User login
Plantar Fibromatosis
The Epiphyseal Scar as a Radiographic Landmark for Retrograde Femoral Nail Insertion
Recurrent pyelonephritis as a sign of ‘sponge kidney’
KEY FEATURES
Medullary sponge kidney causes extensive cystic dilation of medullary collecting tubules.1 It is usually an incidental finding in patients undergoing intravenous urography as part of the evaluation for infection, hematuria, or kidney stones.
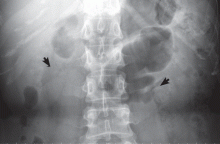
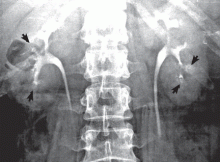
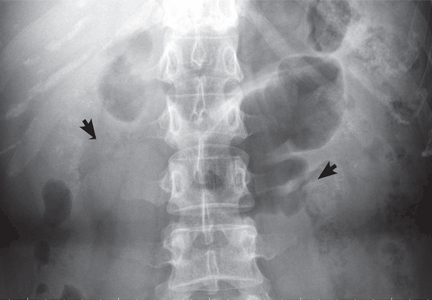
The classic urographic appearance is linear striations with small brushes or “bouquets of flowers,” which represent the collection of contrast material in small papillary cysts.
Medullary sponge kidney has long been considered a congenital disorder, but the genetic defect has not yet been identified, and the pathogenesis is not yet known. It has only rarely been reported in children.2
It is usually asymptomatic, but complications may occur, including nephrocalcinosis or lithiasis, urinary tract infection, renal tubular acidosis, and impaired urine concentrating ability.
RISK OF LITHIASIS
Gambaro et al3 estimated that medullary sponge kidney is found in up to 20% of patients with urolithiasis, and that more than 70% of patients with medullary sponge kidney develop stones.
Cystic dilation of medullary collecting tubules (an anatomic abnormality) inevitably causes urinary stasis. This, combined with hypercalciuria and reduced excretion of urinary citrate and magnesium (metabolic abnormalities), contributes to lithiasis.4 Lithiasis in medullary sponge kidney is a well-known cause of urinary tract infection, and it tends to facilitate infective stone formation after episodes of urinary tract infection.5
The unique anatomic derangement of medullary sponge kidney contributes to the recurrence of pyelonephritis, in addition to the conventional risk factors—frequent sexual intercourse, avoidance of voiding because it is inconvenient, incomplete bladder emptying, ureteropelvic junction obstruction, ectopic ureter, and impaired immunity.6
DIAGNOSIS AND TREATMENT
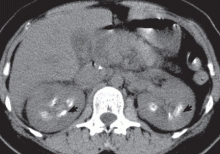
Intravenous urography is the gold standard for the diagnosis of medullary sponge kidney; computed tomography and ultrasonography are generally limited in their ability to clearly show the tubular ectasia.7
Treatment includes antibiotics for acute pyelonephritis and thiazide diuretics and potassium citrate to prevent stone formation and renal tubular acidosis. Due to its silent course, medullary sponge kidney should be considered not only as a cause of nephrocalcinosis and nephrolithiasis, but also as a distinct entity complicating recurrent pyelonephritis.
- Gambaro G, Feltrin GP, Lupo A, Bonfante L, D'Angelo A, Antonello A. Medullary sponge kidney (Lenarduzzi-Cacchi-Ricci disease): a Padua Medical School discovery in the 1930s. Kidney Int 2006; 69:663–670.
- Kasap B, Soylu A, Oren O, Turkmen M, Kavukcu S. Medullary sponge kidney associated with distal renal tubular acidosis in a 5-year-old girl. Eur J Pediatr 2006; 165:648–651.
- Gambaro G, Fabris A, Puliatta D, Lupo A. Lithiasis in cystic kidney disease and malformations of the urinary tract. Urol Res 2006; 34:102–107.
- O'Neill M, Breslau NA, Pak CY. Metabolic evaluation of nephrolithiasis in patients with medullary sponge kidney. JAMA 1981; 245:1233–1236.
- Miano R, Germani S, Vespasiani G. Stones and urinary tract infections. Urol Int 2007; 79(suppl 1):32–36.
- Scholes D, Hooton TM, Roberts PL, Gupta K, Stapleton AE, Stamm WE. Risk factors associated with acute pyelonephritis in healthy women. Ann Intern Med 2005; 142:20–27.
- Maw AM, Megibow AJ, Grasso M, Goldfarb DS. Diagnosis of medullary sponge kidney by computed tomographic urography. Am J Kidney Dis 2007; 50:146–150.
