User login
Glenohumeral Joint Sepsis Caused by Streptococcus mitis: A Case Report
Septic arthritis predominantly involves the weight-bearing joints of the hip and knee, which account for nearly 60% of cases.1 In contrast, the shoulder joint is involved in 10% to 15% of cases, though this number may be higher among intravenous (IV) drug users.2 The most common causative organisms are the Staphylococcus species, followed closely by β-hemolytic streptococci, with these 2 groups accounting for more than 90% of all cases.3 The Streptococcus viridans group belongs to normal oral flora residing predominantly on the surface of teeth. Although well known for its ability to colonize heart valves and frequently cause bacterial endocarditis, this group has rarely been associated with septic arthritis. Furthermore, Streptococcus mitis, a subgroup of S viridans, has been implicated even less commonly.
In this article, we report a case of glenohumeral joint septic arthritis caused by S mitis. To our knowledge, such a case has not been previously reported in the English literature. Given the low virulence of this orally based bacterium, treating physicians must maintain clinical suspicion for the organism in the setting of persistent joint effusion and pain in association with periodontal disease or trauma. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
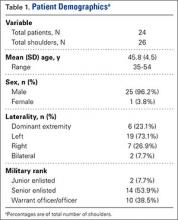
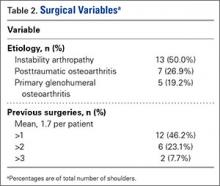
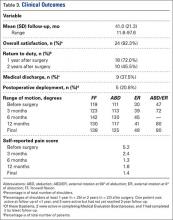
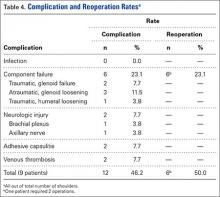
A right-hand-dominant 54-year-old man presented to Dr. Gruson with complaints of persistent right shoulder pain associated with worsening range of motion (ROM). Three weeks earlier, the patient reported being assaulted and noted progressive swelling about the right shoulder. He denied fevers, chills, or prior shoulder problems. Although his past medical history was remarkable for hepatitis C and diabetes, he was not taking any diabetic medications at that time. A review of systems was remarkable for poor dental hygiene, and the patient was missing several teeth, which he said had been knocked out during the assault. Physical examination revealed diffuse tenderness about the right shoulder and severe pain with all passive movement. The shoulder was pseudoparalyzed. There were no subcutaneous collections, wounds, or ecchymosis about the shoulder. Mild calor was noted on the right shoulder relative to the left. Radiographs of the right shoulder showed no acute osseous abnormalities.
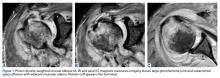
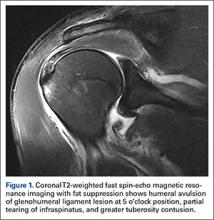
Magnetic resonance imaging (MRI), which was urgently obtained to assess the integrity of the rotator cuff and the location of the effusion, showed a large subacromial and glenohumeral joint effusion as well as diffuse muscular edema (Figures 1A-1C).
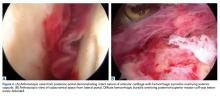
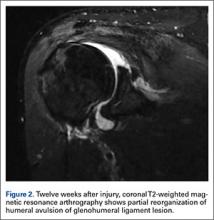
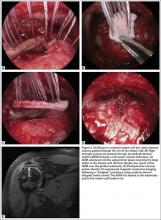
In light of the elevated infection findings of the laboratory tests and the positive culture, urgent arthroscopic irrigation and débridement of the right shoulder were indicated. Given the organism identified, transesophageal echocardiography was performed; there were no valvular vegetations. Creation of the posterior glenohumeral portal resulted in egress of turbid fluid, which was sent for culture. The subacromial space and the glenohumeral joint were thoroughly lavaged and the copious hemorrhagic synovitis débrided (Figures 2A, 2B).
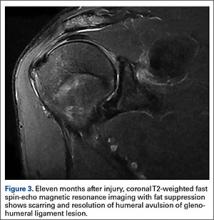
The 8-week course of antibiotics normalized the patient’s ESR to 13 mm/h. Follow-up MRI showed improvement in the soft-tissue edema. Clinically, the patient reported minimal shoulder pain. He was undergoing physical therapy to regain strength and ROM.
Discussion
Staphylococcus aureus is the leading causative organism of septic arthritis, accounting for more than 60% of all cases.4 Conversely, the Streptococcus viridans group is rarely implicated in septic arthritis, accounting for <1% of cases.4S viridans is part of the commensal oral flora and has low virulence. This heterogeneous group is subdivided into S mitis, S salivarius, S anginosus, S mutans, and S bovis. The S mitis group is further subdivided into S sanguinis (formerly known as S sanguis) and S mitis. Infection by an organism of the S viridans group usually occurs on a previously injured focus, and the organism is a causative agent of bacterial endocarditis.5 Reported cases of septic arthritis caused by S viridans have predominantly involved the knee joint—with severe osteoarthritis, poor dental hygiene, and prior IV drug use identified as risk factors.5-7The shoulder joint is seldom involved in septic arthritis; estimated incidence is under 8%.8 Although overall incidence may rise in an increasingly elderly patient population, incidence of shoulder infection remains low.2,9
The main routes for developing septic arthritis include direct inoculation secondary to penetrating trauma or hematologic spread.10 Coatsworth and colleagues11 reported on iatrogenic S mitis septic arthritis of a shoulder arthroplasty during ultrasonography-guided aspiration by a technician who was not wearing a mask. Our institutional policy is to perform joint aspiration under strictly sterile conditions, which were adhered to in the present case. We surmise our patient developed transient bacteremia from the loss of several teeth, particularly given his poor dentition. Yombi and colleagues5 documented 2 cases of septic arthritis caused by Streptococcus gordonii, a relative of S sanguinis. One involved a previously replaced knee, and the other a native knee joint. Other cases of S viridans group septic arthritis have involved the knee,6,7,12,13 the sternoclavicular joint,14-16 and the acromioclavicular joint.17S sanguinis6,7,12,15,16 and S gordonii5 have been implicated in most cases, and an unspeciated S viridans in others.13,14,17 Concomitant periodontal disease has been reported in most cases as well,6,7,12,15 including our patient’s case. In the English-language literature, we found no other reports of S mitis as the causative agent of acute septic glenohumeral joint arthritis from hematogenous spread.
There should be no delay in diagnosing septic arthritis, and infected material should be removed from the joint. In animal models, complete joint destruction occurred only 5 weeks after inoculation with Staphylococcus aureus.10 Garofalo and colleagues18 reported a trend toward improved functional outcomes after earlier operative treatment. The choice of open surgical drainage vs repeat needle aspiration seems to be of little consequence, as both have good long-term outcomes, but open surgical drainage seems to result in better long-term functional ROM.2,9 However, results of a recent study suggested surgical treatment is not always superior to medical treatment for septic arthritis in native joints.19 In some cases involving S viridans species, treatment consisted of a combination of IV antibiotics and onetime or repeat aspiration;6,12-15 treatment in the remaining cases was surgical débridement.5,7,16,17 Given that S viridans is associated with bacterial endocarditis, echocardiography is essential if this organism is to be identified. Medical management and antibiotic treatment should be initiated after consultation with medical and infectious disease specialists.19We have reported a case of septic shoulder caused by S mitis, a low-virulence organism seldom associated with joint infection. The patient’s infection likely resulted from hematogenous spread from the oral cavity (dentition was poor). Urgent aspiration of the joint and baseline infection laboratory tests are recommended. MRI of the shoulder may show an effusion. Urgent arthroscopic irrigation and débridement can yield good clinical outcomes.
Am J Orthop. 2016;45(6):E343-E346. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Mathews CJ, Kingsley G, Field M, et al. Management of septic arthritis: a systematic review. Ann Rheum Dis. 2007;66(4):440-445.
2. Leslie BM, Harris JM 3rd, Driscoll D. Septic arthritis of the shoulder in adults. J Bone Joint Surg Am. 1989;71(10):1516-1522.
3. Gupta MN, Sturrock RD, Field M. A prospective 2-year study of 75 patients with adult-onset septic arthritis. Rheumatology. 2001;40(1):24-30.
4. Dubost JJ, Soubrier M, De Champs C, Ristori JM, Bussiere JL, Sauvezie B. No changes in the distribution of organisms responsible for septic arthritis over a 20 year period. Ann Rheum Dis. 2002;61(3):267-269.
5. Yombi J, Belkhir L, Jonckheere S, et al. Streptococcus gordonii septic arthritis: two cases and review of literature. BMC Infect Dis. 2012;12:215.
6. Papaioannides D, Boniatsi L, Korantzopoulos P, Sinapidis D, Giotis C. Acute septic arthritis due to Streptococcus sanguis. Med Princ Pract. 2006;15(1):77-79.
7. Edson RS, Osmon DR, Berry DJ. Septic arthritis due to Streptococcus sanguis. Mayo Clin Proc. 2002;77(7):709-710.
8. Weston VC, Jones AC, Bradbury N, Fawthrop F, Doherty M. Clinical features and outcome of septic arthritis in a single UK health district 1982-1991. Ann Rheum Dis. 1999;58(4):214-219.
9. Lossos IS, Yossepowitch O, Kandel L, Yardeni D, Arber N. Septic arthritis of the glenohumeral joint. A report of 11 cases and review of the literature. Medicine. 1998;77(3):177-187.
10. Esterhai JL Jr, Gelb I. Adult septic arthritis. Orthop Clin North Am. 1991;22(3):503-514.
11. Coatsworth NR, Huntington PG, Giuffre B, Kotsiou G. The doctor and the mask: iatrogenic septic arthritis caused by Streptoccocus mitis. Med J Aust. 2013;198(5):285-286.
12. Patrick MR, Lewis D. Short of a length: Streptococcus sanguis knee infection from dental source. Br J Rheumatol. 1992;31(8):569.
13. Barbadillo C, Trujillo A, Cuende E, Mazzucchelli R, Mulero J, Andreu JL. Septic arthritis due to Streptococcus viridans. Clin Exp Rheumatol. 1990;8(5):520-521.
14. Mata P, Molins A, de Oya M. Sternal arthritis caused by Streptococcus viridans in a heroin addict [in Spanish]. Med Clin. 1984;83(16):689.
15. Mandac I, Prkacin I, Sabljar Matovinovic M, Sustercic D. Septic arthritis due to Streptococcus sanguis. Coll Antropol. 2010;34(2):661-664.
16. Nitsche JF, Vaughan JH, Williams G, Curd JG. Septic sternoclavicular arthritis with Pasteurella multocida and Streptococcus sanguis. Arthritis Rheum. 1982;25(4):467-469.
17. Blankstein A, Amsallem JL, Rubenstein E, Horoszowski H, Farin I. Septic arthritis of the acromioclavicular joint. Arch Orthop Trauma Surg. 1985;103(6):417-418.
18. Garofalo R, Flanagin B, Cesari E, Vinci E, Conti M, Castagna A. Destructive septic arthritis of shoulder in adults. Musculoskelet Surg. 2014;98(supp 1):S35-S39.
19. Ravindran V, Logan I, Bourke BE. Medical vs surgical treatment for the native joint in septic arthritis: a 6-year, single UK academic centre experience. Rheumatology. 2009;48(10):1320-1322.
Septic arthritis predominantly involves the weight-bearing joints of the hip and knee, which account for nearly 60% of cases.1 In contrast, the shoulder joint is involved in 10% to 15% of cases, though this number may be higher among intravenous (IV) drug users.2 The most common causative organisms are the Staphylococcus species, followed closely by β-hemolytic streptococci, with these 2 groups accounting for more than 90% of all cases.3 The Streptococcus viridans group belongs to normal oral flora residing predominantly on the surface of teeth. Although well known for its ability to colonize heart valves and frequently cause bacterial endocarditis, this group has rarely been associated with septic arthritis. Furthermore, Streptococcus mitis, a subgroup of S viridans, has been implicated even less commonly.
In this article, we report a case of glenohumeral joint septic arthritis caused by S mitis. To our knowledge, such a case has not been previously reported in the English literature. Given the low virulence of this orally based bacterium, treating physicians must maintain clinical suspicion for the organism in the setting of persistent joint effusion and pain in association with periodontal disease or trauma. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A right-hand-dominant 54-year-old man presented to Dr. Gruson with complaints of persistent right shoulder pain associated with worsening range of motion (ROM). Three weeks earlier, the patient reported being assaulted and noted progressive swelling about the right shoulder. He denied fevers, chills, or prior shoulder problems. Although his past medical history was remarkable for hepatitis C and diabetes, he was not taking any diabetic medications at that time. A review of systems was remarkable for poor dental hygiene, and the patient was missing several teeth, which he said had been knocked out during the assault. Physical examination revealed diffuse tenderness about the right shoulder and severe pain with all passive movement. The shoulder was pseudoparalyzed. There were no subcutaneous collections, wounds, or ecchymosis about the shoulder. Mild calor was noted on the right shoulder relative to the left. Radiographs of the right shoulder showed no acute osseous abnormalities.
Magnetic resonance imaging (MRI), which was urgently obtained to assess the integrity of the rotator cuff and the location of the effusion, showed a large subacromial and glenohumeral joint effusion as well as diffuse muscular edema (Figures 1A-1C).
In light of the elevated infection findings of the laboratory tests and the positive culture, urgent arthroscopic irrigation and débridement of the right shoulder were indicated. Given the organism identified, transesophageal echocardiography was performed; there were no valvular vegetations. Creation of the posterior glenohumeral portal resulted in egress of turbid fluid, which was sent for culture. The subacromial space and the glenohumeral joint were thoroughly lavaged and the copious hemorrhagic synovitis débrided (Figures 2A, 2B).
The 8-week course of antibiotics normalized the patient’s ESR to 13 mm/h. Follow-up MRI showed improvement in the soft-tissue edema. Clinically, the patient reported minimal shoulder pain. He was undergoing physical therapy to regain strength and ROM.
Discussion
Staphylococcus aureus is the leading causative organism of septic arthritis, accounting for more than 60% of all cases.4 Conversely, the Streptococcus viridans group is rarely implicated in septic arthritis, accounting for <1% of cases.4S viridans is part of the commensal oral flora and has low virulence. This heterogeneous group is subdivided into S mitis, S salivarius, S anginosus, S mutans, and S bovis. The S mitis group is further subdivided into S sanguinis (formerly known as S sanguis) and S mitis. Infection by an organism of the S viridans group usually occurs on a previously injured focus, and the organism is a causative agent of bacterial endocarditis.5 Reported cases of septic arthritis caused by S viridans have predominantly involved the knee joint—with severe osteoarthritis, poor dental hygiene, and prior IV drug use identified as risk factors.5-7The shoulder joint is seldom involved in septic arthritis; estimated incidence is under 8%.8 Although overall incidence may rise in an increasingly elderly patient population, incidence of shoulder infection remains low.2,9
The main routes for developing septic arthritis include direct inoculation secondary to penetrating trauma or hematologic spread.10 Coatsworth and colleagues11 reported on iatrogenic S mitis septic arthritis of a shoulder arthroplasty during ultrasonography-guided aspiration by a technician who was not wearing a mask. Our institutional policy is to perform joint aspiration under strictly sterile conditions, which were adhered to in the present case. We surmise our patient developed transient bacteremia from the loss of several teeth, particularly given his poor dentition. Yombi and colleagues5 documented 2 cases of septic arthritis caused by Streptococcus gordonii, a relative of S sanguinis. One involved a previously replaced knee, and the other a native knee joint. Other cases of S viridans group septic arthritis have involved the knee,6,7,12,13 the sternoclavicular joint,14-16 and the acromioclavicular joint.17S sanguinis6,7,12,15,16 and S gordonii5 have been implicated in most cases, and an unspeciated S viridans in others.13,14,17 Concomitant periodontal disease has been reported in most cases as well,6,7,12,15 including our patient’s case. In the English-language literature, we found no other reports of S mitis as the causative agent of acute septic glenohumeral joint arthritis from hematogenous spread.
There should be no delay in diagnosing septic arthritis, and infected material should be removed from the joint. In animal models, complete joint destruction occurred only 5 weeks after inoculation with Staphylococcus aureus.10 Garofalo and colleagues18 reported a trend toward improved functional outcomes after earlier operative treatment. The choice of open surgical drainage vs repeat needle aspiration seems to be of little consequence, as both have good long-term outcomes, but open surgical drainage seems to result in better long-term functional ROM.2,9 However, results of a recent study suggested surgical treatment is not always superior to medical treatment for septic arthritis in native joints.19 In some cases involving S viridans species, treatment consisted of a combination of IV antibiotics and onetime or repeat aspiration;6,12-15 treatment in the remaining cases was surgical débridement.5,7,16,17 Given that S viridans is associated with bacterial endocarditis, echocardiography is essential if this organism is to be identified. Medical management and antibiotic treatment should be initiated after consultation with medical and infectious disease specialists.19We have reported a case of septic shoulder caused by S mitis, a low-virulence organism seldom associated with joint infection. The patient’s infection likely resulted from hematogenous spread from the oral cavity (dentition was poor). Urgent aspiration of the joint and baseline infection laboratory tests are recommended. MRI of the shoulder may show an effusion. Urgent arthroscopic irrigation and débridement can yield good clinical outcomes.
Am J Orthop. 2016;45(6):E343-E346. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Septic arthritis predominantly involves the weight-bearing joints of the hip and knee, which account for nearly 60% of cases.1 In contrast, the shoulder joint is involved in 10% to 15% of cases, though this number may be higher among intravenous (IV) drug users.2 The most common causative organisms are the Staphylococcus species, followed closely by β-hemolytic streptococci, with these 2 groups accounting for more than 90% of all cases.3 The Streptococcus viridans group belongs to normal oral flora residing predominantly on the surface of teeth. Although well known for its ability to colonize heart valves and frequently cause bacterial endocarditis, this group has rarely been associated with septic arthritis. Furthermore, Streptococcus mitis, a subgroup of S viridans, has been implicated even less commonly.
In this article, we report a case of glenohumeral joint septic arthritis caused by S mitis. To our knowledge, such a case has not been previously reported in the English literature. Given the low virulence of this orally based bacterium, treating physicians must maintain clinical suspicion for the organism in the setting of persistent joint effusion and pain in association with periodontal disease or trauma. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A right-hand-dominant 54-year-old man presented to Dr. Gruson with complaints of persistent right shoulder pain associated with worsening range of motion (ROM). Three weeks earlier, the patient reported being assaulted and noted progressive swelling about the right shoulder. He denied fevers, chills, or prior shoulder problems. Although his past medical history was remarkable for hepatitis C and diabetes, he was not taking any diabetic medications at that time. A review of systems was remarkable for poor dental hygiene, and the patient was missing several teeth, which he said had been knocked out during the assault. Physical examination revealed diffuse tenderness about the right shoulder and severe pain with all passive movement. The shoulder was pseudoparalyzed. There were no subcutaneous collections, wounds, or ecchymosis about the shoulder. Mild calor was noted on the right shoulder relative to the left. Radiographs of the right shoulder showed no acute osseous abnormalities.
Magnetic resonance imaging (MRI), which was urgently obtained to assess the integrity of the rotator cuff and the location of the effusion, showed a large subacromial and glenohumeral joint effusion as well as diffuse muscular edema (Figures 1A-1C).
In light of the elevated infection findings of the laboratory tests and the positive culture, urgent arthroscopic irrigation and débridement of the right shoulder were indicated. Given the organism identified, transesophageal echocardiography was performed; there were no valvular vegetations. Creation of the posterior glenohumeral portal resulted in egress of turbid fluid, which was sent for culture. The subacromial space and the glenohumeral joint were thoroughly lavaged and the copious hemorrhagic synovitis débrided (Figures 2A, 2B).
The 8-week course of antibiotics normalized the patient’s ESR to 13 mm/h. Follow-up MRI showed improvement in the soft-tissue edema. Clinically, the patient reported minimal shoulder pain. He was undergoing physical therapy to regain strength and ROM.
Discussion
Staphylococcus aureus is the leading causative organism of septic arthritis, accounting for more than 60% of all cases.4 Conversely, the Streptococcus viridans group is rarely implicated in septic arthritis, accounting for <1% of cases.4S viridans is part of the commensal oral flora and has low virulence. This heterogeneous group is subdivided into S mitis, S salivarius, S anginosus, S mutans, and S bovis. The S mitis group is further subdivided into S sanguinis (formerly known as S sanguis) and S mitis. Infection by an organism of the S viridans group usually occurs on a previously injured focus, and the organism is a causative agent of bacterial endocarditis.5 Reported cases of septic arthritis caused by S viridans have predominantly involved the knee joint—with severe osteoarthritis, poor dental hygiene, and prior IV drug use identified as risk factors.5-7The shoulder joint is seldom involved in septic arthritis; estimated incidence is under 8%.8 Although overall incidence may rise in an increasingly elderly patient population, incidence of shoulder infection remains low.2,9
The main routes for developing septic arthritis include direct inoculation secondary to penetrating trauma or hematologic spread.10 Coatsworth and colleagues11 reported on iatrogenic S mitis septic arthritis of a shoulder arthroplasty during ultrasonography-guided aspiration by a technician who was not wearing a mask. Our institutional policy is to perform joint aspiration under strictly sterile conditions, which were adhered to in the present case. We surmise our patient developed transient bacteremia from the loss of several teeth, particularly given his poor dentition. Yombi and colleagues5 documented 2 cases of septic arthritis caused by Streptococcus gordonii, a relative of S sanguinis. One involved a previously replaced knee, and the other a native knee joint. Other cases of S viridans group septic arthritis have involved the knee,6,7,12,13 the sternoclavicular joint,14-16 and the acromioclavicular joint.17S sanguinis6,7,12,15,16 and S gordonii5 have been implicated in most cases, and an unspeciated S viridans in others.13,14,17 Concomitant periodontal disease has been reported in most cases as well,6,7,12,15 including our patient’s case. In the English-language literature, we found no other reports of S mitis as the causative agent of acute septic glenohumeral joint arthritis from hematogenous spread.
There should be no delay in diagnosing septic arthritis, and infected material should be removed from the joint. In animal models, complete joint destruction occurred only 5 weeks after inoculation with Staphylococcus aureus.10 Garofalo and colleagues18 reported a trend toward improved functional outcomes after earlier operative treatment. The choice of open surgical drainage vs repeat needle aspiration seems to be of little consequence, as both have good long-term outcomes, but open surgical drainage seems to result in better long-term functional ROM.2,9 However, results of a recent study suggested surgical treatment is not always superior to medical treatment for septic arthritis in native joints.19 In some cases involving S viridans species, treatment consisted of a combination of IV antibiotics and onetime or repeat aspiration;6,12-15 treatment in the remaining cases was surgical débridement.5,7,16,17 Given that S viridans is associated with bacterial endocarditis, echocardiography is essential if this organism is to be identified. Medical management and antibiotic treatment should be initiated after consultation with medical and infectious disease specialists.19We have reported a case of septic shoulder caused by S mitis, a low-virulence organism seldom associated with joint infection. The patient’s infection likely resulted from hematogenous spread from the oral cavity (dentition was poor). Urgent aspiration of the joint and baseline infection laboratory tests are recommended. MRI of the shoulder may show an effusion. Urgent arthroscopic irrigation and débridement can yield good clinical outcomes.
Am J Orthop. 2016;45(6):E343-E346. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Mathews CJ, Kingsley G, Field M, et al. Management of septic arthritis: a systematic review. Ann Rheum Dis. 2007;66(4):440-445.
2. Leslie BM, Harris JM 3rd, Driscoll D. Septic arthritis of the shoulder in adults. J Bone Joint Surg Am. 1989;71(10):1516-1522.
3. Gupta MN, Sturrock RD, Field M. A prospective 2-year study of 75 patients with adult-onset septic arthritis. Rheumatology. 2001;40(1):24-30.
4. Dubost JJ, Soubrier M, De Champs C, Ristori JM, Bussiere JL, Sauvezie B. No changes in the distribution of organisms responsible for septic arthritis over a 20 year period. Ann Rheum Dis. 2002;61(3):267-269.
5. Yombi J, Belkhir L, Jonckheere S, et al. Streptococcus gordonii septic arthritis: two cases and review of literature. BMC Infect Dis. 2012;12:215.
6. Papaioannides D, Boniatsi L, Korantzopoulos P, Sinapidis D, Giotis C. Acute septic arthritis due to Streptococcus sanguis. Med Princ Pract. 2006;15(1):77-79.
7. Edson RS, Osmon DR, Berry DJ. Septic arthritis due to Streptococcus sanguis. Mayo Clin Proc. 2002;77(7):709-710.
8. Weston VC, Jones AC, Bradbury N, Fawthrop F, Doherty M. Clinical features and outcome of septic arthritis in a single UK health district 1982-1991. Ann Rheum Dis. 1999;58(4):214-219.
9. Lossos IS, Yossepowitch O, Kandel L, Yardeni D, Arber N. Septic arthritis of the glenohumeral joint. A report of 11 cases and review of the literature. Medicine. 1998;77(3):177-187.
10. Esterhai JL Jr, Gelb I. Adult septic arthritis. Orthop Clin North Am. 1991;22(3):503-514.
11. Coatsworth NR, Huntington PG, Giuffre B, Kotsiou G. The doctor and the mask: iatrogenic septic arthritis caused by Streptoccocus mitis. Med J Aust. 2013;198(5):285-286.
12. Patrick MR, Lewis D. Short of a length: Streptococcus sanguis knee infection from dental source. Br J Rheumatol. 1992;31(8):569.
13. Barbadillo C, Trujillo A, Cuende E, Mazzucchelli R, Mulero J, Andreu JL. Septic arthritis due to Streptococcus viridans. Clin Exp Rheumatol. 1990;8(5):520-521.
14. Mata P, Molins A, de Oya M. Sternal arthritis caused by Streptococcus viridans in a heroin addict [in Spanish]. Med Clin. 1984;83(16):689.
15. Mandac I, Prkacin I, Sabljar Matovinovic M, Sustercic D. Septic arthritis due to Streptococcus sanguis. Coll Antropol. 2010;34(2):661-664.
16. Nitsche JF, Vaughan JH, Williams G, Curd JG. Septic sternoclavicular arthritis with Pasteurella multocida and Streptococcus sanguis. Arthritis Rheum. 1982;25(4):467-469.
17. Blankstein A, Amsallem JL, Rubenstein E, Horoszowski H, Farin I. Septic arthritis of the acromioclavicular joint. Arch Orthop Trauma Surg. 1985;103(6):417-418.
18. Garofalo R, Flanagin B, Cesari E, Vinci E, Conti M, Castagna A. Destructive septic arthritis of shoulder in adults. Musculoskelet Surg. 2014;98(supp 1):S35-S39.
19. Ravindran V, Logan I, Bourke BE. Medical vs surgical treatment for the native joint in septic arthritis: a 6-year, single UK academic centre experience. Rheumatology. 2009;48(10):1320-1322.
1. Mathews CJ, Kingsley G, Field M, et al. Management of septic arthritis: a systematic review. Ann Rheum Dis. 2007;66(4):440-445.
2. Leslie BM, Harris JM 3rd, Driscoll D. Septic arthritis of the shoulder in adults. J Bone Joint Surg Am. 1989;71(10):1516-1522.
3. Gupta MN, Sturrock RD, Field M. A prospective 2-year study of 75 patients with adult-onset septic arthritis. Rheumatology. 2001;40(1):24-30.
4. Dubost JJ, Soubrier M, De Champs C, Ristori JM, Bussiere JL, Sauvezie B. No changes in the distribution of organisms responsible for septic arthritis over a 20 year period. Ann Rheum Dis. 2002;61(3):267-269.
5. Yombi J, Belkhir L, Jonckheere S, et al. Streptococcus gordonii septic arthritis: two cases and review of literature. BMC Infect Dis. 2012;12:215.
6. Papaioannides D, Boniatsi L, Korantzopoulos P, Sinapidis D, Giotis C. Acute septic arthritis due to Streptococcus sanguis. Med Princ Pract. 2006;15(1):77-79.
7. Edson RS, Osmon DR, Berry DJ. Septic arthritis due to Streptococcus sanguis. Mayo Clin Proc. 2002;77(7):709-710.
8. Weston VC, Jones AC, Bradbury N, Fawthrop F, Doherty M. Clinical features and outcome of septic arthritis in a single UK health district 1982-1991. Ann Rheum Dis. 1999;58(4):214-219.
9. Lossos IS, Yossepowitch O, Kandel L, Yardeni D, Arber N. Septic arthritis of the glenohumeral joint. A report of 11 cases and review of the literature. Medicine. 1998;77(3):177-187.
10. Esterhai JL Jr, Gelb I. Adult septic arthritis. Orthop Clin North Am. 1991;22(3):503-514.
11. Coatsworth NR, Huntington PG, Giuffre B, Kotsiou G. The doctor and the mask: iatrogenic septic arthritis caused by Streptoccocus mitis. Med J Aust. 2013;198(5):285-286.
12. Patrick MR, Lewis D. Short of a length: Streptococcus sanguis knee infection from dental source. Br J Rheumatol. 1992;31(8):569.
13. Barbadillo C, Trujillo A, Cuende E, Mazzucchelli R, Mulero J, Andreu JL. Septic arthritis due to Streptococcus viridans. Clin Exp Rheumatol. 1990;8(5):520-521.
14. Mata P, Molins A, de Oya M. Sternal arthritis caused by Streptococcus viridans in a heroin addict [in Spanish]. Med Clin. 1984;83(16):689.
15. Mandac I, Prkacin I, Sabljar Matovinovic M, Sustercic D. Septic arthritis due to Streptococcus sanguis. Coll Antropol. 2010;34(2):661-664.
16. Nitsche JF, Vaughan JH, Williams G, Curd JG. Septic sternoclavicular arthritis with Pasteurella multocida and Streptococcus sanguis. Arthritis Rheum. 1982;25(4):467-469.
17. Blankstein A, Amsallem JL, Rubenstein E, Horoszowski H, Farin I. Septic arthritis of the acromioclavicular joint. Arch Orthop Trauma Surg. 1985;103(6):417-418.
18. Garofalo R, Flanagin B, Cesari E, Vinci E, Conti M, Castagna A. Destructive septic arthritis of shoulder in adults. Musculoskelet Surg. 2014;98(supp 1):S35-S39.
19. Ravindran V, Logan I, Bourke BE. Medical vs surgical treatment for the native joint in septic arthritis: a 6-year, single UK academic centre experience. Rheumatology. 2009;48(10):1320-1322.
Pain starting in knee later arises in other joints
People who develop knee pain associated with osteoarthritis often subsequently develop pain in other joints, according to a study of two observational, community-based cohorts that could not discern any pattern of new pain sites.
In the “first investigation of the association of knee pain with pain in multiple other sites,” David T. Felson, MD, of Boston University and his colleagues reported that the regions where pain developed after first appearing in the knee varied from person to person and occurred in both upper and lower extremities, which goes against the hypothesis that adjacent joints are most often affected by knee pain.
The study involved patients from the MOST (Multicenter Osteoarthritis Study) trial, including 281 with knee pain at the index visit (168 unilaterally) and 852 without, as well as patients from OAI (the Osteoarthritis Initiative), including 412 with knee pain at the index visit (241 unilaterally), and 1,941 without. The investigators assessed the patients’ data for 14 total joints outside of the knees: 2 each of feet, ankles, hips, hands, wrists, elbows, and shoulders (Arthritis Rheumatol. 2016 Sep 2. doi: 10.1002/art.39848).
Patients with new-onset knee pain at the index visit reported a mean of 2.3 painful joints outside the knee, compared with a significantly lower number of 1.3 reported by those without knee pain. The mean number of nonknee joints with pain was higher among patients with bilateral knee pain, compared with unilateral knee pain. The percentage of patients who reported pain outside the knee rose with the number of painful knees: 80% for two, 64% for one, and 50% for none.
The patients who developed new unilateral knee pain at the index visit also experienced an increase in prevalent joint pain in multiple joints in upper- and lower-extremity sites. In particular, the investigators noted that ipsilateral prevalent hip joint pain, which they characterized as pain in the groin or front of the thigh, was more than twice as likely to occur among those with new unilateral knee pain at the index visit, but the odds for contralateral hip joint pain did not reach statistical significance. The comparisons were adjusted for age, sex, body mass index, depression at the index visit, study (MOST or OAI), and count of painful upper and lower limb joints at the index visit (excluding knees).
When examining only patients with new-onset joint pain outside of the knee, the odds of patients with new knee pain to later develop new-onset joint pain outside the knee were 30% higher than for those without knee pain. Patients with new knee pain had a mean 2.6 new painful joints out of 12.1 eligible joints, compared with 2.0 new painful joints in those without knee pain out of 12.7 eligible joints. (Joint regions with prevalent symptoms at the index visit were excluded as incident painful sites.) Patients with knee pain also had a consistently higher rate of new-onset pain in nonknee joints when compared with patients without knee pain in at least half of the follow-up visits over the course of the MOST and OAI studies. Sensitivity analyses indicated that the association between knee pain and subsequent pain in other joints was not driven by the inclusion of patients with widespread pain.
“There was no clear-cut predilection for pain in any specific lower-extremity joint region,” the investigators wrote.
The investigators noted that other researchers have suggested that patients with knee pain may be at higher risk for lower-extremity joint pain because of changes to their gait that gradually cause damage to other joints, but evidence in this study doesn’t “necessarily support the argument that in persons with knee pain, aberrant loading by altered movement patterns induces pain in only nearby joints. Our findings suggest that the sites affected are more than just hip and ankle and that there is no special predilection for pain in these locations.”
While the investigators cannot differentiate underlying mechanisms for their study’s finding of multiple co-occurring sites of joint pain in people with new-onset knee pain, they suggested that it “supports either a predilection for osteoarthritic changes at multiple joint sites and/or raises the possibility that nervous system–driven pain sensitization increases the risk not only of widespread pain but even of regional pain. Since symptomatic OA is unusual in some of these painful sites (e.g., elbow, shoulder, ankle), pain sensitization would seem a more likely explanation.”
Some of the study’s limitations described by the investigators included the uncertainty surrounding whether new-onset knee pain was truly new onset or whether it was a reoccurrence, and also the fact that most of the people in the two cohorts had multiple sites of joint pain at both the baseline and the index visit and there were too few people with no sites of pain outside the knee to carry out subanalyses in that group, which “speaks to the high prevalence of multiple joint pains in older adult cohorts.”
The research was supported by grants from the National Institutes of Health. The authors had no disclosures to report.
People who develop knee pain associated with osteoarthritis often subsequently develop pain in other joints, according to a study of two observational, community-based cohorts that could not discern any pattern of new pain sites.
In the “first investigation of the association of knee pain with pain in multiple other sites,” David T. Felson, MD, of Boston University and his colleagues reported that the regions where pain developed after first appearing in the knee varied from person to person and occurred in both upper and lower extremities, which goes against the hypothesis that adjacent joints are most often affected by knee pain.
The study involved patients from the MOST (Multicenter Osteoarthritis Study) trial, including 281 with knee pain at the index visit (168 unilaterally) and 852 without, as well as patients from OAI (the Osteoarthritis Initiative), including 412 with knee pain at the index visit (241 unilaterally), and 1,941 without. The investigators assessed the patients’ data for 14 total joints outside of the knees: 2 each of feet, ankles, hips, hands, wrists, elbows, and shoulders (Arthritis Rheumatol. 2016 Sep 2. doi: 10.1002/art.39848).
Patients with new-onset knee pain at the index visit reported a mean of 2.3 painful joints outside the knee, compared with a significantly lower number of 1.3 reported by those without knee pain. The mean number of nonknee joints with pain was higher among patients with bilateral knee pain, compared with unilateral knee pain. The percentage of patients who reported pain outside the knee rose with the number of painful knees: 80% for two, 64% for one, and 50% for none.
The patients who developed new unilateral knee pain at the index visit also experienced an increase in prevalent joint pain in multiple joints in upper- and lower-extremity sites. In particular, the investigators noted that ipsilateral prevalent hip joint pain, which they characterized as pain in the groin or front of the thigh, was more than twice as likely to occur among those with new unilateral knee pain at the index visit, but the odds for contralateral hip joint pain did not reach statistical significance. The comparisons were adjusted for age, sex, body mass index, depression at the index visit, study (MOST or OAI), and count of painful upper and lower limb joints at the index visit (excluding knees).
When examining only patients with new-onset joint pain outside of the knee, the odds of patients with new knee pain to later develop new-onset joint pain outside the knee were 30% higher than for those without knee pain. Patients with new knee pain had a mean 2.6 new painful joints out of 12.1 eligible joints, compared with 2.0 new painful joints in those without knee pain out of 12.7 eligible joints. (Joint regions with prevalent symptoms at the index visit were excluded as incident painful sites.) Patients with knee pain also had a consistently higher rate of new-onset pain in nonknee joints when compared with patients without knee pain in at least half of the follow-up visits over the course of the MOST and OAI studies. Sensitivity analyses indicated that the association between knee pain and subsequent pain in other joints was not driven by the inclusion of patients with widespread pain.
“There was no clear-cut predilection for pain in any specific lower-extremity joint region,” the investigators wrote.
The investigators noted that other researchers have suggested that patients with knee pain may be at higher risk for lower-extremity joint pain because of changes to their gait that gradually cause damage to other joints, but evidence in this study doesn’t “necessarily support the argument that in persons with knee pain, aberrant loading by altered movement patterns induces pain in only nearby joints. Our findings suggest that the sites affected are more than just hip and ankle and that there is no special predilection for pain in these locations.”
While the investigators cannot differentiate underlying mechanisms for their study’s finding of multiple co-occurring sites of joint pain in people with new-onset knee pain, they suggested that it “supports either a predilection for osteoarthritic changes at multiple joint sites and/or raises the possibility that nervous system–driven pain sensitization increases the risk not only of widespread pain but even of regional pain. Since symptomatic OA is unusual in some of these painful sites (e.g., elbow, shoulder, ankle), pain sensitization would seem a more likely explanation.”
Some of the study’s limitations described by the investigators included the uncertainty surrounding whether new-onset knee pain was truly new onset or whether it was a reoccurrence, and also the fact that most of the people in the two cohorts had multiple sites of joint pain at both the baseline and the index visit and there were too few people with no sites of pain outside the knee to carry out subanalyses in that group, which “speaks to the high prevalence of multiple joint pains in older adult cohorts.”
The research was supported by grants from the National Institutes of Health. The authors had no disclosures to report.
People who develop knee pain associated with osteoarthritis often subsequently develop pain in other joints, according to a study of two observational, community-based cohorts that could not discern any pattern of new pain sites.
In the “first investigation of the association of knee pain with pain in multiple other sites,” David T. Felson, MD, of Boston University and his colleagues reported that the regions where pain developed after first appearing in the knee varied from person to person and occurred in both upper and lower extremities, which goes against the hypothesis that adjacent joints are most often affected by knee pain.
The study involved patients from the MOST (Multicenter Osteoarthritis Study) trial, including 281 with knee pain at the index visit (168 unilaterally) and 852 without, as well as patients from OAI (the Osteoarthritis Initiative), including 412 with knee pain at the index visit (241 unilaterally), and 1,941 without. The investigators assessed the patients’ data for 14 total joints outside of the knees: 2 each of feet, ankles, hips, hands, wrists, elbows, and shoulders (Arthritis Rheumatol. 2016 Sep 2. doi: 10.1002/art.39848).
Patients with new-onset knee pain at the index visit reported a mean of 2.3 painful joints outside the knee, compared with a significantly lower number of 1.3 reported by those without knee pain. The mean number of nonknee joints with pain was higher among patients with bilateral knee pain, compared with unilateral knee pain. The percentage of patients who reported pain outside the knee rose with the number of painful knees: 80% for two, 64% for one, and 50% for none.
The patients who developed new unilateral knee pain at the index visit also experienced an increase in prevalent joint pain in multiple joints in upper- and lower-extremity sites. In particular, the investigators noted that ipsilateral prevalent hip joint pain, which they characterized as pain in the groin or front of the thigh, was more than twice as likely to occur among those with new unilateral knee pain at the index visit, but the odds for contralateral hip joint pain did not reach statistical significance. The comparisons were adjusted for age, sex, body mass index, depression at the index visit, study (MOST or OAI), and count of painful upper and lower limb joints at the index visit (excluding knees).
When examining only patients with new-onset joint pain outside of the knee, the odds of patients with new knee pain to later develop new-onset joint pain outside the knee were 30% higher than for those without knee pain. Patients with new knee pain had a mean 2.6 new painful joints out of 12.1 eligible joints, compared with 2.0 new painful joints in those without knee pain out of 12.7 eligible joints. (Joint regions with prevalent symptoms at the index visit were excluded as incident painful sites.) Patients with knee pain also had a consistently higher rate of new-onset pain in nonknee joints when compared with patients without knee pain in at least half of the follow-up visits over the course of the MOST and OAI studies. Sensitivity analyses indicated that the association between knee pain and subsequent pain in other joints was not driven by the inclusion of patients with widespread pain.
“There was no clear-cut predilection for pain in any specific lower-extremity joint region,” the investigators wrote.
The investigators noted that other researchers have suggested that patients with knee pain may be at higher risk for lower-extremity joint pain because of changes to their gait that gradually cause damage to other joints, but evidence in this study doesn’t “necessarily support the argument that in persons with knee pain, aberrant loading by altered movement patterns induces pain in only nearby joints. Our findings suggest that the sites affected are more than just hip and ankle and that there is no special predilection for pain in these locations.”
While the investigators cannot differentiate underlying mechanisms for their study’s finding of multiple co-occurring sites of joint pain in people with new-onset knee pain, they suggested that it “supports either a predilection for osteoarthritic changes at multiple joint sites and/or raises the possibility that nervous system–driven pain sensitization increases the risk not only of widespread pain but even of regional pain. Since symptomatic OA is unusual in some of these painful sites (e.g., elbow, shoulder, ankle), pain sensitization would seem a more likely explanation.”
Some of the study’s limitations described by the investigators included the uncertainty surrounding whether new-onset knee pain was truly new onset or whether it was a reoccurrence, and also the fact that most of the people in the two cohorts had multiple sites of joint pain at both the baseline and the index visit and there were too few people with no sites of pain outside the knee to carry out subanalyses in that group, which “speaks to the high prevalence of multiple joint pains in older adult cohorts.”
The research was supported by grants from the National Institutes of Health. The authors had no disclosures to report.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point:People with frequently painful knees often develop pain in joints outside the knee, and the sites vary from person to person.
Major finding: The odds of patients with new knee pain to later develop joint pain outside the knee were 30% higher than for those without knee pain.
Data source: A study of 693 persons with index visit knee pain and 2,793 without it from two community-based cohorts.
Disclosures: The research was supported by grants from the National Institutes of Health. The authors had no disclosures to report.
High-Grade Articular, Bursal, and Intratendinous Partial-Thickness Rotator Cuff Tears: A Retrospective Study Comparing Functional Outcomes After Completion and Repair
The Ellman1 classification of partial-thickness rotator cuff tears (PTRCTs) is based on tear location or subtype (A, articular; B, bursal; C, intratendinous) and tear depth (grade 1, <3 mm; grade 2, 3-6 mm; grade 3, >6 mm). Ruotolo and colleagues2 reported that the medial-lateral insertion width of the supraspinatus averaged 12.1 mm, and most authors have indicated that tear depth of 6 mm or more represents 50% tendon thickness. Therefore, Ellman grade 3 tears are considered high-grade (>50% thickness).
Advancements in shoulder arthroscopy, imaging modalities, and clinical research have helped refine our understanding of PTRCTs. Classic teaching based on the retrospective study by Weber3 calls for simple débridement of low-grade (<50%) tears and repair of tears thicker than 50%. According to this standard, Ellman grade 1 and 2 tears should be débrided and grade 3 tears repaired. However, Cordasco and colleagues4 provided evidence supporting an algorithm reformation based on tear location. In their study, results of simple débridement were significantly worse for Ellman grade 2B PTRCTs than for 2A tears, suggesting low-grade bursal tears should also be repaired. Although their study supported a change in operative management for grade 2 tears, to our knowledge no one has investigated the need for differing surgical treatments for grade 3 subtypes based on tear location.
Several studies have demonstrated the efficacy of arthroscopic completion and repair for high-grade PTRCTs of the supraspinatus.5-7 Although all these studies addressed articular- and bursal-sided tears, there has been relative silence with respect to the intratendinous subtype. One explanation is that these tears, given their interstitial nature, pose diagnostic challenges. Histologic research has also shown that they can exist in combination with other tears.8 Despite such challenges, these tears are well documented. They were identified in the seminal study by Ellman1 and were the most common PTRCTs encountered in a well-known cadaveric study (N = 249).9,10 More recently, in 2011, a radiologic study using magnetic resonance arthrography found that 33.8% of PTRCTs were intratendinous (N = 68).11 That study also documented the case of a nonoperatively treated intratendinous tear that progressed to a full-thickness tear within about 6 months.11 Given these facts, it was important for the current PTRCT debate to include an intratendinous group when investigating treatment algorithms for grade 3 tears. Although results of the present study may continue reformation of the 50% algorithm, we hypothesized that arthroscopic completion and repair of all grade 3 PTRCTs will be equally effective, regardless of tear location.
Materials and Methods
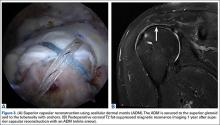
After obtaining Institutional Review Board approval for this study, we retrospectively reviewed the operative reports of a fellowship-trained shoulder surgeon for the period 2008–2010. Patients who underwent arthroscopic completion and repair of a supraspinatus tendon PTRCT were identified. Preoperative identification of PTRCT was made on the basis of physical examination and magnetic resonance imaging (MRI) findings (Figures 1–3).
Patients with low-grade PTRCTs of the supraspinatus, identified at time of arthroscopy, were excluded, as were patients with tears that extended into other rotator cuff tendons and patients with previous rotator cuff repair, glenohumeral instability, or adhesive capsulitis.
During the initial appointment, each patient completed a standard questionnaire that included standardized subjective scales evaluating pain and function. A fellowship-trained surgeon then took the patient’s history and performed a physical examination. Postoperative clinical outcome was determined at a minimum of 12 months. Clinical outcomes were assessed with 3 validated outcome measures: visual analog scale (VAS) score, American Shoulder and Elbow Surgeons (ASES) score, and Constant score.
Surgical Procedure and Rehabilitation
All procedures were performed with the patient under general anesthesia with or without an interscalene block. The patient was positioned in the upright beach-chair position. Diagnostic arthroscopy was used to assess the rotator cuff and associated pathologic conditions. If impingement was noted, subacromial decompression was performed. An acromioplasty was limited to removal of osteophytic bone. Distal clavicle excision and biceps tenotomy or tenodesis were performed if preoperative evaluation warranted these procedures.
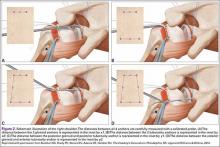
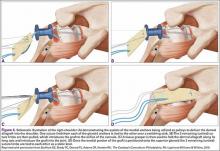
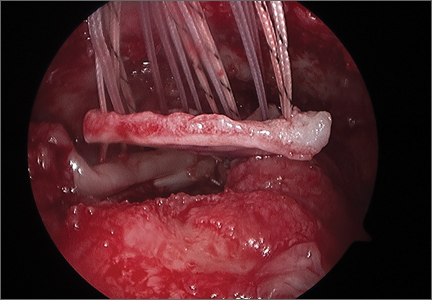
The rotator cuff was assessed from the articular and bursal sides. For articular PTRCTs, a tagging suture was used to identify the lesion from the bursal side. Bursal-sided tears were probed to assess thinning of the tendon and determine tear grade. If preoperative MRI findings suggested an intratendinous tear, a probe was used to confirm thinning of the tendon. An arthroscopic shaver was then carefully used to débride the capsule on either side of the tendon at the location of the suspected tear. The shaver inevitably penetrated the capsule and entered the tear, where any degenerative tissue was further débrided (Figure 4).
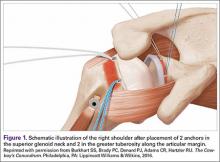
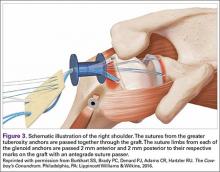
After the PTRCT was completed to full thickness, the rotator cuff footprint on the greater tuberosity was débrided to bleeding cortical bone. Depending on tear length, 1 or 2 Bio-Corkscrew absorbable suture anchors (Arthrex) with 2 No. 2 FiberWire sutures (Arthrex) were then placed in the tuberosity 3 to 5 mm lateral to the articular margin. An arthroscopic suture passer was used to move the 2 sutures through the rotator cuff, such that one was placed in the horizontal mattress and the other was placed in a simple fashion deep to the horizontal mattress. The sutures were then tied with a modified Roeder knot.
A standardized postoperative protocol was used for all patients starting within the first week after surgery. Passive range of motion (ROM) was performed for the first 6 weeks after surgery and was advanced to include active ROM from 6 to 8 weeks after surgery. Strengthening was initiated 8 weeks after surgery.
Statistical Analysis
Power analysis demonstrated that a sample size of 20 in each group was adequate for detecting a medium to large effect size with 80% power. Wilcoxon signed rank test was used to compare the preoperative and postoperative scores for each outcome measure, and analysis of variance (ANOVA) was used to compare the amount of improvement for each of the 3 PTRCT subtypes. Paired t test was used to compare preoperative and postoperative ROM values, and unpaired t tests were used to determine the impact of corticosteroid injections and preoperative PT. For statistical analysis, patients were divided into 2 groups (yes, no) regarding injections and 2 groups (yes, no) regarding PT. Last, multiple linear regression analyses were performed for each outcome measure to determine the impact of potential confounders. Covariates included symptom duration, etiology, age, injection, PT, tear location, percentage of tendon torn (medial-lateral), and tear length (anterior-posterior). P < .05 was considered significant.
Results
Patient Sample and Demographics
Sixty-seven patients underwent arthroscopic repair of a PTRCT—22 grade 3A, 23 grade 3B, and 22 grade 3C. In each of the 3 groups, 20 patients returned for end-of-healing evaluation. Thus, the study population consisted of 60 patients (60 shoulders). The 7 patients who did not return for end-of-healing evaluation or who could not be contacted were excluded from the study.
Table 1 summarizes the key patient demographics. Of the 60 patients, 35 were men and 25 were women.
Range of Motion
The sample as a whole exhibited statistically significant improvement in active ROM (Table 2).
Operative Findings
Operative findings included mean tear thickness of 74% for the sample as a whole and mean anterior-to-posterior tear length of 10.7 mm overall. There was very little variance among the articular, bursal, and intratendinous means with respect to percentage of tear thickness (78.3%, 75.0%, and 68.8%, respectively) and anterior-to-posterior tear thickness (11.5 mm, 11.4 mm, and 9.1 mm, respectively). Each of the 6 tears (3 bursal, 2 articular, 1 intratendinous) that were longer than 15 mm required 2 anchors. Fifty-nine repairs (98%) involved subacromial decompression, 38 (63%) involved acromioclavicular resection, 18 (30%) involved débridement of the superior labrum anterior-to-posterior (SLAP), and 12 (20%) involved biceps tenodesis/tenotomy.
Outcome Measures
In the study population as a whole, and in all 3 tear subtypes, postoperative improvement in VAS, ASES, and Constant scores was statistically significant (Table 3).
Multiple linear regression analyses showed that etiology, symptom duration, and steroid injection were the primary predictors of each outcome. After the other variables were adjusted for, injection (vs noninjection) seemed to be associated with more improvement in ASES (P = .0061), VAS (P = .020), and Constant (P = .067) scores. Insidious (vs traumatic) etiology was significantly associated with more improvement in ASES scores (P = .033) and VAS scores (P = .014) but not Constant scores (P = .50). Longer time from symptom onset to surgery was associated with less improvement, though the coefficient was not statistically significant in any of the models at P = .05. The other possible covariates had no significant impact on outcomes.
Complications
There were no intraoperative or postoperative complications, and there were no incidents of recurrent rotator cuff tear or postoperative stiffness.
Discussion
We investigated the effectiveness of arthroscopic completion and repair of Ellman grade 3 PTRCTs by comparing the functional outcomes for each subtype. Although several studies have analyzed results of PTRCT repair, they all either omitted intratendinous tears or were not grade-specific. In a systematic review, Strauss and colleagues13 discussed 4 PTRCT outcome studies4,6,14,15 in which only articular- and bursal-sided tears were addressed. Of these studies, only 1 (Kamath and colleagues6) focused on grade 3 lesions, and the number of bursal tears was insufficient for comparison with the articular tear group. Cordasco and colleagues4 limited their study to grade 1 and 2 tears but did not include intratendinous lesions.
In other research, Itoi and Tabata16 distinguished among the 3 subtypes but did not measure grade. As we did in our study, Deutsch5 focused on grade 3 lesions and used the completion-and-repair method, but he did not include intratendinous tears. Porat and colleagues17 reviewed grade 3 completion-and-repair results but did not compare them by subtype. Last, Uchiyama and colleagues18 reported strong outcomes for intratendinous tears but did not measure grade and used various surgical methods.
