User login
CRP predicts anti-TNF response in ankylosing spondylitis
Patients with ankylosing spondylitis whose baseline C-reactive protein (CRP) levels were more than three times the upper limit of normal were significantly more likely to respond to 12 weeks of etanercept therapy than were patients with normal baseline CRP levels, investigators reported.
In a post hoc study of 867 patients who received etanercept during clinical trials, the adjusted odds of achieving 20% improvement in Assessment of Spondyloarthritis International Society (ASAS20) at week 12 were 190% higher when CRP levels were “very high” rather than normal at baseline (odds ratio, 2.9; 95% confidence interval, 1.8-4.7). Very-high baseline CRP levels (more than three times the upper limit of normal) also significantly predicted week-12 ASAS50, a change in Ankylosing Spondylitis Disease Activity Score based on CRP (ASDAS-CRP) greater than 1.1 (clinically important improvement), and an ASDAS-CRP less than 1.3 (inactive disease), while a normalization of very-high CRP levels by 2, 4, or 8 weeks of treatment was a significant predictor of achieving week-12 ASDAS inactive disease. “Patient-reported outcomes were less consistent predictors of response,” Xenofon Baraliakos, MD, of Ruhr University Bochum in Herne, Germany, and his coinvestigators wrote in Seminars in Arthritis and Rheumatism.
While the advent of tumor necrosis factor (TNF) antagonists has greatly improved outcomes in ankylosing spondylitis, symptom ambiguity and a lack of objective response criteria still impede early diagnosis and radiographic interpretation. Elevated baseline CRP predicted clinical response to anti-TNF therapy by patients with ankylosing spondylitis in several prior post hoc studies. To further evaluate this finding, the researchers pooled and analyzed data from four randomized, placebo-controlled trials of etanercept in adults with ankylosing spondylitis. In all, 43% of patients had a normal baseline CRP level, 34% had a level that was elevated but did not exceed three times the upper limit of normal, and 23% had a very-high level.
Age of onset, disease duration, and baseline Bath Ankylosing Spondylitis Functional Index also predicted response to etanercept therapy. After controlling for these covariates, a very-high baseline CRP remained a significant predictor for all four week-12 outcomes. This finding points to the value of aggressive, early treatment to normalize CRP in patients with ankylosing spondylitis, the researchers wrote. “Bending the curve of inflammation in the early disease may alter the long-term trajectory of ankylosing spondylitis, which is an opportunity that may not exist in the later stages.”
Thus, CRP, in addition to patient-reported and clinical outcomes, might be useful to help monitor response to anti-TNF therapy, the investigators wrote. It remains unclear whether elevated CRP levels also predict future treatment response in patients who are currently clinically improved or stable.
Pfizer funded the post hoc analysis and acquired the company that had funded the trials (Wyeth). Dr. Baraliakos reported consultancy and speaker fees from Pfizer, AbbVie, Bristol-Myers Squibb, Celgene, Chugai, Janssen, Merck, Novartis, Sandoz, and UCB. Two coinvestigators were Pfizer employees. A third coinvestigator was contracted by Pfizer to provide statistical support.
SOURCE: Baraliakos X et al. Semin Arthritis Rheum. 2018 Nov 2. doi: 10.1016/j.semarthrit.2018.10.019.
Patients with ankylosing spondylitis whose baseline C-reactive protein (CRP) levels were more than three times the upper limit of normal were significantly more likely to respond to 12 weeks of etanercept therapy than were patients with normal baseline CRP levels, investigators reported.
In a post hoc study of 867 patients who received etanercept during clinical trials, the adjusted odds of achieving 20% improvement in Assessment of Spondyloarthritis International Society (ASAS20) at week 12 were 190% higher when CRP levels were “very high” rather than normal at baseline (odds ratio, 2.9; 95% confidence interval, 1.8-4.7). Very-high baseline CRP levels (more than three times the upper limit of normal) also significantly predicted week-12 ASAS50, a change in Ankylosing Spondylitis Disease Activity Score based on CRP (ASDAS-CRP) greater than 1.1 (clinically important improvement), and an ASDAS-CRP less than 1.3 (inactive disease), while a normalization of very-high CRP levels by 2, 4, or 8 weeks of treatment was a significant predictor of achieving week-12 ASDAS inactive disease. “Patient-reported outcomes were less consistent predictors of response,” Xenofon Baraliakos, MD, of Ruhr University Bochum in Herne, Germany, and his coinvestigators wrote in Seminars in Arthritis and Rheumatism.
While the advent of tumor necrosis factor (TNF) antagonists has greatly improved outcomes in ankylosing spondylitis, symptom ambiguity and a lack of objective response criteria still impede early diagnosis and radiographic interpretation. Elevated baseline CRP predicted clinical response to anti-TNF therapy by patients with ankylosing spondylitis in several prior post hoc studies. To further evaluate this finding, the researchers pooled and analyzed data from four randomized, placebo-controlled trials of etanercept in adults with ankylosing spondylitis. In all, 43% of patients had a normal baseline CRP level, 34% had a level that was elevated but did not exceed three times the upper limit of normal, and 23% had a very-high level.
Age of onset, disease duration, and baseline Bath Ankylosing Spondylitis Functional Index also predicted response to etanercept therapy. After controlling for these covariates, a very-high baseline CRP remained a significant predictor for all four week-12 outcomes. This finding points to the value of aggressive, early treatment to normalize CRP in patients with ankylosing spondylitis, the researchers wrote. “Bending the curve of inflammation in the early disease may alter the long-term trajectory of ankylosing spondylitis, which is an opportunity that may not exist in the later stages.”
Thus, CRP, in addition to patient-reported and clinical outcomes, might be useful to help monitor response to anti-TNF therapy, the investigators wrote. It remains unclear whether elevated CRP levels also predict future treatment response in patients who are currently clinically improved or stable.
Pfizer funded the post hoc analysis and acquired the company that had funded the trials (Wyeth). Dr. Baraliakos reported consultancy and speaker fees from Pfizer, AbbVie, Bristol-Myers Squibb, Celgene, Chugai, Janssen, Merck, Novartis, Sandoz, and UCB. Two coinvestigators were Pfizer employees. A third coinvestigator was contracted by Pfizer to provide statistical support.
SOURCE: Baraliakos X et al. Semin Arthritis Rheum. 2018 Nov 2. doi: 10.1016/j.semarthrit.2018.10.019.
Patients with ankylosing spondylitis whose baseline C-reactive protein (CRP) levels were more than three times the upper limit of normal were significantly more likely to respond to 12 weeks of etanercept therapy than were patients with normal baseline CRP levels, investigators reported.
In a post hoc study of 867 patients who received etanercept during clinical trials, the adjusted odds of achieving 20% improvement in Assessment of Spondyloarthritis International Society (ASAS20) at week 12 were 190% higher when CRP levels were “very high” rather than normal at baseline (odds ratio, 2.9; 95% confidence interval, 1.8-4.7). Very-high baseline CRP levels (more than three times the upper limit of normal) also significantly predicted week-12 ASAS50, a change in Ankylosing Spondylitis Disease Activity Score based on CRP (ASDAS-CRP) greater than 1.1 (clinically important improvement), and an ASDAS-CRP less than 1.3 (inactive disease), while a normalization of very-high CRP levels by 2, 4, or 8 weeks of treatment was a significant predictor of achieving week-12 ASDAS inactive disease. “Patient-reported outcomes were less consistent predictors of response,” Xenofon Baraliakos, MD, of Ruhr University Bochum in Herne, Germany, and his coinvestigators wrote in Seminars in Arthritis and Rheumatism.
While the advent of tumor necrosis factor (TNF) antagonists has greatly improved outcomes in ankylosing spondylitis, symptom ambiguity and a lack of objective response criteria still impede early diagnosis and radiographic interpretation. Elevated baseline CRP predicted clinical response to anti-TNF therapy by patients with ankylosing spondylitis in several prior post hoc studies. To further evaluate this finding, the researchers pooled and analyzed data from four randomized, placebo-controlled trials of etanercept in adults with ankylosing spondylitis. In all, 43% of patients had a normal baseline CRP level, 34% had a level that was elevated but did not exceed three times the upper limit of normal, and 23% had a very-high level.
Age of onset, disease duration, and baseline Bath Ankylosing Spondylitis Functional Index also predicted response to etanercept therapy. After controlling for these covariates, a very-high baseline CRP remained a significant predictor for all four week-12 outcomes. This finding points to the value of aggressive, early treatment to normalize CRP in patients with ankylosing spondylitis, the researchers wrote. “Bending the curve of inflammation in the early disease may alter the long-term trajectory of ankylosing spondylitis, which is an opportunity that may not exist in the later stages.”
Thus, CRP, in addition to patient-reported and clinical outcomes, might be useful to help monitor response to anti-TNF therapy, the investigators wrote. It remains unclear whether elevated CRP levels also predict future treatment response in patients who are currently clinically improved or stable.
Pfizer funded the post hoc analysis and acquired the company that had funded the trials (Wyeth). Dr. Baraliakos reported consultancy and speaker fees from Pfizer, AbbVie, Bristol-Myers Squibb, Celgene, Chugai, Janssen, Merck, Novartis, Sandoz, and UCB. Two coinvestigators were Pfizer employees. A third coinvestigator was contracted by Pfizer to provide statistical support.
SOURCE: Baraliakos X et al. Semin Arthritis Rheum. 2018 Nov 2. doi: 10.1016/j.semarthrit.2018.10.019.
FROM SEMINARS IN ARTHRITIS AND RHEUMATISM
Key clinical point:
Major finding: Compared with normal baseline CRP, a CRP level more than three times above the upper limit of normal correlated significantly with all four outcomes at week 12.
Study details: A post hoc analysis of data from 867 patients with ankylosing spondylitis who received etanercept during one of four clinical trials (NCT00421915, NCT00418548, NCT00247962, and NCT00356356).
Disclosures: Pfizer funded the post hoc analysis and acquired the company that had funded the trials (Wyeth). Dr. Baraliakos reported consultancy and speaker fees from Pfizer, AbbVie, Bristol-Myers Squibb, Celgene, Chugai, Janssen, Merck, Novartis, Sandoz, and UCB. Two coinvestigators were Pfizer employees. A third coinvestigator was contracted by Pfizer to provide statistical support.
Source: Baraliakos X et al. Semin Arthritis Rheum. 2018 Nov 2. doi: 10.1016/j.semarthrit.2018.10.019.
Back pain criteria perform well in patients with active axial psoriatic arthritis
A back pain screening questionnaire developed for ankylosing spondylitis performs well for identifying the subset of axial psoriatic arthritis patients who have active symptoms, according to researchers.
The inflammatory back pain criteria didn’t perform as well when patients with established disease but no symptoms were included, though using a lower cutoff point for the questionnaire improved its sensitivity, the researchers reported in the Annals of the Rheumatic Diseases.
Previous investigations showed that the inflammatory back pain criteria, as defined by the Assessment of Spondyloarthritis International Society (ASAS), had low sensitivity and high specificity for axial involvement in psoriatic arthritis.
Those earlier studies may have registered suboptimal performance of the inflammatory back pain criteria by not distinguishing between patients with axial disease in remission and those with active symptoms, according to Muhammad Haroon, PhD, of the division of rheumatology at University Hospital Kerry in Tralee, Ireland, and his coinvestigators.
The present study, which they said represents a much larger cohort than earlier investigations, included 406 patients with psoriatic arthritis, about one-quarter of whom had rheumatologist-diagnosed axial psoriatic arthritis. The mean age of the axial psoriatic arthritis patients was 51 years and 53% were male.
The researchers found that the inflammatory back pain criteria had poor sensitivity but good specificity at 59% and 84%, respectively, in patients with established axial psoriatic arthritis, defined as axial disease regardless of whether it was active or in remission.
By contrast, the criteria had good sensitivity and good specificity at 82% and 88%, respectively, in patients who had active axial psoriatic arthritis, according to the investigators.
The standard cutoff points used by the ASAS inflammatory back pain criteria may be too high for screening for early disease or for evaluating patients already receiving systemic therapies for psoriatic disease, the investigators said.
Looking at a lower cutoff point of three of five ASAS criteria, sensitivity was “quite high” for detecting established axial psoriatic arthritis, they said, increasing from 59% to 84%, while specificity remained relatively high, decreasing from 84% to 80%.
