User login
Report: EHR Implementation Associated with Quality
Hospitals that have made it to the advanced stages of electronic health record (EHR) implementation are significantly more likely to set national benchmarks for quality and safety performance, according to the 2012 HIMSS Analytics Report.
The research (PDF), sponsored by Thomson Reuters and HIMSS Analytics, found a correlation between hospitals that are both ranked in the Thomson Reuters 100 Top Hospitals and at the upper end of the seven-stage HIMMS scale for EHR adoption.
While the link between electronic implementation and quality is important, William Bria, MD, chief medical information officer at Shriners Hospitals for Children in Philadelphia, cautions hospitalists and others from taking too much comfort in it. Simply implementing EHR and other technologies doesn't work, he says; the system has to be crafted in conjunction with its users.
"The best-led organizations in the country are using the metrics of safety and quality of care right alongside the implementation plan of their [health IT] programs," says Dr. Bria. "And the only way this occurs, of course, is if the partnering between executive and technological leadership and clinical leadership occurs."
Dr. Bria views research on the success of EHRs in improving hospital performance as an opportunity for hospitalists to get more involved in both the planning and implementation processes. He urges hospitalists to work with other physicians and IT staffers to learn how best to use their EHR, and not assume they can master complex software systems as easily as they understand smartphones and tablet computers.
"You can buy a piano and bang on it with your fist, and you won't really attract anybody to listen to your music," Dr. Bria says. "On the other hand, if you learn how to play, you study hard, and you learn the nuances of musicianship, you can become a Van Cliburn."
Hospitals that have made it to the advanced stages of electronic health record (EHR) implementation are significantly more likely to set national benchmarks for quality and safety performance, according to the 2012 HIMSS Analytics Report.
The research (PDF), sponsored by Thomson Reuters and HIMSS Analytics, found a correlation between hospitals that are both ranked in the Thomson Reuters 100 Top Hospitals and at the upper end of the seven-stage HIMMS scale for EHR adoption.
While the link between electronic implementation and quality is important, William Bria, MD, chief medical information officer at Shriners Hospitals for Children in Philadelphia, cautions hospitalists and others from taking too much comfort in it. Simply implementing EHR and other technologies doesn't work, he says; the system has to be crafted in conjunction with its users.
"The best-led organizations in the country are using the metrics of safety and quality of care right alongside the implementation plan of their [health IT] programs," says Dr. Bria. "And the only way this occurs, of course, is if the partnering between executive and technological leadership and clinical leadership occurs."
Dr. Bria views research on the success of EHRs in improving hospital performance as an opportunity for hospitalists to get more involved in both the planning and implementation processes. He urges hospitalists to work with other physicians and IT staffers to learn how best to use their EHR, and not assume they can master complex software systems as easily as they understand smartphones and tablet computers.
"You can buy a piano and bang on it with your fist, and you won't really attract anybody to listen to your music," Dr. Bria says. "On the other hand, if you learn how to play, you study hard, and you learn the nuances of musicianship, you can become a Van Cliburn."
Hospitals that have made it to the advanced stages of electronic health record (EHR) implementation are significantly more likely to set national benchmarks for quality and safety performance, according to the 2012 HIMSS Analytics Report.
The research (PDF), sponsored by Thomson Reuters and HIMSS Analytics, found a correlation between hospitals that are both ranked in the Thomson Reuters 100 Top Hospitals and at the upper end of the seven-stage HIMMS scale for EHR adoption.
While the link between electronic implementation and quality is important, William Bria, MD, chief medical information officer at Shriners Hospitals for Children in Philadelphia, cautions hospitalists and others from taking too much comfort in it. Simply implementing EHR and other technologies doesn't work, he says; the system has to be crafted in conjunction with its users.
"The best-led organizations in the country are using the metrics of safety and quality of care right alongside the implementation plan of their [health IT] programs," says Dr. Bria. "And the only way this occurs, of course, is if the partnering between executive and technological leadership and clinical leadership occurs."
Dr. Bria views research on the success of EHRs in improving hospital performance as an opportunity for hospitalists to get more involved in both the planning and implementation processes. He urges hospitalists to work with other physicians and IT staffers to learn how best to use their EHR, and not assume they can master complex software systems as easily as they understand smartphones and tablet computers.
"You can buy a piano and bang on it with your fist, and you won't really attract anybody to listen to your music," Dr. Bria says. "On the other hand, if you learn how to play, you study hard, and you learn the nuances of musicianship, you can become a Van Cliburn."
Training, Leadership, Commitment Integral to HM Improving Stroke Care
Stroke specialists like to say that “time is brain.” With an emphatic focus on those first few critical hours, however, it’s sometimes easy to overlook the vital role that hospitalists play in the days, weeks, and months that follow.
A recent study in The Neurohospitalist suggests that compared to community-based neurologists, practitioners of neurohospital medicine can reduce the length of stay for patients with ischemic stroke.1 A separate study, however, suggests that similar success might have come at a price for their less-specialized hospitalist counterparts.2 Among stroke patients, the latter study found that while the HM model is also associated with a reduced length of stay, it is associated with increased discharges to inpatient rehabilitation centers instead of to home, and higher readmission rates.
In sum, the evidence raises questions about whether rank-and-file hospitalists are adequately equipped to deal with a disease that is a core competency for the profession and ranks among the top sources of adult disability in the United States, at an estimated cost of $34.3 billion in 2008.3
“I think there’s been a mismatch between the training of the average hospitalist and then the expectations for the amount of neurological care they end up delivering once in practice,” says David Likosky, MD, SFHM, director of the stroke program at Evergreen Hospital Medical Center in Kirkland, Wash. “When surveyed, it’s been shown that hospitalists feel that care of stroke is one of the areas with which they’re least comfortable once they get out into practice.” Over the past decade, several studies have reinforced the notion of a training deficit.4,5
Demographic trends suggest that getting up to speed will be imperative, however. “One alarming thing we’re seeing is strokes among individuals that are not in the elderly group, and that group seems to be increasing at an alarming rate,” says Daniel T. Lackland, PhD, professor of epidemiology and neurosciences at the Medical University of South Carolina in Charleston. Hospitals are seeing more ischemic stroke patients in their 40s and 50s, likely a reflection of risk factors such as hypertension, diabetes, and hyperlipidemia that are occurring earlier in life. And because those patients are younger, the aftermath of a stroke could linger for decades.
Although the stroke mortality rate is declining in the U.S., statistics find that about 14% of all patients diagnosed with an initial stroke will have a second one within a year, placing continued strain on a healthcare system already stretched thin.6 Hospitalists, Dr. Lackland says, have an “ideal” opportunity to help build up and improve that system, potentially yielding significant cost savings along with the dramatic improvement in quality of life. Making the most of that opportunity, though, will require a solid understanding of multiple trends that are quickly transforming stroke care delivery.
Time Is of the Essence
Kevin Barrett, MD, MSc, assistant professor of neurology and stroke telemedicine director at the Mayo Clinic in Jacksonville, Fla., says hospitals are focusing more and more on a metric known as “door-to-needle time.” The goal is to treat at least half of incoming ischemic stroke patients with intravenous tissue-type plasminogen activator (IV tPA) within the first 60 minutes after onset of symptoms.
The American Heart Association/American Stroke Association has reinforced the message with its Get With the Guidelines Stroke Program. A recent analysis suggested the program has led to more timely tPA administration and, in turn, better patient outcomes (the program is funded in part through the Bristol-Myers Squib/Sanofi Pharmaceutical Partnership).7
At the same time, clinical research has widened the window for IV tPA delivery from three hours to 4.5 hours for certain patients after the onset of symptoms. Dr. Barrett says “strong evidence” from the European Cooperative Acute Stroke Study III has convinced most clinicians, and the FDA is expected to follow suit in officially approving the extension.8 As more stroke centers become certified, the use of IV tPA has increased accordingly.
Patients who have missed the time window or are not good candidates for IV tPA can still be aided by interarterial tPA at the site of the clot up to six hours after the onset of symptoms. Dr. Likosky says the treatment option should be of particular interest to hospitalists, given that strokes can occur post-operatively and in other patients who cannot receive IV tPA because of bleeding risk.
—Karim Godamunne, MD, MBA, SFHM, medical director, Eagle Hospital Physicians, Roswell, Ga.
For up to eight hours after the onset of symptoms, mechanical clot removal techniques have shown continued efficacy at revascularizing affected areas, with some newer options also offering greater promise of improving patient outcomes. Even with the prospects of declining complication rates, however, “evaluating and initiating treatment in a timely fashion is still going to be one of the most important predictors of outcome,” Dr. Barrett says.
After the initial intervention, hospitalists often are the go-to providers for anticipating and preventing common post-stroke complications, such as aspiration pneumonia, VTE from immobilization, and other infections. The proper use of anti-platelet agents and high-dose statins, also falling solidly within the HM realm, can pay big dividends if used consistently.
Meanwhile, newer studies and clinical observations are widening the scope of considerations that should be on every hospitalist’s radar. Here are a few cited by stroke experts:
Permissive hypertension. After an ischemic stroke, the benefit of permissive hypertension is still widely misunderstood. Perhaps counterintuitively, high blood pressure after a stroke can help protect the area of the brain that is damaged but not yet dead, sometimes called the penumbra. “I highlight this because I think it’s a common mistake, that internists are very used to high blood pressure being a bad thing,” says Andrew Josephson, MD, associate professor of clinical neurology and director of the neurohospitalist program at the University of California at San Francisco (UCSF) Medical Center. “And in general, it is; it’s a cause of stroke. But once somebody has a stroke, in the acute period, it’s important to allow the blood pressure to be high.”
Atrial fibrillation. The accepted role of atrial fibrillation in stroke is evolving. Research suggests that the common but often preventable arrhythmia is an important cause of stroke in about 15% to 20% of cases.9 By the time of hospital discharge, however, Dr. Josephson says physicians haven’t established a cause in about 1 in 4 cases. For these “cryptogenic strokes,” he says, doctors have long suspected that atrial fibrillation not picked up during the initial EKG or by the monitoring with cardiac telemetry could be a major cause.
Recent observations suggest that a longer monitoring period of up to 30 days may uncover atrial fibrillation in a sizable fraction of those patients, highlighting the importance of keeping a close eye on stroke patients both in the hospital and beyond. “It’s very important to identify, because atrial fibrillation changes what we do for folks to prevent a second stroke,” Dr. Josephson explains. Instead of anti-platelet medicine like aspirin, patients with atrial fibrillation often receive anticoagulants like warfarin, or the more recently approved dabigatran and rivaroxaban.
Transient ischemic attack. Improvements in imaging techniques like MRI have likewise begun to shift how stroke patients are treated. For example, Dr. Likosky says, medicine is moving away from a time-based definition of transient ischemic attack (TIA), in which symptoms resolve within 24 hours, to a tissue-based definition. Recent MRI imaging has uncovered evidence of a new infarction in more than half of patients initially diagnosed with TIA.10
“If they do have an infarction on their scan, even if they had symptoms that only lasted for five minutes, that’s a stroke,” Dr. Josephson says. And even a true TIA, he says, represents “a kind of stroke where you got really lucky and you’re not left with deficits, but the risk is still very high.” Accordingly, more patients with TIA are being admitted to the hospital to receive a full workup and preventive treatment. “We think that by evaluating these people urgently, we can reduce the risk of having a stroke by maybe 75% over a three-month time period,” Dr. Josephson says.
Hemorrhagic stroke. To date, the vast majority of patients with hemorrhagic stroke (which accounts for only 13% of all stroke cases) have been managed by neurosurgeons and neurologists. But here, too, Dr. Likosky says the picture could be changing. Recent findings that surgical treatment of intracranial hemorrhaging might not benefit many patients could shift the care paradigm toward a medical management strategy that involves more hospitalists.
Innovations Aplenty
The increasing complexity of stroke care and uneven distribution of resources and expertise have helped fuel several important innovations in delivery, most notably telestroke and neurohospital medicine. Both are being driven, in part, by an increased awareness of time-sensitive interventions and a frequent lack of on-site neurologists at smaller and more rural facilities. If telestroke programs are expanding the reach of neurologists, neurohospitalists are helping to fill the gaps in inpatient stroke care.
Amid the changes, one element is proving a necessary constant: a team approach that relies heavily on the HM emphasis on quality metrics, intensive monitoring, and careful coordination. Who better to lead the charge than hospitalists, says Mary E. Jensen, MD, professor of radiology and neurosurgery at the University of Virginia in Charlottesville. “They’re the ones who are in the hospital, and when these patients go bad, they go bad fast,” she says.
More broadly, Dr. Jensen says, hospitalists should get in on the ground floor when their facility seeks certification as a primary or a comprehensive stroke center. “And they need to make sure that the hospital isn’t just trying to get the sexy elements—the guy with the cath or the gal with the cath who can pull the clot out—but that they have a complete program that involves the care of the patient after they’ve had the procedure done,” she says.
As healthcare reform efforts are making clear, the responsibility doesn’t end after discharge, either. The Affordable Care Act includes a hospital readmission reduction program that will kick in this October, with penalties for hospitals posting unacceptably high 30-day readmission rates. Amy Kind, MD, PhD, assistant professor of medicine in the Division of Geriatrics at the University of Wisconsin School of Medicine and Public Health in Madison, is convinced that a key contributor to high rehospitalization rates among stroke patients may be the woefully incomplete nature of discharge communication.

—Mary E. Jensen, MD, professor of radiology and neurosurgery, University of Virginia, Charlottesville
Dr. Kind, for example, has found a disturbing pattern in communication regarding issues like dysphagia, a common complication among stroke patients and an important risk factor for pneumonia. Countering the risk usually requires such measures as putting patients on a special diet or elevating the head of their bed. “We looked at the quality of the communication of that information in discharge summaries, and it’s just abysmal. It’s absolutely abysmal,” she says. Without clear directives to providers in the next setting of care, such as a skilled-nursing facility, patients could be erroneously put back on a regular diet and aspirate, sending them right back to the hospital.
As one potential solution, Dr. Kind’s team is developing a multidisciplinary stroke discharge summary tool that automatically imports elements like speech-language pathology and dietary recommendations. Although most discharge communication may focus on more visible issues and interventions, Dr. Kind argues that some of the “bread and butter” concerns might ultimately prove just as important for long-term patient outcomes.
Karim Godamunne, MD, MBA, SFHM, vice president of clinical systems integration and medical director of Eagle Hospital Physicians in Atlanta, sees telemedicine as another potential tool to help reach patients after discharge, especially those who haven’t received follow-up care from a primary-care physician (PCP). “We need to be the champions at our hospitals for improving care processes, and we need to work in partnership with the nurses and the other professionals,” Dr. Godamunne says. “As a group, we can really make a difference, and stroke is one of those areas in which we can truly contribute.”
Bryn Nelson is a freelance medical writer in Seattle.
References
- Freeman WD, Dawson SB, Raper C, Thiemann K, et al. Neurohospitalists reduce length of stay for patients with ischemic stroke. The Neurohospitalist. 2011;1(2): 67-70.
- Howrey BT, Kuo Y-F, Goodwin JS. Association of care by hospitalists on discharge destination and 30-day outcomes after acute ischemic stroke. Medical Care. 2011;49(8): 701-707.
- Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125:e2-e220.
- Glasheen JJ, Epstein KR, Siegal E, Kutner JS, Prochazka AV. The spectrum of community-based hospitalist practice: a call to tailor internal medicine residency training. Arch Intern Med. 2007;167(7):727-728.
- Plauth WH, Pantilat SZ, Wachter RM, Fenton CL. Hospitalists’ perceptions of their residency training needs: results of a national survey. Am J Med. 2001; 111(3):247-254.
- Dickerson LM, Carek PJ, Quattlebaum RG. Prevention of recurrent ischemic stroke. Am Fam Physician. 2007; 76(3):382-388.
- Fonarow GC, Smith EE, Saver JL, et al. Timeliness of tissue-type plasminogen activator therapy in acute ischemic stroke: patient characteristics, hospital factors, and outcomes associated with door-to-needle times within 60 minutes. Circulation. 2011;123(7):750-758.
- Hacke W, Kaste M, Bluhmki E, Brozman M, et al. Thrombolysis with Alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317-1329.
- Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121:e91.
- Albers GW, Caplan LR, Easton JD, et al. Transient ischemic attack—proposal for a new definition. N Engl J Med. 2002;347(21):1713-1716.
- Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365:993-1003.
Stroke specialists like to say that “time is brain.” With an emphatic focus on those first few critical hours, however, it’s sometimes easy to overlook the vital role that hospitalists play in the days, weeks, and months that follow.
A recent study in The Neurohospitalist suggests that compared to community-based neurologists, practitioners of neurohospital medicine can reduce the length of stay for patients with ischemic stroke.1 A separate study, however, suggests that similar success might have come at a price for their less-specialized hospitalist counterparts.2 Among stroke patients, the latter study found that while the HM model is also associated with a reduced length of stay, it is associated with increased discharges to inpatient rehabilitation centers instead of to home, and higher readmission rates.
In sum, the evidence raises questions about whether rank-and-file hospitalists are adequately equipped to deal with a disease that is a core competency for the profession and ranks among the top sources of adult disability in the United States, at an estimated cost of $34.3 billion in 2008.3
“I think there’s been a mismatch between the training of the average hospitalist and then the expectations for the amount of neurological care they end up delivering once in practice,” says David Likosky, MD, SFHM, director of the stroke program at Evergreen Hospital Medical Center in Kirkland, Wash. “When surveyed, it’s been shown that hospitalists feel that care of stroke is one of the areas with which they’re least comfortable once they get out into practice.” Over the past decade, several studies have reinforced the notion of a training deficit.4,5
Demographic trends suggest that getting up to speed will be imperative, however. “One alarming thing we’re seeing is strokes among individuals that are not in the elderly group, and that group seems to be increasing at an alarming rate,” says Daniel T. Lackland, PhD, professor of epidemiology and neurosciences at the Medical University of South Carolina in Charleston. Hospitals are seeing more ischemic stroke patients in their 40s and 50s, likely a reflection of risk factors such as hypertension, diabetes, and hyperlipidemia that are occurring earlier in life. And because those patients are younger, the aftermath of a stroke could linger for decades.
Although the stroke mortality rate is declining in the U.S., statistics find that about 14% of all patients diagnosed with an initial stroke will have a second one within a year, placing continued strain on a healthcare system already stretched thin.6 Hospitalists, Dr. Lackland says, have an “ideal” opportunity to help build up and improve that system, potentially yielding significant cost savings along with the dramatic improvement in quality of life. Making the most of that opportunity, though, will require a solid understanding of multiple trends that are quickly transforming stroke care delivery.
Time Is of the Essence
Kevin Barrett, MD, MSc, assistant professor of neurology and stroke telemedicine director at the Mayo Clinic in Jacksonville, Fla., says hospitals are focusing more and more on a metric known as “door-to-needle time.” The goal is to treat at least half of incoming ischemic stroke patients with intravenous tissue-type plasminogen activator (IV tPA) within the first 60 minutes after onset of symptoms.
The American Heart Association/American Stroke Association has reinforced the message with its Get With the Guidelines Stroke Program. A recent analysis suggested the program has led to more timely tPA administration and, in turn, better patient outcomes (the program is funded in part through the Bristol-Myers Squib/Sanofi Pharmaceutical Partnership).7
At the same time, clinical research has widened the window for IV tPA delivery from three hours to 4.5 hours for certain patients after the onset of symptoms. Dr. Barrett says “strong evidence” from the European Cooperative Acute Stroke Study III has convinced most clinicians, and the FDA is expected to follow suit in officially approving the extension.8 As more stroke centers become certified, the use of IV tPA has increased accordingly.
Patients who have missed the time window or are not good candidates for IV tPA can still be aided by interarterial tPA at the site of the clot up to six hours after the onset of symptoms. Dr. Likosky says the treatment option should be of particular interest to hospitalists, given that strokes can occur post-operatively and in other patients who cannot receive IV tPA because of bleeding risk.
—Karim Godamunne, MD, MBA, SFHM, medical director, Eagle Hospital Physicians, Roswell, Ga.
