User login
Direct pharmacy dispensing of naloxone linked to drop in fatal overdoses
investigators reported.
By contrast, state laws that stopped short of allowing pharmacists to directly dispense the opioid antagonist did not appear to impact mortality, according to the report, which appears in JAMA Internal Medicine (2019 May 6. doi: 10.1001/jamainternmed.2019.0272).
The report, based on state-level trends tracked from 2005 to 2016, indicates that fatal opioid overdoses fell by nearly one-third in states that adopted direct dispensing laws as compared with states that adopted other naloxone laws.
That finding suggests that the policy type determines whether a naloxone law is useful in combating fatal opioid overdoses, said Rahi Abouk, PhD, of William Paterson University, Wayne, N.J. and co-authors of the paper.
“Enabling distribution through various sources, or requiring gatekeepers, will not be as beneficial,” Dr. Abouk and co-authors said in their report.
The current rate of deaths from fentanyl, heroin, and prescription analgesic overdose has outpaced all previous drug epidemics on record, and even surpasses the number of deaths in the peak year of the HIV epidemic of the 1980s, Dr. Abouk and colleagues wrote in their paper.
The number of states with naloxone access laws grew from just 2 in 2005 to 47 by 2016, including 9 states that granted direct authority to pharmacists and 38 that granted indirect authority, according to the researchers.
The analysis of overdose trends from 2005 to 2016 was based on naloxone distribution data from state Medicaid agencies and opioid-related mortality data from a national statistics system. Forty percent of nonelderly adults with an opioid addiction are covered by Medicaid, the researchers said.
They found that naloxone laws granting pharmacists direct dispensing authority were linked to a drop in opioid deaths that increased in magnitude over time, according to researchers. The mean number of opioid deaths dropped by 27% in the second year after adoption of direct authority laws, relative to opioid deaths in states with indirect access laws, while in subsequent years, deaths dropped by 34%.
Emergency department visits related to opioids increased by 15% in direct authority states 3 or more years after adoption, as compared to states that did not adopt direct authority laws. According to investigators, that translated into 15 additional opioid-related emergency department visits each month.
That increase suggests that, alongside direct dispensing laws, “useful interventions” and connections to treatment are needed for the emergency department, according to Dr. Abouk and colleagues.
“This is the location where such programs may be the most effective,” they said in their report.
Future research should be done to determine whether removing gatekeepers increases the value of naloxone distribution policies, they concluded in the report.
Dr. Abouk had no disclosures. Co-authors on the study reported funding and conflict of interest disclosures related to the National Institute on Drug Abuse and the Centers for Disease Control and Prevention.
SOURCE: Abouk R, et al. JAMA Intern Med. 2019 May 6. doi:10.1001/jamainternmed.2019.0272.
investigators reported.
By contrast, state laws that stopped short of allowing pharmacists to directly dispense the opioid antagonist did not appear to impact mortality, according to the report, which appears in JAMA Internal Medicine (2019 May 6. doi: 10.1001/jamainternmed.2019.0272).
The report, based on state-level trends tracked from 2005 to 2016, indicates that fatal opioid overdoses fell by nearly one-third in states that adopted direct dispensing laws as compared with states that adopted other naloxone laws.
That finding suggests that the policy type determines whether a naloxone law is useful in combating fatal opioid overdoses, said Rahi Abouk, PhD, of William Paterson University, Wayne, N.J. and co-authors of the paper.
“Enabling distribution through various sources, or requiring gatekeepers, will not be as beneficial,” Dr. Abouk and co-authors said in their report.
The current rate of deaths from fentanyl, heroin, and prescription analgesic overdose has outpaced all previous drug epidemics on record, and even surpasses the number of deaths in the peak year of the HIV epidemic of the 1980s, Dr. Abouk and colleagues wrote in their paper.
The number of states with naloxone access laws grew from just 2 in 2005 to 47 by 2016, including 9 states that granted direct authority to pharmacists and 38 that granted indirect authority, according to the researchers.
The analysis of overdose trends from 2005 to 2016 was based on naloxone distribution data from state Medicaid agencies and opioid-related mortality data from a national statistics system. Forty percent of nonelderly adults with an opioid addiction are covered by Medicaid, the researchers said.
They found that naloxone laws granting pharmacists direct dispensing authority were linked to a drop in opioid deaths that increased in magnitude over time, according to researchers. The mean number of opioid deaths dropped by 27% in the second year after adoption of direct authority laws, relative to opioid deaths in states with indirect access laws, while in subsequent years, deaths dropped by 34%.
Emergency department visits related to opioids increased by 15% in direct authority states 3 or more years after adoption, as compared to states that did not adopt direct authority laws. According to investigators, that translated into 15 additional opioid-related emergency department visits each month.
That increase suggests that, alongside direct dispensing laws, “useful interventions” and connections to treatment are needed for the emergency department, according to Dr. Abouk and colleagues.
“This is the location where such programs may be the most effective,” they said in their report.
Future research should be done to determine whether removing gatekeepers increases the value of naloxone distribution policies, they concluded in the report.
Dr. Abouk had no disclosures. Co-authors on the study reported funding and conflict of interest disclosures related to the National Institute on Drug Abuse and the Centers for Disease Control and Prevention.
SOURCE: Abouk R, et al. JAMA Intern Med. 2019 May 6. doi:10.1001/jamainternmed.2019.0272.
investigators reported.
By contrast, state laws that stopped short of allowing pharmacists to directly dispense the opioid antagonist did not appear to impact mortality, according to the report, which appears in JAMA Internal Medicine (2019 May 6. doi: 10.1001/jamainternmed.2019.0272).
The report, based on state-level trends tracked from 2005 to 2016, indicates that fatal opioid overdoses fell by nearly one-third in states that adopted direct dispensing laws as compared with states that adopted other naloxone laws.
That finding suggests that the policy type determines whether a naloxone law is useful in combating fatal opioid overdoses, said Rahi Abouk, PhD, of William Paterson University, Wayne, N.J. and co-authors of the paper.
“Enabling distribution through various sources, or requiring gatekeepers, will not be as beneficial,” Dr. Abouk and co-authors said in their report.
The current rate of deaths from fentanyl, heroin, and prescription analgesic overdose has outpaced all previous drug epidemics on record, and even surpasses the number of deaths in the peak year of the HIV epidemic of the 1980s, Dr. Abouk and colleagues wrote in their paper.
The number of states with naloxone access laws grew from just 2 in 2005 to 47 by 2016, including 9 states that granted direct authority to pharmacists and 38 that granted indirect authority, according to the researchers.
The analysis of overdose trends from 2005 to 2016 was based on naloxone distribution data from state Medicaid agencies and opioid-related mortality data from a national statistics system. Forty percent of nonelderly adults with an opioid addiction are covered by Medicaid, the researchers said.
They found that naloxone laws granting pharmacists direct dispensing authority were linked to a drop in opioid deaths that increased in magnitude over time, according to researchers. The mean number of opioid deaths dropped by 27% in the second year after adoption of direct authority laws, relative to opioid deaths in states with indirect access laws, while in subsequent years, deaths dropped by 34%.
Emergency department visits related to opioids increased by 15% in direct authority states 3 or more years after adoption, as compared to states that did not adopt direct authority laws. According to investigators, that translated into 15 additional opioid-related emergency department visits each month.
That increase suggests that, alongside direct dispensing laws, “useful interventions” and connections to treatment are needed for the emergency department, according to Dr. Abouk and colleagues.
“This is the location where such programs may be the most effective,” they said in their report.
Future research should be done to determine whether removing gatekeepers increases the value of naloxone distribution policies, they concluded in the report.
Dr. Abouk had no disclosures. Co-authors on the study reported funding and conflict of interest disclosures related to the National Institute on Drug Abuse and the Centers for Disease Control and Prevention.
SOURCE: Abouk R, et al. JAMA Intern Med. 2019 May 6. doi:10.1001/jamainternmed.2019.0272.
FROM JAMA Internal Medicine
Key clinical point: State laws granting pharmacists direct authority to dispense naloxone were linked to significant drops in opioid-related fatal overdoses.
Major finding: The mean number of opioid deaths dropped by 27% in the second year after adoption of direct authority laws relative to opioid deaths in states with indirect access laws, while in subsequent years, deaths dropped by 34%.
Study details: Analysis of naloxone distribution data and opioid-related mortality data from 2005 to 2016 for all 50 states and the District of Columbia.
Disclosures: Study authors reported funding and conflict of interest disclosures related to the National Institute on Drug Abuse and the Centers for Disease Control and Prevention.
Source: Abouk R, et al. JAMA Intern Med. 2019 May 6.
Arthritis joint pain, inactivity vary greatly across U.S.
Almost 31% of the estimated 54 million adults in the United States with arthritis have severe joint pain, according to the Centers for Disease Control and Prevention.

Nationally, the prevalence of severe joint pain was 30.8% in adults with arthritis in 2017, but state-specific, age-standardized prevalences varied from a low of 20.8% in Colorado to 45.2% in Mississippi. Regionally, prevalences of both severe joint pain and physical inactivity in arthritis patients were highest in the Southeast, noted Dana Guglielmo, MPH, of the CDC’s National Center for Chronic Disease Prevention and Health Promotion, Atlanta, and associates (MMWR 2019 May 3;68(17):381-7).
The prevalence of arthritis itself was lowest in the District of Columbia at 15.7% and highest in West Virginia at 34.6%. Alabama, at 30.4%, was the only other state above 30%. Colorado had the lowest physical inactivity rate (23.2%), while Kentucky had the highest (44.4%), the investigators said.
The differences among arthritis patients were demographic as well as geographic in 2017. The prevalence of severe joint pain was 33.0% among those aged 18-44 years and 35.6% in those 45-64 but only 25.1% in those aged 65 and older. Whites had a 27.4% prevalence of severe joint pain, compared with 42.0% for Hispanics and 50.9% for blacks. For arthritis patients with a college degree, the age-standardized prevalence of severe joint pain was 15.1%, compared with 35.5% for high school graduates and 54.1% for those with less than a high school degree, based on data from the Behavioral Risk Factor Surveillance System.
“Although persons with arthritis report that pain, or fear of causing or worsening it, is a substantial barrier to exercising, physical activity is an inexpensive intervention that can reduce pain, prevent or delay disability and limitations, and improve mental health, physical functioning, and quality of life with few adverse effects,” wrote Ms. Guglielmo and associates. Adults with severe joint pain “should engage in regular physical activity according to their abilities and avoid physical inactivity [since] even small amounts of physical activity can improve physical functioning in adults with joint conditions.”
SOURCE: Guglielmo D et al. MMWR 2019 May 3;68(17):381-7.
Almost 31% of the estimated 54 million adults in the United States with arthritis have severe joint pain, according to the Centers for Disease Control and Prevention.

