User login
Reimagining rehabilitation: In-home physical therapy gets a boost
As the aging population grows and telehealth expands in the wake of the COVID-19 pandemic, an emerging trend of in-home care is reshaping how patients access and receive physical therapy services.
Partnerships between hospitals and home health companies are increasing access to rehabilitation services not only for older adults but also for people in rural areas, those without reliable transportation, and patients with injuries that hinder their driving abilities.
“We find more and more that physical therapy at their home, instead of coming to an outpatient facility, is something more and more folks are requesting,” said Bill Benoit, MBA, chief operating officer of University Hospitals, Cleveland. “In this post-COVID environment, people are getting all different types of services in their home when they’re available, and this is one of them. The pandemic sped up the process of us moving away from the traditional brick and mortar hospital.”
UH recently announced a partnership with Luna Physical Therapy, a company founded in 2018 that provides home services. Luna has teamed up with more than two dozen other hospitals in the United States to offer home-based rehabilitation, according to the company.
The process for arranging in-home therapies through hospital-clinic partnerships is like any other inpatient or outpatient rehabilitation, Mr. Benoit said: A patient meets with a specialist or primary care practitioner, they discuss options, and eventually the clinician recommends physical therapy. The only difference here, he said, is rather than going to a separate facility or a hospital, the patient logs onto a mobile app that matches them with a physical therapist on the basis of their location, needs, and the times they are available.
The prescribing physician oversees the patient’s progress through notes provided by the therapist.
“For the primary care physician or surgeon, they’re not going to see much of a difference,” Mr. Benoit said. “This just adds to that list of options for patients.”
Safer, more productive PT
A study, published in the journal Family Practice, found that 76% of patients who are prescribed physical therapy do not initiate the services after it has been recommended.
Aside from the convenience and expanded accessibility for patients, the home therapy option can be more productive, said Denise Wagner, PT, DPT, a physical therapist with Johns Hopkins, Baltimore.
“Home is safer for many patients, but home is also more engaging and motivating,” she said. “Home health clinicians are experts in using whatever they find in the home environment as equipment; many people have stairs in their home, so we can use the rail as something to hold. If patient likes to walk their dog, we can use putting a leash on dog as balance activity.”
Therapy in the home setting helps physical therapists customize programs to fit each patient’s lifestyle, said Gira Shah, PT, a physical therapist with Providence Home Services in Seattle.
For example, patients generally want to know how to function within their own space – navigate their kitchens to make food or get in and out of their bathtubs. Staying in that space allows therapists to focus on those specific goals, Ms. Shah said. “It’s more of a functional therapy. The beauty of this [is that] as therapists we’re trying to assess, ‘what does the patient need to be independent?’ ”
The consulting firm McKinsey predicts that as much as $265 billion in health care services for Medicare recipients will be provided within the home by 2025.
The obvious question is: Why would hospitals partner with clinics rather than offer in-home services on their own?
The answer, like most things in health care, boils down to money.
The billing and documentation system that they use is more efficient than anything hospitals have, said John Brickley, PT, MA, vice president and physical therapist at MedStar Health, a health care system in Maryland and the Washington, D.C., area. MedStar and Luna announced a partnership last June.
“We would financially fall on our face if we tried to use our own billing systems; it would take too much time,” Mr. Brickley said. “Do we need them from a quality-of-care standpoint? No. They have the type of technology that’s not at our disposal.”
Patients should be aware of the difference between home-based PT and other health services for homebound patients, Mr. Brickley said. Medicare considers a patient homebound if they need the help of another person or medical equipment to leave their home or if their doctor believes their condition would worsen with greater mobility.
From the perspective of an insurance company, a home therapy session arranged by a hospital-clinic partnership is an ambulatory appointment and uses the same charging mechanism as most other visits. For a home health care visit, patients must qualify as homebound.
Home-based PT can be used for conditions including neurologic issues, bone and joint problems, balance, and fall deconditioning and prevention. But if a patient needs heavy equipment that cannot be transported, outpatient services are more practical.
That should be determined by the primary care practitioner or specialist evaluating each patient, said Palak Shah, PT, cofounder and head of clinical services at Luna.
“Primary care physicians play a huge role – that’s where patients express their initial concerns,” she said. “It’s up to them to make patients aware about all the options.”
A version of this article first appeared on Medscape.com.
As the aging population grows and telehealth expands in the wake of the COVID-19 pandemic, an emerging trend of in-home care is reshaping how patients access and receive physical therapy services.
Partnerships between hospitals and home health companies are increasing access to rehabilitation services not only for older adults but also for people in rural areas, those without reliable transportation, and patients with injuries that hinder their driving abilities.
“We find more and more that physical therapy at their home, instead of coming to an outpatient facility, is something more and more folks are requesting,” said Bill Benoit, MBA, chief operating officer of University Hospitals, Cleveland. “In this post-COVID environment, people are getting all different types of services in their home when they’re available, and this is one of them. The pandemic sped up the process of us moving away from the traditional brick and mortar hospital.”
UH recently announced a partnership with Luna Physical Therapy, a company founded in 2018 that provides home services. Luna has teamed up with more than two dozen other hospitals in the United States to offer home-based rehabilitation, according to the company.
The process for arranging in-home therapies through hospital-clinic partnerships is like any other inpatient or outpatient rehabilitation, Mr. Benoit said: A patient meets with a specialist or primary care practitioner, they discuss options, and eventually the clinician recommends physical therapy. The only difference here, he said, is rather than going to a separate facility or a hospital, the patient logs onto a mobile app that matches them with a physical therapist on the basis of their location, needs, and the times they are available.
The prescribing physician oversees the patient’s progress through notes provided by the therapist.
“For the primary care physician or surgeon, they’re not going to see much of a difference,” Mr. Benoit said. “This just adds to that list of options for patients.”
Safer, more productive PT
A study, published in the journal Family Practice, found that 76% of patients who are prescribed physical therapy do not initiate the services after it has been recommended.
Aside from the convenience and expanded accessibility for patients, the home therapy option can be more productive, said Denise Wagner, PT, DPT, a physical therapist with Johns Hopkins, Baltimore.
“Home is safer for many patients, but home is also more engaging and motivating,” she said. “Home health clinicians are experts in using whatever they find in the home environment as equipment; many people have stairs in their home, so we can use the rail as something to hold. If patient likes to walk their dog, we can use putting a leash on dog as balance activity.”
Therapy in the home setting helps physical therapists customize programs to fit each patient’s lifestyle, said Gira Shah, PT, a physical therapist with Providence Home Services in Seattle.
For example, patients generally want to know how to function within their own space – navigate their kitchens to make food or get in and out of their bathtubs. Staying in that space allows therapists to focus on those specific goals, Ms. Shah said. “It’s more of a functional therapy. The beauty of this [is that] as therapists we’re trying to assess, ‘what does the patient need to be independent?’ ”
The consulting firm McKinsey predicts that as much as $265 billion in health care services for Medicare recipients will be provided within the home by 2025.
The obvious question is: Why would hospitals partner with clinics rather than offer in-home services on their own?
The answer, like most things in health care, boils down to money.
The billing and documentation system that they use is more efficient than anything hospitals have, said John Brickley, PT, MA, vice president and physical therapist at MedStar Health, a health care system in Maryland and the Washington, D.C., area. MedStar and Luna announced a partnership last June.
“We would financially fall on our face if we tried to use our own billing systems; it would take too much time,” Mr. Brickley said. “Do we need them from a quality-of-care standpoint? No. They have the type of technology that’s not at our disposal.”
Patients should be aware of the difference between home-based PT and other health services for homebound patients, Mr. Brickley said. Medicare considers a patient homebound if they need the help of another person or medical equipment to leave their home or if their doctor believes their condition would worsen with greater mobility.
From the perspective of an insurance company, a home therapy session arranged by a hospital-clinic partnership is an ambulatory appointment and uses the same charging mechanism as most other visits. For a home health care visit, patients must qualify as homebound.
Home-based PT can be used for conditions including neurologic issues, bone and joint problems, balance, and fall deconditioning and prevention. But if a patient needs heavy equipment that cannot be transported, outpatient services are more practical.
That should be determined by the primary care practitioner or specialist evaluating each patient, said Palak Shah, PT, cofounder and head of clinical services at Luna.
“Primary care physicians play a huge role – that’s where patients express their initial concerns,” she said. “It’s up to them to make patients aware about all the options.”
A version of this article first appeared on Medscape.com.
As the aging population grows and telehealth expands in the wake of the COVID-19 pandemic, an emerging trend of in-home care is reshaping how patients access and receive physical therapy services.
Partnerships between hospitals and home health companies are increasing access to rehabilitation services not only for older adults but also for people in rural areas, those without reliable transportation, and patients with injuries that hinder their driving abilities.
“We find more and more that physical therapy at their home, instead of coming to an outpatient facility, is something more and more folks are requesting,” said Bill Benoit, MBA, chief operating officer of University Hospitals, Cleveland. “In this post-COVID environment, people are getting all different types of services in their home when they’re available, and this is one of them. The pandemic sped up the process of us moving away from the traditional brick and mortar hospital.”
UH recently announced a partnership with Luna Physical Therapy, a company founded in 2018 that provides home services. Luna has teamed up with more than two dozen other hospitals in the United States to offer home-based rehabilitation, according to the company.
The process for arranging in-home therapies through hospital-clinic partnerships is like any other inpatient or outpatient rehabilitation, Mr. Benoit said: A patient meets with a specialist or primary care practitioner, they discuss options, and eventually the clinician recommends physical therapy. The only difference here, he said, is rather than going to a separate facility or a hospital, the patient logs onto a mobile app that matches them with a physical therapist on the basis of their location, needs, and the times they are available.
The prescribing physician oversees the patient’s progress through notes provided by the therapist.
“For the primary care physician or surgeon, they’re not going to see much of a difference,” Mr. Benoit said. “This just adds to that list of options for patients.”
Safer, more productive PT
A study, published in the journal Family Practice, found that 76% of patients who are prescribed physical therapy do not initiate the services after it has been recommended.
Aside from the convenience and expanded accessibility for patients, the home therapy option can be more productive, said Denise Wagner, PT, DPT, a physical therapist with Johns Hopkins, Baltimore.
“Home is safer for many patients, but home is also more engaging and motivating,” she said. “Home health clinicians are experts in using whatever they find in the home environment as equipment; many people have stairs in their home, so we can use the rail as something to hold. If patient likes to walk their dog, we can use putting a leash on dog as balance activity.”
Therapy in the home setting helps physical therapists customize programs to fit each patient’s lifestyle, said Gira Shah, PT, a physical therapist with Providence Home Services in Seattle.
For example, patients generally want to know how to function within their own space – navigate their kitchens to make food or get in and out of their bathtubs. Staying in that space allows therapists to focus on those specific goals, Ms. Shah said. “It’s more of a functional therapy. The beauty of this [is that] as therapists we’re trying to assess, ‘what does the patient need to be independent?’ ”
The consulting firm McKinsey predicts that as much as $265 billion in health care services for Medicare recipients will be provided within the home by 2025.
The obvious question is: Why would hospitals partner with clinics rather than offer in-home services on their own?
The answer, like most things in health care, boils down to money.
The billing and documentation system that they use is more efficient than anything hospitals have, said John Brickley, PT, MA, vice president and physical therapist at MedStar Health, a health care system in Maryland and the Washington, D.C., area. MedStar and Luna announced a partnership last June.
“We would financially fall on our face if we tried to use our own billing systems; it would take too much time,” Mr. Brickley said. “Do we need them from a quality-of-care standpoint? No. They have the type of technology that’s not at our disposal.”
Patients should be aware of the difference between home-based PT and other health services for homebound patients, Mr. Brickley said. Medicare considers a patient homebound if they need the help of another person or medical equipment to leave their home or if their doctor believes their condition would worsen with greater mobility.
From the perspective of an insurance company, a home therapy session arranged by a hospital-clinic partnership is an ambulatory appointment and uses the same charging mechanism as most other visits. For a home health care visit, patients must qualify as homebound.
Home-based PT can be used for conditions including neurologic issues, bone and joint problems, balance, and fall deconditioning and prevention. But if a patient needs heavy equipment that cannot be transported, outpatient services are more practical.
That should be determined by the primary care practitioner or specialist evaluating each patient, said Palak Shah, PT, cofounder and head of clinical services at Luna.
“Primary care physicians play a huge role – that’s where patients express their initial concerns,” she said. “It’s up to them to make patients aware about all the options.”
A version of this article first appeared on Medscape.com.
Marijuana use dramatically increases risk of heart problems, stroke
Regularly using marijuana can significantly increase a person’s risk of heart attack, heart failure, and stroke, according to a pair of new studies that will be presented at a major upcoming medical conference.
People who use marijuana daily have a 34% increased risk of heart failure, compared with people who don’t use the drug, according to one of the new studies.
The new findings leverage health data from 157,000 people in the National Institutes of Health “All of Us” research program. Researchers analyzed whether marijuana users were more likely to experience heart failure than nonusers over the course of nearly 4 years. The results indicated that coronary artery disease was behind marijuana users’ increased risk. (Coronary artery disease is the buildup of plaque on the walls of the arteries that supply blood to the heart.)
The research was conducted by a team at Medstar Health, a large Maryland health care system that operates 10 hospitals plus hundreds of clinics. The findings will be presented at the American Heart Association’s Scientific Sessions 2023 in Philadelphia.
“Our results should encourage more researchers to study the use of marijuana to better understand its health implications, especially on cardiovascular risk,” said researcher Yakubu Bene-Alhasan, MD, MPH, a doctor at Medstar Health in Baltimore. “We want to provide the population with high-quality information on marijuana use and to help inform policy decisions at the state level, to educate patients, and to guide health care professionals.”
About one in five people in the United States use marijuana, according to the Centers for Disease Control and Prevention. The majority of U.S. states allow marijuana to be used legally for medical purposes, and more than 20 states have legalized recreational marijuana, a tracker from the National Conference of State Legislatures shows.
A second study that will be presented at the conference shows that older people with any combination of type 2 diabetes, high blood pressure, and high cholesterol who use marijuana have an increased risk for a major heart or brain event, compared with people who never used the drug.
The researchers analyzed data for more than 28,000 people age 65 and older who had health conditions that put them at risk for heart problems and whose medical records showed they were marijuana users but not tobacco users. The results showed at least a 20% increased risk of heart attack, stroke, cardiac arrest, or arrhythmia (irregular heartbeat).
The findings are significant because medical professionals have long said that research on the long-term health effects of using marijuana are limited.
“The latest research about cannabis use indicates that smoking and inhaling cannabis increases concentrations of blood carboxyhemoglobin (carbon monoxide, a poisonous gas), tar (partly burned combustible matter) similar to the effects of inhaling a tobacco cigarette, both of which have been linked to heart muscle disease, chest pain, heart rhythm disturbances, heart attacks and other serious conditions,” said Robert L. Page II, PharmD, MSPH, chair of the volunteer writing group for the 2020 American Heart Association Scientific Statement: Medical Marijuana, Recreational Cannabis, and Cardiovascular Health, in a statement. “Together with the results of these two research studies, the cardiovascular risks of cannabis use are becoming clearer and should be carefully considered and monitored by health care professionals and the public.”
A version of this article first appeared on WebMD.com.
Regularly using marijuana can significantly increase a person’s risk of heart attack, heart failure, and stroke, according to a pair of new studies that will be presented at a major upcoming medical conference.
People who use marijuana daily have a 34% increased risk of heart failure, compared with people who don’t use the drug, according to one of the new studies.
The new findings leverage health data from 157,000 people in the National Institutes of Health “All of Us” research program. Researchers analyzed whether marijuana users were more likely to experience heart failure than nonusers over the course of nearly 4 years. The results indicated that coronary artery disease was behind marijuana users’ increased risk. (Coronary artery disease is the buildup of plaque on the walls of the arteries that supply blood to the heart.)
The research was conducted by a team at Medstar Health, a large Maryland health care system that operates 10 hospitals plus hundreds of clinics. The findings will be presented at the American Heart Association’s Scientific Sessions 2023 in Philadelphia.
“Our results should encourage more researchers to study the use of marijuana to better understand its health implications, especially on cardiovascular risk,” said researcher Yakubu Bene-Alhasan, MD, MPH, a doctor at Medstar Health in Baltimore. “We want to provide the population with high-quality information on marijuana use and to help inform policy decisions at the state level, to educate patients, and to guide health care professionals.”
About one in five people in the United States use marijuana, according to the Centers for Disease Control and Prevention. The majority of U.S. states allow marijuana to be used legally for medical purposes, and more than 20 states have legalized recreational marijuana, a tracker from the National Conference of State Legislatures shows.
A second study that will be presented at the conference shows that older people with any combination of type 2 diabetes, high blood pressure, and high cholesterol who use marijuana have an increased risk for a major heart or brain event, compared with people who never used the drug.
The researchers analyzed data for more than 28,000 people age 65 and older who had health conditions that put them at risk for heart problems and whose medical records showed they were marijuana users but not tobacco users. The results showed at least a 20% increased risk of heart attack, stroke, cardiac arrest, or arrhythmia (irregular heartbeat).
