User login
Does ‘Brain Training’ Really Improve Cognition and Forestall Cognitive Decline?
The concept that cognitive health can be preserved or improved is often expressed as “use it or lose it.” Numerous modifiable risk factors are associated with “losing” cognitive abilities with age, and a cognitively active lifestyle may have a protective effect.
But what is a “cognitively active lifestyle” — do crosswords and Sudoku count?
One popular approach is “brain training.” While not a scientific term with an established definition, it “typically refers to tasks or drills that are designed to strengthen specific aspects of one’s cognitive function,” explained Yuko Hara, PhD, director of Aging and Alzheimer’s Prevention at the Alzheimer’s Drug Discovery Foundation.
Manuel Montero-Odasso, MD, PhD, director of the Gait and Brain Lab, Parkwood Institute, London, Ontario, Canada, elaborated: “Cognitive training involves performing a definitive task or set of tasks where you increase attentional demands to improve focus and concentration and memory. You try to execute the new things that you’ve learned and to remember them.”
In a commentary published by this news organization in 2022, neuroscientist Michael Merzenich, PhD, professor emeritus at University of California San Francisco, said that growing a person’s cognitive reserve and actively managing brain health can play an important role in preventing or delaying Alzheimer’s disease. Important components of this include brain training and physical exercise.
Brain Training: Mechanism of Action
Dr. Montero-Odasso, team leader at the Canadian Consortium on Neurodegeneration in Aging and team co-leader at the Ontario Neurodegenerative Research Initiative, explained that cognitive training creates new synapses in the brain, thus stimulating neuroplasticity.
“When we try to activate networks mainly in the frontal lobe, the prefrontal cortex, a key mechanism underlying this process is enhancement of the synaptic plasticity at excitatory synapses, which connect neurons into networks; in other words, we generate new synapses, and that’s how we enhance brain health and cognitive abilities.”
The more neural connections, the greater the processing speed of the brain, he continued. “Cognitive training creates an anatomical change in the brain.”
Executive functions, which include attention, inhibition, planning, and multitasking, are regulated predominantly by the prefrontal cortex. Damage in this region of the brain is also implicated in dementia. Alterations in the connectivity of this area are associated with cognitive impairment, independent of other structural pathological aberrations (eg, gray matter atrophy). These patterns may precede structural pathological changes associated with cognitive impairment and dementia.
Neuroplasticity changes have been corroborated through neuroimaging, which has demonstrated that after cognitive training, there is more activation in the prefrontal cortex that correlates with new synapses, Dr. Montero-Odasso said.
Henry Mahncke, PhD, CEO of the brain training company Posit Science/BrainHQ, explained that early research was conducted on rodents and monkeys, with Dr. Merzenich as one of the leading pioneers in developing the concept of brain plasticity. Dr. Merzenich cofounded Posit Science and is currently its chief scientific officer.
Dr. Mahncke recounted that as a graduate student, he had worked with Dr. Merzenich researching brain plasticity. When Dr. Merzenich founded Posit Science, he asked Dr. Mahncke to join the company to help develop approaches to enhance brain plasticity — building the brain-training exercises and running the clinical trials.
“It’s now well understood that the brain can rewire itself at any age and in almost any condition,” Dr. Mahncke said. “In kids and in younger and older adults, whether with healthy or unhealthy brains, the fundamental way the brain works is by continually rewiring and rebuilding itself, based on what we ask it to do.”
Dr. Mahncke said.
Unsubstantiated Claims and Controversy
Brain training is not without controversy, Dr. Hara pointed out. “Some manufacturers of brain games have been criticized and even fined for making unsubstantiated claims,” she said.
A 2016 review found that brain-training interventions do improve performance on specific trained tasks, but there is less evidence that they improve performance on closely related tasks and little evidence that training improves everyday cognitive performance. A 2017 review reached similar conclusions, calling evidence regarding prevention or delay of cognitive decline or dementia through brain games “insufficient,” although cognitive training could “improve cognition in the domain trained.”
“The general consensus is that for most brain-training programs, people may get better at specific tasks through practice, but these improvements don’t necessarily translate into improvement in other tasks that require other cognitive domains or prevention of dementia or age-related cognitive decline,” Dr. Hara said.
She noted that most brain-training programs “have not been rigorously tested in clinical trials” — although some, such as those featured in the ACTIVE trial, did show evidence of effectiveness.
Dr. Mahncke agreed. “Asking whether brain training works is like asking whether small molecules improve health,” he said noting that some brain-training programs are nonsense and not evidence based. He believes that his company’s product, BrainHQ, and some others are “backed by robust evidence in their ability to stave off, slow, or even reverse cognitive changes.”
BrainHQ is a web-based brain game suite that can be used independently as an app or in group settings (classes and webinars) and is covered by some Medicare Advantage insurance plans. It encompasses “dozens of individual brain-training exercises, linked by a common thread. Each one is intensively designed to make the brain faster and more accurate,” said Dr. Mahncke.
He explained that human brains “get noisy as people get older, like a radio which is wearing out, so there’s static in the background. This makes the music hard to hear, and in the case of the human brain, it makes it difficult to pay attention.” The exercises are “designed to tamp down the ‘noise,’ speed up the brain, and make information processing more accurate.”
Dr. Mahncke called this a “bottom-up” approach, in contrast to many previous cognitive-training approaches that come from the brain injury rehabilitation field. They teach “top-down” skills and strategies designed to compensate for deficits in specific domains, such as reading, concentration, or fine motor skills.
By contrast, the approach of BrainHQ is “to improve the overall processing system of the brain with speed, attention, working memory, and executive function, which will in turn impact all skills and activities.”
Supporting Evidence
Dr. Mahncke cited several supporting studies. For example, the IMPACT study randomized 487 adults (aged ≥ 65 years) to receive either a brain plasticity–based computerized cognitive training program (BrainHQ) or a novelty- and intensity-matched general cognitive stimulation treatment program (intervention and control group, respectively) for an 8-week period.
Those who underwent brain training showed significantly greater improvement in the repeatable Battery for the Assessment of Neuropsychological Status (RBANS Auditory Memory/Attention) compared with those in the control group (3.9 vs 1.8, respectively; P =.02). The intervention group also showed significant improvements on multiple secondary measures of attention and memory. The magnitude of the effect sizes suggests that the results are clinically significant, according to the authors.
The ACTIVE study tested the effects of different cognitive training programs on cognitive function and time to dementia. The researchers randomized 2802 healthy older adults (mean age, 74 years) to a control group with no cognitive training or one of three brain-training groups comprising:
1. In-person training on verbal memory skills
2. In-person training on reasoning and problem-solving
3. Computer-based speed-of-processing training on visual attention
Participants in the training groups completed 10 sessions, each lasting 60-75 minutes, over a 5- to 6-week period. A random subsample of each training group was selected to receive “booster” sessions, with four-session booster training delivered at 11 and 35 months. All study participants completed follow-up tests of cognition and function after 1, 2, 3, 5, and 10 years.
At the end of 10 years, those assigned to the speed-of-processing training, now part of BrainHQ, had a 29% lower risk for dementia than those in the control group who received no training. No reduction was found in the memory or reasoning training groups. Participants who completed the “booster” sessions had an even greater reduction: Each additional booster session was associated with a 10% lower risk for dementia.
Dr. Montero-Odasso was involved in the SYNERGIC study that randomized 175 participants with mild cognitive impairment (MCI; average age, 73 years) to one of five study arms:
1. Multidomain intervention with exercise, cognitive training, and vitamin D
2. Exercise, cognitive training, and placebo
3. Exercise, sham cognitive training, and vitamin D
4. Exercise, sham cognitive training, and placebo
5. Control group with balance-toning exercise, sham cognitive training, and placebo
“Sham” cognitive training consisted of alternating between two tasks (touristic search and video watching) performed on a tablet, with the same time exposure as the intervention training.
The researchers found that after 6 months of interventions, all active arms with aerobic-resistance exercise showed improvement in the ADAS-Cog-13, an established outcome to evaluate dementia treatments, when compared with the control group — regardless of the addition of cognitive training or vitamin D.
Compared with exercise alone (arms 3 and 4), those who did exercise plus cognitive training (arms 1 and 2) showed greater improvements in their ADAS-Cog-13l score, with a mean difference of −1.45 points (P = .02). The greatest improvement was seen in those who underwent the multidomain intervention in arm 1.
The authors noted that the mean 2.64-point improvement seen in the ADAS-Cog-13 for the multidomain intervention is actually larger than changes seen in previous pharmaceutical trials among individuals with MCI or mild dementia and “approaches” the three points considered clinically meaningful.
“We found that older adults with MCI who received aerobic-resistance exercise with sequential computerized cognitive training significantly improved cognition,” Dr. Montero-Odasso said. “The cognitive training we used was called Neuropeak, a multidomain lifestyle training delivered through a web-based platform developed by our co-leader Louis Bherer at Université de Montréal.”
He explained that the purpose “is to challenge your brain to the point where you need to make an effort to remember things, pay attention, and later to execute tasks. The evidence from clinical trials, including ours, shows this type of brain challenge is effective in slowing and even reversing cognitive decline.”
A follow-up study, SYNERGIC 2.0, is ongoing.
Puzzles, Board Games, and New Challenges
Formal brain-training programs aren’t the only way to improve brain plasticity, Dr. Hara said. Observational studies suggested an association between improved cognitive performance and/or lower dementia risk and engaging in number and word puzzles, such as crosswords, cards, or board games.
Some studies suggested that older adults who use technology might also protect their cognitive reserve. Dr. Hara cited a US longitudinal study of more than 18,000 older adults suggesting that regular Internet users had roughly half the risk for dementia compared to nonregular Internet users. Estimates of daily Internet use suggested a U-shaped relationship with dementia with 0.1-2.0 hours daily (excluding time spent watching television or movies online) associated with the lowest risk. Similar associations between Internet use and a lower risk for cognitive decline have been reported in the United Kingdom and Europe.
“Engaging in mentally stimulating activities can increase ‘cognitive reserve’ — meaning, capacity of the brain to resist the effects of age-related changes or disease-related pathology, such that one can maintain cognitive function for longer,” Dr. Hara said. “Cognitively stimulating activities, regardless of the type, may help delay the onset of cognitive decline.”
She listed several examples of activities that are stimulating to the brain, including learning a new game or puzzle, a new language, or a new dance, and learning how to play a musical instrument.
Dr. Montero-Odasso emphasized that the “newness” is key to increasing and preserving cognitive reserve. “Just surfing the Internet, playing word or board games, or doing crossword puzzles won’t be enough if you’ve been doing these things all your life,” he said. “It won’t hurt, of course, but it won’t necessarily increase your cognitive abilities.
“For example, a person who regularly engages in public speaking may not improve cognition by taking a public-speaking course, but someone who has never spoken before an audience might show cognitive improvements as a result of learning a new skill,” he said. “Or someone who knows several languages already might gain from learning a brand-new language.”
He cited research supporting the benefits of dancing, which he called “an ideal activity because it’s physical, so it provides the exercise that’s been associated with improved cognition. But it also requires learning new steps and moves, which builds the synapses in the brain. And the socialization of dance classes adds another component that can improve cognition.”
Dr. Mahncke hopes that beyond engaging in day-to-day new activities, seniors will participate in computerized brain training. “There’s no reason that evidence-based training can’t be offered in senior and community centers, as yoga and swimming are,” he said. “It doesn’t have to be simply something people do on their own virtually.”
Zoom classes and Medicare reimbursements are “good steps in the right direction, but it’s time to expand this potentially life-transformative intervention so that it reaches the ever-expanding population of seniors in the United States and beyond.”
Dr. Hara reported having no disclosures. Dr. Montero-Odasso reported having no commercial or financial interest related to this topic. He serves as the president of the Canadian Geriatrics Société and is team leader in the Canadian Consortium of Neurodegeneration in Aging. Dr. Mahncke is CEO of the brain training company Posit Science/BrainHQ.
A version of this article appeared on Medscape.com.
The concept that cognitive health can be preserved or improved is often expressed as “use it or lose it.” Numerous modifiable risk factors are associated with “losing” cognitive abilities with age, and a cognitively active lifestyle may have a protective effect.
But what is a “cognitively active lifestyle” — do crosswords and Sudoku count?
One popular approach is “brain training.” While not a scientific term with an established definition, it “typically refers to tasks or drills that are designed to strengthen specific aspects of one’s cognitive function,” explained Yuko Hara, PhD, director of Aging and Alzheimer’s Prevention at the Alzheimer’s Drug Discovery Foundation.
Manuel Montero-Odasso, MD, PhD, director of the Gait and Brain Lab, Parkwood Institute, London, Ontario, Canada, elaborated: “Cognitive training involves performing a definitive task or set of tasks where you increase attentional demands to improve focus and concentration and memory. You try to execute the new things that you’ve learned and to remember them.”
In a commentary published by this news organization in 2022, neuroscientist Michael Merzenich, PhD, professor emeritus at University of California San Francisco, said that growing a person’s cognitive reserve and actively managing brain health can play an important role in preventing or delaying Alzheimer’s disease. Important components of this include brain training and physical exercise.
Brain Training: Mechanism of Action
Dr. Montero-Odasso, team leader at the Canadian Consortium on Neurodegeneration in Aging and team co-leader at the Ontario Neurodegenerative Research Initiative, explained that cognitive training creates new synapses in the brain, thus stimulating neuroplasticity.
“When we try to activate networks mainly in the frontal lobe, the prefrontal cortex, a key mechanism underlying this process is enhancement of the synaptic plasticity at excitatory synapses, which connect neurons into networks; in other words, we generate new synapses, and that’s how we enhance brain health and cognitive abilities.”
The more neural connections, the greater the processing speed of the brain, he continued. “Cognitive training creates an anatomical change in the brain.”
Executive functions, which include attention, inhibition, planning, and multitasking, are regulated predominantly by the prefrontal cortex. Damage in this region of the brain is also implicated in dementia. Alterations in the connectivity of this area are associated with cognitive impairment, independent of other structural pathological aberrations (eg, gray matter atrophy). These patterns may precede structural pathological changes associated with cognitive impairment and dementia.
Neuroplasticity changes have been corroborated through neuroimaging, which has demonstrated that after cognitive training, there is more activation in the prefrontal cortex that correlates with new synapses, Dr. Montero-Odasso said.
Henry Mahncke, PhD, CEO of the brain training company Posit Science/BrainHQ, explained that early research was conducted on rodents and monkeys, with Dr. Merzenich as one of the leading pioneers in developing the concept of brain plasticity. Dr. Merzenich cofounded Posit Science and is currently its chief scientific officer.
Dr. Mahncke recounted that as a graduate student, he had worked with Dr. Merzenich researching brain plasticity. When Dr. Merzenich founded Posit Science, he asked Dr. Mahncke to join the company to help develop approaches to enhance brain plasticity — building the brain-training exercises and running the clinical trials.
“It’s now well understood that the brain can rewire itself at any age and in almost any condition,” Dr. Mahncke said. “In kids and in younger and older adults, whether with healthy or unhealthy brains, the fundamental way the brain works is by continually rewiring and rebuilding itself, based on what we ask it to do.”
Dr. Mahncke said.
Unsubstantiated Claims and Controversy
Brain training is not without controversy, Dr. Hara pointed out. “Some manufacturers of brain games have been criticized and even fined for making unsubstantiated claims,” she said.
A 2016 review found that brain-training interventions do improve performance on specific trained tasks, but there is less evidence that they improve performance on closely related tasks and little evidence that training improves everyday cognitive performance. A 2017 review reached similar conclusions, calling evidence regarding prevention or delay of cognitive decline or dementia through brain games “insufficient,” although cognitive training could “improve cognition in the domain trained.”
“The general consensus is that for most brain-training programs, people may get better at specific tasks through practice, but these improvements don’t necessarily translate into improvement in other tasks that require other cognitive domains or prevention of dementia or age-related cognitive decline,” Dr. Hara said.
She noted that most brain-training programs “have not been rigorously tested in clinical trials” — although some, such as those featured in the ACTIVE trial, did show evidence of effectiveness.
Dr. Mahncke agreed. “Asking whether brain training works is like asking whether small molecules improve health,” he said noting that some brain-training programs are nonsense and not evidence based. He believes that his company’s product, BrainHQ, and some others are “backed by robust evidence in their ability to stave off, slow, or even reverse cognitive changes.”
BrainHQ is a web-based brain game suite that can be used independently as an app or in group settings (classes and webinars) and is covered by some Medicare Advantage insurance plans. It encompasses “dozens of individual brain-training exercises, linked by a common thread. Each one is intensively designed to make the brain faster and more accurate,” said Dr. Mahncke.
He explained that human brains “get noisy as people get older, like a radio which is wearing out, so there’s static in the background. This makes the music hard to hear, and in the case of the human brain, it makes it difficult to pay attention.” The exercises are “designed to tamp down the ‘noise,’ speed up the brain, and make information processing more accurate.”
Dr. Mahncke called this a “bottom-up” approach, in contrast to many previous cognitive-training approaches that come from the brain injury rehabilitation field. They teach “top-down” skills and strategies designed to compensate for deficits in specific domains, such as reading, concentration, or fine motor skills.
By contrast, the approach of BrainHQ is “to improve the overall processing system of the brain with speed, attention, working memory, and executive function, which will in turn impact all skills and activities.”
Supporting Evidence
Dr. Mahncke cited several supporting studies. For example, the IMPACT study randomized 487 adults (aged ≥ 65 years) to receive either a brain plasticity–based computerized cognitive training program (BrainHQ) or a novelty- and intensity-matched general cognitive stimulation treatment program (intervention and control group, respectively) for an 8-week period.
Those who underwent brain training showed significantly greater improvement in the repeatable Battery for the Assessment of Neuropsychological Status (RBANS Auditory Memory/Attention) compared with those in the control group (3.9 vs 1.8, respectively; P =.02). The intervention group also showed significant improvements on multiple secondary measures of attention and memory. The magnitude of the effect sizes suggests that the results are clinically significant, according to the authors.
The ACTIVE study tested the effects of different cognitive training programs on cognitive function and time to dementia. The researchers randomized 2802 healthy older adults (mean age, 74 years) to a control group with no cognitive training or one of three brain-training groups comprising:
1. In-person training on verbal memory skills
2. In-person training on reasoning and problem-solving
3. Computer-based speed-of-processing training on visual attention
Participants in the training groups completed 10 sessions, each lasting 60-75 minutes, over a 5- to 6-week period. A random subsample of each training group was selected to receive “booster” sessions, with four-session booster training delivered at 11 and 35 months. All study participants completed follow-up tests of cognition and function after 1, 2, 3, 5, and 10 years.
At the end of 10 years, those assigned to the speed-of-processing training, now part of BrainHQ, had a 29% lower risk for dementia than those in the control group who received no training. No reduction was found in the memory or reasoning training groups. Participants who completed the “booster” sessions had an even greater reduction: Each additional booster session was associated with a 10% lower risk for dementia.
Dr. Montero-Odasso was involved in the SYNERGIC study that randomized 175 participants with mild cognitive impairment (MCI; average age, 73 years) to one of five study arms:
1. Multidomain intervention with exercise, cognitive training, and vitamin D
2. Exercise, cognitive training, and placebo
3. Exercise, sham cognitive training, and vitamin D
4. Exercise, sham cognitive training, and placebo
5. Control group with balance-toning exercise, sham cognitive training, and placebo
“Sham” cognitive training consisted of alternating between two tasks (touristic search and video watching) performed on a tablet, with the same time exposure as the intervention training.
The researchers found that after 6 months of interventions, all active arms with aerobic-resistance exercise showed improvement in the ADAS-Cog-13, an established outcome to evaluate dementia treatments, when compared with the control group — regardless of the addition of cognitive training or vitamin D.
