User login
Medicare hospital deaths decline, hospice usage increases
Since 2000, Medicare beneficiaries have become less likely to die in hospitals, and more likely to die in their homes or in community health care facilities.
A review of Medicare records also determined that there was a decline in health care transitions in the final 3 days of life for these patients, Joan M. Teno, MD, and her colleagues wrote in JAMA.
It is not possible to identify a specific reason for the shift, wrote Dr. Teno, professor of medicine at the Oregon Health & Science University, Portland. Between the study years of 2000 and 2015, there were several large efforts to improve care at the end of life.
“Since 2009, programs ranging from ensuring informed patient decision making to enhanced care coordination have had the goal of improving care at the end of life. Specific interventions have included promoting conversations about the goals of care, continued growth of hospice services and palliative care, and the debate and passage of the Affordable Care Act … It is difficult to disentangle efforts such as public education, promotion of advance directives through the Patient Self- Determination Act, increased access to hospice and palliative care services, financial incentives of payment policies, and other secular changes.”
The study mined data from the Centers for Medicare & Medicaid Services, and examined end-of-life outcomes among two Medicare groups: Medicare fee-for-service recipients (1,361,870) during 2009-2015, and Medicare Advantage recipients (871,845), comparing 2011 and 2015. The mean age of both cohorts was 82 years.
Outcomes included site of death and “potentially burdensome transitions,” during the last days of life. These were defined as three or more hospitalizations in the previous 3 months, or two or more hospitalizations for pneumonia, urinary tract infection, dehydration, or sepsis during the last 120 days of life. Prolonged mechanical ventilation also was deemed potentially burdensome.
Among fee-for-service recipients, deaths in acute care hospitals declined from 32.6% to19.8%. Deaths in nursing homes remained steady, at 27.2% and 24.9%. Many of these deaths (42.9%) were preceded by a stay in an ICU. There was a transient increase in end-of-life ICU use, around 2009, but by 2015, the percentage was down to 29%, compared with 65.2% in 2000.
Transitions between a nursing home and hospital in the last 90 days of life were 0.49/person in 2000 and 0.33/person in 2015. Hospitalizations for infection or dehydration fell from 14.6% to12.2%. Hospitalization with prolonged ventilation fell from 3.1% to 2.5%.
Dying in hospice care increased from 21.6% to 50.4%, and people were taking advantage of hospice services longer: the proportion using short-term services (3 days or less) fell from 9.8% to 7.7%.
Among Medicare Advantage recipients, the numbers were somewhat different. More than 50% of recipients entered hospice care in both 2011 and 2015; in both years, 8% had services for more than 3 days. About 27% had ICU care in the last days of life, in both years. Compared to fee-for-service recipients, fewer Medicare Advantage patients were in nursing homes at the time of death, and that number declined from 2011 to 2015 (37.7% to 33.2%).
In each year, about 10% of these patients had a hospitalization for dehydration or infection, and 3% had a stay requiring prolonged mechanical ventilation in each year. The mean number of health care transitions remained steady, at 0.23 and 0.21 per person each year.
Dr. Teno had no financial disclosures.
SOURCE: Teno JM et al. JAMA. 2018 Jun 25. doi: 10.1001/jama.2018.8981.
Since 2000, Medicare beneficiaries have become less likely to die in hospitals, and more likely to die in their homes or in community health care facilities.
A review of Medicare records also determined that there was a decline in health care transitions in the final 3 days of life for these patients, Joan M. Teno, MD, and her colleagues wrote in JAMA.
It is not possible to identify a specific reason for the shift, wrote Dr. Teno, professor of medicine at the Oregon Health & Science University, Portland. Between the study years of 2000 and 2015, there were several large efforts to improve care at the end of life.
“Since 2009, programs ranging from ensuring informed patient decision making to enhanced care coordination have had the goal of improving care at the end of life. Specific interventions have included promoting conversations about the goals of care, continued growth of hospice services and palliative care, and the debate and passage of the Affordable Care Act … It is difficult to disentangle efforts such as public education, promotion of advance directives through the Patient Self- Determination Act, increased access to hospice and palliative care services, financial incentives of payment policies, and other secular changes.”
The study mined data from the Centers for Medicare & Medicaid Services, and examined end-of-life outcomes among two Medicare groups: Medicare fee-for-service recipients (1,361,870) during 2009-2015, and Medicare Advantage recipients (871,845), comparing 2011 and 2015. The mean age of both cohorts was 82 years.
Outcomes included site of death and “potentially burdensome transitions,” during the last days of life. These were defined as three or more hospitalizations in the previous 3 months, or two or more hospitalizations for pneumonia, urinary tract infection, dehydration, or sepsis during the last 120 days of life. Prolonged mechanical ventilation also was deemed potentially burdensome.
Among fee-for-service recipients, deaths in acute care hospitals declined from 32.6% to19.8%. Deaths in nursing homes remained steady, at 27.2% and 24.9%. Many of these deaths (42.9%) were preceded by a stay in an ICU. There was a transient increase in end-of-life ICU use, around 2009, but by 2015, the percentage was down to 29%, compared with 65.2% in 2000.
Transitions between a nursing home and hospital in the last 90 days of life were 0.49/person in 2000 and 0.33/person in 2015. Hospitalizations for infection or dehydration fell from 14.6% to12.2%. Hospitalization with prolonged ventilation fell from 3.1% to 2.5%.
Dying in hospice care increased from 21.6% to 50.4%, and people were taking advantage of hospice services longer: the proportion using short-term services (3 days or less) fell from 9.8% to 7.7%.
Among Medicare Advantage recipients, the numbers were somewhat different. More than 50% of recipients entered hospice care in both 2011 and 2015; in both years, 8% had services for more than 3 days. About 27% had ICU care in the last days of life, in both years. Compared to fee-for-service recipients, fewer Medicare Advantage patients were in nursing homes at the time of death, and that number declined from 2011 to 2015 (37.7% to 33.2%).
In each year, about 10% of these patients had a hospitalization for dehydration or infection, and 3% had a stay requiring prolonged mechanical ventilation in each year. The mean number of health care transitions remained steady, at 0.23 and 0.21 per person each year.
Dr. Teno had no financial disclosures.
SOURCE: Teno JM et al. JAMA. 2018 Jun 25. doi: 10.1001/jama.2018.8981.
Since 2000, Medicare beneficiaries have become less likely to die in hospitals, and more likely to die in their homes or in community health care facilities.
A review of Medicare records also determined that there was a decline in health care transitions in the final 3 days of life for these patients, Joan M. Teno, MD, and her colleagues wrote in JAMA.
It is not possible to identify a specific reason for the shift, wrote Dr. Teno, professor of medicine at the Oregon Health & Science University, Portland. Between the study years of 2000 and 2015, there were several large efforts to improve care at the end of life.
“Since 2009, programs ranging from ensuring informed patient decision making to enhanced care coordination have had the goal of improving care at the end of life. Specific interventions have included promoting conversations about the goals of care, continued growth of hospice services and palliative care, and the debate and passage of the Affordable Care Act … It is difficult to disentangle efforts such as public education, promotion of advance directives through the Patient Self- Determination Act, increased access to hospice and palliative care services, financial incentives of payment policies, and other secular changes.”
The study mined data from the Centers for Medicare & Medicaid Services, and examined end-of-life outcomes among two Medicare groups: Medicare fee-for-service recipients (1,361,870) during 2009-2015, and Medicare Advantage recipients (871,845), comparing 2011 and 2015. The mean age of both cohorts was 82 years.
Outcomes included site of death and “potentially burdensome transitions,” during the last days of life. These were defined as three or more hospitalizations in the previous 3 months, or two or more hospitalizations for pneumonia, urinary tract infection, dehydration, or sepsis during the last 120 days of life. Prolonged mechanical ventilation also was deemed potentially burdensome.
Among fee-for-service recipients, deaths in acute care hospitals declined from 32.6% to19.8%. Deaths in nursing homes remained steady, at 27.2% and 24.9%. Many of these deaths (42.9%) were preceded by a stay in an ICU. There was a transient increase in end-of-life ICU use, around 2009, but by 2015, the percentage was down to 29%, compared with 65.2% in 2000.
Transitions between a nursing home and hospital in the last 90 days of life were 0.49/person in 2000 and 0.33/person in 2015. Hospitalizations for infection or dehydration fell from 14.6% to12.2%. Hospitalization with prolonged ventilation fell from 3.1% to 2.5%.
Dying in hospice care increased from 21.6% to 50.4%, and people were taking advantage of hospice services longer: the proportion using short-term services (3 days or less) fell from 9.8% to 7.7%.
Among Medicare Advantage recipients, the numbers were somewhat different. More than 50% of recipients entered hospice care in both 2011 and 2015; in both years, 8% had services for more than 3 days. About 27% had ICU care in the last days of life, in both years. Compared to fee-for-service recipients, fewer Medicare Advantage patients were in nursing homes at the time of death, and that number declined from 2011 to 2015 (37.7% to 33.2%).
In each year, about 10% of these patients had a hospitalization for dehydration or infection, and 3% had a stay requiring prolonged mechanical ventilation in each year. The mean number of health care transitions remained steady, at 0.23 and 0.21 per person each year.
Dr. Teno had no financial disclosures.
SOURCE: Teno JM et al. JAMA. 2018 Jun 25. doi: 10.1001/jama.2018.8981.
FROM JAMA
Key clinical point: During 2000-2015, Medicare recipients became less likely to die in hospitals.
Major finding:
Study details: The retrospective study comprised more than 2.3 million Medicare recipients.
Disclosures: Dr. Teno had no financial disclosures.
Source: Teno JM et al. JAMA. 2018 Jun 25. doi: 10.1001/jama.2018.8981.
Higher BMI tied to lower breast cancer risk in women before menopause
Although obesity increases the risk of breast cancer in postmenopausal women, a large multicenter analysis has confirmed the opposite effect in premenopausal women.
The association had “a greater magnitude than previously shown and across the entire distribution of body mass index,” wrote Minouk J. Schoemaker, PhD, of the Institute of Cancer Research in London, with his associates, on behalf of the Premenopausal Breast Cancer Collaborative Group. The protective effect of adiposity was strongest during young adulthood (ages 18-24 years), when it spanned breast cancer subtypes. “Understanding the biological mechanisms underlying these associations could have important preventive potential,” they wrote in JAMA Oncology.
Prior studies have linked greater body fat with reduced risk of breast cancer in younger women, but the effect has not been well characterized. For this analysis, the investigators pooled data from 19 cohort studies that included a total of 758,592 premenopausal women; median age was 40.6 years (interquartile range, 35.2-45.5 years).
For each 5-unit increase in BMI, the estimated reduction in risk of breast cancer was 23% among women aged 18-24 years (hazard ratio, 0.77; 95% confidence interval, 0.73-0.80), 15% in women aged 25-34 years, 13% in women aged 35-44 years, and 12% in women aged 45-54 years. There was no BMI threshold for risk reduction: the inverse correlation existed even when women were not overweight. Risk also did vary significantly among subgroups stratified by other risk factors for breast cancer. Adiposity was more protective against estrogen receptor-positive and progesterone-receptor positive breast cancers and less protective against hormone receptor–negative breast cancers, which “implies a hormonal mechanism,” the investigators said. “Body mass index at ages 25-54 years was not consistently associated with triple-negative or hormone receptor–negative breast cancer overall.”
Funders included Breast Cancer Now, the Institute of Cancer Research, the National Institutes of Health, and many others. The researchers reported having no relevant conflicts of interest.
SOURCE: Schoemaker MJ et al. JAMA Oncol. 2018; Jun 21. doi: 10.1001/jamaoncol.2018.1771.
Although obesity increases the risk of breast cancer in postmenopausal women, a large multicenter analysis has confirmed the opposite effect in premenopausal women.
The association had “a greater magnitude than previously shown and across the entire distribution of body mass index,” wrote Minouk J. Schoemaker, PhD, of the Institute of Cancer Research in London, with his associates, on behalf of the Premenopausal Breast Cancer Collaborative Group. The protective effect of adiposity was strongest during young adulthood (ages 18-24 years), when it spanned breast cancer subtypes. “Understanding the biological mechanisms underlying these associations could have important preventive potential,” they wrote in JAMA Oncology.
Prior studies have linked greater body fat with reduced risk of breast cancer in younger women, but the effect has not been well characterized. For this analysis, the investigators pooled data from 19 cohort studies that included a total of 758,592 premenopausal women; median age was 40.6 years (interquartile range, 35.2-45.5 years).
For each 5-unit increase in BMI, the estimated reduction in risk of breast cancer was 23% among women aged 18-24 years (hazard ratio, 0.77; 95% confidence interval, 0.73-0.80), 15% in women aged 25-34 years, 13% in women aged 35-44 years, and 12% in women aged 45-54 years. There was no BMI threshold for risk reduction: the inverse correlation existed even when women were not overweight. Risk also did vary significantly among subgroups stratified by other risk factors for breast cancer. Adiposity was more protective against estrogen receptor-positive and progesterone-receptor positive breast cancers and less protective against hormone receptor–negative breast cancers, which “implies a hormonal mechanism,” the investigators said. “Body mass index at ages 25-54 years was not consistently associated with triple-negative or hormone receptor–negative breast cancer overall.”
Funders included Breast Cancer Now, the Institute of Cancer Research, the National Institutes of Health, and many others. The researchers reported having no relevant conflicts of interest.
SOURCE: Schoemaker MJ et al. JAMA Oncol. 2018; Jun 21. doi: 10.1001/jamaoncol.2018.1771.
Although obesity increases the risk of breast cancer in postmenopausal women, a large multicenter analysis has confirmed the opposite effect in premenopausal women.
The association had “a greater magnitude than previously shown and across the entire distribution of body mass index,” wrote Minouk J. Schoemaker, PhD, of the Institute of Cancer Research in London, with his associates, on behalf of the Premenopausal Breast Cancer Collaborative Group. The protective effect of adiposity was strongest during young adulthood (ages 18-24 years), when it spanned breast cancer subtypes. “Understanding the biological mechanisms underlying these associations could have important preventive potential,” they wrote in JAMA Oncology.
Prior studies have linked greater body fat with reduced risk of breast cancer in younger women, but the effect has not been well characterized. For this analysis, the investigators pooled data from 19 cohort studies that included a total of 758,592 premenopausal women; median age was 40.6 years (interquartile range, 35.2-45.5 years).
For each 5-unit increase in BMI, the estimated reduction in risk of breast cancer was 23% among women aged 18-24 years (hazard ratio, 0.77; 95% confidence interval, 0.73-0.80), 15% in women aged 25-34 years, 13% in women aged 35-44 years, and 12% in women aged 45-54 years. There was no BMI threshold for risk reduction: the inverse correlation existed even when women were not overweight. Risk also did vary significantly among subgroups stratified by other risk factors for breast cancer. Adiposity was more protective against estrogen receptor-positive and progesterone-receptor positive breast cancers and less protective against hormone receptor–negative breast cancers, which “implies a hormonal mechanism,” the investigators said. “Body mass index at ages 25-54 years was not consistently associated with triple-negative or hormone receptor–negative breast cancer overall.”
Funders included Breast Cancer Now, the Institute of Cancer Research, the National Institutes of Health, and many others. The researchers reported having no relevant conflicts of interest.
SOURCE: Schoemaker MJ et al. JAMA Oncol. 2018; Jun 21. doi: 10.1001/jamaoncol.2018.1771.
FROM JAMA ONCOLOGY
Key clinical point: In premenopausal women, adiposity inversely correlated with risk of breast cancer, and showed a stronger protective effect than previously documented.
Major finding: For each 5-unit increase in BMI, the estimated reduction in risk of breast cancer was 23% among women aged 18-24 years (hazard ratio, 0.77; 95% confidence interval, 0.73-0.80), 15% in women aged 25-34 years, 13% in women aged 35-44 years, and 12% in women aged 45-54 years.
Study details: Multicenter analysis of 19 cohort studies.
Disclosures: Funders included Breast Cancer Now, the Institute of Cancer Research, the National Institutes of Health, and many others. The researchers reported having no relevant conflicts of interest.
Source: Schoemaker MJ et al. JAMA Oncol. 2018; Jun 21. doi: 10.1001/jamaoncol.2018.1771.
CAR T-cell approvals: multiple myeloma likely next up
The next major approval in the chimeric antigen receptor (CAR) T-cell therapy arena will target multiple myeloma, according to Carl June, MD, the Richard W Vague Professor in Immunotherapy and a pioneer in CAR T-cell research at the University of Pennsylvania, Philadelphia. That approval is anticipated sometime in 2019, and will “completely transform oncology,” Dr June said in a recent interview. “Myeloma is the most common blood cancer in adults, and there’s never been a curative therapy, but now there is a subset of patients who look like they’re cured with CAR T cells.”
Researcher-turned-patient
The first treated patient in a trial of a novel anti–B-cell maturation antigen (BCMA)–specific CAR T-cell therapy (CART-BCMA)1 developed by University of Pennsylvania researchers in collaboration with Novartis is part of that subset. Earlier this year, Woodring Wright, MD, a professor of cell biology and medicine at the University of Texas (UT) Southwestern Medical Center in Dallas, outed himself as that first patient when he announced that CART-BCMA saved his life.2
Dr Wright had been diagnosed with multiple myeloma about 12 years ago and had failed 11 previous chemotherapies before he was enrolled in the CART-BCMA trial. He remains cancer free more than 2 years after receiving CART-BCMA and he’s now conducting CAR T-cell–related research in his UT Southwestern laboratory to broaden the effectiveness of current CAR T-cell therapies. In particular, he is looking at whether the small percentage of patients in whom CAR T-cell therapy does not work might benefit from telomerase to lengthen telomeres, because most patients who fail CAR T-cell therapy are elderly and might have terminally short telomeres. 2
Pharma lines up the trials
An ongoing University of Pennsylvania trial led by Adam D Cohen, MD, director of myeloma immunotherapy at the Abramson Cancer Center, has an overall response rate of 64%; initial phase 1 efficacy and safety results were reported at the 2016 annual meeting of the American Society of Hematology (ASH).3 In addition, multiple companies are pursuing registration trials for CAR T-cell therapies in myeloma, Dr June said.
Among those companies are bluebird bio and Celgene, which together are developing an anti-BCMA CAR T-cell therapy known as bb2121. The product was granted breakthrough therapy designation by the US Food and Drug Administration in November 2017 and will thus receive expedited review by the agency. It has also been fast-tracked in Europe.
The decision to fast-track bb2121 in the United States was based on preliminary results from the CRB-410 trial.4 Updated findings from that trial were presented at the 2017 ASH annual meeting and showed an overall response rate of 94% in 21 patients, with 17 of 18 patients who received doses above 50 x 106 CAR+ T cells having an overall response, and 10 of the 18 achieving complete remission. The progression-free survival rates were 81% at 6 months, and 71% at 9 months, with responses deepening over time. The complete response rates were 27% and 56% in May and October of 2017, respectively.
Responses were durable, lasting more than 1 year in several patients, the investigators reported. Phase 2 of the trial – the global pivotal KarMMA trial – is currently enrolling and will dose patients at between 150 and 350 x 106 CAR+ T cells.5
Janssen Biotech Inc and Legend Biotech USA Inc/ Legend Biotech Ireland Ltd have also joined forces to develop an anti-BCMA CAR T-cell product for multiple myeloma, Dr June said. The companies announced in late 2017 that they had entered into “a worldwide collaboration and license agreement” to develop the CAR T-cell drug candidate, LCAR-B38M.6 It has been accepted for review by the China Food and Drug Administration and is in the planning phase of clinical studies in the United States for multiple myeloma, according to that announcement.
Cost, financial toxicity, and a new therapeutic landscape
The rush for the approval of a CAR T-cell therapy for myeloma will lead to a welcome addition to the treatment armamentarium not just because of the clinical benefits, but because of the possibility of reducing disease-related costs (p. e177). Although myeloma represents only about 2% of all cancers, it is responsible for 7% of cancer costs, Dr June noted, and since many patients live with their disease for a long time, that can mean substantial “financial toxicity” being associated with treatment for the disease. “So CAR T-cell therapy for myeloma will bring a huge change to the practice of oncology,” he added.
Dr June explained that tisagenlecleucel, the first CAR T-cell therapy to be approved (in August 2017; p. e126), was for pediatric acute lymphoblastic leukemia that had relapsed at least twice.7 “That’s only about 600 kids a year in the United States, so it’s an ultra-orphan market,” he said. However, with the subsequent October 2017 approval of axicabtagene ciloleucel for certain cases of large B-cell lymphoma8 and the anticipated myeloma approval, CAR T-cell therapy will move away from that orphan status.
“There are a lot of difficulties whenever you change to something new,” he said, comparing the CAR T-cell therapy evolution to that of bone marrow transplantation in the 1980s, when many voiced concern about the new therapy because it was available at only 2 centers in the United states and required a high level of specialized skill. “But over the years, millions of transplants have been done [and] they’re done at many community centers. And it’s the same thing with CARs.” There are now 30 centers offering CAR T-cell therapy and people have to be trained. “It’s a new skill set, and it will take time,” he said.
Access to trials: balancing demand and availability
That delay can be particularly frustrating because there are many patients who might benefit “in a major way” from CAR T-cell therapy, but who can’t get on a clinical trial, Dr June noted.
“There’s more demand than availability, and it’s going to take a while” for that to change, he said. The solution most likely will involve the complementary use of off-the-shelf CAR T cells in some patients to induce remission and perhaps provide a bridge to another definitive therapy, and ultrapersonalized CAR T-cell therapy in others, as well as combinations that include CAR T cells and targeted agents or checkpoint inhibitors.
CRISPR-Cas9 gene editing is also being considered as a tool for engineering multiple myeloma cellular immunotherapy (and other cancer treatments), as in the Parker Institute-funded NYCE study,9 Dr June said. “We’re actually removing the [programmed death-1] gene and the T-cell receptors ... it shows enormous potential for gene editing. CRISPR is going to be used for a lot of things, but the first use is with T-cell therapies, so we’re really excited about that trial.”
Disclosures. Dr June reported royalties and research funding from Novartis and an ownership interest in Tmunity Therapeutics.
1. University of Pennsylvania. CART-BCMA cells for multiple myeloma. https://clinicaltrials.gov/ct2/show/NCT02546167. NCT02546167. Accessed June 13, 2018.
2. Frisinger C. Cancer researcher's life saved by CAR-T treatment. UT Southwestern Medical Center website. https://www.utsouthwestern.edu/newsroom/articles/year-2018/wright-car-t.html. Published. Accessed June 13, 2018.
3. Cohen AD, Garfall AL, Stadtmauer EA, et al. B-cell maturation antigen (BCMA)-specific chimeric antigen receptor T cells (CART-BCMA) for multiple myeloma (MM): initial safety and efficacy from a phase I study. Blood. 2016;128(22):1147.
4. Berdeja JG, Lin Y, Raje N, et al. Durable clinical responses in heavily pretreated patients with relapsed/refractory multiple myeloma: updated results from a multicenter study of bb2121 anti-BCMA CAR T cell therapy. Blood. 2017;130:740.
5. Celgene. Efficacy and safety study of bb2121 in subjects with relapsed and refractory multiple myeloma (KarMMa) (bb2121). https://clinicaltrials.gov/ct2/show/NCT03361748. NCT03361748. Accessed June 13, 2018.
6. Janssen enters worldwide collaboration and license agreement with Chinese company Legend Biotech to develop investigational CAR-T anti-cancer therapy. https://www.jnj.com/media-center/press-releases/janssen-enters-worldwide-collaboration-and-license-agreement-with-chinese-company-legend-biotech-to-develop-investigational-car-t-anti-cancer-therapy. New Brunswick, NJ: Johnson & Johnson. December 21, 2017. Accessed June 13, 2018.
7. FDA approves tisagenlecleucel for B-cell ALL and tocilizumab for cytokine release syndrome. FDA News Release. August 30, 2017. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm574154.htm. Accessed June 13, 2018.
8. FDA approves axicabtagene ciloleucel for large B-cell lymphoma. FDA News Release. October 18, 2017. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm581296.htm. Accessed June 13, 2018.
9. University of Pennsylvania. NY-ESO-1-redirected CRISPR (TCRendo and PD1) edited T cells (NYCE T Cells). NCT03399448. Accessed June 13, 2018.
The next major approval in the chimeric antigen receptor (CAR) T-cell therapy arena will target multiple myeloma, according to Carl June, MD, the Richard W Vague Professor in Immunotherapy and a pioneer in CAR T-cell research at the University of Pennsylvania, Philadelphia. That approval is anticipated sometime in 2019, and will “completely transform oncology,” Dr June said in a recent interview. “Myeloma is the most common blood cancer in adults, and there’s never been a curative therapy, but now there is a subset of patients who look like they’re cured with CAR T cells.”
Researcher-turned-patient
The first treated patient in a trial of a novel anti–B-cell maturation antigen (BCMA)–specific CAR T-cell therapy (CART-BCMA)1 developed by University of Pennsylvania researchers in collaboration with Novartis is part of that subset. Earlier this year, Woodring Wright, MD, a professor of cell biology and medicine at the University of Texas (UT) Southwestern Medical Center in Dallas, outed himself as that first patient when he announced that CART-BCMA saved his life.2
Dr Wright had been diagnosed with multiple myeloma about 12 years ago and had failed 11 previous chemotherapies before he was enrolled in the CART-BCMA trial. He remains cancer free more than 2 years after receiving CART-BCMA and he’s now conducting CAR T-cell–related research in his UT Southwestern laboratory to broaden the effectiveness of current CAR T-cell therapies. In particular, he is looking at whether the small percentage of patients in whom CAR T-cell therapy does not work might benefit from telomerase to lengthen telomeres, because most patients who fail CAR T-cell therapy are elderly and might have terminally short telomeres. 2
Pharma lines up the trials
An ongoing University of Pennsylvania trial led by Adam D Cohen, MD, director of myeloma immunotherapy at the Abramson Cancer Center, has an overall response rate of 64%; initial phase 1 efficacy and safety results were reported at the 2016 annual meeting of the American Society of Hematology (ASH).3 In addition, multiple companies are pursuing registration trials for CAR T-cell therapies in myeloma, Dr June said.
Among those companies are bluebird bio and Celgene, which together are developing an anti-BCMA CAR T-cell therapy known as bb2121. The product was granted breakthrough therapy designation by the US Food and Drug Administration in November 2017 and will thus receive expedited review by the agency. It has also been fast-tracked in Europe.
The decision to fast-track bb2121 in the United States was based on preliminary results from the CRB-410 trial.4 Updated findings from that trial were presented at the 2017 ASH annual meeting and showed an overall response rate of 94% in 21 patients, with 17 of 18 patients who received doses above 50 x 106 CAR+ T cells having an overall response, and 10 of the 18 achieving complete remission. The progression-free survival rates were 81% at 6 months, and 71% at 9 months, with responses deepening over time. The complete response rates were 27% and 56% in May and October of 2017, respectively.
Responses were durable, lasting more than 1 year in several patients, the investigators reported. Phase 2 of the trial – the global pivotal KarMMA trial – is currently enrolling and will dose patients at between 150 and 350 x 106 CAR+ T cells.5
Janssen Biotech Inc and Legend Biotech USA Inc/ Legend Biotech Ireland Ltd have also joined forces to develop an anti-BCMA CAR T-cell product for multiple myeloma, Dr June said. The companies announced in late 2017 that they had entered into “a worldwide collaboration and license agreement” to develop the CAR T-cell drug candidate, LCAR-B38M.6 It has been accepted for review by the China Food and Drug Administration and is in the planning phase of clinical studies in the United States for multiple myeloma, according to that announcement.
Cost, financial toxicity, and a new therapeutic landscape
The rush for the approval of a CAR T-cell therapy for myeloma will lead to a welcome addition to the treatment armamentarium not just because of the clinical benefits, but because of the possibility of reducing disease-related costs (p. e177). Although myeloma represents only about 2% of all cancers, it is responsible for 7% of cancer costs, Dr June noted, and since many patients live with their disease for a long time, that can mean substantial “financial toxicity” being associated with treatment for the disease. “So CAR T-cell therapy for myeloma will bring a huge change to the practice of oncology,” he added.
Dr June explained that tisagenlecleucel, the first CAR T-cell therapy to be approved (in August 2017; p. e126), was for pediatric acute lymphoblastic leukemia that had relapsed at least twice.7 “That’s only about 600 kids a year in the United States, so it’s an ultra-orphan market,” he said. However, with the subsequent October 2017 approval of axicabtagene ciloleucel for certain cases of large B-cell lymphoma8 and the anticipated myeloma approval, CAR T-cell therapy will move away from that orphan status.
“There are a lot of difficulties whenever you change to something new,” he said, comparing the CAR T-cell therapy evolution to that of bone marrow transplantation in the 1980s, when many voiced concern about the new therapy because it was available at only 2 centers in the United states and required a high level of specialized skill. “But over the years, millions of transplants have been done [and] they’re done at many community centers. And it’s the same thing with CARs.” There are now 30 centers offering CAR T-cell therapy and people have to be trained. “It’s a new skill set, and it will take time,” he said.
Access to trials: balancing demand and availability
That delay can be particularly frustrating because there are many patients who might benefit “in a major way” from CAR T-cell therapy, but who can’t get on a clinical trial, Dr June noted.
“There’s more demand than availability, and it’s going to take a while” for that to change, he said. The solution most likely will involve the complementary use of off-the-shelf CAR T cells in some patients to induce remission and perhaps provide a bridge to another definitive therapy, and ultrapersonalized CAR T-cell therapy in others, as well as combinations that include CAR T cells and targeted agents or checkpoint inhibitors.
CRISPR-Cas9 gene editing is also being considered as a tool for engineering multiple myeloma cellular immunotherapy (and other cancer treatments), as in the Parker Institute-funded NYCE study,9 Dr June said. “We’re actually removing the [programmed death-1] gene and the T-cell receptors ... it shows enormous potential for gene editing. CRISPR is going to be used for a lot of things, but the first use is with T-cell therapies, so we’re really excited about that trial.”
Disclosures. Dr June reported royalties and research funding from Novartis and an ownership interest in Tmunity Therapeutics.
The next major approval in the chimeric antigen receptor (CAR) T-cell therapy arena will target multiple myeloma, according to Carl June, MD, the Richard W Vague Professor in Immunotherapy and a pioneer in CAR T-cell research at the University of Pennsylvania, Philadelphia. That approval is anticipated sometime in 2019, and will “completely transform oncology,” Dr June said in a recent interview. “Myeloma is the most common blood cancer in adults, and there’s never been a curative therapy, but now there is a subset of patients who look like they’re cured with CAR T cells.”
Researcher-turned-patient
The first treated patient in a trial of a novel anti–B-cell maturation antigen (BCMA)–specific CAR T-cell therapy (CART-BCMA)1 developed by University of Pennsylvania researchers in collaboration with Novartis is part of that subset. Earlier this year, Woodring Wright, MD, a professor of cell biology and medicine at the University of Texas (UT) Southwestern Medical Center in Dallas, outed himself as that first patient when he announced that CART-BCMA saved his life.2
Dr Wright had been diagnosed with multiple myeloma about 12 years ago and had failed 11 previous chemotherapies before he was enrolled in the CART-BCMA trial. He remains cancer free more than 2 years after receiving CART-BCMA and he’s now conducting CAR T-cell–related research in his UT Southwestern laboratory to broaden the effectiveness of current CAR T-cell therapies. In particular, he is looking at whether the small percentage of patients in whom CAR T-cell therapy does not work might benefit from telomerase to lengthen telomeres, because most patients who fail CAR T-cell therapy are elderly and might have terminally short telomeres. 2
Pharma lines up the trials
An ongoing University of Pennsylvania trial led by Adam D Cohen, MD, director of myeloma immunotherapy at the Abramson Cancer Center, has an overall response rate of 64%; initial phase 1 efficacy and safety results were reported at the 2016 annual meeting of the American Society of Hematology (ASH).3 In addition, multiple companies are pursuing registration trials for CAR T-cell therapies in myeloma, Dr June said.
Among those companies are bluebird bio and Celgene, which together are developing an anti-BCMA CAR T-cell therapy known as bb2121. The product was granted breakthrough therapy designation by the US Food and Drug Administration in November 2017 and will thus receive expedited review by the agency. It has also been fast-tracked in Europe.
The decision to fast-track bb2121 in the United States was based on preliminary results from the CRB-410 trial.4 Updated findings from that trial were presented at the 2017 ASH annual meeting and showed an overall response rate of 94% in 21 patients, with 17 of 18 patients who received doses above 50 x 106 CAR+ T cells having an overall response, and 10 of the 18 achieving complete remission. The progression-free survival rates were 81% at 6 months, and 71% at 9 months, with responses deepening over time. The complete response rates were 27% and 56% in May and October of 2017, respectively.
Responses were durable, lasting more than 1 year in several patients, the investigators reported. Phase 2 of the trial – the global pivotal KarMMA trial – is currently enrolling and will dose patients at between 150 and 350 x 106 CAR+ T cells.5
Janssen Biotech Inc and Legend Biotech USA Inc/ Legend Biotech Ireland Ltd have also joined forces to develop an anti-BCMA CAR T-cell product for multiple myeloma, Dr June said. The companies announced in late 2017 that they had entered into “a worldwide collaboration and license agreement” to develop the CAR T-cell drug candidate, LCAR-B38M.6 It has been accepted for review by the China Food and Drug Administration and is in the planning phase of clinical studies in the United States for multiple myeloma, according to that announcement.
Cost, financial toxicity, and a new therapeutic landscape
The rush for the approval of a CAR T-cell therapy for myeloma will lead to a welcome addition to the treatment armamentarium not just because of the clinical benefits, but because of the possibility of reducing disease-related costs (p. e177). Although myeloma represents only about 2% of all cancers, it is responsible for 7% of cancer costs, Dr June noted, and since many patients live with their disease for a long time, that can mean substantial “financial toxicity” being associated with treatment for the disease. “So CAR T-cell therapy for myeloma will bring a huge change to the practice of oncology,” he added.
Dr June explained that tisagenlecleucel, the first CAR T-cell therapy to be approved (in August 2017; p. e126), was for pediatric acute lymphoblastic leukemia that had relapsed at least twice.7 “That’s only about 600 kids a year in the United States, so it’s an ultra-orphan market,” he said. However, with the subsequent October 2017 approval of axicabtagene ciloleucel for certain cases of large B-cell lymphoma8 and the anticipated myeloma approval, CAR T-cell therapy will move away from that orphan status.
“There are a lot of difficulties whenever you change to something new,” he said, comparing the CAR T-cell therapy evolution to that of bone marrow transplantation in the 1980s, when many voiced concern about the new therapy because it was available at only 2 centers in the United states and required a high level of specialized skill. “But over the years, millions of transplants have been done [and] they’re done at many community centers. And it’s the same thing with CARs.” There are now 30 centers offering CAR T-cell therapy and people have to be trained. “It’s a new skill set, and it will take time,” he said.
Access to trials: balancing demand and availability
That delay can be particularly frustrating because there are many patients who might benefit “in a major way” from CAR T-cell therapy, but who can’t get on a clinical trial, Dr June noted.
“There’s more demand than availability, and it’s going to take a while” for that to change, he said. The solution most likely will involve the complementary use of off-the-shelf CAR T cells in some patients to induce remission and perhaps provide a bridge to another definitive therapy, and ultrapersonalized CAR T-cell therapy in others, as well as combinations that include CAR T cells and targeted agents or checkpoint inhibitors.
CRISPR-Cas9 gene editing is also being considered as a tool for engineering multiple myeloma cellular immunotherapy (and other cancer treatments), as in the Parker Institute-funded NYCE study,9 Dr June said. “We’re actually removing the [programmed death-1] gene and the T-cell receptors ... it shows enormous potential for gene editing. CRISPR is going to be used for a lot of things, but the first use is with T-cell therapies, so we’re really excited about that trial.”
Disclosures. Dr June reported royalties and research funding from Novartis and an ownership interest in Tmunity Therapeutics.
1. University of Pennsylvania. CART-BCMA cells for multiple myeloma. https://clinicaltrials.gov/ct2/show/NCT02546167. NCT02546167. Accessed June 13, 2018.
2. Frisinger C. Cancer researcher's life saved by CAR-T treatment. UT Southwestern Medical Center website. https://www.utsouthwestern.edu/newsroom/articles/year-2018/wright-car-t.html. Published. Accessed June 13, 2018.
3. Cohen AD, Garfall AL, Stadtmauer EA, et al. B-cell maturation antigen (BCMA)-specific chimeric antigen receptor T cells (CART-BCMA) for multiple myeloma (MM): initial safety and efficacy from a phase I study. Blood. 2016;128(22):1147.
4. Berdeja JG, Lin Y, Raje N, et al. Durable clinical responses in heavily pretreated patients with relapsed/refractory multiple myeloma: updated results from a multicenter study of bb2121 anti-BCMA CAR T cell therapy. Blood. 2017;130:740.
5. Celgene. Efficacy and safety study of bb2121 in subjects with relapsed and refractory multiple myeloma (KarMMa) (bb2121). https://clinicaltrials.gov/ct2/show/NCT03361748. NCT03361748. Accessed June 13, 2018.
6. Janssen enters worldwide collaboration and license agreement with Chinese company Legend Biotech to develop investigational CAR-T anti-cancer therapy. https://www.jnj.com/media-center/press-releases/janssen-enters-worldwide-collaboration-and-license-agreement-with-chinese-company-legend-biotech-to-develop-investigational-car-t-anti-cancer-therapy. New Brunswick, NJ: Johnson & Johnson. December 21, 2017. Accessed June 13, 2018.
7. FDA approves tisagenlecleucel for B-cell ALL and tocilizumab for cytokine release syndrome. FDA News Release. August 30, 2017. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm574154.htm. Accessed June 13, 2018.
8. FDA approves axicabtagene ciloleucel for large B-cell lymphoma. FDA News Release. October 18, 2017. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm581296.htm. Accessed June 13, 2018.
9. University of Pennsylvania. NY-ESO-1-redirected CRISPR (TCRendo and PD1) edited T cells (NYCE T Cells). NCT03399448. Accessed June 13, 2018.
1. University of Pennsylvania. CART-BCMA cells for multiple myeloma. https://clinicaltrials.gov/ct2/show/NCT02546167. NCT02546167. Accessed June 13, 2018.
2. Frisinger C. Cancer researcher's life saved by CAR-T treatment. UT Southwestern Medical Center website. https://www.utsouthwestern.edu/newsroom/articles/year-2018/wright-car-t.html. Published. Accessed June 13, 2018.
3. Cohen AD, Garfall AL, Stadtmauer EA, et al. B-cell maturation antigen (BCMA)-specific chimeric antigen receptor T cells (CART-BCMA) for multiple myeloma (MM): initial safety and efficacy from a phase I study. Blood. 2016;128(22):1147.
4. Berdeja JG, Lin Y, Raje N, et al. Durable clinical responses in heavily pretreated patients with relapsed/refractory multiple myeloma: updated results from a multicenter study of bb2121 anti-BCMA CAR T cell therapy. Blood. 2017;130:740.
5. Celgene. Efficacy and safety study of bb2121 in subjects with relapsed and refractory multiple myeloma (KarMMa) (bb2121). https://clinicaltrials.gov/ct2/show/NCT03361748. NCT03361748. Accessed June 13, 2018.
6. Janssen enters worldwide collaboration and license agreement with Chinese company Legend Biotech to develop investigational CAR-T anti-cancer therapy. https://www.jnj.com/media-center/press-releases/janssen-enters-worldwide-collaboration-and-license-agreement-with-chinese-company-legend-biotech-to-develop-investigational-car-t-anti-cancer-therapy. New Brunswick, NJ: Johnson & Johnson. December 21, 2017. Accessed June 13, 2018.
7. FDA approves tisagenlecleucel for B-cell ALL and tocilizumab for cytokine release syndrome. FDA News Release. August 30, 2017. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm574154.htm. Accessed June 13, 2018.
8. FDA approves axicabtagene ciloleucel for large B-cell lymphoma. FDA News Release. October 18, 2017. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm581296.htm. Accessed June 13, 2018.
9. University of Pennsylvania. NY-ESO-1-redirected CRISPR (TCRendo and PD1) edited T cells (NYCE T Cells). NCT03399448. Accessed June 13, 2018.
Tumor heterogeneity: a central foe in the war on cancer
A major challenge to effective cancer treatment is the astounding level of heterogeneity that tumors display on many different fronts. Here, we discuss how a deeper appreciation of this heterogeneity and its impact is driving research efforts to better understand and tackle it and a radical rethink of treatment paradigms.
A complex and dynamic disease
The nonuniformity of cancer has long been appreciated, reflected most visibly in the variation of response to the same treatment across patients with the same type of tumor (inter-tumor heterogeneity). The extent of tumor heterogeneity is being fully realized only now, with the advent of next-generation sequencing technologies. Even within the same tumor, there can be significant heterogeneity from cell to cell (intra-tumor heterogeneity), yielding substantial complexity in cancer.
Heterogeneity reveals itself on many different levels. Histologically speaking, tumors are composed of a nonhomogenous mass of cells that vary in type and number. In terms of their molecular make-up, there is substantial variation in the types of molecular alterations observed, all the way down to the single cell level. In even more abstract terms, beyond the cancer itself, the microenvironment in which it resides can be highly heterogeneous, composed of a plethora of different supportive and tumor-infiltrating normal cells.
Heterogeneity can manifest spatially, reflecting differences in the composition of the primary tumor and tumors at secondary sites or across regions of the same tumor mass and temporally, at different time points across a tumor’s natural history. Evocative of the second law of thermodynamics, cancers generally become more diverse and complex over time.1-3
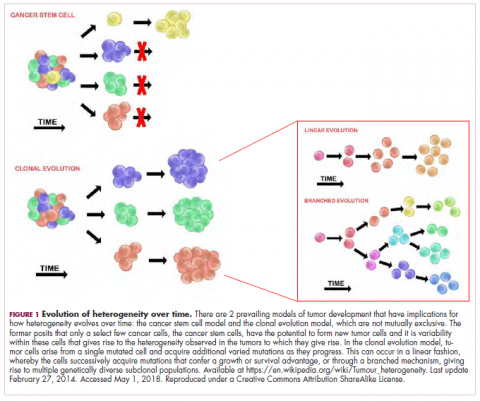
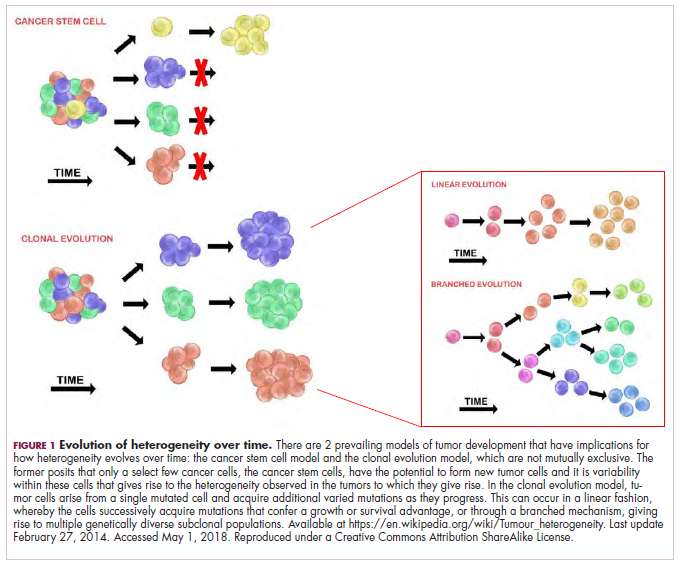
A tale of 2 models
It is widely accepted that the transformation of a normal cell into a malignant one occurs with the acquisition of certain “hallmark” abilities, but there are myriad ways in which these can be attained.