“We suggest that the standard cutoffs for this questionnaire be used for patients with active axial psoriatic arthritis, and the lower cutoffs should be used among patients with established axial psoriatic arthritis, where patients can potentially be in remission or partial remission,” they wrote in their report.
These findings could have important implications for the use of this screening tool in patients with psoriatic arthritis; however, more research is needed to validate the observations, the researchers cautioned.
Dr. Haroon reported competing interests related to AbbVie, Pfizer, and Celgene.
SOURCE: Haroon M et al. Ann Rheum Dis. 2018 Dec 14. doi: 10.1136/annrheumdis-2018-214583.
A back pain screening questionnaire developed for ankylosing spondylitis performs well for identifying the subset of axial psoriatic arthritis patients who have active symptoms, according to researchers.
The inflammatory back pain criteria didn’t perform as well when patients with established disease but no symptoms were included, though using a lower cutoff point for the questionnaire improved its sensitivity, the researchers reported in the Annals of the Rheumatic Diseases.
Previous investigations showed that the inflammatory back pain criteria, as defined by the Assessment of Spondyloarthritis International Society (ASAS), had low sensitivity and high specificity for axial involvement in psoriatic arthritis.
Those earlier studies may have registered suboptimal performance of the inflammatory back pain criteria by not distinguishing between patients with axial disease in remission and those with active symptoms, according to Muhammad Haroon, PhD, of the division of rheumatology at University Hospital Kerry in Tralee, Ireland, and his coinvestigators.
The present study, which they said represents a much larger cohort than earlier investigations, included 406 patients with psoriatic arthritis, about one-quarter of whom had rheumatologist-diagnosed axial psoriatic arthritis. The mean age of the axial psoriatic arthritis patients was 51 years and 53% were male.
The researchers found that the inflammatory back pain criteria had poor sensitivity but good specificity at 59% and 84%, respectively, in patients with established axial psoriatic arthritis, defined as axial disease regardless of whether it was active or in remission.
By contrast, the criteria had good sensitivity and good specificity at 82% and 88%, respectively, in patients who had active axial psoriatic arthritis, according to the investigators.
The standard cutoff points used by the ASAS inflammatory back pain criteria may be too high for screening for early disease or for evaluating patients already receiving systemic therapies for psoriatic disease, the investigators said.
Looking at a lower cutoff point of three of five ASAS criteria, sensitivity was “quite high” for detecting established axial psoriatic arthritis, they said, increasing from 59% to 84%, while specificity remained relatively high, decreasing from 84% to 80%.
“We suggest that the standard cutoffs for this questionnaire be used for patients with active axial psoriatic arthritis, and the lower cutoffs should be used among patients with established axial psoriatic arthritis, where patients can potentially be in remission or partial remission,” they wrote in their report.
These findings could have important implications for the use of this screening tool in patients with psoriatic arthritis; however, more research is needed to validate the observations, the researchers cautioned.
Dr. Haroon reported competing interests related to AbbVie, Pfizer, and Celgene.
SOURCE: Haroon M et al. Ann Rheum Dis. 2018 Dec 14. doi: 10.1136/annrheumdis-2018-214583.
A back pain screening questionnaire developed for ankylosing spondylitis performs well for identifying the subset of axial psoriatic arthritis patients who have active symptoms, according to researchers.
The inflammatory back pain criteria didn’t perform as well when patients with established disease but no symptoms were included, though using a lower cutoff point for the questionnaire improved its sensitivity, the researchers reported in the Annals of the Rheumatic Diseases.
Previous investigations showed that the inflammatory back pain criteria, as defined by the Assessment of Spondyloarthritis International Society (ASAS), had low sensitivity and high specificity for axial involvement in psoriatic arthritis.
Those earlier studies may have registered suboptimal performance of the inflammatory back pain criteria by not distinguishing between patients with axial disease in remission and those with active symptoms, according to Muhammad Haroon, PhD, of the division of rheumatology at University Hospital Kerry in Tralee, Ireland, and his coinvestigators.
The present study, which they said represents a much larger cohort than earlier investigations, included 406 patients with psoriatic arthritis, about one-quarter of whom had rheumatologist-diagnosed axial psoriatic arthritis. The mean age of the axial psoriatic arthritis patients was 51 years and 53% were male.
The researchers found that the inflammatory back pain criteria had poor sensitivity but good specificity at 59% and 84%, respectively, in patients with established axial psoriatic arthritis, defined as axial disease regardless of whether it was active or in remission.
By contrast, the criteria had good sensitivity and good specificity at 82% and 88%, respectively, in patients who had active axial psoriatic arthritis, according to the investigators.
The standard cutoff points used by the ASAS inflammatory back pain criteria may be too high for screening for early disease or for evaluating patients already receiving systemic therapies for psoriatic disease, the investigators said.
Looking at a lower cutoff point of three of five ASAS criteria, sensitivity was “quite high” for detecting established axial psoriatic arthritis, they said, increasing from 59% to 84%, while specificity remained relatively high, decreasing from 84% to 80%.
“We suggest that the standard cutoffs for this questionnaire be used for patients with active axial psoriatic arthritis, and the lower cutoffs should be used among patients with established axial psoriatic arthritis, where patients can potentially be in remission or partial remission,” they wrote in their report.
These findings could have important implications for the use of this screening tool in patients with psoriatic arthritis; however, more research is needed to validate the observations, the researchers cautioned.
Dr. Haroon reported competing interests related to AbbVie, Pfizer, and Celgene.
SOURCE: Haroon M et al. Ann Rheum Dis. 2018 Dec 14. doi: 10.1136/annrheumdis-2018-214583.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point: An inflammatory back pain screening questionnaire, developed for ankylosing spondylitis, performed well in identifying axial psoriatic arthritis in patients with active symptoms.
Major finding: The tool performed suboptimally when patients without active symptoms were included, but had good sensitivity (82%) and specificity (88%) in patients with active axial psoriatic arthritis.
Study details: A study including more than 400 patients with psoriatic arthritis.
Disclosures: The corresponding author reported competing interests related to AbbVie, Pfizer, and Celgene.
Source: Haroon M et al. Ann Rheum Dis. 2018 Dec 14. doi: 10.1136/annrheumdis-2018-214583.
Algorithm proposes approach for managing TNF inhibitor–induced psoriasis
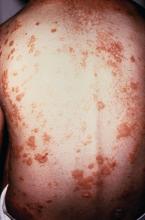
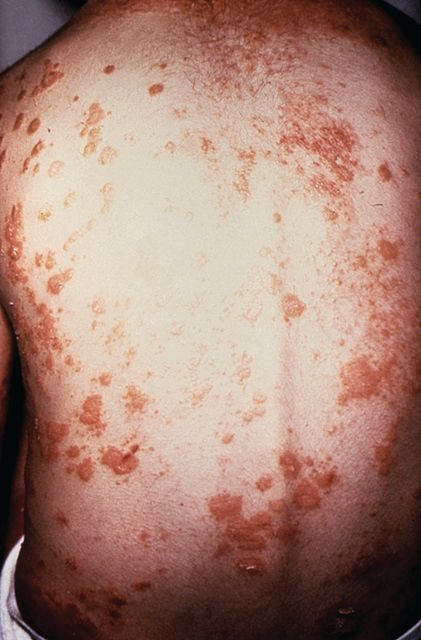
Patients with tumor necrosis factor inhibitor–induced psoriasis could potentially be switched to a different drug class if they have moderate to severe skin eruption or mild skin eruption with an uncontrolled underlying disease such as inflammatory bowel disease, psoriasis, psoriatic arthritis, or rheumatoid arthritis, according to a new treatment algorithm proposed by researchers from Brigham and Women’s Hospital and Harvard Medical School in Boston.
The researchers outlined the prevalence of tumor necrosis factor–alpha inhibitor (TNFi)-induced psoriasis in a literature review of inflammatory bowel disease (IBD), psoriasis, psoriatic arthritis (PsA), and rheumatoid arthritis (RA) and identified an estimated rate of between 2.3% and 5% in patients with RA and between 1.6% and 2.7% in patients with IBD. Although there have been reports of TNFi-induced psoriasis in patients with psoriasis and PsA, the prevalence is unclear, they wrote in the Journal of Psoriasis and Psoriatic Arthritis.
The authors then created an algorithm to manage and treat TNFi-induced psoriasiform skin eruptions with decisions to continue therapy and “treat through” symptoms, switch to a different anti-TNF therapy, or switch to a different drug class based on severity of symptoms, whether the underlying disease is well controlled, and how patients with those underlying diseases have fared with those specific therapies or agents.
“We’ve shifted gears over the past decade, and we’ve gone from having very few agents and trying to keep patients desperately on one or two agents because we didn’t want to have to give up on them for their other comorbid disease, whether it was Crohn’s, colitis, RA, or whatever it may be,” senior author Joseph Merola, MD, director of the Center for Skin and Related Musculoskeletal Diseases at Brigham and Women’s Hospital, Boston, said in an interview. “We’re now in an area where we can have an algorithm like this, and we have so many more mechanistic options to move to.”
Dr. Merola, who is board certified in dermatology and rheumatology, said the algorithm is meant to “open a dialogue” with other specialists in different areas and raise awareness of treatments in related but separate fields. For diseases not often seen by more than one specialty, with the exception of psoriasis and PsA, he said that “the idea is to start a dialogue and increase communication between specialists.”
Dr. Merola noted that while the algorithm in many respects is meant to guide a physician in a specialty in appropriate medication decisions, at the same time he hopes that “it opens a dialogue and communication with the other specialty who tends to oversee this particular disease state or class of medicine to really work together to try to find the right drug for the right person.”
For patients with a mild skin eruption and a controlled underlying disease, the algorithm recommends a “treat through” approach by continuing anti-TNF therapy and treating psoriasis symptoms with topical steroids, ultraviolet therapy, methotrexate, cyclosporine, or acitretin, and to consider dapsone in cases of pustular psoriasis. However, the researchers noted that “treat through” studies have reported complete symptom resolution in 26%-41% of patients.
For patients with recalcitrant or worsening TNFi-induced psoriasis or patients with mild skin eruptions with an uncontrolled underlying disease, the researchers proposed considering switching to a different anti-TNF therapy, although studies have shown complete resolution of symptoms in only 5%-37% of patients.
If patients worsen from there, or if they have moderate to-severe skin eruption with uncontrolled underlying disease, they could be considered for switching to a different drug class and treated based on their underlying disease, along with treatment for psoriasis symptoms. This approach has been shown to completely resolve lesions in up to 64% of cases, they said. IBD patients could benefit from ustekinumab, vedolizumab, 6-mercaptopurine, or azathioprine as an alternative to anti-TNF therapy. Those patients with psoriasis should be considered for guselkumab, while ustekinumab, ixekizumab, secukinumab, and apremilast are effective treatments for patients with psoriasis and PsA. Patients with RA could receive treatment with tocilizumab, rituximab, abatacept, and tofacitinib, the authors wrote.
Dr. Merola reported serving as a consultant and/or investigator for Merck Research Laboratories, AbbVie, Dermavant, Eli Lilly, Novartis, Janssen, UCB, Samumed, Celgene, Sanofi Regeneron, GlaxoSmithKline, Almirall, Sun Pharma, Biogen, Pfizer, Incyte, Aclaris, and Leo Pharma.
SOURCE: Li SJ et al. J Psoriasis Psoriatic Arthritis. 2018 Nov 21. doi: 10.1177/2475530318810851.
Patients with tumor necrosis factor inhibitor–induced psoriasis could potentially be switched to a different drug class if they have moderate to severe skin eruption or mild skin eruption with an uncontrolled underlying disease such as inflammatory bowel disease, psoriasis, psoriatic arthritis, or rheumatoid arthritis, according to a new treatment algorithm proposed by researchers from Brigham and Women’s Hospital and Harvard Medical School in Boston.
The researchers outlined the prevalence of tumor necrosis factor–alpha inhibitor (TNFi)-induced psoriasis in a literature review of inflammatory bowel disease (IBD), psoriasis, psoriatic arthritis (PsA), and rheumatoid arthritis (RA) and identified an estimated rate of between 2.3% and 5% in patients with RA and between 1.6% and 2.7% in patients with IBD. Although there have been reports of TNFi-induced psoriasis in patients with psoriasis and PsA, the prevalence is unclear, they wrote in the Journal of Psoriasis and Psoriatic Arthritis.
The authors then created an algorithm to manage and treat TNFi-induced psoriasiform skin eruptions with decisions to continue therapy and “treat through” symptoms, switch to a different anti-TNF therapy, or switch to a different drug class based on severity of symptoms, whether the underlying disease is well controlled, and how patients with those underlying diseases have fared with those specific therapies or agents.