For up to eight hours after the onset of symptoms, mechanical clot removal techniques have shown continued efficacy at revascularizing affected areas, with some newer options also offering greater promise of improving patient outcomes. Even with the prospects of declining complication rates, however, “evaluating and initiating treatment in a timely fashion is still going to be one of the most important predictors of outcome,” Dr. Barrett says.
After the initial intervention, hospitalists often are the go-to providers for anticipating and preventing common post-stroke complications, such as aspiration pneumonia, VTE from immobilization, and other infections. The proper use of anti-platelet agents and high-dose statins, also falling solidly within the HM realm, can pay big dividends if used consistently.
Meanwhile, newer studies and clinical observations are widening the scope of considerations that should be on every hospitalist’s radar. Here are a few cited by stroke experts:
Permissive hypertension. After an ischemic stroke, the benefit of permissive hypertension is still widely misunderstood. Perhaps counterintuitively, high blood pressure after a stroke can help protect the area of the brain that is damaged but not yet dead, sometimes called the penumbra. “I highlight this because I think it’s a common mistake, that internists are very used to high blood pressure being a bad thing,” says Andrew Josephson, MD, associate professor of clinical neurology and director of the neurohospitalist program at the University of California at San Francisco (UCSF) Medical Center. “And in general, it is; it’s a cause of stroke. But once somebody has a stroke, in the acute period, it’s important to allow the blood pressure to be high.”
Atrial fibrillation. The accepted role of atrial fibrillation in stroke is evolving. Research suggests that the common but often preventable arrhythmia is an important cause of stroke in about 15% to 20% of cases.9 By the time of hospital discharge, however, Dr. Josephson says physicians haven’t established a cause in about 1 in 4 cases. For these “cryptogenic strokes,” he says, doctors have long suspected that atrial fibrillation not picked up during the initial EKG or by the monitoring with cardiac telemetry could be a major cause.
Recent observations suggest that a longer monitoring period of up to 30 days may uncover atrial fibrillation in a sizable fraction of those patients, highlighting the importance of keeping a close eye on stroke patients both in the hospital and beyond. “It’s very important to identify, because atrial fibrillation changes what we do for folks to prevent a second stroke,” Dr. Josephson explains. Instead of anti-platelet medicine like aspirin, patients with atrial fibrillation often receive anticoagulants like warfarin, or the more recently approved dabigatran and rivaroxaban.
Transient ischemic attack. Improvements in imaging techniques like MRI have likewise begun to shift how stroke patients are treated. For example, Dr. Likosky says, medicine is moving away from a time-based definition of transient ischemic attack (TIA), in which symptoms resolve within 24 hours, to a tissue-based definition. Recent MRI imaging has uncovered evidence of a new infarction in more than half of patients initially diagnosed with TIA.10
“If they do have an infarction on their scan, even if they had symptoms that only lasted for five minutes, that’s a stroke,” Dr. Josephson says. And even a true TIA, he says, represents “a kind of stroke where you got really lucky and you’re not left with deficits, but the risk is still very high.” Accordingly, more patients with TIA are being admitted to the hospital to receive a full workup and preventive treatment. “We think that by evaluating these people urgently, we can reduce the risk of having a stroke by maybe 75% over a three-month time period,” Dr. Josephson says.
Hemorrhagic stroke. To date, the vast majority of patients with hemorrhagic stroke (which accounts for only 13% of all stroke cases) have been managed by neurosurgeons and neurologists. But here, too, Dr. Likosky says the picture could be changing. Recent findings that surgical treatment of intracranial hemorrhaging might not benefit many patients could shift the care paradigm toward a medical management strategy that involves more hospitalists.
Innovations Aplenty
The increasing complexity of stroke care and uneven distribution of resources and expertise have helped fuel several important innovations in delivery, most notably telestroke and neurohospital medicine. Both are being driven, in part, by an increased awareness of time-sensitive interventions and a frequent lack of on-site neurologists at smaller and more rural facilities. If telestroke programs are expanding the reach of neurologists, neurohospitalists are helping to fill the gaps in inpatient stroke care.
Amid the changes, one element is proving a necessary constant: a team approach that relies heavily on the HM emphasis on quality metrics, intensive monitoring, and careful coordination. Who better to lead the charge than hospitalists, says Mary E. Jensen, MD, professor of radiology and neurosurgery at the University of Virginia in Charlottesville. “They’re the ones who are in the hospital, and when these patients go bad, they go bad fast,” she says.
More broadly, Dr. Jensen says, hospitalists should get in on the ground floor when their facility seeks certification as a primary or a comprehensive stroke center. “And they need to make sure that the hospital isn’t just trying to get the sexy elements—the guy with the cath or the gal with the cath who can pull the clot out—but that they have a complete program that involves the care of the patient after they’ve had the procedure done,” she says.
As healthcare reform efforts are making clear, the responsibility doesn’t end after discharge, either. The Affordable Care Act includes a hospital readmission reduction program that will kick in this October, with penalties for hospitals posting unacceptably high 30-day readmission rates. Amy Kind, MD, PhD, assistant professor of medicine in the Division of Geriatrics at the University of Wisconsin School of Medicine and Public Health in Madison, is convinced that a key contributor to high rehospitalization rates among stroke patients may be the woefully incomplete nature of discharge communication.

—Mary E. Jensen, MD, professor of radiology and neurosurgery, University of Virginia, Charlottesville
Dr. Kind, for example, has found a disturbing pattern in communication regarding issues like dysphagia, a common complication among stroke patients and an important risk factor for pneumonia. Countering the risk usually requires such measures as putting patients on a special diet or elevating the head of their bed. “We looked at the quality of the communication of that information in discharge summaries, and it’s just abysmal. It’s absolutely abysmal,” she says. Without clear directives to providers in the next setting of care, such as a skilled-nursing facility, patients could be erroneously put back on a regular diet and aspirate, sending them right back to the hospital.
As one potential solution, Dr. Kind’s team is developing a multidisciplinary stroke discharge summary tool that automatically imports elements like speech-language pathology and dietary recommendations. Although most discharge communication may focus on more visible issues and interventions, Dr. Kind argues that some of the “bread and butter” concerns might ultimately prove just as important for long-term patient outcomes.
Karim Godamunne, MD, MBA, SFHM, vice president of clinical systems integration and medical director of Eagle Hospital Physicians in Atlanta, sees telemedicine as another potential tool to help reach patients after discharge, especially those who haven’t received follow-up care from a primary-care physician (PCP). “We need to be the champions at our hospitals for improving care processes, and we need to work in partnership with the nurses and the other professionals,” Dr. Godamunne says. “As a group, we can really make a difference, and stroke is one of those areas in which we can truly contribute.”
Bryn Nelson is a freelance medical writer in Seattle.
References
- Freeman WD, Dawson SB, Raper C, Thiemann K, et al. Neurohospitalists reduce length of stay for patients with ischemic stroke. The Neurohospitalist. 2011;1(2): 67-70.
- Howrey BT, Kuo Y-F, Goodwin JS. Association of care by hospitalists on discharge destination and 30-day outcomes after acute ischemic stroke. Medical Care. 2011;49(8): 701-707.
- Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125:e2-e220.
- Glasheen JJ, Epstein KR, Siegal E, Kutner JS, Prochazka AV. The spectrum of community-based hospitalist practice: a call to tailor internal medicine residency training. Arch Intern Med. 2007;167(7):727-728.
- Plauth WH, Pantilat SZ, Wachter RM, Fenton CL. Hospitalists’ perceptions of their residency training needs: results of a national survey. Am J Med. 2001; 111(3):247-254.
- Dickerson LM, Carek PJ, Quattlebaum RG. Prevention of recurrent ischemic stroke. Am Fam Physician. 2007; 76(3):382-388.
- Fonarow GC, Smith EE, Saver JL, et al. Timeliness of tissue-type plasminogen activator therapy in acute ischemic stroke: patient characteristics, hospital factors, and outcomes associated with door-to-needle times within 60 minutes. Circulation. 2011;123(7):750-758.
- Hacke W, Kaste M, Bluhmki E, Brozman M, et al. Thrombolysis with Alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317-1329.
- Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121:e91.
- Albers GW, Caplan LR, Easton JD, et al. Transient ischemic attack—proposal for a new definition. N Engl J Med. 2002;347(21):1713-1716.
- Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365:993-1003.
Stroke specialists like to say that “time is brain.” With an emphatic focus on those first few critical hours, however, it’s sometimes easy to overlook the vital role that hospitalists play in the days, weeks, and months that follow.
A recent study in The Neurohospitalist suggests that compared to community-based neurologists, practitioners of neurohospital medicine can reduce the length of stay for patients with ischemic stroke.1 A separate study, however, suggests that similar success might have come at a price for their less-specialized hospitalist counterparts.2 Among stroke patients, the latter study found that while the HM model is also associated with a reduced length of stay, it is associated with increased discharges to inpatient rehabilitation centers instead of to home, and higher readmission rates.
In sum, the evidence raises questions about whether rank-and-file hospitalists are adequately equipped to deal with a disease that is a core competency for the profession and ranks among the top sources of adult disability in the United States, at an estimated cost of $34.3 billion in 2008.3
“I think there’s been a mismatch between the training of the average hospitalist and then the expectations for the amount of neurological care they end up delivering once in practice,” says David Likosky, MD, SFHM, director of the stroke program at Evergreen Hospital Medical Center in Kirkland, Wash. “When surveyed, it’s been shown that hospitalists feel that care of stroke is one of the areas with which they’re least comfortable once they get out into practice.” Over the past decade, several studies have reinforced the notion of a training deficit.4,5
Demographic trends suggest that getting up to speed will be imperative, however. “One alarming thing we’re seeing is strokes among individuals that are not in the elderly group, and that group seems to be increasing at an alarming rate,” says Daniel T. Lackland, PhD, professor of epidemiology and neurosciences at the Medical University of South Carolina in Charleston. Hospitals are seeing more ischemic stroke patients in their 40s and 50s, likely a reflection of risk factors such as hypertension, diabetes, and hyperlipidemia that are occurring earlier in life. And because those patients are younger, the aftermath of a stroke could linger for decades.
Although the stroke mortality rate is declining in the U.S., statistics find that about 14% of all patients diagnosed with an initial stroke will have a second one within a year, placing continued strain on a healthcare system already stretched thin.6 Hospitalists, Dr. Lackland says, have an “ideal” opportunity to help build up and improve that system, potentially yielding significant cost savings along with the dramatic improvement in quality of life. Making the most of that opportunity, though, will require a solid understanding of multiple trends that are quickly transforming stroke care delivery.
Time Is of the Essence
Kevin Barrett, MD, MSc, assistant professor of neurology and stroke telemedicine director at the Mayo Clinic in Jacksonville, Fla., says hospitals are focusing more and more on a metric known as “door-to-needle time.” The goal is to treat at least half of incoming ischemic stroke patients with intravenous tissue-type plasminogen activator (IV tPA) within the first 60 minutes after onset of symptoms.
The American Heart Association/American Stroke Association has reinforced the message with its Get With the Guidelines Stroke Program. A recent analysis suggested the program has led to more timely tPA administration and, in turn, better patient outcomes (the program is funded in part through the Bristol-Myers Squib/Sanofi Pharmaceutical Partnership).7
At the same time, clinical research has widened the window for IV tPA delivery from three hours to 4.5 hours for certain patients after the onset of symptoms. Dr. Barrett says “strong evidence” from the European Cooperative Acute Stroke Study III has convinced most clinicians, and the FDA is expected to follow suit in officially approving the extension.8 As more stroke centers become certified, the use of IV tPA has increased accordingly.
Patients who have missed the time window or are not good candidates for IV tPA can still be aided by interarterial tPA at the site of the clot up to six hours after the onset of symptoms. Dr. Likosky says the treatment option should be of particular interest to hospitalists, given that strokes can occur post-operatively and in other patients who cannot receive IV tPA because of bleeding risk.
—Karim Godamunne, MD, MBA, SFHM, medical director, Eagle Hospital Physicians, Roswell, Ga.
For up to eight hours after the onset of symptoms, mechanical clot removal techniques have shown continued efficacy at revascularizing affected areas, with some newer options also offering greater promise of improving patient outcomes. Even with the prospects of declining complication rates, however, “evaluating and initiating treatment in a timely fashion is still going to be one of the most important predictors of outcome,” Dr. Barrett says.
After the initial intervention, hospitalists often are the go-to providers for anticipating and preventing common post-stroke complications, such as aspiration pneumonia, VTE from immobilization, and other infections. The proper use of anti-platelet agents and high-dose statins, also falling solidly within the HM realm, can pay big dividends if used consistently.
Meanwhile, newer studies and clinical observations are widening the scope of considerations that should be on every hospitalist’s radar. Here are a few cited by stroke experts:
Permissive hypertension. After an ischemic stroke, the benefit of permissive hypertension is still widely misunderstood. Perhaps counterintuitively, high blood pressure after a stroke can help protect the area of the brain that is damaged but not yet dead, sometimes called the penumbra. “I highlight this because I think it’s a common mistake, that internists are very used to high blood pressure being a bad thing,” says Andrew Josephson, MD, associate professor of clinical neurology and director of the neurohospitalist program at the University of California at San Francisco (UCSF) Medical Center. “And in general, it is; it’s a cause of stroke. But once somebody has a stroke, in the acute period, it’s important to allow the blood pressure to be high.”
Atrial fibrillation. The accepted role of atrial fibrillation in stroke is evolving. Research suggests that the common but often preventable arrhythmia is an important cause of stroke in about 15% to 20% of cases.9 By the time of hospital discharge, however, Dr. Josephson says physicians haven’t established a cause in about 1 in 4 cases. For these “cryptogenic strokes,” he says, doctors have long suspected that atrial fibrillation not picked up during the initial EKG or by the monitoring with cardiac telemetry could be a major cause.
Recent observations suggest that a longer monitoring period of up to 30 days may uncover atrial fibrillation in a sizable fraction of those patients, highlighting the importance of keeping a close eye on stroke patients both in the hospital and beyond. “It’s very important to identify, because atrial fibrillation changes what we do for folks to prevent a second stroke,” Dr. Josephson explains. Instead of anti-platelet medicine like aspirin, patients with atrial fibrillation often receive anticoagulants like warfarin, or the more recently approved dabigatran and rivaroxaban.
Transient ischemic attack. Improvements in imaging techniques like MRI have likewise begun to shift how stroke patients are treated. For example, Dr. Likosky says, medicine is moving away from a time-based definition of transient ischemic attack (TIA), in which symptoms resolve within 24 hours, to a tissue-based definition. Recent MRI imaging has uncovered evidence of a new infarction in more than half of patients initially diagnosed with TIA.10
“If they do have an infarction on their scan, even if they had symptoms that only lasted for five minutes, that’s a stroke,” Dr. Josephson says. And even a true TIA, he says, represents “a kind of stroke where you got really lucky and you’re not left with deficits, but the risk is still very high.” Accordingly, more patients with TIA are being admitted to the hospital to receive a full workup and preventive treatment. “We think that by evaluating these people urgently, we can reduce the risk of having a stroke by maybe 75% over a three-month time period,” Dr. Josephson says.
Hemorrhagic stroke. To date, the vast majority of patients with hemorrhagic stroke (which accounts for only 13% of all stroke cases) have been managed by neurosurgeons and neurologists. But here, too, Dr. Likosky says the picture could be changing. Recent findings that surgical treatment of intracranial hemorrhaging might not benefit many patients could shift the care paradigm toward a medical management strategy that involves more hospitalists.
Innovations Aplenty
The increasing complexity of stroke care and uneven distribution of resources and expertise have helped fuel several important innovations in delivery, most notably telestroke and neurohospital medicine. Both are being driven, in part, by an increased awareness of time-sensitive interventions and a frequent lack of on-site neurologists at smaller and more rural facilities. If telestroke programs are expanding the reach of neurologists, neurohospitalists are helping to fill the gaps in inpatient stroke care.
Amid the changes, one element is proving a necessary constant: a team approach that relies heavily on the HM emphasis on quality metrics, intensive monitoring, and careful coordination. Who better to lead the charge than hospitalists, says Mary E. Jensen, MD, professor of radiology and neurosurgery at the University of Virginia in Charlottesville. “They’re the ones who are in the hospital, and when these patients go bad, they go bad fast,” she says.
More broadly, Dr. Jensen says, hospitalists should get in on the ground floor when their facility seeks certification as a primary or a comprehensive stroke center. “And they need to make sure that the hospital isn’t just trying to get the sexy elements—the guy with the cath or the gal with the cath who can pull the clot out—but that they have a complete program that involves the care of the patient after they’ve had the procedure done,” she says.
As healthcare reform efforts are making clear, the responsibility doesn’t end after discharge, either. The Affordable Care Act includes a hospital readmission reduction program that will kick in this October, with penalties for hospitals posting unacceptably high 30-day readmission rates. Amy Kind, MD, PhD, assistant professor of medicine in the Division of Geriatrics at the University of Wisconsin School of Medicine and Public Health in Madison, is convinced that a key contributor to high rehospitalization rates among stroke patients may be the woefully incomplete nature of discharge communication.

—Mary E. Jensen, MD, professor of radiology and neurosurgery, University of Virginia, Charlottesville
Dr. Kind, for example, has found a disturbing pattern in communication regarding issues like dysphagia, a common complication among stroke patients and an important risk factor for pneumonia. Countering the risk usually requires such measures as putting patients on a special diet or elevating the head of their bed. “We looked at the quality of the communication of that information in discharge summaries, and it’s just abysmal. It’s absolutely abysmal,” she says. Without clear directives to providers in the next setting of care, such as a skilled-nursing facility, patients could be erroneously put back on a regular diet and aspirate, sending them right back to the hospital.
As one potential solution, Dr. Kind’s team is developing a multidisciplinary stroke discharge summary tool that automatically imports elements like speech-language pathology and dietary recommendations. Although most discharge communication may focus on more visible issues and interventions, Dr. Kind argues that some of the “bread and butter” concerns might ultimately prove just as important for long-term patient outcomes.
Karim Godamunne, MD, MBA, SFHM, vice president of clinical systems integration and medical director of Eagle Hospital Physicians in Atlanta, sees telemedicine as another potential tool to help reach patients after discharge, especially those who haven’t received follow-up care from a primary-care physician (PCP). “We need to be the champions at our hospitals for improving care processes, and we need to work in partnership with the nurses and the other professionals,” Dr. Godamunne says. “As a group, we can really make a difference, and stroke is one of those areas in which we can truly contribute.”
Bryn Nelson is a freelance medical writer in Seattle.
References
- Freeman WD, Dawson SB, Raper C, Thiemann K, et al. Neurohospitalists reduce length of stay for patients with ischemic stroke. The Neurohospitalist. 2011;1(2): 67-70.
- Howrey BT, Kuo Y-F, Goodwin JS. Association of care by hospitalists on discharge destination and 30-day outcomes after acute ischemic stroke. Medical Care. 2011;49(8): 701-707.
- Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125:e2-e220.
- Glasheen JJ, Epstein KR, Siegal E, Kutner JS, Prochazka AV. The spectrum of community-based hospitalist practice: a call to tailor internal medicine residency training. Arch Intern Med. 2007;167(7):727-728.
- Plauth WH, Pantilat SZ, Wachter RM, Fenton CL. Hospitalists’ perceptions of their residency training needs: results of a national survey. Am J Med. 2001; 111(3):247-254.
- Dickerson LM, Carek PJ, Quattlebaum RG. Prevention of recurrent ischemic stroke. Am Fam Physician. 2007; 76(3):382-388.
- Fonarow GC, Smith EE, Saver JL, et al. Timeliness of tissue-type plasminogen activator therapy in acute ischemic stroke: patient characteristics, hospital factors, and outcomes associated with door-to-needle times within 60 minutes. Circulation. 2011;123(7):750-758.
- Hacke W, Kaste M, Bluhmki E, Brozman M, et al. Thrombolysis with Alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317-1329.
- Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121:e91.
- Albers GW, Caplan LR, Easton JD, et al. Transient ischemic attack—proposal for a new definition. N Engl J Med. 2002;347(21):1713-1716.
- Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365:993-1003.
Is Hospitalist Proficiency in Bedside Procedures in Decline?
It’s 3:30 p.m. You’ve seen your old patients, holdovers, and an admission, but you haven’t finished your notes yet. Lunch was an afterthought between emails about schedule changes for the upcoming year. Two pages ring happily from your belt, the first from you-know-who in the ED, and the next from a nurse: “THORA SUPPLIES AT BEDSIDE SINCE THIS AM—WHEN WILL THIS HAPPEN?” The phone number on the wall for the on-call radiologist beckons...
An all-too-familiar situation for hospitalists across the country, this awkward moment raises a series of difficult questions:
Should I set aside time from my day to perform a procedure that could be time-consuming?
- Do I feel confident I can perform this procedure safely?
- Am I really the best physician to provide this service?
- As hospitalists are tasked with an ever-increasing array of responsibilities, answering the call of duty for bedside procedures is becoming more difficult for some.
A Core Competency
“The Core Competencies in Hospital Medicine,” authored by a group of HM thought leaders, was published as a supplement to the January/February 2006 issue of the Journal of Hospital Medicine. The core competencies include such bedside procedures as arthrocentesis, paracentesis, thoracentesis, lumbar puncture, and vascular (arterial and central venous) access (see “Core Competencies in Hospital Medicine: Procedures,” below). Although the authors stressed that the core competencies are to be viewed as a resource rather than as a set of requirements, the inclusion of bedside procedures emphasized the importance of procedural skills for future hospitalists.
“[Hospitalists] are in a perfect spot to continue to perform procedures in a structured manner,” says Joshua Lenchus, DO, RPh, FACP, FHM, associate director of the University of Miami-Jackson Memorial Hospital (UM-JMH) Center for Patient Safety. “As agents of quality and safety, hospitalists should continue to perform this clinically necessary service.”
Jeffrey Barsuk, MD, FHM, associate professor of medicine at Northwestern Feinberg School of Medicine in Chicago and an academic hospitalist at Northwestern Memorial Hospital (NMH), not only agrees that bedside procedures should be a core competency, but he also says hospitalists are the most appropriate providers of these services.
“I think this is part of hospital medicine. We’re in the hospital, [and] that’s what we do,” Dr. Barsuk says. Other providers, such as interventional radiologists, “really don’t understand why I’m doing [a procedure]. They understand it’s safe to do it, but they might not understand all the indications for it, and they certainly don’t understand the interpretation of the tests they’re sending.”
Despite the goals set forth by the core competencies and authorities in procedural safety, the reality of who actually performs bedside procedures is somewhat murky and varies greatly by institution. Many point to HM program setting (urban vs. rural) or structure (academic vs. community) to explain variance, but often it is other factors that determine whether hospitalists are actually preforming bedside procedures regularly.
Where Does HM Perform Procedures?
Community hospitalists, with strong support from interventional radiologists and subspecialists, often find it more efficient—even necessary, considering their patient volumes—to leave procedures to others. Community hospitalists with ICU admitting privileges, intensivists, and other HM subgroups say that being able to perform procedures should be a prerequisite for employment. Hospitalists in rural communities say they are doing procedures because they are “the only game in town.”
“Sometimes you are the only one available, and you are called upon to stretch your abilities,” says Beatrice Szantyr, MD, FAAP, a community hospitalist and pediatrician in Lincoln, Maine, who has practiced most of her career in rural settings.
Academic hospitalists in large, research-based HM programs can, paradoxically, find themselves performing fewer procedures as residents often take the lead on the majority of such cases. Conversely, academic hospitalists in large, nonteaching programs often find themselves called on to perform more bedside procedures.
No matter the setting, the simplicity of being the physician to recognize the need for a procedure, perform it, and interpret the results is undeniably efficient and “clean,” according to authorities on inpatient bedside procedures. Having to consult other physicians, optimize the patient’s lab values to their standards (a common issue with interventional radiologists), and adhere to their work schedules can often delay procedures unnecessarily.
—Joshua Lenchus, DO, RPh, FACP, FHM, associate director, University of Miami-Jackson Memorial Hospital (UM-JMH) Center for Patient Safety
“Hospitalists care for floor and ICU patients in many hospitals, and the inability to perform bedside procedures delays patient care,” says Dr. Nilam Soni, an academic hospitalist at the University of Chicago and a recognized expert on procedural safety.
Dr. Soni notes that when it comes to current techniques, many hospitalists suffer from a knowledge deficit. “The introduction of ultrasound for guidance of bedside procedures has been shown to improve the success and safety of certain procedures,” he says, “but the majority of practicing hospitalists did not learn how to use ultrasound for procedure guidance during residency.”
Heterogeneity of Training, Experience, and Skill
While all hospitalists draw upon different bases of training and experience, the heterogeneity of training, confidence, and inherent skill is greatest when it comes to bedside procedures. Mirroring the heterogeneity at the individual level, hospitalist programs vary greatly on the requirements placed on their staffs in regards to procedural skill and privileging.
Such research-driven programs as Brigham and Women’s Hospital (BWH) in Boston often find requiring maintenance of privileges in bedside procedures to be difficult, says Sally Wang, MD, FHM, director of procedural education at BWH. In fact, a new procedure service being created there will be staffed mainly with ED physicians. On the flipside, most community hospitalist programs leave the task of procedural “policing” to the hospital’s medical staff affairs office.
At the University of California at San Diego (UCSD) Medical Center, the HM group is instituting a division standard in which hospitalists maintain privileging and proficiency in a core group of bedside procedures. Other large hospitalist groups have created “proceduralist” subgroups that shoulder the burden of trainee education, as well as provide a resource for less skilled or less experienced inpatient providers.
“If you have a big group, you could have a dedicated procedure service and have a core group of hospitalists who are experts in procedure,” Dr. Barsuk says. “But it needs to be self-sustaining.” Once started, Dr. Barsuk says, proceduralist groups would continue to provide hospitals with ongoing return-on-investment (ROI) benefit.
Variability in procedure volume and payor mix, however, can make it hard for HM groups to demonstrate to hospital leadership a satisfactory ROI for a proceduralist program. Financial backing from grant support or a high-volume procedure—such as paracentesis in hospitals with large hepatology programs—can nurture starting proceduralist programs until all procedural revenues can justify the costs. Lower ROI can also be justified by showing improvement in quality indices—such as CLABSI rates—reduced time to procedures, and reduced costs compared to other subspecialists offering similar services.
“I’m of the firm belief that we can reduce costs by doing the procedures at the bedside rather than referring them to departments such as interventional radiology (IR),” Dr. Barsuk says. “What you would have to do is show the institution that it costs more money to have IR do [bedside procedures].”
National Response

—Jeffrey Barsuk, MD, MS, FHM, associate professor of medicine, Northwestern Feinberg School of Medicine, academic hospitalist, Northwestern Memorial Hospital, Chicago
Filling in the procedural training gaps found on the local level, such national organizations as SHM have stepped in to provide education and support for hospitalists yearning for training. Since its inception, an SHM annual meeting pre-course that focuses on hand-held ultrasound and invasive procedures has consistently been one of the first to sell out. Other national organizations, such as ACP and its annual meeting, have seen similar interest in their courses on ultrasound-guided procedures.
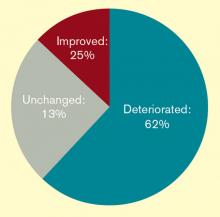
The popularity of this continuing education bears out a worrisome trend: Hospitalists feel they are losing their procedural skills. An online survey conducted by The Hospitalist in May 2011 found that a majority of respondents (62%) had experienced deterioration of their procedural skills in the past five years; only 25% said they experienced improvement over the same period.
Historically, general internists have claimed bedside procedures as their domain. As stated dispassionately in the 1978 book The House of God, “There is no body cavity that cannot be reached with a #14G needle and a good strong arm.”1 Yet much has changed since Samuel Shem’s apocryphal description of medical residency training.
Most notably, the Accreditation Council of Graduate Medical Education (ACGME) has not only progressively restricted inpatient hours and patient loads for residents, but also increased the requirements for outpatient training. Some feel the balance of inpatient and outpatient training has tipped too far toward the latter in medicine residency programs, especially in light of the growing popularity of the hospitalist career path amongst new residency program graduates. This stands in contrast to ED training programs, which have embraced focused procedures training more readily.
“Adult care appears to be diverging into two career tacks as a result of external forces, of which we have limited control over, “ says Michael Beck, MD, a pediatric and adult hospitalist at Milton S. Hershey Medical Center in Hershey, Pa. “With new career choices emerging for graduates, the same square-peg, round-hole residency training should not exist.”
Dr. Beck advocates continuing an ongoing trend of “track” creation in residency programs, which allow trainees to focus training on their planned career path. Hospitalist tracks already exist in many medicine programs, including those at Cleveland Clinic and Northwestern. But many other factors limit the opportunity for trainees to obtain experience with bedside procedures, including competition with nurse practitioners and physician assistants. Even the increasing availability of ancillary phlebotomy and IV-start teams can increase a resident’s anxiety about procedures.
“By the time my residency was over [in 1993] and the work restrictions were beginning, hospital employees were doing all these tasks, making the residents less comfortable with hurting a patient when it was therapeutically necessary,” says Katharine Deiss, MD, assistant clinical professor of medicine at the University of Rochester Medical Center in New York. Interns who came from medical schools without extensive ancillary services in their teaching hospitals, she adds, were more comfortable with invasive procedures.
ACGME has sent a subtle message by decreasing emphasis on procedural skills by eradicating the requirement of showing manual proficiency in most bedside procedures as a requirement for certification. The omission has left individual residency programs and hospitalist groups to determine training and proficiency requirements for more invasive bedside procedures without a national standard.
In an editorial in the March 2007 issue of the Annals of Internal Medicine, F. Daniel Duffy, MD, and Eric Holmboe, MD, wrote that the American Board of Internal Medicine (ABIM) could only give a “qualified ‘yes’” to the question of whether residents should be trained in procedures they may not perform in practice. Although the authors asserted that the relaxed ABIM policy was “an important but small step toward revamping procedure skill training during residency,” others say it portrays an image of the ABIM de-emphasizing the importance of procedural training.
In addition, the recently established Focused Practice in Hospital Medicine (FPHM) pathway to ABIM Maintenance of Certification (MOC) has no requirement to show proficiency in bedside procedures.
“The absence of the procedural requirement in no way constitutes a statement that procedural skills are not important,” says Jeff Wiese, MD, FACP, SFHM, associate professor of medicine and residency program director at Tulane University Health Sciences Center in New Orleans, chair of the ABIM Hospital Medicine MOC Question Writing Committee, and former SHM president. “Rather, it is merely a practical issue with respect to making the MOC process applicable to all physicians engaged in hospital medicine (i.e. many hospitalists do not do procedures) while still making the MOC focused on the skill sets that are common for physicians doing hospital medicine.”
Once released into the world, even if trained well in residency, hospitalists can find it difficult to maintain their skills. In community and nonteaching settings, the pressure to admit and discharge in a timely manner can make procedures seem like the easiest corner to cut. Before long, it has been months since they have laid eyes on a needle of any sort. Many begin to develop performance anxiety.
In teaching hospitals, academic hospitalists often are called upon to participate in quality improvement (QI) and research efforts, which take time away from clinical rotations. Once there, it can be easy for a ward attending to rely upon a well-trained resident to supervise interns doing procedures. The lack of first-hand or even supervisory experience can lead to many academic hospitalists losing facility with procedures, with potentially disastrous results.
“In order to supervise a group of residents, the attending needs to be technically proficient and able to salvage a botched, or failed, procedure,” UM-JMH’s Dr. Lenchus says. “To this end, we strictly limit who can attend on the service.”
So what’s a residency or HM program director to do in the face of wavering support nationally, and sometimes locally, for maintaining procedural skills for hospitalists and trainees? Many hospitalists in teaching hospitals say it’s critical for clinicians to “get their own house in order,” to maintain procedural standards of proficiency with ongoing training, education, and verification.
“The profession now needs to redesign procedural training across the continuum of education and a lifetime of practice,” Drs. Duffy and Holmboe editorialized in the March 2007 Annals paper. “This approach would recognize the varied settings of internal-medicine practice and offer manual skills training to those whose practice settings require such skills.” Hospitalists can partner with medicine residency program leaders to provide procedural education and training to residents, either as a standalone elective or as a more general resource.
Hospitalists in such teaching hospitals as UCSD, Brigham and Women’s, UM-JMH, and Northwestern are leading efforts to provide procedural education to medical students, residents, and attendings. Training takes many forms, including formal procedural electives, required procedure rotations, or even brief one- or two-day courses in procedural skills at a simulation center.
Utilizing simulation training has been shown in many studies to be helpful in establishing procedural skills in learners of all training levels. Dr. Barsuk and his colleagues at Northwestern published studies in the Journal of Hospital Medicine in 2008 and 2009 showing that simulation training of residents was effective in improving skills in thoracentesis and central venous catheterization, respectively.3,4
In the community hospital setting, requirements for procedural skills can vary greatly based on the institution. For those community programs requiring procedural skills of their hospitalists, the clear definition of procedural training and requirements at the time of hiring is critical. Even after vetting a hospitalist’s procedural skills at hire, however, community programs should consider monitoring procedural skills and provide ongoing time and money for CME focused on procedural skills.
Currently, most hospitals depend on the honesty of individual physicians during the privileging process for bedside procedures. Even when the skills of physicians begin to wane, most are reluctant to voluntarily give up their procedure privileges.
“I think it would be pretty unusual for a hospitalist to relinquish their privileges,” Dr. Barsuk admits. But ideally, physicians who relinquish their privileges due to lack of experience could get retrained in simulation centers, then reproctored in order to regain their privileges. Northwestern established the Center for Simulation Technology and Immersive Learning as a resource for simulation training both locally and nationally.
Establishing an environment that supports hospitalists performing bedside procedures is critical. This includes the need to limit hospitalist workload to ensure adequate time to meet the procedural needs of patients. Providing easy access to the tools necessary to perform bedside procedures (e.g. portable ultrasound and pre-packaged procedure trays) helps avoid additional hurdles.
Academic hospitalist programs can serve as a regional resource by developing ongoing procedure mastery programs for hospitalists in their communities, as many smaller institutions do not have the resources to provide ongoing training in bedside procedures. This process can be tedious, but it should not be humiliating.
If the popularity of the SHM pre-course in bedside ultrasound and procedures is any indication, when given the opportunity to receive protected time for procedure training, most hospitalists will likely jump at the chance.
Dr. Chang is an associate clinical professor of medicine in the division of hospital medicine at Diego Medical Center. He is also a member of Team Hospitalist.
References
- Shem S. The House of God. New York: Dell Publishing; 1978.
- Duffy FD, Holmboe ES. What procedures should internists do? Ann Intern Med. 2007;146(5):392-393.
- Wayne DB, Barsuk JH, O’Leary KJ, Fudala MJ, McGaghie WC. Mastery learning of thoracentesis skills by internal medicine residents using simulation technology and deliberate practice. J Hosp Med. 2008;3(1):48-54.
- Barsuk JH, McGaghie WC, Cohen ER, Balachandran JS, Wayne DB. Use of simulation-based mastery learning to improve the quality of central venous catheter placement in a medical intensive care unit. J Hosp Med. 2009;4(7):397–403.
It’s 3:30 p.m. You’ve seen your old patients, holdovers, and an admission, but you haven’t finished your notes yet. Lunch was an afterthought between emails about schedule changes for the upcoming year. Two pages ring happily from your belt, the first from you-know-who in the ED, and the next from a nurse: “THORA SUPPLIES AT BEDSIDE SINCE THIS AM—WHEN WILL THIS HAPPEN?” The phone number on the wall for the on-call radiologist beckons...
An all-too-familiar situation for hospitalists across the country, this awkward moment raises a series of difficult questions:
Should I set aside time from my day to perform a procedure that could be time-consuming?
- Do I feel confident I can perform this procedure safely?
- Am I really the best physician to provide this service?
- As hospitalists are tasked with an ever-increasing array of responsibilities, answering the call of duty for bedside procedures is becoming more difficult for some.
A Core Competency
“The Core Competencies in Hospital Medicine,” authored by a group of HM thought leaders, was published as a supplement to the January/February 2006 issue of the Journal of Hospital Medicine. The core competencies include such bedside procedures as arthrocentesis, paracentesis, thoracentesis, lumbar puncture, and vascular (arterial and central venous) access (see “Core Competencies in Hospital Medicine: Procedures,” below). Although the authors stressed that the core competencies are to be viewed as a resource rather than as a set of requirements, the inclusion of bedside procedures emphasized the importance of procedural skills for future hospitalists.
“[Hospitalists] are in a perfect spot to continue to perform procedures in a structured manner,” says Joshua Lenchus, DO, RPh, FACP, FHM, associate director of the University of Miami-Jackson Memorial Hospital (UM-JMH) Center for Patient Safety. “As agents of quality and safety, hospitalists should continue to perform this clinically necessary service.”
Jeffrey Barsuk, MD, FHM, associate professor of medicine at Northwestern Feinberg School of Medicine in Chicago and an academic hospitalist at Northwestern Memorial Hospital (NMH), not only agrees that bedside procedures should be a core competency, but he also says hospitalists are the most appropriate providers of these services.
“I think this is part of hospital medicine. We’re in the hospital, [and] that’s what we do,” Dr. Barsuk says. Other providers, such as interventional radiologists, “really don’t understand why I’m doing [a procedure]. They understand it’s safe to do it, but they might not understand all the indications for it, and they certainly don’t understand the interpretation of the tests they’re sending.”
Despite the goals set forth by the core competencies and authorities in procedural safety, the reality of who actually performs bedside procedures is somewhat murky and varies greatly by institution. Many point to HM program setting (urban vs. rural) or structure (academic vs. community) to explain variance, but often it is other factors that determine whether hospitalists are actually preforming bedside procedures regularly.
Where Does HM Perform Procedures?
Community hospitalists, with strong support from interventional radiologists and subspecialists, often find it more efficient—even necessary, considering their patient volumes—to leave procedures to others. Community hospitalists with ICU admitting privileges, intensivists, and other HM subgroups say that being able to perform procedures should be a prerequisite for employment. Hospitalists in rural communities say they are doing procedures because they are “the only game in town.”
“Sometimes you are the only one available, and you are called upon to stretch your abilities,” says Beatrice Szantyr, MD, FAAP, a community hospitalist and pediatrician in Lincoln, Maine, who has practiced most of her career in rural settings.
Academic hospitalists in large, research-based HM programs can, paradoxically, find themselves performing fewer procedures as residents often take the lead on the majority of such cases. Conversely, academic hospitalists in large, nonteaching programs often find themselves called on to perform more bedside procedures.
No matter the setting, the simplicity of being the physician to recognize the need for a procedure, perform it, and interpret the results is undeniably efficient and “clean,” according to authorities on inpatient bedside procedures. Having to consult other physicians, optimize the patient’s lab values to their standards (a common issue with interventional radiologists), and adhere to their work schedules can often delay procedures unnecessarily.
—Joshua Lenchus, DO, RPh, FACP, FHM, associate director, University of Miami-Jackson Memorial Hospital (UM-JMH) Center for Patient Safety
“Hospitalists care for floor and ICU patients in many hospitals, and the inability to perform bedside procedures delays patient care,” says Dr. Nilam Soni, an academic hospitalist at the University of Chicago and a recognized expert on procedural safety.
Dr. Soni notes that when it comes to current techniques, many hospitalists suffer from a knowledge deficit. “The introduction of ultrasound for guidance of bedside procedures has been shown to improve the success and safety of certain procedures,” he says, “but the majority of practicing hospitalists did not learn how to use ultrasound for procedure guidance during residency.”
Heterogeneity of Training, Experience, and Skill
While all hospitalists draw upon different bases of training and experience, the heterogeneity of training, confidence, and inherent skill is greatest when it comes to bedside procedures. Mirroring the heterogeneity at the individual level, hospitalist programs vary greatly on the requirements placed on their staffs in regards to procedural skill and privileging.
