User login
Malpractice Counsel: Cervical Spine Injury
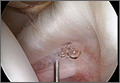
| An 83-year-old man presented to the ED via emergency medical services (EMS) with a chief complaint of neck pain. He was the restrained driver of a car that was struck from behind by another vehicle. The patient denied any head injury, loss of consciousness, chest pain, shortness of breath, or abdominal pain. His medical history was significant for hypertension and coronary artery disease, for which he was taking several medications. Regarding his social history, the patient denied alcohol consumption or cigarette smoking. |
The patient’s physical examination was unremarkable. His vital signs were normal, and there was no obvious external evidence of trauma. The posterior cervical spine was tender to palpation in the midline, but no step-off signs were appreciated. The neurological examination, including strength and sensation in all four extremities, was normal.
Since the patient’s only complaint was neck pain and his physical examination and history were otherwise normal, the emergency physician (EP) ordered radiographs of the cervical spine. The imaging studies were interpreted as showing advanced degenerative changes but no fractures, and the patient was prescribed an analgesic and discharged home.
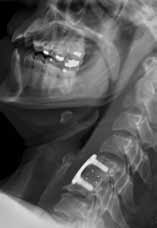
When the patient woke up the next morning, he was unable to move his extremities, and returned to the same ED via EMS. He was placed in a cervical collar and found to have flaccid extremities on examination. A computed tomography (CT) scan of the cervical spine revealed a transverse fracture through the C6 vertebra. Radiology services also reviewed the cervical spine X-rays from the previous day, noting the presence of fracture.
The patient was taken to the operating room by neurosurgery services but remained paralyzed postoperatively. He never recovered from his injury and died 6 months later. His family sued the EP and the hospital for missed diagnosis of cervical spine fracture at the first ED presentation and the resulting paralysis. The case was settled for $1.3 million prior to trial.
Discussion
The evaluation of suspected cervical spine injury secondary to blunt trauma is a frequent and important skill practiced by EPs. Motor vehicle accidents are the most common cause of spinal cord injury in the United States (42%), followed by falls (27%), acts of violence (15%), and sports-related injuries (8%).1 A review by Sekon and Fehlings2 showed that 55% of all spinal injuries involve the cervical spine. Interestingly, the majority of cervical spine injuries occur at the upper or lower ends of the cervical spine; C2 vertebral fractures account for 33%, while C6 and C7 vertebral fractures account for approximately 50%.1
There are two commonly used criteria to clinically clear the cervical spine (ie, no imaging studies necessary) in blunt-trauma patients. The first is the National Emergency X-Radiography Use Study (NEXUS), which has a sensitivity of 99.6% of identifying cervical spine fractures.1 According to the NEXUS criteria, no imaging studies are required if: (1) there is no midline cervical spine tenderness; (2) there are no focal neurological deficits; (3) the patient exhibits a normal level of alertness; (4) the patient is not intoxicated; and (5) there is no distracting injury.1
The other set of criteria used to clear the cervical spine is the Canadian Cervical Spine Rule. In these criteria, a patient is considered at very low risk for cervical spine fracture in the following cases: (1) the patient is fully alert with a Glasgow Coma scale of 15; (2) the patient has no high-risk factors (ie, age >65 years, dangerous mechanism of injury, fall greater than five stairs, axial load to the head, high-speed vehicular crash, bicycle or motorcycle crash, or the presence of paresthesias in the extremities); (3) the patient has low-risk factors (eg, simple vehicle crash, sitting position in the ED, ambulatory at any time, delayed onset of neck pain, and the absence of midline cervical tenderness); and (4) the patient can actively rotate his or her neck 45 degrees to the left and to the right. The Canadian group found the above criteria to have 100% sensitivity for predicting the absence of cervical spine injury.1
The patient in this case failed both sets of criteria (ie, presence of cervical spine tenderness and age >65 years) and therefore required imaging. Historically, cervical spine X-ray (three views, anteroposterior, lateral, and odontoid; or five views, three views plus obliques) has been the imaging study of choice for such patients. Unfortunately, however, cervical spine radiographs have severe limitations in identifying spinal injury. In a large retrospective review, Woodring and Lee,3 found that the standard three-view cervical spine series failed to demonstrate 61% of all fractures and 36% of all subluxation and dislocations. Similarly, in a prospective study of 1,006 patients with 72 injuries, Diaz et al,4 found a 52.3% missed fracture rate when five-view radiographs were used to identify cervical spine injury. In addition, radiographic evaluation of elderly patients was found to be even more challenging in identifying cervical spine injury due to age-related degenerative changes.
Given the abovementioned limitations associated with radiographic imaging, CT scan of the cervical spine has become the imaging study of choice in moderate-to-severe risk patients with blunt cervical spine trauma. This modality has been shown to have a higher sensitivity and specificity for evaluating cervical spine injury compared to plain X-ray films, with CT detecting 97% to 100% of cervical spine fractures.5
In addition to demonstrating a higher sensitivity, CT also has the advantage of speed—especially when the patient is undergoing other CT studies (eg, head, abdomen, pelvis). While some clinicians criticize the higher cost of CT versus plain films, CT has been shown to decrease institutional costs (when settlement costs are taken into account) due to the reduction of the incidence of paralysis resulting from false-negative imaging studies.6
Forgotten Tourniquet
| A 33-year-old woman presented to the ED with a chief complaint of left-sided abdominal and flank pain. She described the onset of pain as abrupt, severe, and lasting approximately 3 hours in duration. She admitted to nausea, but no vomiting. She also denied a history of any previous similar symptoms or recent trauma. The patient’s medical history was unremarkable. Her last menstrual period began 3 days prior to presentation. Regarding social history, she denied any tobacco or alcohol use. |
The patient’s vital signs were: blood pressure, 138/82 mm Hg; heart rate, 102 beats/minute; respiratory rate, 18 breaths/minute; temperature 98.6˚F. Oxygen saturation was 99% on room air.
The patient appeared uncomfortable overall. The physical examination was remarkable only for mild left-sided costovertebral angle tenderness. Her abdomen was soft, nontender, and without guarding or rebound.
The EP ordered the placement of an intravenous (IV) line, through which the patient was administered normal saline and morphine and promethazine, respectively, for pain and nausea. A complete blood count, basic metabolic panel, urinalysis, and urine pregnancy test were ordered. All of the laboratory bloodwork results were normal, and the urine pregnancy test was negative. The urinalysis was remarkable for 50 to 100 red blood cells.
A noncontrast CT scan of the abdomen and pelvis revealed a 3-mm ureteral stone on the left side. When the patient returned from radiology services, her pain was significantly decreased and she felt much improved. She was diagnosed with a kidney stone and discharged home with an analgesic and a strainer, along with instructions to follow-up with urology services. The patient was in the ED for a total of 5 hours.
The plaintiff sued the EP and hospital, claiming that the tourniquet used to start the IV line and draw blood was never removed, which in turn caused nerve damage resulting in reflex sympathetic dystrophy and complex regional pain syndrome. The defense denied all of these allegations, and the ED personnel testified that the tourniquet was removed as soon as the IV was established. The defense cited the plaintiff’s medical records, which contained documentation that the tourniquet had been removed. The defense further argued that if the tourniquet had been left on as the patient alleged, she would have experienced obvious physical signs, such as swelling, redness, infiltration of fluids, pain, and numbness. A defense verdict was returned.
Discussion
It is very tempting to simply dismiss this case as absurd, with nothing to be learned from it. It does defy common sense that no one would have noticed the tourniquet or, at the very least, that the patient would not have spoken up about it during her stay in the ED. While the jury clearly came to the correct conclusion, it does highlight a real problem: forgotten tourniquets.
According to the Pennsylvania Patient Safety Advisory (PPSA), there were 125 reports of tourniquets being left on patients in Pennsylvania healthcare facilities in 1 year alone.1 In 5% of these cases, the tourniquet was discovered within a half hour of application. In approximately 66% of cases, the tourniquet was left on for up to 2 hours, and the remaining were left in place for 2 to 18 hours.
Few locations within the hospital are without risk for this type of accident. The PPSA further noted that approximately 30% of retained tourniquets occurred on medical/surgical units, 14% in the ED, and 14% on inpatient and ambulatory surgical services departments. Approximately 19% were discovered when patients were transferred from one department to another.1
In the analysis of these incidents, contributing factors to forgotten tourniquets included staff failing to follow proper procedures, inadequate staff proficiency, and staff distractions and/or interruptions.1 In addition, some patients appeared to be at increased risk of having a retained tourniquet than others. Sixty percent of 125 patients with a forgotten tourniquet were aged 70 years or older, whereas some patients were younger than age 2 years.1 Not surprisingly, patients who were unable to verbally communicate (eg, patients who were intubated, under anesthesia, had expressive aphasia, severe dementia), were at the highest risk.
In a review of recovery room incidents, Salman and Asfar2 identified two cases of forgotten tourniquets out of approximately 7,000 patients. Potential strategies to avoid this mistake include: (1) only documenting procedures after they have been completed (eg, tourniquet removal); (2) double-checking that the tourniquet has been removed prior to leaving patient bedside; and (3) the use of extra-long tourniquets so the ends are more clearly visible.
Reference - Missed Cervical Spine Injury
- Looby S, Flanders A. Spine trauma. Radiol Clin North Am. 2011;49(1):129-163.
- Sekon LH, Fehlings MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine (Phila Pa 1976). 2001;26(24 Suppl):S2-S12.
- Woodring JH, Lee C. Limitations of cervical radiography in the evaluation of acute cervical trauma. J Trauma. 1993;34(1):32-39.
- Diaz JJ Jr, Gillman C, Morris JA Jr, May AK, Carrillo YM, Guy J. Are five-view plain films of the cervical spine unreliable? A prospective evaluation in blunt trauma patients with altered mental status. J Trauma. 2003;55(4):658-663.
- Parizel PM, Zijden T, Gaudino S, et al. Trauma of the spine and spinal cord: imagining strategies. Eur Spine J. 2010;19(Suppl 1):S8-S17.
- Grogan EL, Morris JA Jr, Dittus RS, et al. Cervical spine evaluation in urban trauma centers: lowering institutional costs and complications through helical CT scan. J Am Coll Surg. 2005;200(2):160-165.
Reference - Forgotten Tourniquet
- Pennsylvania Safety Advisory. Forgotten but not gone: tourniquets left on patients. PA PSRS Patient Saf Advis. 2005;2(2):19-21.
- Salman JM, Asfar SN. Recovery room incidents. Bas J Surg. 2007;24:3.
| An 83-year-old man presented to the ED via emergency medical services (EMS) with a chief complaint of neck pain. He was the restrained driver of a car that was struck from behind by another vehicle. The patient denied any head injury, loss of consciousness, chest pain, shortness of breath, or abdominal pain. His medical history was significant for hypertension and coronary artery disease, for which he was taking several medications. Regarding his social history, the patient denied alcohol consumption or cigarette smoking. |
The patient’s physical examination was unremarkable. His vital signs were normal, and there was no obvious external evidence of trauma. The posterior cervical spine was tender to palpation in the midline, but no step-off signs were appreciated. The neurological examination, including strength and sensation in all four extremities, was normal.
Since the patient’s only complaint was neck pain and his physical examination and history were otherwise normal, the emergency physician (EP) ordered radiographs of the cervical spine. The imaging studies were interpreted as showing advanced degenerative changes but no fractures, and the patient was prescribed an analgesic and discharged home.
When the patient woke up the next morning, he was unable to move his extremities, and returned to the same ED via EMS. He was placed in a cervical collar and found to have flaccid extremities on examination. A computed tomography (CT) scan of the cervical spine revealed a transverse fracture through the C6 vertebra. Radiology services also reviewed the cervical spine X-rays from the previous day, noting the presence of fracture.
The patient was taken to the operating room by neurosurgery services but remained paralyzed postoperatively. He never recovered from his injury and died 6 months later. His family sued the EP and the hospital for missed diagnosis of cervical spine fracture at the first ED presentation and the resulting paralysis. The case was settled for $1.3 million prior to trial.
Discussion
The evaluation of suspected cervical spine injury secondary to blunt trauma is a frequent and important skill practiced by EPs. Motor vehicle accidents are the most common cause of spinal cord injury in the United States (42%), followed by falls (27%), acts of violence (15%), and sports-related injuries (8%).1 A review by Sekon and Fehlings2 showed that 55% of all spinal injuries involve the cervical spine. Interestingly, the majority of cervical spine injuries occur at the upper or lower ends of the cervical spine; C2 vertebral fractures account for 33%, while C6 and C7 vertebral fractures account for approximately 50%.1
There are two commonly used criteria to clinically clear the cervical spine (ie, no imaging studies necessary) in blunt-trauma patients. The first is the National Emergency X-Radiography Use Study (NEXUS), which has a sensitivity of 99.6% of identifying cervical spine fractures.1 According to the NEXUS criteria, no imaging studies are required if: (1) there is no midline cervical spine tenderness; (2) there are no focal neurological deficits; (3) the patient exhibits a normal level of alertness; (4) the patient is not intoxicated; and (5) there is no distracting injury.1
The other set of criteria used to clear the cervical spine is the Canadian Cervical Spine Rule. In these criteria, a patient is considered at very low risk for cervical spine fracture in the following cases: (1) the patient is fully alert with a Glasgow Coma scale of 15; (2) the patient has no high-risk factors (ie, age >65 years, dangerous mechanism of injury, fall greater than five stairs, axial load to the head, high-speed vehicular crash, bicycle or motorcycle crash, or the presence of paresthesias in the extremities); (3) the patient has low-risk factors (eg, simple vehicle crash, sitting position in the ED, ambulatory at any time, delayed onset of neck pain, and the absence of midline cervical tenderness); and (4) the patient can actively rotate his or her neck 45 degrees to the left and to the right. The Canadian group found the above criteria to have 100% sensitivity for predicting the absence of cervical spine injury.1
The patient in this case failed both sets of criteria (ie, presence of cervical spine tenderness and age >65 years) and therefore required imaging. Historically, cervical spine X-ray (three views, anteroposterior, lateral, and odontoid; or five views, three views plus obliques) has been the imaging study of choice for such patients. Unfortunately, however, cervical spine radiographs have severe limitations in identifying spinal injury. In a large retrospective review, Woodring and Lee,3 found that the standard three-view cervical spine series failed to demonstrate 61% of all fractures and 36% of all subluxation and dislocations. Similarly, in a prospective study of 1,006 patients with 72 injuries, Diaz et al,4 found a 52.3% missed fracture rate when five-view radiographs were used to identify cervical spine injury. In addition, radiographic evaluation of elderly patients was found to be even more challenging in identifying cervical spine injury due to age-related degenerative changes.
Given the abovementioned limitations associated with radiographic imaging, CT scan of the cervical spine has become the imaging study of choice in moderate-to-severe risk patients with blunt cervical spine trauma. This modality has been shown to have a higher sensitivity and specificity for evaluating cervical spine injury compared to plain X-ray films, with CT detecting 97% to 100% of cervical spine fractures.5
In addition to demonstrating a higher sensitivity, CT also has the advantage of speed—especially when the patient is undergoing other CT studies (eg, head, abdomen, pelvis). While some clinicians criticize the higher cost of CT versus plain films, CT has been shown to decrease institutional costs (when settlement costs are taken into account) due to the reduction of the incidence of paralysis resulting from false-negative imaging studies.6
Forgotten Tourniquet
| A 33-year-old woman presented to the ED with a chief complaint of left-sided abdominal and flank pain. She described the onset of pain as abrupt, severe, and lasting approximately 3 hours in duration. She admitted to nausea, but no vomiting. She also denied a history of any previous similar symptoms or recent trauma. The patient’s medical history was unremarkable. Her last menstrual period began 3 days prior to presentation. Regarding social history, she denied any tobacco or alcohol use. |
The patient’s vital signs were: blood pressure, 138/82 mm Hg; heart rate, 102 beats/minute; respiratory rate, 18 breaths/minute; temperature 98.6˚F. Oxygen saturation was 99% on room air.
The patient appeared uncomfortable overall. The physical examination was remarkable only for mild left-sided costovertebral angle tenderness. Her abdomen was soft, nontender, and without guarding or rebound.
The EP ordered the placement of an intravenous (IV) line, through which the patient was administered normal saline and morphine and promethazine, respectively, for pain and nausea. A complete blood count, basic metabolic panel, urinalysis, and urine pregnancy test were ordered. All of the laboratory bloodwork results were normal, and the urine pregnancy test was negative. The urinalysis was remarkable for 50 to 100 red blood cells.
A noncontrast CT scan of the abdomen and pelvis revealed a 3-mm ureteral stone on the left side. When the patient returned from radiology services, her pain was significantly decreased and she felt much improved. She was diagnosed with a kidney stone and discharged home with an analgesic and a strainer, along with instructions to follow-up with urology services. The patient was in the ED for a total of 5 hours.
The plaintiff sued the EP and hospital, claiming that the tourniquet used to start the IV line and draw blood was never removed, which in turn caused nerve damage resulting in reflex sympathetic dystrophy and complex regional pain syndrome. The defense denied all of these allegations, and the ED personnel testified that the tourniquet was removed as soon as the IV was established. The defense cited the plaintiff’s medical records, which contained documentation that the tourniquet had been removed. The defense further argued that if the tourniquet had been left on as the patient alleged, she would have experienced obvious physical signs, such as swelling, redness, infiltration of fluids, pain, and numbness. A defense verdict was returned.
Discussion
It is very tempting to simply dismiss this case as absurd, with nothing to be learned from it. It does defy common sense that no one would have noticed the tourniquet or, at the very least, that the patient would not have spoken up about it during her stay in the ED. While the jury clearly came to the correct conclusion, it does highlight a real problem: forgotten tourniquets.
According to the Pennsylvania Patient Safety Advisory (PPSA), there were 125 reports of tourniquets being left on patients in Pennsylvania healthcare facilities in 1 year alone.1 In 5% of these cases, the tourniquet was discovered within a half hour of application. In approximately 66% of cases, the tourniquet was left on for up to 2 hours, and the remaining were left in place for 2 to 18 hours.
Few locations within the hospital are without risk for this type of accident. The PPSA further noted that approximately 30% of retained tourniquets occurred on medical/surgical units, 14% in the ED, and 14% on inpatient and ambulatory surgical services departments. Approximately 19% were discovered when patients were transferred from one department to another.1
In the analysis of these incidents, contributing factors to forgotten tourniquets included staff failing to follow proper procedures, inadequate staff proficiency, and staff distractions and/or interruptions.1 In addition, some patients appeared to be at increased risk of having a retained tourniquet than others. Sixty percent of 125 patients with a forgotten tourniquet were aged 70 years or older, whereas some patients were younger than age 2 years.1 Not surprisingly, patients who were unable to verbally communicate (eg, patients who were intubated, under anesthesia, had expressive aphasia, severe dementia), were at the highest risk.
In a review of recovery room incidents, Salman and Asfar2 identified two cases of forgotten tourniquets out of approximately 7,000 patients. Potential strategies to avoid this mistake include: (1) only documenting procedures after they have been completed (eg, tourniquet removal); (2) double-checking that the tourniquet has been removed prior to leaving patient bedside; and (3) the use of extra-long tourniquets so the ends are more clearly visible.
| An 83-year-old man presented to the ED via emergency medical services (EMS) with a chief complaint of neck pain. He was the restrained driver of a car that was struck from behind by another vehicle. The patient denied any head injury, loss of consciousness, chest pain, shortness of breath, or abdominal pain. His medical history was significant for hypertension and coronary artery disease, for which he was taking several medications. Regarding his social history, the patient denied alcohol consumption or cigarette smoking. |
The patient’s physical examination was unremarkable. His vital signs were normal, and there was no obvious external evidence of trauma. The posterior cervical spine was tender to palpation in the midline, but no step-off signs were appreciated. The neurological examination, including strength and sensation in all four extremities, was normal.
Since the patient’s only complaint was neck pain and his physical examination and history were otherwise normal, the emergency physician (EP) ordered radiographs of the cervical spine. The imaging studies were interpreted as showing advanced degenerative changes but no fractures, and the patient was prescribed an analgesic and discharged home.
When the patient woke up the next morning, he was unable to move his extremities, and returned to the same ED via EMS. He was placed in a cervical collar and found to have flaccid extremities on examination. A computed tomography (CT) scan of the cervical spine revealed a transverse fracture through the C6 vertebra. Radiology services also reviewed the cervical spine X-rays from the previous day, noting the presence of fracture.
The patient was taken to the operating room by neurosurgery services but remained paralyzed postoperatively. He never recovered from his injury and died 6 months later. His family sued the EP and the hospital for missed diagnosis of cervical spine fracture at the first ED presentation and the resulting paralysis. The case was settled for $1.3 million prior to trial.
Discussion
The evaluation of suspected cervical spine injury secondary to blunt trauma is a frequent and important skill practiced by EPs. Motor vehicle accidents are the most common cause of spinal cord injury in the United States (42%), followed by falls (27%), acts of violence (15%), and sports-related injuries (8%).1 A review by Sekon and Fehlings2 showed that 55% of all spinal injuries involve the cervical spine. Interestingly, the majority of cervical spine injuries occur at the upper or lower ends of the cervical spine; C2 vertebral fractures account for 33%, while C6 and C7 vertebral fractures account for approximately 50%.1
There are two commonly used criteria to clinically clear the cervical spine (ie, no imaging studies necessary) in blunt-trauma patients. The first is the National Emergency X-Radiography Use Study (NEXUS), which has a sensitivity of 99.6% of identifying cervical spine fractures.1 According to the NEXUS criteria, no imaging studies are required if: (1) there is no midline cervical spine tenderness; (2) there are no focal neurological deficits; (3) the patient exhibits a normal level of alertness; (4) the patient is not intoxicated; and (5) there is no distracting injury.1
The other set of criteria used to clear the cervical spine is the Canadian Cervical Spine Rule. In these criteria, a patient is considered at very low risk for cervical spine fracture in the following cases: (1) the patient is fully alert with a Glasgow Coma scale of 15; (2) the patient has no high-risk factors (ie, age >65 years, dangerous mechanism of injury, fall greater than five stairs, axial load to the head, high-speed vehicular crash, bicycle or motorcycle crash, or the presence of paresthesias in the extremities); (3) the patient has low-risk factors (eg, simple vehicle crash, sitting position in the ED, ambulatory at any time, delayed onset of neck pain, and the absence of midline cervical tenderness); and (4) the patient can actively rotate his or her neck 45 degrees to the left and to the right. The Canadian group found the above criteria to have 100% sensitivity for predicting the absence of cervical spine injury.1
The patient in this case failed both sets of criteria (ie, presence of cervical spine tenderness and age >65 years) and therefore required imaging. Historically, cervical spine X-ray (three views, anteroposterior, lateral, and odontoid; or five views, three views plus obliques) has been the imaging study of choice for such patients. Unfortunately, however, cervical spine radiographs have severe limitations in identifying spinal injury. In a large retrospective review, Woodring and Lee,3 found that the standard three-view cervical spine series failed to demonstrate 61% of all fractures and 36% of all subluxation and dislocations. Similarly, in a prospective study of 1,006 patients with 72 injuries, Diaz et al,4 found a 52.3% missed fracture rate when five-view radiographs were used to identify cervical spine injury. In addition, radiographic evaluation of elderly patients was found to be even more challenging in identifying cervical spine injury due to age-related degenerative changes.
Given the abovementioned limitations associated with radiographic imaging, CT scan of the cervical spine has become the imaging study of choice in moderate-to-severe risk patients with blunt cervical spine trauma. This modality has been shown to have a higher sensitivity and specificity for evaluating cervical spine injury compared to plain X-ray films, with CT detecting 97% to 100% of cervical spine fractures.5
In addition to demonstrating a higher sensitivity, CT also has the advantage of speed—especially when the patient is undergoing other CT studies (eg, head, abdomen, pelvis). While some clinicians criticize the higher cost of CT versus plain films, CT has been shown to decrease institutional costs (when settlement costs are taken into account) due to the reduction of the incidence of paralysis resulting from false-negative imaging studies.6
Forgotten Tourniquet
| A 33-year-old woman presented to the ED with a chief complaint of left-sided abdominal and flank pain. She described the onset of pain as abrupt, severe, and lasting approximately 3 hours in duration. She admitted to nausea, but no vomiting. She also denied a history of any previous similar symptoms or recent trauma. The patient’s medical history was unremarkable. Her last menstrual period began 3 days prior to presentation. Regarding social history, she denied any tobacco or alcohol use. |
The patient’s vital signs were: blood pressure, 138/82 mm Hg; heart rate, 102 beats/minute; respiratory rate, 18 breaths/minute; temperature 98.6˚F. Oxygen saturation was 99% on room air.
The patient appeared uncomfortable overall. The physical examination was remarkable only for mild left-sided costovertebral angle tenderness. Her abdomen was soft, nontender, and without guarding or rebound.
The EP ordered the placement of an intravenous (IV) line, through which the patient was administered normal saline and morphine and promethazine, respectively, for pain and nausea. A complete blood count, basic metabolic panel, urinalysis, and urine pregnancy test were ordered. All of the laboratory bloodwork results were normal, and the urine pregnancy test was negative. The urinalysis was remarkable for 50 to 100 red blood cells.
A noncontrast CT scan of the abdomen and pelvis revealed a 3-mm ureteral stone on the left side. When the patient returned from radiology services, her pain was significantly decreased and she felt much improved. She was diagnosed with a kidney stone and discharged home with an analgesic and a strainer, along with instructions to follow-up with urology services. The patient was in the ED for a total of 5 hours.
The plaintiff sued the EP and hospital, claiming that the tourniquet used to start the IV line and draw blood was never removed, which in turn caused nerve damage resulting in reflex sympathetic dystrophy and complex regional pain syndrome. The defense denied all of these allegations, and the ED personnel testified that the tourniquet was removed as soon as the IV was established. The defense cited the plaintiff’s medical records, which contained documentation that the tourniquet had been removed. The defense further argued that if the tourniquet had been left on as the patient alleged, she would have experienced obvious physical signs, such as swelling, redness, infiltration of fluids, pain, and numbness. A defense verdict was returned.
Discussion
It is very tempting to simply dismiss this case as absurd, with nothing to be learned from it. It does defy common sense that no one would have noticed the tourniquet or, at the very least, that the patient would not have spoken up about it during her stay in the ED. While the jury clearly came to the correct conclusion, it does highlight a real problem: forgotten tourniquets.
According to the Pennsylvania Patient Safety Advisory (PPSA), there were 125 reports of tourniquets being left on patients in Pennsylvania healthcare facilities in 1 year alone.1 In 5% of these cases, the tourniquet was discovered within a half hour of application. In approximately 66% of cases, the tourniquet was left on for up to 2 hours, and the remaining were left in place for 2 to 18 hours.
Few locations within the hospital are without risk for this type of accident. The PPSA further noted that approximately 30% of retained tourniquets occurred on medical/surgical units, 14% in the ED, and 14% on inpatient and ambulatory surgical services departments. Approximately 19% were discovered when patients were transferred from one department to another.1
In the analysis of these incidents, contributing factors to forgotten tourniquets included staff failing to follow proper procedures, inadequate staff proficiency, and staff distractions and/or interruptions.1 In addition, some patients appeared to be at increased risk of having a retained tourniquet than others. Sixty percent of 125 patients with a forgotten tourniquet were aged 70 years or older, whereas some patients were younger than age 2 years.1 Not surprisingly, patients who were unable to verbally communicate (eg, patients who were intubated, under anesthesia, had expressive aphasia, severe dementia), were at the highest risk.
In a review of recovery room incidents, Salman and Asfar2 identified two cases of forgotten tourniquets out of approximately 7,000 patients. Potential strategies to avoid this mistake include: (1) only documenting procedures after they have been completed (eg, tourniquet removal); (2) double-checking that the tourniquet has been removed prior to leaving patient bedside; and (3) the use of extra-long tourniquets so the ends are more clearly visible.
Reference - Missed Cervical Spine Injury
- Looby S, Flanders A. Spine trauma. Radiol Clin North Am. 2011;49(1):129-163.
- Sekon LH, Fehlings MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine (Phila Pa 1976). 2001;26(24 Suppl):S2-S12.
- Woodring JH, Lee C. Limitations of cervical radiography in the evaluation of acute cervical trauma. J Trauma. 1993;34(1):32-39.
- Diaz JJ Jr, Gillman C, Morris JA Jr, May AK, Carrillo YM, Guy J. Are five-view plain films of the cervical spine unreliable? A prospective evaluation in blunt trauma patients with altered mental status. J Trauma. 2003;55(4):658-663.
- Parizel PM, Zijden T, Gaudino S, et al. Trauma of the spine and spinal cord: imagining strategies. Eur Spine J. 2010;19(Suppl 1):S8-S17.
- Grogan EL, Morris JA Jr, Dittus RS, et al. Cervical spine evaluation in urban trauma centers: lowering institutional costs and complications through helical CT scan. J Am Coll Surg. 2005;200(2):160-165.
Reference - Forgotten Tourniquet
- Pennsylvania Safety Advisory. Forgotten but not gone: tourniquets left on patients. PA PSRS Patient Saf Advis. 2005;2(2):19-21.
- Salman JM, Asfar SN. Recovery room incidents. Bas J Surg. 2007;24:3.
Reference - Missed Cervical Spine Injury
- Looby S, Flanders A. Spine trauma. Radiol Clin North Am. 2011;49(1):129-163.
- Sekon LH, Fehlings MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine (Phila Pa 1976). 2001;26(24 Suppl):S2-S12.
- Woodring JH, Lee C. Limitations of cervical radiography in the evaluation of acute cervical trauma. J Trauma. 1993;34(1):32-39.
- Diaz JJ Jr, Gillman C, Morris JA Jr, May AK, Carrillo YM, Guy J. Are five-view plain films of the cervical spine unreliable? A prospective evaluation in blunt trauma patients with altered mental status. J Trauma. 2003;55(4):658-663.
- Parizel PM, Zijden T, Gaudino S, et al. Trauma of the spine and spinal cord: imagining strategies. Eur Spine J. 2010;19(Suppl 1):S8-S17.
- Grogan EL, Morris JA Jr, Dittus RS, et al. Cervical spine evaluation in urban trauma centers: lowering institutional costs and complications through helical CT scan. J Am Coll Surg. 2005;200(2):160-165.
Reference - Forgotten Tourniquet
- Pennsylvania Safety Advisory. Forgotten but not gone: tourniquets left on patients. PA PSRS Patient Saf Advis. 2005;2(2):19-21.
- Salman JM, Asfar SN. Recovery room incidents. Bas J Surg. 2007;24:3.
Characteristics Associated With Active Defects in Juvenile Spondylolysis
Spondylolysis, a defect in the pars interarticularis, is the single most common identifiable source of persistent low back pain in adolescent athletes.1,2 The diagnosis of spondylolysis is confirmed by radiographic imaging.3 However, there is controversy regarding which imaging modality is preferred—specifically, which to use for first-line advanced imaging after plain radiographs are obtained.3 Single-photon emission computed tomography (SPECT) consistently has been shown to be the most sensitive modality, and it is considered the gold standard.4-7 Patients with a positive SPECT scan are then routinely imaged with computed tomography (CT) for bone detail and staging of the pars defect.8 This imaging or diagnostic sequence yields organ-specific radiation doses (15-30 mSv) as much as 50-fold higher than those of plain radiography.9 Recent epidemiologic studies have shown that this organ dose results in an increased risk of cancer, especially in children.10
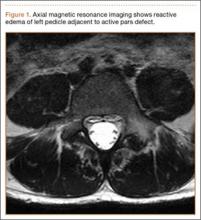
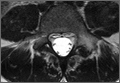
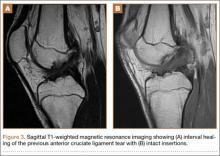
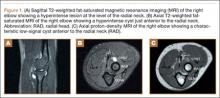
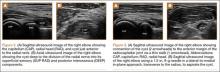
Diagnosis is crucial in early-stage lumbar spondylolysis, as osseous healing can occur with conservative treatment.11,12 High signal change (HSC) in the pedicle or pars interarticularis (Figure 1) on fluid-specific (T2) magnetic resonance imaging (MRI) sequences has been shown to be important in the diagnosis of early spondylolysis and, subsequently, a good predictor of bony healing.13,14 We conducted a study to determine the clinical and radiographic characteristics associated with the diagnosis of early or active spondylolysis.
Materials and Methods
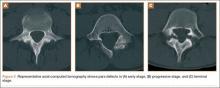
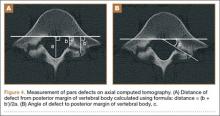
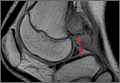
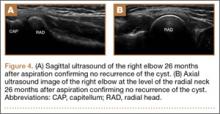
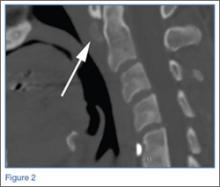
The study was reviewed and approved by the local institutional review board. Using the International Classification of Diseases, Ninth Revision (ICD-9) diagnosis code for spondylolysis (756.11), we retrospectively identified patients (age, 12-21 years) from 2002–2011 billing data from a single specialty spine practice. Baseline data—including height, weight, sex, age, symptom duration, sporting activities, defect location, pain score, and previous treatments—were collected from a standardized patient intake questionnaire and office medical records. We also determined radiographic data, including level, laterality (right vs left, unilateral vs bilateral), presence of listhesis, and slip grade and percentage. CT scans were reviewed to confirm the spondylolysis diagnosis and to measure parameters described by Fujii and colleagues.15 These parameters include spondylolysis chronicity (early, progressive, terminal) (Figure 2), distance from defect to posterior margin of vertebral body, and defect angle relative to posterior margin of vertebral body. We also measured sagittal radiographic parameters, including pelvic incidence and lumbar lordosis.
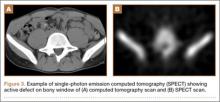
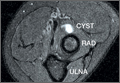
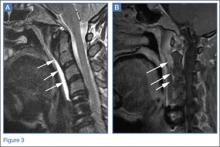
Pars lesions were divided into active and inactive defects16 based on signal characteristics on either MRI or SPECT (Figure 3). Defects with a positive SPECT or HSC on T2 MRI were classified as active; all other defects were classified as inactive. All MRIs were reviewed by a radiologist, and any mention of HSC in the pedicle or pars of the corresponding level was considered positive. For the sake of accuracy, all MRIs were also reviewed by a spine surgeon. All CT measurements were done by 1 of 2 authors. Demographic, clinical, and radiographic characteristics were compared between patients with active defects and patients with inactive defects. Independent t tests and Fisher exact tests were used to compare continuous and categorical variables, respectively. Threshold P was set at .01 to account for the small sample size and multiple concurrent comparisons.
Results
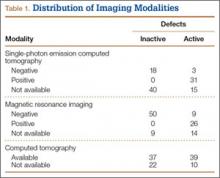
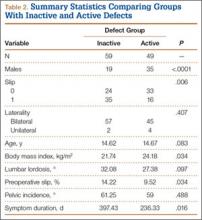
Fifty-seven patients (29 males, 28 females) with a total of 108 pars defects (6 unilateral, 102 bilateral) were identified. Mean age was 14.64 years. Of the 108 defects, 49 were classified as active and 59 as inactive. SPECT results were available for 52 defects, MRI results for 85, and CT results for 76 (Table 1). There was no difference between the active and inactive groups in age (14.7 vs 14.6 years; P = .083), body mass index (24.2 vs 21.7 kg/m2; P = .034), symptom duration (236.3 vs 397.4 days; P = .016), lumbar lordosis (27.4° vs 32.1°; P = .097), pelvic incidence (59.0° vs 61.2°; P = .488), slip percentage (9.5% vs 14.2%; P = .034), and laterality (right vs left, P = .847; unilateral vs bilateral, P = .281) (Table 2). There was a significant difference between the active and inactive groups in sex (35 vs 19 males; P < .0001) and presence of listhesis (16 vs 35; P = .006) (Table 2).
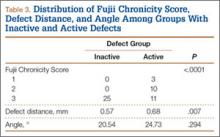
Of the 49 active defects, 3 were graded as early, 10 as progressive, and 11 as terminal (Table 3). There was a statistically significant (P < .0001) difference between active and inactive lesions for each stage. Mean distance from posterior margin of the vertebral body was 0.57 mm and 0.68 mm for inactive and active lesions, respectively (P = .007). There was no significant difference (P = .294) in the posterior angle of the vertebral body and the defect between inactive (20.54°) and active (24.73°) lesions (Table 3).
Subanalysis by sex showed no difference in age (males, 16.4 years vs females, 18.7 years; P = .073), slip percentage (10.4% vs 13.4%; P = .168), or presence or absence of slip (25 vs 26; P > .99) (Table 4).
Discussion
Increasing MRI resolution combined with increasing concern about unnecessary radiation exposure has added to the attractiveness of MRI in the diagnosis of spondylolysis. Spondylolysis progresses on a continuum, starting with a stress reaction (early or active defect) and ending with either healing or nonunion of the pars defect (terminal defect) (Figure 4). Although risk factors for progression are not clearly defined, Fujii and colleagues15 showed that the reaction around the defect is the most important factor for osseous union. It would then make sense that the earlier the spondylolytic defect is identified, the higher the likelihood for union, especially with nonoperative treatment such as rest, activity restriction, and bracing.12,17
There is a lack of consensus regarding MRI use in the diagnosis of spondylolysis. Masci and colleagues18 prospectively evaluated 50 defects in 39 patients using a 1.5-Tesla MRI scanner, concluded MRI is inferior to SPECT/CT, and recommended that SPECT remain the first-line advanced imaging modality. Conversely, Campbell and colleagues4 prospectively evaluated 40 defects in 22 patients using a 1.0-Tesla magnet and concluded that MRI can be used as an effective and reliable first-line advanced imaging modality. These are the only 2 prospective studies conducted within the past decade. Both were underpowered and used outdated technology (newer MRI scanners use 3.0-Tesla magnets). In addition, specific imaging characteristics (eg, edema in pars or pedicle on fluid-specific sequences) that suggest a positive finding—versus overt fracture on T1 MRI—have been recently emphasized. Neither Masci and colleagues18 nor Campbell and colleagues4 detailed what constituted a positive MRI finding. Although an adequately powered prospective study will provide a better analysis of the utility of MRI versus SPECT, such a study is costly and time-consuming. It is important to identify patient and lesion characteristics to help optimize the usefulness of MRI. It is also important to identify the subset of patients most likely to experience osseous healing of active defects,16 as this is the same subset of patients most likely to respond to nonoperative treatment.
We conducted the present study to identify any clinical or radiographic characteristics associated with the diagnosis of early or active spondylolysis. Almost equal numbers of active and inactive defects (49, 59) were identified. There were no differences in patient characteristics, including age, body mass index, and symptom duration. However, there was a significant sex difference—a relatively high proportion of males with active spondylolysis. This finding, which had been reported before,16,19,20 is probably the result of several factors, including males’ lower lumbar spine bone mineral density21; their relatively less spinal flexibility, which affects the distribution of torsional loads on the spine22; and their relatively greater participation in sports, especially sports involving high-velocity, torsional loading of the lumbar spine.23 Studies are needed to delineate the extent to which sex influences the development and persistence of active spondylolytic lesions. Alternatively, a subanalysis revealed an age difference, between our female and male cohorts (18.7 vs 16.4 years), that may have contributed to the high proportion of males with active spondylolysis.
Although the groups’ difference in symptom duration was not significant, it was trending toward significance. As discussed, it could be explained that, along the continuum of disease, earlier defects are more active and either achieve fibrous or osseous union or become chronic and “burn out” to inactive lesions, potentially leading to a listhesis.24 The listhesis association was higher in the inactive group than in the active group (P = .006). The difference in numbers of active and inactive defects at each stage (early, progressive, late) confirms this finding, with no inactive lesions in the early and progressive stages and many fewer active lesions in the terminal stage. Overall, presence of a spondylolisthesis on plain radiographs may obviate the need for SPECT or MRI, as it indicates an inactive chronic lesion—unless new symptoms are suspicious for reactivation or development of previously described adjacent-level pars defects.
No other radiographic parameters were found to be significant—consistent with findings of other studies.2,5,16 Pelvic incidence has been shown to predict progression of spondylisthesis, but under our study parameters it appears not to be associated with development of a slip.
This study had several weaknesses. First, it was retrospective, and imaging parameters were inconsistent, as we included patients who underwent imaging at other facilities. Second, the timing of imaging was inconsistent. Ideally, the same sequence protocol would be used, and all imaging studies (MRI, SPECT, CT) would be performed within a specific period after the initial concern for a spondylolysis was raised. Last, not all patients underwent all 3 advanced imaging modalities; having all 3 would have allowed for a retrospective comparison of MRI and SPECT sensitivity in detecting spondylolysis. Such a comparison would have been interesting, though it was not the goal of this study.
With its technological improvements and lack of radiation exposure, MRI is becoming more attractive as a first-line advanced imaging modality. Although the superiority of MRI over SPECT is yet to be confirmed, clinical use of MRI in the evaluation of spondylolysis seems to be increasing. It is therefore important to characterize the spondylolytic defects that are readily detected with MRI.
Active or early juvenile spondylolysis appears to be associated with males and absence of an associated listhesis. These clinical and radiographic characteristics may be important in the identification of patients with higher potential for osseous healing after nonoperative treatment.
1. Micheli LJ, Wood R. Back pain in young athletes. Significant differences from adults in causes and patterns. Arch Pediatr Adolesc Med. 1995;149(1):15-18.
2. Sakai T, Sairyo K, Suzue N, Kosaka H, Yasui N. Incidence and etiology of lumbar spondylolysis: review of the literature. J Orthop Sci. 2010;15(3):281-288.
3. Standaert CJ, Herring SA. Expert opinion and controversies in sports and musculoskeletal medicine: the diagnosis and treatment of spondylolysis in adolescent athletes. Arch Phys Med Rehabil. 2007;88(4):537-540.
4. Campbell RS, Grainger AJ, Hide IG, Papastefanou S, Greenough CG. Juvenile spondylolysis: a comparative analysis of CT, SPECT and MRI. Skeletal Radiol. 2005;34(2):63-73.
5. Kalichman L, Kim DH, Li L, Guermazi A, Berkin V, Hunter DJ. Spondylolysis and spondylolisthesis: prevalence and association with low back pain in the adult community-based population. Spine. 2009;34(2):199-205.
6. Zukotynski K, Curtis C, Grant FD, Micheli L, Treves ST. The value of SPECT in the detection of stress injury to the pars interarticularis in patients with low back pain. J Orthop Surg Res. 2010;5:13.
7. Leone A, Cianfoni A, Cerase A, Magarelli N, Bonomo L. Lumbar spondylolysis: a review. Skeletal Radiol. 2011;40(6):683-700.
8. Gregory PL, Batt ME, Kerslake RW, Scammell BE, Webb JF. The value of combining single photon emission computerised tomography and computerised tomography in the investigation of spondylolysis. Eur Spine J. 2004;13(6):503-509.
9. Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277-2284.
10. Brenner DJ, Shuryak I, Einstein AJ. Impact of reduced patient life expectancy on potential cancer risks from radiologic imaging. Radiology. 2011;261(1):193-198.
11. Sairyo K, Sakai T, Yasui N, Dezawa A. Conservative treatment for pediatric lumbar spondylolysis to achieve bone healing using a hard brace: what type and how long?: Clinical article. J Neurosurg Spine. 2012;16(6):610-614.
12. Steiner ME, Micheli LJ. Treatment of symptomatic spondylolysis and spondylolisthesis with the modified Boston brace. Spine. 1985;10(10):937-943.
13. Sairyo K, Katoh S, Takata Y, et al. MRI signal changes of the pedicle as an indicator for early diagnosis of spondylolysis in children and adolescents: a clinical and biomechanical study. Spine. 2006;31(2):206-211.
14. Sakai T, Sairyo K, Mima S, Yasui N. Significance of magnetic resonance imaging signal change in the pedicle in the management of pediatric lumbar spondylolysis. Spine. 2010;35(14):E641-E645.
15. Fujii K, Katoh S, Sairyo K, Ikata T, Yasui N. Union of defects in the pars interarticularis of the lumbar spine in children and adolescents. The radiological outcome after conservative treatment. J Bone Joint Surg Br. 2004;86(2):225-231.
16. Gregg CD, Dean S, Schneiders AG. Variables associated with active spondylolysis. Phys Ther Sport. 2009;10(4):121-124.
17. Kobayashi A, Kobayashi T, Kato K, Higuchi H, Takagishi K. Diagnosis of radiographically occult lumbar spondylolysis in young athletes by magnetic resonance imaging. Am J Sports Med. 2013;41(1):169-176.
18. Masci L, Pike J, Malara F, Phillips B, Bennell K, Brukner P. Use of the one-legged hyperextension test and magnetic resonance imaging in the diagnosis of active spondylolysis. Br J Sports Med. 2006;40(11):940-946.
19. Beutler WJ, Fredrickson BE, Murtland A, Sweeney CA, Grant WD, Baker D. The natural history of spondylolysis and spondylolisthesis: 45-year follow-up evaluation. Spine. 2003;28(10):1027-1035.
20. Miller SF, Congeni J, Swanson K. Long-term functional and anatomical follow-up of early detected spondylolysis in young athletes. Am J Sports Med. 2004;32(4):928-933.
21. Zanchetta JR, Plotkin H, Alvarez Filgueira ML. Bone mass in children: normative values for the 2-20-year-old population. Bone. 1995;16(4 suppl):393S-399S.
22. Kondratek M, Krauss J, Stiller C, Olson R. Normative values for active lumbar range of motion in children. Pediatr Phys Ther. 2007;19(3):236-244.
23. Hardcastle P, Annear P, Foster DH, et al. Spinal abnormalities in young fast bowlers. J Bone Joint Surg Br. 1992;74(3):421-425.
24. Fredrickson BE, Baker D, McHolick WJ, Yuan HA, Lubicky JP. The natural history of spondylolysis and spondylolisthesis. J Bone Joint Surg Am. 1984;66(5):699-707.
Spondylolysis, a defect in the pars interarticularis, is the single most common identifiable source of persistent low back pain in adolescent athletes.1,2 The diagnosis of spondylolysis is confirmed by radiographic imaging.3 However, there is controversy regarding which imaging modality is preferred—specifically, which to use for first-line advanced imaging after plain radiographs are obtained.3 Single-photon emission computed tomography (SPECT) consistently has been shown to be the most sensitive modality, and it is considered the gold standard.4-7 Patients with a positive SPECT scan are then routinely imaged with computed tomography (CT) for bone detail and staging of the pars defect.8 This imaging or diagnostic sequence yields organ-specific radiation doses (15-30 mSv) as much as 50-fold higher than those of plain radiography.9 Recent epidemiologic studies have shown that this organ dose results in an increased risk of cancer, especially in children.10
Diagnosis is crucial in early-stage lumbar spondylolysis, as osseous healing can occur with conservative treatment.11,12 High signal change (HSC) in the pedicle or pars interarticularis (Figure 1) on fluid-specific (T2) magnetic resonance imaging (MRI) sequences has been shown to be important in the diagnosis of early spondylolysis and, subsequently, a good predictor of bony healing.13,14 We conducted a study to determine the clinical and radiographic characteristics associated with the diagnosis of early or active spondylolysis.
Materials and Methods
The study was reviewed and approved by the local institutional review board. Using the International Classification of Diseases, Ninth Revision (ICD-9) diagnosis code for spondylolysis (756.11), we retrospectively identified patients (age, 12-21 years) from 2002–2011 billing data from a single specialty spine practice. Baseline data—including height, weight, sex, age, symptom duration, sporting activities, defect location, pain score, and previous treatments—were collected from a standardized patient intake questionnaire and office medical records. We also determined radiographic data, including level, laterality (right vs left, unilateral vs bilateral), presence of listhesis, and slip grade and percentage. CT scans were reviewed to confirm the spondylolysis diagnosis and to measure parameters described by Fujii and colleagues.15 These parameters include spondylolysis chronicity (early, progressive, terminal) (Figure 2), distance from defect to posterior margin of vertebral body, and defect angle relative to posterior margin of vertebral body. We also measured sagittal radiographic parameters, including pelvic incidence and lumbar lordosis.
Pars lesions were divided into active and inactive defects16 based on signal characteristics on either MRI or SPECT (Figure 3). Defects with a positive SPECT or HSC on T2 MRI were classified as active; all other defects were classified as inactive. All MRIs were reviewed by a radiologist, and any mention of HSC in the pedicle or pars of the corresponding level was considered positive. For the sake of accuracy, all MRIs were also reviewed by a spine surgeon. All CT measurements were done by 1 of 2 authors. Demographic, clinical, and radiographic characteristics were compared between patients with active defects and patients with inactive defects. Independent t tests and Fisher exact tests were used to compare continuous and categorical variables, respectively. Threshold P was set at .01 to account for the small sample size and multiple concurrent comparisons.
