User login
Multidisciplinary treatment planning in elderly patients with cancer: a prospective observational study
Background Elderly cancer patients are a special population, and their management should include specialists in oncology, geriatrics, palliative care, and social work. Based on this approach, we designed a multidisciplinary care model (MCM) and prospectively assessed its results.
Objectives To evaluate the applicability of the MCM, to describe the geriatric features of our sample, and to assess the impact of the MCM on treatment choices.
Methods Patients older than 69 years of age with solid tumors were included. The MCM included the following decision algorithm: Patients with an unequivocal condition of frailty, assessed in the corresponding tumor committee, were directly referred to the palliative care team (Group A). In the other cases (Group B), patients over age 79 years underwent the Comprehensive Geriatric Assessment (CGA) and patients aged between 70 and 79 years completed a frailty test. If the frailty test was positive, CGA was also per formed.
Results 295 patients meeting the inclusion criteria were identified during one year. 186 (63%) were included in the MCM. A total of 66 CGA were performed. CGA modified the therapeutic plan in 5 patients older than 80 (13.8%), and in 2 septuagenarian patients (6.6%).
Limitations This study was designed to evaluate the feasibility of a multidisciplinary approach in geriatric oncology patients in a real clinical setting. Therefore, some variables were not fully controlled in the design, such as the willingness of different specialists to refer their patients to the model.
Conclusions MCM in elderly oncology patients is feasible in a general hospital, although several reasons often hinder patient recruitment for this kind of program. CGA can modify the therapeutic plan, especially in the octogenarian population.
Funding/sponsorship This study has been financially supported by a grant from the Fundació Joan Costa Romà.
Click on the PDF icon at the top of this introduction to read the full article.
Background Elderly cancer patients are a special population, and their management should include specialists in oncology, geriatrics, palliative care, and social work. Based on this approach, we designed a multidisciplinary care model (MCM) and prospectively assessed its results.
Objectives To evaluate the applicability of the MCM, to describe the geriatric features of our sample, and to assess the impact of the MCM on treatment choices.
Methods Patients older than 69 years of age with solid tumors were included. The MCM included the following decision algorithm: Patients with an unequivocal condition of frailty, assessed in the corresponding tumor committee, were directly referred to the palliative care team (Group A). In the other cases (Group B), patients over age 79 years underwent the Comprehensive Geriatric Assessment (CGA) and patients aged between 70 and 79 years completed a frailty test. If the frailty test was positive, CGA was also per formed.
Results 295 patients meeting the inclusion criteria were identified during one year. 186 (63%) were included in the MCM. A total of 66 CGA were performed. CGA modified the therapeutic plan in 5 patients older than 80 (13.8%), and in 2 septuagenarian patients (6.6%).
Limitations This study was designed to evaluate the feasibility of a multidisciplinary approach in geriatric oncology patients in a real clinical setting. Therefore, some variables were not fully controlled in the design, such as the willingness of different specialists to refer their patients to the model.
Conclusions MCM in elderly oncology patients is feasible in a general hospital, although several reasons often hinder patient recruitment for this kind of program. CGA can modify the therapeutic plan, especially in the octogenarian population.
Funding/sponsorship This study has been financially supported by a grant from the Fundació Joan Costa Romà.
Click on the PDF icon at the top of this introduction to read the full article.
Background Elderly cancer patients are a special population, and their management should include specialists in oncology, geriatrics, palliative care, and social work. Based on this approach, we designed a multidisciplinary care model (MCM) and prospectively assessed its results.
Objectives To evaluate the applicability of the MCM, to describe the geriatric features of our sample, and to assess the impact of the MCM on treatment choices.
Methods Patients older than 69 years of age with solid tumors were included. The MCM included the following decision algorithm: Patients with an unequivocal condition of frailty, assessed in the corresponding tumor committee, were directly referred to the palliative care team (Group A). In the other cases (Group B), patients over age 79 years underwent the Comprehensive Geriatric Assessment (CGA) and patients aged between 70 and 79 years completed a frailty test. If the frailty test was positive, CGA was also per formed.
Results 295 patients meeting the inclusion criteria were identified during one year. 186 (63%) were included in the MCM. A total of 66 CGA were performed. CGA modified the therapeutic plan in 5 patients older than 80 (13.8%), and in 2 septuagenarian patients (6.6%).
Limitations This study was designed to evaluate the feasibility of a multidisciplinary approach in geriatric oncology patients in a real clinical setting. Therefore, some variables were not fully controlled in the design, such as the willingness of different specialists to refer their patients to the model.
Conclusions MCM in elderly oncology patients is feasible in a general hospital, although several reasons often hinder patient recruitment for this kind of program. CGA can modify the therapeutic plan, especially in the octogenarian population.
Funding/sponsorship This study has been financially supported by a grant from the Fundació Joan Costa Romà.
Click on the PDF icon at the top of this introduction to read the full article.
Outcome of tumor lysis syndrome in pediatric patients with hematologic malignancies – a single-center experience from Pakistan
Background Tumor lysis syndrome (TLS) is serious complication of anticancer chemotherapy, leading to substantial morbidity and mortality in adults and pediatric patients.
Objective To report the incidence and outcomes of TLS in pediatric patients with hematologic malignancies at a center in Pakistan.
Methods Retrospective chart review of 317 pediatric patients with hematologic malignancies during January 2008-December 2013. Demographic features and clinical and laboratory parameters of TLS, with immediate and 6-month outcomes were determined using a semi-structured questionnaire.
