User login
Cavernous gender gap in Medicare payments to cardiologists
Women cardiologists receive dramatically smaller payments from the U.S. Centers for Medicare & Medicaid Services (CMS) than their male counterparts, new research suggests.
An analysis of 2016 claims data revealed male cardiologists received on average 45% more reimbursement than women in the inpatient setting, with the median payment 39% higher ($62,897 vs. $45,288).
In the outpatient setting, men received on average 62% more annual CMS payments, with the median payment 75% higher ($91,053 vs. $51,975; P < .001 for both).
The difference remained significant after the exclusion of the top and bottom 2.5% of earning physicians and cardiology subspecialties, like electrophysiology and interventional cardiology, with high procedural volumes and greater gender imbalances.
“This is one study among others which demonstrates a wage gap between men and women in medicine in cardiology,” lead author Inbar Raber, MD, Beth Israel Deaconess Medical Center, Boston, said in an interview. “I hope by increasing awareness [and] understanding of possible etiologies, it will enable some sustainable solutions, and those include access to additional support staff and equitable models surrounding parental leave and childcare support.”
The study, published online September 8 in JAMA Cardiology, comes on the heels of a recent cross-sectional analysis that put cardiology at the bottom of 13 internal medicine subspecialties with just 21% female faculty representation and one of only three specialties in which women’s median salaries did not reach 90% of men’s.
The new findings build on a 2017 report that showed Medicare payments to women physicians in 2013 were 55% of those to male physicians across all specialties.
“It can be disheartening, especially as an early career woman cardiologist, seeing these differences, but I think the responsibility on all of us is to take these observations and really try to understand more deeply why they exist,” Nosheen Reza, MD, from the University of Pennsylvania, Philadelphia, and coauthor of the cross-sectional analysis, told this news organization.
Several factors could be contributing to the disparity, but “it’s not gender discrimination from Medicare,” Dr. Raber said. “The gap in reimbursement is really driven by the types and the volume of charges submitted.”
Indeed, a direct comparison of the three most common inpatient and outpatient billing codes showed no difference in payments between the sexes.
Men, however, submitted 24% more median inpatient charges to CMS than women (1,190 vs. 959), and 94% more outpatient charges (1,685 vs. 870).
Men also submitted slightly more unique billing codes (median inpatient, 10 vs. 9; median outpatient, 11 vs. 8).
Notably, women made up just 13% of the 17,524 cardiologists who received CMS payments in the inpatient setting in 2016 and 13% of the 16,929 cardiologists who did so in the outpatient setting.
Louisiana had the dubious distinction of having the largest gender gap in mean CMS payments, with male cardiologists earning $145,323 (235%) more than women, whereas women cardiologists in Vermont out-earned men by $31,483 (38%).
Overall, male cardiologists had more years in practice than women cardiologists and cared for slightly older Medicare beneficiaries.
Differences in CMS payments persisted, however, after adjustment for years since graduation, physician subspecialty, number of charges, number of unique billing codes, and patient complexity. The resulting β coefficient was -0.06, which translates into women receiving an average of 94% of the CMS payments received by men.
“The first takeaway, if you were really crass and focused on the bottom line, might be: ‘Hey, let me get a few more male cardiologists because they’re going to bring more into the organization.’ But we shouldn’t do that because, unless you link these data with quality outcomes, they’re an interesting observation and hypothesis-generating,” said Sharonne Hayes, MD, coauthor of the 2017 report and professor of cardiovascular medicine at Mayo Clinic in Rochester, Minn., where she has served as director of diversity and inclusion for a decade.
She noted that there are multiple examples that the style of medicine women practice, on average, may be more effective, may be more outcomes based, and may save lives, as suggested by a recent analysis of hospitalized Medicare beneficiaries.
“The gap was not much different, like within 1% or so, but when you take that over the literally millions of Medicare patients cared for each year by hospitalists, that’s a substantial number of people,” Dr. Hayes said. “So, I think we need to take a step back, and we have to include these observations on studies like this and better understand the compensation gaps.”
She pointed out that the present study lacks data on full-time-equivalent status but that female physicians are more likely to work part-time, thus reducing the volume of claims.
Women might also care for different patient populations. “I practice in a women’s heart clinic and take care of [spontaneous coronary artery dissection] SCAD patients where the average age of SCAD is 42. So, the vast majority of patients I see on a day-to-day basis aren’t going to be Medicare age,” observed Dr. Hayes.
The differences in charges might also reflect the increased obligations in nonreimbursed work that women can have, Dr. Raber said. These can be things like mentoring, teaching roles, and serving on committees, which is a hypothesis supported by a 2021 study that showed women physicians spend more time on these “citizenship tasks” than men.
Finally, there could be organizational barriers that affect women’s clinical volumes, including less access to support from health care personnel. Added support is especially important, though, amid a 100-year pandemic, the women agreed.
“Within the first year of the pandemic, we saw women leaving the workforce in droves across all sectors, including medicine, including academic medicine. And, as the pandemic goes on without any signs of abatement, those threats continue to exist and continue to be amplified,” Dr. Reza said.
The groundswell of support surrounding the importance of diversity, equity, and inclusion initiatives across the board has helped bring attention to the issue, she said. Some institutions, including the National Institutes of Health, are making efforts to extend relief to women with young families, caregivers, or those in academic medicine who, for example, need extensions on grants or bridge funding.
“There’s certainly a lot left to do, but I do think within the last year, there’s been an acceleration of literature that has come out, not only pointing out the disparities, but pointing out that perhaps women physicians do have better outcomes and are better liked by their patients and that losing women in the workforce would be a huge detriment to the field overall,” Dr. Reza said.
Dr. Raber, Dr. Reza, and Dr. Hayes reports no relevant financial relationships. Coauthor conflict of interest disclosures are listed in the paper.
A version of this article first appeared on Medscape.com.
Women cardiologists receive dramatically smaller payments from the U.S. Centers for Medicare & Medicaid Services (CMS) than their male counterparts, new research suggests.
An analysis of 2016 claims data revealed male cardiologists received on average 45% more reimbursement than women in the inpatient setting, with the median payment 39% higher ($62,897 vs. $45,288).
In the outpatient setting, men received on average 62% more annual CMS payments, with the median payment 75% higher ($91,053 vs. $51,975; P < .001 for both).
The difference remained significant after the exclusion of the top and bottom 2.5% of earning physicians and cardiology subspecialties, like electrophysiology and interventional cardiology, with high procedural volumes and greater gender imbalances.
“This is one study among others which demonstrates a wage gap between men and women in medicine in cardiology,” lead author Inbar Raber, MD, Beth Israel Deaconess Medical Center, Boston, said in an interview. “I hope by increasing awareness [and] understanding of possible etiologies, it will enable some sustainable solutions, and those include access to additional support staff and equitable models surrounding parental leave and childcare support.”
The study, published online September 8 in JAMA Cardiology, comes on the heels of a recent cross-sectional analysis that put cardiology at the bottom of 13 internal medicine subspecialties with just 21% female faculty representation and one of only three specialties in which women’s median salaries did not reach 90% of men’s.
The new findings build on a 2017 report that showed Medicare payments to women physicians in 2013 were 55% of those to male physicians across all specialties.
“It can be disheartening, especially as an early career woman cardiologist, seeing these differences, but I think the responsibility on all of us is to take these observations and really try to understand more deeply why they exist,” Nosheen Reza, MD, from the University of Pennsylvania, Philadelphia, and coauthor of the cross-sectional analysis, told this news organization.
Several factors could be contributing to the disparity, but “it’s not gender discrimination from Medicare,” Dr. Raber said. “The gap in reimbursement is really driven by the types and the volume of charges submitted.”
Indeed, a direct comparison of the three most common inpatient and outpatient billing codes showed no difference in payments between the sexes.
Men, however, submitted 24% more median inpatient charges to CMS than women (1,190 vs. 959), and 94% more outpatient charges (1,685 vs. 870).
Men also submitted slightly more unique billing codes (median inpatient, 10 vs. 9; median outpatient, 11 vs. 8).
Notably, women made up just 13% of the 17,524 cardiologists who received CMS payments in the inpatient setting in 2016 and 13% of the 16,929 cardiologists who did so in the outpatient setting.
Louisiana had the dubious distinction of having the largest gender gap in mean CMS payments, with male cardiologists earning $145,323 (235%) more than women, whereas women cardiologists in Vermont out-earned men by $31,483 (38%).
Overall, male cardiologists had more years in practice than women cardiologists and cared for slightly older Medicare beneficiaries.
Differences in CMS payments persisted, however, after adjustment for years since graduation, physician subspecialty, number of charges, number of unique billing codes, and patient complexity. The resulting β coefficient was -0.06, which translates into women receiving an average of 94% of the CMS payments received by men.
“The first takeaway, if you were really crass and focused on the bottom line, might be: ‘Hey, let me get a few more male cardiologists because they’re going to bring more into the organization.’ But we shouldn’t do that because, unless you link these data with quality outcomes, they’re an interesting observation and hypothesis-generating,” said Sharonne Hayes, MD, coauthor of the 2017 report and professor of cardiovascular medicine at Mayo Clinic in Rochester, Minn., where she has served as director of diversity and inclusion for a decade.
She noted that there are multiple examples that the style of medicine women practice, on average, may be more effective, may be more outcomes based, and may save lives, as suggested by a recent analysis of hospitalized Medicare beneficiaries.
“The gap was not much different, like within 1% or so, but when you take that over the literally millions of Medicare patients cared for each year by hospitalists, that’s a substantial number of people,” Dr. Hayes said. “So, I think we need to take a step back, and we have to include these observations on studies like this and better understand the compensation gaps.”
She pointed out that the present study lacks data on full-time-equivalent status but that female physicians are more likely to work part-time, thus reducing the volume of claims.
Women might also care for different patient populations. “I practice in a women’s heart clinic and take care of [spontaneous coronary artery dissection] SCAD patients where the average age of SCAD is 42. So, the vast majority of patients I see on a day-to-day basis aren’t going to be Medicare age,” observed Dr. Hayes.
The differences in charges might also reflect the increased obligations in nonreimbursed work that women can have, Dr. Raber said. These can be things like mentoring, teaching roles, and serving on committees, which is a hypothesis supported by a 2021 study that showed women physicians spend more time on these “citizenship tasks” than men.
Finally, there could be organizational barriers that affect women’s clinical volumes, including less access to support from health care personnel. Added support is especially important, though, amid a 100-year pandemic, the women agreed.
“Within the first year of the pandemic, we saw women leaving the workforce in droves across all sectors, including medicine, including academic medicine. And, as the pandemic goes on without any signs of abatement, those threats continue to exist and continue to be amplified,” Dr. Reza said.
The groundswell of support surrounding the importance of diversity, equity, and inclusion initiatives across the board has helped bring attention to the issue, she said. Some institutions, including the National Institutes of Health, are making efforts to extend relief to women with young families, caregivers, or those in academic medicine who, for example, need extensions on grants or bridge funding.
“There’s certainly a lot left to do, but I do think within the last year, there’s been an acceleration of literature that has come out, not only pointing out the disparities, but pointing out that perhaps women physicians do have better outcomes and are better liked by their patients and that losing women in the workforce would be a huge detriment to the field overall,” Dr. Reza said.
Dr. Raber, Dr. Reza, and Dr. Hayes reports no relevant financial relationships. Coauthor conflict of interest disclosures are listed in the paper.
A version of this article first appeared on Medscape.com.
Women cardiologists receive dramatically smaller payments from the U.S. Centers for Medicare & Medicaid Services (CMS) than their male counterparts, new research suggests.
An analysis of 2016 claims data revealed male cardiologists received on average 45% more reimbursement than women in the inpatient setting, with the median payment 39% higher ($62,897 vs. $45,288).
In the outpatient setting, men received on average 62% more annual CMS payments, with the median payment 75% higher ($91,053 vs. $51,975; P < .001 for both).
The difference remained significant after the exclusion of the top and bottom 2.5% of earning physicians and cardiology subspecialties, like electrophysiology and interventional cardiology, with high procedural volumes and greater gender imbalances.
“This is one study among others which demonstrates a wage gap between men and women in medicine in cardiology,” lead author Inbar Raber, MD, Beth Israel Deaconess Medical Center, Boston, said in an interview. “I hope by increasing awareness [and] understanding of possible etiologies, it will enable some sustainable solutions, and those include access to additional support staff and equitable models surrounding parental leave and childcare support.”
The study, published online September 8 in JAMA Cardiology, comes on the heels of a recent cross-sectional analysis that put cardiology at the bottom of 13 internal medicine subspecialties with just 21% female faculty representation and one of only three specialties in which women’s median salaries did not reach 90% of men’s.
The new findings build on a 2017 report that showed Medicare payments to women physicians in 2013 were 55% of those to male physicians across all specialties.
“It can be disheartening, especially as an early career woman cardiologist, seeing these differences, but I think the responsibility on all of us is to take these observations and really try to understand more deeply why they exist,” Nosheen Reza, MD, from the University of Pennsylvania, Philadelphia, and coauthor of the cross-sectional analysis, told this news organization.
Several factors could be contributing to the disparity, but “it’s not gender discrimination from Medicare,” Dr. Raber said. “The gap in reimbursement is really driven by the types and the volume of charges submitted.”
Indeed, a direct comparison of the three most common inpatient and outpatient billing codes showed no difference in payments between the sexes.
Men, however, submitted 24% more median inpatient charges to CMS than women (1,190 vs. 959), and 94% more outpatient charges (1,685 vs. 870).
Men also submitted slightly more unique billing codes (median inpatient, 10 vs. 9; median outpatient, 11 vs. 8).
Notably, women made up just 13% of the 17,524 cardiologists who received CMS payments in the inpatient setting in 2016 and 13% of the 16,929 cardiologists who did so in the outpatient setting.
Louisiana had the dubious distinction of having the largest gender gap in mean CMS payments, with male cardiologists earning $145,323 (235%) more than women, whereas women cardiologists in Vermont out-earned men by $31,483 (38%).
Overall, male cardiologists had more years in practice than women cardiologists and cared for slightly older Medicare beneficiaries.
Differences in CMS payments persisted, however, after adjustment for years since graduation, physician subspecialty, number of charges, number of unique billing codes, and patient complexity. The resulting β coefficient was -0.06, which translates into women receiving an average of 94% of the CMS payments received by men.
“The first takeaway, if you were really crass and focused on the bottom line, might be: ‘Hey, let me get a few more male cardiologists because they’re going to bring more into the organization.’ But we shouldn’t do that because, unless you link these data with quality outcomes, they’re an interesting observation and hypothesis-generating,” said Sharonne Hayes, MD, coauthor of the 2017 report and professor of cardiovascular medicine at Mayo Clinic in Rochester, Minn., where she has served as director of diversity and inclusion for a decade.
She noted that there are multiple examples that the style of medicine women practice, on average, may be more effective, may be more outcomes based, and may save lives, as suggested by a recent analysis of hospitalized Medicare beneficiaries.
“The gap was not much different, like within 1% or so, but when you take that over the literally millions of Medicare patients cared for each year by hospitalists, that’s a substantial number of people,” Dr. Hayes said. “So, I think we need to take a step back, and we have to include these observations on studies like this and better understand the compensation gaps.”
She pointed out that the present study lacks data on full-time-equivalent status but that female physicians are more likely to work part-time, thus reducing the volume of claims.
Women might also care for different patient populations. “I practice in a women’s heart clinic and take care of [spontaneous coronary artery dissection] SCAD patients where the average age of SCAD is 42. So, the vast majority of patients I see on a day-to-day basis aren’t going to be Medicare age,” observed Dr. Hayes.
The differences in charges might also reflect the increased obligations in nonreimbursed work that women can have, Dr. Raber said. These can be things like mentoring, teaching roles, and serving on committees, which is a hypothesis supported by a 2021 study that showed women physicians spend more time on these “citizenship tasks” than men.
Finally, there could be organizational barriers that affect women’s clinical volumes, including less access to support from health care personnel. Added support is especially important, though, amid a 100-year pandemic, the women agreed.
“Within the first year of the pandemic, we saw women leaving the workforce in droves across all sectors, including medicine, including academic medicine. And, as the pandemic goes on without any signs of abatement, those threats continue to exist and continue to be amplified,” Dr. Reza said.
The groundswell of support surrounding the importance of diversity, equity, and inclusion initiatives across the board has helped bring attention to the issue, she said. Some institutions, including the National Institutes of Health, are making efforts to extend relief to women with young families, caregivers, or those in academic medicine who, for example, need extensions on grants or bridge funding.
“There’s certainly a lot left to do, but I do think within the last year, there’s been an acceleration of literature that has come out, not only pointing out the disparities, but pointing out that perhaps women physicians do have better outcomes and are better liked by their patients and that losing women in the workforce would be a huge detriment to the field overall,” Dr. Reza said.
Dr. Raber, Dr. Reza, and Dr. Hayes reports no relevant financial relationships. Coauthor conflict of interest disclosures are listed in the paper.
A version of this article first appeared on Medscape.com.
Feds slap UPMC, lead cardiothoracic surgeon with fraud lawsuit
Following a 2-year investigation, the U.S. government has filed suit against the University of Pittsburgh Medical Center (UPMC), University of Pittsburgh Physicians (UPP), and James Luketich, MD, for billing related to concurrent surgeries performed by the long-time chair of cardiothoracic surgery.
The lawsuit alleges that UPMC “knowingly allowed” Dr. Luketich to “book and perform three surgeries at the same time, to miss the surgical time outs at the outset of those procedures, to go back-and-forth between operating rooms and even hospital facilities while his surgical patients remain under general anesthesia...”
UPMC, the lawsuit claims, also allowed Dr. Luketich to falsely attest that “he was with his patients throughout the entirety of their surgical procedures or during all ‘key and critical’ portions of those procedures and to unlawfully bill Government Health Benefit Programs for those procedures, all in order to increase surgical volume, maximize UPMC and UPP’s revenue, and/or appease Dr. Luketich.”
These practices violate the statutes and regulations governing the defendants, including those that prohibit “teaching physicians” like Dr. Luketich from performing and billing the U.S. for concurrent surgeries, the Department of Justice said in news release.
The Justice Department contends the defendants “knowingly submitted hundreds of materially false claims for payment” to Medicare, Medicaid, and other government programs over the past 6 years.
“The laws prohibiting ‘concurrent surgeries’ are in place for a reason: To protect patients and ensure they receive appropriate and focused medical care,” Stephen R. Kaufman, Acting U.S. Attorney for the Western District of Pennsylvania, said in the release.
According to the lawsuit, “some of Dr. Luketich’s patients were forced to endure additional surgical procedures and/or extended hospital stays as a result of his unlawful conduct. Numerous patients developed painful pressure ulcers. A few were diagnosed with compartment syndrome. And at least two had to undergo amputations.”
The allegations were originally brought forward under the federal False Claims Act’s whistleblower provisions by Jonathan D’Cunha, MD, PhD, who worked closely with Dr. Luketich from 2012 to 2019 and now chairs the department of cardiothoracic surgery at the Mayo Clinic, Phoenix.
The charges cited in the lawsuit include three counts of violating the False Claims Act, one count of unjust enrichment, and one count of payment by mistake.
The 56-page lawsuit includes numerous case examples and cites an October 2015 Boston Globe Spotlight Team report on the safety of running concurrent operations, which reportedly prompted UPMC to reevaluate its policies and identify physicians or departments in potential violation.
Hospital officials met with Dr. Luketich in March 2016 and devised a “plan” to ensure his availability and “compliance with concurrency rules,” it alleges, but also highlights an email that notes “continued problems” with Dr. Luketich’s schedule.
“UPMC has persistently ignored or minimized complaints by employees and staff regarding Dr. Luketich, his hyper-busy schedule, his refusal to delegate surgeries and surgical tasks” and “protected him from meaningful sanction; refused to curtail his surgical practice; and continued to allow Dr. Luketich to skirt the rules and endanger his patients,” according to the lawsuit.
The suit notes that Dr. Luketich is one of UPMC and UPP’s highest sources of revenue and that UPMC advertises him as a “life-saving pioneer” who routinely performs dramatic, last-ditch procedures on patients who are otherwise hopeless.
In response to an interview request from this news organization, a UPMC spokesperson wrote: “As the government itself concedes in its complaint, many of Dr. Luketich’s surgical patients are elderly, frail, and/or very ill. They include the ‘hopeless’ patients ... who suffer from chronic illness or metastatic cancer, and/or have extensive surgical histories and choose UPMC and Dr. Luketich when other physicians and health care providers have turned them down.”
“Dr. Luketich always performs the most critical portions of every operation he undertakes,” the spokesperson said, adding that no law or regulation prohibits overlapping surgeries or billing for those surgeries, “let alone surgeries conducted by teams of surgeons like those led by Dr. Luketich.”
“The government’s claims are, rather, based on a misapplication or misinterpretation of UPMC’s internal policies and [Centers for Medicare & Medicaid Services] guidance, neither of which can support a claim for fraudulent billing. UPMC and Dr. Luketich plan to vigorously defend against the government’s claims,” the spokesperson concluded.
The claims asserted against the defendants are allegations only; there has been no determination of liability. The government is seeking three times the amount of actual damages suffered as a result of the alleged false claims and/or fraud; a sum of $23,331 (or the maximum penalty, whichever is greater) for each false claim submitted by UPMC, UPP, and/or Dr. Luketich; and costs and expenses associated with the civil suit.
A version of this article first appeared on Medscape.com.