Nationally, the prevalence of severe joint pain was 30.8% in adults with arthritis in 2017, but state-specific, age-standardized prevalences varied from a low of 20.8% in Colorado to 45.2% in Mississippi. Regionally, prevalences of both severe joint pain and physical inactivity in arthritis patients were highest in the Southeast, noted Dana Guglielmo, MPH, of the CDC’s National Center for Chronic Disease Prevention and Health Promotion, Atlanta, and associates (MMWR 2019 May 3;68(17):381-7).
The prevalence of arthritis itself was lowest in the District of Columbia at 15.7% and highest in West Virginia at 34.6%. Alabama, at 30.4%, was the only other state above 30%. Colorado had the lowest physical inactivity rate (23.2%), while Kentucky had the highest (44.4%), the investigators said.
The differences among arthritis patients were demographic as well as geographic in 2017. The prevalence of severe joint pain was 33.0% among those aged 18-44 years and 35.6% in those 45-64 but only 25.1% in those aged 65 and older. Whites had a 27.4% prevalence of severe joint pain, compared with 42.0% for Hispanics and 50.9% for blacks. For arthritis patients with a college degree, the age-standardized prevalence of severe joint pain was 15.1%, compared with 35.5% for high school graduates and 54.1% for those with less than a high school degree, based on data from the Behavioral Risk Factor Surveillance System.
“Although persons with arthritis report that pain, or fear of causing or worsening it, is a substantial barrier to exercising, physical activity is an inexpensive intervention that can reduce pain, prevent or delay disability and limitations, and improve mental health, physical functioning, and quality of life with few adverse effects,” wrote Ms. Guglielmo and associates. Adults with severe joint pain “should engage in regular physical activity according to their abilities and avoid physical inactivity [since] even small amounts of physical activity can improve physical functioning in adults with joint conditions.”
SOURCE: Guglielmo D et al. MMWR 2019 May 3;68(17):381-7.
Almost 31% of the estimated 54 million adults in the United States with arthritis have severe joint pain, according to the Centers for Disease Control and Prevention.

Nationally, the prevalence of severe joint pain was 30.8% in adults with arthritis in 2017, but state-specific, age-standardized prevalences varied from a low of 20.8% in Colorado to 45.2% in Mississippi. Regionally, prevalences of both severe joint pain and physical inactivity in arthritis patients were highest in the Southeast, noted Dana Guglielmo, MPH, of the CDC’s National Center for Chronic Disease Prevention and Health Promotion, Atlanta, and associates (MMWR 2019 May 3;68(17):381-7).
The prevalence of arthritis itself was lowest in the District of Columbia at 15.7% and highest in West Virginia at 34.6%. Alabama, at 30.4%, was the only other state above 30%. Colorado had the lowest physical inactivity rate (23.2%), while Kentucky had the highest (44.4%), the investigators said.
The differences among arthritis patients were demographic as well as geographic in 2017. The prevalence of severe joint pain was 33.0% among those aged 18-44 years and 35.6% in those 45-64 but only 25.1% in those aged 65 and older. Whites had a 27.4% prevalence of severe joint pain, compared with 42.0% for Hispanics and 50.9% for blacks. For arthritis patients with a college degree, the age-standardized prevalence of severe joint pain was 15.1%, compared with 35.5% for high school graduates and 54.1% for those with less than a high school degree, based on data from the Behavioral Risk Factor Surveillance System.
“Although persons with arthritis report that pain, or fear of causing or worsening it, is a substantial barrier to exercising, physical activity is an inexpensive intervention that can reduce pain, prevent or delay disability and limitations, and improve mental health, physical functioning, and quality of life with few adverse effects,” wrote Ms. Guglielmo and associates. Adults with severe joint pain “should engage in regular physical activity according to their abilities and avoid physical inactivity [since] even small amounts of physical activity can improve physical functioning in adults with joint conditions.”
SOURCE: Guglielmo D et al. MMWR 2019 May 3;68(17):381-7.
FROM MMWR
What do patients want in a migraine preventive?
, according to the results of a study published in Headache. When offered hypothetical preventive migraine medicines with a wide array of attributes, patients leaned toward those with a reduction in migraine days and an avoidance of weight gain, according to an analysis of responses to a discrete-choice experiment survey.
“We found that respondents had a significant willingness to pay for medicines with higher efficacy and less-severe adverse events,” wrote Carol Mansfield, PhD, of RTI Health Solutions in North Carolina, and coauthors.
To evaluate patient preferences for theoretical migraine medicine, the researchers conducted a discrete-choice experiment via a web-based survey. Respondents met eligibility criteria if they were adults aged 18 years or older who self-reported 6 or more migraine days per month and completed the survey in full. They were asked to choose between options defined by six attributes: reduction in headache days per month, frequency of limitations with physical activities, cognition problems, weight gain, how the medicine is taken, and monthly out-of-pocket cost.
Of the 300 respondents included in the analysis, 72% indicated that migraines make physical activities difficult all or most of the time, and 81% had taken a prescription migraine preventive in the last 6 months. Respondents reported, on average, approximately 16 headache days per month. Among noncost attributes, respondents valued a change from a 10% reduction in migraine days to a 50% reduction more highly than avoiding the worst levels of adverse events – defined as memory problems and 10% weight gain – but were willing to trade off efficacy for less-severe adverse events. Avoiding memory problems was more important than avoiding thinking problems. Avoiding a 10% weight gain was more important than avoiding thinking and memory problems. Respondents preferred a once-monthly injection or daily pill to twice-monthly injections. Respondents, on average, were willing to pay $116 per month for an improvement from 10% to 50% in reduced headache days (95% confidence interval [CI], $91-$141) and $43 for an improvement from 10% to 25% (95% CI, $34-$53). They were also willing to pay $84 per month to avoid a 10% weight gain (95% CI, $64-$103), $59 per month to avoid memory problems (95% CI, $42-$76), and $32 per month to avoid thinking problems (95% CI, $18-$46).
The coauthors acknowledged their study’s limitations, including all migraine diagnoses being self-reported and the study sample not necessarily being representative of patients with migraine overall. In addition, though the potential medicinal attributes used were prominent in clinical literature and focus groups, they could choose only a limited amount and so their analysis “did not address other attributes that may be important to patients.”
Given their findings, the researchers recommended that “clinicians should work with patients to select treatments that meet each patient’s needs.”
Amgen and Novartis funded the study. The authors reported numerous conflicts of interest, including receiving grants, consulting fees, and royalties from pharmaceutical companies and organizations. During the study, three of the authors were employed at RTI Health Solutions, a non-for-profit organization that conducts research with pharmaceutical companies such as the study’s sponsor.
SOURCE: Mansfield C et al. Headache. 2019 May;59(5):715-26. doi: 10.1111/head.13498.
, according to the results of a study published in Headache. When offered hypothetical preventive migraine medicines with a wide array of attributes, patients leaned toward those with a reduction in migraine days and an avoidance of weight gain, according to an analysis of responses to a discrete-choice experiment survey.
“We found that respondents had a significant willingness to pay for medicines with higher efficacy and less-severe adverse events,” wrote Carol Mansfield, PhD, of RTI Health Solutions in North Carolina, and coauthors.
To evaluate patient preferences for theoretical migraine medicine, the researchers conducted a discrete-choice experiment via a web-based survey. Respondents met eligibility criteria if they were adults aged 18 years or older who self-reported 6 or more migraine days per month and completed the survey in full. They were asked to choose between options defined by six attributes: reduction in headache days per month, frequency of limitations with physical activities, cognition problems, weight gain, how the medicine is taken, and monthly out-of-pocket cost.
Of the 300 respondents included in the analysis, 72% indicated that migraines make physical activities difficult all or most of the time, and 81% had taken a prescription migraine preventive in the last 6 months. Respondents reported, on average, approximately 16 headache days per month. Among noncost attributes, respondents valued a change from a 10% reduction in migraine days to a 50% reduction more highly than avoiding the worst levels of adverse events – defined as memory problems and 10% weight gain – but were willing to trade off efficacy for less-severe adverse events. Avoiding memory problems was more important than avoiding thinking problems. Avoiding a 10% weight gain was more important than avoiding thinking and memory problems. Respondents preferred a once-monthly injection or daily pill to twice-monthly injections. Respondents, on average, were willing to pay $116 per month for an improvement from 10% to 50% in reduced headache days (95% confidence interval [CI], $91-$141) and $43 for an improvement from 10% to 25% (95% CI, $34-$53). They were also willing to pay $84 per month to avoid a 10% weight gain (95% CI, $64-$103), $59 per month to avoid memory problems (95% CI, $42-$76), and $32 per month to avoid thinking problems (95% CI, $18-$46).
The coauthors acknowledged their study’s limitations, including all migraine diagnoses being self-reported and the study sample not necessarily being representative of patients with migraine overall. In addition, though the potential medicinal attributes used were prominent in clinical literature and focus groups, they could choose only a limited amount and so their analysis “did not address other attributes that may be important to patients.”
Given their findings, the researchers recommended that “clinicians should work with patients to select treatments that meet each patient’s needs.”
Amgen and Novartis funded the study. The authors reported numerous conflicts of interest, including receiving grants, consulting fees, and royalties from pharmaceutical companies and organizations. During the study, three of the authors were employed at RTI Health Solutions, a non-for-profit organization that conducts research with pharmaceutical companies such as the study’s sponsor.
SOURCE: Mansfield C et al. Headache. 2019 May;59(5):715-26. doi: 10.1111/head.13498.
, according to the results of a study published in Headache. When offered hypothetical preventive migraine medicines with a wide array of attributes, patients leaned toward those with a reduction in migraine days and an avoidance of weight gain, according to an analysis of responses to a discrete-choice experiment survey.
“We found that respondents had a significant willingness to pay for medicines with higher efficacy and less-severe adverse events,” wrote Carol Mansfield, PhD, of RTI Health Solutions in North Carolina, and coauthors.
To evaluate patient preferences for theoretical migraine medicine, the researchers conducted a discrete-choice experiment via a web-based survey. Respondents met eligibility criteria if they were adults aged 18 years or older who self-reported 6 or more migraine days per month and completed the survey in full. They were asked to choose between options defined by six attributes: reduction in headache days per month, frequency of limitations with physical activities, cognition problems, weight gain, how the medicine is taken, and monthly out-of-pocket cost.
Of the 300 respondents included in the analysis, 72% indicated that migraines make physical activities difficult all or most of the time, and 81% had taken a prescription migraine preventive in the last 6 months. Respondents reported, on average, approximately 16 headache days per month. Among noncost attributes, respondents valued a change from a 10% reduction in migraine days to a 50% reduction more highly than avoiding the worst levels of adverse events – defined as memory problems and 10% weight gain – but were willing to trade off efficacy for less-severe adverse events. Avoiding memory problems was more important than avoiding thinking problems. Avoiding a 10% weight gain was more important than avoiding thinking and memory problems. Respondents preferred a once-monthly injection or daily pill to twice-monthly injections. Respondents, on average, were willing to pay $116 per month for an improvement from 10% to 50% in reduced headache days (95% confidence interval [CI], $91-$141) and $43 for an improvement from 10% to 25% (95% CI, $34-$53). They were also willing to pay $84 per month to avoid a 10% weight gain (95% CI, $64-$103), $59 per month to avoid memory problems (95% CI, $42-$76), and $32 per month to avoid thinking problems (95% CI, $18-$46).