Results
Fifty-seven patients (29 males, 28 females) with a total of 108 pars defects (6 unilateral, 102 bilateral) were identified. Mean age was 14.64 years. Of the 108 defects, 49 were classified as active and 59 as inactive. SPECT results were available for 52 defects, MRI results for 85, and CT results for 76 (Table 1). There was no difference between the active and inactive groups in age (14.7 vs 14.6 years; P = .083), body mass index (24.2 vs 21.7 kg/m2; P = .034), symptom duration (236.3 vs 397.4 days; P = .016), lumbar lordosis (27.4° vs 32.1°; P = .097), pelvic incidence (59.0° vs 61.2°; P = .488), slip percentage (9.5% vs 14.2%; P = .034), and laterality (right vs left, P = .847; unilateral vs bilateral, P = .281) (Table 2). There was a significant difference between the active and inactive groups in sex (35 vs 19 males; P < .0001) and presence of listhesis (16 vs 35; P = .006) (Table 2).
Of the 49 active defects, 3 were graded as early, 10 as progressive, and 11 as terminal (Table 3). There was a statistically significant (P < .0001) difference between active and inactive lesions for each stage. Mean distance from posterior margin of the vertebral body was 0.57 mm and 0.68 mm for inactive and active lesions, respectively (P = .007). There was no significant difference (P = .294) in the posterior angle of the vertebral body and the defect between inactive (20.54°) and active (24.73°) lesions (Table 3).
Subanalysis by sex showed no difference in age (males, 16.4 years vs females, 18.7 years; P = .073), slip percentage (10.4% vs 13.4%; P = .168), or presence or absence of slip (25 vs 26; P > .99) (Table 4).
Discussion
Increasing MRI resolution combined with increasing concern about unnecessary radiation exposure has added to the attractiveness of MRI in the diagnosis of spondylolysis. Spondylolysis progresses on a continuum, starting with a stress reaction (early or active defect) and ending with either healing or nonunion of the pars defect (terminal defect) (Figure 4). Although risk factors for progression are not clearly defined, Fujii and colleagues15 showed that the reaction around the defect is the most important factor for osseous union. It would then make sense that the earlier the spondylolytic defect is identified, the higher the likelihood for union, especially with nonoperative treatment such as rest, activity restriction, and bracing.12,17
There is a lack of consensus regarding MRI use in the diagnosis of spondylolysis. Masci and colleagues18 prospectively evaluated 50 defects in 39 patients using a 1.5-Tesla MRI scanner, concluded MRI is inferior to SPECT/CT, and recommended that SPECT remain the first-line advanced imaging modality. Conversely, Campbell and colleagues4 prospectively evaluated 40 defects in 22 patients using a 1.0-Tesla magnet and concluded that MRI can be used as an effective and reliable first-line advanced imaging modality. These are the only 2 prospective studies conducted within the past decade. Both were underpowered and used outdated technology (newer MRI scanners use 3.0-Tesla magnets). In addition, specific imaging characteristics (eg, edema in pars or pedicle on fluid-specific sequences) that suggest a positive finding—versus overt fracture on T1 MRI—have been recently emphasized. Neither Masci and colleagues18 nor Campbell and colleagues4 detailed what constituted a positive MRI finding. Although an adequately powered prospective study will provide a better analysis of the utility of MRI versus SPECT, such a study is costly and time-consuming. It is important to identify patient and lesion characteristics to help optimize the usefulness of MRI. It is also important to identify the subset of patients most likely to experience osseous healing of active defects,16 as this is the same subset of patients most likely to respond to nonoperative treatment.
We conducted the present study to identify any clinical or radiographic characteristics associated with the diagnosis of early or active spondylolysis. Almost equal numbers of active and inactive defects (49, 59) were identified. There were no differences in patient characteristics, including age, body mass index, and symptom duration. However, there was a significant sex difference—a relatively high proportion of males with active spondylolysis. This finding, which had been reported before,16,19,20 is probably the result of several factors, including males’ lower lumbar spine bone mineral density21; their relatively less spinal flexibility, which affects the distribution of torsional loads on the spine22; and their relatively greater participation in sports, especially sports involving high-velocity, torsional loading of the lumbar spine.23 Studies are needed to delineate the extent to which sex influences the development and persistence of active spondylolytic lesions. Alternatively, a subanalysis revealed an age difference, between our female and male cohorts (18.7 vs 16.4 years), that may have contributed to the high proportion of males with active spondylolysis.
Although the groups’ difference in symptom duration was not significant, it was trending toward significance. As discussed, it could be explained that, along the continuum of disease, earlier defects are more active and either achieve fibrous or osseous union or become chronic and “burn out” to inactive lesions, potentially leading to a listhesis.24 The listhesis association was higher in the inactive group than in the active group (P = .006). The difference in numbers of active and inactive defects at each stage (early, progressive, late) confirms this finding, with no inactive lesions in the early and progressive stages and many fewer active lesions in the terminal stage. Overall, presence of a spondylolisthesis on plain radiographs may obviate the need for SPECT or MRI, as it indicates an inactive chronic lesion—unless new symptoms are suspicious for reactivation or development of previously described adjacent-level pars defects.
No other radiographic parameters were found to be significant—consistent with findings of other studies.2,5,16 Pelvic incidence has been shown to predict progression of spondylisthesis, but under our study parameters it appears not to be associated with development of a slip.
This study had several weaknesses. First, it was retrospective, and imaging parameters were inconsistent, as we included patients who underwent imaging at other facilities. Second, the timing of imaging was inconsistent. Ideally, the same sequence protocol would be used, and all imaging studies (MRI, SPECT, CT) would be performed within a specific period after the initial concern for a spondylolysis was raised. Last, not all patients underwent all 3 advanced imaging modalities; having all 3 would have allowed for a retrospective comparison of MRI and SPECT sensitivity in detecting spondylolysis. Such a comparison would have been interesting, though it was not the goal of this study.
With its technological improvements and lack of radiation exposure, MRI is becoming more attractive as a first-line advanced imaging modality. Although the superiority of MRI over SPECT is yet to be confirmed, clinical use of MRI in the evaluation of spondylolysis seems to be increasing. It is therefore important to characterize the spondylolytic defects that are readily detected with MRI.
Active or early juvenile spondylolysis appears to be associated with males and absence of an associated listhesis. These clinical and radiographic characteristics may be important in the identification of patients with higher potential for osseous healing after nonoperative treatment.
Spondylolysis, a defect in the pars interarticularis, is the single most common identifiable source of persistent low back pain in adolescent athletes.1,2 The diagnosis of spondylolysis is confirmed by radiographic imaging.3 However, there is controversy regarding which imaging modality is preferred—specifically, which to use for first-line advanced imaging after plain radiographs are obtained.3 Single-photon emission computed tomography (SPECT) consistently has been shown to be the most sensitive modality, and it is considered the gold standard.4-7 Patients with a positive SPECT scan are then routinely imaged with computed tomography (CT) for bone detail and staging of the pars defect.8 This imaging or diagnostic sequence yields organ-specific radiation doses (15-30 mSv) as much as 50-fold higher than those of plain radiography.9 Recent epidemiologic studies have shown that this organ dose results in an increased risk of cancer, especially in children.10
Diagnosis is crucial in early-stage lumbar spondylolysis, as osseous healing can occur with conservative treatment.11,12 High signal change (HSC) in the pedicle or pars interarticularis (Figure 1) on fluid-specific (T2) magnetic resonance imaging (MRI) sequences has been shown to be important in the diagnosis of early spondylolysis and, subsequently, a good predictor of bony healing.13,14 We conducted a study to determine the clinical and radiographic characteristics associated with the diagnosis of early or active spondylolysis.
Materials and Methods
The study was reviewed and approved by the local institutional review board. Using the International Classification of Diseases, Ninth Revision (ICD-9) diagnosis code for spondylolysis (756.11), we retrospectively identified patients (age, 12-21 years) from 2002–2011 billing data from a single specialty spine practice. Baseline data—including height, weight, sex, age, symptom duration, sporting activities, defect location, pain score, and previous treatments—were collected from a standardized patient intake questionnaire and office medical records. We also determined radiographic data, including level, laterality (right vs left, unilateral vs bilateral), presence of listhesis, and slip grade and percentage. CT scans were reviewed to confirm the spondylolysis diagnosis and to measure parameters described by Fujii and colleagues.15 These parameters include spondylolysis chronicity (early, progressive, terminal) (Figure 2), distance from defect to posterior margin of vertebral body, and defect angle relative to posterior margin of vertebral body. We also measured sagittal radiographic parameters, including pelvic incidence and lumbar lordosis.
Pars lesions were divided into active and inactive defects16 based on signal characteristics on either MRI or SPECT (Figure 3). Defects with a positive SPECT or HSC on T2 MRI were classified as active; all other defects were classified as inactive. All MRIs were reviewed by a radiologist, and any mention of HSC in the pedicle or pars of the corresponding level was considered positive. For the sake of accuracy, all MRIs were also reviewed by a spine surgeon. All CT measurements were done by 1 of 2 authors. Demographic, clinical, and radiographic characteristics were compared between patients with active defects and patients with inactive defects. Independent t tests and Fisher exact tests were used to compare continuous and categorical variables, respectively. Threshold P was set at .01 to account for the small sample size and multiple concurrent comparisons.
Results
Fifty-seven patients (29 males, 28 females) with a total of 108 pars defects (6 unilateral, 102 bilateral) were identified. Mean age was 14.64 years. Of the 108 defects, 49 were classified as active and 59 as inactive. SPECT results were available for 52 defects, MRI results for 85, and CT results for 76 (Table 1). There was no difference between the active and inactive groups in age (14.7 vs 14.6 years; P = .083), body mass index (24.2 vs 21.7 kg/m2; P = .034), symptom duration (236.3 vs 397.4 days; P = .016), lumbar lordosis (27.4° vs 32.1°; P = .097), pelvic incidence (59.0° vs 61.2°; P = .488), slip percentage (9.5% vs 14.2%; P = .034), and laterality (right vs left, P = .847; unilateral vs bilateral, P = .281) (Table 2). There was a significant difference between the active and inactive groups in sex (35 vs 19 males; P < .0001) and presence of listhesis (16 vs 35; P = .006) (Table 2).
Of the 49 active defects, 3 were graded as early, 10 as progressive, and 11 as terminal (Table 3). There was a statistically significant (P < .0001) difference between active and inactive lesions for each stage. Mean distance from posterior margin of the vertebral body was 0.57 mm and 0.68 mm for inactive and active lesions, respectively (P = .007). There was no significant difference (P = .294) in the posterior angle of the vertebral body and the defect between inactive (20.54°) and active (24.73°) lesions (Table 3).
Subanalysis by sex showed no difference in age (males, 16.4 years vs females, 18.7 years; P = .073), slip percentage (10.4% vs 13.4%; P = .168), or presence or absence of slip (25 vs 26; P > .99) (Table 4).
Discussion
Increasing MRI resolution combined with increasing concern about unnecessary radiation exposure has added to the attractiveness of MRI in the diagnosis of spondylolysis. Spondylolysis progresses on a continuum, starting with a stress reaction (early or active defect) and ending with either healing or nonunion of the pars defect (terminal defect) (Figure 4). Although risk factors for progression are not clearly defined, Fujii and colleagues15 showed that the reaction around the defect is the most important factor for osseous union. It would then make sense that the earlier the spondylolytic defect is identified, the higher the likelihood for union, especially with nonoperative treatment such as rest, activity restriction, and bracing.12,17
There is a lack of consensus regarding MRI use in the diagnosis of spondylolysis. Masci and colleagues18 prospectively evaluated 50 defects in 39 patients using a 1.5-Tesla MRI scanner, concluded MRI is inferior to SPECT/CT, and recommended that SPECT remain the first-line advanced imaging modality. Conversely, Campbell and colleagues4 prospectively evaluated 40 defects in 22 patients using a 1.0-Tesla magnet and concluded that MRI can be used as an effective and reliable first-line advanced imaging modality. These are the only 2 prospective studies conducted within the past decade. Both were underpowered and used outdated technology (newer MRI scanners use 3.0-Tesla magnets). In addition, specific imaging characteristics (eg, edema in pars or pedicle on fluid-specific sequences) that suggest a positive finding—versus overt fracture on T1 MRI—have been recently emphasized. Neither Masci and colleagues18 nor Campbell and colleagues4 detailed what constituted a positive MRI finding. Although an adequately powered prospective study will provide a better analysis of the utility of MRI versus SPECT, such a study is costly and time-consuming. It is important to identify patient and lesion characteristics to help optimize the usefulness of MRI. It is also important to identify the subset of patients most likely to experience osseous healing of active defects,16 as this is the same subset of patients most likely to respond to nonoperative treatment.
We conducted the present study to identify any clinical or radiographic characteristics associated with the diagnosis of early or active spondylolysis. Almost equal numbers of active and inactive defects (49, 59) were identified. There were no differences in patient characteristics, including age, body mass index, and symptom duration. However, there was a significant sex difference—a relatively high proportion of males with active spondylolysis. This finding, which had been reported before,16,19,20 is probably the result of several factors, including males’ lower lumbar spine bone mineral density21; their relatively less spinal flexibility, which affects the distribution of torsional loads on the spine22; and their relatively greater participation in sports, especially sports involving high-velocity, torsional loading of the lumbar spine.23 Studies are needed to delineate the extent to which sex influences the development and persistence of active spondylolytic lesions. Alternatively, a subanalysis revealed an age difference, between our female and male cohorts (18.7 vs 16.4 years), that may have contributed to the high proportion of males with active spondylolysis.
Although the groups’ difference in symptom duration was not significant, it was trending toward significance. As discussed, it could be explained that, along the continuum of disease, earlier defects are more active and either achieve fibrous or osseous union or become chronic and “burn out” to inactive lesions, potentially leading to a listhesis.24 The listhesis association was higher in the inactive group than in the active group (P = .006). The difference in numbers of active and inactive defects at each stage (early, progressive, late) confirms this finding, with no inactive lesions in the early and progressive stages and many fewer active lesions in the terminal stage. Overall, presence of a spondylolisthesis on plain radiographs may obviate the need for SPECT or MRI, as it indicates an inactive chronic lesion—unless new symptoms are suspicious for reactivation or development of previously described adjacent-level pars defects.
No other radiographic parameters were found to be significant—consistent with findings of other studies.2,5,16 Pelvic incidence has been shown to predict progression of spondylisthesis, but under our study parameters it appears not to be associated with development of a slip.
This study had several weaknesses. First, it was retrospective, and imaging parameters were inconsistent, as we included patients who underwent imaging at other facilities. Second, the timing of imaging was inconsistent. Ideally, the same sequence protocol would be used, and all imaging studies (MRI, SPECT, CT) would be performed within a specific period after the initial concern for a spondylolysis was raised. Last, not all patients underwent all 3 advanced imaging modalities; having all 3 would have allowed for a retrospective comparison of MRI and SPECT sensitivity in detecting spondylolysis. Such a comparison would have been interesting, though it was not the goal of this study.
With its technological improvements and lack of radiation exposure, MRI is becoming more attractive as a first-line advanced imaging modality. Although the superiority of MRI over SPECT is yet to be confirmed, clinical use of MRI in the evaluation of spondylolysis seems to be increasing. It is therefore important to characterize the spondylolytic defects that are readily detected with MRI.
Active or early juvenile spondylolysis appears to be associated with males and absence of an associated listhesis. These clinical and radiographic characteristics may be important in the identification of patients with higher potential for osseous healing after nonoperative treatment.
1. Micheli LJ, Wood R. Back pain in young athletes. Significant differences from adults in causes and patterns. Arch Pediatr Adolesc Med. 1995;149(1):15-18.
2. Sakai T, Sairyo K, Suzue N, Kosaka H, Yasui N. Incidence and etiology of lumbar spondylolysis: review of the literature. J Orthop Sci. 2010;15(3):281-288.
3. Standaert CJ, Herring SA. Expert opinion and controversies in sports and musculoskeletal medicine: the diagnosis and treatment of spondylolysis in adolescent athletes. Arch Phys Med Rehabil. 2007;88(4):537-540.
4. Campbell RS, Grainger AJ, Hide IG, Papastefanou S, Greenough CG. Juvenile spondylolysis: a comparative analysis of CT, SPECT and MRI. Skeletal Radiol. 2005;34(2):63-73.
5. Kalichman L, Kim DH, Li L, Guermazi A, Berkin V, Hunter DJ. Spondylolysis and spondylolisthesis: prevalence and association with low back pain in the adult community-based population. Spine. 2009;34(2):199-205.
6. Zukotynski K, Curtis C, Grant FD, Micheli L, Treves ST. The value of SPECT in the detection of stress injury to the pars interarticularis in patients with low back pain. J Orthop Surg Res. 2010;5:13.
7. Leone A, Cianfoni A, Cerase A, Magarelli N, Bonomo L. Lumbar spondylolysis: a review. Skeletal Radiol. 2011;40(6):683-700.
8. Gregory PL, Batt ME, Kerslake RW, Scammell BE, Webb JF. The value of combining single photon emission computerised tomography and computerised tomography in the investigation of spondylolysis. Eur Spine J. 2004;13(6):503-509.
9. Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277-2284.
10. Brenner DJ, Shuryak I, Einstein AJ. Impact of reduced patient life expectancy on potential cancer risks from radiologic imaging. Radiology. 2011;261(1):193-198.
11. Sairyo K, Sakai T, Yasui N, Dezawa A. Conservative treatment for pediatric lumbar spondylolysis to achieve bone healing using a hard brace: what type and how long?: Clinical article. J Neurosurg Spine. 2012;16(6):610-614.
12. Steiner ME, Micheli LJ. Treatment of symptomatic spondylolysis and spondylolisthesis with the modified Boston brace. Spine. 1985;10(10):937-943.
13. Sairyo K, Katoh S, Takata Y, et al. MRI signal changes of the pedicle as an indicator for early diagnosis of spondylolysis in children and adolescents: a clinical and biomechanical study. Spine. 2006;31(2):206-211.
14. Sakai T, Sairyo K, Mima S, Yasui N. Significance of magnetic resonance imaging signal change in the pedicle in the management of pediatric lumbar spondylolysis. Spine. 2010;35(14):E641-E645.
15. Fujii K, Katoh S, Sairyo K, Ikata T, Yasui N. Union of defects in the pars interarticularis of the lumbar spine in children and adolescents. The radiological outcome after conservative treatment. J Bone Joint Surg Br. 2004;86(2):225-231.
16. Gregg CD, Dean S, Schneiders AG. Variables associated with active spondylolysis. Phys Ther Sport. 2009;10(4):121-124.
17. Kobayashi A, Kobayashi T, Kato K, Higuchi H, Takagishi K. Diagnosis of radiographically occult lumbar spondylolysis in young athletes by magnetic resonance imaging. Am J Sports Med. 2013;41(1):169-176.
18. Masci L, Pike J, Malara F, Phillips B, Bennell K, Brukner P. Use of the one-legged hyperextension test and magnetic resonance imaging in the diagnosis of active spondylolysis. Br J Sports Med. 2006;40(11):940-946.
19. Beutler WJ, Fredrickson BE, Murtland A, Sweeney CA, Grant WD, Baker D. The natural history of spondylolysis and spondylolisthesis: 45-year follow-up evaluation. Spine. 2003;28(10):1027-1035.
20. Miller SF, Congeni J, Swanson K. Long-term functional and anatomical follow-up of early detected spondylolysis in young athletes. Am J Sports Med. 2004;32(4):928-933.
21. Zanchetta JR, Plotkin H, Alvarez Filgueira ML. Bone mass in children: normative values for the 2-20-year-old population. Bone. 1995;16(4 suppl):393S-399S.
22. Kondratek M, Krauss J, Stiller C, Olson R. Normative values for active lumbar range of motion in children. Pediatr Phys Ther. 2007;19(3):236-244.
23. Hardcastle P, Annear P, Foster DH, et al. Spinal abnormalities in young fast bowlers. J Bone Joint Surg Br. 1992;74(3):421-425.
24. Fredrickson BE, Baker D, McHolick WJ, Yuan HA, Lubicky JP. The natural history of spondylolysis and spondylolisthesis. J Bone Joint Surg Am. 1984;66(5):699-707.
1. Micheli LJ, Wood R. Back pain in young athletes. Significant differences from adults in causes and patterns. Arch Pediatr Adolesc Med. 1995;149(1):15-18.
2. Sakai T, Sairyo K, Suzue N, Kosaka H, Yasui N. Incidence and etiology of lumbar spondylolysis: review of the literature. J Orthop Sci. 2010;15(3):281-288.
3. Standaert CJ, Herring SA. Expert opinion and controversies in sports and musculoskeletal medicine: the diagnosis and treatment of spondylolysis in adolescent athletes. Arch Phys Med Rehabil. 2007;88(4):537-540.
4. Campbell RS, Grainger AJ, Hide IG, Papastefanou S, Greenough CG. Juvenile spondylolysis: a comparative analysis of CT, SPECT and MRI. Skeletal Radiol. 2005;34(2):63-73.
5. Kalichman L, Kim DH, Li L, Guermazi A, Berkin V, Hunter DJ. Spondylolysis and spondylolisthesis: prevalence and association with low back pain in the adult community-based population. Spine. 2009;34(2):199-205.
6. Zukotynski K, Curtis C, Grant FD, Micheli L, Treves ST. The value of SPECT in the detection of stress injury to the pars interarticularis in patients with low back pain. J Orthop Surg Res. 2010;5:13.
7. Leone A, Cianfoni A, Cerase A, Magarelli N, Bonomo L. Lumbar spondylolysis: a review. Skeletal Radiol. 2011;40(6):683-700.
8. Gregory PL, Batt ME, Kerslake RW, Scammell BE, Webb JF. The value of combining single photon emission computerised tomography and computerised tomography in the investigation of spondylolysis. Eur Spine J. 2004;13(6):503-509.
9. Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277-2284.
10. Brenner DJ, Shuryak I, Einstein AJ. Impact of reduced patient life expectancy on potential cancer risks from radiologic imaging. Radiology. 2011;261(1):193-198.
11. Sairyo K, Sakai T, Yasui N, Dezawa A. Conservative treatment for pediatric lumbar spondylolysis to achieve bone healing using a hard brace: what type and how long?: Clinical article. J Neurosurg Spine. 2012;16(6):610-614.
12. Steiner ME, Micheli LJ. Treatment of symptomatic spondylolysis and spondylolisthesis with the modified Boston brace. Spine. 1985;10(10):937-943.
13. Sairyo K, Katoh S, Takata Y, et al. MRI signal changes of the pedicle as an indicator for early diagnosis of spondylolysis in children and adolescents: a clinical and biomechanical study. Spine. 2006;31(2):206-211.
14. Sakai T, Sairyo K, Mima S, Yasui N. Significance of magnetic resonance imaging signal change in the pedicle in the management of pediatric lumbar spondylolysis. Spine. 2010;35(14):E641-E645.
15. Fujii K, Katoh S, Sairyo K, Ikata T, Yasui N. Union of defects in the pars interarticularis of the lumbar spine in children and adolescents. The radiological outcome after conservative treatment. J Bone Joint Surg Br. 2004;86(2):225-231.
16. Gregg CD, Dean S, Schneiders AG. Variables associated with active spondylolysis. Phys Ther Sport. 2009;10(4):121-124.
17. Kobayashi A, Kobayashi T, Kato K, Higuchi H, Takagishi K. Diagnosis of radiographically occult lumbar spondylolysis in young athletes by magnetic resonance imaging. Am J Sports Med. 2013;41(1):169-176.
18. Masci L, Pike J, Malara F, Phillips B, Bennell K, Brukner P. Use of the one-legged hyperextension test and magnetic resonance imaging in the diagnosis of active spondylolysis. Br J Sports Med. 2006;40(11):940-946.
19. Beutler WJ, Fredrickson BE, Murtland A, Sweeney CA, Grant WD, Baker D. The natural history of spondylolysis and spondylolisthesis: 45-year follow-up evaluation. Spine. 2003;28(10):1027-1035.
20. Miller SF, Congeni J, Swanson K. Long-term functional and anatomical follow-up of early detected spondylolysis in young athletes. Am J Sports Med. 2004;32(4):928-933.
21. Zanchetta JR, Plotkin H, Alvarez Filgueira ML. Bone mass in children: normative values for the 2-20-year-old population. Bone. 1995;16(4 suppl):393S-399S.
22. Kondratek M, Krauss J, Stiller C, Olson R. Normative values for active lumbar range of motion in children. Pediatr Phys Ther. 2007;19(3):236-244.
23. Hardcastle P, Annear P, Foster DH, et al. Spinal abnormalities in young fast bowlers. J Bone Joint Surg Br. 1992;74(3):421-425.
24. Fredrickson BE, Baker D, McHolick WJ, Yuan HA, Lubicky JP. The natural history of spondylolysis and spondylolisthesis. J Bone Joint Surg Am. 1984;66(5):699-707.
Tommy John Surgeries On the Rise In Youth Athletes
ORLANDO—Surgeries related to overuse elbow injuries are more common among youth athletes than previously believed, according to research presented at the 2015 Annual Meeting of the American Orthopaedic Society for Sports Medicine and published in the July issue of the American Journal of Sports Medicine.
“Our results showed that 15- to 19-year-olds accounted for 56.7% of the ulnar collateral ligament reconstruction (UCLR) or Tommy John surgeries performed in the US between 2007 and 2011. This is a significant increase over time with an average increase of 9.12% per year,” said lead study author Brandon Erickson, MD, of Rush University Medical Center in Chicago, Illinois.
Dr. Erickson and his research team performed a retrospective analysis of a private payer database using the PearlDiver Supercomputer to identify UCLR procedures performed throughout the United States.
The overall average annual incidence of the procedure was 3.96 +/-0.38 per 100,000 patients with an annual overall growth rate of 4.2%. There were 695 males and 95 females involved in the analysis. Twenty to 24-year olds accounted for the second highest incident rate at 22.2%.
Other findings from the study included that the southern region of the US performed significantly more UCLR procedures than any other region with 53%. Most of the surgeries were also performed between April and June. Fifty-eight percent of the procedures were performed in an outpatient hospital setting, 40% were performed at a surgical center, and 3% of procedures were performed in an inpatient hospital setting.
“The research numbers suggest that more young athletes believe that having an UCLR procedure performed earlier in their career may lead to the big leagues or a scholarship, even though only one in 200 kids who play high school baseball will make it to Major League Baseball. This paradigm shift needs to be evaluated further to help prevent overuse injuries in kids from the beginning of the season when most issues arise,” said Dr. Erickson.
Suggested Reading
Erickson BJ, Nwachukwu BU, Rosas S, et al. Trends in medial ulnar collateral ligament reconstruction in the United States: a retrospective review of a large private-payer database from 2007 to 2011. Am J Sports Med. 2015;43(7):1770-1774.
ORLANDO—Surgeries related to overuse elbow injuries are more common among youth athletes than previously believed, according to research presented at the 2015 Annual Meeting of the American Orthopaedic Society for Sports Medicine and published in the July issue of the American Journal of Sports Medicine.
“Our results showed that 15- to 19-year-olds accounted for 56.7% of the ulnar collateral ligament reconstruction (UCLR) or Tommy John surgeries performed in the US between 2007 and 2011. This is a significant increase over time with an average increase of 9.12% per year,” said lead study author Brandon Erickson, MD, of Rush University Medical Center in Chicago, Illinois.
Dr. Erickson and his research team performed a retrospective analysis of a private payer database using the PearlDiver Supercomputer to identify UCLR procedures performed throughout the United States.
The overall average annual incidence of the procedure was 3.96 +/-0.38 per 100,000 patients with an annual overall growth rate of 4.2%. There were 695 males and 95 females involved in the analysis. Twenty to 24-year olds accounted for the second highest incident rate at 22.2%.
Other findings from the study included that the southern region of the US performed significantly more UCLR procedures than any other region with 53%. Most of the surgeries were also performed between April and June. Fifty-eight percent of the procedures were performed in an outpatient hospital setting, 40% were performed at a surgical center, and 3% of procedures were performed in an inpatient hospital setting.
“The research numbers suggest that more young athletes believe that having an UCLR procedure performed earlier in their career may lead to the big leagues or a scholarship, even though only one in 200 kids who play high school baseball will make it to Major League Baseball. This paradigm shift needs to be evaluated further to help prevent overuse injuries in kids from the beginning of the season when most issues arise,” said Dr. Erickson.
ORLANDO—Surgeries related to overuse elbow injuries are more common among youth athletes than previously believed, according to research presented at the 2015 Annual Meeting of the American Orthopaedic Society for Sports Medicine and published in the July issue of the American Journal of Sports Medicine.
“Our results showed that 15- to 19-year-olds accounted for 56.7% of the ulnar collateral ligament reconstruction (UCLR) or Tommy John surgeries performed in the US between 2007 and 2011. This is a significant increase over time with an average increase of 9.12% per year,” said lead study author Brandon Erickson, MD, of Rush University Medical Center in Chicago, Illinois.
Dr. Erickson and his research team performed a retrospective analysis of a private payer database using the PearlDiver Supercomputer to identify UCLR procedures performed throughout the United States.
The overall average annual incidence of the procedure was 3.96 +/-0.38 per 100,000 patients with an annual overall growth rate of 4.2%. There were 695 males and 95 females involved in the analysis. Twenty to 24-year olds accounted for the second highest incident rate at 22.2%.
Other findings from the study included that the southern region of the US performed significantly more UCLR procedures than any other region with 53%. Most of the surgeries were also performed between April and June. Fifty-eight percent of the procedures were performed in an outpatient hospital setting, 40% were performed at a surgical center, and 3% of procedures were performed in an inpatient hospital setting.
“The research numbers suggest that more young athletes believe that having an UCLR procedure performed earlier in their career may lead to the big leagues or a scholarship, even though only one in 200 kids who play high school baseball will make it to Major League Baseball. This paradigm shift needs to be evaluated further to help prevent overuse injuries in kids from the beginning of the season when most issues arise,” said Dr. Erickson.
Suggested Reading
Erickson BJ, Nwachukwu BU, Rosas S, et al. Trends in medial ulnar collateral ligament reconstruction in the United States: a retrospective review of a large private-payer database from 2007 to 2011. Am J Sports Med. 2015;43(7):1770-1774.
Suggested Reading
Erickson BJ, Nwachukwu BU, Rosas S, et al. Trends in medial ulnar collateral ligament reconstruction in the United States: a retrospective review of a large private-payer database from 2007 to 2011. Am J Sports Med. 2015;43(7):1770-1774.
Intrinsic Healing of the Anterior Cruciate Ligament in an Adolescent
The anterior cruciate ligament (ACL) restrains anterior translation of the tibia on the femur and controls rotation of the knee. The natural primary healing potential of the ACL has been extremely poor in clinical and experimental studies, and primary suture repair has not provided stability to the joint in most patients.1-8 This has led surgeons to reconstruct the ACL, rather than to attempt nonoperative treatment. Anterior cruciate ligament reconstruction is recommended to help patients maintain activities that place shear and torque forces on the knee or to ameliorate persistent pain due to instability.9 Reconstruction of the ACL in adults is one of the most common procedures performed by orthopedic surgeons. However, reconstruction in the ACL-deficient adolescent remains a controversial subject, with debates surrounding operative timing and surgical technique.
This case report presents a skeletally immature patient who suffered a complete traumatic rupture of his ACL, which intrinsically healed. The patient had a protracted treatment course, complicated by an open tibial fracture with delayed union. He responded to a progressive rehabilitation program and has made a good functional recovery. Review of the literature has demonstrated limited evidence of intrinsic ACL healing, none of which has been shown to occur in a skeletally immature patient. The patient’s mother provided written informed consent for print and electronic publication of this case report.
Case Report
A 12-year-old boy was brought to our level I trauma center by ambulance after being hit by a car while riding a motorized scooter. He presented with a grade IIIB open tibial fracture and a distal fibula fracture of his left lower extremity and was taken to the operating room that night for irrigation and débridement, percutaneous fixation of the fibula, and intramedullary flexible nail fixation of the tibia. On postoperative day 1, he had increasing pain and, once his splint was removed, his compartments were found to be very tense. He was taken emergently to the operating room for 4 compartment fasciotomies of the left lower extremity with wound vacuum-assisted closure (VAC) placement. This was changed on hospital day 4 and was removed with definitive closure on day 7. Examination under anesthesia prior to the final wound VAC change was performed given the patient’s complaints during physical therapy. This showed anterior and posterior ligamentous instability of the knee, and he was placed in a knee immobilizer. He was discharged on hospital day 11.
At 2-week follow-up, the patient was doing well, except that he was nonadherent with the knee immobilizer and unable to fully extend his left knee. On examination, a posterior drawer sign was noted; therefore, the patient was referred for magnetic resonance imaging (MRI) to evaluate his ligaments. His MRI, 9 weeks after injury, showed: (1) complete tears of both the anterior and posterior cruciate ligaments (PCLs) (Figures 1A, 1B); (2) medial meniscus and lateral meniscus tears; (3) 2.0-cm plate-like avulsion fracture of the posterolateral femoral metaphysis involving the insertion of the lateral head of the gastrocnemius muscle, fibular collateral ligament, and popliteus muscle (Figure 2); and (4) left posterior lateral tibial plateau contusion.
The patient was started on a 6-week course of physical therapy with active and active-assisted extension exercises. At follow-up approximately 3½ months after injury, he was found to have a 35º flexion contracture with pain at the end extension. Unfortunately, his tibial fracture showed minimal signs of healing, and the decision was made to delay surgical intervention on the knee until the tibial fracture had healed. He was given a knee orthotic to wear at night to help regain his knee extension.
Six months after injury, the patient underwent open removal of the avulsed bony fragment, posterior knee capsule release, and autograft of the delayed union tibial fracture. He was placed in a straight leg cast postoperatively and was discharged home on postoperative day 2. He transitioned to a knee immobilizer after 2 weeks. Six weeks after the last surgery, he had range of motion of 0º to 130º. Ligamentous examination at this time showed anterior and posterior drawer signs, positive Lachman test, and dial test with 90º of external rotation. He was placed in physical therapy for a total of 10 weeks to work on his quadriceps muscle strength and 15º extension lag.
On 13-month postinjury radiographs, the patient was noted to have adequate healing of his tibial fracture, and ligamentous reconstruction was discussed. At this time, the patient did not have any instability or pain in the knee. Examination demonstrated a very mild effusion of the left knee. Range of motion determined by goniometer was from -3º to 140º, and Lachman test was positive but with solid 2+ endpoint. He also had a positive posterior drawer sign with no endpoint, positive sag sign of his tibia, and positive active quadriceps test of the left leg. His dial test showed some increased external rotation at 90º but was equivocal at 30º when compared with the contralateral knee, demonstrating involvement of the posterolateral corner.
Sixteen months after injury, repeat MRI to further evaluate the posterolateral corner showed: (1) complete medial and lateral meniscal healing without evidence of residual or recurrent tear, and (2) interval healing of the remote ACL and PCL tears with intact insertions (Figures 3A, 3B). This scan showed an end-to-end continuous ACL with homogeneous signal and disappearance of the secondary signs. Physical examination at this time showed a very firm endpoint on Lachman test but some laxity with his posterior drawer. Given these findings, the patient was given a brace and continued in physical therapy to strengthen his quadriceps muscle. By 20 months after injury, he had returned to competitive hockey and had no complaints of pain or instability. His physical examination showed full range of motion in a ligamentously stable knee with firm endpoint. The patient’s condition was unchanged at 29-month follow-up.
Discussion
There is a body of evidence that states a completely ruptured ACL does not heal.3,6,10 In animal models, the ACL has been shown to have poor healing potential.3,11 Some studies have suggested this is secondary to poor blood supply. Blood supply to the ACL is derived from a periligamentous, then endoligamentous, arterial network with a less vascularized area in the middle third of the ACL. Additionally, there is no blood supply from the tibia or femur, meaning the areas of attachment of the ligament are poorly vascularized.12 With a minimal blood supply to the ACL, the supply of undifferentiated mesenchymal cells from the surrounding tissue during the initial healing process is limited. In vitro cell cultures of these cells have showed a reduced potential for proliferation and migration.9 Cells of the ACL have a lower response to growth factors than human medial collateral ligament cells, further suggesting a decreased reparative capacity.7 Joint fluid has been shown to inhibit the proliferation of these cells, further reducing their regenerative potential.13 Additionally, biomechanical factors that alter signaling pathways, sites of ligament reattachment, and injury to proprioceptive structures have been shown to negatively influence the healing response.14-18
Review of the literature on healing of ACLs includes 2 case reports, totaling 3 patients, and 3 level IV therapeutic studies involving 74 patients total.10,19-22 In most cases, the authors of these studies have indicated a nonoperative treatment protocol with bracing and a specific rehabilitation program. Malanga and colleagues10 demonstrated that an ACL torn from its attachment on the femur, with the majority of the ligament in good condition and no compromise in the length, healed back onto the femur. Kurosaka and coauthors20 described case reports of isolated distal or proximal midsubstance tears that have healed spontaneously. However, none of the patients described in the literature were under the age of 20 years.
Treatment for pediatric patients with open physes causes some debate. Nonoperative management of ACL deficiency in adolescents is generally not recommended because the continued instability of the joint leads to intra-articular injury, functional impairment, and joint degeneration.23-25 A recent systematic review found only 1 study that showed no increase in secondary intra-articular injury when surgery was delayed until skeletal maturity.26
Our patient was a 12-year-old boy whose traumatic knee injury with multiple ruptured ligaments healed over the course of 20 months. It is likely that bracing associated with the patient’s second surgery and delayed union of his tibial fracture allowed healing tissue to be protected from excessive stress until it remodeled with sufficient strength. Most would assume that healing would occur early, during the first 6 to 9 months; however, our patient regained his stability between 8 and 13 months. It is possible that the hostile healing environment of the ACL, including the low blood supply, poor response to growth factors, and biomechanical environment, as described previously, played a factor in this delay.7,9,12,13
It is important to recognize that our patient tore his ACL during a traumatic motorized scooter rollover collision, not the more common noncontact twisting injury. Additionally, given the patient’s knee surgery that was performed 6 months after the initial injury, it is possible that intra-articular scar formation contributed to his healing capacity. While this patient did not undergo arthroscopy to visualize the tear in the ACL, or its reconstitution, recent evidence suggests that the accuracy of MRI in diagnosing pediatric ACL injuries is excellent.27,28 The diagnostic accuracy with new MRI machines has sensitivity and specificity approaching 100%.29 Additionally, the patient’s subjective and objective improvements argue for a change in anatomy over a change in the quality of his examination.
Conclusion
The goal of ACL reconstruction in adolescents is to provide long-term stability to the knee while minimizing the risk of growth disturbance. This goal was achieved in our patient through the in situ healing of his ACL. Intrinsic reconstitution of a torn ACL is rare, and it is difficult to speculate which patients may have some healing potential. While this patient was an extreme example, his case demonstrated that protection of the knee from undue stress could favorably alter the environment of the knee to allow for healing of ACL tears. Such information could be valuable in managing select pediatric patients with open physes and ACL injuries nonoperatively, sparing them from the risks associated with surgical treatment. While we do not recommend nonoperative treatment for patients with acute tears of the ACL, we believe more investigation into the healing potential of the ACL, and potential pathways to augment this, is warranted.
1. Noyes FR, Mooar PA, Matthews DS, Butler DL. The symptomatic anterior cruciate-deficient knee. Part I: the long-term functional disability in athletically active individuals. J Bone Joint Surg Am. 1983;65(2):154-162.
2. Nagineni CN, Amiel D, Green MH, Berchuck M, Akeson WH. Characterization of the intrinsic properties of the anterior cruciate and medial collateral ligament cells: an in vitro cell culture study. J Orthop Res. 1992;10(4):465-475.
3. Hefti FL, Kress A, Fasel J, Morscher EW. Healing of the transected anterior cruciate ligament in the rabbit. J Bone Joint Surg Am. 1991;73(3):373-383.
4. Andersson C, Odensten M, Good L, Gillquist J. Surgical or non-surgical treatment of acute rupture of the anterior cruciate ligament. A randomized study with long-term follow-up. J Bone Joint Surg Am. 1989;71(7):965-974.
5. Tang Z, Yang L, Wang Y, et al. Contributions of different intraarticular tissues to the acute phase elevation of synovial fluid MMP-2 following rat ACL rupture. J Orthop Res. 2009;27(2):243-248.
6. Woo SL, Chan SS, Yamaji T. Biomechanics of knee ligament healing, repair and reconstruction. J Biomech. 1997;30(5):431-439.
7. Yoshida M, Fujii K. Differences in cellular properties and responses to growth factors between human ACL and MCL cells. J Orthop Sci. 1999;4(4):293-298.
8. Taylor DC, Posner M, Curl WW, Feagin JA. Isolated tears of the anterior cruciate ligament: over 30-year follow-up of patients treated with arthrotomy and primary repair. Am J Sports Med. 2009;37(1):65-71.
9. Noyes FR, Matthews DS, Mooar PA, Grood ES. The symptomatic anterior cruciate-deficient knee. Part II: the results of rehabilitation, activity modification, and counseling on functional disability. J Bone Joint Surg Am. 1983;65(2):163-174.
10. Malanga GA, Giradi J, Nadler SF. The spontaneous healing of a torn anterior cruciate ligament. Clin J Sport Med. 2001;11(2):118-120.
11. O’Donoghue DH, Rockwood CA Jr, Frank GR, Jack SC, Kenyon R. Repair of the anterior cruciate ligament in dogs. J Bone Joint Surg Am. 1966;48(3):503-519.
12. Guenoun D, Le Corroller T, Amous Z, Pauly V, Sbihi A, Champsaur P. The contribution of MRI to the diagnosis of traumatic tears of the anterior cruciate ligament. Diagn Intervent Imaging. 2012;93(5):331-341.
13. Andrish J, Holmes R. Effects of synovial fluid on fibroblasts in tissue culture. Clin Orthop Relat Res. 1979;(138):279-283.
14. Zimny ML, Schutte M, Dabezies E. Mechanoreceptors in the human anterior cruciate ligament. Anat Rec. 1986;214(2):204-209.
15. Bush-Joseph CA, Cummings JF, Buseck M, et al. Effect of tibial attachment location on the healing of the anterior cruciate ligament freeze model. J Orthop Res. 1996;14(4):534-541.
16. Sung KL, Whittemore DE, Yang L, Amiel D, Akeson WH. Signal pathways and ligament cell adhesiveness. J Orthop Res. 1996;14(5):729-735.
17. Deie M, Ochi M, Ikuta Y. High intrinsic healing potential of human anterior cruciate ligament. Organ culture experiments. Acta Orthop Scand. 1995;66(1):28-32.
18. Voloshin I, Bronstein RD, DeHaven KE. Spontaneous healing of a patellar tendon anterior cruciate ligament graft. A case report. Am J Sports Med. 2002;30(5):751-753.
19. Costa-Paz M, Ayerza MA, Tanoira I, Astoul J, Muscolo DL. Spontaneous healing in complete ACL ruptures: a clinical and MRI study. Clin Orthop Relat Res. 2012;470(4):979-985.
20. Kurosaka M, Yoshiya S, Mizuno T, Mizuno K. Spontaneous healing of a tear of the anterior cruciate ligament. A report of two cases. J Bone Joint Surg Am. 1998;80(8):1200-1203.
21. Fujimoto E, Sumen Y, Ochi M, Ikuta Y. Spontaneous healing of acute anterior cruciate ligament (ACL) injuries - conservative treatment using an extension block soft brace without anterior stabilization. Arch Orthop Trauma Surg. 2002;122(4):212-216.
22. Ihara H, Miwa M, Deya K, Torisu K. MRI of anterior cruciate ligament healing. J Comput Assist Tomogr. 1996;20(2):317-321.
23. Graf BK, Lange RH, Fujisaki CK, Landry GL, Saluja RK. Anterior cruciate ligament tears in skeletally immature patients: meniscal pathology at presentation and after attempted conservative treatment. Arthroscopy. 1992;8(2):229-233.
24. Kannus P, Jarvinen M. Knee ligament injuries in adolescents. Eight year follow-up of conservative management. J Bone Joint Surg Br. 1988;70(5):772-776.
25. Pressman AE, Letts RM, Jarvis JG. Anterior cruciate ligament tears in children: an analysis of operative versus nonoperative treatment. J Pediatr Orthop. 1997;17(4):505-511.
26. Vavken P, Murray MM. Treating anterior cruciate ligament tears in skeletally immature patients. Arthroscopy. 2011;27(5):704-716.
27. Lee K, Siegel MJ, Lau DM, Hildebolt CF, Matava MJ. Anterior cruciate ligament tears: MR imaging-based diagnosis in a pediatric population. Radiology. 1999;213(3):697-704.
28. Major NM, Beard LN Jr, Helms CA. Accuracy of MR imaging of the knee in adolescents. AJR Am J Roentgenol. 2003;180(1):17-19.
29. Sampson MJ, Jackson MP, Moran CJ, Shine S, Moran R, Eustace SJ. Three Tesla MRI for the diagnosis of meniscal and anterior cruciate ligament pathology: a comparison to arthroscopic findings. Clin Radiol. 2008;63(10):1106-1111.
The anterior cruciate ligament (ACL) restrains anterior translation of the tibia on the femur and controls rotation of the knee. The natural primary healing potential of the ACL has been extremely poor in clinical and experimental studies, and primary suture repair has not provided stability to the joint in most patients.1-8 This has led surgeons to reconstruct the ACL, rather than to attempt nonoperative treatment. Anterior cruciate ligament reconstruction is recommended to help patients maintain activities that place shear and torque forces on the knee or to ameliorate persistent pain due to instability.9 Reconstruction of the ACL in adults is one of the most common procedures performed by orthopedic surgeons. However, reconstruction in the ACL-deficient adolescent remains a controversial subject, with debates surrounding operative timing and surgical technique.
This case report presents a skeletally immature patient who suffered a complete traumatic rupture of his ACL, which intrinsically healed. The patient had a protracted treatment course, complicated by an open tibial fracture with delayed union. He responded to a progressive rehabilitation program and has made a good functional recovery. Review of the literature has demonstrated limited evidence of intrinsic ACL healing, none of which has been shown to occur in a skeletally immature patient. The patient’s mother provided written informed consent for print and electronic publication of this case report.
Case Report
A 12-year-old boy was brought to our level I trauma center by ambulance after being hit by a car while riding a motorized scooter. He presented with a grade IIIB open tibial fracture and a distal fibula fracture of his left lower extremity and was taken to the operating room that night for irrigation and débridement, percutaneous fixation of the fibula, and intramedullary flexible nail fixation of the tibia. On postoperative day 1, he had increasing pain and, once his splint was removed, his compartments were found to be very tense. He was taken emergently to the operating room for 4 compartment fasciotomies of the left lower extremity with wound vacuum-assisted closure (VAC) placement. This was changed on hospital day 4 and was removed with definitive closure on day 7. Examination under anesthesia prior to the final wound VAC change was performed given the patient’s complaints during physical therapy. This showed anterior and posterior ligamentous instability of the knee, and he was placed in a knee immobilizer. He was discharged on hospital day 11.
At 2-week follow-up, the patient was doing well, except that he was nonadherent with the knee immobilizer and unable to fully extend his left knee. On examination, a posterior drawer sign was noted; therefore, the patient was referred for magnetic resonance imaging (MRI) to evaluate his ligaments. His MRI, 9 weeks after injury, showed: (1) complete tears of both the anterior and posterior cruciate ligaments (PCLs) (Figures 1A, 1B); (2) medial meniscus and lateral meniscus tears; (3) 2.0-cm plate-like avulsion fracture of the posterolateral femoral metaphysis involving the insertion of the lateral head of the gastrocnemius muscle, fibular collateral ligament, and popliteus muscle (Figure 2); and (4) left posterior lateral tibial plateau contusion.
The patient was started on a 6-week course of physical therapy with active and active-assisted extension exercises. At follow-up approximately 3½ months after injury, he was found to have a 35º flexion contracture with pain at the end extension. Unfortunately, his tibial fracture showed minimal signs of healing, and the decision was made to delay surgical intervention on the knee until the tibial fracture had healed. He was given a knee orthotic to wear at night to help regain his knee extension.
Six months after injury, the patient underwent open removal of the avulsed bony fragment, posterior knee capsule release, and autograft of the delayed union tibial fracture. He was placed in a straight leg cast postoperatively and was discharged home on postoperative day 2. He transitioned to a knee immobilizer after 2 weeks. Six weeks after the last surgery, he had range of motion of 0º to 130º. Ligamentous examination at this time showed anterior and posterior drawer signs, positive Lachman test, and dial test with 90º of external rotation. He was placed in physical therapy for a total of 10 weeks to work on his quadriceps muscle strength and 15º extension lag.
On 13-month postinjury radiographs, the patient was noted to have adequate healing of his tibial fracture, and ligamentous reconstruction was discussed. At this time, the patient did not have any instability or pain in the knee. Examination demonstrated a very mild effusion of the left knee. Range of motion determined by goniometer was from -3º to 140º, and Lachman test was positive but with solid 2+ endpoint. He also had a positive posterior drawer sign with no endpoint, positive sag sign of his tibia, and positive active quadriceps test of the left leg. His dial test showed some increased external rotation at 90º but was equivocal at 30º when compared with the contralateral knee, demonstrating involvement of the posterolateral corner.
Sixteen months after injury, repeat MRI to further evaluate the posterolateral corner showed: (1) complete medial and lateral meniscal healing without evidence of residual or recurrent tear, and (2) interval healing of the remote ACL and PCL tears with intact insertions (Figures 3A, 3B). This scan showed an end-to-end continuous ACL with homogeneous signal and disappearance of the secondary signs. Physical examination at this time showed a very firm endpoint on Lachman test but some laxity with his posterior drawer. Given these findings, the patient was given a brace and continued in physical therapy to strengthen his quadriceps muscle. By 20 months after injury, he had returned to competitive hockey and had no complaints of pain or instability. His physical examination showed full range of motion in a ligamentously stable knee with firm endpoint. The patient’s condition was unchanged at 29-month follow-up.