Compared with exercise alone (arms 3 and 4), those who did exercise plus cognitive training (arms 1 and 2) showed greater improvements in their ADAS-Cog-13l score, with a mean difference of −1.45 points (P = .02). The greatest improvement was seen in those who underwent the multidomain intervention in arm 1.
The authors noted that the mean 2.64-point improvement seen in the ADAS-Cog-13 for the multidomain intervention is actually larger than changes seen in previous pharmaceutical trials among individuals with MCI or mild dementia and “approaches” the three points considered clinically meaningful.
“We found that older adults with MCI who received aerobic-resistance exercise with sequential computerized cognitive training significantly improved cognition,” Dr. Montero-Odasso said. “The cognitive training we used was called Neuropeak, a multidomain lifestyle training delivered through a web-based platform developed by our co-leader Louis Bherer at Université de Montréal.”
He explained that the purpose “is to challenge your brain to the point where you need to make an effort to remember things, pay attention, and later to execute tasks. The evidence from clinical trials, including ours, shows this type of brain challenge is effective in slowing and even reversing cognitive decline.”
A follow-up study, SYNERGIC 2.0, is ongoing.
Puzzles, Board Games, and New Challenges
Formal brain-training programs aren’t the only way to improve brain plasticity, Dr. Hara said. Observational studies suggested an association between improved cognitive performance and/or lower dementia risk and engaging in number and word puzzles, such as crosswords, cards, or board games.
Some studies suggested that older adults who use technology might also protect their cognitive reserve. Dr. Hara cited a US longitudinal study of more than 18,000 older adults suggesting that regular Internet users had roughly half the risk for dementia compared to nonregular Internet users. Estimates of daily Internet use suggested a U-shaped relationship with dementia with 0.1-2.0 hours daily (excluding time spent watching television or movies online) associated with the lowest risk. Similar associations between Internet use and a lower risk for cognitive decline have been reported in the United Kingdom and Europe.
“Engaging in mentally stimulating activities can increase ‘cognitive reserve’ — meaning, capacity of the brain to resist the effects of age-related changes or disease-related pathology, such that one can maintain cognitive function for longer,” Dr. Hara said. “Cognitively stimulating activities, regardless of the type, may help delay the onset of cognitive decline.”
She listed several examples of activities that are stimulating to the brain, including learning a new game or puzzle, a new language, or a new dance, and learning how to play a musical instrument.
Dr. Montero-Odasso emphasized that the “newness” is key to increasing and preserving cognitive reserve. “Just surfing the Internet, playing word or board games, or doing crossword puzzles won’t be enough if you’ve been doing these things all your life,” he said. “It won’t hurt, of course, but it won’t necessarily increase your cognitive abilities.
“For example, a person who regularly engages in public speaking may not improve cognition by taking a public-speaking course, but someone who has never spoken before an audience might show cognitive improvements as a result of learning a new skill,” he said. “Or someone who knows several languages already might gain from learning a brand-new language.”
He cited research supporting the benefits of dancing, which he called “an ideal activity because it’s physical, so it provides the exercise that’s been associated with improved cognition. But it also requires learning new steps and moves, which builds the synapses in the brain. And the socialization of dance classes adds another component that can improve cognition.”
Dr. Mahncke hopes that beyond engaging in day-to-day new activities, seniors will participate in computerized brain training. “There’s no reason that evidence-based training can’t be offered in senior and community centers, as yoga and swimming are,” he said. “It doesn’t have to be simply something people do on their own virtually.”
Zoom classes and Medicare reimbursements are “good steps in the right direction, but it’s time to expand this potentially life-transformative intervention so that it reaches the ever-expanding population of seniors in the United States and beyond.”
Dr. Hara reported having no disclosures. Dr. Montero-Odasso reported having no commercial or financial interest related to this topic. He serves as the president of the Canadian Geriatrics Société and is team leader in the Canadian Consortium of Neurodegeneration in Aging. Dr. Mahncke is CEO of the brain training company Posit Science/BrainHQ.
A version of this article appeared on Medscape.com.
The concept that cognitive health can be preserved or improved is often expressed as “use it or lose it.” Numerous modifiable risk factors are associated with “losing” cognitive abilities with age, and a cognitively active lifestyle may have a protective effect.
But what is a “cognitively active lifestyle” — do crosswords and Sudoku count?
One popular approach is “brain training.” While not a scientific term with an established definition, it “typically refers to tasks or drills that are designed to strengthen specific aspects of one’s cognitive function,” explained Yuko Hara, PhD, director of Aging and Alzheimer’s Prevention at the Alzheimer’s Drug Discovery Foundation.
Manuel Montero-Odasso, MD, PhD, director of the Gait and Brain Lab, Parkwood Institute, London, Ontario, Canada, elaborated: “Cognitive training involves performing a definitive task or set of tasks where you increase attentional demands to improve focus and concentration and memory. You try to execute the new things that you’ve learned and to remember them.”
In a commentary published by this news organization in 2022, neuroscientist Michael Merzenich, PhD, professor emeritus at University of California San Francisco, said that growing a person’s cognitive reserve and actively managing brain health can play an important role in preventing or delaying Alzheimer’s disease. Important components of this include brain training and physical exercise.
Brain Training: Mechanism of Action
Dr. Montero-Odasso, team leader at the Canadian Consortium on Neurodegeneration in Aging and team co-leader at the Ontario Neurodegenerative Research Initiative, explained that cognitive training creates new synapses in the brain, thus stimulating neuroplasticity.
“When we try to activate networks mainly in the frontal lobe, the prefrontal cortex, a key mechanism underlying this process is enhancement of the synaptic plasticity at excitatory synapses, which connect neurons into networks; in other words, we generate new synapses, and that’s how we enhance brain health and cognitive abilities.”
The more neural connections, the greater the processing speed of the brain, he continued. “Cognitive training creates an anatomical change in the brain.”
Executive functions, which include attention, inhibition, planning, and multitasking, are regulated predominantly by the prefrontal cortex. Damage in this region of the brain is also implicated in dementia. Alterations in the connectivity of this area are associated with cognitive impairment, independent of other structural pathological aberrations (eg, gray matter atrophy). These patterns may precede structural pathological changes associated with cognitive impairment and dementia.
Neuroplasticity changes have been corroborated through neuroimaging, which has demonstrated that after cognitive training, there is more activation in the prefrontal cortex that correlates with new synapses, Dr. Montero-Odasso said.
Henry Mahncke, PhD, CEO of the brain training company Posit Science/BrainHQ, explained that early research was conducted on rodents and monkeys, with Dr. Merzenich as one of the leading pioneers in developing the concept of brain plasticity. Dr. Merzenich cofounded Posit Science and is currently its chief scientific officer.
Dr. Mahncke recounted that as a graduate student, he had worked with Dr. Merzenich researching brain plasticity. When Dr. Merzenich founded Posit Science, he asked Dr. Mahncke to join the company to help develop approaches to enhance brain plasticity — building the brain-training exercises and running the clinical trials.
“It’s now well understood that the brain can rewire itself at any age and in almost any condition,” Dr. Mahncke said. “In kids and in younger and older adults, whether with healthy or unhealthy brains, the fundamental way the brain works is by continually rewiring and rebuilding itself, based on what we ask it to do.”
Dr. Mahncke said.
Unsubstantiated Claims and Controversy
Brain training is not without controversy, Dr. Hara pointed out. “Some manufacturers of brain games have been criticized and even fined for making unsubstantiated claims,” she said.
A 2016 review found that brain-training interventions do improve performance on specific trained tasks, but there is less evidence that they improve performance on closely related tasks and little evidence that training improves everyday cognitive performance. A 2017 review reached similar conclusions, calling evidence regarding prevention or delay of cognitive decline or dementia through brain games “insufficient,” although cognitive training could “improve cognition in the domain trained.”
“The general consensus is that for most brain-training programs, people may get better at specific tasks through practice, but these improvements don’t necessarily translate into improvement in other tasks that require other cognitive domains or prevention of dementia or age-related cognitive decline,” Dr. Hara said.
She noted that most brain-training programs “have not been rigorously tested in clinical trials” — although some, such as those featured in the ACTIVE trial, did show evidence of effectiveness.
Dr. Mahncke agreed. “Asking whether brain training works is like asking whether small molecules improve health,” he said noting that some brain-training programs are nonsense and not evidence based. He believes that his company’s product, BrainHQ, and some others are “backed by robust evidence in their ability to stave off, slow, or even reverse cognitive changes.”
BrainHQ is a web-based brain game suite that can be used independently as an app or in group settings (classes and webinars) and is covered by some Medicare Advantage insurance plans. It encompasses “dozens of individual brain-training exercises, linked by a common thread. Each one is intensively designed to make the brain faster and more accurate,” said Dr. Mahncke.
He explained that human brains “get noisy as people get older, like a radio which is wearing out, so there’s static in the background. This makes the music hard to hear, and in the case of the human brain, it makes it difficult to pay attention.” The exercises are “designed to tamp down the ‘noise,’ speed up the brain, and make information processing more accurate.”
Dr. Mahncke called this a “bottom-up” approach, in contrast to many previous cognitive-training approaches that come from the brain injury rehabilitation field. They teach “top-down” skills and strategies designed to compensate for deficits in specific domains, such as reading, concentration, or fine motor skills.
By contrast, the approach of BrainHQ is “to improve the overall processing system of the brain with speed, attention, working memory, and executive function, which will in turn impact all skills and activities.”
Supporting Evidence
Dr. Mahncke cited several supporting studies. For example, the IMPACT study randomized 487 adults (aged ≥ 65 years) to receive either a brain plasticity–based computerized cognitive training program (BrainHQ) or a novelty- and intensity-matched general cognitive stimulation treatment program (intervention and control group, respectively) for an 8-week period.
Those who underwent brain training showed significantly greater improvement in the repeatable Battery for the Assessment of Neuropsychological Status (RBANS Auditory Memory/Attention) compared with those in the control group (3.9 vs 1.8, respectively; P =.02). The intervention group also showed significant improvements on multiple secondary measures of attention and memory. The magnitude of the effect sizes suggests that the results are clinically significant, according to the authors.
The ACTIVE study tested the effects of different cognitive training programs on cognitive function and time to dementia. The researchers randomized 2802 healthy older adults (mean age, 74 years) to a control group with no cognitive training or one of three brain-training groups comprising:
1. In-person training on verbal memory skills
2. In-person training on reasoning and problem-solving
3. Computer-based speed-of-processing training on visual attention
Participants in the training groups completed 10 sessions, each lasting 60-75 minutes, over a 5- to 6-week period. A random subsample of each training group was selected to receive “booster” sessions, with four-session booster training delivered at 11 and 35 months. All study participants completed follow-up tests of cognition and function after 1, 2, 3, 5, and 10 years.
At the end of 10 years, those assigned to the speed-of-processing training, now part of BrainHQ, had a 29% lower risk for dementia than those in the control group who received no training. No reduction was found in the memory or reasoning training groups. Participants who completed the “booster” sessions had an even greater reduction: Each additional booster session was associated with a 10% lower risk for dementia.
Dr. Montero-Odasso was involved in the SYNERGIC study that randomized 175 participants with mild cognitive impairment (MCI; average age, 73 years) to one of five study arms:
1. Multidomain intervention with exercise, cognitive training, and vitamin D
2. Exercise, cognitive training, and placebo
3. Exercise, sham cognitive training, and vitamin D
4. Exercise, sham cognitive training, and placebo
5. Control group with balance-toning exercise, sham cognitive training, and placebo
“Sham” cognitive training consisted of alternating between two tasks (touristic search and video watching) performed on a tablet, with the same time exposure as the intervention training.
The researchers found that after 6 months of interventions, all active arms with aerobic-resistance exercise showed improvement in the ADAS-Cog-13, an established outcome to evaluate dementia treatments, when compared with the control group — regardless of the addition of cognitive training or vitamin D.
Compared with exercise alone (arms 3 and 4), those who did exercise plus cognitive training (arms 1 and 2) showed greater improvements in their ADAS-Cog-13l score, with a mean difference of −1.45 points (P = .02). The greatest improvement was seen in those who underwent the multidomain intervention in arm 1.
The authors noted that the mean 2.64-point improvement seen in the ADAS-Cog-13 for the multidomain intervention is actually larger than changes seen in previous pharmaceutical trials among individuals with MCI or mild dementia and “approaches” the three points considered clinically meaningful.
“We found that older adults with MCI who received aerobic-resistance exercise with sequential computerized cognitive training significantly improved cognition,” Dr. Montero-Odasso said. “The cognitive training we used was called Neuropeak, a multidomain lifestyle training delivered through a web-based platform developed by our co-leader Louis Bherer at Université de Montréal.”
He explained that the purpose “is to challenge your brain to the point where you need to make an effort to remember things, pay attention, and later to execute tasks. The evidence from clinical trials, including ours, shows this type of brain challenge is effective in slowing and even reversing cognitive decline.”
A follow-up study, SYNERGIC 2.0, is ongoing.
Puzzles, Board Games, and New Challenges
Formal brain-training programs aren’t the only way to improve brain plasticity, Dr. Hara said. Observational studies suggested an association between improved cognitive performance and/or lower dementia risk and engaging in number and word puzzles, such as crosswords, cards, or board games.
Some studies suggested that older adults who use technology might also protect their cognitive reserve. Dr. Hara cited a US longitudinal study of more than 18,000 older adults suggesting that regular Internet users had roughly half the risk for dementia compared to nonregular Internet users. Estimates of daily Internet use suggested a U-shaped relationship with dementia with 0.1-2.0 hours daily (excluding time spent watching television or movies online) associated with the lowest risk. Similar associations between Internet use and a lower risk for cognitive decline have been reported in the United Kingdom and Europe.
“Engaging in mentally stimulating activities can increase ‘cognitive reserve’ — meaning, capacity of the brain to resist the effects of age-related changes or disease-related pathology, such that one can maintain cognitive function for longer,” Dr. Hara said. “Cognitively stimulating activities, regardless of the type, may help delay the onset of cognitive decline.”
She listed several examples of activities that are stimulating to the brain, including learning a new game or puzzle, a new language, or a new dance, and learning how to play a musical instrument.
Dr. Montero-Odasso emphasized that the “newness” is key to increasing and preserving cognitive reserve. “Just surfing the Internet, playing word or board games, or doing crossword puzzles won’t be enough if you’ve been doing these things all your life,” he said. “It won’t hurt, of course, but it won’t necessarily increase your cognitive abilities.
“For example, a person who regularly engages in public speaking may not improve cognition by taking a public-speaking course, but someone who has never spoken before an audience might show cognitive improvements as a result of learning a new skill,” he said. “Or someone who knows several languages already might gain from learning a brand-new language.”
He cited research supporting the benefits of dancing, which he called “an ideal activity because it’s physical, so it provides the exercise that’s been associated with improved cognition. But it also requires learning new steps and moves, which builds the synapses in the brain. And the socialization of dance classes adds another component that can improve cognition.”
Dr. Mahncke hopes that beyond engaging in day-to-day new activities, seniors will participate in computerized brain training. “There’s no reason that evidence-based training can’t be offered in senior and community centers, as yoga and swimming are,” he said. “It doesn’t have to be simply something people do on their own virtually.”
Zoom classes and Medicare reimbursements are “good steps in the right direction, but it’s time to expand this potentially life-transformative intervention so that it reaches the ever-expanding population of seniors in the United States and beyond.”
Dr. Hara reported having no disclosures. Dr. Montero-Odasso reported having no commercial or financial interest related to this topic. He serves as the president of the Canadian Geriatrics Société and is team leader in the Canadian Consortium of Neurodegeneration in Aging. Dr. Mahncke is CEO of the brain training company Posit Science/BrainHQ.
A version of this article appeared on Medscape.com.
Could Bedside Training Help End the US Neurologist Shortage?
DENVER — , a new report suggested.
Bedside Rounding Alliance for Internal Medicine and Neurology Residents (BRAINs) moves training from the lecture hall to the bedside, offering instruction on obtaining a focused neurologic history and performing a focused neurologic physical exam for common neurologic symptoms.
Almost 100% of trainees surveyed gave the program a favorable rating, citing patient exposure and bedside training from neurology educators as keys to its success.
As internal medicine providers are often “the first to lay eyes” on patients with a neurology complaint, it’s important they “have a basic level of comfort” in addressing patients’ common questions and concerns, study author Prashanth Rajarajan, MD, PhD, a resident in the Department of Neurology at Brigham and Women’s Hospital, Boston, told this news organization.
The findings were presented at the 2024 annual meeting of the American Academy of Neurology.
Addressing ‘Neurophobia’
Neurology is often viewed by medical trainees as the most difficult subspecialty, Dr. Rajarajan said. Many have what he calls “neurophobia,” which he defines as “a discomfort with assessing and treating neurologic complaints.”
A survey at his institution showed 62% of internal medicine residents lacked the confidence to diagnose and treat neurologic diseases, he reported.
BRAINs is a structured neurology trainee-led, inpatient bedside teaching session for internal medicine residents, medical students, and others that aims to increase trainees’ confidence in assessing patients with common neurologic symptoms.
The program includes a biweekly 45-minute session. Most of the session is spent at the bedside and involves demonstrations and practice of a focused neurologic history and physical exam.
Participants receive feedback from educators, typically neurology residents or fellows in epilepsy, stroke, or some other neurology subspecialty. It also includes a short discussion on pertinent diagnostics, management, and other topics.
Surveys evaluating the program and teaching skill development were completed by 59 residents and 15 neurology educators who participated in BRAINs between 2022 and 2024.
Over 90% of trainees (54) agreed BRAINs sessions met the program’s objective (5 were neutral); 49 agreed it increased confidence in taking a neuro history (9 were neutral and 1 disagreed); 56 felt it boosted their confidence in doing a neuro exam (3 were neutral); and 56 said BRAINs is more effective than traditional lecture-based didactics (3 were neutral).
All the residents rated the material covered as appropriate for their level of training; 88% considered the 45-minute session length appropriate; and 98% had a favorable impression of the program as a whole.
When asked to identify the most helpful aspect of the program, 82% cited more patient exposure and 81% more bedside teaching.
All educators reported that the sessions were an effective way to practice near-peer teaching skills. Most (87%) felt the experience was more effective at accomplishing learning objectives than preparing and giving traditional didactic lectures, and 80% agreed it also gave them an opportunity to get to know their medical colleagues.
Use It or Lose It
Dr. Rajarajan noted that the program doesn’t require significant planning or extra staff, is not resource-intensive, and can be adapted to different services such as emergency departments and other learner populations.
But time will tell if the newfound confidence of those taking the program actually lasts.
“You have to keep using it,” he said. “You use it or lose it when comes to these skills.”
Commenting on the initiative, Denney Zimmerman, DO, Neurocritical Care Faculty, Blount Memorial Hospital, Maryville, Tennessee, and cochair of the AAN session featuring the study, called the program a good example of one way to counteract “neurophobia” and address the widespread neurologist shortage in the United States.
A 2019 AAN report showed that by 2025, almost every state in the United States will have a mismatch between the number of practicing neurologists and the demand from patients with neurologic conditions. The report offered several ways to address the shortage, including more neurology-focused training for internal medicine doctors during their residency.
“They’re usually on the front line, both in the hospital and in the clinics, and can help expedite patients who need to be seen by neurology sooner rather than later,” Dr. Zimmerman said.
Dr. Zimmerman noted that the study assessed how well participants perceived the program but not whether it improved their skills.
He pointed out that different groups may assess different diseases during their training session. “I think it’s important to ensure you’re hitting all the major topics.”
The study received funding from MGB Centers of Expertise Education Grant. Drs. Rajarajan and Zimmerman reported no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
DENVER — , a new report suggested.
