User login
For MD-IQ only
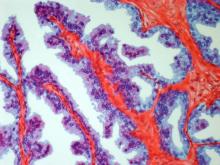
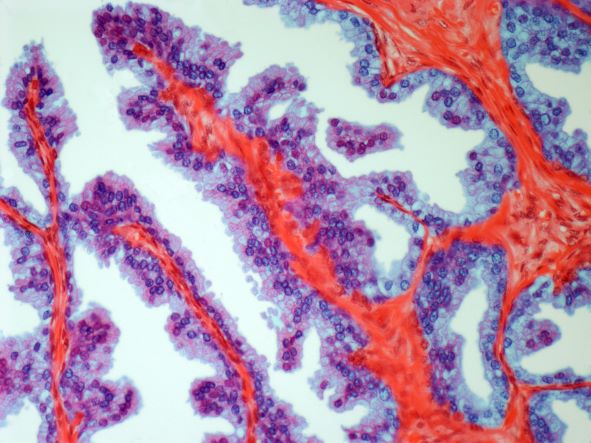
Talazoparib add-on improves outcomes in metastatic prostate cancer
in the TALAPRO-2 trial.
As determined on the basis of imaging, PFS was 37% better for talazoparib plus enzalutamide than for enzalutamide monotherapy. Combination therapy proved superior regardless of homologous recombination repair (HRR) pathway status, noted the authors.
“Not only did the combination therapy delay disease progression, it also significantly delayed progression of PSA [prostate-specific antigen] readings and the time until chemotherapy was needed compared to the control group,” said lead study author Neeraj Agarwal, MD, professor of medicine and director of the genitourinary oncology program at the Huntsman Cancer Institute, University of Utah, Salt Lake City.
“This is important because advanced prostate cancer can be associated with pain, fractures, suffering, and death. The current standard of care treatments were approved almost a decade ago, leaving a huge, unmet need for novel drugs in this setting,” he said.
The new results could pave the way for a prostate cancer indication for talazoparib; the company has said that it will submit these data to regulatory authorities. At present, the drug is approved only for use in BRCA+ breast cancer, an indication that was approved in 2018.
The findings were presented at the 2023 ASCO Genitourinary Cancers Symposium.
Overall, talazoparib plus enzalutamide resulted in a statistically significant and clinically meaningful improvement in PFS over placebo plus enzalutamide. “Results from the primary analysis of the TALAPRO-2 trial support the use of talazoparib plus enzalutamide as a first-line treatment in patients with mCRPC regardless of HRR gene alteration status,” Dr. Agarwal and colleagues concluded.
However, one expert disagreed with the authors’ conclusion regarding HHR pathway status. On the basis of imaging, PFS was 54% better in HHR-deficient patients in the combination therapy group. It was 30% better for patients with HHR-nondeficient tumors or tumors without known HHR status based on imaging and 34% better based on tumor tissue testing.
“There was a huge magnitude in benefit based on HHR, and I think HRR status matters,” commented Elena Castro, MD, PhD, Instituto de Investigación Biomédica de Málaga (Spain), who served as the invited discussant.
“We need to understand the benefit of ARPi [androgen receptor pathway inhibition] and PARP inhibitors better,” she said. “The balance between side effects and benefit depends on HRR status.”
Dr. Castro also noted that the treatment landscape has changed. ARPi is now a standard of care for metastatic prostate cancer, both for hormone-sensitive and castration-resistant disease. “So the question is, does the addition of a PARP inhibitor induce responses after progression to an ARPi in HHR-nondeficient tumors?”
Study details
In the TALAPRO-2 trial, Dr. Agarwal and colleagues randomly assigned 805 patients to receive either talazoparib 0.5 mg or placebo. All patients in the cohort received enzalutamide 160 mg daily.
Participants had mCRPC and were unselected for genetic alterations in DNA damage repair pathways directly or indirectly involved with HRR. They were aged 36-91 years (median age, 71). The cohort was enrolled from 25 countries, including the United States, Canada, Europe, South America, and countries in the Asia-Pacific region.
The men were stratified on the basis of prior use of abiraterone or docetaxel for castration-sensitive prostate cancer and HRR gene alteration status. The study’s primary endpoint was imaging-based PFS (ibPFS) by blinded independent central review (BICR).
Overall, median ibPFS by BICR was significantly improved in the combination group in comparison with the patients who received placebo; it was not reached versus 21.9 months (hazard ratio, 0.63; P < .001). It was also significantly improved among the HRR-deficient subgroup (HR, 0.46; P < .001) as well as in the HRR-nondeficient or unknown (HR, 0.70; P = .004) and HRR-nondeficient patients by tumor tissue testing (HR, 0.66; P = .009).
Talazoparib plus enzalutamide was also favored with regard to other endpoints. Dr. Agarwal noted that, while overall survival data are as yet immature, objective response rates, PSA response of at least 50%, and time to PSA progression and use of subsequent cytotoxic chemotherapy and antineoplastic therapy significantly favored the talazoparib group.
The objective response rate was 61.7% versus 43.9% (P = .005), with 37.5% versus 18.2% complete responses.
“The higher rates of complete response suggest a cooperative effect of talazoparib plus enzalutamide treatment,” he explained.
High rate of adverse events
The rate of treatment-emergent adverse events was higher among patients who received talazoparib plus enzalutamide; 71.9% of the patients who received talazoparib plus enzalutamide experienced grade 3-4 TEAEs versus 40.6%. The most common grade 3 or greater TEAEs in the talazoparib group were anemia, low neutrophil counts, and low platelet counts. Hypertension, anemia, and fatigue were the most common in the placebo group. Talazoparib was discontinued in 19.1% of patients because of TEAEs. Enzalutamide was discontinued in 10.8% of patients in the combination group versus 11.0% in the placebo group.
Dr. Agarwal pointed out that there were TEAEs of special interest for talazoparib. “Myelodysplastic syndrome was reported in one patient during the safety reporting period, and acute myeloid leukemia was reported in one patient during the follow-up period,” he said.
Additionally, pulmonary embolism was reported in 10 (2.5%) patients (grade 3 in 9 patients) in the talazoparib arm and in 3 (0.7%) patients (all grade 3) in the placebo arm.
Results less relevant
Commenting on the study, Matthew Zibelman, MD, associate professor, department of hematology/oncology, Fox Chase Cancer Center, Philadelphia, noted that these results represent an “intriguing finding for men with mCRPC, particularly in conjunction with the previously reported PROPEL study results.
“However, given that many patients receive an androgen receptor inhibitor now for metastatic castration-sensitive prostate cancer, these results are less relevant to current practice,” Dr. Zibelman said.
“Demonstration of an overall survival benefit of the combination would be optimal to change standard of care vs potential sequential therapy.”
The study was sponsored by Pfizer, manufacturer of enzalutamide and talazoparib. Dr. Agarwal has relationships with numerous pharmaceutical companies. Dr. Castro has relationships with Astellas Pharma, AstraZeneca, Bayer, Clovis Oncology, Janssen-Cilag, Merck, MSD Oncology, Novartis and Pfizer, and Roche. Dr. Zibelman has relationships with Bristol-Myers Squibb, Exelixis, Pfizer, Jannsen, EMD Serono, and Blue Earth.
A version of this article first appeared on Medscape.com.
in the TALAPRO-2 trial.
As determined on the basis of imaging, PFS was 37% better for talazoparib plus enzalutamide than for enzalutamide monotherapy. Combination therapy proved superior regardless of homologous recombination repair (HRR) pathway status, noted the authors.
“Not only did the combination therapy delay disease progression, it also significantly delayed progression of PSA [prostate-specific antigen] readings and the time until chemotherapy was needed compared to the control group,” said lead study author Neeraj Agarwal, MD, professor of medicine and director of the genitourinary oncology program at the Huntsman Cancer Institute, University of Utah, Salt Lake City.
“This is important because advanced prostate cancer can be associated with pain, fractures, suffering, and death. The current standard of care treatments were approved almost a decade ago, leaving a huge, unmet need for novel drugs in this setting,” he said.
The new results could pave the way for a prostate cancer indication for talazoparib; the company has said that it will submit these data to regulatory authorities. At present, the drug is approved only for use in BRCA+ breast cancer, an indication that was approved in 2018.
The findings were presented at the 2023 ASCO Genitourinary Cancers Symposium.
Overall, talazoparib plus enzalutamide resulted in a statistically significant and clinically meaningful improvement in PFS over placebo plus enzalutamide. “Results from the primary analysis of the TALAPRO-2 trial support the use of talazoparib plus enzalutamide as a first-line treatment in patients with mCRPC regardless of HRR gene alteration status,” Dr. Agarwal and colleagues concluded.
However, one expert disagreed with the authors’ conclusion regarding HHR pathway status. On the basis of imaging, PFS was 54% better in HHR-deficient patients in the combination therapy group. It was 30% better for patients with HHR-nondeficient tumors or tumors without known HHR status based on imaging and 34% better based on tumor tissue testing.
“There was a huge magnitude in benefit based on HHR, and I think HRR status matters,” commented Elena Castro, MD, PhD, Instituto de Investigación Biomédica de Málaga (Spain), who served as the invited discussant.
“We need to understand the benefit of ARPi [androgen receptor pathway inhibition] and PARP inhibitors better,” she said. “The balance between side effects and benefit depends on HRR status.”
Dr. Castro also noted that the treatment landscape has changed. ARPi is now a standard of care for metastatic prostate cancer, both for hormone-sensitive and castration-resistant disease. “So the question is, does the addition of a PARP inhibitor induce responses after progression to an ARPi in HHR-nondeficient tumors?”
Study details
In the TALAPRO-2 trial, Dr. Agarwal and colleagues randomly assigned 805 patients to receive either talazoparib 0.5 mg or placebo. All patients in the cohort received enzalutamide 160 mg daily.
Participants had mCRPC and were unselected for genetic alterations in DNA damage repair pathways directly or indirectly involved with HRR. They were aged 36-91 years (median age, 71). The cohort was enrolled from 25 countries, including the United States, Canada, Europe, South America, and countries in the Asia-Pacific region.
The men were stratified on the basis of prior use of abiraterone or docetaxel for castration-sensitive prostate cancer and HRR gene alteration status. The study’s primary endpoint was imaging-based PFS (ibPFS) by blinded independent central review (BICR).
Overall, median ibPFS by BICR was significantly improved in the combination group in comparison with the patients who received placebo; it was not reached versus 21.9 months (hazard ratio, 0.63; P < .001). It was also significantly improved among the HRR-deficient subgroup (HR, 0.46; P < .001) as well as in the HRR-nondeficient or unknown (HR, 0.70; P = .004) and HRR-nondeficient patients by tumor tissue testing (HR, 0.66; P = .009).
Talazoparib plus enzalutamide was also favored with regard to other endpoints. Dr. Agarwal noted that, while overall survival data are as yet immature, objective response rates, PSA response of at least 50%, and time to PSA progression and use of subsequent cytotoxic chemotherapy and antineoplastic therapy significantly favored the talazoparib group.
The objective response rate was 61.7% versus 43.9% (P = .005), with 37.5% versus 18.2% complete responses.
“The higher rates of complete response suggest a cooperative effect of talazoparib plus enzalutamide treatment,” he explained.
High rate of adverse events
The rate of treatment-emergent adverse events was higher among patients who received talazoparib plus enzalutamide; 71.9% of the patients who received talazoparib plus enzalutamide experienced grade 3-4 TEAEs versus 40.6%. The most common grade 3 or greater TEAEs in the talazoparib group were anemia, low neutrophil counts, and low platelet counts. Hypertension, anemia, and fatigue were the most common in the placebo group. Talazoparib was discontinued in 19.1% of patients because of TEAEs. Enzalutamide was discontinued in 10.8% of patients in the combination group versus 11.0% in the placebo group.
Dr. Agarwal pointed out that there were TEAEs of special interest for talazoparib. “Myelodysplastic syndrome was reported in one patient during the safety reporting period, and acute myeloid leukemia was reported in one patient during the follow-up period,” he said.
Additionally, pulmonary embolism was reported in 10 (2.5%) patients (grade 3 in 9 patients) in the talazoparib arm and in 3 (0.7%) patients (all grade 3) in the placebo arm.
Results less relevant
Commenting on the study, Matthew Zibelman, MD, associate professor, department of hematology/oncology, Fox Chase Cancer Center, Philadelphia, noted that these results represent an “intriguing finding for men with mCRPC, particularly in conjunction with the previously reported PROPEL study results.
“However, given that many patients receive an androgen receptor inhibitor now for metastatic castration-sensitive prostate cancer, these results are less relevant to current practice,” Dr. Zibelman said.
“Demonstration of an overall survival benefit of the combination would be optimal to change standard of care vs potential sequential therapy.”
The study was sponsored by Pfizer, manufacturer of enzalutamide and talazoparib. Dr. Agarwal has relationships with numerous pharmaceutical companies. Dr. Castro has relationships with Astellas Pharma, AstraZeneca, Bayer, Clovis Oncology, Janssen-Cilag, Merck, MSD Oncology, Novartis and Pfizer, and Roche. Dr. Zibelman has relationships with Bristol-Myers Squibb, Exelixis, Pfizer, Jannsen, EMD Serono, and Blue Earth.
A version of this article first appeared on Medscape.com.
in the TALAPRO-2 trial.
As determined on the basis of imaging, PFS was 37% better for talazoparib plus enzalutamide than for enzalutamide monotherapy. Combination therapy proved superior regardless of homologous recombination repair (HRR) pathway status, noted the authors.
“Not only did the combination therapy delay disease progression, it also significantly delayed progression of PSA [prostate-specific antigen] readings and the time until chemotherapy was needed compared to the control group,” said lead study author Neeraj Agarwal, MD, professor of medicine and director of the genitourinary oncology program at the Huntsman Cancer Institute, University of Utah, Salt Lake City.
“This is important because advanced prostate cancer can be associated with pain, fractures, suffering, and death. The current standard of care treatments were approved almost a decade ago, leaving a huge, unmet need for novel drugs in this setting,” he said.
The new results could pave the way for a prostate cancer indication for talazoparib; the company has said that it will submit these data to regulatory authorities. At present, the drug is approved only for use in BRCA+ breast cancer, an indication that was approved in 2018.
The findings were presented at the 2023 ASCO Genitourinary Cancers Symposium.
Overall, talazoparib plus enzalutamide resulted in a statistically significant and clinically meaningful improvement in PFS over placebo plus enzalutamide. “Results from the primary analysis of the TALAPRO-2 trial support the use of talazoparib plus enzalutamide as a first-line treatment in patients with mCRPC regardless of HRR gene alteration status,” Dr. Agarwal and colleagues concluded.
However, one expert disagreed with the authors’ conclusion regarding HHR pathway status. On the basis of imaging, PFS was 54% better in HHR-deficient patients in the combination therapy group. It was 30% better for patients with HHR-nondeficient tumors or tumors without known HHR status based on imaging and 34% better based on tumor tissue testing.
“There was a huge magnitude in benefit based on HHR, and I think HRR status matters,” commented Elena Castro, MD, PhD, Instituto de Investigación Biomédica de Málaga (Spain), who served as the invited discussant.
“We need to understand the benefit of ARPi [androgen receptor pathway inhibition] and PARP inhibitors better,” she said. “The balance between side effects and benefit depends on HRR status.”
Dr. Castro also noted that the treatment landscape has changed. ARPi is now a standard of care for metastatic prostate cancer, both for hormone-sensitive and castration-resistant disease. “So the question is, does the addition of a PARP inhibitor induce responses after progression to an ARPi in HHR-nondeficient tumors?”
Study details
In the TALAPRO-2 trial, Dr. Agarwal and colleagues randomly assigned 805 patients to receive either talazoparib 0.5 mg or placebo. All patients in the cohort received enzalutamide 160 mg daily.
Participants had mCRPC and were unselected for genetic alterations in DNA damage repair pathways directly or indirectly involved with HRR. They were aged 36-91 years (median age, 71). The cohort was enrolled from 25 countries, including the United States, Canada, Europe, South America, and countries in the Asia-Pacific region.
The men were stratified on the basis of prior use of abiraterone or docetaxel for castration-sensitive prostate cancer and HRR gene alteration status. The study’s primary endpoint was imaging-based PFS (ibPFS) by blinded independent central review (BICR).
Overall, median ibPFS by BICR was significantly improved in the combination group in comparison with the patients who received placebo; it was not reached versus 21.9 months (hazard ratio, 0.63; P < .001). It was also significantly improved among the HRR-deficient subgroup (HR, 0.46; P < .001) as well as in the HRR-nondeficient or unknown (HR, 0.70; P = .004) and HRR-nondeficient patients by tumor tissue testing (HR, 0.66; P = .009).
Talazoparib plus enzalutamide was also favored with regard to other endpoints. Dr. Agarwal noted that, while overall survival data are as yet immature, objective response rates, PSA response of at least 50%, and time to PSA progression and use of subsequent cytotoxic chemotherapy and antineoplastic therapy significantly favored the talazoparib group.
The objective response rate was 61.7% versus 43.9% (P = .005), with 37.5% versus 18.2% complete responses.
“The higher rates of complete response suggest a cooperative effect of talazoparib plus enzalutamide treatment,” he explained.
High rate of adverse events
The rate of treatment-emergent adverse events was higher among patients who received talazoparib plus enzalutamide; 71.9% of the patients who received talazoparib plus enzalutamide experienced grade 3-4 TEAEs versus 40.6%. The most common grade 3 or greater TEAEs in the talazoparib group were anemia, low neutrophil counts, and low platelet counts. Hypertension, anemia, and fatigue were the most common in the placebo group. Talazoparib was discontinued in 19.1% of patients because of TEAEs. Enzalutamide was discontinued in 10.8% of patients in the combination group versus 11.0% in the placebo group.
Dr. Agarwal pointed out that there were TEAEs of special interest for talazoparib. “Myelodysplastic syndrome was reported in one patient during the safety reporting period, and acute myeloid leukemia was reported in one patient during the follow-up period,” he said.
Additionally, pulmonary embolism was reported in 10 (2.5%) patients (grade 3 in 9 patients) in the talazoparib arm and in 3 (0.7%) patients (all grade 3) in the placebo arm.
Results less relevant
Commenting on the study, Matthew Zibelman, MD, associate professor, department of hematology/oncology, Fox Chase Cancer Center, Philadelphia, noted that these results represent an “intriguing finding for men with mCRPC, particularly in conjunction with the previously reported PROPEL study results.
“However, given that many patients receive an androgen receptor inhibitor now for metastatic castration-sensitive prostate cancer, these results are less relevant to current practice,” Dr. Zibelman said.
“Demonstration of an overall survival benefit of the combination would be optimal to change standard of care vs potential sequential therapy.”
The study was sponsored by Pfizer, manufacturer of enzalutamide and talazoparib. Dr. Agarwal has relationships with numerous pharmaceutical companies. Dr. Castro has relationships with Astellas Pharma, AstraZeneca, Bayer, Clovis Oncology, Janssen-Cilag, Merck, MSD Oncology, Novartis and Pfizer, and Roche. Dr. Zibelman has relationships with Bristol-Myers Squibb, Exelixis, Pfizer, Jannsen, EMD Serono, and Blue Earth.
A version of this article first appeared on Medscape.com.
AT ASCO GU 2023
Rucaparib benefit in BRCA+ prostate cancer confirmed
The finding, which comes from the TRITON3 clinical trial, provides evidence of clinical benefit for an indication for rucaparib that was granted an accelerated approval in May 2020.
“Rucaparib reduced the risk of progression or death by half in patients with BRCA alterations,” said lead author Alan H. Bryce, MD, medical director of the Genomic Oncology Clinic at Mayo Clinic Arizona, in Phoenix.
For the subgroup of patients with BRCA alterations, the median PFS was 11.2 months with rucaparib vs. 6.4 months (hazard ratio, 0.50; P < .001) among those who received physician’s choice of therapy, which included docetaxel or a second-generation ARPI, such as abiraterone or enzalutamide.
In another subgroup of patients whose disease had ATM alterations, the median PFS was 8.1 months with rucaparib vs. 6.8 months with physician’s choice of drug. The difference was not statistically significant.
However, the difference was significant in the intention-to-treat (ITT) population (comprising both subgroups), for whom the median PFS was 10.2 months with rucaparib vs. 6.4 months with physician’s choice of drug (HR, 0.61; P < .001 by log-rank test).
Dr. Bryce pointed out that three-quarters of the patients in the physician’s-choice arm who had progressive disease crossed over to rucaparib upon progression and that overall survival (OS) results are immature. At 62 months, median OS did not significantly differ in the BRCA subgroup (24.3 vs. 20.8 months favoring rucaparib; P = .21) or in the ITT group (23.6 vs. 20.9 months; P = .67).
Importantly, rucaparib was well tolerated. In all treatment groups, the most frequent adverse events were asthenia and fatigue, Bryce said. “There were no cases of myelodysplastic syndrome or acute myeloid leukemia reported.”
These results from the TRITON3 trial were presented at the 2023 ASCO Genitourinary Cancers Symposium and were published simultaneously in the New England Journal of Medicine.
Suggested benefit
Rucaparib is the first PARP inhibitor approved for use in patients with mCRPC that harbors deleterious BRCA mutations (germline and/or somatic) who have already been treated with androgen receptor–directed therapy and a taxane-based chemotherapy. This prostate cancer indication was granted an accelerated approval in May 2020 by the U.S. Food and Drug Administration on the basis of response rates and effect on levels of prostate-specific antigen (PSA) from the TRITON2 clinical trial, the forerunner of the current study.
The TRITON2 study was a single-arm clinical trial that involved three cohorts: 62 patients with a BRCA mutation (germline and/or somatic) and measurable disease; 115 patients with a BRCA mutation (germline and/or somatic) and measurable or nonmeasurable disease; and 209 patients with homologous recombination deficiency–positive mCRPC.