Discussion
There is a body of evidence that states a completely ruptured ACL does not heal.3,6,10 In animal models, the ACL has been shown to have poor healing potential.3,11 Some studies have suggested this is secondary to poor blood supply. Blood supply to the ACL is derived from a periligamentous, then endoligamentous, arterial network with a less vascularized area in the middle third of the ACL. Additionally, there is no blood supply from the tibia or femur, meaning the areas of attachment of the ligament are poorly vascularized.12 With a minimal blood supply to the ACL, the supply of undifferentiated mesenchymal cells from the surrounding tissue during the initial healing process is limited. In vitro cell cultures of these cells have showed a reduced potential for proliferation and migration.9 Cells of the ACL have a lower response to growth factors than human medial collateral ligament cells, further suggesting a decreased reparative capacity.7 Joint fluid has been shown to inhibit the proliferation of these cells, further reducing their regenerative potential.13 Additionally, biomechanical factors that alter signaling pathways, sites of ligament reattachment, and injury to proprioceptive structures have been shown to negatively influence the healing response.14-18
Review of the literature on healing of ACLs includes 2 case reports, totaling 3 patients, and 3 level IV therapeutic studies involving 74 patients total.10,19-22 In most cases, the authors of these studies have indicated a nonoperative treatment protocol with bracing and a specific rehabilitation program. Malanga and colleagues10 demonstrated that an ACL torn from its attachment on the femur, with the majority of the ligament in good condition and no compromise in the length, healed back onto the femur. Kurosaka and coauthors20 described case reports of isolated distal or proximal midsubstance tears that have healed spontaneously. However, none of the patients described in the literature were under the age of 20 years.
Treatment for pediatric patients with open physes causes some debate. Nonoperative management of ACL deficiency in adolescents is generally not recommended because the continued instability of the joint leads to intra-articular injury, functional impairment, and joint degeneration.23-25 A recent systematic review found only 1 study that showed no increase in secondary intra-articular injury when surgery was delayed until skeletal maturity.26
Our patient was a 12-year-old boy whose traumatic knee injury with multiple ruptured ligaments healed over the course of 20 months. It is likely that bracing associated with the patient’s second surgery and delayed union of his tibial fracture allowed healing tissue to be protected from excessive stress until it remodeled with sufficient strength. Most would assume that healing would occur early, during the first 6 to 9 months; however, our patient regained his stability between 8 and 13 months. It is possible that the hostile healing environment of the ACL, including the low blood supply, poor response to growth factors, and biomechanical environment, as described previously, played a factor in this delay.7,9,12,13
It is important to recognize that our patient tore his ACL during a traumatic motorized scooter rollover collision, not the more common noncontact twisting injury. Additionally, given the patient’s knee surgery that was performed 6 months after the initial injury, it is possible that intra-articular scar formation contributed to his healing capacity. While this patient did not undergo arthroscopy to visualize the tear in the ACL, or its reconstitution, recent evidence suggests that the accuracy of MRI in diagnosing pediatric ACL injuries is excellent.27,28 The diagnostic accuracy with new MRI machines has sensitivity and specificity approaching 100%.29 Additionally, the patient’s subjective and objective improvements argue for a change in anatomy over a change in the quality of his examination.
Conclusion
The goal of ACL reconstruction in adolescents is to provide long-term stability to the knee while minimizing the risk of growth disturbance. This goal was achieved in our patient through the in situ healing of his ACL. Intrinsic reconstitution of a torn ACL is rare, and it is difficult to speculate which patients may have some healing potential. While this patient was an extreme example, his case demonstrated that protection of the knee from undue stress could favorably alter the environment of the knee to allow for healing of ACL tears. Such information could be valuable in managing select pediatric patients with open physes and ACL injuries nonoperatively, sparing them from the risks associated with surgical treatment. While we do not recommend nonoperative treatment for patients with acute tears of the ACL, we believe more investigation into the healing potential of the ACL, and potential pathways to augment this, is warranted.
The anterior cruciate ligament (ACL) restrains anterior translation of the tibia on the femur and controls rotation of the knee. The natural primary healing potential of the ACL has been extremely poor in clinical and experimental studies, and primary suture repair has not provided stability to the joint in most patients.1-8 This has led surgeons to reconstruct the ACL, rather than to attempt nonoperative treatment. Anterior cruciate ligament reconstruction is recommended to help patients maintain activities that place shear and torque forces on the knee or to ameliorate persistent pain due to instability.9 Reconstruction of the ACL in adults is one of the most common procedures performed by orthopedic surgeons. However, reconstruction in the ACL-deficient adolescent remains a controversial subject, with debates surrounding operative timing and surgical technique.
This case report presents a skeletally immature patient who suffered a complete traumatic rupture of his ACL, which intrinsically healed. The patient had a protracted treatment course, complicated by an open tibial fracture with delayed union. He responded to a progressive rehabilitation program and has made a good functional recovery. Review of the literature has demonstrated limited evidence of intrinsic ACL healing, none of which has been shown to occur in a skeletally immature patient. The patient’s mother provided written informed consent for print and electronic publication of this case report.
Case Report
A 12-year-old boy was brought to our level I trauma center by ambulance after being hit by a car while riding a motorized scooter. He presented with a grade IIIB open tibial fracture and a distal fibula fracture of his left lower extremity and was taken to the operating room that night for irrigation and débridement, percutaneous fixation of the fibula, and intramedullary flexible nail fixation of the tibia. On postoperative day 1, he had increasing pain and, once his splint was removed, his compartments were found to be very tense. He was taken emergently to the operating room for 4 compartment fasciotomies of the left lower extremity with wound vacuum-assisted closure (VAC) placement. This was changed on hospital day 4 and was removed with definitive closure on day 7. Examination under anesthesia prior to the final wound VAC change was performed given the patient’s complaints during physical therapy. This showed anterior and posterior ligamentous instability of the knee, and he was placed in a knee immobilizer. He was discharged on hospital day 11.
At 2-week follow-up, the patient was doing well, except that he was nonadherent with the knee immobilizer and unable to fully extend his left knee. On examination, a posterior drawer sign was noted; therefore, the patient was referred for magnetic resonance imaging (MRI) to evaluate his ligaments. His MRI, 9 weeks after injury, showed: (1) complete tears of both the anterior and posterior cruciate ligaments (PCLs) (Figures 1A, 1B); (2) medial meniscus and lateral meniscus tears; (3) 2.0-cm plate-like avulsion fracture of the posterolateral femoral metaphysis involving the insertion of the lateral head of the gastrocnemius muscle, fibular collateral ligament, and popliteus muscle (Figure 2); and (4) left posterior lateral tibial plateau contusion.
The patient was started on a 6-week course of physical therapy with active and active-assisted extension exercises. At follow-up approximately 3½ months after injury, he was found to have a 35º flexion contracture with pain at the end extension. Unfortunately, his tibial fracture showed minimal signs of healing, and the decision was made to delay surgical intervention on the knee until the tibial fracture had healed. He was given a knee orthotic to wear at night to help regain his knee extension.
Six months after injury, the patient underwent open removal of the avulsed bony fragment, posterior knee capsule release, and autograft of the delayed union tibial fracture. He was placed in a straight leg cast postoperatively and was discharged home on postoperative day 2. He transitioned to a knee immobilizer after 2 weeks. Six weeks after the last surgery, he had range of motion of 0º to 130º. Ligamentous examination at this time showed anterior and posterior drawer signs, positive Lachman test, and dial test with 90º of external rotation. He was placed in physical therapy for a total of 10 weeks to work on his quadriceps muscle strength and 15º extension lag.
On 13-month postinjury radiographs, the patient was noted to have adequate healing of his tibial fracture, and ligamentous reconstruction was discussed. At this time, the patient did not have any instability or pain in the knee. Examination demonstrated a very mild effusion of the left knee. Range of motion determined by goniometer was from -3º to 140º, and Lachman test was positive but with solid 2+ endpoint. He also had a positive posterior drawer sign with no endpoint, positive sag sign of his tibia, and positive active quadriceps test of the left leg. His dial test showed some increased external rotation at 90º but was equivocal at 30º when compared with the contralateral knee, demonstrating involvement of the posterolateral corner.
Sixteen months after injury, repeat MRI to further evaluate the posterolateral corner showed: (1) complete medial and lateral meniscal healing without evidence of residual or recurrent tear, and (2) interval healing of the remote ACL and PCL tears with intact insertions (Figures 3A, 3B). This scan showed an end-to-end continuous ACL with homogeneous signal and disappearance of the secondary signs. Physical examination at this time showed a very firm endpoint on Lachman test but some laxity with his posterior drawer. Given these findings, the patient was given a brace and continued in physical therapy to strengthen his quadriceps muscle. By 20 months after injury, he had returned to competitive hockey and had no complaints of pain or instability. His physical examination showed full range of motion in a ligamentously stable knee with firm endpoint. The patient’s condition was unchanged at 29-month follow-up.
Discussion
There is a body of evidence that states a completely ruptured ACL does not heal.3,6,10 In animal models, the ACL has been shown to have poor healing potential.3,11 Some studies have suggested this is secondary to poor blood supply. Blood supply to the ACL is derived from a periligamentous, then endoligamentous, arterial network with a less vascularized area in the middle third of the ACL. Additionally, there is no blood supply from the tibia or femur, meaning the areas of attachment of the ligament are poorly vascularized.12 With a minimal blood supply to the ACL, the supply of undifferentiated mesenchymal cells from the surrounding tissue during the initial healing process is limited. In vitro cell cultures of these cells have showed a reduced potential for proliferation and migration.9 Cells of the ACL have a lower response to growth factors than human medial collateral ligament cells, further suggesting a decreased reparative capacity.7 Joint fluid has been shown to inhibit the proliferation of these cells, further reducing their regenerative potential.13 Additionally, biomechanical factors that alter signaling pathways, sites of ligament reattachment, and injury to proprioceptive structures have been shown to negatively influence the healing response.14-18
Review of the literature on healing of ACLs includes 2 case reports, totaling 3 patients, and 3 level IV therapeutic studies involving 74 patients total.10,19-22 In most cases, the authors of these studies have indicated a nonoperative treatment protocol with bracing and a specific rehabilitation program. Malanga and colleagues10 demonstrated that an ACL torn from its attachment on the femur, with the majority of the ligament in good condition and no compromise in the length, healed back onto the femur. Kurosaka and coauthors20 described case reports of isolated distal or proximal midsubstance tears that have healed spontaneously. However, none of the patients described in the literature were under the age of 20 years.
Treatment for pediatric patients with open physes causes some debate. Nonoperative management of ACL deficiency in adolescents is generally not recommended because the continued instability of the joint leads to intra-articular injury, functional impairment, and joint degeneration.23-25 A recent systematic review found only 1 study that showed no increase in secondary intra-articular injury when surgery was delayed until skeletal maturity.26
Our patient was a 12-year-old boy whose traumatic knee injury with multiple ruptured ligaments healed over the course of 20 months. It is likely that bracing associated with the patient’s second surgery and delayed union of his tibial fracture allowed healing tissue to be protected from excessive stress until it remodeled with sufficient strength. Most would assume that healing would occur early, during the first 6 to 9 months; however, our patient regained his stability between 8 and 13 months. It is possible that the hostile healing environment of the ACL, including the low blood supply, poor response to growth factors, and biomechanical environment, as described previously, played a factor in this delay.7,9,12,13
It is important to recognize that our patient tore his ACL during a traumatic motorized scooter rollover collision, not the more common noncontact twisting injury. Additionally, given the patient’s knee surgery that was performed 6 months after the initial injury, it is possible that intra-articular scar formation contributed to his healing capacity. While this patient did not undergo arthroscopy to visualize the tear in the ACL, or its reconstitution, recent evidence suggests that the accuracy of MRI in diagnosing pediatric ACL injuries is excellent.27,28 The diagnostic accuracy with new MRI machines has sensitivity and specificity approaching 100%.29 Additionally, the patient’s subjective and objective improvements argue for a change in anatomy over a change in the quality of his examination.
Conclusion
The goal of ACL reconstruction in adolescents is to provide long-term stability to the knee while minimizing the risk of growth disturbance. This goal was achieved in our patient through the in situ healing of his ACL. Intrinsic reconstitution of a torn ACL is rare, and it is difficult to speculate which patients may have some healing potential. While this patient was an extreme example, his case demonstrated that protection of the knee from undue stress could favorably alter the environment of the knee to allow for healing of ACL tears. Such information could be valuable in managing select pediatric patients with open physes and ACL injuries nonoperatively, sparing them from the risks associated with surgical treatment. While we do not recommend nonoperative treatment for patients with acute tears of the ACL, we believe more investigation into the healing potential of the ACL, and potential pathways to augment this, is warranted.
1. Noyes FR, Mooar PA, Matthews DS, Butler DL. The symptomatic anterior cruciate-deficient knee. Part I: the long-term functional disability in athletically active individuals. J Bone Joint Surg Am. 1983;65(2):154-162.
2. Nagineni CN, Amiel D, Green MH, Berchuck M, Akeson WH. Characterization of the intrinsic properties of the anterior cruciate and medial collateral ligament cells: an in vitro cell culture study. J Orthop Res. 1992;10(4):465-475.
3. Hefti FL, Kress A, Fasel J, Morscher EW. Healing of the transected anterior cruciate ligament in the rabbit. J Bone Joint Surg Am. 1991;73(3):373-383.
4. Andersson C, Odensten M, Good L, Gillquist J. Surgical or non-surgical treatment of acute rupture of the anterior cruciate ligament. A randomized study with long-term follow-up. J Bone Joint Surg Am. 1989;71(7):965-974.
5. Tang Z, Yang L, Wang Y, et al. Contributions of different intraarticular tissues to the acute phase elevation of synovial fluid MMP-2 following rat ACL rupture. J Orthop Res. 2009;27(2):243-248.
6. Woo SL, Chan SS, Yamaji T. Biomechanics of knee ligament healing, repair and reconstruction. J Biomech. 1997;30(5):431-439.
7. Yoshida M, Fujii K. Differences in cellular properties and responses to growth factors between human ACL and MCL cells. J Orthop Sci. 1999;4(4):293-298.
8. Taylor DC, Posner M, Curl WW, Feagin JA. Isolated tears of the anterior cruciate ligament: over 30-year follow-up of patients treated with arthrotomy and primary repair. Am J Sports Med. 2009;37(1):65-71.
9. Noyes FR, Matthews DS, Mooar PA, Grood ES. The symptomatic anterior cruciate-deficient knee. Part II: the results of rehabilitation, activity modification, and counseling on functional disability. J Bone Joint Surg Am. 1983;65(2):163-174.
10. Malanga GA, Giradi J, Nadler SF. The spontaneous healing of a torn anterior cruciate ligament. Clin J Sport Med. 2001;11(2):118-120.
11. O’Donoghue DH, Rockwood CA Jr, Frank GR, Jack SC, Kenyon R. Repair of the anterior cruciate ligament in dogs. J Bone Joint Surg Am. 1966;48(3):503-519.
12. Guenoun D, Le Corroller T, Amous Z, Pauly V, Sbihi A, Champsaur P. The contribution of MRI to the diagnosis of traumatic tears of the anterior cruciate ligament. Diagn Intervent Imaging. 2012;93(5):331-341.
13. Andrish J, Holmes R. Effects of synovial fluid on fibroblasts in tissue culture. Clin Orthop Relat Res. 1979;(138):279-283.
14. Zimny ML, Schutte M, Dabezies E. Mechanoreceptors in the human anterior cruciate ligament. Anat Rec. 1986;214(2):204-209.
15. Bush-Joseph CA, Cummings JF, Buseck M, et al. Effect of tibial attachment location on the healing of the anterior cruciate ligament freeze model. J Orthop Res. 1996;14(4):534-541.
16. Sung KL, Whittemore DE, Yang L, Amiel D, Akeson WH. Signal pathways and ligament cell adhesiveness. J Orthop Res. 1996;14(5):729-735.
17. Deie M, Ochi M, Ikuta Y. High intrinsic healing potential of human anterior cruciate ligament. Organ culture experiments. Acta Orthop Scand. 1995;66(1):28-32.
18. Voloshin I, Bronstein RD, DeHaven KE. Spontaneous healing of a patellar tendon anterior cruciate ligament graft. A case report. Am J Sports Med. 2002;30(5):751-753.
19. Costa-Paz M, Ayerza MA, Tanoira I, Astoul J, Muscolo DL. Spontaneous healing in complete ACL ruptures: a clinical and MRI study. Clin Orthop Relat Res. 2012;470(4):979-985.
20. Kurosaka M, Yoshiya S, Mizuno T, Mizuno K. Spontaneous healing of a tear of the anterior cruciate ligament. A report of two cases. J Bone Joint Surg Am. 1998;80(8):1200-1203.
21. Fujimoto E, Sumen Y, Ochi M, Ikuta Y. Spontaneous healing of acute anterior cruciate ligament (ACL) injuries - conservative treatment using an extension block soft brace without anterior stabilization. Arch Orthop Trauma Surg. 2002;122(4):212-216.
22. Ihara H, Miwa M, Deya K, Torisu K. MRI of anterior cruciate ligament healing. J Comput Assist Tomogr. 1996;20(2):317-321.
23. Graf BK, Lange RH, Fujisaki CK, Landry GL, Saluja RK. Anterior cruciate ligament tears in skeletally immature patients: meniscal pathology at presentation and after attempted conservative treatment. Arthroscopy. 1992;8(2):229-233.
24. Kannus P, Jarvinen M. Knee ligament injuries in adolescents. Eight year follow-up of conservative management. J Bone Joint Surg Br. 1988;70(5):772-776.
25. Pressman AE, Letts RM, Jarvis JG. Anterior cruciate ligament tears in children: an analysis of operative versus nonoperative treatment. J Pediatr Orthop. 1997;17(4):505-511.
26. Vavken P, Murray MM. Treating anterior cruciate ligament tears in skeletally immature patients. Arthroscopy. 2011;27(5):704-716.
27. Lee K, Siegel MJ, Lau DM, Hildebolt CF, Matava MJ. Anterior cruciate ligament tears: MR imaging-based diagnosis in a pediatric population. Radiology. 1999;213(3):697-704.
28. Major NM, Beard LN Jr, Helms CA. Accuracy of MR imaging of the knee in adolescents. AJR Am J Roentgenol. 2003;180(1):17-19.
29. Sampson MJ, Jackson MP, Moran CJ, Shine S, Moran R, Eustace SJ. Three Tesla MRI for the diagnosis of meniscal and anterior cruciate ligament pathology: a comparison to arthroscopic findings. Clin Radiol. 2008;63(10):1106-1111.
1. Noyes FR, Mooar PA, Matthews DS, Butler DL. The symptomatic anterior cruciate-deficient knee. Part I: the long-term functional disability in athletically active individuals. J Bone Joint Surg Am. 1983;65(2):154-162.
2. Nagineni CN, Amiel D, Green MH, Berchuck M, Akeson WH. Characterization of the intrinsic properties of the anterior cruciate and medial collateral ligament cells: an in vitro cell culture study. J Orthop Res. 1992;10(4):465-475.
3. Hefti FL, Kress A, Fasel J, Morscher EW. Healing of the transected anterior cruciate ligament in the rabbit. J Bone Joint Surg Am. 1991;73(3):373-383.
4. Andersson C, Odensten M, Good L, Gillquist J. Surgical or non-surgical treatment of acute rupture of the anterior cruciate ligament. A randomized study with long-term follow-up. J Bone Joint Surg Am. 1989;71(7):965-974.
5. Tang Z, Yang L, Wang Y, et al. Contributions of different intraarticular tissues to the acute phase elevation of synovial fluid MMP-2 following rat ACL rupture. J Orthop Res. 2009;27(2):243-248.
6. Woo SL, Chan SS, Yamaji T. Biomechanics of knee ligament healing, repair and reconstruction. J Biomech. 1997;30(5):431-439.
7. Yoshida M, Fujii K. Differences in cellular properties and responses to growth factors between human ACL and MCL cells. J Orthop Sci. 1999;4(4):293-298.
8. Taylor DC, Posner M, Curl WW, Feagin JA. Isolated tears of the anterior cruciate ligament: over 30-year follow-up of patients treated with arthrotomy and primary repair. Am J Sports Med. 2009;37(1):65-71.
9. Noyes FR, Matthews DS, Mooar PA, Grood ES. The symptomatic anterior cruciate-deficient knee. Part II: the results of rehabilitation, activity modification, and counseling on functional disability. J Bone Joint Surg Am. 1983;65(2):163-174.
10. Malanga GA, Giradi J, Nadler SF. The spontaneous healing of a torn anterior cruciate ligament. Clin J Sport Med. 2001;11(2):118-120.
11. O’Donoghue DH, Rockwood CA Jr, Frank GR, Jack SC, Kenyon R. Repair of the anterior cruciate ligament in dogs. J Bone Joint Surg Am. 1966;48(3):503-519.
12. Guenoun D, Le Corroller T, Amous Z, Pauly V, Sbihi A, Champsaur P. The contribution of MRI to the diagnosis of traumatic tears of the anterior cruciate ligament. Diagn Intervent Imaging. 2012;93(5):331-341.
13. Andrish J, Holmes R. Effects of synovial fluid on fibroblasts in tissue culture. Clin Orthop Relat Res. 1979;(138):279-283.
14. Zimny ML, Schutte M, Dabezies E. Mechanoreceptors in the human anterior cruciate ligament. Anat Rec. 1986;214(2):204-209.
15. Bush-Joseph CA, Cummings JF, Buseck M, et al. Effect of tibial attachment location on the healing of the anterior cruciate ligament freeze model. J Orthop Res. 1996;14(4):534-541.
16. Sung KL, Whittemore DE, Yang L, Amiel D, Akeson WH. Signal pathways and ligament cell adhesiveness. J Orthop Res. 1996;14(5):729-735.
17. Deie M, Ochi M, Ikuta Y. High intrinsic healing potential of human anterior cruciate ligament. Organ culture experiments. Acta Orthop Scand. 1995;66(1):28-32.
18. Voloshin I, Bronstein RD, DeHaven KE. Spontaneous healing of a patellar tendon anterior cruciate ligament graft. A case report. Am J Sports Med. 2002;30(5):751-753.
19. Costa-Paz M, Ayerza MA, Tanoira I, Astoul J, Muscolo DL. Spontaneous healing in complete ACL ruptures: a clinical and MRI study. Clin Orthop Relat Res. 2012;470(4):979-985.
20. Kurosaka M, Yoshiya S, Mizuno T, Mizuno K. Spontaneous healing of a tear of the anterior cruciate ligament. A report of two cases. J Bone Joint Surg Am. 1998;80(8):1200-1203.
21. Fujimoto E, Sumen Y, Ochi M, Ikuta Y. Spontaneous healing of acute anterior cruciate ligament (ACL) injuries - conservative treatment using an extension block soft brace without anterior stabilization. Arch Orthop Trauma Surg. 2002;122(4):212-216.
22. Ihara H, Miwa M, Deya K, Torisu K. MRI of anterior cruciate ligament healing. J Comput Assist Tomogr. 1996;20(2):317-321.
23. Graf BK, Lange RH, Fujisaki CK, Landry GL, Saluja RK. Anterior cruciate ligament tears in skeletally immature patients: meniscal pathology at presentation and after attempted conservative treatment. Arthroscopy. 1992;8(2):229-233.
24. Kannus P, Jarvinen M. Knee ligament injuries in adolescents. Eight year follow-up of conservative management. J Bone Joint Surg Br. 1988;70(5):772-776.
25. Pressman AE, Letts RM, Jarvis JG. Anterior cruciate ligament tears in children: an analysis of operative versus nonoperative treatment. J Pediatr Orthop. 1997;17(4):505-511.
26. Vavken P, Murray MM. Treating anterior cruciate ligament tears in skeletally immature patients. Arthroscopy. 2011;27(5):704-716.
27. Lee K, Siegel MJ, Lau DM, Hildebolt CF, Matava MJ. Anterior cruciate ligament tears: MR imaging-based diagnosis in a pediatric population. Radiology. 1999;213(3):697-704.
28. Major NM, Beard LN Jr, Helms CA. Accuracy of MR imaging of the knee in adolescents. AJR Am J Roentgenol. 2003;180(1):17-19.
29. Sampson MJ, Jackson MP, Moran CJ, Shine S, Moran R, Eustace SJ. Three Tesla MRI for the diagnosis of meniscal and anterior cruciate ligament pathology: a comparison to arthroscopic findings. Clin Radiol. 2008;63(10):1106-1111.
Bilateral Superior Labrum Anterior to Posterior (SLAP) Tears With Abnormal Anatomy of Biceps Tendon
The biceps brachii derives its name from the 2 heads of the muscle. The short head originates from the coracoid apex, with the coracobrachialis muscle. The long head of the biceps tendon (LHBT) starts within the capsule of the shoulder joint, running from the supraglenoid tubercle or labrum.1 The tendon typically runs free along its intra-articular course, but it is also extrasynovial and ensheathed by a continuation of the synovial lining of the articular capsule that extends to the inferior-most extent of the bicipital groove.2 Congenital anomalies of the LHBT are uncommon, although several atypical forms have been described. A literature search for anomalous LHBT identified several variations in anatomic descriptions, including Y-shaped variant, complete absence of tendon, extra-articular attachment, and a variety of intracapsular attachments. In all, 8 case reports of aberrant intracapsular attachment of LHBT3-12 were identified. These cases presented with a variety of clinical manifestations and pathologic changes. Often, these anatomic variations are considered innocuous, yet some present with pathologic findings.
We present the clinical, magnetic resonance imaging (MRI), and arthroscopic findings of a relatively young athletic patient who was experiencing symptoms of bilateral superior labrum anterior to posterior (SLAP) tears that were unresponsive to conservative management. A unique anatomic variant of the LHBT that involved confluence of the LHBT with the undersurface of the anterosuperior capsule at the rotator interval, as well as a Buford complex anteriorly, was identified and treated. We believe that the tethering of the biceps tendon to the capsule combined with the Buford complex created increased stress on the superior labrum and biceps anchor variant, leading to the development of bilateral symptomatic type II SLAP tears. Knowledge of this variant, though perhaps rare, may be relevant for diagnostic recognition of young athletic patients who present with recalcitrant shoulder symptoms. The patient and the patient’s parents provided written informed consent for print and electronic publication of this case report.
Case Report
A 15-year-old healthy and active athletic boy presented with pain in the right shoulder without history of trauma. He was active in both swimming and baseball. He complained of pain that was present with activities, such as lifting weights, swimming, and throwing. His treatment prior to the office visit consisted of nonsteroidal anti-inflammatory medication, rest, and a therapy program initiated by his high school athletic trainer.
Physical examination demonstrated tenderness to palpation over the posterior capsule and biceps. Motion was full, cuff strength was normal, and SLAP signs (O’Brien, Speed, and Jobe relocation) were positive. A radiograph showed no sign of fracture or dislocation, and no evidence of bony abnormality.
The patient was sent for an MRI arthrogram, which showed a SLAP tear extending from 1 o’clock anteriorly to 10 o’clock posteriorly without intra-articular displacement. No rotator cuff tear was noted. The biceps tendon was noted to be unremarkable and located within the bicipital groove, although retrospective review of the MRI showed that the intra-articular biceps tendon was somewhat confluent with the adjacent tissues.
The patient underwent right shoulder arthroscopy. The shoulder was stable to ligamentous examination under anesthesia. Arthroscopic evaluation revealed that there was a type II SLAP tear extending from the 11-o’clock to the 2-o’clock positions. The superior glenohumeral ligament was identified as it arose from the upper pole of the glenoid labrum and then ran parallel and inferior to the tendon of the biceps towards the lesser tubercle. Surprisingly, there was a very unusual attachment of the intracapsular LHBT to the undersurface of the rotator interval, which restricted biceps excursion in relation to the rotator cuff. Additionally, there was a thick cord-like middle glenohumeral ligament anteriorly that lacked the normal glenoid attachments, thus representing a Buford complex. Interestingly, the labral tear could not only be displaced with a probe, but placing the shoulder through a range of motion also led to increased displacement of the labrum from the glenoid, likely because the biceps tendon was tethered to the undersurface of the capsule.
At the time of arthroscopy, the LHBT was released from its attachment to the capsule at the rotator interval with a radiofrequency wand and shaver. A labral repair was performed using three 2.9-mm bioabsorbable suture anchors, placing 2 posterior and 1 anterior to the biceps tendon. The integrity of the labral repair was observed while placing the shoulder through range of motion.
Postoperatively, the patient was kept in a sling for 5 weeks. Home exercises were initiated at 2 weeks, and outpatient physical therapy was implemented at 4 weeks. The patient resumed swimming, throwing, and other activities—with minimal discomfort—at 6 months postoperatively.
Three years after his initial visit, the patient returned to the office with a similar complaint of pain and limitation of function in his left shoulder after returning to full athletic competition. Once again, there was no history of injury, and history, physical examination, and MRI arthrogram (Figures 1A, 1B) evaluation proved to be very similar to this young athlete’s right shoulder work-up.
The patient once again underwent shoulder arthroscopy and treatment. Although this was now the left shoulder, the findings were essentially identical to the right shoulder. Once again, the labrum was detached from the 11-o’clock to 2-o’clock positions, and a Buford complex was present anteriorly (Figure 2A). The labral tear was easily displaceable from the glenoid with a probe, and placing the shoulder through a range of motion led to increased displacement of the labrum from the glenoid. There was also confluence of the intra-articular LHBT with the undersurface of the capsule within the rotator interval (Figure 2B). A radiofrequency wand, shaver, and elevator were used to define the biceps tendon and separate it from the undersurface of the capsule. The SLAP repair was performed using three 2.9-mm absorbable suture anchors with 2 posterior and 1 anterior to the biceps tendon insertion. The labral repair was observed while placing the shoulder through range of motion and the shoulder was seen to be free of any undue tension on the labrum.
Postoperatively, the patient’s sling and rehabilitation protocol was identical to that of the right shoulder. The patient progressed well, was released to full activity at 6 months, and has not returned with any further complaints of left or right shoulder pain. Approximately 3 years after treatment the patient was contacted via phone and asked about symptoms, pain, and activity. He denies current symptoms of clicking or instability and has no pain that he can identify as being related to previous pathology or treatment. Since the surgery, he has ceased competitive sports and weight lifting, which he attributes to deconditioning associated with postsurgical immobilization and lack of motivation.
Discussion
Of the 8 case reports in the literature that identified variable intra-articular biceps insertional anatomy, only 2 reports represented confluence of the biceps within the rotator interval.7 Interestingly, of the cases identified, the single case that presented a patient with similar pathology of a type II SLAP lesion had an almost identical anatomical variant presentation consisting of both the anomalous insertion of the LHBT into the undersurface of the rotator interval and a Buford variant of the anterosuperior glenohumeral ligament complex. To our knowledge, our bilateral case of an altered intra-articular biceps insertion and a concomitant SLAP tear supports the theory that this pattern of anomalous insertion may very well have altered the biomechanics of the tendon, resulting in acquired pathology to the superior labrum.
The literature reviewed showed the prevalence of anatomic variations of the LHBT ranged from 1.9% to 7.4%.13,14 These variations are generally considered benign; however, in some cases—as in the cases of the young athletes presented by Wahl and MacGillivray7 and in this report—anatomic variation may play an important role in pathogenesis of different injury patterns. The primary function of the LHBT is the stabilization of the glenohumeral joint during abduction and external rotation.15 When the insertion diverges from normal (eg, when the tendon is tethered to the undersurface of the rotator cuff), the biomechanical stresses on the tendon likely change. As a result of the anomalous position of the LHBT origin, there may be a change in the shoulder joint’s biomechanics, with increased strain on the glenohumeral ligament and its attachment onto the glenoid.16
This case report differs from publications on variable superior glenohumeral ligament attachments because a discrete superior glenohumeral ligament structure was isolated from the biceps tendon. Although a larger case series or patient cohort, as well as more involved biomechanical analysis, would certainly be necessary to prove our hypothesis, we believe that this case suggests certain anatomic LHBT and labral variations can contribute to the develop of SLAP tears in younger individuals.
1. Vangsness CT Jr, Jorgenson SS, Watson T, Johnson DL. The origin of the long head of the biceps from the scapula and glenoid labrum. An anatomical study of 100 shoulders. J Bone Joint Surg Br. 1994;76(6):951-954.
2. Burkhead WZ Jr. The biceps tendon. In: Rockwood CA Jr, Matsen FA III, eds. The Shoulder. Vol. 2. Philadelphia: WB Saunders; 1990:791-836.
3. Parikh SN, Bonnaig N, Zbojniewicz A. Intracapsular origin of the long head biceps tendon with glenoid avulsion of the glenohumeral ligaments. Orthopedics. 2011;34(11):781-784.
4. Gaskin CM, Golish SR, Blount KJ, Diduch DR. Anomalies of the long head of the biceps brachii tendon: clinical significance, MR arthrographic findings, and arthroscopic correlation in two patients. Skeletal Radiol. 2007;36(8):785-789.
5. Yeh L, Pedowitz R, Kwak S, et al. Intracapsular origin of the long head of the biceps tendon. Skeletal Radiol. 1999;28(3):178-181.
6. Richards DP, Schwartz M. Anomalous intraarticular origin of the long head of the biceps brachii. Clin J Sport Med. 2003;13(2):122-124.
7. Wahl CJ, MacGillivray JD. Three congenital variations in the long head of the biceps tendon: a review of the pathoanatomic considerations and case reports. J Shoulder Elbow Surg. 2007;16(6):e25-e30.I
8. Egea JM, Melguizo C, Prados J, Aránega A. Capsular origin of the long head of the biceps tendon: a clinical case. Rom J Morphol Embryol. 2010;51(2):375-377.
9. Hyman JL, Warren RF. Extra-articular origin of biceps brachii. Arthroscopy. 2001;17(7): E29.
10. Enad JG. Bifurcate origin of the long head of the biceps tendon. Arthroscopy. 2004;20(10):1081-1083.
11. Mariani PP, Bellelli A, Botticella C. Arthroscopic absence of the long head of the biceps tendon. Arthroscopy. 1997;13(4):499-501.
12. Koplas MC, Winalski CS, Ulmer WH Jr, Recht M. Bilateral congenital absence of the long head of the biceps tendon. Skeletal Radiol. 2009;38(7):715-719.
13. Kanatli U, Ozturk BY, Eisen E, Bolukbasi S. Intra-articular variations of the long head of the biceps tendon. Knee Surg Sports Traumatol Arthrosc. 2011;19(9):1576-1581.
14. Dierickx C, Ceccarelli E, Conti M, Vanlommel J, Castagna A. Variations of the intra-articular portion of the long head of the biceps tendon: a classification of embryologically explained variations. J Shoulder Elbow Surg. 2009;18(4):556-565.
15. Rodosky MW, Harner CD, Fu FH. The role of the long head of the biceps muscle and superior glenoid labrum in anterior stability of the shoulder. Am J Sports Med. 1994;22(1):121-130.
16. Bigliani LU, Kelkar R, Flatow EL, Pollock RG, Mow VC. Glenohumeral stability. Biomechanical properties of passive and active stabilizers. Clin Orthop Relat Res. 1996;(330):13-30.
The biceps brachii derives its name from the 2 heads of the muscle. The short head originates from the coracoid apex, with the coracobrachialis muscle. The long head of the biceps tendon (LHBT) starts within the capsule of the shoulder joint, running from the supraglenoid tubercle or labrum.1 The tendon typically runs free along its intra-articular course, but it is also extrasynovial and ensheathed by a continuation of the synovial lining of the articular capsule that extends to the inferior-most extent of the bicipital groove.2 Congenital anomalies of the LHBT are uncommon, although several atypical forms have been described. A literature search for anomalous LHBT identified several variations in anatomic descriptions, including Y-shaped variant, complete absence of tendon, extra-articular attachment, and a variety of intracapsular attachments. In all, 8 case reports of aberrant intracapsular attachment of LHBT3-12 were identified. These cases presented with a variety of clinical manifestations and pathologic changes. Often, these anatomic variations are considered innocuous, yet some present with pathologic findings.
We present the clinical, magnetic resonance imaging (MRI), and arthroscopic findings of a relatively young athletic patient who was experiencing symptoms of bilateral superior labrum anterior to posterior (SLAP) tears that were unresponsive to conservative management. A unique anatomic variant of the LHBT that involved confluence of the LHBT with the undersurface of the anterosuperior capsule at the rotator interval, as well as a Buford complex anteriorly, was identified and treated. We believe that the tethering of the biceps tendon to the capsule combined with the Buford complex created increased stress on the superior labrum and biceps anchor variant, leading to the development of bilateral symptomatic type II SLAP tears. Knowledge of this variant, though perhaps rare, may be relevant for diagnostic recognition of young athletic patients who present with recalcitrant shoulder symptoms. The patient and the patient’s parents provided written informed consent for print and electronic publication of this case report.
Case Report
A 15-year-old healthy and active athletic boy presented with pain in the right shoulder without history of trauma. He was active in both swimming and baseball. He complained of pain that was present with activities, such as lifting weights, swimming, and throwing. His treatment prior to the office visit consisted of nonsteroidal anti-inflammatory medication, rest, and a therapy program initiated by his high school athletic trainer.
Physical examination demonstrated tenderness to palpation over the posterior capsule and biceps. Motion was full, cuff strength was normal, and SLAP signs (O’Brien, Speed, and Jobe relocation) were positive. A radiograph showed no sign of fracture or dislocation, and no evidence of bony abnormality.
The patient was sent for an MRI arthrogram, which showed a SLAP tear extending from 1 o’clock anteriorly to 10 o’clock posteriorly without intra-articular displacement. No rotator cuff tear was noted. The biceps tendon was noted to be unremarkable and located within the bicipital groove, although retrospective review of the MRI showed that the intra-articular biceps tendon was somewhat confluent with the adjacent tissues.
The patient underwent right shoulder arthroscopy. The shoulder was stable to ligamentous examination under anesthesia. Arthroscopic evaluation revealed that there was a type II SLAP tear extending from the 11-o’clock to the 2-o’clock positions. The superior glenohumeral ligament was identified as it arose from the upper pole of the glenoid labrum and then ran parallel and inferior to the tendon of the biceps towards the lesser tubercle. Surprisingly, there was a very unusual attachment of the intracapsular LHBT to the undersurface of the rotator interval, which restricted biceps excursion in relation to the rotator cuff. Additionally, there was a thick cord-like middle glenohumeral ligament anteriorly that lacked the normal glenoid attachments, thus representing a Buford complex. Interestingly, the labral tear could not only be displaced with a probe, but placing the shoulder through a range of motion also led to increased displacement of the labrum from the glenoid, likely because the biceps tendon was tethered to the undersurface of the capsule.
At the time of arthroscopy, the LHBT was released from its attachment to the capsule at the rotator interval with a radiofrequency wand and shaver. A labral repair was performed using three 2.9-mm bioabsorbable suture anchors, placing 2 posterior and 1 anterior to the biceps tendon. The integrity of the labral repair was observed while placing the shoulder through range of motion.
Postoperatively, the patient was kept in a sling for 5 weeks. Home exercises were initiated at 2 weeks, and outpatient physical therapy was implemented at 4 weeks. The patient resumed swimming, throwing, and other activities—with minimal discomfort—at 6 months postoperatively.
Three years after his initial visit, the patient returned to the office with a similar complaint of pain and limitation of function in his left shoulder after returning to full athletic competition. Once again, there was no history of injury, and history, physical examination, and MRI arthrogram (Figures 1A, 1B) evaluation proved to be very similar to this young athlete’s right shoulder work-up.
The patient once again underwent shoulder arthroscopy and treatment. Although this was now the left shoulder, the findings were essentially identical to the right shoulder. Once again, the labrum was detached from the 11-o’clock to 2-o’clock positions, and a Buford complex was present anteriorly (Figure 2A). The labral tear was easily displaceable from the glenoid with a probe, and placing the shoulder through a range of motion led to increased displacement of the labrum from the glenoid. There was also confluence of the intra-articular LHBT with the undersurface of the capsule within the rotator interval (Figure 2B). A radiofrequency wand, shaver, and elevator were used to define the biceps tendon and separate it from the undersurface of the capsule. The SLAP repair was performed using three 2.9-mm absorbable suture anchors with 2 posterior and 1 anterior to the biceps tendon insertion. The labral repair was observed while placing the shoulder through range of motion and the shoulder was seen to be free of any undue tension on the labrum.
Postoperatively, the patient’s sling and rehabilitation protocol was identical to that of the right shoulder. The patient progressed well, was released to full activity at 6 months, and has not returned with any further complaints of left or right shoulder pain. Approximately 3 years after treatment the patient was contacted via phone and asked about symptoms, pain, and activity. He denies current symptoms of clicking or instability and has no pain that he can identify as being related to previous pathology or treatment. Since the surgery, he has ceased competitive sports and weight lifting, which he attributes to deconditioning associated with postsurgical immobilization and lack of motivation.
Discussion
Of the 8 case reports in the literature that identified variable intra-articular biceps insertional anatomy, only 2 reports represented confluence of the biceps within the rotator interval.7 Interestingly, of the cases identified, the single case that presented a patient with similar pathology of a type II SLAP lesion had an almost identical anatomical variant presentation consisting of both the anomalous insertion of the LHBT into the undersurface of the rotator interval and a Buford variant of the anterosuperior glenohumeral ligament complex. To our knowledge, our bilateral case of an altered intra-articular biceps insertion and a concomitant SLAP tear supports the theory that this pattern of anomalous insertion may very well have altered the biomechanics of the tendon, resulting in acquired pathology to the superior labrum.
The literature reviewed showed the prevalence of anatomic variations of the LHBT ranged from 1.9% to 7.4%.13,14 These variations are generally considered benign; however, in some cases—as in the cases of the young athletes presented by Wahl and MacGillivray7 and in this report—anatomic variation may play an important role in pathogenesis of different injury patterns. The primary function of the LHBT is the stabilization of the glenohumeral joint during abduction and external rotation.15 When the insertion diverges from normal (eg, when the tendon is tethered to the undersurface of the rotator cuff), the biomechanical stresses on the tendon likely change. As a result of the anomalous position of the LHBT origin, there may be a change in the shoulder joint’s biomechanics, with increased strain on the glenohumeral ligament and its attachment onto the glenoid.16
This case report differs from publications on variable superior glenohumeral ligament attachments because a discrete superior glenohumeral ligament structure was isolated from the biceps tendon. Although a larger case series or patient cohort, as well as more involved biomechanical analysis, would certainly be necessary to prove our hypothesis, we believe that this case suggests certain anatomic LHBT and labral variations can contribute to the develop of SLAP tears in younger individuals.
The biceps brachii derives its name from the 2 heads of the muscle. The short head originates from the coracoid apex, with the coracobrachialis muscle. The long head of the biceps tendon (LHBT) starts within the capsule of the shoulder joint, running from the supraglenoid tubercle or labrum.1 The tendon typically runs free along its intra-articular course, but it is also extrasynovial and ensheathed by a continuation of the synovial lining of the articular capsule that extends to the inferior-most extent of the bicipital groove.2 Congenital anomalies of the LHBT are uncommon, although several atypical forms have been described. A literature search for anomalous LHBT identified several variations in anatomic descriptions, including Y-shaped variant, complete absence of tendon, extra-articular attachment, and a variety of intracapsular attachments. In all, 8 case reports of aberrant intracapsular attachment of LHBT3-12 were identified. These cases presented with a variety of clinical manifestations and pathologic changes. Often, these anatomic variations are considered innocuous, yet some present with pathologic findings.
We present the clinical, magnetic resonance imaging (MRI), and arthroscopic findings of a relatively young athletic patient who was experiencing symptoms of bilateral superior labrum anterior to posterior (SLAP) tears that were unresponsive to conservative management. A unique anatomic variant of the LHBT that involved confluence of the LHBT with the undersurface of the anterosuperior capsule at the rotator interval, as well as a Buford complex anteriorly, was identified and treated. We believe that the tethering of the biceps tendon to the capsule combined with the Buford complex created increased stress on the superior labrum and biceps anchor variant, leading to the development of bilateral symptomatic type II SLAP tears. Knowledge of this variant, though perhaps rare, may be relevant for diagnostic recognition of young athletic patients who present with recalcitrant shoulder symptoms. The patient and the patient’s parents provided written informed consent for print and electronic publication of this case report.
Case Report
A 15-year-old healthy and active athletic boy presented with pain in the right shoulder without history of trauma. He was active in both swimming and baseball. He complained of pain that was present with activities, such as lifting weights, swimming, and throwing. His treatment prior to the office visit consisted of nonsteroidal anti-inflammatory medication, rest, and a therapy program initiated by his high school athletic trainer.
Physical examination demonstrated tenderness to palpation over the posterior capsule and biceps. Motion was full, cuff strength was normal, and SLAP signs (O’Brien, Speed, and Jobe relocation) were positive. A radiograph showed no sign of fracture or dislocation, and no evidence of bony abnormality.
The patient was sent for an MRI arthrogram, which showed a SLAP tear extending from 1 o’clock anteriorly to 10 o’clock posteriorly without intra-articular displacement. No rotator cuff tear was noted. The biceps tendon was noted to be unremarkable and located within the bicipital groove, although retrospective review of the MRI showed that the intra-articular biceps tendon was somewhat confluent with the adjacent tissues.
The patient underwent right shoulder arthroscopy. The shoulder was stable to ligamentous examination under anesthesia. Arthroscopic evaluation revealed that there was a type II SLAP tear extending from the 11-o’clock to the 2-o’clock positions. The superior glenohumeral ligament was identified as it arose from the upper pole of the glenoid labrum and then ran parallel and inferior to the tendon of the biceps towards the lesser tubercle. Surprisingly, there was a very unusual attachment of the intracapsular LHBT to the undersurface of the rotator interval, which restricted biceps excursion in relation to the rotator cuff. Additionally, there was a thick cord-like middle glenohumeral ligament anteriorly that lacked the normal glenoid attachments, thus representing a Buford complex. Interestingly, the labral tear could not only be displaced with a probe, but placing the shoulder through a range of motion also led to increased displacement of the labrum from the glenoid, likely because the biceps tendon was tethered to the undersurface of the capsule.
At the time of arthroscopy, the LHBT was released from its attachment to the capsule at the rotator interval with a radiofrequency wand and shaver. A labral repair was performed using three 2.9-mm bioabsorbable suture anchors, placing 2 posterior and 1 anterior to the biceps tendon. The integrity of the labral repair was observed while placing the shoulder through range of motion.
Postoperatively, the patient was kept in a sling for 5 weeks. Home exercises were initiated at 2 weeks, and outpatient physical therapy was implemented at 4 weeks. The patient resumed swimming, throwing, and other activities—with minimal discomfort—at 6 months postoperatively.
Three years after his initial visit, the patient returned to the office with a similar complaint of pain and limitation of function in his left shoulder after returning to full athletic competition. Once again, there was no history of injury, and history, physical examination, and MRI arthrogram (Figures 1A, 1B) evaluation proved to be very similar to this young athlete’s right shoulder work-up.
The patient once again underwent shoulder arthroscopy and treatment. Although this was now the left shoulder, the findings were essentially identical to the right shoulder. Once again, the labrum was detached from the 11-o’clock to 2-o’clock positions, and a Buford complex was present anteriorly (Figure 2A). The labral tear was easily displaceable from the glenoid with a probe, and placing the shoulder through a range of motion led to increased displacement of the labrum from the glenoid. There was also confluence of the intra-articular LHBT with the undersurface of the capsule within the rotator interval (Figure 2B). A radiofrequency wand, shaver, and elevator were used to define the biceps tendon and separate it from the undersurface of the capsule. The SLAP repair was performed using three 2.9-mm absorbable suture anchors with 2 posterior and 1 anterior to the biceps tendon insertion. The labral repair was observed while placing the shoulder through range of motion and the shoulder was seen to be free of any undue tension on the labrum.
Postoperatively, the patient’s sling and rehabilitation protocol was identical to that of the right shoulder. The patient progressed well, was released to full activity at 6 months, and has not returned with any further complaints of left or right shoulder pain. Approximately 3 years after treatment the patient was contacted via phone and asked about symptoms, pain, and activity. He denies current symptoms of clicking or instability and has no pain that he can identify as being related to previous pathology or treatment. Since the surgery, he has ceased competitive sports and weight lifting, which he attributes to deconditioning associated with postsurgical immobilization and lack of motivation.
Discussion
Of the 8 case reports in the literature that identified variable intra-articular biceps insertional anatomy, only 2 reports represented confluence of the biceps within the rotator interval.7 Interestingly, of the cases identified, the single case that presented a patient with similar pathology of a type II SLAP lesion had an almost identical anatomical variant presentation consisting of both the anomalous insertion of the LHBT into the undersurface of the rotator interval and a Buford variant of the anterosuperior glenohumeral ligament complex. To our knowledge, our bilateral case of an altered intra-articular biceps insertion and a concomitant SLAP tear supports the theory that this pattern of anomalous insertion may very well have altered the biomechanics of the tendon, resulting in acquired pathology to the superior labrum.
