User login
For MD-IQ only
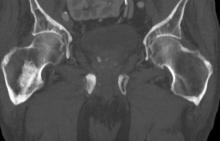
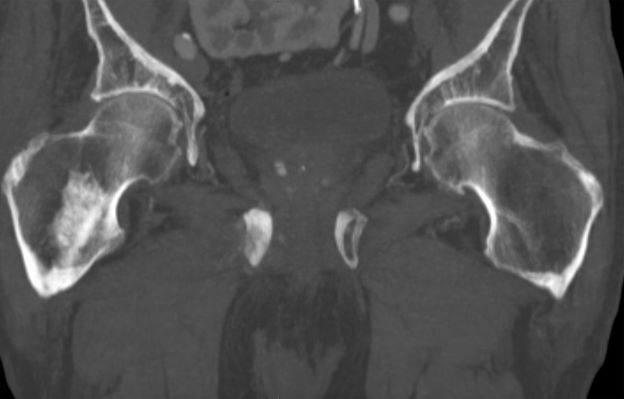
Sudden onset of severe pain in left thigh
On the basis of the patient's physical examination, laboratory findings, and radiographic findings, a diagnosis of de novo metastatic prostate cancer is suspected and later confirmed by transrectal ultrasonography–guided needle biopsy of the prostate.
Prostate cancer is the most common cancer and the second most common cause of cancer-associated death in men in the United States. Among men diagnosed with prostate cancer in the United States, approximately three quarters have localized-stage disease at diagnosis; however, recent data show that an increasing number and percentage of men are being diagnosed with distant-stage prostate cancer. Despite advancements in treatment, less than one third of men survive 5 years after the diagnosis of distant-stage prostate cancer.
Prostate cancer frequently metastasizes to the bone. In fact, as many as 90% of patients with advanced prostate cancer have bone involvement. The morbidity from bone metastases is considerable and includes bone pain, immobility, pathologic fractures, hypercalcemia, hematologic disorders, and spinal cord compression. Bone metastases also have a considerable impact on mortality.
In patients with metastatic prostate cancer, determining the presence and extent of metastatic disease is essential for appropriate treatment to be selected. Studies have shown that the extent of metastatic disease affects treatment response. In a recent exploratory analysis of the STAMPEDE trial, survival benefit associated with prostate radiation therapy decreased continuously as the number of bone metastases rose, with the most benefit being seen in patients with up to three bone metastases.
Guidelines recommend that imaging studies be conducted in all patients with advanced prostate cancer. This may include conventional imaging (ie, CT, bone scan, and/or prostate MRI) and/or next-generation imaging (ie, PET, PET/CT, PET/MRI, whole-body MRI). In cases involving hormone-sensitive disease with obvious metastatic disease on conventional imaging at presentation, next-generation imaging may be useful for illuminating the disease burden and possibly shifting the treatment intent from multimodality management of oligometastatic disease to systemic anticancer therapy, either alone or in combination with targeted therapy for palliative purposes. However, prospective data on this are lacking.
Clinicians should also assess symptoms in patients with metastatic hormone-sensitive prostate cancer at presentation, because symptoms have been shown to have prognostic value. In addition, understanding symptoms related to cancer is essential for optimizing pain and other symptom management in addition to anticancer therapy.
Metastatic prostate cancer remains incurable. Immediate systemic treatment with androgen deprivation therapy (ADT) combined with abiraterone acetate plus prednisone or apalutamide or enzalutamide should be offered to symptomatic patients who have distant metastases on diagnosis, both to alleviate symptoms and to lessen the risk for potential serious complications, such as spinal cord compression. ADT combined with docetaxel can also be offered to patients who are able to tolerate docetaxel.
ADT combined with prostate radiation therapy may be offered to patients with distant metastases and low-volume disease. However, when patients present with high-volume disease, referral to a clinical trial is recommended.
Surgery and/or local radiation therapy can be considered for patients with distant metastases and evidence of impending complications (eg, spinal cord compression or pathologic fracture).
Kyle A. Richards, MD, Assistant Professor, Department of Urology, University of Wisconsin-Madison; Chief of Urology, William S. Middleton Memorial VA Hospital, Madison, Wisconsin.
Kyle A. Richards, MD, has disclosed no relevant financial relationships.
On the basis of the patient's physical examination, laboratory findings, and radiographic findings, a diagnosis of de novo metastatic prostate cancer is suspected and later confirmed by transrectal ultrasonography–guided needle biopsy of the prostate.
Prostate cancer is the most common cancer and the second most common cause of cancer-associated death in men in the United States. Among men diagnosed with prostate cancer in the United States, approximately three quarters have localized-stage disease at diagnosis; however, recent data show that an increasing number and percentage of men are being diagnosed with distant-stage prostate cancer. Despite advancements in treatment, less than one third of men survive 5 years after the diagnosis of distant-stage prostate cancer.
Prostate cancer frequently metastasizes to the bone. In fact, as many as 90% of patients with advanced prostate cancer have bone involvement. The morbidity from bone metastases is considerable and includes bone pain, immobility, pathologic fractures, hypercalcemia, hematologic disorders, and spinal cord compression. Bone metastases also have a considerable impact on mortality.
In patients with metastatic prostate cancer, determining the presence and extent of metastatic disease is essential for appropriate treatment to be selected. Studies have shown that the extent of metastatic disease affects treatment response. In a recent exploratory analysis of the STAMPEDE trial, survival benefit associated with prostate radiation therapy decreased continuously as the number of bone metastases rose, with the most benefit being seen in patients with up to three bone metastases.
Guidelines recommend that imaging studies be conducted in all patients with advanced prostate cancer. This may include conventional imaging (ie, CT, bone scan, and/or prostate MRI) and/or next-generation imaging (ie, PET, PET/CT, PET/MRI, whole-body MRI). In cases involving hormone-sensitive disease with obvious metastatic disease on conventional imaging at presentation, next-generation imaging may be useful for illuminating the disease burden and possibly shifting the treatment intent from multimodality management of oligometastatic disease to systemic anticancer therapy, either alone or in combination with targeted therapy for palliative purposes. However, prospective data on this are lacking.
Clinicians should also assess symptoms in patients with metastatic hormone-sensitive prostate cancer at presentation, because symptoms have been shown to have prognostic value. In addition, understanding symptoms related to cancer is essential for optimizing pain and other symptom management in addition to anticancer therapy.