“We’ve shifted gears over the past decade, and we’ve gone from having very few agents and trying to keep patients desperately on one or two agents because we didn’t want to have to give up on them for their other comorbid disease, whether it was Crohn’s, colitis, RA, or whatever it may be,” senior author Joseph Merola, MD, director of the Center for Skin and Related Musculoskeletal Diseases at Brigham and Women’s Hospital, Boston, said in an interview. “We’re now in an area where we can have an algorithm like this, and we have so many more mechanistic options to move to.”
Dr. Merola, who is board certified in dermatology and rheumatology, said the algorithm is meant to “open a dialogue” with other specialists in different areas and raise awareness of treatments in related but separate fields. For diseases not often seen by more than one specialty, with the exception of psoriasis and PsA, he said that “the idea is to start a dialogue and increase communication between specialists.”
Dr. Merola noted that while the algorithm in many respects is meant to guide a physician in a specialty in appropriate medication decisions, at the same time he hopes that “it opens a dialogue and communication with the other specialty who tends to oversee this particular disease state or class of medicine to really work together to try to find the right drug for the right person.”
For patients with a mild skin eruption and a controlled underlying disease, the algorithm recommends a “treat through” approach by continuing anti-TNF therapy and treating psoriasis symptoms with topical steroids, ultraviolet therapy, methotrexate, cyclosporine, or acitretin, and to consider dapsone in cases of pustular psoriasis. However, the researchers noted that “treat through” studies have reported complete symptom resolution in 26%-41% of patients.
For patients with recalcitrant or worsening TNFi-induced psoriasis or patients with mild skin eruptions with an uncontrolled underlying disease, the researchers proposed considering switching to a different anti-TNF therapy, although studies have shown complete resolution of symptoms in only 5%-37% of patients.
If patients worsen from there, or if they have moderate to-severe skin eruption with uncontrolled underlying disease, they could be considered for switching to a different drug class and treated based on their underlying disease, along with treatment for psoriasis symptoms. This approach has been shown to completely resolve lesions in up to 64% of cases, they said. IBD patients could benefit from ustekinumab, vedolizumab, 6-mercaptopurine, or azathioprine as an alternative to anti-TNF therapy. Those patients with psoriasis should be considered for guselkumab, while ustekinumab, ixekizumab, secukinumab, and apremilast are effective treatments for patients with psoriasis and PsA. Patients with RA could receive treatment with tocilizumab, rituximab, abatacept, and tofacitinib, the authors wrote.
Dr. Merola reported serving as a consultant and/or investigator for Merck Research Laboratories, AbbVie, Dermavant, Eli Lilly, Novartis, Janssen, UCB, Samumed, Celgene, Sanofi Regeneron, GlaxoSmithKline, Almirall, Sun Pharma, Biogen, Pfizer, Incyte, Aclaris, and Leo Pharma.
SOURCE: Li SJ et al. J Psoriasis Psoriatic Arthritis. 2018 Nov 21. doi: 10.1177/2475530318810851.
Patients with tumor necrosis factor inhibitor–induced psoriasis could potentially be switched to a different drug class if they have moderate to severe skin eruption or mild skin eruption with an uncontrolled underlying disease such as inflammatory bowel disease, psoriasis, psoriatic arthritis, or rheumatoid arthritis, according to a new treatment algorithm proposed by researchers from Brigham and Women’s Hospital and Harvard Medical School in Boston.
The researchers outlined the prevalence of tumor necrosis factor–alpha inhibitor (TNFi)-induced psoriasis in a literature review of inflammatory bowel disease (IBD), psoriasis, psoriatic arthritis (PsA), and rheumatoid arthritis (RA) and identified an estimated rate of between 2.3% and 5% in patients with RA and between 1.6% and 2.7% in patients with IBD. Although there have been reports of TNFi-induced psoriasis in patients with psoriasis and PsA, the prevalence is unclear, they wrote in the Journal of Psoriasis and Psoriatic Arthritis.
The authors then created an algorithm to manage and treat TNFi-induced psoriasiform skin eruptions with decisions to continue therapy and “treat through” symptoms, switch to a different anti-TNF therapy, or switch to a different drug class based on severity of symptoms, whether the underlying disease is well controlled, and how patients with those underlying diseases have fared with those specific therapies or agents.
“We’ve shifted gears over the past decade, and we’ve gone from having very few agents and trying to keep patients desperately on one or two agents because we didn’t want to have to give up on them for their other comorbid disease, whether it was Crohn’s, colitis, RA, or whatever it may be,” senior author Joseph Merola, MD, director of the Center for Skin and Related Musculoskeletal Diseases at Brigham and Women’s Hospital, Boston, said in an interview. “We’re now in an area where we can have an algorithm like this, and we have so many more mechanistic options to move to.”
Dr. Merola, who is board certified in dermatology and rheumatology, said the algorithm is meant to “open a dialogue” with other specialists in different areas and raise awareness of treatments in related but separate fields. For diseases not often seen by more than one specialty, with the exception of psoriasis and PsA, he said that “the idea is to start a dialogue and increase communication between specialists.”
Dr. Merola noted that while the algorithm in many respects is meant to guide a physician in a specialty in appropriate medication decisions, at the same time he hopes that “it opens a dialogue and communication with the other specialty who tends to oversee this particular disease state or class of medicine to really work together to try to find the right drug for the right person.”
For patients with a mild skin eruption and a controlled underlying disease, the algorithm recommends a “treat through” approach by continuing anti-TNF therapy and treating psoriasis symptoms with topical steroids, ultraviolet therapy, methotrexate, cyclosporine, or acitretin, and to consider dapsone in cases of pustular psoriasis. However, the researchers noted that “treat through” studies have reported complete symptom resolution in 26%-41% of patients.
For patients with recalcitrant or worsening TNFi-induced psoriasis or patients with mild skin eruptions with an uncontrolled underlying disease, the researchers proposed considering switching to a different anti-TNF therapy, although studies have shown complete resolution of symptoms in only 5%-37% of patients.
If patients worsen from there, or if they have moderate to-severe skin eruption with uncontrolled underlying disease, they could be considered for switching to a different drug class and treated based on their underlying disease, along with treatment for psoriasis symptoms. This approach has been shown to completely resolve lesions in up to 64% of cases, they said. IBD patients could benefit from ustekinumab, vedolizumab, 6-mercaptopurine, or azathioprine as an alternative to anti-TNF therapy. Those patients with psoriasis should be considered for guselkumab, while ustekinumab, ixekizumab, secukinumab, and apremilast are effective treatments for patients with psoriasis and PsA. Patients with RA could receive treatment with tocilizumab, rituximab, abatacept, and tofacitinib, the authors wrote.
Dr. Merola reported serving as a consultant and/or investigator for Merck Research Laboratories, AbbVie, Dermavant, Eli Lilly, Novartis, Janssen, UCB, Samumed, Celgene, Sanofi Regeneron, GlaxoSmithKline, Almirall, Sun Pharma, Biogen, Pfizer, Incyte, Aclaris, and Leo Pharma.
SOURCE: Li SJ et al. J Psoriasis Psoriatic Arthritis. 2018 Nov 21. doi: 10.1177/2475530318810851.
FROM JOURNAL OF PSORIASIS AND PSORIATIC ARTHRITIS
Complications cluster in inflammatory arthritis patients after total knee replacement
CHICAGO – Patients with an inflammatory arthritis had significantly higher rates of infections, transfusions, and readmissions following total knee replacement than did patients without inflammatory arthritis in a study of more than 137,000 Americans who underwent this surgery.
A sampling of U.S. patients who underwent total knee arthroplasty (TKA) during 2007-2016 showed that among the small percentage of these patients who had an inflammatory arthritis (IA), the rate of periprosthetic joint or wound infection while hospitalized or out to 30 days after surgery was a statistically significant 64% higher relative to patients without inflammatory arthritis, after adjustment for several demographic and clinical confounders, including recent glucocorticoid treatment, Susan M. Goodman, MD, said at the annual meeting of the American College of Rheumatology. The analysis also showed a statistically significant 46% higher relative rate of hospital readmission for any cause during the 90 days after surgery, and a significant 39% relative increase in blood transfusions during the 30 days after TKA in the IA patients.
“These results have important implications for evolving bundled payment models” for TKA, said Dr. Goodman, a rheumatologist at the Hospital for Special Surgery in New York. “Hospitals should receive commensurate resources to maintain access to total TKA for patients with IA.”
For this analysis, Dr. Goodman and her associates classified IA as a patient with a recorded diagnosis of rheumatoid arthritis, spondyloarthritis, or systemic lupus erythematosus if the patient had also received treatment during the year before surgery with a disease-modifying antirheumatic drug, a biologic agent, or a drug that treats systemic lupus erythematosus.
Complications following TKA became a particular concern to hospitals starting in 2013 when the Centers for Medicare & Medicaid Services began a program that penalized hospitals for outcomes such as excessive readmissions following selected types of hospitalizations and also with recent steps to bundle TKA reimbursement with related 90-day outcomes.
“My concern is to ensure that patients with IA aren’t penalized and can maintain access” to TKA despite recent policy moves by the CMS. Faced with potential disincentives to treat patients with an IA, “hospitals might cherry pick patients,” Dr. Goodman said in an interview. The new findings “are a reason for administrators to argue for patients with IA to come out of the cost bundle.”
Dr. Goodman expressed hope that future policies will better reflect the higher levels of risk faced by patients with an IA undergoing TKA. CMS “is pretty responsive,” she said.
The study used data collected by Humana for about 25 million American health insurance beneficiaries during 2007-2016, which included 137,550 people who underwent a TKA. Of these, 3,067 (2%) met the study’s definition for IA, and 134,483 did not. Most of those who did not meet the definition likely had osteoarthritis, Dr. Goodman said. This low percentage of U.S. TKA patients with IA was consistent with numbers in prior reports.
The researchers calculated the relative risk of the IA patients, compared with all the others, for nine potential complications, including acute MI, pneumonia, sepsis, pulmonary embolism, and death. The complications with significantly higher rates among the IA patients after confounder adjustment were 30-day infections, 30-day transfusions, and 90-day readmissions.
Dr. Goodman had no relevant disclosures.
SOURCE: Richardson S et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1932.
CHICAGO – Patients with an inflammatory arthritis had significantly higher rates of infections, transfusions, and readmissions following total knee replacement than did patients without inflammatory arthritis in a study of more than 137,000 Americans who underwent this surgery.
A sampling of U.S. patients who underwent total knee arthroplasty (TKA) during 2007-2016 showed that among the small percentage of these patients who had an inflammatory arthritis (IA), the rate of periprosthetic joint or wound infection while hospitalized or out to 30 days after surgery was a statistically significant 64% higher relative to patients without inflammatory arthritis, after adjustment for several demographic and clinical confounders, including recent glucocorticoid treatment, Susan M. Goodman, MD, said at the annual meeting of the American College of Rheumatology. The analysis also showed a statistically significant 46% higher relative rate of hospital readmission for any cause during the 90 days after surgery, and a significant 39% relative increase in blood transfusions during the 30 days after TKA in the IA patients.
“These results have important implications for evolving bundled payment models” for TKA, said Dr. Goodman, a rheumatologist at the Hospital for Special Surgery in New York. “Hospitals should receive commensurate resources to maintain access to total TKA for patients with IA.”
For this analysis, Dr. Goodman and her associates classified IA as a patient with a recorded diagnosis of rheumatoid arthritis, spondyloarthritis, or systemic lupus erythematosus if the patient had also received treatment during the year before surgery with a disease-modifying antirheumatic drug, a biologic agent, or a drug that treats systemic lupus erythematosus.
Complications following TKA became a particular concern to hospitals starting in 2013 when the Centers for Medicare & Medicaid Services began a program that penalized hospitals for outcomes such as excessive readmissions following selected types of hospitalizations and also with recent steps to bundle TKA reimbursement with related 90-day outcomes.
“My concern is to ensure that patients with IA aren’t penalized and can maintain access” to TKA despite recent policy moves by the CMS. Faced with potential disincentives to treat patients with an IA, “hospitals might cherry pick patients,” Dr. Goodman said in an interview. The new findings “are a reason for administrators to argue for patients with IA to come out of the cost bundle.”
