User login
Menstrual Changes Still Top Indicator of Reproductive Aging
Changes to the menstrual cycle remain the most important clinical indicators of women’s reproductive aging, according to new standardized staging criteria.
The changes identified by the most recent Stages of Reproductive Aging Workshop are now defined in 10 stages, whereas the 2001 STRAW criteria had 7. The new criteria, called STRAW-10 and published Feb. 16 in Menopause, offer more detail on the characteristics of flow and cycle length at each stage, along with corresponding endocrine changes. The article was published simultaneously in Climacteric, Fertility and Sterility, and the Journal of Clinical Endocrinology and Metabolism.
For example, the late reproductive stage is now subdivided into two stages, –3b and –3a, instead of only stage –3 as before (Menopause 2012 Feb. 16 [doi:10.1097/gme0b013c31824d8f40]). In stage –3b, flow remains regular, whereas in stage –3a there are subtle changes in flow and length of cycle.
The postmenopausal stage +1, identified in the earlier STRAW criteria, also has been subdivided into three lettered stages, with the endocrine changes and duration of each stage described.
Unlike the previous STRAW criteria, the menstrual changes identified in STRAW-10 are relevant to any healthy woman, regardless of ethnicity, age, body mass index, or lifestyle, researchers said, noting that although factors such as smoking status and BMI may affect the timing of menopause, the bleeding patterns remain reliable indicators of the reproductive stage.
"Despite the availability of blood tests and sonograms, the menstrual cycle remains the single best way to estimate where a woman is along the reproductive path," commented Dr. Margery Gass, one of the coauthors of the new criteria, the executive director of the North American Menopause Society, and the editor of Menopause.
"According to STRAW-10, most women (and their clinicians) can use the changes in their menstrual pattern to determine how close they are to menopause: late reproductive phase (subtle changes in cycle length and blood flow), early menopause transition (menstrual period 7 or more days early or late), and late menopause transition (the occurrence of more than 60 days between cycles)," Dr. Gass said in an interview.
STRAW-10 incorporates three biomarkers – anti-Müllerian hormone (AMH), inhibin B, and antral follicle count – that are not mentioned in the original STRAW criteria, along with follicle-stimulating hormone (FSH), which was included in the original criteria.
In developing the new criteria, an international group of 41 researchers, led by epidemiologist Siobán D. Harlow, Ph.D., of the University of Michigan, Ann Arbor, evaluated data from cohort studies of midlife women with the aim to incorporate the scientific findings of the past decade on ovarian aging and its endocrine and clinical indicators.
Although much has been learned since 2001 with regard to biomarkers, Dr. Harlow and colleagues emphasized that the biomarker criteria outlined in STRAW-10 must still be considered "supportive," in part because more research is needed and in part because of the invasiveness and expense of testing.
Dr. Gass commented that for clinicians, checking hormone levels should not be considered necessary "except in women who have undergone endometrial ablation or hysterectomy, or who have unusual health circumstances."
Women for whom the STRAW-10 criteria are not applicable include those with polycystic ovarian syndrome or hypothalamic amenorrhea. Women with chronic illnesses such as HIV-AIDS, or those who are undergoing certain types of cancer treatments, also are difficult to assess under STRAW-10, as cycles and hormone levels can change in response to medication.
Although the new criteria do not use age in determining reproductive staging, women younger than 40 years who have premature ovarian insufficiency or premature ovarian failure do not fit well under STRAW-10, the researchers noted, as their course of reproductive aging is more variable.
The STRAW-10 meetings received funding from the National Institutes of Health and the Department of Health and Human Services through the National Institute on Aging (NIA) and the Office of Research on Women’s Health, as well as the North American Menopause Society (NAMS), the American Society for Reproductive Medicine (ASRM), the International Menopause Society in Cape Town, South Africa, and the Endocrine Society.
Dr. Gass receives support from NAMS. Dr. Harlow disclosed support from the NIA and NAMS and the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Coauthor Dr. Janet E. Hall disclosed support from NIA and the Endocrine Society. Dr. Roger Lobo is a past president of the ASRM, and Dr. Robert W. Rebar disclosed salary support from ASRM. Pauline Maki, Ph.D., disclosed support from the NIA and other public sources along with board membership on NAMS, a consultant relationship with Noven Pharmaceuticals, and lecture fees from Pfizer and others. Dr. Tobie J. de Villiers disclosed past support from Adcock Ingram, Servier, Pfizer, Bayer, and Amgen without direct bearing on the STRAW-10 work. The remaining two coauthors said they had no relevant financial disclosures.
The main changes [in STRAW-10] have to do with the late reproductive stage and the early postmenopausal stage, which has been subdivided to give more detail, and in turn help understand the physiologic changes which occur at these times. In particular, the late reproductive stage has been subdivided so the measurement of the anti-Müllerian hormone (AMH) and follicle count can be used as a measure of decreased fertility, but the menstrual bleeding is still regular and unchanged. Inhibin B also may be low.
The next stage adds a change in menstrual pattern, usually shorter cycles, and also some variability in FSH levels early in the cycle (days 2-5). Unfortunately, there is no standardization for the AMH assay, so a quantitative measurement is not available.
The bottom line is that these definitions will be helpful mostly for research, but will only give us some guidance to counsel patients about their fertility.
In terms of the late reproductive stages, there now are three subdivisions based on stabilization of key hormones at this time (FSH and estradiol). Perimenopause also is better defined and the addition of symptoms with a description of bleeding patterns will be helpful both to researchers and clinicians. This will lead to more research and understanding of the key physiologic events that lead to reproductive decline and menopausal changes. There also is a description of what happens to reproduction with various dysfunctions, such as chemotherapy and in polycystic ovarian syndrome, which can be helpful both clinically and particularly in research.
Dr. Michelle Warren is professor of medicine and obstetrics and gynecology at Columbia University Medical Center, New York, and medical director of the Center for Menopause, Hormonal Disorders, and Women's Health at Columbia. She commented in an interview regarding the STRAW-10 findings. She disclosed ties with Pfizer and Yoplait, and Ascend Therapeutics.
The main changes [in STRAW-10] have to do with the late reproductive stage and the early postmenopausal stage, which has been subdivided to give more detail, and in turn help understand the physiologic changes which occur at these times. In particular, the late reproductive stage has been subdivided so the measurement of the anti-Müllerian hormone (AMH) and follicle count can be used as a measure of decreased fertility, but the menstrual bleeding is still regular and unchanged. Inhibin B also may be low.
The next stage adds a change in menstrual pattern, usually shorter cycles, and also some variability in FSH levels early in the cycle (days 2-5). Unfortunately, there is no standardization for the AMH assay, so a quantitative measurement is not available.
The bottom line is that these definitions will be helpful mostly for research, but will only give us some guidance to counsel patients about their fertility.
In terms of the late reproductive stages, there now are three subdivisions based on stabilization of key hormones at this time (FSH and estradiol). Perimenopause also is better defined and the addition of symptoms with a description of bleeding patterns will be helpful both to researchers and clinicians. This will lead to more research and understanding of the key physiologic events that lead to reproductive decline and menopausal changes. There also is a description of what happens to reproduction with various dysfunctions, such as chemotherapy and in polycystic ovarian syndrome, which can be helpful both clinically and particularly in research.
Dr. Michelle Warren is professor of medicine and obstetrics and gynecology at Columbia University Medical Center, New York, and medical director of the Center for Menopause, Hormonal Disorders, and Women's Health at Columbia. She commented in an interview regarding the STRAW-10 findings. She disclosed ties with Pfizer and Yoplait, and Ascend Therapeutics.
The main changes [in STRAW-10] have to do with the late reproductive stage and the early postmenopausal stage, which has been subdivided to give more detail, and in turn help understand the physiologic changes which occur at these times. In particular, the late reproductive stage has been subdivided so the measurement of the anti-Müllerian hormone (AMH) and follicle count can be used as a measure of decreased fertility, but the menstrual bleeding is still regular and unchanged. Inhibin B also may be low.
The next stage adds a change in menstrual pattern, usually shorter cycles, and also some variability in FSH levels early in the cycle (days 2-5). Unfortunately, there is no standardization for the AMH assay, so a quantitative measurement is not available.
The bottom line is that these definitions will be helpful mostly for research, but will only give us some guidance to counsel patients about their fertility.
In terms of the late reproductive stages, there now are three subdivisions based on stabilization of key hormones at this time (FSH and estradiol). Perimenopause also is better defined and the addition of symptoms with a description of bleeding patterns will be helpful both to researchers and clinicians. This will lead to more research and understanding of the key physiologic events that lead to reproductive decline and menopausal changes. There also is a description of what happens to reproduction with various dysfunctions, such as chemotherapy and in polycystic ovarian syndrome, which can be helpful both clinically and particularly in research.
Dr. Michelle Warren is professor of medicine and obstetrics and gynecology at Columbia University Medical Center, New York, and medical director of the Center for Menopause, Hormonal Disorders, and Women's Health at Columbia. She commented in an interview regarding the STRAW-10 findings. She disclosed ties with Pfizer and Yoplait, and Ascend Therapeutics.
Changes to the menstrual cycle remain the most important clinical indicators of women’s reproductive aging, according to new standardized staging criteria.
The changes identified by the most recent Stages of Reproductive Aging Workshop are now defined in 10 stages, whereas the 2001 STRAW criteria had 7. The new criteria, called STRAW-10 and published Feb. 16 in Menopause, offer more detail on the characteristics of flow and cycle length at each stage, along with corresponding endocrine changes. The article was published simultaneously in Climacteric, Fertility and Sterility, and the Journal of Clinical Endocrinology and Metabolism.
For example, the late reproductive stage is now subdivided into two stages, –3b and –3a, instead of only stage –3 as before (Menopause 2012 Feb. 16 [doi:10.1097/gme0b013c31824d8f40]). In stage –3b, flow remains regular, whereas in stage –3a there are subtle changes in flow and length of cycle.
The postmenopausal stage +1, identified in the earlier STRAW criteria, also has been subdivided into three lettered stages, with the endocrine changes and duration of each stage described.
Unlike the previous STRAW criteria, the menstrual changes identified in STRAW-10 are relevant to any healthy woman, regardless of ethnicity, age, body mass index, or lifestyle, researchers said, noting that although factors such as smoking status and BMI may affect the timing of menopause, the bleeding patterns remain reliable indicators of the reproductive stage.
"Despite the availability of blood tests and sonograms, the menstrual cycle remains the single best way to estimate where a woman is along the reproductive path," commented Dr. Margery Gass, one of the coauthors of the new criteria, the executive director of the North American Menopause Society, and the editor of Menopause.
"According to STRAW-10, most women (and their clinicians) can use the changes in their menstrual pattern to determine how close they are to menopause: late reproductive phase (subtle changes in cycle length and blood flow), early menopause transition (menstrual period 7 or more days early or late), and late menopause transition (the occurrence of more than 60 days between cycles)," Dr. Gass said in an interview.
STRAW-10 incorporates three biomarkers – anti-Müllerian hormone (AMH), inhibin B, and antral follicle count – that are not mentioned in the original STRAW criteria, along with follicle-stimulating hormone (FSH), which was included in the original criteria.
In developing the new criteria, an international group of 41 researchers, led by epidemiologist Siobán D. Harlow, Ph.D., of the University of Michigan, Ann Arbor, evaluated data from cohort studies of midlife women with the aim to incorporate the scientific findings of the past decade on ovarian aging and its endocrine and clinical indicators.
Although much has been learned since 2001 with regard to biomarkers, Dr. Harlow and colleagues emphasized that the biomarker criteria outlined in STRAW-10 must still be considered "supportive," in part because more research is needed and in part because of the invasiveness and expense of testing.
Dr. Gass commented that for clinicians, checking hormone levels should not be considered necessary "except in women who have undergone endometrial ablation or hysterectomy, or who have unusual health circumstances."
Women for whom the STRAW-10 criteria are not applicable include those with polycystic ovarian syndrome or hypothalamic amenorrhea. Women with chronic illnesses such as HIV-AIDS, or those who are undergoing certain types of cancer treatments, also are difficult to assess under STRAW-10, as cycles and hormone levels can change in response to medication.
Although the new criteria do not use age in determining reproductive staging, women younger than 40 years who have premature ovarian insufficiency or premature ovarian failure do not fit well under STRAW-10, the researchers noted, as their course of reproductive aging is more variable.
The STRAW-10 meetings received funding from the National Institutes of Health and the Department of Health and Human Services through the National Institute on Aging (NIA) and the Office of Research on Women’s Health, as well as the North American Menopause Society (NAMS), the American Society for Reproductive Medicine (ASRM), the International Menopause Society in Cape Town, South Africa, and the Endocrine Society.
Dr. Gass receives support from NAMS. Dr. Harlow disclosed support from the NIA and NAMS and the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Coauthor Dr. Janet E. Hall disclosed support from NIA and the Endocrine Society. Dr. Roger Lobo is a past president of the ASRM, and Dr. Robert W. Rebar disclosed salary support from ASRM. Pauline Maki, Ph.D., disclosed support from the NIA and other public sources along with board membership on NAMS, a consultant relationship with Noven Pharmaceuticals, and lecture fees from Pfizer and others. Dr. Tobie J. de Villiers disclosed past support from Adcock Ingram, Servier, Pfizer, Bayer, and Amgen without direct bearing on the STRAW-10 work. The remaining two coauthors said they had no relevant financial disclosures.
Changes to the menstrual cycle remain the most important clinical indicators of women’s reproductive aging, according to new standardized staging criteria.
The changes identified by the most recent Stages of Reproductive Aging Workshop are now defined in 10 stages, whereas the 2001 STRAW criteria had 7. The new criteria, called STRAW-10 and published Feb. 16 in Menopause, offer more detail on the characteristics of flow and cycle length at each stage, along with corresponding endocrine changes. The article was published simultaneously in Climacteric, Fertility and Sterility, and the Journal of Clinical Endocrinology and Metabolism.
For example, the late reproductive stage is now subdivided into two stages, –3b and –3a, instead of only stage –3 as before (Menopause 2012 Feb. 16 [doi:10.1097/gme0b013c31824d8f40]). In stage –3b, flow remains regular, whereas in stage –3a there are subtle changes in flow and length of cycle.
The postmenopausal stage +1, identified in the earlier STRAW criteria, also has been subdivided into three lettered stages, with the endocrine changes and duration of each stage described.
Unlike the previous STRAW criteria, the menstrual changes identified in STRAW-10 are relevant to any healthy woman, regardless of ethnicity, age, body mass index, or lifestyle, researchers said, noting that although factors such as smoking status and BMI may affect the timing of menopause, the bleeding patterns remain reliable indicators of the reproductive stage.
"Despite the availability of blood tests and sonograms, the menstrual cycle remains the single best way to estimate where a woman is along the reproductive path," commented Dr. Margery Gass, one of the coauthors of the new criteria, the executive director of the North American Menopause Society, and the editor of Menopause.
"According to STRAW-10, most women (and their clinicians) can use the changes in their menstrual pattern to determine how close they are to menopause: late reproductive phase (subtle changes in cycle length and blood flow), early menopause transition (menstrual period 7 or more days early or late), and late menopause transition (the occurrence of more than 60 days between cycles)," Dr. Gass said in an interview.
STRAW-10 incorporates three biomarkers – anti-Müllerian hormone (AMH), inhibin B, and antral follicle count – that are not mentioned in the original STRAW criteria, along with follicle-stimulating hormone (FSH), which was included in the original criteria.
In developing the new criteria, an international group of 41 researchers, led by epidemiologist Siobán D. Harlow, Ph.D., of the University of Michigan, Ann Arbor, evaluated data from cohort studies of midlife women with the aim to incorporate the scientific findings of the past decade on ovarian aging and its endocrine and clinical indicators.
Although much has been learned since 2001 with regard to biomarkers, Dr. Harlow and colleagues emphasized that the biomarker criteria outlined in STRAW-10 must still be considered "supportive," in part because more research is needed and in part because of the invasiveness and expense of testing.
Dr. Gass commented that for clinicians, checking hormone levels should not be considered necessary "except in women who have undergone endometrial ablation or hysterectomy, or who have unusual health circumstances."
Women for whom the STRAW-10 criteria are not applicable include those with polycystic ovarian syndrome or hypothalamic amenorrhea. Women with chronic illnesses such as HIV-AIDS, or those who are undergoing certain types of cancer treatments, also are difficult to assess under STRAW-10, as cycles and hormone levels can change in response to medication.
Although the new criteria do not use age in determining reproductive staging, women younger than 40 years who have premature ovarian insufficiency or premature ovarian failure do not fit well under STRAW-10, the researchers noted, as their course of reproductive aging is more variable.
The STRAW-10 meetings received funding from the National Institutes of Health and the Department of Health and Human Services through the National Institute on Aging (NIA) and the Office of Research on Women’s Health, as well as the North American Menopause Society (NAMS), the American Society for Reproductive Medicine (ASRM), the International Menopause Society in Cape Town, South Africa, and the Endocrine Society.
Dr. Gass receives support from NAMS. Dr. Harlow disclosed support from the NIA and NAMS and the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Coauthor Dr. Janet E. Hall disclosed support from NIA and the Endocrine Society. Dr. Roger Lobo is a past president of the ASRM, and Dr. Robert W. Rebar disclosed salary support from ASRM. Pauline Maki, Ph.D., disclosed support from the NIA and other public sources along with board membership on NAMS, a consultant relationship with Noven Pharmaceuticals, and lecture fees from Pfizer and others. Dr. Tobie J. de Villiers disclosed past support from Adcock Ingram, Servier, Pfizer, Bayer, and Amgen without direct bearing on the STRAW-10 work. The remaining two coauthors said they had no relevant financial disclosures.
FROM MENOPAUSE
Vulvar pain syndromes: Causes and treatment of vestibulodynia
- Part 1: Making the correct diagnosis
(September 2011) - Part 2: A bounty of treatments—but not all of them are proven
(October 2011)
This three-part series concludes with a look at vestibulodynia—pain that is localized to the vulvar vestibule. Much is known about this disorder, compared with our knowledge base in the recent past, but much remains to be discovered. Among the questions explored by the panelists in this article is whether vestibulodynia and generalized vulvodynia are distinct entities—or different manifestations of the same process.
Other questions addressed here:
- Do oral contraceptives (OCs) contribute to vestibulodynia?
- What about herpes and genital warts? Are they causes of vestibular pain?
- Are some women more vulnerable to vestibulodynia than others?
- Is the disorder curable?
- Does vestibulectomy provide definitive treatment?
Part 1 of this series, which appeared in the September 2011 issue, focused on generalized vulvar pain and its causes, features, and diagnosis. Part 2, in the October issue, took as its subject the treatment of vulvar pain. Both are available in the archive at obgmanagement.com.
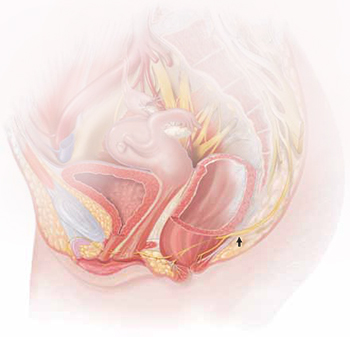
The lower vagina and vulva are richly supplied with peripheral nerves and are, therefore, sensitive to pain, particularly the region of the hymeneal ring. Although the pudendal nerve (arrow) courses through the area, it is an uncommon source of vulvar pain.
What do we know about the causes of vestibulodynia?
Dr. Lonky: What are the causes of provoked vestibulodynia (PVD), also known as vulvar vestibulitis syndrome? And what are the theories behind those causes?
Dr. Haefner: The specific cause is unknown. Most likely, there isn’t a single cause. Theories that have been proposed include abnormalities of embryologic development, infection, inflammation, genetic and immune factors, and nerve pathways.
Patients who have vestibulodynia may also have interstitial cystitis. It has been noted that tissues from the vestibule and bladder have a common embryologic origin and, therefore, are predisposed to similar pathologic responses when challenged.1,2
Candida albicans infection in patients who experience vestibular pain has also been studied. The exact association is difficult to determine because many patients report Candida infections without verified testing for yeast. Bazin and colleagues found a very weak association between infection and pain on the vestibule.3
Inflammation—the “itis” in vestibulitis—has been excluded from the recent International Society for the Study of Vulvovaginal Disease (ISSVD) terminology because studies found no association between excised tissue and inflammation. Bohm-Starke and colleagues found low expression of the inflammatory markers cyclooxygenase 2 and inducible nitric oxide synthase in the vestibular mucosa of women who had localized vestibular pain, as well as in healthy women in the control group.4
Goetsch was one of the first researchers to explore a genetic association with localized vulvar pain.5 Fifteen percent of patients questioned over a 6-month period were found to have localized vestibular pain. Thirty-two percent had a female relative who had dyspareunia or tampon intolerance, raising the issue of a genetic predisposition. Another genetic connection was found in a study evaluating gene coding for interleukin 1-receptor antagonist.6–8
Krantz examined the nerve characteristics of the vulva and vagina.9 The region of the hymeneal ring was richly supplied with free nerve endings. No corpuscular endings of any form were observed. Only free nerve endings were observed in the fossa navicularis. A sparsity of nerve endings occurred in the vagina, as compared with the region of the fourchette, fossa navicularis, and hymeneal ring. More recent studies have analyzed the nerve factors, thermoreceptors, and nociceptors in women with vulvar pain.10,11
Dr. Edwards: I feel strongly that vestibulodynia and generalized vulvodynia are the same process. For example, tension headaches are supposed to be occipital, but some people experience tension headaches that are periorbital. Both are tension headaches despite the different locations. And almost all patients who experience any subset of vulvodynia have provoked vestibular pain. So the only thing that separates vestibulodynia from other patterns of vulvodynia is the option of vestibulectomy for therapy.
I don’t think that vulvodynia and vestibulodynia are “wastebasket” names for undiagnosable vulvar pain; rather, they are specific disease processes produced by pelvic floor dysfunction that predisposes a woman to neuropathic pain with a trigger or to a systemic pain syndrome that includes an abnormal pelvic floor.
Dr. Gunter: There are probably many causes of PVD, as Dr. Haefner suggested. There may be an ignition hypothesis, whereby some outside inflammatory trigger or trauma produces local neurogenic inflammation. However, given the prevalence of other pain disorders, there is probably also a need to have a lowered threshold for these changes to occur—basically, a vulnerable neurologic platform.
For some women, local neural hyperplasia is probably a factor. It is possible that there are different causes for primary and secondary vestibulodynia.
Vulvodynia and depression often travel together. They are such common comorbidities, in fact, that some physicians theorize that vulvodynia may be a symptom of an underlying mood disorder, such as depression, or that depression may be one manifestation of chronic vulvar pain. Suffice it to say that chronic pain and depression are often associated, and it is frequently difficult to determine whether the relationship is one of cause and effect.
Comprehensive care of the patient who has vulvar pain, therefore, should include a thorough history, looking specifically for depression (including sleep disorders) and eliciting information on any suicidal thoughts or intentions.
Although many patients who have vulvodynia are treated with an antidepressant, the dose that relieves pain may not be high enough to attenuate an accompanying mood disorder. My approach is to team up with a psychiatrist or psychologist who is familiar with vulvar pain syndromes. Together, we monitor the patient and fine-tune the therapeutic response.
—Neal M. Lonky, MD, MPH
Do oral contraceptives contribute to vestibulodynia?
Dr. Lonky: Is PVD more prevalent among OC users?
Dr. Haefner: Controversy surrounds the question of whether vestibulodynia and OC use are linked. Some studies suggest no association12–14 and others suggest a possible effect of OCs on vulvodynia.15–17 A study by Reed and colleagues found no association between taking OCs or hormone therapy at enrollment and incident vulvodynia only in the univariable analysis, but not when controlling for age at enrollment.18 This reflects the finding that younger age was associated with incident disease; younger age and use of OCs are similarly associated.
Dr. Gunter: I am not a believer in a cause-and-effect relationship between OC use and vestibulodynia. I do not find the studies demonstrating an association convincing. Given the supraphysiologic levels of hormones during pregnancy, if high hormone levels played a role, we should also see a greater incidence of vestibulodynia among women who have several pregnancies at an early age.
Dr. Edwards: In my practice, stopping, starting, and changing OCs has made no difference for patients. Topical estrogen supplementation in the occasional OC user who has signs of low estrogen has been useful at times.
Do herpes or genital warts contribute to PVD?
Dr. Lonky: Does a history of vulvar herpes or genital warts have any impact on the incidence of PVD?
Dr. Edwards: No.
Dr. Gunter: I agree that it has no impact.
Dr. Haefner: Herpes is sometimes associated with vulvar pain. The lesions resolve, but pain may continue as post-herpetic neuralgia. As with shingles, a low threshold for starting a patient on gabapentin to control pain after herpes may be beneficial.
Genital warts rarely cause vulvar pain—but the treatment may. Patients sometimes feel pain following topical treatment, as well as pain from surgical wart treatment.
Effect of demographic variables
Dr. Lonky: Does race, skin type, or hair or eye color make a difference in the prevalence, manifestation of symptoms, or treatment of PVD?
Dr. Gunter: I am not aware of any studies that confirm an association between vulvodynia and those factors.
Dr. Edwards: I don’t know whether any of these variables make a difference. My own impression—confirmed by informal study in my office—is that vulvodynia patients weigh less than my general dermatology patients and are better educated. I sometimes get the sense that my vulvodynia patients are more likely to be fair.
Dr. Lonky: What age group is most commonly affected by PVD?
Dr. Edwards: In my experience the most common age group is women 25 to 45 years old, probably because they are the most sexually active group old enough and tough enough to pursue this issue.
Dr. Gunter: I believe it affects all women equally, although women in their reproductive years are more likely to visit a gynecologist and, therefore, probably more likely to be given this diagnosis.
Dr. Lonky: Do you believe that PVD and generalized vulvar dysesthesia are curable—or just treatable?
Dr. Gunter: That depends on many variables. It is far more challenging to cure a patient who has multiple pain syndromes (for example, fibromyalgia, migraines, and irritable bowel syndrome) than the woman who simply has vestibulodynia or generalized vulvar pain. In addition, stress, anxiety, coping skills, and depression all play a role. In my opinion, a woman without comorbidity has a good chance of having her symptoms well-controlled. Some will be cured (that is, able to discontinue medications), and others will need ongoing treatment but will not be bothered by their symptoms.
Dr. Haefner: The response to treatment in many pain patients depends on the amount of time that the pain has been present. Someone who has had pain for 30 years will probably not be cured 3 months after starting treatment. However, someone with a short duration of pain often gets good improvement. One hundred percent improvement is rare, however. Many patients are able to approach the 80% improvement mark.
Dr. Edwards: I would say that these conditions are manageable more than curable, although pure vestibulodynia—which is uncommon—is curable with surgery.
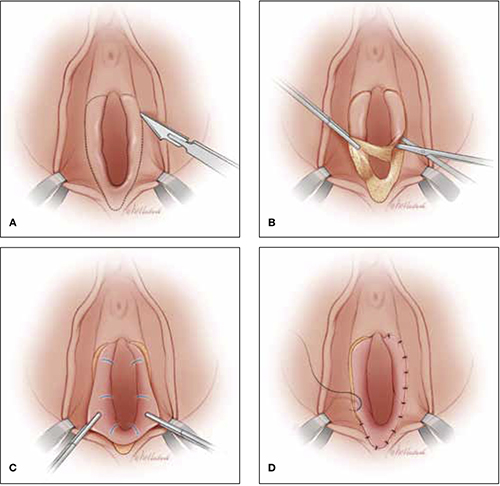
Vestibulectomy technique
(A) Incision. In many cases, the incision needs to extend up to the opening of the Skene’s ducts on the vestibule before it is carried down laterally along Hart’s line to the perianal skin, with the mucosa undermined above the hymeneal ring. (B) Excision. Remove the tissue superior to the hymeneal ring. (C) Advancement of vaginal mucosa. Further undermine the mucosa and advance it to close the defect. (D) Suturing. Close the defect in two layers using absorbable suture.
Is vestibulectomy definitive treatment?
Dr. Lonky: Does vestibulectomy work as a definitive treatment for PVD?
Dr. Gunter: Vestibulectomy—that is, resection of the vestibule and advancement of vaginal mucosa—is well described for women who have localized vestibulodynia. The complication rate is low, with only 3% of women reporting worsening of symptoms after the procedure.19,20 Success rates range from 17% to 89%, although many studies are retrospective reviews or include nonhomogenous populations, or both.19–21 A prospective study (Level II) indicates a 52% reduction in pain scores for women 6 months after vestibulectomy—and the scores continued to improve at evaluation at 2.5 years.22,23
Prospective studies indicate that vestibulectomy improves pain scores more than cognitive behavioral therapy, biofeedback, oral despiramine, and topical lidocaine.22,23 However, even surgery carries a robust placebo response rate, and vulvar biopsy alone has been associated with an improvement in pain scores in 67% of women.24,25
I offer surgery for vestibulodynia after the patient has failed at least two therapies (two topical treatments or one topical and one oral treatment). I offer injections before proceeding, and almost all patients opt to try them. I do not offer vestibulectomy to patients who have unprovoked pain or pain outside of resection margins.
Dr. Haefner: Surgical excision of the vulvar vestibule has met with success, in some studies, in more than 80% of cases, but it should be reserved for women who have longstanding and localized vestibular pain in whom other management options have failed.
The patient should undergo cotton-swab testing to outline areas of pain before anesthesia is administered in the operating room. Often, the incision will need to extend up to the opening of the Skene’s ducts on the vestibule (FIGURE). The incision is carried down laterally along Hart’s line to the perianal skin, and the mucosa is undermined above the hymeneal ring. The specimen is excised superior to the hymeneal ring. The vaginal tissue is further undermined and brought down to close the defect in two layers using absorbable suture. A review of this technique with illustrations has been published.26
Dr. Lonky: Thank you all again for sharing your considerable expertise, experience, and insight.
Harlow BL, Stewart EG. A population-based assessment of chronic unexplained vulvar pain: have we underestimated the prevalence of vulvodynia? J Am Med Womens Assoc. 2003;58(2):82–88.
In this population-based study, 16% of women reported a history of chronic unexplained vulvar pain, and nearly 7% reported current symptoms.
Khandker M, Brady SS, Vitonis AF, Maclehose RF, Stewart EG, Harlow BL. The influence of depression and anxiety on risk of adult onset vulvodynia [published online ahead of print August 8, 2011].
J Womens Health (Larchmt). doi:10.1089/jwh.2010.2661.
Vulvodynia was four times more likely among women who had antecedent mood or anxiety disorders than in women who didn’t. Vulvodynia was also associated with new or recurrent onset of mood or anxiety disorders.
Masheb RM, Wang E, Lozano C, Kerns RD. Prevalence and correlates of depression in treatment-seeking women with vulvodynia. J Obstet Gynaecol. 2005;25(8):786–791.
Comorbid major depressive disorders in women who have vulvodynia are related to greater pain severity and worse functioning.
Reed BD, Haefner HK, Punch MR, Roth RS, Gorenflo DW, Gillespie BW. Psychosocial and sexual functioning in women with vulvodynia and chronic pelvic pain. A comparative evaluation. J Reprod Med. 2000;45(8):624–632.
Women who have vulvodynia are psychologically similar to women who don’t. A primary psychological cause of vulvodynia is not supported.
Tribo MJ, Andion O, Ros S, et al. Clinical characteristics and psychopathological profile of patients with vulvodynia: an observational and descriptive study. Dermatology. 2008;216(1):24–30.
Psychiatric treatment may be a useful option to improve symptoms of vulvodynia.
We want to hear from you! Tell us what you think about this series on Vulvar Pain Syndromes.
1. McCormack WM. Two urogenital sinus syndromes. Interstitial cystitis and focal vulvitis. J Reprod Med. 1990;35(9):873-876.
2. Fitzpatrick CC, DeLancey JP, Elkins TE, McGuire EJ. Vulvar vestibulitis and interstitial cystitis: a disorder of urogenital sinusderived epithelium. Obstet Gynecol. 1993;81(5 Pt 2):860-862.
3. Bazin S, Bouchard C, Brisson J, Morin C, Meisels A, Fortier M. Vulvar vestibulitis syndrome: an exploratory case-control study. Obstet Gynecol. 1994;83(1):47-50.
4. Bohm-Starke N, Falconer C, Rylander E, Hilliges M. The expression of cyclooxygenase 2 and inducible nitric oxide synthase indicates no active inflammation in vulvar vestibulitis. Acta Obstet Gynecol Scand. 2001;80(7):638-644.
5. Goetsch MF. Vulvar vestibulitis: prevalence and historic features in a general gynecologic practice population. Am J Obstet Gynecol. 1991;164(6 Pt 1):1609-1616.
6. Jeremias J, Ledger WJ, Witkin SS. Interleukin 1 receptor antagonist gene polymorphism in women with vulvar vestibulitis. Am J Obstet Gynecol. 2000;182(2):283-285.
7. Witkin SS, Gerber S, Ledger WJ. Differential characterization of women with vulvar vestibulitis syndrome. Am J Obstet Gynecol. 2002;187(3):589-594.
8. Foster DC, Piekarz KH, Murant TI, LaPoint R, Haidaris CG, Phipps RP. Enhanced synthesis of proinflammatory cytokines by vulvar vestibular fibroblasts: implications for vulvar vestibulitis. Am J Obstet Gynecol. 2007;196(4):346. e1-8.
9. Krantz KE. Innervation of the human vulva and vagina: a microscopic study. Obstet Gynecol. 1958;12:382-396.
10. Bohm-Starke N, Hilliges M, Brodda-Jansen G, Rylander E, Torebjork E. Psychophysical evidence of nociceptor sensitization in vulvar vestibulitis syndrome. Pain. 2001;94(2):177-183.
11. Tympanidis P, Terenghi G, Dowd P. Increased innervation of the vulval vestibule in patients with vulvodynia. Br J Dermatol. 2003;148(5):1021-1027.
12. Bachman GA, Rosen R, Pinn VW, et al. Vulvodynia: a state-of-the-art consensus on definitions, diagnosis and management. J Reprod Med. 2006;51(6):447-456.
13. Harlow BL, Vitonis AF, Stewart EG. Influence of oral contraceptive use on the risk of adult-onset vulvodynia. J Reprod Med. 2008;53(2):102-110.
