, said in a news release.
“A team-based approach to patient care that includes the oncology team, cardiologist, primary care clinician, dietitian, endocrinologist, and other health care professionals as appropriate is needed to work with each patient to manage and reduce the increased risk of heart disease and strokes associated with hormonal therapy in breast and prostate cancer treatment,” said Dr. Okwuosa, director of cardio-oncology services, Rush University Medical Center, Chicago.
The scientific statement was published online April 26 in Circulation: Genomic and Precision Medicine.
Hormone-dependent cancers, such as prostate and breast cancer, are the most common noncutaneous cancers in the United States and around the world. As hormonal therapies have markedly improved survival in these patients, CVD has emerged as a leading cause illness and death.
The increased CVD burden might be explained by the increasing average age of cancer survivors, leading to higher rates of age-related CV risk factors and coronary artery disease.
The writing group reviewed existing evidence from observational studies and randomized controlled trials on the cardiovascular impact of anticancer hormonal therapies.
Among the key findings:
- In patients with breast cancer, has been shown to increase the risk for venous thromboembolic events, but to have somewhat protective to neutral effects on CVD risk burden and CVD events. Conversely, aromatase inhibitors have been shown to increase the risk for CVD risk factors and events, including MI and stroke.
- Androgen-deprivation therapy for prostate cancer appears to increase the risk for CV events, although gonadotrophin-releasing hormone (GnRH) antagonists are associated with a lower risk for CV events than are GnRH agonists. The oral antiandrogens appear to be associated with increased CVD risk as well, particularly when used for complete androgen blockade as combination GnRH/anti-androgen therapy.
- The duration of hormonal therapies has a significant impact on CVD risk; the longer patients receive hormonal therapy, the greater the risk. More research is needed to better define the risks associated with duration of treatment.
- The data are mixed on the impact of preexisting CV risk factors and CVD on CV events associated with hormonal therapy. Although the presence of baseline CV risk factors and CVD can increase CV events associated with aromatase inhibitors, it is not clear that tamoxifen does.
- Studies suggest that patients with prostate cancer and baseline CVD and CV risk factors have increased rates of CV events when treated with androgen-deprivation therapy.
- Although the prolonged use of some hormonal therapies worsens CV risk factors and , the effects of the duration of therapy on CV events are less clear.
The writing group noted that there are no definitive guidelines for the monitoring and management of hormonal therapy-related CVD risks.
The authors encourage clinicians to be alert for worsening CV problems in those with preexisting heart disease or risk factors, and to recognize that even patients without preexisting CV problems are at higher risk because of their exposure to hormonal therapies.
“For patients who have two or more cardiovascular risk factors, it is likely that referral to a cardiologist would be appropriate prior to beginning hormone treatment. For patients already receiving hormonal therapies, a discussion with the oncology team can help to determine if a cardiology referral is recommended,” Dr. Okwuosa said in the news release.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Cardio-Oncology Subcommittee of the Council on Clinical Cardiology and the Council on Genomic and Precision Medicine; the Council on Arteriosclerosis, Thrombosis, and Vascular Biology; and the Council on Cardiovascular Radiology and Intervention.
The research had no commercial funding. Dr. Okwuosa has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
VNS plus rehab is a powerful poststroke combination
according to preliminary results of a randomized clinical trial at the 2021 annual meeting of the American Academy of Neurology.
“We believe that vagus nerve stimulation combined with rehabilitation is an acceptable and effective intervention for improving upper-limb impairment and function in people with moderate to severe arm weakness a long time VNS-REHAB pivotal study is a randomized, blinded, controlled trial of 108 people who had upper-extremity weakness after having a stroke at least 9 months before enrollment. The average for the group was 3 years post stroke after ischemic stroke,” said Jesse Dawson, MD, a professor at the University of Glasgow.
The Fifty-three patients were assigned active VNS followed by 6 weeks of in-clinic rehabilitation and then 90 days of home-based rehab. At in-clinic rehab, the therapist initiated a 5-second burst of VNS stimulation during each movement. In home-base treatment, the device was activated by a magnet.
Fifty-five patients were assigned sham VNS. After 90 days, the sham group crossed over to receive VNS for 6 weeks and then 90 days of home exercise. This crossover group was the focus of the data Dr. Dawson presented at AAN 2021. The overall trial results have been published in the Lancet.
“The hypothesis is based on the knowledge that the VNS stimulates the release of proneuroplastic neuromodulators norepinephrine and acetylcholine,” Dr. Dawson said. “By pairing VNS with task-specific movement, we hypothesize that we will increase task-specific neuroplasticity.”
The main study showed “a statistically significant difference across all primary and secondary endpoints at all time points in favor of rehabilitation paired with VNS,” Dr. Dawson said. The primary outcome was improvement in Fugl-Meyer Upper Extremity (FMA-UE) outcome, with the active VNS group having a significantly higher percentage of responders. For example, 47% of the active VNS patients had a greater than 6-point response on FMA-UE improvement versus 27% of the sham group (P = .010).