Metastatic prostate cancer remains incurable. Immediate systemic treatment with androgen deprivation therapy (ADT) combined with abiraterone acetate plus prednisone or apalutamide or enzalutamide should be offered to symptomatic patients who have distant metastases on diagnosis, both to alleviate symptoms and to lessen the risk for potential serious complications, such as spinal cord compression. ADT combined with docetaxel can also be offered to patients who are able to tolerate docetaxel.
ADT combined with prostate radiation therapy may be offered to patients with distant metastases and low-volume disease. However, when patients present with high-volume disease, referral to a clinical trial is recommended.
Surgery and/or local radiation therapy can be considered for patients with distant metastases and evidence of impending complications (eg, spinal cord compression or pathologic fracture).
Kyle A. Richards, MD, Assistant Professor, Department of Urology, University of Wisconsin-Madison; Chief of Urology, William S. Middleton Memorial VA Hospital, Madison, Wisconsin.
Kyle A. Richards, MD, has disclosed no relevant financial relationships.
On the basis of the patient's physical examination, laboratory findings, and radiographic findings, a diagnosis of de novo metastatic prostate cancer is suspected and later confirmed by transrectal ultrasonography–guided needle biopsy of the prostate.
Prostate cancer is the most common cancer and the second most common cause of cancer-associated death in men in the United States. Among men diagnosed with prostate cancer in the United States, approximately three quarters have localized-stage disease at diagnosis; however, recent data show that an increasing number and percentage of men are being diagnosed with distant-stage prostate cancer. Despite advancements in treatment, less than one third of men survive 5 years after the diagnosis of distant-stage prostate cancer.
Prostate cancer frequently metastasizes to the bone. In fact, as many as 90% of patients with advanced prostate cancer have bone involvement. The morbidity from bone metastases is considerable and includes bone pain, immobility, pathologic fractures, hypercalcemia, hematologic disorders, and spinal cord compression. Bone metastases also have a considerable impact on mortality.
In patients with metastatic prostate cancer, determining the presence and extent of metastatic disease is essential for appropriate treatment to be selected. Studies have shown that the extent of metastatic disease affects treatment response. In a recent exploratory analysis of the STAMPEDE trial, survival benefit associated with prostate radiation therapy decreased continuously as the number of bone metastases rose, with the most benefit being seen in patients with up to three bone metastases.
Guidelines recommend that imaging studies be conducted in all patients with advanced prostate cancer. This may include conventional imaging (ie, CT, bone scan, and/or prostate MRI) and/or next-generation imaging (ie, PET, PET/CT, PET/MRI, whole-body MRI). In cases involving hormone-sensitive disease with obvious metastatic disease on conventional imaging at presentation, next-generation imaging may be useful for illuminating the disease burden and possibly shifting the treatment intent from multimodality management of oligometastatic disease to systemic anticancer therapy, either alone or in combination with targeted therapy for palliative purposes. However, prospective data on this are lacking.
Clinicians should also assess symptoms in patients with metastatic hormone-sensitive prostate cancer at presentation, because symptoms have been shown to have prognostic value. In addition, understanding symptoms related to cancer is essential for optimizing pain and other symptom management in addition to anticancer therapy.
Metastatic prostate cancer remains incurable. Immediate systemic treatment with androgen deprivation therapy (ADT) combined with abiraterone acetate plus prednisone or apalutamide or enzalutamide should be offered to symptomatic patients who have distant metastases on diagnosis, both to alleviate symptoms and to lessen the risk for potential serious complications, such as spinal cord compression. ADT combined with docetaxel can also be offered to patients who are able to tolerate docetaxel.
ADT combined with prostate radiation therapy may be offered to patients with distant metastases and low-volume disease. However, when patients present with high-volume disease, referral to a clinical trial is recommended.
Surgery and/or local radiation therapy can be considered for patients with distant metastases and evidence of impending complications (eg, spinal cord compression or pathologic fracture).
Kyle A. Richards, MD, Assistant Professor, Department of Urology, University of Wisconsin-Madison; Chief of Urology, William S. Middleton Memorial VA Hospital, Madison, Wisconsin.
Kyle A. Richards, MD, has disclosed no relevant financial relationships.
A 74-year-old man presents to the emergency department with sudden onset of severe pain in his left thigh, a 3-month history of unexplained weight loss and general weakness, and progressive difficulty in walking for the past 3 weeks. The patient states that he has always been in excellent health and he has not seen a physician in at least 10 years. Cachexia is noted on physical examination. Laboratory findings include hemoglobin, 12.4 g/dL; white blood cells, 8.12 cells/µL; platelets, 310,000 cells/µL; creatinine, 1.4 mg/dL; sodium, 137 mmol/L; potassium, 4.4 mmol/L; calcium, 10.1 mg/dL; prostate-specific antigen, 31 ng/mL; aspartate aminotransferase, 37 IU/L; and gamma-glutamyltransferase, 16 IU/L. Proteinuria and hematuria are detected by urinalysis. CT reveals multiple diffuse osteoblastic lesions in the right proximal femur.