KEY FEATURES
Medullary sponge kidney causes extensive cystic dilation of medullary collecting tubules.1 It is usually an incidental finding in patients undergoing intravenous urography as part of the evaluation for infection, hematuria, or kidney stones.
The classic urographic appearance is linear striations with small brushes or “bouquets of flowers,” which represent the collection of contrast material in small papillary cysts.
Medullary sponge kidney has long been considered a congenital disorder, but the genetic defect has not yet been identified, and the pathogenesis is not yet known. It has only rarely been reported in children.2
It is usually asymptomatic, but complications may occur, including nephrocalcinosis or lithiasis, urinary tract infection, renal tubular acidosis, and impaired urine concentrating ability.
RISK OF LITHIASIS
Gambaro et al3 estimated that medullary sponge kidney is found in up to 20% of patients with urolithiasis, and that more than 70% of patients with medullary sponge kidney develop stones.
Cystic dilation of medullary collecting tubules (an anatomic abnormality) inevitably causes urinary stasis. This, combined with hypercalciuria and reduced excretion of urinary citrate and magnesium (metabolic abnormalities), contributes to lithiasis.4 Lithiasis in medullary sponge kidney is a well-known cause of urinary tract infection, and it tends to facilitate infective stone formation after episodes of urinary tract infection.5
The unique anatomic derangement of medullary sponge kidney contributes to the recurrence of pyelonephritis, in addition to the conventional risk factors—frequent sexual intercourse, avoidance of voiding because it is inconvenient, incomplete bladder emptying, ureteropelvic junction obstruction, ectopic ureter, and impaired immunity.6
DIAGNOSIS AND TREATMENT
Intravenous urography is the gold standard for the diagnosis of medullary sponge kidney; computed tomography and ultrasonography are generally limited in their ability to clearly show the tubular ectasia.7
Treatment includes antibiotics for acute pyelonephritis and thiazide diuretics and potassium citrate to prevent stone formation and renal tubular acidosis. Due to its silent course, medullary sponge kidney should be considered not only as a cause of nephrocalcinosis and nephrolithiasis, but also as a distinct entity complicating recurrent pyelonephritis.
KEY FEATURES
Medullary sponge kidney causes extensive cystic dilation of medullary collecting tubules.1 It is usually an incidental finding in patients undergoing intravenous urography as part of the evaluation for infection, hematuria, or kidney stones.
The classic urographic appearance is linear striations with small brushes or “bouquets of flowers,” which represent the collection of contrast material in small papillary cysts.
Medullary sponge kidney has long been considered a congenital disorder, but the genetic defect has not yet been identified, and the pathogenesis is not yet known. It has only rarely been reported in children.2
It is usually asymptomatic, but complications may occur, including nephrocalcinosis or lithiasis, urinary tract infection, renal tubular acidosis, and impaired urine concentrating ability.
RISK OF LITHIASIS
Gambaro et al3 estimated that medullary sponge kidney is found in up to 20% of patients with urolithiasis, and that more than 70% of patients with medullary sponge kidney develop stones.
Cystic dilation of medullary collecting tubules (an anatomic abnormality) inevitably causes urinary stasis. This, combined with hypercalciuria and reduced excretion of urinary citrate and magnesium (metabolic abnormalities), contributes to lithiasis.4 Lithiasis in medullary sponge kidney is a well-known cause of urinary tract infection, and it tends to facilitate infective stone formation after episodes of urinary tract infection.5
The unique anatomic derangement of medullary sponge kidney contributes to the recurrence of pyelonephritis, in addition to the conventional risk factors—frequent sexual intercourse, avoidance of voiding because it is inconvenient, incomplete bladder emptying, ureteropelvic junction obstruction, ectopic ureter, and impaired immunity.6
DIAGNOSIS AND TREATMENT
Intravenous urography is the gold standard for the diagnosis of medullary sponge kidney; computed tomography and ultrasonography are generally limited in their ability to clearly show the tubular ectasia.7
Treatment includes antibiotics for acute pyelonephritis and thiazide diuretics and potassium citrate to prevent stone formation and renal tubular acidosis. Due to its silent course, medullary sponge kidney should be considered not only as a cause of nephrocalcinosis and nephrolithiasis, but also as a distinct entity complicating recurrent pyelonephritis.
- Gambaro G, Feltrin GP, Lupo A, Bonfante L, D'Angelo A, Antonello A. Medullary sponge kidney (Lenarduzzi-Cacchi-Ricci disease): a Padua Medical School discovery in the 1930s. Kidney Int 2006; 69:663–670.