The findings are significant because medical professionals have long said that research on the long-term health effects of using marijuana are limited.
“The latest research about cannabis use indicates that smoking and inhaling cannabis increases concentrations of blood carboxyhemoglobin (carbon monoxide, a poisonous gas), tar (partly burned combustible matter) similar to the effects of inhaling a tobacco cigarette, both of which have been linked to heart muscle disease, chest pain, heart rhythm disturbances, heart attacks and other serious conditions,” said Robert L. Page II, PharmD, MSPH, chair of the volunteer writing group for the 2020 American Heart Association Scientific Statement: Medical Marijuana, Recreational Cannabis, and Cardiovascular Health, in a statement. “Together with the results of these two research studies, the cardiovascular risks of cannabis use are becoming clearer and should be carefully considered and monitored by health care professionals and the public.”
A version of this article first appeared on WebMD.com.
Regularly using marijuana can significantly increase a person’s risk of heart attack, heart failure, and stroke, according to a pair of new studies that will be presented at a major upcoming medical conference.
People who use marijuana daily have a 34% increased risk of heart failure, compared with people who don’t use the drug, according to one of the new studies.
The new findings leverage health data from 157,000 people in the National Institutes of Health “All of Us” research program. Researchers analyzed whether marijuana users were more likely to experience heart failure than nonusers over the course of nearly 4 years. The results indicated that coronary artery disease was behind marijuana users’ increased risk. (Coronary artery disease is the buildup of plaque on the walls of the arteries that supply blood to the heart.)
The research was conducted by a team at Medstar Health, a large Maryland health care system that operates 10 hospitals plus hundreds of clinics. The findings will be presented at the American Heart Association’s Scientific Sessions 2023 in Philadelphia.
“Our results should encourage more researchers to study the use of marijuana to better understand its health implications, especially on cardiovascular risk,” said researcher Yakubu Bene-Alhasan, MD, MPH, a doctor at Medstar Health in Baltimore. “We want to provide the population with high-quality information on marijuana use and to help inform policy decisions at the state level, to educate patients, and to guide health care professionals.”
About one in five people in the United States use marijuana, according to the Centers for Disease Control and Prevention. The majority of U.S. states allow marijuana to be used legally for medical purposes, and more than 20 states have legalized recreational marijuana, a tracker from the National Conference of State Legislatures shows.
A second study that will be presented at the conference shows that older people with any combination of type 2 diabetes, high blood pressure, and high cholesterol who use marijuana have an increased risk for a major heart or brain event, compared with people who never used the drug.
The researchers analyzed data for more than 28,000 people age 65 and older who had health conditions that put them at risk for heart problems and whose medical records showed they were marijuana users but not tobacco users. The results showed at least a 20% increased risk of heart attack, stroke, cardiac arrest, or arrhythmia (irregular heartbeat).
The findings are significant because medical professionals have long said that research on the long-term health effects of using marijuana are limited.
“The latest research about cannabis use indicates that smoking and inhaling cannabis increases concentrations of blood carboxyhemoglobin (carbon monoxide, a poisonous gas), tar (partly burned combustible matter) similar to the effects of inhaling a tobacco cigarette, both of which have been linked to heart muscle disease, chest pain, heart rhythm disturbances, heart attacks and other serious conditions,” said Robert L. Page II, PharmD, MSPH, chair of the volunteer writing group for the 2020 American Heart Association Scientific Statement: Medical Marijuana, Recreational Cannabis, and Cardiovascular Health, in a statement. “Together with the results of these two research studies, the cardiovascular risks of cannabis use are becoming clearer and should be carefully considered and monitored by health care professionals and the public.”
A version of this article first appeared on WebMD.com.
FROM AHA 2023
Nightmare on CIL Street: A Simulation Series to Increase Confidence and Skill in Responding to Clinical Emergencies
The Central Texas Veteran’s Health Care System (CTVHCS) in Temple, Texas, is a 189-bed teaching hospital. CTVHCS opened the Center for Innovation and Learning (CIL) in 2022. The CIL has about 279 m2 of simulation space that includes high- and low-fidelity simulation equipment and multiple laboratories, which can be used to simulate inpatient and outpatient settings. The CIL high-fidelity manikins and environment allow learners to be immersed in the simulation for maximum realism. Computer and video systems provide clear viewing of training, which allows for more in-depth debriefing and learning. CIL simulation training is used by CTVHCS staff, medical residents, and medical and physician assistant students.
The utility of technology in medical education is rapidly evolving. As noted in many studies, simulation creates an environment that can imitate real patients in the format of a lifelike manikin, anatomic regions stations, clinical tasks, and many real-life circumstances.1 Task trainers for procedure simulation have been widely used and studied. A 2020 study noted that simulation training is effective for developing procedural skills in surgery and prevents the decay of surgical skills.2
In reviewing health care education curriculums, we noted that most of the rapid response situations are learned through active patient experiences. Rapid responses are managed by the intensive care unit and primary care teams during the day but at night are run primarily by the postgraduate year 2 (PGY2) night resident and intern. Knowing these logistics and current studies, we decided to build a rapid response simulation curriculum to improve preparedness for PGY1 residents, medical students, and physician assistant students.
Curriculum Planning
Planning the simulation curriculum began with the CTVHCS internal medicine chief resident and registered nurse (RN) educator. CTVHCS data were reviewed to identify the 3 most common rapid response calls from the past 3 years; research on the most common systems affected by rapid responses also was evaluated.
A 2019 study by Lyons and colleagues evaluated 402,023 rapid response activations across 360 hospitals and found that respiratory scenarios made up 38% and cardiac scenarios made up 37%.3 In addition, the CTVHCS has limited support in stroke neurology. Therefore, the internal medicine chief resident and RN educator decided to run 3 evolving rapid response scenarios per session that included cardiac, respiratory, and neurological scenarios. Capabilities and limitations of different high-fidelity manikins were discussed to identify and use the most appropriate simulator for each situation. Objectives that met both general medicine and site-specific education were discussed, and the program was formulated.
Program Description
Nightmare on CIL Street is a simulation-based program designed for new internal medicine residents and students to encounter difficult situations (late at night, on call, or when resources are limited; ie, weekends/holidays) in a controlled simulation environment. During the simulation, learners will be unable to transfer the patient and no additional help is available. Each learner must determine a differential diagnosis and make appropriate medical interventions with only the assistance of a nurse. Scenarios are derived from common rapid response team calls and low-volume/high-impact situations where clinical decisions must be made quickly to ensure the best patient outcomes. High-fidelity manikins that have abilities to respond to questions, simulate breathing, reproduce pathological heart and breath sounds and more are used to create a realistic patient environment.
This program aligns with 2 national Veterans Health Administration priorities: (1) connect veterans to the soonest and best care; and (2) accelerate the Veterans Health Administration journey to be a high-reliability organization (sensitivity to operations, preoccupation with failure, commitment to resilience, and deference to expertise). Nightmare on CIL Street has 3 clinical episodes: 2 cardiac (A Tell-Tale Heart), respiratory (Don’t Breathe), and neurologic (Brain Scan). Additional clinical episodes will be added based on learner feedback and assessed need.
Each simulation event encompassed all 3 episodes that an individual or a team of 2 learners rotate through in a round-robin fashion. The overarching theme for each episode was a rapid response team call with minimal resources that the learner would have to provide care and stabilization. A literature search for rapid response team training programs found few results, but the literature assisted with providing a foundation for Nightmare on CIL Street.4,5 The goal was to completely envelop the learners in a nightmare scenario that required a solution.
After the safety brief and predata collection, learners received a phone call with minimal information about a patient in need of care. The learners responded to the requested area and provided treatment to the emergency over 25 minutes with the bedside nurse (who is an embedded participant). At the conclusion of the scenario, a physician subject matter expert who has been observing, provided a personalized 10-minute debriefing to the learner, which presented specific learning points and opportunities for the learner’s educational development. After the debriefing, learners returned to a conference room and awaited the next call. After all learners completed the 3 episodes, a group debriefing was conducted using the gather, analyze, summarize debriefing framework. The debriefing begins with an open-ended forum for learners to express their thoughts. Then, each scenario is discussed and broken down by key learning objectives. Starting with cardiac and ending with neurology, the logistics of the cases are discussed based on the trajectory of the learners during the scenarios. Each objective is discussed, and learners are allowed to ask questions before moving to the next scenario. After the debriefing, postevent data were gathered.
Objectives
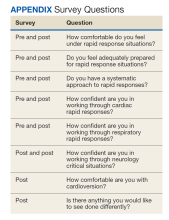
The program objective was to educate residents and students on common rapid response scenarios. We devised each scenario as an evolving simulation where various interventions would improve or worsen vital signs and symptoms. Each scenario had an end goal: cardioversion (cardiac), intubation (respiratory), and transfer (neurologic). Objectives were tailored to the trainees present during the specific simulation (Table).
IMPLEMENTATION
The initial run of the simulation curriculum was implemented on February 22, 2023, and ended on May 17, 2023, with 5 events. Participants included internal medicine PGY1 residents, third-year medical students, and fourth-year physician assistant students. Internal medicine residents ran each scenario with a subject matter expert monitoring; the undergraduate medical trainees partnered with another student. Students were pulled from their ward rotations to attend the simulation, and residents were pulled from electives and wards. Each trainee was able to experience each planned scenario. They were then briefed, participated in each scenario, and ended with a debriefing, discussing each case in detail. Two subject matter experts were always available, and occasionally 4 were present to provide additional knowledge transfer to learners. These included board-certified physicians in internal medicine and pulmonary critical care. Most scenarios were conducted on Wednesday afternoon or Thursday.
The CIL provided 6 staff minimum for every event. The staff controlled the manikins and acted as embedded players for the learners to interact and work with at the bedside. Every embedded RN was provided the same script: They were a new nurse just off orientation and did not know what to do. In addition, they were instructed that no matter who the learner wanted to call/page, that person or service was not answering or unavailable. This forced learners to respond and treat the simulated patient on their own.
Survey Responses
To evaluate the effect of this program on medical education, we administered surveys to the trainees before and after the simulation (Appendix). All questions were evaluated on a 10-point Likert scale (1, minimal comfort; 10, maximum comfort). The postsurvey added an additional Likert scale question and an open-ended question.
Sixteen trainees underwent the simulation curriculum during the 2022 to 2023 academic year, 9 internal medicine PGY1 residents, 4 medical students, and 3 physician assistant students. Postsimulation surveys indicated a mean 2.2 point increase in comfort compared with the presimulation surveys across all questions and participants.
DISCUSSION
The simulation curriculum proved to be successful for all parties, including trainees, medical educators, and simulation staff. Trainees expressed gratitude for the teaching ability of the simulation and the challenge of confronting an evolving scenario. Students also stated that the simulation allowed them to identify knowledge weaknesses.
Medical technology is rapidly advancing. A study evaluating high-fidelity medical simulations between 1969 and 2003 found that they are educationally effective and complement other medical education modalities.6 It is also noted that care provided by junior physicians with a lack of prior exposure to emergencies and unusual clinical syndromes can lead to more adverse effects.7 Simulation curriculums can be used to educate junior physicians as well as trainees on a multitude of medical emergencies, teach systematic approaches to medical scenarios, and increase exposure to unfamiliar experiences.
The goals of this article are to share program details and encourage other training programs with similar capabilities to incorporate simulation into medical education. Using pre- and postsimulation surveys, there was a concrete improvement in the value obtained by participating in this simulation. The Nightmare on CIL Street learners experienced a mean 2.2 point improvement from presimulation survey to postsimulation survey. Some notable improvements were the feelings of preparedness for rapid response situations and developing a systematic approach. As the students who participated in our Nightmare on CIL Street simulation were early in training, we believe the improvement in preparation and developing a systematic approach can be key to their success in their practical environments.
From a site-specific standpoint, improvement in confidence working through cardiac, respiratory, and neurological emergencies will be very useful. The anesthesiology service intubates during respiratory failures and there is no stroke neurologist available at the CTVHCS hospital. Giving trainees experience in these conditions may allow them to better understand their role in coordination during these times and potentially improve patient outcomes. A follow-up questionnaire administered a year after this simulation may be useful in ascertaining the usefulness of the simulation and what items may have been approached differently. We encourage other institutions to build in aspects of their site-specific challenges to improve trainee awareness in approaches to critical scenarios.
Challenges
The greatest challenge for Nightmare on CIL Street was the ability to pull internal medicine residents from their clinical duties to participate in the simulation. As there are many moving parts to their clinical scheduling, residents do not always have sufficient coverage to participate in training. There were also instances where residents needed to cover for another resident preventing them from attending the simulation. In the future, this program will schedule residents months in advance and will have the simulation training built into their rotations.
Medical and physician assistant students were pulled from their ward rotations as well. They rotate on a 2-to-4-week basis and often had already experienced the simulation the week prior, leaving out students for the following week. With more longitudinal planning, students can be pulled on a rotating monthly basis to maximize their participation. Another challenge was deciding whether residents should partner or experience the simulation on their own. After some feedback, it was noted that residents preferred to experience the simulation on their own as this improves their learning value. With the limited resources available, only rotating 3 residents on a scenario limits the number of trainees who can be reached with the program. Running this program throughout an academic year can help to reach more trainees.
CONCLUSIONS
Educating trainees on rapid response scenarios by using a simulation curriculum provides many benefits. Our trainees reported improvement in addressing cardiac, respiratory, and neurological rapid response scenarios after experiencing the simulation. They felt better prepared and had developed a better systematic approach for the future.
Acknowledgments
The authors thank Pawan Sikka, MD, George Martinez, MD and Braden Anderson, MD for participating as physician experts and educating our students. We thank Naomi Devers; Dinetra Jones; Stephanie Garrett; Sara Holton; Evelina Bartnick; Tanelle Smith; Michael Lomax; Shaun Kelemen for their participation as nurses, assistants, and simulation technology experts.
1. Guze PA. Using technology to meet the challenges of medical education. Trans Am Clin Climatol Assoc. 2015;126:260-270.
2. Higgins M, Madan C, Patel R. Development and decay of procedural skills in surgery: a systematic review of the effectiveness of simulation-based medical education interventions. Surgeon. 2021;19(4):e67-e77. doi:10.1016/j.surge.2020.07.013
3. Lyons PG, Edelson DP, Carey KA, et al. Characteristics of rapid response calls in the United States: an analysis of the first 402,023 adult cases from the Get With the Guidelines Resuscitation-Medical Emergency Team registry. Crit Care Med. 2019;47(10):1283-1289. doi:10.1097/CCM.0000000000003912
4. McMurray L, Hall AK, Rich J, Merchant S, Chaplin T. The nightmares course: a longitudinal, multidisciplinary, simulation-based curriculum to train and assess resident competence in resuscitation. J Grad Med Educ. 2017;9(4):503-508. doi:10.4300/JGME-D-16-00462.1
5. Gilic F, Schultz K, Sempowski I, Blagojevic A. “Nightmares-Family Medicine” course is an effective acute care teaching tool for family medicine residents. Simul Healthc. 2019;14(3):157-162. doi:10.1097/SIH.0000000000000355
6. Issenberg SB, McGaghie WC, Petrusa ER, Lee Gordon D, Scalese RJ. Features and uses of high-fidelity medical simulations that lead to effective learning: a BEME systematic review. Med Teach. 2005;27(1):10-28. doi:10.1080/01421590500046924
7. Datta R, Upadhyay K, Jaideep C. Simulation and its role in medical education. Med J Armed Forces India. 2012;68(2):167-172. doi:10.1016/S0377-1237(12)60040-9
The Central Texas Veteran’s Health Care System (CTVHCS) in Temple, Texas, is a 189-bed teaching hospital. CTVHCS opened the Center for Innovation and Learning (CIL) in 2022. The CIL has about 279 m2 of simulation space that includes high- and low-fidelity simulation equipment and multiple laboratories, which can be used to simulate inpatient and outpatient settings. The CIL high-fidelity manikins and environment allow learners to be immersed in the simulation for maximum realism. Computer and video systems provide clear viewing of training, which allows for more in-depth debriefing and learning. CIL simulation training is used by CTVHCS staff, medical residents, and medical and physician assistant students.
The utility of technology in medical education is rapidly evolving. As noted in many studies, simulation creates an environment that can imitate real patients in the format of a lifelike manikin, anatomic regions stations, clinical tasks, and many real-life circumstances.1 Task trainers for procedure simulation have been widely used and studied. A 2020 study noted that simulation training is effective for developing procedural skills in surgery and prevents the decay of surgical skills.2
In reviewing health care education curriculums, we noted that most of the rapid response situations are learned through active patient experiences. Rapid responses are managed by the intensive care unit and primary care teams during the day but at night are run primarily by the postgraduate year 2 (PGY2) night resident and intern. Knowing these logistics and current studies, we decided to build a rapid response simulation curriculum to improve preparedness for PGY1 residents, medical students, and physician assistant students.
Curriculum Planning
Planning the simulation curriculum began with the CTVHCS internal medicine chief resident and registered nurse (RN) educator. CTVHCS data were reviewed to identify the 3 most common rapid response calls from the past 3 years; research on the most common systems affected by rapid responses also was evaluated.
