User login
Real acupuncture beat sham for osteoarthritis knee pain
Electro-acupuncture resulted in significant improvement in pain and function, compared with sham acupuncture, in a randomized trial of more than 400 adults with knee OA.
The socioeconomic burden of knee OA (KOA) remains high, and will likely increase with the aging population and rising rates of obesity, wrote first author Jian-Feng Tu, MD, PhD, of Beijing University of Chinese Medicine and colleagues. “Since no disease-modifying pharmaceutical agents have been approved, current KOA treatments are mainly symptomatic,” and identifying new therapies in addition to pharmacological agents or surgery is a research priority, they added. The research on acupuncture as a treatment for KOA has increased, but remains controversial as researchers attempt to determine the number of sessions needed for effectiveness.
In a study published in Arthritis & Rheumatology, the researchers recruited 480 adults aged 45-75 years with confirmed KOA who reported knee pain for longer than 6 months. Participants were randomized to three groups: electroacupuncture (EA), manual acupuncture (MA), or sham acupuncture (SA). Each group received three treatment sessions per week. In all groups, electrodes were attached to selected acupuncture needles, but the current was turned on only in the EA treatment group.
The primary outcome was the response rate after 8 weeks of treatment, defined as patients who achieved the minimal clinically important improvement (MCII) on both the Numeric Rating Scale and the Western Ontario and McMaster Universities Osteoarthritis Index function subscale.
Overall, response rates at 8 weeks were 60.3%, 58.6%, and 47.3% for the EA, MA, and SA groups, respectively.
Between-group differences were statistically significant for EA versus SA (13%, P = .0234) but not for MA versus SA (11.3%, P = .0507) at 8 weeks; however, both EA and MA groups showed significantly higher response rates, compared with the SA group at 16 and 26 weeks. “Although a clinically meaningful response rate for KOA is not available in the literature, the difference of 11.3%, which indicates the number needed to treat of 9, is acceptable in clinical practices,” the researchers noted.
Adverse events occurred in 11.5% of the EA group, 14.2% of the MA group, and 10.8% of the SA group, and included subcutaneous hematoma, post-needling pain, and pantalgia. All adverse events related to acupuncture resolved within a week and none were serious, the researchers wrote.
The study findings were limited by several factors, including the potential burden on patients of three sessions per week, the limited study population of patients with radiologic grades of II or III only, the use of self-reports, and the lack of blinding for outcome assessors, the researchers noted.
However, the results show persistent effects in reducing pain and improving function with EA or MA, compared with SA, the researchers wrote. The findings were strengthened by “adequate dosage of acupuncture, the use of the primary outcome at an individual level, and the rigorous methodology.” The use of the MCII in the primary outcome “can provide patients and policy makers with more straightforward information to decide whether a treatment should be used.”
Optimal dosing questions remain
Current options for managing KOA are limited by factors that include low efficacy and unwanted side effects, while joint replacements increase the burden on health care systems, wrote David J. Hunter, MBBS, PhD, of the University of Sydney, and Richard E. Harris, PhD, of the University of Michigan, Ann Arbor, in an accompanying editorial. “In this context, development of new treatments or identification of efficacy of existing therapies to address the huge unmet need of pain are strongly desired.” Acupuncture continues to gain popularity in North and South America, but its efficacy for pain and KOA remain controversial.
The question of dose is challenging when assessing acupuncture because the optimal dose and how to classify it remains unknown. “In this study, the authors used three treatments a week, which is more frequent than typical studies done in the West and potentially may not be feasible in some health care settings. A recent systematic review suggests that treatment frequency matters and a dose of three sessions per week may be superior to less frequent treatment,” they emphasized. Acupuncture is generally considered to be safe, but many health systems do not reimburse for it. Patients may have large out-of-pocket expenses because of the number of visits required, which may be a barrier to further implementation in practice.
“Acupuncture is already widely practiced and readily available in many countries and health care systems,” the editorialists said. However, “more research is needed in the areas of dose-response relationships, effects of blinding the acupuncturist, feasibility of three times weekly regimens, and clarifying the mechanism of effect, particularly given the persistence of benefit.”
The study was funded by Beijing Municipal Science & Technology Commission and Beijing Municipal Administration of Hospitals. The researchers had no financial conflicts to disclose. Dr. Hunter disclosed support from a National Health and Medical Research Council Investigator Grant and providing consulting advice for Merck Serono, TLC Bio, Tissuegene, Lilly, and Pfizer.
SOURCE: Tu J-F et al. Arthritis Rheumatol. 2020 Nov 10. doi: 10.1002/art.41584.
Electro-acupuncture resulted in significant improvement in pain and function, compared with sham acupuncture, in a randomized trial of more than 400 adults with knee OA.
The socioeconomic burden of knee OA (KOA) remains high, and will likely increase with the aging population and rising rates of obesity, wrote first author Jian-Feng Tu, MD, PhD, of Beijing University of Chinese Medicine and colleagues. “Since no disease-modifying pharmaceutical agents have been approved, current KOA treatments are mainly symptomatic,” and identifying new therapies in addition to pharmacological agents or surgery is a research priority, they added. The research on acupuncture as a treatment for KOA has increased, but remains controversial as researchers attempt to determine the number of sessions needed for effectiveness.
In a study published in Arthritis & Rheumatology, the researchers recruited 480 adults aged 45-75 years with confirmed KOA who reported knee pain for longer than 6 months. Participants were randomized to three groups: electroacupuncture (EA), manual acupuncture (MA), or sham acupuncture (SA). Each group received three treatment sessions per week. In all groups, electrodes were attached to selected acupuncture needles, but the current was turned on only in the EA treatment group.
The primary outcome was the response rate after 8 weeks of treatment, defined as patients who achieved the minimal clinically important improvement (MCII) on both the Numeric Rating Scale and the Western Ontario and McMaster Universities Osteoarthritis Index function subscale.
Overall, response rates at 8 weeks were 60.3%, 58.6%, and 47.3% for the EA, MA, and SA groups, respectively.
Between-group differences were statistically significant for EA versus SA (13%, P = .0234) but not for MA versus SA (11.3%, P = .0507) at 8 weeks; however, both EA and MA groups showed significantly higher response rates, compared with the SA group at 16 and 26 weeks. “Although a clinically meaningful response rate for KOA is not available in the literature, the difference of 11.3%, which indicates the number needed to treat of 9, is acceptable in clinical practices,” the researchers noted.
Adverse events occurred in 11.5% of the EA group, 14.2% of the MA group, and 10.8% of the SA group, and included subcutaneous hematoma, post-needling pain, and pantalgia. All adverse events related to acupuncture resolved within a week and none were serious, the researchers wrote.
The study findings were limited by several factors, including the potential burden on patients of three sessions per week, the limited study population of patients with radiologic grades of II or III only, the use of self-reports, and the lack of blinding for outcome assessors, the researchers noted.
However, the results show persistent effects in reducing pain and improving function with EA or MA, compared with SA, the researchers wrote. The findings were strengthened by “adequate dosage of acupuncture, the use of the primary outcome at an individual level, and the rigorous methodology.” The use of the MCII in the primary outcome “can provide patients and policy makers with more straightforward information to decide whether a treatment should be used.”
Optimal dosing questions remain
Current options for managing KOA are limited by factors that include low efficacy and unwanted side effects, while joint replacements increase the burden on health care systems, wrote David J. Hunter, MBBS, PhD, of the University of Sydney, and Richard E. Harris, PhD, of the University of Michigan, Ann Arbor, in an accompanying editorial. “In this context, development of new treatments or identification of efficacy of existing therapies to address the huge unmet need of pain are strongly desired.” Acupuncture continues to gain popularity in North and South America, but its efficacy for pain and KOA remain controversial.
The question of dose is challenging when assessing acupuncture because the optimal dose and how to classify it remains unknown. “In this study, the authors used three treatments a week, which is more frequent than typical studies done in the West and potentially may not be feasible in some health care settings. A recent systematic review suggests that treatment frequency matters and a dose of three sessions per week may be superior to less frequent treatment,” they emphasized. Acupuncture is generally considered to be safe, but many health systems do not reimburse for it. Patients may have large out-of-pocket expenses because of the number of visits required, which may be a barrier to further implementation in practice.
“Acupuncture is already widely practiced and readily available in many countries and health care systems,” the editorialists said. However, “more research is needed in the areas of dose-response relationships, effects of blinding the acupuncturist, feasibility of three times weekly regimens, and clarifying the mechanism of effect, particularly given the persistence of benefit.”
The study was funded by Beijing Municipal Science & Technology Commission and Beijing Municipal Administration of Hospitals. The researchers had no financial conflicts to disclose. Dr. Hunter disclosed support from a National Health and Medical Research Council Investigator Grant and providing consulting advice for Merck Serono, TLC Bio, Tissuegene, Lilly, and Pfizer.
SOURCE: Tu J-F et al. Arthritis Rheumatol. 2020 Nov 10. doi: 10.1002/art.41584.
Electro-acupuncture resulted in significant improvement in pain and function, compared with sham acupuncture, in a randomized trial of more than 400 adults with knee OA.
The socioeconomic burden of knee OA (KOA) remains high, and will likely increase with the aging population and rising rates of obesity, wrote first author Jian-Feng Tu, MD, PhD, of Beijing University of Chinese Medicine and colleagues. “Since no disease-modifying pharmaceutical agents have been approved, current KOA treatments are mainly symptomatic,” and identifying new therapies in addition to pharmacological agents or surgery is a research priority, they added. The research on acupuncture as a treatment for KOA has increased, but remains controversial as researchers attempt to determine the number of sessions needed for effectiveness.
In a study published in Arthritis & Rheumatology, the researchers recruited 480 adults aged 45-75 years with confirmed KOA who reported knee pain for longer than 6 months. Participants were randomized to three groups: electroacupuncture (EA), manual acupuncture (MA), or sham acupuncture (SA). Each group received three treatment sessions per week. In all groups, electrodes were attached to selected acupuncture needles, but the current was turned on only in the EA treatment group.
The primary outcome was the response rate after 8 weeks of treatment, defined as patients who achieved the minimal clinically important improvement (MCII) on both the Numeric Rating Scale and the Western Ontario and McMaster Universities Osteoarthritis Index function subscale.
Overall, response rates at 8 weeks were 60.3%, 58.6%, and 47.3% for the EA, MA, and SA groups, respectively.
Between-group differences were statistically significant for EA versus SA (13%, P = .0234) but not for MA versus SA (11.3%, P = .0507) at 8 weeks; however, both EA and MA groups showed significantly higher response rates, compared with the SA group at 16 and 26 weeks. “Although a clinically meaningful response rate for KOA is not available in the literature, the difference of 11.3%, which indicates the number needed to treat of 9, is acceptable in clinical practices,” the researchers noted.
Adverse events occurred in 11.5% of the EA group, 14.2% of the MA group, and 10.8% of the SA group, and included subcutaneous hematoma, post-needling pain, and pantalgia. All adverse events related to acupuncture resolved within a week and none were serious, the researchers wrote.
The study findings were limited by several factors, including the potential burden on patients of three sessions per week, the limited study population of patients with radiologic grades of II or III only, the use of self-reports, and the lack of blinding for outcome assessors, the researchers noted.
However, the results show persistent effects in reducing pain and improving function with EA or MA, compared with SA, the researchers wrote. The findings were strengthened by “adequate dosage of acupuncture, the use of the primary outcome at an individual level, and the rigorous methodology.” The use of the MCII in the primary outcome “can provide patients and policy makers with more straightforward information to decide whether a treatment should be used.”
Optimal dosing questions remain
Current options for managing KOA are limited by factors that include low efficacy and unwanted side effects, while joint replacements increase the burden on health care systems, wrote David J. Hunter, MBBS, PhD, of the University of Sydney, and Richard E. Harris, PhD, of the University of Michigan, Ann Arbor, in an accompanying editorial. “In this context, development of new treatments or identification of efficacy of existing therapies to address the huge unmet need of pain are strongly desired.” Acupuncture continues to gain popularity in North and South America, but its efficacy for pain and KOA remain controversial.
The question of dose is challenging when assessing acupuncture because the optimal dose and how to classify it remains unknown. “In this study, the authors used three treatments a week, which is more frequent than typical studies done in the West and potentially may not be feasible in some health care settings. A recent systematic review suggests that treatment frequency matters and a dose of three sessions per week may be superior to less frequent treatment,” they emphasized. Acupuncture is generally considered to be safe, but many health systems do not reimburse for it. Patients may have large out-of-pocket expenses because of the number of visits required, which may be a barrier to further implementation in practice.
“Acupuncture is already widely practiced and readily available in many countries and health care systems,” the editorialists said. However, “more research is needed in the areas of dose-response relationships, effects of blinding the acupuncturist, feasibility of three times weekly regimens, and clarifying the mechanism of effect, particularly given the persistence of benefit.”
The study was funded by Beijing Municipal Science & Technology Commission and Beijing Municipal Administration of Hospitals. The researchers had no financial conflicts to disclose. Dr. Hunter disclosed support from a National Health and Medical Research Council Investigator Grant and providing consulting advice for Merck Serono, TLC Bio, Tissuegene, Lilly, and Pfizer.
SOURCE: Tu J-F et al. Arthritis Rheumatol. 2020 Nov 10. doi: 10.1002/art.41584.
FROM ARTHRITIS & RHEUMATOLOGY
Cognitive Behavioral Therapy Plus Placebo Is Inferior to NSAID Therapy for Arthritis Pain
Study Overview
Objective. To examine whether discontinuation of nonsteroidal anti-inflammatory drug (NSAID) therapy and initiation of telephone-based cognitive behavioral therapy (CBT) is not worse than continuation of NSAIDs in the management of arthritis pain.
Design. Randomized controlled trial with noninferiority design.
Setting and participants. This study was a multicenter trial conducted across 4 Veterans Affairs health care systems in Boston, Providence, Connecticut, and North Florida/South Georgia that started September 2013 and ended September 2018. Eligibility criteria included being age 20 years or older, radiographic evidence of knee osteoarthritis, and use of an NSAID for knee pain on most days of the month for at least the past 3 months. Exclusion criteria included significant hearing impairments that may impede the conduct of the trial, current opioid prescriptions excluding tramadol, contraindications to NSAID use, recent or scheduled intra-articular injections or surgery, comorbid conditions other than knee pain that limited walking, and bilateral knee replacements or pain only in the replaced knee. Concurrent use of tramadol and other non-NSAID analgesics was permitted.
A total of 490 participants took part in the 2-week run-in period where their NSAID regimen was discontinued and they were started on a standardized dose of the NSAID meloxicam 15 mg daily. During the run-in period, 126 participants were excluded for several reasons, including worsening pain and patient withdrawal, yielding 364 participants who were randomized to continue meloxicam treatment or placebo for 4 weeks with blinding.
Intervention. Subsequent to the 4-week phase 1 placebo controlled trial, participants in the placebo group were given CBT via telephone (unblinded) for 10 weeks, and the meloxicam group continued treatment with meloxicam for phase 2. The CBT group received 10 modules over 10 weeks in 30- to 45-minute telephone contacts with a psychologist using a treatment manual modified for knee osteoarthritis. These modules consisted of 1 introductory module, 8 pain coping skills modules (eg, deep breathing and visual imagery, progressive muscle relaxation, physical activity and bodily mechanics, identifying unhealthy thoughts, balancing unhealthy thoughts, managing stress, time-based pacing, and sleep hygiene), and a final module emphasizing skill consolidation and relapse prevention. Outcomes were assessed at the end of the phase 1 and phase 2 periods.
Main outcome measures. Main study outcome measures included pain as measured with the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) at 4 weeks. Secondary outcomes included the WOMAC pain score, disability score, and global impression of change after treatment at 14 weeks. The WOMAC pain scale ranges from 0 to 20, and consists of 5 questions regarding severity of pain during walking, stair use, lying in bed at night, sitting, and standing, with 0 indicating no pain; 1, mild pain; 2, moderate pain; 3, severe pain; and 4, very severe pain for each item. The WOMAC disability scale measures self-reported difficulty in performing tasks that reflect lower-extremity physical function, including climbing stairs, rising from a chair, walking, and other activities of daily living. The global impression of change after treatment was measured on a 5-point scale (where 1 indicates much better and 5 indicates much worse). The minimum clinically important difference of the WOMAC pain scale is 2, based on prior literature. With the noninferiority design, the margin was set as a score of 1.
Main results. The placebo group consisted of 180 participants, with an average age of 58.2 years (SD, 11.8 years); 89% of them were male. The meloxicam group consisted of 184 participants, with an average age of 58.6 years (SD, 10 years); 84% of them were male. The average body mass index was 33.9 and 33.4 in each group, respectively. For the primary outcome, the placebo group had a worse pain score than the meloxicam group at 4 weeks (difference of 1.4; 95% confidence interval, 0.8- 2.0). At 14 weeks, the placebo group (with CBT) had a worse pain score than the meloxicam group (difference of 0.8; 95% CI, 0.2-1.4). There was no statistically significant difference in the disability score or global impression of change after treatment score between the 2 groups. The observed difference in pain score did not, however, exceed the minimum clinically important difference.
Conclusion. Placebo treatment and CBT are inferior to NSAIDs in managing pain for patients with knee osteoarthritis. The difference in pain may not be clinically important, and there were no differences in function at 14 weeks.
Commentary
Osteoarthritis is a common chronic condition that causes pain and disability and is often treated with oral analgesics. NSAIDs, despite few high-quality trials demonstrating their efficacy, are among the most commonly used treatment for osteoarthritis pain.1 NSAID therapy, however, does have potential side effects, such as gastric reflux and renal dysfunction.2 This withdrawal trial with placebo control contributes further evidence of the effectiveness of NSAIDs on knee osteoarthritis, demonstrating that indeed NSAIDs improve pain scores to a greater degree than placebo treatment. Augmenting placebo treatment with nonpharmacologic CBT was inferior to NSAIDs in pain management. The authors pointed out that the difference in pain score may not be clinically important, and that lower-extremity function was not different between the groups, concluding that, despite the higher pain score, CBT could be a treatment option, particularly for those who may have difficulty tolerating NSAID treatment.
The study population had a number of chronic conditions in addition to having knee arthritis, and thus likely were taking multiple medications for chronic disease management. Use of multiple medications is associated with an increased risk of rug interactions and adverse effects of medications.3 Thus, this attempt to assess whether a nonpharmacologic alternative treatment is noninferior to a drug treatment is a step toward building the evidence base for deprescribing and enhancing medication safety.4 Previous studies have examined other nonpharmacologic treatments for knee arthritis, such as acupuncture,5 and it is worthwhile to consider combining nonpharmacological approaches as an alternative to oral analgesic medication use.
Applications for Clinical Practice
This study advances our understanding of the effect of NSAID use on knee osteoarthritis when compared to placebo with CBT. Although this is a negative study that failed to show that placebo combined with CBT is noninferior to NSAIDs, it did quantify the expected treatment effect of NSAIDs and showed that this effect likely is not clinically important and/or does not alter lower-extremity function. Further studies are needed to identify other nonpharmacologic approaches and test whether combinations of approaches are effective in the management of chronic pain from osteoarthritis.
–William W. Hung, MD, MPH
1. Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis. 2018;9:143-150.
2. Pilotto A, Franceschi M, Leandro G, Di Mario F. NSAID and aspirin use by the elderly in general practice: effect on gastrointestinal symptoms and therapies. Drugs Aging. 2003;20:701-710.
3. Steinman MA. Polypharmacy-time to get beyond numbers. JAMA Intern Med. 2016;176:482-483.
4. Rashid R, Chang C, Niu F, et al. Evaluation of a pharmacist-managed nonsteroidal anti-inflammatory drugs deprescribing program in an integrated health care system. J Manag Care Spec Pharm. 2020;26:918-924.
5. Sun J, Zhao Y, Zhu R, et al. Acupotomy therapy for knee osteoarthritis pain: systematic review and meta-analysis. Evid Based Complement Alternat Med. 2020;2020:2168283.
Study Overview
Objective. To examine whether discontinuation of nonsteroidal anti-inflammatory drug (NSAID) therapy and initiation of telephone-based cognitive behavioral therapy (CBT) is not worse than continuation of NSAIDs in the management of arthritis pain.
Design. Randomized controlled trial with noninferiority design.
Setting and participants. This study was a multicenter trial conducted across 4 Veterans Affairs health care systems in Boston, Providence, Connecticut, and North Florida/South Georgia that started September 2013 and ended September 2018. Eligibility criteria included being age 20 years or older, radiographic evidence of knee osteoarthritis, and use of an NSAID for knee pain on most days of the month for at least the past 3 months. Exclusion criteria included significant hearing impairments that may impede the conduct of the trial, current opioid prescriptions excluding tramadol, contraindications to NSAID use, recent or scheduled intra-articular injections or surgery, comorbid conditions other than knee pain that limited walking, and bilateral knee replacements or pain only in the replaced knee. Concurrent use of tramadol and other non-NSAID analgesics was permitted.
A total of 490 participants took part in the 2-week run-in period where their NSAID regimen was discontinued and they were started on a standardized dose of the NSAID meloxicam 15 mg daily. During the run-in period, 126 participants were excluded for several reasons, including worsening pain and patient withdrawal, yielding 364 participants who were randomized to continue meloxicam treatment or placebo for 4 weeks with blinding.
Intervention. Subsequent to the 4-week phase 1 placebo controlled trial, participants in the placebo group were given CBT via telephone (unblinded) for 10 weeks, and the meloxicam group continued treatment with meloxicam for phase 2. The CBT group received 10 modules over 10 weeks in 30- to 45-minute telephone contacts with a psychologist using a treatment manual modified for knee osteoarthritis. These modules consisted of 1 introductory module, 8 pain coping skills modules (eg, deep breathing and visual imagery, progressive muscle relaxation, physical activity and bodily mechanics, identifying unhealthy thoughts, balancing unhealthy thoughts, managing stress, time-based pacing, and sleep hygiene), and a final module emphasizing skill consolidation and relapse prevention. Outcomes were assessed at the end of the phase 1 and phase 2 periods.
Main outcome measures. Main study outcome measures included pain as measured with the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) at 4 weeks. Secondary outcomes included the WOMAC pain score, disability score, and global impression of change after treatment at 14 weeks. The WOMAC pain scale ranges from 0 to 20, and consists of 5 questions regarding severity of pain during walking, stair use, lying in bed at night, sitting, and standing, with 0 indicating no pain; 1, mild pain; 2, moderate pain; 3, severe pain; and 4, very severe pain for each item. The WOMAC disability scale measures self-reported difficulty in performing tasks that reflect lower-extremity physical function, including climbing stairs, rising from a chair, walking, and other activities of daily living. The global impression of change after treatment was measured on a 5-point scale (where 1 indicates much better and 5 indicates much worse). The minimum clinically important difference of the WOMAC pain scale is 2, based on prior literature. With the noninferiority design, the margin was set as a score of 1.
Main results. The placebo group consisted of 180 participants, with an average age of 58.2 years (SD, 11.8 years); 89% of them were male. The meloxicam group consisted of 184 participants, with an average age of 58.6 years (SD, 10 years); 84% of them were male. The average body mass index was 33.9 and 33.4 in each group, respectively. For the primary outcome, the placebo group had a worse pain score than the meloxicam group at 4 weeks (difference of 1.4; 95% confidence interval, 0.8- 2.0). At 14 weeks, the placebo group (with CBT) had a worse pain score than the meloxicam group (difference of 0.8; 95% CI, 0.2-1.4). There was no statistically significant difference in the disability score or global impression of change after treatment score between the 2 groups. The observed difference in pain score did not, however, exceed the minimum clinically important difference.
Conclusion. Placebo treatment and CBT are inferior to NSAIDs in managing pain for patients with knee osteoarthritis. The difference in pain may not be clinically important, and there were no differences in function at 14 weeks.
Commentary
Osteoarthritis is a common chronic condition that causes pain and disability and is often treated with oral analgesics. NSAIDs, despite few high-quality trials demonstrating their efficacy, are among the most commonly used treatment for osteoarthritis pain.1 NSAID therapy, however, does have potential side effects, such as gastric reflux and renal dysfunction.2 This withdrawal trial with placebo control contributes further evidence of the effectiveness of NSAIDs on knee osteoarthritis, demonstrating that indeed NSAIDs improve pain scores to a greater degree than placebo treatment. Augmenting placebo treatment with nonpharmacologic CBT was inferior to NSAIDs in pain management. The authors pointed out that the difference in pain score may not be clinically important, and that lower-extremity function was not different between the groups, concluding that, despite the higher pain score, CBT could be a treatment option, particularly for those who may have difficulty tolerating NSAID treatment.
The study population had a number of chronic conditions in addition to having knee arthritis, and thus likely were taking multiple medications for chronic disease management. Use of multiple medications is associated with an increased risk of rug interactions and adverse effects of medications.3 Thus, this attempt to assess whether a nonpharmacologic alternative treatment is noninferior to a drug treatment is a step toward building the evidence base for deprescribing and enhancing medication safety.4 Previous studies have examined other nonpharmacologic treatments for knee arthritis, such as acupuncture,5 and it is worthwhile to consider combining nonpharmacological approaches as an alternative to oral analgesic medication use.
Applications for Clinical Practice
This study advances our understanding of the effect of NSAID use on knee osteoarthritis when compared to placebo with CBT. Although this is a negative study that failed to show that placebo combined with CBT is noninferior to NSAIDs, it did quantify the expected treatment effect of NSAIDs and showed that this effect likely is not clinically important and/or does not alter lower-extremity function. Further studies are needed to identify other nonpharmacologic approaches and test whether combinations of approaches are effective in the management of chronic pain from osteoarthritis.
–William W. Hung, MD, MPH
Study Overview
Objective. To examine whether discontinuation of nonsteroidal anti-inflammatory drug (NSAID) therapy and initiation of telephone-based cognitive behavioral therapy (CBT) is not worse than continuation of NSAIDs in the management of arthritis pain.
Design. Randomized controlled trial with noninferiority design.
Setting and participants. This study was a multicenter trial conducted across 4 Veterans Affairs health care systems in Boston, Providence, Connecticut, and North Florida/South Georgia that started September 2013 and ended September 2018. Eligibility criteria included being age 20 years or older, radiographic evidence of knee osteoarthritis, and use of an NSAID for knee pain on most days of the month for at least the past 3 months. Exclusion criteria included significant hearing impairments that may impede the conduct of the trial, current opioid prescriptions excluding tramadol, contraindications to NSAID use, recent or scheduled intra-articular injections or surgery, comorbid conditions other than knee pain that limited walking, and bilateral knee replacements or pain only in the replaced knee. Concurrent use of tramadol and other non-NSAID analgesics was permitted.
A total of 490 participants took part in the 2-week run-in period where their NSAID regimen was discontinued and they were started on a standardized dose of the NSAID meloxicam 15 mg daily. During the run-in period, 126 participants were excluded for several reasons, including worsening pain and patient withdrawal, yielding 364 participants who were randomized to continue meloxicam treatment or placebo for 4 weeks with blinding.
Intervention. Subsequent to the 4-week phase 1 placebo controlled trial, participants in the placebo group were given CBT via telephone (unblinded) for 10 weeks, and the meloxicam group continued treatment with meloxicam for phase 2. The CBT group received 10 modules over 10 weeks in 30- to 45-minute telephone contacts with a psychologist using a treatment manual modified for knee osteoarthritis. These modules consisted of 1 introductory module, 8 pain coping skills modules (eg, deep breathing and visual imagery, progressive muscle relaxation, physical activity and bodily mechanics, identifying unhealthy thoughts, balancing unhealthy thoughts, managing stress, time-based pacing, and sleep hygiene), and a final module emphasizing skill consolidation and relapse prevention. Outcomes were assessed at the end of the phase 1 and phase 2 periods.
Main outcome measures. Main study outcome measures included pain as measured with the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) at 4 weeks. Secondary outcomes included the WOMAC pain score, disability score, and global impression of change after treatment at 14 weeks. The WOMAC pain scale ranges from 0 to 20, and consists of 5 questions regarding severity of pain during walking, stair use, lying in bed at night, sitting, and standing, with 0 indicating no pain; 1, mild pain; 2, moderate pain; 3, severe pain; and 4, very severe pain for each item. The WOMAC disability scale measures self-reported difficulty in performing tasks that reflect lower-extremity physical function, including climbing stairs, rising from a chair, walking, and other activities of daily living. The global impression of change after treatment was measured on a 5-point scale (where 1 indicates much better and 5 indicates much worse). The minimum clinically important difference of the WOMAC pain scale is 2, based on prior literature. With the noninferiority design, the margin was set as a score of 1.
Main results. The placebo group consisted of 180 participants, with an average age of 58.2 years (SD, 11.8 years); 89% of them were male. The meloxicam group consisted of 184 participants, with an average age of 58.6 years (SD, 10 years); 84% of them were male. The average body mass index was 33.9 and 33.4 in each group, respectively. For the primary outcome, the placebo group had a worse pain score than the meloxicam group at 4 weeks (difference of 1.4; 95% confidence interval, 0.8- 2.0). At 14 weeks, the placebo group (with CBT) had a worse pain score than the meloxicam group (difference of 0.8; 95% CI, 0.2-1.4). There was no statistically significant difference in the disability score or global impression of change after treatment score between the 2 groups. The observed difference in pain score did not, however, exceed the minimum clinically important difference.
Conclusion. Placebo treatment and CBT are inferior to NSAIDs in managing pain for patients with knee osteoarthritis. The difference in pain may not be clinically important, and there were no differences in function at 14 weeks.
Commentary
Osteoarthritis is a common chronic condition that causes pain and disability and is often treated with oral analgesics. NSAIDs, despite few high-quality trials demonstrating their efficacy, are among the most commonly used treatment for osteoarthritis pain.1 NSAID therapy, however, does have potential side effects, such as gastric reflux and renal dysfunction.2 This withdrawal trial with placebo control contributes further evidence of the effectiveness of NSAIDs on knee osteoarthritis, demonstrating that indeed NSAIDs improve pain scores to a greater degree than placebo treatment. Augmenting placebo treatment with nonpharmacologic CBT was inferior to NSAIDs in pain management. The authors pointed out that the difference in pain score may not be clinically important, and that lower-extremity function was not different between the groups, concluding that, despite the higher pain score, CBT could be a treatment option, particularly for those who may have difficulty tolerating NSAID treatment.
The study population had a number of chronic conditions in addition to having knee arthritis, and thus likely were taking multiple medications for chronic disease management. Use of multiple medications is associated with an increased risk of rug interactions and adverse effects of medications.3 Thus, this attempt to assess whether a nonpharmacologic alternative treatment is noninferior to a drug treatment is a step toward building the evidence base for deprescribing and enhancing medication safety.4 Previous studies have examined other nonpharmacologic treatments for knee arthritis, such as acupuncture,5 and it is worthwhile to consider combining nonpharmacological approaches as an alternative to oral analgesic medication use.
Applications for Clinical Practice
This study advances our understanding of the effect of NSAID use on knee osteoarthritis when compared to placebo with CBT. Although this is a negative study that failed to show that placebo combined with CBT is noninferior to NSAIDs, it did quantify the expected treatment effect of NSAIDs and showed that this effect likely is not clinically important and/or does not alter lower-extremity function. Further studies are needed to identify other nonpharmacologic approaches and test whether combinations of approaches are effective in the management of chronic pain from osteoarthritis.
–William W. Hung, MD, MPH
1. Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis. 2018;9:143-150.
2. Pilotto A, Franceschi M, Leandro G, Di Mario F. NSAID and aspirin use by the elderly in general practice: effect on gastrointestinal symptoms and therapies. Drugs Aging. 2003;20:701-710.
3. Steinman MA. Polypharmacy-time to get beyond numbers. JAMA Intern Med. 2016;176:482-483.
4. Rashid R, Chang C, Niu F, et al. Evaluation of a pharmacist-managed nonsteroidal anti-inflammatory drugs deprescribing program in an integrated health care system. J Manag Care Spec Pharm. 2020;26:918-924.
5. Sun J, Zhao Y, Zhu R, et al. Acupotomy therapy for knee osteoarthritis pain: systematic review and meta-analysis. Evid Based Complement Alternat Med. 2020;2020:2168283.
1. Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis. 2018;9:143-150.
2. Pilotto A, Franceschi M, Leandro G, Di Mario F. NSAID and aspirin use by the elderly in general practice: effect on gastrointestinal symptoms and therapies. Drugs Aging. 2003;20:701-710.
3. Steinman MA. Polypharmacy-time to get beyond numbers. JAMA Intern Med. 2016;176:482-483.
4. Rashid R, Chang C, Niu F, et al. Evaluation of a pharmacist-managed nonsteroidal anti-inflammatory drugs deprescribing program in an integrated health care system. J Manag Care Spec Pharm. 2020;26:918-924.
5. Sun J, Zhao Y, Zhu R, et al. Acupotomy therapy for knee osteoarthritis pain: systematic review and meta-analysis. Evid Based Complement Alternat Med. 2020;2020:2168283.
A Veteran Presenting With Chronic Progressive Dyspnea on Exertion
Case Presentation: A 45-year-old US Coast Guard veteran with a medical history of asthma and chronic back pain was referred to the VA Boston Healthcare System (VABHS) for evaluation of progressive, unexplained dyspnea. Two years prior to presentation, the patient was an avid outdoorsman and highly active. At the time of his initial primary care physician (PCP) evaluation he reported dyspnea on exertion, and symptoms consistent with an upper respiratory tract infection (URTI) and a recent tick bite with an associated rash. He was treated with intranasal fluticasone and a course of antibiotics. His URTI symptoms and rash improved; however the dyspnea persisted and progressed over the ensuing winter and he was referred for pulmonary function testing. Additional history included a 20 pack-year history of smoking (resolved 10 years prior to the first VABHS clinical encounter) and a family history of premature coronary artery disease (CAD) in his father and 2 paternal uncles. He lived in northern New England where he previously worked as a cemetery groundskeeper.
►Kristopher Clark, MD, Chief Medical Resident, VABHS and Boston University/Boston Medical Center: Dr. Goldstein, how do you approach a patient who presents with progressive dyspnea?
►Ronald Goldstein, MD, Chief of Pulmonary and Critical Care VABHS: The evaluation of dyspnea is a common problem for pulmonary physicians. The sensation of dyspnea may originate from a wide variety of etiologies that involve pulmonary and cardiovascular disorders, neuromuscular impairment, deconditioning, or psychological issues. It is important to characterize the temporal pattern, severity, progression, relation to exertion or other triggers, the smoking history, environmental and occupational exposures to pulmonary toxins, associated symptoms, and the history of pulmonary problems.1
The physical examination may help to identify an airway or parenchymal disorder. Wheezing on chest examination would point to an obstructive defect and crackles to a possible restrictive problem, including pulmonary fibrosis. A cardiac examination should be performed to assess for evidence of heart failure, valvular heart disease, or the presence of loud P2 suggestive of pulmonary hypertension (PH). Laboratory studies, including complete blood counts are indicated.
A more complete pulmonary evaluation usually involves pulmonary function tests (PFTs), oximetry with exertion, and chest imaging. Additional cardiac testing might include electrocardiogram (ECG) and cardiac echocardiogram, followed by an exercise study, if needed. A B-natriuretic peptide determination could be considered if there is concern for congestive heart failure.2
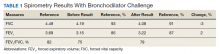
►Dr. Clark: The initial physical examination was normal and laboratory tests were unrevealing. Given his history of asthma, he underwent spirometry testing (Table 1).
Dr. Goldstein, aside from unexplained dyspnea, what are other indications for spirometry and when should we consider ordering a full PFT, including lung volumes and diffusion capacity? Can you interpret this patient’s spirometry results?
►Dr. Goldstein: Spirometry is indicated to evaluate for a suspected obstructive defect. The test is usually performed with and without a bronchodilator to assess airway reactivity. A change in > 12% and > 200 mL suggests acute bronchodilator responsiveness. Periodic spirometry determinations are useful to assess the effect of medications or progression of disease. A reduction in forced vital capacity (FVC) may suggest a restrictive component. This possibility requires measure of lung volumes.
A full set of PFTs (ie, spirometry plus assessment of lung volumes and diffusion capacity) is required to evaluate the abnormalities associated with chronic obstructive pulmonary disease (COPD), interstitial diseases, vascular abnormalities (particularly PH), as well as for certain preoperative assessments. The single breath diffusing capacity for carbon monoxide is a measure of the overall capillary alveolar surface area of the lung. It is decreased in emphysema and interstitial disease as well as pulmonary vascular disorders. It would be particularly useful in this case as the spirometry studies were normal.
In this case, the normal FVC renders a significant restrictive disorder unlikely and his normal forced expiratory volume (FEV1) and FEV1/FVC make a significant obstructive disorder unlikely. He did not show any bronchodilator response; however, this finding does not exclude the presence of underlying asthma or reactive airway disease as patients often will not show a bronchodilator response at time of testing if they are not experiencing active bronchospasm or constriction. Further provocative testing with a methacholine challenge could be used to assess for reactive airway disease.
►Dr. Clark: The patient continued to have dyspnea when he returned to his PCP. Given his family history of premature CAD, an ECG was obtained that showed normal sinus rhythm at a rate of 70 beats per minute. A cardiology consult was placed, and he was referred for cardiac stress testing.
Dr. Maron, there are many forms of cardiac stress tests. In this case, the patient is referred for a stress test due his dyspnea. Does that symptom help you decide which test to order? How often does dyspnea present as an anginal equivalent in the absence of other cardiovascular symptoms or known cardiovascular disease?
►Bradley Maron, MD, Codirector, Pulmonary Vascular Disease Center, VABHS: In this case, stress testing should include a functional (ie, exercise) assessment if possible. Exercise capacity is a critical determinant of prognosis across the spectrum of cardiovascular disease and in a young person can be particularly informative on global health status. Furthermore, the chief complaint from this patient is dyspnea on exertion, and therefore, exercise testing is likely to be needed to reproduce or provoke the main symptom in this case. Estimates for dyspnea as a presenting symptom for ischemic heart disease vary but may be as high as 25%.3 It should be noted that cardiopulmonary exercise testing is useful for evaluating patients with unexplained dyspnea, as exercise hypoxemia, blunted decrease in VD/VT (ventilatory dead space/tidal volume), and evidence of a pulmonary mechanical limit to physical activity can inform the differential diagnosis.
►Dr. Clark: The patient underwent exercise treadmill testing and was able reach the target heart rate (> 85% age-predicted maximal heart rate) and achieve 11 metabolic equivalents. He had no chest pain or diagnostic ECG changes. The report made no mention of whether he experienced dyspnea during the test and was read as negative for exercise-induced ischemia.
He was seen by a cardiologist who noted an increased intensity S2 heart sound on examination without any other cardiopulmonary findings. It was noted that his symptoms occurred when tamping the ground or starting to walk up a hill but resolved with rest. It was also noted that his symptoms did not occur with gradual increased activity such as that performed during an exercise tolerance test. A 2-view chest X-ray was obtained and read as normal. Given the data from this evaluation thus far, the patient was told that his symptoms were most likely a result of his asthma exacerbated by dirt and dust exposure. Continued use of albuterol inhaler therapy was recommended, and no further diagnostic assessment was pursued.
Approximately 11 months later, the patient presented again to his PCP and reported progressive dyspnea. He had delayed seeking further care as he started to “feel like my symptoms were possibly in my head” given his prior negative workup. His symptoms had escalated drastically to the point where he felt short of breath with minimal exertion in addition to feeling sweaty, dizzy, fatigued, and having near-syncope when standing.
He was referred for a transthoracic echocardiogram (TTE) that revealed a left ventricular ejection fraction (LVEF) of 55 to 60% with diastolic relaxation abnormality and a normal-sized left atrium. The TTE also showed (qualitatively) a moderately dilated right ventricle with reduced systolic function, moderately severe tricuspid regurgitation, and severe elevation (> 60 mm Hg) in estimated right ventricular systolic pressure.
Dr. Maron, can you comment on how these findings may explain the patient’s symptoms? What differential diagnoses would you now consider?
►Dr. Maron: These echocardiography results exclude left ventricular systolic dysfunction or primary left-sided valvular disease at rest as a cause of the patient’s symptoms. In light of the patient’s prior normal stress test, high grade coronary disease in the absence of LV systolic dysfunction on echocardiography also seems unlikely. Estimated pulmonary artery systolic pressure > 60 mm Hg by echocardiography is highly suggestive of PH, but in and of itself does not diagnose PH nor inform pulmonary artery wedge pressure or pulmonary vascular resistance. Along with a direct measurement of pulmonary artery (PA) pressure, these data are needed to establish, classify, and prognosticate PH clinically.
►Dr. Clark: The patient was referred to a pulmonologist. His examination included bibasilar crackles and an enhanced P2 heart sound. A comprehensive pulmonary history was obtained, which noted his smoking history, possible asbestos exposure while serving in the Coast Guard, nighttime snoring without witnessed apnea events, and no personal or family history of thromboembolism or connective tissue disease.
Dr. Goldstein, is there anything in this patient’s history that could explain his symptoms and echocardiograph findings? Which tests would you order next?
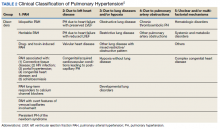
►Dr. Goldstein: PH may be secondary to a wide variety of disorders including left heart disease (Group 2), advanced COPD, interstitial fibrosis, obstructive sleep apnea (OSA), or other lung diseases (Group 3), thromboembolic disorders (Group 4), and other systemic diseases such as sarcoidosis (Group 5). Group 1 is pulmonary arterial hypertension. (Table 2).
