User login
Just the FRAX: National fracture risks estimated
Adults aged 40 years and over have a mean 10-year risk of 5.3% for major osteoporotic fracture, according to the National Center for Health Statistics.
That risk includes a 10-year probability of 0.5% for hip fracture, based on estimates using the FRAX algorithm. These nationally representative estimates, the first to use the FRAX algorithm in this population, are based on 2013-2014 data from the National Health and Nutrition Examination Survey and show that the adjusted mean risks for those aged 50 years and over are 7.4% for major osteoporotic fracture and 0.9% for hip fracture, the NCHS reported.
The risk estimates in this analysis agree in large part with guidelines from the National Osteoporosis Foundation, which “use elevated FRAX scores in combination with low bone mass to define treatment eligibility,” the investigators said. FRAX-based findings, they noted, may provide “a more global evaluation of fracture risk than can be obtained from estimates based on [bone mineral density] alone.”
Adults aged 40 years and over have a mean 10-year risk of 5.3% for major osteoporotic fracture, according to the National Center for Health Statistics.
That risk includes a 10-year probability of 0.5% for hip fracture, based on estimates using the FRAX algorithm. These nationally representative estimates, the first to use the FRAX algorithm in this population, are based on 2013-2014 data from the National Health and Nutrition Examination Survey and show that the adjusted mean risks for those aged 50 years and over are 7.4% for major osteoporotic fracture and 0.9% for hip fracture, the NCHS reported.
The risk estimates in this analysis agree in large part with guidelines from the National Osteoporosis Foundation, which “use elevated FRAX scores in combination with low bone mass to define treatment eligibility,” the investigators said. FRAX-based findings, they noted, may provide “a more global evaluation of fracture risk than can be obtained from estimates based on [bone mineral density] alone.”
Adults aged 40 years and over have a mean 10-year risk of 5.3% for major osteoporotic fracture, according to the National Center for Health Statistics.
That risk includes a 10-year probability of 0.5% for hip fracture, based on estimates using the FRAX algorithm. These nationally representative estimates, the first to use the FRAX algorithm in this population, are based on 2013-2014 data from the National Health and Nutrition Examination Survey and show that the adjusted mean risks for those aged 50 years and over are 7.4% for major osteoporotic fracture and 0.9% for hip fracture, the NCHS reported.
The risk estimates in this analysis agree in large part with guidelines from the National Osteoporosis Foundation, which “use elevated FRAX scores in combination with low bone mass to define treatment eligibility,” the investigators said. FRAX-based findings, they noted, may provide “a more global evaluation of fracture risk than can be obtained from estimates based on [bone mineral density] alone.”
Study shows no adverse events with long-term denosumab in postmenopausal women
Extended follow-up of postmenopausal women taking denosumab for high-risk osteoporosis in the FREEDOM trial showed no increasing rates of either common adverse events or specific adverse events identified in the original study.
“This analysis provides reassuring information regarding safety and tolerability of both short-term and longer-term treatment with denosumab,” said Nelson B. Watts, MD, of Mercy Health, Cincinnati, and his associates.
The average age of the women was 72.3 years, and about 23% had vertebral fractures at baseline. The specific, infrequent adverse events that occurred more commonly in the denosumab group than the placebo group in the original trial were malignancy, eczema/dermatitis, pancreatitis, endocarditis, delayed fracture healing, infections, opportunistic infections, and cellulitis or erysipelas. The more common adverse events associated with the active treatment, and currently listed in the prescribing information for denosumab, were back pain, pain in the extremities, musculoskeletal pain, hypercholesterolemia, and cystitis, the investigators reported (J Bone Mineral Res. 2017 Apr 3. doi: 10.1002/jbmr.3119).
“We found no evidence for a relationship between treatment with denosumab and increasing rates of either low-frequency adverse events or common adverse events in patients who were assigned to placebo in the [original] trial and received 3 years of denosumab in the extension study, and no indication of increased incidence of low-frequency adverse events and common adverse events in patients receiving denosumab in years 4-6, compared with years 1-3,” Dr. Watts and his associates wrote.
This study was funded by Amgen, maker of denosumab (Prolia). Dr. Watts reported ties to Amgen, Shire, AbbVie, Merck, Radius, Sanofi, and Osteodynamics; his associates reported ties to numerous industry sources.
Extended follow-up of postmenopausal women taking denosumab for high-risk osteoporosis in the FREEDOM trial showed no increasing rates of either common adverse events or specific adverse events identified in the original study.
“This analysis provides reassuring information regarding safety and tolerability of both short-term and longer-term treatment with denosumab,” said Nelson B. Watts, MD, of Mercy Health, Cincinnati, and his associates.
The average age of the women was 72.3 years, and about 23% had vertebral fractures at baseline. The specific, infrequent adverse events that occurred more commonly in the denosumab group than the placebo group in the original trial were malignancy, eczema/dermatitis, pancreatitis, endocarditis, delayed fracture healing, infections, opportunistic infections, and cellulitis or erysipelas. The more common adverse events associated with the active treatment, and currently listed in the prescribing information for denosumab, were back pain, pain in the extremities, musculoskeletal pain, hypercholesterolemia, and cystitis, the investigators reported (J Bone Mineral Res. 2017 Apr 3. doi: 10.1002/jbmr.3119).
“We found no evidence for a relationship between treatment with denosumab and increasing rates of either low-frequency adverse events or common adverse events in patients who were assigned to placebo in the [original] trial and received 3 years of denosumab in the extension study, and no indication of increased incidence of low-frequency adverse events and common adverse events in patients receiving denosumab in years 4-6, compared with years 1-3,” Dr. Watts and his associates wrote.
This study was funded by Amgen, maker of denosumab (Prolia). Dr. Watts reported ties to Amgen, Shire, AbbVie, Merck, Radius, Sanofi, and Osteodynamics; his associates reported ties to numerous industry sources.
Extended follow-up of postmenopausal women taking denosumab for high-risk osteoporosis in the FREEDOM trial showed no increasing rates of either common adverse events or specific adverse events identified in the original study.
“This analysis provides reassuring information regarding safety and tolerability of both short-term and longer-term treatment with denosumab,” said Nelson B. Watts, MD, of Mercy Health, Cincinnati, and his associates.
The average age of the women was 72.3 years, and about 23% had vertebral fractures at baseline. The specific, infrequent adverse events that occurred more commonly in the denosumab group than the placebo group in the original trial were malignancy, eczema/dermatitis, pancreatitis, endocarditis, delayed fracture healing, infections, opportunistic infections, and cellulitis or erysipelas. The more common adverse events associated with the active treatment, and currently listed in the prescribing information for denosumab, were back pain, pain in the extremities, musculoskeletal pain, hypercholesterolemia, and cystitis, the investigators reported (J Bone Mineral Res. 2017 Apr 3. doi: 10.1002/jbmr.3119).
“We found no evidence for a relationship between treatment with denosumab and increasing rates of either low-frequency adverse events or common adverse events in patients who were assigned to placebo in the [original] trial and received 3 years of denosumab in the extension study, and no indication of increased incidence of low-frequency adverse events and common adverse events in patients receiving denosumab in years 4-6, compared with years 1-3,” Dr. Watts and his associates wrote.
This study was funded by Amgen, maker of denosumab (Prolia). Dr. Watts reported ties to Amgen, Shire, AbbVie, Merck, Radius, Sanofi, and Osteodynamics; his associates reported ties to numerous industry sources.
FROM THE JOURNAL OF BONE AND MINERAL RESEARCH
Key clinical point: Denosumab, taken long term, is not linked with any adverse events.
Major finding: Extended follow-up of postmenopausal women taking denosumab for high-risk osteoporosis showed no increasing rates of either common adverse events or of specific adverse events identified in the original trial.
Data source: Extended (6-year) follow-up of 4,550 women in the international phase III randomized, double-blind FREEDOM trial.
Disclosures: This study was funded by Amgen, maker of denosumab. Dr. Watts reported ties to Amgen, Shire, AbbVie, Merck, Radius, Sanofi, and Osteodynamics; his associates reported ties to numerous industry sources.
New guidance: Bone health is front and center in functional hypothalamic amenorrhea care
Evaluate bone health no longer than 6 months after onset of functional hypothalamic amenorrhea (FHA), according to new guidance on its diagnosis and management.
The preferred measure of bone mineral density (BMD) is dual-energy x-ray absorptiometry (DXA), guidance lead author Catherine M. Gordon, MD, of Cincinnati Children’s Hospital Medical Center noted in an interview.
“Our group has tried to raise awareness with our guideline about bone health,” said Dr. Gordon. “Bone is unfortunately detrimentally affected in adolescents and adult women with FHA, [so] our guideline now formally recommends bone density screening after 6 months of amenorrhea.”
Among the available therapies to preserve or restore bone density is short-term use of transdermal E2 therapy, with cyclic oral progestin, only after conventional intervention with nurtritional and exercise modification has been attempted for a “reasonable” amount of time but menstrual cycles have not yet been reestablished. Bisphosphonates, denosumab, testosterone, and leptin also can be used to improve bone mineral density (BMD), with recombinant parathyroid hormone 1-34 (rPTH) recommended in rare cases of extremely low BMD.
“Patients should not use oral contraceptives as a way to induce menstruation or improve BMD,” she said.
“We anticipate that some clinicians will be surprised to see that combined oral contraceptive pills do not provide bone protection for patients with FHA,” Dr. Gordon explained. “Our guideline reviews data on this point, which led to our recommendation that short-term transdermal estrogen may be helpful in restoring menses for select patients with longstanding amenorrhea despite efforts to correct their ‘energy deficit.’ ”
Clinicians also should be aware that FHA can mask the signs and symptoms of polycystic ovary syndrome in some young women. It’s therefore important for providers to understand that a patient could carry both diagnoses: Such patients should have a baseline BMD measurement taken, along with “clinical monitoring for hyper-response in those treated with exogenous gonadotropins for infertility,” according to the guidelines.
In general, “FHA is a ‘diagnosis of exclusion,’ meaning that underlying anatomic or organic pathologies must first be ruled out. It is important to consider other etiologies first before the amenorrhea is attributed to inadequate intake, overexercise, or stress,” Dr. Gordon said.
According to the guidelines, patients should only undergo evaluation for FHA if menstrual cycle interval exceeds 45 days on a consistent basis, or if they present with amenorrhea for at least 3 months. Patients with suspected FHA should be screened for psychological stressors that could be inducing anovulation.
When trying to make a diagnosis of FHA, providers should be “obtaining a detailed personal history with a focus on diet; eating disorders; exercise and athletic training; attitudes, such as perfectionism and high need for social approval; ambitions and expectations for self and others; weight fluctuations; sleep patterns; stressors; mood; menstrual pattern; fractures; and substance abuse,” the guidelines state.
Family histories also should be evaluated, and patients should undergo a full physical. Once a diagnosis of FHA is made, however, clinicians should help educate patients about different menstrual cycles while letting women know that an irregular cycle does not necessarily prevent them from conceiving.
The most important aspect of managing FHA is to take a multidiscipinary approach, according to Dr. Gordon and her colleagues (J Clin Endocrinol Metab. May 2017;102[5]:1-27).
“We emphasize that the mainstay of therapy for these adolescents and women is close attention to nutrition, exercise, and alleviating stressors through psychological support, best achieved through a multidisciplinary team,” Dr. Gordon stated.
Any patient diagnosed with FHA who also develops severe bradycardia, hypotension, orthostasis, or electrolyte imbalance should be treated as an inpatient. Energy imbalances can affect the hypothalamic-pituitary-ovarian (HPO) axis function, so correcting that should be considered a priority.
Adolescents and women for whom pregnancy has been excluded are recommended to undergo a progestin challenge, which should indicate chronic estrogen exposure via induced withdrawal bleeding. A brain MRI also can be conducted if the patient complains about chronic headaches, vomiting, or problems with vision or thirst.
FHA patients who wish to conceive have a number of options outlined by the guidelines. Pulsatile gonadotropin-releasing hormone (GnRH) should be the first treatment, followed by gonadotropin therapy, which must be administered as carefully as possible. Clomiphene citrate also can be used in certain situations, and cognitive behavior therapy is also a viable option, although the guidelines note that only one relatively small study offered any evidence of its efficacy. Kisspeptin and leptin should not be used as a treatment for infertility under any circumstances.