14. Danielsson I, Sjoberg I, Stenlund H, Wikman M. Prevalence and incidence of prolonged and severe dyspareunia in women: results from a population study. Scand J Public Health. 2003;31(2):113-118.
15. Bohm-Starke N, Johannesson U, Hilliges M, Rylander E, Torebjork E. Decreased mechanical pain threshold in the vestibular mucosa of women using oral contraceptives: a contributing factor in vulvar vestibulitis? J Reprod Med. 2004;49(11):888-892.
16. Greenstein A, Ben-Aroya Z, Fass O, et al. Vulvar vestibulitis syndrome and estrogen dose of oral contraceptive pills. J Sexual Med. 2007;4(6):1679-1683.
17. Bouchard C, Brisson J, Fortier M, Morin C, Blanchette C. Use of oral contraceptive pills and vulvar vestibulitis: a case-control study. Am J Epidemiol. 2002;156(3):254-261.
18. Reed BD, Haefner HK, Sen A, Gorenflo DW. Vulvodynia incidence and remission rates among adult women: a 2-year follow-up study. Obstet Gynecol. 2008;112(2 Pt 1):231-237.
19. Goldstein AT, Klingman D, Christopher K, Johnson C, Marinoff SC. Surgical treatment of vulvar vestibulitis syndrome: outcome assessment derived from a postoperative questionnaire. J Sex Med. 2006;3(5):923-931.
20. Eva LJ, Narain S, Orakwue CO, Luesley DM. Is modified vestibulectomy for localized provoked vulvodynia an effective long-term treatment? A follow-up study. J Reprod Med. 2008;53(6):435-440.
21. Haefner HK, Collins ME, Davis GD, et al. The vulvodynia guideline. J Lower Genit Tract Dis. 2005;9(1):40-51.
22. Bergeron S, Binik YM, Khalife S, et al. A randomized comparison of group cognitive-behavioral therapy, surface electromyographic biofeedback, and vestibulectomy in the treatment of dyspareunia resulting from vulvar vestibulitis. Pain. 2001;91(3):297-306.
23. Bergeron S, Khalife S, Glazer HI, Binik Y. Surgical and behavioral treatments for vestibulodynia: two-and-one-half-year follow-up and predictors of outcome. Obstet Gynecol. 2008;111(1):159-166.
24. Gunter J. Vulvodynia: new thoughts on a devastating condition. Obstet Gynecol Surv. 2007;62(12):812-819.
25. Tympanidis P, Terenghi G, Dowd P. Increased innervation of the vulvar vestibule in patients with vulvodynia. Br J Dermatol. 2003;148(5):1021-1027.
26. Haefner HK. Critique of new gynecologic surgical procedures: surgery for vulvar vestibulitis. Clin Obstet Gynecol. 2000;43(3):689-700.
- Part 1: Making the correct diagnosis
(September 2011) - Part 2: A bounty of treatments—but not all of them are proven
(October 2011)
This three-part series concludes with a look at vestibulodynia—pain that is localized to the vulvar vestibule. Much is known about this disorder, compared with our knowledge base in the recent past, but much remains to be discovered. Among the questions explored by the panelists in this article is whether vestibulodynia and generalized vulvodynia are distinct entities—or different manifestations of the same process.
Other questions addressed here:
- Do oral contraceptives (OCs) contribute to vestibulodynia?
- What about herpes and genital warts? Are they causes of vestibular pain?
- Are some women more vulnerable to vestibulodynia than others?
- Is the disorder curable?
- Does vestibulectomy provide definitive treatment?
Part 1 of this series, which appeared in the September 2011 issue, focused on generalized vulvar pain and its causes, features, and diagnosis. Part 2, in the October issue, took as its subject the treatment of vulvar pain. Both are available in the archive at obgmanagement.com.

The lower vagina and vulva are richly supplied with peripheral nerves and are, therefore, sensitive to pain, particularly the region of the hymeneal ring. Although the pudendal nerve (arrow) courses through the area, it is an uncommon source of vulvar pain.
What do we know about the causes of vestibulodynia?
Dr. Lonky: What are the causes of provoked vestibulodynia (PVD), also known as vulvar vestibulitis syndrome? And what are the theories behind those causes?
Dr. Haefner: The specific cause is unknown. Most likely, there isn’t a single cause. Theories that have been proposed include abnormalities of embryologic development, infection, inflammation, genetic and immune factors, and nerve pathways.
Patients who have vestibulodynia may also have interstitial cystitis. It has been noted that tissues from the vestibule and bladder have a common embryologic origin and, therefore, are predisposed to similar pathologic responses when challenged.1,2
Candida albicans infection in patients who experience vestibular pain has also been studied. The exact association is difficult to determine because many patients report Candida infections without verified testing for yeast. Bazin and colleagues found a very weak association between infection and pain on the vestibule.3
Inflammation—the “itis” in vestibulitis—has been excluded from the recent International Society for the Study of Vulvovaginal Disease (ISSVD) terminology because studies found no association between excised tissue and inflammation. Bohm-Starke and colleagues found low expression of the inflammatory markers cyclooxygenase 2 and inducible nitric oxide synthase in the vestibular mucosa of women who had localized vestibular pain, as well as in healthy women in the control group.4
Goetsch was one of the first researchers to explore a genetic association with localized vulvar pain.5 Fifteen percent of patients questioned over a 6-month period were found to have localized vestibular pain. Thirty-two percent had a female relative who had dyspareunia or tampon intolerance, raising the issue of a genetic predisposition. Another genetic connection was found in a study evaluating gene coding for interleukin 1-receptor antagonist.6–8
Krantz examined the nerve characteristics of the vulva and vagina.9 The region of the hymeneal ring was richly supplied with free nerve endings. No corpuscular endings of any form were observed. Only free nerve endings were observed in the fossa navicularis. A sparsity of nerve endings occurred in the vagina, as compared with the region of the fourchette, fossa navicularis, and hymeneal ring. More recent studies have analyzed the nerve factors, thermoreceptors, and nociceptors in women with vulvar pain.10,11
Dr. Edwards: I feel strongly that vestibulodynia and generalized vulvodynia are the same process. For example, tension headaches are supposed to be occipital, but some people experience tension headaches that are periorbital. Both are tension headaches despite the different locations. And almost all patients who experience any subset of vulvodynia have provoked vestibular pain. So the only thing that separates vestibulodynia from other patterns of vulvodynia is the option of vestibulectomy for therapy.
I don’t think that vulvodynia and vestibulodynia are “wastebasket” names for undiagnosable vulvar pain; rather, they are specific disease processes produced by pelvic floor dysfunction that predisposes a woman to neuropathic pain with a trigger or to a systemic pain syndrome that includes an abnormal pelvic floor.
Dr. Gunter: There are probably many causes of PVD, as Dr. Haefner suggested. There may be an ignition hypothesis, whereby some outside inflammatory trigger or trauma produces local neurogenic inflammation. However, given the prevalence of other pain disorders, there is probably also a need to have a lowered threshold for these changes to occur—basically, a vulnerable neurologic platform.
For some women, local neural hyperplasia is probably a factor. It is possible that there are different causes for primary and secondary vestibulodynia.
Vulvodynia and depression often travel together. They are such common comorbidities, in fact, that some physicians theorize that vulvodynia may be a symptom of an underlying mood disorder, such as depression, or that depression may be one manifestation of chronic vulvar pain. Suffice it to say that chronic pain and depression are often associated, and it is frequently difficult to determine whether the relationship is one of cause and effect.
Comprehensive care of the patient who has vulvar pain, therefore, should include a thorough history, looking specifically for depression (including sleep disorders) and eliciting information on any suicidal thoughts or intentions.
Although many patients who have vulvodynia are treated with an antidepressant, the dose that relieves pain may not be high enough to attenuate an accompanying mood disorder. My approach is to team up with a psychiatrist or psychologist who is familiar with vulvar pain syndromes. Together, we monitor the patient and fine-tune the therapeutic response.
—Neal M. Lonky, MD, MPH
Do oral contraceptives contribute to vestibulodynia?
Dr. Lonky: Is PVD more prevalent among OC users?
Dr. Haefner: Controversy surrounds the question of whether vestibulodynia and OC use are linked. Some studies suggest no association12–14 and others suggest a possible effect of OCs on vulvodynia.15–17 A study by Reed and colleagues found no association between taking OCs or hormone therapy at enrollment and incident vulvodynia only in the univariable analysis, but not when controlling for age at enrollment.18 This reflects the finding that younger age was associated with incident disease; younger age and use of OCs are similarly associated.
Dr. Gunter: I am not a believer in a cause-and-effect relationship between OC use and vestibulodynia. I do not find the studies demonstrating an association convincing. Given the supraphysiologic levels of hormones during pregnancy, if high hormone levels played a role, we should also see a greater incidence of vestibulodynia among women who have several pregnancies at an early age.
Dr. Edwards: In my practice, stopping, starting, and changing OCs has made no difference for patients. Topical estrogen supplementation in the occasional OC user who has signs of low estrogen has been useful at times.
Do herpes or genital warts contribute to PVD?
Dr. Lonky: Does a history of vulvar herpes or genital warts have any impact on the incidence of PVD?
Dr. Edwards: No.
Dr. Gunter: I agree that it has no impact.
Dr. Haefner: Herpes is sometimes associated with vulvar pain. The lesions resolve, but pain may continue as post-herpetic neuralgia. As with shingles, a low threshold for starting a patient on gabapentin to control pain after herpes may be beneficial.
Genital warts rarely cause vulvar pain—but the treatment may. Patients sometimes feel pain following topical treatment, as well as pain from surgical wart treatment.
Effect of demographic variables
Dr. Lonky: Does race, skin type, or hair or eye color make a difference in the prevalence, manifestation of symptoms, or treatment of PVD?
Dr. Gunter: I am not aware of any studies that confirm an association between vulvodynia and those factors.
Dr. Edwards: I don’t know whether any of these variables make a difference. My own impression—confirmed by informal study in my office—is that vulvodynia patients weigh less than my general dermatology patients and are better educated. I sometimes get the sense that my vulvodynia patients are more likely to be fair.
Dr. Lonky: What age group is most commonly affected by PVD?
Dr. Edwards: In my experience the most common age group is women 25 to 45 years old, probably because they are the most sexually active group old enough and tough enough to pursue this issue.
Dr. Gunter: I believe it affects all women equally, although women in their reproductive years are more likely to visit a gynecologist and, therefore, probably more likely to be given this diagnosis.
Dr. Lonky: Do you believe that PVD and generalized vulvar dysesthesia are curable—or just treatable?
Dr. Gunter: That depends on many variables. It is far more challenging to cure a patient who has multiple pain syndromes (for example, fibromyalgia, migraines, and irritable bowel syndrome) than the woman who simply has vestibulodynia or generalized vulvar pain. In addition, stress, anxiety, coping skills, and depression all play a role. In my opinion, a woman without comorbidity has a good chance of having her symptoms well-controlled. Some will be cured (that is, able to discontinue medications), and others will need ongoing treatment but will not be bothered by their symptoms.
Dr. Haefner: The response to treatment in many pain patients depends on the amount of time that the pain has been present. Someone who has had pain for 30 years will probably not be cured 3 months after starting treatment. However, someone with a short duration of pain often gets good improvement. One hundred percent improvement is rare, however. Many patients are able to approach the 80% improvement mark.
Dr. Edwards: I would say that these conditions are manageable more than curable, although pure vestibulodynia—which is uncommon—is curable with surgery.

Vestibulectomy technique
(A) Incision. In many cases, the incision needs to extend up to the opening of the Skene’s ducts on the vestibule before it is carried down laterally along Hart’s line to the perianal skin, with the mucosa undermined above the hymeneal ring. (B) Excision. Remove the tissue superior to the hymeneal ring. (C) Advancement of vaginal mucosa. Further undermine the mucosa and advance it to close the defect. (D) Suturing. Close the defect in two layers using absorbable suture.
Is vestibulectomy definitive treatment?
Dr. Lonky: Does vestibulectomy work as a definitive treatment for PVD?
Dr. Gunter: Vestibulectomy—that is, resection of the vestibule and advancement of vaginal mucosa—is well described for women who have localized vestibulodynia. The complication rate is low, with only 3% of women reporting worsening of symptoms after the procedure.19,20 Success rates range from 17% to 89%, although many studies are retrospective reviews or include nonhomogenous populations, or both.19–21 A prospective study (Level II) indicates a 52% reduction in pain scores for women 6 months after vestibulectomy—and the scores continued to improve at evaluation at 2.5 years.22,23
Prospective studies indicate that vestibulectomy improves pain scores more than cognitive behavioral therapy, biofeedback, oral despiramine, and topical lidocaine.22,23 However, even surgery carries a robust placebo response rate, and vulvar biopsy alone has been associated with an improvement in pain scores in 67% of women.24,25
I offer surgery for vestibulodynia after the patient has failed at least two therapies (two topical treatments or one topical and one oral treatment). I offer injections before proceeding, and almost all patients opt to try them. I do not offer vestibulectomy to patients who have unprovoked pain or pain outside of resection margins.
Dr. Haefner: Surgical excision of the vulvar vestibule has met with success, in some studies, in more than 80% of cases, but it should be reserved for women who have longstanding and localized vestibular pain in whom other management options have failed.
The patient should undergo cotton-swab testing to outline areas of pain before anesthesia is administered in the operating room. Often, the incision will need to extend up to the opening of the Skene’s ducts on the vestibule (FIGURE). The incision is carried down laterally along Hart’s line to the perianal skin, and the mucosa is undermined above the hymeneal ring. The specimen is excised superior to the hymeneal ring. The vaginal tissue is further undermined and brought down to close the defect in two layers using absorbable suture. A review of this technique with illustrations has been published.26
Dr. Lonky: Thank you all again for sharing your considerable expertise, experience, and insight.
Harlow BL, Stewart EG. A population-based assessment of chronic unexplained vulvar pain: have we underestimated the prevalence of vulvodynia? J Am Med Womens Assoc. 2003;58(2):82–88.
In this population-based study, 16% of women reported a history of chronic unexplained vulvar pain, and nearly 7% reported current symptoms.
Khandker M, Brady SS, Vitonis AF, Maclehose RF, Stewart EG, Harlow BL. The influence of depression and anxiety on risk of adult onset vulvodynia [published online ahead of print August 8, 2011].
J Womens Health (Larchmt). doi:10.1089/jwh.2010.2661.
Vulvodynia was four times more likely among women who had antecedent mood or anxiety disorders than in women who didn’t. Vulvodynia was also associated with new or recurrent onset of mood or anxiety disorders.
Masheb RM, Wang E, Lozano C, Kerns RD. Prevalence and correlates of depression in treatment-seeking women with vulvodynia. J Obstet Gynaecol. 2005;25(8):786–791.
Comorbid major depressive disorders in women who have vulvodynia are related to greater pain severity and worse functioning.
Reed BD, Haefner HK, Punch MR, Roth RS, Gorenflo DW, Gillespie BW. Psychosocial and sexual functioning in women with vulvodynia and chronic pelvic pain. A comparative evaluation. J Reprod Med. 2000;45(8):624–632.
Women who have vulvodynia are psychologically similar to women who don’t. A primary psychological cause of vulvodynia is not supported.
Tribo MJ, Andion O, Ros S, et al. Clinical characteristics and psychopathological profile of patients with vulvodynia: an observational and descriptive study. Dermatology. 2008;216(1):24–30.
Psychiatric treatment may be a useful option to improve symptoms of vulvodynia.
We want to hear from you! Tell us what you think about this series on Vulvar Pain Syndromes.
- Part 1: Making the correct diagnosis
(September 2011) - Part 2: A bounty of treatments—but not all of them are proven
(October 2011)
This three-part series concludes with a look at vestibulodynia—pain that is localized to the vulvar vestibule. Much is known about this disorder, compared with our knowledge base in the recent past, but much remains to be discovered. Among the questions explored by the panelists in this article is whether vestibulodynia and generalized vulvodynia are distinct entities—or different manifestations of the same process.
Other questions addressed here:
- Do oral contraceptives (OCs) contribute to vestibulodynia?
- What about herpes and genital warts? Are they causes of vestibular pain?
- Are some women more vulnerable to vestibulodynia than others?
- Is the disorder curable?
- Does vestibulectomy provide definitive treatment?
Part 1 of this series, which appeared in the September 2011 issue, focused on generalized vulvar pain and its causes, features, and diagnosis. Part 2, in the October issue, took as its subject the treatment of vulvar pain. Both are available in the archive at obgmanagement.com.

The lower vagina and vulva are richly supplied with peripheral nerves and are, therefore, sensitive to pain, particularly the region of the hymeneal ring. Although the pudendal nerve (arrow) courses through the area, it is an uncommon source of vulvar pain.
What do we know about the causes of vestibulodynia?
Dr. Lonky: What are the causes of provoked vestibulodynia (PVD), also known as vulvar vestibulitis syndrome? And what are the theories behind those causes?
Dr. Haefner: The specific cause is unknown. Most likely, there isn’t a single cause. Theories that have been proposed include abnormalities of embryologic development, infection, inflammation, genetic and immune factors, and nerve pathways.
Patients who have vestibulodynia may also have interstitial cystitis. It has been noted that tissues from the vestibule and bladder have a common embryologic origin and, therefore, are predisposed to similar pathologic responses when challenged.1,2
Candida albicans infection in patients who experience vestibular pain has also been studied. The exact association is difficult to determine because many patients report Candida infections without verified testing for yeast. Bazin and colleagues found a very weak association between infection and pain on the vestibule.3
Inflammation—the “itis” in vestibulitis—has been excluded from the recent International Society for the Study of Vulvovaginal Disease (ISSVD) terminology because studies found no association between excised tissue and inflammation. Bohm-Starke and colleagues found low expression of the inflammatory markers cyclooxygenase 2 and inducible nitric oxide synthase in the vestibular mucosa of women who had localized vestibular pain, as well as in healthy women in the control group.4
Goetsch was one of the first researchers to explore a genetic association with localized vulvar pain.5 Fifteen percent of patients questioned over a 6-month period were found to have localized vestibular pain. Thirty-two percent had a female relative who had dyspareunia or tampon intolerance, raising the issue of a genetic predisposition. Another genetic connection was found in a study evaluating gene coding for interleukin 1-receptor antagonist.6–8
Krantz examined the nerve characteristics of the vulva and vagina.9 The region of the hymeneal ring was richly supplied with free nerve endings. No corpuscular endings of any form were observed. Only free nerve endings were observed in the fossa navicularis. A sparsity of nerve endings occurred in the vagina, as compared with the region of the fourchette, fossa navicularis, and hymeneal ring. More recent studies have analyzed the nerve factors, thermoreceptors, and nociceptors in women with vulvar pain.10,11
Dr. Edwards: I feel strongly that vestibulodynia and generalized vulvodynia are the same process. For example, tension headaches are supposed to be occipital, but some people experience tension headaches that are periorbital. Both are tension headaches despite the different locations. And almost all patients who experience any subset of vulvodynia have provoked vestibular pain. So the only thing that separates vestibulodynia from other patterns of vulvodynia is the option of vestibulectomy for therapy.
I don’t think that vulvodynia and vestibulodynia are “wastebasket” names for undiagnosable vulvar pain; rather, they are specific disease processes produced by pelvic floor dysfunction that predisposes a woman to neuropathic pain with a trigger or to a systemic pain syndrome that includes an abnormal pelvic floor.
Dr. Gunter: There are probably many causes of PVD, as Dr. Haefner suggested. There may be an ignition hypothesis, whereby some outside inflammatory trigger or trauma produces local neurogenic inflammation. However, given the prevalence of other pain disorders, there is probably also a need to have a lowered threshold for these changes to occur—basically, a vulnerable neurologic platform.
For some women, local neural hyperplasia is probably a factor. It is possible that there are different causes for primary and secondary vestibulodynia.
Vulvodynia and depression often travel together. They are such common comorbidities, in fact, that some physicians theorize that vulvodynia may be a symptom of an underlying mood disorder, such as depression, or that depression may be one manifestation of chronic vulvar pain. Suffice it to say that chronic pain and depression are often associated, and it is frequently difficult to determine whether the relationship is one of cause and effect.
Comprehensive care of the patient who has vulvar pain, therefore, should include a thorough history, looking specifically for depression (including sleep disorders) and eliciting information on any suicidal thoughts or intentions.
Although many patients who have vulvodynia are treated with an antidepressant, the dose that relieves pain may not be high enough to attenuate an accompanying mood disorder. My approach is to team up with a psychiatrist or psychologist who is familiar with vulvar pain syndromes. Together, we monitor the patient and fine-tune the therapeutic response.
—Neal M. Lonky, MD, MPH
Do oral contraceptives contribute to vestibulodynia?
Dr. Lonky: Is PVD more prevalent among OC users?
Dr. Haefner: Controversy surrounds the question of whether vestibulodynia and OC use are linked. Some studies suggest no association12–14 and others suggest a possible effect of OCs on vulvodynia.15–17 A study by Reed and colleagues found no association between taking OCs or hormone therapy at enrollment and incident vulvodynia only in the univariable analysis, but not when controlling for age at enrollment.18 This reflects the finding that younger age was associated with incident disease; younger age and use of OCs are similarly associated.
Dr. Gunter: I am not a believer in a cause-and-effect relationship between OC use and vestibulodynia. I do not find the studies demonstrating an association convincing. Given the supraphysiologic levels of hormones during pregnancy, if high hormone levels played a role, we should also see a greater incidence of vestibulodynia among women who have several pregnancies at an early age.
Dr. Edwards: In my practice, stopping, starting, and changing OCs has made no difference for patients. Topical estrogen supplementation in the occasional OC user who has signs of low estrogen has been useful at times.
Do herpes or genital warts contribute to PVD?
Dr. Lonky: Does a history of vulvar herpes or genital warts have any impact on the incidence of PVD?
Dr. Edwards: No.
Dr. Gunter: I agree that it has no impact.
Dr. Haefner: Herpes is sometimes associated with vulvar pain. The lesions resolve, but pain may continue as post-herpetic neuralgia. As with shingles, a low threshold for starting a patient on gabapentin to control pain after herpes may be beneficial.
Genital warts rarely cause vulvar pain—but the treatment may. Patients sometimes feel pain following topical treatment, as well as pain from surgical wart treatment.
Effect of demographic variables
Dr. Lonky: Does race, skin type, or hair or eye color make a difference in the prevalence, manifestation of symptoms, or treatment of PVD?
Dr. Gunter: I am not aware of any studies that confirm an association between vulvodynia and those factors.
Dr. Edwards: I don’t know whether any of these variables make a difference. My own impression—confirmed by informal study in my office—is that vulvodynia patients weigh less than my general dermatology patients and are better educated. I sometimes get the sense that my vulvodynia patients are more likely to be fair.
Dr. Lonky: What age group is most commonly affected by PVD?
Dr. Edwards: In my experience the most common age group is women 25 to 45 years old, probably because they are the most sexually active group old enough and tough enough to pursue this issue.
Dr. Gunter: I believe it affects all women equally, although women in their reproductive years are more likely to visit a gynecologist and, therefore, probably more likely to be given this diagnosis.
Dr. Lonky: Do you believe that PVD and generalized vulvar dysesthesia are curable—or just treatable?
Dr. Gunter: That depends on many variables. It is far more challenging to cure a patient who has multiple pain syndromes (for example, fibromyalgia, migraines, and irritable bowel syndrome) than the woman who simply has vestibulodynia or generalized vulvar pain. In addition, stress, anxiety, coping skills, and depression all play a role. In my opinion, a woman without comorbidity has a good chance of having her symptoms well-controlled. Some will be cured (that is, able to discontinue medications), and others will need ongoing treatment but will not be bothered by their symptoms.
Dr. Haefner: The response to treatment in many pain patients depends on the amount of time that the pain has been present. Someone who has had pain for 30 years will probably not be cured 3 months after starting treatment. However, someone with a short duration of pain often gets good improvement. One hundred percent improvement is rare, however. Many patients are able to approach the 80% improvement mark.
Dr. Edwards: I would say that these conditions are manageable more than curable, although pure vestibulodynia—which is uncommon—is curable with surgery.

Vestibulectomy technique
(A) Incision. In many cases, the incision needs to extend up to the opening of the Skene’s ducts on the vestibule before it is carried down laterally along Hart’s line to the perianal skin, with the mucosa undermined above the hymeneal ring. (B) Excision. Remove the tissue superior to the hymeneal ring. (C) Advancement of vaginal mucosa. Further undermine the mucosa and advance it to close the defect. (D) Suturing. Close the defect in two layers using absorbable suture.
Is vestibulectomy definitive treatment?
Dr. Lonky: Does vestibulectomy work as a definitive treatment for PVD?
Dr. Gunter: Vestibulectomy—that is, resection of the vestibule and advancement of vaginal mucosa—is well described for women who have localized vestibulodynia. The complication rate is low, with only 3% of women reporting worsening of symptoms after the procedure.19,20 Success rates range from 17% to 89%, although many studies are retrospective reviews or include nonhomogenous populations, or both.19–21 A prospective study (Level II) indicates a 52% reduction in pain scores for women 6 months after vestibulectomy—and the scores continued to improve at evaluation at 2.5 years.22,23
Prospective studies indicate that vestibulectomy improves pain scores more than cognitive behavioral therapy, biofeedback, oral despiramine, and topical lidocaine.22,23 However, even surgery carries a robust placebo response rate, and vulvar biopsy alone has been associated with an improvement in pain scores in 67% of women.24,25
I offer surgery for vestibulodynia after the patient has failed at least two therapies (two topical treatments or one topical and one oral treatment). I offer injections before proceeding, and almost all patients opt to try them. I do not offer vestibulectomy to patients who have unprovoked pain or pain outside of resection margins.
Dr. Haefner: Surgical excision of the vulvar vestibule has met with success, in some studies, in more than 80% of cases, but it should be reserved for women who have longstanding and localized vestibular pain in whom other management options have failed.
The patient should undergo cotton-swab testing to outline areas of pain before anesthesia is administered in the operating room. Often, the incision will need to extend up to the opening of the Skene’s ducts on the vestibule (FIGURE). The incision is carried down laterally along Hart’s line to the perianal skin, and the mucosa is undermined above the hymeneal ring. The specimen is excised superior to the hymeneal ring. The vaginal tissue is further undermined and brought down to close the defect in two layers using absorbable suture. A review of this technique with illustrations has been published.26
Dr. Lonky: Thank you all again for sharing your considerable expertise, experience, and insight.
Harlow BL, Stewart EG. A population-based assessment of chronic unexplained vulvar pain: have we underestimated the prevalence of vulvodynia? J Am Med Womens Assoc. 2003;58(2):82–88.
In this population-based study, 16% of women reported a history of chronic unexplained vulvar pain, and nearly 7% reported current symptoms.
Khandker M, Brady SS, Vitonis AF, Maclehose RF, Stewart EG, Harlow BL. The influence of depression and anxiety on risk of adult onset vulvodynia [published online ahead of print August 8, 2011].
J Womens Health (Larchmt). doi:10.1089/jwh.2010.2661.
Vulvodynia was four times more likely among women who had antecedent mood or anxiety disorders than in women who didn’t. Vulvodynia was also associated with new or recurrent onset of mood or anxiety disorders.
Masheb RM, Wang E, Lozano C, Kerns RD. Prevalence and correlates of depression in treatment-seeking women with vulvodynia. J Obstet Gynaecol. 2005;25(8):786–791.
Comorbid major depressive disorders in women who have vulvodynia are related to greater pain severity and worse functioning.
Reed BD, Haefner HK, Punch MR, Roth RS, Gorenflo DW, Gillespie BW. Psychosocial and sexual functioning in women with vulvodynia and chronic pelvic pain. A comparative evaluation. J Reprod Med. 2000;45(8):624–632.
Women who have vulvodynia are psychologically similar to women who don’t. A primary psychological cause of vulvodynia is not supported.
Tribo MJ, Andion O, Ros S, et al. Clinical characteristics and psychopathological profile of patients with vulvodynia: an observational and descriptive study. Dermatology. 2008;216(1):24–30.
Psychiatric treatment may be a useful option to improve symptoms of vulvodynia.
We want to hear from you! Tell us what you think about this series on Vulvar Pain Syndromes.
1. McCormack WM. Two urogenital sinus syndromes. Interstitial cystitis and focal vulvitis. J Reprod Med. 1990;35(9):873-876.
2. Fitzpatrick CC, DeLancey JP, Elkins TE, McGuire EJ. Vulvar vestibulitis and interstitial cystitis: a disorder of urogenital sinusderived epithelium. Obstet Gynecol. 1993;81(5 Pt 2):860-862.
3. Bazin S, Bouchard C, Brisson J, Morin C, Meisels A, Fortier M. Vulvar vestibulitis syndrome: an exploratory case-control study. Obstet Gynecol. 1994;83(1):47-50.
4. Bohm-Starke N, Falconer C, Rylander E, Hilliges M. The expression of cyclooxygenase 2 and inducible nitric oxide synthase indicates no active inflammation in vulvar vestibulitis. Acta Obstet Gynecol Scand. 2001;80(7):638-644.
5. Goetsch MF. Vulvar vestibulitis: prevalence and historic features in a general gynecologic practice population. Am J Obstet Gynecol. 1991;164(6 Pt 1):1609-1616.
6. Jeremias J, Ledger WJ, Witkin SS. Interleukin 1 receptor antagonist gene polymorphism in women with vulvar vestibulitis. Am J Obstet Gynecol. 2000;182(2):283-285.
7. Witkin SS, Gerber S, Ledger WJ. Differential characterization of women with vulvar vestibulitis syndrome. Am J Obstet Gynecol. 2002;187(3):589-594.
8. Foster DC, Piekarz KH, Murant TI, LaPoint R, Haidaris CG, Phipps RP. Enhanced synthesis of proinflammatory cytokines by vulvar vestibular fibroblasts: implications for vulvar vestibulitis. Am J Obstet Gynecol. 2007;196(4):346. e1-8.
9. Krantz KE. Innervation of the human vulva and vagina: a microscopic study. Obstet Gynecol. 1958;12:382-396.
10. Bohm-Starke N, Hilliges M, Brodda-Jansen G, Rylander E, Torebjork E. Psychophysical evidence of nociceptor sensitization in vulvar vestibulitis syndrome. Pain. 2001;94(2):177-183.
11. Tympanidis P, Terenghi G, Dowd P. Increased innervation of the vulval vestibule in patients with vulvodynia. Br J Dermatol. 2003;148(5):1021-1027.
12. Bachman GA, Rosen R, Pinn VW, et al. Vulvodynia: a state-of-the-art consensus on definitions, diagnosis and management. J Reprod Med. 2006;51(6):447-456.
13. Harlow BL, Vitonis AF, Stewart EG. Influence of oral contraceptive use on the risk of adult-onset vulvodynia. J Reprod Med. 2008;53(2):102-110.
14. Danielsson I, Sjoberg I, Stenlund H, Wikman M. Prevalence and incidence of prolonged and severe dyspareunia in women: results from a population study. Scand J Public Health. 2003;31(2):113-118.
15. Bohm-Starke N, Johannesson U, Hilliges M, Rylander E, Torebjork E. Decreased mechanical pain threshold in the vestibular mucosa of women using oral contraceptives: a contributing factor in vulvar vestibulitis? J Reprod Med. 2004;49(11):888-892.
16. Greenstein A, Ben-Aroya Z, Fass O, et al. Vulvar vestibulitis syndrome and estrogen dose of oral contraceptive pills. J Sexual Med. 2007;4(6):1679-1683.
17. Bouchard C, Brisson J, Fortier M, Morin C, Blanchette C. Use of oral contraceptive pills and vulvar vestibulitis: a case-control study. Am J Epidemiol. 2002;156(3):254-261.
18. Reed BD, Haefner HK, Sen A, Gorenflo DW. Vulvodynia incidence and remission rates among adult women: a 2-year follow-up study. Obstet Gynecol. 2008;112(2 Pt 1):231-237.
19. Goldstein AT, Klingman D, Christopher K, Johnson C, Marinoff SC. Surgical treatment of vulvar vestibulitis syndrome: outcome assessment derived from a postoperative questionnaire. J Sex Med. 2006;3(5):923-931.
20. Eva LJ, Narain S, Orakwue CO, Luesley DM. Is modified vestibulectomy for localized provoked vulvodynia an effective long-term treatment? A follow-up study. J Reprod Med. 2008;53(6):435-440.
21. Haefner HK, Collins ME, Davis GD, et al. The vulvodynia guideline. J Lower Genit Tract Dis. 2005;9(1):40-51.
22. Bergeron S, Binik YM, Khalife S, et al. A randomized comparison of group cognitive-behavioral therapy, surface electromyographic biofeedback, and vestibulectomy in the treatment of dyspareunia resulting from vulvar vestibulitis. Pain. 2001;91(3):297-306.
23. Bergeron S, Khalife S, Glazer HI, Binik Y. Surgical and behavioral treatments for vestibulodynia: two-and-one-half-year follow-up and predictors of outcome. Obstet Gynecol. 2008;111(1):159-166.
24. Gunter J. Vulvodynia: new thoughts on a devastating condition. Obstet Gynecol Surv. 2007;62(12):812-819.
25. Tympanidis P, Terenghi G, Dowd P. Increased innervation of the vulvar vestibule in patients with vulvodynia. Br J Dermatol. 2003;148(5):1021-1027.
26. Haefner HK. Critique of new gynecologic surgical procedures: surgery for vulvar vestibulitis. Clin Obstet Gynecol. 2000;43(3):689-700.
1. McCormack WM. Two urogenital sinus syndromes. Interstitial cystitis and focal vulvitis. J Reprod Med. 1990;35(9):873-876.
2. Fitzpatrick CC, DeLancey JP, Elkins TE, McGuire EJ. Vulvar vestibulitis and interstitial cystitis: a disorder of urogenital sinusderived epithelium. Obstet Gynecol. 1993;81(5 Pt 2):860-862.
3. Bazin S, Bouchard C, Brisson J, Morin C, Meisels A, Fortier M. Vulvar vestibulitis syndrome: an exploratory case-control study. Obstet Gynecol. 1994;83(1):47-50.