Dr. Goodman expressed hope that future policies will better reflect the higher levels of risk faced by patients with an IA undergoing TKA. CMS “is pretty responsive,” she said.
The study used data collected by Humana for about 25 million American health insurance beneficiaries during 2007-2016, which included 137,550 people who underwent a TKA. Of these, 3,067 (2%) met the study’s definition for IA, and 134,483 did not. Most of those who did not meet the definition likely had osteoarthritis, Dr. Goodman said. This low percentage of U.S. TKA patients with IA was consistent with numbers in prior reports.
The researchers calculated the relative risk of the IA patients, compared with all the others, for nine potential complications, including acute MI, pneumonia, sepsis, pulmonary embolism, and death. The complications with significantly higher rates among the IA patients after confounder adjustment were 30-day infections, 30-day transfusions, and 90-day readmissions.
Dr. Goodman had no relevant disclosures.
SOURCE: Richardson S et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1932.
CHICAGO – Patients with an inflammatory arthritis had significantly higher rates of infections, transfusions, and readmissions following total knee replacement than did patients without inflammatory arthritis in a study of more than 137,000 Americans who underwent this surgery.
A sampling of U.S. patients who underwent total knee arthroplasty (TKA) during 2007-2016 showed that among the small percentage of these patients who had an inflammatory arthritis (IA), the rate of periprosthetic joint or wound infection while hospitalized or out to 30 days after surgery was a statistically significant 64% higher relative to patients without inflammatory arthritis, after adjustment for several demographic and clinical confounders, including recent glucocorticoid treatment, Susan M. Goodman, MD, said at the annual meeting of the American College of Rheumatology. The analysis also showed a statistically significant 46% higher relative rate of hospital readmission for any cause during the 90 days after surgery, and a significant 39% relative increase in blood transfusions during the 30 days after TKA in the IA patients.
“These results have important implications for evolving bundled payment models” for TKA, said Dr. Goodman, a rheumatologist at the Hospital for Special Surgery in New York. “Hospitals should receive commensurate resources to maintain access to total TKA for patients with IA.”
For this analysis, Dr. Goodman and her associates classified IA as a patient with a recorded diagnosis of rheumatoid arthritis, spondyloarthritis, or systemic lupus erythematosus if the patient had also received treatment during the year before surgery with a disease-modifying antirheumatic drug, a biologic agent, or a drug that treats systemic lupus erythematosus.
Complications following TKA became a particular concern to hospitals starting in 2013 when the Centers for Medicare & Medicaid Services began a program that penalized hospitals for outcomes such as excessive readmissions following selected types of hospitalizations and also with recent steps to bundle TKA reimbursement with related 90-day outcomes.
“My concern is to ensure that patients with IA aren’t penalized and can maintain access” to TKA despite recent policy moves by the CMS. Faced with potential disincentives to treat patients with an IA, “hospitals might cherry pick patients,” Dr. Goodman said in an interview. The new findings “are a reason for administrators to argue for patients with IA to come out of the cost bundle.”
Dr. Goodman expressed hope that future policies will better reflect the higher levels of risk faced by patients with an IA undergoing TKA. CMS “is pretty responsive,” she said.
The study used data collected by Humana for about 25 million American health insurance beneficiaries during 2007-2016, which included 137,550 people who underwent a TKA. Of these, 3,067 (2%) met the study’s definition for IA, and 134,483 did not. Most of those who did not meet the definition likely had osteoarthritis, Dr. Goodman said. This low percentage of U.S. TKA patients with IA was consistent with numbers in prior reports.
The researchers calculated the relative risk of the IA patients, compared with all the others, for nine potential complications, including acute MI, pneumonia, sepsis, pulmonary embolism, and death. The complications with significantly higher rates among the IA patients after confounder adjustment were 30-day infections, 30-day transfusions, and 90-day readmissions.
Dr. Goodman had no relevant disclosures.
SOURCE: Richardson S et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1932.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point: Complications were more common after total knee arthroplasty in patients with an inflammatory arthritis.
Major finding: Inflammatory arthritis patients had a 64% higher rate of infections after total knee arthroplasty, compared with patients without inflammatory arthritis.
Study details: Data analysis for 137,550 Americans who underwent total knee arthroplasty during 2007-2016.
Disclosures: Dr. Goodman had no relevant disclosures.
Source: Richardson S et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1932.
FDA approves adalimumab biosimilar Hyrimoz
The Food and Drug Administration has approved the adalimumab biosimilar Hyrimoz (adalimumab-adaz) for a variety of conditions, according to Sandoz, the drug’s manufacturer and a division of Novartis.
FDA approval for Hyrimoz is based on a randomized, double-blind, three-arm, parallel biosimilarity study that demonstrated equivalence for all primary pharmacokinetic parameters, according to the press release. A second study confirmed these results in patients with moderate to severe plaque psoriasis, with Hyrimoz having a safety profile similar to that of adalimumab. Hyrimoz was approved in Europe in July 2018.
Hyrimoz has been approved to treat rheumatoid arthritis, juvenile idiopathic arthritis in patients aged 4 years and older, psoriatic arthritis, ankylosing spondylitis, adult Crohn’s disease, ulcerative colitis, and plaque psoriasis. The most common adverse events associated with the drug, according to the label, are infections, injection site reactions, headache, and rash.
Hyrimoz is the third adalimumab biosimilar approved by the FDA.
“Biosimilars can help people suffering from chronic, debilitating conditions gain expanded access to important medicines that may change the outcome of their disease. With the FDA approval of Hyrimoz, Sandoz is one step closer to offering U.S. patients with autoimmune diseases the same critical access already available in Europe,” Stefan Hendriks, global head of biopharmaceuticals at Sandoz, said in the press release.
Find the full press release on the Novartis website.
The Food and Drug Administration has approved the adalimumab biosimilar Hyrimoz (adalimumab-adaz) for a variety of conditions, according to Sandoz, the drug’s manufacturer and a division of Novartis.
FDA approval for Hyrimoz is based on a randomized, double-blind, three-arm, parallel biosimilarity study that demonstrated equivalence for all primary pharmacokinetic parameters, according to the press release. A second study confirmed these results in patients with moderate to severe plaque psoriasis, with Hyrimoz having a safety profile similar to that of adalimumab. Hyrimoz was approved in Europe in July 2018.
Hyrimoz has been approved to treat rheumatoid arthritis, juvenile idiopathic arthritis in patients aged 4 years and older, psoriatic arthritis, ankylosing spondylitis, adult Crohn’s disease, ulcerative colitis, and plaque psoriasis. The most common adverse events associated with the drug, according to the label, are infections, injection site reactions, headache, and rash.
Hyrimoz is the third adalimumab biosimilar approved by the FDA.
“Biosimilars can help people suffering from chronic, debilitating conditions gain expanded access to important medicines that may change the outcome of their disease. With the FDA approval of Hyrimoz, Sandoz is one step closer to offering U.S. patients with autoimmune diseases the same critical access already available in Europe,” Stefan Hendriks, global head of biopharmaceuticals at Sandoz, said in the press release.
Find the full press release on the Novartis website.
The Food and Drug Administration has approved the adalimumab biosimilar Hyrimoz (adalimumab-adaz) for a variety of conditions, according to Sandoz, the drug’s manufacturer and a division of Novartis.
FDA approval for Hyrimoz is based on a randomized, double-blind, three-arm, parallel biosimilarity study that demonstrated equivalence for all primary pharmacokinetic parameters, according to the press release. A second study confirmed these results in patients with moderate to severe plaque psoriasis, with Hyrimoz having a safety profile similar to that of adalimumab. Hyrimoz was approved in Europe in July 2018.
Hyrimoz has been approved to treat rheumatoid arthritis, juvenile idiopathic arthritis in patients aged 4 years and older, psoriatic arthritis, ankylosing spondylitis, adult Crohn’s disease, ulcerative colitis, and plaque psoriasis. The most common adverse events associated with the drug, according to the label, are infections, injection site reactions, headache, and rash.
Hyrimoz is the third adalimumab biosimilar approved by the FDA.
“Biosimilars can help people suffering from chronic, debilitating conditions gain expanded access to important medicines that may change the outcome of their disease. With the FDA approval of Hyrimoz, Sandoz is one step closer to offering U.S. patients with autoimmune diseases the same critical access already available in Europe,” Stefan Hendriks, global head of biopharmaceuticals at Sandoz, said in the press release.
Find the full press release on the Novartis website.
ACR readies first-ever guidelines on managing reproductive health in rheumatology
CHICAGO – Help is on the way for rheumatologists who may feel out of their depth regarding reproductive health issues in their patients.
for internal review in draft form. Lisa R. Sammaritano, MD, a leader of the expert panel that developed the evidence-based recommendations, shared highlights of the forthcoming guidelines at the annual meeting of the American College of Rheumatology.
“Our patients, fortunately, are pursuing pregnancy more often now than in years past. One of the key messages of the guidelines is that patients really do want to discuss these topics with their rheumatologist, even though that often does not happen now. What patients told us [in the guideline-development process] is their rheumatologist knows them better than their gynecologist or any of their other doctors because we have followed them for a long period of time and we understand their disease and their symptoms. They really want our input on questions about contraception, when to plan a pregnancy, and medication use,” said Dr. Sammaritano of the Hospital for Special Surgery and Cornell University in New York.
The guidelines were created over the course of a year and a half with extensive input from ob.gyns., as well as a patient panel. The project included a systematic review of more than 300 published studies in which guideline panelists attempt to find answers to an initial list of 370 questions. Dr. Sammaritano predicted that the guidelines will prove to be useful not only for rheumatologists, but for their colleagues in ob.gyn. as well. Just as rheumatologists likely haven’t kept up with the sea changes that have occurred in ob.gyn. since their medical school days, most ob.gyns. know little about rheumatic diseases.
“There’s room for education on both sides,” she observed in an interview. “I have had to write letters to gynecologists to get them to put my patients with antiphospholipid antibodies on a contraceptive that includes a progestin because the labeling says, ‘May increase risk of thrombosis.’ And yet if you look at the literature, most of the progestins do not increase the risk of thrombosis, even in patients who are already at increased risk because of a genetic prothrombotic abnormality. I practically had to sign my life away to get a gynecologist to put a progestin-containing IUD in my patient, whereas the risk of thrombosis to my patient with an unplanned pregnancy would have been 10-fold or 100-fold higher. Unplanned pregnancy is dangerous for patients with our diseases.”
And yet, she noted, half of all pregnancies in the United States are unplanned. Among women with rheumatic diseases, the proportion may well be even higher in light of their documented low rate of utilization of effective contraception.
A publication date for the guidelines won’t be set until the review is completed, but the plan is to issue three separate documents. One will address reproductive health outside of pregnancy, with key topics to include contraception, fertility preservation, menopause, and hormone replacement therapy. The second document will focus on pregnancy management, with special attention devoted to women with lupus or antiphospholipid antibodies because they are at particularly high risk of adverse pregnancy outcomes. The third document will be devoted to medications, covering issues including which medications can be continued during pregnancy and when to safely stop the ones that can’t. This section will address both maternal and paternal use of rheumatologic medications, the latter being a topic below the radar of ob.gyns.
The three medications whose paternal use in pregnancy generate the most questions in clinical practice are methotrexate, cyclophosphamide, and sulfasalazine.
“I cannot tell you how many times I’ve been asked whether male patients with rheumatic diseases need to stop their methotrexate before they plan to father a child – that’s been a big one. The answer is they don’t need to stop, but that’s a conditional recommendation because the product label still says to stop it 3 months before. But that’s based on theoretical concerns, and all the data support a lack of teratogenicity for men using methotrexate prior to and during pregnancy,” Dr. Sammaritano said.
Men on cyclophosphamide absolutely have to stop the drug 3 months before pregnancy because the drug causes DNA fragmentation in the sperm. Sulfasalazine is known to impair male fertility. The ACR guidelines will recommend that men continue the drug, but if pregnancy doesn’t occur within a reasonable time, then it’s appropriate to get a semen analysis rather than stopping sulfasalazine unnecessarily.
American College of Obstetricians and Gynecologists guidelines now recommend long-acting reversible contraception, including IUDs and progestin implants, as first-line contraception for all women. The ACR draft guidelines strongly recommend the same.
“That is new. The use of this form of contraception in women with rheumatic diseases is quite low. In general, our patients don’t use contraception as often as other women, and when they do, they don’t use effective contraception. There are many theories as to why that may be: perhaps it’s a focus on the more immediate issues of their rheumatic disease that doesn’t allow their rheumatologist to get to the point of discussing contraception,” according to Dr. Sammaritano.