A right heart catheterization should be done to confirm the PA pressures estimated by echocardiogram. As to a cause, clinically he does not have heart failure. The limited smoking history and spirometry data do not support advanced COPD. He was noted to have crackles on physical examination suggesting an interstitial disorder. To assess the extent of interstitial disease, we would obtain a noncontrast computed tomography (CT) of the chest. The history of snoring suggesting the possibility of OSA indicating the need for overnight oximetry as significant nocturnal hypoxemia is a possible contributing cause to PH. A polysomnogram would be required to fully evaluate a sleep disturbance. The possible asbestos exposure is not likely a contributing factor as asbestosis requires significant exposure. We would obtain a ventilation/perfusion (V/Q) scan to rule out chronic thromboembolic disease. Targeted tests for causes of Group 5 disease should also be done.
►Dr. Clark: The impression from his pulmonologist was that the patient has severe PH, though the specific etiology was not yet known. Dr. Maron, can you review for us the pathophysiology behind PH and describe how the disease is classified?
►Dr. Maron: Elevated mean pulmonary artery pressure (> 20 mm Hg) diagnosed by supine right heart catheterization is the sine qua non of PH.4 However, this alone does not inform pathophysiology. As Dr. Goldstein noted, elevated PA pressure may be due to left heart disease, primary parenchymal lung disease/sleep-disordered breathing, in situ thrombotic remodeling of pulmonary arterioles following prior luminal pulmonary embolism, or in the setting of various specific predisposing conditions, such as sickle cell disease and sarcoidosis among others.5
Alternatively, pulmonary arterial hypertension (PAH) is suspected in patients with no identifiable cause of PH, pulmonary artery wedge pressure 15 mm Hg and pulmonary vascular resistance of 3.0 Wood units.6 Importantly, PAH is not synonymous with PH but is a circumspect PH disease subgroup. In turn, PAH may be idiopathic, hereditary, or associated with other select, predisposing disorders, namely systemic sclerosis. In PAH, the interplay between genetic and molecular factors results in effacement of distal pulmonary arterioles due to plexigenic, fibrotic, and/or concentric hypertrophic remodeling. Increased vascular resistance promotes early right ventricular dilation and impaired systolic function. As a result, patients with PAH are at particularly elevated risk for cor pulmonale.
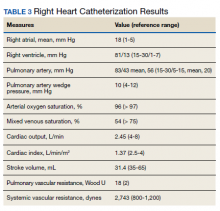
►Dr. Clark: Overnight oximetry revealed baseline oxygen saturation of 94%, an oxygen nadir of 84% with a total of 7 minutes with oxygen < 90%. On a 6-minute walk test, the patient had a max heart rate of 116 and oxygen nadir of 93%. Chest CT with and without contrast showed no evidence of pulmonary emboli but noted mild emphysematous changes. A V/Q revealed no evidence of acute or chronic pulmonary thromboembolic disease. Coronary catheterization showed normal coronary anatomy without significant CAD. A right heart catheterization showed findings consistent with severe PH with normal left-sided filling pressures (Table 3).
The patient returned a normal antinuclear antibody, C-reactive protein, HIV, and liver function panel. Based on these findings, a presumptive diagnosis of group 1 PH (idiopathic PAH) was made. Given the severity of his right heart dysfunction, he was transferred to the cardiac care unit and initiated on epoprostenol.
Dr. Maron, can you review the different treatment options for idiopathic PAH and explain why epoprostenol was chosen for this patient?
►Dr. Maron: There are 14 US Food and Drug Administration-approved drug therapies for patients with PAH, which all target either nitric oxide signaling, endothelin receptors, or the prostacyclin pathway. In the current era, treatment-naïve patients with PAH are generally initiated on calcium channel antagonist therapy if there is evidence of vasoreactivity during right heart catheterization (following nitric oxide administration), dual therapy most often with an endothelin receptor antagonist and phosphodiesterase inhibitor, or parenteral prostacyclin therapy. Since < 5% of patients will demonstrate vasoreactivity, the decision at point of care in incident patients with PAH often focuses on dual oral therapy or initiation of parenteral prostacyclin therapy. In this case, the patient reported presyncope with minimal physical activity (eg, bending over or walking up stairs) and severely decreased functional status (ie, New York Heart Association Functional [NYHA] Class III – IV), and he had a cardiac index within the range of cardiogenic shock (< 2.0 L/min/m2). Collectively, this clinical profile is considered particularly high risk, therefore, a recommendation for parenteral continuous prostacyclin therapy was made.
► Dr. Clark: The patient tolerated epoprostenol and reported improvement in his symptoms. He had a tunneled line catheter placed for continuous epoprostenol infusion. He was discharged home and scheduled for outpatient follow-up in a PH clinic. At 4 months following discharge, he was reporting steady clinical and functional improvement as well as improvement in his dyspnea. A second therapy (oral phosphodiesterase type-V inhibitor) was initiated and tolerated well. Overall, he reported resolution of presyncope, NYHA Functional Class II symptoms, and the absence of important drug effects.
1.. Manning HL, Schwartzstein RM. Pathophysiology of dyspnea. N Engl J Med. 1995;333(23):1547-1553. doi:10.1056/NEJM199512073332307
2. Parshall MB, Schwartzstein RM, Adams L, et al. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185(4):435-452. doi:10.1164/rccm.201111-2042ST
3. Phibbs B, Holmes RW, Lowe CR. Transient myocardial ischemia: the significance of dyspnea. Am J Med Sci. 1968;256(4):210-221. doi:10.1097/00000441-196810000-00002
4. Maron BA, Hess E, Maddox TM, et al. Association of borderline pulmonary hypertension with mortality and hospitalization in a large patient cohort: insights from the veterans affairs clinical assessment, reporting, and tracking program. Circulation. 2016;133(13):1240-1248. doi:10.1161/CIRCULATIONAHA.115.020207
5. Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1):1801913. Published 2019 Jan 24. doi:10.1183/13993003.01913-2018
6. Maron BA, Galiè N. Diagnosis, Treatment, and Clinical Management of Pulmonary Arterial Hypertension in the Contemporary Era: A Review. JAMA Cardiol. 2016;1(9):1056-1065. doi:10.1001/jamacardio.2016.4471
Case Presentation: A 45-year-old US Coast Guard veteran with a medical history of asthma and chronic back pain was referred to the VA Boston Healthcare System (VABHS) for evaluation of progressive, unexplained dyspnea. Two years prior to presentation, the patient was an avid outdoorsman and highly active. At the time of his initial primary care physician (PCP) evaluation he reported dyspnea on exertion, and symptoms consistent with an upper respiratory tract infection (URTI) and a recent tick bite with an associated rash. He was treated with intranasal fluticasone and a course of antibiotics. His URTI symptoms and rash improved; however the dyspnea persisted and progressed over the ensuing winter and he was referred for pulmonary function testing. Additional history included a 20 pack-year history of smoking (resolved 10 years prior to the first VABHS clinical encounter) and a family history of premature coronary artery disease (CAD) in his father and 2 paternal uncles. He lived in northern New England where he previously worked as a cemetery groundskeeper.
►Kristopher Clark, MD, Chief Medical Resident, VABHS and Boston University/Boston Medical Center: Dr. Goldstein, how do you approach a patient who presents with progressive dyspnea?
►Ronald Goldstein, MD, Chief of Pulmonary and Critical Care VABHS: The evaluation of dyspnea is a common problem for pulmonary physicians. The sensation of dyspnea may originate from a wide variety of etiologies that involve pulmonary and cardiovascular disorders, neuromuscular impairment, deconditioning, or psychological issues. It is important to characterize the temporal pattern, severity, progression, relation to exertion or other triggers, the smoking history, environmental and occupational exposures to pulmonary toxins, associated symptoms, and the history of pulmonary problems.1
The physical examination may help to identify an airway or parenchymal disorder. Wheezing on chest examination would point to an obstructive defect and crackles to a possible restrictive problem, including pulmonary fibrosis. A cardiac examination should be performed to assess for evidence of heart failure, valvular heart disease, or the presence of loud P2 suggestive of pulmonary hypertension (PH). Laboratory studies, including complete blood counts are indicated.
A more complete pulmonary evaluation usually involves pulmonary function tests (PFTs), oximetry with exertion, and chest imaging. Additional cardiac testing might include electrocardiogram (ECG) and cardiac echocardiogram, followed by an exercise study, if needed. A B-natriuretic peptide determination could be considered if there is concern for congestive heart failure.2
►Dr. Clark: The initial physical examination was normal and laboratory tests were unrevealing. Given his history of asthma, he underwent spirometry testing (Table 1).
Dr. Goldstein, aside from unexplained dyspnea, what are other indications for spirometry and when should we consider ordering a full PFT, including lung volumes and diffusion capacity? Can you interpret this patient’s spirometry results?
►Dr. Goldstein: Spirometry is indicated to evaluate for a suspected obstructive defect. The test is usually performed with and without a bronchodilator to assess airway reactivity. A change in > 12% and > 200 mL suggests acute bronchodilator responsiveness. Periodic spirometry determinations are useful to assess the effect of medications or progression of disease. A reduction in forced vital capacity (FVC) may suggest a restrictive component. This possibility requires measure of lung volumes.
A full set of PFTs (ie, spirometry plus assessment of lung volumes and diffusion capacity) is required to evaluate the abnormalities associated with chronic obstructive pulmonary disease (COPD), interstitial diseases, vascular abnormalities (particularly PH), as well as for certain preoperative assessments. The single breath diffusing capacity for carbon monoxide is a measure of the overall capillary alveolar surface area of the lung. It is decreased in emphysema and interstitial disease as well as pulmonary vascular disorders. It would be particularly useful in this case as the spirometry studies were normal.
In this case, the normal FVC renders a significant restrictive disorder unlikely and his normal forced expiratory volume (FEV1) and FEV1/FVC make a significant obstructive disorder unlikely. He did not show any bronchodilator response; however, this finding does not exclude the presence of underlying asthma or reactive airway disease as patients often will not show a bronchodilator response at time of testing if they are not experiencing active bronchospasm or constriction. Further provocative testing with a methacholine challenge could be used to assess for reactive airway disease.
►Dr. Clark: The patient continued to have dyspnea when he returned to his PCP. Given his family history of premature CAD, an ECG was obtained that showed normal sinus rhythm at a rate of 70 beats per minute. A cardiology consult was placed, and he was referred for cardiac stress testing.
Dr. Maron, there are many forms of cardiac stress tests. In this case, the patient is referred for a stress test due his dyspnea. Does that symptom help you decide which test to order? How often does dyspnea present as an anginal equivalent in the absence of other cardiovascular symptoms or known cardiovascular disease?
►Bradley Maron, MD, Codirector, Pulmonary Vascular Disease Center, VABHS: In this case, stress testing should include a functional (ie, exercise) assessment if possible. Exercise capacity is a critical determinant of prognosis across the spectrum of cardiovascular disease and in a young person can be particularly informative on global health status. Furthermore, the chief complaint from this patient is dyspnea on exertion, and therefore, exercise testing is likely to be needed to reproduce or provoke the main symptom in this case. Estimates for dyspnea as a presenting symptom for ischemic heart disease vary but may be as high as 25%.3 It should be noted that cardiopulmonary exercise testing is useful for evaluating patients with unexplained dyspnea, as exercise hypoxemia, blunted decrease in VD/VT (ventilatory dead space/tidal volume), and evidence of a pulmonary mechanical limit to physical activity can inform the differential diagnosis.
►Dr. Clark: The patient underwent exercise treadmill testing and was able reach the target heart rate (> 85% age-predicted maximal heart rate) and achieve 11 metabolic equivalents. He had no chest pain or diagnostic ECG changes. The report made no mention of whether he experienced dyspnea during the test and was read as negative for exercise-induced ischemia.
He was seen by a cardiologist who noted an increased intensity S2 heart sound on examination without any other cardiopulmonary findings. It was noted that his symptoms occurred when tamping the ground or starting to walk up a hill but resolved with rest. It was also noted that his symptoms did not occur with gradual increased activity such as that performed during an exercise tolerance test. A 2-view chest X-ray was obtained and read as normal. Given the data from this evaluation thus far, the patient was told that his symptoms were most likely a result of his asthma exacerbated by dirt and dust exposure. Continued use of albuterol inhaler therapy was recommended, and no further diagnostic assessment was pursued.
Approximately 11 months later, the patient presented again to his PCP and reported progressive dyspnea. He had delayed seeking further care as he started to “feel like my symptoms were possibly in my head” given his prior negative workup. His symptoms had escalated drastically to the point where he felt short of breath with minimal exertion in addition to feeling sweaty, dizzy, fatigued, and having near-syncope when standing.
He was referred for a transthoracic echocardiogram (TTE) that revealed a left ventricular ejection fraction (LVEF) of 55 to 60% with diastolic relaxation abnormality and a normal-sized left atrium. The TTE also showed (qualitatively) a moderately dilated right ventricle with reduced systolic function, moderately severe tricuspid regurgitation, and severe elevation (> 60 mm Hg) in estimated right ventricular systolic pressure.
Dr. Maron, can you comment on how these findings may explain the patient’s symptoms? What differential diagnoses would you now consider?
►Dr. Maron: These echocardiography results exclude left ventricular systolic dysfunction or primary left-sided valvular disease at rest as a cause of the patient’s symptoms. In light of the patient’s prior normal stress test, high grade coronary disease in the absence of LV systolic dysfunction on echocardiography also seems unlikely. Estimated pulmonary artery systolic pressure > 60 mm Hg by echocardiography is highly suggestive of PH, but in and of itself does not diagnose PH nor inform pulmonary artery wedge pressure or pulmonary vascular resistance. Along with a direct measurement of pulmonary artery (PA) pressure, these data are needed to establish, classify, and prognosticate PH clinically.
►Dr. Clark: The patient was referred to a pulmonologist. His examination included bibasilar crackles and an enhanced P2 heart sound. A comprehensive pulmonary history was obtained, which noted his smoking history, possible asbestos exposure while serving in the Coast Guard, nighttime snoring without witnessed apnea events, and no personal or family history of thromboembolism or connective tissue disease.
Dr. Goldstein, is there anything in this patient’s history that could explain his symptoms and echocardiograph findings? Which tests would you order next?
►Dr. Goldstein: PH may be secondary to a wide variety of disorders including left heart disease (Group 2), advanced COPD, interstitial fibrosis, obstructive sleep apnea (OSA), or other lung diseases (Group 3), thromboembolic disorders (Group 4), and other systemic diseases such as sarcoidosis (Group 5). Group 1 is pulmonary arterial hypertension. (Table 2).
A right heart catheterization should be done to confirm the PA pressures estimated by echocardiogram. As to a cause, clinically he does not have heart failure. The limited smoking history and spirometry data do not support advanced COPD. He was noted to have crackles on physical examination suggesting an interstitial disorder. To assess the extent of interstitial disease, we would obtain a noncontrast computed tomography (CT) of the chest. The history of snoring suggesting the possibility of OSA indicating the need for overnight oximetry as significant nocturnal hypoxemia is a possible contributing cause to PH. A polysomnogram would be required to fully evaluate a sleep disturbance. The possible asbestos exposure is not likely a contributing factor as asbestosis requires significant exposure. We would obtain a ventilation/perfusion (V/Q) scan to rule out chronic thromboembolic disease. Targeted tests for causes of Group 5 disease should also be done.
►Dr. Clark: The impression from his pulmonologist was that the patient has severe PH, though the specific etiology was not yet known. Dr. Maron, can you review for us the pathophysiology behind PH and describe how the disease is classified?
►Dr. Maron: Elevated mean pulmonary artery pressure (> 20 mm Hg) diagnosed by supine right heart catheterization is the sine qua non of PH.4 However, this alone does not inform pathophysiology. As Dr. Goldstein noted, elevated PA pressure may be due to left heart disease, primary parenchymal lung disease/sleep-disordered breathing, in situ thrombotic remodeling of pulmonary arterioles following prior luminal pulmonary embolism, or in the setting of various specific predisposing conditions, such as sickle cell disease and sarcoidosis among others.5
Alternatively, pulmonary arterial hypertension (PAH) is suspected in patients with no identifiable cause of PH, pulmonary artery wedge pressure 15 mm Hg and pulmonary vascular resistance of 3.0 Wood units.6 Importantly, PAH is not synonymous with PH but is a circumspect PH disease subgroup. In turn, PAH may be idiopathic, hereditary, or associated with other select, predisposing disorders, namely systemic sclerosis. In PAH, the interplay between genetic and molecular factors results in effacement of distal pulmonary arterioles due to plexigenic, fibrotic, and/or concentric hypertrophic remodeling. Increased vascular resistance promotes early right ventricular dilation and impaired systolic function. As a result, patients with PAH are at particularly elevated risk for cor pulmonale.
►Dr. Clark: Overnight oximetry revealed baseline oxygen saturation of 94%, an oxygen nadir of 84% with a total of 7 minutes with oxygen < 90%. On a 6-minute walk test, the patient had a max heart rate of 116 and oxygen nadir of 93%. Chest CT with and without contrast showed no evidence of pulmonary emboli but noted mild emphysematous changes. A V/Q revealed no evidence of acute or chronic pulmonary thromboembolic disease. Coronary catheterization showed normal coronary anatomy without significant CAD. A right heart catheterization showed findings consistent with severe PH with normal left-sided filling pressures (Table 3).
The patient returned a normal antinuclear antibody, C-reactive protein, HIV, and liver function panel. Based on these findings, a presumptive diagnosis of group 1 PH (idiopathic PAH) was made. Given the severity of his right heart dysfunction, he was transferred to the cardiac care unit and initiated on epoprostenol.
Dr. Maron, can you review the different treatment options for idiopathic PAH and explain why epoprostenol was chosen for this patient?
►Dr. Maron: There are 14 US Food and Drug Administration-approved drug therapies for patients with PAH, which all target either nitric oxide signaling, endothelin receptors, or the prostacyclin pathway. In the current era, treatment-naïve patients with PAH are generally initiated on calcium channel antagonist therapy if there is evidence of vasoreactivity during right heart catheterization (following nitric oxide administration), dual therapy most often with an endothelin receptor antagonist and phosphodiesterase inhibitor, or parenteral prostacyclin therapy. Since < 5% of patients will demonstrate vasoreactivity, the decision at point of care in incident patients with PAH often focuses on dual oral therapy or initiation of parenteral prostacyclin therapy. In this case, the patient reported presyncope with minimal physical activity (eg, bending over or walking up stairs) and severely decreased functional status (ie, New York Heart Association Functional [NYHA] Class III – IV), and he had a cardiac index within the range of cardiogenic shock (< 2.0 L/min/m2). Collectively, this clinical profile is considered particularly high risk, therefore, a recommendation for parenteral continuous prostacyclin therapy was made.
► Dr. Clark: The patient tolerated epoprostenol and reported improvement in his symptoms. He had a tunneled line catheter placed for continuous epoprostenol infusion. He was discharged home and scheduled for outpatient follow-up in a PH clinic. At 4 months following discharge, he was reporting steady clinical and functional improvement as well as improvement in his dyspnea. A second therapy (oral phosphodiesterase type-V inhibitor) was initiated and tolerated well. Overall, he reported resolution of presyncope, NYHA Functional Class II symptoms, and the absence of important drug effects.
Case Presentation: A 45-year-old US Coast Guard veteran with a medical history of asthma and chronic back pain was referred to the VA Boston Healthcare System (VABHS) for evaluation of progressive, unexplained dyspnea. Two years prior to presentation, the patient was an avid outdoorsman and highly active. At the time of his initial primary care physician (PCP) evaluation he reported dyspnea on exertion, and symptoms consistent with an upper respiratory tract infection (URTI) and a recent tick bite with an associated rash. He was treated with intranasal fluticasone and a course of antibiotics. His URTI symptoms and rash improved; however the dyspnea persisted and progressed over the ensuing winter and he was referred for pulmonary function testing. Additional history included a 20 pack-year history of smoking (resolved 10 years prior to the first VABHS clinical encounter) and a family history of premature coronary artery disease (CAD) in his father and 2 paternal uncles. He lived in northern New England where he previously worked as a cemetery groundskeeper.
►Kristopher Clark, MD, Chief Medical Resident, VABHS and Boston University/Boston Medical Center: Dr. Goldstein, how do you approach a patient who presents with progressive dyspnea?
►Ronald Goldstein, MD, Chief of Pulmonary and Critical Care VABHS: The evaluation of dyspnea is a common problem for pulmonary physicians. The sensation of dyspnea may originate from a wide variety of etiologies that involve pulmonary and cardiovascular disorders, neuromuscular impairment, deconditioning, or psychological issues. It is important to characterize the temporal pattern, severity, progression, relation to exertion or other triggers, the smoking history, environmental and occupational exposures to pulmonary toxins, associated symptoms, and the history of pulmonary problems.1
The physical examination may help to identify an airway or parenchymal disorder. Wheezing on chest examination would point to an obstructive defect and crackles to a possible restrictive problem, including pulmonary fibrosis. A cardiac examination should be performed to assess for evidence of heart failure, valvular heart disease, or the presence of loud P2 suggestive of pulmonary hypertension (PH). Laboratory studies, including complete blood counts are indicated.
A more complete pulmonary evaluation usually involves pulmonary function tests (PFTs), oximetry with exertion, and chest imaging. Additional cardiac testing might include electrocardiogram (ECG) and cardiac echocardiogram, followed by an exercise study, if needed. A B-natriuretic peptide determination could be considered if there is concern for congestive heart failure.2
►Dr. Clark: The initial physical examination was normal and laboratory tests were unrevealing. Given his history of asthma, he underwent spirometry testing (Table 1).
Dr. Goldstein, aside from unexplained dyspnea, what are other indications for spirometry and when should we consider ordering a full PFT, including lung volumes and diffusion capacity? Can you interpret this patient’s spirometry results?
►Dr. Goldstein: Spirometry is indicated to evaluate for a suspected obstructive defect. The test is usually performed with and without a bronchodilator to assess airway reactivity. A change in > 12% and > 200 mL suggests acute bronchodilator responsiveness. Periodic spirometry determinations are useful to assess the effect of medications or progression of disease. A reduction in forced vital capacity (FVC) may suggest a restrictive component. This possibility requires measure of lung volumes.
A full set of PFTs (ie, spirometry plus assessment of lung volumes and diffusion capacity) is required to evaluate the abnormalities associated with chronic obstructive pulmonary disease (COPD), interstitial diseases, vascular abnormalities (particularly PH), as well as for certain preoperative assessments. The single breath diffusing capacity for carbon monoxide is a measure of the overall capillary alveolar surface area of the lung. It is decreased in emphysema and interstitial disease as well as pulmonary vascular disorders. It would be particularly useful in this case as the spirometry studies were normal.
In this case, the normal FVC renders a significant restrictive disorder unlikely and his normal forced expiratory volume (FEV1) and FEV1/FVC make a significant obstructive disorder unlikely. He did not show any bronchodilator response; however, this finding does not exclude the presence of underlying asthma or reactive airway disease as patients often will not show a bronchodilator response at time of testing if they are not experiencing active bronchospasm or constriction. Further provocative testing with a methacholine challenge could be used to assess for reactive airway disease.
►Dr. Clark: The patient continued to have dyspnea when he returned to his PCP. Given his family history of premature CAD, an ECG was obtained that showed normal sinus rhythm at a rate of 70 beats per minute. A cardiology consult was placed, and he was referred for cardiac stress testing.
Dr. Maron, there are many forms of cardiac stress tests. In this case, the patient is referred for a stress test due his dyspnea. Does that symptom help you decide which test to order? How often does dyspnea present as an anginal equivalent in the absence of other cardiovascular symptoms or known cardiovascular disease?
►Bradley Maron, MD, Codirector, Pulmonary Vascular Disease Center, VABHS: In this case, stress testing should include a functional (ie, exercise) assessment if possible. Exercise capacity is a critical determinant of prognosis across the spectrum of cardiovascular disease and in a young person can be particularly informative on global health status. Furthermore, the chief complaint from this patient is dyspnea on exertion, and therefore, exercise testing is likely to be needed to reproduce or provoke the main symptom in this case. Estimates for dyspnea as a presenting symptom for ischemic heart disease vary but may be as high as 25%.3 It should be noted that cardiopulmonary exercise testing is useful for evaluating patients with unexplained dyspnea, as exercise hypoxemia, blunted decrease in VD/VT (ventilatory dead space/tidal volume), and evidence of a pulmonary mechanical limit to physical activity can inform the differential diagnosis.
►Dr. Clark: The patient underwent exercise treadmill testing and was able reach the target heart rate (> 85% age-predicted maximal heart rate) and achieve 11 metabolic equivalents. He had no chest pain or diagnostic ECG changes. The report made no mention of whether he experienced dyspnea during the test and was read as negative for exercise-induced ischemia.
He was seen by a cardiologist who noted an increased intensity S2 heart sound on examination without any other cardiopulmonary findings. It was noted that his symptoms occurred when tamping the ground or starting to walk up a hill but resolved with rest. It was also noted that his symptoms did not occur with gradual increased activity such as that performed during an exercise tolerance test. A 2-view chest X-ray was obtained and read as normal. Given the data from this evaluation thus far, the patient was told that his symptoms were most likely a result of his asthma exacerbated by dirt and dust exposure. Continued use of albuterol inhaler therapy was recommended, and no further diagnostic assessment was pursued.
Approximately 11 months later, the patient presented again to his PCP and reported progressive dyspnea. He had delayed seeking further care as he started to “feel like my symptoms were possibly in my head” given his prior negative workup. His symptoms had escalated drastically to the point where he felt short of breath with minimal exertion in addition to feeling sweaty, dizzy, fatigued, and having near-syncope when standing.
He was referred for a transthoracic echocardiogram (TTE) that revealed a left ventricular ejection fraction (LVEF) of 55 to 60% with diastolic relaxation abnormality and a normal-sized left atrium. The TTE also showed (qualitatively) a moderately dilated right ventricle with reduced systolic function, moderately severe tricuspid regurgitation, and severe elevation (> 60 mm Hg) in estimated right ventricular systolic pressure.
Dr. Maron, can you comment on how these findings may explain the patient’s symptoms? What differential diagnoses would you now consider?
►Dr. Maron: These echocardiography results exclude left ventricular systolic dysfunction or primary left-sided valvular disease at rest as a cause of the patient’s symptoms. In light of the patient’s prior normal stress test, high grade coronary disease in the absence of LV systolic dysfunction on echocardiography also seems unlikely. Estimated pulmonary artery systolic pressure > 60 mm Hg by echocardiography is highly suggestive of PH, but in and of itself does not diagnose PH nor inform pulmonary artery wedge pressure or pulmonary vascular resistance. Along with a direct measurement of pulmonary artery (PA) pressure, these data are needed to establish, classify, and prognosticate PH clinically.
►Dr. Clark: The patient was referred to a pulmonologist. His examination included bibasilar crackles and an enhanced P2 heart sound. A comprehensive pulmonary history was obtained, which noted his smoking history, possible asbestos exposure while serving in the Coast Guard, nighttime snoring without witnessed apnea events, and no personal or family history of thromboembolism or connective tissue disease.
Dr. Goldstein, is there anything in this patient’s history that could explain his symptoms and echocardiograph findings? Which tests would you order next?
►Dr. Goldstein: PH may be secondary to a wide variety of disorders including left heart disease (Group 2), advanced COPD, interstitial fibrosis, obstructive sleep apnea (OSA), or other lung diseases (Group 3), thromboembolic disorders (Group 4), and other systemic diseases such as sarcoidosis (Group 5). Group 1 is pulmonary arterial hypertension. (Table 2).
A right heart catheterization should be done to confirm the PA pressures estimated by echocardiogram. As to a cause, clinically he does not have heart failure. The limited smoking history and spirometry data do not support advanced COPD. He was noted to have crackles on physical examination suggesting an interstitial disorder. To assess the extent of interstitial disease, we would obtain a noncontrast computed tomography (CT) of the chest. The history of snoring suggesting the possibility of OSA indicating the need for overnight oximetry as significant nocturnal hypoxemia is a possible contributing cause to PH. A polysomnogram would be required to fully evaluate a sleep disturbance. The possible asbestos exposure is not likely a contributing factor as asbestosis requires significant exposure. We would obtain a ventilation/perfusion (V/Q) scan to rule out chronic thromboembolic disease. Targeted tests for causes of Group 5 disease should also be done.
►Dr. Clark: The impression from his pulmonologist was that the patient has severe PH, though the specific etiology was not yet known. Dr. Maron, can you review for us the pathophysiology behind PH and describe how the disease is classified?
►Dr. Maron: Elevated mean pulmonary artery pressure (> 20 mm Hg) diagnosed by supine right heart catheterization is the sine qua non of PH.4 However, this alone does not inform pathophysiology. As Dr. Goldstein noted, elevated PA pressure may be due to left heart disease, primary parenchymal lung disease/sleep-disordered breathing, in situ thrombotic remodeling of pulmonary arterioles following prior luminal pulmonary embolism, or in the setting of various specific predisposing conditions, such as sickle cell disease and sarcoidosis among others.5
Alternatively, pulmonary arterial hypertension (PAH) is suspected in patients with no identifiable cause of PH, pulmonary artery wedge pressure 15 mm Hg and pulmonary vascular resistance of 3.0 Wood units.6 Importantly, PAH is not synonymous with PH but is a circumspect PH disease subgroup. In turn, PAH may be idiopathic, hereditary, or associated with other select, predisposing disorders, namely systemic sclerosis. In PAH, the interplay between genetic and molecular factors results in effacement of distal pulmonary arterioles due to plexigenic, fibrotic, and/or concentric hypertrophic remodeling. Increased vascular resistance promotes early right ventricular dilation and impaired systolic function. As a result, patients with PAH are at particularly elevated risk for cor pulmonale.
►Dr. Clark: Overnight oximetry revealed baseline oxygen saturation of 94%, an oxygen nadir of 84% with a total of 7 minutes with oxygen < 90%. On a 6-minute walk test, the patient had a max heart rate of 116 and oxygen nadir of 93%. Chest CT with and without contrast showed no evidence of pulmonary emboli but noted mild emphysematous changes. A V/Q revealed no evidence of acute or chronic pulmonary thromboembolic disease. Coronary catheterization showed normal coronary anatomy without significant CAD. A right heart catheterization showed findings consistent with severe PH with normal left-sided filling pressures (Table 3).
The patient returned a normal antinuclear antibody, C-reactive protein, HIV, and liver function panel. Based on these findings, a presumptive diagnosis of group 1 PH (idiopathic PAH) was made. Given the severity of his right heart dysfunction, he was transferred to the cardiac care unit and initiated on epoprostenol.
Dr. Maron, can you review the different treatment options for idiopathic PAH and explain why epoprostenol was chosen for this patient?
►Dr. Maron: There are 14 US Food and Drug Administration-approved drug therapies for patients with PAH, which all target either nitric oxide signaling, endothelin receptors, or the prostacyclin pathway. In the current era, treatment-naïve patients with PAH are generally initiated on calcium channel antagonist therapy if there is evidence of vasoreactivity during right heart catheterization (following nitric oxide administration), dual therapy most often with an endothelin receptor antagonist and phosphodiesterase inhibitor, or parenteral prostacyclin therapy. Since < 5% of patients will demonstrate vasoreactivity, the decision at point of care in incident patients with PAH often focuses on dual oral therapy or initiation of parenteral prostacyclin therapy. In this case, the patient reported presyncope with minimal physical activity (eg, bending over or walking up stairs) and severely decreased functional status (ie, New York Heart Association Functional [NYHA] Class III – IV), and he had a cardiac index within the range of cardiogenic shock (< 2.0 L/min/m2). Collectively, this clinical profile is considered particularly high risk, therefore, a recommendation for parenteral continuous prostacyclin therapy was made.
► Dr. Clark: The patient tolerated epoprostenol and reported improvement in his symptoms. He had a tunneled line catheter placed for continuous epoprostenol infusion. He was discharged home and scheduled for outpatient follow-up in a PH clinic. At 4 months following discharge, he was reporting steady clinical and functional improvement as well as improvement in his dyspnea. A second therapy (oral phosphodiesterase type-V inhibitor) was initiated and tolerated well. Overall, he reported resolution of presyncope, NYHA Functional Class II symptoms, and the absence of important drug effects.
1.. Manning HL, Schwartzstein RM. Pathophysiology of dyspnea. N Engl J Med. 1995;333(23):1547-1553. doi:10.1056/NEJM199512073332307
2. Parshall MB, Schwartzstein RM, Adams L, et al. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185(4):435-452. doi:10.1164/rccm.201111-2042ST
3. Phibbs B, Holmes RW, Lowe CR. Transient myocardial ischemia: the significance of dyspnea. Am J Med Sci. 1968;256(4):210-221. doi:10.1097/00000441-196810000-00002
4. Maron BA, Hess E, Maddox TM, et al. Association of borderline pulmonary hypertension with mortality and hospitalization in a large patient cohort: insights from the veterans affairs clinical assessment, reporting, and tracking program. Circulation. 2016;133(13):1240-1248. doi:10.1161/CIRCULATIONAHA.115.020207
5. Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1):1801913. Published 2019 Jan 24. doi:10.1183/13993003.01913-2018
6. Maron BA, Galiè N. Diagnosis, Treatment, and Clinical Management of Pulmonary Arterial Hypertension in the Contemporary Era: A Review. JAMA Cardiol. 2016;1(9):1056-1065. doi:10.1001/jamacardio.2016.4471
1.. Manning HL, Schwartzstein RM. Pathophysiology of dyspnea. N Engl J Med. 1995;333(23):1547-1553. doi:10.1056/NEJM199512073332307
2. Parshall MB, Schwartzstein RM, Adams L, et al. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185(4):435-452. doi:10.1164/rccm.201111-2042ST
3. Phibbs B, Holmes RW, Lowe CR. Transient myocardial ischemia: the significance of dyspnea. Am J Med Sci. 1968;256(4):210-221. doi:10.1097/00000441-196810000-00002
4. Maron BA, Hess E, Maddox TM, et al. Association of borderline pulmonary hypertension with mortality and hospitalization in a large patient cohort: insights from the veterans affairs clinical assessment, reporting, and tracking program. Circulation. 2016;133(13):1240-1248. doi:10.1161/CIRCULATIONAHA.115.020207
5. Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1):1801913. Published 2019 Jan 24. doi:10.1183/13993003.01913-2018
6. Maron BA, Galiè N. Diagnosis, Treatment, and Clinical Management of Pulmonary Arterial Hypertension in the Contemporary Era: A Review. JAMA Cardiol. 2016;1(9):1056-1065. doi:10.1001/jamacardio.2016.4471
Chronic abdominal pain: What to do when a patient presents with it
She reports the pain is about a 7 out of 10, located in the right upper quadrant. The pain does not worsen with food and not relieved with bowel movements. She has no nausea or vomiting. She reports that the pain worsens when she is sitting or standing and is relieved by lying down. Her past medical history includes having had a cholecystectomy in 2016, having hypertension, and having type 2 diabetes mellitus.
The patient’s medications include metformin, lisinopril, and empagliflozin. Her blood pressure was 130/70, and her pulse was 80. An abdominal exam of her found tenderness to palpation in the right upper quadrant, and no rebound tenderness. Her labs found a white blood cell count of 5.4, a hematocrit of 44%, an erythrocyte sedimentation rate of 13, a C-reactive protein of 1.0, a bilirubin of .8, an alkaline phosphatase of 100, an aspartate aminotransferase of 30, and an alanine transaminase of 22.
What is the most appropriate next step?
A) Right side up oblique ultrasound.
B) Abdominal CT scan.
C) Upper endoscopy.
D) More detailed physical exam.
The correct answer here is D, a more detailed physical exam is needed. Given the positional nature of this patient’s abdominal pain, an evaluation for an abdominal wall cause is appropriate.
Abdominal wall pain as a cause of chronic abdominal pain is rarely considered, but it really should be. Costanza and colleagues looked at 2,709 patients referred to gastroenterologists for chronic abdominal pain.1 Chronic abdominal wall pain was diagnosed in 137 patients, with the diagnosis unchanged in 97% of these patients after 4 years. Most of the patients were women (four to one), and the diagnosis was almost always unsuspected by the referring physician. Physical exam was helpful in suggesting the diagnosis of abdominal wall pain.
The use of Carnett sign can be helpful. A positive Carnett sign is when abdominal pain increases or remains unchanged with tensing abdomen or when the examiner palpates the tensed abdomen. Thompson and colleagues looked at the outcome of 72 patients with undiagnosed abdominal pain and a positive Carnett sign.2 Despite multiple diagnostic tests and surgeries done on these patients, very few of them had serious underlying pathology.
Thompson and Frances published another study looking at 120 patients presenting to an ED with undiagnosed abdominal pain.3 Twenty-four of the patients had positive abdominal wall tenderness on exam, and of those, only 1 had intra-abdominal pathology.
In another study, 158 patients admitted to the hospital with abdominal pain were evaluated for the presence of abdominal wall pain.4 Fifty-three patients were diagnosed with appendicitis, and 5 had abdominal wall tenderness on exam. Thirty-eight patients had other intra-abdominal pathology, and none of those had abdominal wall tenderness on exam. Of the 67 patients in the study who had nonspecific abdominal pain, 19 had abdominal wall tenderness on exam.
Most physicians do not include evaluation for abdominal wall tenderness as part of their evaluation of patients with abdominal pain. I think looking for this is helpful and, if positive, may lead to a diagnosis, as well as reduce the likelihood of intra-abdominal diagnoses.
What can we do in regard to therapy for patients with an abdominal wall source of pain?
Many patients with abdominal wall pain have anterior cutaneous nerve entrapment syndrome (ACNES). Patient’s with this often have discrete areas of tenderness on exam, often on the lateral edge of the rectus sheath, frequently on the right side of the abdomen. Anesthetic injection at the point of tenderness provides immediate relief for patients with ACNES, and is helpful in confirming the diagnosis.
Boelens and colleagues did injections in 48 patients suspected of having ACNE, randomizing half to receive lidocaine and half to receive saline placebo.5The majority of the patients receiving lidocaine (54%) had a response, compared with 17% of placebo patients (P less than .007).
Greenbaum and colleagues studied 79 patients with chronic abdominal wall pain.6 In this study, 72 of 79 patients had greater than 50% pain relief with anesthetic injection and were followed for a mean of almost 14 months. Only four of these patients ended up having a visceral cause of pain.
Can using injections help pain from ACNES longer term?
Koop and colleagues looked at all published studies in regards to both immediate and longer-term pain relief with injections.7 Both lidocaine injections and injections with lidocaine plus steroids led to long-term pain relief (40%-50% of patients with multiple lidocaine injections and up to 80% with lidocaine plus steroid injections). I think that injections are certainly worth a try in patients with chronic abdominal wall pain.
Pearl
Consider chronic abdominal wall pain in your differential diagnoses for patients with chronic abdominal pain, and use Carnett sign to help with diagnosis.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Costanza CD et al. Clin Gastroenterol Hepatol. 2004 May;2(5):395-9.
2. Thomson WH et al. Br J Surg. 1991 Feb;78(2):223-5.
3. Thomson H, Francis DM. Lancet. 1977 Nov 19;2(8047):1053-4.
4. Gray DW et al. Ann R Coll Surg Engl. 1988 Jul;70(4):233-4.
5. Boelens OB et al. Br J Surg. 2013 Jan;100(2):217-21.
6. Greenbaum DS et al. Dig Dis Sci. 1994 Sep;39(9):1935-41.
7. Koop H et al. Dtsch Arztebl Int. 2016 Jan 29;113(4):51-7.
She reports the pain is about a 7 out of 10, located in the right upper quadrant. The pain does not worsen with food and not relieved with bowel movements. She has no nausea or vomiting. She reports that the pain worsens when she is sitting or standing and is relieved by lying down. Her past medical history includes having had a cholecystectomy in 2016, having hypertension, and having type 2 diabetes mellitus.
The patient’s medications include metformin, lisinopril, and empagliflozin. Her blood pressure was 130/70, and her pulse was 80. An abdominal exam of her found tenderness to palpation in the right upper quadrant, and no rebound tenderness. Her labs found a white blood cell count of 5.4, a hematocrit of 44%, an erythrocyte sedimentation rate of 13, a C-reactive protein of 1.0, a bilirubin of .8, an alkaline phosphatase of 100, an aspartate aminotransferase of 30, and an alanine transaminase of 22.
What is the most appropriate next step?
A) Right side up oblique ultrasound.
B) Abdominal CT scan.
C) Upper endoscopy.
D) More detailed physical exam.
The correct answer here is D, a more detailed physical exam is needed. Given the positional nature of this patient’s abdominal pain, an evaluation for an abdominal wall cause is appropriate.
Abdominal wall pain as a cause of chronic abdominal pain is rarely considered, but it really should be. Costanza and colleagues looked at 2,709 patients referred to gastroenterologists for chronic abdominal pain.1 Chronic abdominal wall pain was diagnosed in 137 patients, with the diagnosis unchanged in 97% of these patients after 4 years. Most of the patients were women (four to one), and the diagnosis was almost always unsuspected by the referring physician. Physical exam was helpful in suggesting the diagnosis of abdominal wall pain.
The use of Carnett sign can be helpful. A positive Carnett sign is when abdominal pain increases or remains unchanged with tensing abdomen or when the examiner palpates the tensed abdomen. Thompson and colleagues looked at the outcome of 72 patients with undiagnosed abdominal pain and a positive Carnett sign.2 Despite multiple diagnostic tests and surgeries done on these patients, very few of them had serious underlying pathology.
Thompson and Frances published another study looking at 120 patients presenting to an ED with undiagnosed abdominal pain.3 Twenty-four of the patients had positive abdominal wall tenderness on exam, and of those, only 1 had intra-abdominal pathology.
In another study, 158 patients admitted to the hospital with abdominal pain were evaluated for the presence of abdominal wall pain.4 Fifty-three patients were diagnosed with appendicitis, and 5 had abdominal wall tenderness on exam. Thirty-eight patients had other intra-abdominal pathology, and none of those had abdominal wall tenderness on exam. Of the 67 patients in the study who had nonspecific abdominal pain, 19 had abdominal wall tenderness on exam.
Most physicians do not include evaluation for abdominal wall tenderness as part of their evaluation of patients with abdominal pain. I think looking for this is helpful and, if positive, may lead to a diagnosis, as well as reduce the likelihood of intra-abdominal diagnoses.
What can we do in regard to therapy for patients with an abdominal wall source of pain?
Many patients with abdominal wall pain have anterior cutaneous nerve entrapment syndrome (ACNES). Patient’s with this often have discrete areas of tenderness on exam, often on the lateral edge of the rectus sheath, frequently on the right side of the abdomen. Anesthetic injection at the point of tenderness provides immediate relief for patients with ACNES, and is helpful in confirming the diagnosis.
Boelens and colleagues did injections in 48 patients suspected of having ACNE, randomizing half to receive lidocaine and half to receive saline placebo.5The majority of the patients receiving lidocaine (54%) had a response, compared with 17% of placebo patients (P less than .007).
Greenbaum and colleagues studied 79 patients with chronic abdominal wall pain.6 In this study, 72 of 79 patients had greater than 50% pain relief with anesthetic injection and were followed for a mean of almost 14 months. Only four of these patients ended up having a visceral cause of pain.
Can using injections help pain from ACNES longer term?
Koop and colleagues looked at all published studies in regards to both immediate and longer-term pain relief with injections.7 Both lidocaine injections and injections with lidocaine plus steroids led to long-term pain relief (40%-50% of patients with multiple lidocaine injections and up to 80% with lidocaine plus steroid injections). I think that injections are certainly worth a try in patients with chronic abdominal wall pain.