Bedside Rounding Alliance for Internal Medicine and Neurology Residents (BRAINs) moves training from the lecture hall to the bedside, offering instruction on obtaining a focused neurologic history and performing a focused neurologic physical exam for common neurologic symptoms.
Almost 100% of trainees surveyed gave the program a favorable rating, citing patient exposure and bedside training from neurology educators as keys to its success.
As internal medicine providers are often “the first to lay eyes” on patients with a neurology complaint, it’s important they “have a basic level of comfort” in addressing patients’ common questions and concerns, study author Prashanth Rajarajan, MD, PhD, a resident in the Department of Neurology at Brigham and Women’s Hospital, Boston, told this news organization.
The findings were presented at the 2024 annual meeting of the American Academy of Neurology.
Addressing ‘Neurophobia’
Neurology is often viewed by medical trainees as the most difficult subspecialty, Dr. Rajarajan said. Many have what he calls “neurophobia,” which he defines as “a discomfort with assessing and treating neurologic complaints.”
A survey at his institution showed 62% of internal medicine residents lacked the confidence to diagnose and treat neurologic diseases, he reported.
BRAINs is a structured neurology trainee-led, inpatient bedside teaching session for internal medicine residents, medical students, and others that aims to increase trainees’ confidence in assessing patients with common neurologic symptoms.
The program includes a biweekly 45-minute session. Most of the session is spent at the bedside and involves demonstrations and practice of a focused neurologic history and physical exam.
Participants receive feedback from educators, typically neurology residents or fellows in epilepsy, stroke, or some other neurology subspecialty. It also includes a short discussion on pertinent diagnostics, management, and other topics.
Surveys evaluating the program and teaching skill development were completed by 59 residents and 15 neurology educators who participated in BRAINs between 2022 and 2024.
Over 90% of trainees (54) agreed BRAINs sessions met the program’s objective (5 were neutral); 49 agreed it increased confidence in taking a neuro history (9 were neutral and 1 disagreed); 56 felt it boosted their confidence in doing a neuro exam (3 were neutral); and 56 said BRAINs is more effective than traditional lecture-based didactics (3 were neutral).
All the residents rated the material covered as appropriate for their level of training; 88% considered the 45-minute session length appropriate; and 98% had a favorable impression of the program as a whole.
When asked to identify the most helpful aspect of the program, 82% cited more patient exposure and 81% more bedside teaching.
All educators reported that the sessions were an effective way to practice near-peer teaching skills. Most (87%) felt the experience was more effective at accomplishing learning objectives than preparing and giving traditional didactic lectures, and 80% agreed it also gave them an opportunity to get to know their medical colleagues.
Use It or Lose It
Dr. Rajarajan noted that the program doesn’t require significant planning or extra staff, is not resource-intensive, and can be adapted to different services such as emergency departments and other learner populations.
But time will tell if the newfound confidence of those taking the program actually lasts.
“You have to keep using it,” he said. “You use it or lose it when comes to these skills.”
Commenting on the initiative, Denney Zimmerman, DO, Neurocritical Care Faculty, Blount Memorial Hospital, Maryville, Tennessee, and cochair of the AAN session featuring the study, called the program a good example of one way to counteract “neurophobia” and address the widespread neurologist shortage in the United States.
A 2019 AAN report showed that by 2025, almost every state in the United States will have a mismatch between the number of practicing neurologists and the demand from patients with neurologic conditions. The report offered several ways to address the shortage, including more neurology-focused training for internal medicine doctors during their residency.
“They’re usually on the front line, both in the hospital and in the clinics, and can help expedite patients who need to be seen by neurology sooner rather than later,” Dr. Zimmerman said.
Dr. Zimmerman noted that the study assessed how well participants perceived the program but not whether it improved their skills.
He pointed out that different groups may assess different diseases during their training session. “I think it’s important to ensure you’re hitting all the major topics.”
The study received funding from MGB Centers of Expertise Education Grant. Drs. Rajarajan and Zimmerman reported no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
DENVER — , a new report suggested.
Bedside Rounding Alliance for Internal Medicine and Neurology Residents (BRAINs) moves training from the lecture hall to the bedside, offering instruction on obtaining a focused neurologic history and performing a focused neurologic physical exam for common neurologic symptoms.
Almost 100% of trainees surveyed gave the program a favorable rating, citing patient exposure and bedside training from neurology educators as keys to its success.
As internal medicine providers are often “the first to lay eyes” on patients with a neurology complaint, it’s important they “have a basic level of comfort” in addressing patients’ common questions and concerns, study author Prashanth Rajarajan, MD, PhD, a resident in the Department of Neurology at Brigham and Women’s Hospital, Boston, told this news organization.
The findings were presented at the 2024 annual meeting of the American Academy of Neurology.
Addressing ‘Neurophobia’
Neurology is often viewed by medical trainees as the most difficult subspecialty, Dr. Rajarajan said. Many have what he calls “neurophobia,” which he defines as “a discomfort with assessing and treating neurologic complaints.”
A survey at his institution showed 62% of internal medicine residents lacked the confidence to diagnose and treat neurologic diseases, he reported.
BRAINs is a structured neurology trainee-led, inpatient bedside teaching session for internal medicine residents, medical students, and others that aims to increase trainees’ confidence in assessing patients with common neurologic symptoms.
The program includes a biweekly 45-minute session. Most of the session is spent at the bedside and involves demonstrations and practice of a focused neurologic history and physical exam.
Participants receive feedback from educators, typically neurology residents or fellows in epilepsy, stroke, or some other neurology subspecialty. It also includes a short discussion on pertinent diagnostics, management, and other topics.
Surveys evaluating the program and teaching skill development were completed by 59 residents and 15 neurology educators who participated in BRAINs between 2022 and 2024.
Over 90% of trainees (54) agreed BRAINs sessions met the program’s objective (5 were neutral); 49 agreed it increased confidence in taking a neuro history (9 were neutral and 1 disagreed); 56 felt it boosted their confidence in doing a neuro exam (3 were neutral); and 56 said BRAINs is more effective than traditional lecture-based didactics (3 were neutral).
All the residents rated the material covered as appropriate for their level of training; 88% considered the 45-minute session length appropriate; and 98% had a favorable impression of the program as a whole.
When asked to identify the most helpful aspect of the program, 82% cited more patient exposure and 81% more bedside teaching.
All educators reported that the sessions were an effective way to practice near-peer teaching skills. Most (87%) felt the experience was more effective at accomplishing learning objectives than preparing and giving traditional didactic lectures, and 80% agreed it also gave them an opportunity to get to know their medical colleagues.
Use It or Lose It
Dr. Rajarajan noted that the program doesn’t require significant planning or extra staff, is not resource-intensive, and can be adapted to different services such as emergency departments and other learner populations.
But time will tell if the newfound confidence of those taking the program actually lasts.
“You have to keep using it,” he said. “You use it or lose it when comes to these skills.”
Commenting on the initiative, Denney Zimmerman, DO, Neurocritical Care Faculty, Blount Memorial Hospital, Maryville, Tennessee, and cochair of the AAN session featuring the study, called the program a good example of one way to counteract “neurophobia” and address the widespread neurologist shortage in the United States.
A 2019 AAN report showed that by 2025, almost every state in the United States will have a mismatch between the number of practicing neurologists and the demand from patients with neurologic conditions. The report offered several ways to address the shortage, including more neurology-focused training for internal medicine doctors during their residency.
“They’re usually on the front line, both in the hospital and in the clinics, and can help expedite patients who need to be seen by neurology sooner rather than later,” Dr. Zimmerman said.
Dr. Zimmerman noted that the study assessed how well participants perceived the program but not whether it improved their skills.
He pointed out that different groups may assess different diseases during their training session. “I think it’s important to ensure you’re hitting all the major topics.”
The study received funding from MGB Centers of Expertise Education Grant. Drs. Rajarajan and Zimmerman reported no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
FROM AAN 2024
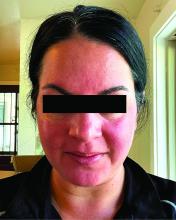
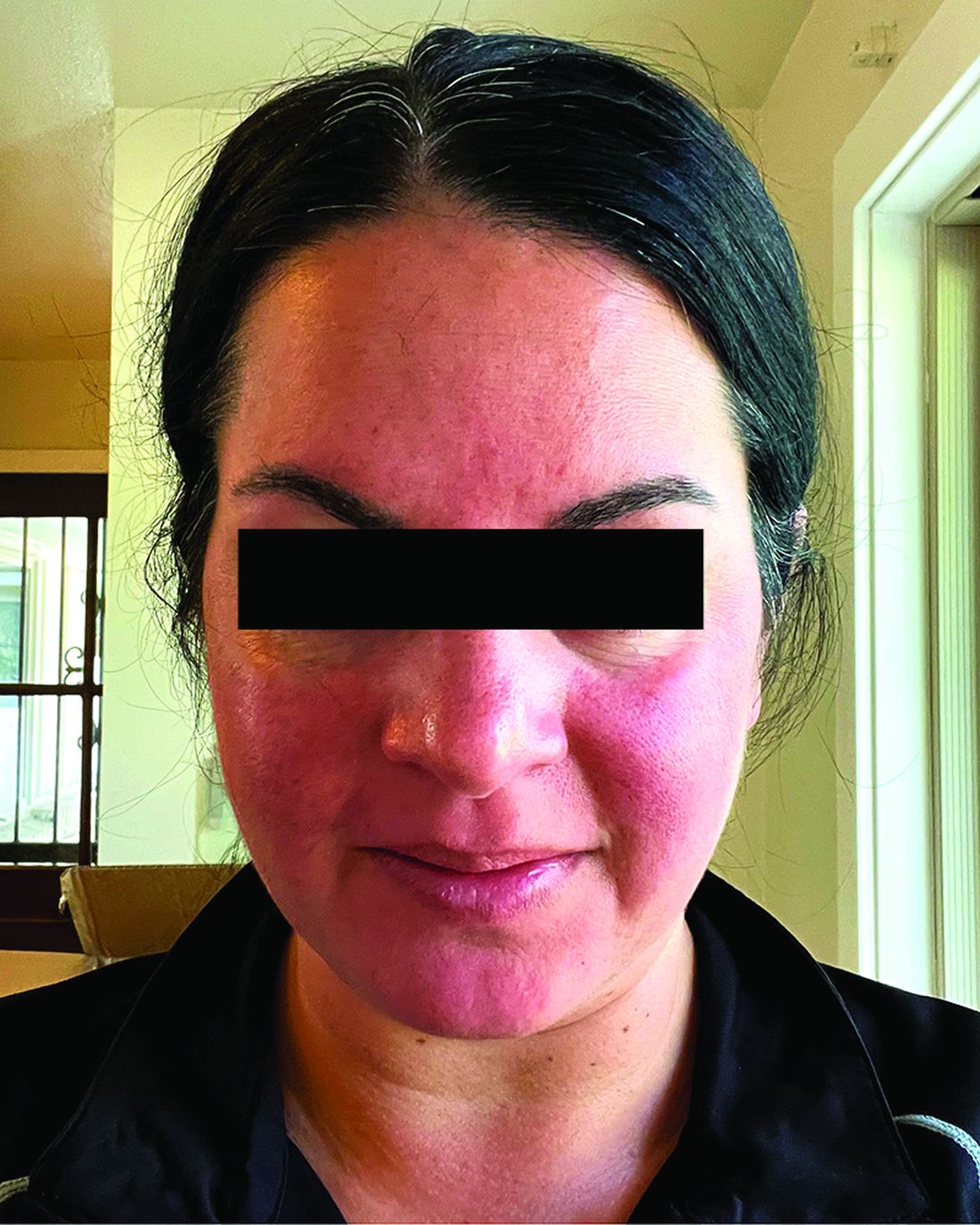
Migraine Drug Reduces Rosacea Flushing, Erythema in Small Study
In. Skin-related quality-of-life (QOL) measures also improved, albeit modestly.
The study was published in JAMA Dermatology.
“The transient erythema of rosacea is one of the most challenging rosacea symptoms to treat,” Emmy Graber, MD, MBA, who was not involved with the study, said in an interview. “As flushing can adversely impact quality of life in our rosacea patients, it is important to find therapeutic options for our patients. This study is exciting, not only because the treatment was successful for a notable number of patients, but also because it involved a drug with a novel mode of action in rosacea.” Dr. Graber practices in Boston and is an affiliate clinical instructor at Northeastern University, Boston.
Guy F. Webster, MD, PhD, clinical professor of dermatology, Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, added, “The interesting thing about this study is that it gives us a new target to think about for therapy. But it’s a long way from saying we can use it tomorrow.” He was not involved with the study but was also asked to comment on the findings.
Spotlight on CGRP
Rosacea’s pathophysiology remains incompletely understood, wrote Nita K.F. Wienholtz, MD, PhD, Department of Dermatology, Bispebjerg and Frederiksberg Hospital, University of Copenhagen, Denmark, and coinvestigators. However, they added, mounting evidence suggests a possible role for CGRP. For example, a study published in JAMA Dermatology in 2015 revealed elevated CGRP levels in facial skin biopsies from patients with rosacea.
For the present study, the investigators enrolled 30 adults (including 23 women) with rosacea who experienced at least 15 days of moderate to severe erythema or extreme flushing during a 4-week, treatment-free run-in period. Most participants (87%) had previously failed one or more rosacea treatments because of a lack of efficacy or adverse reactions, and 43% had failed three or more treatments.
Participants received 3-monthly 140-mg doses of erenumab, which is approved by the Food and Drug Administration for migraine prevention. Patients recorded scores on the Patient Self-Assessment (PSA) and item 2 of the Flushing Assessment Tool online daily and made a final follow-up visit 12 weeks after the third dose.
Among the 27 patients who completed the study, the mean number of days with moderate to severe flushing from week 9 to week 12 fell by 6.9 from 23.6 days over 4 weeks at baseline (P < .001). Patients most severely affected by flushing at baseline experienced an 81% decline in days with severe to extreme flushing. Overall, 26% of patients experienced at least 50% reductions in moderate to extreme flushing days. The number of days with moderate to severe erythema as measured by PSA fell by 8.1 (mean) from baseline, and 56% of patients experienced at least 50% reductions in PSA scores. No unexpected safety signals emerged.
Questions Over QOL Data
“Although there were significant decreases in flushing and erythema,” wrote John S. Barbieri, MD, MBA, in an accompanying Editor’s Note, “the present study had relatively modest improvements in quality of life.” He is director of the Advanced Acne Therapeutics Clinic, Brigham and Women’s Hospital, Boston, and associate editor and evidence-based practice editor of JAMA Dermatology.
Compared with baseline (6.22), mean Dermatology Life Quality Index scores fell 2.08 points and 2.73 points at weeks 8 and 20, respectively (P = .004 and .003). At the same intervals, the mean baseline Rosacea Quality of Life score (48.22) decreased by 2.58 points and 4.14 points, respectively (P = .04 and .02).
No significant changes appeared in gauges of anxiety and depression. These findings, authors wrote, could stem from their decision to omit a follow-up visit at week 12 — where they may have seen mental-health effects which disappeared by week 20 — in response to patients’ logistical concerns.
However, Dr. Webster questioned the value of QOL measurements in rosacea. “Quality-of-life measures are blunt instruments,” he explained, and reducing severe itching or chronic pain improves the lives of affected patients. “But what question are you going to ask to tease out whether being less red-cheeked has made someone’s life easier? It’s not a problem that lends itself to quality-of-life assessments.” Moreover, he said, regulators who increasingly require such measures in clinical trials ignore this point, creating challenges for drug developers and researchers.
Because the study was neither blinded nor controlled, Dr. Webster suggested considering it a tantalizing proof of concept. “If I were putting money into a CGRP inhibitor, I’d want at least a small, placebo-controlled, double-blinded study.”
Study authors and Dr. Barbieri recommended larger randomized studies involving different populations and erenumab doses. For now, Dr. Barbieri wrote, CGRP inhibition represents a promising potential strategy for patients who have rosacea with comorbid migraine or recalcitrant flushing and erythema.
Dr. Wienholtz reported no relevant financial interests. Dr. Barbieri had no related disclosures. Dr. Webster reported no relevant financial interests. Dr. Graber reported no conflicts related to erenumab but consults for other companies with rosacea-related products including Galderma. The study was supported by and conducted in collaboration with Novartis Pharma AG. Additional funding came from the Novo Nordisk Foundation and the Lundbeck Foundation.
A version of this article appeared on Medscape.com.
In. Skin-related quality-of-life (QOL) measures also improved, albeit modestly.
The study was published in JAMA Dermatology.
“The transient erythema of rosacea is one of the most challenging rosacea symptoms to treat,” Emmy Graber, MD, MBA, who was not involved with the study, said in an interview. “As flushing can adversely impact quality of life in our rosacea patients, it is important to find therapeutic options for our patients. This study is exciting, not only because the treatment was successful for a notable number of patients, but also because it involved a drug with a novel mode of action in rosacea.” Dr. Graber practices in Boston and is an affiliate clinical instructor at Northeastern University, Boston.
Guy F. Webster, MD, PhD, clinical professor of dermatology, Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, added, “The interesting thing about this study is that it gives us a new target to think about for therapy. But it’s a long way from saying we can use it tomorrow.” He was not involved with the study but was also asked to comment on the findings.
Spotlight on CGRP
Rosacea’s pathophysiology remains incompletely understood, wrote Nita K.F. Wienholtz, MD, PhD, Department of Dermatology, Bispebjerg and Frederiksberg Hospital, University of Copenhagen, Denmark, and coinvestigators. However, they added, mounting evidence suggests a possible role for CGRP. For example, a study published in JAMA Dermatology in 2015 revealed elevated CGRP levels in facial skin biopsies from patients with rosacea.
For the present study, the investigators enrolled 30 adults (including 23 women) with rosacea who experienced at least 15 days of moderate to severe erythema or extreme flushing during a 4-week, treatment-free run-in period. Most participants (87%) had previously failed one or more rosacea treatments because of a lack of efficacy or adverse reactions, and 43% had failed three or more treatments.
Participants received 3-monthly 140-mg doses of erenumab, which is approved by the Food and Drug Administration for migraine prevention. Patients recorded scores on the Patient Self-Assessment (PSA) and item 2 of the Flushing Assessment Tool online daily and made a final follow-up visit 12 weeks after the third dose.
Among the 27 patients who completed the study, the mean number of days with moderate to severe flushing from week 9 to week 12 fell by 6.9 from 23.6 days over 4 weeks at baseline (P < .001). Patients most severely affected by flushing at baseline experienced an 81% decline in days with severe to extreme flushing. Overall, 26% of patients experienced at least 50% reductions in moderate to extreme flushing days. The number of days with moderate to severe erythema as measured by PSA fell by 8.1 (mean) from baseline, and 56% of patients experienced at least 50% reductions in PSA scores. No unexpected safety signals emerged.
Questions Over QOL Data
“Although there were significant decreases in flushing and erythema,” wrote John S. Barbieri, MD, MBA, in an accompanying Editor’s Note, “the present study had relatively modest improvements in quality of life.” He is director of the Advanced Acne Therapeutics Clinic, Brigham and Women’s Hospital, Boston, and associate editor and evidence-based practice editor of JAMA Dermatology.
Compared with baseline (6.22), mean Dermatology Life Quality Index scores fell 2.08 points and 2.73 points at weeks 8 and 20, respectively (P = .004 and .003). At the same intervals, the mean baseline Rosacea Quality of Life score (48.22) decreased by 2.58 points and 4.14 points, respectively (P = .04 and .02).
No significant changes appeared in gauges of anxiety and depression. These findings, authors wrote, could stem from their decision to omit a follow-up visit at week 12 — where they may have seen mental-health effects which disappeared by week 20 — in response to patients’ logistical concerns.
