User login
FDA safety alert: Face masks with metal can burn during MRI
After a patient’s face was burned in the outline of a mask worn during a 3-Tesla MRI neck scan, the US Food and Drug Administration (FDA) cautioned that face masks containing metal can heat to unsafe temperatures during scanning.
Clinicians have known for years to ask patients to remove all metal jewelry and other objects prior to an MRI. The widespread wearing of face masks during the COVID-19 pandemic, however, adds one more consideration to the list.
The FDA’s December 7 safety communication applies to surgical and nonsurgical face masks and respirators.
The injury risk relates to rapid heating of metal components. Many face masks contain a nose wire or metal clip that helps the product conform to the face. Some masks contain metal nanoparticles, while others feature antimicrobial coatings with silver or copper. Each of these products should be avoided during MRI scanning. Also watch out for staples on headbands, the FDA warned.
If the metal content of a face mask is unknown, the FDA suggests providing the patient with a facial covering that is known not to contain any metal.
Robert E. Watson Jr, MD, PhD, chair of the American College of Radiology (ACR) Committee on MR Safety, agreed. He recommended that facilities “provide patients with masks known to be MRI-safe and not permit patient-owned masks in the MRI.”
Watson suggested this strategy at a time when face masks are required.
“COVID-19 safety protocols require that patients wear masks when being scanned, to decrease infection risk to MRI staff, decrease risk of contaminating the MRI scanner, and to protect themselves from infection,” he told Medscape Medical News. “Any conducting metal that enters the MRI machine is at risk of heating due to the radiofrequency fields inherent to image generation.”
Adverse events related to the metal components of a face mask should be reported to the FDA using the MedWatch voluntary reporting form. In addition, healthcare providers subject to the FDA user facility reporting requirements should follow procedures at their facilities to report such events.
This article first appeared on Medscape.com.
After a patient’s face was burned in the outline of a mask worn during a 3-Tesla MRI neck scan, the US Food and Drug Administration (FDA) cautioned that face masks containing metal can heat to unsafe temperatures during scanning.
Clinicians have known for years to ask patients to remove all metal jewelry and other objects prior to an MRI. The widespread wearing of face masks during the COVID-19 pandemic, however, adds one more consideration to the list.
The FDA’s December 7 safety communication applies to surgical and nonsurgical face masks and respirators.
The injury risk relates to rapid heating of metal components. Many face masks contain a nose wire or metal clip that helps the product conform to the face. Some masks contain metal nanoparticles, while others feature antimicrobial coatings with silver or copper. Each of these products should be avoided during MRI scanning. Also watch out for staples on headbands, the FDA warned.
If the metal content of a face mask is unknown, the FDA suggests providing the patient with a facial covering that is known not to contain any metal.
Robert E. Watson Jr, MD, PhD, chair of the American College of Radiology (ACR) Committee on MR Safety, agreed. He recommended that facilities “provide patients with masks known to be MRI-safe and not permit patient-owned masks in the MRI.”
Watson suggested this strategy at a time when face masks are required.
“COVID-19 safety protocols require that patients wear masks when being scanned, to decrease infection risk to MRI staff, decrease risk of contaminating the MRI scanner, and to protect themselves from infection,” he told Medscape Medical News. “Any conducting metal that enters the MRI machine is at risk of heating due to the radiofrequency fields inherent to image generation.”
Adverse events related to the metal components of a face mask should be reported to the FDA using the MedWatch voluntary reporting form. In addition, healthcare providers subject to the FDA user facility reporting requirements should follow procedures at their facilities to report such events.
This article first appeared on Medscape.com.
After a patient’s face was burned in the outline of a mask worn during a 3-Tesla MRI neck scan, the US Food and Drug Administration (FDA) cautioned that face masks containing metal can heat to unsafe temperatures during scanning.
Clinicians have known for years to ask patients to remove all metal jewelry and other objects prior to an MRI. The widespread wearing of face masks during the COVID-19 pandemic, however, adds one more consideration to the list.
The FDA’s December 7 safety communication applies to surgical and nonsurgical face masks and respirators.
The injury risk relates to rapid heating of metal components. Many face masks contain a nose wire or metal clip that helps the product conform to the face. Some masks contain metal nanoparticles, while others feature antimicrobial coatings with silver or copper. Each of these products should be avoided during MRI scanning. Also watch out for staples on headbands, the FDA warned.
If the metal content of a face mask is unknown, the FDA suggests providing the patient with a facial covering that is known not to contain any metal.
Robert E. Watson Jr, MD, PhD, chair of the American College of Radiology (ACR) Committee on MR Safety, agreed. He recommended that facilities “provide patients with masks known to be MRI-safe and not permit patient-owned masks in the MRI.”
Watson suggested this strategy at a time when face masks are required.
“COVID-19 safety protocols require that patients wear masks when being scanned, to decrease infection risk to MRI staff, decrease risk of contaminating the MRI scanner, and to protect themselves from infection,” he told Medscape Medical News. “Any conducting metal that enters the MRI machine is at risk of heating due to the radiofrequency fields inherent to image generation.”
Adverse events related to the metal components of a face mask should be reported to the FDA using the MedWatch voluntary reporting form. In addition, healthcare providers subject to the FDA user facility reporting requirements should follow procedures at their facilities to report such events.
This article first appeared on Medscape.com.
SCAPIS: Simple questionnaire can identify silent atherosclerosis
Individuals in the general population with high levels of silent coronary atherosclerosis can be successfully identified with a simple questionnaire that they can complete themselves at home, a new study suggests.
The Swedish CardioPulmonary BioImage Study (SCAPIS) found that 40% of middle-aged adults without known heart disease had evidence of coronary atherosclerosis on coronary CT angiography (CCTA), and 13% had extensive atherosclerotic disease.
The authors found that the screening questionnaire could identify individuals who had extensive coronary atherosclerosis with a reasonably high predictive value.
Initial results from the study were presented today at the virtual American Heart Association (AHA) Scientific Sessions 2020.
“Our study is looking to see if we can estimate how many people in the general population have significant coronary atherosclerosis and therefore could benefit from preventative treatment,” lead author, Göran Bergström, MD, explained to Medscape Medical News.
Bergström, who is professor and lead physician at Sahlgrenska Academy, Gothenburg University, Gothenburg, Sweden, said there are no good data on this as yet. “There are studies of atherosclerosis burden in patients who have had a cardiovascular event, but our study was conducted in a random selection of the middle-aged general population who did not have symptoms of heart disease.”
“Our study also suggests that in future we may be able to identify these people with an online questionnaire, and those that reached a certain score could be referred for an imaging test,” he added.
SCAPIS included more than 30,000 men and women, age 50 to 64 years, who had no history of cardiovascular events or cardiac intervention. They were asked questions about sex, age, lifestyle, smoking, body measurements, cholesterol medication, and blood pressure to predict their risk for coronary artery disease.
Researchers then used CCTA images to examine patients’ arteries for the presence of plaque. More than 25,000 individuals from the original sample were successfully imaged.
Results showed that 40% of the middle-aged population had some coronary atherosclerosis and 5% had severe atherosclerosis, defined as the presence of a stenosis blocking 50% or more of blood flow in one of the coronary arteries.
A second aim of the study was to use data from the questionnaire to develop a prediction model to identify people with widespread atherosclerosis — those with any type of stenosis in four different segments of their coronary arteries, who made up 13% of the population.
The questionnaire included data on 120 different variables. Of these variables, around 100 could be assessed by the patients themselves and another 20 measurements could be performed in the clinic, such as blood pressure and cholesterol levels.
