User login
Neurologists’ pay gets a boost, most happy with career choice
findings from the newly released Medscape Neurologist Compensation Report 2020 show.
Neurologists’ average annual income this year rose to $280,000, up from $267,000 last year. More than half of neurologists (53%) feel fairly compensated, similar to last year’s percentage.
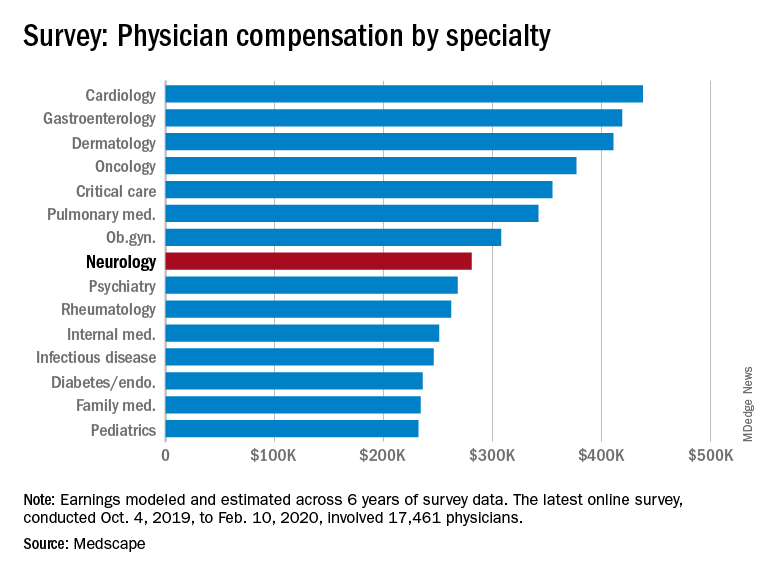
Neurologists are below the middle earners of all physician specialties. At $280,000 in annual compensation for patient care, neurologists rank ninth from the bottom, just below allergists/immunologists ($301,000) but ahead of psychiatrists ($268,000), rheumatologists ($262,000), and internists ($251,000).
Orthopedists are the top earners ($511,000 annual pay), followed by plastic surgeons ($479,000), otolaryngologists ($455,000), and cardiologists ($438,000), according the overall Medscape Physician Compensation Report 2020, which covers U.S. physicians as a whole. The survey included more than 17,000 physicians in over 30 specialties.
COVID-19 impact
An important caveat is that the data for this year’s report were collected prior to Feb. 10, 2020, and therefore reflect physician salary and income prior to the COVID-19 crisis, which has had a huge impact on physicians.
For example, data show that since the start of the crisis, physician practices have seen a 55% dip in revenue and a 60% dip in patient volume on average. Hospitals and physician groups nationwide have implemented layoffs, furloughs, and pay cuts.
In March, 43,000 health care workers were laid off; 9% of independent medical practices reported that they had closed their practices, at least temporarily.
There continues to be a gender pay gap in neurology, with male neurologists earning about 26% more than their female peers ($299,000 vs. $237,000). Among all specialists, men earn 31% more than women, similar to last year’s figure of 33%. There continues to be a 25% gender pay gap among primary care physicians.
More than half of all physicians (56%) say they receive an incentive bonus. Neurologists report that they are eligible for an annual incentive bonus of $35,000. Average annual incentive bonuses are highest among orthopedists ($96,000) and lowest among family medicine physicians ($24,000).
Close to one third of physicians overall who receive incentive bonuses say the prospect of receiving the bonus has encouraged them to work longer hours. A higher percentage of neurologists (41%) say their potential bonus influenced them to increase their work hours.
Fifty-eight percent of neurologists achieve more than three quarters of their potential annual incentive bonus. On average, neurologists achieve about two thirds of their potential bonus, the same proportion as for physicians overall.
However, COVID-19 may change that. Experts who were interviewed recently by Medscape noted that productivity benchmarks for physicians are likely to be lowered in light of plunging patient numbers from COVID-19, and bonuses are expected to take a hit.
Happy at work
On average, male neurologists spend 37.7 hours per week seeing patients, somewhat more hours per week than female neurologists (36.1 hours); the average for all physicians is 37.9 hours per week.
Bureaucratic tasks continue to be a burden for physicians in all specialties. On average, neurologists spend 16.9 hours per week on paperwork and administration, about the same as physicians overall (15.6 hours).
Intensivists top the list regarding such tasks (19.1 hours), followed by internists (18.5), infectious disease physicians (18.5), and psychiatrists (18.3). Anesthesiologists and ophthalmologists spend the least amount of time on paperwork/administration (10.0 and 9.8 hours per week, respectively).
What is most rewarding about being a neurologist? Being good at what they do/finding answers, diagnoses tops the list (33%), followed by making the world a better place/helping others (26%), relationships with and gratitude from patients (18%), and making good money at a job they like (11%). A few cited teaching (5%) and pride in their profession (4%).
The most challenging part of practicing neurology is having to follow so many rules and regulations (26%). Other challenges include having to work long hours (18%), dealing with difficult patients (17%), trouble getting fair reimbursement (13%), and working with electronic health records (10%).
Despite the challenges, if they had to do it all over again, 73% of neurologists would still choose medicine as a career, and 86% would again choose neurology.
Other key findings in the latest report regarding neurologists include the following:
- At 18%, neurologists rank near the middle among physicians with regard to losing money on denied or resubmitted claims. Plastic surgery and emergency medicine have the highest percentage of claims denied or resubmitted (28% and 22%, respectively). One study found that, on average, 63% of denied claims are recoverable, but healthcare professionals spend about $118 per claim on appeals.
- 29% of neurologists say they use physician assistants (PAs) to treat patients in their practices, and 53% use nurse practitioners (NPs); 38% use neither for patient care. Of neurologists who work with PAs and NPs in their offices, 49% say these employees have helped boost profitability.
- Two-thirds of neurologists say they will continue taking new and current Medicare/Medicaid patients; none say they will not take new Medicare patients; and 26% are undecided.
- Neurologists participate in various payment methods; 78% are reimbursed via insurance, 35% have fee-for-service arrangements, and 28% are in accountable care organizations.
- Nearly 40% of neurologists expect to participate in the merit-based incentive payment system option, and 10% expect to participate in alternative payment models.
This article first appeared on Medscape.com.
findings from the newly released Medscape Neurologist Compensation Report 2020 show.
Neurologists’ average annual income this year rose to $280,000, up from $267,000 last year. More than half of neurologists (53%) feel fairly compensated, similar to last year’s percentage.

Neurologists are below the middle earners of all physician specialties. At $280,000 in annual compensation for patient care, neurologists rank ninth from the bottom, just below allergists/immunologists ($301,000) but ahead of psychiatrists ($268,000), rheumatologists ($262,000), and internists ($251,000).
Orthopedists are the top earners ($511,000 annual pay), followed by plastic surgeons ($479,000), otolaryngologists ($455,000), and cardiologists ($438,000), according the overall Medscape Physician Compensation Report 2020, which covers U.S. physicians as a whole. The survey included more than 17,000 physicians in over 30 specialties.
COVID-19 impact
An important caveat is that the data for this year’s report were collected prior to Feb. 10, 2020, and therefore reflect physician salary and income prior to the COVID-19 crisis, which has had a huge impact on physicians.
For example, data show that since the start of the crisis, physician practices have seen a 55% dip in revenue and a 60% dip in patient volume on average. Hospitals and physician groups nationwide have implemented layoffs, furloughs, and pay cuts.
In March, 43,000 health care workers were laid off; 9% of independent medical practices reported that they had closed their practices, at least temporarily.
There continues to be a gender pay gap in neurology, with male neurologists earning about 26% more than their female peers ($299,000 vs. $237,000). Among all specialists, men earn 31% more than women, similar to last year’s figure of 33%. There continues to be a 25% gender pay gap among primary care physicians.
More than half of all physicians (56%) say they receive an incentive bonus. Neurologists report that they are eligible for an annual incentive bonus of $35,000. Average annual incentive bonuses are highest among orthopedists ($96,000) and lowest among family medicine physicians ($24,000).
Close to one third of physicians overall who receive incentive bonuses say the prospect of receiving the bonus has encouraged them to work longer hours. A higher percentage of neurologists (41%) say their potential bonus influenced them to increase their work hours.
Fifty-eight percent of neurologists achieve more than three quarters of their potential annual incentive bonus. On average, neurologists achieve about two thirds of their potential bonus, the same proportion as for physicians overall.
However, COVID-19 may change that. Experts who were interviewed recently by Medscape noted that productivity benchmarks for physicians are likely to be lowered in light of plunging patient numbers from COVID-19, and bonuses are expected to take a hit.
Happy at work
On average, male neurologists spend 37.7 hours per week seeing patients, somewhat more hours per week than female neurologists (36.1 hours); the average for all physicians is 37.9 hours per week.
Bureaucratic tasks continue to be a burden for physicians in all specialties. On average, neurologists spend 16.9 hours per week on paperwork and administration, about the same as physicians overall (15.6 hours).
Intensivists top the list regarding such tasks (19.1 hours), followed by internists (18.5), infectious disease physicians (18.5), and psychiatrists (18.3). Anesthesiologists and ophthalmologists spend the least amount of time on paperwork/administration (10.0 and 9.8 hours per week, respectively).
What is most rewarding about being a neurologist? Being good at what they do/finding answers, diagnoses tops the list (33%), followed by making the world a better place/helping others (26%), relationships with and gratitude from patients (18%), and making good money at a job they like (11%). A few cited teaching (5%) and pride in their profession (4%).
The most challenging part of practicing neurology is having to follow so many rules and regulations (26%). Other challenges include having to work long hours (18%), dealing with difficult patients (17%), trouble getting fair reimbursement (13%), and working with electronic health records (10%).
Despite the challenges, if they had to do it all over again, 73% of neurologists would still choose medicine as a career, and 86% would again choose neurology.
Other key findings in the latest report regarding neurologists include the following:
- At 18%, neurologists rank near the middle among physicians with regard to losing money on denied or resubmitted claims. Plastic surgery and emergency medicine have the highest percentage of claims denied or resubmitted (28% and 22%, respectively). One study found that, on average, 63% of denied claims are recoverable, but healthcare professionals spend about $118 per claim on appeals.
- 29% of neurologists say they use physician assistants (PAs) to treat patients in their practices, and 53% use nurse practitioners (NPs); 38% use neither for patient care. Of neurologists who work with PAs and NPs in their offices, 49% say these employees have helped boost profitability.
- Two-thirds of neurologists say they will continue taking new and current Medicare/Medicaid patients; none say they will not take new Medicare patients; and 26% are undecided.
- Neurologists participate in various payment methods; 78% are reimbursed via insurance, 35% have fee-for-service arrangements, and 28% are in accountable care organizations.
- Nearly 40% of neurologists expect to participate in the merit-based incentive payment system option, and 10% expect to participate in alternative payment models.
This article first appeared on Medscape.com.
findings from the newly released Medscape Neurologist Compensation Report 2020 show.
Neurologists’ average annual income this year rose to $280,000, up from $267,000 last year. More than half of neurologists (53%) feel fairly compensated, similar to last year’s percentage.

Neurologists are below the middle earners of all physician specialties. At $280,000 in annual compensation for patient care, neurologists rank ninth from the bottom, just below allergists/immunologists ($301,000) but ahead of psychiatrists ($268,000), rheumatologists ($262,000), and internists ($251,000).
Orthopedists are the top earners ($511,000 annual pay), followed by plastic surgeons ($479,000), otolaryngologists ($455,000), and cardiologists ($438,000), according the overall Medscape Physician Compensation Report 2020, which covers U.S. physicians as a whole. The survey included more than 17,000 physicians in over 30 specialties.
COVID-19 impact
An important caveat is that the data for this year’s report were collected prior to Feb. 10, 2020, and therefore reflect physician salary and income prior to the COVID-19 crisis, which has had a huge impact on physicians.
For example, data show that since the start of the crisis, physician practices have seen a 55% dip in revenue and a 60% dip in patient volume on average. Hospitals and physician groups nationwide have implemented layoffs, furloughs, and pay cuts.
In March, 43,000 health care workers were laid off; 9% of independent medical practices reported that they had closed their practices, at least temporarily.
There continues to be a gender pay gap in neurology, with male neurologists earning about 26% more than their female peers ($299,000 vs. $237,000). Among all specialists, men earn 31% more than women, similar to last year’s figure of 33%. There continues to be a 25% gender pay gap among primary care physicians.
More than half of all physicians (56%) say they receive an incentive bonus. Neurologists report that they are eligible for an annual incentive bonus of $35,000. Average annual incentive bonuses are highest among orthopedists ($96,000) and lowest among family medicine physicians ($24,000).
Close to one third of physicians overall who receive incentive bonuses say the prospect of receiving the bonus has encouraged them to work longer hours. A higher percentage of neurologists (41%) say their potential bonus influenced them to increase their work hours.
Fifty-eight percent of neurologists achieve more than three quarters of their potential annual incentive bonus. On average, neurologists achieve about two thirds of their potential bonus, the same proportion as for physicians overall.
However, COVID-19 may change that. Experts who were interviewed recently by Medscape noted that productivity benchmarks for physicians are likely to be lowered in light of plunging patient numbers from COVID-19, and bonuses are expected to take a hit.
Happy at work
On average, male neurologists spend 37.7 hours per week seeing patients, somewhat more hours per week than female neurologists (36.1 hours); the average for all physicians is 37.9 hours per week.
Bureaucratic tasks continue to be a burden for physicians in all specialties. On average, neurologists spend 16.9 hours per week on paperwork and administration, about the same as physicians overall (15.6 hours).
Intensivists top the list regarding such tasks (19.1 hours), followed by internists (18.5), infectious disease physicians (18.5), and psychiatrists (18.3). Anesthesiologists and ophthalmologists spend the least amount of time on paperwork/administration (10.0 and 9.8 hours per week, respectively).
What is most rewarding about being a neurologist? Being good at what they do/finding answers, diagnoses tops the list (33%), followed by making the world a better place/helping others (26%), relationships with and gratitude from patients (18%), and making good money at a job they like (11%). A few cited teaching (5%) and pride in their profession (4%).
The most challenging part of practicing neurology is having to follow so many rules and regulations (26%). Other challenges include having to work long hours (18%), dealing with difficult patients (17%), trouble getting fair reimbursement (13%), and working with electronic health records (10%).
Despite the challenges, if they had to do it all over again, 73% of neurologists would still choose medicine as a career, and 86% would again choose neurology.
Other key findings in the latest report regarding neurologists include the following:
- At 18%, neurologists rank near the middle among physicians with regard to losing money on denied or resubmitted claims. Plastic surgery and emergency medicine have the highest percentage of claims denied or resubmitted (28% and 22%, respectively). One study found that, on average, 63% of denied claims are recoverable, but healthcare professionals spend about $118 per claim on appeals.
- 29% of neurologists say they use physician assistants (PAs) to treat patients in their practices, and 53% use nurse practitioners (NPs); 38% use neither for patient care. Of neurologists who work with PAs and NPs in their offices, 49% say these employees have helped boost profitability.
- Two-thirds of neurologists say they will continue taking new and current Medicare/Medicaid patients; none say they will not take new Medicare patients; and 26% are undecided.
- Neurologists participate in various payment methods; 78% are reimbursed via insurance, 35% have fee-for-service arrangements, and 28% are in accountable care organizations.
- Nearly 40% of neurologists expect to participate in the merit-based incentive payment system option, and 10% expect to participate in alternative payment models.
This article first appeared on Medscape.com.
Natalizumab bests fingolimod for relapsing-remitting MS
(RRMS). Use of natalizumab was associated with fewer new T2 lesions (0.7 vs 1.4 with fingolimod) and gadolinium-enhancing lesions (0.03 vs. 0.5, respectively) at 12 months, for example.
