User login
Sleep May Be Target In Treatment of PTSD
BOSTON – Sleep disturbances may be an important target for treating posttraumatic stress disorder, according to Dr. R. Bruce Lydiard of the Medical University of South Carolina in Charleston.
Persistent, severe posttraumatic nightmares, REM sleep fragmentation, insomnia, excessive nocturnal periodic limb movements, and sleep-disordered breathing are frequently experienced by individuals with PTSD, Dr. Lydiard said. Although these sleep problems are often viewed as secondary symptoms of PTSD, “the evidence suggests that after a traumatic event, sleep disruption appears before the onset of PTSD and may be a risk factor for it,” he proposed.
Polysomnographic data from 21 individuals with traumatic injuries showed that the number of REM periods and the (shorter) duration of REM periods within 1 month after the traumatic event were predictive of PTSD symptom severity 6 weeks later (Am. J. Psychiatry 2002;159:1696-701).
Neurobiologically, the association makes sense, Dr. Lydiard said. “Sleep is regulated in part by brain areas in which PTSD-related changes occur,” which suggests that the stress response in PTSD and sleep dysfunction may be biologically linked.
Imaging studies suggest that exposure to trauma-related stimuli leads to hyperactivation in the amygdala and decreased activation in the medial prefrontal cortex/anterior cingulate cortex and hippocampus, with the magnitude of the activation correlating with the clinical severity of PTSD symptoms.
Polysomnographic investigations in patients with PTSD and sleep disturbances have revealed increased REM density, reduced REM duration, and increased motor activity, Dr. Lydiard said.
Together with clinical reports, “these data provide the basis for REM sleep dysregulation as a core feature in PTSD,” whereby increased activity in the amygdala and decreased inhibitory input from the medial prefrontal cortex lead to a persistently overactive noradrenergic system. “As a result, the usual rhythm of REM-NREM sleep is disrupted, and REM sleep is fragmented,” he said.
Based on this model, investigators have hypothesized that targeting noradrenergic signaling during or near REM episodes may normalize REM sleep, which in turn might improve PTSD sleep disturbances and, potentially, other PTSD symptoms, Dr. Lydiard said.
The alpha adrenergic antagonist prazosin has shown promise in multiple case and chart reviews, open-label trials, and placebo-controlled studies.
In one trial of 40 veterans with PTSD sleep disturbance, patients who were randomized to receive a nightly dose of prazosin–originally marketed as an antihypertensive agent–reported significant improvements in sleep quality and significant reductions in trauma nightmares, as well a better overall sense of well-being and improved daily functioning (Biol. Psychiatry 2007;61:928-34).
In another study, investigators evaluated the effect of prazosin vs. placebo on objective sleep parameters in 13 outpatients with chronic civilian trauma PTSD, frequent nightmares, and sleep disturbance. The prazosin group experienced significantly increased total sleep time as well as increased REM sleep time and mean REM period duration (Biol. Psychiatry 2008;63:629-32).
In the various studies, the therapeutic benefit of prazosin has been achieved within 1-2 weeks “with doses as low as 1 mg nightly,” Dr. Lydiard said.
In addition to improving sleep measures, prazosin may be useful for other trauma-related symptoms. In a small study of PTSD subjects whose nightmares were well controlled with the drug, the addition of small daytime doses lessened patients' reactivity to trauma cues during the day, he said (Biol. Psychiatry 2006;59:577-81). This finding “adds to the growing body of evidence that targeting sleep in PTSD is clinically relevant.”
Although some evidence exists to support the use of other antiadrenergic agents such as clonidine and guanfacine–as well as the anticonvulsant gabapentin–in PTSD, “large, randomized controlled trials are needed to clarify the role” of all of these agents, Dr. Lydiard said.
Additional studies also are warranted, he said, to investigate nonpharmacologic approaches to improving PTSD sleep disturbance, such as the use of imagery rehearsal therapy, which has demonstrated efficacy in small studies (J. Trauma Stress 2009;22:236-9).
Dr. Lydiard disclosed receiving honoraria from Reed Medical Education, the logistics collaborator for the Massachusetts General Hospital Psychiatry Academy.
BOSTON – Sleep disturbances may be an important target for treating posttraumatic stress disorder, according to Dr. R. Bruce Lydiard of the Medical University of South Carolina in Charleston.
Persistent, severe posttraumatic nightmares, REM sleep fragmentation, insomnia, excessive nocturnal periodic limb movements, and sleep-disordered breathing are frequently experienced by individuals with PTSD, Dr. Lydiard said. Although these sleep problems are often viewed as secondary symptoms of PTSD, “the evidence suggests that after a traumatic event, sleep disruption appears before the onset of PTSD and may be a risk factor for it,” he proposed.
Polysomnographic data from 21 individuals with traumatic injuries showed that the number of REM periods and the (shorter) duration of REM periods within 1 month after the traumatic event were predictive of PTSD symptom severity 6 weeks later (Am. J. Psychiatry 2002;159:1696-701).
Neurobiologically, the association makes sense, Dr. Lydiard said. “Sleep is regulated in part by brain areas in which PTSD-related changes occur,” which suggests that the stress response in PTSD and sleep dysfunction may be biologically linked.
Imaging studies suggest that exposure to trauma-related stimuli leads to hyperactivation in the amygdala and decreased activation in the medial prefrontal cortex/anterior cingulate cortex and hippocampus, with the magnitude of the activation correlating with the clinical severity of PTSD symptoms.
Polysomnographic investigations in patients with PTSD and sleep disturbances have revealed increased REM density, reduced REM duration, and increased motor activity, Dr. Lydiard said.
Together with clinical reports, “these data provide the basis for REM sleep dysregulation as a core feature in PTSD,” whereby increased activity in the amygdala and decreased inhibitory input from the medial prefrontal cortex lead to a persistently overactive noradrenergic system. “As a result, the usual rhythm of REM-NREM sleep is disrupted, and REM sleep is fragmented,” he said.
Based on this model, investigators have hypothesized that targeting noradrenergic signaling during or near REM episodes may normalize REM sleep, which in turn might improve PTSD sleep disturbances and, potentially, other PTSD symptoms, Dr. Lydiard said.
The alpha adrenergic antagonist prazosin has shown promise in multiple case and chart reviews, open-label trials, and placebo-controlled studies.
In one trial of 40 veterans with PTSD sleep disturbance, patients who were randomized to receive a nightly dose of prazosin–originally marketed as an antihypertensive agent–reported significant improvements in sleep quality and significant reductions in trauma nightmares, as well a better overall sense of well-being and improved daily functioning (Biol. Psychiatry 2007;61:928-34).
In another study, investigators evaluated the effect of prazosin vs. placebo on objective sleep parameters in 13 outpatients with chronic civilian trauma PTSD, frequent nightmares, and sleep disturbance. The prazosin group experienced significantly increased total sleep time as well as increased REM sleep time and mean REM period duration (Biol. Psychiatry 2008;63:629-32).
In the various studies, the therapeutic benefit of prazosin has been achieved within 1-2 weeks “with doses as low as 1 mg nightly,” Dr. Lydiard said.
In addition to improving sleep measures, prazosin may be useful for other trauma-related symptoms. In a small study of PTSD subjects whose nightmares were well controlled with the drug, the addition of small daytime doses lessened patients' reactivity to trauma cues during the day, he said (Biol. Psychiatry 2006;59:577-81). This finding “adds to the growing body of evidence that targeting sleep in PTSD is clinically relevant.”
Although some evidence exists to support the use of other antiadrenergic agents such as clonidine and guanfacine–as well as the anticonvulsant gabapentin–in PTSD, “large, randomized controlled trials are needed to clarify the role” of all of these agents, Dr. Lydiard said.
Additional studies also are warranted, he said, to investigate nonpharmacologic approaches to improving PTSD sleep disturbance, such as the use of imagery rehearsal therapy, which has demonstrated efficacy in small studies (J. Trauma Stress 2009;22:236-9).
Dr. Lydiard disclosed receiving honoraria from Reed Medical Education, the logistics collaborator for the Massachusetts General Hospital Psychiatry Academy.
BOSTON – Sleep disturbances may be an important target for treating posttraumatic stress disorder, according to Dr. R. Bruce Lydiard of the Medical University of South Carolina in Charleston.
Persistent, severe posttraumatic nightmares, REM sleep fragmentation, insomnia, excessive nocturnal periodic limb movements, and sleep-disordered breathing are frequently experienced by individuals with PTSD, Dr. Lydiard said. Although these sleep problems are often viewed as secondary symptoms of PTSD, “the evidence suggests that after a traumatic event, sleep disruption appears before the onset of PTSD and may be a risk factor for it,” he proposed.
Polysomnographic data from 21 individuals with traumatic injuries showed that the number of REM periods and the (shorter) duration of REM periods within 1 month after the traumatic event were predictive of PTSD symptom severity 6 weeks later (Am. J. Psychiatry 2002;159:1696-701).
Neurobiologically, the association makes sense, Dr. Lydiard said. “Sleep is regulated in part by brain areas in which PTSD-related changes occur,” which suggests that the stress response in PTSD and sleep dysfunction may be biologically linked.
Imaging studies suggest that exposure to trauma-related stimuli leads to hyperactivation in the amygdala and decreased activation in the medial prefrontal cortex/anterior cingulate cortex and hippocampus, with the magnitude of the activation correlating with the clinical severity of PTSD symptoms.
Polysomnographic investigations in patients with PTSD and sleep disturbances have revealed increased REM density, reduced REM duration, and increased motor activity, Dr. Lydiard said.
Together with clinical reports, “these data provide the basis for REM sleep dysregulation as a core feature in PTSD,” whereby increased activity in the amygdala and decreased inhibitory input from the medial prefrontal cortex lead to a persistently overactive noradrenergic system. “As a result, the usual rhythm of REM-NREM sleep is disrupted, and REM sleep is fragmented,” he said.
Based on this model, investigators have hypothesized that targeting noradrenergic signaling during or near REM episodes may normalize REM sleep, which in turn might improve PTSD sleep disturbances and, potentially, other PTSD symptoms, Dr. Lydiard said.
The alpha adrenergic antagonist prazosin has shown promise in multiple case and chart reviews, open-label trials, and placebo-controlled studies.
In one trial of 40 veterans with PTSD sleep disturbance, patients who were randomized to receive a nightly dose of prazosin–originally marketed as an antihypertensive agent–reported significant improvements in sleep quality and significant reductions in trauma nightmares, as well a better overall sense of well-being and improved daily functioning (Biol. Psychiatry 2007;61:928-34).
In another study, investigators evaluated the effect of prazosin vs. placebo on objective sleep parameters in 13 outpatients with chronic civilian trauma PTSD, frequent nightmares, and sleep disturbance. The prazosin group experienced significantly increased total sleep time as well as increased REM sleep time and mean REM period duration (Biol. Psychiatry 2008;63:629-32).
In the various studies, the therapeutic benefit of prazosin has been achieved within 1-2 weeks “with doses as low as 1 mg nightly,” Dr. Lydiard said.
In addition to improving sleep measures, prazosin may be useful for other trauma-related symptoms. In a small study of PTSD subjects whose nightmares were well controlled with the drug, the addition of small daytime doses lessened patients' reactivity to trauma cues during the day, he said (Biol. Psychiatry 2006;59:577-81). This finding “adds to the growing body of evidence that targeting sleep in PTSD is clinically relevant.”
Although some evidence exists to support the use of other antiadrenergic agents such as clonidine and guanfacine–as well as the anticonvulsant gabapentin–in PTSD, “large, randomized controlled trials are needed to clarify the role” of all of these agents, Dr. Lydiard said.
Additional studies also are warranted, he said, to investigate nonpharmacologic approaches to improving PTSD sleep disturbance, such as the use of imagery rehearsal therapy, which has demonstrated efficacy in small studies (J. Trauma Stress 2009;22:236-9).
Dr. Lydiard disclosed receiving honoraria from Reed Medical Education, the logistics collaborator for the Massachusetts General Hospital Psychiatry Academy.
Uncooperative and manic
CASE: New-onset mania
Ms. Z, age 69, is admitted to our hospital’s medical unit after developing manic symptoms. Her medical history includes hemodialysis-dependent chronic kidney disease, Parkinson’s disease stabilized by carbidopa/levodopa, 75/300 mg/d, for 4 years, diet-controlled type 2 diabetes mellitus, hypertension, hyperlipidemia, myelodysplasia, and acid reflux. She experiences mild anxiety, which has been stable for many years with escitalopram, 10 mg/d, but has no history of alcohol or drug abuse and no family history of psychiatric illness.
The staff at her assisted living facility reports that 8 days ago Ms. Z was mildly irritable and argumentative regarding her medications and 7 days ago began to refuse all medications. Six days ago she refused dialysis, reportedly because she was angry at the staff. One day later, the staff noticed Ms. Z had developed manic symptoms, including decreased need for sleep (only 2 hours a night), talkativeness, counting things and spelling words rapidly out loud, and making explicit drawings of men. Ms. Z refused her next 2 dialysis treatments and her manic symptoms worsened. She explained that all her medical problems had been “cured.” She inaccurately exclaimed that she can urinate, even though she is anuric, and that she can walk after not having done so for 5 years.
During our interview, Ms. Z is disheveled and exhibits pressured speech, often interrupting the interviewer. Her affect is euphoric and expansive. She perseverates on patenting her cures for diabetes and Parkinson’s disease, endorses hypersexuality, and denies hallucinations. Folstein Mini-Mental State Exam score is 18/28; however, Ms. Z refuses to participate in elements of cognitive testing, including writing a sentence, drawing pentagons, or drawing a clock, all of which would reveal her tremor. We note no disorientation or waxing and waning of attention or consciousness. She is fully oriented to person, place, time, and purpose and can perform serial 7s and spell a word backwards.
The authors’ observations
A number of factors suggest that Ms. Z’s manic symptoms likely are caused by a medical problem (Table 1).1 She has no family history and only minimal personal history of psychiatric illness, and new-onset bipolar disorder in a 69-year-old woman is unusual.2 Given Ms. Z’s acute change in mental status and numerous medical problems, we consider delirium. Because Ms. Z does not exhibit disorientation or waxing and waning of attention or consciousness, we feel delirium is unlikely to be the primary diagnosis.
Table 1
Criteria for mood disorder due to a general medical condition
A. A prominent and persistent disturbance in mood predominates in the clinical picture and is characterized by either (or both) of the following:
|
| B. There is evidence from the history, physical examination, or laboratory findings that the disturbance is the direct physiological consequence of a general medical condition |
| C. The disturbance is not better accounted for by another mental disorder |
| D. The disturbance does not occur exclusively during the course of a delirium |
| E. The symptoms cause clinically significant distress or impairment in social, occupational, or other important areas of functioning. |
| Source: Reference 1 |
EVALUATION: Clues to the cause
Physical exam reveals stable vital signs, and resting tremor and mild cogwheel rigidity in her right upper extremity consistent with Parkinson’s disease. Laboratory results show elevated blood urea nitrogen (65 mg/dL) and creatinine (8 mg/dL) and stably low white cell count (2.9/μL) and platelets (118x103/μL), which are consistent with her known myelodysplasia. Results for urinalysis, B12, folate, thyroid-stimulating hormone, electrolytes, glucose, liver function, antinuclear antibodies, and rapid plasma reagin are unremarkable. Ms. Z’s elevated blood urea nitrogen and creatinine are expected because she recently refused dialysis. We consider that uremia could be causing her manic symptoms; however, with only 2 case reports of uremia-induced mania in the literature over the past century, we want to rule out other potential causes.3,4
A CT of Ms. Z’s brain is normal. The neurology service performs an EEG and results show mild disorganization with a predominantly posterior rhythm of 8 to 9 Hz symmetrically, occasional periods of slowing, and no epileptiform activity or evidence of encephalopathy; these findings are consistent with end-stage renal disease.
The authors’ observations
Although mood disorder due to a general medical condition—in this case, mania secondary to uremia—was our primary consideration, at this point we could not rule out subclinical delirium. In delirium, we would expect EEG to show diffuse slowing of background rhythm, which we did not see with Ms. Z. However, occasional periods of slowing indicate that delirium was a possible factor.
Parkinson’s disease is known to be a rare predisposing factor for mania—possibly related to potential manicogenic properties of dopaminergic medications5—but this would not explain new-onset mania in the context of uremia in a patient whose carbidopa/levodopa dose had been stable for several years. It is possible that Ms. Z’s refusal of dialysis could have led to build-up of carbidopa/levodopa in her blood, thereby contributing to mania; however, when she began feeling irritable, she refused several of her medications, including carbidopa/levodopa. Therefore, it is unlikely that carbidopa/levodopa accumulated to toxic levels.
We carefully evaluated Ms. Z’s complete medication list to determine if other drugs could be contributing factors. She has been taking escitalopram for anxiety for several years. Although Ms. Z had no personal or family history of bipolar disorder and no past hypomania or agitation associated with this medication, we discontinue escitalopram in case it was contributing to her manic symptoms. Ms. Z also receives amlodipine, 5 mg/d for hypertension; atorvastatin, 20 mg/d, for hyperlipidemia; pantoprazole, 40 mg/d, for acid reflux; metoprolol, 100 mg/d, for hypertension; aspirin, 81 mg/d, for cardioprotection; and fish oil, 2000 mg/d, for cardioprotection. We do not feel that any of these medications significantly contribute to her current state.
TREATMENT: Restarting dialysis
We start Ms. Z on olanzapine, 5 mg/d, for manic symptoms 1 day after admission, and resume dialysis treatments 1 day later. Because of concerns that olanzapine could worsen her myelodysplasia, we switch to aripiprazole, titrating up to 30 mg/d, 4 days later. After 2 dialysis treatments, her manic symptoms begin to resolve.
The authors’ observations
A number of factors suggest that uremia likely is causing Ms. Z’s manic symptoms. Her symptoms suddenly developed shortly after her first missed dialysis treatment, but gradually resolved after re-initiating dialysis. It is possible that antipsychotics relieved her manic symptoms, but this does not detract from the factors that make a causal relationship between uremia and mania likely.
Manic symptoms have been reported to be precipitated by a variety of medical problems, including metabolic disturbances, infections such as human immunodeficiency virus brain infection, neurologic disorders, brain neoplasms, or traumatic brain injuries (Table 2).6,7 End-stage renal disease frequently is associated with psychiatric manifestations—including depression, psychosis, delirium, and dementia—but mania is not a typical presentation. It is possible that this condition occurs more often but is not recognized.
Table 2
Common causes of secondary mania
| Metabolic/endocrine disturbances (hyperthyroidism, hyperadrenalism) |
| Infections (HIV) |
| Neurologic disorders (cerebrovascular accident, multiple sclerosis, Parkinson’s disease, epilepsy, Huntington’s disease) |
| Brain neoplasms |
| Traumatic brain injuries |
| Medications (anabolic steroids, antidepressants, corticosteroids, dextromethorphan, dopamine agonists, hypericum, isoniazid, stimulants, ephedrine, zidovudine) |
| Substance abuse (cocaine, amphetamines) |
| HIV: human immunodeficiency virus |
| Source: References 6,7 |
Kidney disease and psychotropics
We considered the effect of dialysis on psychotropics when selecting pharmacotherapy for Ms. Z’s manic symptoms. Haloperidol is not renally cleared so no dosage adjustment is necessary;8 however, this potent dopamine D2-blocker could have worsened Ms. Z’s parkinsonism. Lithium is contraindicated in acute renal failure. Valproic acid clearance is reduced in renal failure, but because it is cleared by hemodialysis, dosage adjustment is not recommended for dialysis patients.8 However, Ms. Z’s myelodysplasia is a contraindication for valproic acid as well as carbamazepine. With atypical antipsychotics as our primary options, we noted that olanzapine, quetiapine, or aripiprazole do not require dosage adjustments for dialysis patients.8,9 Of these, we eventually chose aripiprazole because we felt that it was least likely to exacerbate Ms. Z’s myelodysplasia.10
How uremia might cause mania
The pathophysiology of uremia-induced mania remains speculative. Possible factors include:
- Chronic renal failure can cause an elevation in plasma free tryptophan, a serotonin (5-HT) precursor.11 Postmortem examination of brains of patients who died in uremic coma show elevated 5-HT.12 Moreover, cerebrospinal fluid of patients with chronic renal failure has shown increased 5-hydroxyindoleacetic acid, the major 5-HT metabolite.13 Increased 5-HT could cause mania in some uremic patients, similar to how serotonergic medications can precipitate mania in some patients.
- Circulating ß-endorphin levels are increased in renal failure.14 ß-endorphins increase animal locomotor activity, which is the basis of an animal model of mania.15,16 Therefore, uremia-induced mania could be partly related to elevated ß-endorphin levels.
This case demonstrates that mania could be a psychiatric manifestation of end-stage renal disease. Clinicians should be aware of this possibility, and further study should examine underlying pathophysiologic changes in uremia and other secondary causes of mania that might lead to such a mood state.
OUTCOME: Lasting improvement
At discharge 17 days after admission, Ms. Z is back to her baseline mental state. Her aripiprazole dose is tapered to 20 mg/d with no return of manic symptoms. After 10 weeks, aripiprazole is discontinued, with no recurrence of mania.
Related Resource
- Arora M, Daughton J. Mania in the medically ill. Curr Psychiatry Rep. 2007;9(3):232-235.
Drug Brand Names
- Amlodipine • Norvasc
- Aripiprazole • Abilify
- Atorvastatin • Lipitor
- Carbamazepine • Tegretol
- Carbidopa/levodopa • Sinemet
- Escitalopram • Lexapro
- Haloperidol • Haldol
- Isoniazid • Nydrazid
- Lithium • Eskalith, Lithobid
- Metoprolol • Lopressor
- Olanzapine • Zyprexa
- Pantoprazole • Protonix
- Quetiapine • Seroquel
- Valproic acid • Depakote
- Zidovudine • Retrovir
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
2. Depp CA, Jeste DV. Bipolar disorder in older adults: a critical review. Bipolar Disord. 2004;6:343-367.
3. El-Mallakh RS, Shrader SA, Widger E. Mania as a manifestation of end-stage renal disease. J Nerv Ment Dis. 1987;175:243-245.
4. Thomas CS, Neale TJ. Organic manic syndrome associated with advanced uraemia due to polycystic kidney disease. Br J Psychiatry. 1991;158:119-121.
5. Kim E, Zwil AS, McAllister TW, et al. Treatment of organic bipolar mood disorders in Parkinson’s disease. J Neuropsychiatry Clin Neurosci. 1994;6:181-184.
6. Levenson JL. Psychosis in the medically ill. Primary Psychiatry. 2005;12(8):16-18.
7. Arora M, Daughton J. Mania in the medically ill. Curr Psychiatry Rep. 2007;9(3):232-235.
8. McLaren KD, Marangell LB. Special considerations in the treatment of patients with bipolar disorder and medical comorbidities. Ann Gen Hosp Psychiatry. 2004;3(1):7.-
9. Mallikaarjun S, Shoaf SE, Boulton DW, et al. Effects of hepatic or renal impairment on the pharmacokinetics of aripiprazole. Clin Pharmacokinet. 2008;47(8):533-542.
10. Stip E, Langlois R, Thuot C, et al. Fatal agranulocytosis: the use of olanzapine in a patient with schizophrenia and myelodysplasia. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(1):297-300.
11. de Torrente A, Glazer GB, Gulyassy P. Reduced in vitro binding of tryptophan by plasma in uremia. Kidney Int. 1974;6:222-229.
12. Jellinger E, Irsigler K, Kothbauer P, et al. Brain monoamines in metabolic coma. Excerpta Medica. 1977;427:169.-
13. Sullivan PA, Murnaghan D, Callaghan N, et al. Cerebral transmitter precursors and metabolites in advanced renal disease. J Neurol Neurosurg Psychiatry. 1978;41:581-588.
14. Aronin N, Krieger DT. Plasma immunoreactive beta-endorphin is elevated in uraemia. Clin Endocrinol (Oxf). 1983;18:459-464.
15. Holtzman SG. Behavioral effects of separate and combined administration of naloxone and D-amphetamine. J Pharmacol Exp Ther. 1974;189:51-60.
16. Segal DS, Browne RG, Derrington DC. Characteristics of beta-endorphin induced behavioral activation and immobilization. In: Usdin E, Bunney WE, Kline NS, eds. Endorphins in mental health research. New York, NY: Oxford University Press; 1979.