The coauthors acknowledged their study’s limitations, including all migraine diagnoses being self-reported and the study sample not necessarily being representative of patients with migraine overall. In addition, though the potential medicinal attributes used were prominent in clinical literature and focus groups, they could choose only a limited amount and so their analysis “did not address other attributes that may be important to patients.”
Given their findings, the researchers recommended that “clinicians should work with patients to select treatments that meet each patient’s needs.”
Amgen and Novartis funded the study. The authors reported numerous conflicts of interest, including receiving grants, consulting fees, and royalties from pharmaceutical companies and organizations. During the study, three of the authors were employed at RTI Health Solutions, a non-for-profit organization that conducts research with pharmaceutical companies such as the study’s sponsor.
SOURCE: Mansfield C et al. Headache. 2019 May;59(5):715-26. doi: 10.1111/head.13498.
FROM HEADACHE
Only 1.5% of individuals at high risk of opioid overdose receive naloxone
The vast majority of individuals at high risk for opioid overdose do not receive naloxone, despite numerous opportunities, according to Sarah Follman and associates from the University of Chicago.
In a retrospective study published in JAMA Network Open, the study authors analyzed data from individuals in the Truven Health MarketScan Research Database who had ICD-10 codes related to opioid use, misuse, dependence, and overdose. Data from Oct. 1, 2015, through Dec. 31, 2016, were included; a total of 138,108 high-risk individuals were identified as interacting with the health care system nearly 1.2 million times (88,618 hospitalizations, 229,680 ED visits, 298,058 internal medicine visits, and 568,448 family practice visits).
Of the 138,108 individuals in the study, only 2,135 (1.5%) were prescribed naloxone during the study period. Patients who had prior diagnoses of both opioid misuse/dependence and overdose were significantly more likely to receive naloxone than were those who only had a history of opioid dependence (odds ratio, 2.32; 95% confidence interval, 1.98-2.72; P less than .001). In addition, having a history of overdose alone was associated with a decreased chance of receiving naloxone, compared with those with a history of opioid misuse alone (OR, 0.73; 95% CI, 0.57-0.94; P = .01).
Other factors that significantly reduced the odds of receiving naloxone included being aged 30-44 years and being from the Midwest or West. Factors that reduced the odds include having received treatment for opioid use disorder, visiting a detoxification facility, receiving other substance use disorder treatment; and having received outpatient care from a pain specialist, psychologist, or surgeon.
“Most individuals at high risk of opioid overdose do not receive naloxone through direct prescribing,” Ms. Follman and associates wrote. “Clinicians can address this gap by regularly prescribing naloxone to eligible patients. To address barriers to prescribing, hospital systems and medical schools can support clinicians by improving education on screening and treating substance use disorders, clarifying legal concerns, and developing policies and protocols to guide implementation of increased prescribing.
No conflicts of interest were reported; one coauthor reported receiving a grant from the National Institutes of Health.
SOURCE: Follman S et al. JAMA Netw Open. 2019 May 3. doi: 10.1001/jamanetworkopen.2019.3209.
The vast majority of individuals at high risk for opioid overdose do not receive naloxone, despite numerous opportunities, according to Sarah Follman and associates from the University of Chicago.
In a retrospective study published in JAMA Network Open, the study authors analyzed data from individuals in the Truven Health MarketScan Research Database who had ICD-10 codes related to opioid use, misuse, dependence, and overdose. Data from Oct. 1, 2015, through Dec. 31, 2016, were included; a total of 138,108 high-risk individuals were identified as interacting with the health care system nearly 1.2 million times (88,618 hospitalizations, 229,680 ED visits, 298,058 internal medicine visits, and 568,448 family practice visits).
Of the 138,108 individuals in the study, only 2,135 (1.5%) were prescribed naloxone during the study period. Patients who had prior diagnoses of both opioid misuse/dependence and overdose were significantly more likely to receive naloxone than were those who only had a history of opioid dependence (odds ratio, 2.32; 95% confidence interval, 1.98-2.72; P less than .001). In addition, having a history of overdose alone was associated with a decreased chance of receiving naloxone, compared with those with a history of opioid misuse alone (OR, 0.73; 95% CI, 0.57-0.94; P = .01).
Other factors that significantly reduced the odds of receiving naloxone included being aged 30-44 years and being from the Midwest or West. Factors that reduced the odds include having received treatment for opioid use disorder, visiting a detoxification facility, receiving other substance use disorder treatment; and having received outpatient care from a pain specialist, psychologist, or surgeon.
“Most individuals at high risk of opioid overdose do not receive naloxone through direct prescribing,” Ms. Follman and associates wrote. “Clinicians can address this gap by regularly prescribing naloxone to eligible patients. To address barriers to prescribing, hospital systems and medical schools can support clinicians by improving education on screening and treating substance use disorders, clarifying legal concerns, and developing policies and protocols to guide implementation of increased prescribing.
No conflicts of interest were reported; one coauthor reported receiving a grant from the National Institutes of Health.
SOURCE: Follman S et al. JAMA Netw Open. 2019 May 3. doi: 10.1001/jamanetworkopen.2019.3209.
The vast majority of individuals at high risk for opioid overdose do not receive naloxone, despite numerous opportunities, according to Sarah Follman and associates from the University of Chicago.
In a retrospective study published in JAMA Network Open, the study authors analyzed data from individuals in the Truven Health MarketScan Research Database who had ICD-10 codes related to opioid use, misuse, dependence, and overdose. Data from Oct. 1, 2015, through Dec. 31, 2016, were included; a total of 138,108 high-risk individuals were identified as interacting with the health care system nearly 1.2 million times (88,618 hospitalizations, 229,680 ED visits, 298,058 internal medicine visits, and 568,448 family practice visits).
Of the 138,108 individuals in the study, only 2,135 (1.5%) were prescribed naloxone during the study period. Patients who had prior diagnoses of both opioid misuse/dependence and overdose were significantly more likely to receive naloxone than were those who only had a history of opioid dependence (odds ratio, 2.32; 95% confidence interval, 1.98-2.72; P less than .001). In addition, having a history of overdose alone was associated with a decreased chance of receiving naloxone, compared with those with a history of opioid misuse alone (OR, 0.73; 95% CI, 0.57-0.94; P = .01).
Other factors that significantly reduced the odds of receiving naloxone included being aged 30-44 years and being from the Midwest or West. Factors that reduced the odds include having received treatment for opioid use disorder, visiting a detoxification facility, receiving other substance use disorder treatment; and having received outpatient care from a pain specialist, psychologist, or surgeon.
“Most individuals at high risk of opioid overdose do not receive naloxone through direct prescribing,” Ms. Follman and associates wrote. “Clinicians can address this gap by regularly prescribing naloxone to eligible patients. To address barriers to prescribing, hospital systems and medical schools can support clinicians by improving education on screening and treating substance use disorders, clarifying legal concerns, and developing policies and protocols to guide implementation of increased prescribing.
No conflicts of interest were reported; one coauthor reported receiving a grant from the National Institutes of Health.
SOURCE: Follman S et al. JAMA Netw Open. 2019 May 3. doi: 10.1001/jamanetworkopen.2019.3209.
FROM JAMA NETWORK OPEN
Opioid management program reduced number of narcotics prescribed after breast surgery
An opioid prescription management program implemented at the Cleveland Clinic has led to a reduction in the number of narcotics prescribed to patients after breast surgery, according to research presented in a recent webcast from the annual meeting of the American Society of Breast Surgeons.
“The opioid epidemic has become a critical issue, and narcotic abuse has continued to rise,” Stephanie Valente, DO, FACS, from the Cleveland Clinic, said in her presentation. “Excess narcotic prescriptions may be contributing to this opioid epidemic,” and there are no current narcotic prescribing guidelines for patients after breast surgery, she said. In addition, studies have shown surgeons can overestimate the number of opioid pills a patient needs after surgery for pain control, and any excess pills are at risk of being stolen or inappropriately used, she added.
Dr. Valente and colleagues performed a baseline evaluation of narcotic pills prescribed by surgeons at the Cleveland Clinic for patients who have undergone excisional biopsy or lumpectomy, mastectomy, and mastectomy with reconstruction. They found the median number of narcotics prescribed were 15 pills for excisional biopsy or lumpectomy patients, 20 pills for mastectomy patients and 28 pills for mastectomy with reconstruction patients.
The researchers sought to lower those numbers, and created a departmental change in which they decreased the median number of pills prescribed at discharge from 15 pills to 10 pills for excisional biopsy or lumpectomy patients and from 28 pills to 25 pills for patients who undergo mastectomy with reconstruction. They then examined 100 consecutive patients after a 3-month implementation period to determine whether prescribing numbers had changed and found the surgeons adhered to the prescribing guidelines, which resulted in a statistically significant reduction in median opioid pills prescribed for excisional biopsy or lumpectomy (P less than .01) and mastectomy with reconstruction patients (P less than .01).
“After their departmental plan change, we observed that, as planned, a statistically significant decrease in prescribing practices amongst surgeons was able to be performed, showing that surgeons were able to adhere to these new prescribing practices,” said Dr. Valente.
When they examined the number of pills patients reported they used after surgery, they found excisional biopsy or lumpectomy patients took an average of 1 pill, mastectomy patients took an average of 3 pills, and mastectomy with reconstruction patients took an average of 18 pills. “These were all statistically much less than what was being prescribed even after our purposeful reduction,” said Dr. Valente.
In the study, 40% of patients who underwent breast surgery overall reported that they did not have any postoperative narcotic use at all, with the least narcotic use seen among patients who underwent excisional biopsy or lumpectomy.
“Further directions for opiate reduction can include evaluation of the impact of type and amount of local anesthetic given intraoperatively, and the amount of narcotics used postoperatively … to identify patient factors that contribute to the low narcotic usage postoperatively, and finally, to figure out how to maximize the benefit of adding a formal ERAS [enhanced recovery after surgery] protocol to further reduce patient needs for as many narcotic pills,” said Dr. Valente.
Dr. Valente had no disclosures.
An opioid prescription management program implemented at the Cleveland Clinic has led to a reduction in the number of narcotics prescribed to patients after breast surgery, according to research presented in a recent webcast from the annual meeting of the American Society of Breast Surgeons.
“The opioid epidemic has become a critical issue, and narcotic abuse has continued to rise,” Stephanie Valente, DO, FACS, from the Cleveland Clinic, said in her presentation. “Excess narcotic prescriptions may be contributing to this opioid epidemic,” and there are no current narcotic prescribing guidelines for patients after breast surgery, she said. In addition, studies have shown surgeons can overestimate the number of opioid pills a patient needs after surgery for pain control, and any excess pills are at risk of being stolen or inappropriately used, she added.