“The take-home message is that natalizumab showed significant superiority compared to fingolimod on the primary outcome, which was the proportion of patients reaching NEDA [no evidence of disease activity] at 12 months,” lead author Mikael Cohen, MD, said.
“The difference between both drugs was prominent on MRI parameters, especially regarding the number of gadolinium-enhancing lesions,” added Dr. Cohen, of the Department of Neurology at University Hospital Center in Nice, France.
This research was presented online as part of the 2020 American Academy of Neurology Science Highlights.
Twelve-month results
The design of the Best Escalation Strategy in MS (BEST MS) study makes it unique, Dr. Cohen said. “It was a prospective and standardized study, unlike most other publications comparing efficacy of those two drugs that were based on retrospective analysis of data registries,” he said. Although BEST MS was an open-label, real-life analysis, the neuroradiologist who analyzed MRI images was blinded to treatment arms, he added.
The multicenter study began in France in 2013, when natalizumab and fingolimod were the two most commonly used agents for active RRMS.
Dr. Cohen and colleagues assessed 230 patients with the condition. The mean age was 38 years, and 75% were women. At the discretion of the treating physician, 113 participants received natalizumab, and 117 were treated with fingolimod.
A multivariate analysis confirmed that fingolimod was associated with a lower likelihood of achieving NEDA at 12 months.
Most relapses occurred early, and the annual relapse rate favored natalizumab, the researchers noted. In addition, the number of discontinuations due to adverse events was higher in the fingolimod group.
“We are working to submit the paper for publication,” Dr. Cohen said. It has also been submitted to the ECTRIMS/ACTRIMS Joint Congress in Washington, DC, for presentation in September 2020.
More tesearch warranted
Commenting on the study, Michelle H. Cameron, MD, said the findings are difficult to interpret because “this was not a randomized controlled trial. Treatment choice was at the discretion of the providers.
“It is hard to know what biases this approach introduced – although it is reassuring that the baseline clinical and radiographic characteristics are described as similar,” said Cameron, codirector of the MS Center of Excellence West at the VA Portland Health Care System, Oregon.
In addition, the superior MRI outcomes at 12 months with natalizumab need to be backed up by clinical outcomes, she said, preferably spanning at least 2 years.
“Overall, these results seem to be consistent with the randomized controlled trials of these individual agents,” Dr. Cameron concluded.
BEST MS was an institutional study and was not funded by any pharmaceutical firm. Dr. Cohen has disclosed no relevant financial relationships. Dr. Cameron is a consultant for Greenwich Biosciences and Adamas Pharmaceuticals.
This article first appeared on Medscape.com.
(RRMS). Use of natalizumab was associated with fewer new T2 lesions (0.7 vs 1.4 with fingolimod) and gadolinium-enhancing lesions (0.03 vs. 0.5, respectively) at 12 months, for example.
“The take-home message is that natalizumab showed significant superiority compared to fingolimod on the primary outcome, which was the proportion of patients reaching NEDA [no evidence of disease activity] at 12 months,” lead author Mikael Cohen, MD, said.
“The difference between both drugs was prominent on MRI parameters, especially regarding the number of gadolinium-enhancing lesions,” added Dr. Cohen, of the Department of Neurology at University Hospital Center in Nice, France.
This research was presented online as part of the 2020 American Academy of Neurology Science Highlights.
Twelve-month results
The design of the Best Escalation Strategy in MS (BEST MS) study makes it unique, Dr. Cohen said. “It was a prospective and standardized study, unlike most other publications comparing efficacy of those two drugs that were based on retrospective analysis of data registries,” he said. Although BEST MS was an open-label, real-life analysis, the neuroradiologist who analyzed MRI images was blinded to treatment arms, he added.
The multicenter study began in France in 2013, when natalizumab and fingolimod were the two most commonly used agents for active RRMS.
Dr. Cohen and colleagues assessed 230 patients with the condition. The mean age was 38 years, and 75% were women. At the discretion of the treating physician, 113 participants received natalizumab, and 117 were treated with fingolimod.
A multivariate analysis confirmed that fingolimod was associated with a lower likelihood of achieving NEDA at 12 months.
Most relapses occurred early, and the annual relapse rate favored natalizumab, the researchers noted. In addition, the number of discontinuations due to adverse events was higher in the fingolimod group.
“We are working to submit the paper for publication,” Dr. Cohen said. It has also been submitted to the ECTRIMS/ACTRIMS Joint Congress in Washington, DC, for presentation in September 2020.
More tesearch warranted
Commenting on the study, Michelle H. Cameron, MD, said the findings are difficult to interpret because “this was not a randomized controlled trial. Treatment choice was at the discretion of the providers.
“It is hard to know what biases this approach introduced – although it is reassuring that the baseline clinical and radiographic characteristics are described as similar,” said Cameron, codirector of the MS Center of Excellence West at the VA Portland Health Care System, Oregon.
In addition, the superior MRI outcomes at 12 months with natalizumab need to be backed up by clinical outcomes, she said, preferably spanning at least 2 years.
“Overall, these results seem to be consistent with the randomized controlled trials of these individual agents,” Dr. Cameron concluded.
BEST MS was an institutional study and was not funded by any pharmaceutical firm. Dr. Cohen has disclosed no relevant financial relationships. Dr. Cameron is a consultant for Greenwich Biosciences and Adamas Pharmaceuticals.
This article first appeared on Medscape.com.
(RRMS). Use of natalizumab was associated with fewer new T2 lesions (0.7 vs 1.4 with fingolimod) and gadolinium-enhancing lesions (0.03 vs. 0.5, respectively) at 12 months, for example.
“The take-home message is that natalizumab showed significant superiority compared to fingolimod on the primary outcome, which was the proportion of patients reaching NEDA [no evidence of disease activity] at 12 months,” lead author Mikael Cohen, MD, said.
“The difference between both drugs was prominent on MRI parameters, especially regarding the number of gadolinium-enhancing lesions,” added Dr. Cohen, of the Department of Neurology at University Hospital Center in Nice, France.
This research was presented online as part of the 2020 American Academy of Neurology Science Highlights.
Twelve-month results
The design of the Best Escalation Strategy in MS (BEST MS) study makes it unique, Dr. Cohen said. “It was a prospective and standardized study, unlike most other publications comparing efficacy of those two drugs that were based on retrospective analysis of data registries,” he said. Although BEST MS was an open-label, real-life analysis, the neuroradiologist who analyzed MRI images was blinded to treatment arms, he added.
The multicenter study began in France in 2013, when natalizumab and fingolimod were the two most commonly used agents for active RRMS.
Dr. Cohen and colleagues assessed 230 patients with the condition. The mean age was 38 years, and 75% were women. At the discretion of the treating physician, 113 participants received natalizumab, and 117 were treated with fingolimod.
A multivariate analysis confirmed that fingolimod was associated with a lower likelihood of achieving NEDA at 12 months.
Most relapses occurred early, and the annual relapse rate favored natalizumab, the researchers noted. In addition, the number of discontinuations due to adverse events was higher in the fingolimod group.
“We are working to submit the paper for publication,” Dr. Cohen said. It has also been submitted to the ECTRIMS/ACTRIMS Joint Congress in Washington, DC, for presentation in September 2020.
More tesearch warranted
Commenting on the study, Michelle H. Cameron, MD, said the findings are difficult to interpret because “this was not a randomized controlled trial. Treatment choice was at the discretion of the providers.
“It is hard to know what biases this approach introduced – although it is reassuring that the baseline clinical and radiographic characteristics are described as similar,” said Cameron, codirector of the MS Center of Excellence West at the VA Portland Health Care System, Oregon.
In addition, the superior MRI outcomes at 12 months with natalizumab need to be backed up by clinical outcomes, she said, preferably spanning at least 2 years.
“Overall, these results seem to be consistent with the randomized controlled trials of these individual agents,” Dr. Cameron concluded.
BEST MS was an institutional study and was not funded by any pharmaceutical firm. Dr. Cohen has disclosed no relevant financial relationships. Dr. Cameron is a consultant for Greenwich Biosciences and Adamas Pharmaceuticals.
This article first appeared on Medscape.com.
Satralizumab monotherapy reduces NMOSD relapse rate
according to trial results published in the Lancet Neurology.
The findings help confirm the role of interleukin-6 in the pathobiology of aquaporin-4 autoantibody (AQP4-IgG)–seropositive disease. For patients who are AQP4-IgG seronegative, however, “there is insufficient evidence to indicate a risk reduction” with this drug, the investigators wrote. In addition, satralizumab did not significantly affect pain or fatigue.
“The limitations of the study include the relatively small group sizes and low number of relapses. Despite these limitations, a significant treatment benefit was observed with satralizumab, compared with placebo in the study population, with efficacy and safety comparable to satralizumab used in combination with immunosuppressants,” reported lead author Anthony Traboulsee, MD, professor of neurology at the University of British Columbia, Vancouver, and colleagues.
Satralizumab is a humanized monoclonal antibody targeting the IL-6 receptor. A prior phase 3 study, SakuraSky, found that the drug reduces the risk of NMOSD relapse when added to immunosuppressant therapy. To assess the safety and efficacy of satralizumab monotherapy, Dr. Traboulsee and colleagues conducted SakuraStar, a randomized, double-blind, placebo-controlled trial.
Evaluating drug as monotherapy
The phase 3 trial enrolled 95 patients aged 18-74 years at 44 sites in 13 countries. The investigators included patients with AQP4-IgG–seropositive or –seronegative neuromyelitis optica using the 2006 Wingerchuk criteria or with AQP4-IgG–seropositive NMOSD with at least one event of longitudinally extensive myelitis or optic neuritis. The researchers limited the number of AQP4-IgG–seronegative patients to about 30% of the study population. Eligible participants had at least one NMOSD attack or relapse in the past 12 months and a score of 6.5 or less on the Expanded Disability Status Scale (EDSS). The investigators excluded patients with a clinical relapse in the 30 days before study baseline. Participants were randomly assigned 2:1 to receive satralizumab 120 mg or placebo subcutaneously at weeks 0, 2, 4, and every 4 weeks thereafter. Concomitant immunosuppressant use was prohibited, although corticosteroids and intravenous immunoglobulin were permitted as rescue therapy for the treatment of relapse.
The primary endpoint was time to first relapse, and the trial was designed to continue until 44 relapses occurred or for 1.5 years after the last patient entered the trial, whichever occurred first.
“Because even one NMOSD attack can have serious neurological consequences, this design allowed patients who had an attack on placebo to enter the open-label phase and receive the active drug,” the researchers wrote. “The trial design used unequal randomization to minimize exposure to placebo; because patients were not permitted to receive concomitant immunosuppressant treatment in this trial, the design limited the number of patients not receiving any treatment for the disorder. Placebo was selected with the consideration that no drugs were approved for the treatment of NMOSD when the trial was designed.” Recent trials of eculizumab, inebilizumab, and satralizumab have found that the agents are effective treatments for NMOSD. In 2019, the Food and Drug Administration approved eculizumab, a complement inhibitor, for the treatment of AQP4-IgG–seropositive NMOSD.
For the primary endpoint of SakuraStar, the researchers defined relapses as new or worsening objective neurologic symptoms with at least one of the following:
- Increase of 1 or more EDSS points from a baseline EDSS score of more than 0, or increase of 2 or more EDSS points from a baseline EDSS score of 0
- Increase of 2 or more points on at least one appropriate symptom-specific functional system score for pyramidal, cerebellar, brain stem, sensory, bowel or bladder, or a single eye
- Increase of 1 or more points on more than one symptom-specific functional system score with a baseline of at least 1
- Increase of 1 or more points on a single-eye symptom-specific functional system score with a baseline score of at least 1
In addition, symptoms had to be attributable to NMOSD; persist for more than 24 hours; and not be attributable to confounding clinical factors such as fever, infection, injury, change in mood, or adverse reactions to medications. Researchers assessed EDSS and functional system scores within 7 days of a patient reporting symptoms.
The double-blind treatment period ended 1.5 years after the last enrolled patient was assigned to satralizumab or placebo. More than 80% of the participants were women, including 73% of the satralizumab group and 97% of the placebo group. In all, 95 participants were assigned to a treatment between 2014 and 2017 – 63 to satralizumab and 32 to placebo. Relapses occurred in 19 patients receiving satralizumab (30%) and 16 receiving placebo (50%). The hazard ratio was 0.45.
“Patients treated with placebo showed a shorter time to relapse and a higher withdrawal rate than did patients treated with satralizumab,” wrote Dr. Traboulsee and colleagues. The Kaplan-Meier method suggested that 76% of patients on satralizumab had not relapsed at 48 weeks, compared with 62% of patients on placebo. And at 96 weeks, 72% of patients on satralizumab had not relapsed, compared with 51% of patients on placebo.
Among patients who were AQP4-IgG seropositive, the proportion with protocol-defined relapse was 22% in the satralizumab group versus 57% in the placebo group. Among patients who were AQP4-IgG seronegative, the proportion with a protocol-defined relapse was 46% in the satralizumab group versus 33% in the placebo group.
The most common adverse events were urinary tract infection and upper respiratory tract infection, and most adverse events were mild to moderate. A higher rate of severe adverse events was reported in the satralizumab group than in the placebo group (32.1 vs. 9.9 events per 100 patient-years). The investigators considered most of the severe adverse events unrelated to the study treatment. “None of the severe adverse events led to discontinuation of the study drug except one severe event of pneumonia in the satralizumab group,” the researchers wrote.
Data confirm efficacy of IL-6 blockade
“Satralizumab was well tolerated and no meaningful adverse effects from the drug were reported and no deaths occurred,” said Michael Levy, MD, PhD, director of the NMO clinic and research laboratory at Massachusetts General Hospital, Boston, in an accompanying editorial. “This trial of satralizumab was done shortly after the completion of a parallel trial of satralizumab in patients with NMOSD in which the same dose of satralizumab reduced the risk of relapse by 62%, compared with placebo. The main difference between these two trials is that, in the first published study, participants were permitted to use background immunotherapy; otherwise, the trial designs were nearly identical, and the enrolled participants are comparable. Together, the findings from these studies suggest that background therapy seems to provide only a small additional benefit to satralizumab alone.”
Dr. Levy also discussed findings from a phase 2 study of tocilizumab for the prevention of relapse in patients with NMOSD published in the same issue of the Lancet Neurology. The satralizumab and tocilizumab data have “confirmed that IL-6 blockade reduces the risk of relapse in patients with NMOSD,” Dr. Levy said. “IL-6 is a crucial component of the immune system, but when IL-6 production is altered during autoimmune attacks and sepsis, there can be severe consequences.”
The phase 2 trial of tocilizumab, which was described at the 2019 annual congress of the European Committee for Treatment and Research in Multiple Sclerosis, included 118 patients, 87% of whom were AQP4-IgG seropositive. Patients received intravenous tocilizumab or oral azathioprine for up to 60 weeks. Overall, 14% of patients in the tocilizumab group relapsed, compared with 47% of patients in the azathioprine group.
“The main differences between this trial of tocilizumab and the two satralizumab trials are that the tocilizumab was administered intravenously, rather than subcutaneously, the study duration was approximately 1 year, and the investigators were not masked to the treatment allocation,” Dr. Levy said. “Similar to satralizumab, adverse effects with tocilizumab were mild, including asymptomatic elevations in liver enzymes and an increased incidence of respiratory and urinary infections, with no significant differences identified between the tocilizumab and azathioprine groups.”
Various immunopathologic mechanisms may influence outcomes in NMOSD. While satralizumab and tocilizumab target IL-6, eculizumab is a C5 complement inhibitor and inebilizumab is a CD19 B-cell depleting monoclonal antibody, Dr. Levy said. “The safety concerns regarding these approaches are all substantially outweighed by the benefit of preventing NMOSD relapses.”