CASE: New-onset mania
Ms. Z, age 69, is admitted to our hospital’s medical unit after developing manic symptoms. Her medical history includes hemodialysis-dependent chronic kidney disease, Parkinson’s disease stabilized by carbidopa/levodopa, 75/300 mg/d, for 4 years, diet-controlled type 2 diabetes mellitus, hypertension, hyperlipidemia, myelodysplasia, and acid reflux. She experiences mild anxiety, which has been stable for many years with escitalopram, 10 mg/d, but has no history of alcohol or drug abuse and no family history of psychiatric illness.
The staff at her assisted living facility reports that 8 days ago Ms. Z was mildly irritable and argumentative regarding her medications and 7 days ago began to refuse all medications. Six days ago she refused dialysis, reportedly because she was angry at the staff. One day later, the staff noticed Ms. Z had developed manic symptoms, including decreased need for sleep (only 2 hours a night), talkativeness, counting things and spelling words rapidly out loud, and making explicit drawings of men. Ms. Z refused her next 2 dialysis treatments and her manic symptoms worsened. She explained that all her medical problems had been “cured.” She inaccurately exclaimed that she can urinate, even though she is anuric, and that she can walk after not having done so for 5 years.
During our interview, Ms. Z is disheveled and exhibits pressured speech, often interrupting the interviewer. Her affect is euphoric and expansive. She perseverates on patenting her cures for diabetes and Parkinson’s disease, endorses hypersexuality, and denies hallucinations. Folstein Mini-Mental State Exam score is 18/28; however, Ms. Z refuses to participate in elements of cognitive testing, including writing a sentence, drawing pentagons, or drawing a clock, all of which would reveal her tremor. We note no disorientation or waxing and waning of attention or consciousness. She is fully oriented to person, place, time, and purpose and can perform serial 7s and spell a word backwards.
The authors’ observations
A number of factors suggest that Ms. Z’s manic symptoms likely are caused by a medical problem (Table 1).1 She has no family history and only minimal personal history of psychiatric illness, and new-onset bipolar disorder in a 69-year-old woman is unusual.2 Given Ms. Z’s acute change in mental status and numerous medical problems, we consider delirium. Because Ms. Z does not exhibit disorientation or waxing and waning of attention or consciousness, we feel delirium is unlikely to be the primary diagnosis.
Table 1
Criteria for mood disorder due to a general medical condition
A. A prominent and persistent disturbance in mood predominates in the clinical picture and is characterized by either (or both) of the following:
|
| B. There is evidence from the history, physical examination, or laboratory findings that the disturbance is the direct physiological consequence of a general medical condition |
| C. The disturbance is not better accounted for by another mental disorder |
| D. The disturbance does not occur exclusively during the course of a delirium |
| E. The symptoms cause clinically significant distress or impairment in social, occupational, or other important areas of functioning. |
| Source: Reference 1 |
EVALUATION: Clues to the cause
Physical exam reveals stable vital signs, and resting tremor and mild cogwheel rigidity in her right upper extremity consistent with Parkinson’s disease. Laboratory results show elevated blood urea nitrogen (65 mg/dL) and creatinine (8 mg/dL) and stably low white cell count (2.9/μL) and platelets (118x103/μL), which are consistent with her known myelodysplasia. Results for urinalysis, B12, folate, thyroid-stimulating hormone, electrolytes, glucose, liver function, antinuclear antibodies, and rapid plasma reagin are unremarkable. Ms. Z’s elevated blood urea nitrogen and creatinine are expected because she recently refused dialysis. We consider that uremia could be causing her manic symptoms; however, with only 2 case reports of uremia-induced mania in the literature over the past century, we want to rule out other potential causes.3,4
A CT of Ms. Z’s brain is normal. The neurology service performs an EEG and results show mild disorganization with a predominantly posterior rhythm of 8 to 9 Hz symmetrically, occasional periods of slowing, and no epileptiform activity or evidence of encephalopathy; these findings are consistent with end-stage renal disease.
The authors’ observations
Although mood disorder due to a general medical condition—in this case, mania secondary to uremia—was our primary consideration, at this point we could not rule out subclinical delirium. In delirium, we would expect EEG to show diffuse slowing of background rhythm, which we did not see with Ms. Z. However, occasional periods of slowing indicate that delirium was a possible factor.
Parkinson’s disease is known to be a rare predisposing factor for mania—possibly related to potential manicogenic properties of dopaminergic medications5—but this would not explain new-onset mania in the context of uremia in a patient whose carbidopa/levodopa dose had been stable for several years. It is possible that Ms. Z’s refusal of dialysis could have led to build-up of carbidopa/levodopa in her blood, thereby contributing to mania; however, when she began feeling irritable, she refused several of her medications, including carbidopa/levodopa. Therefore, it is unlikely that carbidopa/levodopa accumulated to toxic levels.
We carefully evaluated Ms. Z’s complete medication list to determine if other drugs could be contributing factors. She has been taking escitalopram for anxiety for several years. Although Ms. Z had no personal or family history of bipolar disorder and no past hypomania or agitation associated with this medication, we discontinue escitalopram in case it was contributing to her manic symptoms. Ms. Z also receives amlodipine, 5 mg/d for hypertension; atorvastatin, 20 mg/d, for hyperlipidemia; pantoprazole, 40 mg/d, for acid reflux; metoprolol, 100 mg/d, for hypertension; aspirin, 81 mg/d, for cardioprotection; and fish oil, 2000 mg/d, for cardioprotection. We do not feel that any of these medications significantly contribute to her current state.
TREATMENT: Restarting dialysis
We start Ms. Z on olanzapine, 5 mg/d, for manic symptoms 1 day after admission, and resume dialysis treatments 1 day later. Because of concerns that olanzapine could worsen her myelodysplasia, we switch to aripiprazole, titrating up to 30 mg/d, 4 days later. After 2 dialysis treatments, her manic symptoms begin to resolve.
The authors’ observations
A number of factors suggest that uremia likely is causing Ms. Z’s manic symptoms. Her symptoms suddenly developed shortly after her first missed dialysis treatment, but gradually resolved after re-initiating dialysis. It is possible that antipsychotics relieved her manic symptoms, but this does not detract from the factors that make a causal relationship between uremia and mania likely.
Manic symptoms have been reported to be precipitated by a variety of medical problems, including metabolic disturbances, infections such as human immunodeficiency virus brain infection, neurologic disorders, brain neoplasms, or traumatic brain injuries (Table 2).6,7 End-stage renal disease frequently is associated with psychiatric manifestations—including depression, psychosis, delirium, and dementia—but mania is not a typical presentation. It is possible that this condition occurs more often but is not recognized.
Table 2
Common causes of secondary mania
| Metabolic/endocrine disturbances (hyperthyroidism, hyperadrenalism) |
| Infections (HIV) |
| Neurologic disorders (cerebrovascular accident, multiple sclerosis, Parkinson’s disease, epilepsy, Huntington’s disease) |
| Brain neoplasms |
| Traumatic brain injuries |
| Medications (anabolic steroids, antidepressants, corticosteroids, dextromethorphan, dopamine agonists, hypericum, isoniazid, stimulants, ephedrine, zidovudine) |
| Substance abuse (cocaine, amphetamines) |
| HIV: human immunodeficiency virus |
| Source: References 6,7 |
Kidney disease and psychotropics
We considered the effect of dialysis on psychotropics when selecting pharmacotherapy for Ms. Z’s manic symptoms. Haloperidol is not renally cleared so no dosage adjustment is necessary;8 however, this potent dopamine D2-blocker could have worsened Ms. Z’s parkinsonism. Lithium is contraindicated in acute renal failure. Valproic acid clearance is reduced in renal failure, but because it is cleared by hemodialysis, dosage adjustment is not recommended for dialysis patients.8 However, Ms. Z’s myelodysplasia is a contraindication for valproic acid as well as carbamazepine. With atypical antipsychotics as our primary options, we noted that olanzapine, quetiapine, or aripiprazole do not require dosage adjustments for dialysis patients.8,9 Of these, we eventually chose aripiprazole because we felt that it was least likely to exacerbate Ms. Z’s myelodysplasia.10
How uremia might cause mania
The pathophysiology of uremia-induced mania remains speculative. Possible factors include:
- Chronic renal failure can cause an elevation in plasma free tryptophan, a serotonin (5-HT) precursor.11 Postmortem examination of brains of patients who died in uremic coma show elevated 5-HT.12 Moreover, cerebrospinal fluid of patients with chronic renal failure has shown increased 5-hydroxyindoleacetic acid, the major 5-HT metabolite.13 Increased 5-HT could cause mania in some uremic patients, similar to how serotonergic medications can precipitate mania in some patients.
- Circulating ß-endorphin levels are increased in renal failure.14 ß-endorphins increase animal locomotor activity, which is the basis of an animal model of mania.15,16 Therefore, uremia-induced mania could be partly related to elevated ß-endorphin levels.
This case demonstrates that mania could be a psychiatric manifestation of end-stage renal disease. Clinicians should be aware of this possibility, and further study should examine underlying pathophysiologic changes in uremia and other secondary causes of mania that might lead to such a mood state.
OUTCOME: Lasting improvement
At discharge 17 days after admission, Ms. Z is back to her baseline mental state. Her aripiprazole dose is tapered to 20 mg/d with no return of manic symptoms. After 10 weeks, aripiprazole is discontinued, with no recurrence of mania.
Related Resource
- Arora M, Daughton J. Mania in the medically ill. Curr Psychiatry Rep. 2007;9(3):232-235.
Drug Brand Names
- Amlodipine • Norvasc
- Aripiprazole • Abilify
- Atorvastatin • Lipitor
- Carbamazepine • Tegretol
- Carbidopa/levodopa • Sinemet
- Escitalopram • Lexapro
- Haloperidol • Haldol
- Isoniazid • Nydrazid
- Lithium • Eskalith, Lithobid
- Metoprolol • Lopressor
- Olanzapine • Zyprexa
- Pantoprazole • Protonix
- Quetiapine • Seroquel
- Valproic acid • Depakote
- Zidovudine • Retrovir
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: New-onset mania
Ms. Z, age 69, is admitted to our hospital’s medical unit after developing manic symptoms. Her medical history includes hemodialysis-dependent chronic kidney disease, Parkinson’s disease stabilized by carbidopa/levodopa, 75/300 mg/d, for 4 years, diet-controlled type 2 diabetes mellitus, hypertension, hyperlipidemia, myelodysplasia, and acid reflux. She experiences mild anxiety, which has been stable for many years with escitalopram, 10 mg/d, but has no history of alcohol or drug abuse and no family history of psychiatric illness.
The staff at her assisted living facility reports that 8 days ago Ms. Z was mildly irritable and argumentative regarding her medications and 7 days ago began to refuse all medications. Six days ago she refused dialysis, reportedly because she was angry at the staff. One day later, the staff noticed Ms. Z had developed manic symptoms, including decreased need for sleep (only 2 hours a night), talkativeness, counting things and spelling words rapidly out loud, and making explicit drawings of men. Ms. Z refused her next 2 dialysis treatments and her manic symptoms worsened. She explained that all her medical problems had been “cured.” She inaccurately exclaimed that she can urinate, even though she is anuric, and that she can walk after not having done so for 5 years.
During our interview, Ms. Z is disheveled and exhibits pressured speech, often interrupting the interviewer. Her affect is euphoric and expansive. She perseverates on patenting her cures for diabetes and Parkinson’s disease, endorses hypersexuality, and denies hallucinations. Folstein Mini-Mental State Exam score is 18/28; however, Ms. Z refuses to participate in elements of cognitive testing, including writing a sentence, drawing pentagons, or drawing a clock, all of which would reveal her tremor. We note no disorientation or waxing and waning of attention or consciousness. She is fully oriented to person, place, time, and purpose and can perform serial 7s and spell a word backwards.
The authors’ observations
A number of factors suggest that Ms. Z’s manic symptoms likely are caused by a medical problem (Table 1).1 She has no family history and only minimal personal history of psychiatric illness, and new-onset bipolar disorder in a 69-year-old woman is unusual.2 Given Ms. Z’s acute change in mental status and numerous medical problems, we consider delirium. Because Ms. Z does not exhibit disorientation or waxing and waning of attention or consciousness, we feel delirium is unlikely to be the primary diagnosis.
Table 1
Criteria for mood disorder due to a general medical condition
A. A prominent and persistent disturbance in mood predominates in the clinical picture and is characterized by either (or both) of the following:
|
| B. There is evidence from the history, physical examination, or laboratory findings that the disturbance is the direct physiological consequence of a general medical condition |
| C. The disturbance is not better accounted for by another mental disorder |
| D. The disturbance does not occur exclusively during the course of a delirium |
| E. The symptoms cause clinically significant distress or impairment in social, occupational, or other important areas of functioning. |
| Source: Reference 1 |
EVALUATION: Clues to the cause
Physical exam reveals stable vital signs, and resting tremor and mild cogwheel rigidity in her right upper extremity consistent with Parkinson’s disease. Laboratory results show elevated blood urea nitrogen (65 mg/dL) and creatinine (8 mg/dL) and stably low white cell count (2.9/μL) and platelets (118x103/μL), which are consistent with her known myelodysplasia. Results for urinalysis, B12, folate, thyroid-stimulating hormone, electrolytes, glucose, liver function, antinuclear antibodies, and rapid plasma reagin are unremarkable. Ms. Z’s elevated blood urea nitrogen and creatinine are expected because she recently refused dialysis. We consider that uremia could be causing her manic symptoms; however, with only 2 case reports of uremia-induced mania in the literature over the past century, we want to rule out other potential causes.3,4
A CT of Ms. Z’s brain is normal. The neurology service performs an EEG and results show mild disorganization with a predominantly posterior rhythm of 8 to 9 Hz symmetrically, occasional periods of slowing, and no epileptiform activity or evidence of encephalopathy; these findings are consistent with end-stage renal disease.
The authors’ observations
Although mood disorder due to a general medical condition—in this case, mania secondary to uremia—was our primary consideration, at this point we could not rule out subclinical delirium. In delirium, we would expect EEG to show diffuse slowing of background rhythm, which we did not see with Ms. Z. However, occasional periods of slowing indicate that delirium was a possible factor.
Parkinson’s disease is known to be a rare predisposing factor for mania—possibly related to potential manicogenic properties of dopaminergic medications5—but this would not explain new-onset mania in the context of uremia in a patient whose carbidopa/levodopa dose had been stable for several years. It is possible that Ms. Z’s refusal of dialysis could have led to build-up of carbidopa/levodopa in her blood, thereby contributing to mania; however, when she began feeling irritable, she refused several of her medications, including carbidopa/levodopa. Therefore, it is unlikely that carbidopa/levodopa accumulated to toxic levels.
We carefully evaluated Ms. Z’s complete medication list to determine if other drugs could be contributing factors. She has been taking escitalopram for anxiety for several years. Although Ms. Z had no personal or family history of bipolar disorder and no past hypomania or agitation associated with this medication, we discontinue escitalopram in case it was contributing to her manic symptoms. Ms. Z also receives amlodipine, 5 mg/d for hypertension; atorvastatin, 20 mg/d, for hyperlipidemia; pantoprazole, 40 mg/d, for acid reflux; metoprolol, 100 mg/d, for hypertension; aspirin, 81 mg/d, for cardioprotection; and fish oil, 2000 mg/d, for cardioprotection. We do not feel that any of these medications significantly contribute to her current state.
TREATMENT: Restarting dialysis
We start Ms. Z on olanzapine, 5 mg/d, for manic symptoms 1 day after admission, and resume dialysis treatments 1 day later. Because of concerns that olanzapine could worsen her myelodysplasia, we switch to aripiprazole, titrating up to 30 mg/d, 4 days later. After 2 dialysis treatments, her manic symptoms begin to resolve.
The authors’ observations
A number of factors suggest that uremia likely is causing Ms. Z’s manic symptoms. Her symptoms suddenly developed shortly after her first missed dialysis treatment, but gradually resolved after re-initiating dialysis. It is possible that antipsychotics relieved her manic symptoms, but this does not detract from the factors that make a causal relationship between uremia and mania likely.
Manic symptoms have been reported to be precipitated by a variety of medical problems, including metabolic disturbances, infections such as human immunodeficiency virus brain infection, neurologic disorders, brain neoplasms, or traumatic brain injuries (Table 2).6,7 End-stage renal disease frequently is associated with psychiatric manifestations—including depression, psychosis, delirium, and dementia—but mania is not a typical presentation. It is possible that this condition occurs more often but is not recognized.
Table 2
Common causes of secondary mania
| Metabolic/endocrine disturbances (hyperthyroidism, hyperadrenalism) |
| Infections (HIV) |
| Neurologic disorders (cerebrovascular accident, multiple sclerosis, Parkinson’s disease, epilepsy, Huntington’s disease) |
| Brain neoplasms |
| Traumatic brain injuries |
| Medications (anabolic steroids, antidepressants, corticosteroids, dextromethorphan, dopamine agonists, hypericum, isoniazid, stimulants, ephedrine, zidovudine) |
| Substance abuse (cocaine, amphetamines) |
| HIV: human immunodeficiency virus |
| Source: References 6,7 |
Kidney disease and psychotropics
We considered the effect of dialysis on psychotropics when selecting pharmacotherapy for Ms. Z’s manic symptoms. Haloperidol is not renally cleared so no dosage adjustment is necessary;8 however, this potent dopamine D2-blocker could have worsened Ms. Z’s parkinsonism. Lithium is contraindicated in acute renal failure. Valproic acid clearance is reduced in renal failure, but because it is cleared by hemodialysis, dosage adjustment is not recommended for dialysis patients.8 However, Ms. Z’s myelodysplasia is a contraindication for valproic acid as well as carbamazepine. With atypical antipsychotics as our primary options, we noted that olanzapine, quetiapine, or aripiprazole do not require dosage adjustments for dialysis patients.8,9 Of these, we eventually chose aripiprazole because we felt that it was least likely to exacerbate Ms. Z’s myelodysplasia.10
How uremia might cause mania
The pathophysiology of uremia-induced mania remains speculative. Possible factors include:
- Chronic renal failure can cause an elevation in plasma free tryptophan, a serotonin (5-HT) precursor.11 Postmortem examination of brains of patients who died in uremic coma show elevated 5-HT.12 Moreover, cerebrospinal fluid of patients with chronic renal failure has shown increased 5-hydroxyindoleacetic acid, the major 5-HT metabolite.13 Increased 5-HT could cause mania in some uremic patients, similar to how serotonergic medications can precipitate mania in some patients.
- Circulating ß-endorphin levels are increased in renal failure.14 ß-endorphins increase animal locomotor activity, which is the basis of an animal model of mania.15,16 Therefore, uremia-induced mania could be partly related to elevated ß-endorphin levels.
This case demonstrates that mania could be a psychiatric manifestation of end-stage renal disease. Clinicians should be aware of this possibility, and further study should examine underlying pathophysiologic changes in uremia and other secondary causes of mania that might lead to such a mood state.
OUTCOME: Lasting improvement
At discharge 17 days after admission, Ms. Z is back to her baseline mental state. Her aripiprazole dose is tapered to 20 mg/d with no return of manic symptoms. After 10 weeks, aripiprazole is discontinued, with no recurrence of mania.
Related Resource
- Arora M, Daughton J. Mania in the medically ill. Curr Psychiatry Rep. 2007;9(3):232-235.
Drug Brand Names
- Amlodipine • Norvasc
- Aripiprazole • Abilify
- Atorvastatin • Lipitor
- Carbamazepine • Tegretol
- Carbidopa/levodopa • Sinemet
- Escitalopram • Lexapro
- Haloperidol • Haldol
- Isoniazid • Nydrazid
- Lithium • Eskalith, Lithobid
- Metoprolol • Lopressor
- Olanzapine • Zyprexa
- Pantoprazole • Protonix
- Quetiapine • Seroquel
- Valproic acid • Depakote
- Zidovudine • Retrovir
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
2. Depp CA, Jeste DV. Bipolar disorder in older adults: a critical review. Bipolar Disord. 2004;6:343-367.
3. El-Mallakh RS, Shrader SA, Widger E. Mania as a manifestation of end-stage renal disease. J Nerv Ment Dis. 1987;175:243-245.
4. Thomas CS, Neale TJ. Organic manic syndrome associated with advanced uraemia due to polycystic kidney disease. Br J Psychiatry. 1991;158:119-121.
5. Kim E, Zwil AS, McAllister TW, et al. Treatment of organic bipolar mood disorders in Parkinson’s disease. J Neuropsychiatry Clin Neurosci. 1994;6:181-184.
6. Levenson JL. Psychosis in the medically ill. Primary Psychiatry. 2005;12(8):16-18.
7. Arora M, Daughton J. Mania in the medically ill. Curr Psychiatry Rep. 2007;9(3):232-235.
8. McLaren KD, Marangell LB. Special considerations in the treatment of patients with bipolar disorder and medical comorbidities. Ann Gen Hosp Psychiatry. 2004;3(1):7.-
9. Mallikaarjun S, Shoaf SE, Boulton DW, et al. Effects of hepatic or renal impairment on the pharmacokinetics of aripiprazole. Clin Pharmacokinet. 2008;47(8):533-542.
10. Stip E, Langlois R, Thuot C, et al. Fatal agranulocytosis: the use of olanzapine in a patient with schizophrenia and myelodysplasia. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(1):297-300.
11. de Torrente A, Glazer GB, Gulyassy P. Reduced in vitro binding of tryptophan by plasma in uremia. Kidney Int. 1974;6:222-229.
12. Jellinger E, Irsigler K, Kothbauer P, et al. Brain monoamines in metabolic coma. Excerpta Medica. 1977;427:169.-
13. Sullivan PA, Murnaghan D, Callaghan N, et al. Cerebral transmitter precursors and metabolites in advanced renal disease. J Neurol Neurosurg Psychiatry. 1978;41:581-588.
14. Aronin N, Krieger DT. Plasma immunoreactive beta-endorphin is elevated in uraemia. Clin Endocrinol (Oxf). 1983;18:459-464.
15. Holtzman SG. Behavioral effects of separate and combined administration of naloxone and D-amphetamine. J Pharmacol Exp Ther. 1974;189:51-60.
16. Segal DS, Browne RG, Derrington DC. Characteristics of beta-endorphin induced behavioral activation and immobilization. In: Usdin E, Bunney WE, Kline NS, eds. Endorphins in mental health research. New York, NY: Oxford University Press; 1979.
1. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
2. Depp CA, Jeste DV. Bipolar disorder in older adults: a critical review. Bipolar Disord. 2004;6:343-367.
3. El-Mallakh RS, Shrader SA, Widger E. Mania as a manifestation of end-stage renal disease. J Nerv Ment Dis. 1987;175:243-245.
4. Thomas CS, Neale TJ. Organic manic syndrome associated with advanced uraemia due to polycystic kidney disease. Br J Psychiatry. 1991;158:119-121.
5. Kim E, Zwil AS, McAllister TW, et al. Treatment of organic bipolar mood disorders in Parkinson’s disease. J Neuropsychiatry Clin Neurosci. 1994;6:181-184.
6. Levenson JL. Psychosis in the medically ill. Primary Psychiatry. 2005;12(8):16-18.
7. Arora M, Daughton J. Mania in the medically ill. Curr Psychiatry Rep. 2007;9(3):232-235.
8. McLaren KD, Marangell LB. Special considerations in the treatment of patients with bipolar disorder and medical comorbidities. Ann Gen Hosp Psychiatry. 2004;3(1):7.-
9. Mallikaarjun S, Shoaf SE, Boulton DW, et al. Effects of hepatic or renal impairment on the pharmacokinetics of aripiprazole. Clin Pharmacokinet. 2008;47(8):533-542.
10. Stip E, Langlois R, Thuot C, et al. Fatal agranulocytosis: the use of olanzapine in a patient with schizophrenia and myelodysplasia. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(1):297-300.
11. de Torrente A, Glazer GB, Gulyassy P. Reduced in vitro binding of tryptophan by plasma in uremia. Kidney Int. 1974;6:222-229.
12. Jellinger E, Irsigler K, Kothbauer P, et al. Brain monoamines in metabolic coma. Excerpta Medica. 1977;427:169.-
13. Sullivan PA, Murnaghan D, Callaghan N, et al. Cerebral transmitter precursors and metabolites in advanced renal disease. J Neurol Neurosurg Psychiatry. 1978;41:581-588.
14. Aronin N, Krieger DT. Plasma immunoreactive beta-endorphin is elevated in uraemia. Clin Endocrinol (Oxf). 1983;18:459-464.
15. Holtzman SG. Behavioral effects of separate and combined administration of naloxone and D-amphetamine. J Pharmacol Exp Ther. 1974;189:51-60.