Such research-driven programs as Brigham and Women’s Hospital (BWH) in Boston often find requiring maintenance of privileges in bedside procedures to be difficult, says Sally Wang, MD, FHM, director of procedural education at BWH. In fact, a new procedure service being created there will be staffed mainly with ED physicians. On the flipside, most community hospitalist programs leave the task of procedural “policing” to the hospital’s medical staff affairs office.
At the University of California at San Diego (UCSD) Medical Center, the HM group is instituting a division standard in which hospitalists maintain privileging and proficiency in a core group of bedside procedures. Other large hospitalist groups have created “proceduralist” subgroups that shoulder the burden of trainee education, as well as provide a resource for less skilled or less experienced inpatient providers.
“If you have a big group, you could have a dedicated procedure service and have a core group of hospitalists who are experts in procedure,” Dr. Barsuk says. “But it needs to be self-sustaining.” Once started, Dr. Barsuk says, proceduralist groups would continue to provide hospitals with ongoing return-on-investment (ROI) benefit.
Variability in procedure volume and payor mix, however, can make it hard for HM groups to demonstrate to hospital leadership a satisfactory ROI for a proceduralist program. Financial backing from grant support or a high-volume procedure—such as paracentesis in hospitals with large hepatology programs—can nurture starting proceduralist programs until all procedural revenues can justify the costs. Lower ROI can also be justified by showing improvement in quality indices—such as CLABSI rates—reduced time to procedures, and reduced costs compared to other subspecialists offering similar services.
“I’m of the firm belief that we can reduce costs by doing the procedures at the bedside rather than referring them to departments such as interventional radiology (IR),” Dr. Barsuk says. “What you would have to do is show the institution that it costs more money to have IR do [bedside procedures].”
National Response

—Jeffrey Barsuk, MD, MS, FHM, associate professor of medicine, Northwestern Feinberg School of Medicine, academic hospitalist, Northwestern Memorial Hospital, Chicago
Filling in the procedural training gaps found on the local level, such national organizations as SHM have stepped in to provide education and support for hospitalists yearning for training. Since its inception, an SHM annual meeting pre-course that focuses on hand-held ultrasound and invasive procedures has consistently been one of the first to sell out. Other national organizations, such as ACP and its annual meeting, have seen similar interest in their courses on ultrasound-guided procedures.
The popularity of this continuing education bears out a worrisome trend: Hospitalists feel they are losing their procedural skills. An online survey conducted by The Hospitalist in May 2011 found that a majority of respondents (62%) had experienced deterioration of their procedural skills in the past five years; only 25% said they experienced improvement over the same period.
Historically, general internists have claimed bedside procedures as their domain. As stated dispassionately in the 1978 book The House of God, “There is no body cavity that cannot be reached with a #14G needle and a good strong arm.”1 Yet much has changed since Samuel Shem’s apocryphal description of medical residency training.
Most notably, the Accreditation Council of Graduate Medical Education (ACGME) has not only progressively restricted inpatient hours and patient loads for residents, but also increased the requirements for outpatient training. Some feel the balance of inpatient and outpatient training has tipped too far toward the latter in medicine residency programs, especially in light of the growing popularity of the hospitalist career path amongst new residency program graduates. This stands in contrast to ED training programs, which have embraced focused procedures training more readily.
“Adult care appears to be diverging into two career tacks as a result of external forces, of which we have limited control over, “ says Michael Beck, MD, a pediatric and adult hospitalist at Milton S. Hershey Medical Center in Hershey, Pa. “With new career choices emerging for graduates, the same square-peg, round-hole residency training should not exist.”
Dr. Beck advocates continuing an ongoing trend of “track” creation in residency programs, which allow trainees to focus training on their planned career path. Hospitalist tracks already exist in many medicine programs, including those at Cleveland Clinic and Northwestern. But many other factors limit the opportunity for trainees to obtain experience with bedside procedures, including competition with nurse practitioners and physician assistants. Even the increasing availability of ancillary phlebotomy and IV-start teams can increase a resident’s anxiety about procedures.
“By the time my residency was over [in 1993] and the work restrictions were beginning, hospital employees were doing all these tasks, making the residents less comfortable with hurting a patient when it was therapeutically necessary,” says Katharine Deiss, MD, assistant clinical professor of medicine at the University of Rochester Medical Center in New York. Interns who came from medical schools without extensive ancillary services in their teaching hospitals, she adds, were more comfortable with invasive procedures.
ACGME has sent a subtle message by decreasing emphasis on procedural skills by eradicating the requirement of showing manual proficiency in most bedside procedures as a requirement for certification. The omission has left individual residency programs and hospitalist groups to determine training and proficiency requirements for more invasive bedside procedures without a national standard.
In an editorial in the March 2007 issue of the Annals of Internal Medicine, F. Daniel Duffy, MD, and Eric Holmboe, MD, wrote that the American Board of Internal Medicine (ABIM) could only give a “qualified ‘yes’” to the question of whether residents should be trained in procedures they may not perform in practice. Although the authors asserted that the relaxed ABIM policy was “an important but small step toward revamping procedure skill training during residency,” others say it portrays an image of the ABIM de-emphasizing the importance of procedural training.
In addition, the recently established Focused Practice in Hospital Medicine (FPHM) pathway to ABIM Maintenance of Certification (MOC) has no requirement to show proficiency in bedside procedures.
“The absence of the procedural requirement in no way constitutes a statement that procedural skills are not important,” says Jeff Wiese, MD, FACP, SFHM, associate professor of medicine and residency program director at Tulane University Health Sciences Center in New Orleans, chair of the ABIM Hospital Medicine MOC Question Writing Committee, and former SHM president. “Rather, it is merely a practical issue with respect to making the MOC process applicable to all physicians engaged in hospital medicine (i.e. many hospitalists do not do procedures) while still making the MOC focused on the skill sets that are common for physicians doing hospital medicine.”
Once released into the world, even if trained well in residency, hospitalists can find it difficult to maintain their skills. In community and nonteaching settings, the pressure to admit and discharge in a timely manner can make procedures seem like the easiest corner to cut. Before long, it has been months since they have laid eyes on a needle of any sort. Many begin to develop performance anxiety.
In teaching hospitals, academic hospitalists often are called upon to participate in quality improvement (QI) and research efforts, which take time away from clinical rotations. Once there, it can be easy for a ward attending to rely upon a well-trained resident to supervise interns doing procedures. The lack of first-hand or even supervisory experience can lead to many academic hospitalists losing facility with procedures, with potentially disastrous results.
“In order to supervise a group of residents, the attending needs to be technically proficient and able to salvage a botched, or failed, procedure,” UM-JMH’s Dr. Lenchus says. “To this end, we strictly limit who can attend on the service.”
So what’s a residency or HM program director to do in the face of wavering support nationally, and sometimes locally, for maintaining procedural skills for hospitalists and trainees? Many hospitalists in teaching hospitals say it’s critical for clinicians to “get their own house in order,” to maintain procedural standards of proficiency with ongoing training, education, and verification.
“The profession now needs to redesign procedural training across the continuum of education and a lifetime of practice,” Drs. Duffy and Holmboe editorialized in the March 2007 Annals paper. “This approach would recognize the varied settings of internal-medicine practice and offer manual skills training to those whose practice settings require such skills.” Hospitalists can partner with medicine residency program leaders to provide procedural education and training to residents, either as a standalone elective or as a more general resource.
Hospitalists in such teaching hospitals as UCSD, Brigham and Women’s, UM-JMH, and Northwestern are leading efforts to provide procedural education to medical students, residents, and attendings. Training takes many forms, including formal procedural electives, required procedure rotations, or even brief one- or two-day courses in procedural skills at a simulation center.
Utilizing simulation training has been shown in many studies to be helpful in establishing procedural skills in learners of all training levels. Dr. Barsuk and his colleagues at Northwestern published studies in the Journal of Hospital Medicine in 2008 and 2009 showing that simulation training of residents was effective in improving skills in thoracentesis and central venous catheterization, respectively.3,4
In the community hospital setting, requirements for procedural skills can vary greatly based on the institution. For those community programs requiring procedural skills of their hospitalists, the clear definition of procedural training and requirements at the time of hiring is critical. Even after vetting a hospitalist’s procedural skills at hire, however, community programs should consider monitoring procedural skills and provide ongoing time and money for CME focused on procedural skills.
Currently, most hospitals depend on the honesty of individual physicians during the privileging process for bedside procedures. Even when the skills of physicians begin to wane, most are reluctant to voluntarily give up their procedure privileges.
“I think it would be pretty unusual for a hospitalist to relinquish their privileges,” Dr. Barsuk admits. But ideally, physicians who relinquish their privileges due to lack of experience could get retrained in simulation centers, then reproctored in order to regain their privileges. Northwestern established the Center for Simulation Technology and Immersive Learning as a resource for simulation training both locally and nationally.
Establishing an environment that supports hospitalists performing bedside procedures is critical. This includes the need to limit hospitalist workload to ensure adequate time to meet the procedural needs of patients. Providing easy access to the tools necessary to perform bedside procedures (e.g. portable ultrasound and pre-packaged procedure trays) helps avoid additional hurdles.
Academic hospitalist programs can serve as a regional resource by developing ongoing procedure mastery programs for hospitalists in their communities, as many smaller institutions do not have the resources to provide ongoing training in bedside procedures. This process can be tedious, but it should not be humiliating.
If the popularity of the SHM pre-course in bedside ultrasound and procedures is any indication, when given the opportunity to receive protected time for procedure training, most hospitalists will likely jump at the chance.
Dr. Chang is an associate clinical professor of medicine in the division of hospital medicine at Diego Medical Center. He is also a member of Team Hospitalist.
References
- Shem S. The House of God. New York: Dell Publishing; 1978.
- Duffy FD, Holmboe ES. What procedures should internists do? Ann Intern Med. 2007;146(5):392-393.
- Wayne DB, Barsuk JH, O’Leary KJ, Fudala MJ, McGaghie WC. Mastery learning of thoracentesis skills by internal medicine residents using simulation technology and deliberate practice. J Hosp Med. 2008;3(1):48-54.
- Barsuk JH, McGaghie WC, Cohen ER, Balachandran JS, Wayne DB. Use of simulation-based mastery learning to improve the quality of central venous catheter placement in a medical intensive care unit. J Hosp Med. 2009;4(7):397–403.
It’s 3:30 p.m. You’ve seen your old patients, holdovers, and an admission, but you haven’t finished your notes yet. Lunch was an afterthought between emails about schedule changes for the upcoming year. Two pages ring happily from your belt, the first from you-know-who in the ED, and the next from a nurse: “THORA SUPPLIES AT BEDSIDE SINCE THIS AM—WHEN WILL THIS HAPPEN?” The phone number on the wall for the on-call radiologist beckons...
An all-too-familiar situation for hospitalists across the country, this awkward moment raises a series of difficult questions:
Should I set aside time from my day to perform a procedure that could be time-consuming?
- Do I feel confident I can perform this procedure safely?
- Am I really the best physician to provide this service?
- As hospitalists are tasked with an ever-increasing array of responsibilities, answering the call of duty for bedside procedures is becoming more difficult for some.
A Core Competency
“The Core Competencies in Hospital Medicine,” authored by a group of HM thought leaders, was published as a supplement to the January/February 2006 issue of the Journal of Hospital Medicine. The core competencies include such bedside procedures as arthrocentesis, paracentesis, thoracentesis, lumbar puncture, and vascular (arterial and central venous) access (see “Core Competencies in Hospital Medicine: Procedures,” below). Although the authors stressed that the core competencies are to be viewed as a resource rather than as a set of requirements, the inclusion of bedside procedures emphasized the importance of procedural skills for future hospitalists.
“[Hospitalists] are in a perfect spot to continue to perform procedures in a structured manner,” says Joshua Lenchus, DO, RPh, FACP, FHM, associate director of the University of Miami-Jackson Memorial Hospital (UM-JMH) Center for Patient Safety. “As agents of quality and safety, hospitalists should continue to perform this clinically necessary service.”
Jeffrey Barsuk, MD, FHM, associate professor of medicine at Northwestern Feinberg School of Medicine in Chicago and an academic hospitalist at Northwestern Memorial Hospital (NMH), not only agrees that bedside procedures should be a core competency, but he also says hospitalists are the most appropriate providers of these services.
“I think this is part of hospital medicine. We’re in the hospital, [and] that’s what we do,” Dr. Barsuk says. Other providers, such as interventional radiologists, “really don’t understand why I’m doing [a procedure]. They understand it’s safe to do it, but they might not understand all the indications for it, and they certainly don’t understand the interpretation of the tests they’re sending.”
Despite the goals set forth by the core competencies and authorities in procedural safety, the reality of who actually performs bedside procedures is somewhat murky and varies greatly by institution. Many point to HM program setting (urban vs. rural) or structure (academic vs. community) to explain variance, but often it is other factors that determine whether hospitalists are actually preforming bedside procedures regularly.
Where Does HM Perform Procedures?
Community hospitalists, with strong support from interventional radiologists and subspecialists, often find it more efficient—even necessary, considering their patient volumes—to leave procedures to others. Community hospitalists with ICU admitting privileges, intensivists, and other HM subgroups say that being able to perform procedures should be a prerequisite for employment. Hospitalists in rural communities say they are doing procedures because they are “the only game in town.”
“Sometimes you are the only one available, and you are called upon to stretch your abilities,” says Beatrice Szantyr, MD, FAAP, a community hospitalist and pediatrician in Lincoln, Maine, who has practiced most of her career in rural settings.
Academic hospitalists in large, research-based HM programs can, paradoxically, find themselves performing fewer procedures as residents often take the lead on the majority of such cases. Conversely, academic hospitalists in large, nonteaching programs often find themselves called on to perform more bedside procedures.
No matter the setting, the simplicity of being the physician to recognize the need for a procedure, perform it, and interpret the results is undeniably efficient and “clean,” according to authorities on inpatient bedside procedures. Having to consult other physicians, optimize the patient’s lab values to their standards (a common issue with interventional radiologists), and adhere to their work schedules can often delay procedures unnecessarily.
—Joshua Lenchus, DO, RPh, FACP, FHM, associate director, University of Miami-Jackson Memorial Hospital (UM-JMH) Center for Patient Safety
“Hospitalists care for floor and ICU patients in many hospitals, and the inability to perform bedside procedures delays patient care,” says Dr. Nilam Soni, an academic hospitalist at the University of Chicago and a recognized expert on procedural safety.
Dr. Soni notes that when it comes to current techniques, many hospitalists suffer from a knowledge deficit. “The introduction of ultrasound for guidance of bedside procedures has been shown to improve the success and safety of certain procedures,” he says, “but the majority of practicing hospitalists did not learn how to use ultrasound for procedure guidance during residency.”
Heterogeneity of Training, Experience, and Skill
While all hospitalists draw upon different bases of training and experience, the heterogeneity of training, confidence, and inherent skill is greatest when it comes to bedside procedures. Mirroring the heterogeneity at the individual level, hospitalist programs vary greatly on the requirements placed on their staffs in regards to procedural skill and privileging.
Such research-driven programs as Brigham and Women’s Hospital (BWH) in Boston often find requiring maintenance of privileges in bedside procedures to be difficult, says Sally Wang, MD, FHM, director of procedural education at BWH. In fact, a new procedure service being created there will be staffed mainly with ED physicians. On the flipside, most community hospitalist programs leave the task of procedural “policing” to the hospital’s medical staff affairs office.
At the University of California at San Diego (UCSD) Medical Center, the HM group is instituting a division standard in which hospitalists maintain privileging and proficiency in a core group of bedside procedures. Other large hospitalist groups have created “proceduralist” subgroups that shoulder the burden of trainee education, as well as provide a resource for less skilled or less experienced inpatient providers.
“If you have a big group, you could have a dedicated procedure service and have a core group of hospitalists who are experts in procedure,” Dr. Barsuk says. “But it needs to be self-sustaining.” Once started, Dr. Barsuk says, proceduralist groups would continue to provide hospitals with ongoing return-on-investment (ROI) benefit.
Variability in procedure volume and payor mix, however, can make it hard for HM groups to demonstrate to hospital leadership a satisfactory ROI for a proceduralist program. Financial backing from grant support or a high-volume procedure—such as paracentesis in hospitals with large hepatology programs—can nurture starting proceduralist programs until all procedural revenues can justify the costs. Lower ROI can also be justified by showing improvement in quality indices—such as CLABSI rates—reduced time to procedures, and reduced costs compared to other subspecialists offering similar services.
“I’m of the firm belief that we can reduce costs by doing the procedures at the bedside rather than referring them to departments such as interventional radiology (IR),” Dr. Barsuk says. “What you would have to do is show the institution that it costs more money to have IR do [bedside procedures].”
National Response

—Jeffrey Barsuk, MD, MS, FHM, associate professor of medicine, Northwestern Feinberg School of Medicine, academic hospitalist, Northwestern Memorial Hospital, Chicago
Filling in the procedural training gaps found on the local level, such national organizations as SHM have stepped in to provide education and support for hospitalists yearning for training. Since its inception, an SHM annual meeting pre-course that focuses on hand-held ultrasound and invasive procedures has consistently been one of the first to sell out. Other national organizations, such as ACP and its annual meeting, have seen similar interest in their courses on ultrasound-guided procedures.
The popularity of this continuing education bears out a worrisome trend: Hospitalists feel they are losing their procedural skills. An online survey conducted by The Hospitalist in May 2011 found that a majority of respondents (62%) had experienced deterioration of their procedural skills in the past five years; only 25% said they experienced improvement over the same period.
Historically, general internists have claimed bedside procedures as their domain. As stated dispassionately in the 1978 book The House of God, “There is no body cavity that cannot be reached with a #14G needle and a good strong arm.”1 Yet much has changed since Samuel Shem’s apocryphal description of medical residency training.
Most notably, the Accreditation Council of Graduate Medical Education (ACGME) has not only progressively restricted inpatient hours and patient loads for residents, but also increased the requirements for outpatient training. Some feel the balance of inpatient and outpatient training has tipped too far toward the latter in medicine residency programs, especially in light of the growing popularity of the hospitalist career path amongst new residency program graduates. This stands in contrast to ED training programs, which have embraced focused procedures training more readily.
“Adult care appears to be diverging into two career tacks as a result of external forces, of which we have limited control over, “ says Michael Beck, MD, a pediatric and adult hospitalist at Milton S. Hershey Medical Center in Hershey, Pa. “With new career choices emerging for graduates, the same square-peg, round-hole residency training should not exist.”
Dr. Beck advocates continuing an ongoing trend of “track” creation in residency programs, which allow trainees to focus training on their planned career path. Hospitalist tracks already exist in many medicine programs, including those at Cleveland Clinic and Northwestern. But many other factors limit the opportunity for trainees to obtain experience with bedside procedures, including competition with nurse practitioners and physician assistants. Even the increasing availability of ancillary phlebotomy and IV-start teams can increase a resident’s anxiety about procedures.
“By the time my residency was over [in 1993] and the work restrictions were beginning, hospital employees were doing all these tasks, making the residents less comfortable with hurting a patient when it was therapeutically necessary,” says Katharine Deiss, MD, assistant clinical professor of medicine at the University of Rochester Medical Center in New York. Interns who came from medical schools without extensive ancillary services in their teaching hospitals, she adds, were more comfortable with invasive procedures.
ACGME has sent a subtle message by decreasing emphasis on procedural skills by eradicating the requirement of showing manual proficiency in most bedside procedures as a requirement for certification. The omission has left individual residency programs and hospitalist groups to determine training and proficiency requirements for more invasive bedside procedures without a national standard.
In an editorial in the March 2007 issue of the Annals of Internal Medicine, F. Daniel Duffy, MD, and Eric Holmboe, MD, wrote that the American Board of Internal Medicine (ABIM) could only give a “qualified ‘yes’” to the question of whether residents should be trained in procedures they may not perform in practice. Although the authors asserted that the relaxed ABIM policy was “an important but small step toward revamping procedure skill training during residency,” others say it portrays an image of the ABIM de-emphasizing the importance of procedural training.