The clonal evolution model
As cells divide, they randomly acquire mutations as a result of DNA damage. The clonal evolution model posits that cancer develops as the result of a multistep accumulation of a series of “driver” mutations that confer a promalignant advantage to the cell and ultimately fuel a cancerous hallmark.
This evolution can occur in a linear fashion, whereby the emergence of a new driver mutation conveys such a potent evolutionary advantage that it outcompetes all previous clones. There is limited evidence for linear evolution in most advanced human cancers; instead, they are thought to evolve predominantly through a process of branching evolution, in which multiple clones can diverge in parallel from a common ancestor through the acquisition of different driver mutations. This results in common clonal mutations that form the trunk of the cancer’s evolutionary tree and are shared by all cells and subclonal mutations, which make up the branches and differ from cell to cell.
More recently, several other mechanisms of clonal evolution have been proposed, including neutral evolution, a type of branching evolution in which there are no selective pressures and evolution occurs by random mutations occurring over time that lead to genetic drift, and punctuated evolution, in which there are short evolutionary bursts of hypermutation.4,5
The CSC model
This model posits that the ability to form and sustain a cancer is restricted to a single cell type – the cancer stem cells – which have the unique capacity for self-renewal and differentiation. Although the forces of evolution are still involved in this model, they act on a hierarchy of cells, with stem cells sitting at the top. A tumor is derived from a single stem cell that has acquired a mutation, and the heterogeneity observed results both from the differentiation and the accumulation of mutations in CSCs.
Accumulated experimental evidence suggests that these models are not mutually exclusive and that they can all contribute to heterogeneity in varied amounts across different tumor types. What is clear is that heterogeneity and evolution are intricately intertwined in cancer development.1,2,6
An unstable genome
Heterogeneity and evolution are fueled by genomic alterations and the genome instability that they foster. This genome instability can range from single base pair substitutions to a doubling of the entire genome and results from both exposure to exogenous mutagens (eg, chemicals and ultraviolet radiation) and genomic alterations that have an impact on important cellular processes (eg, DNA repair or replication).
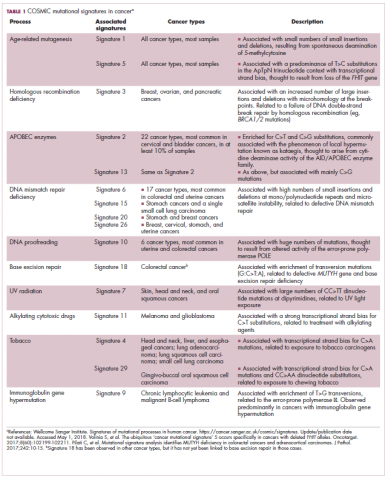
Among the most common causes of genome instability are mutations in the DNA mismatch repair pathway proteins or in the proofreading polymerase enzymes. Genome instability is often associated with unique mutational signatures – characteristic combinations of mutations that arose as the result of the specific biological processes underlying them.7
Genome-wide analyses have begun to reveal these mutational signatures across the spectrum of human cancers. The Wellcome Sanger Institute’s Catalogue of Somatic Mutations in Cancer (COSMIC) database has generated a set of 30 mutational signatures based on analysis of almost 11,000 exomes and more than 1,000 whole genomes spanning 40 different cancer types, some of which have been linked with specific mutagenic processes, such as tobacco, UV radiation, and DNA repair deficiency (Table 1).8
Fueling resistance
Arguably, heterogeneity presents one of the most significant barriers to effective cancer therapy, and this has become increasingly true in the era of personalized medicine in which targeted therapies take aim at specific molecular abnormalities.
It is vital that drugs target the truncal alterations that are present in all cancer cells to ensure that the entire cancer is eradicated. However, it is not always possible to target these alterations, for example, at the present time tumor suppressor proteins like p53 are not druggable.
Even when truncal alterations have been targeted successfully, such as epidermal growth factor receptor (EGFR) mutations and anaplastic lymphoma kinase (ALK) chromosomal rearrangements in non–small-cell lung cancer (NSCLC) and BRAF mutations in melanoma, the long-term efficacy of these drugs is almost invariably limited by the development of resistance.
Tumor heterogeneity and the clonal evolution it fuels are central drivers of resistance. Because tumors are dynamic and continue to evolve, anticancer treatments can act as a strong selective pressure and drive the emergence of drug-resistant subclones that allow the tumor to persist. In fact, study findings have revealed that small populations of resistant cells may be present before treatment. Thus, resistance may also occur as a result of the outgrowth of preexisting treatment-resistant cells that suddenly find that they acquire a survival advantage in the presence of a drug.1,6
Tackling heterogeneity
Despite extensive clinical documentation of the existence of heterogeneity and its underlying mechanisms across a range of tumor types, the development of novel clinical trial designs and therapeutic strategies that account for its effects have only recently begun to be explored.
For the most part, this was because of a lack of effective methods for evaluating intratumor heterogeneity. Multiregion biopsies, in which tissue derived from multiple different regions of a single tumor mass or from distinct cancerous lesions within the same patient, give a snapshot of tumor heterogeneity at a single point in time. The repeated longitudinal sampling required to gain a deeper appreciation of tumor heterogeneity over the course of tumor evolution is often not possible because of the morbidity associated with repeated surgical procedures.
Liquid biopsies, in which DNA sequencing can be performed on tumor components that are found circulating in the blood of cancer patients (including circulating tumor cells and cell-free circulating tumor DNA) have rapidly gained traction in the past several decades and offer an unprecedented opportunity for real-time assessment of evolving tumor heterogeneity.
They have proved to be highly sensitive and specific, with a high degree of concordance with tissue biopsy, they can identify both clonal and subclonal mutations, and they can detect resistance substantially earlier than radiographic imaging, which could permit earlier intervention.10,11 The first liquid biopsy-based companion diagnostic test was approved by the US Food and Drug Administration in 2016, for the detection of EGFR mutations associated with NSCLC.
Yet, even liquid biopsy alone is not able to fully dissect the extent of tumor heterogeneity, especially because it is limited in its ability to assess spatial heterogeneity. Truly effective assessment of tumor heterogeneity is likely to require a combination of liquid biopsy, carefully selected tumor tissue biopsies, imaging diagnostics, and biomarkers.
The ongoing TRACERx (Tracking cancer evolution through therapy [Rx]) trials are evaluating a combination of approaches to follow tumor evolution across the course of treatment. The study in NSCLC began in 2014 with a target enrollment of 842 patients and will follow patients over 6 years. Preliminary data from the first 100 patients were recently published and demonstrated that increased intratumor heterogeneity correlated with increased risk of recurrence or death.12
If patients consent, the TRACERx trials also feed into the PEACE (Posthumous evaluation of advanced cancer environment) trials, which are collecting postmortem biopsies to further evaluate tumor heterogeneity and evolution. TRACERx trials in several other cancer types are now also underway.
Cutting off the source
The main therapeutic strategies for overcoming tumor heterogeneity are focused on the mechanisms of resistance that it drives. It is becoming increasingly apparent that rationally designed combinations of drugs are likely to be required and might need to be administered early in the course of disease to prevent resistance.
However, according to mathematical modeling studies, combinations of at least 3 drugs may be necessary.13 In many cases, this is unlikely to be feasible owing to the unavailability of drugs for certain targets and issues of toxicity, as well as the high cost.
An alternative strategy is to use immunotherapy, because a single treatment can target multiple neoantigens simultaneously. Although immunotherapy has proved to be a highly effective treatment paradigm in multiple tumor types, resistance still arises through varied mechanisms with tumor heterogeneity at their core.14,15
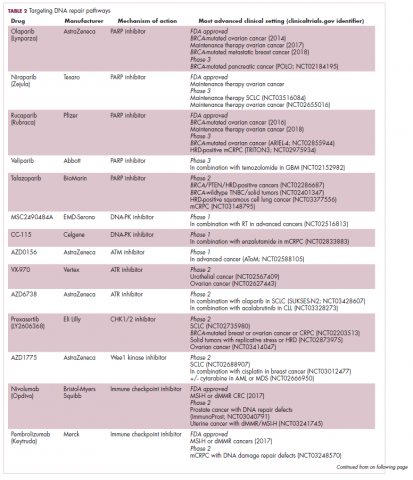
A promising avenue for drug development is to cut off the source of tumor heterogeneity – genomic instability and the mutagenic processes that foster it (Table 2). This is exemplified by the success of poly(ADP-ribose) polymerase (PARP) inhibitors in patients with breast cancer susceptibility (BRCA1/2) gene mutations.
Both germline and somatic mutations in the BRCA1/2 genes are observed in 10% to 15% of patients with ovarian cancer and a substantial number of patients with other types of cancer, including breast, pancreatic, and prostate cancers.16,17
These genes play a central role in the homologous recombination (HR) pathway of DNA repair, which repairs double-strand breaks in DNA. PARP inhibitors target a different DNA repair pathway, base excision repair, which repairs single-strand breaks. The use of PARP inhibitors in patients with BRCA1/2 mutations is designed to create irreparable damage to the DNA repair processes and drive an unsustainable level of genome instability that leads to cell death, whereas normal cells without HR deficiency can survive.18
A growing number of PARP inhibitors are now approved for use in the United States for the treatment of ovarian cancer. In January, olaparib became the first PARP inhibitor approved for patients with BRCA1/2-mutant breast cancer, based on data from the OlympiAD trial in which 302 patients were randomized to receive olaparib 300 mg twice daily or physician’s choice of chemotherapy. Olaparib improved progression-free survival from 4.2 months to 7.0 months (hazard ratio, 0.58; P = .0009), and the most common adverse events included anemia, nausea, fatigue, and vomiting.19
Tumors with other defects in HR have also shown susceptibility to PARP inhibition, shifting interest toward identifying and treating these tumors as a group, independent of histology – about a quarter of all tumors display HR deficiency.20 This novel strategy of targeting mutational processes across a range of tumor types has also been exploited in the development of immunotherapies.
Patients with defects in the mismatch repair (MMR) pathway and microsatellite instability (MSI) – multiple alterations in the length of microsatellite markers within the DNA – are more sensitive to immunotherapy, likely because they are predisposed to a high level of somatic mutations that can serve as neoantigens to provoke a strong anti-tumor immune response.
In 2017, 2 immune checkpoint inhibitors were approved for use in patients with MSI-high or defective MMR (dMMR) cancers. The indication for pembrolizumab (Keytruda) was independent of tumor histology, the first approval of its kind. It was based on the results of 5 clinical trials in which 149 patients with MSI-H or dMMR cancers were given pembrolizumab 200 mg every 3 weeks or 10 mg/kg every 2 weeks for a maximum of 24 months. The overall response rate was 39.6%, including 11 complete responses and 48 partial responses.21
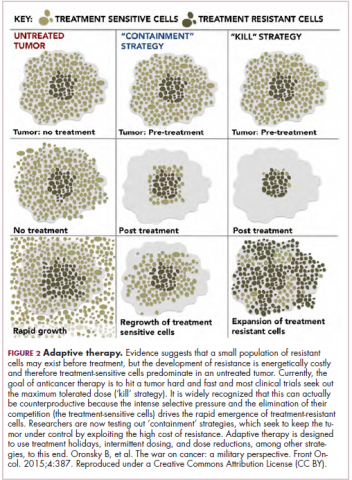
A new paradigm
Treatment of a tumor is one of the major selective pressures that shapes its evolution and recent evidence has emerged that these selective pressures can be highly dynamic. Study findings have shown that there is a cost associated with evolution of resistant subclones and, if the selective pressure of therapy is removed, that cost may become too high, such that resistant subclones are then outcompeted by drug-sensitive ones. There have been reports of reversal of drug resistance when drug treatment is interrupted.
The current treatment paradigm is to try to eliminate tumors by hitting them hard and fast with the maximum tolerated dose (MTD) of a drug. However, there is increasing appreciation that this may be inadvertently fostering more rapid disease progression because it selects for the emergence of resistant cells and eliminates all their competitors (Figure 2).
This is driving a potential paradigm shift, in which researchers are applying concepts from evolutionary biology and the control of invasive species to the treatment of cancer. Instead of completely eliminating a cancer, a strategy of adaptive therapy could be used to set up competition between different subclones and keep tumor growth in check by exploiting the high cost of resistance.22
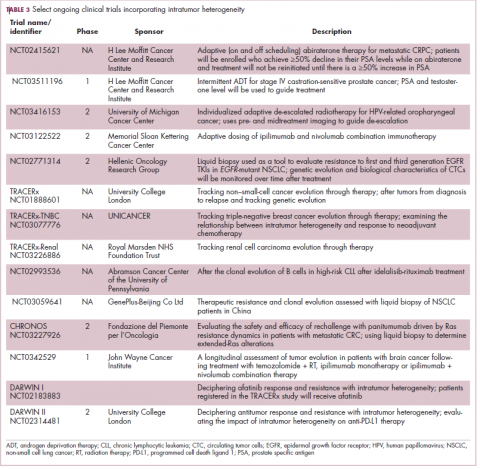
Adaptive therapy involves the use of treatment holidays, intermittent dosing schedules or reduced drug doses, rather than using the MTD. Adaptive therapy was tested recently in mice with triple-negative and estrogen receptor-positive breast cancer. The standard maximum dose of chemotherapy was compared with adaptive therapy with either reduced doses or skipped doses as the tumor responded. Tumor growth initially decreased with all 3 treatment scenarios, but then regrew when chemotherapy was stopped or doses were skipped. However, adaptive therapy with lower doses resulted in long-term stabilization of the tumor where treatment was eventually able to be withdrawn.23 Clinical trials of several different types of adaptive therapy strategies are ongoing (Table 3).
1. Dagogo-Jack I, Shaw AT. Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol. 2018;15(2):81-94.
2. Dzobo K, Senthebane DA, Thomford NE, Rowe A, Dandara C, Parker MI. Not everyone fits the mold: intratumor and intertumor heterogeneity and innovative cancer drug design and development. OMICS. 2018;22(1):17-34.
3. McGranahan N, Swanton C. Clonal heterogeneity and tumor evolution: past, present, and the future. Cell. 2017;168(4):613-628.
4. Davis A, Gao R, Navin N. Tumor evolution: linear, branching, neutral or punctuated? Biochim Biophys Acta. 2017;1867(2):151-161.
5. Amirouchene-Angelozzi N, Swanton C, Bardelli A. Tumor evolution as a therapeutic target. Cancer Discov. Published online first July 20, 2017. Accessed May 23, 2018. doi: 10.1158/2159-8290.CD-17-0343
6. Wu D, Wang DC, Cheng Y, et al. Roles of tumor heterogeneity in the development of drug resistance: a call for precision therapy. Semin Cancer Biol. 2017;42:13-19.
7. Ferguson LR, Chen H, Collins AR, et al. Genomic instability in human cancer: molecular insights and opportunities for therapeutic attack and prevention through diet and nutrition. Semin Cancer Biol. 2015;35(suppl):S5-S24.
8. Forbes SA, Beare D, Gunasekaran P, et al. COSMIC: exploring the world’s knowledge of somatic mutations in human cancer. Nucleic Acids Res. 2015;43(Database issue):D805-811.
9. Rosenthal R, McGranahan N, Herrero J, Swanton C. Deciphering genetic intratumor heterogeneity and its impact on cancer evolution. Ann Rev Cancer Biol. 2017;1(1):223-240.
10. Esposito A, Criscitiello C, Locatelli M, Milano M, Curigliano G. Liquid biopsies for solid tumors: understanding tumor heterogeneity and real time monitoring of early resistance to targeted therapies. Pharmacol Ther. 2016;157:120-124.
11. Venesio T, Siravegna G, Bardelli A, Sapino A. Liquid biopsies for monitoring temporal genomic heterogeneity in breast and colon cancers. Pathobiology. 2018;85(1-2):146-154.
12. Jamal-Hanjani M, Wilson GA, McGranahan N, et al. Tracking the evolution of non–small-cell lung cancer. New Engl J Med. 2017;376(22):2109-2121.
13. Bozic I, Reiter JG, Allen B, et al. Evolutionary dynamics of cancer in response to targeted combination therapy. Elife. 2013;2:e00747.
14. Zugazagoitia J, Guedes C, Ponce S, Ferrer I, Molina-Pinelo S, Paz-Ares L. Current challenges in cancer treatment. Clin Ther. 2016;38(7):1551-1566.
15. Ventola CL. Cancer immunotherapy, Part 3: challenges and future trends. PT. 2017;42(8):514-521.
16. Cavanagh H, Rogers KMA. The role of BRCA1 and BRCA2 mutations in prostate, pancreatic and stomach cancers. Hered Cancer Clin Pract. 2015;13:16.
17. Moschetta M, George A, Kaye SB, Banerjee S. BRCA somatic mutations and epigenetic BRCA modifications in serous ovarian cancer. Ann Oncol. 2016;27(8):1449-1455.
18. Brown JS, O’Carrigan B, Jackson SP, Yap TA. Targeting DNA repair in cancer: beyond PARP inhibitors. Cancer Discov. 2017;7(1):20-37.
19. Robson M, Im S-A, Senkus E, et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. New England Journal of Medicine. 2017;377(6):523-533.
20. Williers H, Pfaffle HN, Zou L. Targeting homologous recombination repair in cancer: molecular targets and clinical applications. In: Kelley M, Fishel M, eds. DNA repair in cancer therapy. 2nd ed: Academic Press; 2016:119-160.
21. U.S. Food and Drug Administration. FDA grants accelerated approval to pembrolizumab for first tissue/site agnostic indication. 2017; https://www.fda.gov/Drugs/InformationOnDrugs/ ApprovedDrugs/ucm560040.htm. Accessed May 1st,, 2018.
22. Gallaher JA, Enriquez-Navas PM, Luddy KA, Gatenby RA, Anderson ARA. Adaptive Therapy For Heterogeneous Cancer: Exploiting Space And Trade-Offs In Drug Scheduling. bioRxiv. 2017.
23. Enriquez-Navas PM, Kam Y, Das T, et al. Exploiting evolutionary principles to prolong tumor control in preclinical models of breast cancer. Sci Transl Med. 2016;8(327):327ra24.
A major challenge to effective cancer treatment is the astounding level of heterogeneity that tumors display on many different fronts. Here, we discuss how a deeper appreciation of this heterogeneity and its impact is driving research efforts to better understand and tackle it and a radical rethink of treatment paradigms.
A complex and dynamic disease
The nonuniformity of cancer has long been appreciated, reflected most visibly in the variation of response to the same treatment across patients with the same type of tumor (inter-tumor heterogeneity). The extent of tumor heterogeneity is being fully realized only now, with the advent of next-generation sequencing technologies. Even within the same tumor, there can be significant heterogeneity from cell to cell (intra-tumor heterogeneity), yielding substantial complexity in cancer.
Heterogeneity reveals itself on many different levels. Histologically speaking, tumors are composed of a nonhomogenous mass of cells that vary in type and number. In terms of their molecular make-up, there is substantial variation in the types of molecular alterations observed, all the way down to the single cell level. In even more abstract terms, beyond the cancer itself, the microenvironment in which it resides can be highly heterogeneous, composed of a plethora of different supportive and tumor-infiltrating normal cells.
Heterogeneity can manifest spatially, reflecting differences in the composition of the primary tumor and tumors at secondary sites or across regions of the same tumor mass and temporally, at different time points across a tumor’s natural history. Evocative of the second law of thermodynamics, cancers generally become more diverse and complex over time.1-3
A tale of 2 models
It is widely accepted that the transformation of a normal cell into a malignant one occurs with the acquisition of certain “hallmark” abilities, but there are myriad ways in which these can be attained.
The clonal evolution model
As cells divide, they randomly acquire mutations as a result of DNA damage. The clonal evolution model posits that cancer develops as the result of a multistep accumulation of a series of “driver” mutations that confer a promalignant advantage to the cell and ultimately fuel a cancerous hallmark.
This evolution can occur in a linear fashion, whereby the emergence of a new driver mutation conveys such a potent evolutionary advantage that it outcompetes all previous clones. There is limited evidence for linear evolution in most advanced human cancers; instead, they are thought to evolve predominantly through a process of branching evolution, in which multiple clones can diverge in parallel from a common ancestor through the acquisition of different driver mutations. This results in common clonal mutations that form the trunk of the cancer’s evolutionary tree and are shared by all cells and subclonal mutations, which make up the branches and differ from cell to cell.
More recently, several other mechanisms of clonal evolution have been proposed, including neutral evolution, a type of branching evolution in which there are no selective pressures and evolution occurs by random mutations occurring over time that lead to genetic drift, and punctuated evolution, in which there are short evolutionary bursts of hypermutation.4,5
The CSC model
This model posits that the ability to form and sustain a cancer is restricted to a single cell type – the cancer stem cells – which have the unique capacity for self-renewal and differentiation. Although the forces of evolution are still involved in this model, they act on a hierarchy of cells, with stem cells sitting at the top. A tumor is derived from a single stem cell that has acquired a mutation, and the heterogeneity observed results both from the differentiation and the accumulation of mutations in CSCs.
Accumulated experimental evidence suggests that these models are not mutually exclusive and that they can all contribute to heterogeneity in varied amounts across different tumor types. What is clear is that heterogeneity and evolution are intricately intertwined in cancer development.1,2,6
An unstable genome
Heterogeneity and evolution are fueled by genomic alterations and the genome instability that they foster. This genome instability can range from single base pair substitutions to a doubling of the entire genome and results from both exposure to exogenous mutagens (eg, chemicals and ultraviolet radiation) and genomic alterations that have an impact on important cellular processes (eg, DNA repair or replication).
Among the most common causes of genome instability are mutations in the DNA mismatch repair pathway proteins or in the proofreading polymerase enzymes. Genome instability is often associated with unique mutational signatures – characteristic combinations of mutations that arose as the result of the specific biological processes underlying them.7
Genome-wide analyses have begun to reveal these mutational signatures across the spectrum of human cancers. The Wellcome Sanger Institute’s Catalogue of Somatic Mutations in Cancer (COSMIC) database has generated a set of 30 mutational signatures based on analysis of almost 11,000 exomes and more than 1,000 whole genomes spanning 40 different cancer types, some of which have been linked with specific mutagenic processes, such as tobacco, UV radiation, and DNA repair deficiency (Table 1).8
Fueling resistance
Arguably, heterogeneity presents one of the most significant barriers to effective cancer therapy, and this has become increasingly true in the era of personalized medicine in which targeted therapies take aim at specific molecular abnormalities.
It is vital that drugs target the truncal alterations that are present in all cancer cells to ensure that the entire cancer is eradicated. However, it is not always possible to target these alterations, for example, at the present time tumor suppressor proteins like p53 are not druggable.
Even when truncal alterations have been targeted successfully, such as epidermal growth factor receptor (EGFR) mutations and anaplastic lymphoma kinase (ALK) chromosomal rearrangements in non–small-cell lung cancer (NSCLC) and BRAF mutations in melanoma, the long-term efficacy of these drugs is almost invariably limited by the development of resistance.
Tumor heterogeneity and the clonal evolution it fuels are central drivers of resistance. Because tumors are dynamic and continue to evolve, anticancer treatments can act as a strong selective pressure and drive the emergence of drug-resistant subclones that allow the tumor to persist. In fact, study findings have revealed that small populations of resistant cells may be present before treatment. Thus, resistance may also occur as a result of the outgrowth of preexisting treatment-resistant cells that suddenly find that they acquire a survival advantage in the presence of a drug.1,6
Tackling heterogeneity
Despite extensive clinical documentation of the existence of heterogeneity and its underlying mechanisms across a range of tumor types, the development of novel clinical trial designs and therapeutic strategies that account for its effects have only recently begun to be explored.
For the most part, this was because of a lack of effective methods for evaluating intratumor heterogeneity. Multiregion biopsies, in which tissue derived from multiple different regions of a single tumor mass or from distinct cancerous lesions within the same patient, give a snapshot of tumor heterogeneity at a single point in time. The repeated longitudinal sampling required to gain a deeper appreciation of tumor heterogeneity over the course of tumor evolution is often not possible because of the morbidity associated with repeated surgical procedures.
Liquid biopsies, in which DNA sequencing can be performed on tumor components that are found circulating in the blood of cancer patients (including circulating tumor cells and cell-free circulating tumor DNA) have rapidly gained traction in the past several decades and offer an unprecedented opportunity for real-time assessment of evolving tumor heterogeneity.
They have proved to be highly sensitive and specific, with a high degree of concordance with tissue biopsy, they can identify both clonal and subclonal mutations, and they can detect resistance substantially earlier than radiographic imaging, which could permit earlier intervention.10,11 The first liquid biopsy-based companion diagnostic test was approved by the US Food and Drug Administration in 2016, for the detection of EGFR mutations associated with NSCLC.
Yet, even liquid biopsy alone is not able to fully dissect the extent of tumor heterogeneity, especially because it is limited in its ability to assess spatial heterogeneity. Truly effective assessment of tumor heterogeneity is likely to require a combination of liquid biopsy, carefully selected tumor tissue biopsies, imaging diagnostics, and biomarkers.
The ongoing TRACERx (Tracking cancer evolution through therapy [Rx]) trials are evaluating a combination of approaches to follow tumor evolution across the course of treatment. The study in NSCLC began in 2014 with a target enrollment of 842 patients and will follow patients over 6 years. Preliminary data from the first 100 patients were recently published and demonstrated that increased intratumor heterogeneity correlated with increased risk of recurrence or death.12
If patients consent, the TRACERx trials also feed into the PEACE (Posthumous evaluation of advanced cancer environment) trials, which are collecting postmortem biopsies to further evaluate tumor heterogeneity and evolution. TRACERx trials in several other cancer types are now also underway.
Cutting off the source
The main therapeutic strategies for overcoming tumor heterogeneity are focused on the mechanisms of resistance that it drives. It is becoming increasingly apparent that rationally designed combinations of drugs are likely to be required and might need to be administered early in the course of disease to prevent resistance.
However, according to mathematical modeling studies, combinations of at least 3 drugs may be necessary.13 In many cases, this is unlikely to be feasible owing to the unavailability of drugs for certain targets and issues of toxicity, as well as the high cost.
An alternative strategy is to use immunotherapy, because a single treatment can target multiple neoantigens simultaneously. Although immunotherapy has proved to be a highly effective treatment paradigm in multiple tumor types, resistance still arises through varied mechanisms with tumor heterogeneity at their core.14,15
A promising avenue for drug development is to cut off the source of tumor heterogeneity – genomic instability and the mutagenic processes that foster it (Table 2). This is exemplified by the success of poly(ADP-ribose) polymerase (PARP) inhibitors in patients with breast cancer susceptibility (BRCA1/2) gene mutations.
Both germline and somatic mutations in the BRCA1/2 genes are observed in 10% to 15% of patients with ovarian cancer and a substantial number of patients with other types of cancer, including breast, pancreatic, and prostate cancers.16,17
These genes play a central role in the homologous recombination (HR) pathway of DNA repair, which repairs double-strand breaks in DNA. PARP inhibitors target a different DNA repair pathway, base excision repair, which repairs single-strand breaks. The use of PARP inhibitors in patients with BRCA1/2 mutations is designed to create irreparable damage to the DNA repair processes and drive an unsustainable level of genome instability that leads to cell death, whereas normal cells without HR deficiency can survive.18
A growing number of PARP inhibitors are now approved for use in the United States for the treatment of ovarian cancer. In January, olaparib became the first PARP inhibitor approved for patients with BRCA1/2-mutant breast cancer, based on data from the OlympiAD trial in which 302 patients were randomized to receive olaparib 300 mg twice daily or physician’s choice of chemotherapy. Olaparib improved progression-free survival from 4.2 months to 7.0 months (hazard ratio, 0.58; P = .0009), and the most common adverse events included anemia, nausea, fatigue, and vomiting.19
Tumors with other defects in HR have also shown susceptibility to PARP inhibition, shifting interest toward identifying and treating these tumors as a group, independent of histology – about a quarter of all tumors display HR deficiency.20 This novel strategy of targeting mutational processes across a range of tumor types has also been exploited in the development of immunotherapies.
Patients with defects in the mismatch repair (MMR) pathway and microsatellite instability (MSI) – multiple alterations in the length of microsatellite markers within the DNA – are more sensitive to immunotherapy, likely because they are predisposed to a high level of somatic mutations that can serve as neoantigens to provoke a strong anti-tumor immune response.
In 2017, 2 immune checkpoint inhibitors were approved for use in patients with MSI-high or defective MMR (dMMR) cancers. The indication for pembrolizumab (Keytruda) was independent of tumor histology, the first approval of its kind. It was based on the results of 5 clinical trials in which 149 patients with MSI-H or dMMR cancers were given pembrolizumab 200 mg every 3 weeks or 10 mg/kg every 2 weeks for a maximum of 24 months. The overall response rate was 39.6%, including 11 complete responses and 48 partial responses.21
A new paradigm
Treatment of a tumor is one of the major selective pressures that shapes its evolution and recent evidence has emerged that these selective pressures can be highly dynamic. Study findings have shown that there is a cost associated with evolution of resistant subclones and, if the selective pressure of therapy is removed, that cost may become too high, such that resistant subclones are then outcompeted by drug-sensitive ones. There have been reports of reversal of drug resistance when drug treatment is interrupted.
The current treatment paradigm is to try to eliminate tumors by hitting them hard and fast with the maximum tolerated dose (MTD) of a drug. However, there is increasing appreciation that this may be inadvertently fostering more rapid disease progression because it selects for the emergence of resistant cells and eliminates all their competitors (Figure 2).
This is driving a potential paradigm shift, in which researchers are applying concepts from evolutionary biology and the control of invasive species to the treatment of cancer. Instead of completely eliminating a cancer, a strategy of adaptive therapy could be used to set up competition between different subclones and keep tumor growth in check by exploiting the high cost of resistance.22
Adaptive therapy involves the use of treatment holidays, intermittent dosing schedules or reduced drug doses, rather than using the MTD. Adaptive therapy was tested recently in mice with triple-negative and estrogen receptor-positive breast cancer. The standard maximum dose of chemotherapy was compared with adaptive therapy with either reduced doses or skipped doses as the tumor responded. Tumor growth initially decreased with all 3 treatment scenarios, but then regrew when chemotherapy was stopped or doses were skipped. However, adaptive therapy with lower doses resulted in long-term stabilization of the tumor where treatment was eventually able to be withdrawn.23 Clinical trials of several different types of adaptive therapy strategies are ongoing (Table 3).
A major challenge to effective cancer treatment is the astounding level of heterogeneity that tumors display on many different fronts. Here, we discuss how a deeper appreciation of this heterogeneity and its impact is driving research efforts to better understand and tackle it and a radical rethink of treatment paradigms.
A complex and dynamic disease
The nonuniformity of cancer has long been appreciated, reflected most visibly in the variation of response to the same treatment across patients with the same type of tumor (inter-tumor heterogeneity). The extent of tumor heterogeneity is being fully realized only now, with the advent of next-generation sequencing technologies. Even within the same tumor, there can be significant heterogeneity from cell to cell (intra-tumor heterogeneity), yielding substantial complexity in cancer.
Heterogeneity reveals itself on many different levels. Histologically speaking, tumors are composed of a nonhomogenous mass of cells that vary in type and number. In terms of their molecular make-up, there is substantial variation in the types of molecular alterations observed, all the way down to the single cell level. In even more abstract terms, beyond the cancer itself, the microenvironment in which it resides can be highly heterogeneous, composed of a plethora of different supportive and tumor-infiltrating normal cells.
Heterogeneity can manifest spatially, reflecting differences in the composition of the primary tumor and tumors at secondary sites or across regions of the same tumor mass and temporally, at different time points across a tumor’s natural history. Evocative of the second law of thermodynamics, cancers generally become more diverse and complex over time.1-3
A tale of 2 models
It is widely accepted that the transformation of a normal cell into a malignant one occurs with the acquisition of certain “hallmark” abilities, but there are myriad ways in which these can be attained.
The clonal evolution model
As cells divide, they randomly acquire mutations as a result of DNA damage. The clonal evolution model posits that cancer develops as the result of a multistep accumulation of a series of “driver” mutations that confer a promalignant advantage to the cell and ultimately fuel a cancerous hallmark.
This evolution can occur in a linear fashion, whereby the emergence of a new driver mutation conveys such a potent evolutionary advantage that it outcompetes all previous clones. There is limited evidence for linear evolution in most advanced human cancers; instead, they are thought to evolve predominantly through a process of branching evolution, in which multiple clones can diverge in parallel from a common ancestor through the acquisition of different driver mutations. This results in common clonal mutations that form the trunk of the cancer’s evolutionary tree and are shared by all cells and subclonal mutations, which make up the branches and differ from cell to cell.
More recently, several other mechanisms of clonal evolution have been proposed, including neutral evolution, a type of branching evolution in which there are no selective pressures and evolution occurs by random mutations occurring over time that lead to genetic drift, and punctuated evolution, in which there are short evolutionary bursts of hypermutation.4,5
The CSC model
This model posits that the ability to form and sustain a cancer is restricted to a single cell type – the cancer stem cells – which have the unique capacity for self-renewal and differentiation. Although the forces of evolution are still involved in this model, they act on a hierarchy of cells, with stem cells sitting at the top. A tumor is derived from a single stem cell that has acquired a mutation, and the heterogeneity observed results both from the differentiation and the accumulation of mutations in CSCs.
Accumulated experimental evidence suggests that these models are not mutually exclusive and that they can all contribute to heterogeneity in varied amounts across different tumor types. What is clear is that heterogeneity and evolution are intricately intertwined in cancer development.1,2,6
An unstable genome
Heterogeneity and evolution are fueled by genomic alterations and the genome instability that they foster. This genome instability can range from single base pair substitutions to a doubling of the entire genome and results from both exposure to exogenous mutagens (eg, chemicals and ultraviolet radiation) and genomic alterations that have an impact on important cellular processes (eg, DNA repair or replication).
Among the most common causes of genome instability are mutations in the DNA mismatch repair pathway proteins or in the proofreading polymerase enzymes. Genome instability is often associated with unique mutational signatures – characteristic combinations of mutations that arose as the result of the specific biological processes underlying them.7
Genome-wide analyses have begun to reveal these mutational signatures across the spectrum of human cancers. The Wellcome Sanger Institute’s Catalogue of Somatic Mutations in Cancer (COSMIC) database has generated a set of 30 mutational signatures based on analysis of almost 11,000 exomes and more than 1,000 whole genomes spanning 40 different cancer types, some of which have been linked with specific mutagenic processes, such as tobacco, UV radiation, and DNA repair deficiency (Table 1).8
Fueling resistance
Arguably, heterogeneity presents one of the most significant barriers to effective cancer therapy, and this has become increasingly true in the era of personalized medicine in which targeted therapies take aim at specific molecular abnormalities.
It is vital that drugs target the truncal alterations that are present in all cancer cells to ensure that the entire cancer is eradicated. However, it is not always possible to target these alterations, for example, at the present time tumor suppressor proteins like p53 are not druggable.
Even when truncal alterations have been targeted successfully, such as epidermal growth factor receptor (EGFR) mutations and anaplastic lymphoma kinase (ALK) chromosomal rearrangements in non–small-cell lung cancer (NSCLC) and BRAF mutations in melanoma, the long-term efficacy of these drugs is almost invariably limited by the development of resistance.
Tumor heterogeneity and the clonal evolution it fuels are central drivers of resistance. Because tumors are dynamic and continue to evolve, anticancer treatments can act as a strong selective pressure and drive the emergence of drug-resistant subclones that allow the tumor to persist. In fact, study findings have revealed that small populations of resistant cells may be present before treatment. Thus, resistance may also occur as a result of the outgrowth of preexisting treatment-resistant cells that suddenly find that they acquire a survival advantage in the presence of a drug.1,6
Tackling heterogeneity
Despite extensive clinical documentation of the existence of heterogeneity and its underlying mechanisms across a range of tumor types, the development of novel clinical trial designs and therapeutic strategies that account for its effects have only recently begun to be explored.
For the most part, this was because of a lack of effective methods for evaluating intratumor heterogeneity. Multiregion biopsies, in which tissue derived from multiple different regions of a single tumor mass or from distinct cancerous lesions within the same patient, give a snapshot of tumor heterogeneity at a single point in time. The repeated longitudinal sampling required to gain a deeper appreciation of tumor heterogeneity over the course of tumor evolution is often not possible because of the morbidity associated with repeated surgical procedures.
Liquid biopsies, in which DNA sequencing can be performed on tumor components that are found circulating in the blood of cancer patients (including circulating tumor cells and cell-free circulating tumor DNA) have rapidly gained traction in the past several decades and offer an unprecedented opportunity for real-time assessment of evolving tumor heterogeneity.
They have proved to be highly sensitive and specific, with a high degree of concordance with tissue biopsy, they can identify both clonal and subclonal mutations, and they can detect resistance substantially earlier than radiographic imaging, which could permit earlier intervention.10,11 The first liquid biopsy-based companion diagnostic test was approved by the US Food and Drug Administration in 2016, for the detection of EGFR mutations associated with NSCLC.
Yet, even liquid biopsy alone is not able to fully dissect the extent of tumor heterogeneity, especially because it is limited in its ability to assess spatial heterogeneity. Truly effective assessment of tumor heterogeneity is likely to require a combination of liquid biopsy, carefully selected tumor tissue biopsies, imaging diagnostics, and biomarkers.
The ongoing TRACERx (Tracking cancer evolution through therapy [Rx]) trials are evaluating a combination of approaches to follow tumor evolution across the course of treatment. The study in NSCLC began in 2014 with a target enrollment of 842 patients and will follow patients over 6 years. Preliminary data from the first 100 patients were recently published and demonstrated that increased intratumor heterogeneity correlated with increased risk of recurrence or death.12
If patients consent, the TRACERx trials also feed into the PEACE (Posthumous evaluation of advanced cancer environment) trials, which are collecting postmortem biopsies to further evaluate tumor heterogeneity and evolution. TRACERx trials in several other cancer types are now also underway.
Cutting off the source
The main therapeutic strategies for overcoming tumor heterogeneity are focused on the mechanisms of resistance that it drives. It is becoming increasingly apparent that rationally designed combinations of drugs are likely to be required and might need to be administered early in the course of disease to prevent resistance.
However, according to mathematical modeling studies, combinations of at least 3 drugs may be necessary.13 In many cases, this is unlikely to be feasible owing to the unavailability of drugs for certain targets and issues of toxicity, as well as the high cost.
An alternative strategy is to use immunotherapy, because a single treatment can target multiple neoantigens simultaneously. Although immunotherapy has proved to be a highly effective treatment paradigm in multiple tumor types, resistance still arises through varied mechanisms with tumor heterogeneity at their core.14,15
A promising avenue for drug development is to cut off the source of tumor heterogeneity – genomic instability and the mutagenic processes that foster it (Table 2). This is exemplified by the success of poly(ADP-ribose) polymerase (PARP) inhibitors in patients with breast cancer susceptibility (BRCA1/2) gene mutations.
Both germline and somatic mutations in the BRCA1/2 genes are observed in 10% to 15% of patients with ovarian cancer and a substantial number of patients with other types of cancer, including breast, pancreatic, and prostate cancers.16,17
These genes play a central role in the homologous recombination (HR) pathway of DNA repair, which repairs double-strand breaks in DNA. PARP inhibitors target a different DNA repair pathway, base excision repair, which repairs single-strand breaks. The use of PARP inhibitors in patients with BRCA1/2 mutations is designed to create irreparable damage to the DNA repair processes and drive an unsustainable level of genome instability that leads to cell death, whereas normal cells without HR deficiency can survive.18
A growing number of PARP inhibitors are now approved for use in the United States for the treatment of ovarian cancer. In January, olaparib became the first PARP inhibitor approved for patients with BRCA1/2-mutant breast cancer, based on data from the OlympiAD trial in which 302 patients were randomized to receive olaparib 300 mg twice daily or physician’s choice of chemotherapy. Olaparib improved progression-free survival from 4.2 months to 7.0 months (hazard ratio, 0.58; P = .0009), and the most common adverse events included anemia, nausea, fatigue, and vomiting.19
Tumors with other defects in HR have also shown susceptibility to PARP inhibition, shifting interest toward identifying and treating these tumors as a group, independent of histology – about a quarter of all tumors display HR deficiency.20 This novel strategy of targeting mutational processes across a range of tumor types has also been exploited in the development of immunotherapies.
Patients with defects in the mismatch repair (MMR) pathway and microsatellite instability (MSI) – multiple alterations in the length of microsatellite markers within the DNA – are more sensitive to immunotherapy, likely because they are predisposed to a high level of somatic mutations that can serve as neoantigens to provoke a strong anti-tumor immune response.
In 2017, 2 immune checkpoint inhibitors were approved for use in patients with MSI-high or defective MMR (dMMR) cancers. The indication for pembrolizumab (Keytruda) was independent of tumor histology, the first approval of its kind. It was based on the results of 5 clinical trials in which 149 patients with MSI-H or dMMR cancers were given pembrolizumab 200 mg every 3 weeks or 10 mg/kg every 2 weeks for a maximum of 24 months. The overall response rate was 39.6%, including 11 complete responses and 48 partial responses.21
A new paradigm
Treatment of a tumor is one of the major selective pressures that shapes its evolution and recent evidence has emerged that these selective pressures can be highly dynamic. Study findings have shown that there is a cost associated with evolution of resistant subclones and, if the selective pressure of therapy is removed, that cost may become too high, such that resistant subclones are then outcompeted by drug-sensitive ones. There have been reports of reversal of drug resistance when drug treatment is interrupted.
The current treatment paradigm is to try to eliminate tumors by hitting them hard and fast with the maximum tolerated dose (MTD) of a drug. However, there is increasing appreciation that this may be inadvertently fostering more rapid disease progression because it selects for the emergence of resistant cells and eliminates all their competitors (Figure 2).
This is driving a potential paradigm shift, in which researchers are applying concepts from evolutionary biology and the control of invasive species to the treatment of cancer. Instead of completely eliminating a cancer, a strategy of adaptive therapy could be used to set up competition between different subclones and keep tumor growth in check by exploiting the high cost of resistance.22
Adaptive therapy involves the use of treatment holidays, intermittent dosing schedules or reduced drug doses, rather than using the MTD. Adaptive therapy was tested recently in mice with triple-negative and estrogen receptor-positive breast cancer. The standard maximum dose of chemotherapy was compared with adaptive therapy with either reduced doses or skipped doses as the tumor responded. Tumor growth initially decreased with all 3 treatment scenarios, but then regrew when chemotherapy was stopped or doses were skipped. However, adaptive therapy with lower doses resulted in long-term stabilization of the tumor where treatment was eventually able to be withdrawn.23 Clinical trials of several different types of adaptive therapy strategies are ongoing (Table 3).
1. Dagogo-Jack I, Shaw AT. Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol. 2018;15(2):81-94.
2. Dzobo K, Senthebane DA, Thomford NE, Rowe A, Dandara C, Parker MI. Not everyone fits the mold: intratumor and intertumor heterogeneity and innovative cancer drug design and development. OMICS. 2018;22(1):17-34.