16. Segal DS, Browne RG, Derrington DC. Characteristics of beta-endorphin induced behavioral activation and immobilization. In: Usdin E, Bunney WE, Kline NS, eds. Endorphins in mental health research. New York, NY: Oxford University Press; 1979.
Excess mortality in patients with mood disorders
Cholesterol, mood, and vascular health: Untangling the relationship
Discuss this article at http://currentpsychiatry.blogspot.com/2010/07/cholesterol-mood-and-vascular-health.html#comments
A growing body of literature examining the putative links among cholesterol, mood disorders, and suicide has produced inconsistent findings and unclear clinical implications that may leave psychiatrists unsure of how to interpret the data. Understanding cholesterol’s role in mood disorders may be relevant to the 2 primary causes of excess deaths in patients with mood disorders: suicide and vascular disease.1
Plausible links
In the early 1990s several studies suggested a link between low cholesterol (<160 mg/dL) and unnatural deaths, including suicide.2-4 Follow-up studies confirmed associations between low cholesterol and suicide attempts, especially violent ones.5 These associations are compelling given the neurobiologic effects of cholesterol, such as a net reduction of serotonergic function (Box 1). Low cholesterol may predispose an individual to aggression, impulsivity, and violence (Table 1).6 Many studies have found that patients with mood disorders have lower cholesterol levels;7 however, other research suggests they are at increased risk of hyperlipidemia, typically hypertriglyceridemia rather than hypercholesterolemia.8
Depression. Several studies have shown an association between low cholesterol and depressive symptoms, although this finding has not been replicated in Asian subjects.9,10 Patients with manic or mixed syndromes have been found to have lower serum cholesterol,11 and individuals with major depression and bipolar disorder have lower cholesterol levels in the brain compared with healthy controls.12 Some studies have observed higher total cholesterol levels after patients receive pharmacotherapy for major depressive symptoms.13 These findings have led to speculation that low serum cholesterol in patients with mood disorders is partially a state-dependent effect of depressive illness.
Suicide. Cohort, case-control, and cross-sectional studies have linked low cholesterol to an increased risk of suicide.2,5 Individuals who attempt suicide by violent means have lower cholesterol compared with those who use less violent methods.5,14 A meta-analysis found statistically significant correlations between low cholesterol and future or past suicidal behavior; however, low cholesterol explained <0.01% of suicidal behavior.15 Studies comparing cholesterol levels of individuals following violent vs nonviolent suicide attempts have demonstrated stronger associations.15
Assessing suicide risk. Current evidence does not support considering low serum cholesterol a risk factor for suicide. One study used cholesterol as a clinical predictor of suicide,16 but this model has not been prospectively validated. As a whole, the evidence does not suggest that cholesterol levels explain a substantial portion of suicidal behaviors.
The neurobiologic effects of low cholesterol—particularly those related to serotonergic hypofunction—are thought to be mediate impulsive, aggressive, and violent behaviors that may predispose an individual to suicide.a,b The CNS contains one-fourth of the body’s free cholesterol,c which is synthesized primarily in situ.
Cholesterol improves membrane stability, reduces permeability, and may influence serotonergic function. Cholesterol depletion may impair function of 5-HT1A and 5-HT7 receptorsd,e and serotonin transporter activity.f Reduced cholesterol after treatment with simvastatin—an HMG-CoA reductase inhibitor that readily crosses the blood-brain barrier—resulted in acute (1-month) increases in serotonin transporter activity followed by subacute (>2 months) decreases.g Lower cholesterol levels may further decrease expression of serotonin receptors and cause a net reduction in serotonergic activity.
In addition, cholesterol is necessary for synapse formation and myelin production. Cholesterol depletion may have more diffuse effects on neurotransmission, such as gamma-aminobutyric acid receptors,hN-methyl-D-aspartate receptors,i opioid signaling,j and excitatory amino acids transport.k
Impulsivity associated with low serotonergic function and low total cholesterol has been suggested as a potential pathway for suicide.l Low cholesterol is associated with self-report measures of impulsivity;m however, increased impulsivity associated with lipid-lowering therapy may be temporary,n which is similar to the time-limited changes in serotonin transporter activity.g Human and animal data have suggested that low cholesterol may be linked to violent behaviors, including suicide.o
Source:
a. Vevera J, Fisar Z, Kvasnicka T, et al. Cholesterol-lowering therapy evokes time-limited changes in serotonergic transmission. Psychiatry Res. 2005;133(2-3):197-203.
b. Kaplan JR, Shively CA, Fontenot MB, et al. Demonstration of an association among dietary cholesterol, central serotonergic activity, and social behavior in monkeys. Psychosom Med. 1994;56(6):479-484.
c. Chattopadhyay A, Paila YD. Lipid-protein interactions, regulation and dysfunction of brain cholesterol. Biochem Biophys Res Commun. 2007;354(3):627-633.
d. Singh P, Paila YD, Chattopadhyay A. Differential effects of cholesterol and 7-dehydrocholesterol on the ligand binding activity of the hippocampal serotonin(1A) receptor: implications in SLOS. Biochem Biophys Res Commun. 2007;358(2):495-499.
e. Sjögren B, Hamblin MW, Svenningsson P. Cholesterol depletion reduces serotonin binding and signaling via human 5-HT(7(a)) receptors. Eur J Pharmacol. 2006;552(1-3):1-10.
f. Scanlon SM, Williams DC, Schloss P. Membrane cholesterol modulates serotonin transporter activity. Biochemistry. 2001;40(35):10507-10513.
g. Vevera J, Fisar Z, Kvasnicka T, et al. Cholesterol-lowering therapy evokes time-limited changes in serotonergic transmission. Psychiatry Res. 2005;133(2-3):197-203.
h. Sooksawate T, Simmonds MA. Effects of membrane cholesterol on the sensitivity of the GABA(A) receptor to GABA in acutely dissociated rat hippocampal neurones. Neuropharmacology. 2001;40(2):178-184.
i. Abulrob A, Tauskela JS, Mealing G, et al. Protection by cholesterol-extracting cyclodextrins: a role for N-methyl-daspartate receptor redistribution. J Neurochem. 2005;92(6):1477-1486.
j. Huang P, Xu W, Yoon SI, et al. Cholesterol reduction by methyl-beta-cyclodextrin attenuates the delta opioid receptor-mediated signaling in neuronal cells but enhances it in non-neuronal cells. Biochem Pharmacol. 2007;73(4):534-549.
k. Butchbach ME, Tian G, Guo H, et al. Association of excitatory amino acid transporters, especially EAAT2, with cholesterol-rich lipid raft microdomains: importance for excitatory amino acid transporter localization and function. J Biol Chem. 2004;279(33):34388-34396.
l. Fawcett J, Busch KA, Jacobs D, et al. Suicide: a four-pathway clinical-biochemical model. Annals N Y Acad Sci. 1997;836:288-301.
m. Garland M, Hickey D, Corvin A, et al. Total serum cholesterol in relation to psychological correlates in parasuicide. Br J Psychiatry. 2000;177:77-83.
n. Ormiston T, Wolkowitz OM, Reus VI, et al. Behavioral implications of lowering cholesterol levels: a double-blind pilot study. Psychosomatics. 2003;44(5):412-414.
o. Golomb BA. Cholesterol and violence: is there a connection? Ann Intern Med. 1998;128(6):478-487.
Table 1
Psychiatric features associated with low cholesterol*
| Symptoms |
| Anxiety, depressed mood, emotional lability, euphoria, impulsivity, irritability, suicidal ideation, aggression |
| Syndromes |
| Anorexia nervosa, bipolar disorder, borderline personality disorder, major depressive disorder, seasonal affective disorder |
| Behaviors |
| Suicide and suicide attempts, violence |
| *Small studies have suggested possible relationships with dissociative and panic disorders |
Effects of lipid-lowering agents
If there is a causal relationship between low cholesterol and mood disorders, then it stands to reason that using cholesterol-lowering drugs would increase the risk of depression and suicide. However, the data do not support that conclusion.
Many case reports have documented adverse psychiatric reactions to statins, including depression, suicidality, emotional lability, agitation, irritability, anxiety, panic, and euphoria.17 In an early analysis of primary prevention trials, patients receiving cholesterol-lowering treatment—mainly non-statins—were estimated to have twice the risk of death by suicide or violence compared with controls.3 However, a more recent meta-analysis of larger clinical trials of lipid-lowering agents including statins and observational studies did not reveal an association between lipid-lowering medications and suicide.15,18
In a large case-control study, statin users had a lower risk of depression (adjusted odds ratio [OR] 0.4, 95% confidence interval [CI], 0.2 to 0.9) than patients taking non-statin lipid-lowering drugs (adjusted OR 1.0, 95% CI, 0.5 to 2.1).19 However, statins reduced cholesterol more (30% to 50%) than non-statin drugs (10% to 20%). A clinical trial of >1,000 patients with stable coronary artery disease treated with pravastatin—an HMG-CoA reductase inhibitor with low lipophilicity that is less likely than other statins to cross the blood-brain barrier—revealed no changes in self-reported anger, impulsiveness, anxiety, or depression.20
This study did not exclude patients with psychiatric illness—who are at greatest risk of suicide—but other trials of lipid-lowering drugs did.21 As a result, the effects of lipid-lowering medications on psychiatric patients are unclear. A clinical trial is underway to assess the effects of pravastatin (low lipophilicity), simvastatin (high lipophilicity), or placebo on mood, sleep, and aggression.21
Low cholesterol: State or trait?
Much of the research linking low cholesterol, mood disorders, and suicidality could be confounded by depressed mood leading to reduced serum cholesterol. There has been considerable debate about whether low cholesterol predisposes patients to suicide or if depression independently leads to poor nutrition and therefore low cholesterol and increased suicide risk.6,22
Some researchers have suggested that depression lowers cholesterol and increases risk of suicide,23 but study designs have limited the ability to discern the directionality of the relationship. Attempts to control for depression-related malnutrition and weight loss—which lowers total cholesterol, low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C)24—suggest the association may be independent of these variables.25-27 These findings suggest that cholesterol may be considered a trait marker and is not entirely state-dependent. However, multiple, large, long-term randomized controlled trials have not shown increased depression and suicide with use of lipid-lowering agents in healthy populations.20
The Figure illustrates known epidemiologic associations of low cholesterol, low serotoninergic function, and suicide and contrasts conceptual models of cholesterol as a state and a trait marker. A case can be made for cholesterol as both a state and a trait marker, and these models could overlap, with depression-induced decreases in cholesterol further mediating changes in serotonergic function and related behavioral sequelae.
Figure
Cholesterol, depression, and suicide: How are they linked? 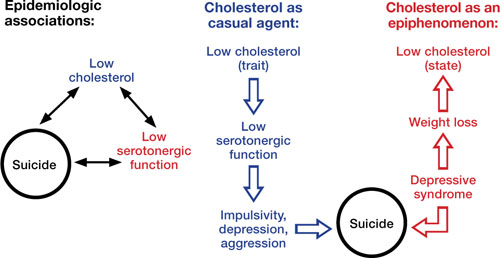
Low cholesterol may be considered a trait marker, predisposing patients to lower serotonergic function and placing them at greater risk for impulsivity, depression, aggression, and suicide. Other models suggest that lower cholesterol is a state-dependent consequence of depression, and not part of a causal chain toward suicide
Improving cardiac health
Limited epidemiologic studies suggest that patients with mood disorders may have lower levels of total cholesterol and LDL-C, but higher rates of hypertriglyceridemia compared with the general population.8 Unfortunately, psychiatric patients—who may be at increased risk of developing cardiovascular disease—may be less likely to be screened and appropriately treated for lipid abnormalities.28 To address this disparity, consider assuming an active role in assessing and managing hyperlipidemia in your patients with mood disorders. Be aware of your patients’ lipid profile and ensure that they follow monitoring recommendations.
The National Cholesterol Education Program recommends screening all adults age >20 for hyperlipidemia every 5 years using measures of total cholesterol, LDL-C, HDL-C, and triglycerides. If LDL-C or triglycerides exceed target values (Table 2), appropriate management includes recommending lifestyle changes and pharmacotherapy (Box 2).
Patients should receive a fasting lipid profile before and 12 weeks after starting any antipsychotic and semiannually thereafter.29 Consider closely monitoring lipids when patients gain weight with psychotropics. Refer patients with hyperlipidemia to a primary care physician, but in the absence of such a provider, mental health clinicians who are familiar with treatment guidelines can manage these patients.30
Closely monitor individuals with mood disorders for changes in behavior or mental status after starting a lipid-lowering agent. Consider discontinuing the drug if a patient develops an adverse reaction. If symptoms return after medication rechallenge, consider other management strategies such as an alternate lipid-lowering agent or re-emphasizing behavioral measures.
Table 2
National Cholesterol Education Program recommended LDL levels
| Risk category* | LDL goal | When to consider medications |
|---|---|---|
| CHD or CHD equivalent | <100 mg/dL | ≥130 mg/dL |
| ≥2 major risk factors | <130 mg/dL | ≥130 to 160 mg/dL (based on 10-year risk) |
| 0 or 1 risk factor | <160 mg/dL | ≥190 mg/dL |
| CHD: coronary heart disease; HDL: high-density lipoprotein; LDL: low-density lipoprotein | ||
| *Risk category is based on the presence of CHD or equivalent and major risk factors for CHD. CHD equivalents include symptomatic carotid artery disease, peripheral artery disease, and abdominal aortic aneurysm. Major risk factors include smoking, hypertension, low HDL, family history, and age. LDL levels to consider medications for those with ≥2 major risk factors vary by 10-year CHD risk | ||
| Source: National Cholesterol Education Program, Adult Treatment Panel III (ATP III) Quick Desk Reference. www.nhlbi.nih.gov/guidelines/cholesterol/atglance.htm | ||
National Cholesterol Education Program guidelines state that when a patient’s low-density lipoprotein cholesterol (LDL-C) exceeds targets (Table 2), first recommend lifestyle changes such as a diet low in saturated fat (<7% of calories) and cholesterol (<200 mg/d), weight management, and exercise. Increases in soluble fiber (10 to 25 g/d) and plant stanols/sterols also may be considered. If LDL-C levels are still too high, pharmacologic therapy such as an HMGCoA reductase inhibitor is suggested.
Treatment of elevated triglycerides (≥150 mg/dL) includes reaching the target LDL-C, intensifying a weight management program, and increasing exercise. Address quitting smoking and limiting alcohol when indicated. If triglyceride levels are ≥200 mg/dL after the LDL-C target is reached, set a secondary goal of reaching a target non-high-density lipoprotein cholesterol (HDL-C) (non-HDL-C; total cholesterol minus HDL-C) 30 mg/dL greater than the LDL goal. This can be achieved by adding an LDL-lowering drug such as a statin, nicotinic acid, or ezetimibe. When triglycerides are ≥500 mg/dL, more aggressive intervention, such as with a fibrate, omega-3 fatty acids, very low-fat diets, and exercise, is required to prevent pancreatitis.
Source: National Heart Lung and Blood Institute. National Cholesterol Education Program. www.nhlbi.nih.gov/guidelines/cholesterol/index.htm
Related Resources
- Fiedorowicz JG, Coryell WH. Cholesterol and suicide attempts: a prospective study of depressed inpatients. Psychiatry Res. 2007;152(1):11-20.
- National Cholesterol Education Program, Adult Treatment Panel III (ATP III) Quick Desk Reference. www.nhlbi.nih.gov/guidelines/cholesterol/atglance.htm.
- Executive Summary of the third report of the national Cholesterol Education Program (nCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486-2497.
Drug Brand Names
- Ezetimibe • Zetia
- Pravastatin • Pravachol
- Simvastatin • Zocor
Acknowledgements
Dr. Fiedorowicz thanks Lois Warren and Miriam Weiner for their editorial assistance.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products. Dr. Fiedorowicz is supported by the national Institutes of Health (1K23MH083695-01A210), nARSAD, and the Institute for Clinical and Translational Science at the University of Iowa (3 UL1 RR024979-03S4). He has received support for participating in a colleague’s investigator-initiated project with Eli Lilly. Dr. Haynes’ research is supported by grants from the national Institutes of Health (nHLBI: HL58972 & HL14388; nCRR CTSA: 1UL1RR024979).
1. Osby U, Brandt L, Correia N, et al. Excess mortality in bipolar and unipolar disorder in Sweden. Arch Gen Psychiatry. 2001;58(9):844-850.
2. Lindberg G, Råstam L, Gullberg B, et al. Low serum cholesterol concentration and short term mortality from injuries in men and women. BMJ. 1992;305(6848):277-279.
3. Muldoon MF, Manuck SB, Matthews KA. Lowering cholesterol concentrations and mortality: a quantitative review of primary prevention trials. BMJ. 1990;301(6747):309-314.
4. Neaton JD, Blackburn H, Jacobs D, et al. Serum cholesterol level and mortality findings for men screened in the Multiple Risk Factor Intervention Trial. Multiple Risk Factor Intervention Trial Research Group. Arch Intern Med. 1992;152(7):1490-1500.
5. Fiedorowicz JG, Coryell WH. Cholesterol and suicide attempts: a prospective study of depressed inpatients. Psychiatry Res. 2007;152(1):11-20.
6. Golomb BA. Cholesterol and violence: is there a connection? Ann Intern Med. 1998;128(6):478-487.
7. Pae CU, Kim JJ, Lee SJ, et al. Aberration of cholesterol level in first-onset bipolar I patients. J Affect Disord. 2004;83(1):79-82.
8. Fiedorowicz JG, Palagummi NM, Forman-Hoffman VL, et al. Elevated prevalence of obesity, metabolic syndrome, and cardiovascular risk factors in bipolar disorder. Ann Clin Psychiatry. 2008;20(3):131-137.
9. Chung KH, Tsai SY, Lee HC. Mood symptoms and serum lipids in acute phase of bipolar disorder in Taiwan. Psychiatry Clin Neurosci. 2007;61(4):428-433.
10. Jow GM, Yang TT, Chen CL. Leptin and cholesterol levels are low in major depressive disorder, but high in schizophrenia. J Affect Disord. 2006;90(1):21-27.
11. Sagud M, Mihaljevic-Peles A, Pivac N, et al. Platelet serotonin and serum lipids in psychotic mania. J Affect Disord. 2007;97(1-3):247-251.
12. Beasley CL, Honer WG, Bergmann K, et al. Reductions in cholesterol and synaptic markers in association cortex in mood disorders. Bipolar Disord. 2005;7(5):449-455.
13. Gabriel A. Changes in plasma cholesterol in mood disorder patients: does treatment make a difference? J Affect Disord. 2007;99(1-3):273-278.
14. Lalovic A, Levy E, Luheshi G, et al. Cholesterol content in brains of suicide completers. Int J Neuropsychopharmacol. 2007;10(2):159-166.
15. Lester D. Serum cholesterol levels and suicide: a meta-analysis. Suicide Life Threat Behav. 2002;32(3):333-346.
16. Coryell W, Schlesser M. Combined biological tests for suicide prediction. Psychiatry Res. 2007;150(2):187-191.
17. Tatley M, Savage R. Psychiatric adverse reactions with statins, fibrates and ezetimibe: implications for the use of lipid-lowering agents. Drug Saf. 2007;30(3):195-201.
18. Callréus T, Agerskov Andersen U, Hallas J, et al. Cardiovascular drugs and the risk of suicide: a nested case-control study. Eur J Clin Pharmacol. 2007;63(6):591-596.
19. Yang CC, Jick SS, Jick H. Lipid-lowering drugs and the risk of depression and suicidal behavior. Arch Intern Med. 2003;163(16):1926-1932.
20. Stewart RA, Sharples KJ, North FM, et al. Long-term assessment of psychological well-being in a randomized placebo-controlled trial of cholesterol reduction with pravastatin. The LIPID Study Investigators. Arch Intern Med. 2000;160(20):3144-3152.
21. Golomb BA, Criqui MH, White HL, et al. The UCSD Statin Study: a randomized controlled trial assessing the impact of statins on selected noncardiac outcomes. Control Clin Trials. 2004;25(2):178-202.
22. Fawcett J, Busch KA, Jacobs D, et al. Suicide: a four-pathway clinical-biochemical model. Annals N Y Acad Sci. 1997;836:288-301.
23. Law MR, Thompson SG, Wald NJ. Assessing possible hazards of reducing serum cholesterol. BMJ. 1994;308(6925):373-379.
24. Dattilo AM, Kris-Etherton PM. Effects of weight reduction on blood lipids and lipoproteins: a meta-analysis. Am J Clin Nutr. 1992;56(2):320-328.
25. Garland M, Hickey D, Corvin A, et al. Total serum cholesterol in relation to psychological correlates in parasuicide. Br J Psychiatry. 2000;177:77-83.
26. Golier JA, Marzuk PM, Leon AC, et al. Low serum cholesterol level and attempted suicide. Am J Psychiatry. 1995;152(3):419-423.
27. Kunugi H, Takei N, Aoki H, et al. Low serum cholesterol in suicide attempters. Biol Psychiatry. 1997;41(2):196-200.
28. Murray DP, Weiner M, Prabhakar M, et al. Mania and mortality: why the excess cardiovascular risk in bipolar disorder? Curr Psychiatry Rep. 2009;11(6):475-480.
29. Sernyak MJ. Implementation of monitoring and management guidelines for second-generation antipsychotics. J Clin Psychiatry. 2007;68(suppl 4):14-18.
30. Marder SR, Essock SM, Miller AL, et al. Physical health monitoring of patients with schizophrenia. Am J Psychiatry. 2004;161(8):1334-1349.
Discuss this article at http://currentpsychiatry.blogspot.com/2010/07/cholesterol-mood-and-vascular-health.html#comments
A growing body of literature examining the putative links among cholesterol, mood disorders, and suicide has produced inconsistent findings and unclear clinical implications that may leave psychiatrists unsure of how to interpret the data. Understanding cholesterol’s role in mood disorders may be relevant to the 2 primary causes of excess deaths in patients with mood disorders: suicide and vascular disease.1
Plausible links
In the early 1990s several studies suggested a link between low cholesterol (<160 mg/dL) and unnatural deaths, including suicide.2-4 Follow-up studies confirmed associations between low cholesterol and suicide attempts, especially violent ones.5 These associations are compelling given the neurobiologic effects of cholesterol, such as a net reduction of serotonergic function (Box 1). Low cholesterol may predispose an individual to aggression, impulsivity, and violence (Table 1).6 Many studies have found that patients with mood disorders have lower cholesterol levels;7 however, other research suggests they are at increased risk of hyperlipidemia, typically hypertriglyceridemia rather than hypercholesterolemia.8
Depression. Several studies have shown an association between low cholesterol and depressive symptoms, although this finding has not been replicated in Asian subjects.9,10 Patients with manic or mixed syndromes have been found to have lower serum cholesterol,11 and individuals with major depression and bipolar disorder have lower cholesterol levels in the brain compared with healthy controls.12 Some studies have observed higher total cholesterol levels after patients receive pharmacotherapy for major depressive symptoms.13 These findings have led to speculation that low serum cholesterol in patients with mood disorders is partially a state-dependent effect of depressive illness.
Suicide. Cohort, case-control, and cross-sectional studies have linked low cholesterol to an increased risk of suicide.2,5 Individuals who attempt suicide by violent means have lower cholesterol compared with those who use less violent methods.5,14 A meta-analysis found statistically significant correlations between low cholesterol and future or past suicidal behavior; however, low cholesterol explained <0.01% of suicidal behavior.15 Studies comparing cholesterol levels of individuals following violent vs nonviolent suicide attempts have demonstrated stronger associations.15
Assessing suicide risk. Current evidence does not support considering low serum cholesterol a risk factor for suicide. One study used cholesterol as a clinical predictor of suicide,16 but this model has not been prospectively validated. As a whole, the evidence does not suggest that cholesterol levels explain a substantial portion of suicidal behaviors.
The neurobiologic effects of low cholesterol—particularly those related to serotonergic hypofunction—are thought to be mediate impulsive, aggressive, and violent behaviors that may predispose an individual to suicide.a,b The CNS contains one-fourth of the body’s free cholesterol,c which is synthesized primarily in situ.
Cholesterol improves membrane stability, reduces permeability, and may influence serotonergic function. Cholesterol depletion may impair function of 5-HT1A and 5-HT7 receptorsd,e and serotonin transporter activity.f Reduced cholesterol after treatment with simvastatin—an HMG-CoA reductase inhibitor that readily crosses the blood-brain barrier—resulted in acute (1-month) increases in serotonin transporter activity followed by subacute (>2 months) decreases.g Lower cholesterol levels may further decrease expression of serotonin receptors and cause a net reduction in serotonergic activity.