The researchers then used artificial intelligence to assess which variables were associated with widespread atherosclerosis. This had an area under the curve (AUC, a measure of the predictive value) of 0.8.
“An AUC of 1.0 would show a perfect prediction, and a value of 0.5 shows no value. A result of 0.8 shows reasonable predictive potential. This is an encouraging result and suggests this strategy could work,” Bergström said.
“We know silent atherosclerosis is a big problem and causes sudden cardiac events in people who have not shown symptoms,” he said.
The goal is to identify these patients before they have an event and offer them preventive treatments. “At present we try and identify patients at high risk of cardiovascular events by using cholesterol and blood pressure measurements and cardiovascular risk scores such as Framingham. But this is not so effective,” Bergström explained.
“Using imaging such as CCTA, where you can actually see atherosclerotic plaque, could be better for prediction, but we can’t image everyone. So, we wanted to see whether we could narrow down the population who should receive imaging with a detailed questionnaire, and it looks like we can.”
The study found that including clinical measurements such as blood pressure and cholesterol did not add much to the predictive value for identifying people with extensive coronary atherosclerosis, a result that Bergström said was surprising.
Which population to target?
Discussant of the study, Pamela Douglas, MD, professor of research in cardiovascular diseases at Duke University, Durham, North Carolina, congratulated the SCAPIS investigators on creating “a very rich data set for current and future study.”
“The SCAPIS study has already yielded novel data on the prevalence of coronary artery disease in the general population, and will address many critical questions over the long term,” she said.
But Douglas suggested that individuals with extensive coronary atherosclerosis were not the most appropriate target population to identify.
“The rationale for choosing this cutpoint is unclear as clinical risk/mortality is higher in all nonobstructive coronary artery disease, starting at one-vessel involvement,” she noted. “Therefore, effective preventive strategies likely need to start with detection and treatment of patients with even minimal plaque.”
Responding to Medscape Medical News, Bergström said this was a valid argument. “We plan to reanalyze our results with different populations as the target — that is something that we can do in the future.
But targeting everyone with just one coronary plaque is going to identify a large group — it was 40% of the population in our study. This will be too many people in whom to perform confirmatory CCTA imaging. It would be impractical to try and conduct cardiac imaging on that many people.”
Bergström noted that more data are needed on the danger of various levels of coronary atherosclerosis in this population who have not had any symptoms.
“We don’t have this information at present, but we are continuing to follow our population and we will have data on cardiac events in a few years’ time. Then we will know which level of atherosclerosis we need to target. It will probably be somewhere in between the extensive levels we used in this first analysis (which occurred in 13% of people) and the 40% of people who showed just one area of plaque.”
This study is the first report from SCAPIS, a collaborative project between six Swedish universities with the following vision statement: to “reduce the risk of cardiovascular and respiratory diseases for generations to come.”
The SCAPIS project is funded by the Swedish Heart and Lung Foundation. Bergström reports no disclosures.
This article first appeared on Medscape.com.
Individuals in the general population with high levels of silent coronary atherosclerosis can be successfully identified with a simple questionnaire that they can complete themselves at home, a new study suggests.
The Swedish CardioPulmonary BioImage Study (SCAPIS) found that 40% of middle-aged adults without known heart disease had evidence of coronary atherosclerosis on coronary CT angiography (CCTA), and 13% had extensive atherosclerotic disease.
The authors found that the screening questionnaire could identify individuals who had extensive coronary atherosclerosis with a reasonably high predictive value.
Initial results from the study were presented today at the virtual American Heart Association (AHA) Scientific Sessions 2020.
“Our study is looking to see if we can estimate how many people in the general population have significant coronary atherosclerosis and therefore could benefit from preventative treatment,” lead author, Göran Bergström, MD, explained to Medscape Medical News.
Bergström, who is professor and lead physician at Sahlgrenska Academy, Gothenburg University, Gothenburg, Sweden, said there are no good data on this as yet. “There are studies of atherosclerosis burden in patients who have had a cardiovascular event, but our study was conducted in a random selection of the middle-aged general population who did not have symptoms of heart disease.”
“Our study also suggests that in future we may be able to identify these people with an online questionnaire, and those that reached a certain score could be referred for an imaging test,” he added.
SCAPIS included more than 30,000 men and women, age 50 to 64 years, who had no history of cardiovascular events or cardiac intervention. They were asked questions about sex, age, lifestyle, smoking, body measurements, cholesterol medication, and blood pressure to predict their risk for coronary artery disease.
Researchers then used CCTA images to examine patients’ arteries for the presence of plaque. More than 25,000 individuals from the original sample were successfully imaged.
Results showed that 40% of the middle-aged population had some coronary atherosclerosis and 5% had severe atherosclerosis, defined as the presence of a stenosis blocking 50% or more of blood flow in one of the coronary arteries.
A second aim of the study was to use data from the questionnaire to develop a prediction model to identify people with widespread atherosclerosis — those with any type of stenosis in four different segments of their coronary arteries, who made up 13% of the population.
The questionnaire included data on 120 different variables. Of these variables, around 100 could be assessed by the patients themselves and another 20 measurements could be performed in the clinic, such as blood pressure and cholesterol levels.
The researchers then used artificial intelligence to assess which variables were associated with widespread atherosclerosis. This had an area under the curve (AUC, a measure of the predictive value) of 0.8.
“An AUC of 1.0 would show a perfect prediction, and a value of 0.5 shows no value. A result of 0.8 shows reasonable predictive potential. This is an encouraging result and suggests this strategy could work,” Bergström said.
“We know silent atherosclerosis is a big problem and causes sudden cardiac events in people who have not shown symptoms,” he said.
The goal is to identify these patients before they have an event and offer them preventive treatments. “At present we try and identify patients at high risk of cardiovascular events by using cholesterol and blood pressure measurements and cardiovascular risk scores such as Framingham. But this is not so effective,” Bergström explained.
“Using imaging such as CCTA, where you can actually see atherosclerotic plaque, could be better for prediction, but we can’t image everyone. So, we wanted to see whether we could narrow down the population who should receive imaging with a detailed questionnaire, and it looks like we can.”
The study found that including clinical measurements such as blood pressure and cholesterol did not add much to the predictive value for identifying people with extensive coronary atherosclerosis, a result that Bergström said was surprising.
Which population to target?
Discussant of the study, Pamela Douglas, MD, professor of research in cardiovascular diseases at Duke University, Durham, North Carolina, congratulated the SCAPIS investigators on creating “a very rich data set for current and future study.”
“The SCAPIS study has already yielded novel data on the prevalence of coronary artery disease in the general population, and will address many critical questions over the long term,” she said.
But Douglas suggested that individuals with extensive coronary atherosclerosis were not the most appropriate target population to identify.
“The rationale for choosing this cutpoint is unclear as clinical risk/mortality is higher in all nonobstructive coronary artery disease, starting at one-vessel involvement,” she noted. “Therefore, effective preventive strategies likely need to start with detection and treatment of patients with even minimal plaque.”
Responding to Medscape Medical News, Bergström said this was a valid argument. “We plan to reanalyze our results with different populations as the target — that is something that we can do in the future.
But targeting everyone with just one coronary plaque is going to identify a large group — it was 40% of the population in our study. This will be too many people in whom to perform confirmatory CCTA imaging. It would be impractical to try and conduct cardiac imaging on that many people.”
Bergström noted that more data are needed on the danger of various levels of coronary atherosclerosis in this population who have not had any symptoms.