These studies have made important contributions to the ongoing PTRCT discussion, but debate about appropriate operative management persists. To limit the influence of external variables and provide the most exhaustive evidence regarding current PTRCT treatment algorithms, we designed the present study to consider outcomes with all 3 Ellman subtypes, only grade 3 lesions of the supraspinatus, only 1 surgical method, and consistent techniques of only 1 fellowship-trained shoulder surgeon.
Results of this chart review confirmed the findings of other grade 3 PTRCT repair studies. For instance, Koh and colleagues15 reported excellent results of 38 grade 3B PTRCTs completed to full thickness and repaired. Specifically, their mean ASES and Constant scores improved 34.1 and 23.7 points, respectively. These results are similar to our ASES and Constant score improvements—38.9 and 24.7 points for the group as a whole and 36 and 25.1 points for the grade 3B cohort. In addition, our ASES scores are nearly identical to the preoperative (46.1) and postoperative (82.1) ASES scores found by Kamath and colleagues.6 Although the mean ASES and VAS score improvements reported by Deutsch5 (51 and 5.7 points, respectively) were slightly better than ours, these results are still comparable and support completion and repair.
Although results of the study by Cordasco and colleagues4 support differing surgical treatments of grade 2 tears based on location, the present findings support the established 50% algorithm for all 3 high-grade PTRCTs. The completion-and-repair method not only produced significant improvements for each PTRCT subtype, but, importantly, there was no significant difference among those outcomes. Unlike previous results for grade 2 tears, the present results confirmed the established algorithm for grade 3 tears.
Our multiple linear regression analyses suggested that etiology, longer duration of symptoms, and steroid injections each had a strong impact on outcomes. The literature on these preoperative factors is often conflicting, and our results continue the trend. For instance, in a study of acute rotator cuff tears, Petersen and Murphy19 studied acute rotator cuff tears and also found tear size had no significant effect on functional outcomes. However, contrary to our findings, they did not find symptom duration to be a significant predictor of results. Also contrary to our findings, Oh and colleagues20 found age and tear size to be significant influences on outcomes for full-thickness tears. The strong correlation of preoperative steroid injection and better outcomes is novel and warrants further investigation.
In this study, we investigated the effectiveness of the completion-and-repair method in treating Ellman grade 3 PTRCTs. Although our findings validate this surgical technique, we acknowledge alternative approaches to high-grade PTRCTs. For instance, the transtendon method, which does not convert PTRCTs to full thickness, has also shown good clinical outcomes.21-23 In fact, the preoperative and postoperative VAS measures used in our study are nearly identical to those used in an Ellman grade 3A transtendon repair study.1 However, we agree with Porat and colleagues17 that the remaining, intact cuff material of PTRCTs is degenerative and may result in poor fixation, increased pain, or retear. In addition, nonoperative treatment typically is attempted before surgery, though little evidence is reported for success specifically in high-grade PTRCTs. One study found that 91% of PTRCT patients were still satisfied 4 years after nonoperative treatment, but it was noted that many of the tears were low-grade.24 To continue an evidence-based discussion on the more effective treatment, we invite advocates of alternative approaches to conduct a similar study on all 3 Ellman grade 3 subtypes.
Study Limitations
Concomitant procedures were not uniform among all patients and therefore may have affected some outcome measurements. Subacromial decompression was nearly universal, as it was performed for surgical visualization in 98% of patients. The additional procedures were also deemed necessary based on the preoperative assessment and arthroscopic findings. Although these procedures may have influenced outcome measurements, similar studies regularly include them as well.5-7,17 Our minimum 12-month follow-up could be considered a restriction, as other studies have cited a 2-year follow-up threshold.5-7 However, Strauss and colleagues13 endorsed a 12-month standard in their systematic review. Last, about 10% (7/67) of our initial patients were lost to follow-up; this percentage, however, is comparable to what has been reported in other PTRCT studies.4-6,14,15,21,22
Conclusion
Our study findings validate use of the current algorithm for Ellman grade 3 PTRCTs of the supraspinatus and advocate their completion and repair, regardless of tear location.
Acknowledgment: The authors thank Lisa Rein, MS, and Sergey Tarima, PhD, of the Division of Biostatistics, Medical College of Wisconsin, for their help in data analysis and manuscript preparation.
Am J Orthop. 2016;45(5):E254-E260. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Ellman H. Diagnosis and treatment of incomplete rotator cuff tears. Clin Orthop Relat Res. 1990;(254):64-74.
2. Ruotolo C, Fow JE, Nottage WM. The supraspinatus footprint: an anatomic study of the supraspinatus insertion. Arthroscopy. 2004;20(3):246-249.
3. Weber SC. Arthroscopic debridement and acromioplasty versus mini-open repair in the treatment of significant partial-thickness rotator cuff tears. Arthroscopy. 1999;15(2):126-131.
4. Cordasco FA, Backer M, Craig EV, Klein D, Warren RF. The partial-thickness rotator cuff tear: is acromioplasty without repair sufficient? Am J Sports Med. 2002;30(2):257-260.
5. Deutsch A. Arthroscopic repair of partial-thickness tears of the rotator cuff. J Shoulder Elbow Surg. 2007;16(2):193-201.
6. Kamath G, Galatz LM, Keener JD, Teefey S, Middleton W, Yamaguchi K. Tendon integrity and functional outcome after arthroscopic repair of high-grade partial-thickness supraspinatus tears. J Bone Joint Surg Am. 2009;91(5):1055-1062.
7. Park JY, Yoo MJ, Kim MH. Comparison of surgical outcome between bursal and articular partial thickness rotator cuff tears. Orthopedics. 2003;26(4):387-390.
8. Fukuda H, Hamada K, Nakajima T, Tomonaga A. Pathology and pathogenesis of the intratendinous tearing of the rotator cuff viewed from en bloc histologic sections. Clin Orthop Relat Res. 1994;(304):60-67.
9. Fukuda H, Mikasa M, Yamanaka K. Incomplete thickness rotator cuff tears diagnosed by subacromial bursography. Clin Orthop Relat Res. 1987;(223):51-58.
10. Yamanaka K, Fukuda H, Hamada K, Mikasa M. Incomplete thickness tears of the rotator cuff [abstract]. Orthop Surg Traumatol (Toyko). 1983;26:713.
11. Schaeffeler C, Mueller D, Kirchhoff C, Wolf P, Rummeny EJ, Woertler K. Tears at the rotator cuff footprint: prevalence and imaging characteristics in 305 MR arthrograms of the shoulder. Eur Radiol. 2011;21(7):1477-1484.
12. Nakagawa S, Yoneda M, Mizuno N, Hayashida K, Mae T, Take Y. Throwing shoulder injury involving the anterior rotator cuff: concealed tears not as uncommon as previously thought. Arthroscopy. 2006;22(12):1298-1303.
13. Strauss EJ, Salata MJ, Kercher J, et al. Multimedia article. The arthroscopic management of partial-thickness rotator cuff tears: a systematic review of the literature. Arthroscopy. 2011;27(4):568-580.
14. Kartus J, Kartus C, Rostgard-Christensen L, Sernert N, Read J, Perko M. Long-term clinical and ultrasound evaluation after arthroscopic acromioplasty in patients with partial rotator cuff tears. Arthroscopy. 2006;22(1):44-49.
15. Koh KH, Shon MS, Lim TK, Yoo JC. Clinical and magnetic resonance imaging results of arthroscopic full-layer repair of bursal-side partial-thickness rotator cuff tears. Am J Sports Med. 2011;39(8):1660-1667.
16. Itoi E, Tabata S. Incomplete rotator cuff tears. Results of operative treatment. Clin Orthop Relat Res. 1992;(284):128-135.
17. Porat S, Nottage WM, Fouse MN. Repair of partial thickness rotator cuff tears: a retrospective review with minimum two-year follow-up. J Shoulder Elbow Surg. 2008;17(5):729-731.
18. Uchiyama Y, Hamada K, Khruekarnchana P, et al. Surgical treatment of confirmed intratendinous rotator cuff tears: retrospective analysis after an average of eight years of follow-up. J Shoulder Elbow Surg. 2010;19(6):837-846.
19. Petersen SA, Murphy TP. The timing of rotator cuff repair for the restoration of function. J Shoulder Elbow Surg. 2011;20(1):62-68.
20. Oh JH, Kim SH, Ji HM, Jo KH, Bin SW, Gong HS. Prognostic factors affecting anatomic outcome of rotator cuff repair and correlation with functional outcome. Arthroscopy. 2009;25(1):30-39.
21. Castagna A, Delle Rose G, Conti M, Snyder SJ, Borroni M, Garofalo R. Predictive factors of subtle residual shoulder symptoms after transtendinous arthroscopic cuff repair: a clinical study. Am J Sports Med. 2009;37(1):103-108.
22. Castricini R, Panfoli N, Nittoli R, Spurio S, Pirani O. Transtendon arthroscopic repair of partial-thickness, articular surface tears of the supraspinatus: results at 2 years. Chir Organi Mov. 2009;93(suppl 1):S49-S54.
23. Spencer EE Jr. Partial-thickness articular surface rotator cuff tears: an all-inside repair technique. Clin Orthop Relat Res. 2010;468(6):1514-1520.
24. Denkers M, Pletsch K, Boorman R, Hollinshead R, Lo IKY. Partial thickness rotator cuff tears: observe or operative. In: Proceedings of the American Academy of Orthopaedic Surgeons Annual Meeting; February 2012; San Francisco, CA.
The Ellman1 classification of partial-thickness rotator cuff tears (PTRCTs) is based on tear location or subtype (A, articular; B, bursal; C, intratendinous) and tear depth (grade 1, <3 mm; grade 2, 3-6 mm; grade 3, >6 mm). Ruotolo and colleagues2 reported that the medial-lateral insertion width of the supraspinatus averaged 12.1 mm, and most authors have indicated that tear depth of 6 mm or more represents 50% tendon thickness. Therefore, Ellman grade 3 tears are considered high-grade (>50% thickness).
Advancements in shoulder arthroscopy, imaging modalities, and clinical research have helped refine our understanding of PTRCTs. Classic teaching based on the retrospective study by Weber3 calls for simple débridement of low-grade (<50%) tears and repair of tears thicker than 50%. According to this standard, Ellman grade 1 and 2 tears should be débrided and grade 3 tears repaired. However, Cordasco and colleagues4 provided evidence supporting an algorithm reformation based on tear location. In their study, results of simple débridement were significantly worse for Ellman grade 2B PTRCTs than for 2A tears, suggesting low-grade bursal tears should also be repaired. Although their study supported a change in operative management for grade 2 tears, to our knowledge no one has investigated the need for differing surgical treatments for grade 3 subtypes based on tear location.
Several studies have demonstrated the efficacy of arthroscopic completion and repair for high-grade PTRCTs of the supraspinatus.5-7 Although all these studies addressed articular- and bursal-sided tears, there has been relative silence with respect to the intratendinous subtype. One explanation is that these tears, given their interstitial nature, pose diagnostic challenges. Histologic research has also shown that they can exist in combination with other tears.8 Despite such challenges, these tears are well documented. They were identified in the seminal study by Ellman1 and were the most common PTRCTs encountered in a well-known cadaveric study (N = 249).9,10 More recently, in 2011, a radiologic study using magnetic resonance arthrography found that 33.8% of PTRCTs were intratendinous (N = 68).11 That study also documented the case of a nonoperatively treated intratendinous tear that progressed to a full-thickness tear within about 6 months.11 Given these facts, it was important for the current PTRCT debate to include an intratendinous group when investigating treatment algorithms for grade 3 tears. Although results of the present study may continue reformation of the 50% algorithm, we hypothesized that arthroscopic completion and repair of all grade 3 PTRCTs will be equally effective, regardless of tear location.
Materials and Methods
After obtaining Institutional Review Board approval for this study, we retrospectively reviewed the operative reports of a fellowship-trained shoulder surgeon for the period 2008–2010. Patients who underwent arthroscopic completion and repair of a supraspinatus tendon PTRCT were identified. Preoperative identification of PTRCT was made on the basis of physical examination and magnetic resonance imaging (MRI) findings (Figures 1–3).
Patients with low-grade PTRCTs of the supraspinatus, identified at time of arthroscopy, were excluded, as were patients with tears that extended into other rotator cuff tendons and patients with previous rotator cuff repair, glenohumeral instability, or adhesive capsulitis.
During the initial appointment, each patient completed a standard questionnaire that included standardized subjective scales evaluating pain and function. A fellowship-trained surgeon then took the patient’s history and performed a physical examination. Postoperative clinical outcome was determined at a minimum of 12 months. Clinical outcomes were assessed with 3 validated outcome measures: visual analog scale (VAS) score, American Shoulder and Elbow Surgeons (ASES) score, and Constant score.
Surgical Procedure and Rehabilitation
All procedures were performed with the patient under general anesthesia with or without an interscalene block. The patient was positioned in the upright beach-chair position. Diagnostic arthroscopy was used to assess the rotator cuff and associated pathologic conditions. If impingement was noted, subacromial decompression was performed. An acromioplasty was limited to removal of osteophytic bone. Distal clavicle excision and biceps tenotomy or tenodesis were performed if preoperative evaluation warranted these procedures.
The rotator cuff was assessed from the articular and bursal sides. For articular PTRCTs, a tagging suture was used to identify the lesion from the bursal side. Bursal-sided tears were probed to assess thinning of the tendon and determine tear grade. If preoperative MRI findings suggested an intratendinous tear, a probe was used to confirm thinning of the tendon. An arthroscopic shaver was then carefully used to débride the capsule on either side of the tendon at the location of the suspected tear. The shaver inevitably penetrated the capsule and entered the tear, where any degenerative tissue was further débrided (Figure 4).
After the PTRCT was completed to full thickness, the rotator cuff footprint on the greater tuberosity was débrided to bleeding cortical bone. Depending on tear length, 1 or 2 Bio-Corkscrew absorbable suture anchors (Arthrex) with 2 No. 2 FiberWire sutures (Arthrex) were then placed in the tuberosity 3 to 5 mm lateral to the articular margin. An arthroscopic suture passer was used to move the 2 sutures through the rotator cuff, such that one was placed in the horizontal mattress and the other was placed in a simple fashion deep to the horizontal mattress. The sutures were then tied with a modified Roeder knot.
A standardized postoperative protocol was used for all patients starting within the first week after surgery. Passive range of motion (ROM) was performed for the first 6 weeks after surgery and was advanced to include active ROM from 6 to 8 weeks after surgery. Strengthening was initiated 8 weeks after surgery.
Statistical Analysis
Power analysis demonstrated that a sample size of 20 in each group was adequate for detecting a medium to large effect size with 80% power. Wilcoxon signed rank test was used to compare the preoperative and postoperative scores for each outcome measure, and analysis of variance (ANOVA) was used to compare the amount of improvement for each of the 3 PTRCT subtypes. Paired t test was used to compare preoperative and postoperative ROM values, and unpaired t tests were used to determine the impact of corticosteroid injections and preoperative PT. For statistical analysis, patients were divided into 2 groups (yes, no) regarding injections and 2 groups (yes, no) regarding PT. Last, multiple linear regression analyses were performed for each outcome measure to determine the impact of potential confounders. Covariates included symptom duration, etiology, age, injection, PT, tear location, percentage of tendon torn (medial-lateral), and tear length (anterior-posterior). P < .05 was considered significant.
Results
Patient Sample and Demographics
Sixty-seven patients underwent arthroscopic repair of a PTRCT—22 grade 3A, 23 grade 3B, and 22 grade 3C. In each of the 3 groups, 20 patients returned for end-of-healing evaluation. Thus, the study population consisted of 60 patients (60 shoulders). The 7 patients who did not return for end-of-healing evaluation or who could not be contacted were excluded from the study.
Table 1 summarizes the key patient demographics. Of the 60 patients, 35 were men and 25 were women.
Range of Motion
The sample as a whole exhibited statistically significant improvement in active ROM (Table 2).
Operative Findings
Operative findings included mean tear thickness of 74% for the sample as a whole and mean anterior-to-posterior tear length of 10.7 mm overall. There was very little variance among the articular, bursal, and intratendinous means with respect to percentage of tear thickness (78.3%, 75.0%, and 68.8%, respectively) and anterior-to-posterior tear thickness (11.5 mm, 11.4 mm, and 9.1 mm, respectively). Each of the 6 tears (3 bursal, 2 articular, 1 intratendinous) that were longer than 15 mm required 2 anchors. Fifty-nine repairs (98%) involved subacromial decompression, 38 (63%) involved acromioclavicular resection, 18 (30%) involved débridement of the superior labrum anterior-to-posterior (SLAP), and 12 (20%) involved biceps tenodesis/tenotomy.
Outcome Measures
In the study population as a whole, and in all 3 tear subtypes, postoperative improvement in VAS, ASES, and Constant scores was statistically significant (Table 3).
Multiple linear regression analyses showed that etiology, symptom duration, and steroid injection were the primary predictors of each outcome. After the other variables were adjusted for, injection (vs noninjection) seemed to be associated with more improvement in ASES (P = .0061), VAS (P = .020), and Constant (P = .067) scores. Insidious (vs traumatic) etiology was significantly associated with more improvement in ASES scores (P = .033) and VAS scores (P = .014) but not Constant scores (P = .50). Longer time from symptom onset to surgery was associated with less improvement, though the coefficient was not statistically significant in any of the models at P = .05. The other possible covariates had no significant impact on outcomes.
Complications
There were no intraoperative or postoperative complications, and there were no incidents of recurrent rotator cuff tear or postoperative stiffness.
Discussion
We investigated the effectiveness of arthroscopic completion and repair of Ellman grade 3 PTRCTs by comparing the functional outcomes for each subtype. Although several studies have analyzed results of PTRCT repair, they all either omitted intratendinous tears or were not grade-specific. In a systematic review, Strauss and colleagues13 discussed 4 PTRCT outcome studies4,6,14,15 in which only articular- and bursal-sided tears were addressed. Of these studies, only 1 (Kamath and colleagues6) focused on grade 3 lesions, and the number of bursal tears was insufficient for comparison with the articular tear group. Cordasco and colleagues4 limited their study to grade 1 and 2 tears but did not include intratendinous lesions.
In other research, Itoi and Tabata16 distinguished among the 3 subtypes but did not measure grade. As we did in our study, Deutsch5 focused on grade 3 lesions and used the completion-and-repair method, but he did not include intratendinous tears. Porat and colleagues17 reviewed grade 3 completion-and-repair results but did not compare them by subtype. Last, Uchiyama and colleagues18 reported strong outcomes for intratendinous tears but did not measure grade and used various surgical methods.
These studies have made important contributions to the ongoing PTRCT discussion, but debate about appropriate operative management persists. To limit the influence of external variables and provide the most exhaustive evidence regarding current PTRCT treatment algorithms, we designed the present study to consider outcomes with all 3 Ellman subtypes, only grade 3 lesions of the supraspinatus, only 1 surgical method, and consistent techniques of only 1 fellowship-trained shoulder surgeon.
Results of this chart review confirmed the findings of other grade 3 PTRCT repair studies. For instance, Koh and colleagues15 reported excellent results of 38 grade 3B PTRCTs completed to full thickness and repaired. Specifically, their mean ASES and Constant scores improved 34.1 and 23.7 points, respectively. These results are similar to our ASES and Constant score improvements—38.9 and 24.7 points for the group as a whole and 36 and 25.1 points for the grade 3B cohort. In addition, our ASES scores are nearly identical to the preoperative (46.1) and postoperative (82.1) ASES scores found by Kamath and colleagues.6 Although the mean ASES and VAS score improvements reported by Deutsch5 (51 and 5.7 points, respectively) were slightly better than ours, these results are still comparable and support completion and repair.
Although results of the study by Cordasco and colleagues4 support differing surgical treatments of grade 2 tears based on location, the present findings support the established 50% algorithm for all 3 high-grade PTRCTs. The completion-and-repair method not only produced significant improvements for each PTRCT subtype, but, importantly, there was no significant difference among those outcomes. Unlike previous results for grade 2 tears, the present results confirmed the established algorithm for grade 3 tears.
Our multiple linear regression analyses suggested that etiology, longer duration of symptoms, and steroid injections each had a strong impact on outcomes. The literature on these preoperative factors is often conflicting, and our results continue the trend. For instance, in a study of acute rotator cuff tears, Petersen and Murphy19 studied acute rotator cuff tears and also found tear size had no significant effect on functional outcomes. However, contrary to our findings, they did not find symptom duration to be a significant predictor of results. Also contrary to our findings, Oh and colleagues20 found age and tear size to be significant influences on outcomes for full-thickness tears. The strong correlation of preoperative steroid injection and better outcomes is novel and warrants further investigation.
In this study, we investigated the effectiveness of the completion-and-repair method in treating Ellman grade 3 PTRCTs. Although our findings validate this surgical technique, we acknowledge alternative approaches to high-grade PTRCTs. For instance, the transtendon method, which does not convert PTRCTs to full thickness, has also shown good clinical outcomes.21-23 In fact, the preoperative and postoperative VAS measures used in our study are nearly identical to those used in an Ellman grade 3A transtendon repair study.1 However, we agree with Porat and colleagues17 that the remaining, intact cuff material of PTRCTs is degenerative and may result in poor fixation, increased pain, or retear. In addition, nonoperative treatment typically is attempted before surgery, though little evidence is reported for success specifically in high-grade PTRCTs. One study found that 91% of PTRCT patients were still satisfied 4 years after nonoperative treatment, but it was noted that many of the tears were low-grade.24 To continue an evidence-based discussion on the more effective treatment, we invite advocates of alternative approaches to conduct a similar study on all 3 Ellman grade 3 subtypes.
Study Limitations
Concomitant procedures were not uniform among all patients and therefore may have affected some outcome measurements. Subacromial decompression was nearly universal, as it was performed for surgical visualization in 98% of patients. The additional procedures were also deemed necessary based on the preoperative assessment and arthroscopic findings. Although these procedures may have influenced outcome measurements, similar studies regularly include them as well.5-7,17 Our minimum 12-month follow-up could be considered a restriction, as other studies have cited a 2-year follow-up threshold.5-7 However, Strauss and colleagues13 endorsed a 12-month standard in their systematic review. Last, about 10% (7/67) of our initial patients were lost to follow-up; this percentage, however, is comparable to what has been reported in other PTRCT studies.4-6,14,15,21,22
Conclusion
Our study findings validate use of the current algorithm for Ellman grade 3 PTRCTs of the supraspinatus and advocate their completion and repair, regardless of tear location.
Acknowledgment: The authors thank Lisa Rein, MS, and Sergey Tarima, PhD, of the Division of Biostatistics, Medical College of Wisconsin, for their help in data analysis and manuscript preparation.
Am J Orthop. 2016;45(5):E254-E260. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
The Ellman1 classification of partial-thickness rotator cuff tears (PTRCTs) is based on tear location or subtype (A, articular; B, bursal; C, intratendinous) and tear depth (grade 1, <3 mm; grade 2, 3-6 mm; grade 3, >6 mm). Ruotolo and colleagues2 reported that the medial-lateral insertion width of the supraspinatus averaged 12.1 mm, and most authors have indicated that tear depth of 6 mm or more represents 50% tendon thickness. Therefore, Ellman grade 3 tears are considered high-grade (>50% thickness).
Advancements in shoulder arthroscopy, imaging modalities, and clinical research have helped refine our understanding of PTRCTs. Classic teaching based on the retrospective study by Weber3 calls for simple débridement of low-grade (<50%) tears and repair of tears thicker than 50%. According to this standard, Ellman grade 1 and 2 tears should be débrided and grade 3 tears repaired. However, Cordasco and colleagues4 provided evidence supporting an algorithm reformation based on tear location. In their study, results of simple débridement were significantly worse for Ellman grade 2B PTRCTs than for 2A tears, suggesting low-grade bursal tears should also be repaired. Although their study supported a change in operative management for grade 2 tears, to our knowledge no one has investigated the need for differing surgical treatments for grade 3 subtypes based on tear location.
Several studies have demonstrated the efficacy of arthroscopic completion and repair for high-grade PTRCTs of the supraspinatus.5-7 Although all these studies addressed articular- and bursal-sided tears, there has been relative silence with respect to the intratendinous subtype. One explanation is that these tears, given their interstitial nature, pose diagnostic challenges. Histologic research has also shown that they can exist in combination with other tears.8 Despite such challenges, these tears are well documented. They were identified in the seminal study by Ellman1 and were the most common PTRCTs encountered in a well-known cadaveric study (N = 249).9,10 More recently, in 2011, a radiologic study using magnetic resonance arthrography found that 33.8% of PTRCTs were intratendinous (N = 68).11 That study also documented the case of a nonoperatively treated intratendinous tear that progressed to a full-thickness tear within about 6 months.11 Given these facts, it was important for the current PTRCT debate to include an intratendinous group when investigating treatment algorithms for grade 3 tears. Although results of the present study may continue reformation of the 50% algorithm, we hypothesized that arthroscopic completion and repair of all grade 3 PTRCTs will be equally effective, regardless of tear location.
Materials and Methods
After obtaining Institutional Review Board approval for this study, we retrospectively reviewed the operative reports of a fellowship-trained shoulder surgeon for the period 2008–2010. Patients who underwent arthroscopic completion and repair of a supraspinatus tendon PTRCT were identified. Preoperative identification of PTRCT was made on the basis of physical examination and magnetic resonance imaging (MRI) findings (Figures 1–3).
Patients with low-grade PTRCTs of the supraspinatus, identified at time of arthroscopy, were excluded, as were patients with tears that extended into other rotator cuff tendons and patients with previous rotator cuff repair, glenohumeral instability, or adhesive capsulitis.
During the initial appointment, each patient completed a standard questionnaire that included standardized subjective scales evaluating pain and function. A fellowship-trained surgeon then took the patient’s history and performed a physical examination. Postoperative clinical outcome was determined at a minimum of 12 months. Clinical outcomes were assessed with 3 validated outcome measures: visual analog scale (VAS) score, American Shoulder and Elbow Surgeons (ASES) score, and Constant score.
Surgical Procedure and Rehabilitation
All procedures were performed with the patient under general anesthesia with or without an interscalene block. The patient was positioned in the upright beach-chair position. Diagnostic arthroscopy was used to assess the rotator cuff and associated pathologic conditions. If impingement was noted, subacromial decompression was performed. An acromioplasty was limited to removal of osteophytic bone. Distal clavicle excision and biceps tenotomy or tenodesis were performed if preoperative evaluation warranted these procedures.
The rotator cuff was assessed from the articular and bursal sides. For articular PTRCTs, a tagging suture was used to identify the lesion from the bursal side. Bursal-sided tears were probed to assess thinning of the tendon and determine tear grade. If preoperative MRI findings suggested an intratendinous tear, a probe was used to confirm thinning of the tendon. An arthroscopic shaver was then carefully used to débride the capsule on either side of the tendon at the location of the suspected tear. The shaver inevitably penetrated the capsule and entered the tear, where any degenerative tissue was further débrided (Figure 4).
After the PTRCT was completed to full thickness, the rotator cuff footprint on the greater tuberosity was débrided to bleeding cortical bone. Depending on tear length, 1 or 2 Bio-Corkscrew absorbable suture anchors (Arthrex) with 2 No. 2 FiberWire sutures (Arthrex) were then placed in the tuberosity 3 to 5 mm lateral to the articular margin. An arthroscopic suture passer was used to move the 2 sutures through the rotator cuff, such that one was placed in the horizontal mattress and the other was placed in a simple fashion deep to the horizontal mattress. The sutures were then tied with a modified Roeder knot.
A standardized postoperative protocol was used for all patients starting within the first week after surgery. Passive range of motion (ROM) was performed for the first 6 weeks after surgery and was advanced to include active ROM from 6 to 8 weeks after surgery. Strengthening was initiated 8 weeks after surgery.
Statistical Analysis
Power analysis demonstrated that a sample size of 20 in each group was adequate for detecting a medium to large effect size with 80% power. Wilcoxon signed rank test was used to compare the preoperative and postoperative scores for each outcome measure, and analysis of variance (ANOVA) was used to compare the amount of improvement for each of the 3 PTRCT subtypes. Paired t test was used to compare preoperative and postoperative ROM values, and unpaired t tests were used to determine the impact of corticosteroid injections and preoperative PT. For statistical analysis, patients were divided into 2 groups (yes, no) regarding injections and 2 groups (yes, no) regarding PT. Last, multiple linear regression analyses were performed for each outcome measure to determine the impact of potential confounders. Covariates included symptom duration, etiology, age, injection, PT, tear location, percentage of tendon torn (medial-lateral), and tear length (anterior-posterior). P < .05 was considered significant.
Results
Patient Sample and Demographics
Sixty-seven patients underwent arthroscopic repair of a PTRCT—22 grade 3A, 23 grade 3B, and 22 grade 3C. In each of the 3 groups, 20 patients returned for end-of-healing evaluation. Thus, the study population consisted of 60 patients (60 shoulders). The 7 patients who did not return for end-of-healing evaluation or who could not be contacted were excluded from the study.
Table 1 summarizes the key patient demographics. Of the 60 patients, 35 were men and 25 were women.
Range of Motion
The sample as a whole exhibited statistically significant improvement in active ROM (Table 2).
Operative Findings
Operative findings included mean tear thickness of 74% for the sample as a whole and mean anterior-to-posterior tear length of 10.7 mm overall. There was very little variance among the articular, bursal, and intratendinous means with respect to percentage of tear thickness (78.3%, 75.0%, and 68.8%, respectively) and anterior-to-posterior tear thickness (11.5 mm, 11.4 mm, and 9.1 mm, respectively). Each of the 6 tears (3 bursal, 2 articular, 1 intratendinous) that were longer than 15 mm required 2 anchors. Fifty-nine repairs (98%) involved subacromial decompression, 38 (63%) involved acromioclavicular resection, 18 (30%) involved débridement of the superior labrum anterior-to-posterior (SLAP), and 12 (20%) involved biceps tenodesis/tenotomy.
Outcome Measures
In the study population as a whole, and in all 3 tear subtypes, postoperative improvement in VAS, ASES, and Constant scores was statistically significant (Table 3).
Multiple linear regression analyses showed that etiology, symptom duration, and steroid injection were the primary predictors of each outcome. After the other variables were adjusted for, injection (vs noninjection) seemed to be associated with more improvement in ASES (P = .0061), VAS (P = .020), and Constant (P = .067) scores. Insidious (vs traumatic) etiology was significantly associated with more improvement in ASES scores (P = .033) and VAS scores (P = .014) but not Constant scores (P = .50). Longer time from symptom onset to surgery was associated with less improvement, though the coefficient was not statistically significant in any of the models at P = .05. The other possible covariates had no significant impact on outcomes.
Complications
There were no intraoperative or postoperative complications, and there were no incidents of recurrent rotator cuff tear or postoperative stiffness.
Discussion
We investigated the effectiveness of arthroscopic completion and repair of Ellman grade 3 PTRCTs by comparing the functional outcomes for each subtype. Although several studies have analyzed results of PTRCT repair, they all either omitted intratendinous tears or were not grade-specific. In a systematic review, Strauss and colleagues13 discussed 4 PTRCT outcome studies4,6,14,15 in which only articular- and bursal-sided tears were addressed. Of these studies, only 1 (Kamath and colleagues6) focused on grade 3 lesions, and the number of bursal tears was insufficient for comparison with the articular tear group. Cordasco and colleagues4 limited their study to grade 1 and 2 tears but did not include intratendinous lesions.
In other research, Itoi and Tabata16 distinguished among the 3 subtypes but did not measure grade. As we did in our study, Deutsch5 focused on grade 3 lesions and used the completion-and-repair method, but he did not include intratendinous tears. Porat and colleagues17 reviewed grade 3 completion-and-repair results but did not compare them by subtype. Last, Uchiyama and colleagues18 reported strong outcomes for intratendinous tears but did not measure grade and used various surgical methods.
These studies have made important contributions to the ongoing PTRCT discussion, but debate about appropriate operative management persists. To limit the influence of external variables and provide the most exhaustive evidence regarding current PTRCT treatment algorithms, we designed the present study to consider outcomes with all 3 Ellman subtypes, only grade 3 lesions of the supraspinatus, only 1 surgical method, and consistent techniques of only 1 fellowship-trained shoulder surgeon.
Results of this chart review confirmed the findings of other grade 3 PTRCT repair studies. For instance, Koh and colleagues15 reported excellent results of 38 grade 3B PTRCTs completed to full thickness and repaired. Specifically, their mean ASES and Constant scores improved 34.1 and 23.7 points, respectively. These results are similar to our ASES and Constant score improvements—38.9 and 24.7 points for the group as a whole and 36 and 25.1 points for the grade 3B cohort. In addition, our ASES scores are nearly identical to the preoperative (46.1) and postoperative (82.1) ASES scores found by Kamath and colleagues.6 Although the mean ASES and VAS score improvements reported by Deutsch5 (51 and 5.7 points, respectively) were slightly better than ours, these results are still comparable and support completion and repair.
Although results of the study by Cordasco and colleagues4 support differing surgical treatments of grade 2 tears based on location, the present findings support the established 50% algorithm for all 3 high-grade PTRCTs. The completion-and-repair method not only produced significant improvements for each PTRCT subtype, but, importantly, there was no significant difference among those outcomes. Unlike previous results for grade 2 tears, the present results confirmed the established algorithm for grade 3 tears.
Our multiple linear regression analyses suggested that etiology, longer duration of symptoms, and steroid injections each had a strong impact on outcomes. The literature on these preoperative factors is often conflicting, and our results continue the trend. For instance, in a study of acute rotator cuff tears, Petersen and Murphy19 studied acute rotator cuff tears and also found tear size had no significant effect on functional outcomes. However, contrary to our findings, they did not find symptom duration to be a significant predictor of results. Also contrary to our findings, Oh and colleagues20 found age and tear size to be significant influences on outcomes for full-thickness tears. The strong correlation of preoperative steroid injection and better outcomes is novel and warrants further investigation.
In this study, we investigated the effectiveness of the completion-and-repair method in treating Ellman grade 3 PTRCTs. Although our findings validate this surgical technique, we acknowledge alternative approaches to high-grade PTRCTs. For instance, the transtendon method, which does not convert PTRCTs to full thickness, has also shown good clinical outcomes.21-23 In fact, the preoperative and postoperative VAS measures used in our study are nearly identical to those used in an Ellman grade 3A transtendon repair study.1 However, we agree with Porat and colleagues17 that the remaining, intact cuff material of PTRCTs is degenerative and may result in poor fixation, increased pain, or retear. In addition, nonoperative treatment typically is attempted before surgery, though little evidence is reported for success specifically in high-grade PTRCTs. One study found that 91% of PTRCT patients were still satisfied 4 years after nonoperative treatment, but it was noted that many of the tears were low-grade.24 To continue an evidence-based discussion on the more effective treatment, we invite advocates of alternative approaches to conduct a similar study on all 3 Ellman grade 3 subtypes.
Study Limitations
Concomitant procedures were not uniform among all patients and therefore may have affected some outcome measurements. Subacromial decompression was nearly universal, as it was performed for surgical visualization in 98% of patients. The additional procedures were also deemed necessary based on the preoperative assessment and arthroscopic findings. Although these procedures may have influenced outcome measurements, similar studies regularly include them as well.5-7,17 Our minimum 12-month follow-up could be considered a restriction, as other studies have cited a 2-year follow-up threshold.5-7 However, Strauss and colleagues13 endorsed a 12-month standard in their systematic review. Last, about 10% (7/67) of our initial patients were lost to follow-up; this percentage, however, is comparable to what has been reported in other PTRCT studies.4-6,14,15,21,22
Conclusion
Our study findings validate use of the current algorithm for Ellman grade 3 PTRCTs of the supraspinatus and advocate their completion and repair, regardless of tear location.
Acknowledgment: The authors thank Lisa Rein, MS, and Sergey Tarima, PhD, of the Division of Biostatistics, Medical College of Wisconsin, for their help in data analysis and manuscript preparation.
Am J Orthop. 2016;45(5):E254-E260. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Ellman H. Diagnosis and treatment of incomplete rotator cuff tears. Clin Orthop Relat Res. 1990;(254):64-74.
2. Ruotolo C, Fow JE, Nottage WM. The supraspinatus footprint: an anatomic study of the supraspinatus insertion. Arthroscopy. 2004;20(3):246-249.
3. Weber SC. Arthroscopic debridement and acromioplasty versus mini-open repair in the treatment of significant partial-thickness rotator cuff tears. Arthroscopy. 1999;15(2):126-131.
4. Cordasco FA, Backer M, Craig EV, Klein D, Warren RF. The partial-thickness rotator cuff tear: is acromioplasty without repair sufficient? Am J Sports Med. 2002;30(2):257-260.
5. Deutsch A. Arthroscopic repair of partial-thickness tears of the rotator cuff. J Shoulder Elbow Surg. 2007;16(2):193-201.
6. Kamath G, Galatz LM, Keener JD, Teefey S, Middleton W, Yamaguchi K. Tendon integrity and functional outcome after arthroscopic repair of high-grade partial-thickness supraspinatus tears. J Bone Joint Surg Am. 2009;91(5):1055-1062.
7. Park JY, Yoo MJ, Kim MH. Comparison of surgical outcome between bursal and articular partial thickness rotator cuff tears. Orthopedics. 2003;26(4):387-390.
8. Fukuda H, Hamada K, Nakajima T, Tomonaga A. Pathology and pathogenesis of the intratendinous tearing of the rotator cuff viewed from en bloc histologic sections. Clin Orthop Relat Res. 1994;(304):60-67.
9. Fukuda H, Mikasa M, Yamanaka K. Incomplete thickness rotator cuff tears diagnosed by subacromial bursography. Clin Orthop Relat Res. 1987;(223):51-58.
10. Yamanaka K, Fukuda H, Hamada K, Mikasa M. Incomplete thickness tears of the rotator cuff [abstract]. Orthop Surg Traumatol (Toyko). 1983;26:713.
11. Schaeffeler C, Mueller D, Kirchhoff C, Wolf P, Rummeny EJ, Woertler K. Tears at the rotator cuff footprint: prevalence and imaging characteristics in 305 MR arthrograms of the shoulder. Eur Radiol. 2011;21(7):1477-1484.
12. Nakagawa S, Yoneda M, Mizuno N, Hayashida K, Mae T, Take Y. Throwing shoulder injury involving the anterior rotator cuff: concealed tears not as uncommon as previously thought. Arthroscopy. 2006;22(12):1298-1303.
13. Strauss EJ, Salata MJ, Kercher J, et al. Multimedia article. The arthroscopic management of partial-thickness rotator cuff tears: a systematic review of the literature. Arthroscopy. 2011;27(4):568-580.
14. Kartus J, Kartus C, Rostgard-Christensen L, Sernert N, Read J, Perko M. Long-term clinical and ultrasound evaluation after arthroscopic acromioplasty in patients with partial rotator cuff tears. Arthroscopy. 2006;22(1):44-49.
15. Koh KH, Shon MS, Lim TK, Yoo JC. Clinical and magnetic resonance imaging results of arthroscopic full-layer repair of bursal-side partial-thickness rotator cuff tears. Am J Sports Med. 2011;39(8):1660-1667.
16. Itoi E, Tabata S. Incomplete rotator cuff tears. Results of operative treatment. Clin Orthop Relat Res. 1992;(284):128-135.
17. Porat S, Nottage WM, Fouse MN. Repair of partial thickness rotator cuff tears: a retrospective review with minimum two-year follow-up. J Shoulder Elbow Surg. 2008;17(5):729-731.
18. Uchiyama Y, Hamada K, Khruekarnchana P, et al. Surgical treatment of confirmed intratendinous rotator cuff tears: retrospective analysis after an average of eight years of follow-up. J Shoulder Elbow Surg. 2010;19(6):837-846.
19. Petersen SA, Murphy TP. The timing of rotator cuff repair for the restoration of function. J Shoulder Elbow Surg. 2011;20(1):62-68.
20. Oh JH, Kim SH, Ji HM, Jo KH, Bin SW, Gong HS. Prognostic factors affecting anatomic outcome of rotator cuff repair and correlation with functional outcome. Arthroscopy. 2009;25(1):30-39.
21. Castagna A, Delle Rose G, Conti M, Snyder SJ, Borroni M, Garofalo R. Predictive factors of subtle residual shoulder symptoms after transtendinous arthroscopic cuff repair: a clinical study. Am J Sports Med. 2009;37(1):103-108.
22. Castricini R, Panfoli N, Nittoli R, Spurio S, Pirani O. Transtendon arthroscopic repair of partial-thickness, articular surface tears of the supraspinatus: results at 2 years. Chir Organi Mov. 2009;93(suppl 1):S49-S54.
23. Spencer EE Jr. Partial-thickness articular surface rotator cuff tears: an all-inside repair technique. Clin Orthop Relat Res. 2010;468(6):1514-1520.
24. Denkers M, Pletsch K, Boorman R, Hollinshead R, Lo IKY. Partial thickness rotator cuff tears: observe or operative. In: Proceedings of the American Academy of Orthopaedic Surgeons Annual Meeting; February 2012; San Francisco, CA.
1. Ellman H. Diagnosis and treatment of incomplete rotator cuff tears. Clin Orthop Relat Res. 1990;(254):64-74.
2. Ruotolo C, Fow JE, Nottage WM. The supraspinatus footprint: an anatomic study of the supraspinatus insertion. Arthroscopy. 2004;20(3):246-249.
3. Weber SC. Arthroscopic debridement and acromioplasty versus mini-open repair in the treatment of significant partial-thickness rotator cuff tears. Arthroscopy. 1999;15(2):126-131.
4. Cordasco FA, Backer M, Craig EV, Klein D, Warren RF. The partial-thickness rotator cuff tear: is acromioplasty without repair sufficient? Am J Sports Med. 2002;30(2):257-260.
5. Deutsch A. Arthroscopic repair of partial-thickness tears of the rotator cuff. J Shoulder Elbow Surg. 2007;16(2):193-201.
6. Kamath G, Galatz LM, Keener JD, Teefey S, Middleton W, Yamaguchi K. Tendon integrity and functional outcome after arthroscopic repair of high-grade partial-thickness supraspinatus tears. J Bone Joint Surg Am. 2009;91(5):1055-1062.
7. Park JY, Yoo MJ, Kim MH. Comparison of surgical outcome between bursal and articular partial thickness rotator cuff tears. Orthopedics. 2003;26(4):387-390.
8. Fukuda H, Hamada K, Nakajima T, Tomonaga A. Pathology and pathogenesis of the intratendinous tearing of the rotator cuff viewed from en bloc histologic sections. Clin Orthop Relat Res. 1994;(304):60-67.
9. Fukuda H, Mikasa M, Yamanaka K. Incomplete thickness rotator cuff tears diagnosed by subacromial bursography. Clin Orthop Relat Res. 1987;(223):51-58.
10. Yamanaka K, Fukuda H, Hamada K, Mikasa M. Incomplete thickness tears of the rotator cuff [abstract]. Orthop Surg Traumatol (Toyko). 1983;26:713.
11. Schaeffeler C, Mueller D, Kirchhoff C, Wolf P, Rummeny EJ, Woertler K. Tears at the rotator cuff footprint: prevalence and imaging characteristics in 305 MR arthrograms of the shoulder. Eur Radiol. 2011;21(7):1477-1484.
12. Nakagawa S, Yoneda M, Mizuno N, Hayashida K, Mae T, Take Y. Throwing shoulder injury involving the anterior rotator cuff: concealed tears not as uncommon as previously thought. Arthroscopy. 2006;22(12):1298-1303.
13. Strauss EJ, Salata MJ, Kercher J, et al. Multimedia article. The arthroscopic management of partial-thickness rotator cuff tears: a systematic review of the literature. Arthroscopy. 2011;27(4):568-580.
14. Kartus J, Kartus C, Rostgard-Christensen L, Sernert N, Read J, Perko M. Long-term clinical and ultrasound evaluation after arthroscopic acromioplasty in patients with partial rotator cuff tears. Arthroscopy. 2006;22(1):44-49.
15. Koh KH, Shon MS, Lim TK, Yoo JC. Clinical and magnetic resonance imaging results of arthroscopic full-layer repair of bursal-side partial-thickness rotator cuff tears. Am J Sports Med. 2011;39(8):1660-1667.
16. Itoi E, Tabata S. Incomplete rotator cuff tears. Results of operative treatment. Clin Orthop Relat Res. 1992;(284):128-135.
17. Porat S, Nottage WM, Fouse MN. Repair of partial thickness rotator cuff tears: a retrospective review with minimum two-year follow-up. J Shoulder Elbow Surg. 2008;17(5):729-731.
18. Uchiyama Y, Hamada K, Khruekarnchana P, et al. Surgical treatment of confirmed intratendinous rotator cuff tears: retrospective analysis after an average of eight years of follow-up. J Shoulder Elbow Surg. 2010;19(6):837-846.
19. Petersen SA, Murphy TP. The timing of rotator cuff repair for the restoration of function. J Shoulder Elbow Surg. 2011;20(1):62-68.
20. Oh JH, Kim SH, Ji HM, Jo KH, Bin SW, Gong HS. Prognostic factors affecting anatomic outcome of rotator cuff repair and correlation with functional outcome. Arthroscopy. 2009;25(1):30-39.
21. Castagna A, Delle Rose G, Conti M, Snyder SJ, Borroni M, Garofalo R. Predictive factors of subtle residual shoulder symptoms after transtendinous arthroscopic cuff repair: a clinical study. Am J Sports Med. 2009;37(1):103-108.
22. Castricini R, Panfoli N, Nittoli R, Spurio S, Pirani O. Transtendon arthroscopic repair of partial-thickness, articular surface tears of the supraspinatus: results at 2 years. Chir Organi Mov. 2009;93(suppl 1):S49-S54.
23. Spencer EE Jr. Partial-thickness articular surface rotator cuff tears: an all-inside repair technique. Clin Orthop Relat Res. 2010;468(6):1514-1520.
24. Denkers M, Pletsch K, Boorman R, Hollinshead R, Lo IKY. Partial thickness rotator cuff tears: observe or operative. In: Proceedings of the American Academy of Orthopaedic Surgeons Annual Meeting; February 2012; San Francisco, CA.
Up in Arms: Bilateral Luxatio Erecta Fracture-Dislocations
Unilateral inferior shoulder dislocation (luxatio erecta) is uncommon, accounting for only 0.5% of all shoulder dislocations.1 Bilateral luxatio erecta is extremely rare, having been described fewer than 20 times in the literature. The most common etiology is hyperabduction causing the humerus to lever on the acromion; less common is axial loading onto a fully abducted arm and an extended elbow.2 Hyperabduction can occur when a person grabs an object in an attempt to stop a fall, as occurred in the present case. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 58-year-old man with a trauma injury presented to our emergency department. For his open right elbow fracture, emergency medical services had given him fentanyl en route, and when he arrived he was less responsive. As the patient reported, he had been on a scaffold 16 feet high when it began to give way. He jumped for another scaffold, 3 to 4 feet away, but came up short and, in an attempt to stop himself from falling, grabbed onto it with arms extended and above his head. His hands and arms were immediately pulled up in full extension. When both shoulders became dislocated, he could not hold on and fell to the ground, landing on a buttock. He did not lose consciousness.
Physical examination revealed both arms abducted at the shoulder, and elbows extended (Figure 1).
Radiographs confirmed the diagnosis and showed bilateral nondisplaced proximal humeral fractures of the greater tuberosity (Figure 2).
For the shoulder reductions, we administered propofol for conscious sedation and fentanyl for analgesia. Then, a sheet was wrapped supraclavicular and pulled across the torso inferiorly to allow countertraction when pulling the arm superiorly on the axial line. Another countertraction sheet was placed on the opposite side. For each arm, the countertraction was pulled inferiorly when the arm was pulled superiorly, both on the longitudinal plane. The arm was then gently rotated in adduction until reduction was achieved.
The right shoulder reduced relatively easily. The left shoulder reduced into an anterior dislocation—a relatively uncommon outcome in in-line traction attempts.3 (Reduction into anterior dislocation can also be a desired result in a specific technique of 2-step reduction, as described by Nho and colleagues.4) The patient’s anterior dislocation was then easily reduced into anatomical position with use of the Kocher technique of arm adduction with elbow flexion, followed by external rotation, and then finally into anatomical position with internal rotation.5 Both arms were then immobilized in full adduction with bilateral slings. The patient was admitted for further treatment of multiple fractures of the arms and vertebrae.
He was discharged in bilateral shoulder slings to an extended-care facility for physical therapy. One month after discharge, he could not elevate his arms and had minimal use of them. Two weeks later, magnetic resonance imaging showed a “comminuted greater tuberosity fracture with new displacement of fragments involving the attachment of the supraspinatus and infraspinatus; posterior subluxation of the glenohumeral joint with evidence of posterior and anterior labral tears; and large glenohumeral joint effusion.” The patient opted for surgical repair and underwent left shoulder arthroscopy with extensive débridement, open rotator cuff repair, open greater tuberosity reduction and internal fixation, and open biceps tenodesis. He was then discharged back to an extended-care facility to continue rehabilitation. One and a half months after surgery, he started the physical therapy phase of the massive rotator cuff repair protocol. He declined reverse total shoulder arthroplasty (RTSA).
Four and a half months after injury (3 months after surgery), the left shoulder demonstrated 20° of flexion and 70° to 110° of abduction (external rotation not tested), and the right shoulder demonstrated 30° of flexion and 70° to 110° of abduction (external rotation not tested). He had no instability and no lag with good external rotation.
Six months after injury, the patient still could not lift his arms above his head. He likely would not be able to do so without RTSA, which he again declined. He continued physical therapy and clinical follow-ups.
Discussion
Although inferior shoulder dislocations are rare, they carry a higher rate of complications, most of which our patient experienced. Our patient had bilateral humeral head fractures, which occur in 80% of cases.6 Postreduction CT showed the degree of his fractures (Figure 3).
Our patient also had reduced sensation in the axillary nerve distribution, which occurs in 60% of inferior dislocations.6 Axillary nerve injuries produce numbness in the lateral arm or posterior shoulder and weakness with shoulder flexion, abduction, and external rotation.7 In our patient’s case, sensation returned after reduction, which is typical (most patients have a positive prognosis).8 As the shoulder dislocates inferiorly, the humeral head tears the glenohumeral capsule inferiorly, which can damage the axillary artery. This artery becomes the brachial and eventually the radial and ulnar arteries, which can have decreased or absent pulses with injury.
Inferior dislocations are also associated with abundant soft-tissue injuries, including torn rotator cuff, shoulder capsule avulsion, and disruption of adjacent muscles (supraspinatus, infraspinatus, teres minor, subscapularis, pectoralis major).9Luxatio erecta is relatively easy to diagnose given the unmistakable arm positioning. The key for the physician is first to assess for the many possible complications, then to administer the proper sedation and analgesia for reduction, and finally to reassess for complications.
Am J Orthop. 2016;45(6):E328-E330. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Camarda L, Martorana U, D’Arienzo M. A case of bilateral luxatio erecta. J Orthop Traumatol. 2009;10(2):97-99.
2. Musmeci E, Gaspari D, Sandri A, Regis D, Bartolozzi P. Bilateral luxatio erecta humeri associated with a unilateral brachial plexus and bilateral rotator cuff injuries: a case report. J Orthop Trauma. 2008;22(7):498-500.
3. Lam AC, Shih RD. Luxatio erecta complicated by anterior shoulder dislocation during reduction. West J Emerg Med. 2010;11(1):28-30.
4. Nho SJ, Dodson CC, Bardzik KF, Brophy RH, Domb BG, MacGillivray JD. The two-step maneuver for closed reduction of inferior glenohumeral dislocation (luxatio erecta to anterior dislocation to reduction). J Orthop Trauma. 2006;20(5):354-357.
5. Beattie TF, Steedman DJ, McGowan A, Robertson CE. A comparison of the Milch and Kocher techniques for acute anterior dislocation of the shoulder. Injury. 1986;17(5):349-352.
6. Mallon WJ, Bassett FH 3rd, Goldner RD. Luxatio erecta: the inferior glenohumeral dislocation. J Orthop Trauma. 1990;4(1):19-24.
7. Miller T. Peripheral nerve injuries at the shoulder. J Manipulative Physiol Ther. 1998;6(4):170-183.
8. Groh GI, Wirth MA, Rockwood CA Jr. Results of treatment of luxatio erecta (inferior shoulder dislocation). J Shoulder Elbow Surg. 2010;19(3):423-426.
9. Garcia R, Ponsky T, Brody F, Long J. Bilateral luxatio erecta complicated by venous thrombosis. J Trauma. 2006;60(5):1132-1134.