Dr. Valente and colleagues performed a baseline evaluation of narcotic pills prescribed by surgeons at the Cleveland Clinic for patients who have undergone excisional biopsy or lumpectomy, mastectomy, and mastectomy with reconstruction. They found the median number of narcotics prescribed were 15 pills for excisional biopsy or lumpectomy patients, 20 pills for mastectomy patients and 28 pills for mastectomy with reconstruction patients.
The researchers sought to lower those numbers, and created a departmental change in which they decreased the median number of pills prescribed at discharge from 15 pills to 10 pills for excisional biopsy or lumpectomy patients and from 28 pills to 25 pills for patients who undergo mastectomy with reconstruction. They then examined 100 consecutive patients after a 3-month implementation period to determine whether prescribing numbers had changed and found the surgeons adhered to the prescribing guidelines, which resulted in a statistically significant reduction in median opioid pills prescribed for excisional biopsy or lumpectomy (P less than .01) and mastectomy with reconstruction patients (P less than .01).
“After their departmental plan change, we observed that, as planned, a statistically significant decrease in prescribing practices amongst surgeons was able to be performed, showing that surgeons were able to adhere to these new prescribing practices,” said Dr. Valente.
When they examined the number of pills patients reported they used after surgery, they found excisional biopsy or lumpectomy patients took an average of 1 pill, mastectomy patients took an average of 3 pills, and mastectomy with reconstruction patients took an average of 18 pills. “These were all statistically much less than what was being prescribed even after our purposeful reduction,” said Dr. Valente.
In the study, 40% of patients who underwent breast surgery overall reported that they did not have any postoperative narcotic use at all, with the least narcotic use seen among patients who underwent excisional biopsy or lumpectomy.
“Further directions for opiate reduction can include evaluation of the impact of type and amount of local anesthetic given intraoperatively, and the amount of narcotics used postoperatively … to identify patient factors that contribute to the low narcotic usage postoperatively, and finally, to figure out how to maximize the benefit of adding a formal ERAS [enhanced recovery after surgery] protocol to further reduce patient needs for as many narcotic pills,” said Dr. Valente.
Dr. Valente had no disclosures.
An opioid prescription management program implemented at the Cleveland Clinic has led to a reduction in the number of narcotics prescribed to patients after breast surgery, according to research presented in a recent webcast from the annual meeting of the American Society of Breast Surgeons.
“The opioid epidemic has become a critical issue, and narcotic abuse has continued to rise,” Stephanie Valente, DO, FACS, from the Cleveland Clinic, said in her presentation. “Excess narcotic prescriptions may be contributing to this opioid epidemic,” and there are no current narcotic prescribing guidelines for patients after breast surgery, she said. In addition, studies have shown surgeons can overestimate the number of opioid pills a patient needs after surgery for pain control, and any excess pills are at risk of being stolen or inappropriately used, she added.
Dr. Valente and colleagues performed a baseline evaluation of narcotic pills prescribed by surgeons at the Cleveland Clinic for patients who have undergone excisional biopsy or lumpectomy, mastectomy, and mastectomy with reconstruction. They found the median number of narcotics prescribed were 15 pills for excisional biopsy or lumpectomy patients, 20 pills for mastectomy patients and 28 pills for mastectomy with reconstruction patients.
The researchers sought to lower those numbers, and created a departmental change in which they decreased the median number of pills prescribed at discharge from 15 pills to 10 pills for excisional biopsy or lumpectomy patients and from 28 pills to 25 pills for patients who undergo mastectomy with reconstruction. They then examined 100 consecutive patients after a 3-month implementation period to determine whether prescribing numbers had changed and found the surgeons adhered to the prescribing guidelines, which resulted in a statistically significant reduction in median opioid pills prescribed for excisional biopsy or lumpectomy (P less than .01) and mastectomy with reconstruction patients (P less than .01).
“After their departmental plan change, we observed that, as planned, a statistically significant decrease in prescribing practices amongst surgeons was able to be performed, showing that surgeons were able to adhere to these new prescribing practices,” said Dr. Valente.
When they examined the number of pills patients reported they used after surgery, they found excisional biopsy or lumpectomy patients took an average of 1 pill, mastectomy patients took an average of 3 pills, and mastectomy with reconstruction patients took an average of 18 pills. “These were all statistically much less than what was being prescribed even after our purposeful reduction,” said Dr. Valente.
In the study, 40% of patients who underwent breast surgery overall reported that they did not have any postoperative narcotic use at all, with the least narcotic use seen among patients who underwent excisional biopsy or lumpectomy.
“Further directions for opiate reduction can include evaluation of the impact of type and amount of local anesthetic given intraoperatively, and the amount of narcotics used postoperatively … to identify patient factors that contribute to the low narcotic usage postoperatively, and finally, to figure out how to maximize the benefit of adding a formal ERAS [enhanced recovery after surgery] protocol to further reduce patient needs for as many narcotic pills,” said Dr. Valente.
Dr. Valente had no disclosures.
REPORTING FROM ASBS 2019
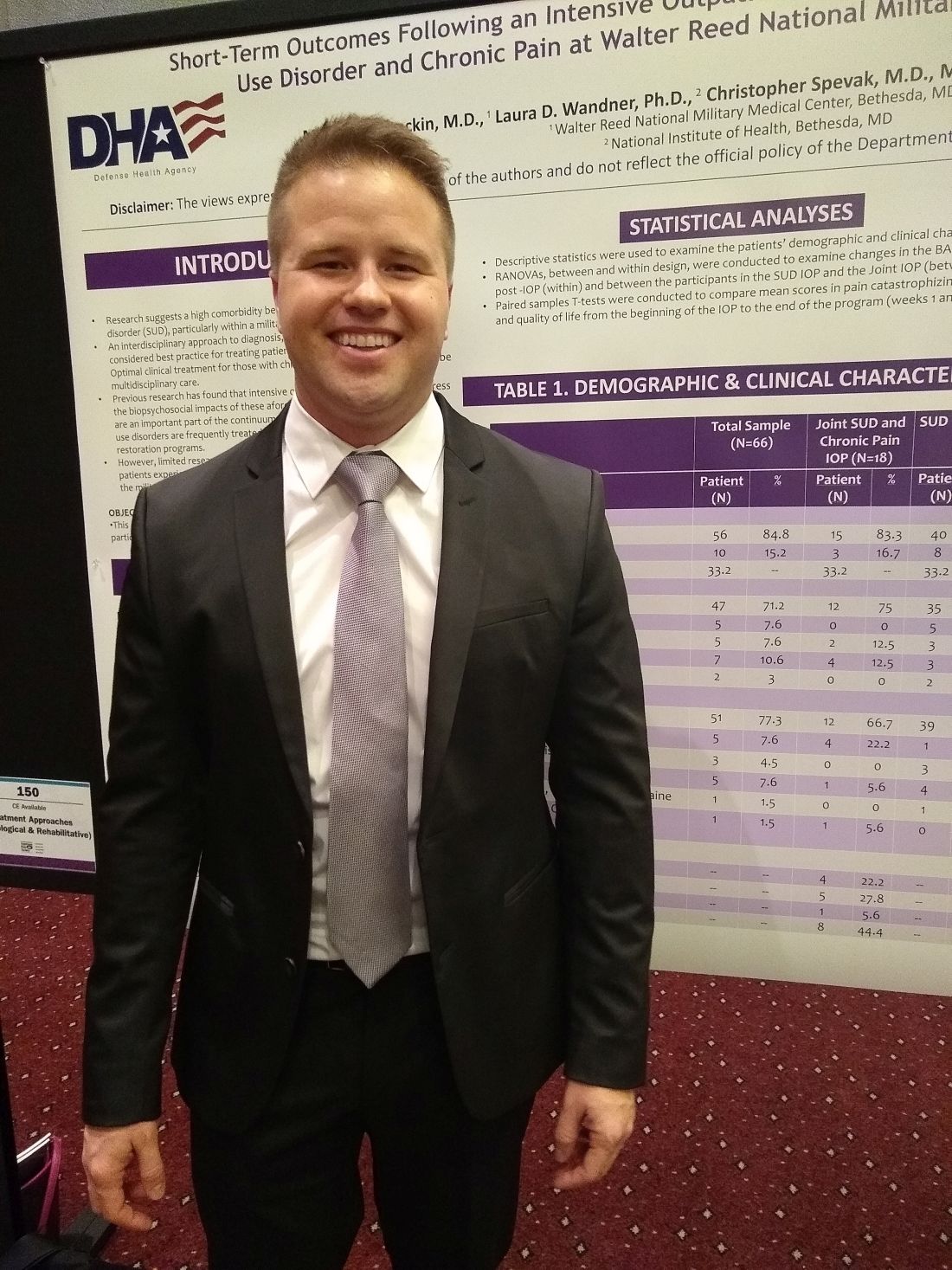
Outpatient program successfully tackles substance use and chronic pain
MILWAUKEE – An interdisciplinary intensive outpatient treatment program addressing chronic pain and substance use disorder effectively addressed both diagnoses in a military population.
Intensive outpatient programs (IOPs) frequently address these conditions within a biopsychosocial format, but it’s not common for IOPs to have this dual focus on chronic pain and substance use disorder (SUD), said Michael Stockin, MD, speaking in an interview at the scientific meeting of the American Pain Society.
Dr. Stockin said he and his collaborators recognized that, especially among a military population, the two conditions have considerable overlap, so it made sense to integrate behavioral treatment for both conditions in an intensive outpatient program. “Our hypothesis was that if you can use an intensive outpatient program to address substance use disorder, maybe you can actually add a chronic pain curriculum – like a functional restoration program to it.
“As a result of our study, we did find that there were significant differences in worst pain scores as a result of the program. In the people who took both the substance use disorder and chronic pain curriculum, we found significant reductions in total impairment, worst pain, and they also had less … substance use as well,” said Dr. Stockin.
In a quality improvement project, Dr. Stockin and collaborators compared short-term outcomes for patients who received IOP treatment addressing both chronic pain and SUD with those receiving SUD-only IOP.
For those participating in the joint IOP, scores indicating worst pain on the 0-10 numeric rating scale were reduced significantly, from 7.55 to 6.23 (P = .013). Scores on a functional measure of impairment, the Pain Outcomes Questionnaire Short Form (POQ-SF) also dropped significantly, from 84.92 to 63.50 (P = .034). The vitality domain of the POQ-SF also showed that patients had less impairment after participation in the joint IOP, with scores in that domain dropping from 20.17 to 17.25 (P = .024).
Looking at the total cohort, patient scores on the Brief Addiction Monitor (BAM) dropped significantly from baseline to the end of the intervention, indicating reduced substance use (P = .041). Mean scores for participants in the joint IOP were higher at baseline than for those in the SUD-only IOP (1.000 vs. 0.565). However, those participating in the joint IOP had lower mean postintervention BAM scores than the SUD-only cohort (0.071 vs. 0.174).