Following a 2-year investigation, the U.S. government has filed suit against the University of Pittsburgh Medical Center (UPMC), University of Pittsburgh Physicians (UPP), and James Luketich, MD, for billing related to concurrent surgeries performed by the long-time chair of cardiothoracic surgery.
The lawsuit alleges that UPMC “knowingly allowed” Dr. Luketich to “book and perform three surgeries at the same time, to miss the surgical time outs at the outset of those procedures, to go back-and-forth between operating rooms and even hospital facilities while his surgical patients remain under general anesthesia...”
UPMC, the lawsuit claims, also allowed Dr. Luketich to falsely attest that “he was with his patients throughout the entirety of their surgical procedures or during all ‘key and critical’ portions of those procedures and to unlawfully bill Government Health Benefit Programs for those procedures, all in order to increase surgical volume, maximize UPMC and UPP’s revenue, and/or appease Dr. Luketich.”
These practices violate the statutes and regulations governing the defendants, including those that prohibit “teaching physicians” like Dr. Luketich from performing and billing the U.S. for concurrent surgeries, the Department of Justice said in news release.
The Justice Department contends the defendants “knowingly submitted hundreds of materially false claims for payment” to Medicare, Medicaid, and other government programs over the past 6 years.
“The laws prohibiting ‘concurrent surgeries’ are in place for a reason: To protect patients and ensure they receive appropriate and focused medical care,” Stephen R. Kaufman, Acting U.S. Attorney for the Western District of Pennsylvania, said in the release.
According to the lawsuit, “some of Dr. Luketich’s patients were forced to endure additional surgical procedures and/or extended hospital stays as a result of his unlawful conduct. Numerous patients developed painful pressure ulcers. A few were diagnosed with compartment syndrome. And at least two had to undergo amputations.”
The allegations were originally brought forward under the federal False Claims Act’s whistleblower provisions by Jonathan D’Cunha, MD, PhD, who worked closely with Dr. Luketich from 2012 to 2019 and now chairs the department of cardiothoracic surgery at the Mayo Clinic, Phoenix.
The charges cited in the lawsuit include three counts of violating the False Claims Act, one count of unjust enrichment, and one count of payment by mistake.
The 56-page lawsuit includes numerous case examples and cites an October 2015 Boston Globe Spotlight Team report on the safety of running concurrent operations, which reportedly prompted UPMC to reevaluate its policies and identify physicians or departments in potential violation.
Hospital officials met with Dr. Luketich in March 2016 and devised a “plan” to ensure his availability and “compliance with concurrency rules,” it alleges, but also highlights an email that notes “continued problems” with Dr. Luketich’s schedule.
“UPMC has persistently ignored or minimized complaints by employees and staff regarding Dr. Luketich, his hyper-busy schedule, his refusal to delegate surgeries and surgical tasks” and “protected him from meaningful sanction; refused to curtail his surgical practice; and continued to allow Dr. Luketich to skirt the rules and endanger his patients,” according to the lawsuit.
The suit notes that Dr. Luketich is one of UPMC and UPP’s highest sources of revenue and that UPMC advertises him as a “life-saving pioneer” who routinely performs dramatic, last-ditch procedures on patients who are otherwise hopeless.
In response to an interview request from this news organization, a UPMC spokesperson wrote: “As the government itself concedes in its complaint, many of Dr. Luketich’s surgical patients are elderly, frail, and/or very ill. They include the ‘hopeless’ patients ... who suffer from chronic illness or metastatic cancer, and/or have extensive surgical histories and choose UPMC and Dr. Luketich when other physicians and health care providers have turned them down.”
“Dr. Luketich always performs the most critical portions of every operation he undertakes,” the spokesperson said, adding that no law or regulation prohibits overlapping surgeries or billing for those surgeries, “let alone surgeries conducted by teams of surgeons like those led by Dr. Luketich.”
“The government’s claims are, rather, based on a misapplication or misinterpretation of UPMC’s internal policies and [Centers for Medicare & Medicaid Services] guidance, neither of which can support a claim for fraudulent billing. UPMC and Dr. Luketich plan to vigorously defend against the government’s claims,” the spokesperson concluded.
The claims asserted against the defendants are allegations only; there has been no determination of liability. The government is seeking three times the amount of actual damages suffered as a result of the alleged false claims and/or fraud; a sum of $23,331 (or the maximum penalty, whichever is greater) for each false claim submitted by UPMC, UPP, and/or Dr. Luketich; and costs and expenses associated with the civil suit.
A version of this article first appeared on Medscape.com.
Following a 2-year investigation, the U.S. government has filed suit against the University of Pittsburgh Medical Center (UPMC), University of Pittsburgh Physicians (UPP), and James Luketich, MD, for billing related to concurrent surgeries performed by the long-time chair of cardiothoracic surgery.
The lawsuit alleges that UPMC “knowingly allowed” Dr. Luketich to “book and perform three surgeries at the same time, to miss the surgical time outs at the outset of those procedures, to go back-and-forth between operating rooms and even hospital facilities while his surgical patients remain under general anesthesia...”
UPMC, the lawsuit claims, also allowed Dr. Luketich to falsely attest that “he was with his patients throughout the entirety of their surgical procedures or during all ‘key and critical’ portions of those procedures and to unlawfully bill Government Health Benefit Programs for those procedures, all in order to increase surgical volume, maximize UPMC and UPP’s revenue, and/or appease Dr. Luketich.”
These practices violate the statutes and regulations governing the defendants, including those that prohibit “teaching physicians” like Dr. Luketich from performing and billing the U.S. for concurrent surgeries, the Department of Justice said in news release.
The Justice Department contends the defendants “knowingly submitted hundreds of materially false claims for payment” to Medicare, Medicaid, and other government programs over the past 6 years.
“The laws prohibiting ‘concurrent surgeries’ are in place for a reason: To protect patients and ensure they receive appropriate and focused medical care,” Stephen R. Kaufman, Acting U.S. Attorney for the Western District of Pennsylvania, said in the release.
According to the lawsuit, “some of Dr. Luketich’s patients were forced to endure additional surgical procedures and/or extended hospital stays as a result of his unlawful conduct. Numerous patients developed painful pressure ulcers. A few were diagnosed with compartment syndrome. And at least two had to undergo amputations.”
The allegations were originally brought forward under the federal False Claims Act’s whistleblower provisions by Jonathan D’Cunha, MD, PhD, who worked closely with Dr. Luketich from 2012 to 2019 and now chairs the department of cardiothoracic surgery at the Mayo Clinic, Phoenix.
The charges cited in the lawsuit include three counts of violating the False Claims Act, one count of unjust enrichment, and one count of payment by mistake.
The 56-page lawsuit includes numerous case examples and cites an October 2015 Boston Globe Spotlight Team report on the safety of running concurrent operations, which reportedly prompted UPMC to reevaluate its policies and identify physicians or departments in potential violation.
Hospital officials met with Dr. Luketich in March 2016 and devised a “plan” to ensure his availability and “compliance with concurrency rules,” it alleges, but also highlights an email that notes “continued problems” with Dr. Luketich’s schedule.
“UPMC has persistently ignored or minimized complaints by employees and staff regarding Dr. Luketich, his hyper-busy schedule, his refusal to delegate surgeries and surgical tasks” and “protected him from meaningful sanction; refused to curtail his surgical practice; and continued to allow Dr. Luketich to skirt the rules and endanger his patients,” according to the lawsuit.
The suit notes that Dr. Luketich is one of UPMC and UPP’s highest sources of revenue and that UPMC advertises him as a “life-saving pioneer” who routinely performs dramatic, last-ditch procedures on patients who are otherwise hopeless.
In response to an interview request from this news organization, a UPMC spokesperson wrote: “As the government itself concedes in its complaint, many of Dr. Luketich’s surgical patients are elderly, frail, and/or very ill. They include the ‘hopeless’ patients ... who suffer from chronic illness or metastatic cancer, and/or have extensive surgical histories and choose UPMC and Dr. Luketich when other physicians and health care providers have turned them down.”
“Dr. Luketich always performs the most critical portions of every operation he undertakes,” the spokesperson said, adding that no law or regulation prohibits overlapping surgeries or billing for those surgeries, “let alone surgeries conducted by teams of surgeons like those led by Dr. Luketich.”
“The government’s claims are, rather, based on a misapplication or misinterpretation of UPMC’s internal policies and [Centers for Medicare & Medicaid Services] guidance, neither of which can support a claim for fraudulent billing. UPMC and Dr. Luketich plan to vigorously defend against the government’s claims,” the spokesperson concluded.
The claims asserted against the defendants are allegations only; there has been no determination of liability. The government is seeking three times the amount of actual damages suffered as a result of the alleged false claims and/or fraud; a sum of $23,331 (or the maximum penalty, whichever is greater) for each false claim submitted by UPMC, UPP, and/or Dr. Luketich; and costs and expenses associated with the civil suit.
A version of this article first appeared on Medscape.com.
STOP-DAPT 2 ACS: 1 month of DAPT proves inadequate for patients with recent ACS
One month of dual antiplatelet therapy followed by 11 months of clopidogrel monotherapy failed to prove noninferiority to 12 unbroken months of DAPT for net clinical benefit in a multicenter Japanese trial that randomized more than 4,000 patients who underwent percutaneous coronary intervention (PCI) after a recent acute coronary syndrome episode.
The outcomes showed that while truncating DAPT duration could, as expected, cut major bleeding episodes roughly in half, it also led to a significant near doubling of myocardial infarction and showed a strong trend toward also increasing a composite tally of several types of ischemic events. These data were reported this week by Hirotoshi Watanabe, MD, PhD, at the virtual annual congress of the European Society of Cardiology. All study patients had undergone PCI with cobalt-chromium everolimus-eluting (CCEE) coronary stents (Xience).
These findings from the STOPDAPT-2 ACS trial highlighted the limits of minimizing DAPT after PCI in patients at high ischemic risk, such as after an acute coronary syndrome (ACS) event.
It also was a counterpoint to a somewhat similar study also reported at the congress, MASTER DAPT, which showed that 1 month of DAPT was noninferior to 3 or more months of DAPT for net clinical benefit in a distinctly different population of patients undergoing PCI (and using a different type of coronary stent) – those at high bleeding risk and with only about half the patients having had a recent ACS.
The results of STOPDAPT-2 ACS “do not support use of 1 month of DAPT followed by P2Y12 inhibitor monotherapy with clopidogrel compared with standard DAPT,” commented Robert A. Byrne, MBBCh, PhD, designated discussant for the report and professor at the RCSI University of Medicine and Health Sciences in Dublin.
“Although major bleeding was significantly reduced with this approach, there appeared to be a significant increase in adverse ischemic events, and there was a clear signal in relation to overall mortality, the ultimate arbiter of net clinical benefit,” added Dr. Byrne, who is also director of cardiology at Mater Private Hospital in Dublin.
He suggested that a mechanistic explanation for the signal of harm seem in STOPDAPT-2 ACS was the relatively low potency of clopidogrel (Plavix) as an antiplatelet agent, compared with other P2Y12 inhibitors such as prasugrel (Effient) and ticagrelor (Brilinta), as well as the genetically driven variability in response to clopidogrel that’s also absent with alternative agents.
These between-agent differences are of “particular clinical relevance in the early aftermath of an ACS event,” Dr. Byrne said.
12-month DAPT remains standard for PCI patients with recent ACS
The totality of clinical evidence “continues to support a standard 12-month duration of DAPT – using aspirin and either prasugrel or ticagrelor – as the preferred default approach,” Dr. Byrne concluded.
He acknowledged that an abbreviated duration of DAPT followed by P2Y12 inhibitor monotherapy “might be considered as an alternative.” In patients following an ACS event who do not have high risk for bleeding, he said, the minimum duration of DAPT should be at least 3 months and with preferential use of a more potent P2Y12 inhibitor.
Twelve months of DAPT treatment with aspirin and a P2Y12 inhibitor for patients following PCI “remains the standard of care in guidelines,” noted Marco Roffi, MD, a second discussant at the congress. But several questions remain, he added, such as which P2Y12 inhibitors work best and whether DAPT can be less than 12 months.
“The investigators [for STOPDAPT-2 ACS] pushed these questions to the limit with 1 month of DAPT and clopidogrel monotherapy,” said Dr. Roffi, professor and director of interventional cardiology at University Hospital, Geneva.
“This was a risky bet, and the investigators lost by not proving noninferiority and with excess ischemic events,” he commented.
First came STOPDAPT-2
Dr. Watanabe and colleagues designed STOPDAPT-2 ACS as a follow-up to their prior STOPDAPT-2 trial, which randomly assigned slightly more than 3000 patients at 90 Japanese centers to the identical two treatment options: 1 month of DAPT followed by 11 months of clopidogrel monotherapy or 12 months of DAPT, except the trial enrolled all types of patients undergoing PCI. This meant that a minority, 38%, had a recent ACS event, while the remaining patients had chronic coronary artery disease. As in STOPDAPT-2 ACS, all patients in STOPDAPT-2 had received a CCEE stent.
STOPDAPT-2 also used the same primary endpoint to tally net clinical benefit as STOPDAPT-2 ACS: cardiovascular death, MI, stroke of any type, definite stent thrombosis, or TIMI major or minor bleeding classification.
In STOPDAPT-2, using the mixed population with both recent ACS and chronic coronary disease, the regimen of 1 month of DAPT followed by 11 months of clopidogrel monotherapy was both noninferior to and superior to 12 months of DAPT, reducing the net adverse-event tally by 36% relative to 12-month DAPT and by an absolute reduction of 1.34%, as reported in 2019.
Despite this superiority, the results from STOPDAPT-2 had little impact on global practice, commented Kurt Huber, MD, professor and director of the cardiology ICU at the Medical University of Vienna.
“STOP-DAPT-2 did not give us a clear message with respect to reducing antiplatelet treatment after 1 month. I thought that for ACS patients 1 month might be too short,” Dr. Huber said during a press briefing.
Focusing on post-ACS
To directly address this issue, the investigators launched STOPDAPT-2 ACS, which used the same design as the preceding study but only enrolled patients soon after an ACS event. The trial included for its main analysis 3,008 newly enrolled patients with recent ACS, and 1,161 patients who had a recent ACS event and had been randomly assigned in STOPDAPT-2, creating a total study cohort for the new analysis of 4136 patients treated and followed for the study’s full 12 months.
The patients averaged 67 years old, 79% were men, and 30% had diabetes. About 56% had a recent ST-elevation MI, about 20% a recent non–ST-elevation MI, and the remaining 24% had unstable angina. For their unspecified P2Y12 inhibition, roughly half the patients received clopidogrel and the rest received prasugrel. Adherence to the two assigned treatment regimens was very good, with a very small number of patients not adhering to their assigned protocol.
The composite adverse event incidence over 12 months was 3.2% among those who received 1-month DAPT and 2.83% in those on DAPT for 12 months, a difference that failed to achieve the prespecified definition of noninferiority for 1-month DAPT, reported Dr. Watanabe, an interventional cardiologist at Kyoto University.
The ischemic event composite was 50% lower among those on 12-month DAPT, compared with 1 month of DAPT, a difference that just missed significance. The rate of MI was 91% higher with 1-month DAPT, compared with 12 months, a significant difference.
One-month DAPT also significantly reduced the primary measure of bleeding events – the combination of TIMI major and minor bleeds – by a significant 54%, compared with 12-month DAPT. A second metric of clinically meaningful bleeds, those that meet either the type 3 or 5 definition of the Bleeding Academic Research Consortium, were reduced by a significant 59% by 1-month DAPT, compared with 12 months of DAPT.
The new findings from STOPDAPT-2 ACS contrasted with those from MASTER DAPT, but in an explicable way that related to different patient types, different P2Y12 inhibitors, different treatment durations, and different stents.
“We’ve seen in MASTER DAPT that if you use the right stent and use ticagrelor for monotherapy there may be some ability to shorten DAPT, but we still do not know what would happen in patients with very high ischemic risk,” concluded Dr. Huber.
“A reduction in DAPT duration might work in patients without high bleeding risk, but I would exclude patients with very high ischemic risk,” he added. “I also can’t tell you whether 1 month or 3 months is the right approach, and I think clopidogrel is not the right drug to use for monotherapy after ACS.”
STOPDAPT-2 and STOPDAPT-2 ACS were both sponsored by Abbott Vascular, which markets the CCEE (Xience) stents used in both studies. Dr. Watanabe has received lecture fees from Abbott and from Daiichi-Sankyo. Dr. Byrne has received research funding from Abbott Vascular as well as from Biosensors, Biotronik, and Boston Scientific. Roffi has received research funding from Biotronik, Boston Scientific, GE Healthcare, Medtronic, and Terumo. Dr. Huber has received lecture fees from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, Eli Lilly, Pfizer, Sanofi-Aventis, and The Medicines Company.
A version of this article first appeared on Medscape.com.
One month of dual antiplatelet therapy followed by 11 months of clopidogrel monotherapy failed to prove noninferiority to 12 unbroken months of DAPT for net clinical benefit in a multicenter Japanese trial that randomized more than 4,000 patients who underwent percutaneous coronary intervention (PCI) after a recent acute coronary syndrome episode.
The outcomes showed that while truncating DAPT duration could, as expected, cut major bleeding episodes roughly in half, it also led to a significant near doubling of myocardial infarction and showed a strong trend toward also increasing a composite tally of several types of ischemic events. These data were reported this week by Hirotoshi Watanabe, MD, PhD, at the virtual annual congress of the European Society of Cardiology. All study patients had undergone PCI with cobalt-chromium everolimus-eluting (CCEE) coronary stents (Xience).
These findings from the STOPDAPT-2 ACS trial highlighted the limits of minimizing DAPT after PCI in patients at high ischemic risk, such as after an acute coronary syndrome (ACS) event.
It also was a counterpoint to a somewhat similar study also reported at the congress, MASTER DAPT, which showed that 1 month of DAPT was noninferior to 3 or more months of DAPT for net clinical benefit in a distinctly different population of patients undergoing PCI (and using a different type of coronary stent) – those at high bleeding risk and with only about half the patients having had a recent ACS.
The results of STOPDAPT-2 ACS “do not support use of 1 month of DAPT followed by P2Y12 inhibitor monotherapy with clopidogrel compared with standard DAPT,” commented Robert A. Byrne, MBBCh, PhD, designated discussant for the report and professor at the RCSI University of Medicine and Health Sciences in Dublin.
“Although major bleeding was significantly reduced with this approach, there appeared to be a significant increase in adverse ischemic events, and there was a clear signal in relation to overall mortality, the ultimate arbiter of net clinical benefit,” added Dr. Byrne, who is also director of cardiology at Mater Private Hospital in Dublin.
He suggested that a mechanistic explanation for the signal of harm seem in STOPDAPT-2 ACS was the relatively low potency of clopidogrel (Plavix) as an antiplatelet agent, compared with other P2Y12 inhibitors such as prasugrel (Effient) and ticagrelor (Brilinta), as well as the genetically driven variability in response to clopidogrel that’s also absent with alternative agents.
These between-agent differences are of “particular clinical relevance in the early aftermath of an ACS event,” Dr. Byrne said.
12-month DAPT remains standard for PCI patients with recent ACS
The totality of clinical evidence “continues to support a standard 12-month duration of DAPT – using aspirin and either prasugrel or ticagrelor – as the preferred default approach,” Dr. Byrne concluded.
He acknowledged that an abbreviated duration of DAPT followed by P2Y12 inhibitor monotherapy “might be considered as an alternative.” In patients following an ACS event who do not have high risk for bleeding, he said, the minimum duration of DAPT should be at least 3 months and with preferential use of a more potent P2Y12 inhibitor.
Twelve months of DAPT treatment with aspirin and a P2Y12 inhibitor for patients following PCI “remains the standard of care in guidelines,” noted Marco Roffi, MD, a second discussant at the congress. But several questions remain, he added, such as which P2Y12 inhibitors work best and whether DAPT can be less than 12 months.
“The investigators [for STOPDAPT-2 ACS] pushed these questions to the limit with 1 month of DAPT and clopidogrel monotherapy,” said Dr. Roffi, professor and director of interventional cardiology at University Hospital, Geneva.
“This was a risky bet, and the investigators lost by not proving noninferiority and with excess ischemic events,” he commented.
First came STOPDAPT-2
Dr. Watanabe and colleagues designed STOPDAPT-2 ACS as a follow-up to their prior STOPDAPT-2 trial, which randomly assigned slightly more than 3000 patients at 90 Japanese centers to the identical two treatment options: 1 month of DAPT followed by 11 months of clopidogrel monotherapy or 12 months of DAPT, except the trial enrolled all types of patients undergoing PCI. This meant that a minority, 38%, had a recent ACS event, while the remaining patients had chronic coronary artery disease. As in STOPDAPT-2 ACS, all patients in STOPDAPT-2 had received a CCEE stent.
STOPDAPT-2 also used the same primary endpoint to tally net clinical benefit as STOPDAPT-2 ACS: cardiovascular death, MI, stroke of any type, definite stent thrombosis, or TIMI major or minor bleeding classification.
In STOPDAPT-2, using the mixed population with both recent ACS and chronic coronary disease, the regimen of 1 month of DAPT followed by 11 months of clopidogrel monotherapy was both noninferior to and superior to 12 months of DAPT, reducing the net adverse-event tally by 36% relative to 12-month DAPT and by an absolute reduction of 1.34%, as reported in 2019.
Despite this superiority, the results from STOPDAPT-2 had little impact on global practice, commented Kurt Huber, MD, professor and director of the cardiology ICU at the Medical University of Vienna.