However, Dr. Webster questioned the value of QOL measurements in rosacea. “Quality-of-life measures are blunt instruments,” he explained, and reducing severe itching or chronic pain improves the lives of affected patients. “But what question are you going to ask to tease out whether being less red-cheeked has made someone’s life easier? It’s not a problem that lends itself to quality-of-life assessments.” Moreover, he said, regulators who increasingly require such measures in clinical trials ignore this point, creating challenges for drug developers and researchers.
Because the study was neither blinded nor controlled, Dr. Webster suggested considering it a tantalizing proof of concept. “If I were putting money into a CGRP inhibitor, I’d want at least a small, placebo-controlled, double-blinded study.”
Study authors and Dr. Barbieri recommended larger randomized studies involving different populations and erenumab doses. For now, Dr. Barbieri wrote, CGRP inhibition represents a promising potential strategy for patients who have rosacea with comorbid migraine or recalcitrant flushing and erythema.
Dr. Wienholtz reported no relevant financial interests. Dr. Barbieri had no related disclosures. Dr. Webster reported no relevant financial interests. Dr. Graber reported no conflicts related to erenumab but consults for other companies with rosacea-related products including Galderma. The study was supported by and conducted in collaboration with Novartis Pharma AG. Additional funding came from the Novo Nordisk Foundation and the Lundbeck Foundation.
A version of this article appeared on Medscape.com.
In. Skin-related quality-of-life (QOL) measures also improved, albeit modestly.
The study was published in JAMA Dermatology.
“The transient erythema of rosacea is one of the most challenging rosacea symptoms to treat,” Emmy Graber, MD, MBA, who was not involved with the study, said in an interview. “As flushing can adversely impact quality of life in our rosacea patients, it is important to find therapeutic options for our patients. This study is exciting, not only because the treatment was successful for a notable number of patients, but also because it involved a drug with a novel mode of action in rosacea.” Dr. Graber practices in Boston and is an affiliate clinical instructor at Northeastern University, Boston.
Guy F. Webster, MD, PhD, clinical professor of dermatology, Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, added, “The interesting thing about this study is that it gives us a new target to think about for therapy. But it’s a long way from saying we can use it tomorrow.” He was not involved with the study but was also asked to comment on the findings.
Spotlight on CGRP
Rosacea’s pathophysiology remains incompletely understood, wrote Nita K.F. Wienholtz, MD, PhD, Department of Dermatology, Bispebjerg and Frederiksberg Hospital, University of Copenhagen, Denmark, and coinvestigators. However, they added, mounting evidence suggests a possible role for CGRP. For example, a study published in JAMA Dermatology in 2015 revealed elevated CGRP levels in facial skin biopsies from patients with rosacea.
For the present study, the investigators enrolled 30 adults (including 23 women) with rosacea who experienced at least 15 days of moderate to severe erythema or extreme flushing during a 4-week, treatment-free run-in period. Most participants (87%) had previously failed one or more rosacea treatments because of a lack of efficacy or adverse reactions, and 43% had failed three or more treatments.
Participants received 3-monthly 140-mg doses of erenumab, which is approved by the Food and Drug Administration for migraine prevention. Patients recorded scores on the Patient Self-Assessment (PSA) and item 2 of the Flushing Assessment Tool online daily and made a final follow-up visit 12 weeks after the third dose.
Among the 27 patients who completed the study, the mean number of days with moderate to severe flushing from week 9 to week 12 fell by 6.9 from 23.6 days over 4 weeks at baseline (P < .001). Patients most severely affected by flushing at baseline experienced an 81% decline in days with severe to extreme flushing. Overall, 26% of patients experienced at least 50% reductions in moderate to extreme flushing days. The number of days with moderate to severe erythema as measured by PSA fell by 8.1 (mean) from baseline, and 56% of patients experienced at least 50% reductions in PSA scores. No unexpected safety signals emerged.
Questions Over QOL Data
“Although there were significant decreases in flushing and erythema,” wrote John S. Barbieri, MD, MBA, in an accompanying Editor’s Note, “the present study had relatively modest improvements in quality of life.” He is director of the Advanced Acne Therapeutics Clinic, Brigham and Women’s Hospital, Boston, and associate editor and evidence-based practice editor of JAMA Dermatology.
Compared with baseline (6.22), mean Dermatology Life Quality Index scores fell 2.08 points and 2.73 points at weeks 8 and 20, respectively (P = .004 and .003). At the same intervals, the mean baseline Rosacea Quality of Life score (48.22) decreased by 2.58 points and 4.14 points, respectively (P = .04 and .02).
No significant changes appeared in gauges of anxiety and depression. These findings, authors wrote, could stem from their decision to omit a follow-up visit at week 12 — where they may have seen mental-health effects which disappeared by week 20 — in response to patients’ logistical concerns.
However, Dr. Webster questioned the value of QOL measurements in rosacea. “Quality-of-life measures are blunt instruments,” he explained, and reducing severe itching or chronic pain improves the lives of affected patients. “But what question are you going to ask to tease out whether being less red-cheeked has made someone’s life easier? It’s not a problem that lends itself to quality-of-life assessments.” Moreover, he said, regulators who increasingly require such measures in clinical trials ignore this point, creating challenges for drug developers and researchers.
Because the study was neither blinded nor controlled, Dr. Webster suggested considering it a tantalizing proof of concept. “If I were putting money into a CGRP inhibitor, I’d want at least a small, placebo-controlled, double-blinded study.”
Study authors and Dr. Barbieri recommended larger randomized studies involving different populations and erenumab doses. For now, Dr. Barbieri wrote, CGRP inhibition represents a promising potential strategy for patients who have rosacea with comorbid migraine or recalcitrant flushing and erythema.
Dr. Wienholtz reported no relevant financial interests. Dr. Barbieri had no related disclosures. Dr. Webster reported no relevant financial interests. Dr. Graber reported no conflicts related to erenumab but consults for other companies with rosacea-related products including Galderma. The study was supported by and conducted in collaboration with Novartis Pharma AG. Additional funding came from the Novo Nordisk Foundation and the Lundbeck Foundation.
A version of this article appeared on Medscape.com.
FROM JAMA DERMATOLOGY
Teleneurology for Suspected Stroke Speeds Treatment
, new research showed.
“This preliminary evidence supports adopting teleneurology prenotification as a best practice within health systems that have telestroke capabilities,” said study investigator Mark McDonald, MD, a neurologist at TeleSpecialists, Fort Myers, Florida.
The findings were presented at the 2024 annual meeting of the American Academy of Neurology.
Best Practices
The impact of emergency medical services prenotification, which refers to paramedics alerting receiving hospital emergency departments (EDs) of a suspected stroke on the way for appropriate preparations to be made, is well-defined, said Dr. McDonald.
“What we’re proposing as a best practice is not only should the ED or ED provider be aware, but there needs to be a system in place for standardizing communication to the neurology team so they’re aware, too.”
Prenotification allows a neurologist to “get on the screen to begin coordinating with the ED team to adequately prepare for the possibility of thrombolytic treatment,” he added.
Currently, teleneurology prenotification, he said, is variable and its benefits unclear.
Dr. McDonald said “his organization, TeleSpecialists, maintains a large detailed medical records database for emergency-related, teleneurology, and other cases. For stroke, it recommends 15 best practices” for facilities including prenotification of teleneurology.
Other best practices include evaluating and administering thrombolysis in the CT imaging suite, a preassembled stroke kit that includes antihypertensives and thrombolytic agents, ensuring a weigh bed is available to determine the exact dose of thrombolysis treatment, and implementing “mock” stroke alerts, said Dr. McDonald.
From the database, researchers extracted acute telestroke consultations seen in the ED in 103 facilities in 15 states. Facilities that did not adhere to the 14 best practices other than teleneurologist prenotification were excluded from the analysis.
Of 9290 patients included in the study, 731 were treated with thrombolysis at prenotification facilities (median age, 69 years; median National Institutes of Health Stroke Score [NIHSS], 8) and 31 were treated at facilities without prenotification (median age, 63 years; median NIHSS score, 4). The thrombolytic treatment rate was 8.5% at prenotification facilities versus 4.8% at facilities without prenotification — a difference that was statistically significant.
Prenotification facilities had a significantly shorter median door-to-needle (DTN) time than those without such a process at 35 versus 43 minutes. In addition, there was a statistically significant difference in the percentage of patients with times less than 60 minutes at approximately 88% at prenotification facilities versus about 68% at the facilities without prenotification.
Case-Level Analysis
However, just because a facility adheres to teleneurology prenotification as a whole, doesn’t mean it occurs in every case. Researchers explored the impact of teleneurology prenotification at the case level rather than the facility level.
“That gave us a bit more insight into the real impact because it’s not just being at a facility with the best practice; it’s actually working case by case to see whether it happened or not and that’s where we get the most compelling findings,” said Dr. McDonald.
Of 761 treatment cases, there was prenotification to the neurology team in 401 cases. In 360 cases, prenotification did not occur.
The median DTN time was 29 minutes in the group with actual prenotification vs 41.5 minutes in the group without actual prenotification, a difference that was statistically significant, Dr. McDonald said.
As for treatment within 30 minutes of arrival, 50.4% of patients in the teleneurology prenotification group versus 18.9% in the no prenotification group — a statistically significant difference.
DTN time of less than 30 minutes is increasingly used as a target. “Being treated within this time frame improves outcomes and reduces length of hospital stay,” said Dr. McDonald.
The prenotification group also had a statistically significant higher percentage of treatment within 60 minutes of hospital arrival (93.5% vs 80%).
These new findings should help convince health and telestroke systems that teleneurology prenotification is worth implementing. “We want to achieve consensus on this as a best practice,” said Dr. McDonald.
Prenotification, he added, “coordinates the process and eliminates unnecessary and time-consuming steps.”
Dr. McDonald plans to prospectively study prenotification by collecting data on a facility before and after implementing a prenotification process.
Compelling Evidence
Commenting on the research, David L. Tirschwell, MD, Harborview Medical Center, Department of Neurology, Seattle, who cochaired the AAN session featuring the research, said the study provides compelling evidence that teleneurologist prenotification improves DTN time.
“Prenotifications are often standard of care in many healthcare settings and should likely be considered a best practice. When possible, extending such prenotification to a teleconsultant would make sense, and these preliminary data support that approach.”
However, more details are needed “to consider whether the intervention is possibly generalizable to other telestroke practices across the United States,” said Dr. Tirschwell.
Dr. McDonald reported receiving personal compensation for serving as a consultant for Syntrillo Inc. and has stock in Syntrillo Inc. Dr. Tirschwell reported no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
, new research showed.
“This preliminary evidence supports adopting teleneurology prenotification as a best practice within health systems that have telestroke capabilities,” said study investigator Mark McDonald, MD, a neurologist at TeleSpecialists, Fort Myers, Florida.
The findings were presented at the 2024 annual meeting of the American Academy of Neurology.
Best Practices
The impact of emergency medical services prenotification, which refers to paramedics alerting receiving hospital emergency departments (EDs) of a suspected stroke on the way for appropriate preparations to be made, is well-defined, said Dr. McDonald.
“What we’re proposing as a best practice is not only should the ED or ED provider be aware, but there needs to be a system in place for standardizing communication to the neurology team so they’re aware, too.”
Prenotification allows a neurologist to “get on the screen to begin coordinating with the ED team to adequately prepare for the possibility of thrombolytic treatment,” he added.
Currently, teleneurology prenotification, he said, is variable and its benefits unclear.
Dr. McDonald said “his organization, TeleSpecialists, maintains a large detailed medical records database for emergency-related, teleneurology, and other cases. For stroke, it recommends 15 best practices” for facilities including prenotification of teleneurology.
Other best practices include evaluating and administering thrombolysis in the CT imaging suite, a preassembled stroke kit that includes antihypertensives and thrombolytic agents, ensuring a weigh bed is available to determine the exact dose of thrombolysis treatment, and implementing “mock” stroke alerts, said Dr. McDonald.
From the database, researchers extracted acute telestroke consultations seen in the ED in 103 facilities in 15 states. Facilities that did not adhere to the 14 best practices other than teleneurologist prenotification were excluded from the analysis.
Of 9290 patients included in the study, 731 were treated with thrombolysis at prenotification facilities (median age, 69 years; median National Institutes of Health Stroke Score [NIHSS], 8) and 31 were treated at facilities without prenotification (median age, 63 years; median NIHSS score, 4). The thrombolytic treatment rate was 8.5% at prenotification facilities versus 4.8% at facilities without prenotification — a difference that was statistically significant.
Prenotification facilities had a significantly shorter median door-to-needle (DTN) time than those without such a process at 35 versus 43 minutes. In addition, there was a statistically significant difference in the percentage of patients with times less than 60 minutes at approximately 88% at prenotification facilities versus about 68% at the facilities without prenotification.
Case-Level Analysis
However, just because a facility adheres to teleneurology prenotification as a whole, doesn’t mean it occurs in every case. Researchers explored the impact of teleneurology prenotification at the case level rather than the facility level.
“That gave us a bit more insight into the real impact because it’s not just being at a facility with the best practice; it’s actually working case by case to see whether it happened or not and that’s where we get the most compelling findings,” said Dr. McDonald.
Of 761 treatment cases, there was prenotification to the neurology team in 401 cases. In 360 cases, prenotification did not occur.
The median DTN time was 29 minutes in the group with actual prenotification vs 41.5 minutes in the group without actual prenotification, a difference that was statistically significant, Dr. McDonald said.
As for treatment within 30 minutes of arrival, 50.4% of patients in the teleneurology prenotification group versus 18.9% in the no prenotification group — a statistically significant difference.
DTN time of less than 30 minutes is increasingly used as a target. “Being treated within this time frame improves outcomes and reduces length of hospital stay,” said Dr. McDonald.
The prenotification group also had a statistically significant higher percentage of treatment within 60 minutes of hospital arrival (93.5% vs 80%).
These new findings should help convince health and telestroke systems that teleneurology prenotification is worth implementing. “We want to achieve consensus on this as a best practice,” said Dr. McDonald.
Prenotification, he added, “coordinates the process and eliminates unnecessary and time-consuming steps.”
Dr. McDonald plans to prospectively study prenotification by collecting data on a facility before and after implementing a prenotification process.
Compelling Evidence
Commenting on the research, David L. Tirschwell, MD, Harborview Medical Center, Department of Neurology, Seattle, who cochaired the AAN session featuring the research, said the study provides compelling evidence that teleneurologist prenotification improves DTN time.
“Prenotifications are often standard of care in many healthcare settings and should likely be considered a best practice. When possible, extending such prenotification to a teleconsultant would make sense, and these preliminary data support that approach.”
However, more details are needed “to consider whether the intervention is possibly generalizable to other telestroke practices across the United States,” said Dr. Tirschwell.
Dr. McDonald reported receiving personal compensation for serving as a consultant for Syntrillo Inc. and has stock in Syntrillo Inc. Dr. Tirschwell reported no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
, new research showed.
“This preliminary evidence supports adopting teleneurology prenotification as a best practice within health systems that have telestroke capabilities,” said study investigator Mark McDonald, MD, a neurologist at TeleSpecialists, Fort Myers, Florida.
The findings were presented at the 2024 annual meeting of the American Academy of Neurology.
Best Practices
The impact of emergency medical services prenotification, which refers to paramedics alerting receiving hospital emergency departments (EDs) of a suspected stroke on the way for appropriate preparations to be made, is well-defined, said Dr. McDonald.
“What we’re proposing as a best practice is not only should the ED or ED provider be aware, but there needs to be a system in place for standardizing communication to the neurology team so they’re aware, too.”
Prenotification allows a neurologist to “get on the screen to begin coordinating with the ED team to adequately prepare for the possibility of thrombolytic treatment,” he added.
Currently, teleneurology prenotification, he said, is variable and its benefits unclear.
Dr. McDonald said “his organization, TeleSpecialists, maintains a large detailed medical records database for emergency-related, teleneurology, and other cases. For stroke, it recommends 15 best practices” for facilities including prenotification of teleneurology.
Other best practices include evaluating and administering thrombolysis in the CT imaging suite, a preassembled stroke kit that includes antihypertensives and thrombolytic agents, ensuring a weigh bed is available to determine the exact dose of thrombolysis treatment, and implementing “mock” stroke alerts, said Dr. McDonald.
From the database, researchers extracted acute telestroke consultations seen in the ED in 103 facilities in 15 states. Facilities that did not adhere to the 14 best practices other than teleneurologist prenotification were excluded from the analysis.
Of 9290 patients included in the study, 731 were treated with thrombolysis at prenotification facilities (median age, 69 years; median National Institutes of Health Stroke Score [NIHSS], 8) and 31 were treated at facilities without prenotification (median age, 63 years; median NIHSS score, 4). The thrombolytic treatment rate was 8.5% at prenotification facilities versus 4.8% at facilities without prenotification — a difference that was statistically significant.
Prenotification facilities had a significantly shorter median door-to-needle (DTN) time than those without such a process at 35 versus 43 minutes. In addition, there was a statistically significant difference in the percentage of patients with times less than 60 minutes at approximately 88% at prenotification facilities versus about 68% at the facilities without prenotification.
Case-Level Analysis
However, just because a facility adheres to teleneurology prenotification as a whole, doesn’t mean it occurs in every case. Researchers explored the impact of teleneurology prenotification at the case level rather than the facility level.
“That gave us a bit more insight into the real impact because it’s not just being at a facility with the best practice; it’s actually working case by case to see whether it happened or not and that’s where we get the most compelling findings,” said Dr. McDonald.
Of 761 treatment cases, there was prenotification to the neurology team in 401 cases. In 360 cases, prenotification did not occur.
The median DTN time was 29 minutes in the group with actual prenotification vs 41.5 minutes in the group without actual prenotification, a difference that was statistically significant, Dr. McDonald said.
As for treatment within 30 minutes of arrival, 50.4% of patients in the teleneurology prenotification group versus 18.9% in the no prenotification group — a statistically significant difference.
DTN time of less than 30 minutes is increasingly used as a target. “Being treated within this time frame improves outcomes and reduces length of hospital stay,” said Dr. McDonald.
The prenotification group also had a statistically significant higher percentage of treatment within 60 minutes of hospital arrival (93.5% vs 80%).
These new findings should help convince health and telestroke systems that teleneurology prenotification is worth implementing. “We want to achieve consensus on this as a best practice,” said Dr. McDonald.
Prenotification, he added, “coordinates the process and eliminates unnecessary and time-consuming steps.”
Dr. McDonald plans to prospectively study prenotification by collecting data on a facility before and after implementing a prenotification process.
Compelling Evidence
Commenting on the research, David L. Tirschwell, MD, Harborview Medical Center, Department of Neurology, Seattle, who cochaired the AAN session featuring the research, said the study provides compelling evidence that teleneurologist prenotification improves DTN time.
“Prenotifications are often standard of care in many healthcare settings and should likely be considered a best practice. When possible, extending such prenotification to a teleconsultant would make sense, and these preliminary data support that approach.”
However, more details are needed “to consider whether the intervention is possibly generalizable to other telestroke practices across the United States,” said Dr. Tirschwell.
Dr. McDonald reported receiving personal compensation for serving as a consultant for Syntrillo Inc. and has stock in Syntrillo Inc. Dr. Tirschwell reported no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
FROM AAN 2024
Novel Agent Curbs Alzheimer’s-Related Agitation
DENVER —
More than half of participants in the open-label extension period of the randomized clinical trial responded to the medication, which was associated with a 3.6-fold lower risk for relapse compared with placebo.
“The positive efficacy and favorable safety results with AXS-05 support its potential to fulfill a high unmet need for the treatment of Alzheimer’s disease agitation,” said Anton P. Porsteinsson, MD, director of the Alzheimer’s Disease Care, Research and Education Program, University of Rochester, New York.