Results Median age at diagnosis was 9 years, with the 79.2% patients being male. Laboratory TLS was present in 36 patients (11.4%), with 27 (8.5%) developing clinical TLS and 13 (4.1%) requiring intensive care support. Hyperphosphatemia was the most frequent metabolic abnormality (14.2%), followed by hypocalcemia (13.9%), hyperuricemia (12.6%), and hyperkalemia (1.3%). 45 patients (14.2%) developed acute kidney injury (AKI). Patients who developed TLS had a signficantly higher white blood cell count at initiation of chemotherapy (142.0 x 109/L [SD, 173.1] vs 31.5 x 109/L [SD, 58.0]; P = .01) and a higher incidence of AKI (58.3% vs 8.5% of patients; P < .001).
Limitations Retrospective design of study, high rate of loss to follow-up, and unavailability of lactate dehydrogenase levels in a majority of patients.
Conclusion The incidence of TLS pediatric hematologic malignancies was 11.4% at our center. The main cause of death was sepsis. Hyperphosphatemia was the common metabolic derangement and hyperkalemia was the least common. TLS warrants intensive supportive care to prevent further morbidity and decrease mortality.
Click on the PDF icon at the top of this introduction to read the full article.
Background Tumor lysis syndrome (TLS) is serious complication of anticancer chemotherapy, leading to substantial morbidity and mortality in adults and pediatric patients.
Objective To report the incidence and outcomes of TLS in pediatric patients with hematologic malignancies at a center in Pakistan.
Methods Retrospective chart review of 317 pediatric patients with hematologic malignancies during January 2008-December 2013. Demographic features and clinical and laboratory parameters of TLS, with immediate and 6-month outcomes were determined using a semi-structured questionnaire.
Results Median age at diagnosis was 9 years, with the 79.2% patients being male. Laboratory TLS was present in 36 patients (11.4%), with 27 (8.5%) developing clinical TLS and 13 (4.1%) requiring intensive care support. Hyperphosphatemia was the most frequent metabolic abnormality (14.2%), followed by hypocalcemia (13.9%), hyperuricemia (12.6%), and hyperkalemia (1.3%). 45 patients (14.2%) developed acute kidney injury (AKI). Patients who developed TLS had a signficantly higher white blood cell count at initiation of chemotherapy (142.0 x 109/L [SD, 173.1] vs 31.5 x 109/L [SD, 58.0]; P = .01) and a higher incidence of AKI (58.3% vs 8.5% of patients; P < .001).
Limitations Retrospective design of study, high rate of loss to follow-up, and unavailability of lactate dehydrogenase levels in a majority of patients.
Conclusion The incidence of TLS pediatric hematologic malignancies was 11.4% at our center. The main cause of death was sepsis. Hyperphosphatemia was the common metabolic derangement and hyperkalemia was the least common. TLS warrants intensive supportive care to prevent further morbidity and decrease mortality.
Click on the PDF icon at the top of this introduction to read the full article.
Background Tumor lysis syndrome (TLS) is serious complication of anticancer chemotherapy, leading to substantial morbidity and mortality in adults and pediatric patients.
Objective To report the incidence and outcomes of TLS in pediatric patients with hematologic malignancies at a center in Pakistan.
Methods Retrospective chart review of 317 pediatric patients with hematologic malignancies during January 2008-December 2013. Demographic features and clinical and laboratory parameters of TLS, with immediate and 6-month outcomes were determined using a semi-structured questionnaire.
Results Median age at diagnosis was 9 years, with the 79.2% patients being male. Laboratory TLS was present in 36 patients (11.4%), with 27 (8.5%) developing clinical TLS and 13 (4.1%) requiring intensive care support. Hyperphosphatemia was the most frequent metabolic abnormality (14.2%), followed by hypocalcemia (13.9%), hyperuricemia (12.6%), and hyperkalemia (1.3%). 45 patients (14.2%) developed acute kidney injury (AKI). Patients who developed TLS had a signficantly higher white blood cell count at initiation of chemotherapy (142.0 x 109/L [SD, 173.1] vs 31.5 x 109/L [SD, 58.0]; P = .01) and a higher incidence of AKI (58.3% vs 8.5% of patients; P < .001).
Limitations Retrospective design of study, high rate of loss to follow-up, and unavailability of lactate dehydrogenase levels in a majority of patients.
Conclusion The incidence of TLS pediatric hematologic malignancies was 11.4% at our center. The main cause of death was sepsis. Hyperphosphatemia was the common metabolic derangement and hyperkalemia was the least common. TLS warrants intensive supportive care to prevent further morbidity and decrease mortality.
Click on the PDF icon at the top of this introduction to read the full article.
Value-based cancer care and the patient perspective
The business of cancer care is in transition. Driven by the Centers for Medicare & Medicaid Services’ (CMS) Oncology Care Model (OCM) program, practices around the country are working to re-engineer the way they provide services, and the way they charge for those services. The implicit goal of all this is to manage (as in reduce) the overall cost of cancer care. A more frequently stated goal is to improve value, typically defined as outcome (numerator) relative to cost (denominator). Alternative payment models are challenged to assess the value of transformational improvement in cancer care.
Click on the PDF icon at the top of this introduction to read the full article.