“STOP-DAPT-2 did not give us a clear message with respect to reducing antiplatelet treatment after 1 month. I thought that for ACS patients 1 month might be too short,” Dr. Huber said during a press briefing.
Focusing on post-ACS
To directly address this issue, the investigators launched STOPDAPT-2 ACS, which used the same design as the preceding study but only enrolled patients soon after an ACS event. The trial included for its main analysis 3,008 newly enrolled patients with recent ACS, and 1,161 patients who had a recent ACS event and had been randomly assigned in STOPDAPT-2, creating a total study cohort for the new analysis of 4136 patients treated and followed for the study’s full 12 months.
The patients averaged 67 years old, 79% were men, and 30% had diabetes. About 56% had a recent ST-elevation MI, about 20% a recent non–ST-elevation MI, and the remaining 24% had unstable angina. For their unspecified P2Y12 inhibition, roughly half the patients received clopidogrel and the rest received prasugrel. Adherence to the two assigned treatment regimens was very good, with a very small number of patients not adhering to their assigned protocol.
The composite adverse event incidence over 12 months was 3.2% among those who received 1-month DAPT and 2.83% in those on DAPT for 12 months, a difference that failed to achieve the prespecified definition of noninferiority for 1-month DAPT, reported Dr. Watanabe, an interventional cardiologist at Kyoto University.
The ischemic event composite was 50% lower among those on 12-month DAPT, compared with 1 month of DAPT, a difference that just missed significance. The rate of MI was 91% higher with 1-month DAPT, compared with 12 months, a significant difference.
One-month DAPT also significantly reduced the primary measure of bleeding events – the combination of TIMI major and minor bleeds – by a significant 54%, compared with 12-month DAPT. A second metric of clinically meaningful bleeds, those that meet either the type 3 or 5 definition of the Bleeding Academic Research Consortium, were reduced by a significant 59% by 1-month DAPT, compared with 12 months of DAPT.
The new findings from STOPDAPT-2 ACS contrasted with those from MASTER DAPT, but in an explicable way that related to different patient types, different P2Y12 inhibitors, different treatment durations, and different stents.
“We’ve seen in MASTER DAPT that if you use the right stent and use ticagrelor for monotherapy there may be some ability to shorten DAPT, but we still do not know what would happen in patients with very high ischemic risk,” concluded Dr. Huber.
“A reduction in DAPT duration might work in patients without high bleeding risk, but I would exclude patients with very high ischemic risk,” he added. “I also can’t tell you whether 1 month or 3 months is the right approach, and I think clopidogrel is not the right drug to use for monotherapy after ACS.”
STOPDAPT-2 and STOPDAPT-2 ACS were both sponsored by Abbott Vascular, which markets the CCEE (Xience) stents used in both studies. Dr. Watanabe has received lecture fees from Abbott and from Daiichi-Sankyo. Dr. Byrne has received research funding from Abbott Vascular as well as from Biosensors, Biotronik, and Boston Scientific. Roffi has received research funding from Biotronik, Boston Scientific, GE Healthcare, Medtronic, and Terumo. Dr. Huber has received lecture fees from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, Eli Lilly, Pfizer, Sanofi-Aventis, and The Medicines Company.
A version of this article first appeared on Medscape.com.
One month of dual antiplatelet therapy followed by 11 months of clopidogrel monotherapy failed to prove noninferiority to 12 unbroken months of DAPT for net clinical benefit in a multicenter Japanese trial that randomized more than 4,000 patients who underwent percutaneous coronary intervention (PCI) after a recent acute coronary syndrome episode.
The outcomes showed that while truncating DAPT duration could, as expected, cut major bleeding episodes roughly in half, it also led to a significant near doubling of myocardial infarction and showed a strong trend toward also increasing a composite tally of several types of ischemic events. These data were reported this week by Hirotoshi Watanabe, MD, PhD, at the virtual annual congress of the European Society of Cardiology. All study patients had undergone PCI with cobalt-chromium everolimus-eluting (CCEE) coronary stents (Xience).
These findings from the STOPDAPT-2 ACS trial highlighted the limits of minimizing DAPT after PCI in patients at high ischemic risk, such as after an acute coronary syndrome (ACS) event.
It also was a counterpoint to a somewhat similar study also reported at the congress, MASTER DAPT, which showed that 1 month of DAPT was noninferior to 3 or more months of DAPT for net clinical benefit in a distinctly different population of patients undergoing PCI (and using a different type of coronary stent) – those at high bleeding risk and with only about half the patients having had a recent ACS.
The results of STOPDAPT-2 ACS “do not support use of 1 month of DAPT followed by P2Y12 inhibitor monotherapy with clopidogrel compared with standard DAPT,” commented Robert A. Byrne, MBBCh, PhD, designated discussant for the report and professor at the RCSI University of Medicine and Health Sciences in Dublin.
“Although major bleeding was significantly reduced with this approach, there appeared to be a significant increase in adverse ischemic events, and there was a clear signal in relation to overall mortality, the ultimate arbiter of net clinical benefit,” added Dr. Byrne, who is also director of cardiology at Mater Private Hospital in Dublin.
He suggested that a mechanistic explanation for the signal of harm seem in STOPDAPT-2 ACS was the relatively low potency of clopidogrel (Plavix) as an antiplatelet agent, compared with other P2Y12 inhibitors such as prasugrel (Effient) and ticagrelor (Brilinta), as well as the genetically driven variability in response to clopidogrel that’s also absent with alternative agents.
These between-agent differences are of “particular clinical relevance in the early aftermath of an ACS event,” Dr. Byrne said.
12-month DAPT remains standard for PCI patients with recent ACS
The totality of clinical evidence “continues to support a standard 12-month duration of DAPT – using aspirin and either prasugrel or ticagrelor – as the preferred default approach,” Dr. Byrne concluded.
He acknowledged that an abbreviated duration of DAPT followed by P2Y12 inhibitor monotherapy “might be considered as an alternative.” In patients following an ACS event who do not have high risk for bleeding, he said, the minimum duration of DAPT should be at least 3 months and with preferential use of a more potent P2Y12 inhibitor.
Twelve months of DAPT treatment with aspirin and a P2Y12 inhibitor for patients following PCI “remains the standard of care in guidelines,” noted Marco Roffi, MD, a second discussant at the congress. But several questions remain, he added, such as which P2Y12 inhibitors work best and whether DAPT can be less than 12 months.
“The investigators [for STOPDAPT-2 ACS] pushed these questions to the limit with 1 month of DAPT and clopidogrel monotherapy,” said Dr. Roffi, professor and director of interventional cardiology at University Hospital, Geneva.
“This was a risky bet, and the investigators lost by not proving noninferiority and with excess ischemic events,” he commented.
First came STOPDAPT-2
Dr. Watanabe and colleagues designed STOPDAPT-2 ACS as a follow-up to their prior STOPDAPT-2 trial, which randomly assigned slightly more than 3000 patients at 90 Japanese centers to the identical two treatment options: 1 month of DAPT followed by 11 months of clopidogrel monotherapy or 12 months of DAPT, except the trial enrolled all types of patients undergoing PCI. This meant that a minority, 38%, had a recent ACS event, while the remaining patients had chronic coronary artery disease. As in STOPDAPT-2 ACS, all patients in STOPDAPT-2 had received a CCEE stent.
STOPDAPT-2 also used the same primary endpoint to tally net clinical benefit as STOPDAPT-2 ACS: cardiovascular death, MI, stroke of any type, definite stent thrombosis, or TIMI major or minor bleeding classification.
In STOPDAPT-2, using the mixed population with both recent ACS and chronic coronary disease, the regimen of 1 month of DAPT followed by 11 months of clopidogrel monotherapy was both noninferior to and superior to 12 months of DAPT, reducing the net adverse-event tally by 36% relative to 12-month DAPT and by an absolute reduction of 1.34%, as reported in 2019.
Despite this superiority, the results from STOPDAPT-2 had little impact on global practice, commented Kurt Huber, MD, professor and director of the cardiology ICU at the Medical University of Vienna.
“STOP-DAPT-2 did not give us a clear message with respect to reducing antiplatelet treatment after 1 month. I thought that for ACS patients 1 month might be too short,” Dr. Huber said during a press briefing.
Focusing on post-ACS
To directly address this issue, the investigators launched STOPDAPT-2 ACS, which used the same design as the preceding study but only enrolled patients soon after an ACS event. The trial included for its main analysis 3,008 newly enrolled patients with recent ACS, and 1,161 patients who had a recent ACS event and had been randomly assigned in STOPDAPT-2, creating a total study cohort for the new analysis of 4136 patients treated and followed for the study’s full 12 months.
The patients averaged 67 years old, 79% were men, and 30% had diabetes. About 56% had a recent ST-elevation MI, about 20% a recent non–ST-elevation MI, and the remaining 24% had unstable angina. For their unspecified P2Y12 inhibition, roughly half the patients received clopidogrel and the rest received prasugrel. Adherence to the two assigned treatment regimens was very good, with a very small number of patients not adhering to their assigned protocol.
The composite adverse event incidence over 12 months was 3.2% among those who received 1-month DAPT and 2.83% in those on DAPT for 12 months, a difference that failed to achieve the prespecified definition of noninferiority for 1-month DAPT, reported Dr. Watanabe, an interventional cardiologist at Kyoto University.
The ischemic event composite was 50% lower among those on 12-month DAPT, compared with 1 month of DAPT, a difference that just missed significance. The rate of MI was 91% higher with 1-month DAPT, compared with 12 months, a significant difference.
One-month DAPT also significantly reduced the primary measure of bleeding events – the combination of TIMI major and minor bleeds – by a significant 54%, compared with 12-month DAPT. A second metric of clinically meaningful bleeds, those that meet either the type 3 or 5 definition of the Bleeding Academic Research Consortium, were reduced by a significant 59% by 1-month DAPT, compared with 12 months of DAPT.
The new findings from STOPDAPT-2 ACS contrasted with those from MASTER DAPT, but in an explicable way that related to different patient types, different P2Y12 inhibitors, different treatment durations, and different stents.
“We’ve seen in MASTER DAPT that if you use the right stent and use ticagrelor for monotherapy there may be some ability to shorten DAPT, but we still do not know what would happen in patients with very high ischemic risk,” concluded Dr. Huber.
“A reduction in DAPT duration might work in patients without high bleeding risk, but I would exclude patients with very high ischemic risk,” he added. “I also can’t tell you whether 1 month or 3 months is the right approach, and I think clopidogrel is not the right drug to use for monotherapy after ACS.”
STOPDAPT-2 and STOPDAPT-2 ACS were both sponsored by Abbott Vascular, which markets the CCEE (Xience) stents used in both studies. Dr. Watanabe has received lecture fees from Abbott and from Daiichi-Sankyo. Dr. Byrne has received research funding from Abbott Vascular as well as from Biosensors, Biotronik, and Boston Scientific. Roffi has received research funding from Biotronik, Boston Scientific, GE Healthcare, Medtronic, and Terumo. Dr. Huber has received lecture fees from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, Eli Lilly, Pfizer, Sanofi-Aventis, and The Medicines Company.
A version of this article first appeared on Medscape.com.
New valvular heart disease guidelines change several repair indications
Antithrombotic recommendations also altered
New European guidelines for the management of valvular heart disease offer more than 45 revised or completely new recommendations relative to the previous version published in 2017, according to members of the writing committee who presented the changes during the annual congress of the European Society of Cardiology.
With their emphasis on early diagnosis and expansion of indications, these 2021 ESC/European Association for Cardio-Thoracic Surgery guidelines are likely to further accelerate the already steep growth in this area of interventional cardiology, according to Alec Vahanian, MD, a professor of cardiology at the University of Paris.
“Valvular heart disease is too often undetected, and these guidelines stress the importance of clinical examination and the best strategies for diagnosis as well as treatment,” he said.
Of the multiple sections and subsections, which follow the same format of the previous guidelines, the greatest number of revisions and new additions involve perioperative antithrombotic therapy, according to the document, which was published in conjunction with the ESC Congress.
Eleven new guidelines for anticoagulants
On the basis of evidence published since the previous guidelines, there are 11 completely new recommendations regarding the use of anticoagulants or antiplatelet therapies. The majority of these have received a grade I indication, which signifies “recommended” or “indicated.” These include indications for stopping or starting anticoagulants and which anticoagulants or antiplatelet drugs to consider in specific patient populations.
The next most common focus of new or revised recommendations involves when to consider surgical aortic valve repair (SAVR) relative to transcatheter aortic valve implantation (TAVI) in severe aortic stenosis. Most of these represent revisions from the previous guidelines, but almost all are also grade I recommendations.
“SAVR and TAVI are both excellent options in appropriate patients, but they are not interchangeable,” explained Bernard D. Prendergast, BMedSci, MD, director of the cardiac structural intervention program, Guy’s and St Thomas’ Hospital, London.
While the previous guidelines generally reserved TAVI for those not suitable for SAVR, the new guidelines are more nuanced.
As a rule, SAVR is generally preferred for younger patients. The reason, according to Dr. Prendergast, is concern that younger patients might outlive the expected lifespan of the prosthetic TAVI device.
No single criterion for selecting SAVR over TAVI
However, there are many exceptions and additional considerations beyond age. When both SAVR and TAVI are otherwise suitable options, but TAVI cannot be performed with a transfemoral access, Dr. Prendergast pointed out that SAVR might be a better choice.
Transfemoral access is the preferred strategy in TAVI, but Dr. Prendergast emphasized that a collaborative “heart team” should help patients select the most appropriate option. In fact, there is a grade IIb recommendation (“usefulness or efficacy is less well established”) to consider other access sites in patients at high surgical risk with contraindications for transfemoral TAVI.
Of new recommendations in the area of severe aortic stenosis, valvular repair may now be considered in asymptomatic patients with a left ventricular ejection fraction of less than 55%, according to a grade IIb recommendation. The 2017 guidelines did not address this issue.
Grade I recommendation for bioprostheses
A substantial number of the revisions involve minor clarifications without a change in the grade, but a revision regarding bioprosthetics is substantial. In the 2017 guidelines, there was a grade IIa recommendation (“weight of evidence is in favor”) to consider bioprosthetics in patients with a life expectancy less than the expected durability of the device. The 2021 guidelines have changed this to a grade I indication, adding an indication for bioprostheses in patients contraindicated for or unlikely to achieve good-quality anticoagulation.
On this same topic, there is a new grade IIb recommendation to consider bioprosthesis over alternative devices in patients already on a long-term non–vitamin K oral anticoagulant because of the high risk of thromboembolism.
There are two new recommendations in the realm of severe secondary mitral regurgitation, reported Fabien Praz, MD, an interventional cardiologist at the University of Bern (Switzerland). The first, which is perhaps the most significant, is a grade I recommendation for valve surgery in patients with severe secondary mitral regurgitation who remain symptomatic despite optimized medical therapy.
The second is a grade IIa recommendation for invasive procedures, such as a percutaneous coronary intervention, for patients with symptomatic secondary mitral regurgitation and coexisting coronary artery disease. In both cases, however, Dr. Praz emphasized language in the guidelines that calls for a collaborative heart team to agree on the suitability of these treatments.
Ultimately, none of these recommendations can be divorced from patient expectations and values, according to Friedhelm Beyersdorf, MD, chairman of the department of cardiovascular surgery at the Heart Center of the University of Freiburg (Germany).
Even if treatment is not expected to prolong life, “symptom relief on its own may justify intervention,” said Dr. Beyersdorf. However, he emphasized that “thoroughly informed” patients are an essential part of the process in selecting a treatment strategy most likely to satisfy patient goals.
Dr. Vahanian and Dr. Prendergast reported no conflicts of interest relevant to these guidelines. Dr. Praz reported a financial relationship with Edwards Lifesciences. Dr. Beyersdorf reported a financial relationship with Resuscitec.
Antithrombotic recommendations also altered
Antithrombotic recommendations also altered
New European guidelines for the management of valvular heart disease offer more than 45 revised or completely new recommendations relative to the previous version published in 2017, according to members of the writing committee who presented the changes during the annual congress of the European Society of Cardiology.
With their emphasis on early diagnosis and expansion of indications, these 2021 ESC/European Association for Cardio-Thoracic Surgery guidelines are likely to further accelerate the already steep growth in this area of interventional cardiology, according to Alec Vahanian, MD, a professor of cardiology at the University of Paris.
“Valvular heart disease is too often undetected, and these guidelines stress the importance of clinical examination and the best strategies for diagnosis as well as treatment,” he said.
Of the multiple sections and subsections, which follow the same format of the previous guidelines, the greatest number of revisions and new additions involve perioperative antithrombotic therapy, according to the document, which was published in conjunction with the ESC Congress.
Eleven new guidelines for anticoagulants
On the basis of evidence published since the previous guidelines, there are 11 completely new recommendations regarding the use of anticoagulants or antiplatelet therapies. The majority of these have received a grade I indication, which signifies “recommended” or “indicated.” These include indications for stopping or starting anticoagulants and which anticoagulants or antiplatelet drugs to consider in specific patient populations.
The next most common focus of new or revised recommendations involves when to consider surgical aortic valve repair (SAVR) relative to transcatheter aortic valve implantation (TAVI) in severe aortic stenosis. Most of these represent revisions from the previous guidelines, but almost all are also grade I recommendations.
“SAVR and TAVI are both excellent options in appropriate patients, but they are not interchangeable,” explained Bernard D. Prendergast, BMedSci, MD, director of the cardiac structural intervention program, Guy’s and St Thomas’ Hospital, London.
While the previous guidelines generally reserved TAVI for those not suitable for SAVR, the new guidelines are more nuanced.
As a rule, SAVR is generally preferred for younger patients. The reason, according to Dr. Prendergast, is concern that younger patients might outlive the expected lifespan of the prosthetic TAVI device.
No single criterion for selecting SAVR over TAVI
However, there are many exceptions and additional considerations beyond age. When both SAVR and TAVI are otherwise suitable options, but TAVI cannot be performed with a transfemoral access, Dr. Prendergast pointed out that SAVR might be a better choice.
Transfemoral access is the preferred strategy in TAVI, but Dr. Prendergast emphasized that a collaborative “heart team” should help patients select the most appropriate option. In fact, there is a grade IIb recommendation (“usefulness or efficacy is less well established”) to consider other access sites in patients at high surgical risk with contraindications for transfemoral TAVI.
Of new recommendations in the area of severe aortic stenosis, valvular repair may now be considered in asymptomatic patients with a left ventricular ejection fraction of less than 55%, according to a grade IIb recommendation. The 2017 guidelines did not address this issue.
Grade I recommendation for bioprostheses
A substantial number of the revisions involve minor clarifications without a change in the grade, but a revision regarding bioprosthetics is substantial. In the 2017 guidelines, there was a grade IIa recommendation (“weight of evidence is in favor”) to consider bioprosthetics in patients with a life expectancy less than the expected durability of the device. The 2021 guidelines have changed this to a grade I indication, adding an indication for bioprostheses in patients contraindicated for or unlikely to achieve good-quality anticoagulation.
On this same topic, there is a new grade IIb recommendation to consider bioprosthesis over alternative devices in patients already on a long-term non–vitamin K oral anticoagulant because of the high risk of thromboembolism.
There are two new recommendations in the realm of severe secondary mitral regurgitation, reported Fabien Praz, MD, an interventional cardiologist at the University of Bern (Switzerland). The first, which is perhaps the most significant, is a grade I recommendation for valve surgery in patients with severe secondary mitral regurgitation who remain symptomatic despite optimized medical therapy.
The second is a grade IIa recommendation for invasive procedures, such as a percutaneous coronary intervention, for patients with symptomatic secondary mitral regurgitation and coexisting coronary artery disease. In both cases, however, Dr. Praz emphasized language in the guidelines that calls for a collaborative heart team to agree on the suitability of these treatments.
Ultimately, none of these recommendations can be divorced from patient expectations and values, according to Friedhelm Beyersdorf, MD, chairman of the department of cardiovascular surgery at the Heart Center of the University of Freiburg (Germany).
Even if treatment is not expected to prolong life, “symptom relief on its own may justify intervention,” said Dr. Beyersdorf. However, he emphasized that “thoroughly informed” patients are an essential part of the process in selecting a treatment strategy most likely to satisfy patient goals.
Dr. Vahanian and Dr. Prendergast reported no conflicts of interest relevant to these guidelines. Dr. Praz reported a financial relationship with Edwards Lifesciences. Dr. Beyersdorf reported a financial relationship with Resuscitec.
New European guidelines for the management of valvular heart disease offer more than 45 revised or completely new recommendations relative to the previous version published in 2017, according to members of the writing committee who presented the changes during the annual congress of the European Society of Cardiology.
With their emphasis on early diagnosis and expansion of indications, these 2021 ESC/European Association for Cardio-Thoracic Surgery guidelines are likely to further accelerate the already steep growth in this area of interventional cardiology, according to Alec Vahanian, MD, a professor of cardiology at the University of Paris.
“Valvular heart disease is too often undetected, and these guidelines stress the importance of clinical examination and the best strategies for diagnosis as well as treatment,” he said.
Of the multiple sections and subsections, which follow the same format of the previous guidelines, the greatest number of revisions and new additions involve perioperative antithrombotic therapy, according to the document, which was published in conjunction with the ESC Congress.
Eleven new guidelines for anticoagulants
On the basis of evidence published since the previous guidelines, there are 11 completely new recommendations regarding the use of anticoagulants or antiplatelet therapies. The majority of these have received a grade I indication, which signifies “recommended” or “indicated.” These include indications for stopping or starting anticoagulants and which anticoagulants or antiplatelet drugs to consider in specific patient populations.