“We don’t have this information at present, but we are continuing to follow our population and we will have data on cardiac events in a few years’ time. Then we will know which level of atherosclerosis we need to target. It will probably be somewhere in between the extensive levels we used in this first analysis (which occurred in 13% of people) and the 40% of people who showed just one area of plaque.”
This study is the first report from SCAPIS, a collaborative project between six Swedish universities with the following vision statement: to “reduce the risk of cardiovascular and respiratory diseases for generations to come.”
The SCAPIS project is funded by the Swedish Heart and Lung Foundation. Bergström reports no disclosures.
This article first appeared on Medscape.com.
Individuals in the general population with high levels of silent coronary atherosclerosis can be successfully identified with a simple questionnaire that they can complete themselves at home, a new study suggests.
The Swedish CardioPulmonary BioImage Study (SCAPIS) found that 40% of middle-aged adults without known heart disease had evidence of coronary atherosclerosis on coronary CT angiography (CCTA), and 13% had extensive atherosclerotic disease.
The authors found that the screening questionnaire could identify individuals who had extensive coronary atherosclerosis with a reasonably high predictive value.
Initial results from the study were presented today at the virtual American Heart Association (AHA) Scientific Sessions 2020.
“Our study is looking to see if we can estimate how many people in the general population have significant coronary atherosclerosis and therefore could benefit from preventative treatment,” lead author, Göran Bergström, MD, explained to Medscape Medical News.
Bergström, who is professor and lead physician at Sahlgrenska Academy, Gothenburg University, Gothenburg, Sweden, said there are no good data on this as yet. “There are studies of atherosclerosis burden in patients who have had a cardiovascular event, but our study was conducted in a random selection of the middle-aged general population who did not have symptoms of heart disease.”
“Our study also suggests that in future we may be able to identify these people with an online questionnaire, and those that reached a certain score could be referred for an imaging test,” he added.
SCAPIS included more than 30,000 men and women, age 50 to 64 years, who had no history of cardiovascular events or cardiac intervention. They were asked questions about sex, age, lifestyle, smoking, body measurements, cholesterol medication, and blood pressure to predict their risk for coronary artery disease.
Researchers then used CCTA images to examine patients’ arteries for the presence of plaque. More than 25,000 individuals from the original sample were successfully imaged.
Results showed that 40% of the middle-aged population had some coronary atherosclerosis and 5% had severe atherosclerosis, defined as the presence of a stenosis blocking 50% or more of blood flow in one of the coronary arteries.
A second aim of the study was to use data from the questionnaire to develop a prediction model to identify people with widespread atherosclerosis — those with any type of stenosis in four different segments of their coronary arteries, who made up 13% of the population.
The questionnaire included data on 120 different variables. Of these variables, around 100 could be assessed by the patients themselves and another 20 measurements could be performed in the clinic, such as blood pressure and cholesterol levels.
The researchers then used artificial intelligence to assess which variables were associated with widespread atherosclerosis. This had an area under the curve (AUC, a measure of the predictive value) of 0.8.
“An AUC of 1.0 would show a perfect prediction, and a value of 0.5 shows no value. A result of 0.8 shows reasonable predictive potential. This is an encouraging result and suggests this strategy could work,” Bergström said.
“We know silent atherosclerosis is a big problem and causes sudden cardiac events in people who have not shown symptoms,” he said.
The goal is to identify these patients before they have an event and offer them preventive treatments. “At present we try and identify patients at high risk of cardiovascular events by using cholesterol and blood pressure measurements and cardiovascular risk scores such as Framingham. But this is not so effective,” Bergström explained.
“Using imaging such as CCTA, where you can actually see atherosclerotic plaque, could be better for prediction, but we can’t image everyone. So, we wanted to see whether we could narrow down the population who should receive imaging with a detailed questionnaire, and it looks like we can.”
The study found that including clinical measurements such as blood pressure and cholesterol did not add much to the predictive value for identifying people with extensive coronary atherosclerosis, a result that Bergström said was surprising.
Which population to target?
Discussant of the study, Pamela Douglas, MD, professor of research in cardiovascular diseases at Duke University, Durham, North Carolina, congratulated the SCAPIS investigators on creating “a very rich data set for current and future study.”
“The SCAPIS study has already yielded novel data on the prevalence of coronary artery disease in the general population, and will address many critical questions over the long term,” she said.
But Douglas suggested that individuals with extensive coronary atherosclerosis were not the most appropriate target population to identify.
“The rationale for choosing this cutpoint is unclear as clinical risk/mortality is higher in all nonobstructive coronary artery disease, starting at one-vessel involvement,” she noted. “Therefore, effective preventive strategies likely need to start with detection and treatment of patients with even minimal plaque.”
Responding to Medscape Medical News, Bergström said this was a valid argument. “We plan to reanalyze our results with different populations as the target — that is something that we can do in the future.
But targeting everyone with just one coronary plaque is going to identify a large group — it was 40% of the population in our study. This will be too many people in whom to perform confirmatory CCTA imaging. It would be impractical to try and conduct cardiac imaging on that many people.”
Bergström noted that more data are needed on the danger of various levels of coronary atherosclerosis in this population who have not had any symptoms.
“We don’t have this information at present, but we are continuing to follow our population and we will have data on cardiac events in a few years’ time. Then we will know which level of atherosclerosis we need to target. It will probably be somewhere in between the extensive levels we used in this first analysis (which occurred in 13% of people) and the 40% of people who showed just one area of plaque.”
This study is the first report from SCAPIS, a collaborative project between six Swedish universities with the following vision statement: to “reduce the risk of cardiovascular and respiratory diseases for generations to come.”
The SCAPIS project is funded by the Swedish Heart and Lung Foundation. Bergström reports no disclosures.
This article first appeared on Medscape.com.
Combined OCT, cardiac MRI unravels root cause in most MINOCA
Optical CT (OCT) plus cardiac MRI (CMR) provides a more specific diagnosis in the majority of women presenting with myocardial infarction with nonobstructive coronary arteries (MINOCA).
The multimodal imaging strategy identified the underlying cause of MINOCA in 85% of women in the HARP-MINOCA study. Overall, 64% of women had a true MI and 21% had an alternate nonischemic diagnosis, most commonly myocarditis.
“OCTCMR findings correlated well with OCT culprit lesions, demonstrating that nonobstructive culprit lesions frequently cause MINOCA,” said study author Harmony Reynolds, MD, director of New York University Langone’s Sarah Ross Soter Center for Women’s Cardiovascular Research.
The results were presented at the virtual American Heart Association (AHA) Scientific Sessions 2020 and published simultaneously in Circulation.
MINOCA occurs in up to 15% of patients with MI and is defined as MI meeting the universal definition but with less than 50% stenosis in all major epicardial arteries on angiography and no specific alternate diagnosis to explain the presentation.
It is three times more common in women than in men and also disproportionately affects Black, Hispanic, Maori, and Pacific persons. MINOCA has several causes, leading to uncertainty in diagnostic testing and treatment.
“Different doctors tell patients different messages about MINOCA and may incorrectly say the event wasn’t a heart attack,” Dr. Reynolds said in an earlier press briefing. “I had a patient who was told ‘your arteries are open,’ and they gave her Xanax.”
As part of the Women’s Heart Attack Research Program (HARP), researchers enrolled 301 women with a clinical diagnosis of MI, of whom 170 were diagnosed with MINOCA during angiography and underwent OCT at that time, followed by CMR within 1 week of the acute presentation.
All images were interpreted by an independent core laboratory blinded to results of the other tests and clinical information. The final cohort included 145 women with interpretable OCT images.