Pearl
Consider chronic abdominal wall pain in your differential diagnoses for patients with chronic abdominal pain, and use Carnett sign to help with diagnosis.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Costanza CD et al. Clin Gastroenterol Hepatol. 2004 May;2(5):395-9.
2. Thomson WH et al. Br J Surg. 1991 Feb;78(2):223-5.
3. Thomson H, Francis DM. Lancet. 1977 Nov 19;2(8047):1053-4.
4. Gray DW et al. Ann R Coll Surg Engl. 1988 Jul;70(4):233-4.
5. Boelens OB et al. Br J Surg. 2013 Jan;100(2):217-21.
6. Greenbaum DS et al. Dig Dis Sci. 1994 Sep;39(9):1935-41.
7. Koop H et al. Dtsch Arztebl Int. 2016 Jan 29;113(4):51-7.
She reports the pain is about a 7 out of 10, located in the right upper quadrant. The pain does not worsen with food and not relieved with bowel movements. She has no nausea or vomiting. She reports that the pain worsens when she is sitting or standing and is relieved by lying down. Her past medical history includes having had a cholecystectomy in 2016, having hypertension, and having type 2 diabetes mellitus.
The patient’s medications include metformin, lisinopril, and empagliflozin. Her blood pressure was 130/70, and her pulse was 80. An abdominal exam of her found tenderness to palpation in the right upper quadrant, and no rebound tenderness. Her labs found a white blood cell count of 5.4, a hematocrit of 44%, an erythrocyte sedimentation rate of 13, a C-reactive protein of 1.0, a bilirubin of .8, an alkaline phosphatase of 100, an aspartate aminotransferase of 30, and an alanine transaminase of 22.
What is the most appropriate next step?
A) Right side up oblique ultrasound.
B) Abdominal CT scan.
C) Upper endoscopy.
D) More detailed physical exam.
The correct answer here is D, a more detailed physical exam is needed. Given the positional nature of this patient’s abdominal pain, an evaluation for an abdominal wall cause is appropriate.
Abdominal wall pain as a cause of chronic abdominal pain is rarely considered, but it really should be. Costanza and colleagues looked at 2,709 patients referred to gastroenterologists for chronic abdominal pain.1 Chronic abdominal wall pain was diagnosed in 137 patients, with the diagnosis unchanged in 97% of these patients after 4 years. Most of the patients were women (four to one), and the diagnosis was almost always unsuspected by the referring physician. Physical exam was helpful in suggesting the diagnosis of abdominal wall pain.
The use of Carnett sign can be helpful. A positive Carnett sign is when abdominal pain increases or remains unchanged with tensing abdomen or when the examiner palpates the tensed abdomen. Thompson and colleagues looked at the outcome of 72 patients with undiagnosed abdominal pain and a positive Carnett sign.2 Despite multiple diagnostic tests and surgeries done on these patients, very few of them had serious underlying pathology.
Thompson and Frances published another study looking at 120 patients presenting to an ED with undiagnosed abdominal pain.3 Twenty-four of the patients had positive abdominal wall tenderness on exam, and of those, only 1 had intra-abdominal pathology.
In another study, 158 patients admitted to the hospital with abdominal pain were evaluated for the presence of abdominal wall pain.4 Fifty-three patients were diagnosed with appendicitis, and 5 had abdominal wall tenderness on exam. Thirty-eight patients had other intra-abdominal pathology, and none of those had abdominal wall tenderness on exam. Of the 67 patients in the study who had nonspecific abdominal pain, 19 had abdominal wall tenderness on exam.
Most physicians do not include evaluation for abdominal wall tenderness as part of their evaluation of patients with abdominal pain. I think looking for this is helpful and, if positive, may lead to a diagnosis, as well as reduce the likelihood of intra-abdominal diagnoses.
What can we do in regard to therapy for patients with an abdominal wall source of pain?
Many patients with abdominal wall pain have anterior cutaneous nerve entrapment syndrome (ACNES). Patient’s with this often have discrete areas of tenderness on exam, often on the lateral edge of the rectus sheath, frequently on the right side of the abdomen. Anesthetic injection at the point of tenderness provides immediate relief for patients with ACNES, and is helpful in confirming the diagnosis.
Boelens and colleagues did injections in 48 patients suspected of having ACNE, randomizing half to receive lidocaine and half to receive saline placebo.5The majority of the patients receiving lidocaine (54%) had a response, compared with 17% of placebo patients (P less than .007).
Greenbaum and colleagues studied 79 patients with chronic abdominal wall pain.6 In this study, 72 of 79 patients had greater than 50% pain relief with anesthetic injection and were followed for a mean of almost 14 months. Only four of these patients ended up having a visceral cause of pain.
Can using injections help pain from ACNES longer term?
Koop and colleagues looked at all published studies in regards to both immediate and longer-term pain relief with injections.7 Both lidocaine injections and injections with lidocaine plus steroids led to long-term pain relief (40%-50% of patients with multiple lidocaine injections and up to 80% with lidocaine plus steroid injections). I think that injections are certainly worth a try in patients with chronic abdominal wall pain.
Pearl
Consider chronic abdominal wall pain in your differential diagnoses for patients with chronic abdominal pain, and use Carnett sign to help with diagnosis.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Costanza CD et al. Clin Gastroenterol Hepatol. 2004 May;2(5):395-9.
2. Thomson WH et al. Br J Surg. 1991 Feb;78(2):223-5.
3. Thomson H, Francis DM. Lancet. 1977 Nov 19;2(8047):1053-4.
4. Gray DW et al. Ann R Coll Surg Engl. 1988 Jul;70(4):233-4.
5. Boelens OB et al. Br J Surg. 2013 Jan;100(2):217-21.
6. Greenbaum DS et al. Dig Dis Sci. 1994 Sep;39(9):1935-41.
7. Koop H et al. Dtsch Arztebl Int. 2016 Jan 29;113(4):51-7.
Sleep apnea found to impact pain severity in younger adults
Sleep specialists might want to take a closer look at the connections between obstructive sleep apnea, chronic pain, and reported pain intensity in younger patients. Young adults with a diagnosis of obstructive sleep apnea (OSA) are more likely to report moderate to severe pain intensity, compared with their peers who do not have the diagnosis, results from a large cross-sectional analysis of veterans showed.
“Because of the high burden of chronic pain conditions in younger adults, this study highlights the need to understand the impact of OSA diagnosis and treatment on pain intensity,” researchers led by Wardah Athar, a graduate student at Yale University, New Haven, Conn., and Lori A. Bastian, MD, MPH, a professor of internal medicine at Yale, wrote in an article published in the Annals of the American Thoracic Society. “This understanding would then help inform the development of interventions to promote screening for OSA among young adults with chronic pain and pain management among those with diagnosed OSA.”
In an effort to assess whether young adults with diagnosed OSA are more likely to report higher pain intensity, compared with those without OSA, the researchers drew from a sample of 858,226 veterans from Operation Enduring Freedom, Operation Iraqi Freedom, and Operation New Dawn who had at least one visit to a VA clinic between 2001 and 2014. They used ICD-9 codes to identify OSA and assessed self-reported responses to pain measures on a 0-10 numeric scale which were recorded in each veteran’s EMR. Next, they averaged pain intensity responses over a 12-month period and categorized them as none (0), mild (1-3), and moderate/severe (4–10). Covariates included age, sex, education, race, mental health diagnoses, headache diagnoses, pain diagnoses, hypertension, diabetes, body mass index, and smoking status. The researchers used multivariate logistic regression models and multiple imputation to generate values for missing variables.
The mean age of the patients was 30 years, 64% were White, 17% were Black, 12% were Hispanic, and remainder were other/unknown race/ethnicity. Ninety percent were male, and 20% had greater than a high school education. Of the 858,226 patients, 91,244 (11%) had a diagnosis of OSA. Compared with patients who had no diagnosis of OSA, the unadjusted odds of reporting moderate/severe pain was 48% higher among those with OSA (odds ratio 1.48; P < .0001). After the researchers adjusted for all covariates in the model, the association between OSA and moderate/severe pain remained significant though attenuated, with an adjusted odds ratio of 1.09 (P < .0001).
Several characteristics were different between those who had a diagnosis of OSA and those who did not, including age (a mean of 36 vs. 26 years, respectively) and having the following diagnoses: pain (36% vs. 16%), headache (28% vs. 14%), diabetes (12% vs. 2%), hypertension (40% vs. 12%), and a body mass index of 30 kg/m2 or greater (69% vs. 35%). Certain psychiatric disorders were also common among patients with OSA, including major depressive disorder (20% vs. 10%), posttraumatic stress disorder (50% vs. 30%), and substance use disorder (26% vs. 17%). Patients with OSA were also more likely to have been prescribed benzodiazepines or opioids within 90 days of their OSA diagnosis. Although men were more likely to have a diagnosis of OSA, no differences related to sex in the association of OSA and pain were observed in sex-based stratified analyses.
“Based on these results, we suggest more thorough and more frequent pain intensity screening in patients with OSA, particularly in those patients who are younger than 60 years old without significant comorbid illness,” the researchers concluded. “Furthermore, we also recommend increased OSA screening for patients with moderate/severe pain intensity and pain diagnoses.” One tool they recommend is the STOP-Bang (Snoring, Tiredness, Observed Apnea, Blood Pressure, Body Mass Index, Age, Neck Circumference, and Gender) questionnaire, which has been validated in multiple settings.
Commenting on the findings of this study, Krishna M. Sundar, MD, FCCP, medical director of the Sleep-Wake Center at the University of Utah, Salt Lake City, commended the study design. “One of the problems with sleep apnea studies is that there are always confounding effects, especially from BMI. This is a population that has a significant medical burden of disease, but I think this is a well-done study to look at the relationship between pain and OSA in a younger population. The authors tried to adjust for all these confounders and they still found a significant association. This indicates that sleep affects one’s pain threshold. And sleep apnea, by mechanisms still yet to be defined, also alters that pain threshold. It may also affect the expression of pain or management of pain, making treatment more problematic in this population,” he said in an interview.
A key limitation of the study, he continued, was the fact it evaluated only one aspect of sleep: OSA. “They didn’t look at duration of sleep, comorbid insomnia, or fragmentation of sleep from apnea or from other causes,” Dr. Sundar said. “We have multiple ways of treating sleep apnea. Clearly, we need studies of treating sleep apnea with [continuous positive airway pressure] and how that affects the occurrence of pain. The relevant practical aspect of this is that there are pain clinics all over the country that should screen for sleep apnea. Along the same lines, sleep practitioners should be aware that pain has an important association with sleep apnea.”
The study was supported by the Health Services Research & Development in the Department of Veterans Affairs of the Veterans Health Administration, the Yale School of Medicine Medical Student Fellowship, and the U.S. National Institutes of Health.
SOURCE: Athar W et al. Ann Am Thorac Soc. 2020;17(10):1273-48.
Correction, 10/28/20: An earlier version of this article misstated Wardah Athar's name in the photo caption.
Sleep specialists might want to take a closer look at the connections between obstructive sleep apnea, chronic pain, and reported pain intensity in younger patients. Young adults with a diagnosis of obstructive sleep apnea (OSA) are more likely to report moderate to severe pain intensity, compared with their peers who do not have the diagnosis, results from a large cross-sectional analysis of veterans showed.
“Because of the high burden of chronic pain conditions in younger adults, this study highlights the need to understand the impact of OSA diagnosis and treatment on pain intensity,” researchers led by Wardah Athar, a graduate student at Yale University, New Haven, Conn., and Lori A. Bastian, MD, MPH, a professor of internal medicine at Yale, wrote in an article published in the Annals of the American Thoracic Society. “This understanding would then help inform the development of interventions to promote screening for OSA among young adults with chronic pain and pain management among those with diagnosed OSA.”
In an effort to assess whether young adults with diagnosed OSA are more likely to report higher pain intensity, compared with those without OSA, the researchers drew from a sample of 858,226 veterans from Operation Enduring Freedom, Operation Iraqi Freedom, and Operation New Dawn who had at least one visit to a VA clinic between 2001 and 2014. They used ICD-9 codes to identify OSA and assessed self-reported responses to pain measures on a 0-10 numeric scale which were recorded in each veteran’s EMR. Next, they averaged pain intensity responses over a 12-month period and categorized them as none (0), mild (1-3), and moderate/severe (4–10). Covariates included age, sex, education, race, mental health diagnoses, headache diagnoses, pain diagnoses, hypertension, diabetes, body mass index, and smoking status. The researchers used multivariate logistic regression models and multiple imputation to generate values for missing variables.
The mean age of the patients was 30 years, 64% were White, 17% were Black, 12% were Hispanic, and remainder were other/unknown race/ethnicity. Ninety percent were male, and 20% had greater than a high school education. Of the 858,226 patients, 91,244 (11%) had a diagnosis of OSA. Compared with patients who had no diagnosis of OSA, the unadjusted odds of reporting moderate/severe pain was 48% higher among those with OSA (odds ratio 1.48; P < .0001). After the researchers adjusted for all covariates in the model, the association between OSA and moderate/severe pain remained significant though attenuated, with an adjusted odds ratio of 1.09 (P < .0001).
Several characteristics were different between those who had a diagnosis of OSA and those who did not, including age (a mean of 36 vs. 26 years, respectively) and having the following diagnoses: pain (36% vs. 16%), headache (28% vs. 14%), diabetes (12% vs. 2%), hypertension (40% vs. 12%), and a body mass index of 30 kg/m2 or greater (69% vs. 35%). Certain psychiatric disorders were also common among patients with OSA, including major depressive disorder (20% vs. 10%), posttraumatic stress disorder (50% vs. 30%), and substance use disorder (26% vs. 17%). Patients with OSA were also more likely to have been prescribed benzodiazepines or opioids within 90 days of their OSA diagnosis. Although men were more likely to have a diagnosis of OSA, no differences related to sex in the association of OSA and pain were observed in sex-based stratified analyses.
“Based on these results, we suggest more thorough and more frequent pain intensity screening in patients with OSA, particularly in those patients who are younger than 60 years old without significant comorbid illness,” the researchers concluded. “Furthermore, we also recommend increased OSA screening for patients with moderate/severe pain intensity and pain diagnoses.” One tool they recommend is the STOP-Bang (Snoring, Tiredness, Observed Apnea, Blood Pressure, Body Mass Index, Age, Neck Circumference, and Gender) questionnaire, which has been validated in multiple settings.
Commenting on the findings of this study, Krishna M. Sundar, MD, FCCP, medical director of the Sleep-Wake Center at the University of Utah, Salt Lake City, commended the study design. “One of the problems with sleep apnea studies is that there are always confounding effects, especially from BMI. This is a population that has a significant medical burden of disease, but I think this is a well-done study to look at the relationship between pain and OSA in a younger population. The authors tried to adjust for all these confounders and they still found a significant association. This indicates that sleep affects one’s pain threshold. And sleep apnea, by mechanisms still yet to be defined, also alters that pain threshold. It may also affect the expression of pain or management of pain, making treatment more problematic in this population,” he said in an interview.
A key limitation of the study, he continued, was the fact it evaluated only one aspect of sleep: OSA. “They didn’t look at duration of sleep, comorbid insomnia, or fragmentation of sleep from apnea or from other causes,” Dr. Sundar said. “We have multiple ways of treating sleep apnea. Clearly, we need studies of treating sleep apnea with [continuous positive airway pressure] and how that affects the occurrence of pain. The relevant practical aspect of this is that there are pain clinics all over the country that should screen for sleep apnea. Along the same lines, sleep practitioners should be aware that pain has an important association with sleep apnea.”
The study was supported by the Health Services Research & Development in the Department of Veterans Affairs of the Veterans Health Administration, the Yale School of Medicine Medical Student Fellowship, and the U.S. National Institutes of Health.
SOURCE: Athar W et al. Ann Am Thorac Soc. 2020;17(10):1273-48.
Correction, 10/28/20: An earlier version of this article misstated Wardah Athar's name in the photo caption.
Sleep specialists might want to take a closer look at the connections between obstructive sleep apnea, chronic pain, and reported pain intensity in younger patients. Young adults with a diagnosis of obstructive sleep apnea (OSA) are more likely to report moderate to severe pain intensity, compared with their peers who do not have the diagnosis, results from a large cross-sectional analysis of veterans showed.
“Because of the high burden of chronic pain conditions in younger adults, this study highlights the need to understand the impact of OSA diagnosis and treatment on pain intensity,” researchers led by Wardah Athar, a graduate student at Yale University, New Haven, Conn., and Lori A. Bastian, MD, MPH, a professor of internal medicine at Yale, wrote in an article published in the Annals of the American Thoracic Society. “This understanding would then help inform the development of interventions to promote screening for OSA among young adults with chronic pain and pain management among those with diagnosed OSA.”
In an effort to assess whether young adults with diagnosed OSA are more likely to report higher pain intensity, compared with those without OSA, the researchers drew from a sample of 858,226 veterans from Operation Enduring Freedom, Operation Iraqi Freedom, and Operation New Dawn who had at least one visit to a VA clinic between 2001 and 2014. They used ICD-9 codes to identify OSA and assessed self-reported responses to pain measures on a 0-10 numeric scale which were recorded in each veteran’s EMR. Next, they averaged pain intensity responses over a 12-month period and categorized them as none (0), mild (1-3), and moderate/severe (4–10). Covariates included age, sex, education, race, mental health diagnoses, headache diagnoses, pain diagnoses, hypertension, diabetes, body mass index, and smoking status. The researchers used multivariate logistic regression models and multiple imputation to generate values for missing variables.
The mean age of the patients was 30 years, 64% were White, 17% were Black, 12% were Hispanic, and remainder were other/unknown race/ethnicity. Ninety percent were male, and 20% had greater than a high school education. Of the 858,226 patients, 91,244 (11%) had a diagnosis of OSA. Compared with patients who had no diagnosis of OSA, the unadjusted odds of reporting moderate/severe pain was 48% higher among those with OSA (odds ratio 1.48; P < .0001). After the researchers adjusted for all covariates in the model, the association between OSA and moderate/severe pain remained significant though attenuated, with an adjusted odds ratio of 1.09 (P < .0001).
Several characteristics were different between those who had a diagnosis of OSA and those who did not, including age (a mean of 36 vs. 26 years, respectively) and having the following diagnoses: pain (36% vs. 16%), headache (28% vs. 14%), diabetes (12% vs. 2%), hypertension (40% vs. 12%), and a body mass index of 30 kg/m2 or greater (69% vs. 35%). Certain psychiatric disorders were also common among patients with OSA, including major depressive disorder (20% vs. 10%), posttraumatic stress disorder (50% vs. 30%), and substance use disorder (26% vs. 17%). Patients with OSA were also more likely to have been prescribed benzodiazepines or opioids within 90 days of their OSA diagnosis. Although men were more likely to have a diagnosis of OSA, no differences related to sex in the association of OSA and pain were observed in sex-based stratified analyses.
“Based on these results, we suggest more thorough and more frequent pain intensity screening in patients with OSA, particularly in those patients who are younger than 60 years old without significant comorbid illness,” the researchers concluded. “Furthermore, we also recommend increased OSA screening for patients with moderate/severe pain intensity and pain diagnoses.” One tool they recommend is the STOP-Bang (Snoring, Tiredness, Observed Apnea, Blood Pressure, Body Mass Index, Age, Neck Circumference, and Gender) questionnaire, which has been validated in multiple settings.
Commenting on the findings of this study, Krishna M. Sundar, MD, FCCP, medical director of the Sleep-Wake Center at the University of Utah, Salt Lake City, commended the study design. “One of the problems with sleep apnea studies is that there are always confounding effects, especially from BMI. This is a population that has a significant medical burden of disease, but I think this is a well-done study to look at the relationship between pain and OSA in a younger population. The authors tried to adjust for all these confounders and they still found a significant association. This indicates that sleep affects one’s pain threshold. And sleep apnea, by mechanisms still yet to be defined, also alters that pain threshold. It may also affect the expression of pain or management of pain, making treatment more problematic in this population,” he said in an interview.
A key limitation of the study, he continued, was the fact it evaluated only one aspect of sleep: OSA. “They didn’t look at duration of sleep, comorbid insomnia, or fragmentation of sleep from apnea or from other causes,” Dr. Sundar said. “We have multiple ways of treating sleep apnea. Clearly, we need studies of treating sleep apnea with [continuous positive airway pressure] and how that affects the occurrence of pain. The relevant practical aspect of this is that there are pain clinics all over the country that should screen for sleep apnea. Along the same lines, sleep practitioners should be aware that pain has an important association with sleep apnea.”
The study was supported by the Health Services Research & Development in the Department of Veterans Affairs of the Veterans Health Administration, the Yale School of Medicine Medical Student Fellowship, and the U.S. National Institutes of Health.
SOURCE: Athar W et al. Ann Am Thorac Soc. 2020;17(10):1273-48.
Correction, 10/28/20: An earlier version of this article misstated Wardah Athar's name in the photo caption.
FROM ANNALS OF THE AMERICAN THORACIC SOCIETY
Diabetic neuropathic pain linked to brain bioenergic anomalies
Abnormal mitochondrial activity in pain-processing areas of the brain may explain why some persons with type 2 diabetes experience painful peripheral neuropathy while others do not, new U.K. study findings have suggested.
A greater ratio of adenosine triphosphate (ATP) – “the cellular energy currency of all life” – to phosphocreatine (PCr) was observed in the somatosensory cortex and right thalamus in those with painful diabetic peripheral neuropathy (DPN). Importantly, this correlated with neuropathic pain symptom intensity as measured by the Neuropathic Pain Symptom Inventory (NPSI) and the Doleur Neuroathique en 4 (DN4).
The findings suggest that altered cerebral phosphorus metabolite ratios may serve as a biomarker of DPN, said the study’s investigators.
“Normally the ATP:Cr ratio will be unaltered, but there’s stress to the brain that might change,” Gordon Sloan, a clinical research fellow within the Diabetes Research Unit at the Royal Hallamshire Hospital in Sheffield (England) said at the virtual annual meeting of the European Association for the Study of Diabetes.
DPN affects around a quarter of patients with type 2 diabetes but treatments are “inadequate”, and “unfortunately fewer than a third of individuals receive 50% or greater pain relief from current neuropathic pain treatments,” Mr. Sloan said. “Ultimately, this lack of understanding of the pathophysiology of the condition is therefore clear rationale to investigate the disease mechanisms further and to find novel targets for treatments,” he added.
Brain metabolites offer clues to neuropathic pain levels
The thalamus and primary somatosensory cortex are two key areas of the brain that are involved in the perception of painful stimuli, Mr. Sloan explained. “The thalamus receives most of the slowest sensory impulses from the peripheral nervous system modulating and processing them for relaying the signals to the rest of the pain matrix, including the somatosensory cortex where these sensations are interpreted and localized.”
Prior imaging work by Mr. Sloan’s group and others have shown that there are alterations in the functioning of both these brain areas in those with painful DPN versus healthy volunteers and those with type 2 diabetes but no DPN. So for their current study, Mr. Sloan and associates from Sheffield University and Sheffield Teaching Hospitals National Health Service Trust, used an advanced imaging method – phosphorus magnetic resonance spectroscopy (MRS) – to scan the thalamus and somatosensory cortex of 43 persons with type 2 diabetes and 12 healthy volunteers. Of those with diabetes, 11 had no DPN, 12 had DPN but were not currently in pain, and 20 had painful DPN.
From the scans, three phosphorus metabolite ratios were calculated, which gave an indication of mitochondrial activity: first, the ATP to PCr ratio, which gives a measure of cellular energy status; second, the ATP to inorganic phosphate (Pi) ratio, which measures oxidative phosphorylation; and third, the ratio of phosphomonoesters (PME) to phosphodiesters (PDE), which gives a measure of cell membrane turnover.
“We have measured the ratio of high-energy phosphate levels which are an indirect representation of the balance between energy generation, reserve and usage in the brain,” Mr. Sloan said.
The subjects studied were of a similar age, around 63 years on average, and well matched in terms of their sex and body mass index. Those with diabetes of course had higher blood glucose and glycated hemoglobin than did the healthy volunteers during the scans. Among those with diabetes, those with DPN were significantly more likely to have a longer duration of diabetes (12.5 years for painful DPN and 15.8 years for nonpainful DPN) than were those with no DPN (8.7 years).
Furthermore, those with DPN had higher scores on the Neuropathic Pain Symptom Inventory (NPSI) than did those without, although there was not much difference between those with painful or nonpainful DPN. On the other had, those with painful DPN were more likely to have higher scores when using the Doleur Neuroathique en 4 (DN4) to assess their pain level.
Results showed significant changes in cerebral cellular bioenergetics in the pain processing regions of the brain in those with painful DPN. The ATP:PCr at the thalamus and at the somatosensory cortex was significantly higher in those with painful DPN, compared with healthy volunteers. The other measures of phosphorus metabolite levels (ATP:Pi and PME:PDE) were unaltered.
“We hypothesize that the findings of the study are suggestive of increased energy demands in regions of pain perception due to increased neuronal activity” said Dr. Sloan.
The study’s results add further evidence for cerebral alterations playing a key role in the generation and maintenance of pain in painful DPN.
SOURCE: Sloan S et al. EASD 2020, oral presentation 181.
Abnormal mitochondrial activity in pain-processing areas of the brain may explain why some persons with type 2 diabetes experience painful peripheral neuropathy while others do not, new U.K. study findings have suggested.
A greater ratio of adenosine triphosphate (ATP) – “the cellular energy currency of all life” – to phosphocreatine (PCr) was observed in the somatosensory cortex and right thalamus in those with painful diabetic peripheral neuropathy (DPN). Importantly, this correlated with neuropathic pain symptom intensity as measured by the Neuropathic Pain Symptom Inventory (NPSI) and the Doleur Neuroathique en 4 (DN4).
The findings suggest that altered cerebral phosphorus metabolite ratios may serve as a biomarker of DPN, said the study’s investigators.
“Normally the ATP:Cr ratio will be unaltered, but there’s stress to the brain that might change,” Gordon Sloan, a clinical research fellow within the Diabetes Research Unit at the Royal Hallamshire Hospital in Sheffield (England) said at the virtual annual meeting of the European Association for the Study of Diabetes.
DPN affects around a quarter of patients with type 2 diabetes but treatments are “inadequate”, and “unfortunately fewer than a third of individuals receive 50% or greater pain relief from current neuropathic pain treatments,” Mr. Sloan said. “Ultimately, this lack of understanding of the pathophysiology of the condition is therefore clear rationale to investigate the disease mechanisms further and to find novel targets for treatments,” he added.
Brain metabolites offer clues to neuropathic pain levels
The thalamus and primary somatosensory cortex are two key areas of the brain that are involved in the perception of painful stimuli, Mr. Sloan explained. “The thalamus receives most of the slowest sensory impulses from the peripheral nervous system modulating and processing them for relaying the signals to the rest of the pain matrix, including the somatosensory cortex where these sensations are interpreted and localized.”
Prior imaging work by Mr. Sloan’s group and others have shown that there are alterations in the functioning of both these brain areas in those with painful DPN versus healthy volunteers and those with type 2 diabetes but no DPN. So for their current study, Mr. Sloan and associates from Sheffield University and Sheffield Teaching Hospitals National Health Service Trust, used an advanced imaging method – phosphorus magnetic resonance spectroscopy (MRS) – to scan the thalamus and somatosensory cortex of 43 persons with type 2 diabetes and 12 healthy volunteers. Of those with diabetes, 11 had no DPN, 12 had DPN but were not currently in pain, and 20 had painful DPN.
From the scans, three phosphorus metabolite ratios were calculated, which gave an indication of mitochondrial activity: first, the ATP to PCr ratio, which gives a measure of cellular energy status; second, the ATP to inorganic phosphate (Pi) ratio, which measures oxidative phosphorylation; and third, the ratio of phosphomonoesters (PME) to phosphodiesters (PDE), which gives a measure of cell membrane turnover.
“We have measured the ratio of high-energy phosphate levels which are an indirect representation of the balance between energy generation, reserve and usage in the brain,” Mr. Sloan said.
The subjects studied were of a similar age, around 63 years on average, and well matched in terms of their sex and body mass index. Those with diabetes of course had higher blood glucose and glycated hemoglobin than did the healthy volunteers during the scans. Among those with diabetes, those with DPN were significantly more likely to have a longer duration of diabetes (12.5 years for painful DPN and 15.8 years for nonpainful DPN) than were those with no DPN (8.7 years).
Furthermore, those with DPN had higher scores on the Neuropathic Pain Symptom Inventory (NPSI) than did those without, although there was not much difference between those with painful or nonpainful DPN. On the other had, those with painful DPN were more likely to have higher scores when using the Doleur Neuroathique en 4 (DN4) to assess their pain level.
Results showed significant changes in cerebral cellular bioenergetics in the pain processing regions of the brain in those with painful DPN. The ATP:PCr at the thalamus and at the somatosensory cortex was significantly higher in those with painful DPN, compared with healthy volunteers. The other measures of phosphorus metabolite levels (ATP:Pi and PME:PDE) were unaltered.
“We hypothesize that the findings of the study are suggestive of increased energy demands in regions of pain perception due to increased neuronal activity” said Dr. Sloan.
The study’s results add further evidence for cerebral alterations playing a key role in the generation and maintenance of pain in painful DPN.
SOURCE: Sloan S et al. EASD 2020, oral presentation 181.
Abnormal mitochondrial activity in pain-processing areas of the brain may explain why some persons with type 2 diabetes experience painful peripheral neuropathy while others do not, new U.K. study findings have suggested.
A greater ratio of adenosine triphosphate (ATP) – “the cellular energy currency of all life” – to phosphocreatine (PCr) was observed in the somatosensory cortex and right thalamus in those with painful diabetic peripheral neuropathy (DPN). Importantly, this correlated with neuropathic pain symptom intensity as measured by the Neuropathic Pain Symptom Inventory (NPSI) and the Doleur Neuroathique en 4 (DN4).
The findings suggest that altered cerebral phosphorus metabolite ratios may serve as a biomarker of DPN, said the study’s investigators.
“Normally the ATP:Cr ratio will be unaltered, but there’s stress to the brain that might change,” Gordon Sloan, a clinical research fellow within the Diabetes Research Unit at the Royal Hallamshire Hospital in Sheffield (England) said at the virtual annual meeting of the European Association for the Study of Diabetes.
DPN affects around a quarter of patients with type 2 diabetes but treatments are “inadequate”, and “unfortunately fewer than a third of individuals receive 50% or greater pain relief from current neuropathic pain treatments,” Mr. Sloan said. “Ultimately, this lack of understanding of the pathophysiology of the condition is therefore clear rationale to investigate the disease mechanisms further and to find novel targets for treatments,” he added.
Brain metabolites offer clues to neuropathic pain levels
The thalamus and primary somatosensory cortex are two key areas of the brain that are involved in the perception of painful stimuli, Mr. Sloan explained. “The thalamus receives most of the slowest sensory impulses from the peripheral nervous system modulating and processing them for relaying the signals to the rest of the pain matrix, including the somatosensory cortex where these sensations are interpreted and localized.”
Prior imaging work by Mr. Sloan’s group and others have shown that there are alterations in the functioning of both these brain areas in those with painful DPN versus healthy volunteers and those with type 2 diabetes but no DPN. So for their current study, Mr. Sloan and associates from Sheffield University and Sheffield Teaching Hospitals National Health Service Trust, used an advanced imaging method – phosphorus magnetic resonance spectroscopy (MRS) – to scan the thalamus and somatosensory cortex of 43 persons with type 2 diabetes and 12 healthy volunteers. Of those with diabetes, 11 had no DPN, 12 had DPN but were not currently in pain, and 20 had painful DPN.
From the scans, three phosphorus metabolite ratios were calculated, which gave an indication of mitochondrial activity: first, the ATP to PCr ratio, which gives a measure of cellular energy status; second, the ATP to inorganic phosphate (Pi) ratio, which measures oxidative phosphorylation; and third, the ratio of phosphomonoesters (PME) to phosphodiesters (PDE), which gives a measure of cell membrane turnover.
“We have measured the ratio of high-energy phosphate levels which are an indirect representation of the balance between energy generation, reserve and usage in the brain,” Mr. Sloan said.
The subjects studied were of a similar age, around 63 years on average, and well matched in terms of their sex and body mass index. Those with diabetes of course had higher blood glucose and glycated hemoglobin than did the healthy volunteers during the scans. Among those with diabetes, those with DPN were significantly more likely to have a longer duration of diabetes (12.5 years for painful DPN and 15.8 years for nonpainful DPN) than were those with no DPN (8.7 years).
Furthermore, those with DPN had higher scores on the Neuropathic Pain Symptom Inventory (NPSI) than did those without, although there was not much difference between those with painful or nonpainful DPN. On the other had, those with painful DPN were more likely to have higher scores when using the Doleur Neuroathique en 4 (DN4) to assess their pain level.
Results showed significant changes in cerebral cellular bioenergetics in the pain processing regions of the brain in those with painful DPN. The ATP:PCr at the thalamus and at the somatosensory cortex was significantly higher in those with painful DPN, compared with healthy volunteers. The other measures of phosphorus metabolite levels (ATP:Pi and PME:PDE) were unaltered.
“We hypothesize that the findings of the study are suggestive of increased energy demands in regions of pain perception due to increased neuronal activity” said Dr. Sloan.
The study’s results add further evidence for cerebral alterations playing a key role in the generation and maintenance of pain in painful DPN.
SOURCE: Sloan S et al. EASD 2020, oral presentation 181.
FROM EASD 2020
FDA issues new NSAIDs warning for second half of pregnancy
The U.S. Food and Drug Administration released new warnings Oct. 15 that most nonsteroidal anti-inflammatory agents (NSAIDs) carry an elevated risk for kidney complications in unborn children when taken around weeks 20 or later in pregnancy.
Citing newly available research, the agency states the risk of low amniotic fluid (known as oligohydramnios) can occur, which in turn can cause rare but serious kidney problems in the offspring. Pregnancy complications also can result.
The FDA action expands on earlier warnings about agents in this drug class, which the FDA previously cautioned about taking after week 30 of pregnancy because of heart-related risks.
Manufacturers of both over-the-counter and prescription NSAIDs – including ibuprofen, naproxen, diclofenac, and celecoxib – will be required to update their labeling with the new warning.
Low-dose (81-mg) aspirin is excluded from this warning.
“Low-dose aspirin may be an important treatment for some women during pregnancy and should be taken under the direction of a healthcare professional,” the agency stated in a news release.
“It is important that women understand the benefits and risks of the medications they may take over the course of their pregnancy,” Patrizia Cavazzoni, MD, acting director of FDA’s Center for Drug Evaluation and Research, states in the release. “To this end, the agency is using its regulatory authority to inform women and their healthcare providers about the risks if NSAIDs are used after around 20 weeks of pregnancy and beyond.”
Oligohydramnios can arise quickly – in as little as 2 days – or weeks after starting regular NSAID use in this patient population. The condition usually resolves if a pregnant woman stops taking the NSAID, the agency notes.
If a health care provider believes NSAIDs are necessary between about 20 and 30 weeks of pregnancy, use should be limited to the lowest effective dose and shortest duration possible, the Drug Safety Communication notes.
As a reminder, health care professionals and patients should report side effects from NSAIDs to the FDA’s MedWatch program.
A version of this article originally appeared on Medscape.com.
The U.S. Food and Drug Administration released new warnings Oct. 15 that most nonsteroidal anti-inflammatory agents (NSAIDs) carry an elevated risk for kidney complications in unborn children when taken around weeks 20 or later in pregnancy.
Citing newly available research, the agency states the risk of low amniotic fluid (known as oligohydramnios) can occur, which in turn can cause rare but serious kidney problems in the offspring. Pregnancy complications also can result.
The FDA action expands on earlier warnings about agents in this drug class, which the FDA previously cautioned about taking after week 30 of pregnancy because of heart-related risks.
Manufacturers of both over-the-counter and prescription NSAIDs – including ibuprofen, naproxen, diclofenac, and celecoxib – will be required to update their labeling with the new warning.
Low-dose (81-mg) aspirin is excluded from this warning.
“Low-dose aspirin may be an important treatment for some women during pregnancy and should be taken under the direction of a healthcare professional,” the agency stated in a news release.
“It is important that women understand the benefits and risks of the medications they may take over the course of their pregnancy,” Patrizia Cavazzoni, MD, acting director of FDA’s Center for Drug Evaluation and Research, states in the release. “To this end, the agency is using its regulatory authority to inform women and their healthcare providers about the risks if NSAIDs are used after around 20 weeks of pregnancy and beyond.”
Oligohydramnios can arise quickly – in as little as 2 days – or weeks after starting regular NSAID use in this patient population. The condition usually resolves if a pregnant woman stops taking the NSAID, the agency notes.
If a health care provider believes NSAIDs are necessary between about 20 and 30 weeks of pregnancy, use should be limited to the lowest effective dose and shortest duration possible, the Drug Safety Communication notes.
As a reminder, health care professionals and patients should report side effects from NSAIDs to the FDA’s MedWatch program.
A version of this article originally appeared on Medscape.com.
The U.S. Food and Drug Administration released new warnings Oct. 15 that most nonsteroidal anti-inflammatory agents (NSAIDs) carry an elevated risk for kidney complications in unborn children when taken around weeks 20 or later in pregnancy.
Citing newly available research, the agency states the risk of low amniotic fluid (known as oligohydramnios) can occur, which in turn can cause rare but serious kidney problems in the offspring. Pregnancy complications also can result.
The FDA action expands on earlier warnings about agents in this drug class, which the FDA previously cautioned about taking after week 30 of pregnancy because of heart-related risks.
Manufacturers of both over-the-counter and prescription NSAIDs – including ibuprofen, naproxen, diclofenac, and celecoxib – will be required to update their labeling with the new warning.
Low-dose (81-mg) aspirin is excluded from this warning.
“Low-dose aspirin may be an important treatment for some women during pregnancy and should be taken under the direction of a healthcare professional,” the agency stated in a news release.
“It is important that women understand the benefits and risks of the medications they may take over the course of their pregnancy,” Patrizia Cavazzoni, MD, acting director of FDA’s Center for Drug Evaluation and Research, states in the release. “To this end, the agency is using its regulatory authority to inform women and their healthcare providers about the risks if NSAIDs are used after around 20 weeks of pregnancy and beyond.”
Oligohydramnios can arise quickly – in as little as 2 days – or weeks after starting regular NSAID use in this patient population. The condition usually resolves if a pregnant woman stops taking the NSAID, the agency notes.
If a health care provider believes NSAIDs are necessary between about 20 and 30 weeks of pregnancy, use should be limited to the lowest effective dose and shortest duration possible, the Drug Safety Communication notes.
As a reminder, health care professionals and patients should report side effects from NSAIDs to the FDA’s MedWatch program.
A version of this article originally appeared on Medscape.com.
Low back pain in youth: Recognizing red flags
Low back pain in not uncommon in children and adolescents.1-3 Although the prevalence of low back pain in children < 7 years is low, it increases with age, with studies reporting lifetime prevalence at age 12 years between 16% and 18% and rates as high as 66% by 16 years of age.4,5 Although children and adolescents usually have pain that is transient and benign without a defined cause, structural causes of low back pain should be considered in school-aged children with pain that persists for > 3 to 6 weeks. 4 The most common structural causes of adolescent low back pain are reviewed here.
Etiology: A mixed bag
Back pain in school-aged children is most commonly due to muscular strain, overuse, or poor posture. The pain is often transient in nature and responds to rest and postural education.4,6 A herniated disc is an uncommon finding in younger school-aged children, but incidence increases slightly among older adolescents, particularly those who are active in collision sports and/or weight-lifting.7,8 Pain caused by a herniated disc often radiates along the distribution of the sciatic nerve and worsens during lumbar flexion.
Spondylolysis and spondylolisthesis are important causes of back pain in children. Spondylolysis is defined as a defect or abnormality of the pars interarticularis and surrounding lamina and pedicle. Spondylolisthesis, which is less common, is defined as the translation or “slippage” of one vertebral segment in relation to the next caudal segment. These conditions commonly occur as a result of repetitive stress.
In a prospective study of adolescents < 19 years with low back pain for > 2 weeks, the prevalence of spondylolysis was 39.7%.9 Adolescent athletes with symptomatic low back pain are more likely to have spondylolysis than nonathletes (32% vs 2%, respectively).2,10 Pain is often made worse by extension of the spine. Spondylolysis and spondylolisthesis can be congenital or acquired, and both can be asymptomatic. Children and teens who are athletes are at higher risk for symptomatic spondylolysis and spondylolisthesis.10-12 This is especially true for those involved in gymnastics, dance, football, and/or volleyball, where a repetitive load is placed onto an extended spine.
Idiopathic scoliosis is an abnormal lateral curvature of the spine that usually develops during adolescence and worsens with growth. Historically, painful scoliosis was considered rare, but more recently researchers determined that children with scoliosis have a higher rate of pain compared to their peers.13,14 School-aged children with scoliosis were found to be at 2 times the risk of low back pain compared to those without scoliosis.13 It is important to identify scoliosis in adolescents so that progression can be monitored.
Screening for scoliosis in primary care is somewhat controversial. The US Preventive Services Task Force (USPSTF) finds insufficient evidence for screening asymptomatic adolescents for scoliosis.15 This recommendation is based on the fact that there is little evidence on the effect of screening on long-term outcomes. Screening may also lead to unnecessary radiation. Conversely, a position statement released by the Scoliosis Research Society, the Pediatric Orthopedic Society of North America, the American Association of Orthopedic Surgeons, and the American Academy of Pediatrics recommends scoliosis screening during routine pediatric office visits.16 Screening for girls is recommended at ages 10 and 12 years, and for boys, once between ages 13 and 14 years. The statement highlights evidence showing that focused screening by appropriate personnel has value in detecting a clinically significant curve (> 20°).
Scheuermann disease is a rare cause of back pain in children that usually develops during adolescence and results in increasing thoracic kyphosis. An autosomal dominant mutation plays a role in this disease of the growth cartilage endplate; repetitive strain on the growth cartilage is also a contributing factor.17,18 An atypical variant manifests with kyphosis in the thoracolumbar region.17
Continue to: Other causes of low back pain
Other causes of low back pain—including inflammatory arthritis, infection (eg, discitis), and tumor—are rare in children but must always be considered, especially in the setting of persistent symptoms.4,19-21 More on the features of these conditions is listed in TABLE 1.1-7,13-15,17-30