Many rheumatologists will be pleasantly surprised to learn that the problem of increased risk of pelvic inflammatory disease associated with earlier-generation IUDs is no longer an issue with the current devices. And contrary to a misconception among some ob.gyns., autoimmune disease will not cause a woman to reject her IUD.
The ACR guidelines recommend continuing hydroxychloroquine in lupus patients during pregnancy – and considering starting the drug in those not already on it – because of strong evidence supporting both safety and benefit for mother and baby.
“We are recommending the use of low-dose aspirin for patients with lupus and antiphospholipid antibodies because those two conditions increase the risk for preeclampsia, and the ob.gyns. routinely use low-dose aspirin starting toward the end of the first trimester as preventive therapy. Large studies show that it reduces the risk,” she continued.
Dr. Sammaritano cautioned that the literature on the use of rheumatologic medications in pregnancy and breast feeding is generally weak – and in the case of the new oral small molecule JAK inhibitors, essentially nonexistent.
“A lot of our recommendations are conditional because we did not feel that the data support a strong recommendation. But you have to do something. As long as you communicate the idea that we’re doing the best we can with what information is available, I think patients will respond to that,” the rheumatologist said.
She reported having no financial conflicts regarding her presentation.
CHICAGO – Help is on the way for rheumatologists who may feel out of their depth regarding reproductive health issues in their patients.
for internal review in draft form. Lisa R. Sammaritano, MD, a leader of the expert panel that developed the evidence-based recommendations, shared highlights of the forthcoming guidelines at the annual meeting of the American College of Rheumatology.
“Our patients, fortunately, are pursuing pregnancy more often now than in years past. One of the key messages of the guidelines is that patients really do want to discuss these topics with their rheumatologist, even though that often does not happen now. What patients told us [in the guideline-development process] is their rheumatologist knows them better than their gynecologist or any of their other doctors because we have followed them for a long period of time and we understand their disease and their symptoms. They really want our input on questions about contraception, when to plan a pregnancy, and medication use,” said Dr. Sammaritano of the Hospital for Special Surgery and Cornell University in New York.
The guidelines were created over the course of a year and a half with extensive input from ob.gyns., as well as a patient panel. The project included a systematic review of more than 300 published studies in which guideline panelists attempt to find answers to an initial list of 370 questions. Dr. Sammaritano predicted that the guidelines will prove to be useful not only for rheumatologists, but for their colleagues in ob.gyn. as well. Just as rheumatologists likely haven’t kept up with the sea changes that have occurred in ob.gyn. since their medical school days, most ob.gyns. know little about rheumatic diseases.
“There’s room for education on both sides,” she observed in an interview. “I have had to write letters to gynecologists to get them to put my patients with antiphospholipid antibodies on a contraceptive that includes a progestin because the labeling says, ‘May increase risk of thrombosis.’ And yet if you look at the literature, most of the progestins do not increase the risk of thrombosis, even in patients who are already at increased risk because of a genetic prothrombotic abnormality. I practically had to sign my life away to get a gynecologist to put a progestin-containing IUD in my patient, whereas the risk of thrombosis to my patient with an unplanned pregnancy would have been 10-fold or 100-fold higher. Unplanned pregnancy is dangerous for patients with our diseases.”
And yet, she noted, half of all pregnancies in the United States are unplanned. Among women with rheumatic diseases, the proportion may well be even higher in light of their documented low rate of utilization of effective contraception.
A publication date for the guidelines won’t be set until the review is completed, but the plan is to issue three separate documents. One will address reproductive health outside of pregnancy, with key topics to include contraception, fertility preservation, menopause, and hormone replacement therapy. The second document will focus on pregnancy management, with special attention devoted to women with lupus or antiphospholipid antibodies because they are at particularly high risk of adverse pregnancy outcomes. The third document will be devoted to medications, covering issues including which medications can be continued during pregnancy and when to safely stop the ones that can’t. This section will address both maternal and paternal use of rheumatologic medications, the latter being a topic below the radar of ob.gyns.
The three medications whose paternal use in pregnancy generate the most questions in clinical practice are methotrexate, cyclophosphamide, and sulfasalazine.
“I cannot tell you how many times I’ve been asked whether male patients with rheumatic diseases need to stop their methotrexate before they plan to father a child – that’s been a big one. The answer is they don’t need to stop, but that’s a conditional recommendation because the product label still says to stop it 3 months before. But that’s based on theoretical concerns, and all the data support a lack of teratogenicity for men using methotrexate prior to and during pregnancy,” Dr. Sammaritano said.
Men on cyclophosphamide absolutely have to stop the drug 3 months before pregnancy because the drug causes DNA fragmentation in the sperm. Sulfasalazine is known to impair male fertility. The ACR guidelines will recommend that men continue the drug, but if pregnancy doesn’t occur within a reasonable time, then it’s appropriate to get a semen analysis rather than stopping sulfasalazine unnecessarily.
American College of Obstetricians and Gynecologists guidelines now recommend long-acting reversible contraception, including IUDs and progestin implants, as first-line contraception for all women. The ACR draft guidelines strongly recommend the same.
“That is new. The use of this form of contraception in women with rheumatic diseases is quite low. In general, our patients don’t use contraception as often as other women, and when they do, they don’t use effective contraception. There are many theories as to why that may be: perhaps it’s a focus on the more immediate issues of their rheumatic disease that doesn’t allow their rheumatologist to get to the point of discussing contraception,” according to Dr. Sammaritano.
Many rheumatologists will be pleasantly surprised to learn that the problem of increased risk of pelvic inflammatory disease associated with earlier-generation IUDs is no longer an issue with the current devices. And contrary to a misconception among some ob.gyns., autoimmune disease will not cause a woman to reject her IUD.
The ACR guidelines recommend continuing hydroxychloroquine in lupus patients during pregnancy – and considering starting the drug in those not already on it – because of strong evidence supporting both safety and benefit for mother and baby.
“We are recommending the use of low-dose aspirin for patients with lupus and antiphospholipid antibodies because those two conditions increase the risk for preeclampsia, and the ob.gyns. routinely use low-dose aspirin starting toward the end of the first trimester as preventive therapy. Large studies show that it reduces the risk,” she continued.
Dr. Sammaritano cautioned that the literature on the use of rheumatologic medications in pregnancy and breast feeding is generally weak – and in the case of the new oral small molecule JAK inhibitors, essentially nonexistent.
“A lot of our recommendations are conditional because we did not feel that the data support a strong recommendation. But you have to do something. As long as you communicate the idea that we’re doing the best we can with what information is available, I think patients will respond to that,” the rheumatologist said.
She reported having no financial conflicts regarding her presentation.
CHICAGO – Help is on the way for rheumatologists who may feel out of their depth regarding reproductive health issues in their patients.
for internal review in draft form. Lisa R. Sammaritano, MD, a leader of the expert panel that developed the evidence-based recommendations, shared highlights of the forthcoming guidelines at the annual meeting of the American College of Rheumatology.
“Our patients, fortunately, are pursuing pregnancy more often now than in years past. One of the key messages of the guidelines is that patients really do want to discuss these topics with their rheumatologist, even though that often does not happen now. What patients told us [in the guideline-development process] is their rheumatologist knows them better than their gynecologist or any of their other doctors because we have followed them for a long period of time and we understand their disease and their symptoms. They really want our input on questions about contraception, when to plan a pregnancy, and medication use,” said Dr. Sammaritano of the Hospital for Special Surgery and Cornell University in New York.
The guidelines were created over the course of a year and a half with extensive input from ob.gyns., as well as a patient panel. The project included a systematic review of more than 300 published studies in which guideline panelists attempt to find answers to an initial list of 370 questions. Dr. Sammaritano predicted that the guidelines will prove to be useful not only for rheumatologists, but for their colleagues in ob.gyn. as well. Just as rheumatologists likely haven’t kept up with the sea changes that have occurred in ob.gyn. since their medical school days, most ob.gyns. know little about rheumatic diseases.
“There’s room for education on both sides,” she observed in an interview. “I have had to write letters to gynecologists to get them to put my patients with antiphospholipid antibodies on a contraceptive that includes a progestin because the labeling says, ‘May increase risk of thrombosis.’ And yet if you look at the literature, most of the progestins do not increase the risk of thrombosis, even in patients who are already at increased risk because of a genetic prothrombotic abnormality. I practically had to sign my life away to get a gynecologist to put a progestin-containing IUD in my patient, whereas the risk of thrombosis to my patient with an unplanned pregnancy would have been 10-fold or 100-fold higher. Unplanned pregnancy is dangerous for patients with our diseases.”
And yet, she noted, half of all pregnancies in the United States are unplanned. Among women with rheumatic diseases, the proportion may well be even higher in light of their documented low rate of utilization of effective contraception.
A publication date for the guidelines won’t be set until the review is completed, but the plan is to issue three separate documents. One will address reproductive health outside of pregnancy, with key topics to include contraception, fertility preservation, menopause, and hormone replacement therapy. The second document will focus on pregnancy management, with special attention devoted to women with lupus or antiphospholipid antibodies because they are at particularly high risk of adverse pregnancy outcomes. The third document will be devoted to medications, covering issues including which medications can be continued during pregnancy and when to safely stop the ones that can’t. This section will address both maternal and paternal use of rheumatologic medications, the latter being a topic below the radar of ob.gyns.
The three medications whose paternal use in pregnancy generate the most questions in clinical practice are methotrexate, cyclophosphamide, and sulfasalazine.
“I cannot tell you how many times I’ve been asked whether male patients with rheumatic diseases need to stop their methotrexate before they plan to father a child – that’s been a big one. The answer is they don’t need to stop, but that’s a conditional recommendation because the product label still says to stop it 3 months before. But that’s based on theoretical concerns, and all the data support a lack of teratogenicity for men using methotrexate prior to and during pregnancy,” Dr. Sammaritano said.
Men on cyclophosphamide absolutely have to stop the drug 3 months before pregnancy because the drug causes DNA fragmentation in the sperm. Sulfasalazine is known to impair male fertility. The ACR guidelines will recommend that men continue the drug, but if pregnancy doesn’t occur within a reasonable time, then it’s appropriate to get a semen analysis rather than stopping sulfasalazine unnecessarily.
American College of Obstetricians and Gynecologists guidelines now recommend long-acting reversible contraception, including IUDs and progestin implants, as first-line contraception for all women. The ACR draft guidelines strongly recommend the same.
“That is new. The use of this form of contraception in women with rheumatic diseases is quite low. In general, our patients don’t use contraception as often as other women, and when they do, they don’t use effective contraception. There are many theories as to why that may be: perhaps it’s a focus on the more immediate issues of their rheumatic disease that doesn’t allow their rheumatologist to get to the point of discussing contraception,” according to Dr. Sammaritano.
Many rheumatologists will be pleasantly surprised to learn that the problem of increased risk of pelvic inflammatory disease associated with earlier-generation IUDs is no longer an issue with the current devices. And contrary to a misconception among some ob.gyns., autoimmune disease will not cause a woman to reject her IUD.
The ACR guidelines recommend continuing hydroxychloroquine in lupus patients during pregnancy – and considering starting the drug in those not already on it – because of strong evidence supporting both safety and benefit for mother and baby.
“We are recommending the use of low-dose aspirin for patients with lupus and antiphospholipid antibodies because those two conditions increase the risk for preeclampsia, and the ob.gyns. routinely use low-dose aspirin starting toward the end of the first trimester as preventive therapy. Large studies show that it reduces the risk,” she continued.
Dr. Sammaritano cautioned that the literature on the use of rheumatologic medications in pregnancy and breast feeding is generally weak – and in the case of the new oral small molecule JAK inhibitors, essentially nonexistent.
“A lot of our recommendations are conditional because we did not feel that the data support a strong recommendation. But you have to do something. As long as you communicate the idea that we’re doing the best we can with what information is available, I think patients will respond to that,” the rheumatologist said.
She reported having no financial conflicts regarding her presentation.
REPORTING FROM THE ACR ANNUAL MEETING
Low BMD and spinal syndesmophytes predict radiographic progression in axSpA
Low bone mineral density and the presence of existing spinal syndesmophytes predict the formation of new spinal bony growths and radiographic progression in axial spondyloarthritis (axSpA) at 2 years, according to research conducted by Hyoung Rae Kim of the Catholic University of Korea, Seoul, South Korea, and colleagues.