In addition, the recently established Focused Practice in Hospital Medicine (FPHM) pathway to ABIM Maintenance of Certification (MOC) has no requirement to show proficiency in bedside procedures.
“The absence of the procedural requirement in no way constitutes a statement that procedural skills are not important,” says Jeff Wiese, MD, FACP, SFHM, associate professor of medicine and residency program director at Tulane University Health Sciences Center in New Orleans, chair of the ABIM Hospital Medicine MOC Question Writing Committee, and former SHM president. “Rather, it is merely a practical issue with respect to making the MOC process applicable to all physicians engaged in hospital medicine (i.e. many hospitalists do not do procedures) while still making the MOC focused on the skill sets that are common for physicians doing hospital medicine.”
Once released into the world, even if trained well in residency, hospitalists can find it difficult to maintain their skills. In community and nonteaching settings, the pressure to admit and discharge in a timely manner can make procedures seem like the easiest corner to cut. Before long, it has been months since they have laid eyes on a needle of any sort. Many begin to develop performance anxiety.
In teaching hospitals, academic hospitalists often are called upon to participate in quality improvement (QI) and research efforts, which take time away from clinical rotations. Once there, it can be easy for a ward attending to rely upon a well-trained resident to supervise interns doing procedures. The lack of first-hand or even supervisory experience can lead to many academic hospitalists losing facility with procedures, with potentially disastrous results.
“In order to supervise a group of residents, the attending needs to be technically proficient and able to salvage a botched, or failed, procedure,” UM-JMH’s Dr. Lenchus says. “To this end, we strictly limit who can attend on the service.”
So what’s a residency or HM program director to do in the face of wavering support nationally, and sometimes locally, for maintaining procedural skills for hospitalists and trainees? Many hospitalists in teaching hospitals say it’s critical for clinicians to “get their own house in order,” to maintain procedural standards of proficiency with ongoing training, education, and verification.
“The profession now needs to redesign procedural training across the continuum of education and a lifetime of practice,” Drs. Duffy and Holmboe editorialized in the March 2007 Annals paper. “This approach would recognize the varied settings of internal-medicine practice and offer manual skills training to those whose practice settings require such skills.” Hospitalists can partner with medicine residency program leaders to provide procedural education and training to residents, either as a standalone elective or as a more general resource.
Hospitalists in such teaching hospitals as UCSD, Brigham and Women’s, UM-JMH, and Northwestern are leading efforts to provide procedural education to medical students, residents, and attendings. Training takes many forms, including formal procedural electives, required procedure rotations, or even brief one- or two-day courses in procedural skills at a simulation center.
Utilizing simulation training has been shown in many studies to be helpful in establishing procedural skills in learners of all training levels. Dr. Barsuk and his colleagues at Northwestern published studies in the Journal of Hospital Medicine in 2008 and 2009 showing that simulation training of residents was effective in improving skills in thoracentesis and central venous catheterization, respectively.3,4
In the community hospital setting, requirements for procedural skills can vary greatly based on the institution. For those community programs requiring procedural skills of their hospitalists, the clear definition of procedural training and requirements at the time of hiring is critical. Even after vetting a hospitalist’s procedural skills at hire, however, community programs should consider monitoring procedural skills and provide ongoing time and money for CME focused on procedural skills.
Currently, most hospitals depend on the honesty of individual physicians during the privileging process for bedside procedures. Even when the skills of physicians begin to wane, most are reluctant to voluntarily give up their procedure privileges.
“I think it would be pretty unusual for a hospitalist to relinquish their privileges,” Dr. Barsuk admits. But ideally, physicians who relinquish their privileges due to lack of experience could get retrained in simulation centers, then reproctored in order to regain their privileges. Northwestern established the Center for Simulation Technology and Immersive Learning as a resource for simulation training both locally and nationally.
Establishing an environment that supports hospitalists performing bedside procedures is critical. This includes the need to limit hospitalist workload to ensure adequate time to meet the procedural needs of patients. Providing easy access to the tools necessary to perform bedside procedures (e.g. portable ultrasound and pre-packaged procedure trays) helps avoid additional hurdles.
Academic hospitalist programs can serve as a regional resource by developing ongoing procedure mastery programs for hospitalists in their communities, as many smaller institutions do not have the resources to provide ongoing training in bedside procedures. This process can be tedious, but it should not be humiliating.
If the popularity of the SHM pre-course in bedside ultrasound and procedures is any indication, when given the opportunity to receive protected time for procedure training, most hospitalists will likely jump at the chance.
Dr. Chang is an associate clinical professor of medicine in the division of hospital medicine at Diego Medical Center. He is also a member of Team Hospitalist.
References
- Shem S. The House of God. New York: Dell Publishing; 1978.
- Duffy FD, Holmboe ES. What procedures should internists do? Ann Intern Med. 2007;146(5):392-393.
- Wayne DB, Barsuk JH, O’Leary KJ, Fudala MJ, McGaghie WC. Mastery learning of thoracentesis skills by internal medicine residents using simulation technology and deliberate practice. J Hosp Med. 2008;3(1):48-54.
- Barsuk JH, McGaghie WC, Cohen ER, Balachandran JS, Wayne DB. Use of simulation-based mastery learning to improve the quality of central venous catheter placement in a medical intensive care unit. J Hosp Med. 2009;4(7):397–403.
Project BOOST Helps California Hospital Improve Care
Soon, hospitals with unnecessary 30-day readmissions will be penalized. Some proactive hospitals are already tackling care transitions to reduce readmissions, improve patient care, and reduce costs.
Lodi Memorial Hospital in Northern California is one such hospital. The 214-bed facility has made preventing readmissions a key strategic goal for improving care, especially for vulnerable, frail elderly patients.
Lodi executives say readmissions are a challenge at the hospital because many different specialties and house staff are involved in the discharge process.
With many options from which to choose, Lodi selected SHM’s Project BOOST (Better Outcomes for Older Adults through Safe Transitions). Project BOOST, unlike other programs, focuses on first identifying system deficiencies, then building cohesive multidisciplinary teams.
“If you layer a clinical intervention onto a broken system,” says BOOST mentor Stephanie Rennke, MD, “success isn’t likely. Project BOOST helps the team map current processes, find deficiencies, assess resources, and redefine a culture of safety.
“BOOST starts with an assessment of how the system functions, and identifies its strengths and weaknesses so you can focus your efforts on critical areas for improvement and tailor the BOOST intervention to fit the unique dynamics of an institution,” she says.
Search for a Solution
No two institutions are exactly the same, especially when analyzing patient-safety culture. Project BOOST is customized to the needs of each BOOST site, which has proven useful to Lodi, as it found it had different needs than other hospitals.
“We chose Project BOOST because it seemed workable and implementable, while offering a less expensive and less complicated solution than competitors’ products,” says Valerie Cronin, Lodi’s director of utilization.
The BOOST mentor, key to this customization, provided Lodi with the reassurance that comes with working directly with someone who has faced similar challenges—not just theoretically, but also on the floor—and was willing to work with Lodi to develop practical solutions. The mentor provided reassurance, support, and perspective as Lodi walked through the BOOST implementation process.
Keys to Success
—Valerie Cronin, director of utilization, Lodi Memorial Hospital, California
Team-focused care, clear communication, and administrative support were keys to successful BOOST implementation at Lodi, says Cronin.
The house staff was overwhelmed, and adding a quality-improvement (QI) project to implement and manage might have seemed like an impossible challenge. By developing multidisciplinary teams, however, Lodi was able to distribute the tasks of implementation and began to recognize the value and benefit of Project BOOST, which already had strong support from hospital executives.
A 14-member multidisciplinary team was formed to oversee the Project BOOST implementation. The team then was divided into sub-groups to work on the main components of Project BOOST: Passport to Care Form, Target Assessment Tool, the Teachback process, and Follow-Up Phone Calls. The sub-groups’ main objectives were to ensure that BOOST effectively changed processes and work practices for a stronger and safer discharge process.
To support these teams and foster communication laterally among healthcare providers and vertically with hospital administration, Lodi established a structured meeting format, delegated task assignments for accountability, and appointed an implementation champion.
Capitalizing on the experience of its BOOST mentor, the Lodi multidisciplinary teams mapped out the process to assess threats to the system and opportunities for improvement, and began moving forward with implementable solutions for sustainable change.
“Evaluating the whole discharge process allowed us to see the gaps and discrepancies in the discharge process,” Cronin says. “Each discipline had their own set of procedures and materials, which proved lacking and inconsistent for our patients. It was an essential and eye-opening experience for Lodi to make change.”
One of the biggest revelations was learning how broken the discharge process was for the nurses. When Lodi looked at its current process of using case managers to handle high-risk patients and leaving the remaining patient discharges to the nurses, they found that the process was not strong enough to support patient load.
Knowledge Is Power
Cronin says Lodi has standardized its patient educational materials and started the patient education process as soon as patients are admitted. This step optimizes a patient’s understanding of diagnoses and care instructions when the time comes for discharge.
Through the Project BOOST assessment, Lodi ascertained that many of its patients were being discharged to skilled-nursing facilities, which heightens the complexity of post-care and introduces the potential for increased risks. Lodi is now working to better communicate with the skilled-nursing facilities using its Project BOOST training to streamline the discharge process.
The implementation of the Teach Back communication strategy has been critical in increasing patient knowledge and adherence to care instructions, official say. Based on the success of Project BOOST implementation, Teach Back has been incorporated into mandatory nurse training throughout the hospital.
Improved Care
More than 80 patients were discharged through the BOOST process in the first 90 days of implementation. Lodi is already experiencing the benefits of Project BOOST organizationally and
expects to see the financial impacts soon through a lower 30-day readmission rate.
“Lodi has experienced a shift in patient safety culture with improved communication through a team approach to care,” Cronin says. “Lodi expects high success through Project BOOST with the goal of implementing Project BOOST across all disease states and every discipline using Teachback.
“Project BOOST has been the ideal program to align with our strategic organizational goals to improve transitions of care and re-create the patient safety culture to make systemwide sustainable change,” she says.
For more information about Project BOOST, visit www.hospitalmedicine.org/boost.
Jacqui Petock, marketing project manager
Soon, hospitals with unnecessary 30-day readmissions will be penalized. Some proactive hospitals are already tackling care transitions to reduce readmissions, improve patient care, and reduce costs.
Lodi Memorial Hospital in Northern California is one such hospital. The 214-bed facility has made preventing readmissions a key strategic goal for improving care, especially for vulnerable, frail elderly patients.
Lodi executives say readmissions are a challenge at the hospital because many different specialties and house staff are involved in the discharge process.
With many options from which to choose, Lodi selected SHM’s Project BOOST (Better Outcomes for Older Adults through Safe Transitions). Project BOOST, unlike other programs, focuses on first identifying system deficiencies, then building cohesive multidisciplinary teams.
“If you layer a clinical intervention onto a broken system,” says BOOST mentor Stephanie Rennke, MD, “success isn’t likely. Project BOOST helps the team map current processes, find deficiencies, assess resources, and redefine a culture of safety.
“BOOST starts with an assessment of how the system functions, and identifies its strengths and weaknesses so you can focus your efforts on critical areas for improvement and tailor the BOOST intervention to fit the unique dynamics of an institution,” she says.
Search for a Solution
No two institutions are exactly the same, especially when analyzing patient-safety culture. Project BOOST is customized to the needs of each BOOST site, which has proven useful to Lodi, as it found it had different needs than other hospitals.
“We chose Project BOOST because it seemed workable and implementable, while offering a less expensive and less complicated solution than competitors’ products,” says Valerie Cronin, Lodi’s director of utilization.
The BOOST mentor, key to this customization, provided Lodi with the reassurance that comes with working directly with someone who has faced similar challenges—not just theoretically, but also on the floor—and was willing to work with Lodi to develop practical solutions. The mentor provided reassurance, support, and perspective as Lodi walked through the BOOST implementation process.
Keys to Success
—Valerie Cronin, director of utilization, Lodi Memorial Hospital, California
Team-focused care, clear communication, and administrative support were keys to successful BOOST implementation at Lodi, says Cronin.
The house staff was overwhelmed, and adding a quality-improvement (QI) project to implement and manage might have seemed like an impossible challenge. By developing multidisciplinary teams, however, Lodi was able to distribute the tasks of implementation and began to recognize the value and benefit of Project BOOST, which already had strong support from hospital executives.
A 14-member multidisciplinary team was formed to oversee the Project BOOST implementation. The team then was divided into sub-groups to work on the main components of Project BOOST: Passport to Care Form, Target Assessment Tool, the Teachback process, and Follow-Up Phone Calls. The sub-groups’ main objectives were to ensure that BOOST effectively changed processes and work practices for a stronger and safer discharge process.
To support these teams and foster communication laterally among healthcare providers and vertically with hospital administration, Lodi established a structured meeting format, delegated task assignments for accountability, and appointed an implementation champion.
Capitalizing on the experience of its BOOST mentor, the Lodi multidisciplinary teams mapped out the process to assess threats to the system and opportunities for improvement, and began moving forward with implementable solutions for sustainable change.
“Evaluating the whole discharge process allowed us to see the gaps and discrepancies in the discharge process,” Cronin says. “Each discipline had their own set of procedures and materials, which proved lacking and inconsistent for our patients. It was an essential and eye-opening experience for Lodi to make change.”
One of the biggest revelations was learning how broken the discharge process was for the nurses. When Lodi looked at its current process of using case managers to handle high-risk patients and leaving the remaining patient discharges to the nurses, they found that the process was not strong enough to support patient load.
Knowledge Is Power
Cronin says Lodi has standardized its patient educational materials and started the patient education process as soon as patients are admitted. This step optimizes a patient’s understanding of diagnoses and care instructions when the time comes for discharge.
Through the Project BOOST assessment, Lodi ascertained that many of its patients were being discharged to skilled-nursing facilities, which heightens the complexity of post-care and introduces the potential for increased risks. Lodi is now working to better communicate with the skilled-nursing facilities using its Project BOOST training to streamline the discharge process.
The implementation of the Teach Back communication strategy has been critical in increasing patient knowledge and adherence to care instructions, official say. Based on the success of Project BOOST implementation, Teach Back has been incorporated into mandatory nurse training throughout the hospital.
Improved Care
More than 80 patients were discharged through the BOOST process in the first 90 days of implementation. Lodi is already experiencing the benefits of Project BOOST organizationally and
expects to see the financial impacts soon through a lower 30-day readmission rate.
“Lodi has experienced a shift in patient safety culture with improved communication through a team approach to care,” Cronin says. “Lodi expects high success through Project BOOST with the goal of implementing Project BOOST across all disease states and every discipline using Teachback.
“Project BOOST has been the ideal program to align with our strategic organizational goals to improve transitions of care and re-create the patient safety culture to make systemwide sustainable change,” she says.
For more information about Project BOOST, visit www.hospitalmedicine.org/boost.
Jacqui Petock, marketing project manager
Soon, hospitals with unnecessary 30-day readmissions will be penalized. Some proactive hospitals are already tackling care transitions to reduce readmissions, improve patient care, and reduce costs.
Lodi Memorial Hospital in Northern California is one such hospital. The 214-bed facility has made preventing readmissions a key strategic goal for improving care, especially for vulnerable, frail elderly patients.
Lodi executives say readmissions are a challenge at the hospital because many different specialties and house staff are involved in the discharge process.
With many options from which to choose, Lodi selected SHM’s Project BOOST (Better Outcomes for Older Adults through Safe Transitions). Project BOOST, unlike other programs, focuses on first identifying system deficiencies, then building cohesive multidisciplinary teams.
“If you layer a clinical intervention onto a broken system,” says BOOST mentor Stephanie Rennke, MD, “success isn’t likely. Project BOOST helps the team map current processes, find deficiencies, assess resources, and redefine a culture of safety.
“BOOST starts with an assessment of how the system functions, and identifies its strengths and weaknesses so you can focus your efforts on critical areas for improvement and tailor the BOOST intervention to fit the unique dynamics of an institution,” she says.
Search for a Solution
No two institutions are exactly the same, especially when analyzing patient-safety culture. Project BOOST is customized to the needs of each BOOST site, which has proven useful to Lodi, as it found it had different needs than other hospitals.
“We chose Project BOOST because it seemed workable and implementable, while offering a less expensive and less complicated solution than competitors’ products,” says Valerie Cronin, Lodi’s director of utilization.
The BOOST mentor, key to this customization, provided Lodi with the reassurance that comes with working directly with someone who has faced similar challenges—not just theoretically, but also on the floor—and was willing to work with Lodi to develop practical solutions. The mentor provided reassurance, support, and perspective as Lodi walked through the BOOST implementation process.
Keys to Success
—Valerie Cronin, director of utilization, Lodi Memorial Hospital, California
Team-focused care, clear communication, and administrative support were keys to successful BOOST implementation at Lodi, says Cronin.
The house staff was overwhelmed, and adding a quality-improvement (QI) project to implement and manage might have seemed like an impossible challenge. By developing multidisciplinary teams, however, Lodi was able to distribute the tasks of implementation and began to recognize the value and benefit of Project BOOST, which already had strong support from hospital executives.
A 14-member multidisciplinary team was formed to oversee the Project BOOST implementation. The team then was divided into sub-groups to work on the main components of Project BOOST: Passport to Care Form, Target Assessment Tool, the Teachback process, and Follow-Up Phone Calls. The sub-groups’ main objectives were to ensure that BOOST effectively changed processes and work practices for a stronger and safer discharge process.
To support these teams and foster communication laterally among healthcare providers and vertically with hospital administration, Lodi established a structured meeting format, delegated task assignments for accountability, and appointed an implementation champion.
Capitalizing on the experience of its BOOST mentor, the Lodi multidisciplinary teams mapped out the process to assess threats to the system and opportunities for improvement, and began moving forward with implementable solutions for sustainable change.
“Evaluating the whole discharge process allowed us to see the gaps and discrepancies in the discharge process,” Cronin says. “Each discipline had their own set of procedures and materials, which proved lacking and inconsistent for our patients. It was an essential and eye-opening experience for Lodi to make change.”
One of the biggest revelations was learning how broken the discharge process was for the nurses. When Lodi looked at its current process of using case managers to handle high-risk patients and leaving the remaining patient discharges to the nurses, they found that the process was not strong enough to support patient load.
Knowledge Is Power
Cronin says Lodi has standardized its patient educational materials and started the patient education process as soon as patients are admitted. This step optimizes a patient’s understanding of diagnoses and care instructions when the time comes for discharge.
Through the Project BOOST assessment, Lodi ascertained that many of its patients were being discharged to skilled-nursing facilities, which heightens the complexity of post-care and introduces the potential for increased risks. Lodi is now working to better communicate with the skilled-nursing facilities using its Project BOOST training to streamline the discharge process.
The implementation of the Teach Back communication strategy has been critical in increasing patient knowledge and adherence to care instructions, official say. Based on the success of Project BOOST implementation, Teach Back has been incorporated into mandatory nurse training throughout the hospital.
Improved Care
More than 80 patients were discharged through the BOOST process in the first 90 days of implementation. Lodi is already experiencing the benefits of Project BOOST organizationally and
expects to see the financial impacts soon through a lower 30-day readmission rate.
“Lodi has experienced a shift in patient safety culture with improved communication through a team approach to care,” Cronin says. “Lodi expects high success through Project BOOST with the goal of implementing Project BOOST across all disease states and every discipline using Teachback.
“Project BOOST has been the ideal program to align with our strategic organizational goals to improve transitions of care and re-create the patient safety culture to make systemwide sustainable change,” she says.
For more information about Project BOOST, visit www.hospitalmedicine.org/boost.
Jacqui Petock, marketing project manager
A Winnable Battle
Redness, pain, and drainage from the skin around a catheter. Bloody, cloudy, or pungent urine and mental confusion. Severe diarrhea and a dilated colon: The potential symptoms of some of the most common hospital-acquired infections in the United States aren’t particularly pleasant.
The numbers don’t paint a pretty picture, either.