The findings were presented at the 2024 annual meeting of the American Academy of Neurology.
Common and Disruptive
Agitation is reported in up to 70% of patients with Alzheimer’s disease and is characterized by emotional distress, aggressive behaviors, disruptive irritability, and disinhibition. Alzheimer’s disease-related agitation has been associated with increased caregiver burden, decreased functioning, accelerated cognitive decline, earlier nursing home placement, and increased mortality.
A previous phase 2/3 study of AXS-05 showed that the investigative agent led to rapid and significantly improvement in Alzheimer’s disease agitation, as measured by the Cohen-Mansfield Agitation Inventory (CMAI) total score, compared with placebo.
ACCORD was a phase 3, randomized, double-blind, placebo-controlled withdrawal trial evaluating the efficacy and safety of AXS-05 in patients with Alzheimer’s disease agitation.
In the open-label period, 178 adults with probable Alzheimer’s disease and clinically significant agitation received AXS-05 (titrated to 45 mg dextromethorphan/105 mg bupropion twice daily) for up to 9 weeks.
A total of 108 (61%) patients had a sustained response, with 30% or more improvement from baseline in the CMAI total score and improvement on the Patient Global Impression of Change that were both maintained for 4 or more consecutive weeks. These patients entered the double-blind phase and were randomly allocated to receive twice-daily AXS-05 or placebo for up to 26 weeks.
In the double-blind period, AXS-05 “substantially and statistically” increased the time to relapse of agitation symptoms compared with placebo (hazard ratio [HR], 0.275; P = .014).
“The risk of relapse was 3.6-fold lower with AXS-05 compared with placebo,” Dr. Porsteinsson reported.
AXS-05 was also associated with a significantly lower relapse rate compared with placebo (7.5% vs 25.9%; P = .018).
Rates of discontinuation in the double-blind period owing to adverse events (AEs) were low (0% for AXS-05 and 1.9% for placebo). Three serious AEs were reported: one in the AXS-05 group (fecaloma), which was not related to study medication, and two in the placebo group (cardiac arrest, femur fracture).
Falls were reported in four participants in the AXS-05 group, none of which were related to study medication or associated with serious AEs, and in two participants in the placebo group, one of which was associated with femur fracture.
One death was reported in the placebo group. There was no evidence of cognitive decline with AXS-05, and treatment was not associated with sedation.
Promising Agent
Commenting on this research, Glen R. Finney, MD, director of the Geisinger Memory and Cognition Clinic in Wilkes-Barre, Pennsylvania, said the data “look promising as a safe way to help address acute agitation and reduce agitation reoccurrence.
“Agitation is a common, distressing, and sometimes safety issue for people fighting Alzheimer’s disease, and there’s very little evidence for efficacy and significant side effect issues for current medical management of agitation in Alzheimer’s disease,” said Dr. Finney, who was not part of the study.
He noted that first-line strategies for addressing agitation involve behavioral and environmental interventions.
“See if there’s a reason for the agitation and address that. Look for triggers for agitation and avoid those. Find places, things, and interactions that help people with Alzheimer’s disease avoid agitation: familiar locations, music, simple engaging activities. Reassurance, redirection, and distraction can help de-escalate agitation. Provide a safe environment that reduces safety risks,” Dr. Finney explained.
The next step, when medically appropriate, is trying acetylcholinesterase inhibitors such as donepezil, rivastigmine, and galantamine, and then adding memantine, a weak N-methyl-D-aspartate receptor antagonist.
“These medications can help reduce the risk of agitation,” Dr. Finney said.
“Beyond that, the evidence becomes weaker for any specific treatments, and that is where treatments with emerging evidence of efficacy and safety like dextromethorphan-bupropion become important,” Dr. Finney added.
Last May, the US Food and Drug Administration (FDA) approved the antipsychotic brexpiprazole (Rexulti) for Alzheimer’s disease-related agitation, making it the first FDA-approved drug for this indication.
The drug includes a boxed warning for medications in this class that older patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk for death.
“There’s certainly a need to have multiple options for treating agitation in individuals with Alzheimer’s disease,” said Rebecca Edelmayer, PhD, senior director of scientific engagement for the Alzheimer’s Association.
Dr. Edelmayer, who was not part of the study, noted that in the ACCORD study, AXS-05 “significantly delayed the relapse or prevented the relapse with Alzheimer’s disease agitation compared with the placebo group and it was generally well tolerated, but it will be important to make sure that there’s more thorough review of the data overall to be sure that it’s both safe and effective.”
The study was funded by Axsome Therapeutics, the manufacturer of AXS-05. Dr. Porsteinsson has disclosed no relevant conflicts of interest. Dr. Finney and Dr. Edelmayer have no relevant disclosures.
A version of this article appeared on Medscape.com.
DENVER —
More than half of participants in the open-label extension period of the randomized clinical trial responded to the medication, which was associated with a 3.6-fold lower risk for relapse compared with placebo.
“The positive efficacy and favorable safety results with AXS-05 support its potential to fulfill a high unmet need for the treatment of Alzheimer’s disease agitation,” said Anton P. Porsteinsson, MD, director of the Alzheimer’s Disease Care, Research and Education Program, University of Rochester, New York.
The findings were presented at the 2024 annual meeting of the American Academy of Neurology.
Common and Disruptive
Agitation is reported in up to 70% of patients with Alzheimer’s disease and is characterized by emotional distress, aggressive behaviors, disruptive irritability, and disinhibition. Alzheimer’s disease-related agitation has been associated with increased caregiver burden, decreased functioning, accelerated cognitive decline, earlier nursing home placement, and increased mortality.
A previous phase 2/3 study of AXS-05 showed that the investigative agent led to rapid and significantly improvement in Alzheimer’s disease agitation, as measured by the Cohen-Mansfield Agitation Inventory (CMAI) total score, compared with placebo.
ACCORD was a phase 3, randomized, double-blind, placebo-controlled withdrawal trial evaluating the efficacy and safety of AXS-05 in patients with Alzheimer’s disease agitation.
In the open-label period, 178 adults with probable Alzheimer’s disease and clinically significant agitation received AXS-05 (titrated to 45 mg dextromethorphan/105 mg bupropion twice daily) for up to 9 weeks.
A total of 108 (61%) patients had a sustained response, with 30% or more improvement from baseline in the CMAI total score and improvement on the Patient Global Impression of Change that were both maintained for 4 or more consecutive weeks. These patients entered the double-blind phase and were randomly allocated to receive twice-daily AXS-05 or placebo for up to 26 weeks.
In the double-blind period, AXS-05 “substantially and statistically” increased the time to relapse of agitation symptoms compared with placebo (hazard ratio [HR], 0.275; P = .014).
“The risk of relapse was 3.6-fold lower with AXS-05 compared with placebo,” Dr. Porsteinsson reported.
AXS-05 was also associated with a significantly lower relapse rate compared with placebo (7.5% vs 25.9%; P = .018).
Rates of discontinuation in the double-blind period owing to adverse events (AEs) were low (0% for AXS-05 and 1.9% for placebo). Three serious AEs were reported: one in the AXS-05 group (fecaloma), which was not related to study medication, and two in the placebo group (cardiac arrest, femur fracture).
Falls were reported in four participants in the AXS-05 group, none of which were related to study medication or associated with serious AEs, and in two participants in the placebo group, one of which was associated with femur fracture.
One death was reported in the placebo group. There was no evidence of cognitive decline with AXS-05, and treatment was not associated with sedation.
Promising Agent
Commenting on this research, Glen R. Finney, MD, director of the Geisinger Memory and Cognition Clinic in Wilkes-Barre, Pennsylvania, said the data “look promising as a safe way to help address acute agitation and reduce agitation reoccurrence.
“Agitation is a common, distressing, and sometimes safety issue for people fighting Alzheimer’s disease, and there’s very little evidence for efficacy and significant side effect issues for current medical management of agitation in Alzheimer’s disease,” said Dr. Finney, who was not part of the study.
He noted that first-line strategies for addressing agitation involve behavioral and environmental interventions.
“See if there’s a reason for the agitation and address that. Look for triggers for agitation and avoid those. Find places, things, and interactions that help people with Alzheimer’s disease avoid agitation: familiar locations, music, simple engaging activities. Reassurance, redirection, and distraction can help de-escalate agitation. Provide a safe environment that reduces safety risks,” Dr. Finney explained.
The next step, when medically appropriate, is trying acetylcholinesterase inhibitors such as donepezil, rivastigmine, and galantamine, and then adding memantine, a weak N-methyl-D-aspartate receptor antagonist.
“These medications can help reduce the risk of agitation,” Dr. Finney said.
“Beyond that, the evidence becomes weaker for any specific treatments, and that is where treatments with emerging evidence of efficacy and safety like dextromethorphan-bupropion become important,” Dr. Finney added.
Last May, the US Food and Drug Administration (FDA) approved the antipsychotic brexpiprazole (Rexulti) for Alzheimer’s disease-related agitation, making it the first FDA-approved drug for this indication.
The drug includes a boxed warning for medications in this class that older patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk for death.
“There’s certainly a need to have multiple options for treating agitation in individuals with Alzheimer’s disease,” said Rebecca Edelmayer, PhD, senior director of scientific engagement for the Alzheimer’s Association.
Dr. Edelmayer, who was not part of the study, noted that in the ACCORD study, AXS-05 “significantly delayed the relapse or prevented the relapse with Alzheimer’s disease agitation compared with the placebo group and it was generally well tolerated, but it will be important to make sure that there’s more thorough review of the data overall to be sure that it’s both safe and effective.”
The study was funded by Axsome Therapeutics, the manufacturer of AXS-05. Dr. Porsteinsson has disclosed no relevant conflicts of interest. Dr. Finney and Dr. Edelmayer have no relevant disclosures.
A version of this article appeared on Medscape.com.
DENVER —
More than half of participants in the open-label extension period of the randomized clinical trial responded to the medication, which was associated with a 3.6-fold lower risk for relapse compared with placebo.
“The positive efficacy and favorable safety results with AXS-05 support its potential to fulfill a high unmet need for the treatment of Alzheimer’s disease agitation,” said Anton P. Porsteinsson, MD, director of the Alzheimer’s Disease Care, Research and Education Program, University of Rochester, New York.
The findings were presented at the 2024 annual meeting of the American Academy of Neurology.
Common and Disruptive
Agitation is reported in up to 70% of patients with Alzheimer’s disease and is characterized by emotional distress, aggressive behaviors, disruptive irritability, and disinhibition. Alzheimer’s disease-related agitation has been associated with increased caregiver burden, decreased functioning, accelerated cognitive decline, earlier nursing home placement, and increased mortality.
A previous phase 2/3 study of AXS-05 showed that the investigative agent led to rapid and significantly improvement in Alzheimer’s disease agitation, as measured by the Cohen-Mansfield Agitation Inventory (CMAI) total score, compared with placebo.
ACCORD was a phase 3, randomized, double-blind, placebo-controlled withdrawal trial evaluating the efficacy and safety of AXS-05 in patients with Alzheimer’s disease agitation.
In the open-label period, 178 adults with probable Alzheimer’s disease and clinically significant agitation received AXS-05 (titrated to 45 mg dextromethorphan/105 mg bupropion twice daily) for up to 9 weeks.
A total of 108 (61%) patients had a sustained response, with 30% or more improvement from baseline in the CMAI total score and improvement on the Patient Global Impression of Change that were both maintained for 4 or more consecutive weeks. These patients entered the double-blind phase and were randomly allocated to receive twice-daily AXS-05 or placebo for up to 26 weeks.
In the double-blind period, AXS-05 “substantially and statistically” increased the time to relapse of agitation symptoms compared with placebo (hazard ratio [HR], 0.275; P = .014).
“The risk of relapse was 3.6-fold lower with AXS-05 compared with placebo,” Dr. Porsteinsson reported.
AXS-05 was also associated with a significantly lower relapse rate compared with placebo (7.5% vs 25.9%; P = .018).
Rates of discontinuation in the double-blind period owing to adverse events (AEs) were low (0% for AXS-05 and 1.9% for placebo). Three serious AEs were reported: one in the AXS-05 group (fecaloma), which was not related to study medication, and two in the placebo group (cardiac arrest, femur fracture).
Falls were reported in four participants in the AXS-05 group, none of which were related to study medication or associated with serious AEs, and in two participants in the placebo group, one of which was associated with femur fracture.
One death was reported in the placebo group. There was no evidence of cognitive decline with AXS-05, and treatment was not associated with sedation.
Promising Agent
Commenting on this research, Glen R. Finney, MD, director of the Geisinger Memory and Cognition Clinic in Wilkes-Barre, Pennsylvania, said the data “look promising as a safe way to help address acute agitation and reduce agitation reoccurrence.
“Agitation is a common, distressing, and sometimes safety issue for people fighting Alzheimer’s disease, and there’s very little evidence for efficacy and significant side effect issues for current medical management of agitation in Alzheimer’s disease,” said Dr. Finney, who was not part of the study.
He noted that first-line strategies for addressing agitation involve behavioral and environmental interventions.
“See if there’s a reason for the agitation and address that. Look for triggers for agitation and avoid those. Find places, things, and interactions that help people with Alzheimer’s disease avoid agitation: familiar locations, music, simple engaging activities. Reassurance, redirection, and distraction can help de-escalate agitation. Provide a safe environment that reduces safety risks,” Dr. Finney explained.
The next step, when medically appropriate, is trying acetylcholinesterase inhibitors such as donepezil, rivastigmine, and galantamine, and then adding memantine, a weak N-methyl-D-aspartate receptor antagonist.
“These medications can help reduce the risk of agitation,” Dr. Finney said.
“Beyond that, the evidence becomes weaker for any specific treatments, and that is where treatments with emerging evidence of efficacy and safety like dextromethorphan-bupropion become important,” Dr. Finney added.
Last May, the US Food and Drug Administration (FDA) approved the antipsychotic brexpiprazole (Rexulti) for Alzheimer’s disease-related agitation, making it the first FDA-approved drug for this indication.
The drug includes a boxed warning for medications in this class that older patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk for death.
“There’s certainly a need to have multiple options for treating agitation in individuals with Alzheimer’s disease,” said Rebecca Edelmayer, PhD, senior director of scientific engagement for the Alzheimer’s Association.
Dr. Edelmayer, who was not part of the study, noted that in the ACCORD study, AXS-05 “significantly delayed the relapse or prevented the relapse with Alzheimer’s disease agitation compared with the placebo group and it was generally well tolerated, but it will be important to make sure that there’s more thorough review of the data overall to be sure that it’s both safe and effective.”
The study was funded by Axsome Therapeutics, the manufacturer of AXS-05. Dr. Porsteinsson has disclosed no relevant conflicts of interest. Dr. Finney and Dr. Edelmayer have no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM AAN 2024
Pediatric Patients With MS May Do Best on High-Efficacy DMTs
DENVER — Patients with pediatric-onset multiple sclerosis (POMS) are often prescribed low-efficacy disease-modifying therapies (DMTs), but a new retrospective analysis suggests that, like adults, this patient population may benefit from early treatment with high-efficacy DMTs.
“I think it’s very important to highlight that we are seeing that traditionally, kids are just started on lower-efficacy treatments and they keep relapsing. If we can show that when they get transitioned to high-efficacy treatments, the relapses are lessening, I’m hoping that can then push for better clinical trials with pediatric patients included,” said Frederick Bassal, DO, who presented the study during a poster session at the 2024 annual meeting of the American Academy of Neurology. He is a pediatric neurologist at University of California, Davis.
The first line for POMS is generally low-efficacy DMTs like interferon-beta and glatiramer acetate, but these medications may not effectively control disease progression, according to the study authors, and this could lead to pediatric patients being changed to more potent therapies. That can include moderate-efficacy drugs like S1P inhibitors and fumarates, or high-efficacy DMTS such as B cell depletors and alpha 4 integrin receptor antibodies.
Treatment Strategies
“Right now what we’re seeing is the conservative approach — starting low and working up with the younger and adolescent patients. I’m speculating, and I want to look more into it. Is [it maybe] because of insurance approval?” said study coauthor Amara Miller, a medical student at the University of Arizona College of Medicine in Phoenix.
The findings aren’t surprising, according to Barbara Giesser, MD, who was asked to comment on the study. “It is in line with what we think we know about people with adult MS — that if you start early on with a more effective therapy, you tend to have better outcomes,” said Dr. Giesser, director of the MS program at the Pacific Neuroscience Institute.
Another reason to consider higher-efficacy DMTs is that children with MS can have cognitive problems and delays. “There’s a suggestion that if you treat with highly-effective DMT that you might be able to abrogate some of that,” said Dr. Giesser.
Among the approximately two dozen FDA-approved disease-modifying therapies for MS, only fingolimod (Gilenya, Novartis) is approved for children and adolescents. “All of the others are used off label, but I think perhaps, if you have more studies that [show] that children do better if you treat with more effective therapies early on, perhaps we might see more on-label indications for use in a pediatric population,” said Dr. Giesser.
The finding that obesity was associated with a higher likelihood of having multiple therapies is also interesting, she said. “We’re beginning to see that obesity in adults as well seems to portend less favorable neurologic outcomes.”
Study Methodology
The researchers analyzed data from 135 POMS patients between 2012 and 2023.
The mean age of participants was 15 years, 60% were female, and 120 of 135 were White, while 76 were of Hispanic ethnicity. Overweight and obesity were common, with 36 and 43 participants in each category. The initial therapy was a low-efficacy DMT in 23.0% of participants, moderate-efficacy in 37.0%, and high-efficacy in 24.4%, while 1.5% received some other kind of medication and 14.1% received no medication. The annualized relapse rate was 0.932, and the mean EDSS score was 0.88.
Patients who underwent three or medication changes had lower EDSS scores than those who underwent zero to 2 (P = .00607).
Over the course of the study, the percentage of patients who received high-efficacy DMTs rose from 25.9% to 48.9%, largely due to changes in medication. Of those initially prescribed low-efficacy DMTs, 77.4% were eventually switched to high-efficacy DMTs.
Every patient who underwent three or more medication changes was initially prescribed a low-efficacy DMT.
Patients started on low-efficacy drugs had a mean of 1.42 medication changes, compared with 0.94 in the moderate-efficacy group and 0.51 in the high-efficacy group. The reasons for changing from the first medication included relapse (36), side effects (11), patient choice or compliance (8), and pregnancy (2).
Patients 10 years or younger were more likely to be initially prescribed a low-efficacy therapy (odds ratio [OR], 5.72; P = .0366), while older patients were more likely to be prescribed moderate- or high-efficacy therapies (OR, 14.44; P = .0012).
There were more total medication changes in the low-efficacy group than the high initial DMT group (P = .000305).
Asked what advice they would give to physicians treating POMS patients, Ms. Miller suggested a top-down approach. “We want to look at if maybe we can start with higher efficacy DMT’s and maybe titering it down. That may be an option,” said Ms. Miller.
Dr. Bassal highlighted the importance of shared decision-making. “We want to go over the options, that we recommend higher-efficacy [DMTs] for these reasons. But every individual is different. And there may be fears that are very reasonable that families have. I think in those cases, it’s also reasonable to make a shared decision. And if that means going with something like an oral, moderate- to lower-efficacy [therapy], that’s okay, because compliance is key, and if you start something where the family is afraid of side effects, or there are side effects, then you kind of lost that opportunity,” he said.
Dr. Bassal, Dr. Giesser, and Ms. Miller have no relevant financial disclosures.
DENVER — Patients with pediatric-onset multiple sclerosis (POMS) are often prescribed low-efficacy disease-modifying therapies (DMTs), but a new retrospective analysis suggests that, like adults, this patient population may benefit from early treatment with high-efficacy DMTs.
