User login
Premature menopause a ‘warning sign’ for greater ASCVD risk
Premature menopause is well known to be linked to cardiovascular disease in women, but it may not carry as much weight as more traditional cardiovascular risk factors in determining a patient’s 10-year risk of having a heart attack or stroke in this population, a cohort study that evaluated the veracity of premature menopause found.
Premature menopause can serve as a “marker or warning sign” that cardiologists should pay closer attention to traditional atherosclerotic cardiovascular disease (ASCVD) risk factors, lead study author Sadiya S. Khan, MD, MS, said in an interview. “When we looked at the addition of premature menopause into the risk-prediction equation, we did not see that it meaningfully improved the ability of the risk predictions of pooled cohort equations [PCEs] to identify who developed cardiovascular disease,” said Dr. Khan, a cardiologist at Northwestern University, Chicago.
The cohort study included 5,466 Black women and 10,584 White women from seven U.S. population-based cohorts, including the Women’s Health Initiative, of whom 951 and 1,039, respectively, self-reported early menopause. The cohort study researchers noted that the 2019 American College of Cardiology/American Heart Association guideline for prevention of CVD acknowledged premature menopause as risk-enhancing factor in the CVD assessment in women younger than 40.
The cohort study found that Black women had almost twice the rate of premature menopause than White women, 17.4% and 9.8%, respectively. And it found that premature menopause was significantly linked with ASCVD in both populations independent of traditional risk factors – a 24% greater risk for Black women and 28% greater risk for White women.
‘Surprising’ finding
However, when premature menopause was added to the pooled cohort equations per the 2013 ACC/AHA guideline, the researchers found no incremental benefit, a finding Dr. Khan called “really surprising to us.”
She added, “If we look at the differences in the characteristics of women who have premature menopause, compared with those who didn’t, there were slight differences in terms of higher blood pressure, higher body mass index, and slightly higher glucose. So maybe what we’re seeing – and this is more speculative – is that risk factors are developing after early menopause, and the focus should be earlier in the patient’s life course to try to prevent hypertension, diabetes, and obesity.”
Dr. Khan emphasized that the findings don’t obviate the value of premature menopause in assessing ASCVD risk in women. “We still know that this is an important marker for women and their risk for heart disease, and it should be a warning sign to pay close attention to those other risk factors and what other preventive measures can be taken,” she said.
Christie Ballantyne, MD, said it’s important to note that the study did not dismiss the relevance of premature menopause in shared decision-making for postmenopausal women. “It certainly doesn’t mean that premature menopause is not a risk,” Dr. Ballantyne said in an interview. “Premature menopause may cause a worsening of traditional CVD risk factors, so that’s one possible explanation for it. The other possible explanation is that women with worse ASCVD risk factors – who are more overweight, have higher blood pressure, and have more diabetes and insulin resistance – are more likely to have earlier menopause.” Dr. Ballantyne is chief of cardiology at Baylor College of Medicine and director of cardiovascular disease prevention at Methodist DeBakey Heart Center, both in Houston.
“You should still look very carefully at the patient’s risk factors, calculate the pooled cohort equations, and make sure you get a recommendation,” he said. “If their risks are up, give recommendations on how to improve diet and exercise. Consider if you need to treat lipids or treat blood pressure with more than diet and exercise because there’s nothing magical about 7.5%”, the threshold for lipid-lowering therapy in the ASCVD risk calculator.
Dr. Khan and coauthors disclosed receiving grants from the National Institutes of Health and the American Heart Association. One coauthor reported a financial relationship with HGM Biopharmaceuticals. Dr. Ballantyne is a lead investigator of the Atherosclerosis Risk in Communities study, one of the population-based cohorts used in the cohort study. He has no other relevant relationships to disclose.
Premature menopause is well known to be linked to cardiovascular disease in women, but it may not carry as much weight as more traditional cardiovascular risk factors in determining a patient’s 10-year risk of having a heart attack or stroke in this population, a cohort study that evaluated the veracity of premature menopause found.
Premature menopause can serve as a “marker or warning sign” that cardiologists should pay closer attention to traditional atherosclerotic cardiovascular disease (ASCVD) risk factors, lead study author Sadiya S. Khan, MD, MS, said in an interview. “When we looked at the addition of premature menopause into the risk-prediction equation, we did not see that it meaningfully improved the ability of the risk predictions of pooled cohort equations [PCEs] to identify who developed cardiovascular disease,” said Dr. Khan, a cardiologist at Northwestern University, Chicago.
The cohort study included 5,466 Black women and 10,584 White women from seven U.S. population-based cohorts, including the Women’s Health Initiative, of whom 951 and 1,039, respectively, self-reported early menopause. The cohort study researchers noted that the 2019 American College of Cardiology/American Heart Association guideline for prevention of CVD acknowledged premature menopause as risk-enhancing factor in the CVD assessment in women younger than 40.
The cohort study found that Black women had almost twice the rate of premature menopause than White women, 17.4% and 9.8%, respectively. And it found that premature menopause was significantly linked with ASCVD in both populations independent of traditional risk factors – a 24% greater risk for Black women and 28% greater risk for White women.
‘Surprising’ finding
However, when premature menopause was added to the pooled cohort equations per the 2013 ACC/AHA guideline, the researchers found no incremental benefit, a finding Dr. Khan called “really surprising to us.”
She added, “If we look at the differences in the characteristics of women who have premature menopause, compared with those who didn’t, there were slight differences in terms of higher blood pressure, higher body mass index, and slightly higher glucose. So maybe what we’re seeing – and this is more speculative – is that risk factors are developing after early menopause, and the focus should be earlier in the patient’s life course to try to prevent hypertension, diabetes, and obesity.”
Dr. Khan emphasized that the findings don’t obviate the value of premature menopause in assessing ASCVD risk in women. “We still know that this is an important marker for women and their risk for heart disease, and it should be a warning sign to pay close attention to those other risk factors and what other preventive measures can be taken,” she said.
Christie Ballantyne, MD, said it’s important to note that the study did not dismiss the relevance of premature menopause in shared decision-making for postmenopausal women. “It certainly doesn’t mean that premature menopause is not a risk,” Dr. Ballantyne said in an interview. “Premature menopause may cause a worsening of traditional CVD risk factors, so that’s one possible explanation for it. The other possible explanation is that women with worse ASCVD risk factors – who are more overweight, have higher blood pressure, and have more diabetes and insulin resistance – are more likely to have earlier menopause.” Dr. Ballantyne is chief of cardiology at Baylor College of Medicine and director of cardiovascular disease prevention at Methodist DeBakey Heart Center, both in Houston.
“You should still look very carefully at the patient’s risk factors, calculate the pooled cohort equations, and make sure you get a recommendation,” he said. “If their risks are up, give recommendations on how to improve diet and exercise. Consider if you need to treat lipids or treat blood pressure with more than diet and exercise because there’s nothing magical about 7.5%”, the threshold for lipid-lowering therapy in the ASCVD risk calculator.
Dr. Khan and coauthors disclosed receiving grants from the National Institutes of Health and the American Heart Association. One coauthor reported a financial relationship with HGM Biopharmaceuticals. Dr. Ballantyne is a lead investigator of the Atherosclerosis Risk in Communities study, one of the population-based cohorts used in the cohort study. He has no other relevant relationships to disclose.
Premature menopause is well known to be linked to cardiovascular disease in women, but it may not carry as much weight as more traditional cardiovascular risk factors in determining a patient’s 10-year risk of having a heart attack or stroke in this population, a cohort study that evaluated the veracity of premature menopause found.
Premature menopause can serve as a “marker or warning sign” that cardiologists should pay closer attention to traditional atherosclerotic cardiovascular disease (ASCVD) risk factors, lead study author Sadiya S. Khan, MD, MS, said in an interview. “When we looked at the addition of premature menopause into the risk-prediction equation, we did not see that it meaningfully improved the ability of the risk predictions of pooled cohort equations [PCEs] to identify who developed cardiovascular disease,” said Dr. Khan, a cardiologist at Northwestern University, Chicago.
The cohort study included 5,466 Black women and 10,584 White women from seven U.S. population-based cohorts, including the Women’s Health Initiative, of whom 951 and 1,039, respectively, self-reported early menopause. The cohort study researchers noted that the 2019 American College of Cardiology/American Heart Association guideline for prevention of CVD acknowledged premature menopause as risk-enhancing factor in the CVD assessment in women younger than 40.
The cohort study found that Black women had almost twice the rate of premature menopause than White women, 17.4% and 9.8%, respectively. And it found that premature menopause was significantly linked with ASCVD in both populations independent of traditional risk factors – a 24% greater risk for Black women and 28% greater risk for White women.
‘Surprising’ finding
However, when premature menopause was added to the pooled cohort equations per the 2013 ACC/AHA guideline, the researchers found no incremental benefit, a finding Dr. Khan called “really surprising to us.”
She added, “If we look at the differences in the characteristics of women who have premature menopause, compared with those who didn’t, there were slight differences in terms of higher blood pressure, higher body mass index, and slightly higher glucose. So maybe what we’re seeing – and this is more speculative – is that risk factors are developing after early menopause, and the focus should be earlier in the patient’s life course to try to prevent hypertension, diabetes, and obesity.”
Dr. Khan emphasized that the findings don’t obviate the value of premature menopause in assessing ASCVD risk in women. “We still know that this is an important marker for women and their risk for heart disease, and it should be a warning sign to pay close attention to those other risk factors and what other preventive measures can be taken,” she said.
Christie Ballantyne, MD, said it’s important to note that the study did not dismiss the relevance of premature menopause in shared decision-making for postmenopausal women. “It certainly doesn’t mean that premature menopause is not a risk,” Dr. Ballantyne said in an interview. “Premature menopause may cause a worsening of traditional CVD risk factors, so that’s one possible explanation for it. The other possible explanation is that women with worse ASCVD risk factors – who are more overweight, have higher blood pressure, and have more diabetes and insulin resistance – are more likely to have earlier menopause.” Dr. Ballantyne is chief of cardiology at Baylor College of Medicine and director of cardiovascular disease prevention at Methodist DeBakey Heart Center, both in Houston.
“You should still look very carefully at the patient’s risk factors, calculate the pooled cohort equations, and make sure you get a recommendation,” he said. “If their risks are up, give recommendations on how to improve diet and exercise. Consider if you need to treat lipids or treat blood pressure with more than diet and exercise because there’s nothing magical about 7.5%”, the threshold for lipid-lowering therapy in the ASCVD risk calculator.
Dr. Khan and coauthors disclosed receiving grants from the National Institutes of Health and the American Heart Association. One coauthor reported a financial relationship with HGM Biopharmaceuticals. Dr. Ballantyne is a lead investigator of the Atherosclerosis Risk in Communities study, one of the population-based cohorts used in the cohort study. He has no other relevant relationships to disclose.
FROM JAMA CARDIOLOGY
2021 Update on female sexual health
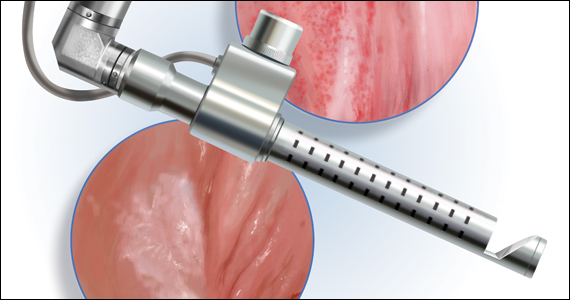
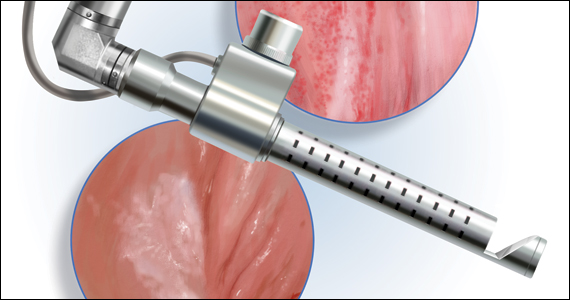
The approach to diagnosis and treatment of female sexual function continues to be a challenge for women’s health professionals. The search for a female “little blue pill” remains elusive as researchers struggle to understand the mechanisms that underlie the complex aspects of female sexual health. This Update will review the recent literature on the use of fractional CO2 laser for treatment of female sexual dysfunction and vulvovaginal symptoms. Bottom line: While the quality of the studies is poor overall, fractional CO2 laser treatment seems to temporarily improve symptoms of genitourinary syndrome of menopause (GSM). The duration of response, cost, and the overall long-term impact on sexual health remain in question.
A retrospective look at CO2 laser and postmenopausal GSM
Filippini M, Luvero D, Salvatore S, et al. Efficacy of fractional CO2 laser treatment in postmenopausal women with genitourinary syndrome: a multicenter study. Menopause. 2019;27:43-49. doi: 10.1097/GME. 0000000000001428.
Researchers conducted a retrospective, multicenter study of postmenopausal women with at least one symptom of GSM, including itching, burning, dyspareunia with penetration, and dryness.
Study details
A total of 171 of the 645 women (26.5%) were oncology patients. Women were excluded from analysis if they used any form of topical therapy within 15 days; had prolapse stage 2 or greater; or had any infection, abscess, or anatomical deformity precluding treatment with the laser.
Patients underwent gynecologic examination and were given a questionnaire to assess vulvovaginal symptoms. Exams occurred monthly during treatment (average, 6.5 months), at 6- and 12-months posttreatment, and then annually. No topical therapy was advised during or after treatment.
Patients received either 3 or 4 fractional CO2 laser treatments to the vulva and/or vagina depending on symptom location and type. Higher power settings of the same laser were used to treat vaginal symptoms (40W; 1,000 microseconds) versus vulvar symptoms (25W; 500 microseconds). Treatment sessions were 5 to 6 minutes. The study authors used a visual analog rating scale (VAS) for “atrophy and related symptoms,” tested vaginal pH, and completed the Vaginal Health Index Score. VAS scores were obtained from the patients prior to the initial laser intervention and 1 month after the final treatment.
Results
There were statistically significant improvements in dryness, vaginal orifice pain, dyspareunia, itching, and burning for both the 3-treatment and 4-treatment cohorts. The delta of improvement was then compared for the 2 subgroups; curiously, there was greater improvement of symptoms such as dryness (65% vs 61%), itching (78% vs 72%), burning (72% vs 67%), and vaginal orifice pain (67% vs 60%) in the group that received 3 cycles than in the group that received 4 cycles.
With regard to vaginal pH improvement, the 4-cycle group performed better than the 3-cycle group (1% improvement in the 4-cycle group vs 6% in the 3-cycle group). Although vaginal pH reduction was somewhat better in the group that received 4 treatments, and the pre versus posttreatment percentages were statistically significantly different, the clinical significance of a pH difference between 5.72 and 5.53 is questionable, especially since there was a greater difference in baseline pH between the two cohorts (6.08 in the 4-cycle group vs 5.59 in the 3-cycle group).
There were no reported adverse events related to the fractional laser treatments, and 6% of the patients underwent additional laser treatments during the followup timeframe of 8 to 20 months.
This was a retrospective study with no control or comparison group and short-term follow-up. The VAS scores were obtained 1 month after the final treatment. Failure to request additional treatment at 8 to 20 months cannot be used to infer that the therapeutic improvements recorded at 1 month were enduring. In addition, although the large number of patients in this study may lead to statistical significance, clinical significance is still questionable. Given the lack of a comparison group and the very short follow-up, it is hard to draw any scientifically valid conclusions from this study.
Continue to: Randomized data on CO2 laser vs Kegels for sexual dysfunction...
Randomized data on CO2 laser vs Kegels for sexual dysfunction
Lou W, Chen F, Xu T, et al. A randomized controlled study of vaginal fractional CO2 laser therapy for female sexual dysfunction. Lasers Med Sci. March 15, 2021. doi: 10.1007/s10103-021-03260-x.
In a small randomized controlled trial (RCT) conducted in China, Lou and colleagues identified premenopausal women at “high risk” for sexual dysfunction as determined by the Chinese version of the Female Sexual Function Index (CFSFI).
Details of the study
A total of 84 women (mean age, 36.5 years) were included in the study. All the participants were heterosexual and married or with a long-term partner. The domain of sexual dysfunction was not considered. Women were excluded if they had no current heterosexual partner; had genital malformation, urinary incontinence, or prolapse stage 2 or higher; a history of pelvic floor mesh treatment; current gynecologic malignancy; abnormal cervical cytology; or were currently pregnant or postpartum. In addition, women were excluded if they had been treated previously for sexual dysfunction or mental “disease.” The cohort was randomized to receive fractional CO2 laser treatments (three 15-minute treatments 1 month apart at 60W, 1,000 microseconds) or coached Kegel exercises (10 exercises repeated twice daily at least 3 times/week and monitored by physical therapists at biweekly clinic visits). Sexual distress was evaluated by using the Female Sexual Distress Scale-Revised (FSDSR). Outcomes measured were pelvic floor muscle strength and scores on the CFSFI and FSDSR. Data were obtained at 3, 6, 9, and 12 months after initiation of therapy.
Both groups showed improvement
The laser cohort showed slightly more improvement in scale scores at 6 and 12 months. Specifically, the laser group had better scores on lubrication and overall satisfaction, with moderate effect size; neither group had improvements in arousal, desire, or orgasm. The Kegel group showed a significant improvement in pelvic floor strength and orgasm at 12 months, an improvement not seen in the laser cohort. Both groups showed gradual improvement in the FSDSR, with the laser group reporting a lower score (10.0) at 12 months posttreatment relative to the Kegel group (11.1). Again, these were modest effects as baseline scores for both cohorts were around 12.5. There were minimal safety signals in the laser group, with 22.5% of women reporting scant bloody discharge posttreatment and 72.5% describing mild discomfort (1 on a 1–10 VAS scale) during the procedure.
This study is problematic in several areas. Although it was a prospective, randomized trial, it was not blinded, and the therapeutic interventions were markedly different in nature and requirement for individual patient motivation. The experiences of sexual dysfunction among the participants were not stratified by type—arousal, desire, lubrication, orgasm, or pain. All patients had regular cyclic menses; however, the authors do not report on contraceptive methods, hormonal therapy, or other comorbid conditions that could impact sexual health. The cohorts may or may not have been similar in baseline types of sexual dissatisfaction.
CO2 laser for lichen sclerosus: Is it effective?
Pagano T, Conforti A, Buonfantino C, et al. Effect of rescue fractional microablative CO2 laser on symptoms and sexual dysfunction in women affected by vulvar lichen sclerosus resistant to long-term use of topic corticosteroid: a prospective longitudinal study. Menopause. 2020;27:418-422. doi: 10.1097 /GME.0000000000001482.
Burkett LS, Siddique M, Zeymo A, et al. Clobetasol compared with fractionated carbon dioxide laser for lichen sclerosus: a randomized controlled trial. Obstet Gynecol. 2021;137:968-978. doi: 10.1097 /AOG.0000000000004332.
Mitchell L, Goldstein AT, Heller D, et al. Fractionated carbon dioxide laser for the treatment of vulvar lichen sclerosus: a randomized controlled trial. Obstet Gynecol. 2021;137:979-987. doi: 10.1097 /AOG.0000000000004409.
High potency corticosteroid ointment is the current standard treatment for lichen sclerosus. Alternative options for disease that is refractory to steroids are limited. Three studies published in the past year explored the CO2 laser’s ability to treat lichen sclerosus symptoms and resultant sexual dysfunction—Pagano and colleagues conducted a small prospective study and Burkett and colleagues and Mitchell et al conducted small RCTs.
Details of the Pagano study
Three premenopausal and 37 postmenopausal women with refractory lichen sclerosus (defined as no improvement after 4 cycles of ultra-high potency steroids) were included in the study. Lichen sclerosus was uniformly biopsy confirmed. Women using topical or systemic hormones were excluded. VAS was administered prior to initial treatment and after each of 2 fractional CO2 treatments (25–30 W; 1,000 microseconds) 30 to 40 days apart to determine severity of vulvar itching, dyspareunia with penetration, vulvar dryness, sexual dysfunction, and procedure discomfort. Follow-up was conducted at 1 month after the final treatment. VAS score for the primary outcome of vulvar itching declined from 8 pretreatment to 6 after the first treatment and to 3 after the second. There was no significant treatment-related pain reported.
The authors acknowledged the limitations of their study; it was a relatively small sample size, nonrandomized and had short-term follow-up of a mixed patient population and no sham or control group. The short-term improvements reported in the study patients may not be sustained without ongoing treatment for a lifelong chronic disease, and the long-term potential for development of squamous cell carcinoma may or may not be ameliorated.
Continue to: Burkett et al: RCT study 1...
Burkett et al: RCT study 1
A total of 52 postmenopausal patients with biopsy-proven lichen sclerosus were randomly assigned to clobetasol or CO2 laser; 51 women completed 6-month follow-up. The outcomes were stratified by prior high-potency steroid use. The steroid cohort used clobetasol 0.05% nightly for 1 month, 3 times per week for 2 months, then as needed. The laser cohort received 3 treatments (26 W; 800 microseconds) 4 to 6 weeks apart. Overall adherence was only 75% in the clobetasol group, compared with 96% in the laser group. The authors found treatment efficacy of CO2 laser therapy only in the group of patients who had prior treatment with high potency topical corticosteroids. They conclude that, …“Despite previously optimistic results in well designed clinical trials of fractionated CO2 for genitourinary syndrome of menopause, and in noncontrolled case series for vulvar lichen sclerosus, our study failed to show any significant benefit of monotherapy of fractionated CO2 for vulvar lichen sclerosus. There may be a role for fractionated CO2 as an adjuvant therapy along with topical ultrapotent corticosteroids in vulvar lichen sclerosus.”
Mitchell et al: RCT study 2
This was a double blind, placebo-controlled, and histologically validated study of fractional CO2 for treatment of lichen sclerosus in 35 women; 17 in the treatment arm and 18 in the sham laser encounters. At least a 4-week no treatment period of topical steroids was required before monotherapy with CO2 laser was initiated.
The authors found no difference in their primary outcome—histopathology scale scores—after 5 treatments over 24 weeks. Secondary endpoints were changes in the CSS (Clinical Scoring System for Vulvar Lichen Sclerosus), a validated instrument that includes both a clinician’s examination of the severity of disease and a patient’s report of the severity of her symptoms. The patient score is the total of 4 domains: itching, soreness, burning, and dyspareunia. The clinician objective examination documents fissures, erosions, hyperkeratosis, agglutination, stenosis, and atrophy. At the conclusion of treatment there were no significant differences in the patient reported symptoms or the clinical findings between the active treatment and sham groups.
As a monotherapy, CO2 laser therapy is not effective in treating lichen sclerosus, although it may help improve symptoms as an adjunct to high potency steroid therapy when topical treatment alone has failed to provide adequate response.
Conclusion
The quality of evidence to support the use of the CO2 laser for improvement in sexual dysfunction is poor. Although patient satisfaction scores improved overall, and most specifically for symptoms related to GSM, the lack of blinding; inappropriate or no control groups; the very short-term outcomes; and for one of the studies, the lack of a clear definition of sexual dysfunction, make it difficult to draw meaningful conclusions for clinical care.
For GSM, we know that topical estrogen therapy works—and with little to no systemic absorption. The CO2 laser should be studied in comparison to this gold standard, with consideration of costs and potential long-term harms in addition to patient satisfaction and short-term measures of improvement. In addition, and very importantly, it is our professional responsibility to present the evidence for safety of topical estrogens to our professional colleagues as well as to our patients with estrogen-dependent cancers so that they understand the value of estrogen as a safe and appropriate alternative to expensive and potentially short-term interventions such as CO2 laser treatment. ●

Cheryl Iglesia, MD
Dr. Iglesia is Director, Section of Female Pelvic Medicine and Reconstructive Surgery, MedStar Washington Hospital Center, and Professor, Departments of ObGyn and Urology, Georgetown University School of Medicine, Washington, DC. She is a member of the OBG Management Board of Editors.
Barbara Levy, MD: Cheryl, you have more experience with use of the energy-based cosmetic laser than most ObGyns, and I thought that speaking with you about this technology would be of benefit, not only to me in learning more about the hands-on experience of a lead researcher and practitioner but also readers who are hearing more and more about the growth of cosmetic gynecology in general. Thank you for taking the time today.
Cheryl Iglesia, MD: I’m happy to speak about this with you, Barbara.
Dr. Levy: Specifically, I would like to talk about use of these technologies for sexual dysfunction. In the last few years some of the available data have been on the CO2 laser versus physical therapy, which is not an appropriate comparison.1
Dr. Iglesia: There have been limited data, and less randomized, controlled data, on laser and radiofrequency energies for cosmetic gynecology, and in fact these devices remain unapproved for any gynecologic indication. In 2018 the US Food and Drug Administration (FDA) issued a Safety Communication about the use of energy-based devices to perform vaginal rejuvenation or cosmetic procedures. The International Urogynecological Association (IUGA) issued a consensus statement echoing concerns about the devices, and an International Continence Society/International Society for the Study of Vulvovaginal Disease Best Practice Consensus Statement did not recommend the laser for “routine treatment of vaginal atrophy or urinary incontinence unless treatment is part of a well-designed trial or with special arrangements for clinical governance, consent, and audit.”2
In May 2020, as evidence remains limited (although 522 studies are ongoing in coordination with the FDA), the American Urogynecologic Society (AUGS) published a clinical consensus statement from a panel of experts in female pelvic medicine and reconstructive surgery. The panel had about 90% consensus that there is short-term efficacy for the laser with GSM and dyspareunia. But we only have outcomes data that lasts a maximum of 1 year.2
A problem with our VeLVET trial,3 which was published in Menopause, and the Cruz and colleagues’ trial from South America,4 both of which compared the CO2 laser to estrogen and had randomized groups, was that they were limited by the outcome measures used, none of which were consistently validated. But these studies also had small numbers of participants and short-term follow-up. So I don’t think there are much existing data that are promising for supporting energy-based treatment for GSM.
We also have just-published data on the laser for lichen sclerosus.5 For the AUGS panel, there was about 80% consensus for energy-based-device use and lichen sclerosus.2 According to Mitchell et al, who conducted a small, randomized, sham-controlled trial, CO2 laser resulted in no significant difference in histopathology scale score between active and sham arms.5
Future trials may want to assess laser as a mechanism for improved local drug delivery (eg, use of combined laser plus local estrogen for GSM or combined laser plus topical steroid for lichen sclerosus). I am also aware that properly designed laser versus sham studies are underway.
Dr. Levy: What about for stress urinary incontinence (SUI)? I don’t think these technologies are going to work.