These guidelines were created in conjunction with the American Society for Reproductive Medicine, the European Society of Endocrinology, and the Pediatric Endocrine Society. An evidence-based approach was undertaken by a panel of eight experts, a methodologist, and a medical writer.
“There is currently a lack of consistency in clinical practice regarding the evaluation for patients with FHA,” said Dr. Gordon. “Our guideline attempts to clarify for clinicians what the appropriate general, endocrine, and imaging work-up would entail.”
The creation of these guidelines was funded by the Endocrine Society.
Evaluate bone health no longer than 6 months after onset of functional hypothalamic amenorrhea (FHA), according to new guidance on its diagnosis and management.
The preferred measure of bone mineral density (BMD) is dual-energy x-ray absorptiometry (DXA), guidance lead author Catherine M. Gordon, MD, of Cincinnati Children’s Hospital Medical Center noted in an interview.
“Our group has tried to raise awareness with our guideline about bone health,” said Dr. Gordon. “Bone is unfortunately detrimentally affected in adolescents and adult women with FHA, [so] our guideline now formally recommends bone density screening after 6 months of amenorrhea.”
Among the available therapies to preserve or restore bone density is short-term use of transdermal E2 therapy, with cyclic oral progestin, only after conventional intervention with nurtritional and exercise modification has been attempted for a “reasonable” amount of time but menstrual cycles have not yet been reestablished. Bisphosphonates, denosumab, testosterone, and leptin also can be used to improve bone mineral density (BMD), with recombinant parathyroid hormone 1-34 (rPTH) recommended in rare cases of extremely low BMD.
“Patients should not use oral contraceptives as a way to induce menstruation or improve BMD,” she said.
“We anticipate that some clinicians will be surprised to see that combined oral contraceptive pills do not provide bone protection for patients with FHA,” Dr. Gordon explained. “Our guideline reviews data on this point, which led to our recommendation that short-term transdermal estrogen may be helpful in restoring menses for select patients with longstanding amenorrhea despite efforts to correct their ‘energy deficit.’ ”
Clinicians also should be aware that FHA can mask the signs and symptoms of polycystic ovary syndrome in some young women. It’s therefore important for providers to understand that a patient could carry both diagnoses: Such patients should have a baseline BMD measurement taken, along with “clinical monitoring for hyper-response in those treated with exogenous gonadotropins for infertility,” according to the guidelines.
In general, “FHA is a ‘diagnosis of exclusion,’ meaning that underlying anatomic or organic pathologies must first be ruled out. It is important to consider other etiologies first before the amenorrhea is attributed to inadequate intake, overexercise, or stress,” Dr. Gordon said.
According to the guidelines, patients should only undergo evaluation for FHA if menstrual cycle interval exceeds 45 days on a consistent basis, or if they present with amenorrhea for at least 3 months. Patients with suspected FHA should be screened for psychological stressors that could be inducing anovulation.
When trying to make a diagnosis of FHA, providers should be “obtaining a detailed personal history with a focus on diet; eating disorders; exercise and athletic training; attitudes, such as perfectionism and high need for social approval; ambitions and expectations for self and others; weight fluctuations; sleep patterns; stressors; mood; menstrual pattern; fractures; and substance abuse,” the guidelines state.
Family histories also should be evaluated, and patients should undergo a full physical. Once a diagnosis of FHA is made, however, clinicians should help educate patients about different menstrual cycles while letting women know that an irregular cycle does not necessarily prevent them from conceiving.
The most important aspect of managing FHA is to take a multidiscipinary approach, according to Dr. Gordon and her colleagues (J Clin Endocrinol Metab. May 2017;102[5]:1-27).
“We emphasize that the mainstay of therapy for these adolescents and women is close attention to nutrition, exercise, and alleviating stressors through psychological support, best achieved through a multidisciplinary team,” Dr. Gordon stated.
Any patient diagnosed with FHA who also develops severe bradycardia, hypotension, orthostasis, or electrolyte imbalance should be treated as an inpatient. Energy imbalances can affect the hypothalamic-pituitary-ovarian (HPO) axis function, so correcting that should be considered a priority.
Adolescents and women for whom pregnancy has been excluded are recommended to undergo a progestin challenge, which should indicate chronic estrogen exposure via induced withdrawal bleeding. A brain MRI also can be conducted if the patient complains about chronic headaches, vomiting, or problems with vision or thirst.
FHA patients who wish to conceive have a number of options outlined by the guidelines. Pulsatile gonadotropin-releasing hormone (GnRH) should be the first treatment, followed by gonadotropin therapy, which must be administered as carefully as possible. Clomiphene citrate also can be used in certain situations, and cognitive behavior therapy is also a viable option, although the guidelines note that only one relatively small study offered any evidence of its efficacy. Kisspeptin and leptin should not be used as a treatment for infertility under any circumstances.
These guidelines were created in conjunction with the American Society for Reproductive Medicine, the European Society of Endocrinology, and the Pediatric Endocrine Society. An evidence-based approach was undertaken by a panel of eight experts, a methodologist, and a medical writer.
“There is currently a lack of consistency in clinical practice regarding the evaluation for patients with FHA,” said Dr. Gordon. “Our guideline attempts to clarify for clinicians what the appropriate general, endocrine, and imaging work-up would entail.”
The creation of these guidelines was funded by the Endocrine Society.
Evaluate bone health no longer than 6 months after onset of functional hypothalamic amenorrhea (FHA), according to new guidance on its diagnosis and management.
The preferred measure of bone mineral density (BMD) is dual-energy x-ray absorptiometry (DXA), guidance lead author Catherine M. Gordon, MD, of Cincinnati Children’s Hospital Medical Center noted in an interview.
“Our group has tried to raise awareness with our guideline about bone health,” said Dr. Gordon. “Bone is unfortunately detrimentally affected in adolescents and adult women with FHA, [so] our guideline now formally recommends bone density screening after 6 months of amenorrhea.”
Among the available therapies to preserve or restore bone density is short-term use of transdermal E2 therapy, with cyclic oral progestin, only after conventional intervention with nurtritional and exercise modification has been attempted for a “reasonable” amount of time but menstrual cycles have not yet been reestablished. Bisphosphonates, denosumab, testosterone, and leptin also can be used to improve bone mineral density (BMD), with recombinant parathyroid hormone 1-34 (rPTH) recommended in rare cases of extremely low BMD.
“Patients should not use oral contraceptives as a way to induce menstruation or improve BMD,” she said.
“We anticipate that some clinicians will be surprised to see that combined oral contraceptive pills do not provide bone protection for patients with FHA,” Dr. Gordon explained. “Our guideline reviews data on this point, which led to our recommendation that short-term transdermal estrogen may be helpful in restoring menses for select patients with longstanding amenorrhea despite efforts to correct their ‘energy deficit.’ ”
Clinicians also should be aware that FHA can mask the signs and symptoms of polycystic ovary syndrome in some young women. It’s therefore important for providers to understand that a patient could carry both diagnoses: Such patients should have a baseline BMD measurement taken, along with “clinical monitoring for hyper-response in those treated with exogenous gonadotropins for infertility,” according to the guidelines.
In general, “FHA is a ‘diagnosis of exclusion,’ meaning that underlying anatomic or organic pathologies must first be ruled out. It is important to consider other etiologies first before the amenorrhea is attributed to inadequate intake, overexercise, or stress,” Dr. Gordon said.
According to the guidelines, patients should only undergo evaluation for FHA if menstrual cycle interval exceeds 45 days on a consistent basis, or if they present with amenorrhea for at least 3 months. Patients with suspected FHA should be screened for psychological stressors that could be inducing anovulation.
When trying to make a diagnosis of FHA, providers should be “obtaining a detailed personal history with a focus on diet; eating disorders; exercise and athletic training; attitudes, such as perfectionism and high need for social approval; ambitions and expectations for self and others; weight fluctuations; sleep patterns; stressors; mood; menstrual pattern; fractures; and substance abuse,” the guidelines state.
Family histories also should be evaluated, and patients should undergo a full physical. Once a diagnosis of FHA is made, however, clinicians should help educate patients about different menstrual cycles while letting women know that an irregular cycle does not necessarily prevent them from conceiving.
The most important aspect of managing FHA is to take a multidiscipinary approach, according to Dr. Gordon and her colleagues (J Clin Endocrinol Metab. May 2017;102[5]:1-27).
“We emphasize that the mainstay of therapy for these adolescents and women is close attention to nutrition, exercise, and alleviating stressors through psychological support, best achieved through a multidisciplinary team,” Dr. Gordon stated.
Any patient diagnosed with FHA who also develops severe bradycardia, hypotension, orthostasis, or electrolyte imbalance should be treated as an inpatient. Energy imbalances can affect the hypothalamic-pituitary-ovarian (HPO) axis function, so correcting that should be considered a priority.
Adolescents and women for whom pregnancy has been excluded are recommended to undergo a progestin challenge, which should indicate chronic estrogen exposure via induced withdrawal bleeding. A brain MRI also can be conducted if the patient complains about chronic headaches, vomiting, or problems with vision or thirst.
FHA patients who wish to conceive have a number of options outlined by the guidelines. Pulsatile gonadotropin-releasing hormone (GnRH) should be the first treatment, followed by gonadotropin therapy, which must be administered as carefully as possible. Clomiphene citrate also can be used in certain situations, and cognitive behavior therapy is also a viable option, although the guidelines note that only one relatively small study offered any evidence of its efficacy. Kisspeptin and leptin should not be used as a treatment for infertility under any circumstances.
These guidelines were created in conjunction with the American Society for Reproductive Medicine, the European Society of Endocrinology, and the Pediatric Endocrine Society. An evidence-based approach was undertaken by a panel of eight experts, a methodologist, and a medical writer.
“There is currently a lack of consistency in clinical practice regarding the evaluation for patients with FHA,” said Dr. Gordon. “Our guideline attempts to clarify for clinicians what the appropriate general, endocrine, and imaging work-up would entail.”
The creation of these guidelines was funded by the Endocrine Society.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY AND METABOLISM
Large, intermittent vitamin D doses may increase fracture, fall risk in elderly
ORLANDO – The vitamin that keeps bones strong and protects against colon cancer also can, in large doses, increase the risk of falls and fractures.
Studies of high-dose vitamin D keep turning up the same concerns, Dr. Martin Weinstock said at the annual meeting of the American Academy of Dermatology. The most striking of these findings are the significantly increased risks of falls and fractures associated with vitamin D megadoses, said Dr. Weinstock of Brown University, Providence, R.I. These trials examined very-large intermittent doses of the vitamin given to elderly patients.
That amount of vitamin D, taken all at once, may seem like a therapeutic outlier to clinicians who are used to giving 2,000-4,000 IU/day, as recommended by the 2011 Institute of Medicine report.
Not so fast, said Dr. Weinstock.
“If you do the math, if you’re taking 2,000 IU every day, that’s 60,000 IU per month. That’s a high dose,” he said. However, he noted, daily supplements in that range appear safe. “It seems that this intermittent dosing in these studies might be the problem.”
The IOM report, an exhaustive, 1,000-page article summarizing the extant data on vitamin D – is considered the optimal dosing guide. For healthy patients, it recommends the following as minimum effective and maximum safe doses:
- 400-1,000 IU/day for infants younger than 6 month.
- 400-1,500 IU/day for infants aged 6-12 months.
- 600-2,000 IU/day for children aged 1-3 years.
- 600-3,000 IU/day for children aged 4-8 years.
- 600-4,000 IU/day for everyone aged 9 years and older.
But dosing of this fat-soluble vitamin should, in some cases, be individualized. For example, obese patients may have persistently low levels despite supplementation, as fat can sequester the vitamin. Patients with fat-metabolizing disorders may not absorb it well. And patients who have had gastric bypass may face the same issues of malabsorption, but from a mechanical, not a metabolic, standpoint.
Elderly people are particularly susceptible to vitamin D deficiency for a couple of reasons, Dr. Weinstock said. They may be less mobile, so lack exposure to sunlight. Age also can decrease the ability to convert 25-hydroxyvitamin D to the biologically active vitamin.