“I think it’s very important to highlight that we are seeing that traditionally, kids are just started on lower-efficacy treatments and they keep relapsing. If we can show that when they get transitioned to high-efficacy treatments, the relapses are lessening, I’m hoping that can then push for better clinical trials with pediatric patients included,” said Frederick Bassal, DO, who presented the study during a poster session at the 2024 annual meeting of the American Academy of Neurology. He is a pediatric neurologist at University of California, Davis.
The first line for POMS is generally low-efficacy DMTs like interferon-beta and glatiramer acetate, but these medications may not effectively control disease progression, according to the study authors, and this could lead to pediatric patients being changed to more potent therapies. That can include moderate-efficacy drugs like S1P inhibitors and fumarates, or high-efficacy DMTS such as B cell depletors and alpha 4 integrin receptor antibodies.
Treatment Strategies
“Right now what we’re seeing is the conservative approach — starting low and working up with the younger and adolescent patients. I’m speculating, and I want to look more into it. Is [it maybe] because of insurance approval?” said study coauthor Amara Miller, a medical student at the University of Arizona College of Medicine in Phoenix.
The findings aren’t surprising, according to Barbara Giesser, MD, who was asked to comment on the study. “It is in line with what we think we know about people with adult MS — that if you start early on with a more effective therapy, you tend to have better outcomes,” said Dr. Giesser, director of the MS program at the Pacific Neuroscience Institute.
Another reason to consider higher-efficacy DMTs is that children with MS can have cognitive problems and delays. “There’s a suggestion that if you treat with highly-effective DMT that you might be able to abrogate some of that,” said Dr. Giesser.
Among the approximately two dozen FDA-approved disease-modifying therapies for MS, only fingolimod (Gilenya, Novartis) is approved for children and adolescents. “All of the others are used off label, but I think perhaps, if you have more studies that [show] that children do better if you treat with more effective therapies early on, perhaps we might see more on-label indications for use in a pediatric population,” said Dr. Giesser.
The finding that obesity was associated with a higher likelihood of having multiple therapies is also interesting, she said. “We’re beginning to see that obesity in adults as well seems to portend less favorable neurologic outcomes.”
Study Methodology
The researchers analyzed data from 135 POMS patients between 2012 and 2023.
The mean age of participants was 15 years, 60% were female, and 120 of 135 were White, while 76 were of Hispanic ethnicity. Overweight and obesity were common, with 36 and 43 participants in each category. The initial therapy was a low-efficacy DMT in 23.0% of participants, moderate-efficacy in 37.0%, and high-efficacy in 24.4%, while 1.5% received some other kind of medication and 14.1% received no medication. The annualized relapse rate was 0.932, and the mean EDSS score was 0.88.
Patients who underwent three or medication changes had lower EDSS scores than those who underwent zero to 2 (P = .00607).
Over the course of the study, the percentage of patients who received high-efficacy DMTs rose from 25.9% to 48.9%, largely due to changes in medication. Of those initially prescribed low-efficacy DMTs, 77.4% were eventually switched to high-efficacy DMTs.
Every patient who underwent three or more medication changes was initially prescribed a low-efficacy DMT.
Patients started on low-efficacy drugs had a mean of 1.42 medication changes, compared with 0.94 in the moderate-efficacy group and 0.51 in the high-efficacy group. The reasons for changing from the first medication included relapse (36), side effects (11), patient choice or compliance (8), and pregnancy (2).
Patients 10 years or younger were more likely to be initially prescribed a low-efficacy therapy (odds ratio [OR], 5.72; P = .0366), while older patients were more likely to be prescribed moderate- or high-efficacy therapies (OR, 14.44; P = .0012).
There were more total medication changes in the low-efficacy group than the high initial DMT group (P = .000305).
Asked what advice they would give to physicians treating POMS patients, Ms. Miller suggested a top-down approach. “We want to look at if maybe we can start with higher efficacy DMT’s and maybe titering it down. That may be an option,” said Ms. Miller.
Dr. Bassal highlighted the importance of shared decision-making. “We want to go over the options, that we recommend higher-efficacy [DMTs] for these reasons. But every individual is different. And there may be fears that are very reasonable that families have. I think in those cases, it’s also reasonable to make a shared decision. And if that means going with something like an oral, moderate- to lower-efficacy [therapy], that’s okay, because compliance is key, and if you start something where the family is afraid of side effects, or there are side effects, then you kind of lost that opportunity,” he said.
Dr. Bassal, Dr. Giesser, and Ms. Miller have no relevant financial disclosures.
DENVER — Patients with pediatric-onset multiple sclerosis (POMS) are often prescribed low-efficacy disease-modifying therapies (DMTs), but a new retrospective analysis suggests that, like adults, this patient population may benefit from early treatment with high-efficacy DMTs.
“I think it’s very important to highlight that we are seeing that traditionally, kids are just started on lower-efficacy treatments and they keep relapsing. If we can show that when they get transitioned to high-efficacy treatments, the relapses are lessening, I’m hoping that can then push for better clinical trials with pediatric patients included,” said Frederick Bassal, DO, who presented the study during a poster session at the 2024 annual meeting of the American Academy of Neurology. He is a pediatric neurologist at University of California, Davis.
The first line for POMS is generally low-efficacy DMTs like interferon-beta and glatiramer acetate, but these medications may not effectively control disease progression, according to the study authors, and this could lead to pediatric patients being changed to more potent therapies. That can include moderate-efficacy drugs like S1P inhibitors and fumarates, or high-efficacy DMTS such as B cell depletors and alpha 4 integrin receptor antibodies.
Treatment Strategies
“Right now what we’re seeing is the conservative approach — starting low and working up with the younger and adolescent patients. I’m speculating, and I want to look more into it. Is [it maybe] because of insurance approval?” said study coauthor Amara Miller, a medical student at the University of Arizona College of Medicine in Phoenix.
The findings aren’t surprising, according to Barbara Giesser, MD, who was asked to comment on the study. “It is in line with what we think we know about people with adult MS — that if you start early on with a more effective therapy, you tend to have better outcomes,” said Dr. Giesser, director of the MS program at the Pacific Neuroscience Institute.
Another reason to consider higher-efficacy DMTs is that children with MS can have cognitive problems and delays. “There’s a suggestion that if you treat with highly-effective DMT that you might be able to abrogate some of that,” said Dr. Giesser.
Among the approximately two dozen FDA-approved disease-modifying therapies for MS, only fingolimod (Gilenya, Novartis) is approved for children and adolescents. “All of the others are used off label, but I think perhaps, if you have more studies that [show] that children do better if you treat with more effective therapies early on, perhaps we might see more on-label indications for use in a pediatric population,” said Dr. Giesser.
The finding that obesity was associated with a higher likelihood of having multiple therapies is also interesting, she said. “We’re beginning to see that obesity in adults as well seems to portend less favorable neurologic outcomes.”
Study Methodology
The researchers analyzed data from 135 POMS patients between 2012 and 2023.
The mean age of participants was 15 years, 60% were female, and 120 of 135 were White, while 76 were of Hispanic ethnicity. Overweight and obesity were common, with 36 and 43 participants in each category. The initial therapy was a low-efficacy DMT in 23.0% of participants, moderate-efficacy in 37.0%, and high-efficacy in 24.4%, while 1.5% received some other kind of medication and 14.1% received no medication. The annualized relapse rate was 0.932, and the mean EDSS score was 0.88.
Patients who underwent three or medication changes had lower EDSS scores than those who underwent zero to 2 (P = .00607).
Over the course of the study, the percentage of patients who received high-efficacy DMTs rose from 25.9% to 48.9%, largely due to changes in medication. Of those initially prescribed low-efficacy DMTs, 77.4% were eventually switched to high-efficacy DMTs.
Every patient who underwent three or more medication changes was initially prescribed a low-efficacy DMT.
Patients started on low-efficacy drugs had a mean of 1.42 medication changes, compared with 0.94 in the moderate-efficacy group and 0.51 in the high-efficacy group. The reasons for changing from the first medication included relapse (36), side effects (11), patient choice or compliance (8), and pregnancy (2).
Patients 10 years or younger were more likely to be initially prescribed a low-efficacy therapy (odds ratio [OR], 5.72; P = .0366), while older patients were more likely to be prescribed moderate- or high-efficacy therapies (OR, 14.44; P = .0012).
There were more total medication changes in the low-efficacy group than the high initial DMT group (P = .000305).
Asked what advice they would give to physicians treating POMS patients, Ms. Miller suggested a top-down approach. “We want to look at if maybe we can start with higher efficacy DMT’s and maybe titering it down. That may be an option,” said Ms. Miller.
Dr. Bassal highlighted the importance of shared decision-making. “We want to go over the options, that we recommend higher-efficacy [DMTs] for these reasons. But every individual is different. And there may be fears that are very reasonable that families have. I think in those cases, it’s also reasonable to make a shared decision. And if that means going with something like an oral, moderate- to lower-efficacy [therapy], that’s okay, because compliance is key, and if you start something where the family is afraid of side effects, or there are side effects, then you kind of lost that opportunity,” he said.
Dr. Bassal, Dr. Giesser, and Ms. Miller have no relevant financial disclosures.
FROM AAN 2024
First Consensus Statement on Improving Healthcare for Children with Neurodevelopmental Disabilities
The statement was published in Pediatrics.
The disparities in healthcare culture, mindset, and practice often start in childhood for young people with conditions including autism spectrum disorder (ASD), intellectual disability, and attention-deficit/hyperactivity disorder (ADHD), wrote co–first authors Carol Weitzman, MD, co-director of the Autism Spectrum Center at Boston Children’s Hospital, Boston, Massachusetts, and Cy Nadler, PhD, section chief of Autism Psychology at Children’s Mercy in Kansas City, Missouri, and colleagues.
Without better access to safe and appropriate care, people with NDDs experience more seclusion, accidents, restraints, and injury in healthcare encounters, the researchers wrote.
‘Accessible, Humane, Effective Care’
“At the heart of this consensus statement is an affirmation that all people are entitled to healthcare that is accessible, humane, and effective,” they wrote.
The consensus statement was developed as part of the Supporting Access for Everyone (SAFE) Initiative, launched by the Developmental Behavioral Pediatric Research Network and the Association of University Centers on Disability. The consensus panel comprised professionals, caregivers, and adults with NDDs. After a 2-day public forum, the consensus panel held a conference and developed a statement on SAFE care, an NDD Health Care Bill of Rights and Transition Considerations. They developed 10 statements across five domains: training; communication; access and planning; diversity, equity, inclusion, belonging, and anti-ableism; and policy and structural change.
Asking the Patient ‘What do You Need?’
One theme in the statement that may have the most impact is “the importance of asking the person in front of you what they need,” and building a care plan around that, said senior author Marilyn Augustyn, MD, Director of the Division of Developmental and Behavioral Pediatrics at Boston University Chobanian & Avedisian School of Medicine, Boston, Massachusetts. “The medical community hasn’t done that very well for individuals with neurodevelopmental disabilities.”
Dr. Weitzman added: “Traditionally in healthcare settings, we’ve asked people to check their disabilities at the door.” Many people with neurodevelopmental disabilities often have “invisible disabilities,” she said, explaining that patients may have accommodation needs that aren’t immediately obvious, but could improve their access to care, so asking them what they need is critical.
Examples of ‘Ableism’
The consensus statement also calls attention to structural “ableism” or policies or practices that favor able-bodied people over those with disabilities and details the need for more training and changed policies.
The paper gives some examples of ableism, such as inappropriately excluding people with NDDs from research; staff assuming nonspeaking patients have no capacity for communication; or lack of awareness of sensory needs before using cold stethoscopes or flashing direct light into eyes.
Dr. Weitzman says this work is just the beginning of a complex process. It is intended to be the driver for developing curriculum to train all clinicians and others working with patients about neurodevelopmental disabilities. The hope is it will lead to more research to formalize best practices and make policies mandatory rather than optional.
The urgency in highlighting these issues is partly related to the prevalence of children and adolescents with neurodevelopmental disabilities, which the paper states is approximately 1 in 6.
But there are personal reasons as well for the team who developed the statement.
“We just believe that it is just a human right,” Dr. Weitzman said. “Having a neurodevelopmental disability does not make you any less entitled to good care. “
Dr. Augustyn added, “The children I’ve had the honor of caring for for the last 30 years deserve all this care and more. I think it’s time.”
This work was supported by the Developmental Behavioral Pediatric Research Network and the Association of University Centers on Disability. Dr. Weitzman is a past consultant for Helios/Meliora. The other authors report no relevant financial relationships.
The statement was published in Pediatrics.
The disparities in healthcare culture, mindset, and practice often start in childhood for young people with conditions including autism spectrum disorder (ASD), intellectual disability, and attention-deficit/hyperactivity disorder (ADHD), wrote co–first authors Carol Weitzman, MD, co-director of the Autism Spectrum Center at Boston Children’s Hospital, Boston, Massachusetts, and Cy Nadler, PhD, section chief of Autism Psychology at Children’s Mercy in Kansas City, Missouri, and colleagues.
Without better access to safe and appropriate care, people with NDDs experience more seclusion, accidents, restraints, and injury in healthcare encounters, the researchers wrote.
‘Accessible, Humane, Effective Care’
“At the heart of this consensus statement is an affirmation that all people are entitled to healthcare that is accessible, humane, and effective,” they wrote.
The consensus statement was developed as part of the Supporting Access for Everyone (SAFE) Initiative, launched by the Developmental Behavioral Pediatric Research Network and the Association of University Centers on Disability. The consensus panel comprised professionals, caregivers, and adults with NDDs. After a 2-day public forum, the consensus panel held a conference and developed a statement on SAFE care, an NDD Health Care Bill of Rights and Transition Considerations. They developed 10 statements across five domains: training; communication; access and planning; diversity, equity, inclusion, belonging, and anti-ableism; and policy and structural change.
Asking the Patient ‘What do You Need?’
One theme in the statement that may have the most impact is “the importance of asking the person in front of you what they need,” and building a care plan around that, said senior author Marilyn Augustyn, MD, Director of the Division of Developmental and Behavioral Pediatrics at Boston University Chobanian & Avedisian School of Medicine, Boston, Massachusetts. “The medical community hasn’t done that very well for individuals with neurodevelopmental disabilities.”
Dr. Weitzman added: “Traditionally in healthcare settings, we’ve asked people to check their disabilities at the door.” Many people with neurodevelopmental disabilities often have “invisible disabilities,” she said, explaining that patients may have accommodation needs that aren’t immediately obvious, but could improve their access to care, so asking them what they need is critical.
Examples of ‘Ableism’
The consensus statement also calls attention to structural “ableism” or policies or practices that favor able-bodied people over those with disabilities and details the need for more training and changed policies.
The paper gives some examples of ableism, such as inappropriately excluding people with NDDs from research; staff assuming nonspeaking patients have no capacity for communication; or lack of awareness of sensory needs before using cold stethoscopes or flashing direct light into eyes.
Dr. Weitzman says this work is just the beginning of a complex process. It is intended to be the driver for developing curriculum to train all clinicians and others working with patients about neurodevelopmental disabilities. The hope is it will lead to more research to formalize best practices and make policies mandatory rather than optional.
The urgency in highlighting these issues is partly related to the prevalence of children and adolescents with neurodevelopmental disabilities, which the paper states is approximately 1 in 6.
But there are personal reasons as well for the team who developed the statement.
“We just believe that it is just a human right,” Dr. Weitzman said. “Having a neurodevelopmental disability does not make you any less entitled to good care. “
Dr. Augustyn added, “The children I’ve had the honor of caring for for the last 30 years deserve all this care and more. I think it’s time.”
This work was supported by the Developmental Behavioral Pediatric Research Network and the Association of University Centers on Disability. Dr. Weitzman is a past consultant for Helios/Meliora. The other authors report no relevant financial relationships.
The statement was published in Pediatrics.
The disparities in healthcare culture, mindset, and practice often start in childhood for young people with conditions including autism spectrum disorder (ASD), intellectual disability, and attention-deficit/hyperactivity disorder (ADHD), wrote co–first authors Carol Weitzman, MD, co-director of the Autism Spectrum Center at Boston Children’s Hospital, Boston, Massachusetts, and Cy Nadler, PhD, section chief of Autism Psychology at Children’s Mercy in Kansas City, Missouri, and colleagues.
Without better access to safe and appropriate care, people with NDDs experience more seclusion, accidents, restraints, and injury in healthcare encounters, the researchers wrote.
‘Accessible, Humane, Effective Care’
“At the heart of this consensus statement is an affirmation that all people are entitled to healthcare that is accessible, humane, and effective,” they wrote.
The consensus statement was developed as part of the Supporting Access for Everyone (SAFE) Initiative, launched by the Developmental Behavioral Pediatric Research Network and the Association of University Centers on Disability. The consensus panel comprised professionals, caregivers, and adults with NDDs. After a 2-day public forum, the consensus panel held a conference and developed a statement on SAFE care, an NDD Health Care Bill of Rights and Transition Considerations. They developed 10 statements across five domains: training; communication; access and planning; diversity, equity, inclusion, belonging, and anti-ableism; and policy and structural change.
Asking the Patient ‘What do You Need?’
One theme in the statement that may have the most impact is “the importance of asking the person in front of you what they need,” and building a care plan around that, said senior author Marilyn Augustyn, MD, Director of the Division of Developmental and Behavioral Pediatrics at Boston University Chobanian & Avedisian School of Medicine, Boston, Massachusetts. “The medical community hasn’t done that very well for individuals with neurodevelopmental disabilities.”
Dr. Weitzman added: “Traditionally in healthcare settings, we’ve asked people to check their disabilities at the door.” Many people with neurodevelopmental disabilities often have “invisible disabilities,” she said, explaining that patients may have accommodation needs that aren’t immediately obvious, but could improve their access to care, so asking them what they need is critical.
Examples of ‘Ableism’
The consensus statement also calls attention to structural “ableism” or policies or practices that favor able-bodied people over those with disabilities and details the need for more training and changed policies.
The paper gives some examples of ableism, such as inappropriately excluding people with NDDs from research; staff assuming nonspeaking patients have no capacity for communication; or lack of awareness of sensory needs before using cold stethoscopes or flashing direct light into eyes.
Dr. Weitzman says this work is just the beginning of a complex process. It is intended to be the driver for developing curriculum to train all clinicians and others working with patients about neurodevelopmental disabilities. The hope is it will lead to more research to formalize best practices and make policies mandatory rather than optional.
The urgency in highlighting these issues is partly related to the prevalence of children and adolescents with neurodevelopmental disabilities, which the paper states is approximately 1 in 6.
But there are personal reasons as well for the team who developed the statement.
“We just believe that it is just a human right,” Dr. Weitzman said. “Having a neurodevelopmental disability does not make you any less entitled to good care. “
Dr. Augustyn added, “The children I’ve had the honor of caring for for the last 30 years deserve all this care and more. I think it’s time.”
This work was supported by the Developmental Behavioral Pediatric Research Network and the Association of University Centers on Disability. Dr. Weitzman is a past consultant for Helios/Meliora. The other authors report no relevant financial relationships.
Lidocaine Nerve Block Effective for Severe, Refractory Migraine in Children
DENVER — , results of a randomized controlled trial show.
Investigators found children receiving bilateral occipital nerve blocks with 2% lidocaine had significantly greater pain relief than that of peers receiving saline injections.
Cases series have shown a benefit of peripheral nerve blocks (PNBs) — injections of local anesthetics over branches of the occipital or trigeminal nerve — for severe, refractory headache in children.
Although 80% of pediatric headache specialists use PNBs, there is “inconsistent insurance coverage” for this treatment, which had not been tested in a randomized controlled trial in children before now, lead investigator Christina Szperka, MD, with the Pediatric Headache Program, Department of Neurology, University of Pennsylvania Perelman School of Medicine, Philadelphia, told delegates attending the 2024 annual meeting of the American Academy of Neurology.