- Kasap B, Soylu A, Oren O, Turkmen M, Kavukcu S. Medullary sponge kidney associated with distal renal tubular acidosis in a 5-year-old girl. Eur J Pediatr 2006; 165:648–651.
- Gambaro G, Fabris A, Puliatta D, Lupo A. Lithiasis in cystic kidney disease and malformations of the urinary tract. Urol Res 2006; 34:102–107.
- O'Neill M, Breslau NA, Pak CY. Metabolic evaluation of nephrolithiasis in patients with medullary sponge kidney. JAMA 1981; 245:1233–1236.
- Miano R, Germani S, Vespasiani G. Stones and urinary tract infections. Urol Int 2007; 79(suppl 1):32–36.
- Scholes D, Hooton TM, Roberts PL, Gupta K, Stapleton AE, Stamm WE. Risk factors associated with acute pyelonephritis in healthy women. Ann Intern Med 2005; 142:20–27.
- Maw AM, Megibow AJ, Grasso M, Goldfarb DS. Diagnosis of medullary sponge kidney by computed tomographic urography. Am J Kidney Dis 2007; 50:146–150.
- Gambaro G, Feltrin GP, Lupo A, Bonfante L, D'Angelo A, Antonello A. Medullary sponge kidney (Lenarduzzi-Cacchi-Ricci disease): a Padua Medical School discovery in the 1930s. Kidney Int 2006; 69:663–670.
- Kasap B, Soylu A, Oren O, Turkmen M, Kavukcu S. Medullary sponge kidney associated with distal renal tubular acidosis in a 5-year-old girl. Eur J Pediatr 2006; 165:648–651.
- Gambaro G, Fabris A, Puliatta D, Lupo A. Lithiasis in cystic kidney disease and malformations of the urinary tract. Urol Res 2006; 34:102–107.
- O'Neill M, Breslau NA, Pak CY. Metabolic evaluation of nephrolithiasis in patients with medullary sponge kidney. JAMA 1981; 245:1233–1236.
- Miano R, Germani S, Vespasiani G. Stones and urinary tract infections. Urol Int 2007; 79(suppl 1):32–36.
- Scholes D, Hooton TM, Roberts PL, Gupta K, Stapleton AE, Stamm WE. Risk factors associated with acute pyelonephritis in healthy women. Ann Intern Med 2005; 142:20–27.
- Maw AM, Megibow AJ, Grasso M, Goldfarb DS. Diagnosis of medullary sponge kidney by computed tomographic urography. Am J Kidney Dis 2007; 50:146–150.
Severe Pain on Bottom of Foot
Two Patients With Pain and Wrist Deformity
Chest Pain, Cough, and General Weakness
DE MRI Predicts Atrial Fib Recurrence Risk
BOSTON — Researchers recently devised a way to visualize fibrotic tissue within the left atrial wall noninvasively with MRI. Results from a new study that took this analysis a step further showed that patients with atrial fibrillation whose left atrium had high levels of fibrosis also faced a significantly increased risk of failing treatment by pulmonary vein isolation and septal debulking and revert to fibrillation.
The new method of left-atrial assessment with delayed-enhancement (DE) MRI may identify patients at the highest risk of early recurrence of atrial fibrillation following a noninvasive, pulmonary-vein isolation procedure, Dr. Saul Kalvaitis said at the Heart Rhythm Society's annual meeting.
Although this early finding needs replication by other groups, it has significant therapeutic implications, said Dr. Melvin M. Scheinman, professor and cardiac electrophysiologist at the University of California, San Francisco.
The report suggests that DE MRI may identify patients with atrial fibrillation who are not good candidates for ablation therapy because of high fibrosis content within the atrial wall. Recent research findings by other groups suggest that certain drug treatments reverse fibrosis. If such treatments prove effective, potentially nonresponsive atrial fibrillation patients might benefit from ablation, he added.
DE MRI is now a standard method for assessing ventricular scar, but Dr. Kalvaitis and coworkers at the University of Utah, Salt Lake City, are the first to apply the method to left atrial assessment, Dr. Scheinman said in an interview. A published report of the Utah group's success with DE MRI for left-atrial assessment appeared in April (Circulation 2009;119:1758–67).
DE MRI involves infusing gadolinium contrast into the patient. Uptake of the contrast into fibrotic tissue occurs at a different rate compared with its entry into healthy tissue, and this difference allows assessment of the amount and location of fibrotic scar within the heart wall.