In an analysis of 115 patients with a deleterious BRCA mutation (germline and/or somatic) and measurable or nonmeasurable disease, the confirmed PSA response rate was 55%. For the patients with measurable disease and a BRCA mutation, the objective response rate was 44%. The objective response rate was similar for those with a germline BRCA mutation.
Study details
The current phase 3 randomized TRITON3 clinical trial was conducted to confirm the earlier findings and to expand upon the data in mCRPC. The participants in this trial were patients with mCRPC who had specific gene alterations, including BRCA and ATM alterations, who had experienced disease progression after androgen receptor–directed therapy but who had not yet received chemotherapy.
A total of 270 men were assigned to receive rucaparib (600 mg twice daily); 135 patients received their physician’s choice of medication. Within the two study arms, 302 patients had a BRCA alteration, and 103 patients had an ATM alteration. The ITT population consisted of all the patients who had been randomly assigned to either of the two groups. A prespecified subgroup included patients with a BRCA alteration.
The primary outcome was the median duration of imaging-based PSF, as determined through independent review. Key secondary outcomes were overall survival and objective response rate.
The most common adverse events in the rucaparib group were fatigue, nausea, and anemia or decreased hemoglobin. In the control group, the most common adverse events were fatigue, diarrhea, and neuropathy. The most common events of grade 3 or higher were anemia or decreased hemoglobin, neutropenia or a decreased neutrophil count, and fatigue in the rucaparib group, and fatigue and neutropenia or a decreased neutrophil count among control patients.
No changes in standard of care
In a discussion of the study, Elena Castro, MD, PhD, of the Instituto de Investigación Biomédica de Málaga, Campanillas, Spain, emphasized that there is a clear benefit from the use of PARP inhibitors (such as rucaparib) for patients with BRCA alterations.
However, she highlighted the absence of convincing overall survival data and the absence of a clear benefit on PFS in the subgroup of patients with ATM alterations.
“These data raise several questions,” she noted, “such as, do patients with ATM alterations benefit at all? And should PARP inhibitors [such as rucaparib] precede or follow docetaxel therapy?”
Because of the high crossover rate, it may be possible to evaluate the directionality of docetaxel followed by PARP inhibitors and the other way around, she suggested.
Dr. Castro said that patients with BRCA alterations benefit from PARP inhibitors and are likely to derive more benefit from them than from taxanes.
“But those with ATM alterations are unlikely to benefit from rucaparib more than from taxanes,” she said.
In a comment, Hank Ng, MD, medical oncologist, NYU Langone Perlmutter Cancer Center, New York, said he is not convinced that the findings from TRITON 3 represent a new standard of care in BRCA 1/2 mutations or ATM.
“Currently, we know that, for patients with prostate cancer with BRCA1/2 or ATM, the standard of care is an androgen receptor pathway inhibitor (ARPI), such as abiraterone or enzalutamide, then docetaxel, and then a PARP inhibitor like rucaparib,” he said.
(Currently, rucaparib is indicated for use in patients with mCRPC with BRCA alterations after they have already received an ARPI and taxane-based chemotherapy.)
Dr. Ng also questioned the control arm of the TRITON 3 trial. All the participants in the trial had already experienced disease progression after treatment with a second-generation ARPI. But the physician’s choice of therapy allowed them to move on to another ARPI or to docetaxel.
Dr. NG commented that, “in almost all cases, after progression of one ARPI, switching to another ARPI does not provide much benefit – from what is visible from this abstract – and only 56% patients received docetaxel, and thus 44% received a not-beneficial treatment,” he said.
“I am not sure what the docetaxel subgroup showed, but potentially, if those numbers are convincing, we could move this [rucaparib] ahead of docetaxel,” he speculated.
However, he also pointed out that an overall survival benefit has not yet been shown; so far, the benefit that has been shown is with respect to imaging-based PFS.
Dr. Ng does agree that rucaparib is indicated in the second line after progression with one ARPI for patients who are not candidates for chemotherapy. “But this has not yet shown me that we should absolutely be offering rucaparib before docetaxel,” he said.
TRITON3 was supported by Clovis Oncology, manufacturer of rucaparib. Dr. Bryce has relationships with Bayer, Foundation Medicine, Janssen, Merck, Myovant Sciences, and Novartis and holds a patent for therapeutic targeting of cancer patients with NRG1 rearrangements. Dr. Castro has relationships with Astellas Pharma, AstraZeneca, Bayer, Clovis Oncology, Janssen-Cilag, Merck, MSD Oncology, Novartis, Pfizer, and Roche.
A version of this article first appeared on Medscape.com.
The finding, which comes from the TRITON3 clinical trial, provides evidence of clinical benefit for an indication for rucaparib that was granted an accelerated approval in May 2020.
“Rucaparib reduced the risk of progression or death by half in patients with BRCA alterations,” said lead author Alan H. Bryce, MD, medical director of the Genomic Oncology Clinic at Mayo Clinic Arizona, in Phoenix.
For the subgroup of patients with BRCA alterations, the median PFS was 11.2 months with rucaparib vs. 6.4 months (hazard ratio, 0.50; P < .001) among those who received physician’s choice of therapy, which included docetaxel or a second-generation ARPI, such as abiraterone or enzalutamide.
In another subgroup of patients whose disease had ATM alterations, the median PFS was 8.1 months with rucaparib vs. 6.8 months with physician’s choice of drug. The difference was not statistically significant.
However, the difference was significant in the intention-to-treat (ITT) population (comprising both subgroups), for whom the median PFS was 10.2 months with rucaparib vs. 6.4 months with physician’s choice of drug (HR, 0.61; P < .001 by log-rank test).
Dr. Bryce pointed out that three-quarters of the patients in the physician’s-choice arm who had progressive disease crossed over to rucaparib upon progression and that overall survival (OS) results are immature. At 62 months, median OS did not significantly differ in the BRCA subgroup (24.3 vs. 20.8 months favoring rucaparib; P = .21) or in the ITT group (23.6 vs. 20.9 months; P = .67).
Importantly, rucaparib was well tolerated. In all treatment groups, the most frequent adverse events were asthenia and fatigue, Bryce said. “There were no cases of myelodysplastic syndrome or acute myeloid leukemia reported.”
These results from the TRITON3 trial were presented at the 2023 ASCO Genitourinary Cancers Symposium and were published simultaneously in the New England Journal of Medicine.
Suggested benefit
Rucaparib is the first PARP inhibitor approved for use in patients with mCRPC that harbors deleterious BRCA mutations (germline and/or somatic) who have already been treated with androgen receptor–directed therapy and a taxane-based chemotherapy. This prostate cancer indication was granted an accelerated approval in May 2020 by the U.S. Food and Drug Administration on the basis of response rates and effect on levels of prostate-specific antigen (PSA) from the TRITON2 clinical trial, the forerunner of the current study.
The TRITON2 study was a single-arm clinical trial that involved three cohorts: 62 patients with a BRCA mutation (germline and/or somatic) and measurable disease; 115 patients with a BRCA mutation (germline and/or somatic) and measurable or nonmeasurable disease; and 209 patients with homologous recombination deficiency–positive mCRPC.
In an analysis of 115 patients with a deleterious BRCA mutation (germline and/or somatic) and measurable or nonmeasurable disease, the confirmed PSA response rate was 55%. For the patients with measurable disease and a BRCA mutation, the objective response rate was 44%. The objective response rate was similar for those with a germline BRCA mutation.
Study details
The current phase 3 randomized TRITON3 clinical trial was conducted to confirm the earlier findings and to expand upon the data in mCRPC. The participants in this trial were patients with mCRPC who had specific gene alterations, including BRCA and ATM alterations, who had experienced disease progression after androgen receptor–directed therapy but who had not yet received chemotherapy.
A total of 270 men were assigned to receive rucaparib (600 mg twice daily); 135 patients received their physician’s choice of medication. Within the two study arms, 302 patients had a BRCA alteration, and 103 patients had an ATM alteration. The ITT population consisted of all the patients who had been randomly assigned to either of the two groups. A prespecified subgroup included patients with a BRCA alteration.
The primary outcome was the median duration of imaging-based PSF, as determined through independent review. Key secondary outcomes were overall survival and objective response rate.
The most common adverse events in the rucaparib group were fatigue, nausea, and anemia or decreased hemoglobin. In the control group, the most common adverse events were fatigue, diarrhea, and neuropathy. The most common events of grade 3 or higher were anemia or decreased hemoglobin, neutropenia or a decreased neutrophil count, and fatigue in the rucaparib group, and fatigue and neutropenia or a decreased neutrophil count among control patients.
No changes in standard of care
In a discussion of the study, Elena Castro, MD, PhD, of the Instituto de Investigación Biomédica de Málaga, Campanillas, Spain, emphasized that there is a clear benefit from the use of PARP inhibitors (such as rucaparib) for patients with BRCA alterations.
However, she highlighted the absence of convincing overall survival data and the absence of a clear benefit on PFS in the subgroup of patients with ATM alterations.
“These data raise several questions,” she noted, “such as, do patients with ATM alterations benefit at all? And should PARP inhibitors [such as rucaparib] precede or follow docetaxel therapy?”
Because of the high crossover rate, it may be possible to evaluate the directionality of docetaxel followed by PARP inhibitors and the other way around, she suggested.
Dr. Castro said that patients with BRCA alterations benefit from PARP inhibitors and are likely to derive more benefit from them than from taxanes.
“But those with ATM alterations are unlikely to benefit from rucaparib more than from taxanes,” she said.
In a comment, Hank Ng, MD, medical oncologist, NYU Langone Perlmutter Cancer Center, New York, said he is not convinced that the findings from TRITON 3 represent a new standard of care in BRCA 1/2 mutations or ATM.
“Currently, we know that, for patients with prostate cancer with BRCA1/2 or ATM, the standard of care is an androgen receptor pathway inhibitor (ARPI), such as abiraterone or enzalutamide, then docetaxel, and then a PARP inhibitor like rucaparib,” he said.
(Currently, rucaparib is indicated for use in patients with mCRPC with BRCA alterations after they have already received an ARPI and taxane-based chemotherapy.)
Dr. Ng also questioned the control arm of the TRITON 3 trial. All the participants in the trial had already experienced disease progression after treatment with a second-generation ARPI. But the physician’s choice of therapy allowed them to move on to another ARPI or to docetaxel.
Dr. NG commented that, “in almost all cases, after progression of one ARPI, switching to another ARPI does not provide much benefit – from what is visible from this abstract – and only 56% patients received docetaxel, and thus 44% received a not-beneficial treatment,” he said.
“I am not sure what the docetaxel subgroup showed, but potentially, if those numbers are convincing, we could move this [rucaparib] ahead of docetaxel,” he speculated.
However, he also pointed out that an overall survival benefit has not yet been shown; so far, the benefit that has been shown is with respect to imaging-based PFS.
Dr. Ng does agree that rucaparib is indicated in the second line after progression with one ARPI for patients who are not candidates for chemotherapy. “But this has not yet shown me that we should absolutely be offering rucaparib before docetaxel,” he said.
TRITON3 was supported by Clovis Oncology, manufacturer of rucaparib. Dr. Bryce has relationships with Bayer, Foundation Medicine, Janssen, Merck, Myovant Sciences, and Novartis and holds a patent for therapeutic targeting of cancer patients with NRG1 rearrangements. Dr. Castro has relationships with Astellas Pharma, AstraZeneca, Bayer, Clovis Oncology, Janssen-Cilag, Merck, MSD Oncology, Novartis, Pfizer, and Roche.
A version of this article first appeared on Medscape.com.
The finding, which comes from the TRITON3 clinical trial, provides evidence of clinical benefit for an indication for rucaparib that was granted an accelerated approval in May 2020.
“Rucaparib reduced the risk of progression or death by half in patients with BRCA alterations,” said lead author Alan H. Bryce, MD, medical director of the Genomic Oncology Clinic at Mayo Clinic Arizona, in Phoenix.
For the subgroup of patients with BRCA alterations, the median PFS was 11.2 months with rucaparib vs. 6.4 months (hazard ratio, 0.50; P < .001) among those who received physician’s choice of therapy, which included docetaxel or a second-generation ARPI, such as abiraterone or enzalutamide.
In another subgroup of patients whose disease had ATM alterations, the median PFS was 8.1 months with rucaparib vs. 6.8 months with physician’s choice of drug. The difference was not statistically significant.
However, the difference was significant in the intention-to-treat (ITT) population (comprising both subgroups), for whom the median PFS was 10.2 months with rucaparib vs. 6.4 months with physician’s choice of drug (HR, 0.61; P < .001 by log-rank test).
Dr. Bryce pointed out that three-quarters of the patients in the physician’s-choice arm who had progressive disease crossed over to rucaparib upon progression and that overall survival (OS) results are immature. At 62 months, median OS did not significantly differ in the BRCA subgroup (24.3 vs. 20.8 months favoring rucaparib; P = .21) or in the ITT group (23.6 vs. 20.9 months; P = .67).
Importantly, rucaparib was well tolerated. In all treatment groups, the most frequent adverse events were asthenia and fatigue, Bryce said. “There were no cases of myelodysplastic syndrome or acute myeloid leukemia reported.”
These results from the TRITON3 trial were presented at the 2023 ASCO Genitourinary Cancers Symposium and were published simultaneously in the New England Journal of Medicine.
Suggested benefit
Rucaparib is the first PARP inhibitor approved for use in patients with mCRPC that harbors deleterious BRCA mutations (germline and/or somatic) who have already been treated with androgen receptor–directed therapy and a taxane-based chemotherapy. This prostate cancer indication was granted an accelerated approval in May 2020 by the U.S. Food and Drug Administration on the basis of response rates and effect on levels of prostate-specific antigen (PSA) from the TRITON2 clinical trial, the forerunner of the current study.
The TRITON2 study was a single-arm clinical trial that involved three cohorts: 62 patients with a BRCA mutation (germline and/or somatic) and measurable disease; 115 patients with a BRCA mutation (germline and/or somatic) and measurable or nonmeasurable disease; and 209 patients with homologous recombination deficiency–positive mCRPC.
In an analysis of 115 patients with a deleterious BRCA mutation (germline and/or somatic) and measurable or nonmeasurable disease, the confirmed PSA response rate was 55%. For the patients with measurable disease and a BRCA mutation, the objective response rate was 44%. The objective response rate was similar for those with a germline BRCA mutation.
Study details
The current phase 3 randomized TRITON3 clinical trial was conducted to confirm the earlier findings and to expand upon the data in mCRPC. The participants in this trial were patients with mCRPC who had specific gene alterations, including BRCA and ATM alterations, who had experienced disease progression after androgen receptor–directed therapy but who had not yet received chemotherapy.
A total of 270 men were assigned to receive rucaparib (600 mg twice daily); 135 patients received their physician’s choice of medication. Within the two study arms, 302 patients had a BRCA alteration, and 103 patients had an ATM alteration. The ITT population consisted of all the patients who had been randomly assigned to either of the two groups. A prespecified subgroup included patients with a BRCA alteration.
The primary outcome was the median duration of imaging-based PSF, as determined through independent review. Key secondary outcomes were overall survival and objective response rate.
The most common adverse events in the rucaparib group were fatigue, nausea, and anemia or decreased hemoglobin. In the control group, the most common adverse events were fatigue, diarrhea, and neuropathy. The most common events of grade 3 or higher were anemia or decreased hemoglobin, neutropenia or a decreased neutrophil count, and fatigue in the rucaparib group, and fatigue and neutropenia or a decreased neutrophil count among control patients.
No changes in standard of care
In a discussion of the study, Elena Castro, MD, PhD, of the Instituto de Investigación Biomédica de Málaga, Campanillas, Spain, emphasized that there is a clear benefit from the use of PARP inhibitors (such as rucaparib) for patients with BRCA alterations.
However, she highlighted the absence of convincing overall survival data and the absence of a clear benefit on PFS in the subgroup of patients with ATM alterations.
“These data raise several questions,” she noted, “such as, do patients with ATM alterations benefit at all? And should PARP inhibitors [such as rucaparib] precede or follow docetaxel therapy?”
Because of the high crossover rate, it may be possible to evaluate the directionality of docetaxel followed by PARP inhibitors and the other way around, she suggested.
Dr. Castro said that patients with BRCA alterations benefit from PARP inhibitors and are likely to derive more benefit from them than from taxanes.
“But those with ATM alterations are unlikely to benefit from rucaparib more than from taxanes,” she said.
In a comment, Hank Ng, MD, medical oncologist, NYU Langone Perlmutter Cancer Center, New York, said he is not convinced that the findings from TRITON 3 represent a new standard of care in BRCA 1/2 mutations or ATM.
“Currently, we know that, for patients with prostate cancer with BRCA1/2 or ATM, the standard of care is an androgen receptor pathway inhibitor (ARPI), such as abiraterone or enzalutamide, then docetaxel, and then a PARP inhibitor like rucaparib,” he said.
(Currently, rucaparib is indicated for use in patients with mCRPC with BRCA alterations after they have already received an ARPI and taxane-based chemotherapy.)
Dr. Ng also questioned the control arm of the TRITON 3 trial. All the participants in the trial had already experienced disease progression after treatment with a second-generation ARPI. But the physician’s choice of therapy allowed them to move on to another ARPI or to docetaxel.
Dr. NG commented that, “in almost all cases, after progression of one ARPI, switching to another ARPI does not provide much benefit – from what is visible from this abstract – and only 56% patients received docetaxel, and thus 44% received a not-beneficial treatment,” he said.
“I am not sure what the docetaxel subgroup showed, but potentially, if those numbers are convincing, we could move this [rucaparib] ahead of docetaxel,” he speculated.
However, he also pointed out that an overall survival benefit has not yet been shown; so far, the benefit that has been shown is with respect to imaging-based PFS.
Dr. Ng does agree that rucaparib is indicated in the second line after progression with one ARPI for patients who are not candidates for chemotherapy. “But this has not yet shown me that we should absolutely be offering rucaparib before docetaxel,” he said.
TRITON3 was supported by Clovis Oncology, manufacturer of rucaparib. Dr. Bryce has relationships with Bayer, Foundation Medicine, Janssen, Merck, Myovant Sciences, and Novartis and holds a patent for therapeutic targeting of cancer patients with NRG1 rearrangements. Dr. Castro has relationships with Astellas Pharma, AstraZeneca, Bayer, Clovis Oncology, Janssen-Cilag, Merck, MSD Oncology, Novartis, Pfizer, and Roche.
A version of this article first appeared on Medscape.com.
AT ASCO GU 2023
Colorectal cancer treatment outcomes in older adults
A phase 2, multi-institutional feasibility study found a completion rate of 67.3%, while a prospective study found that completion was associated with improved disease-free survival.
Both studies were presented in January at the ASCO Gastrointestinal Cancers Symposium 2023.
In HiSCO-04, Japanese researchers found that of 64 older patients with stage 3A colorectal cancer who underwent adjuvant chemotherapy, 53% completed the treatment with an improvement in disease-free survival. Patients who completed adjuvant chemotherapy had better disease-free survival (P = .03), while the survival was lower among those who did not receive adjuvant chemotherapy, and lowest among those who discontinued adjuvant chemotherapy.
“The results showed that adjuvant chemotherapy is not always recommended for elderly patients, and that patients who are able to complete treatment may have a better prognosis for survival. However, the results do not indicate which patients are unable to complete chemotherapy, and it will be necessary to identify patients who are intolerant of chemotherapy,” said the study’s lead author Manabu Shimomura, MD, PhD, an assistant professor of gastroenterological and transplant surgery at the Hiroshima University Graduate School of Biomedical and Health Sciences in Japan.
The study, which was conducted between 2013 and 2021, enrolled 214 patients (99 men, 115 women, 80-101 years old) who were in stage 3 cancer (27 cases 3A, 158 cases 3B, and 29 cases 3C). A total of 41 patients were ineligible for chemotherapy. Of the remaining patients, 65 received adjuvant chemotherapy and 108 did not receive adjuvant chemotherapy.
The 3-year disease-free survival was 63.6%, the 3-year overall survival was 76.9%, and the 3-year relapse-free survival was 63.1%. Thirty-six patients died because of colorectal cancer, and 30 patients died of other causes. There was recurrence in 58 cases and secondary cancers were observed in 17 cases during the 42.5 months–long follow-up period.
There were few reports of serious adverse events, but some cases of treatment discontinuation were because of adverse events.
In a second study presented by Dr. Shimomura’s group, called HiSCO-03, 65 patients (33 female) underwent curative resection and received five courses of uracil-tegafur and leucovorin (UFT/LV).
The completion rate of 67.3% had a 95% lower bound of 54.9%, which were lower than the predefined thresholds of 75% completion and a lower bound of 60%. “Based on the results of a previous (ACTS-CC phase III) study, we set the expected value of UFT/LV therapy in patients over 80 years of age at 75% and the threshold at 60%. Since the target age group of previous study was 75 years or younger, we concluded from the results of the current study that UFT/LV therapy is less well tolerated in patients 80 years of age and older than in patients 75 years of age and younger,” Dr. Shimomura said.
The treatment completion rate trended higher in males than females (77.6% versus 57.2%; P = .06) and performance status of 0 versus 1 or 2 (74.3% versus 58.9%; P = .10). The most common adverse events were anorexia (33.8%), diarrhea (30.8%), and anemia (24.6%). The median relative dose intensity was 84% for UFT and 100% for LV.
The challenges of treating older patients
If and how older patients with colorectal cancer should be treated is not clear cut. While 20% of patients in the United States who have colorectal cancer are over 80 years old, each case should be evaluated individually, experts say.