Unilateral inferior shoulder dislocation (luxatio erecta) is uncommon, accounting for only 0.5% of all shoulder dislocations.1 Bilateral luxatio erecta is extremely rare, having been described fewer than 20 times in the literature. The most common etiology is hyperabduction causing the humerus to lever on the acromion; less common is axial loading onto a fully abducted arm and an extended elbow.2 Hyperabduction can occur when a person grabs an object in an attempt to stop a fall, as occurred in the present case. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 58-year-old man with a trauma injury presented to our emergency department. For his open right elbow fracture, emergency medical services had given him fentanyl en route, and when he arrived he was less responsive. As the patient reported, he had been on a scaffold 16 feet high when it began to give way. He jumped for another scaffold, 3 to 4 feet away, but came up short and, in an attempt to stop himself from falling, grabbed onto it with arms extended and above his head. His hands and arms were immediately pulled up in full extension. When both shoulders became dislocated, he could not hold on and fell to the ground, landing on a buttock. He did not lose consciousness.
Physical examination revealed both arms abducted at the shoulder, and elbows extended (Figure 1).
Radiographs confirmed the diagnosis and showed bilateral nondisplaced proximal humeral fractures of the greater tuberosity (Figure 2).
For the shoulder reductions, we administered propofol for conscious sedation and fentanyl for analgesia. Then, a sheet was wrapped supraclavicular and pulled across the torso inferiorly to allow countertraction when pulling the arm superiorly on the axial line. Another countertraction sheet was placed on the opposite side. For each arm, the countertraction was pulled inferiorly when the arm was pulled superiorly, both on the longitudinal plane. The arm was then gently rotated in adduction until reduction was achieved.
The right shoulder reduced relatively easily. The left shoulder reduced into an anterior dislocation—a relatively uncommon outcome in in-line traction attempts.3 (Reduction into anterior dislocation can also be a desired result in a specific technique of 2-step reduction, as described by Nho and colleagues.4) The patient’s anterior dislocation was then easily reduced into anatomical position with use of the Kocher technique of arm adduction with elbow flexion, followed by external rotation, and then finally into anatomical position with internal rotation.5 Both arms were then immobilized in full adduction with bilateral slings. The patient was admitted for further treatment of multiple fractures of the arms and vertebrae.
He was discharged in bilateral shoulder slings to an extended-care facility for physical therapy. One month after discharge, he could not elevate his arms and had minimal use of them. Two weeks later, magnetic resonance imaging showed a “comminuted greater tuberosity fracture with new displacement of fragments involving the attachment of the supraspinatus and infraspinatus; posterior subluxation of the glenohumeral joint with evidence of posterior and anterior labral tears; and large glenohumeral joint effusion.” The patient opted for surgical repair and underwent left shoulder arthroscopy with extensive débridement, open rotator cuff repair, open greater tuberosity reduction and internal fixation, and open biceps tenodesis. He was then discharged back to an extended-care facility to continue rehabilitation. One and a half months after surgery, he started the physical therapy phase of the massive rotator cuff repair protocol. He declined reverse total shoulder arthroplasty (RTSA).
Four and a half months after injury (3 months after surgery), the left shoulder demonstrated 20° of flexion and 70° to 110° of abduction (external rotation not tested), and the right shoulder demonstrated 30° of flexion and 70° to 110° of abduction (external rotation not tested). He had no instability and no lag with good external rotation.
Six months after injury, the patient still could not lift his arms above his head. He likely would not be able to do so without RTSA, which he again declined. He continued physical therapy and clinical follow-ups.
Discussion
Although inferior shoulder dislocations are rare, they carry a higher rate of complications, most of which our patient experienced. Our patient had bilateral humeral head fractures, which occur in 80% of cases.6 Postreduction CT showed the degree of his fractures (Figure 3).
Our patient also had reduced sensation in the axillary nerve distribution, which occurs in 60% of inferior dislocations.6 Axillary nerve injuries produce numbness in the lateral arm or posterior shoulder and weakness with shoulder flexion, abduction, and external rotation.7 In our patient’s case, sensation returned after reduction, which is typical (most patients have a positive prognosis).8 As the shoulder dislocates inferiorly, the humeral head tears the glenohumeral capsule inferiorly, which can damage the axillary artery. This artery becomes the brachial and eventually the radial and ulnar arteries, which can have decreased or absent pulses with injury.
Inferior dislocations are also associated with abundant soft-tissue injuries, including torn rotator cuff, shoulder capsule avulsion, and disruption of adjacent muscles (supraspinatus, infraspinatus, teres minor, subscapularis, pectoralis major).9Luxatio erecta is relatively easy to diagnose given the unmistakable arm positioning. The key for the physician is first to assess for the many possible complications, then to administer the proper sedation and analgesia for reduction, and finally to reassess for complications.
Am J Orthop. 2016;45(6):E328-E330. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Unilateral inferior shoulder dislocation (luxatio erecta) is uncommon, accounting for only 0.5% of all shoulder dislocations.1 Bilateral luxatio erecta is extremely rare, having been described fewer than 20 times in the literature. The most common etiology is hyperabduction causing the humerus to lever on the acromion; less common is axial loading onto a fully abducted arm and an extended elbow.2 Hyperabduction can occur when a person grabs an object in an attempt to stop a fall, as occurred in the present case. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 58-year-old man with a trauma injury presented to our emergency department. For his open right elbow fracture, emergency medical services had given him fentanyl en route, and when he arrived he was less responsive. As the patient reported, he had been on a scaffold 16 feet high when it began to give way. He jumped for another scaffold, 3 to 4 feet away, but came up short and, in an attempt to stop himself from falling, grabbed onto it with arms extended and above his head. His hands and arms were immediately pulled up in full extension. When both shoulders became dislocated, he could not hold on and fell to the ground, landing on a buttock. He did not lose consciousness.
Physical examination revealed both arms abducted at the shoulder, and elbows extended (Figure 1).
Radiographs confirmed the diagnosis and showed bilateral nondisplaced proximal humeral fractures of the greater tuberosity (Figure 2).
For the shoulder reductions, we administered propofol for conscious sedation and fentanyl for analgesia. Then, a sheet was wrapped supraclavicular and pulled across the torso inferiorly to allow countertraction when pulling the arm superiorly on the axial line. Another countertraction sheet was placed on the opposite side. For each arm, the countertraction was pulled inferiorly when the arm was pulled superiorly, both on the longitudinal plane. The arm was then gently rotated in adduction until reduction was achieved.
The right shoulder reduced relatively easily. The left shoulder reduced into an anterior dislocation—a relatively uncommon outcome in in-line traction attempts.3 (Reduction into anterior dislocation can also be a desired result in a specific technique of 2-step reduction, as described by Nho and colleagues.4) The patient’s anterior dislocation was then easily reduced into anatomical position with use of the Kocher technique of arm adduction with elbow flexion, followed by external rotation, and then finally into anatomical position with internal rotation.5 Both arms were then immobilized in full adduction with bilateral slings. The patient was admitted for further treatment of multiple fractures of the arms and vertebrae.
He was discharged in bilateral shoulder slings to an extended-care facility for physical therapy. One month after discharge, he could not elevate his arms and had minimal use of them. Two weeks later, magnetic resonance imaging showed a “comminuted greater tuberosity fracture with new displacement of fragments involving the attachment of the supraspinatus and infraspinatus; posterior subluxation of the glenohumeral joint with evidence of posterior and anterior labral tears; and large glenohumeral joint effusion.” The patient opted for surgical repair and underwent left shoulder arthroscopy with extensive débridement, open rotator cuff repair, open greater tuberosity reduction and internal fixation, and open biceps tenodesis. He was then discharged back to an extended-care facility to continue rehabilitation. One and a half months after surgery, he started the physical therapy phase of the massive rotator cuff repair protocol. He declined reverse total shoulder arthroplasty (RTSA).
Four and a half months after injury (3 months after surgery), the left shoulder demonstrated 20° of flexion and 70° to 110° of abduction (external rotation not tested), and the right shoulder demonstrated 30° of flexion and 70° to 110° of abduction (external rotation not tested). He had no instability and no lag with good external rotation.
Six months after injury, the patient still could not lift his arms above his head. He likely would not be able to do so without RTSA, which he again declined. He continued physical therapy and clinical follow-ups.
Discussion
Although inferior shoulder dislocations are rare, they carry a higher rate of complications, most of which our patient experienced. Our patient had bilateral humeral head fractures, which occur in 80% of cases.6 Postreduction CT showed the degree of his fractures (Figure 3).
Our patient also had reduced sensation in the axillary nerve distribution, which occurs in 60% of inferior dislocations.6 Axillary nerve injuries produce numbness in the lateral arm or posterior shoulder and weakness with shoulder flexion, abduction, and external rotation.7 In our patient’s case, sensation returned after reduction, which is typical (most patients have a positive prognosis).8 As the shoulder dislocates inferiorly, the humeral head tears the glenohumeral capsule inferiorly, which can damage the axillary artery. This artery becomes the brachial and eventually the radial and ulnar arteries, which can have decreased or absent pulses with injury.
Inferior dislocations are also associated with abundant soft-tissue injuries, including torn rotator cuff, shoulder capsule avulsion, and disruption of adjacent muscles (supraspinatus, infraspinatus, teres minor, subscapularis, pectoralis major).9Luxatio erecta is relatively easy to diagnose given the unmistakable arm positioning. The key for the physician is first to assess for the many possible complications, then to administer the proper sedation and analgesia for reduction, and finally to reassess for complications.
Am J Orthop. 2016;45(6):E328-E330. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Camarda L, Martorana U, D’Arienzo M. A case of bilateral luxatio erecta. J Orthop Traumatol. 2009;10(2):97-99.
2. Musmeci E, Gaspari D, Sandri A, Regis D, Bartolozzi P. Bilateral luxatio erecta humeri associated with a unilateral brachial plexus and bilateral rotator cuff injuries: a case report. J Orthop Trauma. 2008;22(7):498-500.
3. Lam AC, Shih RD. Luxatio erecta complicated by anterior shoulder dislocation during reduction. West J Emerg Med. 2010;11(1):28-30.
4. Nho SJ, Dodson CC, Bardzik KF, Brophy RH, Domb BG, MacGillivray JD. The two-step maneuver for closed reduction of inferior glenohumeral dislocation (luxatio erecta to anterior dislocation to reduction). J Orthop Trauma. 2006;20(5):354-357.
5. Beattie TF, Steedman DJ, McGowan A, Robertson CE. A comparison of the Milch and Kocher techniques for acute anterior dislocation of the shoulder. Injury. 1986;17(5):349-352.
6. Mallon WJ, Bassett FH 3rd, Goldner RD. Luxatio erecta: the inferior glenohumeral dislocation. J Orthop Trauma. 1990;4(1):19-24.
7. Miller T. Peripheral nerve injuries at the shoulder. J Manipulative Physiol Ther. 1998;6(4):170-183.
8. Groh GI, Wirth MA, Rockwood CA Jr. Results of treatment of luxatio erecta (inferior shoulder dislocation). J Shoulder Elbow Surg. 2010;19(3):423-426.
9. Garcia R, Ponsky T, Brody F, Long J. Bilateral luxatio erecta complicated by venous thrombosis. J Trauma. 2006;60(5):1132-1134.
1. Camarda L, Martorana U, D’Arienzo M. A case of bilateral luxatio erecta. J Orthop Traumatol. 2009;10(2):97-99.
2. Musmeci E, Gaspari D, Sandri A, Regis D, Bartolozzi P. Bilateral luxatio erecta humeri associated with a unilateral brachial plexus and bilateral rotator cuff injuries: a case report. J Orthop Trauma. 2008;22(7):498-500.
3. Lam AC, Shih RD. Luxatio erecta complicated by anterior shoulder dislocation during reduction. West J Emerg Med. 2010;11(1):28-30.
4. Nho SJ, Dodson CC, Bardzik KF, Brophy RH, Domb BG, MacGillivray JD. The two-step maneuver for closed reduction of inferior glenohumeral dislocation (luxatio erecta to anterior dislocation to reduction). J Orthop Trauma. 2006;20(5):354-357.
5. Beattie TF, Steedman DJ, McGowan A, Robertson CE. A comparison of the Milch and Kocher techniques for acute anterior dislocation of the shoulder. Injury. 1986;17(5):349-352.
6. Mallon WJ, Bassett FH 3rd, Goldner RD. Luxatio erecta: the inferior glenohumeral dislocation. J Orthop Trauma. 1990;4(1):19-24.
7. Miller T. Peripheral nerve injuries at the shoulder. J Manipulative Physiol Ther. 1998;6(4):170-183.
8. Groh GI, Wirth MA, Rockwood CA Jr. Results of treatment of luxatio erecta (inferior shoulder dislocation). J Shoulder Elbow Surg. 2010;19(3):423-426.
9. Garcia R, Ponsky T, Brody F, Long J. Bilateral luxatio erecta complicated by venous thrombosis. J Trauma. 2006;60(5):1132-1134.
Quality and Quantity of the Elbow Arthroscopy Literature: A Systematic Review and Meta-Analysis
Although elbow arthroscopy was first described in the 1930s, it has become increasingly popular in the last 30 years.1 While initially considered as a tool for diagnosis and loose body removal, indications have expanded to include treatment of osteochondritis dissecans (OCD), treatment of lateral epicondylitis, fixation of fractures, and others.2-5 Miyake and colleagues6 found a significant improvement in range of motion, both flexion and extension, and outcome scores when elbow arthroscopy was used to remove impinging osteophytes. Babaqi and colleagues7 found significant improvement in pain, satisfaction, and outcome scores in 31 patients who underwent elbow arthroscopy for lateral epicondylitis refractory to nonsurgical management. The technical difficulty of the procedure, lower frequency of pathology amenable to arthroscopic intervention, and potential neurovascular complications make the elbow less frequently evaluated with the arthroscope vs other joints, such as the knee and shoulder.2,8,9
Geographic distribution of subjects undergoing elbow arthroscopy, the indications used, surgical techniques being performed, and their associated clinical outcomes have received little to no recognition in the peer-reviewed literature.10 Differences in the elbow arthroscopy literature include characteristics related to the patient (age, gender, hand dominance, duration of symptoms), study (level of evidence, number of subjects, number of participating centers, design), indication (lateral epicondylitis, loose bodies, olecranon osteophytes, OCD), surgical technique, and outcome. Evidence-based medicine and clinical practice guidelines direct surgeons in clinical decision-making. Payers investigate the cost of surgical interventions and the value that surgery may provide, while following trends in different surgical techniques. Regulatory agencies and associations emphasize subjective patient-reported outcomes as the primary outcome measured in high-quality trials. Thus, in discussion of complex surgical interventions such as elbow arthroscopy, it is important to characterize the studies, subjects, and surgeries across the world to understand the geographic similarities and differences to optimize care in this clinical situation.
The goal of this study was to perform a systematic review and meta-analysis of elbow arthroscopy literature to identify and compare the characteristics of the studies published, the subjects analyzed, and surgical techniques performed across continents and countries to answer these questions: “Across the world, what demographic of patients are undergoing elbow arthroscopy, what are the most common indications for elbow arthroscopy, and how good is the evidence?” The authors hypothesized that patients who undergo elbow arthroscopy will be largely age <40 years, the most common indication for elbow arthroscopy will be a release/débridement, and the evidence for elbow arthroscopy will be poor. Also, no significant differences will exist in elbow arthroscopy publications, subjects, outcomes, and techniques based on continent/country of publication.
Methods
A systematic review was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines using a PRISMA checklist.11 Systematic review registration was performed using the International Prospective Register of Ongoing Systematic Reviews (PROSPERO; registration number, CRD42014010580; registration date, July 15, 2014).12 Two study authors independently conducted the search on June 23, 2014 using the following databases: Medline, Cochrane Central Register of Controlled Trials, SportDiscus, and CINAHL. The electronic search citation algorithm used was: (elbow) AND arthroscopy) NOT shoulder) NOT knee) NOT ankle) NOT wrist) NOT hip) NOT dog) NOT cadaver). English language Level I-IV evidence (2012 update by the Oxford Centre for Evidence-Based Medicine13) clinical studies were eligible for inclusion into this study. Abstracts were ineligible for inclusion. All references in selected studies were cross-referenced for inclusion if they were missed during the initial search. Duplicate subject publications within separate unique studies were not reported twice. The study with longer duration follow-up, higher level of evidence, greater number of subjects, or more detailed subject, surgical technique, or outcome reporting was retained for inclusion. Level V evidence reviews, expert opinion articles, letters to the editor, basic science, biomechanical studies, open elbow surgery, imaging, surgical technique, and classification studies were excluded.
All included patients underwent elbow arthroscopy for either intra- or extra-articular elbow pathology (ulnotrochlear osteoarthritis, lateral epicondylitis, rheumatoid arthritis, post-traumatic contracture, osteonecrosis of the capitellum or radial head, osteoid osteoma, and others). There was no minimum follow-up duration or rehabilitation requirement. The study and subject demographic parameters that we analyzed included year of publication, years of subject enrollment, presence of study financial conflict of interest, number of subjects and elbows, elbow dominance, gender, age, body mass index, diagnoses treated, type of anesthesia (block or general), and surgical positioning. Postoperative splint application and pain management, and whether a continuous passive motion machine was used and whether a drain was placed were recorded. Clinical outcome scores were DASH (Disability of the Arm, Shoulder, and Hand), Morrey score, MEPS (Mayo Elbow Performance Score), Andrews-Carson score, Timmerman-Andrews score, LES (Liverpool Elbow Score), Tegner score, HSS (Hospital for Special Surgery Score), VAS (Visual Analog Scale), EFA (Elbow Functional Assessment), Short Form-12 (SF-12), Short Form-36 (SF-36), Kerlan-Jobe Orthopaedic Clinic (KJOC) Shoulder and Elbow Questionnaire, and MAESS (Modified Andrews Elbow Scoring System). Radiographs, computed tomography (CT), computed tomography arthrography (CTA), magnetic resonance imaging (MRI), and magnetic resonance arthrography (MRA) data were extracted when available. Range of motion (flexion, extension, supination, and pronation) and grip strength data, both preoperative and postoperative, were extracted when available. Study methodological quality was evaluated using the Modified Coleman Methodology Score (MCMS).14
Statistical Analysis
Study descriptive statistics were calculated. Continuous variable data were reported as weighted means ± weighted standard deviations. Categorical variable data were reported as frequencies with percentages. For all statistical analysis either measured and calculated from study data extraction or directly reported from the individual studies, P < .05 was considered statistically significant. Study, subject, and surgical outcomes data were compared using 1-way analysis of variance (ANOVA) tests. Where applicable, study, subject, and surgical outcomes data were also compared using 2-sample and 2-proportion Z-test calculators with α .05 because of the difference in sample sizes between compared groups. To examine trends over time, Pearson’s correlation coefficients were calculated. For the purposes of analysis, the indications of “osteoarthritis,” “arthrofibrosis,” “loose body removal,” “ulnotrochlear osteoarthritis causing stiffness,” “post-traumatic contracture/stiffness,” and “post-operative elbow contracture” were combined into the indication “release and débridement.” For the 3 most common indications for arthroscopy (OCD, lateral epicondylitis, and release and débridement) data were combined into 5-year increments to overcome the smaller sample size within each of these categories, and Pearson’s correlation coefficients were calculated to determine if number of reported cases covaried with year period. Within these 3 diagnoses, ANOVA analyses were performed to determine whether the number of cases differed between continents and countries.
Results
A total of 353 studies were located, and, after implementation of the exclusion criteria, 112 studies were included in the final analysis (Figure 1; 3093 subjects; 3168 elbows; 64% male; mean age, 34.9 ± 14.68 years). There was a mean of 33.4 ± 26.02 months of follow-up, and 75% of surgeries involved the dominant elbow (Table 1). Most studies were level IV evidence (94.6%), had a low MCMS (mean 28.1 ± 8.06; poor rating), and were single-center investigations (94.6%). Most studies did not report financial conflicts of interest (56.3%) (Tables 1 and 2). From 1985 through 2014, the number of publications significantly increased with time (P = .004) among all continents. The MCMS was unchanged over time (P = .247) (Figure 2A), as was the level of evidence (P = .094) (Figure 2B). Conflicts of interest significantly increased with time (P = .025) (Figure 3).
Among continents, North America published the largest number of studies (54), and had the largest number of patients (1395) and elbow surgeries (1425) (Table 1). The United States published the largest number of studies (43%). There were no significant differences between age (P = .331), length of follow-up (P = .403), MCMS (P = .123), and level of evidence (P = .288) between continents. Of the 32 studies that reported the use of preoperative MRI, studies from Asia reported significantly more MRI scans than those from other continents (P = .040); there were no other significant differences between continents in reference to preoperative imaging studies or other demographic information.
The most common surgical indications were OCD (Figure 4), lateral epicondylitis (Figure 5), and release and débridement (Figure 6, Table 3; all studies listed indications). The number of reported cases for these 3 indications significantly increased over time (OCD P = .005, lateral epicondylitis P = .044, release and débridement P = .042) but did not significantly differ between regions (P > .05 in all cases).
Thirty-two (28.6%) studies reported the use of outcome measures (16 different outcome scores were used by the included studies). Asia reported outcome measures in 9 of 23 studies (39%), Europe in 12 of 35 studies (34%) and North America in 11 of 54 (20%) of studies. The MEPS was the most frequently used outcome score in 9.8% of studies, followed by VAS for pain in 5.3% of cases. North American studies reported a significantly higher increase in extension after elbow arthroscopy than Asia (P = .0432) (Figure 7), with no differences in flexion (P = .699), pronation (P = .376), or supination (P = .408). No significant differences were observed between continents in the type of anesthesia chosen (general anesthesia [P = .94] or regional anesthesia [P = .85]). Asia and Europe performed elbow arthroscopy most frequently in the lateral decubitus position, while North American studies most often used the supine position (Table 4).
Twenty (17.9%) studies reported the use of a postoperative splint, 12 (10.7%) studies reported use of a drain, 2 (1.79%) studies reported use of a hinged elbow brace, 9 (8.03%) studies reported use of a continuous passive motion machine postoperatively, and 3 (2.68%) studies reported use of an indwelling axillary catheter for postoperative pain management. Of 130 reported surgical complications (4.1%), the most frequent complication was transient sensory ulnar nerve palsy (1.5%), followed by persistent wound drainage (.76%), and transient sensory radial nerve palsy (.38%). Other reported complications included infection (.22%), transient sensory palsy of the median nerve (.19%), heterotopic ossification (.13%), complete transection of the ulnar nerve (.10%), loose body formation (.06%), hematoma formation (.06%), transient sensory palsy of the posterior interosseous (.06%), or anterior interosseous nerve (.03%), and complete transection of the radial (.03%), or median nerve (.03%).
Discussion
Elbow arthroscopy is an evolving surgical procedure that is used to treat intra- and extra-articular pathologies of the elbow. Outcomes of elbow arthroscopy for certain conditions have generally been reported as good, with improvements seen in pain, functional scores, and range of motion.6,15-17 The authors’ hypotheses were mostly confirmed in that the average age of patients undergoing elbow arthroscopy was <40 years, release/débridement was one of the most common indications (along with lateral epicondylitis and OCD), and the general evidence for elbow arthroscopy was poor. Also, there were almost no differences between continents/countries related to patient indications, preoperative imaging, anesthesia choice, indications, postoperative protocols, and outcomes (although the number of studies that reported outcomes was low and could have skewed the results), with the exception of a higher number of preoperative MRI scans in Asia. Some of the notable findings of this study included: 1) the number of studies published on elbow arthroscopy is significantly increasing with time, despite a lack of improvement in the level of evidence; 2) the majority of studies on elbow arthroscopy do not report a surgical outcome score; and 3) the number of reported cases for the 3 most common indications significantly increased over time (OCD, P = .005; lateral epicondylitis, P = .044; release and débridement, P = .042) but did not differ between regions (P > .05 in all cases).
The indications for elbow arthroscopy have grown dramatically in the past 2 decades to include both intra- and extra-articular pathologies.18 Despite this increase in the number of indications for elbow arthroscopy, the study did not find a significant difference between countries/continents in the indications each used for elbow arthroscopy patients. There was a trend towards an increase in OCD cases in all continents, especially Asia (Figure 4), with time. Interestingly, while not statistically significant, there was variation among countries for surgical indications. In North America, removal of loose bodies accounts for 18% of patients, while in Europe this accounted for only 9% and in Asia for 1%. Post-traumatic stiffness was the indication for elbow arthroscopy in Europe in 19% of patients vs 7% in North America and 10% in Asia. In Asia, OCD accounts for 40% of arthroscopies, 7% in Europe, and 14% in North America (Figure 4) (Table 3).
This study demonstrated that the mean increase in elbow extension gained after surgery in North America was significantly greater when compared with studies from Asia, but the gain in flexion, pronation, and supination was similar across continents. The underlying cause of this difference in improvement in elbow extension between nations is unclear, although differences in diagnosis could account for some variation. This study did not examine differences in rehabilitation protocols, and certainly, it is plausible that protocol variations by country could account for some discrepancy. Furthermore, differences in functional needs may vary by continent and could have driven this result.
This study found no routine reporting of outcome scores by elbow arthroscopy studies from any continent, and that when outcome scores are reported, there is substantial inconsistency with regard to the actual scoring system used. No continent reported outcome scores in more than 40% of the studies published from that area, and the variation of outcome scores used, even from a single region, was large. This makes comparing clinical outcomes between studies difficult, even when performing identical procedures for identical indications, because there is no standardized method of reporting outcomes. To allow comparison of studies and generalizability of the results to different populations, a more standardized approach to outcome reporting needs to be instituted in the elbow arthroscopy literature. To date, there is no standardized score that has been validated for reporting clinical outcomes after elbow arthroscopy.19 Hence, it is not surprising that there were 16 different outcome scores reported throughout the 112 studies analyzed in this review, with the most frequent score, the MEPS, reported in a total of 10 studies. As medicine moves towards pay scales that are based on patient outcomes, it will become more important to define a clear outcome score that can be used to assess these patients, and reliably report scores. This will allow comparison of patients across nations to determine the best surgical treatment for different clinical problems. A validation study comparing these outcome scores to determine which score best summarizes the patient’s level of pain and function after surgery would be beneficial, because this could identify 1 score that could be standardized to allow comparison among reported outcomes.
Limitations
This study had several limitations. Despite having 2 authors search independently for studies, some studies could have been missed during the search process, introducing possible selection bias. Including only published studies could have introduced publication bias. Numerous studies did not report all the variables the authors examined. This could have skewed some results, and had additional variables been reported, could have altered the data to show significant differences in some measured variables. Because this study did not compare outcome measures for varying pathologies, conclusions cannot be drawn on the best treatment options for different indications. Case reports could have lowered the MCMS score and the average in studies reporting outcomes. Furthermore, the poor quality of the underlying data used in this study could limit the validity/generalizability of the results because this is a systematic review, and its level of evidence is only as high as the studies it includes. Because the primary aim was to report on demographics, this study did not examine concomitant pathology at the time of surgery or rehabilitation protocols.
Conclusion
The quantity, but not the quality, of arthroscopic elbow publications has significantly increased over time. Most patients undergo elbow arthroscopy for lateral epicondylitis, OCD, and release and débridement. Pathology and indications do not appear to differ geographically with more men undergoing elbow arthroscopy than women.
1. Khanchandani P. Elbow arthroscopy: review of the literature and case reports. Case Rep Orthop. 2012;2012:478214.
2. Dodson CC, Nho SJ, Williams RJ 3rd, Altchek DW. Elbow arthroscopy. J Am Acad Orthop Surg. 2008;16(10):574-585.
3. Takahara M, Mura N, Sasaki J, Harada M, Ogino T. Classification, treatment, and outcome of osteochondritis dissecans of the humeral capitellum. Surgical technique. J Bone Joint Surg Am. 2008;90(suppl 2 Pt 1):47-62.
4. Kelly EW, Morrey BF, O’Driscoll SW. Complications of elbow arthroscopy. J Bone Joint Surg Am. 2001;83-A(1):25-34.
5. Rajeev A, Pooley J. Lateral compartment cartilage changes and lateral elbow pain. Acta Orthop Belg. 2009;75(1):37-40.
6. Miyake J, Shimada K, Oka K, et al. Arthroscopic debridement in the treatment of patients with osteoarthritis of the elbow, based on computer simulation. Bone Joint J. 2014;96-B(2):237-241.
7. Babaqi AA, Kotb MM, Said HG, AbdelHamid MM, ElKady HA, ElAssal MA. Short-term evaluation of arthroscopic management of tennis elbow; including resection of radio-capitellar capsular complex. J Orthop. 2014;11(2):82-86.
8. Gay DM, Raphael BS, Weiland AJ. Revision arthroscopic contracture release in the elbow resulting in an ulnar nerve transection: a case report. J Bone Joint Surg Am. 2010;92(5):1246-1249.
9. Haapaniemi T, Berggren M, Adolfsson L. Complete transection of the median and radial nerves during arthroscopic release of post-traumatic elbow contracture. Arthroscopy. 1999;15(7):784-787.
10. Yeoh KM, King GJ, Faber KJ, Glazebrook MA, Athwal GS. Evidence-based indications for elbow arthroscopy. Arthroscopy. 2012;28(2):272-282.
11. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. BMJ. 2009;339:b2700.
12. PROSPERO. International Prospective Register of Ongoing Systematic Reviews. The University of York CfRaDP-Iprosr-v. 2013 [cited 2014]. http://www.crd.york.ac.uk/PROSPERO/. Accessed March 17, 2016.
13. Oxford Centre for Evidence-Based Medicine - levels of evidence (March 2009). Centre for Evidence-Based Medicine Web site. http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/. Accessed July 6, 2016.
14. Cowan J, Lozano-Calderόn S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
15. Jones GS, Savoie FH 3rd. Arthroscopic capsular release of flexion contractures (arthrofibrosis) of the elbow. Arthroscopy. 1993;9(3):277-283.
16. O’Brien MJ, Lee Murphy R, Savoie FH 3rd. A preliminary report of acute and subacute arthroscopic repair of the radial ulnohumeral ligament after elbow dislocation in the high-demand patient. Arthroscopy. 2014;30(6):679-687.
17. Rhyou IH, Kim KW. Is posterior synovial plica excision necessary for refractory lateral epicondylitis of the elbow? Clin Orthop Relat Res. 2013;471(1):284-290.
18. Jerosch J, Schunck J. Arthroscopic treatment of lateral epicondylitis: indication, technique and early results. Knee Surg Sports Traumatol Arthrosc. 2006;14(4):379-382.
19. Tijssen M, van Cingel R, van Melick N, de Visser E. Patient-Reported Outcome questionnaires for hip arthroscopy: a systematic review of the psychometric evidence. BMC Musculoskelet Disord. 2011;12:117.
Although elbow arthroscopy was first described in the 1930s, it has become increasingly popular in the last 30 years.1 While initially considered as a tool for diagnosis and loose body removal, indications have expanded to include treatment of osteochondritis dissecans (OCD), treatment of lateral epicondylitis, fixation of fractures, and others.2-5 Miyake and colleagues6 found a significant improvement in range of motion, both flexion and extension, and outcome scores when elbow arthroscopy was used to remove impinging osteophytes. Babaqi and colleagues7 found significant improvement in pain, satisfaction, and outcome scores in 31 patients who underwent elbow arthroscopy for lateral epicondylitis refractory to nonsurgical management. The technical difficulty of the procedure, lower frequency of pathology amenable to arthroscopic intervention, and potential neurovascular complications make the elbow less frequently evaluated with the arthroscope vs other joints, such as the knee and shoulder.2,8,9
Geographic distribution of subjects undergoing elbow arthroscopy, the indications used, surgical techniques being performed, and their associated clinical outcomes have received little to no recognition in the peer-reviewed literature.10 Differences in the elbow arthroscopy literature include characteristics related to the patient (age, gender, hand dominance, duration of symptoms), study (level of evidence, number of subjects, number of participating centers, design), indication (lateral epicondylitis, loose bodies, olecranon osteophytes, OCD), surgical technique, and outcome. Evidence-based medicine and clinical practice guidelines direct surgeons in clinical decision-making. Payers investigate the cost of surgical interventions and the value that surgery may provide, while following trends in different surgical techniques. Regulatory agencies and associations emphasize subjective patient-reported outcomes as the primary outcome measured in high-quality trials. Thus, in discussion of complex surgical interventions such as elbow arthroscopy, it is important to characterize the studies, subjects, and surgeries across the world to understand the geographic similarities and differences to optimize care in this clinical situation.
The goal of this study was to perform a systematic review and meta-analysis of elbow arthroscopy literature to identify and compare the characteristics of the studies published, the subjects analyzed, and surgical techniques performed across continents and countries to answer these questions: “Across the world, what demographic of patients are undergoing elbow arthroscopy, what are the most common indications for elbow arthroscopy, and how good is the evidence?” The authors hypothesized that patients who undergo elbow arthroscopy will be largely age <40 years, the most common indication for elbow arthroscopy will be a release/débridement, and the evidence for elbow arthroscopy will be poor. Also, no significant differences will exist in elbow arthroscopy publications, subjects, outcomes, and techniques based on continent/country of publication.
Methods
A systematic review was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines using a PRISMA checklist.11 Systematic review registration was performed using the International Prospective Register of Ongoing Systematic Reviews (PROSPERO; registration number, CRD42014010580; registration date, July 15, 2014).12 Two study authors independently conducted the search on June 23, 2014 using the following databases: Medline, Cochrane Central Register of Controlled Trials, SportDiscus, and CINAHL. The electronic search citation algorithm used was: (elbow) AND arthroscopy) NOT shoulder) NOT knee) NOT ankle) NOT wrist) NOT hip) NOT dog) NOT cadaver). English language Level I-IV evidence (2012 update by the Oxford Centre for Evidence-Based Medicine13) clinical studies were eligible for inclusion into this study. Abstracts were ineligible for inclusion. All references in selected studies were cross-referenced for inclusion if they were missed during the initial search. Duplicate subject publications within separate unique studies were not reported twice. The study with longer duration follow-up, higher level of evidence, greater number of subjects, or more detailed subject, surgical technique, or outcome reporting was retained for inclusion. Level V evidence reviews, expert opinion articles, letters to the editor, basic science, biomechanical studies, open elbow surgery, imaging, surgical technique, and classification studies were excluded.
All included patients underwent elbow arthroscopy for either intra- or extra-articular elbow pathology (ulnotrochlear osteoarthritis, lateral epicondylitis, rheumatoid arthritis, post-traumatic contracture, osteonecrosis of the capitellum or radial head, osteoid osteoma, and others). There was no minimum follow-up duration or rehabilitation requirement. The study and subject demographic parameters that we analyzed included year of publication, years of subject enrollment, presence of study financial conflict of interest, number of subjects and elbows, elbow dominance, gender, age, body mass index, diagnoses treated, type of anesthesia (block or general), and surgical positioning. Postoperative splint application and pain management, and whether a continuous passive motion machine was used and whether a drain was placed were recorded. Clinical outcome scores were DASH (Disability of the Arm, Shoulder, and Hand), Morrey score, MEPS (Mayo Elbow Performance Score), Andrews-Carson score, Timmerman-Andrews score, LES (Liverpool Elbow Score), Tegner score, HSS (Hospital for Special Surgery Score), VAS (Visual Analog Scale), EFA (Elbow Functional Assessment), Short Form-12 (SF-12), Short Form-36 (SF-36), Kerlan-Jobe Orthopaedic Clinic (KJOC) Shoulder and Elbow Questionnaire, and MAESS (Modified Andrews Elbow Scoring System). Radiographs, computed tomography (CT), computed tomography arthrography (CTA), magnetic resonance imaging (MRI), and magnetic resonance arthrography (MRA) data were extracted when available. Range of motion (flexion, extension, supination, and pronation) and grip strength data, both preoperative and postoperative, were extracted when available. Study methodological quality was evaluated using the Modified Coleman Methodology Score (MCMS).14
Statistical Analysis
Study descriptive statistics were calculated. Continuous variable data were reported as weighted means ± weighted standard deviations. Categorical variable data were reported as frequencies with percentages. For all statistical analysis either measured and calculated from study data extraction or directly reported from the individual studies, P < .05 was considered statistically significant. Study, subject, and surgical outcomes data were compared using 1-way analysis of variance (ANOVA) tests. Where applicable, study, subject, and surgical outcomes data were also compared using 2-sample and 2-proportion Z-test calculators with α .05 because of the difference in sample sizes between compared groups. To examine trends over time, Pearson’s correlation coefficients were calculated. For the purposes of analysis, the indications of “osteoarthritis,” “arthrofibrosis,” “loose body removal,” “ulnotrochlear osteoarthritis causing stiffness,” “post-traumatic contracture/stiffness,” and “post-operative elbow contracture” were combined into the indication “release and débridement.” For the 3 most common indications for arthroscopy (OCD, lateral epicondylitis, and release and débridement) data were combined into 5-year increments to overcome the smaller sample size within each of these categories, and Pearson’s correlation coefficients were calculated to determine if number of reported cases covaried with year period. Within these 3 diagnoses, ANOVA analyses were performed to determine whether the number of cases differed between continents and countries.
Results
A total of 353 studies were located, and, after implementation of the exclusion criteria, 112 studies were included in the final analysis (Figure 1; 3093 subjects; 3168 elbows; 64% male; mean age, 34.9 ± 14.68 years). There was a mean of 33.4 ± 26.02 months of follow-up, and 75% of surgeries involved the dominant elbow (Table 1). Most studies were level IV evidence (94.6%), had a low MCMS (mean 28.1 ± 8.06; poor rating), and were single-center investigations (94.6%). Most studies did not report financial conflicts of interest (56.3%) (Tables 1 and 2). From 1985 through 2014, the number of publications significantly increased with time (P = .004) among all continents. The MCMS was unchanged over time (P = .247) (Figure 2A), as was the level of evidence (P = .094) (Figure 2B). Conflicts of interest significantly increased with time (P = .025) (Figure 3).
Among continents, North America published the largest number of studies (54), and had the largest number of patients (1395) and elbow surgeries (1425) (Table 1). The United States published the largest number of studies (43%). There were no significant differences between age (P = .331), length of follow-up (P = .403), MCMS (P = .123), and level of evidence (P = .288) between continents. Of the 32 studies that reported the use of preoperative MRI, studies from Asia reported significantly more MRI scans than those from other continents (P = .040); there were no other significant differences between continents in reference to preoperative imaging studies or other demographic information.
The most common surgical indications were OCD (Figure 4), lateral epicondylitis (Figure 5), and release and débridement (Figure 6, Table 3; all studies listed indications). The number of reported cases for these 3 indications significantly increased over time (OCD P = .005, lateral epicondylitis P = .044, release and débridement P = .042) but did not significantly differ between regions (P > .05 in all cases).
Thirty-two (28.6%) studies reported the use of outcome measures (16 different outcome scores were used by the included studies). Asia reported outcome measures in 9 of 23 studies (39%), Europe in 12 of 35 studies (34%) and North America in 11 of 54 (20%) of studies. The MEPS was the most frequently used outcome score in 9.8% of studies, followed by VAS for pain in 5.3% of cases. North American studies reported a significantly higher increase in extension after elbow arthroscopy than Asia (P = .0432) (Figure 7), with no differences in flexion (P = .699), pronation (P = .376), or supination (P = .408). No significant differences were observed between continents in the type of anesthesia chosen (general anesthesia [P = .94] or regional anesthesia [P = .85]). Asia and Europe performed elbow arthroscopy most frequently in the lateral decubitus position, while North American studies most often used the supine position (Table 4).
Twenty (17.9%) studies reported the use of a postoperative splint, 12 (10.7%) studies reported use of a drain, 2 (1.79%) studies reported use of a hinged elbow brace, 9 (8.03%) studies reported use of a continuous passive motion machine postoperatively, and 3 (2.68%) studies reported use of an indwelling axillary catheter for postoperative pain management. Of 130 reported surgical complications (4.1%), the most frequent complication was transient sensory ulnar nerve palsy (1.5%), followed by persistent wound drainage (.76%), and transient sensory radial nerve palsy (.38%). Other reported complications included infection (.22%), transient sensory palsy of the median nerve (.19%), heterotopic ossification (.13%), complete transection of the ulnar nerve (.10%), loose body formation (.06%), hematoma formation (.06%), transient sensory palsy of the posterior interosseous (.06%), or anterior interosseous nerve (.03%), and complete transection of the radial (.03%), or median nerve (.03%).
Discussion
Elbow arthroscopy is an evolving surgical procedure that is used to treat intra- and extra-articular pathologies of the elbow. Outcomes of elbow arthroscopy for certain conditions have generally been reported as good, with improvements seen in pain, functional scores, and range of motion.6,15-17 The authors’ hypotheses were mostly confirmed in that the average age of patients undergoing elbow arthroscopy was <40 years, release/débridement was one of the most common indications (along with lateral epicondylitis and OCD), and the general evidence for elbow arthroscopy was poor. Also, there were almost no differences between continents/countries related to patient indications, preoperative imaging, anesthesia choice, indications, postoperative protocols, and outcomes (although the number of studies that reported outcomes was low and could have skewed the results), with the exception of a higher number of preoperative MRI scans in Asia. Some of the notable findings of this study included: 1) the number of studies published on elbow arthroscopy is significantly increasing with time, despite a lack of improvement in the level of evidence; 2) the majority of studies on elbow arthroscopy do not report a surgical outcome score; and 3) the number of reported cases for the 3 most common indications significantly increased over time (OCD, P = .005; lateral epicondylitis, P = .044; release and débridement, P = .042) but did not differ between regions (P > .05 in all cases).
The indications for elbow arthroscopy have grown dramatically in the past 2 decades to include both intra- and extra-articular pathologies.18 Despite this increase in the number of indications for elbow arthroscopy, the study did not find a significant difference between countries/continents in the indications each used for elbow arthroscopy patients. There was a trend towards an increase in OCD cases in all continents, especially Asia (Figure 4), with time. Interestingly, while not statistically significant, there was variation among countries for surgical indications. In North America, removal of loose bodies accounts for 18% of patients, while in Europe this accounted for only 9% and in Asia for 1%. Post-traumatic stiffness was the indication for elbow arthroscopy in Europe in 19% of patients vs 7% in North America and 10% in Asia. In Asia, OCD accounts for 40% of arthroscopies, 7% in Europe, and 14% in North America (Figure 4) (Table 3).
This study demonstrated that the mean increase in elbow extension gained after surgery in North America was significantly greater when compared with studies from Asia, but the gain in flexion, pronation, and supination was similar across continents. The underlying cause of this difference in improvement in elbow extension between nations is unclear, although differences in diagnosis could account for some variation. This study did not examine differences in rehabilitation protocols, and certainly, it is plausible that protocol variations by country could account for some discrepancy. Furthermore, differences in functional needs may vary by continent and could have driven this result.
This study found no routine reporting of outcome scores by elbow arthroscopy studies from any continent, and that when outcome scores are reported, there is substantial inconsistency with regard to the actual scoring system used. No continent reported outcome scores in more than 40% of the studies published from that area, and the variation of outcome scores used, even from a single region, was large. This makes comparing clinical outcomes between studies difficult, even when performing identical procedures for identical indications, because there is no standardized method of reporting outcomes. To allow comparison of studies and generalizability of the results to different populations, a more standardized approach to outcome reporting needs to be instituted in the elbow arthroscopy literature. To date, there is no standardized score that has been validated for reporting clinical outcomes after elbow arthroscopy.19 Hence, it is not surprising that there were 16 different outcome scores reported throughout the 112 studies analyzed in this review, with the most frequent score, the MEPS, reported in a total of 10 studies. As medicine moves towards pay scales that are based on patient outcomes, it will become more important to define a clear outcome score that can be used to assess these patients, and reliably report scores. This will allow comparison of patients across nations to determine the best surgical treatment for different clinical problems. A validation study comparing these outcome scores to determine which score best summarizes the patient’s level of pain and function after surgery would be beneficial, because this could identify 1 score that could be standardized to allow comparison among reported outcomes.
Limitations
This study had several limitations. Despite having 2 authors search independently for studies, some studies could have been missed during the search process, introducing possible selection bias. Including only published studies could have introduced publication bias. Numerous studies did not report all the variables the authors examined. This could have skewed some results, and had additional variables been reported, could have altered the data to show significant differences in some measured variables. Because this study did not compare outcome measures for varying pathologies, conclusions cannot be drawn on the best treatment options for different indications. Case reports could have lowered the MCMS score and the average in studies reporting outcomes. Furthermore, the poor quality of the underlying data used in this study could limit the validity/generalizability of the results because this is a systematic review, and its level of evidence is only as high as the studies it includes. Because the primary aim was to report on demographics, this study did not examine concomitant pathology at the time of surgery or rehabilitation protocols.
Conclusion
The quantity, but not the quality, of arthroscopic elbow publications has significantly increased over time. Most patients undergo elbow arthroscopy for lateral epicondylitis, OCD, and release and débridement. Pathology and indications do not appear to differ geographically with more men undergoing elbow arthroscopy than women.
Although elbow arthroscopy was first described in the 1930s, it has become increasingly popular in the last 30 years.1 While initially considered as a tool for diagnosis and loose body removal, indications have expanded to include treatment of osteochondritis dissecans (OCD), treatment of lateral epicondylitis, fixation of fractures, and others.2-5 Miyake and colleagues6 found a significant improvement in range of motion, both flexion and extension, and outcome scores when elbow arthroscopy was used to remove impinging osteophytes. Babaqi and colleagues7 found significant improvement in pain, satisfaction, and outcome scores in 31 patients who underwent elbow arthroscopy for lateral epicondylitis refractory to nonsurgical management. The technical difficulty of the procedure, lower frequency of pathology amenable to arthroscopic intervention, and potential neurovascular complications make the elbow less frequently evaluated with the arthroscope vs other joints, such as the knee and shoulder.2,8,9
Geographic distribution of subjects undergoing elbow arthroscopy, the indications used, surgical techniques being performed, and their associated clinical outcomes have received little to no recognition in the peer-reviewed literature.10 Differences in the elbow arthroscopy literature include characteristics related to the patient (age, gender, hand dominance, duration of symptoms), study (level of evidence, number of subjects, number of participating centers, design), indication (lateral epicondylitis, loose bodies, olecranon osteophytes, OCD), surgical technique, and outcome. Evidence-based medicine and clinical practice guidelines direct surgeons in clinical decision-making. Payers investigate the cost of surgical interventions and the value that surgery may provide, while following trends in different surgical techniques. Regulatory agencies and associations emphasize subjective patient-reported outcomes as the primary outcome measured in high-quality trials. Thus, in discussion of complex surgical interventions such as elbow arthroscopy, it is important to characterize the studies, subjects, and surgeries across the world to understand the geographic similarities and differences to optimize care in this clinical situation.
The goal of this study was to perform a systematic review and meta-analysis of elbow arthroscopy literature to identify and compare the characteristics of the studies published, the subjects analyzed, and surgical techniques performed across continents and countries to answer these questions: “Across the world, what demographic of patients are undergoing elbow arthroscopy, what are the most common indications for elbow arthroscopy, and how good is the evidence?” The authors hypothesized that patients who undergo elbow arthroscopy will be largely age <40 years, the most common indication for elbow arthroscopy will be a release/débridement, and the evidence for elbow arthroscopy will be poor. Also, no significant differences will exist in elbow arthroscopy publications, subjects, outcomes, and techniques based on continent/country of publication.
Methods
A systematic review was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines using a PRISMA checklist.11 Systematic review registration was performed using the International Prospective Register of Ongoing Systematic Reviews (PROSPERO; registration number, CRD42014010580; registration date, July 15, 2014).12 Two study authors independently conducted the search on June 23, 2014 using the following databases: Medline, Cochrane Central Register of Controlled Trials, SportDiscus, and CINAHL. The electronic search citation algorithm used was: (elbow) AND arthroscopy) NOT shoulder) NOT knee) NOT ankle) NOT wrist) NOT hip) NOT dog) NOT cadaver). English language Level I-IV evidence (2012 update by the Oxford Centre for Evidence-Based Medicine13) clinical studies were eligible for inclusion into this study. Abstracts were ineligible for inclusion. All references in selected studies were cross-referenced for inclusion if they were missed during the initial search. Duplicate subject publications within separate unique studies were not reported twice. The study with longer duration follow-up, higher level of evidence, greater number of subjects, or more detailed subject, surgical technique, or outcome reporting was retained for inclusion. Level V evidence reviews, expert opinion articles, letters to the editor, basic science, biomechanical studies, open elbow surgery, imaging, surgical technique, and classification studies were excluded.
All included patients underwent elbow arthroscopy for either intra- or extra-articular elbow pathology (ulnotrochlear osteoarthritis, lateral epicondylitis, rheumatoid arthritis, post-traumatic contracture, osteonecrosis of the capitellum or radial head, osteoid osteoma, and others). There was no minimum follow-up duration or rehabilitation requirement. The study and subject demographic parameters that we analyzed included year of publication, years of subject enrollment, presence of study financial conflict of interest, number of subjects and elbows, elbow dominance, gender, age, body mass index, diagnoses treated, type of anesthesia (block or general), and surgical positioning. Postoperative splint application and pain management, and whether a continuous passive motion machine was used and whether a drain was placed were recorded. Clinical outcome scores were DASH (Disability of the Arm, Shoulder, and Hand), Morrey score, MEPS (Mayo Elbow Performance Score), Andrews-Carson score, Timmerman-Andrews score, LES (Liverpool Elbow Score), Tegner score, HSS (Hospital for Special Surgery Score), VAS (Visual Analog Scale), EFA (Elbow Functional Assessment), Short Form-12 (SF-12), Short Form-36 (SF-36), Kerlan-Jobe Orthopaedic Clinic (KJOC) Shoulder and Elbow Questionnaire, and MAESS (Modified Andrews Elbow Scoring System). Radiographs, computed tomography (CT), computed tomography arthrography (CTA), magnetic resonance imaging (MRI), and magnetic resonance arthrography (MRA) data were extracted when available. Range of motion (flexion, extension, supination, and pronation) and grip strength data, both preoperative and postoperative, were extracted when available. Study methodological quality was evaluated using the Modified Coleman Methodology Score (MCMS).14
Statistical Analysis
Study descriptive statistics were calculated. Continuous variable data were reported as weighted means ± weighted standard deviations. Categorical variable data were reported as frequencies with percentages. For all statistical analysis either measured and calculated from study data extraction or directly reported from the individual studies, P < .05 was considered statistically significant. Study, subject, and surgical outcomes data were compared using 1-way analysis of variance (ANOVA) tests. Where applicable, study, subject, and surgical outcomes data were also compared using 2-sample and 2-proportion Z-test calculators with α .05 because of the difference in sample sizes between compared groups. To examine trends over time, Pearson’s correlation coefficients were calculated. For the purposes of analysis, the indications of “osteoarthritis,” “arthrofibrosis,” “loose body removal,” “ulnotrochlear osteoarthritis causing stiffness,” “post-traumatic contracture/stiffness,” and “post-operative elbow contracture” were combined into the indication “release and débridement.” For the 3 most common indications for arthroscopy (OCD, lateral epicondylitis, and release and débridement) data were combined into 5-year increments to overcome the smaller sample size within each of these categories, and Pearson’s correlation coefficients were calculated to determine if number of reported cases covaried with year period. Within these 3 diagnoses, ANOVA analyses were performed to determine whether the number of cases differed between continents and countries.
Results
A total of 353 studies were located, and, after implementation of the exclusion criteria, 112 studies were included in the final analysis (Figure 1; 3093 subjects; 3168 elbows; 64% male; mean age, 34.9 ± 14.68 years). There was a mean of 33.4 ± 26.02 months of follow-up, and 75% of surgeries involved the dominant elbow (Table 1). Most studies were level IV evidence (94.6%), had a low MCMS (mean 28.1 ± 8.06; poor rating), and were single-center investigations (94.6%). Most studies did not report financial conflicts of interest (56.3%) (Tables 1 and 2). From 1985 through 2014, the number of publications significantly increased with time (P = .004) among all continents. The MCMS was unchanged over time (P = .247) (Figure 2A), as was the level of evidence (P = .094) (Figure 2B). Conflicts of interest significantly increased with time (P = .025) (Figure 3).
Among continents, North America published the largest number of studies (54), and had the largest number of patients (1395) and elbow surgeries (1425) (Table 1). The United States published the largest number of studies (43%). There were no significant differences between age (P = .331), length of follow-up (P = .403), MCMS (P = .123), and level of evidence (P = .288) between continents. Of the 32 studies that reported the use of preoperative MRI, studies from Asia reported significantly more MRI scans than those from other continents (P = .040); there were no other significant differences between continents in reference to preoperative imaging studies or other demographic information.
The most common surgical indications were OCD (Figure 4), lateral epicondylitis (Figure 5), and release and débridement (Figure 6, Table 3; all studies listed indications). The number of reported cases for these 3 indications significantly increased over time (OCD P = .005, lateral epicondylitis P = .044, release and débridement P = .042) but did not significantly differ between regions (P > .05 in all cases).