Mega-dosing has been an attractive method of raising and maintaining levels in this population. But several studies illustrate the risks that come along with this strategy, he noted.
A 2007 study randomized 9,440 men and women 75 years or older to placebo or to a single, 300,000 IU vitamin D intramuscular injection for 3 years. At the end of that time, the rate of falls had not significantly changed, but the rate of hip fractures had increased by 49% (Rheumatology [Oxford] 2007;46:1852-7).
Fractures occurred in 585 subjects, including 110 hip fractures, 116 wrist fractures, and 37 ankle fractures. This represented a 49% increase in any hip fracture and a 22% increase in wrist fracture over the placebo group.
A similar study, published in 2010, randomized 2,225 elderly women to placebo or a single 500,000 oral dose of vitamin D for 4 years. There were 171 fractures in the active group and 135 in the placebo group – a 26% increased risk (JAMA. 2010;303[18]:1815-22).
The fall rate in the active group was 83.4/100 person-years, compared with 72.7/100 person-years in the placebo group. This represented a significant 26% increased fracture risk and 15% increased fall risk.
Interestingly, most of these incidents occurred within the first 3 months after each dose,” Dr. Weinstock said. Those taking vitamin D were 31% more likely to fall in that time period, but no more likely to fall in the subsequent 9 months after each dose.
In 2016, a third study looked at functional status among 200 elderly men and women in a three-armed randomization: a low-dose control group receiving 24,000 IU of vitamin D; 60,000 IU vitamin D3; or 24,000 IU of vitamin plus 300 mcg calcifediol.
Functional status didn’t differ between the groups at the end of 1 year. But the incidence of falls did differ, with falls occurring in 67% of the 60,000 IU group, 54% of the 24,000 IU/calcifediol group, and 48% of the 24,000 IU control group (JAMA Intern Med. 2016;176[2]:175-83).
This represented a 47% increased fall risk in the high dose group, compared with the two low-dose groups.
Dr. Weinstein had no financial disclosures relevant to his lecture.
[email protected]
On Twitter @Alz_Gal
ORLANDO – The vitamin that keeps bones strong and protects against colon cancer also can, in large doses, increase the risk of falls and fractures.
Studies of high-dose vitamin D keep turning up the same concerns, Dr. Martin Weinstock said at the annual meeting of the American Academy of Dermatology. The most striking of these findings are the significantly increased risks of falls and fractures associated with vitamin D megadoses, said Dr. Weinstock of Brown University, Providence, R.I. These trials examined very-large intermittent doses of the vitamin given to elderly patients.
That amount of vitamin D, taken all at once, may seem like a therapeutic outlier to clinicians who are used to giving 2,000-4,000 IU/day, as recommended by the 2011 Institute of Medicine report.
Not so fast, said Dr. Weinstock.
“If you do the math, if you’re taking 2,000 IU every day, that’s 60,000 IU per month. That’s a high dose,” he said. However, he noted, daily supplements in that range appear safe. “It seems that this intermittent dosing in these studies might be the problem.”
The IOM report, an exhaustive, 1,000-page article summarizing the extant data on vitamin D – is considered the optimal dosing guide. For healthy patients, it recommends the following as minimum effective and maximum safe doses:
- 400-1,000 IU/day for infants younger than 6 month.
- 400-1,500 IU/day for infants aged 6-12 months.
- 600-2,000 IU/day for children aged 1-3 years.
- 600-3,000 IU/day for children aged 4-8 years.
- 600-4,000 IU/day for everyone aged 9 years and older.
But dosing of this fat-soluble vitamin should, in some cases, be individualized. For example, obese patients may have persistently low levels despite supplementation, as fat can sequester the vitamin. Patients with fat-metabolizing disorders may not absorb it well. And patients who have had gastric bypass may face the same issues of malabsorption, but from a mechanical, not a metabolic, standpoint.
Elderly people are particularly susceptible to vitamin D deficiency for a couple of reasons, Dr. Weinstock said. They may be less mobile, so lack exposure to sunlight. Age also can decrease the ability to convert 25-hydroxyvitamin D to the biologically active vitamin.
Mega-dosing has been an attractive method of raising and maintaining levels in this population. But several studies illustrate the risks that come along with this strategy, he noted.
A 2007 study randomized 9,440 men and women 75 years or older to placebo or to a single, 300,000 IU vitamin D intramuscular injection for 3 years. At the end of that time, the rate of falls had not significantly changed, but the rate of hip fractures had increased by 49% (Rheumatology [Oxford] 2007;46:1852-7).
Fractures occurred in 585 subjects, including 110 hip fractures, 116 wrist fractures, and 37 ankle fractures. This represented a 49% increase in any hip fracture and a 22% increase in wrist fracture over the placebo group.
A similar study, published in 2010, randomized 2,225 elderly women to placebo or a single 500,000 oral dose of vitamin D for 4 years. There were 171 fractures in the active group and 135 in the placebo group – a 26% increased risk (JAMA. 2010;303[18]:1815-22).
The fall rate in the active group was 83.4/100 person-years, compared with 72.7/100 person-years in the placebo group. This represented a significant 26% increased fracture risk and 15% increased fall risk.
Interestingly, most of these incidents occurred within the first 3 months after each dose,” Dr. Weinstock said. Those taking vitamin D were 31% more likely to fall in that time period, but no more likely to fall in the subsequent 9 months after each dose.
In 2016, a third study looked at functional status among 200 elderly men and women in a three-armed randomization: a low-dose control group receiving 24,000 IU of vitamin D; 60,000 IU vitamin D3; or 24,000 IU of vitamin plus 300 mcg calcifediol.
Functional status didn’t differ between the groups at the end of 1 year. But the incidence of falls did differ, with falls occurring in 67% of the 60,000 IU group, 54% of the 24,000 IU/calcifediol group, and 48% of the 24,000 IU control group (JAMA Intern Med. 2016;176[2]:175-83).
This represented a 47% increased fall risk in the high dose group, compared with the two low-dose groups.
Dr. Weinstein had no financial disclosures relevant to his lecture.
[email protected]
On Twitter @Alz_Gal
ORLANDO – The vitamin that keeps bones strong and protects against colon cancer also can, in large doses, increase the risk of falls and fractures.
Studies of high-dose vitamin D keep turning up the same concerns, Dr. Martin Weinstock said at the annual meeting of the American Academy of Dermatology. The most striking of these findings are the significantly increased risks of falls and fractures associated with vitamin D megadoses, said Dr. Weinstock of Brown University, Providence, R.I. These trials examined very-large intermittent doses of the vitamin given to elderly patients.
That amount of vitamin D, taken all at once, may seem like a therapeutic outlier to clinicians who are used to giving 2,000-4,000 IU/day, as recommended by the 2011 Institute of Medicine report.
Not so fast, said Dr. Weinstock.
“If you do the math, if you’re taking 2,000 IU every day, that’s 60,000 IU per month. That’s a high dose,” he said. However, he noted, daily supplements in that range appear safe. “It seems that this intermittent dosing in these studies might be the problem.”
The IOM report, an exhaustive, 1,000-page article summarizing the extant data on vitamin D – is considered the optimal dosing guide. For healthy patients, it recommends the following as minimum effective and maximum safe doses:
- 400-1,000 IU/day for infants younger than 6 month.
- 400-1,500 IU/day for infants aged 6-12 months.
- 600-2,000 IU/day for children aged 1-3 years.
- 600-3,000 IU/day for children aged 4-8 years.
- 600-4,000 IU/day for everyone aged 9 years and older.
But dosing of this fat-soluble vitamin should, in some cases, be individualized. For example, obese patients may have persistently low levels despite supplementation, as fat can sequester the vitamin. Patients with fat-metabolizing disorders may not absorb it well. And patients who have had gastric bypass may face the same issues of malabsorption, but from a mechanical, not a metabolic, standpoint.
Elderly people are particularly susceptible to vitamin D deficiency for a couple of reasons, Dr. Weinstock said. They may be less mobile, so lack exposure to sunlight. Age also can decrease the ability to convert 25-hydroxyvitamin D to the biologically active vitamin.
Mega-dosing has been an attractive method of raising and maintaining levels in this population. But several studies illustrate the risks that come along with this strategy, he noted.
A 2007 study randomized 9,440 men and women 75 years or older to placebo or to a single, 300,000 IU vitamin D intramuscular injection for 3 years. At the end of that time, the rate of falls had not significantly changed, but the rate of hip fractures had increased by 49% (Rheumatology [Oxford] 2007;46:1852-7).
Fractures occurred in 585 subjects, including 110 hip fractures, 116 wrist fractures, and 37 ankle fractures. This represented a 49% increase in any hip fracture and a 22% increase in wrist fracture over the placebo group.
A similar study, published in 2010, randomized 2,225 elderly women to placebo or a single 500,000 oral dose of vitamin D for 4 years. There were 171 fractures in the active group and 135 in the placebo group – a 26% increased risk (JAMA. 2010;303[18]:1815-22).
The fall rate in the active group was 83.4/100 person-years, compared with 72.7/100 person-years in the placebo group. This represented a significant 26% increased fracture risk and 15% increased fall risk.
Interestingly, most of these incidents occurred within the first 3 months after each dose,” Dr. Weinstock said. Those taking vitamin D were 31% more likely to fall in that time period, but no more likely to fall in the subsequent 9 months after each dose.
In 2016, a third study looked at functional status among 200 elderly men and women in a three-armed randomization: a low-dose control group receiving 24,000 IU of vitamin D; 60,000 IU vitamin D3; or 24,000 IU of vitamin plus 300 mcg calcifediol.
Functional status didn’t differ between the groups at the end of 1 year. But the incidence of falls did differ, with falls occurring in 67% of the 60,000 IU group, 54% of the 24,000 IU/calcifediol group, and 48% of the 24,000 IU control group (JAMA Intern Med. 2016;176[2]:175-83).
This represented a 47% increased fall risk in the high dose group, compared with the two low-dose groups.
Dr. Weinstein had no financial disclosures relevant to his lecture.
[email protected]
On Twitter @Alz_Gal
Targeting Paget’s bone symptoms supported in long-term trial
Long-term intensive bisphosphonate treatment of patients with Paget’s disease of bone continued to show no benefit over symptomatic treatment in a 3-year extension trial of the PRISM study, leading investigators in the trial to recommend that treatment guidelines focus more on managing the condition’s symptoms rather than normalizing bone turnover biomarkers in hope of reducing disease complications.
“The Paget’s Disease Randomized Trial of Intensive versus Symptomatic Management (PRISM) study showed that intensive bisphosphonate therapy and symptomatic treatment had similar effects on clinical outcome of PDB [Paget’s disease of bone] with respect to the occurrence of fractures, orthopedic procedures, hearing loss, bone pain, quality of life, and adverse events,” wrote the principal investigator of the PRISM-EZ (extension with zoledronic acid) phase of the trial, Stuart H. Ralston, MD, of the University of Edinburgh (Scotland), and his colleagues.
Enrollment in the extension trial, which occurred during January 2007-January 2012, meant that patients kept to the same treatment strategies except for those in the intensive arm, who switched to zoledronic acid as the treatment of first choice. Both groups received analgesics and nonsteroidal anti-inflammatory drugs as needed. Patients in the symptomatic arm received bisphosphonate treatment only if they had bone pain thought to be caused by the increased metabolic activity of PDB, whereas those in the intensive arm were prescribed “bisphosphonates as required to suppress and maintain ALP [alkaline phosphatase] concentrations within the normal range,” according to the investigators. Clinical fracture rate was the primary outcome, but Dr. Ralston and his colleagues also compared orthopedic procedures, serum total ALP concentrations, bone pain, and quality of life in both cohorts.
A higher proportion of patients in the intensive group received zoledronic acid than in the symptomatic group (28.1% vs. 10.3%; P less than .001), whereas fewer patients in the intensive group received pamidronate (4.8% vs. 15.5%; P less than .001).