In the new study, Dr. Kalvaitis and his associates performed DE MRI exams on 62 patients with atrial fibrillation scheduled to undergo pulmonary vein antrum isolation and atrial septum debulking. Their average age was 64 (range 23–84), and two-thirds were men. On average for the entire group, structural modeling affected 17% of the left atrium.
The researchers divided the patients into three subgroups based on the extent of their left-atrial remodeling ratio: less than 15% (27 patients), 15%-35% (28), and more than 35% (7). The amount of left atrial fibrosis closely correlated with the ratio of left atrial remodeling, ranging from an average of 8% fibrosis in patients with the least remodeling to 46% in patients with the most remodeling (see box).
The incidence of an early recurrence of atrial fibrillation, defined as atrial fibrillation recurring within 3 months of the ablation procedure, closely correlated with the extent of left atrial fibrosis. The early recurrence rate was 19% in the subgroup with the lowest level of atrial fibrosis and 39% and 40%, respectively, in the two subgroups with higher amount of fibrosis.
In a multivariate analysis that controlled for baseline differences among the patients, the subgroup assignment of left atrial remodeling ratio and left atrial fibrosis was the only significant determinant of recurrence risk, increasing the risk by more than twofold, Dr. Kalvaitis reported.
Expressed another way, patients with an early recurrence of atrial fibrillation after ablation had an average 24% left-atrial remodeling ratio prior to ablation treatment, compared with an average 14% ratio among those who did not have an early recurrence.
This method for defining recurrence risk may identify patients who would benefit from treatment with an anti-arrhythmic drug after their ablation procedure, Dr. Kalvaitis said. He said that he and his associates had no financial disclosures.
Source ELSEVIER GLOBAL MEDICAL NEWS
Delayed-enhancement magnetic resonance imaging of left atria prior to ablation therapy, which has been processed as color three-dimensional models, indicates regions of abnormal enhancement in fibrotic tissue as green or other colors, and healthy tissue as blue.
Source Courtesy Dr. Nassir F. Marrouche
BOSTON — Researchers recently devised a way to visualize fibrotic tissue within the left atrial wall noninvasively with MRI. Results from a new study that took this analysis a step further showed that patients with atrial fibrillation whose left atrium had high levels of fibrosis also faced a significantly increased risk of failing treatment by pulmonary vein isolation and septal debulking and revert to fibrillation.
The new method of left-atrial assessment with delayed-enhancement (DE) MRI may identify patients at the highest risk of early recurrence of atrial fibrillation following a noninvasive, pulmonary-vein isolation procedure, Dr. Saul Kalvaitis said at the Heart Rhythm Society's annual meeting.
Although this early finding needs replication by other groups, it has significant therapeutic implications, said Dr. Melvin M. Scheinman, professor and cardiac electrophysiologist at the University of California, San Francisco.
The report suggests that DE MRI may identify patients with atrial fibrillation who are not good candidates for ablation therapy because of high fibrosis content within the atrial wall. Recent research findings by other groups suggest that certain drug treatments reverse fibrosis. If such treatments prove effective, potentially nonresponsive atrial fibrillation patients might benefit from ablation, he added.
DE MRI is now a standard method for assessing ventricular scar, but Dr. Kalvaitis and coworkers at the University of Utah, Salt Lake City, are the first to apply the method to left atrial assessment, Dr. Scheinman said in an interview. A published report of the Utah group's success with DE MRI for left-atrial assessment appeared in April (Circulation 2009;119:1758–67).
DE MRI involves infusing gadolinium contrast into the patient. Uptake of the contrast into fibrotic tissue occurs at a different rate compared with its entry into healthy tissue, and this difference allows assessment of the amount and location of fibrotic scar within the heart wall.
In the new study, Dr. Kalvaitis and his associates performed DE MRI exams on 62 patients with atrial fibrillation scheduled to undergo pulmonary vein antrum isolation and atrial septum debulking. Their average age was 64 (range 23–84), and two-thirds were men. On average for the entire group, structural modeling affected 17% of the left atrium.
The researchers divided the patients into three subgroups based on the extent of their left-atrial remodeling ratio: less than 15% (27 patients), 15%-35% (28), and more than 35% (7). The amount of left atrial fibrosis closely correlated with the ratio of left atrial remodeling, ranging from an average of 8% fibrosis in patients with the least remodeling to 46% in patients with the most remodeling (see box).
The incidence of an early recurrence of atrial fibrillation, defined as atrial fibrillation recurring within 3 months of the ablation procedure, closely correlated with the extent of left atrial fibrosis. The early recurrence rate was 19% in the subgroup with the lowest level of atrial fibrosis and 39% and 40%, respectively, in the two subgroups with higher amount of fibrosis.