Thirty-two (28.6%) studies reported the use of outcome measures (16 different outcome scores were used by the included studies). Asia reported outcome measures in 9 of 23 studies (39%), Europe in 12 of 35 studies (34%) and North America in 11 of 54 (20%) of studies. The MEPS was the most frequently used outcome score in 9.8% of studies, followed by VAS for pain in 5.3% of cases. North American studies reported a significantly higher increase in extension after elbow arthroscopy than Asia (P = .0432) (Figure 7), with no differences in flexion (P = .699), pronation (P = .376), or supination (P = .408). No significant differences were observed between continents in the type of anesthesia chosen (general anesthesia [P = .94] or regional anesthesia [P = .85]). Asia and Europe performed elbow arthroscopy most frequently in the lateral decubitus position, while North American studies most often used the supine position (Table 4).
Twenty (17.9%) studies reported the use of a postoperative splint, 12 (10.7%) studies reported use of a drain, 2 (1.79%) studies reported use of a hinged elbow brace, 9 (8.03%) studies reported use of a continuous passive motion machine postoperatively, and 3 (2.68%) studies reported use of an indwelling axillary catheter for postoperative pain management. Of 130 reported surgical complications (4.1%), the most frequent complication was transient sensory ulnar nerve palsy (1.5%), followed by persistent wound drainage (.76%), and transient sensory radial nerve palsy (.38%). Other reported complications included infection (.22%), transient sensory palsy of the median nerve (.19%), heterotopic ossification (.13%), complete transection of the ulnar nerve (.10%), loose body formation (.06%), hematoma formation (.06%), transient sensory palsy of the posterior interosseous (.06%), or anterior interosseous nerve (.03%), and complete transection of the radial (.03%), or median nerve (.03%).
Discussion
Elbow arthroscopy is an evolving surgical procedure that is used to treat intra- and extra-articular pathologies of the elbow. Outcomes of elbow arthroscopy for certain conditions have generally been reported as good, with improvements seen in pain, functional scores, and range of motion.6,15-17 The authors’ hypotheses were mostly confirmed in that the average age of patients undergoing elbow arthroscopy was <40 years, release/débridement was one of the most common indications (along with lateral epicondylitis and OCD), and the general evidence for elbow arthroscopy was poor. Also, there were almost no differences between continents/countries related to patient indications, preoperative imaging, anesthesia choice, indications, postoperative protocols, and outcomes (although the number of studies that reported outcomes was low and could have skewed the results), with the exception of a higher number of preoperative MRI scans in Asia. Some of the notable findings of this study included: 1) the number of studies published on elbow arthroscopy is significantly increasing with time, despite a lack of improvement in the level of evidence; 2) the majority of studies on elbow arthroscopy do not report a surgical outcome score; and 3) the number of reported cases for the 3 most common indications significantly increased over time (OCD, P = .005; lateral epicondylitis, P = .044; release and débridement, P = .042) but did not differ between regions (P > .05 in all cases).
The indications for elbow arthroscopy have grown dramatically in the past 2 decades to include both intra- and extra-articular pathologies.18 Despite this increase in the number of indications for elbow arthroscopy, the study did not find a significant difference between countries/continents in the indications each used for elbow arthroscopy patients. There was a trend towards an increase in OCD cases in all continents, especially Asia (Figure 4), with time. Interestingly, while not statistically significant, there was variation among countries for surgical indications. In North America, removal of loose bodies accounts for 18% of patients, while in Europe this accounted for only 9% and in Asia for 1%. Post-traumatic stiffness was the indication for elbow arthroscopy in Europe in 19% of patients vs 7% in North America and 10% in Asia. In Asia, OCD accounts for 40% of arthroscopies, 7% in Europe, and 14% in North America (Figure 4) (Table 3).
This study demonstrated that the mean increase in elbow extension gained after surgery in North America was significantly greater when compared with studies from Asia, but the gain in flexion, pronation, and supination was similar across continents. The underlying cause of this difference in improvement in elbow extension between nations is unclear, although differences in diagnosis could account for some variation. This study did not examine differences in rehabilitation protocols, and certainly, it is plausible that protocol variations by country could account for some discrepancy. Furthermore, differences in functional needs may vary by continent and could have driven this result.
This study found no routine reporting of outcome scores by elbow arthroscopy studies from any continent, and that when outcome scores are reported, there is substantial inconsistency with regard to the actual scoring system used. No continent reported outcome scores in more than 40% of the studies published from that area, and the variation of outcome scores used, even from a single region, was large. This makes comparing clinical outcomes between studies difficult, even when performing identical procedures for identical indications, because there is no standardized method of reporting outcomes. To allow comparison of studies and generalizability of the results to different populations, a more standardized approach to outcome reporting needs to be instituted in the elbow arthroscopy literature. To date, there is no standardized score that has been validated for reporting clinical outcomes after elbow arthroscopy.19 Hence, it is not surprising that there were 16 different outcome scores reported throughout the 112 studies analyzed in this review, with the most frequent score, the MEPS, reported in a total of 10 studies. As medicine moves towards pay scales that are based on patient outcomes, it will become more important to define a clear outcome score that can be used to assess these patients, and reliably report scores. This will allow comparison of patients across nations to determine the best surgical treatment for different clinical problems. A validation study comparing these outcome scores to determine which score best summarizes the patient’s level of pain and function after surgery would be beneficial, because this could identify 1 score that could be standardized to allow comparison among reported outcomes.
Limitations
This study had several limitations. Despite having 2 authors search independently for studies, some studies could have been missed during the search process, introducing possible selection bias. Including only published studies could have introduced publication bias. Numerous studies did not report all the variables the authors examined. This could have skewed some results, and had additional variables been reported, could have altered the data to show significant differences in some measured variables. Because this study did not compare outcome measures for varying pathologies, conclusions cannot be drawn on the best treatment options for different indications. Case reports could have lowered the MCMS score and the average in studies reporting outcomes. Furthermore, the poor quality of the underlying data used in this study could limit the validity/generalizability of the results because this is a systematic review, and its level of evidence is only as high as the studies it includes. Because the primary aim was to report on demographics, this study did not examine concomitant pathology at the time of surgery or rehabilitation protocols.
Conclusion
The quantity, but not the quality, of arthroscopic elbow publications has significantly increased over time. Most patients undergo elbow arthroscopy for lateral epicondylitis, OCD, and release and débridement. Pathology and indications do not appear to differ geographically with more men undergoing elbow arthroscopy than women.
1. Khanchandani P. Elbow arthroscopy: review of the literature and case reports. Case Rep Orthop. 2012;2012:478214.
2. Dodson CC, Nho SJ, Williams RJ 3rd, Altchek DW. Elbow arthroscopy. J Am Acad Orthop Surg. 2008;16(10):574-585.
3. Takahara M, Mura N, Sasaki J, Harada M, Ogino T. Classification, treatment, and outcome of osteochondritis dissecans of the humeral capitellum. Surgical technique. J Bone Joint Surg Am. 2008;90(suppl 2 Pt 1):47-62.
4. Kelly EW, Morrey BF, O’Driscoll SW. Complications of elbow arthroscopy. J Bone Joint Surg Am. 2001;83-A(1):25-34.
5. Rajeev A, Pooley J. Lateral compartment cartilage changes and lateral elbow pain. Acta Orthop Belg. 2009;75(1):37-40.
6. Miyake J, Shimada K, Oka K, et al. Arthroscopic debridement in the treatment of patients with osteoarthritis of the elbow, based on computer simulation. Bone Joint J. 2014;96-B(2):237-241.
7. Babaqi AA, Kotb MM, Said HG, AbdelHamid MM, ElKady HA, ElAssal MA. Short-term evaluation of arthroscopic management of tennis elbow; including resection of radio-capitellar capsular complex. J Orthop. 2014;11(2):82-86.
8. Gay DM, Raphael BS, Weiland AJ. Revision arthroscopic contracture release in the elbow resulting in an ulnar nerve transection: a case report. J Bone Joint Surg Am. 2010;92(5):1246-1249.
9. Haapaniemi T, Berggren M, Adolfsson L. Complete transection of the median and radial nerves during arthroscopic release of post-traumatic elbow contracture. Arthroscopy. 1999;15(7):784-787.
10. Yeoh KM, King GJ, Faber KJ, Glazebrook MA, Athwal GS. Evidence-based indications for elbow arthroscopy. Arthroscopy. 2012;28(2):272-282.
11. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. BMJ. 2009;339:b2700.
12. PROSPERO. International Prospective Register of Ongoing Systematic Reviews. The University of York CfRaDP-Iprosr-v. 2013 [cited 2014]. http://www.crd.york.ac.uk/PROSPERO/. Accessed March 17, 2016.
13. Oxford Centre for Evidence-Based Medicine - levels of evidence (March 2009). Centre for Evidence-Based Medicine Web site. http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/. Accessed July 6, 2016.
14. Cowan J, Lozano-Calderόn S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
15. Jones GS, Savoie FH 3rd. Arthroscopic capsular release of flexion contractures (arthrofibrosis) of the elbow. Arthroscopy. 1993;9(3):277-283.
16. O’Brien MJ, Lee Murphy R, Savoie FH 3rd. A preliminary report of acute and subacute arthroscopic repair of the radial ulnohumeral ligament after elbow dislocation in the high-demand patient. Arthroscopy. 2014;30(6):679-687.
17. Rhyou IH, Kim KW. Is posterior synovial plica excision necessary for refractory lateral epicondylitis of the elbow? Clin Orthop Relat Res. 2013;471(1):284-290.
18. Jerosch J, Schunck J. Arthroscopic treatment of lateral epicondylitis: indication, technique and early results. Knee Surg Sports Traumatol Arthrosc. 2006;14(4):379-382.
19. Tijssen M, van Cingel R, van Melick N, de Visser E. Patient-Reported Outcome questionnaires for hip arthroscopy: a systematic review of the psychometric evidence. BMC Musculoskelet Disord. 2011;12:117.
1. Khanchandani P. Elbow arthroscopy: review of the literature and case reports. Case Rep Orthop. 2012;2012:478214.
2. Dodson CC, Nho SJ, Williams RJ 3rd, Altchek DW. Elbow arthroscopy. J Am Acad Orthop Surg. 2008;16(10):574-585.
3. Takahara M, Mura N, Sasaki J, Harada M, Ogino T. Classification, treatment, and outcome of osteochondritis dissecans of the humeral capitellum. Surgical technique. J Bone Joint Surg Am. 2008;90(suppl 2 Pt 1):47-62.
4. Kelly EW, Morrey BF, O’Driscoll SW. Complications of elbow arthroscopy. J Bone Joint Surg Am. 2001;83-A(1):25-34.
5. Rajeev A, Pooley J. Lateral compartment cartilage changes and lateral elbow pain. Acta Orthop Belg. 2009;75(1):37-40.
6. Miyake J, Shimada K, Oka K, et al. Arthroscopic debridement in the treatment of patients with osteoarthritis of the elbow, based on computer simulation. Bone Joint J. 2014;96-B(2):237-241.
7. Babaqi AA, Kotb MM, Said HG, AbdelHamid MM, ElKady HA, ElAssal MA. Short-term evaluation of arthroscopic management of tennis elbow; including resection of radio-capitellar capsular complex. J Orthop. 2014;11(2):82-86.
8. Gay DM, Raphael BS, Weiland AJ. Revision arthroscopic contracture release in the elbow resulting in an ulnar nerve transection: a case report. J Bone Joint Surg Am. 2010;92(5):1246-1249.
9. Haapaniemi T, Berggren M, Adolfsson L. Complete transection of the median and radial nerves during arthroscopic release of post-traumatic elbow contracture. Arthroscopy. 1999;15(7):784-787.
10. Yeoh KM, King GJ, Faber KJ, Glazebrook MA, Athwal GS. Evidence-based indications for elbow arthroscopy. Arthroscopy. 2012;28(2):272-282.
11. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. BMJ. 2009;339:b2700.
12. PROSPERO. International Prospective Register of Ongoing Systematic Reviews. The University of York CfRaDP-Iprosr-v. 2013 [cited 2014]. http://www.crd.york.ac.uk/PROSPERO/. Accessed March 17, 2016.
13. Oxford Centre for Evidence-Based Medicine - levels of evidence (March 2009). Centre for Evidence-Based Medicine Web site. http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/. Accessed July 6, 2016.
14. Cowan J, Lozano-Calderόn S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
15. Jones GS, Savoie FH 3rd. Arthroscopic capsular release of flexion contractures (arthrofibrosis) of the elbow. Arthroscopy. 1993;9(3):277-283.
16. O’Brien MJ, Lee Murphy R, Savoie FH 3rd. A preliminary report of acute and subacute arthroscopic repair of the radial ulnohumeral ligament after elbow dislocation in the high-demand patient. Arthroscopy. 2014;30(6):679-687.
17. Rhyou IH, Kim KW. Is posterior synovial plica excision necessary for refractory lateral epicondylitis of the elbow? Clin Orthop Relat Res. 2013;471(1):284-290.
18. Jerosch J, Schunck J. Arthroscopic treatment of lateral epicondylitis: indication, technique and early results. Knee Surg Sports Traumatol Arthrosc. 2006;14(4):379-382.
19. Tijssen M, van Cingel R, van Melick N, de Visser E. Patient-Reported Outcome questionnaires for hip arthroscopy: a systematic review of the psychometric evidence. BMC Musculoskelet Disord. 2011;12:117.
Clinical Outcomes of Anatomical Total Shoulder Arthroplasty in a Young, Active Population
Although total shoulder arthroplasty (TSA) has proved to be a reliable solution in older patients, treatment in younger patients with glenohumeral arthritis remains controversial, and there are still few reliable long-term surgical options.1-8 These options include abrasion arthroplasty and arthroscopic management,9,10 biologic glenoid resurfacing,11,12 and humeral hemiarthroplasty with13 or without14,15 glenoid treatment and anatomical TSA.
In the younger cohort, 20-year TSA survivorship rates up to 84% have been reported, and unsatisfactory subjective outcomes have been unacceptably high.16 In addition, there is a paucity of literature addressing the impact of TSA on return to sport. Recommendations on returning to an athletic life style are based largely on surveys of expert opinion17,18 and heterogeneous studies of either older patients (eg, age >50-55 years) who are active19-21 or younger patients with no defined level of activity.5,7,8,16,22-24
To our knowledge, no one has evaluated the short-term morbidity and clinical outcomes within a young, high-demand patient population, such as the US military. Therefore, we conducted a study to evaluate the clinical success and complications of TSA performed for glenohumeral arthritis in a young, active population. We hypothesized that patients who had undergone TSA would have a low rate of return to duty, an increased rate of component failure, and a higher reoperation rate because of increased upper extremity demands.
Materials and Methods
After obtaining protocol approval from the William Beaumont Army Medical Center Institutional Review Board, we searched the Military Health System (MHS) Management Analysis and Reporting Tool (M2) database to retrospectively review the cases of all tri-service US military service members who had undergone primary anatomical TSA (Current Procedural Terminology code 23472) between January 1, 2007 and June 31, 2014. This was a multisurgeon, multicenter study. Patient exclusion criteria were nonmilitary or retired status at time of surgery; primary surgery consisting of limited glenohumeral resurfacing procedure, hemiarthroplasty, or reverse TSA; surgery for acute proximal humerus fracture; rotator cuff deficiency diagnosed before or during surgery; and insufficient follow-up (eg, <12 months, unless medically separated beforehand).
The M2 database is an established tool that has been used for clinical outcomes research on treatment of a variety of orthopedic conditions.25,26 The Medical Data Repository, which is operated by MHS, is populated by its military healthcare providers. The MHS, which offers worldwide coverage for all beneficiaries either at Department of Defense facilities or purchased using civilian providers, is among the largest known closed healthcare systems.
All active-duty US military service members are uniformly required to adhere to stringent and regularly evaluated physical fitness standards, which typically exceed those of average civilians. Routine physical training is required in the form of aerobic fitness, weight training, tactical field exercises, and core military tasks, such as the ability to march at least 2 miles while carrying heavy fighting loads. In addition to satisfying required height and weight standards, all service members are subject to semiannual service-specific physical fitness evaluations inclusive of timed push-ups, sit-ups, and an aerobic event. Service members may also be required to maintain a level of physical training above these baseline standards, contingent on their branch of service, rank, and military occupational specialty. If a service member is unable to maintain these standards, medical separation may be initiated.
Demographic and occupational data were extracted from the database. These data included age, sex, military rank, and branch of service. Line-by-line analysis of the Armed Forces Health Longitudinal Technology Application (Version 22; 3M) electronic medical record was then performed to confirm the underlying diagnosis, surgical procedure, and surgery date. Further chart review yielded additional patient-based factors (eg, laterality, hand dominance, presence and type of prior shoulder surgeries) and surgical factors (eg, surgery indication, implant design). We evaluated clinical and functional outcomes as well as perioperative complications, including both major and minor systemic and local complications as previously described27,28; preoperative and postoperative range of motion (ROM) and self-reported pain score (SRPS, scale 1-10) as measured by physical therapist and surgeon at follow-up; secondary surgical interventions; timing of return to duty; and postoperative deployment history. The primary outcome measures were revision reoperation after index procedure, and military discharge for persistent shoulder-related disability. Clinical failure was defined as component failure or reoperation. Medical Evaluation Board (MEB) is a formal separation from the military in which it is deemed that a service member is no longer able to fulfill his or her duty because of a medical condition.
Statistical Analysis
Continuous variables were compared using statistical means with 95% confidence intervals (CIs) and/or SDs. Categorical data were reported as frequencies or percentages. Univariate analysis was performed to assess the correlation between possible risk factors and the primary outcome measures. P < .05 was considered statistically significant.
Results
Demographics
We identified 24 service members (26 shoulders) who had undergone anatomical TSA during the study period (Table 1). Mean (SD) age was 45.8 (4.5) years (range, 35-54 years), and the cohort was predominately male (25/26 shoulders; 96.2%). Most cohort members were of senior enlisted rank (14, 58.3%), and the US Army was the predominant branch of military service (13, 54.2%). The right side was the operative extremity in 7 cases (26.9%), and the dominant shoulder was involved in 6 cases (23.1%). Two patients (8.3%) underwent staged bilateral TSA. Most patients (76.9%) underwent TSA on the nondominant extremity.
Surgical Variables
TSA was indicated for post-instability arthropathy in 13 cases (50.0%), posttraumatic osteoarthritis in 7 cases (26.9%), and unspecified glenohumeral arthritis, which includes primary glenohumeral osteoarthritis, in 5 cases (19.2%) (Table 2). One case was attributed to iatrogenically induced chondrolysis secondary to intra-articular lidocaine pump. Twelve patients (46.2%) had at least 1 previous surgery. Of the shoulders with instability, 10 (76.9%) had undergone a total of 14 surgical stabilization procedures—10 anterior labral repairs, 2 posterior labral repairs, and 2 capsular plications. The other shoulders had undergone a total of 18 procedures, which included 4 rotator cuff repairs and 3 cartilage restoration procedures.
Clinical Outcomes
Mean (SD) follow-up was 41.0 (21.3) months (range, 11.6-97.6 months). All but 1 shoulder (96.2%) had follow-up of 12 months or more (the only patient with shorter follow-up was because of MEB), and 76.9% of patients had follow-up of 24 months or more (4 of the 6 patients with follow-up under 24 months were medically separated) (Table 3). In all cases, mean ROM improved with respect to flexion, abduction, and external rotation. At final follow-up, mean (SD) ROM was 138° (36°) forward flexion (range, 60°-180°), 125° (39°) abduction (range, 45°-180°), 48° (19°) external rotation at 0° abduction (range, 20°-90°), and 80° (9.4°) external rotation at 90° abduction (range, 70°-90°). Preoperative flexion, abduction, and external rotation at 0° and 90° abduction were all improved at final follow-up. The most improvement in ROM occurred within 6 months after surgery.
Overall patient satisfaction with surgery was 92.3% (n = 24). Ultimately, 18 (72.0%) of 25 shoulders with follow-up of 1 year or more were able to return to active duty within 1 year after surgery, though only 10 (45.5%) of 22 with follow-up of 2 years or more remained active 2 years after surgery. Furthermore, 5 patients (20.8%) were deployed after surgery, and all were still on active duty at final follow-up. By final follow-up, 9 (37.5%) of 24 service members were unable to return to military function; 7 had been medically discharged from the military for persistent shoulder disability, and 2 were in the process of being medically discharged.
In all cases, SRPS improved from before surgery (5.2 out of 10) to final follow-up (1.4). At final follow-up, 22 patients (88.0%) reported mild pain (0-3), and no one had pain above 6.
Complications
Nine patients had a total of 12 postoperative complications (46.2%): 6 component failures (23.1%), 2 neurologic injuries (7.7%; 1 permanent axillary nerve injury, 1 transient brachial plexus neuritis), 2 cases of adhesive capsulitis (7.7%), and 2 episodes of venous thrombosis (7.7%; 1 superficial, 1 deep) (Table 4). There were no documented infections. Six reoperations (23.1%) were performed for the 6 component failures (2 traumatic dislocations of prosthesis resulting in acute glenoid component failure, 3 cases of atraumatic glenoid loosening, 1 case of humeral stem loosening after periprosthetic fracture). Atraumatic glenoid component loosening occurred a mean (SD) of 40.6 (14.2) months after surgery (range, 20.8-54.2 months).
Surgical Failures
Eight service members underwent MEB. Six patients experienced component failure. Factors contributing to both clinical failure and separation from active duty by means of MEB were evaluated with univariate analysis (Table 5). No statistically significant risk factors, including surgical revision and presence of perioperative complications, were identified.
Discussion
We confirmed that our cohort of young service members (mean age, 45.8 years), who had undergone TSA for glenohumeral arthritis, had a relatively higher rate of component failure (23.1%) and a higher reoperation rate (23.1%) with low rates of return to military duty at short-term to midterm follow-up. Our results parallel those of a limited series with a younger cohort (Table 6).7,16,19,21,23,24 The high demand and increased life expectancy of the younger patients with glenohumeral arthritis potentiates the risk of complications, component loosening, and ultimate failure.29 To our knowledge, the present article is the first to report clinical and functional outcomes and perioperative risk profiles in a homogenously young, active military cohort after TSA.
The mean age of our study population (46 years) is one of the lowest in the literature. TSA in younger patients (age, <50-55 years) and older, active patients (>55 years) has received increased attention as a result of the expanding indications and growing popularity of TSA in these groups. Other studies have upheld the efficacy of TSA in achieving predictable pain relief and functional improvement in a diverse and predominantly elderly population.15,30-34 Alternative treatments, including humeral head resurfacing15,30,35 and soft-tissue interposition,15,36-40 have also shown inferior short- and long-term results in terms of longevity and degree of clinical or functional improvement.31-34,41 In addition, the ream-and-run technique has had promising early results by improving glenohumeral kinematics, pain relief, and shoulder function.13,42,43 However, although implantation of a glenoid component is avoided in young, active people because of reduced longevity and higher rates of component failure, the trade-offs are inadequately treated glenoid disease, suboptimal pain relief, and progression of glenoid arthritis eventually requiring revision. Furthermore, midterm and long-term survivorship of TSA in general is unknown, and there remain few good options for treating end-stage arthritis in young, active patients.
Our cohort had high rates of complications (46.2%) and revisions (23.1%). Two in 5 patients had postoperative complications, most commonly component failure resulting in reoperation. In the literature, complication rates among young patients who underwent TSA are much lower (4.8%-10.9%).16,23,24 Our cohort’s most common complication was component failure (23.1%), which was most often attributed to atraumatic, aseptic glenoid component loosening and required reoperation. Previously reported revision rates in a young population that underwent TSA (0%-11%)16,23,24 were also significantly lower than those in the present analysis (23.1%), underscoring the impact of operative indications, postoperative activity levels, and occupational demands on ultimate failure rates. Interestingly, all revisions in our study were for component failure, whereas previous reports have described a higher rate for infection.22 However, the same studies also found glenoid lucency rates as high as 76% at 10-year follow-up.16 Furthermore, in a review of 136 TSAs with unsatisfactory outcomes, glenoid loosening was the most common reason for presenting to clinic after surgery.44 Specifically, our population had a high rate of glenohumeral arthritis secondary to instability (50.0%) and posttraumatic osteoarthritis (26.9%). For many reasons, outcomes were worse in younger patients with a history of glenohumeral instability33 than in older patients without a high incidence of instability.45 This young cohort with higher demands may have had accelerated polyethylene wear patterns caused by repetitive overhead activity, which may have arisen because of a higher functional profile after surgery and greater patient expectations after arthroplasty. In addition, patients with a history of instability may have altered glenohumeral anatomy, especially with previous arthroscopic or open stabilization procedures. Anatomical changes include excessive posterior glenoid wear, internal rotation contracture, patulous capsular tissue, static or dynamic posterior humeral subluxation, and possible overconstraint after prior stabilization procedures. Almost half of our population had a previous surgery; our patients averaged 1.7 previous surgeries each.
Although estimates of component survivorship at a high-volume civilian tertiary-referral center were as high as 97% at 10 years and 84% at 20 years,7,16 10-year survivorship in patients with a history of instability was only 61%.3 TSA survivorship in our young, active cohort is already foreseeably dramatically reduced, given the 23.1% revision rate at 28.5-month follow-up. This consideration must be addressed during preoperative counseling with the young patient with glenohumeral arthritis and a history of shoulder instability.
Despite the high rates of complications and revisions in our study, 92.3% of patients were satisfied with surgery, 88.0% experienced minimal persistence of pain (mean 3.8-point decrease on SRPS), and 100% maintained improved ROM at final follow-up. Satisfaction in the young population has varied significantly, from 52% to 95%, generally on the basis of physical activity.16,22-24 The reasonable rate of postoperative satisfaction in the present analysis is comparable to what has been reported in patients of a similar age (Table 6).7,16,22 However, despite high satisfaction and pain relief, patients were inconsistently able to return to the upper limits of physical activity required of active-duty military service. In addition, we cannot exclude secondary gain motivations for pursuing medical retirement, similar to that seen in patients receiving worker’s compensation.
Other authors have conversely found more favorable functional outcomes and survivorship rates.23,24 In a retrospective review of 46 TSAs in patients 55 years or younger, Bartelt and colleagues24 found sustained improvements in pain, ROM, and satisfaction at 7-year follow-up.24 Raiss and colleagues23 conducted a prospective study of TSA outcomes in 21 patients with a mean age of 55 years and a mean follow-up of 7 years and reported no revisions and only 1 minor complication, a transient brachial plexus palsy.23 The discrepancy between these studies may reflect different activity levels and underlying pathology between cohorts. The present population is unique in that it represents a particularly difficult confluence of factors for shoulder arthroplasty surgeons. The high activity, significant overhead and lifting occupational demands, and discordant patient expectations of this military cohort place a significant functional burden on the implants, the glenoid component in particular. Furthermore, this patient group has a higher incidence of more complex glenohumeral pathology resulting in instability, posttraumatic, or capsulorrhaphy arthropathy, and multiple prior arthroscopic and open stabilization procedures.
At final follow-up, only 33% of our patients were still on activity duty, 37.5% had completed or were completing medical separation from the military after surgery for persistent shoulder disability, and 37.5% were retired from the military. Five patients (20.8%) deployed after surgery. This young, active cohort of service members who had TSA for glenohumeral arthritis faced a unique set of tremendous physical demands. A retrospective case series investigated return to sport in 100 consecutive patients (mean age, 68.9 years) who were participating in recreational and competitive athletics and underwent unilateral TSA.21 The patients were engaged most commonly in swimming (20.4%), golf (16.3%), cycling (16.3%), and fitness training (16.3%). The authors found that, at a mean follow-up of 2.8 years, 49 patients (89%) were able to continue in sports, though 36.7% thought their sport activity was restricted after TSA. In another retrospective case series (61 TSAs), McCarty and colleagues19 found that 48 patients (71%) were improved in their sports participation, and 50% increased their frequency of participation after surgery.
There are no specific recommendations on returning to military service or high-level sport after surgery. Recommendations on returning to sport after TSA have been based largely on small case series involving specific sports46,47 and surveys of expert opinion.17,18 In a survey on postoperative physical activity in young patients after TSA conducted by Healy and colleagues,17 35 American Shoulder and Elbow Surgeons members recommended avoiding contact and impact sports while permitting return to nonimpact sports, such as swimming, which may still impart significant stress to the glenohumeral joint. In an international survey of 101 shoulder and elbow surgeons, Magnussen and colleagues18 also found that most recommended avoiding a return to impact sports that require intensive upper extremity demands and permitting full return to sports at preoperative levels. This likely is a result of the perception that most of these patients having TSA are older and have less rigorous involvement in sports at the outset and a lower propensity for adverse patient outcomes. However, these recommendations may place a younger, more high-demand patient at significantly greater risk. The active-duty cohort engages in daily physical training, including push-ups and frequent overhead lifting, which could account for the high failure rates and low incidence of postoperative deployment. Although TSA seems to demonstrate good initial results in terms of return to high-demand activities, the return-to-duty profile in our study highlights the potential pitfalls of TSA in active individuals attempting to return to high-demand preoperative function.
Our analysis was limited by the fact that we used a small patient cohort, contributing to underpowered analysis of the potential risk factors predictive of reoperation and medical discharge. Although our minimum follow-up was 12 months, with the exception of 1 patient who was medically separated at 11.6 months because of shoulder disability, we captured 5 patients (19.2%) who underwent medical separation but who would otherwise be excluded. Therefore, this limitation is not major in that, with a longer minimum follow-up, we would be excluding a significant number of patients with such persistent disability after TSA that they would not be able to return to duty at anywhere near their previous level. In this retrospective study, we were additionally limited to analysis of the data in the medical records and could not control for variables such as surgeon technique, implant choice, and experience. Complete radiographic images were not available, limiting analysis of radiographic outcomes. Given the lack of a standardized preoperative imaging protocol, we could not evaluate glenoid version on axial imaging. It is possible that some patients with early aseptic glenoid loosening had posterior subluxation or a Walch B2 glenoid, which has a higher failure rate.48 The strengths of this study include its unique analysis of a homogeneous young, active, high-risk patient cohort within a closed healthcare system. In the military, these patients are subject to intense daily physical and occupational demands. In addition, the clinical and functional outcomes we studied are patient-centered and therefore relevant during preoperative counseling. Further investigations might focus on validated outcome measures and on midterm to long-term TSA outcomes in an active military population vis-à-vis other alternatives for clinical management.
Conclusion
By a mean follow-up of 3.5 years, only a third of the service members had returned to active duty, roughly a third had retired, and more than a third had been medically discharged because of persistent disability attributable to the shoulder. Despite initial improvements in ROM and pain, midterm outcomes were poor. The short-term complication rate (46.2%) and the rate of reoperation for component failure (23.1%) should be emphasized during preoperative counseling.
1. Tokish JM. The mature athlete’s shoulder. Sports Health. 2014;6(1):31-35.
2. Sperling JW, Cofield RH. Revision total shoulder arthroplasty for the treatment of glenoid arthrosis. J Bone Joint Surg Am. 1998;80(6):860-867.
3. Sperling JW, Antuna SA, Sanchez-Sotelo J, Schleck C, Cofield RH. Shoulder arthroplasty for arthritis after instability surgery. J Bone Joint Surg Am. 2002;84(10):1775-1781.
4. Izquierdo R, Voloshin I, Edwards S, et al; American Academy of Orthopaedic Surgeons. Treatment of glenohumeral osteoarthritis. J Am Acad Orthop Surg. 2010;18(6):375-382.
5. Johnson MH, Paxton ES, Green A. Shoulder arthroplasty options in young (<50 years old) patients: review of current concepts. J Shoulder Elbow Surg. 2015;24(2):317-325.
6. Cole BJ, Yanke A, Provencher MT. Nonarthroplasty alternatives for the treatment of glenohumeral arthritis. J Shoulder Elbow Surg. 2007;16(5 suppl):S231-S240.
7. Denard PJ, Raiss P, Sowa B, Walch G. Mid- to long-term follow-up of total shoulder arthroplasty using a keeled glenoid in young adults with primary glenohumeral arthritis. J Shoulder Elbow Surg. 2013;22(7):894-900.
8. Denard PJ, Wirth MA, Orfaly RM. Management of glenohumeral arthritis in the young adult. J Bone Joint Surg Am. 2011;93(9):885-892.
9. Millett PJ, Horan MP, Pennock AT, Rios D. Comprehensive arthroscopic management (CAM) procedure: clinical results of a joint-preserving arthroscopic treatment for young, active patients with advanced shoulder osteoarthritis. Arthroscopy. 2013;29(3):440-448.
10 Millett PJ, Gaskill TR. Arthroscopic management of glenohumeral arthrosis: humeral osteoplasty, capsular release, and arthroscopic axillary nerve release as a joint-preserving approach. Arthroscopy. 2011;27(9):1296-1303.
11. Savoie FH 3rd, Brislin KJ, Argo D. Arthroscopic glenoid resurfacing as a surgical treatment for glenohumeral arthritis in the young patient: midterm results. Arthroscopy. 2009;25(8):864-871.
12. Strauss EJ, Verma NN, Salata MJ, et al. The high failure rate of biologic resurfacing of the glenoid in young patients with glenohumeral arthritis. J Shoulder Elbow Surg. 2014;23(3):409-419.
13. Matsen FA 3rd, Warme WJ, Jackins SE. Can the ream and run procedure improve glenohumeral relationships and function for shoulders with the arthritic triad? Clin Orthop Relat Res. 2015;473(6):2088-2096.
14. Lo IK, Litchfield RB, Griffin S, Faber K, Patterson SD, Kirkley A. Quality-of-life outcome following hemiarthroplasty or total shoulder arthroplasty in patients with osteoarthritis. A prospective, randomized trial. J Bone Joint Surg Am. 2005;87(10):2178-2185.
15. Wirth M, Tapscott RS, Southworth C, Rockwood CA Jr. Treatment of glenohumeral arthritis with a hemiarthroplasty: a minimum five-year follow-up outcome study. J Bone Joint Surg Am. 2006;88(5):964-973.
16. Sperling JW, Cofield RH, Rowland CM. Minimum fifteen-year follow-up of Neer hemiarthroplasty and total shoulder arthroplasty in patients aged fifty years or younger. J Shoulder Elbow Surg. 2004;13(6):604-613.
17. Healy WL, Iorio R, Lemos MJ. Athletic activity after joint replacement. Am J Sports Med. 2001;29(3):377-388.
18. Magnussen RA, Mallon WJ, Willems WJ, Moorman CT 3rd. Long-term activity restrictions after shoulder arthroplasty: an international survey of experienced shoulder surgeons. J Shoulder Elbow Surg. 2011;20(2):281-289.
19. McCarty EC, Marx RG, Maerz D, Altchek D, Warren RF. Sports participation after shoulder replacement surgery. Am J Sports Med. 2008;36(8):1577-1581.
20. Schmidt-Wiethoff R, Wolf P, Lehmann M, Habermeyer P. Physical activity after shoulder arthroplasty [in German]. Sportverletz Sportschaden. 2002;16(1):26-30.
21. Schumann K, Flury MP, Schwyzer HK, Simmen BR, Drerup S, Goldhahn J. Sports activity after anatomical total shoulder arthroplasty. Am J Sports Med. 2010;38(10):2097-2105.
22. Sperling JW, Cofield RH, Rowland CM. Neer hemiarthroplasty and Neer total shoulder arthroplasty in patients fifty years old or less. Long-term results. J Bone Joint Surg Am. 1998;80(4):464-473.
23. Raiss P, Aldinger PR, Kasten P, Rickert M, Loew M. Total shoulder replacement in young and middle-aged patients with glenohumeral osteoarthritis. J Bone Joint Surg Br. 2008;90(6):764-769.
24. Bartelt R, Sperling JW, Schleck CD, Cofield RH. Shoulder arthroplasty in patients aged fifty-five years or younger with osteoarthritis. J Shoulder Elbow Surg. 2011;20(1):123-130.
25. Waterman BR, Burns TC, McCriskin B, Kilcoyne K, Cameron KL, Owens BD. Outcomes after Bankart repair in a military population: predictors for surgical revision and long-term disability. Arthroscopy. 2014;30(2):172-177.
26. Waterman BR, Liu J, Newcomb R, Schoenfeld AJ, Orr JD, Belmont PJ Jr. Risk factors for chronic exertional compartment syndrome in a physically active military population. Am J Sports Med. 2013;41(11):2545-2549.
27. Chalmers PN, Gupta AK, Rahman Z, Bruce B, Romeo AA, Nicholson GP. Predictors of early complications of total shoulder arthroplasty. J Arthroplasty. 2014;29(4):856-860.
28. Dunn JC, Lanzi J, Kusnezov N, Bader J, Waterman BR, Belmont PJ Jr. Predictors of length of stay after elective total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2015;24(5):754-759.
29. Hayes PR, Flatow EL. Total shoulder arthroplasty in the young patient. Instr Course Lect. 2001;50;73-88.
30. Rispoli DM, Sperling JW, Athwal GS, Schleck CD, Cofield RH. Humeral head replacement for the treatment of osteoarthritis. J Bone Joint Surg Am. 2006;88(12):2637-2644.
31. Radnay CS, Setter KJ, Chambers L, Levine WN, Bigliani LU, Ahmad CS. Total shoulder replacement compared with humeral head replacement for the treatment of primary glenohumeral osteoarthritis: a systematic review. J Shoulder Elbow Surg. 2007;16(4):396-402.
32. Gartsman GM, Roddey TS, Hammerman SM. Shoulder arthroplasty with or without resurfacing of the glenoid in patients who have osteoarthritis. J Bone Joint Surg Am. 2000;82(1):26-34.
33. Edwards TB, Kadakia NR, Boulahia A, et al. A comparison of hemiarthroplasty and total shoulder arthroplasty in the treatment of primary glenohumeral osteoarthritis: results of a multicenter study. J Shoulder Elbow Surg. 2003;12(3):
207-213.
34. Bryant D, Litchfield R, Sandow M, Gartsman GM, Guyatt G, Kirkley A. A comparison of pain, strength, range of motion, and functional outcomes after hemiarthroplasty and total shoulder arthroplasty in patients with osteoarthritis of the shoulder. A systematic review and meta-analysis. J Bone Joint Surg Am. 2005;87(9):1947-1956.
35. Bailie DS, Llinas PJ, Ellenbecker TS. Cementless humeral resurfacing arthroplasty in active patients less than fifty-five years of age. J Bone Joint Surg Am. 2008;90(1):110-117.
36. Ball CM, Galatz LM, Yamaguchi K. Meniscal allograft interposition arthroplasty for the arthritic shoulder: description of a new surgical technique. Tech Shoulder Elbow Surg. 2001;2:247-254.
37. Elhassan B, Ozbaydar M, Diller D, Higgins LD, Warner JJ. Soft-tissue resurfacing of the glenoid in the treatment of glenohumeral arthritis in active patients less than fifty years old. J Bone Joint Surg Am. 2009;91(2):419-424.
38. Krishnan SG, Nowinski RJ, Harrison D, Burkhead WZ. Humeral hemiarthroplasty with biologic resurfacing of the glenoid for glenohumeral arthritis. Two to fifteen-year outcomes. J Bone Joint Surg Am. 2007;89(4):727-734.
39. Lee KT, Bell S, Salmon J. Cementless surface replacement arthroplasty of the shoulder with biologic resurfacing of the glenoid. J Shoulder Elbow Surg. 2009;18(6):915-919.
40. Nicholson GP, Goldstein JL, Romeo AA, et al. Lateral meniscus allograft biologic glenoid arthroplasty in total shoulder arthroplasty for young shoulders with degenerative joint disease. J Shoulder Elbow Surg. 2007;16(5 suppl):S261-S266.
41. Carroll RM, Izquierdo R, Vazquez M, Blaine TA, Levine WN, Bigliani LU. Conversion of painful hemiarthroplasty to total shoulder arthroplasty: long-term results. J Shoulder Elbow Surg. 2004;13(6):599-603.
42. Clinton J, Franta AK, Lenters TR, Mounce D, Matsen FA 3rd. Nonprosthetic glenoid arthroplasty with humeral hemiarthroplasty and total shoulder arthroplasty yield similar self-assessed outcomes in the management of comparable patients with glenohumeral arthritis. J Shoulder Elbow Surg. 2007;16(5):534-538.
43. Gilmer BB, Comstock BA, Jette JL, Warme WJ, Jackins SE, Matsen FA. The prognosis for improvement in comfort and function after the ream-and-run arthroplasty for glenohumeral arthritis: an analysis of 176 consecutive cases. J Bone Joint Surg Am. 2012;94(14):e102.
44. Franta AK, Lenters TR, Mounce D, Neradilek B, Matsen FA 3rd. The complex characteristics of 282 unsatisfactory shoulder arthroplasties. J Shoulder Elbow Surg. 2007;16(5):555-562.
45. Godenèche A, Boileau P, Favard L, et al. Prosthetic replacement in the treatment of osteoarthritis of the shoulder: early results of 268 cases. J Shoulder Elbow Surg. 2002;11(1):11-18.
46. Jensen KL, Rockwood CA Jr. Shoulder arthroplasty in recreational golfers. J Shoulder Elbow Surg. 1998;7(4):362-367.
47. Kirchhoff C, Imhoff AB, Hinterwimmer S. Winter sports and shoulder arthroplasty [in German]. Sportverletz Sportschaden. 2008;22(3):153-158.
48. Raiss P, Edwards TB, Deutsch A, et al. Radiographic changes around humeral components in shoulder arthroplasty. J Bone Joint Surg Am. 2014;96(7):e54.
Although total shoulder arthroplasty (TSA) has proved to be a reliable solution in older patients, treatment in younger patients with glenohumeral arthritis remains controversial, and there are still few reliable long-term surgical options.1-8 These options include abrasion arthroplasty and arthroscopic management,9,10 biologic glenoid resurfacing,11,12 and humeral hemiarthroplasty with13 or without14,15 glenoid treatment and anatomical TSA.
In the younger cohort, 20-year TSA survivorship rates up to 84% have been reported, and unsatisfactory subjective outcomes have been unacceptably high.16 In addition, there is a paucity of literature addressing the impact of TSA on return to sport. Recommendations on returning to an athletic life style are based largely on surveys of expert opinion17,18 and heterogeneous studies of either older patients (eg, age >50-55 years) who are active19-21 or younger patients with no defined level of activity.5,7,8,16,22-24
To our knowledge, no one has evaluated the short-term morbidity and clinical outcomes within a young, high-demand patient population, such as the US military. Therefore, we conducted a study to evaluate the clinical success and complications of TSA performed for glenohumeral arthritis in a young, active population. We hypothesized that patients who had undergone TSA would have a low rate of return to duty, an increased rate of component failure, and a higher reoperation rate because of increased upper extremity demands.
Materials and Methods
After obtaining protocol approval from the William Beaumont Army Medical Center Institutional Review Board, we searched the Military Health System (MHS) Management Analysis and Reporting Tool (M2) database to retrospectively review the cases of all tri-service US military service members who had undergone primary anatomical TSA (Current Procedural Terminology code 23472) between January 1, 2007 and June 31, 2014. This was a multisurgeon, multicenter study. Patient exclusion criteria were nonmilitary or retired status at time of surgery; primary surgery consisting of limited glenohumeral resurfacing procedure, hemiarthroplasty, or reverse TSA; surgery for acute proximal humerus fracture; rotator cuff deficiency diagnosed before or during surgery; and insufficient follow-up (eg, <12 months, unless medically separated beforehand).
The M2 database is an established tool that has been used for clinical outcomes research on treatment of a variety of orthopedic conditions.25,26 The Medical Data Repository, which is operated by MHS, is populated by its military healthcare providers. The MHS, which offers worldwide coverage for all beneficiaries either at Department of Defense facilities or purchased using civilian providers, is among the largest known closed healthcare systems.
All active-duty US military service members are uniformly required to adhere to stringent and regularly evaluated physical fitness standards, which typically exceed those of average civilians. Routine physical training is required in the form of aerobic fitness, weight training, tactical field exercises, and core military tasks, such as the ability to march at least 2 miles while carrying heavy fighting loads. In addition to satisfying required height and weight standards, all service members are subject to semiannual service-specific physical fitness evaluations inclusive of timed push-ups, sit-ups, and an aerobic event. Service members may also be required to maintain a level of physical training above these baseline standards, contingent on their branch of service, rank, and military occupational specialty. If a service member is unable to maintain these standards, medical separation may be initiated.
Demographic and occupational data were extracted from the database. These data included age, sex, military rank, and branch of service. Line-by-line analysis of the Armed Forces Health Longitudinal Technology Application (Version 22; 3M) electronic medical record was then performed to confirm the underlying diagnosis, surgical procedure, and surgery date. Further chart review yielded additional patient-based factors (eg, laterality, hand dominance, presence and type of prior shoulder surgeries) and surgical factors (eg, surgery indication, implant design). We evaluated clinical and functional outcomes as well as perioperative complications, including both major and minor systemic and local complications as previously described27,28; preoperative and postoperative range of motion (ROM) and self-reported pain score (SRPS, scale 1-10) as measured by physical therapist and surgeon at follow-up; secondary surgical interventions; timing of return to duty; and postoperative deployment history. The primary outcome measures were revision reoperation after index procedure, and military discharge for persistent shoulder-related disability. Clinical failure was defined as component failure or reoperation. Medical Evaluation Board (MEB) is a formal separation from the military in which it is deemed that a service member is no longer able to fulfill his or her duty because of a medical condition.
Statistical Analysis
Continuous variables were compared using statistical means with 95% confidence intervals (CIs) and/or SDs. Categorical data were reported as frequencies or percentages. Univariate analysis was performed to assess the correlation between possible risk factors and the primary outcome measures. P < .05 was considered statistically significant.
Results
Demographics
We identified 24 service members (26 shoulders) who had undergone anatomical TSA during the study period (Table 1). Mean (SD) age was 45.8 (4.5) years (range, 35-54 years), and the cohort was predominately male (25/26 shoulders; 96.2%). Most cohort members were of senior enlisted rank (14, 58.3%), and the US Army was the predominant branch of military service (13, 54.2%). The right side was the operative extremity in 7 cases (26.9%), and the dominant shoulder was involved in 6 cases (23.1%). Two patients (8.3%) underwent staged bilateral TSA. Most patients (76.9%) underwent TSA on the nondominant extremity.
Surgical Variables
TSA was indicated for post-instability arthropathy in 13 cases (50.0%), posttraumatic osteoarthritis in 7 cases (26.9%), and unspecified glenohumeral arthritis, which includes primary glenohumeral osteoarthritis, in 5 cases (19.2%) (Table 2). One case was attributed to iatrogenically induced chondrolysis secondary to intra-articular lidocaine pump. Twelve patients (46.2%) had at least 1 previous surgery. Of the shoulders with instability, 10 (76.9%) had undergone a total of 14 surgical stabilization procedures—10 anterior labral repairs, 2 posterior labral repairs, and 2 capsular plications. The other shoulders had undergone a total of 18 procedures, which included 4 rotator cuff repairs and 3 cartilage restoration procedures.
Clinical Outcomes
Mean (SD) follow-up was 41.0 (21.3) months (range, 11.6-97.6 months). All but 1 shoulder (96.2%) had follow-up of 12 months or more (the only patient with shorter follow-up was because of MEB), and 76.9% of patients had follow-up of 24 months or more (4 of the 6 patients with follow-up under 24 months were medically separated) (Table 3). In all cases, mean ROM improved with respect to flexion, abduction, and external rotation. At final follow-up, mean (SD) ROM was 138° (36°) forward flexion (range, 60°-180°), 125° (39°) abduction (range, 45°-180°), 48° (19°) external rotation at 0° abduction (range, 20°-90°), and 80° (9.4°) external rotation at 90° abduction (range, 70°-90°). Preoperative flexion, abduction, and external rotation at 0° and 90° abduction were all improved at final follow-up. The most improvement in ROM occurred within 6 months after surgery.
Overall patient satisfaction with surgery was 92.3% (n = 24). Ultimately, 18 (72.0%) of 25 shoulders with follow-up of 1 year or more were able to return to active duty within 1 year after surgery, though only 10 (45.5%) of 22 with follow-up of 2 years or more remained active 2 years after surgery. Furthermore, 5 patients (20.8%) were deployed after surgery, and all were still on active duty at final follow-up. By final follow-up, 9 (37.5%) of 24 service members were unable to return to military function; 7 had been medically discharged from the military for persistent shoulder disability, and 2 were in the process of being medically discharged.
In all cases, SRPS improved from before surgery (5.2 out of 10) to final follow-up (1.4). At final follow-up, 22 patients (88.0%) reported mild pain (0-3), and no one had pain above 6.
Complications
Nine patients had a total of 12 postoperative complications (46.2%): 6 component failures (23.1%), 2 neurologic injuries (7.7%; 1 permanent axillary nerve injury, 1 transient brachial plexus neuritis), 2 cases of adhesive capsulitis (7.7%), and 2 episodes of venous thrombosis (7.7%; 1 superficial, 1 deep) (Table 4). There were no documented infections. Six reoperations (23.1%) were performed for the 6 component failures (2 traumatic dislocations of prosthesis resulting in acute glenoid component failure, 3 cases of atraumatic glenoid loosening, 1 case of humeral stem loosening after periprosthetic fracture). Atraumatic glenoid component loosening occurred a mean (SD) of 40.6 (14.2) months after surgery (range, 20.8-54.2 months).
Surgical Failures
Eight service members underwent MEB. Six patients experienced component failure. Factors contributing to both clinical failure and separation from active duty by means of MEB were evaluated with univariate analysis (Table 5). No statistically significant risk factors, including surgical revision and presence of perioperative complications, were identified.
Discussion
We confirmed that our cohort of young service members (mean age, 45.8 years), who had undergone TSA for glenohumeral arthritis, had a relatively higher rate of component failure (23.1%) and a higher reoperation rate (23.1%) with low rates of return to military duty at short-term to midterm follow-up. Our results parallel those of a limited series with a younger cohort (Table 6).7,16,19,21,23,24 The high demand and increased life expectancy of the younger patients with glenohumeral arthritis potentiates the risk of complications, component loosening, and ultimate failure.29 To our knowledge, the present article is the first to report clinical and functional outcomes and perioperative risk profiles in a homogenously young, active military cohort after TSA.
The mean age of our study population (46 years) is one of the lowest in the literature. TSA in younger patients (age, <50-55 years) and older, active patients (>55 years) has received increased attention as a result of the expanding indications and growing popularity of TSA in these groups. Other studies have upheld the efficacy of TSA in achieving predictable pain relief and functional improvement in a diverse and predominantly elderly population.15,30-34 Alternative treatments, including humeral head resurfacing15,30,35 and soft-tissue interposition,15,36-40 have also shown inferior short- and long-term results in terms of longevity and degree of clinical or functional improvement.31-34,41 In addition, the ream-and-run technique has had promising early results by improving glenohumeral kinematics, pain relief, and shoulder function.13,42,43 However, although implantation of a glenoid component is avoided in young, active people because of reduced longevity and higher rates of component failure, the trade-offs are inadequately treated glenoid disease, suboptimal pain relief, and progression of glenoid arthritis eventually requiring revision. Furthermore, midterm and long-term survivorship of TSA in general is unknown, and there remain few good options for treating end-stage arthritis in young, active patients.
Our cohort had high rates of complications (46.2%) and revisions (23.1%). Two in 5 patients had postoperative complications, most commonly component failure resulting in reoperation. In the literature, complication rates among young patients who underwent TSA are much lower (4.8%-10.9%).16,23,24 Our cohort’s most common complication was component failure (23.1%), which was most often attributed to atraumatic, aseptic glenoid component loosening and required reoperation. Previously reported revision rates in a young population that underwent TSA (0%-11%)16,23,24 were also significantly lower than those in the present analysis (23.1%), underscoring the impact of operative indications, postoperative activity levels, and occupational demands on ultimate failure rates. Interestingly, all revisions in our study were for component failure, whereas previous reports have described a higher rate for infection.22 However, the same studies also found glenoid lucency rates as high as 76% at 10-year follow-up.16 Furthermore, in a review of 136 TSAs with unsatisfactory outcomes, glenoid loosening was the most common reason for presenting to clinic after surgery.44 Specifically, our population had a high rate of glenohumeral arthritis secondary to instability (50.0%) and posttraumatic osteoarthritis (26.9%). For many reasons, outcomes were worse in younger patients with a history of glenohumeral instability33 than in older patients without a high incidence of instability.45 This young cohort with higher demands may have had accelerated polyethylene wear patterns caused by repetitive overhead activity, which may have arisen because of a higher functional profile after surgery and greater patient expectations after arthroplasty. In addition, patients with a history of instability may have altered glenohumeral anatomy, especially with previous arthroscopic or open stabilization procedures. Anatomical changes include excessive posterior glenoid wear, internal rotation contracture, patulous capsular tissue, static or dynamic posterior humeral subluxation, and possible overconstraint after prior stabilization procedures. Almost half of our population had a previous surgery; our patients averaged 1.7 previous surgeries each.
Although estimates of component survivorship at a high-volume civilian tertiary-referral center were as high as 97% at 10 years and 84% at 20 years,7,16 10-year survivorship in patients with a history of instability was only 61%.3 TSA survivorship in our young, active cohort is already foreseeably dramatically reduced, given the 23.1% revision rate at 28.5-month follow-up. This consideration must be addressed during preoperative counseling with the young patient with glenohumeral arthritis and a history of shoulder instability.