3. McGranahan N, Swanton C. Clonal heterogeneity and tumor evolution: past, present, and the future. Cell. 2017;168(4):613-628.
4. Davis A, Gao R, Navin N. Tumor evolution: linear, branching, neutral or punctuated? Biochim Biophys Acta. 2017;1867(2):151-161.
5. Amirouchene-Angelozzi N, Swanton C, Bardelli A. Tumor evolution as a therapeutic target. Cancer Discov. Published online first July 20, 2017. Accessed May 23, 2018. doi: 10.1158/2159-8290.CD-17-0343
6. Wu D, Wang DC, Cheng Y, et al. Roles of tumor heterogeneity in the development of drug resistance: a call for precision therapy. Semin Cancer Biol. 2017;42:13-19.
7. Ferguson LR, Chen H, Collins AR, et al. Genomic instability in human cancer: molecular insights and opportunities for therapeutic attack and prevention through diet and nutrition. Semin Cancer Biol. 2015;35(suppl):S5-S24.
8. Forbes SA, Beare D, Gunasekaran P, et al. COSMIC: exploring the world’s knowledge of somatic mutations in human cancer. Nucleic Acids Res. 2015;43(Database issue):D805-811.
9. Rosenthal R, McGranahan N, Herrero J, Swanton C. Deciphering genetic intratumor heterogeneity and its impact on cancer evolution. Ann Rev Cancer Biol. 2017;1(1):223-240.
10. Esposito A, Criscitiello C, Locatelli M, Milano M, Curigliano G. Liquid biopsies for solid tumors: understanding tumor heterogeneity and real time monitoring of early resistance to targeted therapies. Pharmacol Ther. 2016;157:120-124.
11. Venesio T, Siravegna G, Bardelli A, Sapino A. Liquid biopsies for monitoring temporal genomic heterogeneity in breast and colon cancers. Pathobiology. 2018;85(1-2):146-154.
12. Jamal-Hanjani M, Wilson GA, McGranahan N, et al. Tracking the evolution of non–small-cell lung cancer. New Engl J Med. 2017;376(22):2109-2121.
13. Bozic I, Reiter JG, Allen B, et al. Evolutionary dynamics of cancer in response to targeted combination therapy. Elife. 2013;2:e00747.
14. Zugazagoitia J, Guedes C, Ponce S, Ferrer I, Molina-Pinelo S, Paz-Ares L. Current challenges in cancer treatment. Clin Ther. 2016;38(7):1551-1566.
15. Ventola CL. Cancer immunotherapy, Part 3: challenges and future trends. PT. 2017;42(8):514-521.
16. Cavanagh H, Rogers KMA. The role of BRCA1 and BRCA2 mutations in prostate, pancreatic and stomach cancers. Hered Cancer Clin Pract. 2015;13:16.
17. Moschetta M, George A, Kaye SB, Banerjee S. BRCA somatic mutations and epigenetic BRCA modifications in serous ovarian cancer. Ann Oncol. 2016;27(8):1449-1455.
18. Brown JS, O’Carrigan B, Jackson SP, Yap TA. Targeting DNA repair in cancer: beyond PARP inhibitors. Cancer Discov. 2017;7(1):20-37.
19. Robson M, Im S-A, Senkus E, et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. New England Journal of Medicine. 2017;377(6):523-533.
20. Williers H, Pfaffle HN, Zou L. Targeting homologous recombination repair in cancer: molecular targets and clinical applications. In: Kelley M, Fishel M, eds. DNA repair in cancer therapy. 2nd ed: Academic Press; 2016:119-160.
21. U.S. Food and Drug Administration. FDA grants accelerated approval to pembrolizumab for first tissue/site agnostic indication. 2017; https://www.fda.gov/Drugs/InformationOnDrugs/ ApprovedDrugs/ucm560040.htm. Accessed May 1st,, 2018.
22. Gallaher JA, Enriquez-Navas PM, Luddy KA, Gatenby RA, Anderson ARA. Adaptive Therapy For Heterogeneous Cancer: Exploiting Space And Trade-Offs In Drug Scheduling. bioRxiv. 2017.
23. Enriquez-Navas PM, Kam Y, Das T, et al. Exploiting evolutionary principles to prolong tumor control in preclinical models of breast cancer. Sci Transl Med. 2016;8(327):327ra24.
1. Dagogo-Jack I, Shaw AT. Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol. 2018;15(2):81-94.
2. Dzobo K, Senthebane DA, Thomford NE, Rowe A, Dandara C, Parker MI. Not everyone fits the mold: intratumor and intertumor heterogeneity and innovative cancer drug design and development. OMICS. 2018;22(1):17-34.
3. McGranahan N, Swanton C. Clonal heterogeneity and tumor evolution: past, present, and the future. Cell. 2017;168(4):613-628.
4. Davis A, Gao R, Navin N. Tumor evolution: linear, branching, neutral or punctuated? Biochim Biophys Acta. 2017;1867(2):151-161.
5. Amirouchene-Angelozzi N, Swanton C, Bardelli A. Tumor evolution as a therapeutic target. Cancer Discov. Published online first July 20, 2017. Accessed May 23, 2018. doi: 10.1158/2159-8290.CD-17-0343
6. Wu D, Wang DC, Cheng Y, et al. Roles of tumor heterogeneity in the development of drug resistance: a call for precision therapy. Semin Cancer Biol. 2017;42:13-19.
7. Ferguson LR, Chen H, Collins AR, et al. Genomic instability in human cancer: molecular insights and opportunities for therapeutic attack and prevention through diet and nutrition. Semin Cancer Biol. 2015;35(suppl):S5-S24.
8. Forbes SA, Beare D, Gunasekaran P, et al. COSMIC: exploring the world’s knowledge of somatic mutations in human cancer. Nucleic Acids Res. 2015;43(Database issue):D805-811.
9. Rosenthal R, McGranahan N, Herrero J, Swanton C. Deciphering genetic intratumor heterogeneity and its impact on cancer evolution. Ann Rev Cancer Biol. 2017;1(1):223-240.
10. Esposito A, Criscitiello C, Locatelli M, Milano M, Curigliano G. Liquid biopsies for solid tumors: understanding tumor heterogeneity and real time monitoring of early resistance to targeted therapies. Pharmacol Ther. 2016;157:120-124.
11. Venesio T, Siravegna G, Bardelli A, Sapino A. Liquid biopsies for monitoring temporal genomic heterogeneity in breast and colon cancers. Pathobiology. 2018;85(1-2):146-154.
12. Jamal-Hanjani M, Wilson GA, McGranahan N, et al. Tracking the evolution of non–small-cell lung cancer. New Engl J Med. 2017;376(22):2109-2121.
13. Bozic I, Reiter JG, Allen B, et al. Evolutionary dynamics of cancer in response to targeted combination therapy. Elife. 2013;2:e00747.
14. Zugazagoitia J, Guedes C, Ponce S, Ferrer I, Molina-Pinelo S, Paz-Ares L. Current challenges in cancer treatment. Clin Ther. 2016;38(7):1551-1566.
15. Ventola CL. Cancer immunotherapy, Part 3: challenges and future trends. PT. 2017;42(8):514-521.
16. Cavanagh H, Rogers KMA. The role of BRCA1 and BRCA2 mutations in prostate, pancreatic and stomach cancers. Hered Cancer Clin Pract. 2015;13:16.
17. Moschetta M, George A, Kaye SB, Banerjee S. BRCA somatic mutations and epigenetic BRCA modifications in serous ovarian cancer. Ann Oncol. 2016;27(8):1449-1455.
18. Brown JS, O’Carrigan B, Jackson SP, Yap TA. Targeting DNA repair in cancer: beyond PARP inhibitors. Cancer Discov. 2017;7(1):20-37.
19. Robson M, Im S-A, Senkus E, et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. New England Journal of Medicine. 2017;377(6):523-533.
20. Williers H, Pfaffle HN, Zou L. Targeting homologous recombination repair in cancer: molecular targets and clinical applications. In: Kelley M, Fishel M, eds. DNA repair in cancer therapy. 2nd ed: Academic Press; 2016:119-160.
21. U.S. Food and Drug Administration. FDA grants accelerated approval to pembrolizumab for first tissue/site agnostic indication. 2017; https://www.fda.gov/Drugs/InformationOnDrugs/ ApprovedDrugs/ucm560040.htm. Accessed May 1st,, 2018.
22. Gallaher JA, Enriquez-Navas PM, Luddy KA, Gatenby RA, Anderson ARA. Adaptive Therapy For Heterogeneous Cancer: Exploiting Space And Trade-Offs In Drug Scheduling. bioRxiv. 2017.
23. Enriquez-Navas PM, Kam Y, Das T, et al. Exploiting evolutionary principles to prolong tumor control in preclinical models of breast cancer. Sci Transl Med. 2016;8(327):327ra24.
Recurrence of a small gastric gastrointestinal stromal tumor with high mitotic index
Gastrointestinal stromal tumor (GIST) is the most common soft tissue sarcoma of the gastrointestinal tract, usually arising from the interstitial cells of Cajal or similar cells in the outer wall of the gastrointestinal tract.1,2 Most GISTs have an activating mutation in KIT or platelet-derived growth factor receptor alpha (PDGFRA). Tumor size, mitotic rate, and anatomic site are the most common pathological features used to risk stratify GIST tumors.3-10 It is important to note when using such risk calculators that preoperative imatinib before determining tumor characteristics (such as mitoses per 50 high-power fields [hpf]) often changes the relevant parameters so that the same risk calculations may not apply. Tumors with a mitotic rate ≤5 mitoses per 50 hpf and a size ≤5 cm in greatest dimension have a lower recurrence rate after resection than tumors with a mitotic rate >5 mitoses per 50 hpf and a size >10 cm, and larger tumors can have a recurrence rate of up to 86%.11,12 Findings from a large observational study have suggested that the prognosis of gastric GIST in Korea and Japan may be more favorable compared with that in Western countries.13
The primary treatment of a localized primary GIST is surgical excision, but a cure is limited by recurrence.14,15 Imatinib is useful in the treatment of metastatic or recurrent GIST, and adjuvant treatment with imatinib after surgery has been shown to improve progression-free and overall survival in some cases.3,16-18 Responses to adjuvant imatinib depend on tumor sensitivity to the drug and the risk of recurrence. Drug sensitivity is largely dependent on the presence of mutations in KIT or PDGFRA.3,18 Recurrence risk is highly dependent on tumor size, tumor site, tumor rupture, and mitotic index.1,3,5,6,8,9,18,19 Findings on the use of gene expression patterns to predict recurrence risk have also been reported.20-27 However, recurrence risk is poorly understood for categories in which there are few cases with known outcomes, such as very small gastric GIST with a high mitotic index. For example, few cases of gastric GIST have been reported with a tumor size ≤2 cm, a mitotic rate >5 mitoses per 50 hpf, and adequate clinical follow-up. In such cases, it is difficult to assess the risk of recurrence.6 We report here the long-term outcome of a patient with a 1.8 cm gastric GIST with a mitotic index of 36 mitoses per 50 hpf and a KIT exon 11 mutation.
Case presentation and summary
A 69-year-old man presented with periumbilical and epigastric pain of 6-month duration. His medical history was notable for hyperlipidemia, hypertension, coronary angioplasty, and spinal surgery. He had a 40 pack-year smoking history and consumed 2 to 4 alcoholic drinks per day. The results of a physical examination were unremarkable. A computedtomographic (CT) scan showed no abnormalities. An esophagoduodenoscopy (EGD) revealed gastric ulcers. He was treated successfully with omeprazole 20 mg by mouth daily.
A month later, a follow-up EGD revealed a 1.8 × 1.5 cm submucosal mass 3 cm from the gastroesophageal junction. The patient underwent a fundus wedge resection, and a submucosal mass 1.8 cm in greatest dimension was removed. Pathologic examination revealed a GIST, spindle cell type, with a mitotic rate of 36 mitoses per 50 hpf with negative margins. Immunohistochemistry was positive for CD117. An exon 11 deletion (KVV558-560NV) was present in KIT. The patient’s risk of recurrence was unclear, and his follow-up included CT scans of the abdomen and pelvis every 3 to 4 months for the first 2 years, then every 6 months for the next 2.5 years.
A CT scan about 3.5 years after primary resection revealed small nonspecific liver hypodensities that became more prominent during the next year. About 5 years after primary resection, magnetic resonance imaging (MRI) revealed several liver lesions, the largest of which measured at 1.3 cm in greatest dimension. The patient’s liver metastases were readily identified by MRI (Figure 1) and CT imaging (Figure 2A).
Discussion
Small gastric GISTs are sometimes found by endoscopy performed for unrelated reasons. Recent data suggest that the incidence of gastric GIST may be higher than previously thought. In a Japanese study of patients with gastric cancer in which 100 stomachs were systematically examined pathologically, 50 microscopic GISTs were found in 35 patients.28 Most small gastric GISTs have a low mitotic index. Few cases have been described with a high mitotic index. In a study of 1765 cases of GIST of the stomach, 8 patients had a tumor size less than 2 cm and a mitotic index greater than 5. Of those, only 6 patients had long-term follow-up, and 3 were alive without disease at 2, 17, and 20 years of follow-up.7 These limited data make it impossible to predict outcomes in patients with small gastric GIST with a high mitotic index.
For patients who are at high risk of recurrence after surgery, 3 years of adjuvant imatinib treatment compared with 1 year has been shown to improve overall survival and is the current standard of care.10,17 A study comparing 5 and 3 years of imatinib is ongoing to establish whether a longer period of adjuvant treatment is warranted. In patients with metastatic GIST, lifelong imatinib until lack of benefit is considered optimal treatment.10 All patients should undergo KIT mutation analysis. Those with the PDGFRA D842V mutation, SDH (succinate dehydrogenase) deficiency, or neurofibromatosis-related GIST should not receive adjuvant imatinib.
This case has several unusual features. The small tumor size with a very high mitotic rate is rare. Such cases have not been reported in large numbers and have therefore not been reliably incorporated into risk prediction algorithms. In addition, despite a high mitotic index, the tumor was not FDG avid on PET imaging. The diagnosis of GIST is strongly supported by the KIT mutation and response to imatinib. This particular KIT mutation in larger GISTs is associated with aggressive disease. The present case adds to the data on the biology of small gastric GISTs with a high mitotic index and suggests the mitotic index in these tumors may be a more important predictor than size.
Acknowledgment
The authors thank Michael Franklin, MS, for editorial assistance, and Sabrina Porter for media edits.
1. Corless CL, Barnett CM, Heinrich MC. Gastrointestinal stromal tumours: origin and molecular oncology. Nat Rev Cancer. 2011;11(12):865-878.
2. Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279(5350):577-580.
3. Corless CL, Ballman KV, Antonescu CR, et al. Pathologic and molecular features correlate with long-term outcome after adjuvant therapy of resected primary GI stromal tumor: the ACOSOG Z9001 trial. J Clin Oncol. 2014;32(15):1563-1570.
4. Huang J, Zheng DL, Qin FS, et al. Genetic and epigenetic silencing of SCARA5 may contribute to human hepatocellular carcinoma by activating FAK signaling. J Clin Invest. 2010;120(1):223-241.
5. Joensuu H, Vehtari A, Riihimaki J, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. 2012;13(3):265-274.
6. Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130(10):1466-1478.
7. Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29(1):52-68.
8. Patel S. Navigating risk stratification systems for the management of patients with GIST. Ann Surg Oncol. 2011;18(6):1698-1704.
9. Rossi S, Miceli R, Messerini L, et al. Natural history of imatinib-naive GISTs: a retrospective analysis of 929 cases with long-term follow-up and development of a survival nomogram based on mitotic index and size as continuous variables. Am J Surg Pathol. 2011;35(11):1646-1656.
10. National Comprehensive Cancer Network. Sarcoma. https://www.nccn.org. Accessed March 27, 2018.
11. Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Int J Surg Pathol. 2002;10(2):81-89.
12. Huang HY, Li CF, Huang WW, et al. A modification of NIH consensus criteria to better distinguish the highly lethal subset of primary localized gastrointestinal stromal tumors: a subdivision of the original high-risk group on the basis of outcome. Surgery. 2007;141(6):748-756.
13. Kim MC, Yook JH, Yang HK, et al. Long-term surgical outcome of 1057 gastric GISTs according to 7th UICC/AJCC TNM system: multicenter observational study From Korea and Japan. Medicine (Baltimore). 2015;94(41):e1526.
14. Casali PG, Blay JY; ESMO/CONTICANET/EUROBONET Consensus Panel of experts. Soft tissue sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v198-v203.
15. Joensuu H, DeMatteo RP. The management of gastrointestinal stromal tumors: a model for targeted and multidisciplinary therapy of malignancy. Annu Rev Med. 2012;63:247-258.
16. Dematteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373(9669):1097-1104.
17. Joensuu H, Eriksson M, Sundby Hall K, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA. 2012;307(12):1265-1272.
18. Joensuu H, Rutkowski P, Nishida T, et al. KIT and PDGFRA mutations and the risk of GI stromal tumor recurrence. J Clin Oncol. 2015;33(6):634-642.
19. Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33(5):459-465.
20. Antonescu CR, Viale A, Sarran L, et al. Gene expression in gastrointestinal stromal tumors is distinguished by KIT genotype and anatomic site. Clin Cancer Res. 2004;10(10):3282-3290.
21. Arne G, Kristiansson E, Nerman O, et al. Expression profiling of GIST: CD133 is associated with KIT exon 11 mutations, gastric location and poor prognosis. Int J Cancer. 2011;129(5):1149-1161.
22. Bertucci F, Finetti P, Ostrowski J, et al. Genomic Grade Index predicts postoperative clinical outcome of GIST. Br J Cancer. 2012;107(8):1433-1441.
23. Koon N, Schneider-Stock R, Sarlomo-Rikala M, et al. Molecular targets for tumour progression in gastrointestinal stromal tumours. Gut. 2004;53(2):235-240.
24. Lagarde P, Perot G, Kauffmann A, et al. Mitotic checkpoints and chromosome instability are strong predictors of clinical outcome in gastrointestinal stromal tumors. Clin Cancer Res. 2012;18(3):826-838.
25. Skubitz KM, Geschwind K, Xu WW, Koopmeiners JS, Skubitz AP. Gene expression identifies heterogeneity of metastatic behavior among gastrointestinal stromal tumors. J Transl Med. 2016;14:51.
26. Yamaguchi U, Nakayama R, Honda K, et al. Distinct gene expression-defined classes of gastrointestinal stromal tumor. J Clin Oncol. 2008;26(25):4100-4108.
27. Ylipaa A, Hunt KK, Yang J, et al. Integrative genomic characterization and a genomic staging system for gastrointestinal stromal tumors. Cancer. 2011;117(2):380-389.
28. Kawanowa K, Sakuma Y, Sakurai S, et al. High incidence of microscopic gastrointestinal stromal tumors in the stomach. Hum Pathol. 2006;37(12):1527-1535.
Gastrointestinal stromal tumor (GIST) is the most common soft tissue sarcoma of the gastrointestinal tract, usually arising from the interstitial cells of Cajal or similar cells in the outer wall of the gastrointestinal tract.1,2 Most GISTs have an activating mutation in KIT or platelet-derived growth factor receptor alpha (PDGFRA). Tumor size, mitotic rate, and anatomic site are the most common pathological features used to risk stratify GIST tumors.3-10 It is important to note when using such risk calculators that preoperative imatinib before determining tumor characteristics (such as mitoses per 50 high-power fields [hpf]) often changes the relevant parameters so that the same risk calculations may not apply. Tumors with a mitotic rate ≤5 mitoses per 50 hpf and a size ≤5 cm in greatest dimension have a lower recurrence rate after resection than tumors with a mitotic rate >5 mitoses per 50 hpf and a size >10 cm, and larger tumors can have a recurrence rate of up to 86%.11,12 Findings from a large observational study have suggested that the prognosis of gastric GIST in Korea and Japan may be more favorable compared with that in Western countries.13
The primary treatment of a localized primary GIST is surgical excision, but a cure is limited by recurrence.14,15 Imatinib is useful in the treatment of metastatic or recurrent GIST, and adjuvant treatment with imatinib after surgery has been shown to improve progression-free and overall survival in some cases.3,16-18 Responses to adjuvant imatinib depend on tumor sensitivity to the drug and the risk of recurrence. Drug sensitivity is largely dependent on the presence of mutations in KIT or PDGFRA.3,18 Recurrence risk is highly dependent on tumor size, tumor site, tumor rupture, and mitotic index.1,3,5,6,8,9,18,19 Findings on the use of gene expression patterns to predict recurrence risk have also been reported.20-27 However, recurrence risk is poorly understood for categories in which there are few cases with known outcomes, such as very small gastric GIST with a high mitotic index. For example, few cases of gastric GIST have been reported with a tumor size ≤2 cm, a mitotic rate >5 mitoses per 50 hpf, and adequate clinical follow-up. In such cases, it is difficult to assess the risk of recurrence.6 We report here the long-term outcome of a patient with a 1.8 cm gastric GIST with a mitotic index of 36 mitoses per 50 hpf and a KIT exon 11 mutation.
Case presentation and summary
A 69-year-old man presented with periumbilical and epigastric pain of 6-month duration. His medical history was notable for hyperlipidemia, hypertension, coronary angioplasty, and spinal surgery. He had a 40 pack-year smoking history and consumed 2 to 4 alcoholic drinks per day. The results of a physical examination were unremarkable. A computedtomographic (CT) scan showed no abnormalities. An esophagoduodenoscopy (EGD) revealed gastric ulcers. He was treated successfully with omeprazole 20 mg by mouth daily.
A month later, a follow-up EGD revealed a 1.8 × 1.5 cm submucosal mass 3 cm from the gastroesophageal junction. The patient underwent a fundus wedge resection, and a submucosal mass 1.8 cm in greatest dimension was removed. Pathologic examination revealed a GIST, spindle cell type, with a mitotic rate of 36 mitoses per 50 hpf with negative margins. Immunohistochemistry was positive for CD117. An exon 11 deletion (KVV558-560NV) was present in KIT. The patient’s risk of recurrence was unclear, and his follow-up included CT scans of the abdomen and pelvis every 3 to 4 months for the first 2 years, then every 6 months for the next 2.5 years.
A CT scan about 3.5 years after primary resection revealed small nonspecific liver hypodensities that became more prominent during the next year. About 5 years after primary resection, magnetic resonance imaging (MRI) revealed several liver lesions, the largest of which measured at 1.3 cm in greatest dimension. The patient’s liver metastases were readily identified by MRI (Figure 1) and CT imaging (Figure 2A).
Discussion
Small gastric GISTs are sometimes found by endoscopy performed for unrelated reasons. Recent data suggest that the incidence of gastric GIST may be higher than previously thought. In a Japanese study of patients with gastric cancer in which 100 stomachs were systematically examined pathologically, 50 microscopic GISTs were found in 35 patients.28 Most small gastric GISTs have a low mitotic index. Few cases have been described with a high mitotic index. In a study of 1765 cases of GIST of the stomach, 8 patients had a tumor size less than 2 cm and a mitotic index greater than 5. Of those, only 6 patients had long-term follow-up, and 3 were alive without disease at 2, 17, and 20 years of follow-up.7 These limited data make it impossible to predict outcomes in patients with small gastric GIST with a high mitotic index.
For patients who are at high risk of recurrence after surgery, 3 years of adjuvant imatinib treatment compared with 1 year has been shown to improve overall survival and is the current standard of care.10,17 A study comparing 5 and 3 years of imatinib is ongoing to establish whether a longer period of adjuvant treatment is warranted. In patients with metastatic GIST, lifelong imatinib until lack of benefit is considered optimal treatment.10 All patients should undergo KIT mutation analysis. Those with the PDGFRA D842V mutation, SDH (succinate dehydrogenase) deficiency, or neurofibromatosis-related GIST should not receive adjuvant imatinib.
This case has several unusual features. The small tumor size with a very high mitotic rate is rare. Such cases have not been reported in large numbers and have therefore not been reliably incorporated into risk prediction algorithms. In addition, despite a high mitotic index, the tumor was not FDG avid on PET imaging. The diagnosis of GIST is strongly supported by the KIT mutation and response to imatinib. This particular KIT mutation in larger GISTs is associated with aggressive disease. The present case adds to the data on the biology of small gastric GISTs with a high mitotic index and suggests the mitotic index in these tumors may be a more important predictor than size.
Acknowledgment
The authors thank Michael Franklin, MS, for editorial assistance, and Sabrina Porter for media edits.
Gastrointestinal stromal tumor (GIST) is the most common soft tissue sarcoma of the gastrointestinal tract, usually arising from the interstitial cells of Cajal or similar cells in the outer wall of the gastrointestinal tract.1,2 Most GISTs have an activating mutation in KIT or platelet-derived growth factor receptor alpha (PDGFRA). Tumor size, mitotic rate, and anatomic site are the most common pathological features used to risk stratify GIST tumors.3-10 It is important to note when using such risk calculators that preoperative imatinib before determining tumor characteristics (such as mitoses per 50 high-power fields [hpf]) often changes the relevant parameters so that the same risk calculations may not apply. Tumors with a mitotic rate ≤5 mitoses per 50 hpf and a size ≤5 cm in greatest dimension have a lower recurrence rate after resection than tumors with a mitotic rate >5 mitoses per 50 hpf and a size >10 cm, and larger tumors can have a recurrence rate of up to 86%.11,12 Findings from a large observational study have suggested that the prognosis of gastric GIST in Korea and Japan may be more favorable compared with that in Western countries.13
The primary treatment of a localized primary GIST is surgical excision, but a cure is limited by recurrence.14,15 Imatinib is useful in the treatment of metastatic or recurrent GIST, and adjuvant treatment with imatinib after surgery has been shown to improve progression-free and overall survival in some cases.3,16-18 Responses to adjuvant imatinib depend on tumor sensitivity to the drug and the risk of recurrence. Drug sensitivity is largely dependent on the presence of mutations in KIT or PDGFRA.3,18 Recurrence risk is highly dependent on tumor size, tumor site, tumor rupture, and mitotic index.1,3,5,6,8,9,18,19 Findings on the use of gene expression patterns to predict recurrence risk have also been reported.20-27 However, recurrence risk is poorly understood for categories in which there are few cases with known outcomes, such as very small gastric GIST with a high mitotic index. For example, few cases of gastric GIST have been reported with a tumor size ≤2 cm, a mitotic rate >5 mitoses per 50 hpf, and adequate clinical follow-up. In such cases, it is difficult to assess the risk of recurrence.6 We report here the long-term outcome of a patient with a 1.8 cm gastric GIST with a mitotic index of 36 mitoses per 50 hpf and a KIT exon 11 mutation.
Case presentation and summary
A 69-year-old man presented with periumbilical and epigastric pain of 6-month duration. His medical history was notable for hyperlipidemia, hypertension, coronary angioplasty, and spinal surgery. He had a 40 pack-year smoking history and consumed 2 to 4 alcoholic drinks per day. The results of a physical examination were unremarkable. A computedtomographic (CT) scan showed no abnormalities. An esophagoduodenoscopy (EGD) revealed gastric ulcers. He was treated successfully with omeprazole 20 mg by mouth daily.
A month later, a follow-up EGD revealed a 1.8 × 1.5 cm submucosal mass 3 cm from the gastroesophageal junction. The patient underwent a fundus wedge resection, and a submucosal mass 1.8 cm in greatest dimension was removed. Pathologic examination revealed a GIST, spindle cell type, with a mitotic rate of 36 mitoses per 50 hpf with negative margins. Immunohistochemistry was positive for CD117. An exon 11 deletion (KVV558-560NV) was present in KIT. The patient’s risk of recurrence was unclear, and his follow-up included CT scans of the abdomen and pelvis every 3 to 4 months for the first 2 years, then every 6 months for the next 2.5 years.
A CT scan about 3.5 years after primary resection revealed small nonspecific liver hypodensities that became more prominent during the next year. About 5 years after primary resection, magnetic resonance imaging (MRI) revealed several liver lesions, the largest of which measured at 1.3 cm in greatest dimension. The patient’s liver metastases were readily identified by MRI (Figure 1) and CT imaging (Figure 2A).
Discussion
Small gastric GISTs are sometimes found by endoscopy performed for unrelated reasons. Recent data suggest that the incidence of gastric GIST may be higher than previously thought. In a Japanese study of patients with gastric cancer in which 100 stomachs were systematically examined pathologically, 50 microscopic GISTs were found in 35 patients.28 Most small gastric GISTs have a low mitotic index. Few cases have been described with a high mitotic index. In a study of 1765 cases of GIST of the stomach, 8 patients had a tumor size less than 2 cm and a mitotic index greater than 5. Of those, only 6 patients had long-term follow-up, and 3 were alive without disease at 2, 17, and 20 years of follow-up.7 These limited data make it impossible to predict outcomes in patients with small gastric GIST with a high mitotic index.
For patients who are at high risk of recurrence after surgery, 3 years of adjuvant imatinib treatment compared with 1 year has been shown to improve overall survival and is the current standard of care.10,17 A study comparing 5 and 3 years of imatinib is ongoing to establish whether a longer period of adjuvant treatment is warranted. In patients with metastatic GIST, lifelong imatinib until lack of benefit is considered optimal treatment.10 All patients should undergo KIT mutation analysis. Those with the PDGFRA D842V mutation, SDH (succinate dehydrogenase) deficiency, or neurofibromatosis-related GIST should not receive adjuvant imatinib.
This case has several unusual features. The small tumor size with a very high mitotic rate is rare. Such cases have not been reported in large numbers and have therefore not been reliably incorporated into risk prediction algorithms. In addition, despite a high mitotic index, the tumor was not FDG avid on PET imaging. The diagnosis of GIST is strongly supported by the KIT mutation and response to imatinib. This particular KIT mutation in larger GISTs is associated with aggressive disease. The present case adds to the data on the biology of small gastric GISTs with a high mitotic index and suggests the mitotic index in these tumors may be a more important predictor than size.
Acknowledgment
The authors thank Michael Franklin, MS, for editorial assistance, and Sabrina Porter for media edits.
1. Corless CL, Barnett CM, Heinrich MC. Gastrointestinal stromal tumours: origin and molecular oncology. Nat Rev Cancer. 2011;11(12):865-878.
2. Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279(5350):577-580.
3. Corless CL, Ballman KV, Antonescu CR, et al. Pathologic and molecular features correlate with long-term outcome after adjuvant therapy of resected primary GI stromal tumor: the ACOSOG Z9001 trial. J Clin Oncol. 2014;32(15):1563-1570.
4. Huang J, Zheng DL, Qin FS, et al. Genetic and epigenetic silencing of SCARA5 may contribute to human hepatocellular carcinoma by activating FAK signaling. J Clin Invest. 2010;120(1):223-241.
5. Joensuu H, Vehtari A, Riihimaki J, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. 2012;13(3):265-274.
6. Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130(10):1466-1478.
7. Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29(1):52-68.
8. Patel S. Navigating risk stratification systems for the management of patients with GIST. Ann Surg Oncol. 2011;18(6):1698-1704.
9. Rossi S, Miceli R, Messerini L, et al. Natural history of imatinib-naive GISTs: a retrospective analysis of 929 cases with long-term follow-up and development of a survival nomogram based on mitotic index and size as continuous variables. Am J Surg Pathol. 2011;35(11):1646-1656.
10. National Comprehensive Cancer Network. Sarcoma. https://www.nccn.org. Accessed March 27, 2018.
11. Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Int J Surg Pathol. 2002;10(2):81-89.
12. Huang HY, Li CF, Huang WW, et al. A modification of NIH consensus criteria to better distinguish the highly lethal subset of primary localized gastrointestinal stromal tumors: a subdivision of the original high-risk group on the basis of outcome. Surgery. 2007;141(6):748-756.
13. Kim MC, Yook JH, Yang HK, et al. Long-term surgical outcome of 1057 gastric GISTs according to 7th UICC/AJCC TNM system: multicenter observational study From Korea and Japan. Medicine (Baltimore). 2015;94(41):e1526.
14. Casali PG, Blay JY; ESMO/CONTICANET/EUROBONET Consensus Panel of experts. Soft tissue sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v198-v203.
15. Joensuu H, DeMatteo RP. The management of gastrointestinal stromal tumors: a model for targeted and multidisciplinary therapy of malignancy. Annu Rev Med. 2012;63:247-258.
16. Dematteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373(9669):1097-1104.
17. Joensuu H, Eriksson M, Sundby Hall K, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA. 2012;307(12):1265-1272.
18. Joensuu H, Rutkowski P, Nishida T, et al. KIT and PDGFRA mutations and the risk of GI stromal tumor recurrence. J Clin Oncol. 2015;33(6):634-642.
19. Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33(5):459-465.
20. Antonescu CR, Viale A, Sarran L, et al. Gene expression in gastrointestinal stromal tumors is distinguished by KIT genotype and anatomic site. Clin Cancer Res. 2004;10(10):3282-3290.
21. Arne G, Kristiansson E, Nerman O, et al. Expression profiling of GIST: CD133 is associated with KIT exon 11 mutations, gastric location and poor prognosis. Int J Cancer. 2011;129(5):1149-1161.
22. Bertucci F, Finetti P, Ostrowski J, et al. Genomic Grade Index predicts postoperative clinical outcome of GIST. Br J Cancer. 2012;107(8):1433-1441.
23. Koon N, Schneider-Stock R, Sarlomo-Rikala M, et al. Molecular targets for tumour progression in gastrointestinal stromal tumours. Gut. 2004;53(2):235-240.
24. Lagarde P, Perot G, Kauffmann A, et al. Mitotic checkpoints and chromosome instability are strong predictors of clinical outcome in gastrointestinal stromal tumors. Clin Cancer Res. 2012;18(3):826-838.
25. Skubitz KM, Geschwind K, Xu WW, Koopmeiners JS, Skubitz AP. Gene expression identifies heterogeneity of metastatic behavior among gastrointestinal stromal tumors. J Transl Med. 2016;14:51.
26. Yamaguchi U, Nakayama R, Honda K, et al. Distinct gene expression-defined classes of gastrointestinal stromal tumor. J Clin Oncol. 2008;26(25):4100-4108.
27. Ylipaa A, Hunt KK, Yang J, et al. Integrative genomic characterization and a genomic staging system for gastrointestinal stromal tumors. Cancer. 2011;117(2):380-389.
28. Kawanowa K, Sakuma Y, Sakurai S, et al. High incidence of microscopic gastrointestinal stromal tumors in the stomach. Hum Pathol. 2006;37(12):1527-1535.
1. Corless CL, Barnett CM, Heinrich MC. Gastrointestinal stromal tumours: origin and molecular oncology. Nat Rev Cancer. 2011;11(12):865-878.
2. Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279(5350):577-580.
3. Corless CL, Ballman KV, Antonescu CR, et al. Pathologic and molecular features correlate with long-term outcome after adjuvant therapy of resected primary GI stromal tumor: the ACOSOG Z9001 trial. J Clin Oncol. 2014;32(15):1563-1570.
4. Huang J, Zheng DL, Qin FS, et al. Genetic and epigenetic silencing of SCARA5 may contribute to human hepatocellular carcinoma by activating FAK signaling. J Clin Invest. 2010;120(1):223-241.
5. Joensuu H, Vehtari A, Riihimaki J, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. 2012;13(3):265-274.
6. Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130(10):1466-1478.
7. Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29(1):52-68.
8. Patel S. Navigating risk stratification systems for the management of patients with GIST. Ann Surg Oncol. 2011;18(6):1698-1704.
9. Rossi S, Miceli R, Messerini L, et al. Natural history of imatinib-naive GISTs: a retrospective analysis of 929 cases with long-term follow-up and development of a survival nomogram based on mitotic index and size as continuous variables. Am J Surg Pathol. 2011;35(11):1646-1656.
10. National Comprehensive Cancer Network. Sarcoma. https://www.nccn.org. Accessed March 27, 2018.
11. Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Int J Surg Pathol. 2002;10(2):81-89.
12. Huang HY, Li CF, Huang WW, et al. A modification of NIH consensus criteria to better distinguish the highly lethal subset of primary localized gastrointestinal stromal tumors: a subdivision of the original high-risk group on the basis of outcome. Surgery. 2007;141(6):748-756.
13. Kim MC, Yook JH, Yang HK, et al. Long-term surgical outcome of 1057 gastric GISTs according to 7th UICC/AJCC TNM system: multicenter observational study From Korea and Japan. Medicine (Baltimore). 2015;94(41):e1526.
14. Casali PG, Blay JY; ESMO/CONTICANET/EUROBONET Consensus Panel of experts. Soft tissue sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v198-v203.
15. Joensuu H, DeMatteo RP. The management of gastrointestinal stromal tumors: a model for targeted and multidisciplinary therapy of malignancy. Annu Rev Med. 2012;63:247-258.
16. Dematteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373(9669):1097-1104.
17. Joensuu H, Eriksson M, Sundby Hall K, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA. 2012;307(12):1265-1272.
18. Joensuu H, Rutkowski P, Nishida T, et al. KIT and PDGFRA mutations and the risk of GI stromal tumor recurrence. J Clin Oncol. 2015;33(6):634-642.
19. Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33(5):459-465.
20. Antonescu CR, Viale A, Sarran L, et al. Gene expression in gastrointestinal stromal tumors is distinguished by KIT genotype and anatomic site. Clin Cancer Res. 2004;10(10):3282-3290.
21. Arne G, Kristiansson E, Nerman O, et al. Expression profiling of GIST: CD133 is associated with KIT exon 11 mutations, gastric location and poor prognosis. Int J Cancer. 2011;129(5):1149-1161.
22. Bertucci F, Finetti P, Ostrowski J, et al. Genomic Grade Index predicts postoperative clinical outcome of GIST. Br J Cancer. 2012;107(8):1433-1441.
23. Koon N, Schneider-Stock R, Sarlomo-Rikala M, et al. Molecular targets for tumour progression in gastrointestinal stromal tumours. Gut. 2004;53(2):235-240.
24. Lagarde P, Perot G, Kauffmann A, et al. Mitotic checkpoints and chromosome instability are strong predictors of clinical outcome in gastrointestinal stromal tumors. Clin Cancer Res. 2012;18(3):826-838.
25. Skubitz KM, Geschwind K, Xu WW, Koopmeiners JS, Skubitz AP. Gene expression identifies heterogeneity of metastatic behavior among gastrointestinal stromal tumors. J Transl Med. 2016;14:51.
26. Yamaguchi U, Nakayama R, Honda K, et al. Distinct gene expression-defined classes of gastrointestinal stromal tumor. J Clin Oncol. 2008;26(25):4100-4108.
27. Ylipaa A, Hunt KK, Yang J, et al. Integrative genomic characterization and a genomic staging system for gastrointestinal stromal tumors. Cancer. 2011;117(2):380-389.
28. Kawanowa K, Sakuma Y, Sakurai S, et al. High incidence of microscopic gastrointestinal stromal tumors in the stomach. Hum Pathol. 2006;37(12):1527-1535.
Effective management of severe radiation dermatitis after head and neck radiotherapy
Head and neck cancer is among the most prevalent cancers in developing countries.1 Most of the patients in developing countries present in locally advanced stages, and radical radiation therapy with concurrent chemotherapy is the standard treatment.1 Radiation therapy is associated with radiation dermatitis, which causes severe symptoms in the patient and can lead to disruption of treatment, diminished rates of disease control rates, and impaired patient quality of life.2 The management of advanced radiation dermatitis is difficult and can cause consequential late morbidity to patients.2 We report here the rare case of a patient with locally advanced tonsil carcinoma who developed grade 3 radiation dermatitis while receiving radical chemoradiation. The patient’s radiation dermatitis was effectively managed with the use of a silver-containing antimicrobial dressing that yielded remarkable results, so the patient was able to resume and complete radiation therapy.
Case presentation and summary
A 48-year-old man was diagnosed with squamous cell carcinoma of the right tonsil, with bilateral neck nodes (Stage T4a N2c M0; The American Joint Committee on Cancer staging manual, 7th edition). In view of the locally advanced status of his disease, the patient was scheduled for radical radiation therapy at 70 Gy in 35 fractions over 7 weeks along with weekly chemotherapy (cisplatin 40 mg/m2). During the course of radiation therapy, the patient was monitored twice a week, and symptomatic care was done for radiation-therapy–induced toxicities.
The patient presented with grade 3 radiation dermatitis after receiving 58 Gy in 29 fractions over 5 weeks (grade 0, no change; grades 3 and 4, severe change). The radiation dermatitis involved the anterior and bilateral neck with moist desquamation of the skin (Figure 1).
It was associated with severe pain, difficulty in swallowing, and oral mucositis. The patient was subsequently admitted to the hospital; radiation therapy was stopped, and treatment was initiated to ease the effects of the radiation dermatitis. Analgesics were administered for the pain, and adequate hydration and nutritional support was administered through a nasogastric tube. The patient’s score on the Bates-Jensen Wound Assessment Tool (BWAT) for monitoring wound status was 44, which falls in extreme severity status.
In view of the extreme severity status of the radiation dermatitis, after cleaning the wound with sterile water, we covered it with an antimicrobial dressing that contained silver salt (Mepilex AG; Mölnlycke Health Care, Norcross, GA). The dressing was changed regularly every 4 days. There was a gradual improvement in the radiation dermatitis (Figure 2).
Discussion
Head and neck cancer is one of the most common cancers in developing countries.1 Most patients present with locally advanced disease, so chemoradiation is the standard treatment in these patents. Radiation therapy is associated with acute and chronic toxicities. The common radiation therapy toxicities are directed at skin and mucosa, which leads to radiation dermatitis and radiation mucositis, respectively.2 These toxicities are graded as per the Radiation Therapy Oncology Group (RTOG) criteria (Table 2).3
Acute radiation dermatitis is radiation therapy dose-dependent and manifests within a few days to weeks after starting external beam radiation therapy. Its presentation varies in severity and gradually manifests as erythema, dry or moist desquamation, and ulceration when severe. These can cause severe symptoms in the patient, leading to frequent breaks in treatment, decreased rates of disease control, and impaired patient quality of life.2 Apart from RTOG grading, radiation dermatitis can also be scored using the BWAT. This tool has been validated across many studies to score initial wound status and monitor the subsequent status numerically.4 The radiation dermatitis of the index case was scored and monitored with both RTOG and BWAT scores.The management of advanced radiation dermatitis is difficult, and it causes consequential late morbidity in patients. A range of topical agents and dressings are used to treat radiation dermatitis, but there is minimal evidence to support their use.5 The Multinational Association for Supportive Care in Cancer treatment guidelines for prevention and treatment of radiation dermatitis have also concluded that there is a lack of sufficient evidence in the literature to support the superiority for any specific intervention.6 Management of radiation dermatitis varies among practitioners because of the inconclusive evidence for available treatment options.
The use of silver-based antimicrobial dressings has been reported in the literature in the prevention and treatment of radiation dermatitis, but with mixed results.7 Such dressings absorb exudate, maintain a moist environment that promotes wound healing, fight infection, and minimize the risk for maceration, according to the product information sheet.8 Clinical study findings have shown silver to be effective in fighting many different types of pathogens, including Methicillin-resistant Staphylococcus aureus and other drug-resistant bacteria.