The next most common focus of new or revised recommendations involves when to consider surgical aortic valve repair (SAVR) relative to transcatheter aortic valve implantation (TAVI) in severe aortic stenosis. Most of these represent revisions from the previous guidelines, but almost all are also grade I recommendations.
“SAVR and TAVI are both excellent options in appropriate patients, but they are not interchangeable,” explained Bernard D. Prendergast, BMedSci, MD, director of the cardiac structural intervention program, Guy’s and St Thomas’ Hospital, London.
While the previous guidelines generally reserved TAVI for those not suitable for SAVR, the new guidelines are more nuanced.
As a rule, SAVR is generally preferred for younger patients. The reason, according to Dr. Prendergast, is concern that younger patients might outlive the expected lifespan of the prosthetic TAVI device.
No single criterion for selecting SAVR over TAVI
However, there are many exceptions and additional considerations beyond age. When both SAVR and TAVI are otherwise suitable options, but TAVI cannot be performed with a transfemoral access, Dr. Prendergast pointed out that SAVR might be a better choice.
Transfemoral access is the preferred strategy in TAVI, but Dr. Prendergast emphasized that a collaborative “heart team” should help patients select the most appropriate option. In fact, there is a grade IIb recommendation (“usefulness or efficacy is less well established”) to consider other access sites in patients at high surgical risk with contraindications for transfemoral TAVI.
Of new recommendations in the area of severe aortic stenosis, valvular repair may now be considered in asymptomatic patients with a left ventricular ejection fraction of less than 55%, according to a grade IIb recommendation. The 2017 guidelines did not address this issue.
Grade I recommendation for bioprostheses
A substantial number of the revisions involve minor clarifications without a change in the grade, but a revision regarding bioprosthetics is substantial. In the 2017 guidelines, there was a grade IIa recommendation (“weight of evidence is in favor”) to consider bioprosthetics in patients with a life expectancy less than the expected durability of the device. The 2021 guidelines have changed this to a grade I indication, adding an indication for bioprostheses in patients contraindicated for or unlikely to achieve good-quality anticoagulation.
On this same topic, there is a new grade IIb recommendation to consider bioprosthesis over alternative devices in patients already on a long-term non–vitamin K oral anticoagulant because of the high risk of thromboembolism.
There are two new recommendations in the realm of severe secondary mitral regurgitation, reported Fabien Praz, MD, an interventional cardiologist at the University of Bern (Switzerland). The first, which is perhaps the most significant, is a grade I recommendation for valve surgery in patients with severe secondary mitral regurgitation who remain symptomatic despite optimized medical therapy.
The second is a grade IIa recommendation for invasive procedures, such as a percutaneous coronary intervention, for patients with symptomatic secondary mitral regurgitation and coexisting coronary artery disease. In both cases, however, Dr. Praz emphasized language in the guidelines that calls for a collaborative heart team to agree on the suitability of these treatments.
Ultimately, none of these recommendations can be divorced from patient expectations and values, according to Friedhelm Beyersdorf, MD, chairman of the department of cardiovascular surgery at the Heart Center of the University of Freiburg (Germany).
Even if treatment is not expected to prolong life, “symptom relief on its own may justify intervention,” said Dr. Beyersdorf. However, he emphasized that “thoroughly informed” patients are an essential part of the process in selecting a treatment strategy most likely to satisfy patient goals.
Dr. Vahanian and Dr. Prendergast reported no conflicts of interest relevant to these guidelines. Dr. Praz reported a financial relationship with Edwards Lifesciences. Dr. Beyersdorf reported a financial relationship with Resuscitec.
FROM ESC 2021
ACST-2: Carotid stenting, surgery on par in asymptomatic patients
Carotid artery stenting (CAS) and carotid endarterectomy (CEA) provided comparable outcomes over time in asymptomatic patients receiving good medical therapy in the largest trial to date of what to do with severe carotid artery narrowing that is yet to cause a stroke.
Among more than 3,600 patients, stenting and surgery performed by experienced physicians involved a 1.0% risk for causing disabling stroke or death within 30 days.
The annual rate of fatal or disabling strokes was about 0.5% with either procedure over an average 5 years’ follow-up – essentially halving the annual stroke risk had neither procedure been performed, according to Alison Halliday, MD, principal investigator of the Asymptomatic Carotid Surgery Trial-2 (ACST-2).
The results were reported Aug. 29 in a Hot Line session at the virtual annual congress of the European Society of Cardiology and published simultaneously online in The Lancet.
Session chair Gilles Montalescot, MD, Sorbonne University, Paris, noted that ACST-2 doubled the number of randomly assigned patients with asymptomatic carotid stenosis studied in previous trials, “so, a huge contribution to the evidence base in this field and apparently good news for both revascularization techniques.”
Thirty-day and 5-year outcomes
The trial was conducted in 33 countries between January 2008 and December 2020, enrolling 3,625 patients (70% were male; mean age, 70 years) with carotid stenosis of at least 60% on ultrasonography, in whom stenting or surgery was suitable but both the doctor and patient were “substantially uncertain” which procedure to prefer.
Among the 1,811 patients assigned to stenting, 87% underwent the procedure at a median of 14 days; 6% crossed over to surgery, typically because of a highly calcified lesion or a more tortuous carotid than anticipated; and 6% had no intervention.
Among the 1,814 patients assigned to surgery, 92% had the procedure at a median of 14 days; 3% crossed over to stenting, typically because of patient or doctor preference or reluctance to undergo general anesthesia; and 4% had no intervention.
Patients without complications who had stenting stayed on average 1 day less than did those undergoing surgery.
During an earlier press briefing, Dr. Halliday highlighted the need for procedural competency and said doctors had to submit a record of their CEA or CAS experience and, consistent with current guidelines, had to demonstrate an independently verified stroke or death rate of 6% or less for symptomatic patients and 3% or lower for asymptomatic patients.
The results showed the 30-day risk for death, myocardial infarction (MI), or any stroke was 3.9% with carotid stenting and 3.2% with surgery (P = .26).
But with stenting, there was a slightly higher risk for procedural nondisabling strokes (48 vs. 29; P = .03), including 15 strokes vs. 5 strokes, respectively, that left patients with no residual symptoms. This is “consistent with large, recent nationally representative registry data,” observed Dr. Halliday, of the University of Oxford (England).
For those undergoing surgery, cranial nerve palsies were reported in 5.4% vs. no patients undergoing stenting.
At 5 years, the nonprocedural fatal or disabling stroke rate was 2.5% in each group (rate ratio [RR], 0.98; P = .91), with any nonprocedural stroke occurring in 5.3% of patients with stenting vs. 4.5% with surgery (RR, 1.16; P = .33).
The investigators performed a meta-analysis combining the ACST-2 results with those of eight prior trials (four in asymptomatic and four in symptomatic patients) that yielded a similar nonsignificant result for any nonprocedural stroke (RR, 1.11; P = .21).
Based on the results from ACST-2 plus the major trials, stenting and surgery involve “similar risks and similar benefits,” Dr. Halliday concluded.
Discussant Marco Roffi, MD, University Hospital of Geneva, said, “In centers with documented expertise, carotid artery stenting should be offered as an alternative to carotid endarterectomy in patients with asymptomatic stenosis and suitable anatomy.”
While the trial provides “good news” for patients, he pointed out that a reduction in the sample size from 5,000 to 3,625 limited the statistical power and that enrollment over a long period of time may have introduced confounders, such as changes in equipment technique, and medical therapy.
Also, many centers enrolled few patients, raising the concern over low-volume centers and operators, Dr. Roffi said. “We know that 8% of the centers enrolled 39% of the patients,” and “information on the credentialing and experience of the interventionalists was limited.”
Further, a lack of systematic MI assessment may have favored the surgery group, and more recent developments in stenting with the potential of reducing periprocedural stroke were rarely used, such as proximal emboli protection in only 15% and double-layer stents in 11%.
Friedhelm Beyersdorf, MD, University Hospital of Freiburg, Germany, said that, as a vascular surgeon, he finds it understandable that there might be a higher incidence of nonfatal strokes when treating carotid stenosis with stents, given the vulnerability of these lesions.
“Nevertheless, the main conclusion from the entire study is that carotid artery treatment is extremely safe, it has to be done in order to avoid strokes, and, obviously, there seems to be an advantage for surgery in terms of nondisabling stroke,” he said.
Session chair Dr. Montalescot, however, said that what the study cannot address – and what was the subject of many online audience comments – is whether either intervention should be performed in these patients.
Unlike earlier trials comparing interventions to medical therapy, Dr. Halliday said ACST-2 enrolled patients for whom the decision had been made that revascularization was needed. In addition, 99%-100% were receiving antithrombotic therapy at baseline, 85%-90% were receiving antihypertensives, and about 85% were taking statins.
Longer-term follow-up should provide a better picture of the nonprocedural stroke risk, with patients asked annually about exactly what medications and doses they are taking, she said.
“We will have an enormous list of exactly what’s gone on and the intensity of that therapy, which is, of course, much more intense than when we carried out our first trial. But these were people in whom a procedure was thought to be necessary,” she noted.
When asked during the press conference which procedure she would choose, Dr. Halliday, a surgeon, observed that patient preference is important but that the nature of the lesion itself often determines the optimal choice.
“If you know the competence of the people doing it is equal, then the less invasive procedure – providing it has good long-term viability, and that’s why we’re following for 10 years – is the more important,” she added.
The study was funded by the UK Medical Research Council and Health Technology Assessment Programme. Dr. Halliday reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Carotid artery stenting (CAS) and carotid endarterectomy (CEA) provided comparable outcomes over time in asymptomatic patients receiving good medical therapy in the largest trial to date of what to do with severe carotid artery narrowing that is yet to cause a stroke.
Among more than 3,600 patients, stenting and surgery performed by experienced physicians involved a 1.0% risk for causing disabling stroke or death within 30 days.
The annual rate of fatal or disabling strokes was about 0.5% with either procedure over an average 5 years’ follow-up – essentially halving the annual stroke risk had neither procedure been performed, according to Alison Halliday, MD, principal investigator of the Asymptomatic Carotid Surgery Trial-2 (ACST-2).
The results were reported Aug. 29 in a Hot Line session at the virtual annual congress of the European Society of Cardiology and published simultaneously online in The Lancet.
Session chair Gilles Montalescot, MD, Sorbonne University, Paris, noted that ACST-2 doubled the number of randomly assigned patients with asymptomatic carotid stenosis studied in previous trials, “so, a huge contribution to the evidence base in this field and apparently good news for both revascularization techniques.”
Thirty-day and 5-year outcomes
The trial was conducted in 33 countries between January 2008 and December 2020, enrolling 3,625 patients (70% were male; mean age, 70 years) with carotid stenosis of at least 60% on ultrasonography, in whom stenting or surgery was suitable but both the doctor and patient were “substantially uncertain” which procedure to prefer.
Among the 1,811 patients assigned to stenting, 87% underwent the procedure at a median of 14 days; 6% crossed over to surgery, typically because of a highly calcified lesion or a more tortuous carotid than anticipated; and 6% had no intervention.
Among the 1,814 patients assigned to surgery, 92% had the procedure at a median of 14 days; 3% crossed over to stenting, typically because of patient or doctor preference or reluctance to undergo general anesthesia; and 4% had no intervention.
Patients without complications who had stenting stayed on average 1 day less than did those undergoing surgery.
During an earlier press briefing, Dr. Halliday highlighted the need for procedural competency and said doctors had to submit a record of their CEA or CAS experience and, consistent with current guidelines, had to demonstrate an independently verified stroke or death rate of 6% or less for symptomatic patients and 3% or lower for asymptomatic patients.
The results showed the 30-day risk for death, myocardial infarction (MI), or any stroke was 3.9% with carotid stenting and 3.2% with surgery (P = .26).
But with stenting, there was a slightly higher risk for procedural nondisabling strokes (48 vs. 29; P = .03), including 15 strokes vs. 5 strokes, respectively, that left patients with no residual symptoms. This is “consistent with large, recent nationally representative registry data,” observed Dr. Halliday, of the University of Oxford (England).
For those undergoing surgery, cranial nerve palsies were reported in 5.4% vs. no patients undergoing stenting.
At 5 years, the nonprocedural fatal or disabling stroke rate was 2.5% in each group (rate ratio [RR], 0.98; P = .91), with any nonprocedural stroke occurring in 5.3% of patients with stenting vs. 4.5% with surgery (RR, 1.16; P = .33).
The investigators performed a meta-analysis combining the ACST-2 results with those of eight prior trials (four in asymptomatic and four in symptomatic patients) that yielded a similar nonsignificant result for any nonprocedural stroke (RR, 1.11; P = .21).
Based on the results from ACST-2 plus the major trials, stenting and surgery involve “similar risks and similar benefits,” Dr. Halliday concluded.
Discussant Marco Roffi, MD, University Hospital of Geneva, said, “In centers with documented expertise, carotid artery stenting should be offered as an alternative to carotid endarterectomy in patients with asymptomatic stenosis and suitable anatomy.”
While the trial provides “good news” for patients, he pointed out that a reduction in the sample size from 5,000 to 3,625 limited the statistical power and that enrollment over a long period of time may have introduced confounders, such as changes in equipment technique, and medical therapy.
Also, many centers enrolled few patients, raising the concern over low-volume centers and operators, Dr. Roffi said. “We know that 8% of the centers enrolled 39% of the patients,” and “information on the credentialing and experience of the interventionalists was limited.”
Further, a lack of systematic MI assessment may have favored the surgery group, and more recent developments in stenting with the potential of reducing periprocedural stroke were rarely used, such as proximal emboli protection in only 15% and double-layer stents in 11%.
Friedhelm Beyersdorf, MD, University Hospital of Freiburg, Germany, said that, as a vascular surgeon, he finds it understandable that there might be a higher incidence of nonfatal strokes when treating carotid stenosis with stents, given the vulnerability of these lesions.
“Nevertheless, the main conclusion from the entire study is that carotid artery treatment is extremely safe, it has to be done in order to avoid strokes, and, obviously, there seems to be an advantage for surgery in terms of nondisabling stroke,” he said.
Session chair Dr. Montalescot, however, said that what the study cannot address – and what was the subject of many online audience comments – is whether either intervention should be performed in these patients.
Unlike earlier trials comparing interventions to medical therapy, Dr. Halliday said ACST-2 enrolled patients for whom the decision had been made that revascularization was needed. In addition, 99%-100% were receiving antithrombotic therapy at baseline, 85%-90% were receiving antihypertensives, and about 85% were taking statins.
Longer-term follow-up should provide a better picture of the nonprocedural stroke risk, with patients asked annually about exactly what medications and doses they are taking, she said.
“We will have an enormous list of exactly what’s gone on and the intensity of that therapy, which is, of course, much more intense than when we carried out our first trial. But these were people in whom a procedure was thought to be necessary,” she noted.
When asked during the press conference which procedure she would choose, Dr. Halliday, a surgeon, observed that patient preference is important but that the nature of the lesion itself often determines the optimal choice.
“If you know the competence of the people doing it is equal, then the less invasive procedure – providing it has good long-term viability, and that’s why we’re following for 10 years – is the more important,” she added.
The study was funded by the UK Medical Research Council and Health Technology Assessment Programme. Dr. Halliday reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Carotid artery stenting (CAS) and carotid endarterectomy (CEA) provided comparable outcomes over time in asymptomatic patients receiving good medical therapy in the largest trial to date of what to do with severe carotid artery narrowing that is yet to cause a stroke.
Among more than 3,600 patients, stenting and surgery performed by experienced physicians involved a 1.0% risk for causing disabling stroke or death within 30 days.
The annual rate of fatal or disabling strokes was about 0.5% with either procedure over an average 5 years’ follow-up – essentially halving the annual stroke risk had neither procedure been performed, according to Alison Halliday, MD, principal investigator of the Asymptomatic Carotid Surgery Trial-2 (ACST-2).
The results were reported Aug. 29 in a Hot Line session at the virtual annual congress of the European Society of Cardiology and published simultaneously online in The Lancet.
Session chair Gilles Montalescot, MD, Sorbonne University, Paris, noted that ACST-2 doubled the number of randomly assigned patients with asymptomatic carotid stenosis studied in previous trials, “so, a huge contribution to the evidence base in this field and apparently good news for both revascularization techniques.”
Thirty-day and 5-year outcomes
The trial was conducted in 33 countries between January 2008 and December 2020, enrolling 3,625 patients (70% were male; mean age, 70 years) with carotid stenosis of at least 60% on ultrasonography, in whom stenting or surgery was suitable but both the doctor and patient were “substantially uncertain” which procedure to prefer.
Among the 1,811 patients assigned to stenting, 87% underwent the procedure at a median of 14 days; 6% crossed over to surgery, typically because of a highly calcified lesion or a more tortuous carotid than anticipated; and 6% had no intervention.
Among the 1,814 patients assigned to surgery, 92% had the procedure at a median of 14 days; 3% crossed over to stenting, typically because of patient or doctor preference or reluctance to undergo general anesthesia; and 4% had no intervention.
Patients without complications who had stenting stayed on average 1 day less than did those undergoing surgery.
During an earlier press briefing, Dr. Halliday highlighted the need for procedural competency and said doctors had to submit a record of their CEA or CAS experience and, consistent with current guidelines, had to demonstrate an independently verified stroke or death rate of 6% or less for symptomatic patients and 3% or lower for asymptomatic patients.
The results showed the 30-day risk for death, myocardial infarction (MI), or any stroke was 3.9% with carotid stenting and 3.2% with surgery (P = .26).
But with stenting, there was a slightly higher risk for procedural nondisabling strokes (48 vs. 29; P = .03), including 15 strokes vs. 5 strokes, respectively, that left patients with no residual symptoms. This is “consistent with large, recent nationally representative registry data,” observed Dr. Halliday, of the University of Oxford (England).
For those undergoing surgery, cranial nerve palsies were reported in 5.4% vs. no patients undergoing stenting.
At 5 years, the nonprocedural fatal or disabling stroke rate was 2.5% in each group (rate ratio [RR], 0.98; P = .91), with any nonprocedural stroke occurring in 5.3% of patients with stenting vs. 4.5% with surgery (RR, 1.16; P = .33).
The investigators performed a meta-analysis combining the ACST-2 results with those of eight prior trials (four in asymptomatic and four in symptomatic patients) that yielded a similar nonsignificant result for any nonprocedural stroke (RR, 1.11; P = .21).
Based on the results from ACST-2 plus the major trials, stenting and surgery involve “similar risks and similar benefits,” Dr. Halliday concluded.
Discussant Marco Roffi, MD, University Hospital of Geneva, said, “In centers with documented expertise, carotid artery stenting should be offered as an alternative to carotid endarterectomy in patients with asymptomatic stenosis and suitable anatomy.”
While the trial provides “good news” for patients, he pointed out that a reduction in the sample size from 5,000 to 3,625 limited the statistical power and that enrollment over a long period of time may have introduced confounders, such as changes in equipment technique, and medical therapy.
Also, many centers enrolled few patients, raising the concern over low-volume centers and operators, Dr. Roffi said. “We know that 8% of the centers enrolled 39% of the patients,” and “information on the credentialing and experience of the interventionalists was limited.”
Further, a lack of systematic MI assessment may have favored the surgery group, and more recent developments in stenting with the potential of reducing periprocedural stroke were rarely used, such as proximal emboli protection in only 15% and double-layer stents in 11%.
Friedhelm Beyersdorf, MD, University Hospital of Freiburg, Germany, said that, as a vascular surgeon, he finds it understandable that there might be a higher incidence of nonfatal strokes when treating carotid stenosis with stents, given the vulnerability of these lesions.
“Nevertheless, the main conclusion from the entire study is that carotid artery treatment is extremely safe, it has to be done in order to avoid strokes, and, obviously, there seems to be an advantage for surgery in terms of nondisabling stroke,” he said.
Session chair Dr. Montalescot, however, said that what the study cannot address – and what was the subject of many online audience comments – is whether either intervention should be performed in these patients.
Unlike earlier trials comparing interventions to medical therapy, Dr. Halliday said ACST-2 enrolled patients for whom the decision had been made that revascularization was needed. In addition, 99%-100% were receiving antithrombotic therapy at baseline, 85%-90% were receiving antihypertensives, and about 85% were taking statins.
Longer-term follow-up should provide a better picture of the nonprocedural stroke risk, with patients asked annually about exactly what medications and doses they are taking, she said.
“We will have an enormous list of exactly what’s gone on and the intensity of that therapy, which is, of course, much more intense than when we carried out our first trial. But these were people in whom a procedure was thought to be necessary,” she noted.
When asked during the press conference which procedure she would choose, Dr. Halliday, a surgeon, observed that patient preference is important but that the nature of the lesion itself often determines the optimal choice.
“If you know the competence of the people doing it is equal, then the less invasive procedure – providing it has good long-term viability, and that’s why we’re following for 10 years – is the more important,” she added.
The study was funded by the UK Medical Research Council and Health Technology Assessment Programme. Dr. Halliday reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Angiography can wait for cardiac arrest without ST-elevation
A protocol of immediate angiography provided no mortality benefit over a strategy or delayed or more selective angiography among patients resuscitated from out-of-hospital cardiac arrest and without ST-segment elevation, new randomized results show.
“Among patients with resuscitated out-of-hospital cardiac arrest of possible cardiac origin, with shockable and nonshockable arrest rhythm and no ST-elevation, a strategy of immediate, unselected coronary angiography was not found to be beneficial over a delayed and selective approach with regard to the 30-day risk of all-cause death,” concluded principal investigator Steffen Desch, MD, University of Leipzig (Germany) Heart Center.
