User login
WHI hormone trials offer reassurance on long-term mortality risk
Postmenopausal women treated with conjugated equine estrogens (CEE) plus medroxyprogesterone acetate (MPA) for a median of 5.6 years, or with CEE alone for a median of 7.2 years had no increased risk of all-cause, cardiovascular, or cancer mortality over a cumulative follow-up of 18 years, according to the latest report from the Women’s Health Initiative hormone therapy trials.
All-cause mortality among the 27,347 participants in the two randomized, double-blind, placebo-controlled trials was 27.1% in the hormone therapy group versus 27.6% in the placebo group (hazard ratio, 0.99), JoAnn E. Manson, MD, Harvard Medical School, Boston, and her colleagues reported.
For those who received CEE plus MPA, the hazard ratio was 1.02, while for CEE alone the hazard ratio was 0.94 (JAMA. 2017 Sep;318[10]:927-38).
Cardiovascular mortality among the pooled cohort was 8.9% with hormone therapy and 9.0% with placebo (HR, 1.00), and total cancer mortality was 8.2% and 8.0%, respectively (HR, 1.03). Mortality from other causes was 10.0% with hormone therapy, compared with 10.7% with placebo (HR, 0.95). The results did not differ significantly between the trials, the investigators noted.
An analysis by age showed that women aged 50-59 years tended to have lower hazard ratios for mortality from cardiovascular disease, cancer, and other causes during the intervention phases of the trials, but only the difference for “other” causes in the CEE-alone trial showed a statistically significant trend with age. This was influenced in part by adverse effects of the treatment in women aged 70-79 years. During cumulative follow-up, trends related to mortality across age groups were not statistically significantly different.
The trials included women aged 50-79 years who were enrolled between 1993 and 1998 and followed through 2014. Given the hormone-therapy-related risks identified in the CEE plus MPA and CEE-alone trials – which were stopped early because of increased risk of breast cancer/overall risks exceeding benefits, and for increased stroke risk, respectively – the absence of an increase in all-cause mortality during the intervention and cumulative follow-up phases of the trials is noteworthy, the investigators wrote.
“Although these findings lend support to practice guidelines endorsing use of hormone therapy for recently menopausal women with moderate to severe symptoms, in the absence of contraindications, the attenuation of age differences with longer follow-up and potential health risks of treatment would not support use of hormone therapy for reducing chronic disease or mortality,” they wrote. “Moreover, it is unclear whether benefits would outweigh risks with longer duration of treatment.”
They added that “in clinical decision making, these considerations must be weighed against the evidence linking untreated vasomotor symptoms in midlife women to impaired health and quality of life, disrupted sleep, reduced work productivity, and increased health care expenditures.”
The Women’s Health Initiative is funded by the U.S. Department of Health & Human Services. Study drugs were donated by Wyeth Ayerst. Dr. Manson reported having no financial disclosures. Several of her coauthors reported receiving grants and research funding from the National Institutes of Health, and receiving personal fees, speaking fees, and honoraria from various pharmaceutical companies.
The findings from the Women’s Health Initiative hormone therapy trials as reported by Dr. Manson and her colleagues expand the understanding of the long-term risks and benefits of hormone therapy, which is an important issue for women around the world, Melissa A. McNeil, MD, wrote in an editorial.
The information will be helpful for counseling women considering whether to start hormone therapy, and “hopefully will alleviate concerns that many patients and physicians have about the initiation of hormone therapy,” she said, explaining that the effect of hormone therapy on cancer mortality, and especially on breast cancer mortality, has generated concern and reluctance to prescribe and take hormone therapy for troubling menopausal symptoms.
“The current report ... provides substantial reassurance for patients and physicians about this issue,” she said. “For women with troubling vasomotor symptoms, premature menopause, or early-onset osteoporosis, hormone therapy appears to be both safe and efficacious.”
While several questions remain, including about optimal duration of therapy, whether there is a difference in mortality by age and menopausal status at hormone therapy initiation, and if earlier initiation would provide additional benefits, the data “fully support the newly released 2017 hormone therapy position statement of the North American Menopause Society and are a welcome addition to current knowledge regarding hormone therapy administration,” she wrote.
Dr. McNeil is with the University of Pittsburgh. She reported having no financial disclosures. Her comments come from an accompanying editorial (JAMA. 2017 Sep;318[10]:911-13).
The findings from the Women’s Health Initiative hormone therapy trials as reported by Dr. Manson and her colleagues expand the understanding of the long-term risks and benefits of hormone therapy, which is an important issue for women around the world, Melissa A. McNeil, MD, wrote in an editorial.
The information will be helpful for counseling women considering whether to start hormone therapy, and “hopefully will alleviate concerns that many patients and physicians have about the initiation of hormone therapy,” she said, explaining that the effect of hormone therapy on cancer mortality, and especially on breast cancer mortality, has generated concern and reluctance to prescribe and take hormone therapy for troubling menopausal symptoms.
“The current report ... provides substantial reassurance for patients and physicians about this issue,” she said. “For women with troubling vasomotor symptoms, premature menopause, or early-onset osteoporosis, hormone therapy appears to be both safe and efficacious.”
While several questions remain, including about optimal duration of therapy, whether there is a difference in mortality by age and menopausal status at hormone therapy initiation, and if earlier initiation would provide additional benefits, the data “fully support the newly released 2017 hormone therapy position statement of the North American Menopause Society and are a welcome addition to current knowledge regarding hormone therapy administration,” she wrote.
Dr. McNeil is with the University of Pittsburgh. She reported having no financial disclosures. Her comments come from an accompanying editorial (JAMA. 2017 Sep;318[10]:911-13).
The findings from the Women’s Health Initiative hormone therapy trials as reported by Dr. Manson and her colleagues expand the understanding of the long-term risks and benefits of hormone therapy, which is an important issue for women around the world, Melissa A. McNeil, MD, wrote in an editorial.
The information will be helpful for counseling women considering whether to start hormone therapy, and “hopefully will alleviate concerns that many patients and physicians have about the initiation of hormone therapy,” she said, explaining that the effect of hormone therapy on cancer mortality, and especially on breast cancer mortality, has generated concern and reluctance to prescribe and take hormone therapy for troubling menopausal symptoms.
“The current report ... provides substantial reassurance for patients and physicians about this issue,” she said. “For women with troubling vasomotor symptoms, premature menopause, or early-onset osteoporosis, hormone therapy appears to be both safe and efficacious.”
While several questions remain, including about optimal duration of therapy, whether there is a difference in mortality by age and menopausal status at hormone therapy initiation, and if earlier initiation would provide additional benefits, the data “fully support the newly released 2017 hormone therapy position statement of the North American Menopause Society and are a welcome addition to current knowledge regarding hormone therapy administration,” she wrote.
Dr. McNeil is with the University of Pittsburgh. She reported having no financial disclosures. Her comments come from an accompanying editorial (JAMA. 2017 Sep;318[10]:911-13).
Postmenopausal women treated with conjugated equine estrogens (CEE) plus medroxyprogesterone acetate (MPA) for a median of 5.6 years, or with CEE alone for a median of 7.2 years had no increased risk of all-cause, cardiovascular, or cancer mortality over a cumulative follow-up of 18 years, according to the latest report from the Women’s Health Initiative hormone therapy trials.
All-cause mortality among the 27,347 participants in the two randomized, double-blind, placebo-controlled trials was 27.1% in the hormone therapy group versus 27.6% in the placebo group (hazard ratio, 0.99), JoAnn E. Manson, MD, Harvard Medical School, Boston, and her colleagues reported.
For those who received CEE plus MPA, the hazard ratio was 1.02, while for CEE alone the hazard ratio was 0.94 (JAMA. 2017 Sep;318[10]:927-38).
Cardiovascular mortality among the pooled cohort was 8.9% with hormone therapy and 9.0% with placebo (HR, 1.00), and total cancer mortality was 8.2% and 8.0%, respectively (HR, 1.03). Mortality from other causes was 10.0% with hormone therapy, compared with 10.7% with placebo (HR, 0.95). The results did not differ significantly between the trials, the investigators noted.
An analysis by age showed that women aged 50-59 years tended to have lower hazard ratios for mortality from cardiovascular disease, cancer, and other causes during the intervention phases of the trials, but only the difference for “other” causes in the CEE-alone trial showed a statistically significant trend with age. This was influenced in part by adverse effects of the treatment in women aged 70-79 years. During cumulative follow-up, trends related to mortality across age groups were not statistically significantly different.
The trials included women aged 50-79 years who were enrolled between 1993 and 1998 and followed through 2014. Given the hormone-therapy-related risks identified in the CEE plus MPA and CEE-alone trials – which were stopped early because of increased risk of breast cancer/overall risks exceeding benefits, and for increased stroke risk, respectively – the absence of an increase in all-cause mortality during the intervention and cumulative follow-up phases of the trials is noteworthy, the investigators wrote.
“Although these findings lend support to practice guidelines endorsing use of hormone therapy for recently menopausal women with moderate to severe symptoms, in the absence of contraindications, the attenuation of age differences with longer follow-up and potential health risks of treatment would not support use of hormone therapy for reducing chronic disease or mortality,” they wrote. “Moreover, it is unclear whether benefits would outweigh risks with longer duration of treatment.”
They added that “in clinical decision making, these considerations must be weighed against the evidence linking untreated vasomotor symptoms in midlife women to impaired health and quality of life, disrupted sleep, reduced work productivity, and increased health care expenditures.”
The Women’s Health Initiative is funded by the U.S. Department of Health & Human Services. Study drugs were donated by Wyeth Ayerst. Dr. Manson reported having no financial disclosures. Several of her coauthors reported receiving grants and research funding from the National Institutes of Health, and receiving personal fees, speaking fees, and honoraria from various pharmaceutical companies.
Postmenopausal women treated with conjugated equine estrogens (CEE) plus medroxyprogesterone acetate (MPA) for a median of 5.6 years, or with CEE alone for a median of 7.2 years had no increased risk of all-cause, cardiovascular, or cancer mortality over a cumulative follow-up of 18 years, according to the latest report from the Women’s Health Initiative hormone therapy trials.
All-cause mortality among the 27,347 participants in the two randomized, double-blind, placebo-controlled trials was 27.1% in the hormone therapy group versus 27.6% in the placebo group (hazard ratio, 0.99), JoAnn E. Manson, MD, Harvard Medical School, Boston, and her colleagues reported.
For those who received CEE plus MPA, the hazard ratio was 1.02, while for CEE alone the hazard ratio was 0.94 (JAMA. 2017 Sep;318[10]:927-38).
Cardiovascular mortality among the pooled cohort was 8.9% with hormone therapy and 9.0% with placebo (HR, 1.00), and total cancer mortality was 8.2% and 8.0%, respectively (HR, 1.03). Mortality from other causes was 10.0% with hormone therapy, compared with 10.7% with placebo (HR, 0.95). The results did not differ significantly between the trials, the investigators noted.
An analysis by age showed that women aged 50-59 years tended to have lower hazard ratios for mortality from cardiovascular disease, cancer, and other causes during the intervention phases of the trials, but only the difference for “other” causes in the CEE-alone trial showed a statistically significant trend with age. This was influenced in part by adverse effects of the treatment in women aged 70-79 years. During cumulative follow-up, trends related to mortality across age groups were not statistically significantly different.
The trials included women aged 50-79 years who were enrolled between 1993 and 1998 and followed through 2014. Given the hormone-therapy-related risks identified in the CEE plus MPA and CEE-alone trials – which were stopped early because of increased risk of breast cancer/overall risks exceeding benefits, and for increased stroke risk, respectively – the absence of an increase in all-cause mortality during the intervention and cumulative follow-up phases of the trials is noteworthy, the investigators wrote.
“Although these findings lend support to practice guidelines endorsing use of hormone therapy for recently menopausal women with moderate to severe symptoms, in the absence of contraindications, the attenuation of age differences with longer follow-up and potential health risks of treatment would not support use of hormone therapy for reducing chronic disease or mortality,” they wrote. “Moreover, it is unclear whether benefits would outweigh risks with longer duration of treatment.”
They added that “in clinical decision making, these considerations must be weighed against the evidence linking untreated vasomotor symptoms in midlife women to impaired health and quality of life, disrupted sleep, reduced work productivity, and increased health care expenditures.”
The Women’s Health Initiative is funded by the U.S. Department of Health & Human Services. Study drugs were donated by Wyeth Ayerst. Dr. Manson reported having no financial disclosures. Several of her coauthors reported receiving grants and research funding from the National Institutes of Health, and receiving personal fees, speaking fees, and honoraria from various pharmaceutical companies.
FROM JAMA
Key clinical point:
Major finding: All-cause mortality was 27.1% in the hormone therapy group versus 27.6% in the placebo group (hazard ratio, 0.99).
Data source: The randomized, double-blind, placebo-controlled Women’s Health Initiative hormone therapy trials of 27,347 women.
Disclosures: The Women’s Health Initiative is funded by the U.S. Department of Health & Human Services. Study drugs were donated by Wyeth Ayerst. Dr. Manson reported having no disclosures. Several of her coauthors reported receiving grants and research funding from the National Institutes of Health, and receiving personal fees, speaking fees, and honoraria from various pharmaceutical companies.
Sleep issues vary by menopausal status
Perimenopausal women aged 40-59 years were less likely than were others in the same age group to average at least 7 hours’ sleep each night in 2015, according to the National Center for Health Statistics.
Among the perimenopausal women in that age group, 56% said that they slept less than 7 hours, on average, in a 24-hour period, compared with 40.5% of postmenopausal women and 32.5% of those who were premenopausal. Overall, 35.1% of women aged 40-59 did not average at least 7 hours of sleep per night, the NCHS reported in a data brief released Sept. 7.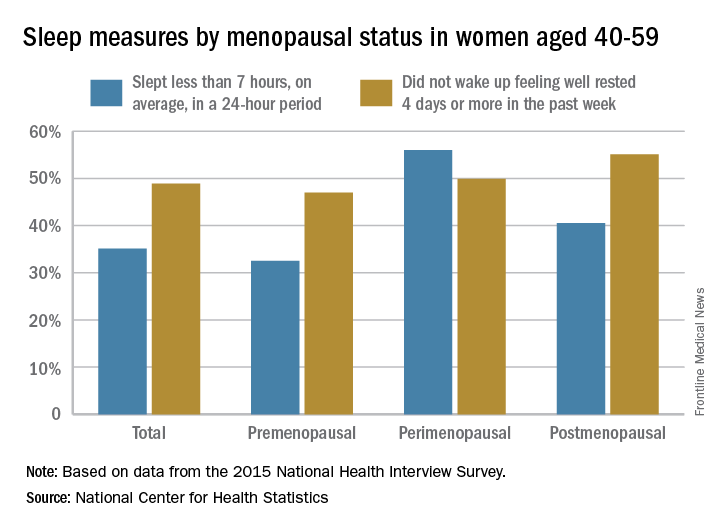
For this analysis, about 74% of the women included were premenopausal (still had a menstrual cycle), 4% were perimenopausal (last menstrual cycle was 1 year before or less), and 22% were postmenopausal (no menstrual cycle for more than 1 year or surgical menopause after removal of their ovaries).
Perimenopausal women aged 40-59 years were less likely than were others in the same age group to average at least 7 hours’ sleep each night in 2015, according to the National Center for Health Statistics.
Among the perimenopausal women in that age group, 56% said that they slept less than 7 hours, on average, in a 24-hour period, compared with 40.5% of postmenopausal women and 32.5% of those who were premenopausal. Overall, 35.1% of women aged 40-59 did not average at least 7 hours of sleep per night, the NCHS reported in a data brief released Sept. 7.
For this analysis, about 74% of the women included were premenopausal (still had a menstrual cycle), 4% were perimenopausal (last menstrual cycle was 1 year before or less), and 22% were postmenopausal (no menstrual cycle for more than 1 year or surgical menopause after removal of their ovaries).
Perimenopausal women aged 40-59 years were less likely than were others in the same age group to average at least 7 hours’ sleep each night in 2015, according to the National Center for Health Statistics.
Among the perimenopausal women in that age group, 56% said that they slept less than 7 hours, on average, in a 24-hour period, compared with 40.5% of postmenopausal women and 32.5% of those who were premenopausal. Overall, 35.1% of women aged 40-59 did not average at least 7 hours of sleep per night, the NCHS reported in a data brief released Sept. 7.
For this analysis, about 74% of the women included were premenopausal (still had a menstrual cycle), 4% were perimenopausal (last menstrual cycle was 1 year before or less), and 22% were postmenopausal (no menstrual cycle for more than 1 year or surgical menopause after removal of their ovaries).
Study finds low risk for jaw osteonecrosis with denosumab for postmenopausal osteoporosis
AT ASBMR
DENVER – Osteonecrosis of the jaw (ONJ) was a rare adverse event in women taking denosumab for postmenopausal osteoporosis, with a 0.7% rate for women who reported an invasive oral procedure or event while taking the drug and a 0.05% rate for women who did not have such procedures, Nelson Watts, MD, reported at the annual meeting of the American Society of Bone and Mineral Research.
The finding comes from a new analysis of a 7-year extension study of denosumab use in 4,550 women who participated in the 3-year, double-blind, phase 3 FREEDOM trial (NCT00089791) that compared denosumab 60 mg and placebo every 6 months. Those who missed 1 dose or fewer and completed visits through year 3 of the initial study were eligible to continue in the 7-year, open-label extension study. Those who had received placebo in the initial trial were crossed over to denosumab for the extension study.
Extension study participants were instructed to chronicle invasive oral procedures and events that had occurred in the initial trial and completed an oral event questionnaire once every 6 months of the extension trial.
All surveys were completed by 3,591 (79%) of the extension study participants, and 45.1% reported at least one invasive oral procedure or event during that time. The frequency of events was similar for the crossover and long-term denosumab groups; these events included scaling or root planing (29.1% and 28.5%), tooth extraction (25.1% and 24.6%), dental implant (5.8% and 6.0%), natural tooth loss (4.2% and 4.0%), and jaw surgery (0.9% and 0.9%). ONJ occurred at a rate of 5.2 cases per 10,000 patient-years of denosumab use, said Nelson Watts, MD, director of osteoporosis and bone health services at Mercy Health Services in Cincinnati, Ohio.
Of the 12 ONJ cases identified in the study, 11 occurred in women who reported an invasive oral procedure or event. This translated to a 0.7% risk of ONJ in women who reported an invasive oral procedure or event (11 in 1,621) and a 0.05% risk in women who did not (1 in 1,970).
The most common inciting event for ONJ appeared to be dental extractions, often of two or three teeth. The next most common dental issue associated with ONJ seemed to be poorly-fitted dentures.
ONJ resolved with treatment in 10 of 12 cases; one case was ongoing at the end of the study and one had an unknown outcome because the subject had withdrawn from the study. “With effective dental therapy, healing is the most likely outcome,” said Dr. Watts.
In clinical trials, ONJ occurred at a rate between 1 and 10 per 10,000 patient-years. A report in 2003, however, described severe ONJ in 36 cancer patients who received bisphosphonates (https://www.ncbi.nlm.nih.gov/pubmed/12966493).
The denosumab doses that cancer patients receive can be 10 to 12 times higher than the typical dose given to a postmenopausal woman being treated for osteoporosis.
“I can’t tell you how many phone calls I get from patients who are worried somehow or worried in situations created by their dentists that whatever procedure they’re going to have is going to end horribly,” Dr. Watts said. “In some cases dentists are telling my patients to either stop the drug that I’m giving them or to wait to get the next dose, and there’s absolutely nothing to support that.”
The study was funded by Amgen, the maker of denosumab (Prolia). Dr. Watts has received research support from Shire and has consulted for Abbvie, Amgen, and Radius. He is on the speakers’ bureau for Amgen, Radius, and Shire.
AT ASBMR
DENVER – Osteonecrosis of the jaw (ONJ) was a rare adverse event in women taking denosumab for postmenopausal osteoporosis, with a 0.7% rate for women who reported an invasive oral procedure or event while taking the drug and a 0.05% rate for women who did not have such procedures, Nelson Watts, MD, reported at the annual meeting of the American Society of Bone and Mineral Research.
The finding comes from a new analysis of a 7-year extension study of denosumab use in 4,550 women who participated in the 3-year, double-blind, phase 3 FREEDOM trial (NCT00089791) that compared denosumab 60 mg and placebo every 6 months. Those who missed 1 dose or fewer and completed visits through year 3 of the initial study were eligible to continue in the 7-year, open-label extension study. Those who had received placebo in the initial trial were crossed over to denosumab for the extension study.
Extension study participants were instructed to chronicle invasive oral procedures and events that had occurred in the initial trial and completed an oral event questionnaire once every 6 months of the extension trial.
All surveys were completed by 3,591 (79%) of the extension study participants, and 45.1% reported at least one invasive oral procedure or event during that time. The frequency of events was similar for the crossover and long-term denosumab groups; these events included scaling or root planing (29.1% and 28.5%), tooth extraction (25.1% and 24.6%), dental implant (5.8% and 6.0%), natural tooth loss (4.2% and 4.0%), and jaw surgery (0.9% and 0.9%). ONJ occurred at a rate of 5.2 cases per 10,000 patient-years of denosumab use, said Nelson Watts, MD, director of osteoporosis and bone health services at Mercy Health Services in Cincinnati, Ohio.
Of the 12 ONJ cases identified in the study, 11 occurred in women who reported an invasive oral procedure or event. This translated to a 0.7% risk of ONJ in women who reported an invasive oral procedure or event (11 in 1,621) and a 0.05% risk in women who did not (1 in 1,970).