Dr. Iglesia: For the AUGS panel, there was only about 70% consensus for energy-based-device use and SUI,2 and I’m one of the naysayers. The pathophysiology of SUI is so multifactorial that it’s hard to believe that laser or radiofrequency wand therapy could have sustained improvements, especially since prior radiofrequency therapy from the last decade (for instance, Renessa, Novasys Medical) did not show long-term efficacy.
Understanding lasers and coordinating care
Dr. Levy: We don’t know what the long-term outcomes are for the CO2 laser and GSM.
Dr. Iglesia: I agree with you, and I think there needs to be an understanding of the mechanism of how lasers work, whether it be erbium (Er:YAG), which is the most common, or CO2. Erbium and CO2 lasers, which are on the far-infrared spectrum, target the chromophore, water. My feeling is that, when you look at results from the Cruz trial,4 or even our trial that compared vaginal estrogen with laser,3 when there is severe GSM and high pH with virtually no water present in the tissues, that laser is not going to properly function. But I don’t think we know exactly what optimal pretreatment is necessary, and that is one of the problems. Furthermore, when intravaginal lasers are done and no adequate speculum exam is conducted prior to introducing the laser, there could be discharge or old creams present that block the mirrors necessary to adequately fire the fractionated laser beams.
Unfortunately, oftentimes these devices are marketed to women with breast cancer, who may be taking aromatase inhibitors, which cause the no-water problem; they dry out everything. They are effective for preventing breast cancer recurrence, but they cause severe atrophy (perhaps worse than many of the other selective estrogen-receptor modulators), with a resultant high vaginal pH. If we can bring that pH level down, closer to the normal 4.5 range so that we could have some level of moisture, and add estrogen first, the overall treatment approach will probably be more effective. We still do not know what happens after 1 year, though, and how often touch-ups need to be performed.
In fact, when working with a patient with breast cancer, I will speak with her oncologist; I will collaborate to put in place a treatment plan that may include initial pretreatment with low-dose vaginal estrogen followed by laser treatment for vaginal atrophy. But I will make sure I use the lowest dose. Sometimes when the patient comes back, the estrogen’s worked so well she’ll say, “Oh, I’m happy, so I don’t need the laser anymore.” A balanced conversation is necessary, especially with cancer survivors.
Informing patients and colleagues
Dr. Levy: I completely agree, and I think one of the key points here is that our purpose is to serve our patients. The data demonstrate that low doses of vaginal estrogen are not harmful for women who are being treated for or who have recovered from breast cancer. It is our ethical obligation to convince these women and their oncologists that ongoing treatment with vaginal estrogen not only will help their GSM but also their overactive bladder and their risk of urinary tract infections and other things. We could be exploiting patients who are really fearful of using any estrogen because of a perceived cancer risk. We could actually be validating their fear by telling them we have an alternative treatment for which they have to pay cash.
Treatment access
Dr. Iglesia: Yes, these are not cosmetic conditions that we are treating. So my goal when evaluating treatment for refractory GSM or lichen sclerosus is to find optimal energy-based therapies with the hope that one day these will be approved gynecologic conditions by the US FDA for laser and wand therapies and that they will ultimately not be out-of-pocket expenses but rather therapies covered by insurance.
Dr. Levy: Great. I understand that AUGS/IUGA have been working on a terminology algorithm to help distinguish between procedures being performed to resolve a medical problem such as prolapse or incontinence versus those designed to be cosmetic.
Dr. Iglesia: Yes, there is a big document from experts in both societies out for public comment right now. It will hopefully be published soon.
Outstanding questions remain
Dr. Levy: Really, we as ObGyns shouldn’t be quick to incorporate these things into our practices without high-quality studies demonstrating value. I have a major concern about these devices in the long term. When you look at fractional CO2 use on the face, for instance, which is a much different type of skin than the vagina, the laser builds collagen—but we don’t have long-term outcome results. The vagina is supposed to be an elastic tissue, so what is the risk of long-term scarring there? Yes, the laser builds collagen in the vaginal epithelium, but what does it do to scarring in the rest of the tissue? We don’t have answers to that.
Dr. Iglesia: And that is the question—how does histology equate with function? Well, I would go with what the patients are reporting.
Dr. Levy: Absolutely. But the thing about vaginal low-dose estrogen is that it is something that the oncologists or the ObGyns could be implementing with patients while they are undergoing cancer therapy, while in their menopausal transition, to preserve vulvovaginal function as opposed to trying to regain it.
Dr. Iglesia: Certainly, although it still needs to be determined when that type of approach would actually be contraindicated.
Dr. Levy: Thank you, Cheryl, for your valuable insights.
Dr. Iglesia: Of course. Thank you. ●
References
1. Lou W, Chen F, Xu T, et al. A randomized controlled study of vaginal fractional CO2 laser therapy for female sexual dysfunction. Lasers Med Sci. March 15, 2021. doi: 10.1007/s10103-021-03260-x.
2. Alshiek J, Garcia B, Minassian V, et al. Vaginal energy-based devices. Female Pelvic Med Reconstr Surg. 2020;26:287-298. doi: 10.1097 /SPV.0000000000000872.
3. Paraiso MF, Ferrando CA, et al. A randomized clinical trial comparing vaginal laser therapy to vaginal estrogen therapy in women with genitourinary syndrome of menopause: the VeLVET Trial. Menopause. 2020;27:50-56. doi: 10.1097/GME.0000000000001416.
4. Cruz VL, Steiner ML, et al. Randomized, double-blind, placebo-controlled clinical trial for evaluating the efficacy of fractional CO2 laser compared with topical estriol in the treatment of vaginal atrophy in postmenopausal women. Menopause. 2018;25:21-28. doi: 10.1097 /GME.0000000000000955.
5. Mitchell L, Goldstein A, Heller D, et al. Fractionated carbon dioxide laser for the treatment of vulvar lichen sclerosus: a randomized controlled trial. Obstet Gynecol. 2021;137:979-987. doi: 10.1097/AOG.0000000000004409.

The approach to diagnosis and treatment of female sexual function continues to be a challenge for women’s health professionals. The search for a female “little blue pill” remains elusive as researchers struggle to understand the mechanisms that underlie the complex aspects of female sexual health. This Update will review the recent literature on the use of fractional CO2 laser for treatment of female sexual dysfunction and vulvovaginal symptoms. Bottom line: While the quality of the studies is poor overall, fractional CO2 laser treatment seems to temporarily improve symptoms of genitourinary syndrome of menopause (GSM). The duration of response, cost, and the overall long-term impact on sexual health remain in question.
A retrospective look at CO2 laser and postmenopausal GSM
Filippini M, Luvero D, Salvatore S, et al. Efficacy of fractional CO2 laser treatment in postmenopausal women with genitourinary syndrome: a multicenter study. Menopause. 2019;27:43-49. doi: 10.1097/GME. 0000000000001428.
Researchers conducted a retrospective, multicenter study of postmenopausal women with at least one symptom of GSM, including itching, burning, dyspareunia with penetration, and dryness.
Study details
A total of 171 of the 645 women (26.5%) were oncology patients. Women were excluded from analysis if they used any form of topical therapy within 15 days; had prolapse stage 2 or greater; or had any infection, abscess, or anatomical deformity precluding treatment with the laser.
Patients underwent gynecologic examination and were given a questionnaire to assess vulvovaginal symptoms. Exams occurred monthly during treatment (average, 6.5 months), at 6- and 12-months posttreatment, and then annually. No topical therapy was advised during or after treatment.
Patients received either 3 or 4 fractional CO2 laser treatments to the vulva and/or vagina depending on symptom location and type. Higher power settings of the same laser were used to treat vaginal symptoms (40W; 1,000 microseconds) versus vulvar symptoms (25W; 500 microseconds). Treatment sessions were 5 to 6 minutes. The study authors used a visual analog rating scale (VAS) for “atrophy and related symptoms,” tested vaginal pH, and completed the Vaginal Health Index Score. VAS scores were obtained from the patients prior to the initial laser intervention and 1 month after the final treatment.
Results
There were statistically significant improvements in dryness, vaginal orifice pain, dyspareunia, itching, and burning for both the 3-treatment and 4-treatment cohorts. The delta of improvement was then compared for the 2 subgroups; curiously, there was greater improvement of symptoms such as dryness (65% vs 61%), itching (78% vs 72%), burning (72% vs 67%), and vaginal orifice pain (67% vs 60%) in the group that received 3 cycles than in the group that received 4 cycles.
With regard to vaginal pH improvement, the 4-cycle group performed better than the 3-cycle group (1% improvement in the 4-cycle group vs 6% in the 3-cycle group). Although vaginal pH reduction was somewhat better in the group that received 4 treatments, and the pre versus posttreatment percentages were statistically significantly different, the clinical significance of a pH difference between 5.72 and 5.53 is questionable, especially since there was a greater difference in baseline pH between the two cohorts (6.08 in the 4-cycle group vs 5.59 in the 3-cycle group).
There were no reported adverse events related to the fractional laser treatments, and 6% of the patients underwent additional laser treatments during the followup timeframe of 8 to 20 months.
This was a retrospective study with no control or comparison group and short-term follow-up. The VAS scores were obtained 1 month after the final treatment. Failure to request additional treatment at 8 to 20 months cannot be used to infer that the therapeutic improvements recorded at 1 month were enduring. In addition, although the large number of patients in this study may lead to statistical significance, clinical significance is still questionable. Given the lack of a comparison group and the very short follow-up, it is hard to draw any scientifically valid conclusions from this study.
Continue to: Randomized data on CO2 laser vs Kegels for sexual dysfunction...
Randomized data on CO2 laser vs Kegels for sexual dysfunction
Lou W, Chen F, Xu T, et al. A randomized controlled study of vaginal fractional CO2 laser therapy for female sexual dysfunction. Lasers Med Sci. March 15, 2021. doi: 10.1007/s10103-021-03260-x.
In a small randomized controlled trial (RCT) conducted in China, Lou and colleagues identified premenopausal women at “high risk” for sexual dysfunction as determined by the Chinese version of the Female Sexual Function Index (CFSFI).
Details of the study
A total of 84 women (mean age, 36.5 years) were included in the study. All the participants were heterosexual and married or with a long-term partner. The domain of sexual dysfunction was not considered. Women were excluded if they had no current heterosexual partner; had genital malformation, urinary incontinence, or prolapse stage 2 or higher; a history of pelvic floor mesh treatment; current gynecologic malignancy; abnormal cervical cytology; or were currently pregnant or postpartum. In addition, women were excluded if they had been treated previously for sexual dysfunction or mental “disease.” The cohort was randomized to receive fractional CO2 laser treatments (three 15-minute treatments 1 month apart at 60W, 1,000 microseconds) or coached Kegel exercises (10 exercises repeated twice daily at least 3 times/week and monitored by physical therapists at biweekly clinic visits). Sexual distress was evaluated by using the Female Sexual Distress Scale-Revised (FSDSR). Outcomes measured were pelvic floor muscle strength and scores on the CFSFI and FSDSR. Data were obtained at 3, 6, 9, and 12 months after initiation of therapy.
Both groups showed improvement
The laser cohort showed slightly more improvement in scale scores at 6 and 12 months. Specifically, the laser group had better scores on lubrication and overall satisfaction, with moderate effect size; neither group had improvements in arousal, desire, or orgasm. The Kegel group showed a significant improvement in pelvic floor strength and orgasm at 12 months, an improvement not seen in the laser cohort. Both groups showed gradual improvement in the FSDSR, with the laser group reporting a lower score (10.0) at 12 months posttreatment relative to the Kegel group (11.1). Again, these were modest effects as baseline scores for both cohorts were around 12.5. There were minimal safety signals in the laser group, with 22.5% of women reporting scant bloody discharge posttreatment and 72.5% describing mild discomfort (1 on a 1–10 VAS scale) during the procedure.
This study is problematic in several areas. Although it was a prospective, randomized trial, it was not blinded, and the therapeutic interventions were markedly different in nature and requirement for individual patient motivation. The experiences of sexual dysfunction among the participants were not stratified by type—arousal, desire, lubrication, orgasm, or pain. All patients had regular cyclic menses; however, the authors do not report on contraceptive methods, hormonal therapy, or other comorbid conditions that could impact sexual health. The cohorts may or may not have been similar in baseline types of sexual dissatisfaction.
CO2 laser for lichen sclerosus: Is it effective?
Pagano T, Conforti A, Buonfantino C, et al. Effect of rescue fractional microablative CO2 laser on symptoms and sexual dysfunction in women affected by vulvar lichen sclerosus resistant to long-term use of topic corticosteroid: a prospective longitudinal study. Menopause. 2020;27:418-422. doi: 10.1097 /GME.0000000000001482.
Burkett LS, Siddique M, Zeymo A, et al. Clobetasol compared with fractionated carbon dioxide laser for lichen sclerosus: a randomized controlled trial. Obstet Gynecol. 2021;137:968-978. doi: 10.1097 /AOG.0000000000004332.
Mitchell L, Goldstein AT, Heller D, et al. Fractionated carbon dioxide laser for the treatment of vulvar lichen sclerosus: a randomized controlled trial. Obstet Gynecol. 2021;137:979-987. doi: 10.1097 /AOG.0000000000004409.
High potency corticosteroid ointment is the current standard treatment for lichen sclerosus. Alternative options for disease that is refractory to steroids are limited. Three studies published in the past year explored the CO2 laser’s ability to treat lichen sclerosus symptoms and resultant sexual dysfunction—Pagano and colleagues conducted a small prospective study and Burkett and colleagues and Mitchell et al conducted small RCTs.
Details of the Pagano study
Three premenopausal and 37 postmenopausal women with refractory lichen sclerosus (defined as no improvement after 4 cycles of ultra-high potency steroids) were included in the study. Lichen sclerosus was uniformly biopsy confirmed. Women using topical or systemic hormones were excluded. VAS was administered prior to initial treatment and after each of 2 fractional CO2 treatments (25–30 W; 1,000 microseconds) 30 to 40 days apart to determine severity of vulvar itching, dyspareunia with penetration, vulvar dryness, sexual dysfunction, and procedure discomfort. Follow-up was conducted at 1 month after the final treatment. VAS score for the primary outcome of vulvar itching declined from 8 pretreatment to 6 after the first treatment and to 3 after the second. There was no significant treatment-related pain reported.
The authors acknowledged the limitations of their study; it was a relatively small sample size, nonrandomized and had short-term follow-up of a mixed patient population and no sham or control group. The short-term improvements reported in the study patients may not be sustained without ongoing treatment for a lifelong chronic disease, and the long-term potential for development of squamous cell carcinoma may or may not be ameliorated.
Continue to: Burkett et al: RCT study 1...
Burkett et al: RCT study 1
A total of 52 postmenopausal patients with biopsy-proven lichen sclerosus were randomly assigned to clobetasol or CO2 laser; 51 women completed 6-month follow-up. The outcomes were stratified by prior high-potency steroid use. The steroid cohort used clobetasol 0.05% nightly for 1 month, 3 times per week for 2 months, then as needed. The laser cohort received 3 treatments (26 W; 800 microseconds) 4 to 6 weeks apart. Overall adherence was only 75% in the clobetasol group, compared with 96% in the laser group. The authors found treatment efficacy of CO2 laser therapy only in the group of patients who had prior treatment with high potency topical corticosteroids. They conclude that, …“Despite previously optimistic results in well designed clinical trials of fractionated CO2 for genitourinary syndrome of menopause, and in noncontrolled case series for vulvar lichen sclerosus, our study failed to show any significant benefit of monotherapy of fractionated CO2 for vulvar lichen sclerosus. There may be a role for fractionated CO2 as an adjuvant therapy along with topical ultrapotent corticosteroids in vulvar lichen sclerosus.”
Mitchell et al: RCT study 2
This was a double blind, placebo-controlled, and histologically validated study of fractional CO2 for treatment of lichen sclerosus in 35 women; 17 in the treatment arm and 18 in the sham laser encounters. At least a 4-week no treatment period of topical steroids was required before monotherapy with CO2 laser was initiated.
The authors found no difference in their primary outcome—histopathology scale scores—after 5 treatments over 24 weeks. Secondary endpoints were changes in the CSS (Clinical Scoring System for Vulvar Lichen Sclerosus), a validated instrument that includes both a clinician’s examination of the severity of disease and a patient’s report of the severity of her symptoms. The patient score is the total of 4 domains: itching, soreness, burning, and dyspareunia. The clinician objective examination documents fissures, erosions, hyperkeratosis, agglutination, stenosis, and atrophy. At the conclusion of treatment there were no significant differences in the patient reported symptoms or the clinical findings between the active treatment and sham groups.
As a monotherapy, CO2 laser therapy is not effective in treating lichen sclerosus, although it may help improve symptoms as an adjunct to high potency steroid therapy when topical treatment alone has failed to provide adequate response.
Conclusion
The quality of evidence to support the use of the CO2 laser for improvement in sexual dysfunction is poor. Although patient satisfaction scores improved overall, and most specifically for symptoms related to GSM, the lack of blinding; inappropriate or no control groups; the very short-term outcomes; and for one of the studies, the lack of a clear definition of sexual dysfunction, make it difficult to draw meaningful conclusions for clinical care.
For GSM, we know that topical estrogen therapy works—and with little to no systemic absorption. The CO2 laser should be studied in comparison to this gold standard, with consideration of costs and potential long-term harms in addition to patient satisfaction and short-term measures of improvement. In addition, and very importantly, it is our professional responsibility to present the evidence for safety of topical estrogens to our professional colleagues as well as to our patients with estrogen-dependent cancers so that they understand the value of estrogen as a safe and appropriate alternative to expensive and potentially short-term interventions such as CO2 laser treatment. ●

Cheryl Iglesia, MD
Dr. Iglesia is Director, Section of Female Pelvic Medicine and Reconstructive Surgery, MedStar Washington Hospital Center, and Professor, Departments of ObGyn and Urology, Georgetown University School of Medicine, Washington, DC. She is a member of the OBG Management Board of Editors.
Barbara Levy, MD: Cheryl, you have more experience with use of the energy-based cosmetic laser than most ObGyns, and I thought that speaking with you about this technology would be of benefit, not only to me in learning more about the hands-on experience of a lead researcher and practitioner but also readers who are hearing more and more about the growth of cosmetic gynecology in general. Thank you for taking the time today.
Cheryl Iglesia, MD: I’m happy to speak about this with you, Barbara.
Dr. Levy: Specifically, I would like to talk about use of these technologies for sexual dysfunction. In the last few years some of the available data have been on the CO2 laser versus physical therapy, which is not an appropriate comparison.1
Dr. Iglesia: There have been limited data, and less randomized, controlled data, on laser and radiofrequency energies for cosmetic gynecology, and in fact these devices remain unapproved for any gynecologic indication. In 2018 the US Food and Drug Administration (FDA) issued a Safety Communication about the use of energy-based devices to perform vaginal rejuvenation or cosmetic procedures. The International Urogynecological Association (IUGA) issued a consensus statement echoing concerns about the devices, and an International Continence Society/International Society for the Study of Vulvovaginal Disease Best Practice Consensus Statement did not recommend the laser for “routine treatment of vaginal atrophy or urinary incontinence unless treatment is part of a well-designed trial or with special arrangements for clinical governance, consent, and audit.”2
In May 2020, as evidence remains limited (although 522 studies are ongoing in coordination with the FDA), the American Urogynecologic Society (AUGS) published a clinical consensus statement from a panel of experts in female pelvic medicine and reconstructive surgery. The panel had about 90% consensus that there is short-term efficacy for the laser with GSM and dyspareunia. But we only have outcomes data that lasts a maximum of 1 year.2
A problem with our VeLVET trial,3 which was published in Menopause, and the Cruz and colleagues’ trial from South America,4 both of which compared the CO2 laser to estrogen and had randomized groups, was that they were limited by the outcome measures used, none of which were consistently validated. But these studies also had small numbers of participants and short-term follow-up. So I don’t think there are much existing data that are promising for supporting energy-based treatment for GSM.
We also have just-published data on the laser for lichen sclerosus.5 For the AUGS panel, there was about 80% consensus for energy-based-device use and lichen sclerosus.2 According to Mitchell et al, who conducted a small, randomized, sham-controlled trial, CO2 laser resulted in no significant difference in histopathology scale score between active and sham arms.5
Future trials may want to assess laser as a mechanism for improved local drug delivery (eg, use of combined laser plus local estrogen for GSM or combined laser plus topical steroid for lichen sclerosus). I am also aware that properly designed laser versus sham studies are underway.
Dr. Levy: What about for stress urinary incontinence (SUI)? I don’t think these technologies are going to work.
Dr. Iglesia: For the AUGS panel, there was only about 70% consensus for energy-based-device use and SUI,2 and I’m one of the naysayers. The pathophysiology of SUI is so multifactorial that it’s hard to believe that laser or radiofrequency wand therapy could have sustained improvements, especially since prior radiofrequency therapy from the last decade (for instance, Renessa, Novasys Medical) did not show long-term efficacy.
Understanding lasers and coordinating care
Dr. Levy: We don’t know what the long-term outcomes are for the CO2 laser and GSM.
Dr. Iglesia: I agree with you, and I think there needs to be an understanding of the mechanism of how lasers work, whether it be erbium (Er:YAG), which is the most common, or CO2. Erbium and CO2 lasers, which are on the far-infrared spectrum, target the chromophore, water. My feeling is that, when you look at results from the Cruz trial,4 or even our trial that compared vaginal estrogen with laser,3 when there is severe GSM and high pH with virtually no water present in the tissues, that laser is not going to properly function. But I don’t think we know exactly what optimal pretreatment is necessary, and that is one of the problems. Furthermore, when intravaginal lasers are done and no adequate speculum exam is conducted prior to introducing the laser, there could be discharge or old creams present that block the mirrors necessary to adequately fire the fractionated laser beams.
Unfortunately, oftentimes these devices are marketed to women with breast cancer, who may be taking aromatase inhibitors, which cause the no-water problem; they dry out everything. They are effective for preventing breast cancer recurrence, but they cause severe atrophy (perhaps worse than many of the other selective estrogen-receptor modulators), with a resultant high vaginal pH. If we can bring that pH level down, closer to the normal 4.5 range so that we could have some level of moisture, and add estrogen first, the overall treatment approach will probably be more effective. We still do not know what happens after 1 year, though, and how often touch-ups need to be performed.
In fact, when working with a patient with breast cancer, I will speak with her oncologist; I will collaborate to put in place a treatment plan that may include initial pretreatment with low-dose vaginal estrogen followed by laser treatment for vaginal atrophy. But I will make sure I use the lowest dose. Sometimes when the patient comes back, the estrogen’s worked so well she’ll say, “Oh, I’m happy, so I don’t need the laser anymore.” A balanced conversation is necessary, especially with cancer survivors.
Informing patients and colleagues
Dr. Levy: I completely agree, and I think one of the key points here is that our purpose is to serve our patients. The data demonstrate that low doses of vaginal estrogen are not harmful for women who are being treated for or who have recovered from breast cancer. It is our ethical obligation to convince these women and their oncologists that ongoing treatment with vaginal estrogen not only will help their GSM but also their overactive bladder and their risk of urinary tract infections and other things. We could be exploiting patients who are really fearful of using any estrogen because of a perceived cancer risk. We could actually be validating their fear by telling them we have an alternative treatment for which they have to pay cash.
Treatment access
Dr. Iglesia: Yes, these are not cosmetic conditions that we are treating. So my goal when evaluating treatment for refractory GSM or lichen sclerosus is to find optimal energy-based therapies with the hope that one day these will be approved gynecologic conditions by the US FDA for laser and wand therapies and that they will ultimately not be out-of-pocket expenses but rather therapies covered by insurance.
Dr. Levy: Great. I understand that AUGS/IUGA have been working on a terminology algorithm to help distinguish between procedures being performed to resolve a medical problem such as prolapse or incontinence versus those designed to be cosmetic.
Dr. Iglesia: Yes, there is a big document from experts in both societies out for public comment right now. It will hopefully be published soon.
Outstanding questions remain
Dr. Levy: Really, we as ObGyns shouldn’t be quick to incorporate these things into our practices without high-quality studies demonstrating value. I have a major concern about these devices in the long term. When you look at fractional CO2 use on the face, for instance, which is a much different type of skin than the vagina, the laser builds collagen—but we don’t have long-term outcome results. The vagina is supposed to be an elastic tissue, so what is the risk of long-term scarring there? Yes, the laser builds collagen in the vaginal epithelium, but what does it do to scarring in the rest of the tissue? We don’t have answers to that.
Dr. Iglesia: And that is the question—how does histology equate with function? Well, I would go with what the patients are reporting.
Dr. Levy: Absolutely. But the thing about vaginal low-dose estrogen is that it is something that the oncologists or the ObGyns could be implementing with patients while they are undergoing cancer therapy, while in their menopausal transition, to preserve vulvovaginal function as opposed to trying to regain it.
Dr. Iglesia: Certainly, although it still needs to be determined when that type of approach would actually be contraindicated.
Dr. Levy: Thank you, Cheryl, for your valuable insights.
Dr. Iglesia: Of course. Thank you. ●
References
1. Lou W, Chen F, Xu T, et al. A randomized controlled study of vaginal fractional CO2 laser therapy for female sexual dysfunction. Lasers Med Sci. March 15, 2021. doi: 10.1007/s10103-021-03260-x.
2. Alshiek J, Garcia B, Minassian V, et al. Vaginal energy-based devices. Female Pelvic Med Reconstr Surg. 2020;26:287-298. doi: 10.1097 /SPV.0000000000000872.
3. Paraiso MF, Ferrando CA, et al. A randomized clinical trial comparing vaginal laser therapy to vaginal estrogen therapy in women with genitourinary syndrome of menopause: the VeLVET Trial. Menopause. 2020;27:50-56. doi: 10.1097/GME.0000000000001416.
4. Cruz VL, Steiner ML, et al. Randomized, double-blind, placebo-controlled clinical trial for evaluating the efficacy of fractional CO2 laser compared with topical estriol in the treatment of vaginal atrophy in postmenopausal women. Menopause. 2018;25:21-28. doi: 10.1097 /GME.0000000000000955.
5. Mitchell L, Goldstein A, Heller D, et al. Fractionated carbon dioxide laser for the treatment of vulvar lichen sclerosus: a randomized controlled trial. Obstet Gynecol. 2021;137:979-987. doi: 10.1097/AOG.0000000000004409.

The approach to diagnosis and treatment of female sexual function continues to be a challenge for women’s health professionals. The search for a female “little blue pill” remains elusive as researchers struggle to understand the mechanisms that underlie the complex aspects of female sexual health. This Update will review the recent literature on the use of fractional CO2 laser for treatment of female sexual dysfunction and vulvovaginal symptoms. Bottom line: While the quality of the studies is poor overall, fractional CO2 laser treatment seems to temporarily improve symptoms of genitourinary syndrome of menopause (GSM). The duration of response, cost, and the overall long-term impact on sexual health remain in question.