Writing in a 2015 review of colorectal cancer treatment in older adults, Monica Millan, MD, PhD, of Joan XXIII University Hospital, Tarragona, Spain, and colleagues, wrote that physiological heterogeneity and coexisting medical conditions make treating older patients with colorectal cancer challenging.
“Age in itself should not be an exclusion criterion for radical treatment, but there will be many elderly patients that will not tolerate or respond well to standard therapies. These patients need to be properly assessed before proposing treatment, and a tailored, individualized approach should be offered in a multidisciplinary setting,” wrote Dr. Millan, who is a colorectal surgeon.
The authors suggest that older patients who are fit could be treated similarly to younger patients, but there remain uncertainties about how to proceed in frail older adults with comorbidities.
“Most elderly patients with cancer will have priorities besides simply prolonging their lives. Surveys have found that their top concerns include avoiding suffering, strengthening relationships with family and friends, being mentally aware, not being a burden on others, and achieving a sense that their life is complete. The treatment plan should be comprehensive: cancer-specific treatment, symptom-specific treatment, supportive treatment modalities, and end-of-life care,” they wrote.
The U.S. Preventive Services Task Force recommends colorectal cancer screening for men and women who are between 45 and 75 years old; however, screening for patients between 76 and 85 years old should be done on a case-by-case basis based on a patient’s overall health, screening history, and the patient’s preferences.
Colorectal cancer incidence rates have been declining since the mid-1980s because of an increase in screening among adults 50 years and older, according to the American Cancer Society. Likewise, mortality rates have dropped from 29.2% in 1970 to 12.6% in 2020 – mostly because of screening.
Dr. Shimomura has no relevant financial disclosures.
The Gastrointestinal Cancers Symposium is sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
A phase 2, multi-institutional feasibility study found a completion rate of 67.3%, while a prospective study found that completion was associated with improved disease-free survival.
Both studies were presented in January at the ASCO Gastrointestinal Cancers Symposium 2023.
In HiSCO-04, Japanese researchers found that of 64 older patients with stage 3A colorectal cancer who underwent adjuvant chemotherapy, 53% completed the treatment with an improvement in disease-free survival. Patients who completed adjuvant chemotherapy had better disease-free survival (P = .03), while the survival was lower among those who did not receive adjuvant chemotherapy, and lowest among those who discontinued adjuvant chemotherapy.
“The results showed that adjuvant chemotherapy is not always recommended for elderly patients, and that patients who are able to complete treatment may have a better prognosis for survival. However, the results do not indicate which patients are unable to complete chemotherapy, and it will be necessary to identify patients who are intolerant of chemotherapy,” said the study’s lead author Manabu Shimomura, MD, PhD, an assistant professor of gastroenterological and transplant surgery at the Hiroshima University Graduate School of Biomedical and Health Sciences in Japan.
The study, which was conducted between 2013 and 2021, enrolled 214 patients (99 men, 115 women, 80-101 years old) who were in stage 3 cancer (27 cases 3A, 158 cases 3B, and 29 cases 3C). A total of 41 patients were ineligible for chemotherapy. Of the remaining patients, 65 received adjuvant chemotherapy and 108 did not receive adjuvant chemotherapy.
The 3-year disease-free survival was 63.6%, the 3-year overall survival was 76.9%, and the 3-year relapse-free survival was 63.1%. Thirty-six patients died because of colorectal cancer, and 30 patients died of other causes. There was recurrence in 58 cases and secondary cancers were observed in 17 cases during the 42.5 months–long follow-up period.
There were few reports of serious adverse events, but some cases of treatment discontinuation were because of adverse events.
In a second study presented by Dr. Shimomura’s group, called HiSCO-03, 65 patients (33 female) underwent curative resection and received five courses of uracil-tegafur and leucovorin (UFT/LV).
The completion rate of 67.3% had a 95% lower bound of 54.9%, which were lower than the predefined thresholds of 75% completion and a lower bound of 60%. “Based on the results of a previous (ACTS-CC phase III) study, we set the expected value of UFT/LV therapy in patients over 80 years of age at 75% and the threshold at 60%. Since the target age group of previous study was 75 years or younger, we concluded from the results of the current study that UFT/LV therapy is less well tolerated in patients 80 years of age and older than in patients 75 years of age and younger,” Dr. Shimomura said.
The treatment completion rate trended higher in males than females (77.6% versus 57.2%; P = .06) and performance status of 0 versus 1 or 2 (74.3% versus 58.9%; P = .10). The most common adverse events were anorexia (33.8%), diarrhea (30.8%), and anemia (24.6%). The median relative dose intensity was 84% for UFT and 100% for LV.
The challenges of treating older patients
If and how older patients with colorectal cancer should be treated is not clear cut. While 20% of patients in the United States who have colorectal cancer are over 80 years old, each case should be evaluated individually, experts say.
Writing in a 2015 review of colorectal cancer treatment in older adults, Monica Millan, MD, PhD, of Joan XXIII University Hospital, Tarragona, Spain, and colleagues, wrote that physiological heterogeneity and coexisting medical conditions make treating older patients with colorectal cancer challenging.
“Age in itself should not be an exclusion criterion for radical treatment, but there will be many elderly patients that will not tolerate or respond well to standard therapies. These patients need to be properly assessed before proposing treatment, and a tailored, individualized approach should be offered in a multidisciplinary setting,” wrote Dr. Millan, who is a colorectal surgeon.
The authors suggest that older patients who are fit could be treated similarly to younger patients, but there remain uncertainties about how to proceed in frail older adults with comorbidities.
“Most elderly patients with cancer will have priorities besides simply prolonging their lives. Surveys have found that their top concerns include avoiding suffering, strengthening relationships with family and friends, being mentally aware, not being a burden on others, and achieving a sense that their life is complete. The treatment plan should be comprehensive: cancer-specific treatment, symptom-specific treatment, supportive treatment modalities, and end-of-life care,” they wrote.
The U.S. Preventive Services Task Force recommends colorectal cancer screening for men and women who are between 45 and 75 years old; however, screening for patients between 76 and 85 years old should be done on a case-by-case basis based on a patient’s overall health, screening history, and the patient’s preferences.
Colorectal cancer incidence rates have been declining since the mid-1980s because of an increase in screening among adults 50 years and older, according to the American Cancer Society. Likewise, mortality rates have dropped from 29.2% in 1970 to 12.6% in 2020 – mostly because of screening.
Dr. Shimomura has no relevant financial disclosures.
The Gastrointestinal Cancers Symposium is sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
A phase 2, multi-institutional feasibility study found a completion rate of 67.3%, while a prospective study found that completion was associated with improved disease-free survival.
Both studies were presented in January at the ASCO Gastrointestinal Cancers Symposium 2023.
In HiSCO-04, Japanese researchers found that of 64 older patients with stage 3A colorectal cancer who underwent adjuvant chemotherapy, 53% completed the treatment with an improvement in disease-free survival. Patients who completed adjuvant chemotherapy had better disease-free survival (P = .03), while the survival was lower among those who did not receive adjuvant chemotherapy, and lowest among those who discontinued adjuvant chemotherapy.
“The results showed that adjuvant chemotherapy is not always recommended for elderly patients, and that patients who are able to complete treatment may have a better prognosis for survival. However, the results do not indicate which patients are unable to complete chemotherapy, and it will be necessary to identify patients who are intolerant of chemotherapy,” said the study’s lead author Manabu Shimomura, MD, PhD, an assistant professor of gastroenterological and transplant surgery at the Hiroshima University Graduate School of Biomedical and Health Sciences in Japan.
The study, which was conducted between 2013 and 2021, enrolled 214 patients (99 men, 115 women, 80-101 years old) who were in stage 3 cancer (27 cases 3A, 158 cases 3B, and 29 cases 3C). A total of 41 patients were ineligible for chemotherapy. Of the remaining patients, 65 received adjuvant chemotherapy and 108 did not receive adjuvant chemotherapy.
The 3-year disease-free survival was 63.6%, the 3-year overall survival was 76.9%, and the 3-year relapse-free survival was 63.1%. Thirty-six patients died because of colorectal cancer, and 30 patients died of other causes. There was recurrence in 58 cases and secondary cancers were observed in 17 cases during the 42.5 months–long follow-up period.
There were few reports of serious adverse events, but some cases of treatment discontinuation were because of adverse events.
In a second study presented by Dr. Shimomura’s group, called HiSCO-03, 65 patients (33 female) underwent curative resection and received five courses of uracil-tegafur and leucovorin (UFT/LV).
The completion rate of 67.3% had a 95% lower bound of 54.9%, which were lower than the predefined thresholds of 75% completion and a lower bound of 60%. “Based on the results of a previous (ACTS-CC phase III) study, we set the expected value of UFT/LV therapy in patients over 80 years of age at 75% and the threshold at 60%. Since the target age group of previous study was 75 years or younger, we concluded from the results of the current study that UFT/LV therapy is less well tolerated in patients 80 years of age and older than in patients 75 years of age and younger,” Dr. Shimomura said.
The treatment completion rate trended higher in males than females (77.6% versus 57.2%; P = .06) and performance status of 0 versus 1 or 2 (74.3% versus 58.9%; P = .10). The most common adverse events were anorexia (33.8%), diarrhea (30.8%), and anemia (24.6%). The median relative dose intensity was 84% for UFT and 100% for LV.
The challenges of treating older patients
If and how older patients with colorectal cancer should be treated is not clear cut. While 20% of patients in the United States who have colorectal cancer are over 80 years old, each case should be evaluated individually, experts say.
Writing in a 2015 review of colorectal cancer treatment in older adults, Monica Millan, MD, PhD, of Joan XXIII University Hospital, Tarragona, Spain, and colleagues, wrote that physiological heterogeneity and coexisting medical conditions make treating older patients with colorectal cancer challenging.
“Age in itself should not be an exclusion criterion for radical treatment, but there will be many elderly patients that will not tolerate or respond well to standard therapies. These patients need to be properly assessed before proposing treatment, and a tailored, individualized approach should be offered in a multidisciplinary setting,” wrote Dr. Millan, who is a colorectal surgeon.
The authors suggest that older patients who are fit could be treated similarly to younger patients, but there remain uncertainties about how to proceed in frail older adults with comorbidities.
“Most elderly patients with cancer will have priorities besides simply prolonging their lives. Surveys have found that their top concerns include avoiding suffering, strengthening relationships with family and friends, being mentally aware, not being a burden on others, and achieving a sense that their life is complete. The treatment plan should be comprehensive: cancer-specific treatment, symptom-specific treatment, supportive treatment modalities, and end-of-life care,” they wrote.
The U.S. Preventive Services Task Force recommends colorectal cancer screening for men and women who are between 45 and 75 years old; however, screening for patients between 76 and 85 years old should be done on a case-by-case basis based on a patient’s overall health, screening history, and the patient’s preferences.
Colorectal cancer incidence rates have been declining since the mid-1980s because of an increase in screening among adults 50 years and older, according to the American Cancer Society. Likewise, mortality rates have dropped from 29.2% in 1970 to 12.6% in 2020 – mostly because of screening.
Dr. Shimomura has no relevant financial disclosures.
The Gastrointestinal Cancers Symposium is sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
FROM ASCO GI 2023
New cancer data spark outcry from patient advocates
The American Cancer Society on Jan. 13 revealed what it called “alarming” news about prostate cancer: After 2 decades of decline, the number of men diagnosed with the disease in the United States rose by 15% from 2014 to 2019.
“Most concerning,” according to the group’s CEO Karen Knudsen, PhD, MBA, is that the increase is being driven by diagnoses of advanced disease.
“Since 2011, the diagnosis of advanced-stage (regional- or distant-stage) prostate cancer has increased by 4%-5% annually and the proportion of men diagnosed with distant-stage disease has doubled,” said Dr. Knudsen at a press conference concerning the figures. “These findings underscore the importance of understanding and reducing this trend.”
The increase, which works out to be an additional 99,000 cases of prostate cancer, did not take the ACS by surprise; the group has been predicting a jump in diagnoses of the disease, which is the most common cancer in men after skin cancer, and the second most common cause of cancer death for that group.
The ACS announced a new action plan, “Improving Mortality from Prostate Cancer Together” – or IMPACT – to address the rise, especially in Black men, and to curb the increasing rate of advanced, difficult-to-treat cases.
“We must address these shifts in prostate cancer, especially in the Black community, since the incidence of prostate cancer in Black men is 70% higher than in White men and prostate cancer mortality rates in Black men are approximately two to four times higher than those in every other racial and ethnic group,” William Dahut, MD, PhD, chief scientific officer for the ACS, said at the press conference.
A study published in JAMA Network Open challenged that claim, finding that, after controlling for socioeconomic factors, race does not appear to be a significant predictor of mortality for prostate cancer.
Dr. Dahut said in an interview that IMPACT “is still [in the] early days for this initiative and more details will be coming out soon.”
Charles Ryan, MD, CEO of the Prostate Cancer Foundation, the world’s largest prostate cancer research charity, called IMPACT “extremely important work. Highlighting the disparities can only serve to benefit all men with prostate cancer, especially Black men.”
Bold action ... or passivity?
Overall cancer mortality has dropped 33% since 1991, averting an estimated 3.8 million deaths, according to ACS. But the story for prostate cancer is different.
The society and advocates had warned as recently as 2 years ago that prostate cancer was poised to rise again, especially advanced cases that may be too late to treat.
Leaders in the prostate cancer advocacy community praised the ACS plan for IMPACT, but some expressed frustration over what they said was ACS’ passivity in the face of long-anticipated increases in cases of the disease.
“I think prostate cancer was not high on their agenda,” said Rick Davis, founder of AnCan, which offers several support groups for patients with prostate cancer. “It’s good to see ACS get back into the prostate cancer game.”
Mr. Davis and patient advocate Darryl Mitteldorf, LCSW, founder of Malecare, another prostate support organization, said ACS dropped patient services for prostate cancer patients a decade ago and has not been a vocal supporter of screening for levels of prostate-specific antigen (PSA) to detect prostate cancer early.
“Early detection is supposed to be their goal,” Mr. Davis said.
In 2012, the U.S. Preventive Services Task Force recommended against PSA screening, giving it a D-rating. The move prompted attacks on the task force from most advocates and many urologists.
Following this criticism, the task force recommended shared decision-making between patient and doctor, while giving PSA screening a C-rating. Now, the ACS recommends men in general at age 50 discuss prostate cancer screening with their doctor and that Black men do the same at age 45.
Mr. Mitteldorf said ACS “owes prostate cancer patients an explanation and analysis of its response to the USPTF’s downgrade of PSA testing and how that response might be related to death and instance rates.”
Mr. Mitteldorf added that male patients lost key support from ACS when the group dismantled its Man to Man group for prostate cancer patients and its Brother to Brother group for Blacks in particular.
Dr. Dahut said Man to Man “sunsetted” and was turned over to any local organization that chose to offer it. He said longtime staff didn’t have “a lot of information about [the demise of] Brother to Brother.”
For Mr. Davis, those smaller cuts add up to a much larger insult.
“Today, in 2023, ACS continues to poke a finger in the eyes of prostate cancer patients,” he said. “Since 2010, they have not given us any respect. ACS dumped its support.”
He pointed to the group’s funding priorities, noting that outlays for prostate cancer have consistently lagged behind those for breast cancer.
The ACS spent $25.3 million on breast cancer research and $6.7 million for prostate cancer in 2018, and in 2023 will designate $126.5 for breast cancer research and $43.9 million for prostate cancer.
ACS has earmarked $62 million this year for lung cancer programs and $61 million for colorectal cancer.
“Parity between breast cancer and prostate cancer would be a good start in sizing the IMPACT program,” Mr. Davis said. “After all, breast cancer and prostate cancer are hardly different in numbers today.”
Dr. Dahut denied any gender bias in research funding. He said the group makes funding decisions “based on finding the most impactful science regardless of tumor type. Our mission includes funding every cancer, every day; thus, we generally do not go into our funding cycle with any set-asides for a particular cancer.”
Mr. Davis also said the ACS data suggest the growing number of prostate cancer cases is even worse than the group has said. Although the society cites a 3% annual increase in prostate cancer diagnoses from 2014 to 2019, since 2019 the annual increase is a much more dramatic 16%. Meanwhile, the number of new cases of the disease is projected to rise from 175,000 per year in 2019 to 288,000 this year.
Dr. Dahut said the society used the 2014-2019 time frame for technical reasons, separating confirmed cases in the earlier period from estimated cases in recent years.
“We discourage comparing projected cases over time because these cases are model-based and subject to fluctuations,” Dr. Dahut said.
A version of this article originally appeared on Medscape.com.
The American Cancer Society on Jan. 13 revealed what it called “alarming” news about prostate cancer: After 2 decades of decline, the number of men diagnosed with the disease in the United States rose by 15% from 2014 to 2019.
“Most concerning,” according to the group’s CEO Karen Knudsen, PhD, MBA, is that the increase is being driven by diagnoses of advanced disease.
“Since 2011, the diagnosis of advanced-stage (regional- or distant-stage) prostate cancer has increased by 4%-5% annually and the proportion of men diagnosed with distant-stage disease has doubled,” said Dr. Knudsen at a press conference concerning the figures. “These findings underscore the importance of understanding and reducing this trend.”
The increase, which works out to be an additional 99,000 cases of prostate cancer, did not take the ACS by surprise; the group has been predicting a jump in diagnoses of the disease, which is the most common cancer in men after skin cancer, and the second most common cause of cancer death for that group.
The ACS announced a new action plan, “Improving Mortality from Prostate Cancer Together” – or IMPACT – to address the rise, especially in Black men, and to curb the increasing rate of advanced, difficult-to-treat cases.
“We must address these shifts in prostate cancer, especially in the Black community, since the incidence of prostate cancer in Black men is 70% higher than in White men and prostate cancer mortality rates in Black men are approximately two to four times higher than those in every other racial and ethnic group,” William Dahut, MD, PhD, chief scientific officer for the ACS, said at the press conference.
A study published in JAMA Network Open challenged that claim, finding that, after controlling for socioeconomic factors, race does not appear to be a significant predictor of mortality for prostate cancer.
Dr. Dahut said in an interview that IMPACT “is still [in the] early days for this initiative and more details will be coming out soon.”
Charles Ryan, MD, CEO of the Prostate Cancer Foundation, the world’s largest prostate cancer research charity, called IMPACT “extremely important work. Highlighting the disparities can only serve to benefit all men with prostate cancer, especially Black men.”
Bold action ... or passivity?
Overall cancer mortality has dropped 33% since 1991, averting an estimated 3.8 million deaths, according to ACS. But the story for prostate cancer is different.
The society and advocates had warned as recently as 2 years ago that prostate cancer was poised to rise again, especially advanced cases that may be too late to treat.
Leaders in the prostate cancer advocacy community praised the ACS plan for IMPACT, but some expressed frustration over what they said was ACS’ passivity in the face of long-anticipated increases in cases of the disease.
“I think prostate cancer was not high on their agenda,” said Rick Davis, founder of AnCan, which offers several support groups for patients with prostate cancer. “It’s good to see ACS get back into the prostate cancer game.”
Mr. Davis and patient advocate Darryl Mitteldorf, LCSW, founder of Malecare, another prostate support organization, said ACS dropped patient services for prostate cancer patients a decade ago and has not been a vocal supporter of screening for levels of prostate-specific antigen (PSA) to detect prostate cancer early.
“Early detection is supposed to be their goal,” Mr. Davis said.
In 2012, the U.S. Preventive Services Task Force recommended against PSA screening, giving it a D-rating. The move prompted attacks on the task force from most advocates and many urologists.
Following this criticism, the task force recommended shared decision-making between patient and doctor, while giving PSA screening a C-rating. Now, the ACS recommends men in general at age 50 discuss prostate cancer screening with their doctor and that Black men do the same at age 45.
Mr. Mitteldorf said ACS “owes prostate cancer patients an explanation and analysis of its response to the USPTF’s downgrade of PSA testing and how that response might be related to death and instance rates.”
Mr. Mitteldorf added that male patients lost key support from ACS when the group dismantled its Man to Man group for prostate cancer patients and its Brother to Brother group for Blacks in particular.
Dr. Dahut said Man to Man “sunsetted” and was turned over to any local organization that chose to offer it. He said longtime staff didn’t have “a lot of information about [the demise of] Brother to Brother.”
For Mr. Davis, those smaller cuts add up to a much larger insult.
“Today, in 2023, ACS continues to poke a finger in the eyes of prostate cancer patients,” he said. “Since 2010, they have not given us any respect. ACS dumped its support.”
He pointed to the group’s funding priorities, noting that outlays for prostate cancer have consistently lagged behind those for breast cancer.
The ACS spent $25.3 million on breast cancer research and $6.7 million for prostate cancer in 2018, and in 2023 will designate $126.5 for breast cancer research and $43.9 million for prostate cancer.
ACS has earmarked $62 million this year for lung cancer programs and $61 million for colorectal cancer.
“Parity between breast cancer and prostate cancer would be a good start in sizing the IMPACT program,” Mr. Davis said. “After all, breast cancer and prostate cancer are hardly different in numbers today.”
Dr. Dahut denied any gender bias in research funding. He said the group makes funding decisions “based on finding the most impactful science regardless of tumor type. Our mission includes funding every cancer, every day; thus, we generally do not go into our funding cycle with any set-asides for a particular cancer.”
Mr. Davis also said the ACS data suggest the growing number of prostate cancer cases is even worse than the group has said. Although the society cites a 3% annual increase in prostate cancer diagnoses from 2014 to 2019, since 2019 the annual increase is a much more dramatic 16%. Meanwhile, the number of new cases of the disease is projected to rise from 175,000 per year in 2019 to 288,000 this year.
Dr. Dahut said the society used the 2014-2019 time frame for technical reasons, separating confirmed cases in the earlier period from estimated cases in recent years.