A need to understand AQP4-IgG–seronegative disease
SakuraStar “provides convincing data for the efficacy of satralizumab monotherapy in NMOSD with subgroup analysis showing that the benefit was seen in AQP4-IgG seropositive patients,” commented Dean M. Wingerchuk, MD, director of the division of multiple sclerosis and autoimmune neurology at the Mayo Clinic in Phoenix. “The results help confirm the key role of IL-6 in the pathobiology of AQP4-IgG–seropositive disease.”
Questions about AQP4-IgG seronegative disease remain. “The results are quite similar to the SakuraSky trial, in which satralizumab was used in conjunction with other background immunosuppressive therapies, suggesting that the primary benefit may be derived primarily from satralizumab. Both trials also showed that satralizumab did not benefit the NMOSD without AQP4-IgG subgroup though the subject numbers are rather small. We need to know more about the clinical and laboratory characteristics of the seronegative patients as they likely comprise a heterogeneous group. For example, did any of them have other autoantibodies such as MOG-IgG? Depending on the results, those details may help us understand the relative role of IL-6 in AQP4-IgG–seronegative subgroups, an important area of further study.”
SakuraStar was funded by Chugai Pharmaceutical, a member of the Roche group. Dr. Traboulsee reported grants, personal fees, and nonfinancial support from Chugai Pharmaceutical during the study, and several coauthors were employees of Chugai Pharmaceutical. Additional coauthors reported personal fees from Chugai, Roche, and other companies. Dr. Levy has received consulting fees from Alexion, Viela Bio, Chugai Pharmaceutical, Quest Diagnostics, and UCB Pharmaceuticals.
SOURCE: Traboulsee A et al. Lancet Neurol. 2020;19(5):402-12.
according to trial results published in the Lancet Neurology.
The findings help confirm the role of interleukin-6 in the pathobiology of aquaporin-4 autoantibody (AQP4-IgG)–seropositive disease. For patients who are AQP4-IgG seronegative, however, “there is insufficient evidence to indicate a risk reduction” with this drug, the investigators wrote. In addition, satralizumab did not significantly affect pain or fatigue.
“The limitations of the study include the relatively small group sizes and low number of relapses. Despite these limitations, a significant treatment benefit was observed with satralizumab, compared with placebo in the study population, with efficacy and safety comparable to satralizumab used in combination with immunosuppressants,” reported lead author Anthony Traboulsee, MD, professor of neurology at the University of British Columbia, Vancouver, and colleagues.
Satralizumab is a humanized monoclonal antibody targeting the IL-6 receptor. A prior phase 3 study, SakuraSky, found that the drug reduces the risk of NMOSD relapse when added to immunosuppressant therapy. To assess the safety and efficacy of satralizumab monotherapy, Dr. Traboulsee and colleagues conducted SakuraStar, a randomized, double-blind, placebo-controlled trial.
Evaluating drug as monotherapy
The phase 3 trial enrolled 95 patients aged 18-74 years at 44 sites in 13 countries. The investigators included patients with AQP4-IgG–seropositive or –seronegative neuromyelitis optica using the 2006 Wingerchuk criteria or with AQP4-IgG–seropositive NMOSD with at least one event of longitudinally extensive myelitis or optic neuritis. The researchers limited the number of AQP4-IgG–seronegative patients to about 30% of the study population. Eligible participants had at least one NMOSD attack or relapse in the past 12 months and a score of 6.5 or less on the Expanded Disability Status Scale (EDSS). The investigators excluded patients with a clinical relapse in the 30 days before study baseline. Participants were randomly assigned 2:1 to receive satralizumab 120 mg or placebo subcutaneously at weeks 0, 2, 4, and every 4 weeks thereafter. Concomitant immunosuppressant use was prohibited, although corticosteroids and intravenous immunoglobulin were permitted as rescue therapy for the treatment of relapse.
The primary endpoint was time to first relapse, and the trial was designed to continue until 44 relapses occurred or for 1.5 years after the last patient entered the trial, whichever occurred first.
“Because even one NMOSD attack can have serious neurological consequences, this design allowed patients who had an attack on placebo to enter the open-label phase and receive the active drug,” the researchers wrote. “The trial design used unequal randomization to minimize exposure to placebo; because patients were not permitted to receive concomitant immunosuppressant treatment in this trial, the design limited the number of patients not receiving any treatment for the disorder. Placebo was selected with the consideration that no drugs were approved for the treatment of NMOSD when the trial was designed.” Recent trials of eculizumab, inebilizumab, and satralizumab have found that the agents are effective treatments for NMOSD. In 2019, the Food and Drug Administration approved eculizumab, a complement inhibitor, for the treatment of AQP4-IgG–seropositive NMOSD.
For the primary endpoint of SakuraStar, the researchers defined relapses as new or worsening objective neurologic symptoms with at least one of the following:
- Increase of 1 or more EDSS points from a baseline EDSS score of more than 0, or increase of 2 or more EDSS points from a baseline EDSS score of 0
- Increase of 2 or more points on at least one appropriate symptom-specific functional system score for pyramidal, cerebellar, brain stem, sensory, bowel or bladder, or a single eye
- Increase of 1 or more points on more than one symptom-specific functional system score with a baseline of at least 1
- Increase of 1 or more points on a single-eye symptom-specific functional system score with a baseline score of at least 1
In addition, symptoms had to be attributable to NMOSD; persist for more than 24 hours; and not be attributable to confounding clinical factors such as fever, infection, injury, change in mood, or adverse reactions to medications. Researchers assessed EDSS and functional system scores within 7 days of a patient reporting symptoms.
The double-blind treatment period ended 1.5 years after the last enrolled patient was assigned to satralizumab or placebo. More than 80% of the participants were women, including 73% of the satralizumab group and 97% of the placebo group. In all, 95 participants were assigned to a treatment between 2014 and 2017 – 63 to satralizumab and 32 to placebo. Relapses occurred in 19 patients receiving satralizumab (30%) and 16 receiving placebo (50%). The hazard ratio was 0.45.
“Patients treated with placebo showed a shorter time to relapse and a higher withdrawal rate than did patients treated with satralizumab,” wrote Dr. Traboulsee and colleagues. The Kaplan-Meier method suggested that 76% of patients on satralizumab had not relapsed at 48 weeks, compared with 62% of patients on placebo. And at 96 weeks, 72% of patients on satralizumab had not relapsed, compared with 51% of patients on placebo.
Among patients who were AQP4-IgG seropositive, the proportion with protocol-defined relapse was 22% in the satralizumab group versus 57% in the placebo group. Among patients who were AQP4-IgG seronegative, the proportion with a protocol-defined relapse was 46% in the satralizumab group versus 33% in the placebo group.
The most common adverse events were urinary tract infection and upper respiratory tract infection, and most adverse events were mild to moderate. A higher rate of severe adverse events was reported in the satralizumab group than in the placebo group (32.1 vs. 9.9 events per 100 patient-years). The investigators considered most of the severe adverse events unrelated to the study treatment. “None of the severe adverse events led to discontinuation of the study drug except one severe event of pneumonia in the satralizumab group,” the researchers wrote.
Data confirm efficacy of IL-6 blockade
“Satralizumab was well tolerated and no meaningful adverse effects from the drug were reported and no deaths occurred,” said Michael Levy, MD, PhD, director of the NMO clinic and research laboratory at Massachusetts General Hospital, Boston, in an accompanying editorial. “This trial of satralizumab was done shortly after the completion of a parallel trial of satralizumab in patients with NMOSD in which the same dose of satralizumab reduced the risk of relapse by 62%, compared with placebo. The main difference between these two trials is that, in the first published study, participants were permitted to use background immunotherapy; otherwise, the trial designs were nearly identical, and the enrolled participants are comparable. Together, the findings from these studies suggest that background therapy seems to provide only a small additional benefit to satralizumab alone.”
Dr. Levy also discussed findings from a phase 2 study of tocilizumab for the prevention of relapse in patients with NMOSD published in the same issue of the Lancet Neurology. The satralizumab and tocilizumab data have “confirmed that IL-6 blockade reduces the risk of relapse in patients with NMOSD,” Dr. Levy said. “IL-6 is a crucial component of the immune system, but when IL-6 production is altered during autoimmune attacks and sepsis, there can be severe consequences.”
The phase 2 trial of tocilizumab, which was described at the 2019 annual congress of the European Committee for Treatment and Research in Multiple Sclerosis, included 118 patients, 87% of whom were AQP4-IgG seropositive. Patients received intravenous tocilizumab or oral azathioprine for up to 60 weeks. Overall, 14% of patients in the tocilizumab group relapsed, compared with 47% of patients in the azathioprine group.
“The main differences between this trial of tocilizumab and the two satralizumab trials are that the tocilizumab was administered intravenously, rather than subcutaneously, the study duration was approximately 1 year, and the investigators were not masked to the treatment allocation,” Dr. Levy said. “Similar to satralizumab, adverse effects with tocilizumab were mild, including asymptomatic elevations in liver enzymes and an increased incidence of respiratory and urinary infections, with no significant differences identified between the tocilizumab and azathioprine groups.”
Various immunopathologic mechanisms may influence outcomes in NMOSD. While satralizumab and tocilizumab target IL-6, eculizumab is a C5 complement inhibitor and inebilizumab is a CD19 B-cell depleting monoclonal antibody, Dr. Levy said. “The safety concerns regarding these approaches are all substantially outweighed by the benefit of preventing NMOSD relapses.”
A need to understand AQP4-IgG–seronegative disease
SakuraStar “provides convincing data for the efficacy of satralizumab monotherapy in NMOSD with subgroup analysis showing that the benefit was seen in AQP4-IgG seropositive patients,” commented Dean M. Wingerchuk, MD, director of the division of multiple sclerosis and autoimmune neurology at the Mayo Clinic in Phoenix. “The results help confirm the key role of IL-6 in the pathobiology of AQP4-IgG–seropositive disease.”
Questions about AQP4-IgG seronegative disease remain. “The results are quite similar to the SakuraSky trial, in which satralizumab was used in conjunction with other background immunosuppressive therapies, suggesting that the primary benefit may be derived primarily from satralizumab. Both trials also showed that satralizumab did not benefit the NMOSD without AQP4-IgG subgroup though the subject numbers are rather small. We need to know more about the clinical and laboratory characteristics of the seronegative patients as they likely comprise a heterogeneous group. For example, did any of them have other autoantibodies such as MOG-IgG? Depending on the results, those details may help us understand the relative role of IL-6 in AQP4-IgG–seronegative subgroups, an important area of further study.”
SakuraStar was funded by Chugai Pharmaceutical, a member of the Roche group. Dr. Traboulsee reported grants, personal fees, and nonfinancial support from Chugai Pharmaceutical during the study, and several coauthors were employees of Chugai Pharmaceutical. Additional coauthors reported personal fees from Chugai, Roche, and other companies. Dr. Levy has received consulting fees from Alexion, Viela Bio, Chugai Pharmaceutical, Quest Diagnostics, and UCB Pharmaceuticals.
SOURCE: Traboulsee A et al. Lancet Neurol. 2020;19(5):402-12.
according to trial results published in the Lancet Neurology.
The findings help confirm the role of interleukin-6 in the pathobiology of aquaporin-4 autoantibody (AQP4-IgG)–seropositive disease. For patients who are AQP4-IgG seronegative, however, “there is insufficient evidence to indicate a risk reduction” with this drug, the investigators wrote. In addition, satralizumab did not significantly affect pain or fatigue.
“The limitations of the study include the relatively small group sizes and low number of relapses. Despite these limitations, a significant treatment benefit was observed with satralizumab, compared with placebo in the study population, with efficacy and safety comparable to satralizumab used in combination with immunosuppressants,” reported lead author Anthony Traboulsee, MD, professor of neurology at the University of British Columbia, Vancouver, and colleagues.
Satralizumab is a humanized monoclonal antibody targeting the IL-6 receptor. A prior phase 3 study, SakuraSky, found that the drug reduces the risk of NMOSD relapse when added to immunosuppressant therapy. To assess the safety and efficacy of satralizumab monotherapy, Dr. Traboulsee and colleagues conducted SakuraStar, a randomized, double-blind, placebo-controlled trial.
Evaluating drug as monotherapy
The phase 3 trial enrolled 95 patients aged 18-74 years at 44 sites in 13 countries. The investigators included patients with AQP4-IgG–seropositive or –seronegative neuromyelitis optica using the 2006 Wingerchuk criteria or with AQP4-IgG–seropositive NMOSD with at least one event of longitudinally extensive myelitis or optic neuritis. The researchers limited the number of AQP4-IgG–seronegative patients to about 30% of the study population. Eligible participants had at least one NMOSD attack or relapse in the past 12 months and a score of 6.5 or less on the Expanded Disability Status Scale (EDSS). The investigators excluded patients with a clinical relapse in the 30 days before study baseline. Participants were randomly assigned 2:1 to receive satralizumab 120 mg or placebo subcutaneously at weeks 0, 2, 4, and every 4 weeks thereafter. Concomitant immunosuppressant use was prohibited, although corticosteroids and intravenous immunoglobulin were permitted as rescue therapy for the treatment of relapse.
The primary endpoint was time to first relapse, and the trial was designed to continue until 44 relapses occurred or for 1.5 years after the last patient entered the trial, whichever occurred first.
“Because even one NMOSD attack can have serious neurological consequences, this design allowed patients who had an attack on placebo to enter the open-label phase and receive the active drug,” the researchers wrote. “The trial design used unequal randomization to minimize exposure to placebo; because patients were not permitted to receive concomitant immunosuppressant treatment in this trial, the design limited the number of patients not receiving any treatment for the disorder. Placebo was selected with the consideration that no drugs were approved for the treatment of NMOSD when the trial was designed.” Recent trials of eculizumab, inebilizumab, and satralizumab have found that the agents are effective treatments for NMOSD. In 2019, the Food and Drug Administration approved eculizumab, a complement inhibitor, for the treatment of AQP4-IgG–seropositive NMOSD.
For the primary endpoint of SakuraStar, the researchers defined relapses as new or worsening objective neurologic symptoms with at least one of the following:
- Increase of 1 or more EDSS points from a baseline EDSS score of more than 0, or increase of 2 or more EDSS points from a baseline EDSS score of 0
- Increase of 2 or more points on at least one appropriate symptom-specific functional system score for pyramidal, cerebellar, brain stem, sensory, bowel or bladder, or a single eye
- Increase of 1 or more points on more than one symptom-specific functional system score with a baseline of at least 1
- Increase of 1 or more points on a single-eye symptom-specific functional system score with a baseline score of at least 1
In addition, symptoms had to be attributable to NMOSD; persist for more than 24 hours; and not be attributable to confounding clinical factors such as fever, infection, injury, change in mood, or adverse reactions to medications. Researchers assessed EDSS and functional system scores within 7 days of a patient reporting symptoms.
The double-blind treatment period ended 1.5 years after the last enrolled patient was assigned to satralizumab or placebo. More than 80% of the participants were women, including 73% of the satralizumab group and 97% of the placebo group. In all, 95 participants were assigned to a treatment between 2014 and 2017 – 63 to satralizumab and 32 to placebo. Relapses occurred in 19 patients receiving satralizumab (30%) and 16 receiving placebo (50%). The hazard ratio was 0.45.
“Patients treated with placebo showed a shorter time to relapse and a higher withdrawal rate than did patients treated with satralizumab,” wrote Dr. Traboulsee and colleagues. The Kaplan-Meier method suggested that 76% of patients on satralizumab had not relapsed at 48 weeks, compared with 62% of patients on placebo. And at 96 weeks, 72% of patients on satralizumab had not relapsed, compared with 51% of patients on placebo.