Of 270 in the intensive cohort, 22 (8.1%) experienced fractures, versus 12 (5.2%) of the 232 in the symptomatic cohort, yielding a hazard ratio of 1.90 (95% confidence interval, 0.91-3.98; P = .087). Of those, fractures in the pagetic bone numbered five and two, respectively. A total of 15 (5.6%) intensive treatment subjects and 7 (3.0%) symptomatic treatment subjects required orthopedic surgery, with a hazard ratio of 1.81 (95% CI, 0.71-4.61; P = .214).
There were no differences in bodily pain, bone pain, or quality of life at year 3 as reported by patients in both cohorts. While there was a statistically significant difference between intensive and symptomatic treatment in bodily pain, physical component summary score, and arthritis-specific health index scores at year 1 of the trial, the investigators noted that the findings were below the clinically significant five-point threshold and did not persist beyond 1 year.
“The results reported here do not support the recommendations made by the Endocrine Society guideline group, which suggested that most patients with PDB should be treated with potent bisphosphonates with the aim of restoring ALP values to within the lower part of the reference range,” the authors concluded. “On the contrary, the PRISM-EZ study demonstrates that this strategy is not associated with clinical benefit and might be harmful, [so] a more appropriate indication for bisphosphonate treatment in PDB is to control bone pain thought to be due to disease activity.”
The study was funded by grants from Arthritis Research UK and the Paget’s Association. Dr. Ralston reported receiving consultancy fees on behalf of his institution from Novartis and Merck and grant support on behalf of his institution from Amgen, Eli Lilly, and UCB. One coauthor reported receiving consultancy fees from Siemens, Becton Dickinson, and Roche, and another disclosed receiving such fees from Internis.
The PRISM-EZ study is the largest and longest study of PDB and provides important information on its long-term management. The results need to be kept in perspective. All patients were treated; hence, there is no untreated group and the results apply to follow-up therapy only. In this group, repeat therapy for active PDB as measured from serum total alkaline phosphatase (ALP) versus therapy for symptoms did not make a difference in a 3-year follow-up, and the authors recommend treating only when symptoms warrant it. Longer follow-ups would be needed to definitively show a difference in a condition that is present for many years.
Roy D. Altman, MD , is professor emeritus of medicine in the division of rheumatology and immunology at the University of California, Los Angeles. He has no relevant disclosures.
The PRISM-EZ study is the largest and longest study of PDB and provides important information on its long-term management. The results need to be kept in perspective. All patients were treated; hence, there is no untreated group and the results apply to follow-up therapy only. In this group, repeat therapy for active PDB as measured from serum total alkaline phosphatase (ALP) versus therapy for symptoms did not make a difference in a 3-year follow-up, and the authors recommend treating only when symptoms warrant it. Longer follow-ups would be needed to definitively show a difference in a condition that is present for many years.
Roy D. Altman, MD , is professor emeritus of medicine in the division of rheumatology and immunology at the University of California, Los Angeles. He has no relevant disclosures.
The PRISM-EZ study is the largest and longest study of PDB and provides important information on its long-term management. The results need to be kept in perspective. All patients were treated; hence, there is no untreated group and the results apply to follow-up therapy only. In this group, repeat therapy for active PDB as measured from serum total alkaline phosphatase (ALP) versus therapy for symptoms did not make a difference in a 3-year follow-up, and the authors recommend treating only when symptoms warrant it. Longer follow-ups would be needed to definitively show a difference in a condition that is present for many years.
Roy D. Altman, MD , is professor emeritus of medicine in the division of rheumatology and immunology at the University of California, Los Angeles. He has no relevant disclosures.
Long-term intensive bisphosphonate treatment of patients with Paget’s disease of bone continued to show no benefit over symptomatic treatment in a 3-year extension trial of the PRISM study, leading investigators in the trial to recommend that treatment guidelines focus more on managing the condition’s symptoms rather than normalizing bone turnover biomarkers in hope of reducing disease complications.
“The Paget’s Disease Randomized Trial of Intensive versus Symptomatic Management (PRISM) study showed that intensive bisphosphonate therapy and symptomatic treatment had similar effects on clinical outcome of PDB [Paget’s disease of bone] with respect to the occurrence of fractures, orthopedic procedures, hearing loss, bone pain, quality of life, and adverse events,” wrote the principal investigator of the PRISM-EZ (extension with zoledronic acid) phase of the trial, Stuart H. Ralston, MD, of the University of Edinburgh (Scotland), and his colleagues.
Enrollment in the extension trial, which occurred during January 2007-January 2012, meant that patients kept to the same treatment strategies except for those in the intensive arm, who switched to zoledronic acid as the treatment of first choice. Both groups received analgesics and nonsteroidal anti-inflammatory drugs as needed. Patients in the symptomatic arm received bisphosphonate treatment only if they had bone pain thought to be caused by the increased metabolic activity of PDB, whereas those in the intensive arm were prescribed “bisphosphonates as required to suppress and maintain ALP [alkaline phosphatase] concentrations within the normal range,” according to the investigators. Clinical fracture rate was the primary outcome, but Dr. Ralston and his colleagues also compared orthopedic procedures, serum total ALP concentrations, bone pain, and quality of life in both cohorts.
A higher proportion of patients in the intensive group received zoledronic acid than in the symptomatic group (28.1% vs. 10.3%; P less than .001), whereas fewer patients in the intensive group received pamidronate (4.8% vs. 15.5%; P less than .001).
Of 270 in the intensive cohort, 22 (8.1%) experienced fractures, versus 12 (5.2%) of the 232 in the symptomatic cohort, yielding a hazard ratio of 1.90 (95% confidence interval, 0.91-3.98; P = .087). Of those, fractures in the pagetic bone numbered five and two, respectively. A total of 15 (5.6%) intensive treatment subjects and 7 (3.0%) symptomatic treatment subjects required orthopedic surgery, with a hazard ratio of 1.81 (95% CI, 0.71-4.61; P = .214).
There were no differences in bodily pain, bone pain, or quality of life at year 3 as reported by patients in both cohorts. While there was a statistically significant difference between intensive and symptomatic treatment in bodily pain, physical component summary score, and arthritis-specific health index scores at year 1 of the trial, the investigators noted that the findings were below the clinically significant five-point threshold and did not persist beyond 1 year.
“The results reported here do not support the recommendations made by the Endocrine Society guideline group, which suggested that most patients with PDB should be treated with potent bisphosphonates with the aim of restoring ALP values to within the lower part of the reference range,” the authors concluded. “On the contrary, the PRISM-EZ study demonstrates that this strategy is not associated with clinical benefit and might be harmful, [so] a more appropriate indication for bisphosphonate treatment in PDB is to control bone pain thought to be due to disease activity.”
The study was funded by grants from Arthritis Research UK and the Paget’s Association. Dr. Ralston reported receiving consultancy fees on behalf of his institution from Novartis and Merck and grant support on behalf of his institution from Amgen, Eli Lilly, and UCB. One coauthor reported receiving consultancy fees from Siemens, Becton Dickinson, and Roche, and another disclosed receiving such fees from Internis.
Long-term intensive bisphosphonate treatment of patients with Paget’s disease of bone continued to show no benefit over symptomatic treatment in a 3-year extension trial of the PRISM study, leading investigators in the trial to recommend that treatment guidelines focus more on managing the condition’s symptoms rather than normalizing bone turnover biomarkers in hope of reducing disease complications.
“The Paget’s Disease Randomized Trial of Intensive versus Symptomatic Management (PRISM) study showed that intensive bisphosphonate therapy and symptomatic treatment had similar effects on clinical outcome of PDB [Paget’s disease of bone] with respect to the occurrence of fractures, orthopedic procedures, hearing loss, bone pain, quality of life, and adverse events,” wrote the principal investigator of the PRISM-EZ (extension with zoledronic acid) phase of the trial, Stuart H. Ralston, MD, of the University of Edinburgh (Scotland), and his colleagues.
Enrollment in the extension trial, which occurred during January 2007-January 2012, meant that patients kept to the same treatment strategies except for those in the intensive arm, who switched to zoledronic acid as the treatment of first choice. Both groups received analgesics and nonsteroidal anti-inflammatory drugs as needed. Patients in the symptomatic arm received bisphosphonate treatment only if they had bone pain thought to be caused by the increased metabolic activity of PDB, whereas those in the intensive arm were prescribed “bisphosphonates as required to suppress and maintain ALP [alkaline phosphatase] concentrations within the normal range,” according to the investigators. Clinical fracture rate was the primary outcome, but Dr. Ralston and his colleagues also compared orthopedic procedures, serum total ALP concentrations, bone pain, and quality of life in both cohorts.
A higher proportion of patients in the intensive group received zoledronic acid than in the symptomatic group (28.1% vs. 10.3%; P less than .001), whereas fewer patients in the intensive group received pamidronate (4.8% vs. 15.5%; P less than .001).
Of 270 in the intensive cohort, 22 (8.1%) experienced fractures, versus 12 (5.2%) of the 232 in the symptomatic cohort, yielding a hazard ratio of 1.90 (95% confidence interval, 0.91-3.98; P = .087). Of those, fractures in the pagetic bone numbered five and two, respectively. A total of 15 (5.6%) intensive treatment subjects and 7 (3.0%) symptomatic treatment subjects required orthopedic surgery, with a hazard ratio of 1.81 (95% CI, 0.71-4.61; P = .214).
There were no differences in bodily pain, bone pain, or quality of life at year 3 as reported by patients in both cohorts. While there was a statistically significant difference between intensive and symptomatic treatment in bodily pain, physical component summary score, and arthritis-specific health index scores at year 1 of the trial, the investigators noted that the findings were below the clinically significant five-point threshold and did not persist beyond 1 year.
“The results reported here do not support the recommendations made by the Endocrine Society guideline group, which suggested that most patients with PDB should be treated with potent bisphosphonates with the aim of restoring ALP values to within the lower part of the reference range,” the authors concluded. “On the contrary, the PRISM-EZ study demonstrates that this strategy is not associated with clinical benefit and might be harmful, [so] a more appropriate indication for bisphosphonate treatment in PDB is to control bone pain thought to be due to disease activity.”
The study was funded by grants from Arthritis Research UK and the Paget’s Association. Dr. Ralston reported receiving consultancy fees on behalf of his institution from Novartis and Merck and grant support on behalf of his institution from Amgen, Eli Lilly, and UCB. One coauthor reported receiving consultancy fees from Siemens, Becton Dickinson, and Roche, and another disclosed receiving such fees from Internis.
FROM JOURNAL OF BONE AND MINERAL RESEARCH
Key clinical point:
Major finding: Intensive bisphosphonate therapy did not yield significant differences in rates of fractures (P = .087), orthopedic procedures (P = .214), and serious adverse events (RR = 1.28).
Data source: An extension study of a randomized trial involving 502 PDB patients during January 2007-January 2012.
Disclosures: The study was funded by grants from Arthritis Research UK and the Paget’s Association. Dr. Ralston reported receiving consultancy fees on behalf of his institution from Novartis and Merck and grant support on behalf of his institution from Amgen, Eli Lilly, and UCB. One coauthor reported receiving consultancy fees from Siemens, Becton Dickinson, and Roche, and another disclosed receiving such fees from Internis.
Bone fractures more likely to occur in psoriasis, PsA patients
Individuals who have psoriasis or psoriatic arthritis are at a significantly higher risk of also suffering bone fractures, particularly in their hip and vertebrae, according to a new study published in the Annals of the Rheumatic Diseases.
“To our knowledge, these are the first population-based estimates of the risk for incident fracture and osteoporosis in patients with psoriasis and/or PsA [psoriatic arthritis] and the first longitudinal cohort study to address this issue,” wrote the authors of the study, led by Alexis Ogdie-Beatty, MD, of the University of Pennsylvania in Philadelphia (Ann Rheum Dis. 2017 Jan 16. doi: 10.1136/annrheumdis-2016-210441).
A total of 9,788 PsA and 158,323 psoriasis patients were included in the study, along with 39,306 RA patients and 821,834 individuals from the general population. Psoriasis patients were divided into groups classified as mild (n = 149,809) or severe (n = 8,514). The average age of each cohort ranged from nearly 47 years to almost 59 years, with all cohorts comprising mostly females, ranging from about 51% to 69%.