The business of cancer care is in transition. Driven by the Centers for Medicare & Medicaid Services’ (CMS) Oncology Care Model (OCM) program, practices around the country are working to re-engineer the way they provide services, and the way they charge for those services. The implicit goal of all this is to manage (as in reduce) the overall cost of cancer care. A more frequently stated goal is to improve value, typically defined as outcome (numerator) relative to cost (denominator). Alternative payment models are challenged to assess the value of transformational improvement in cancer care.
Click on the PDF icon at the top of this introduction to read the full article.
The business of cancer care is in transition. Driven by the Centers for Medicare & Medicaid Services’ (CMS) Oncology Care Model (OCM) program, practices around the country are working to re-engineer the way they provide services, and the way they charge for those services. The implicit goal of all this is to manage (as in reduce) the overall cost of cancer care. A more frequently stated goal is to improve value, typically defined as outcome (numerator) relative to cost (denominator). Alternative payment models are challenged to assess the value of transformational improvement in cancer care.
Click on the PDF icon at the top of this introduction to read the full article.
Primary chest-wall leiomyosarcoma: a rare mimic of a malignant rib lesion
Primary chest-wall leiomyosarcoma (LMS) is an uncommon, malignant, soft-tissue tumor that most often affects the extremities. Malignant LMS originates from mesenchymal cells with smooth muscle differentiation. It is rare in adults, forming only 7% of all soft-tissue sarcomas (STS), but it is the most common STS. In adults, this type of tumor is usually found in the retroperitoneum and extremities.1 Chest-wall LMS is rare and most often occurs in men aged 50-70 years.2 When LMS is associated with rib destruction, it may mimic a primary bone tumor or metastasis. We present here the case of histologically proven chest-wall sarcoma with associated rib destruction that was initially mistaken on imaging for either a metastasis or primary bone tumor.
Case presentation and summary
A 69-year-old man presented to the emergency department complaining of pain over the right side of the chest. The pain, which was pleuritic in nature, had worsened over the previous 6 months and was severe at presentation. The patient had no fever, shortness of breath, or loss of weight. He had no history of chest trauma or chest wall radiation, and nothing noteworthy was discovered in his medical history. Subsequent test results for hemoglobin, white blood cell count, lymphocyte count, and cardiac enzymes were normal.
A frontal chest radiograph showed an osteolytic destructive lesion involving the posterior right 6th rib (Figure 1). A contrast-enhanced computedtomography (CE-CT) scan of the chest showed a heterogeneously enhancing, ovoid, soft-tissue mass of 5.6 x 3.6 cm (2.2 x 1.2 in) centered on the postero- lateral right 6th rib, with associated rib erosion. There was another 2.0-cm (0.8-in) subpleural nodule in the left upper lobe (Figure 2).
Click on the PDF icon below to read the full article.
Primary chest-wall leiomyosarcoma (LMS) is an uncommon, malignant, soft-tissue tumor that most often affects the extremities. Malignant LMS originates from mesenchymal cells with smooth muscle differentiation. It is rare in adults, forming only 7% of all soft-tissue sarcomas (STS), but it is the most common STS. In adults, this type of tumor is usually found in the retroperitoneum and extremities.1 Chest-wall LMS is rare and most often occurs in men aged 50-70 years.2 When LMS is associated with rib destruction, it may mimic a primary bone tumor or metastasis. We present here the case of histologically proven chest-wall sarcoma with associated rib destruction that was initially mistaken on imaging for either a metastasis or primary bone tumor.
Case presentation and summary
A 69-year-old man presented to the emergency department complaining of pain over the right side of the chest. The pain, which was pleuritic in nature, had worsened over the previous 6 months and was severe at presentation. The patient had no fever, shortness of breath, or loss of weight. He had no history of chest trauma or chest wall radiation, and nothing noteworthy was discovered in his medical history. Subsequent test results for hemoglobin, white blood cell count, lymphocyte count, and cardiac enzymes were normal.
A frontal chest radiograph showed an osteolytic destructive lesion involving the posterior right 6th rib (Figure 1). A contrast-enhanced computedtomography (CE-CT) scan of the chest showed a heterogeneously enhancing, ovoid, soft-tissue mass of 5.6 x 3.6 cm (2.2 x 1.2 in) centered on the postero- lateral right 6th rib, with associated rib erosion. There was another 2.0-cm (0.8-in) subpleural nodule in the left upper lobe (Figure 2).
Click on the PDF icon below to read the full article.
Primary chest-wall leiomyosarcoma (LMS) is an uncommon, malignant, soft-tissue tumor that most often affects the extremities. Malignant LMS originates from mesenchymal cells with smooth muscle differentiation. It is rare in adults, forming only 7% of all soft-tissue sarcomas (STS), but it is the most common STS. In adults, this type of tumor is usually found in the retroperitoneum and extremities.1 Chest-wall LMS is rare and most often occurs in men aged 50-70 years.2 When LMS is associated with rib destruction, it may mimic a primary bone tumor or metastasis. We present here the case of histologically proven chest-wall sarcoma with associated rib destruction that was initially mistaken on imaging for either a metastasis or primary bone tumor.
Case presentation and summary
A 69-year-old man presented to the emergency department complaining of pain over the right side of the chest. The pain, which was pleuritic in nature, had worsened over the previous 6 months and was severe at presentation. The patient had no fever, shortness of breath, or loss of weight. He had no history of chest trauma or chest wall radiation, and nothing noteworthy was discovered in his medical history. Subsequent test results for hemoglobin, white blood cell count, lymphocyte count, and cardiac enzymes were normal.