Despite the high rates of complications and revisions in our study, 92.3% of patients were satisfied with surgery, 88.0% experienced minimal persistence of pain (mean 3.8-point decrease on SRPS), and 100% maintained improved ROM at final follow-up. Satisfaction in the young population has varied significantly, from 52% to 95%, generally on the basis of physical activity.16,22-24 The reasonable rate of postoperative satisfaction in the present analysis is comparable to what has been reported in patients of a similar age (Table 6).7,16,22 However, despite high satisfaction and pain relief, patients were inconsistently able to return to the upper limits of physical activity required of active-duty military service. In addition, we cannot exclude secondary gain motivations for pursuing medical retirement, similar to that seen in patients receiving worker’s compensation.
Other authors have conversely found more favorable functional outcomes and survivorship rates.23,24 In a retrospective review of 46 TSAs in patients 55 years or younger, Bartelt and colleagues24 found sustained improvements in pain, ROM, and satisfaction at 7-year follow-up.24 Raiss and colleagues23 conducted a prospective study of TSA outcomes in 21 patients with a mean age of 55 years and a mean follow-up of 7 years and reported no revisions and only 1 minor complication, a transient brachial plexus palsy.23 The discrepancy between these studies may reflect different activity levels and underlying pathology between cohorts. The present population is unique in that it represents a particularly difficult confluence of factors for shoulder arthroplasty surgeons. The high activity, significant overhead and lifting occupational demands, and discordant patient expectations of this military cohort place a significant functional burden on the implants, the glenoid component in particular. Furthermore, this patient group has a higher incidence of more complex glenohumeral pathology resulting in instability, posttraumatic, or capsulorrhaphy arthropathy, and multiple prior arthroscopic and open stabilization procedures.
At final follow-up, only 33% of our patients were still on activity duty, 37.5% had completed or were completing medical separation from the military after surgery for persistent shoulder disability, and 37.5% were retired from the military. Five patients (20.8%) deployed after surgery. This young, active cohort of service members who had TSA for glenohumeral arthritis faced a unique set of tremendous physical demands. A retrospective case series investigated return to sport in 100 consecutive patients (mean age, 68.9 years) who were participating in recreational and competitive athletics and underwent unilateral TSA.21 The patients were engaged most commonly in swimming (20.4%), golf (16.3%), cycling (16.3%), and fitness training (16.3%). The authors found that, at a mean follow-up of 2.8 years, 49 patients (89%) were able to continue in sports, though 36.7% thought their sport activity was restricted after TSA. In another retrospective case series (61 TSAs), McCarty and colleagues19 found that 48 patients (71%) were improved in their sports participation, and 50% increased their frequency of participation after surgery.
There are no specific recommendations on returning to military service or high-level sport after surgery. Recommendations on returning to sport after TSA have been based largely on small case series involving specific sports46,47 and surveys of expert opinion.17,18 In a survey on postoperative physical activity in young patients after TSA conducted by Healy and colleagues,17 35 American Shoulder and Elbow Surgeons members recommended avoiding contact and impact sports while permitting return to nonimpact sports, such as swimming, which may still impart significant stress to the glenohumeral joint. In an international survey of 101 shoulder and elbow surgeons, Magnussen and colleagues18 also found that most recommended avoiding a return to impact sports that require intensive upper extremity demands and permitting full return to sports at preoperative levels. This likely is a result of the perception that most of these patients having TSA are older and have less rigorous involvement in sports at the outset and a lower propensity for adverse patient outcomes. However, these recommendations may place a younger, more high-demand patient at significantly greater risk. The active-duty cohort engages in daily physical training, including push-ups and frequent overhead lifting, which could account for the high failure rates and low incidence of postoperative deployment. Although TSA seems to demonstrate good initial results in terms of return to high-demand activities, the return-to-duty profile in our study highlights the potential pitfalls of TSA in active individuals attempting to return to high-demand preoperative function.
Our analysis was limited by the fact that we used a small patient cohort, contributing to underpowered analysis of the potential risk factors predictive of reoperation and medical discharge. Although our minimum follow-up was 12 months, with the exception of 1 patient who was medically separated at 11.6 months because of shoulder disability, we captured 5 patients (19.2%) who underwent medical separation but who would otherwise be excluded. Therefore, this limitation is not major in that, with a longer minimum follow-up, we would be excluding a significant number of patients with such persistent disability after TSA that they would not be able to return to duty at anywhere near their previous level. In this retrospective study, we were additionally limited to analysis of the data in the medical records and could not control for variables such as surgeon technique, implant choice, and experience. Complete radiographic images were not available, limiting analysis of radiographic outcomes. Given the lack of a standardized preoperative imaging protocol, we could not evaluate glenoid version on axial imaging. It is possible that some patients with early aseptic glenoid loosening had posterior subluxation or a Walch B2 glenoid, which has a higher failure rate.48 The strengths of this study include its unique analysis of a homogeneous young, active, high-risk patient cohort within a closed healthcare system. In the military, these patients are subject to intense daily physical and occupational demands. In addition, the clinical and functional outcomes we studied are patient-centered and therefore relevant during preoperative counseling. Further investigations might focus on validated outcome measures and on midterm to long-term TSA outcomes in an active military population vis-à-vis other alternatives for clinical management.
Conclusion
By a mean follow-up of 3.5 years, only a third of the service members had returned to active duty, roughly a third had retired, and more than a third had been medically discharged because of persistent disability attributable to the shoulder. Despite initial improvements in ROM and pain, midterm outcomes were poor. The short-term complication rate (46.2%) and the rate of reoperation for component failure (23.1%) should be emphasized during preoperative counseling.
Although total shoulder arthroplasty (TSA) has proved to be a reliable solution in older patients, treatment in younger patients with glenohumeral arthritis remains controversial, and there are still few reliable long-term surgical options.1-8 These options include abrasion arthroplasty and arthroscopic management,9,10 biologic glenoid resurfacing,11,12 and humeral hemiarthroplasty with13 or without14,15 glenoid treatment and anatomical TSA.
In the younger cohort, 20-year TSA survivorship rates up to 84% have been reported, and unsatisfactory subjective outcomes have been unacceptably high.16 In addition, there is a paucity of literature addressing the impact of TSA on return to sport. Recommendations on returning to an athletic life style are based largely on surveys of expert opinion17,18 and heterogeneous studies of either older patients (eg, age >50-55 years) who are active19-21 or younger patients with no defined level of activity.5,7,8,16,22-24
To our knowledge, no one has evaluated the short-term morbidity and clinical outcomes within a young, high-demand patient population, such as the US military. Therefore, we conducted a study to evaluate the clinical success and complications of TSA performed for glenohumeral arthritis in a young, active population. We hypothesized that patients who had undergone TSA would have a low rate of return to duty, an increased rate of component failure, and a higher reoperation rate because of increased upper extremity demands.
Materials and Methods
After obtaining protocol approval from the William Beaumont Army Medical Center Institutional Review Board, we searched the Military Health System (MHS) Management Analysis and Reporting Tool (M2) database to retrospectively review the cases of all tri-service US military service members who had undergone primary anatomical TSA (Current Procedural Terminology code 23472) between January 1, 2007 and June 31, 2014. This was a multisurgeon, multicenter study. Patient exclusion criteria were nonmilitary or retired status at time of surgery; primary surgery consisting of limited glenohumeral resurfacing procedure, hemiarthroplasty, or reverse TSA; surgery for acute proximal humerus fracture; rotator cuff deficiency diagnosed before or during surgery; and insufficient follow-up (eg, <12 months, unless medically separated beforehand).
The M2 database is an established tool that has been used for clinical outcomes research on treatment of a variety of orthopedic conditions.25,26 The Medical Data Repository, which is operated by MHS, is populated by its military healthcare providers. The MHS, which offers worldwide coverage for all beneficiaries either at Department of Defense facilities or purchased using civilian providers, is among the largest known closed healthcare systems.
All active-duty US military service members are uniformly required to adhere to stringent and regularly evaluated physical fitness standards, which typically exceed those of average civilians. Routine physical training is required in the form of aerobic fitness, weight training, tactical field exercises, and core military tasks, such as the ability to march at least 2 miles while carrying heavy fighting loads. In addition to satisfying required height and weight standards, all service members are subject to semiannual service-specific physical fitness evaluations inclusive of timed push-ups, sit-ups, and an aerobic event. Service members may also be required to maintain a level of physical training above these baseline standards, contingent on their branch of service, rank, and military occupational specialty. If a service member is unable to maintain these standards, medical separation may be initiated.
Demographic and occupational data were extracted from the database. These data included age, sex, military rank, and branch of service. Line-by-line analysis of the Armed Forces Health Longitudinal Technology Application (Version 22; 3M) electronic medical record was then performed to confirm the underlying diagnosis, surgical procedure, and surgery date. Further chart review yielded additional patient-based factors (eg, laterality, hand dominance, presence and type of prior shoulder surgeries) and surgical factors (eg, surgery indication, implant design). We evaluated clinical and functional outcomes as well as perioperative complications, including both major and minor systemic and local complications as previously described27,28; preoperative and postoperative range of motion (ROM) and self-reported pain score (SRPS, scale 1-10) as measured by physical therapist and surgeon at follow-up; secondary surgical interventions; timing of return to duty; and postoperative deployment history. The primary outcome measures were revision reoperation after index procedure, and military discharge for persistent shoulder-related disability. Clinical failure was defined as component failure or reoperation. Medical Evaluation Board (MEB) is a formal separation from the military in which it is deemed that a service member is no longer able to fulfill his or her duty because of a medical condition.
Statistical Analysis
Continuous variables were compared using statistical means with 95% confidence intervals (CIs) and/or SDs. Categorical data were reported as frequencies or percentages. Univariate analysis was performed to assess the correlation between possible risk factors and the primary outcome measures. P < .05 was considered statistically significant.
Results
Demographics
We identified 24 service members (26 shoulders) who had undergone anatomical TSA during the study period (Table 1). Mean (SD) age was 45.8 (4.5) years (range, 35-54 years), and the cohort was predominately male (25/26 shoulders; 96.2%). Most cohort members were of senior enlisted rank (14, 58.3%), and the US Army was the predominant branch of military service (13, 54.2%). The right side was the operative extremity in 7 cases (26.9%), and the dominant shoulder was involved in 6 cases (23.1%). Two patients (8.3%) underwent staged bilateral TSA. Most patients (76.9%) underwent TSA on the nondominant extremity.
Surgical Variables
TSA was indicated for post-instability arthropathy in 13 cases (50.0%), posttraumatic osteoarthritis in 7 cases (26.9%), and unspecified glenohumeral arthritis, which includes primary glenohumeral osteoarthritis, in 5 cases (19.2%) (Table 2). One case was attributed to iatrogenically induced chondrolysis secondary to intra-articular lidocaine pump. Twelve patients (46.2%) had at least 1 previous surgery. Of the shoulders with instability, 10 (76.9%) had undergone a total of 14 surgical stabilization procedures—10 anterior labral repairs, 2 posterior labral repairs, and 2 capsular plications. The other shoulders had undergone a total of 18 procedures, which included 4 rotator cuff repairs and 3 cartilage restoration procedures.
Clinical Outcomes
Mean (SD) follow-up was 41.0 (21.3) months (range, 11.6-97.6 months). All but 1 shoulder (96.2%) had follow-up of 12 months or more (the only patient with shorter follow-up was because of MEB), and 76.9% of patients had follow-up of 24 months or more (4 of the 6 patients with follow-up under 24 months were medically separated) (Table 3). In all cases, mean ROM improved with respect to flexion, abduction, and external rotation. At final follow-up, mean (SD) ROM was 138° (36°) forward flexion (range, 60°-180°), 125° (39°) abduction (range, 45°-180°), 48° (19°) external rotation at 0° abduction (range, 20°-90°), and 80° (9.4°) external rotation at 90° abduction (range, 70°-90°). Preoperative flexion, abduction, and external rotation at 0° and 90° abduction were all improved at final follow-up. The most improvement in ROM occurred within 6 months after surgery.
Overall patient satisfaction with surgery was 92.3% (n = 24). Ultimately, 18 (72.0%) of 25 shoulders with follow-up of 1 year or more were able to return to active duty within 1 year after surgery, though only 10 (45.5%) of 22 with follow-up of 2 years or more remained active 2 years after surgery. Furthermore, 5 patients (20.8%) were deployed after surgery, and all were still on active duty at final follow-up. By final follow-up, 9 (37.5%) of 24 service members were unable to return to military function; 7 had been medically discharged from the military for persistent shoulder disability, and 2 were in the process of being medically discharged.
In all cases, SRPS improved from before surgery (5.2 out of 10) to final follow-up (1.4). At final follow-up, 22 patients (88.0%) reported mild pain (0-3), and no one had pain above 6.
Complications
Nine patients had a total of 12 postoperative complications (46.2%): 6 component failures (23.1%), 2 neurologic injuries (7.7%; 1 permanent axillary nerve injury, 1 transient brachial plexus neuritis), 2 cases of adhesive capsulitis (7.7%), and 2 episodes of venous thrombosis (7.7%; 1 superficial, 1 deep) (Table 4). There were no documented infections. Six reoperations (23.1%) were performed for the 6 component failures (2 traumatic dislocations of prosthesis resulting in acute glenoid component failure, 3 cases of atraumatic glenoid loosening, 1 case of humeral stem loosening after periprosthetic fracture). Atraumatic glenoid component loosening occurred a mean (SD) of 40.6 (14.2) months after surgery (range, 20.8-54.2 months).
Surgical Failures
Eight service members underwent MEB. Six patients experienced component failure. Factors contributing to both clinical failure and separation from active duty by means of MEB were evaluated with univariate analysis (Table 5). No statistically significant risk factors, including surgical revision and presence of perioperative complications, were identified.
Discussion
We confirmed that our cohort of young service members (mean age, 45.8 years), who had undergone TSA for glenohumeral arthritis, had a relatively higher rate of component failure (23.1%) and a higher reoperation rate (23.1%) with low rates of return to military duty at short-term to midterm follow-up. Our results parallel those of a limited series with a younger cohort (Table 6).7,16,19,21,23,24 The high demand and increased life expectancy of the younger patients with glenohumeral arthritis potentiates the risk of complications, component loosening, and ultimate failure.29 To our knowledge, the present article is the first to report clinical and functional outcomes and perioperative risk profiles in a homogenously young, active military cohort after TSA.
The mean age of our study population (46 years) is one of the lowest in the literature. TSA in younger patients (age, <50-55 years) and older, active patients (>55 years) has received increased attention as a result of the expanding indications and growing popularity of TSA in these groups. Other studies have upheld the efficacy of TSA in achieving predictable pain relief and functional improvement in a diverse and predominantly elderly population.15,30-34 Alternative treatments, including humeral head resurfacing15,30,35 and soft-tissue interposition,15,36-40 have also shown inferior short- and long-term results in terms of longevity and degree of clinical or functional improvement.31-34,41 In addition, the ream-and-run technique has had promising early results by improving glenohumeral kinematics, pain relief, and shoulder function.13,42,43 However, although implantation of a glenoid component is avoided in young, active people because of reduced longevity and higher rates of component failure, the trade-offs are inadequately treated glenoid disease, suboptimal pain relief, and progression of glenoid arthritis eventually requiring revision. Furthermore, midterm and long-term survivorship of TSA in general is unknown, and there remain few good options for treating end-stage arthritis in young, active patients.
Our cohort had high rates of complications (46.2%) and revisions (23.1%). Two in 5 patients had postoperative complications, most commonly component failure resulting in reoperation. In the literature, complication rates among young patients who underwent TSA are much lower (4.8%-10.9%).16,23,24 Our cohort’s most common complication was component failure (23.1%), which was most often attributed to atraumatic, aseptic glenoid component loosening and required reoperation. Previously reported revision rates in a young population that underwent TSA (0%-11%)16,23,24 were also significantly lower than those in the present analysis (23.1%), underscoring the impact of operative indications, postoperative activity levels, and occupational demands on ultimate failure rates. Interestingly, all revisions in our study were for component failure, whereas previous reports have described a higher rate for infection.22 However, the same studies also found glenoid lucency rates as high as 76% at 10-year follow-up.16 Furthermore, in a review of 136 TSAs with unsatisfactory outcomes, glenoid loosening was the most common reason for presenting to clinic after surgery.44 Specifically, our population had a high rate of glenohumeral arthritis secondary to instability (50.0%) and posttraumatic osteoarthritis (26.9%). For many reasons, outcomes were worse in younger patients with a history of glenohumeral instability33 than in older patients without a high incidence of instability.45 This young cohort with higher demands may have had accelerated polyethylene wear patterns caused by repetitive overhead activity, which may have arisen because of a higher functional profile after surgery and greater patient expectations after arthroplasty. In addition, patients with a history of instability may have altered glenohumeral anatomy, especially with previous arthroscopic or open stabilization procedures. Anatomical changes include excessive posterior glenoid wear, internal rotation contracture, patulous capsular tissue, static or dynamic posterior humeral subluxation, and possible overconstraint after prior stabilization procedures. Almost half of our population had a previous surgery; our patients averaged 1.7 previous surgeries each.
Although estimates of component survivorship at a high-volume civilian tertiary-referral center were as high as 97% at 10 years and 84% at 20 years,7,16 10-year survivorship in patients with a history of instability was only 61%.3 TSA survivorship in our young, active cohort is already foreseeably dramatically reduced, given the 23.1% revision rate at 28.5-month follow-up. This consideration must be addressed during preoperative counseling with the young patient with glenohumeral arthritis and a history of shoulder instability.
Despite the high rates of complications and revisions in our study, 92.3% of patients were satisfied with surgery, 88.0% experienced minimal persistence of pain (mean 3.8-point decrease on SRPS), and 100% maintained improved ROM at final follow-up. Satisfaction in the young population has varied significantly, from 52% to 95%, generally on the basis of physical activity.16,22-24 The reasonable rate of postoperative satisfaction in the present analysis is comparable to what has been reported in patients of a similar age (Table 6).7,16,22 However, despite high satisfaction and pain relief, patients were inconsistently able to return to the upper limits of physical activity required of active-duty military service. In addition, we cannot exclude secondary gain motivations for pursuing medical retirement, similar to that seen in patients receiving worker’s compensation.
Other authors have conversely found more favorable functional outcomes and survivorship rates.23,24 In a retrospective review of 46 TSAs in patients 55 years or younger, Bartelt and colleagues24 found sustained improvements in pain, ROM, and satisfaction at 7-year follow-up.24 Raiss and colleagues23 conducted a prospective study of TSA outcomes in 21 patients with a mean age of 55 years and a mean follow-up of 7 years and reported no revisions and only 1 minor complication, a transient brachial plexus palsy.23 The discrepancy between these studies may reflect different activity levels and underlying pathology between cohorts. The present population is unique in that it represents a particularly difficult confluence of factors for shoulder arthroplasty surgeons. The high activity, significant overhead and lifting occupational demands, and discordant patient expectations of this military cohort place a significant functional burden on the implants, the glenoid component in particular. Furthermore, this patient group has a higher incidence of more complex glenohumeral pathology resulting in instability, posttraumatic, or capsulorrhaphy arthropathy, and multiple prior arthroscopic and open stabilization procedures.
At final follow-up, only 33% of our patients were still on activity duty, 37.5% had completed or were completing medical separation from the military after surgery for persistent shoulder disability, and 37.5% were retired from the military. Five patients (20.8%) deployed after surgery. This young, active cohort of service members who had TSA for glenohumeral arthritis faced a unique set of tremendous physical demands. A retrospective case series investigated return to sport in 100 consecutive patients (mean age, 68.9 years) who were participating in recreational and competitive athletics and underwent unilateral TSA.21 The patients were engaged most commonly in swimming (20.4%), golf (16.3%), cycling (16.3%), and fitness training (16.3%). The authors found that, at a mean follow-up of 2.8 years, 49 patients (89%) were able to continue in sports, though 36.7% thought their sport activity was restricted after TSA. In another retrospective case series (61 TSAs), McCarty and colleagues19 found that 48 patients (71%) were improved in their sports participation, and 50% increased their frequency of participation after surgery.
There are no specific recommendations on returning to military service or high-level sport after surgery. Recommendations on returning to sport after TSA have been based largely on small case series involving specific sports46,47 and surveys of expert opinion.17,18 In a survey on postoperative physical activity in young patients after TSA conducted by Healy and colleagues,17 35 American Shoulder and Elbow Surgeons members recommended avoiding contact and impact sports while permitting return to nonimpact sports, such as swimming, which may still impart significant stress to the glenohumeral joint. In an international survey of 101 shoulder and elbow surgeons, Magnussen and colleagues18 also found that most recommended avoiding a return to impact sports that require intensive upper extremity demands and permitting full return to sports at preoperative levels. This likely is a result of the perception that most of these patients having TSA are older and have less rigorous involvement in sports at the outset and a lower propensity for adverse patient outcomes. However, these recommendations may place a younger, more high-demand patient at significantly greater risk. The active-duty cohort engages in daily physical training, including push-ups and frequent overhead lifting, which could account for the high failure rates and low incidence of postoperative deployment. Although TSA seems to demonstrate good initial results in terms of return to high-demand activities, the return-to-duty profile in our study highlights the potential pitfalls of TSA in active individuals attempting to return to high-demand preoperative function.
Our analysis was limited by the fact that we used a small patient cohort, contributing to underpowered analysis of the potential risk factors predictive of reoperation and medical discharge. Although our minimum follow-up was 12 months, with the exception of 1 patient who was medically separated at 11.6 months because of shoulder disability, we captured 5 patients (19.2%) who underwent medical separation but who would otherwise be excluded. Therefore, this limitation is not major in that, with a longer minimum follow-up, we would be excluding a significant number of patients with such persistent disability after TSA that they would not be able to return to duty at anywhere near their previous level. In this retrospective study, we were additionally limited to analysis of the data in the medical records and could not control for variables such as surgeon technique, implant choice, and experience. Complete radiographic images were not available, limiting analysis of radiographic outcomes. Given the lack of a standardized preoperative imaging protocol, we could not evaluate glenoid version on axial imaging. It is possible that some patients with early aseptic glenoid loosening had posterior subluxation or a Walch B2 glenoid, which has a higher failure rate.48 The strengths of this study include its unique analysis of a homogeneous young, active, high-risk patient cohort within a closed healthcare system. In the military, these patients are subject to intense daily physical and occupational demands. In addition, the clinical and functional outcomes we studied are patient-centered and therefore relevant during preoperative counseling. Further investigations might focus on validated outcome measures and on midterm to long-term TSA outcomes in an active military population vis-à-vis other alternatives for clinical management.
Conclusion
By a mean follow-up of 3.5 years, only a third of the service members had returned to active duty, roughly a third had retired, and more than a third had been medically discharged because of persistent disability attributable to the shoulder. Despite initial improvements in ROM and pain, midterm outcomes were poor. The short-term complication rate (46.2%) and the rate of reoperation for component failure (23.1%) should be emphasized during preoperative counseling.
1. Tokish JM. The mature athlete’s shoulder. Sports Health. 2014;6(1):31-35.
2. Sperling JW, Cofield RH. Revision total shoulder arthroplasty for the treatment of glenoid arthrosis. J Bone Joint Surg Am. 1998;80(6):860-867.
3. Sperling JW, Antuna SA, Sanchez-Sotelo J, Schleck C, Cofield RH. Shoulder arthroplasty for arthritis after instability surgery. J Bone Joint Surg Am. 2002;84(10):1775-1781.
4. Izquierdo R, Voloshin I, Edwards S, et al; American Academy of Orthopaedic Surgeons. Treatment of glenohumeral osteoarthritis. J Am Acad Orthop Surg. 2010;18(6):375-382.
5. Johnson MH, Paxton ES, Green A. Shoulder arthroplasty options in young (<50 years old) patients: review of current concepts. J Shoulder Elbow Surg. 2015;24(2):317-325.
6. Cole BJ, Yanke A, Provencher MT. Nonarthroplasty alternatives for the treatment of glenohumeral arthritis. J Shoulder Elbow Surg. 2007;16(5 suppl):S231-S240.
7. Denard PJ, Raiss P, Sowa B, Walch G. Mid- to long-term follow-up of total shoulder arthroplasty using a keeled glenoid in young adults with primary glenohumeral arthritis. J Shoulder Elbow Surg. 2013;22(7):894-900.
8. Denard PJ, Wirth MA, Orfaly RM. Management of glenohumeral arthritis in the young adult. J Bone Joint Surg Am. 2011;93(9):885-892.
9. Millett PJ, Horan MP, Pennock AT, Rios D. Comprehensive arthroscopic management (CAM) procedure: clinical results of a joint-preserving arthroscopic treatment for young, active patients with advanced shoulder osteoarthritis. Arthroscopy. 2013;29(3):440-448.
10 Millett PJ, Gaskill TR. Arthroscopic management of glenohumeral arthrosis: humeral osteoplasty, capsular release, and arthroscopic axillary nerve release as a joint-preserving approach. Arthroscopy. 2011;27(9):1296-1303.
11. Savoie FH 3rd, Brislin KJ, Argo D. Arthroscopic glenoid resurfacing as a surgical treatment for glenohumeral arthritis in the young patient: midterm results. Arthroscopy. 2009;25(8):864-871.
12. Strauss EJ, Verma NN, Salata MJ, et al. The high failure rate of biologic resurfacing of the glenoid in young patients with glenohumeral arthritis. J Shoulder Elbow Surg. 2014;23(3):409-419.
13. Matsen FA 3rd, Warme WJ, Jackins SE. Can the ream and run procedure improve glenohumeral relationships and function for shoulders with the arthritic triad? Clin Orthop Relat Res. 2015;473(6):2088-2096.
14. Lo IK, Litchfield RB, Griffin S, Faber K, Patterson SD, Kirkley A. Quality-of-life outcome following hemiarthroplasty or total shoulder arthroplasty in patients with osteoarthritis. A prospective, randomized trial. J Bone Joint Surg Am. 2005;87(10):2178-2185.
15. Wirth M, Tapscott RS, Southworth C, Rockwood CA Jr. Treatment of glenohumeral arthritis with a hemiarthroplasty: a minimum five-year follow-up outcome study. J Bone Joint Surg Am. 2006;88(5):964-973.
16. Sperling JW, Cofield RH, Rowland CM. Minimum fifteen-year follow-up of Neer hemiarthroplasty and total shoulder arthroplasty in patients aged fifty years or younger. J Shoulder Elbow Surg. 2004;13(6):604-613.
17. Healy WL, Iorio R, Lemos MJ. Athletic activity after joint replacement. Am J Sports Med. 2001;29(3):377-388.
18. Magnussen RA, Mallon WJ, Willems WJ, Moorman CT 3rd. Long-term activity restrictions after shoulder arthroplasty: an international survey of experienced shoulder surgeons. J Shoulder Elbow Surg. 2011;20(2):281-289.
19. McCarty EC, Marx RG, Maerz D, Altchek D, Warren RF. Sports participation after shoulder replacement surgery. Am J Sports Med. 2008;36(8):1577-1581.
20. Schmidt-Wiethoff R, Wolf P, Lehmann M, Habermeyer P. Physical activity after shoulder arthroplasty [in German]. Sportverletz Sportschaden. 2002;16(1):26-30.
21. Schumann K, Flury MP, Schwyzer HK, Simmen BR, Drerup S, Goldhahn J. Sports activity after anatomical total shoulder arthroplasty. Am J Sports Med. 2010;38(10):2097-2105.
22. Sperling JW, Cofield RH, Rowland CM. Neer hemiarthroplasty and Neer total shoulder arthroplasty in patients fifty years old or less. Long-term results. J Bone Joint Surg Am. 1998;80(4):464-473.
23. Raiss P, Aldinger PR, Kasten P, Rickert M, Loew M. Total shoulder replacement in young and middle-aged patients with glenohumeral osteoarthritis. J Bone Joint Surg Br. 2008;90(6):764-769.
24. Bartelt R, Sperling JW, Schleck CD, Cofield RH. Shoulder arthroplasty in patients aged fifty-five years or younger with osteoarthritis. J Shoulder Elbow Surg. 2011;20(1):123-130.
25. Waterman BR, Burns TC, McCriskin B, Kilcoyne K, Cameron KL, Owens BD. Outcomes after Bankart repair in a military population: predictors for surgical revision and long-term disability. Arthroscopy. 2014;30(2):172-177.
26. Waterman BR, Liu J, Newcomb R, Schoenfeld AJ, Orr JD, Belmont PJ Jr. Risk factors for chronic exertional compartment syndrome in a physically active military population. Am J Sports Med. 2013;41(11):2545-2549.
27. Chalmers PN, Gupta AK, Rahman Z, Bruce B, Romeo AA, Nicholson GP. Predictors of early complications of total shoulder arthroplasty. J Arthroplasty. 2014;29(4):856-860.
28. Dunn JC, Lanzi J, Kusnezov N, Bader J, Waterman BR, Belmont PJ Jr. Predictors of length of stay after elective total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2015;24(5):754-759.
29. Hayes PR, Flatow EL. Total shoulder arthroplasty in the young patient. Instr Course Lect. 2001;50;73-88.
30. Rispoli DM, Sperling JW, Athwal GS, Schleck CD, Cofield RH. Humeral head replacement for the treatment of osteoarthritis. J Bone Joint Surg Am. 2006;88(12):2637-2644.
31. Radnay CS, Setter KJ, Chambers L, Levine WN, Bigliani LU, Ahmad CS. Total shoulder replacement compared with humeral head replacement for the treatment of primary glenohumeral osteoarthritis: a systematic review. J Shoulder Elbow Surg. 2007;16(4):396-402.
32. Gartsman GM, Roddey TS, Hammerman SM. Shoulder arthroplasty with or without resurfacing of the glenoid in patients who have osteoarthritis. J Bone Joint Surg Am. 2000;82(1):26-34.
33. Edwards TB, Kadakia NR, Boulahia A, et al. A comparison of hemiarthroplasty and total shoulder arthroplasty in the treatment of primary glenohumeral osteoarthritis: results of a multicenter study. J Shoulder Elbow Surg. 2003;12(3):
207-213.
34. Bryant D, Litchfield R, Sandow M, Gartsman GM, Guyatt G, Kirkley A. A comparison of pain, strength, range of motion, and functional outcomes after hemiarthroplasty and total shoulder arthroplasty in patients with osteoarthritis of the shoulder. A systematic review and meta-analysis. J Bone Joint Surg Am. 2005;87(9):1947-1956.
35. Bailie DS, Llinas PJ, Ellenbecker TS. Cementless humeral resurfacing arthroplasty in active patients less than fifty-five years of age. J Bone Joint Surg Am. 2008;90(1):110-117.
36. Ball CM, Galatz LM, Yamaguchi K. Meniscal allograft interposition arthroplasty for the arthritic shoulder: description of a new surgical technique. Tech Shoulder Elbow Surg. 2001;2:247-254.
37. Elhassan B, Ozbaydar M, Diller D, Higgins LD, Warner JJ. Soft-tissue resurfacing of the glenoid in the treatment of glenohumeral arthritis in active patients less than fifty years old. J Bone Joint Surg Am. 2009;91(2):419-424.
38. Krishnan SG, Nowinski RJ, Harrison D, Burkhead WZ. Humeral hemiarthroplasty with biologic resurfacing of the glenoid for glenohumeral arthritis. Two to fifteen-year outcomes. J Bone Joint Surg Am. 2007;89(4):727-734.
39. Lee KT, Bell S, Salmon J. Cementless surface replacement arthroplasty of the shoulder with biologic resurfacing of the glenoid. J Shoulder Elbow Surg. 2009;18(6):915-919.
40. Nicholson GP, Goldstein JL, Romeo AA, et al. Lateral meniscus allograft biologic glenoid arthroplasty in total shoulder arthroplasty for young shoulders with degenerative joint disease. J Shoulder Elbow Surg. 2007;16(5 suppl):S261-S266.
41. Carroll RM, Izquierdo R, Vazquez M, Blaine TA, Levine WN, Bigliani LU. Conversion of painful hemiarthroplasty to total shoulder arthroplasty: long-term results. J Shoulder Elbow Surg. 2004;13(6):599-603.
42. Clinton J, Franta AK, Lenters TR, Mounce D, Matsen FA 3rd. Nonprosthetic glenoid arthroplasty with humeral hemiarthroplasty and total shoulder arthroplasty yield similar self-assessed outcomes in the management of comparable patients with glenohumeral arthritis. J Shoulder Elbow Surg. 2007;16(5):534-538.
43. Gilmer BB, Comstock BA, Jette JL, Warme WJ, Jackins SE, Matsen FA. The prognosis for improvement in comfort and function after the ream-and-run arthroplasty for glenohumeral arthritis: an analysis of 176 consecutive cases. J Bone Joint Surg Am. 2012;94(14):e102.
44. Franta AK, Lenters TR, Mounce D, Neradilek B, Matsen FA 3rd. The complex characteristics of 282 unsatisfactory shoulder arthroplasties. J Shoulder Elbow Surg. 2007;16(5):555-562.
45. Godenèche A, Boileau P, Favard L, et al. Prosthetic replacement in the treatment of osteoarthritis of the shoulder: early results of 268 cases. J Shoulder Elbow Surg. 2002;11(1):11-18.
46. Jensen KL, Rockwood CA Jr. Shoulder arthroplasty in recreational golfers. J Shoulder Elbow Surg. 1998;7(4):362-367.
47. Kirchhoff C, Imhoff AB, Hinterwimmer S. Winter sports and shoulder arthroplasty [in German]. Sportverletz Sportschaden. 2008;22(3):153-158.
48. Raiss P, Edwards TB, Deutsch A, et al. Radiographic changes around humeral components in shoulder arthroplasty. J Bone Joint Surg Am. 2014;96(7):e54.
1. Tokish JM. The mature athlete’s shoulder. Sports Health. 2014;6(1):31-35.
2. Sperling JW, Cofield RH. Revision total shoulder arthroplasty for the treatment of glenoid arthrosis. J Bone Joint Surg Am. 1998;80(6):860-867.
3. Sperling JW, Antuna SA, Sanchez-Sotelo J, Schleck C, Cofield RH. Shoulder arthroplasty for arthritis after instability surgery. J Bone Joint Surg Am. 2002;84(10):1775-1781.
4. Izquierdo R, Voloshin I, Edwards S, et al; American Academy of Orthopaedic Surgeons. Treatment of glenohumeral osteoarthritis. J Am Acad Orthop Surg. 2010;18(6):375-382.
5. Johnson MH, Paxton ES, Green A. Shoulder arthroplasty options in young (<50 years old) patients: review of current concepts. J Shoulder Elbow Surg. 2015;24(2):317-325.
6. Cole BJ, Yanke A, Provencher MT. Nonarthroplasty alternatives for the treatment of glenohumeral arthritis. J Shoulder Elbow Surg. 2007;16(5 suppl):S231-S240.
7. Denard PJ, Raiss P, Sowa B, Walch G. Mid- to long-term follow-up of total shoulder arthroplasty using a keeled glenoid in young adults with primary glenohumeral arthritis. J Shoulder Elbow Surg. 2013;22(7):894-900.
8. Denard PJ, Wirth MA, Orfaly RM. Management of glenohumeral arthritis in the young adult. J Bone Joint Surg Am. 2011;93(9):885-892.
9. Millett PJ, Horan MP, Pennock AT, Rios D. Comprehensive arthroscopic management (CAM) procedure: clinical results of a joint-preserving arthroscopic treatment for young, active patients with advanced shoulder osteoarthritis. Arthroscopy. 2013;29(3):440-448.
10 Millett PJ, Gaskill TR. Arthroscopic management of glenohumeral arthrosis: humeral osteoplasty, capsular release, and arthroscopic axillary nerve release as a joint-preserving approach. Arthroscopy. 2011;27(9):1296-1303.
11. Savoie FH 3rd, Brislin KJ, Argo D. Arthroscopic glenoid resurfacing as a surgical treatment for glenohumeral arthritis in the young patient: midterm results. Arthroscopy. 2009;25(8):864-871.
12. Strauss EJ, Verma NN, Salata MJ, et al. The high failure rate of biologic resurfacing of the glenoid in young patients with glenohumeral arthritis. J Shoulder Elbow Surg. 2014;23(3):409-419.
13. Matsen FA 3rd, Warme WJ, Jackins SE. Can the ream and run procedure improve glenohumeral relationships and function for shoulders with the arthritic triad? Clin Orthop Relat Res. 2015;473(6):2088-2096.
14. Lo IK, Litchfield RB, Griffin S, Faber K, Patterson SD, Kirkley A. Quality-of-life outcome following hemiarthroplasty or total shoulder arthroplasty in patients with osteoarthritis. A prospective, randomized trial. J Bone Joint Surg Am. 2005;87(10):2178-2185.
15. Wirth M, Tapscott RS, Southworth C, Rockwood CA Jr. Treatment of glenohumeral arthritis with a hemiarthroplasty: a minimum five-year follow-up outcome study. J Bone Joint Surg Am. 2006;88(5):964-973.
16. Sperling JW, Cofield RH, Rowland CM. Minimum fifteen-year follow-up of Neer hemiarthroplasty and total shoulder arthroplasty in patients aged fifty years or younger. J Shoulder Elbow Surg. 2004;13(6):604-613.
17. Healy WL, Iorio R, Lemos MJ. Athletic activity after joint replacement. Am J Sports Med. 2001;29(3):377-388.
18. Magnussen RA, Mallon WJ, Willems WJ, Moorman CT 3rd. Long-term activity restrictions after shoulder arthroplasty: an international survey of experienced shoulder surgeons. J Shoulder Elbow Surg. 2011;20(2):281-289.
19. McCarty EC, Marx RG, Maerz D, Altchek D, Warren RF. Sports participation after shoulder replacement surgery. Am J Sports Med. 2008;36(8):1577-1581.
20. Schmidt-Wiethoff R, Wolf P, Lehmann M, Habermeyer P. Physical activity after shoulder arthroplasty [in German]. Sportverletz Sportschaden. 2002;16(1):26-30.
21. Schumann K, Flury MP, Schwyzer HK, Simmen BR, Drerup S, Goldhahn J. Sports activity after anatomical total shoulder arthroplasty. Am J Sports Med. 2010;38(10):2097-2105.
22. Sperling JW, Cofield RH, Rowland CM. Neer hemiarthroplasty and Neer total shoulder arthroplasty in patients fifty years old or less. Long-term results. J Bone Joint Surg Am. 1998;80(4):464-473.
23. Raiss P, Aldinger PR, Kasten P, Rickert M, Loew M. Total shoulder replacement in young and middle-aged patients with glenohumeral osteoarthritis. J Bone Joint Surg Br. 2008;90(6):764-769.
24. Bartelt R, Sperling JW, Schleck CD, Cofield RH. Shoulder arthroplasty in patients aged fifty-five years or younger with osteoarthritis. J Shoulder Elbow Surg. 2011;20(1):123-130.
25. Waterman BR, Burns TC, McCriskin B, Kilcoyne K, Cameron KL, Owens BD. Outcomes after Bankart repair in a military population: predictors for surgical revision and long-term disability. Arthroscopy. 2014;30(2):172-177.
26. Waterman BR, Liu J, Newcomb R, Schoenfeld AJ, Orr JD, Belmont PJ Jr. Risk factors for chronic exertional compartment syndrome in a physically active military population. Am J Sports Med. 2013;41(11):2545-2549.
27. Chalmers PN, Gupta AK, Rahman Z, Bruce B, Romeo AA, Nicholson GP. Predictors of early complications of total shoulder arthroplasty. J Arthroplasty. 2014;29(4):856-860.
28. Dunn JC, Lanzi J, Kusnezov N, Bader J, Waterman BR, Belmont PJ Jr. Predictors of length of stay after elective total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2015;24(5):754-759.
29. Hayes PR, Flatow EL. Total shoulder arthroplasty in the young patient. Instr Course Lect. 2001;50;73-88.
30. Rispoli DM, Sperling JW, Athwal GS, Schleck CD, Cofield RH. Humeral head replacement for the treatment of osteoarthritis. J Bone Joint Surg Am. 2006;88(12):2637-2644.
31. Radnay CS, Setter KJ, Chambers L, Levine WN, Bigliani LU, Ahmad CS. Total shoulder replacement compared with humeral head replacement for the treatment of primary glenohumeral osteoarthritis: a systematic review. J Shoulder Elbow Surg. 2007;16(4):396-402.
32. Gartsman GM, Roddey TS, Hammerman SM. Shoulder arthroplasty with or without resurfacing of the glenoid in patients who have osteoarthritis. J Bone Joint Surg Am. 2000;82(1):26-34.
33. Edwards TB, Kadakia NR, Boulahia A, et al. A comparison of hemiarthroplasty and total shoulder arthroplasty in the treatment of primary glenohumeral osteoarthritis: results of a multicenter study. J Shoulder Elbow Surg. 2003;12(3):
207-213.
34. Bryant D, Litchfield R, Sandow M, Gartsman GM, Guyatt G, Kirkley A. A comparison of pain, strength, range of motion, and functional outcomes after hemiarthroplasty and total shoulder arthroplasty in patients with osteoarthritis of the shoulder. A systematic review and meta-analysis. J Bone Joint Surg Am. 2005;87(9):1947-1956.
35. Bailie DS, Llinas PJ, Ellenbecker TS. Cementless humeral resurfacing arthroplasty in active patients less than fifty-five years of age. J Bone Joint Surg Am. 2008;90(1):110-117.
36. Ball CM, Galatz LM, Yamaguchi K. Meniscal allograft interposition arthroplasty for the arthritic shoulder: description of a new surgical technique. Tech Shoulder Elbow Surg. 2001;2:247-254.
37. Elhassan B, Ozbaydar M, Diller D, Higgins LD, Warner JJ. Soft-tissue resurfacing of the glenoid in the treatment of glenohumeral arthritis in active patients less than fifty years old. J Bone Joint Surg Am. 2009;91(2):419-424.
38. Krishnan SG, Nowinski RJ, Harrison D, Burkhead WZ. Humeral hemiarthroplasty with biologic resurfacing of the glenoid for glenohumeral arthritis. Two to fifteen-year outcomes. J Bone Joint Surg Am. 2007;89(4):727-734.
39. Lee KT, Bell S, Salmon J. Cementless surface replacement arthroplasty of the shoulder with biologic resurfacing of the glenoid. J Shoulder Elbow Surg. 2009;18(6):915-919.
40. Nicholson GP, Goldstein JL, Romeo AA, et al. Lateral meniscus allograft biologic glenoid arthroplasty in total shoulder arthroplasty for young shoulders with degenerative joint disease. J Shoulder Elbow Surg. 2007;16(5 suppl):S261-S266.
41. Carroll RM, Izquierdo R, Vazquez M, Blaine TA, Levine WN, Bigliani LU. Conversion of painful hemiarthroplasty to total shoulder arthroplasty: long-term results. J Shoulder Elbow Surg. 2004;13(6):599-603.
42. Clinton J, Franta AK, Lenters TR, Mounce D, Matsen FA 3rd. Nonprosthetic glenoid arthroplasty with humeral hemiarthroplasty and total shoulder arthroplasty yield similar self-assessed outcomes in the management of comparable patients with glenohumeral arthritis. J Shoulder Elbow Surg. 2007;16(5):534-538.
43. Gilmer BB, Comstock BA, Jette JL, Warme WJ, Jackins SE, Matsen FA. The prognosis for improvement in comfort and function after the ream-and-run arthroplasty for glenohumeral arthritis: an analysis of 176 consecutive cases. J Bone Joint Surg Am. 2012;94(14):e102.
44. Franta AK, Lenters TR, Mounce D, Neradilek B, Matsen FA 3rd. The complex characteristics of 282 unsatisfactory shoulder arthroplasties. J Shoulder Elbow Surg. 2007;16(5):555-562.
45. Godenèche A, Boileau P, Favard L, et al. Prosthetic replacement in the treatment of osteoarthritis of the shoulder: early results of 268 cases. J Shoulder Elbow Surg. 2002;11(1):11-18.
46. Jensen KL, Rockwood CA Jr. Shoulder arthroplasty in recreational golfers. J Shoulder Elbow Surg. 1998;7(4):362-367.
47. Kirchhoff C, Imhoff AB, Hinterwimmer S. Winter sports and shoulder arthroplasty [in German]. Sportverletz Sportschaden. 2008;22(3):153-158.
48. Raiss P, Edwards TB, Deutsch A, et al. Radiographic changes around humeral components in shoulder arthroplasty. J Bone Joint Surg Am. 2014;96(7):e54.
Successful Nonoperative Management of HAGL (Humeral Avulsion of Glenohumeral Ligament) Lesion With Concurrent Axillary Nerve Injury in an Active-Duty US Navy SEAL
The humeral avulsion of glenohumeral ligament (HAGL) lesion has been recognized as a cause of recurrent shoulder instability. In 1942, Nicola1 was the first to describe this lesion, in a small case series of avulsions of the anterior band of the inferior glenohumeral ligament from the humeral neck secondary to a dislocation injury. In 1988, Bach and colleagues2 described it in 2 patients with recurrent anterior dislocations. Wolf and colleagues3 were the first to apply the term HAGL to the injury, in 1995.
HAGL lesion incidence ranges from 1% to 9%, but many authors think the lesion is underdiagnosed.3-5 It occurs in isolation or in combination with other injuries, and it is commonly identified on recurrence of instability. Bui-Mansfield and colleagues6 found that 11% of patients with a diagnosis of HAGL lesion previously had surgery on the same shoulder, whereas for 62% the lesion was associated with other, concurrent lesions, including labral tears (18, 25%), rotator cuff tears (16, 23%), and Hill-Sachs deformities (12, 17%).
Most young athletes who undergo nonoperative therapy for a HAGL lesion continue to experience pain and/or instability that then requires surgical intervention.4 To our knowledge, there are no reports of return to full function in young competitive athletes or return to manual labor after nonoperative management of a HAGL lesion.
In this article, we report the case of a US Navy SEAL who sustained a traction injury causing an axillary nerve injury and a HAGL lesion. Successful nonoperative management allowed him to return to full duty. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An otherwise healthy 26-year-old Navy SEAL presented with pain and significant weakness in the right (dominant) upper extremity after an injury in a training exercise. The shoulder sustained a traction injury when the man’s fast-moving marine attack craft was in a collision and he was trying not to be thrown off. He reported having a sense of dislocation yet never required a reduction.
Physical examination revealed severe weakness with shoulder abduction, external rotation, and forward flexion; inability to contract the deltoid muscle; and complete numbness along the cutaneous distribution of the axillary nerve. On neurovascular examination, the right upper extremity was otherwise intact. The patient had complete passive range of motion (ROM) with apprehension in abduction with external rotation along with anterior laxity and normal posterior stability.
Standard shoulder radiographs showed no bony abnormalities and a concentrically reduced glenohumeral joint. Magnetic resonance imaging (MRI), reviewed by a staff musculoskeletal radiologist and a sports fellowship–trained orthopedic surgeon, showed a greater tuberosity contusion, a partial tear of the infraspinatus, and a HAGL lesion (Figure 1).
The patient was counseled toward surgical intervention to prevent symptoms of recurrent instability. A detailed discussion ensued about whether to proceed with surgery immediately or to pursue temporary nonoperative treatment to allow for assessment and return of deltoid function. Patient and surgeon decided to delay operative intervention because of concerns about the patient’s ability to effectively rehabilitate while still having a compromised axillary nerve after surgery. The recommendation was to delay initial electromyographic (EMG) and nerve conduction velocity testing at least 4 weeks to allow for completion of Wallerian degeneration and more accurate assessment of the axillary nerve.7 Physical therapy for gentle ROM (excluding external rotation) and isometric rotator cuff exercises were initiated.
Five weeks after injury, the patient left the area to attend a 2-month nonphysical training course and continued rehabilitation and orthopedic follow-up at another military medical facility. Six weeks after injury, initial EMG testing revealed the expected axillary neuropraxia. In addition, some marginal improvement in ROM was noted, but deltoid function was still very limited.
Ten weeks after injury, clinical inspection revealed deltoid wasting. Active shoulder ROM was limited, and deltoid strength was 3/5, though the patient was able to perform a standard push-up without difficulty and showed no sign of laxity or apprehension on shoulder examination. Repeat EMG testing revealed axillary nerve denervation with no sign of regeneration. Twelve weeks after injury, MRA showed reorganization and partial healing of the HAGL lesion relative to the prior study (Figure 2).
On the patient’s return from training, 15 weeks after injury, he had improved active ROM and 4+/5 deltoid strength. Axillary nerve sensation was still decreased but markedly improved. Physical examination revealed no significant shoulder laxity or apprehension, and the patient denied feelings of instability. Activities were advanced to include an organized strengthening program.
Six months after injury, the patient was cleared to return to his unit with only mild physical restriction. Function continued to steadily improve. After 9 months, he was cleared for full, unrestricted duty. Although he still demonstrated slight asymmetric weakness in the right deltoid with continued muscular atrophy, examination findings were otherwise normal, and he was back to full activities without significant symptoms.
Eleven months after injury, MRI showed healing of the HAGL lesion (Figure 3). At 17 months, EMG testing revealed significant interval improvement in axillary motor unit potentials but still about a 50% decrement compared with the noninjured side. The patient denied any motor or sensory deficits and any instability events since his injury. He continued with full function as an active-duty Navy SEAL.
Discussion
Nonoperative management has been used for injuries to the inferior glenohumeral ligament complex when there is no humeral detachment but generally has been reserved for low-demand patients and patients who cannot tolerate surgical intervention.4 Detached lesions may initially be managed nonoperatively with physical therapy and rehabilitation, but the rate of recurrent instability after nonoperative management of a known HAGL lesion remains unknown.4 Most active young people are expected to have persistent pain and/or instability and require surgical intervention. Both arthroscopic and open methods have been used successfully.3,8-15 Persistent instability is often the primary complaint leading to a diagnosis of a HAGL lesion.4 The patient in this case report neither demonstrated nor reported any instability event after his 6-month period of nonoperative management, despite his young age and elite physical requirements.
To our knowledge, there are no reports of successful nonoperative management of a known symptomatic HAGL lesion in a high-demand athlete. Although we do not routinely recommend nonoperative treatment for cases such as the one reported here, the decision to delay this Navy SEAL’s surgical management was made out of concern about potential complications of postoperative rehabilitation given the concurrent axillary nerve injury.
With anterior shoulder dislocations, multiple concomitant shoulder injuries, including a HAGL lesion, are not uncommon.6,16 With HAGL lesions, associated rotator cuff injuries occur at a rate as high as 23%.6 Our patient had a concurrent partial rotator cuff tear but also an axillary nerve traction injury. To our knowledge, the literature has not described axillary nerve injury specifically in association with a HAGL lesion, though it is well documented and maintained as a possible concurrent injury with anterior shoulder instability events.17 Robinson and colleagues16 found a 5.8% incidence of a clinically apparent neurologic deficit after traumatic anterior shoulder dislocation in 3633 dislocations, about 75% of which were isolated axillary nerve injuries. They also reported a 25.7% rate of rotator cuff tear or greater tuberosity fracture, either of which significantly increased the likelihood of a neurologic deficit in their study.
When nerve continuity remains, functional recovery occurs after 3 to 6 months, or within weeks in some cases.18-20 Nerve injuries in continuity but with persistent, severe clinical deficits may require surgical exploration with subsequent neurolysis and/or repair.19-21 Our patient showed gradual axillary nerve recovery from a clinical standpoint. By 6 months after injury, despite continued muscle atrophy and decreased axillary nerve sensation, he had returned to full duty as a Navy SEAL. By 17 months, atrophy was markedly improved, and strength and ROM had subjectively returned, despite there being significant conduction amplitude losses, up to 50% of the contralateral side, on EMG testing.
This case represents a scenario in which likely initial surgical management was precluded by a concomitant injury, and the patient had a serendipitous outcome. It is possible the axillary neuropraxia and subsequent temporary deltoid dysfunction provided a unique environment that was conducive to the healing of the HAGL lesion. With classic Bankart lesions, many surgeons prefer to use aggressive early surgical treatment in first-time dislocators, especially elite athletes, in an attempt to avoid recurrent instability.22-26 However, some have suggested that initial immobilization after acute injury may lead to successful nonoperative management.27 Perhaps our case report raises the question as to whether a prolonged period of initial immobilization can prove successful in management of a HAGL lesion. Prospective studies comparing early surgical and nonoperative treatment of these challenging lesions are warranted.
We have reported a case of successful nonoperative management of a HAGL lesion in an active-duty Navy SEAL with concomitant shoulder injuries. This case could suggest that a trial of initial nonoperative management should be considered for injuries that involve a HAGL lesion when there are concerns about the patient’s ability to complete functional rehabilitation because of the combined injuries of the shoulder.
1. Nicola T. Anterior dislocation of the shoulder: the role of the articular capsule. J Bone Joint Surg. 1942;25:614-616.
2. Bach BR, Warren RF, Fronek J. Disruption of the lateral capsule of the shoulder. A cause of recurrent dislocation. J Bone Joint Surg Br. 1988;70(2):274-276.
