User login
Antibiotic before oral surgery spares endocarditis; study validates guidelines
The strongest evidence yet to support clinical guidelines that recommend that people at high risk of endocarditis, such as those who’ve had previous episode the disease or who have a prosthetic cardiac valve, should take antibiotics before they have a tooth pulled or other types of oral surgery, comes from a new study that used two methodologies.
But it also pointed out that two-thirds of the time they aren’t getting that type of antibiotic coverage.
The researchers conducted a cohort study of almost 8 million retirees with employer-paid Medicare supplemental prescription benefits and dental benefits, then conducted a case-crossover study of 3,774 people from the cohort who’d been hospitalized with infectious endocarditis (IE) and who had invasive dental procedures. The bottom line is that the study supports the clinical guidelines from the American Heart Association and the European Society of Cardiology that recommend antibiotic prophylaxis (AP) before dental procedures for patients at high-risk of IE.
Likewise, lead author Martin Thornhill, MBBS, BDS, PhD, said in an interview, the findings also suggest that existing guidelines in the United Kingdom, which recommend against AP in these patients, “should be reconsidered.”
Those AHA and ESC guidelines, however, are “based on no good quality evidence,” said Dr. Thornhill, professor of translational research in dentistry at the University of Sheffield (England) School of Clinical Dentistry. “Other studies have looked at this, but we’ve done the largest study that has shown the clear association between invasive dental procedures and subsequent development of infective endocarditis.”
In the entire cohort of 7.95 million patients, 3,774 had cases of IE that required hospitalization. The study defined highest risk of IE as meeting one of these six criteria: a previous case of IE; a prosthetic cardiac valve or a valve repair that used prosthetic material; cyanotic congenital heart disease; palliative shunts or conduits to treat CHD; or a congenital heart defect that had been fully repaired, either by surgery or a transcatheter procedure, with prosthetic material or device – the latter within 6 months of the procedure.
Moderate IE risk included patients who had rheumatic heart disease, nonrheumatic valve disease or congenital valve anomalies—including mitral valve prolapse or aortic stenosis—or hypertrophic cardiomyopathy.
Risk classification and poor compliance
Highest-risk patients had significantly higher rates of IE a month after a dental procedure than lower-risk groups: 467.6 cases per 1 million procedures vs. 24.2 for moderate risk and 3.8 for low or unknown risk. A subanalysis found that the odds of IE were significantly increased for two specific dental procedures: extractions, with an odds ratio of 9.22 (95% confidence interval [CI], 5.54-15.88; P < .0001); and other oral surgical procedures, with an OR of 20.18 (95% CI, 11.22-37.74; P < .0001).
The study also found that 32.6% of the high-risk patients undergoing dental procedures got AP. “Clearly that shows a low level of compliance with the guidelines in the U.S.,” Dr. Thornhill said. “That’s something that needs to be addressed.”
The study was unique in that it used both a population cohort study and the case-crossover study. “It didn’t matter which of the two methods we used; we essentially came to the same result, which I think adds further weight to the findings,” Dr. Thornhill said.
This may be the best evidence to support the guidelines that clinicians may get. While the observational nature of this study has its limitations, conducting a randomized clinical trial to further validate the findings would be “logistically impossible,” he said, in that it would require an “absolutely enormous” cohort and coordination between medical and dental databases covering thousands of lives. An RCT would also require not using AP for some patients. “It’s not ethical to keep somebody off of antibiotic prophylaxis when there’s such a high risk of death and severe outcomes,” Dr. Thornhill said.
Ann Bolger, MD, emeritus professor of medicine at the University of California, San Francisco, and coauthor of an editorial comment on the study, said in an interview that this study is noteworthy not only for its dual methodology, but for the quality of the data that matched patients at high risk for IE with prescription and dental records. “The fact that they were able to have those details in enough granularity that they knew whether a dental procedure was likely to meet the criteria for these more invasive exposures really broke it open from my perspective,” she said.
She called the low compliance rate with AHA guidelines “one of the most sobering points of this,” and said it should put clinicians on notice that they must do more to educate and engage with high-risk patients. “The lines of communication here are somewhat fraught; it’s a little bit of a hot potato,” she said. “It’s a really great communications opportunity to get the provider’s attention back on this. You’re a cardiologist; you have to have this conversation when you see your patient with a prosthetic valve or who’s had endocarditis every time they come in. There’s a whole litany, and it takes 3 minutes, but you have to do it.”
The study received funding from Delta Dental of Michigan Research Committee and Renaissance Health Service Corp., and Dr. Thornhill received support from Delta Dental Research and Data Institute for the study. Dr. Bolger participated in the 2007 and 2021 AHA statements on AP to prevent IE.
The strongest evidence yet to support clinical guidelines that recommend that people at high risk of endocarditis, such as those who’ve had previous episode the disease or who have a prosthetic cardiac valve, should take antibiotics before they have a tooth pulled or other types of oral surgery, comes from a new study that used two methodologies.
But it also pointed out that two-thirds of the time they aren’t getting that type of antibiotic coverage.
The researchers conducted a cohort study of almost 8 million retirees with employer-paid Medicare supplemental prescription benefits and dental benefits, then conducted a case-crossover study of 3,774 people from the cohort who’d been hospitalized with infectious endocarditis (IE) and who had invasive dental procedures. The bottom line is that the study supports the clinical guidelines from the American Heart Association and the European Society of Cardiology that recommend antibiotic prophylaxis (AP) before dental procedures for patients at high-risk of IE.
Likewise, lead author Martin Thornhill, MBBS, BDS, PhD, said in an interview, the findings also suggest that existing guidelines in the United Kingdom, which recommend against AP in these patients, “should be reconsidered.”
Those AHA and ESC guidelines, however, are “based on no good quality evidence,” said Dr. Thornhill, professor of translational research in dentistry at the University of Sheffield (England) School of Clinical Dentistry. “Other studies have looked at this, but we’ve done the largest study that has shown the clear association between invasive dental procedures and subsequent development of infective endocarditis.”
In the entire cohort of 7.95 million patients, 3,774 had cases of IE that required hospitalization. The study defined highest risk of IE as meeting one of these six criteria: a previous case of IE; a prosthetic cardiac valve or a valve repair that used prosthetic material; cyanotic congenital heart disease; palliative shunts or conduits to treat CHD; or a congenital heart defect that had been fully repaired, either by surgery or a transcatheter procedure, with prosthetic material or device – the latter within 6 months of the procedure.
Moderate IE risk included patients who had rheumatic heart disease, nonrheumatic valve disease or congenital valve anomalies—including mitral valve prolapse or aortic stenosis—or hypertrophic cardiomyopathy.
Risk classification and poor compliance
Highest-risk patients had significantly higher rates of IE a month after a dental procedure than lower-risk groups: 467.6 cases per 1 million procedures vs. 24.2 for moderate risk and 3.8 for low or unknown risk. A subanalysis found that the odds of IE were significantly increased for two specific dental procedures: extractions, with an odds ratio of 9.22 (95% confidence interval [CI], 5.54-15.88; P < .0001); and other oral surgical procedures, with an OR of 20.18 (95% CI, 11.22-37.74; P < .0001).
The study also found that 32.6% of the high-risk patients undergoing dental procedures got AP. “Clearly that shows a low level of compliance with the guidelines in the U.S.,” Dr. Thornhill said. “That’s something that needs to be addressed.”
The study was unique in that it used both a population cohort study and the case-crossover study. “It didn’t matter which of the two methods we used; we essentially came to the same result, which I think adds further weight to the findings,” Dr. Thornhill said.
This may be the best evidence to support the guidelines that clinicians may get. While the observational nature of this study has its limitations, conducting a randomized clinical trial to further validate the findings would be “logistically impossible,” he said, in that it would require an “absolutely enormous” cohort and coordination between medical and dental databases covering thousands of lives. An RCT would also require not using AP for some patients. “It’s not ethical to keep somebody off of antibiotic prophylaxis when there’s such a high risk of death and severe outcomes,” Dr. Thornhill said.
Ann Bolger, MD, emeritus professor of medicine at the University of California, San Francisco, and coauthor of an editorial comment on the study, said in an interview that this study is noteworthy not only for its dual methodology, but for the quality of the data that matched patients at high risk for IE with prescription and dental records. “The fact that they were able to have those details in enough granularity that they knew whether a dental procedure was likely to meet the criteria for these more invasive exposures really broke it open from my perspective,” she said.
She called the low compliance rate with AHA guidelines “one of the most sobering points of this,” and said it should put clinicians on notice that they must do more to educate and engage with high-risk patients. “The lines of communication here are somewhat fraught; it’s a little bit of a hot potato,” she said. “It’s a really great communications opportunity to get the provider’s attention back on this. You’re a cardiologist; you have to have this conversation when you see your patient with a prosthetic valve or who’s had endocarditis every time they come in. There’s a whole litany, and it takes 3 minutes, but you have to do it.”
The study received funding from Delta Dental of Michigan Research Committee and Renaissance Health Service Corp., and Dr. Thornhill received support from Delta Dental Research and Data Institute for the study. Dr. Bolger participated in the 2007 and 2021 AHA statements on AP to prevent IE.
The strongest evidence yet to support clinical guidelines that recommend that people at high risk of endocarditis, such as those who’ve had previous episode the disease or who have a prosthetic cardiac valve, should take antibiotics before they have a tooth pulled or other types of oral surgery, comes from a new study that used two methodologies.
But it also pointed out that two-thirds of the time they aren’t getting that type of antibiotic coverage.
The researchers conducted a cohort study of almost 8 million retirees with employer-paid Medicare supplemental prescription benefits and dental benefits, then conducted a case-crossover study of 3,774 people from the cohort who’d been hospitalized with infectious endocarditis (IE) and who had invasive dental procedures. The bottom line is that the study supports the clinical guidelines from the American Heart Association and the European Society of Cardiology that recommend antibiotic prophylaxis (AP) before dental procedures for patients at high-risk of IE.
Likewise, lead author Martin Thornhill, MBBS, BDS, PhD, said in an interview, the findings also suggest that existing guidelines in the United Kingdom, which recommend against AP in these patients, “should be reconsidered.”
Those AHA and ESC guidelines, however, are “based on no good quality evidence,” said Dr. Thornhill, professor of translational research in dentistry at the University of Sheffield (England) School of Clinical Dentistry. “Other studies have looked at this, but we’ve done the largest study that has shown the clear association between invasive dental procedures and subsequent development of infective endocarditis.”
In the entire cohort of 7.95 million patients, 3,774 had cases of IE that required hospitalization. The study defined highest risk of IE as meeting one of these six criteria: a previous case of IE; a prosthetic cardiac valve or a valve repair that used prosthetic material; cyanotic congenital heart disease; palliative shunts or conduits to treat CHD; or a congenital heart defect that had been fully repaired, either by surgery or a transcatheter procedure, with prosthetic material or device – the latter within 6 months of the procedure.
Moderate IE risk included patients who had rheumatic heart disease, nonrheumatic valve disease or congenital valve anomalies—including mitral valve prolapse or aortic stenosis—or hypertrophic cardiomyopathy.
Risk classification and poor compliance
Highest-risk patients had significantly higher rates of IE a month after a dental procedure than lower-risk groups: 467.6 cases per 1 million procedures vs. 24.2 for moderate risk and 3.8 for low or unknown risk. A subanalysis found that the odds of IE were significantly increased for two specific dental procedures: extractions, with an odds ratio of 9.22 (95% confidence interval [CI], 5.54-15.88; P < .0001); and other oral surgical procedures, with an OR of 20.18 (95% CI, 11.22-37.74; P < .0001).
The study also found that 32.6% of the high-risk patients undergoing dental procedures got AP. “Clearly that shows a low level of compliance with the guidelines in the U.S.,” Dr. Thornhill said. “That’s something that needs to be addressed.”
The study was unique in that it used both a population cohort study and the case-crossover study. “It didn’t matter which of the two methods we used; we essentially came to the same result, which I think adds further weight to the findings,” Dr. Thornhill said.
This may be the best evidence to support the guidelines that clinicians may get. While the observational nature of this study has its limitations, conducting a randomized clinical trial to further validate the findings would be “logistically impossible,” he said, in that it would require an “absolutely enormous” cohort and coordination between medical and dental databases covering thousands of lives. An RCT would also require not using AP for some patients. “It’s not ethical to keep somebody off of antibiotic prophylaxis when there’s such a high risk of death and severe outcomes,” Dr. Thornhill said.
Ann Bolger, MD, emeritus professor of medicine at the University of California, San Francisco, and coauthor of an editorial comment on the study, said in an interview that this study is noteworthy not only for its dual methodology, but for the quality of the data that matched patients at high risk for IE with prescription and dental records. “The fact that they were able to have those details in enough granularity that they knew whether a dental procedure was likely to meet the criteria for these more invasive exposures really broke it open from my perspective,” she said.
She called the low compliance rate with AHA guidelines “one of the most sobering points of this,” and said it should put clinicians on notice that they must do more to educate and engage with high-risk patients. “The lines of communication here are somewhat fraught; it’s a little bit of a hot potato,” she said. “It’s a really great communications opportunity to get the provider’s attention back on this. You’re a cardiologist; you have to have this conversation when you see your patient with a prosthetic valve or who’s had endocarditis every time they come in. There’s a whole litany, and it takes 3 minutes, but you have to do it.”
The study received funding from Delta Dental of Michigan Research Committee and Renaissance Health Service Corp., and Dr. Thornhill received support from Delta Dental Research and Data Institute for the study. Dr. Bolger participated in the 2007 and 2021 AHA statements on AP to prevent IE.
FROM JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
How nonadherence complicates cardiology, in two trials
Each study adds new twist
Two very different sets of clinical evidence have offered new twists on how nonadherence to cardiovascular medicines not only leads to suboptimal outcomes, but also complicates the data from clinical studies.
One study, a subanalysis of a major trial, outlined how taking more than the assigned therapy – that is, nonadherence by taking too much rather than too little – skewed results. The other was a trial demonstrating that early use of an invasive procedure is not a strategy to compensate for nonadherence to guideline-directed medical therapy (GDMT).
“Both studies provide a fresh reminder that nonadherence is a significant problem in cardiology overall, but also in the trial setting when we are trying to interpret study results,” explained Usam Baber, MD, director of interventional cardiology, University of Oklahoma Health, Oklahoma City, coauthor of an editorial accompanying the two published studies.
Dr. Baber was the first author of a unifying editorial that addressed the issues raised by each. In an interview, Dr. Baber said the studies had unique take-home messages but together highlight important issues of nonadherence.
MASTER DAPT: Too much medicine
The subanalysis was performed on data generated by MASTER DAPT, a study evaluating whether a relatively short course of dual-antiplatelet therapy (DAPT) in patients at high risk of bleeding could preserve protection against major adverse cardiovascular events (MACE) while reducing risk of adverse events. The problem was that nonadherence muddied the primary message.
In MASTER DAPT, 1 month of DAPT was compared with a standard therapy of at least 2 additional months of DAPT following revascularization and placement of a biodegradable polymer stent. Enrollment in the study was restricted to those with a high risk of bleeding, the report of the primary results showed.
The major message of MASTER DAPT was that the abbreviated course of DAPT was noninferior for preventing MACE but resulted in lower rates of clinically relevant bleeding in those patients without an indication for oral anticoagulation (OAC). In the subgroup with an indication for OAC, there was no bleeding benefit.
However, when the results were reexamined in the context of adherence, the benefit of the shorter course was found to be underestimated. Relative to 9.4% in the standard-therapy arm, the nonadherence rate in the experimental arm was 20.2%, most of whom did not stop therapy at 1 month. They instead remained on the antiplatelet therapy, failing to adhere to the study protocol.
This form of nonadherence, taking more DAPT than assigned, was particularly common in the group with an indication for oral anticoagulation (OAC). In this group, nearly 25% assigned to an abbreviated course remained on DAPT for more than 6 months.
In the intention-to-treat analysis, there was no difference between abbreviated and standard DAPT for MACE whether or not patients had an indication for OAC. In other words, the new analysis showed a reduced risk of bleeding among all patients, whether taking OAC or not after controlling for nonadherence.
In addition, this MASTER DAPT analysis found that a high proportion of patients taking OAC did not discontinue their single-antiplatelet therapy (SAPT) after 6 months as specified.
When correcting for this failure to adhere to the MASTER DAPT protocol in a patient population at high bleeding risk, the new analysis “suggests for the first time that discontinuation of SAPT at 6 months after percutaneous intervention is associated with less bleeding without an increase in ischemic events,” Marco Valgimigli, MD, PhD, director of clinical research, Inselspital University Hospital, Bern, Switzerland, reported in the Journal of the American College of Cardiology.
The findings “reinforce the importance of accounting and correcting for nonadherence” in order to reduce bias in the assessment of treatment effects, according to Dr. Valgimigli, principal investigator of MASTER DAPT and this substudy.
“The first interesting message from this study is that clinicians are reluctant to stop SAPT in these patients even in the setting of a randomized controlled trial,” Dr. Valgimigli said in an interview.
In addition, this substudy, which was prespecified in the MASTER DAPT protocol and employed “a very sophisticated methodology” to control for the effect of adherence, extends the value of a conservative approach to those who are candidates for OAC.
“The main clinical message is that SAPT needs to be discontinued after 6 months in OAC patients, and clinicians need to stop being reluctant to do so,” Dr. Valgimigli said. The data show “prolongation of SAPT increases bleeding risk without decreasing ischemic risk.”
In evaluating trial relevance, regulators prefer ITT analyses, but Dr. Baber pointed out that these can obscure the evidence of risk or benefit of a per-protocol analysis when patients take their medicine as prescribed.
“The technical message is that, when we are trying to apply results of a clinical trial to daily practice, we must understand nonadherence,” Dr. Baber said.
Dr. Baber pointed out that the lack of adherence in the case of MASTER DAPT appears to relate more to clinicians managing the patients than to the patients themselves, but it still speaks to the importance of understanding the effects of treatment in the context of the medicine rather than adherence to the medicine.
ISCHEMIA: Reconsidering adherence
In the ISCHEMIA trial, the goal was to evaluate whether an early invasive intervention might compensate to at least some degree for the persistent problem of nonadherence.
“If you are managing a patient that you know is at high risk of noncompliance, many clinicians are tempted to perform early revascularization. This was my bias. The thinking is that by offering an invasive therapy we are at least doing something to control their disease,” John A. Spertus, MD, clinical director of outcomes research, St. Luke’s Mid America Heart Institute, Kansas City, Mo., explained in an interview.
The study did not support the hypothesis. Patients with chronic coronary disease were randomized to a strategy of angiography and, if indicated, revascularization, or to receive GDMT alone. The health status was followed with the Seattle Angina Questionnaire (SAQ-7).