The investigators noted that low bone mass in ankylosing spondyloarthritis (AS) had been linked in previous studies to spinal structural damage yet “despite the identification of this relationship, it is not yet known whether the presence of low bone mass can independently predict radiographic spinal progression in axSpA patients.”
In order to evaluate the association between low bone mass and the formation of new syndesmophytes and to investigate whether low bone mineral density (BMD) independently predicted radiographic progression in axSpA, the researchers enrolled 119 patients with definite axSpA into their study, published in Arthritis Research & Therapy.
They defined low BMD as z scores of less than or equal to –2.0 and spinal radiographic progression as worsening of the modified Stoke Ankylosing Spondylitis Spinal Score (mSASSS) by 2 or more points over the 2-year follow-up period.
Overall, 34 (29%) of the patients had syndesmophytes at baseline, 90 (76%) had radiographic sacroiliitis fulfilling the modified New York criteria for the classification of AS, and 19 (16%) had low BMD.
At 2 years, new syndesmophytes had developed in 22 (21%) of the patients. New syndesmophyte formation occurred in 15% of patients without low BMD and in 37% of patients with low BMD (P = .047).
In the multivariable analysis, current smoking (odds ratio, 3.0; 95% confidence interval, 1.1-7.7), the presence of syndesmophytes (OR, 4.6; 95% CI, 1.8-11.9); and low BMD at baseline (OR, 3.6; 95% CI, 1.2-11.2) were independently associated with significant spinal progression, the investigators reported.
The presence of baseline syndesmophytes and low BMD at any site (OR, 5.5; 95% CI, 2.0-15.2; and OR, 3.6; 95% CI, 1.1-11.8, respectively) was identified by the investigators as significant predictors of new syndesmophytes.
“The presence of a syndesmophyte at baseline and low BMD were predictors of the formation of new syndesmophytes and significant mSASSS progression. … This study is the first to demonstrate that low BMD predicts radiographic progression in axSpA,” they concluded.
The investigators noted that a potential driving mechanism for the ankylosing process was bone loss resulting from chronic inflammation and the associated changes in bone microarchitecture.
“The inflammatory process induces bone loss, which affects the microarchitecture in the trabecular bone, thereby leading to instability. Reduced bone strength triggers a stabilizing anabolic effort that results in bone formation,” they wrote.
“Our results suggest that successful anti-inflammatory treatment reduces inflammation and allows the bone metabolism to normalize, thereby taking away the compensatory anabolic response that leads to new bone formation in the cortical bone of the spine,” they said.
They noted that their study was limited by its small sample and their findings needed confirmation in larger cohorts of axSpA patients.
SOURCE: Arthritis Res Ther. 2018 Oct 16. doi: 10.1186/s13075-018-1731-8.
Low bone mineral density and the presence of existing spinal syndesmophytes predict the formation of new spinal bony growths and radiographic progression in axial spondyloarthritis (axSpA) at 2 years, according to research conducted by Hyoung Rae Kim of the Catholic University of Korea, Seoul, South Korea, and colleagues.
The investigators noted that low bone mass in ankylosing spondyloarthritis (AS) had been linked in previous studies to spinal structural damage yet “despite the identification of this relationship, it is not yet known whether the presence of low bone mass can independently predict radiographic spinal progression in axSpA patients.”
In order to evaluate the association between low bone mass and the formation of new syndesmophytes and to investigate whether low bone mineral density (BMD) independently predicted radiographic progression in axSpA, the researchers enrolled 119 patients with definite axSpA into their study, published in Arthritis Research & Therapy.
They defined low BMD as z scores of less than or equal to –2.0 and spinal radiographic progression as worsening of the modified Stoke Ankylosing Spondylitis Spinal Score (mSASSS) by 2 or more points over the 2-year follow-up period.
Overall, 34 (29%) of the patients had syndesmophytes at baseline, 90 (76%) had radiographic sacroiliitis fulfilling the modified New York criteria for the classification of AS, and 19 (16%) had low BMD.
At 2 years, new syndesmophytes had developed in 22 (21%) of the patients. New syndesmophyte formation occurred in 15% of patients without low BMD and in 37% of patients with low BMD (P = .047).
In the multivariable analysis, current smoking (odds ratio, 3.0; 95% confidence interval, 1.1-7.7), the presence of syndesmophytes (OR, 4.6; 95% CI, 1.8-11.9); and low BMD at baseline (OR, 3.6; 95% CI, 1.2-11.2) were independently associated with significant spinal progression, the investigators reported.
The presence of baseline syndesmophytes and low BMD at any site (OR, 5.5; 95% CI, 2.0-15.2; and OR, 3.6; 95% CI, 1.1-11.8, respectively) was identified by the investigators as significant predictors of new syndesmophytes.
“The presence of a syndesmophyte at baseline and low BMD were predictors of the formation of new syndesmophytes and significant mSASSS progression. … This study is the first to demonstrate that low BMD predicts radiographic progression in axSpA,” they concluded.
The investigators noted that a potential driving mechanism for the ankylosing process was bone loss resulting from chronic inflammation and the associated changes in bone microarchitecture.
“The inflammatory process induces bone loss, which affects the microarchitecture in the trabecular bone, thereby leading to instability. Reduced bone strength triggers a stabilizing anabolic effort that results in bone formation,” they wrote.
“Our results suggest that successful anti-inflammatory treatment reduces inflammation and allows the bone metabolism to normalize, thereby taking away the compensatory anabolic response that leads to new bone formation in the cortical bone of the spine,” they said.
They noted that their study was limited by its small sample and their findings needed confirmation in larger cohorts of axSpA patients.
SOURCE: Arthritis Res Ther. 2018 Oct 16. doi: 10.1186/s13075-018-1731-8.
Low bone mineral density and the presence of existing spinal syndesmophytes predict the formation of new spinal bony growths and radiographic progression in axial spondyloarthritis (axSpA) at 2 years, according to research conducted by Hyoung Rae Kim of the Catholic University of Korea, Seoul, South Korea, and colleagues.
The investigators noted that low bone mass in ankylosing spondyloarthritis (AS) had been linked in previous studies to spinal structural damage yet “despite the identification of this relationship, it is not yet known whether the presence of low bone mass can independently predict radiographic spinal progression in axSpA patients.”
In order to evaluate the association between low bone mass and the formation of new syndesmophytes and to investigate whether low bone mineral density (BMD) independently predicted radiographic progression in axSpA, the researchers enrolled 119 patients with definite axSpA into their study, published in Arthritis Research & Therapy.
They defined low BMD as z scores of less than or equal to –2.0 and spinal radiographic progression as worsening of the modified Stoke Ankylosing Spondylitis Spinal Score (mSASSS) by 2 or more points over the 2-year follow-up period.
Overall, 34 (29%) of the patients had syndesmophytes at baseline, 90 (76%) had radiographic sacroiliitis fulfilling the modified New York criteria for the classification of AS, and 19 (16%) had low BMD.
At 2 years, new syndesmophytes had developed in 22 (21%) of the patients. New syndesmophyte formation occurred in 15% of patients without low BMD and in 37% of patients with low BMD (P = .047).
In the multivariable analysis, current smoking (odds ratio, 3.0; 95% confidence interval, 1.1-7.7), the presence of syndesmophytes (OR, 4.6; 95% CI, 1.8-11.9); and low BMD at baseline (OR, 3.6; 95% CI, 1.2-11.2) were independently associated with significant spinal progression, the investigators reported.
The presence of baseline syndesmophytes and low BMD at any site (OR, 5.5; 95% CI, 2.0-15.2; and OR, 3.6; 95% CI, 1.1-11.8, respectively) was identified by the investigators as significant predictors of new syndesmophytes.
“The presence of a syndesmophyte at baseline and low BMD were predictors of the formation of new syndesmophytes and significant mSASSS progression. … This study is the first to demonstrate that low BMD predicts radiographic progression in axSpA,” they concluded.
The investigators noted that a potential driving mechanism for the ankylosing process was bone loss resulting from chronic inflammation and the associated changes in bone microarchitecture.
“The inflammatory process induces bone loss, which affects the microarchitecture in the trabecular bone, thereby leading to instability. Reduced bone strength triggers a stabilizing anabolic effort that results in bone formation,” they wrote.
“Our results suggest that successful anti-inflammatory treatment reduces inflammation and allows the bone metabolism to normalize, thereby taking away the compensatory anabolic response that leads to new bone formation in the cortical bone of the spine,” they said.
They noted that their study was limited by its small sample and their findings needed confirmation in larger cohorts of axSpA patients.
SOURCE: Arthritis Res Ther. 2018 Oct 16. doi: 10.1186/s13075-018-1731-8.
FROM ARTHRITIS RESEARCH & THERAPY
Key clinical point: Low bone mineral density and existing spinal syndesmophytes are risk factors for new syndesmophytes and radiographic progression in people with axial spondyloarthritis.
Major finding: The presence of syndesmophytes at baseline and low BMD were predictors of new syndesmophytes over the following 2 years (OR, 5.5; 95% CI, 2.0-15.2 and OR, 3.6; 95% CI, 1.1-11.8, respectively).
Study details: A longitudinal observational study of 119 consecutive patients with axial SpA aged under 50 years who fulfilled the imaging arm of the Assessment of SpondyloArthritis International Society axSpA criteria.
Disclosures: No specific funding was received from any public, commercial or not-for-profit bodies. No authors declared any conflicts of interest.
Source: Arthritis Res Ther. 2018 Oct 16. doi: 10.1186/s13075-018-1731-8.
No evidence of subclinical axial involvement seen in skin psoriasis
A study of individuals with longstanding skin psoriasis but no clinical arthritis or spondylitis has found no evidence of subclinical involvement of the sacroiliac joint or spine.
The prevalence of sacroiliac lesions on blinded MRI assessment was similar in 20 patients who had skin psoriasis for a median of 23 years and in 22 healthy controls, and no sacroiliac ankylosis was seen in either group. Similarly, there was no significant difference between the two groups in spinal lesions on MRI, nor in any of the five levels of lesion frequency, Vlad A. Bratu, MD, of the department of radiology at University Hospital Basel (Switzerland) and his coauthors reported in Arthritis Care & Research.
On blinded MRI assessment, five (25%) patients with skin psoriasis and two (9.1%) controls were classified as having inflammation of the sacroiliac joint. Three of these patients in the psoriasis group and one in the control group were older than 50, and the three with psoriasis had had the condition for 26-35 years.
Dr. Bratu and his colleagues said that subclinical peripheral joint inflammation on MRI had previously been a common finding in patients who had skin psoriasis but no clinical signs of psoriatic arthritis. But given the limited evidence of concomitant subclinical axial or spinal inflammation in their study, the authors argued there was no support for routine screening for potential subclinical axial inflammation in patients with longstanding skin psoriasis.
They noted that bone marrow edema lesions in at least two sacroiliac joint quadrants were seen in 35% of patients with psoriasis and 23% of healthy controls, a finding that reflected those seen in other studies in healthy individuals.
“If a specificity threshold for a given MRI lesion of at least 0.9 is applied for axial MRI to discriminate between axial SpA [spondyloarthritis] and background variation in healthy controls or in differential diagnostic conditions, no more than 10% of healthy controls in our study should meet this criterion by an individual level data analysis,” they wrote.
The authors also pointed out the impact of age on lesion frequency, which was more evident in spinal lesions.
“This observation supports the hypothesis that some spinal alterations in higher age may reflect degenerative rather than inflammatory changes,” they wrote. “However, there is a gap in knowledge with virtually no evidence about presence and pattern of degenerative versus inflammatory spinal lesions in subjects beyond 50 years of age.”
The study was supported by the Gottfried and Julia Bangerter-Rhyner Foundation. No conflicts of interest were declared.
SOURCE: Bratu V et al. Arthritis Care Res. 2018 Sep 22. doi: 10.1002/acr.23767.
A study of individuals with longstanding skin psoriasis but no clinical arthritis or spondylitis has found no evidence of subclinical involvement of the sacroiliac joint or spine.
The prevalence of sacroiliac lesions on blinded MRI assessment was similar in 20 patients who had skin psoriasis for a median of 23 years and in 22 healthy controls, and no sacroiliac ankylosis was seen in either group. Similarly, there was no significant difference between the two groups in spinal lesions on MRI, nor in any of the five levels of lesion frequency, Vlad A. Bratu, MD, of the department of radiology at University Hospital Basel (Switzerland) and his coauthors reported in Arthritis Care & Research.