Their median age was 60 years, 49.7% were white non-Hispanic, and 97% presented with a provisional diagnosis of non–ST-segment MI. Their median peak troponin level was 0.94 ng/mL.
OCT identified a definite or probable culprit lesion in 46% of women, most commonly atherosclerosis or thrombosis. On multivariable analysis, having a culprit lesion was associated with older age, abnormal angiography findings at the site, and diabetes, but not peak troponin level or severity of angiographic stenosis.
CMR available in 116 women showed evidence of infarction or regional injury in 69%. Multivariate predictors of an abnormal CMR were higher peak troponin and diastolic blood pressure but not an OCT culprit lesion or angiographic stenosis severity.
When the OCT and CMR results were combined, a cause of MINOCA was identified in 84.5% of women. Three-fourths of the causes were ischemic (64% MI) and one-quarter were nonischemic (15% myocarditis, 3% Takotsubo syndrome, and 3% nonischemic cardiomyopathy). In the remaining 15%, no cause of MINOCA was identified.
To emphasize the effect multimodal imaging can have on treatment, Dr. Reynolds highlighted a 44-year-old woman with no risk factors for coronary artery disease who had chest pain in the context of heavy menstrual bleeding, a low hemoglobin level, and peak troponin level of 3.25 ng/mL.
Unexpectedly, imaging revealed a left anterior descending (LAD) plaque rupture in a thin-cap fibroatheroma, causing a small transmural infarction at the terminus of the LAD.
“Without this diagnosis, it’s unlikely she would have received antiplatelet therapy or statins and might have been given a diagnosis of supply/demand mismatch, when the real diagnosis was MI,” Dr. Reynolds observed.
“Finally we can say this is not just crazy women. There is really something going on,” said panelist Roxana Mehran, MD, of the Icahn School of Medicine at Mount Sinai in New York. “You have now told us this is most likely atherosclerosis for pretty much 85% of the cases. So make the diagnosis and, of course, make sure you treat these patients accordingly for risk factor modification, really thinking about a ruptured plaque.”
Combining OCT and MRI may result in a more specific diagnosis and better treatment but also raises costs and logistical considerations.
“Implementation challenges are that not every form of testing is available in every medical center,” Dr. Reynolds said in an interview. “Many centers have cardiac MRI,” whereas “OCT is not currently available at most medical centers where heart attack patients are treated but is available at specialized centers.”
Asked during the session about the use of CT angiography, invited discussant Martha Gulati, MD, president-elect of the American Society for Preventive Cardiology, said, “For me, CT is helpful when I’m not sure if there’s any plaque because the angiogram looked really normal and there was no opportunity to do intracoronary imaging. And sometimes that will help me, in particular, if a patient doesn’t want to take a statin.”
Dr. Gulati pointed out that the European Society of Cardiology MINOCA guidelines recommend OCT and CMR, whereas the 2019 AHA statement on MINOCA, which she coauthored, also recommends OCT and CMR, but almost as one or the other.
“We already said that you should do cardiac MR to try to make a diagnosis, but I think the combination of the two needs to be emphasized when we next draft these guidelines. It really will help,” Dr. Gulati said in an interview.
“But using OCT, particularly, needs to be in the setting of the MI. I don’t think you want to do a procedure again,” she said. “So we really need it to become more widely available because at the time of an MI, you won’t necessarily know that you’re not going to find an obstructive lesion.”
Dr. Gulati pointed out several unanswered questions, including whether the diagnosis was missed in some patients, because OCT of all three vessels was available in only 59%, and how the use of high-sensitivity troponin, which was left up to the individual institution, might affect the usefulness of OCT and CMR.
It’s also unknown whether the mechanism is different for ST-segment elevation MI, as the trial included very few cases, although MINOCA often occurs in this setting. Future OCT/CMR studies will also need to enroll men to determine potential sex differences, if any.
Commenting on the study, B. Hadley Wilson, MD, Sanger Heart & Vascular Institute in Charlotte, N.C., said, “There would need to be further justification of this invasive interventional procedure to be sure that the benefit outweighed the risk of putting a wire and an OCT catheter down patients without any significant angiographic blockage and to assure interventional cardiologists of its value here.”
He pointed out that noninvasive CMR appears helpful in the diagnosis of nearly three-quarters of these patients and perhaps could be done first to direct which of those with an ischemic cause might benefit from invasive OCT at catheterization. This seems most pertinent in patients with a high suspicion of coronary artery disease or recurrent MINOCA.
“Overall, we need to consider the expense, logistics, and small risk of these combined modalities, particularly in everyday practice, before making recommendations,” Dr. Wilson said. “ Since OCT is much less available than intravascular ultrasound, it would require a challenging marketplace paradigm shift to implement this multimodality imaging strategy regionally and locally in the U.S., including the added costs. However, further study to direct the more judicious use of either CMR and/or combined with OCT is warranted in these patients.”
The study was funded by the AHA through a grant from the Go Red for Women Strategically Focused Research Network. Dr. Reynolds reported in-kind donations from Abbott Vascular and Siemens related to the study and nonfinancial support from BioTelemetry outside the study. Dr. Gulati and Dr. Wilson reported having no relevant disclosures.
A version of this article originally appeared on Medscape.com.
Optical CT (OCT) plus cardiac MRI (CMR) provides a more specific diagnosis in the majority of women presenting with myocardial infarction with nonobstructive coronary arteries (MINOCA).
The multimodal imaging strategy identified the underlying cause of MINOCA in 85% of women in the HARP-MINOCA study. Overall, 64% of women had a true MI and 21% had an alternate nonischemic diagnosis, most commonly myocarditis.
“OCTCMR findings correlated well with OCT culprit lesions, demonstrating that nonobstructive culprit lesions frequently cause MINOCA,” said study author Harmony Reynolds, MD, director of New York University Langone’s Sarah Ross Soter Center for Women’s Cardiovascular Research.
The results were presented at the virtual American Heart Association (AHA) Scientific Sessions 2020 and published simultaneously in Circulation.
MINOCA occurs in up to 15% of patients with MI and is defined as MI meeting the universal definition but with less than 50% stenosis in all major epicardial arteries on angiography and no specific alternate diagnosis to explain the presentation.
It is three times more common in women than in men and also disproportionately affects Black, Hispanic, Maori, and Pacific persons. MINOCA has several causes, leading to uncertainty in diagnostic testing and treatment.
“Different doctors tell patients different messages about MINOCA and may incorrectly say the event wasn’t a heart attack,” Dr. Reynolds said in an earlier press briefing. “I had a patient who was told ‘your arteries are open,’ and they gave her Xanax.”
As part of the Women’s Heart Attack Research Program (HARP), researchers enrolled 301 women with a clinical diagnosis of MI, of whom 170 were diagnosed with MINOCA during angiography and underwent OCT at that time, followed by CMR within 1 week of the acute presentation.
All images were interpreted by an independent core laboratory blinded to results of the other tests and clinical information. The final cohort included 145 women with interpretable OCT images.
Their median age was 60 years, 49.7% were white non-Hispanic, and 97% presented with a provisional diagnosis of non–ST-segment MI. Their median peak troponin level was 0.94 ng/mL.
OCT identified a definite or probable culprit lesion in 46% of women, most commonly atherosclerosis or thrombosis. On multivariable analysis, having a culprit lesion was associated with older age, abnormal angiography findings at the site, and diabetes, but not peak troponin level or severity of angiographic stenosis.
CMR available in 116 women showed evidence of infarction or regional injury in 69%. Multivariate predictors of an abnormal CMR were higher peak troponin and diastolic blood pressure but not an OCT culprit lesion or angiographic stenosis severity.