The literature reviewed showed the prevalence of anatomic variations of the LHBT ranged from 1.9% to 7.4%.13,14 These variations are generally considered benign; however, in some cases—as in the cases of the young athletes presented by Wahl and MacGillivray7 and in this report—anatomic variation may play an important role in pathogenesis of different injury patterns. The primary function of the LHBT is the stabilization of the glenohumeral joint during abduction and external rotation.15 When the insertion diverges from normal (eg, when the tendon is tethered to the undersurface of the rotator cuff), the biomechanical stresses on the tendon likely change. As a result of the anomalous position of the LHBT origin, there may be a change in the shoulder joint’s biomechanics, with increased strain on the glenohumeral ligament and its attachment onto the glenoid.16
This case report differs from publications on variable superior glenohumeral ligament attachments because a discrete superior glenohumeral ligament structure was isolated from the biceps tendon. Although a larger case series or patient cohort, as well as more involved biomechanical analysis, would certainly be necessary to prove our hypothesis, we believe that this case suggests certain anatomic LHBT and labral variations can contribute to the develop of SLAP tears in younger individuals.
1. Vangsness CT Jr, Jorgenson SS, Watson T, Johnson DL. The origin of the long head of the biceps from the scapula and glenoid labrum. An anatomical study of 100 shoulders. J Bone Joint Surg Br. 1994;76(6):951-954.
2. Burkhead WZ Jr. The biceps tendon. In: Rockwood CA Jr, Matsen FA III, eds. The Shoulder. Vol. 2. Philadelphia: WB Saunders; 1990:791-836.
3. Parikh SN, Bonnaig N, Zbojniewicz A. Intracapsular origin of the long head biceps tendon with glenoid avulsion of the glenohumeral ligaments. Orthopedics. 2011;34(11):781-784.
4. Gaskin CM, Golish SR, Blount KJ, Diduch DR. Anomalies of the long head of the biceps brachii tendon: clinical significance, MR arthrographic findings, and arthroscopic correlation in two patients. Skeletal Radiol. 2007;36(8):785-789.
5. Yeh L, Pedowitz R, Kwak S, et al. Intracapsular origin of the long head of the biceps tendon. Skeletal Radiol. 1999;28(3):178-181.
6. Richards DP, Schwartz M. Anomalous intraarticular origin of the long head of the biceps brachii. Clin J Sport Med. 2003;13(2):122-124.
7. Wahl CJ, MacGillivray JD. Three congenital variations in the long head of the biceps tendon: a review of the pathoanatomic considerations and case reports. J Shoulder Elbow Surg. 2007;16(6):e25-e30.I
8. Egea JM, Melguizo C, Prados J, Aránega A. Capsular origin of the long head of the biceps tendon: a clinical case. Rom J Morphol Embryol. 2010;51(2):375-377.
9. Hyman JL, Warren RF. Extra-articular origin of biceps brachii. Arthroscopy. 2001;17(7): E29.
10. Enad JG. Bifurcate origin of the long head of the biceps tendon. Arthroscopy. 2004;20(10):1081-1083.
11. Mariani PP, Bellelli A, Botticella C. Arthroscopic absence of the long head of the biceps tendon. Arthroscopy. 1997;13(4):499-501.
12. Koplas MC, Winalski CS, Ulmer WH Jr, Recht M. Bilateral congenital absence of the long head of the biceps tendon. Skeletal Radiol. 2009;38(7):715-719.
13. Kanatli U, Ozturk BY, Eisen E, Bolukbasi S. Intra-articular variations of the long head of the biceps tendon. Knee Surg Sports Traumatol Arthrosc. 2011;19(9):1576-1581.
14. Dierickx C, Ceccarelli E, Conti M, Vanlommel J, Castagna A. Variations of the intra-articular portion of the long head of the biceps tendon: a classification of embryologically explained variations. J Shoulder Elbow Surg. 2009;18(4):556-565.
15. Rodosky MW, Harner CD, Fu FH. The role of the long head of the biceps muscle and superior glenoid labrum in anterior stability of the shoulder. Am J Sports Med. 1994;22(1):121-130.
16. Bigliani LU, Kelkar R, Flatow EL, Pollock RG, Mow VC. Glenohumeral stability. Biomechanical properties of passive and active stabilizers. Clin Orthop Relat Res. 1996;(330):13-30.
1. Vangsness CT Jr, Jorgenson SS, Watson T, Johnson DL. The origin of the long head of the biceps from the scapula and glenoid labrum. An anatomical study of 100 shoulders. J Bone Joint Surg Br. 1994;76(6):951-954.
2. Burkhead WZ Jr. The biceps tendon. In: Rockwood CA Jr, Matsen FA III, eds. The Shoulder. Vol. 2. Philadelphia: WB Saunders; 1990:791-836.
3. Parikh SN, Bonnaig N, Zbojniewicz A. Intracapsular origin of the long head biceps tendon with glenoid avulsion of the glenohumeral ligaments. Orthopedics. 2011;34(11):781-784.
4. Gaskin CM, Golish SR, Blount KJ, Diduch DR. Anomalies of the long head of the biceps brachii tendon: clinical significance, MR arthrographic findings, and arthroscopic correlation in two patients. Skeletal Radiol. 2007;36(8):785-789.
5. Yeh L, Pedowitz R, Kwak S, et al. Intracapsular origin of the long head of the biceps tendon. Skeletal Radiol. 1999;28(3):178-181.
6. Richards DP, Schwartz M. Anomalous intraarticular origin of the long head of the biceps brachii. Clin J Sport Med. 2003;13(2):122-124.
7. Wahl CJ, MacGillivray JD. Three congenital variations in the long head of the biceps tendon: a review of the pathoanatomic considerations and case reports. J Shoulder Elbow Surg. 2007;16(6):e25-e30.I
8. Egea JM, Melguizo C, Prados J, Aránega A. Capsular origin of the long head of the biceps tendon: a clinical case. Rom J Morphol Embryol. 2010;51(2):375-377.
9. Hyman JL, Warren RF. Extra-articular origin of biceps brachii. Arthroscopy. 2001;17(7): E29.
10. Enad JG. Bifurcate origin of the long head of the biceps tendon. Arthroscopy. 2004;20(10):1081-1083.
11. Mariani PP, Bellelli A, Botticella C. Arthroscopic absence of the long head of the biceps tendon. Arthroscopy. 1997;13(4):499-501.
12. Koplas MC, Winalski CS, Ulmer WH Jr, Recht M. Bilateral congenital absence of the long head of the biceps tendon. Skeletal Radiol. 2009;38(7):715-719.
13. Kanatli U, Ozturk BY, Eisen E, Bolukbasi S. Intra-articular variations of the long head of the biceps tendon. Knee Surg Sports Traumatol Arthrosc. 2011;19(9):1576-1581.
14. Dierickx C, Ceccarelli E, Conti M, Vanlommel J, Castagna A. Variations of the intra-articular portion of the long head of the biceps tendon: a classification of embryologically explained variations. J Shoulder Elbow Surg. 2009;18(4):556-565.
15. Rodosky MW, Harner CD, Fu FH. The role of the long head of the biceps muscle and superior glenoid labrum in anterior stability of the shoulder. Am J Sports Med. 1994;22(1):121-130.
16. Bigliani LU, Kelkar R, Flatow EL, Pollock RG, Mow VC. Glenohumeral stability. Biomechanical properties of passive and active stabilizers. Clin Orthop Relat Res. 1996;(330):13-30.
The Top 100 Cited Articles in Clinical Orthopedic Sports Medicine
Orthopedics and the sports medicine subspecialty are continually evolving fields that depend on research investigation and publication to further knowledge and advance practice. Research has produced new findings that have changed the way we practice sports medicine. In this review, we identify the most widely referenced sports medicine topics and articles, which we believe by their permeative presence in the literature have made lasting contributions to the field.
Many factors can be used to quantify the influence of an academic article on the practice of medicine. Citation analysis is one method that reflects the impact of a publication on the academic medical community.1-3 Total citations record the number of times a journal article has been credited by another study. Therefore, citation count indirectly highlights the articles that are widespread, relevant, and that form the foundation for other investigations on the topic. Related to the impact of the article is the impact of the journal that published the study. We examined journals by impact factor, a score based on the mean number of citations a published article received during the preceding 2 years.
Similar analyses have been performed of publication history in orthopedics and other medical fields. Investigators have examined which historical articles were the most influential in orthopedics as a whole,4 pediatric orthopedics,5,6 shoulder surgery,7 and arthroscopy.8 This influence has also been studied in general surgery,9 otolaryngology,10 plastic surgery,11 dermatology,12 critical care,13 and other disciplines. To our knowledge, the present study is the first bibliometric analysis of the highest-impact articles in orthopedic sports medicine.
Our goal was to identify the 100 articles that have had the highest impact on the clinical orthopedic sports medicine literature. We hypothesized that the most widely recognized articles would be from the highest-impact journals and may also have earlier publication dates. We describe the topics and objectives of these articles to highlight the sports medicine areas on which most research has focused during the past century.
Materials and Methods
Our bibliometric analysis used the Thomson Reuters Web of Knowledge, which consists of all publications from 1900 to the present. This research modality ranks journal articles by frequency of citation. Similar analyses have identified the most often cited articles in pediatric orthopedics,5 shoulder surgery,7 and arthroscopy.8 In our analysis, we included the top 25 journals by impact factor in the field of sports medicine, as rated by the Journal Citation Reports database. Within the highest-impact journals, we sorted all articles by those most often cited, and read them all to identify which ones discuss conditions commonly encountered in the clinical practice of sports medicine. We focused on clinical articles only and therefore excluded related basic science and cadaveric biomechanical studies. The 100 most cited articles were then further evaluated by primary author, journal of publication, institution, country of origin, year of publication, topic, and total number of citations. One-way analysis of variance (ANOVA) and linear regression analyses were used to determine if publication date correlated with mean number of citations.
Results
Eighty authors wrote the top 100 articles in sports medicine, and each publication garnered several hundred citations, ranging from 229 to 1629 with a mean of 408 (Table 114-113). Most of these articles were written in the past 3 decades, with equal distribution from the 1980s, 1990s, and 2000s (Figure 1A). We ran a linear regression to determine if publication date correlated with higher number of citations by virtue of longer time available for citation. The analysis poorly modeled the variability (R2 = 0.05), revealing no correlation between number of citations and publication date. Further, 1-way ANOVA found no significant difference between the number of citations per decade, F(5, 93) = 1.60, P = .17 (Figure 1B). Despite this finding, the oldest cited article, written by Fairbank39 in 1948, ranked high (position 7). Of these top 100 publications, the most recent, written by Knutsen and colleagues69 in 2007, ranked in the second half at position 66.
Seven journals published the top 100 articles, with the American volume of the Journal of Bone and Joint Surgery publishing nearly half (44%) (Table 2). In second place, with 28 articles, was the American Journal of Sports Medicine, followed by the British volume of the Journal of Bone and Joint Surgery, with 10 articles.
Thirty different topics were investigated in this collection of articles, encompassing nearly every major research area of sports medicine. There was a heavy emphasis on anterior cruciate ligament (ACL) injury and reconstruction, knee rating systems, rotator cuff reconstruction, and chondrocyte transplantation (Table 3).
In several cases, an author contributed more than 1 classic article. In fact, 31 of the top 100 articles were by an individual who had coauthored 2 or more of the publications on this list. The researchers with the largest number of first-authored articles were Noyes88-92 (5 articles), Neer81-84 (4 articles), and Rowe,102-104 Daniel,35-37 Peterson,97-99 and Hewett52-54 (3 articles each) (Table 417,19,21-24,29-31,35-37,42,44,45,52-54,58,61-65,69,70,72,74,80-84,87-92,97-99,101-105,107,109,110,113). Articles from authors with multiple publications had a common topic.
Last, these articles originated from a number of different countries and institutions. Of the 15 source countries (Figure 2), the United States contributed the most (61 articles). Other countries had prominent representation: Sweden and Switzerland (8 each), United Kingdom (5), and Canada, France, and Norway (3 each). These articles originated from 69 universities, hospitals, and clinics; 21 institutions had 2 or more articles (Table 5). The 5 institutions with the highest number of articles were Hospital for Special Surgery, University of Bern, Columbia College of Physicians and Surgeons/Columbia-Presbyterian Medical Center, Cincinnati Sports Medicine and Orthopaedic Center, and Massachusetts General Hospital.
Discussion
Several trends can be ascertained from analyzing the top 100 clinical articles cited in sports medicine. The 5 most frequent topics discussed were ACL injury and reconstruction, knee rating systems for injury and function, rotator cuff reconstruction, chondrocyte transplantation, and femoroacetabular impingement (Table 3). Of those 5 topics, only ACL injury and reconstruction falls within the top 10 most common orthopedic surgical procedures performed in the United States reported by one analysis.114 The most common orthopedic surgical procedure, knee arthroscopy, ranks 10th of all topics covered by the top 100 articles, whereas the second most common procedure, shoulder arthroscopy, was not discussed by any of those 100 articles. Also notable is the high frequency of knee rating system studies, which correlates well with the fact that 4 of the most common orthopedic surgical procedures are knee procedures. The prevalence of rating system articles reflects the importance of and need for accurate methods in the diagnosis of injuries in sports medicine.
The most cited sports medicine article was written by Insall and colleagues62 in 1989, more than 2 decades ago. In this article, “Rationale of the Knee Society Clinical Rating System,” they reported on a rigorous system that rates knee function and ability to walk and climb stairs. The second most cited article, “A Clinical Method of Functional Assessment of the Shoulder,” was written in 1987 by Constant and Murley.32 This article discusses another rating system but offers a functional assessment of the shoulder that is highly reproducible and time-efficient. “Rating Systems in the Evaluation of Knee Ligament Injuries,” the third most cited article, was written in 1985 by Tegner and Lysholm.113 This article details the complexities and variable uses of different knee ligament injury rating systems. These top 3 articles were all published in Clinical Orthopaedics and Related Research. In addition, all 3 discussed rating systems, reinforcing the need for accurate scoring systems to standardize the diagnosis of injury across the field of orthopedics and qualify outcomes after injury.
A number of studies have introduced physical examination findings, clinical tests, and rating systems used in the clinical setting of sports medicine (and named after the contributing authors). For example, the Neer sign82 and the Hawkins-Kennedy test51 are used to determine shoulder impingement. In knee ligament injuries, the Tegner knee activity score113 complements other functional scores (eg, Lysholm knee score74). For grading joint cartilage breakdown, the Outerbridge classification system96 is commonly used. The Fairbank test39 is used to gauge knee instability. In evaluating fatty degeneration of rotator cuff muscles through computed tomography scans, the Goutallier classification47 is used. Other metrics, such as the Knee Injury and Osteoarthritis Outcome Score, introduced by Roos and colleagues,101 measure knee injury and osteoarthritis. In other scenarios, studies have improved on surgical techniques—for example, the Neer open modification84 of the Bankart procedure. Many of these rating systems and named clinical findings are so ingrained in the practice and vernacular of orthopedics that it is possible they are in fact undercited in the literature.
As in other bibliometric analyses, one concession made here was to credit the first author listed for making the primary contribution to an article. As a result of journal variability and inconsistency, we were precluded from analyzing senior authors. When analyzed for authorship at any position, 3 of the top authors (Table 4) showed contributions to additional articles in the top 100 list. Noyes was listed as last author on 2 other articles,52,54 raising his total to 7. Daniel was listed as second author on 1 additional article,105 and Beck was listed as third author on 1 other article,42 raising their totals to 4 and 3, respectively.
A criticism of bibliometric analysis is its use of number of citations as an accurate measure of academic contribution. However, other methods for measuring the productivity and impact of researchers (eg, the recently developed Hirsch Index) have their own drawbacks,115,116 including being able to compare authors only at the same point in their careers and self-citation. It is important to note that our analyses focused strictly on publications related to clinical sports medicine, with the exclusion of basic science and cadaveric biomechanical studies.
Through bibliometric citation analysis, we have identified the authors who have made lasting contributions to the field of sports medicine, and we have highlighted the publications that have been cited by hundreds to thousands of authors. This list identifies trends within the articles that have become “classic,” by nature of their deep permeation into subsequent sports medicine literature, and offers guidance for trainees interested in studying the most high-yield sports medicine literature. Given that 69 institutions in 15 countries conducted these studies, we have also shown that orthopedic research can be readily disseminated internationally. Last, our study provides a thorough overview of the sports medicine literature over the past century and provides a strong framework for future research in our field.
1. Adams AB, Simonson D. Publication, citations, and impact factors of leading investigators in critical care medicine. Respir Care. 2004;49(3):276-281.
2. Bhandari M, Busse J, Devereaux PJ, et al. Factors associated with citation rates in the orthopedic literature. Can J Surg. 2007;50(2):119-123.
3. Cheek J, Garnham B, Quan J. What’s in a number? Issues in providing evidence of impact and quality of research(ers). Qual Health Res. 2006;16(3):423-435.
4. Kelly JC, Glynn RW, O’Briain DE, Felle P, McCabe JP. The 100 classic papers of orthopaedic surgery: a bibliometric analysis. J Bone Joint Surg Br. 2010;92(10):1338-1343.
5. Kavanagh RG, Kelly JC, Kelly PM, Moore DP. The 100 classic papers of pediatric orthopaedic surgery: a bibliometric analysis. J Bone Joint Surg Am. 2013;95(18):e134.
6. Mehlman CT, Wenger DR. The top 25 at 25: citation classics in the Journal of Pediatric Orthopaedics. J Pediatr Orthop. 2006;26(5):691-694.
7. Namdari S, Baldwin K, Kovatch K, Huffman GR, Glaser D. Fifty most cited articles in orthopedic shoulder surgery. J Shoulder Elbow Surg. 2012;21(12):1796-1802.
8. Cassar Gheiti AJ, Downey RE, Byrne DP, Molony DC, Mulhall KJ. The 25 most cited articles in arthroscopic orthopaedic surgery. Arthroscopy. 2012;28(4):548-564.
9. Paladugu R, Schein M, Gardezi S, Wise L. One hundred citation classics in general surgical journals. World J Surg. 2002;26(9):1099-1105.
10. Fenton JE, Roy D, Hughes JP, Jones AS. A century of citation classics in otolaryngology-head and neck surgery journals. J Laryngol Otol. 2002;116(7):494-498.
11. Loonen MPJ, Hage JJ, Kon M. Plastic surgery classics: characteristics of 50 top-cited articles in four plastic surgery journals since 1946. Plast Reconstr Surg. 2008;121(5):320e-327e.
12. Dubin D, Hafner AW, Arndt KA. Citation classics in clinical dermatologic journals. Citation analysis, biomedical journals, and landmark articles, 1945–1990. Arch Dermatol. 1993;129(9):1121-1129.
13. Baltussen A, Kindler CH. Citation classics in critical care medicine. Intensive Care Med. 2004;30(5):902-910.
14. Aglietti P, Buzzi R, Zaccherotti G, De Biase P. Patellar tendon versus doubled semitendinosus and gracilis tendons for anterior cruciate ligament reconstruction. Am J Sports Med. 1994;22(2):211-218.
15. Allen PR, Denham RA, Swan AV. Late degenerative changes after meniscectomy. Factors affecting the knee after operation. J Bone Joint Surg Br. 1984;66(5):666-671.
16. Altchek DW, Warren RF, Skyhar MJ, Ortiz G. T-plasty modification of the Bankart procedure for multidirectional instability of the anterior and inferior types. J Bone Joint Surg Am. 1991;73(1):105-112.
17. Andrews JR, Carson WG Jr, McLeod WD. Glenoid labrum tears related to the long head of the biceps. Am J Sports Med. 1985;13(5):337-341.
18. Arendt E, Dick R. Knee injury patterns among men and women in collegiate basketball and soccer. NCAA data and review of literature. Am J Sports Med. 1995;23(6):694-701.
19. Baratta R, Solomonow M, Zhou BH, Letson D, Chuinard R, D’Ambrosia R. Muscular coactivation. The role of the antagonist musculature in maintaining knee stability. Am J Sports Med. 1988;16(2):113-122.
20. Barrack RL, Skinner HB, Buckley SL. Proprioception in the anterior cruciate deficient knee. Am J Sports Med. 1989;17(1):1-6.
21. Bartlett W, Skinner JA, Gooding CR, et al. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br. 2005;87(5):640-645.
22. Beck M, Kalhor M, Leunig M, Ganz R. Hip morphology influences the pattern of damage to the acetabular cartilage: femoroacetabular impingement as a cause of early osteoarthritis of the hip. J Bone Joint Surg Br. 2005;87(7):1012-1018.
23. Beck M, Leunig M, Parvizi J, Boutier V, Wyss D, Ganz R. Anterior femoroacetabular impingement: part II. Midterm results of surgical treatment. Clin Orthop Relat Res. 2004;(418):67-73.
24. Bentley G, Biant LC, Carrington RWJ, et al. A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg Br. 2003;85(2):223-230.
25. Berchuck M, Andriacchi TP, Bach BR, Reider B. Gait adaptations by patients who have a deficient anterior cruciate ligament. J Bone Joint Surg Am. 1990;72(6):871-877.
26. Berndt AL, Harty M. Transchondral fractures (osteochondritis dissecans) of the talus. J Bone Joint Surg Am. 1959;41(6):988-1020.
27. Binkley JM, Stratford PW, Lott SA, Riddle DL, North American Orthopaedic Rehabilitation Research Network. The Lower Extremity Functional Scale (LEFS): scale development, measurement properties, and clinical application. Phys Ther. 1999;79(4):371-383.
28. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
29. Burkhart SS, De Beer JF. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic Bankart repairs: significance of the inverted-pear glenoid and the humeral engaging Hill-Sachs lesion. Arthroscopy. 2000;16(7):677-694.
30. Clancy WG Jr, Nelson DA, Reider B, Narechania RG. Anterior cruciate ligament reconstruction using one-third of the patellar ligament, augmented by extra-articular tendon transfers. J Bone Joint Surg Am. 1982;64(3):352-359.
31. Clancy WG Jr, Shelbourne KD, Zoellner GB, Keene JS, Reider B, Rosenberg TD. Treatment of knee joint instability secondary to rupture of the posterior cruciate ligament. Report of a new procedure. J Bone Joint Surg Am. 1983;65(3):310-322.
32. Constant CR, Murley AHG. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;(214):160-164.
33. Corry IS, Webb JM, Clingeleffer AJ, Pinczewski LA. Arthroscopic reconstruction of the anterior cruciate ligament. A comparison of patellar tendon autograft and four-strand hamstring tendon autograft. Am J Sports Med. 1999;27(3):444-454.
34. Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13(4):456-460.
35. Daniel DM, Malcom LL, Losse G, Stone ML, Sachs R, Burks R. Instrumented measurement of anterior laxity of the knee. J Bone Joint Surg Am. 1985;67(5):720-726.
36. Daniel DM, Stone ML, Dobson BE, Fithian DC, Rossman DJ, Kaufman KR. Fate of the ACL-injured patient. A prospective outcome study. Am J Sports Med. 1994;22(5):632-644.
37. Daniel DM, Stone ML, Sachs R, Malcom L. Instrumented measurement of anterior knee laxity in patients with acute anterior cruciate ligament disruption. Am J Sports Med. 1985;13(6):401-407.
38. Ellman H, Hanker G, Bayer M. Repair of the rotator cuff. End-result study of factors influencing reconstruction. J Bone Joint Surg Am. 1986;68(8):1136-1144.
39. Fairbank TJ. Knee joint changes after meniscectomy. J Bone Joint Surg Br. 1948;30(4):664-670.
40. Freedman KB, D’Amato MJ, Nedeff DD, Kaz A, Bach BR Jr. Arthroscopic anterior cruciate ligament reconstruction: a metaanalysis comparing patellar tendon and hamstring tendon autografts. Am J Sports Med. 2003;31(1):2-11.
41. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
42. Ganz R, Parvizi J, Beck M, Leunig M, Notzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res. 2003;(417):111-119.
43. Gazielly DF, Gleyze P, Montagnon C. Functional and anatomical results after rotator cuff repair. Clin Orthop Relat Res. 1994;(304):43-53.
44. Gerber C, Fuchs B, Hodler J. The results of repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2000;82(4):505-515.
45. Gerber C, Krushell RJ. Isolated rupture of the tendon of the subscapularis muscle. Clinical features in 16 cases. J Bone Joint Surg Br. 1991;73(3):389-394.
46. Glousman R, Jobe F, Tibone J, Moynes D, Antonelli D, Perry J. Dynamic electromyographic analysis of the throwing shoulder with glenohumeral instability. J Bone Joint Surg Am. 1988;70(2):220-226.
47. Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res. 1994;(304):78-83.
48. Guskiewicz KM, Weaver NL, Padua DA, Garrett WE Jr. Epidemiology of concussion in collegiate and high school football players. Am J Sports Med. 2000;28(5):643-650.
49. Hangody L, Fules P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: ten years of experimental and clinical experience. J Bone Joint Surg Am. 2003;85(suppl 2):25-32.
50. Harryman DT 2nd, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FA 3rd. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73(7):982-989.
51. Hawkins RJ, Kennedy JC. Impingement syndrome in athletes. Am J Sports Med. 1980;8(3):151-157.
52. Hewett TE, Lindenfeld TN, Riccobene JV, Noyes FR. The effect of neuromuscular training on the incidence of knee injury in female athletes. A prospective study. Am J Sports Med. 1999;27(6):699-706.
53. Hewett TE, Myer GD, Ford KR, et al. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: a prospective study. Am J Sports Med. 2005;33(4):492-501.
54. Hewett TE, Stroupe AL, Nance TA, Noyes FR. Plyometric training in female athletes. Decreased impact forces and increased hamstring torques. Am J Sports Med. 1996;24(6):765-773.
55. Homminga GN, Bulstra SK, Bouwmeester PSM, van der Linden AJ. Perichondral grafting for cartilage lesions of the knee. J Bone Joint Surg Br. 1990;72(6):1003-1007.
56. Horas U, Pelinkovic D, Herr G, Aigner T, Schnettler R. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint. A prospective, comparative trial. J Bone Joint Surg Am. 2003;85(2):185-192.
57. Hovelius L, Augustini BG, Fredin H, Johansson O, Norlin R, Thorling J. Primary anterior dislocation of the shoulder in young patients. A ten-year prospective study. J Bone Joint Surg Am. 1996;78(11):1677-1684.
58. Hughston JC, Andrews JR, Cross MJ, Moschi A. Classification of knee ligament instabilities. Part I. The medial compartment and cruciate ligaments. J Bone Joint Surg Am. 1976;58(2):159-172.
59. Huston LJ, Wojtys EM. Neuromuscular performance characteristics in elite female athletes. Am J Sports Med. 1996;24(4):427-436.
60. Iannotti JP, Zlatkin MB, Esterhai JL, Kressel HY, Dalinka MK, Spindler KP. Magnetic resonance imaging of the shoulder. Sensitivity, specificity, and predictive value. J Bone Joint Surg Am. 1991;73(1):17-29.
61. Insall J, Falvo KA, Wise DW. Chondromalacia patellae. A prospective study. J Bone Joint Surg Am. 1976;58(1):1-8.
62. Insall JN, Dorr LD, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989;(248):13-14.
63. Irrgang JJ, Anderson AF, Boland AL, et al. Development and validation of the International Knee Documentation Committee subjective knee form. Am J Sports Med. 2001;29(5):600-613.
64. Irrgang JJ, Snyder-Mackler L, Wainner RS, Fu FH, Harner CD. Development of a patient-reported measure of function of the knee. J Bone Joint Surg Am. 1998;80(8):1132-1145.
65. Ito K, Minka MA 2nd, Leunig M, Werlen S, Ganz R. Femoroacetabular impingement and the cam-effect. A MRI-based quantitative anatomical study of the femoral head-neck offset. J Bone Joint Surg Br. 2001;83(2):171-176.
66. Johnson RJ, Kettelkamp DB, Clark W, Leaverton P. Factors affecting late results after meniscectomy. J Bone Joint Surg Am. 1974;56(3):719-729.
67. Jones HH, Priest JD, Hayes WC, Tichenor CC, Nagel DA. Humeral hypertrophy in response to exercise. J Bone Joint Surg Am. 1977;59(2):204-208.
68. Jones KG. Reconstruction of the anterior cruciate ligament: a technique using the central one-third of the patellar ligament. J Bone Joint Surg Am. 1963;45(5):925-932.
69. Knutsen G, Drogset JO, Engebretsen L, et al. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am. 2007;89(10):2105-2112.
70. Knutsen G, Engebretsen L, Ludvigsen TC, et al. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am. 2004;86(3):455-464.
71. Kujala UM, Jaakkola LH, Koskinen SK, Taimela S, Hurme M, Nelimarkka O. Scoring of patellofemoral disorders. Arthroscopy. 1993;9(2):159-163.
72. Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756-1769.
73. Ludewig PM, Cook TM. Alterations in shoulder kinematics and associated muscle activity in people with symptoms of shoulder impingement. Phys Ther. 2000;80(3):276-291.
74. Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10(3):150-154.
75. Mandelbaum BR, Silvers HJ, Watanabe DS, et al. Effectiveness of a neuromuscular and proprioceptive training program in preventing anterior cruciate ligament injuries in female athletes: 2-year follow-up. Am J Sports Med. 2005;33(7):1003-1010.
76. Marder RA, Raskind JR, Carroll M. Prospective evaluation of arthroscopically assisted anterior cruciate ligament reconstruction. Patellar tendon versus semitendinosus and gracilis tendons. Am J Sports Med. 1991;19(5):478-484.
77. Matheson GO, Clement DB, McKenzie DC, Taunton JE, Lloyd-Smith DR, Macintyre JG. Stress fractures in athletes. A study of 320 cases. Am J Sports Med. 1987;15(1):46-58.
78. Matsusue Y, Yamamuro T, Hama H. Arthroscopic multiple osteochondral transplantation to the chondral defect in the knee associated with anterior cruciate ligament disruption. Arthroscopy. 1993;9(3):318-321.
79. McDaniel WJ Jr, Dameron TB Jr. Untreated ruptures of the anterior cruciate ligament. A follow-up study. J Bone Joint Surg Am. 1980;62(5):696-705.
80. Morgan CD, Burkhart SS, Palmeri M, Gillespie M. Type II SLAP lesions: three subtypes and their relationships to superior instability and rotator cuff tears. Arthroscopy. 1998;14(6):553-565.
81. Neer CS 2nd. Anterior acromioplasty for the chronic impingement syndrome in the shoulder: a preliminary report. J Bone Joint Surg Am. 1972;54(1):41-50.
82. Neer CS 2nd. Impingement lesions. Clin Orthop Relat Res. 1983;(173):70-77.
83. Neer CS 2nd, Craig EV, Fukuda H. Cuff-tear arthropathy. J Bone Joint Surg Am. 1983;65(9):1232-1244.
84. Neer CS 2nd, Foster CR. Inferior capsular shift for involuntary inferior and multidirectional instability of the shoulder. A preliminary report. J Bone Joint Surg Am. 1980;62(6):897-908.
85. Nirschl RP, Pettrone FA. Tennis elbow. The surgical treatment of lateral epicondylitis. J Bone Joint Surg Am. 1979;61(6):832-839.
86. Nistor L. Surgical and non-surgical treatment of Achilles tendon rupture. J Bone Joint Surg Am. 1981;63(3):394-399.
87. Notzli HP, Wyss TF, Stoecklin CH, Schmid MR, Treiber K, Hodler J. The contour of the femoral head-neck junction as a predictor for the risk of anterior impingement. J Bone Joint Surg Br. 2002;84(4):556-560.
88. Noyes FR, Barber SD, Mangine RE. Abnormal lower limb symmetry determined by function hop tests after anterior cruciate ligament rupture. Am J Sports Med. 1991;19(5):513-518.
89. Noyes FR, Bassett RW, Grood ES, Butler DL. Arthroscopy in acute traumatic hemarthrosis of the knee. Incidence of anterior cruciate tears and other injuries. J Bone Joint Surg Am. 1980;62(5):687-695, 757.
90. Noyes FR, Matthews DS, Mooar PA, Grood ES. The symptomatic anterior cruciate–deficient knee. Part II: the results of rehabilitation, activity modification, and counseling on functional disability. J Bone Joint Surg Am. 1983;65(2):163-174.
91. Noyes FR, Mooar PA, Matthews DS, Butler DL. The symptomatic anterior cruciate–deficient knee. Part I: the long-term functional disability in athletically active individuals. J Bone Joint Surg Am. 1983;65(2):154-162.
92. Noyes FR, Stabler CL. A system for grading articular cartilage lesions at arthroscopy. Am J Sports Med. 1989;17(4):505-513.
93. O’Brien SJ, Warren RF, Pavlov H, Panariello R, Wickiewicz TL. Reconstruction of the chronically insufficient anterior cruciate ligament with the central third of the patellar ligament. J Bone Joint Surg Am. 1991;73(2):278-286.
94. O’Driscoll SW, Bell DF, Morrey BF. Posterolateral rotatory instability of the elbow. J Bone Joint Surg Am. 1991;73(3):440-446.
95. Olsen OE, Myklebust G, Engebretsen L, Bahr R. Injury mechanisms for anterior cruciate ligament injuries in team handball: a systematic video analysis. Am J Sports Med. 2004;32(4):1002-1012.
96. Outerbridge RE. The etiology of chondromalacia patellae. J Bone Joint Surg Br. 1961;43(4):752-757.
97. Peterson L, Brittberg M, Kiviranta I, Akerlund EL, Lindahl A. Autologous chondrocyte transplantation. Biomechanics and long-term durability. Am J Sports Med. 2002;30(1):2-12.
98. Peterson L, Minas T, Brittberg M, Lindahl A. Treatment of osteochondritis dissecans of the knee with autologous chondrocyte transplantation: results at two to ten years. J Bone Joint Surg Am. 2003;85(suppl 2):17-24.
99. Peterson L, Minas T, Brittberg M, Nilsson A, Sjogren-Jansson E, Lindahl A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000;(374):212-234.
100. Potter HG, Linklater JM, Allen AA, Hannafin JA, Haas SB. Magnetic resonance imaging of articular cartilage in the knee. An evaluation with use of fast-spin-echo imaging. J Bone Joint Surg Am. 1998;80(9):1276-1284.
101. Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS)—development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28(2):88-96.
102. Rowe CR. Prognosis in dislocations of the shoulder. J Bone Joint Surg Am. 1956;38(5):957-977.
103. Rowe CR, Patel D, Southmayd WW. The Bankart procedure: a long-term end-result study. J Bone Joint Surg Am. 1978;60(1):1-16.
104. Rowe CR, Zarins B. Recurrent transient subluxation of the shoulder. J Bone Joint Surg Am. 1981;63(6):863-872.
105. Sachs RA, Daniel DM, Stone ML, Garfein RF. Patellofemoral problems after anterior cruciate ligament reconstruction. Am J Sports Med. 1989;17(6):760-765.
106. Samilson RL, Prieto V. Dislocation arthropathy of the shoulder. J Bone Joint Surg Am. 1983;65(4):456-460.
107. Shelbourne KD, Nitz P. Accelerated rehabilitation after anterior cruciate ligament reconstruction. Am J Sports Med. 1990;18(3):292-299.
108. Sher JS, Uribe JW, Posada A, Murphy BJ, Zlatkin MB. Abnormal findings on magnetic resonance images of asymptomatic shoulders [see comments]. J Bone Joint Surg Am. 1995;77(1):10-15.
109. Siebenrock KA, Schoeniger R, Ganz R. Anterior femoro-acetabular impingement due to acetabular retroversion. Treatment with periacetabular osteotomy. J Bone Joint Surg Am. 2003;85(2):278-286.
110. Solomonow M, Baratta R, Zhou BH, et al. The synergistic action of the anterior cruciate ligament and thigh muscles in maintaining joint stability. Am J Sports Med. 1987;15(3):207-213.
111. Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;19(5):477-484.
112. Tapper EM, Hoover NW. Late results after meniscectomy. J Bone Joint Surg Am. 1969;51(3):517-526.
113. Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985;(198):43-49.
114. Garrett WE Jr, Swiontkowski MF, Weinstein JN, et al. American Board of Orthopaedic Surgery Practice of the Orthopaedic Surgeon: Part-II, certification examination case mix. J Bone Joint Surg Am. 2006;88(3):660-667.
115. Bartneck C, Kokkelmans S. Detecting h-index manipulation through self-citation analysis. Scientometrics. 2011;87(1):85-98.
116. Bornmann L, Daniel HD. The state of h index research. Is the h index the ideal way to measure research performance? EMBO Rep. 2009;10(1):2-6.
Orthopedics and the sports medicine subspecialty are continually evolving fields that depend on research investigation and publication to further knowledge and advance practice. Research has produced new findings that have changed the way we practice sports medicine. In this review, we identify the most widely referenced sports medicine topics and articles, which we believe by their permeative presence in the literature have made lasting contributions to the field.
Many factors can be used to quantify the influence of an academic article on the practice of medicine. Citation analysis is one method that reflects the impact of a publication on the academic medical community.1-3 Total citations record the number of times a journal article has been credited by another study. Therefore, citation count indirectly highlights the articles that are widespread, relevant, and that form the foundation for other investigations on the topic. Related to the impact of the article is the impact of the journal that published the study. We examined journals by impact factor, a score based on the mean number of citations a published article received during the preceding 2 years.
Similar analyses have been performed of publication history in orthopedics and other medical fields. Investigators have examined which historical articles were the most influential in orthopedics as a whole,4 pediatric orthopedics,5,6 shoulder surgery,7 and arthroscopy.8 This influence has also been studied in general surgery,9 otolaryngology,10 plastic surgery,11 dermatology,12 critical care,13 and other disciplines. To our knowledge, the present study is the first bibliometric analysis of the highest-impact articles in orthopedic sports medicine.
Our goal was to identify the 100 articles that have had the highest impact on the clinical orthopedic sports medicine literature. We hypothesized that the most widely recognized articles would be from the highest-impact journals and may also have earlier publication dates. We describe the topics and objectives of these articles to highlight the sports medicine areas on which most research has focused during the past century.
Materials and Methods
Our bibliometric analysis used the Thomson Reuters Web of Knowledge, which consists of all publications from 1900 to the present. This research modality ranks journal articles by frequency of citation. Similar analyses have identified the most often cited articles in pediatric orthopedics,5 shoulder surgery,7 and arthroscopy.8 In our analysis, we included the top 25 journals by impact factor in the field of sports medicine, as rated by the Journal Citation Reports database. Within the highest-impact journals, we sorted all articles by those most often cited, and read them all to identify which ones discuss conditions commonly encountered in the clinical practice of sports medicine. We focused on clinical articles only and therefore excluded related basic science and cadaveric biomechanical studies. The 100 most cited articles were then further evaluated by primary author, journal of publication, institution, country of origin, year of publication, topic, and total number of citations. One-way analysis of variance (ANOVA) and linear regression analyses were used to determine if publication date correlated with mean number of citations.
Results
Eighty authors wrote the top 100 articles in sports medicine, and each publication garnered several hundred citations, ranging from 229 to 1629 with a mean of 408 (Table 114-113). Most of these articles were written in the past 3 decades, with equal distribution from the 1980s, 1990s, and 2000s (Figure 1A). We ran a linear regression to determine if publication date correlated with higher number of citations by virtue of longer time available for citation. The analysis poorly modeled the variability (R2 = 0.05), revealing no correlation between number of citations and publication date. Further, 1-way ANOVA found no significant difference between the number of citations per decade, F(5, 93) = 1.60, P = .17 (Figure 1B). Despite this finding, the oldest cited article, written by Fairbank39 in 1948, ranked high (position 7). Of these top 100 publications, the most recent, written by Knutsen and colleagues69 in 2007, ranked in the second half at position 66.
Seven journals published the top 100 articles, with the American volume of the Journal of Bone and Joint Surgery publishing nearly half (44%) (Table 2). In second place, with 28 articles, was the American Journal of Sports Medicine, followed by the British volume of the Journal of Bone and Joint Surgery, with 10 articles.
Thirty different topics were investigated in this collection of articles, encompassing nearly every major research area of sports medicine. There was a heavy emphasis on anterior cruciate ligament (ACL) injury and reconstruction, knee rating systems, rotator cuff reconstruction, and chondrocyte transplantation (Table 3).
In several cases, an author contributed more than 1 classic article. In fact, 31 of the top 100 articles were by an individual who had coauthored 2 or more of the publications on this list. The researchers with the largest number of first-authored articles were Noyes88-92 (5 articles), Neer81-84 (4 articles), and Rowe,102-104 Daniel,35-37 Peterson,97-99 and Hewett52-54 (3 articles each) (Table 417,19,21-24,29-31,35-37,42,44,45,52-54,58,61-65,69,70,72,74,80-84,87-92,97-99,101-105,107,109,110,113). Articles from authors with multiple publications had a common topic.
Last, these articles originated from a number of different countries and institutions. Of the 15 source countries (Figure 2), the United States contributed the most (61 articles). Other countries had prominent representation: Sweden and Switzerland (8 each), United Kingdom (5), and Canada, France, and Norway (3 each). These articles originated from 69 universities, hospitals, and clinics; 21 institutions had 2 or more articles (Table 5). The 5 institutions with the highest number of articles were Hospital for Special Surgery, University of Bern, Columbia College of Physicians and Surgeons/Columbia-Presbyterian Medical Center, Cincinnati Sports Medicine and Orthopaedic Center, and Massachusetts General Hospital.
Discussion
Several trends can be ascertained from analyzing the top 100 clinical articles cited in sports medicine. The 5 most frequent topics discussed were ACL injury and reconstruction, knee rating systems for injury and function, rotator cuff reconstruction, chondrocyte transplantation, and femoroacetabular impingement (Table 3). Of those 5 topics, only ACL injury and reconstruction falls within the top 10 most common orthopedic surgical procedures performed in the United States reported by one analysis.114 The most common orthopedic surgical procedure, knee arthroscopy, ranks 10th of all topics covered by the top 100 articles, whereas the second most common procedure, shoulder arthroscopy, was not discussed by any of those 100 articles. Also notable is the high frequency of knee rating system studies, which correlates well with the fact that 4 of the most common orthopedic surgical procedures are knee procedures. The prevalence of rating system articles reflects the importance of and need for accurate methods in the diagnosis of injuries in sports medicine.
The most cited sports medicine article was written by Insall and colleagues62 in 1989, more than 2 decades ago. In this article, “Rationale of the Knee Society Clinical Rating System,” they reported on a rigorous system that rates knee function and ability to walk and climb stairs. The second most cited article, “A Clinical Method of Functional Assessment of the Shoulder,” was written in 1987 by Constant and Murley.32 This article discusses another rating system but offers a functional assessment of the shoulder that is highly reproducible and time-efficient. “Rating Systems in the Evaluation of Knee Ligament Injuries,” the third most cited article, was written in 1985 by Tegner and Lysholm.113 This article details the complexities and variable uses of different knee ligament injury rating systems. These top 3 articles were all published in Clinical Orthopaedics and Related Research. In addition, all 3 discussed rating systems, reinforcing the need for accurate scoring systems to standardize the diagnosis of injury across the field of orthopedics and qualify outcomes after injury.
A number of studies have introduced physical examination findings, clinical tests, and rating systems used in the clinical setting of sports medicine (and named after the contributing authors). For example, the Neer sign82 and the Hawkins-Kennedy test51 are used to determine shoulder impingement. In knee ligament injuries, the Tegner knee activity score113 complements other functional scores (eg, Lysholm knee score74). For grading joint cartilage breakdown, the Outerbridge classification system96 is commonly used. The Fairbank test39 is used to gauge knee instability. In evaluating fatty degeneration of rotator cuff muscles through computed tomography scans, the Goutallier classification47 is used. Other metrics, such as the Knee Injury and Osteoarthritis Outcome Score, introduced by Roos and colleagues,101 measure knee injury and osteoarthritis. In other scenarios, studies have improved on surgical techniques—for example, the Neer open modification84 of the Bankart procedure. Many of these rating systems and named clinical findings are so ingrained in the practice and vernacular of orthopedics that it is possible they are in fact undercited in the literature.
As in other bibliometric analyses, one concession made here was to credit the first author listed for making the primary contribution to an article. As a result of journal variability and inconsistency, we were precluded from analyzing senior authors. When analyzed for authorship at any position, 3 of the top authors (Table 4) showed contributions to additional articles in the top 100 list. Noyes was listed as last author on 2 other articles,52,54 raising his total to 7. Daniel was listed as second author on 1 additional article,105 and Beck was listed as third author on 1 other article,42 raising their totals to 4 and 3, respectively.
A criticism of bibliometric analysis is its use of number of citations as an accurate measure of academic contribution. However, other methods for measuring the productivity and impact of researchers (eg, the recently developed Hirsch Index) have their own drawbacks,115,116 including being able to compare authors only at the same point in their careers and self-citation. It is important to note that our analyses focused strictly on publications related to clinical sports medicine, with the exclusion of basic science and cadaveric biomechanical studies.
Through bibliometric citation analysis, we have identified the authors who have made lasting contributions to the field of sports medicine, and we have highlighted the publications that have been cited by hundreds to thousands of authors. This list identifies trends within the articles that have become “classic,” by nature of their deep permeation into subsequent sports medicine literature, and offers guidance for trainees interested in studying the most high-yield sports medicine literature. Given that 69 institutions in 15 countries conducted these studies, we have also shown that orthopedic research can be readily disseminated internationally. Last, our study provides a thorough overview of the sports medicine literature over the past century and provides a strong framework for future research in our field.
Orthopedics and the sports medicine subspecialty are continually evolving fields that depend on research investigation and publication to further knowledge and advance practice. Research has produced new findings that have changed the way we practice sports medicine. In this review, we identify the most widely referenced sports medicine topics and articles, which we believe by their permeative presence in the literature have made lasting contributions to the field.
Many factors can be used to quantify the influence of an academic article on the practice of medicine. Citation analysis is one method that reflects the impact of a publication on the academic medical community.1-3 Total citations record the number of times a journal article has been credited by another study. Therefore, citation count indirectly highlights the articles that are widespread, relevant, and that form the foundation for other investigations on the topic. Related to the impact of the article is the impact of the journal that published the study. We examined journals by impact factor, a score based on the mean number of citations a published article received during the preceding 2 years.
Similar analyses have been performed of publication history in orthopedics and other medical fields. Investigators have examined which historical articles were the most influential in orthopedics as a whole,4 pediatric orthopedics,5,6 shoulder surgery,7 and arthroscopy.8 This influence has also been studied in general surgery,9 otolaryngology,10 plastic surgery,11 dermatology,12 critical care,13 and other disciplines. To our knowledge, the present study is the first bibliometric analysis of the highest-impact articles in orthopedic sports medicine.
Our goal was to identify the 100 articles that have had the highest impact on the clinical orthopedic sports medicine literature. We hypothesized that the most widely recognized articles would be from the highest-impact journals and may also have earlier publication dates. We describe the topics and objectives of these articles to highlight the sports medicine areas on which most research has focused during the past century.
Materials and Methods
Our bibliometric analysis used the Thomson Reuters Web of Knowledge, which consists of all publications from 1900 to the present. This research modality ranks journal articles by frequency of citation. Similar analyses have identified the most often cited articles in pediatric orthopedics,5 shoulder surgery,7 and arthroscopy.8 In our analysis, we included the top 25 journals by impact factor in the field of sports medicine, as rated by the Journal Citation Reports database. Within the highest-impact journals, we sorted all articles by those most often cited, and read them all to identify which ones discuss conditions commonly encountered in the clinical practice of sports medicine. We focused on clinical articles only and therefore excluded related basic science and cadaveric biomechanical studies. The 100 most cited articles were then further evaluated by primary author, journal of publication, institution, country of origin, year of publication, topic, and total number of citations. One-way analysis of variance (ANOVA) and linear regression analyses were used to determine if publication date correlated with mean number of citations.
Results
Eighty authors wrote the top 100 articles in sports medicine, and each publication garnered several hundred citations, ranging from 229 to 1629 with a mean of 408 (Table 114-113). Most of these articles were written in the past 3 decades, with equal distribution from the 1980s, 1990s, and 2000s (Figure 1A). We ran a linear regression to determine if publication date correlated with higher number of citations by virtue of longer time available for citation. The analysis poorly modeled the variability (R2 = 0.05), revealing no correlation between number of citations and publication date. Further, 1-way ANOVA found no significant difference between the number of citations per decade, F(5, 93) = 1.60, P = .17 (Figure 1B). Despite this finding, the oldest cited article, written by Fairbank39 in 1948, ranked high (position 7). Of these top 100 publications, the most recent, written by Knutsen and colleagues69 in 2007, ranked in the second half at position 66.
Seven journals published the top 100 articles, with the American volume of the Journal of Bone and Joint Surgery publishing nearly half (44%) (Table 2). In second place, with 28 articles, was the American Journal of Sports Medicine, followed by the British volume of the Journal of Bone and Joint Surgery, with 10 articles.
Thirty different topics were investigated in this collection of articles, encompassing nearly every major research area of sports medicine. There was a heavy emphasis on anterior cruciate ligament (ACL) injury and reconstruction, knee rating systems, rotator cuff reconstruction, and chondrocyte transplantation (Table 3).