The results support previous results of the Coronary Angiography after Cardiac Arrest (COACT) trial, in patients with shockable rhythms, which also showed no differences in clinical outcomes between immediate and delayed coronary angiography at both 90 days and 1 year, he noted.
“What the clinicians wanted to know is, is it really necessary to get up at 3 a.m. in the morning to perform a coronary angiography on these patients, and that’s certainly out,” Dr. Desch said in an interview. “So, there’s really no room for this strategy anymore. You can take your time and wait a day or 2.”
These findings, from the TOMAHAWK trial, were presented Aug. 29 at the annual congress of the European Society of Cardiology and simultaneously published online in the New England Journal of Medicine.
Larger group without ST-segment elevation
Prognosis after out-of-hospital cardiac arrest is extremely poor, with an overall survival rate of less than 10%, Dr. Desch noted. “Actually, only 20% make it to the hospital; the vast majority of these patients die out in the field, so there’s really a great need in improving treatment.”
Acute coronary syndrome accounts for up to 60% of out-of-hospital arrests in which a cardiac cause has been identified, the authors wrote in their report. ST-segment elevation on postresuscitation electrocardiography “has good positive predictive value” for acute coronary lesions triggering the arrest, but in the far larger subgroup of patients without ST-segment elevation, “the spectrum of underlying causes is considerably broader and includes both cardiac and noncardiac causes.”
In patients with myocardial infarction, early revascularization would prevent negative consequences of myocardial injury, but unselected early coronary angiography would put patients not having an MI at unnecessary risk for procedural complications or delay in the diagnosis of the actual cause of their arrest, they noted.
In this trial, the researchers randomly assigned 554 patients from 31 sites in Germany and Denmark who were successfully resuscitated after cardiac arrest of possible cardiac origin to immediate transfer for coronary angiography or to initial intensive care assessment with delayed or selective angiography after a minimum delay of at least 1 day.
In the end, the average delay in this arm was 2 days, Dr. Desch noted. If the clinical course indicated that a coronary cause was unlikely, angiography might not be performed at all in this group.
No patient had ST-segment elevation on postresuscitation electrocardiography. The primary endpoint was death from any cause at 30 days; secondary end points were death from any cause or severe neurologic deficit at 30 days.
Results showed that 95% of patients in the immediate angiography group actually underwent the procedure, compared with 62% of those in the delayed group, a finding that was “logical” given the study design, he said.
At 30 days, 54% of patients in the immediate angiography group and 46% in the delayed group had died, a nonsignificant difference (P = .06). Because the researchers had performed an interim analysis, Dr. Desch explained, the final P value for significance in this trial was not .05, but rather .034, to account for multiple comparisons.
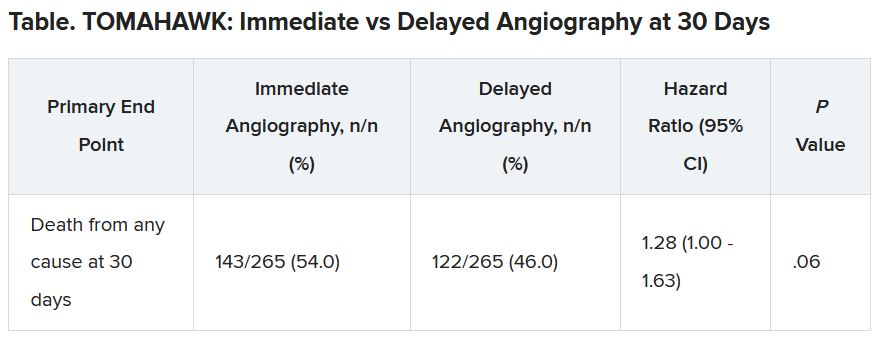
The secondary end point of death from any cause or severe neurologic deficit at 30 days “was actually nominally significant in favor of the delayed group,” he said. “So, this is not corrected for multiple testing, it’s just a hypothesis that’s in the room, but it’s certainly worthy of discussion that the immediate strategy might actually cause harm.”
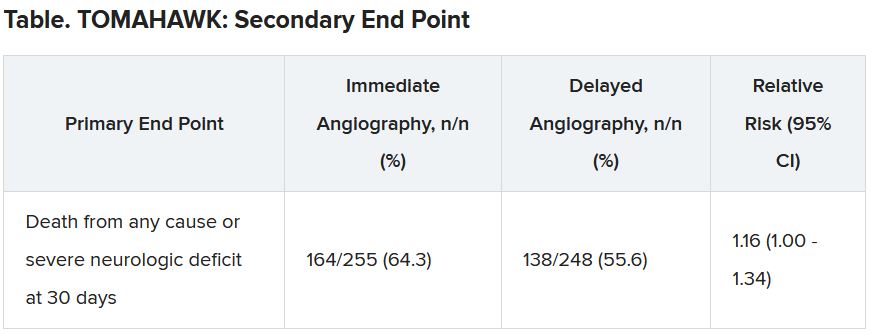
There was no difference between the groups in peak release of myocardial enzymes, or any other safety end points, including bleeding, stroke, or renal failure, Dr. Desch said.
Further analyses showed no large differences between subgroups, including age, diabetes, first monitored rhythm, confirmed MI as the trigger of the arrest, sex, and the time from cardiac arrest to the return of spontaneous circulation, he noted.
Opportunity to minimize harm
Discussant for the results during the presentation was Susanna Price, MBBS, PhD, Royal Brompton Hospital, London.
Dr. Price concluded: “What this means for me, is it gives me information that’s useful regarding the opportunity to minimize harm, which is a lot of what critical care is about, so we don’t necessarily now have to move these patients very acutely when they’ve just come in through the ED [emergency department]. It has implications for resource utilization, but also implications for mobilizing patients around the hospital during COVID-19.”
It’s also important to note that coronary angiography was still carried out in certain patients, “so we still have to have that dialogue with our interventional cardiologists for certain patients who may need to go to the cath lab, and what it should now allow us to do is give appropriate focus to how to manage these patients when they come in to the ED or to our ICUs [intensive care units],” she said.
Dr. Price added, though, that perhaps “the most important slide” in the presentation was that showing 90% of these patients had a witnessed cardiac arrest, “and yet a third of these patients, 168 of them, had no bystander CPR at all.”
She pointed to the “chain of survival” after cardiac arrest, of which Charles D. Deakin, MD, University Hospital Southampton (England), wrote that “not all links are equal.”
“Early recognition and calling for help, early CPR, early defibrillation where appropriate are very, very important, and we need to be addressing all of these, as well as what happens in the cath lab and after admission,” Dr. Price said.
This research was funded by the German Center for Cardiovascular Research. Dr. Desch and Dr. Price reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
A protocol of immediate angiography provided no mortality benefit over a strategy or delayed or more selective angiography among patients resuscitated from out-of-hospital cardiac arrest and without ST-segment elevation, new randomized results show.
“Among patients with resuscitated out-of-hospital cardiac arrest of possible cardiac origin, with shockable and nonshockable arrest rhythm and no ST-elevation, a strategy of immediate, unselected coronary angiography was not found to be beneficial over a delayed and selective approach with regard to the 30-day risk of all-cause death,” concluded principal investigator Steffen Desch, MD, University of Leipzig (Germany) Heart Center.
The results support previous results of the Coronary Angiography after Cardiac Arrest (COACT) trial, in patients with shockable rhythms, which also showed no differences in clinical outcomes between immediate and delayed coronary angiography at both 90 days and 1 year, he noted.
“What the clinicians wanted to know is, is it really necessary to get up at 3 a.m. in the morning to perform a coronary angiography on these patients, and that’s certainly out,” Dr. Desch said in an interview. “So, there’s really no room for this strategy anymore. You can take your time and wait a day or 2.”
These findings, from the TOMAHAWK trial, were presented Aug. 29 at the annual congress of the European Society of Cardiology and simultaneously published online in the New England Journal of Medicine.
Larger group without ST-segment elevation
Prognosis after out-of-hospital cardiac arrest is extremely poor, with an overall survival rate of less than 10%, Dr. Desch noted. “Actually, only 20% make it to the hospital; the vast majority of these patients die out in the field, so there’s really a great need in improving treatment.”
Acute coronary syndrome accounts for up to 60% of out-of-hospital arrests in which a cardiac cause has been identified, the authors wrote in their report. ST-segment elevation on postresuscitation electrocardiography “has good positive predictive value” for acute coronary lesions triggering the arrest, but in the far larger subgroup of patients without ST-segment elevation, “the spectrum of underlying causes is considerably broader and includes both cardiac and noncardiac causes.”
In patients with myocardial infarction, early revascularization would prevent negative consequences of myocardial injury, but unselected early coronary angiography would put patients not having an MI at unnecessary risk for procedural complications or delay in the diagnosis of the actual cause of their arrest, they noted.
In this trial, the researchers randomly assigned 554 patients from 31 sites in Germany and Denmark who were successfully resuscitated after cardiac arrest of possible cardiac origin to immediate transfer for coronary angiography or to initial intensive care assessment with delayed or selective angiography after a minimum delay of at least 1 day.
In the end, the average delay in this arm was 2 days, Dr. Desch noted. If the clinical course indicated that a coronary cause was unlikely, angiography might not be performed at all in this group.
No patient had ST-segment elevation on postresuscitation electrocardiography. The primary endpoint was death from any cause at 30 days; secondary end points were death from any cause or severe neurologic deficit at 30 days.
Results showed that 95% of patients in the immediate angiography group actually underwent the procedure, compared with 62% of those in the delayed group, a finding that was “logical” given the study design, he said.
At 30 days, 54% of patients in the immediate angiography group and 46% in the delayed group had died, a nonsignificant difference (P = .06). Because the researchers had performed an interim analysis, Dr. Desch explained, the final P value for significance in this trial was not .05, but rather .034, to account for multiple comparisons.

The secondary end point of death from any cause or severe neurologic deficit at 30 days “was actually nominally significant in favor of the delayed group,” he said. “So, this is not corrected for multiple testing, it’s just a hypothesis that’s in the room, but it’s certainly worthy of discussion that the immediate strategy might actually cause harm.”

There was no difference between the groups in peak release of myocardial enzymes, or any other safety end points, including bleeding, stroke, or renal failure, Dr. Desch said.
Further analyses showed no large differences between subgroups, including age, diabetes, first monitored rhythm, confirmed MI as the trigger of the arrest, sex, and the time from cardiac arrest to the return of spontaneous circulation, he noted.
Opportunity to minimize harm
Discussant for the results during the presentation was Susanna Price, MBBS, PhD, Royal Brompton Hospital, London.
Dr. Price concluded: “What this means for me, is it gives me information that’s useful regarding the opportunity to minimize harm, which is a lot of what critical care is about, so we don’t necessarily now have to move these patients very acutely when they’ve just come in through the ED [emergency department]. It has implications for resource utilization, but also implications for mobilizing patients around the hospital during COVID-19.”
It’s also important to note that coronary angiography was still carried out in certain patients, “so we still have to have that dialogue with our interventional cardiologists for certain patients who may need to go to the cath lab, and what it should now allow us to do is give appropriate focus to how to manage these patients when they come in to the ED or to our ICUs [intensive care units],” she said.
Dr. Price added, though, that perhaps “the most important slide” in the presentation was that showing 90% of these patients had a witnessed cardiac arrest, “and yet a third of these patients, 168 of them, had no bystander CPR at all.”
She pointed to the “chain of survival” after cardiac arrest, of which Charles D. Deakin, MD, University Hospital Southampton (England), wrote that “not all links are equal.”
“Early recognition and calling for help, early CPR, early defibrillation where appropriate are very, very important, and we need to be addressing all of these, as well as what happens in the cath lab and after admission,” Dr. Price said.
This research was funded by the German Center for Cardiovascular Research. Dr. Desch and Dr. Price reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
A protocol of immediate angiography provided no mortality benefit over a strategy or delayed or more selective angiography among patients resuscitated from out-of-hospital cardiac arrest and without ST-segment elevation, new randomized results show.
“Among patients with resuscitated out-of-hospital cardiac arrest of possible cardiac origin, with shockable and nonshockable arrest rhythm and no ST-elevation, a strategy of immediate, unselected coronary angiography was not found to be beneficial over a delayed and selective approach with regard to the 30-day risk of all-cause death,” concluded principal investigator Steffen Desch, MD, University of Leipzig (Germany) Heart Center.
The results support previous results of the Coronary Angiography after Cardiac Arrest (COACT) trial, in patients with shockable rhythms, which also showed no differences in clinical outcomes between immediate and delayed coronary angiography at both 90 days and 1 year, he noted.
“What the clinicians wanted to know is, is it really necessary to get up at 3 a.m. in the morning to perform a coronary angiography on these patients, and that’s certainly out,” Dr. Desch said in an interview. “So, there’s really no room for this strategy anymore. You can take your time and wait a day or 2.”
These findings, from the TOMAHAWK trial, were presented Aug. 29 at the annual congress of the European Society of Cardiology and simultaneously published online in the New England Journal of Medicine.
Larger group without ST-segment elevation
Prognosis after out-of-hospital cardiac arrest is extremely poor, with an overall survival rate of less than 10%, Dr. Desch noted. “Actually, only 20% make it to the hospital; the vast majority of these patients die out in the field, so there’s really a great need in improving treatment.”
Acute coronary syndrome accounts for up to 60% of out-of-hospital arrests in which a cardiac cause has been identified, the authors wrote in their report. ST-segment elevation on postresuscitation electrocardiography “has good positive predictive value” for acute coronary lesions triggering the arrest, but in the far larger subgroup of patients without ST-segment elevation, “the spectrum of underlying causes is considerably broader and includes both cardiac and noncardiac causes.”
In patients with myocardial infarction, early revascularization would prevent negative consequences of myocardial injury, but unselected early coronary angiography would put patients not having an MI at unnecessary risk for procedural complications or delay in the diagnosis of the actual cause of their arrest, they noted.
In this trial, the researchers randomly assigned 554 patients from 31 sites in Germany and Denmark who were successfully resuscitated after cardiac arrest of possible cardiac origin to immediate transfer for coronary angiography or to initial intensive care assessment with delayed or selective angiography after a minimum delay of at least 1 day.
In the end, the average delay in this arm was 2 days, Dr. Desch noted. If the clinical course indicated that a coronary cause was unlikely, angiography might not be performed at all in this group.
No patient had ST-segment elevation on postresuscitation electrocardiography. The primary endpoint was death from any cause at 30 days; secondary end points were death from any cause or severe neurologic deficit at 30 days.
Results showed that 95% of patients in the immediate angiography group actually underwent the procedure, compared with 62% of those in the delayed group, a finding that was “logical” given the study design, he said.
At 30 days, 54% of patients in the immediate angiography group and 46% in the delayed group had died, a nonsignificant difference (P = .06). Because the researchers had performed an interim analysis, Dr. Desch explained, the final P value for significance in this trial was not .05, but rather .034, to account for multiple comparisons.

The secondary end point of death from any cause or severe neurologic deficit at 30 days “was actually nominally significant in favor of the delayed group,” he said. “So, this is not corrected for multiple testing, it’s just a hypothesis that’s in the room, but it’s certainly worthy of discussion that the immediate strategy might actually cause harm.”

There was no difference between the groups in peak release of myocardial enzymes, or any other safety end points, including bleeding, stroke, or renal failure, Dr. Desch said.
Further analyses showed no large differences between subgroups, including age, diabetes, first monitored rhythm, confirmed MI as the trigger of the arrest, sex, and the time from cardiac arrest to the return of spontaneous circulation, he noted.
Opportunity to minimize harm
Discussant for the results during the presentation was Susanna Price, MBBS, PhD, Royal Brompton Hospital, London.
Dr. Price concluded: “What this means for me, is it gives me information that’s useful regarding the opportunity to minimize harm, which is a lot of what critical care is about, so we don’t necessarily now have to move these patients very acutely when they’ve just come in through the ED [emergency department]. It has implications for resource utilization, but also implications for mobilizing patients around the hospital during COVID-19.”
It’s also important to note that coronary angiography was still carried out in certain patients, “so we still have to have that dialogue with our interventional cardiologists for certain patients who may need to go to the cath lab, and what it should now allow us to do is give appropriate focus to how to manage these patients when they come in to the ED or to our ICUs [intensive care units],” she said.
Dr. Price added, though, that perhaps “the most important slide” in the presentation was that showing 90% of these patients had a witnessed cardiac arrest, “and yet a third of these patients, 168 of them, had no bystander CPR at all.”
She pointed to the “chain of survival” after cardiac arrest, of which Charles D. Deakin, MD, University Hospital Southampton (England), wrote that “not all links are equal.”
“Early recognition and calling for help, early CPR, early defibrillation where appropriate are very, very important, and we need to be addressing all of these, as well as what happens in the cath lab and after admission,” Dr. Price said.
This research was funded by the German Center for Cardiovascular Research. Dr. Desch and Dr. Price reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
LOOP trial undercuts value of long-term continuous ECG screening for AFib
Perhaps short, asymptomatic bouts of atrial fibrillation (AFib) that show up on long-term, continuous monitoring aren’t worth hunting for just so oral anticoagulation (OAC) can be started, even in elderly people with other stroke risk factors.
That’s a potential message from a randomized trial that tested an AFib screening strategy relying on an implantable loop recorder (ILR) in older adults without AFib but with other stroke risk factors who were invited to participate. OAC was recommended to any participant found with even a short bout of the arrhythmia (that is, any lasting 6 minutes or longer).
More than three times as many in the monitoring group compared to a standard-care cohort were found to have AFib, and nearly all were put on OAC. In fact, monitored participants were almost three times as likely to be put on OAC (P < .0001) compared with controls.
But it didn’t make any apparent difference to outcomes. The risk for stroke or systemic embolism did not significantly differ between the two groups over more than 5 years in the trial of about 6,000 participants, called LOOP.
“This result was seen despite a high proportion of atrial fibrillation detection, and a high acceptance of anticoagulation therapy, and might imply that not all atrial fibrillation is worth screening for, and not all screen-detected atrial fibrillation merits anticoagulation,” contend the authors of the LOOP report, simultaneously published in The Lancet and presented Aug. 29 at the virtual European Society of Cardiology (ESC) Congress 2021.
“The rates of bleeding were modest, despite the low threshold for anticoagulation,” and was not significantly different between the two groups, Jesper H. Svendsen, MD, DMSc, Copenhagen University Hospital, Denmark, said at a media briefing before his presentation of the trial at the congress. He is lead author on the Lancet report.
At least 6 minutes of AFib was identified in more than 30% of the ILR-monitored patients, and about 90% of those were started on OAC, Dr. Svendsen observed.
But one take-home message from LOOP, he said in an interview, is that “short-lasting episodes” of AFib do not necessarily pose an untoward risk for stroke compared with AFib revealed by intermittent monitoring, which “primarily identifies longer-lasting atrial fibrillation episodes. So short-lasting episodes are probably not as serious as long-lasting.”
The LOOP trial “teaches us that perhaps short-lasting asymptomatic episodes may not benefit from being screened or found,” said Stefan James, MD, PhD, Uppsala University, Sweden. However, that may not be the case when the monitored individual is symptomatic or has longer-lasting AFib episodes, he said in an interview. “But certainly, this study teaches us that we need to understand much better the relationship between short episodes versus symptoms versus medical outcomes.”
In LOOP, 6,004 people aged 70-90 years without AFib but with at least one other stroke risk factor, which could include hypertension, diabetes, a history of stroke, or heart failure, were implanted with an ILR, the Reveal LINQ (Medtronic).
They were randomly assigned at four centers in Denmark to a monitoring group or a usual care group in a 1:3 ratio. Overwhelmingly, most had hypertension. Almost half the population were women.
OAC was recommended for all persons in the monitoring group who showed an episode of AFib lasting at least 6 minutes.
Atrial fibrillation was diagnosed in 31.8% of the 1,501 participants in the monitored group and 12.2% of the 4,503 assigned to usual care, for a hazard ratio (HR) of 3.17 (95% confidence interval, 2.81-3.59; P < .0001).
OAC was started in 29.7% of monitored participants and 13.1% of the control cohort, for an HR of 2.72 (95% CI, 2.41-3.08; P < .0001).
There were 315 strokes and three systemic arterial embolisms observed in the entire trial, for primary endpoint rates of 4.5% in the ILR monitoring group and 5.6% in the control group (HR, 0.80; 95% CI, 0.61-1.05; P = .11). Adding transient ischemic attack (TIA) or cardiovascular death to the endpoint did not make for a significant difference. The rates of major bleeding were 4.3% and 3.5%, respectively (P = .11).
“In general, the findings were consistent across subgroups,” including by age, sex, diabetes and heart failure status, stroke history, antiplatelet therapy, renal function, and even CHA2DS2–VASc score, Dr. Svendsen noted.
But, he said, participants in the highest tertile for baseline systolic blood pressure (BP), at least 157 mm Hg, “seemed to benefit from being screened,” with a 49% reduction in risk for the primary endpoint (P = .0066). The interaction between systolic BP and outcome was significant (P = .007).
Only 9.3% of participants in LOOP did not have a baseline diagnosis of hypertension and so had to have another risk factor to enroll, the published report notes. However, the significant interaction with systolic BP “suggests that patients with dysregulated hypertension could benefit from this type of screening and concomitant anticoagulation.”
“There is a tight association between our primary endpoint and hypertension,” Dr. Svendsen said in an interview. “But I think it’s very important to say that this subgroup analysis is only hypothesis-generating.”