When the OCT and CMR results were combined, a cause of MINOCA was identified in 84.5% of women. Three-fourths of the causes were ischemic (64% MI) and one-quarter were nonischemic (15% myocarditis, 3% Takotsubo syndrome, and 3% nonischemic cardiomyopathy). In the remaining 15%, no cause of MINOCA was identified.
To emphasize the effect multimodal imaging can have on treatment, Dr. Reynolds highlighted a 44-year-old woman with no risk factors for coronary artery disease who had chest pain in the context of heavy menstrual bleeding, a low hemoglobin level, and peak troponin level of 3.25 ng/mL.
Unexpectedly, imaging revealed a left anterior descending (LAD) plaque rupture in a thin-cap fibroatheroma, causing a small transmural infarction at the terminus of the LAD.
“Without this diagnosis, it’s unlikely she would have received antiplatelet therapy or statins and might have been given a diagnosis of supply/demand mismatch, when the real diagnosis was MI,” Dr. Reynolds observed.
“Finally we can say this is not just crazy women. There is really something going on,” said panelist Roxana Mehran, MD, of the Icahn School of Medicine at Mount Sinai in New York. “You have now told us this is most likely atherosclerosis for pretty much 85% of the cases. So make the diagnosis and, of course, make sure you treat these patients accordingly for risk factor modification, really thinking about a ruptured plaque.”
Combining OCT and MRI may result in a more specific diagnosis and better treatment but also raises costs and logistical considerations.
“Implementation challenges are that not every form of testing is available in every medical center,” Dr. Reynolds said in an interview. “Many centers have cardiac MRI,” whereas “OCT is not currently available at most medical centers where heart attack patients are treated but is available at specialized centers.”
Asked during the session about the use of CT angiography, invited discussant Martha Gulati, MD, president-elect of the American Society for Preventive Cardiology, said, “For me, CT is helpful when I’m not sure if there’s any plaque because the angiogram looked really normal and there was no opportunity to do intracoronary imaging. And sometimes that will help me, in particular, if a patient doesn’t want to take a statin.”
Dr. Gulati pointed out that the European Society of Cardiology MINOCA guidelines recommend OCT and CMR, whereas the 2019 AHA statement on MINOCA, which she coauthored, also recommends OCT and CMR, but almost as one or the other.
“We already said that you should do cardiac MR to try to make a diagnosis, but I think the combination of the two needs to be emphasized when we next draft these guidelines. It really will help,” Dr. Gulati said in an interview.
“But using OCT, particularly, needs to be in the setting of the MI. I don’t think you want to do a procedure again,” she said. “So we really need it to become more widely available because at the time of an MI, you won’t necessarily know that you’re not going to find an obstructive lesion.”
Dr. Gulati pointed out several unanswered questions, including whether the diagnosis was missed in some patients, because OCT of all three vessels was available in only 59%, and how the use of high-sensitivity troponin, which was left up to the individual institution, might affect the usefulness of OCT and CMR.
It’s also unknown whether the mechanism is different for ST-segment elevation MI, as the trial included very few cases, although MINOCA often occurs in this setting. Future OCT/CMR studies will also need to enroll men to determine potential sex differences, if any.
Commenting on the study, B. Hadley Wilson, MD, Sanger Heart & Vascular Institute in Charlotte, N.C., said, “There would need to be further justification of this invasive interventional procedure to be sure that the benefit outweighed the risk of putting a wire and an OCT catheter down patients without any significant angiographic blockage and to assure interventional cardiologists of its value here.”
He pointed out that noninvasive CMR appears helpful in the diagnosis of nearly three-quarters of these patients and perhaps could be done first to direct which of those with an ischemic cause might benefit from invasive OCT at catheterization. This seems most pertinent in patients with a high suspicion of coronary artery disease or recurrent MINOCA.
“Overall, we need to consider the expense, logistics, and small risk of these combined modalities, particularly in everyday practice, before making recommendations,” Dr. Wilson said. “ Since OCT is much less available than intravascular ultrasound, it would require a challenging marketplace paradigm shift to implement this multimodality imaging strategy regionally and locally in the U.S., including the added costs. However, further study to direct the more judicious use of either CMR and/or combined with OCT is warranted in these patients.”
The study was funded by the AHA through a grant from the Go Red for Women Strategically Focused Research Network. Dr. Reynolds reported in-kind donations from Abbott Vascular and Siemens related to the study and nonfinancial support from BioTelemetry outside the study. Dr. Gulati and Dr. Wilson reported having no relevant disclosures.
A version of this article originally appeared on Medscape.com.
Optical CT (OCT) plus cardiac MRI (CMR) provides a more specific diagnosis in the majority of women presenting with myocardial infarction with nonobstructive coronary arteries (MINOCA).
The multimodal imaging strategy identified the underlying cause of MINOCA in 85% of women in the HARP-MINOCA study. Overall, 64% of women had a true MI and 21% had an alternate nonischemic diagnosis, most commonly myocarditis.
“OCTCMR findings correlated well with OCT culprit lesions, demonstrating that nonobstructive culprit lesions frequently cause MINOCA,” said study author Harmony Reynolds, MD, director of New York University Langone’s Sarah Ross Soter Center for Women’s Cardiovascular Research.
The results were presented at the virtual American Heart Association (AHA) Scientific Sessions 2020 and published simultaneously in Circulation.
MINOCA occurs in up to 15% of patients with MI and is defined as MI meeting the universal definition but with less than 50% stenosis in all major epicardial arteries on angiography and no specific alternate diagnosis to explain the presentation.
It is three times more common in women than in men and also disproportionately affects Black, Hispanic, Maori, and Pacific persons. MINOCA has several causes, leading to uncertainty in diagnostic testing and treatment.
“Different doctors tell patients different messages about MINOCA and may incorrectly say the event wasn’t a heart attack,” Dr. Reynolds said in an earlier press briefing. “I had a patient who was told ‘your arteries are open,’ and they gave her Xanax.”
As part of the Women’s Heart Attack Research Program (HARP), researchers enrolled 301 women with a clinical diagnosis of MI, of whom 170 were diagnosed with MINOCA during angiography and underwent OCT at that time, followed by CMR within 1 week of the acute presentation.
All images were interpreted by an independent core laboratory blinded to results of the other tests and clinical information. The final cohort included 145 women with interpretable OCT images.
Their median age was 60 years, 49.7% were white non-Hispanic, and 97% presented with a provisional diagnosis of non–ST-segment MI. Their median peak troponin level was 0.94 ng/mL.
OCT identified a definite or probable culprit lesion in 46% of women, most commonly atherosclerosis or thrombosis. On multivariable analysis, having a culprit lesion was associated with older age, abnormal angiography findings at the site, and diabetes, but not peak troponin level or severity of angiographic stenosis.
CMR available in 116 women showed evidence of infarction or regional injury in 69%. Multivariate predictors of an abnormal CMR were higher peak troponin and diastolic blood pressure but not an OCT culprit lesion or angiographic stenosis severity.
When the OCT and CMR results were combined, a cause of MINOCA was identified in 84.5% of women. Three-fourths of the causes were ischemic (64% MI) and one-quarter were nonischemic (15% myocarditis, 3% Takotsubo syndrome, and 3% nonischemic cardiomyopathy). In the remaining 15%, no cause of MINOCA was identified.
To emphasize the effect multimodal imaging can have on treatment, Dr. Reynolds highlighted a 44-year-old woman with no risk factors for coronary artery disease who had chest pain in the context of heavy menstrual bleeding, a low hemoglobin level, and peak troponin level of 3.25 ng/mL.
