User login
Benefits of peanut desensitization may not last
based on data from a phase 2 randomized trial of individuals with confirmed peanut allergies.
Previous studies have shown that desensitization to peanuts can be successful, but sustained response to oral immunotherapy after treatment reduction or discontinuation has not been well studied, wrote R. Sharon Chinthrajah, MD, of Stanford (Calif.)University, and colleagues.
“We found that OIT with peanut was able to desensitise people with peanut allergy to 4,000 mg of peanut protein, but that discontinuation of peanut, or even a reduction to 300 mg daily, increased the likelihood of regaining clinical reactivity to peanut,” they wrote. “With peanut allergy therapies in varying stages of clinical development, and some nearing [Food and Drug Administration] approval, vital questions remain regarding the durability of treatment effects and the appropriate maintenance doses.”
In the Peanut Oral Immunotherapy Study: Safety Efficacy and Discovery (POISED), published in The Lancet, the researchers randomized 120 participants to three groups:
• 60 patients built up to a maintenance dose of 4,000 mg of peanut protein for 104 weeks followed by total discontinuation (peanut-0).
• 35 patients built up to a maintenance dose of 4,000 mg of peanut protein for 104 weeks followed by a 300-mg maintenance dose of peanut protein in the form of peanut flour (peanut-300).
• 25 patients to an oat flour placebo.
All participants were trained on how and when to use epinephrine autoinjector devices to treat allergic symptoms such as respiratory problems (cough, shortness of breath, or change in voice), widespread hives or erythema, repetitive vomiting, persistent abdominal pain, angioedema of the face, or feeling faint.
The primary outcome was passing a double-blind, placebo-controlled, food challenge (DBPCFC) to 4,000 mg of peanut protein, which was measured at baseline and at weeks 104, 117, 130, 143, and 156.
Overall, 35% of the peanut-0 group passed the challenge at 104 and 117 weeks, compared with 4% of the placebo group. At week 156 after discontinuing OIT, 13% of the peanut-0 group met the DBPCFC challenge, compared with 4% of the placebo group. However, 37% of participants randomized to a reduced peanut protein dose of 300 mg passed the challenge at 156 weeks, suggesting that more data are needed on optimal maintenance dosing strategies.
Baseline demographics were similar across all groups. The median age at study enrollment was 11 years and the median allergy duration was 9 years. The most common adverse events were mild gastrointestinal and respiratory problems. Adverse events decreased over time in all three groups.
“Higher levels of peanut-specific IgE to total IgE ratio, peanut sIgE, Ara h 1, Ara h 2, and Ara h 1 IgE to peanut-specific IgE ratio at baseline in participants were associated with increased frequencies of adverse events during active peanut OIT,” the researchers noted.
The study findings were limited by several factors including the ability of participants to tolerate 4,000 mg of peanut protein after achieving a maintenance dose but conducting serial testing only for those who passed the challenge. In addition, the results may be limited to peanut and not generalizable to other food allergies, the researchers said.
However, the results suggest that OIT remains a promising treatment for peanut allergies, and the association of biomarkers with clinical outcomes “might help the practitioner in identifying good candidates for OIT and those individuals who warrant increased vigilance against allergic reactions during OIT,” they said.
The National Institutes of Health supported the study. The researchers had no financial conflicts to disclose.
SOURCE: Chinthrajah RS et al. Lancet. 2019 Sep 12. doi: 10.1016/S0140-6736(19)31793-3.
based on data from a phase 2 randomized trial of individuals with confirmed peanut allergies.
Previous studies have shown that desensitization to peanuts can be successful, but sustained response to oral immunotherapy after treatment reduction or discontinuation has not been well studied, wrote R. Sharon Chinthrajah, MD, of Stanford (Calif.)University, and colleagues.
“We found that OIT with peanut was able to desensitise people with peanut allergy to 4,000 mg of peanut protein, but that discontinuation of peanut, or even a reduction to 300 mg daily, increased the likelihood of regaining clinical reactivity to peanut,” they wrote. “With peanut allergy therapies in varying stages of clinical development, and some nearing [Food and Drug Administration] approval, vital questions remain regarding the durability of treatment effects and the appropriate maintenance doses.”
In the Peanut Oral Immunotherapy Study: Safety Efficacy and Discovery (POISED), published in The Lancet, the researchers randomized 120 participants to three groups:
• 60 patients built up to a maintenance dose of 4,000 mg of peanut protein for 104 weeks followed by total discontinuation (peanut-0).
• 35 patients built up to a maintenance dose of 4,000 mg of peanut protein for 104 weeks followed by a 300-mg maintenance dose of peanut protein in the form of peanut flour (peanut-300).
• 25 patients to an oat flour placebo.
All participants were trained on how and when to use epinephrine autoinjector devices to treat allergic symptoms such as respiratory problems (cough, shortness of breath, or change in voice), widespread hives or erythema, repetitive vomiting, persistent abdominal pain, angioedema of the face, or feeling faint.
The primary outcome was passing a double-blind, placebo-controlled, food challenge (DBPCFC) to 4,000 mg of peanut protein, which was measured at baseline and at weeks 104, 117, 130, 143, and 156.
Overall, 35% of the peanut-0 group passed the challenge at 104 and 117 weeks, compared with 4% of the placebo group. At week 156 after discontinuing OIT, 13% of the peanut-0 group met the DBPCFC challenge, compared with 4% of the placebo group. However, 37% of participants randomized to a reduced peanut protein dose of 300 mg passed the challenge at 156 weeks, suggesting that more data are needed on optimal maintenance dosing strategies.
Baseline demographics were similar across all groups. The median age at study enrollment was 11 years and the median allergy duration was 9 years. The most common adverse events were mild gastrointestinal and respiratory problems. Adverse events decreased over time in all three groups.
“Higher levels of peanut-specific IgE to total IgE ratio, peanut sIgE, Ara h 1, Ara h 2, and Ara h 1 IgE to peanut-specific IgE ratio at baseline in participants were associated with increased frequencies of adverse events during active peanut OIT,” the researchers noted.
The study findings were limited by several factors including the ability of participants to tolerate 4,000 mg of peanut protein after achieving a maintenance dose but conducting serial testing only for those who passed the challenge. In addition, the results may be limited to peanut and not generalizable to other food allergies, the researchers said.
However, the results suggest that OIT remains a promising treatment for peanut allergies, and the association of biomarkers with clinical outcomes “might help the practitioner in identifying good candidates for OIT and those individuals who warrant increased vigilance against allergic reactions during OIT,” they said.
The National Institutes of Health supported the study. The researchers had no financial conflicts to disclose.
SOURCE: Chinthrajah RS et al. Lancet. 2019 Sep 12. doi: 10.1016/S0140-6736(19)31793-3.
based on data from a phase 2 randomized trial of individuals with confirmed peanut allergies.
Previous studies have shown that desensitization to peanuts can be successful, but sustained response to oral immunotherapy after treatment reduction or discontinuation has not been well studied, wrote R. Sharon Chinthrajah, MD, of Stanford (Calif.)University, and colleagues.
“We found that OIT with peanut was able to desensitise people with peanut allergy to 4,000 mg of peanut protein, but that discontinuation of peanut, or even a reduction to 300 mg daily, increased the likelihood of regaining clinical reactivity to peanut,” they wrote. “With peanut allergy therapies in varying stages of clinical development, and some nearing [Food and Drug Administration] approval, vital questions remain regarding the durability of treatment effects and the appropriate maintenance doses.”
In the Peanut Oral Immunotherapy Study: Safety Efficacy and Discovery (POISED), published in The Lancet, the researchers randomized 120 participants to three groups:
• 60 patients built up to a maintenance dose of 4,000 mg of peanut protein for 104 weeks followed by total discontinuation (peanut-0).
• 35 patients built up to a maintenance dose of 4,000 mg of peanut protein for 104 weeks followed by a 300-mg maintenance dose of peanut protein in the form of peanut flour (peanut-300).
• 25 patients to an oat flour placebo.
All participants were trained on how and when to use epinephrine autoinjector devices to treat allergic symptoms such as respiratory problems (cough, shortness of breath, or change in voice), widespread hives or erythema, repetitive vomiting, persistent abdominal pain, angioedema of the face, or feeling faint.
The primary outcome was passing a double-blind, placebo-controlled, food challenge (DBPCFC) to 4,000 mg of peanut protein, which was measured at baseline and at weeks 104, 117, 130, 143, and 156.
Overall, 35% of the peanut-0 group passed the challenge at 104 and 117 weeks, compared with 4% of the placebo group. At week 156 after discontinuing OIT, 13% of the peanut-0 group met the DBPCFC challenge, compared with 4% of the placebo group. However, 37% of participants randomized to a reduced peanut protein dose of 300 mg passed the challenge at 156 weeks, suggesting that more data are needed on optimal maintenance dosing strategies.
Baseline demographics were similar across all groups. The median age at study enrollment was 11 years and the median allergy duration was 9 years. The most common adverse events were mild gastrointestinal and respiratory problems. Adverse events decreased over time in all three groups.
“Higher levels of peanut-specific IgE to total IgE ratio, peanut sIgE, Ara h 1, Ara h 2, and Ara h 1 IgE to peanut-specific IgE ratio at baseline in participants were associated with increased frequencies of adverse events during active peanut OIT,” the researchers noted.
The study findings were limited by several factors including the ability of participants to tolerate 4,000 mg of peanut protein after achieving a maintenance dose but conducting serial testing only for those who passed the challenge. In addition, the results may be limited to peanut and not generalizable to other food allergies, the researchers said.
However, the results suggest that OIT remains a promising treatment for peanut allergies, and the association of biomarkers with clinical outcomes “might help the practitioner in identifying good candidates for OIT and those individuals who warrant increased vigilance against allergic reactions during OIT,” they said.
The National Institutes of Health supported the study. The researchers had no financial conflicts to disclose.
SOURCE: Chinthrajah RS et al. Lancet. 2019 Sep 12. doi: 10.1016/S0140-6736(19)31793-3.
FROM THE LANCET
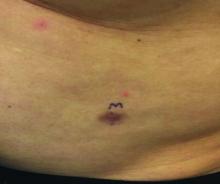
Intense stinging and burning, followed by the development of skin lesions minutes after exposure to a plant
Urticaria from stinging nettle
The stinging nettle (Urtica dioica) is a plant that grows in the United States, Eurasia, Northern Africa, and some parts of South America. It is commonly found in patches along hiking trails and near streams. The leaves are green with a characteristic tapered tip and bear tiny spines or hairs. The spines contain substances such as histamine, serotonin, and acetylcholine. Within seconds of contact with the stinging nettle, sharp stinging and burning will occur. Urticaria and pruritus may appear a few minutes later and may last up to 24 hours. The plant is eaten in some parts of the world and has been used as medicine.
The wood nettle (Laportea canadensis) is a relative of the stinging nettle that often grows in woodlands. Like the stinging nettle, the wood nettle leaves are covered with spines that sting when they come into contact with skin. However, the leaves are shorter and more oval shaped that the stinging nettle, and they lack the tapered tip that is characteristic for the stinging nettle. The reaction from the wood nettle is generally milder than that of the stinging nettle.
Plants can illicit different types of reactions in the skin: urticaria (immunologic and toxin mediated), irritant dermatitis (mechanical and chemical), phototoxic dermatitis (phytophotodermatitis), and allergic contact dermatitis. where anyone coming into contact with the hairs of the plant can be affected. Previous sensitization is not required. The reaction usually occurs immediately after exposure.
The allergic contact dermatitis seen with toxicodendron (poison ivy and poison sumac) appears 48 hours after exposure of a previously sensitized person to the plant. This type of delayed hypersensitivity reaction is known as cell-mediated hypersensitivity. Generally, no reaction is elicited upon the first exposure to the allergen. In fact, it may take years of exposure to allergens for someone to develop an allergic contact dermatitis.
The poison ivy plant can grow anywhere and is characteristically found in “leaves of three.” Skin reactions are often appear as linearly-arranged vesicles a few days after the exposure to the urushiol chemical in the sap of the plant. Poison sumac has red stems with 7-12 green, smooth leaves, and causes a similar skin reaction as poison ivy. It typically grows in wet areas.
Most stings are self-limited. Topical corticosteroid creams may be used if needed.
This case and photo were submitted by Susannah McClain, MD, of Three Rivers Dermatology in Coraopolis, Pa., and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
Urticaria from stinging nettle
The stinging nettle (Urtica dioica) is a plant that grows in the United States, Eurasia, Northern Africa, and some parts of South America. It is commonly found in patches along hiking trails and near streams. The leaves are green with a characteristic tapered tip and bear tiny spines or hairs. The spines contain substances such as histamine, serotonin, and acetylcholine. Within seconds of contact with the stinging nettle, sharp stinging and burning will occur. Urticaria and pruritus may appear a few minutes later and may last up to 24 hours. The plant is eaten in some parts of the world and has been used as medicine.