Among patients who were AQP4-IgG seropositive, the proportion with protocol-defined relapse was 22% in the satralizumab group versus 57% in the placebo group. Among patients who were AQP4-IgG seronegative, the proportion with a protocol-defined relapse was 46% in the satralizumab group versus 33% in the placebo group.
The most common adverse events were urinary tract infection and upper respiratory tract infection, and most adverse events were mild to moderate. A higher rate of severe adverse events was reported in the satralizumab group than in the placebo group (32.1 vs. 9.9 events per 100 patient-years). The investigators considered most of the severe adverse events unrelated to the study treatment. “None of the severe adverse events led to discontinuation of the study drug except one severe event of pneumonia in the satralizumab group,” the researchers wrote.
Data confirm efficacy of IL-6 blockade
“Satralizumab was well tolerated and no meaningful adverse effects from the drug were reported and no deaths occurred,” said Michael Levy, MD, PhD, director of the NMO clinic and research laboratory at Massachusetts General Hospital, Boston, in an accompanying editorial. “This trial of satralizumab was done shortly after the completion of a parallel trial of satralizumab in patients with NMOSD in which the same dose of satralizumab reduced the risk of relapse by 62%, compared with placebo. The main difference between these two trials is that, in the first published study, participants were permitted to use background immunotherapy; otherwise, the trial designs were nearly identical, and the enrolled participants are comparable. Together, the findings from these studies suggest that background therapy seems to provide only a small additional benefit to satralizumab alone.”
Dr. Levy also discussed findings from a phase 2 study of tocilizumab for the prevention of relapse in patients with NMOSD published in the same issue of the Lancet Neurology. The satralizumab and tocilizumab data have “confirmed that IL-6 blockade reduces the risk of relapse in patients with NMOSD,” Dr. Levy said. “IL-6 is a crucial component of the immune system, but when IL-6 production is altered during autoimmune attacks and sepsis, there can be severe consequences.”
The phase 2 trial of tocilizumab, which was described at the 2019 annual congress of the European Committee for Treatment and Research in Multiple Sclerosis, included 118 patients, 87% of whom were AQP4-IgG seropositive. Patients received intravenous tocilizumab or oral azathioprine for up to 60 weeks. Overall, 14% of patients in the tocilizumab group relapsed, compared with 47% of patients in the azathioprine group.
“The main differences between this trial of tocilizumab and the two satralizumab trials are that the tocilizumab was administered intravenously, rather than subcutaneously, the study duration was approximately 1 year, and the investigators were not masked to the treatment allocation,” Dr. Levy said. “Similar to satralizumab, adverse effects with tocilizumab were mild, including asymptomatic elevations in liver enzymes and an increased incidence of respiratory and urinary infections, with no significant differences identified between the tocilizumab and azathioprine groups.”
Various immunopathologic mechanisms may influence outcomes in NMOSD. While satralizumab and tocilizumab target IL-6, eculizumab is a C5 complement inhibitor and inebilizumab is a CD19 B-cell depleting monoclonal antibody, Dr. Levy said. “The safety concerns regarding these approaches are all substantially outweighed by the benefit of preventing NMOSD relapses.”
A need to understand AQP4-IgG–seronegative disease
SakuraStar “provides convincing data for the efficacy of satralizumab monotherapy in NMOSD with subgroup analysis showing that the benefit was seen in AQP4-IgG seropositive patients,” commented Dean M. Wingerchuk, MD, director of the division of multiple sclerosis and autoimmune neurology at the Mayo Clinic in Phoenix. “The results help confirm the key role of IL-6 in the pathobiology of AQP4-IgG–seropositive disease.”
Questions about AQP4-IgG seronegative disease remain. “The results are quite similar to the SakuraSky trial, in which satralizumab was used in conjunction with other background immunosuppressive therapies, suggesting that the primary benefit may be derived primarily from satralizumab. Both trials also showed that satralizumab did not benefit the NMOSD without AQP4-IgG subgroup though the subject numbers are rather small. We need to know more about the clinical and laboratory characteristics of the seronegative patients as they likely comprise a heterogeneous group. For example, did any of them have other autoantibodies such as MOG-IgG? Depending on the results, those details may help us understand the relative role of IL-6 in AQP4-IgG–seronegative subgroups, an important area of further study.”
SakuraStar was funded by Chugai Pharmaceutical, a member of the Roche group. Dr. Traboulsee reported grants, personal fees, and nonfinancial support from Chugai Pharmaceutical during the study, and several coauthors were employees of Chugai Pharmaceutical. Additional coauthors reported personal fees from Chugai, Roche, and other companies. Dr. Levy has received consulting fees from Alexion, Viela Bio, Chugai Pharmaceutical, Quest Diagnostics, and UCB Pharmaceuticals.
SOURCE: Traboulsee A et al. Lancet Neurol. 2020;19(5):402-12.
FROM THE LANCET NEUROLOGY
Depression linked to neuro dysfunction, brain lesions in MS
Depression is associated with decreased neurologic function and new brain lesions in patients with multiple sclerosis (MS), new research suggests.
In an observational study of more than 2500 patients with relapsing-remitting MS (RRMS), participants with self-reported depression were more likely to have worse scores on neuroperformance measures, such as processing speed tests, than their peers without depression.
At baseline, the group with depression also had greater odds of having at least one new contrast-enhancing lesion on MRI.
“Our results suggest that depression is not merely a reactive symptom but indicates increased risk of future MS disease activity,” the investigators note.
Lead author Jenny Feng, MD, clinical associate at the Mellen Center for MS Treatment and Research at Cleveland Clinic, added that depression should be routinely screened for in all patients with MS, something done routinely at her center.
“Every single patient that comes through the door with newly diagnosed MS, we refer to neuropsychology to screen for depression; and if there is depression, then we actively manage it because it does have an effect” on patients, she told Medscape Medical News.
“Depression isn’t just a neuropsychiatric disease,” Feng added. As shown in their study, “it may have effects on MS, especially with regards to performance in neurological function testing.”
The research is presented on AAN.com as part of the American Academy of Neurology 2020 Science Highlights. Because of the COVID-19 pandemic, the AAN had to cancel its 2020 annual meeting.
Associations Have Been “Unclear”
Although inflammatory, psychosocial, and neurodegenerative factors “have been hypothesized as etiologies” for why depression is commonly found in patients with MS,
For the current study, they assessed data from the Partners Advancing Technology and Health Solutions (MS-PATHS) database, an ongoing collaborative network of seven MS centers in the United States and three in Europe.
MS disease history and MRI data were examined, as well as 12-month scores on neuroperformance tests measuring processing speed (Symbol Digit Modalities Test), walking speed (Timed 25-Foot Walk), and manual dexterity (Nine-Hole Peg Test).
Patient-reported outcomes (PROs), as measured with the Quality of Life in Neurological Disorders (Neuro-QoL) and patient-determined disease steps, were also assessed. Depression was defined as a depression T score at baseline greater than “the 50th percentile” on the Neuro-QoL.
In the patient sample, 1333 of the participants with RRMS were classified as “not depressed” (73.7% women; mean age, 45.6 years; disease duration, 13.7 years) while 1172 were “depressed” (78.4% women; mean age, 45.9 years; disease duration, 14.3 years).
“To balance for baseline variances in the observational cohort between group with depression and group without depression, propensity score analysis was used to adjust for potential confounding factors,” the investigators report.
Worse Performance
After adjustment for baseline covariates, results showed that the depressed patients performed worse on the walking speed test (0.48; 95% confidence interval, 0.038-0.918) and processing speed test (–1.899; 95% CI, –3.548 to –0.250).
The depressed group also had increased odds at baseline of having new contrast-enhancing lesions (odds ratio, 5.89; 95% CI, 2.236-15.517). This demonstrated an “association of depression and neuroinflammatory activity” in the central nervous system, the investigators note.
At 12 months, processing speed continued to be worse in the depressed group (–1.68; 95% CI, –3.254 to –0.105).
There were trends, albeit insignificant, for decreased walking speed scores at 12 months and for decreased manual dexterity scores at both baseline and at 12 months for the participants who were depressed.
Interestingly, there were “no significant differences in PROs at month 12, despite worsening neuroperformance,” the investigators report.
“This means that patients themselves may not even realize that they were getting worse,” Feng said.
Underpowered Study?
Further results showed nonsignificant trends for increased T2 lesion volume and white matter fraction and decreased brain volume, gray matter fraction, and cortical gray matter volume at baseline and at 12 months in the depressed group.
The researchers note that study limitations include the unavailability of information on treatment compliance for depression or date of depression onset.
Feng added that because this was an observational study, other missing data included depression status for some patients at year 1 and some MRI metrics.
“So this may have been underpowered to detect some of the results. The power may have been inadequate to detect all changes,” she said.
The investigators write that future research should assess larger sample sizes with longer follow-ups and should use more advanced MRI measures, such as diffusion tensor imaging or functional MRI.
In addition, they will continue examining data from MS-PATHS. “With the newest data cut, we have new patients that we can analyze. So perhaps that can provide sufficient power to detect [more MRI] changes,” Feng said.
Unusual, Intriguing Findings
Commenting on the study for Medscape Medical News, Mark Freedman, MD, professor of neurology at the University of Ottawa and director of the Multiple Sclerosis Research Clinic at the Ottawa Hospital Research Institute, noted that he wasn’t terribly surprised” by the overall findings.
“We’ve known for years that patients who are depressed don’t do as well on our performance methods,” said Freedman, who was not involved with the research.
However, the current investigators “took a huge number of patients in this multicenter study and started using some of the statistical methods we’ve seen in the use of real-world evidence,” he noted.
“So you’re looking at some outcome measures and you have to ask yourself, ‘Why would it influence that?’ and ‘Did it happen by chance or not?’ And you ask why it is that depressed people might actually have more lesions on their MRI, which is something that is unusual,” Freedman said.
“When you start to look at this, even when you’re trying to standardize things for the differences that we know of, there are some stuff that comes out as intriguing. In general, I think those depressed patients did worse on several outcome measures that one would say, ‘That’s somewhat surprising.’ That’s why this group was very careful to not conclude absolutely that depression drives this disease. But it was consistently trending in the direction that it looks like there was more inflammatory activity in these people,” he said.
He echoed the investigators’ note that drug adherence and which depression treatment was used wasn’t controlled for; and he added that depression in the study was not based on receiving a diagnosis of clinical depression but on self-report.
Still, the patients classified as depressed “did worse. They didn’t walk as fast, which was interesting; and we know that cognitive performance is often damped because of poor concentration. But how do you get worse MRIs? This study is raising a question and [the researchers] conclude that it may be that depression might be an independent factor” for that outcome, Freedman said.
“It might be that you could get more out of a particular [MS] medicine if you pay attention to depression; and if that’s the investigators’ conclusion, and I think it is, then I certainly agree with it.”
Freedman noted that, instead of a blanket recommendation that all patients with MS should be screened for depression, he thinks clinicians, especially those at smaller centers, should focus on what’s best for treating all aspects of an individual patient.
“Don’t try to manage them if you’re not going to manage the entire picture. Looking at depression and mood and other things is very important. And if you have the capacity for an official screening, I think it’s wonderful; but not everybody does,” he said.
Feng and Freedman have disclosed no relevant financial relationships. Freedman is currently a member of the Medscape Neurology Advisory Board.
This article appeared on Medscape.com.
Depression is associated with decreased neurologic function and new brain lesions in patients with multiple sclerosis (MS), new research suggests.
In an observational study of more than 2500 patients with relapsing-remitting MS (RRMS), participants with self-reported depression were more likely to have worse scores on neuroperformance measures, such as processing speed tests, than their peers without depression.
At baseline, the group with depression also had greater odds of having at least one new contrast-enhancing lesion on MRI.
“Our results suggest that depression is not merely a reactive symptom but indicates increased risk of future MS disease activity,” the investigators note.
Lead author Jenny Feng, MD, clinical associate at the Mellen Center for MS Treatment and Research at Cleveland Clinic, added that depression should be routinely screened for in all patients with MS, something done routinely at her center.
“Every single patient that comes through the door with newly diagnosed MS, we refer to neuropsychology to screen for depression; and if there is depression, then we actively manage it because it does have an effect” on patients, she told Medscape Medical News.
“Depression isn’t just a neuropsychiatric disease,” Feng added. As shown in their study, “it may have effects on MS, especially with regards to performance in neurological function testing.”
The research is presented on AAN.com as part of the American Academy of Neurology 2020 Science Highlights. Because of the COVID-19 pandemic, the AAN had to cancel its 2020 annual meeting.
Associations Have Been “Unclear”
Although inflammatory, psychosocial, and neurodegenerative factors “have been hypothesized as etiologies” for why depression is commonly found in patients with MS,
For the current study, they assessed data from the Partners Advancing Technology and Health Solutions (MS-PATHS) database, an ongoing collaborative network of seven MS centers in the United States and three in Europe.
MS disease history and MRI data were examined, as well as 12-month scores on neuroperformance tests measuring processing speed (Symbol Digit Modalities Test), walking speed (Timed 25-Foot Walk), and manual dexterity (Nine-Hole Peg Test).
Patient-reported outcomes (PROs), as measured with the Quality of Life in Neurological Disorders (Neuro-QoL) and patient-determined disease steps, were also assessed. Depression was defined as a depression T score at baseline greater than “the 50th percentile” on the Neuro-QoL.
In the patient sample, 1333 of the participants with RRMS were classified as “not depressed” (73.7% women; mean age, 45.6 years; disease duration, 13.7 years) while 1172 were “depressed” (78.4% women; mean age, 45.9 years; disease duration, 14.3 years).
“To balance for baseline variances in the observational cohort between group with depression and group without depression, propensity score analysis was used to adjust for potential confounding factors,” the investigators report.
Worse Performance
After adjustment for baseline covariates, results showed that the depressed patients performed worse on the walking speed test (0.48; 95% confidence interval, 0.038-0.918) and processing speed test (–1.899; 95% CI, –3.548 to –0.250).
The depressed group also had increased odds at baseline of having new contrast-enhancing lesions (odds ratio, 5.89; 95% CI, 2.236-15.517). This demonstrated an “association of depression and neuroinflammatory activity” in the central nervous system, the investigators note.
At 12 months, processing speed continued to be worse in the depressed group (–1.68; 95% CI, –3.254 to –0.105).
There were trends, albeit insignificant, for decreased walking speed scores at 12 months and for decreased manual dexterity scores at both baseline and at 12 months for the participants who were depressed.
Interestingly, there were “no significant differences in PROs at month 12, despite worsening neuroperformance,” the investigators report.
“This means that patients themselves may not even realize that they were getting worse,” Feng said.
Underpowered Study?
Further results showed nonsignificant trends for increased T2 lesion volume and white matter fraction and decreased brain volume, gray matter fraction, and cortical gray matter volume at baseline and at 12 months in the depressed group.
The researchers note that study limitations include the unavailability of information on treatment compliance for depression or date of depression onset.
Feng added that because this was an observational study, other missing data included depression status for some patients at year 1 and some MRI metrics.
“So this may have been underpowered to detect some of the results. The power may have been inadequate to detect all changes,” she said.
The investigators write that future research should assess larger sample sizes with longer follow-ups and should use more advanced MRI measures, such as diffusion tensor imaging or functional MRI.
In addition, they will continue examining data from MS-PATHS. “With the newest data cut, we have new patients that we can analyze. So perhaps that can provide sufficient power to detect [more MRI] changes,” Feng said.
Unusual, Intriguing Findings
Commenting on the study for Medscape Medical News, Mark Freedman, MD, professor of neurology at the University of Ottawa and director of the Multiple Sclerosis Research Clinic at the Ottawa Hospital Research Institute, noted that he wasn’t terribly surprised” by the overall findings.