A 2019 study by Lyons and colleagues evaluated 402,023 rapid response activations across 360 hospitals and found that respiratory scenarios made up 38% and cardiac scenarios made up 37%.3 In addition, the CTVHCS has limited support in stroke neurology. Therefore, the internal medicine chief resident and RN educator decided to run 3 evolving rapid response scenarios per session that included cardiac, respiratory, and neurological scenarios. Capabilities and limitations of different high-fidelity manikins were discussed to identify and use the most appropriate simulator for each situation. Objectives that met both general medicine and site-specific education were discussed, and the program was formulated.
Program Description
Nightmare on CIL Street is a simulation-based program designed for new internal medicine residents and students to encounter difficult situations (late at night, on call, or when resources are limited; ie, weekends/holidays) in a controlled simulation environment. During the simulation, learners will be unable to transfer the patient and no additional help is available. Each learner must determine a differential diagnosis and make appropriate medical interventions with only the assistance of a nurse. Scenarios are derived from common rapid response team calls and low-volume/high-impact situations where clinical decisions must be made quickly to ensure the best patient outcomes. High-fidelity manikins that have abilities to respond to questions, simulate breathing, reproduce pathological heart and breath sounds and more are used to create a realistic patient environment.
This program aligns with 2 national Veterans Health Administration priorities: (1) connect veterans to the soonest and best care; and (2) accelerate the Veterans Health Administration journey to be a high-reliability organization (sensitivity to operations, preoccupation with failure, commitment to resilience, and deference to expertise). Nightmare on CIL Street has 3 clinical episodes: 2 cardiac (A Tell-Tale Heart), respiratory (Don’t Breathe), and neurologic (Brain Scan). Additional clinical episodes will be added based on learner feedback and assessed need.
Each simulation event encompassed all 3 episodes that an individual or a team of 2 learners rotate through in a round-robin fashion. The overarching theme for each episode was a rapid response team call with minimal resources that the learner would have to provide care and stabilization. A literature search for rapid response team training programs found few results, but the literature assisted with providing a foundation for Nightmare on CIL Street.4,5 The goal was to completely envelop the learners in a nightmare scenario that required a solution.
After the safety brief and predata collection, learners received a phone call with minimal information about a patient in need of care. The learners responded to the requested area and provided treatment to the emergency over 25 minutes with the bedside nurse (who is an embedded participant). At the conclusion of the scenario, a physician subject matter expert who has been observing, provided a personalized 10-minute debriefing to the learner, which presented specific learning points and opportunities for the learner’s educational development. After the debriefing, learners returned to a conference room and awaited the next call. After all learners completed the 3 episodes, a group debriefing was conducted using the gather, analyze, summarize debriefing framework. The debriefing begins with an open-ended forum for learners to express their thoughts. Then, each scenario is discussed and broken down by key learning objectives. Starting with cardiac and ending with neurology, the logistics of the cases are discussed based on the trajectory of the learners during the scenarios. Each objective is discussed, and learners are allowed to ask questions before moving to the next scenario. After the debriefing, postevent data were gathered.
Objectives
The program objective was to educate residents and students on common rapid response scenarios. We devised each scenario as an evolving simulation where various interventions would improve or worsen vital signs and symptoms. Each scenario had an end goal: cardioversion (cardiac), intubation (respiratory), and transfer (neurologic). Objectives were tailored to the trainees present during the specific simulation (Table).
IMPLEMENTATION
The initial run of the simulation curriculum was implemented on February 22, 2023, and ended on May 17, 2023, with 5 events. Participants included internal medicine PGY1 residents, third-year medical students, and fourth-year physician assistant students. Internal medicine residents ran each scenario with a subject matter expert monitoring; the undergraduate medical trainees partnered with another student. Students were pulled from their ward rotations to attend the simulation, and residents were pulled from electives and wards. Each trainee was able to experience each planned scenario. They were then briefed, participated in each scenario, and ended with a debriefing, discussing each case in detail. Two subject matter experts were always available, and occasionally 4 were present to provide additional knowledge transfer to learners. These included board-certified physicians in internal medicine and pulmonary critical care. Most scenarios were conducted on Wednesday afternoon or Thursday.
The CIL provided 6 staff minimum for every event. The staff controlled the manikins and acted as embedded players for the learners to interact and work with at the bedside. Every embedded RN was provided the same script: They were a new nurse just off orientation and did not know what to do. In addition, they were instructed that no matter who the learner wanted to call/page, that person or service was not answering or unavailable. This forced learners to respond and treat the simulated patient on their own.
Survey Responses
To evaluate the effect of this program on medical education, we administered surveys to the trainees before and after the simulation (Appendix). All questions were evaluated on a 10-point Likert scale (1, minimal comfort; 10, maximum comfort). The postsurvey added an additional Likert scale question and an open-ended question.
Sixteen trainees underwent the simulation curriculum during the 2022 to 2023 academic year, 9 internal medicine PGY1 residents, 4 medical students, and 3 physician assistant students. Postsimulation surveys indicated a mean 2.2 point increase in comfort compared with the presimulation surveys across all questions and participants.
DISCUSSION
The simulation curriculum proved to be successful for all parties, including trainees, medical educators, and simulation staff. Trainees expressed gratitude for the teaching ability of the simulation and the challenge of confronting an evolving scenario. Students also stated that the simulation allowed them to identify knowledge weaknesses.
Medical technology is rapidly advancing. A study evaluating high-fidelity medical simulations between 1969 and 2003 found that they are educationally effective and complement other medical education modalities.6 It is also noted that care provided by junior physicians with a lack of prior exposure to emergencies and unusual clinical syndromes can lead to more adverse effects.7 Simulation curriculums can be used to educate junior physicians as well as trainees on a multitude of medical emergencies, teach systematic approaches to medical scenarios, and increase exposure to unfamiliar experiences.
The goals of this article are to share program details and encourage other training programs with similar capabilities to incorporate simulation into medical education. Using pre- and postsimulation surveys, there was a concrete improvement in the value obtained by participating in this simulation. The Nightmare on CIL Street learners experienced a mean 2.2 point improvement from presimulation survey to postsimulation survey. Some notable improvements were the feelings of preparedness for rapid response situations and developing a systematic approach. As the students who participated in our Nightmare on CIL Street simulation were early in training, we believe the improvement in preparation and developing a systematic approach can be key to their success in their practical environments.
From a site-specific standpoint, improvement in confidence working through cardiac, respiratory, and neurological emergencies will be very useful. The anesthesiology service intubates during respiratory failures and there is no stroke neurologist available at the CTVHCS hospital. Giving trainees experience in these conditions may allow them to better understand their role in coordination during these times and potentially improve patient outcomes. A follow-up questionnaire administered a year after this simulation may be useful in ascertaining the usefulness of the simulation and what items may have been approached differently. We encourage other institutions to build in aspects of their site-specific challenges to improve trainee awareness in approaches to critical scenarios.
Challenges
The greatest challenge for Nightmare on CIL Street was the ability to pull internal medicine residents from their clinical duties to participate in the simulation. As there are many moving parts to their clinical scheduling, residents do not always have sufficient coverage to participate in training. There were also instances where residents needed to cover for another resident preventing them from attending the simulation. In the future, this program will schedule residents months in advance and will have the simulation training built into their rotations.
Medical and physician assistant students were pulled from their ward rotations as well. They rotate on a 2-to-4-week basis and often had already experienced the simulation the week prior, leaving out students for the following week. With more longitudinal planning, students can be pulled on a rotating monthly basis to maximize their participation. Another challenge was deciding whether residents should partner or experience the simulation on their own. After some feedback, it was noted that residents preferred to experience the simulation on their own as this improves their learning value. With the limited resources available, only rotating 3 residents on a scenario limits the number of trainees who can be reached with the program. Running this program throughout an academic year can help to reach more trainees.
CONCLUSIONS
Educating trainees on rapid response scenarios by using a simulation curriculum provides many benefits. Our trainees reported improvement in addressing cardiac, respiratory, and neurological rapid response scenarios after experiencing the simulation. They felt better prepared and had developed a better systematic approach for the future.
Acknowledgments
The authors thank Pawan Sikka, MD, George Martinez, MD and Braden Anderson, MD for participating as physician experts and educating our students. We thank Naomi Devers; Dinetra Jones; Stephanie Garrett; Sara Holton; Evelina Bartnick; Tanelle Smith; Michael Lomax; Shaun Kelemen for their participation as nurses, assistants, and simulation technology experts.
The Central Texas Veteran’s Health Care System (CTVHCS) in Temple, Texas, is a 189-bed teaching hospital. CTVHCS opened the Center for Innovation and Learning (CIL) in 2022. The CIL has about 279 m2 of simulation space that includes high- and low-fidelity simulation equipment and multiple laboratories, which can be used to simulate inpatient and outpatient settings. The CIL high-fidelity manikins and environment allow learners to be immersed in the simulation for maximum realism. Computer and video systems provide clear viewing of training, which allows for more in-depth debriefing and learning. CIL simulation training is used by CTVHCS staff, medical residents, and medical and physician assistant students.
The utility of technology in medical education is rapidly evolving. As noted in many studies, simulation creates an environment that can imitate real patients in the format of a lifelike manikin, anatomic regions stations, clinical tasks, and many real-life circumstances.1 Task trainers for procedure simulation have been widely used and studied. A 2020 study noted that simulation training is effective for developing procedural skills in surgery and prevents the decay of surgical skills.2
In reviewing health care education curriculums, we noted that most of the rapid response situations are learned through active patient experiences. Rapid responses are managed by the intensive care unit and primary care teams during the day but at night are run primarily by the postgraduate year 2 (PGY2) night resident and intern. Knowing these logistics and current studies, we decided to build a rapid response simulation curriculum to improve preparedness for PGY1 residents, medical students, and physician assistant students.
Curriculum Planning
Planning the simulation curriculum began with the CTVHCS internal medicine chief resident and registered nurse (RN) educator. CTVHCS data were reviewed to identify the 3 most common rapid response calls from the past 3 years; research on the most common systems affected by rapid responses also was evaluated.
A 2019 study by Lyons and colleagues evaluated 402,023 rapid response activations across 360 hospitals and found that respiratory scenarios made up 38% and cardiac scenarios made up 37%.3 In addition, the CTVHCS has limited support in stroke neurology. Therefore, the internal medicine chief resident and RN educator decided to run 3 evolving rapid response scenarios per session that included cardiac, respiratory, and neurological scenarios. Capabilities and limitations of different high-fidelity manikins were discussed to identify and use the most appropriate simulator for each situation. Objectives that met both general medicine and site-specific education were discussed, and the program was formulated.
Program Description
Nightmare on CIL Street is a simulation-based program designed for new internal medicine residents and students to encounter difficult situations (late at night, on call, or when resources are limited; ie, weekends/holidays) in a controlled simulation environment. During the simulation, learners will be unable to transfer the patient and no additional help is available. Each learner must determine a differential diagnosis and make appropriate medical interventions with only the assistance of a nurse. Scenarios are derived from common rapid response team calls and low-volume/high-impact situations where clinical decisions must be made quickly to ensure the best patient outcomes. High-fidelity manikins that have abilities to respond to questions, simulate breathing, reproduce pathological heart and breath sounds and more are used to create a realistic patient environment.
This program aligns with 2 national Veterans Health Administration priorities: (1) connect veterans to the soonest and best care; and (2) accelerate the Veterans Health Administration journey to be a high-reliability organization (sensitivity to operations, preoccupation with failure, commitment to resilience, and deference to expertise). Nightmare on CIL Street has 3 clinical episodes: 2 cardiac (A Tell-Tale Heart), respiratory (Don’t Breathe), and neurologic (Brain Scan). Additional clinical episodes will be added based on learner feedback and assessed need.
Each simulation event encompassed all 3 episodes that an individual or a team of 2 learners rotate through in a round-robin fashion. The overarching theme for each episode was a rapid response team call with minimal resources that the learner would have to provide care and stabilization. A literature search for rapid response team training programs found few results, but the literature assisted with providing a foundation for Nightmare on CIL Street.4,5 The goal was to completely envelop the learners in a nightmare scenario that required a solution.
After the safety brief and predata collection, learners received a phone call with minimal information about a patient in need of care. The learners responded to the requested area and provided treatment to the emergency over 25 minutes with the bedside nurse (who is an embedded participant). At the conclusion of the scenario, a physician subject matter expert who has been observing, provided a personalized 10-minute debriefing to the learner, which presented specific learning points and opportunities for the learner’s educational development. After the debriefing, learners returned to a conference room and awaited the next call. After all learners completed the 3 episodes, a group debriefing was conducted using the gather, analyze, summarize debriefing framework. The debriefing begins with an open-ended forum for learners to express their thoughts. Then, each scenario is discussed and broken down by key learning objectives. Starting with cardiac and ending with neurology, the logistics of the cases are discussed based on the trajectory of the learners during the scenarios. Each objective is discussed, and learners are allowed to ask questions before moving to the next scenario. After the debriefing, postevent data were gathered.
Objectives
The program objective was to educate residents and students on common rapid response scenarios. We devised each scenario as an evolving simulation where various interventions would improve or worsen vital signs and symptoms. Each scenario had an end goal: cardioversion (cardiac), intubation (respiratory), and transfer (neurologic). Objectives were tailored to the trainees present during the specific simulation (Table).
IMPLEMENTATION
The initial run of the simulation curriculum was implemented on February 22, 2023, and ended on May 17, 2023, with 5 events. Participants included internal medicine PGY1 residents, third-year medical students, and fourth-year physician assistant students. Internal medicine residents ran each scenario with a subject matter expert monitoring; the undergraduate medical trainees partnered with another student. Students were pulled from their ward rotations to attend the simulation, and residents were pulled from electives and wards. Each trainee was able to experience each planned scenario. They were then briefed, participated in each scenario, and ended with a debriefing, discussing each case in detail. Two subject matter experts were always available, and occasionally 4 were present to provide additional knowledge transfer to learners. These included board-certified physicians in internal medicine and pulmonary critical care. Most scenarios were conducted on Wednesday afternoon or Thursday.
The CIL provided 6 staff minimum for every event. The staff controlled the manikins and acted as embedded players for the learners to interact and work with at the bedside. Every embedded RN was provided the same script: They were a new nurse just off orientation and did not know what to do. In addition, they were instructed that no matter who the learner wanted to call/page, that person or service was not answering or unavailable. This forced learners to respond and treat the simulated patient on their own.
Survey Responses
To evaluate the effect of this program on medical education, we administered surveys to the trainees before and after the simulation (Appendix). All questions were evaluated on a 10-point Likert scale (1, minimal comfort; 10, maximum comfort). The postsurvey added an additional Likert scale question and an open-ended question.
Sixteen trainees underwent the simulation curriculum during the 2022 to 2023 academic year, 9 internal medicine PGY1 residents, 4 medical students, and 3 physician assistant students. Postsimulation surveys indicated a mean 2.2 point increase in comfort compared with the presimulation surveys across all questions and participants.
DISCUSSION
The simulation curriculum proved to be successful for all parties, including trainees, medical educators, and simulation staff. Trainees expressed gratitude for the teaching ability of the simulation and the challenge of confronting an evolving scenario. Students also stated that the simulation allowed them to identify knowledge weaknesses.
Medical technology is rapidly advancing. A study evaluating high-fidelity medical simulations between 1969 and 2003 found that they are educationally effective and complement other medical education modalities.6 It is also noted that care provided by junior physicians with a lack of prior exposure to emergencies and unusual clinical syndromes can lead to more adverse effects.7 Simulation curriculums can be used to educate junior physicians as well as trainees on a multitude of medical emergencies, teach systematic approaches to medical scenarios, and increase exposure to unfamiliar experiences.
The goals of this article are to share program details and encourage other training programs with similar capabilities to incorporate simulation into medical education. Using pre- and postsimulation surveys, there was a concrete improvement in the value obtained by participating in this simulation. The Nightmare on CIL Street learners experienced a mean 2.2 point improvement from presimulation survey to postsimulation survey. Some notable improvements were the feelings of preparedness for rapid response situations and developing a systematic approach. As the students who participated in our Nightmare on CIL Street simulation were early in training, we believe the improvement in preparation and developing a systematic approach can be key to their success in their practical environments.
From a site-specific standpoint, improvement in confidence working through cardiac, respiratory, and neurological emergencies will be very useful. The anesthesiology service intubates during respiratory failures and there is no stroke neurologist available at the CTVHCS hospital. Giving trainees experience in these conditions may allow them to better understand their role in coordination during these times and potentially improve patient outcomes. A follow-up questionnaire administered a year after this simulation may be useful in ascertaining the usefulness of the simulation and what items may have been approached differently. We encourage other institutions to build in aspects of their site-specific challenges to improve trainee awareness in approaches to critical scenarios.
Challenges
The greatest challenge for Nightmare on CIL Street was the ability to pull internal medicine residents from their clinical duties to participate in the simulation. As there are many moving parts to their clinical scheduling, residents do not always have sufficient coverage to participate in training. There were also instances where residents needed to cover for another resident preventing them from attending the simulation. In the future, this program will schedule residents months in advance and will have the simulation training built into their rotations.