The wood nettle (Laportea canadensis) is a relative of the stinging nettle that often grows in woodlands. Like the stinging nettle, the wood nettle leaves are covered with spines that sting when they come into contact with skin. However, the leaves are shorter and more oval shaped that the stinging nettle, and they lack the tapered tip that is characteristic for the stinging nettle. The reaction from the wood nettle is generally milder than that of the stinging nettle.
Plants can illicit different types of reactions in the skin: urticaria (immunologic and toxin mediated), irritant dermatitis (mechanical and chemical), phototoxic dermatitis (phytophotodermatitis), and allergic contact dermatitis. where anyone coming into contact with the hairs of the plant can be affected. Previous sensitization is not required. The reaction usually occurs immediately after exposure.
The allergic contact dermatitis seen with toxicodendron (poison ivy and poison sumac) appears 48 hours after exposure of a previously sensitized person to the plant. This type of delayed hypersensitivity reaction is known as cell-mediated hypersensitivity. Generally, no reaction is elicited upon the first exposure to the allergen. In fact, it may take years of exposure to allergens for someone to develop an allergic contact dermatitis.
The poison ivy plant can grow anywhere and is characteristically found in “leaves of three.” Skin reactions are often appear as linearly-arranged vesicles a few days after the exposure to the urushiol chemical in the sap of the plant. Poison sumac has red stems with 7-12 green, smooth leaves, and causes a similar skin reaction as poison ivy. It typically grows in wet areas.
Most stings are self-limited. Topical corticosteroid creams may be used if needed.
This case and photo were submitted by Susannah McClain, MD, of Three Rivers Dermatology in Coraopolis, Pa., and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
Urticaria from stinging nettle
The stinging nettle (Urtica dioica) is a plant that grows in the United States, Eurasia, Northern Africa, and some parts of South America. It is commonly found in patches along hiking trails and near streams. The leaves are green with a characteristic tapered tip and bear tiny spines or hairs. The spines contain substances such as histamine, serotonin, and acetylcholine. Within seconds of contact with the stinging nettle, sharp stinging and burning will occur. Urticaria and pruritus may appear a few minutes later and may last up to 24 hours. The plant is eaten in some parts of the world and has been used as medicine.
The wood nettle (Laportea canadensis) is a relative of the stinging nettle that often grows in woodlands. Like the stinging nettle, the wood nettle leaves are covered with spines that sting when they come into contact with skin. However, the leaves are shorter and more oval shaped that the stinging nettle, and they lack the tapered tip that is characteristic for the stinging nettle. The reaction from the wood nettle is generally milder than that of the stinging nettle.
Plants can illicit different types of reactions in the skin: urticaria (immunologic and toxin mediated), irritant dermatitis (mechanical and chemical), phototoxic dermatitis (phytophotodermatitis), and allergic contact dermatitis. where anyone coming into contact with the hairs of the plant can be affected. Previous sensitization is not required. The reaction usually occurs immediately after exposure.
The allergic contact dermatitis seen with toxicodendron (poison ivy and poison sumac) appears 48 hours after exposure of a previously sensitized person to the plant. This type of delayed hypersensitivity reaction is known as cell-mediated hypersensitivity. Generally, no reaction is elicited upon the first exposure to the allergen. In fact, it may take years of exposure to allergens for someone to develop an allergic contact dermatitis.
The poison ivy plant can grow anywhere and is characteristically found in “leaves of three.” Skin reactions are often appear as linearly-arranged vesicles a few days after the exposure to the urushiol chemical in the sap of the plant. Poison sumac has red stems with 7-12 green, smooth leaves, and causes a similar skin reaction as poison ivy. It typically grows in wet areas.
Most stings are self-limited. Topical corticosteroid creams may be used if needed.
This case and photo were submitted by Susannah McClain, MD, of Three Rivers Dermatology in Coraopolis, Pa., and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
Still no standard for HS, but new options increase chance of control
New york – , but the opportunity for disease control and improvements in quality of life in the former are improving, according to an overview presented at the Skin of Color Update 2019.
Two studies published within the last 18 months that included large proportions of black patients have provided evidence that surgery is effective in those inadequately controlled on medical therapies, Theodore Rosen, MD, professor of dermatology at Baylor College of Medicine, Houston, Texas, said at the meeting.
The best results were observed in a study that evaluated the impact of surgery and adjunctive biologic therapy, according to Dr. Rosen. Of the 68 patients, 59 (72%) were black (Int J Dermatol. 2018;57[1]:62-9). For those patients who received both surgery and biologic therapy, there was a nearly three times greater likelihood of achieving a 75% reduction in the active nodule count (AN75) after adjusting for covariates, compared with those who received only surgery (hazard ratio, 2.88; P = .047).
“It did not appear to matter which came first,” said Dr. Rosen, who is also chief of the dermatology service at Michael E. DeBakey Veterans Affairs Medical Center, Houston. He added, “this is a lot of work, and it is a lot of cost. It requires dedicated people, but it probably is the best thing we possibly do in difficult cases.”
In the other study cited by Dr. Rosen, a review of surgical procedures used to treat HS, the authors concluded that en bloc excision of HS was a better approach than less aggressive surgical approaches, such as drainage, because it lowered the risk of recurrences. This evaluation also included a substantial number of black patients, he said.
It is appropriate to consider black patients separately when discussing HS for a number of reasons. One is the evidence that the disease is more common in this population. Although Dr. Rosen cautioned that prevalence studies do not show this consistently, he believes the preponderance of evidence supports this assertion.
In addition, HS appears to be more severe in black patients. Many of the risk factors that predict a difficult course, such as obesity and diabetes, are more prevalent in the black population, Dr. Rosen said. He also cited a study that concluded HS imposes a larger overall negative impact on quality of life in nonwhite than in white patients (Skin Appendage Disord 2015 Sep;1[2]:65-73).
Despite the evidence that surgery plus biologics is an effective strategy for control of severe disease, there remains no standard approach – even for initial therapy, according to Dr. Rosen. Lifestyle modifications, such as weight loss and smoking cessation, are a starting point, but the order of the next best steps are unclear.
At many centers, biologics have been moved forward in the algorithm on the basis of two placebo-controlled trials that associated adalimumab with higher clinical response rates activity in HS (N Engl J Med. 2016 Aug 4;375[5]:422-34). But Dr. Rosen cautioned that this trial did not include many patients with skin of color, and failure rates are not insignificant.
Promising activity has been reported in HS with both anti–interleukin-17 therapies and apremilast, a phosphodiesterase 4 (PDE4) inhibitor, according to Dr. Rosen but this experience is limited overall and more so in black patients. He listed other drugs associated with benefit in cases studies, such as metformin, which deserve consideration when more conventional options fail, but he reiterated that there is no established ladder of therapies to consider in HS patients regardless of skin type.
Overall, treatment strategies are not different in nonwhite patients relative to white patients, but Dr. Rosen believes that the greater severity of HS in black individuals warrants attention. He noted, for example, that the risk of squamous cell carcinoma, which is elevated in HS patients overall, appears to be even higher in black patients with HS.
Although he did not advocate metformin or any of the other off-label treatments associated with efficacy in case studies, he acknowledged that “this may be where you have to go” when the devastating symptoms of HS remain uncontrolled.
Dr. Rosen reports no relevant financial relationships to disclose.
New york – , but the opportunity for disease control and improvements in quality of life in the former are improving, according to an overview presented at the Skin of Color Update 2019.
Two studies published within the last 18 months that included large proportions of black patients have provided evidence that surgery is effective in those inadequately controlled on medical therapies, Theodore Rosen, MD, professor of dermatology at Baylor College of Medicine, Houston, Texas, said at the meeting.
The best results were observed in a study that evaluated the impact of surgery and adjunctive biologic therapy, according to Dr. Rosen. Of the 68 patients, 59 (72%) were black (Int J Dermatol. 2018;57[1]:62-9). For those patients who received both surgery and biologic therapy, there was a nearly three times greater likelihood of achieving a 75% reduction in the active nodule count (AN75) after adjusting for covariates, compared with those who received only surgery (hazard ratio, 2.88; P = .047).
“It did not appear to matter which came first,” said Dr. Rosen, who is also chief of the dermatology service at Michael E. DeBakey Veterans Affairs Medical Center, Houston. He added, “this is a lot of work, and it is a lot of cost. It requires dedicated people, but it probably is the best thing we possibly do in difficult cases.”
In the other study cited by Dr. Rosen, a review of surgical procedures used to treat HS, the authors concluded that en bloc excision of HS was a better approach than less aggressive surgical approaches, such as drainage, because it lowered the risk of recurrences. This evaluation also included a substantial number of black patients, he said.
It is appropriate to consider black patients separately when discussing HS for a number of reasons. One is the evidence that the disease is more common in this population. Although Dr. Rosen cautioned that prevalence studies do not show this consistently, he believes the preponderance of evidence supports this assertion.
In addition, HS appears to be more severe in black patients. Many of the risk factors that predict a difficult course, such as obesity and diabetes, are more prevalent in the black population, Dr. Rosen said. He also cited a study that concluded HS imposes a larger overall negative impact on quality of life in nonwhite than in white patients (Skin Appendage Disord 2015 Sep;1[2]:65-73).
Despite the evidence that surgery plus biologics is an effective strategy for control of severe disease, there remains no standard approach – even for initial therapy, according to Dr. Rosen. Lifestyle modifications, such as weight loss and smoking cessation, are a starting point, but the order of the next best steps are unclear.
At many centers, biologics have been moved forward in the algorithm on the basis of two placebo-controlled trials that associated adalimumab with higher clinical response rates activity in HS (N Engl J Med. 2016 Aug 4;375[5]:422-34). But Dr. Rosen cautioned that this trial did not include many patients with skin of color, and failure rates are not insignificant.
Promising activity has been reported in HS with both anti–interleukin-17 therapies and apremilast, a phosphodiesterase 4 (PDE4) inhibitor, according to Dr. Rosen but this experience is limited overall and more so in black patients. He listed other drugs associated with benefit in cases studies, such as metformin, which deserve consideration when more conventional options fail, but he reiterated that there is no established ladder of therapies to consider in HS patients regardless of skin type.
Overall, treatment strategies are not different in nonwhite patients relative to white patients, but Dr. Rosen believes that the greater severity of HS in black individuals warrants attention. He noted, for example, that the risk of squamous cell carcinoma, which is elevated in HS patients overall, appears to be even higher in black patients with HS.
Although he did not advocate metformin or any of the other off-label treatments associated with efficacy in case studies, he acknowledged that “this may be where you have to go” when the devastating symptoms of HS remain uncontrolled.
Dr. Rosen reports no relevant financial relationships to disclose.
New york – , but the opportunity for disease control and improvements in quality of life in the former are improving, according to an overview presented at the Skin of Color Update 2019.
Two studies published within the last 18 months that included large proportions of black patients have provided evidence that surgery is effective in those inadequately controlled on medical therapies, Theodore Rosen, MD, professor of dermatology at Baylor College of Medicine, Houston, Texas, said at the meeting.
The best results were observed in a study that evaluated the impact of surgery and adjunctive biologic therapy, according to Dr. Rosen. Of the 68 patients, 59 (72%) were black (Int J Dermatol. 2018;57[1]:62-9). For those patients who received both surgery and biologic therapy, there was a nearly three times greater likelihood of achieving a 75% reduction in the active nodule count (AN75) after adjusting for covariates, compared with those who received only surgery (hazard ratio, 2.88; P = .047).
“It did not appear to matter which came first,” said Dr. Rosen, who is also chief of the dermatology service at Michael E. DeBakey Veterans Affairs Medical Center, Houston. He added, “this is a lot of work, and it is a lot of cost. It requires dedicated people, but it probably is the best thing we possibly do in difficult cases.”
In the other study cited by Dr. Rosen, a review of surgical procedures used to treat HS, the authors concluded that en bloc excision of HS was a better approach than less aggressive surgical approaches, such as drainage, because it lowered the risk of recurrences. This evaluation also included a substantial number of black patients, he said.
It is appropriate to consider black patients separately when discussing HS for a number of reasons. One is the evidence that the disease is more common in this population. Although Dr. Rosen cautioned that prevalence studies do not show this consistently, he believes the preponderance of evidence supports this assertion.
In addition, HS appears to be more severe in black patients. Many of the risk factors that predict a difficult course, such as obesity and diabetes, are more prevalent in the black population, Dr. Rosen said. He also cited a study that concluded HS imposes a larger overall negative impact on quality of life in nonwhite than in white patients (Skin Appendage Disord 2015 Sep;1[2]:65-73).
Despite the evidence that surgery plus biologics is an effective strategy for control of severe disease, there remains no standard approach – even for initial therapy, according to Dr. Rosen. Lifestyle modifications, such as weight loss and smoking cessation, are a starting point, but the order of the next best steps are unclear.
At many centers, biologics have been moved forward in the algorithm on the basis of two placebo-controlled trials that associated adalimumab with higher clinical response rates activity in HS (N Engl J Med. 2016 Aug 4;375[5]:422-34). But Dr. Rosen cautioned that this trial did not include many patients with skin of color, and failure rates are not insignificant.