4. Bohm-Starke N, Falconer C, Rylander E, Hilliges M. The expression of cyclooxygenase 2 and inducible nitric oxide synthase indicates no active inflammation in vulvar vestibulitis. Acta Obstet Gynecol Scand. 2001;80(7):638-644.
5. Goetsch MF. Vulvar vestibulitis: prevalence and historic features in a general gynecologic practice population. Am J Obstet Gynecol. 1991;164(6 Pt 1):1609-1616.
6. Jeremias J, Ledger WJ, Witkin SS. Interleukin 1 receptor antagonist gene polymorphism in women with vulvar vestibulitis. Am J Obstet Gynecol. 2000;182(2):283-285.
7. Witkin SS, Gerber S, Ledger WJ. Differential characterization of women with vulvar vestibulitis syndrome. Am J Obstet Gynecol. 2002;187(3):589-594.
8. Foster DC, Piekarz KH, Murant TI, LaPoint R, Haidaris CG, Phipps RP. Enhanced synthesis of proinflammatory cytokines by vulvar vestibular fibroblasts: implications for vulvar vestibulitis. Am J Obstet Gynecol. 2007;196(4):346. e1-8.
9. Krantz KE. Innervation of the human vulva and vagina: a microscopic study. Obstet Gynecol. 1958;12:382-396.
10. Bohm-Starke N, Hilliges M, Brodda-Jansen G, Rylander E, Torebjork E. Psychophysical evidence of nociceptor sensitization in vulvar vestibulitis syndrome. Pain. 2001;94(2):177-183.
11. Tympanidis P, Terenghi G, Dowd P. Increased innervation of the vulval vestibule in patients with vulvodynia. Br J Dermatol. 2003;148(5):1021-1027.
12. Bachman GA, Rosen R, Pinn VW, et al. Vulvodynia: a state-of-the-art consensus on definitions, diagnosis and management. J Reprod Med. 2006;51(6):447-456.
13. Harlow BL, Vitonis AF, Stewart EG. Influence of oral contraceptive use on the risk of adult-onset vulvodynia. J Reprod Med. 2008;53(2):102-110.
14. Danielsson I, Sjoberg I, Stenlund H, Wikman M. Prevalence and incidence of prolonged and severe dyspareunia in women: results from a population study. Scand J Public Health. 2003;31(2):113-118.
15. Bohm-Starke N, Johannesson U, Hilliges M, Rylander E, Torebjork E. Decreased mechanical pain threshold in the vestibular mucosa of women using oral contraceptives: a contributing factor in vulvar vestibulitis? J Reprod Med. 2004;49(11):888-892.
16. Greenstein A, Ben-Aroya Z, Fass O, et al. Vulvar vestibulitis syndrome and estrogen dose of oral contraceptive pills. J Sexual Med. 2007;4(6):1679-1683.
17. Bouchard C, Brisson J, Fortier M, Morin C, Blanchette C. Use of oral contraceptive pills and vulvar vestibulitis: a case-control study. Am J Epidemiol. 2002;156(3):254-261.
18. Reed BD, Haefner HK, Sen A, Gorenflo DW. Vulvodynia incidence and remission rates among adult women: a 2-year follow-up study. Obstet Gynecol. 2008;112(2 Pt 1):231-237.
19. Goldstein AT, Klingman D, Christopher K, Johnson C, Marinoff SC. Surgical treatment of vulvar vestibulitis syndrome: outcome assessment derived from a postoperative questionnaire. J Sex Med. 2006;3(5):923-931.
20. Eva LJ, Narain S, Orakwue CO, Luesley DM. Is modified vestibulectomy for localized provoked vulvodynia an effective long-term treatment? A follow-up study. J Reprod Med. 2008;53(6):435-440.
21. Haefner HK, Collins ME, Davis GD, et al. The vulvodynia guideline. J Lower Genit Tract Dis. 2005;9(1):40-51.
22. Bergeron S, Binik YM, Khalife S, et al. A randomized comparison of group cognitive-behavioral therapy, surface electromyographic biofeedback, and vestibulectomy in the treatment of dyspareunia resulting from vulvar vestibulitis. Pain. 2001;91(3):297-306.
23. Bergeron S, Khalife S, Glazer HI, Binik Y. Surgical and behavioral treatments for vestibulodynia: two-and-one-half-year follow-up and predictors of outcome. Obstet Gynecol. 2008;111(1):159-166.
24. Gunter J. Vulvodynia: new thoughts on a devastating condition. Obstet Gynecol Surv. 2007;62(12):812-819.
25. Tympanidis P, Terenghi G, Dowd P. Increased innervation of the vulvar vestibule in patients with vulvodynia. Br J Dermatol. 2003;148(5):1021-1027.
26. Haefner HK. Critique of new gynecologic surgical procedures: surgery for vulvar vestibulitis. Clin Obstet Gynecol. 2000;43(3):689-700.
Frequent Hot Flashes? Check Lipid Levels : Higher levels of LDL, HDL, and triglycerides were significantly associated with frequent hot flashes.
Major Finding: LDL levels among women with 6 or more days of hot flashes peaked at approximately 125 mg/dL during a 2-week period, compared with a peak of approximately 120 mg/dL among women with 1-5 days of hot flashes and a peak of approximately 118 mg/dL among women with no reported days of hot flashes.
Data Source: Data from 3,201 women enrolled in the Study of Women's Health Across the Nation (SWAN).
Disclosures: The study was supported by a grant from the National Institutes of Health. Dr. Thurston had no financial conflicts to disclose.
NATIONAL HARBOR, MD. – Frequent hot flashes in menopausal women were significantly associated with higher levels of low-density lipoproteins, high-density lipoproteins, and triglycerides during a 7-year follow-up study of 3,201women enrolled in an ongoing longitudinal study.
Previous investigations using the Study of Women's Health Across the Nation (SWAN) database have shown that women with more hot flashes have an elevated risk for subclinical cardiovascular disease, said Rebecca Thurston, Ph.D., of the University of Pittsburgh.
But “there is a lot we don't know about this association, including what could possibly explain this,” she commented.
Dr. Thurston and her colleagues examined hot flashes as they related to lipid profiles in women enrolled in SWAN.
The subjects' median age was 46 years, 48% were white, 46% were in early or perimenopause, and 26% reported hot flashes within the past 2 weeks.
Hot flashes were analyzed in relation to six lipid profiles, after controlling for age, race, menopausal status/cycle day, alcohol use, physical activity, smoking, anxiety, body mass index, cardiovascular disease status and medications, lipid-lowering medications, and estradiol.
Compared with women who reported no hot flashes, women who reported 1-5 days of hot flashes or 6 or more days of hot flashes during the past 2 weeks were significantly more likely to have elevated levels of LDL cholesterol, triglycerides, apolipoprotein B, and apolipoprotein A1.
For example, LDL levels among women with 6 or more days of hot flashes peaked at approximately 125 mg/dL during a 2-week period, compared with a peak of approximately 120 mg/dL among women with 1-5 days of hot flashes and a peak of approximately 118 mg/dL among women with no reported days of hot flashes.
Levels of HDL cholesterol were significantly higher in women who reported 6 or more days of hot flashes during the past 2 weeks, compared with those who reported no hot flashes, but HDL levels were not significantly different between women who reported 1-5 days of hot flashes and those who reported no hot flashes.
By contrast, levels of lipoprotein(a) were not significantly different among women who reported no hot flashes, women who reported 1 to 5 days of hot flashes, and women who reported 6 or more days of hot flashes.
The positive relationship between hot flashes and lipoprotein(a), and between hot flashes and HDL in some women, were surprising, Dr. Thurston commented.
“The cardioprotective nature of HDL may depend on particle size,” she noted.
HDL particles become smaller as women transition through men op ause, she added, which might explain the differences.
Major Finding: LDL levels among women with 6 or more days of hot flashes peaked at approximately 125 mg/dL during a 2-week period, compared with a peak of approximately 120 mg/dL among women with 1-5 days of hot flashes and a peak of approximately 118 mg/dL among women with no reported days of hot flashes.
Data Source: Data from 3,201 women enrolled in the Study of Women's Health Across the Nation (SWAN).
Disclosures: The study was supported by a grant from the National Institutes of Health. Dr. Thurston had no financial conflicts to disclose.
NATIONAL HARBOR, MD. – Frequent hot flashes in menopausal women were significantly associated with higher levels of low-density lipoproteins, high-density lipoproteins, and triglycerides during a 7-year follow-up study of 3,201women enrolled in an ongoing longitudinal study.
Previous investigations using the Study of Women's Health Across the Nation (SWAN) database have shown that women with more hot flashes have an elevated risk for subclinical cardiovascular disease, said Rebecca Thurston, Ph.D., of the University of Pittsburgh.
But “there is a lot we don't know about this association, including what could possibly explain this,” she commented.
Dr. Thurston and her colleagues examined hot flashes as they related to lipid profiles in women enrolled in SWAN.
The subjects' median age was 46 years, 48% were white, 46% were in early or perimenopause, and 26% reported hot flashes within the past 2 weeks.
Hot flashes were analyzed in relation to six lipid profiles, after controlling for age, race, menopausal status/cycle day, alcohol use, physical activity, smoking, anxiety, body mass index, cardiovascular disease status and medications, lipid-lowering medications, and estradiol.
Compared with women who reported no hot flashes, women who reported 1-5 days of hot flashes or 6 or more days of hot flashes during the past 2 weeks were significantly more likely to have elevated levels of LDL cholesterol, triglycerides, apolipoprotein B, and apolipoprotein A1.
For example, LDL levels among women with 6 or more days of hot flashes peaked at approximately 125 mg/dL during a 2-week period, compared with a peak of approximately 120 mg/dL among women with 1-5 days of hot flashes and a peak of approximately 118 mg/dL among women with no reported days of hot flashes.
Levels of HDL cholesterol were significantly higher in women who reported 6 or more days of hot flashes during the past 2 weeks, compared with those who reported no hot flashes, but HDL levels were not significantly different between women who reported 1-5 days of hot flashes and those who reported no hot flashes.
By contrast, levels of lipoprotein(a) were not significantly different among women who reported no hot flashes, women who reported 1 to 5 days of hot flashes, and women who reported 6 or more days of hot flashes.
The positive relationship between hot flashes and lipoprotein(a), and between hot flashes and HDL in some women, were surprising, Dr. Thurston commented.
“The cardioprotective nature of HDL may depend on particle size,” she noted.
HDL particles become smaller as women transition through men op ause, she added, which might explain the differences.
Major Finding: LDL levels among women with 6 or more days of hot flashes peaked at approximately 125 mg/dL during a 2-week period, compared with a peak of approximately 120 mg/dL among women with 1-5 days of hot flashes and a peak of approximately 118 mg/dL among women with no reported days of hot flashes.
Data Source: Data from 3,201 women enrolled in the Study of Women's Health Across the Nation (SWAN).
Disclosures: The study was supported by a grant from the National Institutes of Health. Dr. Thurston had no financial conflicts to disclose.
NATIONAL HARBOR, MD. – Frequent hot flashes in menopausal women were significantly associated with higher levels of low-density lipoproteins, high-density lipoproteins, and triglycerides during a 7-year follow-up study of 3,201women enrolled in an ongoing longitudinal study.
Previous investigations using the Study of Women's Health Across the Nation (SWAN) database have shown that women with more hot flashes have an elevated risk for subclinical cardiovascular disease, said Rebecca Thurston, Ph.D., of the University of Pittsburgh.
But “there is a lot we don't know about this association, including what could possibly explain this,” she commented.
Dr. Thurston and her colleagues examined hot flashes as they related to lipid profiles in women enrolled in SWAN.
The subjects' median age was 46 years, 48% were white, 46% were in early or perimenopause, and 26% reported hot flashes within the past 2 weeks.
Hot flashes were analyzed in relation to six lipid profiles, after controlling for age, race, menopausal status/cycle day, alcohol use, physical activity, smoking, anxiety, body mass index, cardiovascular disease status and medications, lipid-lowering medications, and estradiol.
Compared with women who reported no hot flashes, women who reported 1-5 days of hot flashes or 6 or more days of hot flashes during the past 2 weeks were significantly more likely to have elevated levels of LDL cholesterol, triglycerides, apolipoprotein B, and apolipoprotein A1.
For example, LDL levels among women with 6 or more days of hot flashes peaked at approximately 125 mg/dL during a 2-week period, compared with a peak of approximately 120 mg/dL among women with 1-5 days of hot flashes and a peak of approximately 118 mg/dL among women with no reported days of hot flashes.
Levels of HDL cholesterol were significantly higher in women who reported 6 or more days of hot flashes during the past 2 weeks, compared with those who reported no hot flashes, but HDL levels were not significantly different between women who reported 1-5 days of hot flashes and those who reported no hot flashes.
By contrast, levels of lipoprotein(a) were not significantly different among women who reported no hot flashes, women who reported 1 to 5 days of hot flashes, and women who reported 6 or more days of hot flashes.
The positive relationship between hot flashes and lipoprotein(a), and between hot flashes and HDL in some women, were surprising, Dr. Thurston commented.
“The cardioprotective nature of HDL may depend on particle size,” she noted.
HDL particles become smaller as women transition through men op ause, she added, which might explain the differences.
From the Annual Meeting of the North American Menopause Society
VULVAR PAIN SYNDROMES A bounty of treatments—but not all of them are proven
- Part 1: Making the correct diagnosis
(September 2011) - Part 3: Vestibulodynia
(November 2011)
As we discussed in the first installment of this three-part series in the September issue of OBG Management, the causes of vulvar pain are many, and the diagnosis of this common complaint can be difficult. Once the diagnosis of vulvodynia has been made, however, the challenge shifts to finding an effective treatment. Here, our expert panel discusses the many options available, the data (or lack of it) behind each therapy, and what to do in refractory cases.
In Part 3 of this series, in the November issue, the focus will be vestibulodynia.

The lower vagina and vulva are richly supplied with peripheral nerves and are, therefore, sensitive to pain, particularly the region of the hymeneal ring. Although the pudendal nerve (arrow) courses through the area, it is an uncommon source of vulvar pain.
Management of vulvar pain begins with simple measures
Dr. Lonky: How do you approach treatment of vulvar pain syndromes?
Dr. Haefner: I often advise the patient to begin with simple measures. For example, I recommend that she wear cotton underwear during the day, but no underwear at night. If she perspires with exercise, wicking underwear may be helpful. I also counsel the patient to avoid vulvar irritants, douches, and the application of soap of any kind to the vulva. Cool gel packs are sometimes helpful.
When it comes to intercourse, I recommend adequate lubrication using any of a number of effective products, such as olive oil, vitamin E oil, Replens, Slippery Stuff, Astroglide, KY Liquid, and others.
There is an extensive list of lubricants at http://www.med.umich.edu/sexualhealth/resources/guide.htm
Topical agents might offer relief—but so might placebo
Dr. Lonky: What is the role of topical medications, including anesthetics, for treating vulvar pain syndromes?
Dr. Edwards: I don’t find topical medications to be particularly useful in the treatment of vulvodynia, except for lidocaine 2% jelly, or lidocaine 5% ointment, which tends to burn with application—but I never start a patient on only one medication, so judging the effectiveness of a topical therapy is difficult in that context. Good studies of topical medications in the treatment of vulvar pain syndromes are lacking, other than the recent report on amitriptyline and baclofen.1
Dr. Haefner: For minor degrees of pain, consider lidocaine 5% ointment.
Lidocaine/prilocaine (eutectic mixture of local anesthesia or LMX) may be used but can be irritating.
Doxepin 5% cream can be applied to skin daily, gradually increasing the number of daily applications to as many as four.
Topical amitriptyline 2% with baclofen 2% in a water washable base has also been used for point tenderness (squirt 0.5 cc from a syringe onto the finger and apply it to the affected area three times a day).1
Dr. Gunter: Topical estrogen is prescribed by many providers, but we lack studies supporting its efficacy, except for reversing hypoestrogenic changes in postmenopausal women. Some providers use a high-dose, compounded topical estrogen with lidocaine for vestibulodynia. Certainly, local hypoestrogenic changes should be reversed in postmenopausal women before a diagnosis of vulvodynia or vestibulodynia is given.
As for other topical therapies, they are widely used. Some women report improvement with application of plain petrolatum.2 Response rates of 33% to 46% after use of a topical placebo for vestibulodynia are well described in the literature.3,4
Topical analgesics are used frequently, either sporadically (during pain flares) or regularly (daily application). One method of application for localized vestibulodynia involves liberally coating a cotton ball with lidocaine 5% and then applying it to the vestibule overnight (for at least 8 hours of exposure). In this study, after 7 weeks, 76% of women were able to be sexually active, compared with 36% before the start of treatment. However, a randomized, placebo-controlled trial that included lidocaine 5% cream in one arm identified only a 20% reduction in pain for women who had localized vestibulodynia—although, in this trial, the lidocaine was massaged into the vestibule four times daily.5 In this study, interestingly enough, topical lidocaine was less effective than topical placebo, which produced a 33% response rate.3
Lidocaine gel has also been used, although some women report more local irritation with gel than with ointment.
Dr. Lonky: Do we have any data on topical application of other drugs?
Dr. Gunter: Compounded adjuvant medications have been evaluated. In a retrospective study of topical gabapentin in a Lipoderm base, women who had generalized or localized vulvodynia applied a dose of 2%, 4%, or 6% three times daily. Of these women, 80% experienced a reduction of at least 50% in the pain score. In addition, 67% of patients who had localized vestibular pain were able to resume intercourse.6
A retrospective review of 38 women who used 2% amitriptyline and 2% baclofen in a Lipoderm cream for localized vestibular pain found that 53% experienced an improvement in symptoms of at least 60%, but there was no change in the frequency of sexual intercourse.1
Do tricyclic antidepressants ease chronic pain?
Dr. Lonky: Let’s talk, for a moment, about the use of oral tricyclic antidepressants in the treatment of vulvar pain syndromes. What do we know?
Dr. Haefner: Tricyclic antidepressants are a common treatment for vulvar pain. This group of drugs (including amitriptyline [Elavil], nortriptyline [Pamelor], and desipramine [Norpramin]) has been used to treat many idiopathic chronic pain conditions. Published and presented reports indicate that these drugs elicit about a 60% response rate for various pain conditions. A trial by the National Institutes of Health (NIH) is under way, analyzing the use of antidepressants in women who have vulvar pain.
Although treatment with tricyclic antidepressants has generally been reserved for women who have generalized vulvodynia, recent reports have found these medications to be helpful in the treatment of vestibular pain as well. The mechanism of action is thought to be related to inhibition of the reuptake of transmitters—specifically, norepinephrine and serotonin. However, the mechanism of action may be more closely related to anticholinergic effects. Tricyclics affect sodium channels and the N-methyl-d-aspartate (NMDA) receptor.
If you choose to prescribe one of these medications, consider emphasizing to the patient its effect on the sensation of pain rather than its effect on depression.
Dr. Lonky: Are there any types of patients who should not take a tricyclic?
Dr. Haefner: Yes. A patient should not take a tricyclic if she is pregnant, breastfeeding, or planning to conceive. These medicines also add to the effects of alcohol and other central nervous system depressants.
Dr. Lonky: What dosage is recommended?
Dr. Haefner: The dosage for pain control varies, depending on the age of the patient and the particular agent used. Amitriptyline is often used as a first-line medication. I start the patient on 10 to 25 mg nightly and increase that amount by 10 to 25 mg weekly, not to exceed 150 mg daily. A sample regimen might be 10 mg at bedtime for 1 week. If symptoms persist, increase the dose to 20 mg at bedtime for another week, and so on. Once a dose is established that provides relief, the patient should continue to take that amount nightly. Advise the patient not to discontinue the drug abruptly. Rather, it should be weaned.
In patients who are 60 years or older, I give a starting dose of 5 to 10 mg and increase it by 10 mg weekly.
In all age groups, it is important to advise patients to avoid consuming more than one alcoholic beverage daily while taking this medication. And in reproductive-age women, contraception is critical.
Dr. Edwards: I call these drugs tricyclic medications rather than antidepressants. They are extremely useful in managing the neuropathic component of vulvar pain. Despite a recent, apparently well-conducted study showing a lack of benefit, my 25 years of personal clinical experience with tricyclics convince me that I should wait for follow-up studies before abandoning this therapy.3
The pain literature reports that higher doses than previously reported of tricyclic medications are needed for optimal management of neuropathic pain. Doses from 100 to 150 mg are often required for substantial improvement, and a major design flaw in many studies of the effect of tricyclic medications on vulvodynia is the use of an insufficient dose.
Because of their low cost and their effectiveness, tricyclic medications are my first-line therapy for women who do not suffer severe constipation or dry eyes. The effect on depression is a useful side effect, I find.
Dr. Gunter: Although adjuvant medications, such as antidepressants and anticonvulsants, are considered by more than 80% of practitioners to be effective for vulvodynia, it is important to understand that only one randomized, double-blind, placebo-controlled prospective study has evaluated this approach, and that study found a placebo response rate of 33%.3,7
Randomized studies indicate that low-dose amitriptyline (10–20 mg) and desipramine (150 mg) are ineffective for provoked vestibulodynia.3,8 Cohort and retrospective studies with higher doses of amitriptyline (40 to 60 mg/day) indicate that improvement in pain scores of 50% or more can be achieved for 47% to 59% of women who have localized provoked vestibulodynia and generalized unprovoked vulvodynia.9-11
Tricyclic antidepressants and anticonvulsants should be prescribed with caution for patients 65 years and older because they increase the risk of falls.
I give nortriptyline as a first-line agent to women who have both provoked and unprovoked pain. In general, it has fewer anticholinergic side effects than amitriptyline and is generic—it also is taken once daily. For women who have unprovoked pain, I use gabapentin as a second-line agent.
Dr. Lonky: Are any other antidepressants useful in the treatment of vulvar pain?
Dr. Haefner: I sometimes give duloxetine [Cymbalta], starting with an oral daily dose of 30 mg for 1 week. If symptoms persist, I increase the daily dose to a total of 60 mg. (If the patient is depressed, I have her take 30 mg twice daily; if she isn’t depressed, I have her take the full dose of 60 mg in the morning.) I also occasionally utilize venlafaxine [Effexor XR] for pain control, starting with an oral morning dose of 37.5 mg. This dose can be increased to 75 mg/day.
Dr. Edwards: Literature on venlafaxine for neuropathic pain suggests maximal effects at doses of 150 to 225 mg of the extended release formulation, which is often well tolerated. I start patients on 37.5 mg and increase weekly until I reach the 150-mg threshold.12
Are anticonvulsants effective pain relievers?
Dr. Lonky: How effective are oral anticonvulsants such as gabapentin [Neurontin]?
Dr. Haefner: Gabapentin has been used to treat chronic pain conditions. The drug is available in 100-mg, 300-mg, 400-mg, 600-mg, and 800-mg tablets. It is typically initiated at an oral dose of 300 mg daily for 3 days. The dosage is then increased to 300 mg twice daily for 3 days and, finally, to 300 mg three times daily. If necessary, it can gradually be increased to a total of 3,600 mg daily (usually divided into three doses). No more than 1,200 mg should be administered in a single dose. Side effects include somnolence, mental changes, dizziness, and weight gain.
Dr. Edwards: After tricyclic medications, I find gabapentin to be the most beneficial and easily tolerated agent. I give it to patients who have contraindications to tricyclics and who lack a strong component of depression.
Dr. Gunter: Retrospective reviews have found gabapentin to produce improvement of 80% or more in pain scores for 64% to 82% of women who have generalized unprovoked vulvodynia. And a small open-label, prospective trial of lamotrigine [Lamictal] found that it produced statistically significant improvement for generalized vulvodynia.13-15
Dr. Lonky: What do you know about the use of the anticonvulsants pregabalin [Lyrica] and topiramate [Topamax] to treat vulvar pain syndromes?
Dr. Gunter: Pregabalin was reported to reduce symptoms by 80% for generalized, unprovoked vulvodynia in one case report.16
Dr. Edwards: I find that pregabalin is less well tolerated (and more expensive) than gabapentin, so it is one of the last agents I prescribe. I reported a small, uncontrolled series of patients who were treated with pregabalin. Of those who tolerated the drug, two thirds of the women improved by approximately 62%, as judged by a visual analog scale.
Dr. Haefner: Pregabalin is a relatively new addition to the armamentarium. I give 50 mg orally for 4 days to start. If symptoms persist, I increase the dose to 50 mg twice a day for 4 days. If symptoms still persist, I up the dose again to 50 mg three times daily and gradually increase it to 100 mg three times daily, if necessary. Some reports describe a dose as high as 300 mg twice daily (maximum).
As for topiramate, I have been using it much more frequently for vulvodynia and noticing many fewer side effects than with gabapentin.
you offer them?
A patient who has a short duration of pain often responds to topical medications. In contrast, someone who has experienced pain for years is unlikely to get adequate relief from topical medications alone. These patients often require oral tricyclic antidepressants or anticonvulsants, or both. I often start these medications before deciding whether physical therapy is necessary. If the drugs do not provide adequate relief, then I refer the patient to physical therapy.
In some cases, I begin with physical therapy and add other treatments, if necessary. A patient who has localized vulvodynia who tightens her bulbocavernosus and levator ani muscles upon gentle touch may benefit from starting with physical therapy.
I reserve surgery—vestibulectomy—for the patient who has localized pain that has not responded to numerous treatments.
—Hope K. Haefner, MD
Does capsaicin interrupt the pain circuit?
Dr. Lonky: Capsaicin has been mentioned in the literature as a therapy for vulvar pain. Is it effective? How does it work?
Dr. Haefner: Capsaicin activates A-delta sensory neurons and unmyelinated C fibers. It is a vanillyl amide that evokes the sensation of burning pain. It has been proposed as a means of desensitization, which occurs as an acute reaction mediated by neuropeptides (including substance P).17 Steinberg and colleagues found that topical capsaicin significantly decreased pain with intercourse.17 Patients applied capsaicin 0.025% cream for 20 minutes daily for 12 weeks.
In a study by Murina and colleagues, 33 women were treated with topical capsaicin 0.05%. The capsaicin cream was applied to the vulva twice daily for 30 days, then once daily for 30 days, then twice weekly for 4 months. In this study, however, the response to treatment was only partial.18
Dr. Edwards: I have never had a patient willing to try capsaicin after I describe the therapy to them.
Dr. Gunter: Two studies have evaluated daily applications of capsaicin in concentrations of 0.025% and 0.05%—one of them the study by Murina and colleagues that Dr. Haefner mentioned.2,18 The initial release of substance P causes significant burning on application, so pretreatment with local anesthetic to help the patient tolerate the capsaicin is recommended, which could potentially confound the results. In one study, daily pain scores, as well as pain with intercourse, improved significantly for 59% of participants, but no patient experienced complete resolution of symptoms—and within 2 weeks after capsaicin was discontinued, symptoms returned.2,18 I have had only one patient in 15 years of practice who was willing to try capsaicin and who could get past the initial burning.
Another application for botulinum toxin type A?
Dr. Lonky: Is botulinum toxin type A [Botox] at all effective?
Dr. Haefner: Botulinum toxin type A has been utilized to treat provoked vestibulodynia as well as vaginismus and was beneficial.19-23 It blocks the cholinergic innervation of the target tissue. The therapeutic dose ranges from 20 IU to 300 IU.24
A placebo-controlled trial found that injection of 20 IU of botulinum toxin into the vestibule of women with vestibulodynia did not reduce pain, improve sexual functioning, or impact the quality of life, compared with placebo.25 However, this study utilized a lower dose of botulinum toxin than was used in many of the other studies.
Dr. Edwards: I have only used botulinum toxin type A in a low dose. I injected 6 IU of botulinum toxin A into the periphery of the vestibule at 3, 6, 9, and 12 o’clock in six patients, and half improved modestly. I am not prepared to use electromyography (EMG) localization in my office, but from anecdotal reports, as well as several small series and placebo-controlled trials, I would conclude that some patients improve. Those who have hypertonic pelvic floor muscles are likely to be the best candidates for this treatment.
Because the agent relieves pain only modestly, and because it is not covered by insurance for this application, I refer the patient to a gynecologist in my area who administers the drug under EMG localization.
Dr. Gunter: Given the well-documented effect on muscle spasticity, as well as studies that suggest they are also anti-nociceptive agents, botulinum toxins are certainly an attractive concept for vulvodynia. A small case series and a case report indicated significant improvement with vestibular injections of 20 to 40 U of botulinum toxin. However, a randomized, placebo-controlled, double-blind study indicated no significant improvement for women with localized vestibular pain.25-27
I discuss botulinum toxin A with my patients. I explain that my clinical experience does differ from results published in the literature. I find that many women with vestibulodynia opt to try an injection before proceeding to vestibulectomy.
When combined with pelvic floor physical therapy, botulinum toxins are highly effective at treating muscle spasm and can be very useful for women who have a component to their pain of vaginismus or high-tone pelvic floor dysfunction.
How useful are steroids and nerve blocks?
Dr. Lonky: Is there a role for local injections of glucocorticoids or serial nerve blocks?
Dr. Edwards: The occasional patient with very localized pain (trigger point) responds fairly well in my office to intralesional corticosteroids. I have not used or seen reports describing administration of intralesional corticosteroids into a larger area, although two practitioners have told me informally that it is useful in their hands.
As a dermatologist, I cannot perform blocks. I have referred patients to gyn and pain clinics for this purpose, but neither venue has been willing to administer the blocks.
Dr. Haefner: Patients who present with small, localized areas of pain may benefit from local injections. In small areas—for example, a painful spot 1 cm in diameter—triamcinolone acetonide in combination with bupivacaine may be helpful. It is important to use a small dose of steroid in a small area, however, because tissue erosion or ulceration can occur with too high a dose of steroid in the skin. For large areas, as much as 40 mg of triamcinolone acetonide may be utilized in a single monthly dose. Generally, the dose is repeated monthly, if necessary, as many as three or four times.
For large areas, bupivacaine 0.25% is utilized, and for small areas, bupivacaine 0.5% is injected into the vulva along with the steroid. The steroid should be drawn into the syringe first (because the vial can be used at a later time), followed by bupivacaine, which is a single-dose vial.
Pudendal nerve blocks using bupivacaine have been helpful in some patients—particularly those who have unilateral pain.
Dr. Gunter: Local injections with a variety of agents for localized, provoked vestibulodynia have been described, including steroids, botulinum toxins, and interferon. It is important to interpret these studies with caution, however, as placebo response rates with injection therapy are significant.25
A retrospective review of submucous injections of methylprednisolone and lidocaine found that 68% of women had a complete or marked response, and two case reports describe success with betamethasone.26,28-30 I offer steroid injections to women who have vestibulodynia before proceeding to vestibulectomy; I find that about 50% get at least partial relief. As Dr. Haefner indicated from her own experience, my success seems best with steroid injections in the vestibule when the painful area is smaller.
Using injectable steroids and botulinum toxins, I estimate that I can prevent 33% to 40% of vestibulectomies. Although this may not be better than the placebo response rate and certainly represents biased patient selection (patients are not required to try local injection before vestibulectomy for vestibulodynia), those who are successful are uniformly happy for trying it, and those for whom it did not work are not unhappy that they “gave it a try,” as these women are all motivated to avoid surgery, if possible.
Pudendal nerve blocks with triamcinolone are also described for women who have generalized unprovoked vulvodynia due to suspected pudendal neuralgia. Ganglion impar blocks—steroid injection around the terminal branch of the sympathetic chain in the presacral space—have also been performed with good results for generalized vulvodynia. I have had good success with pudendal nerve blocks for unilateral pain that is suspected to be pudendal in origin and also with ganglion impar blocks for women with generalized vulvodynia, especially postmenopausal women. I perform all of my own nerve blocks (FIGURE).
Ganglion impar block
Ganglion impar blocks—steroid injection around the terminal branch of the sympathetic chain in the presacral space—may provide relief from generalized vulvodynia in some women.
Does physical therapy play a role in easing vulvar pain?
Dr. Lonky: What is the role of physical therapy and pelvic floor muscle rehabilitation?
Dr. Gunter: All women who have high-tone pelvic floor dysfunction should be referred to a physical therapist. Many women who lack muscle spasm but experience vulvar pain can still benefit from physical therapy, as gentle stretching and vibration therapy can sometimes be helpful. A physical therapist can also perform biofeedback.
Dr. Edwards: Physical therapy is crucial. It is my first-line therapy overall, with adjunctive oral medication for neuropathic pain. Besides addressing pelvic floor abnormalities, physical therapy can serve as desensitization therapy and psychological support.
Dr. Haefner: Physical therapy has been successful in the treatment of a number of disorders, including migraine and tension headaches, asthma, and anxiety disorders. It is also used in the treatment of vulvar pain. Physical therapists who have experience in vulvar pain may be extremely helpful, particularly if there is concomitant vaginismus—which isn’t uncommon in this population.
For vulvodynia, techniques include internal (vaginal and rectal) and external soft-tissue mobilization and myofascial release; trigger-point pressure; visceral, urogenital, and joint manipulation; electrical stimulation; therapeutic exercises; active pelvic floor retraining; biofeedback; bladder and bowel retraining; and therapeutic ultrasound.
Biofeedback may be used to assist in developing self-regulation strategies for confronting and reducing pain. Patients who have vestibular pain in general have an increased resting tone and a decreased contraction tone. With the aid of an electronic measurement and amplification system or biofeedback machine, an individual can view a display of numbers on a meter, or colored lights, to assess nerve and muscle tension. In this way, she may be able to develop voluntary control over the biological systems involved in pain, discomfort, and disease.
The duration of physical therapy overall and the frequency of visits varies from person to person. Success rates in the range of 60% to 80% have been reported.
How should we respond when medical treatment fails?
Dr. Lonky: What is the proper approach to the patient who has recalcitrant vulvar pain who fails all medical treatments and is not a candidate for vestibulectomy because her pain is outside the vestibule?
Dr. Edwards: The pain clinic. Actually, in a perfect world, the role of the gynecologist or dermatologist would be to give the patient a diagnosis, after which a pain clinic would offer treatment.
All patients should receive counseling. And clinicians who lack expertise should refer the patient to a vulvodynia specialist.
Dr. Haefner: This type of patient may benefit from physical therapy. Bupivacaine steroid injections could also be considered. A sacral nerve stimulator should be considered if the other measures fail to provide adequate relief.