Significant Results
Investigators enrolled 58 children and adolescents with acute status migrainosus. The mean age was 16 years, and reported gender was female for 44 participants, male for 11 participants, and nonbinary or transgender in 3 participants. Participants had a migraine flare duration of 22 days and had not responded to other treatments.
All participants had topical lidocaine cream applied for 30 minutes as a run-in step and could decline injections if they experienced sufficient benefit from cream alone.
“We used a lidocaine cream lead-in for two reasons. One was to try to see if we could address the issue of high placebo response in pediatric trials in particular, and also to see if we could help with blinding to injection,” said Dr. Szperka.
Topical lidocaine cream led to a small decrease in pain score overall (0.2 point on a 0-10 scale), and all participants proceeded to randomized blinded bilateral greater occipital nerve injection with 2% lidocaine or saline, she reported.
On the primary endpoint — change in pain score at 30 minutes — lidocaine was significantly more effective than saline, achieving a 2.3-point decrease on average (on a 0-10 scale) vs a 1.1-point decrease with saline (P = .01).
A 2-point pain reduction was achieved in 69% of patients in the lidocaine group versus 34% in the saline group.
Three quarters (76%) of patients getting lidocaine reported at least partial relief in severity or location of pain compared with 48% of those getting saline (P = .03). Rates of pain freedom at 30 minutes were 17% and 7%, respectively, and at 24 hours were 14% and 0%, respectively.
The majority of adverse events were mild and fairly equal across groups and included anxiety, worsening headache, injection site pain, dizziness, and numbness (more so with lidocaine). There was one case of anaphylaxis after lidocaine injection.
Quite unexpectedly, said Dr. Szperka, patients rated the saline injection as more painful than the lidocaine injection. “This was not what I expected going in, and I think is relevant for future trials,” she said.
Encouraging Results
Reached for comment, Shaheen Lakhan, MD, a neurologist and researcher based in Miami, said that as a neurologist and pain physician, he sees firsthand the “devastating impact of status migrainosus on children.”
“These debilitating headaches can rob them of precious school days, hindering learning and social interaction,” said Dr. Lakhan. “The constant pain and fear of the next attack can also take a toll on their emotional well-being.”
The impact on families is significant as well, highlighting the need to find more effective treatments, Dr. Lakhan said.
“Traditionally, we’ve relied on case studies to see the benefits of nerve blocks for migraine in younger patients. This is the first randomized controlled trial that shows lidocaine injections can be significantly more effective than a placebo for these unrelenting migraines,” he said.
“It’s important to note that this is a relatively small study, and not without safety concerns, including rare but potentially life-threatening anaphylaxis to lidocaine,” Dr. Lakhan added. “More research is needed, but these findings are encouraging. Lidocaine injections could become a valuable tool for managing treatment-resistant migraines in adolescents and young adults.”
The study was supported by a grant from the National Institute of Neurological Disorders and Stroke. Dr. Szperka is a consultant for AbbVie and Teva; serves on a Data Safety Monitoring Board for Eli Lilly and Upsher-Smith; and is a site principal investigator for AbbVie, Amgen, Biohaven/Pfizer, Teva, and Theranica. Dr. Lakhan had no disclosures.
A version of this article appeared on Medscape.com.
DENVER — , results of a randomized controlled trial show.
Investigators found children receiving bilateral occipital nerve blocks with 2% lidocaine had significantly greater pain relief than that of peers receiving saline injections.
Cases series have shown a benefit of peripheral nerve blocks (PNBs) — injections of local anesthetics over branches of the occipital or trigeminal nerve — for severe, refractory headache in children.
Although 80% of pediatric headache specialists use PNBs, there is “inconsistent insurance coverage” for this treatment, which had not been tested in a randomized controlled trial in children before now, lead investigator Christina Szperka, MD, with the Pediatric Headache Program, Department of Neurology, University of Pennsylvania Perelman School of Medicine, Philadelphia, told delegates attending the 2024 annual meeting of the American Academy of Neurology.
Significant Results
Investigators enrolled 58 children and adolescents with acute status migrainosus. The mean age was 16 years, and reported gender was female for 44 participants, male for 11 participants, and nonbinary or transgender in 3 participants. Participants had a migraine flare duration of 22 days and had not responded to other treatments.
All participants had topical lidocaine cream applied for 30 minutes as a run-in step and could decline injections if they experienced sufficient benefit from cream alone.
“We used a lidocaine cream lead-in for two reasons. One was to try to see if we could address the issue of high placebo response in pediatric trials in particular, and also to see if we could help with blinding to injection,” said Dr. Szperka.
Topical lidocaine cream led to a small decrease in pain score overall (0.2 point on a 0-10 scale), and all participants proceeded to randomized blinded bilateral greater occipital nerve injection with 2% lidocaine or saline, she reported.
On the primary endpoint — change in pain score at 30 minutes — lidocaine was significantly more effective than saline, achieving a 2.3-point decrease on average (on a 0-10 scale) vs a 1.1-point decrease with saline (P = .01).
A 2-point pain reduction was achieved in 69% of patients in the lidocaine group versus 34% in the saline group.
Three quarters (76%) of patients getting lidocaine reported at least partial relief in severity or location of pain compared with 48% of those getting saline (P = .03). Rates of pain freedom at 30 minutes were 17% and 7%, respectively, and at 24 hours were 14% and 0%, respectively.
The majority of adverse events were mild and fairly equal across groups and included anxiety, worsening headache, injection site pain, dizziness, and numbness (more so with lidocaine). There was one case of anaphylaxis after lidocaine injection.
Quite unexpectedly, said Dr. Szperka, patients rated the saline injection as more painful than the lidocaine injection. “This was not what I expected going in, and I think is relevant for future trials,” she said.
Encouraging Results
Reached for comment, Shaheen Lakhan, MD, a neurologist and researcher based in Miami, said that as a neurologist and pain physician, he sees firsthand the “devastating impact of status migrainosus on children.”
“These debilitating headaches can rob them of precious school days, hindering learning and social interaction,” said Dr. Lakhan. “The constant pain and fear of the next attack can also take a toll on their emotional well-being.”
The impact on families is significant as well, highlighting the need to find more effective treatments, Dr. Lakhan said.
“Traditionally, we’ve relied on case studies to see the benefits of nerve blocks for migraine in younger patients. This is the first randomized controlled trial that shows lidocaine injections can be significantly more effective than a placebo for these unrelenting migraines,” he said.
“It’s important to note that this is a relatively small study, and not without safety concerns, including rare but potentially life-threatening anaphylaxis to lidocaine,” Dr. Lakhan added. “More research is needed, but these findings are encouraging. Lidocaine injections could become a valuable tool for managing treatment-resistant migraines in adolescents and young adults.”
The study was supported by a grant from the National Institute of Neurological Disorders and Stroke. Dr. Szperka is a consultant for AbbVie and Teva; serves on a Data Safety Monitoring Board for Eli Lilly and Upsher-Smith; and is a site principal investigator for AbbVie, Amgen, Biohaven/Pfizer, Teva, and Theranica. Dr. Lakhan had no disclosures.
A version of this article appeared on Medscape.com.
DENVER — , results of a randomized controlled trial show.
Investigators found children receiving bilateral occipital nerve blocks with 2% lidocaine had significantly greater pain relief than that of peers receiving saline injections.
Cases series have shown a benefit of peripheral nerve blocks (PNBs) — injections of local anesthetics over branches of the occipital or trigeminal nerve — for severe, refractory headache in children.
Although 80% of pediatric headache specialists use PNBs, there is “inconsistent insurance coverage” for this treatment, which had not been tested in a randomized controlled trial in children before now, lead investigator Christina Szperka, MD, with the Pediatric Headache Program, Department of Neurology, University of Pennsylvania Perelman School of Medicine, Philadelphia, told delegates attending the 2024 annual meeting of the American Academy of Neurology.
Significant Results
Investigators enrolled 58 children and adolescents with acute status migrainosus. The mean age was 16 years, and reported gender was female for 44 participants, male for 11 participants, and nonbinary or transgender in 3 participants. Participants had a migraine flare duration of 22 days and had not responded to other treatments.
All participants had topical lidocaine cream applied for 30 minutes as a run-in step and could decline injections if they experienced sufficient benefit from cream alone.
“We used a lidocaine cream lead-in for two reasons. One was to try to see if we could address the issue of high placebo response in pediatric trials in particular, and also to see if we could help with blinding to injection,” said Dr. Szperka.
Topical lidocaine cream led to a small decrease in pain score overall (0.2 point on a 0-10 scale), and all participants proceeded to randomized blinded bilateral greater occipital nerve injection with 2% lidocaine or saline, she reported.
On the primary endpoint — change in pain score at 30 minutes — lidocaine was significantly more effective than saline, achieving a 2.3-point decrease on average (on a 0-10 scale) vs a 1.1-point decrease with saline (P = .01).
A 2-point pain reduction was achieved in 69% of patients in the lidocaine group versus 34% in the saline group.
Three quarters (76%) of patients getting lidocaine reported at least partial relief in severity or location of pain compared with 48% of those getting saline (P = .03). Rates of pain freedom at 30 minutes were 17% and 7%, respectively, and at 24 hours were 14% and 0%, respectively.
The majority of adverse events were mild and fairly equal across groups and included anxiety, worsening headache, injection site pain, dizziness, and numbness (more so with lidocaine). There was one case of anaphylaxis after lidocaine injection.
Quite unexpectedly, said Dr. Szperka, patients rated the saline injection as more painful than the lidocaine injection. “This was not what I expected going in, and I think is relevant for future trials,” she said.
Encouraging Results
Reached for comment, Shaheen Lakhan, MD, a neurologist and researcher based in Miami, said that as a neurologist and pain physician, he sees firsthand the “devastating impact of status migrainosus on children.”
“These debilitating headaches can rob them of precious school days, hindering learning and social interaction,” said Dr. Lakhan. “The constant pain and fear of the next attack can also take a toll on their emotional well-being.”
The impact on families is significant as well, highlighting the need to find more effective treatments, Dr. Lakhan said.
“Traditionally, we’ve relied on case studies to see the benefits of nerve blocks for migraine in younger patients. This is the first randomized controlled trial that shows lidocaine injections can be significantly more effective than a placebo for these unrelenting migraines,” he said.
“It’s important to note that this is a relatively small study, and not without safety concerns, including rare but potentially life-threatening anaphylaxis to lidocaine,” Dr. Lakhan added. “More research is needed, but these findings are encouraging. Lidocaine injections could become a valuable tool for managing treatment-resistant migraines in adolescents and young adults.”
The study was supported by a grant from the National Institute of Neurological Disorders and Stroke. Dr. Szperka is a consultant for AbbVie and Teva; serves on a Data Safety Monitoring Board for Eli Lilly and Upsher-Smith; and is a site principal investigator for AbbVie, Amgen, Biohaven/Pfizer, Teva, and Theranica. Dr. Lakhan had no disclosures.
A version of this article appeared on Medscape.com.
FROM AAN 2024
IV Ketamine Promising for Severe Refractory Headache in Children
DENVER — , new research suggests. In a retrospective chart review, IV ketamine led to in a 50% reduction in pain at discharge, with “nearly two-thirds” of patients having no recurrence within 30 days, noted lead investigator Scott Rosenthal, MD, from the University of Colorado Anschutz Medical Campus, Aurora.
Dr. Rosenthal reported the findings at the 2024 annual meeting of the American Academy of Neurology.
Statistically Significant Pain Relief
“IV ketamine has shown benefit in nonheadache chronic pain syndromes and refractory mood disorders. Patients with refractory status migraines are often left with ongoing pain and dysfunction after failing typical interventions,” Dr. Rosenthal said.
“Ketamine has emerged as a potential treatment option in this population. However, there’s very little research on the efficacy and tolerability of it in general as well as the pediatric population,” he noted.
Dr. Rosenthal and colleagues took a look back at patients admitted to Children’s Hospital Colorado between 2019 and 2022 for treatment of severe refractory headache who were treated with continuous IV ketamine.
They analyzed 68 encounters of 41 unique patients aged 5-21 years (median age 16 years; 85% girls). Chronic migraine without aura made up 79% of cases.
On presentation, most patients had an exacerbation or ongoing worsening of pain for about 10 days, and all but two were taking a preventive medication. Nearly 70% had a comorbid psychiatric diagnosis such as anxiety or depression, and 60% had a comorbid chronic pain diagnosis separate from their headache diagnosis.
The primary outcome was percent pain reduction at discharge and headache recurrence within 72 hours, with headache recurrence defined as receipt of neurology care via phone, clinic, or hospital encounter.
Patients received IV ketamine at a median dose of 0.25 mg/kg/hr for a median of 3 days.
Overall, the treatment was “safe and well tolerated,” Dr. Rosenthal said.
There were no serious adverse events and no cardiac side effects; 7% (five out of 68) stopped treatment due to side effects. The most common side effects were dizziness (23%), nausea (16%), blurred vision (12%), hallucinations (19%), cognitive fog (7%), vomiting (6%) and dysphoria (4%), worsening headache (4%), and paresthesia and cramping (1.5%).
‘Exciting Starting Point’
At baseline, pain scores were 8 (on a scale of 0-10) and progressively fell (improved) during treatment. Pain scores were 6 on day 1 and were 5 on day 2, with a slight rebound to 5 at discharge, although the pain reduction at discharge (vs baseline) remained statistically significant (P < .001).
“The median percent pain reduction after 3 days of ketamine was about 40%,” Dr. Rosenthal said.
He noted that on the first day of treatment, 16% of patients responded to treatment (with a > 50% reduction in their initial pain); this doubled to 33% on day 2 and increased to 44% at discharge.
In terms of recurrence, 38% had a recurrence within 1 month, “meaning two thirds did not,” Dr. Rosenthal noted. Median time to recurrence was 7 days. There were no recurrences within 72 hours.
The researchers also tried to tease out which patients might respond best to ketamine.
“Surprisingly,” there wasn’t a strong effect of most demographic variables such as age, sex, gender identity, chronic pain, psychiatric comorbidities, duration of headache, or prior interventions, Dr. Rosenthal noted.
“Interestingly,” he said, patients who were on two or more preventive medications had a 50% reduction in their pain at discharge compared with a 33% reduction in patients taking one or no preventive medication. It’s possible that more preventative medications may “prime” a patient’s response to ketamine, Dr. Rosenthal said.
She added that future randomized studies are needed to further assess IV ketamine for refractory headache in children, but these results are “an exciting starting point.”
‘Still an Unknown’
Seniha Nur Ozudogru, MD, assistant professor of clinical neurology at Penn Medicine in Philadelphia, echoed the need for further study.
The role of IV ketamine in refractory pediatric headache is “still an unknown,” said Dr. Ozudogru, who was not involved in the study.
She noted that currently, there is “no standard protocol for ketamine infusion, even for adults. Every institution has their own protocols, which makes it difficult.”
Dr. Ozudogru also wonders how “doable” in-hospital IV infusions over 3 days may be for children.
“Especially for chronic migraine patients, it can be really tricky to manage expectations in that even if they don’t respond and the headache doesn’t go away, they still may have to be discharged. That requires a specific approach and discussion with the patients,” Dr. Ozudogru said.
Intranasal ketamine is another potential option, she said, with a recent study suggesting that intranasal ketamine is an effective treatment for children hospitalized with refractory migraine.
“However, there is some concern about the potential of addiction and the side effects of hallucinations and what the main protocol will be, so this not a standard treatment and has to be studied further,” she said.
The study had no specific funding. Dr. Rosenthal and Dr. Ozudogru have no relevant disclosures.
A version of this article appeared on Medscape.com.
DENVER — , new research suggests. In a retrospective chart review, IV ketamine led to in a 50% reduction in pain at discharge, with “nearly two-thirds” of patients having no recurrence within 30 days, noted lead investigator Scott Rosenthal, MD, from the University of Colorado Anschutz Medical Campus, Aurora.
Dr. Rosenthal reported the findings at the 2024 annual meeting of the American Academy of Neurology.
Statistically Significant Pain Relief
“IV ketamine has shown benefit in nonheadache chronic pain syndromes and refractory mood disorders. Patients with refractory status migraines are often left with ongoing pain and dysfunction after failing typical interventions,” Dr. Rosenthal said.
“Ketamine has emerged as a potential treatment option in this population. However, there’s very little research on the efficacy and tolerability of it in general as well as the pediatric population,” he noted.
Dr. Rosenthal and colleagues took a look back at patients admitted to Children’s Hospital Colorado between 2019 and 2022 for treatment of severe refractory headache who were treated with continuous IV ketamine.
They analyzed 68 encounters of 41 unique patients aged 5-21 years (median age 16 years; 85% girls). Chronic migraine without aura made up 79% of cases.
On presentation, most patients had an exacerbation or ongoing worsening of pain for about 10 days, and all but two were taking a preventive medication. Nearly 70% had a comorbid psychiatric diagnosis such as anxiety or depression, and 60% had a comorbid chronic pain diagnosis separate from their headache diagnosis.
The primary outcome was percent pain reduction at discharge and headache recurrence within 72 hours, with headache recurrence defined as receipt of neurology care via phone, clinic, or hospital encounter.
Patients received IV ketamine at a median dose of 0.25 mg/kg/hr for a median of 3 days.
Overall, the treatment was “safe and well tolerated,” Dr. Rosenthal said.
There were no serious adverse events and no cardiac side effects; 7% (five out of 68) stopped treatment due to side effects. The most common side effects were dizziness (23%), nausea (16%), blurred vision (12%), hallucinations (19%), cognitive fog (7%), vomiting (6%) and dysphoria (4%), worsening headache (4%), and paresthesia and cramping (1.5%).
‘Exciting Starting Point’
At baseline, pain scores were 8 (on a scale of 0-10) and progressively fell (improved) during treatment. Pain scores were 6 on day 1 and were 5 on day 2, with a slight rebound to 5 at discharge, although the pain reduction at discharge (vs baseline) remained statistically significant (P < .001).
“The median percent pain reduction after 3 days of ketamine was about 40%,” Dr. Rosenthal said.
He noted that on the first day of treatment, 16% of patients responded to treatment (with a > 50% reduction in their initial pain); this doubled to 33% on day 2 and increased to 44% at discharge.
In terms of recurrence, 38% had a recurrence within 1 month, “meaning two thirds did not,” Dr. Rosenthal noted. Median time to recurrence was 7 days. There were no recurrences within 72 hours.
The researchers also tried to tease out which patients might respond best to ketamine.
“Surprisingly,” there wasn’t a strong effect of most demographic variables such as age, sex, gender identity, chronic pain, psychiatric comorbidities, duration of headache, or prior interventions, Dr. Rosenthal noted.
“Interestingly,” he said, patients who were on two or more preventive medications had a 50% reduction in their pain at discharge compared with a 33% reduction in patients taking one or no preventive medication. It’s possible that more preventative medications may “prime” a patient’s response to ketamine, Dr. Rosenthal said.
She added that future randomized studies are needed to further assess IV ketamine for refractory headache in children, but these results are “an exciting starting point.”
‘Still an Unknown’
Seniha Nur Ozudogru, MD, assistant professor of clinical neurology at Penn Medicine in Philadelphia, echoed the need for further study.
The role of IV ketamine in refractory pediatric headache is “still an unknown,” said Dr. Ozudogru, who was not involved in the study.
She noted that currently, there is “no standard protocol for ketamine infusion, even for adults. Every institution has their own protocols, which makes it difficult.”
Dr. Ozudogru also wonders how “doable” in-hospital IV infusions over 3 days may be for children.