Promising activity has been reported in HS with both anti–interleukin-17 therapies and apremilast, a phosphodiesterase 4 (PDE4) inhibitor, according to Dr. Rosen but this experience is limited overall and more so in black patients. He listed other drugs associated with benefit in cases studies, such as metformin, which deserve consideration when more conventional options fail, but he reiterated that there is no established ladder of therapies to consider in HS patients regardless of skin type.
Overall, treatment strategies are not different in nonwhite patients relative to white patients, but Dr. Rosen believes that the greater severity of HS in black individuals warrants attention. He noted, for example, that the risk of squamous cell carcinoma, which is elevated in HS patients overall, appears to be even higher in black patients with HS.
Although he did not advocate metformin or any of the other off-label treatments associated with efficacy in case studies, he acknowledged that “this may be where you have to go” when the devastating symptoms of HS remain uncontrolled.
Dr. Rosen reports no relevant financial relationships to disclose.
REPORTING FROM SOC 2019
How to distinguish Stevens-Johnson syndrome from its mimickers
NEW YORK – said Lucia Seminario-Vidal, MD, PhD, speaking at a hospital dermatology–focused session of the summer meeting of the American Academy of Dermatology.
Even before histopathologic results are available, dermatologists can help take some of the panic out of the room when Stevens-Johnson syndrome or toxic epidermal necrolysis (SJS/TEN) is first on the differential diagnosis for the referring physician, she said. Physical findings, a careful review of systems, and past history – especially the introduction of any new medications – can go a long way toward ruling out SJS/TEN among many patients.
In a recent study coauthored by Dr. Seminario-Vidal, of the University of South Florida, Tampa, 208 inpatients with suspected SJS/TEN, 72% were given another diagnosis after a dermatologist consultation (J Am Acad Dermatol. 2019 Sep;81[3]:749-57). The study’s authors, led by Allison Weinkle, MD, a dermatology resident at the University of South Florida, performed a retrospective chart review of 208 patients suspected of having SJS/TEN and received inpatient dermatology consultations.
One surprising finding from the review is that “known risk factors for SJS/TEN such as drug exposure, malignancy, and HIV/AIDS are not helpful to differentiate it from its mimickers,” Dr. Seminario-Vidal said.
However, close attention to the clinical presentation is helpful in differentiating the mimickers from true SJS/TEN. Fever, painful skin, Nikolsky sign, and mucosal involvement were all more common in patients with SJS/TEN. “This was something that was really helpful to discuss with the primary teams at our institutions,” Dr. Seminario-Vidal said.
The most common mimickers in the cases she and her colleagues reviewed were severe cutaneous adverse drug reactions (SCARs) and erythema multiforme; in general, the mimickers were conditions that have severe mucocutaneous involvement.
Patients with SJS/TEN will have fever and pain, typically, and the primary lesions are atypical targets with a positive Nikolsky sign. Hepatic enzyme elevation is seen in 15% of patients, and lymphopenia may be present. Common drugs provoking SJS/TEN include antibiotics, anticonvulsants, NSAIDs, allopurinol, and – importantly – programmed cell death protein 1 (PD-1) inhibitors. Because PD-1s are being used for an ever-expanding range of oncology indications, the rate of SJS/TEN may be expected to rise, Dr. Seminario-Vidal noted.
Other common mimics can include a florid generalized fixed drug eruption, which can also be painful and have atypical targets and a positive Nikolsky sign. Here, large, dusky patches may be present, with perioral, genital, and hand/foot involvement common, but fever is absent. Lesions are sharply localized, round or oval, and they recur at the same site with reexposure to the provoking agent. “Histopathologic studies are diagnostic in these patients, but many times, if you have a good clinical history, you don’t need histopathology,” Dr. Seminario-Vidal said.
As with SJS/TEN, mucosal involvement can be present with fixed drug eruptions. However, hepatic enzymes and the complete blood count will not show abnormalities related to the fixed drug eruption.
Sulfonamides have been associated with up to 75% of fixed drug eruptions reported in some case series, she said, noting that common over-the-counter medications such as nonprescription analgesics, loratadine, and pseudoephedrine can also provoke fixed drug eruptions.
Bullous conditions that can mimic SJS/TEN include linear Immunoglobulin A bullous dermatosis. “We know that the classic presentation is the beautiful ‘string of pearls’ sign, but most patients won’t have this,” Dr. Seminario-Vidal said. The condition often appears in intertriginous locations, and Koebnerization can be a strong clue to this diagnosis, she added.
This painful condition rarely has an associated fever, and will occasionally involve the mucosa, but it doesn’t cause alterations in laboratory values. About 70% of cases of drug-induced linear IgA bullous dermatosis are caused by vancomycin, a consideration when patients may be on several antibiotics. “When I first started, I used to stop everything,” said Dr. Seminario-Vidal. Now, she first discontinues vancomycin and rechecks the patient the next day. In the absence of progression, vancomycin can be presumed to be the culprit, she said.
In bullous pemphigoid, mucosal involvement has been reported in up to 30% of patients, which can initially confuse the diagnosis. “A clue is the presence of tense bullae that initially are very pruritic, but they can become painful over time,” Dr. Seminario-Vidal said. “With tense bullae, with no specific primary lesion, but the patient is very itchy, think bullous pemphigoid.” Pain is sometimes present, but fever is rare. Eosinophilia occurs about 50% of the time, offering an additional diagnostic clue.
Furosemide is a common culprit in drug-induced bullous pemphigoid, as is its cousin bumetanide. Certain analgesics, amoxicillin, ciprofloxacin, and angiotensin converting enzyme inhibitors have also been implicated, said Dr. Seminario-Vidal, though most cases are not drug induced. “Go over the drug history of the patients. It’s something that I cannot emphasize enough … because if you find the drug that causes this disease, you can avoid lengthy systemic treatment.”
Pemphigus foliaceus has a characteristic crusting scale that forms with the rupture of flaccid bullae. Nikolsky’s sign is positive. Mucosal involvement is rare, but is sometimes seen on the occasions when the condition is drug-induced, and laboratory values are usually normal, she said. “The clues to diagnosis of these patients are involvement in the seborrheic areas, including the face; there’s a lot of crust in pemphigus foliaceus.”
Drug-induced pemphigus foliaceus is associated with drugs that have a thiol group, or with sulfur groups that are converted to thiol (–SH) groups. These include both angiotensin converting enzyme inhibitors and angiotensin receptor blockers, penicillins, cephalosporins, and piroxicam. However, said Dr. Seminario-Vidal, penicillamine alone accounts for 50% of drug-induced pemphigus foliaceus.
Dr. Seminario-Vidal reported financial relationships with Eli Lilly, Soligenix, Helsinn, Eisai, Boehringer Ingelheim, Corrona, Akros, Novartis, AbbVie, BMS, Celgene, Glenmark, and Kyowa Kirin.
SOURCE: Seminario-Vidal L. Summer AAD 2019, Session F-025.
NEW YORK – said Lucia Seminario-Vidal, MD, PhD, speaking at a hospital dermatology–focused session of the summer meeting of the American Academy of Dermatology.
Even before histopathologic results are available, dermatologists can help take some of the panic out of the room when Stevens-Johnson syndrome or toxic epidermal necrolysis (SJS/TEN) is first on the differential diagnosis for the referring physician, she said. Physical findings, a careful review of systems, and past history – especially the introduction of any new medications – can go a long way toward ruling out SJS/TEN among many patients.
In a recent study coauthored by Dr. Seminario-Vidal, of the University of South Florida, Tampa, 208 inpatients with suspected SJS/TEN, 72% were given another diagnosis after a dermatologist consultation (J Am Acad Dermatol. 2019 Sep;81[3]:749-57). The study’s authors, led by Allison Weinkle, MD, a dermatology resident at the University of South Florida, performed a retrospective chart review of 208 patients suspected of having SJS/TEN and received inpatient dermatology consultations.
One surprising finding from the review is that “known risk factors for SJS/TEN such as drug exposure, malignancy, and HIV/AIDS are not helpful to differentiate it from its mimickers,” Dr. Seminario-Vidal said.
However, close attention to the clinical presentation is helpful in differentiating the mimickers from true SJS/TEN. Fever, painful skin, Nikolsky sign, and mucosal involvement were all more common in patients with SJS/TEN. “This was something that was really helpful to discuss with the primary teams at our institutions,” Dr. Seminario-Vidal said.
The most common mimickers in the cases she and her colleagues reviewed were severe cutaneous adverse drug reactions (SCARs) and erythema multiforme; in general, the mimickers were conditions that have severe mucocutaneous involvement.
Patients with SJS/TEN will have fever and pain, typically, and the primary lesions are atypical targets with a positive Nikolsky sign. Hepatic enzyme elevation is seen in 15% of patients, and lymphopenia may be present. Common drugs provoking SJS/TEN include antibiotics, anticonvulsants, NSAIDs, allopurinol, and – importantly – programmed cell death protein 1 (PD-1) inhibitors. Because PD-1s are being used for an ever-expanding range of oncology indications, the rate of SJS/TEN may be expected to rise, Dr. Seminario-Vidal noted.
Other common mimics can include a florid generalized fixed drug eruption, which can also be painful and have atypical targets and a positive Nikolsky sign. Here, large, dusky patches may be present, with perioral, genital, and hand/foot involvement common, but fever is absent. Lesions are sharply localized, round or oval, and they recur at the same site with reexposure to the provoking agent. “Histopathologic studies are diagnostic in these patients, but many times, if you have a good clinical history, you don’t need histopathology,” Dr. Seminario-Vidal said.
As with SJS/TEN, mucosal involvement can be present with fixed drug eruptions. However, hepatic enzymes and the complete blood count will not show abnormalities related to the fixed drug eruption.
Sulfonamides have been associated with up to 75% of fixed drug eruptions reported in some case series, she said, noting that common over-the-counter medications such as nonprescription analgesics, loratadine, and pseudoephedrine can also provoke fixed drug eruptions.
Bullous conditions that can mimic SJS/TEN include linear Immunoglobulin A bullous dermatosis. “We know that the classic presentation is the beautiful ‘string of pearls’ sign, but most patients won’t have this,” Dr. Seminario-Vidal said. The condition often appears in intertriginous locations, and Koebnerization can be a strong clue to this diagnosis, she added.
This painful condition rarely has an associated fever, and will occasionally involve the mucosa, but it doesn’t cause alterations in laboratory values. About 70% of cases of drug-induced linear IgA bullous dermatosis are caused by vancomycin, a consideration when patients may be on several antibiotics. “When I first started, I used to stop everything,” said Dr. Seminario-Vidal. Now, she first discontinues vancomycin and rechecks the patient the next day. In the absence of progression, vancomycin can be presumed to be the culprit, she said.
In bullous pemphigoid, mucosal involvement has been reported in up to 30% of patients, which can initially confuse the diagnosis. “A clue is the presence of tense bullae that initially are very pruritic, but they can become painful over time,” Dr. Seminario-Vidal said. “With tense bullae, with no specific primary lesion, but the patient is very itchy, think bullous pemphigoid.” Pain is sometimes present, but fever is rare. Eosinophilia occurs about 50% of the time, offering an additional diagnostic clue.
Furosemide is a common culprit in drug-induced bullous pemphigoid, as is its cousin bumetanide. Certain analgesics, amoxicillin, ciprofloxacin, and angiotensin converting enzyme inhibitors have also been implicated, said Dr. Seminario-Vidal, though most cases are not drug induced. “Go over the drug history of the patients. It’s something that I cannot emphasize enough … because if you find the drug that causes this disease, you can avoid lengthy systemic treatment.”
Pemphigus foliaceus has a characteristic crusting scale that forms with the rupture of flaccid bullae. Nikolsky’s sign is positive. Mucosal involvement is rare, but is sometimes seen on the occasions when the condition is drug-induced, and laboratory values are usually normal, she said. “The clues to diagnosis of these patients are involvement in the seborrheic areas, including the face; there’s a lot of crust in pemphigus foliaceus.”
Drug-induced pemphigus foliaceus is associated with drugs that have a thiol group, or with sulfur groups that are converted to thiol (–SH) groups. These include both angiotensin converting enzyme inhibitors and angiotensin receptor blockers, penicillins, cephalosporins, and piroxicam. However, said Dr. Seminario-Vidal, penicillamine alone accounts for 50% of drug-induced pemphigus foliaceus.
Dr. Seminario-Vidal reported financial relationships with Eli Lilly, Soligenix, Helsinn, Eisai, Boehringer Ingelheim, Corrona, Akros, Novartis, AbbVie, BMS, Celgene, Glenmark, and Kyowa Kirin.
SOURCE: Seminario-Vidal L. Summer AAD 2019, Session F-025.
NEW YORK – said Lucia Seminario-Vidal, MD, PhD, speaking at a hospital dermatology–focused session of the summer meeting of the American Academy of Dermatology.