American veterans experience more severe pain and have a higher prevalence of chronic pain than nonveterans. Similarly, wrote Dr. Stockin, a chronic pain fellow in pain management at Walter Reed National Military Medical Center, Bethesda, Md., and colleagues in the poster presentation.
The project enrolled a total of 66 patients (10 female and 56 male). Of these, 18 participated in the joint SUD–chronic pain program, and 48 received usual treatment of the SUD-only IOP treatment. The mean overall age was 33.2 years, and 71.2% of participants were white.
Overall, 51 patients (77.3%) of participants had alcohol use disorder. Participants included active duty service members, veterans, and their dependents. Opioid and cannabis use disorders were experienced by a total of eight patients, and seven more patients had diagnoses of alcohol use disorder along with other substance use disorders.
All patients completed the BAM and received urine toxicology and alcohol breath testing at enrollment; drug and alcohol screening was completed at other points during the IOP treatment for both groups as well.
The joint IOP ran 3 full days a week, with a substance use curriculum in the morning and a pain management program in the afternoon; the SUD-only participants had three morning sessions weekly. Both interventions lasted 6 weeks, and Dr. Stockin said he and his colleagues would like to acquire longitudinal data to assess the durability of gains seen from the joint IOP.
The multidisciplinary team running the joint IOP was made up of an addiction/pain medicine physician, a clinical health psychologist, a physical therapist, social workers, and a nurse.
“This project is the first of its kind to find a significant reduction in pain burden while concurrently treating addiction and pain in an outpatient military health care setting,” Dr. Stockin and colleagues wrote in the poster accompanying the presentation.
“We had outcomes in both substance use and chronic pain that were positive, so it suggests that in the military health system, people may actually benefit from treating both chronic pain and substance use disorder concurrently. If you could harmonize those programs, you might be able to get good outcomes for soldiers and their families,” Dr. Stockin said.
Dr. Stockin reported no conflicts of interest. The project was funded by the Defense Health Agency.
MILWAUKEE – An interdisciplinary intensive outpatient treatment program addressing chronic pain and substance use disorder effectively addressed both diagnoses in a military population.
Intensive outpatient programs (IOPs) frequently address these conditions within a biopsychosocial format, but it’s not common for IOPs to have this dual focus on chronic pain and substance use disorder (SUD), said Michael Stockin, MD, speaking in an interview at the scientific meeting of the American Pain Society.
Dr. Stockin said he and his collaborators recognized that, especially among a military population, the two conditions have considerable overlap, so it made sense to integrate behavioral treatment for both conditions in an intensive outpatient program. “Our hypothesis was that if you can use an intensive outpatient program to address substance use disorder, maybe you can actually add a chronic pain curriculum – like a functional restoration program to it.
“As a result of our study, we did find that there were significant differences in worst pain scores as a result of the program. In the people who took both the substance use disorder and chronic pain curriculum, we found significant reductions in total impairment, worst pain, and they also had less … substance use as well,” said Dr. Stockin.
In a quality improvement project, Dr. Stockin and collaborators compared short-term outcomes for patients who received IOP treatment addressing both chronic pain and SUD with those receiving SUD-only IOP.
For those participating in the joint IOP, scores indicating worst pain on the 0-10 numeric rating scale were reduced significantly, from 7.55 to 6.23 (P = .013). Scores on a functional measure of impairment, the Pain Outcomes Questionnaire Short Form (POQ-SF) also dropped significantly, from 84.92 to 63.50 (P = .034). The vitality domain of the POQ-SF also showed that patients had less impairment after participation in the joint IOP, with scores in that domain dropping from 20.17 to 17.25 (P = .024).
Looking at the total cohort, patient scores on the Brief Addiction Monitor (BAM) dropped significantly from baseline to the end of the intervention, indicating reduced substance use (P = .041). Mean scores for participants in the joint IOP were higher at baseline than for those in the SUD-only IOP (1.000 vs. 0.565). However, those participating in the joint IOP had lower mean postintervention BAM scores than the SUD-only cohort (0.071 vs. 0.174).
American veterans experience more severe pain and have a higher prevalence of chronic pain than nonveterans. Similarly, wrote Dr. Stockin, a chronic pain fellow in pain management at Walter Reed National Military Medical Center, Bethesda, Md., and colleagues in the poster presentation.
The project enrolled a total of 66 patients (10 female and 56 male). Of these, 18 participated in the joint SUD–chronic pain program, and 48 received usual treatment of the SUD-only IOP treatment. The mean overall age was 33.2 years, and 71.2% of participants were white.
Overall, 51 patients (77.3%) of participants had alcohol use disorder. Participants included active duty service members, veterans, and their dependents. Opioid and cannabis use disorders were experienced by a total of eight patients, and seven more patients had diagnoses of alcohol use disorder along with other substance use disorders.
All patients completed the BAM and received urine toxicology and alcohol breath testing at enrollment; drug and alcohol screening was completed at other points during the IOP treatment for both groups as well.
The joint IOP ran 3 full days a week, with a substance use curriculum in the morning and a pain management program in the afternoon; the SUD-only participants had three morning sessions weekly. Both interventions lasted 6 weeks, and Dr. Stockin said he and his colleagues would like to acquire longitudinal data to assess the durability of gains seen from the joint IOP.
The multidisciplinary team running the joint IOP was made up of an addiction/pain medicine physician, a clinical health psychologist, a physical therapist, social workers, and a nurse.
“This project is the first of its kind to find a significant reduction in pain burden while concurrently treating addiction and pain in an outpatient military health care setting,” Dr. Stockin and colleagues wrote in the poster accompanying the presentation.
“We had outcomes in both substance use and chronic pain that were positive, so it suggests that in the military health system, people may actually benefit from treating both chronic pain and substance use disorder concurrently. If you could harmonize those programs, you might be able to get good outcomes for soldiers and their families,” Dr. Stockin said.
Dr. Stockin reported no conflicts of interest. The project was funded by the Defense Health Agency.
MILWAUKEE – An interdisciplinary intensive outpatient treatment program addressing chronic pain and substance use disorder effectively addressed both diagnoses in a military population.
Intensive outpatient programs (IOPs) frequently address these conditions within a biopsychosocial format, but it’s not common for IOPs to have this dual focus on chronic pain and substance use disorder (SUD), said Michael Stockin, MD, speaking in an interview at the scientific meeting of the American Pain Society.
Dr. Stockin said he and his collaborators recognized that, especially among a military population, the two conditions have considerable overlap, so it made sense to integrate behavioral treatment for both conditions in an intensive outpatient program. “Our hypothesis was that if you can use an intensive outpatient program to address substance use disorder, maybe you can actually add a chronic pain curriculum – like a functional restoration program to it.
“As a result of our study, we did find that there were significant differences in worst pain scores as a result of the program. In the people who took both the substance use disorder and chronic pain curriculum, we found significant reductions in total impairment, worst pain, and they also had less … substance use as well,” said Dr. Stockin.
In a quality improvement project, Dr. Stockin and collaborators compared short-term outcomes for patients who received IOP treatment addressing both chronic pain and SUD with those receiving SUD-only IOP.
For those participating in the joint IOP, scores indicating worst pain on the 0-10 numeric rating scale were reduced significantly, from 7.55 to 6.23 (P = .013). Scores on a functional measure of impairment, the Pain Outcomes Questionnaire Short Form (POQ-SF) also dropped significantly, from 84.92 to 63.50 (P = .034). The vitality domain of the POQ-SF also showed that patients had less impairment after participation in the joint IOP, with scores in that domain dropping from 20.17 to 17.25 (P = .024).
Looking at the total cohort, patient scores on the Brief Addiction Monitor (BAM) dropped significantly from baseline to the end of the intervention, indicating reduced substance use (P = .041). Mean scores for participants in the joint IOP were higher at baseline than for those in the SUD-only IOP (1.000 vs. 0.565). However, those participating in the joint IOP had lower mean postintervention BAM scores than the SUD-only cohort (0.071 vs. 0.174).
American veterans experience more severe pain and have a higher prevalence of chronic pain than nonveterans. Similarly, wrote Dr. Stockin, a chronic pain fellow in pain management at Walter Reed National Military Medical Center, Bethesda, Md., and colleagues in the poster presentation.
The project enrolled a total of 66 patients (10 female and 56 male). Of these, 18 participated in the joint SUD–chronic pain program, and 48 received usual treatment of the SUD-only IOP treatment. The mean overall age was 33.2 years, and 71.2% of participants were white.
Overall, 51 patients (77.3%) of participants had alcohol use disorder. Participants included active duty service members, veterans, and their dependents. Opioid and cannabis use disorders were experienced by a total of eight patients, and seven more patients had diagnoses of alcohol use disorder along with other substance use disorders.
All patients completed the BAM and received urine toxicology and alcohol breath testing at enrollment; drug and alcohol screening was completed at other points during the IOP treatment for both groups as well.
The joint IOP ran 3 full days a week, with a substance use curriculum in the morning and a pain management program in the afternoon; the SUD-only participants had three morning sessions weekly. Both interventions lasted 6 weeks, and Dr. Stockin said he and his colleagues would like to acquire longitudinal data to assess the durability of gains seen from the joint IOP.
The multidisciplinary team running the joint IOP was made up of an addiction/pain medicine physician, a clinical health psychologist, a physical therapist, social workers, and a nurse.
“This project is the first of its kind to find a significant reduction in pain burden while concurrently treating addiction and pain in an outpatient military health care setting,” Dr. Stockin and colleagues wrote in the poster accompanying the presentation.
“We had outcomes in both substance use and chronic pain that were positive, so it suggests that in the military health system, people may actually benefit from treating both chronic pain and substance use disorder concurrently. If you could harmonize those programs, you might be able to get good outcomes for soldiers and their families,” Dr. Stockin said.
Dr. Stockin reported no conflicts of interest. The project was funded by the Defense Health Agency.
REPORTING FROM APS 2019
Key clinical point: An intensive, 6-week joint substance use disorder and chronic pain intensive outpatient program significantly reduced both substance use and pain.
Major finding: Patients had less pain and reduced substance use after completing the program, compared with baseline (P = .013 and .041, respectively).
Study details: A quality improvement project including 66 patients at a military health facility.
Disclosures: The study was sponsored by the Defense Health Agency. Dr. Stockin reported no conflicts of interest.