Medical and physician assistant students were pulled from their ward rotations as well. They rotate on a 2-to-4-week basis and often had already experienced the simulation the week prior, leaving out students for the following week. With more longitudinal planning, students can be pulled on a rotating monthly basis to maximize their participation. Another challenge was deciding whether residents should partner or experience the simulation on their own. After some feedback, it was noted that residents preferred to experience the simulation on their own as this improves their learning value. With the limited resources available, only rotating 3 residents on a scenario limits the number of trainees who can be reached with the program. Running this program throughout an academic year can help to reach more trainees.
CONCLUSIONS
Educating trainees on rapid response scenarios by using a simulation curriculum provides many benefits. Our trainees reported improvement in addressing cardiac, respiratory, and neurological rapid response scenarios after experiencing the simulation. They felt better prepared and had developed a better systematic approach for the future.
Acknowledgments
The authors thank Pawan Sikka, MD, George Martinez, MD and Braden Anderson, MD for participating as physician experts and educating our students. We thank Naomi Devers; Dinetra Jones; Stephanie Garrett; Sara Holton; Evelina Bartnick; Tanelle Smith; Michael Lomax; Shaun Kelemen for their participation as nurses, assistants, and simulation technology experts.
1. Guze PA. Using technology to meet the challenges of medical education. Trans Am Clin Climatol Assoc. 2015;126:260-270.
2. Higgins M, Madan C, Patel R. Development and decay of procedural skills in surgery: a systematic review of the effectiveness of simulation-based medical education interventions. Surgeon. 2021;19(4):e67-e77. doi:10.1016/j.surge.2020.07.013
3. Lyons PG, Edelson DP, Carey KA, et al. Characteristics of rapid response calls in the United States: an analysis of the first 402,023 adult cases from the Get With the Guidelines Resuscitation-Medical Emergency Team registry. Crit Care Med. 2019;47(10):1283-1289. doi:10.1097/CCM.0000000000003912
4. McMurray L, Hall AK, Rich J, Merchant S, Chaplin T. The nightmares course: a longitudinal, multidisciplinary, simulation-based curriculum to train and assess resident competence in resuscitation. J Grad Med Educ. 2017;9(4):503-508. doi:10.4300/JGME-D-16-00462.1
5. Gilic F, Schultz K, Sempowski I, Blagojevic A. “Nightmares-Family Medicine” course is an effective acute care teaching tool for family medicine residents. Simul Healthc. 2019;14(3):157-162. doi:10.1097/SIH.0000000000000355
6. Issenberg SB, McGaghie WC, Petrusa ER, Lee Gordon D, Scalese RJ. Features and uses of high-fidelity medical simulations that lead to effective learning: a BEME systematic review. Med Teach. 2005;27(1):10-28. doi:10.1080/01421590500046924
7. Datta R, Upadhyay K, Jaideep C. Simulation and its role in medical education. Med J Armed Forces India. 2012;68(2):167-172. doi:10.1016/S0377-1237(12)60040-9
1. Guze PA. Using technology to meet the challenges of medical education. Trans Am Clin Climatol Assoc. 2015;126:260-270.
2. Higgins M, Madan C, Patel R. Development and decay of procedural skills in surgery: a systematic review of the effectiveness of simulation-based medical education interventions. Surgeon. 2021;19(4):e67-e77. doi:10.1016/j.surge.2020.07.013
3. Lyons PG, Edelson DP, Carey KA, et al. Characteristics of rapid response calls in the United States: an analysis of the first 402,023 adult cases from the Get With the Guidelines Resuscitation-Medical Emergency Team registry. Crit Care Med. 2019;47(10):1283-1289. doi:10.1097/CCM.0000000000003912
4. McMurray L, Hall AK, Rich J, Merchant S, Chaplin T. The nightmares course: a longitudinal, multidisciplinary, simulation-based curriculum to train and assess resident competence in resuscitation. J Grad Med Educ. 2017;9(4):503-508. doi:10.4300/JGME-D-16-00462.1
5. Gilic F, Schultz K, Sempowski I, Blagojevic A. “Nightmares-Family Medicine” course is an effective acute care teaching tool for family medicine residents. Simul Healthc. 2019;14(3):157-162. doi:10.1097/SIH.0000000000000355
6. Issenberg SB, McGaghie WC, Petrusa ER, Lee Gordon D, Scalese RJ. Features and uses of high-fidelity medical simulations that lead to effective learning: a BEME systematic review. Med Teach. 2005;27(1):10-28. doi:10.1080/01421590500046924
7. Datta R, Upadhyay K, Jaideep C. Simulation and its role in medical education. Med J Armed Forces India. 2012;68(2):167-172. doi:10.1016/S0377-1237(12)60040-9
ILD: Time lost is lung lost
First launched in 2022 in partnership with Three Lakes Foundation, Bridging Specialties™: Timely Diagnosis for ILD is a collaborative initiative hinged on bringing together pulmonary and primary care experts. To shorten the time to diagnosis for interstitial lung diseases (ILDs) like pulmonary fibrosis, The steering committee of experts from both fields created a clinician-facing toolkit that, with support of two quality improvement grants, will be introduced into health care institutions in 2024.
Kavitha Selvan, MD, Pulmonary and Critical Care Fellow at the University of Chicago School of Medicine, and Amirahwaty Abdullah, MBBS, Assistant Professor & Critical Care Medicine Associate Program Director at the West Virginia University School of Medicine, are the recipients of the grants. Each recipient will receive funding to implement strategic quality improvement projects designed to work closely with primary care partners and address the needs of their communities to shorten the time to diagnosis for patients with ILD.
Dr. Selvan’s project leverages the diverse population of Chicago and will engage primary care physicians by working closely with the Medical Director of the Primary Care Group within the University of Chicago. “There is a growing body of research that illustrates vast racial and ethnic disparities in ILD outcomes, including time to diagnosis and survival. The diverse community we serve in Chicago provided the inspiration for our project, which we hope will enable us to take a meaningful step toward achieving equity in health care,” Dr. Selvan said. “Through close collaboration with the dedicated physicians in our Primary Care Group, we aim to increase recognition of signs and symptoms suggestive of ILD earlier in the course of disease and streamline the thoughtful, multidisciplinary care our patients need.”
Affecting 400,000 people in the United States, ILDs are often overlooked as a potential diagnosis given their rarity. A proper diagnosis for this disease is further complicated by ubiquitous presenting symptoms that are common in many other diseases, including asthma, COPD, and cardiac conditions, and often leads to a misdiagnosis. This delay in diagnosis, or an outright misdiagnosis, leads to additional delays in receiving proper treatment and, subsequently, a degradation in the patient’s quality of life. For Dr. Abdullah, the rarity of the disease is not the issue; rather, there is an access issue. Because of this, their project will focus on telemedicine implementation to meet the needs of their area. “While ILD is a rare disease, the state of West Virginia has a disproportionately increased prevalence due to a variety of societal factors,” Dr. Abdullah said. “Despite this prevalence, there is one ILD clinic in the state of West Virginia in comparison to 1,253 primary care providers throughout the state. To address this gap, the project will focus on expanding telemedicine capabilities in order to reach these patients virtually through their primary care physicians who would help us to facilitate the video-assisted visits.”
To learn more about the toolkit they will be implementing, visit the CHEST website.
First launched in 2022 in partnership with Three Lakes Foundation, Bridging Specialties™: Timely Diagnosis for ILD is a collaborative initiative hinged on bringing together pulmonary and primary care experts. To shorten the time to diagnosis for interstitial lung diseases (ILDs) like pulmonary fibrosis, The steering committee of experts from both fields created a clinician-facing toolkit that, with support of two quality improvement grants, will be introduced into health care institutions in 2024.
Kavitha Selvan, MD, Pulmonary and Critical Care Fellow at the University of Chicago School of Medicine, and Amirahwaty Abdullah, MBBS, Assistant Professor & Critical Care Medicine Associate Program Director at the West Virginia University School of Medicine, are the recipients of the grants. Each recipient will receive funding to implement strategic quality improvement projects designed to work closely with primary care partners and address the needs of their communities to shorten the time to diagnosis for patients with ILD.
Dr. Selvan’s project leverages the diverse population of Chicago and will engage primary care physicians by working closely with the Medical Director of the Primary Care Group within the University of Chicago. “There is a growing body of research that illustrates vast racial and ethnic disparities in ILD outcomes, including time to diagnosis and survival. The diverse community we serve in Chicago provided the inspiration for our project, which we hope will enable us to take a meaningful step toward achieving equity in health care,” Dr. Selvan said. “Through close collaboration with the dedicated physicians in our Primary Care Group, we aim to increase recognition of signs and symptoms suggestive of ILD earlier in the course of disease and streamline the thoughtful, multidisciplinary care our patients need.”
Affecting 400,000 people in the United States, ILDs are often overlooked as a potential diagnosis given their rarity. A proper diagnosis for this disease is further complicated by ubiquitous presenting symptoms that are common in many other diseases, including asthma, COPD, and cardiac conditions, and often leads to a misdiagnosis. This delay in diagnosis, or an outright misdiagnosis, leads to additional delays in receiving proper treatment and, subsequently, a degradation in the patient’s quality of life. For Dr. Abdullah, the rarity of the disease is not the issue; rather, there is an access issue. Because of this, their project will focus on telemedicine implementation to meet the needs of their area. “While ILD is a rare disease, the state of West Virginia has a disproportionately increased prevalence due to a variety of societal factors,” Dr. Abdullah said. “Despite this prevalence, there is one ILD clinic in the state of West Virginia in comparison to 1,253 primary care providers throughout the state. To address this gap, the project will focus on expanding telemedicine capabilities in order to reach these patients virtually through their primary care physicians who would help us to facilitate the video-assisted visits.”
To learn more about the toolkit they will be implementing, visit the CHEST website.
First launched in 2022 in partnership with Three Lakes Foundation, Bridging Specialties™: Timely Diagnosis for ILD is a collaborative initiative hinged on bringing together pulmonary and primary care experts. To shorten the time to diagnosis for interstitial lung diseases (ILDs) like pulmonary fibrosis, The steering committee of experts from both fields created a clinician-facing toolkit that, with support of two quality improvement grants, will be introduced into health care institutions in 2024.
Kavitha Selvan, MD, Pulmonary and Critical Care Fellow at the University of Chicago School of Medicine, and Amirahwaty Abdullah, MBBS, Assistant Professor & Critical Care Medicine Associate Program Director at the West Virginia University School of Medicine, are the recipients of the grants. Each recipient will receive funding to implement strategic quality improvement projects designed to work closely with primary care partners and address the needs of their communities to shorten the time to diagnosis for patients with ILD.
Dr. Selvan’s project leverages the diverse population of Chicago and will engage primary care physicians by working closely with the Medical Director of the Primary Care Group within the University of Chicago. “There is a growing body of research that illustrates vast racial and ethnic disparities in ILD outcomes, including time to diagnosis and survival. The diverse community we serve in Chicago provided the inspiration for our project, which we hope will enable us to take a meaningful step toward achieving equity in health care,” Dr. Selvan said. “Through close collaboration with the dedicated physicians in our Primary Care Group, we aim to increase recognition of signs and symptoms suggestive of ILD earlier in the course of disease and streamline the thoughtful, multidisciplinary care our patients need.”
Affecting 400,000 people in the United States, ILDs are often overlooked as a potential diagnosis given their rarity. A proper diagnosis for this disease is further complicated by ubiquitous presenting symptoms that are common in many other diseases, including asthma, COPD, and cardiac conditions, and often leads to a misdiagnosis. This delay in diagnosis, or an outright misdiagnosis, leads to additional delays in receiving proper treatment and, subsequently, a degradation in the patient’s quality of life. For Dr. Abdullah, the rarity of the disease is not the issue; rather, there is an access issue. Because of this, their project will focus on telemedicine implementation to meet the needs of their area. “While ILD is a rare disease, the state of West Virginia has a disproportionately increased prevalence due to a variety of societal factors,” Dr. Abdullah said. “Despite this prevalence, there is one ILD clinic in the state of West Virginia in comparison to 1,253 primary care providers throughout the state. To address this gap, the project will focus on expanding telemedicine capabilities in order to reach these patients virtually through their primary care physicians who would help us to facilitate the video-assisted visits.”
To learn more about the toolkit they will be implementing, visit the CHEST website.
Gen Z is hooked on vaping
Exploring the obstacles to nicotine cessation among teens
Pulmonologist Evan Stepp, MD, FCCP, has a teenage daughter who doesn’t smoke or vape – as far as he knows, Stepp will admit – but the statistics on youth smoking are alarming enough to have him worried.
On one hand, fewer Americans are smoking today than ever before. Since 1992, the percentage of people who told Gallup that they’d had a cigarette in the past week has dropped from 28% to 11%. Meanwhile, the rate of new lung cancer cases declined from 65 per every 100,000 people in 1992 to 34 per 100,000 in 2020, according to the National Cancer Institute.
According to a November 2022 report from the Centers for Disease Control and Prevention (CDC), 1 in 6 high school students and 1 in 20 middle schoolers are using a nicotine product at least once every day.
“It’s a completely different picture for nicotine cessation in youth,” Dr. Stepp, who is an associate professor at National Jewish Health in Denver, said. “Because of the fact that the nicotine addiction is occurring in a developing brain, which raises many other nicotine-related harms.”
Why teens vape
Today’s teens are smoking less actual tobacco, and, instead, overwhelmingly prefer e-cigarettes or vaping. In fact, 85% of high school–aged smokers and 72% of middle school smokers reach for a vape over regular cigarettes or smokeless tobacco, according to the CDC.
It’s not hard to understand why: e-cigarettes use a heating element to turn a nicotine-infused liquid into an aerosol, with no open flame, ash, or lingering smoke. The vapes themselves are easy to conceal, and if someone needed to hide an e-cigarette from particularly perceptive parents or teachers, they can find vapes built into hoodies, fake smartwatches, and USB drives.
Plus, the liquids often come in flavors like fruit, bubble gum, mint, and vanilla, because unflavored nicotine isn’t exactly appealing. “Huge concentrations of nicotine salts are just miserable to breathe in,” Dr. Stepp said. “Flavors are necessary to make these products palatable, and those flavors end up being a huge draw for youth users to get exposed to nicotine addiction.”
Challenges surrounding smoking cessation in youth
The powerful effect of nicotine in youth means the need for effective cessation strategies is both more urgent and more difficult. But while physicians can prescribe to adults the antidepressants varenicline and bupropion, along with nicotine replacement therapy, to help ease withdrawal symptoms, the US Food and Drug Administration (FDA) has not approved those medications for anyone under the age of 18.
Research on cessation medications in young people is limited: A recent meta-analysis found only four studies on people between the ages of 12 and 21. In teens, antidepressants seem to help quitting for the first few weeks but are unproven as a long-term solution.
“That really has been a challenge for the 1 in 6 high school students who are current users of tobacco products,” said pediatrician, Susan Walley, MD, a co-author of the American Academy of Pediatrics’ recent position papers on children and smoking.
“One of the things that is important to keep at the forefront of the conversation is that nicotine addiction is a chronic medical disease, and it’s a form of substance abuse,” Dr. Walley said. “We know that we need more research in adolescent tobacco cessation, and it really is about the funding, about research dollars.”
Without medications, smoking cessation in teens relies largely on counseling strategies. A 2017 review published by Cochrane Library found that group counseling was the most effective quitting method, with teens participating in group sessions 35% more likely to stop using nicotine products up to a year later, compared with teens who did not receive any counseling.
Counseling can help educate teens (and parents) on some of the realities of e-cigarettes, bridging the gap between well-established anti-smoking campaigns and the anti-vape campaigns that have yet to catch up.
“We have done a great job promoting cigarette use as dangerous,” Dr. Walley said. “[But] many teens who would never pick up a cigarette –because they know the health risks – are vaping.”
How to get a teen to quit
Cessation and prevention strategies are closely linked, and interventions can start in middle school-aged children up through high school and young adults. Simply asking a 12-year-old, “Do you know anyone who smokes?” can help start a conversation that leads to an attempt to quit.
Teens may be compelled to smoke through digital advertising and influencer endorsements on social media platforms, but Gen Z is turned off by the idea that it’s being manipulated by the tobacco industry. Juul, for example, is partially owned by Altria, which makes Marlboros, and Vuse is wholly owned by R.J. Reynolds, which makes Camel cigarettes.
“If you can get somebody to understand that Big Tobacco is trying to manipulate you as a young person to want to illegally obtain and use their products, which are incredibly addictive, thus ensuring you will remain a loyal customer, that could be the thing that pushes them over the hump,” Dr. Stepp said. “You push it away like you would push away a parent trying to tell you how to park a car in the driveway.”
And just because a smoker relapses, it doesn’t mean the cessation was a complete failure. The younger someone is when they stop smoking, the less likely they are to suffer from the long-term health consequences of smoking, according to a 2021 study in the Journal of the American Medical Association. “With the right counseling,” Dr. Walley said, “each relapse is an opportunity for losing the habit permanently.”
This article was adapted from the Summer 2023 online issue of CHEST Advocates. For the full article – and to engage with the other content from this issue – visit https://chestnet.org/chest-advocates.