A retrospective look at CO2 laser and postmenopausal GSM
Filippini M, Luvero D, Salvatore S, et al. Efficacy of fractional CO2 laser treatment in postmenopausal women with genitourinary syndrome: a multicenter study. Menopause. 2019;27:43-49. doi: 10.1097/GME. 0000000000001428.
Researchers conducted a retrospective, multicenter study of postmenopausal women with at least one symptom of GSM, including itching, burning, dyspareunia with penetration, and dryness.
Study details
A total of 171 of the 645 women (26.5%) were oncology patients. Women were excluded from analysis if they used any form of topical therapy within 15 days; had prolapse stage 2 or greater; or had any infection, abscess, or anatomical deformity precluding treatment with the laser.
Patients underwent gynecologic examination and were given a questionnaire to assess vulvovaginal symptoms. Exams occurred monthly during treatment (average, 6.5 months), at 6- and 12-months posttreatment, and then annually. No topical therapy was advised during or after treatment.
Patients received either 3 or 4 fractional CO2 laser treatments to the vulva and/or vagina depending on symptom location and type. Higher power settings of the same laser were used to treat vaginal symptoms (40W; 1,000 microseconds) versus vulvar symptoms (25W; 500 microseconds). Treatment sessions were 5 to 6 minutes. The study authors used a visual analog rating scale (VAS) for “atrophy and related symptoms,” tested vaginal pH, and completed the Vaginal Health Index Score. VAS scores were obtained from the patients prior to the initial laser intervention and 1 month after the final treatment.
Results
There were statistically significant improvements in dryness, vaginal orifice pain, dyspareunia, itching, and burning for both the 3-treatment and 4-treatment cohorts. The delta of improvement was then compared for the 2 subgroups; curiously, there was greater improvement of symptoms such as dryness (65% vs 61%), itching (78% vs 72%), burning (72% vs 67%), and vaginal orifice pain (67% vs 60%) in the group that received 3 cycles than in the group that received 4 cycles.
With regard to vaginal pH improvement, the 4-cycle group performed better than the 3-cycle group (1% improvement in the 4-cycle group vs 6% in the 3-cycle group). Although vaginal pH reduction was somewhat better in the group that received 4 treatments, and the pre versus posttreatment percentages were statistically significantly different, the clinical significance of a pH difference between 5.72 and 5.53 is questionable, especially since there was a greater difference in baseline pH between the two cohorts (6.08 in the 4-cycle group vs 5.59 in the 3-cycle group).
There were no reported adverse events related to the fractional laser treatments, and 6% of the patients underwent additional laser treatments during the followup timeframe of 8 to 20 months.
This was a retrospective study with no control or comparison group and short-term follow-up. The VAS scores were obtained 1 month after the final treatment. Failure to request additional treatment at 8 to 20 months cannot be used to infer that the therapeutic improvements recorded at 1 month were enduring. In addition, although the large number of patients in this study may lead to statistical significance, clinical significance is still questionable. Given the lack of a comparison group and the very short follow-up, it is hard to draw any scientifically valid conclusions from this study.
Continue to: Randomized data on CO2 laser vs Kegels for sexual dysfunction...
Randomized data on CO2 laser vs Kegels for sexual dysfunction
Lou W, Chen F, Xu T, et al. A randomized controlled study of vaginal fractional CO2 laser therapy for female sexual dysfunction. Lasers Med Sci. March 15, 2021. doi: 10.1007/s10103-021-03260-x.
In a small randomized controlled trial (RCT) conducted in China, Lou and colleagues identified premenopausal women at “high risk” for sexual dysfunction as determined by the Chinese version of the Female Sexual Function Index (CFSFI).
Details of the study
A total of 84 women (mean age, 36.5 years) were included in the study. All the participants were heterosexual and married or with a long-term partner. The domain of sexual dysfunction was not considered. Women were excluded if they had no current heterosexual partner; had genital malformation, urinary incontinence, or prolapse stage 2 or higher; a history of pelvic floor mesh treatment; current gynecologic malignancy; abnormal cervical cytology; or were currently pregnant or postpartum. In addition, women were excluded if they had been treated previously for sexual dysfunction or mental “disease.” The cohort was randomized to receive fractional CO2 laser treatments (three 15-minute treatments 1 month apart at 60W, 1,000 microseconds) or coached Kegel exercises (10 exercises repeated twice daily at least 3 times/week and monitored by physical therapists at biweekly clinic visits). Sexual distress was evaluated by using the Female Sexual Distress Scale-Revised (FSDSR). Outcomes measured were pelvic floor muscle strength and scores on the CFSFI and FSDSR. Data were obtained at 3, 6, 9, and 12 months after initiation of therapy.
Both groups showed improvement
The laser cohort showed slightly more improvement in scale scores at 6 and 12 months. Specifically, the laser group had better scores on lubrication and overall satisfaction, with moderate effect size; neither group had improvements in arousal, desire, or orgasm. The Kegel group showed a significant improvement in pelvic floor strength and orgasm at 12 months, an improvement not seen in the laser cohort. Both groups showed gradual improvement in the FSDSR, with the laser group reporting a lower score (10.0) at 12 months posttreatment relative to the Kegel group (11.1). Again, these were modest effects as baseline scores for both cohorts were around 12.5. There were minimal safety signals in the laser group, with 22.5% of women reporting scant bloody discharge posttreatment and 72.5% describing mild discomfort (1 on a 1–10 VAS scale) during the procedure.
This study is problematic in several areas. Although it was a prospective, randomized trial, it was not blinded, and the therapeutic interventions were markedly different in nature and requirement for individual patient motivation. The experiences of sexual dysfunction among the participants were not stratified by type—arousal, desire, lubrication, orgasm, or pain. All patients had regular cyclic menses; however, the authors do not report on contraceptive methods, hormonal therapy, or other comorbid conditions that could impact sexual health. The cohorts may or may not have been similar in baseline types of sexual dissatisfaction.
CO2 laser for lichen sclerosus: Is it effective?
Pagano T, Conforti A, Buonfantino C, et al. Effect of rescue fractional microablative CO2 laser on symptoms and sexual dysfunction in women affected by vulvar lichen sclerosus resistant to long-term use of topic corticosteroid: a prospective longitudinal study. Menopause. 2020;27:418-422. doi: 10.1097 /GME.0000000000001482.
Burkett LS, Siddique M, Zeymo A, et al. Clobetasol compared with fractionated carbon dioxide laser for lichen sclerosus: a randomized controlled trial. Obstet Gynecol. 2021;137:968-978. doi: 10.1097 /AOG.0000000000004332.
Mitchell L, Goldstein AT, Heller D, et al. Fractionated carbon dioxide laser for the treatment of vulvar lichen sclerosus: a randomized controlled trial. Obstet Gynecol. 2021;137:979-987. doi: 10.1097 /AOG.0000000000004409.
High potency corticosteroid ointment is the current standard treatment for lichen sclerosus. Alternative options for disease that is refractory to steroids are limited. Three studies published in the past year explored the CO2 laser’s ability to treat lichen sclerosus symptoms and resultant sexual dysfunction—Pagano and colleagues conducted a small prospective study and Burkett and colleagues and Mitchell et al conducted small RCTs.
Details of the Pagano study
Three premenopausal and 37 postmenopausal women with refractory lichen sclerosus (defined as no improvement after 4 cycles of ultra-high potency steroids) were included in the study. Lichen sclerosus was uniformly biopsy confirmed. Women using topical or systemic hormones were excluded. VAS was administered prior to initial treatment and after each of 2 fractional CO2 treatments (25–30 W; 1,000 microseconds) 30 to 40 days apart to determine severity of vulvar itching, dyspareunia with penetration, vulvar dryness, sexual dysfunction, and procedure discomfort. Follow-up was conducted at 1 month after the final treatment. VAS score for the primary outcome of vulvar itching declined from 8 pretreatment to 6 after the first treatment and to 3 after the second. There was no significant treatment-related pain reported.
The authors acknowledged the limitations of their study; it was a relatively small sample size, nonrandomized and had short-term follow-up of a mixed patient population and no sham or control group. The short-term improvements reported in the study patients may not be sustained without ongoing treatment for a lifelong chronic disease, and the long-term potential for development of squamous cell carcinoma may or may not be ameliorated.
Continue to: Burkett et al: RCT study 1...
Burkett et al: RCT study 1
A total of 52 postmenopausal patients with biopsy-proven lichen sclerosus were randomly assigned to clobetasol or CO2 laser; 51 women completed 6-month follow-up. The outcomes were stratified by prior high-potency steroid use. The steroid cohort used clobetasol 0.05% nightly for 1 month, 3 times per week for 2 months, then as needed. The laser cohort received 3 treatments (26 W; 800 microseconds) 4 to 6 weeks apart. Overall adherence was only 75% in the clobetasol group, compared with 96% in the laser group. The authors found treatment efficacy of CO2 laser therapy only in the group of patients who had prior treatment with high potency topical corticosteroids. They conclude that, …“Despite previously optimistic results in well designed clinical trials of fractionated CO2 for genitourinary syndrome of menopause, and in noncontrolled case series for vulvar lichen sclerosus, our study failed to show any significant benefit of monotherapy of fractionated CO2 for vulvar lichen sclerosus. There may be a role for fractionated CO2 as an adjuvant therapy along with topical ultrapotent corticosteroids in vulvar lichen sclerosus.”
Mitchell et al: RCT study 2
This was a double blind, placebo-controlled, and histologically validated study of fractional CO2 for treatment of lichen sclerosus in 35 women; 17 in the treatment arm and 18 in the sham laser encounters. At least a 4-week no treatment period of topical steroids was required before monotherapy with CO2 laser was initiated.
The authors found no difference in their primary outcome—histopathology scale scores—after 5 treatments over 24 weeks. Secondary endpoints were changes in the CSS (Clinical Scoring System for Vulvar Lichen Sclerosus), a validated instrument that includes both a clinician’s examination of the severity of disease and a patient’s report of the severity of her symptoms. The patient score is the total of 4 domains: itching, soreness, burning, and dyspareunia. The clinician objective examination documents fissures, erosions, hyperkeratosis, agglutination, stenosis, and atrophy. At the conclusion of treatment there were no significant differences in the patient reported symptoms or the clinical findings between the active treatment and sham groups.
As a monotherapy, CO2 laser therapy is not effective in treating lichen sclerosus, although it may help improve symptoms as an adjunct to high potency steroid therapy when topical treatment alone has failed to provide adequate response.
Conclusion
The quality of evidence to support the use of the CO2 laser for improvement in sexual dysfunction is poor. Although patient satisfaction scores improved overall, and most specifically for symptoms related to GSM, the lack of blinding; inappropriate or no control groups; the very short-term outcomes; and for one of the studies, the lack of a clear definition of sexual dysfunction, make it difficult to draw meaningful conclusions for clinical care.
For GSM, we know that topical estrogen therapy works—and with little to no systemic absorption. The CO2 laser should be studied in comparison to this gold standard, with consideration of costs and potential long-term harms in addition to patient satisfaction and short-term measures of improvement. In addition, and very importantly, it is our professional responsibility to present the evidence for safety of topical estrogens to our professional colleagues as well as to our patients with estrogen-dependent cancers so that they understand the value of estrogen as a safe and appropriate alternative to expensive and potentially short-term interventions such as CO2 laser treatment. ●

Cheryl Iglesia, MD
Dr. Iglesia is Director, Section of Female Pelvic Medicine and Reconstructive Surgery, MedStar Washington Hospital Center, and Professor, Departments of ObGyn and Urology, Georgetown University School of Medicine, Washington, DC. She is a member of the OBG Management Board of Editors.
Barbara Levy, MD: Cheryl, you have more experience with use of the energy-based cosmetic laser than most ObGyns, and I thought that speaking with you about this technology would be of benefit, not only to me in learning more about the hands-on experience of a lead researcher and practitioner but also readers who are hearing more and more about the growth of cosmetic gynecology in general. Thank you for taking the time today.
Cheryl Iglesia, MD: I’m happy to speak about this with you, Barbara.
Dr. Levy: Specifically, I would like to talk about use of these technologies for sexual dysfunction. In the last few years some of the available data have been on the CO2 laser versus physical therapy, which is not an appropriate comparison.1
Dr. Iglesia: There have been limited data, and less randomized, controlled data, on laser and radiofrequency energies for cosmetic gynecology, and in fact these devices remain unapproved for any gynecologic indication. In 2018 the US Food and Drug Administration (FDA) issued a Safety Communication about the use of energy-based devices to perform vaginal rejuvenation or cosmetic procedures. The International Urogynecological Association (IUGA) issued a consensus statement echoing concerns about the devices, and an International Continence Society/International Society for the Study of Vulvovaginal Disease Best Practice Consensus Statement did not recommend the laser for “routine treatment of vaginal atrophy or urinary incontinence unless treatment is part of a well-designed trial or with special arrangements for clinical governance, consent, and audit.”2
In May 2020, as evidence remains limited (although 522 studies are ongoing in coordination with the FDA), the American Urogynecologic Society (AUGS) published a clinical consensus statement from a panel of experts in female pelvic medicine and reconstructive surgery. The panel had about 90% consensus that there is short-term efficacy for the laser with GSM and dyspareunia. But we only have outcomes data that lasts a maximum of 1 year.2
A problem with our VeLVET trial,3 which was published in Menopause, and the Cruz and colleagues’ trial from South America,4 both of which compared the CO2 laser to estrogen and had randomized groups, was that they were limited by the outcome measures used, none of which were consistently validated. But these studies also had small numbers of participants and short-term follow-up. So I don’t think there are much existing data that are promising for supporting energy-based treatment for GSM.
We also have just-published data on the laser for lichen sclerosus.5 For the AUGS panel, there was about 80% consensus for energy-based-device use and lichen sclerosus.2 According to Mitchell et al, who conducted a small, randomized, sham-controlled trial, CO2 laser resulted in no significant difference in histopathology scale score between active and sham arms.5
Future trials may want to assess laser as a mechanism for improved local drug delivery (eg, use of combined laser plus local estrogen for GSM or combined laser plus topical steroid for lichen sclerosus). I am also aware that properly designed laser versus sham studies are underway.
Dr. Levy: What about for stress urinary incontinence (SUI)? I don’t think these technologies are going to work.
Dr. Iglesia: For the AUGS panel, there was only about 70% consensus for energy-based-device use and SUI,2 and I’m one of the naysayers. The pathophysiology of SUI is so multifactorial that it’s hard to believe that laser or radiofrequency wand therapy could have sustained improvements, especially since prior radiofrequency therapy from the last decade (for instance, Renessa, Novasys Medical) did not show long-term efficacy.
Understanding lasers and coordinating care
Dr. Levy: We don’t know what the long-term outcomes are for the CO2 laser and GSM.
Dr. Iglesia: I agree with you, and I think there needs to be an understanding of the mechanism of how lasers work, whether it be erbium (Er:YAG), which is the most common, or CO2. Erbium and CO2 lasers, which are on the far-infrared spectrum, target the chromophore, water. My feeling is that, when you look at results from the Cruz trial,4 or even our trial that compared vaginal estrogen with laser,3 when there is severe GSM and high pH with virtually no water present in the tissues, that laser is not going to properly function. But I don’t think we know exactly what optimal pretreatment is necessary, and that is one of the problems. Furthermore, when intravaginal lasers are done and no adequate speculum exam is conducted prior to introducing the laser, there could be discharge or old creams present that block the mirrors necessary to adequately fire the fractionated laser beams.
Unfortunately, oftentimes these devices are marketed to women with breast cancer, who may be taking aromatase inhibitors, which cause the no-water problem; they dry out everything. They are effective for preventing breast cancer recurrence, but they cause severe atrophy (perhaps worse than many of the other selective estrogen-receptor modulators), with a resultant high vaginal pH. If we can bring that pH level down, closer to the normal 4.5 range so that we could have some level of moisture, and add estrogen first, the overall treatment approach will probably be more effective. We still do not know what happens after 1 year, though, and how often touch-ups need to be performed.
In fact, when working with a patient with breast cancer, I will speak with her oncologist; I will collaborate to put in place a treatment plan that may include initial pretreatment with low-dose vaginal estrogen followed by laser treatment for vaginal atrophy. But I will make sure I use the lowest dose. Sometimes when the patient comes back, the estrogen’s worked so well she’ll say, “Oh, I’m happy, so I don’t need the laser anymore.” A balanced conversation is necessary, especially with cancer survivors.
Informing patients and colleagues
Dr. Levy: I completely agree, and I think one of the key points here is that our purpose is to serve our patients. The data demonstrate that low doses of vaginal estrogen are not harmful for women who are being treated for or who have recovered from breast cancer. It is our ethical obligation to convince these women and their oncologists that ongoing treatment with vaginal estrogen not only will help their GSM but also their overactive bladder and their risk of urinary tract infections and other things. We could be exploiting patients who are really fearful of using any estrogen because of a perceived cancer risk. We could actually be validating their fear by telling them we have an alternative treatment for which they have to pay cash.
Treatment access
Dr. Iglesia: Yes, these are not cosmetic conditions that we are treating. So my goal when evaluating treatment for refractory GSM or lichen sclerosus is to find optimal energy-based therapies with the hope that one day these will be approved gynecologic conditions by the US FDA for laser and wand therapies and that they will ultimately not be out-of-pocket expenses but rather therapies covered by insurance.
Dr. Levy: Great. I understand that AUGS/IUGA have been working on a terminology algorithm to help distinguish between procedures being performed to resolve a medical problem such as prolapse or incontinence versus those designed to be cosmetic.
Dr. Iglesia: Yes, there is a big document from experts in both societies out for public comment right now. It will hopefully be published soon.
Outstanding questions remain
Dr. Levy: Really, we as ObGyns shouldn’t be quick to incorporate these things into our practices without high-quality studies demonstrating value. I have a major concern about these devices in the long term. When you look at fractional CO2 use on the face, for instance, which is a much different type of skin than the vagina, the laser builds collagen—but we don’t have long-term outcome results. The vagina is supposed to be an elastic tissue, so what is the risk of long-term scarring there? Yes, the laser builds collagen in the vaginal epithelium, but what does it do to scarring in the rest of the tissue? We don’t have answers to that.
Dr. Iglesia: And that is the question—how does histology equate with function? Well, I would go with what the patients are reporting.
Dr. Levy: Absolutely. But the thing about vaginal low-dose estrogen is that it is something that the oncologists or the ObGyns could be implementing with patients while they are undergoing cancer therapy, while in their menopausal transition, to preserve vulvovaginal function as opposed to trying to regain it.
Dr. Iglesia: Certainly, although it still needs to be determined when that type of approach would actually be contraindicated.
Dr. Levy: Thank you, Cheryl, for your valuable insights.
Dr. Iglesia: Of course. Thank you. ●
References
1. Lou W, Chen F, Xu T, et al. A randomized controlled study of vaginal fractional CO2 laser therapy for female sexual dysfunction. Lasers Med Sci. March 15, 2021. doi: 10.1007/s10103-021-03260-x.
2. Alshiek J, Garcia B, Minassian V, et al. Vaginal energy-based devices. Female Pelvic Med Reconstr Surg. 2020;26:287-298. doi: 10.1097 /SPV.0000000000000872.
3. Paraiso MF, Ferrando CA, et al. A randomized clinical trial comparing vaginal laser therapy to vaginal estrogen therapy in women with genitourinary syndrome of menopause: the VeLVET Trial. Menopause. 2020;27:50-56. doi: 10.1097/GME.0000000000001416.
4. Cruz VL, Steiner ML, et al. Randomized, double-blind, placebo-controlled clinical trial for evaluating the efficacy of fractional CO2 laser compared with topical estriol in the treatment of vaginal atrophy in postmenopausal women. Menopause. 2018;25:21-28. doi: 10.1097 /GME.0000000000000955.
5. Mitchell L, Goldstein A, Heller D, et al. Fractionated carbon dioxide laser for the treatment of vulvar lichen sclerosus: a randomized controlled trial. Obstet Gynecol. 2021;137:979-987. doi: 10.1097/AOG.0000000000004409.
Grandmothers, the Friendship Bench, and wisdom
Is this model a blueprint for delivering mental health care?
The 4-year-old boy and his grandmother are out for stroll around the neighborhood, walking hand in hand.
“Let’s sit on the bench and talk,” the boy says.
“Okay,” says the grandmother and they climb up onto the high bench and look out across the quiet road to a small garden beyond.
“What would you like to talk about?” his grandmother asks.
“You first,” he says.
“Okay, let’s see ... the grandmother and the grandson are out for a walk and they see a bench to sit on. They climb up and look around. They see the daffodils and the white clouds in the blue sky. The breeze is blowing gently. It is a happy day. Your turn; what would you like to talk about?”
“Nanna and Papa.”
“Do you miss Papa?”
“Yes.”
“It has been a whole year since he died.”
“A long, long time.”
“He loved you very much.”
“Yes,” the boy replies.
“Nanna must miss him very much. She must be lonely without him.”
The boy nods.
They sit on for a while, watching the occasional car and the occasional bird pass by. The boy and the grandmother are quiet and contemplative.
“Okay, let’s go,” he says and jumps down, ready to continue their walk.
The Friendship Bench
It must have been such an experience that gave Dixon Chibanda, MD, MPH, PhD, a psychiatrist from Zimbabwe, his brilliant idea. He trained grandmothers in evidence-based talk therapy and sat them on a bench in the park with his patients.1,2 He founded the Friendship Bench in 2006 in the Harare township of Mbare with 14 grandmothers. There are more than 300 grandmothers sitting on benches, listening, and providing cognitive-behavioral therapy–informed interventions because he could find no therapists in the community and he found that, with a little training, these grandmothers could provide effective culturally sensitive interventions.
Originally, the sessions were conducted in Shona, the predominant native language in Zimbabwe, but since 2017, the sessions are also in English. By 2017, the Friendship Bench had helped more than 30,000 people. The method has been empirically vetted and expanded to countries beyond, including the United States. Dr. Chibanda’s Friendship Bench serves as a blueprint for any community interested in bringing affordable, accessible, and highly effective mental health services to its residents. Dr. Chibanda said: “Imagine if we could create a global network of grandmothers in every major city in the world.”3 Participants in this study reported that the Friendship Bench had a critical role in helping them accept their HIV status, citing the grandmothers’ empathic attitude, their normalization of the reality of living with HIV, and their encouragement of young people to socialize with peers and be free of guilt. Many recipients also described enhanced health and well-being.
Why grandmothers?
Have you heard of the evolutionary importance of grandmothers? The grandmother hypothesis is an adaptationist explanation for the fact that the human female lifespan extends beyond the period of fertility. A third of the average human female life span is post menopause. Does such a long female postreproductive life span have a reason, inquired Mwenza Blell, PhD.5
Peter B. Medawar, PhD,6 and Kristen Hawkes, PhD,7 suggested that grandparents influence their own fitness by their actions toward their grandchildren. International fieldwork has revealed that the situation is less clear than their hypothesis. In industrialized countries, grandmaternal support is often financial or emotional. Two meta-analyses of largely the same group of studies investigating grandmother effects have come up with differing conclusions. Rebecca Sear, PhD, and Ruth Mace, PhD, conclude that grandmothers are “almost universally” beneficial, while acknowledging some variation in the effects of paternal grandmothers.8 Maternal grandparents appear to invest more in their grandchildren than paternal grandparents. Beverly I. Strassmann, PhD, and Wendy M. Garrard, PhD, concluded that, in patrilineal societies, survival of maternal grandparents is associated with survival of grandchildren and suggest this may represent covert matriliny.9
Examining specific time periods, maternal grandmothers may have greatest effect on survival of grandchildren at the time of weaning, a time when increased pathogenic exposure is a threat to survival. Paternal grandmothers may influence the survival of grandchildren during the early period of life (1-12 months) and to influence the condition of their daughters-in-law during pregnancy. The fact that grandmothers share one X chromosome with their sons’ daughters, none with their sons’ sons, and have a 50% chance of sharing an X chromosome with their daughters’ children is suggested to explain the patterns of survival observed in these studies than a simple maternal/paternal division.
In low- and middle-income countries, grandmothers and older women are seen as owners of traditional knowledge, and influence many decisions about childcare, help with domestic work, and emotional support and advice.10 Studies find a significant positive impact on breastfeeding when grandmothers of the infants had their own breastfeeding experience or were positively inclined toward breastfeeding, although one Chinese study found that highly educated grandmothers were associated with decreased exclusive breastfeeding.11 Despite this, most health programs target individual new mothers, without an understanding of the family and who else influences decisions.
Grandchildren and grandparents benefit from intergenerational activities with improved health and well-being of both generations. When older adults are involved in raising children, there is a significant reduction in the incidence of behavioral problems in childhood and adolescence. Grandparents improve grandchild outcomes, when measured by coresidence, caregiving, financial, and other support. The grandchild outcomes include physical health, socioemotional well-being, and cognitive development.12
Are there ‘grandparent genes?’
Flavio Schwarz, PhD, and colleagues think that variants of APOE and CD33 protect against heart disease and Alzheimer’s disease, allowing older people to live longer with better functioning hearts and brains – thus enabling transfer of wisdom from older to younger generations.13 While this logic may be a bit of a stretch, it does lead to a more interesting question: What has wisdom got to do with it?
When I ask psychiatrists what they think about wisdom, they give a variety of answers. Dilip Jeste, MD, a geriatric psychiatrist who studies successful aging, helped develop a measurable vision of wisdom.14 Wisdom is defined as a “multidimensional human trait that includes good social decision-making and pragmatic knowledge of life, prosocial attitudes and behaviors such as empathy and compassion, emotional homeostasis with a tendency to favor positive emotions, reflection and self-understanding, acknowledgment of and coping effectively with uncertainty, and decisiveness.”15 Others suggest that they include spirituality, openness to new experience, and a sense of humor.16 A scale called the San Diego Wisdom scale (SD-WISE) was created, using 524 community-dwelling adults aged 25-104 years. These subjects comprised a high proportion of White adults and individuals with a higher education, thus lacking diversity. Lack of diversity perpetuates generalizations, and like all sociocultural constructs, truth is specific to the population studied. High scores on the SD-WISE are positively correlated with good mental health, self-ratings of successful aging, mastery, resilience, happiness, and satisfaction with life.
Which brings us back to the grandmothers on the bench: Can someone please give them the SD-WISE scale and confirm several hypotheses? I would like to know whether a pragmatic knowledge of life is a recognized grandmotherly quality, suitable for the bench.
Dr. Heru is professor of psychiatry at the University of Colorado at Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). She has no conflicts of interest.