“Especially for chronic migraine patients, it can be really tricky to manage expectations in that even if they don’t respond and the headache doesn’t go away, they still may have to be discharged. That requires a specific approach and discussion with the patients,” Dr. Ozudogru said.
Intranasal ketamine is another potential option, she said, with a recent study suggesting that intranasal ketamine is an effective treatment for children hospitalized with refractory migraine.
“However, there is some concern about the potential of addiction and the side effects of hallucinations and what the main protocol will be, so this not a standard treatment and has to be studied further,” she said.
The study had no specific funding. Dr. Rosenthal and Dr. Ozudogru have no relevant disclosures.
A version of this article appeared on Medscape.com.
DENVER — , new research suggests. In a retrospective chart review, IV ketamine led to in a 50% reduction in pain at discharge, with “nearly two-thirds” of patients having no recurrence within 30 days, noted lead investigator Scott Rosenthal, MD, from the University of Colorado Anschutz Medical Campus, Aurora.
Dr. Rosenthal reported the findings at the 2024 annual meeting of the American Academy of Neurology.
Statistically Significant Pain Relief
“IV ketamine has shown benefit in nonheadache chronic pain syndromes and refractory mood disorders. Patients with refractory status migraines are often left with ongoing pain and dysfunction after failing typical interventions,” Dr. Rosenthal said.
“Ketamine has emerged as a potential treatment option in this population. However, there’s very little research on the efficacy and tolerability of it in general as well as the pediatric population,” he noted.
Dr. Rosenthal and colleagues took a look back at patients admitted to Children’s Hospital Colorado between 2019 and 2022 for treatment of severe refractory headache who were treated with continuous IV ketamine.
They analyzed 68 encounters of 41 unique patients aged 5-21 years (median age 16 years; 85% girls). Chronic migraine without aura made up 79% of cases.
On presentation, most patients had an exacerbation or ongoing worsening of pain for about 10 days, and all but two were taking a preventive medication. Nearly 70% had a comorbid psychiatric diagnosis such as anxiety or depression, and 60% had a comorbid chronic pain diagnosis separate from their headache diagnosis.
The primary outcome was percent pain reduction at discharge and headache recurrence within 72 hours, with headache recurrence defined as receipt of neurology care via phone, clinic, or hospital encounter.
Patients received IV ketamine at a median dose of 0.25 mg/kg/hr for a median of 3 days.
Overall, the treatment was “safe and well tolerated,” Dr. Rosenthal said.
There were no serious adverse events and no cardiac side effects; 7% (five out of 68) stopped treatment due to side effects. The most common side effects were dizziness (23%), nausea (16%), blurred vision (12%), hallucinations (19%), cognitive fog (7%), vomiting (6%) and dysphoria (4%), worsening headache (4%), and paresthesia and cramping (1.5%).
‘Exciting Starting Point’
At baseline, pain scores were 8 (on a scale of 0-10) and progressively fell (improved) during treatment. Pain scores were 6 on day 1 and were 5 on day 2, with a slight rebound to 5 at discharge, although the pain reduction at discharge (vs baseline) remained statistically significant (P < .001).
“The median percent pain reduction after 3 days of ketamine was about 40%,” Dr. Rosenthal said.
He noted that on the first day of treatment, 16% of patients responded to treatment (with a > 50% reduction in their initial pain); this doubled to 33% on day 2 and increased to 44% at discharge.
In terms of recurrence, 38% had a recurrence within 1 month, “meaning two thirds did not,” Dr. Rosenthal noted. Median time to recurrence was 7 days. There were no recurrences within 72 hours.
The researchers also tried to tease out which patients might respond best to ketamine.
“Surprisingly,” there wasn’t a strong effect of most demographic variables such as age, sex, gender identity, chronic pain, psychiatric comorbidities, duration of headache, or prior interventions, Dr. Rosenthal noted.
“Interestingly,” he said, patients who were on two or more preventive medications had a 50% reduction in their pain at discharge compared with a 33% reduction in patients taking one or no preventive medication. It’s possible that more preventative medications may “prime” a patient’s response to ketamine, Dr. Rosenthal said.
She added that future randomized studies are needed to further assess IV ketamine for refractory headache in children, but these results are “an exciting starting point.”
‘Still an Unknown’
Seniha Nur Ozudogru, MD, assistant professor of clinical neurology at Penn Medicine in Philadelphia, echoed the need for further study.
The role of IV ketamine in refractory pediatric headache is “still an unknown,” said Dr. Ozudogru, who was not involved in the study.
She noted that currently, there is “no standard protocol for ketamine infusion, even for adults. Every institution has their own protocols, which makes it difficult.”
Dr. Ozudogru also wonders how “doable” in-hospital IV infusions over 3 days may be for children.
“Especially for chronic migraine patients, it can be really tricky to manage expectations in that even if they don’t respond and the headache doesn’t go away, they still may have to be discharged. That requires a specific approach and discussion with the patients,” Dr. Ozudogru said.
Intranasal ketamine is another potential option, she said, with a recent study suggesting that intranasal ketamine is an effective treatment for children hospitalized with refractory migraine.
“However, there is some concern about the potential of addiction and the side effects of hallucinations and what the main protocol will be, so this not a standard treatment and has to be studied further,” she said.
The study had no specific funding. Dr. Rosenthal and Dr. Ozudogru have no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM AAN 2024
Tension, Other Headache Types Robustly Linked to Attempted, Completed Suicide
DENVER – , results of a large study suggest.
The risk for suicide attempt was four times higher in people with trigeminal and autonomic cephalalgias (TAC), and the risk for completed suicide was double among those with posttraumatic headache compared with individuals with no headache.
The retrospective analysis included data on more than 100,000 headache patients from a Danish registry.
“The results suggest there’s a unique risk among headache patients for attempted and completed suicide,” lead investigator Holly Elser, MD, MPH, PhD, resident, Department of Neurology, University of Pennsylvania, Philadelphia, said at the 2024 annual meeting of the American Academy of Neurology, where the findings were presented. “This really underscores the potential importance of complementary psychiatric evaluation and treatment for individuals diagnosed with headache.”
Underestimated Problem
Headache disorders affect about half of working-age adults and are among the leading causes of productivity loss, absence from work, and disability.
Prior research suggests headache disorders often co-occur with psychiatric illness including depression, anxiety, posttraumatic stress disorder, and even attempted suicide.
However, previous studies that showed an increased risk for attempted suicide in patients with headache relied heavily on survey data and mostly focused on patients with migraine. There was little information on other headache types and on the risk for completed suicide.
Researchers used Danish registries to identify 64,057 patients with migraine, 40,160 with tension-type headache (TTH), 5743 with TAC, and 4253 with posttraumatic headache, all diagnosed from 1995 to 2019.
Some 5.8% of those with migraine, 6.3% with TAC, 7.2% with TTH, and 7.2% with posttraumatic headache, had a mood disorder (depression and anxiety combined) at baseline.
Those without a headache diagnosis were matched 5:1 to those with a headache diagnosis by sex and birth year.
Across all headache disorders, baseline prevalence of mood disorder was higher among those with headache versus population-matched controls. Dr. Elser emphasized that these are people diagnosed with a mood disorder in the inpatient, emergency department, or outpatient specialist clinic setting, “which means we are almost certainly underestimating the true burden of mood symptoms in our cohort,” she said.
Researchers identified attempted suicides using diagnostic codes. For completed suicide, they determined whether those who attempted suicide died within 30 days of the attempt.
For each headache type, investigators examined both the absolute and relative risk for attempted and completed suicides and estimated the risk at intervals of 5, 10, and 20 years after initial headache diagnosis.
Robust Link
The “power of this study is that we asked a simple, but important question, and answered it with simple, but appropriate, methodologic techniques,” Dr. Elser said.
The estimated risk differences (RDs) for attempted suicide were strongest for TAC and posttraumatic headache and for longer follow-ups. The RDs for completed suicide were largely the same but of a smaller magnitude and were “relatively less precise,” reflecting the “rarity of this outcome,” said Dr. Elser.
After adjusting for sex, age, education, income, comorbidities, and baseline medical and psychiatric diagnoses, researchers found the strongest association or attempted suicide was among those with TAC (adjusted hazard ratio [aHR], 4.25; 95% CI, 2.85-6.33).
“A hazard ratio of 4 is enormous” for this type of comparison, Dr. Elser noted.
For completed suicide, the strongest association was with posttraumatic headache (aHR, 2.19; 95% CI, 0.78-6.16).
The study revealed a robust association with attempted and completed suicide across all headache types, including TTH, noted Dr. Elser. The link between tension headaches and suicide “was the most striking finding to me because I think of that as sort of a benign and common headache disorder,” she said.
The was an observational study, so “it’s not clear whether headache is playing an etiological role in the relationship with suicide,” she said. “It’s possible there are common shared risk factors or confounders that explain the relationship in full or in part that aren’t accounted for in this study.”
Ask About Mood
The results underscore the need for psychiatric evaluations in patients with a headache disorder. “For me, this is just going to make me that much more likely to ask my patients about their mood when I see them in clinic,” Dr. Elser said.
After asking patients with headache about their mood and stress at home and at work, physicians should have a “low threshold to refer to a behavioral health provider,” she added.
Future research should aim to better understand the link between headache and suicide risk, with a focus on the mechanisms behind low- and high-risk subgroups, said Dr. Elser.
A limitation of the study was that headache diagnoses were based on inpatient, emergency department, and outpatient specialist visits but not on visits to primary care practitioners. The study didn’t include information on headache severity or frequency and included only people who sought treatment for their headaches.
Though it’s unlikely the results “are perfectly generalizable” with respect to other geographical or cultural contexts, “I don’t think this relationship is unique to Denmark based on the literature to date,” Dr. Elser said.
Commenting on the study, session co-chair Todd J. Schwedt, MD, professor of neurology, Mayo Clinic Arizona, Phoenix, and president-elect of the American Headache Society, noted that the study offers important findings “that demonstrate the enormous negative impact that headaches can exert.”
It’s “a strong reminder” that clinicians should assess the mental health of their patients with headaches and offer treatment when appropriate, he said.
The study received support from Aarhus University. No relevant conflicts of interest were reported.
A version of this article appeared on Medscape.com.
DENVER – , results of a large study suggest.
The risk for suicide attempt was four times higher in people with trigeminal and autonomic cephalalgias (TAC), and the risk for completed suicide was double among those with posttraumatic headache compared with individuals with no headache.
The retrospective analysis included data on more than 100,000 headache patients from a Danish registry.
“The results suggest there’s a unique risk among headache patients for attempted and completed suicide,” lead investigator Holly Elser, MD, MPH, PhD, resident, Department of Neurology, University of Pennsylvania, Philadelphia, said at the 2024 annual meeting of the American Academy of Neurology, where the findings were presented. “This really underscores the potential importance of complementary psychiatric evaluation and treatment for individuals diagnosed with headache.”
Underestimated Problem
Headache disorders affect about half of working-age adults and are among the leading causes of productivity loss, absence from work, and disability.
Prior research suggests headache disorders often co-occur with psychiatric illness including depression, anxiety, posttraumatic stress disorder, and even attempted suicide.
However, previous studies that showed an increased risk for attempted suicide in patients with headache relied heavily on survey data and mostly focused on patients with migraine. There was little information on other headache types and on the risk for completed suicide.
Researchers used Danish registries to identify 64,057 patients with migraine, 40,160 with tension-type headache (TTH), 5743 with TAC, and 4253 with posttraumatic headache, all diagnosed from 1995 to 2019.
Some 5.8% of those with migraine, 6.3% with TAC, 7.2% with TTH, and 7.2% with posttraumatic headache, had a mood disorder (depression and anxiety combined) at baseline.
Those without a headache diagnosis were matched 5:1 to those with a headache diagnosis by sex and birth year.
Across all headache disorders, baseline prevalence of mood disorder was higher among those with headache versus population-matched controls. Dr. Elser emphasized that these are people diagnosed with a mood disorder in the inpatient, emergency department, or outpatient specialist clinic setting, “which means we are almost certainly underestimating the true burden of mood symptoms in our cohort,” she said.
Researchers identified attempted suicides using diagnostic codes. For completed suicide, they determined whether those who attempted suicide died within 30 days of the attempt.
For each headache type, investigators examined both the absolute and relative risk for attempted and completed suicides and estimated the risk at intervals of 5, 10, and 20 years after initial headache diagnosis.
Robust Link
The “power of this study is that we asked a simple, but important question, and answered it with simple, but appropriate, methodologic techniques,” Dr. Elser said.
The estimated risk differences (RDs) for attempted suicide were strongest for TAC and posttraumatic headache and for longer follow-ups. The RDs for completed suicide were largely the same but of a smaller magnitude and were “relatively less precise,” reflecting the “rarity of this outcome,” said Dr. Elser.
After adjusting for sex, age, education, income, comorbidities, and baseline medical and psychiatric diagnoses, researchers found the strongest association or attempted suicide was among those with TAC (adjusted hazard ratio [aHR], 4.25; 95% CI, 2.85-6.33).
“A hazard ratio of 4 is enormous” for this type of comparison, Dr. Elser noted.
For completed suicide, the strongest association was with posttraumatic headache (aHR, 2.19; 95% CI, 0.78-6.16).
The study revealed a robust association with attempted and completed suicide across all headache types, including TTH, noted Dr. Elser. The link between tension headaches and suicide “was the most striking finding to me because I think of that as sort of a benign and common headache disorder,” she said.
The was an observational study, so “it’s not clear whether headache is playing an etiological role in the relationship with suicide,” she said. “It’s possible there are common shared risk factors or confounders that explain the relationship in full or in part that aren’t accounted for in this study.”
Ask About Mood
The results underscore the need for psychiatric evaluations in patients with a headache disorder. “For me, this is just going to make me that much more likely to ask my patients about their mood when I see them in clinic,” Dr. Elser said.
After asking patients with headache about their mood and stress at home and at work, physicians should have a “low threshold to refer to a behavioral health provider,” she added.
Future research should aim to better understand the link between headache and suicide risk, with a focus on the mechanisms behind low- and high-risk subgroups, said Dr. Elser.
A limitation of the study was that headache diagnoses were based on inpatient, emergency department, and outpatient specialist visits but not on visits to primary care practitioners. The study didn’t include information on headache severity or frequency and included only people who sought treatment for their headaches.
Though it’s unlikely the results “are perfectly generalizable” with respect to other geographical or cultural contexts, “I don’t think this relationship is unique to Denmark based on the literature to date,” Dr. Elser said.
Commenting on the study, session co-chair Todd J. Schwedt, MD, professor of neurology, Mayo Clinic Arizona, Phoenix, and president-elect of the American Headache Society, noted that the study offers important findings “that demonstrate the enormous negative impact that headaches can exert.”
It’s “a strong reminder” that clinicians should assess the mental health of their patients with headaches and offer treatment when appropriate, he said.
The study received support from Aarhus University. No relevant conflicts of interest were reported.
A version of this article appeared on Medscape.com.
DENVER – , results of a large study suggest.
The risk for suicide attempt was four times higher in people with trigeminal and autonomic cephalalgias (TAC), and the risk for completed suicide was double among those with posttraumatic headache compared with individuals with no headache.
The retrospective analysis included data on more than 100,000 headache patients from a Danish registry.
“The results suggest there’s a unique risk among headache patients for attempted and completed suicide,” lead investigator Holly Elser, MD, MPH, PhD, resident, Department of Neurology, University of Pennsylvania, Philadelphia, said at the 2024 annual meeting of the American Academy of Neurology, where the findings were presented. “This really underscores the potential importance of complementary psychiatric evaluation and treatment for individuals diagnosed with headache.”
Underestimated Problem
Headache disorders affect about half of working-age adults and are among the leading causes of productivity loss, absence from work, and disability.
Prior research suggests headache disorders often co-occur with psychiatric illness including depression, anxiety, posttraumatic stress disorder, and even attempted suicide.
However, previous studies that showed an increased risk for attempted suicide in patients with headache relied heavily on survey data and mostly focused on patients with migraine. There was little information on other headache types and on the risk for completed suicide.
Researchers used Danish registries to identify 64,057 patients with migraine, 40,160 with tension-type headache (TTH), 5743 with TAC, and 4253 with posttraumatic headache, all diagnosed from 1995 to 2019.
Some 5.8% of those with migraine, 6.3% with TAC, 7.2% with TTH, and 7.2% with posttraumatic headache, had a mood disorder (depression and anxiety combined) at baseline.
Those without a headache diagnosis were matched 5:1 to those with a headache diagnosis by sex and birth year.
Across all headache disorders, baseline prevalence of mood disorder was higher among those with headache versus population-matched controls. Dr. Elser emphasized that these are people diagnosed with a mood disorder in the inpatient, emergency department, or outpatient specialist clinic setting, “which means we are almost certainly underestimating the true burden of mood symptoms in our cohort,” she said.
Researchers identified attempted suicides using diagnostic codes. For completed suicide, they determined whether those who attempted suicide died within 30 days of the attempt.
For each headache type, investigators examined both the absolute and relative risk for attempted and completed suicides and estimated the risk at intervals of 5, 10, and 20 years after initial headache diagnosis.
Robust Link
The “power of this study is that we asked a simple, but important question, and answered it with simple, but appropriate, methodologic techniques,” Dr. Elser said.
The estimated risk differences (RDs) for attempted suicide were strongest for TAC and posttraumatic headache and for longer follow-ups. The RDs for completed suicide were largely the same but of a smaller magnitude and were “relatively less precise,” reflecting the “rarity of this outcome,” said Dr. Elser.
After adjusting for sex, age, education, income, comorbidities, and baseline medical and psychiatric diagnoses, researchers found the strongest association or attempted suicide was among those with TAC (adjusted hazard ratio [aHR], 4.25; 95% CI, 2.85-6.33).
“A hazard ratio of 4 is enormous” for this type of comparison, Dr. Elser noted.
For completed suicide, the strongest association was with posttraumatic headache (aHR, 2.19; 95% CI, 0.78-6.16).
The study revealed a robust association with attempted and completed suicide across all headache types, including TTH, noted Dr. Elser. The link between tension headaches and suicide “was the most striking finding to me because I think of that as sort of a benign and common headache disorder,” she said.
The was an observational study, so “it’s not clear whether headache is playing an etiological role in the relationship with suicide,” she said. “It’s possible there are common shared risk factors or confounders that explain the relationship in full or in part that aren’t accounted for in this study.”
Ask About Mood
The results underscore the need for psychiatric evaluations in patients with a headache disorder. “For me, this is just going to make me that much more likely to ask my patients about their mood when I see them in clinic,” Dr. Elser said.
After asking patients with headache about their mood and stress at home and at work, physicians should have a “low threshold to refer to a behavioral health provider,” she added.
Future research should aim to better understand the link between headache and suicide risk, with a focus on the mechanisms behind low- and high-risk subgroups, said Dr. Elser.
A limitation of the study was that headache diagnoses were based on inpatient, emergency department, and outpatient specialist visits but not on visits to primary care practitioners. The study didn’t include information on headache severity or frequency and included only people who sought treatment for their headaches.
Though it’s unlikely the results “are perfectly generalizable” with respect to other geographical or cultural contexts, “I don’t think this relationship is unique to Denmark based on the literature to date,” Dr. Elser said.
Commenting on the study, session co-chair Todd J. Schwedt, MD, professor of neurology, Mayo Clinic Arizona, Phoenix, and president-elect of the American Headache Society, noted that the study offers important findings “that demonstrate the enormous negative impact that headaches can exert.”
It’s “a strong reminder” that clinicians should assess the mental health of their patients with headaches and offer treatment when appropriate, he said.
The study received support from Aarhus University. No relevant conflicts of interest were reported.
A version of this article appeared on Medscape.com.
FROM AAN 2024