Exploring the obstacles to nicotine cessation among teens
Exploring the obstacles to nicotine cessation among teens
Pulmonologist Evan Stepp, MD, FCCP, has a teenage daughter who doesn’t smoke or vape – as far as he knows, Stepp will admit – but the statistics on youth smoking are alarming enough to have him worried.
On one hand, fewer Americans are smoking today than ever before. Since 1992, the percentage of people who told Gallup that they’d had a cigarette in the past week has dropped from 28% to 11%. Meanwhile, the rate of new lung cancer cases declined from 65 per every 100,000 people in 1992 to 34 per 100,000 in 2020, according to the National Cancer Institute.
According to a November 2022 report from the Centers for Disease Control and Prevention (CDC), 1 in 6 high school students and 1 in 20 middle schoolers are using a nicotine product at least once every day.
“It’s a completely different picture for nicotine cessation in youth,” Dr. Stepp, who is an associate professor at National Jewish Health in Denver, said. “Because of the fact that the nicotine addiction is occurring in a developing brain, which raises many other nicotine-related harms.”
Why teens vape
Today’s teens are smoking less actual tobacco, and, instead, overwhelmingly prefer e-cigarettes or vaping. In fact, 85% of high school–aged smokers and 72% of middle school smokers reach for a vape over regular cigarettes or smokeless tobacco, according to the CDC.
It’s not hard to understand why: e-cigarettes use a heating element to turn a nicotine-infused liquid into an aerosol, with no open flame, ash, or lingering smoke. The vapes themselves are easy to conceal, and if someone needed to hide an e-cigarette from particularly perceptive parents or teachers, they can find vapes built into hoodies, fake smartwatches, and USB drives.
Plus, the liquids often come in flavors like fruit, bubble gum, mint, and vanilla, because unflavored nicotine isn’t exactly appealing. “Huge concentrations of nicotine salts are just miserable to breathe in,” Dr. Stepp said. “Flavors are necessary to make these products palatable, and those flavors end up being a huge draw for youth users to get exposed to nicotine addiction.”
Challenges surrounding smoking cessation in youth
The powerful effect of nicotine in youth means the need for effective cessation strategies is both more urgent and more difficult. But while physicians can prescribe to adults the antidepressants varenicline and bupropion, along with nicotine replacement therapy, to help ease withdrawal symptoms, the US Food and Drug Administration (FDA) has not approved those medications for anyone under the age of 18.
Research on cessation medications in young people is limited: A recent meta-analysis found only four studies on people between the ages of 12 and 21. In teens, antidepressants seem to help quitting for the first few weeks but are unproven as a long-term solution.
“That really has been a challenge for the 1 in 6 high school students who are current users of tobacco products,” said pediatrician, Susan Walley, MD, a co-author of the American Academy of Pediatrics’ recent position papers on children and smoking.
“One of the things that is important to keep at the forefront of the conversation is that nicotine addiction is a chronic medical disease, and it’s a form of substance abuse,” Dr. Walley said. “We know that we need more research in adolescent tobacco cessation, and it really is about the funding, about research dollars.”
Without medications, smoking cessation in teens relies largely on counseling strategies. A 2017 review published by Cochrane Library found that group counseling was the most effective quitting method, with teens participating in group sessions 35% more likely to stop using nicotine products up to a year later, compared with teens who did not receive any counseling.
Counseling can help educate teens (and parents) on some of the realities of e-cigarettes, bridging the gap between well-established anti-smoking campaigns and the anti-vape campaigns that have yet to catch up.
“We have done a great job promoting cigarette use as dangerous,” Dr. Walley said. “[But] many teens who would never pick up a cigarette –because they know the health risks – are vaping.”
How to get a teen to quit
Cessation and prevention strategies are closely linked, and interventions can start in middle school-aged children up through high school and young adults. Simply asking a 12-year-old, “Do you know anyone who smokes?” can help start a conversation that leads to an attempt to quit.
Teens may be compelled to smoke through digital advertising and influencer endorsements on social media platforms, but Gen Z is turned off by the idea that it’s being manipulated by the tobacco industry. Juul, for example, is partially owned by Altria, which makes Marlboros, and Vuse is wholly owned by R.J. Reynolds, which makes Camel cigarettes.
“If you can get somebody to understand that Big Tobacco is trying to manipulate you as a young person to want to illegally obtain and use their products, which are incredibly addictive, thus ensuring you will remain a loyal customer, that could be the thing that pushes them over the hump,” Dr. Stepp said. “You push it away like you would push away a parent trying to tell you how to park a car in the driveway.”
And just because a smoker relapses, it doesn’t mean the cessation was a complete failure. The younger someone is when they stop smoking, the less likely they are to suffer from the long-term health consequences of smoking, according to a 2021 study in the Journal of the American Medical Association. “With the right counseling,” Dr. Walley said, “each relapse is an opportunity for losing the habit permanently.”
This article was adapted from the Summer 2023 online issue of CHEST Advocates. For the full article – and to engage with the other content from this issue – visit https://chestnet.org/chest-advocates.
Pulmonologist Evan Stepp, MD, FCCP, has a teenage daughter who doesn’t smoke or vape – as far as he knows, Stepp will admit – but the statistics on youth smoking are alarming enough to have him worried.
On one hand, fewer Americans are smoking today than ever before. Since 1992, the percentage of people who told Gallup that they’d had a cigarette in the past week has dropped from 28% to 11%. Meanwhile, the rate of new lung cancer cases declined from 65 per every 100,000 people in 1992 to 34 per 100,000 in 2020, according to the National Cancer Institute.
According to a November 2022 report from the Centers for Disease Control and Prevention (CDC), 1 in 6 high school students and 1 in 20 middle schoolers are using a nicotine product at least once every day.
“It’s a completely different picture for nicotine cessation in youth,” Dr. Stepp, who is an associate professor at National Jewish Health in Denver, said. “Because of the fact that the nicotine addiction is occurring in a developing brain, which raises many other nicotine-related harms.”
Why teens vape
Today’s teens are smoking less actual tobacco, and, instead, overwhelmingly prefer e-cigarettes or vaping. In fact, 85% of high school–aged smokers and 72% of middle school smokers reach for a vape over regular cigarettes or smokeless tobacco, according to the CDC.
It’s not hard to understand why: e-cigarettes use a heating element to turn a nicotine-infused liquid into an aerosol, with no open flame, ash, or lingering smoke. The vapes themselves are easy to conceal, and if someone needed to hide an e-cigarette from particularly perceptive parents or teachers, they can find vapes built into hoodies, fake smartwatches, and USB drives.
Plus, the liquids often come in flavors like fruit, bubble gum, mint, and vanilla, because unflavored nicotine isn’t exactly appealing. “Huge concentrations of nicotine salts are just miserable to breathe in,” Dr. Stepp said. “Flavors are necessary to make these products palatable, and those flavors end up being a huge draw for youth users to get exposed to nicotine addiction.”
Challenges surrounding smoking cessation in youth
The powerful effect of nicotine in youth means the need for effective cessation strategies is both more urgent and more difficult. But while physicians can prescribe to adults the antidepressants varenicline and bupropion, along with nicotine replacement therapy, to help ease withdrawal symptoms, the US Food and Drug Administration (FDA) has not approved those medications for anyone under the age of 18.
Research on cessation medications in young people is limited: A recent meta-analysis found only four studies on people between the ages of 12 and 21. In teens, antidepressants seem to help quitting for the first few weeks but are unproven as a long-term solution.
“That really has been a challenge for the 1 in 6 high school students who are current users of tobacco products,” said pediatrician, Susan Walley, MD, a co-author of the American Academy of Pediatrics’ recent position papers on children and smoking.
“One of the things that is important to keep at the forefront of the conversation is that nicotine addiction is a chronic medical disease, and it’s a form of substance abuse,” Dr. Walley said. “We know that we need more research in adolescent tobacco cessation, and it really is about the funding, about research dollars.”
Without medications, smoking cessation in teens relies largely on counseling strategies. A 2017 review published by Cochrane Library found that group counseling was the most effective quitting method, with teens participating in group sessions 35% more likely to stop using nicotine products up to a year later, compared with teens who did not receive any counseling.
Counseling can help educate teens (and parents) on some of the realities of e-cigarettes, bridging the gap between well-established anti-smoking campaigns and the anti-vape campaigns that have yet to catch up.
“We have done a great job promoting cigarette use as dangerous,” Dr. Walley said. “[But] many teens who would never pick up a cigarette –because they know the health risks – are vaping.”
How to get a teen to quit
Cessation and prevention strategies are closely linked, and interventions can start in middle school-aged children up through high school and young adults. Simply asking a 12-year-old, “Do you know anyone who smokes?” can help start a conversation that leads to an attempt to quit.
Teens may be compelled to smoke through digital advertising and influencer endorsements on social media platforms, but Gen Z is turned off by the idea that it’s being manipulated by the tobacco industry. Juul, for example, is partially owned by Altria, which makes Marlboros, and Vuse is wholly owned by R.J. Reynolds, which makes Camel cigarettes.
“If you can get somebody to understand that Big Tobacco is trying to manipulate you as a young person to want to illegally obtain and use their products, which are incredibly addictive, thus ensuring you will remain a loyal customer, that could be the thing that pushes them over the hump,” Dr. Stepp said. “You push it away like you would push away a parent trying to tell you how to park a car in the driveway.”
And just because a smoker relapses, it doesn’t mean the cessation was a complete failure. The younger someone is when they stop smoking, the less likely they are to suffer from the long-term health consequences of smoking, according to a 2021 study in the Journal of the American Medical Association. “With the right counseling,” Dr. Walley said, “each relapse is an opportunity for losing the habit permanently.”
This article was adapted from the Summer 2023 online issue of CHEST Advocates. For the full article – and to engage with the other content from this issue – visit https://chestnet.org/chest-advocates.
Sedative use in older adults after critical illness
Modifications to medication regimens may include stopping home medications for chronic conditions, dose adjustments for altered organ function, or initiating new treatments for acute illness(es). Common examples of changes to a critically ill patient’s medication regimen are stopping a chronic antihypertensive drug in the setting of shock, holding an oral medication that cannot be crushed or administered through a feeding tube, and initiating sedatives and analgesics to support invasive mechanical ventilation. Medication regimens are especially vulnerable to errors and omissions at transition points (i.e., ICU to ward transfers and home discharge). As critical illness resolves and patients transition to different care teams, the hospital discharge medication regimen may differ from the preadmission list with the omission of prehospital medications and/or the continuation of acute medications no longer needed without thorough medication review and reconciliation.
While admitted to ICU, many critically ill patients – particularly those who are mechanically ventilated – receive intravenous or enteral sedatives such as benzodiazepines and antipsychotics. Sedatives are prescribed to more than two-thirds of critically ill patients for disturbing symptoms of agitation, delirium, anxiety, and insomnia and to facilitate invasive procedures (Burry LD, et al. J Crit Care. 2017;42:268). Current sedation practice guidelines endorse the use of sedatives when indicated for the shortest duration possible, given the known associated serious short- and long-term adverse drug events (Devlin JW, et al. Crit Care Med. 2018;46[9]:e825). Previous research has demonstrated that benzodiazepines initiated in-hospital are often continued on discharge for older adults and that patients from the ICU are at greater risk of benzodiazepine continuation than patients hospitalized without an ICU admission (Scales DC, et al. J Gen Intern Med. 2016;31[2]:196; Bell C, et al. J Gen Intern Med. 2007;22[7]:1024). This is particularly concerning for older adults as sedatives have been associated with serious adverse events in community-dwelling older adults, including falls and cognitive impairment (American Geriatrics Society. J Am Geriatr Soc. 2015;63[11]:2227)
The cohort of patients included all adults aged 66 years or more, who were discharged alive from the hospital and who were sedative-naive prior to hospitalization. Sedative-naive status was defined as no sedative prescription filled for any class, dose, or duration in the 180 days before hospital admission. The proportion of ICU survivors who filled a sedative prescription within 1 week of hospital discharge was the primary outcome. The secondary outcomes were the proportion of patients that filled each sedative class (e.g., antipsychotic, benzodiazepine, nonbenzodiazepine sedative) within 1 week of hospital discharge and persistent sedative prescription (additional prescriptions filled within 6 months after discharge).
The cohort included 250,428 sedative-naive older adults. The mean age was 75.8 years, 61.0% were male, 26.3% received invasive mechanical ventilation, and 14.8% had sepsis. In total, 6.1% (n=15,277) of patients filled a sedative prescription within 1 week of discharge; 57.7% (n = 8824) filled a benzodiazepine, 18.0% (n = 2749) filled a non-benzodiazepine sedative, 17.9% (n = 2745) filled an antipsychotic, and 6.2% (n = 959) filled more than 1 sedative drug class. Most patients filled prescriptions on the day of discharge (median 0 days (interquartile range (IQR) 0-3). The study found considerable variation in the primary outcome across the 153 hospitals: 2.1% (95% confidence interval [CI] 1.2% to 2.8%) to 44.0% (95% CI 3.0% to –57.8%) filled a sedative prescription within a week of hospital discharge. The factors strongly associated with an increased odds of a sedative prescription filled within a week of discharge included: discharge to long-term care (adjusted OR (aOR) 4.00, 95% CI 3.72 to 4.31), receipt of inpatient geriatric (aOR 1.95, 95% CI 1.80 to 2.10) or psychiatry consultation (aOR 2.76, 95% CI 2.62, 2.91), mechanical ventilation (aOR 1.59, 95% CI 1.53 to 1.66), and admitted ≥ 7 days to the ICU (aOR 1.50, 95% CI 1.42 to 1.58). Among hospital factors, a community hospital (vs academic) (aOR 1.40, 95% CI 1.16 to 1.70) and rural location (vs urban) (aOR 1.67, 95% CI 1.36 to 2.05) were also associated with new sedative prescriptions. Even after adjusting for patient and site characteristics, there was considerable remaining variability between sites quantified by the median odds ratio (aMOR) of 1.43. By drug class, there were similar findings with the exception of different associations for sex and frailty. For benzodiazepine prescriptions, female sex was associated with increased odds of a prescription (aOR 1.13, 95% CI 1.08 to 1.18), while frailty was inversely associated (aOR 0.82, 95% CI 0.75 to 0.89). The opposite associations were identified for antipsychotics: female sex (aOR 0.75, 95% CI 0.69 to 0.81) and frailty (aOR 1.41, 95% CI 1.28 to 1.55). No associations were identified for sex and frailty and non-benzodiazepine sedative prescriptions.
Persistent sedative prescription was common as 55% met the definition of persistence, filling a median of 2 prescriptions (IQR 1,3) in the 6 months after hospital discharge. The factors associated with persistent sedative prescriptions were similar to those identified above except female sex was associated with persistent sedative prescription (sHR 1.07, 95% CI 1.02 to 1.13). Those who filled an antipsychotic prescription (sHR 1.45, 95% CI 1.35 to 1.56), a non-benzodiazepine sedative prescription (sHR 1.44, 955 CI 1.34 to 1.53), or prescriptions for more than 1 sedative class filled (sHR 2.16, 95% CI 1.97 to 2.37) were more likely to fill persistent prescriptions compared with those who filled a prescription for a benzodiazepine alone as their first sedative.
In summary, 1 in 15 sedative-naive older adults filled a sedative prescription within a week of hospital discharge following a critical illness, and many continued to fill sedative prescriptions in the next 6 months. We were able to identify factors associated with new sedative prescriptions that could be targeted for stewardship programs or quality improvement projects that focus on medication safety and reconciliation. Medication stewardship and reconciliation processes have been broadly studied in many patient care settings but not the ICU. There is still much to determine regarding de-escalating and discontinuing sedatives as critical illness resolves and patients are liberated from intensive clinical interventions as well as the consequences of sedative exposure after hospital discharge for this population.
Dr. Burry is with the Departments of Pharmacy and Medicine, Sinai Health; Leslie Dan Faculty of Pharmacy and Interdepartmental Division of Critical Care, University of Toronto, Toronto, Canada. Dr. Williamson is with the Faculté de Pharmacie, Université de Montréal; Pharmacy Département, Hôpital du Sacré-Cœur de Montréal; and Research center, CIUSSS du Nord-de-l’Île-de-Montréal, Canada.
Modifications to medication regimens may include stopping home medications for chronic conditions, dose adjustments for altered organ function, or initiating new treatments for acute illness(es). Common examples of changes to a critically ill patient’s medication regimen are stopping a chronic antihypertensive drug in the setting of shock, holding an oral medication that cannot be crushed or administered through a feeding tube, and initiating sedatives and analgesics to support invasive mechanical ventilation. Medication regimens are especially vulnerable to errors and omissions at transition points (i.e., ICU to ward transfers and home discharge). As critical illness resolves and patients transition to different care teams, the hospital discharge medication regimen may differ from the preadmission list with the omission of prehospital medications and/or the continuation of acute medications no longer needed without thorough medication review and reconciliation.