At 12 months, patients who were adherent to GDMT had better SAQ-7 scores than those who were nonadherent, regardless of the arm to which they were randomized. Conversely, there was no difference in SAQ-7 scores between the two groups when the nonadherent subgroups in each arm were compared.
“I think these data suggest that an interventional therapy does not absolve clinicians from the responsibility of educating patients about the importance of adhering to GDMT,” Dr. Spertus said.
In ISCHEMIA, 4,480 patients were randomized. At baseline assessment 27.8% were nonadherent to GDMT. The baselines SAQ-7 scores were worse in these patients relative to those who were adherent. At 12 months, nonadherence still correlated with worse SAQ-7 scores.
“These data dispel the belief that we might be benefiting nonadherent patients by moving more quickly to invasive procedures,” Dr. Spertus said.
In cardiovascular disease, particularly heart failure, adherence to GDMT has been associated numerous times with improved quality of life, according to Dr. Baber. However, he said, the ability of invasive procedures to modify the adverse impact of poor adherence to GDMT has not been well studied. This ISCHEMIA subanalysis only reinforces the message that GDMT adherence is a meaningful predictor of improved quality of life.
However, urging clinicians to work with patients to improve adherence is not a novel idea, according to Dr. Baber. The unmet need is effective and reliable strategies.
“There are so many different reasons that patients are nonadherent, so there are limited gains by focusing on just one of the issues,” Dr. Baber said. “I think the answer is a patient-centric approach in which clinicians deal with the specific issues facing the patient in front of them. I think there are data go suggest this yields better results.”
These two very different studies also show that poor adherence is an insidious issue. While the MASTER DAPT data reveal how nonadherence confuse trial data, the ISCHEMIA trial shows that some assumptions about circumventing the effects of nonadherence might not be accurate.
According to Dr. Baber, effective strategies to reduce nonadherence are available, but the problem deserves to be addressed more proactively in clinical trials and in patient care.
Dr. Baber reported financial relationships with AstraZeneca and Amgen. Dr. Spertus has financial relationships with Abbott, Bayer, Bristol-Myers Squibb, Corvia, Janssen, Merck, Novartis, Pfizer and Terumo. Dr. Valgimigli has financial relationships with more than 15 pharmaceutical companies, including Terumo, which provided funding for the MASTER DAPT trial.
Each study adds new twist
Each study adds new twist
Two very different sets of clinical evidence have offered new twists on how nonadherence to cardiovascular medicines not only leads to suboptimal outcomes, but also complicates the data from clinical studies.
One study, a subanalysis of a major trial, outlined how taking more than the assigned therapy – that is, nonadherence by taking too much rather than too little – skewed results. The other was a trial demonstrating that early use of an invasive procedure is not a strategy to compensate for nonadherence to guideline-directed medical therapy (GDMT).
“Both studies provide a fresh reminder that nonadherence is a significant problem in cardiology overall, but also in the trial setting when we are trying to interpret study results,” explained Usam Baber, MD, director of interventional cardiology, University of Oklahoma Health, Oklahoma City, coauthor of an editorial accompanying the two published studies.
Dr. Baber was the first author of a unifying editorial that addressed the issues raised by each. In an interview, Dr. Baber said the studies had unique take-home messages but together highlight important issues of nonadherence.
MASTER DAPT: Too much medicine
The subanalysis was performed on data generated by MASTER DAPT, a study evaluating whether a relatively short course of dual-antiplatelet therapy (DAPT) in patients at high risk of bleeding could preserve protection against major adverse cardiovascular events (MACE) while reducing risk of adverse events. The problem was that nonadherence muddied the primary message.
In MASTER DAPT, 1 month of DAPT was compared with a standard therapy of at least 2 additional months of DAPT following revascularization and placement of a biodegradable polymer stent. Enrollment in the study was restricted to those with a high risk of bleeding, the report of the primary results showed.
The major message of MASTER DAPT was that the abbreviated course of DAPT was noninferior for preventing MACE but resulted in lower rates of clinically relevant bleeding in those patients without an indication for oral anticoagulation (OAC). In the subgroup with an indication for OAC, there was no bleeding benefit.
However, when the results were reexamined in the context of adherence, the benefit of the shorter course was found to be underestimated. Relative to 9.4% in the standard-therapy arm, the nonadherence rate in the experimental arm was 20.2%, most of whom did not stop therapy at 1 month. They instead remained on the antiplatelet therapy, failing to adhere to the study protocol.
This form of nonadherence, taking more DAPT than assigned, was particularly common in the group with an indication for oral anticoagulation (OAC). In this group, nearly 25% assigned to an abbreviated course remained on DAPT for more than 6 months.
In the intention-to-treat analysis, there was no difference between abbreviated and standard DAPT for MACE whether or not patients had an indication for OAC. In other words, the new analysis showed a reduced risk of bleeding among all patients, whether taking OAC or not after controlling for nonadherence.
In addition, this MASTER DAPT analysis found that a high proportion of patients taking OAC did not discontinue their single-antiplatelet therapy (SAPT) after 6 months as specified.
When correcting for this failure to adhere to the MASTER DAPT protocol in a patient population at high bleeding risk, the new analysis “suggests for the first time that discontinuation of SAPT at 6 months after percutaneous intervention is associated with less bleeding without an increase in ischemic events,” Marco Valgimigli, MD, PhD, director of clinical research, Inselspital University Hospital, Bern, Switzerland, reported in the Journal of the American College of Cardiology.
The findings “reinforce the importance of accounting and correcting for nonadherence” in order to reduce bias in the assessment of treatment effects, according to Dr. Valgimigli, principal investigator of MASTER DAPT and this substudy.
“The first interesting message from this study is that clinicians are reluctant to stop SAPT in these patients even in the setting of a randomized controlled trial,” Dr. Valgimigli said in an interview.
In addition, this substudy, which was prespecified in the MASTER DAPT protocol and employed “a very sophisticated methodology” to control for the effect of adherence, extends the value of a conservative approach to those who are candidates for OAC.
“The main clinical message is that SAPT needs to be discontinued after 6 months in OAC patients, and clinicians need to stop being reluctant to do so,” Dr. Valgimigli said. The data show “prolongation of SAPT increases bleeding risk without decreasing ischemic risk.”
In evaluating trial relevance, regulators prefer ITT analyses, but Dr. Baber pointed out that these can obscure the evidence of risk or benefit of a per-protocol analysis when patients take their medicine as prescribed.
“The technical message is that, when we are trying to apply results of a clinical trial to daily practice, we must understand nonadherence,” Dr. Baber said.
Dr. Baber pointed out that the lack of adherence in the case of MASTER DAPT appears to relate more to clinicians managing the patients than to the patients themselves, but it still speaks to the importance of understanding the effects of treatment in the context of the medicine rather than adherence to the medicine.
ISCHEMIA: Reconsidering adherence
In the ISCHEMIA trial, the goal was to evaluate whether an early invasive intervention might compensate to at least some degree for the persistent problem of nonadherence.
“If you are managing a patient that you know is at high risk of noncompliance, many clinicians are tempted to perform early revascularization. This was my bias. The thinking is that by offering an invasive therapy we are at least doing something to control their disease,” John A. Spertus, MD, clinical director of outcomes research, St. Luke’s Mid America Heart Institute, Kansas City, Mo., explained in an interview.
The study did not support the hypothesis. Patients with chronic coronary disease were randomized to a strategy of angiography and, if indicated, revascularization, or to receive GDMT alone. The health status was followed with the Seattle Angina Questionnaire (SAQ-7).
At 12 months, patients who were adherent to GDMT had better SAQ-7 scores than those who were nonadherent, regardless of the arm to which they were randomized. Conversely, there was no difference in SAQ-7 scores between the two groups when the nonadherent subgroups in each arm were compared.
“I think these data suggest that an interventional therapy does not absolve clinicians from the responsibility of educating patients about the importance of adhering to GDMT,” Dr. Spertus said.
In ISCHEMIA, 4,480 patients were randomized. At baseline assessment 27.8% were nonadherent to GDMT. The baselines SAQ-7 scores were worse in these patients relative to those who were adherent. At 12 months, nonadherence still correlated with worse SAQ-7 scores.
“These data dispel the belief that we might be benefiting nonadherent patients by moving more quickly to invasive procedures,” Dr. Spertus said.
In cardiovascular disease, particularly heart failure, adherence to GDMT has been associated numerous times with improved quality of life, according to Dr. Baber. However, he said, the ability of invasive procedures to modify the adverse impact of poor adherence to GDMT has not been well studied. This ISCHEMIA subanalysis only reinforces the message that GDMT adherence is a meaningful predictor of improved quality of life.
However, urging clinicians to work with patients to improve adherence is not a novel idea, according to Dr. Baber. The unmet need is effective and reliable strategies.
“There are so many different reasons that patients are nonadherent, so there are limited gains by focusing on just one of the issues,” Dr. Baber said. “I think the answer is a patient-centric approach in which clinicians deal with the specific issues facing the patient in front of them. I think there are data go suggest this yields better results.”
These two very different studies also show that poor adherence is an insidious issue. While the MASTER DAPT data reveal how nonadherence confuse trial data, the ISCHEMIA trial shows that some assumptions about circumventing the effects of nonadherence might not be accurate.
According to Dr. Baber, effective strategies to reduce nonadherence are available, but the problem deserves to be addressed more proactively in clinical trials and in patient care.
Dr. Baber reported financial relationships with AstraZeneca and Amgen. Dr. Spertus has financial relationships with Abbott, Bayer, Bristol-Myers Squibb, Corvia, Janssen, Merck, Novartis, Pfizer and Terumo. Dr. Valgimigli has financial relationships with more than 15 pharmaceutical companies, including Terumo, which provided funding for the MASTER DAPT trial.
Two very different sets of clinical evidence have offered new twists on how nonadherence to cardiovascular medicines not only leads to suboptimal outcomes, but also complicates the data from clinical studies.
One study, a subanalysis of a major trial, outlined how taking more than the assigned therapy – that is, nonadherence by taking too much rather than too little – skewed results. The other was a trial demonstrating that early use of an invasive procedure is not a strategy to compensate for nonadherence to guideline-directed medical therapy (GDMT).
“Both studies provide a fresh reminder that nonadherence is a significant problem in cardiology overall, but also in the trial setting when we are trying to interpret study results,” explained Usam Baber, MD, director of interventional cardiology, University of Oklahoma Health, Oklahoma City, coauthor of an editorial accompanying the two published studies.
Dr. Baber was the first author of a unifying editorial that addressed the issues raised by each. In an interview, Dr. Baber said the studies had unique take-home messages but together highlight important issues of nonadherence.
MASTER DAPT: Too much medicine
The subanalysis was performed on data generated by MASTER DAPT, a study evaluating whether a relatively short course of dual-antiplatelet therapy (DAPT) in patients at high risk of bleeding could preserve protection against major adverse cardiovascular events (MACE) while reducing risk of adverse events. The problem was that nonadherence muddied the primary message.
In MASTER DAPT, 1 month of DAPT was compared with a standard therapy of at least 2 additional months of DAPT following revascularization and placement of a biodegradable polymer stent. Enrollment in the study was restricted to those with a high risk of bleeding, the report of the primary results showed.
The major message of MASTER DAPT was that the abbreviated course of DAPT was noninferior for preventing MACE but resulted in lower rates of clinically relevant bleeding in those patients without an indication for oral anticoagulation (OAC). In the subgroup with an indication for OAC, there was no bleeding benefit.
However, when the results were reexamined in the context of adherence, the benefit of the shorter course was found to be underestimated. Relative to 9.4% in the standard-therapy arm, the nonadherence rate in the experimental arm was 20.2%, most of whom did not stop therapy at 1 month. They instead remained on the antiplatelet therapy, failing to adhere to the study protocol.
This form of nonadherence, taking more DAPT than assigned, was particularly common in the group with an indication for oral anticoagulation (OAC). In this group, nearly 25% assigned to an abbreviated course remained on DAPT for more than 6 months.
In the intention-to-treat analysis, there was no difference between abbreviated and standard DAPT for MACE whether or not patients had an indication for OAC. In other words, the new analysis showed a reduced risk of bleeding among all patients, whether taking OAC or not after controlling for nonadherence.
In addition, this MASTER DAPT analysis found that a high proportion of patients taking OAC did not discontinue their single-antiplatelet therapy (SAPT) after 6 months as specified.
When correcting for this failure to adhere to the MASTER DAPT protocol in a patient population at high bleeding risk, the new analysis “suggests for the first time that discontinuation of SAPT at 6 months after percutaneous intervention is associated with less bleeding without an increase in ischemic events,” Marco Valgimigli, MD, PhD, director of clinical research, Inselspital University Hospital, Bern, Switzerland, reported in the Journal of the American College of Cardiology.
The findings “reinforce the importance of accounting and correcting for nonadherence” in order to reduce bias in the assessment of treatment effects, according to Dr. Valgimigli, principal investigator of MASTER DAPT and this substudy.
“The first interesting message from this study is that clinicians are reluctant to stop SAPT in these patients even in the setting of a randomized controlled trial,” Dr. Valgimigli said in an interview.
In addition, this substudy, which was prespecified in the MASTER DAPT protocol and employed “a very sophisticated methodology” to control for the effect of adherence, extends the value of a conservative approach to those who are candidates for OAC.
“The main clinical message is that SAPT needs to be discontinued after 6 months in OAC patients, and clinicians need to stop being reluctant to do so,” Dr. Valgimigli said. The data show “prolongation of SAPT increases bleeding risk without decreasing ischemic risk.”
In evaluating trial relevance, regulators prefer ITT analyses, but Dr. Baber pointed out that these can obscure the evidence of risk or benefit of a per-protocol analysis when patients take their medicine as prescribed.
“The technical message is that, when we are trying to apply results of a clinical trial to daily practice, we must understand nonadherence,” Dr. Baber said.
Dr. Baber pointed out that the lack of adherence in the case of MASTER DAPT appears to relate more to clinicians managing the patients than to the patients themselves, but it still speaks to the importance of understanding the effects of treatment in the context of the medicine rather than adherence to the medicine.
ISCHEMIA: Reconsidering adherence
In the ISCHEMIA trial, the goal was to evaluate whether an early invasive intervention might compensate to at least some degree for the persistent problem of nonadherence.
“If you are managing a patient that you know is at high risk of noncompliance, many clinicians are tempted to perform early revascularization. This was my bias. The thinking is that by offering an invasive therapy we are at least doing something to control their disease,” John A. Spertus, MD, clinical director of outcomes research, St. Luke’s Mid America Heart Institute, Kansas City, Mo., explained in an interview.
The study did not support the hypothesis. Patients with chronic coronary disease were randomized to a strategy of angiography and, if indicated, revascularization, or to receive GDMT alone. The health status was followed with the Seattle Angina Questionnaire (SAQ-7).
At 12 months, patients who were adherent to GDMT had better SAQ-7 scores than those who were nonadherent, regardless of the arm to which they were randomized. Conversely, there was no difference in SAQ-7 scores between the two groups when the nonadherent subgroups in each arm were compared.
“I think these data suggest that an interventional therapy does not absolve clinicians from the responsibility of educating patients about the importance of adhering to GDMT,” Dr. Spertus said.
In ISCHEMIA, 4,480 patients were randomized. At baseline assessment 27.8% were nonadherent to GDMT. The baselines SAQ-7 scores were worse in these patients relative to those who were adherent. At 12 months, nonadherence still correlated with worse SAQ-7 scores.
“These data dispel the belief that we might be benefiting nonadherent patients by moving more quickly to invasive procedures,” Dr. Spertus said.
In cardiovascular disease, particularly heart failure, adherence to GDMT has been associated numerous times with improved quality of life, according to Dr. Baber. However, he said, the ability of invasive procedures to modify the adverse impact of poor adherence to GDMT has not been well studied. This ISCHEMIA subanalysis only reinforces the message that GDMT adherence is a meaningful predictor of improved quality of life.
However, urging clinicians to work with patients to improve adherence is not a novel idea, according to Dr. Baber. The unmet need is effective and reliable strategies.
“There are so many different reasons that patients are nonadherent, so there are limited gains by focusing on just one of the issues,” Dr. Baber said. “I think the answer is a patient-centric approach in which clinicians deal with the specific issues facing the patient in front of them. I think there are data go suggest this yields better results.”
These two very different studies also show that poor adherence is an insidious issue. While the MASTER DAPT data reveal how nonadherence confuse trial data, the ISCHEMIA trial shows that some assumptions about circumventing the effects of nonadherence might not be accurate.
According to Dr. Baber, effective strategies to reduce nonadherence are available, but the problem deserves to be addressed more proactively in clinical trials and in patient care.
Dr. Baber reported financial relationships with AstraZeneca and Amgen. Dr. Spertus has financial relationships with Abbott, Bayer, Bristol-Myers Squibb, Corvia, Janssen, Merck, Novartis, Pfizer and Terumo. Dr. Valgimigli has financial relationships with more than 15 pharmaceutical companies, including Terumo, which provided funding for the MASTER DAPT trial.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Early LV recovery after TAVR tied to 5-year mortality
Early improvement of left ventricular ejection fraction (LVEF) after transcatheter aortic valve replacement (TAVR) is associated with improved all-cause and cardiac death at 5 years in patients with severe aortic stenosis and LVEF less than 50%, new research shows.
Further analyses revealed a significant interaction by sex, with the mortality benefit largely in women.
“It’s absolutely fascinating,” senior author Sammy Elmariah, MD, Massachusetts General Hospital, Boston, said of the finding. “We know that women are more likely to have concentric hypertrophy, that they have lesser degrees of fibrosis, and smaller ventricles, and, of course, they’re in general less affected by coronary artery disease and MIs [myocardial infarctions]. All of those things in my mind, at least that’s what I assumed ahead of time, would make it more likely for women’s hearts to recover.”
“But that’s actually not what we found,” he continued. “We didn’t see a difference between the sexes in terms of likelihood of recovery. But what we saw is that the survival benefit, that associates with improvement in EF, was almost completely driven by women. So women really seem to be reaping that benefit in a manner that is unique and very different from what we saw in men.”
Dr. Elmariah noted that the reason for this benefit is unclear but points to the differences in biology for LV remodeling. “Clearly there are several details there that warrant further attention and more research.”
Suzanne J. Baron, MD, director of interventional cardiology research at Lahey Hospital and Medical Center, Burlington, Mass., said in an email that the finding of a substantial long-term survival benefit was “a bit surprising.”
Several studies have suggested that women may derive a greater benefit from TAVR versus surgical aortic valve replacement, and meta-analyses have demonstrated short and intermediate-term survival after TAVR is better in women, compared with in men, she pointed out. However, the mediating mechanism for this finding has never been clearly elucidated.
“Certainly, the sex differences in LVEF improvement after TAVR observed in this study, which could be related to sex differences in LV remodeling and LV mass regression, may now give us a clue as to why these sex-specific survival differences after TAVR persist,” Dr. Baron said.