A frontal chest radiograph showed an osteolytic destructive lesion involving the posterior right 6th rib (Figure 1). A contrast-enhanced computedtomography (CE-CT) scan of the chest showed a heterogeneously enhancing, ovoid, soft-tissue mass of 5.6 x 3.6 cm (2.2 x 1.2 in) centered on the postero- lateral right 6th rib, with associated rib erosion. There was another 2.0-cm (0.8-in) subpleural nodule in the left upper lobe (Figure 2).
Click on the PDF icon below to read the full article.
Central nervous system manifestations of multiple myeloma: risk and prognostic considerations
Multiple myeloma accounts for about 1% of all cancers and for 10% of hematologic malignancies in the United States. This report describes the cases of 2 patients with multiple myeloma who developed CNS involvement after autologous stem cell transplant in the context of extramedullary disease.
Click on the PDF icon at the top of this introduction to read the full article.
Multiple myeloma accounts for about 1% of all cancers and for 10% of hematologic malignancies in the United States. This report describes the cases of 2 patients with multiple myeloma who developed CNS involvement after autologous stem cell transplant in the context of extramedullary disease.
Click on the PDF icon at the top of this introduction to read the full article.
Multiple myeloma accounts for about 1% of all cancers and for 10% of hematologic malignancies in the United States. This report describes the cases of 2 patients with multiple myeloma who developed CNS involvement after autologous stem cell transplant in the context of extramedullary disease.
Click on the PDF icon at the top of this introduction to read the full article.
Impact of a literacy-sensitive intervention on CRC screening knowledge, attitudes, and intention to screen
Background Colorectal cancer (CRC) screening rates remain low, especially among low-income populations.
Objective To determine if a CRC screening intervention (video, brochure) improves knowledge about CRC and CRC screening, attitudes toward screening, and intention to complete CRC screening among average-risk adults with different health literacy skills, seeking medical care at a Federally Qualified Health Center (FQHC).
Methods Average-risk adults (50 years or older) who were not within CRC screening guidelines completed face-to-face pre- and post-intervention interviews that focused on knowledge about CRC and CRC screening, attitudes toward CRC screening, and intention to complete CRC screening.
Results Of the 270 participants, 64% were women, 72% were black/African American, 86% were not married, 79% had an annual household income of <$20,000, and 57% did not have health insurance. Reading levels by Rapid Estimate of Adult Literacy in Medicine health literacy test were: 3rd grade or lower, 17 participants (6.3%); 4th-6th grade, 27 (10.0%); 7th-8th grade, 101 (37.4 %); and high school, 125 (46.3%). CRC screening knowledge mean score improved, and perceived CRC susceptibility and self-efficacy to complete screening significantly increased, irrespective of health literacy (all P < .01). There were no significant changes in other attitudes or intention to complete screening.
Limitations The study was conducted in a single FQHC, so the results may not be generalizable to other health centers or populations of low-income and minority patients.
Conclusion A CRC screening intervention improved CRC screening knowledge and attitudes across levels of health literacy and may be an important strategy for improving CRC screening in the primary care setting. Funding National Cancer Institute K07 CA107079 (Ohio State University) and P30 CA016058 (Behavioral Measurement Shared Resource at The Ohio State University).
Click on the PDF icon at the top of this introduction to read the full article.
Background Colorectal cancer (CRC) screening rates remain low, especially among low-income populations.
Objective To determine if a CRC screening intervention (video, brochure) improves knowledge about CRC and CRC screening, attitudes toward screening, and intention to complete CRC screening among average-risk adults with different health literacy skills, seeking medical care at a Federally Qualified Health Center (FQHC).
Methods Average-risk adults (50 years or older) who were not within CRC screening guidelines completed face-to-face pre- and post-intervention interviews that focused on knowledge about CRC and CRC screening, attitudes toward CRC screening, and intention to complete CRC screening.
Results Of the 270 participants, 64% were women, 72% were black/African American, 86% were not married, 79% had an annual household income of <$20,000, and 57% did not have health insurance. Reading levels by Rapid Estimate of Adult Literacy in Medicine health literacy test were: 3rd grade or lower, 17 participants (6.3%); 4th-6th grade, 27 (10.0%); 7th-8th grade, 101 (37.4 %); and high school, 125 (46.3%). CRC screening knowledge mean score improved, and perceived CRC susceptibility and self-efficacy to complete screening significantly increased, irrespective of health literacy (all P < .01). There were no significant changes in other attitudes or intention to complete screening.
Limitations The study was conducted in a single FQHC, so the results may not be generalizable to other health centers or populations of low-income and minority patients.
Conclusion A CRC screening intervention improved CRC screening knowledge and attitudes across levels of health literacy and may be an important strategy for improving CRC screening in the primary care setting. Funding National Cancer Institute K07 CA107079 (Ohio State University) and P30 CA016058 (Behavioral Measurement Shared Resource at The Ohio State University).
Click on the PDF icon at the top of this introduction to read the full article.
Background Colorectal cancer (CRC) screening rates remain low, especially among low-income populations.
Objective To determine if a CRC screening intervention (video, brochure) improves knowledge about CRC and CRC screening, attitudes toward screening, and intention to complete CRC screening among average-risk adults with different health literacy skills, seeking medical care at a Federally Qualified Health Center (FQHC).