In addition, cholesterol is necessary for synapse formation and myelin production. Cholesterol depletion may have more diffuse effects on neurotransmission, such as gamma-aminobutyric acid receptors,hN-methyl-D-aspartate receptors,i opioid signaling,j and excitatory amino acids transport.k
Impulsivity associated with low serotonergic function and low total cholesterol has been suggested as a potential pathway for suicide.l Low cholesterol is associated with self-report measures of impulsivity;m however, increased impulsivity associated with lipid-lowering therapy may be temporary,n which is similar to the time-limited changes in serotonin transporter activity.g Human and animal data have suggested that low cholesterol may be linked to violent behaviors, including suicide.o
Source:
a. Vevera J, Fisar Z, Kvasnicka T, et al. Cholesterol-lowering therapy evokes time-limited changes in serotonergic transmission. Psychiatry Res. 2005;133(2-3):197-203.
b. Kaplan JR, Shively CA, Fontenot MB, et al. Demonstration of an association among dietary cholesterol, central serotonergic activity, and social behavior in monkeys. Psychosom Med. 1994;56(6):479-484.
c. Chattopadhyay A, Paila YD. Lipid-protein interactions, regulation and dysfunction of brain cholesterol. Biochem Biophys Res Commun. 2007;354(3):627-633.
d. Singh P, Paila YD, Chattopadhyay A. Differential effects of cholesterol and 7-dehydrocholesterol on the ligand binding activity of the hippocampal serotonin(1A) receptor: implications in SLOS. Biochem Biophys Res Commun. 2007;358(2):495-499.
e. Sjögren B, Hamblin MW, Svenningsson P. Cholesterol depletion reduces serotonin binding and signaling via human 5-HT(7(a)) receptors. Eur J Pharmacol. 2006;552(1-3):1-10.
f. Scanlon SM, Williams DC, Schloss P. Membrane cholesterol modulates serotonin transporter activity. Biochemistry. 2001;40(35):10507-10513.
g. Vevera J, Fisar Z, Kvasnicka T, et al. Cholesterol-lowering therapy evokes time-limited changes in serotonergic transmission. Psychiatry Res. 2005;133(2-3):197-203.
h. Sooksawate T, Simmonds MA. Effects of membrane cholesterol on the sensitivity of the GABA(A) receptor to GABA in acutely dissociated rat hippocampal neurones. Neuropharmacology. 2001;40(2):178-184.
i. Abulrob A, Tauskela JS, Mealing G, et al. Protection by cholesterol-extracting cyclodextrins: a role for N-methyl-daspartate receptor redistribution. J Neurochem. 2005;92(6):1477-1486.
j. Huang P, Xu W, Yoon SI, et al. Cholesterol reduction by methyl-beta-cyclodextrin attenuates the delta opioid receptor-mediated signaling in neuronal cells but enhances it in non-neuronal cells. Biochem Pharmacol. 2007;73(4):534-549.
k. Butchbach ME, Tian G, Guo H, et al. Association of excitatory amino acid transporters, especially EAAT2, with cholesterol-rich lipid raft microdomains: importance for excitatory amino acid transporter localization and function. J Biol Chem. 2004;279(33):34388-34396.
l. Fawcett J, Busch KA, Jacobs D, et al. Suicide: a four-pathway clinical-biochemical model. Annals N Y Acad Sci. 1997;836:288-301.
m. Garland M, Hickey D, Corvin A, et al. Total serum cholesterol in relation to psychological correlates in parasuicide. Br J Psychiatry. 2000;177:77-83.
n. Ormiston T, Wolkowitz OM, Reus VI, et al. Behavioral implications of lowering cholesterol levels: a double-blind pilot study. Psychosomatics. 2003;44(5):412-414.
o. Golomb BA. Cholesterol and violence: is there a connection? Ann Intern Med. 1998;128(6):478-487.
Table 1
Psychiatric features associated with low cholesterol*
| Symptoms |
| Anxiety, depressed mood, emotional lability, euphoria, impulsivity, irritability, suicidal ideation, aggression |
| Syndromes |
| Anorexia nervosa, bipolar disorder, borderline personality disorder, major depressive disorder, seasonal affective disorder |
| Behaviors |
| Suicide and suicide attempts, violence |
| *Small studies have suggested possible relationships with dissociative and panic disorders |
Effects of lipid-lowering agents
If there is a causal relationship between low cholesterol and mood disorders, then it stands to reason that using cholesterol-lowering drugs would increase the risk of depression and suicide. However, the data do not support that conclusion.
Many case reports have documented adverse psychiatric reactions to statins, including depression, suicidality, emotional lability, agitation, irritability, anxiety, panic, and euphoria.17 In an early analysis of primary prevention trials, patients receiving cholesterol-lowering treatment—mainly non-statins—were estimated to have twice the risk of death by suicide or violence compared with controls.3 However, a more recent meta-analysis of larger clinical trials of lipid-lowering agents including statins and observational studies did not reveal an association between lipid-lowering medications and suicide.15,18
In a large case-control study, statin users had a lower risk of depression (adjusted odds ratio [OR] 0.4, 95% confidence interval [CI], 0.2 to 0.9) than patients taking non-statin lipid-lowering drugs (adjusted OR 1.0, 95% CI, 0.5 to 2.1).19 However, statins reduced cholesterol more (30% to 50%) than non-statin drugs (10% to 20%). A clinical trial of >1,000 patients with stable coronary artery disease treated with pravastatin—an HMG-CoA reductase inhibitor with low lipophilicity that is less likely than other statins to cross the blood-brain barrier—revealed no changes in self-reported anger, impulsiveness, anxiety, or depression.20
This study did not exclude patients with psychiatric illness—who are at greatest risk of suicide—but other trials of lipid-lowering drugs did.21 As a result, the effects of lipid-lowering medications on psychiatric patients are unclear. A clinical trial is underway to assess the effects of pravastatin (low lipophilicity), simvastatin (high lipophilicity), or placebo on mood, sleep, and aggression.21
Low cholesterol: State or trait?
Much of the research linking low cholesterol, mood disorders, and suicidality could be confounded by depressed mood leading to reduced serum cholesterol. There has been considerable debate about whether low cholesterol predisposes patients to suicide or if depression independently leads to poor nutrition and therefore low cholesterol and increased suicide risk.6,22
Some researchers have suggested that depression lowers cholesterol and increases risk of suicide,23 but study designs have limited the ability to discern the directionality of the relationship. Attempts to control for depression-related malnutrition and weight loss—which lowers total cholesterol, low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C)24—suggest the association may be independent of these variables.25-27 These findings suggest that cholesterol may be considered a trait marker and is not entirely state-dependent. However, multiple, large, long-term randomized controlled trials have not shown increased depression and suicide with use of lipid-lowering agents in healthy populations.20
The Figure illustrates known epidemiologic associations of low cholesterol, low serotoninergic function, and suicide and contrasts conceptual models of cholesterol as a state and a trait marker. A case can be made for cholesterol as both a state and a trait marker, and these models could overlap, with depression-induced decreases in cholesterol further mediating changes in serotonergic function and related behavioral sequelae.
Figure
Cholesterol, depression, and suicide: How are they linked? 
Low cholesterol may be considered a trait marker, predisposing patients to lower serotonergic function and placing them at greater risk for impulsivity, depression, aggression, and suicide. Other models suggest that lower cholesterol is a state-dependent consequence of depression, and not part of a causal chain toward suicide
Improving cardiac health
Limited epidemiologic studies suggest that patients with mood disorders may have lower levels of total cholesterol and LDL-C, but higher rates of hypertriglyceridemia compared with the general population.8 Unfortunately, psychiatric patients—who may be at increased risk of developing cardiovascular disease—may be less likely to be screened and appropriately treated for lipid abnormalities.28 To address this disparity, consider assuming an active role in assessing and managing hyperlipidemia in your patients with mood disorders. Be aware of your patients’ lipid profile and ensure that they follow monitoring recommendations.
The National Cholesterol Education Program recommends screening all adults age >20 for hyperlipidemia every 5 years using measures of total cholesterol, LDL-C, HDL-C, and triglycerides. If LDL-C or triglycerides exceed target values (Table 2), appropriate management includes recommending lifestyle changes and pharmacotherapy (Box 2).
Patients should receive a fasting lipid profile before and 12 weeks after starting any antipsychotic and semiannually thereafter.29 Consider closely monitoring lipids when patients gain weight with psychotropics. Refer patients with hyperlipidemia to a primary care physician, but in the absence of such a provider, mental health clinicians who are familiar with treatment guidelines can manage these patients.30
Closely monitor individuals with mood disorders for changes in behavior or mental status after starting a lipid-lowering agent. Consider discontinuing the drug if a patient develops an adverse reaction. If symptoms return after medication rechallenge, consider other management strategies such as an alternate lipid-lowering agent or re-emphasizing behavioral measures.
Table 2
National Cholesterol Education Program recommended LDL levels
| Risk category* | LDL goal | When to consider medications |
|---|---|---|
| CHD or CHD equivalent | <100 mg/dL | ≥130 mg/dL |
| ≥2 major risk factors | <130 mg/dL | ≥130 to 160 mg/dL (based on 10-year risk) |
| 0 or 1 risk factor | <160 mg/dL | ≥190 mg/dL |
| CHD: coronary heart disease; HDL: high-density lipoprotein; LDL: low-density lipoprotein | ||
| *Risk category is based on the presence of CHD or equivalent and major risk factors for CHD. CHD equivalents include symptomatic carotid artery disease, peripheral artery disease, and abdominal aortic aneurysm. Major risk factors include smoking, hypertension, low HDL, family history, and age. LDL levels to consider medications for those with ≥2 major risk factors vary by 10-year CHD risk | ||
| Source: National Cholesterol Education Program, Adult Treatment Panel III (ATP III) Quick Desk Reference. www.nhlbi.nih.gov/guidelines/cholesterol/atglance.htm | ||
National Cholesterol Education Program guidelines state that when a patient’s low-density lipoprotein cholesterol (LDL-C) exceeds targets (Table 2), first recommend lifestyle changes such as a diet low in saturated fat (<7% of calories) and cholesterol (<200 mg/d), weight management, and exercise. Increases in soluble fiber (10 to 25 g/d) and plant stanols/sterols also may be considered. If LDL-C levels are still too high, pharmacologic therapy such as an HMGCoA reductase inhibitor is suggested.
Treatment of elevated triglycerides (≥150 mg/dL) includes reaching the target LDL-C, intensifying a weight management program, and increasing exercise. Address quitting smoking and limiting alcohol when indicated. If triglyceride levels are ≥200 mg/dL after the LDL-C target is reached, set a secondary goal of reaching a target non-high-density lipoprotein cholesterol (HDL-C) (non-HDL-C; total cholesterol minus HDL-C) 30 mg/dL greater than the LDL goal. This can be achieved by adding an LDL-lowering drug such as a statin, nicotinic acid, or ezetimibe. When triglycerides are ≥500 mg/dL, more aggressive intervention, such as with a fibrate, omega-3 fatty acids, very low-fat diets, and exercise, is required to prevent pancreatitis.
Source: National Heart Lung and Blood Institute. National Cholesterol Education Program. www.nhlbi.nih.gov/guidelines/cholesterol/index.htm
Related Resources
- Fiedorowicz JG, Coryell WH. Cholesterol and suicide attempts: a prospective study of depressed inpatients. Psychiatry Res. 2007;152(1):11-20.
- National Cholesterol Education Program, Adult Treatment Panel III (ATP III) Quick Desk Reference. www.nhlbi.nih.gov/guidelines/cholesterol/atglance.htm.
- Executive Summary of the third report of the national Cholesterol Education Program (nCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486-2497.
Drug Brand Names
- Ezetimibe • Zetia
- Pravastatin • Pravachol
- Simvastatin • Zocor
Acknowledgements
Dr. Fiedorowicz thanks Lois Warren and Miriam Weiner for their editorial assistance.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products. Dr. Fiedorowicz is supported by the national Institutes of Health (1K23MH083695-01A210), nARSAD, and the Institute for Clinical and Translational Science at the University of Iowa (3 UL1 RR024979-03S4). He has received support for participating in a colleague’s investigator-initiated project with Eli Lilly. Dr. Haynes’ research is supported by grants from the national Institutes of Health (nHLBI: HL58972 & HL14388; nCRR CTSA: 1UL1RR024979).
Discuss this article at http://currentpsychiatry.blogspot.com/2010/07/cholesterol-mood-and-vascular-health.html#comments
A growing body of literature examining the putative links among cholesterol, mood disorders, and suicide has produced inconsistent findings and unclear clinical implications that may leave psychiatrists unsure of how to interpret the data. Understanding cholesterol’s role in mood disorders may be relevant to the 2 primary causes of excess deaths in patients with mood disorders: suicide and vascular disease.1
Plausible links
In the early 1990s several studies suggested a link between low cholesterol (<160 mg/dL) and unnatural deaths, including suicide.2-4 Follow-up studies confirmed associations between low cholesterol and suicide attempts, especially violent ones.5 These associations are compelling given the neurobiologic effects of cholesterol, such as a net reduction of serotonergic function (Box 1). Low cholesterol may predispose an individual to aggression, impulsivity, and violence (Table 1).6 Many studies have found that patients with mood disorders have lower cholesterol levels;7 however, other research suggests they are at increased risk of hyperlipidemia, typically hypertriglyceridemia rather than hypercholesterolemia.8
Depression. Several studies have shown an association between low cholesterol and depressive symptoms, although this finding has not been replicated in Asian subjects.9,10 Patients with manic or mixed syndromes have been found to have lower serum cholesterol,11 and individuals with major depression and bipolar disorder have lower cholesterol levels in the brain compared with healthy controls.12 Some studies have observed higher total cholesterol levels after patients receive pharmacotherapy for major depressive symptoms.13 These findings have led to speculation that low serum cholesterol in patients with mood disorders is partially a state-dependent effect of depressive illness.
Suicide. Cohort, case-control, and cross-sectional studies have linked low cholesterol to an increased risk of suicide.2,5 Individuals who attempt suicide by violent means have lower cholesterol compared with those who use less violent methods.5,14 A meta-analysis found statistically significant correlations between low cholesterol and future or past suicidal behavior; however, low cholesterol explained <0.01% of suicidal behavior.15 Studies comparing cholesterol levels of individuals following violent vs nonviolent suicide attempts have demonstrated stronger associations.15
Assessing suicide risk. Current evidence does not support considering low serum cholesterol a risk factor for suicide. One study used cholesterol as a clinical predictor of suicide,16 but this model has not been prospectively validated. As a whole, the evidence does not suggest that cholesterol levels explain a substantial portion of suicidal behaviors.
The neurobiologic effects of low cholesterol—particularly those related to serotonergic hypofunction—are thought to be mediate impulsive, aggressive, and violent behaviors that may predispose an individual to suicide.a,b The CNS contains one-fourth of the body’s free cholesterol,c which is synthesized primarily in situ.
Cholesterol improves membrane stability, reduces permeability, and may influence serotonergic function. Cholesterol depletion may impair function of 5-HT1A and 5-HT7 receptorsd,e and serotonin transporter activity.f Reduced cholesterol after treatment with simvastatin—an HMG-CoA reductase inhibitor that readily crosses the blood-brain barrier—resulted in acute (1-month) increases in serotonin transporter activity followed by subacute (>2 months) decreases.g Lower cholesterol levels may further decrease expression of serotonin receptors and cause a net reduction in serotonergic activity.
In addition, cholesterol is necessary for synapse formation and myelin production. Cholesterol depletion may have more diffuse effects on neurotransmission, such as gamma-aminobutyric acid receptors,hN-methyl-D-aspartate receptors,i opioid signaling,j and excitatory amino acids transport.k
Impulsivity associated with low serotonergic function and low total cholesterol has been suggested as a potential pathway for suicide.l Low cholesterol is associated with self-report measures of impulsivity;m however, increased impulsivity associated with lipid-lowering therapy may be temporary,n which is similar to the time-limited changes in serotonin transporter activity.g Human and animal data have suggested that low cholesterol may be linked to violent behaviors, including suicide.o
Source:
a. Vevera J, Fisar Z, Kvasnicka T, et al. Cholesterol-lowering therapy evokes time-limited changes in serotonergic transmission. Psychiatry Res. 2005;133(2-3):197-203.
b. Kaplan JR, Shively CA, Fontenot MB, et al. Demonstration of an association among dietary cholesterol, central serotonergic activity, and social behavior in monkeys. Psychosom Med. 1994;56(6):479-484.
c. Chattopadhyay A, Paila YD. Lipid-protein interactions, regulation and dysfunction of brain cholesterol. Biochem Biophys Res Commun. 2007;354(3):627-633.
d. Singh P, Paila YD, Chattopadhyay A. Differential effects of cholesterol and 7-dehydrocholesterol on the ligand binding activity of the hippocampal serotonin(1A) receptor: implications in SLOS. Biochem Biophys Res Commun. 2007;358(2):495-499.
e. Sjögren B, Hamblin MW, Svenningsson P. Cholesterol depletion reduces serotonin binding and signaling via human 5-HT(7(a)) receptors. Eur J Pharmacol. 2006;552(1-3):1-10.
f. Scanlon SM, Williams DC, Schloss P. Membrane cholesterol modulates serotonin transporter activity. Biochemistry. 2001;40(35):10507-10513.
g. Vevera J, Fisar Z, Kvasnicka T, et al. Cholesterol-lowering therapy evokes time-limited changes in serotonergic transmission. Psychiatry Res. 2005;133(2-3):197-203.
h. Sooksawate T, Simmonds MA. Effects of membrane cholesterol on the sensitivity of the GABA(A) receptor to GABA in acutely dissociated rat hippocampal neurones. Neuropharmacology. 2001;40(2):178-184.
i. Abulrob A, Tauskela JS, Mealing G, et al. Protection by cholesterol-extracting cyclodextrins: a role for N-methyl-daspartate receptor redistribution. J Neurochem. 2005;92(6):1477-1486.
j. Huang P, Xu W, Yoon SI, et al. Cholesterol reduction by methyl-beta-cyclodextrin attenuates the delta opioid receptor-mediated signaling in neuronal cells but enhances it in non-neuronal cells. Biochem Pharmacol. 2007;73(4):534-549.
k. Butchbach ME, Tian G, Guo H, et al. Association of excitatory amino acid transporters, especially EAAT2, with cholesterol-rich lipid raft microdomains: importance for excitatory amino acid transporter localization and function. J Biol Chem. 2004;279(33):34388-34396.
l. Fawcett J, Busch KA, Jacobs D, et al. Suicide: a four-pathway clinical-biochemical model. Annals N Y Acad Sci. 1997;836:288-301.
m. Garland M, Hickey D, Corvin A, et al. Total serum cholesterol in relation to psychological correlates in parasuicide. Br J Psychiatry. 2000;177:77-83.
n. Ormiston T, Wolkowitz OM, Reus VI, et al. Behavioral implications of lowering cholesterol levels: a double-blind pilot study. Psychosomatics. 2003;44(5):412-414.
o. Golomb BA. Cholesterol and violence: is there a connection? Ann Intern Med. 1998;128(6):478-487.
Table 1
Psychiatric features associated with low cholesterol*
| Symptoms |
| Anxiety, depressed mood, emotional lability, euphoria, impulsivity, irritability, suicidal ideation, aggression |
| Syndromes |
| Anorexia nervosa, bipolar disorder, borderline personality disorder, major depressive disorder, seasonal affective disorder |
| Behaviors |
| Suicide and suicide attempts, violence |
| *Small studies have suggested possible relationships with dissociative and panic disorders |
Effects of lipid-lowering agents
If there is a causal relationship between low cholesterol and mood disorders, then it stands to reason that using cholesterol-lowering drugs would increase the risk of depression and suicide. However, the data do not support that conclusion.
Many case reports have documented adverse psychiatric reactions to statins, including depression, suicidality, emotional lability, agitation, irritability, anxiety, panic, and euphoria.17 In an early analysis of primary prevention trials, patients receiving cholesterol-lowering treatment—mainly non-statins—were estimated to have twice the risk of death by suicide or violence compared with controls.3 However, a more recent meta-analysis of larger clinical trials of lipid-lowering agents including statins and observational studies did not reveal an association between lipid-lowering medications and suicide.15,18
In a large case-control study, statin users had a lower risk of depression (adjusted odds ratio [OR] 0.4, 95% confidence interval [CI], 0.2 to 0.9) than patients taking non-statin lipid-lowering drugs (adjusted OR 1.0, 95% CI, 0.5 to 2.1).19 However, statins reduced cholesterol more (30% to 50%) than non-statin drugs (10% to 20%). A clinical trial of >1,000 patients with stable coronary artery disease treated with pravastatin—an HMG-CoA reductase inhibitor with low lipophilicity that is less likely than other statins to cross the blood-brain barrier—revealed no changes in self-reported anger, impulsiveness, anxiety, or depression.20
This study did not exclude patients with psychiatric illness—who are at greatest risk of suicide—but other trials of lipid-lowering drugs did.21 As a result, the effects of lipid-lowering medications on psychiatric patients are unclear. A clinical trial is underway to assess the effects of pravastatin (low lipophilicity), simvastatin (high lipophilicity), or placebo on mood, sleep, and aggression.21
Low cholesterol: State or trait?
Much of the research linking low cholesterol, mood disorders, and suicidality could be confounded by depressed mood leading to reduced serum cholesterol. There has been considerable debate about whether low cholesterol predisposes patients to suicide or if depression independently leads to poor nutrition and therefore low cholesterol and increased suicide risk.6,22
Some researchers have suggested that depression lowers cholesterol and increases risk of suicide,23 but study designs have limited the ability to discern the directionality of the relationship. Attempts to control for depression-related malnutrition and weight loss—which lowers total cholesterol, low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C)24—suggest the association may be independent of these variables.25-27 These findings suggest that cholesterol may be considered a trait marker and is not entirely state-dependent. However, multiple, large, long-term randomized controlled trials have not shown increased depression and suicide with use of lipid-lowering agents in healthy populations.20
The Figure illustrates known epidemiologic associations of low cholesterol, low serotoninergic function, and suicide and contrasts conceptual models of cholesterol as a state and a trait marker. A case can be made for cholesterol as both a state and a trait marker, and these models could overlap, with depression-induced decreases in cholesterol further mediating changes in serotonergic function and related behavioral sequelae.
Figure
Cholesterol, depression, and suicide: How are they linked? 
Low cholesterol may be considered a trait marker, predisposing patients to lower serotonergic function and placing them at greater risk for impulsivity, depression, aggression, and suicide. Other models suggest that lower cholesterol is a state-dependent consequence of depression, and not part of a causal chain toward suicide
Improving cardiac health
Limited epidemiologic studies suggest that patients with mood disorders may have lower levels of total cholesterol and LDL-C, but higher rates of hypertriglyceridemia compared with the general population.8 Unfortunately, psychiatric patients—who may be at increased risk of developing cardiovascular disease—may be less likely to be screened and appropriately treated for lipid abnormalities.28 To address this disparity, consider assuming an active role in assessing and managing hyperlipidemia in your patients with mood disorders. Be aware of your patients’ lipid profile and ensure that they follow monitoring recommendations.
The National Cholesterol Education Program recommends screening all adults age >20 for hyperlipidemia every 5 years using measures of total cholesterol, LDL-C, HDL-C, and triglycerides. If LDL-C or triglycerides exceed target values (Table 2), appropriate management includes recommending lifestyle changes and pharmacotherapy (Box 2).
Patients should receive a fasting lipid profile before and 12 weeks after starting any antipsychotic and semiannually thereafter.29 Consider closely monitoring lipids when patients gain weight with psychotropics. Refer patients with hyperlipidemia to a primary care physician, but in the absence of such a provider, mental health clinicians who are familiar with treatment guidelines can manage these patients.30
Closely monitor individuals with mood disorders for changes in behavior or mental status after starting a lipid-lowering agent. Consider discontinuing the drug if a patient develops an adverse reaction. If symptoms return after medication rechallenge, consider other management strategies such as an alternate lipid-lowering agent or re-emphasizing behavioral measures.