References
1. Chibanda D. Bull World Health Organ. 2018 Jun 196(6):376-7.
2. Cavanaugh R. Lancet Psychiatry. 2017 Nov. doi: 10.1016/S2215-0366(17)30420-0.
3. Nuwer R. “How a bench and a team of grandmothers can tackle depression.” BBC. 2020 May 27.
4. Ouansafi I et al. PLoS One. 2021 Apr 22;16(4):e0250074.
5. Blell M. “Grandmother hypothesis, grandmother effect, and residence patterns.” Int Encyclopedia Anthropol. John Wiley & Sons, 2018.
6. Medawar PB. An Unsolved Problem of Biology. Routledge, 1957.
7. Hawkes K et al. Proc Nat Acad Sci. 1998 Feb 395(3):1336-9.
8. Sear R and Mace R. Evol Hum Behav. 2008;29(1):1-18.
9. Strassmann B and Garrard WM. Hum Nat. 2011 Jul;22(1-2):201-22.
10. Aubel J. BMJ Glob Health. 2021;6(2). doi 10.1136/bmjgh-2020-003808.
11. Negin J et al. BMJ Pregnancy Childbirth. 2016 Apr 7. doi: 10.1186/s12884-016-0880-5.
12. Sadruddin AFA. Soc Sci Med. 2019 Aug;239(4):112476.
13. Schwarz F et al. Proc Nat Acad Sci. 2016 Jan 5;113(1):74-9.
14. Jeste DV et al. Psychol Inquiry. 2020 Jun 22;31(2):134-43.
15. Meeks TW and Jeste DV. Arch Gen Psychiatry. 2009 Apr;66(4):355-65.
16. Bangen KJ et al. Am J Geriatr Psychiatry. 2013 Dec;21(12):1254-66.
Is this model a blueprint for delivering mental health care?
Is this model a blueprint for delivering mental health care?
The 4-year-old boy and his grandmother are out for stroll around the neighborhood, walking hand in hand.
“Let’s sit on the bench and talk,” the boy says.
“Okay,” says the grandmother and they climb up onto the high bench and look out across the quiet road to a small garden beyond.
“What would you like to talk about?” his grandmother asks.
“You first,” he says.
“Okay, let’s see ... the grandmother and the grandson are out for a walk and they see a bench to sit on. They climb up and look around. They see the daffodils and the white clouds in the blue sky. The breeze is blowing gently. It is a happy day. Your turn; what would you like to talk about?”
“Nanna and Papa.”
“Do you miss Papa?”
“Yes.”
“It has been a whole year since he died.”
“A long, long time.”
“He loved you very much.”
“Yes,” the boy replies.
“Nanna must miss him very much. She must be lonely without him.”
The boy nods.
They sit on for a while, watching the occasional car and the occasional bird pass by. The boy and the grandmother are quiet and contemplative.
“Okay, let’s go,” he says and jumps down, ready to continue their walk.
The Friendship Bench
It must have been such an experience that gave Dixon Chibanda, MD, MPH, PhD, a psychiatrist from Zimbabwe, his brilliant idea. He trained grandmothers in evidence-based talk therapy and sat them on a bench in the park with his patients.1,2 He founded the Friendship Bench in 2006 in the Harare township of Mbare with 14 grandmothers. There are more than 300 grandmothers sitting on benches, listening, and providing cognitive-behavioral therapy–informed interventions because he could find no therapists in the community and he found that, with a little training, these grandmothers could provide effective culturally sensitive interventions.
Originally, the sessions were conducted in Shona, the predominant native language in Zimbabwe, but since 2017, the sessions are also in English. By 2017, the Friendship Bench had helped more than 30,000 people. The method has been empirically vetted and expanded to countries beyond, including the United States. Dr. Chibanda’s Friendship Bench serves as a blueprint for any community interested in bringing affordable, accessible, and highly effective mental health services to its residents. Dr. Chibanda said: “Imagine if we could create a global network of grandmothers in every major city in the world.”3 Participants in this study reported that the Friendship Bench had a critical role in helping them accept their HIV status, citing the grandmothers’ empathic attitude, their normalization of the reality of living with HIV, and their encouragement of young people to socialize with peers and be free of guilt. Many recipients also described enhanced health and well-being.
Why grandmothers?
Have you heard of the evolutionary importance of grandmothers? The grandmother hypothesis is an adaptationist explanation for the fact that the human female lifespan extends beyond the period of fertility. A third of the average human female life span is post menopause. Does such a long female postreproductive life span have a reason, inquired Mwenza Blell, PhD.5
Peter B. Medawar, PhD,6 and Kristen Hawkes, PhD,7 suggested that grandparents influence their own fitness by their actions toward their grandchildren. International fieldwork has revealed that the situation is less clear than their hypothesis. In industrialized countries, grandmaternal support is often financial or emotional. Two meta-analyses of largely the same group of studies investigating grandmother effects have come up with differing conclusions. Rebecca Sear, PhD, and Ruth Mace, PhD, conclude that grandmothers are “almost universally” beneficial, while acknowledging some variation in the effects of paternal grandmothers.8 Maternal grandparents appear to invest more in their grandchildren than paternal grandparents. Beverly I. Strassmann, PhD, and Wendy M. Garrard, PhD, concluded that, in patrilineal societies, survival of maternal grandparents is associated with survival of grandchildren and suggest this may represent covert matriliny.9
Examining specific time periods, maternal grandmothers may have greatest effect on survival of grandchildren at the time of weaning, a time when increased pathogenic exposure is a threat to survival. Paternal grandmothers may influence the survival of grandchildren during the early period of life (1-12 months) and to influence the condition of their daughters-in-law during pregnancy. The fact that grandmothers share one X chromosome with their sons’ daughters, none with their sons’ sons, and have a 50% chance of sharing an X chromosome with their daughters’ children is suggested to explain the patterns of survival observed in these studies than a simple maternal/paternal division.
In low- and middle-income countries, grandmothers and older women are seen as owners of traditional knowledge, and influence many decisions about childcare, help with domestic work, and emotional support and advice.10 Studies find a significant positive impact on breastfeeding when grandmothers of the infants had their own breastfeeding experience or were positively inclined toward breastfeeding, although one Chinese study found that highly educated grandmothers were associated with decreased exclusive breastfeeding.11 Despite this, most health programs target individual new mothers, without an understanding of the family and who else influences decisions.
Grandchildren and grandparents benefit from intergenerational activities with improved health and well-being of both generations. When older adults are involved in raising children, there is a significant reduction in the incidence of behavioral problems in childhood and adolescence. Grandparents improve grandchild outcomes, when measured by coresidence, caregiving, financial, and other support. The grandchild outcomes include physical health, socioemotional well-being, and cognitive development.12
Are there ‘grandparent genes?’
Flavio Schwarz, PhD, and colleagues think that variants of APOE and CD33 protect against heart disease and Alzheimer’s disease, allowing older people to live longer with better functioning hearts and brains – thus enabling transfer of wisdom from older to younger generations.13 While this logic may be a bit of a stretch, it does lead to a more interesting question: What has wisdom got to do with it?
When I ask psychiatrists what they think about wisdom, they give a variety of answers. Dilip Jeste, MD, a geriatric psychiatrist who studies successful aging, helped develop a measurable vision of wisdom.14 Wisdom is defined as a “multidimensional human trait that includes good social decision-making and pragmatic knowledge of life, prosocial attitudes and behaviors such as empathy and compassion, emotional homeostasis with a tendency to favor positive emotions, reflection and self-understanding, acknowledgment of and coping effectively with uncertainty, and decisiveness.”15 Others suggest that they include spirituality, openness to new experience, and a sense of humor.16 A scale called the San Diego Wisdom scale (SD-WISE) was created, using 524 community-dwelling adults aged 25-104 years. These subjects comprised a high proportion of White adults and individuals with a higher education, thus lacking diversity. Lack of diversity perpetuates generalizations, and like all sociocultural constructs, truth is specific to the population studied. High scores on the SD-WISE are positively correlated with good mental health, self-ratings of successful aging, mastery, resilience, happiness, and satisfaction with life.
Which brings us back to the grandmothers on the bench: Can someone please give them the SD-WISE scale and confirm several hypotheses? I would like to know whether a pragmatic knowledge of life is a recognized grandmotherly quality, suitable for the bench.
Dr. Heru is professor of psychiatry at the University of Colorado at Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). She has no conflicts of interest.
References
1. Chibanda D. Bull World Health Organ. 2018 Jun 196(6):376-7.
2. Cavanaugh R. Lancet Psychiatry. 2017 Nov. doi: 10.1016/S2215-0366(17)30420-0.
3. Nuwer R. “How a bench and a team of grandmothers can tackle depression.” BBC. 2020 May 27.
4. Ouansafi I et al. PLoS One. 2021 Apr 22;16(4):e0250074.
5. Blell M. “Grandmother hypothesis, grandmother effect, and residence patterns.” Int Encyclopedia Anthropol. John Wiley & Sons, 2018.
6. Medawar PB. An Unsolved Problem of Biology. Routledge, 1957.
7. Hawkes K et al. Proc Nat Acad Sci. 1998 Feb 395(3):1336-9.
8. Sear R and Mace R. Evol Hum Behav. 2008;29(1):1-18.
9. Strassmann B and Garrard WM. Hum Nat. 2011 Jul;22(1-2):201-22.
10. Aubel J. BMJ Glob Health. 2021;6(2). doi 10.1136/bmjgh-2020-003808.
11. Negin J et al. BMJ Pregnancy Childbirth. 2016 Apr 7. doi: 10.1186/s12884-016-0880-5.
12. Sadruddin AFA. Soc Sci Med. 2019 Aug;239(4):112476.
13. Schwarz F et al. Proc Nat Acad Sci. 2016 Jan 5;113(1):74-9.
14. Jeste DV et al. Psychol Inquiry. 2020 Jun 22;31(2):134-43.
15. Meeks TW and Jeste DV. Arch Gen Psychiatry. 2009 Apr;66(4):355-65.
16. Bangen KJ et al. Am J Geriatr Psychiatry. 2013 Dec;21(12):1254-66.
The 4-year-old boy and his grandmother are out for stroll around the neighborhood, walking hand in hand.
“Let’s sit on the bench and talk,” the boy says.
“Okay,” says the grandmother and they climb up onto the high bench and look out across the quiet road to a small garden beyond.
“What would you like to talk about?” his grandmother asks.
“You first,” he says.
“Okay, let’s see ... the grandmother and the grandson are out for a walk and they see a bench to sit on. They climb up and look around. They see the daffodils and the white clouds in the blue sky. The breeze is blowing gently. It is a happy day. Your turn; what would you like to talk about?”
“Nanna and Papa.”
“Do you miss Papa?”
“Yes.”
“It has been a whole year since he died.”
“A long, long time.”
“He loved you very much.”
“Yes,” the boy replies.
“Nanna must miss him very much. She must be lonely without him.”
The boy nods.
They sit on for a while, watching the occasional car and the occasional bird pass by. The boy and the grandmother are quiet and contemplative.
“Okay, let’s go,” he says and jumps down, ready to continue their walk.
The Friendship Bench
It must have been such an experience that gave Dixon Chibanda, MD, MPH, PhD, a psychiatrist from Zimbabwe, his brilliant idea. He trained grandmothers in evidence-based talk therapy and sat them on a bench in the park with his patients.1,2 He founded the Friendship Bench in 2006 in the Harare township of Mbare with 14 grandmothers. There are more than 300 grandmothers sitting on benches, listening, and providing cognitive-behavioral therapy–informed interventions because he could find no therapists in the community and he found that, with a little training, these grandmothers could provide effective culturally sensitive interventions.
Originally, the sessions were conducted in Shona, the predominant native language in Zimbabwe, but since 2017, the sessions are also in English. By 2017, the Friendship Bench had helped more than 30,000 people. The method has been empirically vetted and expanded to countries beyond, including the United States. Dr. Chibanda’s Friendship Bench serves as a blueprint for any community interested in bringing affordable, accessible, and highly effective mental health services to its residents. Dr. Chibanda said: “Imagine if we could create a global network of grandmothers in every major city in the world.”3 Participants in this study reported that the Friendship Bench had a critical role in helping them accept their HIV status, citing the grandmothers’ empathic attitude, their normalization of the reality of living with HIV, and their encouragement of young people to socialize with peers and be free of guilt. Many recipients also described enhanced health and well-being.
Why grandmothers?
Have you heard of the evolutionary importance of grandmothers? The grandmother hypothesis is an adaptationist explanation for the fact that the human female lifespan extends beyond the period of fertility. A third of the average human female life span is post menopause. Does such a long female postreproductive life span have a reason, inquired Mwenza Blell, PhD.5
Peter B. Medawar, PhD,6 and Kristen Hawkes, PhD,7 suggested that grandparents influence their own fitness by their actions toward their grandchildren. International fieldwork has revealed that the situation is less clear than their hypothesis. In industrialized countries, grandmaternal support is often financial or emotional. Two meta-analyses of largely the same group of studies investigating grandmother effects have come up with differing conclusions. Rebecca Sear, PhD, and Ruth Mace, PhD, conclude that grandmothers are “almost universally” beneficial, while acknowledging some variation in the effects of paternal grandmothers.8 Maternal grandparents appear to invest more in their grandchildren than paternal grandparents. Beverly I. Strassmann, PhD, and Wendy M. Garrard, PhD, concluded that, in patrilineal societies, survival of maternal grandparents is associated with survival of grandchildren and suggest this may represent covert matriliny.9
Examining specific time periods, maternal grandmothers may have greatest effect on survival of grandchildren at the time of weaning, a time when increased pathogenic exposure is a threat to survival. Paternal grandmothers may influence the survival of grandchildren during the early period of life (1-12 months) and to influence the condition of their daughters-in-law during pregnancy. The fact that grandmothers share one X chromosome with their sons’ daughters, none with their sons’ sons, and have a 50% chance of sharing an X chromosome with their daughters’ children is suggested to explain the patterns of survival observed in these studies than a simple maternal/paternal division.
In low- and middle-income countries, grandmothers and older women are seen as owners of traditional knowledge, and influence many decisions about childcare, help with domestic work, and emotional support and advice.10 Studies find a significant positive impact on breastfeeding when grandmothers of the infants had their own breastfeeding experience or were positively inclined toward breastfeeding, although one Chinese study found that highly educated grandmothers were associated with decreased exclusive breastfeeding.11 Despite this, most health programs target individual new mothers, without an understanding of the family and who else influences decisions.
Grandchildren and grandparents benefit from intergenerational activities with improved health and well-being of both generations. When older adults are involved in raising children, there is a significant reduction in the incidence of behavioral problems in childhood and adolescence. Grandparents improve grandchild outcomes, when measured by coresidence, caregiving, financial, and other support. The grandchild outcomes include physical health, socioemotional well-being, and cognitive development.12
Are there ‘grandparent genes?’
Flavio Schwarz, PhD, and colleagues think that variants of APOE and CD33 protect against heart disease and Alzheimer’s disease, allowing older people to live longer with better functioning hearts and brains – thus enabling transfer of wisdom from older to younger generations.13 While this logic may be a bit of a stretch, it does lead to a more interesting question: What has wisdom got to do with it?
When I ask psychiatrists what they think about wisdom, they give a variety of answers. Dilip Jeste, MD, a geriatric psychiatrist who studies successful aging, helped develop a measurable vision of wisdom.14 Wisdom is defined as a “multidimensional human trait that includes good social decision-making and pragmatic knowledge of life, prosocial attitudes and behaviors such as empathy and compassion, emotional homeostasis with a tendency to favor positive emotions, reflection and self-understanding, acknowledgment of and coping effectively with uncertainty, and decisiveness.”15 Others suggest that they include spirituality, openness to new experience, and a sense of humor.16 A scale called the San Diego Wisdom scale (SD-WISE) was created, using 524 community-dwelling adults aged 25-104 years. These subjects comprised a high proportion of White adults and individuals with a higher education, thus lacking diversity. Lack of diversity perpetuates generalizations, and like all sociocultural constructs, truth is specific to the population studied. High scores on the SD-WISE are positively correlated with good mental health, self-ratings of successful aging, mastery, resilience, happiness, and satisfaction with life.
Which brings us back to the grandmothers on the bench: Can someone please give them the SD-WISE scale and confirm several hypotheses? I would like to know whether a pragmatic knowledge of life is a recognized grandmotherly quality, suitable for the bench.
Dr. Heru is professor of psychiatry at the University of Colorado at Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). She has no conflicts of interest.
References
1. Chibanda D. Bull World Health Organ. 2018 Jun 196(6):376-7.
2. Cavanaugh R. Lancet Psychiatry. 2017 Nov. doi: 10.1016/S2215-0366(17)30420-0.
3. Nuwer R. “How a bench and a team of grandmothers can tackle depression.” BBC. 2020 May 27.
4. Ouansafi I et al. PLoS One. 2021 Apr 22;16(4):e0250074.
5. Blell M. “Grandmother hypothesis, grandmother effect, and residence patterns.” Int Encyclopedia Anthropol. John Wiley & Sons, 2018.
6. Medawar PB. An Unsolved Problem of Biology. Routledge, 1957.
7. Hawkes K et al. Proc Nat Acad Sci. 1998 Feb 395(3):1336-9.
8. Sear R and Mace R. Evol Hum Behav. 2008;29(1):1-18.
9. Strassmann B and Garrard WM. Hum Nat. 2011 Jul;22(1-2):201-22.
10. Aubel J. BMJ Glob Health. 2021;6(2). doi 10.1136/bmjgh-2020-003808.
11. Negin J et al. BMJ Pregnancy Childbirth. 2016 Apr 7. doi: 10.1186/s12884-016-0880-5.
12. Sadruddin AFA. Soc Sci Med. 2019 Aug;239(4):112476.
13. Schwarz F et al. Proc Nat Acad Sci. 2016 Jan 5;113(1):74-9.
14. Jeste DV et al. Psychol Inquiry. 2020 Jun 22;31(2):134-43.
15. Meeks TW and Jeste DV. Arch Gen Psychiatry. 2009 Apr;66(4):355-65.
16. Bangen KJ et al. Am J Geriatr Psychiatry. 2013 Dec;21(12):1254-66.
3 cases of hormone therapy optimized to match the patient problem
There are dozens of medications containing combinations of estrogen and progestin. I am often confused by the bewildering proliferation of generic brand names used to describe the same estrogen-progestin (E-P) regimen. For example, the combination medication containing ethinyl estradiol 20 µg plus norethindrone acetate (NEA) 1 mg is available under at least 5 different names: Lo Estrin 1/20 (Warner Chilcot), Junel 1/20 (Teva Pharmaceuticals), Microgestin Fe 1/20 (Mayne Pharma), Gildess 1/20 (Qualitest Pharmaceuticals), and Larin 1/20 (Novast Laboratories). To reduce the confusion, it is often useful to select a single preferred estrogen and progestin and use the dose combinations that are available to treat a wide range of gynecology problems (TABLE). In this editorial I focus on using various dose combinations of ethinyl estradiol and NEA to treat 3 common gynecologic problems.

CASE 1 Polycystic ovary syndrome
A 19-year-old woman reports 4 spontaneous menses in the past year and bothersome facial hair and acne. Her total testosterone concentration is at the upper limit of normal (0.46 ng/mL) and her sex hormone binding globulin (SHBG) concentration is at the lower limit of normal (35 nM). For treatment of the patient’s menstrual disorder, what is an optimal E-P combination?
Prioritize the use of an estrogen-dominant medication
Based on the Rotterdam criteria this woman has polycystic ovary syndrome (PCOS).1 In women with PCOS, luteinizing hormone (LH) secretion is increased, stimulating excessive ovarian production of testosterone.2 In addition, many women with PCOS have decreased hepatic secretion of SHBG, a binding protein that prevents testosterone from entering cells, resulting in excessive bioavailable testosterone.3 The Endocrine Society recommends that women with PCOS who have menstrual dysfunction or hirsutism be treated initially with a combination E-P hormone medication.1 Combination E-P medications suppress pituitary secretion of LH, thereby reducing ovarian production of testosterone, and ethinyl estradiol increases hepatic secretion of SHBG, reducing bioavailable testosterone. These two goals are best accomplished with an oral E-P hormone medication containing ethinyl estradiol doses of 20 µg to 30 µg per pill. An E-P hormone medication containing pills with an ethinyl estradiol dose ≤ 10 µg-daily may stimulate less hepatic production of SHBG than a pill with an ethinyl estradiol dose of 20 µg or 30 µg daily.4,5 In addition, E-P pills containing levonorgestrel suppress SHBG hormone secretion compared with E-P pills with other progestins.6 Therefore, levonorgestrel-containing E-P pills should not be prioritized for use in women with PCOS because the estrogen-induced increase in SHBG will be blunted by levonorgestrel.
CASE 2 Moderate to severe pelvic pain caused by endometriosis
A 25-year-old woman (G0) with severe dysmenorrhea had a laparoscopy showing endometriosis lesions in the cul-de-sac and a peritoneal window near the left uterosacral ligament. Biopsy showed endometriosis. Postoperatively, the patient was treated with an E-P pill containing 30 µg ethinyl estradiol and 0.15 mg desogestrel per pill using a continuous-dosing protocol. During the year following the laparoscopy, her pelvic pain symptoms gradually increased until they became severe, preventing her from performing daily activities on multiple days per month. She was prescribed elagolix but her insurance did not approve the treatment. What alternative treatment would you prescribe?
Continue to: Use progestin-dominant pills to treat pelvic pain...
Use progestin-dominant pills to treat pelvic pain
Cellular activity in endometriosis lesions is stimulated by estradiol and inhibited by a high concentration of androgenic progestins or androgens. This simplified endocrine paradigm explains the effectiveness of hormonal treatments that suppress ovarian estradiol production, including leuprolide, elagolix, medroxyprogesterone acetate, and NEA. For the woman in the above case, I would advocate for elagolix treatment but, following the insurance denial of the prescription, an alternative treatment for moderate or severe pelvic pain caused by endometriosis would be a progestin-dominant hormone medication (for example, NEA 5 mg daily). Norethindrone acetate 5 mg daily may be associated with bothersome adverse effects including weight gain (16% of patients; mean weight gain, 3.1 kg), acne (10%), mood lability (9%), hot flashes (8%), depression (6%), scalp hair loss (4%), headache (4%), nausea (3%), and deepening of the voice (1%).7
I sometimes see women with moderate to severe pelvic pain caused by endometriosis being treated with norethindrone 0.35 mg daily. This dose of norethindrone is suboptimal for pain treatment because it does not reliably suppress ovarian production of estradiol. In addition, the cells in endometriosis lesions are often resistant to the effects of progesterone, requiring higher dosages to produce secretory or decidual changes. In most situations, I recommend against the use of norethindrone 0.35 mg daily for the treatment of pelvic pain caused by endometriosis.
Patients commonly ask if NEA 5 mg daily has contraceptive efficacy. Although it is not approved at this dosage by the US Food and Drug Administration as a contraceptive,8 norethindrone 0.35 mg daily is approved as a progestin-only contraceptive.9 Norethindrone acetate is rapidly and completely deacetylated to norethindrone and the disposition of oral NEA is indistinguishable from that of norethindrone (which is the FDA-approved dosage mentioned above). Since norethindrone 0.35 mg daily is approved as a contraceptive, it is highly likely that NEA 5 mg daily has contraceptive efficacy, especially if there is good adherence with the daily medication.
CASE 3 Perimenopausal AUB
A 45-year-old woman reports varying menstrual cycle lengths from 24 to 60 days with very heavy menses in some cycles. Pelvic ultrasonography shows no abnormality. Endometrial biopsy shows a proliferative endometrium. Her serum progesterone level, obtained 1 week before the onset of menses, is < 3 ng/mL. She has no past history of heavy menses, easy bruising, excessive bleeding with procedures, or a family history of bleeding problems. She also reports occasional hot flashes that wake her from sleep.
Use an estrogen step-down regimen to manage postmenopause transition
This patient is likely in the perimenopause transition, and the abnormal uterine bleeding (AUB) is caused, in part, by oligo- or anovulation. Perimenopausal women with AUB may have cycles characterized by above normal ovarian estradiol production and below normal progesterone production, or frank anovulation.10 Elevated ovarian estrogen and low progesterone production sets the stage for heavy bleeding in the perimenopause, regardless of the presence of uterine pathology such as fibroids.
For perimenopausal women, one option for treatment of AUB due to anovulation is to prescribe an estrogen step-down regimen. For the 45-year-old woman in this case, initiating treatment with an E-P pill containing ethinyl estradiol 10 µg and NEA 1 mg will likely control the AUB and her occasional hot flash.11 As the woman ages, the ethinyl estradiol dose can be decreased to pills containing 5 µg and then 2.5 µg, covering the transition into postmenopause. Once the woman is in the postmenopause, treatment with transdermal estradiol and oral micronized progesterone is an option to treat menopausal vasomotor symptoms.
Optimize estrogen and progestin treatment for your patients
Many gynecologic problems are effectively treated by estrogen and/or progestin steroids. The dose of estrogen and progestin should be tailored to the specific problem. For PCOS, the estrogen dose selected should be sufficient to safely stimulate hepatic SHBG production. For endometriosis, if a GnRH antagonist is not available to the patient, a high-dose progestin, such as NEA 5 mg, may be an effective treatment. During the perimenopause transition in a woman with AUB, a treatment plan using a sequential E-P step-down program might control symptoms and help smoothly glide the patient into the postmenopause. ●
- Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565-4592. doi: 10.1210/jc.2013-2350.
- Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev. 2016;37:467-520. doi: 10.1210/er.2015-1104.
- Zhu JL, Chen Z, Feng WJ, et al. Sex hormone-binding globulin and polycystic ovary syndrome. Clin Chim Acta. 2019;499:142-148. doi: 10.1016/j.cca.2019.09.010.
- Oner G, Muderris II. A prospective randomized trial comparing low-dose ethinyl estradiol and drospirenone 24/4 combined oral contraceptive vs. ethinyl estradiol and drospirenone 21/7 combined oral contraceptive in the treatment of hirsutism. Contraception. 2011;84:508-511. doi: 10.1016/j.contraception.2011.03.002.
- Boyd RA, Zegarac EA, Posvar EL, et al. Minimal androgenic activity of a new oral contraceptive containing norethindrone acetate and graduated doses of ethinyl estradiol. Contraception. 2001;63:71-76. doi: 10.1016/s0010-7824(01)00179-2.
- Thorneycroft IH, Stanczyk FZ, Bradshaw KD, et al. Effect of low-dose oral contraceptives on androgenic markers and acne. Contraception. 1999;60:255-262. doi: 10.1016/s0010-7824(99)00093-1.