“We found that the risk for any fracture in patients with PsA and severe psoriasis was similar to RA [but] patients with PsA and psoriasis had an increased incidence of fracture compared with the general population by 7%-26%,” the authors explained. “The incidence of vertebral fracture was also increased in patients with severe psoriasis and while hip fracture was elevated in both psoriasis groups, it was only statistically significant in patients with mild psoriasis relative to matched controls after adjusting for risk factors for osteoporosis.”
Dr. Ogdie-Beatty and her colleagues found that all of the conditions conferred an elevated risk for fractures anywhere in the body when compared with the general population, reaching hazard ratios of 1.16 (95% confidence interval, 1.06-1.27) for people with PsA, 1.07 (95% CI, 1.05-1.10) for mild psoriasis, 1.26 for severe psoriasis (95% CI, 1.15-1.39), and 1.23 for RA (95% CI, 1.18-1.28). The risk for hip fractures was only significantly higher for mild (hazard ratio, 1.13; 95% CI, 1.04-1.22) and severe psoriasis (HR, 1.21; 95% CI, 0.88-1.66), and RA (HR, 1.55; 95% CI, 1.40-1.72). Individuals with PsA did not have a significantly higher risk for vertebral fractures (HR, 1.07; 95% CI, 0.66-1.72), whereas those with mild psoriasis (HR, 1.17; 95% CI, 1.03-1.33), severe psoriasis (HR, 2.23; 95% CI, 1.54-3.22), or RA did (HR, 1.53; 95% CI, 1.30-1.80). Each of these models were fully adjusted for multiple different osteoporosis risk factors, although they were all commonly adjusted for age, sex, atrial fibrillation, diabetes, chronic obstructive pulmonary disease, stroke, SSRI use, tricyclic antidepressant use, oral steroids, smoking, and categorical body mass index.
Individual coauthors disclosed receiving funding for their work from the National Institutes of Health, as well as grants from the Department of Veterans Affairs and the Rheumatology Research Foundation. Three of the authors reported receiving payment for continuing medical education work related to psoriatic arthritis or psoriasis.
Individuals who have psoriasis or psoriatic arthritis are at a significantly higher risk of also suffering bone fractures, particularly in their hip and vertebrae, according to a new study published in the Annals of the Rheumatic Diseases.
“To our knowledge, these are the first population-based estimates of the risk for incident fracture and osteoporosis in patients with psoriasis and/or PsA [psoriatic arthritis] and the first longitudinal cohort study to address this issue,” wrote the authors of the study, led by Alexis Ogdie-Beatty, MD, of the University of Pennsylvania in Philadelphia (Ann Rheum Dis. 2017 Jan 16. doi: 10.1136/annrheumdis-2016-210441).
A total of 9,788 PsA and 158,323 psoriasis patients were included in the study, along with 39,306 RA patients and 821,834 individuals from the general population. Psoriasis patients were divided into groups classified as mild (n = 149,809) or severe (n = 8,514). The average age of each cohort ranged from nearly 47 years to almost 59 years, with all cohorts comprising mostly females, ranging from about 51% to 69%.
“We found that the risk for any fracture in patients with PsA and severe psoriasis was similar to RA [but] patients with PsA and psoriasis had an increased incidence of fracture compared with the general population by 7%-26%,” the authors explained. “The incidence of vertebral fracture was also increased in patients with severe psoriasis and while hip fracture was elevated in both psoriasis groups, it was only statistically significant in patients with mild psoriasis relative to matched controls after adjusting for risk factors for osteoporosis.”
Dr. Ogdie-Beatty and her colleagues found that all of the conditions conferred an elevated risk for fractures anywhere in the body when compared with the general population, reaching hazard ratios of 1.16 (95% confidence interval, 1.06-1.27) for people with PsA, 1.07 (95% CI, 1.05-1.10) for mild psoriasis, 1.26 for severe psoriasis (95% CI, 1.15-1.39), and 1.23 for RA (95% CI, 1.18-1.28). The risk for hip fractures was only significantly higher for mild (hazard ratio, 1.13; 95% CI, 1.04-1.22) and severe psoriasis (HR, 1.21; 95% CI, 0.88-1.66), and RA (HR, 1.55; 95% CI, 1.40-1.72). Individuals with PsA did not have a significantly higher risk for vertebral fractures (HR, 1.07; 95% CI, 0.66-1.72), whereas those with mild psoriasis (HR, 1.17; 95% CI, 1.03-1.33), severe psoriasis (HR, 2.23; 95% CI, 1.54-3.22), or RA did (HR, 1.53; 95% CI, 1.30-1.80). Each of these models were fully adjusted for multiple different osteoporosis risk factors, although they were all commonly adjusted for age, sex, atrial fibrillation, diabetes, chronic obstructive pulmonary disease, stroke, SSRI use, tricyclic antidepressant use, oral steroids, smoking, and categorical body mass index.
Individual coauthors disclosed receiving funding for their work from the National Institutes of Health, as well as grants from the Department of Veterans Affairs and the Rheumatology Research Foundation. Three of the authors reported receiving payment for continuing medical education work related to psoriatic arthritis or psoriasis.
Individuals who have psoriasis or psoriatic arthritis are at a significantly higher risk of also suffering bone fractures, particularly in their hip and vertebrae, according to a new study published in the Annals of the Rheumatic Diseases.
“To our knowledge, these are the first population-based estimates of the risk for incident fracture and osteoporosis in patients with psoriasis and/or PsA [psoriatic arthritis] and the first longitudinal cohort study to address this issue,” wrote the authors of the study, led by Alexis Ogdie-Beatty, MD, of the University of Pennsylvania in Philadelphia (Ann Rheum Dis. 2017 Jan 16. doi: 10.1136/annrheumdis-2016-210441).
A total of 9,788 PsA and 158,323 psoriasis patients were included in the study, along with 39,306 RA patients and 821,834 individuals from the general population. Psoriasis patients were divided into groups classified as mild (n = 149,809) or severe (n = 8,514). The average age of each cohort ranged from nearly 47 years to almost 59 years, with all cohorts comprising mostly females, ranging from about 51% to 69%.
“We found that the risk for any fracture in patients with PsA and severe psoriasis was similar to RA [but] patients with PsA and psoriasis had an increased incidence of fracture compared with the general population by 7%-26%,” the authors explained. “The incidence of vertebral fracture was also increased in patients with severe psoriasis and while hip fracture was elevated in both psoriasis groups, it was only statistically significant in patients with mild psoriasis relative to matched controls after adjusting for risk factors for osteoporosis.”
Dr. Ogdie-Beatty and her colleagues found that all of the conditions conferred an elevated risk for fractures anywhere in the body when compared with the general population, reaching hazard ratios of 1.16 (95% confidence interval, 1.06-1.27) for people with PsA, 1.07 (95% CI, 1.05-1.10) for mild psoriasis, 1.26 for severe psoriasis (95% CI, 1.15-1.39), and 1.23 for RA (95% CI, 1.18-1.28). The risk for hip fractures was only significantly higher for mild (hazard ratio, 1.13; 95% CI, 1.04-1.22) and severe psoriasis (HR, 1.21; 95% CI, 0.88-1.66), and RA (HR, 1.55; 95% CI, 1.40-1.72). Individuals with PsA did not have a significantly higher risk for vertebral fractures (HR, 1.07; 95% CI, 0.66-1.72), whereas those with mild psoriasis (HR, 1.17; 95% CI, 1.03-1.33), severe psoriasis (HR, 2.23; 95% CI, 1.54-3.22), or RA did (HR, 1.53; 95% CI, 1.30-1.80). Each of these models were fully adjusted for multiple different osteoporosis risk factors, although they were all commonly adjusted for age, sex, atrial fibrillation, diabetes, chronic obstructive pulmonary disease, stroke, SSRI use, tricyclic antidepressant use, oral steroids, smoking, and categorical body mass index.
Individual coauthors disclosed receiving funding for their work from the National Institutes of Health, as well as grants from the Department of Veterans Affairs and the Rheumatology Research Foundation. Three of the authors reported receiving payment for continuing medical education work related to psoriatic arthritis or psoriasis.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point:
Major finding: The risk of fracture for patients with PsA and psoriasis had a risk of fracture that was increased by 7%-26% in comparison with the general U.K. population.
Data source: Population-based, longitudinal cohort study of 9,788 PsA patients and 158,323 psoriasis patients in the United Kingdom during 1994-2014.
Disclosures: Individual coauthors disclosed receiving funding for their work from the National Institutes of Health, as well as grants from the Department of Veterans Affairs and the Rheumatology Research Foundation. Three of the authors reported receiving payment for continuing medical education work related to psoriatic arthritis or psoriasis.
Osteopenia risk up in men with sarcopenia and COPD
Men experiencing sarcopenia who also have been diagnosed with chronic obstructive pulmonary disease (COPD) are at a significantly higher risk of developing osteopenia and osteoporosis than are men who do not suffer from COPD, according to a new study published in Chest.
“Muscle depletion has been considered a risk factor for low [bone mineral density (BMD)] in the healthy general population [but] data on the association between sarcopenia and osteopenia/osteoporosis in COPD patients are lacking,” wrote the investigators of the study, coauthored by Moo Suk Park, MD, of Yonsei University in Seoul, South Korea (Chest. 2017 Jan. doi: 10.1016/j.chest.2016.12.006).
“Although previous studies showed that loss of fat-free mass (FFM) was related to BMD loss in COPD patients, it is difficult to know the genuine relationship between skeletal muscle mass and BMD because whole body FFM contains a large proportion of water-retaining organs and nonmuscle soft tissue,” the authors continued.
The investigators examined data from the Korean National Health and Nutritional Examination Survey (KNHANES), looking for men at least 20 years of age with COPD who had both pulmonary function test and the dual-energy x-ray absorptiometry (DXA) performed on them during the years 2008-2011. A total of 864 men were deemed eligible for inclusion, and were scored for sarcopenia and osteopenia/osteoporosis; the former was assessed via the appendicular skeletal mass index (ASMI), with the latter done via T-score.
“Sarcopenia and presarcopenia were defined according to the presence of ASMI values that were less than two standard deviations (SDs) and between 2SDs and 1SD, respectively, below the mean value of a young male reference group aged 20-39 years,” according to the investigators. “Osteoporosis, osteopenia, and normal BMD were identified according to the lowest T-score of the three measured locations and were defined according to the World Health Organization criteria.”
“This study affirms the systemic nature of COPD, as it is not merely a disease that manifests as breathlessness and other respiratory complaints, but affects many aspects of a patient’s functionality and overall health,” explained Eric J. Gartman, MD, of Brown University, Providence, Rhode Island. “In clinical practice, this study reminds us that we need to consider these other issues in a COPD patient’s care, since the outcomes from these problems (e.g. hip fractures) can be devastating.”
A critical limitation of this study, however, is the sample population, according to Dr. Gartman. “It is solely made up of Korean men, thus somewhat limiting the generalizability to a larger population [and] especially to women, given that there are several other considerations surrounding effects on BMD.”
No funding sources were disclosed. The authors reported no conflicts of interest.
*This article was updated on 1/20/17 at 1:30 p.m. It misstated the affiliation for Vera Palo, MD, FCCP.
Men experiencing sarcopenia who also have been diagnosed with chronic obstructive pulmonary disease (COPD) are at a significantly higher risk of developing osteopenia and osteoporosis than are men who do not suffer from COPD, according to a new study published in Chest.
“Muscle depletion has been considered a risk factor for low [bone mineral density (BMD)] in the healthy general population [but] data on the association between sarcopenia and osteopenia/osteoporosis in COPD patients are lacking,” wrote the investigators of the study, coauthored by Moo Suk Park, MD, of Yonsei University in Seoul, South Korea (Chest. 2017 Jan. doi: 10.1016/j.chest.2016.12.006).
“Although previous studies showed that loss of fat-free mass (FFM) was related to BMD loss in COPD patients, it is difficult to know the genuine relationship between skeletal muscle mass and BMD because whole body FFM contains a large proportion of water-retaining organs and nonmuscle soft tissue,” the authors continued.