One widely cited study estimates that the nation’s hospitals report 1.7 million healthcare-associated infections (HAIs) every year, along with nearly 100,000 associated deaths.1 All told, the additional healthcare costs could tally $30 billion or more annually, according to a 2009 Centers for Disease Control and Prevention (CDC) analysis. Though some bright spots have emerged, healthcare advocates, including Kevin Kavanagh, MD, MS, FACS, director of Kentucky-based patient advocacy group Health Watch USA, contend that the overall size of the problem is likely underestimated. And unless brought under control, he says, emerging pathogens could make a bad situation worse.
The incredible variety of healthcare settings precludes any across-the-board solutions for hospitalists and other care providers. A recent survey of more than 1,200 healthcare professionals from 33 hospitals, however, revealed some common themes. According to the study authors, the data show that “hand hygiene is consistently identified as the greatest barrier to reducing HAIs, and that sustaining the necessary behavioral change to overcome this barrier is difficult.”2 In fact, the study highlighted a widespread belief that medical staff, and doctors in particular, are not fully engaged or in compliance with HAI reduction efforts. “The data suggest that physicians often do not adhere to protocols and guidelines; moreover, some respondents questioned whether many physicians are open to change,” the authors wrote.
So what can hospitalists do to help their colleagues embrace a culture of positive change and turn back a worsening healthcare epidemic? If simple answers are in short supply, studies aimed at central-line-associated bloodstream infections, catheter-associated urinary tract infections, and Clostridium difficile infections have at least highlighted some keys to a successful intervention.
In Focus: Central Lines
Central-line-associated bloodstream infections (CLABSIs) arguably have received the most attention of any HAI. Not coincidentally, most researchers point to the anti-CLABSI effort as the high point in the struggle to reduce infection rates.
In a landmark 2006 study led by hospitalist Peter Pronovost, MD, more than 100 ICUs in Michigan nearly eliminated CLABSIs over an 18-month period.3 The “remarkably successful” intervention, according to its authors, focused on changing provider behavior through education and collaboration with infection-control specialists. Nationwide, a recent CDC study estimated that absolute CLABSI numbers in ICUs dropped to 18,000 in 2009 from 43,000 in 2001, a 58% decline.4
Sheri Chernetsky Tejedor, MD, SFHM, assistant professor in the division of hospital medicine at Emory University School of Medicine in Atlanta, points out a major caveat in CLABSI prevention efforts, however: To date, she says, most have targeted insertion practices and ICU patients, which do little for the more than half of CLABSIs that occur on hospital wards. These wards thus represent “a ripe opportunity for intervention, especially the low-hanging, ‘low tech’ interventions of removing unnecessary devices,” she says.
Among a small random sample of patients with temporary central venous lines (CVCs), including peripherally inserted central catheters (PICCs), a study led by Dr. Chernetsky Tejedor found that 25% of all CVC days were “idle.”5 In other words, in 1 out of every 4 CVC days, the catheter was retained despite failing to meet standard justification criteria. In particular, the study suggested that PICCs were associated with longer catheter use and more idle days, fueling her group’s suspicion that increased PICC availability has changed CVC use patterns.
Among her group’s interventions, a patient safety checklist has been built, with questions about catheter use entered into a daily electronic progress note used by all services on the inpatient wards. One particularly successful strategy empowered nurses to determine whether continued catheter use seems justified and to ask physicians whether an “idle” CVC could be removed.
With a decline in CLABSI rates in ICUs and a shift in focus to the medical wards, Dr. Chernetsky Tejedor says, hospitalists are in a prime position to help reduce the inappropriate use of catheters and other invasive devices. More broadly, hospitalists may be well-suited to help change the culture of medicine toward a wider acceptance of proven interventions, such as central-line checklists.
Dr. Kavanagh argues that the slow and arduous process to move past what he calls “a huge resistance to checklists” and win universal adoption of such protocols “is not a good chapter in medicine.” He isn’t laying the blame at the feet of doctors alone, however. “In some institutions,” he says, “there needs to be a change from a profit-driven to a patient-centered culture.”
Greg Maynard, MD, MSc, SFHM, director of the University of California at San Diego Center for Innovation and Improvement Science and senior vice president of SHM’s Center for Hospital Innovation and Improvement, acknowledges the integral role of checklists in improvement efforts. He cautions, however, that they should be viewed as only one part of a multipronged approach.
As with hand hygiene, Dr. Maynard concedes that some facilities are still facing resistance from medical staff in integrating checklists into their routines, though he argues that an ingrained anti-checklist medical culture may not be solely at fault. “If you insert a checklist in such a way that it’s very inconvenient, that stops the flow of work,” he says, “you’re not going to have as much success as if you’ve thoughtfully designed it so that the checklist is called into play when it makes sense to bring it into play and made it easy to do, as opposed to difficult and awkward to do.”
In Focus: Catheter-Associated UTIs
Catheter-associated urinary tract infections (CAUTIs) account for roughly 1 in 3 healthcare-associated infections, according to the CDC. And yet many researchers say the “Rodney Dangerfield of HAIs” has long been overlooked.
A frequently cited study led by Sanjay Saint, MD, MPH, FHM, professor of internal medicine at the University of Michigan and the Ann Arbor VA Medical Center, found that nearly 40% of attending physicians were unaware that their patients even had an indwelling urinary catheter.6
Dr. Saint has dubbed the phenomenon “immaculate catheterization” to highlight the glaring discrepancy. “Because we also found, in a significant number of patients, there was no documentation anywhere in the medical record that the catheter existed,” he says. “It’s not in the physician’s notes, it’s not in the nursing notes, but we knew it was there because we could see it coming out of the patient.” Catheters that were inappropriately placed, he found, were more often “forgotten” than appropriate ones.
Perhaps more than anything else, the startling observation underscores the intense need for basic awareness of the two biggest UTI risk factors: whether a catheter is used, and how long it’s left in place. “You can get marked reductions by just taking care of those two factors,” Health Watch USA’s Dr. Kavanagh says.
At the Ann Arbor VA Hospital, one unit’s presence of a “Patient Safety Professional” to help ensure better oversight virtually brought the inappropriate use of indwelling catheters to a standstill. Dr. Saint and his colleagues are now gathering data to determine whether that decrease has translated to a drop in infections.

—Greg Maynard, MD, MSc, SFHM, director, University of California at San Diego Center for Innovation and Improvement Science, senior vice president, SHM Center for Hospital Innovation and Improvement
One fundamental key, he says, is paying close attention to whether a catheter is really in the patient’s best interests. “If we ask that question—‘If this was my family member, what would I want?’—we usually do the right thing,” Dr. Saint explains. Another key is leveraging the hospitalist’s core skill in communicating often and well with nurses to ensure that they are in sync during the “team sport” of CAUTI prevention.
With pockets of success in reducing inappropriate catheterization, the larger question now is how to scale up the individual interventions to achieve nationwide reductions. “How do you take what will work in one hospital, given its culture and microculture, and then apply it to hospitals across the state, or even across the country?” Dr. Saint asks.
Karen Clarke, MD, MS, MPH, a hospitalist and assistant professor of medicine at Emory University Hospital in Atlanta, is in the midst of tackling such issues. Using a bundled approach that included four interventions, she and colleagues reduced CAUTI rates by 70% at 276-bed West Georgia Medical Center in La Grange, Ga.7
The interventions were straightforward and inexpensive, Dr. Clarke says, meaning that they could be widely applied. “The only thing is that there has to be a champion overseeing the interventions to make sure that the steps are followed through on,” she says. Even at cash-strapped facilities, then, a similar approach could prove effective as long as someone assumes responsibility—and hospitalists would be a natural choice.
Based on her study’s promising results, Dr. Clarke hopes to begin implementing the intervention in at least one other hospital starting Jan. 1. If the success can be replicated, she says, the CAUTI-reduction protocol will branch out to include more regional hospitals.
In Focus: C. Diff-Associated Disease
Even as many hospitals are improving their CLABSI and CAUTI rates, hospital-acquired Clostridium difficile infections appear to be getting worse, particularly among older patients. In some facilities, the potentially fatal, diarrhea-causing microbe is now the top pathogen (see “Gut Reaction,” December 2011).
With a timely intervention, however, Kaiser Permanente Medical Center in Santa Clara, Calif., cut its own infection rates by one-third.8 In brainstorming how to improve the medical center’s rates, Susanne Mierendorf, MD, MS, FHM, a hospitalist and associate residency program director for internal medicine, joined colleagues in thinking through the barriers for healthcare providers. “It wasn’t ‘Why don’t they follow the infection-control guidelines?’ It was, ‘Why can’t they?’” Dr. Mierendorf says.
The thought exercise led to some eye-opening observations, including the realization that disposable gowns, gloves, and other personal protective equipment weren’t in the room and were hard to find. To help establish habits, Dr. Mierendorf’s team picked a consistent drawer in each patient’s room to stow the equipment and instructed that a wall-mounted holder be filled with gloves at all times.
The researchers also realized that the rooms of patients with suspected or confirmed C. diff infections had warning signs that were too simplistic at first, then overly wordy. Both were being ignored. The solution was simple signage with yellow color-coding and easily recognizable symbols that readily conveyed the infection-control message to staff: sterile gowns, gloves, hands with soap and water, bleach. Those messages were reinforced through a brief, simple, and mandatory educational module for all hospital workers who might come into contact with the patients.
National Implications
On a national scale, hospitalists have helped compile other educational packets and toolkits to address the spectrum of HAIs, from ventilator-associated pneumonia to methicillin-resistant Staphylococcus aureus (MRSA).
More help may be forthcoming through the American Hospital Association’s Health Research and Education Trust. The broad-based effort, funded by the federal Agency for Healthcare Research and Quality (AHRQ), is partnering with SHM and other professional societies to implement small HAI-reduction successes on ever-wider scales. The University of Michigan’s Dr. Saint, for example, is a key partner in the trust’s national initiative to reduce CAUTI rates and improve patient safety.

—Sanjay Saint, MD, MPH, FHM, professor of internal medicine, University of Michigan, Ann Arbor
Medicare has rolled out its own collection of carrots and sticks to address the problem. The largest carrot, the public-private Partnership for Patients, has joined with SHM, other professional societies, and roughly 3,000 hospitals, so far, and set the ambitious goal of reducing hospital-acquired conditions by 40% by the end of 2013, compared with 2010 tallies. Although applauding the program’s overall goals, physicians, including Dr. Maynard, are taking a wait-and-see attitude, pointing out that many of the details have yet to be ironed out.
Among the sticks, Medicare has begun withholding reimbursement payments for such HAIs as CLABSIs and CAUTIs. Due to intricacies in how hospitals bill under the current DRG system, Dr. Kavanagh says Medicare’s nonpayment policy has recouped relatively little money from its first full year: about $18.8 million in all.
“The policies that cover public reporting, however, do have much more of an impact,” he says. “It’s more of a perceptual sting. Believe me, it is more concerning to have data of bad results that are available to the public than to be penalized by the current financial incentives.”
Other financial policies could carry considerably more weight. The threat of nonpayment for hospital readmissions, Dr. Maynard says, “totally changed the game” by intensifying efforts to reduce those rates, addressing contributing factors such as HAIs in the process. In combination, he says, public accountability through mandated reporting plus financial penalties could prove more powerful than either tactic alone.
Regardless of how federal policy plays out, experts say a new era of accountability and transparency is on its way. As the champions of positive change, hospitalists have a distinct opportunity to help lead the way and bring about a culture that consistently embraces effective interventions—before things get really ugly.
Bryn Nelson is a freelance medical writer based in Seattle.
References
- Klevens RM, Edwards JR, Richards CL, Horan TC, et al. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122:160-166.
- Flanagan ME, Welsh CA, Kiess C, Hoke S, et al. A national collaborative for reducing health care-associated infections: current initiatives, challenges, and opportunities. Am J Infect Control. 2011;39:685-689.
- Pronovost P, Needham D, Berenholtz S, Sinopoli D, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355:2725-2732.
- Srinivasan A, Wise M, Bell M, Cardo D, et al. Vital signs: central line-associated blood stream infections—United States, 2001, 2008, and 2009. MMWR. 2011;60(8):243-248.
- Chernetsky Tejedor S, Tong D, Stein J, Payne C, et al. Temporary central venous catheter utilization patterns in a large tertiary care center: tracking the “idle central venous catheter.” Infect Control Hosp Epidemiol. 2012;33(1): in press.
- Saint S, Wiese J, Amory JK, Bernstein ML, et al. Are physicians aware of which of their patients have indwelling urinary catheters? Am J Med. 2000;109(6):476-480.
- Clarke K, Norrick B, Easley K, Pan Y, et al. Reduction of catheter-associated urinary tract infections through a bundled intervention in a community hospital. J Hosp Med. 2011;6(4):S22.
- Mierendorf S, Rushton M. Decreasing barriers in prevention of hospital-acquired Clostridium difficile colitis. J Hosp Med. 2011;6(4):S50-S51.
Redness, pain, and drainage from the skin around a catheter. Bloody, cloudy, or pungent urine and mental confusion. Severe diarrhea and a dilated colon: The potential symptoms of some of the most common hospital-acquired infections in the United States aren’t particularly pleasant.
The numbers don’t paint a pretty picture, either.
One widely cited study estimates that the nation’s hospitals report 1.7 million healthcare-associated infections (HAIs) every year, along with nearly 100,000 associated deaths.1 All told, the additional healthcare costs could tally $30 billion or more annually, according to a 2009 Centers for Disease Control and Prevention (CDC) analysis. Though some bright spots have emerged, healthcare advocates, including Kevin Kavanagh, MD, MS, FACS, director of Kentucky-based patient advocacy group Health Watch USA, contend that the overall size of the problem is likely underestimated. And unless brought under control, he says, emerging pathogens could make a bad situation worse.
The incredible variety of healthcare settings precludes any across-the-board solutions for hospitalists and other care providers. A recent survey of more than 1,200 healthcare professionals from 33 hospitals, however, revealed some common themes. According to the study authors, the data show that “hand hygiene is consistently identified as the greatest barrier to reducing HAIs, and that sustaining the necessary behavioral change to overcome this barrier is difficult.”2 In fact, the study highlighted a widespread belief that medical staff, and doctors in particular, are not fully engaged or in compliance with HAI reduction efforts. “The data suggest that physicians often do not adhere to protocols and guidelines; moreover, some respondents questioned whether many physicians are open to change,” the authors wrote.
So what can hospitalists do to help their colleagues embrace a culture of positive change and turn back a worsening healthcare epidemic? If simple answers are in short supply, studies aimed at central-line-associated bloodstream infections, catheter-associated urinary tract infections, and Clostridium difficile infections have at least highlighted some keys to a successful intervention.
In Focus: Central Lines
Central-line-associated bloodstream infections (CLABSIs) arguably have received the most attention of any HAI. Not coincidentally, most researchers point to the anti-CLABSI effort as the high point in the struggle to reduce infection rates.
In a landmark 2006 study led by hospitalist Peter Pronovost, MD, more than 100 ICUs in Michigan nearly eliminated CLABSIs over an 18-month period.3 The “remarkably successful” intervention, according to its authors, focused on changing provider behavior through education and collaboration with infection-control specialists. Nationwide, a recent CDC study estimated that absolute CLABSI numbers in ICUs dropped to 18,000 in 2009 from 43,000 in 2001, a 58% decline.4
Sheri Chernetsky Tejedor, MD, SFHM, assistant professor in the division of hospital medicine at Emory University School of Medicine in Atlanta, points out a major caveat in CLABSI prevention efforts, however: To date, she says, most have targeted insertion practices and ICU patients, which do little for the more than half of CLABSIs that occur on hospital wards. These wards thus represent “a ripe opportunity for intervention, especially the low-hanging, ‘low tech’ interventions of removing unnecessary devices,” she says.
Among a small random sample of patients with temporary central venous lines (CVCs), including peripherally inserted central catheters (PICCs), a study led by Dr. Chernetsky Tejedor found that 25% of all CVC days were “idle.”5 In other words, in 1 out of every 4 CVC days, the catheter was retained despite failing to meet standard justification criteria. In particular, the study suggested that PICCs were associated with longer catheter use and more idle days, fueling her group’s suspicion that increased PICC availability has changed CVC use patterns.
Among her group’s interventions, a patient safety checklist has been built, with questions about catheter use entered into a daily electronic progress note used by all services on the inpatient wards. One particularly successful strategy empowered nurses to determine whether continued catheter use seems justified and to ask physicians whether an “idle” CVC could be removed.
With a decline in CLABSI rates in ICUs and a shift in focus to the medical wards, Dr. Chernetsky Tejedor says, hospitalists are in a prime position to help reduce the inappropriate use of catheters and other invasive devices. More broadly, hospitalists may be well-suited to help change the culture of medicine toward a wider acceptance of proven interventions, such as central-line checklists.
Dr. Kavanagh argues that the slow and arduous process to move past what he calls “a huge resistance to checklists” and win universal adoption of such protocols “is not a good chapter in medicine.” He isn’t laying the blame at the feet of doctors alone, however. “In some institutions,” he says, “there needs to be a change from a profit-driven to a patient-centered culture.”
Greg Maynard, MD, MSc, SFHM, director of the University of California at San Diego Center for Innovation and Improvement Science and senior vice president of SHM’s Center for Hospital Innovation and Improvement, acknowledges the integral role of checklists in improvement efforts. He cautions, however, that they should be viewed as only one part of a multipronged approach.
As with hand hygiene, Dr. Maynard concedes that some facilities are still facing resistance from medical staff in integrating checklists into their routines, though he argues that an ingrained anti-checklist medical culture may not be solely at fault. “If you insert a checklist in such a way that it’s very inconvenient, that stops the flow of work,” he says, “you’re not going to have as much success as if you’ve thoughtfully designed it so that the checklist is called into play when it makes sense to bring it into play and made it easy to do, as opposed to difficult and awkward to do.”
In Focus: Catheter-Associated UTIs
Catheter-associated urinary tract infections (CAUTIs) account for roughly 1 in 3 healthcare-associated infections, according to the CDC. And yet many researchers say the “Rodney Dangerfield of HAIs” has long been overlooked.
A frequently cited study led by Sanjay Saint, MD, MPH, FHM, professor of internal medicine at the University of Michigan and the Ann Arbor VA Medical Center, found that nearly 40% of attending physicians were unaware that their patients even had an indwelling urinary catheter.6
Dr. Saint has dubbed the phenomenon “immaculate catheterization” to highlight the glaring discrepancy. “Because we also found, in a significant number of patients, there was no documentation anywhere in the medical record that the catheter existed,” he says. “It’s not in the physician’s notes, it’s not in the nursing notes, but we knew it was there because we could see it coming out of the patient.” Catheters that were inappropriately placed, he found, were more often “forgotten” than appropriate ones.
Perhaps more than anything else, the startling observation underscores the intense need for basic awareness of the two biggest UTI risk factors: whether a catheter is used, and how long it’s left in place. “You can get marked reductions by just taking care of those two factors,” Health Watch USA’s Dr. Kavanagh says.
At the Ann Arbor VA Hospital, one unit’s presence of a “Patient Safety Professional” to help ensure better oversight virtually brought the inappropriate use of indwelling catheters to a standstill. Dr. Saint and his colleagues are now gathering data to determine whether that decrease has translated to a drop in infections.

—Greg Maynard, MD, MSc, SFHM, director, University of California at San Diego Center for Innovation and Improvement Science, senior vice president, SHM Center for Hospital Innovation and Improvement
One fundamental key, he says, is paying close attention to whether a catheter is really in the patient’s best interests. “If we ask that question—‘If this was my family member, what would I want?’—we usually do the right thing,” Dr. Saint explains. Another key is leveraging the hospitalist’s core skill in communicating often and well with nurses to ensure that they are in sync during the “team sport” of CAUTI prevention.
With pockets of success in reducing inappropriate catheterization, the larger question now is how to scale up the individual interventions to achieve nationwide reductions. “How do you take what will work in one hospital, given its culture and microculture, and then apply it to hospitals across the state, or even across the country?” Dr. Saint asks.