Unexpectedly, imaging revealed a left anterior descending (LAD) plaque rupture in a thin-cap fibroatheroma, causing a small transmural infarction at the terminus of the LAD.
“Without this diagnosis, it’s unlikely she would have received antiplatelet therapy or statins and might have been given a diagnosis of supply/demand mismatch, when the real diagnosis was MI,” Dr. Reynolds observed.
“Finally we can say this is not just crazy women. There is really something going on,” said panelist Roxana Mehran, MD, of the Icahn School of Medicine at Mount Sinai in New York. “You have now told us this is most likely atherosclerosis for pretty much 85% of the cases. So make the diagnosis and, of course, make sure you treat these patients accordingly for risk factor modification, really thinking about a ruptured plaque.”
Combining OCT and MRI may result in a more specific diagnosis and better treatment but also raises costs and logistical considerations.
“Implementation challenges are that not every form of testing is available in every medical center,” Dr. Reynolds said in an interview. “Many centers have cardiac MRI,” whereas “OCT is not currently available at most medical centers where heart attack patients are treated but is available at specialized centers.”
Asked during the session about the use of CT angiography, invited discussant Martha Gulati, MD, president-elect of the American Society for Preventive Cardiology, said, “For me, CT is helpful when I’m not sure if there’s any plaque because the angiogram looked really normal and there was no opportunity to do intracoronary imaging. And sometimes that will help me, in particular, if a patient doesn’t want to take a statin.”
Dr. Gulati pointed out that the European Society of Cardiology MINOCA guidelines recommend OCT and CMR, whereas the 2019 AHA statement on MINOCA, which she coauthored, also recommends OCT and CMR, but almost as one or the other.
“We already said that you should do cardiac MR to try to make a diagnosis, but I think the combination of the two needs to be emphasized when we next draft these guidelines. It really will help,” Dr. Gulati said in an interview.
“But using OCT, particularly, needs to be in the setting of the MI. I don’t think you want to do a procedure again,” she said. “So we really need it to become more widely available because at the time of an MI, you won’t necessarily know that you’re not going to find an obstructive lesion.”
Dr. Gulati pointed out several unanswered questions, including whether the diagnosis was missed in some patients, because OCT of all three vessels was available in only 59%, and how the use of high-sensitivity troponin, which was left up to the individual institution, might affect the usefulness of OCT and CMR.
It’s also unknown whether the mechanism is different for ST-segment elevation MI, as the trial included very few cases, although MINOCA often occurs in this setting. Future OCT/CMR studies will also need to enroll men to determine potential sex differences, if any.
Commenting on the study, B. Hadley Wilson, MD, Sanger Heart & Vascular Institute in Charlotte, N.C., said, “There would need to be further justification of this invasive interventional procedure to be sure that the benefit outweighed the risk of putting a wire and an OCT catheter down patients without any significant angiographic blockage and to assure interventional cardiologists of its value here.”
He pointed out that noninvasive CMR appears helpful in the diagnosis of nearly three-quarters of these patients and perhaps could be done first to direct which of those with an ischemic cause might benefit from invasive OCT at catheterization. This seems most pertinent in patients with a high suspicion of coronary artery disease or recurrent MINOCA.
“Overall, we need to consider the expense, logistics, and small risk of these combined modalities, particularly in everyday practice, before making recommendations,” Dr. Wilson said. “ Since OCT is much less available than intravascular ultrasound, it would require a challenging marketplace paradigm shift to implement this multimodality imaging strategy regionally and locally in the U.S., including the added costs. However, further study to direct the more judicious use of either CMR and/or combined with OCT is warranted in these patients.”
The study was funded by the AHA through a grant from the Go Red for Women Strategically Focused Research Network. Dr. Reynolds reported in-kind donations from Abbott Vascular and Siemens related to the study and nonfinancial support from BioTelemetry outside the study. Dr. Gulati and Dr. Wilson reported having no relevant disclosures.
A version of this article originally appeared on Medscape.com.
Radiotherapy planning scans reveal breast cancer patients’ CVD risk
Radiotherapy planning scans may be a rich untapped source of information for estimating the risk of cardiovascular disease (CVD) in breast cancer patients, a large study suggests.
Researchers found that breast cancer patients with a coronary artery calcifications (CAC) score exceeding 400 had nearly four times the adjusted risk of fatal and nonfatal CVD events when compared with patients who had a CAC score of 0.
Patients with scores exceeding 400 also had more than eight times the risk of coronary heart disease events. The associations were especially strong in the subset of patients who received anthracycline-containing chemotherapy.
Helena Verkooijen, MD, PhD, of University Medical Center Utrecht (the Netherlands) presented these findings at the 12th European Breast Cancer Conference.
Dr. Verkooijen noted that, over the past 50 years, breast cancer has dramatically declined as a cause of death among breast cancer survivors, while CVD has continued to account for about 20% of the total deaths in this population.
CACs are sometimes incidentally seen in radiotherapy planning CT scans. “Right now, this information is not often used for patient stratification or informing patients about their cardiovascular risk, and this is a pity, because we know that it is an independent risk factor, and, often, the presence of calcifications can occur in the absence of other cardiovascular risk factors,” Dr. Verkooijen said.
Study details
Dr. Verkooijen and and colleagues from the Bragataston Study Group retrospectively studied 15,919 breast cancer patients who had radiotherapy planning CT scans during 2004-2016 at three Dutch institutions.
The researchers used an automated deep-learning algorithm (described in Radiology) to detect and quantify coronary calcium in planning CT scans and calculate CAC scores, classifying them into five categories.
The median follow-up was 51.6 months. Most women (70%) did not have any calcium detected in their coronary arteries (CAC score of 0), while 3% fell into the highest category (CAC score of >400).
The incidence of nonfatal and fatal CVD events increased with CAC score:
- 5.1% with a score of 0.
- 8.5% with a score of 1-10.
- 13.5% with a score of 11-100.
- 17.6% with a score of 101-400.
- 28.0% with a score greater than 400.
In analyses adjusted for age, laterality of radiation, and receipt of cardiotoxic agents – anthracyclines and trastuzumab – women with a score exceeding 400 had sharply elevated adjusted risks of CVD events (hazard ratio, 3.7), of coronary heart disease events specifically (HR, 8.2), and of death from any cause (HR, 2.8), when compared with peers who had a CAC score of 0.
On further scrutiny of CVD events, the pattern was similar regardless of whether radiation was left- or right-sided. However, the association was stronger among women who received anthracyclines as compared with counterparts who did not, with a nearly six-fold higher risk for those with highest versus lowest CAC scores.
When the women were surveyed, nearly 90% said they wanted to be informed about their CAC score and associated CVD risk, even in the absence of evidence-based risk reduction strategies.
Applying the results
“We believe that this is the first time that anyone has conducted a study on this topic on a scale like this, and we show that it is possible to relatively easily identify women at a very high risk of CVD,” Dr. Verkooijen said. “But what do we do with this information, because these scans are not made to answer this question. … This is information that we get that we haven’t really requested. I think we should only use this information when we have really shown that we can help patients reduce their risk of cardiovascular disease.”
To that end, Dr. Verkooijen and colleagues are planning additional research that will look at the potential benefit of referring high-risk patients for cardioprevention strategies and at the role of using the CAC score to personalize treatment strategies.
“This is an interesting and novel approach to predicting cardiac events for patients undergoing breast cancer treatment,” Meena S. Moran, MD, of Yale University in New Haven, Conn., commented in an interview.