Table 2
National Cholesterol Education Program recommended LDL levels
| Risk category* | LDL goal | When to consider medications |
|---|---|---|
| CHD or CHD equivalent | <100 mg/dL | ≥130 mg/dL |
| ≥2 major risk factors | <130 mg/dL | ≥130 to 160 mg/dL (based on 10-year risk) |
| 0 or 1 risk factor | <160 mg/dL | ≥190 mg/dL |
| CHD: coronary heart disease; HDL: high-density lipoprotein; LDL: low-density lipoprotein | ||
| *Risk category is based on the presence of CHD or equivalent and major risk factors for CHD. CHD equivalents include symptomatic carotid artery disease, peripheral artery disease, and abdominal aortic aneurysm. Major risk factors include smoking, hypertension, low HDL, family history, and age. LDL levels to consider medications for those with ≥2 major risk factors vary by 10-year CHD risk | ||
| Source: National Cholesterol Education Program, Adult Treatment Panel III (ATP III) Quick Desk Reference. www.nhlbi.nih.gov/guidelines/cholesterol/atglance.htm | ||
National Cholesterol Education Program guidelines state that when a patient’s low-density lipoprotein cholesterol (LDL-C) exceeds targets (Table 2), first recommend lifestyle changes such as a diet low in saturated fat (<7% of calories) and cholesterol (<200 mg/d), weight management, and exercise. Increases in soluble fiber (10 to 25 g/d) and plant stanols/sterols also may be considered. If LDL-C levels are still too high, pharmacologic therapy such as an HMGCoA reductase inhibitor is suggested.
Treatment of elevated triglycerides (≥150 mg/dL) includes reaching the target LDL-C, intensifying a weight management program, and increasing exercise. Address quitting smoking and limiting alcohol when indicated. If triglyceride levels are ≥200 mg/dL after the LDL-C target is reached, set a secondary goal of reaching a target non-high-density lipoprotein cholesterol (HDL-C) (non-HDL-C; total cholesterol minus HDL-C) 30 mg/dL greater than the LDL goal. This can be achieved by adding an LDL-lowering drug such as a statin, nicotinic acid, or ezetimibe. When triglycerides are ≥500 mg/dL, more aggressive intervention, such as with a fibrate, omega-3 fatty acids, very low-fat diets, and exercise, is required to prevent pancreatitis.
Source: National Heart Lung and Blood Institute. National Cholesterol Education Program. www.nhlbi.nih.gov/guidelines/cholesterol/index.htm
Related Resources
- Fiedorowicz JG, Coryell WH. Cholesterol and suicide attempts: a prospective study of depressed inpatients. Psychiatry Res. 2007;152(1):11-20.
- National Cholesterol Education Program, Adult Treatment Panel III (ATP III) Quick Desk Reference. www.nhlbi.nih.gov/guidelines/cholesterol/atglance.htm.
- Executive Summary of the third report of the national Cholesterol Education Program (nCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486-2497.
Drug Brand Names
- Ezetimibe • Zetia
- Pravastatin • Pravachol
- Simvastatin • Zocor
Acknowledgements
Dr. Fiedorowicz thanks Lois Warren and Miriam Weiner for their editorial assistance.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products. Dr. Fiedorowicz is supported by the national Institutes of Health (1K23MH083695-01A210), nARSAD, and the Institute for Clinical and Translational Science at the University of Iowa (3 UL1 RR024979-03S4). He has received support for participating in a colleague’s investigator-initiated project with Eli Lilly. Dr. Haynes’ research is supported by grants from the national Institutes of Health (nHLBI: HL58972 & HL14388; nCRR CTSA: 1UL1RR024979).
1. Osby U, Brandt L, Correia N, et al. Excess mortality in bipolar and unipolar disorder in Sweden. Arch Gen Psychiatry. 2001;58(9):844-850.
2. Lindberg G, Råstam L, Gullberg B, et al. Low serum cholesterol concentration and short term mortality from injuries in men and women. BMJ. 1992;305(6848):277-279.
3. Muldoon MF, Manuck SB, Matthews KA. Lowering cholesterol concentrations and mortality: a quantitative review of primary prevention trials. BMJ. 1990;301(6747):309-314.
4. Neaton JD, Blackburn H, Jacobs D, et al. Serum cholesterol level and mortality findings for men screened in the Multiple Risk Factor Intervention Trial. Multiple Risk Factor Intervention Trial Research Group. Arch Intern Med. 1992;152(7):1490-1500.
5. Fiedorowicz JG, Coryell WH. Cholesterol and suicide attempts: a prospective study of depressed inpatients. Psychiatry Res. 2007;152(1):11-20.
6. Golomb BA. Cholesterol and violence: is there a connection? Ann Intern Med. 1998;128(6):478-487.
7. Pae CU, Kim JJ, Lee SJ, et al. Aberration of cholesterol level in first-onset bipolar I patients. J Affect Disord. 2004;83(1):79-82.
8. Fiedorowicz JG, Palagummi NM, Forman-Hoffman VL, et al. Elevated prevalence of obesity, metabolic syndrome, and cardiovascular risk factors in bipolar disorder. Ann Clin Psychiatry. 2008;20(3):131-137.
9. Chung KH, Tsai SY, Lee HC. Mood symptoms and serum lipids in acute phase of bipolar disorder in Taiwan. Psychiatry Clin Neurosci. 2007;61(4):428-433.
10. Jow GM, Yang TT, Chen CL. Leptin and cholesterol levels are low in major depressive disorder, but high in schizophrenia. J Affect Disord. 2006;90(1):21-27.
11. Sagud M, Mihaljevic-Peles A, Pivac N, et al. Platelet serotonin and serum lipids in psychotic mania. J Affect Disord. 2007;97(1-3):247-251.
12. Beasley CL, Honer WG, Bergmann K, et al. Reductions in cholesterol and synaptic markers in association cortex in mood disorders. Bipolar Disord. 2005;7(5):449-455.
13. Gabriel A. Changes in plasma cholesterol in mood disorder patients: does treatment make a difference? J Affect Disord. 2007;99(1-3):273-278.
14. Lalovic A, Levy E, Luheshi G, et al. Cholesterol content in brains of suicide completers. Int J Neuropsychopharmacol. 2007;10(2):159-166.
15. Lester D. Serum cholesterol levels and suicide: a meta-analysis. Suicide Life Threat Behav. 2002;32(3):333-346.
16. Coryell W, Schlesser M. Combined biological tests for suicide prediction. Psychiatry Res. 2007;150(2):187-191.
17. Tatley M, Savage R. Psychiatric adverse reactions with statins, fibrates and ezetimibe: implications for the use of lipid-lowering agents. Drug Saf. 2007;30(3):195-201.
18. Callréus T, Agerskov Andersen U, Hallas J, et al. Cardiovascular drugs and the risk of suicide: a nested case-control study. Eur J Clin Pharmacol. 2007;63(6):591-596.
19. Yang CC, Jick SS, Jick H. Lipid-lowering drugs and the risk of depression and suicidal behavior. Arch Intern Med. 2003;163(16):1926-1932.
20. Stewart RA, Sharples KJ, North FM, et al. Long-term assessment of psychological well-being in a randomized placebo-controlled trial of cholesterol reduction with pravastatin. The LIPID Study Investigators. Arch Intern Med. 2000;160(20):3144-3152.
21. Golomb BA, Criqui MH, White HL, et al. The UCSD Statin Study: a randomized controlled trial assessing the impact of statins on selected noncardiac outcomes. Control Clin Trials. 2004;25(2):178-202.
22. Fawcett J, Busch KA, Jacobs D, et al. Suicide: a four-pathway clinical-biochemical model. Annals N Y Acad Sci. 1997;836:288-301.
23. Law MR, Thompson SG, Wald NJ. Assessing possible hazards of reducing serum cholesterol. BMJ. 1994;308(6925):373-379.
24. Dattilo AM, Kris-Etherton PM. Effects of weight reduction on blood lipids and lipoproteins: a meta-analysis. Am J Clin Nutr. 1992;56(2):320-328.
25. Garland M, Hickey D, Corvin A, et al. Total serum cholesterol in relation to psychological correlates in parasuicide. Br J Psychiatry. 2000;177:77-83.
26. Golier JA, Marzuk PM, Leon AC, et al. Low serum cholesterol level and attempted suicide. Am J Psychiatry. 1995;152(3):419-423.
27. Kunugi H, Takei N, Aoki H, et al. Low serum cholesterol in suicide attempters. Biol Psychiatry. 1997;41(2):196-200.
28. Murray DP, Weiner M, Prabhakar M, et al. Mania and mortality: why the excess cardiovascular risk in bipolar disorder? Curr Psychiatry Rep. 2009;11(6):475-480.
29. Sernyak MJ. Implementation of monitoring and management guidelines for second-generation antipsychotics. J Clin Psychiatry. 2007;68(suppl 4):14-18.
30. Marder SR, Essock SM, Miller AL, et al. Physical health monitoring of patients with schizophrenia. Am J Psychiatry. 2004;161(8):1334-1349.
1. Osby U, Brandt L, Correia N, et al. Excess mortality in bipolar and unipolar disorder in Sweden. Arch Gen Psychiatry. 2001;58(9):844-850.
2. Lindberg G, Råstam L, Gullberg B, et al. Low serum cholesterol concentration and short term mortality from injuries in men and women. BMJ. 1992;305(6848):277-279.
3. Muldoon MF, Manuck SB, Matthews KA. Lowering cholesterol concentrations and mortality: a quantitative review of primary prevention trials. BMJ. 1990;301(6747):309-314.
4. Neaton JD, Blackburn H, Jacobs D, et al. Serum cholesterol level and mortality findings for men screened in the Multiple Risk Factor Intervention Trial. Multiple Risk Factor Intervention Trial Research Group. Arch Intern Med. 1992;152(7):1490-1500.
5. Fiedorowicz JG, Coryell WH. Cholesterol and suicide attempts: a prospective study of depressed inpatients. Psychiatry Res. 2007;152(1):11-20.
6. Golomb BA. Cholesterol and violence: is there a connection? Ann Intern Med. 1998;128(6):478-487.
7. Pae CU, Kim JJ, Lee SJ, et al. Aberration of cholesterol level in first-onset bipolar I patients. J Affect Disord. 2004;83(1):79-82.
8. Fiedorowicz JG, Palagummi NM, Forman-Hoffman VL, et al. Elevated prevalence of obesity, metabolic syndrome, and cardiovascular risk factors in bipolar disorder. Ann Clin Psychiatry. 2008;20(3):131-137.
9. Chung KH, Tsai SY, Lee HC. Mood symptoms and serum lipids in acute phase of bipolar disorder in Taiwan. Psychiatry Clin Neurosci. 2007;61(4):428-433.
10. Jow GM, Yang TT, Chen CL. Leptin and cholesterol levels are low in major depressive disorder, but high in schizophrenia. J Affect Disord. 2006;90(1):21-27.
11. Sagud M, Mihaljevic-Peles A, Pivac N, et al. Platelet serotonin and serum lipids in psychotic mania. J Affect Disord. 2007;97(1-3):247-251.
12. Beasley CL, Honer WG, Bergmann K, et al. Reductions in cholesterol and synaptic markers in association cortex in mood disorders. Bipolar Disord. 2005;7(5):449-455.
13. Gabriel A. Changes in plasma cholesterol in mood disorder patients: does treatment make a difference? J Affect Disord. 2007;99(1-3):273-278.
14. Lalovic A, Levy E, Luheshi G, et al. Cholesterol content in brains of suicide completers. Int J Neuropsychopharmacol. 2007;10(2):159-166.
15. Lester D. Serum cholesterol levels and suicide: a meta-analysis. Suicide Life Threat Behav. 2002;32(3):333-346.
16. Coryell W, Schlesser M. Combined biological tests for suicide prediction. Psychiatry Res. 2007;150(2):187-191.
17. Tatley M, Savage R. Psychiatric adverse reactions with statins, fibrates and ezetimibe: implications for the use of lipid-lowering agents. Drug Saf. 2007;30(3):195-201.
18. Callréus T, Agerskov Andersen U, Hallas J, et al. Cardiovascular drugs and the risk of suicide: a nested case-control study. Eur J Clin Pharmacol. 2007;63(6):591-596.
19. Yang CC, Jick SS, Jick H. Lipid-lowering drugs and the risk of depression and suicidal behavior. Arch Intern Med. 2003;163(16):1926-1932.
20. Stewart RA, Sharples KJ, North FM, et al. Long-term assessment of psychological well-being in a randomized placebo-controlled trial of cholesterol reduction with pravastatin. The LIPID Study Investigators. Arch Intern Med. 2000;160(20):3144-3152.
21. Golomb BA, Criqui MH, White HL, et al. The UCSD Statin Study: a randomized controlled trial assessing the impact of statins on selected noncardiac outcomes. Control Clin Trials. 2004;25(2):178-202.
22. Fawcett J, Busch KA, Jacobs D, et al. Suicide: a four-pathway clinical-biochemical model. Annals N Y Acad Sci. 1997;836:288-301.
23. Law MR, Thompson SG, Wald NJ. Assessing possible hazards of reducing serum cholesterol. BMJ. 1994;308(6925):373-379.
24. Dattilo AM, Kris-Etherton PM. Effects of weight reduction on blood lipids and lipoproteins: a meta-analysis. Am J Clin Nutr. 1992;56(2):320-328.
25. Garland M, Hickey D, Corvin A, et al. Total serum cholesterol in relation to psychological correlates in parasuicide. Br J Psychiatry. 2000;177:77-83.
26. Golier JA, Marzuk PM, Leon AC, et al. Low serum cholesterol level and attempted suicide. Am J Psychiatry. 1995;152(3):419-423.
27. Kunugi H, Takei N, Aoki H, et al. Low serum cholesterol in suicide attempters. Biol Psychiatry. 1997;41(2):196-200.
28. Murray DP, Weiner M, Prabhakar M, et al. Mania and mortality: why the excess cardiovascular risk in bipolar disorder? Curr Psychiatry Rep. 2009;11(6):475-480.
29. Sernyak MJ. Implementation of monitoring and management guidelines for second-generation antipsychotics. J Clin Psychiatry. 2007;68(suppl 4):14-18.
30. Marder SR, Essock SM, Miller AL, et al. Physical health monitoring of patients with schizophrenia. Am J Psychiatry. 2004;161(8):1334-1349.
The truth about treating low back pain
Dr. Lau is chief resident and Dr. Han is residency training director, departments of family and community medicine and psychiatry and behavioral sciences, University of California, Davis, Sacramento, CA.
Principal Source: Chou R, Qaseem A, Snow V, et al. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147(7):478-491.
- Acute low back pain generally has a favorable prognosis.
- Accurate categorizations of symptoms as well as self-education and self-care options are pillars of back pain treatment.
- Reserve imaging for patients with ’red flag’ symptoms.
- First-line pharmacotherapy involves acetaminophen and nonsteroidal anti-inflammatory drugs.
- Nonpharmacologic interventions may be appropriate depending on the duration of symptoms.
Low back pain is a common, unrelenting concern for many patients and accounts for a high percentage of health care visits. Although low back pain has a generally favorable prognosis, some patients develop long-term debilitating symptoms that exacerbate or initiate psychiatric conditions. Chronic low back pain has been associated with depression1 and rising rates of depression may contribute to the increasing prevalence of low back pain.2,3
The recent Stepped Care for Affective Disorders and Musculoskeletal Pain (SCAMP) trial showed that optimization of antidepressants in conjunction with a self-management behavioral program reduced depressive and pain symptoms.4 Understanding current diagnostic and treatment recommendations for physical aspects of low back pain will allow psychiatrists to intervene more effectively in somatic and behavioral aspects of the disease and improve functional outcomes.
This article reviews American College of Physicians guidelines on diagnosing and treating low back pain. Most episodes of acute low back pain are self-limited and do not require medical care, with symptom resolution and functional return occurring within the first month. However, 7.6% adult patients report at least 1 episode of severe acute low back pain over 1 year, and one-third of patients who have suffered an acute back pain episode report persistent, moderately intense symptoms and many suffer functional limitations.
Categorizing pain
Back pain can be grouped into 3 categories:
- non-specific low back pain
- back pain associated with radiculopathy or spinal stenosis
- back pain associated with another specific cause.
Low back pain frequently cannot be attributed to a specific disease or spinal abnormality, and conditions such as cancer, compression fracture, spinal stenosis, herniated disks, spinal infection, and ankylosing spondylitis comprise <10% of diagnosed causes of back pain.5 In the absence of “red flag” symptoms that may indicate more serious conditions (Table 1), there is no need to attribute low back pain symptoms to an anatomical source because often there is no associated improvement in outcomes.
Table 1
Back pain symptoms that may indicate a more serious condition
| Progressive loss of motor or sensory function |
| Bilateral sciatica or leg weakness |
| Saddle anesthesia |
| Urinary or fecal incontinence |
| History of substantial trauma |
| Unrelenting pain at night or during rest |
| Unexplained weight loss |
| No improvement after 6 to 8 weeks of conservative therapy |
When imaging is warranted
Although patients often request imaging as part of their workup, routine imaging or other diagnostic tests do not improve outcomes in patients with nonspecific back pain. When patients present with “red flag” symptoms or you suspect another underlying condition, imaging is warranted. MRI generally is preferred over CT. In patients with possible malignancy but no signs of spinal cord compression, multiple strategies have been proposed but not validated. First check plain radiography or erythrocyte sedimentation rate, followed by MRI if abnormalities are found. For patients with low back pain and signs of radiculopathy or spinal stenosis, MRI or CT is appropriate only if patients are candidates for surgery or epidural steroid injection, because symptoms tend to improve within 4 weeks with conservative, noninvasive management.
Selecting treatment
Education and counseling are essential when treating low back pain. Provide your patient with evidence-based information about low back pain, including self-care options such as support measures for pain relief (applying ice packs and heating or pads/blankets) and back-focused stretching and exercise programs (see Related Resources). Remaining as active as possible is more effective than prolonged (>1 to 2 days) bed rest in promoting return to function.6 Consider recommending self-care educational books such as The back book.7 The prognosis of acute low back pain with or without sciatica generally is favorable, and improvement is likely within the first month.8
Pharmacotherapy for low back pain is used in conjunction with—not in lieu of—back care education. However, there is a relative lack of long-term efficacy and safety. Acetaminophen or nonsteroidal anti-inflammatory drugs are typical first-line options.9 Other medications have moderate, mostly short-term benefits. Opioid analgesics or tramadol should be used occasionally and intermittently. When a patient does not respond to a time-limited opioid trial, reassess the symptoms and consider alternate therapies. Muscle relaxants such as cyclobenzaprine offer short-term relief but are associated with CNS side effects, most commonly drowsiness and dizziness but also fatigue, somnolence, confusion, and irritability. Tricyclic antidepressants are options to relieve chronic low back pain.10
Multiple nonpharmacologic therapies have small-to-moderate benefits for low back pain (Table 2). In acute low back pain (<4 weeks), spinal manipulation often is useful. Subacute low back pain (4 to 8 weeks) may improve with intensive interdisciplinary rehabilitation, including cognitive-behavioral therapy (CBT), and can increase functional status and reduce work absenteeism. For chronic low back pain, CBT or progressive relaxation, spinal manipulation, acupuncture, and other modalities have mild to moderate effectiveness.
Table 2
Nonpharmacologic modalities for low back pain
| Duration of back pain | Treatment modality |
|---|---|
| Acute (<4 weeks) | Spinal manipulation |
| Subacute (4 to 8 weeks) | Intensive interdisciplinary rehabilitation (physician consultation, psychological and physical therapy, social and vocational intervention, cognitive-behavioral therapy [CBT]) |
| Chronic (>8 weeks) | Acupuncture, exercise, massage therapy, yoga, CBT, progressive relaxation, spinal manipulation, intensive interdisciplinary rehabilitation |
| Source: Reference 5 | |
- Last AR, Hulbert K. Chronic low back pain: evaluation and management. Am Fam Physician. 2009;79(12):1067-1074.
- Exercise for a better back. www.backcare.org.uk/CMS/files/702-exercise-for-a-better-back.pdf. Accessed April 12, 2010.
Drug brand names
- Cyclobenzaprine • Flexeril
- Tramadol • Ultram, Ultram ER
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Bair MJ, Robinson RL, Katon W, et al. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163(20):2433-2445.
2. Freburger JK, Holmes GM, Agans RP, et al. The rising prevalence of chronic low back pain. Arch Intern Med. 2009;169(3):251-258.
3. Rush AJ, Polatin P, Gatchel RJ. Depression and chronic low back pain: establishing priorities in treatment. Spine (Phila Pa 1976). 2000;25(20):2566-2571.
4. Kroenke K, Bair MJ, Damush TM, et al. Optimized antidepressant therapy and pain self-management in primary care patients with depression and musculoskeletal pain: a randomized controlled trial. JAMA. 2009;301(20):2099-2110.
5. Chou R, Qaseem A, Snow V, et al. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147(7):478-491.
6. Hagen KB, Hilde G, Jamtvedt G, et al. Bed rest for acute low-back pain and sciatica. Cochrane Database Syst Rev. 2004;(4):CD001254.-
7. Burton AK, Waddell G, Tillotson KM, et al. Information and advice to patients with back pain can have a positive effect. A randomized controlled trial of a novel educational booklet in primary care. Spine (Phila Pa 1976). 1999;24(23):2484-2491.
8. Vroomen PC, de Krom MC, Knottnerus JA. Predicting the outcome of sciatica at short-term follow-up. Br J Gen Pract. 2002;52:119-123.
9. van Tulder MW, Scholten RJ, Koes BW, et al. Nonsteroidal anti-inflammatory drugs for low back pain: a systematic review within the framework of the Cochrane Collaboration Back Review Group. Spine (Phila Pa 1976). 2000;25:2501-2513.
10. Staiger TO, Gaster B, Sullivan MD, et al. Systematic review of antidepressants in the treatment of chronic low back pain. Spine (Phila Pa 1976). 2003;28:2540-2545.
Dr. Lau is chief resident and Dr. Han is residency training director, departments of family and community medicine and psychiatry and behavioral sciences, University of California, Davis, Sacramento, CA.
Principal Source: Chou R, Qaseem A, Snow V, et al. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147(7):478-491.
- Acute low back pain generally has a favorable prognosis.
- Accurate categorizations of symptoms as well as self-education and self-care options are pillars of back pain treatment.
- Reserve imaging for patients with ’red flag’ symptoms.
- First-line pharmacotherapy involves acetaminophen and nonsteroidal anti-inflammatory drugs.
- Nonpharmacologic interventions may be appropriate depending on the duration of symptoms.
Low back pain is a common, unrelenting concern for many patients and accounts for a high percentage of health care visits. Although low back pain has a generally favorable prognosis, some patients develop long-term debilitating symptoms that exacerbate or initiate psychiatric conditions. Chronic low back pain has been associated with depression1 and rising rates of depression may contribute to the increasing prevalence of low back pain.2,3
The recent Stepped Care for Affective Disorders and Musculoskeletal Pain (SCAMP) trial showed that optimization of antidepressants in conjunction with a self-management behavioral program reduced depressive and pain symptoms.4 Understanding current diagnostic and treatment recommendations for physical aspects of low back pain will allow psychiatrists to intervene more effectively in somatic and behavioral aspects of the disease and improve functional outcomes.
This article reviews American College of Physicians guidelines on diagnosing and treating low back pain. Most episodes of acute low back pain are self-limited and do not require medical care, with symptom resolution and functional return occurring within the first month. However, 7.6% adult patients report at least 1 episode of severe acute low back pain over 1 year, and one-third of patients who have suffered an acute back pain episode report persistent, moderately intense symptoms and many suffer functional limitations.
Categorizing pain
Back pain can be grouped into 3 categories:
- non-specific low back pain
- back pain associated with radiculopathy or spinal stenosis
- back pain associated with another specific cause.
Low back pain frequently cannot be attributed to a specific disease or spinal abnormality, and conditions such as cancer, compression fracture, spinal stenosis, herniated disks, spinal infection, and ankylosing spondylitis comprise <10% of diagnosed causes of back pain.5 In the absence of “red flag” symptoms that may indicate more serious conditions (Table 1), there is no need to attribute low back pain symptoms to an anatomical source because often there is no associated improvement in outcomes.
Table 1
Back pain symptoms that may indicate a more serious condition
| Progressive loss of motor or sensory function |
| Bilateral sciatica or leg weakness |
| Saddle anesthesia |
| Urinary or fecal incontinence |
| History of substantial trauma |
| Unrelenting pain at night or during rest |
| Unexplained weight loss |
| No improvement after 6 to 8 weeks of conservative therapy |
When imaging is warranted
Although patients often request imaging as part of their workup, routine imaging or other diagnostic tests do not improve outcomes in patients with nonspecific back pain. When patients present with “red flag” symptoms or you suspect another underlying condition, imaging is warranted. MRI generally is preferred over CT. In patients with possible malignancy but no signs of spinal cord compression, multiple strategies have been proposed but not validated. First check plain radiography or erythrocyte sedimentation rate, followed by MRI if abnormalities are found. For patients with low back pain and signs of radiculopathy or spinal stenosis, MRI or CT is appropriate only if patients are candidates for surgery or epidural steroid injection, because symptoms tend to improve within 4 weeks with conservative, noninvasive management.