History: Focus on onset, timing, and duration of symptoms
As with adults, obtaining a history that includes the onset, timing, and duration of symptoms is key in the evaluation of low back pain in children, as is obtaining a history of the patient’s activities; sports that repetitively load the lumbar spine in an extended position increase the risk of injury.10

Specific risk factors for low back pain in children and adolescents are poorly understood.4,9,31 Pain can be associated with trauma, or it can have a more progressive or insidious onset. Generally, pain that is present for up to 6 weeks and is intermittent or improving has a self-limited course. Pain that persists beyond 3 to 6 weeks or is worsening is more likely to have an anatomical cause that needs further evaluation.2,3,10,21
Identifying exacerbating and alleviating factors can provide useful information. Pain that is worse with lumbar flexion is more likely to come from muscular strain or disc pathology. Pain with extension is more likely due to a structural cause such as spondylolysis/spondylolisthesis, scoliosis, or Scheuermann disease.2,4,10,17,18,21 See TABLE 2 for red flag symptoms that indicate the need for imaging and further work-up.
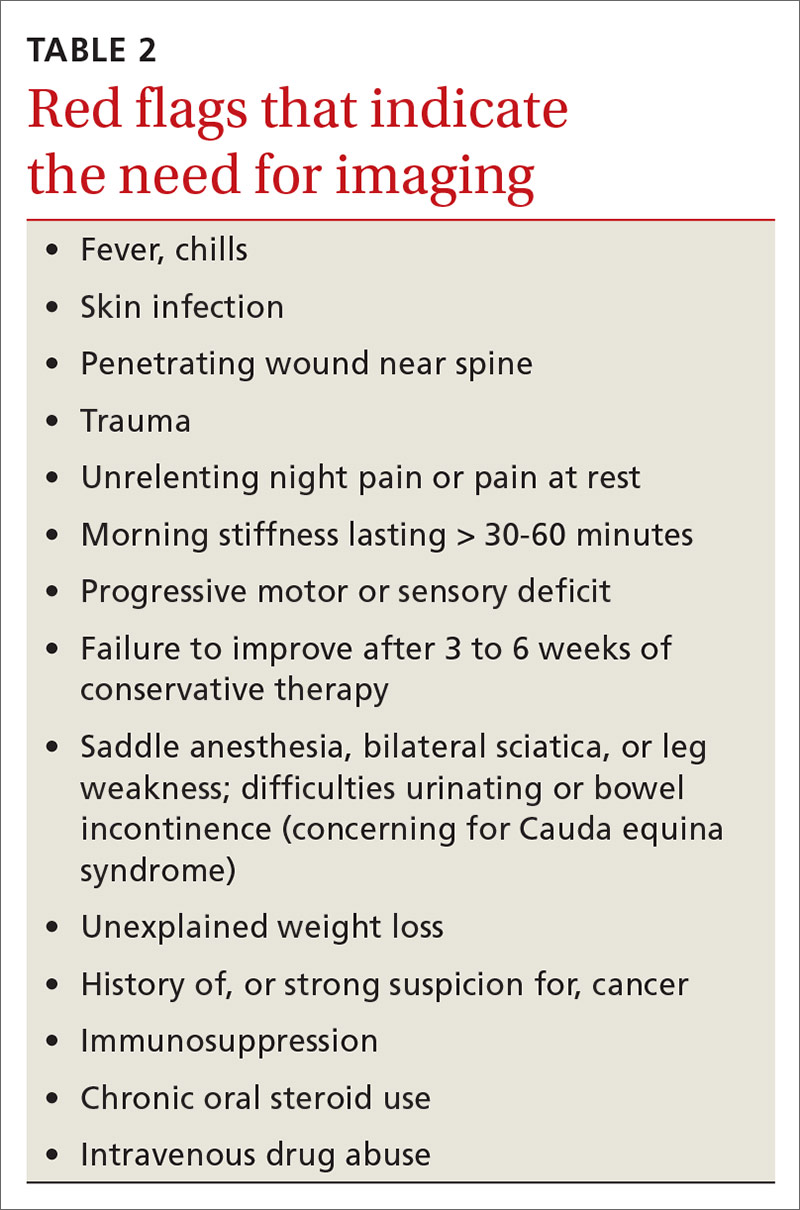
The physical exam: Visualize, assess range of motion, and reproduce pain
The physical examination of any patient with low back pain should include direct visualization and inspection of the back, spine, and pelvis; palpation of the spine and paraspinal regions; assessment of lumbar range of motion and of the lumbar nerve roots, including tests of sensation, strength, and deep tendon reflexes; and an evaluation of the patient’s posture, which can provide clues to underlying causes of pain.
Continue to: Increased thoracic kyphosis...
Increased thoracic kyphosis that is not reversible is concerning for Scheuermann disease.9,17,18 A significant elevation in one shoulder or side of the pelvis can be indicative of scoliosis. Increased lumbar lordosis may predispose a patient to spondylolysis.
In patients with spondylolysis, lumbar extension will usually reproduce pain, which is often unilateral. Hyperextension in a single-leg stance, commonly known as the Stork test, is positive for unilateral spondylolysis when it reproduces pain on the ipsilateral side. The sensitivity of the Stork test for unilateral spondylolysis is approximately 50%.32 (For more information on the Stork test, see www.physio-pedia.com/Stork_test.)
Pain reproduced with lumbar flexion is less concerning for bony pathology and is most often related to soft-tissue strain. Lumbar flexion with concomitant radicular pain is associated with disc pathology.8 Pain with a straight-leg raise is also associated with disk pathology, especially if raising the contralateral leg increases pain.8
Using a scoliometer. Evaluate the flexed spine for the presence of asymmetry, which can indicate scoliosis.33 If asymmetry is present, use a scoliometer to determine the degree of asymmetry. Zero to 5° is considered clinically insignificant; monitor and reevaluate these patients at subsequent visits.34,35 Ten degrees or more of asymmetry with a scoliometer should prompt you to order radiographs.35,36 A smartphone-based scoliometer for iPhones was evaluated in 1 study and was shown to have reasonable reliability and validity for clinical use.37
Deformity of the lower extremities. Because low back pain may be caused by biomechanical or structural deformity of the lower extremities, examine the flexibility of the hip flexors, gluteal musculature, hamstrings, and the iliotibial band.38 In addition, evaluate for leg-length discrepancy and lower-extremity malalignment, such as femoral anteversion, tibial torsion, or pes planus.
Continue to: Imaging
Imaging: Know when it’s needed
Although imaging of the lumbar spine is often unnecessary in the presence of acute low back pain in children, always consider imaging in the setting of bony tenderness, pain that wakes a patient from sleep, and in the setting of other red flag symptoms (see TABLE 2). Low back pain in children that is reproducible with lumbar extension is concerning for spondylolysis or spondylolisthesis. If the pain with extension persists beyond 3 to 6 weeks, order imaging starting with radiographs.2,39
Traditionally, 4 views of the spine—anteroposterior (AP), lateral, and oblique (one right and one left)—were obtained, but recent evidence indicates that 2 views (AP and lateral) have similar sensitivity and specificity to 4 views with significantly reduced radiation exposure.2,39 Because the sensitivity of plain films is relatively low, consider more advanced imaging if spondylolysis or spondylolisthesis is strongly suspected. Recent studies indicate that magnetic resonance imaging (MRI) may be as effective as computed tomography (CT) or bone scan and has the advantage of lower radiation (FIGURE 1).2,22
Similarly, order radiographs if there is > 10° of asymmetry noted on physical exam using a scoliometer.15,23 Calculate the Cobb angle to determine the severity of scoliosis. Refer patients with angles ≥ 20° to a pediatric orthopedist for monitoring of progression and consideration of bracing (FIGURE 2).23,34 For patients with curvatures between 10° and 19°, repeat imaging every 6 to 12 months. Because scoliosis is a risk factor for spondylolysis, evaluate radiographs in the setting of painful scoliosis for the presence of a spondylolysis.34,35
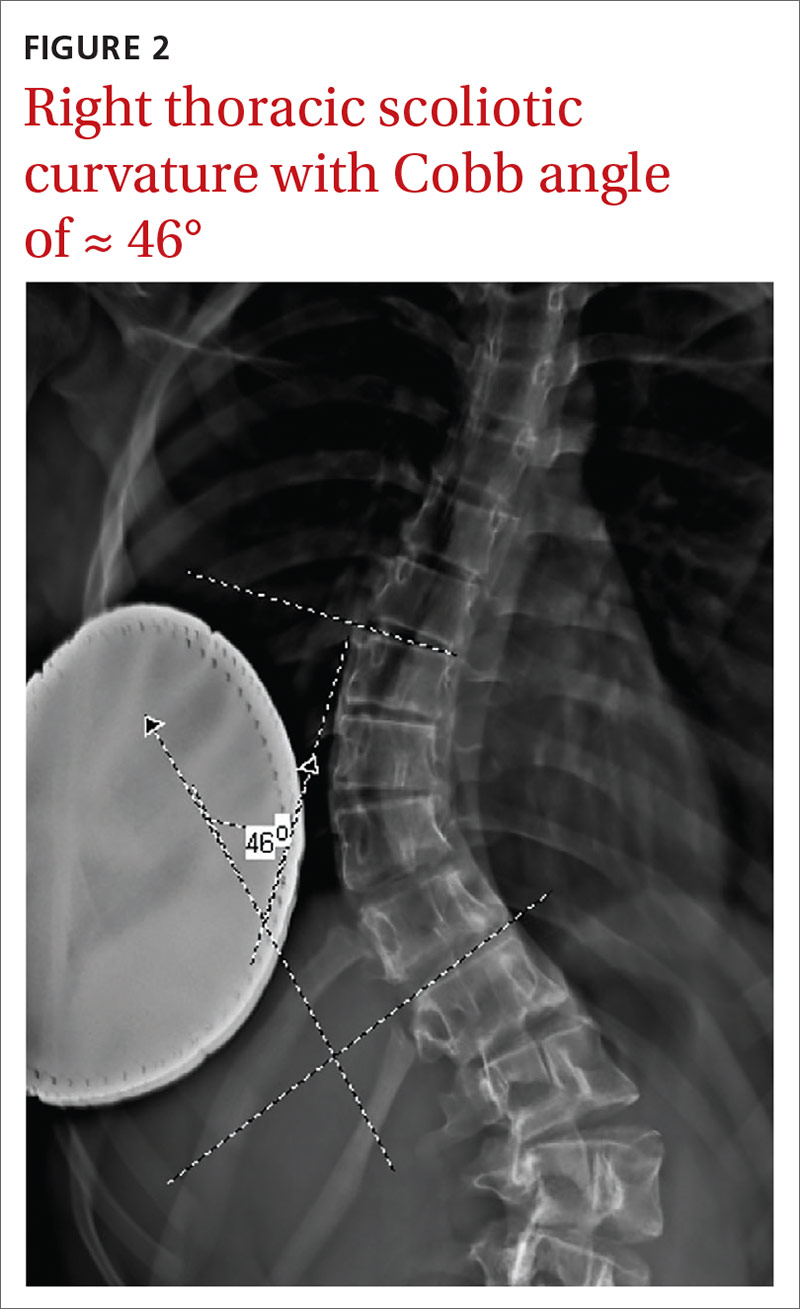
If excessive kyphosis is noted on exam, order radiographs to evaluate for Scheuermann disease. Classic imaging findings include Schmorl nodes, vertebral endplate changes, and anterior wedging (FIGURE 3).17,18
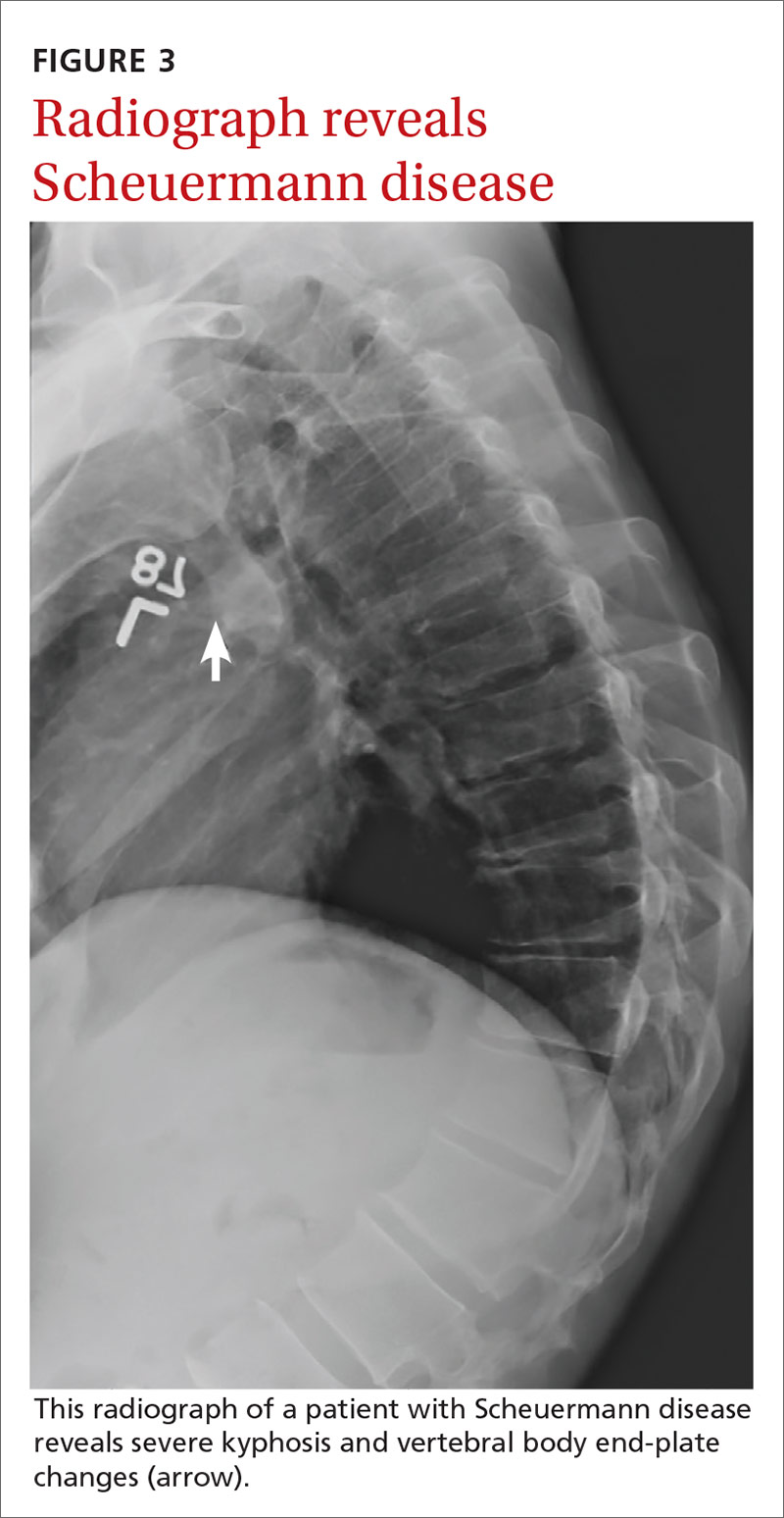
In the absence of the above concerns, defer imaging of the lumbar spine until after adequate rest and rehabilitation have been attempted.
Continue to: Treatment typically involves restor physical therapy
Treatment typically involves restor physical therapy
Most cases of low back pain in children and adolescents are benign and self-limited. Many children with low back pain can be treated with relative rest from the offending activity. For children with more persistent pain, physical therapy (PT) is often indicated. Similar to that for adults, there is little evidence for specific PT programs to help children with low back pain. Rehabilitation should be individualized based on the condition being treated.
Medications. There have been no high-quality studies on the benefit of medications to treat low back pain in children. Studies have shown nonsteroidal anti-inflammatory drugs (NSAIDs) have value in adults, and they are likely safe for use in children,40 but the risk of opiate abuse is significantly increased in adolescents who have been prescribed opiate pain medication prior to 12th grade.41
Lumbar disc herniation. Although still relatively rare, lumbar disc herniation is more common in older children and adolescents than in younger children and is treated similarly to that in adults.8 Range-of-motion exercise to restore lumbar motion is often first-line treatment. Research has shown that exercises that strengthen the abdominal or “core” musculature help prevent the return of low back pain.24,25
In the case of spondylolysis or spondylolisthesis, rest from activity is generally required for a minimum of 4 to 6 weeks. Rehabilitation in the form of range of motion, especially into the lumbar extension, and spinal stabilization exercises are effective for both reducing pain and restoring range-of-motion and strength.42 Have patients avoid heavy backpacks, which can reproduce pain. Children often benefit from leaving a second set of schoolbooks at home. For most patients with spondylolysis, conservative treatment with rehabilitation is equal to or better than surgical intervention in returning the patient to his/her pre-injury activity level.26,43,44 When returning athletes to their sport, aggressive PT, defined as rest for < 10 weeks prior to initiating PT, is superior to delaying PT beyond 10 weeks of rest.27
Idiopathic scoliosis. Much of the literature on the treatment of scoliosis is focused on limiting progression of the scoliotic curvature. Researchers thought that more severe curves were associated with more severe pain, but a recent systematic review showed that back pain can occur in patients with even small curvatures.28 Treatment for patients with smaller degrees of curvature is similar to that for mechanical low back pain. PT may have a role in the treatment of scoliosis, but there is little evidence in the literature of its effectiveness.
Continue to: A Cochrane review showed...
A Cochrane review showed that PT and exercise-based treatments had no effect on back pain or disability in patients with scoliosis.29 And outpatient PT alone, in the absence of bracing, does not arrest progression of the scoliotic curvature.35 One trial did demonstrate that an intensive inpatient treatment program of 4 to 6 weeks for patients with curvature of at least 40° reduced progression of curvature compared to an untreated control group at 1 year.34 The outcomes of functional mobility and pain were not measured. Follow-up data on curve progression beyond 1 year are not available. Unfortunately, intensive inpatient treatment is not readily available or cost-effective for most patients with scoliosis.
Scheuermann disease. The mainstay of treatment for mild Scheuermann disease is advising the patient to avoid repetitive loading of the spine. Patients should avoid sports such as competitive weight-lifting, gymnastics, and football. Lower impact athletics are encouraged. Refer patients with pain to PT to address posture and core stabilization. Patients with severe kyphosis may require surgery.17,18
Bracing: Rarely helpful for low back pain
The use of lumbar braces or corsets is rarely helpful for low back pain in children. Bracing in the setting of spondylolysis is controversial.One study indicated that bracing in combination with activity restriction and lumbar extension exercise is superior to activity restriction and lumbar flexion exercises alone.43 But a meta-analysis did not demonstrate a significant difference in recovery when bracing was added.44 Bracing may help to reduce pain initially in patients with spondylolysis who have pain at rest. Bracing is not recommended for patients with pain that abates with activity modification.
Scoliosis and Scheuermann kyphosis. Treatment of adolescent idiopathic scoliosis usually consists of observation and periodic reevaluation. Bracing is a mainstay of the nonsurgical management of scoliosis and is appropriate for curves of 20° to 40°; studies have reported successful control of curve progression in > 70% of patients.36 According to 1 study, the number of cases of scoliosis needed to treat with bracing to prevent 1 surgery is 3.30 Surgery is often indicated for patients with curvatures > 40°, although this is also debated.33
Bracing is used rarely for Scheuermann kyphosis but may be helpful in more severe or painful cases.17
CORRESPONDENCE
Shawn F. Phillips, MD, MSPT, 500 University Drive H154, Hershey, PA, 17033; [email protected].
1. MacDonald J, Stuart E, Rodenberg R. Musculoskeletal low back pain in school-aged children: a review. JAMA Pediatr. 2017;171:280-287.
2. Tofte JN CarlLee TL, Holte AJ, et al. Imaging pediatric spondylolysis: a systematic review. Spine. 2017;42:777-782.
3. Sakai T, Sairyo K, Suzue N, et al. Incidence and etiology of lumbar spondylolysis: review of the literature. J Orthop Sci. 2010;15:281-288.
4. Calvo-Muñoz I, Gómez-Conesa A, Sánchez-Meca J. Prevalence of low back pain in children and adolescents: a meta-analysis. BMC Pediatrics. 2013;13:14.
5. Bernstein RM, Cozen H. Evaluation of back pain in children and adolescents. Am Fam Physician. 2007;76:1669-1676.
6. Taxter AJ, Chauvin NA, Weiss PF. Diagnosis and treatment of low back pain in the pediatric population. Phys Sportsmed. 2014;42:94-104.
7. Haus BM, Micheli LJ. Back pain in the pediatric and adolescent athlete. Clin Sports Med. 2012;31:423-440.
8. Lavelle WF, Bianco A, Mason R, et al. Pediatric disk herniation. J Am Acad Orthop Surg. 2011;19:649-656.
9. Taimela S, Kujala UM, Salminen JJ, et al. The prevalence of low back pain among children and adolescents: a nationwide, cohort-based questionnaire survey in Finland. Spine. 1997;22:1132-1136.
10. Schroeder GD, LaBella CR, Mendoza M, et al. The role of intense athletic activity on structural lumbar abnormalities in adolescent patients with symptomatic low back pain. Eur Spine J. 2016;25:2842-2848.
11. Waicus KM, Smith BW. Back injuries in the pediatric athlete. Curr Sports Med Rep. 2002;1:52-58.
12. Daniels JM, Pontius G, El-Amin S, et al. Evaluation of low back pain in athletes. Sports Health. 2011;3:336-345.
13. Sato T, Hirano T, Ito T, et al. Back pain in adolescents with idiopathic scoliosis: epidemiological study for 43,630 pupils in Niigata City, Japan. Eur Spine J. 2011;20:274-279.
14. Smorgick Y, Mirovsky Y, Baker KC, et al. Predictors of back pain in adolescent idiopathic scoliosis surgical candidates. J Pediatr Orthop. 2013;33:289-292.
15. US Preventive Services Task Force. Screening for Adolescent Idiopathic Scoliosis. US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:165-172.
16. Hresko MT, Talwalkar VR, Schwend RM. Position statement–Screening for the early detection of idiopathic scoliosis in adolescents. SRS/POSNA/AAOS/AAP Position Statement. 2015. www.srs.org/about-srs/news-and-announcements/position-statement---screening-for-the-early-detection-for-idiopathic-scoliosis-in-adolescents. Accessed September 30, 2020.
17. Palazzo C, Sailhan F, Revel M. Scheuermann’s disease: an update. Joint Bone Spine. 2014;81:209-214.
18. Ali RM, Green DW, Patel TC. Scheuermann’s kyphosis. Curr Opin Pediatr. 1999;11:70-75.
19. de Moraes Barros Fucs PM, Meves R, Yamada HH, et al. Spinal infections in children: a review. Int Orthop. 2012;36:387-395.
20. Joaquim AF, Ghizoni E, Valadares MG, et al. Spinal tumors in children. Revista da Associação Médica Brasileira. 2017;63:459-465.
21. Weiss PF, Colbert RA. Juvenile spondyloarthritis: a distinct form of juvenile arthritis. Pediatr Clin North Am. 2018;65:675-690.
22. Rush JK, Astur N, Scott S, et al. Use of magnetic resonance imaging in the evaluation of spondylolysis. J Pediatr Orthop. 2015;35:271-275.
23. Janicki JA, Alman B. Scoliosis: review of diagnosis and treatment. Pediatr Child Health. 2007;12:771-776.
24. O’Sullivan PB, Phyty GD, Twomey LT, et al. Evaluation of specific stabilizing exercise in the treatment of chronic low back pain with radiologic diagnosis of spondylolysis or spondylolisthesis. Spine.1997;22:2959-2967.
25. Inani SB, Selkar SP. Effect of core stabilization exercises versus conventional exercises on pain and functional status in patients with non-specific low back pain: a randomized clinical trial. J Back Musculoskelet Rehabil. 2013;26:37-43.
26. Garet M, Reiman MP, Mathers J, et al. Nonoperative treatment in lumbar spondylolysis and spondylolisthesis: a systematic review. Sports Health. 2013;5:225-232.
27. Selhorst M, Fischer A, Graft K, et al. Timing of physical therapy referral in adolescent athletes with acute spondylolysis: a retrospective chart review. Clin J Sport Med. 2017;27:296-301.
28. Théroux J, Stomski N, Hodgetts CJ, et al. Prevalence of low back pain in adolescents with idiopathic scoliosis: a systematic review. Chiropr Man Ther. 2017;25:10.
29. Romano M, Minozzi S, Zaina F, et al. Exercises for adolescent idiopathic scoliosis: a Cochrane systematic review. Spine (Phila Pa 1976). 2013;38:E883-E893.
30. Sanders JO, Newton PO, Browne RH, et al. Bracing for idiopathic scoliosis: how many patients require treatment to prevent one surgery? J Bone Joint Surg Am. 2014;96:649-653.
31. Hill JJ, Keating JL. Risk factors for the first episode of low back pain in children are infrequently validated across samples and conditions: a systematic review. J Physiother. 2010;56:237-244.
32. Grødahl LHJ, Fawcett L, Nazareth M, et al. Diagnostic utility of patient history and physical examination data to detect spondylolysis and spondylolisthesis in athletes with low back pain: a systematic review. Man Ther. 2016;24:7-17.
33. Asher MA, Burton DC. Adolescent idiopathic scoliosis: natural history and long term treatment effects. Scoliosis. 2006;1:2.
34. Weiss HR, Weiss G, Petermann F. Incidence of curvature progression in idiopathic scoliosis patients treated with scoliosis inpatient rehabilitation (SIR): an age- and sex-matched controlled study. Pediatr Rehabil. 2003;6:23-30.
35. Gomez JA, Hresko MT, Glotzbecker MP. Nonsurgical management of adolescent idiopathic scoliosis. J Am Acad Orthop Surg. 2016;24:555-564.
36. Weinstein SL, Dolan LA, Wright JG, et al. Effects of bracing in adolescents with idiopathic scoliosis. N Engl J Med. 2013;369:1512-1521.
37. Balg F, Juteau M, Theoret C, et al. Validity and reliability of the iPhone to measure rib hump in scoliosis. J Pediatr Orthop. 2014;34:774-779.
38. Auerbach JD, Ahn J, Zgonis MH, et al. Streamlining the evaluation of low back pain in children. Clin Orthop Relatl Res. 2008;466:1971-1977.
39. Beck NA, Miller R, Baldwin K, et al. Do oblique views add value in the diagnosis of spondylolysis in adolescents? J Bone Joint Surg Am. 2013;95:e65.
40. Roelofs PD, Deyo RA, Koes BW, et al. Nonsteroidal anti-inflammatory drugs for low back pain: an updated Cochrane review. Spine (Phila Pa 1976). 2008;33:1766-1774.
41. Miech R, Johnston L, O’Malley PM, et al. Prescription opioids in adolescence and future opioid misuse. Pediatrics. 2015;136:e1169-e1177.
42. Hu S, Tribus C, Diab M, et al. Spondylolysis and spondylolisthesis. J Bone Joint Surg. 2008;90:655-671.
43. Panteliadis P, Nagra NS, Edwards KL, et al. Athletic population with spondylolysis: review of outcomes following surgical repair or conservative management. Global Spine J. 2016;6:615-625.
44. Klein G, Mehlman CT, McCarty M. Nonoperative treatment of spondylolysis and grade I spondylolisthesis in children and young adults: a meta-analysis of observational studies. J Pediatr Orthop. 2009;29:146-156.
Low back pain in not uncommon in children and adolescents.1-3 Although the prevalence of low back pain in children < 7 years is low, it increases with age, with studies reporting lifetime prevalence at age 12 years between 16% and 18% and rates as high as 66% by 16 years of age.4,5 Although children and adolescents usually have pain that is transient and benign without a defined cause, structural causes of low back pain should be considered in school-aged children with pain that persists for > 3 to 6 weeks. 4 The most common structural causes of adolescent low back pain are reviewed here.
Etiology: A mixed bag
Back pain in school-aged children is most commonly due to muscular strain, overuse, or poor posture. The pain is often transient in nature and responds to rest and postural education.4,6 A herniated disc is an uncommon finding in younger school-aged children, but incidence increases slightly among older adolescents, particularly those who are active in collision sports and/or weight-lifting.7,8 Pain caused by a herniated disc often radiates along the distribution of the sciatic nerve and worsens during lumbar flexion.
Spondylolysis and spondylolisthesis are important causes of back pain in children. Spondylolysis is defined as a defect or abnormality of the pars interarticularis and surrounding lamina and pedicle. Spondylolisthesis, which is less common, is defined as the translation or “slippage” of one vertebral segment in relation to the next caudal segment. These conditions commonly occur as a result of repetitive stress.
In a prospective study of adolescents < 19 years with low back pain for > 2 weeks, the prevalence of spondylolysis was 39.7%.9 Adolescent athletes with symptomatic low back pain are more likely to have spondylolysis than nonathletes (32% vs 2%, respectively).2,10 Pain is often made worse by extension of the spine. Spondylolysis and spondylolisthesis can be congenital or acquired, and both can be asymptomatic. Children and teens who are athletes are at higher risk for symptomatic spondylolysis and spondylolisthesis.10-12 This is especially true for those involved in gymnastics, dance, football, and/or volleyball, where a repetitive load is placed onto an extended spine.
Idiopathic scoliosis is an abnormal lateral curvature of the spine that usually develops during adolescence and worsens with growth. Historically, painful scoliosis was considered rare, but more recently researchers determined that children with scoliosis have a higher rate of pain compared to their peers.13,14 School-aged children with scoliosis were found to be at 2 times the risk of low back pain compared to those without scoliosis.13 It is important to identify scoliosis in adolescents so that progression can be monitored.
Screening for scoliosis in primary care is somewhat controversial. The US Preventive Services Task Force (USPSTF) finds insufficient evidence for screening asymptomatic adolescents for scoliosis.15 This recommendation is based on the fact that there is little evidence on the effect of screening on long-term outcomes. Screening may also lead to unnecessary radiation. Conversely, a position statement released by the Scoliosis Research Society, the Pediatric Orthopedic Society of North America, the American Association of Orthopedic Surgeons, and the American Academy of Pediatrics recommends scoliosis screening during routine pediatric office visits.16 Screening for girls is recommended at ages 10 and 12 years, and for boys, once between ages 13 and 14 years. The statement highlights evidence showing that focused screening by appropriate personnel has value in detecting a clinically significant curve (> 20°).
Scheuermann disease is a rare cause of back pain in children that usually develops during adolescence and results in increasing thoracic kyphosis. An autosomal dominant mutation plays a role in this disease of the growth cartilage endplate; repetitive strain on the growth cartilage is also a contributing factor.17,18 An atypical variant manifests with kyphosis in the thoracolumbar region.17
Continue to: Other causes of low back pain
Other causes of low back pain—including inflammatory arthritis, infection (eg, discitis), and tumor—are rare in children but must always be considered, especially in the setting of persistent symptoms.4,19-21 More on the features of these conditions is listed in TABLE 1.1-7,13-15,17-30