The approach would likely be feasible in typical practice with widespread availability of the automated algorithm and might even alter treatment planning in real time, she said. “From the standpoint of radiation oncology, it would mean running the software to generate a CAC score, which would allow for modifications in decision-making during treatment planning, such as whether or not to include the internal mammary nodal chain in a patient who may be in the ‘gray zone’ for regional nodal radiation. For example, if a patient has a high CAC score, plus if they have received (or are receiving) cardiotoxic drugs, radiation oncologists can use that information as an additional factor to consider in the decision-making of whether or not to include the internal mammary chain, which inevitably can increase the dose delivered to the heart,” Dr. Moran elaborated.
Dr. Verkooijen’s study was supported by the Dutch Cancer Society, the European Commission, the Dutch Digestive Foundation, the Netherlands Organisation for Scientific Research, and Elekta. Dr. Verkooijen and Dr. Moran disclosed no conflicts of interest.
SOURCE: Gal R et al. EBCC-12 Virtual Congress, Abstract 7.
Radiotherapy planning scans may be a rich untapped source of information for estimating the risk of cardiovascular disease (CVD) in breast cancer patients, a large study suggests.
Researchers found that breast cancer patients with a coronary artery calcifications (CAC) score exceeding 400 had nearly four times the adjusted risk of fatal and nonfatal CVD events when compared with patients who had a CAC score of 0.
Patients with scores exceeding 400 also had more than eight times the risk of coronary heart disease events. The associations were especially strong in the subset of patients who received anthracycline-containing chemotherapy.
Helena Verkooijen, MD, PhD, of University Medical Center Utrecht (the Netherlands) presented these findings at the 12th European Breast Cancer Conference.
Dr. Verkooijen noted that, over the past 50 years, breast cancer has dramatically declined as a cause of death among breast cancer survivors, while CVD has continued to account for about 20% of the total deaths in this population.
CACs are sometimes incidentally seen in radiotherapy planning CT scans. “Right now, this information is not often used for patient stratification or informing patients about their cardiovascular risk, and this is a pity, because we know that it is an independent risk factor, and, often, the presence of calcifications can occur in the absence of other cardiovascular risk factors,” Dr. Verkooijen said.
Study details
Dr. Verkooijen and and colleagues from the Bragataston Study Group retrospectively studied 15,919 breast cancer patients who had radiotherapy planning CT scans during 2004-2016 at three Dutch institutions.
The researchers used an automated deep-learning algorithm (described in Radiology) to detect and quantify coronary calcium in planning CT scans and calculate CAC scores, classifying them into five categories.
The median follow-up was 51.6 months. Most women (70%) did not have any calcium detected in their coronary arteries (CAC score of 0), while 3% fell into the highest category (CAC score of >400).
The incidence of nonfatal and fatal CVD events increased with CAC score:
- 5.1% with a score of 0.
- 8.5% with a score of 1-10.
- 13.5% with a score of 11-100.
- 17.6% with a score of 101-400.
- 28.0% with a score greater than 400.
In analyses adjusted for age, laterality of radiation, and receipt of cardiotoxic agents – anthracyclines and trastuzumab – women with a score exceeding 400 had sharply elevated adjusted risks of CVD events (hazard ratio, 3.7), of coronary heart disease events specifically (HR, 8.2), and of death from any cause (HR, 2.8), when compared with peers who had a CAC score of 0.
On further scrutiny of CVD events, the pattern was similar regardless of whether radiation was left- or right-sided. However, the association was stronger among women who received anthracyclines as compared with counterparts who did not, with a nearly six-fold higher risk for those with highest versus lowest CAC scores.
When the women were surveyed, nearly 90% said they wanted to be informed about their CAC score and associated CVD risk, even in the absence of evidence-based risk reduction strategies.
Applying the results
“We believe that this is the first time that anyone has conducted a study on this topic on a scale like this, and we show that it is possible to relatively easily identify women at a very high risk of CVD,” Dr. Verkooijen said. “But what do we do with this information, because these scans are not made to answer this question. … This is information that we get that we haven’t really requested. I think we should only use this information when we have really shown that we can help patients reduce their risk of cardiovascular disease.”
To that end, Dr. Verkooijen and colleagues are planning additional research that will look at the potential benefit of referring high-risk patients for cardioprevention strategies and at the role of using the CAC score to personalize treatment strategies.
“This is an interesting and novel approach to predicting cardiac events for patients undergoing breast cancer treatment,” Meena S. Moran, MD, of Yale University in New Haven, Conn., commented in an interview.
The approach would likely be feasible in typical practice with widespread availability of the automated algorithm and might even alter treatment planning in real time, she said. “From the standpoint of radiation oncology, it would mean running the software to generate a CAC score, which would allow for modifications in decision-making during treatment planning, such as whether or not to include the internal mammary nodal chain in a patient who may be in the ‘gray zone’ for regional nodal radiation. For example, if a patient has a high CAC score, plus if they have received (or are receiving) cardiotoxic drugs, radiation oncologists can use that information as an additional factor to consider in the decision-making of whether or not to include the internal mammary chain, which inevitably can increase the dose delivered to the heart,” Dr. Moran elaborated.
Dr. Verkooijen’s study was supported by the Dutch Cancer Society, the European Commission, the Dutch Digestive Foundation, the Netherlands Organisation for Scientific Research, and Elekta. Dr. Verkooijen and Dr. Moran disclosed no conflicts of interest.
SOURCE: Gal R et al. EBCC-12 Virtual Congress, Abstract 7.
Radiotherapy planning scans may be a rich untapped source of information for estimating the risk of cardiovascular disease (CVD) in breast cancer patients, a large study suggests.
Researchers found that breast cancer patients with a coronary artery calcifications (CAC) score exceeding 400 had nearly four times the adjusted risk of fatal and nonfatal CVD events when compared with patients who had a CAC score of 0.
Patients with scores exceeding 400 also had more than eight times the risk of coronary heart disease events. The associations were especially strong in the subset of patients who received anthracycline-containing chemotherapy.
Helena Verkooijen, MD, PhD, of University Medical Center Utrecht (the Netherlands) presented these findings at the 12th European Breast Cancer Conference.
Dr. Verkooijen noted that, over the past 50 years, breast cancer has dramatically declined as a cause of death among breast cancer survivors, while CVD has continued to account for about 20% of the total deaths in this population.
CACs are sometimes incidentally seen in radiotherapy planning CT scans. “Right now, this information is not often used for patient stratification or informing patients about their cardiovascular risk, and this is a pity, because we know that it is an independent risk factor, and, often, the presence of calcifications can occur in the absence of other cardiovascular risk factors,” Dr. Verkooijen said.
Study details
Dr. Verkooijen and and colleagues from the Bragataston Study Group retrospectively studied 15,919 breast cancer patients who had radiotherapy planning CT scans during 2004-2016 at three Dutch institutions.
The researchers used an automated deep-learning algorithm (described in Radiology) to detect and quantify coronary calcium in planning CT scans and calculate CAC scores, classifying them into five categories.
The median follow-up was 51.6 months. Most women (70%) did not have any calcium detected in their coronary arteries (CAC score of 0), while 3% fell into the highest category (CAC score of >400).
The incidence of nonfatal and fatal CVD events increased with CAC score:
- 5.1% with a score of 0.
- 8.5% with a score of 1-10.
- 13.5% with a score of 11-100.
- 17.6% with a score of 101-400.
- 28.0% with a score greater than 400.
In analyses adjusted for age, laterality of radiation, and receipt of cardiotoxic agents – anthracyclines and trastuzumab – women with a score exceeding 400 had sharply elevated adjusted risks of CVD events (hazard ratio, 3.7), of coronary heart disease events specifically (HR, 8.2), and of death from any cause (HR, 2.8), when compared with peers who had a CAC score of 0.