The investigators examined data from the Korean National Health and Nutritional Examination Survey (KNHANES), looking for men at least 20 years of age with COPD who had both pulmonary function test and the dual-energy x-ray absorptiometry (DXA) performed on them during the years 2008-2011. A total of 864 men were deemed eligible for inclusion, and were scored for sarcopenia and osteopenia/osteoporosis; the former was assessed via the appendicular skeletal mass index (ASMI), with the latter done via T-score.
“Sarcopenia and presarcopenia were defined according to the presence of ASMI values that were less than two standard deviations (SDs) and between 2SDs and 1SD, respectively, below the mean value of a young male reference group aged 20-39 years,” according to the investigators. “Osteoporosis, osteopenia, and normal BMD were identified according to the lowest T-score of the three measured locations and were defined according to the World Health Organization criteria.”
“This study affirms the systemic nature of COPD, as it is not merely a disease that manifests as breathlessness and other respiratory complaints, but affects many aspects of a patient’s functionality and overall health,” explained Eric J. Gartman, MD, of Brown University, Providence, Rhode Island. “In clinical practice, this study reminds us that we need to consider these other issues in a COPD patient’s care, since the outcomes from these problems (e.g. hip fractures) can be devastating.”
A critical limitation of this study, however, is the sample population, according to Dr. Gartman. “It is solely made up of Korean men, thus somewhat limiting the generalizability to a larger population [and] especially to women, given that there are several other considerations surrounding effects on BMD.”
No funding sources were disclosed. The authors reported no conflicts of interest.
*This article was updated on 1/20/17 at 1:30 p.m. It misstated the affiliation for Vera Palo, MD, FCCP.
Men experiencing sarcopenia who also have been diagnosed with chronic obstructive pulmonary disease (COPD) are at a significantly higher risk of developing osteopenia and osteoporosis than are men who do not suffer from COPD, according to a new study published in Chest.
“Muscle depletion has been considered a risk factor for low [bone mineral density (BMD)] in the healthy general population [but] data on the association between sarcopenia and osteopenia/osteoporosis in COPD patients are lacking,” wrote the investigators of the study, coauthored by Moo Suk Park, MD, of Yonsei University in Seoul, South Korea (Chest. 2017 Jan. doi: 10.1016/j.chest.2016.12.006).
“Although previous studies showed that loss of fat-free mass (FFM) was related to BMD loss in COPD patients, it is difficult to know the genuine relationship between skeletal muscle mass and BMD because whole body FFM contains a large proportion of water-retaining organs and nonmuscle soft tissue,” the authors continued.
The investigators examined data from the Korean National Health and Nutritional Examination Survey (KNHANES), looking for men at least 20 years of age with COPD who had both pulmonary function test and the dual-energy x-ray absorptiometry (DXA) performed on them during the years 2008-2011. A total of 864 men were deemed eligible for inclusion, and were scored for sarcopenia and osteopenia/osteoporosis; the former was assessed via the appendicular skeletal mass index (ASMI), with the latter done via T-score.
“Sarcopenia and presarcopenia were defined according to the presence of ASMI values that were less than two standard deviations (SDs) and between 2SDs and 1SD, respectively, below the mean value of a young male reference group aged 20-39 years,” according to the investigators. “Osteoporosis, osteopenia, and normal BMD were identified according to the lowest T-score of the three measured locations and were defined according to the World Health Organization criteria.”
“This study affirms the systemic nature of COPD, as it is not merely a disease that manifests as breathlessness and other respiratory complaints, but affects many aspects of a patient’s functionality and overall health,” explained Eric J. Gartman, MD, of Brown University, Providence, Rhode Island. “In clinical practice, this study reminds us that we need to consider these other issues in a COPD patient’s care, since the outcomes from these problems (e.g. hip fractures) can be devastating.”
A critical limitation of this study, however, is the sample population, according to Dr. Gartman. “It is solely made up of Korean men, thus somewhat limiting the generalizability to a larger population [and] especially to women, given that there are several other considerations surrounding effects on BMD.”
No funding sources were disclosed. The authors reported no conflicts of interest.
*This article was updated on 1/20/17 at 1:30 p.m. It misstated the affiliation for Vera Palo, MD, FCCP.
FROM CHEST
Key clinical point:
Major finding: Sarcopenia in men with COPD carried a significantly higher risk of bone mineral density loss: OR = 2.31 (95% CI 1.53–3.46) (P less than .001).
Data source: Retrospective cross-sectional study of data on 777 men with COPD during 2008-2011.
Disclosures: No funding sources were disclosed. The authors reported no conflicts of interest.
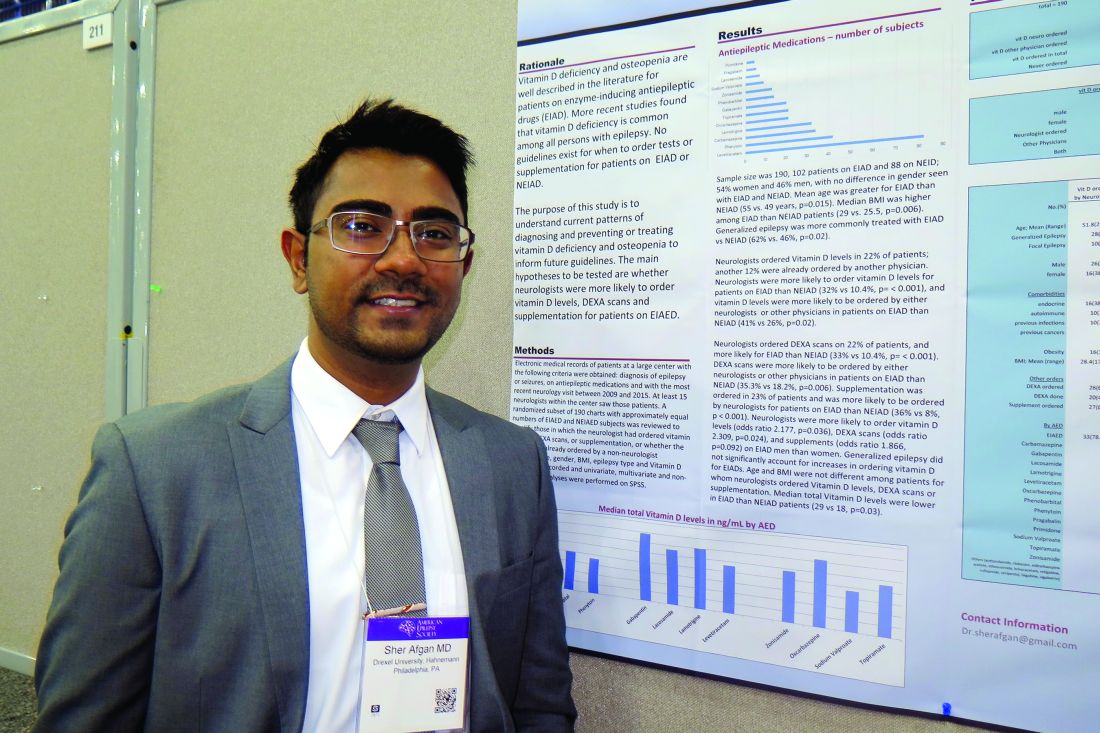
Study highlights need to address vitamin D deficiency in epilepsy patients
HOUSTON – Neurologists and other clinicians ordered vitamin D levels, dual-energy x-ray absorptiometry (DXA) scans, and vitamin D supplementation for epilepsy patients in order to diagnose and prevent vitamin D deficiency and osteopenia, results from a single-center study showed.
Vitamin D deficiency and osteopenia are well described in the literature for patients on enzyme-inducing antiepileptic drugs (EIADs), but no guidelines currently exist for when to order tests or supplementation for patients on EIADs or non–enzyme inducing antiepileptic drugs (NEIADs). “Further studies with larger sample sizes will be helpful in order to establish guidelines for neurologists and other physicians,” Sher Afgan, MD, said in an interview at the annual meeting of the American Epilepsy Society.
Dr. Afgan, a research assistant at Drexel, went on to report that neurologists ordered vitamin D levels in 22% of patients; another 12% had already been ordered by another physician. Neurologists were more likely to order vitamin D levels for patients on EIADs, compared with those on NEIADs (32% vs. 10.4%; P less than .001), and vitamin D levels were more likely to be ordered by either neurologists or other physicians for patients on EIADs, compared with those on NEIADs (41% vs. 26%; P = .02). Neurologists ordered DXA scans in 22% of patients, and more often for those on EIADs, compared with those on NEIADs (33% vs. 10.4%; P less than .001). Similarly, DXA scans were more likely to be ordered by either neurologists or other physicians for patients on EIADs, compared with those on NEIADs (35.3% vs. 18.2%; P = .006). Supplementation was ordered in 23% of patients and was more likely to be ordered by neurologists for patients on EIADs, compared with those on NEIADs (36% vs. 8%; P less than .001).
The researchers also found that neurologists were more likely to order vitamin D levels, DXA scans, and supplements for men on EIADs, compared with women on EIADs (odds ratio, 2.178, P = .03; OR, 2.31, P = .02; OR, 1.87, P = .09, respectively). Generalized epilepsy did not significantly account for increases in ordering vitamin D for EIADs. Median total vitamin D levels were lower in patients on EIADs, compared with those on NEIADs (29 vs. 18 ng/mL; P = .03), but age and body mass index were not different among patients for whom neurologists ordered Vitamin D levels, DXA scans, or supplementation.
Dr. Afgan acknowledged certain limitations of the study, including its retrospective design and small sample size. “Also, type and duration of epilepsy, type and duration of antiepileptic drugs, and comorbidities should be considered in further studies with larger sample sizes,” he said. He reported having no financial disclosures.
HOUSTON – Neurologists and other clinicians ordered vitamin D levels, dual-energy x-ray absorptiometry (DXA) scans, and vitamin D supplementation for epilepsy patients in order to diagnose and prevent vitamin D deficiency and osteopenia, results from a single-center study showed.
Vitamin D deficiency and osteopenia are well described in the literature for patients on enzyme-inducing antiepileptic drugs (EIADs), but no guidelines currently exist for when to order tests or supplementation for patients on EIADs or non–enzyme inducing antiepileptic drugs (NEIADs). “Further studies with larger sample sizes will be helpful in order to establish guidelines for neurologists and other physicians,” Sher Afgan, MD, said in an interview at the annual meeting of the American Epilepsy Society.
Dr. Afgan, a research assistant at Drexel, went on to report that neurologists ordered vitamin D levels in 22% of patients; another 12% had already been ordered by another physician. Neurologists were more likely to order vitamin D levels for patients on EIADs, compared with those on NEIADs (32% vs. 10.4%; P less than .001), and vitamin D levels were more likely to be ordered by either neurologists or other physicians for patients on EIADs, compared with those on NEIADs (41% vs. 26%; P = .02). Neurologists ordered DXA scans in 22% of patients, and more often for those on EIADs, compared with those on NEIADs (33% vs. 10.4%; P less than .001). Similarly, DXA scans were more likely to be ordered by either neurologists or other physicians for patients on EIADs, compared with those on NEIADs (35.3% vs. 18.2%; P = .006). Supplementation was ordered in 23% of patients and was more likely to be ordered by neurologists for patients on EIADs, compared with those on NEIADs (36% vs. 8%; P less than .001).
The researchers also found that neurologists were more likely to order vitamin D levels, DXA scans, and supplements for men on EIADs, compared with women on EIADs (odds ratio, 2.178, P = .03; OR, 2.31, P = .02; OR, 1.87, P = .09, respectively). Generalized epilepsy did not significantly account for increases in ordering vitamin D for EIADs. Median total vitamin D levels were lower in patients on EIADs, compared with those on NEIADs (29 vs. 18 ng/mL; P = .03), but age and body mass index were not different among patients for whom neurologists ordered Vitamin D levels, DXA scans, or supplementation.
Dr. Afgan acknowledged certain limitations of the study, including its retrospective design and small sample size. “Also, type and duration of epilepsy, type and duration of antiepileptic drugs, and comorbidities should be considered in further studies with larger sample sizes,” he said. He reported having no financial disclosures.
HOUSTON – Neurologists and other clinicians ordered vitamin D levels, dual-energy x-ray absorptiometry (DXA) scans, and vitamin D supplementation for epilepsy patients in order to diagnose and prevent vitamin D deficiency and osteopenia, results from a single-center study showed.