- Kaser DJ, Missmer SA, Berry KF, et al. Use of norethindrone acetate alone for postoperative suppression of endometriosis symptoms. J Pediatr Adolesc Gynecol. 2012;25:105-108. doi: 10.1016/j.jpag.2011.09.013.
- Aygestin [package insert]. Pomona, NY: Duramed Pharmaceuticals; 2007.
- Camila [package insert]. Greenville, NC; Mayne Pharma; 2018.
- Santoro N, Brown JR, Adel T, et al. Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab. 1996;81:1495-1501. doi: 10.1210/jcem.81.4.8636357.
- Speroff L, Symons J, Kempfert N, et al; FemHrt Study Investigators. The effect of varying low-dose combinations of norethindrone acetate and ethinyl estradiol (Femhrt) on the frequency and intensity of vasomotor symptoms. Menopause. 2000;7:383-390. doi: 10.1097/00042192-200011000-00003.
There are dozens of medications containing combinations of estrogen and progestin. I am often confused by the bewildering proliferation of generic brand names used to describe the same estrogen-progestin (E-P) regimen. For example, the combination medication containing ethinyl estradiol 20 µg plus norethindrone acetate (NEA) 1 mg is available under at least 5 different names: Lo Estrin 1/20 (Warner Chilcot), Junel 1/20 (Teva Pharmaceuticals), Microgestin Fe 1/20 (Mayne Pharma), Gildess 1/20 (Qualitest Pharmaceuticals), and Larin 1/20 (Novast Laboratories). To reduce the confusion, it is often useful to select a single preferred estrogen and progestin and use the dose combinations that are available to treat a wide range of gynecology problems (TABLE). In this editorial I focus on using various dose combinations of ethinyl estradiol and NEA to treat 3 common gynecologic problems.

CASE 1 Polycystic ovary syndrome
A 19-year-old woman reports 4 spontaneous menses in the past year and bothersome facial hair and acne. Her total testosterone concentration is at the upper limit of normal (0.46 ng/mL) and her sex hormone binding globulin (SHBG) concentration is at the lower limit of normal (35 nM). For treatment of the patient’s menstrual disorder, what is an optimal E-P combination?
Prioritize the use of an estrogen-dominant medication
Based on the Rotterdam criteria this woman has polycystic ovary syndrome (PCOS).1 In women with PCOS, luteinizing hormone (LH) secretion is increased, stimulating excessive ovarian production of testosterone.2 In addition, many women with PCOS have decreased hepatic secretion of SHBG, a binding protein that prevents testosterone from entering cells, resulting in excessive bioavailable testosterone.3 The Endocrine Society recommends that women with PCOS who have menstrual dysfunction or hirsutism be treated initially with a combination E-P hormone medication.1 Combination E-P medications suppress pituitary secretion of LH, thereby reducing ovarian production of testosterone, and ethinyl estradiol increases hepatic secretion of SHBG, reducing bioavailable testosterone. These two goals are best accomplished with an oral E-P hormone medication containing ethinyl estradiol doses of 20 µg to 30 µg per pill. An E-P hormone medication containing pills with an ethinyl estradiol dose ≤ 10 µg-daily may stimulate less hepatic production of SHBG than a pill with an ethinyl estradiol dose of 20 µg or 30 µg daily.4,5 In addition, E-P pills containing levonorgestrel suppress SHBG hormone secretion compared with E-P pills with other progestins.6 Therefore, levonorgestrel-containing E-P pills should not be prioritized for use in women with PCOS because the estrogen-induced increase in SHBG will be blunted by levonorgestrel.
CASE 2 Moderate to severe pelvic pain caused by endometriosis
A 25-year-old woman (G0) with severe dysmenorrhea had a laparoscopy showing endometriosis lesions in the cul-de-sac and a peritoneal window near the left uterosacral ligament. Biopsy showed endometriosis. Postoperatively, the patient was treated with an E-P pill containing 30 µg ethinyl estradiol and 0.15 mg desogestrel per pill using a continuous-dosing protocol. During the year following the laparoscopy, her pelvic pain symptoms gradually increased until they became severe, preventing her from performing daily activities on multiple days per month. She was prescribed elagolix but her insurance did not approve the treatment. What alternative treatment would you prescribe?
Continue to: Use progestin-dominant pills to treat pelvic pain...
Use progestin-dominant pills to treat pelvic pain
Cellular activity in endometriosis lesions is stimulated by estradiol and inhibited by a high concentration of androgenic progestins or androgens. This simplified endocrine paradigm explains the effectiveness of hormonal treatments that suppress ovarian estradiol production, including leuprolide, elagolix, medroxyprogesterone acetate, and NEA. For the woman in the above case, I would advocate for elagolix treatment but, following the insurance denial of the prescription, an alternative treatment for moderate or severe pelvic pain caused by endometriosis would be a progestin-dominant hormone medication (for example, NEA 5 mg daily). Norethindrone acetate 5 mg daily may be associated with bothersome adverse effects including weight gain (16% of patients; mean weight gain, 3.1 kg), acne (10%), mood lability (9%), hot flashes (8%), depression (6%), scalp hair loss (4%), headache (4%), nausea (3%), and deepening of the voice (1%).7
I sometimes see women with moderate to severe pelvic pain caused by endometriosis being treated with norethindrone 0.35 mg daily. This dose of norethindrone is suboptimal for pain treatment because it does not reliably suppress ovarian production of estradiol. In addition, the cells in endometriosis lesions are often resistant to the effects of progesterone, requiring higher dosages to produce secretory or decidual changes. In most situations, I recommend against the use of norethindrone 0.35 mg daily for the treatment of pelvic pain caused by endometriosis.
Patients commonly ask if NEA 5 mg daily has contraceptive efficacy. Although it is not approved at this dosage by the US Food and Drug Administration as a contraceptive,8 norethindrone 0.35 mg daily is approved as a progestin-only contraceptive.9 Norethindrone acetate is rapidly and completely deacetylated to norethindrone and the disposition of oral NEA is indistinguishable from that of norethindrone (which is the FDA-approved dosage mentioned above). Since norethindrone 0.35 mg daily is approved as a contraceptive, it is highly likely that NEA 5 mg daily has contraceptive efficacy, especially if there is good adherence with the daily medication.
CASE 3 Perimenopausal AUB
A 45-year-old woman reports varying menstrual cycle lengths from 24 to 60 days with very heavy menses in some cycles. Pelvic ultrasonography shows no abnormality. Endometrial biopsy shows a proliferative endometrium. Her serum progesterone level, obtained 1 week before the onset of menses, is < 3 ng/mL. She has no past history of heavy menses, easy bruising, excessive bleeding with procedures, or a family history of bleeding problems. She also reports occasional hot flashes that wake her from sleep.
Use an estrogen step-down regimen to manage postmenopause transition
This patient is likely in the perimenopause transition, and the abnormal uterine bleeding (AUB) is caused, in part, by oligo- or anovulation. Perimenopausal women with AUB may have cycles characterized by above normal ovarian estradiol production and below normal progesterone production, or frank anovulation.10 Elevated ovarian estrogen and low progesterone production sets the stage for heavy bleeding in the perimenopause, regardless of the presence of uterine pathology such as fibroids.
For perimenopausal women, one option for treatment of AUB due to anovulation is to prescribe an estrogen step-down regimen. For the 45-year-old woman in this case, initiating treatment with an E-P pill containing ethinyl estradiol 10 µg and NEA 1 mg will likely control the AUB and her occasional hot flash.11 As the woman ages, the ethinyl estradiol dose can be decreased to pills containing 5 µg and then 2.5 µg, covering the transition into postmenopause. Once the woman is in the postmenopause, treatment with transdermal estradiol and oral micronized progesterone is an option to treat menopausal vasomotor symptoms.
Optimize estrogen and progestin treatment for your patients
Many gynecologic problems are effectively treated by estrogen and/or progestin steroids. The dose of estrogen and progestin should be tailored to the specific problem. For PCOS, the estrogen dose selected should be sufficient to safely stimulate hepatic SHBG production. For endometriosis, if a GnRH antagonist is not available to the patient, a high-dose progestin, such as NEA 5 mg, may be an effective treatment. During the perimenopause transition in a woman with AUB, a treatment plan using a sequential E-P step-down program might control symptoms and help smoothly glide the patient into the postmenopause. ●
There are dozens of medications containing combinations of estrogen and progestin. I am often confused by the bewildering proliferation of generic brand names used to describe the same estrogen-progestin (E-P) regimen. For example, the combination medication containing ethinyl estradiol 20 µg plus norethindrone acetate (NEA) 1 mg is available under at least 5 different names: Lo Estrin 1/20 (Warner Chilcot), Junel 1/20 (Teva Pharmaceuticals), Microgestin Fe 1/20 (Mayne Pharma), Gildess 1/20 (Qualitest Pharmaceuticals), and Larin 1/20 (Novast Laboratories). To reduce the confusion, it is often useful to select a single preferred estrogen and progestin and use the dose combinations that are available to treat a wide range of gynecology problems (TABLE). In this editorial I focus on using various dose combinations of ethinyl estradiol and NEA to treat 3 common gynecologic problems.

CASE 1 Polycystic ovary syndrome
A 19-year-old woman reports 4 spontaneous menses in the past year and bothersome facial hair and acne. Her total testosterone concentration is at the upper limit of normal (0.46 ng/mL) and her sex hormone binding globulin (SHBG) concentration is at the lower limit of normal (35 nM). For treatment of the patient’s menstrual disorder, what is an optimal E-P combination?
Prioritize the use of an estrogen-dominant medication
Based on the Rotterdam criteria this woman has polycystic ovary syndrome (PCOS).1 In women with PCOS, luteinizing hormone (LH) secretion is increased, stimulating excessive ovarian production of testosterone.2 In addition, many women with PCOS have decreased hepatic secretion of SHBG, a binding protein that prevents testosterone from entering cells, resulting in excessive bioavailable testosterone.3 The Endocrine Society recommends that women with PCOS who have menstrual dysfunction or hirsutism be treated initially with a combination E-P hormone medication.1 Combination E-P medications suppress pituitary secretion of LH, thereby reducing ovarian production of testosterone, and ethinyl estradiol increases hepatic secretion of SHBG, reducing bioavailable testosterone. These two goals are best accomplished with an oral E-P hormone medication containing ethinyl estradiol doses of 20 µg to 30 µg per pill. An E-P hormone medication containing pills with an ethinyl estradiol dose ≤ 10 µg-daily may stimulate less hepatic production of SHBG than a pill with an ethinyl estradiol dose of 20 µg or 30 µg daily.4,5 In addition, E-P pills containing levonorgestrel suppress SHBG hormone secretion compared with E-P pills with other progestins.6 Therefore, levonorgestrel-containing E-P pills should not be prioritized for use in women with PCOS because the estrogen-induced increase in SHBG will be blunted by levonorgestrel.
CASE 2 Moderate to severe pelvic pain caused by endometriosis
A 25-year-old woman (G0) with severe dysmenorrhea had a laparoscopy showing endometriosis lesions in the cul-de-sac and a peritoneal window near the left uterosacral ligament. Biopsy showed endometriosis. Postoperatively, the patient was treated with an E-P pill containing 30 µg ethinyl estradiol and 0.15 mg desogestrel per pill using a continuous-dosing protocol. During the year following the laparoscopy, her pelvic pain symptoms gradually increased until they became severe, preventing her from performing daily activities on multiple days per month. She was prescribed elagolix but her insurance did not approve the treatment. What alternative treatment would you prescribe?
Continue to: Use progestin-dominant pills to treat pelvic pain...
Use progestin-dominant pills to treat pelvic pain
Cellular activity in endometriosis lesions is stimulated by estradiol and inhibited by a high concentration of androgenic progestins or androgens. This simplified endocrine paradigm explains the effectiveness of hormonal treatments that suppress ovarian estradiol production, including leuprolide, elagolix, medroxyprogesterone acetate, and NEA. For the woman in the above case, I would advocate for elagolix treatment but, following the insurance denial of the prescription, an alternative treatment for moderate or severe pelvic pain caused by endometriosis would be a progestin-dominant hormone medication (for example, NEA 5 mg daily). Norethindrone acetate 5 mg daily may be associated with bothersome adverse effects including weight gain (16% of patients; mean weight gain, 3.1 kg), acne (10%), mood lability (9%), hot flashes (8%), depression (6%), scalp hair loss (4%), headache (4%), nausea (3%), and deepening of the voice (1%).7
I sometimes see women with moderate to severe pelvic pain caused by endometriosis being treated with norethindrone 0.35 mg daily. This dose of norethindrone is suboptimal for pain treatment because it does not reliably suppress ovarian production of estradiol. In addition, the cells in endometriosis lesions are often resistant to the effects of progesterone, requiring higher dosages to produce secretory or decidual changes. In most situations, I recommend against the use of norethindrone 0.35 mg daily for the treatment of pelvic pain caused by endometriosis.
Patients commonly ask if NEA 5 mg daily has contraceptive efficacy. Although it is not approved at this dosage by the US Food and Drug Administration as a contraceptive,8 norethindrone 0.35 mg daily is approved as a progestin-only contraceptive.9 Norethindrone acetate is rapidly and completely deacetylated to norethindrone and the disposition of oral NEA is indistinguishable from that of norethindrone (which is the FDA-approved dosage mentioned above). Since norethindrone 0.35 mg daily is approved as a contraceptive, it is highly likely that NEA 5 mg daily has contraceptive efficacy, especially if there is good adherence with the daily medication.
CASE 3 Perimenopausal AUB
A 45-year-old woman reports varying menstrual cycle lengths from 24 to 60 days with very heavy menses in some cycles. Pelvic ultrasonography shows no abnormality. Endometrial biopsy shows a proliferative endometrium. Her serum progesterone level, obtained 1 week before the onset of menses, is < 3 ng/mL. She has no past history of heavy menses, easy bruising, excessive bleeding with procedures, or a family history of bleeding problems. She also reports occasional hot flashes that wake her from sleep.
Use an estrogen step-down regimen to manage postmenopause transition
This patient is likely in the perimenopause transition, and the abnormal uterine bleeding (AUB) is caused, in part, by oligo- or anovulation. Perimenopausal women with AUB may have cycles characterized by above normal ovarian estradiol production and below normal progesterone production, or frank anovulation.10 Elevated ovarian estrogen and low progesterone production sets the stage for heavy bleeding in the perimenopause, regardless of the presence of uterine pathology such as fibroids.
For perimenopausal women, one option for treatment of AUB due to anovulation is to prescribe an estrogen step-down regimen. For the 45-year-old woman in this case, initiating treatment with an E-P pill containing ethinyl estradiol 10 µg and NEA 1 mg will likely control the AUB and her occasional hot flash.11 As the woman ages, the ethinyl estradiol dose can be decreased to pills containing 5 µg and then 2.5 µg, covering the transition into postmenopause. Once the woman is in the postmenopause, treatment with transdermal estradiol and oral micronized progesterone is an option to treat menopausal vasomotor symptoms.
Optimize estrogen and progestin treatment for your patients
Many gynecologic problems are effectively treated by estrogen and/or progestin steroids. The dose of estrogen and progestin should be tailored to the specific problem. For PCOS, the estrogen dose selected should be sufficient to safely stimulate hepatic SHBG production. For endometriosis, if a GnRH antagonist is not available to the patient, a high-dose progestin, such as NEA 5 mg, may be an effective treatment. During the perimenopause transition in a woman with AUB, a treatment plan using a sequential E-P step-down program might control symptoms and help smoothly glide the patient into the postmenopause. ●
- Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565-4592. doi: 10.1210/jc.2013-2350.
- Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev. 2016;37:467-520. doi: 10.1210/er.2015-1104.
- Zhu JL, Chen Z, Feng WJ, et al. Sex hormone-binding globulin and polycystic ovary syndrome. Clin Chim Acta. 2019;499:142-148. doi: 10.1016/j.cca.2019.09.010.
- Oner G, Muderris II. A prospective randomized trial comparing low-dose ethinyl estradiol and drospirenone 24/4 combined oral contraceptive vs. ethinyl estradiol and drospirenone 21/7 combined oral contraceptive in the treatment of hirsutism. Contraception. 2011;84:508-511. doi: 10.1016/j.contraception.2011.03.002.
- Boyd RA, Zegarac EA, Posvar EL, et al. Minimal androgenic activity of a new oral contraceptive containing norethindrone acetate and graduated doses of ethinyl estradiol. Contraception. 2001;63:71-76. doi: 10.1016/s0010-7824(01)00179-2.
- Thorneycroft IH, Stanczyk FZ, Bradshaw KD, et al. Effect of low-dose oral contraceptives on androgenic markers and acne. Contraception. 1999;60:255-262. doi: 10.1016/s0010-7824(99)00093-1.
- Kaser DJ, Missmer SA, Berry KF, et al. Use of norethindrone acetate alone for postoperative suppression of endometriosis symptoms. J Pediatr Adolesc Gynecol. 2012;25:105-108. doi: 10.1016/j.jpag.2011.09.013.
- Aygestin [package insert]. Pomona, NY: Duramed Pharmaceuticals; 2007.
- Camila [package insert]. Greenville, NC; Mayne Pharma; 2018.
- Santoro N, Brown JR, Adel T, et al. Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab. 1996;81:1495-1501. doi: 10.1210/jcem.81.4.8636357.
- Speroff L, Symons J, Kempfert N, et al; FemHrt Study Investigators. The effect of varying low-dose combinations of norethindrone acetate and ethinyl estradiol (Femhrt) on the frequency and intensity of vasomotor symptoms. Menopause. 2000;7:383-390. doi: 10.1097/00042192-200011000-00003.
- Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565-4592. doi: 10.1210/jc.2013-2350.
- Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev. 2016;37:467-520. doi: 10.1210/er.2015-1104.
- Zhu JL, Chen Z, Feng WJ, et al. Sex hormone-binding globulin and polycystic ovary syndrome. Clin Chim Acta. 2019;499:142-148. doi: 10.1016/j.cca.2019.09.010.
- Oner G, Muderris II. A prospective randomized trial comparing low-dose ethinyl estradiol and drospirenone 24/4 combined oral contraceptive vs. ethinyl estradiol and drospirenone 21/7 combined oral contraceptive in the treatment of hirsutism. Contraception. 2011;84:508-511. doi: 10.1016/j.contraception.2011.03.002.
- Boyd RA, Zegarac EA, Posvar EL, et al. Minimal androgenic activity of a new oral contraceptive containing norethindrone acetate and graduated doses of ethinyl estradiol. Contraception. 2001;63:71-76. doi: 10.1016/s0010-7824(01)00179-2.
- Thorneycroft IH, Stanczyk FZ, Bradshaw KD, et al. Effect of low-dose oral contraceptives on androgenic markers and acne. Contraception. 1999;60:255-262. doi: 10.1016/s0010-7824(99)00093-1.
- Kaser DJ, Missmer SA, Berry KF, et al. Use of norethindrone acetate alone for postoperative suppression of endometriosis symptoms. J Pediatr Adolesc Gynecol. 2012;25:105-108. doi: 10.1016/j.jpag.2011.09.013.
- Aygestin [package insert]. Pomona, NY: Duramed Pharmaceuticals; 2007.
- Camila [package insert]. Greenville, NC; Mayne Pharma; 2018.
- Santoro N, Brown JR, Adel T, et al. Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab. 1996;81:1495-1501. doi: 10.1210/jcem.81.4.8636357.
- Speroff L, Symons J, Kempfert N, et al; FemHrt Study Investigators. The effect of varying low-dose combinations of norethindrone acetate and ethinyl estradiol (Femhrt) on the frequency and intensity of vasomotor symptoms. Menopause. 2000;7:383-390. doi: 10.1097/00042192-200011000-00003.
Hormone pellet safety data ‘not very reassuring at all’ for women
Women who receive pellet hormonal therapy may be significantly more likely to have side effects such as mood swings, anxiety, breast tenderness, hair pattern change, acne, and weight gain, compared with women who receive hormonal treatments that have been approved by the Food and Drug Administration, a study indicates.
In addition, abnormal uterine bleeding may be significantly more common in women who receive pellets than it is in women who receive Food and Drug Administration–approved options, according to the retrospective study, which was published online in Menopause.
Women receiving pellets also were more likely to undergo hysterectomy while on hormonal therapy, and they had higher supraphysiological levels of estradiol and total testosterone during treatment, compared with women on conventional therapy, the study of 539 women shows.
The findings, which had been presented at the North American Menopause Society annual meeting, were highlighted during a lecture at the 2021 virtual meeting of the American College of Obstetricians and Gynecologists.
The data are “not very reassuring at all,” said Robert P. Kauffman, MD, a professor of obstetrics and gynecology at Texas Tech University, Amarillo, who was not involved in the study.
Dr. Kauffman commented on the research during a review of concerns surrounding non–FDA-approved hormone replacement therapies at the ACOG meeting. Concerns include variations in compounded products, a lack of randomized, controlled trial data supporting their use, and ethical dilemmas that may exist if clinicians have financial incentives to provide compounded bioidentical hormone therapy over FDA-approved treatments.
No peer-reviewed studies show that compounded hormone creams or pellets are safer, more efficacious, or less likely to cause adverse effects, compared with FDA-approved products, Dr. Kauffman said.
Data from Pennsylvania
For the retrospective study, Xuezhi (Daniel) Jiang, MD, PhD, and colleagues identified postmenopausal patients in the Reading Hospital Electronic Medical Record System, including 10,801 on FDA-approved hormonal therapy and 1,061 on pellet hormonal therapy. Their analysis focused on data from the medical records of 384 women on pellet hormonal therapy and 155 women on FDA-approved hormonal therapy. Dr. Jiang is affiliated with the department of obstetrics and gynecology at Reading (Pa.) Hospital and Sidney Kimmel Medical College, Philadelphia.
The researchers examined data from 2005 to 2017 for patients in the pellet therapy group, and from 1985 to 2017 for patients in the conventional therapy group.
Patients in the conventional therapy group received 24 brands of FDA-approved hormone products; 4.5% received testosterone or methyltestosterone in addition to estrogen. Patients in the pellet therapy group had pellets prescribed by clinicians at two private practices in the hospital system that use this treatment approach. Patients in the pellet group received compounded estradiol and testosterone pellets made at a pharmacy in Tennessee.* Almost all of the patients in the pellet group received testosterone and estradiol pellets.
Low libido was listed as a reason why women started treatment for 83.5% of the pellet group versus 4.5% of the conventional therapy group.
In all, 57.6% of patients on pellet therapy had side effects, versus 14.8% on FDA-approved therapy, the researchers found. Patients on pellet hormonal therapy reported higher incidence of mood swings (7% vs. 1.9%), anxiety (18.5% vs. 5.8%), breast tenderness (10.1% vs. 2.6%), hair pattern change (13.5% vs. 2.6%), acne (8.6% vs. 1.3%), and weight gain (34.4% vs 4.5%), relative to patients on FDA-approved options.
Among those with an intact uterus when starting therapy (246 of those on pellets and 133 of those on FDA-approved treatments), abnormal uterine bleeding occurred in 55.3% on pellets, compared with 15.2% on FDA-approved treatments (adjusted odds ratio, 7.9). Hysterectomy secondary to abnormal uterine bleeding occurred in 20.3% of the patients on pellets versus 6.3% on FDA-approved treatments (aOR, 3.2).
In many cases, records show that patients chose to have a hysterectomy so they could continue pellet therapy without worrying about abnormal uterine bleeding, Dr. Jiang said in an interview.
Dr. Kauffman has seen patients on pellet therapy, usually implanted by family physicians, develop postmenopausal bleeding because of high levels of estrogen. “Our experience has been too that, if you have pellets, you are more likely to get a hysterectomy for bleeding issues. And I think these are the safety issues that need to be looked at on a broader scope,” he said in an interview.
Although hysterectomy may stop the bleeding, other safety risks may remain with pellet therapy, noted Sharon Winer, MD, MPH, an obstetrician and gynecologist with a subspecialty in reproductive endocrinology and infertility who practices in Beverly Hills, Calif.
Pellets, which are about the size of a grain of rice, typically are implanted in the hip, lower abdomen, or buttock and release hormones over 3-6 months. The pellets are not retrievable. “The question becomes, what if she has a new breast cancer diagnosis or a diagnosis where estrogen is contraindicated? She has got that estrogen already in her system,” Dr. Winer said.
“The hysterectomy may solve the bleeding problem ... but it doesn’t solve the safety problem overall,” said Dr. Winer, who also is a professor of obstetrics and gynecology and codirector of the reproductive endocrinology and infertility clinic at the University of Southern California, Los Angeles.
Elevated levels
Average peak serum estradiol was significantly higher in the pellet treatment group than in the conventional therapy group (237.70 pg/mL vs. 93.45 pg/mL), as was average peak serum testosterone (192.84 ng/dL vs. 15.59 ng/dL), the researchers reported. Patients on FDA-approved treatments were less likely to have had their hormone levels measured. How concentrations of hormone levels correlate with side effects is unclear, Dr. Jiang said.
The study was limited by its single-institution, retrospective design, and some patient characteristics differed between the treatment groups, the authors noted.
Still, “clinicians ought to be mindful of fully counseling patients on side effects identified in the current study,” Dr. Jiang and coauthors concluded. Clinicians also need to discuss potential risks of breast cancer, endometrial cancer, and cardiovascular disease with patients.
Many primary care clinicians rely on outdated information from the Women’s Health Initiative, published in 2002 and 2004, in their understanding of postmenopausal hormonal therapy and its risks and benefits, Dr. Jiang said. And some patients consider custom-compounded hormone therapy to be safer and more natural, “which is totally misleading.”
Pellets and other custom-compounded medicine containing testosterone may make patients feel better and more energetic, Dr. Jiang acknowledged. “That’s a reason why patients ... tend to stay on, even though they have side effects. The only issue is the safety.”
Additional questions remain. The researchers recently started to examine rates of breast cancer and abnormal breast pathology and mammogram results. “It’s a long journey,” he said.
Plenty of approved options
Custom-compounded medicines are not FDA approved and are not recommended by medical menopause societies, Dr. Jiang said. Meanwhile, plenty of approved hormone therapies, including bioidentical treatments, have safety data and are available.
A 2020 consensus study report from the National Academies of Sciences, Engineering, and Medicine that examined the use of compounded hormonal therapy and provides guidance for clinicians is a good start in addressing this major issue, he added.
A committee determined “there is insufficient evidence to support the overall clinical utility of [compounded bioidentical hormone therapies] as treatment for menopause and male hypogonadism symptoms.”
If an FDA-approved option is available, “I would always go with an FDA-approved product before I would go with a compounded product,” Dr. Winer said. A 2012 fungal meningitis outbreak linked to a compounding pharmacy highlighted risks associated with poor quality compounded drugs.