I agree that counseling is extremely helpful in the patient who has vulvodynia. Sexual counseling, with tips on positions for intercourse, lubricants, and control of uncomfortable situations, is of utmost importance.
Dr. Gunter: I offer oral medications and nerve blocks (typically, ganglion impar blocks), and many patients do well.
I also highly recommend advanced programs for mind-body techniques.
Patients who fail all therapies may be candidates for a nerve stimulator, depending on psychiatric comorbidities and response to selective diagnostic nerve blocks.
What’s in the pipeline?
Dr. Lonky: What therapies for vulvar pain are on the horizon?
Dr. Edwards: I believe that cognitive behavioral therapy, sex therapy, and couple counseling will play a larger role in the management of vulvar pain.
Dr. Gunter: Any therapy used in other pain conditions will probably eventually find its way to the management of vulvodynia.
Some investigators believe that Tarlov cysts play a role in vulvar pain and recommend that all women undergo sacral spine and nerve-root magnetic resonance imaging. The problem is that many asymptomatic women have Tarlov cysts, and the surgery to remove them is not at all risk-free. I strongly believe that more research is needed before we can suggest that Tarlov cysts be removed.
Dr. Haefner: Transcutaneous electrical stimulation and biofeedback have been used successfully in the treatment of vulvodynia.31 Some patients benefit from spinal cord stimulators, such as the sacral nerve stimulator, for pain control. Sacral nerve modulation (SNM) works primarily by modulation of the nerve signals to and from the pelvic floor muscles, bladder, and rectum. It applies low-amplitude electrical stimulation to the third sacral nerve via electrodes in a tined lead passing through the S3 foramen, which contains afferent sensory, efferent autonomic motor, and voluntary somatic nerves. Other studies have utilized a different spinal cord level. More studies are needed to demonstrate the full effect of SNM on vulvodynia.
A comprehensive review of the various treatments for vulvodynia can be found in the Journal of Lower Genital Tract Disease.2
Dr. Lonky: Thanks again for your expertise. We’ll focus on provoked vestibulodynia in the final installment of this series on vulvar pain, in the November 2011 issue of OBG Management.
Vulvar pain therapies mentioned in this discussion
| Lifestyle changes Cotton and/or wicking underwear Avoidance of vulvar irritants, douches, soap Use of lubricants during intercourse |
| Physical therapy Internal (vaginal and rectal) and external soft-tissue mobilization and myofascial release Trigger-point pressure Visceral, urogenital, and joint manipulation Electrical stimulation Therapeutic exercises Active pelvic floor retraining Biofeedback Bladder and bowel retraining Therapeutic ultrasound |
| Topical agents Lidocaine 2% jelly Lidocaine 5% ointment Lidocaine/prilocaine Doxepin 5% cream Amitriptyline 2%/baclofen 2% Estrogen Petrolatum Gabapentin |
Oral agents Tricyclic antidepressants
Other antidepressants
Anticonvulsants
|
| Other agents Capsaicin Botulinum toxin type A Corticosteroids Nerve block |
We want to hear from you! Tell us what you think.
1. Nyirjesy P, Lev-Sagie A, Mathew L, Culhane JF. Topical amitriptyline-baclofen cream for the treatment of provoked vestibulodynia. J Lower Genital Tract Dis. 2009;13(4):230-236.
2. Haefner HK, Collins ME, Davis GD, et al. The vulvodynia guideline. J Lower Genital Tract Dis. 2005;9(1):40-51.
3. Foster DC, Kotok MB, Huang L, et al. Oral desipramine and topical lidocaine for vulvodynia: a randomized controlled trial. Obstet Gynecol. 2010;116(3):583-593.
4. Nyirjesy P, Sobel JD, Weitz MV, Leaman DJ, Small MJ, Gelone SP. Cromolyn cream for recalcitrant idiopathic vulvar vestibulitis: results of a placebo-controlled trial. Sex Transm Infect. 2001;77(1):53-57.
5. Zolnoun DA, Hartmann KE, Steege JF. Overnight 5% lidocaine ointment for treatment of vulvar vestibulitis. Obstet Gynecol. 2003;102(1):84-87.
6. Boardman LA, Cooper AS, Blais LR, Raker CA. Topical gabapentin in the treatment of localized and generalized vulvodynia. Obstet Gynecol. 2008;112(3):579-585.
7. Reed BD, Haefner HK, Edwards L. A survey on diagnosis and treatment of vulvodynia researchers and members of the International Society for the Study of Vulvovaginal Disease. J Reprod Med. 2008;53(12):921-929.
8. Brown CS, Wan J, Bachmann G, Rosen R. Self-management amitriptyline, and amitriptyline plus triamcinolone in the management of vulvodynia. J Women Health (Larchmt). 2009;18(2):163-169.
9. Reed BD, Caron AM, Gorenflo DW, Haefner HK. Treatment of vulvodynia with tricyclic antidepressants: efficacy and associated factors. J Low Genit Tract Dis. 2006;10(4):245-251.
10. Munday PE. Response to treatment in dysaesthetic vulvodynia. J Obstet Gynecol. 2001;21(6):610-613.
11. McKay M. Dysesthetic (“essential”) vulvodynia: treatment with amitriptyline. J Reprod Med. 1993;38(1):9-13.
12. Dworkin RH, O’Connor AB, Backonja M, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132(3):237-251.
13. Harris G, Horowitz B, Borgida A. Evaluation of gabapentin in the treatment of generalized vulvodynia unprovoked. J Reprod Med. 2007;52(2):103-106.
14. Ben-David B, Friedman M. Gabapentin therapy for vulvodynia. Anesth Analg. 1999;89(6):1459-1460.
15. Meltzer-Brody SE, Zolnoun D, Steege JF, Rinaldi KL, Leserman J. Open-label trial of lamotrigine focusing on efficacy in vulvodynia. J Reprod Med. 2009;54(3):171-178.
16. Jerome L. Pregabalin-induced remission in a 62-year-old woman with a 20-year history of vulvodynia. Pain Res Manag. 2007;12(3):212-214.
17. Steinberg AC, Oyama IA, Rejba AE, Kellogg-Spadt S, Whitmore KE. Capsaicin for the treatment of vulvar vestibulitis. Am J Obstet Gynecol. 2005;192(5):1549-1553.
18. Murina F, Radici G, Bianco V. Capsaicin and the treatment of vulvar vestibulitis syndrome: a valuable alternative? Med General Med. 2004;6(4):48.-
19. Park AJ, Paraiso MFR. Successful use of botulinum toxin type A in the treatment of refractory postoperative dyspareunia. Obstet Gynecol. 2009;114(2 pt 2):484-487.
20. Brown CS, Glazer HI, Vogt V, Menkes D, Bachmann G. Subjective and objective outcomes of botulinum toxin type A treatment in vestibulodynia: pilot data. J Reprod Med. 2006;51(8):635-641.
21. Dykstra DD, Presthus J. Botulinum toxin type A for the treatment of provoked vestibulodynia: an open-label pilot study. J Reprod Med. 2006;51(6):467-470.
22. Romito S, Bottanelli M, Pellegrini M, Vicentini S, Rizzuto N, Bertolasi L. Botulinum toxin for the treatment of genital pain syndromes. Gynecol Obstet Invest. 2004;58(3):164-167.
23. Bertolasi L, Frasson E, Cappelletti JY, Vicentini S, Bordignon M, Graziottin A. Botulinum neurotoxin type A injections for vaginismus secondary to vulvar vestibulitis syndrome. Obstet Gynecol. 2009;114(5):1008-1016.
24. Presthus JB, Dykstra DD. Botulinum toxin therapy for vulvodynia. NVA News. National Vulvodynia Association. 2007;12(3).
25. Peterson CD, Giraldi A, Lundvall L, Kristensen E. Botulinum toxin type A—a novel treatment for provoked vestibulodynia? Results from a randomized placebo-controlled, double-blinded study. J Sex Med. 2009;6(9):2523-2537.
26. Yoon H, Chung WS, Shim BS. Botulinum toxin A for the management of vulvodynia. Int J Impot Res. 2007;19(1):84-87.
27. Gunter J, Brewer A, Tawfik O. Botulinum toxin A for vulvodynia: a case report. J Pain. 2004;5(4):238-240.
28. Segal D, Tifheret H, Lazr S. Submucous infiltration of betamethasone and lidocaine in the treatment of vulvar vestibulitis. Eur J Obstet Gynecol Reprod Biol. 2003;107(1):105-106.
29. Murina F, Tassan P, Roberti P, Bianco V. Treatment of vulvar vestibulitis with submucous infiltrations of methylprednisolone and lidocaine. An alternative approach. J Reprod Med. 2001;46(8):713-716.
30. Dede M, Yenen MC, Yilmaz A, Baser I. Successful treatment of persistent vulvodynia with submucous infiltration of betamethasone and lidocaine. Eur J Obstet Gynecol Reprod Biol. 2006;124(2):258-259.
31. Dionisi B, Anglana F, Inghirami P, Lippa P, Senatori R. Use of transcutaneous electrical stimulation and biofeedback for the treatment of vulvodynia (vulvar vestibular syndrome): results of 3 years of experience [in Italian]. Minerva Ginecol. 2008;60(6):485-491.
- Part 1: Making the correct diagnosis
(September 2011) - Part 3: Vestibulodynia
(November 2011)
As we discussed in the first installment of this three-part series in the September issue of OBG Management, the causes of vulvar pain are many, and the diagnosis of this common complaint can be difficult. Once the diagnosis of vulvodynia has been made, however, the challenge shifts to finding an effective treatment. Here, our expert panel discusses the many options available, the data (or lack of it) behind each therapy, and what to do in refractory cases.
In Part 3 of this series, in the November issue, the focus will be vestibulodynia.

The lower vagina and vulva are richly supplied with peripheral nerves and are, therefore, sensitive to pain, particularly the region of the hymeneal ring. Although the pudendal nerve (arrow) courses through the area, it is an uncommon source of vulvar pain.
Management of vulvar pain begins with simple measures
Dr. Lonky: How do you approach treatment of vulvar pain syndromes?
Dr. Haefner: I often advise the patient to begin with simple measures. For example, I recommend that she wear cotton underwear during the day, but no underwear at night. If she perspires with exercise, wicking underwear may be helpful. I also counsel the patient to avoid vulvar irritants, douches, and the application of soap of any kind to the vulva. Cool gel packs are sometimes helpful.
When it comes to intercourse, I recommend adequate lubrication using any of a number of effective products, such as olive oil, vitamin E oil, Replens, Slippery Stuff, Astroglide, KY Liquid, and others.
There is an extensive list of lubricants at http://www.med.umich.edu/sexualhealth/resources/guide.htm
Topical agents might offer relief—but so might placebo
Dr. Lonky: What is the role of topical medications, including anesthetics, for treating vulvar pain syndromes?
Dr. Edwards: I don’t find topical medications to be particularly useful in the treatment of vulvodynia, except for lidocaine 2% jelly, or lidocaine 5% ointment, which tends to burn with application—but I never start a patient on only one medication, so judging the effectiveness of a topical therapy is difficult in that context. Good studies of topical medications in the treatment of vulvar pain syndromes are lacking, other than the recent report on amitriptyline and baclofen.1
Dr. Haefner: For minor degrees of pain, consider lidocaine 5% ointment.
Lidocaine/prilocaine (eutectic mixture of local anesthesia or LMX) may be used but can be irritating.
Doxepin 5% cream can be applied to skin daily, gradually increasing the number of daily applications to as many as four.
Topical amitriptyline 2% with baclofen 2% in a water washable base has also been used for point tenderness (squirt 0.5 cc from a syringe onto the finger and apply it to the affected area three times a day).1
Dr. Gunter: Topical estrogen is prescribed by many providers, but we lack studies supporting its efficacy, except for reversing hypoestrogenic changes in postmenopausal women. Some providers use a high-dose, compounded topical estrogen with lidocaine for vestibulodynia. Certainly, local hypoestrogenic changes should be reversed in postmenopausal women before a diagnosis of vulvodynia or vestibulodynia is given.
As for other topical therapies, they are widely used. Some women report improvement with application of plain petrolatum.2 Response rates of 33% to 46% after use of a topical placebo for vestibulodynia are well described in the literature.3,4
Topical analgesics are used frequently, either sporadically (during pain flares) or regularly (daily application). One method of application for localized vestibulodynia involves liberally coating a cotton ball with lidocaine 5% and then applying it to the vestibule overnight (for at least 8 hours of exposure). In this study, after 7 weeks, 76% of women were able to be sexually active, compared with 36% before the start of treatment. However, a randomized, placebo-controlled trial that included lidocaine 5% cream in one arm identified only a 20% reduction in pain for women who had localized vestibulodynia—although, in this trial, the lidocaine was massaged into the vestibule four times daily.5 In this study, interestingly enough, topical lidocaine was less effective than topical placebo, which produced a 33% response rate.3
Lidocaine gel has also been used, although some women report more local irritation with gel than with ointment.
Dr. Lonky: Do we have any data on topical application of other drugs?
Dr. Gunter: Compounded adjuvant medications have been evaluated. In a retrospective study of topical gabapentin in a Lipoderm base, women who had generalized or localized vulvodynia applied a dose of 2%, 4%, or 6% three times daily. Of these women, 80% experienced a reduction of at least 50% in the pain score. In addition, 67% of patients who had localized vestibular pain were able to resume intercourse.6
A retrospective review of 38 women who used 2% amitriptyline and 2% baclofen in a Lipoderm cream for localized vestibular pain found that 53% experienced an improvement in symptoms of at least 60%, but there was no change in the frequency of sexual intercourse.1
Do tricyclic antidepressants ease chronic pain?
Dr. Lonky: Let’s talk, for a moment, about the use of oral tricyclic antidepressants in the treatment of vulvar pain syndromes. What do we know?
Dr. Haefner: Tricyclic antidepressants are a common treatment for vulvar pain. This group of drugs (including amitriptyline [Elavil], nortriptyline [Pamelor], and desipramine [Norpramin]) has been used to treat many idiopathic chronic pain conditions. Published and presented reports indicate that these drugs elicit about a 60% response rate for various pain conditions. A trial by the National Institutes of Health (NIH) is under way, analyzing the use of antidepressants in women who have vulvar pain.
Although treatment with tricyclic antidepressants has generally been reserved for women who have generalized vulvodynia, recent reports have found these medications to be helpful in the treatment of vestibular pain as well. The mechanism of action is thought to be related to inhibition of the reuptake of transmitters—specifically, norepinephrine and serotonin. However, the mechanism of action may be more closely related to anticholinergic effects. Tricyclics affect sodium channels and the N-methyl-d-aspartate (NMDA) receptor.
If you choose to prescribe one of these medications, consider emphasizing to the patient its effect on the sensation of pain rather than its effect on depression.
Dr. Lonky: Are there any types of patients who should not take a tricyclic?
Dr. Haefner: Yes. A patient should not take a tricyclic if she is pregnant, breastfeeding, or planning to conceive. These medicines also add to the effects of alcohol and other central nervous system depressants.
Dr. Lonky: What dosage is recommended?
Dr. Haefner: The dosage for pain control varies, depending on the age of the patient and the particular agent used. Amitriptyline is often used as a first-line medication. I start the patient on 10 to 25 mg nightly and increase that amount by 10 to 25 mg weekly, not to exceed 150 mg daily. A sample regimen might be 10 mg at bedtime for 1 week. If symptoms persist, increase the dose to 20 mg at bedtime for another week, and so on. Once a dose is established that provides relief, the patient should continue to take that amount nightly. Advise the patient not to discontinue the drug abruptly. Rather, it should be weaned.
In patients who are 60 years or older, I give a starting dose of 5 to 10 mg and increase it by 10 mg weekly.
In all age groups, it is important to advise patients to avoid consuming more than one alcoholic beverage daily while taking this medication. And in reproductive-age women, contraception is critical.
Dr. Edwards: I call these drugs tricyclic medications rather than antidepressants. They are extremely useful in managing the neuropathic component of vulvar pain. Despite a recent, apparently well-conducted study showing a lack of benefit, my 25 years of personal clinical experience with tricyclics convince me that I should wait for follow-up studies before abandoning this therapy.3
The pain literature reports that higher doses than previously reported of tricyclic medications are needed for optimal management of neuropathic pain. Doses from 100 to 150 mg are often required for substantial improvement, and a major design flaw in many studies of the effect of tricyclic medications on vulvodynia is the use of an insufficient dose.
Because of their low cost and their effectiveness, tricyclic medications are my first-line therapy for women who do not suffer severe constipation or dry eyes. The effect on depression is a useful side effect, I find.
Dr. Gunter: Although adjuvant medications, such as antidepressants and anticonvulsants, are considered by more than 80% of practitioners to be effective for vulvodynia, it is important to understand that only one randomized, double-blind, placebo-controlled prospective study has evaluated this approach, and that study found a placebo response rate of 33%.3,7
Randomized studies indicate that low-dose amitriptyline (10–20 mg) and desipramine (150 mg) are ineffective for provoked vestibulodynia.3,8 Cohort and retrospective studies with higher doses of amitriptyline (40 to 60 mg/day) indicate that improvement in pain scores of 50% or more can be achieved for 47% to 59% of women who have localized provoked vestibulodynia and generalized unprovoked vulvodynia.9-11
Tricyclic antidepressants and anticonvulsants should be prescribed with caution for patients 65 years and older because they increase the risk of falls.
I give nortriptyline as a first-line agent to women who have both provoked and unprovoked pain. In general, it has fewer anticholinergic side effects than amitriptyline and is generic—it also is taken once daily. For women who have unprovoked pain, I use gabapentin as a second-line agent.
Dr. Lonky: Are any other antidepressants useful in the treatment of vulvar pain?
Dr. Haefner: I sometimes give duloxetine [Cymbalta], starting with an oral daily dose of 30 mg for 1 week. If symptoms persist, I increase the daily dose to a total of 60 mg. (If the patient is depressed, I have her take 30 mg twice daily; if she isn’t depressed, I have her take the full dose of 60 mg in the morning.) I also occasionally utilize venlafaxine [Effexor XR] for pain control, starting with an oral morning dose of 37.5 mg. This dose can be increased to 75 mg/day.
Dr. Edwards: Literature on venlafaxine for neuropathic pain suggests maximal effects at doses of 150 to 225 mg of the extended release formulation, which is often well tolerated. I start patients on 37.5 mg and increase weekly until I reach the 150-mg threshold.12
Are anticonvulsants effective pain relievers?
Dr. Lonky: How effective are oral anticonvulsants such as gabapentin [Neurontin]?
Dr. Haefner: Gabapentin has been used to treat chronic pain conditions. The drug is available in 100-mg, 300-mg, 400-mg, 600-mg, and 800-mg tablets. It is typically initiated at an oral dose of 300 mg daily for 3 days. The dosage is then increased to 300 mg twice daily for 3 days and, finally, to 300 mg three times daily. If necessary, it can gradually be increased to a total of 3,600 mg daily (usually divided into three doses). No more than 1,200 mg should be administered in a single dose. Side effects include somnolence, mental changes, dizziness, and weight gain.
Dr. Edwards: After tricyclic medications, I find gabapentin to be the most beneficial and easily tolerated agent. I give it to patients who have contraindications to tricyclics and who lack a strong component of depression.
Dr. Gunter: Retrospective reviews have found gabapentin to produce improvement of 80% or more in pain scores for 64% to 82% of women who have generalized unprovoked vulvodynia. And a small open-label, prospective trial of lamotrigine [Lamictal] found that it produced statistically significant improvement for generalized vulvodynia.13-15
Dr. Lonky: What do you know about the use of the anticonvulsants pregabalin [Lyrica] and topiramate [Topamax] to treat vulvar pain syndromes?
Dr. Gunter: Pregabalin was reported to reduce symptoms by 80% for generalized, unprovoked vulvodynia in one case report.16
Dr. Edwards: I find that pregabalin is less well tolerated (and more expensive) than gabapentin, so it is one of the last agents I prescribe. I reported a small, uncontrolled series of patients who were treated with pregabalin. Of those who tolerated the drug, two thirds of the women improved by approximately 62%, as judged by a visual analog scale.
Dr. Haefner: Pregabalin is a relatively new addition to the armamentarium. I give 50 mg orally for 4 days to start. If symptoms persist, I increase the dose to 50 mg twice a day for 4 days. If symptoms still persist, I up the dose again to 50 mg three times daily and gradually increase it to 100 mg three times daily, if necessary. Some reports describe a dose as high as 300 mg twice daily (maximum).
As for topiramate, I have been using it much more frequently for vulvodynia and noticing many fewer side effects than with gabapentin.
you offer them?
A patient who has a short duration of pain often responds to topical medications. In contrast, someone who has experienced pain for years is unlikely to get adequate relief from topical medications alone. These patients often require oral tricyclic antidepressants or anticonvulsants, or both. I often start these medications before deciding whether physical therapy is necessary. If the drugs do not provide adequate relief, then I refer the patient to physical therapy.
In some cases, I begin with physical therapy and add other treatments, if necessary. A patient who has localized vulvodynia who tightens her bulbocavernosus and levator ani muscles upon gentle touch may benefit from starting with physical therapy.
I reserve surgery—vestibulectomy—for the patient who has localized pain that has not responded to numerous treatments.
—Hope K. Haefner, MD
Does capsaicin interrupt the pain circuit?
Dr. Lonky: Capsaicin has been mentioned in the literature as a therapy for vulvar pain. Is it effective? How does it work?
Dr. Haefner: Capsaicin activates A-delta sensory neurons and unmyelinated C fibers. It is a vanillyl amide that evokes the sensation of burning pain. It has been proposed as a means of desensitization, which occurs as an acute reaction mediated by neuropeptides (including substance P).17 Steinberg and colleagues found that topical capsaicin significantly decreased pain with intercourse.17 Patients applied capsaicin 0.025% cream for 20 minutes daily for 12 weeks.
In a study by Murina and colleagues, 33 women were treated with topical capsaicin 0.05%. The capsaicin cream was applied to the vulva twice daily for 30 days, then once daily for 30 days, then twice weekly for 4 months. In this study, however, the response to treatment was only partial.18
Dr. Edwards: I have never had a patient willing to try capsaicin after I describe the therapy to them.
Dr. Gunter: Two studies have evaluated daily applications of capsaicin in concentrations of 0.025% and 0.05%—one of them the study by Murina and colleagues that Dr. Haefner mentioned.2,18 The initial release of substance P causes significant burning on application, so pretreatment with local anesthetic to help the patient tolerate the capsaicin is recommended, which could potentially confound the results. In one study, daily pain scores, as well as pain with intercourse, improved significantly for 59% of participants, but no patient experienced complete resolution of symptoms—and within 2 weeks after capsaicin was discontinued, symptoms returned.2,18 I have had only one patient in 15 years of practice who was willing to try capsaicin and who could get past the initial burning.
Another application for botulinum toxin type A?
Dr. Lonky: Is botulinum toxin type A [Botox] at all effective?
Dr. Haefner: Botulinum toxin type A has been utilized to treat provoked vestibulodynia as well as vaginismus and was beneficial.19-23 It blocks the cholinergic innervation of the target tissue. The therapeutic dose ranges from 20 IU to 300 IU.24
A placebo-controlled trial found that injection of 20 IU of botulinum toxin into the vestibule of women with vestibulodynia did not reduce pain, improve sexual functioning, or impact the quality of life, compared with placebo.25 However, this study utilized a lower dose of botulinum toxin than was used in many of the other studies.
Dr. Edwards: I have only used botulinum toxin type A in a low dose. I injected 6 IU of botulinum toxin A into the periphery of the vestibule at 3, 6, 9, and 12 o’clock in six patients, and half improved modestly. I am not prepared to use electromyography (EMG) localization in my office, but from anecdotal reports, as well as several small series and placebo-controlled trials, I would conclude that some patients improve. Those who have hypertonic pelvic floor muscles are likely to be the best candidates for this treatment.
Because the agent relieves pain only modestly, and because it is not covered by insurance for this application, I refer the patient to a gynecologist in my area who administers the drug under EMG localization.
Dr. Gunter: Given the well-documented effect on muscle spasticity, as well as studies that suggest they are also anti-nociceptive agents, botulinum toxins are certainly an attractive concept for vulvodynia. A small case series and a case report indicated significant improvement with vestibular injections of 20 to 40 U of botulinum toxin. However, a randomized, placebo-controlled, double-blind study indicated no significant improvement for women with localized vestibular pain.25-27
I discuss botulinum toxin A with my patients. I explain that my clinical experience does differ from results published in the literature. I find that many women with vestibulodynia opt to try an injection before proceeding to vestibulectomy.
When combined with pelvic floor physical therapy, botulinum toxins are highly effective at treating muscle spasm and can be very useful for women who have a component to their pain of vaginismus or high-tone pelvic floor dysfunction.
How useful are steroids and nerve blocks?
Dr. Lonky: Is there a role for local injections of glucocorticoids or serial nerve blocks?
Dr. Edwards: The occasional patient with very localized pain (trigger point) responds fairly well in my office to intralesional corticosteroids. I have not used or seen reports describing administration of intralesional corticosteroids into a larger area, although two practitioners have told me informally that it is useful in their hands.
As a dermatologist, I cannot perform blocks. I have referred patients to gyn and pain clinics for this purpose, but neither venue has been willing to administer the blocks.
Dr. Haefner: Patients who present with small, localized areas of pain may benefit from local injections. In small areas—for example, a painful spot 1 cm in diameter—triamcinolone acetonide in combination with bupivacaine may be helpful. It is important to use a small dose of steroid in a small area, however, because tissue erosion or ulceration can occur with too high a dose of steroid in the skin. For large areas, as much as 40 mg of triamcinolone acetonide may be utilized in a single monthly dose. Generally, the dose is repeated monthly, if necessary, as many as three or four times.
For large areas, bupivacaine 0.25% is utilized, and for small areas, bupivacaine 0.5% is injected into the vulva along with the steroid. The steroid should be drawn into the syringe first (because the vial can be used at a later time), followed by bupivacaine, which is a single-dose vial.
Pudendal nerve blocks using bupivacaine have been helpful in some patients—particularly those who have unilateral pain.
Dr. Gunter: Local injections with a variety of agents for localized, provoked vestibulodynia have been described, including steroids, botulinum toxins, and interferon. It is important to interpret these studies with caution, however, as placebo response rates with injection therapy are significant.25
A retrospective review of submucous injections of methylprednisolone and lidocaine found that 68% of women had a complete or marked response, and two case reports describe success with betamethasone.26,28-30 I offer steroid injections to women who have vestibulodynia before proceeding to vestibulectomy; I find that about 50% get at least partial relief. As Dr. Haefner indicated from her own experience, my success seems best with steroid injections in the vestibule when the painful area is smaller.
Using injectable steroids and botulinum toxins, I estimate that I can prevent 33% to 40% of vestibulectomies. Although this may not be better than the placebo response rate and certainly represents biased patient selection (patients are not required to try local injection before vestibulectomy for vestibulodynia), those who are successful are uniformly happy for trying it, and those for whom it did not work are not unhappy that they “gave it a try,” as these women are all motivated to avoid surgery, if possible.
Pudendal nerve blocks with triamcinolone are also described for women who have generalized unprovoked vulvodynia due to suspected pudendal neuralgia. Ganglion impar blocks—steroid injection around the terminal branch of the sympathetic chain in the presacral space—have also been performed with good results for generalized vulvodynia. I have had good success with pudendal nerve blocks for unilateral pain that is suspected to be pudendal in origin and also with ganglion impar blocks for women with generalized vulvodynia, especially postmenopausal women. I perform all of my own nerve blocks (FIGURE).

Ganglion impar block
Ganglion impar blocks—steroid injection around the terminal branch of the sympathetic chain in the presacral space—may provide relief from generalized vulvodynia in some women.
Does physical therapy play a role in easing vulvar pain?
Dr. Lonky: What is the role of physical therapy and pelvic floor muscle rehabilitation?
Dr. Gunter: All women who have high-tone pelvic floor dysfunction should be referred to a physical therapist. Many women who lack muscle spasm but experience vulvar pain can still benefit from physical therapy, as gentle stretching and vibration therapy can sometimes be helpful. A physical therapist can also perform biofeedback.
Dr. Edwards: Physical therapy is crucial. It is my first-line therapy overall, with adjunctive oral medication for neuropathic pain. Besides addressing pelvic floor abnormalities, physical therapy can serve as desensitization therapy and psychological support.
Dr. Haefner: Physical therapy has been successful in the treatment of a number of disorders, including migraine and tension headaches, asthma, and anxiety disorders. It is also used in the treatment of vulvar pain. Physical therapists who have experience in vulvar pain may be extremely helpful, particularly if there is concomitant vaginismus—which isn’t uncommon in this population.
For vulvodynia, techniques include internal (vaginal and rectal) and external soft-tissue mobilization and myofascial release; trigger-point pressure; visceral, urogenital, and joint manipulation; electrical stimulation; therapeutic exercises; active pelvic floor retraining; biofeedback; bladder and bowel retraining; and therapeutic ultrasound.
Biofeedback may be used to assist in developing self-regulation strategies for confronting and reducing pain. Patients who have vestibular pain in general have an increased resting tone and a decreased contraction tone. With the aid of an electronic measurement and amplification system or biofeedback machine, an individual can view a display of numbers on a meter, or colored lights, to assess nerve and muscle tension. In this way, she may be able to develop voluntary control over the biological systems involved in pain, discomfort, and disease.
The duration of physical therapy overall and the frequency of visits varies from person to person. Success rates in the range of 60% to 80% have been reported.
How should we respond when medical treatment fails?
Dr. Lonky: What is the proper approach to the patient who has recalcitrant vulvar pain who fails all medical treatments and is not a candidate for vestibulectomy because her pain is outside the vestibule?
Dr. Edwards: The pain clinic. Actually, in a perfect world, the role of the gynecologist or dermatologist would be to give the patient a diagnosis, after which a pain clinic would offer treatment.
All patients should receive counseling. And clinicians who lack expertise should refer the patient to a vulvodynia specialist.
Dr. Haefner: This type of patient may benefit from physical therapy. Bupivacaine steroid injections could also be considered. A sacral nerve stimulator should be considered if the other measures fail to provide adequate relief.
I agree that counseling is extremely helpful in the patient who has vulvodynia. Sexual counseling, with tips on positions for intercourse, lubricants, and control of uncomfortable situations, is of utmost importance.
Dr. Gunter: I offer oral medications and nerve blocks (typically, ganglion impar blocks), and many patients do well.
I also highly recommend advanced programs for mind-body techniques.
Patients who fail all therapies may be candidates for a nerve stimulator, depending on psychiatric comorbidities and response to selective diagnostic nerve blocks.
What’s in the pipeline?
Dr. Lonky: What therapies for vulvar pain are on the horizon?
Dr. Edwards: I believe that cognitive behavioral therapy, sex therapy, and couple counseling will play a larger role in the management of vulvar pain.
Dr. Gunter: Any therapy used in other pain conditions will probably eventually find its way to the management of vulvodynia.
Some investigators believe that Tarlov cysts play a role in vulvar pain and recommend that all women undergo sacral spine and nerve-root magnetic resonance imaging. The problem is that many asymptomatic women have Tarlov cysts, and the surgery to remove them is not at all risk-free. I strongly believe that more research is needed before we can suggest that Tarlov cysts be removed.
Dr. Haefner: Transcutaneous electrical stimulation and biofeedback have been used successfully in the treatment of vulvodynia.31 Some patients benefit from spinal cord stimulators, such as the sacral nerve stimulator, for pain control. Sacral nerve modulation (SNM) works primarily by modulation of the nerve signals to and from the pelvic floor muscles, bladder, and rectum. It applies low-amplitude electrical stimulation to the third sacral nerve via electrodes in a tined lead passing through the S3 foramen, which contains afferent sensory, efferent autonomic motor, and voluntary somatic nerves. Other studies have utilized a different spinal cord level. More studies are needed to demonstrate the full effect of SNM on vulvodynia.
A comprehensive review of the various treatments for vulvodynia can be found in the Journal of Lower Genital Tract Disease.2
Dr. Lonky: Thanks again for your expertise. We’ll focus on provoked vestibulodynia in the final installment of this series on vulvar pain, in the November 2011 issue of OBG Management.
Vulvar pain therapies mentioned in this discussion
| Lifestyle changes Cotton and/or wicking underwear Avoidance of vulvar irritants, douches, soap Use of lubricants during intercourse |
| Physical therapy Internal (vaginal and rectal) and external soft-tissue mobilization and myofascial release Trigger-point pressure Visceral, urogenital, and joint manipulation Electrical stimulation Therapeutic exercises Active pelvic floor retraining Biofeedback Bladder and bowel retraining Therapeutic ultrasound |
| Topical agents Lidocaine 2% jelly Lidocaine 5% ointment Lidocaine/prilocaine Doxepin 5% cream Amitriptyline 2%/baclofen 2% Estrogen Petrolatum Gabapentin |
Oral agents Tricyclic antidepressants
Other antidepressants
Anticonvulsants
|
| Other agents Capsaicin Botulinum toxin type A Corticosteroids Nerve block |
We want to hear from you! Tell us what you think.
- Part 1: Making the correct diagnosis
(September 2011) - Part 3: Vestibulodynia
(November 2011)
As we discussed in the first installment of this three-part series in the September issue of OBG Management, the causes of vulvar pain are many, and the diagnosis of this common complaint can be difficult. Once the diagnosis of vulvodynia has been made, however, the challenge shifts to finding an effective treatment. Here, our expert panel discusses the many options available, the data (or lack of it) behind each therapy, and what to do in refractory cases.
In Part 3 of this series, in the November issue, the focus will be vestibulodynia.

The lower vagina and vulva are richly supplied with peripheral nerves and are, therefore, sensitive to pain, particularly the region of the hymeneal ring. Although the pudendal nerve (arrow) courses through the area, it is an uncommon source of vulvar pain.
Management of vulvar pain begins with simple measures
Dr. Lonky: How do you approach treatment of vulvar pain syndromes?
Dr. Haefner: I often advise the patient to begin with simple measures. For example, I recommend that she wear cotton underwear during the day, but no underwear at night. If she perspires with exercise, wicking underwear may be helpful. I also counsel the patient to avoid vulvar irritants, douches, and the application of soap of any kind to the vulva. Cool gel packs are sometimes helpful.