While admitted to ICU, many critically ill patients – particularly those who are mechanically ventilated – receive intravenous or enteral sedatives such as benzodiazepines and antipsychotics. Sedatives are prescribed to more than two-thirds of critically ill patients for disturbing symptoms of agitation, delirium, anxiety, and insomnia and to facilitate invasive procedures (Burry LD, et al. J Crit Care. 2017;42:268). Current sedation practice guidelines endorse the use of sedatives when indicated for the shortest duration possible, given the known associated serious short- and long-term adverse drug events (Devlin JW, et al. Crit Care Med. 2018;46[9]:e825). Previous research has demonstrated that benzodiazepines initiated in-hospital are often continued on discharge for older adults and that patients from the ICU are at greater risk of benzodiazepine continuation than patients hospitalized without an ICU admission (Scales DC, et al. J Gen Intern Med. 2016;31[2]:196; Bell C, et al. J Gen Intern Med. 2007;22[7]:1024). This is particularly concerning for older adults as sedatives have been associated with serious adverse events in community-dwelling older adults, including falls and cognitive impairment (American Geriatrics Society. J Am Geriatr Soc. 2015;63[11]:2227)
The cohort of patients included all adults aged 66 years or more, who were discharged alive from the hospital and who were sedative-naive prior to hospitalization. Sedative-naive status was defined as no sedative prescription filled for any class, dose, or duration in the 180 days before hospital admission. The proportion of ICU survivors who filled a sedative prescription within 1 week of hospital discharge was the primary outcome. The secondary outcomes were the proportion of patients that filled each sedative class (e.g., antipsychotic, benzodiazepine, nonbenzodiazepine sedative) within 1 week of hospital discharge and persistent sedative prescription (additional prescriptions filled within 6 months after discharge).
The cohort included 250,428 sedative-naive older adults. The mean age was 75.8 years, 61.0% were male, 26.3% received invasive mechanical ventilation, and 14.8% had sepsis. In total, 6.1% (n=15,277) of patients filled a sedative prescription within 1 week of discharge; 57.7% (n = 8824) filled a benzodiazepine, 18.0% (n = 2749) filled a non-benzodiazepine sedative, 17.9% (n = 2745) filled an antipsychotic, and 6.2% (n = 959) filled more than 1 sedative drug class. Most patients filled prescriptions on the day of discharge (median 0 days (interquartile range (IQR) 0-3). The study found considerable variation in the primary outcome across the 153 hospitals: 2.1% (95% confidence interval [CI] 1.2% to 2.8%) to 44.0% (95% CI 3.0% to –57.8%) filled a sedative prescription within a week of hospital discharge. The factors strongly associated with an increased odds of a sedative prescription filled within a week of discharge included: discharge to long-term care (adjusted OR (aOR) 4.00, 95% CI 3.72 to 4.31), receipt of inpatient geriatric (aOR 1.95, 95% CI 1.80 to 2.10) or psychiatry consultation (aOR 2.76, 95% CI 2.62, 2.91), mechanical ventilation (aOR 1.59, 95% CI 1.53 to 1.66), and admitted ≥ 7 days to the ICU (aOR 1.50, 95% CI 1.42 to 1.58). Among hospital factors, a community hospital (vs academic) (aOR 1.40, 95% CI 1.16 to 1.70) and rural location (vs urban) (aOR 1.67, 95% CI 1.36 to 2.05) were also associated with new sedative prescriptions. Even after adjusting for patient and site characteristics, there was considerable remaining variability between sites quantified by the median odds ratio (aMOR) of 1.43. By drug class, there were similar findings with the exception of different associations for sex and frailty. For benzodiazepine prescriptions, female sex was associated with increased odds of a prescription (aOR 1.13, 95% CI 1.08 to 1.18), while frailty was inversely associated (aOR 0.82, 95% CI 0.75 to 0.89). The opposite associations were identified for antipsychotics: female sex (aOR 0.75, 95% CI 0.69 to 0.81) and frailty (aOR 1.41, 95% CI 1.28 to 1.55). No associations were identified for sex and frailty and non-benzodiazepine sedative prescriptions.
Persistent sedative prescription was common as 55% met the definition of persistence, filling a median of 2 prescriptions (IQR 1,3) in the 6 months after hospital discharge. The factors associated with persistent sedative prescriptions were similar to those identified above except female sex was associated with persistent sedative prescription (sHR 1.07, 95% CI 1.02 to 1.13). Those who filled an antipsychotic prescription (sHR 1.45, 95% CI 1.35 to 1.56), a non-benzodiazepine sedative prescription (sHR 1.44, 955 CI 1.34 to 1.53), or prescriptions for more than 1 sedative class filled (sHR 2.16, 95% CI 1.97 to 2.37) were more likely to fill persistent prescriptions compared with those who filled a prescription for a benzodiazepine alone as their first sedative.
In summary, 1 in 15 sedative-naive older adults filled a sedative prescription within a week of hospital discharge following a critical illness, and many continued to fill sedative prescriptions in the next 6 months. We were able to identify factors associated with new sedative prescriptions that could be targeted for stewardship programs or quality improvement projects that focus on medication safety and reconciliation. Medication stewardship and reconciliation processes have been broadly studied in many patient care settings but not the ICU. There is still much to determine regarding de-escalating and discontinuing sedatives as critical illness resolves and patients are liberated from intensive clinical interventions as well as the consequences of sedative exposure after hospital discharge for this population.
Dr. Burry is with the Departments of Pharmacy and Medicine, Sinai Health; Leslie Dan Faculty of Pharmacy and Interdepartmental Division of Critical Care, University of Toronto, Toronto, Canada. Dr. Williamson is with the Faculté de Pharmacie, Université de Montréal; Pharmacy Département, Hôpital du Sacré-Cœur de Montréal; and Research center, CIUSSS du Nord-de-l’Île-de-Montréal, Canada.
Modifications to medication regimens may include stopping home medications for chronic conditions, dose adjustments for altered organ function, or initiating new treatments for acute illness(es). Common examples of changes to a critically ill patient’s medication regimen are stopping a chronic antihypertensive drug in the setting of shock, holding an oral medication that cannot be crushed or administered through a feeding tube, and initiating sedatives and analgesics to support invasive mechanical ventilation. Medication regimens are especially vulnerable to errors and omissions at transition points (i.e., ICU to ward transfers and home discharge). As critical illness resolves and patients transition to different care teams, the hospital discharge medication regimen may differ from the preadmission list with the omission of prehospital medications and/or the continuation of acute medications no longer needed without thorough medication review and reconciliation.
While admitted to ICU, many critically ill patients – particularly those who are mechanically ventilated – receive intravenous or enteral sedatives such as benzodiazepines and antipsychotics. Sedatives are prescribed to more than two-thirds of critically ill patients for disturbing symptoms of agitation, delirium, anxiety, and insomnia and to facilitate invasive procedures (Burry LD, et al. J Crit Care. 2017;42:268). Current sedation practice guidelines endorse the use of sedatives when indicated for the shortest duration possible, given the known associated serious short- and long-term adverse drug events (Devlin JW, et al. Crit Care Med. 2018;46[9]:e825). Previous research has demonstrated that benzodiazepines initiated in-hospital are often continued on discharge for older adults and that patients from the ICU are at greater risk of benzodiazepine continuation than patients hospitalized without an ICU admission (Scales DC, et al. J Gen Intern Med. 2016;31[2]:196; Bell C, et al. J Gen Intern Med. 2007;22[7]:1024). This is particularly concerning for older adults as sedatives have been associated with serious adverse events in community-dwelling older adults, including falls and cognitive impairment (American Geriatrics Society. J Am Geriatr Soc. 2015;63[11]:2227)
The cohort of patients included all adults aged 66 years or more, who were discharged alive from the hospital and who were sedative-naive prior to hospitalization. Sedative-naive status was defined as no sedative prescription filled for any class, dose, or duration in the 180 days before hospital admission. The proportion of ICU survivors who filled a sedative prescription within 1 week of hospital discharge was the primary outcome. The secondary outcomes were the proportion of patients that filled each sedative class (e.g., antipsychotic, benzodiazepine, nonbenzodiazepine sedative) within 1 week of hospital discharge and persistent sedative prescription (additional prescriptions filled within 6 months after discharge).
The cohort included 250,428 sedative-naive older adults. The mean age was 75.8 years, 61.0% were male, 26.3% received invasive mechanical ventilation, and 14.8% had sepsis. In total, 6.1% (n=15,277) of patients filled a sedative prescription within 1 week of discharge; 57.7% (n = 8824) filled a benzodiazepine, 18.0% (n = 2749) filled a non-benzodiazepine sedative, 17.9% (n = 2745) filled an antipsychotic, and 6.2% (n = 959) filled more than 1 sedative drug class. Most patients filled prescriptions on the day of discharge (median 0 days (interquartile range (IQR) 0-3). The study found considerable variation in the primary outcome across the 153 hospitals: 2.1% (95% confidence interval [CI] 1.2% to 2.8%) to 44.0% (95% CI 3.0% to –57.8%) filled a sedative prescription within a week of hospital discharge. The factors strongly associated with an increased odds of a sedative prescription filled within a week of discharge included: discharge to long-term care (adjusted OR (aOR) 4.00, 95% CI 3.72 to 4.31), receipt of inpatient geriatric (aOR 1.95, 95% CI 1.80 to 2.10) or psychiatry consultation (aOR 2.76, 95% CI 2.62, 2.91), mechanical ventilation (aOR 1.59, 95% CI 1.53 to 1.66), and admitted ≥ 7 days to the ICU (aOR 1.50, 95% CI 1.42 to 1.58). Among hospital factors, a community hospital (vs academic) (aOR 1.40, 95% CI 1.16 to 1.70) and rural location (vs urban) (aOR 1.67, 95% CI 1.36 to 2.05) were also associated with new sedative prescriptions. Even after adjusting for patient and site characteristics, there was considerable remaining variability between sites quantified by the median odds ratio (aMOR) of 1.43. By drug class, there were similar findings with the exception of different associations for sex and frailty. For benzodiazepine prescriptions, female sex was associated with increased odds of a prescription (aOR 1.13, 95% CI 1.08 to 1.18), while frailty was inversely associated (aOR 0.82, 95% CI 0.75 to 0.89). The opposite associations were identified for antipsychotics: female sex (aOR 0.75, 95% CI 0.69 to 0.81) and frailty (aOR 1.41, 95% CI 1.28 to 1.55). No associations were identified for sex and frailty and non-benzodiazepine sedative prescriptions.
Persistent sedative prescription was common as 55% met the definition of persistence, filling a median of 2 prescriptions (IQR 1,3) in the 6 months after hospital discharge. The factors associated with persistent sedative prescriptions were similar to those identified above except female sex was associated with persistent sedative prescription (sHR 1.07, 95% CI 1.02 to 1.13). Those who filled an antipsychotic prescription (sHR 1.45, 95% CI 1.35 to 1.56), a non-benzodiazepine sedative prescription (sHR 1.44, 955 CI 1.34 to 1.53), or prescriptions for more than 1 sedative class filled (sHR 2.16, 95% CI 1.97 to 2.37) were more likely to fill persistent prescriptions compared with those who filled a prescription for a benzodiazepine alone as their first sedative.
In summary, 1 in 15 sedative-naive older adults filled a sedative prescription within a week of hospital discharge following a critical illness, and many continued to fill sedative prescriptions in the next 6 months. We were able to identify factors associated with new sedative prescriptions that could be targeted for stewardship programs or quality improvement projects that focus on medication safety and reconciliation. Medication stewardship and reconciliation processes have been broadly studied in many patient care settings but not the ICU. There is still much to determine regarding de-escalating and discontinuing sedatives as critical illness resolves and patients are liberated from intensive clinical interventions as well as the consequences of sedative exposure after hospital discharge for this population.
Dr. Burry is with the Departments of Pharmacy and Medicine, Sinai Health; Leslie Dan Faculty of Pharmacy and Interdepartmental Division of Critical Care, University of Toronto, Toronto, Canada. Dr. Williamson is with the Faculté de Pharmacie, Université de Montréal; Pharmacy Département, Hôpital du Sacré-Cœur de Montréal; and Research center, CIUSSS du Nord-de-l’Île-de-Montréal, Canada.
High school students using less tobacco, vape products, CDC report shows
TOPLINE:
which have been shown to both entice teens and keep them vaping.
METHODOLOGY:
- The MMRW report from the U.S. Centers for Disease Control and Prevention presents data from an annual survey of U.S. middle and high school students of their use of tobacco products, including vapes.
- The survey is a cross-sectional, school-based, self-administered web-based questionnaire that uses a stratified, three-stage cluster sampling procedure to generate a nationally representative sample based off the responses of 22,069 students in 2023.
- The overall response rate was 30.5%.
- “Ever use” was defined as using a product once or twice previously, and “current use” was defined as use in the past 30 days.
- The survey queried students on their use of e-cigarettes, traditional cigarettes, cigars, smokeless tobacco, nicotine pouches, hookahs, pipe tobacco, and other oral nicotine products.
TAKEAWAY:
- The use of tobacco products by high school students decreased by 540,000 people from 2022 to 2023 (2.51 million vs. 1.97 million students).
- From 2022 to 2023, current e-cigarette use among high school students declined from 14.1% to 10.0%.
- Among middle and high school students, e-cigarettes were the most used nicotine product in 2023 (7.7%; 2.13 million), followed by cigarettes (1.6%), cigars (1.6%), nicotine pouches (1.5%), smokeless tobacco (1.2%), other oral nicotine products (1.2%), hookahs (1.1%), heated tobacco products (1.0%), and pipe tobacco (0.5%).
- Among students reporting current e-cigarette use, 89.4% said that they used flavored products, and 25.2% said they used an e-cigarette daily. The most commonly reported brands were Elf Bar, Esco Bar, Vuse, JUUL, and Mr. Fog. Fruit (63.4%) and candy (35%) were the most commonly reported flavors.
IN PRACTICE:
“Sustained efforts to prevent initiation of tobacco product use among young persons and strategies to help young tobacco users quit are critical to reducing U.S. youth tobacco product use,” the report states.
SOURCE:
The report was produced by the CDC and published in the Morbidity and Mortality Weekly Report for Nov. 3, 2023.
LIMITATIONS:
Data were obtained by students self-reporting their tobacco use, which can result in social desirability and recall biases, the report states. In addition, the responses were from students enrolled in school settings and may not be representative of teens who are in detention centers, alternative schools, have dropped out of school or are homeschooled. The response rate for the 2023 survey was also lower than in the previous year (30.5% in 2023 vs. 45.2% in 2022), increasing the potential for higher standard errors and reducing the power to detect significant differences.
DISCLOSURES:
No potential conflicts of interest were disclosed.
A version of this article first appeared on Medscape.com.
TOPLINE:
which have been shown to both entice teens and keep them vaping.
METHODOLOGY:
- The MMRW report from the U.S. Centers for Disease Control and Prevention presents data from an annual survey of U.S. middle and high school students of their use of tobacco products, including vapes.
- The survey is a cross-sectional, school-based, self-administered web-based questionnaire that uses a stratified, three-stage cluster sampling procedure to generate a nationally representative sample based off the responses of 22,069 students in 2023.
- The overall response rate was 30.5%.
- “Ever use” was defined as using a product once or twice previously, and “current use” was defined as use in the past 30 days.
- The survey queried students on their use of e-cigarettes, traditional cigarettes, cigars, smokeless tobacco, nicotine pouches, hookahs, pipe tobacco, and other oral nicotine products.
TAKEAWAY:
- The use of tobacco products by high school students decreased by 540,000 people from 2022 to 2023 (2.51 million vs. 1.97 million students).
- From 2022 to 2023, current e-cigarette use among high school students declined from 14.1% to 10.0%.
- Among middle and high school students, e-cigarettes were the most used nicotine product in 2023 (7.7%; 2.13 million), followed by cigarettes (1.6%), cigars (1.6%), nicotine pouches (1.5%), smokeless tobacco (1.2%), other oral nicotine products (1.2%), hookahs (1.1%), heated tobacco products (1.0%), and pipe tobacco (0.5%).
- Among students reporting current e-cigarette use, 89.4% said that they used flavored products, and 25.2% said they used an e-cigarette daily. The most commonly reported brands were Elf Bar, Esco Bar, Vuse, JUUL, and Mr. Fog. Fruit (63.4%) and candy (35%) were the most commonly reported flavors.
IN PRACTICE:
“Sustained efforts to prevent initiation of tobacco product use among young persons and strategies to help young tobacco users quit are critical to reducing U.S. youth tobacco product use,” the report states.
SOURCE:
The report was produced by the CDC and published in the Morbidity and Mortality Weekly Report for Nov. 3, 2023.
LIMITATIONS:
Data were obtained by students self-reporting their tobacco use, which can result in social desirability and recall biases, the report states. In addition, the responses were from students enrolled in school settings and may not be representative of teens who are in detention centers, alternative schools, have dropped out of school or are homeschooled. The response rate for the 2023 survey was also lower than in the previous year (30.5% in 2023 vs. 45.2% in 2022), increasing the potential for higher standard errors and reducing the power to detect significant differences.
DISCLOSURES:
No potential conflicts of interest were disclosed.
A version of this article first appeared on Medscape.com.
TOPLINE:
which have been shown to both entice teens and keep them vaping.
METHODOLOGY:
- The MMRW report from the U.S. Centers for Disease Control and Prevention presents data from an annual survey of U.S. middle and high school students of their use of tobacco products, including vapes.