An editorial accompanying the LOOP publication suggests, in line with Dr. Svendsen’s proposal, that “shorter atrial fibrillation episodes found by long-term ILRs might not have the same stroke risk as atrial fibrillation detected through single-timepoint or less intense monitoring.”
If much of the paroxysmal AFib observed in LOOP and other studies with similar monitoring methods “is not the actual cause of stroke and is instead predominantly a risk marker, further research is warranted to establish whether a different screening focus and treatment paradigm are required to prevent stroke and other vascular brain injury related to atrial fibrillation,” wrote editorialists Ben Freedman, MBBS, PhD, and Nicole Lowres, BPhty, PhD, University of Sydney, Australia.
LOOP was partially supported by Medtronic. Dr. Svendsen is a member of Medtronic advisory boards and has received speaker honoraria and research grants from Medtronic in relation to this work and outside the submitted work. Disclosures for the other authors are in the report. Dr. Freedman reports grants to the Heart Research Institute, speakers fees and nonfinancial support from the Bristol-Myers Squibb–Pfizer Alliance, speakers fees and nonfinancial support from Daiichi Sankyo, nonfinancial support from AliveCor, and speakers fees and nonfinancial support from Omron unrelated to the topic of the editorial but related to atrial fibrillation and screening for atrial fibrillation. Dr. Lowres reports grants to the Heart Research Institute from the Bristol-Myers Squibb–Pfizer Alliance unrelated to the topic of the editorial but related to atrial fibrillation and screening for atrial fibrillation.
A version of this article first appeared on Medscape.com.
Perhaps short, asymptomatic bouts of atrial fibrillation (AFib) that show up on long-term, continuous monitoring aren’t worth hunting for just so oral anticoagulation (OAC) can be started, even in elderly people with other stroke risk factors.
That’s a potential message from a randomized trial that tested an AFib screening strategy relying on an implantable loop recorder (ILR) in older adults without AFib but with other stroke risk factors who were invited to participate. OAC was recommended to any participant found with even a short bout of the arrhythmia (that is, any lasting 6 minutes or longer).
More than three times as many in the monitoring group compared to a standard-care cohort were found to have AFib, and nearly all were put on OAC. In fact, monitored participants were almost three times as likely to be put on OAC (P < .0001) compared with controls.
But it didn’t make any apparent difference to outcomes. The risk for stroke or systemic embolism did not significantly differ between the two groups over more than 5 years in the trial of about 6,000 participants, called LOOP.
“This result was seen despite a high proportion of atrial fibrillation detection, and a high acceptance of anticoagulation therapy, and might imply that not all atrial fibrillation is worth screening for, and not all screen-detected atrial fibrillation merits anticoagulation,” contend the authors of the LOOP report, simultaneously published in The Lancet and presented Aug. 29 at the virtual European Society of Cardiology (ESC) Congress 2021.
“The rates of bleeding were modest, despite the low threshold for anticoagulation,” and was not significantly different between the two groups, Jesper H. Svendsen, MD, DMSc, Copenhagen University Hospital, Denmark, said at a media briefing before his presentation of the trial at the congress. He is lead author on the Lancet report.
At least 6 minutes of AFib was identified in more than 30% of the ILR-monitored patients, and about 90% of those were started on OAC, Dr. Svendsen observed.
But one take-home message from LOOP, he said in an interview, is that “short-lasting episodes” of AFib do not necessarily pose an untoward risk for stroke compared with AFib revealed by intermittent monitoring, which “primarily identifies longer-lasting atrial fibrillation episodes. So short-lasting episodes are probably not as serious as long-lasting.”
The LOOP trial “teaches us that perhaps short-lasting asymptomatic episodes may not benefit from being screened or found,” said Stefan James, MD, PhD, Uppsala University, Sweden. However, that may not be the case when the monitored individual is symptomatic or has longer-lasting AFib episodes, he said in an interview. “But certainly, this study teaches us that we need to understand much better the relationship between short episodes versus symptoms versus medical outcomes.”
In LOOP, 6,004 people aged 70-90 years without AFib but with at least one other stroke risk factor, which could include hypertension, diabetes, a history of stroke, or heart failure, were implanted with an ILR, the Reveal LINQ (Medtronic).
They were randomly assigned at four centers in Denmark to a monitoring group or a usual care group in a 1:3 ratio. Overwhelmingly, most had hypertension. Almost half the population were women.
OAC was recommended for all persons in the monitoring group who showed an episode of AFib lasting at least 6 minutes.
Atrial fibrillation was diagnosed in 31.8% of the 1,501 participants in the monitored group and 12.2% of the 4,503 assigned to usual care, for a hazard ratio (HR) of 3.17 (95% confidence interval, 2.81-3.59; P < .0001).
OAC was started in 29.7% of monitored participants and 13.1% of the control cohort, for an HR of 2.72 (95% CI, 2.41-3.08; P < .0001).
There were 315 strokes and three systemic arterial embolisms observed in the entire trial, for primary endpoint rates of 4.5% in the ILR monitoring group and 5.6% in the control group (HR, 0.80; 95% CI, 0.61-1.05; P = .11). Adding transient ischemic attack (TIA) or cardiovascular death to the endpoint did not make for a significant difference. The rates of major bleeding were 4.3% and 3.5%, respectively (P = .11).
“In general, the findings were consistent across subgroups,” including by age, sex, diabetes and heart failure status, stroke history, antiplatelet therapy, renal function, and even CHA2DS2–VASc score, Dr. Svendsen noted.
But, he said, participants in the highest tertile for baseline systolic blood pressure (BP), at least 157 mm Hg, “seemed to benefit from being screened,” with a 49% reduction in risk for the primary endpoint (P = .0066). The interaction between systolic BP and outcome was significant (P = .007).
Only 9.3% of participants in LOOP did not have a baseline diagnosis of hypertension and so had to have another risk factor to enroll, the published report notes. However, the significant interaction with systolic BP “suggests that patients with dysregulated hypertension could benefit from this type of screening and concomitant anticoagulation.”
“There is a tight association between our primary endpoint and hypertension,” Dr. Svendsen said in an interview. “But I think it’s very important to say that this subgroup analysis is only hypothesis-generating.”
An editorial accompanying the LOOP publication suggests, in line with Dr. Svendsen’s proposal, that “shorter atrial fibrillation episodes found by long-term ILRs might not have the same stroke risk as atrial fibrillation detected through single-timepoint or less intense monitoring.”
If much of the paroxysmal AFib observed in LOOP and other studies with similar monitoring methods “is not the actual cause of stroke and is instead predominantly a risk marker, further research is warranted to establish whether a different screening focus and treatment paradigm are required to prevent stroke and other vascular brain injury related to atrial fibrillation,” wrote editorialists Ben Freedman, MBBS, PhD, and Nicole Lowres, BPhty, PhD, University of Sydney, Australia.
LOOP was partially supported by Medtronic. Dr. Svendsen is a member of Medtronic advisory boards and has received speaker honoraria and research grants from Medtronic in relation to this work and outside the submitted work. Disclosures for the other authors are in the report. Dr. Freedman reports grants to the Heart Research Institute, speakers fees and nonfinancial support from the Bristol-Myers Squibb–Pfizer Alliance, speakers fees and nonfinancial support from Daiichi Sankyo, nonfinancial support from AliveCor, and speakers fees and nonfinancial support from Omron unrelated to the topic of the editorial but related to atrial fibrillation and screening for atrial fibrillation. Dr. Lowres reports grants to the Heart Research Institute from the Bristol-Myers Squibb–Pfizer Alliance unrelated to the topic of the editorial but related to atrial fibrillation and screening for atrial fibrillation.
A version of this article first appeared on Medscape.com.
Perhaps short, asymptomatic bouts of atrial fibrillation (AFib) that show up on long-term, continuous monitoring aren’t worth hunting for just so oral anticoagulation (OAC) can be started, even in elderly people with other stroke risk factors.
That’s a potential message from a randomized trial that tested an AFib screening strategy relying on an implantable loop recorder (ILR) in older adults without AFib but with other stroke risk factors who were invited to participate. OAC was recommended to any participant found with even a short bout of the arrhythmia (that is, any lasting 6 minutes or longer).
More than three times as many in the monitoring group compared to a standard-care cohort were found to have AFib, and nearly all were put on OAC. In fact, monitored participants were almost three times as likely to be put on OAC (P < .0001) compared with controls.
But it didn’t make any apparent difference to outcomes. The risk for stroke or systemic embolism did not significantly differ between the two groups over more than 5 years in the trial of about 6,000 participants, called LOOP.
“This result was seen despite a high proportion of atrial fibrillation detection, and a high acceptance of anticoagulation therapy, and might imply that not all atrial fibrillation is worth screening for, and not all screen-detected atrial fibrillation merits anticoagulation,” contend the authors of the LOOP report, simultaneously published in The Lancet and presented Aug. 29 at the virtual European Society of Cardiology (ESC) Congress 2021.
“The rates of bleeding were modest, despite the low threshold for anticoagulation,” and was not significantly different between the two groups, Jesper H. Svendsen, MD, DMSc, Copenhagen University Hospital, Denmark, said at a media briefing before his presentation of the trial at the congress. He is lead author on the Lancet report.
At least 6 minutes of AFib was identified in more than 30% of the ILR-monitored patients, and about 90% of those were started on OAC, Dr. Svendsen observed.
But one take-home message from LOOP, he said in an interview, is that “short-lasting episodes” of AFib do not necessarily pose an untoward risk for stroke compared with AFib revealed by intermittent monitoring, which “primarily identifies longer-lasting atrial fibrillation episodes. So short-lasting episodes are probably not as serious as long-lasting.”
The LOOP trial “teaches us that perhaps short-lasting asymptomatic episodes may not benefit from being screened or found,” said Stefan James, MD, PhD, Uppsala University, Sweden. However, that may not be the case when the monitored individual is symptomatic or has longer-lasting AFib episodes, he said in an interview. “But certainly, this study teaches us that we need to understand much better the relationship between short episodes versus symptoms versus medical outcomes.”
In LOOP, 6,004 people aged 70-90 years without AFib but with at least one other stroke risk factor, which could include hypertension, diabetes, a history of stroke, or heart failure, were implanted with an ILR, the Reveal LINQ (Medtronic).
They were randomly assigned at four centers in Denmark to a monitoring group or a usual care group in a 1:3 ratio. Overwhelmingly, most had hypertension. Almost half the population were women.
OAC was recommended for all persons in the monitoring group who showed an episode of AFib lasting at least 6 minutes.
Atrial fibrillation was diagnosed in 31.8% of the 1,501 participants in the monitored group and 12.2% of the 4,503 assigned to usual care, for a hazard ratio (HR) of 3.17 (95% confidence interval, 2.81-3.59; P < .0001).
OAC was started in 29.7% of monitored participants and 13.1% of the control cohort, for an HR of 2.72 (95% CI, 2.41-3.08; P < .0001).
There were 315 strokes and three systemic arterial embolisms observed in the entire trial, for primary endpoint rates of 4.5% in the ILR monitoring group and 5.6% in the control group (HR, 0.80; 95% CI, 0.61-1.05; P = .11). Adding transient ischemic attack (TIA) or cardiovascular death to the endpoint did not make for a significant difference. The rates of major bleeding were 4.3% and 3.5%, respectively (P = .11).
“In general, the findings were consistent across subgroups,” including by age, sex, diabetes and heart failure status, stroke history, antiplatelet therapy, renal function, and even CHA2DS2–VASc score, Dr. Svendsen noted.
But, he said, participants in the highest tertile for baseline systolic blood pressure (BP), at least 157 mm Hg, “seemed to benefit from being screened,” with a 49% reduction in risk for the primary endpoint (P = .0066). The interaction between systolic BP and outcome was significant (P = .007).
Only 9.3% of participants in LOOP did not have a baseline diagnosis of hypertension and so had to have another risk factor to enroll, the published report notes. However, the significant interaction with systolic BP “suggests that patients with dysregulated hypertension could benefit from this type of screening and concomitant anticoagulation.”
“There is a tight association between our primary endpoint and hypertension,” Dr. Svendsen said in an interview. “But I think it’s very important to say that this subgroup analysis is only hypothesis-generating.”
An editorial accompanying the LOOP publication suggests, in line with Dr. Svendsen’s proposal, that “shorter atrial fibrillation episodes found by long-term ILRs might not have the same stroke risk as atrial fibrillation detected through single-timepoint or less intense monitoring.”
If much of the paroxysmal AFib observed in LOOP and other studies with similar monitoring methods “is not the actual cause of stroke and is instead predominantly a risk marker, further research is warranted to establish whether a different screening focus and treatment paradigm are required to prevent stroke and other vascular brain injury related to atrial fibrillation,” wrote editorialists Ben Freedman, MBBS, PhD, and Nicole Lowres, BPhty, PhD, University of Sydney, Australia.
LOOP was partially supported by Medtronic. Dr. Svendsen is a member of Medtronic advisory boards and has received speaker honoraria and research grants from Medtronic in relation to this work and outside the submitted work. Disclosures for the other authors are in the report. Dr. Freedman reports grants to the Heart Research Institute, speakers fees and nonfinancial support from the Bristol-Myers Squibb–Pfizer Alliance, speakers fees and nonfinancial support from Daiichi Sankyo, nonfinancial support from AliveCor, and speakers fees and nonfinancial support from Omron unrelated to the topic of the editorial but related to atrial fibrillation and screening for atrial fibrillation. Dr. Lowres reports grants to the Heart Research Institute from the Bristol-Myers Squibb–Pfizer Alliance unrelated to the topic of the editorial but related to atrial fibrillation and screening for atrial fibrillation.
A version of this article first appeared on Medscape.com.
MASTER DAPT: 1 month DAPT enough after high-bleeding-risk PCI
Another trial has added to the movement toward shortening the duration of dual antiplatelet therapy (DAPT) after percutaneous coronary intervention (PCI).
In the MASTER DAPT trial involving patients at high risk for bleeding who had undergone implantation of a biodegradable-polymer sirolimus-eluting stent, switching from DAPT to single antiplatelet therapy at a median of 34 days after PCI was noninferior to the continuation of DAPT treatment for a median duration of 193 days with regard to the incidence of major adverse cardiac or cerebral events, and was associated with a lower incidence of major or clinically relevant bleeding.
The results of the study were presented by Marco Valgimigli, MD, Cardiocentro Ticino Institute, Lugano, Switzerland, on Aug. 28 at the virtual European Society of Cardiology (ESC) Congress 2021. They were simultaneously published online in the New England Journal of Medicine.
“It has been suggested in previous studies that if patients are at high bleeding risk, then they do not seem to derive ischemic benefit from prolonging DAPT, they just get the increased bleeding risk,” Dr. Valgimigli said. “But this has never been prospectively tested until now.”
He pointed out that patients at high bleeding risk are a large group, representing up to 40% of patients undergoing PCI, and the MASTER DAPT trial included “all-comer” high-bleeding-risk patients with no selection based on ischemic risk.
The trial was very well received by commentators at the ESC Hot Line presentation.
Chair of the session, Roxana Mehran, MD, Icahn School of Medicine at Mount Sinai, New York, described the trial as “practice-changing.”
And Robert Byrne, MD, Mater Private Hospital, Dublin, added: “This is a standout trial. We have become more comfortable with abbreviated DAPT in high-bleeding-risk patients, but definite evidence for this has been lacking until now. This study tells us that just 1 month of DAPT appears to be safe in that there was no increase in ischemic complications and there was a clear reduction in bleeding.”
The MASTER DAPT study involved 4,579 patients at high bleeding risk who had undergone implantation of a biodegradable-polymer sirolimus-eluting coronary stent (Ultimaster, Terumo). Around half the patients had PCI for acute coronary syndrome (ACS) and half had it electively. One month after PCI they were randomly assigned to discontinue DAPT immediately (abbreviated therapy) or to continue it for at least 2 additional months (standard therapy).
The three co-primary outcomes were net adverse clinical events (a composite of death from any cause, myocardial infarction, stroke, or major bleeding), major adverse cardiac or cerebral events (a composite of death from any cause, myocardial infarction, or stroke), and major or clinically relevant nonmajor bleeding, all assessed cumulatively at 335 days. The first two outcomes were assessed for noninferiority in the per-protocol population, and the third outcome for superiority in the intention-to-treat population.
Dual antiplatelet therapy consisted of aspirin plus a P2Y12 inhibitor. The choices of the type of P2Y12 inhibitor for DAPT and the type of monotherapy after the discontinuation of DAPT were at the discretion of the investigator. Clopidogrel was the most popular choice, used as monotherapy in 54% of the patients in the abbreviated-therapy group and as part of DAPT in 79% of patients in the standard-therapy group.
Results showed that net adverse clinical events occurred in 7.5% of the abbreviated-therapy group and in 7.7% of the standard-therapy group (difference, –0.23 percentage points; 95% confidence interval, –1.80 to 1.33 percentage points; P < .001 for noninferiority).
Major adverse cardiac or cerebral events occurred in 6.1% of the abbreviated-therapy group and 5.9% of standard therapy group (difference, 0.11 percentage points; 95% CI, –1.29 to 1.51 percentage points; P = .001 for noninferiority).
Reduction in bleeding driven by BARC-2
Major bleeding or clinically relevant nonmajor bleeding occurred in 6.5% in the abbreviated-therapy group and in 9.4% in the standard-therapy group (difference, –2.82 percentage points; 95% CI, –4.40 to –1.24 percentage points; P < .001 for superiority).
“This is a highly statistically significant reduction in bleeding giving a number needed to treat of 35,” Dr. Valgimigli said.
The lower risk for bleeding in the abbreviated-therapy group was mainly due to the lower incidence of clinically relevant nonmajor bleeding events (BARC type 2) in this group than in the standard-therapy group (4.5% vs. 6.8%).
During the discussion, Dr. Byrne pointed out that the most serious type of bleeding (BARC type 3-5) was not reduced in the abbreviated DAPT group.
Dr. Valgimigli responded that the investigators were surprised about that because previous studies indicated that this most serious bleeding would be reduced, but he suggested that this may be explained by the standard group receiving 3-6 months of DAPT rather than a year or more in previous studies. “Having said that, BARC-2 bleeding is not a trivial event,” he added.
Can results be applied to other stents?
Dr. Byrne also questioned whether the results can be applied to patients receiving other types of stents – not just Ultimaster, which is not available everywhere. Dr. Valgimigli highlighted the low rate of stent thrombosis seen with the Ultimaster stent and said, “I would be scared to assume these results are reproducible with other stents.”
But Dr. Mehran challenged this view, saying, “I’m not so sure about that. I think we can probably extrapolate.”
In an interview, Dr. Mehran added: “I think this is one of the much-needed studies in our field. For the first time, we have a randomized trial on duration of DAPT in high-bleeding-risk patients. The study was inclusive, and enrolled truly high-bleeding-risk patients, including those on oral anticoagulants.
“These results show that, although high-bleeding-risk patients are at high risk of ischemic events, just 1 month of DAPT works well for them regardless, by reducing bleeding, net adverse clinical events, and without increasing ischemic events,” she concluded.
In an editorial accompanying the publication, E. Magnus Ohman, MB, from Duke University, Durham, N.C., pointed out the wide CIs in the results, which he said introduced some uncertainly to the findings.
But he concluded that: “The findings of Dr. Valgimigli and colleagues are important and move us toward a shorter and simpler antithrombotic strategy after PCI.”
In an interview, Dr. Ohman pointed out that the Ultimaster stent is not available in the United States. “We have to think about whether this stent would perform differently to other third- or fourth-generation stents. I wouldn’t have thought so, but it is hard to say for sure.
“All in all, we are looking at shorter periods of DAPT now after PCI. Several trials have now suggested that is the way to go. The forthcoming U.S. PCI guidelines should put all the studies together and come up with recommendations on different patient groups,” he concluded.
Several commentators said they would like to see the data on the patients receiving oral anticoagulants in the study before making firm conclusions on how to translate the results into clinical practice. “This is such an important group. It is difficult to interpret the results without this data,” Dr. Ohman noted. Patients receiving oral anticoagulants, who made up 36% of the study population, will be the subject of a separate report to be presented at the ESC meeting.
The MASTER DAPT trial was supported by Terumo. Dr. Valgimigli reports research grants from Terumo, Abbott, and SMT and consulting or speaker fees from Terumo, Abbott, Daiichi Sankyo, Chiesi, Vesalio, Vifor, Avimedica, Medtronic, Boston Scientific, and AstraZeneca. Dr. Ohman reports grants from Abiomed, grants from Chiesi USA, personal fees from Cara Therapeutics, Genentech, Imbria, Impulse Dynamics, Milestone Pharmaceuticals, XyloCor, Cytokinetics, Dispersol, Otsuka, Pfizer, Cytosorbents, Neurocrine, and Paradigm, outside the submitted work.
A version of this article first appeared on Medscape.com.
Another trial has added to the movement toward shortening the duration of dual antiplatelet therapy (DAPT) after percutaneous coronary intervention (PCI).
In the MASTER DAPT trial involving patients at high risk for bleeding who had undergone implantation of a biodegradable-polymer sirolimus-eluting stent, switching from DAPT to single antiplatelet therapy at a median of 34 days after PCI was noninferior to the continuation of DAPT treatment for a median duration of 193 days with regard to the incidence of major adverse cardiac or cerebral events, and was associated with a lower incidence of major or clinically relevant bleeding.
The results of the study were presented by Marco Valgimigli, MD, Cardiocentro Ticino Institute, Lugano, Switzerland, on Aug. 28 at the virtual European Society of Cardiology (ESC) Congress 2021. They were simultaneously published online in the New England Journal of Medicine.