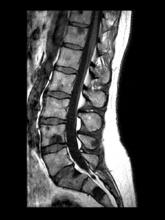
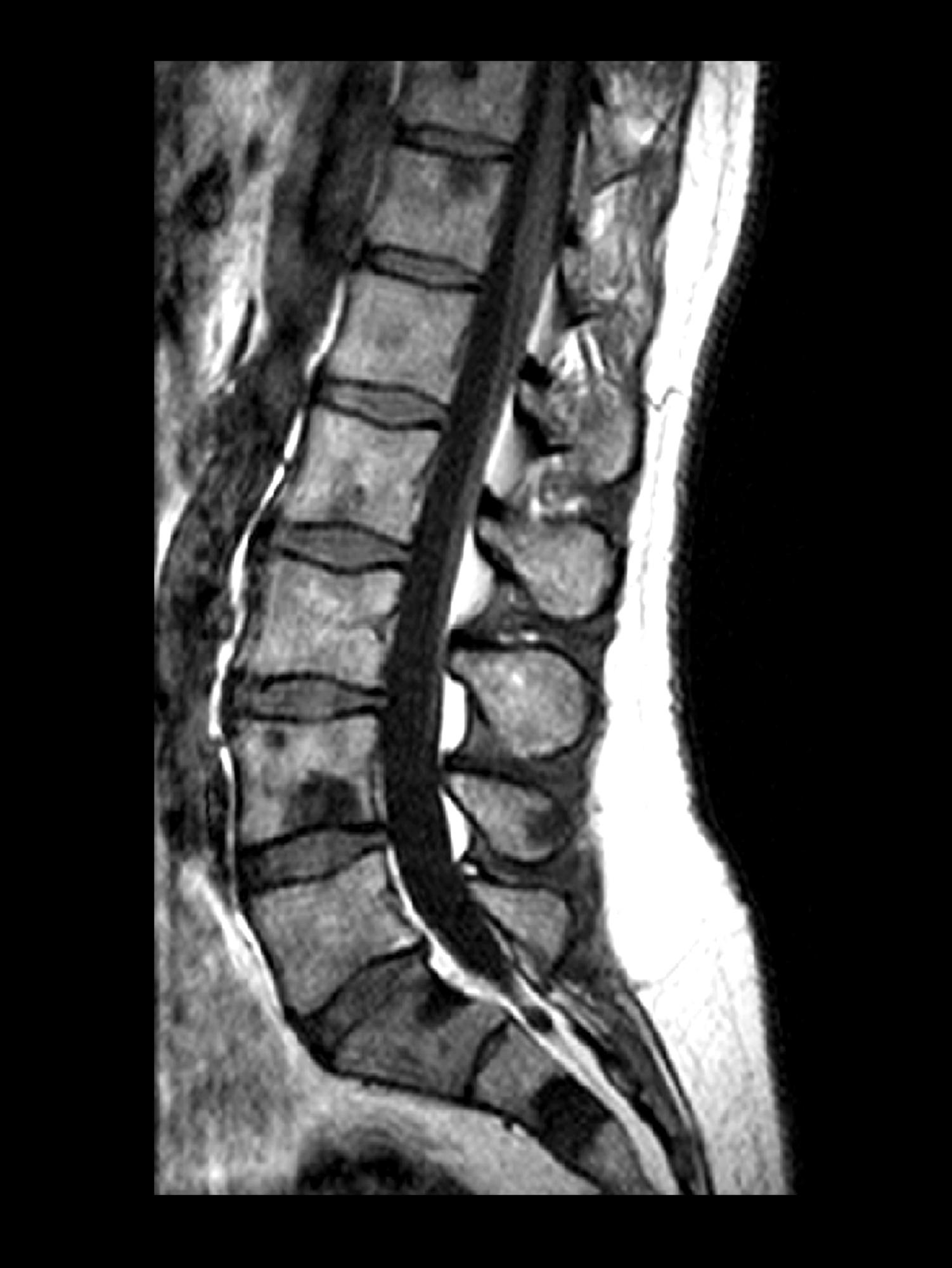
Intermittent fever and gradually progressive low back pain
Transrectal ultrasonography–guided needle biopsy of the prostate confirms a diagnosis of metastatic prostate cancer.
With the exception of nonmelanoma skin cancer, prostate cancer is the most commonly occurring cancer and the second most common cause of cancer-associated mortality in men in the United States. Most patients have localized stage at diagnosis; however, the incidence of distant-stage prostate cancer at diagnosis is steadily increasing. Five-year survival for distant-stage prostate cancer is approximately 32%.
High serum levels of PSA have been associated with bone metastases in men with prostate cancer, and the presence of metastatic disease increases with rising PSA levels. Over the past several decades, PSA levels > 100 ng/mL have been used as a marker for metastatic prostate cancer. However, not all men with metastatic prostate cancer will have elevated PSA levels, and bone imaging is necessary for correct staging and treatment stratification.
Bone metastases occur in approximately 70% of men with advanced prostate cancer, most often in the spine, and are a leading cause of morbidity and mortality. Bone metastases can cause severe pain, particularly in the evening; decreased mobility; pathologic fractures; spinal cord compression; bone marrow aplasia; and hypercalcemia.
The bone marrow represents a fertile soil into which prostate tumors can colonize and proliferate. Such colonization by prostate tumor cells is commonly associated with tumor-induced bone lesions, which typically arise from an imbalance between bone-forming osteoblasts and bone-absorbing osteoclasts generated by prostate cancer cells. Whereas most solid tumors, such as breast cancer and melanoma, have a propensity for causing osteolytic lesions with excessive bone resorption, bone lesions resulting from prostate cancer are largely osteoblastic and are associated with uncontrolled low-quality bone formation. The resultant metastases have a unique bone formation that can be detected by plain radiography, bone scan, bone biopsy, and increased serum alkaline phosphatase levels.
CT; skeletal scintigraphy and PET; and single-photon emission CT (SPECT)/CT, PET/CT, and PET/MRI are recommended diagnostics for men at risk for prostate cancer metastasis. Radiotracer-based PET, which mainly uses altered metabolic activity or explicitly overexpressed receptors, is a promising diagnostic modality. However, the choice of a respective radiotracer must be carefully considered because a single radiotracer is typically insufficient to visualize all clinical stages of prostate cancer. In addition, its use is reliant on the extent of malignant tissue, tumor heterogeneity, and previous treatments.
Systemic androgen-deprivation therapy, with or without docetaxel-based chemotherapy, is the standard of care for metastatic prostate cancer. Treatment is largely directed at preventing skeletal-related events and providing pain management.
Radium-223 is the only available therapy for castrate-resistant prostate cancer that specifically targets bone metastases, delays development of skeletal-related events, and improves survival. Based on the results of the ALSYMPCA study, radium-223 in combination with systemic therapies is now considered an effective, efficient, and well-tolerated therapy for castrate-resistant prostate cancer with bone lesions.
The effects of local radiation therapy for men with metastatic prostate cancer and the optimal combination of systemic therapies in the metastatic setting are still under investigation.
Kyle A. Richards, MD, Assistant Professor, Department of Urology, University of Wisconsin-Madison; Chief of Urology, William S. Middleton Memorial VA Hospital, Madison, Wisconsin.
Kyle A. Richards, MD, has disclosed no relevant financial relationships.
Transrectal ultrasonography–guided needle biopsy of the prostate confirms a diagnosis of metastatic prostate cancer.