In a multivariate analysis that controlled for baseline differences among the patients, the subgroup assignment of left atrial remodeling ratio and left atrial fibrosis was the only significant determinant of recurrence risk, increasing the risk by more than twofold, Dr. Kalvaitis reported.
Expressed another way, patients with an early recurrence of atrial fibrillation after ablation had an average 24% left-atrial remodeling ratio prior to ablation treatment, compared with an average 14% ratio among those who did not have an early recurrence.
This method for defining recurrence risk may identify patients who would benefit from treatment with an anti-arrhythmic drug after their ablation procedure, Dr. Kalvaitis said. He said that he and his associates had no financial disclosures.
Source ELSEVIER GLOBAL MEDICAL NEWS
Delayed-enhancement magnetic resonance imaging of left atria prior to ablation therapy, which has been processed as color three-dimensional models, indicates regions of abnormal enhancement in fibrotic tissue as green or other colors, and healthy tissue as blue.
Source Courtesy Dr. Nassir F. Marrouche
BOSTON — Researchers recently devised a way to visualize fibrotic tissue within the left atrial wall noninvasively with MRI. Results from a new study that took this analysis a step further showed that patients with atrial fibrillation whose left atrium had high levels of fibrosis also faced a significantly increased risk of failing treatment by pulmonary vein isolation and septal debulking and revert to fibrillation.
The new method of left-atrial assessment with delayed-enhancement (DE) MRI may identify patients at the highest risk of early recurrence of atrial fibrillation following a noninvasive, pulmonary-vein isolation procedure, Dr. Saul Kalvaitis said at the Heart Rhythm Society's annual meeting.
Although this early finding needs replication by other groups, it has significant therapeutic implications, said Dr. Melvin M. Scheinman, professor and cardiac electrophysiologist at the University of California, San Francisco.
The report suggests that DE MRI may identify patients with atrial fibrillation who are not good candidates for ablation therapy because of high fibrosis content within the atrial wall. Recent research findings by other groups suggest that certain drug treatments reverse fibrosis. If such treatments prove effective, potentially nonresponsive atrial fibrillation patients might benefit from ablation, he added.
DE MRI is now a standard method for assessing ventricular scar, but Dr. Kalvaitis and coworkers at the University of Utah, Salt Lake City, are the first to apply the method to left atrial assessment, Dr. Scheinman said in an interview. A published report of the Utah group's success with DE MRI for left-atrial assessment appeared in April (Circulation 2009;119:1758–67).
DE MRI involves infusing gadolinium contrast into the patient. Uptake of the contrast into fibrotic tissue occurs at a different rate compared with its entry into healthy tissue, and this difference allows assessment of the amount and location of fibrotic scar within the heart wall.
In the new study, Dr. Kalvaitis and his associates performed DE MRI exams on 62 patients with atrial fibrillation scheduled to undergo pulmonary vein antrum isolation and atrial septum debulking. Their average age was 64 (range 23–84), and two-thirds were men. On average for the entire group, structural modeling affected 17% of the left atrium.
The researchers divided the patients into three subgroups based on the extent of their left-atrial remodeling ratio: less than 15% (27 patients), 15%-35% (28), and more than 35% (7). The amount of left atrial fibrosis closely correlated with the ratio of left atrial remodeling, ranging from an average of 8% fibrosis in patients with the least remodeling to 46% in patients with the most remodeling (see box).
The incidence of an early recurrence of atrial fibrillation, defined as atrial fibrillation recurring within 3 months of the ablation procedure, closely correlated with the extent of left atrial fibrosis. The early recurrence rate was 19% in the subgroup with the lowest level of atrial fibrosis and 39% and 40%, respectively, in the two subgroups with higher amount of fibrosis.
In a multivariate analysis that controlled for baseline differences among the patients, the subgroup assignment of left atrial remodeling ratio and left atrial fibrosis was the only significant determinant of recurrence risk, increasing the risk by more than twofold, Dr. Kalvaitis reported.
Expressed another way, patients with an early recurrence of atrial fibrillation after ablation had an average 24% left-atrial remodeling ratio prior to ablation treatment, compared with an average 14% ratio among those who did not have an early recurrence.
This method for defining recurrence risk may identify patients who would benefit from treatment with an anti-arrhythmic drug after their ablation procedure, Dr. Kalvaitis said. He said that he and his associates had no financial disclosures.
Source ELSEVIER GLOBAL MEDICAL NEWS
Delayed-enhancement magnetic resonance imaging of left atria prior to ablation therapy, which has been processed as color three-dimensional models, indicates regions of abnormal enhancement in fibrotic tissue as green or other colors, and healthy tissue as blue.