More data amassed
Previous research in smaller cohorts with follow-up out to 1 year have shown an association between early LVEF improvement after TAVR and better survival. This includes a 2013 study by the investigators in high-risk patients in PARTNER-1 and a separate 2016 study in patients in the CoreValve extreme and high surgical risk trials.
Now, with longer follow-up amassed, the investigators examined data from 659 high- or intermediate-risk patients with severe stenosis and LVEF less than 50% who underwent transfemoral TAVR in the PARTNER 1, 2, and S3 trials and registries between July 2007 and April 2015.
Their mean age was 82.4 years, 71% were men, and 89.7% were White individuals. During the study period, 55.6% of the cohort died.
As reported in JAMA Cardiology, 32.8% of patients had early LVEF improvement, defined as an increase of at least 10% percentage points at 30 days after TAVR (mean change, 16.4%).
This compares with about 50%-60% of patients in the earlier studies, likely owing to the relatively higher baseline LVEF, especially in the intermediate-risk cohort, the authors suggested.
Independent predictors of lower likelihood of early LVEF improvement were previous MI, diabetes, cancer, higher baseline LVEF, larger LV end-diastolic diameter, and larger aortic valve area (AVA), whereas higher body mass index and higher stroke volume index predicted greater likelihood of LV recovery.
At 5 years, patients with versus without improved early LV improvement had lower risks of all-cause death (50.0% vs. 58.4%; P = .04) and cardiac death (29.5% vs. 38.1%; P = .05).
In multivariable analyses, each 5%-point increase in LVEF after TAVR was associated with a 6% lower risk of all-cause death (hazard ratio [HR], 0.94; P = .04) and 10% lower risk of cardiac death (HR, 0.90; P = .02).
Restricted cubic spline analysis demonstrated an inflection point above a 10% change in LVEF beyond which there was a steep decline in all-cause mortality with increasing degree of LVEF improvement.
There were no significant differences in rehospitalization, New York Heart Association functional class, or Kansas City Cardiomyopathy Questionnaire score at 5 years in patients with and without early LVEF improvement.
“I think what this really gets to is what is the reason behind the LV dysfunction in the first place,” said Dr. Elmariah, soon to be joining the University of California, San Francisco. “We know that TAVR cures aortic stenosis, so if the LV dysfunction is primarily related to the valve itself, hopefully those patients are going to recover.”
On the other hand, if the patient has LV dysfunction because of a prior myocardial infarction or cardiomyopathy and then developed aortic stenosis, “you can treat the aortic stenosis but the heart is still diseased from whatever process was affecting it previously and so it’s not likely to recover in those scenarios,” he added.
The results can be used for counseling patients and highlight the need to optimize goal-directed medical therapy in those with valvular heart disease, Dr. Elmariah suggested.
“Often, patients with aortic stenosis are on miniscule doses of many of the heart failure agents because people are worried about the hemodynamic consequences and they’re worried that patients won’t tolerate these medications,” he said. “But it’s very important for us to aggressively try to treat the heart failure that is affecting these patients in order to hopefully increase the chances that their left ventricles will recover and, hopefully, that they will have improved survival.”
Dr. Baron said that “this study clearly demonstrates that patients with reduced LVEF and severe aortic stenosis can benefit from TAVR and that early improvement in LVEF is an important prognostic marker for this population.”
In Dr. Baron and colleagues’ earlier analysis of 11,000 patients who underwent TAVR as part of the transcatheter valve therapy registry, only low aortic valve gradient but not LV dysfunction was associated with higher adjusted 1-year mortality. Asked about the finding, she noted that patients were evaluated based on LV function at baseline and not for a difference in outcomes based on LVEF improvement after TAVR.
“As such, I think that these two studies are actually complementary,” Dr. Baron said. “Together, they suggest that a low LVEF should not preclude a patient from receiving TAVR and if the patient does experience a 10% increase in LVEF after TAVR, then their 5-year prognosis is improved.”
Dr. Elmariah reports grants from the American Heart Association, National Institutes of Health, Edwards Lifesciences, Medtronic, and Svelte Medical and has received consulting fees from Medtronic and AstraZeneca. Coauthor disclosures are listed in the paper. The PARTNER trials and registries and this analysis were supported by Edwards Lifesciences. Edwards was involved in the design and conduct of the study including collection, management, analysis, and interpretation of the data. Dr. Baron reports receiving research grant funding from Abiomed and Boston Scientific; consulting/medical advisory board fees from Boston Scientific, Shockwave and Biotronik; and speaking honoraria from Medtronic and Zoll.
A version of this article first appeared on Medscape.com.
Early improvement of left ventricular ejection fraction (LVEF) after transcatheter aortic valve replacement (TAVR) is associated with improved all-cause and cardiac death at 5 years in patients with severe aortic stenosis and LVEF less than 50%, new research shows.
Further analyses revealed a significant interaction by sex, with the mortality benefit largely in women.
“It’s absolutely fascinating,” senior author Sammy Elmariah, MD, Massachusetts General Hospital, Boston, said of the finding. “We know that women are more likely to have concentric hypertrophy, that they have lesser degrees of fibrosis, and smaller ventricles, and, of course, they’re in general less affected by coronary artery disease and MIs [myocardial infarctions]. All of those things in my mind, at least that’s what I assumed ahead of time, would make it more likely for women’s hearts to recover.”
“But that’s actually not what we found,” he continued. “We didn’t see a difference between the sexes in terms of likelihood of recovery. But what we saw is that the survival benefit, that associates with improvement in EF, was almost completely driven by women. So women really seem to be reaping that benefit in a manner that is unique and very different from what we saw in men.”
Dr. Elmariah noted that the reason for this benefit is unclear but points to the differences in biology for LV remodeling. “Clearly there are several details there that warrant further attention and more research.”
Suzanne J. Baron, MD, director of interventional cardiology research at Lahey Hospital and Medical Center, Burlington, Mass., said in an email that the finding of a substantial long-term survival benefit was “a bit surprising.”
Several studies have suggested that women may derive a greater benefit from TAVR versus surgical aortic valve replacement, and meta-analyses have demonstrated short and intermediate-term survival after TAVR is better in women, compared with in men, she pointed out. However, the mediating mechanism for this finding has never been clearly elucidated.
“Certainly, the sex differences in LVEF improvement after TAVR observed in this study, which could be related to sex differences in LV remodeling and LV mass regression, may now give us a clue as to why these sex-specific survival differences after TAVR persist,” Dr. Baron said.
More data amassed
Previous research in smaller cohorts with follow-up out to 1 year have shown an association between early LVEF improvement after TAVR and better survival. This includes a 2013 study by the investigators in high-risk patients in PARTNER-1 and a separate 2016 study in patients in the CoreValve extreme and high surgical risk trials.
Now, with longer follow-up amassed, the investigators examined data from 659 high- or intermediate-risk patients with severe stenosis and LVEF less than 50% who underwent transfemoral TAVR in the PARTNER 1, 2, and S3 trials and registries between July 2007 and April 2015.
Their mean age was 82.4 years, 71% were men, and 89.7% were White individuals. During the study period, 55.6% of the cohort died.
As reported in JAMA Cardiology, 32.8% of patients had early LVEF improvement, defined as an increase of at least 10% percentage points at 30 days after TAVR (mean change, 16.4%).
This compares with about 50%-60% of patients in the earlier studies, likely owing to the relatively higher baseline LVEF, especially in the intermediate-risk cohort, the authors suggested.
Independent predictors of lower likelihood of early LVEF improvement were previous MI, diabetes, cancer, higher baseline LVEF, larger LV end-diastolic diameter, and larger aortic valve area (AVA), whereas higher body mass index and higher stroke volume index predicted greater likelihood of LV recovery.
At 5 years, patients with versus without improved early LV improvement had lower risks of all-cause death (50.0% vs. 58.4%; P = .04) and cardiac death (29.5% vs. 38.1%; P = .05).
In multivariable analyses, each 5%-point increase in LVEF after TAVR was associated with a 6% lower risk of all-cause death (hazard ratio [HR], 0.94; P = .04) and 10% lower risk of cardiac death (HR, 0.90; P = .02).
Restricted cubic spline analysis demonstrated an inflection point above a 10% change in LVEF beyond which there was a steep decline in all-cause mortality with increasing degree of LVEF improvement.
There were no significant differences in rehospitalization, New York Heart Association functional class, or Kansas City Cardiomyopathy Questionnaire score at 5 years in patients with and without early LVEF improvement.
“I think what this really gets to is what is the reason behind the LV dysfunction in the first place,” said Dr. Elmariah, soon to be joining the University of California, San Francisco. “We know that TAVR cures aortic stenosis, so if the LV dysfunction is primarily related to the valve itself, hopefully those patients are going to recover.”
On the other hand, if the patient has LV dysfunction because of a prior myocardial infarction or cardiomyopathy and then developed aortic stenosis, “you can treat the aortic stenosis but the heart is still diseased from whatever process was affecting it previously and so it’s not likely to recover in those scenarios,” he added.
The results can be used for counseling patients and highlight the need to optimize goal-directed medical therapy in those with valvular heart disease, Dr. Elmariah suggested.
“Often, patients with aortic stenosis are on miniscule doses of many of the heart failure agents because people are worried about the hemodynamic consequences and they’re worried that patients won’t tolerate these medications,” he said. “But it’s very important for us to aggressively try to treat the heart failure that is affecting these patients in order to hopefully increase the chances that their left ventricles will recover and, hopefully, that they will have improved survival.”
Dr. Baron said that “this study clearly demonstrates that patients with reduced LVEF and severe aortic stenosis can benefit from TAVR and that early improvement in LVEF is an important prognostic marker for this population.”
In Dr. Baron and colleagues’ earlier analysis of 11,000 patients who underwent TAVR as part of the transcatheter valve therapy registry, only low aortic valve gradient but not LV dysfunction was associated with higher adjusted 1-year mortality. Asked about the finding, she noted that patients were evaluated based on LV function at baseline and not for a difference in outcomes based on LVEF improvement after TAVR.
“As such, I think that these two studies are actually complementary,” Dr. Baron said. “Together, they suggest that a low LVEF should not preclude a patient from receiving TAVR and if the patient does experience a 10% increase in LVEF after TAVR, then their 5-year prognosis is improved.”
Dr. Elmariah reports grants from the American Heart Association, National Institutes of Health, Edwards Lifesciences, Medtronic, and Svelte Medical and has received consulting fees from Medtronic and AstraZeneca. Coauthor disclosures are listed in the paper. The PARTNER trials and registries and this analysis were supported by Edwards Lifesciences. Edwards was involved in the design and conduct of the study including collection, management, analysis, and interpretation of the data. Dr. Baron reports receiving research grant funding from Abiomed and Boston Scientific; consulting/medical advisory board fees from Boston Scientific, Shockwave and Biotronik; and speaking honoraria from Medtronic and Zoll.
A version of this article first appeared on Medscape.com.
Early improvement of left ventricular ejection fraction (LVEF) after transcatheter aortic valve replacement (TAVR) is associated with improved all-cause and cardiac death at 5 years in patients with severe aortic stenosis and LVEF less than 50%, new research shows.
Further analyses revealed a significant interaction by sex, with the mortality benefit largely in women.
“It’s absolutely fascinating,” senior author Sammy Elmariah, MD, Massachusetts General Hospital, Boston, said of the finding. “We know that women are more likely to have concentric hypertrophy, that they have lesser degrees of fibrosis, and smaller ventricles, and, of course, they’re in general less affected by coronary artery disease and MIs [myocardial infarctions]. All of those things in my mind, at least that’s what I assumed ahead of time, would make it more likely for women’s hearts to recover.”
“But that’s actually not what we found,” he continued. “We didn’t see a difference between the sexes in terms of likelihood of recovery. But what we saw is that the survival benefit, that associates with improvement in EF, was almost completely driven by women. So women really seem to be reaping that benefit in a manner that is unique and very different from what we saw in men.”
Dr. Elmariah noted that the reason for this benefit is unclear but points to the differences in biology for LV remodeling. “Clearly there are several details there that warrant further attention and more research.”
Suzanne J. Baron, MD, director of interventional cardiology research at Lahey Hospital and Medical Center, Burlington, Mass., said in an email that the finding of a substantial long-term survival benefit was “a bit surprising.”
Several studies have suggested that women may derive a greater benefit from TAVR versus surgical aortic valve replacement, and meta-analyses have demonstrated short and intermediate-term survival after TAVR is better in women, compared with in men, she pointed out. However, the mediating mechanism for this finding has never been clearly elucidated.
“Certainly, the sex differences in LVEF improvement after TAVR observed in this study, which could be related to sex differences in LV remodeling and LV mass regression, may now give us a clue as to why these sex-specific survival differences after TAVR persist,” Dr. Baron said.
More data amassed
Previous research in smaller cohorts with follow-up out to 1 year have shown an association between early LVEF improvement after TAVR and better survival. This includes a 2013 study by the investigators in high-risk patients in PARTNER-1 and a separate 2016 study in patients in the CoreValve extreme and high surgical risk trials.
Now, with longer follow-up amassed, the investigators examined data from 659 high- or intermediate-risk patients with severe stenosis and LVEF less than 50% who underwent transfemoral TAVR in the PARTNER 1, 2, and S3 trials and registries between July 2007 and April 2015.
Their mean age was 82.4 years, 71% were men, and 89.7% were White individuals. During the study period, 55.6% of the cohort died.
As reported in JAMA Cardiology, 32.8% of patients had early LVEF improvement, defined as an increase of at least 10% percentage points at 30 days after TAVR (mean change, 16.4%).
This compares with about 50%-60% of patients in the earlier studies, likely owing to the relatively higher baseline LVEF, especially in the intermediate-risk cohort, the authors suggested.
Independent predictors of lower likelihood of early LVEF improvement were previous MI, diabetes, cancer, higher baseline LVEF, larger LV end-diastolic diameter, and larger aortic valve area (AVA), whereas higher body mass index and higher stroke volume index predicted greater likelihood of LV recovery.
At 5 years, patients with versus without improved early LV improvement had lower risks of all-cause death (50.0% vs. 58.4%; P = .04) and cardiac death (29.5% vs. 38.1%; P = .05).
In multivariable analyses, each 5%-point increase in LVEF after TAVR was associated with a 6% lower risk of all-cause death (hazard ratio [HR], 0.94; P = .04) and 10% lower risk of cardiac death (HR, 0.90; P = .02).
Restricted cubic spline analysis demonstrated an inflection point above a 10% change in LVEF beyond which there was a steep decline in all-cause mortality with increasing degree of LVEF improvement.
There were no significant differences in rehospitalization, New York Heart Association functional class, or Kansas City Cardiomyopathy Questionnaire score at 5 years in patients with and without early LVEF improvement.
“I think what this really gets to is what is the reason behind the LV dysfunction in the first place,” said Dr. Elmariah, soon to be joining the University of California, San Francisco. “We know that TAVR cures aortic stenosis, so if the LV dysfunction is primarily related to the valve itself, hopefully those patients are going to recover.”
On the other hand, if the patient has LV dysfunction because of a prior myocardial infarction or cardiomyopathy and then developed aortic stenosis, “you can treat the aortic stenosis but the heart is still diseased from whatever process was affecting it previously and so it’s not likely to recover in those scenarios,” he added.
The results can be used for counseling patients and highlight the need to optimize goal-directed medical therapy in those with valvular heart disease, Dr. Elmariah suggested.
“Often, patients with aortic stenosis are on miniscule doses of many of the heart failure agents because people are worried about the hemodynamic consequences and they’re worried that patients won’t tolerate these medications,” he said. “But it’s very important for us to aggressively try to treat the heart failure that is affecting these patients in order to hopefully increase the chances that their left ventricles will recover and, hopefully, that they will have improved survival.”
Dr. Baron said that “this study clearly demonstrates that patients with reduced LVEF and severe aortic stenosis can benefit from TAVR and that early improvement in LVEF is an important prognostic marker for this population.”
In Dr. Baron and colleagues’ earlier analysis of 11,000 patients who underwent TAVR as part of the transcatheter valve therapy registry, only low aortic valve gradient but not LV dysfunction was associated with higher adjusted 1-year mortality. Asked about the finding, she noted that patients were evaluated based on LV function at baseline and not for a difference in outcomes based on LVEF improvement after TAVR.
“As such, I think that these two studies are actually complementary,” Dr. Baron said. “Together, they suggest that a low LVEF should not preclude a patient from receiving TAVR and if the patient does experience a 10% increase in LVEF after TAVR, then their 5-year prognosis is improved.”
Dr. Elmariah reports grants from the American Heart Association, National Institutes of Health, Edwards Lifesciences, Medtronic, and Svelte Medical and has received consulting fees from Medtronic and AstraZeneca. Coauthor disclosures are listed in the paper. The PARTNER trials and registries and this analysis were supported by Edwards Lifesciences. Edwards was involved in the design and conduct of the study including collection, management, analysis, and interpretation of the data. Dr. Baron reports receiving research grant funding from Abiomed and Boston Scientific; consulting/medical advisory board fees from Boston Scientific, Shockwave and Biotronik; and speaking honoraria from Medtronic and Zoll.
A version of this article first appeared on Medscape.com.
Hot weather risk for nonfatal MI hinted for antiplatelets, beta-blockers
Patients who take beta-blockers or antiplatelet agents are lowering their risk for cardiovascular events, but the protection may fall short for those who spend time outdoors on hot summer days, hints a limited analysis published as a letter in Nature Cardiovascular Research.
Patients taking either a beta-blocker or antiplatelet, or both medications together, appeared at elevated risk for nonfatal acute MI specifically on days when the weather turned hot, suggests the registry cohort study that covered 14 years of clinical and meteorologic data.
“The take-away message is not that patients should stop using these two medications, by no means. We’re raising cautions for patients taking them, to watch out for themselves during high-heat days,” lead author Kai Chen, PhD, Yale University, New Haven, Conn., said in an interview.
“We’re not giving the message that these drugs have harmful effects” because the nature of the links between the medications and MI in the study, with its potential for confounding, remain unknown, said Dr. Chen, from the department of environmental health sciences and Yale Center on Climate Change and Health.
For example, patients who take beta-blockers or antiplatelets tend to be sicker than patients not on the drugs, which could make heat-related MI more likely, and the drugs wrongly appear to be culprits, he observed. The analysis contained signals that could support either scenario.
The study is based on cases of nonfatal MI in Augsburg, Germany, that are part of the MONICA-KORA MI registry. The odds of a heat-related nonfatal MI, it suggests, were increased 63% among patients taking antiplatelets and by 65% among those on beta-blockers, compared with those not on these drugs. The odds went up by 75% among those on both drug classes, but the risks weren’t raised in patients not taking them.