With the exception of nonmelanoma skin cancer, prostate cancer is the most commonly occurring cancer and the second most common cause of cancer-associated mortality in men in the United States. Most patients have localized stage at diagnosis; however, the incidence of distant-stage prostate cancer at diagnosis is steadily increasing. Five-year survival for distant-stage prostate cancer is approximately 32%.
High serum levels of PSA have been associated with bone metastases in men with prostate cancer, and the presence of metastatic disease increases with rising PSA levels. Over the past several decades, PSA levels > 100 ng/mL have been used as a marker for metastatic prostate cancer. However, not all men with metastatic prostate cancer will have elevated PSA levels, and bone imaging is necessary for correct staging and treatment stratification.
Bone metastases occur in approximately 70% of men with advanced prostate cancer, most often in the spine, and are a leading cause of morbidity and mortality. Bone metastases can cause severe pain, particularly in the evening; decreased mobility; pathologic fractures; spinal cord compression; bone marrow aplasia; and hypercalcemia.
The bone marrow represents a fertile soil into which prostate tumors can colonize and proliferate. Such colonization by prostate tumor cells is commonly associated with tumor-induced bone lesions, which typically arise from an imbalance between bone-forming osteoblasts and bone-absorbing osteoclasts generated by prostate cancer cells. Whereas most solid tumors, such as breast cancer and melanoma, have a propensity for causing osteolytic lesions with excessive bone resorption, bone lesions resulting from prostate cancer are largely osteoblastic and are associated with uncontrolled low-quality bone formation. The resultant metastases have a unique bone formation that can be detected by plain radiography, bone scan, bone biopsy, and increased serum alkaline phosphatase levels.
CT; skeletal scintigraphy and PET; and single-photon emission CT (SPECT)/CT, PET/CT, and PET/MRI are recommended diagnostics for men at risk for prostate cancer metastasis. Radiotracer-based PET, which mainly uses altered metabolic activity or explicitly overexpressed receptors, is a promising diagnostic modality. However, the choice of a respective radiotracer must be carefully considered because a single radiotracer is typically insufficient to visualize all clinical stages of prostate cancer. In addition, its use is reliant on the extent of malignant tissue, tumor heterogeneity, and previous treatments.
Systemic androgen-deprivation therapy, with or without docetaxel-based chemotherapy, is the standard of care for metastatic prostate cancer. Treatment is largely directed at preventing skeletal-related events and providing pain management.
Radium-223 is the only available therapy for castrate-resistant prostate cancer that specifically targets bone metastases, delays development of skeletal-related events, and improves survival. Based on the results of the ALSYMPCA study, radium-223 in combination with systemic therapies is now considered an effective, efficient, and well-tolerated therapy for castrate-resistant prostate cancer with bone lesions.
The effects of local radiation therapy for men with metastatic prostate cancer and the optimal combination of systemic therapies in the metastatic setting are still under investigation.
Kyle A. Richards, MD, Assistant Professor, Department of Urology, University of Wisconsin-Madison; Chief of Urology, William S. Middleton Memorial VA Hospital, Madison, Wisconsin.
Kyle A. Richards, MD, has disclosed no relevant financial relationships.
Transrectal ultrasonography–guided needle biopsy of the prostate confirms a diagnosis of metastatic prostate cancer.
With the exception of nonmelanoma skin cancer, prostate cancer is the most commonly occurring cancer and the second most common cause of cancer-associated mortality in men in the United States. Most patients have localized stage at diagnosis; however, the incidence of distant-stage prostate cancer at diagnosis is steadily increasing. Five-year survival for distant-stage prostate cancer is approximately 32%.
High serum levels of PSA have been associated with bone metastases in men with prostate cancer, and the presence of metastatic disease increases with rising PSA levels. Over the past several decades, PSA levels > 100 ng/mL have been used as a marker for metastatic prostate cancer. However, not all men with metastatic prostate cancer will have elevated PSA levels, and bone imaging is necessary for correct staging and treatment stratification.
Bone metastases occur in approximately 70% of men with advanced prostate cancer, most often in the spine, and are a leading cause of morbidity and mortality. Bone metastases can cause severe pain, particularly in the evening; decreased mobility; pathologic fractures; spinal cord compression; bone marrow aplasia; and hypercalcemia.
The bone marrow represents a fertile soil into which prostate tumors can colonize and proliferate. Such colonization by prostate tumor cells is commonly associated with tumor-induced bone lesions, which typically arise from an imbalance between bone-forming osteoblasts and bone-absorbing osteoclasts generated by prostate cancer cells. Whereas most solid tumors, such as breast cancer and melanoma, have a propensity for causing osteolytic lesions with excessive bone resorption, bone lesions resulting from prostate cancer are largely osteoblastic and are associated with uncontrolled low-quality bone formation. The resultant metastases have a unique bone formation that can be detected by plain radiography, bone scan, bone biopsy, and increased serum alkaline phosphatase levels.
CT; skeletal scintigraphy and PET; and single-photon emission CT (SPECT)/CT, PET/CT, and PET/MRI are recommended diagnostics for men at risk for prostate cancer metastasis. Radiotracer-based PET, which mainly uses altered metabolic activity or explicitly overexpressed receptors, is a promising diagnostic modality. However, the choice of a respective radiotracer must be carefully considered because a single radiotracer is typically insufficient to visualize all clinical stages of prostate cancer. In addition, its use is reliant on the extent of malignant tissue, tumor heterogeneity, and previous treatments.
Systemic androgen-deprivation therapy, with or without docetaxel-based chemotherapy, is the standard of care for metastatic prostate cancer. Treatment is largely directed at preventing skeletal-related events and providing pain management.
Radium-223 is the only available therapy for castrate-resistant prostate cancer that specifically targets bone metastases, delays development of skeletal-related events, and improves survival. Based on the results of the ALSYMPCA study, radium-223 in combination with systemic therapies is now considered an effective, efficient, and well-tolerated therapy for castrate-resistant prostate cancer with bone lesions.
The effects of local radiation therapy for men with metastatic prostate cancer and the optimal combination of systemic therapies in the metastatic setting are still under investigation.
Kyle A. Richards, MD, Assistant Professor, Department of Urology, University of Wisconsin-Madison; Chief of Urology, William S. Middleton Memorial VA Hospital, Madison, Wisconsin.
Kyle A. Richards, MD, has disclosed no relevant financial relationships.