Click for Credit: Migraine & stroke risk; Aspirin for CV events; more
Here are 5 articles from the May issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Subclinical hypothyroidism boosts immediate risk of heart failure
To take the posttest, go to: https://bit.ly/2IK0YiL
Expires January 24, 2020
2. Meta-analysis supports aspirin to reduce cardiovascular events
To take the posttest, go to: https://bit.ly/2GJLgSB
Expires January 24, 2020
3. Age of migraine onset may affect stroke risk
To take the posttest, go to: https://bit.ly/2ZAJ5YR
Expires January 24, 2020
4. Women with RA have reduced chance of live birth after assisted reproduction treatment
To take the posttest, go to: https://bit.ly/2VvKRLF
Expires January 27, 2020
5. New SLE disease activity measure beats SLEDAI-2K
To take the posttest, go to: https://bit.ly/2W8SVPA
Expires January 31, 2020
Here are 5 articles from the May issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Subclinical hypothyroidism boosts immediate risk of heart failure
To take the posttest, go to: https://bit.ly/2IK0YiL
Expires January 24, 2020
2. Meta-analysis supports aspirin to reduce cardiovascular events
To take the posttest, go to: https://bit.ly/2GJLgSB
Expires January 24, 2020
3. Age of migraine onset may affect stroke risk
To take the posttest, go to: https://bit.ly/2ZAJ5YR
Expires January 24, 2020
4. Women with RA have reduced chance of live birth after assisted reproduction treatment
To take the posttest, go to: https://bit.ly/2VvKRLF
Expires January 27, 2020
5. New SLE disease activity measure beats SLEDAI-2K
To take the posttest, go to: https://bit.ly/2W8SVPA
Expires January 31, 2020
Here are 5 articles from the May issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Subclinical hypothyroidism boosts immediate risk of heart failure
To take the posttest, go to: https://bit.ly/2IK0YiL
Expires January 24, 2020
2. Meta-analysis supports aspirin to reduce cardiovascular events
To take the posttest, go to: https://bit.ly/2GJLgSB
Expires January 24, 2020
3. Age of migraine onset may affect stroke risk
To take the posttest, go to: https://bit.ly/2ZAJ5YR
Expires January 24, 2020
4. Women with RA have reduced chance of live birth after assisted reproduction treatment
To take the posttest, go to: https://bit.ly/2VvKRLF
Expires January 27, 2020
5. New SLE disease activity measure beats SLEDAI-2K
To take the posttest, go to: https://bit.ly/2W8SVPA
Expires January 31, 2020
Medical cannabis relieved pain, decreased opioid use in elderly
results of a recent retrospective chart review suggest. Treatment with medical cannabis improved pain, sleep, anxiety, and neuropathy in patients aged 75 years of age and older, and was associated with reduced use of opioids in about one-third of cases, according to authors of the study, which will be presented at the annual meeting of the American Academy of Neurology.
“Our findings are promising and can help fuel further research into medical marijuana as an additional option for this group of people who often have chronic conditions,” said lead investigator Laszlo Mechtler, MD, of Dent Neurologic Institute in Buffalo, N.Y., in a news release. However, additional randomized, placebo-controlled studies are needed to confirm results of this study, Dr. Mechtler added.
The chart review focused on 204 elderly patients who participated in New York State’s medical marijuana program and were followed in a neurologic outpatient setting. The cohort included 129 female and 75 male patients, ranging in age from 75 to 102 years, with a mean age of 81 years. The medical marijuana was taken by mouth as a liquid extract tincture, capsule, or in an electronic vaporizer.
With an average exposure time of 16.8 weeks, 69% of patients experienced symptomatic benefit, according to patient self-report. The most commonly reported benefit was relief of chronic pain in 49%, while improvements in sleep, neuropathy, and anxiety were reported in 18%, 15%, and 10%, respectively. Reductions in opioid pain medication were noted in about one-third of cases, they found.
While 34% of patients had adverse effects on medical marijuana, only 21% reported adverse effects after cannabinoid doses were adjusted, investigators said. Adverse effects led to discontinuation of medical cannabis in seven patients, or 3.4% of the overall cohort. Somnolence, disequilibrium, and gastrointestinal disturbance were the most common adverse effects, occurring in 13%, 7%, and 7% of patients, respectively. Euphoria was reported in 3% of patients.
Among patients who had no reported adverse effects, the most commonly used formulation was a balanced 1:1 tincture of tetrahydrocannabinol to cannabidiol, investigators said.
Further trials could explore optimal dosing of medical cannabis in elderly patients and shed more light on adverse effects such as somnolence and disequilibrium, according to Dr. Mechtler and colleagues.
The study was supported by the Dent Family Foundation.
SOURCE: Bargnes V et al. AAN 2019, Abstract P4.1-014.
results of a recent retrospective chart review suggest. Treatment with medical cannabis improved pain, sleep, anxiety, and neuropathy in patients aged 75 years of age and older, and was associated with reduced use of opioids in about one-third of cases, according to authors of the study, which will be presented at the annual meeting of the American Academy of Neurology.
“Our findings are promising and can help fuel further research into medical marijuana as an additional option for this group of people who often have chronic conditions,” said lead investigator Laszlo Mechtler, MD, of Dent Neurologic Institute in Buffalo, N.Y., in a news release. However, additional randomized, placebo-controlled studies are needed to confirm results of this study, Dr. Mechtler added.
The chart review focused on 204 elderly patients who participated in New York State’s medical marijuana program and were followed in a neurologic outpatient setting. The cohort included 129 female and 75 male patients, ranging in age from 75 to 102 years, with a mean age of 81 years. The medical marijuana was taken by mouth as a liquid extract tincture, capsule, or in an electronic vaporizer.
With an average exposure time of 16.8 weeks, 69% of patients experienced symptomatic benefit, according to patient self-report. The most commonly reported benefit was relief of chronic pain in 49%, while improvements in sleep, neuropathy, and anxiety were reported in 18%, 15%, and 10%, respectively. Reductions in opioid pain medication were noted in about one-third of cases, they found.
While 34% of patients had adverse effects on medical marijuana, only 21% reported adverse effects after cannabinoid doses were adjusted, investigators said. Adverse effects led to discontinuation of medical cannabis in seven patients, or 3.4% of the overall cohort. Somnolence, disequilibrium, and gastrointestinal disturbance were the most common adverse effects, occurring in 13%, 7%, and 7% of patients, respectively. Euphoria was reported in 3% of patients.
Among patients who had no reported adverse effects, the most commonly used formulation was a balanced 1:1 tincture of tetrahydrocannabinol to cannabidiol, investigators said.
Further trials could explore optimal dosing of medical cannabis in elderly patients and shed more light on adverse effects such as somnolence and disequilibrium, according to Dr. Mechtler and colleagues.
The study was supported by the Dent Family Foundation.
SOURCE: Bargnes V et al. AAN 2019, Abstract P4.1-014.
results of a recent retrospective chart review suggest. Treatment with medical cannabis improved pain, sleep, anxiety, and neuropathy in patients aged 75 years of age and older, and was associated with reduced use of opioids in about one-third of cases, according to authors of the study, which will be presented at the annual meeting of the American Academy of Neurology.
“Our findings are promising and can help fuel further research into medical marijuana as an additional option for this group of people who often have chronic conditions,” said lead investigator Laszlo Mechtler, MD, of Dent Neurologic Institute in Buffalo, N.Y., in a news release. However, additional randomized, placebo-controlled studies are needed to confirm results of this study, Dr. Mechtler added.
The chart review focused on 204 elderly patients who participated in New York State’s medical marijuana program and were followed in a neurologic outpatient setting. The cohort included 129 female and 75 male patients, ranging in age from 75 to 102 years, with a mean age of 81 years. The medical marijuana was taken by mouth as a liquid extract tincture, capsule, or in an electronic vaporizer.
With an average exposure time of 16.8 weeks, 69% of patients experienced symptomatic benefit, according to patient self-report. The most commonly reported benefit was relief of chronic pain in 49%, while improvements in sleep, neuropathy, and anxiety were reported in 18%, 15%, and 10%, respectively. Reductions in opioid pain medication were noted in about one-third of cases, they found.
While 34% of patients had adverse effects on medical marijuana, only 21% reported adverse effects after cannabinoid doses were adjusted, investigators said. Adverse effects led to discontinuation of medical cannabis in seven patients, or 3.4% of the overall cohort. Somnolence, disequilibrium, and gastrointestinal disturbance were the most common adverse effects, occurring in 13%, 7%, and 7% of patients, respectively. Euphoria was reported in 3% of patients.
Among patients who had no reported adverse effects, the most commonly used formulation was a balanced 1:1 tincture of tetrahydrocannabinol to cannabidiol, investigators said.
Further trials could explore optimal dosing of medical cannabis in elderly patients and shed more light on adverse effects such as somnolence and disequilibrium, according to Dr. Mechtler and colleagues.
The study was supported by the Dent Family Foundation.
SOURCE: Bargnes V et al. AAN 2019, Abstract P4.1-014.
FROM AAN 2019
CDC warns against misuse of opioid-prescribing guideline
Officials at the Centers for Disease Control and Prevention are warning against the misapplication of the agency’s 2016 guidelines on opioid prescribing, as well as clarifying dosage recommendations for patients starting or stopping pain medications.
In a perspective published in the New England Journal of Medicine on April 24, lead author Deborah Dowell, MD, chief medical officer for the CDC’s National Center for Injury Prevention and Control, conveyed concern that some policies and practices derived from the 2016 CDC Guideline for Prescribing Opioids for Chronic Pain are inconsistent with the recommendations and often go beyond their scope.
Misapplication examples include inappropriately applying the guideline to patients in active cancer treatment, patients experiencing acute sickle cell crises, or patients experiencing postsurgical pain, Dr. Dowell wrote.
The guideline offers guidance to clinicians treating chronic pain in adults who are already receiving opioids long-term at high dosages, she noted. It includes advice on maximizing nonopioid treatment, reviewing risks associated with continuing high-dose opioids, and collaborating with patients who agree to taper dosage, among other guidance.
Any application of the guideline’s dosage recommendation that results in hard limits or “cutting off” opioids is also an incorrect use of the recommendations, according to Dr. Dowell.
While the guideline advises clinicians to start opioids at the lowest effective dosage and avoid increasing dosage to 90 morphine milligram equivalents per day or more, that statement does not suggest discontinuation of opioids already prescribed at high dosages, according to the CDC’s clarification.
The guidance also does not apply to patients receiving or starting medication-assisted treatment for opioid use disorder.
The commentary comes after a trio of organizations raised concerns that insurers are inappropriately applying the recommendations to active cancer patients when making coverage determinations.
The American Society of Clinical Oncology, the National Comprehensive Cancer Network, and the American Society of Hematology, raised the issue in a letter to the CDC in February. In response, Dr. Dowell clarified that the recommendations are not intended to deny clinically appropriate opioid therapy to any patients who suffer chronic pain, but rather to ensure that physicians and patients consider all safe and effective treatment options.
In the perspective, Dr. Dowell wrote that the CDC is evaluating the intended and unintended impact of the 2016 opioid-prescribing guideline on clinician and patient outcomes and that the agency is committed to updating the recommendations when new evidence is available.
Officials at the Centers for Disease Control and Prevention are warning against the misapplication of the agency’s 2016 guidelines on opioid prescribing, as well as clarifying dosage recommendations for patients starting or stopping pain medications.