“We’ve known for years that patients who are depressed don’t do as well on our performance methods,” said Freedman, who was not involved with the research.
However, the current investigators “took a huge number of patients in this multicenter study and started using some of the statistical methods we’ve seen in the use of real-world evidence,” he noted.
“So you’re looking at some outcome measures and you have to ask yourself, ‘Why would it influence that?’ and ‘Did it happen by chance or not?’ And you ask why it is that depressed people might actually have more lesions on their MRI, which is something that is unusual,” Freedman said.
“When you start to look at this, even when you’re trying to standardize things for the differences that we know of, there are some stuff that comes out as intriguing. In general, I think those depressed patients did worse on several outcome measures that one would say, ‘That’s somewhat surprising.’ That’s why this group was very careful to not conclude absolutely that depression drives this disease. But it was consistently trending in the direction that it looks like there was more inflammatory activity in these people,” he said.
He echoed the investigators’ note that drug adherence and which depression treatment was used wasn’t controlled for; and he added that depression in the study was not based on receiving a diagnosis of clinical depression but on self-report.
Still, the patients classified as depressed “did worse. They didn’t walk as fast, which was interesting; and we know that cognitive performance is often damped because of poor concentration. But how do you get worse MRIs? This study is raising a question and [the researchers] conclude that it may be that depression might be an independent factor” for that outcome, Freedman said.
“It might be that you could get more out of a particular [MS] medicine if you pay attention to depression; and if that’s the investigators’ conclusion, and I think it is, then I certainly agree with it.”
Freedman noted that, instead of a blanket recommendation that all patients with MS should be screened for depression, he thinks clinicians, especially those at smaller centers, should focus on what’s best for treating all aspects of an individual patient.
“Don’t try to manage them if you’re not going to manage the entire picture. Looking at depression and mood and other things is very important. And if you have the capacity for an official screening, I think it’s wonderful; but not everybody does,” he said.
Feng and Freedman have disclosed no relevant financial relationships. Freedman is currently a member of the Medscape Neurology Advisory Board.
This article appeared on Medscape.com.
Depression is associated with decreased neurologic function and new brain lesions in patients with multiple sclerosis (MS), new research suggests.
In an observational study of more than 2500 patients with relapsing-remitting MS (RRMS), participants with self-reported depression were more likely to have worse scores on neuroperformance measures, such as processing speed tests, than their peers without depression.
At baseline, the group with depression also had greater odds of having at least one new contrast-enhancing lesion on MRI.
“Our results suggest that depression is not merely a reactive symptom but indicates increased risk of future MS disease activity,” the investigators note.
Lead author Jenny Feng, MD, clinical associate at the Mellen Center for MS Treatment and Research at Cleveland Clinic, added that depression should be routinely screened for in all patients with MS, something done routinely at her center.
“Every single patient that comes through the door with newly diagnosed MS, we refer to neuropsychology to screen for depression; and if there is depression, then we actively manage it because it does have an effect” on patients, she told Medscape Medical News.
“Depression isn’t just a neuropsychiatric disease,” Feng added. As shown in their study, “it may have effects on MS, especially with regards to performance in neurological function testing.”
The research is presented on AAN.com as part of the American Academy of Neurology 2020 Science Highlights. Because of the COVID-19 pandemic, the AAN had to cancel its 2020 annual meeting.
Associations Have Been “Unclear”
Although inflammatory, psychosocial, and neurodegenerative factors “have been hypothesized as etiologies” for why depression is commonly found in patients with MS,
For the current study, they assessed data from the Partners Advancing Technology and Health Solutions (MS-PATHS) database, an ongoing collaborative network of seven MS centers in the United States and three in Europe.
MS disease history and MRI data were examined, as well as 12-month scores on neuroperformance tests measuring processing speed (Symbol Digit Modalities Test), walking speed (Timed 25-Foot Walk), and manual dexterity (Nine-Hole Peg Test).
Patient-reported outcomes (PROs), as measured with the Quality of Life in Neurological Disorders (Neuro-QoL) and patient-determined disease steps, were also assessed. Depression was defined as a depression T score at baseline greater than “the 50th percentile” on the Neuro-QoL.
In the patient sample, 1333 of the participants with RRMS were classified as “not depressed” (73.7% women; mean age, 45.6 years; disease duration, 13.7 years) while 1172 were “depressed” (78.4% women; mean age, 45.9 years; disease duration, 14.3 years).
“To balance for baseline variances in the observational cohort between group with depression and group without depression, propensity score analysis was used to adjust for potential confounding factors,” the investigators report.
Worse Performance
After adjustment for baseline covariates, results showed that the depressed patients performed worse on the walking speed test (0.48; 95% confidence interval, 0.038-0.918) and processing speed test (–1.899; 95% CI, –3.548 to –0.250).
The depressed group also had increased odds at baseline of having new contrast-enhancing lesions (odds ratio, 5.89; 95% CI, 2.236-15.517). This demonstrated an “association of depression and neuroinflammatory activity” in the central nervous system, the investigators note.
At 12 months, processing speed continued to be worse in the depressed group (–1.68; 95% CI, –3.254 to –0.105).
There were trends, albeit insignificant, for decreased walking speed scores at 12 months and for decreased manual dexterity scores at both baseline and at 12 months for the participants who were depressed.
Interestingly, there were “no significant differences in PROs at month 12, despite worsening neuroperformance,” the investigators report.
“This means that patients themselves may not even realize that they were getting worse,” Feng said.
Underpowered Study?
Further results showed nonsignificant trends for increased T2 lesion volume and white matter fraction and decreased brain volume, gray matter fraction, and cortical gray matter volume at baseline and at 12 months in the depressed group.
The researchers note that study limitations include the unavailability of information on treatment compliance for depression or date of depression onset.
Feng added that because this was an observational study, other missing data included depression status for some patients at year 1 and some MRI metrics.
“So this may have been underpowered to detect some of the results. The power may have been inadequate to detect all changes,” she said.
The investigators write that future research should assess larger sample sizes with longer follow-ups and should use more advanced MRI measures, such as diffusion tensor imaging or functional MRI.
In addition, they will continue examining data from MS-PATHS. “With the newest data cut, we have new patients that we can analyze. So perhaps that can provide sufficient power to detect [more MRI] changes,” Feng said.
Unusual, Intriguing Findings
Commenting on the study for Medscape Medical News, Mark Freedman, MD, professor of neurology at the University of Ottawa and director of the Multiple Sclerosis Research Clinic at the Ottawa Hospital Research Institute, noted that he wasn’t terribly surprised” by the overall findings.
“We’ve known for years that patients who are depressed don’t do as well on our performance methods,” said Freedman, who was not involved with the research.
However, the current investigators “took a huge number of patients in this multicenter study and started using some of the statistical methods we’ve seen in the use of real-world evidence,” he noted.
“So you’re looking at some outcome measures and you have to ask yourself, ‘Why would it influence that?’ and ‘Did it happen by chance or not?’ And you ask why it is that depressed people might actually have more lesions on their MRI, which is something that is unusual,” Freedman said.
“When you start to look at this, even when you’re trying to standardize things for the differences that we know of, there are some stuff that comes out as intriguing. In general, I think those depressed patients did worse on several outcome measures that one would say, ‘That’s somewhat surprising.’ That’s why this group was very careful to not conclude absolutely that depression drives this disease. But it was consistently trending in the direction that it looks like there was more inflammatory activity in these people,” he said.
He echoed the investigators’ note that drug adherence and which depression treatment was used wasn’t controlled for; and he added that depression in the study was not based on receiving a diagnosis of clinical depression but on self-report.
Still, the patients classified as depressed “did worse. They didn’t walk as fast, which was interesting; and we know that cognitive performance is often damped because of poor concentration. But how do you get worse MRIs? This study is raising a question and [the researchers] conclude that it may be that depression might be an independent factor” for that outcome, Freedman said.
“It might be that you could get more out of a particular [MS] medicine if you pay attention to depression; and if that’s the investigators’ conclusion, and I think it is, then I certainly agree with it.”
Freedman noted that, instead of a blanket recommendation that all patients with MS should be screened for depression, he thinks clinicians, especially those at smaller centers, should focus on what’s best for treating all aspects of an individual patient.
“Don’t try to manage them if you’re not going to manage the entire picture. Looking at depression and mood and other things is very important. And if you have the capacity for an official screening, I think it’s wonderful; but not everybody does,” he said.
Feng and Freedman have disclosed no relevant financial relationships. Freedman is currently a member of the Medscape Neurology Advisory Board.
This article appeared on Medscape.com.
Serum NfL in early MS can help predict clinical course
research suggests. The study showed that patients with higher sNfL within 5 years of MS diagnosis had a higher risk of long term-clinical disability and higher risk of developing progressive MS. The level of sNfL also predicted the rate of increase over time in the Expanded Disability Status Scale (EDSS).
Serum NfL levels can provide “useful information in both directions, adding to both an overall reassuring picture or worrying picture both at first presentation and then on subsequent visits,” said Simon Thebault, MBBCh, a neurology resident at the University of Ottawa and the Ottawa Hospital Research Institute, Canada.
This research was presented online as part of the 2020 American Academy of Neurology Science Highlights.
Prognostication from day one
Many studies have shown a correlation between MS disease activity (clinical relapses, EDSS progression, MRI lesions) and elevated sNfL. Other studies have also looked at the prognostic value of NfL in serum and cerebrospinal fluid (CSF), but the data are limited by the lack of long-term biobanked samples and subsequent follow-up, Dr. Thebault explained.
The new study took advantage of the Ottawa MS biobank, which contains carefully frozen and stored samples from more than 3,000 patients with MS going back up to 25 years.
The team identified patients with serum collected within 5 years of first MS symptom onset (baseline) who were followed for a median of 18.9 years (range 15.0 to 27.0 years). They quantified levels of sNfL in 67 patients and 37 matched controls.
In patients with MS, the median baseline sNfL level was 10.1 pg/mL – 38.5% higher than the median level in controls (7.26 pg/mL, P = 0.004).
The baseline sNfL level was “most helpful as a sensitive predictive marker to rule out disease progression,” the researchers reported in their meeting abstract.
Patients with baseline sNfL levels less than 7.62 pg/mL were 4.3 times less likely to develop significant disability (EDSS score ≥ 4; P = 0.001) and 7.1 times less likely to develop progressive MS by end of follow-up (P = 0.054).
The most rapid disease progression was seen in patients with the highest baseline NfL levels (3rd-tertile, > 13.2 pg/mL). Higher baseline sNfL level was associated with faster rate of EDSS progression even after adjusting for confounders of age, sex, and disease-modifying treatment.
“We were able to show that serum neurofilament levels collected very early in the disease, usually at the time of first diagnosis, were predictive of the clinical progression [by EDSS score] and the risk of evolving to secondary progressive MS on average 19 years later,” Dr. Thebault said. A baseline level less than 7.6 pg/mL was “reassuring.”
“Prognostication in MS from day one is important,” he emphasized.
“If we know someone is on a bad trajectory, neurologists might recommend more aggressive therapies up front. Equally, if a patient has a very reassuring picture, then maybe it is more appropriate to start with safer treatments [the so called ‘platform therapies’] that may serve a patient well for many years, as they did for many in the years before higher-efficacy therapies were available,” Dr. Thebault said.
“In the hands of an expert MS neurologist who understands both the pearls and pitfalls of this test ... serum neurofilament is already a useful clinical tool, and we have implemented it in our daily practice in Ottawa,” he concluded.
Noteworthy study
Commenting on the study, Asaff Harel, MD, neurologist at Lenox Hill Hospital in New York City, said the findings in this study are “noteworthy, as there is a relative lack of effective prognostic biomarkers in the field of MS.”
“It remains to be seen whether this improves risk stratification of patients above what can be achieved by looking at other prognostic factors, such as age, gender, baseline EDSS, and severity and frequency of relapses during early disease course,” Dr. Harel cautioned.
“This was a relatively small study and further research is necessary,” Dr. Harel added. It’s also worth noting, he said, that out of the 67 patients who met criteria to be included in the study (i.e., those with blood samples taken during “early MS,” more than 15 years ago), almost half were lost to follow-up, which could potentially open the study to error.
It is also “unclear whether early NfL level is a better prognostic marker than severity of early disease course and baseline EDSS, both of which were not addressed in the study, and this will be interesting to determine in the future,” Dr. Harel commented.
Funding for the study was provided by The Ottawa Hospital Pilot Project Grant. Thebault and Harel have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
research suggests. The study showed that patients with higher sNfL within 5 years of MS diagnosis had a higher risk of long term-clinical disability and higher risk of developing progressive MS. The level of sNfL also predicted the rate of increase over time in the Expanded Disability Status Scale (EDSS).
Serum NfL levels can provide “useful information in both directions, adding to both an overall reassuring picture or worrying picture both at first presentation and then on subsequent visits,” said Simon Thebault, MBBCh, a neurology resident at the University of Ottawa and the Ottawa Hospital Research Institute, Canada.
This research was presented online as part of the 2020 American Academy of Neurology Science Highlights.
Prognostication from day one
Many studies have shown a correlation between MS disease activity (clinical relapses, EDSS progression, MRI lesions) and elevated sNfL. Other studies have also looked at the prognostic value of NfL in serum and cerebrospinal fluid (CSF), but the data are limited by the lack of long-term biobanked samples and subsequent follow-up, Dr. Thebault explained.
The new study took advantage of the Ottawa MS biobank, which contains carefully frozen and stored samples from more than 3,000 patients with MS going back up to 25 years.
The team identified patients with serum collected within 5 years of first MS symptom onset (baseline) who were followed for a median of 18.9 years (range 15.0 to 27.0 years). They quantified levels of sNfL in 67 patients and 37 matched controls.
In patients with MS, the median baseline sNfL level was 10.1 pg/mL – 38.5% higher than the median level in controls (7.26 pg/mL, P = 0.004).
The baseline sNfL level was “most helpful as a sensitive predictive marker to rule out disease progression,” the researchers reported in their meeting abstract.
Patients with baseline sNfL levels less than 7.62 pg/mL were 4.3 times less likely to develop significant disability (EDSS score ≥ 4; P = 0.001) and 7.1 times less likely to develop progressive MS by end of follow-up (P = 0.054).
The most rapid disease progression was seen in patients with the highest baseline NfL levels (3rd-tertile, > 13.2 pg/mL). Higher baseline sNfL level was associated with faster rate of EDSS progression even after adjusting for confounders of age, sex, and disease-modifying treatment.
“We were able to show that serum neurofilament levels collected very early in the disease, usually at the time of first diagnosis, were predictive of the clinical progression [by EDSS score] and the risk of evolving to secondary progressive MS on average 19 years later,” Dr. Thebault said. A baseline level less than 7.6 pg/mL was “reassuring.”
“Prognostication in MS from day one is important,” he emphasized.
“If we know someone is on a bad trajectory, neurologists might recommend more aggressive therapies up front. Equally, if a patient has a very reassuring picture, then maybe it is more appropriate to start with safer treatments [the so called ‘platform therapies’] that may serve a patient well for many years, as they did for many in the years before higher-efficacy therapies were available,” Dr. Thebault said.
“In the hands of an expert MS neurologist who understands both the pearls and pitfalls of this test ... serum neurofilament is already a useful clinical tool, and we have implemented it in our daily practice in Ottawa,” he concluded.
Noteworthy study
Commenting on the study, Asaff Harel, MD, neurologist at Lenox Hill Hospital in New York City, said the findings in this study are “noteworthy, as there is a relative lack of effective prognostic biomarkers in the field of MS.”
“It remains to be seen whether this improves risk stratification of patients above what can be achieved by looking at other prognostic factors, such as age, gender, baseline EDSS, and severity and frequency of relapses during early disease course,” Dr. Harel cautioned.