A 71-year-old homeless man presents to the emergency department (ED) with intermittent fever, gradually progressive low back pain restricting physical activities and movement, fatigue, exertional dyspnea, and poor appetite. The patient has been seen in the same ED sporadically over the years for various problems, and his medical history is notable for chronic obstructive pulmonary disease, tobacco use, alcoholism, and foot infections. Physical examination findings include tenderness to percussion over the thoracic and lumbar spine and a mildly enlarged prostate that appears to be smooth, normal in texture, and lacking nodules on digital rectal exam. Complete blood cell count and chemistry panel are normal. Both alkaline phosphatase and prostate-specific antigen (PSA) levels are elevated, at 240 U/L and 115 ng/mL, respectively. Urinalysis shows hematuria. CT shows osteolytic lesions in the patient's lumbar spine and femur.
Clinical Edge Journal Scan Commentary: Prostate Cancer June 2021
Due to the heterogeneity of outcomes and study designs, consensus on definitive prostate cancer risk assessment has been somewhat elusive. Differences in outcomes based on ethnicity and race have been observed, but much data on risk has originally been obtained in populations with lower ethnic and racial diversity, complicating extrapolation to larger populations. Huynh-Le et al developed an updated polygenic hazard score (PHS2) based on a single nucleotide polymorphism (SNP) panel (46 total SNPs) for prostate cancer patients with multiple ethnicities (African, Asian, and European ancestries). This updated PHS2 score stratified men into higher and lower risks for any, aggressive, and fatal prostate cancers in a statistically significant way. Camargo et al took a different approach and evaluated whether 2 SNPs were prognostic in prostate cancer: rs1834306 corresponding to microRNA 100 (miR 100) and rs2910164 from miR 146a. There were no differences in miR 100 or miR 156a between patients with local prostate cancer or a control group of men without prostate cancer. In addition, there were no differences in the chance of particular genotypes between the 2 groups. There was an association between lower presence of rs1834306 (miR 100) and patients with PSA > 10 mg/mL and between a higher amount of the polymorphic allele for rs2910164 (miR 146A).
The 3 studies summarized here demonstrate the ongoing challenges in how to identify nuances that will affect clinical decision-making in PSA screening and to identify prognostic features that associate with particular outcomes. The study by Bergengren confirmed the current state of PSA screening in that balancing diagnosis and potential overtreatment with modest survival outcomes is challenging. While the studies by Huynh-Le et al and Camargo et al have interesting findings, the use of SNPs and miR in prostate cancer prognosis is still not ready for routine clinical use in prostate cancer management.
Due to the heterogeneity of outcomes and study designs, consensus on definitive prostate cancer risk assessment has been somewhat elusive. Differences in outcomes based on ethnicity and race have been observed, but much data on risk has originally been obtained in populations with lower ethnic and racial diversity, complicating extrapolation to larger populations. Huynh-Le et al developed an updated polygenic hazard score (PHS2) based on a single nucleotide polymorphism (SNP) panel (46 total SNPs) for prostate cancer patients with multiple ethnicities (African, Asian, and European ancestries). This updated PHS2 score stratified men into higher and lower risks for any, aggressive, and fatal prostate cancers in a statistically significant way. Camargo et al took a different approach and evaluated whether 2 SNPs were prognostic in prostate cancer: rs1834306 corresponding to microRNA 100 (miR 100) and rs2910164 from miR 146a. There were no differences in miR 100 or miR 156a between patients with local prostate cancer or a control group of men without prostate cancer. In addition, there were no differences in the chance of particular genotypes between the 2 groups. There was an association between lower presence of rs1834306 (miR 100) and patients with PSA > 10 mg/mL and between a higher amount of the polymorphic allele for rs2910164 (miR 146A).
The 3 studies summarized here demonstrate the ongoing challenges in how to identify nuances that will affect clinical decision-making in PSA screening and to identify prognostic features that associate with particular outcomes. The study by Bergengren confirmed the current state of PSA screening in that balancing diagnosis and potential overtreatment with modest survival outcomes is challenging. While the studies by Huynh-Le et al and Camargo et al have interesting findings, the use of SNPs and miR in prostate cancer prognosis is still not ready for routine clinical use in prostate cancer management.
Due to the heterogeneity of outcomes and study designs, consensus on definitive prostate cancer risk assessment has been somewhat elusive. Differences in outcomes based on ethnicity and race have been observed, but much data on risk has originally been obtained in populations with lower ethnic and racial diversity, complicating extrapolation to larger populations. Huynh-Le et al developed an updated polygenic hazard score (PHS2) based on a single nucleotide polymorphism (SNP) panel (46 total SNPs) for prostate cancer patients with multiple ethnicities (African, Asian, and European ancestries). This updated PHS2 score stratified men into higher and lower risks for any, aggressive, and fatal prostate cancers in a statistically significant way. Camargo et al took a different approach and evaluated whether 2 SNPs were prognostic in prostate cancer: rs1834306 corresponding to microRNA 100 (miR 100) and rs2910164 from miR 146a. There were no differences in miR 100 or miR 156a between patients with local prostate cancer or a control group of men without prostate cancer. In addition, there were no differences in the chance of particular genotypes between the 2 groups. There was an association between lower presence of rs1834306 (miR 100) and patients with PSA > 10 mg/mL and between a higher amount of the polymorphic allele for rs2910164 (miR 146A).
The 3 studies summarized here demonstrate the ongoing challenges in how to identify nuances that will affect clinical decision-making in PSA screening and to identify prognostic features that associate with particular outcomes. The study by Bergengren confirmed the current state of PSA screening in that balancing diagnosis and potential overtreatment with modest survival outcomes is challenging. While the studies by Huynh-Le et al and Camargo et al have interesting findings, the use of SNPs and miR in prostate cancer prognosis is still not ready for routine clinical use in prostate cancer management.
SNPs show predictive value for prostate cancer
Key clinical point: Single nucleotide polymorphisms (SNP) can serve as prognostic factors for prostate cancer
Major finding: In patients with prostate cancer, SNP rs2910164 of miR146a was more common in patients with a Gleason score of 7 or higher than those with a Gleason score of less than 7 (P=0.043); in addition, SNP rs1834306 of miR100 was overexpressed in those with pT3 staging compared to pT2 (P = 0.004).