When it comes to intercourse, I recommend adequate lubrication using any of a number of effective products, such as olive oil, vitamin E oil, Replens, Slippery Stuff, Astroglide, KY Liquid, and others.
There is an extensive list of lubricants at http://www.med.umich.edu/sexualhealth/resources/guide.htm
Topical agents might offer relief—but so might placebo
Dr. Lonky: What is the role of topical medications, including anesthetics, for treating vulvar pain syndromes?
Dr. Edwards: I don’t find topical medications to be particularly useful in the treatment of vulvodynia, except for lidocaine 2% jelly, or lidocaine 5% ointment, which tends to burn with application—but I never start a patient on only one medication, so judging the effectiveness of a topical therapy is difficult in that context. Good studies of topical medications in the treatment of vulvar pain syndromes are lacking, other than the recent report on amitriptyline and baclofen.1
Dr. Haefner: For minor degrees of pain, consider lidocaine 5% ointment.
Lidocaine/prilocaine (eutectic mixture of local anesthesia or LMX) may be used but can be irritating.
Doxepin 5% cream can be applied to skin daily, gradually increasing the number of daily applications to as many as four.
Topical amitriptyline 2% with baclofen 2% in a water washable base has also been used for point tenderness (squirt 0.5 cc from a syringe onto the finger and apply it to the affected area three times a day).1
Dr. Gunter: Topical estrogen is prescribed by many providers, but we lack studies supporting its efficacy, except for reversing hypoestrogenic changes in postmenopausal women. Some providers use a high-dose, compounded topical estrogen with lidocaine for vestibulodynia. Certainly, local hypoestrogenic changes should be reversed in postmenopausal women before a diagnosis of vulvodynia or vestibulodynia is given.
As for other topical therapies, they are widely used. Some women report improvement with application of plain petrolatum.2 Response rates of 33% to 46% after use of a topical placebo for vestibulodynia are well described in the literature.3,4
Topical analgesics are used frequently, either sporadically (during pain flares) or regularly (daily application). One method of application for localized vestibulodynia involves liberally coating a cotton ball with lidocaine 5% and then applying it to the vestibule overnight (for at least 8 hours of exposure). In this study, after 7 weeks, 76% of women were able to be sexually active, compared with 36% before the start of treatment. However, a randomized, placebo-controlled trial that included lidocaine 5% cream in one arm identified only a 20% reduction in pain for women who had localized vestibulodynia—although, in this trial, the lidocaine was massaged into the vestibule four times daily.5 In this study, interestingly enough, topical lidocaine was less effective than topical placebo, which produced a 33% response rate.3
Lidocaine gel has also been used, although some women report more local irritation with gel than with ointment.
Dr. Lonky: Do we have any data on topical application of other drugs?
Dr. Gunter: Compounded adjuvant medications have been evaluated. In a retrospective study of topical gabapentin in a Lipoderm base, women who had generalized or localized vulvodynia applied a dose of 2%, 4%, or 6% three times daily. Of these women, 80% experienced a reduction of at least 50% in the pain score. In addition, 67% of patients who had localized vestibular pain were able to resume intercourse.6
A retrospective review of 38 women who used 2% amitriptyline and 2% baclofen in a Lipoderm cream for localized vestibular pain found that 53% experienced an improvement in symptoms of at least 60%, but there was no change in the frequency of sexual intercourse.1
Do tricyclic antidepressants ease chronic pain?
Dr. Lonky: Let’s talk, for a moment, about the use of oral tricyclic antidepressants in the treatment of vulvar pain syndromes. What do we know?
Dr. Haefner: Tricyclic antidepressants are a common treatment for vulvar pain. This group of drugs (including amitriptyline [Elavil], nortriptyline [Pamelor], and desipramine [Norpramin]) has been used to treat many idiopathic chronic pain conditions. Published and presented reports indicate that these drugs elicit about a 60% response rate for various pain conditions. A trial by the National Institutes of Health (NIH) is under way, analyzing the use of antidepressants in women who have vulvar pain.
Although treatment with tricyclic antidepressants has generally been reserved for women who have generalized vulvodynia, recent reports have found these medications to be helpful in the treatment of vestibular pain as well. The mechanism of action is thought to be related to inhibition of the reuptake of transmitters—specifically, norepinephrine and serotonin. However, the mechanism of action may be more closely related to anticholinergic effects. Tricyclics affect sodium channels and the N-methyl-d-aspartate (NMDA) receptor.
If you choose to prescribe one of these medications, consider emphasizing to the patient its effect on the sensation of pain rather than its effect on depression.
Dr. Lonky: Are there any types of patients who should not take a tricyclic?
Dr. Haefner: Yes. A patient should not take a tricyclic if she is pregnant, breastfeeding, or planning to conceive. These medicines also add to the effects of alcohol and other central nervous system depressants.
Dr. Lonky: What dosage is recommended?
Dr. Haefner: The dosage for pain control varies, depending on the age of the patient and the particular agent used. Amitriptyline is often used as a first-line medication. I start the patient on 10 to 25 mg nightly and increase that amount by 10 to 25 mg weekly, not to exceed 150 mg daily. A sample regimen might be 10 mg at bedtime for 1 week. If symptoms persist, increase the dose to 20 mg at bedtime for another week, and so on. Once a dose is established that provides relief, the patient should continue to take that amount nightly. Advise the patient not to discontinue the drug abruptly. Rather, it should be weaned.
In patients who are 60 years or older, I give a starting dose of 5 to 10 mg and increase it by 10 mg weekly.
In all age groups, it is important to advise patients to avoid consuming more than one alcoholic beverage daily while taking this medication. And in reproductive-age women, contraception is critical.
Dr. Edwards: I call these drugs tricyclic medications rather than antidepressants. They are extremely useful in managing the neuropathic component of vulvar pain. Despite a recent, apparently well-conducted study showing a lack of benefit, my 25 years of personal clinical experience with tricyclics convince me that I should wait for follow-up studies before abandoning this therapy.3
The pain literature reports that higher doses than previously reported of tricyclic medications are needed for optimal management of neuropathic pain. Doses from 100 to 150 mg are often required for substantial improvement, and a major design flaw in many studies of the effect of tricyclic medications on vulvodynia is the use of an insufficient dose.
Because of their low cost and their effectiveness, tricyclic medications are my first-line therapy for women who do not suffer severe constipation or dry eyes. The effect on depression is a useful side effect, I find.
Dr. Gunter: Although adjuvant medications, such as antidepressants and anticonvulsants, are considered by more than 80% of practitioners to be effective for vulvodynia, it is important to understand that only one randomized, double-blind, placebo-controlled prospective study has evaluated this approach, and that study found a placebo response rate of 33%.3,7
Randomized studies indicate that low-dose amitriptyline (10–20 mg) and desipramine (150 mg) are ineffective for provoked vestibulodynia.3,8 Cohort and retrospective studies with higher doses of amitriptyline (40 to 60 mg/day) indicate that improvement in pain scores of 50% or more can be achieved for 47% to 59% of women who have localized provoked vestibulodynia and generalized unprovoked vulvodynia.9-11
Tricyclic antidepressants and anticonvulsants should be prescribed with caution for patients 65 years and older because they increase the risk of falls.
I give nortriptyline as a first-line agent to women who have both provoked and unprovoked pain. In general, it has fewer anticholinergic side effects than amitriptyline and is generic—it also is taken once daily. For women who have unprovoked pain, I use gabapentin as a second-line agent.
Dr. Lonky: Are any other antidepressants useful in the treatment of vulvar pain?
Dr. Haefner: I sometimes give duloxetine [Cymbalta], starting with an oral daily dose of 30 mg for 1 week. If symptoms persist, I increase the daily dose to a total of 60 mg. (If the patient is depressed, I have her take 30 mg twice daily; if she isn’t depressed, I have her take the full dose of 60 mg in the morning.) I also occasionally utilize venlafaxine [Effexor XR] for pain control, starting with an oral morning dose of 37.5 mg. This dose can be increased to 75 mg/day.
Dr. Edwards: Literature on venlafaxine for neuropathic pain suggests maximal effects at doses of 150 to 225 mg of the extended release formulation, which is often well tolerated. I start patients on 37.5 mg and increase weekly until I reach the 150-mg threshold.12
Are anticonvulsants effective pain relievers?
Dr. Lonky: How effective are oral anticonvulsants such as gabapentin [Neurontin]?
Dr. Haefner: Gabapentin has been used to treat chronic pain conditions. The drug is available in 100-mg, 300-mg, 400-mg, 600-mg, and 800-mg tablets. It is typically initiated at an oral dose of 300 mg daily for 3 days. The dosage is then increased to 300 mg twice daily for 3 days and, finally, to 300 mg three times daily. If necessary, it can gradually be increased to a total of 3,600 mg daily (usually divided into three doses). No more than 1,200 mg should be administered in a single dose. Side effects include somnolence, mental changes, dizziness, and weight gain.
Dr. Edwards: After tricyclic medications, I find gabapentin to be the most beneficial and easily tolerated agent. I give it to patients who have contraindications to tricyclics and who lack a strong component of depression.
Dr. Gunter: Retrospective reviews have found gabapentin to produce improvement of 80% or more in pain scores for 64% to 82% of women who have generalized unprovoked vulvodynia. And a small open-label, prospective trial of lamotrigine [Lamictal] found that it produced statistically significant improvement for generalized vulvodynia.13-15
Dr. Lonky: What do you know about the use of the anticonvulsants pregabalin [Lyrica] and topiramate [Topamax] to treat vulvar pain syndromes?
Dr. Gunter: Pregabalin was reported to reduce symptoms by 80% for generalized, unprovoked vulvodynia in one case report.16
Dr. Edwards: I find that pregabalin is less well tolerated (and more expensive) than gabapentin, so it is one of the last agents I prescribe. I reported a small, uncontrolled series of patients who were treated with pregabalin. Of those who tolerated the drug, two thirds of the women improved by approximately 62%, as judged by a visual analog scale.
Dr. Haefner: Pregabalin is a relatively new addition to the armamentarium. I give 50 mg orally for 4 days to start. If symptoms persist, I increase the dose to 50 mg twice a day for 4 days. If symptoms still persist, I up the dose again to 50 mg three times daily and gradually increase it to 100 mg three times daily, if necessary. Some reports describe a dose as high as 300 mg twice daily (maximum).
As for topiramate, I have been using it much more frequently for vulvodynia and noticing many fewer side effects than with gabapentin.
you offer them?
A patient who has a short duration of pain often responds to topical medications. In contrast, someone who has experienced pain for years is unlikely to get adequate relief from topical medications alone. These patients often require oral tricyclic antidepressants or anticonvulsants, or both. I often start these medications before deciding whether physical therapy is necessary. If the drugs do not provide adequate relief, then I refer the patient to physical therapy.
In some cases, I begin with physical therapy and add other treatments, if necessary. A patient who has localized vulvodynia who tightens her bulbocavernosus and levator ani muscles upon gentle touch may benefit from starting with physical therapy.
I reserve surgery—vestibulectomy—for the patient who has localized pain that has not responded to numerous treatments.
—Hope K. Haefner, MD
Does capsaicin interrupt the pain circuit?
Dr. Lonky: Capsaicin has been mentioned in the literature as a therapy for vulvar pain. Is it effective? How does it work?
Dr. Haefner: Capsaicin activates A-delta sensory neurons and unmyelinated C fibers. It is a vanillyl amide that evokes the sensation of burning pain. It has been proposed as a means of desensitization, which occurs as an acute reaction mediated by neuropeptides (including substance P).17 Steinberg and colleagues found that topical capsaicin significantly decreased pain with intercourse.17 Patients applied capsaicin 0.025% cream for 20 minutes daily for 12 weeks.
In a study by Murina and colleagues, 33 women were treated with topical capsaicin 0.05%. The capsaicin cream was applied to the vulva twice daily for 30 days, then once daily for 30 days, then twice weekly for 4 months. In this study, however, the response to treatment was only partial.18
Dr. Edwards: I have never had a patient willing to try capsaicin after I describe the therapy to them.
Dr. Gunter: Two studies have evaluated daily applications of capsaicin in concentrations of 0.025% and 0.05%—one of them the study by Murina and colleagues that Dr. Haefner mentioned.2,18 The initial release of substance P causes significant burning on application, so pretreatment with local anesthetic to help the patient tolerate the capsaicin is recommended, which could potentially confound the results. In one study, daily pain scores, as well as pain with intercourse, improved significantly for 59% of participants, but no patient experienced complete resolution of symptoms—and within 2 weeks after capsaicin was discontinued, symptoms returned.2,18 I have had only one patient in 15 years of practice who was willing to try capsaicin and who could get past the initial burning.
Another application for botulinum toxin type A?
Dr. Lonky: Is botulinum toxin type A [Botox] at all effective?
Dr. Haefner: Botulinum toxin type A has been utilized to treat provoked vestibulodynia as well as vaginismus and was beneficial.19-23 It blocks the cholinergic innervation of the target tissue. The therapeutic dose ranges from 20 IU to 300 IU.24
A placebo-controlled trial found that injection of 20 IU of botulinum toxin into the vestibule of women with vestibulodynia did not reduce pain, improve sexual functioning, or impact the quality of life, compared with placebo.25 However, this study utilized a lower dose of botulinum toxin than was used in many of the other studies.
Dr. Edwards: I have only used botulinum toxin type A in a low dose. I injected 6 IU of botulinum toxin A into the periphery of the vestibule at 3, 6, 9, and 12 o’clock in six patients, and half improved modestly. I am not prepared to use electromyography (EMG) localization in my office, but from anecdotal reports, as well as several small series and placebo-controlled trials, I would conclude that some patients improve. Those who have hypertonic pelvic floor muscles are likely to be the best candidates for this treatment.
Because the agent relieves pain only modestly, and because it is not covered by insurance for this application, I refer the patient to a gynecologist in my area who administers the drug under EMG localization.
Dr. Gunter: Given the well-documented effect on muscle spasticity, as well as studies that suggest they are also anti-nociceptive agents, botulinum toxins are certainly an attractive concept for vulvodynia. A small case series and a case report indicated significant improvement with vestibular injections of 20 to 40 U of botulinum toxin. However, a randomized, placebo-controlled, double-blind study indicated no significant improvement for women with localized vestibular pain.25-27
I discuss botulinum toxin A with my patients. I explain that my clinical experience does differ from results published in the literature. I find that many women with vestibulodynia opt to try an injection before proceeding to vestibulectomy.
When combined with pelvic floor physical therapy, botulinum toxins are highly effective at treating muscle spasm and can be very useful for women who have a component to their pain of vaginismus or high-tone pelvic floor dysfunction.
How useful are steroids and nerve blocks?
Dr. Lonky: Is there a role for local injections of glucocorticoids or serial nerve blocks?
Dr. Edwards: The occasional patient with very localized pain (trigger point) responds fairly well in my office to intralesional corticosteroids. I have not used or seen reports describing administration of intralesional corticosteroids into a larger area, although two practitioners have told me informally that it is useful in their hands.
As a dermatologist, I cannot perform blocks. I have referred patients to gyn and pain clinics for this purpose, but neither venue has been willing to administer the blocks.
Dr. Haefner: Patients who present with small, localized areas of pain may benefit from local injections. In small areas—for example, a painful spot 1 cm in diameter—triamcinolone acetonide in combination with bupivacaine may be helpful. It is important to use a small dose of steroid in a small area, however, because tissue erosion or ulceration can occur with too high a dose of steroid in the skin. For large areas, as much as 40 mg of triamcinolone acetonide may be utilized in a single monthly dose. Generally, the dose is repeated monthly, if necessary, as many as three or four times.
For large areas, bupivacaine 0.25% is utilized, and for small areas, bupivacaine 0.5% is injected into the vulva along with the steroid. The steroid should be drawn into the syringe first (because the vial can be used at a later time), followed by bupivacaine, which is a single-dose vial.
Pudendal nerve blocks using bupivacaine have been helpful in some patients—particularly those who have unilateral pain.
Dr. Gunter: Local injections with a variety of agents for localized, provoked vestibulodynia have been described, including steroids, botulinum toxins, and interferon. It is important to interpret these studies with caution, however, as placebo response rates with injection therapy are significant.25
A retrospective review of submucous injections of methylprednisolone and lidocaine found that 68% of women had a complete or marked response, and two case reports describe success with betamethasone.26,28-30 I offer steroid injections to women who have vestibulodynia before proceeding to vestibulectomy; I find that about 50% get at least partial relief. As Dr. Haefner indicated from her own experience, my success seems best with steroid injections in the vestibule when the painful area is smaller.
Using injectable steroids and botulinum toxins, I estimate that I can prevent 33% to 40% of vestibulectomies. Although this may not be better than the placebo response rate and certainly represents biased patient selection (patients are not required to try local injection before vestibulectomy for vestibulodynia), those who are successful are uniformly happy for trying it, and those for whom it did not work are not unhappy that they “gave it a try,” as these women are all motivated to avoid surgery, if possible.
Pudendal nerve blocks with triamcinolone are also described for women who have generalized unprovoked vulvodynia due to suspected pudendal neuralgia. Ganglion impar blocks—steroid injection around the terminal branch of the sympathetic chain in the presacral space—have also been performed with good results for generalized vulvodynia. I have had good success with pudendal nerve blocks for unilateral pain that is suspected to be pudendal in origin and also with ganglion impar blocks for women with generalized vulvodynia, especially postmenopausal women. I perform all of my own nerve blocks (FIGURE).

Ganglion impar block
Ganglion impar blocks—steroid injection around the terminal branch of the sympathetic chain in the presacral space—may provide relief from generalized vulvodynia in some women.
Does physical therapy play a role in easing vulvar pain?
Dr. Lonky: What is the role of physical therapy and pelvic floor muscle rehabilitation?
Dr. Gunter: All women who have high-tone pelvic floor dysfunction should be referred to a physical therapist. Many women who lack muscle spasm but experience vulvar pain can still benefit from physical therapy, as gentle stretching and vibration therapy can sometimes be helpful. A physical therapist can also perform biofeedback.
Dr. Edwards: Physical therapy is crucial. It is my first-line therapy overall, with adjunctive oral medication for neuropathic pain. Besides addressing pelvic floor abnormalities, physical therapy can serve as desensitization therapy and psychological support.
Dr. Haefner: Physical therapy has been successful in the treatment of a number of disorders, including migraine and tension headaches, asthma, and anxiety disorders. It is also used in the treatment of vulvar pain. Physical therapists who have experience in vulvar pain may be extremely helpful, particularly if there is concomitant vaginismus—which isn’t uncommon in this population.
For vulvodynia, techniques include internal (vaginal and rectal) and external soft-tissue mobilization and myofascial release; trigger-point pressure; visceral, urogenital, and joint manipulation; electrical stimulation; therapeutic exercises; active pelvic floor retraining; biofeedback; bladder and bowel retraining; and therapeutic ultrasound.
Biofeedback may be used to assist in developing self-regulation strategies for confronting and reducing pain. Patients who have vestibular pain in general have an increased resting tone and a decreased contraction tone. With the aid of an electronic measurement and amplification system or biofeedback machine, an individual can view a display of numbers on a meter, or colored lights, to assess nerve and muscle tension. In this way, she may be able to develop voluntary control over the biological systems involved in pain, discomfort, and disease.
The duration of physical therapy overall and the frequency of visits varies from person to person. Success rates in the range of 60% to 80% have been reported.
How should we respond when medical treatment fails?
Dr. Lonky: What is the proper approach to the patient who has recalcitrant vulvar pain who fails all medical treatments and is not a candidate for vestibulectomy because her pain is outside the vestibule?
Dr. Edwards: The pain clinic. Actually, in a perfect world, the role of the gynecologist or dermatologist would be to give the patient a diagnosis, after which a pain clinic would offer treatment.
All patients should receive counseling. And clinicians who lack expertise should refer the patient to a vulvodynia specialist.
Dr. Haefner: This type of patient may benefit from physical therapy. Bupivacaine steroid injections could also be considered. A sacral nerve stimulator should be considered if the other measures fail to provide adequate relief.
I agree that counseling is extremely helpful in the patient who has vulvodynia. Sexual counseling, with tips on positions for intercourse, lubricants, and control of uncomfortable situations, is of utmost importance.
Dr. Gunter: I offer oral medications and nerve blocks (typically, ganglion impar blocks), and many patients do well.
I also highly recommend advanced programs for mind-body techniques.
Patients who fail all therapies may be candidates for a nerve stimulator, depending on psychiatric comorbidities and response to selective diagnostic nerve blocks.
What’s in the pipeline?
Dr. Lonky: What therapies for vulvar pain are on the horizon?
Dr. Edwards: I believe that cognitive behavioral therapy, sex therapy, and couple counseling will play a larger role in the management of vulvar pain.
Dr. Gunter: Any therapy used in other pain conditions will probably eventually find its way to the management of vulvodynia.
Some investigators believe that Tarlov cysts play a role in vulvar pain and recommend that all women undergo sacral spine and nerve-root magnetic resonance imaging. The problem is that many asymptomatic women have Tarlov cysts, and the surgery to remove them is not at all risk-free. I strongly believe that more research is needed before we can suggest that Tarlov cysts be removed.
Dr. Haefner: Transcutaneous electrical stimulation and biofeedback have been used successfully in the treatment of vulvodynia.31 Some patients benefit from spinal cord stimulators, such as the sacral nerve stimulator, for pain control. Sacral nerve modulation (SNM) works primarily by modulation of the nerve signals to and from the pelvic floor muscles, bladder, and rectum. It applies low-amplitude electrical stimulation to the third sacral nerve via electrodes in a tined lead passing through the S3 foramen, which contains afferent sensory, efferent autonomic motor, and voluntary somatic nerves. Other studies have utilized a different spinal cord level. More studies are needed to demonstrate the full effect of SNM on vulvodynia.
A comprehensive review of the various treatments for vulvodynia can be found in the Journal of Lower Genital Tract Disease.2
Dr. Lonky: Thanks again for your expertise. We’ll focus on provoked vestibulodynia in the final installment of this series on vulvar pain, in the November 2011 issue of OBG Management.
Vulvar pain therapies mentioned in this discussion
| Lifestyle changes Cotton and/or wicking underwear Avoidance of vulvar irritants, douches, soap Use of lubricants during intercourse |
| Physical therapy Internal (vaginal and rectal) and external soft-tissue mobilization and myofascial release Trigger-point pressure Visceral, urogenital, and joint manipulation Electrical stimulation Therapeutic exercises Active pelvic floor retraining Biofeedback Bladder and bowel retraining Therapeutic ultrasound |
| Topical agents Lidocaine 2% jelly Lidocaine 5% ointment Lidocaine/prilocaine Doxepin 5% cream Amitriptyline 2%/baclofen 2% Estrogen Petrolatum Gabapentin |
Oral agents Tricyclic antidepressants
Other antidepressants
Anticonvulsants
|
| Other agents Capsaicin Botulinum toxin type A Corticosteroids Nerve block |
We want to hear from you! Tell us what you think.
1. Nyirjesy P, Lev-Sagie A, Mathew L, Culhane JF. Topical amitriptyline-baclofen cream for the treatment of provoked vestibulodynia. J Lower Genital Tract Dis. 2009;13(4):230-236.
2. Haefner HK, Collins ME, Davis GD, et al. The vulvodynia guideline. J Lower Genital Tract Dis. 2005;9(1):40-51.
3. Foster DC, Kotok MB, Huang L, et al. Oral desipramine and topical lidocaine for vulvodynia: a randomized controlled trial. Obstet Gynecol. 2010;116(3):583-593.
4. Nyirjesy P, Sobel JD, Weitz MV, Leaman DJ, Small MJ, Gelone SP. Cromolyn cream for recalcitrant idiopathic vulvar vestibulitis: results of a placebo-controlled trial. Sex Transm Infect. 2001;77(1):53-57.
5. Zolnoun DA, Hartmann KE, Steege JF. Overnight 5% lidocaine ointment for treatment of vulvar vestibulitis. Obstet Gynecol. 2003;102(1):84-87.
6. Boardman LA, Cooper AS, Blais LR, Raker CA. Topical gabapentin in the treatment of localized and generalized vulvodynia. Obstet Gynecol. 2008;112(3):579-585.
7. Reed BD, Haefner HK, Edwards L. A survey on diagnosis and treatment of vulvodynia researchers and members of the International Society for the Study of Vulvovaginal Disease. J Reprod Med. 2008;53(12):921-929.
8. Brown CS, Wan J, Bachmann G, Rosen R. Self-management amitriptyline, and amitriptyline plus triamcinolone in the management of vulvodynia. J Women Health (Larchmt). 2009;18(2):163-169.
9. Reed BD, Caron AM, Gorenflo DW, Haefner HK. Treatment of vulvodynia with tricyclic antidepressants: efficacy and associated factors. J Low Genit Tract Dis. 2006;10(4):245-251.
10. Munday PE. Response to treatment in dysaesthetic vulvodynia. J Obstet Gynecol. 2001;21(6):610-613.
11. McKay M. Dysesthetic (“essential”) vulvodynia: treatment with amitriptyline. J Reprod Med. 1993;38(1):9-13.
12. Dworkin RH, O’Connor AB, Backonja M, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132(3):237-251.
13. Harris G, Horowitz B, Borgida A. Evaluation of gabapentin in the treatment of generalized vulvodynia unprovoked. J Reprod Med. 2007;52(2):103-106.
14. Ben-David B, Friedman M. Gabapentin therapy for vulvodynia. Anesth Analg. 1999;89(6):1459-1460.
15. Meltzer-Brody SE, Zolnoun D, Steege JF, Rinaldi KL, Leserman J. Open-label trial of lamotrigine focusing on efficacy in vulvodynia. J Reprod Med. 2009;54(3):171-178.
16. Jerome L. Pregabalin-induced remission in a 62-year-old woman with a 20-year history of vulvodynia. Pain Res Manag. 2007;12(3):212-214.
17. Steinberg AC, Oyama IA, Rejba AE, Kellogg-Spadt S, Whitmore KE. Capsaicin for the treatment of vulvar vestibulitis. Am J Obstet Gynecol. 2005;192(5):1549-1553.
18. Murina F, Radici G, Bianco V. Capsaicin and the treatment of vulvar vestibulitis syndrome: a valuable alternative? Med General Med. 2004;6(4):48.-
19. Park AJ, Paraiso MFR. Successful use of botulinum toxin type A in the treatment of refractory postoperative dyspareunia. Obstet Gynecol. 2009;114(2 pt 2):484-487.
20. Brown CS, Glazer HI, Vogt V, Menkes D, Bachmann G. Subjective and objective outcomes of botulinum toxin type A treatment in vestibulodynia: pilot data. J Reprod Med. 2006;51(8):635-641.
21. Dykstra DD, Presthus J. Botulinum toxin type A for the treatment of provoked vestibulodynia: an open-label pilot study. J Reprod Med. 2006;51(6):467-470.
22. Romito S, Bottanelli M, Pellegrini M, Vicentini S, Rizzuto N, Bertolasi L. Botulinum toxin for the treatment of genital pain syndromes. Gynecol Obstet Invest. 2004;58(3):164-167.
23. Bertolasi L, Frasson E, Cappelletti JY, Vicentini S, Bordignon M, Graziottin A. Botulinum neurotoxin type A injections for vaginismus secondary to vulvar vestibulitis syndrome. Obstet Gynecol. 2009;114(5):1008-1016.
24. Presthus JB, Dykstra DD. Botulinum toxin therapy for vulvodynia. NVA News. National Vulvodynia Association. 2007;12(3).
25. Peterson CD, Giraldi A, Lundvall L, Kristensen E. Botulinum toxin type A—a novel treatment for provoked vestibulodynia? Results from a randomized placebo-controlled, double-blinded study. J Sex Med. 2009;6(9):2523-2537.
26. Yoon H, Chung WS, Shim BS. Botulinum toxin A for the management of vulvodynia. Int J Impot Res. 2007;19(1):84-87.
27. Gunter J, Brewer A, Tawfik O. Botulinum toxin A for vulvodynia: a case report. J Pain. 2004;5(4):238-240.
28. Segal D, Tifheret H, Lazr S. Submucous infiltration of betamethasone and lidocaine in the treatment of vulvar vestibulitis. Eur J Obstet Gynecol Reprod Biol. 2003;107(1):105-106.
29. Murina F, Tassan P, Roberti P, Bianco V. Treatment of vulvar vestibulitis with submucous infiltrations of methylprednisolone and lidocaine. An alternative approach. J Reprod Med. 2001;46(8):713-716.
30. Dede M, Yenen MC, Yilmaz A, Baser I. Successful treatment of persistent vulvodynia with submucous infiltration of betamethasone and lidocaine. Eur J Obstet Gynecol Reprod Biol. 2006;124(2):258-259.
31. Dionisi B, Anglana F, Inghirami P, Lippa P, Senatori R. Use of transcutaneous electrical stimulation and biofeedback for the treatment of vulvodynia (vulvar vestibular syndrome): results of 3 years of experience [in Italian]. Minerva Ginecol. 2008;60(6):485-491.
1. Nyirjesy P, Lev-Sagie A, Mathew L, Culhane JF. Topical amitriptyline-baclofen cream for the treatment of provoked vestibulodynia. J Lower Genital Tract Dis. 2009;13(4):230-236.
2. Haefner HK, Collins ME, Davis GD, et al. The vulvodynia guideline. J Lower Genital Tract Dis. 2005;9(1):40-51.
3. Foster DC, Kotok MB, Huang L, et al. Oral desipramine and topical lidocaine for vulvodynia: a randomized controlled trial. Obstet Gynecol. 2010;116(3):583-593.
4. Nyirjesy P, Sobel JD, Weitz MV, Leaman DJ, Small MJ, Gelone SP. Cromolyn cream for recalcitrant idiopathic vulvar vestibulitis: results of a placebo-controlled trial. Sex Transm Infect. 2001;77(1):53-57.
5. Zolnoun DA, Hartmann KE, Steege JF. Overnight 5% lidocaine ointment for treatment of vulvar vestibulitis. Obstet Gynecol. 2003;102(1):84-87.
6. Boardman LA, Cooper AS, Blais LR, Raker CA. Topical gabapentin in the treatment of localized and generalized vulvodynia. Obstet Gynecol. 2008;112(3):579-585.
7. Reed BD, Haefner HK, Edwards L. A survey on diagnosis and treatment of vulvodynia researchers and members of the International Society for the Study of Vulvovaginal Disease. J Reprod Med. 2008;53(12):921-929.
8. Brown CS, Wan J, Bachmann G, Rosen R. Self-management amitriptyline, and amitriptyline plus triamcinolone in the management of vulvodynia. J Women Health (Larchmt). 2009;18(2):163-169.
9. Reed BD, Caron AM, Gorenflo DW, Haefner HK. Treatment of vulvodynia with tricyclic antidepressants: efficacy and associated factors. J Low Genit Tract Dis. 2006;10(4):245-251.
10. Munday PE. Response to treatment in dysaesthetic vulvodynia. J Obstet Gynecol. 2001;21(6):610-613.
11. McKay M. Dysesthetic (“essential”) vulvodynia: treatment with amitriptyline. J Reprod Med. 1993;38(1):9-13.
12. Dworkin RH, O’Connor AB, Backonja M, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132(3):237-251.
13. Harris G, Horowitz B, Borgida A. Evaluation of gabapentin in the treatment of generalized vulvodynia unprovoked. J Reprod Med. 2007;52(2):103-106.
14. Ben-David B, Friedman M. Gabapentin therapy for vulvodynia. Anesth Analg. 1999;89(6):1459-1460.
15. Meltzer-Brody SE, Zolnoun D, Steege JF, Rinaldi KL, Leserman J. Open-label trial of lamotrigine focusing on efficacy in vulvodynia. J Reprod Med. 2009;54(3):171-178.
16. Jerome L. Pregabalin-induced remission in a 62-year-old woman with a 20-year history of vulvodynia. Pain Res Manag. 2007;12(3):212-214.
17. Steinberg AC, Oyama IA, Rejba AE, Kellogg-Spadt S, Whitmore KE. Capsaicin for the treatment of vulvar vestibulitis. Am J Obstet Gynecol. 2005;192(5):1549-1553.
18. Murina F, Radici G, Bianco V. Capsaicin and the treatment of vulvar vestibulitis syndrome: a valuable alternative? Med General Med. 2004;6(4):48.-
19. Park AJ, Paraiso MFR. Successful use of botulinum toxin type A in the treatment of refractory postoperative dyspareunia. Obstet Gynecol. 2009;114(2 pt 2):484-487.
20. Brown CS, Glazer HI, Vogt V, Menkes D, Bachmann G. Subjective and objective outcomes of botulinum toxin type A treatment in vestibulodynia: pilot data. J Reprod Med. 2006;51(8):635-641.
21. Dykstra DD, Presthus J. Botulinum toxin type A for the treatment of provoked vestibulodynia: an open-label pilot study. J Reprod Med. 2006;51(6):467-470.
22. Romito S, Bottanelli M, Pellegrini M, Vicentini S, Rizzuto N, Bertolasi L. Botulinum toxin for the treatment of genital pain syndromes. Gynecol Obstet Invest. 2004;58(3):164-167.
23. Bertolasi L, Frasson E, Cappelletti JY, Vicentini S, Bordignon M, Graziottin A. Botulinum neurotoxin type A injections for vaginismus secondary to vulvar vestibulitis syndrome. Obstet Gynecol. 2009;114(5):1008-1016.
24. Presthus JB, Dykstra DD. Botulinum toxin therapy for vulvodynia. NVA News. National Vulvodynia Association. 2007;12(3).
25. Peterson CD, Giraldi A, Lundvall L, Kristensen E. Botulinum toxin type A—a novel treatment for provoked vestibulodynia? Results from a randomized placebo-controlled, double-blinded study. J Sex Med. 2009;6(9):2523-2537.
26. Yoon H, Chung WS, Shim BS. Botulinum toxin A for the management of vulvodynia. Int J Impot Res. 2007;19(1):84-87.
27. Gunter J, Brewer A, Tawfik O. Botulinum toxin A for vulvodynia: a case report. J Pain. 2004;5(4):238-240.
28. Segal D, Tifheret H, Lazr S. Submucous infiltration of betamethasone and lidocaine in the treatment of vulvar vestibulitis. Eur J Obstet Gynecol Reprod Biol. 2003;107(1):105-106.