Even before histopathologic results are available, dermatologists can help take some of the panic out of the room when Stevens-Johnson syndrome or toxic epidermal necrolysis (SJS/TEN) is first on the differential diagnosis for the referring physician, she said. Physical findings, a careful review of systems, and past history – especially the introduction of any new medications – can go a long way toward ruling out SJS/TEN among many patients.
In a recent study coauthored by Dr. Seminario-Vidal, of the University of South Florida, Tampa, 208 inpatients with suspected SJS/TEN, 72% were given another diagnosis after a dermatologist consultation (J Am Acad Dermatol. 2019 Sep;81[3]:749-57). The study’s authors, led by Allison Weinkle, MD, a dermatology resident at the University of South Florida, performed a retrospective chart review of 208 patients suspected of having SJS/TEN and received inpatient dermatology consultations.
One surprising finding from the review is that “known risk factors for SJS/TEN such as drug exposure, malignancy, and HIV/AIDS are not helpful to differentiate it from its mimickers,” Dr. Seminario-Vidal said.
However, close attention to the clinical presentation is helpful in differentiating the mimickers from true SJS/TEN. Fever, painful skin, Nikolsky sign, and mucosal involvement were all more common in patients with SJS/TEN. “This was something that was really helpful to discuss with the primary teams at our institutions,” Dr. Seminario-Vidal said.
The most common mimickers in the cases she and her colleagues reviewed were severe cutaneous adverse drug reactions (SCARs) and erythema multiforme; in general, the mimickers were conditions that have severe mucocutaneous involvement.
Patients with SJS/TEN will have fever and pain, typically, and the primary lesions are atypical targets with a positive Nikolsky sign. Hepatic enzyme elevation is seen in 15% of patients, and lymphopenia may be present. Common drugs provoking SJS/TEN include antibiotics, anticonvulsants, NSAIDs, allopurinol, and – importantly – programmed cell death protein 1 (PD-1) inhibitors. Because PD-1s are being used for an ever-expanding range of oncology indications, the rate of SJS/TEN may be expected to rise, Dr. Seminario-Vidal noted.
Other common mimics can include a florid generalized fixed drug eruption, which can also be painful and have atypical targets and a positive Nikolsky sign. Here, large, dusky patches may be present, with perioral, genital, and hand/foot involvement common, but fever is absent. Lesions are sharply localized, round or oval, and they recur at the same site with reexposure to the provoking agent. “Histopathologic studies are diagnostic in these patients, but many times, if you have a good clinical history, you don’t need histopathology,” Dr. Seminario-Vidal said.
As with SJS/TEN, mucosal involvement can be present with fixed drug eruptions. However, hepatic enzymes and the complete blood count will not show abnormalities related to the fixed drug eruption.
Sulfonamides have been associated with up to 75% of fixed drug eruptions reported in some case series, she said, noting that common over-the-counter medications such as nonprescription analgesics, loratadine, and pseudoephedrine can also provoke fixed drug eruptions.
Bullous conditions that can mimic SJS/TEN include linear Immunoglobulin A bullous dermatosis. “We know that the classic presentation is the beautiful ‘string of pearls’ sign, but most patients won’t have this,” Dr. Seminario-Vidal said. The condition often appears in intertriginous locations, and Koebnerization can be a strong clue to this diagnosis, she added.
This painful condition rarely has an associated fever, and will occasionally involve the mucosa, but it doesn’t cause alterations in laboratory values. About 70% of cases of drug-induced linear IgA bullous dermatosis are caused by vancomycin, a consideration when patients may be on several antibiotics. “When I first started, I used to stop everything,” said Dr. Seminario-Vidal. Now, she first discontinues vancomycin and rechecks the patient the next day. In the absence of progression, vancomycin can be presumed to be the culprit, she said.
In bullous pemphigoid, mucosal involvement has been reported in up to 30% of patients, which can initially confuse the diagnosis. “A clue is the presence of tense bullae that initially are very pruritic, but they can become painful over time,” Dr. Seminario-Vidal said. “With tense bullae, with no specific primary lesion, but the patient is very itchy, think bullous pemphigoid.” Pain is sometimes present, but fever is rare. Eosinophilia occurs about 50% of the time, offering an additional diagnostic clue.
Furosemide is a common culprit in drug-induced bullous pemphigoid, as is its cousin bumetanide. Certain analgesics, amoxicillin, ciprofloxacin, and angiotensin converting enzyme inhibitors have also been implicated, said Dr. Seminario-Vidal, though most cases are not drug induced. “Go over the drug history of the patients. It’s something that I cannot emphasize enough … because if you find the drug that causes this disease, you can avoid lengthy systemic treatment.”
Pemphigus foliaceus has a characteristic crusting scale that forms with the rupture of flaccid bullae. Nikolsky’s sign is positive. Mucosal involvement is rare, but is sometimes seen on the occasions when the condition is drug-induced, and laboratory values are usually normal, she said. “The clues to diagnosis of these patients are involvement in the seborrheic areas, including the face; there’s a lot of crust in pemphigus foliaceus.”
Drug-induced pemphigus foliaceus is associated with drugs that have a thiol group, or with sulfur groups that are converted to thiol (–SH) groups. These include both angiotensin converting enzyme inhibitors and angiotensin receptor blockers, penicillins, cephalosporins, and piroxicam. However, said Dr. Seminario-Vidal, penicillamine alone accounts for 50% of drug-induced pemphigus foliaceus.
Dr. Seminario-Vidal reported financial relationships with Eli Lilly, Soligenix, Helsinn, Eisai, Boehringer Ingelheim, Corrona, Akros, Novartis, AbbVie, BMS, Celgene, Glenmark, and Kyowa Kirin.
SOURCE: Seminario-Vidal L. Summer AAD 2019, Session F-025.
EXPERT ANALYSIS FROM SUMMER AAD 2019
New evidence supports immune system involvement in hidradenitis suppurativa
Immune dysregulation is a substantial feature of hidradenitis suppurativa in African American patients, according to data from 16 adults.
The etiology of hidradenitis suppurativa (HS) remains unknown, but neutrophils are prominent in affected areas of skin, wrote Angel S. Byrd, MD, PhD, of Johns Hopkins University, Baltimore, and colleagues.
“Among the several functions of neutrophils, these cells have the ability to form neutrophil extracellular traps (NETs) after exposure to certain microbes or sterile stimuli,” the researchers noted. These NETs are weblike structures that have been shown to activate several types of immune responses and promote inflammation.
In a study published in Science Translational Medicine, the researchers analyzed biospecimens from African American HS patients. They determined that NET structures were present in the lesional skin of HS patients, but not in control skin. Additionally, serum from HS patients did not properly degrade healthy control NETs in an in vitro examination.
A significant correlation (P less than .0001) occurred between the amount of NETs released in hidradenitis suppurativa lesions and hidradenitis suppurativa severity.
“Together, these results indicate that NET formation is enhanced in HS, both systemically and in lesional skin, in association with disease severity and that NET degradation mechanisms may be impaired in this disease,” the researchers wrote.
The researchers also found that total immunoglobulin G was significantly increased in the sera of HS patients, compared with that of healthy volunteers (P = .0011), and that enzymes involved in skin citrullination were elevated in the skin of HS patients, compared with controls’ skin.
Previous studies have shown that NETs can promote type I interferon (IFN) signatures in the blood of patients with autoimmune and chronic inflammatory conditions, and in this study, several type I IFN–regulated genes had increased expression in HS skin, including IFI44L, MX1, CXCL10, RSAD2, and IFI27.
The study findings were limited by the small sample size and homogenous population, the researchers noted. Future research should include “the role that neutrophil/type I IFN dysregulation plays in the clinical manifestations of the disease, and how these abnormalities in immune responses regulate other innate and adaptive immune cell types in HS,” which could affect the development of new treatments, they wrote.
The study was supported in part by the National Institutes of Health, the Johns Hopkins School of Medicine Physician Scientist Training Program, and the Danby Hidradenitis Suppurativa Foundation. Dr. Byrd disclosed participating in the Johns Hopkins Ethnic Skin Fellowship, which was supported in part by Valeant. Dr. Byrd also disclosed serving as a former investigator for Eli Lilly.
SOURCE: Byrd AS et al. Sci Transl Med. 2019 Sep 4. doi: 10.1126/scitranslmed.aav5908.
Immune dysregulation is a substantial feature of hidradenitis suppurativa in African American patients, according to data from 16 adults.
The etiology of hidradenitis suppurativa (HS) remains unknown, but neutrophils are prominent in affected areas of skin, wrote Angel S. Byrd, MD, PhD, of Johns Hopkins University, Baltimore, and colleagues.
“Among the several functions of neutrophils, these cells have the ability to form neutrophil extracellular traps (NETs) after exposure to certain microbes or sterile stimuli,” the researchers noted. These NETs are weblike structures that have been shown to activate several types of immune responses and promote inflammation.
In a study published in Science Translational Medicine, the researchers analyzed biospecimens from African American HS patients. They determined that NET structures were present in the lesional skin of HS patients, but not in control skin. Additionally, serum from HS patients did not properly degrade healthy control NETs in an in vitro examination.
A significant correlation (P less than .0001) occurred between the amount of NETs released in hidradenitis suppurativa lesions and hidradenitis suppurativa severity.
“Together, these results indicate that NET formation is enhanced in HS, both systemically and in lesional skin, in association with disease severity and that NET degradation mechanisms may be impaired in this disease,” the researchers wrote.
The researchers also found that total immunoglobulin G was significantly increased in the sera of HS patients, compared with that of healthy volunteers (P = .0011), and that enzymes involved in skin citrullination were elevated in the skin of HS patients, compared with controls’ skin.
Previous studies have shown that NETs can promote type I interferon (IFN) signatures in the blood of patients with autoimmune and chronic inflammatory conditions, and in this study, several type I IFN–regulated genes had increased expression in HS skin, including IFI44L, MX1, CXCL10, RSAD2, and IFI27.
The study findings were limited by the small sample size and homogenous population, the researchers noted. Future research should include “the role that neutrophil/type I IFN dysregulation plays in the clinical manifestations of the disease, and how these abnormalities in immune responses regulate other innate and adaptive immune cell types in HS,” which could affect the development of new treatments, they wrote.
The study was supported in part by the National Institutes of Health, the Johns Hopkins School of Medicine Physician Scientist Training Program, and the Danby Hidradenitis Suppurativa Foundation. Dr. Byrd disclosed participating in the Johns Hopkins Ethnic Skin Fellowship, which was supported in part by Valeant. Dr. Byrd also disclosed serving as a former investigator for Eli Lilly.
SOURCE: Byrd AS et al. Sci Transl Med. 2019 Sep 4. doi: 10.1126/scitranslmed.aav5908.
Immune dysregulation is a substantial feature of hidradenitis suppurativa in African American patients, according to data from 16 adults.
The etiology of hidradenitis suppurativa (HS) remains unknown, but neutrophils are prominent in affected areas of skin, wrote Angel S. Byrd, MD, PhD, of Johns Hopkins University, Baltimore, and colleagues.
“Among the several functions of neutrophils, these cells have the ability to form neutrophil extracellular traps (NETs) after exposure to certain microbes or sterile stimuli,” the researchers noted. These NETs are weblike structures that have been shown to activate several types of immune responses and promote inflammation.
In a study published in Science Translational Medicine, the researchers analyzed biospecimens from African American HS patients. They determined that NET structures were present in the lesional skin of HS patients, but not in control skin. Additionally, serum from HS patients did not properly degrade healthy control NETs in an in vitro examination.
A significant correlation (P less than .0001) occurred between the amount of NETs released in hidradenitis suppurativa lesions and hidradenitis suppurativa severity.
“Together, these results indicate that NET formation is enhanced in HS, both systemically and in lesional skin, in association with disease severity and that NET degradation mechanisms may be impaired in this disease,” the researchers wrote.
The researchers also found that total immunoglobulin G was significantly increased in the sera of HS patients, compared with that of healthy volunteers (P = .0011), and that enzymes involved in skin citrullination were elevated in the skin of HS patients, compared with controls’ skin.
Previous studies have shown that NETs can promote type I interferon (IFN) signatures in the blood of patients with autoimmune and chronic inflammatory conditions, and in this study, several type I IFN–regulated genes had increased expression in HS skin, including IFI44L, MX1, CXCL10, RSAD2, and IFI27.
The study findings were limited by the small sample size and homogenous population, the researchers noted. Future research should include “the role that neutrophil/type I IFN dysregulation plays in the clinical manifestations of the disease, and how these abnormalities in immune responses regulate other innate and adaptive immune cell types in HS,” which could affect the development of new treatments, they wrote.
The study was supported in part by the National Institutes of Health, the Johns Hopkins School of Medicine Physician Scientist Training Program, and the Danby Hidradenitis Suppurativa Foundation. Dr. Byrd disclosed participating in the Johns Hopkins Ethnic Skin Fellowship, which was supported in part by Valeant. Dr. Byrd also disclosed serving as a former investigator for Eli Lilly.