Rising heat-related MI
Chen said analysis was inspired by a 2019 report – also based on MONICA-KORA, from many of the same authors and using similar methods to track events by daily air temperature – that showed a rising trend for heat-related MI and declining rate for MI related to cold weather from 1987 to 2014. A next step, he figured, would be to determine whether the MI risk trends were associated with any cardiovascular medications.
The current study’s signal of risk related to antiplatelets and beta-blockers did not emerge for ACE inhibitors, calcium-channel blockers, or diuretics. Statins showed a link to increased nonfatal MI risk, but solely among participants aged younger than 60 years, who were also far less likely to have pre-existing coronary heart disease (CHD). He and his colleagues chose not to highlight that finding, Dr. Chen said, because the age subgroup analysis was grossly underpowered.
The overall analysis involved 2,494 cases of nonfatal MI that occurred during the warmer months – May to September – from 2001 to 2014. It was limited to nonfatal cases – those with at least a month of survival after hospital admission – because of insufficient data on medication use associated with fatal MIs, the report states.
Nonfatal MIs were defined as heat-related if they struck on days reaching the 95th percentile for temperature across the 14 years, in this case 24.2 °C (about 75.6 °F), relative to the average temperature of lowest nonfatal MI risk across the cohort, 7.5 °C (about 45.5 °F).
Patients served as both cases and their own controls, in that air temperature exposures on the day of their MI (case day) were compared with the remaining same days of the week in the same calendar month (control days). That approach, the report stated, “automatically controls for long-term time trends, seasonality, day of the week, and time-invariant confounders (for example, pre-existing cardiovascular disease).”
The odds ratio for heat-related MI for patients on antiplatelets was 1.63 (95% confidence interval, 1.07-2.46), and for antiplatelet nonusers was 0.94 (95% CI, 0.68-1.29). The difference between the two ratios was significant (P = .04).
The corresponding OR for patients taking beta-blockers was 1.65 (95% CI, 1.11-2.45), and for nonusers of beta-blockers was 0.90 (95% CI, 0.64-1.26). Again, the OR difference was significant (P = .02).
The ORs for users of both medication classes and nonusers of either med class, respectively, were 1.75 (95% CI, 1.12-2.73) and 0.84 (95% CI, 0.59-1.19). The latter OR was significantly lower than former (P = .01).
In a sign that antiplatelet and beta-blocker use might have been just a marker for sicker patients who were more vulnerable to heat-related MI, Chen said, the nonfatal MI risk was significantly elevated (OR, 2.17; 95% CI, 1.40-3.38) among patients with pre-existing CHD, but not among those free of pre-existing CHD (OR, 0.88; 95% CI, 0.65-1.20); the odds difference was P < .01.
That signal of confounding by indication is somewhat countered, the report states, by variations in nonfatal MI risk by age group. The increased chances of an event seen overall in relation to beta-blockers and antiplatelets were more pronounced among the 39% of patients aged 25-59 years (P < .01). That’s in spite that group’s lower CHD prevalence. The risk elevation solely among the older patients was attenuated and rendered nonsignificant, even with their greater CHD burden, the report noted.
The report speculates on a potential mechanism by which beta-blockers, at least, might conceivably raise the risk for heat-related MI. “Beta-receptor blockers inhibit skin vasodilation, resulting in reduced heat dissipation through convection and, at the same time, could intensify the blood-pressure-lowering effect of other antihypertensive drugs, which then could lead to syncope.”
Beta-blockers, Dr. Chen said, “can mechanistically make people more vulnerable to heat. That’s one potential explanation. Or it could be that these people taking the medications are just sicker. Whatever the reasons, the phenomenon we observed is that these patients taking these two medications are at higher risk during high-temperature days.”
Dr. Chen and the other authors declare no competing interests.
A version of this article first appeared on Medscape.com.
Patients who take beta-blockers or antiplatelet agents are lowering their risk for cardiovascular events, but the protection may fall short for those who spend time outdoors on hot summer days, hints a limited analysis published as a letter in Nature Cardiovascular Research.
Patients taking either a beta-blocker or antiplatelet, or both medications together, appeared at elevated risk for nonfatal acute MI specifically on days when the weather turned hot, suggests the registry cohort study that covered 14 years of clinical and meteorologic data.
“The take-away message is not that patients should stop using these two medications, by no means. We’re raising cautions for patients taking them, to watch out for themselves during high-heat days,” lead author Kai Chen, PhD, Yale University, New Haven, Conn., said in an interview.
“We’re not giving the message that these drugs have harmful effects” because the nature of the links between the medications and MI in the study, with its potential for confounding, remain unknown, said Dr. Chen, from the department of environmental health sciences and Yale Center on Climate Change and Health.
For example, patients who take beta-blockers or antiplatelets tend to be sicker than patients not on the drugs, which could make heat-related MI more likely, and the drugs wrongly appear to be culprits, he observed. The analysis contained signals that could support either scenario.
The study is based on cases of nonfatal MI in Augsburg, Germany, that are part of the MONICA-KORA MI registry. The odds of a heat-related nonfatal MI, it suggests, were increased 63% among patients taking antiplatelets and by 65% among those on beta-blockers, compared with those not on these drugs. The odds went up by 75% among those on both drug classes, but the risks weren’t raised in patients not taking them.
Rising heat-related MI
Chen said analysis was inspired by a 2019 report – also based on MONICA-KORA, from many of the same authors and using similar methods to track events by daily air temperature – that showed a rising trend for heat-related MI and declining rate for MI related to cold weather from 1987 to 2014. A next step, he figured, would be to determine whether the MI risk trends were associated with any cardiovascular medications.
The current study’s signal of risk related to antiplatelets and beta-blockers did not emerge for ACE inhibitors, calcium-channel blockers, or diuretics. Statins showed a link to increased nonfatal MI risk, but solely among participants aged younger than 60 years, who were also far less likely to have pre-existing coronary heart disease (CHD). He and his colleagues chose not to highlight that finding, Dr. Chen said, because the age subgroup analysis was grossly underpowered.
The overall analysis involved 2,494 cases of nonfatal MI that occurred during the warmer months – May to September – from 2001 to 2014. It was limited to nonfatal cases – those with at least a month of survival after hospital admission – because of insufficient data on medication use associated with fatal MIs, the report states.
Nonfatal MIs were defined as heat-related if they struck on days reaching the 95th percentile for temperature across the 14 years, in this case 24.2 °C (about 75.6 °F), relative to the average temperature of lowest nonfatal MI risk across the cohort, 7.5 °C (about 45.5 °F).
Patients served as both cases and their own controls, in that air temperature exposures on the day of their MI (case day) were compared with the remaining same days of the week in the same calendar month (control days). That approach, the report stated, “automatically controls for long-term time trends, seasonality, day of the week, and time-invariant confounders (for example, pre-existing cardiovascular disease).”
The odds ratio for heat-related MI for patients on antiplatelets was 1.63 (95% confidence interval, 1.07-2.46), and for antiplatelet nonusers was 0.94 (95% CI, 0.68-1.29). The difference between the two ratios was significant (P = .04).
The corresponding OR for patients taking beta-blockers was 1.65 (95% CI, 1.11-2.45), and for nonusers of beta-blockers was 0.90 (95% CI, 0.64-1.26). Again, the OR difference was significant (P = .02).
The ORs for users of both medication classes and nonusers of either med class, respectively, were 1.75 (95% CI, 1.12-2.73) and 0.84 (95% CI, 0.59-1.19). The latter OR was significantly lower than former (P = .01).
In a sign that antiplatelet and beta-blocker use might have been just a marker for sicker patients who were more vulnerable to heat-related MI, Chen said, the nonfatal MI risk was significantly elevated (OR, 2.17; 95% CI, 1.40-3.38) among patients with pre-existing CHD, but not among those free of pre-existing CHD (OR, 0.88; 95% CI, 0.65-1.20); the odds difference was P < .01.
That signal of confounding by indication is somewhat countered, the report states, by variations in nonfatal MI risk by age group. The increased chances of an event seen overall in relation to beta-blockers and antiplatelets were more pronounced among the 39% of patients aged 25-59 years (P < .01). That’s in spite that group’s lower CHD prevalence. The risk elevation solely among the older patients was attenuated and rendered nonsignificant, even with their greater CHD burden, the report noted.
The report speculates on a potential mechanism by which beta-blockers, at least, might conceivably raise the risk for heat-related MI. “Beta-receptor blockers inhibit skin vasodilation, resulting in reduced heat dissipation through convection and, at the same time, could intensify the blood-pressure-lowering effect of other antihypertensive drugs, which then could lead to syncope.”
Beta-blockers, Dr. Chen said, “can mechanistically make people more vulnerable to heat. That’s one potential explanation. Or it could be that these people taking the medications are just sicker. Whatever the reasons, the phenomenon we observed is that these patients taking these two medications are at higher risk during high-temperature days.”
Dr. Chen and the other authors declare no competing interests.
A version of this article first appeared on Medscape.com.
Patients who take beta-blockers or antiplatelet agents are lowering their risk for cardiovascular events, but the protection may fall short for those who spend time outdoors on hot summer days, hints a limited analysis published as a letter in Nature Cardiovascular Research.
Patients taking either a beta-blocker or antiplatelet, or both medications together, appeared at elevated risk for nonfatal acute MI specifically on days when the weather turned hot, suggests the registry cohort study that covered 14 years of clinical and meteorologic data.
“The take-away message is not that patients should stop using these two medications, by no means. We’re raising cautions for patients taking them, to watch out for themselves during high-heat days,” lead author Kai Chen, PhD, Yale University, New Haven, Conn., said in an interview.
“We’re not giving the message that these drugs have harmful effects” because the nature of the links between the medications and MI in the study, with its potential for confounding, remain unknown, said Dr. Chen, from the department of environmental health sciences and Yale Center on Climate Change and Health.
For example, patients who take beta-blockers or antiplatelets tend to be sicker than patients not on the drugs, which could make heat-related MI more likely, and the drugs wrongly appear to be culprits, he observed. The analysis contained signals that could support either scenario.
The study is based on cases of nonfatal MI in Augsburg, Germany, that are part of the MONICA-KORA MI registry. The odds of a heat-related nonfatal MI, it suggests, were increased 63% among patients taking antiplatelets and by 65% among those on beta-blockers, compared with those not on these drugs. The odds went up by 75% among those on both drug classes, but the risks weren’t raised in patients not taking them.
Rising heat-related MI
Chen said analysis was inspired by a 2019 report – also based on MONICA-KORA, from many of the same authors and using similar methods to track events by daily air temperature – that showed a rising trend for heat-related MI and declining rate for MI related to cold weather from 1987 to 2014. A next step, he figured, would be to determine whether the MI risk trends were associated with any cardiovascular medications.
The current study’s signal of risk related to antiplatelets and beta-blockers did not emerge for ACE inhibitors, calcium-channel blockers, or diuretics. Statins showed a link to increased nonfatal MI risk, but solely among participants aged younger than 60 years, who were also far less likely to have pre-existing coronary heart disease (CHD). He and his colleagues chose not to highlight that finding, Dr. Chen said, because the age subgroup analysis was grossly underpowered.
The overall analysis involved 2,494 cases of nonfatal MI that occurred during the warmer months – May to September – from 2001 to 2014. It was limited to nonfatal cases – those with at least a month of survival after hospital admission – because of insufficient data on medication use associated with fatal MIs, the report states.
Nonfatal MIs were defined as heat-related if they struck on days reaching the 95th percentile for temperature across the 14 years, in this case 24.2 °C (about 75.6 °F), relative to the average temperature of lowest nonfatal MI risk across the cohort, 7.5 °C (about 45.5 °F).
Patients served as both cases and their own controls, in that air temperature exposures on the day of their MI (case day) were compared with the remaining same days of the week in the same calendar month (control days). That approach, the report stated, “automatically controls for long-term time trends, seasonality, day of the week, and time-invariant confounders (for example, pre-existing cardiovascular disease).”
The odds ratio for heat-related MI for patients on antiplatelets was 1.63 (95% confidence interval, 1.07-2.46), and for antiplatelet nonusers was 0.94 (95% CI, 0.68-1.29). The difference between the two ratios was significant (P = .04).
The corresponding OR for patients taking beta-blockers was 1.65 (95% CI, 1.11-2.45), and for nonusers of beta-blockers was 0.90 (95% CI, 0.64-1.26). Again, the OR difference was significant (P = .02).
The ORs for users of both medication classes and nonusers of either med class, respectively, were 1.75 (95% CI, 1.12-2.73) and 0.84 (95% CI, 0.59-1.19). The latter OR was significantly lower than former (P = .01).
In a sign that antiplatelet and beta-blocker use might have been just a marker for sicker patients who were more vulnerable to heat-related MI, Chen said, the nonfatal MI risk was significantly elevated (OR, 2.17; 95% CI, 1.40-3.38) among patients with pre-existing CHD, but not among those free of pre-existing CHD (OR, 0.88; 95% CI, 0.65-1.20); the odds difference was P < .01.
That signal of confounding by indication is somewhat countered, the report states, by variations in nonfatal MI risk by age group. The increased chances of an event seen overall in relation to beta-blockers and antiplatelets were more pronounced among the 39% of patients aged 25-59 years (P < .01). That’s in spite that group’s lower CHD prevalence. The risk elevation solely among the older patients was attenuated and rendered nonsignificant, even with their greater CHD burden, the report noted.
The report speculates on a potential mechanism by which beta-blockers, at least, might conceivably raise the risk for heat-related MI. “Beta-receptor blockers inhibit skin vasodilation, resulting in reduced heat dissipation through convection and, at the same time, could intensify the blood-pressure-lowering effect of other antihypertensive drugs, which then could lead to syncope.”
Beta-blockers, Dr. Chen said, “can mechanistically make people more vulnerable to heat. That’s one potential explanation. Or it could be that these people taking the medications are just sicker. Whatever the reasons, the phenomenon we observed is that these patients taking these two medications are at higher risk during high-temperature days.”
Dr. Chen and the other authors declare no competing interests.
A version of this article first appeared on Medscape.com.
FROM NATURE CARDIOVASCULAR RESEARCH
‘Staggering’ CVD rise projected in U.S., especially in minorities
A new analysis projects steep increases by 2060 in the prevalence of cardiovascular (CV) risk factors and disease that will disproportionately affect non-White populations who have limited access to health care.
The study by Reza Mohebi, MD, Massachusetts General Hospital and Harvard Medical School, both in Boston, and colleagues was published in the Journal of the American College of Cardiology.
“Even though several assumptions underlie these projections, the importance of this work cannot be overestimated,” Andreas P. Kalogeropoulos, MD, MPH, PhD, and Javed Butler, MD, MPH, MBA, wrote in an accompanying editorial. “The absolute numbers are staggering.”
From 2025 to 2060, the number of people with any one of four CV risk factors – type 2 diabetes, hypertension, dyslipidemia, and obesity – is projected to increase by 15.4 million, to 34.7 million.
And the number of people with of any one of four CV disease types – ischemic heart disease, heart failure, MI, and stroke – is projected to increase by 3.2 million, to 6.8 million.
Although the model predicts that the prevalence of CV risk factors will gradually decrease among White Americans, the highest prevalence of CV risk factors will be among the White population because of its overall size.
Conversely, the projected prevalence of CV risk factors is expected to increase in Black, Hispanic, Asian, and other race/ethnicity populations.
In parallel, the prevalence of CV disease is projected to decrease in the White population and increase among all other race/ethnicities, particularly in the Black and Hispanic populations.
“Our results project a worrisome increase with a particularly ominous increase in risk factors and disease in our most vulnerable patients, including Blacks and Hispanics,” senior author James L. Januzzi Jr., MD, summarized in a video issued by the society.
“The steep rise in CV risk factors and disease reflects the generally higher prevalence in populations projected to increase in the United States, owing to immigration and growth, including Black or Hispanic individuals,” Dr. Januzzi, also from Massachusetts General and Harvard, said in an interview.
“The disproportionate size of the risk is expected in a sense, as minority populations are disproportionately disadvantaged with respect to their health care,” he said. “But whether it is expected or not, the increase in projected prevalence is, nonetheless, concerning and a call to action.”
This study identifies “areas of opportunity for change in the U.S. health care system,” he continued. “Business as usual will result in us encountering a huge number of individuals with CV risk factors and diseases.”
The results from the current analysis assume there will be no modification in health care policies or changes in access to care for at-risk populations, Dr. Mohebi and colleagues noted.
To “stem the rising tide of CV disease in at-risk individuals,” would require strategies such as “emphasis on education regarding CV risk factors, improving access to quality healthcare, and facilitating lower-cost access to effective therapies for treatment of CV risk factors,” according to the researchers.
“Such advances need to be applied in a more equitable way throughout the United States, however,” they cautioned.
Census plus NHANES data
The researchers used 2020 U.S. census data and projected growth and 2013-2018 U.S. National Health and Nutrition Survey data to estimate the number of people with CV risk factors and CV disease from 2025 to 2060.
The estimates are based on a growing population and a fixed frequency.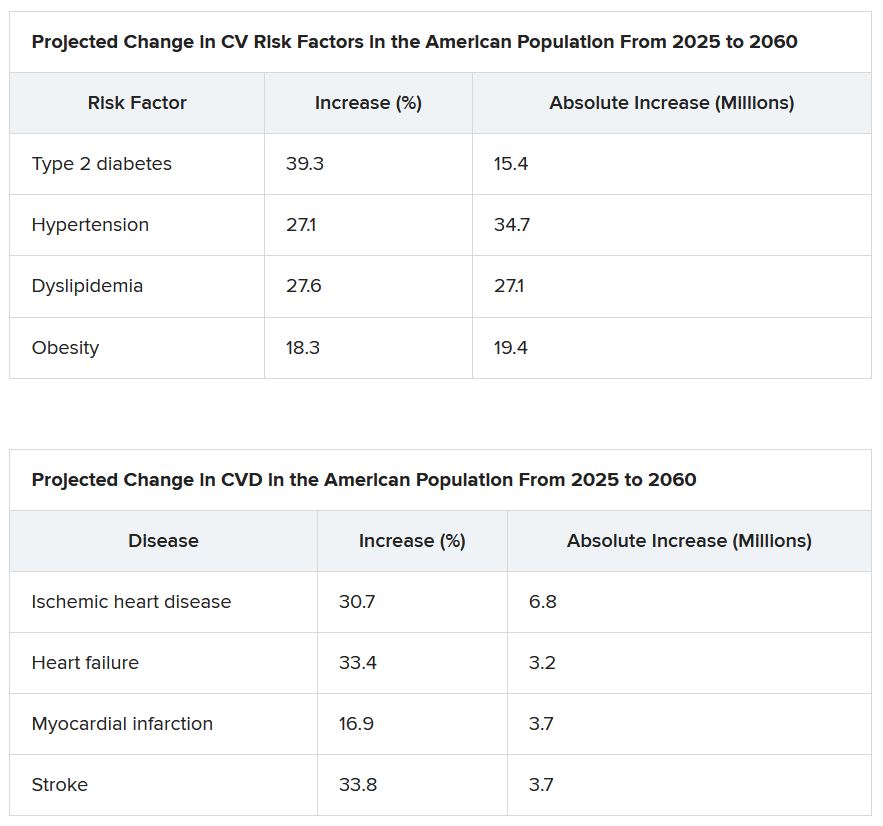
The projected changes in CV risk factors and disease over time were similar in men and women.