“It has been suggested in previous studies that if patients are at high bleeding risk, then they do not seem to derive ischemic benefit from prolonging DAPT, they just get the increased bleeding risk,” Dr. Valgimigli said. “But this has never been prospectively tested until now.”
He pointed out that patients at high bleeding risk are a large group, representing up to 40% of patients undergoing PCI, and the MASTER DAPT trial included “all-comer” high-bleeding-risk patients with no selection based on ischemic risk.
The trial was very well received by commentators at the ESC Hot Line presentation.
Chair of the session, Roxana Mehran, MD, Icahn School of Medicine at Mount Sinai, New York, described the trial as “practice-changing.”
And Robert Byrne, MD, Mater Private Hospital, Dublin, added: “This is a standout trial. We have become more comfortable with abbreviated DAPT in high-bleeding-risk patients, but definite evidence for this has been lacking until now. This study tells us that just 1 month of DAPT appears to be safe in that there was no increase in ischemic complications and there was a clear reduction in bleeding.”
The MASTER DAPT study involved 4,579 patients at high bleeding risk who had undergone implantation of a biodegradable-polymer sirolimus-eluting coronary stent (Ultimaster, Terumo). Around half the patients had PCI for acute coronary syndrome (ACS) and half had it electively. One month after PCI they were randomly assigned to discontinue DAPT immediately (abbreviated therapy) or to continue it for at least 2 additional months (standard therapy).
The three co-primary outcomes were net adverse clinical events (a composite of death from any cause, myocardial infarction, stroke, or major bleeding), major adverse cardiac or cerebral events (a composite of death from any cause, myocardial infarction, or stroke), and major or clinically relevant nonmajor bleeding, all assessed cumulatively at 335 days. The first two outcomes were assessed for noninferiority in the per-protocol population, and the third outcome for superiority in the intention-to-treat population.
Dual antiplatelet therapy consisted of aspirin plus a P2Y12 inhibitor. The choices of the type of P2Y12 inhibitor for DAPT and the type of monotherapy after the discontinuation of DAPT were at the discretion of the investigator. Clopidogrel was the most popular choice, used as monotherapy in 54% of the patients in the abbreviated-therapy group and as part of DAPT in 79% of patients in the standard-therapy group.
Results showed that net adverse clinical events occurred in 7.5% of the abbreviated-therapy group and in 7.7% of the standard-therapy group (difference, –0.23 percentage points; 95% confidence interval, –1.80 to 1.33 percentage points; P < .001 for noninferiority).
Major adverse cardiac or cerebral events occurred in 6.1% of the abbreviated-therapy group and 5.9% of standard therapy group (difference, 0.11 percentage points; 95% CI, –1.29 to 1.51 percentage points; P = .001 for noninferiority).
Reduction in bleeding driven by BARC-2
Major bleeding or clinically relevant nonmajor bleeding occurred in 6.5% in the abbreviated-therapy group and in 9.4% in the standard-therapy group (difference, –2.82 percentage points; 95% CI, –4.40 to –1.24 percentage points; P < .001 for superiority).
“This is a highly statistically significant reduction in bleeding giving a number needed to treat of 35,” Dr. Valgimigli said.
The lower risk for bleeding in the abbreviated-therapy group was mainly due to the lower incidence of clinically relevant nonmajor bleeding events (BARC type 2) in this group than in the standard-therapy group (4.5% vs. 6.8%).
During the discussion, Dr. Byrne pointed out that the most serious type of bleeding (BARC type 3-5) was not reduced in the abbreviated DAPT group.
Dr. Valgimigli responded that the investigators were surprised about that because previous studies indicated that this most serious bleeding would be reduced, but he suggested that this may be explained by the standard group receiving 3-6 months of DAPT rather than a year or more in previous studies. “Having said that, BARC-2 bleeding is not a trivial event,” he added.
Can results be applied to other stents?
Dr. Byrne also questioned whether the results can be applied to patients receiving other types of stents – not just Ultimaster, which is not available everywhere. Dr. Valgimigli highlighted the low rate of stent thrombosis seen with the Ultimaster stent and said, “I would be scared to assume these results are reproducible with other stents.”
But Dr. Mehran challenged this view, saying, “I’m not so sure about that. I think we can probably extrapolate.”
In an interview, Dr. Mehran added: “I think this is one of the much-needed studies in our field. For the first time, we have a randomized trial on duration of DAPT in high-bleeding-risk patients. The study was inclusive, and enrolled truly high-bleeding-risk patients, including those on oral anticoagulants.
“These results show that, although high-bleeding-risk patients are at high risk of ischemic events, just 1 month of DAPT works well for them regardless, by reducing bleeding, net adverse clinical events, and without increasing ischemic events,” she concluded.
In an editorial accompanying the publication, E. Magnus Ohman, MB, from Duke University, Durham, N.C., pointed out the wide CIs in the results, which he said introduced some uncertainly to the findings.
But he concluded that: “The findings of Dr. Valgimigli and colleagues are important and move us toward a shorter and simpler antithrombotic strategy after PCI.”
In an interview, Dr. Ohman pointed out that the Ultimaster stent is not available in the United States. “We have to think about whether this stent would perform differently to other third- or fourth-generation stents. I wouldn’t have thought so, but it is hard to say for sure.
“All in all, we are looking at shorter periods of DAPT now after PCI. Several trials have now suggested that is the way to go. The forthcoming U.S. PCI guidelines should put all the studies together and come up with recommendations on different patient groups,” he concluded.
Several commentators said they would like to see the data on the patients receiving oral anticoagulants in the study before making firm conclusions on how to translate the results into clinical practice. “This is such an important group. It is difficult to interpret the results without this data,” Dr. Ohman noted. Patients receiving oral anticoagulants, who made up 36% of the study population, will be the subject of a separate report to be presented at the ESC meeting.
The MASTER DAPT trial was supported by Terumo. Dr. Valgimigli reports research grants from Terumo, Abbott, and SMT and consulting or speaker fees from Terumo, Abbott, Daiichi Sankyo, Chiesi, Vesalio, Vifor, Avimedica, Medtronic, Boston Scientific, and AstraZeneca. Dr. Ohman reports grants from Abiomed, grants from Chiesi USA, personal fees from Cara Therapeutics, Genentech, Imbria, Impulse Dynamics, Milestone Pharmaceuticals, XyloCor, Cytokinetics, Dispersol, Otsuka, Pfizer, Cytosorbents, Neurocrine, and Paradigm, outside the submitted work.
A version of this article first appeared on Medscape.com.
Another trial has added to the movement toward shortening the duration of dual antiplatelet therapy (DAPT) after percutaneous coronary intervention (PCI).
In the MASTER DAPT trial involving patients at high risk for bleeding who had undergone implantation of a biodegradable-polymer sirolimus-eluting stent, switching from DAPT to single antiplatelet therapy at a median of 34 days after PCI was noninferior to the continuation of DAPT treatment for a median duration of 193 days with regard to the incidence of major adverse cardiac or cerebral events, and was associated with a lower incidence of major or clinically relevant bleeding.
The results of the study were presented by Marco Valgimigli, MD, Cardiocentro Ticino Institute, Lugano, Switzerland, on Aug. 28 at the virtual European Society of Cardiology (ESC) Congress 2021. They were simultaneously published online in the New England Journal of Medicine.
“It has been suggested in previous studies that if patients are at high bleeding risk, then they do not seem to derive ischemic benefit from prolonging DAPT, they just get the increased bleeding risk,” Dr. Valgimigli said. “But this has never been prospectively tested until now.”
He pointed out that patients at high bleeding risk are a large group, representing up to 40% of patients undergoing PCI, and the MASTER DAPT trial included “all-comer” high-bleeding-risk patients with no selection based on ischemic risk.
The trial was very well received by commentators at the ESC Hot Line presentation.
Chair of the session, Roxana Mehran, MD, Icahn School of Medicine at Mount Sinai, New York, described the trial as “practice-changing.”
And Robert Byrne, MD, Mater Private Hospital, Dublin, added: “This is a standout trial. We have become more comfortable with abbreviated DAPT in high-bleeding-risk patients, but definite evidence for this has been lacking until now. This study tells us that just 1 month of DAPT appears to be safe in that there was no increase in ischemic complications and there was a clear reduction in bleeding.”
The MASTER DAPT study involved 4,579 patients at high bleeding risk who had undergone implantation of a biodegradable-polymer sirolimus-eluting coronary stent (Ultimaster, Terumo). Around half the patients had PCI for acute coronary syndrome (ACS) and half had it electively. One month after PCI they were randomly assigned to discontinue DAPT immediately (abbreviated therapy) or to continue it for at least 2 additional months (standard therapy).
The three co-primary outcomes were net adverse clinical events (a composite of death from any cause, myocardial infarction, stroke, or major bleeding), major adverse cardiac or cerebral events (a composite of death from any cause, myocardial infarction, or stroke), and major or clinically relevant nonmajor bleeding, all assessed cumulatively at 335 days. The first two outcomes were assessed for noninferiority in the per-protocol population, and the third outcome for superiority in the intention-to-treat population.
Dual antiplatelet therapy consisted of aspirin plus a P2Y12 inhibitor. The choices of the type of P2Y12 inhibitor for DAPT and the type of monotherapy after the discontinuation of DAPT were at the discretion of the investigator. Clopidogrel was the most popular choice, used as monotherapy in 54% of the patients in the abbreviated-therapy group and as part of DAPT in 79% of patients in the standard-therapy group.
Results showed that net adverse clinical events occurred in 7.5% of the abbreviated-therapy group and in 7.7% of the standard-therapy group (difference, –0.23 percentage points; 95% confidence interval, –1.80 to 1.33 percentage points; P < .001 for noninferiority).
Major adverse cardiac or cerebral events occurred in 6.1% of the abbreviated-therapy group and 5.9% of standard therapy group (difference, 0.11 percentage points; 95% CI, –1.29 to 1.51 percentage points; P = .001 for noninferiority).
Reduction in bleeding driven by BARC-2
Major bleeding or clinically relevant nonmajor bleeding occurred in 6.5% in the abbreviated-therapy group and in 9.4% in the standard-therapy group (difference, –2.82 percentage points; 95% CI, –4.40 to –1.24 percentage points; P < .001 for superiority).
“This is a highly statistically significant reduction in bleeding giving a number needed to treat of 35,” Dr. Valgimigli said.
The lower risk for bleeding in the abbreviated-therapy group was mainly due to the lower incidence of clinically relevant nonmajor bleeding events (BARC type 2) in this group than in the standard-therapy group (4.5% vs. 6.8%).
During the discussion, Dr. Byrne pointed out that the most serious type of bleeding (BARC type 3-5) was not reduced in the abbreviated DAPT group.
Dr. Valgimigli responded that the investigators were surprised about that because previous studies indicated that this most serious bleeding would be reduced, but he suggested that this may be explained by the standard group receiving 3-6 months of DAPT rather than a year or more in previous studies. “Having said that, BARC-2 bleeding is not a trivial event,” he added.
Can results be applied to other stents?
Dr. Byrne also questioned whether the results can be applied to patients receiving other types of stents – not just Ultimaster, which is not available everywhere. Dr. Valgimigli highlighted the low rate of stent thrombosis seen with the Ultimaster stent and said, “I would be scared to assume these results are reproducible with other stents.”
But Dr. Mehran challenged this view, saying, “I’m not so sure about that. I think we can probably extrapolate.”
In an interview, Dr. Mehran added: “I think this is one of the much-needed studies in our field. For the first time, we have a randomized trial on duration of DAPT in high-bleeding-risk patients. The study was inclusive, and enrolled truly high-bleeding-risk patients, including those on oral anticoagulants.
“These results show that, although high-bleeding-risk patients are at high risk of ischemic events, just 1 month of DAPT works well for them regardless, by reducing bleeding, net adverse clinical events, and without increasing ischemic events,” she concluded.
In an editorial accompanying the publication, E. Magnus Ohman, MB, from Duke University, Durham, N.C., pointed out the wide CIs in the results, which he said introduced some uncertainly to the findings.
But he concluded that: “The findings of Dr. Valgimigli and colleagues are important and move us toward a shorter and simpler antithrombotic strategy after PCI.”
In an interview, Dr. Ohman pointed out that the Ultimaster stent is not available in the United States. “We have to think about whether this stent would perform differently to other third- or fourth-generation stents. I wouldn’t have thought so, but it is hard to say for sure.
“All in all, we are looking at shorter periods of DAPT now after PCI. Several trials have now suggested that is the way to go. The forthcoming U.S. PCI guidelines should put all the studies together and come up with recommendations on different patient groups,” he concluded.
Several commentators said they would like to see the data on the patients receiving oral anticoagulants in the study before making firm conclusions on how to translate the results into clinical practice. “This is such an important group. It is difficult to interpret the results without this data,” Dr. Ohman noted. Patients receiving oral anticoagulants, who made up 36% of the study population, will be the subject of a separate report to be presented at the ESC meeting.
The MASTER DAPT trial was supported by Terumo. Dr. Valgimigli reports research grants from Terumo, Abbott, and SMT and consulting or speaker fees from Terumo, Abbott, Daiichi Sankyo, Chiesi, Vesalio, Vifor, Avimedica, Medtronic, Boston Scientific, and AstraZeneca. Dr. Ohman reports grants from Abiomed, grants from Chiesi USA, personal fees from Cara Therapeutics, Genentech, Imbria, Impulse Dynamics, Milestone Pharmaceuticals, XyloCor, Cytokinetics, Dispersol, Otsuka, Pfizer, Cytosorbents, Neurocrine, and Paradigm, outside the submitted work.
A version of this article first appeared on Medscape.com.
In all-comer approach, FFR adds no value to angiography: RIPCORD 2
Study confirms selective application
In patients with coronary artery disease scheduled for a percutaneous intervention (PCI), fractional flow reserve (FFR) assessment at the time of angiography significantly improves outcome, but it has no apparent value as a routine study in all CAD patients, according to the randomized RIPCORD 2 trial.
When compared to angiography alone in an all comer-strategy, the addition of FFR did not significantly change management or lower costs, but it was associated with a longer time for diagnostic assessment and more complications, Nicholas P. Curzen, BM, PhD, reported at the annual congress of the European Society of Cardiology.
As a tool for evaluating stenotic lesions in diseased vessels, FFR, also known as pressure wire assessment, allows interventionalists to target those vessels that induce ischemia without unnecessarily treating vessels with lesions that are hemodynamically nonsignificant. It is guideline recommended for patients with scheduled PCI on the basis of several randomized trials, including the landmark FAME trial.
“The results of these trials were spectacular. The clinical outcomes were significantly better in the FFR group despite less stents being placed and fewer vessels being stented. And there was significantly less resource utilization in the FFR group,” said Dr. Curzen, professor of interventional cardiology, University of Southampton, England.
Hypothesis: All-comers benefit from FFR
This prompted the new trial, called RIPCORD 2. The hypothesis was that systematic FFR early in the diagnosis of CAD would reduce resource utilization and improve quality of life relative to angiography alone. Both were addressed as primary endpoints. A reduction in clinical events at 12 months was a secondary endpoint.
The 1,136 participants, all scheduled for angiographic evaluation for stable angina or non-ST elevated myocardial infarction (NSTEMI), were randomized at 17 participating centers in the United Kingdom. All underwent angiography, but the experimental arm also underwent FFR for all arteries of a size suitable for revascularization.
Resource utilization evaluated through hospital costs at 12 months was somewhat higher in the FFR group, but the difference was not significant (P =.137). There was also no significant difference (P = 0.88) between the groups in quality of life, which was measured with EQ-5D-5L, an instrument for expressing five dimensions of health on a visual analog scale.
No impact from FFR on clinical events
Furthermore, there was no difference in the rate of clinical events, whether measured by a composite endpoint of major adverse cardiac events (MACE) (P = .64) or by the components of death, stroke, myocardial infarction, and unplanned revascularization, according to Dr. Curzen.
Finally, FFR did not appear to influence subsequent management. When the intervention and control groups were compared, the proportions triaged to optimal medical therapy, optimal medical therapy plus PCI, or optimal medical therapy plus bypass grafting did not differ significantly.
Given the lack of significant differences for FFR plus angiography relative to angiography alone for any clinically relevant outcome, the addition of FFR provides "no overall advantage" in this all comer study population, Dr. Curzen concluded.
However, FFR was associated with some relative disadvantages. These included significantly longer mean procedure times (69 vs. 42.4 minutes; P < .001), significantly greater mean use of contrast (206 vs. 146.3 mL; P < .001), and a significantly higher mean radiation dose (6608.7 vs. 5029.7 cGY/cm2; P < .001). There were 10 complications (1.8%) associated with FFR.
RIPCORD 1 results provided study rationale
In the previously published nonrandomized RIPCORD 1 study, interventionalists were asked to develop a management plan on the basis of angiography alone in 200 patients with stable chest pain. When these interventionalists were then provided with FFR results, the new information resulted in a change of management plan in 36% of cases.
According to Dr. Curzen, it was this study that raised all-comer FFR as a “logical and clinically plausible question.” RIPCORD 2 provided the answer.
While he is now conducting an evaluation of a subgroup of RIPCORD 2 patients with more severe disease, “it appears that the atheroma burden on angiography is adequate” to make an appropriate management determination in most or all cases.
The invited discussant for this study, Robert Byrne, MD, BCh, PhD, director of cardiology, Mater Private Hospital, Dublin, pointed out that more angiography-alone patients in RIPCORD 2 required additional evaluation to develop a management strategy (14.7% vs. 1.8%), but he agreed that FFR offered “no reasonable benefit” in the relatively low-risk patients who were enrolled.
Results do not alter FFR indications
However, he emphasized that the lack of an advantage in this trial should in no way diminish the evidence of benefit for selective FFR use as currently recommended in guidelines. This was echoed strongly in remarks by two other interventionalists who served on the same panel after the RIPCORD 2 results were presented.
“I want to make sure that our audience does not walk away thinking that FFR is useless. This is not what was shown,” said Roxana Mehran, MD, director of interventional cardiovascular research at Icahn School of Medicine at Mount Sinai, New York. She emphasized that this was a study that found no value in a low-risk, all-comer population and is not relevant to the populations where it now has an indication.
Marco Roffi, MD, director of the interventional cardiology unit, Geneva University Hospitals, made the same point.
“These results do not take away the value of FFR in a more selected population [than that enrolled in RIPCORD 2],” Dr. Roffi said. He did not rule out the potential for benefit from adding FFR to angiography even in early disease assessment if a benefit can be demonstrated in a higher-risk population.
Dr. Curzen reports financial relationships with Abbott, Beckman Coulter, HeartFlow, and Boston Scientific, which provided funding for RIPCORD 2. Dr. Byrne reported financial relationships with the trial sponsor as well as Abbott, Biosensors, and Biotronik. Dr. Mehran reports financial relationships with more than 15 medical product companies including the sponsor of this trial. Dr. Roffi reports no relevant financial disclosures.
Study confirms selective application
Study confirms selective application
In patients with coronary artery disease scheduled for a percutaneous intervention (PCI), fractional flow reserve (FFR) assessment at the time of angiography significantly improves outcome, but it has no apparent value as a routine study in all CAD patients, according to the randomized RIPCORD 2 trial.
When compared to angiography alone in an all comer-strategy, the addition of FFR did not significantly change management or lower costs, but it was associated with a longer time for diagnostic assessment and more complications, Nicholas P. Curzen, BM, PhD, reported at the annual congress of the European Society of Cardiology.
As a tool for evaluating stenotic lesions in diseased vessels, FFR, also known as pressure wire assessment, allows interventionalists to target those vessels that induce ischemia without unnecessarily treating vessels with lesions that are hemodynamically nonsignificant. It is guideline recommended for patients with scheduled PCI on the basis of several randomized trials, including the landmark FAME trial.
“The results of these trials were spectacular. The clinical outcomes were significantly better in the FFR group despite less stents being placed and fewer vessels being stented. And there was significantly less resource utilization in the FFR group,” said Dr. Curzen, professor of interventional cardiology, University of Southampton, England.
Hypothesis: All-comers benefit from FFR
This prompted the new trial, called RIPCORD 2. The hypothesis was that systematic FFR early in the diagnosis of CAD would reduce resource utilization and improve quality of life relative to angiography alone. Both were addressed as primary endpoints. A reduction in clinical events at 12 months was a secondary endpoint.
The 1,136 participants, all scheduled for angiographic evaluation for stable angina or non-ST elevated myocardial infarction (NSTEMI), were randomized at 17 participating centers in the United Kingdom. All underwent angiography, but the experimental arm also underwent FFR for all arteries of a size suitable for revascularization.
Resource utilization evaluated through hospital costs at 12 months was somewhat higher in the FFR group, but the difference was not significant (P =.137). There was also no significant difference (P = 0.88) between the groups in quality of life, which was measured with EQ-5D-5L, an instrument for expressing five dimensions of health on a visual analog scale.
No impact from FFR on clinical events
Furthermore, there was no difference in the rate of clinical events, whether measured by a composite endpoint of major adverse cardiac events (MACE) (P = .64) or by the components of death, stroke, myocardial infarction, and unplanned revascularization, according to Dr. Curzen.
Finally, FFR did not appear to influence subsequent management. When the intervention and control groups were compared, the proportions triaged to optimal medical therapy, optimal medical therapy plus PCI, or optimal medical therapy plus bypass grafting did not differ significantly.
Given the lack of significant differences for FFR plus angiography relative to angiography alone for any clinically relevant outcome, the addition of FFR provides "no overall advantage" in this all comer study population, Dr. Curzen concluded.