Selecting treatment
Education and counseling are essential when treating low back pain. Provide your patient with evidence-based information about low back pain, including self-care options such as support measures for pain relief (applying ice packs and heating or pads/blankets) and back-focused stretching and exercise programs (see Related Resources). Remaining as active as possible is more effective than prolonged (>1 to 2 days) bed rest in promoting return to function.6 Consider recommending self-care educational books such as The back book.7 The prognosis of acute low back pain with or without sciatica generally is favorable, and improvement is likely within the first month.8
Pharmacotherapy for low back pain is used in conjunction with—not in lieu of—back care education. However, there is a relative lack of long-term efficacy and safety. Acetaminophen or nonsteroidal anti-inflammatory drugs are typical first-line options.9 Other medications have moderate, mostly short-term benefits. Opioid analgesics or tramadol should be used occasionally and intermittently. When a patient does not respond to a time-limited opioid trial, reassess the symptoms and consider alternate therapies. Muscle relaxants such as cyclobenzaprine offer short-term relief but are associated with CNS side effects, most commonly drowsiness and dizziness but also fatigue, somnolence, confusion, and irritability. Tricyclic antidepressants are options to relieve chronic low back pain.10
Multiple nonpharmacologic therapies have small-to-moderate benefits for low back pain (Table 2). In acute low back pain (<4 weeks), spinal manipulation often is useful. Subacute low back pain (4 to 8 weeks) may improve with intensive interdisciplinary rehabilitation, including cognitive-behavioral therapy (CBT), and can increase functional status and reduce work absenteeism. For chronic low back pain, CBT or progressive relaxation, spinal manipulation, acupuncture, and other modalities have mild to moderate effectiveness.
Table 2
Nonpharmacologic modalities for low back pain
| Duration of back pain | Treatment modality |
|---|---|
| Acute (<4 weeks) | Spinal manipulation |
| Subacute (4 to 8 weeks) | Intensive interdisciplinary rehabilitation (physician consultation, psychological and physical therapy, social and vocational intervention, cognitive-behavioral therapy [CBT]) |
| Chronic (>8 weeks) | Acupuncture, exercise, massage therapy, yoga, CBT, progressive relaxation, spinal manipulation, intensive interdisciplinary rehabilitation |
| Source: Reference 5 | |
- Last AR, Hulbert K. Chronic low back pain: evaluation and management. Am Fam Physician. 2009;79(12):1067-1074.
- Exercise for a better back. www.backcare.org.uk/CMS/files/702-exercise-for-a-better-back.pdf. Accessed April 12, 2010.
Drug brand names
- Cyclobenzaprine • Flexeril
- Tramadol • Ultram, Ultram ER
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Lau is chief resident and Dr. Han is residency training director, departments of family and community medicine and psychiatry and behavioral sciences, University of California, Davis, Sacramento, CA.
Principal Source: Chou R, Qaseem A, Snow V, et al. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147(7):478-491.
- Acute low back pain generally has a favorable prognosis.
- Accurate categorizations of symptoms as well as self-education and self-care options are pillars of back pain treatment.
- Reserve imaging for patients with ’red flag’ symptoms.
- First-line pharmacotherapy involves acetaminophen and nonsteroidal anti-inflammatory drugs.
- Nonpharmacologic interventions may be appropriate depending on the duration of symptoms.
Low back pain is a common, unrelenting concern for many patients and accounts for a high percentage of health care visits. Although low back pain has a generally favorable prognosis, some patients develop long-term debilitating symptoms that exacerbate or initiate psychiatric conditions. Chronic low back pain has been associated with depression1 and rising rates of depression may contribute to the increasing prevalence of low back pain.2,3
The recent Stepped Care for Affective Disorders and Musculoskeletal Pain (SCAMP) trial showed that optimization of antidepressants in conjunction with a self-management behavioral program reduced depressive and pain symptoms.4 Understanding current diagnostic and treatment recommendations for physical aspects of low back pain will allow psychiatrists to intervene more effectively in somatic and behavioral aspects of the disease and improve functional outcomes.
This article reviews American College of Physicians guidelines on diagnosing and treating low back pain. Most episodes of acute low back pain are self-limited and do not require medical care, with symptom resolution and functional return occurring within the first month. However, 7.6% adult patients report at least 1 episode of severe acute low back pain over 1 year, and one-third of patients who have suffered an acute back pain episode report persistent, moderately intense symptoms and many suffer functional limitations.
Categorizing pain
Back pain can be grouped into 3 categories:
- non-specific low back pain
- back pain associated with radiculopathy or spinal stenosis
- back pain associated with another specific cause.
Low back pain frequently cannot be attributed to a specific disease or spinal abnormality, and conditions such as cancer, compression fracture, spinal stenosis, herniated disks, spinal infection, and ankylosing spondylitis comprise <10% of diagnosed causes of back pain.5 In the absence of “red flag” symptoms that may indicate more serious conditions (Table 1), there is no need to attribute low back pain symptoms to an anatomical source because often there is no associated improvement in outcomes.
Table 1
Back pain symptoms that may indicate a more serious condition
| Progressive loss of motor or sensory function |
| Bilateral sciatica or leg weakness |
| Saddle anesthesia |
| Urinary or fecal incontinence |
| History of substantial trauma |
| Unrelenting pain at night or during rest |
| Unexplained weight loss |
| No improvement after 6 to 8 weeks of conservative therapy |
When imaging is warranted
Although patients often request imaging as part of their workup, routine imaging or other diagnostic tests do not improve outcomes in patients with nonspecific back pain. When patients present with “red flag” symptoms or you suspect another underlying condition, imaging is warranted. MRI generally is preferred over CT. In patients with possible malignancy but no signs of spinal cord compression, multiple strategies have been proposed but not validated. First check plain radiography or erythrocyte sedimentation rate, followed by MRI if abnormalities are found. For patients with low back pain and signs of radiculopathy or spinal stenosis, MRI or CT is appropriate only if patients are candidates for surgery or epidural steroid injection, because symptoms tend to improve within 4 weeks with conservative, noninvasive management.
Selecting treatment
Education and counseling are essential when treating low back pain. Provide your patient with evidence-based information about low back pain, including self-care options such as support measures for pain relief (applying ice packs and heating or pads/blankets) and back-focused stretching and exercise programs (see Related Resources). Remaining as active as possible is more effective than prolonged (>1 to 2 days) bed rest in promoting return to function.6 Consider recommending self-care educational books such as The back book.7 The prognosis of acute low back pain with or without sciatica generally is favorable, and improvement is likely within the first month.8
Pharmacotherapy for low back pain is used in conjunction with—not in lieu of—back care education. However, there is a relative lack of long-term efficacy and safety. Acetaminophen or nonsteroidal anti-inflammatory drugs are typical first-line options.9 Other medications have moderate, mostly short-term benefits. Opioid analgesics or tramadol should be used occasionally and intermittently. When a patient does not respond to a time-limited opioid trial, reassess the symptoms and consider alternate therapies. Muscle relaxants such as cyclobenzaprine offer short-term relief but are associated with CNS side effects, most commonly drowsiness and dizziness but also fatigue, somnolence, confusion, and irritability. Tricyclic antidepressants are options to relieve chronic low back pain.10
Multiple nonpharmacologic therapies have small-to-moderate benefits for low back pain (Table 2). In acute low back pain (<4 weeks), spinal manipulation often is useful. Subacute low back pain (4 to 8 weeks) may improve with intensive interdisciplinary rehabilitation, including cognitive-behavioral therapy (CBT), and can increase functional status and reduce work absenteeism. For chronic low back pain, CBT or progressive relaxation, spinal manipulation, acupuncture, and other modalities have mild to moderate effectiveness.
Table 2
Nonpharmacologic modalities for low back pain
| Duration of back pain | Treatment modality |
|---|---|
| Acute (<4 weeks) | Spinal manipulation |
| Subacute (4 to 8 weeks) | Intensive interdisciplinary rehabilitation (physician consultation, psychological and physical therapy, social and vocational intervention, cognitive-behavioral therapy [CBT]) |
| Chronic (>8 weeks) | Acupuncture, exercise, massage therapy, yoga, CBT, progressive relaxation, spinal manipulation, intensive interdisciplinary rehabilitation |
| Source: Reference 5 | |
- Last AR, Hulbert K. Chronic low back pain: evaluation and management. Am Fam Physician. 2009;79(12):1067-1074.
- Exercise for a better back. www.backcare.org.uk/CMS/files/702-exercise-for-a-better-back.pdf. Accessed April 12, 2010.
Drug brand names
- Cyclobenzaprine • Flexeril
- Tramadol • Ultram, Ultram ER
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Bair MJ, Robinson RL, Katon W, et al. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163(20):2433-2445.
2. Freburger JK, Holmes GM, Agans RP, et al. The rising prevalence of chronic low back pain. Arch Intern Med. 2009;169(3):251-258.
3. Rush AJ, Polatin P, Gatchel RJ. Depression and chronic low back pain: establishing priorities in treatment. Spine (Phila Pa 1976). 2000;25(20):2566-2571.
4. Kroenke K, Bair MJ, Damush TM, et al. Optimized antidepressant therapy and pain self-management in primary care patients with depression and musculoskeletal pain: a randomized controlled trial. JAMA. 2009;301(20):2099-2110.
5. Chou R, Qaseem A, Snow V, et al. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147(7):478-491.
6. Hagen KB, Hilde G, Jamtvedt G, et al. Bed rest for acute low-back pain and sciatica. Cochrane Database Syst Rev. 2004;(4):CD001254.-
7. Burton AK, Waddell G, Tillotson KM, et al. Information and advice to patients with back pain can have a positive effect. A randomized controlled trial of a novel educational booklet in primary care. Spine (Phila Pa 1976). 1999;24(23):2484-2491.
8. Vroomen PC, de Krom MC, Knottnerus JA. Predicting the outcome of sciatica at short-term follow-up. Br J Gen Pract. 2002;52:119-123.
9. van Tulder MW, Scholten RJ, Koes BW, et al. Nonsteroidal anti-inflammatory drugs for low back pain: a systematic review within the framework of the Cochrane Collaboration Back Review Group. Spine (Phila Pa 1976). 2000;25:2501-2513.
10. Staiger TO, Gaster B, Sullivan MD, et al. Systematic review of antidepressants in the treatment of chronic low back pain. Spine (Phila Pa 1976). 2003;28:2540-2545.
1. Bair MJ, Robinson RL, Katon W, et al. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163(20):2433-2445.
2. Freburger JK, Holmes GM, Agans RP, et al. The rising prevalence of chronic low back pain. Arch Intern Med. 2009;169(3):251-258.
3. Rush AJ, Polatin P, Gatchel RJ. Depression and chronic low back pain: establishing priorities in treatment. Spine (Phila Pa 1976). 2000;25(20):2566-2571.
4. Kroenke K, Bair MJ, Damush TM, et al. Optimized antidepressant therapy and pain self-management in primary care patients with depression and musculoskeletal pain: a randomized controlled trial. JAMA. 2009;301(20):2099-2110.
5. Chou R, Qaseem A, Snow V, et al. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147(7):478-491.
6. Hagen KB, Hilde G, Jamtvedt G, et al. Bed rest for acute low-back pain and sciatica. Cochrane Database Syst Rev. 2004;(4):CD001254.-
7. Burton AK, Waddell G, Tillotson KM, et al. Information and advice to patients with back pain can have a positive effect. A randomized controlled trial of a novel educational booklet in primary care. Spine (Phila Pa 1976). 1999;24(23):2484-2491.
8. Vroomen PC, de Krom MC, Knottnerus JA. Predicting the outcome of sciatica at short-term follow-up. Br J Gen Pract. 2002;52:119-123.
9. van Tulder MW, Scholten RJ, Koes BW, et al. Nonsteroidal anti-inflammatory drugs for low back pain: a systematic review within the framework of the Cochrane Collaboration Back Review Group. Spine (Phila Pa 1976). 2000;25:2501-2513.
10. Staiger TO, Gaster B, Sullivan MD, et al. Systematic review of antidepressants in the treatment of chronic low back pain. Spine (Phila Pa 1976). 2003;28:2540-2545.
Beta blockers, T3, and T4
Propranolol is used to treat thyroid storm specifically because of its action in blocking conversion of prohormone thyroxine (T4) to triiodothyronine (T3) (“Do beta blockers cause depression?” Medicine in Brief, Current Psychiatry, May 2010). Because T3 is the basis of the basal metabolic rate, if T3 were decreased then the only other mechanism for energy is adrenaline. This would cause depression when adrenaline wasn’t in use and anxiety when it was. This sounds like a direct link to depression and anxiety to me. The thyroid function test would show no change in thyroid-stimulating hormone, but an increase in T4 to compensate for the decrease in T3. There are no medical standards to routinely look at T3 and the effect would not be seen anyway. I am not aware of research that explores this connection between beta blockers and depression.
John V. Billings, ARNP
Spokane, WA
Propranolol is used to treat thyroid storm specifically because of its action in blocking conversion of prohormone thyroxine (T4) to triiodothyronine (T3) (“Do beta blockers cause depression?” Medicine in Brief, Current Psychiatry, May 2010). Because T3 is the basis of the basal metabolic rate, if T3 were decreased then the only other mechanism for energy is adrenaline. This would cause depression when adrenaline wasn’t in use and anxiety when it was. This sounds like a direct link to depression and anxiety to me. The thyroid function test would show no change in thyroid-stimulating hormone, but an increase in T4 to compensate for the decrease in T3. There are no medical standards to routinely look at T3 and the effect would not be seen anyway. I am not aware of research that explores this connection between beta blockers and depression.
John V. Billings, ARNP
Spokane, WA
Propranolol is used to treat thyroid storm specifically because of its action in blocking conversion of prohormone thyroxine (T4) to triiodothyronine (T3) (“Do beta blockers cause depression?” Medicine in Brief, Current Psychiatry, May 2010). Because T3 is the basis of the basal metabolic rate, if T3 were decreased then the only other mechanism for energy is adrenaline. This would cause depression when adrenaline wasn’t in use and anxiety when it was. This sounds like a direct link to depression and anxiety to me. The thyroid function test would show no change in thyroid-stimulating hormone, but an increase in T4 to compensate for the decrease in T3. There are no medical standards to routinely look at T3 and the effect would not be seen anyway. I am not aware of research that explores this connection between beta blockers and depression.
John V. Billings, ARNP
Spokane, WA
Is it a mood disorder or menopause?
Consider the neuroendocrinology of menopause when evaluating midlife women for new or worsening mood symptoms. The risk of depression increases during perimenopause, even in women with no history of depression.1 Fluctuating estrogen levels can cause vasomotor symptoms (VMS) and depression, presenting diagnostic and treatment challenges. In addition to conducting a comprehensive psychiatric evaluation, our collaborative rotation between the UCLA-Kern Psychiatry Residency Program and the department of obstetrics and gynecology uses the following approach for women age >40.
Obtain a menstrual history
Ask your patient when her last menstrual period was and if her periods are irregular, heavy, light, or missing. Menopausal transition begins when the length of the menstrual cycle varies and ends with the final menstrual period. Perimenopause begins early in the transition and ends 12 months after the last menses. During this time VMS and mood instability may worsen.
Ask about menopausal symptoms
Hot flashes typically begin as a sudden sensation of heat centered in the upper chest and face that rapidly generalizes. Flashes last 2 to 4 minutes and often are accompanied by profuse perspiration and occasional palpitations. VMS can occur several times during the day and night. Hot flashes—the most common symptom associated with menopausal transition—peak during the 12 months surrounding the last period and can commonly persist up to 5 years or more. Hot flashes affect a woman’s sense of well-being and often are the reason women seek medical attention during midlife.
Insomnia. Sleep disturbance during the menopausal transition is common, sometimes severe, and may be related to nocturnal hot flashes and night sweats. Hot flashes and awakenings are sometimes followed by chills, shivering, anxiety, or panic.
Mood instability. Dysregulation of monoaminergic neurotransmitter systems caused by fluctuating estrogen levels may cause both depression and VMS.2 Perimenopausal women with VMS are more likely to be depressed than those who do not have VMS. VMS may signal the onset or recurrence of major depression.
Sexual changes. Estrogen deficiency may lead to vaginal dryness and urogenital atrophy, resulting in infection, painful intercourse, or decreased sexual desire.
Body aches. Many perimenopausal women complain of stiffness, joint pain, breast pain, menstrual migraines, bladder discomfort, and impaired balance.
Memory changes. Complaints of forgetfulness may reflect aging and effects of sleep disturbance.3
Diagnostic workup
Perimenopause can be diagnosed before clinical symptoms appear if the follicle stimulating hormone (FSH) level is >25 IU/L and estrogen is <40 pg/mL during the early follicular phase (day 3 of the menstrual cycle).3 In women age <45 with irregular bleeding and menopause symptoms, check serum beta human chorionic gonadotropin (to rule out pregnancy), prolactin, thyroid-stimulating hormone, and FSH.
Women of any age with estrogen deficiency—such as those undergoing chemotherapy for breast cancer, treatment with gonadotropin-releasing hormone agonists for endometriosis or in-vitro fertilization, premature ovarian failure, or who have undergone oophorectomy—might experience VMS and other perimenopausal symptoms.
Women age >45 with 12 months of amenorrhea may be diagnosed with menopause clinically without further testing.
Treatment strategies
Fewer women are choosing hormone replacement therapy (HRT) (estrogen alone or estrogen and progesterone) after the landmark Women’s Health Initiative (WHI) study in 2002.4 Reports that HRT may increase the risk of breast cancer and offers no cardiac protection prompted many women to forego or discontinue HRT use. Subsequent interpretation of the WHI data has reduced many of these concerns.5 As a result, estrogen alone currently is the most effective and only FDA-approved treatment for VMS.5 Because of overlap between VMS and depression, treatment for these 2 conditions could be combined. Theoretically, treating VMS could prevent a major depressive episode in vulnerable women and may improve the chance of full remission of depression.1
Although results of studies of HRT for depression are mixed, estrogen alone may be effective for mild depression during perimenopause but not postmenopause. Estrogen also may be appropriate during perimenopause if a depressive disorder represents a first-onset episode of mild to moderate severity.6 Estrogen is not FDA-approved for treating perimenopausal depression. As with all medications, counsel patients on the risks and benefits and administer the medication at the lowest dose and for the shortest time period to effectively treat symptoms.
Consider antidepressants when HRT is contraindicated or declined. Selective norepinephrine reuptake inhibitors such as venlafaxine, desvenlafaxine, and duloxetine have demonstrated efficacy for VMS and depression.2 Selective serotonin reuptake inhibitors (SSRIs) are effective in women age <40 but show inconsistent efficacy for VMS and depression in women age >50. SSRIs combined with estrogen therapy may be useful in postmenopausal women.2
Biopsychosocial factors
Psychosocial attitudes about aging, sexual attractiveness, and children leaving home may contribute to depression during perimenopause. However, many women welcome the freedom from menstrual periods and pregnancy worries.
Some women may not be aware of the impact of menopausal changes on mood. Educating patients with a mood disorder about what to expect and identifying and treating disabling hormonal dysregulation symptoms is an ideal opportunity to enhance the quality of life for patients during menopause and beyond.
1. Cohen LS, Soares CN, Vitonis AF, et al. Risk for new onset of depression during the menopausal transition: the Harvard study of moods and cycles. Arch Gen Psychiatry. 2006;63(4):385-390.
2. Thase ME, Entsuah R, Cantillon M, et al. Relative antidepressant efficacy of venlafaxine and SSRIs: sex-age interactions. J Womens Health (Larchmt). 2005;14:609-616.
3. Aloysi A, Van Dyk K, Sano M. Women’s cognitive and affective health and neuropsychiatry. Mt Sinai J Medicine. 2006;73(7):967-975.
4. Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
5. Santoro N. Symptoms of menopause: hot flushes. Clinical ObGyn. 2008;51(3):539-548.
6. Joffe H, Soares CN, Cohen LS. Assessment and treatment of hot flushes and menopausal mood disturbance. Psychiatr Clin N Am. 2003;26:563-580.
Consider the neuroendocrinology of menopause when evaluating midlife women for new or worsening mood symptoms. The risk of depression increases during perimenopause, even in women with no history of depression.1 Fluctuating estrogen levels can cause vasomotor symptoms (VMS) and depression, presenting diagnostic and treatment challenges. In addition to conducting a comprehensive psychiatric evaluation, our collaborative rotation between the UCLA-Kern Psychiatry Residency Program and the department of obstetrics and gynecology uses the following approach for women age >40.
Obtain a menstrual history
Ask your patient when her last menstrual period was and if her periods are irregular, heavy, light, or missing. Menopausal transition begins when the length of the menstrual cycle varies and ends with the final menstrual period. Perimenopause begins early in the transition and ends 12 months after the last menses. During this time VMS and mood instability may worsen.
Ask about menopausal symptoms
Hot flashes typically begin as a sudden sensation of heat centered in the upper chest and face that rapidly generalizes. Flashes last 2 to 4 minutes and often are accompanied by profuse perspiration and occasional palpitations. VMS can occur several times during the day and night. Hot flashes—the most common symptom associated with menopausal transition—peak during the 12 months surrounding the last period and can commonly persist up to 5 years or more. Hot flashes affect a woman’s sense of well-being and often are the reason women seek medical attention during midlife.
Insomnia. Sleep disturbance during the menopausal transition is common, sometimes severe, and may be related to nocturnal hot flashes and night sweats. Hot flashes and awakenings are sometimes followed by chills, shivering, anxiety, or panic.
Mood instability. Dysregulation of monoaminergic neurotransmitter systems caused by fluctuating estrogen levels may cause both depression and VMS.2 Perimenopausal women with VMS are more likely to be depressed than those who do not have VMS. VMS may signal the onset or recurrence of major depression.
Sexual changes. Estrogen deficiency may lead to vaginal dryness and urogenital atrophy, resulting in infection, painful intercourse, or decreased sexual desire.
Body aches. Many perimenopausal women complain of stiffness, joint pain, breast pain, menstrual migraines, bladder discomfort, and impaired balance.
Memory changes. Complaints of forgetfulness may reflect aging and effects of sleep disturbance.3
Diagnostic workup
Perimenopause can be diagnosed before clinical symptoms appear if the follicle stimulating hormone (FSH) level is >25 IU/L and estrogen is <40 pg/mL during the early follicular phase (day 3 of the menstrual cycle).3 In women age <45 with irregular bleeding and menopause symptoms, check serum beta human chorionic gonadotropin (to rule out pregnancy), prolactin, thyroid-stimulating hormone, and FSH.
Women of any age with estrogen deficiency—such as those undergoing chemotherapy for breast cancer, treatment with gonadotropin-releasing hormone agonists for endometriosis or in-vitro fertilization, premature ovarian failure, or who have undergone oophorectomy—might experience VMS and other perimenopausal symptoms.
Women age >45 with 12 months of amenorrhea may be diagnosed with menopause clinically without further testing.
Treatment strategies
Fewer women are choosing hormone replacement therapy (HRT) (estrogen alone or estrogen and progesterone) after the landmark Women’s Health Initiative (WHI) study in 2002.4 Reports that HRT may increase the risk of breast cancer and offers no cardiac protection prompted many women to forego or discontinue HRT use. Subsequent interpretation of the WHI data has reduced many of these concerns.5 As a result, estrogen alone currently is the most effective and only FDA-approved treatment for VMS.5 Because of overlap between VMS and depression, treatment for these 2 conditions could be combined. Theoretically, treating VMS could prevent a major depressive episode in vulnerable women and may improve the chance of full remission of depression.1
Although results of studies of HRT for depression are mixed, estrogen alone may be effective for mild depression during perimenopause but not postmenopause. Estrogen also may be appropriate during perimenopause if a depressive disorder represents a first-onset episode of mild to moderate severity.6 Estrogen is not FDA-approved for treating perimenopausal depression. As with all medications, counsel patients on the risks and benefits and administer the medication at the lowest dose and for the shortest time period to effectively treat symptoms.