“This was a relatively small study and further research is necessary,” Dr. Harel added. It’s also worth noting, he said, that out of the 67 patients who met criteria to be included in the study (i.e., those with blood samples taken during “early MS,” more than 15 years ago), almost half were lost to follow-up, which could potentially open the study to error.
It is also “unclear whether early NfL level is a better prognostic marker than severity of early disease course and baseline EDSS, both of which were not addressed in the study, and this will be interesting to determine in the future,” Dr. Harel commented.
Funding for the study was provided by The Ottawa Hospital Pilot Project Grant. Thebault and Harel have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
research suggests. The study showed that patients with higher sNfL within 5 years of MS diagnosis had a higher risk of long term-clinical disability and higher risk of developing progressive MS. The level of sNfL also predicted the rate of increase over time in the Expanded Disability Status Scale (EDSS).
Serum NfL levels can provide “useful information in both directions, adding to both an overall reassuring picture or worrying picture both at first presentation and then on subsequent visits,” said Simon Thebault, MBBCh, a neurology resident at the University of Ottawa and the Ottawa Hospital Research Institute, Canada.
This research was presented online as part of the 2020 American Academy of Neurology Science Highlights.
Prognostication from day one
Many studies have shown a correlation between MS disease activity (clinical relapses, EDSS progression, MRI lesions) and elevated sNfL. Other studies have also looked at the prognostic value of NfL in serum and cerebrospinal fluid (CSF), but the data are limited by the lack of long-term biobanked samples and subsequent follow-up, Dr. Thebault explained.
The new study took advantage of the Ottawa MS biobank, which contains carefully frozen and stored samples from more than 3,000 patients with MS going back up to 25 years.
The team identified patients with serum collected within 5 years of first MS symptom onset (baseline) who were followed for a median of 18.9 years (range 15.0 to 27.0 years). They quantified levels of sNfL in 67 patients and 37 matched controls.
In patients with MS, the median baseline sNfL level was 10.1 pg/mL – 38.5% higher than the median level in controls (7.26 pg/mL, P = 0.004).
The baseline sNfL level was “most helpful as a sensitive predictive marker to rule out disease progression,” the researchers reported in their meeting abstract.
Patients with baseline sNfL levels less than 7.62 pg/mL were 4.3 times less likely to develop significant disability (EDSS score ≥ 4; P = 0.001) and 7.1 times less likely to develop progressive MS by end of follow-up (P = 0.054).
The most rapid disease progression was seen in patients with the highest baseline NfL levels (3rd-tertile, > 13.2 pg/mL). Higher baseline sNfL level was associated with faster rate of EDSS progression even after adjusting for confounders of age, sex, and disease-modifying treatment.
“We were able to show that serum neurofilament levels collected very early in the disease, usually at the time of first diagnosis, were predictive of the clinical progression [by EDSS score] and the risk of evolving to secondary progressive MS on average 19 years later,” Dr. Thebault said. A baseline level less than 7.6 pg/mL was “reassuring.”
“Prognostication in MS from day one is important,” he emphasized.
“If we know someone is on a bad trajectory, neurologists might recommend more aggressive therapies up front. Equally, if a patient has a very reassuring picture, then maybe it is more appropriate to start with safer treatments [the so called ‘platform therapies’] that may serve a patient well for many years, as they did for many in the years before higher-efficacy therapies were available,” Dr. Thebault said.
“In the hands of an expert MS neurologist who understands both the pearls and pitfalls of this test ... serum neurofilament is already a useful clinical tool, and we have implemented it in our daily practice in Ottawa,” he concluded.
Noteworthy study
Commenting on the study, Asaff Harel, MD, neurologist at Lenox Hill Hospital in New York City, said the findings in this study are “noteworthy, as there is a relative lack of effective prognostic biomarkers in the field of MS.”
“It remains to be seen whether this improves risk stratification of patients above what can be achieved by looking at other prognostic factors, such as age, gender, baseline EDSS, and severity and frequency of relapses during early disease course,” Dr. Harel cautioned.
“This was a relatively small study and further research is necessary,” Dr. Harel added. It’s also worth noting, he said, that out of the 67 patients who met criteria to be included in the study (i.e., those with blood samples taken during “early MS,” more than 15 years ago), almost half were lost to follow-up, which could potentially open the study to error.
It is also “unclear whether early NfL level is a better prognostic marker than severity of early disease course and baseline EDSS, both of which were not addressed in the study, and this will be interesting to determine in the future,” Dr. Harel commented.
Funding for the study was provided by The Ottawa Hospital Pilot Project Grant. Thebault and Harel have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Initial high-efficacy MS therapy tied to less disability later
new research suggests. However, there is a trade-off: In this study of nearly 300 patients, those treated with initial HET experienced more disease activity in the first 2 years than other participants.
The HET benefit emerged between 2 and 10 years into the study. For example, the mean Expanded Disability Status Scale (EDSS) scores were significantly lower at 6 years in the early, aggressive treatment group than in the later HET group (2.4 vs 3.3, respectively).
“Treatment decisions made around the time of diagnosis will affect long-term outcomes,” said lead author Anna He, MBBS, currently with the Department of Clinical Neuroscience, Karolinska Institute, Stockholm, and the UCL Queen Square Institute of Neurology in London.
Using the most efficacious disease-modifying therapies from the start minimizes disability, “whereas those patients escalating to high-efficacy disease-modifying therapies later do not seem to catch up to those who commenced earlier,” Dr. He said.
“Patients and clinicians should be aware of this when choosing treatment in early MS,” she added.
This research was presented online as part of the 2020 American Academy of Neurology Science Highlights.
Patient-centered outcome
Instead of measures of brain volume, lesion count, serum neurofilament, or other biomarkers that are mainly of interest to clinicians and scientists, “the main outcome of interest to our patients is their disability,” Dr. He said. “The first question they ask at diagnosis is usually along the lines of: ‘What will my disability be in 10 years?’ ”
“This is what matters to patients and is fundamentally what motivated this study,” Dr. He added.
The investigators searched international MS registries for patients with relapsing-remitting MS starting HET, which included rituximab, ocrelizumab, mitoxantrone, alemtuzumab, or natalizumab.
They compared 117 participants who started HET within the first 2 years of clinical disease onset (the early group) with 181 participants who started HET after more than 4 years (the late group). All were followed for a median of 7.4 years (range, 6.4 to 8.6 years).
Difference in EDSS scores from baseline was the primary outcome. Both cohorts began the study with a mean EDSS score of 2.4, but between-group differences were significant at 10 years.
The secondary outcome of cumulative hazard of disability progression was higher in the early-treatment group from baseline to 2 years. Between the period of 2 and 10 years, the inverse was true.
In patients with highly active MS, “early exposure to high efficacy therapies is recommended,” Dr. He noted.
“We can already affect our patients’ lives enormously by utilizing our current toolbox in the most optimal way. It is our task to optimize this in a data-driven manner.”
Going forward, Dr. He plans to look at other outcomes, including patient-reported quality of life and health economic measures, and to take a different approach to future research.
Rather than assess MS outcomes from a disease-biology perspective, “I will be looking at MS outcomes from the perspective of its key stakeholders—the individual and society,” and the factors that influence them, Dr. He said.
Confirmatory evidence?
Commenting on the findings, Robert Gross, MD, a neurologist at the Rocky Mountain MS Center at the University of Colorado Denver in Aurora, said it is “hard to believe we are still having this debate” about earlier versus later HET.
There are now “numerous studies, including head-to-head trials and large cohort studies, showing superiority of highly efficacious agents to older disease-modifying therapies of more limited efficacy, as well as better outcomes with early versus delayed use of high-efficacy therapy,” said Dr. Gross, who was not involved with the current research.
“This study further adds to the evidence that we should be preferentially starting folks with relapsing-remitting MS right away on high-efficacy therapy, rather than waiting for relapses and disease progression to occur,” he added.
Drs. He and Gross have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
new research suggests. However, there is a trade-off: In this study of nearly 300 patients, those treated with initial HET experienced more disease activity in the first 2 years than other participants.
The HET benefit emerged between 2 and 10 years into the study. For example, the mean Expanded Disability Status Scale (EDSS) scores were significantly lower at 6 years in the early, aggressive treatment group than in the later HET group (2.4 vs 3.3, respectively).
“Treatment decisions made around the time of diagnosis will affect long-term outcomes,” said lead author Anna He, MBBS, currently with the Department of Clinical Neuroscience, Karolinska Institute, Stockholm, and the UCL Queen Square Institute of Neurology in London.
Using the most efficacious disease-modifying therapies from the start minimizes disability, “whereas those patients escalating to high-efficacy disease-modifying therapies later do not seem to catch up to those who commenced earlier,” Dr. He said.
“Patients and clinicians should be aware of this when choosing treatment in early MS,” she added.
This research was presented online as part of the 2020 American Academy of Neurology Science Highlights.
Patient-centered outcome
Instead of measures of brain volume, lesion count, serum neurofilament, or other biomarkers that are mainly of interest to clinicians and scientists, “the main outcome of interest to our patients is their disability,” Dr. He said. “The first question they ask at diagnosis is usually along the lines of: ‘What will my disability be in 10 years?’ ”
“This is what matters to patients and is fundamentally what motivated this study,” Dr. He added.
The investigators searched international MS registries for patients with relapsing-remitting MS starting HET, which included rituximab, ocrelizumab, mitoxantrone, alemtuzumab, or natalizumab.
They compared 117 participants who started HET within the first 2 years of clinical disease onset (the early group) with 181 participants who started HET after more than 4 years (the late group). All were followed for a median of 7.4 years (range, 6.4 to 8.6 years).
Difference in EDSS scores from baseline was the primary outcome. Both cohorts began the study with a mean EDSS score of 2.4, but between-group differences were significant at 10 years.
The secondary outcome of cumulative hazard of disability progression was higher in the early-treatment group from baseline to 2 years. Between the period of 2 and 10 years, the inverse was true.
In patients with highly active MS, “early exposure to high efficacy therapies is recommended,” Dr. He noted.
“We can already affect our patients’ lives enormously by utilizing our current toolbox in the most optimal way. It is our task to optimize this in a data-driven manner.”
Going forward, Dr. He plans to look at other outcomes, including patient-reported quality of life and health economic measures, and to take a different approach to future research.
Rather than assess MS outcomes from a disease-biology perspective, “I will be looking at MS outcomes from the perspective of its key stakeholders—the individual and society,” and the factors that influence them, Dr. He said.
Confirmatory evidence?
Commenting on the findings, Robert Gross, MD, a neurologist at the Rocky Mountain MS Center at the University of Colorado Denver in Aurora, said it is “hard to believe we are still having this debate” about earlier versus later HET.
There are now “numerous studies, including head-to-head trials and large cohort studies, showing superiority of highly efficacious agents to older disease-modifying therapies of more limited efficacy, as well as better outcomes with early versus delayed use of high-efficacy therapy,” said Dr. Gross, who was not involved with the current research.
“This study further adds to the evidence that we should be preferentially starting folks with relapsing-remitting MS right away on high-efficacy therapy, rather than waiting for relapses and disease progression to occur,” he added.
Drs. He and Gross have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
new research suggests. However, there is a trade-off: In this study of nearly 300 patients, those treated with initial HET experienced more disease activity in the first 2 years than other participants.
The HET benefit emerged between 2 and 10 years into the study. For example, the mean Expanded Disability Status Scale (EDSS) scores were significantly lower at 6 years in the early, aggressive treatment group than in the later HET group (2.4 vs 3.3, respectively).
“Treatment decisions made around the time of diagnosis will affect long-term outcomes,” said lead author Anna He, MBBS, currently with the Department of Clinical Neuroscience, Karolinska Institute, Stockholm, and the UCL Queen Square Institute of Neurology in London.
Using the most efficacious disease-modifying therapies from the start minimizes disability, “whereas those patients escalating to high-efficacy disease-modifying therapies later do not seem to catch up to those who commenced earlier,” Dr. He said.
“Patients and clinicians should be aware of this when choosing treatment in early MS,” she added.
This research was presented online as part of the 2020 American Academy of Neurology Science Highlights.
Patient-centered outcome
Instead of measures of brain volume, lesion count, serum neurofilament, or other biomarkers that are mainly of interest to clinicians and scientists, “the main outcome of interest to our patients is their disability,” Dr. He said. “The first question they ask at diagnosis is usually along the lines of: ‘What will my disability be in 10 years?’ ”
“This is what matters to patients and is fundamentally what motivated this study,” Dr. He added.
The investigators searched international MS registries for patients with relapsing-remitting MS starting HET, which included rituximab, ocrelizumab, mitoxantrone, alemtuzumab, or natalizumab.
They compared 117 participants who started HET within the first 2 years of clinical disease onset (the early group) with 181 participants who started HET after more than 4 years (the late group). All were followed for a median of 7.4 years (range, 6.4 to 8.6 years).
Difference in EDSS scores from baseline was the primary outcome. Both cohorts began the study with a mean EDSS score of 2.4, but between-group differences were significant at 10 years.
The secondary outcome of cumulative hazard of disability progression was higher in the early-treatment group from baseline to 2 years. Between the period of 2 and 10 years, the inverse was true.
In patients with highly active MS, “early exposure to high efficacy therapies is recommended,” Dr. He noted.
“We can already affect our patients’ lives enormously by utilizing our current toolbox in the most optimal way. It is our task to optimize this in a data-driven manner.”
Going forward, Dr. He plans to look at other outcomes, including patient-reported quality of life and health economic measures, and to take a different approach to future research.
Rather than assess MS outcomes from a disease-biology perspective, “I will be looking at MS outcomes from the perspective of its key stakeholders—the individual and society,” and the factors that influence them, Dr. He said.
Confirmatory evidence?
Commenting on the findings, Robert Gross, MD, a neurologist at the Rocky Mountain MS Center at the University of Colorado Denver in Aurora, said it is “hard to believe we are still having this debate” about earlier versus later HET.
There are now “numerous studies, including head-to-head trials and large cohort studies, showing superiority of highly efficacious agents to older disease-modifying therapies of more limited efficacy, as well as better outcomes with early versus delayed use of high-efficacy therapy,” said Dr. Gross, who was not involved with the current research.
“This study further adds to the evidence that we should be preferentially starting folks with relapsing-remitting MS right away on high-efficacy therapy, rather than waiting for relapses and disease progression to occur,” he added.
Drs. He and Gross have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Cautionary findings on acquired immunodeficiency from anti-CD20 MS therapy
, Brandi L. Vollmer, MPH, reported online as part of the 2020 American Academy of Neurology Science Highlights.
The hypogammaglobulinemia was preceded by an IgM of 40 mg/dL or less in 35% of cases and was accompanied by concurrent development of low IgM in another 39%, added Ms. Vollmer, a professional research assistant at the Rocky Mountain Multiple Sclerosis Center at Anschutz Medical Campus, University of Colorado, Denver.
She presented a retrospective study of 527 randomly selected MS patients and another 17 with neuromyelitis optica spectrum disorder who averaged 44 years of age and a 9.2-year disease duration upon commencing rituximab (Rituxan) with close laboratory monitoring. Their mean cumulative rituximab dose during a mean 30.2 months of therapy was 3,312 mg. Ninety-six MS patients eventually switched to ocrelizumab (Ocrevus), accumulating a total dose of 1,175 mg of that anti-CD20 humanized monoclonal antibody.
Absolute lymphocyte count dropped to 500 cells/mm3 or lower in 10.4% of patients at a mean of 11.3 months into anti-CD20 therapy. Low immunoglobulins came later: The mean time to onset of low IgM in affected patients was 19.7 months, and hypogammaglobulinemia, as defined by an IgG of 500 mg/dL or less, occurred at a mean of 36.1 months. Higher cumulative doses of anti-CD20 agents were associated with increased likelihood of hypogammaglobulinemia.