3. Wolf EM, Cheng JC, Dickson K. Humeral avulsion of glenohumeral ligaments as a cause of anterior shoulder instability. Arthroscopy. 1995;11(5):600-607.
4. George MS, Khazzam M, Kuhn JE. Humeral avulsion of glenohumeral ligaments. J Am Acad Orthop Surg. 2011;19(3):127-133.
5. Tirman PF, Steinbach LS, Feller JF, Stauffer AE. Humeral avulsion of the anterior shoulder stabilizing structures after anterior shoulder dislocation: demonstration by MRI and MR arthrography. Skeletal Radiol. 1996;25(8):743-748.
6. Bui-Mansfield LT, Banks KP, Taylor DC. Humeral avulsion of the glenohumeral ligaments: the HAGL lesion. Am J Sports Med. 2007;35(11):1960-1966.
7. Dumitru D, Zwarts MJ. Needle electromyography. In: Dumitru D, Amato AA, Zwarts MJ, eds. Electrodiagnostic Medicine. 3rd ed. Philadelphia, PA: Hanley & Belfus; 2005:257-292.
8. Parameswaran AD, Provencher MT, Bach BR Jr, Verma N, Romeo AA. Humeral avulsion of the glenohumeral ligament. Injury pattern and arthroscopic repair techniques. Orthopedics. 2008;31(8):773-779.
9. Kon Y, Shiozaki H, Sugaya H. Arthroscopic repair of a humeral avulsion of the glenohumeral ligament lesion. Arthroscopy. 2005;21(5):632.
10. Bokor DJ, Conboy VB, Olson C. Anterior instability of the glenohumeral joint with humeral avulsion of the glenohumeral ligament: a review of 41 cases. J Bone Joint Surg Br. 1999;81(1):93-96.
11. Field LD, Bokor DJ, Savoie FH 3rd. Humeral and glenoid detachment of the anterior inferior glenohumeral ligament: a cause of anterior shoulder instability. J Shoulder Elbow Surg. 1997;6(1):6-10.
12. Arciero RA, Mazzocca AD. Mini-open repair technique of HAGL (humeral avulsion of the glenohumeral ligament) lesion. Arthroscopy. 2005;21(9):1152.
13. Bhatia DN, DeBeer JF, van Rooyen KS. The “subscapularis-sparing” approach: a new mini-open technique to repair a humeral avulsion of the glenohumeral ligament lesion. Arthroscopy. 2009;25(6):686-690.
14. Huberty D, Burkhart S. Arthroscopic repair of anterior humeral avulsion of the glenohumeral ligaments. Tech Shoulder Elbow Surg. 2006;7(4):186-190.
15. Richards DP, Burkhart SS. Arthroscopic humeral avulsion of the glenohumeral ligaments (HAGL) repair. Arthroscopy. 2004;20(suppl 2):134-141.
16. Robinson CM, Shur N, Sharpe T, Ray A, Murray IR. Injuries associated with traumatic anterior glenohumeral dislocations. J Bone Joint Surg Am. 2012;94(1):18-26.
17. Visser CP, Coene LN, Brand R, Tavy DL. The incidence of nerve injury in anterior dislocation of the shoulder and its influence on functional recovery. A prospective clinical and EMG study. J Bone Joint Surg Br. 1999;81(4):679-685.
18. Gumina S, Bertino A, Di Giorgio G, Postacchini F. Injury of the axillary nerve subsequent to recurrence of shoulder dislocation. Clinical and electromyographic study. Chir Organi Mov. 2005;90(2):153-158.
19. Perlmutter GS. Axillary nerve injury. Clin Orthop Relat Res. 1999;(368):28-36.
20. Saragaglia D, Picard F, Le Bredonchel T, Moncenis C, Sardo M, Tourne Y. Acute anterior instability of the shoulder: short- and mid-term outcome after conservative treatment [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2001;87(3):215-220.
21. Kline DG, Kim DH. Axillary nerve repair in 99 patients with 101 stretch injuries. J Neurosurg. 2003;99(4):630-636.
22. Kralinger FS, Golser K, Wischatta R, Wambacher M, Sperner G. Predicting recurrence after primary anterior shoulder dislocation. Am J Sports Med. 2002;30(1):116-120.
23. Bottoni CR, Wilckens JH, DeBerardino TM, et al. A prospective, randomized evaluation of arthroscopic stabilization versus nonoperative treatment in patients with acute, traumatic, first-time shoulder dislocations. Am J Sports Med. 2002;30(4):576-580.
24. Handoll HH, Almaiyah MA, Rangan A. Surgical versus non-surgical treatment for acute anterior shoulder dislocation. Cochrane Database Syst Rev. 2004;(1):CD004325.
25. Jakobsen BW, Johannsen HV, Suder P, Søjbjerg JO. Primary repair versus conservative treatment of first-time traumatic anterior dislocation of the shoulder: a randomized study with 10-year follow-up. Arthroscopy. 2007;23(2):118-123.
26. Kirkley A, Griffin S, Richards C, Miniaci A, Mohtadi N. Prospective randomized clinical trial comparing the effectiveness of immediate arthroscopic stabilization versus immobilization and rehabilitation in first traumatic anterior dislocations of the shoulder. Arthroscopy. 1999;15(5):507-514.
27. Paterson WH, Throckmorton TW, Koester M, Azar FM, Kuhn JE. Position and duration of immobilization after primary anterior shoulder dislocation: a systematic review and meta-analysis of the literature. J Bone Joint Surg Am. 2010;92(18):2924-2933.
The humeral avulsion of glenohumeral ligament (HAGL) lesion has been recognized as a cause of recurrent shoulder instability. In 1942, Nicola1 was the first to describe this lesion, in a small case series of avulsions of the anterior band of the inferior glenohumeral ligament from the humeral neck secondary to a dislocation injury. In 1988, Bach and colleagues2 described it in 2 patients with recurrent anterior dislocations. Wolf and colleagues3 were the first to apply the term HAGL to the injury, in 1995.
HAGL lesion incidence ranges from 1% to 9%, but many authors think the lesion is underdiagnosed.3-5 It occurs in isolation or in combination with other injuries, and it is commonly identified on recurrence of instability. Bui-Mansfield and colleagues6 found that 11% of patients with a diagnosis of HAGL lesion previously had surgery on the same shoulder, whereas for 62% the lesion was associated with other, concurrent lesions, including labral tears (18, 25%), rotator cuff tears (16, 23%), and Hill-Sachs deformities (12, 17%).
Most young athletes who undergo nonoperative therapy for a HAGL lesion continue to experience pain and/or instability that then requires surgical intervention.4 To our knowledge, there are no reports of return to full function in young competitive athletes or return to manual labor after nonoperative management of a HAGL lesion.
In this article, we report the case of a US Navy SEAL who sustained a traction injury causing an axillary nerve injury and a HAGL lesion. Successful nonoperative management allowed him to return to full duty. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An otherwise healthy 26-year-old Navy SEAL presented with pain and significant weakness in the right (dominant) upper extremity after an injury in a training exercise. The shoulder sustained a traction injury when the man’s fast-moving marine attack craft was in a collision and he was trying not to be thrown off. He reported having a sense of dislocation yet never required a reduction.
Physical examination revealed severe weakness with shoulder abduction, external rotation, and forward flexion; inability to contract the deltoid muscle; and complete numbness along the cutaneous distribution of the axillary nerve. On neurovascular examination, the right upper extremity was otherwise intact. The patient had complete passive range of motion (ROM) with apprehension in abduction with external rotation along with anterior laxity and normal posterior stability.
Standard shoulder radiographs showed no bony abnormalities and a concentrically reduced glenohumeral joint. Magnetic resonance imaging (MRI), reviewed by a staff musculoskeletal radiologist and a sports fellowship–trained orthopedic surgeon, showed a greater tuberosity contusion, a partial tear of the infraspinatus, and a HAGL lesion (Figure 1).
The patient was counseled toward surgical intervention to prevent symptoms of recurrent instability. A detailed discussion ensued about whether to proceed with surgery immediately or to pursue temporary nonoperative treatment to allow for assessment and return of deltoid function. Patient and surgeon decided to delay operative intervention because of concerns about the patient’s ability to effectively rehabilitate while still having a compromised axillary nerve after surgery. The recommendation was to delay initial electromyographic (EMG) and nerve conduction velocity testing at least 4 weeks to allow for completion of Wallerian degeneration and more accurate assessment of the axillary nerve.7 Physical therapy for gentle ROM (excluding external rotation) and isometric rotator cuff exercises were initiated.
Five weeks after injury, the patient left the area to attend a 2-month nonphysical training course and continued rehabilitation and orthopedic follow-up at another military medical facility. Six weeks after injury, initial EMG testing revealed the expected axillary neuropraxia. In addition, some marginal improvement in ROM was noted, but deltoid function was still very limited.
Ten weeks after injury, clinical inspection revealed deltoid wasting. Active shoulder ROM was limited, and deltoid strength was 3/5, though the patient was able to perform a standard push-up without difficulty and showed no sign of laxity or apprehension on shoulder examination. Repeat EMG testing revealed axillary nerve denervation with no sign of regeneration. Twelve weeks after injury, MRA showed reorganization and partial healing of the HAGL lesion relative to the prior study (Figure 2).
On the patient’s return from training, 15 weeks after injury, he had improved active ROM and 4+/5 deltoid strength. Axillary nerve sensation was still decreased but markedly improved. Physical examination revealed no significant shoulder laxity or apprehension, and the patient denied feelings of instability. Activities were advanced to include an organized strengthening program.
Six months after injury, the patient was cleared to return to his unit with only mild physical restriction. Function continued to steadily improve. After 9 months, he was cleared for full, unrestricted duty. Although he still demonstrated slight asymmetric weakness in the right deltoid with continued muscular atrophy, examination findings were otherwise normal, and he was back to full activities without significant symptoms.
Eleven months after injury, MRI showed healing of the HAGL lesion (Figure 3). At 17 months, EMG testing revealed significant interval improvement in axillary motor unit potentials but still about a 50% decrement compared with the noninjured side. The patient denied any motor or sensory deficits and any instability events since his injury. He continued with full function as an active-duty Navy SEAL.
Discussion
Nonoperative management has been used for injuries to the inferior glenohumeral ligament complex when there is no humeral detachment but generally has been reserved for low-demand patients and patients who cannot tolerate surgical intervention.4 Detached lesions may initially be managed nonoperatively with physical therapy and rehabilitation, but the rate of recurrent instability after nonoperative management of a known HAGL lesion remains unknown.4 Most active young people are expected to have persistent pain and/or instability and require surgical intervention. Both arthroscopic and open methods have been used successfully.3,8-15 Persistent instability is often the primary complaint leading to a diagnosis of a HAGL lesion.4 The patient in this case report neither demonstrated nor reported any instability event after his 6-month period of nonoperative management, despite his young age and elite physical requirements.
To our knowledge, there are no reports of successful nonoperative management of a known symptomatic HAGL lesion in a high-demand athlete. Although we do not routinely recommend nonoperative treatment for cases such as the one reported here, the decision to delay this Navy SEAL’s surgical management was made out of concern about potential complications of postoperative rehabilitation given the concurrent axillary nerve injury.
With anterior shoulder dislocations, multiple concomitant shoulder injuries, including a HAGL lesion, are not uncommon.6,16 With HAGL lesions, associated rotator cuff injuries occur at a rate as high as 23%.6 Our patient had a concurrent partial rotator cuff tear but also an axillary nerve traction injury. To our knowledge, the literature has not described axillary nerve injury specifically in association with a HAGL lesion, though it is well documented and maintained as a possible concurrent injury with anterior shoulder instability events.17 Robinson and colleagues16 found a 5.8% incidence of a clinically apparent neurologic deficit after traumatic anterior shoulder dislocation in 3633 dislocations, about 75% of which were isolated axillary nerve injuries. They also reported a 25.7% rate of rotator cuff tear or greater tuberosity fracture, either of which significantly increased the likelihood of a neurologic deficit in their study.
When nerve continuity remains, functional recovery occurs after 3 to 6 months, or within weeks in some cases.18-20 Nerve injuries in continuity but with persistent, severe clinical deficits may require surgical exploration with subsequent neurolysis and/or repair.19-21 Our patient showed gradual axillary nerve recovery from a clinical standpoint. By 6 months after injury, despite continued muscle atrophy and decreased axillary nerve sensation, he had returned to full duty as a Navy SEAL. By 17 months, atrophy was markedly improved, and strength and ROM had subjectively returned, despite there being significant conduction amplitude losses, up to 50% of the contralateral side, on EMG testing.
This case represents a scenario in which likely initial surgical management was precluded by a concomitant injury, and the patient had a serendipitous outcome. It is possible the axillary neuropraxia and subsequent temporary deltoid dysfunction provided a unique environment that was conducive to the healing of the HAGL lesion. With classic Bankart lesions, many surgeons prefer to use aggressive early surgical treatment in first-time dislocators, especially elite athletes, in an attempt to avoid recurrent instability.22-26 However, some have suggested that initial immobilization after acute injury may lead to successful nonoperative management.27 Perhaps our case report raises the question as to whether a prolonged period of initial immobilization can prove successful in management of a HAGL lesion. Prospective studies comparing early surgical and nonoperative treatment of these challenging lesions are warranted.
We have reported a case of successful nonoperative management of a HAGL lesion in an active-duty Navy SEAL with concomitant shoulder injuries. This case could suggest that a trial of initial nonoperative management should be considered for injuries that involve a HAGL lesion when there are concerns about the patient’s ability to complete functional rehabilitation because of the combined injuries of the shoulder.
The humeral avulsion of glenohumeral ligament (HAGL) lesion has been recognized as a cause of recurrent shoulder instability. In 1942, Nicola1 was the first to describe this lesion, in a small case series of avulsions of the anterior band of the inferior glenohumeral ligament from the humeral neck secondary to a dislocation injury. In 1988, Bach and colleagues2 described it in 2 patients with recurrent anterior dislocations. Wolf and colleagues3 were the first to apply the term HAGL to the injury, in 1995.
HAGL lesion incidence ranges from 1% to 9%, but many authors think the lesion is underdiagnosed.3-5 It occurs in isolation or in combination with other injuries, and it is commonly identified on recurrence of instability. Bui-Mansfield and colleagues6 found that 11% of patients with a diagnosis of HAGL lesion previously had surgery on the same shoulder, whereas for 62% the lesion was associated with other, concurrent lesions, including labral tears (18, 25%), rotator cuff tears (16, 23%), and Hill-Sachs deformities (12, 17%).
Most young athletes who undergo nonoperative therapy for a HAGL lesion continue to experience pain and/or instability that then requires surgical intervention.4 To our knowledge, there are no reports of return to full function in young competitive athletes or return to manual labor after nonoperative management of a HAGL lesion.
In this article, we report the case of a US Navy SEAL who sustained a traction injury causing an axillary nerve injury and a HAGL lesion. Successful nonoperative management allowed him to return to full duty. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An otherwise healthy 26-year-old Navy SEAL presented with pain and significant weakness in the right (dominant) upper extremity after an injury in a training exercise. The shoulder sustained a traction injury when the man’s fast-moving marine attack craft was in a collision and he was trying not to be thrown off. He reported having a sense of dislocation yet never required a reduction.
Physical examination revealed severe weakness with shoulder abduction, external rotation, and forward flexion; inability to contract the deltoid muscle; and complete numbness along the cutaneous distribution of the axillary nerve. On neurovascular examination, the right upper extremity was otherwise intact. The patient had complete passive range of motion (ROM) with apprehension in abduction with external rotation along with anterior laxity and normal posterior stability.
Standard shoulder radiographs showed no bony abnormalities and a concentrically reduced glenohumeral joint. Magnetic resonance imaging (MRI), reviewed by a staff musculoskeletal radiologist and a sports fellowship–trained orthopedic surgeon, showed a greater tuberosity contusion, a partial tear of the infraspinatus, and a HAGL lesion (Figure 1).
The patient was counseled toward surgical intervention to prevent symptoms of recurrent instability. A detailed discussion ensued about whether to proceed with surgery immediately or to pursue temporary nonoperative treatment to allow for assessment and return of deltoid function. Patient and surgeon decided to delay operative intervention because of concerns about the patient’s ability to effectively rehabilitate while still having a compromised axillary nerve after surgery. The recommendation was to delay initial electromyographic (EMG) and nerve conduction velocity testing at least 4 weeks to allow for completion of Wallerian degeneration and more accurate assessment of the axillary nerve.7 Physical therapy for gentle ROM (excluding external rotation) and isometric rotator cuff exercises were initiated.
Five weeks after injury, the patient left the area to attend a 2-month nonphysical training course and continued rehabilitation and orthopedic follow-up at another military medical facility. Six weeks after injury, initial EMG testing revealed the expected axillary neuropraxia. In addition, some marginal improvement in ROM was noted, but deltoid function was still very limited.
Ten weeks after injury, clinical inspection revealed deltoid wasting. Active shoulder ROM was limited, and deltoid strength was 3/5, though the patient was able to perform a standard push-up without difficulty and showed no sign of laxity or apprehension on shoulder examination. Repeat EMG testing revealed axillary nerve denervation with no sign of regeneration. Twelve weeks after injury, MRA showed reorganization and partial healing of the HAGL lesion relative to the prior study (Figure 2).
On the patient’s return from training, 15 weeks after injury, he had improved active ROM and 4+/5 deltoid strength. Axillary nerve sensation was still decreased but markedly improved. Physical examination revealed no significant shoulder laxity or apprehension, and the patient denied feelings of instability. Activities were advanced to include an organized strengthening program.
Six months after injury, the patient was cleared to return to his unit with only mild physical restriction. Function continued to steadily improve. After 9 months, he was cleared for full, unrestricted duty. Although he still demonstrated slight asymmetric weakness in the right deltoid with continued muscular atrophy, examination findings were otherwise normal, and he was back to full activities without significant symptoms.
Eleven months after injury, MRI showed healing of the HAGL lesion (Figure 3). At 17 months, EMG testing revealed significant interval improvement in axillary motor unit potentials but still about a 50% decrement compared with the noninjured side. The patient denied any motor or sensory deficits and any instability events since his injury. He continued with full function as an active-duty Navy SEAL.
Discussion
Nonoperative management has been used for injuries to the inferior glenohumeral ligament complex when there is no humeral detachment but generally has been reserved for low-demand patients and patients who cannot tolerate surgical intervention.4 Detached lesions may initially be managed nonoperatively with physical therapy and rehabilitation, but the rate of recurrent instability after nonoperative management of a known HAGL lesion remains unknown.4 Most active young people are expected to have persistent pain and/or instability and require surgical intervention. Both arthroscopic and open methods have been used successfully.3,8-15 Persistent instability is often the primary complaint leading to a diagnosis of a HAGL lesion.4 The patient in this case report neither demonstrated nor reported any instability event after his 6-month period of nonoperative management, despite his young age and elite physical requirements.
To our knowledge, there are no reports of successful nonoperative management of a known symptomatic HAGL lesion in a high-demand athlete. Although we do not routinely recommend nonoperative treatment for cases such as the one reported here, the decision to delay this Navy SEAL’s surgical management was made out of concern about potential complications of postoperative rehabilitation given the concurrent axillary nerve injury.
With anterior shoulder dislocations, multiple concomitant shoulder injuries, including a HAGL lesion, are not uncommon.6,16 With HAGL lesions, associated rotator cuff injuries occur at a rate as high as 23%.6 Our patient had a concurrent partial rotator cuff tear but also an axillary nerve traction injury. To our knowledge, the literature has not described axillary nerve injury specifically in association with a HAGL lesion, though it is well documented and maintained as a possible concurrent injury with anterior shoulder instability events.17 Robinson and colleagues16 found a 5.8% incidence of a clinically apparent neurologic deficit after traumatic anterior shoulder dislocation in 3633 dislocations, about 75% of which were isolated axillary nerve injuries. They also reported a 25.7% rate of rotator cuff tear or greater tuberosity fracture, either of which significantly increased the likelihood of a neurologic deficit in their study.
When nerve continuity remains, functional recovery occurs after 3 to 6 months, or within weeks in some cases.18-20 Nerve injuries in continuity but with persistent, severe clinical deficits may require surgical exploration with subsequent neurolysis and/or repair.19-21 Our patient showed gradual axillary nerve recovery from a clinical standpoint. By 6 months after injury, despite continued muscle atrophy and decreased axillary nerve sensation, he had returned to full duty as a Navy SEAL. By 17 months, atrophy was markedly improved, and strength and ROM had subjectively returned, despite there being significant conduction amplitude losses, up to 50% of the contralateral side, on EMG testing.
This case represents a scenario in which likely initial surgical management was precluded by a concomitant injury, and the patient had a serendipitous outcome. It is possible the axillary neuropraxia and subsequent temporary deltoid dysfunction provided a unique environment that was conducive to the healing of the HAGL lesion. With classic Bankart lesions, many surgeons prefer to use aggressive early surgical treatment in first-time dislocators, especially elite athletes, in an attempt to avoid recurrent instability.22-26 However, some have suggested that initial immobilization after acute injury may lead to successful nonoperative management.27 Perhaps our case report raises the question as to whether a prolonged period of initial immobilization can prove successful in management of a HAGL lesion. Prospective studies comparing early surgical and nonoperative treatment of these challenging lesions are warranted.
We have reported a case of successful nonoperative management of a HAGL lesion in an active-duty Navy SEAL with concomitant shoulder injuries. This case could suggest that a trial of initial nonoperative management should be considered for injuries that involve a HAGL lesion when there are concerns about the patient’s ability to complete functional rehabilitation because of the combined injuries of the shoulder.
1. Nicola T. Anterior dislocation of the shoulder: the role of the articular capsule. J Bone Joint Surg. 1942;25:614-616.
2. Bach BR, Warren RF, Fronek J. Disruption of the lateral capsule of the shoulder. A cause of recurrent dislocation. J Bone Joint Surg Br. 1988;70(2):274-276.
3. Wolf EM, Cheng JC, Dickson K. Humeral avulsion of glenohumeral ligaments as a cause of anterior shoulder instability. Arthroscopy. 1995;11(5):600-607.
4. George MS, Khazzam M, Kuhn JE. Humeral avulsion of glenohumeral ligaments. J Am Acad Orthop Surg. 2011;19(3):127-133.
5. Tirman PF, Steinbach LS, Feller JF, Stauffer AE. Humeral avulsion of the anterior shoulder stabilizing structures after anterior shoulder dislocation: demonstration by MRI and MR arthrography. Skeletal Radiol. 1996;25(8):743-748.
6. Bui-Mansfield LT, Banks KP, Taylor DC. Humeral avulsion of the glenohumeral ligaments: the HAGL lesion. Am J Sports Med. 2007;35(11):1960-1966.
7. Dumitru D, Zwarts MJ. Needle electromyography. In: Dumitru D, Amato AA, Zwarts MJ, eds. Electrodiagnostic Medicine. 3rd ed. Philadelphia, PA: Hanley & Belfus; 2005:257-292.
8. Parameswaran AD, Provencher MT, Bach BR Jr, Verma N, Romeo AA. Humeral avulsion of the glenohumeral ligament. Injury pattern and arthroscopic repair techniques. Orthopedics. 2008;31(8):773-779.
9. Kon Y, Shiozaki H, Sugaya H. Arthroscopic repair of a humeral avulsion of the glenohumeral ligament lesion. Arthroscopy. 2005;21(5):632.
10. Bokor DJ, Conboy VB, Olson C. Anterior instability of the glenohumeral joint with humeral avulsion of the glenohumeral ligament: a review of 41 cases. J Bone Joint Surg Br. 1999;81(1):93-96.
11. Field LD, Bokor DJ, Savoie FH 3rd. Humeral and glenoid detachment of the anterior inferior glenohumeral ligament: a cause of anterior shoulder instability. J Shoulder Elbow Surg. 1997;6(1):6-10.
12. Arciero RA, Mazzocca AD. Mini-open repair technique of HAGL (humeral avulsion of the glenohumeral ligament) lesion. Arthroscopy. 2005;21(9):1152.
13. Bhatia DN, DeBeer JF, van Rooyen KS. The “subscapularis-sparing” approach: a new mini-open technique to repair a humeral avulsion of the glenohumeral ligament lesion. Arthroscopy. 2009;25(6):686-690.
14. Huberty D, Burkhart S. Arthroscopic repair of anterior humeral avulsion of the glenohumeral ligaments. Tech Shoulder Elbow Surg. 2006;7(4):186-190.
15. Richards DP, Burkhart SS. Arthroscopic humeral avulsion of the glenohumeral ligaments (HAGL) repair. Arthroscopy. 2004;20(suppl 2):134-141.
16. Robinson CM, Shur N, Sharpe T, Ray A, Murray IR. Injuries associated with traumatic anterior glenohumeral dislocations. J Bone Joint Surg Am. 2012;94(1):18-26.
17. Visser CP, Coene LN, Brand R, Tavy DL. The incidence of nerve injury in anterior dislocation of the shoulder and its influence on functional recovery. A prospective clinical and EMG study. J Bone Joint Surg Br. 1999;81(4):679-685.
18. Gumina S, Bertino A, Di Giorgio G, Postacchini F. Injury of the axillary nerve subsequent to recurrence of shoulder dislocation. Clinical and electromyographic study. Chir Organi Mov. 2005;90(2):153-158.
19. Perlmutter GS. Axillary nerve injury. Clin Orthop Relat Res. 1999;(368):28-36.
20. Saragaglia D, Picard F, Le Bredonchel T, Moncenis C, Sardo M, Tourne Y. Acute anterior instability of the shoulder: short- and mid-term outcome after conservative treatment [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2001;87(3):215-220.
21. Kline DG, Kim DH. Axillary nerve repair in 99 patients with 101 stretch injuries. J Neurosurg. 2003;99(4):630-636.
22. Kralinger FS, Golser K, Wischatta R, Wambacher M, Sperner G. Predicting recurrence after primary anterior shoulder dislocation. Am J Sports Med. 2002;30(1):116-120.
23. Bottoni CR, Wilckens JH, DeBerardino TM, et al. A prospective, randomized evaluation of arthroscopic stabilization versus nonoperative treatment in patients with acute, traumatic, first-time shoulder dislocations. Am J Sports Med. 2002;30(4):576-580.
24. Handoll HH, Almaiyah MA, Rangan A. Surgical versus non-surgical treatment for acute anterior shoulder dislocation. Cochrane Database Syst Rev. 2004;(1):CD004325.
25. Jakobsen BW, Johannsen HV, Suder P, Søjbjerg JO. Primary repair versus conservative treatment of first-time traumatic anterior dislocation of the shoulder: a randomized study with 10-year follow-up. Arthroscopy. 2007;23(2):118-123.
26. Kirkley A, Griffin S, Richards C, Miniaci A, Mohtadi N. Prospective randomized clinical trial comparing the effectiveness of immediate arthroscopic stabilization versus immobilization and rehabilitation in first traumatic anterior dislocations of the shoulder. Arthroscopy. 1999;15(5):507-514.
27. Paterson WH, Throckmorton TW, Koester M, Azar FM, Kuhn JE. Position and duration of immobilization after primary anterior shoulder dislocation: a systematic review and meta-analysis of the literature. J Bone Joint Surg Am. 2010;92(18):2924-2933.
1. Nicola T. Anterior dislocation of the shoulder: the role of the articular capsule. J Bone Joint Surg. 1942;25:614-616.
2. Bach BR, Warren RF, Fronek J. Disruption of the lateral capsule of the shoulder. A cause of recurrent dislocation. J Bone Joint Surg Br. 1988;70(2):274-276.
3. Wolf EM, Cheng JC, Dickson K. Humeral avulsion of glenohumeral ligaments as a cause of anterior shoulder instability. Arthroscopy. 1995;11(5):600-607.
4. George MS, Khazzam M, Kuhn JE. Humeral avulsion of glenohumeral ligaments. J Am Acad Orthop Surg. 2011;19(3):127-133.
5. Tirman PF, Steinbach LS, Feller JF, Stauffer AE. Humeral avulsion of the anterior shoulder stabilizing structures after anterior shoulder dislocation: demonstration by MRI and MR arthrography. Skeletal Radiol. 1996;25(8):743-748.
6. Bui-Mansfield LT, Banks KP, Taylor DC. Humeral avulsion of the glenohumeral ligaments: the HAGL lesion. Am J Sports Med. 2007;35(11):1960-1966.
7. Dumitru D, Zwarts MJ. Needle electromyography. In: Dumitru D, Amato AA, Zwarts MJ, eds. Electrodiagnostic Medicine. 3rd ed. Philadelphia, PA: Hanley & Belfus; 2005:257-292.
8. Parameswaran AD, Provencher MT, Bach BR Jr, Verma N, Romeo AA. Humeral avulsion of the glenohumeral ligament. Injury pattern and arthroscopic repair techniques. Orthopedics. 2008;31(8):773-779.
9. Kon Y, Shiozaki H, Sugaya H. Arthroscopic repair of a humeral avulsion of the glenohumeral ligament lesion. Arthroscopy. 2005;21(5):632.
10. Bokor DJ, Conboy VB, Olson C. Anterior instability of the glenohumeral joint with humeral avulsion of the glenohumeral ligament: a review of 41 cases. J Bone Joint Surg Br. 1999;81(1):93-96.
11. Field LD, Bokor DJ, Savoie FH 3rd. Humeral and glenoid detachment of the anterior inferior glenohumeral ligament: a cause of anterior shoulder instability. J Shoulder Elbow Surg. 1997;6(1):6-10.
12. Arciero RA, Mazzocca AD. Mini-open repair technique of HAGL (humeral avulsion of the glenohumeral ligament) lesion. Arthroscopy. 2005;21(9):1152.
13. Bhatia DN, DeBeer JF, van Rooyen KS. The “subscapularis-sparing” approach: a new mini-open technique to repair a humeral avulsion of the glenohumeral ligament lesion. Arthroscopy. 2009;25(6):686-690.
14. Huberty D, Burkhart S. Arthroscopic repair of anterior humeral avulsion of the glenohumeral ligaments. Tech Shoulder Elbow Surg. 2006;7(4):186-190.
15. Richards DP, Burkhart SS. Arthroscopic humeral avulsion of the glenohumeral ligaments (HAGL) repair. Arthroscopy. 2004;20(suppl 2):134-141.
16. Robinson CM, Shur N, Sharpe T, Ray A, Murray IR. Injuries associated with traumatic anterior glenohumeral dislocations. J Bone Joint Surg Am. 2012;94(1):18-26.
17. Visser CP, Coene LN, Brand R, Tavy DL. The incidence of nerve injury in anterior dislocation of the shoulder and its influence on functional recovery. A prospective clinical and EMG study. J Bone Joint Surg Br. 1999;81(4):679-685.
18. Gumina S, Bertino A, Di Giorgio G, Postacchini F. Injury of the axillary nerve subsequent to recurrence of shoulder dislocation. Clinical and electromyographic study. Chir Organi Mov. 2005;90(2):153-158.
19. Perlmutter GS. Axillary nerve injury. Clin Orthop Relat Res. 1999;(368):28-36.
20. Saragaglia D, Picard F, Le Bredonchel T, Moncenis C, Sardo M, Tourne Y. Acute anterior instability of the shoulder: short- and mid-term outcome after conservative treatment [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2001;87(3):215-220.
21. Kline DG, Kim DH. Axillary nerve repair in 99 patients with 101 stretch injuries. J Neurosurg. 2003;99(4):630-636.
22. Kralinger FS, Golser K, Wischatta R, Wambacher M, Sperner G. Predicting recurrence after primary anterior shoulder dislocation. Am J Sports Med. 2002;30(1):116-120.
23. Bottoni CR, Wilckens JH, DeBerardino TM, et al. A prospective, randomized evaluation of arthroscopic stabilization versus nonoperative treatment in patients with acute, traumatic, first-time shoulder dislocations. Am J Sports Med. 2002;30(4):576-580.
24. Handoll HH, Almaiyah MA, Rangan A. Surgical versus non-surgical treatment for acute anterior shoulder dislocation. Cochrane Database Syst Rev. 2004;(1):CD004325.
25. Jakobsen BW, Johannsen HV, Suder P, Søjbjerg JO. Primary repair versus conservative treatment of first-time traumatic anterior dislocation of the shoulder: a randomized study with 10-year follow-up. Arthroscopy. 2007;23(2):118-123.
26. Kirkley A, Griffin S, Richards C, Miniaci A, Mohtadi N. Prospective randomized clinical trial comparing the effectiveness of immediate arthroscopic stabilization versus immobilization and rehabilitation in first traumatic anterior dislocations of the shoulder. Arthroscopy. 1999;15(5):507-514.
27. Paterson WH, Throckmorton TW, Koester M, Azar FM, Kuhn JE. Position and duration of immobilization after primary anterior shoulder dislocation: a systematic review and meta-analysis of the literature. J Bone Joint Surg Am. 2010;92(18):2924-2933.
The Arthroscopic Superior Capsular Reconstruction
Rotator cuff tears are very common, and 250,000 to 500,000 rotator cuff repairs are performed in the United States each year.1,2 In most cases, a complete repair of even large or massive tears can be achieved. However, a subset of patients exist in whom the glenohumeral joint has minimal degenerative changes and the rotator cuff tendon is either irreparable or very poor quality and unlikely to heal (ie, failed previous cuff repair). Some authors have advocated for reverse shoulder arthroplasty (RSA) in these patients despite the lack of glenohumeral arthritis. However, due to the permanent destruction of the glenohumeral articular surfaces, complication rates, and concerns about implant longevity with RSA, we believe the superior capsular reconstruction (SCR) is a viable alternative in patients in whom joint preservation is appropriate based on age limitations and/or activity requirements.3
The SCR was first described by Mihata and colleagues4 as a means to reconstruct the superior capsule in shoulders with large, irreparable posterosuperior rotator cuff tears. Originally described using a fascia lata autograft, our technique has been adapted to incorporate a dermal allograft, which limits donor site morbidity and operative time. In most cases, the dermal allograft is fixed to the normal anatomic attachments of the superior glenoid just medial to the superior labrum, laterally to the greater tuberosity, and posteriorly with side-to-side sutures to the remaining rotator cuff. If there is a robust band of “comma” tissue anteriorly, we fix the anterior margin of the dermal graft to this with side-to-side sutures. The comma tissue represents the medial sling of the biceps tendon and connects the upper subscapularis tendon to the anterior supraspinatus. In most cases, this tissue is intact after repair of the subscapularis tendon.
Technique
The patient is positioned in either the lateral decubitus or beach chair position. The arm is positioned in 20° to 30° of abduction and 20° to 30° of forward flexion. A diagnostic arthroscopy is performed through a posterior glenohumeral viewing portal. The subscapularis is visualized and repaired if torn. A biceps tenodesis is performed in most cases, as there is often a tear of the subscapularis, tear or instability of the biceps tendon, and/or a compromised attachment of the biceps root.
Attention is turned to the subacromial space. Posterior viewing and lateral working portals are established. A 10-mm flexible cannula (PassPort; Arthrex) is placed in the lateral portal to aid with suture management and graft passage. A limited subacromial decompression is performed that preserves the coracoacromial arch. The rotator cuff is carefully dissected and freed from the internal deltoid fascia. The scapular spine is identified to visualize the raphé between the supraspinatus and infraspinatus. The infraspinatus is mobilized and repaired as much as possible.
If we think that the tear might be reparable by gaining added excursion from a posterior interval slide, or if it is clearly not reparable but the remaining rim of rotator cuff obscures clear visualization of the superior glenoid, we perform a posterior interval slide. If the additional excursion that is achieved by the posterior slide is adequate for a complete repair, we proceed with the repair. However, if the tear is not reparable even after the posterior interval slide, we have found that the exposure and preparation of the superior glenoid is greatly improved after the posterior slide. After fixation of the dermal graft, we typically perform a partial side-to-side repair of the supraspinatus to the infraspinatus over the top of the graft.
The bone beds of the greater tuberosity and just medial to the superior glenoid labrum are prepared with a shaver and motorized burr. Two anchors (3.0-mm BioComposite SutureTak; Arthrex) are placed in the superior glenoid neck at about the 10 o’clock and 2 o’clock positions approximately 5 mm medial to the superior labrum. Note: the placement medial to the labrum is chosen because this is the normal origin of the superior capsule and because of the angle of approach, these percutaneous portals are often more medial than typical portals for placing anchors during SLAP (superior labral anterior to posterior) repair. Next, 2 threaded anchors (4.75-mm BioComposite SwiveLock; Arthrex) preloaded with suture tape are placed in the greater tuberosity along the articular margin (Figure 1). However, if a biceps tenodesis with an interference screw is placed at the top of the bicipital groove, this anchor preloaded with suture tape can also serve as the anteromedial anchor in the greater tuberosity footprint. The distances between all 4 anchors are carefully measured with a calibrated probe (Figures 2A-2D).
We use a 3.0-mm acellular dermal allograft (ArthroFlex; Arthrex) to reconstruct the superior capsule. The positions of the 4 anchors are carefully marked on the dermal allograft. We routinely add an additional 5 mm of tissue to the medial, anterior, and posterior margins to decrease the risk of suture cut out. An additional 10 mm of tissue is added laterally to cover the greater tuberosity. The final contoured graft is typically trapezoidal in shape.
The sutures from the 4 anchors are then sequentially retrieved through the lateral cannula. The sutures from the greater tuberosity anchors are passed through their respective holes in the graft. However, the suture limbs from each of the glenoid anchors are individually passed 2 mm anterior and 2 mm posterior to their respective marks on the graft with an antegrade suture passer (Figure 3). It is important to have an assistant apply tension to each of the sutures after they are passed through the graft to decrease the chance of crossing and tangling the sutures.
The eyelets of the medial anchors are utilized as pulleys to deliver the dermal allograft into the shoulder. One suture limb from each of the glenoid anchors is tied to the other over a switching stick (Figure 4A). The 2 remaining (untied) suture limbs are then pulled, which introduces the graft to the orifice of the cannula (Figure 4B). A tissue grasper is then used to fold the dermal allograft along its long axis and introduce the graft into the joint (Figure 4C). Once the medial portion of the graft is positioned onto the superior glenoid the 2 remaining (untied) suture limbs are tied to each other as a static knot in the subacromial space (Figure 4D).
The redundancy in the suture tapes can be removed by sequentially sliding a retriever down each suture and tensioning the suture as the nose of the instrument pushes the dermal graft down to the tuberosity bone bed. The suture tapes are crisscrossed and secured laterally with 2 additional knotless threaded anchors (Figure 5). One may also place cinch stitches at the anterolateral and posterolateral corners of the graft that are incorporated into the lateral anchors. These sutures can be useful for pulling the graft back out of the subacromial space in the event of any suture tangles, and can be used for controlling the lateral aspect of the graft during lateral anchor placement.
At this point in the procedure, additional glenoid anchors can be placed both anterior and posterior to the superior glenoid anchors if additional glenoid fixation is desired. Finally, 2 to 3 side-to-side sutures are placed posteriorly attaching the anterior aspect of the infraspinatus to the posterior aspect of the dermal allograft (Figures 6A-6C). If rotator interval tissue (comma tissue) is present, anterior side-to-side sutures may be placed. However, we do not recommend placing anterior side-to-side sutures directly from the dermal allograft to the subscapularis as this may deform the graft, over- constrain the shoulder, and restrict motion.
Discussion
Reconstruction of the superior capsule has been shown to restore the normal restraint to superior translation of the humeral head and reestablish a stable fulcrum at the glenohumeral joint.5 It should be mentioned that we do not perform the SCR in patients with advanced glenohumeral arthritis. The short-term results of this novel procedure have been encouraging, including our own series of patients, in which most patients have had a significant reduction in pain, improvement in function, and very few complications (P. J. Denard, MD, S. S. Burkhart, MD, P. C. Brady, MD, J. Tokish, MD, C. R. Adams, MD, unpublished data, May 2016).
The early success of this procedure suggests that a robust superior capsule is necessary, in addition to functional muscle-tendon units, to restore the stable fulcrum and force couples that are necessary for normal shoulder function. Perhaps we have not paid enough attention to the integrity of the superior capsule in the past. In cases of revision cuff repair, we pay special attention to the quality of the capsular layer deep to the cuff tendon. If the capsule is poor quality, we sometimes reconstruct the capsule with a dermal allograft (SCR) and then do a rotator cuff repair (partial or complete) over the top of the SCR to maintain the normal anatomic deep to superficial layering of the capsule and rotator cuff.
We are very conservative with our postoperative rehabilitation program after a SCR. We know that the rate of stiffness with a conservative program after an arthroscopic rotator cuff repair, even in the revision setting, is very low.6 Furthermore, both basic science on healing of soft tissue to bone and radiographic analysis of healing after postoperative rotator cuff repairs support a slow rehabilitation program.7,8 A canine model specifically evaluating acellular dermal allografts in the shoulder suggests that these grafts undergo significant remodeling and become weaker before they get stronger.9 We would rather err on the side of healing of the SCR with potentially a slight increase in the rate of shoulder stiffness than to regain early motion at the expense of graft failure. Therefore, we have the patient wear a sling with no shoulder motion for 6 weeks. Passive motion is started at 6 weeks postoperative and strengthening is delayed until 12 to 16 weeks postoperative.
1. Orr SB, Chainani A, Hippensteel KJ, et al. Aligned multilayered electrospun scaffolds for rotator cuff tendon tissue engineering. Acta Biomater. 2015;24:117-126.
2. Austin L, Black EM, Lombardi NJ, Pepe MD, Lazarus M. Arthroscopic transosseous rotator cuff repair. A prospective study on cost savings, surgical time, and outcomes. Ortho J Sports Med. 2015;3(2 Suppl). doi:10.1177/2325967115S00156.
3. Denard PJ, Lädermann A, Jiwani AZ, Burkhart SS. Functional outcome after arthroscopic repair of massive rotator cuff tears in individuals with pseudoparalysis. Arthroscopy. 2012;28(9):1214-1219.
4. Mihata T, Lee TQ, Watanabe C, et al. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy. 2013;29(3):459-470.
5. Mihata T, McGarry MH, Pirolo JM, Kinoshita M, Lee TQ. Superior capsule reconstruction to restore superior stability in irreparable rotator cuff tears: a biomechanical cadaveric study. Am J Sports Med. 2012;40(10):2248-2255.
6. Huberty DP, Schoolfield JD, Brady PC, Vadala AP, Arrigoni P, Burkhart SS. Incidence and treatment of postoperative stiffness following arthroscopic rotator cuff repair. Arthroscopy. 2009;25(8):880-890.
7. Sonnabend DH, Howlett CR, Young AA. Histological evaluation of repair of the rotator cuff in a primate model. J Bone Joint Surg Br. 2010;92(4):586-594.
8. Lee BG, Cho NS, Rhee YG. Effect of two rehabilitation protocols on range of motion and healing rates after arthroscopic rotator cuff repair: aggressive versus limited early passive exercises. Arthroscopy. 2012;28(1):34-42.
9. Adams JE, Zobitz ME, Reach JS Jr, An KN, Steinmann SP. Rotator cuff repair using an acellular dermal matrix graft: an in vivo study in a canine model. Arthroscopy. 2006;22(7):700-709.
Rotator cuff tears are very common, and 250,000 to 500,000 rotator cuff repairs are performed in the United States each year.1,2 In most cases, a complete repair of even large or massive tears can be achieved. However, a subset of patients exist in whom the glenohumeral joint has minimal degenerative changes and the rotator cuff tendon is either irreparable or very poor quality and unlikely to heal (ie, failed previous cuff repair). Some authors have advocated for reverse shoulder arthroplasty (RSA) in these patients despite the lack of glenohumeral arthritis. However, due to the permanent destruction of the glenohumeral articular surfaces, complication rates, and concerns about implant longevity with RSA, we believe the superior capsular reconstruction (SCR) is a viable alternative in patients in whom joint preservation is appropriate based on age limitations and/or activity requirements.3
The SCR was first described by Mihata and colleagues4 as a means to reconstruct the superior capsule in shoulders with large, irreparable posterosuperior rotator cuff tears. Originally described using a fascia lata autograft, our technique has been adapted to incorporate a dermal allograft, which limits donor site morbidity and operative time. In most cases, the dermal allograft is fixed to the normal anatomic attachments of the superior glenoid just medial to the superior labrum, laterally to the greater tuberosity, and posteriorly with side-to-side sutures to the remaining rotator cuff. If there is a robust band of “comma” tissue anteriorly, we fix the anterior margin of the dermal graft to this with side-to-side sutures. The comma tissue represents the medial sling of the biceps tendon and connects the upper subscapularis tendon to the anterior supraspinatus. In most cases, this tissue is intact after repair of the subscapularis tendon.
Technique
The patient is positioned in either the lateral decubitus or beach chair position. The arm is positioned in 20° to 30° of abduction and 20° to 30° of forward flexion. A diagnostic arthroscopy is performed through a posterior glenohumeral viewing portal. The subscapularis is visualized and repaired if torn. A biceps tenodesis is performed in most cases, as there is often a tear of the subscapularis, tear or instability of the biceps tendon, and/or a compromised attachment of the biceps root.
Attention is turned to the subacromial space. Posterior viewing and lateral working portals are established. A 10-mm flexible cannula (PassPort; Arthrex) is placed in the lateral portal to aid with suture management and graft passage. A limited subacromial decompression is performed that preserves the coracoacromial arch. The rotator cuff is carefully dissected and freed from the internal deltoid fascia. The scapular spine is identified to visualize the raphé between the supraspinatus and infraspinatus. The infraspinatus is mobilized and repaired as much as possible.
If we think that the tear might be reparable by gaining added excursion from a posterior interval slide, or if it is clearly not reparable but the remaining rim of rotator cuff obscures clear visualization of the superior glenoid, we perform a posterior interval slide. If the additional excursion that is achieved by the posterior slide is adequate for a complete repair, we proceed with the repair. However, if the tear is not reparable even after the posterior interval slide, we have found that the exposure and preparation of the superior glenoid is greatly improved after the posterior slide. After fixation of the dermal graft, we typically perform a partial side-to-side repair of the supraspinatus to the infraspinatus over the top of the graft.
The bone beds of the greater tuberosity and just medial to the superior glenoid labrum are prepared with a shaver and motorized burr. Two anchors (3.0-mm BioComposite SutureTak; Arthrex) are placed in the superior glenoid neck at about the 10 o’clock and 2 o’clock positions approximately 5 mm medial to the superior labrum. Note: the placement medial to the labrum is chosen because this is the normal origin of the superior capsule and because of the angle of approach, these percutaneous portals are often more medial than typical portals for placing anchors during SLAP (superior labral anterior to posterior) repair. Next, 2 threaded anchors (4.75-mm BioComposite SwiveLock; Arthrex) preloaded with suture tape are placed in the greater tuberosity along the articular margin (Figure 1). However, if a biceps tenodesis with an interference screw is placed at the top of the bicipital groove, this anchor preloaded with suture tape can also serve as the anteromedial anchor in the greater tuberosity footprint. The distances between all 4 anchors are carefully measured with a calibrated probe (Figures 2A-2D).
We use a 3.0-mm acellular dermal allograft (ArthroFlex; Arthrex) to reconstruct the superior capsule. The positions of the 4 anchors are carefully marked on the dermal allograft. We routinely add an additional 5 mm of tissue to the medial, anterior, and posterior margins to decrease the risk of suture cut out. An additional 10 mm of tissue is added laterally to cover the greater tuberosity. The final contoured graft is typically trapezoidal in shape.
The sutures from the 4 anchors are then sequentially retrieved through the lateral cannula. The sutures from the greater tuberosity anchors are passed through their respective holes in the graft. However, the suture limbs from each of the glenoid anchors are individually passed 2 mm anterior and 2 mm posterior to their respective marks on the graft with an antegrade suture passer (Figure 3). It is important to have an assistant apply tension to each of the sutures after they are passed through the graft to decrease the chance of crossing and tangling the sutures.
The eyelets of the medial anchors are utilized as pulleys to deliver the dermal allograft into the shoulder. One suture limb from each of the glenoid anchors is tied to the other over a switching stick (Figure 4A). The 2 remaining (untied) suture limbs are then pulled, which introduces the graft to the orifice of the cannula (Figure 4B). A tissue grasper is then used to fold the dermal allograft along its long axis and introduce the graft into the joint (Figure 4C). Once the medial portion of the graft is positioned onto the superior glenoid the 2 remaining (untied) suture limbs are tied to each other as a static knot in the subacromial space (Figure 4D).
The redundancy in the suture tapes can be removed by sequentially sliding a retriever down each suture and tensioning the suture as the nose of the instrument pushes the dermal graft down to the tuberosity bone bed. The suture tapes are crisscrossed and secured laterally with 2 additional knotless threaded anchors (Figure 5). One may also place cinch stitches at the anterolateral and posterolateral corners of the graft that are incorporated into the lateral anchors. These sutures can be useful for pulling the graft back out of the subacromial space in the event of any suture tangles, and can be used for controlling the lateral aspect of the graft during lateral anchor placement.
At this point in the procedure, additional glenoid anchors can be placed both anterior and posterior to the superior glenoid anchors if additional glenoid fixation is desired. Finally, 2 to 3 side-to-side sutures are placed posteriorly attaching the anterior aspect of the infraspinatus to the posterior aspect of the dermal allograft (Figures 6A-6C). If rotator interval tissue (comma tissue) is present, anterior side-to-side sutures may be placed. However, we do not recommend placing anterior side-to-side sutures directly from the dermal allograft to the subscapularis as this may deform the graft, over- constrain the shoulder, and restrict motion.
Discussion
Reconstruction of the superior capsule has been shown to restore the normal restraint to superior translation of the humeral head and reestablish a stable fulcrum at the glenohumeral joint.5 It should be mentioned that we do not perform the SCR in patients with advanced glenohumeral arthritis. The short-term results of this novel procedure have been encouraging, including our own series of patients, in which most patients have had a significant reduction in pain, improvement in function, and very few complications (P. J. Denard, MD, S. S. Burkhart, MD, P. C. Brady, MD, J. Tokish, MD, C. R. Adams, MD, unpublished data, May 2016).
The early success of this procedure suggests that a robust superior capsule is necessary, in addition to functional muscle-tendon units, to restore the stable fulcrum and force couples that are necessary for normal shoulder function. Perhaps we have not paid enough attention to the integrity of the superior capsule in the past. In cases of revision cuff repair, we pay special attention to the quality of the capsular layer deep to the cuff tendon. If the capsule is poor quality, we sometimes reconstruct the capsule with a dermal allograft (SCR) and then do a rotator cuff repair (partial or complete) over the top of the SCR to maintain the normal anatomic deep to superficial layering of the capsule and rotator cuff.
We are very conservative with our postoperative rehabilitation program after a SCR. We know that the rate of stiffness with a conservative program after an arthroscopic rotator cuff repair, even in the revision setting, is very low.6 Furthermore, both basic science on healing of soft tissue to bone and radiographic analysis of healing after postoperative rotator cuff repairs support a slow rehabilitation program.7,8 A canine model specifically evaluating acellular dermal allografts in the shoulder suggests that these grafts undergo significant remodeling and become weaker before they get stronger.9 We would rather err on the side of healing of the SCR with potentially a slight increase in the rate of shoulder stiffness than to regain early motion at the expense of graft failure. Therefore, we have the patient wear a sling with no shoulder motion for 6 weeks. Passive motion is started at 6 weeks postoperative and strengthening is delayed until 12 to 16 weeks postoperative.
Rotator cuff tears are very common, and 250,000 to 500,000 rotator cuff repairs are performed in the United States each year.1,2 In most cases, a complete repair of even large or massive tears can be achieved. However, a subset of patients exist in whom the glenohumeral joint has minimal degenerative changes and the rotator cuff tendon is either irreparable or very poor quality and unlikely to heal (ie, failed previous cuff repair). Some authors have advocated for reverse shoulder arthroplasty (RSA) in these patients despite the lack of glenohumeral arthritis. However, due to the permanent destruction of the glenohumeral articular surfaces, complication rates, and concerns about implant longevity with RSA, we believe the superior capsular reconstruction (SCR) is a viable alternative in patients in whom joint preservation is appropriate based on age limitations and/or activity requirements.3
The SCR was first described by Mihata and colleagues4 as a means to reconstruct the superior capsule in shoulders with large, irreparable posterosuperior rotator cuff tears. Originally described using a fascia lata autograft, our technique has been adapted to incorporate a dermal allograft, which limits donor site morbidity and operative time. In most cases, the dermal allograft is fixed to the normal anatomic attachments of the superior glenoid just medial to the superior labrum, laterally to the greater tuberosity, and posteriorly with side-to-side sutures to the remaining rotator cuff. If there is a robust band of “comma” tissue anteriorly, we fix the anterior margin of the dermal graft to this with side-to-side sutures. The comma tissue represents the medial sling of the biceps tendon and connects the upper subscapularis tendon to the anterior supraspinatus. In most cases, this tissue is intact after repair of the subscapularis tendon.