- The survey is a cross-sectional, school-based, self-administered web-based questionnaire that uses a stratified, three-stage cluster sampling procedure to generate a nationally representative sample based off the responses of 22,069 students in 2023.
- The overall response rate was 30.5%.
- “Ever use” was defined as using a product once or twice previously, and “current use” was defined as use in the past 30 days.
- The survey queried students on their use of e-cigarettes, traditional cigarettes, cigars, smokeless tobacco, nicotine pouches, hookahs, pipe tobacco, and other oral nicotine products.
TAKEAWAY:
- The use of tobacco products by high school students decreased by 540,000 people from 2022 to 2023 (2.51 million vs. 1.97 million students).
- From 2022 to 2023, current e-cigarette use among high school students declined from 14.1% to 10.0%.
- Among middle and high school students, e-cigarettes were the most used nicotine product in 2023 (7.7%; 2.13 million), followed by cigarettes (1.6%), cigars (1.6%), nicotine pouches (1.5%), smokeless tobacco (1.2%), other oral nicotine products (1.2%), hookahs (1.1%), heated tobacco products (1.0%), and pipe tobacco (0.5%).
- Among students reporting current e-cigarette use, 89.4% said that they used flavored products, and 25.2% said they used an e-cigarette daily. The most commonly reported brands were Elf Bar, Esco Bar, Vuse, JUUL, and Mr. Fog. Fruit (63.4%) and candy (35%) were the most commonly reported flavors.
IN PRACTICE:
“Sustained efforts to prevent initiation of tobacco product use among young persons and strategies to help young tobacco users quit are critical to reducing U.S. youth tobacco product use,” the report states.
SOURCE:
The report was produced by the CDC and published in the Morbidity and Mortality Weekly Report for Nov. 3, 2023.
LIMITATIONS:
Data were obtained by students self-reporting their tobacco use, which can result in social desirability and recall biases, the report states. In addition, the responses were from students enrolled in school settings and may not be representative of teens who are in detention centers, alternative schools, have dropped out of school or are homeschooled. The response rate for the 2023 survey was also lower than in the previous year (30.5% in 2023 vs. 45.2% in 2022), increasing the potential for higher standard errors and reducing the power to detect significant differences.
DISCLOSURES:
No potential conflicts of interest were disclosed.
A version of this article first appeared on Medscape.com.
ACS expands lung cancer screening eligibility
The American Cancer Society has updated its screening guidelines for lung cancer, the leading cause of cancer-specific deaths in the United States and the largest driver of potential years of life lost from cancer.
The 2023 screening guidance, aimed principally at reducing lung cancer mortality in asymptomatic but high-risk, tobacco-exposed individuals, expands the age eligibility and lowers both the former smoking history and the years since quitting threshold for screening with low-dose CT (LDCT).
It is based on the most recent evidence on the efficacy and effectiveness of screening and lung cancer risk in persons who formerly smoked, wrote the ACS’s Guideline Development Group led by Robert A. Smith, PhD, senior vice president of early cancer detection science. The new guidelines, which replace the 2013 statement, appear in CA: A Cancer Journal for Physicians.
The primary evidence source for the update was a systematic review of LDCT lung cancer screening conducted for the U.S. Preventive Services Task Force and published in 2021.
The new guideline continues a trend of expanding eligibility for lung cancer screening, which has had low uptake, to prevent more deaths. “Recent studies have shown that extending the age for persons who smoked and formerly smoked, eliminating the ‘years since quitting’ requirement, and lowering the pack-per-year recommendation could make a real difference in saving lives,” Dr. Smith said. “The relative risk of developing lung cancer in people who have smoked most of their life compared to people who never smoked is very high – about 70 times the risk.” Although lung cancer is the third most common malignancy in the United States, it accounts for more deaths than colorectal, breast, prostate, and cervical cancers combined.
The recommendation for annual LDCT for at-risk persons remains unchanged from 2013.
Among the 2023 eligibility changes:
- Age: Expanded to 50-80 years from 55-74 years.
- Smoking status: Changed to current or previous smoker from current smoker or smoker who quit within past 15 years (number of years since quitting no longer a criterion to start or stop screening). Dr. Smith noted that both the 2013 guidelines and other groups’ updated recommendations retained the eligibility cutoff of 15 years since smoking cessation. “But had their risk declined to a level that just did not justify continuing screening?” he asked. “There wasn’t an answer to that question, so we needed to look carefully at the absolute risk of lung cancer in persons who formerly smoked compared with people who currently smoked and people who never smoked.”
- Smoking history: Reduced to 20 or more pack-years (average of 20 cigarettes a day) versus 30 or more pack-years.
- Exclusions: Expanded to health conditions that may increase harm or hinder further evaluation, surgery, or treatment; comorbidities limiting life expectancy to fewer than 5 years; unwillingness to accept treatment for screen‐detected cancer, which was changed from 2013’s life‐limiting comorbid conditions, metallic implants or devices in the chest or back, home oxygen supplementation.
In addition, decision-making should be a shared process with a health professional providing the patient with information on the benefits, limitations, and harms of LDCT screening, as well as prescreening advice on smoking cessation and the offer of assistive counseling and pharmocotherapy.
“Overall, lung cancer screening remains one of the least used early cancer detection modalities in clinical practice. The new guidance opens up lung cancer screening to all former smokers regardless of time of cessation,” said internist William E. Golden, MD, MACP, a professor of medicine and public health at the University of Arkansas for Medical Sciences, Little Rock. “This may promote greater uptake in concert with greater availability of low-radiation CT scanning.”
While agreeing the expanded criteria will enfranchise nearly 5 million current and former U.S. smokers for screening and may reduce deaths, internist Aarati D. Didwania, MD, MMSCI, MACP, a professor of medicine and medical education at Northwestern University, Chicago, warned that increasing actual uptake may be an uphill battle. “The practical part of the equation is seeing that the scans get done. There is often a lag between a recommendation of a yearly test and getting insurance coverage for it, and many disadvantaged people face barriers.” Then there’s the knowledge gap. “Patients and doctors have to know what the new guidelines are and who has access,” she said.
Reaching the target population in rural areas is particularly challenging with the greater distances to imaging centers. Another barrier is that most electronic health records do not identify eligible patients based on smoking and pack‐year history.
In Dr. Didwania’s view, professional medical societies have an important role to play in educating their members, and through them, patients. “Disseminating information about the new recommendations is the first step and would be incredibly helpful.”
A brief history of lung cancer screening
1950s: By mid-20th century, the causal association between tobacco exposure and lung cancer became clear and by the late 1950s attempts were made to develop a lung cancer screening strategy for high‐risk individuals, commonly with the combination of sputum cytology and chest x-ray.
1970s: The ACS recommended annual testing for current or former smokers with chest x-ray (and sometimes sputum cytology).
1980: The ACS withdrew the above recommendation for regular radiographic screening after randomized controlled trials failed to yield convincing evidence that such screening saved lives.
2013: After the National Lung Screening Trial found three annual LDCT screenings were associated with a 20% relative mortality reduction, compared with annual chest x-ray, the ACS issued a recommendation for annual screening with LDCT: in persons 55-74 years with a pack‐year history of 30 or more who currently smoke or formerly smoked but had not exceeded 15 years since quitting and had no life-limiting morbidity.
Future mortality
Although tobacco controls are expected to reduce age‐adjusted lung cancer mortality in the United States by 79% from 2015 to 2065, 4.4 million lung cancer deaths are projected to occur in this period, the authors stated. “A large fraction of these deaths can be prevented if we embrace the urgent challenge to improve our ability to identify the population at risk and apply our knowledge to achieve high rates of participation in regular [lung cancer screening].”
The study was funded by the American Cancer Society Guideline Development Group and the National Comprehensive Cancer Network. The authors disclosed no relevant competing interests. Dr. Golden and Dr. Didwania had no relevant conflicts of interest to declare with regard to their comments.
The American Cancer Society has updated its screening guidelines for lung cancer, the leading cause of cancer-specific deaths in the United States and the largest driver of potential years of life lost from cancer.
The 2023 screening guidance, aimed principally at reducing lung cancer mortality in asymptomatic but high-risk, tobacco-exposed individuals, expands the age eligibility and lowers both the former smoking history and the years since quitting threshold for screening with low-dose CT (LDCT).
It is based on the most recent evidence on the efficacy and effectiveness of screening and lung cancer risk in persons who formerly smoked, wrote the ACS’s Guideline Development Group led by Robert A. Smith, PhD, senior vice president of early cancer detection science. The new guidelines, which replace the 2013 statement, appear in CA: A Cancer Journal for Physicians.
The primary evidence source for the update was a systematic review of LDCT lung cancer screening conducted for the U.S. Preventive Services Task Force and published in 2021.
The new guideline continues a trend of expanding eligibility for lung cancer screening, which has had low uptake, to prevent more deaths. “Recent studies have shown that extending the age for persons who smoked and formerly smoked, eliminating the ‘years since quitting’ requirement, and lowering the pack-per-year recommendation could make a real difference in saving lives,” Dr. Smith said. “The relative risk of developing lung cancer in people who have smoked most of their life compared to people who never smoked is very high – about 70 times the risk.” Although lung cancer is the third most common malignancy in the United States, it accounts for more deaths than colorectal, breast, prostate, and cervical cancers combined.
The recommendation for annual LDCT for at-risk persons remains unchanged from 2013.
Among the 2023 eligibility changes:
- Age: Expanded to 50-80 years from 55-74 years.
- Smoking status: Changed to current or previous smoker from current smoker or smoker who quit within past 15 years (number of years since quitting no longer a criterion to start or stop screening). Dr. Smith noted that both the 2013 guidelines and other groups’ updated recommendations retained the eligibility cutoff of 15 years since smoking cessation. “But had their risk declined to a level that just did not justify continuing screening?” he asked. “There wasn’t an answer to that question, so we needed to look carefully at the absolute risk of lung cancer in persons who formerly smoked compared with people who currently smoked and people who never smoked.”
- Smoking history: Reduced to 20 or more pack-years (average of 20 cigarettes a day) versus 30 or more pack-years.
- Exclusions: Expanded to health conditions that may increase harm or hinder further evaluation, surgery, or treatment; comorbidities limiting life expectancy to fewer than 5 years; unwillingness to accept treatment for screen‐detected cancer, which was changed from 2013’s life‐limiting comorbid conditions, metallic implants or devices in the chest or back, home oxygen supplementation.
In addition, decision-making should be a shared process with a health professional providing the patient with information on the benefits, limitations, and harms of LDCT screening, as well as prescreening advice on smoking cessation and the offer of assistive counseling and pharmocotherapy.
“Overall, lung cancer screening remains one of the least used early cancer detection modalities in clinical practice. The new guidance opens up lung cancer screening to all former smokers regardless of time of cessation,” said internist William E. Golden, MD, MACP, a professor of medicine and public health at the University of Arkansas for Medical Sciences, Little Rock. “This may promote greater uptake in concert with greater availability of low-radiation CT scanning.”
While agreeing the expanded criteria will enfranchise nearly 5 million current and former U.S. smokers for screening and may reduce deaths, internist Aarati D. Didwania, MD, MMSCI, MACP, a professor of medicine and medical education at Northwestern University, Chicago, warned that increasing actual uptake may be an uphill battle. “The practical part of the equation is seeing that the scans get done. There is often a lag between a recommendation of a yearly test and getting insurance coverage for it, and many disadvantaged people face barriers.” Then there’s the knowledge gap. “Patients and doctors have to know what the new guidelines are and who has access,” she said.
Reaching the target population in rural areas is particularly challenging with the greater distances to imaging centers. Another barrier is that most electronic health records do not identify eligible patients based on smoking and pack‐year history.
In Dr. Didwania’s view, professional medical societies have an important role to play in educating their members, and through them, patients. “Disseminating information about the new recommendations is the first step and would be incredibly helpful.”
A brief history of lung cancer screening
1950s: By mid-20th century, the causal association between tobacco exposure and lung cancer became clear and by the late 1950s attempts were made to develop a lung cancer screening strategy for high‐risk individuals, commonly with the combination of sputum cytology and chest x-ray.
1970s: The ACS recommended annual testing for current or former smokers with chest x-ray (and sometimes sputum cytology).
1980: The ACS withdrew the above recommendation for regular radiographic screening after randomized controlled trials failed to yield convincing evidence that such screening saved lives.
2013: After the National Lung Screening Trial found three annual LDCT screenings were associated with a 20% relative mortality reduction, compared with annual chest x-ray, the ACS issued a recommendation for annual screening with LDCT: in persons 55-74 years with a pack‐year history of 30 or more who currently smoke or formerly smoked but had not exceeded 15 years since quitting and had no life-limiting morbidity.
Future mortality
Although tobacco controls are expected to reduce age‐adjusted lung cancer mortality in the United States by 79% from 2015 to 2065, 4.4 million lung cancer deaths are projected to occur in this period, the authors stated. “A large fraction of these deaths can be prevented if we embrace the urgent challenge to improve our ability to identify the population at risk and apply our knowledge to achieve high rates of participation in regular [lung cancer screening].”
The study was funded by the American Cancer Society Guideline Development Group and the National Comprehensive Cancer Network. The authors disclosed no relevant competing interests. Dr. Golden and Dr. Didwania had no relevant conflicts of interest to declare with regard to their comments.
The American Cancer Society has updated its screening guidelines for lung cancer, the leading cause of cancer-specific deaths in the United States and the largest driver of potential years of life lost from cancer.
The 2023 screening guidance, aimed principally at reducing lung cancer mortality in asymptomatic but high-risk, tobacco-exposed individuals, expands the age eligibility and lowers both the former smoking history and the years since quitting threshold for screening with low-dose CT (LDCT).
It is based on the most recent evidence on the efficacy and effectiveness of screening and lung cancer risk in persons who formerly smoked, wrote the ACS’s Guideline Development Group led by Robert A. Smith, PhD, senior vice president of early cancer detection science. The new guidelines, which replace the 2013 statement, appear in CA: A Cancer Journal for Physicians.
The primary evidence source for the update was a systematic review of LDCT lung cancer screening conducted for the U.S. Preventive Services Task Force and published in 2021.
The new guideline continues a trend of expanding eligibility for lung cancer screening, which has had low uptake, to prevent more deaths. “Recent studies have shown that extending the age for persons who smoked and formerly smoked, eliminating the ‘years since quitting’ requirement, and lowering the pack-per-year recommendation could make a real difference in saving lives,” Dr. Smith said. “The relative risk of developing lung cancer in people who have smoked most of their life compared to people who never smoked is very high – about 70 times the risk.” Although lung cancer is the third most common malignancy in the United States, it accounts for more deaths than colorectal, breast, prostate, and cervical cancers combined.
The recommendation for annual LDCT for at-risk persons remains unchanged from 2013.
Among the 2023 eligibility changes:
- Age: Expanded to 50-80 years from 55-74 years.
- Smoking status: Changed to current or previous smoker from current smoker or smoker who quit within past 15 years (number of years since quitting no longer a criterion to start or stop screening). Dr. Smith noted that both the 2013 guidelines and other groups’ updated recommendations retained the eligibility cutoff of 15 years since smoking cessation. “But had their risk declined to a level that just did not justify continuing screening?” he asked. “There wasn’t an answer to that question, so we needed to look carefully at the absolute risk of lung cancer in persons who formerly smoked compared with people who currently smoked and people who never smoked.”
- Smoking history: Reduced to 20 or more pack-years (average of 20 cigarettes a day) versus 30 or more pack-years.
- Exclusions: Expanded to health conditions that may increase harm or hinder further evaluation, surgery, or treatment; comorbidities limiting life expectancy to fewer than 5 years; unwillingness to accept treatment for screen‐detected cancer, which was changed from 2013’s life‐limiting comorbid conditions, metallic implants or devices in the chest or back, home oxygen supplementation.
In addition, decision-making should be a shared process with a health professional providing the patient with information on the benefits, limitations, and harms of LDCT screening, as well as prescreening advice on smoking cessation and the offer of assistive counseling and pharmocotherapy.
“Overall, lung cancer screening remains one of the least used early cancer detection modalities in clinical practice. The new guidance opens up lung cancer screening to all former smokers regardless of time of cessation,” said internist William E. Golden, MD, MACP, a professor of medicine and public health at the University of Arkansas for Medical Sciences, Little Rock. “This may promote greater uptake in concert with greater availability of low-radiation CT scanning.”
While agreeing the expanded criteria will enfranchise nearly 5 million current and former U.S. smokers for screening and may reduce deaths, internist Aarati D. Didwania, MD, MMSCI, MACP, a professor of medicine and medical education at Northwestern University, Chicago, warned that increasing actual uptake may be an uphill battle. “The practical part of the equation is seeing that the scans get done. There is often a lag between a recommendation of a yearly test and getting insurance coverage for it, and many disadvantaged people face barriers.” Then there’s the knowledge gap. “Patients and doctors have to know what the new guidelines are and who has access,” she said.
Reaching the target population in rural areas is particularly challenging with the greater distances to imaging centers. Another barrier is that most electronic health records do not identify eligible patients based on smoking and pack‐year history.
In Dr. Didwania’s view, professional medical societies have an important role to play in educating their members, and through them, patients. “Disseminating information about the new recommendations is the first step and would be incredibly helpful.”