“I think at least now it is recognized that compounding is an issue that has got to be dealt with,” Dr. Winer said. “It is just that it is so widespread and it is sometimes under the radar ... that I think it is really hard for the FDA to get a handle on it.”
Dr. Winer has seen patients on compounded treatments who are underdosed and patients who are overdosed. “I’ve also seen patients who do quite well with it, but I’m not happy continuing it because tomorrow there may be inconsistency in potency or quality resulting in a different clinical response,” she said.
Nevertheless, compounded pharmacies are needed, Dr. Winer said. If she wants to give natural progesterone that is FDA approved but happens to be made with peanut oil, she will have a compounding pharmacy make it with canola oil instead if a patient has a peanut allergy, for example. Other patients need dosages that are so low that they are not available as FDA-approved products.
Dr. Jiang and Dr. Kauffman had no relevant financial disclosures. Dr. Winer has done work with AbbVie (related to endometriosis), TherapeuticsMD (related to a menopause bioidentical hormonal pill and vaginal estrogen product), and Biogix (related to an antioxidant supplement for menopause symptoms).
*This story was updated on 6/22/2021.
Women who receive pellet hormonal therapy may be significantly more likely to have side effects such as mood swings, anxiety, breast tenderness, hair pattern change, acne, and weight gain, compared with women who receive hormonal treatments that have been approved by the Food and Drug Administration, a study indicates.
In addition, abnormal uterine bleeding may be significantly more common in women who receive pellets than it is in women who receive Food and Drug Administration–approved options, according to the retrospective study, which was published online in Menopause.
Women receiving pellets also were more likely to undergo hysterectomy while on hormonal therapy, and they had higher supraphysiological levels of estradiol and total testosterone during treatment, compared with women on conventional therapy, the study of 539 women shows.
The findings, which had been presented at the North American Menopause Society annual meeting, were highlighted during a lecture at the 2021 virtual meeting of the American College of Obstetricians and Gynecologists.
The data are “not very reassuring at all,” said Robert P. Kauffman, MD, a professor of obstetrics and gynecology at Texas Tech University, Amarillo, who was not involved in the study.
Dr. Kauffman commented on the research during a review of concerns surrounding non–FDA-approved hormone replacement therapies at the ACOG meeting. Concerns include variations in compounded products, a lack of randomized, controlled trial data supporting their use, and ethical dilemmas that may exist if clinicians have financial incentives to provide compounded bioidentical hormone therapy over FDA-approved treatments.
No peer-reviewed studies show that compounded hormone creams or pellets are safer, more efficacious, or less likely to cause adverse effects, compared with FDA-approved products, Dr. Kauffman said.
Data from Pennsylvania
For the retrospective study, Xuezhi (Daniel) Jiang, MD, PhD, and colleagues identified postmenopausal patients in the Reading Hospital Electronic Medical Record System, including 10,801 on FDA-approved hormonal therapy and 1,061 on pellet hormonal therapy. Their analysis focused on data from the medical records of 384 women on pellet hormonal therapy and 155 women on FDA-approved hormonal therapy. Dr. Jiang is affiliated with the department of obstetrics and gynecology at Reading (Pa.) Hospital and Sidney Kimmel Medical College, Philadelphia.
The researchers examined data from 2005 to 2017 for patients in the pellet therapy group, and from 1985 to 2017 for patients in the conventional therapy group.
Patients in the conventional therapy group received 24 brands of FDA-approved hormone products; 4.5% received testosterone or methyltestosterone in addition to estrogen. Patients in the pellet therapy group had pellets prescribed by clinicians at two private practices in the hospital system that use this treatment approach. Patients in the pellet group received compounded estradiol and testosterone pellets made at a pharmacy in Tennessee.* Almost all of the patients in the pellet group received testosterone and estradiol pellets.
Low libido was listed as a reason why women started treatment for 83.5% of the pellet group versus 4.5% of the conventional therapy group.
In all, 57.6% of patients on pellet therapy had side effects, versus 14.8% on FDA-approved therapy, the researchers found. Patients on pellet hormonal therapy reported higher incidence of mood swings (7% vs. 1.9%), anxiety (18.5% vs. 5.8%), breast tenderness (10.1% vs. 2.6%), hair pattern change (13.5% vs. 2.6%), acne (8.6% vs. 1.3%), and weight gain (34.4% vs 4.5%), relative to patients on FDA-approved options.
Among those with an intact uterus when starting therapy (246 of those on pellets and 133 of those on FDA-approved treatments), abnormal uterine bleeding occurred in 55.3% on pellets, compared with 15.2% on FDA-approved treatments (adjusted odds ratio, 7.9). Hysterectomy secondary to abnormal uterine bleeding occurred in 20.3% of the patients on pellets versus 6.3% on FDA-approved treatments (aOR, 3.2).
In many cases, records show that patients chose to have a hysterectomy so they could continue pellet therapy without worrying about abnormal uterine bleeding, Dr. Jiang said in an interview.
Dr. Kauffman has seen patients on pellet therapy, usually implanted by family physicians, develop postmenopausal bleeding because of high levels of estrogen. “Our experience has been too that, if you have pellets, you are more likely to get a hysterectomy for bleeding issues. And I think these are the safety issues that need to be looked at on a broader scope,” he said in an interview.
Although hysterectomy may stop the bleeding, other safety risks may remain with pellet therapy, noted Sharon Winer, MD, MPH, an obstetrician and gynecologist with a subspecialty in reproductive endocrinology and infertility who practices in Beverly Hills, Calif.
Pellets, which are about the size of a grain of rice, typically are implanted in the hip, lower abdomen, or buttock and release hormones over 3-6 months. The pellets are not retrievable. “The question becomes, what if she has a new breast cancer diagnosis or a diagnosis where estrogen is contraindicated? She has got that estrogen already in her system,” Dr. Winer said.
“The hysterectomy may solve the bleeding problem ... but it doesn’t solve the safety problem overall,” said Dr. Winer, who also is a professor of obstetrics and gynecology and codirector of the reproductive endocrinology and infertility clinic at the University of Southern California, Los Angeles.
Elevated levels
Average peak serum estradiol was significantly higher in the pellet treatment group than in the conventional therapy group (237.70 pg/mL vs. 93.45 pg/mL), as was average peak serum testosterone (192.84 ng/dL vs. 15.59 ng/dL), the researchers reported. Patients on FDA-approved treatments were less likely to have had their hormone levels measured. How concentrations of hormone levels correlate with side effects is unclear, Dr. Jiang said.
The study was limited by its single-institution, retrospective design, and some patient characteristics differed between the treatment groups, the authors noted.
Still, “clinicians ought to be mindful of fully counseling patients on side effects identified in the current study,” Dr. Jiang and coauthors concluded. Clinicians also need to discuss potential risks of breast cancer, endometrial cancer, and cardiovascular disease with patients.
Many primary care clinicians rely on outdated information from the Women’s Health Initiative, published in 2002 and 2004, in their understanding of postmenopausal hormonal therapy and its risks and benefits, Dr. Jiang said. And some patients consider custom-compounded hormone therapy to be safer and more natural, “which is totally misleading.”
Pellets and other custom-compounded medicine containing testosterone may make patients feel better and more energetic, Dr. Jiang acknowledged. “That’s a reason why patients ... tend to stay on, even though they have side effects. The only issue is the safety.”
Additional questions remain. The researchers recently started to examine rates of breast cancer and abnormal breast pathology and mammogram results. “It’s a long journey,” he said.
Plenty of approved options
Custom-compounded medicines are not FDA approved and are not recommended by medical menopause societies, Dr. Jiang said. Meanwhile, plenty of approved hormone therapies, including bioidentical treatments, have safety data and are available.
A 2020 consensus study report from the National Academies of Sciences, Engineering, and Medicine that examined the use of compounded hormonal therapy and provides guidance for clinicians is a good start in addressing this major issue, he added.
A committee determined “there is insufficient evidence to support the overall clinical utility of [compounded bioidentical hormone therapies] as treatment for menopause and male hypogonadism symptoms.”
If an FDA-approved option is available, “I would always go with an FDA-approved product before I would go with a compounded product,” Dr. Winer said. A 2012 fungal meningitis outbreak linked to a compounding pharmacy highlighted risks associated with poor quality compounded drugs.
“I think at least now it is recognized that compounding is an issue that has got to be dealt with,” Dr. Winer said. “It is just that it is so widespread and it is sometimes under the radar ... that I think it is really hard for the FDA to get a handle on it.”
Dr. Winer has seen patients on compounded treatments who are underdosed and patients who are overdosed. “I’ve also seen patients who do quite well with it, but I’m not happy continuing it because tomorrow there may be inconsistency in potency or quality resulting in a different clinical response,” she said.
Nevertheless, compounded pharmacies are needed, Dr. Winer said. If she wants to give natural progesterone that is FDA approved but happens to be made with peanut oil, she will have a compounding pharmacy make it with canola oil instead if a patient has a peanut allergy, for example. Other patients need dosages that are so low that they are not available as FDA-approved products.
Dr. Jiang and Dr. Kauffman had no relevant financial disclosures. Dr. Winer has done work with AbbVie (related to endometriosis), TherapeuticsMD (related to a menopause bioidentical hormonal pill and vaginal estrogen product), and Biogix (related to an antioxidant supplement for menopause symptoms).
*This story was updated on 6/22/2021.
Women who receive pellet hormonal therapy may be significantly more likely to have side effects such as mood swings, anxiety, breast tenderness, hair pattern change, acne, and weight gain, compared with women who receive hormonal treatments that have been approved by the Food and Drug Administration, a study indicates.
In addition, abnormal uterine bleeding may be significantly more common in women who receive pellets than it is in women who receive Food and Drug Administration–approved options, according to the retrospective study, which was published online in Menopause.
Women receiving pellets also were more likely to undergo hysterectomy while on hormonal therapy, and they had higher supraphysiological levels of estradiol and total testosterone during treatment, compared with women on conventional therapy, the study of 539 women shows.
The findings, which had been presented at the North American Menopause Society annual meeting, were highlighted during a lecture at the 2021 virtual meeting of the American College of Obstetricians and Gynecologists.
The data are “not very reassuring at all,” said Robert P. Kauffman, MD, a professor of obstetrics and gynecology at Texas Tech University, Amarillo, who was not involved in the study.
Dr. Kauffman commented on the research during a review of concerns surrounding non–FDA-approved hormone replacement therapies at the ACOG meeting. Concerns include variations in compounded products, a lack of randomized, controlled trial data supporting their use, and ethical dilemmas that may exist if clinicians have financial incentives to provide compounded bioidentical hormone therapy over FDA-approved treatments.
No peer-reviewed studies show that compounded hormone creams or pellets are safer, more efficacious, or less likely to cause adverse effects, compared with FDA-approved products, Dr. Kauffman said.
Data from Pennsylvania
For the retrospective study, Xuezhi (Daniel) Jiang, MD, PhD, and colleagues identified postmenopausal patients in the Reading Hospital Electronic Medical Record System, including 10,801 on FDA-approved hormonal therapy and 1,061 on pellet hormonal therapy. Their analysis focused on data from the medical records of 384 women on pellet hormonal therapy and 155 women on FDA-approved hormonal therapy. Dr. Jiang is affiliated with the department of obstetrics and gynecology at Reading (Pa.) Hospital and Sidney Kimmel Medical College, Philadelphia.
The researchers examined data from 2005 to 2017 for patients in the pellet therapy group, and from 1985 to 2017 for patients in the conventional therapy group.
Patients in the conventional therapy group received 24 brands of FDA-approved hormone products; 4.5% received testosterone or methyltestosterone in addition to estrogen. Patients in the pellet therapy group had pellets prescribed by clinicians at two private practices in the hospital system that use this treatment approach. Patients in the pellet group received compounded estradiol and testosterone pellets made at a pharmacy in Tennessee.* Almost all of the patients in the pellet group received testosterone and estradiol pellets.
Low libido was listed as a reason why women started treatment for 83.5% of the pellet group versus 4.5% of the conventional therapy group.
In all, 57.6% of patients on pellet therapy had side effects, versus 14.8% on FDA-approved therapy, the researchers found. Patients on pellet hormonal therapy reported higher incidence of mood swings (7% vs. 1.9%), anxiety (18.5% vs. 5.8%), breast tenderness (10.1% vs. 2.6%), hair pattern change (13.5% vs. 2.6%), acne (8.6% vs. 1.3%), and weight gain (34.4% vs 4.5%), relative to patients on FDA-approved options.
Among those with an intact uterus when starting therapy (246 of those on pellets and 133 of those on FDA-approved treatments), abnormal uterine bleeding occurred in 55.3% on pellets, compared with 15.2% on FDA-approved treatments (adjusted odds ratio, 7.9). Hysterectomy secondary to abnormal uterine bleeding occurred in 20.3% of the patients on pellets versus 6.3% on FDA-approved treatments (aOR, 3.2).
In many cases, records show that patients chose to have a hysterectomy so they could continue pellet therapy without worrying about abnormal uterine bleeding, Dr. Jiang said in an interview.
Dr. Kauffman has seen patients on pellet therapy, usually implanted by family physicians, develop postmenopausal bleeding because of high levels of estrogen. “Our experience has been too that, if you have pellets, you are more likely to get a hysterectomy for bleeding issues. And I think these are the safety issues that need to be looked at on a broader scope,” he said in an interview.
Although hysterectomy may stop the bleeding, other safety risks may remain with pellet therapy, noted Sharon Winer, MD, MPH, an obstetrician and gynecologist with a subspecialty in reproductive endocrinology and infertility who practices in Beverly Hills, Calif.
Pellets, which are about the size of a grain of rice, typically are implanted in the hip, lower abdomen, or buttock and release hormones over 3-6 months. The pellets are not retrievable. “The question becomes, what if she has a new breast cancer diagnosis or a diagnosis where estrogen is contraindicated? She has got that estrogen already in her system,” Dr. Winer said.
“The hysterectomy may solve the bleeding problem ... but it doesn’t solve the safety problem overall,” said Dr. Winer, who also is a professor of obstetrics and gynecology and codirector of the reproductive endocrinology and infertility clinic at the University of Southern California, Los Angeles.
Elevated levels
Average peak serum estradiol was significantly higher in the pellet treatment group than in the conventional therapy group (237.70 pg/mL vs. 93.45 pg/mL), as was average peak serum testosterone (192.84 ng/dL vs. 15.59 ng/dL), the researchers reported. Patients on FDA-approved treatments were less likely to have had their hormone levels measured. How concentrations of hormone levels correlate with side effects is unclear, Dr. Jiang said.
The study was limited by its single-institution, retrospective design, and some patient characteristics differed between the treatment groups, the authors noted.
Still, “clinicians ought to be mindful of fully counseling patients on side effects identified in the current study,” Dr. Jiang and coauthors concluded. Clinicians also need to discuss potential risks of breast cancer, endometrial cancer, and cardiovascular disease with patients.
Many primary care clinicians rely on outdated information from the Women’s Health Initiative, published in 2002 and 2004, in their understanding of postmenopausal hormonal therapy and its risks and benefits, Dr. Jiang said. And some patients consider custom-compounded hormone therapy to be safer and more natural, “which is totally misleading.”
Pellets and other custom-compounded medicine containing testosterone may make patients feel better and more energetic, Dr. Jiang acknowledged. “That’s a reason why patients ... tend to stay on, even though they have side effects. The only issue is the safety.”
Additional questions remain. The researchers recently started to examine rates of breast cancer and abnormal breast pathology and mammogram results. “It’s a long journey,” he said.
Plenty of approved options
Custom-compounded medicines are not FDA approved and are not recommended by medical menopause societies, Dr. Jiang said. Meanwhile, plenty of approved hormone therapies, including bioidentical treatments, have safety data and are available.
A 2020 consensus study report from the National Academies of Sciences, Engineering, and Medicine that examined the use of compounded hormonal therapy and provides guidance for clinicians is a good start in addressing this major issue, he added.
A committee determined “there is insufficient evidence to support the overall clinical utility of [compounded bioidentical hormone therapies] as treatment for menopause and male hypogonadism symptoms.”
If an FDA-approved option is available, “I would always go with an FDA-approved product before I would go with a compounded product,” Dr. Winer said. A 2012 fungal meningitis outbreak linked to a compounding pharmacy highlighted risks associated with poor quality compounded drugs.
“I think at least now it is recognized that compounding is an issue that has got to be dealt with,” Dr. Winer said. “It is just that it is so widespread and it is sometimes under the radar ... that I think it is really hard for the FDA to get a handle on it.”
Dr. Winer has seen patients on compounded treatments who are underdosed and patients who are overdosed. “I’ve also seen patients who do quite well with it, but I’m not happy continuing it because tomorrow there may be inconsistency in potency or quality resulting in a different clinical response,” she said.
Nevertheless, compounded pharmacies are needed, Dr. Winer said. If she wants to give natural progesterone that is FDA approved but happens to be made with peanut oil, she will have a compounding pharmacy make it with canola oil instead if a patient has a peanut allergy, for example. Other patients need dosages that are so low that they are not available as FDA-approved products.
Dr. Jiang and Dr. Kauffman had no relevant financial disclosures. Dr. Winer has done work with AbbVie (related to endometriosis), TherapeuticsMD (related to a menopause bioidentical hormonal pill and vaginal estrogen product), and Biogix (related to an antioxidant supplement for menopause symptoms).
*This story was updated on 6/22/2021.
FROM ACOG 2021
Pilot study: Hybrid laser found effective for treating genitourinary syndrome of menopause
, results from a pilot trial showed.
“The genitourinary syndrome of menopause causes suffering in breast cancer survivors and postmenopausal women,” Jill S. Waibel, MD, said during the annual conference of the American Society for Laser Medicine and Surgery. A common side effect for breast cancer survivors is early onset of menopause that is brought on by treatment, specifically aromatase-inhibitor therapies, she noted.
The symptoms of GSM include discomfort during sex, impaired sexual function, burning or sensation or irritation of the genital area, vaginal constriction, frequent urinary tract infections, urinary incontinence, and vaginal laxity, said Dr. Waibel, owner and medical director of the Miami Dermatology and Laser Institute. Nonhormonal treatments have included OTC vaginal lubricants, OTC moisturizers, low-dose vaginal estrogen – which increases the risk of breast cancer – and systemic estrogen therapy, which also can increase the risk of breast and endometrial cancer. “So, we need a healthy, nondrug option,” she said.
The objective of the pilot study was to determine the safety and efficacy of the diVa hybrid fractional laser as a treatment for symptoms of genitourinary syndrome of menopause, early menopause after breast cancer, or vaginal atrophy. The laser applies tunable nonablative (1,470-nm) and ablative (2,940-nm) wavelengths to the same microscopic treatment zone to maximize results and reduce downtime. The device features a motorized precision guidance system and calibrated rotation for homogeneous pulsing.
“The 2,940-nm wavelength is used to ablate to a depth of 0-800 micrometers while the 1,470-nm wavelength is used to coagulate the epithelium and the lamina propria at a depth of 100-700 micrometers,” said Dr. Waibel, who is also subsection chief of dermatology at Baptist Hospital of Miami. “This combination is used for epithelial tissue to heal quickly and the lamina propria to remodel slowly over time, laying down more collagen in tissue.” Each procedure is delivered via a single-use dilator, which expands the vaginal canal for increased treatment area. “The tip length is 5.5 cm and the diameter is 1 cm,” she said. “The clear tip acts as a hygienic barrier between the tip and the handpiece.”
Study participants included 25 women between the ages of 40 and 70 with early menopause after breast cancer or vaginal atrophy: 20 in the treatment arm and 5 in the sham-treatment arm. Dr. Waibel performed three procedures 2 weeks apart. An ob.gyn. assessed the primary endpoints, which included the Vaginal Health Index Scale (VHIS), the Vaginal Maturation Index (VMI), the Female Sexual Function Index (FSFI) questionnaire, and the Day-to-Day Impact of Vaginal Aging (DIVA) questionnaire. Secondary endpoints were histology and a satisfaction questionnaire.
Of the women in the treated group, there were data available for 19 at 3 months follow-up and 17 at 6 months follow-up. Based on the results in these patients, there were statistically significant improvements in nearly all domains of the FSFI treatment arm at 3 and 6 months when compared to baseline, especially arousal (P values of .05 at 3 months and .01 at 6 months) and lubrication (P values of .009 at three months and .001 at 6 months).
Between 3 and 6 months, patients in the treatment arm experienced improvements in four dimensions of the DIVA questionnaire: daily activities (P value of .01 at 3 months to .010 at 6 months), emotional well-being (P value of .06 at 3 months to .014 at 6 months), sexual function (P value of .30 at 3 months to .003 at 6 months), and self-concept/body image (P value of .002 at 3 months to .001 at 6 months).
As for satisfaction, a majority of those in the treatment arm were “somewhat satisfied” with the treatment and would “somewhat likely” repeat and recommend the treatment to friends and family, Dr. Waibel said. Results among the women in the control arm, who were also surveyed, were in the similar range, she noted. (No other results for women in the control arm were available.)
Following treatments, histology revealed that the collagen was denser, fibroblasts were more dense, and vascularity was more notable. No adverse events were observed. “The hybrid fractional laser is safe and effective for treating GSM, early menopause after breast cancer, or vaginal atrophy,” Dr. Waibel concluded. Further studies are important to improve the understanding of “laser dosimetry, frequency of treatments, and longevity of effect. Collaboration between ob.gyns. and dermatologists is important as we learn about laser therapy in GSM.”
Dr. Waibel disclosed that she is a member of the advisory board of Sciton, which manufactures the diVa laser. She has also conducted clinical trials for many other device and pharmaceutical companies.
, results from a pilot trial showed.
“The genitourinary syndrome of menopause causes suffering in breast cancer survivors and postmenopausal women,” Jill S. Waibel, MD, said during the annual conference of the American Society for Laser Medicine and Surgery. A common side effect for breast cancer survivors is early onset of menopause that is brought on by treatment, specifically aromatase-inhibitor therapies, she noted.
The symptoms of GSM include discomfort during sex, impaired sexual function, burning or sensation or irritation of the genital area, vaginal constriction, frequent urinary tract infections, urinary incontinence, and vaginal laxity, said Dr. Waibel, owner and medical director of the Miami Dermatology and Laser Institute. Nonhormonal treatments have included OTC vaginal lubricants, OTC moisturizers, low-dose vaginal estrogen – which increases the risk of breast cancer – and systemic estrogen therapy, which also can increase the risk of breast and endometrial cancer. “So, we need a healthy, nondrug option,” she said.
The objective of the pilot study was to determine the safety and efficacy of the diVa hybrid fractional laser as a treatment for symptoms of genitourinary syndrome of menopause, early menopause after breast cancer, or vaginal atrophy. The laser applies tunable nonablative (1,470-nm) and ablative (2,940-nm) wavelengths to the same microscopic treatment zone to maximize results and reduce downtime. The device features a motorized precision guidance system and calibrated rotation for homogeneous pulsing.
“The 2,940-nm wavelength is used to ablate to a depth of 0-800 micrometers while the 1,470-nm wavelength is used to coagulate the epithelium and the lamina propria at a depth of 100-700 micrometers,” said Dr. Waibel, who is also subsection chief of dermatology at Baptist Hospital of Miami. “This combination is used for epithelial tissue to heal quickly and the lamina propria to remodel slowly over time, laying down more collagen in tissue.” Each procedure is delivered via a single-use dilator, which expands the vaginal canal for increased treatment area. “The tip length is 5.5 cm and the diameter is 1 cm,” she said. “The clear tip acts as a hygienic barrier between the tip and the handpiece.”
Study participants included 25 women between the ages of 40 and 70 with early menopause after breast cancer or vaginal atrophy: 20 in the treatment arm and 5 in the sham-treatment arm. Dr. Waibel performed three procedures 2 weeks apart. An ob.gyn. assessed the primary endpoints, which included the Vaginal Health Index Scale (VHIS), the Vaginal Maturation Index (VMI), the Female Sexual Function Index (FSFI) questionnaire, and the Day-to-Day Impact of Vaginal Aging (DIVA) questionnaire. Secondary endpoints were histology and a satisfaction questionnaire.
Of the women in the treated group, there were data available for 19 at 3 months follow-up and 17 at 6 months follow-up. Based on the results in these patients, there were statistically significant improvements in nearly all domains of the FSFI treatment arm at 3 and 6 months when compared to baseline, especially arousal (P values of .05 at 3 months and .01 at 6 months) and lubrication (P values of .009 at three months and .001 at 6 months).
Between 3 and 6 months, patients in the treatment arm experienced improvements in four dimensions of the DIVA questionnaire: daily activities (P value of .01 at 3 months to .010 at 6 months), emotional well-being (P value of .06 at 3 months to .014 at 6 months), sexual function (P value of .30 at 3 months to .003 at 6 months), and self-concept/body image (P value of .002 at 3 months to .001 at 6 months).
As for satisfaction, a majority of those in the treatment arm were “somewhat satisfied” with the treatment and would “somewhat likely” repeat and recommend the treatment to friends and family, Dr. Waibel said. Results among the women in the control arm, who were also surveyed, were in the similar range, she noted. (No other results for women in the control arm were available.)
Following treatments, histology revealed that the collagen was denser, fibroblasts were more dense, and vascularity was more notable. No adverse events were observed. “The hybrid fractional laser is safe and effective for treating GSM, early menopause after breast cancer, or vaginal atrophy,” Dr. Waibel concluded. Further studies are important to improve the understanding of “laser dosimetry, frequency of treatments, and longevity of effect. Collaboration between ob.gyns. and dermatologists is important as we learn about laser therapy in GSM.”
Dr. Waibel disclosed that she is a member of the advisory board of Sciton, which manufactures the diVa laser. She has also conducted clinical trials for many other device and pharmaceutical companies.
, results from a pilot trial showed.
“The genitourinary syndrome of menopause causes suffering in breast cancer survivors and postmenopausal women,” Jill S. Waibel, MD, said during the annual conference of the American Society for Laser Medicine and Surgery. A common side effect for breast cancer survivors is early onset of menopause that is brought on by treatment, specifically aromatase-inhibitor therapies, she noted.
The symptoms of GSM include discomfort during sex, impaired sexual function, burning or sensation or irritation of the genital area, vaginal constriction, frequent urinary tract infections, urinary incontinence, and vaginal laxity, said Dr. Waibel, owner and medical director of the Miami Dermatology and Laser Institute. Nonhormonal treatments have included OTC vaginal lubricants, OTC moisturizers, low-dose vaginal estrogen – which increases the risk of breast cancer – and systemic estrogen therapy, which also can increase the risk of breast and endometrial cancer. “So, we need a healthy, nondrug option,” she said.