History: Focus on onset, timing, and duration of symptoms
As with adults, obtaining a history that includes the onset, timing, and duration of symptoms is key in the evaluation of low back pain in children, as is obtaining a history of the patient’s activities; sports that repetitively load the lumbar spine in an extended position increase the risk of injury.10

Specific risk factors for low back pain in children and adolescents are poorly understood.4,9,31 Pain can be associated with trauma, or it can have a more progressive or insidious onset. Generally, pain that is present for up to 6 weeks and is intermittent or improving has a self-limited course. Pain that persists beyond 3 to 6 weeks or is worsening is more likely to have an anatomical cause that needs further evaluation.2,3,10,21
Identifying exacerbating and alleviating factors can provide useful information. Pain that is worse with lumbar flexion is more likely to come from muscular strain or disc pathology. Pain with extension is more likely due to a structural cause such as spondylolysis/spondylolisthesis, scoliosis, or Scheuermann disease.2,4,10,17,18,21 See TABLE 2 for red flag symptoms that indicate the need for imaging and further work-up.

The physical exam: Visualize, assess range of motion, and reproduce pain
The physical examination of any patient with low back pain should include direct visualization and inspection of the back, spine, and pelvis; palpation of the spine and paraspinal regions; assessment of lumbar range of motion and of the lumbar nerve roots, including tests of sensation, strength, and deep tendon reflexes; and an evaluation of the patient’s posture, which can provide clues to underlying causes of pain.
Continue to: Increased thoracic kyphosis...
Increased thoracic kyphosis that is not reversible is concerning for Scheuermann disease.9,17,18 A significant elevation in one shoulder or side of the pelvis can be indicative of scoliosis. Increased lumbar lordosis may predispose a patient to spondylolysis.
In patients with spondylolysis, lumbar extension will usually reproduce pain, which is often unilateral. Hyperextension in a single-leg stance, commonly known as the Stork test, is positive for unilateral spondylolysis when it reproduces pain on the ipsilateral side. The sensitivity of the Stork test for unilateral spondylolysis is approximately 50%.32 (For more information on the Stork test, see www.physio-pedia.com/Stork_test.)
Pain reproduced with lumbar flexion is less concerning for bony pathology and is most often related to soft-tissue strain. Lumbar flexion with concomitant radicular pain is associated with disc pathology.8 Pain with a straight-leg raise is also associated with disk pathology, especially if raising the contralateral leg increases pain.8
Using a scoliometer. Evaluate the flexed spine for the presence of asymmetry, which can indicate scoliosis.33 If asymmetry is present, use a scoliometer to determine the degree of asymmetry. Zero to 5° is considered clinically insignificant; monitor and reevaluate these patients at subsequent visits.34,35 Ten degrees or more of asymmetry with a scoliometer should prompt you to order radiographs.35,36 A smartphone-based scoliometer for iPhones was evaluated in 1 study and was shown to have reasonable reliability and validity for clinical use.37
Deformity of the lower extremities. Because low back pain may be caused by biomechanical or structural deformity of the lower extremities, examine the flexibility of the hip flexors, gluteal musculature, hamstrings, and the iliotibial band.38 In addition, evaluate for leg-length discrepancy and lower-extremity malalignment, such as femoral anteversion, tibial torsion, or pes planus.
Continue to: Imaging
Imaging: Know when it’s needed
Although imaging of the lumbar spine is often unnecessary in the presence of acute low back pain in children, always consider imaging in the setting of bony tenderness, pain that wakes a patient from sleep, and in the setting of other red flag symptoms (see TABLE 2). Low back pain in children that is reproducible with lumbar extension is concerning for spondylolysis or spondylolisthesis. If the pain with extension persists beyond 3 to 6 weeks, order imaging starting with radiographs.2,39
Traditionally, 4 views of the spine—anteroposterior (AP), lateral, and oblique (one right and one left)—were obtained, but recent evidence indicates that 2 views (AP and lateral) have similar sensitivity and specificity to 4 views with significantly reduced radiation exposure.2,39 Because the sensitivity of plain films is relatively low, consider more advanced imaging if spondylolysis or spondylolisthesis is strongly suspected. Recent studies indicate that magnetic resonance imaging (MRI) may be as effective as computed tomography (CT) or bone scan and has the advantage of lower radiation (FIGURE 1).2,22

Similarly, order radiographs if there is > 10° of asymmetry noted on physical exam using a scoliometer.15,23 Calculate the Cobb angle to determine the severity of scoliosis. Refer patients with angles ≥ 20° to a pediatric orthopedist for monitoring of progression and consideration of bracing (FIGURE 2).23,34 For patients with curvatures between 10° and 19°, repeat imaging every 6 to 12 months. Because scoliosis is a risk factor for spondylolysis, evaluate radiographs in the setting of painful scoliosis for the presence of a spondylolysis.34,35

If excessive kyphosis is noted on exam, order radiographs to evaluate for Scheuermann disease. Classic imaging findings include Schmorl nodes, vertebral endplate changes, and anterior wedging (FIGURE 3).17,18

In the absence of the above concerns, defer imaging of the lumbar spine until after adequate rest and rehabilitation have been attempted.
Continue to: Treatment typically involves restor physical therapy
Treatment typically involves restor physical therapy
Most cases of low back pain in children and adolescents are benign and self-limited. Many children with low back pain can be treated with relative rest from the offending activity. For children with more persistent pain, physical therapy (PT) is often indicated. Similar to that for adults, there is little evidence for specific PT programs to help children with low back pain. Rehabilitation should be individualized based on the condition being treated.
Medications. There have been no high-quality studies on the benefit of medications to treat low back pain in children. Studies have shown nonsteroidal anti-inflammatory drugs (NSAIDs) have value in adults, and they are likely safe for use in children,40 but the risk of opiate abuse is significantly increased in adolescents who have been prescribed opiate pain medication prior to 12th grade.41
Lumbar disc herniation. Although still relatively rare, lumbar disc herniation is more common in older children and adolescents than in younger children and is treated similarly to that in adults.8 Range-of-motion exercise to restore lumbar motion is often first-line treatment. Research has shown that exercises that strengthen the abdominal or “core” musculature help prevent the return of low back pain.24,25
In the case of spondylolysis or spondylolisthesis, rest from activity is generally required for a minimum of 4 to 6 weeks. Rehabilitation in the form of range of motion, especially into the lumbar extension, and spinal stabilization exercises are effective for both reducing pain and restoring range-of-motion and strength.42 Have patients avoid heavy backpacks, which can reproduce pain. Children often benefit from leaving a second set of schoolbooks at home. For most patients with spondylolysis, conservative treatment with rehabilitation is equal to or better than surgical intervention in returning the patient to his/her pre-injury activity level.26,43,44 When returning athletes to their sport, aggressive PT, defined as rest for < 10 weeks prior to initiating PT, is superior to delaying PT beyond 10 weeks of rest.27
Idiopathic scoliosis. Much of the literature on the treatment of scoliosis is focused on limiting progression of the scoliotic curvature. Researchers thought that more severe curves were associated with more severe pain, but a recent systematic review showed that back pain can occur in patients with even small curvatures.28 Treatment for patients with smaller degrees of curvature is similar to that for mechanical low back pain. PT may have a role in the treatment of scoliosis, but there is little evidence in the literature of its effectiveness.
Continue to: A Cochrane review showed...
A Cochrane review showed that PT and exercise-based treatments had no effect on back pain or disability in patients with scoliosis.29 And outpatient PT alone, in the absence of bracing, does not arrest progression of the scoliotic curvature.35 One trial did demonstrate that an intensive inpatient treatment program of 4 to 6 weeks for patients with curvature of at least 40° reduced progression of curvature compared to an untreated control group at 1 year.34 The outcomes of functional mobility and pain were not measured. Follow-up data on curve progression beyond 1 year are not available. Unfortunately, intensive inpatient treatment is not readily available or cost-effective for most patients with scoliosis.
Scheuermann disease. The mainstay of treatment for mild Scheuermann disease is advising the patient to avoid repetitive loading of the spine. Patients should avoid sports such as competitive weight-lifting, gymnastics, and football. Lower impact athletics are encouraged. Refer patients with pain to PT to address posture and core stabilization. Patients with severe kyphosis may require surgery.17,18
Bracing: Rarely helpful for low back pain
The use of lumbar braces or corsets is rarely helpful for low back pain in children. Bracing in the setting of spondylolysis is controversial.One study indicated that bracing in combination with activity restriction and lumbar extension exercise is superior to activity restriction and lumbar flexion exercises alone.43 But a meta-analysis did not demonstrate a significant difference in recovery when bracing was added.44 Bracing may help to reduce pain initially in patients with spondylolysis who have pain at rest. Bracing is not recommended for patients with pain that abates with activity modification.
Scoliosis and Scheuermann kyphosis. Treatment of adolescent idiopathic scoliosis usually consists of observation and periodic reevaluation. Bracing is a mainstay of the nonsurgical management of scoliosis and is appropriate for curves of 20° to 40°; studies have reported successful control of curve progression in > 70% of patients.36 According to 1 study, the number of cases of scoliosis needed to treat with bracing to prevent 1 surgery is 3.30 Surgery is often indicated for patients with curvatures > 40°, although this is also debated.33
Bracing is used rarely for Scheuermann kyphosis but may be helpful in more severe or painful cases.17
CORRESPONDENCE
Shawn F. Phillips, MD, MSPT, 500 University Drive H154, Hershey, PA, 17033; [email protected].
Low back pain in not uncommon in children and adolescents.1-3 Although the prevalence of low back pain in children < 7 years is low, it increases with age, with studies reporting lifetime prevalence at age 12 years between 16% and 18% and rates as high as 66% by 16 years of age.4,5 Although children and adolescents usually have pain that is transient and benign without a defined cause, structural causes of low back pain should be considered in school-aged children with pain that persists for > 3 to 6 weeks. 4 The most common structural causes of adolescent low back pain are reviewed here.
Etiology: A mixed bag
Back pain in school-aged children is most commonly due to muscular strain, overuse, or poor posture. The pain is often transient in nature and responds to rest and postural education.4,6 A herniated disc is an uncommon finding in younger school-aged children, but incidence increases slightly among older adolescents, particularly those who are active in collision sports and/or weight-lifting.7,8 Pain caused by a herniated disc often radiates along the distribution of the sciatic nerve and worsens during lumbar flexion.
Spondylolysis and spondylolisthesis are important causes of back pain in children. Spondylolysis is defined as a defect or abnormality of the pars interarticularis and surrounding lamina and pedicle. Spondylolisthesis, which is less common, is defined as the translation or “slippage” of one vertebral segment in relation to the next caudal segment. These conditions commonly occur as a result of repetitive stress.
In a prospective study of adolescents < 19 years with low back pain for > 2 weeks, the prevalence of spondylolysis was 39.7%.9 Adolescent athletes with symptomatic low back pain are more likely to have spondylolysis than nonathletes (32% vs 2%, respectively).2,10 Pain is often made worse by extension of the spine. Spondylolysis and spondylolisthesis can be congenital or acquired, and both can be asymptomatic. Children and teens who are athletes are at higher risk for symptomatic spondylolysis and spondylolisthesis.10-12 This is especially true for those involved in gymnastics, dance, football, and/or volleyball, where a repetitive load is placed onto an extended spine.
Idiopathic scoliosis is an abnormal lateral curvature of the spine that usually develops during adolescence and worsens with growth. Historically, painful scoliosis was considered rare, but more recently researchers determined that children with scoliosis have a higher rate of pain compared to their peers.13,14 School-aged children with scoliosis were found to be at 2 times the risk of low back pain compared to those without scoliosis.13 It is important to identify scoliosis in adolescents so that progression can be monitored.
Screening for scoliosis in primary care is somewhat controversial. The US Preventive Services Task Force (USPSTF) finds insufficient evidence for screening asymptomatic adolescents for scoliosis.15 This recommendation is based on the fact that there is little evidence on the effect of screening on long-term outcomes. Screening may also lead to unnecessary radiation. Conversely, a position statement released by the Scoliosis Research Society, the Pediatric Orthopedic Society of North America, the American Association of Orthopedic Surgeons, and the American Academy of Pediatrics recommends scoliosis screening during routine pediatric office visits.16 Screening for girls is recommended at ages 10 and 12 years, and for boys, once between ages 13 and 14 years. The statement highlights evidence showing that focused screening by appropriate personnel has value in detecting a clinically significant curve (> 20°).
Scheuermann disease is a rare cause of back pain in children that usually develops during adolescence and results in increasing thoracic kyphosis. An autosomal dominant mutation plays a role in this disease of the growth cartilage endplate; repetitive strain on the growth cartilage is also a contributing factor.17,18 An atypical variant manifests with kyphosis in the thoracolumbar region.17
Continue to: Other causes of low back pain
Other causes of low back pain—including inflammatory arthritis, infection (eg, discitis), and tumor—are rare in children but must always be considered, especially in the setting of persistent symptoms.4,19-21 More on the features of these conditions is listed in TABLE 1.1-7,13-15,17-30

History: Focus on onset, timing, and duration of symptoms
As with adults, obtaining a history that includes the onset, timing, and duration of symptoms is key in the evaluation of low back pain in children, as is obtaining a history of the patient’s activities; sports that repetitively load the lumbar spine in an extended position increase the risk of injury.10

Specific risk factors for low back pain in children and adolescents are poorly understood.4,9,31 Pain can be associated with trauma, or it can have a more progressive or insidious onset. Generally, pain that is present for up to 6 weeks and is intermittent or improving has a self-limited course. Pain that persists beyond 3 to 6 weeks or is worsening is more likely to have an anatomical cause that needs further evaluation.2,3,10,21
Identifying exacerbating and alleviating factors can provide useful information. Pain that is worse with lumbar flexion is more likely to come from muscular strain or disc pathology. Pain with extension is more likely due to a structural cause such as spondylolysis/spondylolisthesis, scoliosis, or Scheuermann disease.2,4,10,17,18,21 See TABLE 2 for red flag symptoms that indicate the need for imaging and further work-up.