However, FFR was associated with some relative disadvantages. These included significantly longer mean procedure times (69 vs. 42.4 minutes; P < .001), significantly greater mean use of contrast (206 vs. 146.3 mL; P < .001), and a significantly higher mean radiation dose (6608.7 vs. 5029.7 cGY/cm2; P < .001). There were 10 complications (1.8%) associated with FFR.
RIPCORD 1 results provided study rationale
In the previously published nonrandomized RIPCORD 1 study, interventionalists were asked to develop a management plan on the basis of angiography alone in 200 patients with stable chest pain. When these interventionalists were then provided with FFR results, the new information resulted in a change of management plan in 36% of cases.
According to Dr. Curzen, it was this study that raised all-comer FFR as a “logical and clinically plausible question.” RIPCORD 2 provided the answer.
While he is now conducting an evaluation of a subgroup of RIPCORD 2 patients with more severe disease, “it appears that the atheroma burden on angiography is adequate” to make an appropriate management determination in most or all cases.
The invited discussant for this study, Robert Byrne, MD, BCh, PhD, director of cardiology, Mater Private Hospital, Dublin, pointed out that more angiography-alone patients in RIPCORD 2 required additional evaluation to develop a management strategy (14.7% vs. 1.8%), but he agreed that FFR offered “no reasonable benefit” in the relatively low-risk patients who were enrolled.
Results do not alter FFR indications
However, he emphasized that the lack of an advantage in this trial should in no way diminish the evidence of benefit for selective FFR use as currently recommended in guidelines. This was echoed strongly in remarks by two other interventionalists who served on the same panel after the RIPCORD 2 results were presented.
“I want to make sure that our audience does not walk away thinking that FFR is useless. This is not what was shown,” said Roxana Mehran, MD, director of interventional cardiovascular research at Icahn School of Medicine at Mount Sinai, New York. She emphasized that this was a study that found no value in a low-risk, all-comer population and is not relevant to the populations where it now has an indication.
Marco Roffi, MD, director of the interventional cardiology unit, Geneva University Hospitals, made the same point.
“These results do not take away the value of FFR in a more selected population [than that enrolled in RIPCORD 2],” Dr. Roffi said. He did not rule out the potential for benefit from adding FFR to angiography even in early disease assessment if a benefit can be demonstrated in a higher-risk population.
Dr. Curzen reports financial relationships with Abbott, Beckman Coulter, HeartFlow, and Boston Scientific, which provided funding for RIPCORD 2. Dr. Byrne reported financial relationships with the trial sponsor as well as Abbott, Biosensors, and Biotronik. Dr. Mehran reports financial relationships with more than 15 medical product companies including the sponsor of this trial. Dr. Roffi reports no relevant financial disclosures.
In patients with coronary artery disease scheduled for a percutaneous intervention (PCI), fractional flow reserve (FFR) assessment at the time of angiography significantly improves outcome, but it has no apparent value as a routine study in all CAD patients, according to the randomized RIPCORD 2 trial.
When compared to angiography alone in an all comer-strategy, the addition of FFR did not significantly change management or lower costs, but it was associated with a longer time for diagnostic assessment and more complications, Nicholas P. Curzen, BM, PhD, reported at the annual congress of the European Society of Cardiology.
As a tool for evaluating stenotic lesions in diseased vessels, FFR, also known as pressure wire assessment, allows interventionalists to target those vessels that induce ischemia without unnecessarily treating vessels with lesions that are hemodynamically nonsignificant. It is guideline recommended for patients with scheduled PCI on the basis of several randomized trials, including the landmark FAME trial.
“The results of these trials were spectacular. The clinical outcomes were significantly better in the FFR group despite less stents being placed and fewer vessels being stented. And there was significantly less resource utilization in the FFR group,” said Dr. Curzen, professor of interventional cardiology, University of Southampton, England.
Hypothesis: All-comers benefit from FFR
This prompted the new trial, called RIPCORD 2. The hypothesis was that systematic FFR early in the diagnosis of CAD would reduce resource utilization and improve quality of life relative to angiography alone. Both were addressed as primary endpoints. A reduction in clinical events at 12 months was a secondary endpoint.
The 1,136 participants, all scheduled for angiographic evaluation for stable angina or non-ST elevated myocardial infarction (NSTEMI), were randomized at 17 participating centers in the United Kingdom. All underwent angiography, but the experimental arm also underwent FFR for all arteries of a size suitable for revascularization.
Resource utilization evaluated through hospital costs at 12 months was somewhat higher in the FFR group, but the difference was not significant (P =.137). There was also no significant difference (P = 0.88) between the groups in quality of life, which was measured with EQ-5D-5L, an instrument for expressing five dimensions of health on a visual analog scale.
No impact from FFR on clinical events
Furthermore, there was no difference in the rate of clinical events, whether measured by a composite endpoint of major adverse cardiac events (MACE) (P = .64) or by the components of death, stroke, myocardial infarction, and unplanned revascularization, according to Dr. Curzen.
Finally, FFR did not appear to influence subsequent management. When the intervention and control groups were compared, the proportions triaged to optimal medical therapy, optimal medical therapy plus PCI, or optimal medical therapy plus bypass grafting did not differ significantly.
Given the lack of significant differences for FFR plus angiography relative to angiography alone for any clinically relevant outcome, the addition of FFR provides "no overall advantage" in this all comer study population, Dr. Curzen concluded.
However, FFR was associated with some relative disadvantages. These included significantly longer mean procedure times (69 vs. 42.4 minutes; P < .001), significantly greater mean use of contrast (206 vs. 146.3 mL; P < .001), and a significantly higher mean radiation dose (6608.7 vs. 5029.7 cGY/cm2; P < .001). There were 10 complications (1.8%) associated with FFR.
RIPCORD 1 results provided study rationale
In the previously published nonrandomized RIPCORD 1 study, interventionalists were asked to develop a management plan on the basis of angiography alone in 200 patients with stable chest pain. When these interventionalists were then provided with FFR results, the new information resulted in a change of management plan in 36% of cases.
According to Dr. Curzen, it was this study that raised all-comer FFR as a “logical and clinically plausible question.” RIPCORD 2 provided the answer.
While he is now conducting an evaluation of a subgroup of RIPCORD 2 patients with more severe disease, “it appears that the atheroma burden on angiography is adequate” to make an appropriate management determination in most or all cases.
The invited discussant for this study, Robert Byrne, MD, BCh, PhD, director of cardiology, Mater Private Hospital, Dublin, pointed out that more angiography-alone patients in RIPCORD 2 required additional evaluation to develop a management strategy (14.7% vs. 1.8%), but he agreed that FFR offered “no reasonable benefit” in the relatively low-risk patients who were enrolled.
Results do not alter FFR indications
However, he emphasized that the lack of an advantage in this trial should in no way diminish the evidence of benefit for selective FFR use as currently recommended in guidelines. This was echoed strongly in remarks by two other interventionalists who served on the same panel after the RIPCORD 2 results were presented.
“I want to make sure that our audience does not walk away thinking that FFR is useless. This is not what was shown,” said Roxana Mehran, MD, director of interventional cardiovascular research at Icahn School of Medicine at Mount Sinai, New York. She emphasized that this was a study that found no value in a low-risk, all-comer population and is not relevant to the populations where it now has an indication.
Marco Roffi, MD, director of the interventional cardiology unit, Geneva University Hospitals, made the same point.
“These results do not take away the value of FFR in a more selected population [than that enrolled in RIPCORD 2],” Dr. Roffi said. He did not rule out the potential for benefit from adding FFR to angiography even in early disease assessment if a benefit can be demonstrated in a higher-risk population.
Dr. Curzen reports financial relationships with Abbott, Beckman Coulter, HeartFlow, and Boston Scientific, which provided funding for RIPCORD 2. Dr. Byrne reported financial relationships with the trial sponsor as well as Abbott, Biosensors, and Biotronik. Dr. Mehran reports financial relationships with more than 15 medical product companies including the sponsor of this trial. Dr. Roffi reports no relevant financial disclosures.
FROM ESC CONGRESS 2021
APAF-CRT: ‘Ablate and pace’ cuts mortality in narrow-QRS HF, permanent AFib
When a patient has permanent atrial fibrillation (AFib) and advanced heart failure (HF), rate control therapy is an option but an “ablate-and-pace” strategy may be better at improving symptoms. The ablate-and-pace approach, compared to pharmacologic rate control, may even prolong survival in a subset of such patients when the accompanying pacemaker provides cardiac resynchronization therapy (CRT), suggests a new randomized trial.
In the APAF-CRT trial, mortality fell more than 70% over 4 years for such patients with HF and narrow QRS intervals who were assigned to ablate-and-pace – that is, CRT after creation of heart block by atrioventricular (AV) junction ablation – compared to those managed medically.
The benefit was seen regardless of left ventricular ejection fraction (LVEF) at the start of the trial and probably stemmed from “the combination of strict rate control and rate regulation achieved by AV-junction ablation together with biventricular pacing,” said Michele Brignole, MD, Istituto Auxologico Italiano, Ospedale San Luca, Milan. The CRT substitution for a standard pacemaker, he explained, is thought to “counteract” the adverse remodeling effects of apical right ventricular (RV) pacing.
Dr. Brignole delivered the remarks at a media presentation before his presentation of the APAF-CRT during the virtual annual congress of the European Society of Cardiology.
The results “support ablation-CRT as a first-line therapy in patients with permanent AFib and narrow QRS who were hospitalized for heart failure,” regardless of ejection fraction, said Dr. Brignole, lead author on the study’s same-day publication in the European Heart Journal.
“The results are not surprising. They are in line with prior studies with shorter follow-up, and they justify a relatively common practice today, to implant CRT in these patients. It has previously been shown to improve heart failure and quality of life, and is now proven to improve survival because of the longer follow-up,” Michael Glikson, MD, Shaare Zedek Medical Center, Jerusalem, said at the media briefing.
“The APAF-CRT mortality trial makes an important contribution to establishment of AV-nodal ablation with CRT as first-line therapy of resistant atrial fibrillation with heart failure, mostly in patients with reduced ejection fraction,” said Dr. Glikson, who was not part of the trial.
However, he added, “the advantage of CRT over RV pacing is still somewhat unclear in patients with normal or preserved ejection fraction,” who were relatively few in APAF-CRT and in whom RV apical pacing after AV nodal ablation has not been shown to make a big difference to ventricular function.
The new analysis covered the trial’s second phase, which featured a mortality primary endpoint, in contrast to the previously reported initial stage that followed the first 102 patients over 2 years for death, worsening HF, or HF hospitalization.
The first phase had halted enrollment before reaching its planned target of 280 patients when an interim analysis showed a significant benefit for ablate and pace. The mortality trial continued to recruit at 11 centers in Europe, reaching 133 patients, who were followed for up to 4 years, the report notes. But its enrollment had also been suspended after an interim analysis saw superiority in the ablate-and-pace arm.
APAF-CRT entered patients with severely symptomatic permanent AFib for longer than 6 months, with a QRS interval no greater than 110 ms, who had at least one HF hospitalization in the last year and were considered poor candidates for AFib ablation. Their mean age was 73 years, and almost half, 47%, were women.
They were randomly assigned to ablate-and-pace with CRT or pharmacologic rate control therapy, 63 and 70 patients, respectively. Patients in either group could be given an implantable defibrillator at physician discretion.
Patients had been followed a median of 29 months when the trial was stopped for efficacy. The hazard ratio (HR) for death from any cause, ablate-and-pace vs. rate control, was 0.26 (95% confidence interval, 0.10-0.65; P = .004), with a number needed to treat to prevent an event of 3.7. The HR was 0.40 (95% CI, 0.22-0.73; P = .002) for the secondary endpoint of death or HF hospitalization.
The new ESC guidelines on cardiac pacing and cardiac resynchronization therapy recommend “that if the ejection fraction is subnormal, they should receive a CRT as the first choice,” Dr. Glikson said. “However, for patients who are undergoing AV nodal ablation and have normal ejection fractions, we thought that RV apical pacing should be okay,” so that was the main recommendation, he said.
“I think that the APAF-CRT study does not really change this approach” because the study was small and there were few data on such patients.
APAF-CRT was an investigator-initiated independent clinical trial, sponsored by a nonprofit organization, Centro Prevenzione Malattie Cardiorespiratorie ‘Nuccia e Vittore Corbella’, Rapallo, Italy, which received an unrestricted research grant from the Boston Scientific Investigator Sponsored Research (ISR) Committee. Dr. Brignole declared no conflicts. Disclosures for the other authors are in the report. Dr. Glikson had no disclosures.
A version of this article first appeared on Medscape.com.
When a patient has permanent atrial fibrillation (AFib) and advanced heart failure (HF), rate control therapy is an option but an “ablate-and-pace” strategy may be better at improving symptoms. The ablate-and-pace approach, compared to pharmacologic rate control, may even prolong survival in a subset of such patients when the accompanying pacemaker provides cardiac resynchronization therapy (CRT), suggests a new randomized trial.
In the APAF-CRT trial, mortality fell more than 70% over 4 years for such patients with HF and narrow QRS intervals who were assigned to ablate-and-pace – that is, CRT after creation of heart block by atrioventricular (AV) junction ablation – compared to those managed medically.
The benefit was seen regardless of left ventricular ejection fraction (LVEF) at the start of the trial and probably stemmed from “the combination of strict rate control and rate regulation achieved by AV-junction ablation together with biventricular pacing,” said Michele Brignole, MD, Istituto Auxologico Italiano, Ospedale San Luca, Milan. The CRT substitution for a standard pacemaker, he explained, is thought to “counteract” the adverse remodeling effects of apical right ventricular (RV) pacing.
Dr. Brignole delivered the remarks at a media presentation before his presentation of the APAF-CRT during the virtual annual congress of the European Society of Cardiology.
The results “support ablation-CRT as a first-line therapy in patients with permanent AFib and narrow QRS who were hospitalized for heart failure,” regardless of ejection fraction, said Dr. Brignole, lead author on the study’s same-day publication in the European Heart Journal.
“The results are not surprising. They are in line with prior studies with shorter follow-up, and they justify a relatively common practice today, to implant CRT in these patients. It has previously been shown to improve heart failure and quality of life, and is now proven to improve survival because of the longer follow-up,” Michael Glikson, MD, Shaare Zedek Medical Center, Jerusalem, said at the media briefing.
“The APAF-CRT mortality trial makes an important contribution to establishment of AV-nodal ablation with CRT as first-line therapy of resistant atrial fibrillation with heart failure, mostly in patients with reduced ejection fraction,” said Dr. Glikson, who was not part of the trial.
However, he added, “the advantage of CRT over RV pacing is still somewhat unclear in patients with normal or preserved ejection fraction,” who were relatively few in APAF-CRT and in whom RV apical pacing after AV nodal ablation has not been shown to make a big difference to ventricular function.
The new analysis covered the trial’s second phase, which featured a mortality primary endpoint, in contrast to the previously reported initial stage that followed the first 102 patients over 2 years for death, worsening HF, or HF hospitalization.
The first phase had halted enrollment before reaching its planned target of 280 patients when an interim analysis showed a significant benefit for ablate and pace. The mortality trial continued to recruit at 11 centers in Europe, reaching 133 patients, who were followed for up to 4 years, the report notes. But its enrollment had also been suspended after an interim analysis saw superiority in the ablate-and-pace arm.
APAF-CRT entered patients with severely symptomatic permanent AFib for longer than 6 months, with a QRS interval no greater than 110 ms, who had at least one HF hospitalization in the last year and were considered poor candidates for AFib ablation. Their mean age was 73 years, and almost half, 47%, were women.
They were randomly assigned to ablate-and-pace with CRT or pharmacologic rate control therapy, 63 and 70 patients, respectively. Patients in either group could be given an implantable defibrillator at physician discretion.
Patients had been followed a median of 29 months when the trial was stopped for efficacy. The hazard ratio (HR) for death from any cause, ablate-and-pace vs. rate control, was 0.26 (95% confidence interval, 0.10-0.65; P = .004), with a number needed to treat to prevent an event of 3.7. The HR was 0.40 (95% CI, 0.22-0.73; P = .002) for the secondary endpoint of death or HF hospitalization.
The new ESC guidelines on cardiac pacing and cardiac resynchronization therapy recommend “that if the ejection fraction is subnormal, they should receive a CRT as the first choice,” Dr. Glikson said. “However, for patients who are undergoing AV nodal ablation and have normal ejection fractions, we thought that RV apical pacing should be okay,” so that was the main recommendation, he said.
“I think that the APAF-CRT study does not really change this approach” because the study was small and there were few data on such patients.
APAF-CRT was an investigator-initiated independent clinical trial, sponsored by a nonprofit organization, Centro Prevenzione Malattie Cardiorespiratorie ‘Nuccia e Vittore Corbella’, Rapallo, Italy, which received an unrestricted research grant from the Boston Scientific Investigator Sponsored Research (ISR) Committee. Dr. Brignole declared no conflicts. Disclosures for the other authors are in the report. Dr. Glikson had no disclosures.
A version of this article first appeared on Medscape.com.
When a patient has permanent atrial fibrillation (AFib) and advanced heart failure (HF), rate control therapy is an option but an “ablate-and-pace” strategy may be better at improving symptoms. The ablate-and-pace approach, compared to pharmacologic rate control, may even prolong survival in a subset of such patients when the accompanying pacemaker provides cardiac resynchronization therapy (CRT), suggests a new randomized trial.
In the APAF-CRT trial, mortality fell more than 70% over 4 years for such patients with HF and narrow QRS intervals who were assigned to ablate-and-pace – that is, CRT after creation of heart block by atrioventricular (AV) junction ablation – compared to those managed medically.
The benefit was seen regardless of left ventricular ejection fraction (LVEF) at the start of the trial and probably stemmed from “the combination of strict rate control and rate regulation achieved by AV-junction ablation together with biventricular pacing,” said Michele Brignole, MD, Istituto Auxologico Italiano, Ospedale San Luca, Milan. The CRT substitution for a standard pacemaker, he explained, is thought to “counteract” the adverse remodeling effects of apical right ventricular (RV) pacing.
Dr. Brignole delivered the remarks at a media presentation before his presentation of the APAF-CRT during the virtual annual congress of the European Society of Cardiology.
The results “support ablation-CRT as a first-line therapy in patients with permanent AFib and narrow QRS who were hospitalized for heart failure,” regardless of ejection fraction, said Dr. Brignole, lead author on the study’s same-day publication in the European Heart Journal.
“The results are not surprising. They are in line with prior studies with shorter follow-up, and they justify a relatively common practice today, to implant CRT in these patients. It has previously been shown to improve heart failure and quality of life, and is now proven to improve survival because of the longer follow-up,” Michael Glikson, MD, Shaare Zedek Medical Center, Jerusalem, said at the media briefing.
“The APAF-CRT mortality trial makes an important contribution to establishment of AV-nodal ablation with CRT as first-line therapy of resistant atrial fibrillation with heart failure, mostly in patients with reduced ejection fraction,” said Dr. Glikson, who was not part of the trial.
However, he added, “the advantage of CRT over RV pacing is still somewhat unclear in patients with normal or preserved ejection fraction,” who were relatively few in APAF-CRT and in whom RV apical pacing after AV nodal ablation has not been shown to make a big difference to ventricular function.
The new analysis covered the trial’s second phase, which featured a mortality primary endpoint, in contrast to the previously reported initial stage that followed the first 102 patients over 2 years for death, worsening HF, or HF hospitalization.
The first phase had halted enrollment before reaching its planned target of 280 patients when an interim analysis showed a significant benefit for ablate and pace. The mortality trial continued to recruit at 11 centers in Europe, reaching 133 patients, who were followed for up to 4 years, the report notes. But its enrollment had also been suspended after an interim analysis saw superiority in the ablate-and-pace arm.
APAF-CRT entered patients with severely symptomatic permanent AFib for longer than 6 months, with a QRS interval no greater than 110 ms, who had at least one HF hospitalization in the last year and were considered poor candidates for AFib ablation. Their mean age was 73 years, and almost half, 47%, were women.
They were randomly assigned to ablate-and-pace with CRT or pharmacologic rate control therapy, 63 and 70 patients, respectively. Patients in either group could be given an implantable defibrillator at physician discretion.
Patients had been followed a median of 29 months when the trial was stopped for efficacy. The hazard ratio (HR) for death from any cause, ablate-and-pace vs. rate control, was 0.26 (95% confidence interval, 0.10-0.65; P = .004), with a number needed to treat to prevent an event of 3.7. The HR was 0.40 (95% CI, 0.22-0.73; P = .002) for the secondary endpoint of death or HF hospitalization.
The new ESC guidelines on cardiac pacing and cardiac resynchronization therapy recommend “that if the ejection fraction is subnormal, they should receive a CRT as the first choice,” Dr. Glikson said. “However, for patients who are undergoing AV nodal ablation and have normal ejection fractions, we thought that RV apical pacing should be okay,” so that was the main recommendation, he said.
“I think that the APAF-CRT study does not really change this approach” because the study was small and there were few data on such patients.
APAF-CRT was an investigator-initiated independent clinical trial, sponsored by a nonprofit organization, Centro Prevenzione Malattie Cardiorespiratorie ‘Nuccia e Vittore Corbella’, Rapallo, Italy, which received an unrestricted research grant from the Boston Scientific Investigator Sponsored Research (ISR) Committee. Dr. Brignole declared no conflicts. Disclosures for the other authors are in the report. Dr. Glikson had no disclosures.
A version of this article first appeared on Medscape.com.