The researchers acknowledge that study limitations include the assumption that the prevalence patterns for CV risk factors and disease will be stable.
“To the extent the frequency of risk factors and disease are not likely to remain static, that assumption may reduce the accuracy of the projections,” Dr. Januzzi said. “However, we would point out that the goals of our analysis were to set general trends, and not to seek to project exact figures.”
Also, they did not take into account the effect of COVID-19. CV diseases were also based on self-report and CV risk factors could have been underestimated in minority populations that do not access health care.
Changing demographic landscape
It is “striking” that the numbers of non-White individuals with CV risk factors is projected to surpass the number of White individuals over time, and the number of non-White individuals with CV disease will be almost as many as White individuals by the year 2060, the editorialists noted.
“From a policy perspective, this means that unless appropriate, targeted action is taken, disparities in the burden of cardiovascular disease are only going to be exacerbated over time,” wrote Dr. Kalogeropoulos, from Stony Brook (N.Y.) University, and Dr. Butler, from Baylor College of Medicine, Dallas.
“On the positive side,” they continued, “the absolute increase in the percent prevalence of cardiovascular risk factors and conditions is projected to lie within a manageable range,” assuming that specific prevention policies are implemented.
“This is an opportunity for professional societies, including the cardiovascular care community, to re-evaluate priorities and strategies, for both training and practice, to best match the growing demands of a changing demographic landscape in the United States,” Dr. Kalogeropoulos and Dr. Butler concluded.
Dr. Mohebi is supported by the Barry Fellowship. Dr. Januzzi is supported by the Hutter Family Professorship; is a Trustee of the American College of Cardiology; is a board member of Imbria Pharmaceuticals; has received grant support from Abbott Diagnostics, Applied Therapeutics, Innolife, and Novartis; has received consulting income from Abbott Diagnostics, Boehringer Ingelheim, Janssen, Novartis, and Roche Diagnostics; and participates in clinical endpoint committees/data safety monitoring boards for AbbVie, Siemens, Takeda, and Vifor. Dr. Kalogeropoulos has received research funding from the National Heart, Lung, and Blood Institute; the American Heart Association; and the Centers for Disease Control and Prevention. Dr. Butler has been a consultant for numerous pharmaceutical companies.
A version of this article first appeared on Medscape.com.
A new analysis projects steep increases by 2060 in the prevalence of cardiovascular (CV) risk factors and disease that will disproportionately affect non-White populations who have limited access to health care.
The study by Reza Mohebi, MD, Massachusetts General Hospital and Harvard Medical School, both in Boston, and colleagues was published in the Journal of the American College of Cardiology.
“Even though several assumptions underlie these projections, the importance of this work cannot be overestimated,” Andreas P. Kalogeropoulos, MD, MPH, PhD, and Javed Butler, MD, MPH, MBA, wrote in an accompanying editorial. “The absolute numbers are staggering.”
From 2025 to 2060, the number of people with any one of four CV risk factors – type 2 diabetes, hypertension, dyslipidemia, and obesity – is projected to increase by 15.4 million, to 34.7 million.
And the number of people with of any one of four CV disease types – ischemic heart disease, heart failure, MI, and stroke – is projected to increase by 3.2 million, to 6.8 million.
Although the model predicts that the prevalence of CV risk factors will gradually decrease among White Americans, the highest prevalence of CV risk factors will be among the White population because of its overall size.
Conversely, the projected prevalence of CV risk factors is expected to increase in Black, Hispanic, Asian, and other race/ethnicity populations.
In parallel, the prevalence of CV disease is projected to decrease in the White population and increase among all other race/ethnicities, particularly in the Black and Hispanic populations.
“Our results project a worrisome increase with a particularly ominous increase in risk factors and disease in our most vulnerable patients, including Blacks and Hispanics,” senior author James L. Januzzi Jr., MD, summarized in a video issued by the society.
“The steep rise in CV risk factors and disease reflects the generally higher prevalence in populations projected to increase in the United States, owing to immigration and growth, including Black or Hispanic individuals,” Dr. Januzzi, also from Massachusetts General and Harvard, said in an interview.
“The disproportionate size of the risk is expected in a sense, as minority populations are disproportionately disadvantaged with respect to their health care,” he said. “But whether it is expected or not, the increase in projected prevalence is, nonetheless, concerning and a call to action.”
This study identifies “areas of opportunity for change in the U.S. health care system,” he continued. “Business as usual will result in us encountering a huge number of individuals with CV risk factors and diseases.”
The results from the current analysis assume there will be no modification in health care policies or changes in access to care for at-risk populations, Dr. Mohebi and colleagues noted.
To “stem the rising tide of CV disease in at-risk individuals,” would require strategies such as “emphasis on education regarding CV risk factors, improving access to quality healthcare, and facilitating lower-cost access to effective therapies for treatment of CV risk factors,” according to the researchers.
“Such advances need to be applied in a more equitable way throughout the United States, however,” they cautioned.
Census plus NHANES data
The researchers used 2020 U.S. census data and projected growth and 2013-2018 U.S. National Health and Nutrition Survey data to estimate the number of people with CV risk factors and CV disease from 2025 to 2060.
The estimates are based on a growing population and a fixed frequency.
The projected changes in CV risk factors and disease over time were similar in men and women.
The researchers acknowledge that study limitations include the assumption that the prevalence patterns for CV risk factors and disease will be stable.
“To the extent the frequency of risk factors and disease are not likely to remain static, that assumption may reduce the accuracy of the projections,” Dr. Januzzi said. “However, we would point out that the goals of our analysis were to set general trends, and not to seek to project exact figures.”
Also, they did not take into account the effect of COVID-19. CV diseases were also based on self-report and CV risk factors could have been underestimated in minority populations that do not access health care.
Changing demographic landscape
It is “striking” that the numbers of non-White individuals with CV risk factors is projected to surpass the number of White individuals over time, and the number of non-White individuals with CV disease will be almost as many as White individuals by the year 2060, the editorialists noted.
“From a policy perspective, this means that unless appropriate, targeted action is taken, disparities in the burden of cardiovascular disease are only going to be exacerbated over time,” wrote Dr. Kalogeropoulos, from Stony Brook (N.Y.) University, and Dr. Butler, from Baylor College of Medicine, Dallas.
“On the positive side,” they continued, “the absolute increase in the percent prevalence of cardiovascular risk factors and conditions is projected to lie within a manageable range,” assuming that specific prevention policies are implemented.
“This is an opportunity for professional societies, including the cardiovascular care community, to re-evaluate priorities and strategies, for both training and practice, to best match the growing demands of a changing demographic landscape in the United States,” Dr. Kalogeropoulos and Dr. Butler concluded.
Dr. Mohebi is supported by the Barry Fellowship. Dr. Januzzi is supported by the Hutter Family Professorship; is a Trustee of the American College of Cardiology; is a board member of Imbria Pharmaceuticals; has received grant support from Abbott Diagnostics, Applied Therapeutics, Innolife, and Novartis; has received consulting income from Abbott Diagnostics, Boehringer Ingelheim, Janssen, Novartis, and Roche Diagnostics; and participates in clinical endpoint committees/data safety monitoring boards for AbbVie, Siemens, Takeda, and Vifor. Dr. Kalogeropoulos has received research funding from the National Heart, Lung, and Blood Institute; the American Heart Association; and the Centers for Disease Control and Prevention. Dr. Butler has been a consultant for numerous pharmaceutical companies.
A version of this article first appeared on Medscape.com.
A new analysis projects steep increases by 2060 in the prevalence of cardiovascular (CV) risk factors and disease that will disproportionately affect non-White populations who have limited access to health care.
The study by Reza Mohebi, MD, Massachusetts General Hospital and Harvard Medical School, both in Boston, and colleagues was published in the Journal of the American College of Cardiology.
“Even though several assumptions underlie these projections, the importance of this work cannot be overestimated,” Andreas P. Kalogeropoulos, MD, MPH, PhD, and Javed Butler, MD, MPH, MBA, wrote in an accompanying editorial. “The absolute numbers are staggering.”
From 2025 to 2060, the number of people with any one of four CV risk factors – type 2 diabetes, hypertension, dyslipidemia, and obesity – is projected to increase by 15.4 million, to 34.7 million.
And the number of people with of any one of four CV disease types – ischemic heart disease, heart failure, MI, and stroke – is projected to increase by 3.2 million, to 6.8 million.
Although the model predicts that the prevalence of CV risk factors will gradually decrease among White Americans, the highest prevalence of CV risk factors will be among the White population because of its overall size.
Conversely, the projected prevalence of CV risk factors is expected to increase in Black, Hispanic, Asian, and other race/ethnicity populations.
In parallel, the prevalence of CV disease is projected to decrease in the White population and increase among all other race/ethnicities, particularly in the Black and Hispanic populations.
“Our results project a worrisome increase with a particularly ominous increase in risk factors and disease in our most vulnerable patients, including Blacks and Hispanics,” senior author James L. Januzzi Jr., MD, summarized in a video issued by the society.
“The steep rise in CV risk factors and disease reflects the generally higher prevalence in populations projected to increase in the United States, owing to immigration and growth, including Black or Hispanic individuals,” Dr. Januzzi, also from Massachusetts General and Harvard, said in an interview.
“The disproportionate size of the risk is expected in a sense, as minority populations are disproportionately disadvantaged with respect to their health care,” he said. “But whether it is expected or not, the increase in projected prevalence is, nonetheless, concerning and a call to action.”
This study identifies “areas of opportunity for change in the U.S. health care system,” he continued. “Business as usual will result in us encountering a huge number of individuals with CV risk factors and diseases.”
The results from the current analysis assume there will be no modification in health care policies or changes in access to care for at-risk populations, Dr. Mohebi and colleagues noted.
To “stem the rising tide of CV disease in at-risk individuals,” would require strategies such as “emphasis on education regarding CV risk factors, improving access to quality healthcare, and facilitating lower-cost access to effective therapies for treatment of CV risk factors,” according to the researchers.
“Such advances need to be applied in a more equitable way throughout the United States, however,” they cautioned.
Census plus NHANES data
The researchers used 2020 U.S. census data and projected growth and 2013-2018 U.S. National Health and Nutrition Survey data to estimate the number of people with CV risk factors and CV disease from 2025 to 2060.
The estimates are based on a growing population and a fixed frequency.
The projected changes in CV risk factors and disease over time were similar in men and women.
The researchers acknowledge that study limitations include the assumption that the prevalence patterns for CV risk factors and disease will be stable.
“To the extent the frequency of risk factors and disease are not likely to remain static, that assumption may reduce the accuracy of the projections,” Dr. Januzzi said. “However, we would point out that the goals of our analysis were to set general trends, and not to seek to project exact figures.”
Also, they did not take into account the effect of COVID-19. CV diseases were also based on self-report and CV risk factors could have been underestimated in minority populations that do not access health care.
Changing demographic landscape
It is “striking” that the numbers of non-White individuals with CV risk factors is projected to surpass the number of White individuals over time, and the number of non-White individuals with CV disease will be almost as many as White individuals by the year 2060, the editorialists noted.
“From a policy perspective, this means that unless appropriate, targeted action is taken, disparities in the burden of cardiovascular disease are only going to be exacerbated over time,” wrote Dr. Kalogeropoulos, from Stony Brook (N.Y.) University, and Dr. Butler, from Baylor College of Medicine, Dallas.
“On the positive side,” they continued, “the absolute increase in the percent prevalence of cardiovascular risk factors and conditions is projected to lie within a manageable range,” assuming that specific prevention policies are implemented.
“This is an opportunity for professional societies, including the cardiovascular care community, to re-evaluate priorities and strategies, for both training and practice, to best match the growing demands of a changing demographic landscape in the United States,” Dr. Kalogeropoulos and Dr. Butler concluded.
Dr. Mohebi is supported by the Barry Fellowship. Dr. Januzzi is supported by the Hutter Family Professorship; is a Trustee of the American College of Cardiology; is a board member of Imbria Pharmaceuticals; has received grant support from Abbott Diagnostics, Applied Therapeutics, Innolife, and Novartis; has received consulting income from Abbott Diagnostics, Boehringer Ingelheim, Janssen, Novartis, and Roche Diagnostics; and participates in clinical endpoint committees/data safety monitoring boards for AbbVie, Siemens, Takeda, and Vifor. Dr. Kalogeropoulos has received research funding from the National Heart, Lung, and Blood Institute; the American Heart Association; and the Centers for Disease Control and Prevention. Dr. Butler has been a consultant for numerous pharmaceutical companies.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF AMERICAN COLLEGE OF CARDIOLOGY
Is Lp(a) a marker for aortic calcium onset?
Lipoprotein(a) has long been thought to be a potential marker of aortic valve disease, and the population-based Rotterdam Study in the Netherlands has reported that Lp(a) has a strong association with new-onset aortic valve calcium (AVC), but not necessarily with progression of aortic valve disease.
Reporting in the European Heart Journal, the study authors analyzed data on 922 participants in the Rotterdam Study whose Lp(a) was measured along with a computed tomography scan upon enrollment, followed by CT scan 14 years later. At baseline, 702 participants didn’t have AVC, but the follow-up scan identified new-onset AVC in 415 (59.1%).
The investigators found an association between Lp(a) concentration and baseline AVC, with an odds ratio of 1.43 for each 50 mg/dL higher Lp(a) (95% confidence interval, 1.15-1.79), as well as new-onset AVC, with an OR of 1.30 for each 50 mg/dL increase in Lp(a) (95% CI, 1.02-1.65). However, the study found no association between rising Lp(a) levels and AVC progression; it found only an association between baseline AVC score and progression (P < .001).
‘Trigger’ for calcification but not progression
“This suggests that Lp(a) is an important trigger in the initiation of aortic valve calcification, but once the valve is calcified, disease progression may be primarily driven by other factors such as the baseline calcium burden of the valve and likely other unknown factors,” senior study author Daniel Bos, MD, PhD, said in e-mailed comments.
Dr. Bos and coauthors claim this is the first study to show that even minor AVC progresses independently of Lp(a).
“There are previous studies that showed a possible relationship between Lp(a) [and] progression of aortic valve calcium,” he said. “Our study suggests that the most meaningful benefit of Lp(a) lowering may actually be prior to the onset of aortic valve calcification.”
While no treatments have been approved for lowering Lp(a), the study findings could be meaningful if trials, including the ongoing phase 3 Lp(a) HORIZON trial of the investigational antisense agent pelacarsen (NCT04023552), show promising results, Dr. Bos said. Citing Lp(a) HORIZON, he said, “If the study shows Lp(a) lowering leads to a reduction in incident cardiovascular disease, similar strategies may be applied to prevent, rather than slow down, progression of aortic valve calcification.”
Dr. Bos called the Rotterdam Study results “an important first pointer into that direction.” He added, “We will need randomized trials to provide a definitive answer to the question whether Lp(a) lowering may prevent aortic valve calcium.”
Focus on AVC is study ‘weakness’
The study findings raise a key question for clinical trials of investigative Lp(a)-lowering therapies as well as how to use those therapies to treat aortic valve disease, said Christie Ballantyne, MD, chief of cardiology at Baylor College of Medicine in Houston.
The findings could be “problematic” for these clinical trials, he said. “This study is just looking at calcium progression,” Dr. Ballantyne noted. “What we want to know about clinically is the progression to aortic stenosis, and then in particular to progression from mild disease to moderate or severe disease, because once you get into more severe disease, one has to do an intervention with either surgery or TAVR [transcatheter aortic valve replacement].”
He considered the study’s focus on AVC rather than aortic valve function a weakness and noted that only 14 study participants had TAVR. “We’re going to need much bigger numbers to look into this question of progression, including progression to severe diseases,” he said.
However, the Rotterdam Study showed the importance of CT in evaluating AVC, which can easily be done in other trials to further explore the association between Lp(a) and AVC, Dr. Ballantyne said.
Dr. Bos has no relevant disclosures. Study coauthors disclosed relationships with Amgen, Sanofi, Reservlogix, Athera, Experio, Novartis and Ionis Pharmaceuticals. Dr. Ballantyne disclosed relationships with Amgen and Novartis.
Lipoprotein(a) has long been thought to be a potential marker of aortic valve disease, and the population-based Rotterdam Study in the Netherlands has reported that Lp(a) has a strong association with new-onset aortic valve calcium (AVC), but not necessarily with progression of aortic valve disease.
Reporting in the European Heart Journal, the study authors analyzed data on 922 participants in the Rotterdam Study whose Lp(a) was measured along with a computed tomography scan upon enrollment, followed by CT scan 14 years later. At baseline, 702 participants didn’t have AVC, but the follow-up scan identified new-onset AVC in 415 (59.1%).
The investigators found an association between Lp(a) concentration and baseline AVC, with an odds ratio of 1.43 for each 50 mg/dL higher Lp(a) (95% confidence interval, 1.15-1.79), as well as new-onset AVC, with an OR of 1.30 for each 50 mg/dL increase in Lp(a) (95% CI, 1.02-1.65). However, the study found no association between rising Lp(a) levels and AVC progression; it found only an association between baseline AVC score and progression (P < .001).
‘Trigger’ for calcification but not progression
“This suggests that Lp(a) is an important trigger in the initiation of aortic valve calcification, but once the valve is calcified, disease progression may be primarily driven by other factors such as the baseline calcium burden of the valve and likely other unknown factors,” senior study author Daniel Bos, MD, PhD, said in e-mailed comments.
Dr. Bos and coauthors claim this is the first study to show that even minor AVC progresses independently of Lp(a).
“There are previous studies that showed a possible relationship between Lp(a) [and] progression of aortic valve calcium,” he said. “Our study suggests that the most meaningful benefit of Lp(a) lowering may actually be prior to the onset of aortic valve calcification.”
While no treatments have been approved for lowering Lp(a), the study findings could be meaningful if trials, including the ongoing phase 3 Lp(a) HORIZON trial of the investigational antisense agent pelacarsen (NCT04023552), show promising results, Dr. Bos said. Citing Lp(a) HORIZON, he said, “If the study shows Lp(a) lowering leads to a reduction in incident cardiovascular disease, similar strategies may be applied to prevent, rather than slow down, progression of aortic valve calcification.”