Aquino-Parsons and colleagues studied 196 patients with breast cancer who were undergoing whole-breast radiation therapy.9 They showed that there was no benefit of silver-containing foam dressings for the prevention of acute grade 3 radiation dermatitis compared with patients who received standard skin care (with moisturizing cream, topical steroids, saline compress, and silver sulfadiazine cream). However, the incidence of itching in the last week of radiation and 1 week after treatment completion was lower among the patients who used the dressings.
Diggelmann and colleagues studied 24 patients with breast cancer who were undergoing radiation therapy.10 Each of the erythematous areas (n = 34) was randomly divided into 2 groups; 1 group was treated with Mepilex Lite dressing and the other with standard aqueous cream. There was a significant reduction in the severity of acute radiation dermatitis in the areas on which Mepilex Lite dressings were used compared with the areas on which standard aqueous cream was used.
The patient in the present case had severe grade 3 acute radiation dermatitis with a BWAT score indicative of extreme severity. After cleaning the wound with sterile water, instead of using the standard aqueous cream on the wounds, we used Mepilex AG, an antimicrobial dressing that contains silver salt. The results were remarkable (Figure 2 and Table 2). The patient was able to restart radiation therapy, and he completed his scheduled doses.
This case highlights the effectiveness of a silver-based antimicrobial dressing in the management of advanced and severe radiation dermatitis. Further large and randomized studies are needed to test the routine use of the dressing in the management of radiation dermatitis.
1. Simard EP, Torre LA, Jemal A. International trends in head and neck cancer incidence rates: differences by country, sex and anatomic site. Oral Oncol. 2014;50(5):387-403.
2. Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol. 2006;54(1):28-46.
3. Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31(5):1341-1346.
4. Harris C, Bates-Jensen B, Parslow N, Raizman R, Singh M, Ketchen R. Bates‐Jensen wound assessment tool: pictorial guide validation project. J Wound Ostomy Continence Nurs. 2010;37(3):253-259.
5. Lucey P, Zouzias C, Franco L, Chennupati SK, Kalnicki S, McLellan BN. Practice patterns for the prophylaxis and treatment of acute radiation dermatitis in the United States. Support Care Cancer. 2017;25(9):2857-2862.
6. Wong RK, Bensadoun RJ, Boers-Doets CB, et al. Clinical practice guidelines for the prevention and treatment of acute and late radiation reactions from the MASCC Skin Toxicity Study Group. Support Care Cancer. 2013;21(10):2933-2948.
7. Vavassis P, Gelinas M, Chabot Tr J, Nguyen-Tân PF. Phase 2 study of silver leaf dressing for treatment of radiation-induced dermatitis in patients receiving radiotherapy to the head and neck. J Otolaryngology Head Neck Surg. 2008;37(1):124-129.
8. Mepilex Ag product information. Mölnlycke Health Care website. http://www.molnlycke.us/advanced-wound-care-products/antimicrobial-products/mepilex-ag/#confirm. Accessed May 3, 2018.
9. Aquino-Parsons C, Lomas S, Smith K, et al. Phase III study of silver leaf nylon dressing vs standard care for reduction of inframammary moist desquamation in patients undergoing adjuvant whole breast radiation therapy. J Med Imaging Radiat Sci. 2010;41(4):215-221.
10. Diggelmann KV, Zytkovicz AE, Tuaine JM, Bennett NC, Kelly LE, Herst PM. Mepilex Lite dressings for the management of radiation-induced erythema: a systematic inpatient controlled clinical trial. Br J Radiol. 2010;83(995):971-978.
Head and neck cancer is among the most prevalent cancers in developing countries.1 Most of the patients in developing countries present in locally advanced stages, and radical radiation therapy with concurrent chemotherapy is the standard treatment.1 Radiation therapy is associated with radiation dermatitis, which causes severe symptoms in the patient and can lead to disruption of treatment, diminished rates of disease control rates, and impaired patient quality of life.2 The management of advanced radiation dermatitis is difficult and can cause consequential late morbidity to patients.2 We report here the rare case of a patient with locally advanced tonsil carcinoma who developed grade 3 radiation dermatitis while receiving radical chemoradiation. The patient’s radiation dermatitis was effectively managed with the use of a silver-containing antimicrobial dressing that yielded remarkable results, so the patient was able to resume and complete radiation therapy.
Case presentation and summary
A 48-year-old man was diagnosed with squamous cell carcinoma of the right tonsil, with bilateral neck nodes (Stage T4a N2c M0; The American Joint Committee on Cancer staging manual, 7th edition). In view of the locally advanced status of his disease, the patient was scheduled for radical radiation therapy at 70 Gy in 35 fractions over 7 weeks along with weekly chemotherapy (cisplatin 40 mg/m2). During the course of radiation therapy, the patient was monitored twice a week, and symptomatic care was done for radiation-therapy–induced toxicities.
The patient presented with grade 3 radiation dermatitis after receiving 58 Gy in 29 fractions over 5 weeks (grade 0, no change; grades 3 and 4, severe change). The radiation dermatitis involved the anterior and bilateral neck with moist desquamation of the skin (Figure 1).
It was associated with severe pain, difficulty in swallowing, and oral mucositis. The patient was subsequently admitted to the hospital; radiation therapy was stopped, and treatment was initiated to ease the effects of the radiation dermatitis. Analgesics were administered for the pain, and adequate hydration and nutritional support was administered through a nasogastric tube. The patient’s score on the Bates-Jensen Wound Assessment Tool (BWAT) for monitoring wound status was 44, which falls in extreme severity status.
In view of the extreme severity status of the radiation dermatitis, after cleaning the wound with sterile water, we covered it with an antimicrobial dressing that contained silver salt (Mepilex AG; Mölnlycke Health Care, Norcross, GA). The dressing was changed regularly every 4 days. There was a gradual improvement in the radiation dermatitis (Figure 2).
Discussion
Head and neck cancer is one of the most common cancers in developing countries.1 Most patients present with locally advanced disease, so chemoradiation is the standard treatment in these patents. Radiation therapy is associated with acute and chronic toxicities. The common radiation therapy toxicities are directed at skin and mucosa, which leads to radiation dermatitis and radiation mucositis, respectively.2 These toxicities are graded as per the Radiation Therapy Oncology Group (RTOG) criteria (Table 2).3
Acute radiation dermatitis is radiation therapy dose-dependent and manifests within a few days to weeks after starting external beam radiation therapy. Its presentation varies in severity and gradually manifests as erythema, dry or moist desquamation, and ulceration when severe. These can cause severe symptoms in the patient, leading to frequent breaks in treatment, decreased rates of disease control, and impaired patient quality of life.2 Apart from RTOG grading, radiation dermatitis can also be scored using the BWAT. This tool has been validated across many studies to score initial wound status and monitor the subsequent status numerically.4 The radiation dermatitis of the index case was scored and monitored with both RTOG and BWAT scores.The management of advanced radiation dermatitis is difficult, and it causes consequential late morbidity in patients. A range of topical agents and dressings are used to treat radiation dermatitis, but there is minimal evidence to support their use.5 The Multinational Association for Supportive Care in Cancer treatment guidelines for prevention and treatment of radiation dermatitis have also concluded that there is a lack of sufficient evidence in the literature to support the superiority for any specific intervention.6 Management of radiation dermatitis varies among practitioners because of the inconclusive evidence for available treatment options.
The use of silver-based antimicrobial dressings has been reported in the literature in the prevention and treatment of radiation dermatitis, but with mixed results.7 Such dressings absorb exudate, maintain a moist environment that promotes wound healing, fight infection, and minimize the risk for maceration, according to the product information sheet.8 Clinical study findings have shown silver to be effective in fighting many different types of pathogens, including Methicillin-resistant Staphylococcus aureus and other drug-resistant bacteria.
Aquino-Parsons and colleagues studied 196 patients with breast cancer who were undergoing whole-breast radiation therapy.9 They showed that there was no benefit of silver-containing foam dressings for the prevention of acute grade 3 radiation dermatitis compared with patients who received standard skin care (with moisturizing cream, topical steroids, saline compress, and silver sulfadiazine cream). However, the incidence of itching in the last week of radiation and 1 week after treatment completion was lower among the patients who used the dressings.
Diggelmann and colleagues studied 24 patients with breast cancer who were undergoing radiation therapy.10 Each of the erythematous areas (n = 34) was randomly divided into 2 groups; 1 group was treated with Mepilex Lite dressing and the other with standard aqueous cream. There was a significant reduction in the severity of acute radiation dermatitis in the areas on which Mepilex Lite dressings were used compared with the areas on which standard aqueous cream was used.
The patient in the present case had severe grade 3 acute radiation dermatitis with a BWAT score indicative of extreme severity. After cleaning the wound with sterile water, instead of using the standard aqueous cream on the wounds, we used Mepilex AG, an antimicrobial dressing that contains silver salt. The results were remarkable (Figure 2 and Table 2). The patient was able to restart radiation therapy, and he completed his scheduled doses.
This case highlights the effectiveness of a silver-based antimicrobial dressing in the management of advanced and severe radiation dermatitis. Further large and randomized studies are needed to test the routine use of the dressing in the management of radiation dermatitis.
Head and neck cancer is among the most prevalent cancers in developing countries.1 Most of the patients in developing countries present in locally advanced stages, and radical radiation therapy with concurrent chemotherapy is the standard treatment.1 Radiation therapy is associated with radiation dermatitis, which causes severe symptoms in the patient and can lead to disruption of treatment, diminished rates of disease control rates, and impaired patient quality of life.2 The management of advanced radiation dermatitis is difficult and can cause consequential late morbidity to patients.2 We report here the rare case of a patient with locally advanced tonsil carcinoma who developed grade 3 radiation dermatitis while receiving radical chemoradiation. The patient’s radiation dermatitis was effectively managed with the use of a silver-containing antimicrobial dressing that yielded remarkable results, so the patient was able to resume and complete radiation therapy.
Case presentation and summary
A 48-year-old man was diagnosed with squamous cell carcinoma of the right tonsil, with bilateral neck nodes (Stage T4a N2c M0; The American Joint Committee on Cancer staging manual, 7th edition). In view of the locally advanced status of his disease, the patient was scheduled for radical radiation therapy at 70 Gy in 35 fractions over 7 weeks along with weekly chemotherapy (cisplatin 40 mg/m2). During the course of radiation therapy, the patient was monitored twice a week, and symptomatic care was done for radiation-therapy–induced toxicities.
The patient presented with grade 3 radiation dermatitis after receiving 58 Gy in 29 fractions over 5 weeks (grade 0, no change; grades 3 and 4, severe change). The radiation dermatitis involved the anterior and bilateral neck with moist desquamation of the skin (Figure 1).
It was associated with severe pain, difficulty in swallowing, and oral mucositis. The patient was subsequently admitted to the hospital; radiation therapy was stopped, and treatment was initiated to ease the effects of the radiation dermatitis. Analgesics were administered for the pain, and adequate hydration and nutritional support was administered through a nasogastric tube. The patient’s score on the Bates-Jensen Wound Assessment Tool (BWAT) for monitoring wound status was 44, which falls in extreme severity status.
In view of the extreme severity status of the radiation dermatitis, after cleaning the wound with sterile water, we covered it with an antimicrobial dressing that contained silver salt (Mepilex AG; Mölnlycke Health Care, Norcross, GA). The dressing was changed regularly every 4 days. There was a gradual improvement in the radiation dermatitis (Figure 2).
Discussion
Head and neck cancer is one of the most common cancers in developing countries.1 Most patients present with locally advanced disease, so chemoradiation is the standard treatment in these patents. Radiation therapy is associated with acute and chronic toxicities. The common radiation therapy toxicities are directed at skin and mucosa, which leads to radiation dermatitis and radiation mucositis, respectively.2 These toxicities are graded as per the Radiation Therapy Oncology Group (RTOG) criteria (Table 2).3
Acute radiation dermatitis is radiation therapy dose-dependent and manifests within a few days to weeks after starting external beam radiation therapy. Its presentation varies in severity and gradually manifests as erythema, dry or moist desquamation, and ulceration when severe. These can cause severe symptoms in the patient, leading to frequent breaks in treatment, decreased rates of disease control, and impaired patient quality of life.2 Apart from RTOG grading, radiation dermatitis can also be scored using the BWAT. This tool has been validated across many studies to score initial wound status and monitor the subsequent status numerically.4 The radiation dermatitis of the index case was scored and monitored with both RTOG and BWAT scores.The management of advanced radiation dermatitis is difficult, and it causes consequential late morbidity in patients. A range of topical agents and dressings are used to treat radiation dermatitis, but there is minimal evidence to support their use.5 The Multinational Association for Supportive Care in Cancer treatment guidelines for prevention and treatment of radiation dermatitis have also concluded that there is a lack of sufficient evidence in the literature to support the superiority for any specific intervention.6 Management of radiation dermatitis varies among practitioners because of the inconclusive evidence for available treatment options.
The use of silver-based antimicrobial dressings has been reported in the literature in the prevention and treatment of radiation dermatitis, but with mixed results.7 Such dressings absorb exudate, maintain a moist environment that promotes wound healing, fight infection, and minimize the risk for maceration, according to the product information sheet.8 Clinical study findings have shown silver to be effective in fighting many different types of pathogens, including Methicillin-resistant Staphylococcus aureus and other drug-resistant bacteria.
Aquino-Parsons and colleagues studied 196 patients with breast cancer who were undergoing whole-breast radiation therapy.9 They showed that there was no benefit of silver-containing foam dressings for the prevention of acute grade 3 radiation dermatitis compared with patients who received standard skin care (with moisturizing cream, topical steroids, saline compress, and silver sulfadiazine cream). However, the incidence of itching in the last week of radiation and 1 week after treatment completion was lower among the patients who used the dressings.
Diggelmann and colleagues studied 24 patients with breast cancer who were undergoing radiation therapy.10 Each of the erythematous areas (n = 34) was randomly divided into 2 groups; 1 group was treated with Mepilex Lite dressing and the other with standard aqueous cream. There was a significant reduction in the severity of acute radiation dermatitis in the areas on which Mepilex Lite dressings were used compared with the areas on which standard aqueous cream was used.
The patient in the present case had severe grade 3 acute radiation dermatitis with a BWAT score indicative of extreme severity. After cleaning the wound with sterile water, instead of using the standard aqueous cream on the wounds, we used Mepilex AG, an antimicrobial dressing that contains silver salt. The results were remarkable (Figure 2 and Table 2). The patient was able to restart radiation therapy, and he completed his scheduled doses.
This case highlights the effectiveness of a silver-based antimicrobial dressing in the management of advanced and severe radiation dermatitis. Further large and randomized studies are needed to test the routine use of the dressing in the management of radiation dermatitis.
1. Simard EP, Torre LA, Jemal A. International trends in head and neck cancer incidence rates: differences by country, sex and anatomic site. Oral Oncol. 2014;50(5):387-403.
2. Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol. 2006;54(1):28-46.
3. Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31(5):1341-1346.
4. Harris C, Bates-Jensen B, Parslow N, Raizman R, Singh M, Ketchen R. Bates‐Jensen wound assessment tool: pictorial guide validation project. J Wound Ostomy Continence Nurs. 2010;37(3):253-259.
5. Lucey P, Zouzias C, Franco L, Chennupati SK, Kalnicki S, McLellan BN. Practice patterns for the prophylaxis and treatment of acute radiation dermatitis in the United States. Support Care Cancer. 2017;25(9):2857-2862.
6. Wong RK, Bensadoun RJ, Boers-Doets CB, et al. Clinical practice guidelines for the prevention and treatment of acute and late radiation reactions from the MASCC Skin Toxicity Study Group. Support Care Cancer. 2013;21(10):2933-2948.
7. Vavassis P, Gelinas M, Chabot Tr J, Nguyen-Tân PF. Phase 2 study of silver leaf dressing for treatment of radiation-induced dermatitis in patients receiving radiotherapy to the head and neck. J Otolaryngology Head Neck Surg. 2008;37(1):124-129.
8. Mepilex Ag product information. Mölnlycke Health Care website. http://www.molnlycke.us/advanced-wound-care-products/antimicrobial-products/mepilex-ag/#confirm. Accessed May 3, 2018.
9. Aquino-Parsons C, Lomas S, Smith K, et al. Phase III study of silver leaf nylon dressing vs standard care for reduction of inframammary moist desquamation in patients undergoing adjuvant whole breast radiation therapy. J Med Imaging Radiat Sci. 2010;41(4):215-221.
10. Diggelmann KV, Zytkovicz AE, Tuaine JM, Bennett NC, Kelly LE, Herst PM. Mepilex Lite dressings for the management of radiation-induced erythema: a systematic inpatient controlled clinical trial. Br J Radiol. 2010;83(995):971-978.
1. Simard EP, Torre LA, Jemal A. International trends in head and neck cancer incidence rates: differences by country, sex and anatomic site. Oral Oncol. 2014;50(5):387-403.
2. Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol. 2006;54(1):28-46.
3. Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31(5):1341-1346.
4. Harris C, Bates-Jensen B, Parslow N, Raizman R, Singh M, Ketchen R. Bates‐Jensen wound assessment tool: pictorial guide validation project. J Wound Ostomy Continence Nurs. 2010;37(3):253-259.
5. Lucey P, Zouzias C, Franco L, Chennupati SK, Kalnicki S, McLellan BN. Practice patterns for the prophylaxis and treatment of acute radiation dermatitis in the United States. Support Care Cancer. 2017;25(9):2857-2862.
6. Wong RK, Bensadoun RJ, Boers-Doets CB, et al. Clinical practice guidelines for the prevention and treatment of acute and late radiation reactions from the MASCC Skin Toxicity Study Group. Support Care Cancer. 2013;21(10):2933-2948.
7. Vavassis P, Gelinas M, Chabot Tr J, Nguyen-Tân PF. Phase 2 study of silver leaf dressing for treatment of radiation-induced dermatitis in patients receiving radiotherapy to the head and neck. J Otolaryngology Head Neck Surg. 2008;37(1):124-129.
8. Mepilex Ag product information. Mölnlycke Health Care website. http://www.molnlycke.us/advanced-wound-care-products/antimicrobial-products/mepilex-ag/#confirm. Accessed May 3, 2018.
9. Aquino-Parsons C, Lomas S, Smith K, et al. Phase III study of silver leaf nylon dressing vs standard care for reduction of inframammary moist desquamation in patients undergoing adjuvant whole breast radiation therapy. J Med Imaging Radiat Sci. 2010;41(4):215-221.
10. Diggelmann KV, Zytkovicz AE, Tuaine JM, Bennett NC, Kelly LE, Herst PM. Mepilex Lite dressings for the management of radiation-induced erythema: a systematic inpatient controlled clinical trial. Br J Radiol. 2010;83(995):971-978.
The long-term effects of posttreatment exercise on pain in young women with breast cancer
Breast cancer is one of the most prevalent cancers in women worldwide, with more than 1 million new cases diagnosed annually.1 Prognosis for the disease has improved significantly, but 25% to 60% of women living with breast cancer experience some level of pain ranging from mild to severe, the nature of which can evolve from acute to chronic.2 Pre-, intra-, and post-treatment risk factors have been found to correlate with the development of acute and chronic pain and include young age, type of breast surgery (lumpectomy or total mastectomy), axillary node dissection, radiation therapy, and hormonal therapy.3-5 Chemotherapy, particularly anthracycline- and taxane-based regimens, has also been shown to induce pain, arthralgia, myalgia, and peripheral neuropathy during treatment.6 In particular, postradiation pain may result from subcutaneous fibrosis with fixation to underlying musculature and the development of fibrous flaps in the internal axilla.7 These tissue changes are commonly subclinical, occurring 4 to 12 months postradiation,8 and can progress undetected until pain and upper-limb disability develop.
The presence of persistent pain has a considerable impact on the quality of life in survivors of breast cancer: psychological distress is prevalent (anxiety, depression, worry, fear), the performance of daily activities is diminished (eg, bathing, dressing, preparing meals, shopping), and economic independence is compromised by the inability to work or reduced employment and income. These factors directly and indirectly contribute to an increase in the use of health care services.9,10
The management of pain is often characterized by pharmacologic-related treatment, such as the use of opioids and nonsteroidal anti-inflammatory medications, and nonpharmacologic-related treatment, such as exercise. Empirical evidence has shown that rehabilitative exercise programs, which commonly include a combination of resistance training and aerobic exercises, can effectively reduce pain in breast cancer survivors.10-12 Women living with breast cancer who are directed to rehabilitative exercise programs experience an improvement not only in pain levels but also in their ability to engage in activities of daily living, in their psychological health, and in their overall quality of life.13-15 However, despite evidence to support exercise programs to reduce pain related to breast cancer treatment, residual pain and upper-limb discomfort are common complaints in breast cancer survivors, and there is little focus on the duration of effectiveness of such programs for reducing pain after treatment for breast cancer. The objective of this study was to determine if an exercise program initiated postradiation would improve long-term pain levels in a carefully selected population of young women who were living with breast cancer and had no history of shoulder pathology or significant treatment complications.
Methods
Design
We used a pilot randomized control trial to compare the long-term effectiveness of a 12-week postradiation exercise program versus standard care on residual pain levels in young women (aged 18-45 years) living with breast cancer. The program was initiated 3 to 4 weeks postradiation to allow for acute inflammatory reactions to subside. Pain severity and interference were assessed using the Brief Pain Inventory-Short Form (BPI-SF), a tool for assessing cancer pain.16,17 Pain levels for isolated shoulder movements were also recorded on examination by a physical therapist. All measures were collected at 6 time points (T1-T6): postsurgery and preradiation (T1, baseline), postradiation and preintervention (T2), and 4 points during an 18-month period postradiation (T3-T6 at 3, 6, 12, and 18 months postradiation).
Sample
Young women living with breast cancer who met our eligibility criteria were identified from 2 clinics at the Jewish General Hospital – the Segal Cancer Center and the Department of Radiation Oncology in Montréal, Québec, Canada. Inclusion criteria included women with a diagnosis of stage I to stage III breast cancer, who were 18 to 45 years old, were scheduled for postoperative adjuvant radiation therapy, had an Eastern Cooperative Oncology Group Performance Status of 0 or 1 (normal ambulatory function, minimal symptoms), and who consented to participate in the study. Exclusion criteria included women with a metastatic (stage IV) diagnosis; significant musculoskeletal, cardiac, pulmonary, or metabolic comorbidities that would not allow for participation in physical activity; a previous breast cancer diagnosis with treatment to the ipsilateral or contralateral sides; postsurgical lymphedema; postsurgical capsulitis, tendonitis, or other shoulder inflammatory complications; and any contraindication to exercise. The recruitment goal was outlined as 50 patients per group; however, a protracted accrual time because of the stringent study criteria yielded a sample of 29 and 30 patients for the intervention and control groups, respectively, which was sufficient for significant testing of differences between the 2 study groups.18
Variables and measures
Clinical characteristics. We used standardized questions and chart review to document the participants’ clinical characteristics and to capture information on the following: the stage and subtype of breast cancer, hormonal and human epidermal growth factor receptors (HER2) (estrogen receptor, progesterone receptor, and HER2 status), extent of surgery (lumpectomy or total mastectomy), and other modalities of treatment (eg, chemotherapy, radiation therapy).
Pain assessment. The BPI-SF was used to assess participants’ cancer-related pain. Pain severity ranged from 0 (no pain), 1 to 4 (mild pain), 5 to 6 (moderate pain), to 7 to 10 (severe pain).18,19 The questionnaire also identifies the pain interference in daily activities using a Likert scale ranging from 0 (Does not interfere) to 10 (Completely interferes) in the following 7 domains or subscales: General Activity, Walking, Mood, Sleep, Work, Relations with Others, and Enjoyment of Life.16 For the purpose of this study, mean scores were tabulated using both pain intensity and interference scales.
Another important component of the BPI-SF instructs participants to localize pain by means of a body diagram. For purpose of analysis, 3 pain regions were established: shoulder girdle/chest wall on the affected side; neck and other upper extremity, including hand(s), forearm(s), wrist(s), and finger(s); and other regions, including abdominal discomfort, leg(s), hip(s), knee(s), ankle(s), lower back, and feet. In addition, pain levels on movement (Yes/No) were recorded for isolated shoulder flexion, abduction, and horizontal abduction (sitting and standing). The measurements were completed by a single physical therapist throughout the course of the study to minimize variance.
Procedure
The study protocol was approved by the Research Ethics Board at the Jewish General Hospital. Recruitment occurred from 2011 through 2015. The research was in accordance with the ethical standards of the responsible committee on human experimentation. Eligible women were recruited by the research coordinator who described the purpose, risks, and benefits of the study; advised on confidentiality, data collection, and intervention allocation procedures; and highlighted voluntary participation. The research coordinator addressed any concerns on the part of the participants before obtaining their written informed consent. Random allocation to the intervention and control groups was established using a web-based randomization plan generator (www.randomization.com). A single individual was responsible for the randomization process, and treatment assignments were revealed after each participant’s name had been entered. A physical therapist performed 6 sequential evaluations (T1-T6) at the time of participants’ medical follow-up appointments.
Intervention
The 12-week exercise intervention started 3 weeks postradiation and was composed of an initial 6-week program of low-level cardiovascular and resistance exercises that progressed to a set of more advanced exercises for the remaining 6 weeks. Participants were instructed to warm up for at least 10 minutes with a cardiovascular exercise of their choice (eg, a recumbent cross trainer, walking, or stairs) before doing a combined strength, endurance, and stretching exercise program for the upper body.20 The final portion of the exercise intervention included a period of light cool-down. Weight training resistance levels were based on a maximum 8 to 10 repetitions for strength and a maximum of 20 repetitions for endurance training exercises, which progressed gradually over the course of the 12-week exercise program to ensure participant safety.21,22 Participants in the intervention group were supervised at least once a week by an exercise physiologist at a center for oncology patients (Hope & Cope Wellness Centre), and patients were encouraged to perform the program at home 2 to 3 times a week. Those who were not able to exercise consistently at the center were provided with equipment and instructed on how to do the program safely at home.
By comparison, the control group received standard care, which included advice on the benefits of an active lifestyle, including exercise, but without a specific intervention. Participants were not restricted in their physical activity and/or sport participation levels, and their weekly activity levels were calculated using the Metabolic Equivalent of Task and recorded at each of the 6 time points.
Statistical analysis
Descriptive statistics were used to examine participant characteristics. The quantitative data collected through the BPI-SF measures were analyzed with JMP software (version 11.2; SAS Institute, Cary, NC). Continuous variables were tested for statistical significance (P ≤ .05) through the chi-square (categorical), analysis of variance, and nonparametric Wilcoxon tests. The analyses did not include missing data.
Results
A total of 59 young women were randomized into the intervention (n = 29) and control (n = 30) groups. Of those, 2 participants dropped out of the study because of family and time constraints, and 3 participants died, 2 from the control and 1 from the intervention group, after subsequently developing metastatic disease. Baseline data including comparative tumor characteristics, surgical interventions, and treatment interventions have been published in relation to other elements of this study.23,24 The participants had a mean age of 39.2 years (standard deviation [SD], 5.0). More than half of them had an invasive ductal carcinoma (69.5%) and were estrogen positive (78.0%), progesterone positive (74.6%), or HER2 positive (20.3%), whereas 10.2% were triple negative. Most of the participants had undergone breast-sparing procedures (86.4% lumpectomy), and 18.6% had a total mastectomy. By random chance, the intervention group had higher rates of total mastectomy (24.4% and 13.3%, respectively) and surgical reconstruction (12.2% and 6.7%, respectively) compared with the control group. Most of the women (71.2%) received chemotherapy, and all received radiation therapy. In the intervention group, 37.2% received radiation therapy localized to the axilla, and 88% received a boost of radiation to the surgical bed. Self-reported exercise diaries were returned by 15 of the 29 intervention participants, and training frequencies among them varied significantly (1-6 times a week).
The findings showed that there was little variance between the intervention and control groups in BPI-SF severity scores from T1 to T6, so the means and SDs of the BPI-SF scores were grouped at 6 time points (Table 1). There was no statistically significant difference between baseline measures at T1 (1.68; SD, 1.17) and measures at 18 months postintervention (T6: 1.46; SD, 1.37). At baseline, 87.7% of the women reported no pain (31.5%) or mild levels of pain (55.6%), and 13% reported moderate or severe pain. Over the duration of the study from T1 to T6, these primarily low levels of pain (BPI-SF, 0-4) remained consistent with a favorable shift toward having no pain (T1: 31.5%; T6: 24.4%). By 18 months postintervention, 95.7% of women reported no or mild pain, with 4.9% reporting moderate pain.
Similarly, there was little variance over time (T1-T6) and no statistically significant differences between the 2 groups in BPI-SF–measured levels of pain interference in daily activities (Table 2). Moreover, a domain analysis showed that there were no statistically significant differences in pain interference scores when comparing the type and extent of surgery (total mastectomy: 0.59 [1.17]; lumpectomy: 0.94 [1.96]). By chance – and not related directly to the objectives of this study – there was a statistically significant difference between the intervention and control groups in the interference of pain on the Enjoyment of Life domain in favor of the control group.
The sites of pain captured by the BPI-SF shed light on the preceding findings (Figure 1). At baseline (T1, postsurgery and preradiation), 37.0% of participants reported pain in the shoulder girdle–chest wall region, whereas 20.4% reported pain in the general neck–upper extremity region and 50% in other regions. Postradiation, shoulder girdle–chest wall pain was identified as the highest reported site of pain (49.1%; T2, postradiation and preintervention) and remained elevated at 3 months (T3) and 6 months (T4) postradiation (46.9% and 45.5%, respectively). At 12 and 18 months postradiation (T5 and T6), the principal focus of pain shifted once again to “other” regions at 30% and 32.5%, respectively, and the neck–upper extremity region at 10% and 15%, respectively. Shoulder girdle–chest wall pain concomitantly improved at those time points (15% and 25% respectively) but was not eliminated.
Pain levels recorded on physical examination for isolated shoulder range of movements were recently published,24 and they have been abbreviated and reproduced in this paper (Figure 2) to allow for a comparison of findings between the exercise intervention group and the control group to help determine the sensitivity of these tools for use in breast cancer patients. At baseline, pain levels with active movement were noted to be slightly greater in the intervention group for flexion and abduction.
Following the intervention, at 3 and 6 months postradiation (T3 and T4), the intervention group showed a steady decrease in pain levels in flexion and abduction, whereas the control group showed a 5-fold increase in pain with horizontal abduction. Furthermore, participants in the intervention group reported having no pain on movement 12 months postradiation (T5); however, recurrence of pain was apparent with all shoulder movements by 18 months postradiation (T6) in both the intervention and control groups.
Discussion
Previous studies have hypothesized that younger age (18-39 years), adjuvant radiotherapy, and axillary node dissection are risk factors for chronic pain in breast cancer survivors.22,25 Persistent pain is prevalent in 12% to 51% of breast cancer survivors, with up to one-third experiencing some pain more than 5 years after treatment,26,27 and our study outcomes concur with those findings. In our study, pain, as measured by the BPI-SF, was found to persist for most participants (75.6%) after the 18-month follow-up. The results of our trial showed that a 12-week exercise intervention administered postsurgery and postradiation had no statistically significant effect on long-term (18 months) pain severity and its interference in daily life. It is worth noting that body regions that had not been directly related to either surgical or radiation treatment for breast cancer were commonly identified as areas of pain but were not specifically targeted by our intervention. However, focusing on pain severity (BPI-SF), our findings suggest that the benefits of targeted upper-extremity exercise on pain in the intermediate time course of follow-up (T3, T4, and T5) was notable compared with the control group, which received standard care. The apparent recurrence of pain at 18 months in both groups was not anticipated and needs to be further investigated.
More specific objective assessments of pain on active shoulder movement identified distinct patterns of pain that could not be isolated using the BPI-SF alone. The incidence and localization of pain on movement differed between the population of women who received a specific exercise intervention and those who received standard care (Figure 2). Patterns of pain over time fluctuated in the control group, whereas the intervention group reported a linear decrease in pain. Residual pain on shoulder movement remained apparent in both groups at 18-months postradiation, but that finding was not reflected in the BPI-SF results. The literature supports our findings on persistent pain among breast cancer survivors,3,7,8,28-30 and in our study of young women carefully screened and excluded for pre-existent shoulder conditions or comorbid medical conditions, recurrent articular pain was nonetheless prevalent. It seems that unidentified or multiple factors may be part of the etiology of pain in this young adult cohort.
Although the BPI-SF is a generic measurement tool commonly used to assess and measure cancer patients’ pain levels, the lack of variance in our BPI-SF severity and interference outcomes over time (T1-T6) (Table 1, Table 2), the variety of “other” unrelated regions (Figure 1) identified by the BPI-SF, and the contrast in our findings on specific physical examination emphasize the potential limitations of this clinical tool.
Moreover, the BPI-SF has not been validated specifically for breast cancer. Harrington and colleagues have recommended using the BPI-SF to assess pain in women with breast cancer,31 but the use of a more multidimensional measurement tool that evaluates axillary, chest, trunk, and upper-limb pain may prove to be more valuable in this population.
Limitations
Recruitment of young adult women was difficult because of our stringent inclusion criteria, the long-term follow-up, and the relatively small population of breast cancer patients in this age demographic. Therefore, the duration of the recruitment phase, despite our having access to a specialized young adult and adolescent clinic in our institute, greatly surpassed the expectations we had when we designed the study. In addition, there remains an inherent bias in participants who accept participation in a study that includes exercise interventions. Potential participants who exercise regularly or have a positive inclination toward doing exercise are more likely to participate. Despite the prescription of a targeted 12-week upper-limb intervention in this study, the general activity levels of both groups may have had an impact on the significance of this study. In addition, the low adherence to the use of self-reported logs failed to capture the true compliance rates of our participants because their lack of tracking does not indicate failure to comply with the program. The use of weekly or biweekly telephone calls to monitor compliance rates of activity more vigilantly may be used in future studies.
Conclusions
Advances in clinical management of breast cancer have improved survival outcomes, and morbidity over recent years, yet symptoms such as pain remain prevalent in this population. The results of this study showed that a targeted, 12-week upper-limb exercise intervention postradiation transiently improved levels of shoulder pain without a concomitant impact on chronic pain or any positive influence on activities of daily living 18 months posttreatment. Furthermore, future studies should use a variety of measurement tools to evaluate trunk and upper-limb pain in women with breast cancer and investigate the optimal timing of postradiation exercise interventions.
Acknowledgments
The authors thank Hope & Cope, the CURE foundation, and the Jewish General Hospital Foundation/Weekend to End Breast Cancer for providing the financial resources needed to sustain this research study. They also thank the McGill Adolescent and Young Adult program for its continued support. Previous oral presentations of research Muanza TM, et al. Randomized clinical trial of a progressive exercise program for young women with breast cancer undergoing radiation therapy. Int J Radiat Oncol Biol Phys. 2015;93(3):s35-s36.
1. World Health Organization. Breast cancer: prevention and control. www.who.int/cancer/detection/breastcancer/en/. Updated 2017. Accessed September 16, 2016.
2. Andersen KG, Kehlet H. Persistent pain after breast cancer treatment: a critical review of risk factors and strategies for prevention. J Pain. 2011;12(7):725-746.
3. Ernst MF, Voogd AC, Balder W, Klinkenbijl JH, Roukema JA. Early and late morbidity associated with axillary levels I-III dissection in breast cancer. J Surg Oncol. 2002;79(3):151-155; discussion 156.
4. Gulluoglu BM, Cingi A, Cakir T, Gercek A, Barlas A, Eti Z. Factors related to post-treatment chronic pain in breast cancer survivors: the interference of pain with life functions. Int J Fertil Womens Med. 2006;51(2):75-82.
5. Jung BF, Ahrendt GM, Oaklander AL, Dworkin RH. Neuropathic pain following breast cancer surgery: proposed classification and research update. Pain. 2003;104(1-2):1-13.
6. Saibil S, Fitzgerald B, Freedman OC, et al. Incidence of taxane-induced pain and distress in patients receiving chemotherapy for early-stage breast cancer: a retrospective, outcomes-based survey. Curr Oncol. 2010;17(4):42-47.
7. Tengrup I, Tennvall-Nittby L, Christiansson I, Laurin M. Arm morbidity after breast-conserving therapy for breast cancer. Acta Oncol. 2000;39(3):393-397.
8. Johansen J, Overgaard J, Blichert-Toft M, Overgaard M. Treatment of morbidity associated with the management of the axilla in breast-conserving therapy. Acta Oncol. 2000;39(3):349-354.
9. Mittmann N, Porter JM, Rangrej J, et al. Health system costs for stage-specific breast cancer: a population-based approach. Curr Oncol. 2014;21(6):281-293.
10. Page A. Keeping patients safe: transforming the work environment of nurses. Washington, DC: National Academies Press; 2004.
11. McNeely ML, Campbell K, Ospina M, et al. Exercise interventions for upper-limb dysfunction due to breast cancer treatment. Cochrane Database Syst Rev. 2010;(6):CD005211. doi:10.1002/14651858.CD005211.pub2
12. Wong P, Muanza T, Hijal T, et al. Effect of exercise in reducing breast and chest-wall pain in patients with breast cancer: a pilot study. Curr Oncol. 2012;19(3):e129-e135.
13. Fernández-Lao C, Cantarero-Villanueva I, Fernández-de-Las-Peñas C, del Moral-Ávila R, Castro-Sánchez AM, Arroyo-Morales M. Effectiveness of a multidimensional physical therapy program on pain, pressure hypersensitivity, and trigger points in breast cancer survivors: a randomized controlled clinical trial. Clin J Pain. 2012;28(2):113-121.
14. Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: cardiopulmonary and quality of life outcomes. J Clin Oncol. 2003;21(9):1660-1668.
15. Segal R, Evans W, Johnson D, et al. Structured exercise improves physical functioning in women with stages I and II breast cancer: results of a randomized controlled trial. J Clin Oncol. 2001;19(3):657-665.
16. Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129-138.
17. Kumar SP. Utilization of Brief Pain Inventory as an assessment tool for pain in patients with cancer: a focused review. Indian J Palliat Care. 2011;17(2):108-115.
18. Van Voorhis CRW, Morgan BL. Understanding power and rules of thumb for determining sample sizes. Tutor Quant Methods Psychol. 2007;3(2):43-50.
19. Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, Cleeland CS. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61(2):277-284.
20. Lee TS, Kilbreath SL, Refshauge KM, Pendlebury SC, Beith JM, Lee MJ. Pectoral stretching program for women undergoing radiotherapy for breast cancer. Breast Cancer Res Treat. 2007;102(3):313-321.
21. Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409-1426.
22. Pollock ML, Gaesser GA, Butcher JD, et al. ACSM position stand: the recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc. 1998;30(6):975-991.
23. Ibrahim M, Muanza T, Smirnow N, et al. Time course of upper limb function and return-to-work post-radiotherapy in young adults with breast cancer: a pilot randomized control trial on effects of targeted exercise program. J Cancer Surviv. 2017;11(6):791-799.
24. Ibrahim M, Muanza T, Smirnow N, et al. A pilot randomized controlled trial on the effects of a progressive exercise program on the range of motion and upper extremity grip strength in young adults with breast cancer. Clin Breast Cancer. 2018;18(1):e55-e64.
25. Gärtner R, Jensen MB, Nielsen J, Ewertz M, Kroman N, Kehlet H. Prevalence of and factors associated with persistent pain following breast cancer surgery. JAMA. 2009;302(18):1985-1992.
26. Hayes SC, Johansson K, Stout NL, et al. Upper-body morbidity after breast cancer: incidence and evidence for evaluation, prevention, and management within a prospective surveillance model of care. Cancer. 2012;118(suppl 8):2237-2249.
27. Kärki A, Simonen R, Mälkiä E, Selfe J. Impairments, activity limitations and participation restrictions 6 and 12 months after breast cancer operation. J Rehabil Med. 2005;37(3):180-188.
28. Katz J, Poleshuck EL, Andrus CH, et al. Risk factors for acute pain and its persistence following breast cancer surgery. Pain. 2005;119(1-3):16-25.
29. Tasmuth T, von Smitten K, Hietanen P, Kataja M, Kalso E. Pain and other symptoms after different treatment modalities of breast cancer. Ann Oncol. 1995;6(5):453-459.
30. Whelan TJ, Levine M, Julian J, Kirkbride P, Skingley P. The effects of radiation therapy on quality of life of women with breast carcinoma: results of a randomized trial. Ontario Clinical Oncology Group. Cancer. 2000;88(10):2260-2266.
31. Harrington S, Gilchrist L, Sander A. Breast cancer EDGE task force outcomes: clinical measures of pain. Rehabil Oncol. 2014;32(1):13-21.
Breast cancer is one of the most prevalent cancers in women worldwide, with more than 1 million new cases diagnosed annually.1 Prognosis for the disease has improved significantly, but 25% to 60% of women living with breast cancer experience some level of pain ranging from mild to severe, the nature of which can evolve from acute to chronic.2 Pre-, intra-, and post-treatment risk factors have been found to correlate with the development of acute and chronic pain and include young age, type of breast surgery (lumpectomy or total mastectomy), axillary node dissection, radiation therapy, and hormonal therapy.3-5 Chemotherapy, particularly anthracycline- and taxane-based regimens, has also been shown to induce pain, arthralgia, myalgia, and peripheral neuropathy during treatment.6 In particular, postradiation pain may result from subcutaneous fibrosis with fixation to underlying musculature and the development of fibrous flaps in the internal axilla.7 These tissue changes are commonly subclinical, occurring 4 to 12 months postradiation,8 and can progress undetected until pain and upper-limb disability develop.
The presence of persistent pain has a considerable impact on the quality of life in survivors of breast cancer: psychological distress is prevalent (anxiety, depression, worry, fear), the performance of daily activities is diminished (eg, bathing, dressing, preparing meals, shopping), and economic independence is compromised by the inability to work or reduced employment and income. These factors directly and indirectly contribute to an increase in the use of health care services.9,10
The management of pain is often characterized by pharmacologic-related treatment, such as the use of opioids and nonsteroidal anti-inflammatory medications, and nonpharmacologic-related treatment, such as exercise. Empirical evidence has shown that rehabilitative exercise programs, which commonly include a combination of resistance training and aerobic exercises, can effectively reduce pain in breast cancer survivors.10-12 Women living with breast cancer who are directed to rehabilitative exercise programs experience an improvement not only in pain levels but also in their ability to engage in activities of daily living, in their psychological health, and in their overall quality of life.13-15 However, despite evidence to support exercise programs to reduce pain related to breast cancer treatment, residual pain and upper-limb discomfort are common complaints in breast cancer survivors, and there is little focus on the duration of effectiveness of such programs for reducing pain after treatment for breast cancer. The objective of this study was to determine if an exercise program initiated postradiation would improve long-term pain levels in a carefully selected population of young women who were living with breast cancer and had no history of shoulder pathology or significant treatment complications.
Methods
Design
We used a pilot randomized control trial to compare the long-term effectiveness of a 12-week postradiation exercise program versus standard care on residual pain levels in young women (aged 18-45 years) living with breast cancer. The program was initiated 3 to 4 weeks postradiation to allow for acute inflammatory reactions to subside. Pain severity and interference were assessed using the Brief Pain Inventory-Short Form (BPI-SF), a tool for assessing cancer pain.16,17 Pain levels for isolated shoulder movements were also recorded on examination by a physical therapist. All measures were collected at 6 time points (T1-T6): postsurgery and preradiation (T1, baseline), postradiation and preintervention (T2), and 4 points during an 18-month period postradiation (T3-T6 at 3, 6, 12, and 18 months postradiation).