The most common inciting event for ONJ appeared to be dental extractions, often of two or three teeth. The next most common dental issue associated with ONJ seemed to be poorly-fitted dentures.
ONJ resolved with treatment in 10 of 12 cases; one case was ongoing at the end of the study and one had an unknown outcome because the subject had withdrawn from the study. “With effective dental therapy, healing is the most likely outcome,” said Dr. Watts.
In clinical trials, ONJ occurred at a rate between 1 and 10 per 10,000 patient-years. A report in 2003, however, described severe ONJ in 36 cancer patients who received bisphosphonates (https://www.ncbi.nlm.nih.gov/pubmed/12966493).
The denosumab doses that cancer patients receive can be 10 to 12 times higher than the typical dose given to a postmenopausal woman being treated for osteoporosis.
“I can’t tell you how many phone calls I get from patients who are worried somehow or worried in situations created by their dentists that whatever procedure they’re going to have is going to end horribly,” Dr. Watts said. “In some cases dentists are telling my patients to either stop the drug that I’m giving them or to wait to get the next dose, and there’s absolutely nothing to support that.”
The study was funded by Amgen, the maker of denosumab (Prolia). Dr. Watts has received research support from Shire and has consulted for Abbvie, Amgen, and Radius. He is on the speakers’ bureau for Amgen, Radius, and Shire.
AT ASBMR
DENVER – Osteonecrosis of the jaw (ONJ) was a rare adverse event in women taking denosumab for postmenopausal osteoporosis, with a 0.7% rate for women who reported an invasive oral procedure or event while taking the drug and a 0.05% rate for women who did not have such procedures, Nelson Watts, MD, reported at the annual meeting of the American Society of Bone and Mineral Research.
The finding comes from a new analysis of a 7-year extension study of denosumab use in 4,550 women who participated in the 3-year, double-blind, phase 3 FREEDOM trial (NCT00089791) that compared denosumab 60 mg and placebo every 6 months. Those who missed 1 dose or fewer and completed visits through year 3 of the initial study were eligible to continue in the 7-year, open-label extension study. Those who had received placebo in the initial trial were crossed over to denosumab for the extension study.
Extension study participants were instructed to chronicle invasive oral procedures and events that had occurred in the initial trial and completed an oral event questionnaire once every 6 months of the extension trial.
All surveys were completed by 3,591 (79%) of the extension study participants, and 45.1% reported at least one invasive oral procedure or event during that time. The frequency of events was similar for the crossover and long-term denosumab groups; these events included scaling or root planing (29.1% and 28.5%), tooth extraction (25.1% and 24.6%), dental implant (5.8% and 6.0%), natural tooth loss (4.2% and 4.0%), and jaw surgery (0.9% and 0.9%). ONJ occurred at a rate of 5.2 cases per 10,000 patient-years of denosumab use, said Nelson Watts, MD, director of osteoporosis and bone health services at Mercy Health Services in Cincinnati, Ohio.
Of the 12 ONJ cases identified in the study, 11 occurred in women who reported an invasive oral procedure or event. This translated to a 0.7% risk of ONJ in women who reported an invasive oral procedure or event (11 in 1,621) and a 0.05% risk in women who did not (1 in 1,970).
The most common inciting event for ONJ appeared to be dental extractions, often of two or three teeth. The next most common dental issue associated with ONJ seemed to be poorly-fitted dentures.
ONJ resolved with treatment in 10 of 12 cases; one case was ongoing at the end of the study and one had an unknown outcome because the subject had withdrawn from the study. “With effective dental therapy, healing is the most likely outcome,” said Dr. Watts.
In clinical trials, ONJ occurred at a rate between 1 and 10 per 10,000 patient-years. A report in 2003, however, described severe ONJ in 36 cancer patients who received bisphosphonates (https://www.ncbi.nlm.nih.gov/pubmed/12966493).
The denosumab doses that cancer patients receive can be 10 to 12 times higher than the typical dose given to a postmenopausal woman being treated for osteoporosis.
“I can’t tell you how many phone calls I get from patients who are worried somehow or worried in situations created by their dentists that whatever procedure they’re going to have is going to end horribly,” Dr. Watts said. “In some cases dentists are telling my patients to either stop the drug that I’m giving them or to wait to get the next dose, and there’s absolutely nothing to support that.”
The study was funded by Amgen, the maker of denosumab (Prolia). Dr. Watts has received research support from Shire and has consulted for Abbvie, Amgen, and Radius. He is on the speakers’ bureau for Amgen, Radius, and Shire.
2017 Update on female sexual dysfunction
Sexual function is a complex, multifaceted process mediated by neurologic functions, hormonal regulation, and ps
As it turns out, quite a lot. Female sexual dysfunction is a common, vastly undertreated sexual health problem that can have wide-reaching effects on a woman’s life. These effects may include impaired body image, self-confidence, and self-worth. Sexual dysfunction also can contribute to relationship dissatisfaction and leave one feeling less connected with her partner.1,2 Studies have shown women with sexual dysfunction have higher health care expenditures3 and that depression and fatigue are common comorbidities, as is frequently seen in other chronic conditions such as diabetes and back pain.4
Understanding the pathogenesis of female sexual dysfunction helps to guide our approach to its management. Indeed, increased understanding of its pathology has helped to usher in new and emerging treatment options, as well as a personalized, biopsychosocial approach to its management.
Related article:
2016 Update on female sexual dysfunction
In this Update, I discuss the interplay of physiologic and psychological factors that affect female sexual function as well as the latest options for its management. I have also assembled a panel of experts to discuss 2 cases representative of sexual dysfunction that you may encounter in your clinical practice and how prescribing decisions are made for their management.
Read about factors that impact sexual function and agents to help manage dysfunction.
Multiple transmitters in the brain can increase or decrease sexual desire and function
Neurotransmitters involved in sexual excitation include brain dopamine, melanocortin, oxytocin, vasopressin, and norepinephrine, whereas brain opioids, serotonin, prolactin, and endocannabinoids function as sexual inhibitors. Inhibitory transmitters are activated normally during sexual refractoriness but also from primary aversion or secondary avoidance disorders.1 Drugs or conditions that reduce brain dopamine levels, increase the action of brain serotonin, or enhance brain opioid pathways have been shown to inhibit sexual desire, while those that increase hypothalamic and mesolimbic dopamine or decrease serotonin release have been shown to stimulate sexual desire.1
Estradiol and progesterone can impact sexual function and desire
In addition to the neurotransmitters, hormones are important modulators of female sexual function. Decreasing levels of circulating estrogen after menopause lead to physiologic, biologic, and clinical changes in the urogenital tissues, such as decreased elastin, thinning of the epithelium, reduced vaginal blood flow, diminished lubrication, and decreased flexibility and elasticity. These changes result in the symptoms of genitourinary syndrome of menopause (GSM), which affects as many as half of all menopausal women.5,6 In clinical trials, dyspareunia and vaginal dryness are the most bothersome GSM symptoms reported.7
The role of hormonal regulation in sexual dysfunction among premenopausal women is not yet fully understood, but we do know that estradiol has been shown to improve sexual desire, progesterone tends to dampen sexual desire, and that testosterone at physiological levels has been shown in most studies to have a neutral effect on sexual desire in a well-estrogenized patient.8
Related article:
Focus on treating genital atrophy symptoms
Experience and behavior modulate or reinforce sexual dysfunction
The most common psychological factors that trigger or amplify female sexual dysfunction are depression, anxiety, distraction, negative body image, sexual abuse, and emotional neglect.9 Contextual or sociocultural factors, such as relationship discord, life-stage stressors (the empty nest syndrome or anxiety and sleep deprivation from a new baby), as well as cultural or religious values that suppress sexuality, also should be considered.9 Experience-based neuroplasticity (changes in brain pathways that become solidified by negative or positive experiences) may elucidate how a multimodal approach, utilizing medical and psychological treatment, can be beneficial for patients, particularly those with hypoactive sexual desire disorder (HSDD).1
New and emerging approaches to managing female sexual dysfunction
Three agents, one of which has been available for prescription for some time, one that is newly available, and one in the pipeline, are or may soon be in the gynecologist's armamentarium.
Flibanserin
Medications that target excitatory pathways or blunt inhibitory pathways are in development, and one, flibanserin (Addyi), has been US Food and Drug Administration (FDA)-approved for the treatment of acquired, generalized HSDD in premenopausal women.1,10 Flibanserin is a nonhormonal, centrally acting, postsynaptic serotonin 1A receptor agonist and a serotonin 2A receptor antagonist that is taken daily at bedtime (100 mg); several weeks are usually needed before any effects are noted.1 It is not approved for postmenopausal women and has a boxed warning about the risks of hypotension and syncope; its use is contraindicated in women who drink alcohol, in those who have hepatic impairment, and with the use of moderate or strong CYP3A4 inhibitors.11
Also keep in mind that flibanserin is only available through a Risk Evaluation and Mitigation Strategy program, so clinicians who wish to prescribe it must enroll in and complete training to become certified providers.9
Related article:
What you need to know (and do) to prescribe the new drug flibanserin
Prasterone
Prasterone (Intrarosa), a once-daily intravaginal dehydroepiandrosterone (DHEA) product, is a prohormone that increases local estrogen and testosterone and has the advantage of improved sexual function, desire, arousal, lubrication, orgasm, satisfaction, as well as pain at sexual activity.12 It was approved by the FDA in November 2016 to treat moderate to severe dyspareunia and has been available for prescribing since July 2017. Its cost is comparable to topical estrogen products, with a $25 copay program.
Because prasterone is not an estrogen, it does not have the boxed warning that all estrogen products are mandated by the FDA to have. This may make it more acceptable to patients, who often decline to use an estrogen product after seeing the boxed warning on the package. The Centers for Medicare and Medicaid Services (CMS) does not have prasterone on its list of potentially hazardous drugs for the elderly. However, keep in mind that because its label is for dyspareunia and not specifically for GSM, CMS considers it a drug of choice--in other words, like sildenafil (Viagra), a lifestyle choice and not for treatment of a medical condition. As such, at the present time, Medicare does not cover it.
Bremelanotide
Late-stage trials of bremelanotide, a melanocortin receptor agonist, are underway. Its mechanism of action is somewhat like that of flibanserin in that both drugs increase dopamine and norepinephrine levels. The advantage of bremelanotide is that it is used as needed. It is dosed subcutaneously (1.75 mg) and it can be used as often as a woman would like to use it. The FDA is expected to consider it for approval in about a year. Unpublished data from poster sessions at recent meetings show that, in a phase 3 study of 1,247 premenopausal women with HSDD (who had already been screened for depression and were found to have a physiologic condition), improvements in desire, arousal, lubrication, and orgasm were shown with bremelanotide. About 18% of women stopped using the drug because of adverse effects (nausea, vomiting, flushing, or headache) versus 2% for placebo. Like flibanserin, it is expected to be approved for premenopausal women only.
Read how 3 experts would manage differing GSM symptoms.
What would you prescribe for these patients?
CASE Genitourinary syndrome of menopause (GSM) in a 55-year-old woman
A 55-year-old widow is beginning a new relationship. She has not had partnered sexual activity for several years, but she recently has begun a relationship. She describes pain with attempted penetration with her new partner. Her last menstrual period was 3 years ago and she has experienced very minor menopausal symptoms, which are not bothersome. On examination, the vulva and vagina are pale, with thin epithelium and absent rugae. The tissue lacks elasticity. A virginal speculum is needed to visualize the cervix.
How would you go about deciding which of the many options for management of GSM you will recommend for this patient? What do you weigh as you consider DHEA versus estrogen and topical versus oral therapy?
JoAnn V. Pinkerton, MD: Vulvovaginal atrophy (VVA), part of GSM, is associated with postmenopausal estrogen deficiency and includes the signs and symptoms seen on this patient's physical exam: vaginal narrowing, pallor, loss of elasticity, as well as pain with intercourse.6 Estrogen therapy is the most effective treatment for vaginal atrophy.13 Since she does not have significant menopausal symptoms, low-dose vaginal estrogen preparations are effective and generally safe treatments for VVA; these include creams, tablets containing estradiol or conjugated equine estrogen (CEE), and a low-dose vaginal estradiol ring--all available at doses that result in minimal systemic absorption.
Choice is usually made based on patient desire and likely adherence. If the patient prefers nonestrogen therapies that improve VVA and have been approved for relief of dyspareunia in postmenopausal women, I would discuss with the patient the oral selective estrogen receptor modulator ospemifene,14 and the new intravaginal DHEA suppositories, prasterone.15 Ospemifene is taken daily as an oral tablet, has a small risk of blood clots, and is my choice for women who do not need systemic hormone therapy and prefer to avoid vaginal therapy.
Andrew M. Kaunitz, MD: GSM is prevalent in menopausal women and, if not treated, causes progressive vaginal dryness and sexual discomfort. When the main indication for hormonal management in a menopausal woman is GSM (as opposed to treatment of vasomotor symptoms or prevention of osteoporosis), the treatment of choice is low-dose local vaginal estrogen, ospemifene, or prasterone (DHEA). Prasterone is a vaginally administered nonestrogen steroid that was approved by the FDA to treat dyspareunia associated with GSM. DHEA is an endogenous inactive steroid that is converted locally into androgens and estrogens; one vaginal insert is placed nightly.16,17
This 55-year-old widow has not been sexually active for some time. The facts that attempted penetration was painful and only an ultrathin (virginal) speculum could be used for examination indicate that contraction of the pelvic floor muscles is likely present. Simply starting medical management may not lead to comfortable/successful penetrative sex for this woman. In addition to medical management, she would likely benefit from referral for physical therapy. Using dilators and other strategies, along with the positive impact that medical management will have on the vaginal mucosa, a woman's physical therapist can work with this patient to help the pelvic floor muscles relax and facilitate comfortable penetrative sex.
James A. Simon, MD: With only minor vasomotor symptoms, I would assess the other potential benefits of a systemic therapy. These might include cardiovascular risk reduction (systemic estrogens or estrogens/progesterone in some), breast cancer risk reduction (some data suggesting ospemifene can accomplish this), osteoporosis prevention (systemic estrogens and estrogen/androgens), etc. If there is an option for a treatment to address more than one symptom, in this case GSM, assessing the risks/benefits of each of these therapies should be estimated for this specific patient.
If there are no systemic benefits to be had, then any of the local treatments should be helpful. As there are no head-to-head comparisons available, local estrogen cream, tablets, rings, local DHEA, or systemic ospemifene each should be considered possible treatments. I also feel this patient may benefit from supplementary self-dilation and/or physical therapy.
Related article:
2017 Update on menopause
CASE Dyspareunia and vasomotor symptoms in a 42-year-old breast cancer survivor
A 42-year-old woman with a BRCA1 mutation has undergone prophylactic mastectomies as well as hysterectomy with bilateral salpingo-oophorectomy. She reports mild to moderate hot flashes and bothersome vaginal dryness and dyspareunia. Examination confirms GSM.
Would you advise systemic hormone therapy for this patient? What would your recommendation be for management of her GSM symptoms?
Dr. Simon: While one's gut reaction would be to avoid systemic estrogen therapy in a patient with a BRCA1 mutation, the scientific information confirming this fear is lacking.18 Such patients may benefit significantly from systemic estrogen therapy (reduced risk of cardiovascular disease and cognitive decline, etc.), and with both breasts and both ovaries removed, estrogen's breast cancer risks, if any in this population, are largely avoided. The patient also may benefit from additional local therapy with either estrogens or DHEA.
Dr. Kaunitz: Due to her high lifetime risk of breast and ovarian cancer, this woman has proceeded with risk-reducing breast and gynecologic surgery. As more BRCA mutation carriers are being identified and undergo risk-reducing bilateral mastectomy (usually with reconstruction) and salpingo-oophorectomy, clinicians and mutation carriers more frequently face decisions regarding use of systemic hormone therapy.
Mutation carriers who have undergone bilateral risk-reducing mastectomy experience a very low baseline future risk for breast cancer; accordingly, concerns regarding this disease should not prevent use of systemic hormone therapy. Furthermore, without hormone replacement, induced menopause in women this age is associated with an elevated risk of osteoporosis, persistent vasomotor symptoms, cardiovascular disease, stroke, mood changes, dementia, Parkinson disease, and overall mortality. Recognizing the safety of estrogen therapy in this setting, this 42-year-old BRCA1 mutation carrier can initiate estrogen therapy. Standard dose estrogen therapy refers to oral estradiol 1.0 mg, conjugated equine estrogen 0.625 mg,or transdermal estradiol 0.05 mg. In younger women like this 42-year-old with surgically induced menopause, higher than standard replacement doses of estrogen are often appropriate.17
Due to concerns the hormone therapy might further increase future risk of breast cancer, some mutation carriers may delay or avoid risk-reducing bilateral salpingo-oophorectomy, a potentially lifesaving surgery which reduces not only future risk of ovarian cancer but also future risk for breast cancer.
Among mutation carriers with intact breasts, several studies address risk of breast cancer with use of systemic hormone therapy. Although limited in numbers of participants and years of follow-up, in aggregate, these studies provide reassurance that short-term use of systemic hormone therapy does not increase breast cancer risk in women with BRCA1 or BRCA2 mutations and intact breasts.19
Dr. Pinkerton: For this woman with early surgical menopause and hysterectomy, estrogen therapy could improve her vasomotor symptoms and decrease her risk of bone loss and GSM.17 In the Women's Health Initiative trial, there were 7 fewer breast cancers per 10,000 women-years in the estrogen-onlyarm.20 Observational studies suggest that hormone therapy, when given to the average age of menopause, decreases the risks of heart disease, Parkinson disease, and dementia.21 Limited observational evidence suggests that hormone therapy use does not further increase risk of breast cancer in women following oophorectomy for BRCA1 or BRCA2 gene mutation.22
The absolute risks observed with hormone therapy tended to be small, especially in younger, healthy women. Systemic hormone therapy could treat her hot flashes and her GSM symptoms and potentially decrease health risks associated with premature estrogen deficiency. Nonestrogen therapies for hot flashes include low-dose antidepressants, gabapentin, and mind-body options, such as cognitive behavioral therapy and hypnosis, but these would not decrease her health risks or treat her GSM.
If she only requests treatment of her GSM symptoms, she would be a candidate for low-dose vaginal estrogen therapy, given as a cream, tablet, or ring depending on her choice. I would not choose ospemifene as my first choice as she is having hot flashes, and there are no data yet on the drug's health benefits in early menopause. If she prefers nonestrogen vaginal therapy, the new intravaginal DHEA might be a good choice as both estrogen and testosterone are increased locally in the vagina while hormone levels remain in the postmenopausal range. There is no boxed warning on the patient insert, although safety in women with breast cancer or in those with elevated risk of breast cancer has not been tested.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Goldstein I, Kim NN, Clayton AH, et al. Hypoactive Sexual Desire Disorder: International Society for the Study of Women’s Sexual Health (ISSWSH) Expert Consensus Panel Review. Mayo Clin Proc. 2017;92(1):114–128.
- Kingsberg SA. Attitudinal survey of women living with low sexual desire. J Womens Health (Larchmt). 2014;23(10):817–823.
- Foley K, Foley D, Johnson BH. Healthcare resource utilization and expenditures of women diagnosed with hypoactive sexual desire disorder. J Med Econ. 2010;13(4):583–590.
- Biddle AK, West SL, D’Aloisio AA, Wheeler SB, Borisov NN, Thorp J. Hypoactive sexual desire disorder in postmenopausal women: quality of life and health burden. Value Health. 2009;12(5):763–772.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1063–1068.
- Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause. 2013;20(9):888–902.
- Ettinger B, Hait H, Reape KZ, Shu H. Measuring symptom relief in studies of vaginal and vulvar atrophy: the most bothersome symptom approach. Menopause. 2008;15(5):885–889.
- Dennerstein L, Randolph J, Taffe J, Dudley E, Burger H. Hormones, mood, sexuality, and the menopausal transition. Fertil Steril. 2002;77(suppl 4):S42–S48.
- Brotto LA, Bitzer J, Laan E, Leiblum S, Luria M. Women’s sexual desire and arousal disorders [published correction appears in J Sex Med. 2010;7(2 pt 1):856]. J Sex Med. 2010;7(1 pt 2):586–614.
- US Food and Drug Administration website. FDA approves first treatment for sexual desire disorder. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm458734.htm. Accessed August 14, 2017.
- Addyi (flibanserin) [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals North America, LLC; 2016.
- Labrie F, Derogatis L, Archer DF, et al; Members of the VVA Prasterone Research Group. Effect of intravaginal prasterone on sexual dysfunction in postmenopausal women with vulvovaginal atrophy. J Sex Med. 2015;12(12):2401–2412.
- Lethaby A, Ayeleke RO, Roberts H. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2016;8:CD001500.
- Portman DJ, Bachmann GA, Simon JA; Ospemifene Study Group. Ospemifene, a novel selective estrogen receptor modulator for treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy. Menopause. 2013;20(6):623–630.
- Labrie F, Archer DF, Koltun, W, et al; VVA Prasterone Research Group. Efficacy of intravaginal dehydroepiandrosterone (DHEA) on moderate to severe dyspareunia and vaginal dryness, symptoms of vulvovaginal atrophy, and of the genitourinary syndrome of menopause. Menopause. 2016;23(3):243–256.
- Kaunitz AM. Focus on treating genital atrophy symptoms. OBG Manag. 2017;29(1):14, 16–17.
- The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–753.
- Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the Women’s Health Initiative Observational Study. Menopause. August 14, 2017. doi:10.1097/GME.0000000000000956.