“We discourage comparing projected cases over time because these cases are model-based and subject to fluctuations,” Dr. Dahut said.
A version of this article originally appeared on Medscape.com.
The American Cancer Society on Jan. 13 revealed what it called “alarming” news about prostate cancer: After 2 decades of decline, the number of men diagnosed with the disease in the United States rose by 15% from 2014 to 2019.
“Most concerning,” according to the group’s CEO Karen Knudsen, PhD, MBA, is that the increase is being driven by diagnoses of advanced disease.
“Since 2011, the diagnosis of advanced-stage (regional- or distant-stage) prostate cancer has increased by 4%-5% annually and the proportion of men diagnosed with distant-stage disease has doubled,” said Dr. Knudsen at a press conference concerning the figures. “These findings underscore the importance of understanding and reducing this trend.”
The increase, which works out to be an additional 99,000 cases of prostate cancer, did not take the ACS by surprise; the group has been predicting a jump in diagnoses of the disease, which is the most common cancer in men after skin cancer, and the second most common cause of cancer death for that group.
The ACS announced a new action plan, “Improving Mortality from Prostate Cancer Together” – or IMPACT – to address the rise, especially in Black men, and to curb the increasing rate of advanced, difficult-to-treat cases.
“We must address these shifts in prostate cancer, especially in the Black community, since the incidence of prostate cancer in Black men is 70% higher than in White men and prostate cancer mortality rates in Black men are approximately two to four times higher than those in every other racial and ethnic group,” William Dahut, MD, PhD, chief scientific officer for the ACS, said at the press conference.
A study published in JAMA Network Open challenged that claim, finding that, after controlling for socioeconomic factors, race does not appear to be a significant predictor of mortality for prostate cancer.
Dr. Dahut said in an interview that IMPACT “is still [in the] early days for this initiative and more details will be coming out soon.”
Charles Ryan, MD, CEO of the Prostate Cancer Foundation, the world’s largest prostate cancer research charity, called IMPACT “extremely important work. Highlighting the disparities can only serve to benefit all men with prostate cancer, especially Black men.”
Bold action ... or passivity?
Overall cancer mortality has dropped 33% since 1991, averting an estimated 3.8 million deaths, according to ACS. But the story for prostate cancer is different.
The society and advocates had warned as recently as 2 years ago that prostate cancer was poised to rise again, especially advanced cases that may be too late to treat.
Leaders in the prostate cancer advocacy community praised the ACS plan for IMPACT, but some expressed frustration over what they said was ACS’ passivity in the face of long-anticipated increases in cases of the disease.
“I think prostate cancer was not high on their agenda,” said Rick Davis, founder of AnCan, which offers several support groups for patients with prostate cancer. “It’s good to see ACS get back into the prostate cancer game.”
Mr. Davis and patient advocate Darryl Mitteldorf, LCSW, founder of Malecare, another prostate support organization, said ACS dropped patient services for prostate cancer patients a decade ago and has not been a vocal supporter of screening for levels of prostate-specific antigen (PSA) to detect prostate cancer early.
“Early detection is supposed to be their goal,” Mr. Davis said.
In 2012, the U.S. Preventive Services Task Force recommended against PSA screening, giving it a D-rating. The move prompted attacks on the task force from most advocates and many urologists.
Following this criticism, the task force recommended shared decision-making between patient and doctor, while giving PSA screening a C-rating. Now, the ACS recommends men in general at age 50 discuss prostate cancer screening with their doctor and that Black men do the same at age 45.
Mr. Mitteldorf said ACS “owes prostate cancer patients an explanation and analysis of its response to the USPTF’s downgrade of PSA testing and how that response might be related to death and instance rates.”
Mr. Mitteldorf added that male patients lost key support from ACS when the group dismantled its Man to Man group for prostate cancer patients and its Brother to Brother group for Blacks in particular.
Dr. Dahut said Man to Man “sunsetted” and was turned over to any local organization that chose to offer it. He said longtime staff didn’t have “a lot of information about [the demise of] Brother to Brother.”
For Mr. Davis, those smaller cuts add up to a much larger insult.
“Today, in 2023, ACS continues to poke a finger in the eyes of prostate cancer patients,” he said. “Since 2010, they have not given us any respect. ACS dumped its support.”
He pointed to the group’s funding priorities, noting that outlays for prostate cancer have consistently lagged behind those for breast cancer.
The ACS spent $25.3 million on breast cancer research and $6.7 million for prostate cancer in 2018, and in 2023 will designate $126.5 for breast cancer research and $43.9 million for prostate cancer.
ACS has earmarked $62 million this year for lung cancer programs and $61 million for colorectal cancer.
“Parity between breast cancer and prostate cancer would be a good start in sizing the IMPACT program,” Mr. Davis said. “After all, breast cancer and prostate cancer are hardly different in numbers today.”
Dr. Dahut denied any gender bias in research funding. He said the group makes funding decisions “based on finding the most impactful science regardless of tumor type. Our mission includes funding every cancer, every day; thus, we generally do not go into our funding cycle with any set-asides for a particular cancer.”
Mr. Davis also said the ACS data suggest the growing number of prostate cancer cases is even worse than the group has said. Although the society cites a 3% annual increase in prostate cancer diagnoses from 2014 to 2019, since 2019 the annual increase is a much more dramatic 16%. Meanwhile, the number of new cases of the disease is projected to rise from 175,000 per year in 2019 to 288,000 this year.
Dr. Dahut said the society used the 2014-2019 time frame for technical reasons, separating confirmed cases in the earlier period from estimated cases in recent years.
“We discourage comparing projected cases over time because these cases are model-based and subject to fluctuations,” Dr. Dahut said.
A version of this article originally appeared on Medscape.com.
‘Low-value’ prostate cancer screening prevalent in primary care
Yet a new study shows that testing for prostate-specific antigen (PSA) and also digital rectal examinations (DRE) are both carried out frequently in older men, even when there is no indication for such testing.
“As a man ages, the risk for a false-positive result increases,” said lead author Chris Gillette, PhD, associate professor of physician assistant studies at Wake Forest University, Winston-Salem, N.C., in a statement
The study authors looked at primary care visits for men who were age 70 or older, and found that, per 100 visits, there were 6.7 PSA tests and 1.6 DRE performed.
Dr. Gillette and colleagues emphasized the importance of their findings. Whereas prior studies have relied on commercially insured men or patient-reported rates of PSA testing, they used a nationally representative clinical dataset that is much more inclusive, as it includes men who are also uninsured or insured through traditional Medicare.
The study was published online in the Journal of the American Board of Family Medicine.
Screening for prostate cancer has been much debated, and the guidelines have changed in recent years. In the period 2012-2018, the U.S. Preventive Services Task Force recommended against PSA-based screening in all men, but then the guidelines changed, and the USPSTF subsequently endorsed individualized screening in those aged 55-69 years after a shared decision-making discussion. That same 2018 update also recommends against PSA screening in men over the age of 70.
In addition, the American Urological Association has recommended against PSA-based prostate cancer screening for men over the age of 70 since 2013.
Previous studies have shown that clinicians are not adhering to the guidelines. An analysis conducted in March 2022 found that about one in four accredited U.S. cancer centers fails to follow national guidelines for PSA testing to screen for prostate cancer. Contrary to national guidelines, which advocate shared decision-making, 22% of centers recommend all men universally initiate PSA screening at either age 50 or 55 and another 4% of centers recommend this before age 50, earlier than the guidelines advise.
In the current study, Dr. Gillette and colleagues conducted a secondary analysis of the National Ambulatory Medical Care Survey datasets from 2013 to 2016 and 2018. The dataset is a nationally representative sample of visits to nonfederal, office-based physician clinics. This analysis was restricted to male patients aged 70 years and older who visited a primary care clinic.
The team found that health care professionals who order a lot of tests are more likely to order low-value screening such as PSA and DRE.
The data also showed that when there were a higher number of services ordered/provided, the patients were significantly more likely to receive a low-value PSA (odds ratio, 1.49) and a low-value DRE (OR, 1.37). In contrast, patients who had more previous visits to the clinician were less likely to receive a low-value DRE (OR, 0.92).
Overall, there a decline in low-value PSA screening after 2014, but this trend was not seen for DRE during primary care visits.
Speculating as to why these low-value tests are being carried out, Dr. Gillette suggested that health care professionals might be responding to patient requests when ordering these screening tests, or they may be using what’s known as a “shotgun” approach to medical testing where all possible tests are ordered during a medical visit.
“However, as health care systems move toward a more value-based care system – where the benefit of services provided outweighs any risks – clinicians need to engage patients in these discussions on the complexity of this testing,” he commented. “Ultimately, when and if to screen is a decision best left between a provider and the patient.”
There was no outside funding and the authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Yet a new study shows that testing for prostate-specific antigen (PSA) and also digital rectal examinations (DRE) are both carried out frequently in older men, even when there is no indication for such testing.
“As a man ages, the risk for a false-positive result increases,” said lead author Chris Gillette, PhD, associate professor of physician assistant studies at Wake Forest University, Winston-Salem, N.C., in a statement
The study authors looked at primary care visits for men who were age 70 or older, and found that, per 100 visits, there were 6.7 PSA tests and 1.6 DRE performed.
Dr. Gillette and colleagues emphasized the importance of their findings. Whereas prior studies have relied on commercially insured men or patient-reported rates of PSA testing, they used a nationally representative clinical dataset that is much more inclusive, as it includes men who are also uninsured or insured through traditional Medicare.
The study was published online in the Journal of the American Board of Family Medicine.
Screening for prostate cancer has been much debated, and the guidelines have changed in recent years. In the period 2012-2018, the U.S. Preventive Services Task Force recommended against PSA-based screening in all men, but then the guidelines changed, and the USPSTF subsequently endorsed individualized screening in those aged 55-69 years after a shared decision-making discussion. That same 2018 update also recommends against PSA screening in men over the age of 70.
In addition, the American Urological Association has recommended against PSA-based prostate cancer screening for men over the age of 70 since 2013.
Previous studies have shown that clinicians are not adhering to the guidelines. An analysis conducted in March 2022 found that about one in four accredited U.S. cancer centers fails to follow national guidelines for PSA testing to screen for prostate cancer. Contrary to national guidelines, which advocate shared decision-making, 22% of centers recommend all men universally initiate PSA screening at either age 50 or 55 and another 4% of centers recommend this before age 50, earlier than the guidelines advise.
In the current study, Dr. Gillette and colleagues conducted a secondary analysis of the National Ambulatory Medical Care Survey datasets from 2013 to 2016 and 2018. The dataset is a nationally representative sample of visits to nonfederal, office-based physician clinics. This analysis was restricted to male patients aged 70 years and older who visited a primary care clinic.
The team found that health care professionals who order a lot of tests are more likely to order low-value screening such as PSA and DRE.
The data also showed that when there were a higher number of services ordered/provided, the patients were significantly more likely to receive a low-value PSA (odds ratio, 1.49) and a low-value DRE (OR, 1.37). In contrast, patients who had more previous visits to the clinician were less likely to receive a low-value DRE (OR, 0.92).
Overall, there a decline in low-value PSA screening after 2014, but this trend was not seen for DRE during primary care visits.
Speculating as to why these low-value tests are being carried out, Dr. Gillette suggested that health care professionals might be responding to patient requests when ordering these screening tests, or they may be using what’s known as a “shotgun” approach to medical testing where all possible tests are ordered during a medical visit.
“However, as health care systems move toward a more value-based care system – where the benefit of services provided outweighs any risks – clinicians need to engage patients in these discussions on the complexity of this testing,” he commented. “Ultimately, when and if to screen is a decision best left between a provider and the patient.”
There was no outside funding and the authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Yet a new study shows that testing for prostate-specific antigen (PSA) and also digital rectal examinations (DRE) are both carried out frequently in older men, even when there is no indication for such testing.
“As a man ages, the risk for a false-positive result increases,” said lead author Chris Gillette, PhD, associate professor of physician assistant studies at Wake Forest University, Winston-Salem, N.C., in a statement
The study authors looked at primary care visits for men who were age 70 or older, and found that, per 100 visits, there were 6.7 PSA tests and 1.6 DRE performed.
Dr. Gillette and colleagues emphasized the importance of their findings. Whereas prior studies have relied on commercially insured men or patient-reported rates of PSA testing, they used a nationally representative clinical dataset that is much more inclusive, as it includes men who are also uninsured or insured through traditional Medicare.
The study was published online in the Journal of the American Board of Family Medicine.
Screening for prostate cancer has been much debated, and the guidelines have changed in recent years. In the period 2012-2018, the U.S. Preventive Services Task Force recommended against PSA-based screening in all men, but then the guidelines changed, and the USPSTF subsequently endorsed individualized screening in those aged 55-69 years after a shared decision-making discussion. That same 2018 update also recommends against PSA screening in men over the age of 70.
In addition, the American Urological Association has recommended against PSA-based prostate cancer screening for men over the age of 70 since 2013.
Previous studies have shown that clinicians are not adhering to the guidelines. An analysis conducted in March 2022 found that about one in four accredited U.S. cancer centers fails to follow national guidelines for PSA testing to screen for prostate cancer. Contrary to national guidelines, which advocate shared decision-making, 22% of centers recommend all men universally initiate PSA screening at either age 50 or 55 and another 4% of centers recommend this before age 50, earlier than the guidelines advise.
In the current study, Dr. Gillette and colleagues conducted a secondary analysis of the National Ambulatory Medical Care Survey datasets from 2013 to 2016 and 2018. The dataset is a nationally representative sample of visits to nonfederal, office-based physician clinics. This analysis was restricted to male patients aged 70 years and older who visited a primary care clinic.
The team found that health care professionals who order a lot of tests are more likely to order low-value screening such as PSA and DRE.
The data also showed that when there were a higher number of services ordered/provided, the patients were significantly more likely to receive a low-value PSA (odds ratio, 1.49) and a low-value DRE (OR, 1.37). In contrast, patients who had more previous visits to the clinician were less likely to receive a low-value DRE (OR, 0.92).
Overall, there a decline in low-value PSA screening after 2014, but this trend was not seen for DRE during primary care visits.
Speculating as to why these low-value tests are being carried out, Dr. Gillette suggested that health care professionals might be responding to patient requests when ordering these screening tests, or they may be using what’s known as a “shotgun” approach to medical testing where all possible tests are ordered during a medical visit.
“However, as health care systems move toward a more value-based care system – where the benefit of services provided outweighs any risks – clinicians need to engage patients in these discussions on the complexity of this testing,” he commented. “Ultimately, when and if to screen is a decision best left between a provider and the patient.”
There was no outside funding and the authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN BOARD OF FAMILY MEDICINE
Improving Bone Health in Patients With Advanced Prostate Cancer With the Use of Algorithm-Based Clinical Practice Tool at Salt Lake City VA
Background
The bone health of patients with locally advanced and metastatic prostate cancer is at risk both from treatment-related loss of bone density and skeletal-related events from metastasis to bones. Evidence-based guidelines recommend the use of denosumab or zoledronic acid at bone metastasis-indicated dosages in the setting of castration-resistant prostate cancer with bone metastases, and at the osteoporosis-indicated dosages in the hormone-sensitive setting in patients with a significant risk of fragility fracture. For the concerns of jaw osteonecrosis, a dental evaluation is recommended before starting bone modifying agents. The literature review suggests that there is a limited evidence-based practice for bone health with prostate cancer in the real world. Both underdosing and overdosing on bone remodeling therapies place additional risk on bone health. An incomplete dental workup before starting bone modifying agents increases the risk of osteonecrosis of the jaw.
Methods
To minimize the deviation from evidencebased guidelines at VA Salt Lake City Health Care, and to provide appropriate bone health care to our patients, we created an algorithm-based clinical practice tool. This order set was incorporated into the electronic medical record system to be used while ordering a bone remodeling agent for prostate cancer. The tool prompts the clinicians to follow the appropriate algorithm in a stepwise manner to ensure a pretreatment dental evaluation and use of the correct dosage of drugs.
Results
We analyzed the data from Sept 2019 to April 2022 following the incorporation of this tool. 0/35 (0%) patients were placed on inappropriate bone modifying agent dosing and dental health was addressed on every patient before initiating treatment. We noted a significant change in the clinician’s practice while prescribing denosumab/zoledronate before and after implementation of this tool (24/41 vs 0/35, P < .00001); and an improvement in pretreatment dental checkups before and after implementation of the tool was noted to be 12/41 vs 0/35 (P < .00001).
Conclusions
We found that incorporating an evidence-based algorithm in the order set while prescribing bone remodeling agents led to a significant improvement in our institutional clinical practice to provide high-quality evidence-based care to our patients with prostate cancer.
Background
The bone health of patients with locally advanced and metastatic prostate cancer is at risk both from treatment-related loss of bone density and skeletal-related events from metastasis to bones. Evidence-based guidelines recommend the use of denosumab or zoledronic acid at bone metastasis-indicated dosages in the setting of castration-resistant prostate cancer with bone metastases, and at the osteoporosis-indicated dosages in the hormone-sensitive setting in patients with a significant risk of fragility fracture. For the concerns of jaw osteonecrosis, a dental evaluation is recommended before starting bone modifying agents. The literature review suggests that there is a limited evidence-based practice for bone health with prostate cancer in the real world. Both underdosing and overdosing on bone remodeling therapies place additional risk on bone health. An incomplete dental workup before starting bone modifying agents increases the risk of osteonecrosis of the jaw.
Methods
To minimize the deviation from evidencebased guidelines at VA Salt Lake City Health Care, and to provide appropriate bone health care to our patients, we created an algorithm-based clinical practice tool. This order set was incorporated into the electronic medical record system to be used while ordering a bone remodeling agent for prostate cancer. The tool prompts the clinicians to follow the appropriate algorithm in a stepwise manner to ensure a pretreatment dental evaluation and use of the correct dosage of drugs.
Results
We analyzed the data from Sept 2019 to April 2022 following the incorporation of this tool. 0/35 (0%) patients were placed on inappropriate bone modifying agent dosing and dental health was addressed on every patient before initiating treatment. We noted a significant change in the clinician’s practice while prescribing denosumab/zoledronate before and after implementation of this tool (24/41 vs 0/35, P < .00001); and an improvement in pretreatment dental checkups before and after implementation of the tool was noted to be 12/41 vs 0/35 (P < .00001).
Conclusions
We found that incorporating an evidence-based algorithm in the order set while prescribing bone remodeling agents led to a significant improvement in our institutional clinical practice to provide high-quality evidence-based care to our patients with prostate cancer.
Background
The bone health of patients with locally advanced and metastatic prostate cancer is at risk both from treatment-related loss of bone density and skeletal-related events from metastasis to bones. Evidence-based guidelines recommend the use of denosumab or zoledronic acid at bone metastasis-indicated dosages in the setting of castration-resistant prostate cancer with bone metastases, and at the osteoporosis-indicated dosages in the hormone-sensitive setting in patients with a significant risk of fragility fracture. For the concerns of jaw osteonecrosis, a dental evaluation is recommended before starting bone modifying agents. The literature review suggests that there is a limited evidence-based practice for bone health with prostate cancer in the real world. Both underdosing and overdosing on bone remodeling therapies place additional risk on bone health. An incomplete dental workup before starting bone modifying agents increases the risk of osteonecrosis of the jaw.
Methods
To minimize the deviation from evidencebased guidelines at VA Salt Lake City Health Care, and to provide appropriate bone health care to our patients, we created an algorithm-based clinical practice tool. This order set was incorporated into the electronic medical record system to be used while ordering a bone remodeling agent for prostate cancer. The tool prompts the clinicians to follow the appropriate algorithm in a stepwise manner to ensure a pretreatment dental evaluation and use of the correct dosage of drugs.
Results
We analyzed the data from Sept 2019 to April 2022 following the incorporation of this tool. 0/35 (0%) patients were placed on inappropriate bone modifying agent dosing and dental health was addressed on every patient before initiating treatment. We noted a significant change in the clinician’s practice while prescribing denosumab/zoledronate before and after implementation of this tool (24/41 vs 0/35, P < .00001); and an improvement in pretreatment dental checkups before and after implementation of the tool was noted to be 12/41 vs 0/35 (P < .00001).
Conclusions
We found that incorporating an evidence-based algorithm in the order set while prescribing bone remodeling agents led to a significant improvement in our institutional clinical practice to provide high-quality evidence-based care to our patients with prostate cancer.
Palliative Care Disparities in Small Cell Carcinoma of the Prostate: An Analysis of the National Cancer Database
Purpose
This study addresses a gap in knowledge regarding palliative care utilization patterns in smallcell carcinoma of the prostate.
Background
Prostate cancer is the most common cancer affecting males. One of the most aggressive malignancies of the prostate is small cell carcinoma (SCC) of the prostate. Almost 70% of patients diagnosed with SCC present with the disseminated disease with a low 5-year survival rate of less than 2%. The role of palliative care can be beneficial in metastatic prostate cancer given its largely incurable course. Despite evidence favoring palliative care for prostate cancer in several patient populations, it remains under-utilized. Palliative care utilization patterns in SCC of the prostate have not yet been studied.
Methods
This is a retrospective study of patients diagnosed with all subtypes of AJCC staged metastatic SCC of the prostate between 2004 and 2017 in the National Cancer Database (NCDB) to determine palliative care usage (n = 615). Exclusion criteria included missing data.
Data Analysis
Variables were evaluated for significance (P < .05) in relation to the receipt of palliative care using Pearson Chi-Square, ANOVA, and Kaplan- Meier tests. Multivariate analysis was performed via binary logistics regression.