Study details: The data come from a review of 100 patients with clinically localized prostate cancer who underwent radical prostatectomy and 68 controls without prostate cancer.
Disclosures: The study was supported in part by a grant from the Sao Paulo Research Foundation. The researchers had no financial conflicts to disclose.
Source: Camargo JA et al. Int J Biol Markers. 2021 May 25. doi: 10.1177/172460082199746.
Key clinical point: Single nucleotide polymorphisms (SNP) can serve as prognostic factors for prostate cancer
Major finding: In patients with prostate cancer, SNP rs2910164 of miR146a was more common in patients with a Gleason score of 7 or higher than those with a Gleason score of less than 7 (P=0.043); in addition, SNP rs1834306 of miR100 was overexpressed in those with pT3 staging compared to pT2 (P = 0.004).
Study details: The data come from a review of 100 patients with clinically localized prostate cancer who underwent radical prostatectomy and 68 controls without prostate cancer.
Disclosures: The study was supported in part by a grant from the Sao Paulo Research Foundation. The researchers had no financial conflicts to disclose.
Source: Camargo JA et al. Int J Biol Markers. 2021 May 25. doi: 10.1177/172460082199746.
Key clinical point: Single nucleotide polymorphisms (SNP) can serve as prognostic factors for prostate cancer
Major finding: In patients with prostate cancer, SNP rs2910164 of miR146a was more common in patients with a Gleason score of 7 or higher than those with a Gleason score of less than 7 (P=0.043); in addition, SNP rs1834306 of miR100 was overexpressed in those with pT3 staging compared to pT2 (P = 0.004).
Study details: The data come from a review of 100 patients with clinically localized prostate cancer who underwent radical prostatectomy and 68 controls without prostate cancer.
Disclosures: The study was supported in part by a grant from the Sao Paulo Research Foundation. The researchers had no financial conflicts to disclose.
Source: Camargo JA et al. Int J Biol Markers. 2021 May 25. doi: 10.1177/172460082199746.
Greater bone involvement curbs survival after radioligand prostate cancer therapy
Key clinical point: Increased bone involvement was negatively correlated with overall survival after radioligand therapy for prostate cancer.
Major finding: The median overall survival for prostate cancer patients with less than 6 bone lesions, 6-20 lesions, more than 20 lesions, and diffuse lesions was 18 months, 13 months, 11 months, and 8 months, respectively.
Study details: The data come from 319 men with progressive metastatic castration-resistant prostate cancer who underwent radioligand therapy with lutetium prostate-specific membrane antigen (Lu-PSMA-617).
Disclosures: The study received no outside funding. The researchers had no financial conflicts to disclose.
Source: Ahmadzadehfar H et al. Eur J Nucl Med Mol Imaging. 2021 May 25. doi: 10.1007/s00259-021-05383-3.
Key clinical point: Increased bone involvement was negatively correlated with overall survival after radioligand therapy for prostate cancer.
Major finding: The median overall survival for prostate cancer patients with less than 6 bone lesions, 6-20 lesions, more than 20 lesions, and diffuse lesions was 18 months, 13 months, 11 months, and 8 months, respectively.
Study details: The data come from 319 men with progressive metastatic castration-resistant prostate cancer who underwent radioligand therapy with lutetium prostate-specific membrane antigen (Lu-PSMA-617).
Disclosures: The study received no outside funding. The researchers had no financial conflicts to disclose.
Source: Ahmadzadehfar H et al. Eur J Nucl Med Mol Imaging. 2021 May 25. doi: 10.1007/s00259-021-05383-3.
Key clinical point: Increased bone involvement was negatively correlated with overall survival after radioligand therapy for prostate cancer.
Major finding: The median overall survival for prostate cancer patients with less than 6 bone lesions, 6-20 lesions, more than 20 lesions, and diffuse lesions was 18 months, 13 months, 11 months, and 8 months, respectively.
Study details: The data come from 319 men with progressive metastatic castration-resistant prostate cancer who underwent radioligand therapy with lutetium prostate-specific membrane antigen (Lu-PSMA-617).
Disclosures: The study received no outside funding. The researchers had no financial conflicts to disclose.
Source: Ahmadzadehfar H et al. Eur J Nucl Med Mol Imaging. 2021 May 25. doi: 10.1007/s00259-021-05383-3.
Polygenic hazard score predicts prostate cancer
Key clinical point: The Polygenic Hazard Score (PHS2) was effective for risk-stratifying men for prostate cancer in a large, multiethnic data set.
Major finding: The Polygenic Hazard Score (PHS2) indicated hazard ratios for prostate cancer, aggressive cancer, and prostate cancer-specific death of 5.32, 5.88, and 5.68, respectively, when researchers compared the 80th and 20th PHS2 percentiles.
Study details: The data come from 80,491 men enrolled in the OncoArray genetic project; researchers tested the polygenic hazard score (PHS2, adapted for OncoArray) for association with age at diagnosis of any and aggressive prostate cancer.
Disclosures: The study was supported in part by the National Institutes of Health/National Institute of Biomedical Imaging and Bioengineering, the United States Department of Defense, the University of California, the Research Council of Norway, K.G. Jebsen Stiftelsen, and South East Norway Health Authority. Lead author Dr. Huynh-Le had no financial conflicts to disclose.
Source: Huynh-Le M-P et al. Nat Commun. 2021 Feb 23. doi: 10.1038/s41467-021-21287-0.
Key clinical point: The Polygenic Hazard Score (PHS2) was effective for risk-stratifying men for prostate cancer in a large, multiethnic data set.
Major finding: The Polygenic Hazard Score (PHS2) indicated hazard ratios for prostate cancer, aggressive cancer, and prostate cancer-specific death of 5.32, 5.88, and 5.68, respectively, when researchers compared the 80th and 20th PHS2 percentiles.
Study details: The data come from 80,491 men enrolled in the OncoArray genetic project; researchers tested the polygenic hazard score (PHS2, adapted for OncoArray) for association with age at diagnosis of any and aggressive prostate cancer.
Disclosures: The study was supported in part by the National Institutes of Health/National Institute of Biomedical Imaging and Bioengineering, the United States Department of Defense, the University of California, the Research Council of Norway, K.G. Jebsen Stiftelsen, and South East Norway Health Authority. Lead author Dr. Huynh-Le had no financial conflicts to disclose.