A brief history of lung cancer screening
1950s: By mid-20th century, the causal association between tobacco exposure and lung cancer became clear and by the late 1950s attempts were made to develop a lung cancer screening strategy for high‐risk individuals, commonly with the combination of sputum cytology and chest x-ray.
1970s: The ACS recommended annual testing for current or former smokers with chest x-ray (and sometimes sputum cytology).
1980: The ACS withdrew the above recommendation for regular radiographic screening after randomized controlled trials failed to yield convincing evidence that such screening saved lives.
2013: After the National Lung Screening Trial found three annual LDCT screenings were associated with a 20% relative mortality reduction, compared with annual chest x-ray, the ACS issued a recommendation for annual screening with LDCT: in persons 55-74 years with a pack‐year history of 30 or more who currently smoke or formerly smoked but had not exceeded 15 years since quitting and had no life-limiting morbidity.
Future mortality
Although tobacco controls are expected to reduce age‐adjusted lung cancer mortality in the United States by 79% from 2015 to 2065, 4.4 million lung cancer deaths are projected to occur in this period, the authors stated. “A large fraction of these deaths can be prevented if we embrace the urgent challenge to improve our ability to identify the population at risk and apply our knowledge to achieve high rates of participation in regular [lung cancer screening].”
The study was funded by the American Cancer Society Guideline Development Group and the National Comprehensive Cancer Network. The authors disclosed no relevant competing interests. Dr. Golden and Dr. Didwania had no relevant conflicts of interest to declare with regard to their comments.
FROM CA: A CANCER JOURNAL FOR PHYSICIANS
Race-specific lung-function values may skew IPF testing
HONOLULU – Old habits die hard, especially when it comes to pulmonary function testing in a diverse population of patients with interstitial lung disease (ILD).
Specifically, pulmonary care clinicians may be habitually relying on outdated and inaccurate race-specific reference values when evaluating respiratory impairment in persons of African and Hispanic/Latino ancestry, which can result in underrecognition, underdiagnosis, and undertreatment, reported Ayodeji Adegunsoye, MD, from the University of Chicago, and colleagues.
“Our results make a compelling case for re-evaluating the use of race as a physiological variable, and highlight the need to offer equitable and optimal care for all patients, regardless of their race or ethnicity,” Dr. Adegunsoye said in an oral abstract session at the annual meeting of the American College of Chest Physicians (CHEST).
Flawed assumptions
In an interview, Dr. Adegunsoye noted that race-specific notions, such as the automatic assumption that Black people have less lung capacity than White people, are baked into clinical practice and passed on as clinical wisdom from one generation of clinicians to the next.
Pulmonary function reference values that are used to make a diagnosis of idiopathic pulmonary fibrosis in Black or Hispanic/Latino patients “appear flawed when we use race-specific values. And beyond the diagnosis, it also appears to impact eligibility for key interventional strategies for managing the disease itself,” he said.
The use of race-specific equations can falsely inflate percent-predicted pulmonary function values in non-White patients, and make it seem as if a patient has normal lung function when in fact he may be have impaired function.
For example, using race-based reference values a Black patient and a White patient may appear to have the same absolute forced vital capacity readings, but different FVC percent predicted (FVCpp), which can mean a missed diagnosis.
Investigators who studied the association between self-identified race and visually identified emphysema among 2,674 participants in the Coronary Artery Risk Development in Young Adults study found that using standard equations to adjust for racial differences in lung-function measures appeared to miss emphysema in a significant proportion of Black patients.
PF registry study
In the current study, to see whether the use of race-neutral equations for evaluating FVCpp could change access to health care in patients with ILD, Dr. Adegunsoye and colleagues used both race-specific and race-neutral equations to calculate FVCpp values among separate cohorts of Black, Hispanic/Latino, and White patients enrolled in the Pulmonary Fibrosis Foundation Patient Registry who had pulmonary functions test within about 90 days of enrollment.
The race-specific equations used to calculate FVCpp was that published in 1999 by Hankinson and colleagues in American Journal of Respiratory and Critical Care Medicine. The race-neutral Global Lung Function Initiative (GLI) equations by Bowerman and colleagues were developed in 2022 and published in March 2023 in the same journal.
The investigators defined access to care as enrollment in ILD clinical trials for patients with FVCpp greater than 45% but less than 90%, and US payer access to antifibrotic therapy for patients with FVCpp of greater than 55% but less than 82%.
They found that 22% of Black patients were misclassified in their eligibility for clinical trials in each of two scenarios – those who would be excluded from trials using the 1999 criteria but included using the 2022 criteria, and vice versa, that is included with 1999 criteria but excluded by the 2022 GLI criteria. In contrast, 14% of Hispanic Latino patients and 12% of White patients were misclassified.
Using the 1999 criteria to exclude patients because their values were ostensibly higher than the upper cutoff meant that 10.3% of Black patients who might benefit would be ineligible for clinical trial, compared with 0% of Hispanic/Latinos and 0.1% of Whites.
Similarly, 11.5% of Black patients but no Hispanic/Latino or White patients would be considered eligible for clinical trials using the old criteria but ineligible under the new criteria.
Regarding antifibrotic therapy eligibility, the respective misclassification rates were 21%, 17%, and 19%.
“Our study showed that use of race-specific equations may confound lung function tests, potentially leading to misclassification, delayed diagnosis, and inadequate treatment provision. While our study suggests potential disparities in access to health care for patients with interstitial lung disease facilitated by race-specific equations, further research is required to fully comprehend the implications,” the investigators wrote.
ATS statement
In an interview, Juan Wisnievsky, MD, DrPh, from Mount Sinai Medical Center, New York, who also chairs the Health Equity and Diversity Committee for the American Thoracic Society, pointed to a recent ATS statement he coauthored citing evidence for replacing race and ethnicity-specific equations with race-neutral average reference equations.
“This use of race and ethnicity may contribute to health disparities by norming differences in pulmonary function. In the United States and globally, race serves as a social construct that is based on appearance and reflects social values, structures, and practices. Classification of people into racial and ethnic groups differs geographically and temporally. These considerations challenge the notion that racial and ethnic categories have biological meaning and question the use of race in PFT interpretation,” the statement authors wrote.
“There is some agreement that race-based equations shouldn’t be used, but all the potential consequences of doing that and which equations would be the best ones to use to replace them is a bit unclear,” Dr. Wisnievsky said.
He was not involved in the study by Dr. Adegunsoye and colleagues.
Data used in the study were derived from research sponsored by F. Hoffman–La Roche and Genentech. Dr. Adegunsoye disclosed consultancy fees from AbbVie, Inogen, F. Hoffman–La Roche, Medscape, and PatientMpower; speaking/advisory fees from Boehringer Ingelheim; and grants/award from the CHEST Foundation, Pulmonary Fibrosis Foundation, and National Institutes of Health. Dr. Wisnievsky had no relevant disclosures.
HONOLULU – Old habits die hard, especially when it comes to pulmonary function testing in a diverse population of patients with interstitial lung disease (ILD).
Specifically, pulmonary care clinicians may be habitually relying on outdated and inaccurate race-specific reference values when evaluating respiratory impairment in persons of African and Hispanic/Latino ancestry, which can result in underrecognition, underdiagnosis, and undertreatment, reported Ayodeji Adegunsoye, MD, from the University of Chicago, and colleagues.
“Our results make a compelling case for re-evaluating the use of race as a physiological variable, and highlight the need to offer equitable and optimal care for all patients, regardless of their race or ethnicity,” Dr. Adegunsoye said in an oral abstract session at the annual meeting of the American College of Chest Physicians (CHEST).
Flawed assumptions
In an interview, Dr. Adegunsoye noted that race-specific notions, such as the automatic assumption that Black people have less lung capacity than White people, are baked into clinical practice and passed on as clinical wisdom from one generation of clinicians to the next.
Pulmonary function reference values that are used to make a diagnosis of idiopathic pulmonary fibrosis in Black or Hispanic/Latino patients “appear flawed when we use race-specific values. And beyond the diagnosis, it also appears to impact eligibility for key interventional strategies for managing the disease itself,” he said.
The use of race-specific equations can falsely inflate percent-predicted pulmonary function values in non-White patients, and make it seem as if a patient has normal lung function when in fact he may be have impaired function.
For example, using race-based reference values a Black patient and a White patient may appear to have the same absolute forced vital capacity readings, but different FVC percent predicted (FVCpp), which can mean a missed diagnosis.
Investigators who studied the association between self-identified race and visually identified emphysema among 2,674 participants in the Coronary Artery Risk Development in Young Adults study found that using standard equations to adjust for racial differences in lung-function measures appeared to miss emphysema in a significant proportion of Black patients.
PF registry study
In the current study, to see whether the use of race-neutral equations for evaluating FVCpp could change access to health care in patients with ILD, Dr. Adegunsoye and colleagues used both race-specific and race-neutral equations to calculate FVCpp values among separate cohorts of Black, Hispanic/Latino, and White patients enrolled in the Pulmonary Fibrosis Foundation Patient Registry who had pulmonary functions test within about 90 days of enrollment.
The race-specific equations used to calculate FVCpp was that published in 1999 by Hankinson and colleagues in American Journal of Respiratory and Critical Care Medicine. The race-neutral Global Lung Function Initiative (GLI) equations by Bowerman and colleagues were developed in 2022 and published in March 2023 in the same journal.
The investigators defined access to care as enrollment in ILD clinical trials for patients with FVCpp greater than 45% but less than 90%, and US payer access to antifibrotic therapy for patients with FVCpp of greater than 55% but less than 82%.
They found that 22% of Black patients were misclassified in their eligibility for clinical trials in each of two scenarios – those who would be excluded from trials using the 1999 criteria but included using the 2022 criteria, and vice versa, that is included with 1999 criteria but excluded by the 2022 GLI criteria. In contrast, 14% of Hispanic Latino patients and 12% of White patients were misclassified.
Using the 1999 criteria to exclude patients because their values were ostensibly higher than the upper cutoff meant that 10.3% of Black patients who might benefit would be ineligible for clinical trial, compared with 0% of Hispanic/Latinos and 0.1% of Whites.
Similarly, 11.5% of Black patients but no Hispanic/Latino or White patients would be considered eligible for clinical trials using the old criteria but ineligible under the new criteria.
Regarding antifibrotic therapy eligibility, the respective misclassification rates were 21%, 17%, and 19%.
“Our study showed that use of race-specific equations may confound lung function tests, potentially leading to misclassification, delayed diagnosis, and inadequate treatment provision. While our study suggests potential disparities in access to health care for patients with interstitial lung disease facilitated by race-specific equations, further research is required to fully comprehend the implications,” the investigators wrote.
ATS statement
In an interview, Juan Wisnievsky, MD, DrPh, from Mount Sinai Medical Center, New York, who also chairs the Health Equity and Diversity Committee for the American Thoracic Society, pointed to a recent ATS statement he coauthored citing evidence for replacing race and ethnicity-specific equations with race-neutral average reference equations.
“This use of race and ethnicity may contribute to health disparities by norming differences in pulmonary function. In the United States and globally, race serves as a social construct that is based on appearance and reflects social values, structures, and practices. Classification of people into racial and ethnic groups differs geographically and temporally. These considerations challenge the notion that racial and ethnic categories have biological meaning and question the use of race in PFT interpretation,” the statement authors wrote.
“There is some agreement that race-based equations shouldn’t be used, but all the potential consequences of doing that and which equations would be the best ones to use to replace them is a bit unclear,” Dr. Wisnievsky said.
He was not involved in the study by Dr. Adegunsoye and colleagues.
Data used in the study were derived from research sponsored by F. Hoffman–La Roche and Genentech. Dr. Adegunsoye disclosed consultancy fees from AbbVie, Inogen, F. Hoffman–La Roche, Medscape, and PatientMpower; speaking/advisory fees from Boehringer Ingelheim; and grants/award from the CHEST Foundation, Pulmonary Fibrosis Foundation, and National Institutes of Health. Dr. Wisnievsky had no relevant disclosures.
HONOLULU – Old habits die hard, especially when it comes to pulmonary function testing in a diverse population of patients with interstitial lung disease (ILD).
Specifically, pulmonary care clinicians may be habitually relying on outdated and inaccurate race-specific reference values when evaluating respiratory impairment in persons of African and Hispanic/Latino ancestry, which can result in underrecognition, underdiagnosis, and undertreatment, reported Ayodeji Adegunsoye, MD, from the University of Chicago, and colleagues.
“Our results make a compelling case for re-evaluating the use of race as a physiological variable, and highlight the need to offer equitable and optimal care for all patients, regardless of their race or ethnicity,” Dr. Adegunsoye said in an oral abstract session at the annual meeting of the American College of Chest Physicians (CHEST).
Flawed assumptions
In an interview, Dr. Adegunsoye noted that race-specific notions, such as the automatic assumption that Black people have less lung capacity than White people, are baked into clinical practice and passed on as clinical wisdom from one generation of clinicians to the next.
Pulmonary function reference values that are used to make a diagnosis of idiopathic pulmonary fibrosis in Black or Hispanic/Latino patients “appear flawed when we use race-specific values. And beyond the diagnosis, it also appears to impact eligibility for key interventional strategies for managing the disease itself,” he said.
The use of race-specific equations can falsely inflate percent-predicted pulmonary function values in non-White patients, and make it seem as if a patient has normal lung function when in fact he may be have impaired function.
For example, using race-based reference values a Black patient and a White patient may appear to have the same absolute forced vital capacity readings, but different FVC percent predicted (FVCpp), which can mean a missed diagnosis.
Investigators who studied the association between self-identified race and visually identified emphysema among 2,674 participants in the Coronary Artery Risk Development in Young Adults study found that using standard equations to adjust for racial differences in lung-function measures appeared to miss emphysema in a significant proportion of Black patients.
PF registry study
In the current study, to see whether the use of race-neutral equations for evaluating FVCpp could change access to health care in patients with ILD, Dr. Adegunsoye and colleagues used both race-specific and race-neutral equations to calculate FVCpp values among separate cohorts of Black, Hispanic/Latino, and White patients enrolled in the Pulmonary Fibrosis Foundation Patient Registry who had pulmonary functions test within about 90 days of enrollment.
The race-specific equations used to calculate FVCpp was that published in 1999 by Hankinson and colleagues in American Journal of Respiratory and Critical Care Medicine. The race-neutral Global Lung Function Initiative (GLI) equations by Bowerman and colleagues were developed in 2022 and published in March 2023 in the same journal.
The investigators defined access to care as enrollment in ILD clinical trials for patients with FVCpp greater than 45% but less than 90%, and US payer access to antifibrotic therapy for patients with FVCpp of greater than 55% but less than 82%.
They found that 22% of Black patients were misclassified in their eligibility for clinical trials in each of two scenarios – those who would be excluded from trials using the 1999 criteria but included using the 2022 criteria, and vice versa, that is included with 1999 criteria but excluded by the 2022 GLI criteria. In contrast, 14% of Hispanic Latino patients and 12% of White patients were misclassified.
Using the 1999 criteria to exclude patients because their values were ostensibly higher than the upper cutoff meant that 10.3% of Black patients who might benefit would be ineligible for clinical trial, compared with 0% of Hispanic/Latinos and 0.1% of Whites.
Similarly, 11.5% of Black patients but no Hispanic/Latino or White patients would be considered eligible for clinical trials using the old criteria but ineligible under the new criteria.
Regarding antifibrotic therapy eligibility, the respective misclassification rates were 21%, 17%, and 19%.
“Our study showed that use of race-specific equations may confound lung function tests, potentially leading to misclassification, delayed diagnosis, and inadequate treatment provision. While our study suggests potential disparities in access to health care for patients with interstitial lung disease facilitated by race-specific equations, further research is required to fully comprehend the implications,” the investigators wrote.
ATS statement
In an interview, Juan Wisnievsky, MD, DrPh, from Mount Sinai Medical Center, New York, who also chairs the Health Equity and Diversity Committee for the American Thoracic Society, pointed to a recent ATS statement he coauthored citing evidence for replacing race and ethnicity-specific equations with race-neutral average reference equations.
“This use of race and ethnicity may contribute to health disparities by norming differences in pulmonary function. In the United States and globally, race serves as a social construct that is based on appearance and reflects social values, structures, and practices. Classification of people into racial and ethnic groups differs geographically and temporally. These considerations challenge the notion that racial and ethnic categories have biological meaning and question the use of race in PFT interpretation,” the statement authors wrote.
“There is some agreement that race-based equations shouldn’t be used, but all the potential consequences of doing that and which equations would be the best ones to use to replace them is a bit unclear,” Dr. Wisnievsky said.
He was not involved in the study by Dr. Adegunsoye and colleagues.
Data used in the study were derived from research sponsored by F. Hoffman–La Roche and Genentech. Dr. Adegunsoye disclosed consultancy fees from AbbVie, Inogen, F. Hoffman–La Roche, Medscape, and PatientMpower; speaking/advisory fees from Boehringer Ingelheim; and grants/award from the CHEST Foundation, Pulmonary Fibrosis Foundation, and National Institutes of Health. Dr. Wisnievsky had no relevant disclosures.
AT CHEST 2023