Results
Among the 961 patients diagnosed with SCC of the prostate, 64% had metastatic disease (n = 615). The metastatic cohort was more likely to receive palliative care than those that did not have distant metastasis (24.2% vs 5.7%, P < .001). Palliative care use has grown between 2004 (n = 6) and 2017 (n = 20). Patients that were uninsured were more likely than insured patients to receive palliative care (50% vs 23.5%, P = .003; 95% CI, 0.051- 0.546). Non-Hispanic patients were also more likely than Hispanic patients to receive palliative care (P = .033; 95% CI, 1.154-28.140). New England locations had the highest utilization of palliative care (43.%, P = .009). Factors that impacted palliative care use included facility region, insurance status, and Hispanic status. As palliative care continues to be utilized more frequently, we hope that this study can provide a starting point in studying and preventing palliative treatment disparities.
Purpose
This study addresses a gap in knowledge regarding palliative care utilization patterns in smallcell carcinoma of the prostate.
Background
Prostate cancer is the most common cancer affecting males. One of the most aggressive malignancies of the prostate is small cell carcinoma (SCC) of the prostate. Almost 70% of patients diagnosed with SCC present with the disseminated disease with a low 5-year survival rate of less than 2%. The role of palliative care can be beneficial in metastatic prostate cancer given its largely incurable course. Despite evidence favoring palliative care for prostate cancer in several patient populations, it remains under-utilized. Palliative care utilization patterns in SCC of the prostate have not yet been studied.
Methods
This is a retrospective study of patients diagnosed with all subtypes of AJCC staged metastatic SCC of the prostate between 2004 and 2017 in the National Cancer Database (NCDB) to determine palliative care usage (n = 615). Exclusion criteria included missing data.
Data Analysis
Variables were evaluated for significance (P < .05) in relation to the receipt of palliative care using Pearson Chi-Square, ANOVA, and Kaplan- Meier tests. Multivariate analysis was performed via binary logistics regression.
Results
Among the 961 patients diagnosed with SCC of the prostate, 64% had metastatic disease (n = 615). The metastatic cohort was more likely to receive palliative care than those that did not have distant metastasis (24.2% vs 5.7%, P < .001). Palliative care use has grown between 2004 (n = 6) and 2017 (n = 20). Patients that were uninsured were more likely than insured patients to receive palliative care (50% vs 23.5%, P = .003; 95% CI, 0.051- 0.546). Non-Hispanic patients were also more likely than Hispanic patients to receive palliative care (P = .033; 95% CI, 1.154-28.140). New England locations had the highest utilization of palliative care (43.%, P = .009). Factors that impacted palliative care use included facility region, insurance status, and Hispanic status. As palliative care continues to be utilized more frequently, we hope that this study can provide a starting point in studying and preventing palliative treatment disparities.
Purpose
This study addresses a gap in knowledge regarding palliative care utilization patterns in smallcell carcinoma of the prostate.
Background
Prostate cancer is the most common cancer affecting males. One of the most aggressive malignancies of the prostate is small cell carcinoma (SCC) of the prostate. Almost 70% of patients diagnosed with SCC present with the disseminated disease with a low 5-year survival rate of less than 2%. The role of palliative care can be beneficial in metastatic prostate cancer given its largely incurable course. Despite evidence favoring palliative care for prostate cancer in several patient populations, it remains under-utilized. Palliative care utilization patterns in SCC of the prostate have not yet been studied.
Methods
This is a retrospective study of patients diagnosed with all subtypes of AJCC staged metastatic SCC of the prostate between 2004 and 2017 in the National Cancer Database (NCDB) to determine palliative care usage (n = 615). Exclusion criteria included missing data.
Data Analysis
Variables were evaluated for significance (P < .05) in relation to the receipt of palliative care using Pearson Chi-Square, ANOVA, and Kaplan- Meier tests. Multivariate analysis was performed via binary logistics regression.
Results
Among the 961 patients diagnosed with SCC of the prostate, 64% had metastatic disease (n = 615). The metastatic cohort was more likely to receive palliative care than those that did not have distant metastasis (24.2% vs 5.7%, P < .001). Palliative care use has grown between 2004 (n = 6) and 2017 (n = 20). Patients that were uninsured were more likely than insured patients to receive palliative care (50% vs 23.5%, P = .003; 95% CI, 0.051- 0.546). Non-Hispanic patients were also more likely than Hispanic patients to receive palliative care (P = .033; 95% CI, 1.154-28.140). New England locations had the highest utilization of palliative care (43.%, P = .009). Factors that impacted palliative care use included facility region, insurance status, and Hispanic status. As palliative care continues to be utilized more frequently, we hope that this study can provide a starting point in studying and preventing palliative treatment disparities.
Utilization and Clinical Benefit of Immune Checkpoint Inhibitor in Veterans With Microsatellite Instability-High Prostate Cancer
Background
The utilization of immune checkpoint inhibitors (ICI) in prostate cancer (PC) can be very effective for patients with mismatch repair-deficiency (as identified by MSI-H by PCR/NGS or dMMR IHC). The use of ICI in this patient population has been associated with high rates of durable response. There is limited published data on factors that may influence patient response and outcomes. The aim of this study is to describe the utilization of and tumor response to ICI in this patient population.
Methods
This is a retrospective study of men with MSI-H PC reported by somatic genomic testing from April 1, 2015 to March 31, 2022 through the VA National Precision Oncology Program (NPOP), who received at least one dose of ICI. The primary objectives are to describe the incidence of MSI-H PC and the utilization of ICI. Descriptive statistics and Kaplan- Meier estimator were used for secondary objectives to determine the prostate-specific antigen decline of at least 50% (PSA50), clinical progression free survival (cPFS), time on ICI as a function of number of prior therapies, the extent of metastasis prior to initiation of ICI, and the correlation of MMR genetic alterations with treatment response.
Results
66 patients with MSI-H PC were identified (1.5% of a total of 4267 patients with PC tested through NPOP). 23 patients (35%) received at least one dose of ICI. 12 of 23 patients (52%) had PSA response. PSA50 responses occurred in 6 patients (50%) and 5 continued to have durable PSA50 at six months. Median cPFS was 280 days (95% CI: 105 days-not reached) and the estimated PFS at six months was 72.2% (95% CI: 35.7%-90.2%). 8 of 12 (67%) responders have received multiple lines of therapy for M1 PC. 8 of 12 patients (67%) had high-volume disease at ICI initiation. Of those patients with a MMR genetic alteration, patients with MLH1 (3/3) and MSH2 (6/8) alterations responded more frequently than those with MSH6 alterations (1/4).
Conclusions
MSI-H PC is rare but response rates to ICI are high and durable. Patients with MLH1 and MSH2 alterations appeared to respond more frequently than those with MSH6. Additional follow-up is ongoing.
Background
The utilization of immune checkpoint inhibitors (ICI) in prostate cancer (PC) can be very effective for patients with mismatch repair-deficiency (as identified by MSI-H by PCR/NGS or dMMR IHC). The use of ICI in this patient population has been associated with high rates of durable response. There is limited published data on factors that may influence patient response and outcomes. The aim of this study is to describe the utilization of and tumor response to ICI in this patient population.
Methods
This is a retrospective study of men with MSI-H PC reported by somatic genomic testing from April 1, 2015 to March 31, 2022 through the VA National Precision Oncology Program (NPOP), who received at least one dose of ICI. The primary objectives are to describe the incidence of MSI-H PC and the utilization of ICI. Descriptive statistics and Kaplan- Meier estimator were used for secondary objectives to determine the prostate-specific antigen decline of at least 50% (PSA50), clinical progression free survival (cPFS), time on ICI as a function of number of prior therapies, the extent of metastasis prior to initiation of ICI, and the correlation of MMR genetic alterations with treatment response.
Results
66 patients with MSI-H PC were identified (1.5% of a total of 4267 patients with PC tested through NPOP). 23 patients (35%) received at least one dose of ICI. 12 of 23 patients (52%) had PSA response. PSA50 responses occurred in 6 patients (50%) and 5 continued to have durable PSA50 at six months. Median cPFS was 280 days (95% CI: 105 days-not reached) and the estimated PFS at six months was 72.2% (95% CI: 35.7%-90.2%). 8 of 12 (67%) responders have received multiple lines of therapy for M1 PC. 8 of 12 patients (67%) had high-volume disease at ICI initiation. Of those patients with a MMR genetic alteration, patients with MLH1 (3/3) and MSH2 (6/8) alterations responded more frequently than those with MSH6 alterations (1/4).
Conclusions
MSI-H PC is rare but response rates to ICI are high and durable. Patients with MLH1 and MSH2 alterations appeared to respond more frequently than those with MSH6. Additional follow-up is ongoing.
Background
The utilization of immune checkpoint inhibitors (ICI) in prostate cancer (PC) can be very effective for patients with mismatch repair-deficiency (as identified by MSI-H by PCR/NGS or dMMR IHC). The use of ICI in this patient population has been associated with high rates of durable response. There is limited published data on factors that may influence patient response and outcomes. The aim of this study is to describe the utilization of and tumor response to ICI in this patient population.
Methods
This is a retrospective study of men with MSI-H PC reported by somatic genomic testing from April 1, 2015 to March 31, 2022 through the VA National Precision Oncology Program (NPOP), who received at least one dose of ICI. The primary objectives are to describe the incidence of MSI-H PC and the utilization of ICI. Descriptive statistics and Kaplan- Meier estimator were used for secondary objectives to determine the prostate-specific antigen decline of at least 50% (PSA50), clinical progression free survival (cPFS), time on ICI as a function of number of prior therapies, the extent of metastasis prior to initiation of ICI, and the correlation of MMR genetic alterations with treatment response.
Results
66 patients with MSI-H PC were identified (1.5% of a total of 4267 patients with PC tested through NPOP). 23 patients (35%) received at least one dose of ICI. 12 of 23 patients (52%) had PSA response. PSA50 responses occurred in 6 patients (50%) and 5 continued to have durable PSA50 at six months. Median cPFS was 280 days (95% CI: 105 days-not reached) and the estimated PFS at six months was 72.2% (95% CI: 35.7%-90.2%). 8 of 12 (67%) responders have received multiple lines of therapy for M1 PC. 8 of 12 patients (67%) had high-volume disease at ICI initiation. Of those patients with a MMR genetic alteration, patients with MLH1 (3/3) and MSH2 (6/8) alterations responded more frequently than those with MSH6 alterations (1/4).
Conclusions
MSI-H PC is rare but response rates to ICI are high and durable. Patients with MLH1 and MSH2 alterations appeared to respond more frequently than those with MSH6. Additional follow-up is ongoing.
Impact of Race on Outcomes of High-Risk Patients With Prostate Cancer Treated With Moderately Hypofractionated Radiotherapy in an Equal Access Setting
Although moderately hypofractionated radiotherapy (MHRT) is an accepted treatment for localized prostate cancer, its adaptation remains limited in the United States.1,2 MHRT theoretically exploits α/β ratio differences between the prostate (1.5 Gy), bladder (5-10 Gy), and rectum (3 Gy), thereby reducing late treatment-related adverse effects compared with those of conventional fractionation at biologically equivalent doses.3-8 Multiple randomized noninferiority trials have demonstrated equivalent outcomes between MHRT and conventional fraction with no appreciable increase in patient-reported toxicity.9-14 Although these studies have led to the acceptance of MHRT as a standard treatment, the majority of these trials involve individuals with low- and intermediate-risk disease.
There are less phase 3 data addressing MHRT for high-risk prostate cancer (HRPC).10,12,14-17 Only 2 studies examined predominately high-risk populations, accounting for 83 and 292 patients, respectively.15,16 Additional phase 3 trials with small proportions of high-risk patients (n = 126, 12%; n = 53, 35%) offer limited additional information regarding clinical outcomes and toxicity rates specific to high-risk disease.10-12 Numerous phase 1 and 2 studies report various field designs and fractionation plans for MHRT in the context of high-risk disease, although the applicability of these data to off-trial populations remains limited.18-20
Furthermore, African American individuals are underrepresented in the trials establishing the role of MHRT despite higher rates of prostate cancer incidence, more advanced disease stage at diagnosis, and higher rates of prostate cancer–specific survival (PCSS) when compared with White patients.21 Racial disparities across patients with prostate cancer and their management are multifactorial across health care literacy, education level, access to care (including transportation issues), and issues of adherence and distrust.22-25 Correlation of patient race to prostate cancer outcomes varies greatly across health care systems, with the US Department of Veterans Affairs (VA) equal access system providing robust mental health services and transportation services for some patients, while demonstrating similar rates of stage-adjusted PCSS between African American and White patients across a broad range of treatment modalities.26-28 Given the paucity of data exploring outcomes following MHRT for African American patients with HRPC, the present analysis provides long-term clinical outcomes and toxicity profiles for an off-trial majority African American population with HRPC treated with MHRT within the VA.
Methods
Records were retrospectively reviewed under an institutional review board–approved protocol for all patients with HRPC treated with definitive MHRT at the Durham Veterans Affairs Healthcare System in North Carolina between November 2008 and August 2018. Exclusion criteria included < 12 months of follow-up or elective nodal irradiation. Demographic variables obtained included age at diagnosis, race, clinical T stage, pre-MHRT prostate-specific antigen (PSA), Gleason grade group at diagnosis, favorable vs unfavorable high-risk disease, pre-MHRT international prostate symptom score (IPSS), and pre-MHRT urinary medication usage (yes/no).29
Concurrent androgen deprivation therapy (ADT) was initiated 6 to 8 weeks before MHRT unless medically contraindicated per the discretion of the treating radiation oncologist. Patients generally received 18 to 24 months of ADT, with those with favorable HRPC (ie, T1c disease with either Gleason 4+4 and PSA < 10 mg/mL or Gleason 3+3 and PSA > 20 ng/mL) receiving 6 months after 2015.29 Patients were simulated supine in either standard or custom immobilization with a full bladder and empty rectum. MHRT fractionation plans included 70 Gy at 2.5 Gy per fraction and 60 Gy at 3 Gy per fraction. Radiotherapy targets included the prostate and seminal vesicles without elective nodal coverage per institutional practice. Treatments were delivered following image guidance, either prostate matching with cone beam computed tomography or fiducial matching with kilo voltage imaging. All patients received intensity-modulated radiotherapy. For plans delivering 70 Gy at 2.5 Gy per fraction, constraints included bladder V (volume receiving) 70 < 10 cc, V65 ≤ 15%, V40 ≤ 35%, rectum V70 < 10 cc, V65 ≤ 10%, V40 ≤ 35%, femoral heads maximum point dose ≤ 40 Gy, penile bulb mean dose ≤ 50 Gy, and small bowel V40 ≤ 1%. For plans delivering 60 Gy at 3 Gy per fraction, constraints included rectum V57 ≤ 15%, V46 ≤ 30%, V37 ≤ 50%, bladder V60 ≤ 5%, V46 ≤ 30%, V37 ≤ 50%, and femoral heads V43 ≤ 5%.
Gastrointestinal (GI) and genitourinary (GU) toxicities were graded using Common Terminology Criteria for Adverse Events (CTCAE), version 5.0, with acute toxicity defined as on-treatment < 3 months following completion of MHRT. Late toxicity was defined as ≥ 3 months following completion of MHRT. Individuals were seen in follow-up at 6 weeks and 3 months with PSA and testosterone after MHRT completion, then every 6 to 12 months for 5 years and annually thereafter. Each follow-up visit included history, physical examination, IPSS, and CTCAE grading for GI and GU toxicity.
The Wilcoxon rank sum test and χ2 test were used to compare differences in demographic data, dosimetric parameters, and frequency of toxicity events with respect to patient race. Clinical endpoints including biochemical recurrence-free survival (BRFS; defined by Phoenix criteria as 2.0 above PSA nadir), distant metastases-free survival (DMFS), PCSS, and overall survival (OS) were estimated from time of radiotherapy completion by the Kaplan-Meier method and compared between African American and White race by log-rank testing.30 Late GI and GU toxicity-free survival were estimated by Kaplan-Meier plots and compared between African American and White patients by the log-rank test. Statistical analysis was performed using SAS 9.4.
Results
We identified 143 patients with HRPC treated with definitive MHRT between November 2008 and August 2018 (Table 1). Mean age was 65 years (range, 36-80 years); 57% were African American men. Eighty percent of individuals had unfavorable high-risk disease. Median (IQR) PSA was 14.4 (7.8-28.6). Twenty-six percent had grade group 1-3 disease, 47% had grade group 4 disease, and 27% had grade group 5 disease. African American patients had significantly lower pre-MHRT IPSS scores than White patients (mean IPSS, 11 vs 14, respectively; P = .02) despite similar rates of preradiotherapy urinary medication usage (66% and 66%, respectively).
Eighty-six percent received 70 Gy over 28 fractions, with institutional protocol shifting to 60 Gy over 20 fractions (14%) in June 2017. The median (IQR) duration of radiotherapy was 39 (38-42) days, with 97% of individuals undergoing ADT for a median (IQR) duration of 24 (24-36) months. The median follow-up time was 38 months, with 57 (40%) patients followed for at least 60 months.
Grade 3 GI and GU acute toxicity events were observed in 1% and 4% of all individuals, respectively (Table 2). No acute GI or GU grade 4+ events were observed. No significant differences in acute GU or GI toxicity were observed between African American and White patients.
No significant differences between African American and White patients were observed for late grade 2+ GI (P = .19) or GU (P = .55) toxicity. Late grade 2+ GI toxicity was observed in 17 (12%) patients overall (Figure 1A). One grade 3 and 1 grade 4 late GI event were observed following MHRT completion: The latter involved hospitalization for bleeding secondary to radiation proctitis in the context of cirrhosis predating MHRT. Late grade 2+ GU toxicity was observed in 80 (56%) patients, with late grade 2 events steadily increasing over time (Figure 1B). Nine late grade 3 GU toxicity events were observed at a median of 13 months following completion of MHRT, 2 of which occurred more than 24 months after MHRT completion. No late grade 4 or 5 GU events were observed. IPSS values both before MHRT and at time of last follow-up were available for 65 (40%) patients, with a median (IQR) IPSS of 10 (6-16) before MHRT and 12 (8-16) at last follow-up at a median (IQR) interval of 36 months (26-76) from radiation completion.
No significant differences were observed between African American and White patients with respect to BRFS, DMFS, PCSS, or OS (Figure 2). Overall, 21 of 143 (15%) patients experienced biochemical recurrence: 5-year BRFS was 77% (95% CI, 67%-85%) for all patients, 83% (95% CI, 70%-91%) for African American patients, and 71% (95% CI, 53%-82%) for White patients. Five-year DMFS was 87% (95% CI, 77%-92%) for all individuals, 91% (95% CI, 80%-96%) for African American patients, and 81% (95% CI, 62%-91%) for White patients. Five-year PCSS was 89% (95% CI, 80%-94%) for all patients, with 5-year PCSS rates of 90% (95% CI, 79%-95%) for African American patients and 87% (95% CI, 70%-95%) for White patients. Five-year OS was 75% overall (95% CI, 64%-82%), with 5-year OS rates of 73% (95% CI, 58%-83%) for African American patients and 77% (95% CI, 60%-87%) for White patients.
Discussion
In this study, we reported acute and late GI and GU toxicity rates as well as clinical outcomes for a majority African American population with predominately unfavorable HRPC treated with MHRT in an equal access health care environment. We found that MHRT was well tolerated with high rates of biochemical control, PCSS, and OS. Additionally, outcomes were not significantly different across patient race. To our knowledge, this is the first report of MHRT for HRPC in a majority African American population.
We found that MHRT was an effective treatment for patients with HRPC, in particular those with unfavorable high-risk disease. While prior prospective and randomized studies have investigated the use of MHRT, our series was larger than most and had a predominately unfavorable high-risk population.12,15-17 Our biochemical and PCSS rates compare favorably with those of HRPC trial populations, particularly given the high proportion of unfavorable high-risk disease.12,15,16 Despite similar rates of biochemical control, OS was lower in the present cohort than in HRPC trial populations, even with a younger median age at diagnosis. The similarly high rates of non–HRPC-related death across race may reflect differences in baseline comorbidities compared with trial populations as well as reported differences between individuals in the VA and the private sector.31 This suggests that MHRT can be an effective treatment for patients with unfavorable HRPC.
We did not find any differences in outcomes between African American and White individuals with HRPC treated with MHRT. Furthermore, our study demonstrates long-term rates of BRFS and PCSS in a majority African American population with predominately unfavorable HRPC that are comparable with those of prior randomized MHRT studies in high-risk, predominately White populations.12,15,16 Prior reports have found that African American men with HRPC may be at increased risk for inferior clinical outcomes due to a number of socioeconomic, biologic, and cultural mediators.26,27,32 Such individuals may disproportionally benefit from shorter treatment courses that improve access to radiotherapy, a well-documented disparity for African American men with localized prostate cancer.33-36 The VA is an ideal system for studying racial disparities within prostate cancer, as accessibility of mental health and transportation services, income, and insurance status are not barriers to preventative or acute care.37 Our results are concordant with those previously seen for African American patients with prostate cancer seen in the VA, which similarly demonstrate equal outcomes with those of other races.28,36 Incorporation of the earlier mentioned VA services into oncologic care across other health care systems could better characterize determinants of racial disparities in prostate cancer, including the prognostic significance of shortening treatment duration and number of patient visits via MHRT.