SOURCE: Byrd AS et al. Sci Transl Med. 2019 Sep 4. doi: 10.1126/scitranslmed.aav5908.
FROM SCIENCE TRANSLATIONAL MEDICINE
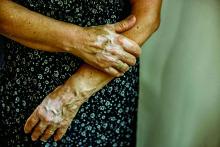
Recent progress in vitiligo treatment might be heading to vitiligo cure
NEW YORK – but also might be leading to a strategy that will prevent the inevitable relapse that occurs after treatment is stopped, according to an update at the American Academy of Dermatology summer meeting.
Recently, trial results with a Janus kinase (JAK) pathway inhibitor have shown promise for treatment of vitiligo, but the ultimate fix for this recurring autoimmune disease might be elimination of resident-memory T cells, according to John Harris, MD, PhD, of the department of dermatology at the University of Massachusetts, Worcester.
In a murine vitiligo model, targeting interleukin-15, a cytokine thought to be essential for maintaining memory T cells, produced rapid and durable repigmentation without apparent adverse effects in a series of studies sufficiently promising that clinical trials are now being actively planned, Dr. Harris said. The ongoing work to eliminate resident-memory T cells to prevent relapse of vitiligo comes at the end of other recent advances that have provided major insights into the pathophysiology of vitiligo.
As outlined by Dr. Harris, vitiligo involves an autoimmune sequence that includes up-regulation of interferon-gamma, activation of the JAK signaling pathway, and mobilization of the cytokine CXCl10, all of which are part of the sequence of events culminating in activation of T cells that attack the melanocyte. The process can be stopped when any of these events are targeted, according to the experimental studies. These findings have already been translated into new drug development.
“There are now three ongoing clinical trials with JAK inhibitors. This is a tremendous advance in a disease for which there have been no clinical trials for decades,” Dr. Harris said. He cited highly positive data with the JAK inhibitor ruxolitinib, which were reported just weeks earlier at the World Congress of Dermatology, to confirm that this principle of intervention is viable.
However, relapse after discontinuation of ruxolitinib, like other treatments for vitiligo, is high. The observation that relapses typically occur in the exact spot where skin lesions occurred previously created the framework of a new potential wave of advances, according to Dr. Harris, director of the Vitiligo Clinic and Research Center at the University of Massachusetts, Worcester.
These advances involve progress in understanding the role of resident-memory T cells in driving autoimmune disease relapse.
In principle, memory-resident T cells are left behind in order to stimulate a rapid immune response in the event of a recurrence of a virus or another pathogen. According to work performed in animal models of vitiligo, they also appear to play a critical role in reactivation of this autoimmune disease, Dr. Harris said.
This role was not surprising, but the potential breakthrough in vitiligo surrounds evidence that the cytokine IL-15 is essential to the creation and maintenance of these memory cells. Evidence suggests vitiligo in animal models does not recur in the absence of IL-15, making it a potential target for treatment.
Initially, there was concern that inhibition of IL-15 would have off-target effects, but this concern has diminished with antibodies designed to inhibit IL-15 signaling in the animal model.
“It turns out that autoreactive cells are much more dependent on the cytokine than other T cells,” he said.
In the animal model, repigmentation has occurred more rapidly with anti-IL-15 therapy than with any other treatment tested to date, but more importantly, these mice then appear to be protected from vitiligo recurrence for extended periods, Dr. Harris noted.
Studies conducted with human tissue have provided strong evidence that the same mechanisms are in play. There are now several approaches to blocking IL-15 signaling, including a monoclonal antibody targeted at the IL-15 receptor, in development. This latter approach is now the focus of a company formed by Dr. Harris.
It is not yet clear if one approach to the inhibition of IL-15 will be superior to another, but Dr. Harris is highly optimistic that this will be a viable approach to control of vitiligo. Noting that good results have been achieved in experimental models by skin injections, thereby avoiding systemic exposure, he is also optimistic that this approach will be well tolerated.
“Based on these data, we are expecting clinical trials soon,” he said.
Dr. Harris reported serving as a consultant and/or investigator for multiple pharmaceutical companies including Aclaris Therapeutics, Celgene, EMD Serono, Genzyme, Incyte, and Janssen Biotech.
NEW YORK – but also might be leading to a strategy that will prevent the inevitable relapse that occurs after treatment is stopped, according to an update at the American Academy of Dermatology summer meeting.
Recently, trial results with a Janus kinase (JAK) pathway inhibitor have shown promise for treatment of vitiligo, but the ultimate fix for this recurring autoimmune disease might be elimination of resident-memory T cells, according to John Harris, MD, PhD, of the department of dermatology at the University of Massachusetts, Worcester.
In a murine vitiligo model, targeting interleukin-15, a cytokine thought to be essential for maintaining memory T cells, produced rapid and durable repigmentation without apparent adverse effects in a series of studies sufficiently promising that clinical trials are now being actively planned, Dr. Harris said. The ongoing work to eliminate resident-memory T cells to prevent relapse of vitiligo comes at the end of other recent advances that have provided major insights into the pathophysiology of vitiligo.
As outlined by Dr. Harris, vitiligo involves an autoimmune sequence that includes up-regulation of interferon-gamma, activation of the JAK signaling pathway, and mobilization of the cytokine CXCl10, all of which are part of the sequence of events culminating in activation of T cells that attack the melanocyte. The process can be stopped when any of these events are targeted, according to the experimental studies. These findings have already been translated into new drug development.
“There are now three ongoing clinical trials with JAK inhibitors. This is a tremendous advance in a disease for which there have been no clinical trials for decades,” Dr. Harris said. He cited highly positive data with the JAK inhibitor ruxolitinib, which were reported just weeks earlier at the World Congress of Dermatology, to confirm that this principle of intervention is viable.
However, relapse after discontinuation of ruxolitinib, like other treatments for vitiligo, is high. The observation that relapses typically occur in the exact spot where skin lesions occurred previously created the framework of a new potential wave of advances, according to Dr. Harris, director of the Vitiligo Clinic and Research Center at the University of Massachusetts, Worcester.
These advances involve progress in understanding the role of resident-memory T cells in driving autoimmune disease relapse.
In principle, memory-resident T cells are left behind in order to stimulate a rapid immune response in the event of a recurrence of a virus or another pathogen. According to work performed in animal models of vitiligo, they also appear to play a critical role in reactivation of this autoimmune disease, Dr. Harris said.
This role was not surprising, but the potential breakthrough in vitiligo surrounds evidence that the cytokine IL-15 is essential to the creation and maintenance of these memory cells. Evidence suggests vitiligo in animal models does not recur in the absence of IL-15, making it a potential target for treatment.
Initially, there was concern that inhibition of IL-15 would have off-target effects, but this concern has diminished with antibodies designed to inhibit IL-15 signaling in the animal model.
“It turns out that autoreactive cells are much more dependent on the cytokine than other T cells,” he said.
In the animal model, repigmentation has occurred more rapidly with anti-IL-15 therapy than with any other treatment tested to date, but more importantly, these mice then appear to be protected from vitiligo recurrence for extended periods, Dr. Harris noted.
Studies conducted with human tissue have provided strong evidence that the same mechanisms are in play. There are now several approaches to blocking IL-15 signaling, including a monoclonal antibody targeted at the IL-15 receptor, in development. This latter approach is now the focus of a company formed by Dr. Harris.
It is not yet clear if one approach to the inhibition of IL-15 will be superior to another, but Dr. Harris is highly optimistic that this will be a viable approach to control of vitiligo. Noting that good results have been achieved in experimental models by skin injections, thereby avoiding systemic exposure, he is also optimistic that this approach will be well tolerated.
“Based on these data, we are expecting clinical trials soon,” he said.
Dr. Harris reported serving as a consultant and/or investigator for multiple pharmaceutical companies including Aclaris Therapeutics, Celgene, EMD Serono, Genzyme, Incyte, and Janssen Biotech.
NEW YORK – but also might be leading to a strategy that will prevent the inevitable relapse that occurs after treatment is stopped, according to an update at the American Academy of Dermatology summer meeting.
Recently, trial results with a Janus kinase (JAK) pathway inhibitor have shown promise for treatment of vitiligo, but the ultimate fix for this recurring autoimmune disease might be elimination of resident-memory T cells, according to John Harris, MD, PhD, of the department of dermatology at the University of Massachusetts, Worcester.
In a murine vitiligo model, targeting interleukin-15, a cytokine thought to be essential for maintaining memory T cells, produced rapid and durable repigmentation without apparent adverse effects in a series of studies sufficiently promising that clinical trials are now being actively planned, Dr. Harris said. The ongoing work to eliminate resident-memory T cells to prevent relapse of vitiligo comes at the end of other recent advances that have provided major insights into the pathophysiology of vitiligo.
As outlined by Dr. Harris, vitiligo involves an autoimmune sequence that includes up-regulation of interferon-gamma, activation of the JAK signaling pathway, and mobilization of the cytokine CXCl10, all of which are part of the sequence of events culminating in activation of T cells that attack the melanocyte. The process can be stopped when any of these events are targeted, according to the experimental studies. These findings have already been translated into new drug development.
“There are now three ongoing clinical trials with JAK inhibitors. This is a tremendous advance in a disease for which there have been no clinical trials for decades,” Dr. Harris said. He cited highly positive data with the JAK inhibitor ruxolitinib, which were reported just weeks earlier at the World Congress of Dermatology, to confirm that this principle of intervention is viable.
However, relapse after discontinuation of ruxolitinib, like other treatments for vitiligo, is high. The observation that relapses typically occur in the exact spot where skin lesions occurred previously created the framework of a new potential wave of advances, according to Dr. Harris, director of the Vitiligo Clinic and Research Center at the University of Massachusetts, Worcester.
These advances involve progress in understanding the role of resident-memory T cells in driving autoimmune disease relapse.
In principle, memory-resident T cells are left behind in order to stimulate a rapid immune response in the event of a recurrence of a virus or another pathogen. According to work performed in animal models of vitiligo, they also appear to play a critical role in reactivation of this autoimmune disease, Dr. Harris said.
This role was not surprising, but the potential breakthrough in vitiligo surrounds evidence that the cytokine IL-15 is essential to the creation and maintenance of these memory cells. Evidence suggests vitiligo in animal models does not recur in the absence of IL-15, making it a potential target for treatment.
Initially, there was concern that inhibition of IL-15 would have off-target effects, but this concern has diminished with antibodies designed to inhibit IL-15 signaling in the animal model.
“It turns out that autoreactive cells are much more dependent on the cytokine than other T cells,” he said.
In the animal model, repigmentation has occurred more rapidly with anti-IL-15 therapy than with any other treatment tested to date, but more importantly, these mice then appear to be protected from vitiligo recurrence for extended periods, Dr. Harris noted.
Studies conducted with human tissue have provided strong evidence that the same mechanisms are in play. There are now several approaches to blocking IL-15 signaling, including a monoclonal antibody targeted at the IL-15 receptor, in development. This latter approach is now the focus of a company formed by Dr. Harris.
It is not yet clear if one approach to the inhibition of IL-15 will be superior to another, but Dr. Harris is highly optimistic that this will be a viable approach to control of vitiligo. Noting that good results have been achieved in experimental models by skin injections, thereby avoiding systemic exposure, he is also optimistic that this approach will be well tolerated.
“Based on these data, we are expecting clinical trials soon,” he said.
Dr. Harris reported serving as a consultant and/or investigator for multiple pharmaceutical companies including Aclaris Therapeutics, Celgene, EMD Serono, Genzyme, Incyte, and Janssen Biotech.
EXPERT ANALYSIS FROM SUMMER AAD 2019
Dermatologists urged to take ownership of preventable AEs
NEW YORK – according to Galen T. Foulke, MD, who discussed avoidable clinical disasters in practice at the American Academy of Dermatology summer meeting.
Of a list of known and predictable risks that “fall under the purview of ‘You Should Have Known Better,’ ” Dr. Foulke focused on glucocorticoid-associated osteoporosis and infections associated with biologics.
Prior to delivering advice about preventing osteoporosis in patients taking glucocorticoids, he polled the audience about what they considered appropriate prophylaxis in this setting. The vast majority opted for vitamin D and calcium supplementation.
“This is a common answer, but it is the wrong answer,” said Dr. Foulke, a dermatologist affiliated with Penn State Milton S. Hershey Medical Center in Hershey, Pennsylvania.
The available data show that patients on vitamin D and calcium supplementation will continue to lose bone density on therapeutic doses of steroids, according to Dr. Foulke. This is true even when the daily doses for these supplements exceed 800 units and 900 mg, respectively. Moreover, calcium supplementation is associated with an increased risk of heart disease in patients without a calcium deficiency, he noted.
The correct answer is a bisphosphonate, said Dr. Foulke. Of the bisphosphonates, he recommended alendronate as one that is particularly well tolerated and readily reimbursed.