In several cases, an author contributed more than 1 classic article. In fact, 31 of the top 100 articles were by an individual who had coauthored 2 or more of the publications on this list. The researchers with the largest number of first-authored articles were Noyes88-92 (5 articles), Neer81-84 (4 articles), and Rowe,102-104 Daniel,35-37 Peterson,97-99 and Hewett52-54 (3 articles each) (Table 417,19,21-24,29-31,35-37,42,44,45,52-54,58,61-65,69,70,72,74,80-84,87-92,97-99,101-105,107,109,110,113). Articles from authors with multiple publications had a common topic.
Last, these articles originated from a number of different countries and institutions. Of the 15 source countries (Figure 2), the United States contributed the most (61 articles). Other countries had prominent representation: Sweden and Switzerland (8 each), United Kingdom (5), and Canada, France, and Norway (3 each). These articles originated from 69 universities, hospitals, and clinics; 21 institutions had 2 or more articles (Table 5). The 5 institutions with the highest number of articles were Hospital for Special Surgery, University of Bern, Columbia College of Physicians and Surgeons/Columbia-Presbyterian Medical Center, Cincinnati Sports Medicine and Orthopaedic Center, and Massachusetts General Hospital.
Discussion
Several trends can be ascertained from analyzing the top 100 clinical articles cited in sports medicine. The 5 most frequent topics discussed were ACL injury and reconstruction, knee rating systems for injury and function, rotator cuff reconstruction, chondrocyte transplantation, and femoroacetabular impingement (Table 3). Of those 5 topics, only ACL injury and reconstruction falls within the top 10 most common orthopedic surgical procedures performed in the United States reported by one analysis.114 The most common orthopedic surgical procedure, knee arthroscopy, ranks 10th of all topics covered by the top 100 articles, whereas the second most common procedure, shoulder arthroscopy, was not discussed by any of those 100 articles. Also notable is the high frequency of knee rating system studies, which correlates well with the fact that 4 of the most common orthopedic surgical procedures are knee procedures. The prevalence of rating system articles reflects the importance of and need for accurate methods in the diagnosis of injuries in sports medicine.
The most cited sports medicine article was written by Insall and colleagues62 in 1989, more than 2 decades ago. In this article, “Rationale of the Knee Society Clinical Rating System,” they reported on a rigorous system that rates knee function and ability to walk and climb stairs. The second most cited article, “A Clinical Method of Functional Assessment of the Shoulder,” was written in 1987 by Constant and Murley.32 This article discusses another rating system but offers a functional assessment of the shoulder that is highly reproducible and time-efficient. “Rating Systems in the Evaluation of Knee Ligament Injuries,” the third most cited article, was written in 1985 by Tegner and Lysholm.113 This article details the complexities and variable uses of different knee ligament injury rating systems. These top 3 articles were all published in Clinical Orthopaedics and Related Research. In addition, all 3 discussed rating systems, reinforcing the need for accurate scoring systems to standardize the diagnosis of injury across the field of orthopedics and qualify outcomes after injury.
A number of studies have introduced physical examination findings, clinical tests, and rating systems used in the clinical setting of sports medicine (and named after the contributing authors). For example, the Neer sign82 and the Hawkins-Kennedy test51 are used to determine shoulder impingement. In knee ligament injuries, the Tegner knee activity score113 complements other functional scores (eg, Lysholm knee score74). For grading joint cartilage breakdown, the Outerbridge classification system96 is commonly used. The Fairbank test39 is used to gauge knee instability. In evaluating fatty degeneration of rotator cuff muscles through computed tomography scans, the Goutallier classification47 is used. Other metrics, such as the Knee Injury and Osteoarthritis Outcome Score, introduced by Roos and colleagues,101 measure knee injury and osteoarthritis. In other scenarios, studies have improved on surgical techniques—for example, the Neer open modification84 of the Bankart procedure. Many of these rating systems and named clinical findings are so ingrained in the practice and vernacular of orthopedics that it is possible they are in fact undercited in the literature.
As in other bibliometric analyses, one concession made here was to credit the first author listed for making the primary contribution to an article. As a result of journal variability and inconsistency, we were precluded from analyzing senior authors. When analyzed for authorship at any position, 3 of the top authors (Table 4) showed contributions to additional articles in the top 100 list. Noyes was listed as last author on 2 other articles,52,54 raising his total to 7. Daniel was listed as second author on 1 additional article,105 and Beck was listed as third author on 1 other article,42 raising their totals to 4 and 3, respectively.
A criticism of bibliometric analysis is its use of number of citations as an accurate measure of academic contribution. However, other methods for measuring the productivity and impact of researchers (eg, the recently developed Hirsch Index) have their own drawbacks,115,116 including being able to compare authors only at the same point in their careers and self-citation. It is important to note that our analyses focused strictly on publications related to clinical sports medicine, with the exclusion of basic science and cadaveric biomechanical studies.
Through bibliometric citation analysis, we have identified the authors who have made lasting contributions to the field of sports medicine, and we have highlighted the publications that have been cited by hundreds to thousands of authors. This list identifies trends within the articles that have become “classic,” by nature of their deep permeation into subsequent sports medicine literature, and offers guidance for trainees interested in studying the most high-yield sports medicine literature. Given that 69 institutions in 15 countries conducted these studies, we have also shown that orthopedic research can be readily disseminated internationally. Last, our study provides a thorough overview of the sports medicine literature over the past century and provides a strong framework for future research in our field.
1. Adams AB, Simonson D. Publication, citations, and impact factors of leading investigators in critical care medicine. Respir Care. 2004;49(3):276-281.
2. Bhandari M, Busse J, Devereaux PJ, et al. Factors associated with citation rates in the orthopedic literature. Can J Surg. 2007;50(2):119-123.
3. Cheek J, Garnham B, Quan J. What’s in a number? Issues in providing evidence of impact and quality of research(ers). Qual Health Res. 2006;16(3):423-435.
4. Kelly JC, Glynn RW, O’Briain DE, Felle P, McCabe JP. The 100 classic papers of orthopaedic surgery: a bibliometric analysis. J Bone Joint Surg Br. 2010;92(10):1338-1343.
5. Kavanagh RG, Kelly JC, Kelly PM, Moore DP. The 100 classic papers of pediatric orthopaedic surgery: a bibliometric analysis. J Bone Joint Surg Am. 2013;95(18):e134.
6. Mehlman CT, Wenger DR. The top 25 at 25: citation classics in the Journal of Pediatric Orthopaedics. J Pediatr Orthop. 2006;26(5):691-694.
7. Namdari S, Baldwin K, Kovatch K, Huffman GR, Glaser D. Fifty most cited articles in orthopedic shoulder surgery. J Shoulder Elbow Surg. 2012;21(12):1796-1802.
8. Cassar Gheiti AJ, Downey RE, Byrne DP, Molony DC, Mulhall KJ. The 25 most cited articles in arthroscopic orthopaedic surgery. Arthroscopy. 2012;28(4):548-564.
9. Paladugu R, Schein M, Gardezi S, Wise L. One hundred citation classics in general surgical journals. World J Surg. 2002;26(9):1099-1105.
10. Fenton JE, Roy D, Hughes JP, Jones AS. A century of citation classics in otolaryngology-head and neck surgery journals. J Laryngol Otol. 2002;116(7):494-498.
11. Loonen MPJ, Hage JJ, Kon M. Plastic surgery classics: characteristics of 50 top-cited articles in four plastic surgery journals since 1946. Plast Reconstr Surg. 2008;121(5):320e-327e.
12. Dubin D, Hafner AW, Arndt KA. Citation classics in clinical dermatologic journals. Citation analysis, biomedical journals, and landmark articles, 1945–1990. Arch Dermatol. 1993;129(9):1121-1129.
13. Baltussen A, Kindler CH. Citation classics in critical care medicine. Intensive Care Med. 2004;30(5):902-910.
14. Aglietti P, Buzzi R, Zaccherotti G, De Biase P. Patellar tendon versus doubled semitendinosus and gracilis tendons for anterior cruciate ligament reconstruction. Am J Sports Med. 1994;22(2):211-218.
15. Allen PR, Denham RA, Swan AV. Late degenerative changes after meniscectomy. Factors affecting the knee after operation. J Bone Joint Surg Br. 1984;66(5):666-671.
16. Altchek DW, Warren RF, Skyhar MJ, Ortiz G. T-plasty modification of the Bankart procedure for multidirectional instability of the anterior and inferior types. J Bone Joint Surg Am. 1991;73(1):105-112.
17. Andrews JR, Carson WG Jr, McLeod WD. Glenoid labrum tears related to the long head of the biceps. Am J Sports Med. 1985;13(5):337-341.
18. Arendt E, Dick R. Knee injury patterns among men and women in collegiate basketball and soccer. NCAA data and review of literature. Am J Sports Med. 1995;23(6):694-701.
19. Baratta R, Solomonow M, Zhou BH, Letson D, Chuinard R, D’Ambrosia R. Muscular coactivation. The role of the antagonist musculature in maintaining knee stability. Am J Sports Med. 1988;16(2):113-122.
20. Barrack RL, Skinner HB, Buckley SL. Proprioception in the anterior cruciate deficient knee. Am J Sports Med. 1989;17(1):1-6.
21. Bartlett W, Skinner JA, Gooding CR, et al. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br. 2005;87(5):640-645.
22. Beck M, Kalhor M, Leunig M, Ganz R. Hip morphology influences the pattern of damage to the acetabular cartilage: femoroacetabular impingement as a cause of early osteoarthritis of the hip. J Bone Joint Surg Br. 2005;87(7):1012-1018.
23. Beck M, Leunig M, Parvizi J, Boutier V, Wyss D, Ganz R. Anterior femoroacetabular impingement: part II. Midterm results of surgical treatment. Clin Orthop Relat Res. 2004;(418):67-73.
24. Bentley G, Biant LC, Carrington RWJ, et al. A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg Br. 2003;85(2):223-230.
25. Berchuck M, Andriacchi TP, Bach BR, Reider B. Gait adaptations by patients who have a deficient anterior cruciate ligament. J Bone Joint Surg Am. 1990;72(6):871-877.
26. Berndt AL, Harty M. Transchondral fractures (osteochondritis dissecans) of the talus. J Bone Joint Surg Am. 1959;41(6):988-1020.
27. Binkley JM, Stratford PW, Lott SA, Riddle DL, North American Orthopaedic Rehabilitation Research Network. The Lower Extremity Functional Scale (LEFS): scale development, measurement properties, and clinical application. Phys Ther. 1999;79(4):371-383.
28. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
29. Burkhart SS, De Beer JF. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic Bankart repairs: significance of the inverted-pear glenoid and the humeral engaging Hill-Sachs lesion. Arthroscopy. 2000;16(7):677-694.
30. Clancy WG Jr, Nelson DA, Reider B, Narechania RG. Anterior cruciate ligament reconstruction using one-third of the patellar ligament, augmented by extra-articular tendon transfers. J Bone Joint Surg Am. 1982;64(3):352-359.
31. Clancy WG Jr, Shelbourne KD, Zoellner GB, Keene JS, Reider B, Rosenberg TD. Treatment of knee joint instability secondary to rupture of the posterior cruciate ligament. Report of a new procedure. J Bone Joint Surg Am. 1983;65(3):310-322.
32. Constant CR, Murley AHG. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;(214):160-164.
33. Corry IS, Webb JM, Clingeleffer AJ, Pinczewski LA. Arthroscopic reconstruction of the anterior cruciate ligament. A comparison of patellar tendon autograft and four-strand hamstring tendon autograft. Am J Sports Med. 1999;27(3):444-454.
34. Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13(4):456-460.
35. Daniel DM, Malcom LL, Losse G, Stone ML, Sachs R, Burks R. Instrumented measurement of anterior laxity of the knee. J Bone Joint Surg Am. 1985;67(5):720-726.
36. Daniel DM, Stone ML, Dobson BE, Fithian DC, Rossman DJ, Kaufman KR. Fate of the ACL-injured patient. A prospective outcome study. Am J Sports Med. 1994;22(5):632-644.
37. Daniel DM, Stone ML, Sachs R, Malcom L. Instrumented measurement of anterior knee laxity in patients with acute anterior cruciate ligament disruption. Am J Sports Med. 1985;13(6):401-407.
38. Ellman H, Hanker G, Bayer M. Repair of the rotator cuff. End-result study of factors influencing reconstruction. J Bone Joint Surg Am. 1986;68(8):1136-1144.
39. Fairbank TJ. Knee joint changes after meniscectomy. J Bone Joint Surg Br. 1948;30(4):664-670.
40. Freedman KB, D’Amato MJ, Nedeff DD, Kaz A, Bach BR Jr. Arthroscopic anterior cruciate ligament reconstruction: a metaanalysis comparing patellar tendon and hamstring tendon autografts. Am J Sports Med. 2003;31(1):2-11.
41. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
42. Ganz R, Parvizi J, Beck M, Leunig M, Notzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res. 2003;(417):111-119.
43. Gazielly DF, Gleyze P, Montagnon C. Functional and anatomical results after rotator cuff repair. Clin Orthop Relat Res. 1994;(304):43-53.
44. Gerber C, Fuchs B, Hodler J. The results of repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2000;82(4):505-515.
45. Gerber C, Krushell RJ. Isolated rupture of the tendon of the subscapularis muscle. Clinical features in 16 cases. J Bone Joint Surg Br. 1991;73(3):389-394.
46. Glousman R, Jobe F, Tibone J, Moynes D, Antonelli D, Perry J. Dynamic electromyographic analysis of the throwing shoulder with glenohumeral instability. J Bone Joint Surg Am. 1988;70(2):220-226.
47. Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res. 1994;(304):78-83.
48. Guskiewicz KM, Weaver NL, Padua DA, Garrett WE Jr. Epidemiology of concussion in collegiate and high school football players. Am J Sports Med. 2000;28(5):643-650.
49. Hangody L, Fules P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: ten years of experimental and clinical experience. J Bone Joint Surg Am. 2003;85(suppl 2):25-32.
50. Harryman DT 2nd, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FA 3rd. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73(7):982-989.
51. Hawkins RJ, Kennedy JC. Impingement syndrome in athletes. Am J Sports Med. 1980;8(3):151-157.
52. Hewett TE, Lindenfeld TN, Riccobene JV, Noyes FR. The effect of neuromuscular training on the incidence of knee injury in female athletes. A prospective study. Am J Sports Med. 1999;27(6):699-706.
53. Hewett TE, Myer GD, Ford KR, et al. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: a prospective study. Am J Sports Med. 2005;33(4):492-501.
54. Hewett TE, Stroupe AL, Nance TA, Noyes FR. Plyometric training in female athletes. Decreased impact forces and increased hamstring torques. Am J Sports Med. 1996;24(6):765-773.
55. Homminga GN, Bulstra SK, Bouwmeester PSM, van der Linden AJ. Perichondral grafting for cartilage lesions of the knee. J Bone Joint Surg Br. 1990;72(6):1003-1007.
56. Horas U, Pelinkovic D, Herr G, Aigner T, Schnettler R. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint. A prospective, comparative trial. J Bone Joint Surg Am. 2003;85(2):185-192.
57. Hovelius L, Augustini BG, Fredin H, Johansson O, Norlin R, Thorling J. Primary anterior dislocation of the shoulder in young patients. A ten-year prospective study. J Bone Joint Surg Am. 1996;78(11):1677-1684.
58. Hughston JC, Andrews JR, Cross MJ, Moschi A. Classification of knee ligament instabilities. Part I. The medial compartment and cruciate ligaments. J Bone Joint Surg Am. 1976;58(2):159-172.
59. Huston LJ, Wojtys EM. Neuromuscular performance characteristics in elite female athletes. Am J Sports Med. 1996;24(4):427-436.
60. Iannotti JP, Zlatkin MB, Esterhai JL, Kressel HY, Dalinka MK, Spindler KP. Magnetic resonance imaging of the shoulder. Sensitivity, specificity, and predictive value. J Bone Joint Surg Am. 1991;73(1):17-29.
61. Insall J, Falvo KA, Wise DW. Chondromalacia patellae. A prospective study. J Bone Joint Surg Am. 1976;58(1):1-8.
62. Insall JN, Dorr LD, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989;(248):13-14.
63. Irrgang JJ, Anderson AF, Boland AL, et al. Development and validation of the International Knee Documentation Committee subjective knee form. Am J Sports Med. 2001;29(5):600-613.
64. Irrgang JJ, Snyder-Mackler L, Wainner RS, Fu FH, Harner CD. Development of a patient-reported measure of function of the knee. J Bone Joint Surg Am. 1998;80(8):1132-1145.
65. Ito K, Minka MA 2nd, Leunig M, Werlen S, Ganz R. Femoroacetabular impingement and the cam-effect. A MRI-based quantitative anatomical study of the femoral head-neck offset. J Bone Joint Surg Br. 2001;83(2):171-176.
66. Johnson RJ, Kettelkamp DB, Clark W, Leaverton P. Factors affecting late results after meniscectomy. J Bone Joint Surg Am. 1974;56(3):719-729.
67. Jones HH, Priest JD, Hayes WC, Tichenor CC, Nagel DA. Humeral hypertrophy in response to exercise. J Bone Joint Surg Am. 1977;59(2):204-208.
68. Jones KG. Reconstruction of the anterior cruciate ligament: a technique using the central one-third of the patellar ligament. J Bone Joint Surg Am. 1963;45(5):925-932.
69. Knutsen G, Drogset JO, Engebretsen L, et al. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am. 2007;89(10):2105-2112.
70. Knutsen G, Engebretsen L, Ludvigsen TC, et al. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am. 2004;86(3):455-464.
71. Kujala UM, Jaakkola LH, Koskinen SK, Taimela S, Hurme M, Nelimarkka O. Scoring of patellofemoral disorders. Arthroscopy. 1993;9(2):159-163.
72. Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756-1769.
73. Ludewig PM, Cook TM. Alterations in shoulder kinematics and associated muscle activity in people with symptoms of shoulder impingement. Phys Ther. 2000;80(3):276-291.
74. Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10(3):150-154.
75. Mandelbaum BR, Silvers HJ, Watanabe DS, et al. Effectiveness of a neuromuscular and proprioceptive training program in preventing anterior cruciate ligament injuries in female athletes: 2-year follow-up. Am J Sports Med. 2005;33(7):1003-1010.
76. Marder RA, Raskind JR, Carroll M. Prospective evaluation of arthroscopically assisted anterior cruciate ligament reconstruction. Patellar tendon versus semitendinosus and gracilis tendons. Am J Sports Med. 1991;19(5):478-484.
77. Matheson GO, Clement DB, McKenzie DC, Taunton JE, Lloyd-Smith DR, Macintyre JG. Stress fractures in athletes. A study of 320 cases. Am J Sports Med. 1987;15(1):46-58.
78. Matsusue Y, Yamamuro T, Hama H. Arthroscopic multiple osteochondral transplantation to the chondral defect in the knee associated with anterior cruciate ligament disruption. Arthroscopy. 1993;9(3):318-321.
79. McDaniel WJ Jr, Dameron TB Jr. Untreated ruptures of the anterior cruciate ligament. A follow-up study. J Bone Joint Surg Am. 1980;62(5):696-705.
80. Morgan CD, Burkhart SS, Palmeri M, Gillespie M. Type II SLAP lesions: three subtypes and their relationships to superior instability and rotator cuff tears. Arthroscopy. 1998;14(6):553-565.
81. Neer CS 2nd. Anterior acromioplasty for the chronic impingement syndrome in the shoulder: a preliminary report. J Bone Joint Surg Am. 1972;54(1):41-50.
82. Neer CS 2nd. Impingement lesions. Clin Orthop Relat Res. 1983;(173):70-77.
83. Neer CS 2nd, Craig EV, Fukuda H. Cuff-tear arthropathy. J Bone Joint Surg Am. 1983;65(9):1232-1244.
84. Neer CS 2nd, Foster CR. Inferior capsular shift for involuntary inferior and multidirectional instability of the shoulder. A preliminary report. J Bone Joint Surg Am. 1980;62(6):897-908.
85. Nirschl RP, Pettrone FA. Tennis elbow. The surgical treatment of lateral epicondylitis. J Bone Joint Surg Am. 1979;61(6):832-839.
86. Nistor L. Surgical and non-surgical treatment of Achilles tendon rupture. J Bone Joint Surg Am. 1981;63(3):394-399.
87. Notzli HP, Wyss TF, Stoecklin CH, Schmid MR, Treiber K, Hodler J. The contour of the femoral head-neck junction as a predictor for the risk of anterior impingement. J Bone Joint Surg Br. 2002;84(4):556-560.
88. Noyes FR, Barber SD, Mangine RE. Abnormal lower limb symmetry determined by function hop tests after anterior cruciate ligament rupture. Am J Sports Med. 1991;19(5):513-518.
89. Noyes FR, Bassett RW, Grood ES, Butler DL. Arthroscopy in acute traumatic hemarthrosis of the knee. Incidence of anterior cruciate tears and other injuries. J Bone Joint Surg Am. 1980;62(5):687-695, 757.
90. Noyes FR, Matthews DS, Mooar PA, Grood ES. The symptomatic anterior cruciate–deficient knee. Part II: the results of rehabilitation, activity modification, and counseling on functional disability. J Bone Joint Surg Am. 1983;65(2):163-174.
91. Noyes FR, Mooar PA, Matthews DS, Butler DL. The symptomatic anterior cruciate–deficient knee. Part I: the long-term functional disability in athletically active individuals. J Bone Joint Surg Am. 1983;65(2):154-162.
92. Noyes FR, Stabler CL. A system for grading articular cartilage lesions at arthroscopy. Am J Sports Med. 1989;17(4):505-513.
93. O’Brien SJ, Warren RF, Pavlov H, Panariello R, Wickiewicz TL. Reconstruction of the chronically insufficient anterior cruciate ligament with the central third of the patellar ligament. J Bone Joint Surg Am. 1991;73(2):278-286.
94. O’Driscoll SW, Bell DF, Morrey BF. Posterolateral rotatory instability of the elbow. J Bone Joint Surg Am. 1991;73(3):440-446.
95. Olsen OE, Myklebust G, Engebretsen L, Bahr R. Injury mechanisms for anterior cruciate ligament injuries in team handball: a systematic video analysis. Am J Sports Med. 2004;32(4):1002-1012.
96. Outerbridge RE. The etiology of chondromalacia patellae. J Bone Joint Surg Br. 1961;43(4):752-757.
97. Peterson L, Brittberg M, Kiviranta I, Akerlund EL, Lindahl A. Autologous chondrocyte transplantation. Biomechanics and long-term durability. Am J Sports Med. 2002;30(1):2-12.
98. Peterson L, Minas T, Brittberg M, Lindahl A. Treatment of osteochondritis dissecans of the knee with autologous chondrocyte transplantation: results at two to ten years. J Bone Joint Surg Am. 2003;85(suppl 2):17-24.
99. Peterson L, Minas T, Brittberg M, Nilsson A, Sjogren-Jansson E, Lindahl A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000;(374):212-234.
100. Potter HG, Linklater JM, Allen AA, Hannafin JA, Haas SB. Magnetic resonance imaging of articular cartilage in the knee. An evaluation with use of fast-spin-echo imaging. J Bone Joint Surg Am. 1998;80(9):1276-1284.
101. Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS)—development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28(2):88-96.
102. Rowe CR. Prognosis in dislocations of the shoulder. J Bone Joint Surg Am. 1956;38(5):957-977.
103. Rowe CR, Patel D, Southmayd WW. The Bankart procedure: a long-term end-result study. J Bone Joint Surg Am. 1978;60(1):1-16.
104. Rowe CR, Zarins B. Recurrent transient subluxation of the shoulder. J Bone Joint Surg Am. 1981;63(6):863-872.
105. Sachs RA, Daniel DM, Stone ML, Garfein RF. Patellofemoral problems after anterior cruciate ligament reconstruction. Am J Sports Med. 1989;17(6):760-765.
106. Samilson RL, Prieto V. Dislocation arthropathy of the shoulder. J Bone Joint Surg Am. 1983;65(4):456-460.
107. Shelbourne KD, Nitz P. Accelerated rehabilitation after anterior cruciate ligament reconstruction. Am J Sports Med. 1990;18(3):292-299.
108. Sher JS, Uribe JW, Posada A, Murphy BJ, Zlatkin MB. Abnormal findings on magnetic resonance images of asymptomatic shoulders [see comments]. J Bone Joint Surg Am. 1995;77(1):10-15.
109. Siebenrock KA, Schoeniger R, Ganz R. Anterior femoro-acetabular impingement due to acetabular retroversion. Treatment with periacetabular osteotomy. J Bone Joint Surg Am. 2003;85(2):278-286.
110. Solomonow M, Baratta R, Zhou BH, et al. The synergistic action of the anterior cruciate ligament and thigh muscles in maintaining joint stability. Am J Sports Med. 1987;15(3):207-213.
111. Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;19(5):477-484.
112. Tapper EM, Hoover NW. Late results after meniscectomy. J Bone Joint Surg Am. 1969;51(3):517-526.
113. Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985;(198):43-49.
114. Garrett WE Jr, Swiontkowski MF, Weinstein JN, et al. American Board of Orthopaedic Surgery Practice of the Orthopaedic Surgeon: Part-II, certification examination case mix. J Bone Joint Surg Am. 2006;88(3):660-667.
115. Bartneck C, Kokkelmans S. Detecting h-index manipulation through self-citation analysis. Scientometrics. 2011;87(1):85-98.
116. Bornmann L, Daniel HD. The state of h index research. Is the h index the ideal way to measure research performance? EMBO Rep. 2009;10(1):2-6.
1. Adams AB, Simonson D. Publication, citations, and impact factors of leading investigators in critical care medicine. Respir Care. 2004;49(3):276-281.
2. Bhandari M, Busse J, Devereaux PJ, et al. Factors associated with citation rates in the orthopedic literature. Can J Surg. 2007;50(2):119-123.
3. Cheek J, Garnham B, Quan J. What’s in a number? Issues in providing evidence of impact and quality of research(ers). Qual Health Res. 2006;16(3):423-435.
4. Kelly JC, Glynn RW, O’Briain DE, Felle P, McCabe JP. The 100 classic papers of orthopaedic surgery: a bibliometric analysis. J Bone Joint Surg Br. 2010;92(10):1338-1343.
5. Kavanagh RG, Kelly JC, Kelly PM, Moore DP. The 100 classic papers of pediatric orthopaedic surgery: a bibliometric analysis. J Bone Joint Surg Am. 2013;95(18):e134.
6. Mehlman CT, Wenger DR. The top 25 at 25: citation classics in the Journal of Pediatric Orthopaedics. J Pediatr Orthop. 2006;26(5):691-694.
7. Namdari S, Baldwin K, Kovatch K, Huffman GR, Glaser D. Fifty most cited articles in orthopedic shoulder surgery. J Shoulder Elbow Surg. 2012;21(12):1796-1802.
8. Cassar Gheiti AJ, Downey RE, Byrne DP, Molony DC, Mulhall KJ. The 25 most cited articles in arthroscopic orthopaedic surgery. Arthroscopy. 2012;28(4):548-564.
9. Paladugu R, Schein M, Gardezi S, Wise L. One hundred citation classics in general surgical journals. World J Surg. 2002;26(9):1099-1105.
10. Fenton JE, Roy D, Hughes JP, Jones AS. A century of citation classics in otolaryngology-head and neck surgery journals. J Laryngol Otol. 2002;116(7):494-498.
11. Loonen MPJ, Hage JJ, Kon M. Plastic surgery classics: characteristics of 50 top-cited articles in four plastic surgery journals since 1946. Plast Reconstr Surg. 2008;121(5):320e-327e.
12. Dubin D, Hafner AW, Arndt KA. Citation classics in clinical dermatologic journals. Citation analysis, biomedical journals, and landmark articles, 1945–1990. Arch Dermatol. 1993;129(9):1121-1129.
13. Baltussen A, Kindler CH. Citation classics in critical care medicine. Intensive Care Med. 2004;30(5):902-910.
14. Aglietti P, Buzzi R, Zaccherotti G, De Biase P. Patellar tendon versus doubled semitendinosus and gracilis tendons for anterior cruciate ligament reconstruction. Am J Sports Med. 1994;22(2):211-218.
15. Allen PR, Denham RA, Swan AV. Late degenerative changes after meniscectomy. Factors affecting the knee after operation. J Bone Joint Surg Br. 1984;66(5):666-671.
16. Altchek DW, Warren RF, Skyhar MJ, Ortiz G. T-plasty modification of the Bankart procedure for multidirectional instability of the anterior and inferior types. J Bone Joint Surg Am. 1991;73(1):105-112.
17. Andrews JR, Carson WG Jr, McLeod WD. Glenoid labrum tears related to the long head of the biceps. Am J Sports Med. 1985;13(5):337-341.
18. Arendt E, Dick R. Knee injury patterns among men and women in collegiate basketball and soccer. NCAA data and review of literature. Am J Sports Med. 1995;23(6):694-701.
19. Baratta R, Solomonow M, Zhou BH, Letson D, Chuinard R, D’Ambrosia R. Muscular coactivation. The role of the antagonist musculature in maintaining knee stability. Am J Sports Med. 1988;16(2):113-122.
20. Barrack RL, Skinner HB, Buckley SL. Proprioception in the anterior cruciate deficient knee. Am J Sports Med. 1989;17(1):1-6.
21. Bartlett W, Skinner JA, Gooding CR, et al. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br. 2005;87(5):640-645.
22. Beck M, Kalhor M, Leunig M, Ganz R. Hip morphology influences the pattern of damage to the acetabular cartilage: femoroacetabular impingement as a cause of early osteoarthritis of the hip. J Bone Joint Surg Br. 2005;87(7):1012-1018.
23. Beck M, Leunig M, Parvizi J, Boutier V, Wyss D, Ganz R. Anterior femoroacetabular impingement: part II. Midterm results of surgical treatment. Clin Orthop Relat Res. 2004;(418):67-73.
24. Bentley G, Biant LC, Carrington RWJ, et al. A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg Br. 2003;85(2):223-230.
25. Berchuck M, Andriacchi TP, Bach BR, Reider B. Gait adaptations by patients who have a deficient anterior cruciate ligament. J Bone Joint Surg Am. 1990;72(6):871-877.
26. Berndt AL, Harty M. Transchondral fractures (osteochondritis dissecans) of the talus. J Bone Joint Surg Am. 1959;41(6):988-1020.
27. Binkley JM, Stratford PW, Lott SA, Riddle DL, North American Orthopaedic Rehabilitation Research Network. The Lower Extremity Functional Scale (LEFS): scale development, measurement properties, and clinical application. Phys Ther. 1999;79(4):371-383.
28. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
29. Burkhart SS, De Beer JF. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic Bankart repairs: significance of the inverted-pear glenoid and the humeral engaging Hill-Sachs lesion. Arthroscopy. 2000;16(7):677-694.
30. Clancy WG Jr, Nelson DA, Reider B, Narechania RG. Anterior cruciate ligament reconstruction using one-third of the patellar ligament, augmented by extra-articular tendon transfers. J Bone Joint Surg Am. 1982;64(3):352-359.
31. Clancy WG Jr, Shelbourne KD, Zoellner GB, Keene JS, Reider B, Rosenberg TD. Treatment of knee joint instability secondary to rupture of the posterior cruciate ligament. Report of a new procedure. J Bone Joint Surg Am. 1983;65(3):310-322.
32. Constant CR, Murley AHG. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;(214):160-164.
33. Corry IS, Webb JM, Clingeleffer AJ, Pinczewski LA. Arthroscopic reconstruction of the anterior cruciate ligament. A comparison of patellar tendon autograft and four-strand hamstring tendon autograft. Am J Sports Med. 1999;27(3):444-454.
34. Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13(4):456-460.
35. Daniel DM, Malcom LL, Losse G, Stone ML, Sachs R, Burks R. Instrumented measurement of anterior laxity of the knee. J Bone Joint Surg Am. 1985;67(5):720-726.
36. Daniel DM, Stone ML, Dobson BE, Fithian DC, Rossman DJ, Kaufman KR. Fate of the ACL-injured patient. A prospective outcome study. Am J Sports Med. 1994;22(5):632-644.
37. Daniel DM, Stone ML, Sachs R, Malcom L. Instrumented measurement of anterior knee laxity in patients with acute anterior cruciate ligament disruption. Am J Sports Med. 1985;13(6):401-407.
38. Ellman H, Hanker G, Bayer M. Repair of the rotator cuff. End-result study of factors influencing reconstruction. J Bone Joint Surg Am. 1986;68(8):1136-1144.
39. Fairbank TJ. Knee joint changes after meniscectomy. J Bone Joint Surg Br. 1948;30(4):664-670.
40. Freedman KB, D’Amato MJ, Nedeff DD, Kaz A, Bach BR Jr. Arthroscopic anterior cruciate ligament reconstruction: a metaanalysis comparing patellar tendon and hamstring tendon autografts. Am J Sports Med. 2003;31(1):2-11.
41. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
42. Ganz R, Parvizi J, Beck M, Leunig M, Notzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res. 2003;(417):111-119.
43. Gazielly DF, Gleyze P, Montagnon C. Functional and anatomical results after rotator cuff repair. Clin Orthop Relat Res. 1994;(304):43-53.
44. Gerber C, Fuchs B, Hodler J. The results of repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2000;82(4):505-515.
45. Gerber C, Krushell RJ. Isolated rupture of the tendon of the subscapularis muscle. Clinical features in 16 cases. J Bone Joint Surg Br. 1991;73(3):389-394.
46. Glousman R, Jobe F, Tibone J, Moynes D, Antonelli D, Perry J. Dynamic electromyographic analysis of the throwing shoulder with glenohumeral instability. J Bone Joint Surg Am. 1988;70(2):220-226.
47. Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res. 1994;(304):78-83.
48. Guskiewicz KM, Weaver NL, Padua DA, Garrett WE Jr. Epidemiology of concussion in collegiate and high school football players. Am J Sports Med. 2000;28(5):643-650.
49. Hangody L, Fules P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: ten years of experimental and clinical experience. J Bone Joint Surg Am. 2003;85(suppl 2):25-32.
50. Harryman DT 2nd, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FA 3rd. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73(7):982-989.
51. Hawkins RJ, Kennedy JC. Impingement syndrome in athletes. Am J Sports Med. 1980;8(3):151-157.
52. Hewett TE, Lindenfeld TN, Riccobene JV, Noyes FR. The effect of neuromuscular training on the incidence of knee injury in female athletes. A prospective study. Am J Sports Med. 1999;27(6):699-706.
53. Hewett TE, Myer GD, Ford KR, et al. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: a prospective study. Am J Sports Med. 2005;33(4):492-501.
54. Hewett TE, Stroupe AL, Nance TA, Noyes FR. Plyometric training in female athletes. Decreased impact forces and increased hamstring torques. Am J Sports Med. 1996;24(6):765-773.
55. Homminga GN, Bulstra SK, Bouwmeester PSM, van der Linden AJ. Perichondral grafting for cartilage lesions of the knee. J Bone Joint Surg Br. 1990;72(6):1003-1007.
56. Horas U, Pelinkovic D, Herr G, Aigner T, Schnettler R. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint. A prospective, comparative trial. J Bone Joint Surg Am. 2003;85(2):185-192.
57. Hovelius L, Augustini BG, Fredin H, Johansson O, Norlin R, Thorling J. Primary anterior dislocation of the shoulder in young patients. A ten-year prospective study. J Bone Joint Surg Am. 1996;78(11):1677-1684.
58. Hughston JC, Andrews JR, Cross MJ, Moschi A. Classification of knee ligament instabilities. Part I. The medial compartment and cruciate ligaments. J Bone Joint Surg Am. 1976;58(2):159-172.
59. Huston LJ, Wojtys EM. Neuromuscular performance characteristics in elite female athletes. Am J Sports Med. 1996;24(4):427-436.
60. Iannotti JP, Zlatkin MB, Esterhai JL, Kressel HY, Dalinka MK, Spindler KP. Magnetic resonance imaging of the shoulder. Sensitivity, specificity, and predictive value. J Bone Joint Surg Am. 1991;73(1):17-29.
61. Insall J, Falvo KA, Wise DW. Chondromalacia patellae. A prospective study. J Bone Joint Surg Am. 1976;58(1):1-8.
62. Insall JN, Dorr LD, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989;(248):13-14.
63. Irrgang JJ, Anderson AF, Boland AL, et al. Development and validation of the International Knee Documentation Committee subjective knee form. Am J Sports Med. 2001;29(5):600-613.
64. Irrgang JJ, Snyder-Mackler L, Wainner RS, Fu FH, Harner CD. Development of a patient-reported measure of function of the knee. J Bone Joint Surg Am. 1998;80(8):1132-1145.
65. Ito K, Minka MA 2nd, Leunig M, Werlen S, Ganz R. Femoroacetabular impingement and the cam-effect. A MRI-based quantitative anatomical study of the femoral head-neck offset. J Bone Joint Surg Br. 2001;83(2):171-176.
66. Johnson RJ, Kettelkamp DB, Clark W, Leaverton P. Factors affecting late results after meniscectomy. J Bone Joint Surg Am. 1974;56(3):719-729.
67. Jones HH, Priest JD, Hayes WC, Tichenor CC, Nagel DA. Humeral hypertrophy in response to exercise. J Bone Joint Surg Am. 1977;59(2):204-208.
68. Jones KG. Reconstruction of the anterior cruciate ligament: a technique using the central one-third of the patellar ligament. J Bone Joint Surg Am. 1963;45(5):925-932.
69. Knutsen G, Drogset JO, Engebretsen L, et al. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am. 2007;89(10):2105-2112.
70. Knutsen G, Engebretsen L, Ludvigsen TC, et al. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am. 2004;86(3):455-464.
71. Kujala UM, Jaakkola LH, Koskinen SK, Taimela S, Hurme M, Nelimarkka O. Scoring of patellofemoral disorders. Arthroscopy. 1993;9(2):159-163.
72. Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756-1769.
73. Ludewig PM, Cook TM. Alterations in shoulder kinematics and associated muscle activity in people with symptoms of shoulder impingement. Phys Ther. 2000;80(3):276-291.
74. Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10(3):150-154.
75. Mandelbaum BR, Silvers HJ, Watanabe DS, et al. Effectiveness of a neuromuscular and proprioceptive training program in preventing anterior cruciate ligament injuries in female athletes: 2-year follow-up. Am J Sports Med. 2005;33(7):1003-1010.
76. Marder RA, Raskind JR, Carroll M. Prospective evaluation of arthroscopically assisted anterior cruciate ligament reconstruction. Patellar tendon versus semitendinosus and gracilis tendons. Am J Sports Med. 1991;19(5):478-484.
77. Matheson GO, Clement DB, McKenzie DC, Taunton JE, Lloyd-Smith DR, Macintyre JG. Stress fractures in athletes. A study of 320 cases. Am J Sports Med. 1987;15(1):46-58.
78. Matsusue Y, Yamamuro T, Hama H. Arthroscopic multiple osteochondral transplantation to the chondral defect in the knee associated with anterior cruciate ligament disruption. Arthroscopy. 1993;9(3):318-321.
79. McDaniel WJ Jr, Dameron TB Jr. Untreated ruptures of the anterior cruciate ligament. A follow-up study. J Bone Joint Surg Am. 1980;62(5):696-705.
80. Morgan CD, Burkhart SS, Palmeri M, Gillespie M. Type II SLAP lesions: three subtypes and their relationships to superior instability and rotator cuff tears. Arthroscopy. 1998;14(6):553-565.
81. Neer CS 2nd. Anterior acromioplasty for the chronic impingement syndrome in the shoulder: a preliminary report. J Bone Joint Surg Am. 1972;54(1):41-50.
82. Neer CS 2nd. Impingement lesions. Clin Orthop Relat Res. 1983;(173):70-77.
83. Neer CS 2nd, Craig EV, Fukuda H. Cuff-tear arthropathy. J Bone Joint Surg Am. 1983;65(9):1232-1244.
84. Neer CS 2nd, Foster CR. Inferior capsular shift for involuntary inferior and multidirectional instability of the shoulder. A preliminary report. J Bone Joint Surg Am. 1980;62(6):897-908.
85. Nirschl RP, Pettrone FA. Tennis elbow. The surgical treatment of lateral epicondylitis. J Bone Joint Surg Am. 1979;61(6):832-839.
86. Nistor L. Surgical and non-surgical treatment of Achilles tendon rupture. J Bone Joint Surg Am. 1981;63(3):394-399.
87. Notzli HP, Wyss TF, Stoecklin CH, Schmid MR, Treiber K, Hodler J. The contour of the femoral head-neck junction as a predictor for the risk of anterior impingement. J Bone Joint Surg Br. 2002;84(4):556-560.
88. Noyes FR, Barber SD, Mangine RE. Abnormal lower limb symmetry determined by function hop tests after anterior cruciate ligament rupture. Am J Sports Med. 1991;19(5):513-518.
89. Noyes FR, Bassett RW, Grood ES, Butler DL. Arthroscopy in acute traumatic hemarthrosis of the knee. Incidence of anterior cruciate tears and other injuries. J Bone Joint Surg Am. 1980;62(5):687-695, 757.
90. Noyes FR, Matthews DS, Mooar PA, Grood ES. The symptomatic anterior cruciate–deficient knee. Part II: the results of rehabilitation, activity modification, and counseling on functional disability. J Bone Joint Surg Am. 1983;65(2):163-174.
91. Noyes FR, Mooar PA, Matthews DS, Butler DL. The symptomatic anterior cruciate–deficient knee. Part I: the long-term functional disability in athletically active individuals. J Bone Joint Surg Am. 1983;65(2):154-162.
92. Noyes FR, Stabler CL. A system for grading articular cartilage lesions at arthroscopy. Am J Sports Med. 1989;17(4):505-513.
93. O’Brien SJ, Warren RF, Pavlov H, Panariello R, Wickiewicz TL. Reconstruction of the chronically insufficient anterior cruciate ligament with the central third of the patellar ligament. J Bone Joint Surg Am. 1991;73(2):278-286.
94. O’Driscoll SW, Bell DF, Morrey BF. Posterolateral rotatory instability of the elbow. J Bone Joint Surg Am. 1991;73(3):440-446.
95. Olsen OE, Myklebust G, Engebretsen L, Bahr R. Injury mechanisms for anterior cruciate ligament injuries in team handball: a systematic video analysis. Am J Sports Med. 2004;32(4):1002-1012.
96. Outerbridge RE. The etiology of chondromalacia patellae. J Bone Joint Surg Br. 1961;43(4):752-757.
97. Peterson L, Brittberg M, Kiviranta I, Akerlund EL, Lindahl A. Autologous chondrocyte transplantation. Biomechanics and long-term durability. Am J Sports Med. 2002;30(1):2-12.
98. Peterson L, Minas T, Brittberg M, Lindahl A. Treatment of osteochondritis dissecans of the knee with autologous chondrocyte transplantation: results at two to ten years. J Bone Joint Surg Am. 2003;85(suppl 2):17-24.
99. Peterson L, Minas T, Brittberg M, Nilsson A, Sjogren-Jansson E, Lindahl A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000;(374):212-234.
100. Potter HG, Linklater JM, Allen AA, Hannafin JA, Haas SB. Magnetic resonance imaging of articular cartilage in the knee. An evaluation with use of fast-spin-echo imaging. J Bone Joint Surg Am. 1998;80(9):1276-1284.
101. Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS)—development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28(2):88-96.
102. Rowe CR. Prognosis in dislocations of the shoulder. J Bone Joint Surg Am. 1956;38(5):957-977.
103. Rowe CR, Patel D, Southmayd WW. The Bankart procedure: a long-term end-result study. J Bone Joint Surg Am. 1978;60(1):1-16.
104. Rowe CR, Zarins B. Recurrent transient subluxation of the shoulder. J Bone Joint Surg Am. 1981;63(6):863-872.
105. Sachs RA, Daniel DM, Stone ML, Garfein RF. Patellofemoral problems after anterior cruciate ligament reconstruction. Am J Sports Med. 1989;17(6):760-765.
106. Samilson RL, Prieto V. Dislocation arthropathy of the shoulder. J Bone Joint Surg Am. 1983;65(4):456-460.
107. Shelbourne KD, Nitz P. Accelerated rehabilitation after anterior cruciate ligament reconstruction. Am J Sports Med. 1990;18(3):292-299.
108. Sher JS, Uribe JW, Posada A, Murphy BJ, Zlatkin MB. Abnormal findings on magnetic resonance images of asymptomatic shoulders [see comments]. J Bone Joint Surg Am. 1995;77(1):10-15.
109. Siebenrock KA, Schoeniger R, Ganz R. Anterior femoro-acetabular impingement due to acetabular retroversion. Treatment with periacetabular osteotomy. J Bone Joint Surg Am. 2003;85(2):278-286.
110. Solomonow M, Baratta R, Zhou BH, et al. The synergistic action of the anterior cruciate ligament and thigh muscles in maintaining joint stability. Am J Sports Med. 1987;15(3):207-213.
111. Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;19(5):477-484.
112. Tapper EM, Hoover NW. Late results after meniscectomy. J Bone Joint Surg Am. 1969;51(3):517-526.
113. Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985;(198):43-49.
114. Garrett WE Jr, Swiontkowski MF, Weinstein JN, et al. American Board of Orthopaedic Surgery Practice of the Orthopaedic Surgeon: Part-II, certification examination case mix. J Bone Joint Surg Am. 2006;88(3):660-667.
115. Bartneck C, Kokkelmans S. Detecting h-index manipulation through self-citation analysis. Scientometrics. 2011;87(1):85-98.
116. Bornmann L, Daniel HD. The state of h index research. Is the h index the ideal way to measure research performance? EMBO Rep. 2009;10(1):2-6.
Shoulder Examination of the Overhead Athlete
The overhead athlete’s shoulder is exposed to extremes of stress and range of motion (ROM), predisposing this joint to unique injury patterns. Prompt diagnosis and management begin with a comprehensive history and a physical examination, supplemented by imaging studies as needed. Furthermore, the throwing shoulder undergoes adaptive changes, such as partial undersurface rotator cuff tears and capsular laxity. Imaging studies typically demonstrate abnormalities in asymptomatic throwers. Therefore, clinicians must be skilled in history taking and physical examination in throwing athletes to accurately determine the cause of symptoms and provide optimal treatment. This primer provides orthopedic surgeons with the key points in performing a thorough physical examination of the shoulder in overhead athletes.
When working with overhead athletes, surgeons must elicit the precise nature of symptoms. For example, it is important to distinguish pain from fatigue, as well as complaints related purely to decline in performance. Often, collaboration with the player’s parent or coach may help clarify the chief complaint. In addition, surgeons must have an intricate knowledge of the various stages of the overhead motion, as symptoms in specific stages (late cocking/early acceleration) may raise suspicion for distinctive pathology (labral/biceps complex). Last, it is imperative to understand that the shoulder represents only one part of the kinetic chain in overhead athletes. Successful throwing relies on integrity of the entire kinetic chain, starting with the lower extremity and trunk, extending through the spine, scapula, and shoulder, and terminating with the hand and fingers. Pathology anywhere in the chain must be evaluated and addressed.
When examining the shoulder in overhead athletes, surgeons must address several anatomical structures, both bony and soft tissue. Proper examination begins with comprehensive assessment of the ROM and strength of the various muscles around the shoulder, along with visual inspection to identify any asymmetry of these structures. In addition, the scapulothoracic structures must be examined in detail to rule out underlying dyskinesis. The capsular and ligamentous components of the shoulder joint must be further assessed to note any capsular contracture causing glenohumeral internal rotation deficit (GIRD) or any pathology with the rotator cuff or labral/biceps complex. Last, a comprehensive neurovascular examination should be performed to rule out any compression or neuropathy affecting the shoulder and overhead motion. Findings from the physical examination may then require further imaging to correlate the history and physical examination findings.
1. Inspection, palpation, strength testing
Every examination of the shoulder must begin with visual inspection, along with assessment of basic ROM and strength. The patient must be positioned and exposed adequately to promote visualization of the entire shoulder and scapular girdle, from both anterior and posterior. Visual inspection focuses on identifying any areas of asymmetry, such as position of the bony prominences or bulk of the muscular fossae. Asymmetry of the bony architecture may indicate prior trauma, and atrophy of the muscular fossae may indicate nerve compression. For example, atrophy of the infraspinatus fossa may be caused by compression of the suprascapular nerve at the spinoglenoid notch (likely by a cyst, often associated with labral pathology, but infraspinatus atrophy can result even without the presence of a compressive cyst1). Alternatively, atrophy of both the supraspinatus and infraspinatus fossae may indicate underlying compression of the suprascapular nerve at the suprascapular notch (either by a cyst or by the transverse scapular ligament). Static and dynamic observation of the posterior aspect of the shoulder may help identify gross pathology with scapular positioning or retraction, indicating underlying dyskinesis (discussed later). Deformity of the acromioclavicular joint may indicate prior trauma or separation. Last, all prior surgical scars should be noted.
Selective palpation may help identify pathology in the shoulder of the throwing athlete. Tenderness at the acromioclavicular joint may be especially common in patients who have had prior sprains of this joint or who have degenerative changes. Tenderness along the biceps tendon may be present in those with biceps tendinitis or partial tear. In addition, tenderness at the coracoid may be present in those with scapular dyskinesis. Posteriorly, palpation at the inferomedial aspect of the scapula (Figure 1), as with palpation along the medial border of the scapula, may elicit tenderness in those with scapulothoracic bursitis.