Despite widespread acceptance in prostate cancer radiotherapy guidelines, routine use of MHRT seems limited across all stages of localized prostate cancer.1,2 Late toxicity is a frequently noted concern regarding MHRT use. Higher rates of late grade 2+ GI toxicity were observed in the hypofractionation arm of the HYPRO trial.17 While RTOG 0415 did not include patients with HRPC, significantly higher rates of physician-reported (but not patient-reported) late grade 2+ GI and GU toxicity were observed using the same MHRT fractionation regimen used for the majority of individuals in our cohort.9 In our study, the steady increase in late grade 2 GU toxicity is consistent with what is seen following conventionally fractionated radiotherapy and is likely multifactorial.38 The mean IPSS difference of 2/35 from pre-MHRT baseline to the time of last follow-up suggests minimal quality of life decline. The relatively stable IPSSs over time alongside the > 50% prevalence of late grade 2 GU toxicity per CTCAE grading seems consistent with the discrepancy noted in RTOG 0415 between increased physician-reported late toxicity and favorable patient-reported quality of life scores.9 Moreover, significant variance exists in toxicity grading across scoring systems, revised editions of CTCAE, and physician-specific toxicity classification, particularly with regard to the use of adrenergic receptor blocker medications. In light of these factors, the high rate of late grade 2 GU toxicity in our study should be interpreted in the context of largely stable post-MHRT IPSSs and favorable rates of late GI grade 2+ and late GU grade 3+ toxicity.
Limitations
This study has several inherent limitations. While the size of the current HRPC cohort is notably larger than similar populations within the majority of phase 3 MHRT trials, these data derive from a single VA hospital. It is unclear whether these outcomes would be representative in a similar high-risk population receiving care outside of the VA equal access system. Follow-up data beyond 5 years was available for less than half of patients, partially due to nonprostate cancer–related mortality at a higher rate than observed in HRPC trial populations.12,15,16 Furthermore, all GI toxicity events were exclusively physician reported, and GU toxicity reporting was limited in the off-trial setting with not all patients routinely completing IPSS questionnaires following MHRT completion. However, all patients were treated similarly, and radiation quality was verified over the treatment period with mandated accreditation, frequent standardized output checks, and systematic treatment review.39
Conclusions
Patients with HRPC treated with MHRT in an equal access, off-trial setting demonstrated favorable rates of biochemical control with acceptable rates of acute and late GI and GU toxicities. Clinical outcomes, including biochemical control, were not significantly different between African American and White patients, which may reflect equal access to care within the VA irrespective of income and insurance status. Incorporating VA services, such as access to primary care, mental health services, and transportation across other health care systems may aid in characterizing and mitigating racial and gender disparities in oncologic care.
Acknowledgments
Portions of this work were presented at the November 2020 ASTRO conference. 40
1. Stokes WA, Kavanagh BD, Raben D, Pugh TJ. Implementation of hypofractionated prostate radiation therapy in the United States: a National Cancer Database analysis. Pract Radiat Oncol. 2017;7:270-278. doi:10.1016/j.prro.2017.03.011
2. Jaworski L, Dominello MM, Heimburger DK, et al. Contemporary practice patterns for intact and post-operative prostate cancer: results from a statewide collaborative. Int J Radiat Oncol Biol Phys. 2019;105(1):E282. doi:10.1016/j.ijrobp.2019.06.1915
3. Miralbell R, Roberts SA, Zubizarreta E, Hendry JH. Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5,969 patients in seven international institutional datasets: α/β = 1.4 (0.9-2.2) Gy. Int J Radiat Oncol Biol Phys. 2012;82(1):e17-e24. doi:10.1016/j.ijrobp.2010.10.075
4. Tree AC, Khoo VS, van As NJ, Partridge M. Is biochemical relapse-free survival after profoundly hypofractionated radiotherapy consistent with current radiobiological models? Clin Oncol (R Coll Radiol). 2014;26(4):216-229. doi:10.1016/j.clon.2014.01.008
5. Brenner DJ. Fractionation and late rectal toxicity. Int J Radiat Oncol Biol Phys. 2004;60(4):1013-1015. doi:10.1016/j.ijrobp.2004.04.014
6. Tucker SL, Thames HD, Michalski JM, et al. Estimation of α/β for late rectal toxicity based on RTOG 94-06. Int J Radiat Oncol Biol Phys. 2011;81(2):600-605. doi:10.1016/j.ijrobp.2010.11.080
7. Dasu A, Toma-Dasu I. Prostate alpha/beta revisited—an analysis of clinical results from 14 168 patients. Acta Oncol. 2012;51(8):963-974. doi:10.3109/0284186X.2012.719635 start
8. Proust-Lima C, Taylor JMG, Sécher S, et al. Confirmation of a Low α/β ratio for prostate cancer treated by external beam radiation therapy alone using a post-treatment repeated-measures model for PSA dynamics. Int J Radiat Oncol Biol Phys. 2011;79(1):195-201. doi:10.1016/j.ijrobp.2009.10.008
9. Lee WR, Dignam JJ, Amin MB, et al. Randomized phase III noninferiority study comparing two radiotherapy fractionation schedules in patients with low-risk prostate cancer. J Clin Oncol. 2016;34(20): 2325-2332. doi:10.1200/JCO.2016.67.0448
10. Dearnaley D, Syndikus I, Mossop H, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17(8):1047-1060. doi:10.1016/S1470-2045(16)30102-4
11. Catton CN, Lukka H, Gu C-S, et al. Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J Clin Oncol. 2017;35(17):1884-1890. doi:10.1200/JCO.2016.71.7397
12. Pollack A, Walker G, Horwitz EM, et al. Randomized trial of hypofractionated external-beam radiotherapy for prostate cancer. J Clin Oncol. 2013;31(31):3860-3868. doi:10.1200/JCO.2013.51.1972
13. Hoffman KE, Voong KR, Levy LB, et al. Randomized trial of hypofractionated, dose-escalated, intensity-modulated radiation therapy (IMRT) versus conventionally fractionated IMRT for localized prostate cancer. J Clin Oncol. 2018;36(29):2943-2949. doi:10.1200/JCO.2018.77.9868
14. Wilkins A, Mossop H, Syndikus I, et al. Hypofractionated radiotherapy versus conventionally fractionated radiotherapy for patients with intermediate-risk localised prostate cancer: 2-year patient-reported outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2015;16(16):1605-1616. doi:10.1016/S1470-2045(15)00280-6
15. Incrocci L, Wortel RC, Alemayehu WG, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with localised prostate cancer (HYPRO): final efficacy results from a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2016;17(8):1061-1069. doi.10.1016/S1470-2045(16)30070-5
16. Arcangeli G, Saracino B, Arcangeli S, et al. Moderate hypofractionation in high-risk, organ-confined prostate cancer: final results of a phase III randomized trial. J Clin Oncol. 2017;35(17):1891-1897. doi:10.1200/JCO.2016.70.4189
17. Aluwini S, Pos F, Schimmel E, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): late toxicity results from a randomised, non-inferiority, phase 3 trial. Lancet Oncol. 2016;17(4):464-474. doi:10.1016/S1470-2045(15)00567-7
18. Pervez N, Small C, MacKenzie M, et al. Acute toxicity in high-risk prostate cancer patients treated with androgen suppression and hypofractionated intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;76(1):57-64. doi:10.1016/j.ijrobp.2009.01.048
19. Magli A, Moretti E, Tullio A, Giannarini G. Hypofractionated simultaneous integrated boost (IMRT- cancer: results of a prospective phase II trial SIB) with pelvic nodal irradiation and concurrent androgen deprivation therapy for high-risk prostate cancer: results of a prospective phase II trial. Prostate Cancer Prostatic Dis. 2018;21(2):269-276. doi:10.1038/s41391-018-0034-0
20. Di Muzio NG, Fodor A, Noris Chiorda B, et al. Moderate hypofractionation with simultaneous integrated boost in prostate cancer: long-term results of a phase I–II study. Clin Oncol (R Coll Radiol). 2016;28(8):490-500. doi:10.1016/j.clon.2016.02.005
21. DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin. 2019;69(3):21-233. doi:10.3322/caac.21555
22. Wolf MS, Knight SJ, Lyons EA, et al. Literacy, race, and PSA level among low-income men newly diagnosed with prostate cancer. Urology. 2006(1);68:89-93. doi:10.1016/j.urology.2006.01.064
23. Rebbeck TR. Prostate cancer disparities by race and ethnicity: from nucleotide to neighborhood. Cold Spring Harb Perspect Med. 2018;8(9):a030387. doi:10.1101/cshperspect.a030387
24. Guidry JJ, Aday LA, Zhang D, Winn RJ. Transportation as a barrier to cancer treatment. Cancer Pract. 1997;5(6):361-366.
25. Friedman DB, Corwin SJ, Dominick GM, Rose ID. African American men’s understanding and perceptions about prostate cancer: why multiple dimensions of health literacy are important in cancer communication. J Community Health. 2009;34(5):449-460. doi:10.1007/s10900-009-9167-3
26. Connell PP, Ignacio L, Haraf D, et al. Equivalent racial outcome after conformal radiotherapy for prostate cancer: a single departmental experience. J Clin Oncol. 2001;19(1):54-61. doi:10.1200/JCO.2001.19.1.54
27. Dess RT, Hartman HE, Mahal BA, et al. Association of black race with prostate cancer-specific and other-cause mortality. JAMA Oncol. 2019;5(1):975-983. doi:10.1200/JCO.2001.19.1.54
28. McKay RR, Sarkar RR, Kumar A, et al. Outcomes of Black men with prostate cancer treated with radiation therapy in the Veterans Health Administration. Cancer. 2021;127(3):403-411. doi:10.1002/cncr.33224

29. Muralidhar V, Chen M-H, Reznor G, et al. Definition and validation of “favorable high-risk prostate cancer”: implications for personalizing treatment of radiation-managed patients. Int J Radiat Oncol Biol Phys. 2015;93(4):828-835. doi:10.1016/j.ijrobp.2015.07.2281
30. Roach M 3rd, Hanks G, Thames H Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65(4):965-974. doi:10.1016/j.ijrobp.2006.04.029
31. Freeman VL, Durazo-Arvizu R, Arozullah AM, Keys LC. Determinants of mortality following a diagnosis of prostate cancer in Veterans Affairs and private sector health care systems. Am J Public Health. 2003;93(100):1706-1712. doi:10.2105/ajph.93.10.1706
32. Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54(2):78-93. doi:10.3322/canjclin.54.2.78
33. Zemplenyi AT, Kaló Z, Kovacs G, et al. Cost-effectiveness analysis of intensity-modulated radiation therapy with normal and hypofractionated schemes for the treatment of localised prostate cancer. Eur J Cancer Care. 2018;27(1):e12430. doi:10.1111/ecc.12430
34. Klabunde CN, Potosky AL, Harlan LC, Kramer BS. Trends and black/white differences in treatment for nonmetastatic prostate cancer. Med Care. 1998;36(9):1337-1348. doi:10.1097/00005650-199809000-00006
35. Harlan L, Brawley O, Pommerenke F, Wali P, Kramer B. Geographic, age, and racial variation in the treatment of local/regional carcinoma of the prostate. J Clin Oncol. 1995;13(1):93-100. doi:10.1200/JCO.1995.13.1.93
36. Riviere P, Luterstein E, Kumar A, et al. Racial equity among African-American and non-Hispanic white men diagnosed with prostate cancer in the veterans affairs healthcare system. Int J Radiat Oncol Biol Phys. 2019;105:E305.
37. Peterson K, Anderson J, Boundy E, Ferguson L, McCleery E, Waldrip K. Mortality disparities in racial/ethnic minority groups in the Veterans Health Administration: an evidence review and map. Am J Public Health. 2018;108(3):e1-e11. doi:10.2105/AJPH.2017.304246
38. Zietman AL, DeSilvio ML, Slater JD, et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA. 2005;294(10):1233-1239. doi:10.1001/jama.294.10.1233
39. Hagan M, Kapoor R, Michalski J, et al. VA-Radiation Oncology Quality Surveillance program. Int J Radiat Oncol Biol Phys. 2020;106(3):639-647. doi.10.1016/j.ijrobp.2019.08.064
40. Carpenter DJ, Natesan D, Floyd W, et al. Long-term experience in an equal access health care system using moderately hypofractionated radiotherapy for high risk prostate cancer in a predominately African American population with unfavorable disease. Int J Radiat Oncol Biol Phys. 2020;108(3):E417. https://www.redjournal.org/article/S0360-3016(20)33923-7/fulltext
Although moderately hypofractionated radiotherapy (MHRT) is an accepted treatment for localized prostate cancer, its adaptation remains limited in the United States.1,2 MHRT theoretically exploits α/β ratio differences between the prostate (1.5 Gy), bladder (5-10 Gy), and rectum (3 Gy), thereby reducing late treatment-related adverse effects compared with those of conventional fractionation at biologically equivalent doses.3-8 Multiple randomized noninferiority trials have demonstrated equivalent outcomes between MHRT and conventional fraction with no appreciable increase in patient-reported toxicity.9-14 Although these studies have led to the acceptance of MHRT as a standard treatment, the majority of these trials involve individuals with low- and intermediate-risk disease.
There are less phase 3 data addressing MHRT for high-risk prostate cancer (HRPC).10,12,14-17 Only 2 studies examined predominately high-risk populations, accounting for 83 and 292 patients, respectively.15,16 Additional phase 3 trials with small proportions of high-risk patients (n = 126, 12%; n = 53, 35%) offer limited additional information regarding clinical outcomes and toxicity rates specific to high-risk disease.10-12 Numerous phase 1 and 2 studies report various field designs and fractionation plans for MHRT in the context of high-risk disease, although the applicability of these data to off-trial populations remains limited.18-20
Furthermore, African American individuals are underrepresented in the trials establishing the role of MHRT despite higher rates of prostate cancer incidence, more advanced disease stage at diagnosis, and higher rates of prostate cancer–specific survival (PCSS) when compared with White patients.21 Racial disparities across patients with prostate cancer and their management are multifactorial across health care literacy, education level, access to care (including transportation issues), and issues of adherence and distrust.22-25 Correlation of patient race to prostate cancer outcomes varies greatly across health care systems, with the US Department of Veterans Affairs (VA) equal access system providing robust mental health services and transportation services for some patients, while demonstrating similar rates of stage-adjusted PCSS between African American and White patients across a broad range of treatment modalities.26-28 Given the paucity of data exploring outcomes following MHRT for African American patients with HRPC, the present analysis provides long-term clinical outcomes and toxicity profiles for an off-trial majority African American population with HRPC treated with MHRT within the VA.
Methods
Records were retrospectively reviewed under an institutional review board–approved protocol for all patients with HRPC treated with definitive MHRT at the Durham Veterans Affairs Healthcare System in North Carolina between November 2008 and August 2018. Exclusion criteria included < 12 months of follow-up or elective nodal irradiation. Demographic variables obtained included age at diagnosis, race, clinical T stage, pre-MHRT prostate-specific antigen (PSA), Gleason grade group at diagnosis, favorable vs unfavorable high-risk disease, pre-MHRT international prostate symptom score (IPSS), and pre-MHRT urinary medication usage (yes/no).29
Concurrent androgen deprivation therapy (ADT) was initiated 6 to 8 weeks before MHRT unless medically contraindicated per the discretion of the treating radiation oncologist. Patients generally received 18 to 24 months of ADT, with those with favorable HRPC (ie, T1c disease with either Gleason 4+4 and PSA < 10 mg/mL or Gleason 3+3 and PSA > 20 ng/mL) receiving 6 months after 2015.29 Patients were simulated supine in either standard or custom immobilization with a full bladder and empty rectum. MHRT fractionation plans included 70 Gy at 2.5 Gy per fraction and 60 Gy at 3 Gy per fraction. Radiotherapy targets included the prostate and seminal vesicles without elective nodal coverage per institutional practice. Treatments were delivered following image guidance, either prostate matching with cone beam computed tomography or fiducial matching with kilo voltage imaging. All patients received intensity-modulated radiotherapy. For plans delivering 70 Gy at 2.5 Gy per fraction, constraints included bladder V (volume receiving) 70 < 10 cc, V65 ≤ 15%, V40 ≤ 35%, rectum V70 < 10 cc, V65 ≤ 10%, V40 ≤ 35%, femoral heads maximum point dose ≤ 40 Gy, penile bulb mean dose ≤ 50 Gy, and small bowel V40 ≤ 1%. For plans delivering 60 Gy at 3 Gy per fraction, constraints included rectum V57 ≤ 15%, V46 ≤ 30%, V37 ≤ 50%, bladder V60 ≤ 5%, V46 ≤ 30%, V37 ≤ 50%, and femoral heads V43 ≤ 5%.
Gastrointestinal (GI) and genitourinary (GU) toxicities were graded using Common Terminology Criteria for Adverse Events (CTCAE), version 5.0, with acute toxicity defined as on-treatment < 3 months following completion of MHRT. Late toxicity was defined as ≥ 3 months following completion of MHRT. Individuals were seen in follow-up at 6 weeks and 3 months with PSA and testosterone after MHRT completion, then every 6 to 12 months for 5 years and annually thereafter. Each follow-up visit included history, physical examination, IPSS, and CTCAE grading for GI and GU toxicity.
The Wilcoxon rank sum test and χ2 test were used to compare differences in demographic data, dosimetric parameters, and frequency of toxicity events with respect to patient race. Clinical endpoints including biochemical recurrence-free survival (BRFS; defined by Phoenix criteria as 2.0 above PSA nadir), distant metastases-free survival (DMFS), PCSS, and overall survival (OS) were estimated from time of radiotherapy completion by the Kaplan-Meier method and compared between African American and White race by log-rank testing.30 Late GI and GU toxicity-free survival were estimated by Kaplan-Meier plots and compared between African American and White patients by the log-rank test. Statistical analysis was performed using SAS 9.4.
Results
We identified 143 patients with HRPC treated with definitive MHRT between November 2008 and August 2018 (Table 1). Mean age was 65 years (range, 36-80 years); 57% were African American men. Eighty percent of individuals had unfavorable high-risk disease. Median (IQR) PSA was 14.4 (7.8-28.6). Twenty-six percent had grade group 1-3 disease, 47% had grade group 4 disease, and 27% had grade group 5 disease. African American patients had significantly lower pre-MHRT IPSS scores than White patients (mean IPSS, 11 vs 14, respectively; P = .02) despite similar rates of preradiotherapy urinary medication usage (66% and 66%, respectively).
Eighty-six percent received 70 Gy over 28 fractions, with institutional protocol shifting to 60 Gy over 20 fractions (14%) in June 2017. The median (IQR) duration of radiotherapy was 39 (38-42) days, with 97% of individuals undergoing ADT for a median (IQR) duration of 24 (24-36) months. The median follow-up time was 38 months, with 57 (40%) patients followed for at least 60 months.
Grade 3 GI and GU acute toxicity events were observed in 1% and 4% of all individuals, respectively (Table 2). No acute GI or GU grade 4+ events were observed. No significant differences in acute GU or GI toxicity were observed between African American and White patients.
No significant differences between African American and White patients were observed for late grade 2+ GI (P = .19) or GU (P = .55) toxicity. Late grade 2+ GI toxicity was observed in 17 (12%) patients overall (Figure 1A). One grade 3 and 1 grade 4 late GI event were observed following MHRT completion: The latter involved hospitalization for bleeding secondary to radiation proctitis in the context of cirrhosis predating MHRT. Late grade 2+ GU toxicity was observed in 80 (56%) patients, with late grade 2 events steadily increasing over time (Figure 1B). Nine late grade 3 GU toxicity events were observed at a median of 13 months following completion of MHRT, 2 of which occurred more than 24 months after MHRT completion. No late grade 4 or 5 GU events were observed. IPSS values both before MHRT and at time of last follow-up were available for 65 (40%) patients, with a median (IQR) IPSS of 10 (6-16) before MHRT and 12 (8-16) at last follow-up at a median (IQR) interval of 36 months (26-76) from radiation completion.
No significant differences were observed between African American and White patients with respect to BRFS, DMFS, PCSS, or OS (Figure 2). Overall, 21 of 143 (15%) patients experienced biochemical recurrence: 5-year BRFS was 77% (95% CI, 67%-85%) for all patients, 83% (95% CI, 70%-91%) for African American patients, and 71% (95% CI, 53%-82%) for White patients. Five-year DMFS was 87% (95% CI, 77%-92%) for all individuals, 91% (95% CI, 80%-96%) for African American patients, and 81% (95% CI, 62%-91%) for White patients. Five-year PCSS was 89% (95% CI, 80%-94%) for all patients, with 5-year PCSS rates of 90% (95% CI, 79%-95%) for African American patients and 87% (95% CI, 70%-95%) for White patients. Five-year OS was 75% overall (95% CI, 64%-82%), with 5-year OS rates of 73% (95% CI, 58%-83%) for African American patients and 77% (95% CI, 60%-87%) for White patients.
Discussion
In this study, we reported acute and late GI and GU toxicity rates as well as clinical outcomes for a majority African American population with predominately unfavorable HRPC treated with MHRT in an equal access health care environment. We found that MHRT was well tolerated with high rates of biochemical control, PCSS, and OS. Additionally, outcomes were not significantly different across patient race. To our knowledge, this is the first report of MHRT for HRPC in a majority African American population.
We found that MHRT was an effective treatment for patients with HRPC, in particular those with unfavorable high-risk disease. While prior prospective and randomized studies have investigated the use of MHRT, our series was larger than most and had a predominately unfavorable high-risk population.12,15-17 Our biochemical and PCSS rates compare favorably with those of HRPC trial populations, particularly given the high proportion of unfavorable high-risk disease.12,15,16 Despite similar rates of biochemical control, OS was lower in the present cohort than in HRPC trial populations, even with a younger median age at diagnosis. The similarly high rates of non–HRPC-related death across race may reflect differences in baseline comorbidities compared with trial populations as well as reported differences between individuals in the VA and the private sector.31 This suggests that MHRT can be an effective treatment for patients with unfavorable HRPC.