- Domchek S, Kaunitz AM. Use of systemic hormone therapy in BRCA mutation carriers. Menopause. 2016;23(9):1026–1027.
- Anderson GL, Limacher M, Assaf AR, et al; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701–1712.
- Faubion SS, Kuhle CL, Shuster LT, Rocca WA. Long-term health consequences of premature or early menopause and considerations for management. Climacteric. 2015;18(4):483–491.
- Gabriel CA, Tigges-Cardwell J, Stopfer J, Erlichman J, Nathanson K, Domchek SM. Use of total abdominal hysterectomy and hormone replacement therapy in BRCA1 and BRCA2 mutation carriers undergoing risk-reducing salpingo-oophorectomy. Fam Cancer. 2009;8(1):23-28.
Sexual function is a complex, multifaceted process mediated by neurologic functions, hormonal regulation, and ps
As it turns out, quite a lot. Female sexual dysfunction is a common, vastly undertreated sexual health problem that can have wide-reaching effects on a woman’s life. These effects may include impaired body image, self-confidence, and self-worth. Sexual dysfunction also can contribute to relationship dissatisfaction and leave one feeling less connected with her partner.1,2 Studies have shown women with sexual dysfunction have higher health care expenditures3 and that depression and fatigue are common comorbidities, as is frequently seen in other chronic conditions such as diabetes and back pain.4
Understanding the pathogenesis of female sexual dysfunction helps to guide our approach to its management. Indeed, increased understanding of its pathology has helped to usher in new and emerging treatment options, as well as a personalized, biopsychosocial approach to its management.
Related article:
2016 Update on female sexual dysfunction
In this Update, I discuss the interplay of physiologic and psychological factors that affect female sexual function as well as the latest options for its management. I have also assembled a panel of experts to discuss 2 cases representative of sexual dysfunction that you may encounter in your clinical practice and how prescribing decisions are made for their management.
Read about factors that impact sexual function and agents to help manage dysfunction.
Multiple transmitters in the brain can increase or decrease sexual desire and function
Neurotransmitters involved in sexual excitation include brain dopamine, melanocortin, oxytocin, vasopressin, and norepinephrine, whereas brain opioids, serotonin, prolactin, and endocannabinoids function as sexual inhibitors. Inhibitory transmitters are activated normally during sexual refractoriness but also from primary aversion or secondary avoidance disorders.1 Drugs or conditions that reduce brain dopamine levels, increase the action of brain serotonin, or enhance brain opioid pathways have been shown to inhibit sexual desire, while those that increase hypothalamic and mesolimbic dopamine or decrease serotonin release have been shown to stimulate sexual desire.1
Estradiol and progesterone can impact sexual function and desire
In addition to the neurotransmitters, hormones are important modulators of female sexual function. Decreasing levels of circulating estrogen after menopause lead to physiologic, biologic, and clinical changes in the urogenital tissues, such as decreased elastin, thinning of the epithelium, reduced vaginal blood flow, diminished lubrication, and decreased flexibility and elasticity. These changes result in the symptoms of genitourinary syndrome of menopause (GSM), which affects as many as half of all menopausal women.5,6 In clinical trials, dyspareunia and vaginal dryness are the most bothersome GSM symptoms reported.7
The role of hormonal regulation in sexual dysfunction among premenopausal women is not yet fully understood, but we do know that estradiol has been shown to improve sexual desire, progesterone tends to dampen sexual desire, and that testosterone at physiological levels has been shown in most studies to have a neutral effect on sexual desire in a well-estrogenized patient.8
Related article:
Focus on treating genital atrophy symptoms
Experience and behavior modulate or reinforce sexual dysfunction
The most common psychological factors that trigger or amplify female sexual dysfunction are depression, anxiety, distraction, negative body image, sexual abuse, and emotional neglect.9 Contextual or sociocultural factors, such as relationship discord, life-stage stressors (the empty nest syndrome or anxiety and sleep deprivation from a new baby), as well as cultural or religious values that suppress sexuality, also should be considered.9 Experience-based neuroplasticity (changes in brain pathways that become solidified by negative or positive experiences) may elucidate how a multimodal approach, utilizing medical and psychological treatment, can be beneficial for patients, particularly those with hypoactive sexual desire disorder (HSDD).1
New and emerging approaches to managing female sexual dysfunction
Three agents, one of which has been available for prescription for some time, one that is newly available, and one in the pipeline, are or may soon be in the gynecologist's armamentarium.
Flibanserin
Medications that target excitatory pathways or blunt inhibitory pathways are in development, and one, flibanserin (Addyi), has been US Food and Drug Administration (FDA)-approved for the treatment of acquired, generalized HSDD in premenopausal women.1,10 Flibanserin is a nonhormonal, centrally acting, postsynaptic serotonin 1A receptor agonist and a serotonin 2A receptor antagonist that is taken daily at bedtime (100 mg); several weeks are usually needed before any effects are noted.1 It is not approved for postmenopausal women and has a boxed warning about the risks of hypotension and syncope; its use is contraindicated in women who drink alcohol, in those who have hepatic impairment, and with the use of moderate or strong CYP3A4 inhibitors.11
Also keep in mind that flibanserin is only available through a Risk Evaluation and Mitigation Strategy program, so clinicians who wish to prescribe it must enroll in and complete training to become certified providers.9
Related article:
What you need to know (and do) to prescribe the new drug flibanserin
Prasterone
Prasterone (Intrarosa), a once-daily intravaginal dehydroepiandrosterone (DHEA) product, is a prohormone that increases local estrogen and testosterone and has the advantage of improved sexual function, desire, arousal, lubrication, orgasm, satisfaction, as well as pain at sexual activity.12 It was approved by the FDA in November 2016 to treat moderate to severe dyspareunia and has been available for prescribing since July 2017. Its cost is comparable to topical estrogen products, with a $25 copay program.
Because prasterone is not an estrogen, it does not have the boxed warning that all estrogen products are mandated by the FDA to have. This may make it more acceptable to patients, who often decline to use an estrogen product after seeing the boxed warning on the package. The Centers for Medicare and Medicaid Services (CMS) does not have prasterone on its list of potentially hazardous drugs for the elderly. However, keep in mind that because its label is for dyspareunia and not specifically for GSM, CMS considers it a drug of choice--in other words, like sildenafil (Viagra), a lifestyle choice and not for treatment of a medical condition. As such, at the present time, Medicare does not cover it.
Bremelanotide
Late-stage trials of bremelanotide, a melanocortin receptor agonist, are underway. Its mechanism of action is somewhat like that of flibanserin in that both drugs increase dopamine and norepinephrine levels. The advantage of bremelanotide is that it is used as needed. It is dosed subcutaneously (1.75 mg) and it can be used as often as a woman would like to use it. The FDA is expected to consider it for approval in about a year. Unpublished data from poster sessions at recent meetings show that, in a phase 3 study of 1,247 premenopausal women with HSDD (who had already been screened for depression and were found to have a physiologic condition), improvements in desire, arousal, lubrication, and orgasm were shown with bremelanotide. About 18% of women stopped using the drug because of adverse effects (nausea, vomiting, flushing, or headache) versus 2% for placebo. Like flibanserin, it is expected to be approved for premenopausal women only.
Read how 3 experts would manage differing GSM symptoms.
What would you prescribe for these patients?
CASE Genitourinary syndrome of menopause (GSM) in a 55-year-old woman
A 55-year-old widow is beginning a new relationship. She has not had partnered sexual activity for several years, but she recently has begun a relationship. She describes pain with attempted penetration with her new partner. Her last menstrual period was 3 years ago and she has experienced very minor menopausal symptoms, which are not bothersome. On examination, the vulva and vagina are pale, with thin epithelium and absent rugae. The tissue lacks elasticity. A virginal speculum is needed to visualize the cervix.
How would you go about deciding which of the many options for management of GSM you will recommend for this patient? What do you weigh as you consider DHEA versus estrogen and topical versus oral therapy?
JoAnn V. Pinkerton, MD: Vulvovaginal atrophy (VVA), part of GSM, is associated with postmenopausal estrogen deficiency and includes the signs and symptoms seen on this patient's physical exam: vaginal narrowing, pallor, loss of elasticity, as well as pain with intercourse.6 Estrogen therapy is the most effective treatment for vaginal atrophy.13 Since she does not have significant menopausal symptoms, low-dose vaginal estrogen preparations are effective and generally safe treatments for VVA; these include creams, tablets containing estradiol or conjugated equine estrogen (CEE), and a low-dose vaginal estradiol ring--all available at doses that result in minimal systemic absorption.
Choice is usually made based on patient desire and likely adherence. If the patient prefers nonestrogen therapies that improve VVA and have been approved for relief of dyspareunia in postmenopausal women, I would discuss with the patient the oral selective estrogen receptor modulator ospemifene,14 and the new intravaginal DHEA suppositories, prasterone.15 Ospemifene is taken daily as an oral tablet, has a small risk of blood clots, and is my choice for women who do not need systemic hormone therapy and prefer to avoid vaginal therapy.
Andrew M. Kaunitz, MD: GSM is prevalent in menopausal women and, if not treated, causes progressive vaginal dryness and sexual discomfort. When the main indication for hormonal management in a menopausal woman is GSM (as opposed to treatment of vasomotor symptoms or prevention of osteoporosis), the treatment of choice is low-dose local vaginal estrogen, ospemifene, or prasterone (DHEA). Prasterone is a vaginally administered nonestrogen steroid that was approved by the FDA to treat dyspareunia associated with GSM. DHEA is an endogenous inactive steroid that is converted locally into androgens and estrogens; one vaginal insert is placed nightly.16,17
This 55-year-old widow has not been sexually active for some time. The facts that attempted penetration was painful and only an ultrathin (virginal) speculum could be used for examination indicate that contraction of the pelvic floor muscles is likely present. Simply starting medical management may not lead to comfortable/successful penetrative sex for this woman. In addition to medical management, she would likely benefit from referral for physical therapy. Using dilators and other strategies, along with the positive impact that medical management will have on the vaginal mucosa, a woman's physical therapist can work with this patient to help the pelvic floor muscles relax and facilitate comfortable penetrative sex.
James A. Simon, MD: With only minor vasomotor symptoms, I would assess the other potential benefits of a systemic therapy. These might include cardiovascular risk reduction (systemic estrogens or estrogens/progesterone in some), breast cancer risk reduction (some data suggesting ospemifene can accomplish this), osteoporosis prevention (systemic estrogens and estrogen/androgens), etc. If there is an option for a treatment to address more than one symptom, in this case GSM, assessing the risks/benefits of each of these therapies should be estimated for this specific patient.
If there are no systemic benefits to be had, then any of the local treatments should be helpful. As there are no head-to-head comparisons available, local estrogen cream, tablets, rings, local DHEA, or systemic ospemifene each should be considered possible treatments. I also feel this patient may benefit from supplementary self-dilation and/or physical therapy.
Related article:
2017 Update on menopause
CASE Dyspareunia and vasomotor symptoms in a 42-year-old breast cancer survivor
A 42-year-old woman with a BRCA1 mutation has undergone prophylactic mastectomies as well as hysterectomy with bilateral salpingo-oophorectomy. She reports mild to moderate hot flashes and bothersome vaginal dryness and dyspareunia. Examination confirms GSM.
Would you advise systemic hormone therapy for this patient? What would your recommendation be for management of her GSM symptoms?
Dr. Simon: While one's gut reaction would be to avoid systemic estrogen therapy in a patient with a BRCA1 mutation, the scientific information confirming this fear is lacking.18 Such patients may benefit significantly from systemic estrogen therapy (reduced risk of cardiovascular disease and cognitive decline, etc.), and with both breasts and both ovaries removed, estrogen's breast cancer risks, if any in this population, are largely avoided. The patient also may benefit from additional local therapy with either estrogens or DHEA.
Dr. Kaunitz: Due to her high lifetime risk of breast and ovarian cancer, this woman has proceeded with risk-reducing breast and gynecologic surgery. As more BRCA mutation carriers are being identified and undergo risk-reducing bilateral mastectomy (usually with reconstruction) and salpingo-oophorectomy, clinicians and mutation carriers more frequently face decisions regarding use of systemic hormone therapy.
Mutation carriers who have undergone bilateral risk-reducing mastectomy experience a very low baseline future risk for breast cancer; accordingly, concerns regarding this disease should not prevent use of systemic hormone therapy. Furthermore, without hormone replacement, induced menopause in women this age is associated with an elevated risk of osteoporosis, persistent vasomotor symptoms, cardiovascular disease, stroke, mood changes, dementia, Parkinson disease, and overall mortality. Recognizing the safety of estrogen therapy in this setting, this 42-year-old BRCA1 mutation carrier can initiate estrogen therapy. Standard dose estrogen therapy refers to oral estradiol 1.0 mg, conjugated equine estrogen 0.625 mg,or transdermal estradiol 0.05 mg. In younger women like this 42-year-old with surgically induced menopause, higher than standard replacement doses of estrogen are often appropriate.17
Due to concerns the hormone therapy might further increase future risk of breast cancer, some mutation carriers may delay or avoid risk-reducing bilateral salpingo-oophorectomy, a potentially lifesaving surgery which reduces not only future risk of ovarian cancer but also future risk for breast cancer.
Among mutation carriers with intact breasts, several studies address risk of breast cancer with use of systemic hormone therapy. Although limited in numbers of participants and years of follow-up, in aggregate, these studies provide reassurance that short-term use of systemic hormone therapy does not increase breast cancer risk in women with BRCA1 or BRCA2 mutations and intact breasts.19
Dr. Pinkerton: For this woman with early surgical menopause and hysterectomy, estrogen therapy could improve her vasomotor symptoms and decrease her risk of bone loss and GSM.17 In the Women's Health Initiative trial, there were 7 fewer breast cancers per 10,000 women-years in the estrogen-onlyarm.20 Observational studies suggest that hormone therapy, when given to the average age of menopause, decreases the risks of heart disease, Parkinson disease, and dementia.21 Limited observational evidence suggests that hormone therapy use does not further increase risk of breast cancer in women following oophorectomy for BRCA1 or BRCA2 gene mutation.22
The absolute risks observed with hormone therapy tended to be small, especially in younger, healthy women. Systemic hormone therapy could treat her hot flashes and her GSM symptoms and potentially decrease health risks associated with premature estrogen deficiency. Nonestrogen therapies for hot flashes include low-dose antidepressants, gabapentin, and mind-body options, such as cognitive behavioral therapy and hypnosis, but these would not decrease her health risks or treat her GSM.
If she only requests treatment of her GSM symptoms, she would be a candidate for low-dose vaginal estrogen therapy, given as a cream, tablet, or ring depending on her choice. I would not choose ospemifene as my first choice as she is having hot flashes, and there are no data yet on the drug's health benefits in early menopause. If she prefers nonestrogen vaginal therapy, the new intravaginal DHEA might be a good choice as both estrogen and testosterone are increased locally in the vagina while hormone levels remain in the postmenopausal range. There is no boxed warning on the patient insert, although safety in women with breast cancer or in those with elevated risk of breast cancer has not been tested.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Sexual function is a complex, multifaceted process mediated by neurologic functions, hormonal regulation, and ps
As it turns out, quite a lot. Female sexual dysfunction is a common, vastly undertreated sexual health problem that can have wide-reaching effects on a woman’s life. These effects may include impaired body image, self-confidence, and self-worth. Sexual dysfunction also can contribute to relationship dissatisfaction and leave one feeling less connected with her partner.1,2 Studies have shown women with sexual dysfunction have higher health care expenditures3 and that depression and fatigue are common comorbidities, as is frequently seen in other chronic conditions such as diabetes and back pain.4
Understanding the pathogenesis of female sexual dysfunction helps to guide our approach to its management. Indeed, increased understanding of its pathology has helped to usher in new and emerging treatment options, as well as a personalized, biopsychosocial approach to its management.
Related article:
2016 Update on female sexual dysfunction
In this Update, I discuss the interplay of physiologic and psychological factors that affect female sexual function as well as the latest options for its management. I have also assembled a panel of experts to discuss 2 cases representative of sexual dysfunction that you may encounter in your clinical practice and how prescribing decisions are made for their management.
Read about factors that impact sexual function and agents to help manage dysfunction.
Multiple transmitters in the brain can increase or decrease sexual desire and function
Neurotransmitters involved in sexual excitation include brain dopamine, melanocortin, oxytocin, vasopressin, and norepinephrine, whereas brain opioids, serotonin, prolactin, and endocannabinoids function as sexual inhibitors. Inhibitory transmitters are activated normally during sexual refractoriness but also from primary aversion or secondary avoidance disorders.1 Drugs or conditions that reduce brain dopamine levels, increase the action of brain serotonin, or enhance brain opioid pathways have been shown to inhibit sexual desire, while those that increase hypothalamic and mesolimbic dopamine or decrease serotonin release have been shown to stimulate sexual desire.1
Estradiol and progesterone can impact sexual function and desire
In addition to the neurotransmitters, hormones are important modulators of female sexual function. Decreasing levels of circulating estrogen after menopause lead to physiologic, biologic, and clinical changes in the urogenital tissues, such as decreased elastin, thinning of the epithelium, reduced vaginal blood flow, diminished lubrication, and decreased flexibility and elasticity. These changes result in the symptoms of genitourinary syndrome of menopause (GSM), which affects as many as half of all menopausal women.5,6 In clinical trials, dyspareunia and vaginal dryness are the most bothersome GSM symptoms reported.7
The role of hormonal regulation in sexual dysfunction among premenopausal women is not yet fully understood, but we do know that estradiol has been shown to improve sexual desire, progesterone tends to dampen sexual desire, and that testosterone at physiological levels has been shown in most studies to have a neutral effect on sexual desire in a well-estrogenized patient.8
Related article:
Focus on treating genital atrophy symptoms
Experience and behavior modulate or reinforce sexual dysfunction
The most common psychological factors that trigger or amplify female sexual dysfunction are depression, anxiety, distraction, negative body image, sexual abuse, and emotional neglect.9 Contextual or sociocultural factors, such as relationship discord, life-stage stressors (the empty nest syndrome or anxiety and sleep deprivation from a new baby), as well as cultural or religious values that suppress sexuality, also should be considered.9 Experience-based neuroplasticity (changes in brain pathways that become solidified by negative or positive experiences) may elucidate how a multimodal approach, utilizing medical and psychological treatment, can be beneficial for patients, particularly those with hypoactive sexual desire disorder (HSDD).1
New and emerging approaches to managing female sexual dysfunction
Three agents, one of which has been available for prescription for some time, one that is newly available, and one in the pipeline, are or may soon be in the gynecologist's armamentarium.
Flibanserin
Medications that target excitatory pathways or blunt inhibitory pathways are in development, and one, flibanserin (Addyi), has been US Food and Drug Administration (FDA)-approved for the treatment of acquired, generalized HSDD in premenopausal women.1,10 Flibanserin is a nonhormonal, centrally acting, postsynaptic serotonin 1A receptor agonist and a serotonin 2A receptor antagonist that is taken daily at bedtime (100 mg); several weeks are usually needed before any effects are noted.1 It is not approved for postmenopausal women and has a boxed warning about the risks of hypotension and syncope; its use is contraindicated in women who drink alcohol, in those who have hepatic impairment, and with the use of moderate or strong CYP3A4 inhibitors.11
Also keep in mind that flibanserin is only available through a Risk Evaluation and Mitigation Strategy program, so clinicians who wish to prescribe it must enroll in and complete training to become certified providers.9
Related article:
What you need to know (and do) to prescribe the new drug flibanserin
Prasterone
Prasterone (Intrarosa), a once-daily intravaginal dehydroepiandrosterone (DHEA) product, is a prohormone that increases local estrogen and testosterone and has the advantage of improved sexual function, desire, arousal, lubrication, orgasm, satisfaction, as well as pain at sexual activity.12 It was approved by the FDA in November 2016 to treat moderate to severe dyspareunia and has been available for prescribing since July 2017. Its cost is comparable to topical estrogen products, with a $25 copay program.
Because prasterone is not an estrogen, it does not have the boxed warning that all estrogen products are mandated by the FDA to have. This may make it more acceptable to patients, who often decline to use an estrogen product after seeing the boxed warning on the package. The Centers for Medicare and Medicaid Services (CMS) does not have prasterone on its list of potentially hazardous drugs for the elderly. However, keep in mind that because its label is for dyspareunia and not specifically for GSM, CMS considers it a drug of choice--in other words, like sildenafil (Viagra), a lifestyle choice and not for treatment of a medical condition. As such, at the present time, Medicare does not cover it.
Bremelanotide
Late-stage trials of bremelanotide, a melanocortin receptor agonist, are underway. Its mechanism of action is somewhat like that of flibanserin in that both drugs increase dopamine and norepinephrine levels. The advantage of bremelanotide is that it is used as needed. It is dosed subcutaneously (1.75 mg) and it can be used as often as a woman would like to use it. The FDA is expected to consider it for approval in about a year. Unpublished data from poster sessions at recent meetings show that, in a phase 3 study of 1,247 premenopausal women with HSDD (who had already been screened for depression and were found to have a physiologic condition), improvements in desire, arousal, lubrication, and orgasm were shown with bremelanotide. About 18% of women stopped using the drug because of adverse effects (nausea, vomiting, flushing, or headache) versus 2% for placebo. Like flibanserin, it is expected to be approved for premenopausal women only.
Read how 3 experts would manage differing GSM symptoms.
What would you prescribe for these patients?