He believes that dermatologists prescribing glucocorticoids should not overlook the substantial risk of osteoporosis or their responsibility to discuss strategies for risk mitigation. Moreover, he believes dermatologists should consider prescribing alendronate in the appropriate candidates, not just refer to another specialist.
“In the first year of steroid use, substantial bone loss is a risk even at doses below 5 mg,” Dr. Foulke warned. At higher doses, patients can lose up to 25% of their bone density, he added.
Of preventable risks of biologics, Dr. Foulke focused on infection. In particular, he urged dermatologists who prescribe these drugs to routinely inform patients about the role and safety of vaccines in infection prevention.
“An immunosuppressant suppresses the immune system, increasing the risk of infection. It is our responsibility to protect patients from the known risks of the therapies we offer them,” he said.
Several organizations recommend the pneumococcal vaccine series and the annual influenza vaccine for patients taking biologics. Although Dr. Foulke was unable to find reliable data on vaccination rates among patients prescribed a biologic for a dermatologic indication, he cited rheumatology practice data to suggest that less than half of patients receive this protection.
A major reason for the low rate of vaccination was failure of the biologic prescriber to assume responsibility for this recommended step, according to Dr. Foulke. Although he does not believe that biologic prescribers need to administer the vaccine, and he acknowledged that he does not stock vaccines in his clinic, he does believe they should inform patients when vaccination is safe and appropriate.
Live attenuated vaccines, in his opinion, are not safe. Although he reported that this is an area of controversy, he takes a conservative approach, advising candidates for a live attenuated vaccine to undergo vaccination prior to starting the biologic or during a break from biologic therapy.
When he polled the audience about which vaccines are live attenuated vaccines, several failed to recognize that the MMR vaccine falls into this category. However, he also noted that the majority of vaccines are not live attenuated and should be considered. He specifically singled out the recombinant herpes zoster as a vaccine recommended by the American College of Rheumatology in patients on biologics.
“We do not have to give the shots, but we should be the ones who start the discussion,” said Dr. Foulke, referring to vaccines in patients on a biologic. He called these important steps “to protect patients from preventable disasters.”
Dr. Foulke reported no potential conflicts of interest.
SOURCE: Summer AAD 2019, Session F02.
NEW YORK – according to Galen T. Foulke, MD, who discussed avoidable clinical disasters in practice at the American Academy of Dermatology summer meeting.
Of a list of known and predictable risks that “fall under the purview of ‘You Should Have Known Better,’ ” Dr. Foulke focused on glucocorticoid-associated osteoporosis and infections associated with biologics.
Prior to delivering advice about preventing osteoporosis in patients taking glucocorticoids, he polled the audience about what they considered appropriate prophylaxis in this setting. The vast majority opted for vitamin D and calcium supplementation.
“This is a common answer, but it is the wrong answer,” said Dr. Foulke, a dermatologist affiliated with Penn State Milton S. Hershey Medical Center in Hershey, Pennsylvania.
The available data show that patients on vitamin D and calcium supplementation will continue to lose bone density on therapeutic doses of steroids, according to Dr. Foulke. This is true even when the daily doses for these supplements exceed 800 units and 900 mg, respectively. Moreover, calcium supplementation is associated with an increased risk of heart disease in patients without a calcium deficiency, he noted.
The correct answer is a bisphosphonate, said Dr. Foulke. Of the bisphosphonates, he recommended alendronate as one that is particularly well tolerated and readily reimbursed.
He believes that dermatologists prescribing glucocorticoids should not overlook the substantial risk of osteoporosis or their responsibility to discuss strategies for risk mitigation. Moreover, he believes dermatologists should consider prescribing alendronate in the appropriate candidates, not just refer to another specialist.
“In the first year of steroid use, substantial bone loss is a risk even at doses below 5 mg,” Dr. Foulke warned. At higher doses, patients can lose up to 25% of their bone density, he added.
Of preventable risks of biologics, Dr. Foulke focused on infection. In particular, he urged dermatologists who prescribe these drugs to routinely inform patients about the role and safety of vaccines in infection prevention.
“An immunosuppressant suppresses the immune system, increasing the risk of infection. It is our responsibility to protect patients from the known risks of the therapies we offer them,” he said.
Several organizations recommend the pneumococcal vaccine series and the annual influenza vaccine for patients taking biologics. Although Dr. Foulke was unable to find reliable data on vaccination rates among patients prescribed a biologic for a dermatologic indication, he cited rheumatology practice data to suggest that less than half of patients receive this protection.
A major reason for the low rate of vaccination was failure of the biologic prescriber to assume responsibility for this recommended step, according to Dr. Foulke. Although he does not believe that biologic prescribers need to administer the vaccine, and he acknowledged that he does not stock vaccines in his clinic, he does believe they should inform patients when vaccination is safe and appropriate.
Live attenuated vaccines, in his opinion, are not safe. Although he reported that this is an area of controversy, he takes a conservative approach, advising candidates for a live attenuated vaccine to undergo vaccination prior to starting the biologic or during a break from biologic therapy.
When he polled the audience about which vaccines are live attenuated vaccines, several failed to recognize that the MMR vaccine falls into this category. However, he also noted that the majority of vaccines are not live attenuated and should be considered. He specifically singled out the recombinant herpes zoster as a vaccine recommended by the American College of Rheumatology in patients on biologics.
“We do not have to give the shots, but we should be the ones who start the discussion,” said Dr. Foulke, referring to vaccines in patients on a biologic. He called these important steps “to protect patients from preventable disasters.”
Dr. Foulke reported no potential conflicts of interest.
SOURCE: Summer AAD 2019, Session F02.
NEW YORK – according to Galen T. Foulke, MD, who discussed avoidable clinical disasters in practice at the American Academy of Dermatology summer meeting.
Of a list of known and predictable risks that “fall under the purview of ‘You Should Have Known Better,’ ” Dr. Foulke focused on glucocorticoid-associated osteoporosis and infections associated with biologics.
Prior to delivering advice about preventing osteoporosis in patients taking glucocorticoids, he polled the audience about what they considered appropriate prophylaxis in this setting. The vast majority opted for vitamin D and calcium supplementation.
“This is a common answer, but it is the wrong answer,” said Dr. Foulke, a dermatologist affiliated with Penn State Milton S. Hershey Medical Center in Hershey, Pennsylvania.
The available data show that patients on vitamin D and calcium supplementation will continue to lose bone density on therapeutic doses of steroids, according to Dr. Foulke. This is true even when the daily doses for these supplements exceed 800 units and 900 mg, respectively. Moreover, calcium supplementation is associated with an increased risk of heart disease in patients without a calcium deficiency, he noted.
The correct answer is a bisphosphonate, said Dr. Foulke. Of the bisphosphonates, he recommended alendronate as one that is particularly well tolerated and readily reimbursed.
He believes that dermatologists prescribing glucocorticoids should not overlook the substantial risk of osteoporosis or their responsibility to discuss strategies for risk mitigation. Moreover, he believes dermatologists should consider prescribing alendronate in the appropriate candidates, not just refer to another specialist.
“In the first year of steroid use, substantial bone loss is a risk even at doses below 5 mg,” Dr. Foulke warned. At higher doses, patients can lose up to 25% of their bone density, he added.
Of preventable risks of biologics, Dr. Foulke focused on infection. In particular, he urged dermatologists who prescribe these drugs to routinely inform patients about the role and safety of vaccines in infection prevention.
“An immunosuppressant suppresses the immune system, increasing the risk of infection. It is our responsibility to protect patients from the known risks of the therapies we offer them,” he said.
Several organizations recommend the pneumococcal vaccine series and the annual influenza vaccine for patients taking biologics. Although Dr. Foulke was unable to find reliable data on vaccination rates among patients prescribed a biologic for a dermatologic indication, he cited rheumatology practice data to suggest that less than half of patients receive this protection.
A major reason for the low rate of vaccination was failure of the biologic prescriber to assume responsibility for this recommended step, according to Dr. Foulke. Although he does not believe that biologic prescribers need to administer the vaccine, and he acknowledged that he does not stock vaccines in his clinic, he does believe they should inform patients when vaccination is safe and appropriate.
Live attenuated vaccines, in his opinion, are not safe. Although he reported that this is an area of controversy, he takes a conservative approach, advising candidates for a live attenuated vaccine to undergo vaccination prior to starting the biologic or during a break from biologic therapy.
When he polled the audience about which vaccines are live attenuated vaccines, several failed to recognize that the MMR vaccine falls into this category. However, he also noted that the majority of vaccines are not live attenuated and should be considered. He specifically singled out the recombinant herpes zoster as a vaccine recommended by the American College of Rheumatology in patients on biologics.
“We do not have to give the shots, but we should be the ones who start the discussion,” said Dr. Foulke, referring to vaccines in patients on a biologic. He called these important steps “to protect patients from preventable disasters.”
Dr. Foulke reported no potential conflicts of interest.
SOURCE: Summer AAD 2019, Session F02.
EXPERT ANALYSIS FROM SUMMER AAD 2019
Hidradenitis suppurativa linked to higher NAFLD risk
independent of other metabolic risk factors, a study has found.
The results of the case-control study of 70 individuals with hidradenitis suppurativa (HS) and 150 age- and gender-matched controls were published in the Journal of the European Academy of Dermatology and Venereology. Using hepatic ultrasonography and transient elastography, the investigators found that 51 (72.9%) the participants with HS also had nonalcoholic fatty liver disease (NAFLD), compared with 37 (24.7%) of the controls (P less than .001).
Those with HS and NAFLD were more likely to be obese, have more central adiposity, and meet more of the criteria for metabolic syndrome than those with HS but without NAFLD. They also showed higher serum ALT levels, higher triglycerides, and higher controlled attenuation parameter scores, which is a surrogate marker of liver steatosis.
However, the HS plus NAFLD group had similar rates of active smoking, diabetes, dyslipidemia, hypertension, and cardiovascular events, compared with those who had HS only. They also showed no differences in hemoglobin A1c, serum insulin, insulin resistance, or liver stiffness, compared with the HS-only group.
When researchers compared the participants with HS plus NAFLD with controls with NAFLD, they found the HS group had significantly higher levels of liver stiffness measurement, which is a surrogate marker of liver fibrosis and severity, but there were no differences in the degree of hepatic steatosis.
The individuals with HS plus NAFLD had significantly lower serum albumin, but significantly higher serum gamma–glutamyl transpeptidase and ferritin, compared with controls who had NAFLD. They were also more likely to have metabolic risk factors such as hypertension, dyslipidemia, and metabolic syndrome.
The multivariate analysis also showed that male sex was a protective factor, because the prevalence of obesity was higher in women.
After adjusting for classic cardiovascular and steatosis risk factors, the researchers calculated that HS was a significant and independent risk factor for NAFLD, with an odds ratio of 7.75 (P less than .001). The results provide “the first evidence that patients with HS have a significant high prevalence of NAFLD, which is independent of classic metabolic risk factors and, according to our results, probably not related to the severity of the disease,” wrote Carlos Durán-Vian, MD, from the department of dermatology at the University of Cantabria, Santander, Spain, and coauthors.
“We think that our findings might have potential clinical implications, and physicians involved in the care of patients with HS should be aware of the link between this entity and NAFLD, in order to improve the overall management of these patients,” they wrote, noting that HS is often associated with the same metabolic disorders that can promote fatty liver disease, such as obesity and metabolic syndrome. But the discovery that it is an independent risk factor demands other hypotheses to explain the association between the two conditions.
“In this sense, a possible explanation to deeper understanding the link between HS and NAFLD could be the presence of chronic inflammation due to persistent and abnormal secretion of adipokines (i.e. adiponectin, leptin, resistin) and several proinflammatory cytokines,” the authors wrote, pointing out that NAFLD is also common among people with immune-mediated inflammatory disorders.
The study had the limitation of being an observational, cross-sectional design, and the authors acknowledged that the cohort was relatively small. They also were unable to use liver biopsies to confirm the NAFLD diagnosis.
No funding or conflicts of interest were reported.
SOURCE: Durán-Vian C et al. J Eur Acad Dermatol Venereol. 2019 Jul 1. doi: 10.1111/jdv.15764.
independent of other metabolic risk factors, a study has found.
The results of the case-control study of 70 individuals with hidradenitis suppurativa (HS) and 150 age- and gender-matched controls were published in the Journal of the European Academy of Dermatology and Venereology. Using hepatic ultrasonography and transient elastography, the investigators found that 51 (72.9%) the participants with HS also had nonalcoholic fatty liver disease (NAFLD), compared with 37 (24.7%) of the controls (P less than .001).
Those with HS and NAFLD were more likely to be obese, have more central adiposity, and meet more of the criteria for metabolic syndrome than those with HS but without NAFLD. They also showed higher serum ALT levels, higher triglycerides, and higher controlled attenuation parameter scores, which is a surrogate marker of liver steatosis.