Methods Average-risk adults (50 years or older) who were not within CRC screening guidelines completed face-to-face pre- and post-intervention interviews that focused on knowledge about CRC and CRC screening, attitudes toward CRC screening, and intention to complete CRC screening.
Results Of the 270 participants, 64% were women, 72% were black/African American, 86% were not married, 79% had an annual household income of <$20,000, and 57% did not have health insurance. Reading levels by Rapid Estimate of Adult Literacy in Medicine health literacy test were: 3rd grade or lower, 17 participants (6.3%); 4th-6th grade, 27 (10.0%); 7th-8th grade, 101 (37.4 %); and high school, 125 (46.3%). CRC screening knowledge mean score improved, and perceived CRC susceptibility and self-efficacy to complete screening significantly increased, irrespective of health literacy (all P < .01). There were no significant changes in other attitudes or intention to complete screening.
Limitations The study was conducted in a single FQHC, so the results may not be generalizable to other health centers or populations of low-income and minority patients.
Conclusion A CRC screening intervention improved CRC screening knowledge and attitudes across levels of health literacy and may be an important strategy for improving CRC screening in the primary care setting. Funding National Cancer Institute K07 CA107079 (Ohio State University) and P30 CA016058 (Behavioral Measurement Shared Resource at The Ohio State University).
Click on the PDF icon at the top of this introduction to read the full article.
Renal cell carcinoma approval adds another notch to cabozantinib’s belt
In April this year, the US Food and Drug Administration awarded regulatory approval to cabozantinib for the treatment of advanced renal cell carcinoma patients previously treated with anti-angiogenic therapy.1 The small-molecule inhibitor, which targets multiple kinases, including the vascular endothelial growth factor receptors (VEGFRs) and the hepatocyte growth factor receptor (MET), had previously been approved for the treatment of medullary thyroid carcinoma in 2012.
Click on the PDF icon below for the full article.
In April this year, the US Food and Drug Administration awarded regulatory approval to cabozantinib for the treatment of advanced renal cell carcinoma patients previously treated with anti-angiogenic therapy.1 The small-molecule inhibitor, which targets multiple kinases, including the vascular endothelial growth factor receptors (VEGFRs) and the hepatocyte growth factor receptor (MET), had previously been approved for the treatment of medullary thyroid carcinoma in 2012.
Click on the PDF icon below for the full article.
In April this year, the US Food and Drug Administration awarded regulatory approval to cabozantinib for the treatment of advanced renal cell carcinoma patients previously treated with anti-angiogenic therapy.1 The small-molecule inhibitor, which targets multiple kinases, including the vascular endothelial growth factor receptors (VEGFRs) and the hepatocyte growth factor receptor (MET), had previously been approved for the treatment of medullary thyroid carcinoma in 2012.
Click on the PDF icon below for the full article.
Toxicity analysis of docetaxel, cisplatin, and 5- fluorouracil neoadjuvant chemotherapy in Indian patients with head and neck cancers
Background There is a lack of data that systematically address toxicity with docetaxel, cisplatin, and 5-fluorouracil (TPF) regimen in routine care.
Objective To detect, profile, and quantify the toxicity in Indian patients with head and neck cancers who received neoadjuvant TPF chemotherapy in a routine clinical practice (non-trial setting).
Methods 58 patients with locally advanced head and neck cancer who received TPF chemotherapy were selected for this analysis. They received 2 cycles of TPF chemotherapy every 21 days. The patients were monitored for the occurrence of adverse drug reactions in accordance with Common Terminology Criteria for Adverse Events (version 4.03) during the hospitalization (median length of stay in cycle 1, 10 days), daily (at least until day 8 after chemotherapy initiation), then at days 15 and 20. Descriptive statistics was done and factors predicting for toxicity were identified using logistic regression analysis.
Results The cumulative rate of grade ¦3 anemia, neutropenia, and thrombocytopenia were 12.1%, 56.9%, and 5.2%, respectively. The cumulative incidence of febrile neutropenia was 20.7% (12 of 58 patients). The cumulative incidences of mucositis and diarrhea were 67.2% and 74.1%, respectively. There was no mortality associated with induction chemotherapy, and all of the patients completed the planned 2 cycles of TPF. None of the tested factors predicted for any of the adverse events considered in the study.
Limitations Small, single-center study
Conclusion The incidence of TPF-related toxicity in Indian patients in routine practice is high, and the toxicities differ substantially from the toxicities seen in trial settings.
Click on the PDF icon at the top of this introduction to read the full article.
Background There is a lack of data that systematically address toxicity with docetaxel, cisplatin, and 5-fluorouracil (TPF) regimen in routine care.
Objective To detect, profile, and quantify the toxicity in Indian patients with head and neck cancers who received neoadjuvant TPF chemotherapy in a routine clinical practice (non-trial setting).
Methods 58 patients with locally advanced head and neck cancer who received TPF chemotherapy were selected for this analysis. They received 2 cycles of TPF chemotherapy every 21 days. The patients were monitored for the occurrence of adverse drug reactions in accordance with Common Terminology Criteria for Adverse Events (version 4.03) during the hospitalization (median length of stay in cycle 1, 10 days), daily (at least until day 8 after chemotherapy initiation), then at days 15 and 20. Descriptive statistics was done and factors predicting for toxicity were identified using logistic regression analysis.