CASE Genitourinary syndrome of menopause (GSM) in a 55-year-old woman
A 55-year-old widow is beginning a new relationship. She has not had partnered sexual activity for several years, but she recently has begun a relationship. She describes pain with attempted penetration with her new partner. Her last menstrual period was 3 years ago and she has experienced very minor menopausal symptoms, which are not bothersome. On examination, the vulva and vagina are pale, with thin epithelium and absent rugae. The tissue lacks elasticity. A virginal speculum is needed to visualize the cervix.
How would you go about deciding which of the many options for management of GSM you will recommend for this patient? What do you weigh as you consider DHEA versus estrogen and topical versus oral therapy?
JoAnn V. Pinkerton, MD: Vulvovaginal atrophy (VVA), part of GSM, is associated with postmenopausal estrogen deficiency and includes the signs and symptoms seen on this patient's physical exam: vaginal narrowing, pallor, loss of elasticity, as well as pain with intercourse.6 Estrogen therapy is the most effective treatment for vaginal atrophy.13 Since she does not have significant menopausal symptoms, low-dose vaginal estrogen preparations are effective and generally safe treatments for VVA; these include creams, tablets containing estradiol or conjugated equine estrogen (CEE), and a low-dose vaginal estradiol ring--all available at doses that result in minimal systemic absorption.
Choice is usually made based on patient desire and likely adherence. If the patient prefers nonestrogen therapies that improve VVA and have been approved for relief of dyspareunia in postmenopausal women, I would discuss with the patient the oral selective estrogen receptor modulator ospemifene,14 and the new intravaginal DHEA suppositories, prasterone.15 Ospemifene is taken daily as an oral tablet, has a small risk of blood clots, and is my choice for women who do not need systemic hormone therapy and prefer to avoid vaginal therapy.
Andrew M. Kaunitz, MD: GSM is prevalent in menopausal women and, if not treated, causes progressive vaginal dryness and sexual discomfort. When the main indication for hormonal management in a menopausal woman is GSM (as opposed to treatment of vasomotor symptoms or prevention of osteoporosis), the treatment of choice is low-dose local vaginal estrogen, ospemifene, or prasterone (DHEA). Prasterone is a vaginally administered nonestrogen steroid that was approved by the FDA to treat dyspareunia associated with GSM. DHEA is an endogenous inactive steroid that is converted locally into androgens and estrogens; one vaginal insert is placed nightly.16,17
This 55-year-old widow has not been sexually active for some time. The facts that attempted penetration was painful and only an ultrathin (virginal) speculum could be used for examination indicate that contraction of the pelvic floor muscles is likely present. Simply starting medical management may not lead to comfortable/successful penetrative sex for this woman. In addition to medical management, she would likely benefit from referral for physical therapy. Using dilators and other strategies, along with the positive impact that medical management will have on the vaginal mucosa, a woman's physical therapist can work with this patient to help the pelvic floor muscles relax and facilitate comfortable penetrative sex.
James A. Simon, MD: With only minor vasomotor symptoms, I would assess the other potential benefits of a systemic therapy. These might include cardiovascular risk reduction (systemic estrogens or estrogens/progesterone in some), breast cancer risk reduction (some data suggesting ospemifene can accomplish this), osteoporosis prevention (systemic estrogens and estrogen/androgens), etc. If there is an option for a treatment to address more than one symptom, in this case GSM, assessing the risks/benefits of each of these therapies should be estimated for this specific patient.
If there are no systemic benefits to be had, then any of the local treatments should be helpful. As there are no head-to-head comparisons available, local estrogen cream, tablets, rings, local DHEA, or systemic ospemifene each should be considered possible treatments. I also feel this patient may benefit from supplementary self-dilation and/or physical therapy.
Related article:
2017 Update on menopause
CASE Dyspareunia and vasomotor symptoms in a 42-year-old breast cancer survivor
A 42-year-old woman with a BRCA1 mutation has undergone prophylactic mastectomies as well as hysterectomy with bilateral salpingo-oophorectomy. She reports mild to moderate hot flashes and bothersome vaginal dryness and dyspareunia. Examination confirms GSM.
Would you advise systemic hormone therapy for this patient? What would your recommendation be for management of her GSM symptoms?
Dr. Simon: While one's gut reaction would be to avoid systemic estrogen therapy in a patient with a BRCA1 mutation, the scientific information confirming this fear is lacking.18 Such patients may benefit significantly from systemic estrogen therapy (reduced risk of cardiovascular disease and cognitive decline, etc.), and with both breasts and both ovaries removed, estrogen's breast cancer risks, if any in this population, are largely avoided. The patient also may benefit from additional local therapy with either estrogens or DHEA.
Dr. Kaunitz: Due to her high lifetime risk of breast and ovarian cancer, this woman has proceeded with risk-reducing breast and gynecologic surgery. As more BRCA mutation carriers are being identified and undergo risk-reducing bilateral mastectomy (usually with reconstruction) and salpingo-oophorectomy, clinicians and mutation carriers more frequently face decisions regarding use of systemic hormone therapy.
Mutation carriers who have undergone bilateral risk-reducing mastectomy experience a very low baseline future risk for breast cancer; accordingly, concerns regarding this disease should not prevent use of systemic hormone therapy. Furthermore, without hormone replacement, induced menopause in women this age is associated with an elevated risk of osteoporosis, persistent vasomotor symptoms, cardiovascular disease, stroke, mood changes, dementia, Parkinson disease, and overall mortality. Recognizing the safety of estrogen therapy in this setting, this 42-year-old BRCA1 mutation carrier can initiate estrogen therapy. Standard dose estrogen therapy refers to oral estradiol 1.0 mg, conjugated equine estrogen 0.625 mg,or transdermal estradiol 0.05 mg. In younger women like this 42-year-old with surgically induced menopause, higher than standard replacement doses of estrogen are often appropriate.17
Due to concerns the hormone therapy might further increase future risk of breast cancer, some mutation carriers may delay or avoid risk-reducing bilateral salpingo-oophorectomy, a potentially lifesaving surgery which reduces not only future risk of ovarian cancer but also future risk for breast cancer.
Among mutation carriers with intact breasts, several studies address risk of breast cancer with use of systemic hormone therapy. Although limited in numbers of participants and years of follow-up, in aggregate, these studies provide reassurance that short-term use of systemic hormone therapy does not increase breast cancer risk in women with BRCA1 or BRCA2 mutations and intact breasts.19
Dr. Pinkerton: For this woman with early surgical menopause and hysterectomy, estrogen therapy could improve her vasomotor symptoms and decrease her risk of bone loss and GSM.17 In the Women's Health Initiative trial, there were 7 fewer breast cancers per 10,000 women-years in the estrogen-onlyarm.20 Observational studies suggest that hormone therapy, when given to the average age of menopause, decreases the risks of heart disease, Parkinson disease, and dementia.21 Limited observational evidence suggests that hormone therapy use does not further increase risk of breast cancer in women following oophorectomy for BRCA1 or BRCA2 gene mutation.22
The absolute risks observed with hormone therapy tended to be small, especially in younger, healthy women. Systemic hormone therapy could treat her hot flashes and her GSM symptoms and potentially decrease health risks associated with premature estrogen deficiency. Nonestrogen therapies for hot flashes include low-dose antidepressants, gabapentin, and mind-body options, such as cognitive behavioral therapy and hypnosis, but these would not decrease her health risks or treat her GSM.
If she only requests treatment of her GSM symptoms, she would be a candidate for low-dose vaginal estrogen therapy, given as a cream, tablet, or ring depending on her choice. I would not choose ospemifene as my first choice as she is having hot flashes, and there are no data yet on the drug's health benefits in early menopause. If she prefers nonestrogen vaginal therapy, the new intravaginal DHEA might be a good choice as both estrogen and testosterone are increased locally in the vagina while hormone levels remain in the postmenopausal range. There is no boxed warning on the patient insert, although safety in women with breast cancer or in those with elevated risk of breast cancer has not been tested.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Goldstein I, Kim NN, Clayton AH, et al. Hypoactive Sexual Desire Disorder: International Society for the Study of Women’s Sexual Health (ISSWSH) Expert Consensus Panel Review. Mayo Clin Proc. 2017;92(1):114–128.
- Kingsberg SA. Attitudinal survey of women living with low sexual desire. J Womens Health (Larchmt). 2014;23(10):817–823.
- Foley K, Foley D, Johnson BH. Healthcare resource utilization and expenditures of women diagnosed with hypoactive sexual desire disorder. J Med Econ. 2010;13(4):583–590.
- Biddle AK, West SL, D’Aloisio AA, Wheeler SB, Borisov NN, Thorp J. Hypoactive sexual desire disorder in postmenopausal women: quality of life and health burden. Value Health. 2009;12(5):763–772.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1063–1068.
- Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause. 2013;20(9):888–902.
- Ettinger B, Hait H, Reape KZ, Shu H. Measuring symptom relief in studies of vaginal and vulvar atrophy: the most bothersome symptom approach. Menopause. 2008;15(5):885–889.
- Dennerstein L, Randolph J, Taffe J, Dudley E, Burger H. Hormones, mood, sexuality, and the menopausal transition. Fertil Steril. 2002;77(suppl 4):S42–S48.
- Brotto LA, Bitzer J, Laan E, Leiblum S, Luria M. Women’s sexual desire and arousal disorders [published correction appears in J Sex Med. 2010;7(2 pt 1):856]. J Sex Med. 2010;7(1 pt 2):586–614.
- US Food and Drug Administration website. FDA approves first treatment for sexual desire disorder. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm458734.htm. Accessed August 14, 2017.
- Addyi (flibanserin) [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals North America, LLC; 2016.
- Labrie F, Derogatis L, Archer DF, et al; Members of the VVA Prasterone Research Group. Effect of intravaginal prasterone on sexual dysfunction in postmenopausal women with vulvovaginal atrophy. J Sex Med. 2015;12(12):2401–2412.
- Lethaby A, Ayeleke RO, Roberts H. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2016;8:CD001500.
- Portman DJ, Bachmann GA, Simon JA; Ospemifene Study Group. Ospemifene, a novel selective estrogen receptor modulator for treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy. Menopause. 2013;20(6):623–630.
- Labrie F, Archer DF, Koltun, W, et al; VVA Prasterone Research Group. Efficacy of intravaginal dehydroepiandrosterone (DHEA) on moderate to severe dyspareunia and vaginal dryness, symptoms of vulvovaginal atrophy, and of the genitourinary syndrome of menopause. Menopause. 2016;23(3):243–256.
- Kaunitz AM. Focus on treating genital atrophy symptoms. OBG Manag. 2017;29(1):14, 16–17.
- The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–753.
- Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the Women’s Health Initiative Observational Study. Menopause. August 14, 2017. doi:10.1097/GME.0000000000000956.
- Domchek S, Kaunitz AM. Use of systemic hormone therapy in BRCA mutation carriers. Menopause. 2016;23(9):1026–1027.
- Anderson GL, Limacher M, Assaf AR, et al; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701–1712.
- Faubion SS, Kuhle CL, Shuster LT, Rocca WA. Long-term health consequences of premature or early menopause and considerations for management. Climacteric. 2015;18(4):483–491.
- Gabriel CA, Tigges-Cardwell J, Stopfer J, Erlichman J, Nathanson K, Domchek SM. Use of total abdominal hysterectomy and hormone replacement therapy in BRCA1 and BRCA2 mutation carriers undergoing risk-reducing salpingo-oophorectomy. Fam Cancer. 2009;8(1):23-28.
- Goldstein I, Kim NN, Clayton AH, et al. Hypoactive Sexual Desire Disorder: International Society for the Study of Women’s Sexual Health (ISSWSH) Expert Consensus Panel Review. Mayo Clin Proc. 2017;92(1):114–128.
- Kingsberg SA. Attitudinal survey of women living with low sexual desire. J Womens Health (Larchmt). 2014;23(10):817–823.
- Foley K, Foley D, Johnson BH. Healthcare resource utilization and expenditures of women diagnosed with hypoactive sexual desire disorder. J Med Econ. 2010;13(4):583–590.
- Biddle AK, West SL, D’Aloisio AA, Wheeler SB, Borisov NN, Thorp J. Hypoactive sexual desire disorder in postmenopausal women: quality of life and health burden. Value Health. 2009;12(5):763–772.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1063–1068.
- Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause. 2013;20(9):888–902.
- Ettinger B, Hait H, Reape KZ, Shu H. Measuring symptom relief in studies of vaginal and vulvar atrophy: the most bothersome symptom approach. Menopause. 2008;15(5):885–889.
- Dennerstein L, Randolph J, Taffe J, Dudley E, Burger H. Hormones, mood, sexuality, and the menopausal transition. Fertil Steril. 2002;77(suppl 4):S42–S48.
- Brotto LA, Bitzer J, Laan E, Leiblum S, Luria M. Women’s sexual desire and arousal disorders [published correction appears in J Sex Med. 2010;7(2 pt 1):856]. J Sex Med. 2010;7(1 pt 2):586–614.
- US Food and Drug Administration website. FDA approves first treatment for sexual desire disorder. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm458734.htm. Accessed August 14, 2017.
- Addyi (flibanserin) [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals North America, LLC; 2016.
- Labrie F, Derogatis L, Archer DF, et al; Members of the VVA Prasterone Research Group. Effect of intravaginal prasterone on sexual dysfunction in postmenopausal women with vulvovaginal atrophy. J Sex Med. 2015;12(12):2401–2412.
- Lethaby A, Ayeleke RO, Roberts H. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2016;8:CD001500.
- Portman DJ, Bachmann GA, Simon JA; Ospemifene Study Group. Ospemifene, a novel selective estrogen receptor modulator for treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy. Menopause. 2013;20(6):623–630.
- Labrie F, Archer DF, Koltun, W, et al; VVA Prasterone Research Group. Efficacy of intravaginal dehydroepiandrosterone (DHEA) on moderate to severe dyspareunia and vaginal dryness, symptoms of vulvovaginal atrophy, and of the genitourinary syndrome of menopause. Menopause. 2016;23(3):243–256.
- Kaunitz AM. Focus on treating genital atrophy symptoms. OBG Manag. 2017;29(1):14, 16–17.
- The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–753.
- Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the Women’s Health Initiative Observational Study. Menopause. August 14, 2017. doi:10.1097/GME.0000000000000956.
- Domchek S, Kaunitz AM. Use of systemic hormone therapy in BRCA mutation carriers. Menopause. 2016;23(9):1026–1027.
- Anderson GL, Limacher M, Assaf AR, et al; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701–1712.
- Faubion SS, Kuhle CL, Shuster LT, Rocca WA. Long-term health consequences of premature or early menopause and considerations for management. Climacteric. 2015;18(4):483–491.
- Gabriel CA, Tigges-Cardwell J, Stopfer J, Erlichman J, Nathanson K, Domchek SM. Use of total abdominal hysterectomy and hormone replacement therapy in BRCA1 and BRCA2 mutation carriers undergoing risk-reducing salpingo-oophorectomy. Fam Cancer. 2009;8(1):23-28.
Fast Tracks
- Although not fully understood how, estradiol can improve sexual desire, progesterone tends to dampen sexual desire, and testosterone has a neutral effect in premenopausal women
- Newly available since July 2017, prasterone is a once-daily intravaginal agent that treats moderate to severe dyspareunia and has costs similar to topical estrogens
- Estrogen therapy may be considered in a breast cancer mutation carrier who has undergone prophylactic mastectomies and bilateral salpingo-oophorectomy
Focus on lifestyle to manage menopause symptoms after breast cancer
Lifestyle modification, rather than hormone therapy, should form the basis for managing estrogen-depletion symptoms and associated clinical problems in breast cancer survivors, according to a review of available evidence.
The review, conducted by the writing group for the Endocrine Society’s guidelines on management of menopausal symptoms, was prompted by the paucity of both randomized controlled trials in breast cancer survivors with estrogen deficiency issues and guidelines that sufficiently focus on treatment of this subgroup of women.
“A large proportion of women experience menopausal symptoms or clinical manifestations of estrogen deficiency during treatment of their breast cancer or after completion of therapy. The specific symptoms and clinical challenges differ based on menopausal status prior to initiation of cancer treatment and therapeutic agents used,” the researchers wrote in a report published in the Journal of Clinical Endocrinology & Metabolism (2017 Aug 2. doi: 10.1210/jc.2017-01138).
For instance, among premenopausal women treated with chemotherapy, ovarian insufficiency, severe menopausal symptoms, and infertility can result. Postmenopasual women treated with aromatase inhibitors may experience arthralgia, accelerated bone loss, and osteoporotic fractures, as well as severe vulvovaginal atrophy, they explained, noting that both premenopausal and postmenopausal survivors can experience moderate-to-severe vasomotor symptoms and sleep disturbance with related fatigue, depressive symptoms, and mood changes.
“Less common problems include weight gain, symptomatic osteoarthritis and intervertebral disk degeneration, degenerative skin changes, radiation and chemotherapy-related cardiovascular disease, and reduced quality of life,” the researchers wrote.
Based on a review of randomized controlled clinical trials, observational studies, evidence-based guidelines, and expert opinion from professional societies, the writing group concluded that individualized lifestyle modifications and nonpharmacologic therapies are recommended for the treatment of these symptoms.
Specifically, the writing group recommended smoking cessation, weight loss when indicated, limited alcohol intake, maintenance of adequate vitamin D and calcium levels, a healthy diet, and regular physical activity for all women with prior breast cancer.
They also recommended nonpharmacologic therapies for vasomotor symptoms, and noted that cognitive behavioral therapy, hypnosis, and acupuncture are among the approaches that may be helpful.
Vaginal lubricants and moisturizers can also be helpful for mild vulvovaginal atrophy, they wrote. For women with more severe symptoms or signs of estrogen deficiency, pharmacologic agents are available to relieve vasomotor symptoms and vulvovaginal atrophy, and to prevent and treat fractures, they wrote, adding that “therapy must be individualized based on each woman’s needs and goals for therapy.”
Among emerging approaches to treatment of symptoms are selective estrogen receptor modulators (SERMs), tissue selective estrogen complex (TSEC) therapy, estetrol, and neurokinin B inhibitors, which show promise for expanding options for symptom relief with less breast cancer risk. However, these have not yet been tested in women with prior breast cancer, the researchers noted.
Dr. Santen reported receiving research funding from Panterhei Bioscience. Other authors received research funding from Therapeutics MD and Lawley Pharmaceuticals, and honoraria from Abbott, Besins Health Care, and Pfizer.
Lifestyle modification, rather than hormone therapy, should form the basis for managing estrogen-depletion symptoms and associated clinical problems in breast cancer survivors, according to a review of available evidence.
The review, conducted by the writing group for the Endocrine Society’s guidelines on management of menopausal symptoms, was prompted by the paucity of both randomized controlled trials in breast cancer survivors with estrogen deficiency issues and guidelines that sufficiently focus on treatment of this subgroup of women.
“A large proportion of women experience menopausal symptoms or clinical manifestations of estrogen deficiency during treatment of their breast cancer or after completion of therapy. The specific symptoms and clinical challenges differ based on menopausal status prior to initiation of cancer treatment and therapeutic agents used,” the researchers wrote in a report published in the Journal of Clinical Endocrinology & Metabolism (2017 Aug 2. doi: 10.1210/jc.2017-01138).
For instance, among premenopausal women treated with chemotherapy, ovarian insufficiency, severe menopausal symptoms, and infertility can result. Postmenopasual women treated with aromatase inhibitors may experience arthralgia, accelerated bone loss, and osteoporotic fractures, as well as severe vulvovaginal atrophy, they explained, noting that both premenopausal and postmenopausal survivors can experience moderate-to-severe vasomotor symptoms and sleep disturbance with related fatigue, depressive symptoms, and mood changes.
“Less common problems include weight gain, symptomatic osteoarthritis and intervertebral disk degeneration, degenerative skin changes, radiation and chemotherapy-related cardiovascular disease, and reduced quality of life,” the researchers wrote.
Based on a review of randomized controlled clinical trials, observational studies, evidence-based guidelines, and expert opinion from professional societies, the writing group concluded that individualized lifestyle modifications and nonpharmacologic therapies are recommended for the treatment of these symptoms.
Specifically, the writing group recommended smoking cessation, weight loss when indicated, limited alcohol intake, maintenance of adequate vitamin D and calcium levels, a healthy diet, and regular physical activity for all women with prior breast cancer.
They also recommended nonpharmacologic therapies for vasomotor symptoms, and noted that cognitive behavioral therapy, hypnosis, and acupuncture are among the approaches that may be helpful.
Vaginal lubricants and moisturizers can also be helpful for mild vulvovaginal atrophy, they wrote. For women with more severe symptoms or signs of estrogen deficiency, pharmacologic agents are available to relieve vasomotor symptoms and vulvovaginal atrophy, and to prevent and treat fractures, they wrote, adding that “therapy must be individualized based on each woman’s needs and goals for therapy.”
Among emerging approaches to treatment of symptoms are selective estrogen receptor modulators (SERMs), tissue selective estrogen complex (TSEC) therapy, estetrol, and neurokinin B inhibitors, which show promise for expanding options for symptom relief with less breast cancer risk. However, these have not yet been tested in women with prior breast cancer, the researchers noted.
Dr. Santen reported receiving research funding from Panterhei Bioscience. Other authors received research funding from Therapeutics MD and Lawley Pharmaceuticals, and honoraria from Abbott, Besins Health Care, and Pfizer.
Lifestyle modification, rather than hormone therapy, should form the basis for managing estrogen-depletion symptoms and associated clinical problems in breast cancer survivors, according to a review of available evidence.
The review, conducted by the writing group for the Endocrine Society’s guidelines on management of menopausal symptoms, was prompted by the paucity of both randomized controlled trials in breast cancer survivors with estrogen deficiency issues and guidelines that sufficiently focus on treatment of this subgroup of women.