The physical exam: Visualize, assess range of motion, and reproduce pain
The physical examination of any patient with low back pain should include direct visualization and inspection of the back, spine, and pelvis; palpation of the spine and paraspinal regions; assessment of lumbar range of motion and of the lumbar nerve roots, including tests of sensation, strength, and deep tendon reflexes; and an evaluation of the patient’s posture, which can provide clues to underlying causes of pain.
Continue to: Increased thoracic kyphosis...
Increased thoracic kyphosis that is not reversible is concerning for Scheuermann disease.9,17,18 A significant elevation in one shoulder or side of the pelvis can be indicative of scoliosis. Increased lumbar lordosis may predispose a patient to spondylolysis.
In patients with spondylolysis, lumbar extension will usually reproduce pain, which is often unilateral. Hyperextension in a single-leg stance, commonly known as the Stork test, is positive for unilateral spondylolysis when it reproduces pain on the ipsilateral side. The sensitivity of the Stork test for unilateral spondylolysis is approximately 50%.32 (For more information on the Stork test, see www.physio-pedia.com/Stork_test.)
Pain reproduced with lumbar flexion is less concerning for bony pathology and is most often related to soft-tissue strain. Lumbar flexion with concomitant radicular pain is associated with disc pathology.8 Pain with a straight-leg raise is also associated with disk pathology, especially if raising the contralateral leg increases pain.8
Using a scoliometer. Evaluate the flexed spine for the presence of asymmetry, which can indicate scoliosis.33 If asymmetry is present, use a scoliometer to determine the degree of asymmetry. Zero to 5° is considered clinically insignificant; monitor and reevaluate these patients at subsequent visits.34,35 Ten degrees or more of asymmetry with a scoliometer should prompt you to order radiographs.35,36 A smartphone-based scoliometer for iPhones was evaluated in 1 study and was shown to have reasonable reliability and validity for clinical use.37
Deformity of the lower extremities. Because low back pain may be caused by biomechanical or structural deformity of the lower extremities, examine the flexibility of the hip flexors, gluteal musculature, hamstrings, and the iliotibial band.38 In addition, evaluate for leg-length discrepancy and lower-extremity malalignment, such as femoral anteversion, tibial torsion, or pes planus.
Continue to: Imaging
Imaging: Know when it’s needed
Although imaging of the lumbar spine is often unnecessary in the presence of acute low back pain in children, always consider imaging in the setting of bony tenderness, pain that wakes a patient from sleep, and in the setting of other red flag symptoms (see TABLE 2). Low back pain in children that is reproducible with lumbar extension is concerning for spondylolysis or spondylolisthesis. If the pain with extension persists beyond 3 to 6 weeks, order imaging starting with radiographs.2,39
Traditionally, 4 views of the spine—anteroposterior (AP), lateral, and oblique (one right and one left)—were obtained, but recent evidence indicates that 2 views (AP and lateral) have similar sensitivity and specificity to 4 views with significantly reduced radiation exposure.2,39 Because the sensitivity of plain films is relatively low, consider more advanced imaging if spondylolysis or spondylolisthesis is strongly suspected. Recent studies indicate that magnetic resonance imaging (MRI) may be as effective as computed tomography (CT) or bone scan and has the advantage of lower radiation (FIGURE 1).2,22

Similarly, order radiographs if there is > 10° of asymmetry noted on physical exam using a scoliometer.15,23 Calculate the Cobb angle to determine the severity of scoliosis. Refer patients with angles ≥ 20° to a pediatric orthopedist for monitoring of progression and consideration of bracing (FIGURE 2).23,34 For patients with curvatures between 10° and 19°, repeat imaging every 6 to 12 months. Because scoliosis is a risk factor for spondylolysis, evaluate radiographs in the setting of painful scoliosis for the presence of a spondylolysis.34,35

If excessive kyphosis is noted on exam, order radiographs to evaluate for Scheuermann disease. Classic imaging findings include Schmorl nodes, vertebral endplate changes, and anterior wedging (FIGURE 3).17,18

In the absence of the above concerns, defer imaging of the lumbar spine until after adequate rest and rehabilitation have been attempted.
Continue to: Treatment typically involves restor physical therapy
Treatment typically involves restor physical therapy
Most cases of low back pain in children and adolescents are benign and self-limited. Many children with low back pain can be treated with relative rest from the offending activity. For children with more persistent pain, physical therapy (PT) is often indicated. Similar to that for adults, there is little evidence for specific PT programs to help children with low back pain. Rehabilitation should be individualized based on the condition being treated.
Medications. There have been no high-quality studies on the benefit of medications to treat low back pain in children. Studies have shown nonsteroidal anti-inflammatory drugs (NSAIDs) have value in adults, and they are likely safe for use in children,40 but the risk of opiate abuse is significantly increased in adolescents who have been prescribed opiate pain medication prior to 12th grade.41
Lumbar disc herniation. Although still relatively rare, lumbar disc herniation is more common in older children and adolescents than in younger children and is treated similarly to that in adults.8 Range-of-motion exercise to restore lumbar motion is often first-line treatment. Research has shown that exercises that strengthen the abdominal or “core” musculature help prevent the return of low back pain.24,25
In the case of spondylolysis or spondylolisthesis, rest from activity is generally required for a minimum of 4 to 6 weeks. Rehabilitation in the form of range of motion, especially into the lumbar extension, and spinal stabilization exercises are effective for both reducing pain and restoring range-of-motion and strength.42 Have patients avoid heavy backpacks, which can reproduce pain. Children often benefit from leaving a second set of schoolbooks at home. For most patients with spondylolysis, conservative treatment with rehabilitation is equal to or better than surgical intervention in returning the patient to his/her pre-injury activity level.26,43,44 When returning athletes to their sport, aggressive PT, defined as rest for < 10 weeks prior to initiating PT, is superior to delaying PT beyond 10 weeks of rest.27
Idiopathic scoliosis. Much of the literature on the treatment of scoliosis is focused on limiting progression of the scoliotic curvature. Researchers thought that more severe curves were associated with more severe pain, but a recent systematic review showed that back pain can occur in patients with even small curvatures.28 Treatment for patients with smaller degrees of curvature is similar to that for mechanical low back pain. PT may have a role in the treatment of scoliosis, but there is little evidence in the literature of its effectiveness.
Continue to: A Cochrane review showed...
A Cochrane review showed that PT and exercise-based treatments had no effect on back pain or disability in patients with scoliosis.29 And outpatient PT alone, in the absence of bracing, does not arrest progression of the scoliotic curvature.35 One trial did demonstrate that an intensive inpatient treatment program of 4 to 6 weeks for patients with curvature of at least 40° reduced progression of curvature compared to an untreated control group at 1 year.34 The outcomes of functional mobility and pain were not measured. Follow-up data on curve progression beyond 1 year are not available. Unfortunately, intensive inpatient treatment is not readily available or cost-effective for most patients with scoliosis.
Scheuermann disease. The mainstay of treatment for mild Scheuermann disease is advising the patient to avoid repetitive loading of the spine. Patients should avoid sports such as competitive weight-lifting, gymnastics, and football. Lower impact athletics are encouraged. Refer patients with pain to PT to address posture and core stabilization. Patients with severe kyphosis may require surgery.17,18
Bracing: Rarely helpful for low back pain
The use of lumbar braces or corsets is rarely helpful for low back pain in children. Bracing in the setting of spondylolysis is controversial.One study indicated that bracing in combination with activity restriction and lumbar extension exercise is superior to activity restriction and lumbar flexion exercises alone.43 But a meta-analysis did not demonstrate a significant difference in recovery when bracing was added.44 Bracing may help to reduce pain initially in patients with spondylolysis who have pain at rest. Bracing is not recommended for patients with pain that abates with activity modification.
Scoliosis and Scheuermann kyphosis. Treatment of adolescent idiopathic scoliosis usually consists of observation and periodic reevaluation. Bracing is a mainstay of the nonsurgical management of scoliosis and is appropriate for curves of 20° to 40°; studies have reported successful control of curve progression in > 70% of patients.36 According to 1 study, the number of cases of scoliosis needed to treat with bracing to prevent 1 surgery is 3.30 Surgery is often indicated for patients with curvatures > 40°, although this is also debated.33
Bracing is used rarely for Scheuermann kyphosis but may be helpful in more severe or painful cases.17
CORRESPONDENCE
Shawn F. Phillips, MD, MSPT, 500 University Drive H154, Hershey, PA, 17033; [email protected].
1. MacDonald J, Stuart E, Rodenberg R. Musculoskeletal low back pain in school-aged children: a review. JAMA Pediatr. 2017;171:280-287.
2. Tofte JN CarlLee TL, Holte AJ, et al. Imaging pediatric spondylolysis: a systematic review. Spine. 2017;42:777-782.
3. Sakai T, Sairyo K, Suzue N, et al. Incidence and etiology of lumbar spondylolysis: review of the literature. J Orthop Sci. 2010;15:281-288.
4. Calvo-Muñoz I, Gómez-Conesa A, Sánchez-Meca J. Prevalence of low back pain in children and adolescents: a meta-analysis. BMC Pediatrics. 2013;13:14.
5. Bernstein RM, Cozen H. Evaluation of back pain in children and adolescents. Am Fam Physician. 2007;76:1669-1676.
6. Taxter AJ, Chauvin NA, Weiss PF. Diagnosis and treatment of low back pain in the pediatric population. Phys Sportsmed. 2014;42:94-104.
7. Haus BM, Micheli LJ. Back pain in the pediatric and adolescent athlete. Clin Sports Med. 2012;31:423-440.
8. Lavelle WF, Bianco A, Mason R, et al. Pediatric disk herniation. J Am Acad Orthop Surg. 2011;19:649-656.
9. Taimela S, Kujala UM, Salminen JJ, et al. The prevalence of low back pain among children and adolescents: a nationwide, cohort-based questionnaire survey in Finland. Spine. 1997;22:1132-1136.
10. Schroeder GD, LaBella CR, Mendoza M, et al. The role of intense athletic activity on structural lumbar abnormalities in adolescent patients with symptomatic low back pain. Eur Spine J. 2016;25:2842-2848.
11. Waicus KM, Smith BW. Back injuries in the pediatric athlete. Curr Sports Med Rep. 2002;1:52-58.
12. Daniels JM, Pontius G, El-Amin S, et al. Evaluation of low back pain in athletes. Sports Health. 2011;3:336-345.
13. Sato T, Hirano T, Ito T, et al. Back pain in adolescents with idiopathic scoliosis: epidemiological study for 43,630 pupils in Niigata City, Japan. Eur Spine J. 2011;20:274-279.
14. Smorgick Y, Mirovsky Y, Baker KC, et al. Predictors of back pain in adolescent idiopathic scoliosis surgical candidates. J Pediatr Orthop. 2013;33:289-292.
15. US Preventive Services Task Force. Screening for Adolescent Idiopathic Scoliosis. US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:165-172.
16. Hresko MT, Talwalkar VR, Schwend RM. Position statement–Screening for the early detection of idiopathic scoliosis in adolescents. SRS/POSNA/AAOS/AAP Position Statement. 2015. www.srs.org/about-srs/news-and-announcements/position-statement---screening-for-the-early-detection-for-idiopathic-scoliosis-in-adolescents. Accessed September 30, 2020.
17. Palazzo C, Sailhan F, Revel M. Scheuermann’s disease: an update. Joint Bone Spine. 2014;81:209-214.
18. Ali RM, Green DW, Patel TC. Scheuermann’s kyphosis. Curr Opin Pediatr. 1999;11:70-75.
19. de Moraes Barros Fucs PM, Meves R, Yamada HH, et al. Spinal infections in children: a review. Int Orthop. 2012;36:387-395.
20. Joaquim AF, Ghizoni E, Valadares MG, et al. Spinal tumors in children. Revista da Associação Médica Brasileira. 2017;63:459-465.
21. Weiss PF, Colbert RA. Juvenile spondyloarthritis: a distinct form of juvenile arthritis. Pediatr Clin North Am. 2018;65:675-690.
22. Rush JK, Astur N, Scott S, et al. Use of magnetic resonance imaging in the evaluation of spondylolysis. J Pediatr Orthop. 2015;35:271-275.
23. Janicki JA, Alman B. Scoliosis: review of diagnosis and treatment. Pediatr Child Health. 2007;12:771-776.
24. O’Sullivan PB, Phyty GD, Twomey LT, et al. Evaluation of specific stabilizing exercise in the treatment of chronic low back pain with radiologic diagnosis of spondylolysis or spondylolisthesis. Spine.1997;22:2959-2967.
25. Inani SB, Selkar SP. Effect of core stabilization exercises versus conventional exercises on pain and functional status in patients with non-specific low back pain: a randomized clinical trial. J Back Musculoskelet Rehabil. 2013;26:37-43.
26. Garet M, Reiman MP, Mathers J, et al. Nonoperative treatment in lumbar spondylolysis and spondylolisthesis: a systematic review. Sports Health. 2013;5:225-232.
27. Selhorst M, Fischer A, Graft K, et al. Timing of physical therapy referral in adolescent athletes with acute spondylolysis: a retrospective chart review. Clin J Sport Med. 2017;27:296-301.
28. Théroux J, Stomski N, Hodgetts CJ, et al. Prevalence of low back pain in adolescents with idiopathic scoliosis: a systematic review. Chiropr Man Ther. 2017;25:10.
29. Romano M, Minozzi S, Zaina F, et al. Exercises for adolescent idiopathic scoliosis: a Cochrane systematic review. Spine (Phila Pa 1976). 2013;38:E883-E893.
30. Sanders JO, Newton PO, Browne RH, et al. Bracing for idiopathic scoliosis: how many patients require treatment to prevent one surgery? J Bone Joint Surg Am. 2014;96:649-653.
31. Hill JJ, Keating JL. Risk factors for the first episode of low back pain in children are infrequently validated across samples and conditions: a systematic review. J Physiother. 2010;56:237-244.
32. Grødahl LHJ, Fawcett L, Nazareth M, et al. Diagnostic utility of patient history and physical examination data to detect spondylolysis and spondylolisthesis in athletes with low back pain: a systematic review. Man Ther. 2016;24:7-17.
33. Asher MA, Burton DC. Adolescent idiopathic scoliosis: natural history and long term treatment effects. Scoliosis. 2006;1:2.
34. Weiss HR, Weiss G, Petermann F. Incidence of curvature progression in idiopathic scoliosis patients treated with scoliosis inpatient rehabilitation (SIR): an age- and sex-matched controlled study. Pediatr Rehabil. 2003;6:23-30.
35. Gomez JA, Hresko MT, Glotzbecker MP. Nonsurgical management of adolescent idiopathic scoliosis. J Am Acad Orthop Surg. 2016;24:555-564.
36. Weinstein SL, Dolan LA, Wright JG, et al. Effects of bracing in adolescents with idiopathic scoliosis. N Engl J Med. 2013;369:1512-1521.
37. Balg F, Juteau M, Theoret C, et al. Validity and reliability of the iPhone to measure rib hump in scoliosis. J Pediatr Orthop. 2014;34:774-779.
38. Auerbach JD, Ahn J, Zgonis MH, et al. Streamlining the evaluation of low back pain in children. Clin Orthop Relatl Res. 2008;466:1971-1977.
39. Beck NA, Miller R, Baldwin K, et al. Do oblique views add value in the diagnosis of spondylolysis in adolescents? J Bone Joint Surg Am. 2013;95:e65.
40. Roelofs PD, Deyo RA, Koes BW, et al. Nonsteroidal anti-inflammatory drugs for low back pain: an updated Cochrane review. Spine (Phila Pa 1976). 2008;33:1766-1774.
41. Miech R, Johnston L, O’Malley PM, et al. Prescription opioids in adolescence and future opioid misuse. Pediatrics. 2015;136:e1169-e1177.
42. Hu S, Tribus C, Diab M, et al. Spondylolysis and spondylolisthesis. J Bone Joint Surg. 2008;90:655-671.
43. Panteliadis P, Nagra NS, Edwards KL, et al. Athletic population with spondylolysis: review of outcomes following surgical repair or conservative management. Global Spine J. 2016;6:615-625.
44. Klein G, Mehlman CT, McCarty M. Nonoperative treatment of spondylolysis and grade I spondylolisthesis in children and young adults: a meta-analysis of observational studies. J Pediatr Orthop. 2009;29:146-156.
1. MacDonald J, Stuart E, Rodenberg R. Musculoskeletal low back pain in school-aged children: a review. JAMA Pediatr. 2017;171:280-287.
2. Tofte JN CarlLee TL, Holte AJ, et al. Imaging pediatric spondylolysis: a systematic review. Spine. 2017;42:777-782.
3. Sakai T, Sairyo K, Suzue N, et al. Incidence and etiology of lumbar spondylolysis: review of the literature. J Orthop Sci. 2010;15:281-288.
4. Calvo-Muñoz I, Gómez-Conesa A, Sánchez-Meca J. Prevalence of low back pain in children and adolescents: a meta-analysis. BMC Pediatrics. 2013;13:14.
5. Bernstein RM, Cozen H. Evaluation of back pain in children and adolescents. Am Fam Physician. 2007;76:1669-1676.
6. Taxter AJ, Chauvin NA, Weiss PF. Diagnosis and treatment of low back pain in the pediatric population. Phys Sportsmed. 2014;42:94-104.
7. Haus BM, Micheli LJ. Back pain in the pediatric and adolescent athlete. Clin Sports Med. 2012;31:423-440.
8. Lavelle WF, Bianco A, Mason R, et al. Pediatric disk herniation. J Am Acad Orthop Surg. 2011;19:649-656.
9. Taimela S, Kujala UM, Salminen JJ, et al. The prevalence of low back pain among children and adolescents: a nationwide, cohort-based questionnaire survey in Finland. Spine. 1997;22:1132-1136.
10. Schroeder GD, LaBella CR, Mendoza M, et al. The role of intense athletic activity on structural lumbar abnormalities in adolescent patients with symptomatic low back pain. Eur Spine J. 2016;25:2842-2848.
11. Waicus KM, Smith BW. Back injuries in the pediatric athlete. Curr Sports Med Rep. 2002;1:52-58.
12. Daniels JM, Pontius G, El-Amin S, et al. Evaluation of low back pain in athletes. Sports Health. 2011;3:336-345.
13. Sato T, Hirano T, Ito T, et al. Back pain in adolescents with idiopathic scoliosis: epidemiological study for 43,630 pupils in Niigata City, Japan. Eur Spine J. 2011;20:274-279.
14. Smorgick Y, Mirovsky Y, Baker KC, et al. Predictors of back pain in adolescent idiopathic scoliosis surgical candidates. J Pediatr Orthop. 2013;33:289-292.
15. US Preventive Services Task Force. Screening for Adolescent Idiopathic Scoliosis. US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:165-172.
16. Hresko MT, Talwalkar VR, Schwend RM. Position statement–Screening for the early detection of idiopathic scoliosis in adolescents. SRS/POSNA/AAOS/AAP Position Statement. 2015. www.srs.org/about-srs/news-and-announcements/position-statement---screening-for-the-early-detection-for-idiopathic-scoliosis-in-adolescents. Accessed September 30, 2020.
17. Palazzo C, Sailhan F, Revel M. Scheuermann’s disease: an update. Joint Bone Spine. 2014;81:209-214.
18. Ali RM, Green DW, Patel TC. Scheuermann’s kyphosis. Curr Opin Pediatr. 1999;11:70-75.
19. de Moraes Barros Fucs PM, Meves R, Yamada HH, et al. Spinal infections in children: a review. Int Orthop. 2012;36:387-395.
20. Joaquim AF, Ghizoni E, Valadares MG, et al. Spinal tumors in children. Revista da Associação Médica Brasileira. 2017;63:459-465.
21. Weiss PF, Colbert RA. Juvenile spondyloarthritis: a distinct form of juvenile arthritis. Pediatr Clin North Am. 2018;65:675-690.
22. Rush JK, Astur N, Scott S, et al. Use of magnetic resonance imaging in the evaluation of spondylolysis. J Pediatr Orthop. 2015;35:271-275.
23. Janicki JA, Alman B. Scoliosis: review of diagnosis and treatment. Pediatr Child Health. 2007;12:771-776.
24. O’Sullivan PB, Phyty GD, Twomey LT, et al. Evaluation of specific stabilizing exercise in the treatment of chronic low back pain with radiologic diagnosis of spondylolysis or spondylolisthesis. Spine.1997;22:2959-2967.
25. Inani SB, Selkar SP. Effect of core stabilization exercises versus conventional exercises on pain and functional status in patients with non-specific low back pain: a randomized clinical trial. J Back Musculoskelet Rehabil. 2013;26:37-43.
26. Garet M, Reiman MP, Mathers J, et al. Nonoperative treatment in lumbar spondylolysis and spondylolisthesis: a systematic review. Sports Health. 2013;5:225-232.
27. Selhorst M, Fischer A, Graft K, et al. Timing of physical therapy referral in adolescent athletes with acute spondylolysis: a retrospective chart review. Clin J Sport Med. 2017;27:296-301.
28. Théroux J, Stomski N, Hodgetts CJ, et al. Prevalence of low back pain in adolescents with idiopathic scoliosis: a systematic review. Chiropr Man Ther. 2017;25:10.
29. Romano M, Minozzi S, Zaina F, et al. Exercises for adolescent idiopathic scoliosis: a Cochrane systematic review. Spine (Phila Pa 1976). 2013;38:E883-E893.
30. Sanders JO, Newton PO, Browne RH, et al. Bracing for idiopathic scoliosis: how many patients require treatment to prevent one surgery? J Bone Joint Surg Am. 2014;96:649-653.
31. Hill JJ, Keating JL. Risk factors for the first episode of low back pain in children are infrequently validated across samples and conditions: a systematic review. J Physiother. 2010;56:237-244.
32. Grødahl LHJ, Fawcett L, Nazareth M, et al. Diagnostic utility of patient history and physical examination data to detect spondylolysis and spondylolisthesis in athletes with low back pain: a systematic review. Man Ther. 2016;24:7-17.
33. Asher MA, Burton DC. Adolescent idiopathic scoliosis: natural history and long term treatment effects. Scoliosis. 2006;1:2.
34. Weiss HR, Weiss G, Petermann F. Incidence of curvature progression in idiopathic scoliosis patients treated with scoliosis inpatient rehabilitation (SIR): an age- and sex-matched controlled study. Pediatr Rehabil. 2003;6:23-30.
35. Gomez JA, Hresko MT, Glotzbecker MP. Nonsurgical management of adolescent idiopathic scoliosis. J Am Acad Orthop Surg. 2016;24:555-564.
36. Weinstein SL, Dolan LA, Wright JG, et al. Effects of bracing in adolescents with idiopathic scoliosis. N Engl J Med. 2013;369:1512-1521.
37. Balg F, Juteau M, Theoret C, et al. Validity and reliability of the iPhone to measure rib hump in scoliosis. J Pediatr Orthop. 2014;34:774-779.
38. Auerbach JD, Ahn J, Zgonis MH, et al. Streamlining the evaluation of low back pain in children. Clin Orthop Relatl Res. 2008;466:1971-1977.
39. Beck NA, Miller R, Baldwin K, et al. Do oblique views add value in the diagnosis of spondylolysis in adolescents? J Bone Joint Surg Am. 2013;95:e65.
40. Roelofs PD, Deyo RA, Koes BW, et al. Nonsteroidal anti-inflammatory drugs for low back pain: an updated Cochrane review. Spine (Phila Pa 1976). 2008;33:1766-1774.
41. Miech R, Johnston L, O’Malley PM, et al. Prescription opioids in adolescence and future opioid misuse. Pediatrics. 2015;136:e1169-e1177.
42. Hu S, Tribus C, Diab M, et al. Spondylolysis and spondylolisthesis. J Bone Joint Surg. 2008;90:655-671.
43. Panteliadis P, Nagra NS, Edwards KL, et al. Athletic population with spondylolysis: review of outcomes following surgical repair or conservative management. Global Spine J. 2016;6:615-625.
44. Klein G, Mehlman CT, McCarty M. Nonoperative treatment of spondylolysis and grade I spondylolisthesis in children and young adults: a meta-analysis of observational studies. J Pediatr Orthop. 2009;29:146-156.
PRACTICE RECOMMENDATIONS
› Be aware that low back pain is rare in children < 7 years but increases in incidence as children near adolescence. A
› Consider imaging in the setting of bony tenderness, pain that awakens the patient from sleep, or in the presence of other “red flag” symptoms. A
› Consider spondylolysis and spondylolisthesis in adolescent athletes with low back pain lasting longer than 3 to 6 weeks. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Putting an end to chronic opioid prescriptions
Thanks to Dr. Linn et al for “Tips and tools for safe opioid prescribing” (J Fam Pract. 2020;69:280-292), which addressed an important topic: the risks of, and poor evidence for, chronic opioids in noncancer pain.
Pain management is challenging, and it is easy to prescribe opioids from a desire to help. However, we must translate the evidence of chronic opioids’ poor benefit and real harms into practice. No studies show a long-term benefit of opioids for chronic noncancer pain, but they do demonstrate abundant findings of harm. As a family medicine community, we should be practicing at the highest level of evidence and addressing legacy opioid prescribing for chronic noncancer pain.
Increasing opioid doses for pain only offers short-term benefits and can result in rapid tolerance and withdrawal. We should not be starting people on opioids for knee and back pain. We do not need more ways to initiate opioids or tables on how to dose long-acting opioids—drugs that increase mortality.1 Let’s stop using poorly validated tools like DIRE to ignore the evidence against opioids (validated with 61 retrospective chart reviews; 81% sensitivity, 76% specificity for predicting efficacy of opioids).2,3
A 2018 randomized controlled trial of 240 patients with back, knee, or hip osteoarthritis found opioids were not superior to nonopioid medication for pain-related function at 12 months and had more adverse effects.4 A 2015 systematic review concluded there was insufficient evidence of long-term benefits of opioids but a dose-dependent risk of serious harm.5 Just 1 year of taking low-dose opioids can increase the risk of opioid use disorder by 0.7%, compared with 0.004% with no opioids.5
Practical approaches exist. Excellent examples of modern pain care have been developed by the Department of Veterans Affairs/Department of Defense, the Department of Health and Human Services, and state-level initiatives such as the Oregon Pain Guidance.6-8 All use a similar clinical algorithm (FIGURE). If pain is poorly controlled, a slow medically supervised tapering of opioids is indicated.
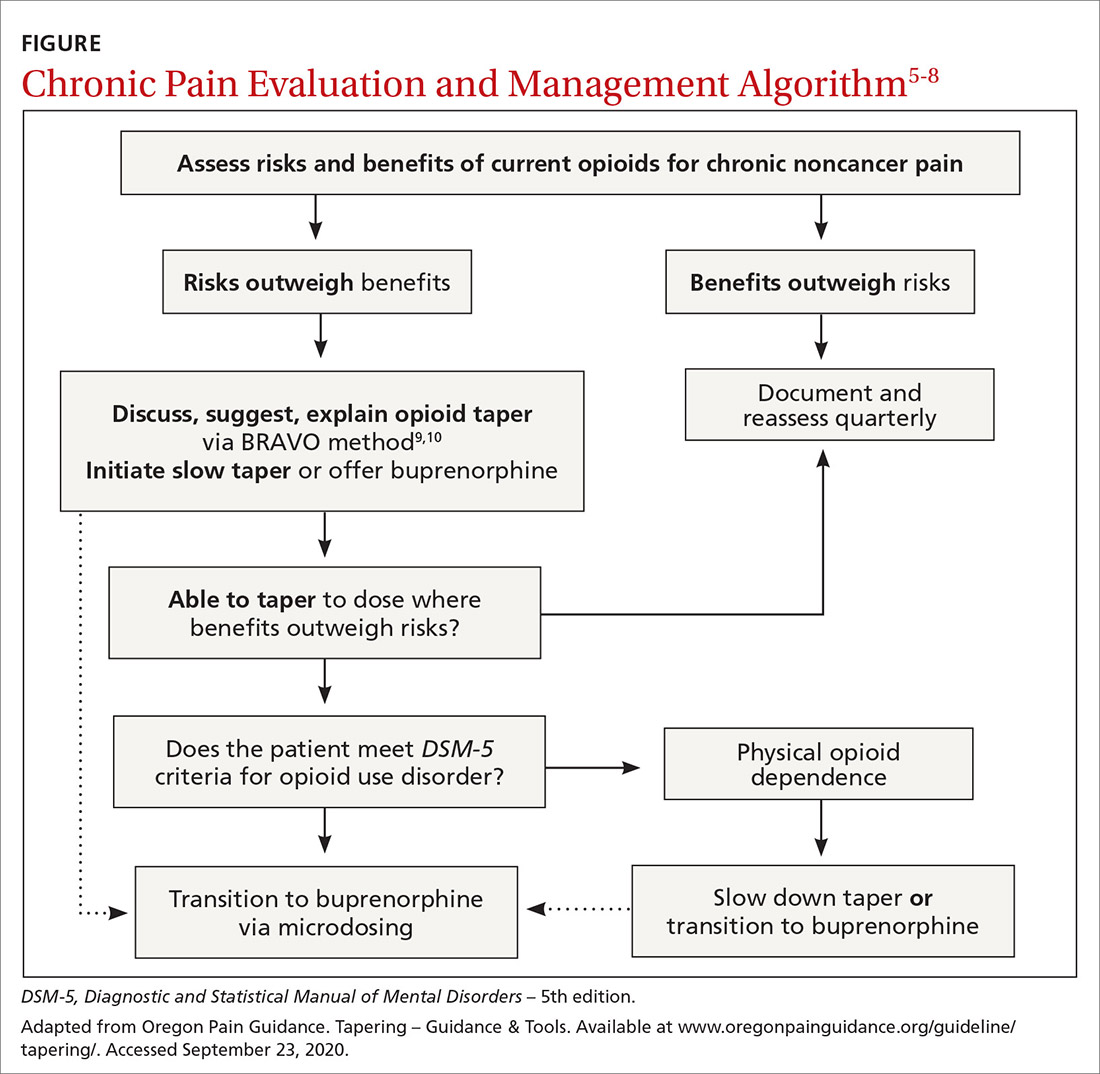
It can be challenging to raise the subject of opioid tapering with patients; I use Stanford’s BRAVO method to guide these conversations.9,10 At my facility, we are tapering about 50 legacy opioid patients, and most are surprised to find that their pain is the same or better with reduced to no opioids, with fewer adverse effects. Many are happier on sublingual buprenorphine, a safer opioid analgesic.11 The algorithm shown in the FIGURE and the BRAVO method should be more widely used within our specialty for a safe and patient-centered approach to chronic pain.
Above all, let the patient know that you are with them on this journey to safe pain management. Start the conversation: “I’ve been thinking a lot about your chronic pain and how best to help you with it. Our understanding of what opioids do for pain has changed, and I worry they’re causing more harm than good now. This is a scary thing to talk about, but I’ll be with you every step of the way.”
Matt Perez, MD
Neighborcare Health
Seattle
1. Ray WA, Chung CP, Murray KT, et al. Prescription of long-acting opioids and mortality in patients with chronic noncancer pain. JAMA. 2016;315:2415-23.
2. Belgrade MJ, Schamber CD, Lindgren BR. The DIRE score: predicting outcomes of opioid prescribing for chronic pain. J Pain. 2006;7:671-681.
3. Brennan MJ. Letter to the editor. J Pain. 2007;8:185.
4. Krebs EE, Gravely A, Nugent S, et al. Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain: the SPACE randomized clinical trial. JAMA 2018;319:872-882.
5. Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015;162:276-286.
6. Oldfield BJ, Edens EL, Agnoli A, et al. Multimodal treatment options, including rotating to buprenorphine, within a multidisciplinary pain clinic for patients on risky opioid regimens: a quality improvement study. Pain Med. 2018;19(suppl 1):S38–S45.
7. HHS guide for clinicians on the appropriate dosage reduction or discontinuation of long-term opioid analgesics. US Department of Health of Human Services Web site. www.hhs.gov/opioids/sites/default/files/2019-10/Dosage_Reduction_Discontinuation.pdf. October 2019. Accessed September 29, 2020.
8. Pain treatment guidelines. Oregon Pain Guidance Web site. www.oregonpainguidance.org/pain-treatment-guidelines/. Accessed September 29, 2020.
9. Tapering – BRAVO – a collaborative approach clinical update March 2020. Oregon Pain Guidance Web site. www.oregonpainguidance.org/guideline/tapering/. Accessed September 29, 2020.
10. How to taper patients off of chronic opioid therapy. Stanford Center for Continuing Medical Education Web site. https://stanford.cloud-cme.com/default.aspx?P=0&EID=20909. Accessed September 29, 2020.
11. Chou R, Ballantyne J, Lembke A, et al. Rethinking opioid dose tapering, prescription opioid dependence, and indications for buprenorphine. Ann Intern Med. 2019;171:427-429.
Thanks to Dr. Linn et al for “Tips and tools for safe opioid prescribing” (J Fam Pract. 2020;69:280-292), which addressed an important topic: the risks of, and poor evidence for, chronic opioids in noncancer pain.
Pain management is challenging, and it is easy to prescribe opioids from a desire to help. However, we must translate the evidence of chronic opioids’ poor benefit and real harms into practice. No studies show a long-term benefit of opioids for chronic noncancer pain, but they do demonstrate abundant findings of harm. As a family medicine community, we should be practicing at the highest level of evidence and addressing legacy opioid prescribing for chronic noncancer pain.
Increasing opioid doses for pain only offers short-term benefits and can result in rapid tolerance and withdrawal. We should not be starting people on opioids for knee and back pain. We do not need more ways to initiate opioids or tables on how to dose long-acting opioids—drugs that increase mortality.1 Let’s stop using poorly validated tools like DIRE to ignore the evidence against opioids (validated with 61 retrospective chart reviews; 81% sensitivity, 76% specificity for predicting efficacy of opioids).2,3
A 2018 randomized controlled trial of 240 patients with back, knee, or hip osteoarthritis found opioids were not superior to nonopioid medication for pain-related function at 12 months and had more adverse effects.4 A 2015 systematic review concluded there was insufficient evidence of long-term benefits of opioids but a dose-dependent risk of serious harm.5 Just 1 year of taking low-dose opioids can increase the risk of opioid use disorder by 0.7%, compared with 0.004% with no opioids.5
Practical approaches exist. Excellent examples of modern pain care have been developed by the Department of Veterans Affairs/Department of Defense, the Department of Health and Human Services, and state-level initiatives such as the Oregon Pain Guidance.6-8 All use a similar clinical algorithm (FIGURE). If pain is poorly controlled, a slow medically supervised tapering of opioids is indicated.