Asked to comment on the research findings, neurologist Nida Laurin, MD, said the Colorado study provides helpful insights into the timing of onset of acquired immunodeficiency in patients on B-cell-targeted therapy.
“This paper informs us that we should monitor our patients much closer for signs of hypogammaglobulinemia and lymphopenia starting with year 2 on therapy, and switch treatment when the threshold is reached. I do expect production of gamma globulins and lymphocytes to recover with discontinuation of anti-CD20 therapy, maybe over a period of 6-10 months. It might also recover with lower-dose therapy because the effect on B cells is dose-dependent,” observed Dr. Laurin, an MS specialist at the Banner Health–University Medicine Neuroscience Institute in Phoenix and the University of Arizona in Tucson.
Her colleague Barry Hendin, MD, noted that there is no consensus regarding the best response to all these changes.
“Some clinicians add IVIG, some change therapies, and some observe only,” said Dr. Hendin, a neurologist at Banner Health–University Medical Center, Phoenix, and clinical professor of neurology at the University of Arizona in Tucson.
However, Dr. Laurin asserted that it would be a mistake for physicians and patients to shrug off anti-CD20 therapy–induced lymphopenia in light of studies demonstrating that lymphopenia and older age are two main risk factors for progressive multifocal leukoencephalopathy in patients on disease-modifying therapies.
“More cases of PML can be expected with continuous use of anti-CD20 therapies if lymphopenia is ignored,” she cautioned.
Depressed levels of IgM and IgG have been associated with increased risk of serious infections. In light of the COVID-19 pandemic and the eventual prospect of a vaccine, it is especially important to avoid putting patients with MS in harm’s way via treatment-induced acquired immunodeficiency, Dr. Laurin said.
Ms. Vollmer reported having no financial conflicts regarding her study. Dr. Laurin reported serving as a speaker or consultant for Alexion, Allergan, Biogen, Bristol-Myers Squibb, EMD Serono, Genentech, Lundbeck, and Sanofi Genzyme. Dr. Hendin serves as a consultant to Biogen, Genentech, Genzyme, EMD Serono, Novartis, and Bristol-Myers Squibb.
SOURCE: Vollmer BL et al. AAN 2020. Abstract S29.002.
, Brandi L. Vollmer, MPH, reported online as part of the 2020 American Academy of Neurology Science Highlights.
The hypogammaglobulinemia was preceded by an IgM of 40 mg/dL or less in 35% of cases and was accompanied by concurrent development of low IgM in another 39%, added Ms. Vollmer, a professional research assistant at the Rocky Mountain Multiple Sclerosis Center at Anschutz Medical Campus, University of Colorado, Denver.
She presented a retrospective study of 527 randomly selected MS patients and another 17 with neuromyelitis optica spectrum disorder who averaged 44 years of age and a 9.2-year disease duration upon commencing rituximab (Rituxan) with close laboratory monitoring. Their mean cumulative rituximab dose during a mean 30.2 months of therapy was 3,312 mg. Ninety-six MS patients eventually switched to ocrelizumab (Ocrevus), accumulating a total dose of 1,175 mg of that anti-CD20 humanized monoclonal antibody.
Absolute lymphocyte count dropped to 500 cells/mm3 or lower in 10.4% of patients at a mean of 11.3 months into anti-CD20 therapy. Low immunoglobulins came later: The mean time to onset of low IgM in affected patients was 19.7 months, and hypogammaglobulinemia, as defined by an IgG of 500 mg/dL or less, occurred at a mean of 36.1 months. Higher cumulative doses of anti-CD20 agents were associated with increased likelihood of hypogammaglobulinemia.
Asked to comment on the research findings, neurologist Nida Laurin, MD, said the Colorado study provides helpful insights into the timing of onset of acquired immunodeficiency in patients on B-cell-targeted therapy.
“This paper informs us that we should monitor our patients much closer for signs of hypogammaglobulinemia and lymphopenia starting with year 2 on therapy, and switch treatment when the threshold is reached. I do expect production of gamma globulins and lymphocytes to recover with discontinuation of anti-CD20 therapy, maybe over a period of 6-10 months. It might also recover with lower-dose therapy because the effect on B cells is dose-dependent,” observed Dr. Laurin, an MS specialist at the Banner Health–University Medicine Neuroscience Institute in Phoenix and the University of Arizona in Tucson.
Her colleague Barry Hendin, MD, noted that there is no consensus regarding the best response to all these changes.
“Some clinicians add IVIG, some change therapies, and some observe only,” said Dr. Hendin, a neurologist at Banner Health–University Medical Center, Phoenix, and clinical professor of neurology at the University of Arizona in Tucson.
However, Dr. Laurin asserted that it would be a mistake for physicians and patients to shrug off anti-CD20 therapy–induced lymphopenia in light of studies demonstrating that lymphopenia and older age are two main risk factors for progressive multifocal leukoencephalopathy in patients on disease-modifying therapies.
“More cases of PML can be expected with continuous use of anti-CD20 therapies if lymphopenia is ignored,” she cautioned.
Depressed levels of IgM and IgG have been associated with increased risk of serious infections. In light of the COVID-19 pandemic and the eventual prospect of a vaccine, it is especially important to avoid putting patients with MS in harm’s way via treatment-induced acquired immunodeficiency, Dr. Laurin said.
Ms. Vollmer reported having no financial conflicts regarding her study. Dr. Laurin reported serving as a speaker or consultant for Alexion, Allergan, Biogen, Bristol-Myers Squibb, EMD Serono, Genentech, Lundbeck, and Sanofi Genzyme. Dr. Hendin serves as a consultant to Biogen, Genentech, Genzyme, EMD Serono, Novartis, and Bristol-Myers Squibb.
SOURCE: Vollmer BL et al. AAN 2020. Abstract S29.002.
, Brandi L. Vollmer, MPH, reported online as part of the 2020 American Academy of Neurology Science Highlights.
The hypogammaglobulinemia was preceded by an IgM of 40 mg/dL or less in 35% of cases and was accompanied by concurrent development of low IgM in another 39%, added Ms. Vollmer, a professional research assistant at the Rocky Mountain Multiple Sclerosis Center at Anschutz Medical Campus, University of Colorado, Denver.
She presented a retrospective study of 527 randomly selected MS patients and another 17 with neuromyelitis optica spectrum disorder who averaged 44 years of age and a 9.2-year disease duration upon commencing rituximab (Rituxan) with close laboratory monitoring. Their mean cumulative rituximab dose during a mean 30.2 months of therapy was 3,312 mg. Ninety-six MS patients eventually switched to ocrelizumab (Ocrevus), accumulating a total dose of 1,175 mg of that anti-CD20 humanized monoclonal antibody.
Absolute lymphocyte count dropped to 500 cells/mm3 or lower in 10.4% of patients at a mean of 11.3 months into anti-CD20 therapy. Low immunoglobulins came later: The mean time to onset of low IgM in affected patients was 19.7 months, and hypogammaglobulinemia, as defined by an IgG of 500 mg/dL or less, occurred at a mean of 36.1 months. Higher cumulative doses of anti-CD20 agents were associated with increased likelihood of hypogammaglobulinemia.
Asked to comment on the research findings, neurologist Nida Laurin, MD, said the Colorado study provides helpful insights into the timing of onset of acquired immunodeficiency in patients on B-cell-targeted therapy.
“This paper informs us that we should monitor our patients much closer for signs of hypogammaglobulinemia and lymphopenia starting with year 2 on therapy, and switch treatment when the threshold is reached. I do expect production of gamma globulins and lymphocytes to recover with discontinuation of anti-CD20 therapy, maybe over a period of 6-10 months. It might also recover with lower-dose therapy because the effect on B cells is dose-dependent,” observed Dr. Laurin, an MS specialist at the Banner Health–University Medicine Neuroscience Institute in Phoenix and the University of Arizona in Tucson.
Her colleague Barry Hendin, MD, noted that there is no consensus regarding the best response to all these changes.
“Some clinicians add IVIG, some change therapies, and some observe only,” said Dr. Hendin, a neurologist at Banner Health–University Medical Center, Phoenix, and clinical professor of neurology at the University of Arizona in Tucson.
However, Dr. Laurin asserted that it would be a mistake for physicians and patients to shrug off anti-CD20 therapy–induced lymphopenia in light of studies demonstrating that lymphopenia and older age are two main risk factors for progressive multifocal leukoencephalopathy in patients on disease-modifying therapies.
“More cases of PML can be expected with continuous use of anti-CD20 therapies if lymphopenia is ignored,” she cautioned.
Depressed levels of IgM and IgG have been associated with increased risk of serious infections. In light of the COVID-19 pandemic and the eventual prospect of a vaccine, it is especially important to avoid putting patients with MS in harm’s way via treatment-induced acquired immunodeficiency, Dr. Laurin said.
Ms. Vollmer reported having no financial conflicts regarding her study. Dr. Laurin reported serving as a speaker or consultant for Alexion, Allergan, Biogen, Bristol-Myers Squibb, EMD Serono, Genentech, Lundbeck, and Sanofi Genzyme. Dr. Hendin serves as a consultant to Biogen, Genentech, Genzyme, EMD Serono, Novartis, and Bristol-Myers Squibb.
SOURCE: Vollmer BL et al. AAN 2020. Abstract S29.002.
REPORTING FROM AAN 2020
Five-year siponimod data support early MS treatment
Among and had a lower annualized relapse rate up to 5 years later, compared with patients who initially received placebo and switched to siponimod. This research was presented online as part of the 2020 American Academy of Neurology Science Highlights.
Benefits of siponimod gained during the controlled period were “sustained for up to 5 years, suggesting a continuous effect of siponimod and underlining the advantages of early treatment initiation with siponimod,” the researchers said. Incidence rates of adverse events during the extension study were consistent with those during the controlled treatment period.
The results “highlight the critical importance of early treatment intervention ... to ensure the best possible long-term outcomes for patients with MS who are experiencing progression,” study investigator Bruce Cree, MD, PhD, said in a news release.
“It’s never too early to stay ahead of progression in MS, since the early identification of physical and cognitive changes – even subtle ones – can indicate MS disease progression and therefore allow for timely intervention.” said Dr. Cree, who is clinical research director and George A. Zimmermann Endowed Professor in Multiple Sclerosis at the University of California, San Francisco.
Siponimod, marketed as Mayzent, is a sphingosine 1-phosphate receptor modulator that selectively binds to S1P1 and S1P5 receptors. The oral drug was approved by the Food and Drug Administration in 2019 for the treatment of relapsing forms of MS, including clinically isolated syndrome, relapsing remitting disease, and active secondary progressive disease in adults.
To assess the long-term efficacy and safety of siponimod in patients with secondary progressive MS, Dr. Cree and colleagues analyzed data from patients in the controlled and extension parts of the EXPAND trial. Patients could have had been in the study for as long as 5 years at the data cutoff in April 2019. Efficacy analyses included time to 3-month confirmed disability progression on the Expanded Disability Status Scale (EDSS), time to 6-month confirmed disability progression, time to 6-month confirmed worsening of 4 or more points on the Symbol Digit Modalities Test (SDMT), and annualized relapse rate. In EXPAND, the researchers defined confirmed disability progression as a 1-point increase in EDSS if the baseline score was 3.0-5.0, or a 0.5-point increase if the baseline score was 5.5-6.5.
“Of the 1,224 (74% of 1,651 randomized) patients entering the extension, 878 (72%) were ongoing,” the researchers reported. Patients who received siponimod continuously were less likely to experience 3-month confirmed disability progression and 6-month confirmed disability progression, relative to patients who switched from placebo. In addition, patients who received continuous siponimod treatment had a prolonged time to 6-month confirmed disability progression, compared with patients who switched from placebo. For the 25th percentile of patients, continuous siponimod treatment corresponded to a delay of 54% (21 months vs. 13.6 months). Risk of worsening on the SDMT was reduced by 23% in the continuous siponimod–treatment group. For the 25th percentile of patients, this reduced risk corresponded to a delay of 62% (29.6 months vs. 18.3 months). ARR was 0.054 in the continuous-siponimod group, compared with 0.097 in the group that switched to siponimod from placebo, a reduction of 52%.
Dr. Cree has received personal compensation from Novartis, which markets siponimod, as well as Akili, Alexion, Atara, Biogen, EMD Serono, and TG Therapeutics. His coauthors reported receiving research support and personal compensation from Novartis and other pharmaceutical companies. Several coauthors were Novartis employees.
SOURCE: Kappos L et al. AAN 2020, Abstract S40.003.
Among and had a lower annualized relapse rate up to 5 years later, compared with patients who initially received placebo and switched to siponimod. This research was presented online as part of the 2020 American Academy of Neurology Science Highlights.
Benefits of siponimod gained during the controlled period were “sustained for up to 5 years, suggesting a continuous effect of siponimod and underlining the advantages of early treatment initiation with siponimod,” the researchers said. Incidence rates of adverse events during the extension study were consistent with those during the controlled treatment period.
The results “highlight the critical importance of early treatment intervention ... to ensure the best possible long-term outcomes for patients with MS who are experiencing progression,” study investigator Bruce Cree, MD, PhD, said in a news release.
“It’s never too early to stay ahead of progression in MS, since the early identification of physical and cognitive changes – even subtle ones – can indicate MS disease progression and therefore allow for timely intervention.” said Dr. Cree, who is clinical research director and George A. Zimmermann Endowed Professor in Multiple Sclerosis at the University of California, San Francisco.
Siponimod, marketed as Mayzent, is a sphingosine 1-phosphate receptor modulator that selectively binds to S1P1 and S1P5 receptors. The oral drug was approved by the Food and Drug Administration in 2019 for the treatment of relapsing forms of MS, including clinically isolated syndrome, relapsing remitting disease, and active secondary progressive disease in adults.
To assess the long-term efficacy and safety of siponimod in patients with secondary progressive MS, Dr. Cree and colleagues analyzed data from patients in the controlled and extension parts of the EXPAND trial. Patients could have had been in the study for as long as 5 years at the data cutoff in April 2019. Efficacy analyses included time to 3-month confirmed disability progression on the Expanded Disability Status Scale (EDSS), time to 6-month confirmed disability progression, time to 6-month confirmed worsening of 4 or more points on the Symbol Digit Modalities Test (SDMT), and annualized relapse rate. In EXPAND, the researchers defined confirmed disability progression as a 1-point increase in EDSS if the baseline score was 3.0-5.0, or a 0.5-point increase if the baseline score was 5.5-6.5.
“Of the 1,224 (74% of 1,651 randomized) patients entering the extension, 878 (72%) were ongoing,” the researchers reported. Patients who received siponimod continuously were less likely to experience 3-month confirmed disability progression and 6-month confirmed disability progression, relative to patients who switched from placebo. In addition, patients who received continuous siponimod treatment had a prolonged time to 6-month confirmed disability progression, compared with patients who switched from placebo. For the 25th percentile of patients, continuous siponimod treatment corresponded to a delay of 54% (21 months vs. 13.6 months). Risk of worsening on the SDMT was reduced by 23% in the continuous siponimod–treatment group. For the 25th percentile of patients, this reduced risk corresponded to a delay of 62% (29.6 months vs. 18.3 months). ARR was 0.054 in the continuous-siponimod group, compared with 0.097 in the group that switched to siponimod from placebo, a reduction of 52%.
Dr. Cree has received personal compensation from Novartis, which markets siponimod, as well as Akili, Alexion, Atara, Biogen, EMD Serono, and TG Therapeutics. His coauthors reported receiving research support and personal compensation from Novartis and other pharmaceutical companies. Several coauthors were Novartis employees.
SOURCE: Kappos L et al. AAN 2020, Abstract S40.003.