Source Courtesy Dr. Nassir F. Marrouche
In reply: Radiologic workup of a palpable breast mass
The authors thank Dr. Keller for his readership. (On a personal note, Dr. Chellman-Jeffers spent her childhood in the Los Angeles area near his practice.) Dr. Keller brings up several interesting points regarding breast MRI, a subject that fills entire subspecialty textbooks.
On the subject of a palpable abnormality, a breast MRI’s field of view encompasses the entire breast, and although breast MRI is quite sensitive, it is known to have a lower specificity than other modalities.1 This means that more findings—which may or may not be related to the actual palpable abnormality—will lead to more studies and more biopsies, with proportionately fewer cancers found.
As for regions of tissue coverage with mammography, the axillary tail is actually more consistently imaged with mammography and ultrasonography than with MRI because of the cardiac pulsation artifact in the plane of the heart, as well as the breastcoil image centering on the breast. MRI-guided biopsy in the axilla is also generally not possible. These limitations are typical for breast MRI equipment. The expense of breast MRI is indeed considerable, but cost is not the main reason for the preference of other modalities.
In contrast, targeted ultrasonography is exquisitely suited to specifically image a palpable abnormality. With its small field of view (4 cm and smaller), a very high percentage of palpable masses can be seen. It is also more personal and comfortable and can be patient-directed. You can ask the patient to physically show you what is being felt and then scan it in real time. Needle biopsy can then be performed, often during the same visit (at many facilities), using ultrasonography as a real-time guidance tool in any location within the breast, including the axilla.
In the algorithm implied by your question, the patient feels a lump and has a negative diagnostic mammogram (including specific, problem-directed views) and targeted ultrasonography, which, again, is more focused than MRI and more capable of imaging the axilla or areas out of the breast coil for this purpose. Then, based on clinical suspicion or patient anxiety, these two very good tests are disregarded or not believed. At this point, the patient should be seen by a specialist, usually a surgeon, for evaluation for palpation-guided biopsy. It is true that some palpable masses are not identified by mammography and ultrasonography. But it is also true that MRI does not find every cancer, and it can find many more lesions that are not cancerous and that have a dubious relation to the original area of concern. This can easily turn into the proverbial wild-goose chase. No matter the outcome of the MRI, the patient still needs to be seen by a surgeon.
Our two major indications for breast MRI are currently in the preoperative extent-of-disease workup for known breast cancer and as an additional screening examination for high-risk patients (lifetime risk greater than 20%–25% by BRCAPRO, Gail, or other model method per the 2007 American Cancer Society guidelines2). We always require a comparative review of mammography in the completed interpretation of breast MRI and, as such, do not consider MRI a viable (or statistically proven) substitute for screening mammography for patients with sensitive breasts. Breast MRI is in fact more physically challenging for most patients than mammography, because the patient needs to remain motionless in a prone position in an enclosed space for an extended period of time (our protocol is 17 minutes). Gadolinium contrast must also be given, which requires renal function laboratory tests and intravenous access. The study must also be scheduled in all premenopausal patients in the postmenstrual phase of her cycle (around days 7–14) to avoid diffuse hormonally related enhancement and to minimize false-positive results.
- Orel S. Who should have breast magnetic resonance imaging evaluation? J Clin Oncol 2008; 26:703–711.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast cancer screening with MRI as an adjunct to mammography, CA Cancer J Clin 2007; 57:75–89. Erratum in: CA Cancer J Clin 2007; 57:185.
The authors thank Dr. Keller for his readership. (On a personal note, Dr. Chellman-Jeffers spent her childhood in the Los Angeles area near his practice.) Dr. Keller brings up several interesting points regarding breast MRI, a subject that fills entire subspecialty textbooks.
On the subject of a palpable abnormality, a breast MRI’s field of view encompasses the entire breast, and although breast MRI is quite sensitive, it is known to have a lower specificity than other modalities.1 This means that more findings—which may or may not be related to the actual palpable abnormality—will lead to more studies and more biopsies, with proportionately fewer cancers found.
As for regions of tissue coverage with mammography, the axillary tail is actually more consistently imaged with mammography and ultrasonography than with MRI because of the cardiac pulsation artifact in the plane of the heart, as well as the breastcoil image centering on the breast. MRI-guided biopsy in the axilla is also generally not possible. These limitations are typical for breast MRI equipment. The expense of breast MRI is indeed considerable, but cost is not the main reason for the preference of other modalities.