It can be challenging to raise the subject of opioid tapering with patients; I use Stanford’s BRAVO method to guide these conversations.9,10 At my facility, we are tapering about 50 legacy opioid patients, and most are surprised to find that their pain is the same or better with reduced to no opioids, with fewer adverse effects. Many are happier on sublingual buprenorphine, a safer opioid analgesic.11 The algorithm shown in the FIGURE and the BRAVO method should be more widely used within our specialty for a safe and patient-centered approach to chronic pain.
Above all, let the patient know that you are with them on this journey to safe pain management. Start the conversation: “I’ve been thinking a lot about your chronic pain and how best to help you with it. Our understanding of what opioids do for pain has changed, and I worry they’re causing more harm than good now. This is a scary thing to talk about, but I’ll be with you every step of the way.”
Matt Perez, MD
Neighborcare Health
Seattle
Thanks to Dr. Linn et al for “Tips and tools for safe opioid prescribing” (J Fam Pract. 2020;69:280-292), which addressed an important topic: the risks of, and poor evidence for, chronic opioids in noncancer pain.
Pain management is challenging, and it is easy to prescribe opioids from a desire to help. However, we must translate the evidence of chronic opioids’ poor benefit and real harms into practice. No studies show a long-term benefit of opioids for chronic noncancer pain, but they do demonstrate abundant findings of harm. As a family medicine community, we should be practicing at the highest level of evidence and addressing legacy opioid prescribing for chronic noncancer pain.
Increasing opioid doses for pain only offers short-term benefits and can result in rapid tolerance and withdrawal. We should not be starting people on opioids for knee and back pain. We do not need more ways to initiate opioids or tables on how to dose long-acting opioids—drugs that increase mortality.1 Let’s stop using poorly validated tools like DIRE to ignore the evidence against opioids (validated with 61 retrospective chart reviews; 81% sensitivity, 76% specificity for predicting efficacy of opioids).2,3
A 2018 randomized controlled trial of 240 patients with back, knee, or hip osteoarthritis found opioids were not superior to nonopioid medication for pain-related function at 12 months and had more adverse effects.4 A 2015 systematic review concluded there was insufficient evidence of long-term benefits of opioids but a dose-dependent risk of serious harm.5 Just 1 year of taking low-dose opioids can increase the risk of opioid use disorder by 0.7%, compared with 0.004% with no opioids.5
Practical approaches exist. Excellent examples of modern pain care have been developed by the Department of Veterans Affairs/Department of Defense, the Department of Health and Human Services, and state-level initiatives such as the Oregon Pain Guidance.6-8 All use a similar clinical algorithm (FIGURE). If pain is poorly controlled, a slow medically supervised tapering of opioids is indicated.

It can be challenging to raise the subject of opioid tapering with patients; I use Stanford’s BRAVO method to guide these conversations.9,10 At my facility, we are tapering about 50 legacy opioid patients, and most are surprised to find that their pain is the same or better with reduced to no opioids, with fewer adverse effects. Many are happier on sublingual buprenorphine, a safer opioid analgesic.11 The algorithm shown in the FIGURE and the BRAVO method should be more widely used within our specialty for a safe and patient-centered approach to chronic pain.
Above all, let the patient know that you are with them on this journey to safe pain management. Start the conversation: “I’ve been thinking a lot about your chronic pain and how best to help you with it. Our understanding of what opioids do for pain has changed, and I worry they’re causing more harm than good now. This is a scary thing to talk about, but I’ll be with you every step of the way.”
Matt Perez, MD
Neighborcare Health
Seattle
1. Ray WA, Chung CP, Murray KT, et al. Prescription of long-acting opioids and mortality in patients with chronic noncancer pain. JAMA. 2016;315:2415-23.
2. Belgrade MJ, Schamber CD, Lindgren BR. The DIRE score: predicting outcomes of opioid prescribing for chronic pain. J Pain. 2006;7:671-681.
3. Brennan MJ. Letter to the editor. J Pain. 2007;8:185.
4. Krebs EE, Gravely A, Nugent S, et al. Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain: the SPACE randomized clinical trial. JAMA 2018;319:872-882.
5. Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015;162:276-286.
6. Oldfield BJ, Edens EL, Agnoli A, et al. Multimodal treatment options, including rotating to buprenorphine, within a multidisciplinary pain clinic for patients on risky opioid regimens: a quality improvement study. Pain Med. 2018;19(suppl 1):S38–S45.
7. HHS guide for clinicians on the appropriate dosage reduction or discontinuation of long-term opioid analgesics. US Department of Health of Human Services Web site. www.hhs.gov/opioids/sites/default/files/2019-10/Dosage_Reduction_Discontinuation.pdf. October 2019. Accessed September 29, 2020.
8. Pain treatment guidelines. Oregon Pain Guidance Web site. www.oregonpainguidance.org/pain-treatment-guidelines/. Accessed September 29, 2020.
9. Tapering – BRAVO – a collaborative approach clinical update March 2020. Oregon Pain Guidance Web site. www.oregonpainguidance.org/guideline/tapering/. Accessed September 29, 2020.
10. How to taper patients off of chronic opioid therapy. Stanford Center for Continuing Medical Education Web site. https://stanford.cloud-cme.com/default.aspx?P=0&EID=20909. Accessed September 29, 2020.
11. Chou R, Ballantyne J, Lembke A, et al. Rethinking opioid dose tapering, prescription opioid dependence, and indications for buprenorphine. Ann Intern Med. 2019;171:427-429.
1. Ray WA, Chung CP, Murray KT, et al. Prescription of long-acting opioids and mortality in patients with chronic noncancer pain. JAMA. 2016;315:2415-23.
2. Belgrade MJ, Schamber CD, Lindgren BR. The DIRE score: predicting outcomes of opioid prescribing for chronic pain. J Pain. 2006;7:671-681.
3. Brennan MJ. Letter to the editor. J Pain. 2007;8:185.
4. Krebs EE, Gravely A, Nugent S, et al. Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain: the SPACE randomized clinical trial. JAMA 2018;319:872-882.
5. Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015;162:276-286.
6. Oldfield BJ, Edens EL, Agnoli A, et al. Multimodal treatment options, including rotating to buprenorphine, within a multidisciplinary pain clinic for patients on risky opioid regimens: a quality improvement study. Pain Med. 2018;19(suppl 1):S38–S45.
7. HHS guide for clinicians on the appropriate dosage reduction or discontinuation of long-term opioid analgesics. US Department of Health of Human Services Web site. www.hhs.gov/opioids/sites/default/files/2019-10/Dosage_Reduction_Discontinuation.pdf. October 2019. Accessed September 29, 2020.
8. Pain treatment guidelines. Oregon Pain Guidance Web site. www.oregonpainguidance.org/pain-treatment-guidelines/. Accessed September 29, 2020.
9. Tapering – BRAVO – a collaborative approach clinical update March 2020. Oregon Pain Guidance Web site. www.oregonpainguidance.org/guideline/tapering/. Accessed September 29, 2020.
10. How to taper patients off of chronic opioid therapy. Stanford Center for Continuing Medical Education Web site. https://stanford.cloud-cme.com/default.aspx?P=0&EID=20909. Accessed September 29, 2020.
11. Chou R, Ballantyne J, Lembke A, et al. Rethinking opioid dose tapering, prescription opioid dependence, and indications for buprenorphine. Ann Intern Med. 2019;171:427-429.
Choosing Wisely: 10 practices to stop—or adopt—to reduce overuse in health care
When medical care is based on consistent, good-quality evidence, most physicians adopt it. However, not all care is well supported by the literature and may, in fact, be overused without offering benefit to patients. Choosing Wisely, at www.choosingwisely.org, is a health care initiative that highlights screening and testing recommendations from specialty societies in an effort to encourage patients and clinicians to talk about how to make high-value, effective health care decisions and avoid overuse. (See “Test and Tx overutilization: A bigger problem than you might think"1-3).
SIDEBAR
Test and Tx overutilization: A bigger problem than you might think
Care that isn’t backed up by the medical literature is adopted by some physicians and not adopted by others, leading to practice variations. Some variation is to be expected, since no 2 patients require exactly the same care, but substantial variations may be a clue to overuse.
A 2006 analysis of inpatient lab studies found that doctors ordered an average of 2.96 studies per patient per day, but only 29% of these tests (0.95 test/patient/day) contributed to management.1 A 2016 systematic review found more than 800 studies on overuse were published in a single year.2 One study of thyroid nodules followed almost 1000 patients with nodules as they underwent routine follow-up imaging. At the end of the study, 7 were found to have cancer, but of those, only 3 had enlarging or changing nodules that would have been detected with the follow-up imaging being studied. Three of the cancers were stable in size and 1 was found incidentally.3
Enabling physician and patient dialogue. The initiative began in 2010 when the American Board of Internal Medicine convened a panel of experts to identify low-value tests and therapies. Their list took the form of a “Top Five Things” that may not be high value in patient care, and it used language tailored to patients and physicians so that they could converse meaningfully. Physicians could use the evidence to make a clinical decision, and patients could feel empowered to ask informed questions about recommendations they received. The initiative has now expanded to include ways that health care systems can reduce low-value interventions.
Scope of participation. Since the first Choosing Wisely recommendations were published in 2013, more than 80 professional associations have contributed lists of their own. Professional societies participate voluntarily. The American Academy of Family Physicians (AAFP), Society of General Internal Medicine, and American Academy of Pediatrics (AAP) have contributed lists relevant to primary care. All Choosing Wisely recommendations can be searched or sorted by specialty organization. Recommendations are reviewed and revised regularly. If the evidence becomes conflicted or contradictory, recommendations are withdrawn.
Making meaningful improvements by Choosing Wisely
Several studies have shown that health care systems can implement Choosing Wisely recommendations to reduce overuse of unnecessary tests. A 2015 study examined the effect of applying a Choosing Wisely recommendation to reduce the use of continuous pulse oximetry in pediatric inpatients with asthma, wheezing, or bronchiolitis. The recommendation, from the Society of Hospital Medicine–Pediatric Hospital Medicine, advises against continuous pulse oximetry in children with acute respiratory illnesses unless the child is using supplemental oxygen.4 This study, done at the Cincinnati Children’s Hospital Medical Center, found that within 3 months of initiating a protocol on all general pediatrics floors, the average time on pulse oximetry after meeting clinical goals decreased from 10.7 hours to 3.1 hours. In addition, the percentage of patients who had their continuous pulse oximetry stopped within 2 hours of clinical stability (a goal time) increased from 25% to 46%.5
Patients are important drivers of health care utilization. A 2003 study showed that physicians are more likely to order referrals, tests, and prescriptions when patients ask for them, and that nearly 1 in 4 patients did so.6 A 2002 study found that physicians granted all but 3% of patient’s requests for orders or tests, and that fulfilling requests correlated with patient satisfaction in the specialty office studied (cardiology) but not in the primary care (internal medicine) office.7
From its inception, Choosing Wisely has considered patients as full partners in conversations about health care utilization. Choosing Wisely partners with Consumer Reports to create and disseminate plain-language summaries of recommendations. Community groups and physician organizations have also participated in implementation efforts. In 2018, Choosing Wisely secured a grant to expand outreach to diverse or underserved communities.
Choosing Wisely recommendations are not guidelines or mandates. They are intended to be evidence-based advice from a specialty society to its members and to patients about care that is often unnecessary. The goal is to create a conversation and not to eliminate these services from ever being offered or used.
Continue to: Improve your practice with these 10 primary care recommendations
Improve your practice with these 10 primary care recommendations
1 Avoid imaging studies in early acute low back pain without red flags.
Both the AAFP and the American Society of Anesthesiologists recommend against routine X-rays, magnetic resonance imaging, and computed tomography (CT) scans in the first 6 weeks of acute low back pain (LBP).8,9 The American College of Emergency Physicians (ACEP) recommends against routine lumbar spine imaging for emergency department (ED) patients.10 In all cases, imaging is indicated if the patient has any signs or symptoms of neurologic deficits or other indications, such as signs of spinal infection or fracture. However, as ACEP notes, diagnostic imaging does not typically help identify the cause of acute LBP, and when it does, it does not reduce the time to symptom improvement.10
2 Prescribe oral contraceptives on the basis of a medical history and a blood pressure measurement. No routine pelvic exam or other physical exam is necessary.
This AAFP recommendation11 is based on clinical practice guidelines from the American College of Obstetricians and Gynecologists (ACOG) and other research.12 The ACOG practice guideline supports provision of hormonal contraception without a pelvic exam, cervical cancer (Pap) testing, urine pregnancy testing, or testing for sexually transmitted infections. ACOG guidelines also support over-the-counter provision of hormonal contraceptives, including combined oral contraceptives.12
3 Stop recommending daily self-glucose monitoring for patients with diabetes who are not using insulin.
Both the AAFP and the Society for General Internal Medicine recommend against daily blood sugar checks for people who do not use insulin.13,14 A Cochrane review of 9 trials (3300 patients) found that after 6 months, hemoglobin A1C was reduced by 0.3% in people who checked their sugar daily compared with those who did not, but this difference was not significant after a year.15 Hypoglycemic episodes were more common in the “checking” group, and there were no differences in quality of life. A qualitative study found that blood sugar results had little impact on patients’ motivation to change behavior.16
4 Don’t screen for herpes simplex virus (HSV) infection in asymptomatic adults, even those who are pregnant.
This AAFP recommendation17 comes from a US Preventive Services Task Force (USPSTF) Grade D recommendation.18 Most people with positive HSV-2 serology have had an outbreak; even those who do not think they have had one will realize that they had the symptoms once they hear them described.18 With available tests, 1 in 2 positive results for HSV-2 among asymptomatic people will be a false-positive.18
There is no known cure, intervention, or reduction in transmission for infected patients who do not have symptoms.18 Also, serologically detected HSV-2 does not reliably predict genital herpes; and HSV-1 has been found to cause an increasing percentage of genital infection cases.18
Continue to: 5 Don't screen for testicular cancer in asymptomatic individuals
5 Don’t screen for testicular cancer in asymptomatic individuals.
This AAFP recommendation19 also comes from a USPSTF Grade D recommendation.20 A 2010 systematic review found no evidence to support screening of asymptomatic people with a physical exam or ultrasound. All available studies involved symptomatic patients.20
6 Stop recommending cough and cold medicines for children younger than 4 years.
The AAP recommends that clinicians discourage the use of any cough or cold medicine for children in this age-group.21 A 2008 study found that more than 7000 children annually presented to EDs for adverse events from cough and cold medicines.22 Previous studies found no benefit in reducing symptoms.23 In children older than 12 months, a Cochrane review found that honey has a modest benefit for cough in single-night trials.24
7 Avoid performing serum allergy panels.
The American Academy of Allergy, Asthma, and Immunology discourages the use of serum panel testing when patients present with allergy symptoms.25 A patient can have a strong positive immunoglobulin E (IgE) serum result to an allergen and have no clinical allergic symptoms or can have a weak positive serum result and a strong clinical reaction. Targeted skin or serum IgE testing—for example, testing for cashew allergy in a patient known to have had a reaction after eating one—is reasonable.26
8 Avoid routine electroencephalography (EEG), head CT, and carotid ultrasound as initial work-up for simple syncope in adults.
These recommendations, from the American Epilepsy Society,27 ACEP,28 American College of Physicians,29 and American Academy of Neurology (AAN),30 emphasize the low yield of routine work-ups for patients with simple syncope. The AAN notes that 40% of people will experience syncope during adulthood and most will not have carotid disease, which generally manifests with stroke-like symptoms rather than syncope. One study found that approximately 1 in 8 patients referred to an epilepsy clinic had neurocardiogenic syncope rather than epilepsy.31
EEGs have high false-negative and false-positive rates, and history-taking is a better tool with which to make a diagnosis. CT scans performed in the ED were found to contribute to the diagnosis of simple syncope in fewer than 2% of cases of syncope, compared with orthostatic blood pressure (25% of cases).32
Continue to: 9 Wait to refer children with umbilical hernias to pediatric surgery until they are 4 to 5 years of age
9 Wait to refer children with umbilical hernias to pediatric surgery until they are 4 to 5 years of age.
The AAP Section on Surgery offers evidence that the risk-benefit analysis strongly favors waiting on intervention.33 About 1 in 4 children will have an umbilical hernia, and about 85% of cases will resolve by age 5. The strangulation rate with umbilical hernias is very low, and although the risk of infection with surgery is likewise low, the risk of recurrence following surgery before the age of 4 is as high as 2.4%.34 The AAP Section on Surgery recommends against strapping or restraining the hernia, as well.
10 Avoid using appetite stimulants, such as megesterol, and high-calorie nutritional supplements to treat anorexia and cachexia in older adults.
Instead, the American Geriatrics Society recommends that physicians encourage caregivers to serve appealing food, provide support with eating, and remove barriers to appetite and nutrition.35 A Cochrane review showed that high-calorie supplements, such as Boost or Ensure, are associated with very modest weight gain—about 2% of weight—but are not associated with an increased life expectancy or improved quality of life.36
Prescription appetite stimulants are associated with adverse effects and yield inconsistent benefits in older adults. Megesterol, for example, was associated with headache, gastrointestinal adverse effects, insomnia, weakness, and fatigue. Mirtazapine is associated with sedation and fatigue.37
CORRESPONDENCE
Kathleen Rowland, MD, MS, Rush Copley Family Medicine Residency, Rush Medical College, 600 South Paulina, Kidston House Room 605, Chicago IL 60612; [email protected].
1. Miyakis S, Karamanof G, Liontos M, et al. Factors contributing to inappropriate ordering of tests in an academic medical department and the effect of an educational feedback strategy. Postgrad Med J. 2006;82:823-829.
2. Morgan DJ, Dhruva SS, Wright SM, et al. Update on medical overuse: a systematic review. JAMA Intern Med. 2016;176:1687-1692.
3. Durante C, Costante G, Lucisano G, et al. The natural history of benign thyroid nodules. JAMA. 2015;313:926-935.
4. Choosing Wisely. Society of Hospital Medicine—Pediatric hospital medicine. Don’t use continuous pulse oximetry routinely in children with acute respiratory illness unless they are on supplemental oxygen. www.choosingwisely.org/clinician-lists/society-hospital-medicine-pediatric-continuous-pulse-oximetry-in-children-with-acute-respiratory-illness/. Accessed September 28, 2020.
5. Schondelmeyer AC, Simmons JM, Statile AM, et al. Using quality improvement to reduce continuous pulse oximetry use in children with wheezing. Pediatrics. 2015;135:e1044-e1051.
6. Kravitz RL, Bell RA, Azari R, et al. Direct observation of requests for clinical services in office practice: what do patients want and do they get it? Arch Intern Med. 2003;163:1673-1681.
7. Kravitz RL, Bell RA, Franz CE, et al. Characterizing patient requests and physician responses in office practice. Health Serv Res. 2002;37:217-238.
8. Choosing Wisely. American Academy of Family Physicians. Don’t do imaging for low back pain within the first six weeks, unless red flags are present. www.choosingwisely.org/clinician-lists/american-academy-family-physicians-imaging-low-back-pain/. Accessed September 28, 2020.
9. Choosing Wisely. American Society of Anesthesiologists–Pain Medicine. Avoid imaging studies (MRI, CT or X-rays) for acute low back pain without specific indications. www.choosingwisely.org/clinician-lists/american-society-anesthesiologists-imaging-studies-for-acute-low-back-pain/. Accessed September 28, 2020.
10. Choosing Wisely. American College of Emergency Physicians. Avoid lumbar spine imaging in the emergency department for adults with non-traumatic back pain unless the patient has severe or progressive neurologic deficits or is suspected of having a serious underlying condition (such as vertebral infection, cauda equina syndrome, or cancer with bony metastasis). www.choosingwisely.org/clinician-lists/acep-lumbar-spine-imaging-in-the-ed/. Accessed September 28, 2020.
11. Choosing Wisely. American Academy of Family Physicians. Don’t require a pelvic exam or other physical exam to prescribe oral contraceptive medications. www.choosingwisely.org/clinician-lists/american-academy-family-physicians-pelvic-or-physical-exams-to-prescribe-oral-contraceptives/. Accessed September 28, 2020.
12. Over-the-counter access to hormonal contraception. ACOG Committee Opinion, Number 788. Obstet Gynecol. 2019;134:e96-e105. https://journals.lww.com/greenjournal/Fulltext/2019/10000/Over_the_Counter_Access_to_Hormonal_Contraception_.46.aspx. Accessed September 28, 2020.
13. Choosing Wisely. American Academy of Family Physicians. Don’t routinely recommend daily home glucose monitoring for patients who have Type 2 diabetes mellitus and are not using insulin. www.choosingwisely.org/clinician-lists/aafp-daily-home-glucose-monitoring-for-patients-with-type-2-diabetes. Accessed September 28, 2020.
14. Choosing Wisely. Society of General Internal Medicine. Don’t recommend daily home finger glucose testing in patients with Type 2 diabetes mellitus not using insulin. www.choosingwisely.org/clinician-lists/society-general-internal-medicine-daily-home-finger-glucose-testing-type-2-diabetes-mellitus/. Accessed September 28, 2020.
15. Malanda UL, Welschen LM, Riphagen II, et al. Self‐monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. Cochrane Database Syst Rev. 2012(1):CD005060.
16. Peel E, Douglas M, Lawton J. Self monitoring of blood glucose in type 2 diabetes: longitudinal qualitative study of patients’ perspectives. BMJ. 2007;335:493.
17. Choosing Wisely. American Academy of Family Physicians. Don’t screen for genital herpes simplex virus infection (HSV) in asymptomatic adults, including pregnant women. www.choosingwisely.org/clinician-lists/aafp-genital-herpes-screening-in-asymptomatic-adults/. Accessed September 28, 2020.
18. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Serologic screening for genital herpes infection: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:2525-2530.
19. Choosing Wisely. American Academy of Family Physicians. Don’t screen for testicular cancer in asymptomatic adolescent and adult males. www.choosingwisely.org/clinician-lists/aafp-testicular-cancer-screening-in-asymptomatic-adolescent-and-adult-men/. Accessed September 28, 2020.
20. Lin K, Sharangpani R. Screening for testicular cancer: an evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2010;153:396-399.
21. Choosing Wisely. American Academy of Pediatrics. Cough and cold medicines should not be prescribed, recommended or used for respiratory illnesses in young children. www.choosingwisely.org/clinician-lists/american-academy-pediatrics-cough-and-cold-medicines-for-children-under-four/. Accessed September 28, 2020.
22. Schaefer MK, Shehab N, Cohen AL, et al. Adverse events from cough and cold medications in children. Pediatrics. 2008;121:783-787.
23. Carr BC. Efficacy, abuse, and toxicity of over-the-counter cough and cold medicines in the pediatric population. Curr Opin Pediatr. 2006;18:184-188.
24. Oduwole O, Udoh EE, Oyo‐Ita A, et al. Honey for acute cough in children. Cochrane Database Syst Rev. 2018(4):CD007094.
25. Choosing Wisely. American Academy of Allergy, Asthma & Immunology. Don’t perform unproven diagnostic tests, such as immunoglobulin G(lgG) testing or an indiscriminate battery of immunoglobulin E(lgE) tests, in the evaluation of allergy. www.choosingwisely.org/clinician-lists/american-academy-allergy-asthma-immunology-diagnostic-tests-for-allergy-evaluation/. Accessed September 28, 2020.
26. Cox L, Williams B, Sicherer S, et al. Pearls and pitfalls of allergy diagnostic testing: report from the American College of Allergy, Asthma and Immunology Specific IgE Test Task Force. Ann Allergy Asthma Immunol. 2008;101:580-592.
27. Choosing Wisely. American Epilepsy Society. Do not routinely order electroencephalogram (EEG) as part of initial syncope work-up. www.choosingwisely.org/clinician-lists/aes-eeg-as-part-of-initial-syncope-work-up/. Accessed September 28, 2020.
28. Choosing Wisely. American College of Emergency Physicians. Avoid CT of the head in asymptomatic adult patients in the emergency department with syncope, insignificant trauma and a normal neurological evaluation. www.choosingwisely.org/clinician-lists/acep-avoid-head-ct-for-asymptomatic-adults-with-syncope/. Accessed September 28, 2020.
29. Choosing Wisely. American College of Physicians. In the evaluation of simple syncope and a normal neurological examination, don’t obtain brain imaging studies (CT or MRI). www.choosingwisely.org/clinician-lists/american-college-physicians-brain-imaging-to-evaluate-simple-syncope/. Accessed September 28, 2020.
30. Choosing Wisely. American Academy of Neurology. Don’t perform imaging of the carotid arteries for simple syncope without other neurologic symptoms. www.choosingwisely.org/clinician-lists/american-academy-neurology-carotid-artery-imaging-for-simple-syncope/. Accessed September 28, 2020.
31. Josephson CB, Rahey S, Sadler RM. Neurocardiogenic syncope: frequency and consequences of its misdiagnosis as epilepsy. Can J Neurol Sci. 2007;34:221-224.
32. Mendu ML, McAvay G, Lampert R, et al. Yield of diagnostic tests in evaluating syncopal episodes in older patients. Arch Intern Med. 2009;169:1299-1305.
33. Choosing Wisely. American Academy of Pediatrics–Section on Surgery. Avoid referring most children with umbilical hernias to a pediatric surgeon until around age 4-5 years. www.choosingwisely.org/clinician-lists/aap-sosu-avoid-surgery-referral-for-umbilical-hernias-until-age-4-5/. Accessed September 28, 2020.
34. Antonoff MB, Kreykes NS, Saltzman DA, et al. American Academy of Pediatrics Section on Surgery hernia survey revisited. J Pediatr Surg. 2005;40:1009-1014.
35. Choosing Wisely. American Geriatrics Society. Avoid using prescription appetite stimulants or high-calorie supplements for treatment of anorexia or cachexia in older adults; instead, optimize social supports, discontinue medications that may interfere with eating, provide appealing food and feeding assistance, and clarify patient goals and expectations. www.choosingwisely.org/clinician-lists/american-geriatrics-society-prescription-appetite-stimulants-to-treat-anorexia-cachexia-in-elderly/. Accessed September 28, 2020.
36. Milne AC, Potter J, Vivanti A, et al. Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane Database Sys Rev. 2009(2):CD003288.
37. Fox CB, Treadway AK, Blaszczyk AT, et al. Megestrol acetate and mirtazapine for the treatment of unplanned weight loss in the elderly. Pharmacotherapy. 2009;29:383-397.
When medical care is based on consistent, good-quality evidence, most physicians adopt it. However, not all care is well supported by the literature and may, in fact, be overused without offering benefit to patients. Choosing Wisely, at www.choosingwisely.org, is a health care initiative that highlights screening and testing recommendations from specialty societies in an effort to encourage patients and clinicians to talk about how to make high-value, effective health care decisions and avoid overuse. (See “Test and Tx overutilization: A bigger problem than you might think"1-3).
SIDEBAR
Test and Tx overutilization: A bigger problem than you might think
Care that isn’t backed up by the medical literature is adopted by some physicians and not adopted by others, leading to practice variations. Some variation is to be expected, since no 2 patients require exactly the same care, but substantial variations may be a clue to overuse.
A 2006 analysis of inpatient lab studies found that doctors ordered an average of 2.96 studies per patient per day, but only 29% of these tests (0.95 test/patient/day) contributed to management.1 A 2016 systematic review found more than 800 studies on overuse were published in a single year.2 One study of thyroid nodules followed almost 1000 patients with nodules as they underwent routine follow-up imaging. At the end of the study, 7 were found to have cancer, but of those, only 3 had enlarging or changing nodules that would have been detected with the follow-up imaging being studied. Three of the cancers were stable in size and 1 was found incidentally.3
Enabling physician and patient dialogue. The initiative began in 2010 when the American Board of Internal Medicine convened a panel of experts to identify low-value tests and therapies. Their list took the form of a “Top Five Things” that may not be high value in patient care, and it used language tailored to patients and physicians so that they could converse meaningfully. Physicians could use the evidence to make a clinical decision, and patients could feel empowered to ask informed questions about recommendations they received. The initiative has now expanded to include ways that health care systems can reduce low-value interventions.