Results The cumulative rate of grade ¦3 anemia, neutropenia, and thrombocytopenia were 12.1%, 56.9%, and 5.2%, respectively. The cumulative incidence of febrile neutropenia was 20.7% (12 of 58 patients). The cumulative incidences of mucositis and diarrhea were 67.2% and 74.1%, respectively. There was no mortality associated with induction chemotherapy, and all of the patients completed the planned 2 cycles of TPF. None of the tested factors predicted for any of the adverse events considered in the study.
Limitations Small, single-center study
Conclusion The incidence of TPF-related toxicity in Indian patients in routine practice is high, and the toxicities differ substantially from the toxicities seen in trial settings.
Click on the PDF icon at the top of this introduction to read the full article.
Background There is a lack of data that systematically address toxicity with docetaxel, cisplatin, and 5-fluorouracil (TPF) regimen in routine care.
Objective To detect, profile, and quantify the toxicity in Indian patients with head and neck cancers who received neoadjuvant TPF chemotherapy in a routine clinical practice (non-trial setting).
Methods 58 patients with locally advanced head and neck cancer who received TPF chemotherapy were selected for this analysis. They received 2 cycles of TPF chemotherapy every 21 days. The patients were monitored for the occurrence of adverse drug reactions in accordance with Common Terminology Criteria for Adverse Events (version 4.03) during the hospitalization (median length of stay in cycle 1, 10 days), daily (at least until day 8 after chemotherapy initiation), then at days 15 and 20. Descriptive statistics was done and factors predicting for toxicity were identified using logistic regression analysis.
Results The cumulative rate of grade ¦3 anemia, neutropenia, and thrombocytopenia were 12.1%, 56.9%, and 5.2%, respectively. The cumulative incidence of febrile neutropenia was 20.7% (12 of 58 patients). The cumulative incidences of mucositis and diarrhea were 67.2% and 74.1%, respectively. There was no mortality associated with induction chemotherapy, and all of the patients completed the planned 2 cycles of TPF. None of the tested factors predicted for any of the adverse events considered in the study.
Limitations Small, single-center study
Conclusion The incidence of TPF-related toxicity in Indian patients in routine practice is high, and the toxicities differ substantially from the toxicities seen in trial settings.
Click on the PDF icon at the top of this introduction to read the full article.
High protein intake moderately associated with improved breast cancer survival
Higher protein intake, particularly protein from animal sources, is associated with a modest but lower risk of breast cancer recurrence and death, regardless of insulin receptor status.
Using information gathered through biennial questionnaires from 6,348 women who were diagnosed with stage I to III breast cancer between 1976 and 2004, investigators found a significant inverse association between total protein intake and distant breast cancer recurrence (P = .02). This association was driven specifically by protein from animal sources (P = .003) rather than vegetable sources.
Pathology records were reviewed and histology samples were analyzed for insulin receptor and estrogen receptor expression. Associations between breast cancer recurrence and protein intake, amino acids, or protein-containing food groups did not differ by tumor receptor status or body mass index at time of cancer diagnosis. Given that the association between protein intake and recurrence was not confined to tumors expressing insulin receptors, “It is difficult to invoke the insulin pathway as a mechanism to explain these findings,” the investigators wrote.
Given the only “modest survival advantage” of higher protein intake among women with breast cancer, and given the “challenges involved in randomized trials of diet, this association is unlikely to ever be definitively tested in a randomized trial,” Dr. Holmes and her associates wrote.
“However, the modest survival advantage with higher protein intake has been found in several studies, and we feel it is important that patients with breast cancer and their clinicians know this. At the least, it may provide reassurance that consuming protein-containing foods is not likely to increase the risk of breast cancer recurrence,” the researchers concluded.
This study was sponsored by grants from the National Institutes of Health. Dr. Holmes and one other investigator reported receiving financial compensation from Bayer HealthCare Pharmaceuticals.
[email protected]
On Twitter @jessnicolecraig
Higher protein intake, particularly protein from animal sources, is associated with a modest but lower risk of breast cancer recurrence and death, regardless of insulin receptor status.
Using information gathered through biennial questionnaires from 6,348 women who were diagnosed with stage I to III breast cancer between 1976 and 2004, investigators found a significant inverse association between total protein intake and distant breast cancer recurrence (P = .02). This association was driven specifically by protein from animal sources (P = .003) rather than vegetable sources.
Pathology records were reviewed and histology samples were analyzed for insulin receptor and estrogen receptor expression. Associations between breast cancer recurrence and protein intake, amino acids, or protein-containing food groups did not differ by tumor receptor status or body mass index at time of cancer diagnosis. Given that the association between protein intake and recurrence was not confined to tumors expressing insulin receptors, “It is difficult to invoke the insulin pathway as a mechanism to explain these findings,” the investigators wrote.
Given the only “modest survival advantage” of higher protein intake among women with breast cancer, and given the “challenges involved in randomized trials of diet, this association is unlikely to ever be definitively tested in a randomized trial,” Dr. Holmes and her associates wrote.
“However, the modest survival advantage with higher protein intake has been found in several studies, and we feel it is important that patients with breast cancer and their clinicians know this. At the least, it may provide reassurance that consuming protein-containing foods is not likely to increase the risk of breast cancer recurrence,” the researchers concluded.
This study was sponsored by grants from the National Institutes of Health. Dr. Holmes and one other investigator reported receiving financial compensation from Bayer HealthCare Pharmaceuticals.
[email protected]
On Twitter @jessnicolecraig
Higher protein intake, particularly protein from animal sources, is associated with a modest but lower risk of breast cancer recurrence and death, regardless of insulin receptor status.