We did not find any differences in outcomes between African American and White individuals with HRPC treated with MHRT. Furthermore, our study demonstrates long-term rates of BRFS and PCSS in a majority African American population with predominately unfavorable HRPC that are comparable with those of prior randomized MHRT studies in high-risk, predominately White populations.12,15,16 Prior reports have found that African American men with HRPC may be at increased risk for inferior clinical outcomes due to a number of socioeconomic, biologic, and cultural mediators.26,27,32 Such individuals may disproportionally benefit from shorter treatment courses that improve access to radiotherapy, a well-documented disparity for African American men with localized prostate cancer.33-36 The VA is an ideal system for studying racial disparities within prostate cancer, as accessibility of mental health and transportation services, income, and insurance status are not barriers to preventative or acute care.37 Our results are concordant with those previously seen for African American patients with prostate cancer seen in the VA, which similarly demonstrate equal outcomes with those of other races.28,36 Incorporation of the earlier mentioned VA services into oncologic care across other health care systems could better characterize determinants of racial disparities in prostate cancer, including the prognostic significance of shortening treatment duration and number of patient visits via MHRT.
Despite widespread acceptance in prostate cancer radiotherapy guidelines, routine use of MHRT seems limited across all stages of localized prostate cancer.1,2 Late toxicity is a frequently noted concern regarding MHRT use. Higher rates of late grade 2+ GI toxicity were observed in the hypofractionation arm of the HYPRO trial.17 While RTOG 0415 did not include patients with HRPC, significantly higher rates of physician-reported (but not patient-reported) late grade 2+ GI and GU toxicity were observed using the same MHRT fractionation regimen used for the majority of individuals in our cohort.9 In our study, the steady increase in late grade 2 GU toxicity is consistent with what is seen following conventionally fractionated radiotherapy and is likely multifactorial.38 The mean IPSS difference of 2/35 from pre-MHRT baseline to the time of last follow-up suggests minimal quality of life decline. The relatively stable IPSSs over time alongside the > 50% prevalence of late grade 2 GU toxicity per CTCAE grading seems consistent with the discrepancy noted in RTOG 0415 between increased physician-reported late toxicity and favorable patient-reported quality of life scores.9 Moreover, significant variance exists in toxicity grading across scoring systems, revised editions of CTCAE, and physician-specific toxicity classification, particularly with regard to the use of adrenergic receptor blocker medications. In light of these factors, the high rate of late grade 2 GU toxicity in our study should be interpreted in the context of largely stable post-MHRT IPSSs and favorable rates of late GI grade 2+ and late GU grade 3+ toxicity.
Limitations
This study has several inherent limitations. While the size of the current HRPC cohort is notably larger than similar populations within the majority of phase 3 MHRT trials, these data derive from a single VA hospital. It is unclear whether these outcomes would be representative in a similar high-risk population receiving care outside of the VA equal access system. Follow-up data beyond 5 years was available for less than half of patients, partially due to nonprostate cancer–related mortality at a higher rate than observed in HRPC trial populations.12,15,16 Furthermore, all GI toxicity events were exclusively physician reported, and GU toxicity reporting was limited in the off-trial setting with not all patients routinely completing IPSS questionnaires following MHRT completion. However, all patients were treated similarly, and radiation quality was verified over the treatment period with mandated accreditation, frequent standardized output checks, and systematic treatment review.39
Conclusions
Patients with HRPC treated with MHRT in an equal access, off-trial setting demonstrated favorable rates of biochemical control with acceptable rates of acute and late GI and GU toxicities. Clinical outcomes, including biochemical control, were not significantly different between African American and White patients, which may reflect equal access to care within the VA irrespective of income and insurance status. Incorporating VA services, such as access to primary care, mental health services, and transportation across other health care systems may aid in characterizing and mitigating racial and gender disparities in oncologic care.
Acknowledgments
Portions of this work were presented at the November 2020 ASTRO conference. 40
Although moderately hypofractionated radiotherapy (MHRT) is an accepted treatment for localized prostate cancer, its adaptation remains limited in the United States.1,2 MHRT theoretically exploits α/β ratio differences between the prostate (1.5 Gy), bladder (5-10 Gy), and rectum (3 Gy), thereby reducing late treatment-related adverse effects compared with those of conventional fractionation at biologically equivalent doses.3-8 Multiple randomized noninferiority trials have demonstrated equivalent outcomes between MHRT and conventional fraction with no appreciable increase in patient-reported toxicity.9-14 Although these studies have led to the acceptance of MHRT as a standard treatment, the majority of these trials involve individuals with low- and intermediate-risk disease.
There are less phase 3 data addressing MHRT for high-risk prostate cancer (HRPC).10,12,14-17 Only 2 studies examined predominately high-risk populations, accounting for 83 and 292 patients, respectively.15,16 Additional phase 3 trials with small proportions of high-risk patients (n = 126, 12%; n = 53, 35%) offer limited additional information regarding clinical outcomes and toxicity rates specific to high-risk disease.10-12 Numerous phase 1 and 2 studies report various field designs and fractionation plans for MHRT in the context of high-risk disease, although the applicability of these data to off-trial populations remains limited.18-20
Furthermore, African American individuals are underrepresented in the trials establishing the role of MHRT despite higher rates of prostate cancer incidence, more advanced disease stage at diagnosis, and higher rates of prostate cancer–specific survival (PCSS) when compared with White patients.21 Racial disparities across patients with prostate cancer and their management are multifactorial across health care literacy, education level, access to care (including transportation issues), and issues of adherence and distrust.22-25 Correlation of patient race to prostate cancer outcomes varies greatly across health care systems, with the US Department of Veterans Affairs (VA) equal access system providing robust mental health services and transportation services for some patients, while demonstrating similar rates of stage-adjusted PCSS between African American and White patients across a broad range of treatment modalities.26-28 Given the paucity of data exploring outcomes following MHRT for African American patients with HRPC, the present analysis provides long-term clinical outcomes and toxicity profiles for an off-trial majority African American population with HRPC treated with MHRT within the VA.
Methods
Records were retrospectively reviewed under an institutional review board–approved protocol for all patients with HRPC treated with definitive MHRT at the Durham Veterans Affairs Healthcare System in North Carolina between November 2008 and August 2018. Exclusion criteria included < 12 months of follow-up or elective nodal irradiation. Demographic variables obtained included age at diagnosis, race, clinical T stage, pre-MHRT prostate-specific antigen (PSA), Gleason grade group at diagnosis, favorable vs unfavorable high-risk disease, pre-MHRT international prostate symptom score (IPSS), and pre-MHRT urinary medication usage (yes/no).29
Concurrent androgen deprivation therapy (ADT) was initiated 6 to 8 weeks before MHRT unless medically contraindicated per the discretion of the treating radiation oncologist. Patients generally received 18 to 24 months of ADT, with those with favorable HRPC (ie, T1c disease with either Gleason 4+4 and PSA < 10 mg/mL or Gleason 3+3 and PSA > 20 ng/mL) receiving 6 months after 2015.29 Patients were simulated supine in either standard or custom immobilization with a full bladder and empty rectum. MHRT fractionation plans included 70 Gy at 2.5 Gy per fraction and 60 Gy at 3 Gy per fraction. Radiotherapy targets included the prostate and seminal vesicles without elective nodal coverage per institutional practice. Treatments were delivered following image guidance, either prostate matching with cone beam computed tomography or fiducial matching with kilo voltage imaging. All patients received intensity-modulated radiotherapy. For plans delivering 70 Gy at 2.5 Gy per fraction, constraints included bladder V (volume receiving) 70 < 10 cc, V65 ≤ 15%, V40 ≤ 35%, rectum V70 < 10 cc, V65 ≤ 10%, V40 ≤ 35%, femoral heads maximum point dose ≤ 40 Gy, penile bulb mean dose ≤ 50 Gy, and small bowel V40 ≤ 1%. For plans delivering 60 Gy at 3 Gy per fraction, constraints included rectum V57 ≤ 15%, V46 ≤ 30%, V37 ≤ 50%, bladder V60 ≤ 5%, V46 ≤ 30%, V37 ≤ 50%, and femoral heads V43 ≤ 5%.
Gastrointestinal (GI) and genitourinary (GU) toxicities were graded using Common Terminology Criteria for Adverse Events (CTCAE), version 5.0, with acute toxicity defined as on-treatment < 3 months following completion of MHRT. Late toxicity was defined as ≥ 3 months following completion of MHRT. Individuals were seen in follow-up at 6 weeks and 3 months with PSA and testosterone after MHRT completion, then every 6 to 12 months for 5 years and annually thereafter. Each follow-up visit included history, physical examination, IPSS, and CTCAE grading for GI and GU toxicity.
The Wilcoxon rank sum test and χ2 test were used to compare differences in demographic data, dosimetric parameters, and frequency of toxicity events with respect to patient race. Clinical endpoints including biochemical recurrence-free survival (BRFS; defined by Phoenix criteria as 2.0 above PSA nadir), distant metastases-free survival (DMFS), PCSS, and overall survival (OS) were estimated from time of radiotherapy completion by the Kaplan-Meier method and compared between African American and White race by log-rank testing.30 Late GI and GU toxicity-free survival were estimated by Kaplan-Meier plots and compared between African American and White patients by the log-rank test. Statistical analysis was performed using SAS 9.4.
Results
We identified 143 patients with HRPC treated with definitive MHRT between November 2008 and August 2018 (Table 1). Mean age was 65 years (range, 36-80 years); 57% were African American men. Eighty percent of individuals had unfavorable high-risk disease. Median (IQR) PSA was 14.4 (7.8-28.6). Twenty-six percent had grade group 1-3 disease, 47% had grade group 4 disease, and 27% had grade group 5 disease. African American patients had significantly lower pre-MHRT IPSS scores than White patients (mean IPSS, 11 vs 14, respectively; P = .02) despite similar rates of preradiotherapy urinary medication usage (66% and 66%, respectively).
Eighty-six percent received 70 Gy over 28 fractions, with institutional protocol shifting to 60 Gy over 20 fractions (14%) in June 2017. The median (IQR) duration of radiotherapy was 39 (38-42) days, with 97% of individuals undergoing ADT for a median (IQR) duration of 24 (24-36) months. The median follow-up time was 38 months, with 57 (40%) patients followed for at least 60 months.
Grade 3 GI and GU acute toxicity events were observed in 1% and 4% of all individuals, respectively (Table 2). No acute GI or GU grade 4+ events were observed. No significant differences in acute GU or GI toxicity were observed between African American and White patients.
No significant differences between African American and White patients were observed for late grade 2+ GI (P = .19) or GU (P = .55) toxicity. Late grade 2+ GI toxicity was observed in 17 (12%) patients overall (Figure 1A). One grade 3 and 1 grade 4 late GI event were observed following MHRT completion: The latter involved hospitalization for bleeding secondary to radiation proctitis in the context of cirrhosis predating MHRT. Late grade 2+ GU toxicity was observed in 80 (56%) patients, with late grade 2 events steadily increasing over time (Figure 1B). Nine late grade 3 GU toxicity events were observed at a median of 13 months following completion of MHRT, 2 of which occurred more than 24 months after MHRT completion. No late grade 4 or 5 GU events were observed. IPSS values both before MHRT and at time of last follow-up were available for 65 (40%) patients, with a median (IQR) IPSS of 10 (6-16) before MHRT and 12 (8-16) at last follow-up at a median (IQR) interval of 36 months (26-76) from radiation completion.
No significant differences were observed between African American and White patients with respect to BRFS, DMFS, PCSS, or OS (Figure 2). Overall, 21 of 143 (15%) patients experienced biochemical recurrence: 5-year BRFS was 77% (95% CI, 67%-85%) for all patients, 83% (95% CI, 70%-91%) for African American patients, and 71% (95% CI, 53%-82%) for White patients. Five-year DMFS was 87% (95% CI, 77%-92%) for all individuals, 91% (95% CI, 80%-96%) for African American patients, and 81% (95% CI, 62%-91%) for White patients. Five-year PCSS was 89% (95% CI, 80%-94%) for all patients, with 5-year PCSS rates of 90% (95% CI, 79%-95%) for African American patients and 87% (95% CI, 70%-95%) for White patients. Five-year OS was 75% overall (95% CI, 64%-82%), with 5-year OS rates of 73% (95% CI, 58%-83%) for African American patients and 77% (95% CI, 60%-87%) for White patients.
Discussion
In this study, we reported acute and late GI and GU toxicity rates as well as clinical outcomes for a majority African American population with predominately unfavorable HRPC treated with MHRT in an equal access health care environment. We found that MHRT was well tolerated with high rates of biochemical control, PCSS, and OS. Additionally, outcomes were not significantly different across patient race. To our knowledge, this is the first report of MHRT for HRPC in a majority African American population.
We found that MHRT was an effective treatment for patients with HRPC, in particular those with unfavorable high-risk disease. While prior prospective and randomized studies have investigated the use of MHRT, our series was larger than most and had a predominately unfavorable high-risk population.12,15-17 Our biochemical and PCSS rates compare favorably with those of HRPC trial populations, particularly given the high proportion of unfavorable high-risk disease.12,15,16 Despite similar rates of biochemical control, OS was lower in the present cohort than in HRPC trial populations, even with a younger median age at diagnosis. The similarly high rates of non–HRPC-related death across race may reflect differences in baseline comorbidities compared with trial populations as well as reported differences between individuals in the VA and the private sector.31 This suggests that MHRT can be an effective treatment for patients with unfavorable HRPC.
We did not find any differences in outcomes between African American and White individuals with HRPC treated with MHRT. Furthermore, our study demonstrates long-term rates of BRFS and PCSS in a majority African American population with predominately unfavorable HRPC that are comparable with those of prior randomized MHRT studies in high-risk, predominately White populations.12,15,16 Prior reports have found that African American men with HRPC may be at increased risk for inferior clinical outcomes due to a number of socioeconomic, biologic, and cultural mediators.26,27,32 Such individuals may disproportionally benefit from shorter treatment courses that improve access to radiotherapy, a well-documented disparity for African American men with localized prostate cancer.33-36 The VA is an ideal system for studying racial disparities within prostate cancer, as accessibility of mental health and transportation services, income, and insurance status are not barriers to preventative or acute care.37 Our results are concordant with those previously seen for African American patients with prostate cancer seen in the VA, which similarly demonstrate equal outcomes with those of other races.28,36 Incorporation of the earlier mentioned VA services into oncologic care across other health care systems could better characterize determinants of racial disparities in prostate cancer, including the prognostic significance of shortening treatment duration and number of patient visits via MHRT.
Despite widespread acceptance in prostate cancer radiotherapy guidelines, routine use of MHRT seems limited across all stages of localized prostate cancer.1,2 Late toxicity is a frequently noted concern regarding MHRT use. Higher rates of late grade 2+ GI toxicity were observed in the hypofractionation arm of the HYPRO trial.17 While RTOG 0415 did not include patients with HRPC, significantly higher rates of physician-reported (but not patient-reported) late grade 2+ GI and GU toxicity were observed using the same MHRT fractionation regimen used for the majority of individuals in our cohort.9 In our study, the steady increase in late grade 2 GU toxicity is consistent with what is seen following conventionally fractionated radiotherapy and is likely multifactorial.38 The mean IPSS difference of 2/35 from pre-MHRT baseline to the time of last follow-up suggests minimal quality of life decline. The relatively stable IPSSs over time alongside the > 50% prevalence of late grade 2 GU toxicity per CTCAE grading seems consistent with the discrepancy noted in RTOG 0415 between increased physician-reported late toxicity and favorable patient-reported quality of life scores.9 Moreover, significant variance exists in toxicity grading across scoring systems, revised editions of CTCAE, and physician-specific toxicity classification, particularly with regard to the use of adrenergic receptor blocker medications. In light of these factors, the high rate of late grade 2 GU toxicity in our study should be interpreted in the context of largely stable post-MHRT IPSSs and favorable rates of late GI grade 2+ and late GU grade 3+ toxicity.
Limitations
This study has several inherent limitations. While the size of the current HRPC cohort is notably larger than similar populations within the majority of phase 3 MHRT trials, these data derive from a single VA hospital. It is unclear whether these outcomes would be representative in a similar high-risk population receiving care outside of the VA equal access system. Follow-up data beyond 5 years was available for less than half of patients, partially due to nonprostate cancer–related mortality at a higher rate than observed in HRPC trial populations.12,15,16 Furthermore, all GI toxicity events were exclusively physician reported, and GU toxicity reporting was limited in the off-trial setting with not all patients routinely completing IPSS questionnaires following MHRT completion. However, all patients were treated similarly, and radiation quality was verified over the treatment period with mandated accreditation, frequent standardized output checks, and systematic treatment review.39
Conclusions
Patients with HRPC treated with MHRT in an equal access, off-trial setting demonstrated favorable rates of biochemical control with acceptable rates of acute and late GI and GU toxicities. Clinical outcomes, including biochemical control, were not significantly different between African American and White patients, which may reflect equal access to care within the VA irrespective of income and insurance status. Incorporating VA services, such as access to primary care, mental health services, and transportation across other health care systems may aid in characterizing and mitigating racial and gender disparities in oncologic care.
Acknowledgments
Portions of this work were presented at the November 2020 ASTRO conference. 40
1. Stokes WA, Kavanagh BD, Raben D, Pugh TJ. Implementation of hypofractionated prostate radiation therapy in the United States: a National Cancer Database analysis. Pract Radiat Oncol. 2017;7:270-278. doi:10.1016/j.prro.2017.03.011
2. Jaworski L, Dominello MM, Heimburger DK, et al. Contemporary practice patterns for intact and post-operative prostate cancer: results from a statewide collaborative. Int J Radiat Oncol Biol Phys. 2019;105(1):E282. doi:10.1016/j.ijrobp.2019.06.1915
3. Miralbell R, Roberts SA, Zubizarreta E, Hendry JH. Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5,969 patients in seven international institutional datasets: α/β = 1.4 (0.9-2.2) Gy. Int J Radiat Oncol Biol Phys. 2012;82(1):e17-e24. doi:10.1016/j.ijrobp.2010.10.075
4. Tree AC, Khoo VS, van As NJ, Partridge M. Is biochemical relapse-free survival after profoundly hypofractionated radiotherapy consistent with current radiobiological models? Clin Oncol (R Coll Radiol). 2014;26(4):216-229. doi:10.1016/j.clon.2014.01.008
5. Brenner DJ. Fractionation and late rectal toxicity. Int J Radiat Oncol Biol Phys. 2004;60(4):1013-1015. doi:10.1016/j.ijrobp.2004.04.014
6. Tucker SL, Thames HD, Michalski JM, et al. Estimation of α/β for late rectal toxicity based on RTOG 94-06. Int J Radiat Oncol Biol Phys. 2011;81(2):600-605. doi:10.1016/j.ijrobp.2010.11.080
7. Dasu A, Toma-Dasu I. Prostate alpha/beta revisited—an analysis of clinical results from 14 168 patients. Acta Oncol. 2012;51(8):963-974. doi:10.3109/0284186X.2012.719635 start
8. Proust-Lima C, Taylor JMG, Sécher S, et al. Confirmation of a Low α/β ratio for prostate cancer treated by external beam radiation therapy alone using a post-treatment repeated-measures model for PSA dynamics. Int J Radiat Oncol Biol Phys. 2011;79(1):195-201. doi:10.1016/j.ijrobp.2009.10.008
9. Lee WR, Dignam JJ, Amin MB, et al. Randomized phase III noninferiority study comparing two radiotherapy fractionation schedules in patients with low-risk prostate cancer. J Clin Oncol. 2016;34(20): 2325-2332. doi:10.1200/JCO.2016.67.0448
10. Dearnaley D, Syndikus I, Mossop H, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17(8):1047-1060. doi:10.1016/S1470-2045(16)30102-4
11. Catton CN, Lukka H, Gu C-S, et al. Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J Clin Oncol. 2017;35(17):1884-1890. doi:10.1200/JCO.2016.71.7397
12. Pollack A, Walker G, Horwitz EM, et al. Randomized trial of hypofractionated external-beam radiotherapy for prostate cancer. J Clin Oncol. 2013;31(31):3860-3868. doi:10.1200/JCO.2013.51.1972
13. Hoffman KE, Voong KR, Levy LB, et al. Randomized trial of hypofractionated, dose-escalated, intensity-modulated radiation therapy (IMRT) versus conventionally fractionated IMRT for localized prostate cancer. J Clin Oncol. 2018;36(29):2943-2949. doi:10.1200/JCO.2018.77.9868
14. Wilkins A, Mossop H, Syndikus I, et al. Hypofractionated radiotherapy versus conventionally fractionated radiotherapy for patients with intermediate-risk localised prostate cancer: 2-year patient-reported outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2015;16(16):1605-1616. doi:10.1016/S1470-2045(15)00280-6
15. Incrocci L, Wortel RC, Alemayehu WG, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with localised prostate cancer (HYPRO): final efficacy results from a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2016;17(8):1061-1069. doi.10.1016/S1470-2045(16)30070-5
16. Arcangeli G, Saracino B, Arcangeli S, et al. Moderate hypofractionation in high-risk, organ-confined prostate cancer: final results of a phase III randomized trial. J Clin Oncol. 2017;35(17):1891-1897. doi:10.1200/JCO.2016.70.4189
17. Aluwini S, Pos F, Schimmel E, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): late toxicity results from a randomised, non-inferiority, phase 3 trial. Lancet Oncol. 2016;17(4):464-474. doi:10.1016/S1470-2045(15)00567-7
18. Pervez N, Small C, MacKenzie M, et al. Acute toxicity in high-risk prostate cancer patients treated with androgen suppression and hypofractionated intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;76(1):57-64. doi:10.1016/j.ijrobp.2009.01.048
19. Magli A, Moretti E, Tullio A, Giannarini G. Hypofractionated simultaneous integrated boost (IMRT- cancer: results of a prospective phase II trial SIB) with pelvic nodal irradiation and concurrent androgen deprivation therapy for high-risk prostate cancer: results of a prospective phase II trial. Prostate Cancer Prostatic Dis. 2018;21(2):269-276. doi:10.1038/s41391-018-0034-0
20. Di Muzio NG, Fodor A, Noris Chiorda B, et al. Moderate hypofractionation with simultaneous integrated boost in prostate cancer: long-term results of a phase I–II study. Clin Oncol (R Coll Radiol). 2016;28(8):490-500. doi:10.1016/j.clon.2016.02.005
21. DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin. 2019;69(3):21-233. doi:10.3322/caac.21555
22. Wolf MS, Knight SJ, Lyons EA, et al. Literacy, race, and PSA level among low-income men newly diagnosed with prostate cancer. Urology. 2006(1);68:89-93. doi:10.1016/j.urology.2006.01.064
23. Rebbeck TR. Prostate cancer disparities by race and ethnicity: from nucleotide to neighborhood. Cold Spring Harb Perspect Med. 2018;8(9):a030387. doi:10.1101/cshperspect.a030387
24. Guidry JJ, Aday LA, Zhang D, Winn RJ. Transportation as a barrier to cancer treatment. Cancer Pract. 1997;5(6):361-366.