Sample
Young women living with breast cancer who met our eligibility criteria were identified from 2 clinics at the Jewish General Hospital – the Segal Cancer Center and the Department of Radiation Oncology in Montréal, Québec, Canada. Inclusion criteria included women with a diagnosis of stage I to stage III breast cancer, who were 18 to 45 years old, were scheduled for postoperative adjuvant radiation therapy, had an Eastern Cooperative Oncology Group Performance Status of 0 or 1 (normal ambulatory function, minimal symptoms), and who consented to participate in the study. Exclusion criteria included women with a metastatic (stage IV) diagnosis; significant musculoskeletal, cardiac, pulmonary, or metabolic comorbidities that would not allow for participation in physical activity; a previous breast cancer diagnosis with treatment to the ipsilateral or contralateral sides; postsurgical lymphedema; postsurgical capsulitis, tendonitis, or other shoulder inflammatory complications; and any contraindication to exercise. The recruitment goal was outlined as 50 patients per group; however, a protracted accrual time because of the stringent study criteria yielded a sample of 29 and 30 patients for the intervention and control groups, respectively, which was sufficient for significant testing of differences between the 2 study groups.18
Variables and measures
Clinical characteristics. We used standardized questions and chart review to document the participants’ clinical characteristics and to capture information on the following: the stage and subtype of breast cancer, hormonal and human epidermal growth factor receptors (HER2) (estrogen receptor, progesterone receptor, and HER2 status), extent of surgery (lumpectomy or total mastectomy), and other modalities of treatment (eg, chemotherapy, radiation therapy).
Pain assessment. The BPI-SF was used to assess participants’ cancer-related pain. Pain severity ranged from 0 (no pain), 1 to 4 (mild pain), 5 to 6 (moderate pain), to 7 to 10 (severe pain).18,19 The questionnaire also identifies the pain interference in daily activities using a Likert scale ranging from 0 (Does not interfere) to 10 (Completely interferes) in the following 7 domains or subscales: General Activity, Walking, Mood, Sleep, Work, Relations with Others, and Enjoyment of Life.16 For the purpose of this study, mean scores were tabulated using both pain intensity and interference scales.
Another important component of the BPI-SF instructs participants to localize pain by means of a body diagram. For purpose of analysis, 3 pain regions were established: shoulder girdle/chest wall on the affected side; neck and other upper extremity, including hand(s), forearm(s), wrist(s), and finger(s); and other regions, including abdominal discomfort, leg(s), hip(s), knee(s), ankle(s), lower back, and feet. In addition, pain levels on movement (Yes/No) were recorded for isolated shoulder flexion, abduction, and horizontal abduction (sitting and standing). The measurements were completed by a single physical therapist throughout the course of the study to minimize variance.
Procedure
The study protocol was approved by the Research Ethics Board at the Jewish General Hospital. Recruitment occurred from 2011 through 2015. The research was in accordance with the ethical standards of the responsible committee on human experimentation. Eligible women were recruited by the research coordinator who described the purpose, risks, and benefits of the study; advised on confidentiality, data collection, and intervention allocation procedures; and highlighted voluntary participation. The research coordinator addressed any concerns on the part of the participants before obtaining their written informed consent. Random allocation to the intervention and control groups was established using a web-based randomization plan generator (www.randomization.com). A single individual was responsible for the randomization process, and treatment assignments were revealed after each participant’s name had been entered. A physical therapist performed 6 sequential evaluations (T1-T6) at the time of participants’ medical follow-up appointments.
Intervention
The 12-week exercise intervention started 3 weeks postradiation and was composed of an initial 6-week program of low-level cardiovascular and resistance exercises that progressed to a set of more advanced exercises for the remaining 6 weeks. Participants were instructed to warm up for at least 10 minutes with a cardiovascular exercise of their choice (eg, a recumbent cross trainer, walking, or stairs) before doing a combined strength, endurance, and stretching exercise program for the upper body.20 The final portion of the exercise intervention included a period of light cool-down. Weight training resistance levels were based on a maximum 8 to 10 repetitions for strength and a maximum of 20 repetitions for endurance training exercises, which progressed gradually over the course of the 12-week exercise program to ensure participant safety.21,22 Participants in the intervention group were supervised at least once a week by an exercise physiologist at a center for oncology patients (Hope & Cope Wellness Centre), and patients were encouraged to perform the program at home 2 to 3 times a week. Those who were not able to exercise consistently at the center were provided with equipment and instructed on how to do the program safely at home.
By comparison, the control group received standard care, which included advice on the benefits of an active lifestyle, including exercise, but without a specific intervention. Participants were not restricted in their physical activity and/or sport participation levels, and their weekly activity levels were calculated using the Metabolic Equivalent of Task and recorded at each of the 6 time points.
Statistical analysis
Descriptive statistics were used to examine participant characteristics. The quantitative data collected through the BPI-SF measures were analyzed with JMP software (version 11.2; SAS Institute, Cary, NC). Continuous variables were tested for statistical significance (P ≤ .05) through the chi-square (categorical), analysis of variance, and nonparametric Wilcoxon tests. The analyses did not include missing data.
Results
A total of 59 young women were randomized into the intervention (n = 29) and control (n = 30) groups. Of those, 2 participants dropped out of the study because of family and time constraints, and 3 participants died, 2 from the control and 1 from the intervention group, after subsequently developing metastatic disease. Baseline data including comparative tumor characteristics, surgical interventions, and treatment interventions have been published in relation to other elements of this study.23,24 The participants had a mean age of 39.2 years (standard deviation [SD], 5.0). More than half of them had an invasive ductal carcinoma (69.5%) and were estrogen positive (78.0%), progesterone positive (74.6%), or HER2 positive (20.3%), whereas 10.2% were triple negative. Most of the participants had undergone breast-sparing procedures (86.4% lumpectomy), and 18.6% had a total mastectomy. By random chance, the intervention group had higher rates of total mastectomy (24.4% and 13.3%, respectively) and surgical reconstruction (12.2% and 6.7%, respectively) compared with the control group. Most of the women (71.2%) received chemotherapy, and all received radiation therapy. In the intervention group, 37.2% received radiation therapy localized to the axilla, and 88% received a boost of radiation to the surgical bed. Self-reported exercise diaries were returned by 15 of the 29 intervention participants, and training frequencies among them varied significantly (1-6 times a week).
The findings showed that there was little variance between the intervention and control groups in BPI-SF severity scores from T1 to T6, so the means and SDs of the BPI-SF scores were grouped at 6 time points (Table 1). There was no statistically significant difference between baseline measures at T1 (1.68; SD, 1.17) and measures at 18 months postintervention (T6: 1.46; SD, 1.37). At baseline, 87.7% of the women reported no pain (31.5%) or mild levels of pain (55.6%), and 13% reported moderate or severe pain. Over the duration of the study from T1 to T6, these primarily low levels of pain (BPI-SF, 0-4) remained consistent with a favorable shift toward having no pain (T1: 31.5%; T6: 24.4%). By 18 months postintervention, 95.7% of women reported no or mild pain, with 4.9% reporting moderate pain.
Similarly, there was little variance over time (T1-T6) and no statistically significant differences between the 2 groups in BPI-SF–measured levels of pain interference in daily activities (Table 2). Moreover, a domain analysis showed that there were no statistically significant differences in pain interference scores when comparing the type and extent of surgery (total mastectomy: 0.59 [1.17]; lumpectomy: 0.94 [1.96]). By chance – and not related directly to the objectives of this study – there was a statistically significant difference between the intervention and control groups in the interference of pain on the Enjoyment of Life domain in favor of the control group.
The sites of pain captured by the BPI-SF shed light on the preceding findings (Figure 1). At baseline (T1, postsurgery and preradiation), 37.0% of participants reported pain in the shoulder girdle–chest wall region, whereas 20.4% reported pain in the general neck–upper extremity region and 50% in other regions. Postradiation, shoulder girdle–chest wall pain was identified as the highest reported site of pain (49.1%; T2, postradiation and preintervention) and remained elevated at 3 months (T3) and 6 months (T4) postradiation (46.9% and 45.5%, respectively). At 12 and 18 months postradiation (T5 and T6), the principal focus of pain shifted once again to “other” regions at 30% and 32.5%, respectively, and the neck–upper extremity region at 10% and 15%, respectively. Shoulder girdle–chest wall pain concomitantly improved at those time points (15% and 25% respectively) but was not eliminated.
Pain levels recorded on physical examination for isolated shoulder range of movements were recently published,24 and they have been abbreviated and reproduced in this paper (Figure 2) to allow for a comparison of findings between the exercise intervention group and the control group to help determine the sensitivity of these tools for use in breast cancer patients. At baseline, pain levels with active movement were noted to be slightly greater in the intervention group for flexion and abduction.
Following the intervention, at 3 and 6 months postradiation (T3 and T4), the intervention group showed a steady decrease in pain levels in flexion and abduction, whereas the control group showed a 5-fold increase in pain with horizontal abduction. Furthermore, participants in the intervention group reported having no pain on movement 12 months postradiation (T5); however, recurrence of pain was apparent with all shoulder movements by 18 months postradiation (T6) in both the intervention and control groups.
Discussion
Previous studies have hypothesized that younger age (18-39 years), adjuvant radiotherapy, and axillary node dissection are risk factors for chronic pain in breast cancer survivors.22,25 Persistent pain is prevalent in 12% to 51% of breast cancer survivors, with up to one-third experiencing some pain more than 5 years after treatment,26,27 and our study outcomes concur with those findings. In our study, pain, as measured by the BPI-SF, was found to persist for most participants (75.6%) after the 18-month follow-up. The results of our trial showed that a 12-week exercise intervention administered postsurgery and postradiation had no statistically significant effect on long-term (18 months) pain severity and its interference in daily life. It is worth noting that body regions that had not been directly related to either surgical or radiation treatment for breast cancer were commonly identified as areas of pain but were not specifically targeted by our intervention. However, focusing on pain severity (BPI-SF), our findings suggest that the benefits of targeted upper-extremity exercise on pain in the intermediate time course of follow-up (T3, T4, and T5) was notable compared with the control group, which received standard care. The apparent recurrence of pain at 18 months in both groups was not anticipated and needs to be further investigated.
More specific objective assessments of pain on active shoulder movement identified distinct patterns of pain that could not be isolated using the BPI-SF alone. The incidence and localization of pain on movement differed between the population of women who received a specific exercise intervention and those who received standard care (Figure 2). Patterns of pain over time fluctuated in the control group, whereas the intervention group reported a linear decrease in pain. Residual pain on shoulder movement remained apparent in both groups at 18-months postradiation, but that finding was not reflected in the BPI-SF results. The literature supports our findings on persistent pain among breast cancer survivors,3,7,8,28-30 and in our study of young women carefully screened and excluded for pre-existent shoulder conditions or comorbid medical conditions, recurrent articular pain was nonetheless prevalent. It seems that unidentified or multiple factors may be part of the etiology of pain in this young adult cohort.
Although the BPI-SF is a generic measurement tool commonly used to assess and measure cancer patients’ pain levels, the lack of variance in our BPI-SF severity and interference outcomes over time (T1-T6) (Table 1, Table 2), the variety of “other” unrelated regions (Figure 1) identified by the BPI-SF, and the contrast in our findings on specific physical examination emphasize the potential limitations of this clinical tool.
Moreover, the BPI-SF has not been validated specifically for breast cancer. Harrington and colleagues have recommended using the BPI-SF to assess pain in women with breast cancer,31 but the use of a more multidimensional measurement tool that evaluates axillary, chest, trunk, and upper-limb pain may prove to be more valuable in this population.
Limitations
Recruitment of young adult women was difficult because of our stringent inclusion criteria, the long-term follow-up, and the relatively small population of breast cancer patients in this age demographic. Therefore, the duration of the recruitment phase, despite our having access to a specialized young adult and adolescent clinic in our institute, greatly surpassed the expectations we had when we designed the study. In addition, there remains an inherent bias in participants who accept participation in a study that includes exercise interventions. Potential participants who exercise regularly or have a positive inclination toward doing exercise are more likely to participate. Despite the prescription of a targeted 12-week upper-limb intervention in this study, the general activity levels of both groups may have had an impact on the significance of this study. In addition, the low adherence to the use of self-reported logs failed to capture the true compliance rates of our participants because their lack of tracking does not indicate failure to comply with the program. The use of weekly or biweekly telephone calls to monitor compliance rates of activity more vigilantly may be used in future studies.
Conclusions
Advances in clinical management of breast cancer have improved survival outcomes, and morbidity over recent years, yet symptoms such as pain remain prevalent in this population. The results of this study showed that a targeted, 12-week upper-limb exercise intervention postradiation transiently improved levels of shoulder pain without a concomitant impact on chronic pain or any positive influence on activities of daily living 18 months posttreatment. Furthermore, future studies should use a variety of measurement tools to evaluate trunk and upper-limb pain in women with breast cancer and investigate the optimal timing of postradiation exercise interventions.
Acknowledgments
The authors thank Hope & Cope, the CURE foundation, and the Jewish General Hospital Foundation/Weekend to End Breast Cancer for providing the financial resources needed to sustain this research study. They also thank the McGill Adolescent and Young Adult program for its continued support. Previous oral presentations of research Muanza TM, et al. Randomized clinical trial of a progressive exercise program for young women with breast cancer undergoing radiation therapy. Int J Radiat Oncol Biol Phys. 2015;93(3):s35-s36.
Breast cancer is one of the most prevalent cancers in women worldwide, with more than 1 million new cases diagnosed annually.1 Prognosis for the disease has improved significantly, but 25% to 60% of women living with breast cancer experience some level of pain ranging from mild to severe, the nature of which can evolve from acute to chronic.2 Pre-, intra-, and post-treatment risk factors have been found to correlate with the development of acute and chronic pain and include young age, type of breast surgery (lumpectomy or total mastectomy), axillary node dissection, radiation therapy, and hormonal therapy.3-5 Chemotherapy, particularly anthracycline- and taxane-based regimens, has also been shown to induce pain, arthralgia, myalgia, and peripheral neuropathy during treatment.6 In particular, postradiation pain may result from subcutaneous fibrosis with fixation to underlying musculature and the development of fibrous flaps in the internal axilla.7 These tissue changes are commonly subclinical, occurring 4 to 12 months postradiation,8 and can progress undetected until pain and upper-limb disability develop.
The presence of persistent pain has a considerable impact on the quality of life in survivors of breast cancer: psychological distress is prevalent (anxiety, depression, worry, fear), the performance of daily activities is diminished (eg, bathing, dressing, preparing meals, shopping), and economic independence is compromised by the inability to work or reduced employment and income. These factors directly and indirectly contribute to an increase in the use of health care services.9,10
The management of pain is often characterized by pharmacologic-related treatment, such as the use of opioids and nonsteroidal anti-inflammatory medications, and nonpharmacologic-related treatment, such as exercise. Empirical evidence has shown that rehabilitative exercise programs, which commonly include a combination of resistance training and aerobic exercises, can effectively reduce pain in breast cancer survivors.10-12 Women living with breast cancer who are directed to rehabilitative exercise programs experience an improvement not only in pain levels but also in their ability to engage in activities of daily living, in their psychological health, and in their overall quality of life.13-15 However, despite evidence to support exercise programs to reduce pain related to breast cancer treatment, residual pain and upper-limb discomfort are common complaints in breast cancer survivors, and there is little focus on the duration of effectiveness of such programs for reducing pain after treatment for breast cancer. The objective of this study was to determine if an exercise program initiated postradiation would improve long-term pain levels in a carefully selected population of young women who were living with breast cancer and had no history of shoulder pathology or significant treatment complications.
Methods
Design
We used a pilot randomized control trial to compare the long-term effectiveness of a 12-week postradiation exercise program versus standard care on residual pain levels in young women (aged 18-45 years) living with breast cancer. The program was initiated 3 to 4 weeks postradiation to allow for acute inflammatory reactions to subside. Pain severity and interference were assessed using the Brief Pain Inventory-Short Form (BPI-SF), a tool for assessing cancer pain.16,17 Pain levels for isolated shoulder movements were also recorded on examination by a physical therapist. All measures were collected at 6 time points (T1-T6): postsurgery and preradiation (T1, baseline), postradiation and preintervention (T2), and 4 points during an 18-month period postradiation (T3-T6 at 3, 6, 12, and 18 months postradiation).
Sample
Young women living with breast cancer who met our eligibility criteria were identified from 2 clinics at the Jewish General Hospital – the Segal Cancer Center and the Department of Radiation Oncology in Montréal, Québec, Canada. Inclusion criteria included women with a diagnosis of stage I to stage III breast cancer, who were 18 to 45 years old, were scheduled for postoperative adjuvant radiation therapy, had an Eastern Cooperative Oncology Group Performance Status of 0 or 1 (normal ambulatory function, minimal symptoms), and who consented to participate in the study. Exclusion criteria included women with a metastatic (stage IV) diagnosis; significant musculoskeletal, cardiac, pulmonary, or metabolic comorbidities that would not allow for participation in physical activity; a previous breast cancer diagnosis with treatment to the ipsilateral or contralateral sides; postsurgical lymphedema; postsurgical capsulitis, tendonitis, or other shoulder inflammatory complications; and any contraindication to exercise. The recruitment goal was outlined as 50 patients per group; however, a protracted accrual time because of the stringent study criteria yielded a sample of 29 and 30 patients for the intervention and control groups, respectively, which was sufficient for significant testing of differences between the 2 study groups.18
Variables and measures
Clinical characteristics. We used standardized questions and chart review to document the participants’ clinical characteristics and to capture information on the following: the stage and subtype of breast cancer, hormonal and human epidermal growth factor receptors (HER2) (estrogen receptor, progesterone receptor, and HER2 status), extent of surgery (lumpectomy or total mastectomy), and other modalities of treatment (eg, chemotherapy, radiation therapy).
Pain assessment. The BPI-SF was used to assess participants’ cancer-related pain. Pain severity ranged from 0 (no pain), 1 to 4 (mild pain), 5 to 6 (moderate pain), to 7 to 10 (severe pain).18,19 The questionnaire also identifies the pain interference in daily activities using a Likert scale ranging from 0 (Does not interfere) to 10 (Completely interferes) in the following 7 domains or subscales: General Activity, Walking, Mood, Sleep, Work, Relations with Others, and Enjoyment of Life.16 For the purpose of this study, mean scores were tabulated using both pain intensity and interference scales.
Another important component of the BPI-SF instructs participants to localize pain by means of a body diagram. For purpose of analysis, 3 pain regions were established: shoulder girdle/chest wall on the affected side; neck and other upper extremity, including hand(s), forearm(s), wrist(s), and finger(s); and other regions, including abdominal discomfort, leg(s), hip(s), knee(s), ankle(s), lower back, and feet. In addition, pain levels on movement (Yes/No) were recorded for isolated shoulder flexion, abduction, and horizontal abduction (sitting and standing). The measurements were completed by a single physical therapist throughout the course of the study to minimize variance.
Procedure
The study protocol was approved by the Research Ethics Board at the Jewish General Hospital. Recruitment occurred from 2011 through 2015. The research was in accordance with the ethical standards of the responsible committee on human experimentation. Eligible women were recruited by the research coordinator who described the purpose, risks, and benefits of the study; advised on confidentiality, data collection, and intervention allocation procedures; and highlighted voluntary participation. The research coordinator addressed any concerns on the part of the participants before obtaining their written informed consent. Random allocation to the intervention and control groups was established using a web-based randomization plan generator (www.randomization.com). A single individual was responsible for the randomization process, and treatment assignments were revealed after each participant’s name had been entered. A physical therapist performed 6 sequential evaluations (T1-T6) at the time of participants’ medical follow-up appointments.
Intervention
The 12-week exercise intervention started 3 weeks postradiation and was composed of an initial 6-week program of low-level cardiovascular and resistance exercises that progressed to a set of more advanced exercises for the remaining 6 weeks. Participants were instructed to warm up for at least 10 minutes with a cardiovascular exercise of their choice (eg, a recumbent cross trainer, walking, or stairs) before doing a combined strength, endurance, and stretching exercise program for the upper body.20 The final portion of the exercise intervention included a period of light cool-down. Weight training resistance levels were based on a maximum 8 to 10 repetitions for strength and a maximum of 20 repetitions for endurance training exercises, which progressed gradually over the course of the 12-week exercise program to ensure participant safety.21,22 Participants in the intervention group were supervised at least once a week by an exercise physiologist at a center for oncology patients (Hope & Cope Wellness Centre), and patients were encouraged to perform the program at home 2 to 3 times a week. Those who were not able to exercise consistently at the center were provided with equipment and instructed on how to do the program safely at home.
By comparison, the control group received standard care, which included advice on the benefits of an active lifestyle, including exercise, but without a specific intervention. Participants were not restricted in their physical activity and/or sport participation levels, and their weekly activity levels were calculated using the Metabolic Equivalent of Task and recorded at each of the 6 time points.
Statistical analysis
Descriptive statistics were used to examine participant characteristics. The quantitative data collected through the BPI-SF measures were analyzed with JMP software (version 11.2; SAS Institute, Cary, NC). Continuous variables were tested for statistical significance (P ≤ .05) through the chi-square (categorical), analysis of variance, and nonparametric Wilcoxon tests. The analyses did not include missing data.
Results
A total of 59 young women were randomized into the intervention (n = 29) and control (n = 30) groups. Of those, 2 participants dropped out of the study because of family and time constraints, and 3 participants died, 2 from the control and 1 from the intervention group, after subsequently developing metastatic disease. Baseline data including comparative tumor characteristics, surgical interventions, and treatment interventions have been published in relation to other elements of this study.23,24 The participants had a mean age of 39.2 years (standard deviation [SD], 5.0). More than half of them had an invasive ductal carcinoma (69.5%) and were estrogen positive (78.0%), progesterone positive (74.6%), or HER2 positive (20.3%), whereas 10.2% were triple negative. Most of the participants had undergone breast-sparing procedures (86.4% lumpectomy), and 18.6% had a total mastectomy. By random chance, the intervention group had higher rates of total mastectomy (24.4% and 13.3%, respectively) and surgical reconstruction (12.2% and 6.7%, respectively) compared with the control group. Most of the women (71.2%) received chemotherapy, and all received radiation therapy. In the intervention group, 37.2% received radiation therapy localized to the axilla, and 88% received a boost of radiation to the surgical bed. Self-reported exercise diaries were returned by 15 of the 29 intervention participants, and training frequencies among them varied significantly (1-6 times a week).
The findings showed that there was little variance between the intervention and control groups in BPI-SF severity scores from T1 to T6, so the means and SDs of the BPI-SF scores were grouped at 6 time points (Table 1). There was no statistically significant difference between baseline measures at T1 (1.68; SD, 1.17) and measures at 18 months postintervention (T6: 1.46; SD, 1.37). At baseline, 87.7% of the women reported no pain (31.5%) or mild levels of pain (55.6%), and 13% reported moderate or severe pain. Over the duration of the study from T1 to T6, these primarily low levels of pain (BPI-SF, 0-4) remained consistent with a favorable shift toward having no pain (T1: 31.5%; T6: 24.4%). By 18 months postintervention, 95.7% of women reported no or mild pain, with 4.9% reporting moderate pain.
Similarly, there was little variance over time (T1-T6) and no statistically significant differences between the 2 groups in BPI-SF–measured levels of pain interference in daily activities (Table 2). Moreover, a domain analysis showed that there were no statistically significant differences in pain interference scores when comparing the type and extent of surgery (total mastectomy: 0.59 [1.17]; lumpectomy: 0.94 [1.96]). By chance – and not related directly to the objectives of this study – there was a statistically significant difference between the intervention and control groups in the interference of pain on the Enjoyment of Life domain in favor of the control group.
The sites of pain captured by the BPI-SF shed light on the preceding findings (Figure 1). At baseline (T1, postsurgery and preradiation), 37.0% of participants reported pain in the shoulder girdle–chest wall region, whereas 20.4% reported pain in the general neck–upper extremity region and 50% in other regions. Postradiation, shoulder girdle–chest wall pain was identified as the highest reported site of pain (49.1%; T2, postradiation and preintervention) and remained elevated at 3 months (T3) and 6 months (T4) postradiation (46.9% and 45.5%, respectively). At 12 and 18 months postradiation (T5 and T6), the principal focus of pain shifted once again to “other” regions at 30% and 32.5%, respectively, and the neck–upper extremity region at 10% and 15%, respectively. Shoulder girdle–chest wall pain concomitantly improved at those time points (15% and 25% respectively) but was not eliminated.
Pain levels recorded on physical examination for isolated shoulder range of movements were recently published,24 and they have been abbreviated and reproduced in this paper (Figure 2) to allow for a comparison of findings between the exercise intervention group and the control group to help determine the sensitivity of these tools for use in breast cancer patients. At baseline, pain levels with active movement were noted to be slightly greater in the intervention group for flexion and abduction.
Following the intervention, at 3 and 6 months postradiation (T3 and T4), the intervention group showed a steady decrease in pain levels in flexion and abduction, whereas the control group showed a 5-fold increase in pain with horizontal abduction. Furthermore, participants in the intervention group reported having no pain on movement 12 months postradiation (T5); however, recurrence of pain was apparent with all shoulder movements by 18 months postradiation (T6) in both the intervention and control groups.
Discussion
Previous studies have hypothesized that younger age (18-39 years), adjuvant radiotherapy, and axillary node dissection are risk factors for chronic pain in breast cancer survivors.22,25 Persistent pain is prevalent in 12% to 51% of breast cancer survivors, with up to one-third experiencing some pain more than 5 years after treatment,26,27 and our study outcomes concur with those findings. In our study, pain, as measured by the BPI-SF, was found to persist for most participants (75.6%) after the 18-month follow-up. The results of our trial showed that a 12-week exercise intervention administered postsurgery and postradiation had no statistically significant effect on long-term (18 months) pain severity and its interference in daily life. It is worth noting that body regions that had not been directly related to either surgical or radiation treatment for breast cancer were commonly identified as areas of pain but were not specifically targeted by our intervention. However, focusing on pain severity (BPI-SF), our findings suggest that the benefits of targeted upper-extremity exercise on pain in the intermediate time course of follow-up (T3, T4, and T5) was notable compared with the control group, which received standard care. The apparent recurrence of pain at 18 months in both groups was not anticipated and needs to be further investigated.
More specific objective assessments of pain on active shoulder movement identified distinct patterns of pain that could not be isolated using the BPI-SF alone. The incidence and localization of pain on movement differed between the population of women who received a specific exercise intervention and those who received standard care (Figure 2). Patterns of pain over time fluctuated in the control group, whereas the intervention group reported a linear decrease in pain. Residual pain on shoulder movement remained apparent in both groups at 18-months postradiation, but that finding was not reflected in the BPI-SF results. The literature supports our findings on persistent pain among breast cancer survivors,3,7,8,28-30 and in our study of young women carefully screened and excluded for pre-existent shoulder conditions or comorbid medical conditions, recurrent articular pain was nonetheless prevalent. It seems that unidentified or multiple factors may be part of the etiology of pain in this young adult cohort.
Although the BPI-SF is a generic measurement tool commonly used to assess and measure cancer patients’ pain levels, the lack of variance in our BPI-SF severity and interference outcomes over time (T1-T6) (Table 1, Table 2), the variety of “other” unrelated regions (Figure 1) identified by the BPI-SF, and the contrast in our findings on specific physical examination emphasize the potential limitations of this clinical tool.
Moreover, the BPI-SF has not been validated specifically for breast cancer. Harrington and colleagues have recommended using the BPI-SF to assess pain in women with breast cancer,31 but the use of a more multidimensional measurement tool that evaluates axillary, chest, trunk, and upper-limb pain may prove to be more valuable in this population.
Limitations
Recruitment of young adult women was difficult because of our stringent inclusion criteria, the long-term follow-up, and the relatively small population of breast cancer patients in this age demographic. Therefore, the duration of the recruitment phase, despite our having access to a specialized young adult and adolescent clinic in our institute, greatly surpassed the expectations we had when we designed the study. In addition, there remains an inherent bias in participants who accept participation in a study that includes exercise interventions. Potential participants who exercise regularly or have a positive inclination toward doing exercise are more likely to participate. Despite the prescription of a targeted 12-week upper-limb intervention in this study, the general activity levels of both groups may have had an impact on the significance of this study. In addition, the low adherence to the use of self-reported logs failed to capture the true compliance rates of our participants because their lack of tracking does not indicate failure to comply with the program. The use of weekly or biweekly telephone calls to monitor compliance rates of activity more vigilantly may be used in future studies.
Conclusions
Advances in clinical management of breast cancer have improved survival outcomes, and morbidity over recent years, yet symptoms such as pain remain prevalent in this population. The results of this study showed that a targeted, 12-week upper-limb exercise intervention postradiation transiently improved levels of shoulder pain without a concomitant impact on chronic pain or any positive influence on activities of daily living 18 months posttreatment. Furthermore, future studies should use a variety of measurement tools to evaluate trunk and upper-limb pain in women with breast cancer and investigate the optimal timing of postradiation exercise interventions.
Acknowledgments
The authors thank Hope & Cope, the CURE foundation, and the Jewish General Hospital Foundation/Weekend to End Breast Cancer for providing the financial resources needed to sustain this research study. They also thank the McGill Adolescent and Young Adult program for its continued support. Previous oral presentations of research Muanza TM, et al. Randomized clinical trial of a progressive exercise program for young women with breast cancer undergoing radiation therapy. Int J Radiat Oncol Biol Phys. 2015;93(3):s35-s36.
1. World Health Organization. Breast cancer: prevention and control. www.who.int/cancer/detection/breastcancer/en/. Updated 2017. Accessed September 16, 2016.
2. Andersen KG, Kehlet H. Persistent pain after breast cancer treatment: a critical review of risk factors and strategies for prevention. J Pain. 2011;12(7):725-746.
3. Ernst MF, Voogd AC, Balder W, Klinkenbijl JH, Roukema JA. Early and late morbidity associated with axillary levels I-III dissection in breast cancer. J Surg Oncol. 2002;79(3):151-155; discussion 156.
4. Gulluoglu BM, Cingi A, Cakir T, Gercek A, Barlas A, Eti Z. Factors related to post-treatment chronic pain in breast cancer survivors: the interference of pain with life functions. Int J Fertil Womens Med. 2006;51(2):75-82.
5. Jung BF, Ahrendt GM, Oaklander AL, Dworkin RH. Neuropathic pain following breast cancer surgery: proposed classification and research update. Pain. 2003;104(1-2):1-13.
6. Saibil S, Fitzgerald B, Freedman OC, et al. Incidence of taxane-induced pain and distress in patients receiving chemotherapy for early-stage breast cancer: a retrospective, outcomes-based survey. Curr Oncol. 2010;17(4):42-47.
7. Tengrup I, Tennvall-Nittby L, Christiansson I, Laurin M. Arm morbidity after breast-conserving therapy for breast cancer. Acta Oncol. 2000;39(3):393-397.
8. Johansen J, Overgaard J, Blichert-Toft M, Overgaard M. Treatment of morbidity associated with the management of the axilla in breast-conserving therapy. Acta Oncol. 2000;39(3):349-354.
9. Mittmann N, Porter JM, Rangrej J, et al. Health system costs for stage-specific breast cancer: a population-based approach. Curr Oncol. 2014;21(6):281-293.
10. Page A. Keeping patients safe: transforming the work environment of nurses. Washington, DC: National Academies Press; 2004.
11. McNeely ML, Campbell K, Ospina M, et al. Exercise interventions for upper-limb dysfunction due to breast cancer treatment. Cochrane Database Syst Rev. 2010;(6):CD005211. doi:10.1002/14651858.CD005211.pub2
12. Wong P, Muanza T, Hijal T, et al. Effect of exercise in reducing breast and chest-wall pain in patients with breast cancer: a pilot study. Curr Oncol. 2012;19(3):e129-e135.
13. Fernández-Lao C, Cantarero-Villanueva I, Fernández-de-Las-Peñas C, del Moral-Ávila R, Castro-Sánchez AM, Arroyo-Morales M. Effectiveness of a multidimensional physical therapy program on pain, pressure hypersensitivity, and trigger points in breast cancer survivors: a randomized controlled clinical trial. Clin J Pain. 2012;28(2):113-121.
14. Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: cardiopulmonary and quality of life outcomes. J Clin Oncol. 2003;21(9):1660-1668.
15. Segal R, Evans W, Johnson D, et al. Structured exercise improves physical functioning in women with stages I and II breast cancer: results of a randomized controlled trial. J Clin Oncol. 2001;19(3):657-665.
16. Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129-138.
17. Kumar SP. Utilization of Brief Pain Inventory as an assessment tool for pain in patients with cancer: a focused review. Indian J Palliat Care. 2011;17(2):108-115.
18. Van Voorhis CRW, Morgan BL. Understanding power and rules of thumb for determining sample sizes. Tutor Quant Methods Psychol. 2007;3(2):43-50.
19. Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, Cleeland CS. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61(2):277-284.
20. Lee TS, Kilbreath SL, Refshauge KM, Pendlebury SC, Beith JM, Lee MJ. Pectoral stretching program for women undergoing radiotherapy for breast cancer. Breast Cancer Res Treat. 2007;102(3):313-321.
21. Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409-1426.
22. Pollock ML, Gaesser GA, Butcher JD, et al. ACSM position stand: the recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc. 1998;30(6):975-991.
23. Ibrahim M, Muanza T, Smirnow N, et al. Time course of upper limb function and return-to-work post-radiotherapy in young adults with breast cancer: a pilot randomized control trial on effects of targeted exercise program. J Cancer Surviv. 2017;11(6):791-799.
24. Ibrahim M, Muanza T, Smirnow N, et al. A pilot randomized controlled trial on the effects of a progressive exercise program on the range of motion and upper extremity grip strength in young adults with breast cancer. Clin Breast Cancer. 2018;18(1):e55-e64.
25. Gärtner R, Jensen MB, Nielsen J, Ewertz M, Kroman N, Kehlet H. Prevalence of and factors associated with persistent pain following breast cancer surgery. JAMA. 2009;302(18):1985-1992.
26. Hayes SC, Johansson K, Stout NL, et al. Upper-body morbidity after breast cancer: incidence and evidence for evaluation, prevention, and management within a prospective surveillance model of care. Cancer. 2012;118(suppl 8):2237-2249.
27. Kärki A, Simonen R, Mälkiä E, Selfe J. Impairments, activity limitations and participation restrictions 6 and 12 months after breast cancer operation. J Rehabil Med. 2005;37(3):180-188.
28. Katz J, Poleshuck EL, Andrus CH, et al. Risk factors for acute pain and its persistence following breast cancer surgery. Pain. 2005;119(1-3):16-25.
29. Tasmuth T, von Smitten K, Hietanen P, Kataja M, Kalso E. Pain and other symptoms after different treatment modalities of breast cancer. Ann Oncol. 1995;6(5):453-459.
30. Whelan TJ, Levine M, Julian J, Kirkbride P, Skingley P. The effects of radiation therapy on quality of life of women with breast carcinoma: results of a randomized trial. Ontario Clinical Oncology Group. Cancer. 2000;88(10):2260-2266.
31. Harrington S, Gilchrist L, Sander A. Breast cancer EDGE task force outcomes: clinical measures of pain. Rehabil Oncol. 2014;32(1):13-21.
1. World Health Organization. Breast cancer: prevention and control. www.who.int/cancer/detection/breastcancer/en/. Updated 2017. Accessed September 16, 2016.
2. Andersen KG, Kehlet H. Persistent pain after breast cancer treatment: a critical review of risk factors and strategies for prevention. J Pain. 2011;12(7):725-746.
3. Ernst MF, Voogd AC, Balder W, Klinkenbijl JH, Roukema JA. Early and late morbidity associated with axillary levels I-III dissection in breast cancer. J Surg Oncol. 2002;79(3):151-155; discussion 156.
4. Gulluoglu BM, Cingi A, Cakir T, Gercek A, Barlas A, Eti Z. Factors related to post-treatment chronic pain in breast cancer survivors: the interference of pain with life functions. Int J Fertil Womens Med. 2006;51(2):75-82.
5. Jung BF, Ahrendt GM, Oaklander AL, Dworkin RH. Neuropathic pain following breast cancer surgery: proposed classification and research update. Pain. 2003;104(1-2):1-13.
6. Saibil S, Fitzgerald B, Freedman OC, et al. Incidence of taxane-induced pain and distress in patients receiving chemotherapy for early-stage breast cancer: a retrospective, outcomes-based survey. Curr Oncol. 2010;17(4):42-47.
7. Tengrup I, Tennvall-Nittby L, Christiansson I, Laurin M. Arm morbidity after breast-conserving therapy for breast cancer. Acta Oncol. 2000;39(3):393-397.
8. Johansen J, Overgaard J, Blichert-Toft M, Overgaard M. Treatment of morbidity associated with the management of the axilla in breast-conserving therapy. Acta Oncol. 2000;39(3):349-354.
9. Mittmann N, Porter JM, Rangrej J, et al. Health system costs for stage-specific breast cancer: a population-based approach. Curr Oncol. 2014;21(6):281-293.
10. Page A. Keeping patients safe: transforming the work environment of nurses. Washington, DC: National Academies Press; 2004.
11. McNeely ML, Campbell K, Ospina M, et al. Exercise interventions for upper-limb dysfunction due to breast cancer treatment. Cochrane Database Syst Rev. 2010;(6):CD005211. doi:10.1002/14651858.CD005211.pub2
12. Wong P, Muanza T, Hijal T, et al. Effect of exercise in reducing breast and chest-wall pain in patients with breast cancer: a pilot study. Curr Oncol. 2012;19(3):e129-e135.
13. Fernández-Lao C, Cantarero-Villanueva I, Fernández-de-Las-Peñas C, del Moral-Ávila R, Castro-Sánchez AM, Arroyo-Morales M. Effectiveness of a multidimensional physical therapy program on pain, pressure hypersensitivity, and trigger points in breast cancer survivors: a randomized controlled clinical trial. Clin J Pain. 2012;28(2):113-121.
14. Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: cardiopulmonary and quality of life outcomes. J Clin Oncol. 2003;21(9):1660-1668.
15. Segal R, Evans W, Johnson D, et al. Structured exercise improves physical functioning in women with stages I and II breast cancer: results of a randomized controlled trial. J Clin Oncol. 2001;19(3):657-665.
16. Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129-138.
17. Kumar SP. Utilization of Brief Pain Inventory as an assessment tool for pain in patients with cancer: a focused review. Indian J Palliat Care. 2011;17(2):108-115.
18. Van Voorhis CRW, Morgan BL. Understanding power and rules of thumb for determining sample sizes. Tutor Quant Methods Psychol. 2007;3(2):43-50.
19. Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, Cleeland CS. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61(2):277-284.
20. Lee TS, Kilbreath SL, Refshauge KM, Pendlebury SC, Beith JM, Lee MJ. Pectoral stretching program for women undergoing radiotherapy for breast cancer. Breast Cancer Res Treat. 2007;102(3):313-321.
21. Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409-1426.
22. Pollock ML, Gaesser GA, Butcher JD, et al. ACSM position stand: the recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc. 1998;30(6):975-991.
23. Ibrahim M, Muanza T, Smirnow N, et al. Time course of upper limb function and return-to-work post-radiotherapy in young adults with breast cancer: a pilot randomized control trial on effects of targeted exercise program. J Cancer Surviv. 2017;11(6):791-799.
24. Ibrahim M, Muanza T, Smirnow N, et al. A pilot randomized controlled trial on the effects of a progressive exercise program on the range of motion and upper extremity grip strength in young adults with breast cancer. Clin Breast Cancer. 2018;18(1):e55-e64.
25. Gärtner R, Jensen MB, Nielsen J, Ewertz M, Kroman N, Kehlet H. Prevalence of and factors associated with persistent pain following breast cancer surgery. JAMA. 2009;302(18):1985-1992.
26. Hayes SC, Johansson K, Stout NL, et al. Upper-body morbidity after breast cancer: incidence and evidence for evaluation, prevention, and management within a prospective surveillance model of care. Cancer. 2012;118(suppl 8):2237-2249.
27. Kärki A, Simonen R, Mälkiä E, Selfe J. Impairments, activity limitations and participation restrictions 6 and 12 months after breast cancer operation. J Rehabil Med. 2005;37(3):180-188.
28. Katz J, Poleshuck EL, Andrus CH, et al. Risk factors for acute pain and its persistence following breast cancer surgery. Pain. 2005;119(1-3):16-25.
29. Tasmuth T, von Smitten K, Hietanen P, Kataja M, Kalso E. Pain and other symptoms after different treatment modalities of breast cancer. Ann Oncol. 1995;6(5):453-459.
30. Whelan TJ, Levine M, Julian J, Kirkbride P, Skingley P. The effects of radiation therapy on quality of life of women with breast carcinoma: results of a randomized trial. Ontario Clinical Oncology Group. Cancer. 2000;88(10):2260-2266.
31. Harrington S, Gilchrist L, Sander A. Breast cancer EDGE task force outcomes: clinical measures of pain. Rehabil Oncol. 2014;32(1):13-21.
The impact of inpatient rehabilitation on outcomes for patients with cancer
The American Cancer Society reports that 1.6 million people are diagnosed with cancer each year, of whom 78% are aged 55 years or older. The 5-year survival rate for cancer is 68%.1 Almost 15.5 million living Americans have been diagnosed with cancer.2 Many patients with cancer have difficulty walking and with activities of daily living. Patients with primary brain tumors or tumors metastatic to the brain may present with focal weakness or cognitive deficits similar to patients with stroke. Patients with tumors metastatic to the spine may have the same deficits as a patient with a traumatic spinal cord injury. Patients with metastasis to bone may have pathologic fractures of the hip or long bones. Patients may develop peripheral neuropathy associated with a paraneoplastic syndrome, chemotherapy, or critical illness neuropathy. Lehmann and colleagues evaluated 805 patients admitted to hospitals affiliated with the University of Washington Medical School with a diagnosis of cancer and found that 15% had difficulty walking and 20% had difficulty with activities of daily living.3
Many patients with cancer can benefit from inpatient rehabilitation.4,5 Study findings have shown that patients with impairments in function related to cancer are often not referred for rehabilitation. Among the reasons mentioned for that are that oncologists are more focused on treating the patients’ cancer than on their functional deficits and that specialists in rehabilitation medicine do not want to be involved with patients with complex medical problems. Rehabilitation facilities may not want to incur the costs associated with caring for patients with cancer.6
The present paper looks at the outcomes of 61 consecutive patients with cancer who were admitted to an inpatient rehabilitation facility (IRF) and received radiation therapy concurrent with rehabilitation. It compares the outcomes of the cancer patients with the outcomes of patients without cancer who were admitted with stroke or spinal cord injury, conditions more commonly treated at an IRF.
Methods
We reviewed electronic medical records of all patients with cancer admitted to the IRF from 2008 through 2013 who received radiation therapy while at the facility. We also reviewed the data of all patients without cancer admitted with a diagnosis of stroke in 2013 and all patients admitted with a diagnosis of traumatic spinal cord injury in 2012 and 2013. No patients were excluded from stroke and traumatic spinal cord injury groups.