29. Murina F, Tassan P, Roberti P, Bianco V. Treatment of vulvar vestibulitis with submucous infiltrations of methylprednisolone and lidocaine. An alternative approach. J Reprod Med. 2001;46(8):713-716.
30. Dede M, Yenen MC, Yilmaz A, Baser I. Successful treatment of persistent vulvodynia with submucous infiltration of betamethasone and lidocaine. Eur J Obstet Gynecol Reprod Biol. 2006;124(2):258-259.
31. Dionisi B, Anglana F, Inghirami P, Lippa P, Senatori R. Use of transcutaneous electrical stimulation and biofeedback for the treatment of vulvodynia (vulvar vestibular syndrome): results of 3 years of experience [in Italian]. Minerva Ginecol. 2008;60(6):485-491.
Raloxifene Did Not Increase Lupus Activity in Study
LONDON – Raloxifene did not increase lupus activity or flares in a 1-year trial of post-menopausal women who had systemic lupus erythematosus without hypercoagulability risk factors, Dr. Chi Chiu Mok reported.
Raloxifene has been shown in previous studies to preserve bone mineral density (BMD) after menopause in women with systemic lupus erythematosus (SLE). However, mild to moderate disease flares occurred in some patients in these small studies. Lupus flares are rare in postmenopausal women, so this finding suggested a possible association between raloxifene (Evista) and disease activity, explained Dr. Mok of Tuen Mun Hospital in Hong Kong.
Dr. Mok and his colleagues analyzed subgroup data from a 12-month randomized controlled trial that assessed bone turnover and BMD in 62 postmenopausal women who had no risk factors for arterial or venous thromboembolism and were being treated with glucocorticoids. The women were randomized to receive either 60 mg/day of raloxifene (30) or placebo (32) in addition to 1,000 mg/day of calcium and 0.25 mcg/day of calcitriol, Dr. Mok explained. “The primary study outcome was bone mineral density of the hip and spine, and secondary outcomes were bone-turnover markers and new vertebral fractures at 12 months,” he said.
In the overall study population, the mean duration of menopause was 7.2 years, the mean duration of treatment with prednisolone (mean daily dose of 6.8 mg) was 87 months, and the mean Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) score at entry was 1.8, Dr. Mok said. The basic clinical characteristics were similar between the two groups, he noted.
After 12 months, “there was a significant gain in bone mineral density at the lumbar spine in the raloxifene patients but not in the placebo group. And there was a gain, though not significant, in the bone mineral density of the hip in the raloxifene-treated patients,” Dr. Mok reported. Similarly, markers of bone resorption and formation decreased significantly in the treatment group, he said.
At 12 months, there was no significant difference in the mean area under the curve SLEDAI scores, which were 18.7 in the raloxifene group and 20.3 in the placebo group, or in the mean area under the curve patient global assessments, which were 2.2 for raloxifene patients and 2.3 for placebo patients, Dr. Mok said.
Regarding disease flares, “there were three episodes of mild to moderate flares in the raloxifene patients, compared with nine in the placebo group,” he stated.
The researchers also assessed the effect of raloxifene on homocysteine and high-sensitivity C-reactive protein (hsCRP) levels and changes in atherosclerotic risk factors. They observed a trend of decrease in homocysteine level in raloxifene patients only, no statistically significant changes in hsCRP levels in either group, significant increases in total and LDL cholesterol in the placebo-treated patients only, and no significant changes in systolic and diastolic blood pressure values in either group, Dr. Mok stated. “In the treatment group, [raloxifene] was well tolerated and there were no thromboembolic complications,” he said.
“As previous studies have shown, raloxifene offers protection against bone mineral density loss in postmenopausal women with lupus receiving long-term glucocorticoids,” Dr. Mok said in an interview. “These results show that it's safe and well tolerated in this population and does not increase lupus disease activity or disease flares.”
Dr. Mok disclosed having no financial conflicts of interest.
LONDON – Raloxifene did not increase lupus activity or flares in a 1-year trial of post-menopausal women who had systemic lupus erythematosus without hypercoagulability risk factors, Dr. Chi Chiu Mok reported.
Raloxifene has been shown in previous studies to preserve bone mineral density (BMD) after menopause in women with systemic lupus erythematosus (SLE). However, mild to moderate disease flares occurred in some patients in these small studies. Lupus flares are rare in postmenopausal women, so this finding suggested a possible association between raloxifene (Evista) and disease activity, explained Dr. Mok of Tuen Mun Hospital in Hong Kong.
Dr. Mok and his colleagues analyzed subgroup data from a 12-month randomized controlled trial that assessed bone turnover and BMD in 62 postmenopausal women who had no risk factors for arterial or venous thromboembolism and were being treated with glucocorticoids. The women were randomized to receive either 60 mg/day of raloxifene (30) or placebo (32) in addition to 1,000 mg/day of calcium and 0.25 mcg/day of calcitriol, Dr. Mok explained. “The primary study outcome was bone mineral density of the hip and spine, and secondary outcomes were bone-turnover markers and new vertebral fractures at 12 months,” he said.
In the overall study population, the mean duration of menopause was 7.2 years, the mean duration of treatment with prednisolone (mean daily dose of 6.8 mg) was 87 months, and the mean Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) score at entry was 1.8, Dr. Mok said. The basic clinical characteristics were similar between the two groups, he noted.
After 12 months, “there was a significant gain in bone mineral density at the lumbar spine in the raloxifene patients but not in the placebo group. And there was a gain, though not significant, in the bone mineral density of the hip in the raloxifene-treated patients,” Dr. Mok reported. Similarly, markers of bone resorption and formation decreased significantly in the treatment group, he said.
At 12 months, there was no significant difference in the mean area under the curve SLEDAI scores, which were 18.7 in the raloxifene group and 20.3 in the placebo group, or in the mean area under the curve patient global assessments, which were 2.2 for raloxifene patients and 2.3 for placebo patients, Dr. Mok said.
Regarding disease flares, “there were three episodes of mild to moderate flares in the raloxifene patients, compared with nine in the placebo group,” he stated.
The researchers also assessed the effect of raloxifene on homocysteine and high-sensitivity C-reactive protein (hsCRP) levels and changes in atherosclerotic risk factors. They observed a trend of decrease in homocysteine level in raloxifene patients only, no statistically significant changes in hsCRP levels in either group, significant increases in total and LDL cholesterol in the placebo-treated patients only, and no significant changes in systolic and diastolic blood pressure values in either group, Dr. Mok stated. “In the treatment group, [raloxifene] was well tolerated and there were no thromboembolic complications,” he said.
“As previous studies have shown, raloxifene offers protection against bone mineral density loss in postmenopausal women with lupus receiving long-term glucocorticoids,” Dr. Mok said in an interview. “These results show that it's safe and well tolerated in this population and does not increase lupus disease activity or disease flares.”
Dr. Mok disclosed having no financial conflicts of interest.
LONDON – Raloxifene did not increase lupus activity or flares in a 1-year trial of post-menopausal women who had systemic lupus erythematosus without hypercoagulability risk factors, Dr. Chi Chiu Mok reported.
Raloxifene has been shown in previous studies to preserve bone mineral density (BMD) after menopause in women with systemic lupus erythematosus (SLE). However, mild to moderate disease flares occurred in some patients in these small studies. Lupus flares are rare in postmenopausal women, so this finding suggested a possible association between raloxifene (Evista) and disease activity, explained Dr. Mok of Tuen Mun Hospital in Hong Kong.
Dr. Mok and his colleagues analyzed subgroup data from a 12-month randomized controlled trial that assessed bone turnover and BMD in 62 postmenopausal women who had no risk factors for arterial or venous thromboembolism and were being treated with glucocorticoids. The women were randomized to receive either 60 mg/day of raloxifene (30) or placebo (32) in addition to 1,000 mg/day of calcium and 0.25 mcg/day of calcitriol, Dr. Mok explained. “The primary study outcome was bone mineral density of the hip and spine, and secondary outcomes were bone-turnover markers and new vertebral fractures at 12 months,” he said.
In the overall study population, the mean duration of menopause was 7.2 years, the mean duration of treatment with prednisolone (mean daily dose of 6.8 mg) was 87 months, and the mean Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) score at entry was 1.8, Dr. Mok said. The basic clinical characteristics were similar between the two groups, he noted.
After 12 months, “there was a significant gain in bone mineral density at the lumbar spine in the raloxifene patients but not in the placebo group. And there was a gain, though not significant, in the bone mineral density of the hip in the raloxifene-treated patients,” Dr. Mok reported. Similarly, markers of bone resorption and formation decreased significantly in the treatment group, he said.
At 12 months, there was no significant difference in the mean area under the curve SLEDAI scores, which were 18.7 in the raloxifene group and 20.3 in the placebo group, or in the mean area under the curve patient global assessments, which were 2.2 for raloxifene patients and 2.3 for placebo patients, Dr. Mok said.
Regarding disease flares, “there were three episodes of mild to moderate flares in the raloxifene patients, compared with nine in the placebo group,” he stated.
The researchers also assessed the effect of raloxifene on homocysteine and high-sensitivity C-reactive protein (hsCRP) levels and changes in atherosclerotic risk factors. They observed a trend of decrease in homocysteine level in raloxifene patients only, no statistically significant changes in hsCRP levels in either group, significant increases in total and LDL cholesterol in the placebo-treated patients only, and no significant changes in systolic and diastolic blood pressure values in either group, Dr. Mok stated. “In the treatment group, [raloxifene] was well tolerated and there were no thromboembolic complications,” he said.
“As previous studies have shown, raloxifene offers protection against bone mineral density loss in postmenopausal women with lupus receiving long-term glucocorticoids,” Dr. Mok said in an interview. “These results show that it's safe and well tolerated in this population and does not increase lupus disease activity or disease flares.”
Dr. Mok disclosed having no financial conflicts of interest.
FROM THE ANNUAL EUROPEAN CONGRESS OF RHEUMATOLOGY
Oral Calcitonin Scores for Osteoporosis in Phase III
SAN DIEGO – A novel once-daily oral calcitonin tablet hit all of its efficacy and safety end points in a phase III clinical trial for treatment of postmenopausal osteoporosis.
The oral formulation of recombinant salmon calcitonin, known as Ostora, proved significantly more effective at building bone mineral density than conventional nasal calcitonin in the 18-site, multinational, double-blind, double-placebo ORACAL trial, Dr. Neil Binkley reported at the meeting.
ORACAL involved 565 postmenopausal women with osteoporosis and a mean age of 66 years. They were randomized 4:3:2 to once-daily bedtime therapy with oral calcitonin at 200 mcg, conventional nasal calcitonin at 200 IU, or placebo.
The absolute change in lumbar spine bone mineral density at 48 weeks – the primary study end point – showed a relatively modest 1.5% increase in the oral calcitonin group. But this was significantly greater than the 0.8% increase with the commercially available nasal calcitonin, which in turn was significantly better than the placebo response.
Potential safety issues with the bisphosphonates and other currently available medications have led many osteoporosis patients to decline therapy, while other women stop treatment due to side effects. Calcitonin, in contrast, has a 30-year history of safe prescription use via the intranasal and injectable routes. An oral formulation “might improve convenience and compliance with calcitonin therapy,” observed Dr. Binkley, an endocrinologist at the University of Wisconsin, Madison.
Salmon calcitonin is an analog of human calcitonin that's 30- to 50-fold more potent in suppressing osteoclast resorption.
In the ORACAL trial, levels of CTx (C-terminal telopeptide), a biomarker of bone resorption, dropped by 30% in the oral calcitonin group at 48 weeks, compared with 11% reductions in the nasal calcitonin and placebo arms.
Only 6.5% of women assigned to oral calcitonin developed anticalcitonin antibodies, compared with 32.5% of those on nasal calcitonin.
This suggests delivery of the agent via the gastrointestinal tract is less immunogenic than the nasal mucosa. In any case, the production of antibodies had no apparent impact on safety or efficacy, the endocrinologist continued.
The side effect picture in the oral and nasal calcitonin groups mirrored that in placebo-treated controls.
Tarsa Therapeutics, which is developing oral calcitonin, estimates at least 2 million American women have discontinued osteoporosis treatment. The company plans to file with the Food and Drug Administration for marketing approval later this year and with the European regulatory agency next year. In addition, the company is developing oral calcitonin for the prevention of osteoporosis in postmenopausal women with osteopenia. A double-blind Phase II trial is underway.
Dr. Binkley disclosed he has received a research grant from Tarsa, which sponsored the Phase III trial.
An oral formulation 'might improve convenience and compliance with calcitonin therapy.'
Source DR. BINKLEY
SAN DIEGO – A novel once-daily oral calcitonin tablet hit all of its efficacy and safety end points in a phase III clinical trial for treatment of postmenopausal osteoporosis.
The oral formulation of recombinant salmon calcitonin, known as Ostora, proved significantly more effective at building bone mineral density than conventional nasal calcitonin in the 18-site, multinational, double-blind, double-placebo ORACAL trial, Dr. Neil Binkley reported at the meeting.
ORACAL involved 565 postmenopausal women with osteoporosis and a mean age of 66 years. They were randomized 4:3:2 to once-daily bedtime therapy with oral calcitonin at 200 mcg, conventional nasal calcitonin at 200 IU, or placebo.
The absolute change in lumbar spine bone mineral density at 48 weeks – the primary study end point – showed a relatively modest 1.5% increase in the oral calcitonin group. But this was significantly greater than the 0.8% increase with the commercially available nasal calcitonin, which in turn was significantly better than the placebo response.
Potential safety issues with the bisphosphonates and other currently available medications have led many osteoporosis patients to decline therapy, while other women stop treatment due to side effects. Calcitonin, in contrast, has a 30-year history of safe prescription use via the intranasal and injectable routes. An oral formulation “might improve convenience and compliance with calcitonin therapy,” observed Dr. Binkley, an endocrinologist at the University of Wisconsin, Madison.
Salmon calcitonin is an analog of human calcitonin that's 30- to 50-fold more potent in suppressing osteoclast resorption.
In the ORACAL trial, levels of CTx (C-terminal telopeptide), a biomarker of bone resorption, dropped by 30% in the oral calcitonin group at 48 weeks, compared with 11% reductions in the nasal calcitonin and placebo arms.
Only 6.5% of women assigned to oral calcitonin developed anticalcitonin antibodies, compared with 32.5% of those on nasal calcitonin.
This suggests delivery of the agent via the gastrointestinal tract is less immunogenic than the nasal mucosa. In any case, the production of antibodies had no apparent impact on safety or efficacy, the endocrinologist continued.
The side effect picture in the oral and nasal calcitonin groups mirrored that in placebo-treated controls.
Tarsa Therapeutics, which is developing oral calcitonin, estimates at least 2 million American women have discontinued osteoporosis treatment. The company plans to file with the Food and Drug Administration for marketing approval later this year and with the European regulatory agency next year. In addition, the company is developing oral calcitonin for the prevention of osteoporosis in postmenopausal women with osteopenia. A double-blind Phase II trial is underway.
Dr. Binkley disclosed he has received a research grant from Tarsa, which sponsored the Phase III trial.
An oral formulation 'might improve convenience and compliance with calcitonin therapy.'
Source DR. BINKLEY
SAN DIEGO – A novel once-daily oral calcitonin tablet hit all of its efficacy and safety end points in a phase III clinical trial for treatment of postmenopausal osteoporosis.
The oral formulation of recombinant salmon calcitonin, known as Ostora, proved significantly more effective at building bone mineral density than conventional nasal calcitonin in the 18-site, multinational, double-blind, double-placebo ORACAL trial, Dr. Neil Binkley reported at the meeting.
ORACAL involved 565 postmenopausal women with osteoporosis and a mean age of 66 years. They were randomized 4:3:2 to once-daily bedtime therapy with oral calcitonin at 200 mcg, conventional nasal calcitonin at 200 IU, or placebo.
The absolute change in lumbar spine bone mineral density at 48 weeks – the primary study end point – showed a relatively modest 1.5% increase in the oral calcitonin group. But this was significantly greater than the 0.8% increase with the commercially available nasal calcitonin, which in turn was significantly better than the placebo response.
Potential safety issues with the bisphosphonates and other currently available medications have led many osteoporosis patients to decline therapy, while other women stop treatment due to side effects. Calcitonin, in contrast, has a 30-year history of safe prescription use via the intranasal and injectable routes. An oral formulation “might improve convenience and compliance with calcitonin therapy,” observed Dr. Binkley, an endocrinologist at the University of Wisconsin, Madison.
Salmon calcitonin is an analog of human calcitonin that's 30- to 50-fold more potent in suppressing osteoclast resorption.
In the ORACAL trial, levels of CTx (C-terminal telopeptide), a biomarker of bone resorption, dropped by 30% in the oral calcitonin group at 48 weeks, compared with 11% reductions in the nasal calcitonin and placebo arms.
Only 6.5% of women assigned to oral calcitonin developed anticalcitonin antibodies, compared with 32.5% of those on nasal calcitonin.
This suggests delivery of the agent via the gastrointestinal tract is less immunogenic than the nasal mucosa. In any case, the production of antibodies had no apparent impact on safety or efficacy, the endocrinologist continued.
The side effect picture in the oral and nasal calcitonin groups mirrored that in placebo-treated controls.
Tarsa Therapeutics, which is developing oral calcitonin, estimates at least 2 million American women have discontinued osteoporosis treatment. The company plans to file with the Food and Drug Administration for marketing approval later this year and with the European regulatory agency next year. In addition, the company is developing oral calcitonin for the prevention of osteoporosis in postmenopausal women with osteopenia. A double-blind Phase II trial is underway.
Dr. Binkley disclosed he has received a research grant from Tarsa, which sponsored the Phase III trial.
An oral formulation 'might improve convenience and compliance with calcitonin therapy.'
Source DR. BINKLEY
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR BONE AND MINERAL RESEARCH
Denosumab's Effect Greater in Selected Patients
SAN FRANCISCO – The pivotal clinical trial of denosumab showed a 20% decrease in nonvertebral fractures compared with placebo treatment, but a new subgroup analysis shows the protective effect is significantly higher in patients with femoral neck osteoporosis.
The preplanned subgroup analysis of data from the FREEDOM trial found that denosumab decreased nonvertebral fractures by 35% in patients with a femoral neck bone mineral density T score of −2.5 or lower and by only 3% in patients with higher femoral neck T scores, compared with patients in those subgroups who received placebo, Dr. Steven R. Cummings said at the meeting.
The report of a 20% reduction in nonvertebral fractures in the overall trial for denosumab “underestimates its efficacy for those patients that we're most interested in treating with this drug – those with osteoporosis,” he said at the conference, sponsored by the University of California, San Francisco.
The findings have been submitted for publication. The analysis is one of several preplanned subgroup analyses being conducted, though this one is “the most interesting result for clinical care,” said Dr. Cummings, emeritus professor of medicine and epidemiology and biostatistics at the university.
The original FREEDOM study (Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months) enrolled 7,808 postmenopausal women aged 60–80 years with osteoporosis to receive every 6 months either a subcutaneous injection of denosumab (60 mg) or placebo along with daily calcium and vitamin D supplements.
All of the subjects had bone mineral density T scores that were less than −2.5 but not less than −4.0 at the lumbar spine or total hip.
At 36 months, denosumab was associated with reductions of 68% in vertebral fracture and 40% in hip fracture (N. Engl. J. Med. 2009;361:756–65).
The FREEDOM results were the basis of the Food and Drug Administration's approval of denosumab in June 2010.
Data for 2,343 patients who continued denosumab for another 2 years and 2,207 patients who switched from placebo to denosumab in an ongoing extension of the trial suggest that the incidence of nonvertebral fractures continues to decline in the first 5 years of denosumab use. The 5-year results have been submitted for publication, he said.
For nonvertebral fractures, the incidence decreased from 2.6% in the denosumab group in the first year of the FREEDOM trial to 2.1% in year 2 and 2.2% in year 3. Nonvertebral fractures were seen in 1.4% of patients in year 4 and 1.1% of patients in year 5, extension study data show.
Similar rates were seen for vertebral fractures.
Dr. Cummings has been a consultant to Amgen Pharmaceuticals, which markets denosumab; to Merck, which markets alendronate; and to Eli Lilly & Company.
SAN FRANCISCO – The pivotal clinical trial of denosumab showed a 20% decrease in nonvertebral fractures compared with placebo treatment, but a new subgroup analysis shows the protective effect is significantly higher in patients with femoral neck osteoporosis.
The preplanned subgroup analysis of data from the FREEDOM trial found that denosumab decreased nonvertebral fractures by 35% in patients with a femoral neck bone mineral density T score of −2.5 or lower and by only 3% in patients with higher femoral neck T scores, compared with patients in those subgroups who received placebo, Dr. Steven R. Cummings said at the meeting.
The report of a 20% reduction in nonvertebral fractures in the overall trial for denosumab “underestimates its efficacy for those patients that we're most interested in treating with this drug – those with osteoporosis,” he said at the conference, sponsored by the University of California, San Francisco.
The findings have been submitted for publication. The analysis is one of several preplanned subgroup analyses being conducted, though this one is “the most interesting result for clinical care,” said Dr. Cummings, emeritus professor of medicine and epidemiology and biostatistics at the university.
The original FREEDOM study (Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months) enrolled 7,808 postmenopausal women aged 60–80 years with osteoporosis to receive every 6 months either a subcutaneous injection of denosumab (60 mg) or placebo along with daily calcium and vitamin D supplements.
All of the subjects had bone mineral density T scores that were less than −2.5 but not less than −4.0 at the lumbar spine or total hip.
At 36 months, denosumab was associated with reductions of 68% in vertebral fracture and 40% in hip fracture (N. Engl. J. Med. 2009;361:756–65).
The FREEDOM results were the basis of the Food and Drug Administration's approval of denosumab in June 2010.
Data for 2,343 patients who continued denosumab for another 2 years and 2,207 patients who switched from placebo to denosumab in an ongoing extension of the trial suggest that the incidence of nonvertebral fractures continues to decline in the first 5 years of denosumab use. The 5-year results have been submitted for publication, he said.
For nonvertebral fractures, the incidence decreased from 2.6% in the denosumab group in the first year of the FREEDOM trial to 2.1% in year 2 and 2.2% in year 3. Nonvertebral fractures were seen in 1.4% of patients in year 4 and 1.1% of patients in year 5, extension study data show.
Similar rates were seen for vertebral fractures.
Dr. Cummings has been a consultant to Amgen Pharmaceuticals, which markets denosumab; to Merck, which markets alendronate; and to Eli Lilly & Company.
SAN FRANCISCO – The pivotal clinical trial of denosumab showed a 20% decrease in nonvertebral fractures compared with placebo treatment, but a new subgroup analysis shows the protective effect is significantly higher in patients with femoral neck osteoporosis.
The preplanned subgroup analysis of data from the FREEDOM trial found that denosumab decreased nonvertebral fractures by 35% in patients with a femoral neck bone mineral density T score of −2.5 or lower and by only 3% in patients with higher femoral neck T scores, compared with patients in those subgroups who received placebo, Dr. Steven R. Cummings said at the meeting.
The report of a 20% reduction in nonvertebral fractures in the overall trial for denosumab “underestimates its efficacy for those patients that we're most interested in treating with this drug – those with osteoporosis,” he said at the conference, sponsored by the University of California, San Francisco.
The findings have been submitted for publication. The analysis is one of several preplanned subgroup analyses being conducted, though this one is “the most interesting result for clinical care,” said Dr. Cummings, emeritus professor of medicine and epidemiology and biostatistics at the university.
The original FREEDOM study (Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months) enrolled 7,808 postmenopausal women aged 60–80 years with osteoporosis to receive every 6 months either a subcutaneous injection of denosumab (60 mg) or placebo along with daily calcium and vitamin D supplements.
All of the subjects had bone mineral density T scores that were less than −2.5 but not less than −4.0 at the lumbar spine or total hip.
At 36 months, denosumab was associated with reductions of 68% in vertebral fracture and 40% in hip fracture (N. Engl. J. Med. 2009;361:756–65).
The FREEDOM results were the basis of the Food and Drug Administration's approval of denosumab in June 2010.
Data for 2,343 patients who continued denosumab for another 2 years and 2,207 patients who switched from placebo to denosumab in an ongoing extension of the trial suggest that the incidence of nonvertebral fractures continues to decline in the first 5 years of denosumab use. The 5-year results have been submitted for publication, he said.
For nonvertebral fractures, the incidence decreased from 2.6% in the denosumab group in the first year of the FREEDOM trial to 2.1% in year 2 and 2.2% in year 3. Nonvertebral fractures were seen in 1.4% of patients in year 4 and 1.1% of patients in year 5, extension study data show.
Similar rates were seen for vertebral fractures.
Dr. Cummings has been a consultant to Amgen Pharmaceuticals, which markets denosumab; to Merck, which markets alendronate; and to Eli Lilly & Company.
EXPERT ANALYSIS FROM A MEETING ON OSTEOPOROSIS
Postmenopausal Obesity Doesn't Cut Fracture Risk
DENVER – New data indicate that obesity in postmenopausal women doesn't protect against fractures, contrary to the conventional wisdom.
Indeed, postmenopausal obesity actually appears to be a risk factor for fractures at selected sites, Dr. Juliet E. Compston reported at the meeting.
This is a finding with major public health implications because of the growing obesity epidemic. The ramifications are especially pressing in light of new evidence that obese postmenopausal women who experience a fracture are far less likely than nonobese women to be placed on bone-protective medication, said Dr. Compston, professor of bone medicine at the University of Cambridge (England).
She reported on a study of 44,534 postmenopausal women (mean age, 67 years) in the United States and nine other countries who are participating in the ongoing, prospective, observational GLOW (Global Longitudinal Study of Osteoporosis in Women). At enrollment, 23.4% of the women had a body mass index of 30 kg/m
The prevalence of fracture at baseline was 23% in obese women, 24% in nonobese women, and 32% in the underweight study population. The incidence of one or more new fractures during 2 years of follow-up was 6.4% in the obese and similar at 6.8% in the nonobese women, compared with 7.3% in the underweight group.
Thus, nearly one in four postmenopausal women with a fracture is obese. As the obesity rate continues to climb in the developed world, fractures in the obese will increasingly contribute to the overall burden of fractures in the postmenopausal population, Dr. Compston observed.
The higher prevalence and incidence of fracture in underweight postmenopausal women comes as no surprise; low BMI is recognized as a major risk factor for fracture. What was surprising, though, was that only 27% of obese GLOW participants with an incident fracture were placed on bone-protective medication for secondary prevention.
In contrast, the treatment rate in nonobese women with an incident fracture was 41%, and in underweight women it was 57%. The likely explanation for the markedly lower treatment rate in the obese group is the widespread belief that obesity protects against fractures, according to Dr. Compston.
Incident fractures of the ankle and tibia were significantly more common and wrist fractures were less common in obese women, compared with nonobese study participants.
The obese subjects with an incident fracture had significantly higher rates of several comorbid conditions – asthma, emphysema, and type 1 diabetes – than did nonobese women. They also were more likely than nonobese participants with an incident fracture to have a baseline history of two or more falls within the past 2 years. They had more mobility issues as well, as reflected in their increased rate of self-reported need for arm assistance in standing up. It's likely that reduced mobility and increased risk of falling play an important role in the pathogenesis of fractures in obese postmenopausal women, although this is an issue that needs further study, she observed.
In a separate presentation, Helena Johansson presented an analysis of BMI and fracture risk in 281,637 women drawn from 27 prospective, population-based cohort studies that were conducted in more than two dozen countries. The women were aged 20–105 years (mean age, 63 years).
A total of 18,441 osteoporotic fractures occurred in the study population during a mean 4.8 years of follow-up. After adjustment for bone mineral density, obesity proved to be a risk factor for fracture.
For example, women with a BMI of 34 had an adjusted 14% increased risk of osteoporotic fracture, compared with those having a BMI of 26, an elevation in risk that, while modest, was statistically significant.
And women having a BMI of 34 had a more impressive adjusted 60% increased risk of humerus or elbow fracture, added Ms. Johansson, a statistician at the University of Gothenburg (Sweden).
Dr. Compston declared having no relevant financial disclosures. GLOW is supported by grants from Sanofi-Aventis and Warner Chilcott.
Ms. Johansson declared having no relevant financial disclosures.
DENVER – New data indicate that obesity in postmenopausal women doesn't protect against fractures, contrary to the conventional wisdom.
Indeed, postmenopausal obesity actually appears to be a risk factor for fractures at selected sites, Dr. Juliet E. Compston reported at the meeting.
This is a finding with major public health implications because of the growing obesity epidemic. The ramifications are especially pressing in light of new evidence that obese postmenopausal women who experience a fracture are far less likely than nonobese women to be placed on bone-protective medication, said Dr. Compston, professor of bone medicine at the University of Cambridge (England).
She reported on a study of 44,534 postmenopausal women (mean age, 67 years) in the United States and nine other countries who are participating in the ongoing, prospective, observational GLOW (Global Longitudinal Study of Osteoporosis in Women). At enrollment, 23.4% of the women had a body mass index of 30 kg/m
The prevalence of fracture at baseline was 23% in obese women, 24% in nonobese women, and 32% in the underweight study population. The incidence of one or more new fractures during 2 years of follow-up was 6.4% in the obese and similar at 6.8% in the nonobese women, compared with 7.3% in the underweight group.
Thus, nearly one in four postmenopausal women with a fracture is obese. As the obesity rate continues to climb in the developed world, fractures in the obese will increasingly contribute to the overall burden of fractures in the postmenopausal population, Dr. Compston observed.
The higher prevalence and incidence of fracture in underweight postmenopausal women comes as no surprise; low BMI is recognized as a major risk factor for fracture. What was surprising, though, was that only 27% of obese GLOW participants with an incident fracture were placed on bone-protective medication for secondary prevention.
In contrast, the treatment rate in nonobese women with an incident fracture was 41%, and in underweight women it was 57%. The likely explanation for the markedly lower treatment rate in the obese group is the widespread belief that obesity protects against fractures, according to Dr. Compston.
Incident fractures of the ankle and tibia were significantly more common and wrist fractures were less common in obese women, compared with nonobese study participants.
The obese subjects with an incident fracture had significantly higher rates of several comorbid conditions – asthma, emphysema, and type 1 diabetes – than did nonobese women. They also were more likely than nonobese participants with an incident fracture to have a baseline history of two or more falls within the past 2 years. They had more mobility issues as well, as reflected in their increased rate of self-reported need for arm assistance in standing up. It's likely that reduced mobility and increased risk of falling play an important role in the pathogenesis of fractures in obese postmenopausal women, although this is an issue that needs further study, she observed.
In a separate presentation, Helena Johansson presented an analysis of BMI and fracture risk in 281,637 women drawn from 27 prospective, population-based cohort studies that were conducted in more than two dozen countries. The women were aged 20–105 years (mean age, 63 years).
A total of 18,441 osteoporotic fractures occurred in the study population during a mean 4.8 years of follow-up. After adjustment for bone mineral density, obesity proved to be a risk factor for fracture.
For example, women with a BMI of 34 had an adjusted 14% increased risk of osteoporotic fracture, compared with those having a BMI of 26, an elevation in risk that, while modest, was statistically significant.
And women having a BMI of 34 had a more impressive adjusted 60% increased risk of humerus or elbow fracture, added Ms. Johansson, a statistician at the University of Gothenburg (Sweden).
Dr. Compston declared having no relevant financial disclosures. GLOW is supported by grants from Sanofi-Aventis and Warner Chilcott.
Ms. Johansson declared having no relevant financial disclosures.
DENVER – New data indicate that obesity in postmenopausal women doesn't protect against fractures, contrary to the conventional wisdom.
Indeed, postmenopausal obesity actually appears to be a risk factor for fractures at selected sites, Dr. Juliet E. Compston reported at the meeting.
This is a finding with major public health implications because of the growing obesity epidemic. The ramifications are especially pressing in light of new evidence that obese postmenopausal women who experience a fracture are far less likely than nonobese women to be placed on bone-protective medication, said Dr. Compston, professor of bone medicine at the University of Cambridge (England).
She reported on a study of 44,534 postmenopausal women (mean age, 67 years) in the United States and nine other countries who are participating in the ongoing, prospective, observational GLOW (Global Longitudinal Study of Osteoporosis in Women). At enrollment, 23.4% of the women had a body mass index of 30 kg/m
The prevalence of fracture at baseline was 23% in obese women, 24% in nonobese women, and 32% in the underweight study population. The incidence of one or more new fractures during 2 years of follow-up was 6.4% in the obese and similar at 6.8% in the nonobese women, compared with 7.3% in the underweight group.
Thus, nearly one in four postmenopausal women with a fracture is obese. As the obesity rate continues to climb in the developed world, fractures in the obese will increasingly contribute to the overall burden of fractures in the postmenopausal population, Dr. Compston observed.
The higher prevalence and incidence of fracture in underweight postmenopausal women comes as no surprise; low BMI is recognized as a major risk factor for fracture. What was surprising, though, was that only 27% of obese GLOW participants with an incident fracture were placed on bone-protective medication for secondary prevention.
In contrast, the treatment rate in nonobese women with an incident fracture was 41%, and in underweight women it was 57%. The likely explanation for the markedly lower treatment rate in the obese group is the widespread belief that obesity protects against fractures, according to Dr. Compston.
Incident fractures of the ankle and tibia were significantly more common and wrist fractures were less common in obese women, compared with nonobese study participants.
The obese subjects with an incident fracture had significantly higher rates of several comorbid conditions – asthma, emphysema, and type 1 diabetes – than did nonobese women. They also were more likely than nonobese participants with an incident fracture to have a baseline history of two or more falls within the past 2 years. They had more mobility issues as well, as reflected in their increased rate of self-reported need for arm assistance in standing up. It's likely that reduced mobility and increased risk of falling play an important role in the pathogenesis of fractures in obese postmenopausal women, although this is an issue that needs further study, she observed.
In a separate presentation, Helena Johansson presented an analysis of BMI and fracture risk in 281,637 women drawn from 27 prospective, population-based cohort studies that were conducted in more than two dozen countries. The women were aged 20–105 years (mean age, 63 years).