Dr. Bos called the Rotterdam Study results “an important first pointer into that direction.” He added, “We will need randomized trials to provide a definitive answer to the question whether Lp(a) lowering may prevent aortic valve calcium.”
Focus on AVC is study ‘weakness’
The study findings raise a key question for clinical trials of investigative Lp(a)-lowering therapies as well as how to use those therapies to treat aortic valve disease, said Christie Ballantyne, MD, chief of cardiology at Baylor College of Medicine in Houston.
The findings could be “problematic” for these clinical trials, he said. “This study is just looking at calcium progression,” Dr. Ballantyne noted. “What we want to know about clinically is the progression to aortic stenosis, and then in particular to progression from mild disease to moderate or severe disease, because once you get into more severe disease, one has to do an intervention with either surgery or TAVR [transcatheter aortic valve replacement].”
He considered the study’s focus on AVC rather than aortic valve function a weakness and noted that only 14 study participants had TAVR. “We’re going to need much bigger numbers to look into this question of progression, including progression to severe diseases,” he said.
However, the Rotterdam Study showed the importance of CT in evaluating AVC, which can easily be done in other trials to further explore the association between Lp(a) and AVC, Dr. Ballantyne said.
Dr. Bos has no relevant disclosures. Study coauthors disclosed relationships with Amgen, Sanofi, Reservlogix, Athera, Experio, Novartis and Ionis Pharmaceuticals. Dr. Ballantyne disclosed relationships with Amgen and Novartis.
Lipoprotein(a) has long been thought to be a potential marker of aortic valve disease, and the population-based Rotterdam Study in the Netherlands has reported that Lp(a) has a strong association with new-onset aortic valve calcium (AVC), but not necessarily with progression of aortic valve disease.
Reporting in the European Heart Journal, the study authors analyzed data on 922 participants in the Rotterdam Study whose Lp(a) was measured along with a computed tomography scan upon enrollment, followed by CT scan 14 years later. At baseline, 702 participants didn’t have AVC, but the follow-up scan identified new-onset AVC in 415 (59.1%).
The investigators found an association between Lp(a) concentration and baseline AVC, with an odds ratio of 1.43 for each 50 mg/dL higher Lp(a) (95% confidence interval, 1.15-1.79), as well as new-onset AVC, with an OR of 1.30 for each 50 mg/dL increase in Lp(a) (95% CI, 1.02-1.65). However, the study found no association between rising Lp(a) levels and AVC progression; it found only an association between baseline AVC score and progression (P < .001).
‘Trigger’ for calcification but not progression
“This suggests that Lp(a) is an important trigger in the initiation of aortic valve calcification, but once the valve is calcified, disease progression may be primarily driven by other factors such as the baseline calcium burden of the valve and likely other unknown factors,” senior study author Daniel Bos, MD, PhD, said in e-mailed comments.
Dr. Bos and coauthors claim this is the first study to show that even minor AVC progresses independently of Lp(a).
“There are previous studies that showed a possible relationship between Lp(a) [and] progression of aortic valve calcium,” he said. “Our study suggests that the most meaningful benefit of Lp(a) lowering may actually be prior to the onset of aortic valve calcification.”
While no treatments have been approved for lowering Lp(a), the study findings could be meaningful if trials, including the ongoing phase 3 Lp(a) HORIZON trial of the investigational antisense agent pelacarsen (NCT04023552), show promising results, Dr. Bos said. Citing Lp(a) HORIZON, he said, “If the study shows Lp(a) lowering leads to a reduction in incident cardiovascular disease, similar strategies may be applied to prevent, rather than slow down, progression of aortic valve calcification.”
Dr. Bos called the Rotterdam Study results “an important first pointer into that direction.” He added, “We will need randomized trials to provide a definitive answer to the question whether Lp(a) lowering may prevent aortic valve calcium.”
Focus on AVC is study ‘weakness’
The study findings raise a key question for clinical trials of investigative Lp(a)-lowering therapies as well as how to use those therapies to treat aortic valve disease, said Christie Ballantyne, MD, chief of cardiology at Baylor College of Medicine in Houston.
The findings could be “problematic” for these clinical trials, he said. “This study is just looking at calcium progression,” Dr. Ballantyne noted. “What we want to know about clinically is the progression to aortic stenosis, and then in particular to progression from mild disease to moderate or severe disease, because once you get into more severe disease, one has to do an intervention with either surgery or TAVR [transcatheter aortic valve replacement].”
He considered the study’s focus on AVC rather than aortic valve function a weakness and noted that only 14 study participants had TAVR. “We’re going to need much bigger numbers to look into this question of progression, including progression to severe diseases,” he said.
However, the Rotterdam Study showed the importance of CT in evaluating AVC, which can easily be done in other trials to further explore the association between Lp(a) and AVC, Dr. Ballantyne said.
Dr. Bos has no relevant disclosures. Study coauthors disclosed relationships with Amgen, Sanofi, Reservlogix, Athera, Experio, Novartis and Ionis Pharmaceuticals. Dr. Ballantyne disclosed relationships with Amgen and Novartis.
FROM THE EUROPEAN HEART JOURNAL
RADIANCE II: Positive signal for ultrasound renal denervation
Top-line results released on July 26 from the RADIANCE II trial show the Paradise ultrasound renal denervation system significantly reduces daytime ambulatory systolic blood pressure, compared with a sham procedure at 2 months in patients with mild to moderate uncontrolled hypertension.
The trial was conducted in 224 patients who were previously treated with up to two medications and were randomized while off medication at more than 60 centers in 8 countries. No further details or results were provided.
The pivotal RADIANCE II trial, required for FDA approval, is the third and largest randomized, sham-controlled study following positive results reported by RADIANCE-HTN SOLO and RADIANCE-HTN TRIO, ReCor Medical and its subsidiary Otsuka Medical Devices noted in the announcement.
The field of renal denervation fell out of favor after the largest trial in 535 patients, SYMPLICITY HTN-3, failed to show a significant reduction in systolic blood pressure at 6 months, compared with sham control in resistant hypertension.
A version of this article first appeared on Medscape.com.
Top-line results released on July 26 from the RADIANCE II trial show the Paradise ultrasound renal denervation system significantly reduces daytime ambulatory systolic blood pressure, compared with a sham procedure at 2 months in patients with mild to moderate uncontrolled hypertension.
The trial was conducted in 224 patients who were previously treated with up to two medications and were randomized while off medication at more than 60 centers in 8 countries. No further details or results were provided.
The pivotal RADIANCE II trial, required for FDA approval, is the third and largest randomized, sham-controlled study following positive results reported by RADIANCE-HTN SOLO and RADIANCE-HTN TRIO, ReCor Medical and its subsidiary Otsuka Medical Devices noted in the announcement.
The field of renal denervation fell out of favor after the largest trial in 535 patients, SYMPLICITY HTN-3, failed to show a significant reduction in systolic blood pressure at 6 months, compared with sham control in resistant hypertension.
A version of this article first appeared on Medscape.com.
Top-line results released on July 26 from the RADIANCE II trial show the Paradise ultrasound renal denervation system significantly reduces daytime ambulatory systolic blood pressure, compared with a sham procedure at 2 months in patients with mild to moderate uncontrolled hypertension.
The trial was conducted in 224 patients who were previously treated with up to two medications and were randomized while off medication at more than 60 centers in 8 countries. No further details or results were provided.
The pivotal RADIANCE II trial, required for FDA approval, is the third and largest randomized, sham-controlled study following positive results reported by RADIANCE-HTN SOLO and RADIANCE-HTN TRIO, ReCor Medical and its subsidiary Otsuka Medical Devices noted in the announcement.
The field of renal denervation fell out of favor after the largest trial in 535 patients, SYMPLICITY HTN-3, failed to show a significant reduction in systolic blood pressure at 6 months, compared with sham control in resistant hypertension.
A version of this article first appeared on Medscape.com.
‘Case closed’: Bridging thrombolysis remains ‘gold standard’ in stroke thrombectomy
Two new noninferiority trials address the controversial question of whether thrombolytic therapy can be omitted for acute ischemic stroke in patients undergoing endovascular thrombectomy for large-vessel occlusion.
Both trials show better outcomes when standard bridging thrombolytic therapy is used before thrombectomy, with comparable safety.
The results of SWIFT-DIRECT and DIRECT-SAFE were published online June 22 in The Lancet.
“The case appears closed. Bypass intravenous thrombolysis is highly unlikely to be noninferior to standard care by a clinically acceptable margin for most patients,” writes Pooja Khatri, MD, MSc, department of neurology, University of Cincinnati, in a linked comment.
SWIFT-DIRECT
SWIFT-DIRECT enrolled 408 patients (median age 72; 51% women) with acute stroke due to large vessel occlusion admitted to stroke centers in Europe and Canada. Half were randomly allocated to thrombectomy alone and half to intravenous alteplase and thrombectomy.
Successful reperfusion was less common in patients who had thrombectomy alone (91% vs. 96%; risk difference −5.1%; 95% confidence interval, −10.2 to 0.0, P = .047).
With combination therapy, more patients achieved functional independence with a modified Rankin scale score of 0-2 at 90 days (65% vs. 57%; adjusted risk difference −7.3%; 95% CI, −16·6 to 2·1, lower limit of one-sided 95% CI, −15·1%, crossing the noninferiority margin of −12%).
“Despite a very liberal noninferiority margin and strict inclusion and exclusion criteria aimed at studying a population most likely to benefit from thrombectomy alone, point estimates directionally favored intravenous thrombolysis plus thrombectomy,” Urs Fischer, MD, cochair of the Stroke Center, University Hospital Basel, Switzerland, told this news organization.
“Furthermore, we could demonstrate that overall reperfusion rates were extremely high and yet significantly better in patients receiving intravenous thrombolysis plus thrombectomy than in patients treated with thrombectomy alone, a finding which has not been shown before,” Dr. Fischer said.
There was no significant difference in the risk of symptomatic intracranial bleeding (3% with combination therapy and 2% with thrombectomy alone).
Based on the results, in patients suitable for thrombolysis, skipping it before thrombectomy “is not justified,” the study team concludes.
DIRECT-SAFE
DIRECT-SAFE enrolled 295 patients (median age 69; 43% women) with stroke and large vessel occlusion from Australia, New Zealand, China, and Vietnam, with half undergoing direct thrombectomy and half bridging therapy first.
Functional independence (modified Rankin Scale 0-2 or return to baseline at 90 days) was more common in the bridging group (61% vs. 55%).
Safety outcomes were similar between groups. Symptomatic intracerebral hemorrhage occurred in 2 (1%) patients in the direct group and 1 (1%) patient in the bridging group. There were 22 (15%) deaths in the direct group and 24 in the bridging group.
“There has been concern across the world regarding cost of treatment, together with fears of increasing bleeding risk or clot migration with intravenous thrombolytic,” lead investigator Peter Mitchell, MBBS, director, NeuroIntervention Service, The Royal Melbourne Hospital, Parkville, Victoria, Australia, told this news organization.
“We showed that patients in the bridging treatment arm had better outcomes across the entire study, especially in Asian region patients” and therefore remains “the gold standard,” Dr. Mitchell said.
To date, six published trials have addressed this question of endovascular therapy alone or with thrombolysis – SKIP, DIRECT-MT, MR CLEAN NO IV, SWIFT-DIRECT, and DIRECT-SAFE.
Dr. Fischer said the SWIFT-DIRECT study group plans to perform an individual participant data meta-analysis known as Improving Reperfusion Strategies in Ischemic Stroke (IRIS) of all six trials to see whether there are subgroups of patients in whom thrombectomy alone is as effective as thrombolysis plus thrombectomy.
Subgroups of interest, he said, include patients with early ischemic signs on imaging, those at increased risk for hemorrhagic complications, and patients with a high clot burden.
SWIFT-DIRECT was funding by Medtronic and University Hospital Bern. DIRECT-SAFE was funded by Australian National Health and Medical Research Council and Stryker USA. A complete list of author disclosures is available with the original articles.
A version of this article first appeared on Medscape.com.
Two new noninferiority trials address the controversial question of whether thrombolytic therapy can be omitted for acute ischemic stroke in patients undergoing endovascular thrombectomy for large-vessel occlusion.
Both trials show better outcomes when standard bridging thrombolytic therapy is used before thrombectomy, with comparable safety.
The results of SWIFT-DIRECT and DIRECT-SAFE were published online June 22 in The Lancet.
“The case appears closed. Bypass intravenous thrombolysis is highly unlikely to be noninferior to standard care by a clinically acceptable margin for most patients,” writes Pooja Khatri, MD, MSc, department of neurology, University of Cincinnati, in a linked comment.
SWIFT-DIRECT
SWIFT-DIRECT enrolled 408 patients (median age 72; 51% women) with acute stroke due to large vessel occlusion admitted to stroke centers in Europe and Canada. Half were randomly allocated to thrombectomy alone and half to intravenous alteplase and thrombectomy.
Successful reperfusion was less common in patients who had thrombectomy alone (91% vs. 96%; risk difference −5.1%; 95% confidence interval, −10.2 to 0.0, P = .047).
With combination therapy, more patients achieved functional independence with a modified Rankin scale score of 0-2 at 90 days (65% vs. 57%; adjusted risk difference −7.3%; 95% CI, −16·6 to 2·1, lower limit of one-sided 95% CI, −15·1%, crossing the noninferiority margin of −12%).
“Despite a very liberal noninferiority margin and strict inclusion and exclusion criteria aimed at studying a population most likely to benefit from thrombectomy alone, point estimates directionally favored intravenous thrombolysis plus thrombectomy,” Urs Fischer, MD, cochair of the Stroke Center, University Hospital Basel, Switzerland, told this news organization.
“Furthermore, we could demonstrate that overall reperfusion rates were extremely high and yet significantly better in patients receiving intravenous thrombolysis plus thrombectomy than in patients treated with thrombectomy alone, a finding which has not been shown before,” Dr. Fischer said.
There was no significant difference in the risk of symptomatic intracranial bleeding (3% with combination therapy and 2% with thrombectomy alone).
Based on the results, in patients suitable for thrombolysis, skipping it before thrombectomy “is not justified,” the study team concludes.
DIRECT-SAFE
DIRECT-SAFE enrolled 295 patients (median age 69; 43% women) with stroke and large vessel occlusion from Australia, New Zealand, China, and Vietnam, with half undergoing direct thrombectomy and half bridging therapy first.
Functional independence (modified Rankin Scale 0-2 or return to baseline at 90 days) was more common in the bridging group (61% vs. 55%).
Safety outcomes were similar between groups. Symptomatic intracerebral hemorrhage occurred in 2 (1%) patients in the direct group and 1 (1%) patient in the bridging group. There were 22 (15%) deaths in the direct group and 24 in the bridging group.
“There has been concern across the world regarding cost of treatment, together with fears of increasing bleeding risk or clot migration with intravenous thrombolytic,” lead investigator Peter Mitchell, MBBS, director, NeuroIntervention Service, The Royal Melbourne Hospital, Parkville, Victoria, Australia, told this news organization.
“We showed that patients in the bridging treatment arm had better outcomes across the entire study, especially in Asian region patients” and therefore remains “the gold standard,” Dr. Mitchell said.
To date, six published trials have addressed this question of endovascular therapy alone or with thrombolysis – SKIP, DIRECT-MT, MR CLEAN NO IV, SWIFT-DIRECT, and DIRECT-SAFE.
Dr. Fischer said the SWIFT-DIRECT study group plans to perform an individual participant data meta-analysis known as Improving Reperfusion Strategies in Ischemic Stroke (IRIS) of all six trials to see whether there are subgroups of patients in whom thrombectomy alone is as effective as thrombolysis plus thrombectomy.
Subgroups of interest, he said, include patients with early ischemic signs on imaging, those at increased risk for hemorrhagic complications, and patients with a high clot burden.
SWIFT-DIRECT was funding by Medtronic and University Hospital Bern. DIRECT-SAFE was funded by Australian National Health and Medical Research Council and Stryker USA. A complete list of author disclosures is available with the original articles.
A version of this article first appeared on Medscape.com.
Two new noninferiority trials address the controversial question of whether thrombolytic therapy can be omitted for acute ischemic stroke in patients undergoing endovascular thrombectomy for large-vessel occlusion.
Both trials show better outcomes when standard bridging thrombolytic therapy is used before thrombectomy, with comparable safety.
The results of SWIFT-DIRECT and DIRECT-SAFE were published online June 22 in The Lancet.
“The case appears closed. Bypass intravenous thrombolysis is highly unlikely to be noninferior to standard care by a clinically acceptable margin for most patients,” writes Pooja Khatri, MD, MSc, department of neurology, University of Cincinnati, in a linked comment.
SWIFT-DIRECT
SWIFT-DIRECT enrolled 408 patients (median age 72; 51% women) with acute stroke due to large vessel occlusion admitted to stroke centers in Europe and Canada. Half were randomly allocated to thrombectomy alone and half to intravenous alteplase and thrombectomy.
Successful reperfusion was less common in patients who had thrombectomy alone (91% vs. 96%; risk difference −5.1%; 95% confidence interval, −10.2 to 0.0, P = .047).
With combination therapy, more patients achieved functional independence with a modified Rankin scale score of 0-2 at 90 days (65% vs. 57%; adjusted risk difference −7.3%; 95% CI, −16·6 to 2·1, lower limit of one-sided 95% CI, −15·1%, crossing the noninferiority margin of −12%).
“Despite a very liberal noninferiority margin and strict inclusion and exclusion criteria aimed at studying a population most likely to benefit from thrombectomy alone, point estimates directionally favored intravenous thrombolysis plus thrombectomy,” Urs Fischer, MD, cochair of the Stroke Center, University Hospital Basel, Switzerland, told this news organization.
“Furthermore, we could demonstrate that overall reperfusion rates were extremely high and yet significantly better in patients receiving intravenous thrombolysis plus thrombectomy than in patients treated with thrombectomy alone, a finding which has not been shown before,” Dr. Fischer said.
There was no significant difference in the risk of symptomatic intracranial bleeding (3% with combination therapy and 2% with thrombectomy alone).
Based on the results, in patients suitable for thrombolysis, skipping it before thrombectomy “is not justified,” the study team concludes.
DIRECT-SAFE
DIRECT-SAFE enrolled 295 patients (median age 69; 43% women) with stroke and large vessel occlusion from Australia, New Zealand, China, and Vietnam, with half undergoing direct thrombectomy and half bridging therapy first.
Functional independence (modified Rankin Scale 0-2 or return to baseline at 90 days) was more common in the bridging group (61% vs. 55%).
Safety outcomes were similar between groups. Symptomatic intracerebral hemorrhage occurred in 2 (1%) patients in the direct group and 1 (1%) patient in the bridging group. There were 22 (15%) deaths in the direct group and 24 in the bridging group.