Consider antidepressants when HRT is contraindicated or declined. Selective norepinephrine reuptake inhibitors such as venlafaxine, desvenlafaxine, and duloxetine have demonstrated efficacy for VMS and depression.2 Selective serotonin reuptake inhibitors (SSRIs) are effective in women age <40 but show inconsistent efficacy for VMS and depression in women age >50. SSRIs combined with estrogen therapy may be useful in postmenopausal women.2
Biopsychosocial factors
Psychosocial attitudes about aging, sexual attractiveness, and children leaving home may contribute to depression during perimenopause. However, many women welcome the freedom from menstrual periods and pregnancy worries.
Some women may not be aware of the impact of menopausal changes on mood. Educating patients with a mood disorder about what to expect and identifying and treating disabling hormonal dysregulation symptoms is an ideal opportunity to enhance the quality of life for patients during menopause and beyond.
Consider the neuroendocrinology of menopause when evaluating midlife women for new or worsening mood symptoms. The risk of depression increases during perimenopause, even in women with no history of depression.1 Fluctuating estrogen levels can cause vasomotor symptoms (VMS) and depression, presenting diagnostic and treatment challenges. In addition to conducting a comprehensive psychiatric evaluation, our collaborative rotation between the UCLA-Kern Psychiatry Residency Program and the department of obstetrics and gynecology uses the following approach for women age >40.
Obtain a menstrual history
Ask your patient when her last menstrual period was and if her periods are irregular, heavy, light, or missing. Menopausal transition begins when the length of the menstrual cycle varies and ends with the final menstrual period. Perimenopause begins early in the transition and ends 12 months after the last menses. During this time VMS and mood instability may worsen.
Ask about menopausal symptoms
Hot flashes typically begin as a sudden sensation of heat centered in the upper chest and face that rapidly generalizes. Flashes last 2 to 4 minutes and often are accompanied by profuse perspiration and occasional palpitations. VMS can occur several times during the day and night. Hot flashes—the most common symptom associated with menopausal transition—peak during the 12 months surrounding the last period and can commonly persist up to 5 years or more. Hot flashes affect a woman’s sense of well-being and often are the reason women seek medical attention during midlife.
Insomnia. Sleep disturbance during the menopausal transition is common, sometimes severe, and may be related to nocturnal hot flashes and night sweats. Hot flashes and awakenings are sometimes followed by chills, shivering, anxiety, or panic.
Mood instability. Dysregulation of monoaminergic neurotransmitter systems caused by fluctuating estrogen levels may cause both depression and VMS.2 Perimenopausal women with VMS are more likely to be depressed than those who do not have VMS. VMS may signal the onset or recurrence of major depression.
Sexual changes. Estrogen deficiency may lead to vaginal dryness and urogenital atrophy, resulting in infection, painful intercourse, or decreased sexual desire.
Body aches. Many perimenopausal women complain of stiffness, joint pain, breast pain, menstrual migraines, bladder discomfort, and impaired balance.
Memory changes. Complaints of forgetfulness may reflect aging and effects of sleep disturbance.3
Diagnostic workup
Perimenopause can be diagnosed before clinical symptoms appear if the follicle stimulating hormone (FSH) level is >25 IU/L and estrogen is <40 pg/mL during the early follicular phase (day 3 of the menstrual cycle).3 In women age <45 with irregular bleeding and menopause symptoms, check serum beta human chorionic gonadotropin (to rule out pregnancy), prolactin, thyroid-stimulating hormone, and FSH.
Women of any age with estrogen deficiency—such as those undergoing chemotherapy for breast cancer, treatment with gonadotropin-releasing hormone agonists for endometriosis or in-vitro fertilization, premature ovarian failure, or who have undergone oophorectomy—might experience VMS and other perimenopausal symptoms.
Women age >45 with 12 months of amenorrhea may be diagnosed with menopause clinically without further testing.
Treatment strategies
Fewer women are choosing hormone replacement therapy (HRT) (estrogen alone or estrogen and progesterone) after the landmark Women’s Health Initiative (WHI) study in 2002.4 Reports that HRT may increase the risk of breast cancer and offers no cardiac protection prompted many women to forego or discontinue HRT use. Subsequent interpretation of the WHI data has reduced many of these concerns.5 As a result, estrogen alone currently is the most effective and only FDA-approved treatment for VMS.5 Because of overlap between VMS and depression, treatment for these 2 conditions could be combined. Theoretically, treating VMS could prevent a major depressive episode in vulnerable women and may improve the chance of full remission of depression.1
Although results of studies of HRT for depression are mixed, estrogen alone may be effective for mild depression during perimenopause but not postmenopause. Estrogen also may be appropriate during perimenopause if a depressive disorder represents a first-onset episode of mild to moderate severity.6 Estrogen is not FDA-approved for treating perimenopausal depression. As with all medications, counsel patients on the risks and benefits and administer the medication at the lowest dose and for the shortest time period to effectively treat symptoms.
Consider antidepressants when HRT is contraindicated or declined. Selective norepinephrine reuptake inhibitors such as venlafaxine, desvenlafaxine, and duloxetine have demonstrated efficacy for VMS and depression.2 Selective serotonin reuptake inhibitors (SSRIs) are effective in women age <40 but show inconsistent efficacy for VMS and depression in women age >50. SSRIs combined with estrogen therapy may be useful in postmenopausal women.2
Biopsychosocial factors
Psychosocial attitudes about aging, sexual attractiveness, and children leaving home may contribute to depression during perimenopause. However, many women welcome the freedom from menstrual periods and pregnancy worries.
Some women may not be aware of the impact of menopausal changes on mood. Educating patients with a mood disorder about what to expect and identifying and treating disabling hormonal dysregulation symptoms is an ideal opportunity to enhance the quality of life for patients during menopause and beyond.
1. Cohen LS, Soares CN, Vitonis AF, et al. Risk for new onset of depression during the menopausal transition: the Harvard study of moods and cycles. Arch Gen Psychiatry. 2006;63(4):385-390.
2. Thase ME, Entsuah R, Cantillon M, et al. Relative antidepressant efficacy of venlafaxine and SSRIs: sex-age interactions. J Womens Health (Larchmt). 2005;14:609-616.
3. Aloysi A, Van Dyk K, Sano M. Women’s cognitive and affective health and neuropsychiatry. Mt Sinai J Medicine. 2006;73(7):967-975.
4. Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
5. Santoro N. Symptoms of menopause: hot flushes. Clinical ObGyn. 2008;51(3):539-548.
6. Joffe H, Soares CN, Cohen LS. Assessment and treatment of hot flushes and menopausal mood disturbance. Psychiatr Clin N Am. 2003;26:563-580.
1. Cohen LS, Soares CN, Vitonis AF, et al. Risk for new onset of depression during the menopausal transition: the Harvard study of moods and cycles. Arch Gen Psychiatry. 2006;63(4):385-390.
2. Thase ME, Entsuah R, Cantillon M, et al. Relative antidepressant efficacy of venlafaxine and SSRIs: sex-age interactions. J Womens Health (Larchmt). 2005;14:609-616.
3. Aloysi A, Van Dyk K, Sano M. Women’s cognitive and affective health and neuropsychiatry. Mt Sinai J Medicine. 2006;73(7):967-975.
4. Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
5. Santoro N. Symptoms of menopause: hot flushes. Clinical ObGyn. 2008;51(3):539-548.
6. Joffe H, Soares CN, Cohen LS. Assessment and treatment of hot flushes and menopausal mood disturbance. Psychiatr Clin N Am. 2003;26:563-580.
Aspirin and GI bleeding
In “Aspirin to prevent cardiovascular events,” (Medicine in Brief, Current Psychiatry, February 2010), the authors emphasize the risk of gastrointestinal (GI) bleeding. Because about 80% of strokes are ischemic but 20% represent a CNS bleed, shouldn’t the risk of hemorrhagic stroke be considered, especially in patients without known heart disease or those who have never had a heart attack before taking daily aspirin?
Bryan D. Spader, MD
Kinston, NC
The authors respond
We appreciate Dr. Spader’s question about the risk of hemorrhagic stroke in addition to GI bleeding with daily aspirin. The Women’s Health Study shows increases in hemorrhagic strokes in the aspirin group are not statistically significant (relative risk [RR] 1.24, confidence interval [CI] 0.82 to 1.87). This is confirmed by the meta-analysis that is the basis for the U.S. Preventive Services Task Force recommendations.1 Hemorrhagic stroke was not significantly higher in women taking aspirin than controls, but was higher in men (odds ratio [OR] 1.69, [CI, 1.04 to 2.73]). However, the same study concluded, “Aspirin does not seem to affect CVD (cardiovascular disease) mortality or all-cause mortality in either men or women. Aspirin use for the primary prevention of CVD events probably provides more benefits than harms to men at increased risk for myocardial infarction and women at increased risk for ischemic stroke.”1 Recent estimates indicate that the risk of hemorrhagic stroke is small, at about 0.2 per 1,000 patient-years of aspirin exposure. For every 1 hemorrhagic stroke over 5 years, approximately 14 myocardial infarctions are prevented in individuals with moderate cardiac risks.2
However, we found a dearth of follow-up studies showing individuals having hemorrhagic strokes when taking aspirin. One study examined 204 hemorrhagic stroke patients who were later placed on aspirin to reduce ischemic events and showed that aspirin use is not associated with intracerebral hemorrhage recurrence in survivors of either lobar hemorrhage or deep hemorrhage.3 Nevertheless, the median time to aspirin initiation is 5.4 months after index hemorrhagic stroke. Until more evidence emerges, use of aspirin for hemorrhagic stroke patients should be made on an individual basis after considering the benefits, controlling hypertension, and assessing other risk factors.
Glen L. Xiong, MD
Assistant clinical professor
University of California, Davis
Sacramento, CA
Christopher A. Kenedi, MD, MPH
Adjunct professor of psychiatry
Duke University Medical Center
Durham, NC
Reference
1. Wolff T, Miller T, Ko S. Aspirin for the primary prevention of cardiovascular events: an update of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;150:405-410.
2. Gorelick PB, Weisman SM. Risk of hemorrhagic stroke with aspirin use: an update. Stroke. 2005;36:1801-1817.
3. Viswanathan A, Rakich SM, Engel C, et al. Anti-platelet use after intracerebral hemorrhage. Neurology. 2006;66:206-209.
In “Aspirin to prevent cardiovascular events,” (Medicine in Brief, Current Psychiatry, February 2010), the authors emphasize the risk of gastrointestinal (GI) bleeding. Because about 80% of strokes are ischemic but 20% represent a CNS bleed, shouldn’t the risk of hemorrhagic stroke be considered, especially in patients without known heart disease or those who have never had a heart attack before taking daily aspirin?
Bryan D. Spader, MD
Kinston, NC
The authors respond
We appreciate Dr. Spader’s question about the risk of hemorrhagic stroke in addition to GI bleeding with daily aspirin. The Women’s Health Study shows increases in hemorrhagic strokes in the aspirin group are not statistically significant (relative risk [RR] 1.24, confidence interval [CI] 0.82 to 1.87). This is confirmed by the meta-analysis that is the basis for the U.S. Preventive Services Task Force recommendations.1 Hemorrhagic stroke was not significantly higher in women taking aspirin than controls, but was higher in men (odds ratio [OR] 1.69, [CI, 1.04 to 2.73]). However, the same study concluded, “Aspirin does not seem to affect CVD (cardiovascular disease) mortality or all-cause mortality in either men or women. Aspirin use for the primary prevention of CVD events probably provides more benefits than harms to men at increased risk for myocardial infarction and women at increased risk for ischemic stroke.”1 Recent estimates indicate that the risk of hemorrhagic stroke is small, at about 0.2 per 1,000 patient-years of aspirin exposure. For every 1 hemorrhagic stroke over 5 years, approximately 14 myocardial infarctions are prevented in individuals with moderate cardiac risks.2
However, we found a dearth of follow-up studies showing individuals having hemorrhagic strokes when taking aspirin. One study examined 204 hemorrhagic stroke patients who were later placed on aspirin to reduce ischemic events and showed that aspirin use is not associated with intracerebral hemorrhage recurrence in survivors of either lobar hemorrhage or deep hemorrhage.3 Nevertheless, the median time to aspirin initiation is 5.4 months after index hemorrhagic stroke. Until more evidence emerges, use of aspirin for hemorrhagic stroke patients should be made on an individual basis after considering the benefits, controlling hypertension, and assessing other risk factors.
Glen L. Xiong, MD
Assistant clinical professor
University of California, Davis
Sacramento, CA
Christopher A. Kenedi, MD, MPH
Adjunct professor of psychiatry
Duke University Medical Center
Durham, NC
In “Aspirin to prevent cardiovascular events,” (Medicine in Brief, Current Psychiatry, February 2010), the authors emphasize the risk of gastrointestinal (GI) bleeding. Because about 80% of strokes are ischemic but 20% represent a CNS bleed, shouldn’t the risk of hemorrhagic stroke be considered, especially in patients without known heart disease or those who have never had a heart attack before taking daily aspirin?
Bryan D. Spader, MD
Kinston, NC
The authors respond
We appreciate Dr. Spader’s question about the risk of hemorrhagic stroke in addition to GI bleeding with daily aspirin. The Women’s Health Study shows increases in hemorrhagic strokes in the aspirin group are not statistically significant (relative risk [RR] 1.24, confidence interval [CI] 0.82 to 1.87). This is confirmed by the meta-analysis that is the basis for the U.S. Preventive Services Task Force recommendations.1 Hemorrhagic stroke was not significantly higher in women taking aspirin than controls, but was higher in men (odds ratio [OR] 1.69, [CI, 1.04 to 2.73]). However, the same study concluded, “Aspirin does not seem to affect CVD (cardiovascular disease) mortality or all-cause mortality in either men or women. Aspirin use for the primary prevention of CVD events probably provides more benefits than harms to men at increased risk for myocardial infarction and women at increased risk for ischemic stroke.”1 Recent estimates indicate that the risk of hemorrhagic stroke is small, at about 0.2 per 1,000 patient-years of aspirin exposure. For every 1 hemorrhagic stroke over 5 years, approximately 14 myocardial infarctions are prevented in individuals with moderate cardiac risks.2
However, we found a dearth of follow-up studies showing individuals having hemorrhagic strokes when taking aspirin. One study examined 204 hemorrhagic stroke patients who were later placed on aspirin to reduce ischemic events and showed that aspirin use is not associated with intracerebral hemorrhage recurrence in survivors of either lobar hemorrhage or deep hemorrhage.3 Nevertheless, the median time to aspirin initiation is 5.4 months after index hemorrhagic stroke. Until more evidence emerges, use of aspirin for hemorrhagic stroke patients should be made on an individual basis after considering the benefits, controlling hypertension, and assessing other risk factors.
Glen L. Xiong, MD
Assistant clinical professor
University of California, Davis
Sacramento, CA
Christopher A. Kenedi, MD, MPH
Adjunct professor of psychiatry
Duke University Medical Center
Durham, NC
Reference
1. Wolff T, Miller T, Ko S. Aspirin for the primary prevention of cardiovascular events: an update of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;150:405-410.
2. Gorelick PB, Weisman SM. Risk of hemorrhagic stroke with aspirin use: an update. Stroke. 2005;36:1801-1817.
3. Viswanathan A, Rakich SM, Engel C, et al. Anti-platelet use after intracerebral hemorrhage. Neurology. 2006;66:206-209.
Reference
1. Wolff T, Miller T, Ko S. Aspirin for the primary prevention of cardiovascular events: an update of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;150:405-410.
2. Gorelick PB, Weisman SM. Risk of hemorrhagic stroke with aspirin use: an update. Stroke. 2005;36:1801-1817.
3. Viswanathan A, Rakich SM, Engel C, et al. Anti-platelet use after intracerebral hemorrhage. Neurology. 2006;66:206-209.
Chamomile Eases Anxiety, Depressive Symptoms
BALTIMORE – Chamomile extract therapy demonstrated both anxiolytic and antidepressive effects in a two-part randomized, controlled, blinded study of 57 patients with mild to moderate generalized anxiety disorder.
The initial study, published in 2009, is thought to be the first controlled clinical trial of oral chamomile (Matricaria recutita) extract for GAD. A substudy presented in a poster at the annual meeting of the Anxiety Disorders Association of America (ADAA), investigated the effect of chamomile on depressive symptoms in GAD patients who had comorbid depression, a history of depression, or no depression.
Because not all patients are willing or able to use psychopharmacologic treatment, “the identification of a safe and effective herbal remedy for treating anxious and depressive symptoms would be of public health relevance,” Matthew A. Shore and his associates said in their poster.
Chamomile has long been used as a traditional remedy for its calming effect and has demonstrated pharmacologic activity in animal models of anxiety. Its anxiolytic and antidepressive properties may relate to modulation of central noradrenalin, dopamine, and serotonin, and gamma-aminobutyric acid neurotransmission and hypothalamic-pituitary-adrenocorticalaxis activity, said Mr. Shore and his associates, of the University of Pennsylvania, Philadelphia.
The original study, led by Dr. Jay D. Amsterdam, was summarized by coauthor Irene Soeller, a nurse practitioner, in a session on alternative/complementary medicine at the ADAA meeting. The 57 GAD patients all had minimum baseline Hamilton Anxiety Rating (HAM-A) scores of 9 or more. Patients with other DSM-IV axis 1 disorders, such as minor depression, were not excluded as long as the comorbid condition was not the primary diagnosis.
Those with major depressive disorder, bipolar disorder, or other serious psychiatric diagnoses were excluded (J. Clin. Psychopharmacol. 2009;29:378–82).
Twenty-eight of the patients were randomized to chamomile extract and 29 to placebo for 8 weeks. Identically appearing and smelling capsules contained either pharmaceutical-grade chamomile extract or placebo. Initial dose was one capsule (220 mg for the chamomile) daily for the first week, increasing to two capsules daily for week two. After that, patients with a 50% or less reduction in HAM-A scores at each week were increased to three capsules at week 3 and four at week 4, and then up to five capsules at weeks 5-8 if response was still less than 50%.
At 8 weeks, there was a significantly greater reduction in the mean total HAM-A score for chamomile versus placebo–the primary outcome–with a mean difference of 3.17 points between the two groups.
There was also a somewhat greater proportion of overall HAM-A responders to chamomile versus placebo (57% vs. 38%), and the overall percentage change was numerically greater for chamomile than placebo on the HAM-A (53% vs. 35%), the Beck Anxiety Index (42% vs. 21%) and the Psychological General Well-Being Index (28% vs. 18%).
In all three groups combined, there was a significantly greater reduction over time in total Hamilton Depression Rating (HAM-D) 17 scores and in core HAM-D depression items (including depressed mood, guilt, and suicidal ideation) for chamomile versus placebo, with a P value of less than .05 on both measures.
These studies were funded by grants from the National Center for Complementary and Alternative Medicine (part of the National Institutes of Health). Both Mr. Shore and Ms. Soeller stated that they have no other financial disclosures.
Dr. Amsterdam received grant support from Stanley Medical Research Institute, Lilly Research Laboratories, Sanofi Aventis Inc., and Novartis Inc. Dr. Amsterdam is not a member of any industry-sponsored advisory board or speakers bureau and has no significant financial interest in any pharmaceutical company.
BALTIMORE – Chamomile extract therapy demonstrated both anxiolytic and antidepressive effects in a two-part randomized, controlled, blinded study of 57 patients with mild to moderate generalized anxiety disorder.
The initial study, published in 2009, is thought to be the first controlled clinical trial of oral chamomile (Matricaria recutita) extract for GAD. A substudy presented in a poster at the annual meeting of the Anxiety Disorders Association of America (ADAA), investigated the effect of chamomile on depressive symptoms in GAD patients who had comorbid depression, a history of depression, or no depression.
Because not all patients are willing or able to use psychopharmacologic treatment, “the identification of a safe and effective herbal remedy for treating anxious and depressive symptoms would be of public health relevance,” Matthew A. Shore and his associates said in their poster.
Chamomile has long been used as a traditional remedy for its calming effect and has demonstrated pharmacologic activity in animal models of anxiety. Its anxiolytic and antidepressive properties may relate to modulation of central noradrenalin, dopamine, and serotonin, and gamma-aminobutyric acid neurotransmission and hypothalamic-pituitary-adrenocorticalaxis activity, said Mr. Shore and his associates, of the University of Pennsylvania, Philadelphia.
The original study, led by Dr. Jay D. Amsterdam, was summarized by coauthor Irene Soeller, a nurse practitioner, in a session on alternative/complementary medicine at the ADAA meeting. The 57 GAD patients all had minimum baseline Hamilton Anxiety Rating (HAM-A) scores of 9 or more. Patients with other DSM-IV axis 1 disorders, such as minor depression, were not excluded as long as the comorbid condition was not the primary diagnosis.
Those with major depressive disorder, bipolar disorder, or other serious psychiatric diagnoses were excluded (J. Clin. Psychopharmacol. 2009;29:378–82).
Twenty-eight of the patients were randomized to chamomile extract and 29 to placebo for 8 weeks. Identically appearing and smelling capsules contained either pharmaceutical-grade chamomile extract or placebo. Initial dose was one capsule (220 mg for the chamomile) daily for the first week, increasing to two capsules daily for week two. After that, patients with a 50% or less reduction in HAM-A scores at each week were increased to three capsules at week 3 and four at week 4, and then up to five capsules at weeks 5-8 if response was still less than 50%.
At 8 weeks, there was a significantly greater reduction in the mean total HAM-A score for chamomile versus placebo–the primary outcome–with a mean difference of 3.17 points between the two groups.
There was also a somewhat greater proportion of overall HAM-A responders to chamomile versus placebo (57% vs. 38%), and the overall percentage change was numerically greater for chamomile than placebo on the HAM-A (53% vs. 35%), the Beck Anxiety Index (42% vs. 21%) and the Psychological General Well-Being Index (28% vs. 18%).
In all three groups combined, there was a significantly greater reduction over time in total Hamilton Depression Rating (HAM-D) 17 scores and in core HAM-D depression items (including depressed mood, guilt, and suicidal ideation) for chamomile versus placebo, with a P value of less than .05 on both measures.
These studies were funded by grants from the National Center for Complementary and Alternative Medicine (part of the National Institutes of Health). Both Mr. Shore and Ms. Soeller stated that they have no other financial disclosures.
Dr. Amsterdam received grant support from Stanley Medical Research Institute, Lilly Research Laboratories, Sanofi Aventis Inc., and Novartis Inc. Dr. Amsterdam is not a member of any industry-sponsored advisory board or speakers bureau and has no significant financial interest in any pharmaceutical company.
BALTIMORE – Chamomile extract therapy demonstrated both anxiolytic and antidepressive effects in a two-part randomized, controlled, blinded study of 57 patients with mild to moderate generalized anxiety disorder.
The initial study, published in 2009, is thought to be the first controlled clinical trial of oral chamomile (Matricaria recutita) extract for GAD. A substudy presented in a poster at the annual meeting of the Anxiety Disorders Association of America (ADAA), investigated the effect of chamomile on depressive symptoms in GAD patients who had comorbid depression, a history of depression, or no depression.
Because not all patients are willing or able to use psychopharmacologic treatment, “the identification of a safe and effective herbal remedy for treating anxious and depressive symptoms would be of public health relevance,” Matthew A. Shore and his associates said in their poster.
Chamomile has long been used as a traditional remedy for its calming effect and has demonstrated pharmacologic activity in animal models of anxiety. Its anxiolytic and antidepressive properties may relate to modulation of central noradrenalin, dopamine, and serotonin, and gamma-aminobutyric acid neurotransmission and hypothalamic-pituitary-adrenocorticalaxis activity, said Mr. Shore and his associates, of the University of Pennsylvania, Philadelphia.
The original study, led by Dr. Jay D. Amsterdam, was summarized by coauthor Irene Soeller, a nurse practitioner, in a session on alternative/complementary medicine at the ADAA meeting. The 57 GAD patients all had minimum baseline Hamilton Anxiety Rating (HAM-A) scores of 9 or more. Patients with other DSM-IV axis 1 disorders, such as minor depression, were not excluded as long as the comorbid condition was not the primary diagnosis.
Those with major depressive disorder, bipolar disorder, or other serious psychiatric diagnoses were excluded (J. Clin. Psychopharmacol. 2009;29:378–82).
Twenty-eight of the patients were randomized to chamomile extract and 29 to placebo for 8 weeks. Identically appearing and smelling capsules contained either pharmaceutical-grade chamomile extract or placebo. Initial dose was one capsule (220 mg for the chamomile) daily for the first week, increasing to two capsules daily for week two. After that, patients with a 50% or less reduction in HAM-A scores at each week were increased to three capsules at week 3 and four at week 4, and then up to five capsules at weeks 5-8 if response was still less than 50%.