Scope of participation. Since the first Choosing Wisely recommendations were published in 2013, more than 80 professional associations have contributed lists of their own. Professional societies participate voluntarily. The American Academy of Family Physicians (AAFP), Society of General Internal Medicine, and American Academy of Pediatrics (AAP) have contributed lists relevant to primary care. All Choosing Wisely recommendations can be searched or sorted by specialty organization. Recommendations are reviewed and revised regularly. If the evidence becomes conflicted or contradictory, recommendations are withdrawn.
Making meaningful improvements by Choosing Wisely
Several studies have shown that health care systems can implement Choosing Wisely recommendations to reduce overuse of unnecessary tests. A 2015 study examined the effect of applying a Choosing Wisely recommendation to reduce the use of continuous pulse oximetry in pediatric inpatients with asthma, wheezing, or bronchiolitis. The recommendation, from the Society of Hospital Medicine–Pediatric Hospital Medicine, advises against continuous pulse oximetry in children with acute respiratory illnesses unless the child is using supplemental oxygen.4 This study, done at the Cincinnati Children’s Hospital Medical Center, found that within 3 months of initiating a protocol on all general pediatrics floors, the average time on pulse oximetry after meeting clinical goals decreased from 10.7 hours to 3.1 hours. In addition, the percentage of patients who had their continuous pulse oximetry stopped within 2 hours of clinical stability (a goal time) increased from 25% to 46%.5
Patients are important drivers of health care utilization. A 2003 study showed that physicians are more likely to order referrals, tests, and prescriptions when patients ask for them, and that nearly 1 in 4 patients did so.6 A 2002 study found that physicians granted all but 3% of patient’s requests for orders or tests, and that fulfilling requests correlated with patient satisfaction in the specialty office studied (cardiology) but not in the primary care (internal medicine) office.7
From its inception, Choosing Wisely has considered patients as full partners in conversations about health care utilization. Choosing Wisely partners with Consumer Reports to create and disseminate plain-language summaries of recommendations. Community groups and physician organizations have also participated in implementation efforts. In 2018, Choosing Wisely secured a grant to expand outreach to diverse or underserved communities.
Choosing Wisely recommendations are not guidelines or mandates. They are intended to be evidence-based advice from a specialty society to its members and to patients about care that is often unnecessary. The goal is to create a conversation and not to eliminate these services from ever being offered or used.
Continue to: Improve your practice with these 10 primary care recommendations
Improve your practice with these 10 primary care recommendations
1 Avoid imaging studies in early acute low back pain without red flags.
Both the AAFP and the American Society of Anesthesiologists recommend against routine X-rays, magnetic resonance imaging, and computed tomography (CT) scans in the first 6 weeks of acute low back pain (LBP).8,9 The American College of Emergency Physicians (ACEP) recommends against routine lumbar spine imaging for emergency department (ED) patients.10 In all cases, imaging is indicated if the patient has any signs or symptoms of neurologic deficits or other indications, such as signs of spinal infection or fracture. However, as ACEP notes, diagnostic imaging does not typically help identify the cause of acute LBP, and when it does, it does not reduce the time to symptom improvement.10
2 Prescribe oral contraceptives on the basis of a medical history and a blood pressure measurement. No routine pelvic exam or other physical exam is necessary.
This AAFP recommendation11 is based on clinical practice guidelines from the American College of Obstetricians and Gynecologists (ACOG) and other research.12 The ACOG practice guideline supports provision of hormonal contraception without a pelvic exam, cervical cancer (Pap) testing, urine pregnancy testing, or testing for sexually transmitted infections. ACOG guidelines also support over-the-counter provision of hormonal contraceptives, including combined oral contraceptives.12
3 Stop recommending daily self-glucose monitoring for patients with diabetes who are not using insulin.
Both the AAFP and the Society for General Internal Medicine recommend against daily blood sugar checks for people who do not use insulin.13,14 A Cochrane review of 9 trials (3300 patients) found that after 6 months, hemoglobin A1C was reduced by 0.3% in people who checked their sugar daily compared with those who did not, but this difference was not significant after a year.15 Hypoglycemic episodes were more common in the “checking” group, and there were no differences in quality of life. A qualitative study found that blood sugar results had little impact on patients’ motivation to change behavior.16
4 Don’t screen for herpes simplex virus (HSV) infection in asymptomatic adults, even those who are pregnant.
This AAFP recommendation17 comes from a US Preventive Services Task Force (USPSTF) Grade D recommendation.18 Most people with positive HSV-2 serology have had an outbreak; even those who do not think they have had one will realize that they had the symptoms once they hear them described.18 With available tests, 1 in 2 positive results for HSV-2 among asymptomatic people will be a false-positive.18
There is no known cure, intervention, or reduction in transmission for infected patients who do not have symptoms.18 Also, serologically detected HSV-2 does not reliably predict genital herpes; and HSV-1 has been found to cause an increasing percentage of genital infection cases.18
Continue to: 5 Don't screen for testicular cancer in asymptomatic individuals
5 Don’t screen for testicular cancer in asymptomatic individuals.
This AAFP recommendation19 also comes from a USPSTF Grade D recommendation.20 A 2010 systematic review found no evidence to support screening of asymptomatic people with a physical exam or ultrasound. All available studies involved symptomatic patients.20
6 Stop recommending cough and cold medicines for children younger than 4 years.
The AAP recommends that clinicians discourage the use of any cough or cold medicine for children in this age-group.21 A 2008 study found that more than 7000 children annually presented to EDs for adverse events from cough and cold medicines.22 Previous studies found no benefit in reducing symptoms.23 In children older than 12 months, a Cochrane review found that honey has a modest benefit for cough in single-night trials.24
7 Avoid performing serum allergy panels.
The American Academy of Allergy, Asthma, and Immunology discourages the use of serum panel testing when patients present with allergy symptoms.25 A patient can have a strong positive immunoglobulin E (IgE) serum result to an allergen and have no clinical allergic symptoms or can have a weak positive serum result and a strong clinical reaction. Targeted skin or serum IgE testing—for example, testing for cashew allergy in a patient known to have had a reaction after eating one—is reasonable.26
8 Avoid routine electroencephalography (EEG), head CT, and carotid ultrasound as initial work-up for simple syncope in adults.
These recommendations, from the American Epilepsy Society,27 ACEP,28 American College of Physicians,29 and American Academy of Neurology (AAN),30 emphasize the low yield of routine work-ups for patients with simple syncope. The AAN notes that 40% of people will experience syncope during adulthood and most will not have carotid disease, which generally manifests with stroke-like symptoms rather than syncope. One study found that approximately 1 in 8 patients referred to an epilepsy clinic had neurocardiogenic syncope rather than epilepsy.31
EEGs have high false-negative and false-positive rates, and history-taking is a better tool with which to make a diagnosis. CT scans performed in the ED were found to contribute to the diagnosis of simple syncope in fewer than 2% of cases of syncope, compared with orthostatic blood pressure (25% of cases).32
Continue to: 9 Wait to refer children with umbilical hernias to pediatric surgery until they are 4 to 5 years of age
9 Wait to refer children with umbilical hernias to pediatric surgery until they are 4 to 5 years of age.
The AAP Section on Surgery offers evidence that the risk-benefit analysis strongly favors waiting on intervention.33 About 1 in 4 children will have an umbilical hernia, and about 85% of cases will resolve by age 5. The strangulation rate with umbilical hernias is very low, and although the risk of infection with surgery is likewise low, the risk of recurrence following surgery before the age of 4 is as high as 2.4%.34 The AAP Section on Surgery recommends against strapping or restraining the hernia, as well.
10 Avoid using appetite stimulants, such as megesterol, and high-calorie nutritional supplements to treat anorexia and cachexia in older adults.
Instead, the American Geriatrics Society recommends that physicians encourage caregivers to serve appealing food, provide support with eating, and remove barriers to appetite and nutrition.35 A Cochrane review showed that high-calorie supplements, such as Boost or Ensure, are associated with very modest weight gain—about 2% of weight—but are not associated with an increased life expectancy or improved quality of life.36
Prescription appetite stimulants are associated with adverse effects and yield inconsistent benefits in older adults. Megesterol, for example, was associated with headache, gastrointestinal adverse effects, insomnia, weakness, and fatigue. Mirtazapine is associated with sedation and fatigue.37
CORRESPONDENCE
Kathleen Rowland, MD, MS, Rush Copley Family Medicine Residency, Rush Medical College, 600 South Paulina, Kidston House Room 605, Chicago IL 60612; [email protected].
When medical care is based on consistent, good-quality evidence, most physicians adopt it. However, not all care is well supported by the literature and may, in fact, be overused without offering benefit to patients. Choosing Wisely, at www.choosingwisely.org, is a health care initiative that highlights screening and testing recommendations from specialty societies in an effort to encourage patients and clinicians to talk about how to make high-value, effective health care decisions and avoid overuse. (See “Test and Tx overutilization: A bigger problem than you might think"1-3).
SIDEBAR
Test and Tx overutilization: A bigger problem than you might think
Care that isn’t backed up by the medical literature is adopted by some physicians and not adopted by others, leading to practice variations. Some variation is to be expected, since no 2 patients require exactly the same care, but substantial variations may be a clue to overuse.
A 2006 analysis of inpatient lab studies found that doctors ordered an average of 2.96 studies per patient per day, but only 29% of these tests (0.95 test/patient/day) contributed to management.1 A 2016 systematic review found more than 800 studies on overuse were published in a single year.2 One study of thyroid nodules followed almost 1000 patients with nodules as they underwent routine follow-up imaging. At the end of the study, 7 were found to have cancer, but of those, only 3 had enlarging or changing nodules that would have been detected with the follow-up imaging being studied. Three of the cancers were stable in size and 1 was found incidentally.3
Enabling physician and patient dialogue. The initiative began in 2010 when the American Board of Internal Medicine convened a panel of experts to identify low-value tests and therapies. Their list took the form of a “Top Five Things” that may not be high value in patient care, and it used language tailored to patients and physicians so that they could converse meaningfully. Physicians could use the evidence to make a clinical decision, and patients could feel empowered to ask informed questions about recommendations they received. The initiative has now expanded to include ways that health care systems can reduce low-value interventions.

Scope of participation. Since the first Choosing Wisely recommendations were published in 2013, more than 80 professional associations have contributed lists of their own. Professional societies participate voluntarily. The American Academy of Family Physicians (AAFP), Society of General Internal Medicine, and American Academy of Pediatrics (AAP) have contributed lists relevant to primary care. All Choosing Wisely recommendations can be searched or sorted by specialty organization. Recommendations are reviewed and revised regularly. If the evidence becomes conflicted or contradictory, recommendations are withdrawn.
Making meaningful improvements by Choosing Wisely
Several studies have shown that health care systems can implement Choosing Wisely recommendations to reduce overuse of unnecessary tests. A 2015 study examined the effect of applying a Choosing Wisely recommendation to reduce the use of continuous pulse oximetry in pediatric inpatients with asthma, wheezing, or bronchiolitis. The recommendation, from the Society of Hospital Medicine–Pediatric Hospital Medicine, advises against continuous pulse oximetry in children with acute respiratory illnesses unless the child is using supplemental oxygen.4 This study, done at the Cincinnati Children’s Hospital Medical Center, found that within 3 months of initiating a protocol on all general pediatrics floors, the average time on pulse oximetry after meeting clinical goals decreased from 10.7 hours to 3.1 hours. In addition, the percentage of patients who had their continuous pulse oximetry stopped within 2 hours of clinical stability (a goal time) increased from 25% to 46%.5
Patients are important drivers of health care utilization. A 2003 study showed that physicians are more likely to order referrals, tests, and prescriptions when patients ask for them, and that nearly 1 in 4 patients did so.6 A 2002 study found that physicians granted all but 3% of patient’s requests for orders or tests, and that fulfilling requests correlated with patient satisfaction in the specialty office studied (cardiology) but not in the primary care (internal medicine) office.7
From its inception, Choosing Wisely has considered patients as full partners in conversations about health care utilization. Choosing Wisely partners with Consumer Reports to create and disseminate plain-language summaries of recommendations. Community groups and physician organizations have also participated in implementation efforts. In 2018, Choosing Wisely secured a grant to expand outreach to diverse or underserved communities.
Choosing Wisely recommendations are not guidelines or mandates. They are intended to be evidence-based advice from a specialty society to its members and to patients about care that is often unnecessary. The goal is to create a conversation and not to eliminate these services from ever being offered or used.
Continue to: Improve your practice with these 10 primary care recommendations
Improve your practice with these 10 primary care recommendations
1 Avoid imaging studies in early acute low back pain without red flags.
Both the AAFP and the American Society of Anesthesiologists recommend against routine X-rays, magnetic resonance imaging, and computed tomography (CT) scans in the first 6 weeks of acute low back pain (LBP).8,9 The American College of Emergency Physicians (ACEP) recommends against routine lumbar spine imaging for emergency department (ED) patients.10 In all cases, imaging is indicated if the patient has any signs or symptoms of neurologic deficits or other indications, such as signs of spinal infection or fracture. However, as ACEP notes, diagnostic imaging does not typically help identify the cause of acute LBP, and when it does, it does not reduce the time to symptom improvement.10
2 Prescribe oral contraceptives on the basis of a medical history and a blood pressure measurement. No routine pelvic exam or other physical exam is necessary.
This AAFP recommendation11 is based on clinical practice guidelines from the American College of Obstetricians and Gynecologists (ACOG) and other research.12 The ACOG practice guideline supports provision of hormonal contraception without a pelvic exam, cervical cancer (Pap) testing, urine pregnancy testing, or testing for sexually transmitted infections. ACOG guidelines also support over-the-counter provision of hormonal contraceptives, including combined oral contraceptives.12
3 Stop recommending daily self-glucose monitoring for patients with diabetes who are not using insulin.
Both the AAFP and the Society for General Internal Medicine recommend against daily blood sugar checks for people who do not use insulin.13,14 A Cochrane review of 9 trials (3300 patients) found that after 6 months, hemoglobin A1C was reduced by 0.3% in people who checked their sugar daily compared with those who did not, but this difference was not significant after a year.15 Hypoglycemic episodes were more common in the “checking” group, and there were no differences in quality of life. A qualitative study found that blood sugar results had little impact on patients’ motivation to change behavior.16
4 Don’t screen for herpes simplex virus (HSV) infection in asymptomatic adults, even those who are pregnant.
This AAFP recommendation17 comes from a US Preventive Services Task Force (USPSTF) Grade D recommendation.18 Most people with positive HSV-2 serology have had an outbreak; even those who do not think they have had one will realize that they had the symptoms once they hear them described.18 With available tests, 1 in 2 positive results for HSV-2 among asymptomatic people will be a false-positive.18
There is no known cure, intervention, or reduction in transmission for infected patients who do not have symptoms.18 Also, serologically detected HSV-2 does not reliably predict genital herpes; and HSV-1 has been found to cause an increasing percentage of genital infection cases.18
Continue to: 5 Don't screen for testicular cancer in asymptomatic individuals
5 Don’t screen for testicular cancer in asymptomatic individuals.
This AAFP recommendation19 also comes from a USPSTF Grade D recommendation.20 A 2010 systematic review found no evidence to support screening of asymptomatic people with a physical exam or ultrasound. All available studies involved symptomatic patients.20
6 Stop recommending cough and cold medicines for children younger than 4 years.
The AAP recommends that clinicians discourage the use of any cough or cold medicine for children in this age-group.21 A 2008 study found that more than 7000 children annually presented to EDs for adverse events from cough and cold medicines.22 Previous studies found no benefit in reducing symptoms.23 In children older than 12 months, a Cochrane review found that honey has a modest benefit for cough in single-night trials.24
7 Avoid performing serum allergy panels.
The American Academy of Allergy, Asthma, and Immunology discourages the use of serum panel testing when patients present with allergy symptoms.25 A patient can have a strong positive immunoglobulin E (IgE) serum result to an allergen and have no clinical allergic symptoms or can have a weak positive serum result and a strong clinical reaction. Targeted skin or serum IgE testing—for example, testing for cashew allergy in a patient known to have had a reaction after eating one—is reasonable.26
8 Avoid routine electroencephalography (EEG), head CT, and carotid ultrasound as initial work-up for simple syncope in adults.
These recommendations, from the American Epilepsy Society,27 ACEP,28 American College of Physicians,29 and American Academy of Neurology (AAN),30 emphasize the low yield of routine work-ups for patients with simple syncope. The AAN notes that 40% of people will experience syncope during adulthood and most will not have carotid disease, which generally manifests with stroke-like symptoms rather than syncope. One study found that approximately 1 in 8 patients referred to an epilepsy clinic had neurocardiogenic syncope rather than epilepsy.31
EEGs have high false-negative and false-positive rates, and history-taking is a better tool with which to make a diagnosis. CT scans performed in the ED were found to contribute to the diagnosis of simple syncope in fewer than 2% of cases of syncope, compared with orthostatic blood pressure (25% of cases).32
Continue to: 9 Wait to refer children with umbilical hernias to pediatric surgery until they are 4 to 5 years of age
9 Wait to refer children with umbilical hernias to pediatric surgery until they are 4 to 5 years of age.
The AAP Section on Surgery offers evidence that the risk-benefit analysis strongly favors waiting on intervention.33 About 1 in 4 children will have an umbilical hernia, and about 85% of cases will resolve by age 5. The strangulation rate with umbilical hernias is very low, and although the risk of infection with surgery is likewise low, the risk of recurrence following surgery before the age of 4 is as high as 2.4%.34 The AAP Section on Surgery recommends against strapping or restraining the hernia, as well.
10 Avoid using appetite stimulants, such as megesterol, and high-calorie nutritional supplements to treat anorexia and cachexia in older adults.
Instead, the American Geriatrics Society recommends that physicians encourage caregivers to serve appealing food, provide support with eating, and remove barriers to appetite and nutrition.35 A Cochrane review showed that high-calorie supplements, such as Boost or Ensure, are associated with very modest weight gain—about 2% of weight—but are not associated with an increased life expectancy or improved quality of life.36
Prescription appetite stimulants are associated with adverse effects and yield inconsistent benefits in older adults. Megesterol, for example, was associated with headache, gastrointestinal adverse effects, insomnia, weakness, and fatigue. Mirtazapine is associated with sedation and fatigue.37
CORRESPONDENCE
Kathleen Rowland, MD, MS, Rush Copley Family Medicine Residency, Rush Medical College, 600 South Paulina, Kidston House Room 605, Chicago IL 60612; [email protected].
1. Miyakis S, Karamanof G, Liontos M, et al. Factors contributing to inappropriate ordering of tests in an academic medical department and the effect of an educational feedback strategy. Postgrad Med J. 2006;82:823-829.
2. Morgan DJ, Dhruva SS, Wright SM, et al. Update on medical overuse: a systematic review. JAMA Intern Med. 2016;176:1687-1692.
3. Durante C, Costante G, Lucisano G, et al. The natural history of benign thyroid nodules. JAMA. 2015;313:926-935.
4. Choosing Wisely. Society of Hospital Medicine—Pediatric hospital medicine. Don’t use continuous pulse oximetry routinely in children with acute respiratory illness unless they are on supplemental oxygen. www.choosingwisely.org/clinician-lists/society-hospital-medicine-pediatric-continuous-pulse-oximetry-in-children-with-acute-respiratory-illness/. Accessed September 28, 2020.
5. Schondelmeyer AC, Simmons JM, Statile AM, et al. Using quality improvement to reduce continuous pulse oximetry use in children with wheezing. Pediatrics. 2015;135:e1044-e1051.
6. Kravitz RL, Bell RA, Azari R, et al. Direct observation of requests for clinical services in office practice: what do patients want and do they get it? Arch Intern Med. 2003;163:1673-1681.
7. Kravitz RL, Bell RA, Franz CE, et al. Characterizing patient requests and physician responses in office practice. Health Serv Res. 2002;37:217-238.
8. Choosing Wisely. American Academy of Family Physicians. Don’t do imaging for low back pain within the first six weeks, unless red flags are present. www.choosingwisely.org/clinician-lists/american-academy-family-physicians-imaging-low-back-pain/. Accessed September 28, 2020.
9. Choosing Wisely. American Society of Anesthesiologists–Pain Medicine. Avoid imaging studies (MRI, CT or X-rays) for acute low back pain without specific indications. www.choosingwisely.org/clinician-lists/american-society-anesthesiologists-imaging-studies-for-acute-low-back-pain/. Accessed September 28, 2020.
10. Choosing Wisely. American College of Emergency Physicians. Avoid lumbar spine imaging in the emergency department for adults with non-traumatic back pain unless the patient has severe or progressive neurologic deficits or is suspected of having a serious underlying condition (such as vertebral infection, cauda equina syndrome, or cancer with bony metastasis). www.choosingwisely.org/clinician-lists/acep-lumbar-spine-imaging-in-the-ed/. Accessed September 28, 2020.
11. Choosing Wisely. American Academy of Family Physicians. Don’t require a pelvic exam or other physical exam to prescribe oral contraceptive medications. www.choosingwisely.org/clinician-lists/american-academy-family-physicians-pelvic-or-physical-exams-to-prescribe-oral-contraceptives/. Accessed September 28, 2020.
12. Over-the-counter access to hormonal contraception. ACOG Committee Opinion, Number 788. Obstet Gynecol. 2019;134:e96-e105. https://journals.lww.com/greenjournal/Fulltext/2019/10000/Over_the_Counter_Access_to_Hormonal_Contraception_.46.aspx. Accessed September 28, 2020.
13. Choosing Wisely. American Academy of Family Physicians. Don’t routinely recommend daily home glucose monitoring for patients who have Type 2 diabetes mellitus and are not using insulin. www.choosingwisely.org/clinician-lists/aafp-daily-home-glucose-monitoring-for-patients-with-type-2-diabetes. Accessed September 28, 2020.
14. Choosing Wisely. Society of General Internal Medicine. Don’t recommend daily home finger glucose testing in patients with Type 2 diabetes mellitus not using insulin. www.choosingwisely.org/clinician-lists/society-general-internal-medicine-daily-home-finger-glucose-testing-type-2-diabetes-mellitus/. Accessed September 28, 2020.
15. Malanda UL, Welschen LM, Riphagen II, et al. Self‐monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. Cochrane Database Syst Rev. 2012(1):CD005060.
16. Peel E, Douglas M, Lawton J. Self monitoring of blood glucose in type 2 diabetes: longitudinal qualitative study of patients’ perspectives. BMJ. 2007;335:493.
17. Choosing Wisely. American Academy of Family Physicians. Don’t screen for genital herpes simplex virus infection (HSV) in asymptomatic adults, including pregnant women. www.choosingwisely.org/clinician-lists/aafp-genital-herpes-screening-in-asymptomatic-adults/. Accessed September 28, 2020.
18. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Serologic screening for genital herpes infection: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:2525-2530.
19. Choosing Wisely. American Academy of Family Physicians. Don’t screen for testicular cancer in asymptomatic adolescent and adult males. www.choosingwisely.org/clinician-lists/aafp-testicular-cancer-screening-in-asymptomatic-adolescent-and-adult-men/. Accessed September 28, 2020.
20. Lin K, Sharangpani R. Screening for testicular cancer: an evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2010;153:396-399.
21. Choosing Wisely. American Academy of Pediatrics. Cough and cold medicines should not be prescribed, recommended or used for respiratory illnesses in young children. www.choosingwisely.org/clinician-lists/american-academy-pediatrics-cough-and-cold-medicines-for-children-under-four/. Accessed September 28, 2020.
22. Schaefer MK, Shehab N, Cohen AL, et al. Adverse events from cough and cold medications in children. Pediatrics. 2008;121:783-787.
23. Carr BC. Efficacy, abuse, and toxicity of over-the-counter cough and cold medicines in the pediatric population. Curr Opin Pediatr. 2006;18:184-188.
24. Oduwole O, Udoh EE, Oyo‐Ita A, et al. Honey for acute cough in children. Cochrane Database Syst Rev. 2018(4):CD007094.
25. Choosing Wisely. American Academy of Allergy, Asthma & Immunology. Don’t perform unproven diagnostic tests, such as immunoglobulin G(lgG) testing or an indiscriminate battery of immunoglobulin E(lgE) tests, in the evaluation of allergy. www.choosingwisely.org/clinician-lists/american-academy-allergy-asthma-immunology-diagnostic-tests-for-allergy-evaluation/. Accessed September 28, 2020.
26. Cox L, Williams B, Sicherer S, et al. Pearls and pitfalls of allergy diagnostic testing: report from the American College of Allergy, Asthma and Immunology Specific IgE Test Task Force. Ann Allergy Asthma Immunol. 2008;101:580-592.
27. Choosing Wisely. American Epilepsy Society. Do not routinely order electroencephalogram (EEG) as part of initial syncope work-up. www.choosingwisely.org/clinician-lists/aes-eeg-as-part-of-initial-syncope-work-up/. Accessed September 28, 2020.
28. Choosing Wisely. American College of Emergency Physicians. Avoid CT of the head in asymptomatic adult patients in the emergency department with syncope, insignificant trauma and a normal neurological evaluation. www.choosingwisely.org/clinician-lists/acep-avoid-head-ct-for-asymptomatic-adults-with-syncope/. Accessed September 28, 2020.
29. Choosing Wisely. American College of Physicians. In the evaluation of simple syncope and a normal neurological examination, don’t obtain brain imaging studies (CT or MRI). www.choosingwisely.org/clinician-lists/american-college-physicians-brain-imaging-to-evaluate-simple-syncope/. Accessed September 28, 2020.
30. Choosing Wisely. American Academy of Neurology. Don’t perform imaging of the carotid arteries for simple syncope without other neurologic symptoms. www.choosingwisely.org/clinician-lists/american-academy-neurology-carotid-artery-imaging-for-simple-syncope/. Accessed September 28, 2020.
31. Josephson CB, Rahey S, Sadler RM. Neurocardiogenic syncope: frequency and consequences of its misdiagnosis as epilepsy. Can J Neurol Sci. 2007;34:221-224.
32. Mendu ML, McAvay G, Lampert R, et al. Yield of diagnostic tests in evaluating syncopal episodes in older patients. Arch Intern Med. 2009;169:1299-1305.
33. Choosing Wisely. American Academy of Pediatrics–Section on Surgery. Avoid referring most children with umbilical hernias to a pediatric surgeon until around age 4-5 years. www.choosingwisely.org/clinician-lists/aap-sosu-avoid-surgery-referral-for-umbilical-hernias-until-age-4-5/. Accessed September 28, 2020.
34. Antonoff MB, Kreykes NS, Saltzman DA, et al. American Academy of Pediatrics Section on Surgery hernia survey revisited. J Pediatr Surg. 2005;40:1009-1014.
35. Choosing Wisely. American Geriatrics Society. Avoid using prescription appetite stimulants or high-calorie supplements for treatment of anorexia or cachexia in older adults; instead, optimize social supports, discontinue medications that may interfere with eating, provide appealing food and feeding assistance, and clarify patient goals and expectations. www.choosingwisely.org/clinician-lists/american-geriatrics-society-prescription-appetite-stimulants-to-treat-anorexia-cachexia-in-elderly/. Accessed September 28, 2020.
36. Milne AC, Potter J, Vivanti A, et al. Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane Database Sys Rev. 2009(2):CD003288.
37. Fox CB, Treadway AK, Blaszczyk AT, et al. Megestrol acetate and mirtazapine for the treatment of unplanned weight loss in the elderly. Pharmacotherapy. 2009;29:383-397.
1. Miyakis S, Karamanof G, Liontos M, et al. Factors contributing to inappropriate ordering of tests in an academic medical department and the effect of an educational feedback strategy. Postgrad Med J. 2006;82:823-829.
2. Morgan DJ, Dhruva SS, Wright SM, et al. Update on medical overuse: a systematic review. JAMA Intern Med. 2016;176:1687-1692.
3. Durante C, Costante G, Lucisano G, et al. The natural history of benign thyroid nodules. JAMA. 2015;313:926-935.
4. Choosing Wisely. Society of Hospital Medicine—Pediatric hospital medicine. Don’t use continuous pulse oximetry routinely in children with acute respiratory illness unless they are on supplemental oxygen. www.choosingwisely.org/clinician-lists/society-hospital-medicine-pediatric-continuous-pulse-oximetry-in-children-with-acute-respiratory-illness/. Accessed September 28, 2020.
5. Schondelmeyer AC, Simmons JM, Statile AM, et al. Using quality improvement to reduce continuous pulse oximetry use in children with wheezing. Pediatrics. 2015;135:e1044-e1051.
6. Kravitz RL, Bell RA, Azari R, et al. Direct observation of requests for clinical services in office practice: what do patients want and do they get it? Arch Intern Med. 2003;163:1673-1681.
7. Kravitz RL, Bell RA, Franz CE, et al. Characterizing patient requests and physician responses in office practice. Health Serv Res. 2002;37:217-238.
8. Choosing Wisely. American Academy of Family Physicians. Don’t do imaging for low back pain within the first six weeks, unless red flags are present. www.choosingwisely.org/clinician-lists/american-academy-family-physicians-imaging-low-back-pain/. Accessed September 28, 2020.
9. Choosing Wisely. American Society of Anesthesiologists–Pain Medicine. Avoid imaging studies (MRI, CT or X-rays) for acute low back pain without specific indications. www.choosingwisely.org/clinician-lists/american-society-anesthesiologists-imaging-studies-for-acute-low-back-pain/. Accessed September 28, 2020.
10. Choosing Wisely. American College of Emergency Physicians. Avoid lumbar spine imaging in the emergency department for adults with non-traumatic back pain unless the patient has severe or progressive neurologic deficits or is suspected of having a serious underlying condition (such as vertebral infection, cauda equina syndrome, or cancer with bony metastasis). www.choosingwisely.org/clinician-lists/acep-lumbar-spine-imaging-in-the-ed/. Accessed September 28, 2020.
11. Choosing Wisely. American Academy of Family Physicians. Don’t require a pelvic exam or other physical exam to prescribe oral contraceptive medications. www.choosingwisely.org/clinician-lists/american-academy-family-physicians-pelvic-or-physical-exams-to-prescribe-oral-contraceptives/. Accessed September 28, 2020.
12. Over-the-counter access to hormonal contraception. ACOG Committee Opinion, Number 788. Obstet Gynecol. 2019;134:e96-e105. https://journals.lww.com/greenjournal/Fulltext/2019/10000/Over_the_Counter_Access_to_Hormonal_Contraception_.46.aspx. Accessed September 28, 2020.
13. Choosing Wisely. American Academy of Family Physicians. Don’t routinely recommend daily home glucose monitoring for patients who have Type 2 diabetes mellitus and are not using insulin. www.choosingwisely.org/clinician-lists/aafp-daily-home-glucose-monitoring-for-patients-with-type-2-diabetes. Accessed September 28, 2020.
14. Choosing Wisely. Society of General Internal Medicine. Don’t recommend daily home finger glucose testing in patients with Type 2 diabetes mellitus not using insulin. www.choosingwisely.org/clinician-lists/society-general-internal-medicine-daily-home-finger-glucose-testing-type-2-diabetes-mellitus/. Accessed September 28, 2020.
15. Malanda UL, Welschen LM, Riphagen II, et al. Self‐monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. Cochrane Database Syst Rev. 2012(1):CD005060.
16. Peel E, Douglas M, Lawton J. Self monitoring of blood glucose in type 2 diabetes: longitudinal qualitative study of patients’ perspectives. BMJ. 2007;335:493.
17. Choosing Wisely. American Academy of Family Physicians. Don’t screen for genital herpes simplex virus infection (HSV) in asymptomatic adults, including pregnant women. www.choosingwisely.org/clinician-lists/aafp-genital-herpes-screening-in-asymptomatic-adults/. Accessed September 28, 2020.
18. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Serologic screening for genital herpes infection: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:2525-2530.
19. Choosing Wisely. American Academy of Family Physicians. Don’t screen for testicular cancer in asymptomatic adolescent and adult males. www.choosingwisely.org/clinician-lists/aafp-testicular-cancer-screening-in-asymptomatic-adolescent-and-adult-men/. Accessed September 28, 2020.
20. Lin K, Sharangpani R. Screening for testicular cancer: an evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2010;153:396-399.
21. Choosing Wisely. American Academy of Pediatrics. Cough and cold medicines should not be prescribed, recommended or used for respiratory illnesses in young children. www.choosingwisely.org/clinician-lists/american-academy-pediatrics-cough-and-cold-medicines-for-children-under-four/. Accessed September 28, 2020.
22. Schaefer MK, Shehab N, Cohen AL, et al. Adverse events from cough and cold medications in children. Pediatrics. 2008;121:783-787.
23. Carr BC. Efficacy, abuse, and toxicity of over-the-counter cough and cold medicines in the pediatric population. Curr Opin Pediatr. 2006;18:184-188.
24. Oduwole O, Udoh EE, Oyo‐Ita A, et al. Honey for acute cough in children. Cochrane Database Syst Rev. 2018(4):CD007094.
25. Choosing Wisely. American Academy of Allergy, Asthma & Immunology. Don’t perform unproven diagnostic tests, such as immunoglobulin G(lgG) testing or an indiscriminate battery of immunoglobulin E(lgE) tests, in the evaluation of allergy. www.choosingwisely.org/clinician-lists/american-academy-allergy-asthma-immunology-diagnostic-tests-for-allergy-evaluation/. Accessed September 28, 2020.
26. Cox L, Williams B, Sicherer S, et al. Pearls and pitfalls of allergy diagnostic testing: report from the American College of Allergy, Asthma and Immunology Specific IgE Test Task Force. Ann Allergy Asthma Immunol. 2008;101:580-592.
27. Choosing Wisely. American Epilepsy Society. Do not routinely order electroencephalogram (EEG) as part of initial syncope work-up. www.choosingwisely.org/clinician-lists/aes-eeg-as-part-of-initial-syncope-work-up/. Accessed September 28, 2020.
28. Choosing Wisely. American College of Emergency Physicians. Avoid CT of the head in asymptomatic adult patients in the emergency department with syncope, insignificant trauma and a normal neurological evaluation. www.choosingwisely.org/clinician-lists/acep-avoid-head-ct-for-asymptomatic-adults-with-syncope/. Accessed September 28, 2020.
29. Choosing Wisely. American College of Physicians. In the evaluation of simple syncope and a normal neurological examination, don’t obtain brain imaging studies (CT or MRI). www.choosingwisely.org/clinician-lists/american-college-physicians-brain-imaging-to-evaluate-simple-syncope/. Accessed September 28, 2020.
30. Choosing Wisely. American Academy of Neurology. Don’t perform imaging of the carotid arteries for simple syncope without other neurologic symptoms. www.choosingwisely.org/clinician-lists/american-academy-neurology-carotid-artery-imaging-for-simple-syncope/. Accessed September 28, 2020.
31. Josephson CB, Rahey S, Sadler RM. Neurocardiogenic syncope: frequency and consequences of its misdiagnosis as epilepsy. Can J Neurol Sci. 2007;34:221-224.
32. Mendu ML, McAvay G, Lampert R, et al. Yield of diagnostic tests in evaluating syncopal episodes in older patients. Arch Intern Med. 2009;169:1299-1305.
33. Choosing Wisely. American Academy of Pediatrics–Section on Surgery. Avoid referring most children with umbilical hernias to a pediatric surgeon until around age 4-5 years. www.choosingwisely.org/clinician-lists/aap-sosu-avoid-surgery-referral-for-umbilical-hernias-until-age-4-5/. Accessed September 28, 2020.
34. Antonoff MB, Kreykes NS, Saltzman DA, et al. American Academy of Pediatrics Section on Surgery hernia survey revisited. J Pediatr Surg. 2005;40:1009-1014.
35. Choosing Wisely. American Geriatrics Society. Avoid using prescription appetite stimulants or high-calorie supplements for treatment of anorexia or cachexia in older adults; instead, optimize social supports, discontinue medications that may interfere with eating, provide appealing food and feeding assistance, and clarify patient goals and expectations. www.choosingwisely.org/clinician-lists/american-geriatrics-society-prescription-appetite-stimulants-to-treat-anorexia-cachexia-in-elderly/. Accessed September 28, 2020.
36. Milne AC, Potter J, Vivanti A, et al. Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane Database Sys Rev. 2009(2):CD003288.
37. Fox CB, Treadway AK, Blaszczyk AT, et al. Megestrol acetate and mirtazapine for the treatment of unplanned weight loss in the elderly. Pharmacotherapy. 2009;29:383-397.