“A large proportion of women experience menopausal symptoms or clinical manifestations of estrogen deficiency during treatment of their breast cancer or after completion of therapy. The specific symptoms and clinical challenges differ based on menopausal status prior to initiation of cancer treatment and therapeutic agents used,” the researchers wrote in a report published in the Journal of Clinical Endocrinology & Metabolism (2017 Aug 2. doi: 10.1210/jc.2017-01138).
For instance, among premenopausal women treated with chemotherapy, ovarian insufficiency, severe menopausal symptoms, and infertility can result. Postmenopasual women treated with aromatase inhibitors may experience arthralgia, accelerated bone loss, and osteoporotic fractures, as well as severe vulvovaginal atrophy, they explained, noting that both premenopausal and postmenopausal survivors can experience moderate-to-severe vasomotor symptoms and sleep disturbance with related fatigue, depressive symptoms, and mood changes.
“Less common problems include weight gain, symptomatic osteoarthritis and intervertebral disk degeneration, degenerative skin changes, radiation and chemotherapy-related cardiovascular disease, and reduced quality of life,” the researchers wrote.
Based on a review of randomized controlled clinical trials, observational studies, evidence-based guidelines, and expert opinion from professional societies, the writing group concluded that individualized lifestyle modifications and nonpharmacologic therapies are recommended for the treatment of these symptoms.
Specifically, the writing group recommended smoking cessation, weight loss when indicated, limited alcohol intake, maintenance of adequate vitamin D and calcium levels, a healthy diet, and regular physical activity for all women with prior breast cancer.
They also recommended nonpharmacologic therapies for vasomotor symptoms, and noted that cognitive behavioral therapy, hypnosis, and acupuncture are among the approaches that may be helpful.
Vaginal lubricants and moisturizers can also be helpful for mild vulvovaginal atrophy, they wrote. For women with more severe symptoms or signs of estrogen deficiency, pharmacologic agents are available to relieve vasomotor symptoms and vulvovaginal atrophy, and to prevent and treat fractures, they wrote, adding that “therapy must be individualized based on each woman’s needs and goals for therapy.”
Among emerging approaches to treatment of symptoms are selective estrogen receptor modulators (SERMs), tissue selective estrogen complex (TSEC) therapy, estetrol, and neurokinin B inhibitors, which show promise for expanding options for symptom relief with less breast cancer risk. However, these have not yet been tested in women with prior breast cancer, the researchers noted.
Dr. Santen reported receiving research funding from Panterhei Bioscience. Other authors received research funding from Therapeutics MD and Lawley Pharmaceuticals, and honoraria from Abbott, Besins Health Care, and Pfizer.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
Anabolic agents for osteoporosis have limited role so far
SAN FRANCISCO – Parathyroid hormone and parathyroid hormone–related protein analogs show promise for treating osteoporosis, but, for now, they’re reserved for the most severe cases.
Teriparatide, a parathyroid hormone analog, is the first treatment that stimulates bone formation instead of inhibiting bone resorption, but studies to date are too small to evaluate its effect on hip fractures, Jeffrey A. Tice, MD, said at the UCSF Annual Advances in Internal Medicine meeting.
Abaloparatide, a parathyroid hormone-related protein analog approved in April 2017, requires subcutaneous dosing of 80 mcg daily for up to 2 years and has been found to increase bone density in the spine by 11% and in the hip by 4%, compared with placebo, over 18 months. In women with pre-existing vertebral fractures, the agent was found to reduce the incidence of vertebral fractures by 86% and nonvertebral fragility fractures by 43%, but data are not adequate to evaluate its effect on hip fractures. Adverse reactions include hypercalciuria, nausea, hypercalcemia, orthostatic hypotension, tachycardia, and injection site reactions.
Studies to date have shown that combining either teriparatide or abaloparatide with an antiresorptive drug is less effective than either agent alone. However, both agents must be followed by an antiresorptive agent. “These drugs are expensive,” Dr. Tice said. “Right now, they’re reserved for patients with severe osteoporosis.”
The lifetime risk for osteoporotic fractures is 50% in women and 20% in men, and they cause significant morbidity and mortality. Among U.S. hospitalizations for women 55 years and older between 2000 and 2012, Dr. Tice noted, 4.9 million were for osteoporotic fractures, 3 million were for stroke, and 2.9 million were for myocardial infarction.
“Osteoporosis is a very common condition, but it’s under-recognized, in part because it’s a silent disease,” he said. “Only 20%-30% of patients with bone mineral densities less than –2.5 are treated in this country. And, 12 months after hip fracture, only 2% had a dual-energy x-ray absorptiometry [DXA] test, and only 15% were treated with an appropriate drug. We should be asking about fracture history, note vertebral fractures, treat those patients, and we should remember to use chart reminders for DXA.”
Clinical guidelines from the American College of Physicians and other groups recommend alendronate, risedronate, zoledronic acid, or denosumab as first-line therapy for women with osteoporosis. “There are no head to head trials to indicate which agent is best,” Dr. Tice said. “They reduce the fracture risk by 30%-50%, primarily in patients with an existing vertebral fracture or a T score below –2.5.” The use of hormone therapy and raloxifene are generally not recommended.
In a review published in the New England Journal of Medicine, researchers Dennis M. Black, MD, and Clifford J. Rosen, MD, estimated the number of patients needed to treat for 3 years to prevent various fractures (N Engl J Med. 2016 Jan 21;374[3]:254-62). They found that, for nonvertebral fractures, including hip, 35 patients need to be treated for 3 years to prevent one of those fractures, 90 patients for 3 years to prevent one hip fracture, and 14 patients for 3 years to prevent one vertebral fracture.
In the FIT trial, 20% of women taking alendronate lost BMD during the first year (JAMA. 1998 Dec 23-30;280[24]:2077-82). However, those same patients had a 50% fracture reduction, and 92% regained the lost bone mineral density (BMD) by the next measurement. “Using DXA to measure BMD after 1 year of therapy does not accurately predict what will happen over time or reflect fracture reduction,” Dr. Tice said. “Effective treatment for osteoporosis should not be changed because of loss of BMD during the first year of use.”
A rare but potential harm from treatment with bisphosphonates includes osteonecrosis of the jaw, which is believed to affect only 1 in 10,000 patients who are treated for 10 years. Most of these cases (94%) were caused by IV bisphosphonates. Only 4% of cases were being treated for osteoporosis, and 60% were caused by tooth extraction. Because of this, a dental exam is recommended before starting patients on bisphosphonate therapy, but this small risk of osteonecrosis of the jaw “is not a reason to stop bisphosphonate therapy,” Dr. Tice said.
Other concerns include an increased risk for atrial fibrillation that has been observed in randomized, controlled trials of zoledronate and alendronate. “There has been no association in other trials,” Dr. Tice said. “This is likely a chance finding, likely spurious.”
Reports of an increased risk of esophageal cancer from bisphosphonate use have also been suggested, but these come from case series and from two conflicting cohorts of data. “This is most likely not a real finding but is a concern because of clinical reports of esophagitis associated with oral bisphosphonates,” he said.
Another concern from long-term bisphosphonate use is the development of atypical femoral fractures, a subtrochanteric fracture with atypical features. “These are transverse fractures of the femur and are likely a real effect of bisphosphonates,” Dr. Tice said. “They are characterized by transverse fractures, cortical thickening, and the 10-year risk is about 1:200. It’s similar to a stress fracture, and it’s often preceded by pain.”
To bring perspective on the risk of developing an atypical femoral fracture, Dr. Tice offered a comparison. If 1,000 women are treated for 3 years with zoledronic acid, it will prevent 71 vertebral fractures, 11 hip fractures, and 18 other fractures. “So, the best estimate is that you would cause 0.1 atypical femoral fractures,” he said. “That really means that, for every one atypical femoral fracture caused, you’ve prevented 110 hip fractures. So, yes, there’s a risk of harm – but there is so much more benefit.”
Dr. Tice reported having no relevant financial disclosures.
SAN FRANCISCO – Parathyroid hormone and parathyroid hormone–related protein analogs show promise for treating osteoporosis, but, for now, they’re reserved for the most severe cases.
Teriparatide, a parathyroid hormone analog, is the first treatment that stimulates bone formation instead of inhibiting bone resorption, but studies to date are too small to evaluate its effect on hip fractures, Jeffrey A. Tice, MD, said at the UCSF Annual Advances in Internal Medicine meeting.
Abaloparatide, a parathyroid hormone-related protein analog approved in April 2017, requires subcutaneous dosing of 80 mcg daily for up to 2 years and has been found to increase bone density in the spine by 11% and in the hip by 4%, compared with placebo, over 18 months. In women with pre-existing vertebral fractures, the agent was found to reduce the incidence of vertebral fractures by 86% and nonvertebral fragility fractures by 43%, but data are not adequate to evaluate its effect on hip fractures. Adverse reactions include hypercalciuria, nausea, hypercalcemia, orthostatic hypotension, tachycardia, and injection site reactions.
Studies to date have shown that combining either teriparatide or abaloparatide with an antiresorptive drug is less effective than either agent alone. However, both agents must be followed by an antiresorptive agent. “These drugs are expensive,” Dr. Tice said. “Right now, they’re reserved for patients with severe osteoporosis.”
The lifetime risk for osteoporotic fractures is 50% in women and 20% in men, and they cause significant morbidity and mortality. Among U.S. hospitalizations for women 55 years and older between 2000 and 2012, Dr. Tice noted, 4.9 million were for osteoporotic fractures, 3 million were for stroke, and 2.9 million were for myocardial infarction.
“Osteoporosis is a very common condition, but it’s under-recognized, in part because it’s a silent disease,” he said. “Only 20%-30% of patients with bone mineral densities less than –2.5 are treated in this country. And, 12 months after hip fracture, only 2% had a dual-energy x-ray absorptiometry [DXA] test, and only 15% were treated with an appropriate drug. We should be asking about fracture history, note vertebral fractures, treat those patients, and we should remember to use chart reminders for DXA.”
Clinical guidelines from the American College of Physicians and other groups recommend alendronate, risedronate, zoledronic acid, or denosumab as first-line therapy for women with osteoporosis. “There are no head to head trials to indicate which agent is best,” Dr. Tice said. “They reduce the fracture risk by 30%-50%, primarily in patients with an existing vertebral fracture or a T score below –2.5.” The use of hormone therapy and raloxifene are generally not recommended.
In a review published in the New England Journal of Medicine, researchers Dennis M. Black, MD, and Clifford J. Rosen, MD, estimated the number of patients needed to treat for 3 years to prevent various fractures (N Engl J Med. 2016 Jan 21;374[3]:254-62). They found that, for nonvertebral fractures, including hip, 35 patients need to be treated for 3 years to prevent one of those fractures, 90 patients for 3 years to prevent one hip fracture, and 14 patients for 3 years to prevent one vertebral fracture.
In the FIT trial, 20% of women taking alendronate lost BMD during the first year (JAMA. 1998 Dec 23-30;280[24]:2077-82). However, those same patients had a 50% fracture reduction, and 92% regained the lost bone mineral density (BMD) by the next measurement. “Using DXA to measure BMD after 1 year of therapy does not accurately predict what will happen over time or reflect fracture reduction,” Dr. Tice said. “Effective treatment for osteoporosis should not be changed because of loss of BMD during the first year of use.”
A rare but potential harm from treatment with bisphosphonates includes osteonecrosis of the jaw, which is believed to affect only 1 in 10,000 patients who are treated for 10 years. Most of these cases (94%) were caused by IV bisphosphonates. Only 4% of cases were being treated for osteoporosis, and 60% were caused by tooth extraction. Because of this, a dental exam is recommended before starting patients on bisphosphonate therapy, but this small risk of osteonecrosis of the jaw “is not a reason to stop bisphosphonate therapy,” Dr. Tice said.
Other concerns include an increased risk for atrial fibrillation that has been observed in randomized, controlled trials of zoledronate and alendronate. “There has been no association in other trials,” Dr. Tice said. “This is likely a chance finding, likely spurious.”
Reports of an increased risk of esophageal cancer from bisphosphonate use have also been suggested, but these come from case series and from two conflicting cohorts of data. “This is most likely not a real finding but is a concern because of clinical reports of esophagitis associated with oral bisphosphonates,” he said.
Another concern from long-term bisphosphonate use is the development of atypical femoral fractures, a subtrochanteric fracture with atypical features. “These are transverse fractures of the femur and are likely a real effect of bisphosphonates,” Dr. Tice said. “They are characterized by transverse fractures, cortical thickening, and the 10-year risk is about 1:200. It’s similar to a stress fracture, and it’s often preceded by pain.”
To bring perspective on the risk of developing an atypical femoral fracture, Dr. Tice offered a comparison. If 1,000 women are treated for 3 years with zoledronic acid, it will prevent 71 vertebral fractures, 11 hip fractures, and 18 other fractures. “So, the best estimate is that you would cause 0.1 atypical femoral fractures,” he said. “That really means that, for every one atypical femoral fracture caused, you’ve prevented 110 hip fractures. So, yes, there’s a risk of harm – but there is so much more benefit.”
Dr. Tice reported having no relevant financial disclosures.
SAN FRANCISCO – Parathyroid hormone and parathyroid hormone–related protein analogs show promise for treating osteoporosis, but, for now, they’re reserved for the most severe cases.
Teriparatide, a parathyroid hormone analog, is the first treatment that stimulates bone formation instead of inhibiting bone resorption, but studies to date are too small to evaluate its effect on hip fractures, Jeffrey A. Tice, MD, said at the UCSF Annual Advances in Internal Medicine meeting.
Abaloparatide, a parathyroid hormone-related protein analog approved in April 2017, requires subcutaneous dosing of 80 mcg daily for up to 2 years and has been found to increase bone density in the spine by 11% and in the hip by 4%, compared with placebo, over 18 months. In women with pre-existing vertebral fractures, the agent was found to reduce the incidence of vertebral fractures by 86% and nonvertebral fragility fractures by 43%, but data are not adequate to evaluate its effect on hip fractures. Adverse reactions include hypercalciuria, nausea, hypercalcemia, orthostatic hypotension, tachycardia, and injection site reactions.
Studies to date have shown that combining either teriparatide or abaloparatide with an antiresorptive drug is less effective than either agent alone. However, both agents must be followed by an antiresorptive agent. “These drugs are expensive,” Dr. Tice said. “Right now, they’re reserved for patients with severe osteoporosis.”
The lifetime risk for osteoporotic fractures is 50% in women and 20% in men, and they cause significant morbidity and mortality. Among U.S. hospitalizations for women 55 years and older between 2000 and 2012, Dr. Tice noted, 4.9 million were for osteoporotic fractures, 3 million were for stroke, and 2.9 million were for myocardial infarction.
“Osteoporosis is a very common condition, but it’s under-recognized, in part because it’s a silent disease,” he said. “Only 20%-30% of patients with bone mineral densities less than –2.5 are treated in this country. And, 12 months after hip fracture, only 2% had a dual-energy x-ray absorptiometry [DXA] test, and only 15% were treated with an appropriate drug. We should be asking about fracture history, note vertebral fractures, treat those patients, and we should remember to use chart reminders for DXA.”
Clinical guidelines from the American College of Physicians and other groups recommend alendronate, risedronate, zoledronic acid, or denosumab as first-line therapy for women with osteoporosis. “There are no head to head trials to indicate which agent is best,” Dr. Tice said. “They reduce the fracture risk by 30%-50%, primarily in patients with an existing vertebral fracture or a T score below –2.5.” The use of hormone therapy and raloxifene are generally not recommended.
In a review published in the New England Journal of Medicine, researchers Dennis M. Black, MD, and Clifford J. Rosen, MD, estimated the number of patients needed to treat for 3 years to prevent various fractures (N Engl J Med. 2016 Jan 21;374[3]:254-62). They found that, for nonvertebral fractures, including hip, 35 patients need to be treated for 3 years to prevent one of those fractures, 90 patients for 3 years to prevent one hip fracture, and 14 patients for 3 years to prevent one vertebral fracture.
In the FIT trial, 20% of women taking alendronate lost BMD during the first year (JAMA. 1998 Dec 23-30;280[24]:2077-82). However, those same patients had a 50% fracture reduction, and 92% regained the lost bone mineral density (BMD) by the next measurement. “Using DXA to measure BMD after 1 year of therapy does not accurately predict what will happen over time or reflect fracture reduction,” Dr. Tice said. “Effective treatment for osteoporosis should not be changed because of loss of BMD during the first year of use.”
A rare but potential harm from treatment with bisphosphonates includes osteonecrosis of the jaw, which is believed to affect only 1 in 10,000 patients who are treated for 10 years. Most of these cases (94%) were caused by IV bisphosphonates. Only 4% of cases were being treated for osteoporosis, and 60% were caused by tooth extraction. Because of this, a dental exam is recommended before starting patients on bisphosphonate therapy, but this small risk of osteonecrosis of the jaw “is not a reason to stop bisphosphonate therapy,” Dr. Tice said.
Other concerns include an increased risk for atrial fibrillation that has been observed in randomized, controlled trials of zoledronate and alendronate. “There has been no association in other trials,” Dr. Tice said. “This is likely a chance finding, likely spurious.”
Reports of an increased risk of esophageal cancer from bisphosphonate use have also been suggested, but these come from case series and from two conflicting cohorts of data. “This is most likely not a real finding but is a concern because of clinical reports of esophagitis associated with oral bisphosphonates,” he said.
Another concern from long-term bisphosphonate use is the development of atypical femoral fractures, a subtrochanteric fracture with atypical features. “These are transverse fractures of the femur and are likely a real effect of bisphosphonates,” Dr. Tice said. “They are characterized by transverse fractures, cortical thickening, and the 10-year risk is about 1:200. It’s similar to a stress fracture, and it’s often preceded by pain.”
To bring perspective on the risk of developing an atypical femoral fracture, Dr. Tice offered a comparison. If 1,000 women are treated for 3 years with zoledronic acid, it will prevent 71 vertebral fractures, 11 hip fractures, and 18 other fractures. “So, the best estimate is that you would cause 0.1 atypical femoral fractures,” he said. “That really means that, for every one atypical femoral fracture caused, you’ve prevented 110 hip fractures. So, yes, there’s a risk of harm – but there is so much more benefit.”
Dr. Tice reported having no relevant financial disclosures.
EXPERT ANALYSIS FROM THE ANNUAL ADVANCES IN INTERNAL MEDICINE
Product Update: Intrarosa, ZEJULA, Signia Stapling System, TYMLOS, Videssa Breast
NONESTROGEN PRODUCT FOR DYSPAREUNIA
FOR MORE INFORMATION, VISIT: http://www.amagpharma.com
ORAL MAINTENANCE TX FOR RECURRENT CANCER
FOR MORE INFORMATION, VISIT: http://www.zejula.com
MIGS STAPLING SYSTEM WITH REAL-TIME FEEDBACK
FOR MORE INFORMATION, VISIT: http://www.medtronic.com
BONE BUILDING AGENT
FOR MORE INFORMATION, VISIT: http://www.radiuspharm.com
PROTEOMIC BREAST CANCER ASSAY
FOR MORE INFORMATION, VISIT: http://www.provistadx.com
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
NONESTROGEN PRODUCT FOR DYSPAREUNIA
FOR MORE INFORMATION, VISIT: http://www.amagpharma.com
ORAL MAINTENANCE TX FOR RECURRENT CANCER
FOR MORE INFORMATION, VISIT: http://www.zejula.com
MIGS STAPLING SYSTEM WITH REAL-TIME FEEDBACK
FOR MORE INFORMATION, VISIT: http://www.medtronic.com
BONE BUILDING AGENT
FOR MORE INFORMATION, VISIT: http://www.radiuspharm.com
PROTEOMIC BREAST CANCER ASSAY
FOR MORE INFORMATION, VISIT: http://www.provistadx.com
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
NONESTROGEN PRODUCT FOR DYSPAREUNIA
FOR MORE INFORMATION, VISIT: http://www.amagpharma.com
ORAL MAINTENANCE TX FOR RECURRENT CANCER
FOR MORE INFORMATION, VISIT: http://www.zejula.com
MIGS STAPLING SYSTEM WITH REAL-TIME FEEDBACK
FOR MORE INFORMATION, VISIT: http://www.medtronic.com
BONE BUILDING AGENT
FOR MORE INFORMATION, VISIT: http://www.radiuspharm.com
PROTEOMIC BREAST CANCER ASSAY
FOR MORE INFORMATION, VISIT: http://www.provistadx.com
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
2017 Update on menopause
Since publication of initial findings of the Women’s Health Initiative (WHI) in 2002, use of systemic menopausal hormone therapy (HT) has declined by some 80% among US women.1 Against this backdrop, this year’s Menopause Update highlights the “hot off the press” updated position statement on menopausal HT from The North American Menopause Society (NAMS), summarized by Dr. JoAnn V. Pinkerton. Although this guidance is chock full of practical, evidence-based guidance, the take-home message that Dr. Pinkerton and I would like to leave readers of OBG
Related Article:
Dr. Andrew M. Kaunitz on prescribing systemic HT to older women
Although menopausal vasomotor and related symptoms improve as women age, in untreated women, vulvovaginal atrophy (VVA, also known as genitourinary syndrome of menopause, or GSM) tends to progress, causing vaginal dryness and sexual dysfunction, among other symptoms. When symptomatic GSM represents the only indication for treatment, low-dose local vaginal estrogen, ospemifene, or dehydroepiandrosterone (DHEA; prasterone) is safe and effective. However, as with systemic HT, specific treatments for GSM are substantially underutilized.2 The current package labeling for low-dose vaginal estrogen deters many appropriate candidates from using this safe, effective treatment. In this Update, Dr. JoAnn E. Manson reviews the rationale for updating this labeling as well as recent efforts to accomplish the task.