We recorded the sex, age, diagnostic group, Functional Independence Measure (FIM) admission score, FIM discharge score, length of stay (LoS) in the IRF, place of discharge of each patient (eg, home, acute care, or subacute care), and calculated the FIM efficiency score (change in FIM/LoS) for each patient. The FIM is an instrument that has 18 items measuring mobility, participation in activities of daily living, ability to communicate, and cognitive function.7 Each item is scored from 1 to 7, with 1 denoting that the patient cannot perform the task and 7 that the activity can be performed independently. The minimum score is 18 (complete dependence), and the maximum score is 126 (independent function). Thirteen items compose the motor FIM score: eating, grooming, bathing, dressing upper body, dressing lower body, toileting, bladder management, management of bowel, transfer to bed or wheelchair, transfer to toilet, tub transfer, walking (or wheelchair use), and climbing stairs. Five items – comprehension, expression, social interaction, problem solving, and memory – compose the cognitive FIM score.
We used a 1-way analysis of variance to evaluate differences between age and cancer type, age and diagnostic group, admission FIM score and cancer type, discharge FIM score and cancer type, change in FIM and cancer type, LoS and cancer type, and LoS and diagnostic group. The Pearson chi-square test was used to test the goodness of fit between the place of disposition and diagnostic group. The paired t test was used to evaluate the improvement in FIM of the patients who were in the cancer groups. The Tukey Simultaneous Tests for Differences of Means was used to compare the FIM efficiency scores of the groups. A 2-sample t test was used to evaluate the factors associated with the need for transfer from the IRF to the acute medical service.
Results
The demographic characteristics of the patients in the study and the admission and discharge FIM scores are reported in Table 1. There were initially 62 cancer patients in the radiation group, which was further divided into 4 subgroups based on the site of the primary tumor or metastasis. In all, 23 had a primary malignant brain tumor and received radiation and temozolomide. Sixteen patients had malignancies metastatic to the brain, 15 patients had tumors metastatic to the spine, and 7 had tumors metastatic to the long bones. One patient had laryngeal cancer and was excluded from the study because we did not think that we could do an analysis of a group with only 1 patient. The final number of patients in the cancer group was therefore 61. There were 69 patients in the stroke group and 23 in the spinal cord injury group.
We report improvement in total FIM, motor FIM, and cognitive FIM scores and were able to identify all 18 of the items of the FIM score on 60 of the 61 patients in the cancer group. Improvement in total FIM of the 61 patients in the cancer groups was significant at P P P = .05. Just over 75% of the patients in the cancer group had sufficient enough improvement in their level of function that they were able to return to their homes (Table 1). The average FIM score at the time of discharge was 83.08. This was not significantly different than the level of function of patients discharged after stroke (87.52) or traumatic spinal cord injury (89.13).
The patients with primary brain tumors were younger than the patients with cancer metastatic to the brain (P = .013). The patients with a primary brain tumor had lower admission FIM scores than patients with tumors metastatic to the brain (P = .027). The patients with a primary brain tumor had a greater increase in FIM score than patients with metastasis to the brain (P = .043; Table 2). There was not a significant difference between these 2 groups in FIM score at discharge or in the likelihood of discharge to home (Table 1). The FIM efficiency score was 1.12 for the patients in the primary brain tumor group and .80 in those with metastasis to the brain. This difference was not significant P = .96.
There were 69 patients in the stroke group. We compared the 39 patients with primary or metastatic brain lesion to the stroke group. The patients with primary or metastatic cancer of the brain were younger than the patients with stroke, 60.4 years old versus 69.1 years old (P = .004). The patients in the combined cancer group had a higher admission FIM score compared with the stroke patients (68.4 vs 63.12; P = .05). The discharge FIM scores were 83.3 in the combined cancer group and 87.5 in the stroke group (Table 1). This difference was not significant, but the improvement in the combined cancer group (14.6) was less than the improvement in the stroke group (24.40; P = .002) (Table 3).
The average LoS in the IRF was 18.7 days in the combined cancer group and 16.8 days in the stroke group. This difference was not significant. An average of 82% of the patients in the primary tumor or brain metastasis group and 85.5% of the patients in the stroke group were discharged to home. This difference was not significant. The FIM efficiency score of the patients in the stroke group was 2.0. This was significantly greater than the score for the patients in the metastasis to the brain group (0.80; P = .044) but not significantly greater than the primary brain cancer group (1.19; P = .22).
There were 23 patients in the traumatic spinal cord injury group. A comparison of the patients with tumors metastatic to the spine and patients with traumatic spinal cord injury showed that the patients in the cancer group were older (60.27 and 42.70 years, respectively; P = .001). In all, 80% of patients with tumors metastatic to the spine were men. This was not significantly different from the percentage of men in the traumatic spinal cord injury group (82.6%; Table 1). The admission FIM score of the patients with cancer was 66.5 (standard deviation [SD], 13.3) and 58.03 (SD, 15.1) in the patients with a traumatic spinal cord injury (Table 1). The FIM score at discharge was 80.4 (SD, 19.1) in the patients with cancer and 89.1 (SD, 20.3) in the patients with a traumatic spinal cord injury (Table 1). Neither of these were statistically significant. The improvement in patients with cancer was 13.9 (SD, 12.2) and 31.1 (SD, 13.9) in the traumatic spinal cord injured patients. This difference was significant (P
The median LoS was 18.98 days in the cancer metastasis to spine group (interquartile range [IQR] is the 25th-75th percentile, 12-30 days). In the traumatic group the median LoS was 23 days (IQR, 16-50 days). This difference was not significant (P = .14 Mann-Whitney test). The mean FIM efficiency score was 1.46 in the traumatic spinal cord injury group and .78 in the group with cancer metastatic to the spine. This difference was not significant (P = .72). Sixty percent of the patients in the cancer group were discharged to home, and 87% of patients in the traumatic spinal cord group were discharged to home. This difference was not significant (P = .12; Fisher exact test).
As far as we can ascertain, this is the first paper that has looked at the outcomes of patients receiving rehabilitation concurrent with radiation of the long bones. The average improvement in FIM was 12.4 (Table 1). The LoS was 11.6 days, and the FIM efficiency was 1.25. In all, 71.4% made enough progress to go home.
Of the total number of cancer patients, 18% were transferred to the acute medical service of the hospital (Table 1). Neither age, sex, type of cancer, nor admission FIM score were associated with the need for transfer to acute hospital care. Change in FIM score was inversely associated with transfer to acute hospital care (P = .027). Patients whose function did not improve with rehabilitation were most likely to be transferred back to acute hospital care.
Discussion
Radiation therapy is considered a service that is provided to people who come for treatment as an outpatient. Caregivers may have difficulty transporting patients to radiation if the patient has deficits in mobility. This may be particularly true if the patient is heavy, the caregivers are frail, or perhaps if they live in rural settings where there is no wheelchair-accessible public transportation. There are many factors that help determine whether a patient with functional deficits can be discharged to his or her home. These include sex, age, marital status, family and/or community support, income, and insurance.8 The FIM is an instrument that indicates how much help a patient needs with mobility and self-care skills. It also correlates with the amount of time that caregivers must spend helping a patient.9 Study findings have shown that the FIM score is an important determinant of whether a patient can be discharged to home. The total FIM score is as useful as an analysis of the components of the FIM score in predicting whether a patient can return to the community.10,11 Reistetter and colleagues found a total FIM score of 78 to be the score that best separates patients who are likely to be able to go home and patients who are likely to need long-term care.11 Bottemiller and colleagues10 reported that 37% of patients with total discharge FIM scores of less than 40 were discharged to home. They reported that 62% of patients with FIM scores between 40 and 79 were discharged to home, and 88% of patients with scores of 80 or above were discharged to home.10 The goal in bringing patients to the IRF was to accept and treat patients with reasonable community support and potential to achieve a functional level compatible with discharge to the community. Most patients in each of the cancer groups were able to reach an FIM score of 78 to 80 and to be discharged to home.
Most of the patients in the cancer groups had underlying problems that are not considered curable. The primary goal was to enable the patients to have some time at home with their families before requiring readmission to a hospital or hospice care. Reasonable LoS and rate of progress are now expected or required by third-party payors and hospital administrators. Physicians at the Mayo Clinic have indicated that a rehabilitation service should aim for an FIM efficiency score of at least .6 points per day.10 The FIM efficiency of patients in each of the 4 cancer subgroups in this study was higher than this level.
J. Herbert Dietz, Jr was an early advocate of the need to provide comprehensive rehabilitation services for patients with cancer. He first described his work in 1969.12 Since that time, there have been many papers that have documented the benefits of IRF for patients with cancer. O’Toole and Golden have shown outcomes of a large series of patients from an IRF. They reported that at the time of admission, 14% of patients could ambulate, but at discharge, 80% could ambulate without hands-on assistance. They reported significant improvements in continence, FIM score, and score on the Karnofsky Performance Scale.13 Marciniak,14 Hunter,15 Shin,16 and Cole,17 and their respective colleagues have all shown that patients with many different types of cancer benefit from rehabilitation at the IRF level. Gallegos-Kearin and colleagues4 reported on the care of 115,570 patients admitted to IRF with cancer from 2002 to 2014. Patients had significant improvement in function, with more than 70% of patients discharged to home.4 Ng and colleagues studied a group of 200 patients who received IRF care and found there was significant improvement in function. Ninety-four percent of patients rated their stay as either extremely good or very good.5
Metastasis to the spine is a common problem. It is found in 30% of cancer patients at autopsy. The most common sources of metastasis to the spine are breast, lung, prostate, kidney, and thyroid.18 Multiple myeloma and lymphoma may also involve the spine. Several authors have shown that these patients benefit from inpatient rehabilitation. Mckinley and colleagues19 have noted that patients with metastasis to the spine make significant improvement with care at an IRF. Compared with patients with a traumatic spinal cord injury, the cancer patients had shorter LoS, smaller improvement in FIM, equal FIM efficiency (FIM gain/LoS), and equal success in making enough progress to be discharged to home.19 Eriks and colleagues showed that patients at an IRF in Amsterdam made significant improvement in function as measured by the Barthel’s Index.20 Tang .,21 and Parsch22 and their respective colleagues, Murray,23 and New24 and colleagues have published findings confirming that patients with spinal cord injury caused by metastasis to the spine make significant progress with inpatient rehabilitation programs. The present study adds to the literature by showing that patients with metastasis to the spine who are receiving radiation can make progress and be discharged to the community.
There are 24,000 new cases of primary malignant brain tumors in the United States each year.25 The incidence of metastatic cancer to the brain has been estimated to be 100,000 cases per year in the United States. The most common cancer sources are lung, breast, melanoma, kidney, and colon.26,27 The first study of patients admitted to an IRF for treatment of brain tumors was published in 1998 by Huang and colleagues28 who compared the outcomes of 63 patients with brain tumors with the outcomes of 63 patients with stroke. They reported that the patients with the brain tumors made significant improvement in function. There was not a significant difference between the 2 groups of patients in improvement in function, FIM efficiency, or success in discharging the patients to home.28 Greenberg29 and Bartolo30 and their respective colleagues compared the outcomes of patients admitted with brain tumors and patients with stroke and found that improvement in function and discharge to home was similar in the 2 groups. In 2000, Huang and his same colleagues31 compared a group of patients with brain tumors to a group of patients with traumatic brain injury. They found significant improvement in the function of the patients with brain tumors. Patients in the traumatic brain injury group made more progress but had longer LoS. FIM efficiency was not significantly different between the groups.31
Three papers have reported outcomes of patients who received radiation concurrent with inpatient rehabilitation. Tang and colleagues32 reported 63 patients, of whom 48% percent received radiation concurrent with rehabilitation. The patients who received radiation made significant gains in function, and more than 70% were discharged to home. There was no difference in the outcomes of the patients in the radiation and nonradiation groups.32 Marciniak33 and O’Dell34 and their colleagues also reported that patients with brain tumors that required radiation therapy can benefit from inpatient rehabilitation. The present paper is the fourth (with the largest patient group) to show that patients with primary and metastatic tumors to the brain can benefit from a program that provides radiation concurrent with inpatient rehabilitation. We have shown that patients can achieve functional levels and rates of discharge to home that are not significantly different from those of the most commonly admitted group of patients to IRF – patients with stroke.
In the present study, 18% of all of the cancer patients were transferred to medical services and/or acute hospital care (Table 1). This is consistent with a paper by Asher and colleagues35 who reported that 17.4% of patients at an IRF with a diagnosis of cancer required transfer back to medical service, and that low admission motor FIM score correlated with the likelihood of transfer back to medical service. In the present paper, the total admission FIM score was not related to the likelihood of return to medical service, although a lack of improvement in the FIM score did correlate with transfer to medical service.
All of the papers we reviewed found that appropriately selected patients with cancer make significant improvement in function with treatment at an IRF. Tang and colleagues have also shown that for patients with malignant brain tumors and metastasis to the spine, improvement in function correlates with increased survival.32 Our paper confirms that patients with primary malignant brain tumors, malignant tumors metastatic to the brain or spine, and tumors metastatic to long bones may benefit from rehabilitation concurrent with radiation. Rehabilitation units are traditionally associated with treating patients with stroke and spinal cord injury. The patients in our study had cancer and were receiving radiation therapy. They had significant improvement in function and FIM efficiency scores that are not below the threshold set as expected for care at an IRF. Most patients in our study achieved a functional level consistent with what is needed to go home.
There is a prospective payment or reimbursement system for rehabilitation units.36 The payments are based on the admitting diagnosis, the admission FIM score, the age of the patient, and comorbidities. There are 4 tiers for comorbidities with no additional payments for patients in tier 0 but with additional payments for patients with conditions that qualify for tiers 1 through 3. The highest payments are for patients in tier 1. Examples of conditions that can increase payment include morbid obesity, congestive heart failure, vocal cord paralysis, and the need for hemodialysis. There is no increased payment for provision of radiation therapy. There are no reports on the feasibility, in terms of finances, of providing radiation on an IRF. We asked the finance office of the Albany Medical Center to comment on the cost to the hospital of providing radiation therapy to patients on the rehabilitation unit. The hospital’s finance department reviewed available data and reported that the variable cost of providing radiation therapy is about 6.5% of the revenue collected from third-party payors for caring for patients who receive that service (personal communication from the finance office of Albany Medical Center to George Forrest, 2015). Our findings suggest that the Centers for Medicare & Medicaid Services should make an adjustment to the payment system to support the cost of providing radiation to patients at an IRF. Even under the current payment system, for a hospital that has the equipment and personnel to provide radiation treatments, the variable cost of 6.5% of revenue should not be an absolute barrier to providing this service.
Limitations
This study reports on the experience of only 1 facility. The number of patients in the radiation group is greater than the number of patients in any previous report of people receiving radiation at an IRF, but the statistician does not think it is large enough to allow statistical analysis of covariates such as age, sex, and comorbid conditions. In addition, we did not investigate all of the factors that influence the type of care patients are offered and their LoS, such as hospital policy, insurance coverage, income, and family structure.
Conclusions
Acute care medical units are now challenged to both reduce LoS and reduce the number of patients who are readmitted to the hospital. Rehabilitation units are challenged to maintain census, as government and private payors are shifting patients from acute rehabilitation units to subacute rehabilitation units. We found that patients with cancer who need radiation are a population of patients who are seen by payors as needing to be in a facility with excellent nursing, therapy, and comprehensive physician services. A comprehensive cancer care program within a rehabilitation unit can be a great benefit to the acute care services, the IRF, and, most importantly, patients and their families.
1. American Cancer Society. Cancer facts & figures 2016. Atlanta, GA: American Cancer Society; 2016.
2. National Cancer Institute: Office of cancer survivorship: statistics. https://cancercontrol.cancer.gov/ocs/statistics/statistics.html. Updated October 17, 2016. Accessed April 21, 2018.
3. Lehmann JF, DeLisa JA, Warren CG, deLateur BJ, Bryant PL, Nicholson CG. Cancer rehabilitation: assessment of need, development and evaluation of a model of care. Arch Phys Med Rehabil. 1978;59(9):410-419.
4. Gallegos-Kearin V, Knowlton SE, Goldstein R, et al. Outcome trends of adult cancer patients receiving inpatient rehabilitation: a 13-year review [published online Feb 21, 2018]. Am J Phys Med Rehabil. doi:10.1097/PHM.0000000000000911
5. Ng AH, Gupta E, Fontillas RC, et al. Patient-reported usefulness of acute cancer rehabilitation. PM R. 2017;9(11):1135-1143.
6. Cheville AL, Kornblith AB, Basford JR. An examination of the causes for the underutilization of rehabilitation services among people with advanced cancer. Am J Phys Med Rehabil. 2011;90(5 suppl 1):S27-S37.
7. Cohen ME, Marino RJ. The tools of disability outcomes research functional status measures. Arch Phys Med Rehabil. 2000;81(12 suppl 2):S21-S29.
8. Nguyen VQ, PrvuBettger J, Guerrier T, et al. Factors associated with discharge to home versus discharge to institutional care after inpatient stroke rehabilitation. Arch Phys Med Rehabil. 2015;96(7):1297-1303.
9. Forrest G, Schwam A, Cohen E. Time of care required by patients discharged from a rehabilitation unit. Am J Phys Med Rehabil. 2002;81(1):57-62.
10. Bottemiller KL, Bieber PL, Basford JR, Harris M. FIM scores, FIM efficiency and discharge following inpatient stroke rehabilitation. Rehabil Nurs. 2006;31(1):22-25.
11. Reistetter TA, Graham JE, Deutsch A, Granger CV, Markello S, Ottenbacher KJ. Utility of functional status for classifying community versus institutional discharges after inpatient rehabilitation for stroke. Arch Phys Med Rehabil. 2010;91(3):345-350.
12. Dietz JH Jr. Rehabilitation of the cancer patient. Med Clin North Am. 1969;53(3):607-624.
13. O'Toole DM, Golden AM. Evaluating cancer patients for rehabilitation potential. West J Med. 1991;155(4):384-387.
14. Marciniak CM, Sliwa JA, Spill G, Heinemann AW, Semik PE. Functional outcome following rehabilitation of the cancer patient. Arch Phys Med Rehabil. 1996;77(1):54-57.
15. Hunter EG, Baltisberger J. Functional outcomes by age for inpatient cancer rehabilitation: a retrospective chart review. J Appl Gerontol. 2013;32(4):443-456.
16. Shin KY, Guo Y, Konzen B, Fu J, Yadav R, Bruera E. Inpatient cancer rehabilitation: the experience of a national comprehensive cancer center. Am J Phys Med Rehabil. 2011;90(5 suppl 1):S63-S68.
17. Cole RP, Scialla S, Bednarz L. Functional recovery in cancer rehabilitation. Arch Phys Med Rehabil. 2000;81(5):623-627.
18. White AP, Kwon BK, Lindskog DM, Friedlaender GE, Grauer JN. Metastatic disease of the spine. J Am Acad Orthop Surg. 2006;14(11):587-598.
19. McKinley WO, Huang ME, Tewksbury MA. Neoplastic vs traumatic spinal cord injury: an inpatient rehabilitation comparison. Am J Phys Med Rehabil. 2000;79(2):138-144.
20. Eriks IE, Angenot EL, Lankhorst GJ. Epidural metastatic spinal cord compression: functional outcome and survival after inpatient rehabilitation. Spinal Cord. 2004;42(4):235-239.
21. Tang V, Harvey D, Park Dorsay J, Jiang S, Rathbone MP. Prognostic indicators in metastatic spinal cord compression: using functional independence measure and Tokuhashi scale to optimize rehabilitation planning. Spinal Cord. 2007;45(10):671-677.
22. Parsch D, Mikut R, Abel R. Postacute management of patients with spinal cord injury due to metastatic tumor disease: survival and efficacy of rehabilitation. Spinal Cord. 2003;41:205-210.
23. Murray PK. Functional outcome and survival in spinal cord injury secondary to neoplasia. Cancer. 1985;55:197-201.
24. New PW. Functional outcomes and disability after nontraumatic spinal cord injury rehabilitation: results from a retrospective study. Arch Phys Med Rehabil. 2005;86(2):250-261
25. Central Brain Tumor Registry of the United States: 2016 CBTRUS fact sheet. www.cbtrus.org/factsheet/factsheet.html. Updated 2017. Accessed May 28, 2016.
26. Memorial Sloan Kettering Cancer Center: Metastatic brain tumors & secondary brain cancer. https://www.mskcc.org/cancer-care/types/brain-tumors-metastatic. Updated 2018. Accessed April 21, 2018.
27. Bruckner JC, Brown PD, O'Neill BP, Meyer FB, Wetmore CJ, Uhm JH. Central nervous system tumors. Mayo Clin Proc. 2007;82(10):1271-1286.
28. Huang ME, Cifu DX, Keyser-Marcus L. Functional outcome after brain tumor and acute stroke: a comparative analysis. Arch Phys Med Rehabil. 1998;79(11):1386-1390.
29. Greenberg E, Treger I, Ring H. Rehabilitation outcomes in patients with brain tumors and acute stroke: comparative study of inpatient rehabilitation. Am J Phys Med Rehabil. 2006;85(7):568-573.
30. Bartolo M, Zucchella C, Pace A, et al. Early rehabilitation after surgery improves functional outcomes in inpatients with brain tumours. J Neurooncol. 2012;107(3);537-544.
31. Huang ME, Cifu DX, Keyser-Marcus L. Functional outcomes in patients with brain tumor after inpatient rehabilitation: comparison with traumatic brain injury. Am J Phys Med Rehabil. 2000;79(4):327-335.
32. Tang V, Rathbone M, Park Dorsay J, Jiang S, Harvey D. Rehabilitation in primary and metastatic brain tumours: impact of functional outcomes on survival. J Neurol. 2008;255(6):820-827.
33. Marciniak CM, Sliwa JA, Heinemann AW, Semik PE. Functional outcomes of persons with brain tumors after inpatient rehabilitation. Arch Phys Med Rehabil. 2001;82(4):457-463.
34. O'Dell MW, Barr K, Spanier D, Warnick RE. Functional outcome of inpatient rehabilitation in persons with brain tumors. Arch Phys Med Rehabil. 1998;79(12):1530-1534.
35. Asher A, Roberts PS, Bresee C, Zabel G, Riggs RV, Rogatko A. Transferring inpatient rehabilitation facility cancer patients back to acute care (TRIPBAC). PM R. 2014;6(9):808-813.
36. Centers for Medicare and Medicaid Services: Inpatient rehabilitation facilities. https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/CertificationandComplianc/InpatientRehab.html. Published March 5, 2012. Accessed May 21, 2018.
The American Cancer Society reports that 1.6 million people are diagnosed with cancer each year, of whom 78% are aged 55 years or older. The 5-year survival rate for cancer is 68%.1 Almost 15.5 million living Americans have been diagnosed with cancer.2 Many patients with cancer have difficulty walking and with activities of daily living. Patients with primary brain tumors or tumors metastatic to the brain may present with focal weakness or cognitive deficits similar to patients with stroke. Patients with tumors metastatic to the spine may have the same deficits as a patient with a traumatic spinal cord injury. Patients with metastasis to bone may have pathologic fractures of the hip or long bones. Patients may develop peripheral neuropathy associated with a paraneoplastic syndrome, chemotherapy, or critical illness neuropathy. Lehmann and colleagues evaluated 805 patients admitted to hospitals affiliated with the University of Washington Medical School with a diagnosis of cancer and found that 15% had difficulty walking and 20% had difficulty with activities of daily living.3
Many patients with cancer can benefit from inpatient rehabilitation.4,5 Study findings have shown that patients with impairments in function related to cancer are often not referred for rehabilitation. Among the reasons mentioned for that are that oncologists are more focused on treating the patients’ cancer than on their functional deficits and that specialists in rehabilitation medicine do not want to be involved with patients with complex medical problems. Rehabilitation facilities may not want to incur the costs associated with caring for patients with cancer.6
The present paper looks at the outcomes of 61 consecutive patients with cancer who were admitted to an inpatient rehabilitation facility (IRF) and received radiation therapy concurrent with rehabilitation. It compares the outcomes of the cancer patients with the outcomes of patients without cancer who were admitted with stroke or spinal cord injury, conditions more commonly treated at an IRF.
Methods
We reviewed electronic medical records of all patients with cancer admitted to the IRF from 2008 through 2013 who received radiation therapy while at the facility. We also reviewed the data of all patients without cancer admitted with a diagnosis of stroke in 2013 and all patients admitted with a diagnosis of traumatic spinal cord injury in 2012 and 2013. No patients were excluded from stroke and traumatic spinal cord injury groups.
We recorded the sex, age, diagnostic group, Functional Independence Measure (FIM) admission score, FIM discharge score, length of stay (LoS) in the IRF, place of discharge of each patient (eg, home, acute care, or subacute care), and calculated the FIM efficiency score (change in FIM/LoS) for each patient. The FIM is an instrument that has 18 items measuring mobility, participation in activities of daily living, ability to communicate, and cognitive function.7 Each item is scored from 1 to 7, with 1 denoting that the patient cannot perform the task and 7 that the activity can be performed independently. The minimum score is 18 (complete dependence), and the maximum score is 126 (independent function). Thirteen items compose the motor FIM score: eating, grooming, bathing, dressing upper body, dressing lower body, toileting, bladder management, management of bowel, transfer to bed or wheelchair, transfer to toilet, tub transfer, walking (or wheelchair use), and climbing stairs. Five items – comprehension, expression, social interaction, problem solving, and memory – compose the cognitive FIM score.
We used a 1-way analysis of variance to evaluate differences between age and cancer type, age and diagnostic group, admission FIM score and cancer type, discharge FIM score and cancer type, change in FIM and cancer type, LoS and cancer type, and LoS and diagnostic group. The Pearson chi-square test was used to test the goodness of fit between the place of disposition and diagnostic group. The paired t test was used to evaluate the improvement in FIM of the patients who were in the cancer groups. The Tukey Simultaneous Tests for Differences of Means was used to compare the FIM efficiency scores of the groups. A 2-sample t test was used to evaluate the factors associated with the need for transfer from the IRF to the acute medical service.
Results
The demographic characteristics of the patients in the study and the admission and discharge FIM scores are reported in Table 1. There were initially 62 cancer patients in the radiation group, which was further divided into 4 subgroups based on the site of the primary tumor or metastasis. In all, 23 had a primary malignant brain tumor and received radiation and temozolomide. Sixteen patients had malignancies metastatic to the brain, 15 patients had tumors metastatic to the spine, and 7 had tumors metastatic to the long bones. One patient had laryngeal cancer and was excluded from the study because we did not think that we could do an analysis of a group with only 1 patient. The final number of patients in the cancer group was therefore 61. There were 69 patients in the stroke group and 23 in the spinal cord injury group.
We report improvement in total FIM, motor FIM, and cognitive FIM scores and were able to identify all 18 of the items of the FIM score on 60 of the 61 patients in the cancer group. Improvement in total FIM of the 61 patients in the cancer groups was significant at P P P = .05. Just over 75% of the patients in the cancer group had sufficient enough improvement in their level of function that they were able to return to their homes (Table 1). The average FIM score at the time of discharge was 83.08. This was not significantly different than the level of function of patients discharged after stroke (87.52) or traumatic spinal cord injury (89.13).
The patients with primary brain tumors were younger than the patients with cancer metastatic to the brain (P = .013). The patients with a primary brain tumor had lower admission FIM scores than patients with tumors metastatic to the brain (P = .027). The patients with a primary brain tumor had a greater increase in FIM score than patients with metastasis to the brain (P = .043; Table 2). There was not a significant difference between these 2 groups in FIM score at discharge or in the likelihood of discharge to home (Table 1). The FIM efficiency score was 1.12 for the patients in the primary brain tumor group and .80 in those with metastasis to the brain. This difference was not significant P = .96.
There were 69 patients in the stroke group. We compared the 39 patients with primary or metastatic brain lesion to the stroke group. The patients with primary or metastatic cancer of the brain were younger than the patients with stroke, 60.4 years old versus 69.1 years old (P = .004). The patients in the combined cancer group had a higher admission FIM score compared with the stroke patients (68.4 vs 63.12; P = .05). The discharge FIM scores were 83.3 in the combined cancer group and 87.5 in the stroke group (Table 1). This difference was not significant, but the improvement in the combined cancer group (14.6) was less than the improvement in the stroke group (24.40; P = .002) (Table 3).
The average LoS in the IRF was 18.7 days in the combined cancer group and 16.8 days in the stroke group. This difference was not significant. An average of 82% of the patients in the primary tumor or brain metastasis group and 85.5% of the patients in the stroke group were discharged to home. This difference was not significant. The FIM efficiency score of the patients in the stroke group was 2.0. This was significantly greater than the score for the patients in the metastasis to the brain group (0.80; P = .044) but not significantly greater than the primary brain cancer group (1.19; P = .22).
There were 23 patients in the traumatic spinal cord injury group. A comparison of the patients with tumors metastatic to the spine and patients with traumatic spinal cord injury showed that the patients in the cancer group were older (60.27 and 42.70 years, respectively; P = .001). In all, 80% of patients with tumors metastatic to the spine were men. This was not significantly different from the percentage of men in the traumatic spinal cord injury group (82.6%; Table 1). The admission FIM score of the patients with cancer was 66.5 (standard deviation [SD], 13.3) and 58.03 (SD, 15.1) in the patients with a traumatic spinal cord injury (Table 1). The FIM score at discharge was 80.4 (SD, 19.1) in the patients with cancer and 89.1 (SD, 20.3) in the patients with a traumatic spinal cord injury (Table 1). Neither of these were statistically significant. The improvement in patients with cancer was 13.9 (SD, 12.2) and 31.1 (SD, 13.9) in the traumatic spinal cord injured patients. This difference was significant (P
The median LoS was 18.98 days in the cancer metastasis to spine group (interquartile range [IQR] is the 25th-75th percentile, 12-30 days). In the traumatic group the median LoS was 23 days (IQR, 16-50 days). This difference was not significant (P = .14 Mann-Whitney test). The mean FIM efficiency score was 1.46 in the traumatic spinal cord injury group and .78 in the group with cancer metastatic to the spine. This difference was not significant (P = .72). Sixty percent of the patients in the cancer group were discharged to home, and 87% of patients in the traumatic spinal cord group were discharged to home. This difference was not significant (P = .12; Fisher exact test).
As far as we can ascertain, this is the first paper that has looked at the outcomes of patients receiving rehabilitation concurrent with radiation of the long bones. The average improvement in FIM was 12.4 (Table 1). The LoS was 11.6 days, and the FIM efficiency was 1.25. In all, 71.4% made enough progress to go home.
Of the total number of cancer patients, 18% were transferred to the acute medical service of the hospital (Table 1). Neither age, sex, type of cancer, nor admission FIM score were associated with the need for transfer to acute hospital care. Change in FIM score was inversely associated with transfer to acute hospital care (P = .027). Patients whose function did not improve with rehabilitation were most likely to be transferred back to acute hospital care.
Discussion
Radiation therapy is considered a service that is provided to people who come for treatment as an outpatient. Caregivers may have difficulty transporting patients to radiation if the patient has deficits in mobility. This may be particularly true if the patient is heavy, the caregivers are frail, or perhaps if they live in rural settings where there is no wheelchair-accessible public transportation. There are many factors that help determine whether a patient with functional deficits can be discharged to his or her home. These include sex, age, marital status, family and/or community support, income, and insurance.8 The FIM is an instrument that indicates how much help a patient needs with mobility and self-care skills. It also correlates with the amount of time that caregivers must spend helping a patient.9 Study findings have shown that the FIM score is an important determinant of whether a patient can be discharged to home. The total FIM score is as useful as an analysis of the components of the FIM score in predicting whether a patient can return to the community.10,11 Reistetter and colleagues found a total FIM score of 78 to be the score that best separates patients who are likely to be able to go home and patients who are likely to need long-term care.11 Bottemiller and colleagues10 reported that 37% of patients with total discharge FIM scores of less than 40 were discharged to home. They reported that 62% of patients with FIM scores between 40 and 79 were discharged to home, and 88% of patients with scores of 80 or above were discharged to home.10 The goal in bringing patients to the IRF was to accept and treat patients with reasonable community support and potential to achieve a functional level compatible with discharge to the community. Most patients in each of the cancer groups were able to reach an FIM score of 78 to 80 and to be discharged to home.
Most of the patients in the cancer groups had underlying problems that are not considered curable. The primary goal was to enable the patients to have some time at home with their families before requiring readmission to a hospital or hospice care. Reasonable LoS and rate of progress are now expected or required by third-party payors and hospital administrators. Physicians at the Mayo Clinic have indicated that a rehabilitation service should aim for an FIM efficiency score of at least .6 points per day.10 The FIM efficiency of patients in each of the 4 cancer subgroups in this study was higher than this level.
J. Herbert Dietz, Jr was an early advocate of the need to provide comprehensive rehabilitation services for patients with cancer. He first described his work in 1969.12 Since that time, there have been many papers that have documented the benefits of IRF for patients with cancer. O’Toole and Golden have shown outcomes of a large series of patients from an IRF. They reported that at the time of admission, 14% of patients could ambulate, but at discharge, 80% could ambulate without hands-on assistance. They reported significant improvements in continence, FIM score, and score on the Karnofsky Performance Scale.13 Marciniak,14 Hunter,15 Shin,16 and Cole,17 and their respective colleagues have all shown that patients with many different types of cancer benefit from rehabilitation at the IRF level. Gallegos-Kearin and colleagues4 reported on the care of 115,570 patients admitted to IRF with cancer from 2002 to 2014. Patients had significant improvement in function, with more than 70% of patients discharged to home.4 Ng and colleagues studied a group of 200 patients who received IRF care and found there was significant improvement in function. Ninety-four percent of patients rated their stay as either extremely good or very good.5
Metastasis to the spine is a common problem. It is found in 30% of cancer patients at autopsy. The most common sources of metastasis to the spine are breast, lung, prostate, kidney, and thyroid.18 Multiple myeloma and lymphoma may also involve the spine. Several authors have shown that these patients benefit from inpatient rehabilitation. Mckinley and colleagues19 have noted that patients with metastasis to the spine make significant improvement with care at an IRF. Compared with patients with a traumatic spinal cord injury, the cancer patients had shorter LoS, smaller improvement in FIM, equal FIM efficiency (FIM gain/LoS), and equal success in making enough progress to be discharged to home.19 Eriks and colleagues showed that patients at an IRF in Amsterdam made significant improvement in function as measured by the Barthel’s Index.20 Tang .,21 and Parsch22 and their respective colleagues, Murray,23 and New24 and colleagues have published findings confirming that patients with spinal cord injury caused by metastasis to the spine make significant progress with inpatient rehabilitation programs. The present study adds to the literature by showing that patients with metastasis to the spine who are receiving radiation can make progress and be discharged to the community.
There are 24,000 new cases of primary malignant brain tumors in the United States each year.25 The incidence of metastatic cancer to the brain has been estimated to be 100,000 cases per year in the United States. The most common cancer sources are lung, breast, melanoma, kidney, and colon.26,27 The first study of patients admitted to an IRF for treatment of brain tumors was published in 1998 by Huang and colleagues28 who compared the outcomes of 63 patients with brain tumors with the outcomes of 63 patients with stroke. They reported that the patients with the brain tumors made significant improvement in function. There was not a significant difference between the 2 groups of patients in improvement in function, FIM efficiency, or success in discharging the patients to home.28 Greenberg29 and Bartolo30 and their respective colleagues compared the outcomes of patients admitted with brain tumors and patients with stroke and found that improvement in function and discharge to home was similar in the 2 groups. In 2000, Huang and his same colleagues31 compared a group of patients with brain tumors to a group of patients with traumatic brain injury. They found significant improvement in the function of the patients with brain tumors. Patients in the traumatic brain injury group made more progress but had longer LoS. FIM efficiency was not significantly different between the groups.31
Three papers have reported outcomes of patients who received radiation concurrent with inpatient rehabilitation. Tang and colleagues32 reported 63 patients, of whom 48% percent received radiation concurrent with rehabilitation. The patients who received radiation made significant gains in function, and more than 70% were discharged to home. There was no difference in the outcomes of the patients in the radiation and nonradiation groups.32 Marciniak33 and O’Dell34 and their colleagues also reported that patients with brain tumors that required radiation therapy can benefit from inpatient rehabilitation. The present paper is the fourth (with the largest patient group) to show that patients with primary and metastatic tumors to the brain can benefit from a program that provides radiation concurrent with inpatient rehabilitation. We have shown that patients can achieve functional levels and rates of discharge to home that are not significantly different from those of the most commonly admitted group of patients to IRF – patients with stroke.
In the present study, 18% of all of the cancer patients were transferred to medical services and/or acute hospital care (Table 1). This is consistent with a paper by Asher and colleagues35 who reported that 17.4% of patients at an IRF with a diagnosis of cancer required transfer back to medical service, and that low admission motor FIM score correlated with the likelihood of transfer back to medical service. In the present paper, the total admission FIM score was not related to the likelihood of return to medical service, although a lack of improvement in the FIM score did correlate with transfer to medical service.
All of the papers we reviewed found that appropriately selected patients with cancer make significant improvement in function with treatment at an IRF. Tang and colleagues have also shown that for patients with malignant brain tumors and metastasis to the spine, improvement in function correlates with increased survival.32 Our paper confirms that patients with primary malignant brain tumors, malignant tumors metastatic to the brain or spine, and tumors metastatic to long bones may benefit from rehabilitation concurrent with radiation. Rehabilitation units are traditionally associated with treating patients with stroke and spinal cord injury. The patients in our study had cancer and were receiving radiation therapy. They had significant improvement in function and FIM efficiency scores that are not below the threshold set as expected for care at an IRF. Most patients in our study achieved a functional level consistent with what is needed to go home.
There is a prospective payment or reimbursement system for rehabilitation units.36 The payments are based on the admitting diagnosis, the admission FIM score, the age of the patient, and comorbidities. There are 4 tiers for comorbidities with no additional payments for patients in tier 0 but with additional payments for patients with conditions that qualify for tiers 1 through 3. The highest payments are for patients in tier 1. Examples of conditions that can increase payment include morbid obesity, congestive heart failure, vocal cord paralysis, and the need for hemodialysis. There is no increased payment for provision of radiation therapy. There are no reports on the feasibility, in terms of finances, of providing radiation on an IRF. We asked the finance office of the Albany Medical Center to comment on the cost to the hospital of providing radiation therapy to patients on the rehabilitation unit. The hospital’s finance department reviewed available data and reported that the variable cost of providing radiation therapy is about 6.5% of the revenue collected from third-party payors for caring for patients who receive that service (personal communication from the finance office of Albany Medical Center to George Forrest, 2015). Our findings suggest that the Centers for Medicare & Medicaid Services should make an adjustment to the payment system to support the cost of providing radiation to patients at an IRF. Even under the current payment system, for a hospital that has the equipment and personnel to provide radiation treatments, the variable cost of 6.5% of revenue should not be an absolute barrier to providing this service.
Limitations
This study reports on the experience of only 1 facility. The number of patients in the radiation group is greater than the number of patients in any previous report of people receiving radiation at an IRF, but the statistician does not think it is large enough to allow statistical analysis of covariates such as age, sex, and comorbid conditions. In addition, we did not investigate all of the factors that influence the type of care patients are offered and their LoS, such as hospital policy, insurance coverage, income, and family structure.
Conclusions
Acute care medical units are now challenged to both reduce LoS and reduce the number of patients who are readmitted to the hospital. Rehabilitation units are challenged to maintain census, as government and private payors are shifting patients from acute rehabilitation units to subacute rehabilitation units. We found that patients with cancer who need radiation are a population of patients who are seen by payors as needing to be in a facility with excellent nursing, therapy, and comprehensive physician services. A comprehensive cancer care program within a rehabilitation unit can be a great benefit to the acute care services, the IRF, and, most importantly, patients and their families.
The American Cancer Society reports that 1.6 million people are diagnosed with cancer each year, of whom 78% are aged 55 years or older. The 5-year survival rate for cancer is 68%.1 Almost 15.5 million living Americans have been diagnosed with cancer.2 Many patients with cancer have difficulty walking and with activities of daily living. Patients with primary brain tumors or tumors metastatic to the brain may present with focal weakness or cognitive deficits similar to patients with stroke. Patients with tumors metastatic to the spine may have the same deficits as a patient with a traumatic spinal cord injury. Patients with metastasis to bone may have pathologic fractures of the hip or long bones. Patients may develop peripheral neuropathy associated with a paraneoplastic syndrome, chemotherapy, or critical illness neuropathy. Lehmann and colleagues evaluated 805 patients admitted to hospitals affiliated with the University of Washington Medical School with a diagnosis of cancer and found that 15% had difficulty walking and 20% had difficulty with activities of daily living.3
Many patients with cancer can benefit from inpatient rehabilitation.4,5 Study findings have shown that patients with impairments in function related to cancer are often not referred for rehabilitation. Among the reasons mentioned for that are that oncologists are more focused on treating the patients’ cancer than on their functional deficits and that specialists in rehabilitation medicine do not want to be involved with patients with complex medical problems. Rehabilitation facilities may not want to incur the costs associated with caring for patients with cancer.6
The present paper looks at the outcomes of 61 consecutive patients with cancer who were admitted to an inpatient rehabilitation facility (IRF) and received radiation therapy concurrent with rehabilitation. It compares the outcomes of the cancer patients with the outcomes of patients without cancer who were admitted with stroke or spinal cord injury, conditions more commonly treated at an IRF.
Methods
We reviewed electronic medical records of all patients with cancer admitted to the IRF from 2008 through 2013 who received radiation therapy while at the facility. We also reviewed the data of all patients without cancer admitted with a diagnosis of stroke in 2013 and all patients admitted with a diagnosis of traumatic spinal cord injury in 2012 and 2013. No patients were excluded from stroke and traumatic spinal cord injury groups.
We recorded the sex, age, diagnostic group, Functional Independence Measure (FIM) admission score, FIM discharge score, length of stay (LoS) in the IRF, place of discharge of each patient (eg, home, acute care, or subacute care), and calculated the FIM efficiency score (change in FIM/LoS) for each patient. The FIM is an instrument that has 18 items measuring mobility, participation in activities of daily living, ability to communicate, and cognitive function.7 Each item is scored from 1 to 7, with 1 denoting that the patient cannot perform the task and 7 that the activity can be performed independently. The minimum score is 18 (complete dependence), and the maximum score is 126 (independent function). Thirteen items compose the motor FIM score: eating, grooming, bathing, dressing upper body, dressing lower body, toileting, bladder management, management of bowel, transfer to bed or wheelchair, transfer to toilet, tub transfer, walking (or wheelchair use), and climbing stairs. Five items – comprehension, expression, social interaction, problem solving, and memory – compose the cognitive FIM score.
We used a 1-way analysis of variance to evaluate differences between age and cancer type, age and diagnostic group, admission FIM score and cancer type, discharge FIM score and cancer type, change in FIM and cancer type, LoS and cancer type, and LoS and diagnostic group. The Pearson chi-square test was used to test the goodness of fit between the place of disposition and diagnostic group. The paired t test was used to evaluate the improvement in FIM of the patients who were in the cancer groups. The Tukey Simultaneous Tests for Differences of Means was used to compare the FIM efficiency scores of the groups. A 2-sample t test was used to evaluate the factors associated with the need for transfer from the IRF to the acute medical service.