Karen Clarke, MD, MS, MPH, a hospitalist and assistant professor of medicine at Emory University Hospital in Atlanta, is in the midst of tackling such issues. Using a bundled approach that included four interventions, she and colleagues reduced CAUTI rates by 70% at 276-bed West Georgia Medical Center in La Grange, Ga.7
The interventions were straightforward and inexpensive, Dr. Clarke says, meaning that they could be widely applied. “The only thing is that there has to be a champion overseeing the interventions to make sure that the steps are followed through on,” she says. Even at cash-strapped facilities, then, a similar approach could prove effective as long as someone assumes responsibility—and hospitalists would be a natural choice.
Based on her study’s promising results, Dr. Clarke hopes to begin implementing the intervention in at least one other hospital starting Jan. 1. If the success can be replicated, she says, the CAUTI-reduction protocol will branch out to include more regional hospitals.
In Focus: C. Diff-Associated Disease
Even as many hospitals are improving their CLABSI and CAUTI rates, hospital-acquired Clostridium difficile infections appear to be getting worse, particularly among older patients. In some facilities, the potentially fatal, diarrhea-causing microbe is now the top pathogen (see “Gut Reaction,” December 2011).
With a timely intervention, however, Kaiser Permanente Medical Center in Santa Clara, Calif., cut its own infection rates by one-third.8 In brainstorming how to improve the medical center’s rates, Susanne Mierendorf, MD, MS, FHM, a hospitalist and associate residency program director for internal medicine, joined colleagues in thinking through the barriers for healthcare providers. “It wasn’t ‘Why don’t they follow the infection-control guidelines?’ It was, ‘Why can’t they?’” Dr. Mierendorf says.
The thought exercise led to some eye-opening observations, including the realization that disposable gowns, gloves, and other personal protective equipment weren’t in the room and were hard to find. To help establish habits, Dr. Mierendorf’s team picked a consistent drawer in each patient’s room to stow the equipment and instructed that a wall-mounted holder be filled with gloves at all times.
The researchers also realized that the rooms of patients with suspected or confirmed C. diff infections had warning signs that were too simplistic at first, then overly wordy. Both were being ignored. The solution was simple signage with yellow color-coding and easily recognizable symbols that readily conveyed the infection-control message to staff: sterile gowns, gloves, hands with soap and water, bleach. Those messages were reinforced through a brief, simple, and mandatory educational module for all hospital workers who might come into contact with the patients.
National Implications
On a national scale, hospitalists have helped compile other educational packets and toolkits to address the spectrum of HAIs, from ventilator-associated pneumonia to methicillin-resistant Staphylococcus aureus (MRSA).
More help may be forthcoming through the American Hospital Association’s Health Research and Education Trust. The broad-based effort, funded by the federal Agency for Healthcare Research and Quality (AHRQ), is partnering with SHM and other professional societies to implement small HAI-reduction successes on ever-wider scales. The University of Michigan’s Dr. Saint, for example, is a key partner in the trust’s national initiative to reduce CAUTI rates and improve patient safety.

—Sanjay Saint, MD, MPH, FHM, professor of internal medicine, University of Michigan, Ann Arbor
Medicare has rolled out its own collection of carrots and sticks to address the problem. The largest carrot, the public-private Partnership for Patients, has joined with SHM, other professional societies, and roughly 3,000 hospitals, so far, and set the ambitious goal of reducing hospital-acquired conditions by 40% by the end of 2013, compared with 2010 tallies. Although applauding the program’s overall goals, physicians, including Dr. Maynard, are taking a wait-and-see attitude, pointing out that many of the details have yet to be ironed out.
Among the sticks, Medicare has begun withholding reimbursement payments for such HAIs as CLABSIs and CAUTIs. Due to intricacies in how hospitals bill under the current DRG system, Dr. Kavanagh says Medicare’s nonpayment policy has recouped relatively little money from its first full year: about $18.8 million in all.
“The policies that cover public reporting, however, do have much more of an impact,” he says. “It’s more of a perceptual sting. Believe me, it is more concerning to have data of bad results that are available to the public than to be penalized by the current financial incentives.”
Other financial policies could carry considerably more weight. The threat of nonpayment for hospital readmissions, Dr. Maynard says, “totally changed the game” by intensifying efforts to reduce those rates, addressing contributing factors such as HAIs in the process. In combination, he says, public accountability through mandated reporting plus financial penalties could prove more powerful than either tactic alone.
Regardless of how federal policy plays out, experts say a new era of accountability and transparency is on its way. As the champions of positive change, hospitalists have a distinct opportunity to help lead the way and bring about a culture that consistently embraces effective interventions—before things get really ugly.
Bryn Nelson is a freelance medical writer based in Seattle.
References
- Klevens RM, Edwards JR, Richards CL, Horan TC, et al. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122:160-166.
- Flanagan ME, Welsh CA, Kiess C, Hoke S, et al. A national collaborative for reducing health care-associated infections: current initiatives, challenges, and opportunities. Am J Infect Control. 2011;39:685-689.
- Pronovost P, Needham D, Berenholtz S, Sinopoli D, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355:2725-2732.
- Srinivasan A, Wise M, Bell M, Cardo D, et al. Vital signs: central line-associated blood stream infections—United States, 2001, 2008, and 2009. MMWR. 2011;60(8):243-248.
- Chernetsky Tejedor S, Tong D, Stein J, Payne C, et al. Temporary central venous catheter utilization patterns in a large tertiary care center: tracking the “idle central venous catheter.” Infect Control Hosp Epidemiol. 2012;33(1): in press.
- Saint S, Wiese J, Amory JK, Bernstein ML, et al. Are physicians aware of which of their patients have indwelling urinary catheters? Am J Med. 2000;109(6):476-480.
- Clarke K, Norrick B, Easley K, Pan Y, et al. Reduction of catheter-associated urinary tract infections through a bundled intervention in a community hospital. J Hosp Med. 2011;6(4):S22.
- Mierendorf S, Rushton M. Decreasing barriers in prevention of hospital-acquired Clostridium difficile colitis. J Hosp Med. 2011;6(4):S50-S51.
Redness, pain, and drainage from the skin around a catheter. Bloody, cloudy, or pungent urine and mental confusion. Severe diarrhea and a dilated colon: The potential symptoms of some of the most common hospital-acquired infections in the United States aren’t particularly pleasant.
The numbers don’t paint a pretty picture, either.
One widely cited study estimates that the nation’s hospitals report 1.7 million healthcare-associated infections (HAIs) every year, along with nearly 100,000 associated deaths.1 All told, the additional healthcare costs could tally $30 billion or more annually, according to a 2009 Centers for Disease Control and Prevention (CDC) analysis. Though some bright spots have emerged, healthcare advocates, including Kevin Kavanagh, MD, MS, FACS, director of Kentucky-based patient advocacy group Health Watch USA, contend that the overall size of the problem is likely underestimated. And unless brought under control, he says, emerging pathogens could make a bad situation worse.
The incredible variety of healthcare settings precludes any across-the-board solutions for hospitalists and other care providers. A recent survey of more than 1,200 healthcare professionals from 33 hospitals, however, revealed some common themes. According to the study authors, the data show that “hand hygiene is consistently identified as the greatest barrier to reducing HAIs, and that sustaining the necessary behavioral change to overcome this barrier is difficult.”2 In fact, the study highlighted a widespread belief that medical staff, and doctors in particular, are not fully engaged or in compliance with HAI reduction efforts. “The data suggest that physicians often do not adhere to protocols and guidelines; moreover, some respondents questioned whether many physicians are open to change,” the authors wrote.
So what can hospitalists do to help their colleagues embrace a culture of positive change and turn back a worsening healthcare epidemic? If simple answers are in short supply, studies aimed at central-line-associated bloodstream infections, catheter-associated urinary tract infections, and Clostridium difficile infections have at least highlighted some keys to a successful intervention.
In Focus: Central Lines
Central-line-associated bloodstream infections (CLABSIs) arguably have received the most attention of any HAI. Not coincidentally, most researchers point to the anti-CLABSI effort as the high point in the struggle to reduce infection rates.
In a landmark 2006 study led by hospitalist Peter Pronovost, MD, more than 100 ICUs in Michigan nearly eliminated CLABSIs over an 18-month period.3 The “remarkably successful” intervention, according to its authors, focused on changing provider behavior through education and collaboration with infection-control specialists. Nationwide, a recent CDC study estimated that absolute CLABSI numbers in ICUs dropped to 18,000 in 2009 from 43,000 in 2001, a 58% decline.4
Sheri Chernetsky Tejedor, MD, SFHM, assistant professor in the division of hospital medicine at Emory University School of Medicine in Atlanta, points out a major caveat in CLABSI prevention efforts, however: To date, she says, most have targeted insertion practices and ICU patients, which do little for the more than half of CLABSIs that occur on hospital wards. These wards thus represent “a ripe opportunity for intervention, especially the low-hanging, ‘low tech’ interventions of removing unnecessary devices,” she says.
Among a small random sample of patients with temporary central venous lines (CVCs), including peripherally inserted central catheters (PICCs), a study led by Dr. Chernetsky Tejedor found that 25% of all CVC days were “idle.”5 In other words, in 1 out of every 4 CVC days, the catheter was retained despite failing to meet standard justification criteria. In particular, the study suggested that PICCs were associated with longer catheter use and more idle days, fueling her group’s suspicion that increased PICC availability has changed CVC use patterns.
Among her group’s interventions, a patient safety checklist has been built, with questions about catheter use entered into a daily electronic progress note used by all services on the inpatient wards. One particularly successful strategy empowered nurses to determine whether continued catheter use seems justified and to ask physicians whether an “idle” CVC could be removed.
With a decline in CLABSI rates in ICUs and a shift in focus to the medical wards, Dr. Chernetsky Tejedor says, hospitalists are in a prime position to help reduce the inappropriate use of catheters and other invasive devices. More broadly, hospitalists may be well-suited to help change the culture of medicine toward a wider acceptance of proven interventions, such as central-line checklists.
Dr. Kavanagh argues that the slow and arduous process to move past what he calls “a huge resistance to checklists” and win universal adoption of such protocols “is not a good chapter in medicine.” He isn’t laying the blame at the feet of doctors alone, however. “In some institutions,” he says, “there needs to be a change from a profit-driven to a patient-centered culture.”
Greg Maynard, MD, MSc, SFHM, director of the University of California at San Diego Center for Innovation and Improvement Science and senior vice president of SHM’s Center for Hospital Innovation and Improvement, acknowledges the integral role of checklists in improvement efforts. He cautions, however, that they should be viewed as only one part of a multipronged approach.
As with hand hygiene, Dr. Maynard concedes that some facilities are still facing resistance from medical staff in integrating checklists into their routines, though he argues that an ingrained anti-checklist medical culture may not be solely at fault. “If you insert a checklist in such a way that it’s very inconvenient, that stops the flow of work,” he says, “you’re not going to have as much success as if you’ve thoughtfully designed it so that the checklist is called into play when it makes sense to bring it into play and made it easy to do, as opposed to difficult and awkward to do.”
In Focus: Catheter-Associated UTIs
Catheter-associated urinary tract infections (CAUTIs) account for roughly 1 in 3 healthcare-associated infections, according to the CDC. And yet many researchers say the “Rodney Dangerfield of HAIs” has long been overlooked.
A frequently cited study led by Sanjay Saint, MD, MPH, FHM, professor of internal medicine at the University of Michigan and the Ann Arbor VA Medical Center, found that nearly 40% of attending physicians were unaware that their patients even had an indwelling urinary catheter.6
Dr. Saint has dubbed the phenomenon “immaculate catheterization” to highlight the glaring discrepancy. “Because we also found, in a significant number of patients, there was no documentation anywhere in the medical record that the catheter existed,” he says. “It’s not in the physician’s notes, it’s not in the nursing notes, but we knew it was there because we could see it coming out of the patient.” Catheters that were inappropriately placed, he found, were more often “forgotten” than appropriate ones.
Perhaps more than anything else, the startling observation underscores the intense need for basic awareness of the two biggest UTI risk factors: whether a catheter is used, and how long it’s left in place. “You can get marked reductions by just taking care of those two factors,” Health Watch USA’s Dr. Kavanagh says.
At the Ann Arbor VA Hospital, one unit’s presence of a “Patient Safety Professional” to help ensure better oversight virtually brought the inappropriate use of indwelling catheters to a standstill. Dr. Saint and his colleagues are now gathering data to determine whether that decrease has translated to a drop in infections.

—Greg Maynard, MD, MSc, SFHM, director, University of California at San Diego Center for Innovation and Improvement Science, senior vice president, SHM Center for Hospital Innovation and Improvement
One fundamental key, he says, is paying close attention to whether a catheter is really in the patient’s best interests. “If we ask that question—‘If this was my family member, what would I want?’—we usually do the right thing,” Dr. Saint explains. Another key is leveraging the hospitalist’s core skill in communicating often and well with nurses to ensure that they are in sync during the “team sport” of CAUTI prevention.
With pockets of success in reducing inappropriate catheterization, the larger question now is how to scale up the individual interventions to achieve nationwide reductions. “How do you take what will work in one hospital, given its culture and microculture, and then apply it to hospitals across the state, or even across the country?” Dr. Saint asks.
Karen Clarke, MD, MS, MPH, a hospitalist and assistant professor of medicine at Emory University Hospital in Atlanta, is in the midst of tackling such issues. Using a bundled approach that included four interventions, she and colleagues reduced CAUTI rates by 70% at 276-bed West Georgia Medical Center in La Grange, Ga.7
The interventions were straightforward and inexpensive, Dr. Clarke says, meaning that they could be widely applied. “The only thing is that there has to be a champion overseeing the interventions to make sure that the steps are followed through on,” she says. Even at cash-strapped facilities, then, a similar approach could prove effective as long as someone assumes responsibility—and hospitalists would be a natural choice.
Based on her study’s promising results, Dr. Clarke hopes to begin implementing the intervention in at least one other hospital starting Jan. 1. If the success can be replicated, she says, the CAUTI-reduction protocol will branch out to include more regional hospitals.
In Focus: C. Diff-Associated Disease
Even as many hospitals are improving their CLABSI and CAUTI rates, hospital-acquired Clostridium difficile infections appear to be getting worse, particularly among older patients. In some facilities, the potentially fatal, diarrhea-causing microbe is now the top pathogen (see “Gut Reaction,” December 2011).
With a timely intervention, however, Kaiser Permanente Medical Center in Santa Clara, Calif., cut its own infection rates by one-third.8 In brainstorming how to improve the medical center’s rates, Susanne Mierendorf, MD, MS, FHM, a hospitalist and associate residency program director for internal medicine, joined colleagues in thinking through the barriers for healthcare providers. “It wasn’t ‘Why don’t they follow the infection-control guidelines?’ It was, ‘Why can’t they?’” Dr. Mierendorf says.
The thought exercise led to some eye-opening observations, including the realization that disposable gowns, gloves, and other personal protective equipment weren’t in the room and were hard to find. To help establish habits, Dr. Mierendorf’s team picked a consistent drawer in each patient’s room to stow the equipment and instructed that a wall-mounted holder be filled with gloves at all times.
The researchers also realized that the rooms of patients with suspected or confirmed C. diff infections had warning signs that were too simplistic at first, then overly wordy. Both were being ignored. The solution was simple signage with yellow color-coding and easily recognizable symbols that readily conveyed the infection-control message to staff: sterile gowns, gloves, hands with soap and water, bleach. Those messages were reinforced through a brief, simple, and mandatory educational module for all hospital workers who might come into contact with the patients.
National Implications
On a national scale, hospitalists have helped compile other educational packets and toolkits to address the spectrum of HAIs, from ventilator-associated pneumonia to methicillin-resistant Staphylococcus aureus (MRSA).
More help may be forthcoming through the American Hospital Association’s Health Research and Education Trust. The broad-based effort, funded by the federal Agency for Healthcare Research and Quality (AHRQ), is partnering with SHM and other professional societies to implement small HAI-reduction successes on ever-wider scales. The University of Michigan’s Dr. Saint, for example, is a key partner in the trust’s national initiative to reduce CAUTI rates and improve patient safety.

—Sanjay Saint, MD, MPH, FHM, professor of internal medicine, University of Michigan, Ann Arbor
Medicare has rolled out its own collection of carrots and sticks to address the problem. The largest carrot, the public-private Partnership for Patients, has joined with SHM, other professional societies, and roughly 3,000 hospitals, so far, and set the ambitious goal of reducing hospital-acquired conditions by 40% by the end of 2013, compared with 2010 tallies. Although applauding the program’s overall goals, physicians, including Dr. Maynard, are taking a wait-and-see attitude, pointing out that many of the details have yet to be ironed out.
Among the sticks, Medicare has begun withholding reimbursement payments for such HAIs as CLABSIs and CAUTIs. Due to intricacies in how hospitals bill under the current DRG system, Dr. Kavanagh says Medicare’s nonpayment policy has recouped relatively little money from its first full year: about $18.8 million in all.
“The policies that cover public reporting, however, do have much more of an impact,” he says. “It’s more of a perceptual sting. Believe me, it is more concerning to have data of bad results that are available to the public than to be penalized by the current financial incentives.”
Other financial policies could carry considerably more weight. The threat of nonpayment for hospital readmissions, Dr. Maynard says, “totally changed the game” by intensifying efforts to reduce those rates, addressing contributing factors such as HAIs in the process. In combination, he says, public accountability through mandated reporting plus financial penalties could prove more powerful than either tactic alone.
Regardless of how federal policy plays out, experts say a new era of accountability and transparency is on its way. As the champions of positive change, hospitalists have a distinct opportunity to help lead the way and bring about a culture that consistently embraces effective interventions—before things get really ugly.
Bryn Nelson is a freelance medical writer based in Seattle.
References
- Klevens RM, Edwards JR, Richards CL, Horan TC, et al. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122:160-166.
- Flanagan ME, Welsh CA, Kiess C, Hoke S, et al. A national collaborative for reducing health care-associated infections: current initiatives, challenges, and opportunities. Am J Infect Control. 2011;39:685-689.
- Pronovost P, Needham D, Berenholtz S, Sinopoli D, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355:2725-2732.
- Srinivasan A, Wise M, Bell M, Cardo D, et al. Vital signs: central line-associated blood stream infections—United States, 2001, 2008, and 2009. MMWR. 2011;60(8):243-248.
- Chernetsky Tejedor S, Tong D, Stein J, Payne C, et al. Temporary central venous catheter utilization patterns in a large tertiary care center: tracking the “idle central venous catheter.” Infect Control Hosp Epidemiol. 2012;33(1): in press.
- Saint S, Wiese J, Amory JK, Bernstein ML, et al. Are physicians aware of which of their patients have indwelling urinary catheters? Am J Med. 2000;109(6):476-480.
- Clarke K, Norrick B, Easley K, Pan Y, et al. Reduction of catheter-associated urinary tract infections through a bundled intervention in a community hospital. J Hosp Med. 2011;6(4):S22.
- Mierendorf S, Rushton M. Decreasing barriers in prevention of hospital-acquired Clostridium difficile colitis. J Hosp Med. 2011;6(4):S50-S51.
Policy Corner: An Inside Look at the Most Pressing Policy Issues
In early November, the Institute of Medicine (IOM) released a report on the current status of health information technology (HIT). Although the report was developed at the request of the Office of the National Coordinator (ONC), the arm within the Department of Health and Human Services (HHS) responsible for promoting the use of HIT, not everything in the report was positive—and could leave the impression that HIT is not quite as successful as some think.
The report recommends that the ONC should work with the private and public sectors to make comparative user experiences across vendors publicly available.
Many hospitalists have developed significant expertise with HIT, played significant roles in its effective implementation and use, and are acutely aware of implementation pitfalls. This practical experience could be very helpful in working with the ONC to develop solutions. It is for this reason that hospitalists should reach out to the ONC and offer their expertise instead of waiting for the ONC to act.
The report, “Patient Safety and Health IT: Building Safer Systems for Better Care,” did praise HIT’s potential for eventual cost savings and increased patient safety but stopped short of being a ringing endorsement of the pace HM is taking toward implementation initiatives, such as meaningful use. An overall theme of the report is that greater oversight of HIT is needed to protect patients from potential medical errors associated with its use.
A few of the recommendations given by the IOM to achieve a greater level of safety range from the establishment of a mechanism for vendors and users to report health IT-related deaths, injuries, or unsafe conditions to possible FDA regulation of the systems themselves.