Vitamin D deficiency and osteopenia are well described in the literature for patients on enzyme-inducing antiepileptic drugs (EIADs), but no guidelines currently exist for when to order tests or supplementation for patients on EIADs or non–enzyme inducing antiepileptic drugs (NEIADs). “Further studies with larger sample sizes will be helpful in order to establish guidelines for neurologists and other physicians,” Sher Afgan, MD, said in an interview at the annual meeting of the American Epilepsy Society.
Dr. Afgan, a research assistant at Drexel, went on to report that neurologists ordered vitamin D levels in 22% of patients; another 12% had already been ordered by another physician. Neurologists were more likely to order vitamin D levels for patients on EIADs, compared with those on NEIADs (32% vs. 10.4%; P less than .001), and vitamin D levels were more likely to be ordered by either neurologists or other physicians for patients on EIADs, compared with those on NEIADs (41% vs. 26%; P = .02). Neurologists ordered DXA scans in 22% of patients, and more often for those on EIADs, compared with those on NEIADs (33% vs. 10.4%; P less than .001). Similarly, DXA scans were more likely to be ordered by either neurologists or other physicians for patients on EIADs, compared with those on NEIADs (35.3% vs. 18.2%; P = .006). Supplementation was ordered in 23% of patients and was more likely to be ordered by neurologists for patients on EIADs, compared with those on NEIADs (36% vs. 8%; P less than .001).
The researchers also found that neurologists were more likely to order vitamin D levels, DXA scans, and supplements for men on EIADs, compared with women on EIADs (odds ratio, 2.178, P = .03; OR, 2.31, P = .02; OR, 1.87, P = .09, respectively). Generalized epilepsy did not significantly account for increases in ordering vitamin D for EIADs. Median total vitamin D levels were lower in patients on EIADs, compared with those on NEIADs (29 vs. 18 ng/mL; P = .03), but age and body mass index were not different among patients for whom neurologists ordered Vitamin D levels, DXA scans, or supplementation.
Dr. Afgan acknowledged certain limitations of the study, including its retrospective design and small sample size. “Also, type and duration of epilepsy, type and duration of antiepileptic drugs, and comorbidities should be considered in further studies with larger sample sizes,” he said. He reported having no financial disclosures.
AT AES 2016
Key clinical point:
Major finding: Neurologists ordered vitamin D levels in 22% of patients; another 12% were already ordered by another physician.
Data source: A retrospective review of 190 patients who had a diagnosis of epilepsy or seizures, were currently on antiepileptic medications, and whose most recent neurology visit occurred between 2009 and 2015.
Disclosures: Dr. Afgan reported having no financial disclosures.
VIDEO: Denosumab trumps risedronate in bone building for glucocorticoid-induced osteoporosis
WASHINGTON – Denosumab built significantly more bone at the hip and lumbar spine than did risedronate when given for 1 year to patients with glucocorticoid-induced osteoporosis in an ongoing 2-year, head-to-head, randomized trial.
Denosumab (Prolia) is currently approved for the treatment of postmenopausal osteoporosis, and it performed so well in the trial that it could be put forward for the indication of glucocorticoid-induced osteoporosis as well, Kenneth Saag, MD, said at the annual meeting of the American College of Rheumatology.
“I would say there is definitely potential for this as a new therapeutic option for these patients,” he said in a video interview about the trial’s primary outcome of denosumab’s noninferiority to risedronate in percentage change in bone mineral density (BMD) at the lumbar spine after 1 year and secondary outcomes of the superiority of denosumab over risedronate in total hip and lumbar spine BMD at 1 year.
Denosumab is a particularly intriguing treatment option for patients with glucocorticoid-induced osteoporosis. They experience a double hit on bone health: increased RANKL, a protein that stimulates osteoclast development, and decreased osteoprotegerin, a protein that inhibits osteoclasts. Denosumab is a RANKL-inhibitor and, as such, tamps down on osteoclastic bone remodeling, said Dr. Saag, vice chair of the department of medicine and director of the Center for Education and Research on Therapeutics at the University of Alabama at Birmingham.
The phase III trial comprised 795 patients who were taking corticosteroids for a variety of rheumatic diseases, including rheumatoid arthritis, polymyalgia rheumatica, and systemic lupus erythematosus, and randomized them to denosumab or risedronate, which is already FDA approved for glucocorticoid-induced bone loss. Patients were randomized to 24 months of subcutaneous denosumab 60 mg given every 6 months or oral risedronate 5-mg daily. The study is still ongoing to test secondary outcomes at 24 months.
The patients were split into those who were continuing glucocorticoid therapy (505) and those who were just initiating it (290). Patients’ mean age ranged from 61 to 67 years, with the glucocorticoid-initiating group (GC-I) being somewhat older. The mean daily prednisone-equivalent dose was 16 mg in that group and 12 mg in the glucocorticoid-continuing group (GC-C). The mean BMD T-scores in the GC-C group were –1.96 at the lumbar spine and –1.56 at the total hip. In the GC-I group, BMD T-scores were –1.06 at the lumbar spine and –0.98 at the total hip.
In the GC-C group, denosumab increased BMD significantly more than risedronate at both spine and hip. At the lumbar spine, denosumab was associated with a mean increase of 4.4% over baseline, compared with a 2.3% increase with risedronate. Total hip BMD increased 2.1% with denosumab and 0.6% with risedronate.
The results were similar in the GC-I group. Denosumab increased lumbar spine BMD by 3.8% over baseline, compared with an increase of 0.8% with risedronate. Total hip BMD increased 1.7% with denosumab and 0.2% with risedronate.
Denosumab was also associated with significantly greater increases in femoral neck BMD in both groups, Dr. Saag noted. There were no significant differences in markers of bone turnover between the treatment groups. Adverse events, including pneumonia, diverticulitis, and bronchitis, were similar.
Amgen, manufacturer of denosumab, is sponsoring the 24-month study. Dr. Saag has been a consultant for Amgen. One coauthor is an employee of Amgen, and others disclosed financial relationships with Amgen and other pharmaceutical companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @alz_gal
WASHINGTON – Denosumab built significantly more bone at the hip and lumbar spine than did risedronate when given for 1 year to patients with glucocorticoid-induced osteoporosis in an ongoing 2-year, head-to-head, randomized trial.
Denosumab (Prolia) is currently approved for the treatment of postmenopausal osteoporosis, and it performed so well in the trial that it could be put forward for the indication of glucocorticoid-induced osteoporosis as well, Kenneth Saag, MD, said at the annual meeting of the American College of Rheumatology.
“I would say there is definitely potential for this as a new therapeutic option for these patients,” he said in a video interview about the trial’s primary outcome of denosumab’s noninferiority to risedronate in percentage change in bone mineral density (BMD) at the lumbar spine after 1 year and secondary outcomes of the superiority of denosumab over risedronate in total hip and lumbar spine BMD at 1 year.
Denosumab is a particularly intriguing treatment option for patients with glucocorticoid-induced osteoporosis. They experience a double hit on bone health: increased RANKL, a protein that stimulates osteoclast development, and decreased osteoprotegerin, a protein that inhibits osteoclasts. Denosumab is a RANKL-inhibitor and, as such, tamps down on osteoclastic bone remodeling, said Dr. Saag, vice chair of the department of medicine and director of the Center for Education and Research on Therapeutics at the University of Alabama at Birmingham.
The phase III trial comprised 795 patients who were taking corticosteroids for a variety of rheumatic diseases, including rheumatoid arthritis, polymyalgia rheumatica, and systemic lupus erythematosus, and randomized them to denosumab or risedronate, which is already FDA approved for glucocorticoid-induced bone loss. Patients were randomized to 24 months of subcutaneous denosumab 60 mg given every 6 months or oral risedronate 5-mg daily. The study is still ongoing to test secondary outcomes at 24 months.
The patients were split into those who were continuing glucocorticoid therapy (505) and those who were just initiating it (290). Patients’ mean age ranged from 61 to 67 years, with the glucocorticoid-initiating group (GC-I) being somewhat older. The mean daily prednisone-equivalent dose was 16 mg in that group and 12 mg in the glucocorticoid-continuing group (GC-C). The mean BMD T-scores in the GC-C group were –1.96 at the lumbar spine and –1.56 at the total hip. In the GC-I group, BMD T-scores were –1.06 at the lumbar spine and –0.98 at the total hip.
In the GC-C group, denosumab increased BMD significantly more than risedronate at both spine and hip. At the lumbar spine, denosumab was associated with a mean increase of 4.4% over baseline, compared with a 2.3% increase with risedronate. Total hip BMD increased 2.1% with denosumab and 0.6% with risedronate.
The results were similar in the GC-I group. Denosumab increased lumbar spine BMD by 3.8% over baseline, compared with an increase of 0.8% with risedronate. Total hip BMD increased 1.7% with denosumab and 0.2% with risedronate.
Denosumab was also associated with significantly greater increases in femoral neck BMD in both groups, Dr. Saag noted. There were no significant differences in markers of bone turnover between the treatment groups. Adverse events, including pneumonia, diverticulitis, and bronchitis, were similar.
Amgen, manufacturer of denosumab, is sponsoring the 24-month study. Dr. Saag has been a consultant for Amgen. One coauthor is an employee of Amgen, and others disclosed financial relationships with Amgen and other pharmaceutical companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @alz_gal
WASHINGTON – Denosumab built significantly more bone at the hip and lumbar spine than did risedronate when given for 1 year to patients with glucocorticoid-induced osteoporosis in an ongoing 2-year, head-to-head, randomized trial.
Denosumab (Prolia) is currently approved for the treatment of postmenopausal osteoporosis, and it performed so well in the trial that it could be put forward for the indication of glucocorticoid-induced osteoporosis as well, Kenneth Saag, MD, said at the annual meeting of the American College of Rheumatology.
“I would say there is definitely potential for this as a new therapeutic option for these patients,” he said in a video interview about the trial’s primary outcome of denosumab’s noninferiority to risedronate in percentage change in bone mineral density (BMD) at the lumbar spine after 1 year and secondary outcomes of the superiority of denosumab over risedronate in total hip and lumbar spine BMD at 1 year.
Denosumab is a particularly intriguing treatment option for patients with glucocorticoid-induced osteoporosis. They experience a double hit on bone health: increased RANKL, a protein that stimulates osteoclast development, and decreased osteoprotegerin, a protein that inhibits osteoclasts. Denosumab is a RANKL-inhibitor and, as such, tamps down on osteoclastic bone remodeling, said Dr. Saag, vice chair of the department of medicine and director of the Center for Education and Research on Therapeutics at the University of Alabama at Birmingham.
The phase III trial comprised 795 patients who were taking corticosteroids for a variety of rheumatic diseases, including rheumatoid arthritis, polymyalgia rheumatica, and systemic lupus erythematosus, and randomized them to denosumab or risedronate, which is already FDA approved for glucocorticoid-induced bone loss. Patients were randomized to 24 months of subcutaneous denosumab 60 mg given every 6 months or oral risedronate 5-mg daily. The study is still ongoing to test secondary outcomes at 24 months.
The patients were split into those who were continuing glucocorticoid therapy (505) and those who were just initiating it (290). Patients’ mean age ranged from 61 to 67 years, with the glucocorticoid-initiating group (GC-I) being somewhat older. The mean daily prednisone-equivalent dose was 16 mg in that group and 12 mg in the glucocorticoid-continuing group (GC-C). The mean BMD T-scores in the GC-C group were –1.96 at the lumbar spine and –1.56 at the total hip. In the GC-I group, BMD T-scores were –1.06 at the lumbar spine and –0.98 at the total hip.
In the GC-C group, denosumab increased BMD significantly more than risedronate at both spine and hip. At the lumbar spine, denosumab was associated with a mean increase of 4.4% over baseline, compared with a 2.3% increase with risedronate. Total hip BMD increased 2.1% with denosumab and 0.6% with risedronate.
The results were similar in the GC-I group. Denosumab increased lumbar spine BMD by 3.8% over baseline, compared with an increase of 0.8% with risedronate. Total hip BMD increased 1.7% with denosumab and 0.2% with risedronate.