“There has been concern across the world regarding cost of treatment, together with fears of increasing bleeding risk or clot migration with intravenous thrombolytic,” lead investigator Peter Mitchell, MBBS, director, NeuroIntervention Service, The Royal Melbourne Hospital, Parkville, Victoria, Australia, told this news organization.
“We showed that patients in the bridging treatment arm had better outcomes across the entire study, especially in Asian region patients” and therefore remains “the gold standard,” Dr. Mitchell said.
To date, six published trials have addressed this question of endovascular therapy alone or with thrombolysis – SKIP, DIRECT-MT, MR CLEAN NO IV, SWIFT-DIRECT, and DIRECT-SAFE.
Dr. Fischer said the SWIFT-DIRECT study group plans to perform an individual participant data meta-analysis known as Improving Reperfusion Strategies in Ischemic Stroke (IRIS) of all six trials to see whether there are subgroups of patients in whom thrombectomy alone is as effective as thrombolysis plus thrombectomy.
Subgroups of interest, he said, include patients with early ischemic signs on imaging, those at increased risk for hemorrhagic complications, and patients with a high clot burden.
SWIFT-DIRECT was funding by Medtronic and University Hospital Bern. DIRECT-SAFE was funded by Australian National Health and Medical Research Council and Stryker USA. A complete list of author disclosures is available with the original articles.
A version of this article first appeared on Medscape.com.
FROM THE LANCET
‘Stunning variation’ in CV test, procedure costs revealed at top U.S. hospitals
Wide variation in the cost of common cardiovascular (CV) tests and procedures, from stress tests to coronary interventions, was revealed in a cross-sectional analysis based on publicly available data from 20 top-ranked hospitals in the United States.
The analysis also suggested a low level of compliance with the 2021 Hospital Price Transparency Final Rule among the 20 centers.
“The variation we found in payer-negotiated prices for identical cardiovascular tests and procedures was stunning,” Rishi K. Wadhera, MD, MPP, MPhil, Beth Israel Deaconess Medical Center, Boston, told this news organization.
“For example, there was a 10-fold difference in the median price of an echocardiogram, and these differences were even larger for common procedures” such as percutaneous coronary intervention (PCI) and pacemaker implantation, he said. “It’s hard to argue that this variation reflects quality of care, given that we looked at a top group of highly ranked hospitals.”
“Even more striking was how the price of a cardiovascular test within the very same hospital could differ across commercial insurance companies,” he said. “For example, the price of a stress test varied 5-fold in one hospital, and in another hospital, more than 4-fold for a coronary angiogram.”
Dr. Wadhera is senior author on the study published online as a research letter in JAMA Internal Medicine, with lead author Andrew S. Oseran, MD, MBA, also from Beth Israel Deaconess Medical Center.
Difficulties with data, interpretation
The researchers looked at payer and self-pay cash prices for noninvasive and invasive CV tests and procedures at the U.S. News & World Report 2021 top 20–ranked U.S. hospitals, based in part on Current Procedural Terminology codes.
Price differences among the hospitals were derived from median negotiated prices for each test and procedure at the centers across all payers. The interquartile ratio (IQR) of prices for each test or procedure across payers was used to evaluate within-hospital price variation.
“Only 80% of the hospitals reported prices for some cardiovascular tests and procedures,” Dr. Wadhera said. “For the most part, even among the hospitals that did report this information, it was extremely challenging to navigate and interpret the data provided.”
Further, the team found that only 7 of the 20 hospitals reported prices for all CV tests and procedures. Centers that did not post prices for some tests or procedures are named in the report’s Figure 1 and Figure 2.
The number of insurance plans listed for each test or procedure ranged from 1 to 432 in the analysis. Median prices ranged from $204 to $2,588 for an echocardiogram, $463 to $3,230 for a stress test, $2,821 to $9,382 for right heart catheterization, $2,868 to $9,203 for a coronary angiogram, $657 to $25,521 for a PCI, and $506 to $20,002 for pacemaker implantation, the report states.
A similar pattern was seen for self-pay cash prices.
Within-hospital variation also ranged broadly. For example, the widest IQR ranges were $3,143-$12,926 for a right heart catheterization, $4,011-$14,486 for a coronary angiogram, $11,325-$23,392 for a PCI, and $8,474-$22,694 for pacemaker implantation.
The report cites a number of limitations to the analysis, among those, the need to rely on the hospitals themselves for data quality and accuracy.
‘More needed besides transparency’
“As a means to better understand health care costs, many opined that full price transparency would leverage market dynamics and result in lower costs,” observed Clyde W. Yancy, MD, MSc, professor of medicine and chief of cardiology at Northwestern Medicine, Chicago. The findings “by an expert group of outcomes scientists make clear that more is needed besides price transparency to lower cost,” he said in an interview.
That said, he added, “there are sufficient variations and allowances made for data collection that it is preferable to hold the current findings circumspect at best. Importantly, the voice of the hospitals does not appear.”
Although “price variation among the top 20 hospitals is substantial,” he observed, “without a better assessment of root cause, actual charge capture, prevailing market dynamics – especially nursing and ancillary staff costs – and the general influence of inflation, it is too difficult to emerge with a precise interpretation.”
Across the 20 hospitals, “there are likely to be 20 different business models,” he added, with negotiated prices reflecting “at least regional, if not institutional, variations.”
“These are complex issues. The several-fold price differences in standard procedures are a concern and an area worth further study with the intention of lowering health care costs,” Dr. Yancy said. “But clearly our next efforts should not address lowering prices per se but understanding how prices are set [and] the connection with reimbursement and actual payments.”
Dr. Wadhera discloses receiving personal fees from Abbott and CVS Health unrelated to the current study; disclosures for the other authors are in the report. Dr. Yancy is deputy editor of JAMA Cardiology.
A version of this article first appeared on Medscape.com.
Wide variation in the cost of common cardiovascular (CV) tests and procedures, from stress tests to coronary interventions, was revealed in a cross-sectional analysis based on publicly available data from 20 top-ranked hospitals in the United States.
The analysis also suggested a low level of compliance with the 2021 Hospital Price Transparency Final Rule among the 20 centers.
“The variation we found in payer-negotiated prices for identical cardiovascular tests and procedures was stunning,” Rishi K. Wadhera, MD, MPP, MPhil, Beth Israel Deaconess Medical Center, Boston, told this news organization.
“For example, there was a 10-fold difference in the median price of an echocardiogram, and these differences were even larger for common procedures” such as percutaneous coronary intervention (PCI) and pacemaker implantation, he said. “It’s hard to argue that this variation reflects quality of care, given that we looked at a top group of highly ranked hospitals.”
“Even more striking was how the price of a cardiovascular test within the very same hospital could differ across commercial insurance companies,” he said. “For example, the price of a stress test varied 5-fold in one hospital, and in another hospital, more than 4-fold for a coronary angiogram.”
Dr. Wadhera is senior author on the study published online as a research letter in JAMA Internal Medicine, with lead author Andrew S. Oseran, MD, MBA, also from Beth Israel Deaconess Medical Center.
Difficulties with data, interpretation
The researchers looked at payer and self-pay cash prices for noninvasive and invasive CV tests and procedures at the U.S. News & World Report 2021 top 20–ranked U.S. hospitals, based in part on Current Procedural Terminology codes.
Price differences among the hospitals were derived from median negotiated prices for each test and procedure at the centers across all payers. The interquartile ratio (IQR) of prices for each test or procedure across payers was used to evaluate within-hospital price variation.
“Only 80% of the hospitals reported prices for some cardiovascular tests and procedures,” Dr. Wadhera said. “For the most part, even among the hospitals that did report this information, it was extremely challenging to navigate and interpret the data provided.”
Further, the team found that only 7 of the 20 hospitals reported prices for all CV tests and procedures. Centers that did not post prices for some tests or procedures are named in the report’s Figure 1 and Figure 2.
The number of insurance plans listed for each test or procedure ranged from 1 to 432 in the analysis. Median prices ranged from $204 to $2,588 for an echocardiogram, $463 to $3,230 for a stress test, $2,821 to $9,382 for right heart catheterization, $2,868 to $9,203 for a coronary angiogram, $657 to $25,521 for a PCI, and $506 to $20,002 for pacemaker implantation, the report states.
A similar pattern was seen for self-pay cash prices.
Within-hospital variation also ranged broadly. For example, the widest IQR ranges were $3,143-$12,926 for a right heart catheterization, $4,011-$14,486 for a coronary angiogram, $11,325-$23,392 for a PCI, and $8,474-$22,694 for pacemaker implantation.
The report cites a number of limitations to the analysis, among those, the need to rely on the hospitals themselves for data quality and accuracy.
‘More needed besides transparency’
“As a means to better understand health care costs, many opined that full price transparency would leverage market dynamics and result in lower costs,” observed Clyde W. Yancy, MD, MSc, professor of medicine and chief of cardiology at Northwestern Medicine, Chicago. The findings “by an expert group of outcomes scientists make clear that more is needed besides price transparency to lower cost,” he said in an interview.
That said, he added, “there are sufficient variations and allowances made for data collection that it is preferable to hold the current findings circumspect at best. Importantly, the voice of the hospitals does not appear.”
Although “price variation among the top 20 hospitals is substantial,” he observed, “without a better assessment of root cause, actual charge capture, prevailing market dynamics – especially nursing and ancillary staff costs – and the general influence of inflation, it is too difficult to emerge with a precise interpretation.”
Across the 20 hospitals, “there are likely to be 20 different business models,” he added, with negotiated prices reflecting “at least regional, if not institutional, variations.”
“These are complex issues. The several-fold price differences in standard procedures are a concern and an area worth further study with the intention of lowering health care costs,” Dr. Yancy said. “But clearly our next efforts should not address lowering prices per se but understanding how prices are set [and] the connection with reimbursement and actual payments.”
Dr. Wadhera discloses receiving personal fees from Abbott and CVS Health unrelated to the current study; disclosures for the other authors are in the report. Dr. Yancy is deputy editor of JAMA Cardiology.
A version of this article first appeared on Medscape.com.
Wide variation in the cost of common cardiovascular (CV) tests and procedures, from stress tests to coronary interventions, was revealed in a cross-sectional analysis based on publicly available data from 20 top-ranked hospitals in the United States.
The analysis also suggested a low level of compliance with the 2021 Hospital Price Transparency Final Rule among the 20 centers.
“The variation we found in payer-negotiated prices for identical cardiovascular tests and procedures was stunning,” Rishi K. Wadhera, MD, MPP, MPhil, Beth Israel Deaconess Medical Center, Boston, told this news organization.
“For example, there was a 10-fold difference in the median price of an echocardiogram, and these differences were even larger for common procedures” such as percutaneous coronary intervention (PCI) and pacemaker implantation, he said. “It’s hard to argue that this variation reflects quality of care, given that we looked at a top group of highly ranked hospitals.”
“Even more striking was how the price of a cardiovascular test within the very same hospital could differ across commercial insurance companies,” he said. “For example, the price of a stress test varied 5-fold in one hospital, and in another hospital, more than 4-fold for a coronary angiogram.”
Dr. Wadhera is senior author on the study published online as a research letter in JAMA Internal Medicine, with lead author Andrew S. Oseran, MD, MBA, also from Beth Israel Deaconess Medical Center.
Difficulties with data, interpretation
The researchers looked at payer and self-pay cash prices for noninvasive and invasive CV tests and procedures at the U.S. News & World Report 2021 top 20–ranked U.S. hospitals, based in part on Current Procedural Terminology codes.
Price differences among the hospitals were derived from median negotiated prices for each test and procedure at the centers across all payers. The interquartile ratio (IQR) of prices for each test or procedure across payers was used to evaluate within-hospital price variation.
“Only 80% of the hospitals reported prices for some cardiovascular tests and procedures,” Dr. Wadhera said. “For the most part, even among the hospitals that did report this information, it was extremely challenging to navigate and interpret the data provided.”
Further, the team found that only 7 of the 20 hospitals reported prices for all CV tests and procedures. Centers that did not post prices for some tests or procedures are named in the report’s Figure 1 and Figure 2.
The number of insurance plans listed for each test or procedure ranged from 1 to 432 in the analysis. Median prices ranged from $204 to $2,588 for an echocardiogram, $463 to $3,230 for a stress test, $2,821 to $9,382 for right heart catheterization, $2,868 to $9,203 for a coronary angiogram, $657 to $25,521 for a PCI, and $506 to $20,002 for pacemaker implantation, the report states.
A similar pattern was seen for self-pay cash prices.
Within-hospital variation also ranged broadly. For example, the widest IQR ranges were $3,143-$12,926 for a right heart catheterization, $4,011-$14,486 for a coronary angiogram, $11,325-$23,392 for a PCI, and $8,474-$22,694 for pacemaker implantation.
The report cites a number of limitations to the analysis, among those, the need to rely on the hospitals themselves for data quality and accuracy.
‘More needed besides transparency’
“As a means to better understand health care costs, many opined that full price transparency would leverage market dynamics and result in lower costs,” observed Clyde W. Yancy, MD, MSc, professor of medicine and chief of cardiology at Northwestern Medicine, Chicago. The findings “by an expert group of outcomes scientists make clear that more is needed besides price transparency to lower cost,” he said in an interview.
That said, he added, “there are sufficient variations and allowances made for data collection that it is preferable to hold the current findings circumspect at best. Importantly, the voice of the hospitals does not appear.”
Although “price variation among the top 20 hospitals is substantial,” he observed, “without a better assessment of root cause, actual charge capture, prevailing market dynamics – especially nursing and ancillary staff costs – and the general influence of inflation, it is too difficult to emerge with a precise interpretation.”
Across the 20 hospitals, “there are likely to be 20 different business models,” he added, with negotiated prices reflecting “at least regional, if not institutional, variations.”
“These are complex issues. The several-fold price differences in standard procedures are a concern and an area worth further study with the intention of lowering health care costs,” Dr. Yancy said. “But clearly our next efforts should not address lowering prices per se but understanding how prices are set [and] the connection with reimbursement and actual payments.”
Dr. Wadhera discloses receiving personal fees from Abbott and CVS Health unrelated to the current study; disclosures for the other authors are in the report. Dr. Yancy is deputy editor of JAMA Cardiology.
A version of this article first appeared on Medscape.com.
Pig heart transplants and the ethical challenges that lie ahead
The long-struggling field of cardiac xenotransplantation has had a very good year.
In January, the University of Maryland made history by keeping a 57-year-old man deemed too sick for a human heart transplant alive for 2 months with a genetically engineered pig heart. On July 12, New York University surgeons reported that heart function was “completely normal with excellent contractility” in two brain-dead patients with pig hearts beating in their chests for 72 hours.
The NYU team approached the project with a decedent model in mind and, after discussions with their IRB equivalent, settled on a 72-hour window because that’s the time they typically keep people ventilated when trying to place their organs, explained Robert A. Montgomery, MD, DPhil, director of the NYU Langone Transplant Institute.
“There’s no real ethical argument for that,” he said in an interview. The consideration is what the family is willing to do when trying to balance doing “something very altruistic and good versus having closure.”
Some families have religious beliefs that burial or interment has to occur very rapidly, whereas others, including one of the family donors, were willing to have the research go on much longer, Dr. Montgomery said. Indeed, the next protocol is being written to consider maintaining the bodies for 2-4 weeks.
“People do vary and you have to kind of accommodate that variation,” he said. “For some people, this isn’t going to be what they’re going to want and that’s why you have to go through the consent process.”
Informed authorization
Arthur L. Caplan, PhD, director of medical ethics at the NYU Langone Medical Center, said the Uniform Anatomical Gift Act recognizes an individual’s right to be an organ donor for transplant and research, but it “mentions nothing about maintaining you in a dead state artificially for research purposes.”
“It’s a major shift in what people are thinking about doing when they die or their relatives die,” he said.
Because organ donation is controlled at the state, not federal, level, the possibility of donating organs for xenotransplantation, like medical aid in dying, will vary between states, observed Dr. Caplan. The best way to ensure that patients whose organs are found to be unsuitable for transplantation have the option is to change state laws.
He noted that cases are already springing up where people are requesting postmortem sperm or egg donations without direct consents from the person who died. “So we have this new area opening up of handling the use of the dead body and we need to bring the law into sync with the possibilities that are out there.”
In terms of informed authorization (informed consent is reserved for the living), Dr. Caplan said there should be written evidence the person wanted to be a donor and, while not required by law, all survivors should give their permission and understand what’s going to be done in terms of the experiment, such as the use of animal parts, when the body will be returned, and the possibility of zoonotic viral infection.
“They have to fully accept that the person is dead and we’re just maintaining them artificially,” he said. “There’s no maintaining anyone who’s alive. That’s a source of a lot of confusion.”
Special committees also need to be appointed with voices from people in organ procurement, law, theology, and patient groups to monitor practice to ensure people who have given permission understood the process, that families have their questions answered independent of the research team, and that clear limits are set on how long experiments will last.
As to what those limits should be: “I think in terms of a week or 2,” Dr. Caplan said. “Obviously we could maintain bodies longer and people have. But I think, culturally in our society, going much past that starts to perhaps stress emotionally, psychologically, family and friends about getting closure.”
“I’m not as comfortable when people say things like, ‘How about 2 months?’ ” he said. “That’s a long time to sort of accept the fact that somebody has died but you can’t complete all the things that go along with the death.”
Dr. Caplan is also uncomfortable with the use of one-off emergency authorizations, as used for Maryland resident David Bennett Sr., who was rejected for standard heart transplantation and required mechanical circulatory support to stay alive.
“It’s too premature, I believe, even to try and rescue someone,” he said. “We need to learn more from the deceased models.”
A better model
Dr. Montgomery noted that primates are very imperfect models for predicting what’s going to happen in humans, and that in order to do xenotransplantation in living humans, there are only two pathways – the one-off emergency authorization or a clinical phase 1 trial.
The decedent model, he said, “will make human trials safer because it’s an intermediate step. You don’t have a living human’s life on the line when you’re trying to do iterative changes and improve the procedure.”
The team, for example, omitted a perfusion pump that was used in the Maryland case and would likely have made its way into phase 1 trials based on baboon data that suggested it was important to have the heart on the pump for hours before it was transplanted, he said. “We didn’t do any of that. We just did it like we would do a regular heart transplant and it started right up, immediately, and started to work.”
The researchers did not release details on the immunosuppression regimen, but noted that, unlike Maryland, they also did not use the experimental anti-CD40 antibody to tamp down the recipients’ immune system.
Although Mr. Bennett’s autopsy did not show any conventional sign of graft rejection, the transplanted pig heart was infected with porcine cytomegalovirus (PCMV) and Mr. Bennett showed traces of DNA from PCMV in his circulation.
Nailing down safety
Dr. Montgomery said he wouldn’t rule out xenotransplantation in a living human, but that the safety issues need to be nailed down. “I think that the tests used on the pig that was the donor for the Bennett case were not sensitive enough for latent virus, and that’s how it slipped through. So there was a bit of going back to the drawing board, really looking at each of the tests, and being sure we had the sensitivity to pick up a latent virus.”