Using information gathered through biennial questionnaires from 6,348 women who were diagnosed with stage I to III breast cancer between 1976 and 2004, investigators found a significant inverse association between total protein intake and distant breast cancer recurrence (P = .02). This association was driven specifically by protein from animal sources (P = .003) rather than vegetable sources.
Pathology records were reviewed and histology samples were analyzed for insulin receptor and estrogen receptor expression. Associations between breast cancer recurrence and protein intake, amino acids, or protein-containing food groups did not differ by tumor receptor status or body mass index at time of cancer diagnosis. Given that the association between protein intake and recurrence was not confined to tumors expressing insulin receptors, “It is difficult to invoke the insulin pathway as a mechanism to explain these findings,” the investigators wrote.
Given the only “modest survival advantage” of higher protein intake among women with breast cancer, and given the “challenges involved in randomized trials of diet, this association is unlikely to ever be definitively tested in a randomized trial,” Dr. Holmes and her associates wrote.
“However, the modest survival advantage with higher protein intake has been found in several studies, and we feel it is important that patients with breast cancer and their clinicians know this. At the least, it may provide reassurance that consuming protein-containing foods is not likely to increase the risk of breast cancer recurrence,” the researchers concluded.
This study was sponsored by grants from the National Institutes of Health. Dr. Holmes and one other investigator reported receiving financial compensation from Bayer HealthCare Pharmaceuticals.
[email protected]
On Twitter @jessnicolecraig
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point:
Major finding: The 5-year recurrence-free survival for women in the highest quintile of protein consumption was 94.0%, while those in the lowest quintile of protein consumption had 5-year recurrence-free survival of 92.1%.
Data source: Biennial questionnaires for 6,348 women diagnosed with any stage breast cancer between 1976 and 2004.
Disclosures: This study was sponsored by grants from the National Institutes of Health. Dr. Holmes and one coinvestigator reported receiving financial compensation from Bayer HealthCare Pharmaceuticals.
Cancer type, age at time of diagnosis implicated in risk of CVD-related deaths
Survivorship data derived from a U.K. cancer registry make it possible to more closely pinpoint the risk of cardiovascular disease in patients treated for cancer as adolescents and young adults.
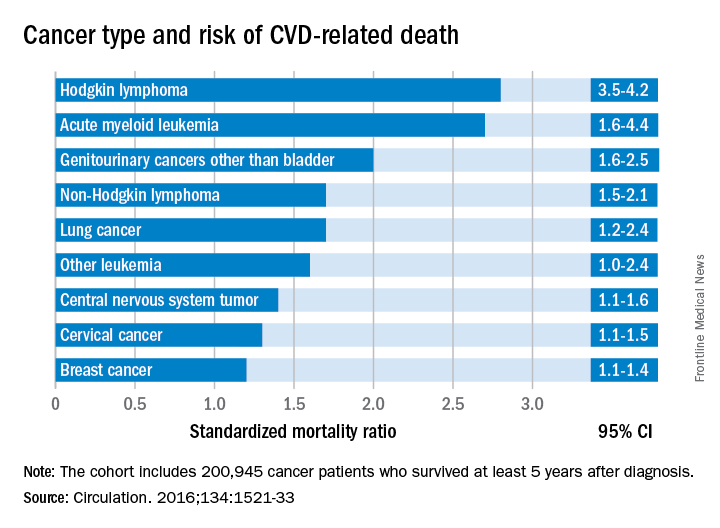
Researchers report that 6% of the 2,016 deaths occurring in 200,945 cancer survivors diagnosed between the ages of 15 and 39 years were directly related to cardiovascular disease. A multivariable Poisson regression analysis of data from the Teenage and Young Adult Cancer Survivor Study also showed that survivors who were diagnosed between the ages of 15 and 19 years had 4.2 times the risk (95% confidence interval, 3.4-5.2) of death from cardiovascular disease, compared with their peers in the general population. But for survivors who were aged 35-39 years when diagnosed, that risk decreased to 1.2 times (95% CI, 1.1-1.3) that of their general population peers (P less than .0001). The standardized mortality ratios and absolute excess risks for ischemic heart disease, valvular heart disease, and cardiomyopathy were similar (Circulation. 2016;134:1521-33).
The findings should help clinicians craft more effective after-cancer care, according to Mike Hawkins, DPhil. “It helps them focus the most intensive follow-up care on those most at risk,” Dr. Hawkins, an epidemiology professor and director of the Centre for Childhood Cancer Survivor Studies at the University of Birmingham (England), said in a statement. “It is important for survivors because it empowers them by providing them with their long-term chances of a specific side effect of cancer treatment.”
The most significant relationship between cardiovascular disease and cancer occurred in those diagnosed with Hodgkin lymphoma, and at an earlier age. Overall, Hodgkin lymphoma survivors had a 3.8 times higher risk of cardiovascular disease–related death than their peers not diagnosed with any cancer. In those diagnosed at age 15-19 years, 6.9% had died from cardiovascular disease by age 55 years, compared with 2% of those who’d been diagnosed at age 35-39 years. Among these two age groups in the general population, fewer than 1% typically die from cardiovascular disease–related deaths. In Hodgkin lymphoma survivors aged 60 years or older, 27.5% of excess deaths were from cardiovascular disease.
Although not stratified by treatment, the study includes risk estimates for other cancers diagnosed in the teen and young adult years, stratified by the age at diagnosis, something the authors of the study noted is “a considerable advance on previous knowledge.”