Technique
The patient is positioned in either the lateral decubitus or beach chair position. The arm is positioned in 20° to 30° of abduction and 20° to 30° of forward flexion. A diagnostic arthroscopy is performed through a posterior glenohumeral viewing portal. The subscapularis is visualized and repaired if torn. A biceps tenodesis is performed in most cases, as there is often a tear of the subscapularis, tear or instability of the biceps tendon, and/or a compromised attachment of the biceps root.
Attention is turned to the subacromial space. Posterior viewing and lateral working portals are established. A 10-mm flexible cannula (PassPort; Arthrex) is placed in the lateral portal to aid with suture management and graft passage. A limited subacromial decompression is performed that preserves the coracoacromial arch. The rotator cuff is carefully dissected and freed from the internal deltoid fascia. The scapular spine is identified to visualize the raphé between the supraspinatus and infraspinatus. The infraspinatus is mobilized and repaired as much as possible.
If we think that the tear might be reparable by gaining added excursion from a posterior interval slide, or if it is clearly not reparable but the remaining rim of rotator cuff obscures clear visualization of the superior glenoid, we perform a posterior interval slide. If the additional excursion that is achieved by the posterior slide is adequate for a complete repair, we proceed with the repair. However, if the tear is not reparable even after the posterior interval slide, we have found that the exposure and preparation of the superior glenoid is greatly improved after the posterior slide. After fixation of the dermal graft, we typically perform a partial side-to-side repair of the supraspinatus to the infraspinatus over the top of the graft.
The bone beds of the greater tuberosity and just medial to the superior glenoid labrum are prepared with a shaver and motorized burr. Two anchors (3.0-mm BioComposite SutureTak; Arthrex) are placed in the superior glenoid neck at about the 10 o’clock and 2 o’clock positions approximately 5 mm medial to the superior labrum. Note: the placement medial to the labrum is chosen because this is the normal origin of the superior capsule and because of the angle of approach, these percutaneous portals are often more medial than typical portals for placing anchors during SLAP (superior labral anterior to posterior) repair. Next, 2 threaded anchors (4.75-mm BioComposite SwiveLock; Arthrex) preloaded with suture tape are placed in the greater tuberosity along the articular margin (Figure 1). However, if a biceps tenodesis with an interference screw is placed at the top of the bicipital groove, this anchor preloaded with suture tape can also serve as the anteromedial anchor in the greater tuberosity footprint. The distances between all 4 anchors are carefully measured with a calibrated probe (Figures 2A-2D).
We use a 3.0-mm acellular dermal allograft (ArthroFlex; Arthrex) to reconstruct the superior capsule. The positions of the 4 anchors are carefully marked on the dermal allograft. We routinely add an additional 5 mm of tissue to the medial, anterior, and posterior margins to decrease the risk of suture cut out. An additional 10 mm of tissue is added laterally to cover the greater tuberosity. The final contoured graft is typically trapezoidal in shape.
The sutures from the 4 anchors are then sequentially retrieved through the lateral cannula. The sutures from the greater tuberosity anchors are passed through their respective holes in the graft. However, the suture limbs from each of the glenoid anchors are individually passed 2 mm anterior and 2 mm posterior to their respective marks on the graft with an antegrade suture passer (Figure 3). It is important to have an assistant apply tension to each of the sutures after they are passed through the graft to decrease the chance of crossing and tangling the sutures.
The eyelets of the medial anchors are utilized as pulleys to deliver the dermal allograft into the shoulder. One suture limb from each of the glenoid anchors is tied to the other over a switching stick (Figure 4A). The 2 remaining (untied) suture limbs are then pulled, which introduces the graft to the orifice of the cannula (Figure 4B). A tissue grasper is then used to fold the dermal allograft along its long axis and introduce the graft into the joint (Figure 4C). Once the medial portion of the graft is positioned onto the superior glenoid the 2 remaining (untied) suture limbs are tied to each other as a static knot in the subacromial space (Figure 4D).
The redundancy in the suture tapes can be removed by sequentially sliding a retriever down each suture and tensioning the suture as the nose of the instrument pushes the dermal graft down to the tuberosity bone bed. The suture tapes are crisscrossed and secured laterally with 2 additional knotless threaded anchors (Figure 5). One may also place cinch stitches at the anterolateral and posterolateral corners of the graft that are incorporated into the lateral anchors. These sutures can be useful for pulling the graft back out of the subacromial space in the event of any suture tangles, and can be used for controlling the lateral aspect of the graft during lateral anchor placement.
At this point in the procedure, additional glenoid anchors can be placed both anterior and posterior to the superior glenoid anchors if additional glenoid fixation is desired. Finally, 2 to 3 side-to-side sutures are placed posteriorly attaching the anterior aspect of the infraspinatus to the posterior aspect of the dermal allograft (Figures 6A-6C). If rotator interval tissue (comma tissue) is present, anterior side-to-side sutures may be placed. However, we do not recommend placing anterior side-to-side sutures directly from the dermal allograft to the subscapularis as this may deform the graft, over- constrain the shoulder, and restrict motion.
Discussion
Reconstruction of the superior capsule has been shown to restore the normal restraint to superior translation of the humeral head and reestablish a stable fulcrum at the glenohumeral joint.5 It should be mentioned that we do not perform the SCR in patients with advanced glenohumeral arthritis. The short-term results of this novel procedure have been encouraging, including our own series of patients, in which most patients have had a significant reduction in pain, improvement in function, and very few complications (P. J. Denard, MD, S. S. Burkhart, MD, P. C. Brady, MD, J. Tokish, MD, C. R. Adams, MD, unpublished data, May 2016).
The early success of this procedure suggests that a robust superior capsule is necessary, in addition to functional muscle-tendon units, to restore the stable fulcrum and force couples that are necessary for normal shoulder function. Perhaps we have not paid enough attention to the integrity of the superior capsule in the past. In cases of revision cuff repair, we pay special attention to the quality of the capsular layer deep to the cuff tendon. If the capsule is poor quality, we sometimes reconstruct the capsule with a dermal allograft (SCR) and then do a rotator cuff repair (partial or complete) over the top of the SCR to maintain the normal anatomic deep to superficial layering of the capsule and rotator cuff.
We are very conservative with our postoperative rehabilitation program after a SCR. We know that the rate of stiffness with a conservative program after an arthroscopic rotator cuff repair, even in the revision setting, is very low.6 Furthermore, both basic science on healing of soft tissue to bone and radiographic analysis of healing after postoperative rotator cuff repairs support a slow rehabilitation program.7,8 A canine model specifically evaluating acellular dermal allografts in the shoulder suggests that these grafts undergo significant remodeling and become weaker before they get stronger.9 We would rather err on the side of healing of the SCR with potentially a slight increase in the rate of shoulder stiffness than to regain early motion at the expense of graft failure. Therefore, we have the patient wear a sling with no shoulder motion for 6 weeks. Passive motion is started at 6 weeks postoperative and strengthening is delayed until 12 to 16 weeks postoperative.
1. Orr SB, Chainani A, Hippensteel KJ, et al. Aligned multilayered electrospun scaffolds for rotator cuff tendon tissue engineering. Acta Biomater. 2015;24:117-126.
2. Austin L, Black EM, Lombardi NJ, Pepe MD, Lazarus M. Arthroscopic transosseous rotator cuff repair. A prospective study on cost savings, surgical time, and outcomes. Ortho J Sports Med. 2015;3(2 Suppl). doi:10.1177/2325967115S00156.
3. Denard PJ, Lädermann A, Jiwani AZ, Burkhart SS. Functional outcome after arthroscopic repair of massive rotator cuff tears in individuals with pseudoparalysis. Arthroscopy. 2012;28(9):1214-1219.
4. Mihata T, Lee TQ, Watanabe C, et al. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy. 2013;29(3):459-470.
5. Mihata T, McGarry MH, Pirolo JM, Kinoshita M, Lee TQ. Superior capsule reconstruction to restore superior stability in irreparable rotator cuff tears: a biomechanical cadaveric study. Am J Sports Med. 2012;40(10):2248-2255.
6. Huberty DP, Schoolfield JD, Brady PC, Vadala AP, Arrigoni P, Burkhart SS. Incidence and treatment of postoperative stiffness following arthroscopic rotator cuff repair. Arthroscopy. 2009;25(8):880-890.
7. Sonnabend DH, Howlett CR, Young AA. Histological evaluation of repair of the rotator cuff in a primate model. J Bone Joint Surg Br. 2010;92(4):586-594.
8. Lee BG, Cho NS, Rhee YG. Effect of two rehabilitation protocols on range of motion and healing rates after arthroscopic rotator cuff repair: aggressive versus limited early passive exercises. Arthroscopy. 2012;28(1):34-42.
9. Adams JE, Zobitz ME, Reach JS Jr, An KN, Steinmann SP. Rotator cuff repair using an acellular dermal matrix graft: an in vivo study in a canine model. Arthroscopy. 2006;22(7):700-709.
1. Orr SB, Chainani A, Hippensteel KJ, et al. Aligned multilayered electrospun scaffolds for rotator cuff tendon tissue engineering. Acta Biomater. 2015;24:117-126.
2. Austin L, Black EM, Lombardi NJ, Pepe MD, Lazarus M. Arthroscopic transosseous rotator cuff repair. A prospective study on cost savings, surgical time, and outcomes. Ortho J Sports Med. 2015;3(2 Suppl). doi:10.1177/2325967115S00156.
3. Denard PJ, Lädermann A, Jiwani AZ, Burkhart SS. Functional outcome after arthroscopic repair of massive rotator cuff tears in individuals with pseudoparalysis. Arthroscopy. 2012;28(9):1214-1219.
4. Mihata T, Lee TQ, Watanabe C, et al. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy. 2013;29(3):459-470.
5. Mihata T, McGarry MH, Pirolo JM, Kinoshita M, Lee TQ. Superior capsule reconstruction to restore superior stability in irreparable rotator cuff tears: a biomechanical cadaveric study. Am J Sports Med. 2012;40(10):2248-2255.
6. Huberty DP, Schoolfield JD, Brady PC, Vadala AP, Arrigoni P, Burkhart SS. Incidence and treatment of postoperative stiffness following arthroscopic rotator cuff repair. Arthroscopy. 2009;25(8):880-890.
7. Sonnabend DH, Howlett CR, Young AA. Histological evaluation of repair of the rotator cuff in a primate model. J Bone Joint Surg Br. 2010;92(4):586-594.
8. Lee BG, Cho NS, Rhee YG. Effect of two rehabilitation protocols on range of motion and healing rates after arthroscopic rotator cuff repair: aggressive versus limited early passive exercises. Arthroscopy. 2012;28(1):34-42.
9. Adams JE, Zobitz ME, Reach JS Jr, An KN, Steinmann SP. Rotator cuff repair using an acellular dermal matrix graft: an in vivo study in a canine model. Arthroscopy. 2006;22(7):700-709.
Platelet-Rich Plasma Can Be Used to Successfully Treat Elbow Ulnar Collateral Ligament Insufficiency in High-Level Throwers
For overhead athletes, elbow ulnar collateral ligament (UCL) insufficiency is a potential career-ending injury. Baseball players with UCL insufficiency typically complain of medial-sided elbow pain that affects their ability to throw. Loss of velocity, loss of control, difficulty warming up, and pain while throwing are all symptoms of UCL injury.
Classically, nonoperative treatment of UCL injuries involves activity modification, use of anti-inflammatory medication, and a structured physical therapy program. Asymptomatic players can return to throwing after a structured interval throwing program. Rettig and colleagues1 found a 42% rate of success in conservatively treating UCL injuries in throwing athletes. UCL reconstruction is reserved for players with complete tears of the UCL or with partial tears after failed conservative treatment. Several techniques have been used to reconstruct the ligament, but successful outcomes depend on a long rehabilitation process. According to most published series, 85% to 90% of athletes who had UCL reconstruction returned to their previous level of play, but it took, on average, 9 to 12 months.2,3 This prolonged recovery period is one reason that some older professional baseball players, as well as casual high school and college players, elect to forgo surgery.
Over the past few years, platelet-rich plasma (PRP) has garnered attention as a bridge between conservative treatment and surgery. PRP refers to a sample of autologous blood that contains a platelet concentration higher than baseline levels. This sample often has a 3 to 5 times increase in growth factor concentration.4-6 Initial studies focused on its ability to successfully treat lateral epicondylitis.7-9 More recent clinical work has shown that PRP can potentially enhance healing after anterior cruciate ligament reconstruction,10-14 rotator cuff repair,15-17 and subacromial decompression.11,18-23 If PRP could be used to successfully treat UCL insufficiency that is refractory to conservative treatment, then year-long recovery periods could be avoided. This could potentially prolong certain athletes’ careers or, at the very least, allow them to return to play much sooner. In the present case series, we hypothesized that PRP injections could be used to successfully treat partial UCL tears in high-level throwing athletes, obviating the need for surgery and its associated prolonged recovery period.
Materials and Methods
Institutional Review Board approval was obtained for this retrospective study of 44 baseball players treated with PRP injections for partial-thickness UCL tears.
Patients provided written informed consent. They were diagnosed with UCL insufficiency by physical examination, and findings were confirmed by magnetic resonance imaging (MRI). After diagnosis, all throwers underwent a trial of conservative treatment that included rest, activity modification, use of anti-inflammatory medication, and physical therapy followed by an attempt to return to throwing using an interval throwing program.
Study inclusion criteria were physical examinations and MRI results consistent with UCL insufficiency, and failure of the conservative treatment plan described.
Patients were injected using the Autologous Conditioned Plasma system (Arthrex). PRP solutions were prepared according to manufacturer guidelines. After the elbow was prepared sterilely, the UCL was injected at the location of the tear. Typically, 3 mL of PRP was injected into the elbow. Sixteen patients had 1 injection, 6 had 2, and 22 had 3. Repeat injections were considered for recalcitrant pain after 3 weeks.
After injection, patients used acetaminophen and ice for pain control. Anti-inflammatory medications were avoided for a minimum of 2 weeks after injection. Typical postinjection therapy protocol consisted of rest followed by progressive stretching and strengthening for about 4 to 6 weeks before the start of an interval throwing program. Although there is no well-defined postinjection recovery protocol, as a general rule rest was prescribed for the first 2 weeks, followed by a progressive stretching and strengthening program for the next month. Patients who were asymptomatic subjectively and clinically—negative moving valgus stress test, negative milking maneuver, no pain with valgus stress—were started on an interval throwing program.
Final follow-up involved a physical examination. Results were classified according to a modified version of the Conway Scale12,24-26: excellent (return to preinjury level of competition or performance), good (return to play at a lower level of competition or performance or, specifically for baseball players, ability to throw in daily batting practice), fair (able to play recreationally), and poor (unable to return to previous sport at any level).
By final follow-up, all patients had completed their postoperative rehabilitation protocol, and all had at least tried to return to their previous activities. No patients were lost to follow-up.
Results
Of the 44 baseball players, 6 were professional, 14 were in college, and 24 were in high school. There were 36 pitchers and 8 position players. Mean age was 17.3 years (range, 16-28 years). All patients were available for follow-up after injection (mean, 11 months). Fifteen of the 44 players had an excellent outcome (34%), 17 had a good outcome, 2 had a fair outcome, and 10 had a poor outcome. After injection, 4 (67%) of the 6 professional baseball players returned to professional play. Five (36%) of the 14 college players had an excellent outcome, and 4 (17%) of the 24 high school players had an excellent outcome. Of the 8 position players, 4 had an excellent outcome, 3 had a good outcome, and 1 had a poor outcome.
Before treatment, all patients had medial-sided elbow pain over the UCL inhibiting their ability to throw. Mean duration of symptoms before injection was 8.8 months (range, 1-36 months). There was no correlation between symptom duration and any outcome measure. On MRI, 29 patients showed partial tears: 22 proximally based and 7 distally based. The other 15 patients had diffuse signal without partial tear. All 7 patients with distally based partial tears and 3 of the patients with proximally based partial tears had a poor outcome. Overall, there were 6 excellent, 7 good, and 2 fair outcomes in the partial-tear group. In the patients with diffuse signal without partial tear, there were 9 excellent and 10 good outcomes.
Mean time from injection to return to throwing was 5 weeks, and mean time to return to competition was 12 weeks (range, 5-24 weeks). The 1 player who returned at 5 weeks was a professional relief pitcher whose team was in the playoffs. He has now pitched for an additional 2 baseball seasons without elbow difficulty.
There were no injection-related complications.
Discussion
To our knowledge, this is the first report documenting successful PRP treatment of UCL insufficiency. In this study, 73% of players who had failed a course of conservative treatment had good to excellent outcomes with PRP injection.
Data on successful nonoperative treatment of UCL injuries are limited. Rettig and colleagues1 treated 31 throwing athletes’ UCL injuries with a supervised rehabilitation program. Treatment included rest, use of anti-inflammatory medication, progressive strengthening, and an interval throwing program. Only 41% of the athletes returned to their previous level of play, and it took, on average, 24.5 weeks. There was no significant difference in age or in duration or acuity of symptoms between those who returned to play and those whose conservative treatment failed.
Surgical reconstruction of UCL injuries has been very successful, with upward of 90% of athletes returning to previous level of play.3,27The procedure, however, is not without associated complications, including retear of the ligament, stiffness, ulnar nerve injury, and fracture.27-29 In addition, even when successful, the procedure requires that athletes take 9 to 12 months to recover before returning to competition at their previous level.
Savoie and colleagues,30 in their recent study on UCL repairs, highlighted an important fact that is often overlooked when reviewing the literature on UCL tears. Most of the literature on these injuries focuses on college and professional baseball players in whom ligament damage is often extensive, precluding repair. In contrast to prior reports, Savoie and colleagues30 found excellent results in 93% of their young athletes who underwent UCL repair. It is possible that their results can be attributed to the fact that many of their athletes had tears isolated to one area of the ligament, as opposed to generalized ligament incompetence. Our improved results vis-à-vis other reports on conservative management may be attributable to the same phenomenon.
PRP has garnered much attention in the literature and media because of its potential to enhance healing of tendons and ligaments; in some cases, it can obviate the need for surgery. After failure of other nonoperative measures in 15 patients with elbow epicondylitis, Mishra and Pavelko8 treated each patient with a single PRP injection. They prepared the PRP using the GPS III system (Biomet). At final follow-up, 93% improvement was seen. Clearly, their experiment had design flaws: It was nonblinded, and 3 of the 5 patients in the control group treated with bupivacaine injection withdrew from the experiment. Despite its shortcomings, their study became the impetus for several other studies.
A larger, double-blinded, randomized controlled trial comparing PRP and cortisone injections for lateral epicondylitis in 100 patients is under way, and preliminary results have been published.9 A minimum of 6 months after injection, patients who received PRP showed more improvement in visual analog scale (VAS) pain scores and Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire scores. In another large, double-blinded, randomized controlled trial, patients with chronic lateral epicondylitis had significant improvements in VAS pain scores and DASH scores relative to patients injected with corticosteroids with a 2-year follow-up.31 Similarly, Thanasas and colleagues32 found significantly reduced VAS pain scores in patients injected with PRP versus autologous whole blood. Another study demonstrated improved tendon morphology using ultrasound imaging 6 months after PRP injection.33
Contrary to these positive results, Krogh and colleagues34 found that a single injection of PRP or glucocorticoid was not significantly superior to a saline injection for reducing pain and disability over a 3-month period in patients with lateral epicondylitis. Their study, however, had major flaws. Its original design called for a 12-month follow-up, but there was massive dropout in all 3 treatment arms, necessitating reporting of only 3-month data. In addition, 60% of the patients in the glucocorticoid group were not naïve to this treatment, so definitive conclusions about the efficacy of glucocorticoids could not be made.
In the present study, we successfully treated partial ligament tears with PRP injections. Sixty-seven percent of our baseball players returned to play at a mean of 4 months, much earlier than the 9 to 12 months typically required after ligament reconstruction. Many athletes, such as high school baseball players or aging veteran professional baseball players, do not have the luxury of 12 months for recovery. Therefore, this select group of patients clearly has a limited window of opportunity to return to play. In fact, these patients might be ideal candidates for PRP injections for UCL injuries. Return-to-play rates, however, differed significantly among professional players and nonprofessional players. The difference may be attributable to professional players’ conditioning, quality of physical therapy, extrinsic motivation, and other intangible factors. Four (67%) of our 6 professional baseball players returned to professional play after injection, whereas only 36% of college players and 17% of high school players had excellent outcomes.
Limitations
The present study had several weaknesses, several of which are inherent to PRP studies conducted so far. It was not a prospective, randomized controlled trial. It is important to note that PRP treatment in diseased tissue may have some drawbacks, as its success depends on the ability of healing tissue to use concentrated growth factors and cytokines to proliferate.35 Thus, a chronically injured ligament with depleted active cells may have a diminished response to PRP. Another limitation of this study is that we evaluated outcomes based on return to play using the Conway Scale, which is well reported but not validated. Despite the potential weaknesses of this outcome scale, it has become the benchmark for measuring the success of outcomes of UCL reconstruction. Furthermore, we did not measure patients’ satisfaction with the treatment. Players who could not return to their preinjury level of play may have considered the treatment a failure regardless of their ability to continue throwing. Last, MRI was not repeated to document ligament healing. We did not routinely perform a second MRI because we thought it would not affect treatment. Several series have found a high incidence of abnormal signal in baseball players’ UCLs. In this group of patients, the most important outcome is return to previous level of competition.
This study raised several questions. Is one PRP brand better than another? Should more than 1 injection be given? What is the ideal postinjection protocol? Clearly, larger, prospective, randomized controlled studies are needed to truly elucidate the potential role of PRP in the treatment algorithm for UCL injury. Nevertheless, in certain cases in which traditional conservative measures have failed and patients do not have the luxury of rehabilitating for 9 to 12 months after surgery, PRP may be a viable treatment option.
Conclusion
In this study, use of PRP in the treatment of UCL insufficiency produced outcomes much better than earlier reported outcomes of conservative treatment of these injuries. PRP injections may be particularly beneficial in young athletes who have sustained acute damage to an isolated part of the ligament and in athletes unwilling or unable to undergo the extended rehabilitation required after surgical reconstruction of the ligament.
1. Rettig AC, Sherrill C, Snead DS, Mendler JC, Mieling P. Nonoperative treatment of ulnar collateral ligament injuries in throwing athletes. Am J Sports Med. 2001;29(1):15-17.
2. Eygendaal D, Rahussen FT, Diercks RL. Biomechanics of the elbow joint in tennis players and relation to pathology. Br J Sports Med. 2007;41(11):820-823.
3. Bowers AL, Dines JS, Dines DM, Altchek DW. Elbow medial ulnar collateral ligament reconstruction: clinical relevance and the docking technique. J Shoulder Elbow Surg. 2010;19(2):110-117.
5. Kibler WB. Biomechanical analysis of the shoulder during tennis activities. Clin Sports Med. 1995;14(1):79-85.
5. Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62(4):489-496.
6. Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10(4):225-228.
7. Elliott B, Fleisig G, Nicholls R, Escamilia R. Technique effects on upper limb loading in the tennis serve. J Sci Med Sport. 2003;6(1):76-87.
8. Mishra A, Pavelko T. Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am J Sports Med. 2006;34(11):1774-1778.
9. Mishra A, Woodall J Jr, Vieira A. Treatment of tendon and muscle using platelet-rich plasma. Clin Sports Med. 2009;28(1):113-125.
10. Kovacs MS. Applied physiology of tennis performance. Br J Sports Med. 2006;40(5):381-386.
11. Xie X, Wu H, Zhao S, Xie G, Huangfu X, Zhao J. The effect of platelet-rich plasma on patterns of gene expression in a dog model of anterior cruciate ligament reconstruction. J Surg Res. 2013;180(1):80-88.
12. Pluim BM, Staal JB, Windler GE, Jayanthi N. Tennis injuries: occurrence, aetiology, and prevention. Br J Sports Med. 2006;40(5):415-423.
13. Xie X, Zhao S, Wu H, et al. Platelet-rich plasma enhances autograft revascularization and reinnervation in a dog model of anterior cruciate ligament reconstruction. J Surg Res. 2013;183(1):214-222.
14. Lopez-Vidriero E, Goulding KA, Simon DA, Sanchez M, Johnson DH. The use of platelet-rich plasma in arthroscopy and sports medicine: optimizing the healing environment. Arthroscopy. 2010;26(2):269-278.
15. Jo CH, Shin JS, Shin WH, Lee SY, Yoon KS, Shin S. Platelet-rich plasma for arthroscopic repair of medium to large rotator cuff tears: a randomized controlled trial. Am J Sports Med. 2015;43(9):2102-2110.
16. Jo CH, Shin JS, Lee YG, et al. Platelet-rich plasma for arthroscopic repair of large to massive rotator cuff tears: a randomized, single-blinded, parallel-group trial. Am J Sports Med. 2013;41(10):2240-2248.
17. Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet-rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20(4):518-528.
18. Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20(4):518-528.
19. Barber FA, Hrnack SA, Snyder SJ, Hapa O. Rotator cuff repair healing influenced by platelet-rich plasma construct augmentation. Arthroscopy. 2011;27(8):1029-1035.
20. Jo CH, Kim JE, Yoon KS, et al. Does platelet-rich plasma accelerate recovery after rotator cuff repair? A prospective cohort study. Am J Sports Med. 2011;39(10):2082-2090.
21. Jo CH, Kim JE, Yoon KS, Shin S. Platelet-rich plasma stimulates cell proliferation and enhances matrix gene expression and synthesis in tenocytes from human rotator cuff tendons with degenerative tears. Am J Sports Med. 2012;40(5):1035-1045.
22. Chahal J, Van Thiel GS, Mall N, et al. The role of platelet-rich plasma in arthroscopic rotator cuff repair: a systematic review with quantitative synthesis. Arthroscopy. 2012;28(11):1718-1727.
23. Mei-Dan O, Carmont MR. The role of platelet-rich plasma in rotator cuff repair. Sports Med Arthrosc Rev. 2011;19(3):244-250.
24. Dines JS, ElAttrache NS, Conway JE, Smith W, Ahmad CS. Clinical outcomes of the DANE TJ technique to treat ulnar collateral ligament insufficiency of the elbow. Am J Sports Med. 2007;35(12):2039-2044.
25. Hutchinson MR, Laprade RF, Burnett QM 2nd, Moss R, Terpstra J. Injury surveillance at the USTA boys’ tennis championships: a 6-yr study. Med Sci Sports Exerc. 1995;27(6):826-830.
26. Winge S, Jørgensen U, Nielsen A. Epidemiology of injuries in Danish championship tennis. Int J Sports Med. 1989;10(5):368-371.
27. Safran MR, Hutchinson MR, Moss R, Albrandt J. A comparison of injuries in elite boys and girls tennis players. Paper presented at: 9th Annual Meeting of the Society of Tennis Medicine and Science; March 1999; Indian Wells, CA.
28. Cain EL, Andrews JR, Dugas JR, et al. Outcome of ulnar collateral ligament reconstruction of the elbow in 1281 athletes: results in 743 athletes with minimum 2-year follow-up. Am J Sports Med. 2010;38(12):2426-2434.
29. Dines JS, Yocum LA, Frank JB, ElAttrache NS, Gambardella RA, Jobe FW. Revision surgery for failed elbow medial collateral ligament reconstruction. Am J Sports Med. 2008;36(6):1061-1065.
30. Savoie FH, Trenhaile SW, Roberts J, Field LD, Ramsey JR. Primary repair of ulnar collateral ligament injuries of the elbow in young athletes: a case series of injuries to the proximal and distal ends of the ligament. Am J Sports Med. 2008;36(6):1066-1072.
31. Gosens T, Peerbooms JC, van Laar W, Oudsten den BL. Ongoing positive effect of platelet-rich plasma versus corticosteroid injection in lateral epicondylitis: a double-blind randomized controlled trial with 2-year follow-up. Am J Sports Med. 2011;39(6):1200-1208.
32. Thanasas C, Papadimitriou G, Charalambidis C, Paraskevopoulos I, Papanikolaou A. Platelet-rich plasma versus autologous whole blood for the treatment of chronic lateral elbow epicondylitis: a randomized controlled clinical trial. Am J Sports Med. 2011;39(10):2130-2134.
33. Chaudhury S, La Lama de M, Adler RS, et al. Platelet-rich plasma for the treatment of lateral epicondylitis: sonographic assessment of tendon morphology and vascularity (pilot study). Skeletal Radiol. 2013;42(1):91-97.
34. Krogh TP, Fredberg U, Stengaard-Pedersen K, Christensen R, Jensen P, Ellingsen T. Treatment of lateral epicondylitis with platelet-rich plasma, glucocorticoid, or saline: a randomized, double-blind, placebo-controlled trial. Am J Sports Med. 2013;41(3):625-635.
35. Anz AW, Hackel JG, Nilssen EC, Andrews JR. Application of biologics in the treatment of the rotator cuff, meniscus, cartilage, and osteoarthritis. J Am Acad Orthop Surg. 2014;22(2):68-79.
For overhead athletes, elbow ulnar collateral ligament (UCL) insufficiency is a potential career-ending injury. Baseball players with UCL insufficiency typically complain of medial-sided elbow pain that affects their ability to throw. Loss of velocity, loss of control, difficulty warming up, and pain while throwing are all symptoms of UCL injury.
Classically, nonoperative treatment of UCL injuries involves activity modification, use of anti-inflammatory medication, and a structured physical therapy program. Asymptomatic players can return to throwing after a structured interval throwing program. Rettig and colleagues1 found a 42% rate of success in conservatively treating UCL injuries in throwing athletes. UCL reconstruction is reserved for players with complete tears of the UCL or with partial tears after failed conservative treatment. Several techniques have been used to reconstruct the ligament, but successful outcomes depend on a long rehabilitation process. According to most published series, 85% to 90% of athletes who had UCL reconstruction returned to their previous level of play, but it took, on average, 9 to 12 months.2,3 This prolonged recovery period is one reason that some older professional baseball players, as well as casual high school and college players, elect to forgo surgery.
Over the past few years, platelet-rich plasma (PRP) has garnered attention as a bridge between conservative treatment and surgery. PRP refers to a sample of autologous blood that contains a platelet concentration higher than baseline levels. This sample often has a 3 to 5 times increase in growth factor concentration.4-6 Initial studies focused on its ability to successfully treat lateral epicondylitis.7-9 More recent clinical work has shown that PRP can potentially enhance healing after anterior cruciate ligament reconstruction,10-14 rotator cuff repair,15-17 and subacromial decompression.11,18-23 If PRP could be used to successfully treat UCL insufficiency that is refractory to conservative treatment, then year-long recovery periods could be avoided. This could potentially prolong certain athletes’ careers or, at the very least, allow them to return to play much sooner. In the present case series, we hypothesized that PRP injections could be used to successfully treat partial UCL tears in high-level throwing athletes, obviating the need for surgery and its associated prolonged recovery period.
Materials and Methods
Institutional Review Board approval was obtained for this retrospective study of 44 baseball players treated with PRP injections for partial-thickness UCL tears.
Patients provided written informed consent. They were diagnosed with UCL insufficiency by physical examination, and findings were confirmed by magnetic resonance imaging (MRI). After diagnosis, all throwers underwent a trial of conservative treatment that included rest, activity modification, use of anti-inflammatory medication, and physical therapy followed by an attempt to return to throwing using an interval throwing program.
Study inclusion criteria were physical examinations and MRI results consistent with UCL insufficiency, and failure of the conservative treatment plan described.
Patients were injected using the Autologous Conditioned Plasma system (Arthrex). PRP solutions were prepared according to manufacturer guidelines. After the elbow was prepared sterilely, the UCL was injected at the location of the tear. Typically, 3 mL of PRP was injected into the elbow. Sixteen patients had 1 injection, 6 had 2, and 22 had 3. Repeat injections were considered for recalcitrant pain after 3 weeks.
After injection, patients used acetaminophen and ice for pain control. Anti-inflammatory medications were avoided for a minimum of 2 weeks after injection. Typical postinjection therapy protocol consisted of rest followed by progressive stretching and strengthening for about 4 to 6 weeks before the start of an interval throwing program. Although there is no well-defined postinjection recovery protocol, as a general rule rest was prescribed for the first 2 weeks, followed by a progressive stretching and strengthening program for the next month. Patients who were asymptomatic subjectively and clinically—negative moving valgus stress test, negative milking maneuver, no pain with valgus stress—were started on an interval throwing program.
Final follow-up involved a physical examination. Results were classified according to a modified version of the Conway Scale12,24-26: excellent (return to preinjury level of competition or performance), good (return to play at a lower level of competition or performance or, specifically for baseball players, ability to throw in daily batting practice), fair (able to play recreationally), and poor (unable to return to previous sport at any level).
By final follow-up, all patients had completed their postoperative rehabilitation protocol, and all had at least tried to return to their previous activities. No patients were lost to follow-up.
Results
Of the 44 baseball players, 6 were professional, 14 were in college, and 24 were in high school. There were 36 pitchers and 8 position players. Mean age was 17.3 years (range, 16-28 years). All patients were available for follow-up after injection (mean, 11 months). Fifteen of the 44 players had an excellent outcome (34%), 17 had a good outcome, 2 had a fair outcome, and 10 had a poor outcome. After injection, 4 (67%) of the 6 professional baseball players returned to professional play. Five (36%) of the 14 college players had an excellent outcome, and 4 (17%) of the 24 high school players had an excellent outcome. Of the 8 position players, 4 had an excellent outcome, 3 had a good outcome, and 1 had a poor outcome.
Before treatment, all patients had medial-sided elbow pain over the UCL inhibiting their ability to throw. Mean duration of symptoms before injection was 8.8 months (range, 1-36 months). There was no correlation between symptom duration and any outcome measure. On MRI, 29 patients showed partial tears: 22 proximally based and 7 distally based. The other 15 patients had diffuse signal without partial tear. All 7 patients with distally based partial tears and 3 of the patients with proximally based partial tears had a poor outcome. Overall, there were 6 excellent, 7 good, and 2 fair outcomes in the partial-tear group. In the patients with diffuse signal without partial tear, there were 9 excellent and 10 good outcomes.
Mean time from injection to return to throwing was 5 weeks, and mean time to return to competition was 12 weeks (range, 5-24 weeks). The 1 player who returned at 5 weeks was a professional relief pitcher whose team was in the playoffs. He has now pitched for an additional 2 baseball seasons without elbow difficulty.
There were no injection-related complications.
Discussion
To our knowledge, this is the first report documenting successful PRP treatment of UCL insufficiency. In this study, 73% of players who had failed a course of conservative treatment had good to excellent outcomes with PRP injection.
Data on successful nonoperative treatment of UCL injuries are limited. Rettig and colleagues1 treated 31 throwing athletes’ UCL injuries with a supervised rehabilitation program. Treatment included rest, use of anti-inflammatory medication, progressive strengthening, and an interval throwing program. Only 41% of the athletes returned to their previous level of play, and it took, on average, 24.5 weeks. There was no significant difference in age or in duration or acuity of symptoms between those who returned to play and those whose conservative treatment failed.
Surgical reconstruction of UCL injuries has been very successful, with upward of 90% of athletes returning to previous level of play.3,27The procedure, however, is not without associated complications, including retear of the ligament, stiffness, ulnar nerve injury, and fracture.27-29 In addition, even when successful, the procedure requires that athletes take 9 to 12 months to recover before returning to competition at their previous level.
Savoie and colleagues,30 in their recent study on UCL repairs, highlighted an important fact that is often overlooked when reviewing the literature on UCL tears. Most of the literature on these injuries focuses on college and professional baseball players in whom ligament damage is often extensive, precluding repair. In contrast to prior reports, Savoie and colleagues30 found excellent results in 93% of their young athletes who underwent UCL repair. It is possible that their results can be attributed to the fact that many of their athletes had tears isolated to one area of the ligament, as opposed to generalized ligament incompetence. Our improved results vis-à-vis other reports on conservative management may be attributable to the same phenomenon.
PRP has garnered much attention in the literature and media because of its potential to enhance healing of tendons and ligaments; in some cases, it can obviate the need for surgery. After failure of other nonoperative measures in 15 patients with elbow epicondylitis, Mishra and Pavelko8 treated each patient with a single PRP injection. They prepared the PRP using the GPS III system (Biomet). At final follow-up, 93% improvement was seen. Clearly, their experiment had design flaws: It was nonblinded, and 3 of the 5 patients in the control group treated with bupivacaine injection withdrew from the experiment. Despite its shortcomings, their study became the impetus for several other studies.
A larger, double-blinded, randomized controlled trial comparing PRP and cortisone injections for lateral epicondylitis in 100 patients is under way, and preliminary results have been published.9 A minimum of 6 months after injection, patients who received PRP showed more improvement in visual analog scale (VAS) pain scores and Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire scores. In another large, double-blinded, randomized controlled trial, patients with chronic lateral epicondylitis had significant improvements in VAS pain scores and DASH scores relative to patients injected with corticosteroids with a 2-year follow-up.31 Similarly, Thanasas and colleagues32 found significantly reduced VAS pain scores in patients injected with PRP versus autologous whole blood. Another study demonstrated improved tendon morphology using ultrasound imaging 6 months after PRP injection.33
Contrary to these positive results, Krogh and colleagues34 found that a single injection of PRP or glucocorticoid was not significantly superior to a saline injection for reducing pain and disability over a 3-month period in patients with lateral epicondylitis. Their study, however, had major flaws. Its original design called for a 12-month follow-up, but there was massive dropout in all 3 treatment arms, necessitating reporting of only 3-month data. In addition, 60% of the patients in the glucocorticoid group were not naïve to this treatment, so definitive conclusions about the efficacy of glucocorticoids could not be made.
In the present study, we successfully treated partial ligament tears with PRP injections. Sixty-seven percent of our baseball players returned to play at a mean of 4 months, much earlier than the 9 to 12 months typically required after ligament reconstruction. Many athletes, such as high school baseball players or aging veteran professional baseball players, do not have the luxury of 12 months for recovery. Therefore, this select group of patients clearly has a limited window of opportunity to return to play. In fact, these patients might be ideal candidates for PRP injections for UCL injuries. Return-to-play rates, however, differed significantly among professional players and nonprofessional players. The difference may be attributable to professional players’ conditioning, quality of physical therapy, extrinsic motivation, and other intangible factors. Four (67%) of our 6 professional baseball players returned to professional play after injection, whereas only 36% of college players and 17% of high school players had excellent outcomes.
Limitations
The present study had several weaknesses, several of which are inherent to PRP studies conducted so far. It was not a prospective, randomized controlled trial. It is important to note that PRP treatment in diseased tissue may have some drawbacks, as its success depends on the ability of healing tissue to use concentrated growth factors and cytokines to proliferate.35 Thus, a chronically injured ligament with depleted active cells may have a diminished response to PRP. Another limitation of this study is that we evaluated outcomes based on return to play using the Conway Scale, which is well reported but not validated. Despite the potential weaknesses of this outcome scale, it has become the benchmark for measuring the success of outcomes of UCL reconstruction. Furthermore, we did not measure patients’ satisfaction with the treatment. Players who could not return to their preinjury level of play may have considered the treatment a failure regardless of their ability to continue throwing. Last, MRI was not repeated to document ligament healing. We did not routinely perform a second MRI because we thought it would not affect treatment. Several series have found a high incidence of abnormal signal in baseball players’ UCLs. In this group of patients, the most important outcome is return to previous level of competition.
This study raised several questions. Is one PRP brand better than another? Should more than 1 injection be given? What is the ideal postinjection protocol? Clearly, larger, prospective, randomized controlled studies are needed to truly elucidate the potential role of PRP in the treatment algorithm for UCL injury. Nevertheless, in certain cases in which traditional conservative measures have failed and patients do not have the luxury of rehabilitating for 9 to 12 months after surgery, PRP may be a viable treatment option.
Conclusion
In this study, use of PRP in the treatment of UCL insufficiency produced outcomes much better than earlier reported outcomes of conservative treatment of these injuries. PRP injections may be particularly beneficial in young athletes who have sustained acute damage to an isolated part of the ligament and in athletes unwilling or unable to undergo the extended rehabilitation required after surgical reconstruction of the ligament.
For overhead athletes, elbow ulnar collateral ligament (UCL) insufficiency is a potential career-ending injury. Baseball players with UCL insufficiency typically complain of medial-sided elbow pain that affects their ability to throw. Loss of velocity, loss of control, difficulty warming up, and pain while throwing are all symptoms of UCL injury.
Classically, nonoperative treatment of UCL injuries involves activity modification, use of anti-inflammatory medication, and a structured physical therapy program. Asymptomatic players can return to throwing after a structured interval throwing program. Rettig and colleagues1 found a 42% rate of success in conservatively treating UCL injuries in throwing athletes. UCL reconstruction is reserved for players with complete tears of the UCL or with partial tears after failed conservative treatment. Several techniques have been used to reconstruct the ligament, but successful outcomes depend on a long rehabilitation process. According to most published series, 85% to 90% of athletes who had UCL reconstruction returned to their previous level of play, but it took, on average, 9 to 12 months.2,3 This prolonged recovery period is one reason that some older professional baseball players, as well as casual high school and college players, elect to forgo surgery.
Over the past few years, platelet-rich plasma (PRP) has garnered attention as a bridge between conservative treatment and surgery. PRP refers to a sample of autologous blood that contains a platelet concentration higher than baseline levels. This sample often has a 3 to 5 times increase in growth factor concentration.4-6 Initial studies focused on its ability to successfully treat lateral epicondylitis.7-9 More recent clinical work has shown that PRP can potentially enhance healing after anterior cruciate ligament reconstruction,10-14 rotator cuff repair,15-17 and subacromial decompression.11,18-23 If PRP could be used to successfully treat UCL insufficiency that is refractory to conservative treatment, then year-long recovery periods could be avoided. This could potentially prolong certain athletes’ careers or, at the very least, allow them to return to play much sooner. In the present case series, we hypothesized that PRP injections could be used to successfully treat partial UCL tears in high-level throwing athletes, obviating the need for surgery and its associated prolonged recovery period.
Materials and Methods
Institutional Review Board approval was obtained for this retrospective study of 44 baseball players treated with PRP injections for partial-thickness UCL tears.
Patients provided written informed consent. They were diagnosed with UCL insufficiency by physical examination, and findings were confirmed by magnetic resonance imaging (MRI). After diagnosis, all throwers underwent a trial of conservative treatment that included rest, activity modification, use of anti-inflammatory medication, and physical therapy followed by an attempt to return to throwing using an interval throwing program.
Study inclusion criteria were physical examinations and MRI results consistent with UCL insufficiency, and failure of the conservative treatment plan described.
Patients were injected using the Autologous Conditioned Plasma system (Arthrex). PRP solutions were prepared according to manufacturer guidelines. After the elbow was prepared sterilely, the UCL was injected at the location of the tear. Typically, 3 mL of PRP was injected into the elbow. Sixteen patients had 1 injection, 6 had 2, and 22 had 3. Repeat injections were considered for recalcitrant pain after 3 weeks.
After injection, patients used acetaminophen and ice for pain control. Anti-inflammatory medications were avoided for a minimum of 2 weeks after injection. Typical postinjection therapy protocol consisted of rest followed by progressive stretching and strengthening for about 4 to 6 weeks before the start of an interval throwing program. Although there is no well-defined postinjection recovery protocol, as a general rule rest was prescribed for the first 2 weeks, followed by a progressive stretching and strengthening program for the next month. Patients who were asymptomatic subjectively and clinically—negative moving valgus stress test, negative milking maneuver, no pain with valgus stress—were started on an interval throwing program.
Final follow-up involved a physical examination. Results were classified according to a modified version of the Conway Scale12,24-26: excellent (return to preinjury level of competition or performance), good (return to play at a lower level of competition or performance or, specifically for baseball players, ability to throw in daily batting practice), fair (able to play recreationally), and poor (unable to return to previous sport at any level).
By final follow-up, all patients had completed their postoperative rehabilitation protocol, and all had at least tried to return to their previous activities. No patients were lost to follow-up.
Results
Of the 44 baseball players, 6 were professional, 14 were in college, and 24 were in high school. There were 36 pitchers and 8 position players. Mean age was 17.3 years (range, 16-28 years). All patients were available for follow-up after injection (mean, 11 months). Fifteen of the 44 players had an excellent outcome (34%), 17 had a good outcome, 2 had a fair outcome, and 10 had a poor outcome. After injection, 4 (67%) of the 6 professional baseball players returned to professional play. Five (36%) of the 14 college players had an excellent outcome, and 4 (17%) of the 24 high school players had an excellent outcome. Of the 8 position players, 4 had an excellent outcome, 3 had a good outcome, and 1 had a poor outcome.
Before treatment, all patients had medial-sided elbow pain over the UCL inhibiting their ability to throw. Mean duration of symptoms before injection was 8.8 months (range, 1-36 months). There was no correlation between symptom duration and any outcome measure. On MRI, 29 patients showed partial tears: 22 proximally based and 7 distally based. The other 15 patients had diffuse signal without partial tear. All 7 patients with distally based partial tears and 3 of the patients with proximally based partial tears had a poor outcome. Overall, there were 6 excellent, 7 good, and 2 fair outcomes in the partial-tear group. In the patients with diffuse signal without partial tear, there were 9 excellent and 10 good outcomes.
Mean time from injection to return to throwing was 5 weeks, and mean time to return to competition was 12 weeks (range, 5-24 weeks). The 1 player who returned at 5 weeks was a professional relief pitcher whose team was in the playoffs. He has now pitched for an additional 2 baseball seasons without elbow difficulty.
There were no injection-related complications.
Discussion
To our knowledge, this is the first report documenting successful PRP treatment of UCL insufficiency. In this study, 73% of players who had failed a course of conservative treatment had good to excellent outcomes with PRP injection.
Data on successful nonoperative treatment of UCL injuries are limited. Rettig and colleagues1 treated 31 throwing athletes’ UCL injuries with a supervised rehabilitation program. Treatment included rest, use of anti-inflammatory medication, progressive strengthening, and an interval throwing program. Only 41% of the athletes returned to their previous level of play, and it took, on average, 24.5 weeks. There was no significant difference in age or in duration or acuity of symptoms between those who returned to play and those whose conservative treatment failed.
Surgical reconstruction of UCL injuries has been very successful, with upward of 90% of athletes returning to previous level of play.3,27The procedure, however, is not without associated complications, including retear of the ligament, stiffness, ulnar nerve injury, and fracture.27-29 In addition, even when successful, the procedure requires that athletes take 9 to 12 months to recover before returning to competition at their previous level.
Savoie and colleagues,30 in their recent study on UCL repairs, highlighted an important fact that is often overlooked when reviewing the literature on UCL tears. Most of the literature on these injuries focuses on college and professional baseball players in whom ligament damage is often extensive, precluding repair. In contrast to prior reports, Savoie and colleagues30 found excellent results in 93% of their young athletes who underwent UCL repair. It is possible that their results can be attributed to the fact that many of their athletes had tears isolated to one area of the ligament, as opposed to generalized ligament incompetence. Our improved results vis-à-vis other reports on conservative management may be attributable to the same phenomenon.
PRP has garnered much attention in the literature and media because of its potential to enhance healing of tendons and ligaments; in some cases, it can obviate the need for surgery. After failure of other nonoperative measures in 15 patients with elbow epicondylitis, Mishra and Pavelko8 treated each patient with a single PRP injection. They prepared the PRP using the GPS III system (Biomet). At final follow-up, 93% improvement was seen. Clearly, their experiment had design flaws: It was nonblinded, and 3 of the 5 patients in the control group treated with bupivacaine injection withdrew from the experiment. Despite its shortcomings, their study became the impetus for several other studies.
A larger, double-blinded, randomized controlled trial comparing PRP and cortisone injections for lateral epicondylitis in 100 patients is under way, and preliminary results have been published.9 A minimum of 6 months after injection, patients who received PRP showed more improvement in visual analog scale (VAS) pain scores and Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire scores. In another large, double-blinded, randomized controlled trial, patients with chronic lateral epicondylitis had significant improvements in VAS pain scores and DASH scores relative to patients injected with corticosteroids with a 2-year follow-up.31 Similarly, Thanasas and colleagues32 found significantly reduced VAS pain scores in patients injected with PRP versus autologous whole blood. Another study demonstrated improved tendon morphology using ultrasound imaging 6 months after PRP injection.33
Contrary to these positive results, Krogh and colleagues34 found that a single injection of PRP or glucocorticoid was not significantly superior to a saline injection for reducing pain and disability over a 3-month period in patients with lateral epicondylitis. Their study, however, had major flaws. Its original design called for a 12-month follow-up, but there was massive dropout in all 3 treatment arms, necessitating reporting of only 3-month data. In addition, 60% of the patients in the glucocorticoid group were not naïve to this treatment, so definitive conclusions about the efficacy of glucocorticoids could not be made.
In the present study, we successfully treated partial ligament tears with PRP injections. Sixty-seven percent of our baseball players returned to play at a mean of 4 months, much earlier than the 9 to 12 months typically required after ligament reconstruction. Many athletes, such as high school baseball players or aging veteran professional baseball players, do not have the luxury of 12 months for recovery. Therefore, this select group of patients clearly has a limited window of opportunity to return to play. In fact, these patients might be ideal candidates for PRP injections for UCL injuries. Return-to-play rates, however, differed significantly among professional players and nonprofessional players. The difference may be attributable to professional players’ conditioning, quality of physical therapy, extrinsic motivation, and other intangible factors. Four (67%) of our 6 professional baseball players returned to professional play after injection, whereas only 36% of college players and 17% of high school players had excellent outcomes.
Limitations
The present study had several weaknesses, several of which are inherent to PRP studies conducted so far. It was not a prospective, randomized controlled trial. It is important to note that PRP treatment in diseased tissue may have some drawbacks, as its success depends on the ability of healing tissue to use concentrated growth factors and cytokines to proliferate.35 Thus, a chronically injured ligament with depleted active cells may have a diminished response to PRP. Another limitation of this study is that we evaluated outcomes based on return to play using the Conway Scale, which is well reported but not validated. Despite the potential weaknesses of this outcome scale, it has become the benchmark for measuring the success of outcomes of UCL reconstruction. Furthermore, we did not measure patients’ satisfaction with the treatment. Players who could not return to their preinjury level of play may have considered the treatment a failure regardless of their ability to continue throwing. Last, MRI was not repeated to document ligament healing. We did not routinely perform a second MRI because we thought it would not affect treatment. Several series have found a high incidence of abnormal signal in baseball players’ UCLs. In this group of patients, the most important outcome is return to previous level of competition.
This study raised several questions. Is one PRP brand better than another? Should more than 1 injection be given? What is the ideal postinjection protocol? Clearly, larger, prospective, randomized controlled studies are needed to truly elucidate the potential role of PRP in the treatment algorithm for UCL injury. Nevertheless, in certain cases in which traditional conservative measures have failed and patients do not have the luxury of rehabilitating for 9 to 12 months after surgery, PRP may be a viable treatment option.
Conclusion
In this study, use of PRP in the treatment of UCL insufficiency produced outcomes much better than earlier reported outcomes of conservative treatment of these injuries. PRP injections may be particularly beneficial in young athletes who have sustained acute damage to an isolated part of the ligament and in athletes unwilling or unable to undergo the extended rehabilitation required after surgical reconstruction of the ligament.
1. Rettig AC, Sherrill C, Snead DS, Mendler JC, Mieling P. Nonoperative treatment of ulnar collateral ligament injuries in throwing athletes. Am J Sports Med. 2001;29(1):15-17.
2. Eygendaal D, Rahussen FT, Diercks RL. Biomechanics of the elbow joint in tennis players and relation to pathology. Br J Sports Med. 2007;41(11):820-823.
3. Bowers AL, Dines JS, Dines DM, Altchek DW. Elbow medial ulnar collateral ligament reconstruction: clinical relevance and the docking technique. J Shoulder Elbow Surg. 2010;19(2):110-117.
5. Kibler WB. Biomechanical analysis of the shoulder during tennis activities. Clin Sports Med. 1995;14(1):79-85.
5. Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62(4):489-496.
6. Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10(4):225-228.
7. Elliott B, Fleisig G, Nicholls R, Escamilia R. Technique effects on upper limb loading in the tennis serve. J Sci Med Sport. 2003;6(1):76-87.
8. Mishra A, Pavelko T. Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am J Sports Med. 2006;34(11):1774-1778.
9. Mishra A, Woodall J Jr, Vieira A. Treatment of tendon and muscle using platelet-rich plasma. Clin Sports Med. 2009;28(1):113-125.
10. Kovacs MS. Applied physiology of tennis performance. Br J Sports Med. 2006;40(5):381-386.
11. Xie X, Wu H, Zhao S, Xie G, Huangfu X, Zhao J. The effect of platelet-rich plasma on patterns of gene expression in a dog model of anterior cruciate ligament reconstruction. J Surg Res. 2013;180(1):80-88.
12. Pluim BM, Staal JB, Windler GE, Jayanthi N. Tennis injuries: occurrence, aetiology, and prevention. Br J Sports Med. 2006;40(5):415-423.
13. Xie X, Zhao S, Wu H, et al. Platelet-rich plasma enhances autograft revascularization and reinnervation in a dog model of anterior cruciate ligament reconstruction. J Surg Res. 2013;183(1):214-222.