In a perspective published in the New England Journal of Medicine on April 24, lead author Deborah Dowell, MD, chief medical officer for the CDC’s National Center for Injury Prevention and Control, conveyed concern that some policies and practices derived from the 2016 CDC Guideline for Prescribing Opioids for Chronic Pain are inconsistent with the recommendations and often go beyond their scope.
Misapplication examples include inappropriately applying the guideline to patients in active cancer treatment, patients experiencing acute sickle cell crises, or patients experiencing postsurgical pain, Dr. Dowell wrote.
The guideline offers guidance to clinicians treating chronic pain in adults who are already receiving opioids long-term at high dosages, she noted. It includes advice on maximizing nonopioid treatment, reviewing risks associated with continuing high-dose opioids, and collaborating with patients who agree to taper dosage, among other guidance.
Any application of the guideline’s dosage recommendation that results in hard limits or “cutting off” opioids is also an incorrect use of the recommendations, according to Dr. Dowell.
While the guideline advises clinicians to start opioids at the lowest effective dosage and avoid increasing dosage to 90 morphine milligram equivalents per day or more, that statement does not suggest discontinuation of opioids already prescribed at high dosages, according to the CDC’s clarification.
The guidance also does not apply to patients receiving or starting medication-assisted treatment for opioid use disorder.
The commentary comes after a trio of organizations raised concerns that insurers are inappropriately applying the recommendations to active cancer patients when making coverage determinations.
The American Society of Clinical Oncology, the National Comprehensive Cancer Network, and the American Society of Hematology, raised the issue in a letter to the CDC in February. In response, Dr. Dowell clarified that the recommendations are not intended to deny clinically appropriate opioid therapy to any patients who suffer chronic pain, but rather to ensure that physicians and patients consider all safe and effective treatment options.
In the perspective, Dr. Dowell wrote that the CDC is evaluating the intended and unintended impact of the 2016 opioid-prescribing guideline on clinician and patient outcomes and that the agency is committed to updating the recommendations when new evidence is available.
Officials at the Centers for Disease Control and Prevention are warning against the misapplication of the agency’s 2016 guidelines on opioid prescribing, as well as clarifying dosage recommendations for patients starting or stopping pain medications.
In a perspective published in the New England Journal of Medicine on April 24, lead author Deborah Dowell, MD, chief medical officer for the CDC’s National Center for Injury Prevention and Control, conveyed concern that some policies and practices derived from the 2016 CDC Guideline for Prescribing Opioids for Chronic Pain are inconsistent with the recommendations and often go beyond their scope.
Misapplication examples include inappropriately applying the guideline to patients in active cancer treatment, patients experiencing acute sickle cell crises, or patients experiencing postsurgical pain, Dr. Dowell wrote.
The guideline offers guidance to clinicians treating chronic pain in adults who are already receiving opioids long-term at high dosages, she noted. It includes advice on maximizing nonopioid treatment, reviewing risks associated with continuing high-dose opioids, and collaborating with patients who agree to taper dosage, among other guidance.
Any application of the guideline’s dosage recommendation that results in hard limits or “cutting off” opioids is also an incorrect use of the recommendations, according to Dr. Dowell.
While the guideline advises clinicians to start opioids at the lowest effective dosage and avoid increasing dosage to 90 morphine milligram equivalents per day or more, that statement does not suggest discontinuation of opioids already prescribed at high dosages, according to the CDC’s clarification.
The guidance also does not apply to patients receiving or starting medication-assisted treatment for opioid use disorder.
The commentary comes after a trio of organizations raised concerns that insurers are inappropriately applying the recommendations to active cancer patients when making coverage determinations.
The American Society of Clinical Oncology, the National Comprehensive Cancer Network, and the American Society of Hematology, raised the issue in a letter to the CDC in February. In response, Dr. Dowell clarified that the recommendations are not intended to deny clinically appropriate opioid therapy to any patients who suffer chronic pain, but rather to ensure that physicians and patients consider all safe and effective treatment options.
In the perspective, Dr. Dowell wrote that the CDC is evaluating the intended and unintended impact of the 2016 opioid-prescribing guideline on clinician and patient outcomes and that the agency is committed to updating the recommendations when new evidence is available.
In chronic pain, catastrophizing contributes to disrupted brain circuitry
MILWAUKEE – When a patient with acute pain tumbles into a chronic pain state, many factors are at play, according to the widely accepted biopsychosocial theory of pain. Emotional, cognitive, and environmental components all contribute to the persistent and recalcitrant symptoms chronic pain patients experience.
Now, modern neuroimaging techniques show how for some, pain signals hijack the brain’s regulatory networks, allowing rumination and catastrophizing to intrude on the exteroception that’s critical to how humans interact with one another and the world. Interrupting catastrophizing with nonpharmacologic techniques yields measurable improvements – and there’s promise that a single treatment session can make a lasting difference.
“Psychosocial phenotypes, such as catastrophizing, are part of a complex biopsychosocial web of contributors to chronic pain. ,” said Robert R. Edwards, PhD, a psychologist at Brigham and Women’s Hospital/Harvard Medical School (Boston) Pain Management Center. Dr. Edwards moderated a session focused on catastrophizing at the scientific meeting of the American Pain Society.
Through magnetic resonance imaging techniques that measure functional connectivity, researchers can now see how nodes in the brain form connected networks that are differentially activated.
For example, the brain’s salience network (SLN) responds to stimuli that merit attention, such as evoked or clinical pain, Vitaly Napadow, PhD, said during his presentation. Key nodes in the SLN include the anterior cingulate cortex, the anterior insula, and the anterior temporoparietal junction. One function of the salience network, he said, is to regulate switching between the default mode network (DMN) – an interoceptive network – and the central executive network, usually active in exteroceptive tasks.
“The default mode network has been found to play an important role in pain processing,” Dr. Napadow said. These brain regions are more active in self-referential cognition – thinking about oneself – than when performing external tasks, he said. Consistently, studies have found decreased DMN deactivation in patients with chronic pain; essentially, the constant low hum of pain-focused DMN activity never turns off in a chronic pain state.
For patients with chronic pain, high levels of catastrophizing mean greater impact on functional brain connectivity, said Dr. Napadow, director of the Center for Integrative Pain NeuroImaging at the Martino Center for Biomedical Imaging at Massachusetts General Hospital and Harvard Medical School, Boston.
Looking at patients with chronic low back pain, he and his research team looked for connections between the DMN and the insula, which has a central role in pain processing. This connectivity was increased only in patients with high catastrophizing scores, said Dr. Napadow, with increased DMN-insula connectivity associated with increased pain scores only for this subgroup (Pain. 2019 Mar 4. doi: 10.1097/j.pain.0000000000001541).
“The model that we’re moving toward is that chronic pain leads to a blurring in the canonical network” of brain connectivity, Dr. Napadow said. “The speculation here is that the DMN-SLN linkage could be a sort of neural substrate for a common perception that chronic pain patients have – that their pain becomes part of who they are. Their interoceptive state becomes linked to the pain they are feeling: They are their pain.”
Where to turn with this information, which has large clinical implications? “Catastrophizing is a consistent risk factor for poor pain treatment outcomes, especially when we’re talking about pharmacologic treatments,” Dr. Edwards said. Also, chronic pain patients with the highest catastrophizing scores have the most opioid-related side effects, he said.
“Cognitive-behavioral therapy is potentially the most effective at reducing this risk factor,” said Dr. Edwards, noting that long-term effects were seen at 6 and 12 months post treatment. “These are significant, moderate-sized effects; there is some evidence that effects are largest in those with the highest baseline pain catastrophizing scores.”
“CBT is considered the gold standard, mainly because it’s the best studied” among treatment modalities, psychologist Beth Darnall, PhD, pointed out in her presentation. There’s evidence that other nonpharmacologic interventions can reduce catastrophizing: Psychology-informed yoga practices, physical therapy, and certain medical devices, such as high-frequency transcutaneous electric nerve stimulation units, may all have efficacy against catastrophizing and the downward spiral of chronic pain.
Still, a randomized controlled trial of CBT for pain in patients with fibromyalgia showed that the benefit, measured as reduction in pain interference with daily functioning, was almost twice as high in the high-catastrophizing group, “suggesting the potential utility of this method for patients at greatest risk,” said Dr. Edwards.
“We see a specific pattern of alterations in chronic pain similar to that seen in anxiety disorder; this suggests that some individuals are primed for the experience of pain,” said Dr. Darnall, clinical professor of anesthesiology, perioperative medicine, and pain medicine at Stanford (Calif.) University. “We are not born with the understanding of how to modulate pain and the distress it causes us.”
When she talks to patients, Dr. Darnall said: “I describe pain as being our ‘harm alarm.’ ... I like to describe it to people that ‘you have a very protective nervous system.’ ”
Dr. Darnall and her colleagues reported success with a pilot study of a single 2.5-hour-long session that addressed pain catastrophizing. From a baseline score of 26.1 on the Pain Catastrophizing Scale to a score of 13.8 at week 4, the 57 participants saw a significant decrease in mean scores on the scale (d [effect size] = 1.15).
On the strength of these early findings, Dr. Darnall and her collaborators are embarking on a randomized controlled trial ; the 3-arm comparative effectiveness study will compare a single-session intervention against 8 weeks of CBT or education-only classes for individuals with catastrophizing and chronic pain. The trial is structured to test the hypothesis that the single-session intervention will be noninferior to the full 8 weeks of CBT, Dr. Darnall said.
Building on the importance of avoiding stigmatizing and pejorative terms when talking about pain and catastrophizing, Dr. Darnall said she’s moved away from using the term “catastrophizing” in patient interactions. The one-session intervention is called “Empowered Relief – Train Your Brain Away from Pain.”
There’s a practical promise to a single-session class: Dr. Darnall has taught up to 85 patients at once, she said, adding, “This is a low-cost and scalable intervention.”
Dr. Edwards and Dr. Napadow reported funding from the National Institutes of Health, and they reported no conflicts of interest. Dr. Darnall reported funding from the NIH and the Patient-Centered Outcomes Research Institute. She serves on the scientific advisory board of Axial Healthcare and has several commercial publications about pain.
MILWAUKEE – When a patient with acute pain tumbles into a chronic pain state, many factors are at play, according to the widely accepted biopsychosocial theory of pain. Emotional, cognitive, and environmental components all contribute to the persistent and recalcitrant symptoms chronic pain patients experience.
Now, modern neuroimaging techniques show how for some, pain signals hijack the brain’s regulatory networks, allowing rumination and catastrophizing to intrude on the exteroception that’s critical to how humans interact with one another and the world. Interrupting catastrophizing with nonpharmacologic techniques yields measurable improvements – and there’s promise that a single treatment session can make a lasting difference.
“Psychosocial phenotypes, such as catastrophizing, are part of a complex biopsychosocial web of contributors to chronic pain. ,” said Robert R. Edwards, PhD, a psychologist at Brigham and Women’s Hospital/Harvard Medical School (Boston) Pain Management Center. Dr. Edwards moderated a session focused on catastrophizing at the scientific meeting of the American Pain Society.