The objective of the pilot study was to determine the safety and efficacy of the diVa hybrid fractional laser as a treatment for symptoms of genitourinary syndrome of menopause, early menopause after breast cancer, or vaginal atrophy. The laser applies tunable nonablative (1,470-nm) and ablative (2,940-nm) wavelengths to the same microscopic treatment zone to maximize results and reduce downtime. The device features a motorized precision guidance system and calibrated rotation for homogeneous pulsing.
“The 2,940-nm wavelength is used to ablate to a depth of 0-800 micrometers while the 1,470-nm wavelength is used to coagulate the epithelium and the lamina propria at a depth of 100-700 micrometers,” said Dr. Waibel, who is also subsection chief of dermatology at Baptist Hospital of Miami. “This combination is used for epithelial tissue to heal quickly and the lamina propria to remodel slowly over time, laying down more collagen in tissue.” Each procedure is delivered via a single-use dilator, which expands the vaginal canal for increased treatment area. “The tip length is 5.5 cm and the diameter is 1 cm,” she said. “The clear tip acts as a hygienic barrier between the tip and the handpiece.”
Study participants included 25 women between the ages of 40 and 70 with early menopause after breast cancer or vaginal atrophy: 20 in the treatment arm and 5 in the sham-treatment arm. Dr. Waibel performed three procedures 2 weeks apart. An ob.gyn. assessed the primary endpoints, which included the Vaginal Health Index Scale (VHIS), the Vaginal Maturation Index (VMI), the Female Sexual Function Index (FSFI) questionnaire, and the Day-to-Day Impact of Vaginal Aging (DIVA) questionnaire. Secondary endpoints were histology and a satisfaction questionnaire.
Of the women in the treated group, there were data available for 19 at 3 months follow-up and 17 at 6 months follow-up. Based on the results in these patients, there were statistically significant improvements in nearly all domains of the FSFI treatment arm at 3 and 6 months when compared to baseline, especially arousal (P values of .05 at 3 months and .01 at 6 months) and lubrication (P values of .009 at three months and .001 at 6 months).
Between 3 and 6 months, patients in the treatment arm experienced improvements in four dimensions of the DIVA questionnaire: daily activities (P value of .01 at 3 months to .010 at 6 months), emotional well-being (P value of .06 at 3 months to .014 at 6 months), sexual function (P value of .30 at 3 months to .003 at 6 months), and self-concept/body image (P value of .002 at 3 months to .001 at 6 months).
As for satisfaction, a majority of those in the treatment arm were “somewhat satisfied” with the treatment and would “somewhat likely” repeat and recommend the treatment to friends and family, Dr. Waibel said. Results among the women in the control arm, who were also surveyed, were in the similar range, she noted. (No other results for women in the control arm were available.)
Following treatments, histology revealed that the collagen was denser, fibroblasts were more dense, and vascularity was more notable. No adverse events were observed. “The hybrid fractional laser is safe and effective for treating GSM, early menopause after breast cancer, or vaginal atrophy,” Dr. Waibel concluded. Further studies are important to improve the understanding of “laser dosimetry, frequency of treatments, and longevity of effect. Collaboration between ob.gyns. and dermatologists is important as we learn about laser therapy in GSM.”
Dr. Waibel disclosed that she is a member of the advisory board of Sciton, which manufactures the diVa laser. She has also conducted clinical trials for many other device and pharmaceutical companies.
FROM ASLMS 2021
Primary ovarian insufficiency requires long-term management of sequelae
Primary ovarian insufficiency is not your mother’s early menopause, according to Laurie McKenzie, MD, a reproductive endocrinologist and associate professor of ob.gyn. at the University of Texas MD Anderson Cancer Center with a joint appointment at Baylor College of Medicine, both in Houston.
Known previously as primary ovarian failure, the syndrome of primary ovarian insufficiency (POI) no longer refers to a failure in part because of the term’s negative connotations but mostly because it’s not precisely accurate, Dr. McKenzie told attendees at the 2021 annual meeting of the American College of Obstetricians and Gynecologists on May 1.
“Many of these women, especially early on in diagnosis, may be experiencing some intermittent ovarian function, so it may not be a complete failure of the ovaries,” Dr. McKenzie said.
Although the condition is not common, affecting about 1% of the female population, “it’s the kind of thing that when a gynecologist has someone who has this walk into their office, you really need to know how to address it because these women are understandably very distressed.” Lauren Streicher, MD, a clinical professor of obstetrics and gynecology at Northwestern University, Chicago, said in an interview after attending the talk.
Women who develop POI lose ovarian activity before age 40, characterized by menstrual disturbance with raised gonadotropins and low estradiol. Symptoms include the hot flushes and night sweats characteristic of estrogen deficiency as well as vaginal symptoms, including dyspareunia and dryness. Other symptoms can include sleep disturbance, mood changes, poor concentration, stiffness, dry eyes, altered urinary frequency, low libido, and lack of energy.
Dr. McKenzie urged doctors to ask women about their symptoms if they present with amenorrhea because young women with primary amenorrhea rarely experience symptoms at presentation, “implying that these symptoms are due to estrogen withdrawal rather than estrogen deficiency,” she said. Diagnosis involves confirmation of 4-6 months of amenorrhea or oligomenorrhea and two measurements of elevated follicle-stimulating hormone (FSH). Following this work-up, clinicians should seek the cause of the condition.
Etiology of POI and associated conditions
A wide range of conditions or genetic factors can cause POI or be more likely in patients with POI, Dr. McKenzie said. Many women diagnosed with POI have chromosomal abnormalities, and there is no cutoff for genetic testing, she said. Most of these genetic causes (94%) are X chromosome abnormalities, including Turners-associated dysmorphic features, gonadal dysgenesis, and FMR1 anomalies. Autosomal gene mutations could also play a role in POI.
Although women with the full FMR1 mutation (Fragile X syndrome) do not have an increased risk of POI, those with the premutation (55-200 repeats) have a 13%-26% increased risk of developing POI, albeit no increased risk of intellectual disability. About 0.8%-7.5% of women with sporadic POI and up to 13% of women with a family history of POI have this genetic anomaly.
Autoimmune conditions may also develop or be related to POI, including hypothyroidism and adrenal insufficiency, Dr. McKenzie said. About 20% of adults with POI will develop hypothyroidism, so testing every 1-2 years is reasonable, though no formal screening guidelines exist. In women whose cause of POI is unknown or in whom you suspect an immune disorder, clinicians may consider screening for 21OH-Ab or adrenocortical antibodies. Patients with a positive 21OH-Ab or adrenocortical antibodies test should be referred to an endocrinologist to test adrenal function and rule out Addison disease.
Though diabetes mellitus has been linked to POI, not enough evidence exists to recommend screening women with POI for diabetes. There’s similarly no indication for infection screening, but infections can cause POI. Mumps oophoritis, for example, accounts for 3%-7% of POI cases. Cancer therapy, including radiotherapy and chemotherapy, and surgical treatment for cancer can result in POI.
“Smoking, alcohol, nutrition, and exposure to endocrine disruptors are implicated as influencing the age of menopause but are not readily diagnosable causes of POI,” Dr. McKenzie said. “Although not proven to cause POI, cigarette smoking is toxic to the ovaries and has been linked to an earlier age at menopause.” Then there are many women whose cause of POI is unknown.
To take all these possibilities into account, Dr. McKenzie described the complete diagnostic work-up recommended by ACOG:
- Menstrual irregularity for at least 3-4 months
- Test FSH and estradiol
- Test hCG, TSH, and prolactin
- If diagnosis is confirmed, test karyotype, FMR1 premutation, adrenal antibodies, and a pelvic sonogram.
However, she added during the Q&A after her talk, she is not sure why a sonogram is recommended or what additional information it might provide.
Long-term consequences of POI
Dr McKenzie noted that one study found a 2-year reduction in life expectancy among women who developed menopause before age 40. The reduced life expectancy linked to untreated POI is primarily caused by cardiovascular disease, she said. Women who undergo menopause aged between 35 and 40 years have a 50% greater risk of death related to ischemic heart disease than those ages 49-51, after adjusting for other comorbidities and confounders.
“Women with primary ovarian insufficiency should be advised on how to reduce cardiovascular risk factors by not smoking, taking regular exercise, and maintaining a healthy weight,” Dr McKenzie said.
No interventions have been shown to increase ovarian activity
Though fertility is substantially reduced in women with POI, it may not be completely gone. Several studies have found pregnancy rates ranging from 1.5% to 4.8%, and one study found that 25% of women with idiopathic POI had some evidence of ovarian function. Clinicians should therefore recommend women with POI use contraception if they do not want to conceive. Egg donation is an option for preserving fertility in women with POI but only before POI is solidly established.
“No interventions have been reliably shown to increase ovarian activity and natural conception rates,” Dr. McKenzie said.
For women who survive childhood or adolescent cancer and become pregnant, no evidence has shown an increased risk of congenital anomalies, but risk of low birth weight is elevated in babies whose mothers received anthracyclines. Treatment with anthracyclines and mediastinal radiotherapy have also been linked with cardiomyopathy and heart failure, so an echocardiogram prior to pregnancy is indicated in women with exposure to these or high-dose cyclophosphamide.
Abdominopelvic radiotherapy, however, has been linked to poor uterine function with a greater risk of late miscarriage, prematurity, low birth weight, stillbirth, neonatal hemorrhage, and postpartum hemorrhage.
“Pregnancies in women with Turner syndrome are very high risk and may have a maternal mortality as high as 3.5%,” Dr. McKenzie said, so these pregnancies require involvement of a cardiologist.
Other sequelae of POI can include increased bone resorption, net loss of bone (2%-3% annually soon after menopause) and reduced bone mineral density. Women should be getting 1,000 mg/day of calcium and 800 IU/day of vitamin D, but bone screening remains controversial in the field. Finally, providers should not ignore psychosocial effects of POI, including grief, diminished self esteem, and sadness, even more so, potentially, among adolescents.
Treatment of POI
Managing POI involves a two-pronged strategy of providing enough estrogen (estradiol, ethinyl estradiol, or conjugated equine estrogens) to mimic normal physiology and enough progestogen (synthetic or progesterone) to protect the endometrium from the mitogenic effect of estrogen.
The two primary options are hormone therapy and combination oral contraceptives. Hormone therapy might allow ovulation and pregnancy in some women, but combination oral contraceptive may feel less stigmatized in those who are still young, albeit with a potential risk for venous thromboembolism.
Continuous treatment tends to be easier and can involve breakthrough bleeding in younger patients; in postmenopausal women, breast cancer risk is higher but endometrial cancer risk is lower. Cyclic treatment mimics the endometrium’s normal function, resulting in bleeding that may help some women feel more “normal” and aids in knowing about a pregnancy. Those wanting to avoid bleeds and use contraception can use the levonorgestrel IUD off label.
Dr. Streicher said in an interview, “Not only is it critically important to recognize [long-term consequences] in this small group of women, but the lessons learned from young women who go though menopause can absolutely be extrapolated to women who go through menopause at an appropriate time.”
Dr. McKenzie had no disclosures. Dr. Streicher has consulted for Astellas Pharma and Church & Dwight, and she owns investments in InControl Medical and Sermonix Pharmaceutical.
Primary ovarian insufficiency is not your mother’s early menopause, according to Laurie McKenzie, MD, a reproductive endocrinologist and associate professor of ob.gyn. at the University of Texas MD Anderson Cancer Center with a joint appointment at Baylor College of Medicine, both in Houston.
Known previously as primary ovarian failure, the syndrome of primary ovarian insufficiency (POI) no longer refers to a failure in part because of the term’s negative connotations but mostly because it’s not precisely accurate, Dr. McKenzie told attendees at the 2021 annual meeting of the American College of Obstetricians and Gynecologists on May 1.
“Many of these women, especially early on in diagnosis, may be experiencing some intermittent ovarian function, so it may not be a complete failure of the ovaries,” Dr. McKenzie said.
Although the condition is not common, affecting about 1% of the female population, “it’s the kind of thing that when a gynecologist has someone who has this walk into their office, you really need to know how to address it because these women are understandably very distressed.” Lauren Streicher, MD, a clinical professor of obstetrics and gynecology at Northwestern University, Chicago, said in an interview after attending the talk.
Women who develop POI lose ovarian activity before age 40, characterized by menstrual disturbance with raised gonadotropins and low estradiol. Symptoms include the hot flushes and night sweats characteristic of estrogen deficiency as well as vaginal symptoms, including dyspareunia and dryness. Other symptoms can include sleep disturbance, mood changes, poor concentration, stiffness, dry eyes, altered urinary frequency, low libido, and lack of energy.
Dr. McKenzie urged doctors to ask women about their symptoms if they present with amenorrhea because young women with primary amenorrhea rarely experience symptoms at presentation, “implying that these symptoms are due to estrogen withdrawal rather than estrogen deficiency,” she said. Diagnosis involves confirmation of 4-6 months of amenorrhea or oligomenorrhea and two measurements of elevated follicle-stimulating hormone (FSH). Following this work-up, clinicians should seek the cause of the condition.
Etiology of POI and associated conditions
A wide range of conditions or genetic factors can cause POI or be more likely in patients with POI, Dr. McKenzie said. Many women diagnosed with POI have chromosomal abnormalities, and there is no cutoff for genetic testing, she said. Most of these genetic causes (94%) are X chromosome abnormalities, including Turners-associated dysmorphic features, gonadal dysgenesis, and FMR1 anomalies. Autosomal gene mutations could also play a role in POI.
Although women with the full FMR1 mutation (Fragile X syndrome) do not have an increased risk of POI, those with the premutation (55-200 repeats) have a 13%-26% increased risk of developing POI, albeit no increased risk of intellectual disability. About 0.8%-7.5% of women with sporadic POI and up to 13% of women with a family history of POI have this genetic anomaly.
Autoimmune conditions may also develop or be related to POI, including hypothyroidism and adrenal insufficiency, Dr. McKenzie said. About 20% of adults with POI will develop hypothyroidism, so testing every 1-2 years is reasonable, though no formal screening guidelines exist. In women whose cause of POI is unknown or in whom you suspect an immune disorder, clinicians may consider screening for 21OH-Ab or adrenocortical antibodies. Patients with a positive 21OH-Ab or adrenocortical antibodies test should be referred to an endocrinologist to test adrenal function and rule out Addison disease.
Though diabetes mellitus has been linked to POI, not enough evidence exists to recommend screening women with POI for diabetes. There’s similarly no indication for infection screening, but infections can cause POI. Mumps oophoritis, for example, accounts for 3%-7% of POI cases. Cancer therapy, including radiotherapy and chemotherapy, and surgical treatment for cancer can result in POI.
“Smoking, alcohol, nutrition, and exposure to endocrine disruptors are implicated as influencing the age of menopause but are not readily diagnosable causes of POI,” Dr. McKenzie said. “Although not proven to cause POI, cigarette smoking is toxic to the ovaries and has been linked to an earlier age at menopause.” Then there are many women whose cause of POI is unknown.
To take all these possibilities into account, Dr. McKenzie described the complete diagnostic work-up recommended by ACOG:
- Menstrual irregularity for at least 3-4 months
- Test FSH and estradiol
- Test hCG, TSH, and prolactin
- If diagnosis is confirmed, test karyotype, FMR1 premutation, adrenal antibodies, and a pelvic sonogram.
However, she added during the Q&A after her talk, she is not sure why a sonogram is recommended or what additional information it might provide.
Long-term consequences of POI
Dr McKenzie noted that one study found a 2-year reduction in life expectancy among women who developed menopause before age 40. The reduced life expectancy linked to untreated POI is primarily caused by cardiovascular disease, she said. Women who undergo menopause aged between 35 and 40 years have a 50% greater risk of death related to ischemic heart disease than those ages 49-51, after adjusting for other comorbidities and confounders.
“Women with primary ovarian insufficiency should be advised on how to reduce cardiovascular risk factors by not smoking, taking regular exercise, and maintaining a healthy weight,” Dr McKenzie said.
No interventions have been shown to increase ovarian activity
Though fertility is substantially reduced in women with POI, it may not be completely gone. Several studies have found pregnancy rates ranging from 1.5% to 4.8%, and one study found that 25% of women with idiopathic POI had some evidence of ovarian function. Clinicians should therefore recommend women with POI use contraception if they do not want to conceive. Egg donation is an option for preserving fertility in women with POI but only before POI is solidly established.
“No interventions have been reliably shown to increase ovarian activity and natural conception rates,” Dr. McKenzie said.
For women who survive childhood or adolescent cancer and become pregnant, no evidence has shown an increased risk of congenital anomalies, but risk of low birth weight is elevated in babies whose mothers received anthracyclines. Treatment with anthracyclines and mediastinal radiotherapy have also been linked with cardiomyopathy and heart failure, so an echocardiogram prior to pregnancy is indicated in women with exposure to these or high-dose cyclophosphamide.
Abdominopelvic radiotherapy, however, has been linked to poor uterine function with a greater risk of late miscarriage, prematurity, low birth weight, stillbirth, neonatal hemorrhage, and postpartum hemorrhage.
“Pregnancies in women with Turner syndrome are very high risk and may have a maternal mortality as high as 3.5%,” Dr. McKenzie said, so these pregnancies require involvement of a cardiologist.
Other sequelae of POI can include increased bone resorption, net loss of bone (2%-3% annually soon after menopause) and reduced bone mineral density. Women should be getting 1,000 mg/day of calcium and 800 IU/day of vitamin D, but bone screening remains controversial in the field. Finally, providers should not ignore psychosocial effects of POI, including grief, diminished self esteem, and sadness, even more so, potentially, among adolescents.
Treatment of POI
Managing POI involves a two-pronged strategy of providing enough estrogen (estradiol, ethinyl estradiol, or conjugated equine estrogens) to mimic normal physiology and enough progestogen (synthetic or progesterone) to protect the endometrium from the mitogenic effect of estrogen.
The two primary options are hormone therapy and combination oral contraceptives. Hormone therapy might allow ovulation and pregnancy in some women, but combination oral contraceptive may feel less stigmatized in those who are still young, albeit with a potential risk for venous thromboembolism.
Continuous treatment tends to be easier and can involve breakthrough bleeding in younger patients; in postmenopausal women, breast cancer risk is higher but endometrial cancer risk is lower. Cyclic treatment mimics the endometrium’s normal function, resulting in bleeding that may help some women feel more “normal” and aids in knowing about a pregnancy. Those wanting to avoid bleeds and use contraception can use the levonorgestrel IUD off label.
Dr. Streicher said in an interview, “Not only is it critically important to recognize [long-term consequences] in this small group of women, but the lessons learned from young women who go though menopause can absolutely be extrapolated to women who go through menopause at an appropriate time.”
Dr. McKenzie had no disclosures. Dr. Streicher has consulted for Astellas Pharma and Church & Dwight, and she owns investments in InControl Medical and Sermonix Pharmaceutical.
Primary ovarian insufficiency is not your mother’s early menopause, according to Laurie McKenzie, MD, a reproductive endocrinologist and associate professor of ob.gyn. at the University of Texas MD Anderson Cancer Center with a joint appointment at Baylor College of Medicine, both in Houston.
Known previously as primary ovarian failure, the syndrome of primary ovarian insufficiency (POI) no longer refers to a failure in part because of the term’s negative connotations but mostly because it’s not precisely accurate, Dr. McKenzie told attendees at the 2021 annual meeting of the American College of Obstetricians and Gynecologists on May 1.
“Many of these women, especially early on in diagnosis, may be experiencing some intermittent ovarian function, so it may not be a complete failure of the ovaries,” Dr. McKenzie said.
Although the condition is not common, affecting about 1% of the female population, “it’s the kind of thing that when a gynecologist has someone who has this walk into their office, you really need to know how to address it because these women are understandably very distressed.” Lauren Streicher, MD, a clinical professor of obstetrics and gynecology at Northwestern University, Chicago, said in an interview after attending the talk.
Women who develop POI lose ovarian activity before age 40, characterized by menstrual disturbance with raised gonadotropins and low estradiol. Symptoms include the hot flushes and night sweats characteristic of estrogen deficiency as well as vaginal symptoms, including dyspareunia and dryness. Other symptoms can include sleep disturbance, mood changes, poor concentration, stiffness, dry eyes, altered urinary frequency, low libido, and lack of energy.
Dr. McKenzie urged doctors to ask women about their symptoms if they present with amenorrhea because young women with primary amenorrhea rarely experience symptoms at presentation, “implying that these symptoms are due to estrogen withdrawal rather than estrogen deficiency,” she said. Diagnosis involves confirmation of 4-6 months of amenorrhea or oligomenorrhea and two measurements of elevated follicle-stimulating hormone (FSH). Following this work-up, clinicians should seek the cause of the condition.
Etiology of POI and associated conditions
A wide range of conditions or genetic factors can cause POI or be more likely in patients with POI, Dr. McKenzie said. Many women diagnosed with POI have chromosomal abnormalities, and there is no cutoff for genetic testing, she said. Most of these genetic causes (94%) are X chromosome abnormalities, including Turners-associated dysmorphic features, gonadal dysgenesis, and FMR1 anomalies. Autosomal gene mutations could also play a role in POI.
Although women with the full FMR1 mutation (Fragile X syndrome) do not have an increased risk of POI, those with the premutation (55-200 repeats) have a 13%-26% increased risk of developing POI, albeit no increased risk of intellectual disability. About 0.8%-7.5% of women with sporadic POI and up to 13% of women with a family history of POI have this genetic anomaly.
Autoimmune conditions may also develop or be related to POI, including hypothyroidism and adrenal insufficiency, Dr. McKenzie said. About 20% of adults with POI will develop hypothyroidism, so testing every 1-2 years is reasonable, though no formal screening guidelines exist. In women whose cause of POI is unknown or in whom you suspect an immune disorder, clinicians may consider screening for 21OH-Ab or adrenocortical antibodies. Patients with a positive 21OH-Ab or adrenocortical antibodies test should be referred to an endocrinologist to test adrenal function and rule out Addison disease.
Though diabetes mellitus has been linked to POI, not enough evidence exists to recommend screening women with POI for diabetes. There’s similarly no indication for infection screening, but infections can cause POI. Mumps oophoritis, for example, accounts for 3%-7% of POI cases. Cancer therapy, including radiotherapy and chemotherapy, and surgical treatment for cancer can result in POI.
“Smoking, alcohol, nutrition, and exposure to endocrine disruptors are implicated as influencing the age of menopause but are not readily diagnosable causes of POI,” Dr. McKenzie said. “Although not proven to cause POI, cigarette smoking is toxic to the ovaries and has been linked to an earlier age at menopause.” Then there are many women whose cause of POI is unknown.
To take all these possibilities into account, Dr. McKenzie described the complete diagnostic work-up recommended by ACOG:
- Menstrual irregularity for at least 3-4 months
- Test FSH and estradiol
- Test hCG, TSH, and prolactin
- If diagnosis is confirmed, test karyotype, FMR1 premutation, adrenal antibodies, and a pelvic sonogram.
However, she added during the Q&A after her talk, she is not sure why a sonogram is recommended or what additional information it might provide.
Long-term consequences of POI
Dr McKenzie noted that one study found a 2-year reduction in life expectancy among women who developed menopause before age 40. The reduced life expectancy linked to untreated POI is primarily caused by cardiovascular disease, she said. Women who undergo menopause aged between 35 and 40 years have a 50% greater risk of death related to ischemic heart disease than those ages 49-51, after adjusting for other comorbidities and confounders.
“Women with primary ovarian insufficiency should be advised on how to reduce cardiovascular risk factors by not smoking, taking regular exercise, and maintaining a healthy weight,” Dr McKenzie said.
No interventions have been shown to increase ovarian activity
Though fertility is substantially reduced in women with POI, it may not be completely gone. Several studies have found pregnancy rates ranging from 1.5% to 4.8%, and one study found that 25% of women with idiopathic POI had some evidence of ovarian function. Clinicians should therefore recommend women with POI use contraception if they do not want to conceive. Egg donation is an option for preserving fertility in women with POI but only before POI is solidly established.
“No interventions have been reliably shown to increase ovarian activity and natural conception rates,” Dr. McKenzie said.
For women who survive childhood or adolescent cancer and become pregnant, no evidence has shown an increased risk of congenital anomalies, but risk of low birth weight is elevated in babies whose mothers received anthracyclines. Treatment with anthracyclines and mediastinal radiotherapy have also been linked with cardiomyopathy and heart failure, so an echocardiogram prior to pregnancy is indicated in women with exposure to these or high-dose cyclophosphamide.
Abdominopelvic radiotherapy, however, has been linked to poor uterine function with a greater risk of late miscarriage, prematurity, low birth weight, stillbirth, neonatal hemorrhage, and postpartum hemorrhage.
“Pregnancies in women with Turner syndrome are very high risk and may have a maternal mortality as high as 3.5%,” Dr. McKenzie said, so these pregnancies require involvement of a cardiologist.
Other sequelae of POI can include increased bone resorption, net loss of bone (2%-3% annually soon after menopause) and reduced bone mineral density. Women should be getting 1,000 mg/day of calcium and 800 IU/day of vitamin D, but bone screening remains controversial in the field. Finally, providers should not ignore psychosocial effects of POI, including grief, diminished self esteem, and sadness, even more so, potentially, among adolescents.
Treatment of POI
Managing POI involves a two-pronged strategy of providing enough estrogen (estradiol, ethinyl estradiol, or conjugated equine estrogens) to mimic normal physiology and enough progestogen (synthetic or progesterone) to protect the endometrium from the mitogenic effect of estrogen.
The two primary options are hormone therapy and combination oral contraceptives. Hormone therapy might allow ovulation and pregnancy in some women, but combination oral contraceptive may feel less stigmatized in those who are still young, albeit with a potential risk for venous thromboembolism.
Continuous treatment tends to be easier and can involve breakthrough bleeding in younger patients; in postmenopausal women, breast cancer risk is higher but endometrial cancer risk is lower. Cyclic treatment mimics the endometrium’s normal function, resulting in bleeding that may help some women feel more “normal” and aids in knowing about a pregnancy. Those wanting to avoid bleeds and use contraception can use the levonorgestrel IUD off label.
Dr. Streicher said in an interview, “Not only is it critically important to recognize [long-term consequences] in this small group of women, but the lessons learned from young women who go though menopause can absolutely be extrapolated to women who go through menopause at an appropriate time.”
Dr. McKenzie had no disclosures. Dr. Streicher has consulted for Astellas Pharma and Church & Dwight, and she owns investments in InControl Medical and Sermonix Pharmaceutical.