Results
The demographic characteristics of the patients in the study and the admission and discharge FIM scores are reported in Table 1. There were initially 62 cancer patients in the radiation group, which was further divided into 4 subgroups based on the site of the primary tumor or metastasis. In all, 23 had a primary malignant brain tumor and received radiation and temozolomide. Sixteen patients had malignancies metastatic to the brain, 15 patients had tumors metastatic to the spine, and 7 had tumors metastatic to the long bones. One patient had laryngeal cancer and was excluded from the study because we did not think that we could do an analysis of a group with only 1 patient. The final number of patients in the cancer group was therefore 61. There were 69 patients in the stroke group and 23 in the spinal cord injury group.
We report improvement in total FIM, motor FIM, and cognitive FIM scores and were able to identify all 18 of the items of the FIM score on 60 of the 61 patients in the cancer group. Improvement in total FIM of the 61 patients in the cancer groups was significant at P P P = .05. Just over 75% of the patients in the cancer group had sufficient enough improvement in their level of function that they were able to return to their homes (Table 1). The average FIM score at the time of discharge was 83.08. This was not significantly different than the level of function of patients discharged after stroke (87.52) or traumatic spinal cord injury (89.13).
The patients with primary brain tumors were younger than the patients with cancer metastatic to the brain (P = .013). The patients with a primary brain tumor had lower admission FIM scores than patients with tumors metastatic to the brain (P = .027). The patients with a primary brain tumor had a greater increase in FIM score than patients with metastasis to the brain (P = .043; Table 2). There was not a significant difference between these 2 groups in FIM score at discharge or in the likelihood of discharge to home (Table 1). The FIM efficiency score was 1.12 for the patients in the primary brain tumor group and .80 in those with metastasis to the brain. This difference was not significant P = .96.
There were 69 patients in the stroke group. We compared the 39 patients with primary or metastatic brain lesion to the stroke group. The patients with primary or metastatic cancer of the brain were younger than the patients with stroke, 60.4 years old versus 69.1 years old (P = .004). The patients in the combined cancer group had a higher admission FIM score compared with the stroke patients (68.4 vs 63.12; P = .05). The discharge FIM scores were 83.3 in the combined cancer group and 87.5 in the stroke group (Table 1). This difference was not significant, but the improvement in the combined cancer group (14.6) was less than the improvement in the stroke group (24.40; P = .002) (Table 3).
The average LoS in the IRF was 18.7 days in the combined cancer group and 16.8 days in the stroke group. This difference was not significant. An average of 82% of the patients in the primary tumor or brain metastasis group and 85.5% of the patients in the stroke group were discharged to home. This difference was not significant. The FIM efficiency score of the patients in the stroke group was 2.0. This was significantly greater than the score for the patients in the metastasis to the brain group (0.80; P = .044) but not significantly greater than the primary brain cancer group (1.19; P = .22).
There were 23 patients in the traumatic spinal cord injury group. A comparison of the patients with tumors metastatic to the spine and patients with traumatic spinal cord injury showed that the patients in the cancer group were older (60.27 and 42.70 years, respectively; P = .001). In all, 80% of patients with tumors metastatic to the spine were men. This was not significantly different from the percentage of men in the traumatic spinal cord injury group (82.6%; Table 1). The admission FIM score of the patients with cancer was 66.5 (standard deviation [SD], 13.3) and 58.03 (SD, 15.1) in the patients with a traumatic spinal cord injury (Table 1). The FIM score at discharge was 80.4 (SD, 19.1) in the patients with cancer and 89.1 (SD, 20.3) in the patients with a traumatic spinal cord injury (Table 1). Neither of these were statistically significant. The improvement in patients with cancer was 13.9 (SD, 12.2) and 31.1 (SD, 13.9) in the traumatic spinal cord injured patients. This difference was significant (P
The median LoS was 18.98 days in the cancer metastasis to spine group (interquartile range [IQR] is the 25th-75th percentile, 12-30 days). In the traumatic group the median LoS was 23 days (IQR, 16-50 days). This difference was not significant (P = .14 Mann-Whitney test). The mean FIM efficiency score was 1.46 in the traumatic spinal cord injury group and .78 in the group with cancer metastatic to the spine. This difference was not significant (P = .72). Sixty percent of the patients in the cancer group were discharged to home, and 87% of patients in the traumatic spinal cord group were discharged to home. This difference was not significant (P = .12; Fisher exact test).
As far as we can ascertain, this is the first paper that has looked at the outcomes of patients receiving rehabilitation concurrent with radiation of the long bones. The average improvement in FIM was 12.4 (Table 1). The LoS was 11.6 days, and the FIM efficiency was 1.25. In all, 71.4% made enough progress to go home.
Of the total number of cancer patients, 18% were transferred to the acute medical service of the hospital (Table 1). Neither age, sex, type of cancer, nor admission FIM score were associated with the need for transfer to acute hospital care. Change in FIM score was inversely associated with transfer to acute hospital care (P = .027). Patients whose function did not improve with rehabilitation were most likely to be transferred back to acute hospital care.
Discussion
Radiation therapy is considered a service that is provided to people who come for treatment as an outpatient. Caregivers may have difficulty transporting patients to radiation if the patient has deficits in mobility. This may be particularly true if the patient is heavy, the caregivers are frail, or perhaps if they live in rural settings where there is no wheelchair-accessible public transportation. There are many factors that help determine whether a patient with functional deficits can be discharged to his or her home. These include sex, age, marital status, family and/or community support, income, and insurance.8 The FIM is an instrument that indicates how much help a patient needs with mobility and self-care skills. It also correlates with the amount of time that caregivers must spend helping a patient.9 Study findings have shown that the FIM score is an important determinant of whether a patient can be discharged to home. The total FIM score is as useful as an analysis of the components of the FIM score in predicting whether a patient can return to the community.10,11 Reistetter and colleagues found a total FIM score of 78 to be the score that best separates patients who are likely to be able to go home and patients who are likely to need long-term care.11 Bottemiller and colleagues10 reported that 37% of patients with total discharge FIM scores of less than 40 were discharged to home. They reported that 62% of patients with FIM scores between 40 and 79 were discharged to home, and 88% of patients with scores of 80 or above were discharged to home.10 The goal in bringing patients to the IRF was to accept and treat patients with reasonable community support and potential to achieve a functional level compatible with discharge to the community. Most patients in each of the cancer groups were able to reach an FIM score of 78 to 80 and to be discharged to home.
Most of the patients in the cancer groups had underlying problems that are not considered curable. The primary goal was to enable the patients to have some time at home with their families before requiring readmission to a hospital or hospice care. Reasonable LoS and rate of progress are now expected or required by third-party payors and hospital administrators. Physicians at the Mayo Clinic have indicated that a rehabilitation service should aim for an FIM efficiency score of at least .6 points per day.10 The FIM efficiency of patients in each of the 4 cancer subgroups in this study was higher than this level.
J. Herbert Dietz, Jr was an early advocate of the need to provide comprehensive rehabilitation services for patients with cancer. He first described his work in 1969.12 Since that time, there have been many papers that have documented the benefits of IRF for patients with cancer. O’Toole and Golden have shown outcomes of a large series of patients from an IRF. They reported that at the time of admission, 14% of patients could ambulate, but at discharge, 80% could ambulate without hands-on assistance. They reported significant improvements in continence, FIM score, and score on the Karnofsky Performance Scale.13 Marciniak,14 Hunter,15 Shin,16 and Cole,17 and their respective colleagues have all shown that patients with many different types of cancer benefit from rehabilitation at the IRF level. Gallegos-Kearin and colleagues4 reported on the care of 115,570 patients admitted to IRF with cancer from 2002 to 2014. Patients had significant improvement in function, with more than 70% of patients discharged to home.4 Ng and colleagues studied a group of 200 patients who received IRF care and found there was significant improvement in function. Ninety-four percent of patients rated their stay as either extremely good or very good.5
Metastasis to the spine is a common problem. It is found in 30% of cancer patients at autopsy. The most common sources of metastasis to the spine are breast, lung, prostate, kidney, and thyroid.18 Multiple myeloma and lymphoma may also involve the spine. Several authors have shown that these patients benefit from inpatient rehabilitation. Mckinley and colleagues19 have noted that patients with metastasis to the spine make significant improvement with care at an IRF. Compared with patients with a traumatic spinal cord injury, the cancer patients had shorter LoS, smaller improvement in FIM, equal FIM efficiency (FIM gain/LoS), and equal success in making enough progress to be discharged to home.19 Eriks and colleagues showed that patients at an IRF in Amsterdam made significant improvement in function as measured by the Barthel’s Index.20 Tang .,21 and Parsch22 and their respective colleagues, Murray,23 and New24 and colleagues have published findings confirming that patients with spinal cord injury caused by metastasis to the spine make significant progress with inpatient rehabilitation programs. The present study adds to the literature by showing that patients with metastasis to the spine who are receiving radiation can make progress and be discharged to the community.
There are 24,000 new cases of primary malignant brain tumors in the United States each year.25 The incidence of metastatic cancer to the brain has been estimated to be 100,000 cases per year in the United States. The most common cancer sources are lung, breast, melanoma, kidney, and colon.26,27 The first study of patients admitted to an IRF for treatment of brain tumors was published in 1998 by Huang and colleagues28 who compared the outcomes of 63 patients with brain tumors with the outcomes of 63 patients with stroke. They reported that the patients with the brain tumors made significant improvement in function. There was not a significant difference between the 2 groups of patients in improvement in function, FIM efficiency, or success in discharging the patients to home.28 Greenberg29 and Bartolo30 and their respective colleagues compared the outcomes of patients admitted with brain tumors and patients with stroke and found that improvement in function and discharge to home was similar in the 2 groups. In 2000, Huang and his same colleagues31 compared a group of patients with brain tumors to a group of patients with traumatic brain injury. They found significant improvement in the function of the patients with brain tumors. Patients in the traumatic brain injury group made more progress but had longer LoS. FIM efficiency was not significantly different between the groups.31
Three papers have reported outcomes of patients who received radiation concurrent with inpatient rehabilitation. Tang and colleagues32 reported 63 patients, of whom 48% percent received radiation concurrent with rehabilitation. The patients who received radiation made significant gains in function, and more than 70% were discharged to home. There was no difference in the outcomes of the patients in the radiation and nonradiation groups.32 Marciniak33 and O’Dell34 and their colleagues also reported that patients with brain tumors that required radiation therapy can benefit from inpatient rehabilitation. The present paper is the fourth (with the largest patient group) to show that patients with primary and metastatic tumors to the brain can benefit from a program that provides radiation concurrent with inpatient rehabilitation. We have shown that patients can achieve functional levels and rates of discharge to home that are not significantly different from those of the most commonly admitted group of patients to IRF – patients with stroke.
In the present study, 18% of all of the cancer patients were transferred to medical services and/or acute hospital care (Table 1). This is consistent with a paper by Asher and colleagues35 who reported that 17.4% of patients at an IRF with a diagnosis of cancer required transfer back to medical service, and that low admission motor FIM score correlated with the likelihood of transfer back to medical service. In the present paper, the total admission FIM score was not related to the likelihood of return to medical service, although a lack of improvement in the FIM score did correlate with transfer to medical service.
All of the papers we reviewed found that appropriately selected patients with cancer make significant improvement in function with treatment at an IRF. Tang and colleagues have also shown that for patients with malignant brain tumors and metastasis to the spine, improvement in function correlates with increased survival.32 Our paper confirms that patients with primary malignant brain tumors, malignant tumors metastatic to the brain or spine, and tumors metastatic to long bones may benefit from rehabilitation concurrent with radiation. Rehabilitation units are traditionally associated with treating patients with stroke and spinal cord injury. The patients in our study had cancer and were receiving radiation therapy. They had significant improvement in function and FIM efficiency scores that are not below the threshold set as expected for care at an IRF. Most patients in our study achieved a functional level consistent with what is needed to go home.
There is a prospective payment or reimbursement system for rehabilitation units.36 The payments are based on the admitting diagnosis, the admission FIM score, the age of the patient, and comorbidities. There are 4 tiers for comorbidities with no additional payments for patients in tier 0 but with additional payments for patients with conditions that qualify for tiers 1 through 3. The highest payments are for patients in tier 1. Examples of conditions that can increase payment include morbid obesity, congestive heart failure, vocal cord paralysis, and the need for hemodialysis. There is no increased payment for provision of radiation therapy. There are no reports on the feasibility, in terms of finances, of providing radiation on an IRF. We asked the finance office of the Albany Medical Center to comment on the cost to the hospital of providing radiation therapy to patients on the rehabilitation unit. The hospital’s finance department reviewed available data and reported that the variable cost of providing radiation therapy is about 6.5% of the revenue collected from third-party payors for caring for patients who receive that service (personal communication from the finance office of Albany Medical Center to George Forrest, 2015). Our findings suggest that the Centers for Medicare & Medicaid Services should make an adjustment to the payment system to support the cost of providing radiation to patients at an IRF. Even under the current payment system, for a hospital that has the equipment and personnel to provide radiation treatments, the variable cost of 6.5% of revenue should not be an absolute barrier to providing this service.
Limitations
This study reports on the experience of only 1 facility. The number of patients in the radiation group is greater than the number of patients in any previous report of people receiving radiation at an IRF, but the statistician does not think it is large enough to allow statistical analysis of covariates such as age, sex, and comorbid conditions. In addition, we did not investigate all of the factors that influence the type of care patients are offered and their LoS, such as hospital policy, insurance coverage, income, and family structure.
Conclusions
Acute care medical units are now challenged to both reduce LoS and reduce the number of patients who are readmitted to the hospital. Rehabilitation units are challenged to maintain census, as government and private payors are shifting patients from acute rehabilitation units to subacute rehabilitation units. We found that patients with cancer who need radiation are a population of patients who are seen by payors as needing to be in a facility with excellent nursing, therapy, and comprehensive physician services. A comprehensive cancer care program within a rehabilitation unit can be a great benefit to the acute care services, the IRF, and, most importantly, patients and their families.
1. American Cancer Society. Cancer facts & figures 2016. Atlanta, GA: American Cancer Society; 2016.
2. National Cancer Institute: Office of cancer survivorship: statistics. https://cancercontrol.cancer.gov/ocs/statistics/statistics.html. Updated October 17, 2016. Accessed April 21, 2018.
3. Lehmann JF, DeLisa JA, Warren CG, deLateur BJ, Bryant PL, Nicholson CG. Cancer rehabilitation: assessment of need, development and evaluation of a model of care. Arch Phys Med Rehabil. 1978;59(9):410-419.
4. Gallegos-Kearin V, Knowlton SE, Goldstein R, et al. Outcome trends of adult cancer patients receiving inpatient rehabilitation: a 13-year review [published online Feb 21, 2018]. Am J Phys Med Rehabil. doi:10.1097/PHM.0000000000000911
5. Ng AH, Gupta E, Fontillas RC, et al. Patient-reported usefulness of acute cancer rehabilitation. PM R. 2017;9(11):1135-1143.
6. Cheville AL, Kornblith AB, Basford JR. An examination of the causes for the underutilization of rehabilitation services among people with advanced cancer. Am J Phys Med Rehabil. 2011;90(5 suppl 1):S27-S37.
7. Cohen ME, Marino RJ. The tools of disability outcomes research functional status measures. Arch Phys Med Rehabil. 2000;81(12 suppl 2):S21-S29.
8. Nguyen VQ, PrvuBettger J, Guerrier T, et al. Factors associated with discharge to home versus discharge to institutional care after inpatient stroke rehabilitation. Arch Phys Med Rehabil. 2015;96(7):1297-1303.
9. Forrest G, Schwam A, Cohen E. Time of care required by patients discharged from a rehabilitation unit. Am J Phys Med Rehabil. 2002;81(1):57-62.
10. Bottemiller KL, Bieber PL, Basford JR, Harris M. FIM scores, FIM efficiency and discharge following inpatient stroke rehabilitation. Rehabil Nurs. 2006;31(1):22-25.
11. Reistetter TA, Graham JE, Deutsch A, Granger CV, Markello S, Ottenbacher KJ. Utility of functional status for classifying community versus institutional discharges after inpatient rehabilitation for stroke. Arch Phys Med Rehabil. 2010;91(3):345-350.
12. Dietz JH Jr. Rehabilitation of the cancer patient. Med Clin North Am. 1969;53(3):607-624.
13. O'Toole DM, Golden AM. Evaluating cancer patients for rehabilitation potential. West J Med. 1991;155(4):384-387.
14. Marciniak CM, Sliwa JA, Spill G, Heinemann AW, Semik PE. Functional outcome following rehabilitation of the cancer patient. Arch Phys Med Rehabil. 1996;77(1):54-57.
15. Hunter EG, Baltisberger J. Functional outcomes by age for inpatient cancer rehabilitation: a retrospective chart review. J Appl Gerontol. 2013;32(4):443-456.
16. Shin KY, Guo Y, Konzen B, Fu J, Yadav R, Bruera E. Inpatient cancer rehabilitation: the experience of a national comprehensive cancer center. Am J Phys Med Rehabil. 2011;90(5 suppl 1):S63-S68.
17. Cole RP, Scialla S, Bednarz L. Functional recovery in cancer rehabilitation. Arch Phys Med Rehabil. 2000;81(5):623-627.
18. White AP, Kwon BK, Lindskog DM, Friedlaender GE, Grauer JN. Metastatic disease of the spine. J Am Acad Orthop Surg. 2006;14(11):587-598.
19. McKinley WO, Huang ME, Tewksbury MA. Neoplastic vs traumatic spinal cord injury: an inpatient rehabilitation comparison. Am J Phys Med Rehabil. 2000;79(2):138-144.
20. Eriks IE, Angenot EL, Lankhorst GJ. Epidural metastatic spinal cord compression: functional outcome and survival after inpatient rehabilitation. Spinal Cord. 2004;42(4):235-239.
21. Tang V, Harvey D, Park Dorsay J, Jiang S, Rathbone MP. Prognostic indicators in metastatic spinal cord compression: using functional independence measure and Tokuhashi scale to optimize rehabilitation planning. Spinal Cord. 2007;45(10):671-677.
22. Parsch D, Mikut R, Abel R. Postacute management of patients with spinal cord injury due to metastatic tumor disease: survival and efficacy of rehabilitation. Spinal Cord. 2003;41:205-210.
23. Murray PK. Functional outcome and survival in spinal cord injury secondary to neoplasia. Cancer. 1985;55:197-201.
24. New PW. Functional outcomes and disability after nontraumatic spinal cord injury rehabilitation: results from a retrospective study. Arch Phys Med Rehabil. 2005;86(2):250-261
25. Central Brain Tumor Registry of the United States: 2016 CBTRUS fact sheet. www.cbtrus.org/factsheet/factsheet.html. Updated 2017. Accessed May 28, 2016.
26. Memorial Sloan Kettering Cancer Center: Metastatic brain tumors & secondary brain cancer. https://www.mskcc.org/cancer-care/types/brain-tumors-metastatic. Updated 2018. Accessed April 21, 2018.
27. Bruckner JC, Brown PD, O'Neill BP, Meyer FB, Wetmore CJ, Uhm JH. Central nervous system tumors. Mayo Clin Proc. 2007;82(10):1271-1286.
28. Huang ME, Cifu DX, Keyser-Marcus L. Functional outcome after brain tumor and acute stroke: a comparative analysis. Arch Phys Med Rehabil. 1998;79(11):1386-1390.
29. Greenberg E, Treger I, Ring H. Rehabilitation outcomes in patients with brain tumors and acute stroke: comparative study of inpatient rehabilitation. Am J Phys Med Rehabil. 2006;85(7):568-573.
30. Bartolo M, Zucchella C, Pace A, et al. Early rehabilitation after surgery improves functional outcomes in inpatients with brain tumours. J Neurooncol. 2012;107(3);537-544.
31. Huang ME, Cifu DX, Keyser-Marcus L. Functional outcomes in patients with brain tumor after inpatient rehabilitation: comparison with traumatic brain injury. Am J Phys Med Rehabil. 2000;79(4):327-335.
32. Tang V, Rathbone M, Park Dorsay J, Jiang S, Harvey D. Rehabilitation in primary and metastatic brain tumours: impact of functional outcomes on survival. J Neurol. 2008;255(6):820-827.
33. Marciniak CM, Sliwa JA, Heinemann AW, Semik PE. Functional outcomes of persons with brain tumors after inpatient rehabilitation. Arch Phys Med Rehabil. 2001;82(4):457-463.
34. O'Dell MW, Barr K, Spanier D, Warnick RE. Functional outcome of inpatient rehabilitation in persons with brain tumors. Arch Phys Med Rehabil. 1998;79(12):1530-1534.
35. Asher A, Roberts PS, Bresee C, Zabel G, Riggs RV, Rogatko A. Transferring inpatient rehabilitation facility cancer patients back to acute care (TRIPBAC). PM R. 2014;6(9):808-813.
36. Centers for Medicare and Medicaid Services: Inpatient rehabilitation facilities. https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/CertificationandComplianc/InpatientRehab.html. Published March 5, 2012. Accessed May 21, 2018.
1. American Cancer Society. Cancer facts & figures 2016. Atlanta, GA: American Cancer Society; 2016.
2. National Cancer Institute: Office of cancer survivorship: statistics. https://cancercontrol.cancer.gov/ocs/statistics/statistics.html. Updated October 17, 2016. Accessed April 21, 2018.
3. Lehmann JF, DeLisa JA, Warren CG, deLateur BJ, Bryant PL, Nicholson CG. Cancer rehabilitation: assessment of need, development and evaluation of a model of care. Arch Phys Med Rehabil. 1978;59(9):410-419.
4. Gallegos-Kearin V, Knowlton SE, Goldstein R, et al. Outcome trends of adult cancer patients receiving inpatient rehabilitation: a 13-year review [published online Feb 21, 2018]. Am J Phys Med Rehabil. doi:10.1097/PHM.0000000000000911
5. Ng AH, Gupta E, Fontillas RC, et al. Patient-reported usefulness of acute cancer rehabilitation. PM R. 2017;9(11):1135-1143.
6. Cheville AL, Kornblith AB, Basford JR. An examination of the causes for the underutilization of rehabilitation services among people with advanced cancer. Am J Phys Med Rehabil. 2011;90(5 suppl 1):S27-S37.
7. Cohen ME, Marino RJ. The tools of disability outcomes research functional status measures. Arch Phys Med Rehabil. 2000;81(12 suppl 2):S21-S29.
8. Nguyen VQ, PrvuBettger J, Guerrier T, et al. Factors associated with discharge to home versus discharge to institutional care after inpatient stroke rehabilitation. Arch Phys Med Rehabil. 2015;96(7):1297-1303.
9. Forrest G, Schwam A, Cohen E. Time of care required by patients discharged from a rehabilitation unit. Am J Phys Med Rehabil. 2002;81(1):57-62.
10. Bottemiller KL, Bieber PL, Basford JR, Harris M. FIM scores, FIM efficiency and discharge following inpatient stroke rehabilitation. Rehabil Nurs. 2006;31(1):22-25.
11. Reistetter TA, Graham JE, Deutsch A, Granger CV, Markello S, Ottenbacher KJ. Utility of functional status for classifying community versus institutional discharges after inpatient rehabilitation for stroke. Arch Phys Med Rehabil. 2010;91(3):345-350.
12. Dietz JH Jr. Rehabilitation of the cancer patient. Med Clin North Am. 1969;53(3):607-624.
13. O'Toole DM, Golden AM. Evaluating cancer patients for rehabilitation potential. West J Med. 1991;155(4):384-387.
14. Marciniak CM, Sliwa JA, Spill G, Heinemann AW, Semik PE. Functional outcome following rehabilitation of the cancer patient. Arch Phys Med Rehabil. 1996;77(1):54-57.
15. Hunter EG, Baltisberger J. Functional outcomes by age for inpatient cancer rehabilitation: a retrospective chart review. J Appl Gerontol. 2013;32(4):443-456.
16. Shin KY, Guo Y, Konzen B, Fu J, Yadav R, Bruera E. Inpatient cancer rehabilitation: the experience of a national comprehensive cancer center. Am J Phys Med Rehabil. 2011;90(5 suppl 1):S63-S68.
17. Cole RP, Scialla S, Bednarz L. Functional recovery in cancer rehabilitation. Arch Phys Med Rehabil. 2000;81(5):623-627.
18. White AP, Kwon BK, Lindskog DM, Friedlaender GE, Grauer JN. Metastatic disease of the spine. J Am Acad Orthop Surg. 2006;14(11):587-598.
19. McKinley WO, Huang ME, Tewksbury MA. Neoplastic vs traumatic spinal cord injury: an inpatient rehabilitation comparison. Am J Phys Med Rehabil. 2000;79(2):138-144.
20. Eriks IE, Angenot EL, Lankhorst GJ. Epidural metastatic spinal cord compression: functional outcome and survival after inpatient rehabilitation. Spinal Cord. 2004;42(4):235-239.
21. Tang V, Harvey D, Park Dorsay J, Jiang S, Rathbone MP. Prognostic indicators in metastatic spinal cord compression: using functional independence measure and Tokuhashi scale to optimize rehabilitation planning. Spinal Cord. 2007;45(10):671-677.
22. Parsch D, Mikut R, Abel R. Postacute management of patients with spinal cord injury due to metastatic tumor disease: survival and efficacy of rehabilitation. Spinal Cord. 2003;41:205-210.
23. Murray PK. Functional outcome and survival in spinal cord injury secondary to neoplasia. Cancer. 1985;55:197-201.
24. New PW. Functional outcomes and disability after nontraumatic spinal cord injury rehabilitation: results from a retrospective study. Arch Phys Med Rehabil. 2005;86(2):250-261
25. Central Brain Tumor Registry of the United States: 2016 CBTRUS fact sheet. www.cbtrus.org/factsheet/factsheet.html. Updated 2017. Accessed May 28, 2016.
26. Memorial Sloan Kettering Cancer Center: Metastatic brain tumors & secondary brain cancer. https://www.mskcc.org/cancer-care/types/brain-tumors-metastatic. Updated 2018. Accessed April 21, 2018.
27. Bruckner JC, Brown PD, O'Neill BP, Meyer FB, Wetmore CJ, Uhm JH. Central nervous system tumors. Mayo Clin Proc. 2007;82(10):1271-1286.
28. Huang ME, Cifu DX, Keyser-Marcus L. Functional outcome after brain tumor and acute stroke: a comparative analysis. Arch Phys Med Rehabil. 1998;79(11):1386-1390.
29. Greenberg E, Treger I, Ring H. Rehabilitation outcomes in patients with brain tumors and acute stroke: comparative study of inpatient rehabilitation. Am J Phys Med Rehabil. 2006;85(7):568-573.
30. Bartolo M, Zucchella C, Pace A, et al. Early rehabilitation after surgery improves functional outcomes in inpatients with brain tumours. J Neurooncol. 2012;107(3);537-544.
31. Huang ME, Cifu DX, Keyser-Marcus L. Functional outcomes in patients with brain tumor after inpatient rehabilitation: comparison with traumatic brain injury. Am J Phys Med Rehabil. 2000;79(4):327-335.
32. Tang V, Rathbone M, Park Dorsay J, Jiang S, Harvey D. Rehabilitation in primary and metastatic brain tumours: impact of functional outcomes on survival. J Neurol. 2008;255(6):820-827.
33. Marciniak CM, Sliwa JA, Heinemann AW, Semik PE. Functional outcomes of persons with brain tumors after inpatient rehabilitation. Arch Phys Med Rehabil. 2001;82(4):457-463.
34. O'Dell MW, Barr K, Spanier D, Warnick RE. Functional outcome of inpatient rehabilitation in persons with brain tumors. Arch Phys Med Rehabil. 1998;79(12):1530-1534.
35. Asher A, Roberts PS, Bresee C, Zabel G, Riggs RV, Rogatko A. Transferring inpatient rehabilitation facility cancer patients back to acute care (TRIPBAC). PM R. 2014;6(9):808-813.
36. Centers for Medicare and Medicaid Services: Inpatient rehabilitation facilities. https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/CertificationandComplianc/InpatientRehab.html. Published March 5, 2012. Accessed May 21, 2018.
Psychosocial factors and treatment satisfaction after radical prostatectomy
More than 164,690 men are expected to be diagnosed with prostate cancer in the United States in 2018.1 Men with prostate cancer face not only stress associated with the diagnosis but also decisional conflict regarding different treatment options.2 Most men diagnosed with clinically localized prostate cancer receive 1 or more of the following treatments: radical prostatectomy, external-beam radiation therapy, and/or brachytherapy, all of which are associated with posttreatment urological or sexual side effects including bowel, urinary, or erectile dysfunction.3-5 Men who choose active surveillance may experience increased anxiety associated with the constant vigilance and monitoring of their tumor status along with the uncertainty of not definitively removing or radiating their prostate.6 In addition to direct functional limitations of sexual and urological side effects, treatment can also lead to secondary psychosocial effects, including depression, self-blame, embarrassment, guilt, lower masculine self-esteem, increased reticence to participate socially or engage in sexual activity, and relationship distress.7-9 Therefore, health-related quality of life (HRQoL) and treatment satisfaction are important for this population.
Urological and sexual side effects of prostate cancer treatments are often a primary focus during treatment decision making between patients and providers. However, little prospective empirical data exist regarding the role of HRQoL and other nonurological physical and psychosocial outcomes on overall treatment satisfaction. The purpose of this study was to prospectively evaluate the role of both urological and nonurological outcomes on overall treatment satisfaction in men diagnosed with prostate cancer. We hypothesize that such an understanding can help describe changes in physical and psychosocial factors that are important to men beyond traditional urological outcomes, including their association with overall treatment satisfaction.
Methods
This was a prospective longitudinal assessment of patients from the Department of Urology at Northwestern University’s Feinberg School of Medicine in Chicago. Patients were eligible if they met the following inclusion criteria: they had been diagnosed with clinically localized or locally advanced prostate cancer; they had not yet received a primary treatment (eg, surgery, radiation, active surveillance) before their baseline assessment; they were 18 years or older; and they were able to read, write, speak, and understand English. Patients were excluded if they had a physical debilitation that would make participation not feasible or would create undue hardship, or if they had a history of diagnosed severe mental illness or hospitalization for chronic psychiatric reasons, as identified by referring physicians.
Eligible participants were approached before their treatment decision (if any). Patient enrollment occurred in 2 ways. For patients invited to participate during their clinic visit, the research assistant explained the study and obtained written informed consent for interested patients. A unique user identification and password was created for each patient, and they practiced using the touch screen computer while the research assistant observed and provided guidance as needed. When the patients were ready to start their pretreatment online interview, they completed the questionnaires by themselves. For patients who were invited to participate but were not scheduled to return in the foreseeable future, enrollment was carried out differently. In those cases, participating physicians contacted eligible patients who were not scheduled for a visit and informed them of the study opportunity. Interested patients were contacted by the research assistant who provided them with the study website address, which directed them to the online consent form. After a patient had completed the consent form, he was prompted to self-register. He received a unique user identification and password that could be used to complete the baseline assessment and subsequent assessments. However, for interested patients who did not have access to a computer or Internet connection, the research assistant provided them with paper consent forms and paper versions of all study assessments. After participants had completed the baseline assessment, the research assistant provided them with a written schedule of future assessments, which were expected to occur at 1 month posttreatment, 3 months posttreatment, 6 months posttreatment, and 12 months posttreatment.
For all follow-up appointments, participants could complete assessments either at clinic visits or from home using a secure online assessment platform called Assessment Center.10 The research assistant used a patient log to track participants and their progress in the study, which included study number, patient name (or initials), registration date, date of birth, sex, and timeline of completed or future assessments. The research assistant called or emailed participants (depending on patient preference) about a week before each of their follow-up assessments to facilitate adherence. If the participant did not log into the system by the target day, the research assistant contacted him the following day (target day +1) with a phone or email reminder to log into the system and complete the assessments. If the participant did not log in by midnight 1 day after the target day, the research assistant attempted to contact him one last time (target day +2) with either a reminder to log into the system or to ascertain his status that might be related to his noncompletion. Overall, a participant was called or e-mailed 1 to 3 times to remind him of his assessment. If he was unresponsive after 3 attempts, he was recorded as having withdrawn for an unknown reason.
At baseline and each follow-up time point, study participants completed a battery of patient-reported outcome measures, with most coming from the Patient-Reported Outcomes Measurement Information System (PROMIS)11 and the Surgical Outcomes Measurement System (SOMS).12 PROMIS is a National Institutes of Health (NIH) funded measurement system that has helped standardize and improve self-reported assessment of health status, symptoms, side effects, and different aspects of HRQoL, including physical, emotional, cognitive, and social health. SOMS is a suite of patient-reported outcome measures assessing important aspects of HRQoL after surgery. It was developed with feedback from surgeons, postoperative patients, and surgical nurses. PROMIS items were directly incorporated into numerous SOMS measures to facilitate easier comparisons and score crosswalks across measures and patient populations. In addition to PROMIS and SOMS measures, we also administered several well-known instruments of urological and sexual function, including the International Index of Erectile Function (IIEF) and American Urological Association Symptom Score Index (AUASS).13,14
Outcome measures were compared across sociodemographic and clinical variables at each time point using t tests for numerical variables (age) and with chi-square or Fisher exact tests for categorical variables; those variables with significant differences were used as covariates in statistical models. To examine differences in patient-reported scores over time, we used repeated measures analysis of covariance with general linear modeling methods. We used Pearson correlation coefficients to evaluate for correlations between quality-of-life outcomes and treatment satisfaction.
Not all participants completed each of the follow-up surveys, and reasons for dropout were prospectively documented. Most participants elected surgical resection as their primary treatment compared with the fewer than 10% of patients who chose radiation or chemotherapy as their primary treatment and about 20% of men who chose active surveillance after their initial diagnosis. Therefore, our analysis focused on patients who elected surgical resection. For comparison purposes, we included the HRQoL results from active surveillance patients.
Results
A total of 105 patients diagnosed with prostate cancer were enrolled in the study. Response rates decreased throughout the study (n = 75 at 1 month; n = 71 at 3 months; n = 64 at 6 months; n = 54 at 12 months). Sociodemographic and clinical characteristics of participants are shown in Table 1. The mean change from pretreatment (baseline) scores for each measure in patients treated with surgery is shown in Table 2, and the mean change from pretreatment scores in patients who elected active surveillance is shown in Table 3 (in both tables, a negative score denotes worsened function, and a positive change denotes improvement).
After surgery, patients reported significantly lower erectile function and sexual satisfaction scores. These included statistically significant decreases for IIEF Erectile Function, IIEF Overall Satisfaction, PROMIS Sexual Satisfaction, PROMIS Sexual Interest, and PROMIS Orgasm. In patients treated with surgery, there were significant improvements in anxiety observed for patients at each follow-up time, whereas significantly worse bladder problems were observed on SOMS Bladder at 1 and 3 months but returned to baseline by 12 months after surgery. AUASS was worse at 1 month but significantly improved at 6 and 12 months. Fatigue scores significantly worsened at 1 month but were no longer significant at 6 and 12 months. Physical Function was worsened at 1 month but not throughout the rest of the study. Bowel Problems (SOMS) were significantly worse at 1 month, but changes became nonsignificant on subsequent assessments. The only 2 domains that did not demonstrate any significant changes o
In active surveillance patients, sexual function domains were generally unchanged over the course of the study. However, unlike treated patients, there was no significant improvement in anxiety, depression, pain, fatigue, or sleep. In fact, most of these domains demonstrated worsened functioning, although these were not statistically significant. Urinary domains generally remained unchanged.
Pearson correlation coefficients between HRQoL measures and overall treatment satisfaction (assessed by the question, Are you satisfied with the results of your operation?) at each follow-up time point in patients treated with surgery are shown in Table 4. Relations between treatment satisfaction and sexual outcomes were generally statistically insignificant (r, .08-.56). However, sleep disturbance, depression, pain interference, fatigue, embarrassment, and bladder problems all demonstrated statistically significant positive associations with treatment satisfaction, with coefficients ranging from small to medium in magnitude (r, .32-.61). Other outcomes such as anxiety, physical function, and bowel problems demonstrated small to medium statistically significant associations with treatment satisfaction (r, .04-.60) but not at every time point. We performed t tests to examine treatment satisfaction in patients with detectable initial posttreatment prostate-specific antigen (PSA; >0.01 ng/mL). We found no difference in treatment satisfaction between patients with detectable PSA values and those with undetectable PSA at each time point.
When the patients were asked, Compared with what you expected, how do you rate the results of your operation?, most of those treated with surgery reported that the results of their operation were better than they had expected (Figure 1A; p. e137). More than 75% of the patients had results that were as expected or better than expected. When asked, Compared with what you expected, how do you rate your side effects of the operation?, almost 70% of patients reported side effects no worse than expected (Figure 1B). When asked, Are you satisfied with the results of your operation?, most patients reported that overall, they were satisfied with the results of their operation (Figure 1C).
At 12 months, none of the patients reported overall dissatisfaction with their treatment choice. More than 90% of patients were mostly or completely satisfied with the results of their operation.
Discussion
This prospective study assessed the HRQoL from pretreatment through 12 months posttreatment in men diagnosed with clinically localized prostate cancer that had been treated with surgery. Although the indicators of sexual function significantly decreased over time, they were not meaningfully associated with overall treatment satisfaction. Instead, a host of other factors, including psychosocial (eg, anxiety, depression, body image dissatisfaction, embarrassment), nonurological physical symptoms (pain interference, physical function, sleep disturbance, fatigue), and bladder problems, were significantly related to overall treatment satisfaction. Although this may not be surprising in other clinical oncology paradigms, the sheer surfeit of focus and attention on sexual function has overshadowed aspects of HRQoL that many men report are important to them, despite worsened sexual function outcomes.
Understanding potential treatment-related changes in HRQoL can be challenging for men when choosing providers and different therapeutic options. The increasing complexity of treatment in prostate cancer has created an opportunity to not only understand efficacy on cancer control but also focus on meaningful patient-reported outcomes. Hospitals and medical groups are increasingly aware of the importance of improving the patient care experience. Objective measures of patient satisfaction for health care providers, such as the Press-Ganey and Net Promoter score, exist to measure and improve patient experience. In prostate cancer, clinicians and large groups, including governmental agencies such as the US Preventive Services Task Force, have often focused on declines in urinary and erectile function15 without considering the full impact of prostate cancer treatment on global HRQoL. Our study was a prospective, longitudinal, self-reported examination of the impact, positive and negative, of prostate cancer treatment over a 12-month period.
Numerous studies have documented the treatment-related side effects of erectile, urinary, and bowel dysfunction in patients treated for prostate cancer, which may occur after definitive local therapies.5,16-18 The present study shows a similar impact on urinary, bowel, and erectile domains after treatment. Although erectile function scores remained lower through the course of the 12-month study, bowel and bladder domains returned to baseline by month 12. Unlike other studies, we also examined psychosocial and nonurological aspects of prostate cancer treatment. We found that there was a measurable and significant positive impact on other HRQoL measurements such as decreased anxiety. Despite a variety of declines across HRQoL domains, most patients reported that their results were largely as they had expected, and their side effects were the same or better than they had expected. No patient in the cohort reported being dissatisfied with his overall treatment, and more than 90% of patients were mostly or completely satisfied with their treatment choice. This highlights the point that while sexual and other urological domains of HRQoL are important, impairments in these areas do not necessarily reflect how many patients perceive success or satisfaction with their treatment choice. We also showed correlations between treatment satisfaction and improvement in sleep, anxiety, depression, and fatigue. It is worth noting that although there were decreases in the erectile and sexual function domains after treatment, those factors were not correlated with overall treatment satisfaction. Those factors may not routinely be assessed before, during, and after treatment for prostate cancer in most clinical encounters. However, because they were strongly associated with satisfaction with treatment outcomes in this study, identification in impairments may lead to opportunities to intervene and improve the patient experience. Therefore, important “teachable moments” may be missed (for both patients and providers) during treatment decision-making encounters if other factors beyond sexual and urological outcomes are not adequately considered and addressed. Furthermore, the results of our study may help clinicians counsel patients on their expectations for their recovery after surgery and identify particular issues related to HRQoL to pay close attention to in follow-up visits.
Strengths of our study include its prospective nature, which allowed evaluation of HRQoL outcomes at multiple time points throughout the first year after treatment. In addition, we used existing patient-reported outcome tools validated by the NIH to assess changes in HRQoL. PROMIS is an NIH-supported tool that can be leveraged in the pre- and posttreatment periods to identify patients who have impairments with HRQoL. It can provide clinicians with a unique opportunity to detect and intervene in setbacks and side effects to improve patient satisfaction and HRQoL.
Limitations of the current study include that most patients selected surgery for their treatment choice and that not all patients completed all longitudinal questionnaires, although this is expected in longitudinal studies of this nature. Although all the patients were approached and encouraged to participate, many did not participate and were not captured. In addition, not all patients completed end-of-study surveys. These factors may have biased our results because of unmeasurable factors related to nonparticipation or dropout. Our study encompassed the preoperative period up to 12 months postoperatively, which may fail to identify improvements or declines in HRQoL that may occur more than 12 months postoperatively, particularly related to continence and erectile function. The participants were enrolled by 6 surgeons, and we were not able to standardize the preoperative counseling either preoperatively or postoperatively, which may have biased our results. Finally, our study population consisted of predominantly white, married men of higher socioeconomic status; therefore, our results may not be generalizable to newly diagnosed prostate cancer patients overall.
Conclusions
By using validated self-administered questionnaires, we found that despite decreased sexual and urinary function, patients treated for prostate cancer were satisfied with their treatment choice. Correlates to higher patient satisfaction included decreased anxiety, depression, fatigue, and sleep disturbances.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7‐30.
2. Berry DL, Ellis WJ, Woods NF, Schwien C, Mullen KH, Yang C. Treatment decision-making by men with localized prostate cancer: the influence of personal factors. Urol Oncol. 2003;21(2):93-100.
3. Dubbelman YD, Dohle GR, Schröder FH. Sexual function before and after radical retropubic prostatectomy: a systematic review of prognostic indicators for a successful outcome. Eur Urol. 2006;50(4):711-718; discussion 718-720.
4. McCullough AR. Sexual dysfunction after radical prostatectomy. Rev Urol. 2005;7(2 suppl):S3-S10.
5. Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358(12):1250-1261.
6. Latini DM, Hart SL, Knight SJ, et al. The relationship between anxiety and time to treatment for patients with prostate cancer on surveillance. J Urol. 2007;178(3, pt 1):826-831; discussion 831-832.
7. Meyer JP, Gillatt DA, Lockyer R, Macdonagh R. The effect of erectile dysfunction on the quality of life of men after radical prostatectomy. BJU Int. 2003;92(9):929-931.
8. Casey RG, Corcoran NM, Goldenberg SL. Quality of life issues in men undergoing androgen deprivation therapy: a review. Asian J Androl. 2012;14(2):226-231.
9. Segrin C, Badger TA, Harrington J. Interdependent psychological quality of life in dyads adjusting to prostate cancer. Health Psychol. 2012;31(1):70-79.
10. Gershon RC, Rothrock N, Hanrahan R, Bass M, Cella D. The use of PROMIS and assessment center to deliver patient-reported outcome measures in clinical research. J Appl Meas. 2010;11(3):304-314.