14. Lopez-Vidriero E, Goulding KA, Simon DA, Sanchez M, Johnson DH. The use of platelet-rich plasma in arthroscopy and sports medicine: optimizing the healing environment. Arthroscopy. 2010;26(2):269-278.
15. Jo CH, Shin JS, Shin WH, Lee SY, Yoon KS, Shin S. Platelet-rich plasma for arthroscopic repair of medium to large rotator cuff tears: a randomized controlled trial. Am J Sports Med. 2015;43(9):2102-2110.
16. Jo CH, Shin JS, Lee YG, et al. Platelet-rich plasma for arthroscopic repair of large to massive rotator cuff tears: a randomized, single-blinded, parallel-group trial. Am J Sports Med. 2013;41(10):2240-2248.
17. Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet-rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20(4):518-528.
18. Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20(4):518-528.
19. Barber FA, Hrnack SA, Snyder SJ, Hapa O. Rotator cuff repair healing influenced by platelet-rich plasma construct augmentation. Arthroscopy. 2011;27(8):1029-1035.
20. Jo CH, Kim JE, Yoon KS, et al. Does platelet-rich plasma accelerate recovery after rotator cuff repair? A prospective cohort study. Am J Sports Med. 2011;39(10):2082-2090.
21. Jo CH, Kim JE, Yoon KS, Shin S. Platelet-rich plasma stimulates cell proliferation and enhances matrix gene expression and synthesis in tenocytes from human rotator cuff tendons with degenerative tears. Am J Sports Med. 2012;40(5):1035-1045.
22. Chahal J, Van Thiel GS, Mall N, et al. The role of platelet-rich plasma in arthroscopic rotator cuff repair: a systematic review with quantitative synthesis. Arthroscopy. 2012;28(11):1718-1727.
23. Mei-Dan O, Carmont MR. The role of platelet-rich plasma in rotator cuff repair. Sports Med Arthrosc Rev. 2011;19(3):244-250.
24. Dines JS, ElAttrache NS, Conway JE, Smith W, Ahmad CS. Clinical outcomes of the DANE TJ technique to treat ulnar collateral ligament insufficiency of the elbow. Am J Sports Med. 2007;35(12):2039-2044.
25. Hutchinson MR, Laprade RF, Burnett QM 2nd, Moss R, Terpstra J. Injury surveillance at the USTA boys’ tennis championships: a 6-yr study. Med Sci Sports Exerc. 1995;27(6):826-830.
26. Winge S, Jørgensen U, Nielsen A. Epidemiology of injuries in Danish championship tennis. Int J Sports Med. 1989;10(5):368-371.
27. Safran MR, Hutchinson MR, Moss R, Albrandt J. A comparison of injuries in elite boys and girls tennis players. Paper presented at: 9th Annual Meeting of the Society of Tennis Medicine and Science; March 1999; Indian Wells, CA.
28. Cain EL, Andrews JR, Dugas JR, et al. Outcome of ulnar collateral ligament reconstruction of the elbow in 1281 athletes: results in 743 athletes with minimum 2-year follow-up. Am J Sports Med. 2010;38(12):2426-2434.
29. Dines JS, Yocum LA, Frank JB, ElAttrache NS, Gambardella RA, Jobe FW. Revision surgery for failed elbow medial collateral ligament reconstruction. Am J Sports Med. 2008;36(6):1061-1065.
30. Savoie FH, Trenhaile SW, Roberts J, Field LD, Ramsey JR. Primary repair of ulnar collateral ligament injuries of the elbow in young athletes: a case series of injuries to the proximal and distal ends of the ligament. Am J Sports Med. 2008;36(6):1066-1072.
31. Gosens T, Peerbooms JC, van Laar W, Oudsten den BL. Ongoing positive effect of platelet-rich plasma versus corticosteroid injection in lateral epicondylitis: a double-blind randomized controlled trial with 2-year follow-up. Am J Sports Med. 2011;39(6):1200-1208.
32. Thanasas C, Papadimitriou G, Charalambidis C, Paraskevopoulos I, Papanikolaou A. Platelet-rich plasma versus autologous whole blood for the treatment of chronic lateral elbow epicondylitis: a randomized controlled clinical trial. Am J Sports Med. 2011;39(10):2130-2134.
33. Chaudhury S, La Lama de M, Adler RS, et al. Platelet-rich plasma for the treatment of lateral epicondylitis: sonographic assessment of tendon morphology and vascularity (pilot study). Skeletal Radiol. 2013;42(1):91-97.
34. Krogh TP, Fredberg U, Stengaard-Pedersen K, Christensen R, Jensen P, Ellingsen T. Treatment of lateral epicondylitis with platelet-rich plasma, glucocorticoid, or saline: a randomized, double-blind, placebo-controlled trial. Am J Sports Med. 2013;41(3):625-635.
35. Anz AW, Hackel JG, Nilssen EC, Andrews JR. Application of biologics in the treatment of the rotator cuff, meniscus, cartilage, and osteoarthritis. J Am Acad Orthop Surg. 2014;22(2):68-79.
1. Rettig AC, Sherrill C, Snead DS, Mendler JC, Mieling P. Nonoperative treatment of ulnar collateral ligament injuries in throwing athletes. Am J Sports Med. 2001;29(1):15-17.
2. Eygendaal D, Rahussen FT, Diercks RL. Biomechanics of the elbow joint in tennis players and relation to pathology. Br J Sports Med. 2007;41(11):820-823.
3. Bowers AL, Dines JS, Dines DM, Altchek DW. Elbow medial ulnar collateral ligament reconstruction: clinical relevance and the docking technique. J Shoulder Elbow Surg. 2010;19(2):110-117.
5. Kibler WB. Biomechanical analysis of the shoulder during tennis activities. Clin Sports Med. 1995;14(1):79-85.
5. Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62(4):489-496.
6. Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10(4):225-228.
7. Elliott B, Fleisig G, Nicholls R, Escamilia R. Technique effects on upper limb loading in the tennis serve. J Sci Med Sport. 2003;6(1):76-87.
8. Mishra A, Pavelko T. Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am J Sports Med. 2006;34(11):1774-1778.
9. Mishra A, Woodall J Jr, Vieira A. Treatment of tendon and muscle using platelet-rich plasma. Clin Sports Med. 2009;28(1):113-125.
10. Kovacs MS. Applied physiology of tennis performance. Br J Sports Med. 2006;40(5):381-386.
11. Xie X, Wu H, Zhao S, Xie G, Huangfu X, Zhao J. The effect of platelet-rich plasma on patterns of gene expression in a dog model of anterior cruciate ligament reconstruction. J Surg Res. 2013;180(1):80-88.
12. Pluim BM, Staal JB, Windler GE, Jayanthi N. Tennis injuries: occurrence, aetiology, and prevention. Br J Sports Med. 2006;40(5):415-423.
13. Xie X, Zhao S, Wu H, et al. Platelet-rich plasma enhances autograft revascularization and reinnervation in a dog model of anterior cruciate ligament reconstruction. J Surg Res. 2013;183(1):214-222.
14. Lopez-Vidriero E, Goulding KA, Simon DA, Sanchez M, Johnson DH. The use of platelet-rich plasma in arthroscopy and sports medicine: optimizing the healing environment. Arthroscopy. 2010;26(2):269-278.
15. Jo CH, Shin JS, Shin WH, Lee SY, Yoon KS, Shin S. Platelet-rich plasma for arthroscopic repair of medium to large rotator cuff tears: a randomized controlled trial. Am J Sports Med. 2015;43(9):2102-2110.
16. Jo CH, Shin JS, Lee YG, et al. Platelet-rich plasma for arthroscopic repair of large to massive rotator cuff tears: a randomized, single-blinded, parallel-group trial. Am J Sports Med. 2013;41(10):2240-2248.
17. Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet-rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20(4):518-528.
18. Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20(4):518-528.
19. Barber FA, Hrnack SA, Snyder SJ, Hapa O. Rotator cuff repair healing influenced by platelet-rich plasma construct augmentation. Arthroscopy. 2011;27(8):1029-1035.
20. Jo CH, Kim JE, Yoon KS, et al. Does platelet-rich plasma accelerate recovery after rotator cuff repair? A prospective cohort study. Am J Sports Med. 2011;39(10):2082-2090.
21. Jo CH, Kim JE, Yoon KS, Shin S. Platelet-rich plasma stimulates cell proliferation and enhances matrix gene expression and synthesis in tenocytes from human rotator cuff tendons with degenerative tears. Am J Sports Med. 2012;40(5):1035-1045.
22. Chahal J, Van Thiel GS, Mall N, et al. The role of platelet-rich plasma in arthroscopic rotator cuff repair: a systematic review with quantitative synthesis. Arthroscopy. 2012;28(11):1718-1727.
23. Mei-Dan O, Carmont MR. The role of platelet-rich plasma in rotator cuff repair. Sports Med Arthrosc Rev. 2011;19(3):244-250.
24. Dines JS, ElAttrache NS, Conway JE, Smith W, Ahmad CS. Clinical outcomes of the DANE TJ technique to treat ulnar collateral ligament insufficiency of the elbow. Am J Sports Med. 2007;35(12):2039-2044.
25. Hutchinson MR, Laprade RF, Burnett QM 2nd, Moss R, Terpstra J. Injury surveillance at the USTA boys’ tennis championships: a 6-yr study. Med Sci Sports Exerc. 1995;27(6):826-830.
26. Winge S, Jørgensen U, Nielsen A. Epidemiology of injuries in Danish championship tennis. Int J Sports Med. 1989;10(5):368-371.
27. Safran MR, Hutchinson MR, Moss R, Albrandt J. A comparison of injuries in elite boys and girls tennis players. Paper presented at: 9th Annual Meeting of the Society of Tennis Medicine and Science; March 1999; Indian Wells, CA.
28. Cain EL, Andrews JR, Dugas JR, et al. Outcome of ulnar collateral ligament reconstruction of the elbow in 1281 athletes: results in 743 athletes with minimum 2-year follow-up. Am J Sports Med. 2010;38(12):2426-2434.
29. Dines JS, Yocum LA, Frank JB, ElAttrache NS, Gambardella RA, Jobe FW. Revision surgery for failed elbow medial collateral ligament reconstruction. Am J Sports Med. 2008;36(6):1061-1065.
30. Savoie FH, Trenhaile SW, Roberts J, Field LD, Ramsey JR. Primary repair of ulnar collateral ligament injuries of the elbow in young athletes: a case series of injuries to the proximal and distal ends of the ligament. Am J Sports Med. 2008;36(6):1066-1072.
31. Gosens T, Peerbooms JC, van Laar W, Oudsten den BL. Ongoing positive effect of platelet-rich plasma versus corticosteroid injection in lateral epicondylitis: a double-blind randomized controlled trial with 2-year follow-up. Am J Sports Med. 2011;39(6):1200-1208.
32. Thanasas C, Papadimitriou G, Charalambidis C, Paraskevopoulos I, Papanikolaou A. Platelet-rich plasma versus autologous whole blood for the treatment of chronic lateral elbow epicondylitis: a randomized controlled clinical trial. Am J Sports Med. 2011;39(10):2130-2134.
33. Chaudhury S, La Lama de M, Adler RS, et al. Platelet-rich plasma for the treatment of lateral epicondylitis: sonographic assessment of tendon morphology and vascularity (pilot study). Skeletal Radiol. 2013;42(1):91-97.
34. Krogh TP, Fredberg U, Stengaard-Pedersen K, Christensen R, Jensen P, Ellingsen T. Treatment of lateral epicondylitis with platelet-rich plasma, glucocorticoid, or saline: a randomized, double-blind, placebo-controlled trial. Am J Sports Med. 2013;41(3):625-635.
35. Anz AW, Hackel JG, Nilssen EC, Andrews JR. Application of biologics in the treatment of the rotator cuff, meniscus, cartilage, and osteoarthritis. J Am Acad Orthop Surg. 2014;22(2):68-79.
Acellular Dermal Matrix in Rotator Cuff Surgery
Rotator cuff repairs (RCRs) can be challenging due to poor tendon quality and the inability of tendon to heal to bone. Smoking, age over 63 years, fatty infiltration, and massive cuff tears are all factors implicated in increased failure rates.1-3 Tears >3 cm have a structural failure rate ranging from 11% to 95% in the literature.1-5 Massive tears (tears >5 cm or involving 2 or more tendons) are even more complex and have failure rates of 20% to 90%.5,6 The weakest link in the RCR construct is the suture-tendon interface, and suture pullout through the tendon is thought to be the most common method of failure.6 The purpose of this review is to examine whether literature supports the use of acellular dermal matrices (ADMs) in rotator cuff surgery.
The high rate of structural failures after RCR has led surgeons to seek means to augment repairs and new means of reconstruction for irreparable tears. Freeze dried allograft tendons have been used historically with mixed results, including reports of complete graft failures and foreign body reaction.7-10 Porcine intestinal submucosal membrane “patches” gained popularity due to off-the- shelf availability of the graft. However, these were found to have poor outcomes with early graft rejection and intense inflammatory reaction.11,12 Recently, ADMs have gained significant interest due to favorable biomechanical properties and clinical outcomes.13-19
An ADM is an allograft composed of mostly type I collagen that is processed to remove donor cells while preserving the extracellular matrix. There are several commercially available ADMs with different methods of processing and sterilization, as well as handling characteristics.20,21 In vivo studies have demonstrated that removing the cellular components allows infiltration of native cellular agents, such as fibroblasts, vascular tissue, and tenocytes, while causing minimal host inflammatory reaction.21-23 In addition, superior suture pullout strength has been demonstrated by multiple benchtop and preclinical studies.23,24 Therefore, ADMs play a dual role of strengthening the repair while allowing infiltration of host cells and growth factors to potentially promote healing at the repair site.
Emerging Evidence
Multiple biomechanical studies have evaluated ADMs in RC models.24-28 Barber and colleagues24 demonstrated that ADM had significantly higher loads to failure (229 N) than porcine skin (128 N), bovine skin (76 N), and porcine small intestine submucosa (32 N) (P < .001). In another study, Barber and colleagues25 subsequently demonstrated, in a cadaver RC tear model, an increase in mean failure strength in augmented repairs with ADM (325 N) compared to cadaveric controls (273 N) (P = .047).
A subsequent study by Barber and Aziz-Jacobo26 compared ADMs to a control model of allograft RC. The ADMs had significantly higher tensile modulus (P < .001) and higher suture retention measure by a single-pull destructive test of a simple vertical stitch (P < .05) than the RC allograft. The ultimate load to failure of the ADM model was higher than the RC allograft control (523±154 N vs 208±115 N); however, this difference did not reach statistical significance.26 Beitzel and colleagues27 evaluated ADM augmentation in a cadaver RC model and found a statistically significant increase in load to failure in ADM augmented repairs vs nonaugmented controls, (575.8 N vs 348.9 N, P = .025). Ely and colleagues28 also demonstrated that repairs augmented with ADM had a higher load to failure (643 N vs 551 N) and less gap formation (2.2 mm vs 2.8 mm) compared to controls, although this difference was not statistically significant.
These biomechanical studies have been translated to clinical findings. A level II, prospective, randomized controlled study by Barber and colleagues29 evaluated 42 patients with >3 cm, 2-tendon RCTs repaired arthroscopically.Twenty-two patients were randomized to single-row arthroscopic repair, and 20 patients to single-row arthroscopic repair augmented by ADM by an onlay technique (Figure 1) as described by Labbé.30 At average follow-up of 24 months, 85% of the augmented repairs were intact on magnetic resonance imaging (MRI) at follow-up, compared to 40% in the control group (P < .05). Agrawal31 retrospectively reviewed 14 patients with either RCTs >3 cm or recurrent RCT (may be <3 cm) that were arthroscopically repaired with a double-row technique with ADM augmentation. Postoperative MRI obtained at average of 16.8 months revealed 85.7% of repairs to be intact, with 14.3% having recurrent tears of <1 cm. Rotini and colleagues32 evaluated a smaller subset of 5 patients with large/massive primary cuff tears, arthroscopically repaired with double-row technique and ADM augmentation. Follow-up MRI at an average of 1 year demonstrated 3 intact repairs, 1 partial recurrence, and 1 complete recurrence. These clinical studies demonstrate that RCRs augmented with ADM have a much higher rate of structural integrity on postoperative imaging compared to what has been previously reported in the literature.1-6
Although an “off-label” indication, the use of ADM in massive RC tears has been described with good clinical results.14,17,19,33 The ADM is used to bridge the gap by suturing it to the edge of the retracted tendon and anchoring it to the tuberosity (Figures 2A-2E). Improvement in pain, function, and active range of motion can be achieved. Burkhead and colleagues14 obtained postoperative MRIs at average follow-up of 1.2 years and found only 3 of 11 repairs with evidence of re-tear, all noted to be smaller than preoperative tears. Gupta and colleagues17 obtained postoperative ultrasounds in 24 patients at average 3 years and showed 76% of tears to be fully intact, with the remaining 24% having only a partial tear, and 0% with full re-tears. Venouziou and colleagues19 evaluated 14 patients with minimum 18-month follow-up and Kokkalis and colleagues33 evaluated 21 patients with a 29-month follow-up; both described successful clinical outcomes but did not provide postoperative imaging evaluation. Multiple studies have adapted this technique to a fully arthroscopic method and have had similarly positive results clinically and with MRI.13,16,18,34,35 Bond and colleagues13 reported 16 cases with massive irreparable tears repaired arthroscopically with ADM to span the tendon gap. At an average follow-up of 26.8 months, 75% had good or excellent clinical results, and at an average of 1 year postoperatively 13 of 16 cases had an intact repair on gadolinium enhanced MRI.13 These studies suggest that ADM can be used for bridging massive irreparable RC tears with good clinical and radiographic outcomes.
Superior capsule reconstruction is a biomechanically proven concept that has been described in previous studies.36,37 In the original technique, autologous tensor fascia lata (TFL) is anchored from the glenoid margin to the greater tuberosity footprint to restore the superior stability of the glenohumeral joint, without altering the native glenohumeral contact forces.38 This concept has gained popularity in the United States, but with the use of an ADM instead of harvesting TFL (Figures 3A, 3B). However, there are no published biomechanical or clinical studies with the use of ADM in superior capsular reconstruction.
Conclusion
The use of ADM is an emerging solution for augmenting primary RCRs and the treatment of irreparable RC tears. The biomechanical and clinical studies summarized support the use of ADM in RC surgery. Further randomized studies are needed to add to the growing evidence on the use of ADMs.
1. Green A. Chronic massive rotator cuff tears: evaluation and management. J Am Acad Orthop Surg. 2003;11(5):321-331.
2. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
3. Iannotti JP, Deutsch A, Green A, et al. Time to failure after rotator cuff repair: a prospective imaging study. J Bone Joint Surg Am. 2013;95(11):965-971.
4. Karas EH, Iannotti JP. Failed repair of the rotator cuff: evaluation and treatment of complications. Instr Course Lect. 1998;47:87-95.
5. Burkhart SS. Biomechanics of rotator cuff repair: converting the ritual to a science. Instr Course Lect. 1998;47:43-50.
6. Derwin KA, Badylak SF, Steinmann SP, Iannotti JP. Extracellular matrix scaffold devices for rotator cuff repair. J Shoulder Elbow Surg. 2010;19:467-476.
7. Neviaser JS, Neviaser RJ, Neviaser TJ. The repair of chronic massive ruptures of the rotator cuff of the shoulder by use of a freeze-dried rotator cuff. J Bone Joint Surg Am. 1978;60(5):681-684.
8. Ito J, Morioka T. Surgical treatment for large and massive tears of the rotator cuff. Int Orthop. 2003;27(4):228-231.
9. Nasca RJ. The use of freeze-dried allografts in the management of global rotator cuff tears. Clin Orthop Related Res. 1988;228:218-226.
10. Moore DR, Cain EL, Schwartz ML, Clancy WG Jr. Allograft reconstruction for massive, irreparable rotator cuff tears. Am J Sports Med. 2006;34(3):392-396.
11. Walton JR, Bowman NK, Khatib Y, Linklater J, Murrell GA. Restore orthobiologic implant: not recommended for augmentation of rotator cuff repairs. J Bone Joint Surg Am. 2007;89(4):786-791.
12. Iannotti JP, Codsi MJ, Kwon YW, Derwin K, Ciccone J, Brems JJ. Porcine small intestine submucosa augmentation of surgical repair of chronic two-tendon rotator cuff tears. A randomized, controlled trial. J Bone Joint Surg Am. 2006;88(6):1238-1244.
13. Bond JL, Dopirak RM, Higgins J, Burns J, Snyder SJ. Arthroscopic replacement of massive, irreparable rotator cuff tears using a GraftJacket allograft: technique and preliminary results. Arthroscopy. 2008;24(4):403-409.
14. Burkhead WZ Jr, Schiffern SC, Krishnan SG. Use of Graft Jacket as an augmentation for massive rotator cuff tears. Semin Arthoplasty. 2007;18(1):11-18.
15. Dehler T, Pennings AL, ElMaraghy AW. Dermal allograft reconstruction of a chronic pectoralis major tear. J Shoulder Elbow Surg. 2013;22(10):e18-e22.
16. Dopirak R, Bond JL, Snyder SJ. Arthroscopic total rotator cuff replacement with an acellular dermal allograft matrix. Int J Shoulder Surg. 2007;1(1):7-15.
17. Gupta AK, Hug K, Berkoff DJ, et al. Dermal tissue allograft for the repair of massive irreparable rotator cuff tears. Am J Sports Med. 2012;40(1):141-147.
18. Modi A, Singh HP, Pandey R, Armstrong A. Management of irreparable rotator cuff tears with the GraftJacket allograft as an interpositional graft. Shoulder Elbow. 2013;5(3):188-194.
19. Venouziou AI, Kokkalis ZT, Sotereanos DG. Human dermal allograft interposition for the reconstruction of massive irreparable rotator cuff tears. Am J Orthop. 2013;42(2):63-70.
20. Acevedo DC, Shore B, Mirzayan R. Orthopedic applications of acellular human dermal allograft for shoulder and elbow surgery. Orthop Clin North Am. 2015;46(3):377-388.
21. Beniker D, McQuillan D, Livesey S, et al. The use of acellular dermal matrix as a scaffold for periosteum replacement. Orthopedics. 2003;26(5 Suppl):s591-s596.
22. Smith RD, Carr A, Dakin SG, Snelling SJ, Yapp C, Hakimi O. The response of tenocytes to commercial scaffolds used for rotator cuff repair. Eur Cell Mater. 2016;31:107-118.
23. Adams JE, Zobitz ME, Reach JS Jr, An KN, Steinmann SP. Rotator cuff repair using an acellular dermal matrix graft: an in vivo study in a canine model. Arthroscopy. 2006;22(7):700-709.
24. Barber FA, Herbert MA, Coons DA. Tendon augmentation grafts: biomechanical failure loads and failure patterns. Arthroscopy. 2006;22(5):534-538.
25. Barber FA, Herbert MA, Boothby MH. Ultimate tensile failure loads of a human dermal allograft rotator cuff augmentation. Arthroscopy. 2008;24(1):20-24.
26. Barber AF, Aziz-Jacobo J. Biomechanical testing of commercially available soft-tissue augmentation materials. Arthroscopy. 2009;25(11):1233-1239.
27. Beitzel K, Chowaniec DM, McCarthy MB, et al. Stability of double-row rotator cuff repair is not adversely affected by scaffold interposition between tendon and bone. Am J Sports Med. 2012;40(5):1148-1154.
28. Ely EE, Figueroa NM, Gilot GJ. Biomechanical analysis of rotator cuff repairs with extraccellular matrix graft augmentation. Orthopedics. 2014;37(9):608-614.
29. Barber AF, Burns JP, Deutsch A, Labbé MR, Litchfield RB. A prospective, randomized evaluation of acellular human dermal matrix augmentation for arthroscopic rotator cuff repair. Arthroscopy. 2012;28(1):8-15.
30. Labbé MR. Arthroscopic technique for patch augmentation of rotator cuff repairs. Arthroscopy. 2006;22(1):1136.e1-e6.
31. Agrawal V. Healing rates for challenging rotator cuff tears utilizing an acellular human dermal reinforcement graft. Int J Shoulder Surg. 2012;6(2):36-44.
32. Rotini R, Marinelli A, Guerra E, et al. Human dermal matrix scaffold augmentation for large and massive rotator cuff repairs: preliminary clinical and MRI results at 1-year follow-up. Musculoskelet Surg. 2011;95 Suppl 1:S13-S23.
33. Kokkalis ZT, Mavrogenis AF, Scarlat M, et al. Human dermal allograft for massive rotator cuff tears. Orthopedics. 2014;37(12):e1108-e1116.
34. Wong I, Burns J, Snyder S. Arthroscopic GraftJacket repair of rotator cuff tears. J Shoulder Elbow Surg. 2010;19(2 Suppl):104-109.
35. Snyder SJ, Bond JL. Technique for arthroscopic replacement of severely damaged rotator cuff using “GraftJacket” allograft. Oper Tech Sports Med. 2007;15(2):86-94.
36. Mihata T, McGarry MH, Pirolo JM, Kinoshita M, Lee TQ. Superior capsule reconstruction to restore superior stability in irreparable rotator cuff tears: a biomechanical cadaveric study. Am J Sports Med. 2012;40(10):2248-2255.
37. Mihata T, McGarry MH, Kahn T, Goldberg I, Neo M, Lee TQ. Biomechanical role of capsular continuity in superior capsule reconstruction for irreparable tears of the supraspinatus tendon. Am J Sports Med. 2016;44(6):1423-1430.
38. Mihata T, Lee TQ, Watanabe C, et al. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy. 2013;29(3):459-470.
Rotator cuff repairs (RCRs) can be challenging due to poor tendon quality and the inability of tendon to heal to bone. Smoking, age over 63 years, fatty infiltration, and massive cuff tears are all factors implicated in increased failure rates.1-3 Tears >3 cm have a structural failure rate ranging from 11% to 95% in the literature.1-5 Massive tears (tears >5 cm or involving 2 or more tendons) are even more complex and have failure rates of 20% to 90%.5,6 The weakest link in the RCR construct is the suture-tendon interface, and suture pullout through the tendon is thought to be the most common method of failure.6 The purpose of this review is to examine whether literature supports the use of acellular dermal matrices (ADMs) in rotator cuff surgery.
The high rate of structural failures after RCR has led surgeons to seek means to augment repairs and new means of reconstruction for irreparable tears. Freeze dried allograft tendons have been used historically with mixed results, including reports of complete graft failures and foreign body reaction.7-10 Porcine intestinal submucosal membrane “patches” gained popularity due to off-the- shelf availability of the graft. However, these were found to have poor outcomes with early graft rejection and intense inflammatory reaction.11,12 Recently, ADMs have gained significant interest due to favorable biomechanical properties and clinical outcomes.13-19
An ADM is an allograft composed of mostly type I collagen that is processed to remove donor cells while preserving the extracellular matrix. There are several commercially available ADMs with different methods of processing and sterilization, as well as handling characteristics.20,21 In vivo studies have demonstrated that removing the cellular components allows infiltration of native cellular agents, such as fibroblasts, vascular tissue, and tenocytes, while causing minimal host inflammatory reaction.21-23 In addition, superior suture pullout strength has been demonstrated by multiple benchtop and preclinical studies.23,24 Therefore, ADMs play a dual role of strengthening the repair while allowing infiltration of host cells and growth factors to potentially promote healing at the repair site.
Emerging Evidence
Multiple biomechanical studies have evaluated ADMs in RC models.24-28 Barber and colleagues24 demonstrated that ADM had significantly higher loads to failure (229 N) than porcine skin (128 N), bovine skin (76 N), and porcine small intestine submucosa (32 N) (P < .001). In another study, Barber and colleagues25 subsequently demonstrated, in a cadaver RC tear model, an increase in mean failure strength in augmented repairs with ADM (325 N) compared to cadaveric controls (273 N) (P = .047).
A subsequent study by Barber and Aziz-Jacobo26 compared ADMs to a control model of allograft RC. The ADMs had significantly higher tensile modulus (P < .001) and higher suture retention measure by a single-pull destructive test of a simple vertical stitch (P < .05) than the RC allograft. The ultimate load to failure of the ADM model was higher than the RC allograft control (523±154 N vs 208±115 N); however, this difference did not reach statistical significance.26 Beitzel and colleagues27 evaluated ADM augmentation in a cadaver RC model and found a statistically significant increase in load to failure in ADM augmented repairs vs nonaugmented controls, (575.8 N vs 348.9 N, P = .025). Ely and colleagues28 also demonstrated that repairs augmented with ADM had a higher load to failure (643 N vs 551 N) and less gap formation (2.2 mm vs 2.8 mm) compared to controls, although this difference was not statistically significant.
These biomechanical studies have been translated to clinical findings. A level II, prospective, randomized controlled study by Barber and colleagues29 evaluated 42 patients with >3 cm, 2-tendon RCTs repaired arthroscopically.Twenty-two patients were randomized to single-row arthroscopic repair, and 20 patients to single-row arthroscopic repair augmented by ADM by an onlay technique (Figure 1) as described by Labbé.30 At average follow-up of 24 months, 85% of the augmented repairs were intact on magnetic resonance imaging (MRI) at follow-up, compared to 40% in the control group (P < .05). Agrawal31 retrospectively reviewed 14 patients with either RCTs >3 cm or recurrent RCT (may be <3 cm) that were arthroscopically repaired with a double-row technique with ADM augmentation. Postoperative MRI obtained at average of 16.8 months revealed 85.7% of repairs to be intact, with 14.3% having recurrent tears of <1 cm. Rotini and colleagues32 evaluated a smaller subset of 5 patients with large/massive primary cuff tears, arthroscopically repaired with double-row technique and ADM augmentation. Follow-up MRI at an average of 1 year demonstrated 3 intact repairs, 1 partial recurrence, and 1 complete recurrence. These clinical studies demonstrate that RCRs augmented with ADM have a much higher rate of structural integrity on postoperative imaging compared to what has been previously reported in the literature.1-6
Although an “off-label” indication, the use of ADM in massive RC tears has been described with good clinical results.14,17,19,33 The ADM is used to bridge the gap by suturing it to the edge of the retracted tendon and anchoring it to the tuberosity (Figures 2A-2E). Improvement in pain, function, and active range of motion can be achieved. Burkhead and colleagues14 obtained postoperative MRIs at average follow-up of 1.2 years and found only 3 of 11 repairs with evidence of re-tear, all noted to be smaller than preoperative tears. Gupta and colleagues17 obtained postoperative ultrasounds in 24 patients at average 3 years and showed 76% of tears to be fully intact, with the remaining 24% having only a partial tear, and 0% with full re-tears. Venouziou and colleagues19 evaluated 14 patients with minimum 18-month follow-up and Kokkalis and colleagues33 evaluated 21 patients with a 29-month follow-up; both described successful clinical outcomes but did not provide postoperative imaging evaluation. Multiple studies have adapted this technique to a fully arthroscopic method and have had similarly positive results clinically and with MRI.13,16,18,34,35 Bond and colleagues13 reported 16 cases with massive irreparable tears repaired arthroscopically with ADM to span the tendon gap. At an average follow-up of 26.8 months, 75% had good or excellent clinical results, and at an average of 1 year postoperatively 13 of 16 cases had an intact repair on gadolinium enhanced MRI.13 These studies suggest that ADM can be used for bridging massive irreparable RC tears with good clinical and radiographic outcomes.
Superior capsule reconstruction is a biomechanically proven concept that has been described in previous studies.36,37 In the original technique, autologous tensor fascia lata (TFL) is anchored from the glenoid margin to the greater tuberosity footprint to restore the superior stability of the glenohumeral joint, without altering the native glenohumeral contact forces.38 This concept has gained popularity in the United States, but with the use of an ADM instead of harvesting TFL (Figures 3A, 3B). However, there are no published biomechanical or clinical studies with the use of ADM in superior capsular reconstruction.
Conclusion
The use of ADM is an emerging solution for augmenting primary RCRs and the treatment of irreparable RC tears. The biomechanical and clinical studies summarized support the use of ADM in RC surgery. Further randomized studies are needed to add to the growing evidence on the use of ADMs.
Rotator cuff repairs (RCRs) can be challenging due to poor tendon quality and the inability of tendon to heal to bone. Smoking, age over 63 years, fatty infiltration, and massive cuff tears are all factors implicated in increased failure rates.1-3 Tears >3 cm have a structural failure rate ranging from 11% to 95% in the literature.1-5 Massive tears (tears >5 cm or involving 2 or more tendons) are even more complex and have failure rates of 20% to 90%.5,6 The weakest link in the RCR construct is the suture-tendon interface, and suture pullout through the tendon is thought to be the most common method of failure.6 The purpose of this review is to examine whether literature supports the use of acellular dermal matrices (ADMs) in rotator cuff surgery.
The high rate of structural failures after RCR has led surgeons to seek means to augment repairs and new means of reconstruction for irreparable tears. Freeze dried allograft tendons have been used historically with mixed results, including reports of complete graft failures and foreign body reaction.7-10 Porcine intestinal submucosal membrane “patches” gained popularity due to off-the- shelf availability of the graft. However, these were found to have poor outcomes with early graft rejection and intense inflammatory reaction.11,12 Recently, ADMs have gained significant interest due to favorable biomechanical properties and clinical outcomes.13-19
An ADM is an allograft composed of mostly type I collagen that is processed to remove donor cells while preserving the extracellular matrix. There are several commercially available ADMs with different methods of processing and sterilization, as well as handling characteristics.20,21 In vivo studies have demonstrated that removing the cellular components allows infiltration of native cellular agents, such as fibroblasts, vascular tissue, and tenocytes, while causing minimal host inflammatory reaction.21-23 In addition, superior suture pullout strength has been demonstrated by multiple benchtop and preclinical studies.23,24 Therefore, ADMs play a dual role of strengthening the repair while allowing infiltration of host cells and growth factors to potentially promote healing at the repair site.
Emerging Evidence
Multiple biomechanical studies have evaluated ADMs in RC models.24-28 Barber and colleagues24 demonstrated that ADM had significantly higher loads to failure (229 N) than porcine skin (128 N), bovine skin (76 N), and porcine small intestine submucosa (32 N) (P < .001). In another study, Barber and colleagues25 subsequently demonstrated, in a cadaver RC tear model, an increase in mean failure strength in augmented repairs with ADM (325 N) compared to cadaveric controls (273 N) (P = .047).
A subsequent study by Barber and Aziz-Jacobo26 compared ADMs to a control model of allograft RC. The ADMs had significantly higher tensile modulus (P < .001) and higher suture retention measure by a single-pull destructive test of a simple vertical stitch (P < .05) than the RC allograft. The ultimate load to failure of the ADM model was higher than the RC allograft control (523±154 N vs 208±115 N); however, this difference did not reach statistical significance.26 Beitzel and colleagues27 evaluated ADM augmentation in a cadaver RC model and found a statistically significant increase in load to failure in ADM augmented repairs vs nonaugmented controls, (575.8 N vs 348.9 N, P = .025). Ely and colleagues28 also demonstrated that repairs augmented with ADM had a higher load to failure (643 N vs 551 N) and less gap formation (2.2 mm vs 2.8 mm) compared to controls, although this difference was not statistically significant.
These biomechanical studies have been translated to clinical findings. A level II, prospective, randomized controlled study by Barber and colleagues29 evaluated 42 patients with >3 cm, 2-tendon RCTs repaired arthroscopically.Twenty-two patients were randomized to single-row arthroscopic repair, and 20 patients to single-row arthroscopic repair augmented by ADM by an onlay technique (Figure 1) as described by Labbé.30 At average follow-up of 24 months, 85% of the augmented repairs were intact on magnetic resonance imaging (MRI) at follow-up, compared to 40% in the control group (P < .05). Agrawal31 retrospectively reviewed 14 patients with either RCTs >3 cm or recurrent RCT (may be <3 cm) that were arthroscopically repaired with a double-row technique with ADM augmentation. Postoperative MRI obtained at average of 16.8 months revealed 85.7% of repairs to be intact, with 14.3% having recurrent tears of <1 cm. Rotini and colleagues32 evaluated a smaller subset of 5 patients with large/massive primary cuff tears, arthroscopically repaired with double-row technique and ADM augmentation. Follow-up MRI at an average of 1 year demonstrated 3 intact repairs, 1 partial recurrence, and 1 complete recurrence. These clinical studies demonstrate that RCRs augmented with ADM have a much higher rate of structural integrity on postoperative imaging compared to what has been previously reported in the literature.1-6
Although an “off-label” indication, the use of ADM in massive RC tears has been described with good clinical results.14,17,19,33 The ADM is used to bridge the gap by suturing it to the edge of the retracted tendon and anchoring it to the tuberosity (Figures 2A-2E). Improvement in pain, function, and active range of motion can be achieved. Burkhead and colleagues14 obtained postoperative MRIs at average follow-up of 1.2 years and found only 3 of 11 repairs with evidence of re-tear, all noted to be smaller than preoperative tears. Gupta and colleagues17 obtained postoperative ultrasounds in 24 patients at average 3 years and showed 76% of tears to be fully intact, with the remaining 24% having only a partial tear, and 0% with full re-tears. Venouziou and colleagues19 evaluated 14 patients with minimum 18-month follow-up and Kokkalis and colleagues33 evaluated 21 patients with a 29-month follow-up; both described successful clinical outcomes but did not provide postoperative imaging evaluation. Multiple studies have adapted this technique to a fully arthroscopic method and have had similarly positive results clinically and with MRI.13,16,18,34,35 Bond and colleagues13 reported 16 cases with massive irreparable tears repaired arthroscopically with ADM to span the tendon gap. At an average follow-up of 26.8 months, 75% had good or excellent clinical results, and at an average of 1 year postoperatively 13 of 16 cases had an intact repair on gadolinium enhanced MRI.13 These studies suggest that ADM can be used for bridging massive irreparable RC tears with good clinical and radiographic outcomes.
Superior capsule reconstruction is a biomechanically proven concept that has been described in previous studies.36,37 In the original technique, autologous tensor fascia lata (TFL) is anchored from the glenoid margin to the greater tuberosity footprint to restore the superior stability of the glenohumeral joint, without altering the native glenohumeral contact forces.38 This concept has gained popularity in the United States, but with the use of an ADM instead of harvesting TFL (Figures 3A, 3B). However, there are no published biomechanical or clinical studies with the use of ADM in superior capsular reconstruction.
Conclusion
The use of ADM is an emerging solution for augmenting primary RCRs and the treatment of irreparable RC tears. The biomechanical and clinical studies summarized support the use of ADM in RC surgery. Further randomized studies are needed to add to the growing evidence on the use of ADMs.
1. Green A. Chronic massive rotator cuff tears: evaluation and management. J Am Acad Orthop Surg. 2003;11(5):321-331.
2. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
3. Iannotti JP, Deutsch A, Green A, et al. Time to failure after rotator cuff repair: a prospective imaging study. J Bone Joint Surg Am. 2013;95(11):965-971.
4. Karas EH, Iannotti JP. Failed repair of the rotator cuff: evaluation and treatment of complications. Instr Course Lect. 1998;47:87-95.
5. Burkhart SS. Biomechanics of rotator cuff repair: converting the ritual to a science. Instr Course Lect. 1998;47:43-50.
6. Derwin KA, Badylak SF, Steinmann SP, Iannotti JP. Extracellular matrix scaffold devices for rotator cuff repair. J Shoulder Elbow Surg. 2010;19:467-476.
7. Neviaser JS, Neviaser RJ, Neviaser TJ. The repair of chronic massive ruptures of the rotator cuff of the shoulder by use of a freeze-dried rotator cuff. J Bone Joint Surg Am. 1978;60(5):681-684.
8. Ito J, Morioka T. Surgical treatment for large and massive tears of the rotator cuff. Int Orthop. 2003;27(4):228-231.
9. Nasca RJ. The use of freeze-dried allografts in the management of global rotator cuff tears. Clin Orthop Related Res. 1988;228:218-226.
10. Moore DR, Cain EL, Schwartz ML, Clancy WG Jr. Allograft reconstruction for massive, irreparable rotator cuff tears. Am J Sports Med. 2006;34(3):392-396.
11. Walton JR, Bowman NK, Khatib Y, Linklater J, Murrell GA. Restore orthobiologic implant: not recommended for augmentation of rotator cuff repairs. J Bone Joint Surg Am. 2007;89(4):786-791.
12. Iannotti JP, Codsi MJ, Kwon YW, Derwin K, Ciccone J, Brems JJ. Porcine small intestine submucosa augmentation of surgical repair of chronic two-tendon rotator cuff tears. A randomized, controlled trial. J Bone Joint Surg Am. 2006;88(6):1238-1244.
13. Bond JL, Dopirak RM, Higgins J, Burns J, Snyder SJ. Arthroscopic replacement of massive, irreparable rotator cuff tears using a GraftJacket allograft: technique and preliminary results. Arthroscopy. 2008;24(4):403-409.
14. Burkhead WZ Jr, Schiffern SC, Krishnan SG. Use of Graft Jacket as an augmentation for massive rotator cuff tears. Semin Arthoplasty. 2007;18(1):11-18.
15. Dehler T, Pennings AL, ElMaraghy AW. Dermal allograft reconstruction of a chronic pectoralis major tear. J Shoulder Elbow Surg. 2013;22(10):e18-e22.
16. Dopirak R, Bond JL, Snyder SJ. Arthroscopic total rotator cuff replacement with an acellular dermal allograft matrix. Int J Shoulder Surg. 2007;1(1):7-15.
17. Gupta AK, Hug K, Berkoff DJ, et al. Dermal tissue allograft for the repair of massive irreparable rotator cuff tears. Am J Sports Med. 2012;40(1):141-147.
18. Modi A, Singh HP, Pandey R, Armstrong A. Management of irreparable rotator cuff tears with the GraftJacket allograft as an interpositional graft. Shoulder Elbow. 2013;5(3):188-194.
19. Venouziou AI, Kokkalis ZT, Sotereanos DG. Human dermal allograft interposition for the reconstruction of massive irreparable rotator cuff tears. Am J Orthop. 2013;42(2):63-70.
20. Acevedo DC, Shore B, Mirzayan R. Orthopedic applications of acellular human dermal allograft for shoulder and elbow surgery. Orthop Clin North Am. 2015;46(3):377-388.
21. Beniker D, McQuillan D, Livesey S, et al. The use of acellular dermal matrix as a scaffold for periosteum replacement. Orthopedics. 2003;26(5 Suppl):s591-s596.
22. Smith RD, Carr A, Dakin SG, Snelling SJ, Yapp C, Hakimi O. The response of tenocytes to commercial scaffolds used for rotator cuff repair. Eur Cell Mater. 2016;31:107-118.
23. Adams JE, Zobitz ME, Reach JS Jr, An KN, Steinmann SP. Rotator cuff repair using an acellular dermal matrix graft: an in vivo study in a canine model. Arthroscopy. 2006;22(7):700-709.
24. Barber FA, Herbert MA, Coons DA. Tendon augmentation grafts: biomechanical failure loads and failure patterns. Arthroscopy. 2006;22(5):534-538.
25. Barber FA, Herbert MA, Boothby MH. Ultimate tensile failure loads of a human dermal allograft rotator cuff augmentation. Arthroscopy. 2008;24(1):20-24.
26. Barber AF, Aziz-Jacobo J. Biomechanical testing of commercially available soft-tissue augmentation materials. Arthroscopy. 2009;25(11):1233-1239.
27. Beitzel K, Chowaniec DM, McCarthy MB, et al. Stability of double-row rotator cuff repair is not adversely affected by scaffold interposition between tendon and bone. Am J Sports Med. 2012;40(5):1148-1154.
28. Ely EE, Figueroa NM, Gilot GJ. Biomechanical analysis of rotator cuff repairs with extraccellular matrix graft augmentation. Orthopedics. 2014;37(9):608-614.
29. Barber AF, Burns JP, Deutsch A, Labbé MR, Litchfield RB. A prospective, randomized evaluation of acellular human dermal matrix augmentation for arthroscopic rotator cuff repair. Arthroscopy. 2012;28(1):8-15.
30. Labbé MR. Arthroscopic technique for patch augmentation of rotator cuff repairs. Arthroscopy. 2006;22(1):1136.e1-e6.
31. Agrawal V. Healing rates for challenging rotator cuff tears utilizing an acellular human dermal reinforcement graft. Int J Shoulder Surg. 2012;6(2):36-44.
32. Rotini R, Marinelli A, Guerra E, et al. Human dermal matrix scaffold augmentation for large and massive rotator cuff repairs: preliminary clinical and MRI results at 1-year follow-up. Musculoskelet Surg. 2011;95 Suppl 1:S13-S23.
33. Kokkalis ZT, Mavrogenis AF, Scarlat M, et al. Human dermal allograft for massive rotator cuff tears. Orthopedics. 2014;37(12):e1108-e1116.
34. Wong I, Burns J, Snyder S. Arthroscopic GraftJacket repair of rotator cuff tears. J Shoulder Elbow Surg. 2010;19(2 Suppl):104-109.
35. Snyder SJ, Bond JL. Technique for arthroscopic replacement of severely damaged rotator cuff using “GraftJacket” allograft. Oper Tech Sports Med. 2007;15(2):86-94.
36. Mihata T, McGarry MH, Pirolo JM, Kinoshita M, Lee TQ. Superior capsule reconstruction to restore superior stability in irreparable rotator cuff tears: a biomechanical cadaveric study. Am J Sports Med. 2012;40(10):2248-2255.
37. Mihata T, McGarry MH, Kahn T, Goldberg I, Neo M, Lee TQ. Biomechanical role of capsular continuity in superior capsule reconstruction for irreparable tears of the supraspinatus tendon. Am J Sports Med. 2016;44(6):1423-1430.
38. Mihata T, Lee TQ, Watanabe C, et al. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy. 2013;29(3):459-470.
1. Green A. Chronic massive rotator cuff tears: evaluation and management. J Am Acad Orthop Surg. 2003;11(5):321-331.
2. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
3. Iannotti JP, Deutsch A, Green A, et al. Time to failure after rotator cuff repair: a prospective imaging study. J Bone Joint Surg Am. 2013;95(11):965-971.
4. Karas EH, Iannotti JP. Failed repair of the rotator cuff: evaluation and treatment of complications. Instr Course Lect. 1998;47:87-95.
5. Burkhart SS. Biomechanics of rotator cuff repair: converting the ritual to a science. Instr Course Lect. 1998;47:43-50.
6. Derwin KA, Badylak SF, Steinmann SP, Iannotti JP. Extracellular matrix scaffold devices for rotator cuff repair. J Shoulder Elbow Surg. 2010;19:467-476.
7. Neviaser JS, Neviaser RJ, Neviaser TJ. The repair of chronic massive ruptures of the rotator cuff of the shoulder by use of a freeze-dried rotator cuff. J Bone Joint Surg Am. 1978;60(5):681-684.
8. Ito J, Morioka T. Surgical treatment for large and massive tears of the rotator cuff. Int Orthop. 2003;27(4):228-231.
9. Nasca RJ. The use of freeze-dried allografts in the management of global rotator cuff tears. Clin Orthop Related Res. 1988;228:218-226.
10. Moore DR, Cain EL, Schwartz ML, Clancy WG Jr. Allograft reconstruction for massive, irreparable rotator cuff tears. Am J Sports Med. 2006;34(3):392-396.
11. Walton JR, Bowman NK, Khatib Y, Linklater J, Murrell GA. Restore orthobiologic implant: not recommended for augmentation of rotator cuff repairs. J Bone Joint Surg Am. 2007;89(4):786-791.
12. Iannotti JP, Codsi MJ, Kwon YW, Derwin K, Ciccone J, Brems JJ. Porcine small intestine submucosa augmentation of surgical repair of chronic two-tendon rotator cuff tears. A randomized, controlled trial. J Bone Joint Surg Am. 2006;88(6):1238-1244.
13. Bond JL, Dopirak RM, Higgins J, Burns J, Snyder SJ. Arthroscopic replacement of massive, irreparable rotator cuff tears using a GraftJacket allograft: technique and preliminary results. Arthroscopy. 2008;24(4):403-409.
14. Burkhead WZ Jr, Schiffern SC, Krishnan SG. Use of Graft Jacket as an augmentation for massive rotator cuff tears. Semin Arthoplasty. 2007;18(1):11-18.
15. Dehler T, Pennings AL, ElMaraghy AW. Dermal allograft reconstruction of a chronic pectoralis major tear. J Shoulder Elbow Surg. 2013;22(10):e18-e22.
16. Dopirak R, Bond JL, Snyder SJ. Arthroscopic total rotator cuff replacement with an acellular dermal allograft matrix. Int J Shoulder Surg. 2007;1(1):7-15.
17. Gupta AK, Hug K, Berkoff DJ, et al. Dermal tissue allograft for the repair of massive irreparable rotator cuff tears. Am J Sports Med. 2012;40(1):141-147.
18. Modi A, Singh HP, Pandey R, Armstrong A. Management of irreparable rotator cuff tears with the GraftJacket allograft as an interpositional graft. Shoulder Elbow. 2013;5(3):188-194.
19. Venouziou AI, Kokkalis ZT, Sotereanos DG. Human dermal allograft interposition for the reconstruction of massive irreparable rotator cuff tears. Am J Orthop. 2013;42(2):63-70.
20. Acevedo DC, Shore B, Mirzayan R. Orthopedic applications of acellular human dermal allograft for shoulder and elbow surgery. Orthop Clin North Am. 2015;46(3):377-388.
21. Beniker D, McQuillan D, Livesey S, et al. The use of acellular dermal matrix as a scaffold for periosteum replacement. Orthopedics. 2003;26(5 Suppl):s591-s596.
22. Smith RD, Carr A, Dakin SG, Snelling SJ, Yapp C, Hakimi O. The response of tenocytes to commercial scaffolds used for rotator cuff repair. Eur Cell Mater. 2016;31:107-118.
23. Adams JE, Zobitz ME, Reach JS Jr, An KN, Steinmann SP. Rotator cuff repair using an acellular dermal matrix graft: an in vivo study in a canine model. Arthroscopy. 2006;22(7):700-709.
24. Barber FA, Herbert MA, Coons DA. Tendon augmentation grafts: biomechanical failure loads and failure patterns. Arthroscopy. 2006;22(5):534-538.
25. Barber FA, Herbert MA, Boothby MH. Ultimate tensile failure loads of a human dermal allograft rotator cuff augmentation. Arthroscopy. 2008;24(1):20-24.
26. Barber AF, Aziz-Jacobo J. Biomechanical testing of commercially available soft-tissue augmentation materials. Arthroscopy. 2009;25(11):1233-1239.
27. Beitzel K, Chowaniec DM, McCarthy MB, et al. Stability of double-row rotator cuff repair is not adversely affected by scaffold interposition between tendon and bone. Am J Sports Med. 2012;40(5):1148-1154.
28. Ely EE, Figueroa NM, Gilot GJ. Biomechanical analysis of rotator cuff repairs with extraccellular matrix graft augmentation. Orthopedics. 2014;37(9):608-614.
29. Barber AF, Burns JP, Deutsch A, Labbé MR, Litchfield RB. A prospective, randomized evaluation of acellular human dermal matrix augmentation for arthroscopic rotator cuff repair. Arthroscopy. 2012;28(1):8-15.
30. Labbé MR. Arthroscopic technique for patch augmentation of rotator cuff repairs. Arthroscopy. 2006;22(1):1136.e1-e6.
31. Agrawal V. Healing rates for challenging rotator cuff tears utilizing an acellular human dermal reinforcement graft. Int J Shoulder Surg. 2012;6(2):36-44.
32. Rotini R, Marinelli A, Guerra E, et al. Human dermal matrix scaffold augmentation for large and massive rotator cuff repairs: preliminary clinical and MRI results at 1-year follow-up. Musculoskelet Surg. 2011;95 Suppl 1:S13-S23.
33. Kokkalis ZT, Mavrogenis AF, Scarlat M, et al. Human dermal allograft for massive rotator cuff tears. Orthopedics. 2014;37(12):e1108-e1116.
34. Wong I, Burns J, Snyder S. Arthroscopic GraftJacket repair of rotator cuff tears. J Shoulder Elbow Surg. 2010;19(2 Suppl):104-109.
35. Snyder SJ, Bond JL. Technique for arthroscopic replacement of severely damaged rotator cuff using “GraftJacket” allograft. Oper Tech Sports Med. 2007;15(2):86-94.
36. Mihata T, McGarry MH, Pirolo JM, Kinoshita M, Lee TQ. Superior capsule reconstruction to restore superior stability in irreparable rotator cuff tears: a biomechanical cadaveric study. Am J Sports Med. 2012;40(10):2248-2255.
37. Mihata T, McGarry MH, Kahn T, Goldberg I, Neo M, Lee TQ. Biomechanical role of capsular continuity in superior capsule reconstruction for irreparable tears of the supraspinatus tendon. Am J Sports Med. 2016;44(6):1423-1430.
38. Mihata T, Lee TQ, Watanabe C, et al. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy. 2013;29(3):459-470.