FROM ACOG 2021
FDA and power morcellation, gel for vaginal odor, and an intrauterine electrosurgery system
FDA guidance for power morcellation
“The FDA has granted marketing authorization for one containment system and continues to encourage innovation in this area” said the report. Olympus’ Pneumoliner is the only FDA cleared containment device to provide a laparoscopic option for appropriately identified patients undergoing myomectomy and hysterectomy. The containment system is sold with Olympus’ PK Morcellator, but the company says that it has made the Pneumoliner available to physicians choosing an alternate to the PK Morcellator, provided that there is device compatibility. The Pneumoliner “reduces the spread of benign tissue into the abdominal cavity, in which pathologies, like fibroids, may regrow when tissue or cells are inadvertently left behind,” according to Olympus.
Vaginal odor elimination gel
The gel is sold in 7 single-day applications, with a single tube used per day at bedtime to eliminate unwanted odor. To maintain freshness and comfort, a single tube of Relactagel can be used for 2 to 3 days after a woman’s menstrual cycle, says Kora Healthcare. The company warns that mild irritation can occur with product use during fungal infections or when small tears are present in the vaginal tissue and that use should be discontinued if irritation occurs. In addition, if trying to become pregnant Relatagel should not be used, advises Kora Healthcare, although the gel is not a contraceptive.
Intrauterine electrosurgery system
FDA guidance for power morcellation
“The FDA has granted marketing authorization for one containment system and continues to encourage innovation in this area” said the report. Olympus’ Pneumoliner is the only FDA cleared containment device to provide a laparoscopic option for appropriately identified patients undergoing myomectomy and hysterectomy. The containment system is sold with Olympus’ PK Morcellator, but the company says that it has made the Pneumoliner available to physicians choosing an alternate to the PK Morcellator, provided that there is device compatibility. The Pneumoliner “reduces the spread of benign tissue into the abdominal cavity, in which pathologies, like fibroids, may regrow when tissue or cells are inadvertently left behind,” according to Olympus.
Vaginal odor elimination gel
The gel is sold in 7 single-day applications, with a single tube used per day at bedtime to eliminate unwanted odor. To maintain freshness and comfort, a single tube of Relactagel can be used for 2 to 3 days after a woman’s menstrual cycle, says Kora Healthcare. The company warns that mild irritation can occur with product use during fungal infections or when small tears are present in the vaginal tissue and that use should be discontinued if irritation occurs. In addition, if trying to become pregnant Relatagel should not be used, advises Kora Healthcare, although the gel is not a contraceptive.
Intrauterine electrosurgery system
FDA guidance for power morcellation
“The FDA has granted marketing authorization for one containment system and continues to encourage innovation in this area” said the report. Olympus’ Pneumoliner is the only FDA cleared containment device to provide a laparoscopic option for appropriately identified patients undergoing myomectomy and hysterectomy. The containment system is sold with Olympus’ PK Morcellator, but the company says that it has made the Pneumoliner available to physicians choosing an alternate to the PK Morcellator, provided that there is device compatibility. The Pneumoliner “reduces the spread of benign tissue into the abdominal cavity, in which pathologies, like fibroids, may regrow when tissue or cells are inadvertently left behind,” according to Olympus.
Vaginal odor elimination gel
The gel is sold in 7 single-day applications, with a single tube used per day at bedtime to eliminate unwanted odor. To maintain freshness and comfort, a single tube of Relactagel can be used for 2 to 3 days after a woman’s menstrual cycle, says Kora Healthcare. The company warns that mild irritation can occur with product use during fungal infections or when small tears are present in the vaginal tissue and that use should be discontinued if irritation occurs. In addition, if trying to become pregnant Relatagel should not be used, advises Kora Healthcare, although the gel is not a contraceptive.
Intrauterine electrosurgery system
Pregnancy after pioneering treatment for early menopause
A novel therapy combining platelet-rich plasma (PRP) with follicle-stimulating hormone that is injected directly into the ovaries has the potential to restore ovarian function for women who experience early menopause, possibly allowing for pregnancy without the need for donor eggs.
“The resumption of ovarian function in our participants means women with early menopause could have the opportunity to pursue pregnancy through IVF [in vitro fertilization] using their own eggs,” the authors of the groundbreaking pilot study report.
In the small study, published online March 29 in Menopause, menstruation resumed within a mean of about 5 weeks for 11 of 12 patients with early menopause who were treated with the technique. One patient achieved a clinical pregnancy.
In commenting on the study, Stephanie S. Faubion, MD, medical director of the North American Menopause Society, was cautious in her interpretation, noting the need for more research in larger samples.
“Any pregnancy that results from a regenerative therapy is novel,” she told this news organization. “Still, we are a long way away from this being a standard therapy for women with premature ovarian insufficiency.”
Pilot study: Platelet-rich plasma combination with FSH
Early menopause is the cessation of ovarian function at or before the age of 45 years. It is estimated that 12.2% of women experience early menopause. For these women, currently, the only chance of becoming pregnant is with donor eggs.
PRP, an autologous plasma preparation containing more than 10 times the concentration of growth factors and active metabolites than normal plasma, has recently been shown to have the potential to restore the menstrual cycles in perimenopausal women, allowing IVF. It has also been shown to benefit women with premature ovarian insufficiency (POI). However, there have been few reports of pregnancies or live births.
Chao Chin Hsu, MD, PhD, of the National Taiwan University Hospital, Taipei, and colleagues investigated whether the combination of the activated PRP treatment with gondatrophins such as FSH could provide a more robust effect so as to sufficiently stimulate follicles. They used the intraovarian injection of the combination to treat a 38-year-old woman with POI.
The effort was successful, and the woman gave birth to healthy twins.
To further evaluate the approach, the authors conducted a pilot study involving 12 women with early menopause (mean age, 44.4 years) between November 2018 and November 2019.
The women received intraovarian injection with PRP prepared from 40 mL of autologous peripheral blood combined with recombinant FSH.
Following the treatment, 11 of the 12 women experienced resumption of menstruation within a mean of 37 days. For seven patients, menstruation resumed within a month; for three, it resumed within about 2 months; and for one, it resumed after approximately 3 months.
Of note, the menstrual cycles were mostly irregular, with an interval of about 45.6 days.
The women’s average serum FSH level dropped significantly from 70.5 IU/L at baseline to 26.2 IU/L within days of treatment, as did the average luteinizing hormone level (34.8 before and 14.3 IU/L after treatment), indicative of improved ovary function.
For six participants, 10 oocyte retrieval procedures were performed after a mean of about 2 months. Thirteen mature eggs were retrieved, and fertilization via intracytoplasmic sperm injection was attempted, resulting in 10 fertilized oocytes.
Cleavage-stage embryos were transferred into two of the participants. One achieved a clinical pregnancy, defined as a pregnancy that was confirmed by ultrasound and by the presence of a fetal heartbeat. The pregnancy ended in miscarriage at 7 weeks’ gestation.
The length of controlled ovarian stimulation necessary for follicle growth ranged from 8 to 14 days, which the authors note is similar to that seen with women of normal reproductive age.
“Although the use of PRP in reproductive medicine is considered experimental, we demonstrated the restoration of ovarian function in early menopausal women who adopted whole dimension subcortical ovarian injection of PRP/gonadotropin,” the authors write.
“Most remarkably, an early menopausal woman achieved pregnancy after the treatment followed by IVF with her mature ovulating follicle,” they report.
Mechanisms, caveats
The mechanisms thought to underlie the success of the approach include increases in ovarian vascularization and stromal cell proliferation and reductions in oxidative stress and cell death in ovaries, the authors explain.
Key caveats with the treatment include the fact that anesthesia and laparoscopy are required, and precise administration is required at 15 injection sites in 1-2 mm of the ovarian subcortical area, which can be difficult to achieve, Dr. Hsu said in an interview.
“If a new instrument could be developed in which physicians can carry out this treatment through a vaginal approach, like the transvaginal retrieval of eggs in IVF treatments,” the approach could become more acceptable, Dr. Hsu added.
The authors call for studies with larger sample sizes and say it will also be interesting to determine effects in different groups: For example, women with cancer who have undergone chemotherapy.
Dr. Faubion, who is director of the Mayo Clinic Women’s Health, Rochester, Minn., says the causes of early menopause could be important in determining the treatment’s efficacy.
“[The therapy’s] success may depend on the reason the woman experienced early menopause: For instance, due to chemotherapy, radiation, virus, autoimmune disease, genetic mutation, or other cause,” she said.
She also noted that cost could be an important factor.
“I don’t see a cost estimate, but it will be substantial,” she said. “So, even if the success rate improves as this technique is further studied, cost and the invasive nature of the treatment may prove to be substantial barriers to this therapy becoming mainstream,” she said.
The authors and Dr. Faubion have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A novel therapy combining platelet-rich plasma (PRP) with follicle-stimulating hormone that is injected directly into the ovaries has the potential to restore ovarian function for women who experience early menopause, possibly allowing for pregnancy without the need for donor eggs.
“The resumption of ovarian function in our participants means women with early menopause could have the opportunity to pursue pregnancy through IVF [in vitro fertilization] using their own eggs,” the authors of the groundbreaking pilot study report.
In the small study, published online March 29 in Menopause, menstruation resumed within a mean of about 5 weeks for 11 of 12 patients with early menopause who were treated with the technique. One patient achieved a clinical pregnancy.
In commenting on the study, Stephanie S. Faubion, MD, medical director of the North American Menopause Society, was cautious in her interpretation, noting the need for more research in larger samples.
“Any pregnancy that results from a regenerative therapy is novel,” she told this news organization. “Still, we are a long way away from this being a standard therapy for women with premature ovarian insufficiency.”
Pilot study: Platelet-rich plasma combination with FSH
Early menopause is the cessation of ovarian function at or before the age of 45 years. It is estimated that 12.2% of women experience early menopause. For these women, currently, the only chance of becoming pregnant is with donor eggs.
PRP, an autologous plasma preparation containing more than 10 times the concentration of growth factors and active metabolites than normal plasma, has recently been shown to have the potential to restore the menstrual cycles in perimenopausal women, allowing IVF. It has also been shown to benefit women with premature ovarian insufficiency (POI). However, there have been few reports of pregnancies or live births.
Chao Chin Hsu, MD, PhD, of the National Taiwan University Hospital, Taipei, and colleagues investigated whether the combination of the activated PRP treatment with gondatrophins such as FSH could provide a more robust effect so as to sufficiently stimulate follicles. They used the intraovarian injection of the combination to treat a 38-year-old woman with POI.
The effort was successful, and the woman gave birth to healthy twins.
To further evaluate the approach, the authors conducted a pilot study involving 12 women with early menopause (mean age, 44.4 years) between November 2018 and November 2019.
The women received intraovarian injection with PRP prepared from 40 mL of autologous peripheral blood combined with recombinant FSH.
Following the treatment, 11 of the 12 women experienced resumption of menstruation within a mean of 37 days. For seven patients, menstruation resumed within a month; for three, it resumed within about 2 months; and for one, it resumed after approximately 3 months.
Of note, the menstrual cycles were mostly irregular, with an interval of about 45.6 days.
The women’s average serum FSH level dropped significantly from 70.5 IU/L at baseline to 26.2 IU/L within days of treatment, as did the average luteinizing hormone level (34.8 before and 14.3 IU/L after treatment), indicative of improved ovary function.
For six participants, 10 oocyte retrieval procedures were performed after a mean of about 2 months. Thirteen mature eggs were retrieved, and fertilization via intracytoplasmic sperm injection was attempted, resulting in 10 fertilized oocytes.
Cleavage-stage embryos were transferred into two of the participants. One achieved a clinical pregnancy, defined as a pregnancy that was confirmed by ultrasound and by the presence of a fetal heartbeat. The pregnancy ended in miscarriage at 7 weeks’ gestation.
The length of controlled ovarian stimulation necessary for follicle growth ranged from 8 to 14 days, which the authors note is similar to that seen with women of normal reproductive age.
“Although the use of PRP in reproductive medicine is considered experimental, we demonstrated the restoration of ovarian function in early menopausal women who adopted whole dimension subcortical ovarian injection of PRP/gonadotropin,” the authors write.
“Most remarkably, an early menopausal woman achieved pregnancy after the treatment followed by IVF with her mature ovulating follicle,” they report.
Mechanisms, caveats
The mechanisms thought to underlie the success of the approach include increases in ovarian vascularization and stromal cell proliferation and reductions in oxidative stress and cell death in ovaries, the authors explain.
Key caveats with the treatment include the fact that anesthesia and laparoscopy are required, and precise administration is required at 15 injection sites in 1-2 mm of the ovarian subcortical area, which can be difficult to achieve, Dr. Hsu said in an interview.
“If a new instrument could be developed in which physicians can carry out this treatment through a vaginal approach, like the transvaginal retrieval of eggs in IVF treatments,” the approach could become more acceptable, Dr. Hsu added.
The authors call for studies with larger sample sizes and say it will also be interesting to determine effects in different groups: For example, women with cancer who have undergone chemotherapy.
Dr. Faubion, who is director of the Mayo Clinic Women’s Health, Rochester, Minn., says the causes of early menopause could be important in determining the treatment’s efficacy.
“[The therapy’s] success may depend on the reason the woman experienced early menopause: For instance, due to chemotherapy, radiation, virus, autoimmune disease, genetic mutation, or other cause,” she said.
She also noted that cost could be an important factor.
“I don’t see a cost estimate, but it will be substantial,” she said. “So, even if the success rate improves as this technique is further studied, cost and the invasive nature of the treatment may prove to be substantial barriers to this therapy becoming mainstream,” she said.
The authors and Dr. Faubion have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A novel therapy combining platelet-rich plasma (PRP) with follicle-stimulating hormone that is injected directly into the ovaries has the potential to restore ovarian function for women who experience early menopause, possibly allowing for pregnancy without the need for donor eggs.
“The resumption of ovarian function in our participants means women with early menopause could have the opportunity to pursue pregnancy through IVF [in vitro fertilization] using their own eggs,” the authors of the groundbreaking pilot study report.
In the small study, published online March 29 in Menopause, menstruation resumed within a mean of about 5 weeks for 11 of 12 patients with early menopause who were treated with the technique. One patient achieved a clinical pregnancy.
In commenting on the study, Stephanie S. Faubion, MD, medical director of the North American Menopause Society, was cautious in her interpretation, noting the need for more research in larger samples.
“Any pregnancy that results from a regenerative therapy is novel,” she told this news organization. “Still, we are a long way away from this being a standard therapy for women with premature ovarian insufficiency.”
Pilot study: Platelet-rich plasma combination with FSH
Early menopause is the cessation of ovarian function at or before the age of 45 years. It is estimated that 12.2% of women experience early menopause. For these women, currently, the only chance of becoming pregnant is with donor eggs.
PRP, an autologous plasma preparation containing more than 10 times the concentration of growth factors and active metabolites than normal plasma, has recently been shown to have the potential to restore the menstrual cycles in perimenopausal women, allowing IVF. It has also been shown to benefit women with premature ovarian insufficiency (POI). However, there have been few reports of pregnancies or live births.
Chao Chin Hsu, MD, PhD, of the National Taiwan University Hospital, Taipei, and colleagues investigated whether the combination of the activated PRP treatment with gondatrophins such as FSH could provide a more robust effect so as to sufficiently stimulate follicles. They used the intraovarian injection of the combination to treat a 38-year-old woman with POI.
The effort was successful, and the woman gave birth to healthy twins.
To further evaluate the approach, the authors conducted a pilot study involving 12 women with early menopause (mean age, 44.4 years) between November 2018 and November 2019.
The women received intraovarian injection with PRP prepared from 40 mL of autologous peripheral blood combined with recombinant FSH.
Following the treatment, 11 of the 12 women experienced resumption of menstruation within a mean of 37 days. For seven patients, menstruation resumed within a month; for three, it resumed within about 2 months; and for one, it resumed after approximately 3 months.
Of note, the menstrual cycles were mostly irregular, with an interval of about 45.6 days.
The women’s average serum FSH level dropped significantly from 70.5 IU/L at baseline to 26.2 IU/L within days of treatment, as did the average luteinizing hormone level (34.8 before and 14.3 IU/L after treatment), indicative of improved ovary function.
For six participants, 10 oocyte retrieval procedures were performed after a mean of about 2 months. Thirteen mature eggs were retrieved, and fertilization via intracytoplasmic sperm injection was attempted, resulting in 10 fertilized oocytes.
Cleavage-stage embryos were transferred into two of the participants. One achieved a clinical pregnancy, defined as a pregnancy that was confirmed by ultrasound and by the presence of a fetal heartbeat. The pregnancy ended in miscarriage at 7 weeks’ gestation.
The length of controlled ovarian stimulation necessary for follicle growth ranged from 8 to 14 days, which the authors note is similar to that seen with women of normal reproductive age.
“Although the use of PRP in reproductive medicine is considered experimental, we demonstrated the restoration of ovarian function in early menopausal women who adopted whole dimension subcortical ovarian injection of PRP/gonadotropin,” the authors write.
“Most remarkably, an early menopausal woman achieved pregnancy after the treatment followed by IVF with her mature ovulating follicle,” they report.
Mechanisms, caveats
The mechanisms thought to underlie the success of the approach include increases in ovarian vascularization and stromal cell proliferation and reductions in oxidative stress and cell death in ovaries, the authors explain.
Key caveats with the treatment include the fact that anesthesia and laparoscopy are required, and precise administration is required at 15 injection sites in 1-2 mm of the ovarian subcortical area, which can be difficult to achieve, Dr. Hsu said in an interview.
“If a new instrument could be developed in which physicians can carry out this treatment through a vaginal approach, like the transvaginal retrieval of eggs in IVF treatments,” the approach could become more acceptable, Dr. Hsu added.
The authors call for studies with larger sample sizes and say it will also be interesting to determine effects in different groups: For example, women with cancer who have undergone chemotherapy.
Dr. Faubion, who is director of the Mayo Clinic Women’s Health, Rochester, Minn., says the causes of early menopause could be important in determining the treatment’s efficacy.
“[The therapy’s] success may depend on the reason the woman experienced early menopause: For instance, due to chemotherapy, radiation, virus, autoimmune disease, genetic mutation, or other cause,” she said.
She also noted that cost could be an important factor.
“I don’t see a cost estimate, but it will be substantial,” she said. “So, even if the success rate improves as this technique is further studied, cost and the invasive nature of the treatment may prove to be substantial barriers to this therapy becoming mainstream,” she said.
The authors and Dr. Faubion have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Can supplementary estrogen relieve MS symptoms in menopausal women?
, a neurologist told colleagues at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
This kind of research should explore the effects of aging, including in the brain, and “focus on what is preventable – this dramatic and abrupt loss of estrogen in women with MS,” said Rhonda Voskuhl, MD, of the Brain Research Institute at the University of California, Los Angeles.
“This is a call to action. There’s a huge gap that needs to be filled,” she added in an interview. “Not enough attention has been paid to menopause and cognitive issues in MS and even in healthy women.”
Research has found that many women with MS experience a decline in function during menopause, she said. “They’re having a worsening of their preexisting disabilities,” she noted, due to neurodegeneration.
Dr. Voskuhl highlighted a 2016 study, for instance, that found postmenopausal women with MS on hormone replacement therapy reported better physical function and quality of life than did their counterparts after adjustment for covariates. She also pointed to a 2019 study that concluded that “natural menopause seems to be a turning point to a more progressive phase of MS.”
Estrogen appears to play a significant role. “It’s involved in synaptic plasticity,” she said. “That’s why the disabilities are worsening.”
Dr. Voskuhl supports a year-long, randomized and controlled study of estrogen supplementation in 150-200 participants. The goal, she said, is “not just to prevent loss and bad things from happening but also make improvements.”
In healthy patients, she said, outcomes should include cognitive decline in menopause, cognitive domain outcomes, and region-specific biomarkers in the frontal cortex and hippocampus instead of global cognition and global brain volume. In patients with MS, she said, the focus should be on worsening of disability with emphasis on specific disabilities such as walking and region-specific biomarkers for the motor cortex and spinal cord.
“We need to be looking at cortical gray matter, which we know is responsive to estrogen,” Dr. Voskuhl said. She led a 2018 placebo-controlled study that found women with MS who took estrogen supplements appeared to experience localized sparing of progressive gray matter, which the researchers linked to improved results in cognitive testing. The findings, the study authors wrote, suggest “a clinically relevant, disability-specific biomarker for clinical trials of candidate neuroprotective treatments in MS.”
What about men? Does hormone loss worsen their MS? Dr. Voskuhl said there seems to be a connection between lower levels of testosterone and more disability in men with MS. But their situation is different. Loss of testosterone in men is gradual and happens over decades instead of over the short period of menopause in women, she said.
Jennifer Graves, MD, a neurologist at the University of California, San Diego, agreed that it’s time for further research into estrogen supplementation in MS. As she noted, “we don’t know the exact biological mechanism that might link perimenopause with developing a more progressive type of MS.”
She added: “An overall decrease in estrogen may be at play but there are other biological changes around menopause. We must also take care in studies to try to separate out what might be due to ovarian aging versus other types of aging processes that might be happening at the same time.”
Dr. Voskuhl disclosed that she is an inventor on university patents for use of estriol and estrogen receptor–beta ligands as treatments. Dr. Graves reports no relevant disclosures.
, a neurologist told colleagues at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
This kind of research should explore the effects of aging, including in the brain, and “focus on what is preventable – this dramatic and abrupt loss of estrogen in women with MS,” said Rhonda Voskuhl, MD, of the Brain Research Institute at the University of California, Los Angeles.
“This is a call to action. There’s a huge gap that needs to be filled,” she added in an interview. “Not enough attention has been paid to menopause and cognitive issues in MS and even in healthy women.”
Research has found that many women with MS experience a decline in function during menopause, she said. “They’re having a worsening of their preexisting disabilities,” she noted, due to neurodegeneration.
Dr. Voskuhl highlighted a 2016 study, for instance, that found postmenopausal women with MS on hormone replacement therapy reported better physical function and quality of life than did their counterparts after adjustment for covariates. She also pointed to a 2019 study that concluded that “natural menopause seems to be a turning point to a more progressive phase of MS.”
Estrogen appears to play a significant role. “It’s involved in synaptic plasticity,” she said. “That’s why the disabilities are worsening.”
Dr. Voskuhl supports a year-long, randomized and controlled study of estrogen supplementation in 150-200 participants. The goal, she said, is “not just to prevent loss and bad things from happening but also make improvements.”
In healthy patients, she said, outcomes should include cognitive decline in menopause, cognitive domain outcomes, and region-specific biomarkers in the frontal cortex and hippocampus instead of global cognition and global brain volume. In patients with MS, she said, the focus should be on worsening of disability with emphasis on specific disabilities such as walking and region-specific biomarkers for the motor cortex and spinal cord.
“We need to be looking at cortical gray matter, which we know is responsive to estrogen,” Dr. Voskuhl said. She led a 2018 placebo-controlled study that found women with MS who took estrogen supplements appeared to experience localized sparing of progressive gray matter, which the researchers linked to improved results in cognitive testing. The findings, the study authors wrote, suggest “a clinically relevant, disability-specific biomarker for clinical trials of candidate neuroprotective treatments in MS.”
What about men? Does hormone loss worsen their MS? Dr. Voskuhl said there seems to be a connection between lower levels of testosterone and more disability in men with MS. But their situation is different. Loss of testosterone in men is gradual and happens over decades instead of over the short period of menopause in women, she said.
Jennifer Graves, MD, a neurologist at the University of California, San Diego, agreed that it’s time for further research into estrogen supplementation in MS. As she noted, “we don’t know the exact biological mechanism that might link perimenopause with developing a more progressive type of MS.”
She added: “An overall decrease in estrogen may be at play but there are other biological changes around menopause. We must also take care in studies to try to separate out what might be due to ovarian aging versus other types of aging processes that might be happening at the same time.”
Dr. Voskuhl disclosed that she is an inventor on university patents for use of estriol and estrogen receptor–beta ligands as treatments. Dr. Graves reports no relevant disclosures.
, a neurologist told colleagues at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
This kind of research should explore the effects of aging, including in the brain, and “focus on what is preventable – this dramatic and abrupt loss of estrogen in women with MS,” said Rhonda Voskuhl, MD, of the Brain Research Institute at the University of California, Los Angeles.
“This is a call to action. There’s a huge gap that needs to be filled,” she added in an interview. “Not enough attention has been paid to menopause and cognitive issues in MS and even in healthy women.”
Research has found that many women with MS experience a decline in function during menopause, she said. “They’re having a worsening of their preexisting disabilities,” she noted, due to neurodegeneration.
Dr. Voskuhl highlighted a 2016 study, for instance, that found postmenopausal women with MS on hormone replacement therapy reported better physical function and quality of life than did their counterparts after adjustment for covariates. She also pointed to a 2019 study that concluded that “natural menopause seems to be a turning point to a more progressive phase of MS.”
Estrogen appears to play a significant role. “It’s involved in synaptic plasticity,” she said. “That’s why the disabilities are worsening.”
Dr. Voskuhl supports a year-long, randomized and controlled study of estrogen supplementation in 150-200 participants. The goal, she said, is “not just to prevent loss and bad things from happening but also make improvements.”
In healthy patients, she said, outcomes should include cognitive decline in menopause, cognitive domain outcomes, and region-specific biomarkers in the frontal cortex and hippocampus instead of global cognition and global brain volume. In patients with MS, she said, the focus should be on worsening of disability with emphasis on specific disabilities such as walking and region-specific biomarkers for the motor cortex and spinal cord.
“We need to be looking at cortical gray matter, which we know is responsive to estrogen,” Dr. Voskuhl said. She led a 2018 placebo-controlled study that found women with MS who took estrogen supplements appeared to experience localized sparing of progressive gray matter, which the researchers linked to improved results in cognitive testing. The findings, the study authors wrote, suggest “a clinically relevant, disability-specific biomarker for clinical trials of candidate neuroprotective treatments in MS.”
What about men? Does hormone loss worsen their MS? Dr. Voskuhl said there seems to be a connection between lower levels of testosterone and more disability in men with MS. But their situation is different. Loss of testosterone in men is gradual and happens over decades instead of over the short period of menopause in women, she said.
Jennifer Graves, MD, a neurologist at the University of California, San Diego, agreed that it’s time for further research into estrogen supplementation in MS. As she noted, “we don’t know the exact biological mechanism that might link perimenopause with developing a more progressive type of MS.”
She added: “An overall decrease in estrogen may be at play but there are other biological changes around menopause. We must also take care in studies to try to separate out what might be due to ovarian aging versus other types of aging processes that might be happening at the same time.”
Dr. Voskuhl disclosed that she is an inventor on university patents for use of estriol and estrogen receptor–beta ligands as treatments. Dr. Graves reports no relevant disclosures.
FROM ACTRIMS FORUM 2021