11. Cella D, Yount S, Rothrock N, et al. The patient-reported outcomes measurement information system (PROMIS): progress of an NIH roadmap cooperative group during its first two years. Med Care. 2007;45(5 suppl 1):S3-S11.
12. Zapf M, Denham W, Barrera E, et al. Patient-centered outcomes after laparoscopic cholecystectomy. Surg Endosc. 2013;27(12):4491-4498.
13. Barry MJ, Fowler FJ Jr, O'Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148(5):1549-1557; discussion 1564.
14. Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49(6):822-830.
15. United States Preventive Services Task Force. Final update summary: prostate cancer: screening. http:// www.uspreventiveservicestaskforce.org/Page/ Document/UpdateSummaryFinal/prostate-cancer-screening. Updated July 2015. Accessed April 14, 2017
16. Litwin MS, Gore JL, Kwan L, et al. Quality of life after surgery, external beam irradiation, or brachytherapy for early-stage prostate cancer. Cancer. 2007;109(11):2239-2247.
17. Miwa S, Mizokami A, Konaka H, et al. Prospective longitudinal comparative study of health-related quality of life and treatment satisfaction in patients treated with hormone therapy, radical retropubic prostatectomy, and high or low dose rate brachytherapy for prostate cancer. Prostate Int. 2013;1(3):117-124.
18. Miller DC, Sanda MG, Dunn RL et al. Long-term outcomes among localized prostate cancer survivors: health-related quality-of-life changes after radical prostatectomy, external radiation, and brachytherapy. J Clin Oncol. 2005;23(12):2772-2780.
More than 164,690 men are expected to be diagnosed with prostate cancer in the United States in 2018.1 Men with prostate cancer face not only stress associated with the diagnosis but also decisional conflict regarding different treatment options.2 Most men diagnosed with clinically localized prostate cancer receive 1 or more of the following treatments: radical prostatectomy, external-beam radiation therapy, and/or brachytherapy, all of which are associated with posttreatment urological or sexual side effects including bowel, urinary, or erectile dysfunction.3-5 Men who choose active surveillance may experience increased anxiety associated with the constant vigilance and monitoring of their tumor status along with the uncertainty of not definitively removing or radiating their prostate.6 In addition to direct functional limitations of sexual and urological side effects, treatment can also lead to secondary psychosocial effects, including depression, self-blame, embarrassment, guilt, lower masculine self-esteem, increased reticence to participate socially or engage in sexual activity, and relationship distress.7-9 Therefore, health-related quality of life (HRQoL) and treatment satisfaction are important for this population.
Urological and sexual side effects of prostate cancer treatments are often a primary focus during treatment decision making between patients and providers. However, little prospective empirical data exist regarding the role of HRQoL and other nonurological physical and psychosocial outcomes on overall treatment satisfaction. The purpose of this study was to prospectively evaluate the role of both urological and nonurological outcomes on overall treatment satisfaction in men diagnosed with prostate cancer. We hypothesize that such an understanding can help describe changes in physical and psychosocial factors that are important to men beyond traditional urological outcomes, including their association with overall treatment satisfaction.
Methods
This was a prospective longitudinal assessment of patients from the Department of Urology at Northwestern University’s Feinberg School of Medicine in Chicago. Patients were eligible if they met the following inclusion criteria: they had been diagnosed with clinically localized or locally advanced prostate cancer; they had not yet received a primary treatment (eg, surgery, radiation, active surveillance) before their baseline assessment; they were 18 years or older; and they were able to read, write, speak, and understand English. Patients were excluded if they had a physical debilitation that would make participation not feasible or would create undue hardship, or if they had a history of diagnosed severe mental illness or hospitalization for chronic psychiatric reasons, as identified by referring physicians.
Eligible participants were approached before their treatment decision (if any). Patient enrollment occurred in 2 ways. For patients invited to participate during their clinic visit, the research assistant explained the study and obtained written informed consent for interested patients. A unique user identification and password was created for each patient, and they practiced using the touch screen computer while the research assistant observed and provided guidance as needed. When the patients were ready to start their pretreatment online interview, they completed the questionnaires by themselves. For patients who were invited to participate but were not scheduled to return in the foreseeable future, enrollment was carried out differently. In those cases, participating physicians contacted eligible patients who were not scheduled for a visit and informed them of the study opportunity. Interested patients were contacted by the research assistant who provided them with the study website address, which directed them to the online consent form. After a patient had completed the consent form, he was prompted to self-register. He received a unique user identification and password that could be used to complete the baseline assessment and subsequent assessments. However, for interested patients who did not have access to a computer or Internet connection, the research assistant provided them with paper consent forms and paper versions of all study assessments. After participants had completed the baseline assessment, the research assistant provided them with a written schedule of future assessments, which were expected to occur at 1 month posttreatment, 3 months posttreatment, 6 months posttreatment, and 12 months posttreatment.
For all follow-up appointments, participants could complete assessments either at clinic visits or from home using a secure online assessment platform called Assessment Center.10 The research assistant used a patient log to track participants and their progress in the study, which included study number, patient name (or initials), registration date, date of birth, sex, and timeline of completed or future assessments. The research assistant called or emailed participants (depending on patient preference) about a week before each of their follow-up assessments to facilitate adherence. If the participant did not log into the system by the target day, the research assistant contacted him the following day (target day +1) with a phone or email reminder to log into the system and complete the assessments. If the participant did not log in by midnight 1 day after the target day, the research assistant attempted to contact him one last time (target day +2) with either a reminder to log into the system or to ascertain his status that might be related to his noncompletion. Overall, a participant was called or e-mailed 1 to 3 times to remind him of his assessment. If he was unresponsive after 3 attempts, he was recorded as having withdrawn for an unknown reason.
At baseline and each follow-up time point, study participants completed a battery of patient-reported outcome measures, with most coming from the Patient-Reported Outcomes Measurement Information System (PROMIS)11 and the Surgical Outcomes Measurement System (SOMS).12 PROMIS is a National Institutes of Health (NIH) funded measurement system that has helped standardize and improve self-reported assessment of health status, symptoms, side effects, and different aspects of HRQoL, including physical, emotional, cognitive, and social health. SOMS is a suite of patient-reported outcome measures assessing important aspects of HRQoL after surgery. It was developed with feedback from surgeons, postoperative patients, and surgical nurses. PROMIS items were directly incorporated into numerous SOMS measures to facilitate easier comparisons and score crosswalks across measures and patient populations. In addition to PROMIS and SOMS measures, we also administered several well-known instruments of urological and sexual function, including the International Index of Erectile Function (IIEF) and American Urological Association Symptom Score Index (AUASS).13,14
Outcome measures were compared across sociodemographic and clinical variables at each time point using t tests for numerical variables (age) and with chi-square or Fisher exact tests for categorical variables; those variables with significant differences were used as covariates in statistical models. To examine differences in patient-reported scores over time, we used repeated measures analysis of covariance with general linear modeling methods. We used Pearson correlation coefficients to evaluate for correlations between quality-of-life outcomes and treatment satisfaction.
Not all participants completed each of the follow-up surveys, and reasons for dropout were prospectively documented. Most participants elected surgical resection as their primary treatment compared with the fewer than 10% of patients who chose radiation or chemotherapy as their primary treatment and about 20% of men who chose active surveillance after their initial diagnosis. Therefore, our analysis focused on patients who elected surgical resection. For comparison purposes, we included the HRQoL results from active surveillance patients.
Results
A total of 105 patients diagnosed with prostate cancer were enrolled in the study. Response rates decreased throughout the study (n = 75 at 1 month; n = 71 at 3 months; n = 64 at 6 months; n = 54 at 12 months). Sociodemographic and clinical characteristics of participants are shown in Table 1. The mean change from pretreatment (baseline) scores for each measure in patients treated with surgery is shown in Table 2, and the mean change from pretreatment scores in patients who elected active surveillance is shown in Table 3 (in both tables, a negative score denotes worsened function, and a positive change denotes improvement).
After surgery, patients reported significantly lower erectile function and sexual satisfaction scores. These included statistically significant decreases for IIEF Erectile Function, IIEF Overall Satisfaction, PROMIS Sexual Satisfaction, PROMIS Sexual Interest, and PROMIS Orgasm. In patients treated with surgery, there were significant improvements in anxiety observed for patients at each follow-up time, whereas significantly worse bladder problems were observed on SOMS Bladder at 1 and 3 months but returned to baseline by 12 months after surgery. AUASS was worse at 1 month but significantly improved at 6 and 12 months. Fatigue scores significantly worsened at 1 month but were no longer significant at 6 and 12 months. Physical Function was worsened at 1 month but not throughout the rest of the study. Bowel Problems (SOMS) were significantly worse at 1 month, but changes became nonsignificant on subsequent assessments. The only 2 domains that did not demonstrate any significant changes o
In active surveillance patients, sexual function domains were generally unchanged over the course of the study. However, unlike treated patients, there was no significant improvement in anxiety, depression, pain, fatigue, or sleep. In fact, most of these domains demonstrated worsened functioning, although these were not statistically significant. Urinary domains generally remained unchanged.
Pearson correlation coefficients between HRQoL measures and overall treatment satisfaction (assessed by the question, Are you satisfied with the results of your operation?) at each follow-up time point in patients treated with surgery are shown in Table 4. Relations between treatment satisfaction and sexual outcomes were generally statistically insignificant (r, .08-.56). However, sleep disturbance, depression, pain interference, fatigue, embarrassment, and bladder problems all demonstrated statistically significant positive associations with treatment satisfaction, with coefficients ranging from small to medium in magnitude (r, .32-.61). Other outcomes such as anxiety, physical function, and bowel problems demonstrated small to medium statistically significant associations with treatment satisfaction (r, .04-.60) but not at every time point. We performed t tests to examine treatment satisfaction in patients with detectable initial posttreatment prostate-specific antigen (PSA; >0.01 ng/mL). We found no difference in treatment satisfaction between patients with detectable PSA values and those with undetectable PSA at each time point.
When the patients were asked, Compared with what you expected, how do you rate the results of your operation?, most of those treated with surgery reported that the results of their operation were better than they had expected (Figure 1A; p. e137). More than 75% of the patients had results that were as expected or better than expected. When asked, Compared with what you expected, how do you rate your side effects of the operation?, almost 70% of patients reported side effects no worse than expected (Figure 1B). When asked, Are you satisfied with the results of your operation?, most patients reported that overall, they were satisfied with the results of their operation (Figure 1C).
At 12 months, none of the patients reported overall dissatisfaction with their treatment choice. More than 90% of patients were mostly or completely satisfied with the results of their operation.
Discussion
This prospective study assessed the HRQoL from pretreatment through 12 months posttreatment in men diagnosed with clinically localized prostate cancer that had been treated with surgery. Although the indicators of sexual function significantly decreased over time, they were not meaningfully associated with overall treatment satisfaction. Instead, a host of other factors, including psychosocial (eg, anxiety, depression, body image dissatisfaction, embarrassment), nonurological physical symptoms (pain interference, physical function, sleep disturbance, fatigue), and bladder problems, were significantly related to overall treatment satisfaction. Although this may not be surprising in other clinical oncology paradigms, the sheer surfeit of focus and attention on sexual function has overshadowed aspects of HRQoL that many men report are important to them, despite worsened sexual function outcomes.
Understanding potential treatment-related changes in HRQoL can be challenging for men when choosing providers and different therapeutic options. The increasing complexity of treatment in prostate cancer has created an opportunity to not only understand efficacy on cancer control but also focus on meaningful patient-reported outcomes. Hospitals and medical groups are increasingly aware of the importance of improving the patient care experience. Objective measures of patient satisfaction for health care providers, such as the Press-Ganey and Net Promoter score, exist to measure and improve patient experience. In prostate cancer, clinicians and large groups, including governmental agencies such as the US Preventive Services Task Force, have often focused on declines in urinary and erectile function15 without considering the full impact of prostate cancer treatment on global HRQoL. Our study was a prospective, longitudinal, self-reported examination of the impact, positive and negative, of prostate cancer treatment over a 12-month period.
Numerous studies have documented the treatment-related side effects of erectile, urinary, and bowel dysfunction in patients treated for prostate cancer, which may occur after definitive local therapies.5,16-18 The present study shows a similar impact on urinary, bowel, and erectile domains after treatment. Although erectile function scores remained lower through the course of the 12-month study, bowel and bladder domains returned to baseline by month 12. Unlike other studies, we also examined psychosocial and nonurological aspects of prostate cancer treatment. We found that there was a measurable and significant positive impact on other HRQoL measurements such as decreased anxiety. Despite a variety of declines across HRQoL domains, most patients reported that their results were largely as they had expected, and their side effects were the same or better than they had expected. No patient in the cohort reported being dissatisfied with his overall treatment, and more than 90% of patients were mostly or completely satisfied with their treatment choice. This highlights the point that while sexual and other urological domains of HRQoL are important, impairments in these areas do not necessarily reflect how many patients perceive success or satisfaction with their treatment choice. We also showed correlations between treatment satisfaction and improvement in sleep, anxiety, depression, and fatigue. It is worth noting that although there were decreases in the erectile and sexual function domains after treatment, those factors were not correlated with overall treatment satisfaction. Those factors may not routinely be assessed before, during, and after treatment for prostate cancer in most clinical encounters. However, because they were strongly associated with satisfaction with treatment outcomes in this study, identification in impairments may lead to opportunities to intervene and improve the patient experience. Therefore, important “teachable moments” may be missed (for both patients and providers) during treatment decision-making encounters if other factors beyond sexual and urological outcomes are not adequately considered and addressed. Furthermore, the results of our study may help clinicians counsel patients on their expectations for their recovery after surgery and identify particular issues related to HRQoL to pay close attention to in follow-up visits.
Strengths of our study include its prospective nature, which allowed evaluation of HRQoL outcomes at multiple time points throughout the first year after treatment. In addition, we used existing patient-reported outcome tools validated by the NIH to assess changes in HRQoL. PROMIS is an NIH-supported tool that can be leveraged in the pre- and posttreatment periods to identify patients who have impairments with HRQoL. It can provide clinicians with a unique opportunity to detect and intervene in setbacks and side effects to improve patient satisfaction and HRQoL.
Limitations of the current study include that most patients selected surgery for their treatment choice and that not all patients completed all longitudinal questionnaires, although this is expected in longitudinal studies of this nature. Although all the patients were approached and encouraged to participate, many did not participate and were not captured. In addition, not all patients completed end-of-study surveys. These factors may have biased our results because of unmeasurable factors related to nonparticipation or dropout. Our study encompassed the preoperative period up to 12 months postoperatively, which may fail to identify improvements or declines in HRQoL that may occur more than 12 months postoperatively, particularly related to continence and erectile function. The participants were enrolled by 6 surgeons, and we were not able to standardize the preoperative counseling either preoperatively or postoperatively, which may have biased our results. Finally, our study population consisted of predominantly white, married men of higher socioeconomic status; therefore, our results may not be generalizable to newly diagnosed prostate cancer patients overall.
Conclusions
By using validated self-administered questionnaires, we found that despite decreased sexual and urinary function, patients treated for prostate cancer were satisfied with their treatment choice. Correlates to higher patient satisfaction included decreased anxiety, depression, fatigue, and sleep disturbances.
More than 164,690 men are expected to be diagnosed with prostate cancer in the United States in 2018.1 Men with prostate cancer face not only stress associated with the diagnosis but also decisional conflict regarding different treatment options.2 Most men diagnosed with clinically localized prostate cancer receive 1 or more of the following treatments: radical prostatectomy, external-beam radiation therapy, and/or brachytherapy, all of which are associated with posttreatment urological or sexual side effects including bowel, urinary, or erectile dysfunction.3-5 Men who choose active surveillance may experience increased anxiety associated with the constant vigilance and monitoring of their tumor status along with the uncertainty of not definitively removing or radiating their prostate.6 In addition to direct functional limitations of sexual and urological side effects, treatment can also lead to secondary psychosocial effects, including depression, self-blame, embarrassment, guilt, lower masculine self-esteem, increased reticence to participate socially or engage in sexual activity, and relationship distress.7-9 Therefore, health-related quality of life (HRQoL) and treatment satisfaction are important for this population.
Urological and sexual side effects of prostate cancer treatments are often a primary focus during treatment decision making between patients and providers. However, little prospective empirical data exist regarding the role of HRQoL and other nonurological physical and psychosocial outcomes on overall treatment satisfaction. The purpose of this study was to prospectively evaluate the role of both urological and nonurological outcomes on overall treatment satisfaction in men diagnosed with prostate cancer. We hypothesize that such an understanding can help describe changes in physical and psychosocial factors that are important to men beyond traditional urological outcomes, including their association with overall treatment satisfaction.
Methods
This was a prospective longitudinal assessment of patients from the Department of Urology at Northwestern University’s Feinberg School of Medicine in Chicago. Patients were eligible if they met the following inclusion criteria: they had been diagnosed with clinically localized or locally advanced prostate cancer; they had not yet received a primary treatment (eg, surgery, radiation, active surveillance) before their baseline assessment; they were 18 years or older; and they were able to read, write, speak, and understand English. Patients were excluded if they had a physical debilitation that would make participation not feasible or would create undue hardship, or if they had a history of diagnosed severe mental illness or hospitalization for chronic psychiatric reasons, as identified by referring physicians.
Eligible participants were approached before their treatment decision (if any). Patient enrollment occurred in 2 ways. For patients invited to participate during their clinic visit, the research assistant explained the study and obtained written informed consent for interested patients. A unique user identification and password was created for each patient, and they practiced using the touch screen computer while the research assistant observed and provided guidance as needed. When the patients were ready to start their pretreatment online interview, they completed the questionnaires by themselves. For patients who were invited to participate but were not scheduled to return in the foreseeable future, enrollment was carried out differently. In those cases, participating physicians contacted eligible patients who were not scheduled for a visit and informed them of the study opportunity. Interested patients were contacted by the research assistant who provided them with the study website address, which directed them to the online consent form. After a patient had completed the consent form, he was prompted to self-register. He received a unique user identification and password that could be used to complete the baseline assessment and subsequent assessments. However, for interested patients who did not have access to a computer or Internet connection, the research assistant provided them with paper consent forms and paper versions of all study assessments. After participants had completed the baseline assessment, the research assistant provided them with a written schedule of future assessments, which were expected to occur at 1 month posttreatment, 3 months posttreatment, 6 months posttreatment, and 12 months posttreatment.
For all follow-up appointments, participants could complete assessments either at clinic visits or from home using a secure online assessment platform called Assessment Center.10 The research assistant used a patient log to track participants and their progress in the study, which included study number, patient name (or initials), registration date, date of birth, sex, and timeline of completed or future assessments. The research assistant called or emailed participants (depending on patient preference) about a week before each of their follow-up assessments to facilitate adherence. If the participant did not log into the system by the target day, the research assistant contacted him the following day (target day +1) with a phone or email reminder to log into the system and complete the assessments. If the participant did not log in by midnight 1 day after the target day, the research assistant attempted to contact him one last time (target day +2) with either a reminder to log into the system or to ascertain his status that might be related to his noncompletion. Overall, a participant was called or e-mailed 1 to 3 times to remind him of his assessment. If he was unresponsive after 3 attempts, he was recorded as having withdrawn for an unknown reason.
At baseline and each follow-up time point, study participants completed a battery of patient-reported outcome measures, with most coming from the Patient-Reported Outcomes Measurement Information System (PROMIS)11 and the Surgical Outcomes Measurement System (SOMS).12 PROMIS is a National Institutes of Health (NIH) funded measurement system that has helped standardize and improve self-reported assessment of health status, symptoms, side effects, and different aspects of HRQoL, including physical, emotional, cognitive, and social health. SOMS is a suite of patient-reported outcome measures assessing important aspects of HRQoL after surgery. It was developed with feedback from surgeons, postoperative patients, and surgical nurses. PROMIS items were directly incorporated into numerous SOMS measures to facilitate easier comparisons and score crosswalks across measures and patient populations. In addition to PROMIS and SOMS measures, we also administered several well-known instruments of urological and sexual function, including the International Index of Erectile Function (IIEF) and American Urological Association Symptom Score Index (AUASS).13,14
Outcome measures were compared across sociodemographic and clinical variables at each time point using t tests for numerical variables (age) and with chi-square or Fisher exact tests for categorical variables; those variables with significant differences were used as covariates in statistical models. To examine differences in patient-reported scores over time, we used repeated measures analysis of covariance with general linear modeling methods. We used Pearson correlation coefficients to evaluate for correlations between quality-of-life outcomes and treatment satisfaction.
Not all participants completed each of the follow-up surveys, and reasons for dropout were prospectively documented. Most participants elected surgical resection as their primary treatment compared with the fewer than 10% of patients who chose radiation or chemotherapy as their primary treatment and about 20% of men who chose active surveillance after their initial diagnosis. Therefore, our analysis focused on patients who elected surgical resection. For comparison purposes, we included the HRQoL results from active surveillance patients.
Results
A total of 105 patients diagnosed with prostate cancer were enrolled in the study. Response rates decreased throughout the study (n = 75 at 1 month; n = 71 at 3 months; n = 64 at 6 months; n = 54 at 12 months). Sociodemographic and clinical characteristics of participants are shown in Table 1. The mean change from pretreatment (baseline) scores for each measure in patients treated with surgery is shown in Table 2, and the mean change from pretreatment scores in patients who elected active surveillance is shown in Table 3 (in both tables, a negative score denotes worsened function, and a positive change denotes improvement).
After surgery, patients reported significantly lower erectile function and sexual satisfaction scores. These included statistically significant decreases for IIEF Erectile Function, IIEF Overall Satisfaction, PROMIS Sexual Satisfaction, PROMIS Sexual Interest, and PROMIS Orgasm. In patients treated with surgery, there were significant improvements in anxiety observed for patients at each follow-up time, whereas significantly worse bladder problems were observed on SOMS Bladder at 1 and 3 months but returned to baseline by 12 months after surgery. AUASS was worse at 1 month but significantly improved at 6 and 12 months. Fatigue scores significantly worsened at 1 month but were no longer significant at 6 and 12 months. Physical Function was worsened at 1 month but not throughout the rest of the study. Bowel Problems (SOMS) were significantly worse at 1 month, but changes became nonsignificant on subsequent assessments. The only 2 domains that did not demonstrate any significant changes o
In active surveillance patients, sexual function domains were generally unchanged over the course of the study. However, unlike treated patients, there was no significant improvement in anxiety, depression, pain, fatigue, or sleep. In fact, most of these domains demonstrated worsened functioning, although these were not statistically significant. Urinary domains generally remained unchanged.
Pearson correlation coefficients between HRQoL measures and overall treatment satisfaction (assessed by the question, Are you satisfied with the results of your operation?) at each follow-up time point in patients treated with surgery are shown in Table 4. Relations between treatment satisfaction and sexual outcomes were generally statistically insignificant (r, .08-.56). However, sleep disturbance, depression, pain interference, fatigue, embarrassment, and bladder problems all demonstrated statistically significant positive associations with treatment satisfaction, with coefficients ranging from small to medium in magnitude (r, .32-.61). Other outcomes such as anxiety, physical function, and bowel problems demonstrated small to medium statistically significant associations with treatment satisfaction (r, .04-.60) but not at every time point. We performed t tests to examine treatment satisfaction in patients with detectable initial posttreatment prostate-specific antigen (PSA; >0.01 ng/mL). We found no difference in treatment satisfaction between patients with detectable PSA values and those with undetectable PSA at each time point.
When the patients were asked, Compared with what you expected, how do you rate the results of your operation?, most of those treated with surgery reported that the results of their operation were better than they had expected (Figure 1A; p. e137). More than 75% of the patients had results that were as expected or better than expected. When asked, Compared with what you expected, how do you rate your side effects of the operation?, almost 70% of patients reported side effects no worse than expected (Figure 1B). When asked, Are you satisfied with the results of your operation?, most patients reported that overall, they were satisfied with the results of their operation (Figure 1C).
At 12 months, none of the patients reported overall dissatisfaction with their treatment choice. More than 90% of patients were mostly or completely satisfied with the results of their operation.
Discussion
This prospective study assessed the HRQoL from pretreatment through 12 months posttreatment in men diagnosed with clinically localized prostate cancer that had been treated with surgery. Although the indicators of sexual function significantly decreased over time, they were not meaningfully associated with overall treatment satisfaction. Instead, a host of other factors, including psychosocial (eg, anxiety, depression, body image dissatisfaction, embarrassment), nonurological physical symptoms (pain interference, physical function, sleep disturbance, fatigue), and bladder problems, were significantly related to overall treatment satisfaction. Although this may not be surprising in other clinical oncology paradigms, the sheer surfeit of focus and attention on sexual function has overshadowed aspects of HRQoL that many men report are important to them, despite worsened sexual function outcomes.
Understanding potential treatment-related changes in HRQoL can be challenging for men when choosing providers and different therapeutic options. The increasing complexity of treatment in prostate cancer has created an opportunity to not only understand efficacy on cancer control but also focus on meaningful patient-reported outcomes. Hospitals and medical groups are increasingly aware of the importance of improving the patient care experience. Objective measures of patient satisfaction for health care providers, such as the Press-Ganey and Net Promoter score, exist to measure and improve patient experience. In prostate cancer, clinicians and large groups, including governmental agencies such as the US Preventive Services Task Force, have often focused on declines in urinary and erectile function15 without considering the full impact of prostate cancer treatment on global HRQoL. Our study was a prospective, longitudinal, self-reported examination of the impact, positive and negative, of prostate cancer treatment over a 12-month period.
Numerous studies have documented the treatment-related side effects of erectile, urinary, and bowel dysfunction in patients treated for prostate cancer, which may occur after definitive local therapies.5,16-18 The present study shows a similar impact on urinary, bowel, and erectile domains after treatment. Although erectile function scores remained lower through the course of the 12-month study, bowel and bladder domains returned to baseline by month 12. Unlike other studies, we also examined psychosocial and nonurological aspects of prostate cancer treatment. We found that there was a measurable and significant positive impact on other HRQoL measurements such as decreased anxiety. Despite a variety of declines across HRQoL domains, most patients reported that their results were largely as they had expected, and their side effects were the same or better than they had expected. No patient in the cohort reported being dissatisfied with his overall treatment, and more than 90% of patients were mostly or completely satisfied with their treatment choice. This highlights the point that while sexual and other urological domains of HRQoL are important, impairments in these areas do not necessarily reflect how many patients perceive success or satisfaction with their treatment choice. We also showed correlations between treatment satisfaction and improvement in sleep, anxiety, depression, and fatigue. It is worth noting that although there were decreases in the erectile and sexual function domains after treatment, those factors were not correlated with overall treatment satisfaction. Those factors may not routinely be assessed before, during, and after treatment for prostate cancer in most clinical encounters. However, because they were strongly associated with satisfaction with treatment outcomes in this study, identification in impairments may lead to opportunities to intervene and improve the patient experience. Therefore, important “teachable moments” may be missed (for both patients and providers) during treatment decision-making encounters if other factors beyond sexual and urological outcomes are not adequately considered and addressed. Furthermore, the results of our study may help clinicians counsel patients on their expectations for their recovery after surgery and identify particular issues related to HRQoL to pay close attention to in follow-up visits.
Strengths of our study include its prospective nature, which allowed evaluation of HRQoL outcomes at multiple time points throughout the first year after treatment. In addition, we used existing patient-reported outcome tools validated by the NIH to assess changes in HRQoL. PROMIS is an NIH-supported tool that can be leveraged in the pre- and posttreatment periods to identify patients who have impairments with HRQoL. It can provide clinicians with a unique opportunity to detect and intervene in setbacks and side effects to improve patient satisfaction and HRQoL.
Limitations of the current study include that most patients selected surgery for their treatment choice and that not all patients completed all longitudinal questionnaires, although this is expected in longitudinal studies of this nature. Although all the patients were approached and encouraged to participate, many did not participate and were not captured. In addition, not all patients completed end-of-study surveys. These factors may have biased our results because of unmeasurable factors related to nonparticipation or dropout. Our study encompassed the preoperative period up to 12 months postoperatively, which may fail to identify improvements or declines in HRQoL that may occur more than 12 months postoperatively, particularly related to continence and erectile function. The participants were enrolled by 6 surgeons, and we were not able to standardize the preoperative counseling either preoperatively or postoperatively, which may have biased our results. Finally, our study population consisted of predominantly white, married men of higher socioeconomic status; therefore, our results may not be generalizable to newly diagnosed prostate cancer patients overall.
Conclusions
By using validated self-administered questionnaires, we found that despite decreased sexual and urinary function, patients treated for prostate cancer were satisfied with their treatment choice. Correlates to higher patient satisfaction included decreased anxiety, depression, fatigue, and sleep disturbances.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7‐30.
2. Berry DL, Ellis WJ, Woods NF, Schwien C, Mullen KH, Yang C. Treatment decision-making by men with localized prostate cancer: the influence of personal factors. Urol Oncol. 2003;21(2):93-100.
3. Dubbelman YD, Dohle GR, Schröder FH. Sexual function before and after radical retropubic prostatectomy: a systematic review of prognostic indicators for a successful outcome. Eur Urol. 2006;50(4):711-718; discussion 718-720.
4. McCullough AR. Sexual dysfunction after radical prostatectomy. Rev Urol. 2005;7(2 suppl):S3-S10.
5. Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358(12):1250-1261.
6. Latini DM, Hart SL, Knight SJ, et al. The relationship between anxiety and time to treatment for patients with prostate cancer on surveillance. J Urol. 2007;178(3, pt 1):826-831; discussion 831-832.
7. Meyer JP, Gillatt DA, Lockyer R, Macdonagh R. The effect of erectile dysfunction on the quality of life of men after radical prostatectomy. BJU Int. 2003;92(9):929-931.
8. Casey RG, Corcoran NM, Goldenberg SL. Quality of life issues in men undergoing androgen deprivation therapy: a review. Asian J Androl. 2012;14(2):226-231.
9. Segrin C, Badger TA, Harrington J. Interdependent psychological quality of life in dyads adjusting to prostate cancer. Health Psychol. 2012;31(1):70-79.
10. Gershon RC, Rothrock N, Hanrahan R, Bass M, Cella D. The use of PROMIS and assessment center to deliver patient-reported outcome measures in clinical research. J Appl Meas. 2010;11(3):304-314.
11. Cella D, Yount S, Rothrock N, et al. The patient-reported outcomes measurement information system (PROMIS): progress of an NIH roadmap cooperative group during its first two years. Med Care. 2007;45(5 suppl 1):S3-S11.
12. Zapf M, Denham W, Barrera E, et al. Patient-centered outcomes after laparoscopic cholecystectomy. Surg Endosc. 2013;27(12):4491-4498.
13. Barry MJ, Fowler FJ Jr, O'Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148(5):1549-1557; discussion 1564.
14. Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49(6):822-830.
15. United States Preventive Services Task Force. Final update summary: prostate cancer: screening. http:// www.uspreventiveservicestaskforce.org/Page/ Document/UpdateSummaryFinal/prostate-cancer-screening. Updated July 2015. Accessed April 14, 2017
16. Litwin MS, Gore JL, Kwan L, et al. Quality of life after surgery, external beam irradiation, or brachytherapy for early-stage prostate cancer. Cancer. 2007;109(11):2239-2247.
17. Miwa S, Mizokami A, Konaka H, et al. Prospective longitudinal comparative study of health-related quality of life and treatment satisfaction in patients treated with hormone therapy, radical retropubic prostatectomy, and high or low dose rate brachytherapy for prostate cancer. Prostate Int. 2013;1(3):117-124.
18. Miller DC, Sanda MG, Dunn RL et al. Long-term outcomes among localized prostate cancer survivors: health-related quality-of-life changes after radical prostatectomy, external radiation, and brachytherapy. J Clin Oncol. 2005;23(12):2772-2780.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7‐30.
2. Berry DL, Ellis WJ, Woods NF, Schwien C, Mullen KH, Yang C. Treatment decision-making by men with localized prostate cancer: the influence of personal factors. Urol Oncol. 2003;21(2):93-100.
3. Dubbelman YD, Dohle GR, Schröder FH. Sexual function before and after radical retropubic prostatectomy: a systematic review of prognostic indicators for a successful outcome. Eur Urol. 2006;50(4):711-718; discussion 718-720.
4. McCullough AR. Sexual dysfunction after radical prostatectomy. Rev Urol. 2005;7(2 suppl):S3-S10.
5. Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358(12):1250-1261.
6. Latini DM, Hart SL, Knight SJ, et al. The relationship between anxiety and time to treatment for patients with prostate cancer on surveillance. J Urol. 2007;178(3, pt 1):826-831; discussion 831-832.
7. Meyer JP, Gillatt DA, Lockyer R, Macdonagh R. The effect of erectile dysfunction on the quality of life of men after radical prostatectomy. BJU Int. 2003;92(9):929-931.
8. Casey RG, Corcoran NM, Goldenberg SL. Quality of life issues in men undergoing androgen deprivation therapy: a review. Asian J Androl. 2012;14(2):226-231.
9. Segrin C, Badger TA, Harrington J. Interdependent psychological quality of life in dyads adjusting to prostate cancer. Health Psychol. 2012;31(1):70-79.
10. Gershon RC, Rothrock N, Hanrahan R, Bass M, Cella D. The use of PROMIS and assessment center to deliver patient-reported outcome measures in clinical research. J Appl Meas. 2010;11(3):304-314.
11. Cella D, Yount S, Rothrock N, et al. The patient-reported outcomes measurement information system (PROMIS): progress of an NIH roadmap cooperative group during its first two years. Med Care. 2007;45(5 suppl 1):S3-S11.
12. Zapf M, Denham W, Barrera E, et al. Patient-centered outcomes after laparoscopic cholecystectomy. Surg Endosc. 2013;27(12):4491-4498.
13. Barry MJ, Fowler FJ Jr, O'Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148(5):1549-1557; discussion 1564.
14. Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49(6):822-830.
15. United States Preventive Services Task Force. Final update summary: prostate cancer: screening. http:// www.uspreventiveservicestaskforce.org/Page/ Document/UpdateSummaryFinal/prostate-cancer-screening. Updated July 2015. Accessed April 14, 2017
16. Litwin MS, Gore JL, Kwan L, et al. Quality of life after surgery, external beam irradiation, or brachytherapy for early-stage prostate cancer. Cancer. 2007;109(11):2239-2247.
17. Miwa S, Mizokami A, Konaka H, et al. Prospective longitudinal comparative study of health-related quality of life and treatment satisfaction in patients treated with hormone therapy, radical retropubic prostatectomy, and high or low dose rate brachytherapy for prostate cancer. Prostate Int. 2013;1(3):117-124.
18. Miller DC, Sanda MG, Dunn RL et al. Long-term outcomes among localized prostate cancer survivors: health-related quality-of-life changes after radical prostatectomy, external radiation, and brachytherapy. J Clin Oncol. 2005;23(12):2772-2780.
Rural cancer patients report faster care than urban counterparts
in a survey of 6,826 Medicare beneficiaries.
Taken as a whole, a similar quality of care was reported between the two groups, but the picture changed when racial/ethnic subgroups were considered. Non-Hispanic black and Hispanic patients in rural locations reported inferior care to their urban counterparts, investigators wrote in Cancer.
“Cancer patients living in rural areas are vulnerable and have unique health care needs,” wrote lead author Michelle A. Mollica, PhD, of the National Cancer Institute, and her colleagues. “To our knowledge, this is the first study to explore the patient’s perception of the timeliness of care in such a large, multiregion sample of cancer patients.”
In 2003, the National Academy of Medicine concluded that living in a rural environment was associated with poorer health. Existing research surrounding cancer has echoed this concern, showing that rural patients have higher rates of cancer and mortality, longer delays in diagnosis, and limited access to care.
The current, retrospective study involved 6,140 urban and 686 rural Medicare beneficiaries who were aged at least 65 years when diagnosed with either breast, lung, colorectal, or prostate cancer. Consumer Assessment of Healthcare Providers and Systems surveys were conducted between 1998 and 2013, then linked with data from the Surveillance, Epidemiology, and End Results registry program.
Surveys were conducted within 12 months of diagnosis, during which time patients were asked about their access to care as defined by two composites: “Getting Needed Care” and “Getting Care Quickly.” Getting Needed Care included ease of making appointments and receiving treatments and Getting Care Quickly questions asked about appointment delays and time spent waiting at the doctor’s office. Answers were converted to a numerical score from 0 to 100, with 0 being the worst and 100 being the best.
For both composites, mean scores for urban and rural locations were greater than 85 out of 100.
In contrast to previous studies, urban patients reported longer delays in care, scoring Getting Care Quickly 2.27 points lower than rural patients (P = .02). Pacific Islanders and non-Hispanic Asian patients from rural places reported even faster care, ranking about 8 points higher than urban patients of the same race/ethnicity.
Locality did not have a significant impact on Getting Needed Care unless race/ethnicity was also considered (P = .04). Non-Hispanic white patients from rural locations scored Getting Needed Care about 2 points higher than urban white patients, while Hispanic and non-Hispanic black patients had an opposite trend, with this rural cohort ranking Getting Needed Care lower than urban patients of the same race/ethnicity.
“Geographic residence is but one important factor in cancer care delivery,” the authors noted. “There is a need for fine-grained research looking at specific barriers for urban residents, experiences of racial/ethnic minority survivors residing in rural areas, and rural-urban differences in the clinic settings in which medical care is delivered.”
The authors had no disclosures to report.
SOURCE: Mollica MA et al. Cancer. 2018 Jun 7. doi: 10.1002/cncr.31541.
in a survey of 6,826 Medicare beneficiaries.
Taken as a whole, a similar quality of care was reported between the two groups, but the picture changed when racial/ethnic subgroups were considered. Non-Hispanic black and Hispanic patients in rural locations reported inferior care to their urban counterparts, investigators wrote in Cancer.
“Cancer patients living in rural areas are vulnerable and have unique health care needs,” wrote lead author Michelle A. Mollica, PhD, of the National Cancer Institute, and her colleagues. “To our knowledge, this is the first study to explore the patient’s perception of the timeliness of care in such a large, multiregion sample of cancer patients.”
In 2003, the National Academy of Medicine concluded that living in a rural environment was associated with poorer health. Existing research surrounding cancer has echoed this concern, showing that rural patients have higher rates of cancer and mortality, longer delays in diagnosis, and limited access to care.
The current, retrospective study involved 6,140 urban and 686 rural Medicare beneficiaries who were aged at least 65 years when diagnosed with either breast, lung, colorectal, or prostate cancer. Consumer Assessment of Healthcare Providers and Systems surveys were conducted between 1998 and 2013, then linked with data from the Surveillance, Epidemiology, and End Results registry program.
Surveys were conducted within 12 months of diagnosis, during which time patients were asked about their access to care as defined by two composites: “Getting Needed Care” and “Getting Care Quickly.” Getting Needed Care included ease of making appointments and receiving treatments and Getting Care Quickly questions asked about appointment delays and time spent waiting at the doctor’s office. Answers were converted to a numerical score from 0 to 100, with 0 being the worst and 100 being the best.
For both composites, mean scores for urban and rural locations were greater than 85 out of 100.
In contrast to previous studies, urban patients reported longer delays in care, scoring Getting Care Quickly 2.27 points lower than rural patients (P = .02). Pacific Islanders and non-Hispanic Asian patients from rural places reported even faster care, ranking about 8 points higher than urban patients of the same race/ethnicity.
Locality did not have a significant impact on Getting Needed Care unless race/ethnicity was also considered (P = .04). Non-Hispanic white patients from rural locations scored Getting Needed Care about 2 points higher than urban white patients, while Hispanic and non-Hispanic black patients had an opposite trend, with this rural cohort ranking Getting Needed Care lower than urban patients of the same race/ethnicity.
“Geographic residence is but one important factor in cancer care delivery,” the authors noted. “There is a need for fine-grained research looking at specific barriers for urban residents, experiences of racial/ethnic minority survivors residing in rural areas, and rural-urban differences in the clinic settings in which medical care is delivered.”
The authors had no disclosures to report.
SOURCE: Mollica MA et al. Cancer. 2018 Jun 7. doi: 10.1002/cncr.31541.
in a survey of 6,826 Medicare beneficiaries.
Taken as a whole, a similar quality of care was reported between the two groups, but the picture changed when racial/ethnic subgroups were considered. Non-Hispanic black and Hispanic patients in rural locations reported inferior care to their urban counterparts, investigators wrote in Cancer.
“Cancer patients living in rural areas are vulnerable and have unique health care needs,” wrote lead author Michelle A. Mollica, PhD, of the National Cancer Institute, and her colleagues. “To our knowledge, this is the first study to explore the patient’s perception of the timeliness of care in such a large, multiregion sample of cancer patients.”
In 2003, the National Academy of Medicine concluded that living in a rural environment was associated with poorer health. Existing research surrounding cancer has echoed this concern, showing that rural patients have higher rates of cancer and mortality, longer delays in diagnosis, and limited access to care.
The current, retrospective study involved 6,140 urban and 686 rural Medicare beneficiaries who were aged at least 65 years when diagnosed with either breast, lung, colorectal, or prostate cancer. Consumer Assessment of Healthcare Providers and Systems surveys were conducted between 1998 and 2013, then linked with data from the Surveillance, Epidemiology, and End Results registry program.
Surveys were conducted within 12 months of diagnosis, during which time patients were asked about their access to care as defined by two composites: “Getting Needed Care” and “Getting Care Quickly.” Getting Needed Care included ease of making appointments and receiving treatments and Getting Care Quickly questions asked about appointment delays and time spent waiting at the doctor’s office. Answers were converted to a numerical score from 0 to 100, with 0 being the worst and 100 being the best.
For both composites, mean scores for urban and rural locations were greater than 85 out of 100.
In contrast to previous studies, urban patients reported longer delays in care, scoring Getting Care Quickly 2.27 points lower than rural patients (P = .02). Pacific Islanders and non-Hispanic Asian patients from rural places reported even faster care, ranking about 8 points higher than urban patients of the same race/ethnicity.
Locality did not have a significant impact on Getting Needed Care unless race/ethnicity was also considered (P = .04). Non-Hispanic white patients from rural locations scored Getting Needed Care about 2 points higher than urban white patients, while Hispanic and non-Hispanic black patients had an opposite trend, with this rural cohort ranking Getting Needed Care lower than urban patients of the same race/ethnicity.
“Geographic residence is but one important factor in cancer care delivery,” the authors noted. “There is a need for fine-grained research looking at specific barriers for urban residents, experiences of racial/ethnic minority survivors residing in rural areas, and rural-urban differences in the clinic settings in which medical care is delivered.”
The authors had no disclosures to report.
SOURCE: Mollica MA et al. Cancer. 2018 Jun 7. doi: 10.1002/cncr.31541.
FROM CANCER
Key clinical point: Cancer patients living in rural areas reported more timely care than urban patients.
Major finding: In a Consumer Assessment of Healthcare Providers and Systems (CAHPS) survey, urban patients rated “Getting Care Quickly” 2.27 points lower than rural patients (P = .02).
Study details: A retrospective study of 6,140 urban and 686 rural Medicare beneficiaries who were aged at least 65 years when diagnosed with either breast, lung, colorectal, or prostate cancer. CAHPS patient experience surveys were conducted between 1998 and 2013, then linked with Surveillance, Epidemiology, and End Results data.
Disclosures: The authors had no disclosures to report.
Source: Mollica MA et al. Cancer. 2018 Jun 7. doi: 10.1002/cncr.31541.