At 8 weeks, there was a significantly greater reduction in the mean total HAM-A score for chamomile versus placebo–the primary outcome–with a mean difference of 3.17 points between the two groups.
There was also a somewhat greater proportion of overall HAM-A responders to chamomile versus placebo (57% vs. 38%), and the overall percentage change was numerically greater for chamomile than placebo on the HAM-A (53% vs. 35%), the Beck Anxiety Index (42% vs. 21%) and the Psychological General Well-Being Index (28% vs. 18%).
In all three groups combined, there was a significantly greater reduction over time in total Hamilton Depression Rating (HAM-D) 17 scores and in core HAM-D depression items (including depressed mood, guilt, and suicidal ideation) for chamomile versus placebo, with a P value of less than .05 on both measures.
These studies were funded by grants from the National Center for Complementary and Alternative Medicine (part of the National Institutes of Health). Both Mr. Shore and Ms. Soeller stated that they have no other financial disclosures.
Dr. Amsterdam received grant support from Stanley Medical Research Institute, Lilly Research Laboratories, Sanofi Aventis Inc., and Novartis Inc. Dr. Amsterdam is not a member of any industry-sponsored advisory board or speakers bureau and has no significant financial interest in any pharmaceutical company.
Hope Can Play a Transformative Role in Cancer
NEW ORLEANS – Hope plays an important role in the experience of cancer patients, especially those with poor prognoses, and it often follows an unexpected trajectory.
These were the findings of several studies presented at the annual conference of the American Psychosocial Oncology Society.
“While patients have a hard time defining hope, they almost always know exactly what it means to them, and they usually define its opposite as 'giving up,'” said Amy Pearson of the Lung Cancer Alliance in Washington. Her study was conducted with the National Brain Tumor Society and the Pancreatic Cancer Action Network.
Meredith Cammarata and colleagues from Mount Sinai Hospital in New York added that hope has been described as the ability to acquire belief in one's ability to control one's circumstances, a positive expectation for goal attainment, belief in possibilities for the future, and belief that one's present situation can be modified–that there is a way out of difficulties.
Others have suggested that hope is an experiential process; a relational process; a rational process; or a spiritual and transcendent process that might be determined by one's faith and belief or one's life experiences, her poster noted.
Studies further indicate that hope exists along a continuum, with goals ranging from cure to comfortable death; that hope is fluid and changes throughout the course of the illness; and that hope is dynamic, beginning with one's reaction to a diagnosis, according to Ms. Pearson's study, which examined this “hope trajectory” in 15 long-term survivors of lung, brain, and pancreatic cancers.
Although the 5-year survival rates for these cancers are approximately 30%, 15%, and 5%, respectively, the subjects in the study had survival that was double the median survival time for their tumor type. Therefore, the lung cancer survivors were required to live at least 34 months, but actually lived 4-12 years; the brain tumor survivors were required to live at least 30 months, but lived 8-21 years; and the pancreatic cancer survivors were required live at least 1 year, but actually lived 3-14 years. “We sought to better understand the meaning of hope, the role hope plays, and what contributes to hope or takes it away from these patients,” she said.
The research was based on semistructured 1-hour interviews. Patients also completed an online version of the Herth Hope Index, a validated 12-item scale. From their analysis, three major themes emerged: taking control, having faith, and finding meaning.
All of the patients took at least one action involving treatment decision making. Ten sought second opinions, five researched clinical trials (and three participated), three insisted on off-label treatment, and two performed research to confirm protocols and doctors' decisions. Several continued to work and take other measures to “normalize” their lives. They protected themselves through avoidance of “negative people” and avoidance of negative information. Some made healthy lifestyle changes, which they later attributed to saving their lives.
Family, Faith Are the Main Sources
One-third identified faith as the most important factor in finding hope and in coping, and the majority called faith important. Ten said that their diagnosis had changed their lives for the better or for “a reason.” Virtually all became part of a peer-support network to engender hope in other patients.
The most frequently mentioned sources of hope were family members, church and/or faith, and the medical personnel who treated them. Things that seemed to “take hope away” included dismal research statistics, negative medical personnel, death of other survivors, and setbacks in disease status.
The study validated that patients want to maintain hope–and can do so, especially when the oncology team understands the individual patient's beliefs and helps foster that patient's version of hope. (See box below.)
Other investigators illustrated how the patient's “trajectory of hope” does not necessarily correspond with their prognosis or treatment response.
Strong religious affiliation, a supportive family, cancer prognosis, and treatment plan are “not always associated with hope in the manner in which we would expect them to be,” said Ms. Cammarata. She and her colleagues presented the following cases to illustrate:
▸ Patient No. 1 had acute myeloid leukemia and expressed minimal hope from the time of diagnosis. “Instead of focusing on getting better, she ruminated on her symptoms and the possibility of relapse,” the researchers noted. As the treatment plan and bone marrow transplant team became positive about her diagnosis, she remained hopeless. Even in remission, she refused to leave the house and obsessed over relapse. Despite having a loving support system, she was unable to accept and benefit from their support.” The hope trajectory, which plotted the patient's expression of hope against the treatment course, showed that her hope plummeted continuously from baseline, with the curve continuing to fall even when the transplant appeared to be working.
▸ Patient No. 2 had acute lymphoblastic leukemia. Although she underwent an allogeneic transplant from her HLA-matched sister, she relapsed and died 1 year later. Her experience of hope closely matched her treatment plan, with the curve of her hope trajectory paralleling her treatment's ups and downs. “Because of the match, she was hopeful for a good response, but when she experienced chemotherapy side effects, she became depressed and difficult to engage. After the transplant, she enjoyed a brief state of remission and felt hopeful about regaining a normal life, but she began to be continuously fatigued, and along with this came the fear that she would never feel better. She relapsed within 3 months and was offered a second transplant, but a slim chance for prolonged survival.
“She refused the transplant and chose to live her precious last days as positively as she could, surrounded by family and friends, even giving herself a going-away party,” Ms. Cammarata and colleagues reported. “Her hope trajectory completely mirrored her disease and, surprisingly, the curve even rose as she approached death and treatments failed.”
▸ Patient No. 3 expressed “endless hope,” in spite of a poor prognosis, the death of a friend who also had leukemia, and ultimately his debilitating graft-vs.-host disease. “He had a tremendous amount of optimism from the time of diagnosis,” the authors wrote in the poster. “He felt the transplant made him a better person, and he became closer than ever with his family.” In this case, the trajectory of hope was higher than one would expect, and remained high even in the face of life-threatening complications.
Multiple aspects of hope can be fostered, the investigators suggested, not only for the patient but for the medical team and family. These can influence the already complex and confusing role that hope plays in the mind of a bone marrow transplant patient.
Go Carefully With Informed Consent
Dr. Carl G. Kardinal of the University of Missouri in Columbia suggested that Phase II trials offer patients with advanced disease hope that might not otherwise be available. He and his colleagues evaluated the hope trajectory of 50 consecutive patients who consented to participate in phase II cooperative trials. Patients were interviewed by a psychiatric social worker who was not directly involved in their care.
All 50 patients stated that hope of therapeutic benefit, however small, was their primary motivation to join the trial. Other motivating factors were altruism (29), avoidance of regret that later they should have participated (19), lack of other treatment alternatives (14), and trust that their oncologist thinks this trial might help (10), Dr. Kardinal reported.
He pointed out that this is a vulnerable patient population for whom “truly informed consent” might not be possible. He further maintained that the current informed-consent process is too cumbersome and should be simplified.
“Hope of a treatment response is the overwhelming motivation of cancer patients to participate in phase II trials. This places an even greater responsibility on the physician-investigator to protect these human subjects,” he said.
Physicians Can Create a Space for Hope
Health care providers can foster hope in the following ways:
▸ Even in cancers of poor prognosis, patients can survive. When physicians deliver the diagnosis, they can create a space for hope.
▸ “What can I control?” is an important question for patients. Assess what level of information the patient wants, and communicate accordingly. For patients who believe that a healthy lifestyle might make a difference, foster this behavior.
▸ Psychosocial and support resources might have a positive impact. Inform patients about support resources and peer support programs. Connecting with other patients might help survivors find meaning.
▸ Cancer is an existential crisis. Some patients search for the meaning of it while their faith, spirituality, and personal beliefs might be challenged. If the patient uses faith or spirituality to gain hope, find ways to support this tool. If the patient's questioning of his or her faith results in a loss of hope, consider helping the patient connect with a spiritual community or adviser.
Source: Ms. Pearson
My Take
Seeing the Future as Half Full
A diagnosis like cancer calls the future into question and causes us to peer anxiously ahead. Hope is a way of seeing our future as half full, rather than half empty. Unrealistic hope can be a form of denial, and many cancer patients find themselves caught in the “prison of positive thinking,” urged to be upbeat and positive no matter how bad their prognosis. On the other hand, hopelessness is a symptom of depression, and a uniformly down-beat view is demoralizing to patient, family, and medical staff. The real question is: Hope for what?” Even a very short future can be more than half full.
Dr. David Spiegel is the Jack, Lulu and Sam Willson-professor in the School of Medicine at Stanford (Calif.) University. He also serves as associate chair of psychiatry and behavioral sciences at the university.
NEW ORLEANS – Hope plays an important role in the experience of cancer patients, especially those with poor prognoses, and it often follows an unexpected trajectory.
These were the findings of several studies presented at the annual conference of the American Psychosocial Oncology Society.
“While patients have a hard time defining hope, they almost always know exactly what it means to them, and they usually define its opposite as 'giving up,'” said Amy Pearson of the Lung Cancer Alliance in Washington. Her study was conducted with the National Brain Tumor Society and the Pancreatic Cancer Action Network.
Meredith Cammarata and colleagues from Mount Sinai Hospital in New York added that hope has been described as the ability to acquire belief in one's ability to control one's circumstances, a positive expectation for goal attainment, belief in possibilities for the future, and belief that one's present situation can be modified–that there is a way out of difficulties.
Others have suggested that hope is an experiential process; a relational process; a rational process; or a spiritual and transcendent process that might be determined by one's faith and belief or one's life experiences, her poster noted.
Studies further indicate that hope exists along a continuum, with goals ranging from cure to comfortable death; that hope is fluid and changes throughout the course of the illness; and that hope is dynamic, beginning with one's reaction to a diagnosis, according to Ms. Pearson's study, which examined this “hope trajectory” in 15 long-term survivors of lung, brain, and pancreatic cancers.
Although the 5-year survival rates for these cancers are approximately 30%, 15%, and 5%, respectively, the subjects in the study had survival that was double the median survival time for their tumor type. Therefore, the lung cancer survivors were required to live at least 34 months, but actually lived 4-12 years; the brain tumor survivors were required to live at least 30 months, but lived 8-21 years; and the pancreatic cancer survivors were required live at least 1 year, but actually lived 3-14 years. “We sought to better understand the meaning of hope, the role hope plays, and what contributes to hope or takes it away from these patients,” she said.
The research was based on semistructured 1-hour interviews. Patients also completed an online version of the Herth Hope Index, a validated 12-item scale. From their analysis, three major themes emerged: taking control, having faith, and finding meaning.
All of the patients took at least one action involving treatment decision making. Ten sought second opinions, five researched clinical trials (and three participated), three insisted on off-label treatment, and two performed research to confirm protocols and doctors' decisions. Several continued to work and take other measures to “normalize” their lives. They protected themselves through avoidance of “negative people” and avoidance of negative information. Some made healthy lifestyle changes, which they later attributed to saving their lives.
Family, Faith Are the Main Sources
One-third identified faith as the most important factor in finding hope and in coping, and the majority called faith important. Ten said that their diagnosis had changed their lives for the better or for “a reason.” Virtually all became part of a peer-support network to engender hope in other patients.
The most frequently mentioned sources of hope were family members, church and/or faith, and the medical personnel who treated them. Things that seemed to “take hope away” included dismal research statistics, negative medical personnel, death of other survivors, and setbacks in disease status.
The study validated that patients want to maintain hope–and can do so, especially when the oncology team understands the individual patient's beliefs and helps foster that patient's version of hope. (See box below.)
Other investigators illustrated how the patient's “trajectory of hope” does not necessarily correspond with their prognosis or treatment response.
Strong religious affiliation, a supportive family, cancer prognosis, and treatment plan are “not always associated with hope in the manner in which we would expect them to be,” said Ms. Cammarata. She and her colleagues presented the following cases to illustrate:
▸ Patient No. 1 had acute myeloid leukemia and expressed minimal hope from the time of diagnosis. “Instead of focusing on getting better, she ruminated on her symptoms and the possibility of relapse,” the researchers noted. As the treatment plan and bone marrow transplant team became positive about her diagnosis, she remained hopeless. Even in remission, she refused to leave the house and obsessed over relapse. Despite having a loving support system, she was unable to accept and benefit from their support.” The hope trajectory, which plotted the patient's expression of hope against the treatment course, showed that her hope plummeted continuously from baseline, with the curve continuing to fall even when the transplant appeared to be working.
▸ Patient No. 2 had acute lymphoblastic leukemia. Although she underwent an allogeneic transplant from her HLA-matched sister, she relapsed and died 1 year later. Her experience of hope closely matched her treatment plan, with the curve of her hope trajectory paralleling her treatment's ups and downs. “Because of the match, she was hopeful for a good response, but when she experienced chemotherapy side effects, she became depressed and difficult to engage. After the transplant, she enjoyed a brief state of remission and felt hopeful about regaining a normal life, but she began to be continuously fatigued, and along with this came the fear that she would never feel better. She relapsed within 3 months and was offered a second transplant, but a slim chance for prolonged survival.
“She refused the transplant and chose to live her precious last days as positively as she could, surrounded by family and friends, even giving herself a going-away party,” Ms. Cammarata and colleagues reported. “Her hope trajectory completely mirrored her disease and, surprisingly, the curve even rose as she approached death and treatments failed.”
▸ Patient No. 3 expressed “endless hope,” in spite of a poor prognosis, the death of a friend who also had leukemia, and ultimately his debilitating graft-vs.-host disease. “He had a tremendous amount of optimism from the time of diagnosis,” the authors wrote in the poster. “He felt the transplant made him a better person, and he became closer than ever with his family.” In this case, the trajectory of hope was higher than one would expect, and remained high even in the face of life-threatening complications.
Multiple aspects of hope can be fostered, the investigators suggested, not only for the patient but for the medical team and family. These can influence the already complex and confusing role that hope plays in the mind of a bone marrow transplant patient.
Go Carefully With Informed Consent
Dr. Carl G. Kardinal of the University of Missouri in Columbia suggested that Phase II trials offer patients with advanced disease hope that might not otherwise be available. He and his colleagues evaluated the hope trajectory of 50 consecutive patients who consented to participate in phase II cooperative trials. Patients were interviewed by a psychiatric social worker who was not directly involved in their care.
All 50 patients stated that hope of therapeutic benefit, however small, was their primary motivation to join the trial. Other motivating factors were altruism (29), avoidance of regret that later they should have participated (19), lack of other treatment alternatives (14), and trust that their oncologist thinks this trial might help (10), Dr. Kardinal reported.
He pointed out that this is a vulnerable patient population for whom “truly informed consent” might not be possible. He further maintained that the current informed-consent process is too cumbersome and should be simplified.
“Hope of a treatment response is the overwhelming motivation of cancer patients to participate in phase II trials. This places an even greater responsibility on the physician-investigator to protect these human subjects,” he said.
Physicians Can Create a Space for Hope
Health care providers can foster hope in the following ways:
▸ Even in cancers of poor prognosis, patients can survive. When physicians deliver the diagnosis, they can create a space for hope.
▸ “What can I control?” is an important question for patients. Assess what level of information the patient wants, and communicate accordingly. For patients who believe that a healthy lifestyle might make a difference, foster this behavior.
▸ Psychosocial and support resources might have a positive impact. Inform patients about support resources and peer support programs. Connecting with other patients might help survivors find meaning.
▸ Cancer is an existential crisis. Some patients search for the meaning of it while their faith, spirituality, and personal beliefs might be challenged. If the patient uses faith or spirituality to gain hope, find ways to support this tool. If the patient's questioning of his or her faith results in a loss of hope, consider helping the patient connect with a spiritual community or adviser.
Source: Ms. Pearson
My Take
Seeing the Future as Half Full
A diagnosis like cancer calls the future into question and causes us to peer anxiously ahead. Hope is a way of seeing our future as half full, rather than half empty. Unrealistic hope can be a form of denial, and many cancer patients find themselves caught in the “prison of positive thinking,” urged to be upbeat and positive no matter how bad their prognosis. On the other hand, hopelessness is a symptom of depression, and a uniformly down-beat view is demoralizing to patient, family, and medical staff. The real question is: Hope for what?” Even a very short future can be more than half full.
Dr. David Spiegel is the Jack, Lulu and Sam Willson-professor in the School of Medicine at Stanford (Calif.) University. He also serves as associate chair of psychiatry and behavioral sciences at the university.
NEW ORLEANS – Hope plays an important role in the experience of cancer patients, especially those with poor prognoses, and it often follows an unexpected trajectory.
These were the findings of several studies presented at the annual conference of the American Psychosocial Oncology Society.
“While patients have a hard time defining hope, they almost always know exactly what it means to them, and they usually define its opposite as 'giving up,'” said Amy Pearson of the Lung Cancer Alliance in Washington. Her study was conducted with the National Brain Tumor Society and the Pancreatic Cancer Action Network.
Meredith Cammarata and colleagues from Mount Sinai Hospital in New York added that hope has been described as the ability to acquire belief in one's ability to control one's circumstances, a positive expectation for goal attainment, belief in possibilities for the future, and belief that one's present situation can be modified–that there is a way out of difficulties.
Others have suggested that hope is an experiential process; a relational process; a rational process; or a spiritual and transcendent process that might be determined by one's faith and belief or one's life experiences, her poster noted.
Studies further indicate that hope exists along a continuum, with goals ranging from cure to comfortable death; that hope is fluid and changes throughout the course of the illness; and that hope is dynamic, beginning with one's reaction to a diagnosis, according to Ms. Pearson's study, which examined this “hope trajectory” in 15 long-term survivors of lung, brain, and pancreatic cancers.
Although the 5-year survival rates for these cancers are approximately 30%, 15%, and 5%, respectively, the subjects in the study had survival that was double the median survival time for their tumor type. Therefore, the lung cancer survivors were required to live at least 34 months, but actually lived 4-12 years; the brain tumor survivors were required to live at least 30 months, but lived 8-21 years; and the pancreatic cancer survivors were required live at least 1 year, but actually lived 3-14 years. “We sought to better understand the meaning of hope, the role hope plays, and what contributes to hope or takes it away from these patients,” she said.
The research was based on semistructured 1-hour interviews. Patients also completed an online version of the Herth Hope Index, a validated 12-item scale. From their analysis, three major themes emerged: taking control, having faith, and finding meaning.
All of the patients took at least one action involving treatment decision making. Ten sought second opinions, five researched clinical trials (and three participated), three insisted on off-label treatment, and two performed research to confirm protocols and doctors' decisions. Several continued to work and take other measures to “normalize” their lives. They protected themselves through avoidance of “negative people” and avoidance of negative information. Some made healthy lifestyle changes, which they later attributed to saving their lives.
Family, Faith Are the Main Sources
One-third identified faith as the most important factor in finding hope and in coping, and the majority called faith important. Ten said that their diagnosis had changed their lives for the better or for “a reason.” Virtually all became part of a peer-support network to engender hope in other patients.
The most frequently mentioned sources of hope were family members, church and/or faith, and the medical personnel who treated them. Things that seemed to “take hope away” included dismal research statistics, negative medical personnel, death of other survivors, and setbacks in disease status.
The study validated that patients want to maintain hope–and can do so, especially when the oncology team understands the individual patient's beliefs and helps foster that patient's version of hope. (See box below.)
Other investigators illustrated how the patient's “trajectory of hope” does not necessarily correspond with their prognosis or treatment response.
Strong religious affiliation, a supportive family, cancer prognosis, and treatment plan are “not always associated with hope in the manner in which we would expect them to be,” said Ms. Cammarata. She and her colleagues presented the following cases to illustrate:
▸ Patient No. 1 had acute myeloid leukemia and expressed minimal hope from the time of diagnosis. “Instead of focusing on getting better, she ruminated on her symptoms and the possibility of relapse,” the researchers noted. As the treatment plan and bone marrow transplant team became positive about her diagnosis, she remained hopeless. Even in remission, she refused to leave the house and obsessed over relapse. Despite having a loving support system, she was unable to accept and benefit from their support.” The hope trajectory, which plotted the patient's expression of hope against the treatment course, showed that her hope plummeted continuously from baseline, with the curve continuing to fall even when the transplant appeared to be working.
▸ Patient No. 2 had acute lymphoblastic leukemia. Although she underwent an allogeneic transplant from her HLA-matched sister, she relapsed and died 1 year later. Her experience of hope closely matched her treatment plan, with the curve of her hope trajectory paralleling her treatment's ups and downs. “Because of the match, she was hopeful for a good response, but when she experienced chemotherapy side effects, she became depressed and difficult to engage. After the transplant, she enjoyed a brief state of remission and felt hopeful about regaining a normal life, but she began to be continuously fatigued, and along with this came the fear that she would never feel better. She relapsed within 3 months and was offered a second transplant, but a slim chance for prolonged survival.
“She refused the transplant and chose to live her precious last days as positively as she could, surrounded by family and friends, even giving herself a going-away party,” Ms. Cammarata and colleagues reported. “Her hope trajectory completely mirrored her disease and, surprisingly, the curve even rose as she approached death and treatments failed.”
▸ Patient No. 3 expressed “endless hope,” in spite of a poor prognosis, the death of a friend who also had leukemia, and ultimately his debilitating graft-vs.-host disease. “He had a tremendous amount of optimism from the time of diagnosis,” the authors wrote in the poster. “He felt the transplant made him a better person, and he became closer than ever with his family.” In this case, the trajectory of hope was higher than one would expect, and remained high even in the face of life-threatening complications.
Multiple aspects of hope can be fostered, the investigators suggested, not only for the patient but for the medical team and family. These can influence the already complex and confusing role that hope plays in the mind of a bone marrow transplant patient.
Go Carefully With Informed Consent
Dr. Carl G. Kardinal of the University of Missouri in Columbia suggested that Phase II trials offer patients with advanced disease hope that might not otherwise be available. He and his colleagues evaluated the hope trajectory of 50 consecutive patients who consented to participate in phase II cooperative trials. Patients were interviewed by a psychiatric social worker who was not directly involved in their care.
All 50 patients stated that hope of therapeutic benefit, however small, was their primary motivation to join the trial. Other motivating factors were altruism (29), avoidance of regret that later they should have participated (19), lack of other treatment alternatives (14), and trust that their oncologist thinks this trial might help (10), Dr. Kardinal reported.
He pointed out that this is a vulnerable patient population for whom “truly informed consent” might not be possible. He further maintained that the current informed-consent process is too cumbersome and should be simplified.
“Hope of a treatment response is the overwhelming motivation of cancer patients to participate in phase II trials. This places an even greater responsibility on the physician-investigator to protect these human subjects,” he said.
Physicians Can Create a Space for Hope
Health care providers can foster hope in the following ways:
▸ Even in cancers of poor prognosis, patients can survive. When physicians deliver the diagnosis, they can create a space for hope.
▸ “What can I control?” is an important question for patients. Assess what level of information the patient wants, and communicate accordingly. For patients who believe that a healthy lifestyle might make a difference, foster this behavior.
▸ Psychosocial and support resources might have a positive impact. Inform patients about support resources and peer support programs. Connecting with other patients might help survivors find meaning.
▸ Cancer is an existential crisis. Some patients search for the meaning of it while their faith, spirituality, and personal beliefs might be challenged. If the patient uses faith or spirituality to gain hope, find ways to support this tool. If the patient's questioning of his or her faith results in a loss of hope, consider helping the patient connect with a spiritual community or adviser.
Source: Ms. Pearson
My Take
Seeing the Future as Half Full
A diagnosis like cancer calls the future into question and causes us to peer anxiously ahead. Hope is a way of seeing our future as half full, rather than half empty. Unrealistic hope can be a form of denial, and many cancer patients find themselves caught in the “prison of positive thinking,” urged to be upbeat and positive no matter how bad their prognosis. On the other hand, hopelessness is a symptom of depression, and a uniformly down-beat view is demoralizing to patient, family, and medical staff. The real question is: Hope for what?” Even a very short future can be more than half full.
Dr. David Spiegel is the Jack, Lulu and Sam Willson-professor in the School of Medicine at Stanford (Calif.) University. He also serves as associate chair of psychiatry and behavioral sciences at the university.