Among and had a lower annualized relapse rate up to 5 years later, compared with patients who initially received placebo and switched to siponimod. This research was presented online as part of the 2020 American Academy of Neurology Science Highlights.
Benefits of siponimod gained during the controlled period were “sustained for up to 5 years, suggesting a continuous effect of siponimod and underlining the advantages of early treatment initiation with siponimod,” the researchers said. Incidence rates of adverse events during the extension study were consistent with those during the controlled treatment period.
The results “highlight the critical importance of early treatment intervention ... to ensure the best possible long-term outcomes for patients with MS who are experiencing progression,” study investigator Bruce Cree, MD, PhD, said in a news release.
“It’s never too early to stay ahead of progression in MS, since the early identification of physical and cognitive changes – even subtle ones – can indicate MS disease progression and therefore allow for timely intervention.” said Dr. Cree, who is clinical research director and George A. Zimmermann Endowed Professor in Multiple Sclerosis at the University of California, San Francisco.
Siponimod, marketed as Mayzent, is a sphingosine 1-phosphate receptor modulator that selectively binds to S1P1 and S1P5 receptors. The oral drug was approved by the Food and Drug Administration in 2019 for the treatment of relapsing forms of MS, including clinically isolated syndrome, relapsing remitting disease, and active secondary progressive disease in adults.
To assess the long-term efficacy and safety of siponimod in patients with secondary progressive MS, Dr. Cree and colleagues analyzed data from patients in the controlled and extension parts of the EXPAND trial. Patients could have had been in the study for as long as 5 years at the data cutoff in April 2019. Efficacy analyses included time to 3-month confirmed disability progression on the Expanded Disability Status Scale (EDSS), time to 6-month confirmed disability progression, time to 6-month confirmed worsening of 4 or more points on the Symbol Digit Modalities Test (SDMT), and annualized relapse rate. In EXPAND, the researchers defined confirmed disability progression as a 1-point increase in EDSS if the baseline score was 3.0-5.0, or a 0.5-point increase if the baseline score was 5.5-6.5.
“Of the 1,224 (74% of 1,651 randomized) patients entering the extension, 878 (72%) were ongoing,” the researchers reported. Patients who received siponimod continuously were less likely to experience 3-month confirmed disability progression and 6-month confirmed disability progression, relative to patients who switched from placebo. In addition, patients who received continuous siponimod treatment had a prolonged time to 6-month confirmed disability progression, compared with patients who switched from placebo. For the 25th percentile of patients, continuous siponimod treatment corresponded to a delay of 54% (21 months vs. 13.6 months). Risk of worsening on the SDMT was reduced by 23% in the continuous siponimod–treatment group. For the 25th percentile of patients, this reduced risk corresponded to a delay of 62% (29.6 months vs. 18.3 months). ARR was 0.054 in the continuous-siponimod group, compared with 0.097 in the group that switched to siponimod from placebo, a reduction of 52%.
Dr. Cree has received personal compensation from Novartis, which markets siponimod, as well as Akili, Alexion, Atara, Biogen, EMD Serono, and TG Therapeutics. His coauthors reported receiving research support and personal compensation from Novartis and other pharmaceutical companies. Several coauthors were Novartis employees.
SOURCE: Kappos L et al. AAN 2020, Abstract S40.003.
FROM AAN 2020
Report details first case of PML with ocrelizumab alone
The case report was presented online as part of the 2020 American Academy of Neurology Science Highlights.
PML, an opportunistic infection of the brain caused by reactivation of the John Cunningham (JC) virus, has occurred with rituximab, another anti-CD20 therapy, in rare cases. Eight other cases of PML diagnosed after ocrelizumab initiation are considered carry-over cases related to prior treatment with natalizumab or fingolimod, according to Genentech, the manufacturer of ocrelizumab. No other PML cases have been associated with ocrelizumab alone.
The case without prior MS treatment was in a 78-year-old man. He presented with 2 weeks of progressive visual disturbance and confusion, said Marc L. Gordon, MD, chief of neurology at the Zucker Hillside Hospital in Glen Oaks, N.Y., and professor of neurology and psychiatry at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell in Hempstead, N.Y.
The patient had a right homonymous hemianopia. “MRI revealed an enlarging non-enhancing left parietal lesion without mass effect,” reported Dr. Gordon and colleagues. “CSF PCR revealed 1,000 copies/mL of JCV, confirming the diagnosis of PML. Blood work upon diagnosis revealed grade-2 lymphopenia ... and negative HIV serology.”
“The patient’s symptoms progressed over weeks to involve bilateral visual loss, right facial droop, and dysphasia,” they said. “Ocrelizumab was discontinued and off-label pembrolizumab treatment was initiated.” The patient did not respond to therapy and became bedbound, Dr. Gordon said in an interview. The patient received palliative care and died. An autopsy is pending.
“PML occurrence may have been multifactorial, due to a combination of the immunomodulatory function of ocrelizumab, possible immune senescence, and preceding mild lymphopenia,” Dr. Gordon and coauthors said. “This case emphasizes the importance of a thorough discussion of the risks and benefits of ocrelizumab, especially in patients at higher risk for infections, such as the elderly.”
The patient, who was Dr. Gordon’s patient for 20 years, had monitored updates in drug development and had looked forward to starting ocrelizumab, the first therapy approved for primary progressive MS, when it became available after its approval in 2017. The patient was concerned about progressively worsening gait impairment and related falls caused by MS.
Antibodies indicated that the patient had prior exposure to JCV, but Dr. Gordon considered the risk of PML to be relatively small. Prior to treatment, the patient’s absolute lymphocyte count was normal or indicated mild lymphocytopenia, which Dr. Gordon did not consider clinically significant. The patient received ocrelizumab for 2 years without incident.
In an information sheet for health care professionals about ocrelizumab and PML prepared in February 2020, Genentech says the patient’s age and low absolute lymphocyte count are confounding factors, which distort “the assessment of association between exposure to a drug and an adverse event.
“As of January 31, 2020, no unconfounded PML cases associated with ocrelizumab therapy have been reported,” according to the document. “Out of more than 150,000 patients treated globally (clinical trials and post-marketing experience), there have been nine confirmed, confounded cases of PML in patients treated with ocrelizumab, of which eight were carry-over cases from a prior DMT.”
The prescribing information for the drug notes that no cases of PML were identified in ocrelizumab clinical trials, but that PML has been observed in patients treated with other anti-CD20 antibodies and other MS therapies. In addition, PML “has been associated with some risk factors (eg, immunocompromised patients, polytherapy with immunosuppressants).
“At the first sign or symptom suggestive of PML, withhold ocrelizumab and perform an appropriate diagnostic evaluation,” the prescribing information says. “MRI findings may be apparent before clinical signs or symptoms. Typical symptoms associated with PML are diverse, progress over days to weeks, and include progressive weakness on one side of the body or clumsiness of limbs, disturbance of vision, and changes in thinking, memory, and orientation leading to confusion and personality changes.”
“It is important for people to recognize that this is at least a possibility,” Dr. Gordon said. Any change in clinical symptomatology may warrant imaging, and CSF testing may be warranted if an MRI raises concerns about PML, he said.
Dr. Gordon has received research support from MSD (Merck), Eisai, AbbVie, and Janssen.
SOURCE: Sul J et al. AAN 2020. Abstract S29.001.
The case report was presented online as part of the 2020 American Academy of Neurology Science Highlights.
PML, an opportunistic infection of the brain caused by reactivation of the John Cunningham (JC) virus, has occurred with rituximab, another anti-CD20 therapy, in rare cases. Eight other cases of PML diagnosed after ocrelizumab initiation are considered carry-over cases related to prior treatment with natalizumab or fingolimod, according to Genentech, the manufacturer of ocrelizumab. No other PML cases have been associated with ocrelizumab alone.
The case without prior MS treatment was in a 78-year-old man. He presented with 2 weeks of progressive visual disturbance and confusion, said Marc L. Gordon, MD, chief of neurology at the Zucker Hillside Hospital in Glen Oaks, N.Y., and professor of neurology and psychiatry at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell in Hempstead, N.Y.
The patient had a right homonymous hemianopia. “MRI revealed an enlarging non-enhancing left parietal lesion without mass effect,” reported Dr. Gordon and colleagues. “CSF PCR revealed 1,000 copies/mL of JCV, confirming the diagnosis of PML. Blood work upon diagnosis revealed grade-2 lymphopenia ... and negative HIV serology.”
“The patient’s symptoms progressed over weeks to involve bilateral visual loss, right facial droop, and dysphasia,” they said. “Ocrelizumab was discontinued and off-label pembrolizumab treatment was initiated.” The patient did not respond to therapy and became bedbound, Dr. Gordon said in an interview. The patient received palliative care and died. An autopsy is pending.
“PML occurrence may have been multifactorial, due to a combination of the immunomodulatory function of ocrelizumab, possible immune senescence, and preceding mild lymphopenia,” Dr. Gordon and coauthors said. “This case emphasizes the importance of a thorough discussion of the risks and benefits of ocrelizumab, especially in patients at higher risk for infections, such as the elderly.”
The patient, who was Dr. Gordon’s patient for 20 years, had monitored updates in drug development and had looked forward to starting ocrelizumab, the first therapy approved for primary progressive MS, when it became available after its approval in 2017. The patient was concerned about progressively worsening gait impairment and related falls caused by MS.
Antibodies indicated that the patient had prior exposure to JCV, but Dr. Gordon considered the risk of PML to be relatively small. Prior to treatment, the patient’s absolute lymphocyte count was normal or indicated mild lymphocytopenia, which Dr. Gordon did not consider clinically significant. The patient received ocrelizumab for 2 years without incident.
In an information sheet for health care professionals about ocrelizumab and PML prepared in February 2020, Genentech says the patient’s age and low absolute lymphocyte count are confounding factors, which distort “the assessment of association between exposure to a drug and an adverse event.
“As of January 31, 2020, no unconfounded PML cases associated with ocrelizumab therapy have been reported,” according to the document. “Out of more than 150,000 patients treated globally (clinical trials and post-marketing experience), there have been nine confirmed, confounded cases of PML in patients treated with ocrelizumab, of which eight were carry-over cases from a prior DMT.”
The prescribing information for the drug notes that no cases of PML were identified in ocrelizumab clinical trials, but that PML has been observed in patients treated with other anti-CD20 antibodies and other MS therapies. In addition, PML “has been associated with some risk factors (eg, immunocompromised patients, polytherapy with immunosuppressants).
“At the first sign or symptom suggestive of PML, withhold ocrelizumab and perform an appropriate diagnostic evaluation,” the prescribing information says. “MRI findings may be apparent before clinical signs or symptoms. Typical symptoms associated with PML are diverse, progress over days to weeks, and include progressive weakness on one side of the body or clumsiness of limbs, disturbance of vision, and changes in thinking, memory, and orientation leading to confusion and personality changes.”
“It is important for people to recognize that this is at least a possibility,” Dr. Gordon said. Any change in clinical symptomatology may warrant imaging, and CSF testing may be warranted if an MRI raises concerns about PML, he said.
Dr. Gordon has received research support from MSD (Merck), Eisai, AbbVie, and Janssen.
SOURCE: Sul J et al. AAN 2020. Abstract S29.001.
The case report was presented online as part of the 2020 American Academy of Neurology Science Highlights.
PML, an opportunistic infection of the brain caused by reactivation of the John Cunningham (JC) virus, has occurred with rituximab, another anti-CD20 therapy, in rare cases. Eight other cases of PML diagnosed after ocrelizumab initiation are considered carry-over cases related to prior treatment with natalizumab or fingolimod, according to Genentech, the manufacturer of ocrelizumab. No other PML cases have been associated with ocrelizumab alone.
The case without prior MS treatment was in a 78-year-old man. He presented with 2 weeks of progressive visual disturbance and confusion, said Marc L. Gordon, MD, chief of neurology at the Zucker Hillside Hospital in Glen Oaks, N.Y., and professor of neurology and psychiatry at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell in Hempstead, N.Y.
The patient had a right homonymous hemianopia. “MRI revealed an enlarging non-enhancing left parietal lesion without mass effect,” reported Dr. Gordon and colleagues. “CSF PCR revealed 1,000 copies/mL of JCV, confirming the diagnosis of PML. Blood work upon diagnosis revealed grade-2 lymphopenia ... and negative HIV serology.”
“The patient’s symptoms progressed over weeks to involve bilateral visual loss, right facial droop, and dysphasia,” they said. “Ocrelizumab was discontinued and off-label pembrolizumab treatment was initiated.” The patient did not respond to therapy and became bedbound, Dr. Gordon said in an interview. The patient received palliative care and died. An autopsy is pending.
“PML occurrence may have been multifactorial, due to a combination of the immunomodulatory function of ocrelizumab, possible immune senescence, and preceding mild lymphopenia,” Dr. Gordon and coauthors said. “This case emphasizes the importance of a thorough discussion of the risks and benefits of ocrelizumab, especially in patients at higher risk for infections, such as the elderly.”
The patient, who was Dr. Gordon’s patient for 20 years, had monitored updates in drug development and had looked forward to starting ocrelizumab, the first therapy approved for primary progressive MS, when it became available after its approval in 2017. The patient was concerned about progressively worsening gait impairment and related falls caused by MS.
Antibodies indicated that the patient had prior exposure to JCV, but Dr. Gordon considered the risk of PML to be relatively small. Prior to treatment, the patient’s absolute lymphocyte count was normal or indicated mild lymphocytopenia, which Dr. Gordon did not consider clinically significant. The patient received ocrelizumab for 2 years without incident.
In an information sheet for health care professionals about ocrelizumab and PML prepared in February 2020, Genentech says the patient’s age and low absolute lymphocyte count are confounding factors, which distort “the assessment of association between exposure to a drug and an adverse event.
“As of January 31, 2020, no unconfounded PML cases associated with ocrelizumab therapy have been reported,” according to the document. “Out of more than 150,000 patients treated globally (clinical trials and post-marketing experience), there have been nine confirmed, confounded cases of PML in patients treated with ocrelizumab, of which eight were carry-over cases from a prior DMT.”
The prescribing information for the drug notes that no cases of PML were identified in ocrelizumab clinical trials, but that PML has been observed in patients treated with other anti-CD20 antibodies and other MS therapies. In addition, PML “has been associated with some risk factors (eg, immunocompromised patients, polytherapy with immunosuppressants).
“At the first sign or symptom suggestive of PML, withhold ocrelizumab and perform an appropriate diagnostic evaluation,” the prescribing information says. “MRI findings may be apparent before clinical signs or symptoms. Typical symptoms associated with PML are diverse, progress over days to weeks, and include progressive weakness on one side of the body or clumsiness of limbs, disturbance of vision, and changes in thinking, memory, and orientation leading to confusion and personality changes.”
“It is important for people to recognize that this is at least a possibility,” Dr. Gordon said. Any change in clinical symptomatology may warrant imaging, and CSF testing may be warranted if an MRI raises concerns about PML, he said.
Dr. Gordon has received research support from MSD (Merck), Eisai, AbbVie, and Janssen.
SOURCE: Sul J et al. AAN 2020. Abstract S29.001.
FROM AAN 2020
MS Highlights from ACTRIMS/ECTRIMS
This supplement to Neurology Reviews compiles news briefs from the 35th Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS), held in Stockholm, Sweden, and the 5th annual Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum, held in West Palm Beach, Florida.
Click here to read the supplement
This supplement to Neurology Reviews compiles news briefs from the 35th Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS), held in Stockholm, Sweden, and the 5th annual Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum, held in West Palm Beach, Florida.
Click here to read the supplement
This supplement to Neurology Reviews compiles news briefs from the 35th Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS), held in Stockholm, Sweden, and the 5th annual Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum, held in West Palm Beach, Florida.
Click here to read the supplement