In contrast, targeted ultrasonography is exquisitely suited to specifically image a palpable abnormality. With its small field of view (4 cm and smaller), a very high percentage of palpable masses can be seen. It is also more personal and comfortable and can be patient-directed. You can ask the patient to physically show you what is being felt and then scan it in real time. Needle biopsy can then be performed, often during the same visit (at many facilities), using ultrasonography as a real-time guidance tool in any location within the breast, including the axilla.
In the algorithm implied by your question, the patient feels a lump and has a negative diagnostic mammogram (including specific, problem-directed views) and targeted ultrasonography, which, again, is more focused than MRI and more capable of imaging the axilla or areas out of the breast coil for this purpose. Then, based on clinical suspicion or patient anxiety, these two very good tests are disregarded or not believed. At this point, the patient should be seen by a specialist, usually a surgeon, for evaluation for palpation-guided biopsy. It is true that some palpable masses are not identified by mammography and ultrasonography. But it is also true that MRI does not find every cancer, and it can find many more lesions that are not cancerous and that have a dubious relation to the original area of concern. This can easily turn into the proverbial wild-goose chase. No matter the outcome of the MRI, the patient still needs to be seen by a surgeon.
Our two major indications for breast MRI are currently in the preoperative extent-of-disease workup for known breast cancer and as an additional screening examination for high-risk patients (lifetime risk greater than 20%–25% by BRCAPRO, Gail, or other model method per the 2007 American Cancer Society guidelines2). We always require a comparative review of mammography in the completed interpretation of breast MRI and, as such, do not consider MRI a viable (or statistically proven) substitute for screening mammography for patients with sensitive breasts. Breast MRI is in fact more physically challenging for most patients than mammography, because the patient needs to remain motionless in a prone position in an enclosed space for an extended period of time (our protocol is 17 minutes). Gadolinium contrast must also be given, which requires renal function laboratory tests and intravenous access. The study must also be scheduled in all premenopausal patients in the postmenstrual phase of her cycle (around days 7–14) to avoid diffuse hormonally related enhancement and to minimize false-positive results.
The authors thank Dr. Keller for his readership. (On a personal note, Dr. Chellman-Jeffers spent her childhood in the Los Angeles area near his practice.) Dr. Keller brings up several interesting points regarding breast MRI, a subject that fills entire subspecialty textbooks.
On the subject of a palpable abnormality, a breast MRI’s field of view encompasses the entire breast, and although breast MRI is quite sensitive, it is known to have a lower specificity than other modalities.1 This means that more findings—which may or may not be related to the actual palpable abnormality—will lead to more studies and more biopsies, with proportionately fewer cancers found.
As for regions of tissue coverage with mammography, the axillary tail is actually more consistently imaged with mammography and ultrasonography than with MRI because of the cardiac pulsation artifact in the plane of the heart, as well as the breastcoil image centering on the breast. MRI-guided biopsy in the axilla is also generally not possible. These limitations are typical for breast MRI equipment. The expense of breast MRI is indeed considerable, but cost is not the main reason for the preference of other modalities.
In contrast, targeted ultrasonography is exquisitely suited to specifically image a palpable abnormality. With its small field of view (4 cm and smaller), a very high percentage of palpable masses can be seen. It is also more personal and comfortable and can be patient-directed. You can ask the patient to physically show you what is being felt and then scan it in real time. Needle biopsy can then be performed, often during the same visit (at many facilities), using ultrasonography as a real-time guidance tool in any location within the breast, including the axilla.
In the algorithm implied by your question, the patient feels a lump and has a negative diagnostic mammogram (including specific, problem-directed views) and targeted ultrasonography, which, again, is more focused than MRI and more capable of imaging the axilla or areas out of the breast coil for this purpose. Then, based on clinical suspicion or patient anxiety, these two very good tests are disregarded or not believed. At this point, the patient should be seen by a specialist, usually a surgeon, for evaluation for palpation-guided biopsy. It is true that some palpable masses are not identified by mammography and ultrasonography. But it is also true that MRI does not find every cancer, and it can find many more lesions that are not cancerous and that have a dubious relation to the original area of concern. This can easily turn into the proverbial wild-goose chase. No matter the outcome of the MRI, the patient still needs to be seen by a surgeon.