When the sham group crossed over to active VNS, the improvement in arm function matched that of the treatment group in the main study, Dr. Dawson said. “If you look at specifically what happened after they completed the control phase, there was a further small increase in Fugl-Meyer score, but, more importantly between 20% and 35% achieved a clinically important response on the Fugl-Meyer assessment or the Wolf Motor Function Test, giving a number need to treat ranging from three to five,” he said.
Dr. Dawson said that data on adverse events was presented in the Lancet publication. “These were observed at expected frequencies,” he said.
In an interview, he explained the significance of reporting the number to treat. “The number needed to treat helps give an idea of how many times you need to do something to achieve the desired outcome. So for VNS paired with rehab versus rehab alone, you need to treat four people to get one extra clinically important response, compared with just doing therapy.”
The next steps for his group’s research, he said, “will be to try and explore whether we can predict who responds best, and we would like to see if people with other types of stroke benefit.”
In providing comment on the study, Andreas Luft, MD, a professor at the University Hospital Zürich, noted that the FME-UE score improvements reported “are significant and meaningful. ... However, they may also be achieved by increasing the intensity of training. Many medical systems offer their patients high rehabilitation intensities and achieve similar improvements. Whether VNS can further boost higher-intensity training ‘beyond its limits’ is probable but remains to be demonstrated.”
Dr. Luft noted the study advances the knowledge of combining a therapeutic approach with training. “More such approaches are necessary to increase the therapeutic instrumentation of neurorehabilitation,” he said.
Dr. Dawson reported a financial relationship with MicroTransponder. His coauthors reported relationships with MicroTransponder, SanBio, Fujifilm Toyoma Chemical, Medtronic, TRCare, SAEBO, Allergan/AbbVie, Ipsen, Merz, Ottobock/Hangar Orthopedics, Parker Hannifin, Revance Therapeutics, ReWallk, and Sword Health. Three coauthors are employees of MicroTransponder. Dr. Luft has no relevant relationships to disclose.
according to preliminary results of a randomized clinical trial at the 2021 annual meeting of the American Academy of Neurology.
“We believe that vagus nerve stimulation combined with rehabilitation is an acceptable and effective intervention for improving upper-limb impairment and function in people with moderate to severe arm weakness a long time VNS-REHAB pivotal study is a randomized, blinded, controlled trial of 108 people who had upper-extremity weakness after having a stroke at least 9 months before enrollment. The average for the group was 3 years post stroke after ischemic stroke,” said Jesse Dawson, MD, a professor at the University of Glasgow.
The Fifty-three patients were assigned active VNS followed by 6 weeks of in-clinic rehabilitation and then 90 days of home-based rehab. At in-clinic rehab, the therapist initiated a 5-second burst of VNS stimulation during each movement. In home-base treatment, the device was activated by a magnet.
Fifty-five patients were assigned sham VNS. After 90 days, the sham group crossed over to receive VNS for 6 weeks and then 90 days of home exercise. This crossover group was the focus of the data Dr. Dawson presented at AAN 2021. The overall trial results have been published in the Lancet.
“The hypothesis is based on the knowledge that the VNS stimulates the release of proneuroplastic neuromodulators norepinephrine and acetylcholine,” Dr. Dawson said. “By pairing VNS with task-specific movement, we hypothesize that we will increase task-specific neuroplasticity.”
The main study showed “a statistically significant difference across all primary and secondary endpoints at all time points in favor of rehabilitation paired with VNS,” Dr. Dawson said. The primary outcome was improvement in Fugl-Meyer Upper Extremity (FMA-UE) outcome, with the active VNS group having a significantly higher percentage of responders. For example, 47% of the active VNS patients had a greater than 6-point response on FMA-UE improvement versus 27% of the sham group (P = .010).
When the sham group crossed over to active VNS, the improvement in arm function matched that of the treatment group in the main study, Dr. Dawson said. “If you look at specifically what happened after they completed the control phase, there was a further small increase in Fugl-Meyer score, but, more importantly between 20% and 35% achieved a clinically important response on the Fugl-Meyer assessment or the Wolf Motor Function Test, giving a number need to treat ranging from three to five,” he said.
Dr. Dawson said that data on adverse events was presented in the Lancet publication. “These were observed at expected frequencies,” he said.
In an interview, he explained the significance of reporting the number to treat. “The number needed to treat helps give an idea of how many times you need to do something to achieve the desired outcome. So for VNS paired with rehab versus rehab alone, you need to treat four people to get one extra clinically important response, compared with just doing therapy.”
The next steps for his group’s research, he said, “will be to try and explore whether we can predict who responds best, and we would like to see if people with other types of stroke benefit.”