Read about updated NAMS guidelines on HT
Guidelines on HT have been updated by The North American Menopause Society
The North American Menopause Society Hormone Therapy (HT) Position Statement Advisory Panel, composed of more than 20 experts in menopausal women's HT, including clinicians, researchers, and epidemiologists, reviewed the 2012 HT Position Statement, evaluated prior and new literature and used levels of evidence to identify the quality of the evidence and strength of the recommendations and to find consensus for the guidelines. The following information comes from the NAMS 2017 Hormone Therapy Position Statement.3
What are the major findings?
HT is the most effective treatment for vasomotor symptoms (VMS) and GSM and has been shown to prevent bone loss and fracture. Risks of HT may differ for women depending on type, dose, duration, route of administration, and timing of initiation and whether or not a progestogen is needed. Treatment should be individualized using the best available evidence to maximize benefits and minimize risks, with periodic reevaluation about benefits and risks of continuing or discontinuing HT.
For women who are younger than age 60 or within 10 years of menopause and have no contraindication, the clearest benefit of HT is for the treatment of VMS and prevention of bone loss in those at elevated risk.
The clinical guidelines were presented to NAMS audience at the 2016 annual clinical meeting, where NAMS recommended "determining the most appropriate type, dose, formulation, and duration of HT."4
When to initiate HT and duration of use
In its now-published 2017 guidelines on HT, NAMS affirms the safety and efficacy of HT for symptomatic menopausal women or those at high risk for bone loss who are under age 60 or within 10 years of menopause. NAMS encourages practitioners to employ shared decision making with their patients to find the appropriate type, dose, formulation, and duration of HT, making individualized decisions based on evidence-based information, the unique health risks of women, and with periodic reassessment.
In the clinical guidelines presented in the 2016 NAMS annual meeting,4 key recommendations taken from the 2017 Hormone Therapy Position Statement3 include the following: For women who are aged younger than 60 years or within 10 years of menopause and have no contraindications, the benefit/risk ratio appears favorable for treatment of bothersome VMS and in those at elevated risk for bone loss or fracture.
For women who initiate HT more than 10 years from menopause or after age 60, this benefit/risk ratio appears less favorable because of greater absolute risks of coronary heart disease, stroke, venous thromboembolism, and dementia.
What about extended use of hormone therapy? There is no evidence to support routine discontinuation of HT after age 65. Decisions about longer durations of HT should be individualized and considered for indications such as persistent VMS or bone loss, with shared decision making, documentation, and periodic reevaluation. Longer duration is more favorable for estrogen therapy than for estrogen-progestin therapy, based on the Women's Health Initiative (WHI) randomized controlled trials.5
What about only vaginal symptoms? For bothersome GSM not relieved with over-the-counter therapies and without indications for use of systemic HT, low-dose vaginal estrogen therapy or other therapies are recommended and can be continued as long as indicated since there is minimal systemic absorption of estrogen, with serum levels remaining within the normal postmenopausal range.6,7 For women with estrogen sensitive cancer, oncologists should be included in decision making, particularly for women on aromatase inhibitors.
Considerations for special populations Early menopause. For women with hypoestrogenism, primary ovarian insufficiency, or premature surgical menopause without contraindications, HT is recommended until at least the median age of menopause (52 years), as studies suggest that benefits outweigh the risks for effects on bone, heart, cognition, GSM, sexual function, and mood.8
Family history of breast cancer. Observational evidence suggests that use of HT does not further alter the risk for breast cancer in women with a family history of breast cancer. Family history is one risk, among others, that should be assessed when counseling women regarding HT.
Women who are BRCA-positive without breast cancer. For women who are BRCA-positive (higher genetic risk of breast cancer, primarily estrogen-receptor-negative), and have undergone surgical menopause (bilateral salpingo-oophorectomy), the benefits of estrogen to decrease health risks caused by premature loss of estrogen need to be considered on an individual basis.9 On the basis of limited observational studies, consider offering systemic HT until the median age of menopause (52 years) with longer use individualized.3
Related Article:
Is menopausal hormone therapy safe when your patient carries a BRCA mutation?
Survivors of endometrial and breast cancer with bothersome VMS. For women with prior estrogen-sensitive cancers, non-HTs should be considered first, particularly those agents studied through randomized controlled trials in this population and found to be effective. If systemic estrogen is considered for persistent symptoms after non-HT or complementary options have been unsuccessful, decisions should be made for compelling reasons and after detailed counseling, with shared decision making and in conjunction with their oncologist.3
Bothersome GSM. On the basis of limited observational data, there appears to be minimal to no demonstrated elevation in risk for recurrence of endometrial or breast cancer using low-dose vaginal estrogen,3,10 but decisions should be made in conjunction with an oncologist.
Related Article:
Focus on treating genital atrophy symptoms
The importance of relaying the new guidelines to patients
It is important for clinicians to talk to women about their menopausal symptoms and their options for relief of symptoms or prevention of bone loss. Discussion should take into account age and time from menopause, include evidence-based information11-13 about benefits and risks of different types of therapy, and employ shared decision making to choose the most appropriate therapy to maximize benefits and minimize risks for the individual woman.
Following the WHI initial release in 2002, both women and providers became fearful of HT and believed media hype and celebrities that compounded bioidentical HT was safer than FDA-approved HTs. However, compounded products lack safety and efficacy data, are not monitored or regulated by the FDA, and have unique risks associated with compounding, including concerns about sterility, impurities, and overdosing or underdosing, which could increase cancer risk.3
- Hormone therapy for symptomatic menopausal women is safe and effective for those under age 60 or within 10 years of menopause.
- Identify the most appropriate type, dose, formulation, and duration of hormone therapy for an individual woman based on evidence.
- We want to remove the fear of using hormone therapy for healthy symptomatic women who are under age 60 or within 10 years of menopause.
- Age at initiation of hormone therapy matters.
- NAMS endorses use of FDA-approved hormone therapy over compounded therapies.
Read about modifying low-dose vaginal estrogen’s black box warning
Physicians continue to underwhelmingly prescribe low-dose vaginal estrogen for GSM
Kingsberg SA, Krychman M, Graham S, Bernick B, Mirkin S. The Women's EMPOWER survey: identifying women's perceptions on vulvar and vaginal atrophy and its treatment. J Sex Med. 2017;14(3):413-424.
GSM is seriously underrecognized and undertreated.2,8,14 It has a major impact on women's lives--a silent epidemic affecting women's quality of life, sexual health, interpersonal relationships, and even physical health in terms of increased risk of urinary tract infections and urinary symptoms. Unfortunately, patients are reluctant to mention the problem to their clinicians, and they do not clearly recognize it as a medical condition that has available treatment options. Clinicians also rarely receive adequate training in the management of this condition and how to discuss it with their patients. Given busy schedules and time constraints, addressing this topic often falls through the cracks, representing a missed opportunity for helping our patients with safe and effective treatments. In a recent study by Kingsberg and colleagues, an astoundingly low percentageof women with GSM symptoms received treatment.
Details of the study
The study authors evaluated women's perceptions of GSM and available treatment options. US women aged 45 and older who reported GSM symptoms were surveyed. Of 1,858 women with a median age of 58 (range, 45-90), the study authors found that 50% had never used any treatment; 25% used over-the-counter medications; 18% were former users of GSM treatments; and 7% currently used prescribed GSM therapies.
When GSM was discussed, women were more likely than their clinicians to initiate the conversation. The main reason for women not mentioning their symptoms was the perception that GSM symptoms were a natural and inevitable part of aging. Hormonal products were perceived by women as having several downsides, including risk of systemic absorption, messiness of local creams, and the need to reuse an applicator. Overall, clinicians recommended vaginal estrogen therapy to only 23% and oral HTs to 18% of women.
The results of the study are consistent with results of earlier surveys of menopausal women. Although the survey included nearly 2,000 women, it has the potential for selection biases inherent to most Internet-based surveys. In addition, the respondents tended to be white and have higher socieconomic status, with limited representation from other groups.
Calls for the current boxed warning to be revised
GSM is highly prevalent among postmenopausal women; the condition has adverse effects on quality of life and sexual health.2,8,14 Safe and effective treatments are available but are underutilized.1,8,15,16 A current boxed warning appears on low-dose vaginal estrogen--class labeling that appears on all medications in the class of estrogen or HT, regardless of dose or route of administration. These warnings are based on findings from the WHI and other studies of systemic estrogen or estrogen plus progestin, which demonstrated a complex pattern of risks and benefits of HT (including increased risk of venous thrombosis or pulmonary embolism, stroke, and breast cancer [with estrogen plus progestin]).
These findings, however, do not appear to be relevant to low-dose vaginal estrogen, given minimal if any systemic absorption and much lower blood levels of hormones than found with systemic HT. Blood levels of estradiol with low-dose vaginal estrogen remain in the normal postmenopausal range, compared to several-fold elevations in hormone levels with systemic HT.8,15,16 Additionally, observational studies of low-dose vaginal estrogen, as well as short-term randomized clinical trials, show no evidence of an increased risk of venous thromboembolic events, heart disease, stroke, breast cancer, or dementia--the listed possible adverse effects in the boxed warning. The current warning is based on extrapolating findings from systemic HT, which is inappropriate and not evidence-based for low-dose vaginal estrogen.15
The inappropriate boxed warning contributes to the problem of undertreatment of GSM in women by discouraging clinicians from prescribing the medication and dissuading patients from taking it even after purchase. Testimonials from many clinicians caring for these women have underscored that women will fill their prescription, but after seeing the boxed warning will often become alarmed and decide not to take the medication. Clinicians reported that patients often say at their next appointment: "No, I never took it. I got very scared when I saw the boxed warning." As a result, clinicians often have to spend a great deal of time explaining the limitations of, and lack of evidence for, the boxed warning on low-dose vaginal estrogen.
Related Article:
2016 Update on menopause
Recommended label revisions
A modified label, without a boxed warning, would be safer for women because the key messages would not be obscured by the large amount of irrelevant information. Our Working Group recommended that the label explain that the listed risks were found in studies of systemic HT and their relevance to low-dose vaginal estrogen is unknown. The Group also recommended that warning text should be added in bold font to advise patients to seek medical attention if they have vaginal bleeding or spotting while taking the medication. In addition, patients who have a history of breast cancer or other hormone-sensitive cancer should discuss the use of the medication with their oncologist.
Status update on efforts to revise label. A citizen's petition was filed in the Spring of 2016, with signatures from more than 600 clinicians and patients and representatives of medical and professional organizations endorsing a more appropriate evidence-based label for low-dose vaginal estrogen. The FDA is continuing to review and deliberate on these issues but has not yet made a final decision.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Manson JM, Kaunitz AM. Menopause management—Getting clinical care back on track. N Engl J Med. 2016;374(9):803–806.
- Parish SJ, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437–447.
- The 2017 hormone therapy position statement of The North American Menopause Society [published online ahead of print June 2017]. Menopause.
- Pinkerton JV. Hormone therapy: 2016 NAMS position statement [abstract]. Menopause. 2016;23:1365.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368.
- Lethaby A, Ayeleke RO, Roberts H. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Sys Rev. 2016;8:CD001500.
- Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause. 2013;20(9):888–902.
- Faubion SS, Kuhle CL, Shuster LT, Rocca WA. Long-term health consequences of premature or early menopause and considerations for management. Climacteric. 2015;18(4):483–491.
- Chai X, Domchek S, Kauff N, Rebbeck T, Chen J. RE: Breast cancer risk after salpingo-oophorectomy in healthy BRCA1/2 mutation carriers: revisiting the evidence for risk reduction. J Natl Cancer Inst. 2015;107(9).
- Farrell R; American College of Obstetricians and Gynecologists’ Committee on Gynecologic Practice. ACOG Committee Opinion No. 659 summary: The use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol. 2016;127(3):618–619.
- Hodis HN, Mack WJ, Henderson VW, et al; ELITE Research Group. Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med. 2016;374(13):1221–1231.
- Marjoribanks J, Farquhar C, Roberts H, Lethaby A, Lee J. Long-term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst Rev. 2017;1:CD004143.
- Boardman HM, Hartley L, Eisinga A, et al. Hormone therapy for preventing cardiovascular disease in post-menopausal women. Cochrane Database Syst Rev. 2015;(3):CD002229.
- Parish S, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437–447.
- Manson JE, Goldstein SR, Kagan R, et al; Working Group on Women’s Health and Well-Being in Menopause. Why the product labeling for low-dose vaginal estrogen should be changed. Menopause. 2014;21(9):911–916.
- Kaunitz AM, Manson JE. Management of menopausal symptoms. Obstet Gynecol. 2015;126(4):859-876.
Since publication of initial findings of the Women’s Health Initiative (WHI) in 2002, use of systemic menopausal hormone therapy (HT) has declined by some 80% among US women.1 Against this backdrop, this year’s Menopause Update highlights the “hot off the press” updated position statement on menopausal HT from The North American Menopause Society (NAMS), summarized by Dr. JoAnn V. Pinkerton. Although this guidance is chock full of practical, evidence-based guidance, the take-home message that Dr. Pinkerton and I would like to leave readers of OBG
Related Article:
Dr. Andrew M. Kaunitz on prescribing systemic HT to older women
Although menopausal vasomotor and related symptoms improve as women age, in untreated women, vulvovaginal atrophy (VVA, also known as genitourinary syndrome of menopause, or GSM) tends to progress, causing vaginal dryness and sexual dysfunction, among other symptoms. When symptomatic GSM represents the only indication for treatment, low-dose local vaginal estrogen, ospemifene, or dehydroepiandrosterone (DHEA; prasterone) is safe and effective. However, as with systemic HT, specific treatments for GSM are substantially underutilized.2 The current package labeling for low-dose vaginal estrogen deters many appropriate candidates from using this safe, effective treatment. In this Update, Dr. JoAnn E. Manson reviews the rationale for updating this labeling as well as recent efforts to accomplish the task.
Read about updated NAMS guidelines on HT
Guidelines on HT have been updated by The North American Menopause Society
The North American Menopause Society Hormone Therapy (HT) Position Statement Advisory Panel, composed of more than 20 experts in menopausal women's HT, including clinicians, researchers, and epidemiologists, reviewed the 2012 HT Position Statement, evaluated prior and new literature and used levels of evidence to identify the quality of the evidence and strength of the recommendations and to find consensus for the guidelines. The following information comes from the NAMS 2017 Hormone Therapy Position Statement.3
What are the major findings?
HT is the most effective treatment for vasomotor symptoms (VMS) and GSM and has been shown to prevent bone loss and fracture. Risks of HT may differ for women depending on type, dose, duration, route of administration, and timing of initiation and whether or not a progestogen is needed. Treatment should be individualized using the best available evidence to maximize benefits and minimize risks, with periodic reevaluation about benefits and risks of continuing or discontinuing HT.
For women who are younger than age 60 or within 10 years of menopause and have no contraindication, the clearest benefit of HT is for the treatment of VMS and prevention of bone loss in those at elevated risk.
The clinical guidelines were presented to NAMS audience at the 2016 annual clinical meeting, where NAMS recommended "determining the most appropriate type, dose, formulation, and duration of HT."4
When to initiate HT and duration of use
In its now-published 2017 guidelines on HT, NAMS affirms the safety and efficacy of HT for symptomatic menopausal women or those at high risk for bone loss who are under age 60 or within 10 years of menopause. NAMS encourages practitioners to employ shared decision making with their patients to find the appropriate type, dose, formulation, and duration of HT, making individualized decisions based on evidence-based information, the unique health risks of women, and with periodic reassessment.
In the clinical guidelines presented in the 2016 NAMS annual meeting,4 key recommendations taken from the 2017 Hormone Therapy Position Statement3 include the following: For women who are aged younger than 60 years or within 10 years of menopause and have no contraindications, the benefit/risk ratio appears favorable for treatment of bothersome VMS and in those at elevated risk for bone loss or fracture.
For women who initiate HT more than 10 years from menopause or after age 60, this benefit/risk ratio appears less favorable because of greater absolute risks of coronary heart disease, stroke, venous thromboembolism, and dementia.
What about extended use of hormone therapy? There is no evidence to support routine discontinuation of HT after age 65. Decisions about longer durations of HT should be individualized and considered for indications such as persistent VMS or bone loss, with shared decision making, documentation, and periodic reevaluation. Longer duration is more favorable for estrogen therapy than for estrogen-progestin therapy, based on the Women's Health Initiative (WHI) randomized controlled trials.5
What about only vaginal symptoms? For bothersome GSM not relieved with over-the-counter therapies and without indications for use of systemic HT, low-dose vaginal estrogen therapy or other therapies are recommended and can be continued as long as indicated since there is minimal systemic absorption of estrogen, with serum levels remaining within the normal postmenopausal range.6,7 For women with estrogen sensitive cancer, oncologists should be included in decision making, particularly for women on aromatase inhibitors.
Considerations for special populations Early menopause. For women with hypoestrogenism, primary ovarian insufficiency, or premature surgical menopause without contraindications, HT is recommended until at least the median age of menopause (52 years), as studies suggest that benefits outweigh the risks for effects on bone, heart, cognition, GSM, sexual function, and mood.8
Family history of breast cancer. Observational evidence suggests that use of HT does not further alter the risk for breast cancer in women with a family history of breast cancer. Family history is one risk, among others, that should be assessed when counseling women regarding HT.
Women who are BRCA-positive without breast cancer. For women who are BRCA-positive (higher genetic risk of breast cancer, primarily estrogen-receptor-negative), and have undergone surgical menopause (bilateral salpingo-oophorectomy), the benefits of estrogen to decrease health risks caused by premature loss of estrogen need to be considered on an individual basis.9 On the basis of limited observational studies, consider offering systemic HT until the median age of menopause (52 years) with longer use individualized.3
Related Article:
Is menopausal hormone therapy safe when your patient carries a BRCA mutation?
Survivors of endometrial and breast cancer with bothersome VMS. For women with prior estrogen-sensitive cancers, non-HTs should be considered first, particularly those agents studied through randomized controlled trials in this population and found to be effective. If systemic estrogen is considered for persistent symptoms after non-HT or complementary options have been unsuccessful, decisions should be made for compelling reasons and after detailed counseling, with shared decision making and in conjunction with their oncologist.3
Bothersome GSM. On the basis of limited observational data, there appears to be minimal to no demonstrated elevation in risk for recurrence of endometrial or breast cancer using low-dose vaginal estrogen,3,10 but decisions should be made in conjunction with an oncologist.
Related Article:
Focus on treating genital atrophy symptoms
The importance of relaying the new guidelines to patients
It is important for clinicians to talk to women about their menopausal symptoms and their options for relief of symptoms or prevention of bone loss. Discussion should take into account age and time from menopause, include evidence-based information11-13 about benefits and risks of different types of therapy, and employ shared decision making to choose the most appropriate therapy to maximize benefits and minimize risks for the individual woman.
Following the WHI initial release in 2002, both women and providers became fearful of HT and believed media hype and celebrities that compounded bioidentical HT was safer than FDA-approved HTs. However, compounded products lack safety and efficacy data, are not monitored or regulated by the FDA, and have unique risks associated with compounding, including concerns about sterility, impurities, and overdosing or underdosing, which could increase cancer risk.3
- Hormone therapy for symptomatic menopausal women is safe and effective for those under age 60 or within 10 years of menopause.
- Identify the most appropriate type, dose, formulation, and duration of hormone therapy for an individual woman based on evidence.
- We want to remove the fear of using hormone therapy for healthy symptomatic women who are under age 60 or within 10 years of menopause.
- Age at initiation of hormone therapy matters.
- NAMS endorses use of FDA-approved hormone therapy over compounded therapies.
Read about modifying low-dose vaginal estrogen’s black box warning
Physicians continue to underwhelmingly prescribe low-dose vaginal estrogen for GSM
Kingsberg SA, Krychman M, Graham S, Bernick B, Mirkin S. The Women's EMPOWER survey: identifying women's perceptions on vulvar and vaginal atrophy and its treatment. J Sex Med. 2017;14(3):413-424.
GSM is seriously underrecognized and undertreated.2,8,14 It has a major impact on women's lives--a silent epidemic affecting women's quality of life, sexual health, interpersonal relationships, and even physical health in terms of increased risk of urinary tract infections and urinary symptoms. Unfortunately, patients are reluctant to mention the problem to their clinicians, and they do not clearly recognize it as a medical condition that has available treatment options. Clinicians also rarely receive adequate training in the management of this condition and how to discuss it with their patients. Given busy schedules and time constraints, addressing this topic often falls through the cracks, representing a missed opportunity for helping our patients with safe and effective treatments. In a recent study by Kingsberg and colleagues, an astoundingly low percentageof women with GSM symptoms received treatment.
Details of the study
The study authors evaluated women's perceptions of GSM and available treatment options. US women aged 45 and older who reported GSM symptoms were surveyed. Of 1,858 women with a median age of 58 (range, 45-90), the study authors found that 50% had never used any treatment; 25% used over-the-counter medications; 18% were former users of GSM treatments; and 7% currently used prescribed GSM therapies.
When GSM was discussed, women were more likely than their clinicians to initiate the conversation. The main reason for women not mentioning their symptoms was the perception that GSM symptoms were a natural and inevitable part of aging. Hormonal products were perceived by women as having several downsides, including risk of systemic absorption, messiness of local creams, and the need to reuse an applicator. Overall, clinicians recommended vaginal estrogen therapy to only 23% and oral HTs to 18% of women.