Strength testing in the shoulder is performed to elicit any deficiencies of the rotator cuff/musculature or surrounding structures. Weakness in forward elevation may indicate pathology in the supraspinatus, whereas weakness in external rotation may reflect deficiency in the infraspinatus or teres minor. Teres minor deficiency may be more isolated with weakness in a position of shoulder abduction to 90°. Last, weakness in internal rotation may indicate subscapularis deficiency. Lag signs and other provocative maneuvers are similarly elicited but typically are positive only in the event of large tears of the rotator cuff. These signs and maneuvers include the internal rotation lag sign or belly press test for subscapularis integrity, the drop-arm sign for supraspinatus function, the external rotation lag sign for infraspinatus function, and the hornblower sign for teres minor integrity. Supporting muscles of the shoulder may also be tested. Latissimus strength may be tested with resisted downward rotation of the arm with the shoulder in abduction and the elbow flexed to 90°.
2. ROM and GIRD assessment
After inspection and palpation, the shoulder should be ranged in all relevant planes of motion. Our standard examination includes forward elevation in the frontal and scapular planes, along with external rotation at the side and at 90° of abduction, as well as internal rotation behind the back with documentation of the highest spinal level that the patient can reach. This examination may be performed with the patient upright, but supine positioning can help stabilize the scapula and provide more accurate views of motion. Deficits of internal rotation may be a common finding in overhead athletes, and the degree of this deficit should be quantitatively noted.
Bony and soft-tissue remodeling of the shoulder (and associated structures) in the overhead athlete can lead to contracture of the posterior capsule. This contracture can cause excessive external rotation and subsequent decrease in internal rotation, leading to pain and anterior instability in the throwing shoulder.2 For precise measurements of the internal and external rotation arc, the scapula must be stabilized. This can be done with the patient supine on the examining table or seated upright with manual stabilization of the scapula by the examiner. Once the scapula is stabilized, the arc of internal and external rotation (with the arm in about 90° of abduction) can be measured with a goniometer, with maximum values obtained as the scapula begins to move along the posterior chest wall.2 The difference in internal rotation between the dominant and nondominant arms defines the extent of the athlete’s GIRD. Internal rotation can also be qualitatively assessed by having the athlete internally rotate each arm and reach up the spine while the examiner notes the difference in level achieved. However, this does not provide a quantitative assessment of the patient’s GIRD.
In general, the sum of the internal and external rotation arcs on the 2 sides should be symmetric. Consequently, in GIRD, excessive external rotation is balanced by decreased internal rotation. Symptomatic GIRD may be present when there is more than 25° of discrepancy in internal rotation between the athlete’s dominant and nondominant arms.2 The goal is to reduce this discrepancy to less than 20°.
3. Internal impingement: rotator cuff and labrum
In overhead athletes, an intricate relationship involving rotator cuff, labrum, and biceps tendon allows for efficient, pain-free force delivery at the shoulder. However, because of the significant external rotation and abduction required in the overhead motion, there may be internal impingement of the posterosuperior rotator cuff (infraspinatus and posterior aspect of supraspinatus) between the posterior labrum and the greater tuberosity. Detailed examination of these structures must be performed in any assessment of an overhead athlete. Symptomatic patients may complain of pain during the throwing cycle, particularly in late cocking and early acceleration.
The modified relocation examination is a common maneuver to detect internal impingement.3 In this examination, the patient’s arm is brought into a position of maximal external rotation and abduction mimicking that found in late cocking or early acceleration. In this position, a patient with internal impingement complains of pain in the posterior shoulder. A posteriorly directed force on the humerus relieves this pain.
There are also many examinations for detecting labral pathology, specifically a SLAP (superior labrum, anterior to posterior) lesion, which is commonly found in patients with internal impingement. One commonly tested maneuver is the O’Brien active compression test (Figures 2A, 2B), which has excellent sensitivity and specificity in detecting type II SLAP lesions.4 In this examination, the patient holds the arm in about 15° of adduction and 90° of forward elevation. A downward force is applied with the forearm pronated and subsequently supinated. If pain is noted on the force applied to the pronated arm, and if this pain decreases in the supinated examination, the test is positive for labral pathology.
Anterior instability is routinely found in these patients. Translation is measured with the anterior load and shift test. Anterior translation is tested with the patient supine, with the arm in abduction and external rotation, and with the examiner placing an anteriorly directed force on the humeral head. Translation is compared with the contralateral side and graded on a 3-point scale (1+ is translation to glenoid rim, 2+ is translation over glenoid rim but reduces, 3+ is translation over glenoid and locking). We also use the anterior release test, in which the patient is supine, the arm is brought into abduction and external rotation, and the examiner places a posteriorly directed force on the humeral head. When the examiner removes this force, the patient notices symptoms of instability caused by subluxation (Figures 3A, 3B).
Biceps tendon testing should also be performed to help elicit signs of labral pathology. The Speed test is performed by placing a downward force on the patient’s arm, which is held in 90° forward elevation, and with elbows in extension and forearm in supination. Pain in the long head of the biceps tendon is considered a positive sign and suggestive of SLAP lesion. Although not commonly found in these athletes, external impingement should also be elicited through both the Neer test and the Hawkins test. In the Neer test, the patient’s arm is brought to maximal forward elevation with the forearm supinated and elbow extended, while the scapula is stabilized by the examiner. Pain in the shoulder indicates a positive examination. In the Hawkins test, the patient’s arm is brought into a position of forward elevation, internal rotation, and elbow flexion. The arm is then further internally rotated, and shoulder pain defines a positive examination.
Any of these findings can be concomitant with scapular dyskinesis. Moreover, symptoms related to internal impingement may be exacerbated by concomitant scapular pathology, and therefore proper assessment of scapulothoracic motion must also be performed.
4. Scapulothoracic examination
Motion coupled between the scapula and the rest of the arm (scapular rhythm) allows for efficient use of the shoulder girdle. The scapula helps transfer the force generated by the core so that the hand can efficiently deliver it. Therefore, scapular pathology (or dyskinesis) results in inefficient functioning of the arm, which can be especially debilitating in an overhead athlete.
Scapular assessment begins with visual inspection of the patient, typically from the posterior view, which allows for assessment of the resting position of the scapula. Evidence of prominence of the medial or inferomedial border, coracoid malposition (or pain on palpation), or general scapular malposition should be noted. On active ROM, as the patient forward-elevates the arm, any asymmetric prominence of the inferomedial border of the scapula should be noted. Such asymmetry may indicate underlying scapular dyskinesis. In another important test, the lateral scapular slide test (described by Kibler5), the distance from the inferomedial angle of the scapula to the thoracic spine should be measured for both sides and in 3 difference positions, noting any asymmetry between the affected and nonaffected sides. These 3 positions (Figures 4A–4C) are with arms at side, with hands on hips (internal rotation of humerus in 45° abduction), and in 90° of shoulder abduction. Last, medial and lateral scapular winging—caused by long thoracic nerve and spinal accessory nerve pathology, respectively—can be detected by asking the patient to do a “push-up” against the wall while the examiner views from posterior.
After assessment of scapular position at rest and through motion, a series of provocative maneuvers6 may aid in the diagnosis of scapular dyskinesis. The first maneuver is the scapular assistance test, in which the examiner provides a gentle force at the inferomedial angle of the scapula, promoting upward rotation and posterior tilt as the patient elevates the arm (Figures 5A, 5B). If the patient experiences a decrease or absence of symptoms through this arc, the test is considered positive. The second maneuver is the scapular retraction test, in which strength testing of the supraspinatus is performed before and after retraction stabilization of the scapula. In the baseline state, the strength of the supraspinatus is tested in standard fashion, with resisted elevation of the internally rotated and abducted arm. The strength is then tested with the scapula stabilized in retraction (the examiner medially stabilizes the scapula). With scapular stabilization, an increase in strength or a decrease in symptoms is considered a positive test.
5. Neurovascular examination
It is essential to perform a comprehensive neurovascular examination in all overhead athletes. This includes basic cervical spine testing for any motor or sensory deficits, along with assessment of scapular winging to detect long thoracic or spinal accessory nerve palsy for medial and lateral winging, respectively. Although neurovascular injury may be a rare finding in the overhead athlete, a detailed examination must still be performed to rule it out.
Thoracic outlet syndrome
Thoracic syndrome is a compressive neuropathy of nerves and vasculature exiting the thorax and entering the upper extremity. Common symptoms include pain and tingling (sometimes vague) in the neck and upper extremity. These symptoms may be positional as well.
Diagnosis of thoracic outlet syndrome begins with visual inspection of the involved upper extremity, noting atrophy or asymmetry. Weakness may also be present. Additional provocative maneuvers can be used to detect decrease or loss of pulses, along with reproduction of symptoms, during a provocative maneuver with subsequent return of pulses and resolution of symptoms after the maneuver is completed.
One examination that can be used to detect thoracic outlet syndrome is the Adson test.7 During this maneuver, the radial pulse is palpated with the arm at rest on the patient’s side. The patient then turns to the symptomatic side, hyperextends the arm, and holds inspiration. A positive test coincides with both decreased pulse and reproduction of symptoms, indicating compression within the scalene triangle. In the Wright test,7 the pulse is again palpated at rest with the arm at the side. The patient then holds inspiration and places the arm in a position of abduction and external rotation. If the pulses decrease with this maneuver, the test is considered positive, indicating compression in the sub–pectoralis minor region deep to the coracoid. In a third test, the costoclavicular test, again pulses are measured before and during the provocative maneuver, which is with the shoulders thrust backward and depressed downward. A positive test indicates compression between the clavicle and the first rib. In our practice, we use a modified Wright test in which the arm is held in abduction and external rotation while radial pulses are palpated. The fist is then opened and clenched rapidly, and diminution of radial pulses is considered a positive examination (Figures 6A, 6B).
Effort thrombosis
Overhead athletes are at increased risk for developing effort thrombosis8 (Paget-Schroetter syndrome). This thrombosis, which results from repetitive motion involving the upper extremity, is not limited to overhead sports; it may be caused by underlying compression of or microtrauma to the venous infrastructure. On physical examination, there may be swelling of the affected limb, along with diffuse pain and fatigue, as well as dermatologic changes. Positive findings warrant further testing, such as coagulation profile testing and advanced imaging or venography.
Arterial aneurysm
Although rare, arterial aneurysms, especially of the axillary artery, must be ruled out in the overhead athlete with vague upper extremity pain (especially distally) and without clear diagnosis.9 Aneurysm of the axillary artery can result from repetitive microtrauma related to repetitive overhead motion of the upper extremity. This condition may cause showering of emboli distally to the vasculature of the hand and fingers (Figure 7). Patients may complain of pain in the fingers, difficulty with grip, cyanosis, or cold sensation. On examination, the sufficiency of the radial and ulnar arteries should be assessed, as with detailed sensorimotor examination of the fingers. The fingernails should be examined for splinter hemorrhages.
Conclusion
Overhead athletes place extreme stress on the shoulder during the throwing motion and are at high risk for injury because of repetitive stress on the shoulder girdle. When examining overhead athletes with shoulder pain, surgeons must consider the entire kinetic chain, as inefficiencies anywhere along the chain can lead to altered mechanics and pathology in the shoulder.
1. Cummins CA, Messer TM, Schafer MF. Infraspinatus muscle atrophy in professional baseball players. Am J Sports Med. 2004;32(1):116-120.
2. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
3. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part II: evaluation and treatment of SLAP lesions in throwers. Arthroscopy. 2003;19(5):531-539.
4. O’Brien SJ, Pagnani MJ, Fealy S, McGlynn SR, Wilson JB. The active compression test: a new and effective test for diagnosing labral tears and acromioclavicular joint abnormality. Am J Sports Med. 1998;26(5):610-613.
5. Kibler WB. The role of the scapula in athletic shoulder function. Am J Sports Med. 1998;26(2):325-337.
6. Kibler WB, Sciascia A, Wilkes T. Scapular dyskinesis and its relation to shoulder injury. J Am Acad Orthop Surg. 2012;20(6):364-372.
7. Leffert RD. Thoracic outlet syndrome. J Am Acad Orthop Surg. 1994;2(6):317-325.
8. Alla VM, Natarajan N, Kaushik M, Warrier R, Nair CK. Paget-Schroetter syndrome: review of pathogenesis and treatment of effort thrombosis. West J Emerg Med. 2010;11(4):358-362.
9. Baumgarten KM, Dines JS, Winchester PA, et al. Axillary artery aneurysm with distal embolization in a Major League Baseball pitcher. Am J Sports Med. 2007;35(4):650-653.
The overhead athlete’s shoulder is exposed to extremes of stress and range of motion (ROM), predisposing this joint to unique injury patterns. Prompt diagnosis and management begin with a comprehensive history and a physical examination, supplemented by imaging studies as needed. Furthermore, the throwing shoulder undergoes adaptive changes, such as partial undersurface rotator cuff tears and capsular laxity. Imaging studies typically demonstrate abnormalities in asymptomatic throwers. Therefore, clinicians must be skilled in history taking and physical examination in throwing athletes to accurately determine the cause of symptoms and provide optimal treatment. This primer provides orthopedic surgeons with the key points in performing a thorough physical examination of the shoulder in overhead athletes.
When working with overhead athletes, surgeons must elicit the precise nature of symptoms. For example, it is important to distinguish pain from fatigue, as well as complaints related purely to decline in performance. Often, collaboration with the player’s parent or coach may help clarify the chief complaint. In addition, surgeons must have an intricate knowledge of the various stages of the overhead motion, as symptoms in specific stages (late cocking/early acceleration) may raise suspicion for distinctive pathology (labral/biceps complex). Last, it is imperative to understand that the shoulder represents only one part of the kinetic chain in overhead athletes. Successful throwing relies on integrity of the entire kinetic chain, starting with the lower extremity and trunk, extending through the spine, scapula, and shoulder, and terminating with the hand and fingers. Pathology anywhere in the chain must be evaluated and addressed.
When examining the shoulder in overhead athletes, surgeons must address several anatomical structures, both bony and soft tissue. Proper examination begins with comprehensive assessment of the ROM and strength of the various muscles around the shoulder, along with visual inspection to identify any asymmetry of these structures. In addition, the scapulothoracic structures must be examined in detail to rule out underlying dyskinesis. The capsular and ligamentous components of the shoulder joint must be further assessed to note any capsular contracture causing glenohumeral internal rotation deficit (GIRD) or any pathology with the rotator cuff or labral/biceps complex. Last, a comprehensive neurovascular examination should be performed to rule out any compression or neuropathy affecting the shoulder and overhead motion. Findings from the physical examination may then require further imaging to correlate the history and physical examination findings.
1. Inspection, palpation, strength testing
Every examination of the shoulder must begin with visual inspection, along with assessment of basic ROM and strength. The patient must be positioned and exposed adequately to promote visualization of the entire shoulder and scapular girdle, from both anterior and posterior. Visual inspection focuses on identifying any areas of asymmetry, such as position of the bony prominences or bulk of the muscular fossae. Asymmetry of the bony architecture may indicate prior trauma, and atrophy of the muscular fossae may indicate nerve compression. For example, atrophy of the infraspinatus fossa may be caused by compression of the suprascapular nerve at the spinoglenoid notch (likely by a cyst, often associated with labral pathology, but infraspinatus atrophy can result even without the presence of a compressive cyst1). Alternatively, atrophy of both the supraspinatus and infraspinatus fossae may indicate underlying compression of the suprascapular nerve at the suprascapular notch (either by a cyst or by the transverse scapular ligament). Static and dynamic observation of the posterior aspect of the shoulder may help identify gross pathology with scapular positioning or retraction, indicating underlying dyskinesis (discussed later). Deformity of the acromioclavicular joint may indicate prior trauma or separation. Last, all prior surgical scars should be noted.
Selective palpation may help identify pathology in the shoulder of the throwing athlete. Tenderness at the acromioclavicular joint may be especially common in patients who have had prior sprains of this joint or who have degenerative changes. Tenderness along the biceps tendon may be present in those with biceps tendinitis or partial tear. In addition, tenderness at the coracoid may be present in those with scapular dyskinesis. Posteriorly, palpation at the inferomedial aspect of the scapula (Figure 1), as with palpation along the medial border of the scapula, may elicit tenderness in those with scapulothoracic bursitis.
Strength testing in the shoulder is performed to elicit any deficiencies of the rotator cuff/musculature or surrounding structures. Weakness in forward elevation may indicate pathology in the supraspinatus, whereas weakness in external rotation may reflect deficiency in the infraspinatus or teres minor. Teres minor deficiency may be more isolated with weakness in a position of shoulder abduction to 90°. Last, weakness in internal rotation may indicate subscapularis deficiency. Lag signs and other provocative maneuvers are similarly elicited but typically are positive only in the event of large tears of the rotator cuff. These signs and maneuvers include the internal rotation lag sign or belly press test for subscapularis integrity, the drop-arm sign for supraspinatus function, the external rotation lag sign for infraspinatus function, and the hornblower sign for teres minor integrity. Supporting muscles of the shoulder may also be tested. Latissimus strength may be tested with resisted downward rotation of the arm with the shoulder in abduction and the elbow flexed to 90°.
2. ROM and GIRD assessment
After inspection and palpation, the shoulder should be ranged in all relevant planes of motion. Our standard examination includes forward elevation in the frontal and scapular planes, along with external rotation at the side and at 90° of abduction, as well as internal rotation behind the back with documentation of the highest spinal level that the patient can reach. This examination may be performed with the patient upright, but supine positioning can help stabilize the scapula and provide more accurate views of motion. Deficits of internal rotation may be a common finding in overhead athletes, and the degree of this deficit should be quantitatively noted.
Bony and soft-tissue remodeling of the shoulder (and associated structures) in the overhead athlete can lead to contracture of the posterior capsule. This contracture can cause excessive external rotation and subsequent decrease in internal rotation, leading to pain and anterior instability in the throwing shoulder.2 For precise measurements of the internal and external rotation arc, the scapula must be stabilized. This can be done with the patient supine on the examining table or seated upright with manual stabilization of the scapula by the examiner. Once the scapula is stabilized, the arc of internal and external rotation (with the arm in about 90° of abduction) can be measured with a goniometer, with maximum values obtained as the scapula begins to move along the posterior chest wall.2 The difference in internal rotation between the dominant and nondominant arms defines the extent of the athlete’s GIRD. Internal rotation can also be qualitatively assessed by having the athlete internally rotate each arm and reach up the spine while the examiner notes the difference in level achieved. However, this does not provide a quantitative assessment of the patient’s GIRD.
In general, the sum of the internal and external rotation arcs on the 2 sides should be symmetric. Consequently, in GIRD, excessive external rotation is balanced by decreased internal rotation. Symptomatic GIRD may be present when there is more than 25° of discrepancy in internal rotation between the athlete’s dominant and nondominant arms.2 The goal is to reduce this discrepancy to less than 20°.
3. Internal impingement: rotator cuff and labrum
In overhead athletes, an intricate relationship involving rotator cuff, labrum, and biceps tendon allows for efficient, pain-free force delivery at the shoulder. However, because of the significant external rotation and abduction required in the overhead motion, there may be internal impingement of the posterosuperior rotator cuff (infraspinatus and posterior aspect of supraspinatus) between the posterior labrum and the greater tuberosity. Detailed examination of these structures must be performed in any assessment of an overhead athlete. Symptomatic patients may complain of pain during the throwing cycle, particularly in late cocking and early acceleration.
The modified relocation examination is a common maneuver to detect internal impingement.3 In this examination, the patient’s arm is brought into a position of maximal external rotation and abduction mimicking that found in late cocking or early acceleration. In this position, a patient with internal impingement complains of pain in the posterior shoulder. A posteriorly directed force on the humerus relieves this pain.
There are also many examinations for detecting labral pathology, specifically a SLAP (superior labrum, anterior to posterior) lesion, which is commonly found in patients with internal impingement. One commonly tested maneuver is the O’Brien active compression test (Figures 2A, 2B), which has excellent sensitivity and specificity in detecting type II SLAP lesions.4 In this examination, the patient holds the arm in about 15° of adduction and 90° of forward elevation. A downward force is applied with the forearm pronated and subsequently supinated. If pain is noted on the force applied to the pronated arm, and if this pain decreases in the supinated examination, the test is positive for labral pathology.
Anterior instability is routinely found in these patients. Translation is measured with the anterior load and shift test. Anterior translation is tested with the patient supine, with the arm in abduction and external rotation, and with the examiner placing an anteriorly directed force on the humeral head. Translation is compared with the contralateral side and graded on a 3-point scale (1+ is translation to glenoid rim, 2+ is translation over glenoid rim but reduces, 3+ is translation over glenoid and locking). We also use the anterior release test, in which the patient is supine, the arm is brought into abduction and external rotation, and the examiner places a posteriorly directed force on the humeral head. When the examiner removes this force, the patient notices symptoms of instability caused by subluxation (Figures 3A, 3B).
Biceps tendon testing should also be performed to help elicit signs of labral pathology. The Speed test is performed by placing a downward force on the patient’s arm, which is held in 90° forward elevation, and with elbows in extension and forearm in supination. Pain in the long head of the biceps tendon is considered a positive sign and suggestive of SLAP lesion. Although not commonly found in these athletes, external impingement should also be elicited through both the Neer test and the Hawkins test. In the Neer test, the patient’s arm is brought to maximal forward elevation with the forearm supinated and elbow extended, while the scapula is stabilized by the examiner. Pain in the shoulder indicates a positive examination. In the Hawkins test, the patient’s arm is brought into a position of forward elevation, internal rotation, and elbow flexion. The arm is then further internally rotated, and shoulder pain defines a positive examination.
Any of these findings can be concomitant with scapular dyskinesis. Moreover, symptoms related to internal impingement may be exacerbated by concomitant scapular pathology, and therefore proper assessment of scapulothoracic motion must also be performed.
4. Scapulothoracic examination
Motion coupled between the scapula and the rest of the arm (scapular rhythm) allows for efficient use of the shoulder girdle. The scapula helps transfer the force generated by the core so that the hand can efficiently deliver it. Therefore, scapular pathology (or dyskinesis) results in inefficient functioning of the arm, which can be especially debilitating in an overhead athlete.
Scapular assessment begins with visual inspection of the patient, typically from the posterior view, which allows for assessment of the resting position of the scapula. Evidence of prominence of the medial or inferomedial border, coracoid malposition (or pain on palpation), or general scapular malposition should be noted. On active ROM, as the patient forward-elevates the arm, any asymmetric prominence of the inferomedial border of the scapula should be noted. Such asymmetry may indicate underlying scapular dyskinesis. In another important test, the lateral scapular slide test (described by Kibler5), the distance from the inferomedial angle of the scapula to the thoracic spine should be measured for both sides and in 3 difference positions, noting any asymmetry between the affected and nonaffected sides. These 3 positions (Figures 4A–4C) are with arms at side, with hands on hips (internal rotation of humerus in 45° abduction), and in 90° of shoulder abduction. Last, medial and lateral scapular winging—caused by long thoracic nerve and spinal accessory nerve pathology, respectively—can be detected by asking the patient to do a “push-up” against the wall while the examiner views from posterior.
After assessment of scapular position at rest and through motion, a series of provocative maneuvers6 may aid in the diagnosis of scapular dyskinesis. The first maneuver is the scapular assistance test, in which the examiner provides a gentle force at the inferomedial angle of the scapula, promoting upward rotation and posterior tilt as the patient elevates the arm (Figures 5A, 5B). If the patient experiences a decrease or absence of symptoms through this arc, the test is considered positive. The second maneuver is the scapular retraction test, in which strength testing of the supraspinatus is performed before and after retraction stabilization of the scapula. In the baseline state, the strength of the supraspinatus is tested in standard fashion, with resisted elevation of the internally rotated and abducted arm. The strength is then tested with the scapula stabilized in retraction (the examiner medially stabilizes the scapula). With scapular stabilization, an increase in strength or a decrease in symptoms is considered a positive test.
5. Neurovascular examination
It is essential to perform a comprehensive neurovascular examination in all overhead athletes. This includes basic cervical spine testing for any motor or sensory deficits, along with assessment of scapular winging to detect long thoracic or spinal accessory nerve palsy for medial and lateral winging, respectively. Although neurovascular injury may be a rare finding in the overhead athlete, a detailed examination must still be performed to rule it out.
Thoracic outlet syndrome
Thoracic syndrome is a compressive neuropathy of nerves and vasculature exiting the thorax and entering the upper extremity. Common symptoms include pain and tingling (sometimes vague) in the neck and upper extremity. These symptoms may be positional as well.
Diagnosis of thoracic outlet syndrome begins with visual inspection of the involved upper extremity, noting atrophy or asymmetry. Weakness may also be present. Additional provocative maneuvers can be used to detect decrease or loss of pulses, along with reproduction of symptoms, during a provocative maneuver with subsequent return of pulses and resolution of symptoms after the maneuver is completed.
One examination that can be used to detect thoracic outlet syndrome is the Adson test.7 During this maneuver, the radial pulse is palpated with the arm at rest on the patient’s side. The patient then turns to the symptomatic side, hyperextends the arm, and holds inspiration. A positive test coincides with both decreased pulse and reproduction of symptoms, indicating compression within the scalene triangle. In the Wright test,7 the pulse is again palpated at rest with the arm at the side. The patient then holds inspiration and places the arm in a position of abduction and external rotation. If the pulses decrease with this maneuver, the test is considered positive, indicating compression in the sub–pectoralis minor region deep to the coracoid. In a third test, the costoclavicular test, again pulses are measured before and during the provocative maneuver, which is with the shoulders thrust backward and depressed downward. A positive test indicates compression between the clavicle and the first rib. In our practice, we use a modified Wright test in which the arm is held in abduction and external rotation while radial pulses are palpated. The fist is then opened and clenched rapidly, and diminution of radial pulses is considered a positive examination (Figures 6A, 6B).
Effort thrombosis
Overhead athletes are at increased risk for developing effort thrombosis8 (Paget-Schroetter syndrome). This thrombosis, which results from repetitive motion involving the upper extremity, is not limited to overhead sports; it may be caused by underlying compression of or microtrauma to the venous infrastructure. On physical examination, there may be swelling of the affected limb, along with diffuse pain and fatigue, as well as dermatologic changes. Positive findings warrant further testing, such as coagulation profile testing and advanced imaging or venography.
Arterial aneurysm
Although rare, arterial aneurysms, especially of the axillary artery, must be ruled out in the overhead athlete with vague upper extremity pain (especially distally) and without clear diagnosis.9 Aneurysm of the axillary artery can result from repetitive microtrauma related to repetitive overhead motion of the upper extremity. This condition may cause showering of emboli distally to the vasculature of the hand and fingers (Figure 7). Patients may complain of pain in the fingers, difficulty with grip, cyanosis, or cold sensation. On examination, the sufficiency of the radial and ulnar arteries should be assessed, as with detailed sensorimotor examination of the fingers. The fingernails should be examined for splinter hemorrhages.
Conclusion
Overhead athletes place extreme stress on the shoulder during the throwing motion and are at high risk for injury because of repetitive stress on the shoulder girdle. When examining overhead athletes with shoulder pain, surgeons must consider the entire kinetic chain, as inefficiencies anywhere along the chain can lead to altered mechanics and pathology in the shoulder.
The overhead athlete’s shoulder is exposed to extremes of stress and range of motion (ROM), predisposing this joint to unique injury patterns. Prompt diagnosis and management begin with a comprehensive history and a physical examination, supplemented by imaging studies as needed. Furthermore, the throwing shoulder undergoes adaptive changes, such as partial undersurface rotator cuff tears and capsular laxity. Imaging studies typically demonstrate abnormalities in asymptomatic throwers. Therefore, clinicians must be skilled in history taking and physical examination in throwing athletes to accurately determine the cause of symptoms and provide optimal treatment. This primer provides orthopedic surgeons with the key points in performing a thorough physical examination of the shoulder in overhead athletes.
When working with overhead athletes, surgeons must elicit the precise nature of symptoms. For example, it is important to distinguish pain from fatigue, as well as complaints related purely to decline in performance. Often, collaboration with the player’s parent or coach may help clarify the chief complaint. In addition, surgeons must have an intricate knowledge of the various stages of the overhead motion, as symptoms in specific stages (late cocking/early acceleration) may raise suspicion for distinctive pathology (labral/biceps complex). Last, it is imperative to understand that the shoulder represents only one part of the kinetic chain in overhead athletes. Successful throwing relies on integrity of the entire kinetic chain, starting with the lower extremity and trunk, extending through the spine, scapula, and shoulder, and terminating with the hand and fingers. Pathology anywhere in the chain must be evaluated and addressed.
When examining the shoulder in overhead athletes, surgeons must address several anatomical structures, both bony and soft tissue. Proper examination begins with comprehensive assessment of the ROM and strength of the various muscles around the shoulder, along with visual inspection to identify any asymmetry of these structures. In addition, the scapulothoracic structures must be examined in detail to rule out underlying dyskinesis. The capsular and ligamentous components of the shoulder joint must be further assessed to note any capsular contracture causing glenohumeral internal rotation deficit (GIRD) or any pathology with the rotator cuff or labral/biceps complex. Last, a comprehensive neurovascular examination should be performed to rule out any compression or neuropathy affecting the shoulder and overhead motion. Findings from the physical examination may then require further imaging to correlate the history and physical examination findings.
1. Inspection, palpation, strength testing
Every examination of the shoulder must begin with visual inspection, along with assessment of basic ROM and strength. The patient must be positioned and exposed adequately to promote visualization of the entire shoulder and scapular girdle, from both anterior and posterior. Visual inspection focuses on identifying any areas of asymmetry, such as position of the bony prominences or bulk of the muscular fossae. Asymmetry of the bony architecture may indicate prior trauma, and atrophy of the muscular fossae may indicate nerve compression. For example, atrophy of the infraspinatus fossa may be caused by compression of the suprascapular nerve at the spinoglenoid notch (likely by a cyst, often associated with labral pathology, but infraspinatus atrophy can result even without the presence of a compressive cyst1). Alternatively, atrophy of both the supraspinatus and infraspinatus fossae may indicate underlying compression of the suprascapular nerve at the suprascapular notch (either by a cyst or by the transverse scapular ligament). Static and dynamic observation of the posterior aspect of the shoulder may help identify gross pathology with scapular positioning or retraction, indicating underlying dyskinesis (discussed later). Deformity of the acromioclavicular joint may indicate prior trauma or separation. Last, all prior surgical scars should be noted.
Selective palpation may help identify pathology in the shoulder of the throwing athlete. Tenderness at the acromioclavicular joint may be especially common in patients who have had prior sprains of this joint or who have degenerative changes. Tenderness along the biceps tendon may be present in those with biceps tendinitis or partial tear. In addition, tenderness at the coracoid may be present in those with scapular dyskinesis. Posteriorly, palpation at the inferomedial aspect of the scapula (Figure 1), as with palpation along the medial border of the scapula, may elicit tenderness in those with scapulothoracic bursitis.
Strength testing in the shoulder is performed to elicit any deficiencies of the rotator cuff/musculature or surrounding structures. Weakness in forward elevation may indicate pathology in the supraspinatus, whereas weakness in external rotation may reflect deficiency in the infraspinatus or teres minor. Teres minor deficiency may be more isolated with weakness in a position of shoulder abduction to 90°. Last, weakness in internal rotation may indicate subscapularis deficiency. Lag signs and other provocative maneuvers are similarly elicited but typically are positive only in the event of large tears of the rotator cuff. These signs and maneuvers include the internal rotation lag sign or belly press test for subscapularis integrity, the drop-arm sign for supraspinatus function, the external rotation lag sign for infraspinatus function, and the hornblower sign for teres minor integrity. Supporting muscles of the shoulder may also be tested. Latissimus strength may be tested with resisted downward rotation of the arm with the shoulder in abduction and the elbow flexed to 90°.
2. ROM and GIRD assessment
After inspection and palpation, the shoulder should be ranged in all relevant planes of motion. Our standard examination includes forward elevation in the frontal and scapular planes, along with external rotation at the side and at 90° of abduction, as well as internal rotation behind the back with documentation of the highest spinal level that the patient can reach. This examination may be performed with the patient upright, but supine positioning can help stabilize the scapula and provide more accurate views of motion. Deficits of internal rotation may be a common finding in overhead athletes, and the degree of this deficit should be quantitatively noted.
Bony and soft-tissue remodeling of the shoulder (and associated structures) in the overhead athlete can lead to contracture of the posterior capsule. This contracture can cause excessive external rotation and subsequent decrease in internal rotation, leading to pain and anterior instability in the throwing shoulder.2 For precise measurements of the internal and external rotation arc, the scapula must be stabilized. This can be done with the patient supine on the examining table or seated upright with manual stabilization of the scapula by the examiner. Once the scapula is stabilized, the arc of internal and external rotation (with the arm in about 90° of abduction) can be measured with a goniometer, with maximum values obtained as the scapula begins to move along the posterior chest wall.2 The difference in internal rotation between the dominant and nondominant arms defines the extent of the athlete’s GIRD. Internal rotation can also be qualitatively assessed by having the athlete internally rotate each arm and reach up the spine while the examiner notes the difference in level achieved. However, this does not provide a quantitative assessment of the patient’s GIRD.
In general, the sum of the internal and external rotation arcs on the 2 sides should be symmetric. Consequently, in GIRD, excessive external rotation is balanced by decreased internal rotation. Symptomatic GIRD may be present when there is more than 25° of discrepancy in internal rotation between the athlete’s dominant and nondominant arms.2 The goal is to reduce this discrepancy to less than 20°.
3. Internal impingement: rotator cuff and labrum
In overhead athletes, an intricate relationship involving rotator cuff, labrum, and biceps tendon allows for efficient, pain-free force delivery at the shoulder. However, because of the significant external rotation and abduction required in the overhead motion, there may be internal impingement of the posterosuperior rotator cuff (infraspinatus and posterior aspect of supraspinatus) between the posterior labrum and the greater tuberosity. Detailed examination of these structures must be performed in any assessment of an overhead athlete. Symptomatic patients may complain of pain during the throwing cycle, particularly in late cocking and early acceleration.
The modified relocation examination is a common maneuver to detect internal impingement.3 In this examination, the patient’s arm is brought into a position of maximal external rotation and abduction mimicking that found in late cocking or early acceleration. In this position, a patient with internal impingement complains of pain in the posterior shoulder. A posteriorly directed force on the humerus relieves this pain.
There are also many examinations for detecting labral pathology, specifically a SLAP (superior labrum, anterior to posterior) lesion, which is commonly found in patients with internal impingement. One commonly tested maneuver is the O’Brien active compression test (Figures 2A, 2B), which has excellent sensitivity and specificity in detecting type II SLAP lesions.4 In this examination, the patient holds the arm in about 15° of adduction and 90° of forward elevation. A downward force is applied with the forearm pronated and subsequently supinated. If pain is noted on the force applied to the pronated arm, and if this pain decreases in the supinated examination, the test is positive for labral pathology.
Anterior instability is routinely found in these patients. Translation is measured with the anterior load and shift test. Anterior translation is tested with the patient supine, with the arm in abduction and external rotation, and with the examiner placing an anteriorly directed force on the humeral head. Translation is compared with the contralateral side and graded on a 3-point scale (1+ is translation to glenoid rim, 2+ is translation over glenoid rim but reduces, 3+ is translation over glenoid and locking). We also use the anterior release test, in which the patient is supine, the arm is brought into abduction and external rotation, and the examiner places a posteriorly directed force on the humeral head. When the examiner removes this force, the patient notices symptoms of instability caused by subluxation (Figures 3A, 3B).
Biceps tendon testing should also be performed to help elicit signs of labral pathology. The Speed test is performed by placing a downward force on the patient’s arm, which is held in 90° forward elevation, and with elbows in extension and forearm in supination. Pain in the long head of the biceps tendon is considered a positive sign and suggestive of SLAP lesion. Although not commonly found in these athletes, external impingement should also be elicited through both the Neer test and the Hawkins test. In the Neer test, the patient’s arm is brought to maximal forward elevation with the forearm supinated and elbow extended, while the scapula is stabilized by the examiner. Pain in the shoulder indicates a positive examination. In the Hawkins test, the patient’s arm is brought into a position of forward elevation, internal rotation, and elbow flexion. The arm is then further internally rotated, and shoulder pain defines a positive examination.
Any of these findings can be concomitant with scapular dyskinesis. Moreover, symptoms related to internal impingement may be exacerbated by concomitant scapular pathology, and therefore proper assessment of scapulothoracic motion must also be performed.
4. Scapulothoracic examination
Motion coupled between the scapula and the rest of the arm (scapular rhythm) allows for efficient use of the shoulder girdle. The scapula helps transfer the force generated by the core so that the hand can efficiently deliver it. Therefore, scapular pathology (or dyskinesis) results in inefficient functioning of the arm, which can be especially debilitating in an overhead athlete.
Scapular assessment begins with visual inspection of the patient, typically from the posterior view, which allows for assessment of the resting position of the scapula. Evidence of prominence of the medial or inferomedial border, coracoid malposition (or pain on palpation), or general scapular malposition should be noted. On active ROM, as the patient forward-elevates the arm, any asymmetric prominence of the inferomedial border of the scapula should be noted. Such asymmetry may indicate underlying scapular dyskinesis. In another important test, the lateral scapular slide test (described by Kibler5), the distance from the inferomedial angle of the scapula to the thoracic spine should be measured for both sides and in 3 difference positions, noting any asymmetry between the affected and nonaffected sides. These 3 positions (Figures 4A–4C) are with arms at side, with hands on hips (internal rotation of humerus in 45° abduction), and in 90° of shoulder abduction. Last, medial and lateral scapular winging—caused by long thoracic nerve and spinal accessory nerve pathology, respectively—can be detected by asking the patient to do a “push-up” against the wall while the examiner views from posterior.
After assessment of scapular position at rest and through motion, a series of provocative maneuvers6 may aid in the diagnosis of scapular dyskinesis. The first maneuver is the scapular assistance test, in which the examiner provides a gentle force at the inferomedial angle of the scapula, promoting upward rotation and posterior tilt as the patient elevates the arm (Figures 5A, 5B). If the patient experiences a decrease or absence of symptoms through this arc, the test is considered positive. The second maneuver is the scapular retraction test, in which strength testing of the supraspinatus is performed before and after retraction stabilization of the scapula. In the baseline state, the strength of the supraspinatus is tested in standard fashion, with resisted elevation of the internally rotated and abducted arm. The strength is then tested with the scapula stabilized in retraction (the examiner medially stabilizes the scapula). With scapular stabilization, an increase in strength or a decrease in symptoms is considered a positive test.
5. Neurovascular examination
It is essential to perform a comprehensive neurovascular examination in all overhead athletes. This includes basic cervical spine testing for any motor or sensory deficits, along with assessment of scapular winging to detect long thoracic or spinal accessory nerve palsy for medial and lateral winging, respectively. Although neurovascular injury may be a rare finding in the overhead athlete, a detailed examination must still be performed to rule it out.
Thoracic outlet syndrome
Thoracic syndrome is a compressive neuropathy of nerves and vasculature exiting the thorax and entering the upper extremity. Common symptoms include pain and tingling (sometimes vague) in the neck and upper extremity. These symptoms may be positional as well.
Diagnosis of thoracic outlet syndrome begins with visual inspection of the involved upper extremity, noting atrophy or asymmetry. Weakness may also be present. Additional provocative maneuvers can be used to detect decrease or loss of pulses, along with reproduction of symptoms, during a provocative maneuver with subsequent return of pulses and resolution of symptoms after the maneuver is completed.
One examination that can be used to detect thoracic outlet syndrome is the Adson test.7 During this maneuver, the radial pulse is palpated with the arm at rest on the patient’s side. The patient then turns to the symptomatic side, hyperextends the arm, and holds inspiration. A positive test coincides with both decreased pulse and reproduction of symptoms, indicating compression within the scalene triangle. In the Wright test,7 the pulse is again palpated at rest with the arm at the side. The patient then holds inspiration and places the arm in a position of abduction and external rotation. If the pulses decrease with this maneuver, the test is considered positive, indicating compression in the sub–pectoralis minor region deep to the coracoid. In a third test, the costoclavicular test, again pulses are measured before and during the provocative maneuver, which is with the shoulders thrust backward and depressed downward. A positive test indicates compression between the clavicle and the first rib. In our practice, we use a modified Wright test in which the arm is held in abduction and external rotation while radial pulses are palpated. The fist is then opened and clenched rapidly, and diminution of radial pulses is considered a positive examination (Figures 6A, 6B).
Effort thrombosis
Overhead athletes are at increased risk for developing effort thrombosis8 (Paget-Schroetter syndrome). This thrombosis, which results from repetitive motion involving the upper extremity, is not limited to overhead sports; it may be caused by underlying compression of or microtrauma to the venous infrastructure. On physical examination, there may be swelling of the affected limb, along with diffuse pain and fatigue, as well as dermatologic changes. Positive findings warrant further testing, such as coagulation profile testing and advanced imaging or venography.
Arterial aneurysm
Although rare, arterial aneurysms, especially of the axillary artery, must be ruled out in the overhead athlete with vague upper extremity pain (especially distally) and without clear diagnosis.9 Aneurysm of the axillary artery can result from repetitive microtrauma related to repetitive overhead motion of the upper extremity. This condition may cause showering of emboli distally to the vasculature of the hand and fingers (Figure 7). Patients may complain of pain in the fingers, difficulty with grip, cyanosis, or cold sensation. On examination, the sufficiency of the radial and ulnar arteries should be assessed, as with detailed sensorimotor examination of the fingers. The fingernails should be examined for splinter hemorrhages.
Conclusion
Overhead athletes place extreme stress on the shoulder during the throwing motion and are at high risk for injury because of repetitive stress on the shoulder girdle. When examining overhead athletes with shoulder pain, surgeons must consider the entire kinetic chain, as inefficiencies anywhere along the chain can lead to altered mechanics and pathology in the shoulder.
1. Cummins CA, Messer TM, Schafer MF. Infraspinatus muscle atrophy in professional baseball players. Am J Sports Med. 2004;32(1):116-120.
2. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
3. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part II: evaluation and treatment of SLAP lesions in throwers. Arthroscopy. 2003;19(5):531-539.
4. O’Brien SJ, Pagnani MJ, Fealy S, McGlynn SR, Wilson JB. The active compression test: a new and effective test for diagnosing labral tears and acromioclavicular joint abnormality. Am J Sports Med. 1998;26(5):610-613.
5. Kibler WB. The role of the scapula in athletic shoulder function. Am J Sports Med. 1998;26(2):325-337.
6. Kibler WB, Sciascia A, Wilkes T. Scapular dyskinesis and its relation to shoulder injury. J Am Acad Orthop Surg. 2012;20(6):364-372.
7. Leffert RD. Thoracic outlet syndrome. J Am Acad Orthop Surg. 1994;2(6):317-325.
8. Alla VM, Natarajan N, Kaushik M, Warrier R, Nair CK. Paget-Schroetter syndrome: review of pathogenesis and treatment of effort thrombosis. West J Emerg Med. 2010;11(4):358-362.
9. Baumgarten KM, Dines JS, Winchester PA, et al. Axillary artery aneurysm with distal embolization in a Major League Baseball pitcher. Am J Sports Med. 2007;35(4):650-653.
1. Cummins CA, Messer TM, Schafer MF. Infraspinatus muscle atrophy in professional baseball players. Am J Sports Med. 2004;32(1):116-120.
2. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
3. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part II: evaluation and treatment of SLAP lesions in throwers. Arthroscopy. 2003;19(5):531-539.
4. O’Brien SJ, Pagnani MJ, Fealy S, McGlynn SR, Wilson JB. The active compression test: a new and effective test for diagnosing labral tears and acromioclavicular joint abnormality. Am J Sports Med. 1998;26(5):610-613.
5. Kibler WB. The role of the scapula in athletic shoulder function. Am J Sports Med. 1998;26(2):325-337.
6. Kibler WB, Sciascia A, Wilkes T. Scapular dyskinesis and its relation to shoulder injury. J Am Acad Orthop Surg. 2012;20(6):364-372.
7. Leffert RD. Thoracic outlet syndrome. J Am Acad Orthop Surg. 1994;2(6):317-325.
8. Alla VM, Natarajan N, Kaushik M, Warrier R, Nair CK. Paget-Schroetter syndrome: review of pathogenesis and treatment of effort thrombosis. West J Emerg Med. 2010;11(4):358-362.
9. Baumgarten KM, Dines JS, Winchester PA, et al. Axillary artery aneurysm with distal embolization in a Major League Baseball pitcher. Am J Sports Med. 2007;35(4):650-653.
Supinator Cyst in a Young Female Softball Player Successfully Treated With Aspiration
Ganglion cysts around the elbow joint are unusual, with fewer than 25 citations (most of which are case reports) in the English-language literature. Among the many causes of elbow pain, cysts are chiefly diagnosed by advanced imaging. When an elbow ganglion or perineural cyst is symptomatic, treatment has ranged from nonoperative to surgical intervention. Our case report is the first documented ultrasound-guided aspiration and cortisone injection to successfully alleviate a patient’s symptoms. The procedures and outcomes of minimally invasive ultrasound-guided aspiration and steroid injections have not been described for cysts around the elbow. The patient and patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 14-year-old female freshman varsity softball pitcher on multiple teams presented with 6 months of vague right elbow pain. She was unable to pitch and had intermittent sharp pain localized to the lateral proximal forearm. She was, however, able to bat without pain and denied any radiating paresthesias. Despite a reduction in sports activities, the symptoms did not improve.
On physical examination, there was preserved strength that was symmetric with the contralateral side of all major muscles innervated by the radial nerve in the right arm, including full wrist, thumb, and finger extension. Sensation was intact to light touch in all major nervous distributions of the right and left upper extremities. She was tender to palpation at the radiocapitellar joint anteriorly, as well as just distally. The patient was also tender with motion through the proximal radial head. She had pain with resisted finger extension; however, resisted supination elicited no discomfort or pain.
The initial diagnostic workup included radiographs of the right elbow, a magnetic resonance imaging (MRI) scan, and an ultrasound. Elbow radiographs revealed no abnormalities. The MRI scan showed a well-circumscribed ovoid T2-hyperintense structure within the supinator muscle measuring 0.6×0.6×0.4 cm (longitudinal × anteroposterior × transverse), just deep to the split of the superficial and deep radial nerves (Figures 1A-1C). A musculoskeletal ultrasound was performed to further characterize and determine the relationship to neurovascular structures. Longitudinal (Figure 2A) and transverse (Figure 2B) images showed a hypoechoic cystic structure, separate from any local nerve, and without Doppler flow, consistent with what was seen on MRI. Additionally, there was an apparent stalk communicating with the anterior margin of the radiocapitellar articulation, seen on longitudinal images, suggesting an extension of the joint capsule (Figure 3A).
We diagnosed the patient with a radiocapitellar ganglion cyst. Her symptoms continued despite several sessions of physical therapy and cessation from all throwing. Given the ultrasound and MRI findings, and continuation of the symptoms despite conservative treatment, alternative treatment plans were discussed with the patient. These included continued activity modification and nonoperative treatment, open excision of the cyst, or aspiration of the cyst under ultrasound guidance. All appropriate risks and benefits were discussed, including possibility of nerve damage given the proximity of the cyst to the radial nerve branches. After a thorough discussion with both patient and family, a plan was made to undergo aspiration under ultrasound guidance. This was carried out using a lateral-to-medial in-plane approach, transverse to the radius. Using a 19-g, 1.5-inch needle (Figure 3B), 1 mL of serosanguinous fluid was aspirated from the cyst, followed by injection of 40 mg methylprednisolone sodium succinate.
The patient made a dramatic recovery within 8 days after aspiration. On examination, she had full strength to resisted flexion, extension, pronation, and supination; had no tenderness to palpation over the supinator; and no pain with resisted finger extension. She began dedicated physical therapy and a gradual return to throwing. She was able to return to her original level of softball activities 2 months after the aspiration. The patient continued to be symptom-free 26 months after the aspiration/injection. There was no evidence of recurrence of the ganglion on repeat ultrasound at her most recent follow-up (Figures 4A, 4B).
Discussion
Our review of the English-language literature identified 23 reports of cysts in and around the supinator muscle. Ganglion cysts are benign lesions that are uncommonly seen about the elbow. This highlights the rarity of this diagnosis, as well as the need for recognition of its existence. Cysts located in the substance of the nerve1-5 and extraneural ganglia causing symptomatic nerve compression have been described. These extraneural ganglia have been reported to cause compression of the ulnar nerve,1-4,6 posterior interosseous nerve (PIN),5,7-12 and radial nerve,13 and isolated compression of the radial sensory branch.14-17 Ganglion cyst compression in the elbow can result in pain, decreased motor function, and decreased sensation. The PIN syndrome is primarily a motor deficiency, whereas isolated compression of the sensory branches of the radial nerve presents as pain along the radial tunnel and extensor muscle mass.17
Most ganglion cysts are formed when joint fluid extrudes through a defect in the joint capsule; they have also been described originating from a nonunion site.18 When conservative treatment fails, surgical excision has been recommended.5,6,8-10,12-16 We present the first known case of successful ultrasound-guided aspiration and injection of a ganglion cyst from the proximal radiocapitellar joint.