Our two major indications for breast MRI are currently in the preoperative extent-of-disease workup for known breast cancer and as an additional screening examination for high-risk patients (lifetime risk greater than 20%–25% by BRCAPRO, Gail, or other model method per the 2007 American Cancer Society guidelines2). We always require a comparative review of mammography in the completed interpretation of breast MRI and, as such, do not consider MRI a viable (or statistically proven) substitute for screening mammography for patients with sensitive breasts. Breast MRI is in fact more physically challenging for most patients than mammography, because the patient needs to remain motionless in a prone position in an enclosed space for an extended period of time (our protocol is 17 minutes). Gadolinium contrast must also be given, which requires renal function laboratory tests and intravenous access. The study must also be scheduled in all premenopausal patients in the postmenstrual phase of her cycle (around days 7–14) to avoid diffuse hormonally related enhancement and to minimize false-positive results.
- Orel S. Who should have breast magnetic resonance imaging evaluation? J Clin Oncol 2008; 26:703–711.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast cancer screening with MRI as an adjunct to mammography, CA Cancer J Clin 2007; 57:75–89. Erratum in: CA Cancer J Clin 2007; 57:185.
- Orel S. Who should have breast magnetic resonance imaging evaluation? J Clin Oncol 2008; 26:703–711.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast cancer screening with MRI as an adjunct to mammography, CA Cancer J Clin 2007; 57:75–89. Erratum in: CA Cancer J Clin 2007; 57:185.
Radiologic workup of a palpable breast mass
To the Editor: Thank you for the excellent review, “The radiologic workup of a palpable breast mass” in your March 2009 issue.1
The authors stated that magnetic resonance imaging (MRI) of the breast “does not currently have a role in the workup of a palpable abnormality.” This may be true in general, because breast MRI is more expensive than mammography plus or minus ultrasonography. However, breast surgeons are currently ordering preoperative MRI to evaluate biopsy-proven breast cancer to help them plan the surgery. This is because MRI provides superior three-dimensional spatial resolution and image quality as compared with ultrasonography or mammography.
My question is whether breast MRI might be useful in the prebiopsy diagnostic workup of breast masses in special cases. For example, some women have very sensitive breasts and refuse to undergo mammography, which requires compression of the breast. Another special case is when the palpable mass is located in a portion of the breast which is not amenable to mammography, such as in the axillary tail of the breast. In these cases, MRI might be helpful if the palpable mass is not definitively imaged with ultrasonography. Would the authors care to comment?
- Stein L, Chellman-Jeffers M. Radiologic workup of a palpable breast mass. Cleve Clin J Med 2009; 76:175–180.
To the Editor: Thank you for the excellent review, “The radiologic workup of a palpable breast mass” in your March 2009 issue.1
The authors stated that magnetic resonance imaging (MRI) of the breast “does not currently have a role in the workup of a palpable abnormality.” This may be true in general, because breast MRI is more expensive than mammography plus or minus ultrasonography. However, breast surgeons are currently ordering preoperative MRI to evaluate biopsy-proven breast cancer to help them plan the surgery. This is because MRI provides superior three-dimensional spatial resolution and image quality as compared with ultrasonography or mammography.
My question is whether breast MRI might be useful in the prebiopsy diagnostic workup of breast masses in special cases. For example, some women have very sensitive breasts and refuse to undergo mammography, which requires compression of the breast. Another special case is when the palpable mass is located in a portion of the breast which is not amenable to mammography, such as in the axillary tail of the breast. In these cases, MRI might be helpful if the palpable mass is not definitively imaged with ultrasonography. Would the authors care to comment?
To the Editor: Thank you for the excellent review, “The radiologic workup of a palpable breast mass” in your March 2009 issue.1
The authors stated that magnetic resonance imaging (MRI) of the breast “does not currently have a role in the workup of a palpable abnormality.” This may be true in general, because breast MRI is more expensive than mammography plus or minus ultrasonography. However, breast surgeons are currently ordering preoperative MRI to evaluate biopsy-proven breast cancer to help them plan the surgery. This is because MRI provides superior three-dimensional spatial resolution and image quality as compared with ultrasonography or mammography.
My question is whether breast MRI might be useful in the prebiopsy diagnostic workup of breast masses in special cases. For example, some women have very sensitive breasts and refuse to undergo mammography, which requires compression of the breast. Another special case is when the palpable mass is located in a portion of the breast which is not amenable to mammography, such as in the axillary tail of the breast. In these cases, MRI might be helpful if the palpable mass is not definitively imaged with ultrasonography. Would the authors care to comment?
- Stein L, Chellman-Jeffers M. Radiologic workup of a palpable breast mass. Cleve Clin J Med 2009; 76:175–180.
- Stein L, Chellman-Jeffers M. Radiologic workup of a palpable breast mass. Cleve Clin J Med 2009; 76:175–180.