On blinded MRI assessment, five (25%) patients with skin psoriasis and two (9.1%) controls were classified as having inflammation of the sacroiliac joint. Three of these patients in the psoriasis group and one in the control group were older than 50, and the three with psoriasis had had the condition for 26-35 years.
Dr. Bratu and his colleagues said that subclinical peripheral joint inflammation on MRI had previously been a common finding in patients who had skin psoriasis but no clinical signs of psoriatic arthritis. But given the limited evidence of concomitant subclinical axial or spinal inflammation in their study, the authors argued there was no support for routine screening for potential subclinical axial inflammation in patients with longstanding skin psoriasis.
They noted that bone marrow edema lesions in at least two sacroiliac joint quadrants were seen in 35% of patients with psoriasis and 23% of healthy controls, a finding that reflected those seen in other studies in healthy individuals.
“If a specificity threshold for a given MRI lesion of at least 0.9 is applied for axial MRI to discriminate between axial SpA [spondyloarthritis] and background variation in healthy controls or in differential diagnostic conditions, no more than 10% of healthy controls in our study should meet this criterion by an individual level data analysis,” they wrote.
The authors also pointed out the impact of age on lesion frequency, which was more evident in spinal lesions.
“This observation supports the hypothesis that some spinal alterations in higher age may reflect degenerative rather than inflammatory changes,” they wrote. “However, there is a gap in knowledge with virtually no evidence about presence and pattern of degenerative versus inflammatory spinal lesions in subjects beyond 50 years of age.”
The study was supported by the Gottfried and Julia Bangerter-Rhyner Foundation. No conflicts of interest were declared.
SOURCE: Bratu V et al. Arthritis Care Res. 2018 Sep 22. doi: 10.1002/acr.23767.
A study of individuals with longstanding skin psoriasis but no clinical arthritis or spondylitis has found no evidence of subclinical involvement of the sacroiliac joint or spine.
The prevalence of sacroiliac lesions on blinded MRI assessment was similar in 20 patients who had skin psoriasis for a median of 23 years and in 22 healthy controls, and no sacroiliac ankylosis was seen in either group. Similarly, there was no significant difference between the two groups in spinal lesions on MRI, nor in any of the five levels of lesion frequency, Vlad A. Bratu, MD, of the department of radiology at University Hospital Basel (Switzerland) and his coauthors reported in Arthritis Care & Research.
On blinded MRI assessment, five (25%) patients with skin psoriasis and two (9.1%) controls were classified as having inflammation of the sacroiliac joint. Three of these patients in the psoriasis group and one in the control group were older than 50, and the three with psoriasis had had the condition for 26-35 years.
Dr. Bratu and his colleagues said that subclinical peripheral joint inflammation on MRI had previously been a common finding in patients who had skin psoriasis but no clinical signs of psoriatic arthritis. But given the limited evidence of concomitant subclinical axial or spinal inflammation in their study, the authors argued there was no support for routine screening for potential subclinical axial inflammation in patients with longstanding skin psoriasis.
They noted that bone marrow edema lesions in at least two sacroiliac joint quadrants were seen in 35% of patients with psoriasis and 23% of healthy controls, a finding that reflected those seen in other studies in healthy individuals.
“If a specificity threshold for a given MRI lesion of at least 0.9 is applied for axial MRI to discriminate between axial SpA [spondyloarthritis] and background variation in healthy controls or in differential diagnostic conditions, no more than 10% of healthy controls in our study should meet this criterion by an individual level data analysis,” they wrote.
The authors also pointed out the impact of age on lesion frequency, which was more evident in spinal lesions.
“This observation supports the hypothesis that some spinal alterations in higher age may reflect degenerative rather than inflammatory changes,” they wrote. “However, there is a gap in knowledge with virtually no evidence about presence and pattern of degenerative versus inflammatory spinal lesions in subjects beyond 50 years of age.”
The study was supported by the Gottfried and Julia Bangerter-Rhyner Foundation. No conflicts of interest were declared.
SOURCE: Bratu V et al. Arthritis Care Res. 2018 Sep 22. doi: 10.1002/acr.23767.
FROM ARTHRITIS CARE & RESEARCH
Key clinical point:
Major finding: The prevalence of sacroiliac bone marrow lesions was similar between patients with skin psoriasis and healthy controls.
Study details: Case-control study in 20 patients with skin psoriasis and 22 healthy controls.
Disclosures: The study was supported by the Gottfried and Julia Bangerter-Rhyner Foundation. No conflicts of interest were declared.
Source: Bratu V et al. Arthritis Care Res. 2018 Sep 22. doi: 10.1002/acr.23767.
Arthritis prevalent in older adults with any degree of depression
Arthritis is highly prevalent in older adults with any degree of depression, results of a recent study suggest.
Doctor-diagnosed arthritis was reported by more than 50% of older adults with mild depression, according to results of the study, which was based on data from the National Health and Nutrition Examination Survey (NHANES).
The prevalence of arthritis exceeded 60% in participants with moderate depression, and approached 70% for those with severe depression, according to the study, published in the International Journal of Geriatric Psychiatry.
Based on those findings, arthritis and depression need to be viewed as frequently co-occurring physical and psychosocial issues, reported Jessica M. Brooks, PhD, of the department of psychiatry at Dartmouth College, Lebanon, N.H., and her coauthors.
“It may be critical for mental health care providers to provide regular arthritis-related pain assessments and evidence‐based treatments for co‐occurring arthritis in older adults with or at risk for depression,” Dr. Brooks and her colleagues said in their report.
Their analysis was based on 2,483 women and 2,309 men aged 50 years and older (mean age, 64.5 years) who had participated in the NHANES survey between 2011 and 2014. Out of that sample, 2,094 participants (43.7%) said they had been told by a doctor that they had arthritis, the researchers said.
The rate of arthritis was 38.2% for participants with no depressive symptoms as indicated by a 0-4 score on the 9-item Patient Health Questionnaire (PHQ-9). By comparison, rates of arthritis were 55.0%, 62.9%, and 67.8% for those with mild, moderate, or severe depression by PHQ-9.
Individuals with arthritis had a significantly higher mean PHQ-9 score, at 4.6, compared with 2.6 for those without arthritis (P less than .001), the investigators said.
that controlled for age, gender, comorbid conditions, and other factors such as smoking history.
Establishing prevalence rates of arthritis in older adults with depression is an “important step” toward informing mental health professionals on the need to identify and treat arthritis-related pain, Dr. Brooks and her coauthors said.
“Addressing arthritis in mental health treatment and behavioral medicine may also help to reduce the overlapping cognitive, behavioral, and somatic symptoms in older adults with depressive symptoms and arthritis, which may be difficult for providers to disentangle through brief screening procedures and treat through conventional depression care,” they wrote.
The investigators cited several limitations. For example, the cross-sectional nature of the study makes it difficult to draw conclusions about causality. In addition, Dr. Brooks and her colleagues did not distinguish between different types of arthritis.
The researchers declared no conflicts of interest. The study was supported by several U.S. institutes, including the National Institute of Mental Health, and by numerous entities related to Dartmouth, including the Dartmouth Health Promotion and Disease Prevention Research Center. The Howard and Phyllis Schwartz Philanthropic Fund also provided funding.
SOURCE: Brooks JM et al. Int J Geriatr Psychiatry. 2018 Sep 19. doi: 10.1022/gps.4971.
Arthritis is highly prevalent in older adults with any degree of depression, results of a recent study suggest.
Doctor-diagnosed arthritis was reported by more than 50% of older adults with mild depression, according to results of the study, which was based on data from the National Health and Nutrition Examination Survey (NHANES).
The prevalence of arthritis exceeded 60% in participants with moderate depression, and approached 70% for those with severe depression, according to the study, published in the International Journal of Geriatric Psychiatry.
Based on those findings, arthritis and depression need to be viewed as frequently co-occurring physical and psychosocial issues, reported Jessica M. Brooks, PhD, of the department of psychiatry at Dartmouth College, Lebanon, N.H., and her coauthors.
“It may be critical for mental health care providers to provide regular arthritis-related pain assessments and evidence‐based treatments for co‐occurring arthritis in older adults with or at risk for depression,” Dr. Brooks and her colleagues said in their report.
Their analysis was based on 2,483 women and 2,309 men aged 50 years and older (mean age, 64.5 years) who had participated in the NHANES survey between 2011 and 2014. Out of that sample, 2,094 participants (43.7%) said they had been told by a doctor that they had arthritis, the researchers said.
The rate of arthritis was 38.2% for participants with no depressive symptoms as indicated by a 0-4 score on the 9-item Patient Health Questionnaire (PHQ-9). By comparison, rates of arthritis were 55.0%, 62.9%, and 67.8% for those with mild, moderate, or severe depression by PHQ-9.
Individuals with arthritis had a significantly higher mean PHQ-9 score, at 4.6, compared with 2.6 for those without arthritis (P less than .001), the investigators said.
that controlled for age, gender, comorbid conditions, and other factors such as smoking history.
Establishing prevalence rates of arthritis in older adults with depression is an “important step” toward informing mental health professionals on the need to identify and treat arthritis-related pain, Dr. Brooks and her coauthors said.
“Addressing arthritis in mental health treatment and behavioral medicine may also help to reduce the overlapping cognitive, behavioral, and somatic symptoms in older adults with depressive symptoms and arthritis, which may be difficult for providers to disentangle through brief screening procedures and treat through conventional depression care,” they wrote.
The investigators cited several limitations. For example, the cross-sectional nature of the study makes it difficult to draw conclusions about causality. In addition, Dr. Brooks and her colleagues did not distinguish between different types of arthritis.
The researchers declared no conflicts of interest. The study was supported by several U.S. institutes, including the National Institute of Mental Health, and by numerous entities related to Dartmouth, including the Dartmouth Health Promotion and Disease Prevention Research Center. The Howard and Phyllis Schwartz Philanthropic Fund also provided funding.
SOURCE: Brooks JM et al. Int J Geriatr Psychiatry. 2018 Sep 19. doi: 10.1022/gps.4971.
Arthritis is highly prevalent in older adults with any degree of depression, results of a recent study suggest.
Doctor-diagnosed arthritis was reported by more than 50% of older adults with mild depression, according to results of the study, which was based on data from the National Health and Nutrition Examination Survey (NHANES).
The prevalence of arthritis exceeded 60% in participants with moderate depression, and approached 70% for those with severe depression, according to the study, published in the International Journal of Geriatric Psychiatry.
Based on those findings, arthritis and depression need to be viewed as frequently co-occurring physical and psychosocial issues, reported Jessica M. Brooks, PhD, of the department of psychiatry at Dartmouth College, Lebanon, N.H., and her coauthors.
“It may be critical for mental health care providers to provide regular arthritis-related pain assessments and evidence‐based treatments for co‐occurring arthritis in older adults with or at risk for depression,” Dr. Brooks and her colleagues said in their report.
Their analysis was based on 2,483 women and 2,309 men aged 50 years and older (mean age, 64.5 years) who had participated in the NHANES survey between 2011 and 2014. Out of that sample, 2,094 participants (43.7%) said they had been told by a doctor that they had arthritis, the researchers said.
The rate of arthritis was 38.2% for participants with no depressive symptoms as indicated by a 0-4 score on the 9-item Patient Health Questionnaire (PHQ-9). By comparison, rates of arthritis were 55.0%, 62.9%, and 67.8% for those with mild, moderate, or severe depression by PHQ-9.
Individuals with arthritis had a significantly higher mean PHQ-9 score, at 4.6, compared with 2.6 for those without arthritis (P less than .001), the investigators said.
that controlled for age, gender, comorbid conditions, and other factors such as smoking history.
Establishing prevalence rates of arthritis in older adults with depression is an “important step” toward informing mental health professionals on the need to identify and treat arthritis-related pain, Dr. Brooks and her coauthors said.
“Addressing arthritis in mental health treatment and behavioral medicine may also help to reduce the overlapping cognitive, behavioral, and somatic symptoms in older adults with depressive symptoms and arthritis, which may be difficult for providers to disentangle through brief screening procedures and treat through conventional depression care,” they wrote.
The investigators cited several limitations. For example, the cross-sectional nature of the study makes it difficult to draw conclusions about causality. In addition, Dr. Brooks and her colleagues did not distinguish between different types of arthritis.
The researchers declared no conflicts of interest. The study was supported by several U.S. institutes, including the National Institute of Mental Health, and by numerous entities related to Dartmouth, including the Dartmouth Health Promotion and Disease Prevention Research Center. The Howard and Phyllis Schwartz Philanthropic Fund also provided funding.