The results of the study are consistent with results of earlier surveys of menopausal women. Although the survey included nearly 2,000 women, it has the potential for selection biases inherent to most Internet-based surveys. In addition, the respondents tended to be white and have higher socieconomic status, with limited representation from other groups.
Calls for the current boxed warning to be revised
GSM is highly prevalent among postmenopausal women; the condition has adverse effects on quality of life and sexual health.2,8,14 Safe and effective treatments are available but are underutilized.1,8,15,16 A current boxed warning appears on low-dose vaginal estrogen--class labeling that appears on all medications in the class of estrogen or HT, regardless of dose or route of administration. These warnings are based on findings from the WHI and other studies of systemic estrogen or estrogen plus progestin, which demonstrated a complex pattern of risks and benefits of HT (including increased risk of venous thrombosis or pulmonary embolism, stroke, and breast cancer [with estrogen plus progestin]).
These findings, however, do not appear to be relevant to low-dose vaginal estrogen, given minimal if any systemic absorption and much lower blood levels of hormones than found with systemic HT. Blood levels of estradiol with low-dose vaginal estrogen remain in the normal postmenopausal range, compared to several-fold elevations in hormone levels with systemic HT.8,15,16 Additionally, observational studies of low-dose vaginal estrogen, as well as short-term randomized clinical trials, show no evidence of an increased risk of venous thromboembolic events, heart disease, stroke, breast cancer, or dementia--the listed possible adverse effects in the boxed warning. The current warning is based on extrapolating findings from systemic HT, which is inappropriate and not evidence-based for low-dose vaginal estrogen.15
The inappropriate boxed warning contributes to the problem of undertreatment of GSM in women by discouraging clinicians from prescribing the medication and dissuading patients from taking it even after purchase. Testimonials from many clinicians caring for these women have underscored that women will fill their prescription, but after seeing the boxed warning will often become alarmed and decide not to take the medication. Clinicians reported that patients often say at their next appointment: "No, I never took it. I got very scared when I saw the boxed warning." As a result, clinicians often have to spend a great deal of time explaining the limitations of, and lack of evidence for, the boxed warning on low-dose vaginal estrogen.
Related Article:
2016 Update on menopause
Recommended label revisions
A modified label, without a boxed warning, would be safer for women because the key messages would not be obscured by the large amount of irrelevant information. Our Working Group recommended that the label explain that the listed risks were found in studies of systemic HT and their relevance to low-dose vaginal estrogen is unknown. The Group also recommended that warning text should be added in bold font to advise patients to seek medical attention if they have vaginal bleeding or spotting while taking the medication. In addition, patients who have a history of breast cancer or other hormone-sensitive cancer should discuss the use of the medication with their oncologist.
Status update on efforts to revise label. A citizen's petition was filed in the Spring of 2016, with signatures from more than 600 clinicians and patients and representatives of medical and professional organizations endorsing a more appropriate evidence-based label for low-dose vaginal estrogen. The FDA is continuing to review and deliberate on these issues but has not yet made a final decision.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Since publication of initial findings of the Women’s Health Initiative (WHI) in 2002, use of systemic menopausal hormone therapy (HT) has declined by some 80% among US women.1 Against this backdrop, this year’s Menopause Update highlights the “hot off the press” updated position statement on menopausal HT from The North American Menopause Society (NAMS), summarized by Dr. JoAnn V. Pinkerton. Although this guidance is chock full of practical, evidence-based guidance, the take-home message that Dr. Pinkerton and I would like to leave readers of OBG
Related Article:
Dr. Andrew M. Kaunitz on prescribing systemic HT to older women
Although menopausal vasomotor and related symptoms improve as women age, in untreated women, vulvovaginal atrophy (VVA, also known as genitourinary syndrome of menopause, or GSM) tends to progress, causing vaginal dryness and sexual dysfunction, among other symptoms. When symptomatic GSM represents the only indication for treatment, low-dose local vaginal estrogen, ospemifene, or dehydroepiandrosterone (DHEA; prasterone) is safe and effective. However, as with systemic HT, specific treatments for GSM are substantially underutilized.2 The current package labeling for low-dose vaginal estrogen deters many appropriate candidates from using this safe, effective treatment. In this Update, Dr. JoAnn E. Manson reviews the rationale for updating this labeling as well as recent efforts to accomplish the task.
Read about updated NAMS guidelines on HT
Guidelines on HT have been updated by The North American Menopause Society
The North American Menopause Society Hormone Therapy (HT) Position Statement Advisory Panel, composed of more than 20 experts in menopausal women's HT, including clinicians, researchers, and epidemiologists, reviewed the 2012 HT Position Statement, evaluated prior and new literature and used levels of evidence to identify the quality of the evidence and strength of the recommendations and to find consensus for the guidelines. The following information comes from the NAMS 2017 Hormone Therapy Position Statement.3
What are the major findings?
HT is the most effective treatment for vasomotor symptoms (VMS) and GSM and has been shown to prevent bone loss and fracture. Risks of HT may differ for women depending on type, dose, duration, route of administration, and timing of initiation and whether or not a progestogen is needed. Treatment should be individualized using the best available evidence to maximize benefits and minimize risks, with periodic reevaluation about benefits and risks of continuing or discontinuing HT.
For women who are younger than age 60 or within 10 years of menopause and have no contraindication, the clearest benefit of HT is for the treatment of VMS and prevention of bone loss in those at elevated risk.
The clinical guidelines were presented to NAMS audience at the 2016 annual clinical meeting, where NAMS recommended "determining the most appropriate type, dose, formulation, and duration of HT."4
When to initiate HT and duration of use
In its now-published 2017 guidelines on HT, NAMS affirms the safety and efficacy of HT for symptomatic menopausal women or those at high risk for bone loss who are under age 60 or within 10 years of menopause. NAMS encourages practitioners to employ shared decision making with their patients to find the appropriate type, dose, formulation, and duration of HT, making individualized decisions based on evidence-based information, the unique health risks of women, and with periodic reassessment.
In the clinical guidelines presented in the 2016 NAMS annual meeting,4 key recommendations taken from the 2017 Hormone Therapy Position Statement3 include the following: For women who are aged younger than 60 years or within 10 years of menopause and have no contraindications, the benefit/risk ratio appears favorable for treatment of bothersome VMS and in those at elevated risk for bone loss or fracture.
For women who initiate HT more than 10 years from menopause or after age 60, this benefit/risk ratio appears less favorable because of greater absolute risks of coronary heart disease, stroke, venous thromboembolism, and dementia.
What about extended use of hormone therapy? There is no evidence to support routine discontinuation of HT after age 65. Decisions about longer durations of HT should be individualized and considered for indications such as persistent VMS or bone loss, with shared decision making, documentation, and periodic reevaluation. Longer duration is more favorable for estrogen therapy than for estrogen-progestin therapy, based on the Women's Health Initiative (WHI) randomized controlled trials.5
What about only vaginal symptoms? For bothersome GSM not relieved with over-the-counter therapies and without indications for use of systemic HT, low-dose vaginal estrogen therapy or other therapies are recommended and can be continued as long as indicated since there is minimal systemic absorption of estrogen, with serum levels remaining within the normal postmenopausal range.6,7 For women with estrogen sensitive cancer, oncologists should be included in decision making, particularly for women on aromatase inhibitors.
Considerations for special populations Early menopause. For women with hypoestrogenism, primary ovarian insufficiency, or premature surgical menopause without contraindications, HT is recommended until at least the median age of menopause (52 years), as studies suggest that benefits outweigh the risks for effects on bone, heart, cognition, GSM, sexual function, and mood.8
Family history of breast cancer. Observational evidence suggests that use of HT does not further alter the risk for breast cancer in women with a family history of breast cancer. Family history is one risk, among others, that should be assessed when counseling women regarding HT.
Women who are BRCA-positive without breast cancer. For women who are BRCA-positive (higher genetic risk of breast cancer, primarily estrogen-receptor-negative), and have undergone surgical menopause (bilateral salpingo-oophorectomy), the benefits of estrogen to decrease health risks caused by premature loss of estrogen need to be considered on an individual basis.9 On the basis of limited observational studies, consider offering systemic HT until the median age of menopause (52 years) with longer use individualized.3
Related Article:
Is menopausal hormone therapy safe when your patient carries a BRCA mutation?
Survivors of endometrial and breast cancer with bothersome VMS. For women with prior estrogen-sensitive cancers, non-HTs should be considered first, particularly those agents studied through randomized controlled trials in this population and found to be effective. If systemic estrogen is considered for persistent symptoms after non-HT or complementary options have been unsuccessful, decisions should be made for compelling reasons and after detailed counseling, with shared decision making and in conjunction with their oncologist.3
Bothersome GSM. On the basis of limited observational data, there appears to be minimal to no demonstrated elevation in risk for recurrence of endometrial or breast cancer using low-dose vaginal estrogen,3,10 but decisions should be made in conjunction with an oncologist.
Related Article:
Focus on treating genital atrophy symptoms
The importance of relaying the new guidelines to patients
It is important for clinicians to talk to women about their menopausal symptoms and their options for relief of symptoms or prevention of bone loss. Discussion should take into account age and time from menopause, include evidence-based information11-13 about benefits and risks of different types of therapy, and employ shared decision making to choose the most appropriate therapy to maximize benefits and minimize risks for the individual woman.
Following the WHI initial release in 2002, both women and providers became fearful of HT and believed media hype and celebrities that compounded bioidentical HT was safer than FDA-approved HTs. However, compounded products lack safety and efficacy data, are not monitored or regulated by the FDA, and have unique risks associated with compounding, including concerns about sterility, impurities, and overdosing or underdosing, which could increase cancer risk.3
- Hormone therapy for symptomatic menopausal women is safe and effective for those under age 60 or within 10 years of menopause.
- Identify the most appropriate type, dose, formulation, and duration of hormone therapy for an individual woman based on evidence.
- We want to remove the fear of using hormone therapy for healthy symptomatic women who are under age 60 or within 10 years of menopause.
- Age at initiation of hormone therapy matters.
- NAMS endorses use of FDA-approved hormone therapy over compounded therapies.
Read about modifying low-dose vaginal estrogen’s black box warning
Physicians continue to underwhelmingly prescribe low-dose vaginal estrogen for GSM
Kingsberg SA, Krychman M, Graham S, Bernick B, Mirkin S. The Women's EMPOWER survey: identifying women's perceptions on vulvar and vaginal atrophy and its treatment. J Sex Med. 2017;14(3):413-424.
GSM is seriously underrecognized and undertreated.2,8,14 It has a major impact on women's lives--a silent epidemic affecting women's quality of life, sexual health, interpersonal relationships, and even physical health in terms of increased risk of urinary tract infections and urinary symptoms. Unfortunately, patients are reluctant to mention the problem to their clinicians, and they do not clearly recognize it as a medical condition that has available treatment options. Clinicians also rarely receive adequate training in the management of this condition and how to discuss it with their patients. Given busy schedules and time constraints, addressing this topic often falls through the cracks, representing a missed opportunity for helping our patients with safe and effective treatments. In a recent study by Kingsberg and colleagues, an astoundingly low percentageof women with GSM symptoms received treatment.
Details of the study
The study authors evaluated women's perceptions of GSM and available treatment options. US women aged 45 and older who reported GSM symptoms were surveyed. Of 1,858 women with a median age of 58 (range, 45-90), the study authors found that 50% had never used any treatment; 25% used over-the-counter medications; 18% were former users of GSM treatments; and 7% currently used prescribed GSM therapies.
When GSM was discussed, women were more likely than their clinicians to initiate the conversation. The main reason for women not mentioning their symptoms was the perception that GSM symptoms were a natural and inevitable part of aging. Hormonal products were perceived by women as having several downsides, including risk of systemic absorption, messiness of local creams, and the need to reuse an applicator. Overall, clinicians recommended vaginal estrogen therapy to only 23% and oral HTs to 18% of women.
The results of the study are consistent with results of earlier surveys of menopausal women. Although the survey included nearly 2,000 women, it has the potential for selection biases inherent to most Internet-based surveys. In addition, the respondents tended to be white and have higher socieconomic status, with limited representation from other groups.
Calls for the current boxed warning to be revised
GSM is highly prevalent among postmenopausal women; the condition has adverse effects on quality of life and sexual health.2,8,14 Safe and effective treatments are available but are underutilized.1,8,15,16 A current boxed warning appears on low-dose vaginal estrogen--class labeling that appears on all medications in the class of estrogen or HT, regardless of dose or route of administration. These warnings are based on findings from the WHI and other studies of systemic estrogen or estrogen plus progestin, which demonstrated a complex pattern of risks and benefits of HT (including increased risk of venous thrombosis or pulmonary embolism, stroke, and breast cancer [with estrogen plus progestin]).
These findings, however, do not appear to be relevant to low-dose vaginal estrogen, given minimal if any systemic absorption and much lower blood levels of hormones than found with systemic HT. Blood levels of estradiol with low-dose vaginal estrogen remain in the normal postmenopausal range, compared to several-fold elevations in hormone levels with systemic HT.8,15,16 Additionally, observational studies of low-dose vaginal estrogen, as well as short-term randomized clinical trials, show no evidence of an increased risk of venous thromboembolic events, heart disease, stroke, breast cancer, or dementia--the listed possible adverse effects in the boxed warning. The current warning is based on extrapolating findings from systemic HT, which is inappropriate and not evidence-based for low-dose vaginal estrogen.15
The inappropriate boxed warning contributes to the problem of undertreatment of GSM in women by discouraging clinicians from prescribing the medication and dissuading patients from taking it even after purchase. Testimonials from many clinicians caring for these women have underscored that women will fill their prescription, but after seeing the boxed warning will often become alarmed and decide not to take the medication. Clinicians reported that patients often say at their next appointment: "No, I never took it. I got very scared when I saw the boxed warning." As a result, clinicians often have to spend a great deal of time explaining the limitations of, and lack of evidence for, the boxed warning on low-dose vaginal estrogen.
Related Article:
2016 Update on menopause
Recommended label revisions
A modified label, without a boxed warning, would be safer for women because the key messages would not be obscured by the large amount of irrelevant information. Our Working Group recommended that the label explain that the listed risks were found in studies of systemic HT and their relevance to low-dose vaginal estrogen is unknown. The Group also recommended that warning text should be added in bold font to advise patients to seek medical attention if they have vaginal bleeding or spotting while taking the medication. In addition, patients who have a history of breast cancer or other hormone-sensitive cancer should discuss the use of the medication with their oncologist.
Status update on efforts to revise label. A citizen's petition was filed in the Spring of 2016, with signatures from more than 600 clinicians and patients and representatives of medical and professional organizations endorsing a more appropriate evidence-based label for low-dose vaginal estrogen. The FDA is continuing to review and deliberate on these issues but has not yet made a final decision.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Manson JM, Kaunitz AM. Menopause management—Getting clinical care back on track. N Engl J Med. 2016;374(9):803–806.
- Parish SJ, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437–447.
- The 2017 hormone therapy position statement of The North American Menopause Society [published online ahead of print June 2017]. Menopause.
- Pinkerton JV. Hormone therapy: 2016 NAMS position statement [abstract]. Menopause. 2016;23:1365.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368.
- Lethaby A, Ayeleke RO, Roberts H. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Sys Rev. 2016;8:CD001500.
- Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause. 2013;20(9):888–902.
- Faubion SS, Kuhle CL, Shuster LT, Rocca WA. Long-term health consequences of premature or early menopause and considerations for management. Climacteric. 2015;18(4):483–491.
- Chai X, Domchek S, Kauff N, Rebbeck T, Chen J. RE: Breast cancer risk after salpingo-oophorectomy in healthy BRCA1/2 mutation carriers: revisiting the evidence for risk reduction. J Natl Cancer Inst. 2015;107(9).
- Farrell R; American College of Obstetricians and Gynecologists’ Committee on Gynecologic Practice. ACOG Committee Opinion No. 659 summary: The use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol. 2016;127(3):618–619.
- Hodis HN, Mack WJ, Henderson VW, et al; ELITE Research Group. Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med. 2016;374(13):1221–1231.
- Marjoribanks J, Farquhar C, Roberts H, Lethaby A, Lee J. Long-term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst Rev. 2017;1:CD004143.
- Boardman HM, Hartley L, Eisinga A, et al. Hormone therapy for preventing cardiovascular disease in post-menopausal women. Cochrane Database Syst Rev. 2015;(3):CD002229.
- Parish S, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437–447.
- Manson JE, Goldstein SR, Kagan R, et al; Working Group on Women’s Health and Well-Being in Menopause. Why the product labeling for low-dose vaginal estrogen should be changed. Menopause. 2014;21(9):911–916.
- Kaunitz AM, Manson JE. Management of menopausal symptoms. Obstet Gynecol. 2015;126(4):859-876.
- Manson JM, Kaunitz AM. Menopause management—Getting clinical care back on track. N Engl J Med. 2016;374(9):803–806.
- Parish SJ, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437–447.
- The 2017 hormone therapy position statement of The North American Menopause Society [published online ahead of print June 2017]. Menopause.
- Pinkerton JV. Hormone therapy: 2016 NAMS position statement [abstract]. Menopause. 2016;23:1365.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368.
- Lethaby A, Ayeleke RO, Roberts H. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Sys Rev. 2016;8:CD001500.
- Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause. 2013;20(9):888–902.
- Faubion SS, Kuhle CL, Shuster LT, Rocca WA. Long-term health consequences of premature or early menopause and considerations for management. Climacteric. 2015;18(4):483–491.
- Chai X, Domchek S, Kauff N, Rebbeck T, Chen J. RE: Breast cancer risk after salpingo-oophorectomy in healthy BRCA1/2 mutation carriers: revisiting the evidence for risk reduction. J Natl Cancer Inst. 2015;107(9).
- Farrell R; American College of Obstetricians and Gynecologists’ Committee on Gynecologic Practice. ACOG Committee Opinion No. 659 summary: The use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol. 2016;127(3):618–619.
- Hodis HN, Mack WJ, Henderson VW, et al; ELITE Research Group. Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med. 2016;374(13):1221–1231.
- Marjoribanks J, Farquhar C, Roberts H, Lethaby A, Lee J. Long-term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst Rev. 2017;1:CD004143.
- Boardman HM, Hartley L, Eisinga A, et al. Hormone therapy for preventing cardiovascular disease in post-menopausal women. Cochrane Database Syst Rev. 2015;(3):CD002229.
- Parish S, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437–447.
- Manson JE, Goldstein SR, Kagan R, et al; Working Group on Women’s Health and Well-Being in Menopause. Why the product labeling for low-dose vaginal estrogen should be changed. Menopause. 2014;21(9):911–916.
- Kaunitz AM, Manson JE. Management of menopausal symptoms. Obstet Gynecol. 2015;126(4):859-876.
Romosozumab cuts new vertebral fracture risk by 73%, but safety data are concerning
MADRID – Romosozumab, an investigational bone-building agent, reduced new vertebral fractures by 73% among postmenopausal women with osteoporosis.
Compared with placebo, the monoclonal antibody also reduced the risk of a clinical fracture by 36% after 12 months of treatment, Piet Geusens, MD, said at the European Congress of Rheumatology. The effect was maintained at 24 months, with a 50% reduction in fracture risk, said Dr. Geusens of Maastricht University, the Netherlands.
Romosozumab also significantly reduced clinical and nonvertebral fractures and increased bone mineral density at the total hip, femoral neck, and lumbar spine in the phase III FRAME study, cosponsored by Amgen and UCB Pharma.
But recently, the finding of increased cardiovascular events in another highly anticipated phase III study of romosozumab cast a cloud of doubt over its rising star. During an interview at EULAR, a UCB company spokesman said the company no longer anticipates a 2017 Food and Drug Administration approval.
FRAME randomized 7,180 postmenopausal women with osteoporosis to monthly injections of romosozumab 210 mg or placebo for 12 months; after that, patients who had been taking placebo switched to denosumab. Dr. Geusens presented only the first year’s placebo-controlled portion. The full FRAME study was published in the New England Journal of Medicine last September (doi: 10.1056/NEJMoa1607948).
At baseline, the women had a mean bone mineral density T score of –2.7 at the lumbar spine, –2.4 at the total hip, and –2.7 at the femoral neck. Mean age was 71 years. About 20% of the women had a previous vertebral fracture, and 22% a previous nonvertebral fracture. The mean FRAX (fracture risk assessment tool) score was 13.4 in both groups.
After 12 months, a new vertebral fracture had occurred in 59 women taking placebo and 16 taking romosozumab (1.8% vs. 0.5%). This amounted to a 73% risk reduction, Dr. Geusens said. Although he did not present 24-month data, the published article cited the antibody’s sustained effect, with a 50% risk reduction evident after the 12-month comparison with denosumab.
The drug also exerted its benefit quickly, Dr. Geusens said. Most of the fractures in the active group occurred in the first 6 months of treatment, with only two additional fractures later.
Romosozumab also was associated with a 36% decrease in the risk of clinical fracture by 12 months (1.6% vs. 2.5% placebo). There was also a positive effect on nonvertebral fractures, which constituted more than 85% of the clinical fractures. Nonvertebral fractures occurred in 56 of those taking the antibody and 75 of those taking placebo (1.6% vs. 2.1%; hazard ratio [HR], .75).
By 12 months, bone mineral density had increased in the romosozumab group by 13% more than in the placebo group at the lumbar spine, by 7% more at the total hip, and by 6% more at the femoral neck.