In the earliest described case in 1955, Broomhead19 noted exploration was essential to establish the diagnosis of nerve palsy. In 1966, Bowen and Stone7 were the first to report PIN compression by a ganglion and that compression was likely where nerves pass through confined spaces. In keeping with the known potential for compression of the common peroneal nerve around the fibular head, Bowen and Stone7 posited that the same could be true of the PIN coursing through the supinator and around the radial neck.
Many authors have noted that nerve palsy either improves with rest or worsens with heavy manual work.3,20,21 These observations suggest that dynamic factors in addition to compression of the nerve by the ganglion may influence the occurrence of the nerve palsy.14 This is in line with our patient whose symptoms worsened after pitching.
Ogino and colleagues20 reported on the first use of ultrasonography as a screening examination for a ganglion, particularly when palpation was difficult. Ultrasound allows a detailed assessment of peripheral nerve continuity with a mass, differentiating an intraneural lesion from an adjacent extrinsic ganglion.13 Tonkin10 published the first description of MRI used for the diagnosis of an elbow cyst, and its use has been supported by others.5,8,20 The typical appearance of ganglion cysts on MRI include low signal on T1-weighted images and very high signal on T2-weighted images. Only the periphery of the mass is enhanced by gadolinium, if used.
As recently as 2009, Jou and associates13 suggested that surgical excision should be performed promptly to ensure optimal recovery from a nerve palsy. Many authors agree that early diagnosis and careful surgical excision is associated with a satisfactory outcome without recurrence of the cyst.5,6,8-10,12-15 There are only 4 published case reports14-17 of ganglions causing isolated compression of the superficial radial sensory nerve, as in our case. Their patients had pain with exertional trauma14 as did our patient, a positive Tinel sign,15 and resolution of symptoms after surgical excision without recurrence.14-16 Mileti and colleagues16 state that standard management for resistant radial tunnel syndrome is open decompression of the radial nerve.
In the last decade, a few reports of arthroscopic excision being a viable and safe alternative to open excision have been published.16,22,23 In 2000, Feldman22 described the benefits of an arthroscopic approach as decreased soft-tissue dissection, increased ability to identify intra-articular pathology, and similar recurrence rates to open procedures. He reported 1 transient neurapraxia of the superficial radial nerve from the arthroscopy, highlighting a risk of arthroscopic treatment.
An alternative to open or arthroscopic cyst decompression is aspiration. The only mention of aspiration in the literature comes from Broomhead19 in 1955 when he described 2 patients in whom treatment by aspiration was unsuccessful in relieving their symptoms. Yamazaki and colleagues12 noted that 1 of their 14 patients with PIN palsies caused by ganglions at the elbow underwent puncture of the ganglion with recovery of the paralysis. With the aid of ultrasound guidance, we were able to accurately locate the ganglion cyst, aspirate its contents, and inject methylprednisolone sodium succinate. Our patient continued to be symptom-free and was an active pitcher on a varsity softball team 26 months after aspiration.
Conclusion
This case report describes a rare location for a ganglion cyst in a high-level softball player. To our knowledge, successful treatment with ultrasound-guided aspiration and injection of a supinator cyst has not been reported in the literature. This case report highlights the importance of a careful diagnosis of this condition and an alternative treatment algorithm.
1. Boursinos LA, Dimitriou CG. Ulnar nerve compression in the cubital tunnel by an epineural ganglion: a case report. Hand (N Y). 2007;2(1):12-15.
2. Ferlic DC, Ries MD. Epineural ganglion of the ulnar nerve at the elbow. J Hand Surg Am. 1990;15(6):996-998.
3. Ming Chan K, Thompson S, Amirjani N, Satkunam L, Strohschlein FJ, Lobay GL. Compression of the ulnar nerve at the elbow by an intraneural ganglion. J Clin Neurosci. 2003;10(2):245-248.
4. Sharma RR, Pawar SJ, Delmendo A, Mahapatra AK. Symptomatic epineural ganglion cyst of the ulnar nerve in the cubital tunnel: a case report and brief review of the literature. J Clin Neurosci. 2000;7(6):542-543.
5. Hashizume H, Nishida K, Nanba Y, Inoue H, Konishiike T. Intraneural ganglion of the posterior interosseous nerve with lateral elbow pain. J Hand Surg Br. 1995;20(5):649-651.
6. Kato H, Hirayama T, Minami A, Iwasaki N, Hirachi K. Cubital tunnel syndrome associated with medial elbow Ganglia and osteoarthritis of the elbow. J Bone Joint Surg Am. 2002;84(8):1413-1419.
7. Bowen TL, Stone KH. Posterior interosseous nerve paralysis caused by a ganglion at the elbow. J Bone Joint Surg Br. 1966;48(4):774-776.
8. Ly JQ, Barrett TJ, Beall DP, Bertagnolli R. MRI diagnosis of occult ganglion compression of the posterior interosseous nerve and associated supinator muscle pathology. Clin Imaging. 2005;29(5):362-363.
9. McCollam SM, Corley FG, Green DP. Posterior interosseous nerve palsy caused by ganglions of the proximal radioulnar joint. J Hand Surg Am. 1988;13(5):725-728.
10. Tonkin MA. Posterior interosseous nerve axonotmesis from compression by a ganglion. J Hand Surg Br. 1990;15(4):491-493.
11. Tuygun H, Kose O, Gorgec M. Partial paralysis of the posterior interosseous nerve caused by a ganglion. J Hand Surg Eur. 2008;33(4):540-541.
12. Yamazaki H, Kato H, Hata Y, Murakami N, Saitoh S. The two locations of ganglions causing radial nerve palsy. J Hand Surg Eur. 2007;32(3):341-345.
13. Jou IM, Wang HN, Wang PH, Yong IS, Su WR. Compression of the radial nerve at the elbow by a ganglion: two case reports. J Med Case Rep. 2009;3:7258.
14. Hermansdorfer JD, Greider JL, Dell PC. A case report of a compressive neuropathy of the radial sensory nerve caused by a ganglion cyst at the elbow. Orthopedics. 1986;9(7):1005-1006.
15. McFarlane J, Trehan R, Olivera M, Jones C, Blease S, Davey P. A ganglion cyst at the elbow causing superficial radial nerve compression: a case report. J Med Case Rep. 2008;2:122.
16. Mileti J, Largacha M, O’Driscoll SW. Radial tunnel syndrome caused by ganglion cyst: treatment by arthroscopic cyst decompression. Arthroscopy. 2004;20(5):e39-e44.
17. Plancher KD, Peterson RK, Steichen JB. Compressive neuropathies and tendinopathies in the athletic elbow and wrist. Clin Sports Med. 1996;15(2):331-371.
18. Chim H, Yam AK, Teoh LC. Elbow ganglion arising from medial epicondyle pseudarthrosis. Hand Surg. 2007;12(3):155-158.
19. Broomhead IW. Ganglia associated with elbow and knee joints. Lancet. 1955;269(6885):317-319.
20. Ogino T, Minami A, Kato H. Diagnosis of radial nerve palsy caused by ganglion with use of different imaging techniques. J Hand Surg Am. 1991;16(2):230-235.
21. Spinner M, Spencer PS. Nerve compression lesions of the upper extremity. A clinical and experimental review. Clin Orthop Relat Res. 1974;(104):46-67.
22. Feldman MD. Arthroscopic excision of a ganglion cyst from the elbow. Arthroscopy. 2000;16(6):661-664.
23. Kirpalani PA, Lee HK, Lee YS, Han CW. Transarticular arthroscopic excision of an elbow cyst. Acta Orthop Belg. 2005;71(4):477-480.
Ganglion cysts around the elbow joint are unusual, with fewer than 25 citations (most of which are case reports) in the English-language literature. Among the many causes of elbow pain, cysts are chiefly diagnosed by advanced imaging. When an elbow ganglion or perineural cyst is symptomatic, treatment has ranged from nonoperative to surgical intervention. Our case report is the first documented ultrasound-guided aspiration and cortisone injection to successfully alleviate a patient’s symptoms. The procedures and outcomes of minimally invasive ultrasound-guided aspiration and steroid injections have not been described for cysts around the elbow. The patient and patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 14-year-old female freshman varsity softball pitcher on multiple teams presented with 6 months of vague right elbow pain. She was unable to pitch and had intermittent sharp pain localized to the lateral proximal forearm. She was, however, able to bat without pain and denied any radiating paresthesias. Despite a reduction in sports activities, the symptoms did not improve.
On physical examination, there was preserved strength that was symmetric with the contralateral side of all major muscles innervated by the radial nerve in the right arm, including full wrist, thumb, and finger extension. Sensation was intact to light touch in all major nervous distributions of the right and left upper extremities. She was tender to palpation at the radiocapitellar joint anteriorly, as well as just distally. The patient was also tender with motion through the proximal radial head. She had pain with resisted finger extension; however, resisted supination elicited no discomfort or pain.
The initial diagnostic workup included radiographs of the right elbow, a magnetic resonance imaging (MRI) scan, and an ultrasound. Elbow radiographs revealed no abnormalities. The MRI scan showed a well-circumscribed ovoid T2-hyperintense structure within the supinator muscle measuring 0.6×0.6×0.4 cm (longitudinal × anteroposterior × transverse), just deep to the split of the superficial and deep radial nerves (Figures 1A-1C). A musculoskeletal ultrasound was performed to further characterize and determine the relationship to neurovascular structures. Longitudinal (Figure 2A) and transverse (Figure 2B) images showed a hypoechoic cystic structure, separate from any local nerve, and without Doppler flow, consistent with what was seen on MRI. Additionally, there was an apparent stalk communicating with the anterior margin of the radiocapitellar articulation, seen on longitudinal images, suggesting an extension of the joint capsule (Figure 3A).
We diagnosed the patient with a radiocapitellar ganglion cyst. Her symptoms continued despite several sessions of physical therapy and cessation from all throwing. Given the ultrasound and MRI findings, and continuation of the symptoms despite conservative treatment, alternative treatment plans were discussed with the patient. These included continued activity modification and nonoperative treatment, open excision of the cyst, or aspiration of the cyst under ultrasound guidance. All appropriate risks and benefits were discussed, including possibility of nerve damage given the proximity of the cyst to the radial nerve branches. After a thorough discussion with both patient and family, a plan was made to undergo aspiration under ultrasound guidance. This was carried out using a lateral-to-medial in-plane approach, transverse to the radius. Using a 19-g, 1.5-inch needle (Figure 3B), 1 mL of serosanguinous fluid was aspirated from the cyst, followed by injection of 40 mg methylprednisolone sodium succinate.
The patient made a dramatic recovery within 8 days after aspiration. On examination, she had full strength to resisted flexion, extension, pronation, and supination; had no tenderness to palpation over the supinator; and no pain with resisted finger extension. She began dedicated physical therapy and a gradual return to throwing. She was able to return to her original level of softball activities 2 months after the aspiration. The patient continued to be symptom-free 26 months after the aspiration/injection. There was no evidence of recurrence of the ganglion on repeat ultrasound at her most recent follow-up (Figures 4A, 4B).
Discussion
Our review of the English-language literature identified 23 reports of cysts in and around the supinator muscle. Ganglion cysts are benign lesions that are uncommonly seen about the elbow. This highlights the rarity of this diagnosis, as well as the need for recognition of its existence. Cysts located in the substance of the nerve1-5 and extraneural ganglia causing symptomatic nerve compression have been described. These extraneural ganglia have been reported to cause compression of the ulnar nerve,1-4,6 posterior interosseous nerve (PIN),5,7-12 and radial nerve,13 and isolated compression of the radial sensory branch.14-17 Ganglion cyst compression in the elbow can result in pain, decreased motor function, and decreased sensation. The PIN syndrome is primarily a motor deficiency, whereas isolated compression of the sensory branches of the radial nerve presents as pain along the radial tunnel and extensor muscle mass.17
Most ganglion cysts are formed when joint fluid extrudes through a defect in the joint capsule; they have also been described originating from a nonunion site.18 When conservative treatment fails, surgical excision has been recommended.5,6,8-10,12-16 We present the first known case of successful ultrasound-guided aspiration and injection of a ganglion cyst from the proximal radiocapitellar joint.
In the earliest described case in 1955, Broomhead19 noted exploration was essential to establish the diagnosis of nerve palsy. In 1966, Bowen and Stone7 were the first to report PIN compression by a ganglion and that compression was likely where nerves pass through confined spaces. In keeping with the known potential for compression of the common peroneal nerve around the fibular head, Bowen and Stone7 posited that the same could be true of the PIN coursing through the supinator and around the radial neck.
Many authors have noted that nerve palsy either improves with rest or worsens with heavy manual work.3,20,21 These observations suggest that dynamic factors in addition to compression of the nerve by the ganglion may influence the occurrence of the nerve palsy.14 This is in line with our patient whose symptoms worsened after pitching.
Ogino and colleagues20 reported on the first use of ultrasonography as a screening examination for a ganglion, particularly when palpation was difficult. Ultrasound allows a detailed assessment of peripheral nerve continuity with a mass, differentiating an intraneural lesion from an adjacent extrinsic ganglion.13 Tonkin10 published the first description of MRI used for the diagnosis of an elbow cyst, and its use has been supported by others.5,8,20 The typical appearance of ganglion cysts on MRI include low signal on T1-weighted images and very high signal on T2-weighted images. Only the periphery of the mass is enhanced by gadolinium, if used.
As recently as 2009, Jou and associates13 suggested that surgical excision should be performed promptly to ensure optimal recovery from a nerve palsy. Many authors agree that early diagnosis and careful surgical excision is associated with a satisfactory outcome without recurrence of the cyst.5,6,8-10,12-15 There are only 4 published case reports14-17 of ganglions causing isolated compression of the superficial radial sensory nerve, as in our case. Their patients had pain with exertional trauma14 as did our patient, a positive Tinel sign,15 and resolution of symptoms after surgical excision without recurrence.14-16 Mileti and colleagues16 state that standard management for resistant radial tunnel syndrome is open decompression of the radial nerve.
In the last decade, a few reports of arthroscopic excision being a viable and safe alternative to open excision have been published.16,22,23 In 2000, Feldman22 described the benefits of an arthroscopic approach as decreased soft-tissue dissection, increased ability to identify intra-articular pathology, and similar recurrence rates to open procedures. He reported 1 transient neurapraxia of the superficial radial nerve from the arthroscopy, highlighting a risk of arthroscopic treatment.
An alternative to open or arthroscopic cyst decompression is aspiration. The only mention of aspiration in the literature comes from Broomhead19 in 1955 when he described 2 patients in whom treatment by aspiration was unsuccessful in relieving their symptoms. Yamazaki and colleagues12 noted that 1 of their 14 patients with PIN palsies caused by ganglions at the elbow underwent puncture of the ganglion with recovery of the paralysis. With the aid of ultrasound guidance, we were able to accurately locate the ganglion cyst, aspirate its contents, and inject methylprednisolone sodium succinate. Our patient continued to be symptom-free and was an active pitcher on a varsity softball team 26 months after aspiration.
Conclusion
This case report describes a rare location for a ganglion cyst in a high-level softball player. To our knowledge, successful treatment with ultrasound-guided aspiration and injection of a supinator cyst has not been reported in the literature. This case report highlights the importance of a careful diagnosis of this condition and an alternative treatment algorithm.
Ganglion cysts around the elbow joint are unusual, with fewer than 25 citations (most of which are case reports) in the English-language literature. Among the many causes of elbow pain, cysts are chiefly diagnosed by advanced imaging. When an elbow ganglion or perineural cyst is symptomatic, treatment has ranged from nonoperative to surgical intervention. Our case report is the first documented ultrasound-guided aspiration and cortisone injection to successfully alleviate a patient’s symptoms. The procedures and outcomes of minimally invasive ultrasound-guided aspiration and steroid injections have not been described for cysts around the elbow. The patient and patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 14-year-old female freshman varsity softball pitcher on multiple teams presented with 6 months of vague right elbow pain. She was unable to pitch and had intermittent sharp pain localized to the lateral proximal forearm. She was, however, able to bat without pain and denied any radiating paresthesias. Despite a reduction in sports activities, the symptoms did not improve.
On physical examination, there was preserved strength that was symmetric with the contralateral side of all major muscles innervated by the radial nerve in the right arm, including full wrist, thumb, and finger extension. Sensation was intact to light touch in all major nervous distributions of the right and left upper extremities. She was tender to palpation at the radiocapitellar joint anteriorly, as well as just distally. The patient was also tender with motion through the proximal radial head. She had pain with resisted finger extension; however, resisted supination elicited no discomfort or pain.
The initial diagnostic workup included radiographs of the right elbow, a magnetic resonance imaging (MRI) scan, and an ultrasound. Elbow radiographs revealed no abnormalities. The MRI scan showed a well-circumscribed ovoid T2-hyperintense structure within the supinator muscle measuring 0.6×0.6×0.4 cm (longitudinal × anteroposterior × transverse), just deep to the split of the superficial and deep radial nerves (Figures 1A-1C). A musculoskeletal ultrasound was performed to further characterize and determine the relationship to neurovascular structures. Longitudinal (Figure 2A) and transverse (Figure 2B) images showed a hypoechoic cystic structure, separate from any local nerve, and without Doppler flow, consistent with what was seen on MRI. Additionally, there was an apparent stalk communicating with the anterior margin of the radiocapitellar articulation, seen on longitudinal images, suggesting an extension of the joint capsule (Figure 3A).
We diagnosed the patient with a radiocapitellar ganglion cyst. Her symptoms continued despite several sessions of physical therapy and cessation from all throwing. Given the ultrasound and MRI findings, and continuation of the symptoms despite conservative treatment, alternative treatment plans were discussed with the patient. These included continued activity modification and nonoperative treatment, open excision of the cyst, or aspiration of the cyst under ultrasound guidance. All appropriate risks and benefits were discussed, including possibility of nerve damage given the proximity of the cyst to the radial nerve branches. After a thorough discussion with both patient and family, a plan was made to undergo aspiration under ultrasound guidance. This was carried out using a lateral-to-medial in-plane approach, transverse to the radius. Using a 19-g, 1.5-inch needle (Figure 3B), 1 mL of serosanguinous fluid was aspirated from the cyst, followed by injection of 40 mg methylprednisolone sodium succinate.
The patient made a dramatic recovery within 8 days after aspiration. On examination, she had full strength to resisted flexion, extension, pronation, and supination; had no tenderness to palpation over the supinator; and no pain with resisted finger extension. She began dedicated physical therapy and a gradual return to throwing. She was able to return to her original level of softball activities 2 months after the aspiration. The patient continued to be symptom-free 26 months after the aspiration/injection. There was no evidence of recurrence of the ganglion on repeat ultrasound at her most recent follow-up (Figures 4A, 4B).
Discussion
Our review of the English-language literature identified 23 reports of cysts in and around the supinator muscle. Ganglion cysts are benign lesions that are uncommonly seen about the elbow. This highlights the rarity of this diagnosis, as well as the need for recognition of its existence. Cysts located in the substance of the nerve1-5 and extraneural ganglia causing symptomatic nerve compression have been described. These extraneural ganglia have been reported to cause compression of the ulnar nerve,1-4,6 posterior interosseous nerve (PIN),5,7-12 and radial nerve,13 and isolated compression of the radial sensory branch.14-17 Ganglion cyst compression in the elbow can result in pain, decreased motor function, and decreased sensation. The PIN syndrome is primarily a motor deficiency, whereas isolated compression of the sensory branches of the radial nerve presents as pain along the radial tunnel and extensor muscle mass.17
Most ganglion cysts are formed when joint fluid extrudes through a defect in the joint capsule; they have also been described originating from a nonunion site.18 When conservative treatment fails, surgical excision has been recommended.5,6,8-10,12-16 We present the first known case of successful ultrasound-guided aspiration and injection of a ganglion cyst from the proximal radiocapitellar joint.
In the earliest described case in 1955, Broomhead19 noted exploration was essential to establish the diagnosis of nerve palsy. In 1966, Bowen and Stone7 were the first to report PIN compression by a ganglion and that compression was likely where nerves pass through confined spaces. In keeping with the known potential for compression of the common peroneal nerve around the fibular head, Bowen and Stone7 posited that the same could be true of the PIN coursing through the supinator and around the radial neck.
Many authors have noted that nerve palsy either improves with rest or worsens with heavy manual work.3,20,21 These observations suggest that dynamic factors in addition to compression of the nerve by the ganglion may influence the occurrence of the nerve palsy.14 This is in line with our patient whose symptoms worsened after pitching.
Ogino and colleagues20 reported on the first use of ultrasonography as a screening examination for a ganglion, particularly when palpation was difficult. Ultrasound allows a detailed assessment of peripheral nerve continuity with a mass, differentiating an intraneural lesion from an adjacent extrinsic ganglion.13 Tonkin10 published the first description of MRI used for the diagnosis of an elbow cyst, and its use has been supported by others.5,8,20 The typical appearance of ganglion cysts on MRI include low signal on T1-weighted images and very high signal on T2-weighted images. Only the periphery of the mass is enhanced by gadolinium, if used.
As recently as 2009, Jou and associates13 suggested that surgical excision should be performed promptly to ensure optimal recovery from a nerve palsy. Many authors agree that early diagnosis and careful surgical excision is associated with a satisfactory outcome without recurrence of the cyst.5,6,8-10,12-15 There are only 4 published case reports14-17 of ganglions causing isolated compression of the superficial radial sensory nerve, as in our case. Their patients had pain with exertional trauma14 as did our patient, a positive Tinel sign,15 and resolution of symptoms after surgical excision without recurrence.14-16 Mileti and colleagues16 state that standard management for resistant radial tunnel syndrome is open decompression of the radial nerve.
In the last decade, a few reports of arthroscopic excision being a viable and safe alternative to open excision have been published.16,22,23 In 2000, Feldman22 described the benefits of an arthroscopic approach as decreased soft-tissue dissection, increased ability to identify intra-articular pathology, and similar recurrence rates to open procedures. He reported 1 transient neurapraxia of the superficial radial nerve from the arthroscopy, highlighting a risk of arthroscopic treatment.
An alternative to open or arthroscopic cyst decompression is aspiration. The only mention of aspiration in the literature comes from Broomhead19 in 1955 when he described 2 patients in whom treatment by aspiration was unsuccessful in relieving their symptoms. Yamazaki and colleagues12 noted that 1 of their 14 patients with PIN palsies caused by ganglions at the elbow underwent puncture of the ganglion with recovery of the paralysis. With the aid of ultrasound guidance, we were able to accurately locate the ganglion cyst, aspirate its contents, and inject methylprednisolone sodium succinate. Our patient continued to be symptom-free and was an active pitcher on a varsity softball team 26 months after aspiration.
Conclusion
This case report describes a rare location for a ganglion cyst in a high-level softball player. To our knowledge, successful treatment with ultrasound-guided aspiration and injection of a supinator cyst has not been reported in the literature. This case report highlights the importance of a careful diagnosis of this condition and an alternative treatment algorithm.
1. Boursinos LA, Dimitriou CG. Ulnar nerve compression in the cubital tunnel by an epineural ganglion: a case report. Hand (N Y). 2007;2(1):12-15.
2. Ferlic DC, Ries MD. Epineural ganglion of the ulnar nerve at the elbow. J Hand Surg Am. 1990;15(6):996-998.
3. Ming Chan K, Thompson S, Amirjani N, Satkunam L, Strohschlein FJ, Lobay GL. Compression of the ulnar nerve at the elbow by an intraneural ganglion. J Clin Neurosci. 2003;10(2):245-248.
4. Sharma RR, Pawar SJ, Delmendo A, Mahapatra AK. Symptomatic epineural ganglion cyst of the ulnar nerve in the cubital tunnel: a case report and brief review of the literature. J Clin Neurosci. 2000;7(6):542-543.
5. Hashizume H, Nishida K, Nanba Y, Inoue H, Konishiike T. Intraneural ganglion of the posterior interosseous nerve with lateral elbow pain. J Hand Surg Br. 1995;20(5):649-651.
6. Kato H, Hirayama T, Minami A, Iwasaki N, Hirachi K. Cubital tunnel syndrome associated with medial elbow Ganglia and osteoarthritis of the elbow. J Bone Joint Surg Am. 2002;84(8):1413-1419.
7. Bowen TL, Stone KH. Posterior interosseous nerve paralysis caused by a ganglion at the elbow. J Bone Joint Surg Br. 1966;48(4):774-776.
8. Ly JQ, Barrett TJ, Beall DP, Bertagnolli R. MRI diagnosis of occult ganglion compression of the posterior interosseous nerve and associated supinator muscle pathology. Clin Imaging. 2005;29(5):362-363.
9. McCollam SM, Corley FG, Green DP. Posterior interosseous nerve palsy caused by ganglions of the proximal radioulnar joint. J Hand Surg Am. 1988;13(5):725-728.
10. Tonkin MA. Posterior interosseous nerve axonotmesis from compression by a ganglion. J Hand Surg Br. 1990;15(4):491-493.
11. Tuygun H, Kose O, Gorgec M. Partial paralysis of the posterior interosseous nerve caused by a ganglion. J Hand Surg Eur. 2008;33(4):540-541.
12. Yamazaki H, Kato H, Hata Y, Murakami N, Saitoh S. The two locations of ganglions causing radial nerve palsy. J Hand Surg Eur. 2007;32(3):341-345.
13. Jou IM, Wang HN, Wang PH, Yong IS, Su WR. Compression of the radial nerve at the elbow by a ganglion: two case reports. J Med Case Rep. 2009;3:7258.
14. Hermansdorfer JD, Greider JL, Dell PC. A case report of a compressive neuropathy of the radial sensory nerve caused by a ganglion cyst at the elbow. Orthopedics. 1986;9(7):1005-1006.
15. McFarlane J, Trehan R, Olivera M, Jones C, Blease S, Davey P. A ganglion cyst at the elbow causing superficial radial nerve compression: a case report. J Med Case Rep. 2008;2:122.
16. Mileti J, Largacha M, O’Driscoll SW. Radial tunnel syndrome caused by ganglion cyst: treatment by arthroscopic cyst decompression. Arthroscopy. 2004;20(5):e39-e44.
17. Plancher KD, Peterson RK, Steichen JB. Compressive neuropathies and tendinopathies in the athletic elbow and wrist. Clin Sports Med. 1996;15(2):331-371.
18. Chim H, Yam AK, Teoh LC. Elbow ganglion arising from medial epicondyle pseudarthrosis. Hand Surg. 2007;12(3):155-158.
19. Broomhead IW. Ganglia associated with elbow and knee joints. Lancet. 1955;269(6885):317-319.
20. Ogino T, Minami A, Kato H. Diagnosis of radial nerve palsy caused by ganglion with use of different imaging techniques. J Hand Surg Am. 1991;16(2):230-235.
21. Spinner M, Spencer PS. Nerve compression lesions of the upper extremity. A clinical and experimental review. Clin Orthop Relat Res. 1974;(104):46-67.
22. Feldman MD. Arthroscopic excision of a ganglion cyst from the elbow. Arthroscopy. 2000;16(6):661-664.
23. Kirpalani PA, Lee HK, Lee YS, Han CW. Transarticular arthroscopic excision of an elbow cyst. Acta Orthop Belg. 2005;71(4):477-480.
1. Boursinos LA, Dimitriou CG. Ulnar nerve compression in the cubital tunnel by an epineural ganglion: a case report. Hand (N Y). 2007;2(1):12-15.
2. Ferlic DC, Ries MD. Epineural ganglion of the ulnar nerve at the elbow. J Hand Surg Am. 1990;15(6):996-998.
3. Ming Chan K, Thompson S, Amirjani N, Satkunam L, Strohschlein FJ, Lobay GL. Compression of the ulnar nerve at the elbow by an intraneural ganglion. J Clin Neurosci. 2003;10(2):245-248.
4. Sharma RR, Pawar SJ, Delmendo A, Mahapatra AK. Symptomatic epineural ganglion cyst of the ulnar nerve in the cubital tunnel: a case report and brief review of the literature. J Clin Neurosci. 2000;7(6):542-543.
5. Hashizume H, Nishida K, Nanba Y, Inoue H, Konishiike T. Intraneural ganglion of the posterior interosseous nerve with lateral elbow pain. J Hand Surg Br. 1995;20(5):649-651.
6. Kato H, Hirayama T, Minami A, Iwasaki N, Hirachi K. Cubital tunnel syndrome associated with medial elbow Ganglia and osteoarthritis of the elbow. J Bone Joint Surg Am. 2002;84(8):1413-1419.
7. Bowen TL, Stone KH. Posterior interosseous nerve paralysis caused by a ganglion at the elbow. J Bone Joint Surg Br. 1966;48(4):774-776.
8. Ly JQ, Barrett TJ, Beall DP, Bertagnolli R. MRI diagnosis of occult ganglion compression of the posterior interosseous nerve and associated supinator muscle pathology. Clin Imaging. 2005;29(5):362-363.
9. McCollam SM, Corley FG, Green DP. Posterior interosseous nerve palsy caused by ganglions of the proximal radioulnar joint. J Hand Surg Am. 1988;13(5):725-728.
10. Tonkin MA. Posterior interosseous nerve axonotmesis from compression by a ganglion. J Hand Surg Br. 1990;15(4):491-493.
11. Tuygun H, Kose O, Gorgec M. Partial paralysis of the posterior interosseous nerve caused by a ganglion. J Hand Surg Eur. 2008;33(4):540-541.
12. Yamazaki H, Kato H, Hata Y, Murakami N, Saitoh S. The two locations of ganglions causing radial nerve palsy. J Hand Surg Eur. 2007;32(3):341-345.
13. Jou IM, Wang HN, Wang PH, Yong IS, Su WR. Compression of the radial nerve at the elbow by a ganglion: two case reports. J Med Case Rep. 2009;3:7258.
14. Hermansdorfer JD, Greider JL, Dell PC. A case report of a compressive neuropathy of the radial sensory nerve caused by a ganglion cyst at the elbow. Orthopedics. 1986;9(7):1005-1006.
15. McFarlane J, Trehan R, Olivera M, Jones C, Blease S, Davey P. A ganglion cyst at the elbow causing superficial radial nerve compression: a case report. J Med Case Rep. 2008;2:122.
16. Mileti J, Largacha M, O’Driscoll SW. Radial tunnel syndrome caused by ganglion cyst: treatment by arthroscopic cyst decompression. Arthroscopy. 2004;20(5):e39-e44.
17. Plancher KD, Peterson RK, Steichen JB. Compressive neuropathies and tendinopathies in the athletic elbow and wrist. Clin Sports Med. 1996;15(2):331-371.
18. Chim H, Yam AK, Teoh LC. Elbow ganglion arising from medial epicondyle pseudarthrosis. Hand Surg. 2007;12(3):155-158.
19. Broomhead IW. Ganglia associated with elbow and knee joints. Lancet. 1955;269(6885):317-319.
20. Ogino T, Minami A, Kato H. Diagnosis of radial nerve palsy caused by ganglion with use of different imaging techniques. J Hand Surg Am. 1991;16(2):230-235.
21. Spinner M, Spencer PS. Nerve compression lesions of the upper extremity. A clinical and experimental review. Clin Orthop Relat Res. 1974;(104):46-67.
22. Feldman MD. Arthroscopic excision of a ganglion cyst from the elbow. Arthroscopy. 2000;16(6):661-664.
23. Kirpalani PA, Lee HK, Lee YS, Han CW. Transarticular arthroscopic excision of an elbow cyst. Acta Orthop Belg. 2005;71(4):477-480.
Emergency Imaging
Case
A 41-year-old woman presented to the ED with a 3-day history of posterior neck pain that radiated to the occipital region. The patient stated that the day prior to presentation, the pain had worsened to what she rated as a “9” on a pain scale of 0 to 10. The patient’s surgical history included a remote prior cervical fusion at C5-C6. A lateral radiograph of the cervical spine is shown above (Figure 1a).
What is the diagnosis? Is additional imaging necessary? If so, why?
Answer
The lateral view of the cervical spine demonstrated soft tissue swelling in the upper prevertebral region (red arrowheads, Figure 1b). (The soft tissues at this level in an adult normally measure less than 3 mm between the airway and the vertebral body.) The radiograph also showed an area of calcification within the prevertebral soft tissues (white arrow, Figure 1b).
The patient denied fevers, chills, dysphagia, visual changes, and nasal congestion. Physical examination demonstrated tenderness to palpation of the posterior neck, but there was no evidence of palpable mass or lymphadenopathy. Neck extension and lateral movement to the left were severely limited due to pain; neck flexion and lateral movement range of motion to the right were mildly decreased due to pain. The physical examination was otherwise normal.
Longus colli (prevertebral) calcific tendinitis is a self-limiting condition that typically lasts 1 to 3 weeks, though it can be very painful during the acute phase.1,2 Treatment is typically conservative, and the patient in this case received a course of oral corticosteroids and nonsteroidal anti-inflammatory drugs to help alleviate the associated inflammation.
Dr Bartolotta is an assistant professor of radiology at Weill Cornell Medical College in New York City and assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical Center. Dr Baradaran is a resident in the department of radiology at Weill Cornell Medical College in New York City. Dr Hentel is an associate professor of clinical radiology at Weill Cornell Medical College, New York. He is also chief of emergency/musculoskeletal imaging and executive vice-chairman for the department of radiology at New York-Presbyterian Hospital/Weill Cornell Medical Center; and associate editor, imaging, of the EMERGENCY MEDICINE editorial board.
- Coulier B, Macsim M, Desgain O. Retropharyngeal calcific tendinitis--longus colli tendinitis--an unusual cause of acute dysphagia. Emerg Radiol. 2011;18(5):449-451.
- Silva CF, Soffia PS, Pruzzo E. Acute prevertebral calcific tendinitis: a source of non-surgical acute cervical pain. Acta Radiologica. 2014;55(1):91-94.
- Zibis AH, Giannis D, Malizos KN, Kitsioulis P, Arvanitis DL. Acute calcific tendinitis of the longus colli muscle: case report and review of the literature. Eur Spine J. 2013;22(Suppl 3):S434-S438.
Case
A 41-year-old woman presented to the ED with a 3-day history of posterior neck pain that radiated to the occipital region. The patient stated that the day prior to presentation, the pain had worsened to what she rated as a “9” on a pain scale of 0 to 10. The patient’s surgical history included a remote prior cervical fusion at C5-C6. A lateral radiograph of the cervical spine is shown above (Figure 1a).
What is the diagnosis? Is additional imaging necessary? If so, why?
Answer
The lateral view of the cervical spine demonstrated soft tissue swelling in the upper prevertebral region (red arrowheads, Figure 1b). (The soft tissues at this level in an adult normally measure less than 3 mm between the airway and the vertebral body.) The radiograph also showed an area of calcification within the prevertebral soft tissues (white arrow, Figure 1b).
The patient denied fevers, chills, dysphagia, visual changes, and nasal congestion. Physical examination demonstrated tenderness to palpation of the posterior neck, but there was no evidence of palpable mass or lymphadenopathy. Neck extension and lateral movement to the left were severely limited due to pain; neck flexion and lateral movement range of motion to the right were mildly decreased due to pain. The physical examination was otherwise normal.
Longus colli (prevertebral) calcific tendinitis is a self-limiting condition that typically lasts 1 to 3 weeks, though it can be very painful during the acute phase.1,2 Treatment is typically conservative, and the patient in this case received a course of oral corticosteroids and nonsteroidal anti-inflammatory drugs to help alleviate the associated inflammation.
Dr Bartolotta is an assistant professor of radiology at Weill Cornell Medical College in New York City and assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical Center. Dr Baradaran is a resident in the department of radiology at Weill Cornell Medical College in New York City. Dr Hentel is an associate professor of clinical radiology at Weill Cornell Medical College, New York. He is also chief of emergency/musculoskeletal imaging and executive vice-chairman for the department of radiology at New York-Presbyterian Hospital/Weill Cornell Medical Center; and associate editor, imaging, of the EMERGENCY MEDICINE editorial board.
Case
A 41-year-old woman presented to the ED with a 3-day history of posterior neck pain that radiated to the occipital region. The patient stated that the day prior to presentation, the pain had worsened to what she rated as a “9” on a pain scale of 0 to 10. The patient’s surgical history included a remote prior cervical fusion at C5-C6. A lateral radiograph of the cervical spine is shown above (Figure 1a).
What is the diagnosis? Is additional imaging necessary? If so, why?
Answer
The lateral view of the cervical spine demonstrated soft tissue swelling in the upper prevertebral region (red arrowheads, Figure 1b). (The soft tissues at this level in an adult normally measure less than 3 mm between the airway and the vertebral body.) The radiograph also showed an area of calcification within the prevertebral soft tissues (white arrow, Figure 1b).
The patient denied fevers, chills, dysphagia, visual changes, and nasal congestion. Physical examination demonstrated tenderness to palpation of the posterior neck, but there was no evidence of palpable mass or lymphadenopathy. Neck extension and lateral movement to the left were severely limited due to pain; neck flexion and lateral movement range of motion to the right were mildly decreased due to pain. The physical examination was otherwise normal.
Longus colli (prevertebral) calcific tendinitis is a self-limiting condition that typically lasts 1 to 3 weeks, though it can be very painful during the acute phase.1,2 Treatment is typically conservative, and the patient in this case received a course of oral corticosteroids and nonsteroidal anti-inflammatory drugs to help alleviate the associated inflammation.
Dr Bartolotta is an assistant professor of radiology at Weill Cornell Medical College in New York City and assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical Center. Dr Baradaran is a resident in the department of radiology at Weill Cornell Medical College in New York City. Dr Hentel is an associate professor of clinical radiology at Weill Cornell Medical College, New York. He is also chief of emergency/musculoskeletal imaging and executive vice-chairman for the department of radiology at New York-Presbyterian Hospital/Weill Cornell Medical Center; and associate editor, imaging, of the EMERGENCY MEDICINE editorial board.
- Coulier B, Macsim M, Desgain O. Retropharyngeal calcific tendinitis--longus colli tendinitis--an unusual cause of acute dysphagia. Emerg Radiol. 2011;18(5):449-451.
- Silva CF, Soffia PS, Pruzzo E. Acute prevertebral calcific tendinitis: a source of non-surgical acute cervical pain. Acta Radiologica. 2014;55(1):91-94.
- Zibis AH, Giannis D, Malizos KN, Kitsioulis P, Arvanitis DL. Acute calcific tendinitis of the longus colli muscle: case report and review of the literature. Eur Spine J. 2013;22(Suppl 3):S434-S438.
- Coulier B, Macsim M, Desgain O. Retropharyngeal calcific tendinitis--longus colli tendinitis--an unusual cause of acute dysphagia. Emerg Radiol. 2011;18(5):449-451.
- Silva CF, Soffia PS, Pruzzo E. Acute prevertebral calcific tendinitis: a source of non-surgical acute cervical pain. Acta Radiologica. 2014;55(1):91-94.
- Zibis AH, Giannis D, Malizos KN, Kitsioulis P, Arvanitis DL. Acute calcific tendinitis of the longus colli muscle: case report and review of the literature. Eur Spine J. 2013;22(Suppl 3):S434-S438.
Arthroscopic Posterior-Inferior Capsular Release in the Treatment of Overhead Athletes
Glenohumeral internal rotation deficit (GIRD) can be observed in overhead athletes and is thought to play a role in generating pain and rotator cuff weakness in the dominant shoulder with sport. It is unclear what is an acceptable value of GIRD in a population of overhead athletes and whether it should be based solely on internal rotation deficit or should include total range of motion (ROM) deficit.1,2 Acquired GIRD in the athlete’s throwing shoulder has been thoroughly documented in the literature as a loss of internal rotation relative to the nonthrowing shoulder, with etiologies including bony adaptations (increased humeral retroversion), muscular tightness, and posterior capsular tightness.1,3-11 In particular, the repetitive torsional stresses acting on the throwing shoulder of baseball players is thought to produce, over the long term, structural adaptations such as increased humeral retroversion.5,12-14 Further, for shoulders with posterior-inferior capsular tightness, cadaveric studies have shown increased contact pressure at the coracoacromial arch during simulated follow-through.15 Athletes of other overhead and throwing sports, such as football, softball, tennis, and volleyball, may show similar adaptations in overhead motion.9,16,17
GIRD has been associated with a variety of pathologic conditions, including scapular dyskinesis, internal and secondary impingement, partial articular-sided rotator cuff tears, damage to the biceps–labral complex, and ulnar collateral ligament insufficiency.10,12,18-22
Restriction from engaging in exacerbating activities (eg, throwing) and compliance with a specific stretching program reduces or eliminates GIRD in the majority of cases.1,23-28 In the few cases in which conservative management fails, operative intervention may be indicated.1,23,29,30 Few investigators have detailed an operative technique for selective arthroscopic capsular release of the posterior-inferior capsule or evaluated the ability of athletes to return to sport after such surgery.
In this article, we present our technique for arthroscopic posterior-inferior capsular release and report the results of applying this technique in a population of athletes with symptomatic GIRD that was unresponsive to nonoperative treatment and was preventing them from returning to sport.
We hypothesized that selective arthroscopic surgical release of the posterior-inferior capsule would improve symptomatic GIRD and result in a return to sport in the majority of cases unresponsive to nonoperative treatment.
Materials and Methods
Patients
After obtaining institutional review board approval, we retrospectively reviewed patient charts and collected data. Study inclusion criteria were arthroscopic selective posterior-inferior capsular release between 2004 and 2008; failure to resume sport after minimum 3 months of physical therapy, including use of sleeper stretch, active joint mobilization by licensed physical therapist, and sport-specific restriction from exacerbating activities (eg, throwing for baseball players); and active participation in overhead sport.1,27 Exclusion criteria were generalized adhesive capsulitis, labral pathology producing glenohumeral joint instability (Bankart or reverse Bankart lesion), high-grade or full-thickness tearing of rotator cuff, and clinically significant partial-thickness tearing or instability of long head of biceps tendon.
Assessment
One of 3 authors (Dr. Buss, Dr. Codding, or Dr. Dahm) used a bubble goniometer to measure passive internal rotation. Patients were positioned supine with 90° of thoracohumeral abduction and 90° of elbow flexion. The examiner’s hand stabilized the scapula against the examination table, in accordance with published techniques.1,26 Active internal rotation was measured at 0° of thoracohumeral abduction by noting the most superior spinal segment reached. Before and after surgery, passive internal rotation measurements were taken on both arms. GIRD was determined by the difference between dominant and nondominant arm measurements; segmental differences were obtained by subtracting segments achieved between the dominant and nondominant arms.
Before surgery and at minimum 2-year follow-up after surgery, patients completed a subjective questionnaire, which included the American Shoulder and Elbow Surgeons (ASES) Standardized Shoulder Assessment Form, for assessment of both arms. ASES scores are reliable, valid, and responsive in evaluating shoulder pain and function.15,31 Patients also answered questions about their ability to return to play, their level of play after surgery, and whether they would undergo the procedure again.
Surgical Technique
After induction of general anesthesia and standard preparation and draping, the patient is placed in a standard beach-chair position and examined. Diagnostic arthroscopy is then performed. In all patients, intra-articular evaluation revealed a thickened, contracted posterior band of the inferior glenohumeral ligament. This finding is consistent with other studies of patients with significant GIRD.1,14,22,30
On completion of the diagnostic portion of the arthroscopy, attention is turned to the selective posterior-inferior capsular release. Key to proper execution of the release is establishing a posterior-inferior accessory portal. This is accomplished while viewing from a standard posterior (“soft spot”) portal and determining the appropriate location and angle of entry by spinal needle localization. Typically, an entry point is selected about 4 cm distal and 1 cm lateral to the standard posterior portal. An 18-gauge spinal needle introduced at this location is angled about 15° superiorly and about 20° medially. Once the appropriate vector is determined, a skin incision is made, and a Wissinger rod is introduced, over which a small-diameter cannula is passed. A hooked-tip electrocautery device is used to divide the posterior capsule from the glenoid labrum between the 8- and 6-o’clock positions in the right shoulder (Figure). Care is taken to perform the release immediately adjacent to the glenoid labrum and using short bursts of cautery in order to minimize risk of injury to the teres minor branch of the axillary nerve. Adequate release is confirmed by reassessing passive internal rotation under anesthesia. Additional procedures are performed, if necessary, after completion of the capsular release.
Postoperative rehabilitation consists initially of pendulum exercises and scapular retraction starting on postoperative day 1. Once the swelling from the surgical procedure subsides, typically within 1 week, passive and active-assisted ROM and gentle posterior capsular mobilization are initiated under the direction of a licensed physical therapist. Active ROM is allowed once the patient regains normal scapulothoracic rhythm. Strengthening consists initially of isometrics followed by light resistance strengthening for the rotator cuff and scapular stabilizers once active ROM and scapulothoracic rhythm return to normal. Passive internal rotation stretching, including use of the sleeper stretch, is implemented as soon as tolerated and continues throughout the rehabilitation process.32
Statistical Analysis
Statistical analysis was performed with Stata Release 11 (StataCorp, College Station, Texas). Paired t tests were used to assess preoperative and postoperative mean differences in ASES scores, in passive glenohumeral internal rotation, and in active glenohumeral internal rotation; independent-samples t tests were used to assess side-to-side differences. Significance was set at P < .05.
Results
Fifteen overhead athletes met the study inclusion criteria. Two were lost to follow-up. Of the remaining 13 patients, 6 underwent isolated arthroscopic posterior-inferior capsular release, and 7 had concomitant procedures (6 subacromial decompressions, 1 superior labrum anterior-posterior [SLAP] repair). There were 11 male athletes and 2 female athletes. Twelve of the 13 patients were right-hand–dominant. Mean age at time of surgery was 21 years (range, 16-33 years). There were 10 baseball players (6 pitchers, 4 position players); the other 3 patients played softball (1), volleyball (1), or tennis (1). Six patients played at high school level, 5 at college level, 1 at professional level, and 1 at amateur level. All 13 patients underwent a minimum of 3 months of comprehensive rehabilitation, which included use of the sleeper stretch, active joint mobilization by a licensed physical therapist, and sport-specific restriction from exacerbating activities. Mean duration of symptoms before surgery was 18 months (range, 4-48 months). Mean postoperative follow-up was 31 months (range, 24-59 months). Mean ASES score was 71.5 (range, 33-95) before surgery and 86.9 (range, 60-100) after surgery (P < .001). Mean GIRD improved from 43.1° (range, 30°-60°) before surgery to 9.7° (range, –7° to 40°) after surgery (P < .001). Mean active internal rotation difference improved from 3.8 vertebral segments before surgery to 2.6 vertebral segments after surgery; this difference was not statistically significant (P = .459). Ten (77%) of the 13 patients returned to their preoperative level of play or a higher level; the other 3 (23%) did not return to their preoperative level of play but continued to compete in a different position (Table). Eleven patients (85%) stated they would repeat the procedure. One of the 2 patients who would not repeat the procedure was in the isolated posterior-inferior capsular release group; the other was in the concomitant-procedure group (subacromial decompression). Total glenohumeral ROM of dominant arm was 122° before surgery and 136° after surgery (P = .04). There was no significant difference in total ROM between dominant and nondominant arms after surgery (136° and 141°; P = .12), but the preoperative difference was significant (122° vs 141°; P = .022).
Discussion
GIRD has been associated with various pathologic conditions of the upper extremity. In 1991, Verna28 found that a majority of 39 professional baseball pitchers with significant GIRD had shoulder problems that affected playing time. More recently, GIRD has been associated with a progression of injuries, including scapular dyskinesia, internal and secondary impingement, articular-sided partial rotator cuff tears, rotator cuff weakness, damage to the biceps–labral complex, and ulnar collateral ligament insufficiency.12,18-22 In a cadaveric study of humeral head translation, Harryman and colleagues33 noted an anterosuperior migration of the humeral head during flexion and concluded it resulted from a loose anterior and tight posterior glenohumeral capsule, leading to loss of glenohumeral internal rotation. More recently, posterosuperior migration of the humeral head has been postulated, with GIRD secondary to an essential posterior capsular contracture.1 Tyler and colleagues34 clinically linked posterior capsular tightness with GIRD, and both cadaveric and magnetic resonance imaging studies have supported the finding that posterior capsular contracture leads to posterosuperior humeral head migration in association with GIRD.14,20 Such a disruption in normal glenohumeral joint mechanics could produce phenomena of internal or secondary acromiohumeral impingement and pain.
More recently, in a large cohort of professional baseball pitchers, a significant correlation was found between the incidence of rotator cuff strength deficits and GIRD.35 More than 40% of the pitchers with GIRD of at least 35° had a measureable rotator cuff strength deficit in the throwing shoulder.
Burkhart and colleagues23 concluded that the shoulder most at risk for developing “dead arm” has GIRD and an advanced form of scapular dyskinesia known as SICK scapula (the phenomenon involves Scapula malposition, Inferior medial border prominence, Coracoid pain and malposition, and dysKinesis of scapular movement).
Most athletes with symptoms attributed to GIRD respond to conservative management. A posterior-inferior capsular stretching program focused on regaining internal rotation in the throwing arm has been shown to return about 90% of athletes to play.1 Numerous studies have indicated that enrollment in a compliant stretching program reduces GIRD.1,23-27 However, nonoperative treatment fails in a reported 10% of patients with GIRD; these patients may respond to operative treatment.1
More specifically, for patients who do not respond to conservative treatment, a posterior-inferior capsular release may be indicated.1,29 Ticker and colleagues22 identified 9 patients who had lost internal rotation and had a posterior capsular contracture at arthroscopy. That study, however, was not performed on overhead or throwing athletes. Yoneda and colleagues30 followed 16 overhead throwing athletes after arthroscopic posterior-inferior capsular release and found favorable preliminary clinical results. Eleven of the 16 patients returned to their preinjury level of performance; the other 5 returned to a lower level. In addition, all 4 patients who underwent isolated arthroscopic capsular release had throwing power restored to between 90% and 100%.