Source: Huynh-Le M-P et al. Nat Commun. 2021 Feb 23. doi: 10.1038/s41467-021-21287-0.
Key clinical point: The Polygenic Hazard Score (PHS2) was effective for risk-stratifying men for prostate cancer in a large, multiethnic data set.
Major finding: The Polygenic Hazard Score (PHS2) indicated hazard ratios for prostate cancer, aggressive cancer, and prostate cancer-specific death of 5.32, 5.88, and 5.68, respectively, when researchers compared the 80th and 20th PHS2 percentiles.
Study details: The data come from 80,491 men enrolled in the OncoArray genetic project; researchers tested the polygenic hazard score (PHS2, adapted for OncoArray) for association with age at diagnosis of any and aggressive prostate cancer.
Disclosures: The study was supported in part by the National Institutes of Health/National Institute of Biomedical Imaging and Bioengineering, the United States Department of Defense, the University of California, the Research Council of Norway, K.G. Jebsen Stiftelsen, and South East Norway Health Authority. Lead author Dr. Huynh-Le had no financial conflicts to disclose.
Source: Huynh-Le M-P et al. Nat Commun. 2021 Feb 23. doi: 10.1038/s41467-021-21287-0.
Simulation shows impact of increased PSA testing on prostate cancer diagnosis
Key clinical point: Increased use of PSA testing and diagnostic activity increased the number of men diagnosed with low and intermediate-risk prostate cancer.
Major finding: The number of men diagnosed with prostate cancer increased by 48% in the high diagnostic activity model compared to the low diagnostic activity model (423 cases per 100,000 me per year vs. 286 cases per 100,000 men per year).
Study details: The data come from a cohort study of 188,884 men aged 64-77 years diagnosed with prostate cancer between 1996 and 2016 in Sweden. The researchers used a simulation model to compare scenarios of high and low diagnostic activity for prostate cancer.
Disclosures: The study was funded by the Swedish Cancer Society. The researchers had no financial conflicts to disclose.
Source: Bergengren O et al. JAMA Netw Open. 2021 May 17. doi: 10.1001/jamanetworkopen.2021.9444.
Key clinical point: Increased use of PSA testing and diagnostic activity increased the number of men diagnosed with low and intermediate-risk prostate cancer.
Major finding: The number of men diagnosed with prostate cancer increased by 48% in the high diagnostic activity model compared to the low diagnostic activity model (423 cases per 100,000 me per year vs. 286 cases per 100,000 men per year).
Study details: The data come from a cohort study of 188,884 men aged 64-77 years diagnosed with prostate cancer between 1996 and 2016 in Sweden. The researchers used a simulation model to compare scenarios of high and low diagnostic activity for prostate cancer.
Disclosures: The study was funded by the Swedish Cancer Society. The researchers had no financial conflicts to disclose.
Source: Bergengren O et al. JAMA Netw Open. 2021 May 17. doi: 10.1001/jamanetworkopen.2021.9444.
Key clinical point: Increased use of PSA testing and diagnostic activity increased the number of men diagnosed with low and intermediate-risk prostate cancer.
Major finding: The number of men diagnosed with prostate cancer increased by 48% in the high diagnostic activity model compared to the low diagnostic activity model (423 cases per 100,000 me per year vs. 286 cases per 100,000 men per year).
Study details: The data come from a cohort study of 188,884 men aged 64-77 years diagnosed with prostate cancer between 1996 and 2016 in Sweden. The researchers used a simulation model to compare scenarios of high and low diagnostic activity for prostate cancer.
Disclosures: The study was funded by the Swedish Cancer Society. The researchers had no financial conflicts to disclose.
Source: Bergengren O et al. JAMA Netw Open. 2021 May 17. doi: 10.1001/jamanetworkopen.2021.9444.
PSMA therapy promotes similar prostate cancer survival regardless of regimen
Key clinical point: Treatment with lutetium prostate-specific membrane antigen (Lu-PSMA) therapy at 6.0 GBq and 7.4 GBq yielded similar PSA response rates and overall survival for patients with progressive metastatic castrate resistant prostate cancer.
Major finding: The primary endpoint of PSA response of at least a 50% reduction from baseline after 2 treatment cycles was met in 28% of the whole cohort, and 46% and 19%, respectively, for treatment regimens of 6.0 GBq and 7.4 GBq.
Study details: The data come from a prospective, phase II trial of 71 men with progressive, metastatic castrate resistant prostate cancer. The patients were randomized to Lu-PSMA therapy at doses of either 6.0 vs 7.4 GBq.
Disclosures: The original study was supported by Endocyte. The researchers had no financial conflicts to disclose.
Source: Calais J. et al. J Nucl Med. 2021 May 20. doi: 10.2967/jnumed.121.261982.
Key clinical point: Treatment with lutetium prostate-specific membrane antigen (Lu-PSMA) therapy at 6.0 GBq and 7.4 GBq yielded similar PSA response rates and overall survival for patients with progressive metastatic castrate resistant prostate cancer.
Major finding: The primary endpoint of PSA response of at least a 50% reduction from baseline after 2 treatment cycles was met in 28% of the whole cohort, and 46% and 19%, respectively, for treatment regimens of 6.0 GBq and 7.4 GBq.
Study details: The data come from a prospective, phase II trial of 71 men with progressive, metastatic castrate resistant prostate cancer. The patients were randomized to Lu-PSMA therapy at doses of either 6.0 vs 7.4 GBq.
Disclosures: The original study was supported by Endocyte. The researchers had no financial conflicts to disclose.
Source: Calais J. et al. J Nucl Med. 2021 May 20. doi: 10.2967/jnumed.121.261982.
Key clinical point: Treatment with lutetium prostate-specific membrane antigen (Lu-PSMA) therapy at 6.0 GBq and 7.4 GBq yielded similar PSA response rates and overall survival for patients with progressive metastatic castrate resistant prostate cancer.
Major finding: The primary endpoint of PSA response of at least a 50% reduction from baseline after 2 treatment cycles was met in 28% of the whole cohort, and 46% and 19%, respectively, for treatment regimens of 6.0 GBq and 7.4 GBq.
Study details: The data come from a prospective, phase II trial of 71 men with progressive, metastatic castrate resistant prostate cancer. The patients were randomized to Lu-PSMA therapy at doses of either 6.0 vs 7.4 GBq.