Survivors of all age groups in the cohort diagnosed with a variety of cancers experienced a greater risk of death from heart disease, compared with their peers in the general population.
[email protected]
On Twitter @whitneymcknight
Survivorship data derived from a U.K. cancer registry make it possible to more closely pinpoint the risk of cardiovascular disease in patients treated for cancer as adolescents and young adults.
Researchers report that 6% of the 2,016 deaths occurring in 200,945 cancer survivors diagnosed between the ages of 15 and 39 years were directly related to cardiovascular disease. A multivariable Poisson regression analysis of data from the Teenage and Young Adult Cancer Survivor Study also showed that survivors who were diagnosed between the ages of 15 and 19 years had 4.2 times the risk (95% confidence interval, 3.4-5.2) of death from cardiovascular disease, compared with their peers in the general population. But for survivors who were aged 35-39 years when diagnosed, that risk decreased to 1.2 times (95% CI, 1.1-1.3) that of their general population peers (P less than .0001). The standardized mortality ratios and absolute excess risks for ischemic heart disease, valvular heart disease, and cardiomyopathy were similar (Circulation. 2016;134:1521-33).
The findings should help clinicians craft more effective after-cancer care, according to Mike Hawkins, DPhil. “It helps them focus the most intensive follow-up care on those most at risk,” Dr. Hawkins, an epidemiology professor and director of the Centre for Childhood Cancer Survivor Studies at the University of Birmingham (England), said in a statement. “It is important for survivors because it empowers them by providing them with their long-term chances of a specific side effect of cancer treatment.”
The most significant relationship between cardiovascular disease and cancer occurred in those diagnosed with Hodgkin lymphoma, and at an earlier age. Overall, Hodgkin lymphoma survivors had a 3.8 times higher risk of cardiovascular disease–related death than their peers not diagnosed with any cancer. In those diagnosed at age 15-19 years, 6.9% had died from cardiovascular disease by age 55 years, compared with 2% of those who’d been diagnosed at age 35-39 years. Among these two age groups in the general population, fewer than 1% typically die from cardiovascular disease–related deaths. In Hodgkin lymphoma survivors aged 60 years or older, 27.5% of excess deaths were from cardiovascular disease.
Although not stratified by treatment, the study includes risk estimates for other cancers diagnosed in the teen and young adult years, stratified by the age at diagnosis, something the authors of the study noted is “a considerable advance on previous knowledge.”
Survivors of all age groups in the cohort diagnosed with a variety of cancers experienced a greater risk of death from heart disease, compared with their peers in the general population.
[email protected]
On Twitter @whitneymcknight
Survivorship data derived from a U.K. cancer registry make it possible to more closely pinpoint the risk of cardiovascular disease in patients treated for cancer as adolescents and young adults.
Researchers report that 6% of the 2,016 deaths occurring in 200,945 cancer survivors diagnosed between the ages of 15 and 39 years were directly related to cardiovascular disease. A multivariable Poisson regression analysis of data from the Teenage and Young Adult Cancer Survivor Study also showed that survivors who were diagnosed between the ages of 15 and 19 years had 4.2 times the risk (95% confidence interval, 3.4-5.2) of death from cardiovascular disease, compared with their peers in the general population. But for survivors who were aged 35-39 years when diagnosed, that risk decreased to 1.2 times (95% CI, 1.1-1.3) that of their general population peers (P less than .0001). The standardized mortality ratios and absolute excess risks for ischemic heart disease, valvular heart disease, and cardiomyopathy were similar (Circulation. 2016;134:1521-33).
The findings should help clinicians craft more effective after-cancer care, according to Mike Hawkins, DPhil. “It helps them focus the most intensive follow-up care on those most at risk,” Dr. Hawkins, an epidemiology professor and director of the Centre for Childhood Cancer Survivor Studies at the University of Birmingham (England), said in a statement. “It is important for survivors because it empowers them by providing them with their long-term chances of a specific side effect of cancer treatment.”
The most significant relationship between cardiovascular disease and cancer occurred in those diagnosed with Hodgkin lymphoma, and at an earlier age. Overall, Hodgkin lymphoma survivors had a 3.8 times higher risk of cardiovascular disease–related death than their peers not diagnosed with any cancer. In those diagnosed at age 15-19 years, 6.9% had died from cardiovascular disease by age 55 years, compared with 2% of those who’d been diagnosed at age 35-39 years. Among these two age groups in the general population, fewer than 1% typically die from cardiovascular disease–related deaths. In Hodgkin lymphoma survivors aged 60 years or older, 27.5% of excess deaths were from cardiovascular disease.
Although not stratified by treatment, the study includes risk estimates for other cancers diagnosed in the teen and young adult years, stratified by the age at diagnosis, something the authors of the study noted is “a considerable advance on previous knowledge.”
Survivors of all age groups in the cohort diagnosed with a variety of cancers experienced a greater risk of death from heart disease, compared with their peers in the general population.
[email protected]
On Twitter @whitneymcknight
FROM CIRCULATION
Key clinical point:
Major finding: Cancer survivors who were diagnosed at age 15-19 years had 4.2 times the risk of death from cardiovascular disease than did their peers in the general population.
Data source: A U.K. cancer registry of 200,945 persons between 15 and 39 years at time of diagnosis.
Disclosures: This study was supported by the National Institute for Health Research in the United Kingdom. The authors had no relevant disclosures.