Denosumab was also associated with significantly greater increases in femoral neck BMD in both groups, Dr. Saag noted. There were no significant differences in markers of bone turnover between the treatment groups. Adverse events, including pneumonia, diverticulitis, and bronchitis, were similar.
Amgen, manufacturer of denosumab, is sponsoring the 24-month study. Dr. Saag has been a consultant for Amgen. One coauthor is an employee of Amgen, and others disclosed financial relationships with Amgen and other pharmaceutical companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @alz_gal
AT THE ACR ANNUAL MEETING
Key clinical point:
Major finding: In patients on continuous glucocorticoid therapy, denosumab increased BMD by 4.4% at the lumbar spine and 2.1% at the total hip, compared with increases of 2.3% and 0.6% with risedronate.
Data source: 12-month results of the 24-month, phase III study of 795 patients.
Disclosures: Amgen sponsored the study. Dr. Saag has been a consultant for the company. One coauthor is an employee of Amgen, and others disclosed financial relationships with Amgen and other pharmaceutical companies.
VIDEO: ACR recommendations for glucocorticoid-induced osteoporosis unveiled
WASHINGTON – New American College of Rheumatology recommendations for glucocorticoid-induced osteoporosis prevention and treatment include refinements in risk assessment and treatment.
“These are draft recommendations not yet accepted by ACR,” said Lenore M. Buckley, MD, of Yale University, New Haven, Conn. “They are intended to be dynamic, because risk factors change for patients over time,” she added.
The draft recommendations build upon the 2010 ACR recommendations.
“About 1% of the United States population is on glucocorticoid treatment. Fracture is the most common adverse event, and trabecular bone in the spine is the most vulnerable,” Dr. Buckley explained in her presentation of the recommendations at the annual meeting of the American College of Rheumatology. “The risk of glucocorticoid (GC)-induced fracture is related to dose level and cumulative dose.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The draft recommendations were developed via GRADE (Grading of Assessment, Development, and Evaluation) methodology by a core team of internists, rheumatologists, a GRADE expert, a voting panel, and an expert panel.
Recommendations for risk assessment
Risk assessment for GC-induced osteoporosis is individualized. “You need to know the patient in front of you. Fracture risk is not just related to GC use, but also to bone mass, age, and race. Older age, female gender, Caucasian race all increase risk, and these factors need to be brought in to assessment,” Dr. Buckley explained.
For men and women over age 40, the Fracture Risk Assessment Tool (FRAX), which calculates the 10-year fracture risk in adults over age 40, should be used for risk assessment, incorporating GC use as a risk factor, she said.
“Adjust risk of FRAX according to dose of glucocorticoid. For 2.5-7.5 mg/day, the FRAX risk is fine, but if the patient is on higher doses, adjust the FRAX accordingly,” she said.
FRAX is not valid for women and men under age 40, she continued. A new recommendation is the inclusion of a moderate risk group based on very low bone mass score (Z score less than –3 below the standard deviation of the mean) and/or rapid bone loss (greater than 10% in 1 year). Patients who had prior GC-associated fracture under 40 years are considered high risk.
A thorough history and physical exam are necessary for all patients, and risk assessment should be done within 6 months of GC initiation. Physical exam should be repeated annually, and bone mineral density (BMD) should be assessed every 2-3 years for patients who continue on GC.
Treatment
The proposed treatment recommendations were not age dependent. Patients with moderate to high risk should be treated, in descending order, with oral bisphosphonates, intravenous bisphosphonates, teriparatide (Forteo), and denosumab (Prolia). The order of preference for these treatments was based on cost, efficacy, toxicity, and patient preference.
All patients should take calcium and vitamin D, regardless of risk level.
The proposed recommendations also addressed four special groups considered at significant risk: women of childbearing potential, organ transplant recipients, children, and people on very high doses of GC (greater than 30 mg/day, cumulative dose of 5 g/year).
“For women of childbearing potential, there are emerging data suggesting that bisphosphonates are safe. For this group, consider oral bisphosphonate and teriparatide as a second choice. Animal data suggest that IV bisphosphonates and denosumab are harmful to the fetus,” Dr. Buckley said.
For organ transplant recipients, general recommendations can be followed with two provisions: Kidney transplant recipients should have a work-up for metabolic bone disease, and denosumab should not be used in people on multiple immunosuppressants.
Optimize calcium and vitamin D for children, and treat with oral bisphosphonate, Dr. Buckley continued. If oral bisphosphonates are contraindicated, IV bisphosphonates can be used.
Patients on very high GC dose should be treated with oral bisphosphonates if they are age 30 or older.
Osteoporosis medications can be discontinued in low-risk patients who stop taking GC, but should be continued for those at moderate to high risk.
Patients who benefit from osteoporosis medications but remain at moderate to high risk at the end of 3 years should continue GC treatment.
The authors and sponsors had no relevant financial disclosures.
WASHINGTON – New American College of Rheumatology recommendations for glucocorticoid-induced osteoporosis prevention and treatment include refinements in risk assessment and treatment.
“These are draft recommendations not yet accepted by ACR,” said Lenore M. Buckley, MD, of Yale University, New Haven, Conn. “They are intended to be dynamic, because risk factors change for patients over time,” she added.
The draft recommendations build upon the 2010 ACR recommendations.
“About 1% of the United States population is on glucocorticoid treatment. Fracture is the most common adverse event, and trabecular bone in the spine is the most vulnerable,” Dr. Buckley explained in her presentation of the recommendations at the annual meeting of the American College of Rheumatology. “The risk of glucocorticoid (GC)-induced fracture is related to dose level and cumulative dose.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The draft recommendations were developed via GRADE (Grading of Assessment, Development, and Evaluation) methodology by a core team of internists, rheumatologists, a GRADE expert, a voting panel, and an expert panel.
Recommendations for risk assessment
Risk assessment for GC-induced osteoporosis is individualized. “You need to know the patient in front of you. Fracture risk is not just related to GC use, but also to bone mass, age, and race. Older age, female gender, Caucasian race all increase risk, and these factors need to be brought in to assessment,” Dr. Buckley explained.
For men and women over age 40, the Fracture Risk Assessment Tool (FRAX), which calculates the 10-year fracture risk in adults over age 40, should be used for risk assessment, incorporating GC use as a risk factor, she said.
“Adjust risk of FRAX according to dose of glucocorticoid. For 2.5-7.5 mg/day, the FRAX risk is fine, but if the patient is on higher doses, adjust the FRAX accordingly,” she said.
FRAX is not valid for women and men under age 40, she continued. A new recommendation is the inclusion of a moderate risk group based on very low bone mass score (Z score less than –3 below the standard deviation of the mean) and/or rapid bone loss (greater than 10% in 1 year). Patients who had prior GC-associated fracture under 40 years are considered high risk.
A thorough history and physical exam are necessary for all patients, and risk assessment should be done within 6 months of GC initiation. Physical exam should be repeated annually, and bone mineral density (BMD) should be assessed every 2-3 years for patients who continue on GC.
Treatment
The proposed treatment recommendations were not age dependent. Patients with moderate to high risk should be treated, in descending order, with oral bisphosphonates, intravenous bisphosphonates, teriparatide (Forteo), and denosumab (Prolia). The order of preference for these treatments was based on cost, efficacy, toxicity, and patient preference.
All patients should take calcium and vitamin D, regardless of risk level.
The proposed recommendations also addressed four special groups considered at significant risk: women of childbearing potential, organ transplant recipients, children, and people on very high doses of GC (greater than 30 mg/day, cumulative dose of 5 g/year).
“For women of childbearing potential, there are emerging data suggesting that bisphosphonates are safe. For this group, consider oral bisphosphonate and teriparatide as a second choice. Animal data suggest that IV bisphosphonates and denosumab are harmful to the fetus,” Dr. Buckley said.
For organ transplant recipients, general recommendations can be followed with two provisions: Kidney transplant recipients should have a work-up for metabolic bone disease, and denosumab should not be used in people on multiple immunosuppressants.
Optimize calcium and vitamin D for children, and treat with oral bisphosphonate, Dr. Buckley continued. If oral bisphosphonates are contraindicated, IV bisphosphonates can be used.
Patients on very high GC dose should be treated with oral bisphosphonates if they are age 30 or older.
Osteoporosis medications can be discontinued in low-risk patients who stop taking GC, but should be continued for those at moderate to high risk.
Patients who benefit from osteoporosis medications but remain at moderate to high risk at the end of 3 years should continue GC treatment.
The authors and sponsors had no relevant financial disclosures.
WASHINGTON – New American College of Rheumatology recommendations for glucocorticoid-induced osteoporosis prevention and treatment include refinements in risk assessment and treatment.
“These are draft recommendations not yet accepted by ACR,” said Lenore M. Buckley, MD, of Yale University, New Haven, Conn. “They are intended to be dynamic, because risk factors change for patients over time,” she added.
The draft recommendations build upon the 2010 ACR recommendations.
“About 1% of the United States population is on glucocorticoid treatment. Fracture is the most common adverse event, and trabecular bone in the spine is the most vulnerable,” Dr. Buckley explained in her presentation of the recommendations at the annual meeting of the American College of Rheumatology. “The risk of glucocorticoid (GC)-induced fracture is related to dose level and cumulative dose.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The draft recommendations were developed via GRADE (Grading of Assessment, Development, and Evaluation) methodology by a core team of internists, rheumatologists, a GRADE expert, a voting panel, and an expert panel.
Recommendations for risk assessment
Risk assessment for GC-induced osteoporosis is individualized. “You need to know the patient in front of you. Fracture risk is not just related to GC use, but also to bone mass, age, and race. Older age, female gender, Caucasian race all increase risk, and these factors need to be brought in to assessment,” Dr. Buckley explained.
For men and women over age 40, the Fracture Risk Assessment Tool (FRAX), which calculates the 10-year fracture risk in adults over age 40, should be used for risk assessment, incorporating GC use as a risk factor, she said.
“Adjust risk of FRAX according to dose of glucocorticoid. For 2.5-7.5 mg/day, the FRAX risk is fine, but if the patient is on higher doses, adjust the FRAX accordingly,” she said.
FRAX is not valid for women and men under age 40, she continued. A new recommendation is the inclusion of a moderate risk group based on very low bone mass score (Z score less than –3 below the standard deviation of the mean) and/or rapid bone loss (greater than 10% in 1 year). Patients who had prior GC-associated fracture under 40 years are considered high risk.
A thorough history and physical exam are necessary for all patients, and risk assessment should be done within 6 months of GC initiation. Physical exam should be repeated annually, and bone mineral density (BMD) should be assessed every 2-3 years for patients who continue on GC.
Treatment
The proposed treatment recommendations were not age dependent. Patients with moderate to high risk should be treated, in descending order, with oral bisphosphonates, intravenous bisphosphonates, teriparatide (Forteo), and denosumab (Prolia). The order of preference for these treatments was based on cost, efficacy, toxicity, and patient preference.
All patients should take calcium and vitamin D, regardless of risk level.
The proposed recommendations also addressed four special groups considered at significant risk: women of childbearing potential, organ transplant recipients, children, and people on very high doses of GC (greater than 30 mg/day, cumulative dose of 5 g/year).
“For women of childbearing potential, there are emerging data suggesting that bisphosphonates are safe. For this group, consider oral bisphosphonate and teriparatide as a second choice. Animal data suggest that IV bisphosphonates and denosumab are harmful to the fetus,” Dr. Buckley said.
For organ transplant recipients, general recommendations can be followed with two provisions: Kidney transplant recipients should have a work-up for metabolic bone disease, and denosumab should not be used in people on multiple immunosuppressants.
Optimize calcium and vitamin D for children, and treat with oral bisphosphonate, Dr. Buckley continued. If oral bisphosphonates are contraindicated, IV bisphosphonates can be used.
Patients on very high GC dose should be treated with oral bisphosphonates if they are age 30 or older.
Osteoporosis medications can be discontinued in low-risk patients who stop taking GC, but should be continued for those at moderate to high risk.
Patients who benefit from osteoporosis medications but remain at moderate to high risk at the end of 3 years should continue GC treatment.
The authors and sponsors had no relevant financial disclosures.
AT THE ACR ANNUAL MEETING