Dr. Geusens did not address the adverse event profile during his talk. However, according to the published study, romosozumab was generally well tolerated. Serious hypersensitivity events occurred in seven romosozumab patients. Injection site reactions were mostly mild and occurred in 5% of the active group and 3% of the placebo group.
Two patients taking romosozumab experienced osteonecrosis of the jaw; both incidences occurred during the second 12 months and in conjunction with dental issues (tooth extraction and poorly fitted dentures). Anti-romosozumab antibodies developed in 18% and neutralizing antibodies in 0.7%.
Serious cardiovascular events occurred in about 1% of each treatment group, with 17 among those taking romosozumab and15 cardiovascular deaths among those taking placebo – not a significant difference.
UCB and Amgen were pleased with FRAME’s results and, last July, submitted a Biologics License Application to the FDA based on the positive data. A 2017 approval was anticipated, UCB spokesman Scott Fleming said in an interview. But in May, the primary safety analysis of another phase III study, ARCH, threw a monkey wrench in the works.
ARCH compared romosozumab to alendronate in 4,100 postmenopausal women with osteoporosis. ARCH met its primary and secondary endpoints, reducing the incidence of new vertebral fractures by 50%, clinical fractures by 27%, and nonvertebral fractures by 19%. But significantly more women taking the antibody experienced an adjudicated serious cardiovascular event (2.5% vs. 1.9% on alendronate).
On May 21, the companies said these new data would delay romosozumab’s progress toward approval, despite the fact that the submission was based on FRAMES’s positive safety and efficacy data.
Mr. Fleming confirmed this in an interview.
“Amgen has agreed with the FDA that the ARCH data should be considered in the regulatory review prior to the initial marketing authorization, and as a result we do not expect approval of romosozumab in the U.S. to occur in 2017,” he said. “Patient safety is of utmost importance and whilst the cardiac imbalance observed in ARCH was not seen in FRAME, it is important and our responsibility to better understand this imbalance. Further analysis of the ARCH study data is ongoing and will be submitted to a future medical conference and for publication.”
Dr. Geusens refused to comment on the cardiovascular adverse events, saying he had not seen the ARCH data; nor did he explain the cardiovascular events that did occur in FRAME.
Romosozumab also is being reviewed in Canada and Japan; those processes are still underway. Mr. Fleming said the companies are preparing a European Medicines Agency application as well. “The preparation for the European regulatory submission will continue as planned – second half of 2017,” he said.
Dr. Geusens has received research support from Amgen and other pharmaceutical companies. He is a consultant for Amgen and a member of its speakers bureau.
[email protected]
On Twitter @Alz_gal
MADRID – Romosozumab, an investigational bone-building agent, reduced new vertebral fractures by 73% among postmenopausal women with osteoporosis.
Compared with placebo, the monoclonal antibody also reduced the risk of a clinical fracture by 36% after 12 months of treatment, Piet Geusens, MD, said at the European Congress of Rheumatology. The effect was maintained at 24 months, with a 50% reduction in fracture risk, said Dr. Geusens of Maastricht University, the Netherlands.
Romosozumab also significantly reduced clinical and nonvertebral fractures and increased bone mineral density at the total hip, femoral neck, and lumbar spine in the phase III FRAME study, cosponsored by Amgen and UCB Pharma.
But recently, the finding of increased cardiovascular events in another highly anticipated phase III study of romosozumab cast a cloud of doubt over its rising star. During an interview at EULAR, a UCB company spokesman said the company no longer anticipates a 2017 Food and Drug Administration approval.
FRAME randomized 7,180 postmenopausal women with osteoporosis to monthly injections of romosozumab 210 mg or placebo for 12 months; after that, patients who had been taking placebo switched to denosumab. Dr. Geusens presented only the first year’s placebo-controlled portion. The full FRAME study was published in the New England Journal of Medicine last September (doi: 10.1056/NEJMoa1607948).
At baseline, the women had a mean bone mineral density T score of –2.7 at the lumbar spine, –2.4 at the total hip, and –2.7 at the femoral neck. Mean age was 71 years. About 20% of the women had a previous vertebral fracture, and 22% a previous nonvertebral fracture. The mean FRAX (fracture risk assessment tool) score was 13.4 in both groups.
After 12 months, a new vertebral fracture had occurred in 59 women taking placebo and 16 taking romosozumab (1.8% vs. 0.5%). This amounted to a 73% risk reduction, Dr. Geusens said. Although he did not present 24-month data, the published article cited the antibody’s sustained effect, with a 50% risk reduction evident after the 12-month comparison with denosumab.
The drug also exerted its benefit quickly, Dr. Geusens said. Most of the fractures in the active group occurred in the first 6 months of treatment, with only two additional fractures later.
Romosozumab also was associated with a 36% decrease in the risk of clinical fracture by 12 months (1.6% vs. 2.5% placebo). There was also a positive effect on nonvertebral fractures, which constituted more than 85% of the clinical fractures. Nonvertebral fractures occurred in 56 of those taking the antibody and 75 of those taking placebo (1.6% vs. 2.1%; hazard ratio [HR], .75).
By 12 months, bone mineral density had increased in the romosozumab group by 13% more than in the placebo group at the lumbar spine, by 7% more at the total hip, and by 6% more at the femoral neck.
Dr. Geusens did not address the adverse event profile during his talk. However, according to the published study, romosozumab was generally well tolerated. Serious hypersensitivity events occurred in seven romosozumab patients. Injection site reactions were mostly mild and occurred in 5% of the active group and 3% of the placebo group.
Two patients taking romosozumab experienced osteonecrosis of the jaw; both incidences occurred during the second 12 months and in conjunction with dental issues (tooth extraction and poorly fitted dentures). Anti-romosozumab antibodies developed in 18% and neutralizing antibodies in 0.7%.
Serious cardiovascular events occurred in about 1% of each treatment group, with 17 among those taking romosozumab and15 cardiovascular deaths among those taking placebo – not a significant difference.
UCB and Amgen were pleased with FRAME’s results and, last July, submitted a Biologics License Application to the FDA based on the positive data. A 2017 approval was anticipated, UCB spokesman Scott Fleming said in an interview. But in May, the primary safety analysis of another phase III study, ARCH, threw a monkey wrench in the works.
ARCH compared romosozumab to alendronate in 4,100 postmenopausal women with osteoporosis. ARCH met its primary and secondary endpoints, reducing the incidence of new vertebral fractures by 50%, clinical fractures by 27%, and nonvertebral fractures by 19%. But significantly more women taking the antibody experienced an adjudicated serious cardiovascular event (2.5% vs. 1.9% on alendronate).
On May 21, the companies said these new data would delay romosozumab’s progress toward approval, despite the fact that the submission was based on FRAMES’s positive safety and efficacy data.
Mr. Fleming confirmed this in an interview.
“Amgen has agreed with the FDA that the ARCH data should be considered in the regulatory review prior to the initial marketing authorization, and as a result we do not expect approval of romosozumab in the U.S. to occur in 2017,” he said. “Patient safety is of utmost importance and whilst the cardiac imbalance observed in ARCH was not seen in FRAME, it is important and our responsibility to better understand this imbalance. Further analysis of the ARCH study data is ongoing and will be submitted to a future medical conference and for publication.”
Dr. Geusens refused to comment on the cardiovascular adverse events, saying he had not seen the ARCH data; nor did he explain the cardiovascular events that did occur in FRAME.
Romosozumab also is being reviewed in Canada and Japan; those processes are still underway. Mr. Fleming said the companies are preparing a European Medicines Agency application as well. “The preparation for the European regulatory submission will continue as planned – second half of 2017,” he said.
Dr. Geusens has received research support from Amgen and other pharmaceutical companies. He is a consultant for Amgen and a member of its speakers bureau.
[email protected]
On Twitter @Alz_gal
MADRID – Romosozumab, an investigational bone-building agent, reduced new vertebral fractures by 73% among postmenopausal women with osteoporosis.
Compared with placebo, the monoclonal antibody also reduced the risk of a clinical fracture by 36% after 12 months of treatment, Piet Geusens, MD, said at the European Congress of Rheumatology. The effect was maintained at 24 months, with a 50% reduction in fracture risk, said Dr. Geusens of Maastricht University, the Netherlands.
Romosozumab also significantly reduced clinical and nonvertebral fractures and increased bone mineral density at the total hip, femoral neck, and lumbar spine in the phase III FRAME study, cosponsored by Amgen and UCB Pharma.
But recently, the finding of increased cardiovascular events in another highly anticipated phase III study of romosozumab cast a cloud of doubt over its rising star. During an interview at EULAR, a UCB company spokesman said the company no longer anticipates a 2017 Food and Drug Administration approval.
FRAME randomized 7,180 postmenopausal women with osteoporosis to monthly injections of romosozumab 210 mg or placebo for 12 months; after that, patients who had been taking placebo switched to denosumab. Dr. Geusens presented only the first year’s placebo-controlled portion. The full FRAME study was published in the New England Journal of Medicine last September (doi: 10.1056/NEJMoa1607948).
At baseline, the women had a mean bone mineral density T score of –2.7 at the lumbar spine, –2.4 at the total hip, and –2.7 at the femoral neck. Mean age was 71 years. About 20% of the women had a previous vertebral fracture, and 22% a previous nonvertebral fracture. The mean FRAX (fracture risk assessment tool) score was 13.4 in both groups.
After 12 months, a new vertebral fracture had occurred in 59 women taking placebo and 16 taking romosozumab (1.8% vs. 0.5%). This amounted to a 73% risk reduction, Dr. Geusens said. Although he did not present 24-month data, the published article cited the antibody’s sustained effect, with a 50% risk reduction evident after the 12-month comparison with denosumab.
The drug also exerted its benefit quickly, Dr. Geusens said. Most of the fractures in the active group occurred in the first 6 months of treatment, with only two additional fractures later.
Romosozumab also was associated with a 36% decrease in the risk of clinical fracture by 12 months (1.6% vs. 2.5% placebo). There was also a positive effect on nonvertebral fractures, which constituted more than 85% of the clinical fractures. Nonvertebral fractures occurred in 56 of those taking the antibody and 75 of those taking placebo (1.6% vs. 2.1%; hazard ratio [HR], .75).
By 12 months, bone mineral density had increased in the romosozumab group by 13% more than in the placebo group at the lumbar spine, by 7% more at the total hip, and by 6% more at the femoral neck.
Dr. Geusens did not address the adverse event profile during his talk. However, according to the published study, romosozumab was generally well tolerated. Serious hypersensitivity events occurred in seven romosozumab patients. Injection site reactions were mostly mild and occurred in 5% of the active group and 3% of the placebo group.
Two patients taking romosozumab experienced osteonecrosis of the jaw; both incidences occurred during the second 12 months and in conjunction with dental issues (tooth extraction and poorly fitted dentures). Anti-romosozumab antibodies developed in 18% and neutralizing antibodies in 0.7%.
Serious cardiovascular events occurred in about 1% of each treatment group, with 17 among those taking romosozumab and15 cardiovascular deaths among those taking placebo – not a significant difference.
UCB and Amgen were pleased with FRAME’s results and, last July, submitted a Biologics License Application to the FDA based on the positive data. A 2017 approval was anticipated, UCB spokesman Scott Fleming said in an interview. But in May, the primary safety analysis of another phase III study, ARCH, threw a monkey wrench in the works.
ARCH compared romosozumab to alendronate in 4,100 postmenopausal women with osteoporosis. ARCH met its primary and secondary endpoints, reducing the incidence of new vertebral fractures by 50%, clinical fractures by 27%, and nonvertebral fractures by 19%. But significantly more women taking the antibody experienced an adjudicated serious cardiovascular event (2.5% vs. 1.9% on alendronate).
On May 21, the companies said these new data would delay romosozumab’s progress toward approval, despite the fact that the submission was based on FRAMES’s positive safety and efficacy data.
Mr. Fleming confirmed this in an interview.
“Amgen has agreed with the FDA that the ARCH data should be considered in the regulatory review prior to the initial marketing authorization, and as a result we do not expect approval of romosozumab in the U.S. to occur in 2017,” he said. “Patient safety is of utmost importance and whilst the cardiac imbalance observed in ARCH was not seen in FRAME, it is important and our responsibility to better understand this imbalance. Further analysis of the ARCH study data is ongoing and will be submitted to a future medical conference and for publication.”
Dr. Geusens refused to comment on the cardiovascular adverse events, saying he had not seen the ARCH data; nor did he explain the cardiovascular events that did occur in FRAME.
Romosozumab also is being reviewed in Canada and Japan; those processes are still underway. Mr. Fleming said the companies are preparing a European Medicines Agency application as well. “The preparation for the European regulatory submission will continue as planned – second half of 2017,” he said.
Dr. Geusens has received research support from Amgen and other pharmaceutical companies. He is a consultant for Amgen and a member of its speakers bureau.
[email protected]
On Twitter @Alz_gal
AT THE EULAR 2017 CONGRESS
Key clinical point:
Major finding: Compared with placebo, the antibody reduced the risk of a new-onset vertebral fracture by 73% over 1 year.
Data source: FRAME, which randomized 7,180 women to monthly injections of 210 mg romosozumab or placebo for 12 months; for an additional 12 months, those taking placebo switched to denosumab.
Disclosures: Amgen and UCB Pharma are codeveloping the drug. Dr. Geusens is a consultant and speaker for Amgen, and has received research from the company.
Endometriosis after menopause: Weigh the treatment risks
VANCOUVER – Endometriosis, while generally considered a premenopausal condition, can also occur in women following surgical or natural menopause, and can undergo malignant transformation, although this risk is likely very small.
That was the main message from a new meta-analysis presented at the World Congress on Endometriosis. “We wanted to synthesize the case reports out there to show some common factors so physicians can be aware of them,” said Laura Gemmell, a second-year medical student at Case Western Reserve University, Cleveland, who presented the research.
The researchers surveyed the literature for studies in postmenopausal women with a confirmed or clinically suspected history of endometriosis, and who discussed the management of their menopausal symptoms. They included 33 case reports and case series (42 patients, 36 surgical menopause, 4 natural, 2 presumed natural with later oophorectomy), as well as 6 observational studies and clinical trials.
In the case reports, patients were on HT for a mean of 7.8 years, and 17 of 42 women experienced a recurrence of endometriosis. Also, 25 women had a malignant transformation and there was some overlap with the recurrence group.
Among 17 patients with recurrence, 6 had “severe” or “extensive” endometriosis, and 14 had surgical menopause, with a mean of 7.1 years between surgical menopause and presentation. Twelve of 17 received unopposed estrogen. Following surgical excision (16 of 17 cases), 10 had symptom regression without relapses.
When the researchers looked at the 25 cases of malignant transformation, they found that 13 women had endometriosis at more than one site, 22 had undergone surgical menopause, 19 were on unopposed estrogen, and the mean duration of HT was 6.7 years. Seven women presented with vaginal bleeding and nine with masses. Three died from the disease. These three women had severe endometriosis complicating factors, including older age and multiple malignancies.
The analysis also included six observational studies and clinical trials that explored recurrence of endometriosis, and whether HT should be given to women with a history of endometriosis, whether it should be given immediately after surgical menopause, and the most appropriate menopause treatments.
Predictably, the evidence could not be summed up neatly, but Ms. Gemmell emphasized the need to individually weigh the risks and benefits of HT in each patient, with consideration of characteristics such as age, previous disease severity, family history, comorbidities, and body mass index.
She also suggested that patients should be active participants in decision making.
Finally, if the decision is to go forward or continue with HT, she suggested that clinicians consider a combined treatment rather than estrogen-only, though she pointed out the increased risk for breast cancer that this presents.
Tommaso Falcone, MD, chairman of obstetrics and gynecology at the Cleveland Clinic, sounded a note of caution about the use of progestins during the question-and-answer session. “The data are not strong that it actually prevents the development of cancer in the residual disease, if there is any. Even if you take the hypothesis that progestins are going to prevent cancer of residual disease, which is a low-level risk, the main worry that women have is breast cancer, and progestin is strongly associated with breast cancer,” Dr. Falcone said in an interview.
Ms. Gemmell and Dr. Falcone reported having no financial disclosures.
VANCOUVER – Endometriosis, while generally considered a premenopausal condition, can also occur in women following surgical or natural menopause, and can undergo malignant transformation, although this risk is likely very small.
That was the main message from a new meta-analysis presented at the World Congress on Endometriosis. “We wanted to synthesize the case reports out there to show some common factors so physicians can be aware of them,” said Laura Gemmell, a second-year medical student at Case Western Reserve University, Cleveland, who presented the research.
The researchers surveyed the literature for studies in postmenopausal women with a confirmed or clinically suspected history of endometriosis, and who discussed the management of their menopausal symptoms. They included 33 case reports and case series (42 patients, 36 surgical menopause, 4 natural, 2 presumed natural with later oophorectomy), as well as 6 observational studies and clinical trials.
In the case reports, patients were on HT for a mean of 7.8 years, and 17 of 42 women experienced a recurrence of endometriosis. Also, 25 women had a malignant transformation and there was some overlap with the recurrence group.
Among 17 patients with recurrence, 6 had “severe” or “extensive” endometriosis, and 14 had surgical menopause, with a mean of 7.1 years between surgical menopause and presentation. Twelve of 17 received unopposed estrogen. Following surgical excision (16 of 17 cases), 10 had symptom regression without relapses.
When the researchers looked at the 25 cases of malignant transformation, they found that 13 women had endometriosis at more than one site, 22 had undergone surgical menopause, 19 were on unopposed estrogen, and the mean duration of HT was 6.7 years. Seven women presented with vaginal bleeding and nine with masses. Three died from the disease. These three women had severe endometriosis complicating factors, including older age and multiple malignancies.
The analysis also included six observational studies and clinical trials that explored recurrence of endometriosis, and whether HT should be given to women with a history of endometriosis, whether it should be given immediately after surgical menopause, and the most appropriate menopause treatments.
Predictably, the evidence could not be summed up neatly, but Ms. Gemmell emphasized the need to individually weigh the risks and benefits of HT in each patient, with consideration of characteristics such as age, previous disease severity, family history, comorbidities, and body mass index.
She also suggested that patients should be active participants in decision making.
Finally, if the decision is to go forward or continue with HT, she suggested that clinicians consider a combined treatment rather than estrogen-only, though she pointed out the increased risk for breast cancer that this presents.
Tommaso Falcone, MD, chairman of obstetrics and gynecology at the Cleveland Clinic, sounded a note of caution about the use of progestins during the question-and-answer session. “The data are not strong that it actually prevents the development of cancer in the residual disease, if there is any. Even if you take the hypothesis that progestins are going to prevent cancer of residual disease, which is a low-level risk, the main worry that women have is breast cancer, and progestin is strongly associated with breast cancer,” Dr. Falcone said in an interview.
Ms. Gemmell and Dr. Falcone reported having no financial disclosures.
VANCOUVER – Endometriosis, while generally considered a premenopausal condition, can also occur in women following surgical or natural menopause, and can undergo malignant transformation, although this risk is likely very small.
That was the main message from a new meta-analysis presented at the World Congress on Endometriosis. “We wanted to synthesize the case reports out there to show some common factors so physicians can be aware of them,” said Laura Gemmell, a second-year medical student at Case Western Reserve University, Cleveland, who presented the research.
The researchers surveyed the literature for studies in postmenopausal women with a confirmed or clinically suspected history of endometriosis, and who discussed the management of their menopausal symptoms. They included 33 case reports and case series (42 patients, 36 surgical menopause, 4 natural, 2 presumed natural with later oophorectomy), as well as 6 observational studies and clinical trials.
In the case reports, patients were on HT for a mean of 7.8 years, and 17 of 42 women experienced a recurrence of endometriosis. Also, 25 women had a malignant transformation and there was some overlap with the recurrence group.
Among 17 patients with recurrence, 6 had “severe” or “extensive” endometriosis, and 14 had surgical menopause, with a mean of 7.1 years between surgical menopause and presentation. Twelve of 17 received unopposed estrogen. Following surgical excision (16 of 17 cases), 10 had symptom regression without relapses.
When the researchers looked at the 25 cases of malignant transformation, they found that 13 women had endometriosis at more than one site, 22 had undergone surgical menopause, 19 were on unopposed estrogen, and the mean duration of HT was 6.7 years. Seven women presented with vaginal bleeding and nine with masses. Three died from the disease. These three women had severe endometriosis complicating factors, including older age and multiple malignancies.
The analysis also included six observational studies and clinical trials that explored recurrence of endometriosis, and whether HT should be given to women with a history of endometriosis, whether it should be given immediately after surgical menopause, and the most appropriate menopause treatments.
Predictably, the evidence could not be summed up neatly, but Ms. Gemmell emphasized the need to individually weigh the risks and benefits of HT in each patient, with consideration of characteristics such as age, previous disease severity, family history, comorbidities, and body mass index.
She also suggested that patients should be active participants in decision making.
Finally, if the decision is to go forward or continue with HT, she suggested that clinicians consider a combined treatment rather than estrogen-only, though she pointed out the increased risk for breast cancer that this presents.
Tommaso Falcone, MD, chairman of obstetrics and gynecology at the Cleveland Clinic, sounded a note of caution about the use of progestins during the question-and-answer session. “The data are not strong that it actually prevents the development of cancer in the residual disease, if there is any. Even if you take the hypothesis that progestins are going to prevent cancer of residual disease, which is a low-level risk, the main worry that women have is breast cancer, and progestin is strongly associated with breast cancer,” Dr. Falcone said in an interview.
Ms. Gemmell and Dr. Falcone reported having no financial disclosures.
EXPERT ANALYSIS AT WCE 2017