He noted that United Therapeutics, which funded the research and provided the engineered pigs through its subsidiary Revivicor, has created and validated a more sensitive polymerase chain reaction test that covers some 35 different pathogens, microbes, and parasites. NYU has also developed its own platform to repeat the testing and for monitoring after the transplant. “The ones that we’re currently using would have picked up the virus.”
Stuart Russell, MD, a professor of medicine who specializes in advanced HF at Duke University, Durham, N.C., said “the biggest thing from my perspective is those two amazing families that were willing let this happen. ... If 20 years from now, this is what we’re doing, it’s related to these families being this generous at a really tough time in their lives.”
Dr. Russell said he awaits publication of the data on what the pathology of the heart looks like, but that the experiments “help to give us a lot of reassurance that we don’t need to worry about hyperacute rejection,” which by definition is going to happen in the first 24-48 hours.
That said, longer-term data is essential to potential safety issues. Notably, among the 10 genetic modifications made to the pigs, four were porcine gene knockouts, including a growth hormone receptor knockout to prevent abnormal organ growth inside the recipient’s chest. As a result, the organs seem to be small for the age of the pig and just don’t grow that well, admitted Dr. Montgomery, who said they are currently analyzing this with echocardiography.
Dr. Russell said this may create a sizing issue, but also “if you have a heart that’s more stressed in the pig, from the point of being a donor, maybe it’s not as good a heart as if it was growing normally. But that kind of stuff, I think, is going to take more than two cases and longer-term data to sort out.”
Sharon Hunt, MD, professor emerita, Stanford (Calif.) University Medical Center, and past president of the International Society for Heart Lung Transplantation, said it’s not the technical aspects, but the biology of xenotransplantation that’s really daunting.
“It’s not the physical act of doing it, like they needed a bigger heart or a smaller heart. Those are technical problems but they’ll manage them,” she said. “The big problem is biological – and the bottom line is we don’t really know. We may have overcome hyperacute rejection, which is great, but the rest remains to be seen.”
Dr. Hunt, who worked with heart transplantation pioneer Norman Shumway, MD, and spent decades caring for patients after transplantation, said most families will consent to 24 or 48 hours or even a week of experimentation on a brain-dead loved one, but what the transplant community wants to know is whether this is workable for many months.
“So the fact that the xenotransplant works for 72 hours, yeah, that’s groovy. But, you know, the answer is kind of ‘so what,’ ” she said. “I’d like to see this go for months, like they were trying to do in the human in Maryland.”
For phase 1 trials, even longer-term survival with or without rejection or with rejection that’s treatable is needed, Dr. Hunt suggested.
“We haven’t seen that yet. The Maryland people were very valiant but they lost the cause,” she said. “There’s just so much more to do before we have a viable model to start anything like a phase 1 trial. I’d love it if that happens in my lifetime, but I’m not sure it’s going to.”
Dr. Russell and Dr. Hunt reported no relevant financial relationships. Dr. Caplan reported serving as a director, officer, partner, employee, advisor, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position) and is a contributing author and adviser for Medscape.
A version of this article first appeared on Medscape.com.
The long-struggling field of cardiac xenotransplantation has had a very good year.
In January, the University of Maryland made history by keeping a 57-year-old man deemed too sick for a human heart transplant alive for 2 months with a genetically engineered pig heart. On July 12, New York University surgeons reported that heart function was “completely normal with excellent contractility” in two brain-dead patients with pig hearts beating in their chests for 72 hours.
The NYU team approached the project with a decedent model in mind and, after discussions with their IRB equivalent, settled on a 72-hour window because that’s the time they typically keep people ventilated when trying to place their organs, explained Robert A. Montgomery, MD, DPhil, director of the NYU Langone Transplant Institute.
“There’s no real ethical argument for that,” he said in an interview. The consideration is what the family is willing to do when trying to balance doing “something very altruistic and good versus having closure.”
Some families have religious beliefs that burial or interment has to occur very rapidly, whereas others, including one of the family donors, were willing to have the research go on much longer, Dr. Montgomery said. Indeed, the next protocol is being written to consider maintaining the bodies for 2-4 weeks.
“People do vary and you have to kind of accommodate that variation,” he said. “For some people, this isn’t going to be what they’re going to want and that’s why you have to go through the consent process.”
Informed authorization
Arthur L. Caplan, PhD, director of medical ethics at the NYU Langone Medical Center, said the Uniform Anatomical Gift Act recognizes an individual’s right to be an organ donor for transplant and research, but it “mentions nothing about maintaining you in a dead state artificially for research purposes.”
“It’s a major shift in what people are thinking about doing when they die or their relatives die,” he said.
Because organ donation is controlled at the state, not federal, level, the possibility of donating organs for xenotransplantation, like medical aid in dying, will vary between states, observed Dr. Caplan. The best way to ensure that patients whose organs are found to be unsuitable for transplantation have the option is to change state laws.
He noted that cases are already springing up where people are requesting postmortem sperm or egg donations without direct consents from the person who died. “So we have this new area opening up of handling the use of the dead body and we need to bring the law into sync with the possibilities that are out there.”
In terms of informed authorization (informed consent is reserved for the living), Dr. Caplan said there should be written evidence the person wanted to be a donor and, while not required by law, all survivors should give their permission and understand what’s going to be done in terms of the experiment, such as the use of animal parts, when the body will be returned, and the possibility of zoonotic viral infection.
“They have to fully accept that the person is dead and we’re just maintaining them artificially,” he said. “There’s no maintaining anyone who’s alive. That’s a source of a lot of confusion.”
Special committees also need to be appointed with voices from people in organ procurement, law, theology, and patient groups to monitor practice to ensure people who have given permission understood the process, that families have their questions answered independent of the research team, and that clear limits are set on how long experiments will last.
As to what those limits should be: “I think in terms of a week or 2,” Dr. Caplan said. “Obviously we could maintain bodies longer and people have. But I think, culturally in our society, going much past that starts to perhaps stress emotionally, psychologically, family and friends about getting closure.”
“I’m not as comfortable when people say things like, ‘How about 2 months?’ ” he said. “That’s a long time to sort of accept the fact that somebody has died but you can’t complete all the things that go along with the death.”
Dr. Caplan is also uncomfortable with the use of one-off emergency authorizations, as used for Maryland resident David Bennett Sr., who was rejected for standard heart transplantation and required mechanical circulatory support to stay alive.
“It’s too premature, I believe, even to try and rescue someone,” he said. “We need to learn more from the deceased models.”
A better model
Dr. Montgomery noted that primates are very imperfect models for predicting what’s going to happen in humans, and that in order to do xenotransplantation in living humans, there are only two pathways – the one-off emergency authorization or a clinical phase 1 trial.
The decedent model, he said, “will make human trials safer because it’s an intermediate step. You don’t have a living human’s life on the line when you’re trying to do iterative changes and improve the procedure.”

The team, for example, omitted a perfusion pump that was used in the Maryland case and would likely have made its way into phase 1 trials based on baboon data that suggested it was important to have the heart on the pump for hours before it was transplanted, he said. “We didn’t do any of that. We just did it like we would do a regular heart transplant and it started right up, immediately, and started to work.”
The researchers did not release details on the immunosuppression regimen, but noted that, unlike Maryland, they also did not use the experimental anti-CD40 antibody to tamp down the recipients’ immune system.
Although Mr. Bennett’s autopsy did not show any conventional sign of graft rejection, the transplanted pig heart was infected with porcine cytomegalovirus (PCMV) and Mr. Bennett showed traces of DNA from PCMV in his circulation.
Nailing down safety
Dr. Montgomery said he wouldn’t rule out xenotransplantation in a living human, but that the safety issues need to be nailed down. “I think that the tests used on the pig that was the donor for the Bennett case were not sensitive enough for latent virus, and that’s how it slipped through. So there was a bit of going back to the drawing board, really looking at each of the tests, and being sure we had the sensitivity to pick up a latent virus.”
He noted that United Therapeutics, which funded the research and provided the engineered pigs through its subsidiary Revivicor, has created and validated a more sensitive polymerase chain reaction test that covers some 35 different pathogens, microbes, and parasites. NYU has also developed its own platform to repeat the testing and for monitoring after the transplant. “The ones that we’re currently using would have picked up the virus.”
Stuart Russell, MD, a professor of medicine who specializes in advanced HF at Duke University, Durham, N.C., said “the biggest thing from my perspective is those two amazing families that were willing let this happen. ... If 20 years from now, this is what we’re doing, it’s related to these families being this generous at a really tough time in their lives.”
Dr. Russell said he awaits publication of the data on what the pathology of the heart looks like, but that the experiments “help to give us a lot of reassurance that we don’t need to worry about hyperacute rejection,” which by definition is going to happen in the first 24-48 hours.
That said, longer-term data is essential to potential safety issues. Notably, among the 10 genetic modifications made to the pigs, four were porcine gene knockouts, including a growth hormone receptor knockout to prevent abnormal organ growth inside the recipient’s chest. As a result, the organs seem to be small for the age of the pig and just don’t grow that well, admitted Dr. Montgomery, who said they are currently analyzing this with echocardiography.
Dr. Russell said this may create a sizing issue, but also “if you have a heart that’s more stressed in the pig, from the point of being a donor, maybe it’s not as good a heart as if it was growing normally. But that kind of stuff, I think, is going to take more than two cases and longer-term data to sort out.”
Sharon Hunt, MD, professor emerita, Stanford (Calif.) University Medical Center, and past president of the International Society for Heart Lung Transplantation, said it’s not the technical aspects, but the biology of xenotransplantation that’s really daunting.
“It’s not the physical act of doing it, like they needed a bigger heart or a smaller heart. Those are technical problems but they’ll manage them,” she said. “The big problem is biological – and the bottom line is we don’t really know. We may have overcome hyperacute rejection, which is great, but the rest remains to be seen.”
Dr. Hunt, who worked with heart transplantation pioneer Norman Shumway, MD, and spent decades caring for patients after transplantation, said most families will consent to 24 or 48 hours or even a week of experimentation on a brain-dead loved one, but what the transplant community wants to know is whether this is workable for many months.
“So the fact that the xenotransplant works for 72 hours, yeah, that’s groovy. But, you know, the answer is kind of ‘so what,’ ” she said. “I’d like to see this go for months, like they were trying to do in the human in Maryland.”
For phase 1 trials, even longer-term survival with or without rejection or with rejection that’s treatable is needed, Dr. Hunt suggested.
“We haven’t seen that yet. The Maryland people were very valiant but they lost the cause,” she said. “There’s just so much more to do before we have a viable model to start anything like a phase 1 trial. I’d love it if that happens in my lifetime, but I’m not sure it’s going to.”
Dr. Russell and Dr. Hunt reported no relevant financial relationships. Dr. Caplan reported serving as a director, officer, partner, employee, advisor, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position) and is a contributing author and adviser for Medscape.
A version of this article first appeared on Medscape.com.
The long-struggling field of cardiac xenotransplantation has had a very good year.
In January, the University of Maryland made history by keeping a 57-year-old man deemed too sick for a human heart transplant alive for 2 months with a genetically engineered pig heart. On July 12, New York University surgeons reported that heart function was “completely normal with excellent contractility” in two brain-dead patients with pig hearts beating in their chests for 72 hours.
The NYU team approached the project with a decedent model in mind and, after discussions with their IRB equivalent, settled on a 72-hour window because that’s the time they typically keep people ventilated when trying to place their organs, explained Robert A. Montgomery, MD, DPhil, director of the NYU Langone Transplant Institute.
“There’s no real ethical argument for that,” he said in an interview. The consideration is what the family is willing to do when trying to balance doing “something very altruistic and good versus having closure.”
Some families have religious beliefs that burial or interment has to occur very rapidly, whereas others, including one of the family donors, were willing to have the research go on much longer, Dr. Montgomery said. Indeed, the next protocol is being written to consider maintaining the bodies for 2-4 weeks.
“People do vary and you have to kind of accommodate that variation,” he said. “For some people, this isn’t going to be what they’re going to want and that’s why you have to go through the consent process.”
Informed authorization
Arthur L. Caplan, PhD, director of medical ethics at the NYU Langone Medical Center, said the Uniform Anatomical Gift Act recognizes an individual’s right to be an organ donor for transplant and research, but it “mentions nothing about maintaining you in a dead state artificially for research purposes.”
“It’s a major shift in what people are thinking about doing when they die or their relatives die,” he said.
Because organ donation is controlled at the state, not federal, level, the possibility of donating organs for xenotransplantation, like medical aid in dying, will vary between states, observed Dr. Caplan. The best way to ensure that patients whose organs are found to be unsuitable for transplantation have the option is to change state laws.
He noted that cases are already springing up where people are requesting postmortem sperm or egg donations without direct consents from the person who died. “So we have this new area opening up of handling the use of the dead body and we need to bring the law into sync with the possibilities that are out there.”
In terms of informed authorization (informed consent is reserved for the living), Dr. Caplan said there should be written evidence the person wanted to be a donor and, while not required by law, all survivors should give their permission and understand what’s going to be done in terms of the experiment, such as the use of animal parts, when the body will be returned, and the possibility of zoonotic viral infection.
“They have to fully accept that the person is dead and we’re just maintaining them artificially,” he said. “There’s no maintaining anyone who’s alive. That’s a source of a lot of confusion.”
Special committees also need to be appointed with voices from people in organ procurement, law, theology, and patient groups to monitor practice to ensure people who have given permission understood the process, that families have their questions answered independent of the research team, and that clear limits are set on how long experiments will last.
As to what those limits should be: “I think in terms of a week or 2,” Dr. Caplan said. “Obviously we could maintain bodies longer and people have. But I think, culturally in our society, going much past that starts to perhaps stress emotionally, psychologically, family and friends about getting closure.”
“I’m not as comfortable when people say things like, ‘How about 2 months?’ ” he said. “That’s a long time to sort of accept the fact that somebody has died but you can’t complete all the things that go along with the death.”
Dr. Caplan is also uncomfortable with the use of one-off emergency authorizations, as used for Maryland resident David Bennett Sr., who was rejected for standard heart transplantation and required mechanical circulatory support to stay alive.
“It’s too premature, I believe, even to try and rescue someone,” he said. “We need to learn more from the deceased models.”
A better model
Dr. Montgomery noted that primates are very imperfect models for predicting what’s going to happen in humans, and that in order to do xenotransplantation in living humans, there are only two pathways – the one-off emergency authorization or a clinical phase 1 trial.
The decedent model, he said, “will make human trials safer because it’s an intermediate step. You don’t have a living human’s life on the line when you’re trying to do iterative changes and improve the procedure.”

The team, for example, omitted a perfusion pump that was used in the Maryland case and would likely have made its way into phase 1 trials based on baboon data that suggested it was important to have the heart on the pump for hours before it was transplanted, he said. “We didn’t do any of that. We just did it like we would do a regular heart transplant and it started right up, immediately, and started to work.”
The researchers did not release details on the immunosuppression regimen, but noted that, unlike Maryland, they also did not use the experimental anti-CD40 antibody to tamp down the recipients’ immune system.
Although Mr. Bennett’s autopsy did not show any conventional sign of graft rejection, the transplanted pig heart was infected with porcine cytomegalovirus (PCMV) and Mr. Bennett showed traces of DNA from PCMV in his circulation.
Nailing down safety
Dr. Montgomery said he wouldn’t rule out xenotransplantation in a living human, but that the safety issues need to be nailed down. “I think that the tests used on the pig that was the donor for the Bennett case were not sensitive enough for latent virus, and that’s how it slipped through. So there was a bit of going back to the drawing board, really looking at each of the tests, and being sure we had the sensitivity to pick up a latent virus.”
He noted that United Therapeutics, which funded the research and provided the engineered pigs through its subsidiary Revivicor, has created and validated a more sensitive polymerase chain reaction test that covers some 35 different pathogens, microbes, and parasites. NYU has also developed its own platform to repeat the testing and for monitoring after the transplant. “The ones that we’re currently using would have picked up the virus.”
Stuart Russell, MD, a professor of medicine who specializes in advanced HF at Duke University, Durham, N.C., said “the biggest thing from my perspective is those two amazing families that were willing let this happen. ... If 20 years from now, this is what we’re doing, it’s related to these families being this generous at a really tough time in their lives.”
Dr. Russell said he awaits publication of the data on what the pathology of the heart looks like, but that the experiments “help to give us a lot of reassurance that we don’t need to worry about hyperacute rejection,” which by definition is going to happen in the first 24-48 hours.
That said, longer-term data is essential to potential safety issues. Notably, among the 10 genetic modifications made to the pigs, four were porcine gene knockouts, including a growth hormone receptor knockout to prevent abnormal organ growth inside the recipient’s chest. As a result, the organs seem to be small for the age of the pig and just don’t grow that well, admitted Dr. Montgomery, who said they are currently analyzing this with echocardiography.
Dr. Russell said this may create a sizing issue, but also “if you have a heart that’s more stressed in the pig, from the point of being a donor, maybe it’s not as good a heart as if it was growing normally. But that kind of stuff, I think, is going to take more than two cases and longer-term data to sort out.”
Sharon Hunt, MD, professor emerita, Stanford (Calif.) University Medical Center, and past president of the International Society for Heart Lung Transplantation, said it’s not the technical aspects, but the biology of xenotransplantation that’s really daunting.
“It’s not the physical act of doing it, like they needed a bigger heart or a smaller heart. Those are technical problems but they’ll manage them,” she said. “The big problem is biological – and the bottom line is we don’t really know. We may have overcome hyperacute rejection, which is great, but the rest remains to be seen.”
Dr. Hunt, who worked with heart transplantation pioneer Norman Shumway, MD, and spent decades caring for patients after transplantation, said most families will consent to 24 or 48 hours or even a week of experimentation on a brain-dead loved one, but what the transplant community wants to know is whether this is workable for many months.
“So the fact that the xenotransplant works for 72 hours, yeah, that’s groovy. But, you know, the answer is kind of ‘so what,’ ” she said. “I’d like to see this go for months, like they were trying to do in the human in Maryland.”
For phase 1 trials, even longer-term survival with or without rejection or with rejection that’s treatable is needed, Dr. Hunt suggested.
“We haven’t seen that yet. The Maryland people were very valiant but they lost the cause,” she said. “There’s just so much more to do before we have a viable model to start anything like a phase 1 trial. I’d love it if that happens in my lifetime, but I’m not sure it’s going to.”
Dr. Russell and Dr. Hunt reported no relevant financial relationships. Dr. Caplan reported serving as a director, officer, partner, employee, advisor, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position) and is a contributing author and adviser for Medscape.
A version of this article first appeared on Medscape.com.