A total of 18,441 osteoporotic fractures occurred in the study population during a mean 4.8 years of follow-up. After adjustment for bone mineral density, obesity proved to be a risk factor for fracture.
For example, women with a BMI of 34 had an adjusted 14% increased risk of osteoporotic fracture, compared with those having a BMI of 26, an elevation in risk that, while modest, was statistically significant.
And women having a BMI of 34 had a more impressive adjusted 60% increased risk of humerus or elbow fracture, added Ms. Johansson, a statistician at the University of Gothenburg (Sweden).
Dr. Compston declared having no relevant financial disclosures. GLOW is supported by grants from Sanofi-Aventis and Warner Chilcott.
Ms. Johansson declared having no relevant financial disclosures.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR BONE AND MINERAL RESEARCH
Vulvar pain syndromes: Making the correct diagnosis
Although the incidence of vulvar pain has increased over the past decade—thanks to both greater awareness and increasing numbers of affected women—the phenomenon is not a recent development. As early as 1874, T. Galliard Thomas wrote, “[T]his disorder, although fortunately not very frequent, is by no means very rare.”1 He went on to express “surprise” that it had not been “more generally and fully described.”
Despite the focus Thomas directed to the issue, vulvar pain did not get much attention until the 21st century, when a number of studies began to gauge its prevalence. For example, in a study in Boston of about 5,000 women, the lifetime prevalence of chronic vulvar pain was 16%.2 And in a study in Texas, the prevalence of vulvar pain in an urban, largely minority population was estimated to be 11%.3 The Boston study also reported that “nearly 40% of women chose not to seek treatment, and, of those who did, 60% saw three or more doctors, many of whom could not provide a diagnosis.”2
Clearly, there is a need for comprehensive information on vulvar pain and its causes, symptoms, diagnosis, and treatment. To address the lack of guidance, OBG Management Contributing Editor Neal M. Lonky, MD, assembled a panel of experts on vulvar pain syndromes and invited them to share their considerable knowledge. The ensuing discussion, presented in three parts, offers a gold mine of information.
In this opening article, the panel focuses on causes, symptomatology, and diagnosis of this common complaint. In Part 2, which will appear in the October issue of this journal, the focus is the bounty of treatment options. Part 3 follows in November, when the discussion shifts to vestibulodynia.
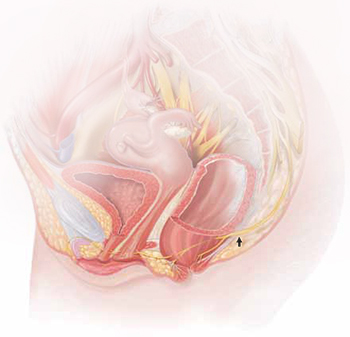
The lower vagina and vulva are richly supplied with peripheral nerves and are, therefore, sensitive to pain, particularly the region of the hymeneal ring. Although the pudendal nerve (arrow) courses through the area, it is an uncommon source of vulvar pain.
Common diagnoses—and misdiagnoses
Dr. Lonky: What are the most common diagnoses when vulvar pain is the complaint?
Dr. Gunter: The most common cause of chronic vulvar pain is vulvodynia, although lichen simplex chronicus, chronic yeast infections, and non-neoplastic epithelial disorders, such as lichen sclerosus and lichen planus, can also produce irritation and pain. In postmenopausal women, atrophic vaginitis can also cause a burning pain, although symptoms are typically more vaginal than vulvar. Yeast and lichen simplex chronicus typically produce itching, although sometimes they can present with irritation and pain, so they must be considered in the differential diagnosis. It is important to remember that many women with vulvodynia have used multiple topical agents and may have developed complex hygiene rituals in an attempt to treat their symptoms, which can result in a secondary lichen simplex chronicus.
That said, there is a high frequency of misdiagnosis with yeast. For example, in a study by Nyirjesy and colleagues, two thirds of women who were referred to a tertiary clinic for chronic vulvovaginal candidiasis were found to have a noninfectious entity instead—most commonly lichen simplex chronicus and vulvodynia.4
Dr. Edwards: The most common “diagnosis” for vulvar pain is vulvodynia. However, the definition of vulvodynia is pain—i.e., burning, rawness, irritation, soreness, aching, or stabbing or stinging sensations—in the absence of skin disease, infection, or specific neurologic disease. Therefore, even though the usual cause of vulvar pain is vulvodynia, it is a diagnosis of exclusion, and skin disease, infection, and neurologic disease must be ruled out.
In regard to infection, Candida albicans and bacterial vaginosis (BV) are usually the first conditions that are considered when a patient complains of vulvar pain, but they are not common causes of vulvar pain and are never causes of chronic vulvar pain. Very rarely they may cause recurrent pain that clears, at least briefly, with treatment.
Candida albicans is usually primarily pruritic, and BV produces discharge and odor, sometimes with minor symptoms. Non-albicans Candida (e.g., Candida glabrata) is nearly always asymptomatic, but it occasionally causes irritation and burning.
Group B streptococcus is another infectious entity that very, very occasionally causes irritation and dyspareunia but is usually only a colonizer.
Herpes simplex virus is a cause of recurrent but not chronic pain.
Chronic pain is more likely to be caused by skin disease than by infection. Lichen simplex chronicus causes itching; any pain is due to erosions from scratching.
Dr. Haefner: Several other infectious conditions or their treatments can cause vulvar pain. For example, herpes (particularly primary herpes infection) is classically associated with vulvar pain. The pain is so great that, at times, the patient requires admission for pain control. Surprisingly, despite the known pain of herpes, approximately 80% of patients who have it are unaware of their diagnosis.
Although condyloma is generally a painless condition, many patients complain of pain following treatment for it, whether treatment involves topical medications or laser surgery.
Chancroid is a painful vulvar ulcer. Trichomonas can sometimes be associated with vulvar pain.
Dr. Lonky: What terminology do we use when we discuss vulvar pain?
Dr. Haefner: The current terminology used to describe vulvar pain was published in 2004, after years of debate over nomenclature within the International Society for the Study of Vulvovaginal Disease.5 The terminology lists two major categories of vulvar pain:
- pain related to a specific disorder. This category encompasses numerous conditions that feature an abnormal appearance of the vulva (Table 1).
TABLE 1
Terminology and classification of vulvar pain from the International Society for the Study of Vulvovaginal Disease
|
| SOURCE: Moyal-Barracco and Lynch.5 Reproduced with permission from the Journal of Reproductive Medicine. |
- vulvodynia, in which the vulva appears normal, other than occasional erythema, which is most prominent at the duct openings (vestibular ducts—Bartholin’s and Skene’s).
As for vulvar pain, there are two major forms:
- hyperalgesia (a low threshold for pain)
- allodynia (pain in response to light touch).
Some diseases that are associated with vulvar pain do not qualify for the diagnosis of vulvodynia (Table 2) because they are associated with an abnormal appearance of the vulva.
TABLE 2
Conditions other than vulvodynia that are associated with vulvar pain
| Acute irritant contact dermatitis (e.g., erosion due to podofilox, imiquimod, cantharidin, fluorouracil, or podophyllin toxin) |
| Aphthous ulcer |
| Atrophy |
| Bartholin’s abscess |
| Candidiasis |
| Carcinoma |
| Chronic irritant contact dermatitis |
| Endometriosis |
| Herpes (simplex and zoster) |
| Immunobullous diseases (including cicatricial pemphigoid, pemphigus vulgaris, linear immunoglobulin A disease, etc.) |
| Lichen planus |
| Lichen sclerosus |
| Podophyllin overdose (see above) |
| Prolapsed urethra |
| Sjögren’s syndrome |
| Trauma |
| Trichomoniasis |
| Vulvar intraepithelial neoplasia |
What needs to be ruled out for a diagnosis of vulvodynia?
Dr. Lonky: What skin diseases need to be ruled out before vulvodynia can be diagnosed?
Dr. Edwards: Skin diseases that affect the vulva are usually pruritic—pain is a later sign. Lichen simplex chronicus (also known as eczema) is pruritus caused by any irritant; any pain that arises is produced by visible excoriations from scratching.
Lichen sclerosus manifests as white epithelium that has a crinkling, shiny, or waxy texture. It can produce pain, especially dyspareunia. The pain is caused by erosions that arise from fragility and introital narrowing and inelasticity.
Vulvovaginal lichen planus is usually erosive and preferentially affects mucous membranes, especially the vestibule; it sometimes affects the vagina and mouth, as well.
Desquamative inflammatory vaginitis is most likely a skin disease that affects only the vagina. It involves introital redness and a clinically and microscopically purulent vaginal discharge that also reveals parabasal cells and absent lactobacilli.
Dr. Lonky: You mentioned that neurologic diseases can sometimes cause vulvar pain. Which ones?
Dr. Edwards: Pudendal neuralgia, diabetic neuropathy, and post-herpetic neuralgia are the most common specific neurologic causes of vulvar pain. Multiple sclerosis can also produce pain syndromes. Post-herpetic neuralgia follows herpes zoster—not herpes simplex—virus infection.
Dr. Lonky: Any other conditions that can cause vulvar pain?
Dr. Haefner: Aphthous ulcers are common and are often flared by stress.
Non-neoplastic epithelial disorders are also seen frequently in health-care providers’ offices; many patients who experience them report pain on the vulva.
It is always important to consider cancer when a patient has an abnormal vulvar appearance and pain that has persisted despite treatment.
What are the most common vulvar pain syndromes?
Dr. Lonky: If you were to rank vulvar pain syndromes according to their prevalence, what would the most common syndromes be?
Dr. Gunter: Given the misdiagnosis of many women, who are told they have chronic yeast infection, as I mentioned, it’s hard to know which vulvar pain syndromes are most prevalent. I suspect that lichen simplex chronicus is most common, followed by vulvodynia, with chronic yeast infection a distant third.
My experience reflects what Nyirjesy and colleagues4 found: 65% to 75% of women referred to my clinic with chronic yeast actually have lichen simplex chronicus or vulvodynia. In postmenopausal women, atrophic vaginitis is also a consideration; it’s becoming more common now that the use of systemic hormone replacement therapy is decreasing.
Dr. Lonky: What about subsets of vulvodynia? Which ones are most common?
Dr. Edwards: There is good evidence of marked overlap among subsets of vulvodynia. The vast majority of women who have vulvodynia experience primarily provoked vestibular pain, regardless of age. However, I find that almost all patients also report pain that extends beyond the vestibule at times, as well as occasional unprovoked pain.
The diagnosis requires the exclusion of other causes of vulvar pain, and the subset is identified by the location of pain (that is, is it strictly localized or generalized or even migratory?) and its provoked or unprovoked nature.
Localized clitoral pain and vulvar pain localized to one side of the vulva are extremely uncommon, but they do occur. And although I rarely encounter teenagers and prepubertal children who have vulvodynia, I do have patients in both age groups who have vulvodynia.
Dr. Lonky: Are there racial differences in the prevalence of vulvodynia?
Dr. Edwards: Although several good studies show that women of African descent and white patients are equally likely to experience vulvodynia, the vast majority (99%) of my patients who have vulvodynia are white. My patients of African descent consult me primarily for itching or discharge.
My local demographics prevent me from judging the likelihood of Asians having vulvodynia, and our Hispanic population has limited access to health care.
In general, I don’t think that demographics are useful in making the diagnosis of vulvodynia.
Do women who have vulvar pain tend to have comorbidities?
Dr. Lonky: Do your patients who have vulvodynia or another vulvar pain syndrome tend to have comorbidities? If so, is this information helpful in establishing the diagnosis and planning therapy?
Dr. Haefner: Women who have vulvodynia often have other medical problems as well. In my practice, when new patients who have vulvodynia complete their intake survey, they often report a history of headache, irritable bowel syndrome, interstitial cystitis, fibromyalgia,6 chronic fatigue syndrome, back pain, and temporomandibular joint (TMJ) disorder. These comorbidities are not particularly helpful in establishing the diagnosis of vulvodynia, but they are an important consideration when choosing therapy for the patient. Often, the medications chosen to treat one condition will also benefit another condition. However, it’s important to check for potential interactions between drugs before prescribing a new treatment.
Dr. Gunter: A significant number of women who have vulvodynia also have other chronic pain syndromes. For example, the incidence of bladder pain syndrome–interstitial cystitis is 68% to 82% among women who have vulvodynia, compared with a baseline rate among all women of 6% to 11%.7-10 The rate of irritable bowel syndrome is more than doubled among women who have vulvodynia, compared with the general population (27% versus 12%).8 Another common comorbidity, hypertonic somatic dysfunction of the pelvic floor, is identified in 10% to 90% of women who have chronic vulvar pain.8,11,12 These women also have a higher incidence of nongenital pain syndromes, such as fibromyalgia, migraine, and TMJ dysfunction, than the general population, as Dr. Haefner noted.8,12,13
Many studies have evaluated psychological and emotional contributions to chronic vulvar pain. Pain and depression are intimately related—the incidence of depression among all people who experience chronic pain ranges from 27% to 54%, compared with 5% to 17% among the general population.14-16 The relationship is complex because chronic illness in general is associated with depression. Nevertheless, several studies have noted an increase in anxiety, stress, and depression among women who have vulvodynia.17-19
I screen every patient for depression using a Patient Health Questionnaire (PHQ-9); I also screen for anxiety. I find that a significant percentage of patients in my clinic are depressed or have an anxiety disorder. Failure to address these comorbidities makes treatment very difficult. I typically prescribe citalopram (Celexa), although there is some question whether it can safely be combined with a tricyclic antidepressant. We also offer stress-reduction classes, teach every patient the value of diaphragmatic breathing, offer mind-body classes for anxiety and stress, and provide intensive programs where the patient can learn important self-care skills, such as pacing (spacing activities throughout the day in a manner that avoids aggravating the pain), and address her anxiety and stress in a more guided manner. We also have a psychologist who specializes in pain for any patient who may need one-on-one counseling.
Dr. Edwards: The presence of comorbidities is somewhat useful in making the diagnosis of vulvodynia. I question my diagnosis, in fact, when a patient who has vulvodynia does not have headaches, low energy, depression, anxiety, irritable bowel syndrome, constipation, fibromyalgia, chronic fatigue, sensitivity to medications, TMJ dysfunction, or urinary symptoms.
How common is pudendal neuralgia?
Dr. Lonky: How prevalent is a finding of pudendal neuralgia?
Dr. Edwards: The prevalence and incidence of pudendal neuralgia are not known. Those who specialize in this condition think it is relatively common. I do not identify or suspect it very often. Its definitive diagnosis and management are outside the purview of the general gynecologist, but the general gynecologist should recognize the symptoms of pudendal neuralgia and refer the patient for evaluation and therapy.
Dr. Lonky: What are those symptoms?
Dr. Haefner: Pudendal neuralgia often occurs following trauma to the pudendal nerve. The pudendal nerve arises from sacral nerves, generally sacral nerves 2 to 4. Several tests can be utilized to diagnose this condition, including quantitative sensor tests, pudendal nerve motor latency tests, electromyography (EMG), and pudendal nerve blocks.20
Nantes Criteria allow for making a diagnosis of pudendal neuralgia (Table 3).21
TABLE 3
Nantes Criteria for pudendal neuralgia by pudendal nerve entrapment
Essential criteria
|
Complementary diagnostic criteria
|
Exclusion criteria
|
Associated signs not excluding the diagnosis
|
| SOURCE: Labat et al.21 Reproduced with permission from Neurology and Urodynamics. |
Initial treatments for pudendal neuralgia should be conservative. Treatments consist of lifestyle changes to prevent flare of disease. Physical therapy, medical management, nerve blocks, and alternative treatments may be beneficial.
Pudendal nerve entrapment is often exacerbated by sitting (not on a toilet seat, however) and is reduced in a standing position. It tends to increase in intensity throughout the day.22 The final treatment for pudendal nerve entrapment is surgery if the nerve is compressed. By this time, the generalist is not generally the provider who performs the surgery.
Dr. Gunter: I believe pudendal neuralgia is sometimes overdiagnosed. EMG studies of the pudendal nerve, often touted as a diagnostic tool, are unreliable (they can be abnormal after vaginal delivery or vaginal hysterectomy, for example). In my experience, bilateral pain is less likely to be pudendal neuralgia; spontaneous bilateral compression neuropathy at exactly the same level is not a common phenomenon in chronic pain.
I reserve the diagnosis of pudendal neuralgia for women who have allodynia in the distribution of the pudendal nerve with severe pain on sitting, and who have exquisite tenderness when pressure is applied over the pudendal nerve (at the level of the ischial spine on vaginal examination). Typically, the vaginal sidewall on the affected side is very sensitive to light touch. I do see pudendal nerve pain after vaginal surgery when there has been some compromise of the pudendal nerve or the sacral plexus. This is typically unilateral pain.
Dr. Lonky: Thank you all. We’ll continue our discussion, with a focus on treatment, in the October 2011 issue.
- Part 2: A bounty of treatment options
(October 2011) - Part 3: Vestibulodynia
(November 2011)
We want to hear from you! Tell us what you think.
1. Thomas TG. Practical Treatise on the Diseases of Women. Philadelphia Pa: Henry C. Lea; 1874.
2. Harlow BL, Stewart EG. A population-based assessment of chronic unexplained vulvar pain: have we underestimated the prevalence of vulvodynia? J Am Med Womens Assoc. 2003;58(2):82-88.
3. Lavy RJ, Hynan LS, Haley RW. Prevalence of vulvar pain in an urban minority population. J Reprod Med. 2007;52(1):59-62.
4. Nyirjesy P, Peyton C, Weitz MV, Mathew L, Culhane JF. Causes of chronic vaginitis: analysis of a prospective database of affected women. Obstet Gynecol. 2006;108(5):1185-1191.
5. Moyal-Barracco M, Lynch PJ. 2003 ISSVD terminology and classification of vulvodynia: a historical perspective. J Reprod Med. 2004;49(10):772-777.
6. Yunas MB. Fibromyalgia and overlapping disorders: the unifying concept of central sensitivity syndromes. Semin Arthritis Rheum. 2007;36(6):339-356.
7. Kahn BS, Tatro C, Parsons CL, Willems JJ. Prevalence of interstitial cystitis in vulvodynia patients detected by bladder potassium sensitivity. J Sex Med. 2010;7(2 Pt 2):996-1002.
8. Arnold JD, Bachman GS, Rosen R, Kelly S, Rhoads GG. Vulvodynia: characteristics and associations with comorbidities and quality of life. Obstet Gynecol. 2006;107(3):617-624.
9. Parsons CL, Dell J, Stanford EJ, et al. The prevalence of interstitial cystitis in gynecologic patients with pelvic pain, as detected by intravesical potassium sensitivity. Am J Obstet Gynecol. 2002;187(5):1395-1400.
10. Clemens JQ, Meenan RT, O’Keefe Rosetti MC, et al. Prevalence of interstitial cystitis symptoms in a managed care population. J Urol. 2005;174(2):576-580.
11. Engman M, Lindehammar H, Wijma B. Surface electromyography diagnostics in women with partial vaginismus with or without vulvar vestibulitis and in asymptomatic women. J Psychosom Obstet Gynecol. 2004;25(3-4):281-294.
12. Gunter J. Vulvodynia: new thoughts on a devastating condition. Obstet Gynecol Surv. 2007;62(12):812-819.
13. Gordon AS, Panahlan-Jand M, McComb F, Melegari C, Sharp S. Characteristics of women with vulvar pain disorders: a Web-based survey. J Sex Marital Ther. 2003;29(suppl 1):45.-
14. Whitten CE, Cristobal K. Chronic pain is a chronic condition not just a symptom. Permanente J. 2005;9(3):43.-
15. Manchikanti L, Fellows B, Pampati V, et al. Comparison of psychological status of chronic pain patients and the general population. Pain Physician. 2002;5(1):40-48.
16. Banks SM, Kerns RD. Explaining the high rates of depression in chronic pain: a diathesis-stress framework. Psychological Bulletin. 1996;119(1):95-110.
17. Sadownik LA. Clinical correlates of vulvodynia patients. A prospective study of 300 patients. J Reprod Med. 2000;5:40-48.Editor found in PubMed: Sadownik LA. Clinical profile of vulvodynia patients. A prospective study of 300 patients. J Reprod Med. 2000;45(8):679–684. Could not find the citation listed. Please confirm.
18. Reed BD, Haefner HK, Punch MR, Roth RS, Gorenflo DW, Gillespie BW. Psychosocial and sexual functioning in women with vulvodynia and chronic pelvic pain. A comparative evaluation. J Reprod Med. 2000;45(8):624-632.
19. Landry T, Bergeron S. Biopsychosocial factors associated with dyspareunia in a community sample of adolescent girls. Arch Sex Behav. 2011;June 22.
20. Goldstein A, Pukall C, Goldstein I. When Sex Hurts: A Woman’s Guide to Banishing Sexual Pain. Cambridge Mass: Da Capo Lifelong Books; 2011;117-126.
21. Labat JJ, Riant T, Robert R, Amarenco G, Lefaucheur JP, Rigaud J. Diagnostic criteria for pudendal neuralgia by pudendal nerve entrapment (Nantes criteria). Neurol Urodyn. 2008;27(4):306-310.
22. Popeney C, Answell V, Renney K. Pudendal entrapment as an etiology of chronic perineal pain: Diagnosis and treatment. Neurol Urodyn. 2007;26(6):820-827.
Although the incidence of vulvar pain has increased over the past decade—thanks to both greater awareness and increasing numbers of affected women—the phenomenon is not a recent development. As early as 1874, T. Galliard Thomas wrote, “[T]his disorder, although fortunately not very frequent, is by no means very rare.”1 He went on to express “surprise” that it had not been “more generally and fully described.”
Despite the focus Thomas directed to the issue, vulvar pain did not get much attention until the 21st century, when a number of studies began to gauge its prevalence. For example, in a study in Boston of about 5,000 women, the lifetime prevalence of chronic vulvar pain was 16%.2 And in a study in Texas, the prevalence of vulvar pain in an urban, largely minority population was estimated to be 11%.3 The Boston study also reported that “nearly 40% of women chose not to seek treatment, and, of those who did, 60% saw three or more doctors, many of whom could not provide a diagnosis.”2
Clearly, there is a need for comprehensive information on vulvar pain and its causes, symptoms, diagnosis, and treatment. To address the lack of guidance, OBG Management Contributing Editor Neal M. Lonky, MD, assembled a panel of experts on vulvar pain syndromes and invited them to share their considerable knowledge. The ensuing discussion, presented in three parts, offers a gold mine of information.
In this opening article, the panel focuses on causes, symptomatology, and diagnosis of this common complaint. In Part 2, which will appear in the October issue of this journal, the focus is the bounty of treatment options. Part 3 follows in November, when the discussion shifts to vestibulodynia.

The lower vagina and vulva are richly supplied with peripheral nerves and are, therefore, sensitive to pain, particularly the region of the hymeneal ring. Although the pudendal nerve (arrow) courses through the area, it is an uncommon source of vulvar pain.
Common diagnoses—and misdiagnoses
Dr. Lonky: What are the most common diagnoses when vulvar pain is the complaint?
Dr. Gunter: The most common cause of chronic vulvar pain is vulvodynia, although lichen simplex chronicus, chronic yeast infections, and non-neoplastic epithelial disorders, such as lichen sclerosus and lichen planus, can also produce irritation and pain. In postmenopausal women, atrophic vaginitis can also cause a burning pain, although symptoms are typically more vaginal than vulvar. Yeast and lichen simplex chronicus typically produce itching, although sometimes they can present with irritation and pain, so they must be considered in the differential diagnosis. It is important to remember that many women with vulvodynia have used multiple topical agents and may have developed complex hygiene rituals in an attempt to treat their symptoms, which can result in a secondary lichen simplex chronicus.
That said, there is a high frequency of misdiagnosis with yeast. For example, in a study by Nyirjesy and colleagues, two thirds of women who were referred to a tertiary clinic for chronic vulvovaginal candidiasis were found to have a noninfectious entity instead—most commonly lichen simplex chronicus and vulvodynia.4
Dr. Edwards: The most common “diagnosis” for vulvar pain is vulvodynia. However, the definition of vulvodynia is pain—i.e., burning, rawness, irritation, soreness, aching, or stabbing or stinging sensations—in the absence of skin disease, infection, or specific neurologic disease. Therefore, even though the usual cause of vulvar pain is vulvodynia, it is a diagnosis of exclusion, and skin disease, infection, and neurologic disease must be ruled out.
In regard to infection, Candida albicans and bacterial vaginosis (BV) are usually the first conditions that are considered when a patient complains of vulvar pain, but they are not common causes of vulvar pain and are never causes of chronic vulvar pain. Very rarely they may cause recurrent pain that clears, at least briefly, with treatment.
Candida albicans is usually primarily pruritic, and BV produces discharge and odor, sometimes with minor symptoms. Non-albicans Candida (e.g., Candida glabrata) is nearly always asymptomatic, but it occasionally causes irritation and burning.
Group B streptococcus is another infectious entity that very, very occasionally causes irritation and dyspareunia but is usually only a colonizer.
Herpes simplex virus is a cause of recurrent but not chronic pain.
Chronic pain is more likely to be caused by skin disease than by infection. Lichen simplex chronicus causes itching; any pain is due to erosions from scratching.
Dr. Haefner: Several other infectious conditions or their treatments can cause vulvar pain. For example, herpes (particularly primary herpes infection) is classically associated with vulvar pain. The pain is so great that, at times, the patient requires admission for pain control. Surprisingly, despite the known pain of herpes, approximately 80% of patients who have it are unaware of their diagnosis.
Although condyloma is generally a painless condition, many patients complain of pain following treatment for it, whether treatment involves topical medications or laser surgery.
Chancroid is a painful vulvar ulcer. Trichomonas can sometimes be associated with vulvar pain.
Dr. Lonky: What terminology do we use when we discuss vulvar pain?
Dr. Haefner: The current terminology used to describe vulvar pain was published in 2004, after years of debate over nomenclature within the International Society for the Study of Vulvovaginal Disease.5 The terminology lists two major categories of vulvar pain:
- pain related to a specific disorder. This category encompasses numerous conditions that feature an abnormal appearance of the vulva (Table 1).
TABLE 1
Terminology and classification of vulvar pain from the International Society for the Study of Vulvovaginal Disease
|
| SOURCE: Moyal-Barracco and Lynch.5 Reproduced with permission from the Journal of Reproductive Medicine. |
- vulvodynia, in which the vulva appears normal, other than occasional erythema, which is most prominent at the duct openings (vestibular ducts—Bartholin’s and Skene’s).
As for vulvar pain, there are two major forms:
- hyperalgesia (a low threshold for pain)
- allodynia (pain in response to light touch).
Some diseases that are associated with vulvar pain do not qualify for the diagnosis of vulvodynia (Table 2) because they are associated with an abnormal appearance of the vulva.
TABLE 2
Conditions other than vulvodynia that are associated with vulvar pain
| Acute irritant contact dermatitis (e.g., erosion due to podofilox, imiquimod, cantharidin, fluorouracil, or podophyllin toxin) |
| Aphthous ulcer |
| Atrophy |
| Bartholin’s abscess |
| Candidiasis |
| Carcinoma |
| Chronic irritant contact dermatitis |
| Endometriosis |
| Herpes (simplex and zoster) |
| Immunobullous diseases (including cicatricial pemphigoid, pemphigus vulgaris, linear immunoglobulin A disease, etc.) |
| Lichen planus |
| Lichen sclerosus |
| Podophyllin overdose (see above) |
| Prolapsed urethra |
| Sjögren’s syndrome |
| Trauma |
| Trichomoniasis |
| Vulvar intraepithelial neoplasia |
What needs to be ruled out for a diagnosis of vulvodynia?
Dr. Lonky: What skin diseases need to be ruled out before vulvodynia can be diagnosed?
Dr. Edwards: Skin diseases that affect the vulva are usually pruritic—pain is a later sign. Lichen simplex chronicus (also known as eczema) is pruritus caused by any irritant; any pain that arises is produced by visible excoriations from scratching.
Lichen sclerosus manifests as white epithelium that has a crinkling, shiny, or waxy texture. It can produce pain, especially dyspareunia. The pain is caused by erosions that arise from fragility and introital narrowing and inelasticity.
Vulvovaginal lichen planus is usually erosive and preferentially affects mucous membranes, especially the vestibule; it sometimes affects the vagina and mouth, as well.
Desquamative inflammatory vaginitis is most likely a skin disease that affects only the vagina. It involves introital redness and a clinically and microscopically purulent vaginal discharge that also reveals parabasal cells and absent lactobacilli.
Dr. Lonky: You mentioned that neurologic diseases can sometimes cause vulvar pain. Which ones?
Dr. Edwards: Pudendal neuralgia, diabetic neuropathy, and post-herpetic neuralgia are the most common specific neurologic causes of vulvar pain. Multiple sclerosis can also produce pain syndromes. Post-herpetic neuralgia follows herpes zoster—not herpes simplex—virus infection.
Dr. Lonky: Any other conditions that can cause vulvar pain?
Dr. Haefner: Aphthous ulcers are common and are often flared by stress.
Non-neoplastic epithelial disorders are also seen frequently in health-care providers’ offices; many patients who experience them report pain on the vulva.
It is always important to consider cancer when a patient has an abnormal vulvar appearance and pain that has persisted despite treatment.
What are the most common vulvar pain syndromes?
Dr. Lonky: If you were to rank vulvar pain syndromes according to their prevalence, what would the most common syndromes be?
Dr. Gunter: Given the misdiagnosis of many women, who are told they have chronic yeast infection, as I mentioned, it’s hard to know which vulvar pain syndromes are most prevalent. I suspect that lichen simplex chronicus is most common, followed by vulvodynia, with chronic yeast infection a distant third.
My experience reflects what Nyirjesy and colleagues4 found: 65% to 75% of women referred to my clinic with chronic yeast actually have lichen simplex chronicus or vulvodynia. In postmenopausal women, atrophic vaginitis is also a consideration; it’s becoming more common now that the use of systemic hormone replacement therapy is decreasing.
Dr. Lonky: What about subsets of vulvodynia? Which ones are most common?
Dr. Edwards: There is good evidence of marked overlap among subsets of vulvodynia. The vast majority of women who have vulvodynia experience primarily provoked vestibular pain, regardless of age. However, I find that almost all patients also report pain that extends beyond the vestibule at times, as well as occasional unprovoked pain.
The diagnosis requires the exclusion of other causes of vulvar pain, and the subset is identified by the location of pain (that is, is it strictly localized or generalized or even migratory?) and its provoked or unprovoked nature.
Localized clitoral pain and vulvar pain localized to one side of the vulva are extremely uncommon, but they do occur. And although I rarely encounter teenagers and prepubertal children who have vulvodynia, I do have patients in both age groups who have vulvodynia.
Dr. Lonky: Are there racial differences in the prevalence of vulvodynia?
Dr. Edwards: Although several good studies show that women of African descent and white patients are equally likely to experience vulvodynia, the vast majority (99%) of my patients who have vulvodynia are white. My patients of African descent consult me primarily for itching or discharge.
My local demographics prevent me from judging the likelihood of Asians having vulvodynia, and our Hispanic population has limited access to health care.
In general, I don’t think that demographics are useful in making the diagnosis of vulvodynia.
Do women who have vulvar pain tend to have comorbidities?
Dr. Lonky: Do your patients who have vulvodynia or another vulvar pain syndrome tend to have comorbidities? If so, is this information helpful in establishing the diagnosis and planning therapy?
Dr. Haefner: Women who have vulvodynia often have other medical problems as well. In my practice, when new patients who have vulvodynia complete their intake survey, they often report a history of headache, irritable bowel syndrome, interstitial cystitis, fibromyalgia,6 chronic fatigue syndrome, back pain, and temporomandibular joint (TMJ) disorder. These comorbidities are not particularly helpful in establishing the diagnosis of vulvodynia, but they are an important consideration when choosing therapy for the patient. Often, the medications chosen to treat one condition will also benefit another condition. However, it’s important to check for potential interactions between drugs before prescribing a new treatment.
Dr. Gunter: A significant number of women who have vulvodynia also have other chronic pain syndromes. For example, the incidence of bladder pain syndrome–interstitial cystitis is 68% to 82% among women who have vulvodynia, compared with a baseline rate among all women of 6% to 11%.7-10 The rate of irritable bowel syndrome is more than doubled among women who have vulvodynia, compared with the general population (27% versus 12%).8 Another common comorbidity, hypertonic somatic dysfunction of the pelvic floor, is identified in 10% to 90% of women who have chronic vulvar pain.8,11,12 These women also have a higher incidence of nongenital pain syndromes, such as fibromyalgia, migraine, and TMJ dysfunction, than the general population, as Dr. Haefner noted.8,12,13
Many studies have evaluated psychological and emotional contributions to chronic vulvar pain. Pain and depression are intimately related—the incidence of depression among all people who experience chronic pain ranges from 27% to 54%, compared with 5% to 17% among the general population.14-16 The relationship is complex because chronic illness in general is associated with depression. Nevertheless, several studies have noted an increase in anxiety, stress, and depression among women who have vulvodynia.17-19
I screen every patient for depression using a Patient Health Questionnaire (PHQ-9); I also screen for anxiety. I find that a significant percentage of patients in my clinic are depressed or have an anxiety disorder. Failure to address these comorbidities makes treatment very difficult. I typically prescribe citalopram (Celexa), although there is some question whether it can safely be combined with a tricyclic antidepressant. We also offer stress-reduction classes, teach every patient the value of diaphragmatic breathing, offer mind-body classes for anxiety and stress, and provide intensive programs where the patient can learn important self-care skills, such as pacing (spacing activities throughout the day in a manner that avoids aggravating the pain), and address her anxiety and stress in a more guided manner. We also have a psychologist who specializes in pain for any patient who may need one-on-one counseling.
Dr. Edwards: The presence of comorbidities is somewhat useful in making the diagnosis of vulvodynia. I question my diagnosis, in fact, when a patient who has vulvodynia does not have headaches, low energy, depression, anxiety, irritable bowel syndrome, constipation, fibromyalgia, chronic fatigue, sensitivity to medications, TMJ dysfunction, or urinary symptoms.
How common is pudendal neuralgia?
Dr. Lonky: How prevalent is a finding of pudendal neuralgia?