However, the HS plus NAFLD group had similar rates of active smoking, diabetes, dyslipidemia, hypertension, and cardiovascular events, compared with those who had HS only. They also showed no differences in hemoglobin A1c, serum insulin, insulin resistance, or liver stiffness, compared with the HS-only group.
When researchers compared the participants with HS plus NAFLD with controls with NAFLD, they found the HS group had significantly higher levels of liver stiffness measurement, which is a surrogate marker of liver fibrosis and severity, but there were no differences in the degree of hepatic steatosis.
The individuals with HS plus NAFLD had significantly lower serum albumin, but significantly higher serum gamma–glutamyl transpeptidase and ferritin, compared with controls who had NAFLD. They were also more likely to have metabolic risk factors such as hypertension, dyslipidemia, and metabolic syndrome.
The multivariate analysis also showed that male sex was a protective factor, because the prevalence of obesity was higher in women.
After adjusting for classic cardiovascular and steatosis risk factors, the researchers calculated that HS was a significant and independent risk factor for NAFLD, with an odds ratio of 7.75 (P less than .001). The results provide “the first evidence that patients with HS have a significant high prevalence of NAFLD, which is independent of classic metabolic risk factors and, according to our results, probably not related to the severity of the disease,” wrote Carlos Durán-Vian, MD, from the department of dermatology at the University of Cantabria, Santander, Spain, and coauthors.
“We think that our findings might have potential clinical implications, and physicians involved in the care of patients with HS should be aware of the link between this entity and NAFLD, in order to improve the overall management of these patients,” they wrote, noting that HS is often associated with the same metabolic disorders that can promote fatty liver disease, such as obesity and metabolic syndrome. But the discovery that it is an independent risk factor demands other hypotheses to explain the association between the two conditions.
“In this sense, a possible explanation to deeper understanding the link between HS and NAFLD could be the presence of chronic inflammation due to persistent and abnormal secretion of adipokines (i.e. adiponectin, leptin, resistin) and several proinflammatory cytokines,” the authors wrote, pointing out that NAFLD is also common among people with immune-mediated inflammatory disorders.
The study had the limitation of being an observational, cross-sectional design, and the authors acknowledged that the cohort was relatively small. They also were unable to use liver biopsies to confirm the NAFLD diagnosis.
No funding or conflicts of interest were reported.
SOURCE: Durán-Vian C et al. J Eur Acad Dermatol Venereol. 2019 Jul 1. doi: 10.1111/jdv.15764.
independent of other metabolic risk factors, a study has found.
The results of the case-control study of 70 individuals with hidradenitis suppurativa (HS) and 150 age- and gender-matched controls were published in the Journal of the European Academy of Dermatology and Venereology. Using hepatic ultrasonography and transient elastography, the investigators found that 51 (72.9%) the participants with HS also had nonalcoholic fatty liver disease (NAFLD), compared with 37 (24.7%) of the controls (P less than .001).
Those with HS and NAFLD were more likely to be obese, have more central adiposity, and meet more of the criteria for metabolic syndrome than those with HS but without NAFLD. They also showed higher serum ALT levels, higher triglycerides, and higher controlled attenuation parameter scores, which is a surrogate marker of liver steatosis.
However, the HS plus NAFLD group had similar rates of active smoking, diabetes, dyslipidemia, hypertension, and cardiovascular events, compared with those who had HS only. They also showed no differences in hemoglobin A1c, serum insulin, insulin resistance, or liver stiffness, compared with the HS-only group.
When researchers compared the participants with HS plus NAFLD with controls with NAFLD, they found the HS group had significantly higher levels of liver stiffness measurement, which is a surrogate marker of liver fibrosis and severity, but there were no differences in the degree of hepatic steatosis.
The individuals with HS plus NAFLD had significantly lower serum albumin, but significantly higher serum gamma–glutamyl transpeptidase and ferritin, compared with controls who had NAFLD. They were also more likely to have metabolic risk factors such as hypertension, dyslipidemia, and metabolic syndrome.
The multivariate analysis also showed that male sex was a protective factor, because the prevalence of obesity was higher in women.
After adjusting for classic cardiovascular and steatosis risk factors, the researchers calculated that HS was a significant and independent risk factor for NAFLD, with an odds ratio of 7.75 (P less than .001). The results provide “the first evidence that patients with HS have a significant high prevalence of NAFLD, which is independent of classic metabolic risk factors and, according to our results, probably not related to the severity of the disease,” wrote Carlos Durán-Vian, MD, from the department of dermatology at the University of Cantabria, Santander, Spain, and coauthors.
“We think that our findings might have potential clinical implications, and physicians involved in the care of patients with HS should be aware of the link between this entity and NAFLD, in order to improve the overall management of these patients,” they wrote, noting that HS is often associated with the same metabolic disorders that can promote fatty liver disease, such as obesity and metabolic syndrome. But the discovery that it is an independent risk factor demands other hypotheses to explain the association between the two conditions.
“In this sense, a possible explanation to deeper understanding the link between HS and NAFLD could be the presence of chronic inflammation due to persistent and abnormal secretion of adipokines (i.e. adiponectin, leptin, resistin) and several proinflammatory cytokines,” the authors wrote, pointing out that NAFLD is also common among people with immune-mediated inflammatory disorders.
The study had the limitation of being an observational, cross-sectional design, and the authors acknowledged that the cohort was relatively small. They also were unable to use liver biopsies to confirm the NAFLD diagnosis.
No funding or conflicts of interest were reported.
SOURCE: Durán-Vian C et al. J Eur Acad Dermatol Venereol. 2019 Jul 1. doi: 10.1111/jdv.15764.
FROM THE JOURNAL OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
An asymptomatic reddish-brown plaque in a healthy adult man
Chromosomal translocation abnormalities in the tumor cells involving chromosomes 17 and 22 resulting in the fusion gene COL1A1-PDGFB have been reported in DFSP. This translocation causes an overproduction of the protein platelet-derived growth factor, resulting in tumor growth.
Lesions are most common in the trunk and proximal extremities. Less commonly, the head and neck may be involved. Lesions present as painless slow-growing red-brown nodules that may become painful as they enlarge. The differential diagnosis for early DFSP includes large dermatofibroma, keloid, dermatomyofibroma, and morphea. DFSP in childhood tends to appear more atrophic. It may be difficult to diagnose DFSP if the initial biopsy is superficial. If clinical suspicion is high, rebiopsy, ideally into the fat, is recommended.
Histologically, there is a cellular proliferation of thin spindled fibroblasts and collagen in the dermis that extend into the fat, often in a multilayered pattern. Adnexal structures can be obliterated. Fibroblasts may form a cartwheel or storiform pattern. There is mild cytologic atypia. Fibrosarcomatous change may signal increased risk of metastasis. CD34 is often positive and factor XIIIa is negative, unlike in dermatofibroma, which is opposite. Forms of DFSP that can be seen histologically include atrophic DFSP (flat rather than nodular), myxoid DFSP, and pigmented DFSP (also known as Bednar tumor).
DFSP can have irregular shapes with extensions into the fat. Subsequently, DFSP has a high recurrence rate with traditional surgical removal. Mohs surgery is now the treatment of choice. Recurrent tumors should be resected. As metastasis is rare, further work-up is not routinely indicated unless history and physical examination warrant it. Imatinib mesylate (Gleevec), which targets the platelet-derived growth factor receptor, has been tried with patients with inoperable or metastatic DFSP with some success. Radiation may also be used as an adjuvant after surgery. Regular follow-up exams with examination of the surgical site for possible recurrence should be performed every 6-12 months.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
Chromosomal translocation abnormalities in the tumor cells involving chromosomes 17 and 22 resulting in the fusion gene COL1A1-PDGFB have been reported in DFSP. This translocation causes an overproduction of the protein platelet-derived growth factor, resulting in tumor growth.
Lesions are most common in the trunk and proximal extremities. Less commonly, the head and neck may be involved. Lesions present as painless slow-growing red-brown nodules that may become painful as they enlarge. The differential diagnosis for early DFSP includes large dermatofibroma, keloid, dermatomyofibroma, and morphea. DFSP in childhood tends to appear more atrophic. It may be difficult to diagnose DFSP if the initial biopsy is superficial. If clinical suspicion is high, rebiopsy, ideally into the fat, is recommended.
Histologically, there is a cellular proliferation of thin spindled fibroblasts and collagen in the dermis that extend into the fat, often in a multilayered pattern. Adnexal structures can be obliterated. Fibroblasts may form a cartwheel or storiform pattern. There is mild cytologic atypia. Fibrosarcomatous change may signal increased risk of metastasis. CD34 is often positive and factor XIIIa is negative, unlike in dermatofibroma, which is opposite. Forms of DFSP that can be seen histologically include atrophic DFSP (flat rather than nodular), myxoid DFSP, and pigmented DFSP (also known as Bednar tumor).
DFSP can have irregular shapes with extensions into the fat. Subsequently, DFSP has a high recurrence rate with traditional surgical removal. Mohs surgery is now the treatment of choice. Recurrent tumors should be resected. As metastasis is rare, further work-up is not routinely indicated unless history and physical examination warrant it. Imatinib mesylate (Gleevec), which targets the platelet-derived growth factor receptor, has been tried with patients with inoperable or metastatic DFSP with some success. Radiation may also be used as an adjuvant after surgery. Regular follow-up exams with examination of the surgical site for possible recurrence should be performed every 6-12 months.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
Chromosomal translocation abnormalities in the tumor cells involving chromosomes 17 and 22 resulting in the fusion gene COL1A1-PDGFB have been reported in DFSP. This translocation causes an overproduction of the protein platelet-derived growth factor, resulting in tumor growth.
Lesions are most common in the trunk and proximal extremities. Less commonly, the head and neck may be involved. Lesions present as painless slow-growing red-brown nodules that may become painful as they enlarge. The differential diagnosis for early DFSP includes large dermatofibroma, keloid, dermatomyofibroma, and morphea. DFSP in childhood tends to appear more atrophic. It may be difficult to diagnose DFSP if the initial biopsy is superficial. If clinical suspicion is high, rebiopsy, ideally into the fat, is recommended.
Histologically, there is a cellular proliferation of thin spindled fibroblasts and collagen in the dermis that extend into the fat, often in a multilayered pattern. Adnexal structures can be obliterated. Fibroblasts may form a cartwheel or storiform pattern. There is mild cytologic atypia. Fibrosarcomatous change may signal increased risk of metastasis. CD34 is often positive and factor XIIIa is negative, unlike in dermatofibroma, which is opposite. Forms of DFSP that can be seen histologically include atrophic DFSP (flat rather than nodular), myxoid DFSP, and pigmented DFSP (also known as Bednar tumor).
DFSP can have irregular shapes with extensions into the fat. Subsequently, DFSP has a high recurrence rate with traditional surgical removal. Mohs surgery is now the treatment of choice. Recurrent tumors should be resected. As metastasis is rare, further work-up is not routinely indicated unless history and physical examination warrant it. Imatinib mesylate (Gleevec), which targets the platelet-derived growth factor receptor, has been tried with patients with inoperable or metastatic DFSP with some success. Radiation may also be used as an adjuvant after surgery. Regular follow-up exams with examination of the surgical site for possible recurrence should be performed every 6-12 months.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
Clues to eczematous cheilitis may lie in the history
NEW YORK – , but patients may be slow to seek help, Bethanee Schlosser, MD, PhD, said at the American Academy of Dermatology summer meeting.
One of the challenges in helping patients with lip problems is that lips are constantly in motion and constantly interacting with the outside world, said Dr. Schlosser, of the department of dermatology, Northwestern University, Chicago. There’s ongoing low-level trauma with phonation, eating, drinking, and general environmental exposure, she said. Eczematous cheilitis will present with scaling and erythema of the vermilion lips, with lower lip involvement often more pronounced than symptoms on the upper lip. Fissuring and erosion are sometimes, but not always, present as well.
In addition to flaking and redness, Dr. Schlosser noted that patients will complain of dry lips, irritation, itching, and sometimes tingling.
Sorting out the etiology of eczematous cheilitis requires a thorough history. “Ask about habits, such as lip licking, picking, or biting,” she said. Recent dental work, braces, or other appliances for alignment or temporomandibular joint problems can introduce both mechanical irritation and potential allergens, and even musical instruments can be culprits, such as when an oboe reed causes an allergic reaction.
Personal hygiene products, cosmetics, gum chewing, and candy consumption can be the irritant culprits, noted Dr. Schlosser. Careful questioning of patients and examination of the products used can provide clues, since dyes and pigments in cosmetics and gum may provoke reactions.
History taking should also include questions about tobacco in all forms, marijuana, and prescription medication, which can cause lip problems. And it’s important to ask about skin disease in general, to determine if symptoms are present in other anatomic locations, and to ask about any family history of skin disease, she said.