Through magnetic resonance imaging techniques that measure functional connectivity, researchers can now see how nodes in the brain form connected networks that are differentially activated.
For example, the brain’s salience network (SLN) responds to stimuli that merit attention, such as evoked or clinical pain, Vitaly Napadow, PhD, said during his presentation. Key nodes in the SLN include the anterior cingulate cortex, the anterior insula, and the anterior temporoparietal junction. One function of the salience network, he said, is to regulate switching between the default mode network (DMN) – an interoceptive network – and the central executive network, usually active in exteroceptive tasks.
“The default mode network has been found to play an important role in pain processing,” Dr. Napadow said. These brain regions are more active in self-referential cognition – thinking about oneself – than when performing external tasks, he said. Consistently, studies have found decreased DMN deactivation in patients with chronic pain; essentially, the constant low hum of pain-focused DMN activity never turns off in a chronic pain state.
For patients with chronic pain, high levels of catastrophizing mean greater impact on functional brain connectivity, said Dr. Napadow, director of the Center for Integrative Pain NeuroImaging at the Martino Center for Biomedical Imaging at Massachusetts General Hospital and Harvard Medical School, Boston.
Looking at patients with chronic low back pain, he and his research team looked for connections between the DMN and the insula, which has a central role in pain processing. This connectivity was increased only in patients with high catastrophizing scores, said Dr. Napadow, with increased DMN-insula connectivity associated with increased pain scores only for this subgroup (Pain. 2019 Mar 4. doi: 10.1097/j.pain.0000000000001541).
“The model that we’re moving toward is that chronic pain leads to a blurring in the canonical network” of brain connectivity, Dr. Napadow said. “The speculation here is that the DMN-SLN linkage could be a sort of neural substrate for a common perception that chronic pain patients have – that their pain becomes part of who they are. Their interoceptive state becomes linked to the pain they are feeling: They are their pain.”
Where to turn with this information, which has large clinical implications? “Catastrophizing is a consistent risk factor for poor pain treatment outcomes, especially when we’re talking about pharmacologic treatments,” Dr. Edwards said. Also, chronic pain patients with the highest catastrophizing scores have the most opioid-related side effects, he said.
“Cognitive-behavioral therapy is potentially the most effective at reducing this risk factor,” said Dr. Edwards, noting that long-term effects were seen at 6 and 12 months post treatment. “These are significant, moderate-sized effects; there is some evidence that effects are largest in those with the highest baseline pain catastrophizing scores.”
“CBT is considered the gold standard, mainly because it’s the best studied” among treatment modalities, psychologist Beth Darnall, PhD, pointed out in her presentation. There’s evidence that other nonpharmacologic interventions can reduce catastrophizing: Psychology-informed yoga practices, physical therapy, and certain medical devices, such as high-frequency transcutaneous electric nerve stimulation units, may all have efficacy against catastrophizing and the downward spiral of chronic pain.
Still, a randomized controlled trial of CBT for pain in patients with fibromyalgia showed that the benefit, measured as reduction in pain interference with daily functioning, was almost twice as high in the high-catastrophizing group, “suggesting the potential utility of this method for patients at greatest risk,” said Dr. Edwards.
“We see a specific pattern of alterations in chronic pain similar to that seen in anxiety disorder; this suggests that some individuals are primed for the experience of pain,” said Dr. Darnall, clinical professor of anesthesiology, perioperative medicine, and pain medicine at Stanford (Calif.) University. “We are not born with the understanding of how to modulate pain and the distress it causes us.”
When she talks to patients, Dr. Darnall said: “I describe pain as being our ‘harm alarm.’ ... I like to describe it to people that ‘you have a very protective nervous system.’ ”
Dr. Darnall and her colleagues reported success with a pilot study of a single 2.5-hour-long session that addressed pain catastrophizing. From a baseline score of 26.1 on the Pain Catastrophizing Scale to a score of 13.8 at week 4, the 57 participants saw a significant decrease in mean scores on the scale (d [effect size] = 1.15).
On the strength of these early findings, Dr. Darnall and her collaborators are embarking on a randomized controlled trial ; the 3-arm comparative effectiveness study will compare a single-session intervention against 8 weeks of CBT or education-only classes for individuals with catastrophizing and chronic pain. The trial is structured to test the hypothesis that the single-session intervention will be noninferior to the full 8 weeks of CBT, Dr. Darnall said.
Building on the importance of avoiding stigmatizing and pejorative terms when talking about pain and catastrophizing, Dr. Darnall said she’s moved away from using the term “catastrophizing” in patient interactions. The one-session intervention is called “Empowered Relief – Train Your Brain Away from Pain.”
There’s a practical promise to a single-session class: Dr. Darnall has taught up to 85 patients at once, she said, adding, “This is a low-cost and scalable intervention.”
Dr. Edwards and Dr. Napadow reported funding from the National Institutes of Health, and they reported no conflicts of interest. Dr. Darnall reported funding from the NIH and the Patient-Centered Outcomes Research Institute. She serves on the scientific advisory board of Axial Healthcare and has several commercial publications about pain.
MILWAUKEE – When a patient with acute pain tumbles into a chronic pain state, many factors are at play, according to the widely accepted biopsychosocial theory of pain. Emotional, cognitive, and environmental components all contribute to the persistent and recalcitrant symptoms chronic pain patients experience.
Now, modern neuroimaging techniques show how for some, pain signals hijack the brain’s regulatory networks, allowing rumination and catastrophizing to intrude on the exteroception that’s critical to how humans interact with one another and the world. Interrupting catastrophizing with nonpharmacologic techniques yields measurable improvements – and there’s promise that a single treatment session can make a lasting difference.
“Psychosocial phenotypes, such as catastrophizing, are part of a complex biopsychosocial web of contributors to chronic pain. ,” said Robert R. Edwards, PhD, a psychologist at Brigham and Women’s Hospital/Harvard Medical School (Boston) Pain Management Center. Dr. Edwards moderated a session focused on catastrophizing at the scientific meeting of the American Pain Society.
Through magnetic resonance imaging techniques that measure functional connectivity, researchers can now see how nodes in the brain form connected networks that are differentially activated.
For example, the brain’s salience network (SLN) responds to stimuli that merit attention, such as evoked or clinical pain, Vitaly Napadow, PhD, said during his presentation. Key nodes in the SLN include the anterior cingulate cortex, the anterior insula, and the anterior temporoparietal junction. One function of the salience network, he said, is to regulate switching between the default mode network (DMN) – an interoceptive network – and the central executive network, usually active in exteroceptive tasks.
“The default mode network has been found to play an important role in pain processing,” Dr. Napadow said. These brain regions are more active in self-referential cognition – thinking about oneself – than when performing external tasks, he said. Consistently, studies have found decreased DMN deactivation in patients with chronic pain; essentially, the constant low hum of pain-focused DMN activity never turns off in a chronic pain state.
For patients with chronic pain, high levels of catastrophizing mean greater impact on functional brain connectivity, said Dr. Napadow, director of the Center for Integrative Pain NeuroImaging at the Martino Center for Biomedical Imaging at Massachusetts General Hospital and Harvard Medical School, Boston.
Looking at patients with chronic low back pain, he and his research team looked for connections between the DMN and the insula, which has a central role in pain processing. This connectivity was increased only in patients with high catastrophizing scores, said Dr. Napadow, with increased DMN-insula connectivity associated with increased pain scores only for this subgroup (Pain. 2019 Mar 4. doi: 10.1097/j.pain.0000000000001541).
“The model that we’re moving toward is that chronic pain leads to a blurring in the canonical network” of brain connectivity, Dr. Napadow said. “The speculation here is that the DMN-SLN linkage could be a sort of neural substrate for a common perception that chronic pain patients have – that their pain becomes part of who they are. Their interoceptive state becomes linked to the pain they are feeling: They are their pain.”
Where to turn with this information, which has large clinical implications? “Catastrophizing is a consistent risk factor for poor pain treatment outcomes, especially when we’re talking about pharmacologic treatments,” Dr. Edwards said. Also, chronic pain patients with the highest catastrophizing scores have the most opioid-related side effects, he said.
“Cognitive-behavioral therapy is potentially the most effective at reducing this risk factor,” said Dr. Edwards, noting that long-term effects were seen at 6 and 12 months post treatment. “These are significant, moderate-sized effects; there is some evidence that effects are largest in those with the highest baseline pain catastrophizing scores.”
“CBT is considered the gold standard, mainly because it’s the best studied” among treatment modalities, psychologist Beth Darnall, PhD, pointed out in her presentation. There’s evidence that other nonpharmacologic interventions can reduce catastrophizing: Psychology-informed yoga practices, physical therapy, and certain medical devices, such as high-frequency transcutaneous electric nerve stimulation units, may all have efficacy against catastrophizing and the downward spiral of chronic pain.
Still, a randomized controlled trial of CBT for pain in patients with fibromyalgia showed that the benefit, measured as reduction in pain interference with daily functioning, was almost twice as high in the high-catastrophizing group, “suggesting the potential utility of this method for patients at greatest risk,” said Dr. Edwards.
“We see a specific pattern of alterations in chronic pain similar to that seen in anxiety disorder; this suggests that some individuals are primed for the experience of pain,” said Dr. Darnall, clinical professor of anesthesiology, perioperative medicine, and pain medicine at Stanford (Calif.) University. “We are not born with the understanding of how to modulate pain and the distress it causes us.”
When she talks to patients, Dr. Darnall said: “I describe pain as being our ‘harm alarm.’ ... I like to describe it to people that ‘you have a very protective nervous system.’ ”
Dr. Darnall and her colleagues reported success with a pilot study of a single 2.5-hour-long session that addressed pain catastrophizing. From a baseline score of 26.1 on the Pain Catastrophizing Scale to a score of 13.8 at week 4, the 57 participants saw a significant decrease in mean scores on the scale (d [effect size] = 1.15).
On the strength of these early findings, Dr. Darnall and her collaborators are embarking on a randomized controlled trial ; the 3-arm comparative effectiveness study will compare a single-session intervention against 8 weeks of CBT or education-only classes for individuals with catastrophizing and chronic pain. The trial is structured to test the hypothesis that the single-session intervention will be noninferior to the full 8 weeks of CBT, Dr. Darnall said.
Building on the importance of avoiding stigmatizing and pejorative terms when talking about pain and catastrophizing, Dr. Darnall said she’s moved away from using the term “catastrophizing” in patient interactions. The one-session intervention is called “Empowered Relief – Train Your Brain Away from Pain.”
There’s a practical promise to a single-session class: Dr. Darnall has taught up to 85 patients at once, she said, adding, “This is a low-cost and scalable intervention.”
Dr. Edwards and Dr. Napadow reported funding from the National Institutes of Health, and they reported no conflicts of interest. Dr. Darnall reported funding from the NIH and the Patient-Centered Outcomes Research Institute. She serves on the scientific advisory board of Axial Healthcare and has several commercial publications about pain.
REPORTING FROM APS 2019