Information-sharing and reporting in a nonpunitive environment, as recommended by the IOM, would go a long way when it comes to remedying or avoiding IT-related problems, and hospitalists probably have some ideas about how this could be done.
Unfortunately, IT vendor contracts often prevent the open sharing of information, so working toward doing away with such contract terms might be a worthy step before making a push toward overall FDA regulation and the unintended consequences that may come with it.
At first glance, FDA regulation seems like the easiest solution because the FDA can theoretically control every aspect of what might go wrong with HIT, but at what cost would such regulation come? FDA approval can be long, complicated and expensive. The whole process could result in cutting-edge technology becoming outdated by the time approval is granted or innovations being overlooked entirely because of a negative cost-benefit analysis. Furthermore, the expense associated with FDA approval could in turn increase the cost of already costly electronic health records (EHR).
Despite the myriad problems that can arise if implementation moves too fast, HIT holds promise and has shown success when done well.
SHM is currently working to position hospitalists as a resource for the ONC, so hospitalists with expertise in this area should not hesitate to come forward with ideas on how to make HIT work better and more safely. HIT is not going to go away, so the best option is to help make it better.
In early November, the Institute of Medicine (IOM) released a report on the current status of health information technology (HIT). Although the report was developed at the request of the Office of the National Coordinator (ONC), the arm within the Department of Health and Human Services (HHS) responsible for promoting the use of HIT, not everything in the report was positive—and could leave the impression that HIT is not quite as successful as some think.
The report recommends that the ONC should work with the private and public sectors to make comparative user experiences across vendors publicly available.
Many hospitalists have developed significant expertise with HIT, played significant roles in its effective implementation and use, and are acutely aware of implementation pitfalls. This practical experience could be very helpful in working with the ONC to develop solutions. It is for this reason that hospitalists should reach out to the ONC and offer their expertise instead of waiting for the ONC to act.
The report, “Patient Safety and Health IT: Building Safer Systems for Better Care,” did praise HIT’s potential for eventual cost savings and increased patient safety but stopped short of being a ringing endorsement of the pace HM is taking toward implementation initiatives, such as meaningful use. An overall theme of the report is that greater oversight of HIT is needed to protect patients from potential medical errors associated with its use.
A few of the recommendations given by the IOM to achieve a greater level of safety range from the establishment of a mechanism for vendors and users to report health IT-related deaths, injuries, or unsafe conditions to possible FDA regulation of the systems themselves.
Information-sharing and reporting in a nonpunitive environment, as recommended by the IOM, would go a long way when it comes to remedying or avoiding IT-related problems, and hospitalists probably have some ideas about how this could be done.
Unfortunately, IT vendor contracts often prevent the open sharing of information, so working toward doing away with such contract terms might be a worthy step before making a push toward overall FDA regulation and the unintended consequences that may come with it.
At first glance, FDA regulation seems like the easiest solution because the FDA can theoretically control every aspect of what might go wrong with HIT, but at what cost would such regulation come? FDA approval can be long, complicated and expensive. The whole process could result in cutting-edge technology becoming outdated by the time approval is granted or innovations being overlooked entirely because of a negative cost-benefit analysis. Furthermore, the expense associated with FDA approval could in turn increase the cost of already costly electronic health records (EHR).
Despite the myriad problems that can arise if implementation moves too fast, HIT holds promise and has shown success when done well.
SHM is currently working to position hospitalists as a resource for the ONC, so hospitalists with expertise in this area should not hesitate to come forward with ideas on how to make HIT work better and more safely. HIT is not going to go away, so the best option is to help make it better.
In early November, the Institute of Medicine (IOM) released a report on the current status of health information technology (HIT). Although the report was developed at the request of the Office of the National Coordinator (ONC), the arm within the Department of Health and Human Services (HHS) responsible for promoting the use of HIT, not everything in the report was positive—and could leave the impression that HIT is not quite as successful as some think.
The report recommends that the ONC should work with the private and public sectors to make comparative user experiences across vendors publicly available.
Many hospitalists have developed significant expertise with HIT, played significant roles in its effective implementation and use, and are acutely aware of implementation pitfalls. This practical experience could be very helpful in working with the ONC to develop solutions. It is for this reason that hospitalists should reach out to the ONC and offer their expertise instead of waiting for the ONC to act.
The report, “Patient Safety and Health IT: Building Safer Systems for Better Care,” did praise HIT’s potential for eventual cost savings and increased patient safety but stopped short of being a ringing endorsement of the pace HM is taking toward implementation initiatives, such as meaningful use. An overall theme of the report is that greater oversight of HIT is needed to protect patients from potential medical errors associated with its use.
A few of the recommendations given by the IOM to achieve a greater level of safety range from the establishment of a mechanism for vendors and users to report health IT-related deaths, injuries, or unsafe conditions to possible FDA regulation of the systems themselves.
Information-sharing and reporting in a nonpunitive environment, as recommended by the IOM, would go a long way when it comes to remedying or avoiding IT-related problems, and hospitalists probably have some ideas about how this could be done.
Unfortunately, IT vendor contracts often prevent the open sharing of information, so working toward doing away with such contract terms might be a worthy step before making a push toward overall FDA regulation and the unintended consequences that may come with it.
At first glance, FDA regulation seems like the easiest solution because the FDA can theoretically control every aspect of what might go wrong with HIT, but at what cost would such regulation come? FDA approval can be long, complicated and expensive. The whole process could result in cutting-edge technology becoming outdated by the time approval is granted or innovations being overlooked entirely because of a negative cost-benefit analysis. Furthermore, the expense associated with FDA approval could in turn increase the cost of already costly electronic health records (EHR).
Despite the myriad problems that can arise if implementation moves too fast, HIT holds promise and has shown success when done well.
SHM is currently working to position hospitalists as a resource for the ONC, so hospitalists with expertise in this area should not hesitate to come forward with ideas on how to make HIT work better and more safely. HIT is not going to go away, so the best option is to help make it better.
Professional Development Program Advances Hospitalist Leadership Skills
Akin to other doctors, hospitalists seek clinical and nonclinical continuing medical education (CME) opportunities in subjects that they hope will improve their professional skill set. But Emory School of Medicine’s Division of Hospital Medicine has tried to make this training more systematic for its 110 members. Since 2005, competitively awarded grants have supported faculty development training in the areas of administrative leadership, quality improvement and research, and education and training.
According to an abstract presented at HM11, Emory’s faculty development program has helped train 36 HM physicians. The upshot of the program: Thirty-three hospitalists now fill formal leadership positions in six Emory-affiliated hospitals. Examples include hospital chief medical officers, chief quality officers, and medical directors for care coordination.
“Hospital medicine is a young field, and we had a young group of clinicians lacking experience that other physicians might get in the course of a career,” says Daniel Dressler, MD, MSc, SFHM, director of education for the hospital medicine division of the Atlanta-based group. “If we were going to be asked to do things, leadershipwise, in the hospital, we needed to build a program to help individuals get additional training for them.”
The physicians pick courses in areas where they want to better themselves, either local educational offerings or national conferences. A committee applies a structured process for reviewing their applications, with funding coming from the department. “We ask the doctors to come back and report on what they learned,” says Dr. Dressler, an SHM board member.
Akin to other doctors, hospitalists seek clinical and nonclinical continuing medical education (CME) opportunities in subjects that they hope will improve their professional skill set. But Emory School of Medicine’s Division of Hospital Medicine has tried to make this training more systematic for its 110 members. Since 2005, competitively awarded grants have supported faculty development training in the areas of administrative leadership, quality improvement and research, and education and training.
According to an abstract presented at HM11, Emory’s faculty development program has helped train 36 HM physicians. The upshot of the program: Thirty-three hospitalists now fill formal leadership positions in six Emory-affiliated hospitals. Examples include hospital chief medical officers, chief quality officers, and medical directors for care coordination.
“Hospital medicine is a young field, and we had a young group of clinicians lacking experience that other physicians might get in the course of a career,” says Daniel Dressler, MD, MSc, SFHM, director of education for the hospital medicine division of the Atlanta-based group. “If we were going to be asked to do things, leadershipwise, in the hospital, we needed to build a program to help individuals get additional training for them.”
The physicians pick courses in areas where they want to better themselves, either local educational offerings or national conferences. A committee applies a structured process for reviewing their applications, with funding coming from the department. “We ask the doctors to come back and report on what they learned,” says Dr. Dressler, an SHM board member.
Akin to other doctors, hospitalists seek clinical and nonclinical continuing medical education (CME) opportunities in subjects that they hope will improve their professional skill set. But Emory School of Medicine’s Division of Hospital Medicine has tried to make this training more systematic for its 110 members. Since 2005, competitively awarded grants have supported faculty development training in the areas of administrative leadership, quality improvement and research, and education and training.
According to an abstract presented at HM11, Emory’s faculty development program has helped train 36 HM physicians. The upshot of the program: Thirty-three hospitalists now fill formal leadership positions in six Emory-affiliated hospitals. Examples include hospital chief medical officers, chief quality officers, and medical directors for care coordination.
“Hospital medicine is a young field, and we had a young group of clinicians lacking experience that other physicians might get in the course of a career,” says Daniel Dressler, MD, MSc, SFHM, director of education for the hospital medicine division of the Atlanta-based group. “If we were going to be asked to do things, leadershipwise, in the hospital, we needed to build a program to help individuals get additional training for them.”
The physicians pick courses in areas where they want to better themselves, either local educational offerings or national conferences. A committee applies a structured process for reviewing their applications, with funding coming from the department. “We ask the doctors to come back and report on what they learned,” says Dr. Dressler, an SHM board member.
IOM Report Outlines Health IT Concerns
The Institute of Medicine in November issued a new report, “Patient Safety and Health IT (HIT): Building Safer Systems for Better Care,” which identifies potential harm that could stem from a digital healthcare system and proposes 10 recommendations. Many of the suggestions are directed at the U.S. Secretary of Health and Human Services, urging the office to work with the private sector and research groups on patient safety, ensure the free exchange of information on healthcare information technology (HIT) issues, and create a process for reporting HIT-related deaths and injuries.
“Concerns about potential harm are emerging as providers increasingly rely on electronic medical records, secure patient portals, and other technologies to deliver care,” the report states, but there is a lack of published research quantifying the risks. For more on the HIT report, check out the Policy Corner.
The Institute of Medicine in November issued a new report, “Patient Safety and Health IT (HIT): Building Safer Systems for Better Care,” which identifies potential harm that could stem from a digital healthcare system and proposes 10 recommendations. Many of the suggestions are directed at the U.S. Secretary of Health and Human Services, urging the office to work with the private sector and research groups on patient safety, ensure the free exchange of information on healthcare information technology (HIT) issues, and create a process for reporting HIT-related deaths and injuries.
“Concerns about potential harm are emerging as providers increasingly rely on electronic medical records, secure patient portals, and other technologies to deliver care,” the report states, but there is a lack of published research quantifying the risks. For more on the HIT report, check out the Policy Corner.
The Institute of Medicine in November issued a new report, “Patient Safety and Health IT (HIT): Building Safer Systems for Better Care,” which identifies potential harm that could stem from a digital healthcare system and proposes 10 recommendations. Many of the suggestions are directed at the U.S. Secretary of Health and Human Services, urging the office to work with the private sector and research groups on patient safety, ensure the free exchange of information on healthcare information technology (HIT) issues, and create a process for reporting HIT-related deaths and injuries.
“Concerns about potential harm are emerging as providers increasingly rely on electronic medical records, secure patient portals, and other technologies to deliver care,” the report states, but there is a lack of published research quantifying the risks. For more on the HIT report, check out the Policy Corner.
HM’s Role in Helping Hospitals Profit
A new report shows that 1 in 5 community hospitals operates in the red, but the chief strategy officer of the firm that conducted the survey thinks hospitals can help change that.
The second annual survey from healthcare information technology (HIT) provider Anthelio and leadership group Community Hospital 100 found that 22% of community hospitals operate with margins below 2%; another 38% operate below 1%. Rick Kneipper, Anthelio’s cofounder and chief strategy officer, says that hospitalists can be at the forefront “of the creative changes needed” to reduce costs and improve profitability.
“Hospital medicine groups and hospitals could free up significant funds to devote to improved patient-care services if they focus on their core competency of patient care and farm out their non-core, back-office services to experts who can use leverage to provide more efficient services at significantly reduced costs,” Kneipper wrote in an email to The Hospitalist. “Financial pressures have historically forced most industries to stop trying to be vertically integrated [trying to be ‘all things to all people’] and instead to focus on their core competencies—it’s time for healthcare to do the same.”
—Rick Kneipper, cofounder, chief strategy officer, Anthelio
HM’s foothold at the intersection of clinical care and safety and QI positions the specialty to “respond to the new challenges of readmission penalties, evidenced-based medicine requirements, EMR implementation, and operation challenges,” Kneipper wrote.
For the full survey, please visit www.antheliohealth.com and search “survey.”
A new report shows that 1 in 5 community hospitals operates in the red, but the chief strategy officer of the firm that conducted the survey thinks hospitals can help change that.
The second annual survey from healthcare information technology (HIT) provider Anthelio and leadership group Community Hospital 100 found that 22% of community hospitals operate with margins below 2%; another 38% operate below 1%. Rick Kneipper, Anthelio’s cofounder and chief strategy officer, says that hospitalists can be at the forefront “of the creative changes needed” to reduce costs and improve profitability.
“Hospital medicine groups and hospitals could free up significant funds to devote to improved patient-care services if they focus on their core competency of patient care and farm out their non-core, back-office services to experts who can use leverage to provide more efficient services at significantly reduced costs,” Kneipper wrote in an email to The Hospitalist. “Financial pressures have historically forced most industries to stop trying to be vertically integrated [trying to be ‘all things to all people’] and instead to focus on their core competencies—it’s time for healthcare to do the same.”
—Rick Kneipper, cofounder, chief strategy officer, Anthelio
HM’s foothold at the intersection of clinical care and safety and QI positions the specialty to “respond to the new challenges of readmission penalties, evidenced-based medicine requirements, EMR implementation, and operation challenges,” Kneipper wrote.
For the full survey, please visit www.antheliohealth.com and search “survey.”
A new report shows that 1 in 5 community hospitals operates in the red, but the chief strategy officer of the firm that conducted the survey thinks hospitals can help change that.
The second annual survey from healthcare information technology (HIT) provider Anthelio and leadership group Community Hospital 100 found that 22% of community hospitals operate with margins below 2%; another 38% operate below 1%. Rick Kneipper, Anthelio’s cofounder and chief strategy officer, says that hospitalists can be at the forefront “of the creative changes needed” to reduce costs and improve profitability.
“Hospital medicine groups and hospitals could free up significant funds to devote to improved patient-care services if they focus on their core competency of patient care and farm out their non-core, back-office services to experts who can use leverage to provide more efficient services at significantly reduced costs,” Kneipper wrote in an email to The Hospitalist. “Financial pressures have historically forced most industries to stop trying to be vertically integrated [trying to be ‘all things to all people’] and instead to focus on their core competencies—it’s time for healthcare to do the same.”
—Rick Kneipper, cofounder, chief strategy officer, Anthelio
HM’s foothold at the intersection of clinical care and safety and QI positions the specialty to “respond to the new challenges of readmission penalties, evidenced-based medicine requirements, EMR implementation, and operation challenges,” Kneipper wrote.
For the full survey, please visit www.antheliohealth.com and search “survey.”
Six Ways You Can Help Reduce HAIs in Your Hospital
- Encourage good hand hygiene. This should be obvious, but hospitals are struggling to achieve compliance rates of even 50%. One study has found significant improvement by appealing to medical providers’ altruistic sense: “Hand hygiene prevents patients from catching diseases.”1
- Embrace checklists. If they work for airline pilots, they can work for you. Study after study has supported their effectiveness, particularly in preventing CLABSIs and CAUTIs when well-integrated into a multifaceted approach.
- Bundle up. A bundled approach that emphasized proper hand hygiene, disinfection, catheter avoidance, and timely removal cut CLABSI rates by morethan half, on average, in Veterans Administration ICUs throughout the U.S.2
- Team up. For a C. diff-reduction effort at Kaiser Permanente Medical Center in Santa Clara, Calif., success meant getting doctors, nurses, specialists, and administrators on board, both to brainstorm and to sustain momentum.
- Be a role model. Consistently following HAI-prevention protocols, such as contact precautions, can make adherence contagious—in a very good way.
- Be an innovator. By virtue of being ubiquitous in inpatient wards, hospitalists know what works and what doesn’t; your insight can be particularly valuable for a team-based, HAI-reduction effort.
References
- Grant AM, Hofmann DA. It’s not all about me: Motivating hospital hand hygiene by focusing on patients. Psychol Sci. 2011;22:1494-1499.
- Render ML, Hasselbeck R, Freyberg RW, Hofer TP, et al. Reduction of central line infections in Veterans Administration intensive care units: an observational cohort using a central infrastructure to support learning and improvement. BMJ Qual Saf. 2011;20(8):725-732.
- Encourage good hand hygiene. This should be obvious, but hospitals are struggling to achieve compliance rates of even 50%. One study has found significant improvement by appealing to medical providers’ altruistic sense: “Hand hygiene prevents patients from catching diseases.”1
- Embrace checklists. If they work for airline pilots, they can work for you. Study after study has supported their effectiveness, particularly in preventing CLABSIs and CAUTIs when well-integrated into a multifaceted approach.
- Bundle up. A bundled approach that emphasized proper hand hygiene, disinfection, catheter avoidance, and timely removal cut CLABSI rates by morethan half, on average, in Veterans Administration ICUs throughout the U.S.2
- Team up. For a C. diff-reduction effort at Kaiser Permanente Medical Center in Santa Clara, Calif., success meant getting doctors, nurses, specialists, and administrators on board, both to brainstorm and to sustain momentum.
- Be a role model. Consistently following HAI-prevention protocols, such as contact precautions, can make adherence contagious—in a very good way.
- Be an innovator. By virtue of being ubiquitous in inpatient wards, hospitalists know what works and what doesn’t; your insight can be particularly valuable for a team-based, HAI-reduction effort.
References
- Grant AM, Hofmann DA. It’s not all about me: Motivating hospital hand hygiene by focusing on patients. Psychol Sci. 2011;22:1494-1499.
- Render ML, Hasselbeck R, Freyberg RW, Hofer TP, et al. Reduction of central line infections in Veterans Administration intensive care units: an observational cohort using a central infrastructure to support learning and improvement. BMJ Qual Saf. 2011;20(8):725-732.
- Encourage good hand hygiene. This should be obvious, but hospitals are struggling to achieve compliance rates of even 50%. One study has found significant improvement by appealing to medical providers’ altruistic sense: “Hand hygiene prevents patients from catching diseases.”1
- Embrace checklists. If they work for airline pilots, they can work for you. Study after study has supported their effectiveness, particularly in preventing CLABSIs and CAUTIs when well-integrated into a multifaceted approach.
- Bundle up. A bundled approach that emphasized proper hand hygiene, disinfection, catheter avoidance, and timely removal cut CLABSI rates by morethan half, on average, in Veterans Administration ICUs throughout the U.S.2
- Team up. For a C. diff-reduction effort at Kaiser Permanente Medical Center in Santa Clara, Calif., success meant getting doctors, nurses, specialists, and administrators on board, both to brainstorm and to sustain momentum.
- Be a role model. Consistently following HAI-prevention protocols, such as contact precautions, can make adherence contagious—in a very good way.
- Be an innovator. By virtue of being ubiquitous in inpatient wards, hospitalists know what works and what doesn’t; your insight can be particularly valuable for a team-based, HAI-reduction effort.
References
- Grant AM, Hofmann DA. It’s not all about me: Motivating hospital hand hygiene by focusing on patients. Psychol Sci. 2011;22:1494-1499.
- Render ML, Hasselbeck R, Freyberg RW, Hofer TP, et al. Reduction of central line infections in Veterans Administration intensive care units: an observational cohort using a central infrastructure to support learning and improvement. BMJ Qual Saf. 2011;20(8):725-732.