In the present study, 10 of 13 patients who underwent arthroscopic posterior-inferior capsular release returned to their preoperative level of play or a higher level. Mean passive GIRD improved significantly from before surgery to after surgery. ASES scores likewise were significantly improved from before surgery to after surgery. The active internal rotation difference as measured by vertebral segment level was not significantly changed after surgery. This lack of improvement may stem from the more complex musculoligamentous interactions governing active internal rotation versus isolated, passive internal rotation. Another possible explanation for lack of improvement is that the interobserver and intraobserver reliability of this method is lower.36
At 2-year follow-up, the patient who had undergone concomitant SLAP repair demonstrated a 23% improvement in ASES score and more internal rotation on the dominant arm relative to the nondominant arm. This patient returned to a level of play at least as good as his preoperative level. Although we could not determine its statistical significance, this patient’s improvement suggests that the SLAP repair did not reduce the efficacy of the posterior-inferior capsular release.
Limitations of this study include its relatively small cohort (precluded statistical comparisons between groups), the proportion of patients (7/13) who had concomitant surgeries, and the limited options for patient outcome scores. Although the ASES score is a validated outcome score, the Kerlan-Jobe Orthopaedic Clinic Shoulder and Elbow (KJOC) score or the Disabilities of the Arm, Shoulder, and Hand (DASH) score may be more appropriate in an athletic population. In addition, although all study patients had GIRD that was unresponsive to a concerted trial of nonoperative management, we did not have a control group (nonoperatively treated patients) for comparison. Finally, we did not obtain computed tomography scans or account for the potential contribution of humeral retroversion to GIRD in this group of patients.
Conclusion
Selective arthroscopic posterior-inferior capsular release can be recommended as a reasonable operative solution for overhead athletes with symptomatic GIRD that has not responded to conservative management. In the present study, ASES scores improved significantly, and 77% of our athlete-patients returned to sport at their preoperative level of play or a higher level.
1. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
2. Wilk KE, Macrina LC, Fleisig GS, et al. Correlation of glenohumeral internal rotation deficit and total rotational motion to shoulder injuries in professional baseball pitchers. Am J Sports Med. 2011;39(2):329-335.
3. Bigliani LU, Codd TP, Connor PM, Levine WN, Littlefield MA, Hershon SJ. Shoulder motion and laxity in the professional baseball player. Am J Sports Med. 1997;25(5):609-613.
4. Brown LP, Niehues SL, Harrah A, Yavorsky P, Hirshman HP. Upper extremity range of motion and isokinetic strength of the internal and external shoulder rotators in Major League baseball players. Am J Sports Med. 1988;16(6):577-585.
5. Crockett HC, Gross LB, Wilk KE, et al. Osseous adaptation and range of motion at the glenohumeral joint in professional baseball pitchers. Am J Sports Med. 2002;30(1):20-26.
6. Kibler WB, Chandler TJ, Livingston BP, Roetert EP. Shoulder range of motion in elite tennis players. Effect of age and years of tournament play. Am J Sports Med. 1996;24(3):279-285.
7. Meister K. Injuries to the shoulder in the throwing athlete. Part one: biomechanics/pathophysiology/classification of injury. Am J Sports Med. 2000;28(2):265-275.
8. Osbahr DC, Cannon DL, Speer KP. Retroversion of the humerus in the throwing shoulder of college baseball pitchers. Am J Sports Med. 2002;30(3):347-353.
9. Torres RR, Gomes JL. Measurement of glenohumeral internal rotation in asymptomatic tennis players and swimmers. Am J Sports Med. 2009;37(5):1017-1023.
10. Tyler TF, Nicholas SJ, Lee SJ, Mullaney M, McHugh MP. Correction of posterior shoulder tightness is associated with symptom resolution in patients with internal impingement. Am J Sports Med. 2010;28(1):114-119.
11. Wilk KE, Meister K, Andrews JR. Current concepts in the rehabilitation of the overhead throwing athlete. Am J Sports Med. 2002;30(1):136-151.
12. Braun S, Kokmeyer D, Millett PJ. Shoulder injuries in the throwing athlete. J Bone Joint Surg Am. 2009;91(4):966-978.
13. Reagan KM, Meister K, Horodyski MB, Werner DW, Carruthers C, Wilk K. Humeral retroversion and its relationship to glenohumeral rotation in the shoulder of college baseball players. Am J Sports Med. 2002;30(3):354-360.
14. Tehranzadeh AD, Fronek J, Resnick D. Posterior capsular fibrosis in professional baseball pitchers: case series of MR arthrographic findings in six patients with glenohumeral internal rotational deficit. Clin Imaging. 2007;31(5):343-348.
15. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
16. Curtis AS, Deshmukh R. Throwing injuries: diagnosis and treatment. Arthroscopy. 2003;19(suppl 1):80-85.
17. Lajtai G, Pfirrmann CW, Aitzetmuller G, Pirkl C, Gerber C, Jost B. The shoulders of fully competitive professional beach volleyball players: high prevalence of infraspinatus atrophy. Am J Sports Med. 2009;37(7):1375-1383.
18. Burkhart SS, Morgan CD. The peel-back mechanism: its role in producing and extending posterior type II SLAP lesions and its effect on SLAP repair rehabilitation. Arthroscopy. 1998;14(6):637-640.
19. Dines JS, Frank JB, Akerman M, Yocum LA. Glenohumeral internal rotation deficits in baseball players with ulnar collateral ligament insufficiency. Am J Sports Med. 2009;37(3):566-570.
20. Grossman MG, Tibone JE, McGarry MH, Schneider DJ, Veneziani S, Lee TQ. A cadaveric model of the throwing shoulder: a possible etiology of superior labrum anterior-to-posterior lesions. J Bone Joint Surg Am. 2005;87(4):824-831.
21. Myers JB, Laudner KG, Pasquale MR, Bradley JP, Lephart SM. Glenohumeral range of motion deficits and posterior shoulder tightness in throwers with pathologic internal impingement. Am J Sports Med. 2006;34(3):385-391.
22. Ticker JB, Beim GM, Warner JJ. Recognition and treatment of refractory posterior capsular contracture of the shoulder. Arthroscopy. 2000;16(1):27-34.
23. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology part III: the SICK scapula, scapular dyskinesis, the kinetic chain, and rehabilitation. Arthroscopy. 2003;19(6):641-661.
24. Kibler WB, McMullen J. Scapular dyskinesis and its relation to shoulder pain. J Am Acad Orthop Surg. 2003;11(2):142-151.
25. Kibler WB. The relationship of glenohumeral internal rotation deficit to shoulder and elbow injuries in tennis players: a prospective evaluation of posterior capsular stretching. Presented at: American Shoulder and Elbow Surgeons 15th Annual Closed Meeting; November 6, 1998; New York, NY.
26. Lintner D, Mayol M, Uzodinma O, Jones R, Labossiere D. Glenohumeral internal rotation deficits in professional pitchers enrolled in an internal rotation stretching program. Am J Sports Med. 2007;35(4):617-621.
27. McClure P, Balaicuis J, Heiland D, Broersma ME, Thorndike CK, Wood A. A randomized controlled comparison of stretching procedures for posterior shoulder tightness. J Orthop Sports Phys Ther. 2007;37(3):108-114.
28. Verna C. Shoulder flexibility to reduce impingement. Presented at: 3rd Annual Professional Baseball Athletic Trainer Society Meeting; March 1991; Mesa, AZ.
29. Bach HG, Goldberg BA. Posterior capsular contracture of the shoulder. J Am Acad Orthop Surg. 2006;14(5):265-277.
30. Yoneda M, Nakagawa S, Mizuno N, et al. Arthroscopic capsular release for painful throwing shoulder with posterior capsular tightness. Arthroscopy. 2006;22(7):801e1-801e5.
31. Kocher MS, Horan MP, Briggs KK, Richardson TR, O’Holleran J, Hawkins RJ. Reliability, validity, and responsiveness of the American Shoulder and Elbow Surgeons subjective shoulder scale in patients with shoulder instability, rotator cuff disease, and glenohumeral arthritis. J Bone Joint Surg Am. 2005;87(9):2006-2011.
32. Johansen RL, Callis M, Potts J, Shall LM. A modified internal rotation stretching technique for overhand and throwing athletes. J Orthop Sports Phys Ther. 1995;21(4):216-219.
33. Harryman DT 2nd, Sidles JA, Clark JM, McQuade KJ, Gibb TD, Matsen FA 3rd. Translation of the humeral head on the glenoid with passive glenohumeral motion. J Bone Joint Surg Am. 1990;72(9):1334-1343.
34. Tyler TF, Nicholas SJ, Roy T, Gleim GW. Quantification of posterior capsule tightness and motion loss in patients with shoulder impingement. Am J Sports Med. 2000;28(5):668-673.
35. McCarty LP, Buss DD, Giveans MR. Correlation between throwing arm strength deficit and glenohumeral internal rotation deficit in professional baseball pitchers, and differences between Latino and non-Latino pitchers. Presented at: American Academy of Orthopaedic Surgeons Annual Meeting; February 2012; San Francisco, CA.
36. Edwards TB, Bostick RD, Greene CC, Baratta RV, Drez D. Interobserver and intraobserver reliability of the measurement of shoulder internal rotation by vertebral level. J Shoulder Elbow Surg. 2002;11(1):40-42.
Glenohumeral internal rotation deficit (GIRD) can be observed in overhead athletes and is thought to play a role in generating pain and rotator cuff weakness in the dominant shoulder with sport. It is unclear what is an acceptable value of GIRD in a population of overhead athletes and whether it should be based solely on internal rotation deficit or should include total range of motion (ROM) deficit.1,2 Acquired GIRD in the athlete’s throwing shoulder has been thoroughly documented in the literature as a loss of internal rotation relative to the nonthrowing shoulder, with etiologies including bony adaptations (increased humeral retroversion), muscular tightness, and posterior capsular tightness.1,3-11 In particular, the repetitive torsional stresses acting on the throwing shoulder of baseball players is thought to produce, over the long term, structural adaptations such as increased humeral retroversion.5,12-14 Further, for shoulders with posterior-inferior capsular tightness, cadaveric studies have shown increased contact pressure at the coracoacromial arch during simulated follow-through.15 Athletes of other overhead and throwing sports, such as football, softball, tennis, and volleyball, may show similar adaptations in overhead motion.9,16,17
GIRD has been associated with a variety of pathologic conditions, including scapular dyskinesis, internal and secondary impingement, partial articular-sided rotator cuff tears, damage to the biceps–labral complex, and ulnar collateral ligament insufficiency.10,12,18-22
Restriction from engaging in exacerbating activities (eg, throwing) and compliance with a specific stretching program reduces or eliminates GIRD in the majority of cases.1,23-28 In the few cases in which conservative management fails, operative intervention may be indicated.1,23,29,30 Few investigators have detailed an operative technique for selective arthroscopic capsular release of the posterior-inferior capsule or evaluated the ability of athletes to return to sport after such surgery.
In this article, we present our technique for arthroscopic posterior-inferior capsular release and report the results of applying this technique in a population of athletes with symptomatic GIRD that was unresponsive to nonoperative treatment and was preventing them from returning to sport.
We hypothesized that selective arthroscopic surgical release of the posterior-inferior capsule would improve symptomatic GIRD and result in a return to sport in the majority of cases unresponsive to nonoperative treatment.
Materials and Methods
Patients
After obtaining institutional review board approval, we retrospectively reviewed patient charts and collected data. Study inclusion criteria were arthroscopic selective posterior-inferior capsular release between 2004 and 2008; failure to resume sport after minimum 3 months of physical therapy, including use of sleeper stretch, active joint mobilization by licensed physical therapist, and sport-specific restriction from exacerbating activities (eg, throwing for baseball players); and active participation in overhead sport.1,27 Exclusion criteria were generalized adhesive capsulitis, labral pathology producing glenohumeral joint instability (Bankart or reverse Bankart lesion), high-grade or full-thickness tearing of rotator cuff, and clinically significant partial-thickness tearing or instability of long head of biceps tendon.
Assessment
One of 3 authors (Dr. Buss, Dr. Codding, or Dr. Dahm) used a bubble goniometer to measure passive internal rotation. Patients were positioned supine with 90° of thoracohumeral abduction and 90° of elbow flexion. The examiner’s hand stabilized the scapula against the examination table, in accordance with published techniques.1,26 Active internal rotation was measured at 0° of thoracohumeral abduction by noting the most superior spinal segment reached. Before and after surgery, passive internal rotation measurements were taken on both arms. GIRD was determined by the difference between dominant and nondominant arm measurements; segmental differences were obtained by subtracting segments achieved between the dominant and nondominant arms.
Before surgery and at minimum 2-year follow-up after surgery, patients completed a subjective questionnaire, which included the American Shoulder and Elbow Surgeons (ASES) Standardized Shoulder Assessment Form, for assessment of both arms. ASES scores are reliable, valid, and responsive in evaluating shoulder pain and function.15,31 Patients also answered questions about their ability to return to play, their level of play after surgery, and whether they would undergo the procedure again.
Surgical Technique
After induction of general anesthesia and standard preparation and draping, the patient is placed in a standard beach-chair position and examined. Diagnostic arthroscopy is then performed. In all patients, intra-articular evaluation revealed a thickened, contracted posterior band of the inferior glenohumeral ligament. This finding is consistent with other studies of patients with significant GIRD.1,14,22,30
On completion of the diagnostic portion of the arthroscopy, attention is turned to the selective posterior-inferior capsular release. Key to proper execution of the release is establishing a posterior-inferior accessory portal. This is accomplished while viewing from a standard posterior (“soft spot”) portal and determining the appropriate location and angle of entry by spinal needle localization. Typically, an entry point is selected about 4 cm distal and 1 cm lateral to the standard posterior portal. An 18-gauge spinal needle introduced at this location is angled about 15° superiorly and about 20° medially. Once the appropriate vector is determined, a skin incision is made, and a Wissinger rod is introduced, over which a small-diameter cannula is passed. A hooked-tip electrocautery device is used to divide the posterior capsule from the glenoid labrum between the 8- and 6-o’clock positions in the right shoulder (Figure). Care is taken to perform the release immediately adjacent to the glenoid labrum and using short bursts of cautery in order to minimize risk of injury to the teres minor branch of the axillary nerve. Adequate release is confirmed by reassessing passive internal rotation under anesthesia. Additional procedures are performed, if necessary, after completion of the capsular release.
Postoperative rehabilitation consists initially of pendulum exercises and scapular retraction starting on postoperative day 1. Once the swelling from the surgical procedure subsides, typically within 1 week, passive and active-assisted ROM and gentle posterior capsular mobilization are initiated under the direction of a licensed physical therapist. Active ROM is allowed once the patient regains normal scapulothoracic rhythm. Strengthening consists initially of isometrics followed by light resistance strengthening for the rotator cuff and scapular stabilizers once active ROM and scapulothoracic rhythm return to normal. Passive internal rotation stretching, including use of the sleeper stretch, is implemented as soon as tolerated and continues throughout the rehabilitation process.32
Statistical Analysis
Statistical analysis was performed with Stata Release 11 (StataCorp, College Station, Texas). Paired t tests were used to assess preoperative and postoperative mean differences in ASES scores, in passive glenohumeral internal rotation, and in active glenohumeral internal rotation; independent-samples t tests were used to assess side-to-side differences. Significance was set at P < .05.
Results
Fifteen overhead athletes met the study inclusion criteria. Two were lost to follow-up. Of the remaining 13 patients, 6 underwent isolated arthroscopic posterior-inferior capsular release, and 7 had concomitant procedures (6 subacromial decompressions, 1 superior labrum anterior-posterior [SLAP] repair). There were 11 male athletes and 2 female athletes. Twelve of the 13 patients were right-hand–dominant. Mean age at time of surgery was 21 years (range, 16-33 years). There were 10 baseball players (6 pitchers, 4 position players); the other 3 patients played softball (1), volleyball (1), or tennis (1). Six patients played at high school level, 5 at college level, 1 at professional level, and 1 at amateur level. All 13 patients underwent a minimum of 3 months of comprehensive rehabilitation, which included use of the sleeper stretch, active joint mobilization by a licensed physical therapist, and sport-specific restriction from exacerbating activities. Mean duration of symptoms before surgery was 18 months (range, 4-48 months). Mean postoperative follow-up was 31 months (range, 24-59 months). Mean ASES score was 71.5 (range, 33-95) before surgery and 86.9 (range, 60-100) after surgery (P < .001). Mean GIRD improved from 43.1° (range, 30°-60°) before surgery to 9.7° (range, –7° to 40°) after surgery (P < .001). Mean active internal rotation difference improved from 3.8 vertebral segments before surgery to 2.6 vertebral segments after surgery; this difference was not statistically significant (P = .459). Ten (77%) of the 13 patients returned to their preoperative level of play or a higher level; the other 3 (23%) did not return to their preoperative level of play but continued to compete in a different position (Table). Eleven patients (85%) stated they would repeat the procedure. One of the 2 patients who would not repeat the procedure was in the isolated posterior-inferior capsular release group; the other was in the concomitant-procedure group (subacromial decompression). Total glenohumeral ROM of dominant arm was 122° before surgery and 136° after surgery (P = .04). There was no significant difference in total ROM between dominant and nondominant arms after surgery (136° and 141°; P = .12), but the preoperative difference was significant (122° vs 141°; P = .022).
Discussion
GIRD has been associated with various pathologic conditions of the upper extremity. In 1991, Verna28 found that a majority of 39 professional baseball pitchers with significant GIRD had shoulder problems that affected playing time. More recently, GIRD has been associated with a progression of injuries, including scapular dyskinesia, internal and secondary impingement, articular-sided partial rotator cuff tears, rotator cuff weakness, damage to the biceps–labral complex, and ulnar collateral ligament insufficiency.12,18-22 In a cadaveric study of humeral head translation, Harryman and colleagues33 noted an anterosuperior migration of the humeral head during flexion and concluded it resulted from a loose anterior and tight posterior glenohumeral capsule, leading to loss of glenohumeral internal rotation. More recently, posterosuperior migration of the humeral head has been postulated, with GIRD secondary to an essential posterior capsular contracture.1 Tyler and colleagues34 clinically linked posterior capsular tightness with GIRD, and both cadaveric and magnetic resonance imaging studies have supported the finding that posterior capsular contracture leads to posterosuperior humeral head migration in association with GIRD.14,20 Such a disruption in normal glenohumeral joint mechanics could produce phenomena of internal or secondary acromiohumeral impingement and pain.
More recently, in a large cohort of professional baseball pitchers, a significant correlation was found between the incidence of rotator cuff strength deficits and GIRD.35 More than 40% of the pitchers with GIRD of at least 35° had a measureable rotator cuff strength deficit in the throwing shoulder.
Burkhart and colleagues23 concluded that the shoulder most at risk for developing “dead arm” has GIRD and an advanced form of scapular dyskinesia known as SICK scapula (the phenomenon involves Scapula malposition, Inferior medial border prominence, Coracoid pain and malposition, and dysKinesis of scapular movement).
Most athletes with symptoms attributed to GIRD respond to conservative management. A posterior-inferior capsular stretching program focused on regaining internal rotation in the throwing arm has been shown to return about 90% of athletes to play.1 Numerous studies have indicated that enrollment in a compliant stretching program reduces GIRD.1,23-27 However, nonoperative treatment fails in a reported 10% of patients with GIRD; these patients may respond to operative treatment.1
More specifically, for patients who do not respond to conservative treatment, a posterior-inferior capsular release may be indicated.1,29 Ticker and colleagues22 identified 9 patients who had lost internal rotation and had a posterior capsular contracture at arthroscopy. That study, however, was not performed on overhead or throwing athletes. Yoneda and colleagues30 followed 16 overhead throwing athletes after arthroscopic posterior-inferior capsular release and found favorable preliminary clinical results. Eleven of the 16 patients returned to their preinjury level of performance; the other 5 returned to a lower level. In addition, all 4 patients who underwent isolated arthroscopic capsular release had throwing power restored to between 90% and 100%.
In the present study, 10 of 13 patients who underwent arthroscopic posterior-inferior capsular release returned to their preoperative level of play or a higher level. Mean passive GIRD improved significantly from before surgery to after surgery. ASES scores likewise were significantly improved from before surgery to after surgery. The active internal rotation difference as measured by vertebral segment level was not significantly changed after surgery. This lack of improvement may stem from the more complex musculoligamentous interactions governing active internal rotation versus isolated, passive internal rotation. Another possible explanation for lack of improvement is that the interobserver and intraobserver reliability of this method is lower.36
At 2-year follow-up, the patient who had undergone concomitant SLAP repair demonstrated a 23% improvement in ASES score and more internal rotation on the dominant arm relative to the nondominant arm. This patient returned to a level of play at least as good as his preoperative level. Although we could not determine its statistical significance, this patient’s improvement suggests that the SLAP repair did not reduce the efficacy of the posterior-inferior capsular release.
Limitations of this study include its relatively small cohort (precluded statistical comparisons between groups), the proportion of patients (7/13) who had concomitant surgeries, and the limited options for patient outcome scores. Although the ASES score is a validated outcome score, the Kerlan-Jobe Orthopaedic Clinic Shoulder and Elbow (KJOC) score or the Disabilities of the Arm, Shoulder, and Hand (DASH) score may be more appropriate in an athletic population. In addition, although all study patients had GIRD that was unresponsive to a concerted trial of nonoperative management, we did not have a control group (nonoperatively treated patients) for comparison. Finally, we did not obtain computed tomography scans or account for the potential contribution of humeral retroversion to GIRD in this group of patients.
Conclusion
Selective arthroscopic posterior-inferior capsular release can be recommended as a reasonable operative solution for overhead athletes with symptomatic GIRD that has not responded to conservative management. In the present study, ASES scores improved significantly, and 77% of our athlete-patients returned to sport at their preoperative level of play or a higher level.
Glenohumeral internal rotation deficit (GIRD) can be observed in overhead athletes and is thought to play a role in generating pain and rotator cuff weakness in the dominant shoulder with sport. It is unclear what is an acceptable value of GIRD in a population of overhead athletes and whether it should be based solely on internal rotation deficit or should include total range of motion (ROM) deficit.1,2 Acquired GIRD in the athlete’s throwing shoulder has been thoroughly documented in the literature as a loss of internal rotation relative to the nonthrowing shoulder, with etiologies including bony adaptations (increased humeral retroversion), muscular tightness, and posterior capsular tightness.1,3-11 In particular, the repetitive torsional stresses acting on the throwing shoulder of baseball players is thought to produce, over the long term, structural adaptations such as increased humeral retroversion.5,12-14 Further, for shoulders with posterior-inferior capsular tightness, cadaveric studies have shown increased contact pressure at the coracoacromial arch during simulated follow-through.15 Athletes of other overhead and throwing sports, such as football, softball, tennis, and volleyball, may show similar adaptations in overhead motion.9,16,17
GIRD has been associated with a variety of pathologic conditions, including scapular dyskinesis, internal and secondary impingement, partial articular-sided rotator cuff tears, damage to the biceps–labral complex, and ulnar collateral ligament insufficiency.10,12,18-22
Restriction from engaging in exacerbating activities (eg, throwing) and compliance with a specific stretching program reduces or eliminates GIRD in the majority of cases.1,23-28 In the few cases in which conservative management fails, operative intervention may be indicated.1,23,29,30 Few investigators have detailed an operative technique for selective arthroscopic capsular release of the posterior-inferior capsule or evaluated the ability of athletes to return to sport after such surgery.
In this article, we present our technique for arthroscopic posterior-inferior capsular release and report the results of applying this technique in a population of athletes with symptomatic GIRD that was unresponsive to nonoperative treatment and was preventing them from returning to sport.
We hypothesized that selective arthroscopic surgical release of the posterior-inferior capsule would improve symptomatic GIRD and result in a return to sport in the majority of cases unresponsive to nonoperative treatment.
Materials and Methods
Patients
After obtaining institutional review board approval, we retrospectively reviewed patient charts and collected data. Study inclusion criteria were arthroscopic selective posterior-inferior capsular release between 2004 and 2008; failure to resume sport after minimum 3 months of physical therapy, including use of sleeper stretch, active joint mobilization by licensed physical therapist, and sport-specific restriction from exacerbating activities (eg, throwing for baseball players); and active participation in overhead sport.1,27 Exclusion criteria were generalized adhesive capsulitis, labral pathology producing glenohumeral joint instability (Bankart or reverse Bankart lesion), high-grade or full-thickness tearing of rotator cuff, and clinically significant partial-thickness tearing or instability of long head of biceps tendon.
Assessment
One of 3 authors (Dr. Buss, Dr. Codding, or Dr. Dahm) used a bubble goniometer to measure passive internal rotation. Patients were positioned supine with 90° of thoracohumeral abduction and 90° of elbow flexion. The examiner’s hand stabilized the scapula against the examination table, in accordance with published techniques.1,26 Active internal rotation was measured at 0° of thoracohumeral abduction by noting the most superior spinal segment reached. Before and after surgery, passive internal rotation measurements were taken on both arms. GIRD was determined by the difference between dominant and nondominant arm measurements; segmental differences were obtained by subtracting segments achieved between the dominant and nondominant arms.
Before surgery and at minimum 2-year follow-up after surgery, patients completed a subjective questionnaire, which included the American Shoulder and Elbow Surgeons (ASES) Standardized Shoulder Assessment Form, for assessment of both arms. ASES scores are reliable, valid, and responsive in evaluating shoulder pain and function.15,31 Patients also answered questions about their ability to return to play, their level of play after surgery, and whether they would undergo the procedure again.
Surgical Technique
After induction of general anesthesia and standard preparation and draping, the patient is placed in a standard beach-chair position and examined. Diagnostic arthroscopy is then performed. In all patients, intra-articular evaluation revealed a thickened, contracted posterior band of the inferior glenohumeral ligament. This finding is consistent with other studies of patients with significant GIRD.1,14,22,30
On completion of the diagnostic portion of the arthroscopy, attention is turned to the selective posterior-inferior capsular release. Key to proper execution of the release is establishing a posterior-inferior accessory portal. This is accomplished while viewing from a standard posterior (“soft spot”) portal and determining the appropriate location and angle of entry by spinal needle localization. Typically, an entry point is selected about 4 cm distal and 1 cm lateral to the standard posterior portal. An 18-gauge spinal needle introduced at this location is angled about 15° superiorly and about 20° medially. Once the appropriate vector is determined, a skin incision is made, and a Wissinger rod is introduced, over which a small-diameter cannula is passed. A hooked-tip electrocautery device is used to divide the posterior capsule from the glenoid labrum between the 8- and 6-o’clock positions in the right shoulder (Figure). Care is taken to perform the release immediately adjacent to the glenoid labrum and using short bursts of cautery in order to minimize risk of injury to the teres minor branch of the axillary nerve. Adequate release is confirmed by reassessing passive internal rotation under anesthesia. Additional procedures are performed, if necessary, after completion of the capsular release.
Postoperative rehabilitation consists initially of pendulum exercises and scapular retraction starting on postoperative day 1. Once the swelling from the surgical procedure subsides, typically within 1 week, passive and active-assisted ROM and gentle posterior capsular mobilization are initiated under the direction of a licensed physical therapist. Active ROM is allowed once the patient regains normal scapulothoracic rhythm. Strengthening consists initially of isometrics followed by light resistance strengthening for the rotator cuff and scapular stabilizers once active ROM and scapulothoracic rhythm return to normal. Passive internal rotation stretching, including use of the sleeper stretch, is implemented as soon as tolerated and continues throughout the rehabilitation process.32
Statistical Analysis
Statistical analysis was performed with Stata Release 11 (StataCorp, College Station, Texas). Paired t tests were used to assess preoperative and postoperative mean differences in ASES scores, in passive glenohumeral internal rotation, and in active glenohumeral internal rotation; independent-samples t tests were used to assess side-to-side differences. Significance was set at P < .05.
Results
Fifteen overhead athletes met the study inclusion criteria. Two were lost to follow-up. Of the remaining 13 patients, 6 underwent isolated arthroscopic posterior-inferior capsular release, and 7 had concomitant procedures (6 subacromial decompressions, 1 superior labrum anterior-posterior [SLAP] repair). There were 11 male athletes and 2 female athletes. Twelve of the 13 patients were right-hand–dominant. Mean age at time of surgery was 21 years (range, 16-33 years). There were 10 baseball players (6 pitchers, 4 position players); the other 3 patients played softball (1), volleyball (1), or tennis (1). Six patients played at high school level, 5 at college level, 1 at professional level, and 1 at amateur level. All 13 patients underwent a minimum of 3 months of comprehensive rehabilitation, which included use of the sleeper stretch, active joint mobilization by a licensed physical therapist, and sport-specific restriction from exacerbating activities. Mean duration of symptoms before surgery was 18 months (range, 4-48 months). Mean postoperative follow-up was 31 months (range, 24-59 months). Mean ASES score was 71.5 (range, 33-95) before surgery and 86.9 (range, 60-100) after surgery (P < .001). Mean GIRD improved from 43.1° (range, 30°-60°) before surgery to 9.7° (range, –7° to 40°) after surgery (P < .001). Mean active internal rotation difference improved from 3.8 vertebral segments before surgery to 2.6 vertebral segments after surgery; this difference was not statistically significant (P = .459). Ten (77%) of the 13 patients returned to their preoperative level of play or a higher level; the other 3 (23%) did not return to their preoperative level of play but continued to compete in a different position (Table). Eleven patients (85%) stated they would repeat the procedure. One of the 2 patients who would not repeat the procedure was in the isolated posterior-inferior capsular release group; the other was in the concomitant-procedure group (subacromial decompression). Total glenohumeral ROM of dominant arm was 122° before surgery and 136° after surgery (P = .04). There was no significant difference in total ROM between dominant and nondominant arms after surgery (136° and 141°; P = .12), but the preoperative difference was significant (122° vs 141°; P = .022).
Discussion
GIRD has been associated with various pathologic conditions of the upper extremity. In 1991, Verna28 found that a majority of 39 professional baseball pitchers with significant GIRD had shoulder problems that affected playing time. More recently, GIRD has been associated with a progression of injuries, including scapular dyskinesia, internal and secondary impingement, articular-sided partial rotator cuff tears, rotator cuff weakness, damage to the biceps–labral complex, and ulnar collateral ligament insufficiency.12,18-22 In a cadaveric study of humeral head translation, Harryman and colleagues33 noted an anterosuperior migration of the humeral head during flexion and concluded it resulted from a loose anterior and tight posterior glenohumeral capsule, leading to loss of glenohumeral internal rotation. More recently, posterosuperior migration of the humeral head has been postulated, with GIRD secondary to an essential posterior capsular contracture.1 Tyler and colleagues34 clinically linked posterior capsular tightness with GIRD, and both cadaveric and magnetic resonance imaging studies have supported the finding that posterior capsular contracture leads to posterosuperior humeral head migration in association with GIRD.14,20 Such a disruption in normal glenohumeral joint mechanics could produce phenomena of internal or secondary acromiohumeral impingement and pain.
More recently, in a large cohort of professional baseball pitchers, a significant correlation was found between the incidence of rotator cuff strength deficits and GIRD.35 More than 40% of the pitchers with GIRD of at least 35° had a measureable rotator cuff strength deficit in the throwing shoulder.
Burkhart and colleagues23 concluded that the shoulder most at risk for developing “dead arm” has GIRD and an advanced form of scapular dyskinesia known as SICK scapula (the phenomenon involves Scapula malposition, Inferior medial border prominence, Coracoid pain and malposition, and dysKinesis of scapular movement).
Most athletes with symptoms attributed to GIRD respond to conservative management. A posterior-inferior capsular stretching program focused on regaining internal rotation in the throwing arm has been shown to return about 90% of athletes to play.1 Numerous studies have indicated that enrollment in a compliant stretching program reduces GIRD.1,23-27 However, nonoperative treatment fails in a reported 10% of patients with GIRD; these patients may respond to operative treatment.1
More specifically, for patients who do not respond to conservative treatment, a posterior-inferior capsular release may be indicated.1,29 Ticker and colleagues22 identified 9 patients who had lost internal rotation and had a posterior capsular contracture at arthroscopy. That study, however, was not performed on overhead or throwing athletes. Yoneda and colleagues30 followed 16 overhead throwing athletes after arthroscopic posterior-inferior capsular release and found favorable preliminary clinical results. Eleven of the 16 patients returned to their preinjury level of performance; the other 5 returned to a lower level. In addition, all 4 patients who underwent isolated arthroscopic capsular release had throwing power restored to between 90% and 100%.
In the present study, 10 of 13 patients who underwent arthroscopic posterior-inferior capsular release returned to their preoperative level of play or a higher level. Mean passive GIRD improved significantly from before surgery to after surgery. ASES scores likewise were significantly improved from before surgery to after surgery. The active internal rotation difference as measured by vertebral segment level was not significantly changed after surgery. This lack of improvement may stem from the more complex musculoligamentous interactions governing active internal rotation versus isolated, passive internal rotation. Another possible explanation for lack of improvement is that the interobserver and intraobserver reliability of this method is lower.36
At 2-year follow-up, the patient who had undergone concomitant SLAP repair demonstrated a 23% improvement in ASES score and more internal rotation on the dominant arm relative to the nondominant arm. This patient returned to a level of play at least as good as his preoperative level. Although we could not determine its statistical significance, this patient’s improvement suggests that the SLAP repair did not reduce the efficacy of the posterior-inferior capsular release.
Limitations of this study include its relatively small cohort (precluded statistical comparisons between groups), the proportion of patients (7/13) who had concomitant surgeries, and the limited options for patient outcome scores. Although the ASES score is a validated outcome score, the Kerlan-Jobe Orthopaedic Clinic Shoulder and Elbow (KJOC) score or the Disabilities of the Arm, Shoulder, and Hand (DASH) score may be more appropriate in an athletic population. In addition, although all study patients had GIRD that was unresponsive to a concerted trial of nonoperative management, we did not have a control group (nonoperatively treated patients) for comparison. Finally, we did not obtain computed tomography scans or account for the potential contribution of humeral retroversion to GIRD in this group of patients.
Conclusion
Selective arthroscopic posterior-inferior capsular release can be recommended as a reasonable operative solution for overhead athletes with symptomatic GIRD that has not responded to conservative management. In the present study, ASES scores improved significantly, and 77% of our athlete-patients returned to sport at their preoperative level of play or a higher level.
1. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
2. Wilk KE, Macrina LC, Fleisig GS, et al. Correlation of glenohumeral internal rotation deficit and total rotational motion to shoulder injuries in professional baseball pitchers. Am J Sports Med. 2011;39(2):329-335.
3. Bigliani LU, Codd TP, Connor PM, Levine WN, Littlefield MA, Hershon SJ. Shoulder motion and laxity in the professional baseball player. Am J Sports Med. 1997;25(5):609-613.
4. Brown LP, Niehues SL, Harrah A, Yavorsky P, Hirshman HP. Upper extremity range of motion and isokinetic strength of the internal and external shoulder rotators in Major League baseball players. Am J Sports Med. 1988;16(6):577-585.
5. Crockett HC, Gross LB, Wilk KE, et al. Osseous adaptation and range of motion at the glenohumeral joint in professional baseball pitchers. Am J Sports Med. 2002;30(1):20-26.
6. Kibler WB, Chandler TJ, Livingston BP, Roetert EP. Shoulder range of motion in elite tennis players. Effect of age and years of tournament play. Am J Sports Med. 1996;24(3):279-285.
7. Meister K. Injuries to the shoulder in the throwing athlete. Part one: biomechanics/pathophysiology/classification of injury. Am J Sports Med. 2000;28(2):265-275.
8. Osbahr DC, Cannon DL, Speer KP. Retroversion of the humerus in the throwing shoulder of college baseball pitchers. Am J Sports Med. 2002;30(3):347-353.
9. Torres RR, Gomes JL. Measurement of glenohumeral internal rotation in asymptomatic tennis players and swimmers. Am J Sports Med. 2009;37(5):1017-1023.
10. Tyler TF, Nicholas SJ, Lee SJ, Mullaney M, McHugh MP. Correction of posterior shoulder tightness is associated with symptom resolution in patients with internal impingement. Am J Sports Med. 2010;28(1):114-119.
11. Wilk KE, Meister K, Andrews JR. Current concepts in the rehabilitation of the overhead throwing athlete. Am J Sports Med. 2002;30(1):136-151.
12. Braun S, Kokmeyer D, Millett PJ. Shoulder injuries in the throwing athlete. J Bone Joint Surg Am. 2009;91(4):966-978.
13. Reagan KM, Meister K, Horodyski MB, Werner DW, Carruthers C, Wilk K. Humeral retroversion and its relationship to glenohumeral rotation in the shoulder of college baseball players. Am J Sports Med. 2002;30(3):354-360.
14. Tehranzadeh AD, Fronek J, Resnick D. Posterior capsular fibrosis in professional baseball pitchers: case series of MR arthrographic findings in six patients with glenohumeral internal rotational deficit. Clin Imaging. 2007;31(5):343-348.
15. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
16. Curtis AS, Deshmukh R. Throwing injuries: diagnosis and treatment. Arthroscopy. 2003;19(suppl 1):80-85.
17. Lajtai G, Pfirrmann CW, Aitzetmuller G, Pirkl C, Gerber C, Jost B. The shoulders of fully competitive professional beach volleyball players: high prevalence of infraspinatus atrophy. Am J Sports Med. 2009;37(7):1375-1383.
18. Burkhart SS, Morgan CD. The peel-back mechanism: its role in producing and extending posterior type II SLAP lesions and its effect on SLAP repair rehabilitation. Arthroscopy. 1998;14(6):637-640.
19. Dines JS, Frank JB, Akerman M, Yocum LA. Glenohumeral internal rotation deficits in baseball players with ulnar collateral ligament insufficiency. Am J Sports Med. 2009;37(3):566-570.
20. Grossman MG, Tibone JE, McGarry MH, Schneider DJ, Veneziani S, Lee TQ. A cadaveric model of the throwing shoulder: a possible etiology of superior labrum anterior-to-posterior lesions. J Bone Joint Surg Am. 2005;87(4):824-831.
21. Myers JB, Laudner KG, Pasquale MR, Bradley JP, Lephart SM. Glenohumeral range of motion deficits and posterior shoulder tightness in throwers with pathologic internal impingement. Am J Sports Med. 2006;34(3):385-391.
22. Ticker JB, Beim GM, Warner JJ. Recognition and treatment of refractory posterior capsular contracture of the shoulder. Arthroscopy. 2000;16(1):27-34.
23. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology part III: the SICK scapula, scapular dyskinesis, the kinetic chain, and rehabilitation. Arthroscopy. 2003;19(6):641-661.
24. Kibler WB, McMullen J. Scapular dyskinesis and its relation to shoulder pain. J Am Acad Orthop Surg. 2003;11(2):142-151.
25. Kibler WB. The relationship of glenohumeral internal rotation deficit to shoulder and elbow injuries in tennis players: a prospective evaluation of posterior capsular stretching. Presented at: American Shoulder and Elbow Surgeons 15th Annual Closed Meeting; November 6, 1998; New York, NY.
26. Lintner D, Mayol M, Uzodinma O, Jones R, Labossiere D. Glenohumeral internal rotation deficits in professional pitchers enrolled in an internal rotation stretching program. Am J Sports Med. 2007;35(4):617-621.
27. McClure P, Balaicuis J, Heiland D, Broersma ME, Thorndike CK, Wood A. A randomized controlled comparison of stretching procedures for posterior shoulder tightness. J Orthop Sports Phys Ther. 2007;37(3):108-114.
28. Verna C. Shoulder flexibility to reduce impingement. Presented at: 3rd Annual Professional Baseball Athletic Trainer Society Meeting; March 1991; Mesa, AZ.
29. Bach HG, Goldberg BA. Posterior capsular contracture of the shoulder. J Am Acad Orthop Surg. 2006;14(5):265-277.
30. Yoneda M, Nakagawa S, Mizuno N, et al. Arthroscopic capsular release for painful throwing shoulder with posterior capsular tightness. Arthroscopy. 2006;22(7):801e1-801e5.
31. Kocher MS, Horan MP, Briggs KK, Richardson TR, O’Holleran J, Hawkins RJ. Reliability, validity, and responsiveness of the American Shoulder and Elbow Surgeons subjective shoulder scale in patients with shoulder instability, rotator cuff disease, and glenohumeral arthritis. J Bone Joint Surg Am. 2005;87(9):2006-2011.
32. Johansen RL, Callis M, Potts J, Shall LM. A modified internal rotation stretching technique for overhand and throwing athletes. J Orthop Sports Phys Ther. 1995;21(4):216-219.
33. Harryman DT 2nd, Sidles JA, Clark JM, McQuade KJ, Gibb TD, Matsen FA 3rd. Translation of the humeral head on the glenoid with passive glenohumeral motion. J Bone Joint Surg Am. 1990;72(9):1334-1343.
34. Tyler TF, Nicholas SJ, Roy T, Gleim GW. Quantification of posterior capsule tightness and motion loss in patients with shoulder impingement. Am J Sports Med. 2000;28(5):668-673.
35. McCarty LP, Buss DD, Giveans MR. Correlation between throwing arm strength deficit and glenohumeral internal rotation deficit in professional baseball pitchers, and differences between Latino and non-Latino pitchers. Presented at: American Academy of Orthopaedic Surgeons Annual Meeting; February 2012; San Francisco, CA.
36. Edwards TB, Bostick RD, Greene CC, Baratta RV, Drez D. Interobserver and intraobserver reliability of the measurement of shoulder internal rotation by vertebral level. J Shoulder Elbow Surg. 2002;11(1):40-42.
1. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
2. Wilk KE, Macrina LC, Fleisig GS, et al. Correlation of glenohumeral internal rotation deficit and total rotational motion to shoulder injuries in professional baseball pitchers. Am J Sports Med. 2011;39(2):329-335.
3. Bigliani LU, Codd TP, Connor PM, Levine WN, Littlefield MA, Hershon SJ. Shoulder motion and laxity in the professional baseball player. Am J Sports Med. 1997;25(5):609-613.
4. Brown LP, Niehues SL, Harrah A, Yavorsky P, Hirshman HP. Upper extremity range of motion and isokinetic strength of the internal and external shoulder rotators in Major League baseball players. Am J Sports Med. 1988;16(6):577-585.
5. Crockett HC, Gross LB, Wilk KE, et al. Osseous adaptation and range of motion at the glenohumeral joint in professional baseball pitchers. Am J Sports Med. 2002;30(1):20-26.
6. Kibler WB, Chandler TJ, Livingston BP, Roetert EP. Shoulder range of motion in elite tennis players. Effect of age and years of tournament play. Am J Sports Med. 1996;24(3):279-285.
7. Meister K. Injuries to the shoulder in the throwing athlete. Part one: biomechanics/pathophysiology/classification of injury. Am J Sports Med. 2000;28(2):265-275.
8. Osbahr DC, Cannon DL, Speer KP. Retroversion of the humerus in the throwing shoulder of college baseball pitchers. Am J Sports Med. 2002;30(3):347-353.
9. Torres RR, Gomes JL. Measurement of glenohumeral internal rotation in asymptomatic tennis players and swimmers. Am J Sports Med. 2009;37(5):1017-1023.
10. Tyler TF, Nicholas SJ, Lee SJ, Mullaney M, McHugh MP. Correction of posterior shoulder tightness is associated with symptom resolution in patients with internal impingement. Am J Sports Med. 2010;28(1):114-119.
11. Wilk KE, Meister K, Andrews JR. Current concepts in the rehabilitation of the overhead throwing athlete. Am J Sports Med. 2002;30(1):136-151.
12. Braun S, Kokmeyer D, Millett PJ. Shoulder injuries in the throwing athlete. J Bone Joint Surg Am. 2009;91(4):966-978.
13. Reagan KM, Meister K, Horodyski MB, Werner DW, Carruthers C, Wilk K. Humeral retroversion and its relationship to glenohumeral rotation in the shoulder of college baseball players. Am J Sports Med. 2002;30(3):354-360.
14. Tehranzadeh AD, Fronek J, Resnick D. Posterior capsular fibrosis in professional baseball pitchers: case series of MR arthrographic findings in six patients with glenohumeral internal rotational deficit. Clin Imaging. 2007;31(5):343-348.
15. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
16. Curtis AS, Deshmukh R. Throwing injuries: diagnosis and treatment. Arthroscopy. 2003;19(suppl 1):80-85.
17. Lajtai G, Pfirrmann CW, Aitzetmuller G, Pirkl C, Gerber C, Jost B. The shoulders of fully competitive professional beach volleyball players: high prevalence of infraspinatus atrophy. Am J Sports Med. 2009;37(7):1375-1383.
18. Burkhart SS, Morgan CD. The peel-back mechanism: its role in producing and extending posterior type II SLAP lesions and its effect on SLAP repair rehabilitation. Arthroscopy. 1998;14(6):637-640.
19. Dines JS, Frank JB, Akerman M, Yocum LA. Glenohumeral internal rotation deficits in baseball players with ulnar collateral ligament insufficiency. Am J Sports Med. 2009;37(3):566-570.
20. Grossman MG, Tibone JE, McGarry MH, Schneider DJ, Veneziani S, Lee TQ. A cadaveric model of the throwing shoulder: a possible etiology of superior labrum anterior-to-posterior lesions. J Bone Joint Surg Am. 2005;87(4):824-831.
21. Myers JB, Laudner KG, Pasquale MR, Bradley JP, Lephart SM. Glenohumeral range of motion deficits and posterior shoulder tightness in throwers with pathologic internal impingement. Am J Sports Med. 2006;34(3):385-391.
22. Ticker JB, Beim GM, Warner JJ. Recognition and treatment of refractory posterior capsular contracture of the shoulder. Arthroscopy. 2000;16(1):27-34.
23. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology part III: the SICK scapula, scapular dyskinesis, the kinetic chain, and rehabilitation. Arthroscopy. 2003;19(6):641-661.
24. Kibler WB, McMullen J. Scapular dyskinesis and its relation to shoulder pain. J Am Acad Orthop Surg. 2003;11(2):142-151.
25. Kibler WB. The relationship of glenohumeral internal rotation deficit to shoulder and elbow injuries in tennis players: a prospective evaluation of posterior capsular stretching. Presented at: American Shoulder and Elbow Surgeons 15th Annual Closed Meeting; November 6, 1998; New York, NY.
26. Lintner D, Mayol M, Uzodinma O, Jones R, Labossiere D. Glenohumeral internal rotation deficits in professional pitchers enrolled in an internal rotation stretching program. Am J Sports Med. 2007;35(4):617-621.
27. McClure P, Balaicuis J, Heiland D, Broersma ME, Thorndike CK, Wood A. A randomized controlled comparison of stretching procedures for posterior shoulder tightness. J Orthop Sports Phys Ther. 2007;37(3):108-114.
28. Verna C. Shoulder flexibility to reduce impingement. Presented at: 3rd Annual Professional Baseball Athletic Trainer Society Meeting; March 1991; Mesa, AZ.
29. Bach HG, Goldberg BA. Posterior capsular contracture of the shoulder. J Am Acad Orthop Surg. 2006;14(5):265-277.
30. Yoneda M, Nakagawa S, Mizuno N, et al. Arthroscopic capsular release for painful throwing shoulder with posterior capsular tightness. Arthroscopy. 2006;22(7):801e1-801e5.
31. Kocher MS, Horan MP, Briggs KK, Richardson TR, O’Holleran J, Hawkins RJ. Reliability, validity, and responsiveness of the American Shoulder and Elbow Surgeons subjective shoulder scale in patients with shoulder instability, rotator cuff disease, and glenohumeral arthritis. J Bone Joint Surg Am. 2005;87(9):2006-2011.
32. Johansen RL, Callis M, Potts J, Shall LM. A modified internal rotation stretching technique for overhand and throwing athletes. J Orthop Sports Phys Ther. 1995;21(4):216-219.
33. Harryman DT 2nd, Sidles JA, Clark JM, McQuade KJ, Gibb TD, Matsen FA 3rd. Translation of the humeral head on the glenoid with passive glenohumeral motion. J Bone Joint Surg Am. 1990;72(9):1334-1343.
34. Tyler TF, Nicholas SJ, Roy T, Gleim GW. Quantification of posterior capsule tightness and motion loss in patients with shoulder impingement. Am J Sports Med. 2000;28(5):668-673.
35. McCarty LP, Buss DD, Giveans MR. Correlation between throwing arm strength deficit and glenohumeral internal rotation deficit in professional baseball pitchers, and differences between Latino and non-Latino pitchers. Presented at: American Academy of Orthopaedic Surgeons Annual Meeting; February 2012; San Francisco, CA.
36. Edwards TB, Bostick RD, Greene CC, Baratta RV, Drez D. Interobserver and intraobserver reliability of the measurement of shoulder internal rotation by vertebral level. J Shoulder Elbow Surg. 2002;11(1):40-42.