Disclosures: The original study was supported by Endocyte. The researchers had no financial conflicts to disclose.
Source: Calais J. et al. J Nucl Med. 2021 May 20. doi: 10.2967/jnumed.121.261982.
Serum metabolic profiling improves prostate cancer diagnosis
Key clinical point: A serum metabolic panel was more effective than prostate-specific antigen at differentiating prostate cancer patients from patients with negative prostate biopsy and healthy controls.
Major finding: The metabolic panel showed a higher diagnostic performance than prostate-specific antigen in distinguishing PCa from control patients, with an area under the curve of 0.823 for the metabolic panel vs. 0.712 for PSA (P <0.001).
Study details: The data come from a logistic regression analysis of 134 individuals, 39 prostate cancer patients, 45 controls with a negative prostate biopsy, and 50 healthy controls.
Disclosures: The study was funded by the National Natural Science Foundation of China, Science and Technology Support Project in the field of biomedicine of Shanghai Science and Technology Action Plan, Clinical Research Project of Shanghai Municipal Commission of Health and Family Planning, Precision Medicine Program of Second Military Medical University, Youth Startup Program of Second Military Medical University, and Jiangsu Provincial Medical Youth Talent. The researchers had no financial conflicts to disclose.
Source: Xu H et al. Front Oncol. 2021 May 7. doi: 10.3389/fonc.2021.666320.
Key clinical point: A serum metabolic panel was more effective than prostate-specific antigen at differentiating prostate cancer patients from patients with negative prostate biopsy and healthy controls.
Major finding: The metabolic panel showed a higher diagnostic performance than prostate-specific antigen in distinguishing PCa from control patients, with an area under the curve of 0.823 for the metabolic panel vs. 0.712 for PSA (P <0.001).
Study details: The data come from a logistic regression analysis of 134 individuals, 39 prostate cancer patients, 45 controls with a negative prostate biopsy, and 50 healthy controls.
Disclosures: The study was funded by the National Natural Science Foundation of China, Science and Technology Support Project in the field of biomedicine of Shanghai Science and Technology Action Plan, Clinical Research Project of Shanghai Municipal Commission of Health and Family Planning, Precision Medicine Program of Second Military Medical University, Youth Startup Program of Second Military Medical University, and Jiangsu Provincial Medical Youth Talent. The researchers had no financial conflicts to disclose.
Source: Xu H et al. Front Oncol. 2021 May 7. doi: 10.3389/fonc.2021.666320.
Key clinical point: A serum metabolic panel was more effective than prostate-specific antigen at differentiating prostate cancer patients from patients with negative prostate biopsy and healthy controls.
Major finding: The metabolic panel showed a higher diagnostic performance than prostate-specific antigen in distinguishing PCa from control patients, with an area under the curve of 0.823 for the metabolic panel vs. 0.712 for PSA (P <0.001).
Study details: The data come from a logistic regression analysis of 134 individuals, 39 prostate cancer patients, 45 controls with a negative prostate biopsy, and 50 healthy controls.
Disclosures: The study was funded by the National Natural Science Foundation of China, Science and Technology Support Project in the field of biomedicine of Shanghai Science and Technology Action Plan, Clinical Research Project of Shanghai Municipal Commission of Health and Family Planning, Precision Medicine Program of Second Military Medical University, Youth Startup Program of Second Military Medical University, and Jiangsu Provincial Medical Youth Talent. The researchers had no financial conflicts to disclose.
Source: Xu H et al. Front Oncol. 2021 May 7. doi: 10.3389/fonc.2021.666320.
Abiraterone enhances progression-free survival in prostate cancer
Key clinical point: Overall survival among men with castration sensitive prostate cancer was similar whether they were treated with docetaxel or abiraterone, but progression-free survival favored abiraterone patients.
Major finding: Progression-free survival at 12 months was greater among men who received ABI compared to those who received DOC (79.7% vs. 67.1%). Overall survival rates at 12 months were similar between the ABI and DOC groups (92.7% and 98.7%, respectively).
Study details: The data come from a retrospective analysis of 121 men with castration sensitive prostate cancer (mCSPC) who were treated at a single center between December 2014 and March 2021; 79 received docetaxel and 42 received abiraterone in addition to androgen deprivation therapy.
Disclosures: The study was supported by the Joseph and Silvana Melara Cancer Research Fund. Lead author Dr. Briones had no financial conflicts to disclose.
Source: Briones J et al. Front Oncol. 2021 May 7. doi: 10.3389/fonc.2021.658331.
Key clinical point: Overall survival among men with castration sensitive prostate cancer was similar whether they were treated with docetaxel or abiraterone, but progression-free survival favored abiraterone patients.
Major finding: Progression-free survival at 12 months was greater among men who received ABI compared to those who received DOC (79.7% vs. 67.1%). Overall survival rates at 12 months were similar between the ABI and DOC groups (92.7% and 98.7%, respectively).
Study details: The data come from a retrospective analysis of 121 men with castration sensitive prostate cancer (mCSPC) who were treated at a single center between December 2014 and March 2021; 79 received docetaxel and 42 received abiraterone in addition to androgen deprivation therapy.
Disclosures: The study was supported by the Joseph and Silvana Melara Cancer Research Fund. Lead author Dr. Briones had no financial conflicts to disclose.
Source: Briones J et al. Front Oncol. 2021 May 7. doi: 10.3389/fonc.2021.658331.
Key clinical point: Overall survival among men with castration sensitive prostate cancer was similar whether they were treated with docetaxel or abiraterone, but progression-free survival favored abiraterone patients.
Major finding: Progression-free survival at 12 months was greater among men who received ABI compared to those who received DOC (79.7% vs. 67.1%). Overall survival rates at 12 months were similar between the ABI and DOC groups (92.7% and 98.7%, respectively).
Study details: The data come from a retrospective analysis of 121 men with castration sensitive prostate cancer (mCSPC) who were treated at a single center between December 2014 and March 2021; 79 received docetaxel and 42 received abiraterone in addition to androgen deprivation therapy.
Disclosures: The study was supported by the Joseph and Silvana Melara Cancer Research Fund. Lead author Dr. Briones had no financial conflicts to disclose.
Source: Briones J et al. Front Oncol. 2021 May 7. doi: 10.3389/fonc.2021.658331.