25. Friedman DB, Corwin SJ, Dominick GM, Rose ID. African American men’s understanding and perceptions about prostate cancer: why multiple dimensions of health literacy are important in cancer communication. J Community Health. 2009;34(5):449-460. doi:10.1007/s10900-009-9167-3
26. Connell PP, Ignacio L, Haraf D, et al. Equivalent racial outcome after conformal radiotherapy for prostate cancer: a single departmental experience. J Clin Oncol. 2001;19(1):54-61. doi:10.1200/JCO.2001.19.1.54
27. Dess RT, Hartman HE, Mahal BA, et al. Association of black race with prostate cancer-specific and other-cause mortality. JAMA Oncol. 2019;5(1):975-983. doi:10.1200/JCO.2001.19.1.54
28. McKay RR, Sarkar RR, Kumar A, et al. Outcomes of Black men with prostate cancer treated with radiation therapy in the Veterans Health Administration. Cancer. 2021;127(3):403-411. doi:10.1002/cncr.33224

29. Muralidhar V, Chen M-H, Reznor G, et al. Definition and validation of “favorable high-risk prostate cancer”: implications for personalizing treatment of radiation-managed patients. Int J Radiat Oncol Biol Phys. 2015;93(4):828-835. doi:10.1016/j.ijrobp.2015.07.2281
30. Roach M 3rd, Hanks G, Thames H Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65(4):965-974. doi:10.1016/j.ijrobp.2006.04.029
31. Freeman VL, Durazo-Arvizu R, Arozullah AM, Keys LC. Determinants of mortality following a diagnosis of prostate cancer in Veterans Affairs and private sector health care systems. Am J Public Health. 2003;93(100):1706-1712. doi:10.2105/ajph.93.10.1706
32. Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54(2):78-93. doi:10.3322/canjclin.54.2.78
33. Zemplenyi AT, Kaló Z, Kovacs G, et al. Cost-effectiveness analysis of intensity-modulated radiation therapy with normal and hypofractionated schemes for the treatment of localised prostate cancer. Eur J Cancer Care. 2018;27(1):e12430. doi:10.1111/ecc.12430
34. Klabunde CN, Potosky AL, Harlan LC, Kramer BS. Trends and black/white differences in treatment for nonmetastatic prostate cancer. Med Care. 1998;36(9):1337-1348. doi:10.1097/00005650-199809000-00006
35. Harlan L, Brawley O, Pommerenke F, Wali P, Kramer B. Geographic, age, and racial variation in the treatment of local/regional carcinoma of the prostate. J Clin Oncol. 1995;13(1):93-100. doi:10.1200/JCO.1995.13.1.93
36. Riviere P, Luterstein E, Kumar A, et al. Racial equity among African-American and non-Hispanic white men diagnosed with prostate cancer in the veterans affairs healthcare system. Int J Radiat Oncol Biol Phys. 2019;105:E305.
37. Peterson K, Anderson J, Boundy E, Ferguson L, McCleery E, Waldrip K. Mortality disparities in racial/ethnic minority groups in the Veterans Health Administration: an evidence review and map. Am J Public Health. 2018;108(3):e1-e11. doi:10.2105/AJPH.2017.304246
38. Zietman AL, DeSilvio ML, Slater JD, et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA. 2005;294(10):1233-1239. doi:10.1001/jama.294.10.1233
39. Hagan M, Kapoor R, Michalski J, et al. VA-Radiation Oncology Quality Surveillance program. Int J Radiat Oncol Biol Phys. 2020;106(3):639-647. doi.10.1016/j.ijrobp.2019.08.064
40. Carpenter DJ, Natesan D, Floyd W, et al. Long-term experience in an equal access health care system using moderately hypofractionated radiotherapy for high risk prostate cancer in a predominately African American population with unfavorable disease. Int J Radiat Oncol Biol Phys. 2020;108(3):E417. https://www.redjournal.org/article/S0360-3016(20)33923-7/fulltext
1. Stokes WA, Kavanagh BD, Raben D, Pugh TJ. Implementation of hypofractionated prostate radiation therapy in the United States: a National Cancer Database analysis. Pract Radiat Oncol. 2017;7:270-278. doi:10.1016/j.prro.2017.03.011
2. Jaworski L, Dominello MM, Heimburger DK, et al. Contemporary practice patterns for intact and post-operative prostate cancer: results from a statewide collaborative. Int J Radiat Oncol Biol Phys. 2019;105(1):E282. doi:10.1016/j.ijrobp.2019.06.1915
3. Miralbell R, Roberts SA, Zubizarreta E, Hendry JH. Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5,969 patients in seven international institutional datasets: α/β = 1.4 (0.9-2.2) Gy. Int J Radiat Oncol Biol Phys. 2012;82(1):e17-e24. doi:10.1016/j.ijrobp.2010.10.075
4. Tree AC, Khoo VS, van As NJ, Partridge M. Is biochemical relapse-free survival after profoundly hypofractionated radiotherapy consistent with current radiobiological models? Clin Oncol (R Coll Radiol). 2014;26(4):216-229. doi:10.1016/j.clon.2014.01.008
5. Brenner DJ. Fractionation and late rectal toxicity. Int J Radiat Oncol Biol Phys. 2004;60(4):1013-1015. doi:10.1016/j.ijrobp.2004.04.014
6. Tucker SL, Thames HD, Michalski JM, et al. Estimation of α/β for late rectal toxicity based on RTOG 94-06. Int J Radiat Oncol Biol Phys. 2011;81(2):600-605. doi:10.1016/j.ijrobp.2010.11.080
7. Dasu A, Toma-Dasu I. Prostate alpha/beta revisited—an analysis of clinical results from 14 168 patients. Acta Oncol. 2012;51(8):963-974. doi:10.3109/0284186X.2012.719635 start
8. Proust-Lima C, Taylor JMG, Sécher S, et al. Confirmation of a Low α/β ratio for prostate cancer treated by external beam radiation therapy alone using a post-treatment repeated-measures model for PSA dynamics. Int J Radiat Oncol Biol Phys. 2011;79(1):195-201. doi:10.1016/j.ijrobp.2009.10.008
9. Lee WR, Dignam JJ, Amin MB, et al. Randomized phase III noninferiority study comparing two radiotherapy fractionation schedules in patients with low-risk prostate cancer. J Clin Oncol. 2016;34(20): 2325-2332. doi:10.1200/JCO.2016.67.0448
10. Dearnaley D, Syndikus I, Mossop H, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17(8):1047-1060. doi:10.1016/S1470-2045(16)30102-4
11. Catton CN, Lukka H, Gu C-S, et al. Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J Clin Oncol. 2017;35(17):1884-1890. doi:10.1200/JCO.2016.71.7397
12. Pollack A, Walker G, Horwitz EM, et al. Randomized trial of hypofractionated external-beam radiotherapy for prostate cancer. J Clin Oncol. 2013;31(31):3860-3868. doi:10.1200/JCO.2013.51.1972
13. Hoffman KE, Voong KR, Levy LB, et al. Randomized trial of hypofractionated, dose-escalated, intensity-modulated radiation therapy (IMRT) versus conventionally fractionated IMRT for localized prostate cancer. J Clin Oncol. 2018;36(29):2943-2949. doi:10.1200/JCO.2018.77.9868
14. Wilkins A, Mossop H, Syndikus I, et al. Hypofractionated radiotherapy versus conventionally fractionated radiotherapy for patients with intermediate-risk localised prostate cancer: 2-year patient-reported outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2015;16(16):1605-1616. doi:10.1016/S1470-2045(15)00280-6
15. Incrocci L, Wortel RC, Alemayehu WG, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with localised prostate cancer (HYPRO): final efficacy results from a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2016;17(8):1061-1069. doi.10.1016/S1470-2045(16)30070-5
16. Arcangeli G, Saracino B, Arcangeli S, et al. Moderate hypofractionation in high-risk, organ-confined prostate cancer: final results of a phase III randomized trial. J Clin Oncol. 2017;35(17):1891-1897. doi:10.1200/JCO.2016.70.4189
17. Aluwini S, Pos F, Schimmel E, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): late toxicity results from a randomised, non-inferiority, phase 3 trial. Lancet Oncol. 2016;17(4):464-474. doi:10.1016/S1470-2045(15)00567-7
18. Pervez N, Small C, MacKenzie M, et al. Acute toxicity in high-risk prostate cancer patients treated with androgen suppression and hypofractionated intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;76(1):57-64. doi:10.1016/j.ijrobp.2009.01.048
19. Magli A, Moretti E, Tullio A, Giannarini G. Hypofractionated simultaneous integrated boost (IMRT- cancer: results of a prospective phase II trial SIB) with pelvic nodal irradiation and concurrent androgen deprivation therapy for high-risk prostate cancer: results of a prospective phase II trial. Prostate Cancer Prostatic Dis. 2018;21(2):269-276. doi:10.1038/s41391-018-0034-0
20. Di Muzio NG, Fodor A, Noris Chiorda B, et al. Moderate hypofractionation with simultaneous integrated boost in prostate cancer: long-term results of a phase I–II study. Clin Oncol (R Coll Radiol). 2016;28(8):490-500. doi:10.1016/j.clon.2016.02.005
21. DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin. 2019;69(3):21-233. doi:10.3322/caac.21555
22. Wolf MS, Knight SJ, Lyons EA, et al. Literacy, race, and PSA level among low-income men newly diagnosed with prostate cancer. Urology. 2006(1);68:89-93. doi:10.1016/j.urology.2006.01.064
23. Rebbeck TR. Prostate cancer disparities by race and ethnicity: from nucleotide to neighborhood. Cold Spring Harb Perspect Med. 2018;8(9):a030387. doi:10.1101/cshperspect.a030387
24. Guidry JJ, Aday LA, Zhang D, Winn RJ. Transportation as a barrier to cancer treatment. Cancer Pract. 1997;5(6):361-366.
25. Friedman DB, Corwin SJ, Dominick GM, Rose ID. African American men’s understanding and perceptions about prostate cancer: why multiple dimensions of health literacy are important in cancer communication. J Community Health. 2009;34(5):449-460. doi:10.1007/s10900-009-9167-3
26. Connell PP, Ignacio L, Haraf D, et al. Equivalent racial outcome after conformal radiotherapy for prostate cancer: a single departmental experience. J Clin Oncol. 2001;19(1):54-61. doi:10.1200/JCO.2001.19.1.54
27. Dess RT, Hartman HE, Mahal BA, et al. Association of black race with prostate cancer-specific and other-cause mortality. JAMA Oncol. 2019;5(1):975-983. doi:10.1200/JCO.2001.19.1.54
28. McKay RR, Sarkar RR, Kumar A, et al. Outcomes of Black men with prostate cancer treated with radiation therapy in the Veterans Health Administration. Cancer. 2021;127(3):403-411. doi:10.1002/cncr.33224

29. Muralidhar V, Chen M-H, Reznor G, et al. Definition and validation of “favorable high-risk prostate cancer”: implications for personalizing treatment of radiation-managed patients. Int J Radiat Oncol Biol Phys. 2015;93(4):828-835. doi:10.1016/j.ijrobp.2015.07.2281
30. Roach M 3rd, Hanks G, Thames H Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65(4):965-974. doi:10.1016/j.ijrobp.2006.04.029
31. Freeman VL, Durazo-Arvizu R, Arozullah AM, Keys LC. Determinants of mortality following a diagnosis of prostate cancer in Veterans Affairs and private sector health care systems. Am J Public Health. 2003;93(100):1706-1712. doi:10.2105/ajph.93.10.1706
32. Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54(2):78-93. doi:10.3322/canjclin.54.2.78
33. Zemplenyi AT, Kaló Z, Kovacs G, et al. Cost-effectiveness analysis of intensity-modulated radiation therapy with normal and hypofractionated schemes for the treatment of localised prostate cancer. Eur J Cancer Care. 2018;27(1):e12430. doi:10.1111/ecc.12430
34. Klabunde CN, Potosky AL, Harlan LC, Kramer BS. Trends and black/white differences in treatment for nonmetastatic prostate cancer. Med Care. 1998;36(9):1337-1348. doi:10.1097/00005650-199809000-00006
35. Harlan L, Brawley O, Pommerenke F, Wali P, Kramer B. Geographic, age, and racial variation in the treatment of local/regional carcinoma of the prostate. J Clin Oncol. 1995;13(1):93-100. doi:10.1200/JCO.1995.13.1.93
36. Riviere P, Luterstein E, Kumar A, et al. Racial equity among African-American and non-Hispanic white men diagnosed with prostate cancer in the veterans affairs healthcare system. Int J Radiat Oncol Biol Phys. 2019;105:E305.
37. Peterson K, Anderson J, Boundy E, Ferguson L, McCleery E, Waldrip K. Mortality disparities in racial/ethnic minority groups in the Veterans Health Administration: an evidence review and map. Am J Public Health. 2018;108(3):e1-e11. doi:10.2105/AJPH.2017.304246
38. Zietman AL, DeSilvio ML, Slater JD, et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA. 2005;294(10):1233-1239. doi:10.1001/jama.294.10.1233
39. Hagan M, Kapoor R, Michalski J, et al. VA-Radiation Oncology Quality Surveillance program. Int J Radiat Oncol Biol Phys. 2020;106(3):639-647. doi.10.1016/j.ijrobp.2019.08.064
40. Carpenter DJ, Natesan D, Floyd W, et al. Long-term experience in an equal access health care system using moderately hypofractionated radiotherapy for high risk prostate cancer in a predominately African American population with unfavorable disease. Int J Radiat Oncol Biol Phys. 2020;108(3):E417. https://www.redjournal.org/article/S0360-3016(20)33923-7/fulltext
Urinating multiple times per night
On the basis of the patient's history and presentation, this is likely a case of adenocarcinoma of the prostate. Although most patients with prostate cancer are diagnosed on screening, when localized symptoms do occur, they may include urinary frequency, decreased urine stream, urinary urgency, and hematuria. In some cases, these signs and symptoms may well be related to age-associated prostate enlargement or other conditions; benign prostatic hyperplasia, for example, can manifest in urinary symptoms and even elevate PSA (but because this patient does not report pain, nonbacterial prostatitis is unlikely). Symptomatic patients older than 50 years, such as the one in this case, should be screened for prostate cancer. Those with a PSA > 10 ng/mL are more than 50% likely to have prostate cancer.
National Comprehensive Cancer Network guidelines advise that needle biopsy of the prostate is indicated for tissue diagnosis in those with elevated PSA levels, preferably via a transrectal ultrasound. MRI can be used to assess lesions that are concerning for prostate cancer prior to biopsy. Lesions are then assigned Prostate Imaging Reporting and Data System (PI-RADS) scores depending on their location within the prostatic zones. A pathologic evaluation of the biopsy specimen will determine the patient's Gleason score. PSA density and PSA doubling time should be collected as well. The clinician should ask about high-risk germline mutations and estimate life expectancy because course of treatment is largely based on risk assessment.
Standard treatments for clinically localized prostate cancer include watchful waiting, active surveillance, radical prostatectomy, and radiation therapy. Active surveillance is often recommended for those who have very-low-risk disease because of the slow growth of certain types of prostate cancer. Radical prostatectomy is a viable option for any patient with localized disease that can be completely excised surgically, provided the patient has a life expectancy of 10 or more years and no serious comorbidities. In some patients, radical prostatectomy may be followed by radiation with or without a short course of hormone treatment, depending on risk factors for recurrence. Radiation therapy is also potentially curative in localized prostate cancer and may be delivered in the form of external-beam radiation therapy or brachytherapy. For asymptomatic patients who are older and/or have other serious underlying conditions, observation may be recommended.
Chad R. Tracy, MD, Professor; Director, Minimally Invasive Surgery, Department of Urology, University of Iowa Hospitals and Clinics, Iowa City, Iowa
Chad R. Tracy, MD, has disclosed the following relevant financial relationships:
Serve(d) as a consultant for: CVICO Medical Solutions.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
On the basis of the patient's history and presentation, this is likely a case of adenocarcinoma of the prostate. Although most patients with prostate cancer are diagnosed on screening, when localized symptoms do occur, they may include urinary frequency, decreased urine stream, urinary urgency, and hematuria. In some cases, these signs and symptoms may well be related to age-associated prostate enlargement or other conditions; benign prostatic hyperplasia, for example, can manifest in urinary symptoms and even elevate PSA (but because this patient does not report pain, nonbacterial prostatitis is unlikely). Symptomatic patients older than 50 years, such as the one in this case, should be screened for prostate cancer. Those with a PSA > 10 ng/mL are more than 50% likely to have prostate cancer.
National Comprehensive Cancer Network guidelines advise that needle biopsy of the prostate is indicated for tissue diagnosis in those with elevated PSA levels, preferably via a transrectal ultrasound. MRI can be used to assess lesions that are concerning for prostate cancer prior to biopsy. Lesions are then assigned Prostate Imaging Reporting and Data System (PI-RADS) scores depending on their location within the prostatic zones. A pathologic evaluation of the biopsy specimen will determine the patient's Gleason score. PSA density and PSA doubling time should be collected as well. The clinician should ask about high-risk germline mutations and estimate life expectancy because course of treatment is largely based on risk assessment.
Standard treatments for clinically localized prostate cancer include watchful waiting, active surveillance, radical prostatectomy, and radiation therapy. Active surveillance is often recommended for those who have very-low-risk disease because of the slow growth of certain types of prostate cancer. Radical prostatectomy is a viable option for any patient with localized disease that can be completely excised surgically, provided the patient has a life expectancy of 10 or more years and no serious comorbidities. In some patients, radical prostatectomy may be followed by radiation with or without a short course of hormone treatment, depending on risk factors for recurrence. Radiation therapy is also potentially curative in localized prostate cancer and may be delivered in the form of external-beam radiation therapy or brachytherapy. For asymptomatic patients who are older and/or have other serious underlying conditions, observation may be recommended.
Chad R. Tracy, MD, Professor; Director, Minimally Invasive Surgery, Department of Urology, University of Iowa Hospitals and Clinics, Iowa City, Iowa
Chad R. Tracy, MD, has disclosed the following relevant financial relationships:
Serve(d) as a consultant for: CVICO Medical Solutions.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
On the basis of the patient's history and presentation, this is likely a case of adenocarcinoma of the prostate. Although most patients with prostate cancer are diagnosed on screening, when localized symptoms do occur, they may include urinary frequency, decreased urine stream, urinary urgency, and hematuria. In some cases, these signs and symptoms may well be related to age-associated prostate enlargement or other conditions; benign prostatic hyperplasia, for example, can manifest in urinary symptoms and even elevate PSA (but because this patient does not report pain, nonbacterial prostatitis is unlikely). Symptomatic patients older than 50 years, such as the one in this case, should be screened for prostate cancer. Those with a PSA > 10 ng/mL are more than 50% likely to have prostate cancer.
National Comprehensive Cancer Network guidelines advise that needle biopsy of the prostate is indicated for tissue diagnosis in those with elevated PSA levels, preferably via a transrectal ultrasound. MRI can be used to assess lesions that are concerning for prostate cancer prior to biopsy. Lesions are then assigned Prostate Imaging Reporting and Data System (PI-RADS) scores depending on their location within the prostatic zones. A pathologic evaluation of the biopsy specimen will determine the patient's Gleason score. PSA density and PSA doubling time should be collected as well. The clinician should ask about high-risk germline mutations and estimate life expectancy because course of treatment is largely based on risk assessment.
Standard treatments for clinically localized prostate cancer include watchful waiting, active surveillance, radical prostatectomy, and radiation therapy. Active surveillance is often recommended for those who have very-low-risk disease because of the slow growth of certain types of prostate cancer. Radical prostatectomy is a viable option for any patient with localized disease that can be completely excised surgically, provided the patient has a life expectancy of 10 or more years and no serious comorbidities. In some patients, radical prostatectomy may be followed by radiation with or without a short course of hormone treatment, depending on risk factors for recurrence. Radiation therapy is also potentially curative in localized prostate cancer and may be delivered in the form of external-beam radiation therapy or brachytherapy. For asymptomatic patients who are older and/or have other serious underlying conditions, observation may be recommended.
Chad R. Tracy, MD, Professor; Director, Minimally Invasive Surgery, Department of Urology, University of Iowa Hospitals and Clinics, Iowa City, Iowa
Chad R. Tracy, MD, has disclosed the following relevant financial relationships:
Serve(d) as a consultant for: CVICO Medical Solutions.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 62-year-old man presents for routine prostate cancer screening. He notes that he has not been sleeping well as a result of getting up to urinate multiple times per night for the past few months. The patient underwent a prostate cancer screening about 26 months ago, and results were normal. On examination, digital rectal examination is normal, but prostate-specific antigen (PSA) levels are elevated at 10.2 ng/mL.