SOURCE: Brooks JM et al. Int J Geriatr Psychiatry. 2018 Sep 19. doi: 10.1022/gps.4971.
FROM THE INTERNATIONAL JOURNAL OF GERIATRIC PSYCHIATRY
Key clinical point: “It may be critical for mental health care providers to provide regular arthritis-related pain assessments” for older adults with or at risk for depression.
Major finding: Rates of arthritis were 55.0%, 62.9%, and 67.8% for those with mild, moderate, or severe depression, respectively, according to the 9-item Patient Health Questionnaire-9 scores.
Study details: Findings on 2,483 women and 2,309 men aged 50 years and older who participated in the National Health and Nutrition Examination Survey.
Disclosures: Dr. Brooks and her coauthors declared no conflicts of interest. The study was supported by several U.S. institutes, including the National Institute of Mental Health, and by numerous entities related to Dartmouth, including the Dartmouth Health Promotion and Disease Prevention Research Center. The Howard and Phyllis Schwartz Philanthropic Fund also provided funding.
Source: Brooks JM et al. Int J Geriatr Psychiatry. 2018 Sep 19. doi: 10.1002/gps.4971.
Repeat MRI not useful in suspected axial spondyloarthritis
Patients with chronic back pain suspected of having axial spondyloarthritis (axSpA) should not undergo follow-up MRI of the sacroiliac joint (MRI-SI) within a year if they had a negative baseline MRI-SI, according to investigators.
It is very unlikely that a follow-up MRI-SI will yield new results, reported Pauline A. Bakker, MD, of the department of rheumatology at Leiden University Medical Centre, the Netherlands, and her colleagues.
Since gender and HLA-B27 status were closely associated with MRI-SI positivity, these factors should be considered when deciding to repeat an MRI-SI.
“Over the last decade, MRI rapidly gained ground and proved to be an important imaging technique in the diagnostic process of [nonradiographic] axial spondyloarthritis,” the investigators wrote in Arthritis and Rheumatology. “It has been shown that MRI can detect the early inflammatory stages of sacroiliitis months to years before structural damage can be detected on a conventional radiograph.”
Although MRI represents a diagnostic leap forward, questions of clinical application remain. “For example… if an MRI is completely normal and there is still a clinical suspicion of axSpA, should the MRI be repeated? And if so, after what period of follow-up? Or does this not contribute to the diagnostic process?”
To answer these questions, the investigators observed the “evolution of MRI lesions over a 3-month and 1-year time frame” in patients with early chronic back pain and suspected axSpA.
The prospective study involved 188 patients from the Spondyloarthritis Caught Early (SPACE) cohort. The authors commented that this is “an ideal cohort… since it includes a population of patients with back pain of short duration referred to rheumatologists with a suspicion of SpA [but without the mandatory presence of a single or multiple SpA features].”
Enrolled patients had chronic back pain lasting between 3 months and 2 years, with onset beginning between 16 and 45 years of age. Each underwent physical examination (including evaluation of other SpA features), MRI and radiographs of the sacroiliac joints, and HLA-B27 testing. If patients fulfilled axSpA criteria or had possible axSpA (at least one SpA feature), then they proceeded into the follow-up phase of the study, which included repeat MRI-SI at 3 months and 1 year.
Among enrolled patients, slightly more than one-third were male, almost half were HLA-B27 positive, and about three-quarters had Assessment of Spondyloarthritis International Society–defined inflammatory back pain. Mean age was 31 years; 31 (16.5%) patients were MRI-SI positive.
Of patients that were MRI-SI positive at baseline, 11.1% and 37.9% had a negative MRI-SI at 3 months and 1 year, respectively. The authors noted that this change “was partly induced by the start of anti-TNF [tumor necrosis factor] therapy.” In patients who were MRI-SI negative at baseline, 4.3% and 7.2% were positive at 3 months and 1 year, respectively.
“A very small percentage of patients become positive… which indicates that the usefulness of repeating an MRI-SI in the diagnostic process after 3 months or 1 year is very limited,” the authors wrote.
Gender and HLA-B27 status were independently associated with a positive MRI-SI at any point in time. About 43% of HLA-B27-positive men had a positive MRI-SI, compared with just 7% of HLA-B27-negative women. The investigators advised that these associations be considered when deciding upon repeat MRI-SI.
“If a clinical suspicion [of axSpA] remains [for example, a patient develops other SpA features] it might be worthwhile to consider redoing an MRI in HLA-B27 positive patients,” the investigators wrote. “Likewise, there is a statistically significant difference between male and female patients with a negative baseline MRI, namely that in male patients more often a positive MRI at follow-up is seen [difference: 12% in men, 3% in women].”
The researchers reported having no conflicts of interest.
SOURCE: Bakker PA et al. Arthritis Rheumatol. 2018 Sep 11. doi: 10.1002/art.40718.
Patients with chronic back pain suspected of having axial spondyloarthritis (axSpA) should not undergo follow-up MRI of the sacroiliac joint (MRI-SI) within a year if they had a negative baseline MRI-SI, according to investigators.
It is very unlikely that a follow-up MRI-SI will yield new results, reported Pauline A. Bakker, MD, of the department of rheumatology at Leiden University Medical Centre, the Netherlands, and her colleagues.
Since gender and HLA-B27 status were closely associated with MRI-SI positivity, these factors should be considered when deciding to repeat an MRI-SI.
“Over the last decade, MRI rapidly gained ground and proved to be an important imaging technique in the diagnostic process of [nonradiographic] axial spondyloarthritis,” the investigators wrote in Arthritis and Rheumatology. “It has been shown that MRI can detect the early inflammatory stages of sacroiliitis months to years before structural damage can be detected on a conventional radiograph.”
Although MRI represents a diagnostic leap forward, questions of clinical application remain. “For example… if an MRI is completely normal and there is still a clinical suspicion of axSpA, should the MRI be repeated? And if so, after what period of follow-up? Or does this not contribute to the diagnostic process?”
To answer these questions, the investigators observed the “evolution of MRI lesions over a 3-month and 1-year time frame” in patients with early chronic back pain and suspected axSpA.
The prospective study involved 188 patients from the Spondyloarthritis Caught Early (SPACE) cohort. The authors commented that this is “an ideal cohort… since it includes a population of patients with back pain of short duration referred to rheumatologists with a suspicion of SpA [but without the mandatory presence of a single or multiple SpA features].”
Enrolled patients had chronic back pain lasting between 3 months and 2 years, with onset beginning between 16 and 45 years of age. Each underwent physical examination (including evaluation of other SpA features), MRI and radiographs of the sacroiliac joints, and HLA-B27 testing. If patients fulfilled axSpA criteria or had possible axSpA (at least one SpA feature), then they proceeded into the follow-up phase of the study, which included repeat MRI-SI at 3 months and 1 year.
Among enrolled patients, slightly more than one-third were male, almost half were HLA-B27 positive, and about three-quarters had Assessment of Spondyloarthritis International Society–defined inflammatory back pain. Mean age was 31 years; 31 (16.5%) patients were MRI-SI positive.
Of patients that were MRI-SI positive at baseline, 11.1% and 37.9% had a negative MRI-SI at 3 months and 1 year, respectively. The authors noted that this change “was partly induced by the start of anti-TNF [tumor necrosis factor] therapy.” In patients who were MRI-SI negative at baseline, 4.3% and 7.2% were positive at 3 months and 1 year, respectively.
“A very small percentage of patients become positive… which indicates that the usefulness of repeating an MRI-SI in the diagnostic process after 3 months or 1 year is very limited,” the authors wrote.
Gender and HLA-B27 status were independently associated with a positive MRI-SI at any point in time. About 43% of HLA-B27-positive men had a positive MRI-SI, compared with just 7% of HLA-B27-negative women. The investigators advised that these associations be considered when deciding upon repeat MRI-SI.
“If a clinical suspicion [of axSpA] remains [for example, a patient develops other SpA features] it might be worthwhile to consider redoing an MRI in HLA-B27 positive patients,” the investigators wrote. “Likewise, there is a statistically significant difference between male and female patients with a negative baseline MRI, namely that in male patients more often a positive MRI at follow-up is seen [difference: 12% in men, 3% in women].”
The researchers reported having no conflicts of interest.
SOURCE: Bakker PA et al. Arthritis Rheumatol. 2018 Sep 11. doi: 10.1002/art.40718.
Patients with chronic back pain suspected of having axial spondyloarthritis (axSpA) should not undergo follow-up MRI of the sacroiliac joint (MRI-SI) within a year if they had a negative baseline MRI-SI, according to investigators.
It is very unlikely that a follow-up MRI-SI will yield new results, reported Pauline A. Bakker, MD, of the department of rheumatology at Leiden University Medical Centre, the Netherlands, and her colleagues.
Since gender and HLA-B27 status were closely associated with MRI-SI positivity, these factors should be considered when deciding to repeat an MRI-SI.
“Over the last decade, MRI rapidly gained ground and proved to be an important imaging technique in the diagnostic process of [nonradiographic] axial spondyloarthritis,” the investigators wrote in Arthritis and Rheumatology. “It has been shown that MRI can detect the early inflammatory stages of sacroiliitis months to years before structural damage can be detected on a conventional radiograph.”
Although MRI represents a diagnostic leap forward, questions of clinical application remain. “For example… if an MRI is completely normal and there is still a clinical suspicion of axSpA, should the MRI be repeated? And if so, after what period of follow-up? Or does this not contribute to the diagnostic process?”
To answer these questions, the investigators observed the “evolution of MRI lesions over a 3-month and 1-year time frame” in patients with early chronic back pain and suspected axSpA.
The prospective study involved 188 patients from the Spondyloarthritis Caught Early (SPACE) cohort. The authors commented that this is “an ideal cohort… since it includes a population of patients with back pain of short duration referred to rheumatologists with a suspicion of SpA [but without the mandatory presence of a single or multiple SpA features].”
Enrolled patients had chronic back pain lasting between 3 months and 2 years, with onset beginning between 16 and 45 years of age. Each underwent physical examination (including evaluation of other SpA features), MRI and radiographs of the sacroiliac joints, and HLA-B27 testing. If patients fulfilled axSpA criteria or had possible axSpA (at least one SpA feature), then they proceeded into the follow-up phase of the study, which included repeat MRI-SI at 3 months and 1 year.
Among enrolled patients, slightly more than one-third were male, almost half were HLA-B27 positive, and about three-quarters had Assessment of Spondyloarthritis International Society–defined inflammatory back pain. Mean age was 31 years; 31 (16.5%) patients were MRI-SI positive.
Of patients that were MRI-SI positive at baseline, 11.1% and 37.9% had a negative MRI-SI at 3 months and 1 year, respectively. The authors noted that this change “was partly induced by the start of anti-TNF [tumor necrosis factor] therapy.” In patients who were MRI-SI negative at baseline, 4.3% and 7.2% were positive at 3 months and 1 year, respectively.
“A very small percentage of patients become positive… which indicates that the usefulness of repeating an MRI-SI in the diagnostic process after 3 months or 1 year is very limited,” the authors wrote.
Gender and HLA-B27 status were independently associated with a positive MRI-SI at any point in time. About 43% of HLA-B27-positive men had a positive MRI-SI, compared with just 7% of HLA-B27-negative women. The investigators advised that these associations be considered when deciding upon repeat MRI-SI.
“If a clinical suspicion [of axSpA] remains [for example, a patient develops other SpA features] it might be worthwhile to consider redoing an MRI in HLA-B27 positive patients,” the investigators wrote. “Likewise, there is a statistically significant difference between male and female patients with a negative baseline MRI, namely that in male patients more often a positive MRI at follow-up is seen [difference: 12% in men, 3% in women].”
The researchers reported having no conflicts of interest.
SOURCE: Bakker PA et al. Arthritis Rheumatol. 2018 Sep 11. doi: 10.1002/art.40718.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point:
Major finding: In patients who had a negative baseline MRI of the sacroiliac joints, the maximum likelihood of a follow-up MRI being positive was 7%.
Study details: A prospective study involving 188 patients (from the Spondyloarthritis Caught Early cohort) with early chronic back pain suspected of axial spondyloarthritis.
Disclosures: The researchers reported having no financial disclosures.
Source: Bakker PA et al. Arthritis Rheumatol. 2018 Sep 11. doi: 10.1002/art.40718.