In providing comment on the study, Andreas Luft, MD, a professor at the University Hospital Zürich, noted that the FME-UE score improvements reported “are significant and meaningful. ... However, they may also be achieved by increasing the intensity of training. Many medical systems offer their patients high rehabilitation intensities and achieve similar improvements. Whether VNS can further boost higher-intensity training ‘beyond its limits’ is probable but remains to be demonstrated.”
Dr. Luft noted the study advances the knowledge of combining a therapeutic approach with training. “More such approaches are necessary to increase the therapeutic instrumentation of neurorehabilitation,” he said.
Dr. Dawson reported a financial relationship with MicroTransponder. His coauthors reported relationships with MicroTransponder, SanBio, Fujifilm Toyoma Chemical, Medtronic, TRCare, SAEBO, Allergan/AbbVie, Ipsen, Merz, Ottobock/Hangar Orthopedics, Parker Hannifin, Revance Therapeutics, ReWallk, and Sword Health. Three coauthors are employees of MicroTransponder. Dr. Luft has no relevant relationships to disclose.
according to preliminary results of a randomized clinical trial at the 2021 annual meeting of the American Academy of Neurology.
“We believe that vagus nerve stimulation combined with rehabilitation is an acceptable and effective intervention for improving upper-limb impairment and function in people with moderate to severe arm weakness a long time VNS-REHAB pivotal study is a randomized, blinded, controlled trial of 108 people who had upper-extremity weakness after having a stroke at least 9 months before enrollment. The average for the group was 3 years post stroke after ischemic stroke,” said Jesse Dawson, MD, a professor at the University of Glasgow.
The Fifty-three patients were assigned active VNS followed by 6 weeks of in-clinic rehabilitation and then 90 days of home-based rehab. At in-clinic rehab, the therapist initiated a 5-second burst of VNS stimulation during each movement. In home-base treatment, the device was activated by a magnet.
Fifty-five patients were assigned sham VNS. After 90 days, the sham group crossed over to receive VNS for 6 weeks and then 90 days of home exercise. This crossover group was the focus of the data Dr. Dawson presented at AAN 2021. The overall trial results have been published in the Lancet.
“The hypothesis is based on the knowledge that the VNS stimulates the release of proneuroplastic neuromodulators norepinephrine and acetylcholine,” Dr. Dawson said. “By pairing VNS with task-specific movement, we hypothesize that we will increase task-specific neuroplasticity.”
The main study showed “a statistically significant difference across all primary and secondary endpoints at all time points in favor of rehabilitation paired with VNS,” Dr. Dawson said. The primary outcome was improvement in Fugl-Meyer Upper Extremity (FMA-UE) outcome, with the active VNS group having a significantly higher percentage of responders. For example, 47% of the active VNS patients had a greater than 6-point response on FMA-UE improvement versus 27% of the sham group (P = .010).
When the sham group crossed over to active VNS, the improvement in arm function matched that of the treatment group in the main study, Dr. Dawson said. “If you look at specifically what happened after they completed the control phase, there was a further small increase in Fugl-Meyer score, but, more importantly between 20% and 35% achieved a clinically important response on the Fugl-Meyer assessment or the Wolf Motor Function Test, giving a number need to treat ranging from three to five,” he said.
Dr. Dawson said that data on adverse events was presented in the Lancet publication. “These were observed at expected frequencies,” he said.
In an interview, he explained the significance of reporting the number to treat. “The number needed to treat helps give an idea of how many times you need to do something to achieve the desired outcome. So for VNS paired with rehab versus rehab alone, you need to treat four people to get one extra clinically important response, compared with just doing therapy.”
The next steps for his group’s research, he said, “will be to try and explore whether we can predict who responds best, and we would like to see if people with other types of stroke benefit.”
In providing comment on the study, Andreas Luft, MD, a professor at the University Hospital Zürich, noted that the FME-UE score improvements reported “are significant and meaningful. ... However, they may also be achieved by increasing the intensity of training. Many medical systems offer their patients high rehabilitation intensities and achieve similar improvements. Whether VNS can further boost higher-intensity training ‘beyond its limits’ is probable but remains to be demonstrated.”
Dr. Luft noted the study advances the knowledge of combining a therapeutic approach with training. “More such approaches are necessary to increase the therapeutic instrumentation of neurorehabilitation,” he said.
Dr. Dawson reported a financial relationship with MicroTransponder. His coauthors reported relationships with MicroTransponder, SanBio, Fujifilm Toyoma Chemical, Medtronic, TRCare, SAEBO, Allergan/AbbVie, Ipsen, Merz, Ottobock/Hangar Orthopedics, Parker Hannifin, Revance Therapeutics, ReWallk, and Sword Health. Three coauthors are employees of MicroTransponder. Dr. Luft has no relevant relationships to disclose.
FROM AAN 2021