Endogenous contributors can include true atopic dermatitis, psoriasis, and nutritional deficiencies. Psoriatic cheilitis can have prominent crusting and exfoliation. In a Brazilian study that evaluated patents with cutaneous psoriasis and age-, race-, and sex-matched controls with no history of skin disease, psoriasis was associated with geographic tongue, with an odds ratio of 5.0 (95% CI 1.5-16.8). Geographic stomatitis can also be seen, said Dr. Schlosser. Tongue fissures were also more common among those with psoriasis cheilitis (OR 2.7, 95% confidence interval, 1.3-5.6) in the same study (Med Oral Patol Oral Cir Bucal. 2009 Aug 1;14[8]:e371-5).
For psoriatic cheilitis, looking beyond the lips can help refine the diagnosis, she noted. There may be intra-oral signs or signs of extra-oral involvement, especially on the scalp, ears, and genitalia. Koebnerization may be difficult to detect on the lips, but may be present elsewhere. A family history of psoriasis may also tip the scales toward this diagnosis.
Exogenous causes of eczematous cheilitis are much more common and can include contact with irritants and allergens, factitial cheilitis, and cheilitis medicamentosa, Dr Schlosser pointed out.
Allergic contact dermatitis can come from local exposure (to cosmetics and other personal care items, for example) or from incidental exposures. Components of saliva can become concentrated when saliva dries outside the oral cavity, so for chronic lip lickers, saliva alone can be sufficiently irritating to provoke a cheilitis, Dr. Schlosser said.
Transfer of an irritant or allergen is also possible from other body sites, as when a nail-chewer develops allergic cheilitis from an ingredient in nail polish. Transfer from products used on other facial areas and the hair is also possible, as is “connubial transfer,” when an allergen is transferred from an intimate partner.
Cutaneous patch tests can be helpful in pinpointing the offending agent, or agents, according to Dr. Schlosser. She cited a study of 91 patients (77% of whom were female) who underwent patch testing for eczematous cheilitis. The researchers determined that 45% of patients had allergic contact cheilitis (Int J Dermatol. 2016 Jul;55[7]:e386-91).
The patch testing revealed that fragrances, balsam of Peru (Myroxylon pereirae resin), preservatives, and even metals such as nickel and gold were common allergens. The findings echo those in another database review that showed fragrances, M. pereirae, and nickel as the top three allergens on patch testing for lip cheilitis.
Dr. Schlosser said that the most common offending sources are lipsticks, makeup, other cosmetic products, and moisturizer, which are responsible for 10% or more of reactions.
Whatever the etiology, the treatment of eczematous cheilitis can be divided conceptually into two phases. During the induction phase, use of a low- to mid-potency topical corticosteroid ointment quiets inflammation. Examples include alclometasone 0.05%, desonide 0.05%, fluticasone 0.005%, or triamcinolone 0.1%. “Ointment formulations are preferred,” said Dr. Schlosser, since they won’t dissolve so easily with lip licking and will adhere well to the surface of the vermilion lip.
Next, a topical calcineurin inhibitor such as tacrolimus 0.1% can be used for maintenance. Other topical medications, especially topical anesthetics, should be used with caution, she said.
For psoriatic cheilitis, induction with 5% salicylic acid ointment can be followed by the topical calcineurin inhibitor phase, said Dr. Schlosser.
Dr. Schlosser disclosed financial relationships with Beiersdorf, Decision Support in Medicine, and UpToDate.
[email protected]
NEW YORK – , but patients may be slow to seek help, Bethanee Schlosser, MD, PhD, said at the American Academy of Dermatology summer meeting.
One of the challenges in helping patients with lip problems is that lips are constantly in motion and constantly interacting with the outside world, said Dr. Schlosser, of the department of dermatology, Northwestern University, Chicago. There’s ongoing low-level trauma with phonation, eating, drinking, and general environmental exposure, she said. Eczematous cheilitis will present with scaling and erythema of the vermilion lips, with lower lip involvement often more pronounced than symptoms on the upper lip. Fissuring and erosion are sometimes, but not always, present as well.
In addition to flaking and redness, Dr. Schlosser noted that patients will complain of dry lips, irritation, itching, and sometimes tingling.
Sorting out the etiology of eczematous cheilitis requires a thorough history. “Ask about habits, such as lip licking, picking, or biting,” she said. Recent dental work, braces, or other appliances for alignment or temporomandibular joint problems can introduce both mechanical irritation and potential allergens, and even musical instruments can be culprits, such as when an oboe reed causes an allergic reaction.
Personal hygiene products, cosmetics, gum chewing, and candy consumption can be the irritant culprits, noted Dr. Schlosser. Careful questioning of patients and examination of the products used can provide clues, since dyes and pigments in cosmetics and gum may provoke reactions.
History taking should also include questions about tobacco in all forms, marijuana, and prescription medication, which can cause lip problems. And it’s important to ask about skin disease in general, to determine if symptoms are present in other anatomic locations, and to ask about any family history of skin disease, she said.
Endogenous contributors can include true atopic dermatitis, psoriasis, and nutritional deficiencies. Psoriatic cheilitis can have prominent crusting and exfoliation. In a Brazilian study that evaluated patents with cutaneous psoriasis and age-, race-, and sex-matched controls with no history of skin disease, psoriasis was associated with geographic tongue, with an odds ratio of 5.0 (95% CI 1.5-16.8). Geographic stomatitis can also be seen, said Dr. Schlosser. Tongue fissures were also more common among those with psoriasis cheilitis (OR 2.7, 95% confidence interval, 1.3-5.6) in the same study (Med Oral Patol Oral Cir Bucal. 2009 Aug 1;14[8]:e371-5).
For psoriatic cheilitis, looking beyond the lips can help refine the diagnosis, she noted. There may be intra-oral signs or signs of extra-oral involvement, especially on the scalp, ears, and genitalia. Koebnerization may be difficult to detect on the lips, but may be present elsewhere. A family history of psoriasis may also tip the scales toward this diagnosis.
Exogenous causes of eczematous cheilitis are much more common and can include contact with irritants and allergens, factitial cheilitis, and cheilitis medicamentosa, Dr Schlosser pointed out.
Allergic contact dermatitis can come from local exposure (to cosmetics and other personal care items, for example) or from incidental exposures. Components of saliva can become concentrated when saliva dries outside the oral cavity, so for chronic lip lickers, saliva alone can be sufficiently irritating to provoke a cheilitis, Dr. Schlosser said.
Transfer of an irritant or allergen is also possible from other body sites, as when a nail-chewer develops allergic cheilitis from an ingredient in nail polish. Transfer from products used on other facial areas and the hair is also possible, as is “connubial transfer,” when an allergen is transferred from an intimate partner.
Cutaneous patch tests can be helpful in pinpointing the offending agent, or agents, according to Dr. Schlosser. She cited a study of 91 patients (77% of whom were female) who underwent patch testing for eczematous cheilitis. The researchers determined that 45% of patients had allergic contact cheilitis (Int J Dermatol. 2016 Jul;55[7]:e386-91).
The patch testing revealed that fragrances, balsam of Peru (Myroxylon pereirae resin), preservatives, and even metals such as nickel and gold were common allergens. The findings echo those in another database review that showed fragrances, M. pereirae, and nickel as the top three allergens on patch testing for lip cheilitis.
Dr. Schlosser said that the most common offending sources are lipsticks, makeup, other cosmetic products, and moisturizer, which are responsible for 10% or more of reactions.
Whatever the etiology, the treatment of eczematous cheilitis can be divided conceptually into two phases. During the induction phase, use of a low- to mid-potency topical corticosteroid ointment quiets inflammation. Examples include alclometasone 0.05%, desonide 0.05%, fluticasone 0.005%, or triamcinolone 0.1%. “Ointment formulations are preferred,” said Dr. Schlosser, since they won’t dissolve so easily with lip licking and will adhere well to the surface of the vermilion lip.
Next, a topical calcineurin inhibitor such as tacrolimus 0.1% can be used for maintenance. Other topical medications, especially topical anesthetics, should be used with caution, she said.
For psoriatic cheilitis, induction with 5% salicylic acid ointment can be followed by the topical calcineurin inhibitor phase, said Dr. Schlosser.
Dr. Schlosser disclosed financial relationships with Beiersdorf, Decision Support in Medicine, and UpToDate.
[email protected]
NEW YORK – , but patients may be slow to seek help, Bethanee Schlosser, MD, PhD, said at the American Academy of Dermatology summer meeting.
One of the challenges in helping patients with lip problems is that lips are constantly in motion and constantly interacting with the outside world, said Dr. Schlosser, of the department of dermatology, Northwestern University, Chicago. There’s ongoing low-level trauma with phonation, eating, drinking, and general environmental exposure, she said. Eczematous cheilitis will present with scaling and erythema of the vermilion lips, with lower lip involvement often more pronounced than symptoms on the upper lip. Fissuring and erosion are sometimes, but not always, present as well.
In addition to flaking and redness, Dr. Schlosser noted that patients will complain of dry lips, irritation, itching, and sometimes tingling.
Sorting out the etiology of eczematous cheilitis requires a thorough history. “Ask about habits, such as lip licking, picking, or biting,” she said. Recent dental work, braces, or other appliances for alignment or temporomandibular joint problems can introduce both mechanical irritation and potential allergens, and even musical instruments can be culprits, such as when an oboe reed causes an allergic reaction.
Personal hygiene products, cosmetics, gum chewing, and candy consumption can be the irritant culprits, noted Dr. Schlosser. Careful questioning of patients and examination of the products used can provide clues, since dyes and pigments in cosmetics and gum may provoke reactions.
History taking should also include questions about tobacco in all forms, marijuana, and prescription medication, which can cause lip problems. And it’s important to ask about skin disease in general, to determine if symptoms are present in other anatomic locations, and to ask about any family history of skin disease, she said.
Endogenous contributors can include true atopic dermatitis, psoriasis, and nutritional deficiencies. Psoriatic cheilitis can have prominent crusting and exfoliation. In a Brazilian study that evaluated patents with cutaneous psoriasis and age-, race-, and sex-matched controls with no history of skin disease, psoriasis was associated with geographic tongue, with an odds ratio of 5.0 (95% CI 1.5-16.8). Geographic stomatitis can also be seen, said Dr. Schlosser. Tongue fissures were also more common among those with psoriasis cheilitis (OR 2.7, 95% confidence interval, 1.3-5.6) in the same study (Med Oral Patol Oral Cir Bucal. 2009 Aug 1;14[8]:e371-5).
For psoriatic cheilitis, looking beyond the lips can help refine the diagnosis, she noted. There may be intra-oral signs or signs of extra-oral involvement, especially on the scalp, ears, and genitalia. Koebnerization may be difficult to detect on the lips, but may be present elsewhere. A family history of psoriasis may also tip the scales toward this diagnosis.
Exogenous causes of eczematous cheilitis are much more common and can include contact with irritants and allergens, factitial cheilitis, and cheilitis medicamentosa, Dr Schlosser pointed out.
Allergic contact dermatitis can come from local exposure (to cosmetics and other personal care items, for example) or from incidental exposures. Components of saliva can become concentrated when saliva dries outside the oral cavity, so for chronic lip lickers, saliva alone can be sufficiently irritating to provoke a cheilitis, Dr. Schlosser said.
Transfer of an irritant or allergen is also possible from other body sites, as when a nail-chewer develops allergic cheilitis from an ingredient in nail polish. Transfer from products used on other facial areas and the hair is also possible, as is “connubial transfer,” when an allergen is transferred from an intimate partner.
Cutaneous patch tests can be helpful in pinpointing the offending agent, or agents, according to Dr. Schlosser. She cited a study of 91 patients (77% of whom were female) who underwent patch testing for eczematous cheilitis. The researchers determined that 45% of patients had allergic contact cheilitis (Int J Dermatol. 2016 Jul;55[7]:e386-91).
The patch testing revealed that fragrances, balsam of Peru (Myroxylon pereirae resin), preservatives, and even metals such as nickel and gold were common allergens. The findings echo those in another database review that showed fragrances, M. pereirae, and nickel as the top three allergens on patch testing for lip cheilitis.
Dr. Schlosser said that the most common offending sources are lipsticks, makeup, other cosmetic products, and moisturizer, which are responsible for 10% or more of reactions.
Whatever the etiology, the treatment of eczematous cheilitis can be divided conceptually into two phases. During the induction phase, use of a low- to mid-potency topical corticosteroid ointment quiets inflammation. Examples include alclometasone 0.05%, desonide 0.05%, fluticasone 0.005%, or triamcinolone 0.1%. “Ointment formulations are preferred,” said Dr. Schlosser, since they won’t dissolve so easily with lip licking and will adhere well to the surface of the vermilion lip.
Next, a topical calcineurin inhibitor such as tacrolimus 0.1% can be used for maintenance. Other topical medications, especially topical anesthetics, should be used with caution, she said.
For psoriatic cheilitis, induction with 5% salicylic acid ointment can be followed by the topical calcineurin inhibitor phase, said Dr. Schlosser.
Dr. Schlosser disclosed financial relationships with Beiersdorf, Decision Support in Medicine, and UpToDate.
[email protected]
EXPERT ANALYSIS FROM SUMMER AAD 2019