Dr. Edwards: The prevalence and incidence of pudendal neuralgia are not known. Those who specialize in this condition think it is relatively common. I do not identify or suspect it very often. Its definitive diagnosis and management are outside the purview of the general gynecologist, but the general gynecologist should recognize the symptoms of pudendal neuralgia and refer the patient for evaluation and therapy.
Dr. Lonky: What are those symptoms?
Dr. Haefner: Pudendal neuralgia often occurs following trauma to the pudendal nerve. The pudendal nerve arises from sacral nerves, generally sacral nerves 2 to 4. Several tests can be utilized to diagnose this condition, including quantitative sensor tests, pudendal nerve motor latency tests, electromyography (EMG), and pudendal nerve blocks.20
Nantes Criteria allow for making a diagnosis of pudendal neuralgia (Table 3).21
TABLE 3
Nantes Criteria for pudendal neuralgia by pudendal nerve entrapment
Essential criteria
|
Complementary diagnostic criteria
|
Exclusion criteria
|
Associated signs not excluding the diagnosis
|
| SOURCE: Labat et al.21 Reproduced with permission from Neurology and Urodynamics. |
Initial treatments for pudendal neuralgia should be conservative. Treatments consist of lifestyle changes to prevent flare of disease. Physical therapy, medical management, nerve blocks, and alternative treatments may be beneficial.
Pudendal nerve entrapment is often exacerbated by sitting (not on a toilet seat, however) and is reduced in a standing position. It tends to increase in intensity throughout the day.22 The final treatment for pudendal nerve entrapment is surgery if the nerve is compressed. By this time, the generalist is not generally the provider who performs the surgery.
Dr. Gunter: I believe pudendal neuralgia is sometimes overdiagnosed. EMG studies of the pudendal nerve, often touted as a diagnostic tool, are unreliable (they can be abnormal after vaginal delivery or vaginal hysterectomy, for example). In my experience, bilateral pain is less likely to be pudendal neuralgia; spontaneous bilateral compression neuropathy at exactly the same level is not a common phenomenon in chronic pain.
I reserve the diagnosis of pudendal neuralgia for women who have allodynia in the distribution of the pudendal nerve with severe pain on sitting, and who have exquisite tenderness when pressure is applied over the pudendal nerve (at the level of the ischial spine on vaginal examination). Typically, the vaginal sidewall on the affected side is very sensitive to light touch. I do see pudendal nerve pain after vaginal surgery when there has been some compromise of the pudendal nerve or the sacral plexus. This is typically unilateral pain.
Dr. Lonky: Thank you all. We’ll continue our discussion, with a focus on treatment, in the October 2011 issue.
- Part 2: A bounty of treatment options
(October 2011) - Part 3: Vestibulodynia
(November 2011)
We want to hear from you! Tell us what you think.
Although the incidence of vulvar pain has increased over the past decade—thanks to both greater awareness and increasing numbers of affected women—the phenomenon is not a recent development. As early as 1874, T. Galliard Thomas wrote, “[T]his disorder, although fortunately not very frequent, is by no means very rare.”1 He went on to express “surprise” that it had not been “more generally and fully described.”
Despite the focus Thomas directed to the issue, vulvar pain did not get much attention until the 21st century, when a number of studies began to gauge its prevalence. For example, in a study in Boston of about 5,000 women, the lifetime prevalence of chronic vulvar pain was 16%.2 And in a study in Texas, the prevalence of vulvar pain in an urban, largely minority population was estimated to be 11%.3 The Boston study also reported that “nearly 40% of women chose not to seek treatment, and, of those who did, 60% saw three or more doctors, many of whom could not provide a diagnosis.”2
Clearly, there is a need for comprehensive information on vulvar pain and its causes, symptoms, diagnosis, and treatment. To address the lack of guidance, OBG Management Contributing Editor Neal M. Lonky, MD, assembled a panel of experts on vulvar pain syndromes and invited them to share their considerable knowledge. The ensuing discussion, presented in three parts, offers a gold mine of information.
In this opening article, the panel focuses on causes, symptomatology, and diagnosis of this common complaint. In Part 2, which will appear in the October issue of this journal, the focus is the bounty of treatment options. Part 3 follows in November, when the discussion shifts to vestibulodynia.

The lower vagina and vulva are richly supplied with peripheral nerves and are, therefore, sensitive to pain, particularly the region of the hymeneal ring. Although the pudendal nerve (arrow) courses through the area, it is an uncommon source of vulvar pain.
Common diagnoses—and misdiagnoses
Dr. Lonky: What are the most common diagnoses when vulvar pain is the complaint?
Dr. Gunter: The most common cause of chronic vulvar pain is vulvodynia, although lichen simplex chronicus, chronic yeast infections, and non-neoplastic epithelial disorders, such as lichen sclerosus and lichen planus, can also produce irritation and pain. In postmenopausal women, atrophic vaginitis can also cause a burning pain, although symptoms are typically more vaginal than vulvar. Yeast and lichen simplex chronicus typically produce itching, although sometimes they can present with irritation and pain, so they must be considered in the differential diagnosis. It is important to remember that many women with vulvodynia have used multiple topical agents and may have developed complex hygiene rituals in an attempt to treat their symptoms, which can result in a secondary lichen simplex chronicus.
That said, there is a high frequency of misdiagnosis with yeast. For example, in a study by Nyirjesy and colleagues, two thirds of women who were referred to a tertiary clinic for chronic vulvovaginal candidiasis were found to have a noninfectious entity instead—most commonly lichen simplex chronicus and vulvodynia.4
Dr. Edwards: The most common “diagnosis” for vulvar pain is vulvodynia. However, the definition of vulvodynia is pain—i.e., burning, rawness, irritation, soreness, aching, or stabbing or stinging sensations—in the absence of skin disease, infection, or specific neurologic disease. Therefore, even though the usual cause of vulvar pain is vulvodynia, it is a diagnosis of exclusion, and skin disease, infection, and neurologic disease must be ruled out.
In regard to infection, Candida albicans and bacterial vaginosis (BV) are usually the first conditions that are considered when a patient complains of vulvar pain, but they are not common causes of vulvar pain and are never causes of chronic vulvar pain. Very rarely they may cause recurrent pain that clears, at least briefly, with treatment.
Candida albicans is usually primarily pruritic, and BV produces discharge and odor, sometimes with minor symptoms. Non-albicans Candida (e.g., Candida glabrata) is nearly always asymptomatic, but it occasionally causes irritation and burning.
Group B streptococcus is another infectious entity that very, very occasionally causes irritation and dyspareunia but is usually only a colonizer.
Herpes simplex virus is a cause of recurrent but not chronic pain.
Chronic pain is more likely to be caused by skin disease than by infection. Lichen simplex chronicus causes itching; any pain is due to erosions from scratching.
Dr. Haefner: Several other infectious conditions or their treatments can cause vulvar pain. For example, herpes (particularly primary herpes infection) is classically associated with vulvar pain. The pain is so great that, at times, the patient requires admission for pain control. Surprisingly, despite the known pain of herpes, approximately 80% of patients who have it are unaware of their diagnosis.
Although condyloma is generally a painless condition, many patients complain of pain following treatment for it, whether treatment involves topical medications or laser surgery.
Chancroid is a painful vulvar ulcer. Trichomonas can sometimes be associated with vulvar pain.
Dr. Lonky: What terminology do we use when we discuss vulvar pain?
Dr. Haefner: The current terminology used to describe vulvar pain was published in 2004, after years of debate over nomenclature within the International Society for the Study of Vulvovaginal Disease.5 The terminology lists two major categories of vulvar pain:
- pain related to a specific disorder. This category encompasses numerous conditions that feature an abnormal appearance of the vulva (Table 1).
TABLE 1
Terminology and classification of vulvar pain from the International Society for the Study of Vulvovaginal Disease
|
| SOURCE: Moyal-Barracco and Lynch.5 Reproduced with permission from the Journal of Reproductive Medicine. |
- vulvodynia, in which the vulva appears normal, other than occasional erythema, which is most prominent at the duct openings (vestibular ducts—Bartholin’s and Skene’s).
As for vulvar pain, there are two major forms:
- hyperalgesia (a low threshold for pain)
- allodynia (pain in response to light touch).
Some diseases that are associated with vulvar pain do not qualify for the diagnosis of vulvodynia (Table 2) because they are associated with an abnormal appearance of the vulva.
TABLE 2
Conditions other than vulvodynia that are associated with vulvar pain
| Acute irritant contact dermatitis (e.g., erosion due to podofilox, imiquimod, cantharidin, fluorouracil, or podophyllin toxin) |
| Aphthous ulcer |
| Atrophy |
| Bartholin’s abscess |
| Candidiasis |
| Carcinoma |
| Chronic irritant contact dermatitis |
| Endometriosis |
| Herpes (simplex and zoster) |
| Immunobullous diseases (including cicatricial pemphigoid, pemphigus vulgaris, linear immunoglobulin A disease, etc.) |
| Lichen planus |
| Lichen sclerosus |
| Podophyllin overdose (see above) |
| Prolapsed urethra |
| Sjögren’s syndrome |
| Trauma |
| Trichomoniasis |
| Vulvar intraepithelial neoplasia |
What needs to be ruled out for a diagnosis of vulvodynia?
Dr. Lonky: What skin diseases need to be ruled out before vulvodynia can be diagnosed?
Dr. Edwards: Skin diseases that affect the vulva are usually pruritic—pain is a later sign. Lichen simplex chronicus (also known as eczema) is pruritus caused by any irritant; any pain that arises is produced by visible excoriations from scratching.
Lichen sclerosus manifests as white epithelium that has a crinkling, shiny, or waxy texture. It can produce pain, especially dyspareunia. The pain is caused by erosions that arise from fragility and introital narrowing and inelasticity.
Vulvovaginal lichen planus is usually erosive and preferentially affects mucous membranes, especially the vestibule; it sometimes affects the vagina and mouth, as well.
Desquamative inflammatory vaginitis is most likely a skin disease that affects only the vagina. It involves introital redness and a clinically and microscopically purulent vaginal discharge that also reveals parabasal cells and absent lactobacilli.
Dr. Lonky: You mentioned that neurologic diseases can sometimes cause vulvar pain. Which ones?
Dr. Edwards: Pudendal neuralgia, diabetic neuropathy, and post-herpetic neuralgia are the most common specific neurologic causes of vulvar pain. Multiple sclerosis can also produce pain syndromes. Post-herpetic neuralgia follows herpes zoster—not herpes simplex—virus infection.
Dr. Lonky: Any other conditions that can cause vulvar pain?
Dr. Haefner: Aphthous ulcers are common and are often flared by stress.
Non-neoplastic epithelial disorders are also seen frequently in health-care providers’ offices; many patients who experience them report pain on the vulva.
It is always important to consider cancer when a patient has an abnormal vulvar appearance and pain that has persisted despite treatment.
What are the most common vulvar pain syndromes?
Dr. Lonky: If you were to rank vulvar pain syndromes according to their prevalence, what would the most common syndromes be?
Dr. Gunter: Given the misdiagnosis of many women, who are told they have chronic yeast infection, as I mentioned, it’s hard to know which vulvar pain syndromes are most prevalent. I suspect that lichen simplex chronicus is most common, followed by vulvodynia, with chronic yeast infection a distant third.
My experience reflects what Nyirjesy and colleagues4 found: 65% to 75% of women referred to my clinic with chronic yeast actually have lichen simplex chronicus or vulvodynia. In postmenopausal women, atrophic vaginitis is also a consideration; it’s becoming more common now that the use of systemic hormone replacement therapy is decreasing.
Dr. Lonky: What about subsets of vulvodynia? Which ones are most common?
Dr. Edwards: There is good evidence of marked overlap among subsets of vulvodynia. The vast majority of women who have vulvodynia experience primarily provoked vestibular pain, regardless of age. However, I find that almost all patients also report pain that extends beyond the vestibule at times, as well as occasional unprovoked pain.
The diagnosis requires the exclusion of other causes of vulvar pain, and the subset is identified by the location of pain (that is, is it strictly localized or generalized or even migratory?) and its provoked or unprovoked nature.
Localized clitoral pain and vulvar pain localized to one side of the vulva are extremely uncommon, but they do occur. And although I rarely encounter teenagers and prepubertal children who have vulvodynia, I do have patients in both age groups who have vulvodynia.
Dr. Lonky: Are there racial differences in the prevalence of vulvodynia?
Dr. Edwards: Although several good studies show that women of African descent and white patients are equally likely to experience vulvodynia, the vast majority (99%) of my patients who have vulvodynia are white. My patients of African descent consult me primarily for itching or discharge.
My local demographics prevent me from judging the likelihood of Asians having vulvodynia, and our Hispanic population has limited access to health care.
In general, I don’t think that demographics are useful in making the diagnosis of vulvodynia.
Do women who have vulvar pain tend to have comorbidities?
Dr. Lonky: Do your patients who have vulvodynia or another vulvar pain syndrome tend to have comorbidities? If so, is this information helpful in establishing the diagnosis and planning therapy?
Dr. Haefner: Women who have vulvodynia often have other medical problems as well. In my practice, when new patients who have vulvodynia complete their intake survey, they often report a history of headache, irritable bowel syndrome, interstitial cystitis, fibromyalgia,6 chronic fatigue syndrome, back pain, and temporomandibular joint (TMJ) disorder. These comorbidities are not particularly helpful in establishing the diagnosis of vulvodynia, but they are an important consideration when choosing therapy for the patient. Often, the medications chosen to treat one condition will also benefit another condition. However, it’s important to check for potential interactions between drugs before prescribing a new treatment.
Dr. Gunter: A significant number of women who have vulvodynia also have other chronic pain syndromes. For example, the incidence of bladder pain syndrome–interstitial cystitis is 68% to 82% among women who have vulvodynia, compared with a baseline rate among all women of 6% to 11%.7-10 The rate of irritable bowel syndrome is more than doubled among women who have vulvodynia, compared with the general population (27% versus 12%).8 Another common comorbidity, hypertonic somatic dysfunction of the pelvic floor, is identified in 10% to 90% of women who have chronic vulvar pain.8,11,12 These women also have a higher incidence of nongenital pain syndromes, such as fibromyalgia, migraine, and TMJ dysfunction, than the general population, as Dr. Haefner noted.8,12,13
Many studies have evaluated psychological and emotional contributions to chronic vulvar pain. Pain and depression are intimately related—the incidence of depression among all people who experience chronic pain ranges from 27% to 54%, compared with 5% to 17% among the general population.14-16 The relationship is complex because chronic illness in general is associated with depression. Nevertheless, several studies have noted an increase in anxiety, stress, and depression among women who have vulvodynia.17-19
I screen every patient for depression using a Patient Health Questionnaire (PHQ-9); I also screen for anxiety. I find that a significant percentage of patients in my clinic are depressed or have an anxiety disorder. Failure to address these comorbidities makes treatment very difficult. I typically prescribe citalopram (Celexa), although there is some question whether it can safely be combined with a tricyclic antidepressant. We also offer stress-reduction classes, teach every patient the value of diaphragmatic breathing, offer mind-body classes for anxiety and stress, and provide intensive programs where the patient can learn important self-care skills, such as pacing (spacing activities throughout the day in a manner that avoids aggravating the pain), and address her anxiety and stress in a more guided manner. We also have a psychologist who specializes in pain for any patient who may need one-on-one counseling.
Dr. Edwards: The presence of comorbidities is somewhat useful in making the diagnosis of vulvodynia. I question my diagnosis, in fact, when a patient who has vulvodynia does not have headaches, low energy, depression, anxiety, irritable bowel syndrome, constipation, fibromyalgia, chronic fatigue, sensitivity to medications, TMJ dysfunction, or urinary symptoms.
How common is pudendal neuralgia?
Dr. Lonky: How prevalent is a finding of pudendal neuralgia?
Dr. Edwards: The prevalence and incidence of pudendal neuralgia are not known. Those who specialize in this condition think it is relatively common. I do not identify or suspect it very often. Its definitive diagnosis and management are outside the purview of the general gynecologist, but the general gynecologist should recognize the symptoms of pudendal neuralgia and refer the patient for evaluation and therapy.
Dr. Lonky: What are those symptoms?
Dr. Haefner: Pudendal neuralgia often occurs following trauma to the pudendal nerve. The pudendal nerve arises from sacral nerves, generally sacral nerves 2 to 4. Several tests can be utilized to diagnose this condition, including quantitative sensor tests, pudendal nerve motor latency tests, electromyography (EMG), and pudendal nerve blocks.20
Nantes Criteria allow for making a diagnosis of pudendal neuralgia (Table 3).21
TABLE 3
Nantes Criteria for pudendal neuralgia by pudendal nerve entrapment
Essential criteria
|
Complementary diagnostic criteria
|
Exclusion criteria
|
Associated signs not excluding the diagnosis
|
| SOURCE: Labat et al.21 Reproduced with permission from Neurology and Urodynamics. |
Initial treatments for pudendal neuralgia should be conservative. Treatments consist of lifestyle changes to prevent flare of disease. Physical therapy, medical management, nerve blocks, and alternative treatments may be beneficial.
Pudendal nerve entrapment is often exacerbated by sitting (not on a toilet seat, however) and is reduced in a standing position. It tends to increase in intensity throughout the day.22 The final treatment for pudendal nerve entrapment is surgery if the nerve is compressed. By this time, the generalist is not generally the provider who performs the surgery.
Dr. Gunter: I believe pudendal neuralgia is sometimes overdiagnosed. EMG studies of the pudendal nerve, often touted as a diagnostic tool, are unreliable (they can be abnormal after vaginal delivery or vaginal hysterectomy, for example). In my experience, bilateral pain is less likely to be pudendal neuralgia; spontaneous bilateral compression neuropathy at exactly the same level is not a common phenomenon in chronic pain.
I reserve the diagnosis of pudendal neuralgia for women who have allodynia in the distribution of the pudendal nerve with severe pain on sitting, and who have exquisite tenderness when pressure is applied over the pudendal nerve (at the level of the ischial spine on vaginal examination). Typically, the vaginal sidewall on the affected side is very sensitive to light touch. I do see pudendal nerve pain after vaginal surgery when there has been some compromise of the pudendal nerve or the sacral plexus. This is typically unilateral pain.
Dr. Lonky: Thank you all. We’ll continue our discussion, with a focus on treatment, in the October 2011 issue.
- Part 2: A bounty of treatment options
(October 2011) - Part 3: Vestibulodynia
(November 2011)
We want to hear from you! Tell us what you think.
1. Thomas TG. Practical Treatise on the Diseases of Women. Philadelphia Pa: Henry C. Lea; 1874.
2. Harlow BL, Stewart EG. A population-based assessment of chronic unexplained vulvar pain: have we underestimated the prevalence of vulvodynia? J Am Med Womens Assoc. 2003;58(2):82-88.
3. Lavy RJ, Hynan LS, Haley RW. Prevalence of vulvar pain in an urban minority population. J Reprod Med. 2007;52(1):59-62.
4. Nyirjesy P, Peyton C, Weitz MV, Mathew L, Culhane JF. Causes of chronic vaginitis: analysis of a prospective database of affected women. Obstet Gynecol. 2006;108(5):1185-1191.
5. Moyal-Barracco M, Lynch PJ. 2003 ISSVD terminology and classification of vulvodynia: a historical perspective. J Reprod Med. 2004;49(10):772-777.
6. Yunas MB. Fibromyalgia and overlapping disorders: the unifying concept of central sensitivity syndromes. Semin Arthritis Rheum. 2007;36(6):339-356.
7. Kahn BS, Tatro C, Parsons CL, Willems JJ. Prevalence of interstitial cystitis in vulvodynia patients detected by bladder potassium sensitivity. J Sex Med. 2010;7(2 Pt 2):996-1002.
8. Arnold JD, Bachman GS, Rosen R, Kelly S, Rhoads GG. Vulvodynia: characteristics and associations with comorbidities and quality of life. Obstet Gynecol. 2006;107(3):617-624.
9. Parsons CL, Dell J, Stanford EJ, et al. The prevalence of interstitial cystitis in gynecologic patients with pelvic pain, as detected by intravesical potassium sensitivity. Am J Obstet Gynecol. 2002;187(5):1395-1400.
10. Clemens JQ, Meenan RT, O’Keefe Rosetti MC, et al. Prevalence of interstitial cystitis symptoms in a managed care population. J Urol. 2005;174(2):576-580.
11. Engman M, Lindehammar H, Wijma B. Surface electromyography diagnostics in women with partial vaginismus with or without vulvar vestibulitis and in asymptomatic women. J Psychosom Obstet Gynecol. 2004;25(3-4):281-294.
12. Gunter J. Vulvodynia: new thoughts on a devastating condition. Obstet Gynecol Surv. 2007;62(12):812-819.
13. Gordon AS, Panahlan-Jand M, McComb F, Melegari C, Sharp S. Characteristics of women with vulvar pain disorders: a Web-based survey. J Sex Marital Ther. 2003;29(suppl 1):45.-
14. Whitten CE, Cristobal K. Chronic pain is a chronic condition not just a symptom. Permanente J. 2005;9(3):43.-
15. Manchikanti L, Fellows B, Pampati V, et al. Comparison of psychological status of chronic pain patients and the general population. Pain Physician. 2002;5(1):40-48.
16. Banks SM, Kerns RD. Explaining the high rates of depression in chronic pain: a diathesis-stress framework. Psychological Bulletin. 1996;119(1):95-110.
17. Sadownik LA. Clinical correlates of vulvodynia patients. A prospective study of 300 patients. J Reprod Med. 2000;5:40-48.Editor found in PubMed: Sadownik LA. Clinical profile of vulvodynia patients. A prospective study of 300 patients. J Reprod Med. 2000;45(8):679–684. Could not find the citation listed. Please confirm.
18. Reed BD, Haefner HK, Punch MR, Roth RS, Gorenflo DW, Gillespie BW. Psychosocial and sexual functioning in women with vulvodynia and chronic pelvic pain. A comparative evaluation. J Reprod Med. 2000;45(8):624-632.
19. Landry T, Bergeron S. Biopsychosocial factors associated with dyspareunia in a community sample of adolescent girls. Arch Sex Behav. 2011;June 22.
20. Goldstein A, Pukall C, Goldstein I. When Sex Hurts: A Woman’s Guide to Banishing Sexual Pain. Cambridge Mass: Da Capo Lifelong Books; 2011;117-126.
21. Labat JJ, Riant T, Robert R, Amarenco G, Lefaucheur JP, Rigaud J. Diagnostic criteria for pudendal neuralgia by pudendal nerve entrapment (Nantes criteria). Neurol Urodyn. 2008;27(4):306-310.
22. Popeney C, Answell V, Renney K. Pudendal entrapment as an etiology of chronic perineal pain: Diagnosis and treatment. Neurol Urodyn. 2007;26(6):820-827.
1. Thomas TG. Practical Treatise on the Diseases of Women. Philadelphia Pa: Henry C. Lea; 1874.
2. Harlow BL, Stewart EG. A population-based assessment of chronic unexplained vulvar pain: have we underestimated the prevalence of vulvodynia? J Am Med Womens Assoc. 2003;58(2):82-88.
3. Lavy RJ, Hynan LS, Haley RW. Prevalence of vulvar pain in an urban minority population. J Reprod Med. 2007;52(1):59-62.
4. Nyirjesy P, Peyton C, Weitz MV, Mathew L, Culhane JF. Causes of chronic vaginitis: analysis of a prospective database of affected women. Obstet Gynecol. 2006;108(5):1185-1191.
5. Moyal-Barracco M, Lynch PJ. 2003 ISSVD terminology and classification of vulvodynia: a historical perspective. J Reprod Med. 2004;49(10):772-777.
6. Yunas MB. Fibromyalgia and overlapping disorders: the unifying concept of central sensitivity syndromes. Semin Arthritis Rheum. 2007;36(6):339-356.
7. Kahn BS, Tatro C, Parsons CL, Willems JJ. Prevalence of interstitial cystitis in vulvodynia patients detected by bladder potassium sensitivity. J Sex Med. 2010;7(2 Pt 2):996-1002.
8. Arnold JD, Bachman GS, Rosen R, Kelly S, Rhoads GG. Vulvodynia: characteristics and associations with comorbidities and quality of life. Obstet Gynecol. 2006;107(3):617-624.
9. Parsons CL, Dell J, Stanford EJ, et al. The prevalence of interstitial cystitis in gynecologic patients with pelvic pain, as detected by intravesical potassium sensitivity. Am J Obstet Gynecol. 2002;187(5):1395-1400.
10. Clemens JQ, Meenan RT, O’Keefe Rosetti MC, et al. Prevalence of interstitial cystitis symptoms in a managed care population. J Urol. 2005;174(2):576-580.
11. Engman M, Lindehammar H, Wijma B. Surface electromyography diagnostics in women with partial vaginismus with or without vulvar vestibulitis and in asymptomatic women. J Psychosom Obstet Gynecol. 2004;25(3-4):281-294.
12. Gunter J. Vulvodynia: new thoughts on a devastating condition. Obstet Gynecol Surv. 2007;62(12):812-819.
13. Gordon AS, Panahlan-Jand M, McComb F, Melegari C, Sharp S. Characteristics of women with vulvar pain disorders: a Web-based survey. J Sex Marital Ther. 2003;29(suppl 1):45.-
14. Whitten CE, Cristobal K. Chronic pain is a chronic condition not just a symptom. Permanente J. 2005;9(3):43.-
15. Manchikanti L, Fellows B, Pampati V, et al. Comparison of psychological status of chronic pain patients and the general population. Pain Physician. 2002;5(1):40-48.
16. Banks SM, Kerns RD. Explaining the high rates of depression in chronic pain: a diathesis-stress framework. Psychological Bulletin. 1996;119(1):95-110.
17. Sadownik LA. Clinical correlates of vulvodynia patients. A prospective study of 300 patients. J Reprod Med. 2000;5:40-48.Editor found in PubMed: Sadownik LA. Clinical profile of vulvodynia patients. A prospective study of 300 patients. J Reprod Med. 2000;45(8):679–684. Could not find the citation listed. Please confirm.
18. Reed BD, Haefner HK, Punch MR, Roth RS, Gorenflo DW, Gillespie BW. Psychosocial and sexual functioning in women with vulvodynia and chronic pelvic pain. A comparative evaluation. J Reprod Med. 2000;45(8):624-632.
19. Landry T, Bergeron S. Biopsychosocial factors associated with dyspareunia in a community sample of adolescent girls. Arch Sex Behav. 2011;June 22.
20. Goldstein A, Pukall C, Goldstein I. When Sex Hurts: A Woman’s Guide to Banishing Sexual Pain. Cambridge Mass: Da Capo Lifelong Books; 2011;117-126.
21. Labat JJ, Riant T, Robert R, Amarenco G, Lefaucheur JP, Rigaud J. Diagnostic criteria for pudendal neuralgia by pudendal nerve entrapment (Nantes criteria). Neurol Urodyn. 2008;27(4):306-310.
22. Popeney C, Answell V, Renney K. Pudendal entrapment as an etiology of chronic perineal pain: Diagnosis and treatment. Neurol Urodyn. 2007;26(6):820-827.
IN THIS ARTICLE
Asymptomatic Older Women Not on HRT May Have Polyps
Major Finding: Polyps were suspected in 101 (6.7%) of these patients based on the ultrasound appearance of the endometrial lining.
Data Source: A prospective study of 1,500 consecutive asymptomatic women.
Disclosures: Dr. Hartman said that he had no relevant financial disclosures.
WASHINGTON – A small but important percentage of postmenopausal women not taking hormone replacement therapy have an endometrial lining that is suspicious for polyps, according to a prospective study of 1,500 consecutive asymptomatic women.
“We found no suspicion of polyps in the vast majority of asymptomatic postmenopausal women not taking HRT [hormone replacement therapy]; but we did find a suspicion of polyps in 6.7% of all patients. The appearance of a nonhomogeneous endometrial lining on ultrasound increased our suspicion of polyp,” Dr. Michael Hartman of Memorial University of Newfoundland, St. John's, Canada, said in a poster presentation at the meeting. “The number of women with suspicion of polyps was higher than expected and indicates there are a large number of asymptomatic postmenopausal women with endometrial polyps.”
Dr. Hartman explained in an interview: “Any woman can develop polyps, whether or not she is on exogenous hormones. Estrogen can affect the lining of the uterus after menopause since it is produced in fat cells and not just the ovaries.”
In a study of women aged 45–95 years (mean age, 62.7 years) who underwent transvaginal ultrasound from January to August 2010, 77.1% had an endometrial thickness of less than or equal to 4 mm, and 92% had an endometrial thickness of less than 5 mm. Polyps were suspected in 101 (6.7%) of these patients based on the ultrasound appearance of the endometrial lining.
Independent t-tests of age and endometrial thickening were performed, comparing the patients with a normal-appearing endometrium with those whose endometrial thickening was suspicious for polyps. A significant difference was observed between the groups, with older age and mean endometrial thickness having a significant association with suspicion of polyps. Polyps were significantly more likely to be found in older patients, with a mean age of 67.7 years, than in younger patients (mean age of 62 years). Patients with a lining suspicious for polyps had a thicker endometrium (mean of 8.02 mm) than did patients who did not have a lining suspicious for a polyp (mean of 3.40 mm).
These findings do not support routine ultrasound screening of older asymptomatic women. “In fact, I would say that the finding of polyps in 6.7% of women does not necessarily signal the presence of cancerous or precancerous growths. Further clinical investigation is required to determine the natural history of these polyps,” Dr. Hartman stated.
Major Finding: Polyps were suspected in 101 (6.7%) of these patients based on the ultrasound appearance of the endometrial lining.
Data Source: A prospective study of 1,500 consecutive asymptomatic women.
Disclosures: Dr. Hartman said that he had no relevant financial disclosures.
WASHINGTON – A small but important percentage of postmenopausal women not taking hormone replacement therapy have an endometrial lining that is suspicious for polyps, according to a prospective study of 1,500 consecutive asymptomatic women.
“We found no suspicion of polyps in the vast majority of asymptomatic postmenopausal women not taking HRT [hormone replacement therapy]; but we did find a suspicion of polyps in 6.7% of all patients. The appearance of a nonhomogeneous endometrial lining on ultrasound increased our suspicion of polyp,” Dr. Michael Hartman of Memorial University of Newfoundland, St. John's, Canada, said in a poster presentation at the meeting. “The number of women with suspicion of polyps was higher than expected and indicates there are a large number of asymptomatic postmenopausal women with endometrial polyps.”
Dr. Hartman explained in an interview: “Any woman can develop polyps, whether or not she is on exogenous hormones. Estrogen can affect the lining of the uterus after menopause since it is produced in fat cells and not just the ovaries.”
In a study of women aged 45–95 years (mean age, 62.7 years) who underwent transvaginal ultrasound from January to August 2010, 77.1% had an endometrial thickness of less than or equal to 4 mm, and 92% had an endometrial thickness of less than 5 mm. Polyps were suspected in 101 (6.7%) of these patients based on the ultrasound appearance of the endometrial lining.
Independent t-tests of age and endometrial thickening were performed, comparing the patients with a normal-appearing endometrium with those whose endometrial thickening was suspicious for polyps. A significant difference was observed between the groups, with older age and mean endometrial thickness having a significant association with suspicion of polyps. Polyps were significantly more likely to be found in older patients, with a mean age of 67.7 years, than in younger patients (mean age of 62 years). Patients with a lining suspicious for polyps had a thicker endometrium (mean of 8.02 mm) than did patients who did not have a lining suspicious for a polyp (mean of 3.40 mm).
These findings do not support routine ultrasound screening of older asymptomatic women. “In fact, I would say that the finding of polyps in 6.7% of women does not necessarily signal the presence of cancerous or precancerous growths. Further clinical investigation is required to determine the natural history of these polyps,” Dr. Hartman stated.
Major Finding: Polyps were suspected in 101 (6.7%) of these patients based on the ultrasound appearance of the endometrial lining.
Data Source: A prospective study of 1,500 consecutive asymptomatic women.
Disclosures: Dr. Hartman said that he had no relevant financial disclosures.
WASHINGTON – A small but important percentage of postmenopausal women not taking hormone replacement therapy have an endometrial lining that is suspicious for polyps, according to a prospective study of 1,500 consecutive asymptomatic women.
“We found no suspicion of polyps in the vast majority of asymptomatic postmenopausal women not taking HRT [hormone replacement therapy]; but we did find a suspicion of polyps in 6.7% of all patients. The appearance of a nonhomogeneous endometrial lining on ultrasound increased our suspicion of polyp,” Dr. Michael Hartman of Memorial University of Newfoundland, St. John's, Canada, said in a poster presentation at the meeting. “The number of women with suspicion of polyps was higher than expected and indicates there are a large number of asymptomatic postmenopausal women with endometrial polyps.”
Dr. Hartman explained in an interview: “Any woman can develop polyps, whether or not she is on exogenous hormones. Estrogen can affect the lining of the uterus after menopause since it is produced in fat cells and not just the ovaries.”
In a study of women aged 45–95 years (mean age, 62.7 years) who underwent transvaginal ultrasound from January to August 2010, 77.1% had an endometrial thickness of less than or equal to 4 mm, and 92% had an endometrial thickness of less than 5 mm. Polyps were suspected in 101 (6.7%) of these patients based on the ultrasound appearance of the endometrial lining.
Independent t-tests of age and endometrial thickening were performed, comparing the patients with a normal-appearing endometrium with those whose endometrial thickening was suspicious for polyps. A significant difference was observed between the groups, with older age and mean endometrial thickness having a significant association with suspicion of polyps. Polyps were significantly more likely to be found in older patients, with a mean age of 67.7 years, than in younger patients (mean age of 62 years). Patients with a lining suspicious for polyps had a thicker endometrium (mean of 8.02 mm) than did patients who did not have a lining suspicious for a polyp (mean of 3.40 mm).
These findings do not support routine ultrasound screening of older asymptomatic women. “In fact, I would say that the finding of polyps in 6.7% of women does not necessarily signal the presence of cancerous or precancerous growths. Further clinical investigation is required to determine the natural history of these polyps,” Dr. Hartman stated.
From the Annual Meeting of the American College of Obstetricians and Gynecolgists