On further scrutiny of CVD events, the pattern was similar regardless of whether radiation was left- or right-sided. However, the association was stronger among women who received anthracyclines as compared with counterparts who did not, with a nearly six-fold higher risk for those with highest versus lowest CAC scores.
When the women were surveyed, nearly 90% said they wanted to be informed about their CAC score and associated CVD risk, even in the absence of evidence-based risk reduction strategies.
Applying the results
“We believe that this is the first time that anyone has conducted a study on this topic on a scale like this, and we show that it is possible to relatively easily identify women at a very high risk of CVD,” Dr. Verkooijen said. “But what do we do with this information, because these scans are not made to answer this question. … This is information that we get that we haven’t really requested. I think we should only use this information when we have really shown that we can help patients reduce their risk of cardiovascular disease.”
To that end, Dr. Verkooijen and colleagues are planning additional research that will look at the potential benefit of referring high-risk patients for cardioprevention strategies and at the role of using the CAC score to personalize treatment strategies.
“This is an interesting and novel approach to predicting cardiac events for patients undergoing breast cancer treatment,” Meena S. Moran, MD, of Yale University in New Haven, Conn., commented in an interview.
The approach would likely be feasible in typical practice with widespread availability of the automated algorithm and might even alter treatment planning in real time, she said. “From the standpoint of radiation oncology, it would mean running the software to generate a CAC score, which would allow for modifications in decision-making during treatment planning, such as whether or not to include the internal mammary nodal chain in a patient who may be in the ‘gray zone’ for regional nodal radiation. For example, if a patient has a high CAC score, plus if they have received (or are receiving) cardiotoxic drugs, radiation oncologists can use that information as an additional factor to consider in the decision-making of whether or not to include the internal mammary chain, which inevitably can increase the dose delivered to the heart,” Dr. Moran elaborated.
Dr. Verkooijen’s study was supported by the Dutch Cancer Society, the European Commission, the Dutch Digestive Foundation, the Netherlands Organisation for Scientific Research, and Elekta. Dr. Verkooijen and Dr. Moran disclosed no conflicts of interest.
SOURCE: Gal R et al. EBCC-12 Virtual Congress, Abstract 7.
FROM EBCC-12 VIRTUAL CONFERENCE
Non-COVID-19 clinical trials grind to a halt during pandemic
The COVID-19 pandemic has created unique and unprecedented challenges for the clinical research world, with potentially long-lasting consequences.
A new analysis of the extent of disruption shows that the average rate of stopped trials nearly doubled during the first 5 months of 2020, compared with the 2 previous years.
“Typically, clinical research precedes clinical practice by several years, so this disruption we’re seeing now will be felt for many years to come,” said Mario Guadino, MD, of Weill Cornell Medicine, New York.
The analysis was published online July 31 in the Journal of the American College of Cardiology.
The researchers used Python software to query meta-data from all trials reported on ClinicalTrials.gov. Of 321,218 non-COVID-19 trials queried, 28,672 (8.9%) were reported as stopped, defined as a switch in trial status from “recruiting” to “active and not recruiting,” “completed,” “suspended,” “terminated,” or “withdrawn.”
The average rate of discontinuation was 638 trials/month from January 2017 to December 2019, rising to 1,147 trials/month between January 2020 and May 2020 (P < .001 for trend).
Once stopped (as opposed to paused), restarting a trial is a tricky prospect, said Dr. Guadino. “You can’t stop and restart a trial because it creates a lot of issues, so we should expect many of these stopped trials to never be completed.”
He said these figures likely represent an underestimate of the true impact of the pandemic because there is typically a delay in the updating of the status of a trial on ClinicalTrials.gov.
“We are likely looking only at the tip of the iceberg,” he added. “My impression is that the number of trials that will be affected and even canceled will be very high.”
As for cardiology trials, one of the report’s authors, Deepak Bhatt, MD, Brigham and Women’s Hospital, Boston, without naming specific trials, had this to say: “Several cardiovascular trials were paused, and some were permanently discontinued. It may be a while before we fully appreciate just how much information was lost and how much might be salvaged.”
He’s not worried, however, that upcoming cardiology meetings, which have moved online for the foreseeable future, might get a bit boring. “Fortunately, there is enough good work going on in the cardiovascular and cardiometabolic space that I believe there will still be ample randomized and observational data of high quality to present at the major meetings,” Dr. Bhatt said in an email.
The researchers found a weak correlation between the national population-adjusted numbers of COVID-19 cases and the proportion of non-COVID-19 trials stopped by country.
Even for trials that stopped recruiting for a period of time but are continuing, there are myriad issues involving compliance, data integrity, statistical interpretability, etc.
“Even if there is just a temporary disruption, that will most likely lead to reduced enrollment, missing follow-up visits, and protocol deviations, all things that would be red flags during normal times and impact the quality of the clinical trial,” said Dr. Guadino.
“And if your outcome of interest is mortality, well, how exactly do you measure that during a pandemic?” he added.
Stopped for lack of funding
Besides the logistical issues, another reason trials may be in jeopardy is funding. A warning early in the pandemic from the research community in Canada that funding was quickly drying up, leaving both jobs and data at risk, led to an aid package from the government to keep the lights on.
The National Institutes of Health (NIH), the Canadian Institutes of Health Research, and similar groups “have devoted large sums of money to research in COVID, which is of course very appropriate, but that clearly reduces the amount of funding that is available for other researchers,” said Dr. Guadino.
Some funding agencies around the world have canceled or put on hold all non-COVID-19 clinical trials still at the design state, Dr. Guadino said in an interview.
The NIH, he stressed, has not canceled funding and has been “extremely open and cooperative” in trying to help trialists navigate the many COVID-generated issues. They’ve even issued guidance on how to manage trials during COVID-19.
Of note, in the survey, the majority of the trials stopped (95.4%) had nongovernmental funding.
“The data are not very granular, so we’re only able to make some very simple, descriptive comments, but it does seem like the more fragile trials – those that are smaller and industry-funded – are the ones more likely to be disrupted,” said Dr. Guadino.
In some cases, he said, priorities have shifted to COVID-19. “If a small company is sponsoring a trial and they decide they want to sponsor something related to COVID, or they realize that because of the slow enrollment, the trial becomes too expensive to complete, they may opt to just abandon it,” said Dr. Guadino.
At what cost? It will take years to sort that out, he said.
This study received no funding. Dr. Guadino and Dr. Bhatt are both active trialists, participating in both industry- and government-sponsored clinical research.
A version of this article originally appeared on Medscape.com.
The COVID-19 pandemic has created unique and unprecedented challenges for the clinical research world, with potentially long-lasting consequences.
A new analysis of the extent of disruption shows that the average rate of stopped trials nearly doubled during the first 5 months of 2020, compared with the 2 previous years.
“Typically, clinical research precedes clinical practice by several years, so this disruption we’re seeing now will be felt for many years to come,” said Mario Guadino, MD, of Weill Cornell Medicine, New York.
The analysis was published online July 31 in the Journal of the American College of Cardiology.
The researchers used Python software to query meta-data from all trials reported on ClinicalTrials.gov. Of 321,218 non-COVID-19 trials queried, 28,672 (8.9%) were reported as stopped, defined as a switch in trial status from “recruiting” to “active and not recruiting,” “completed,” “suspended,” “terminated,” or “withdrawn.”
The average rate of discontinuation was 638 trials/month from January 2017 to December 2019, rising to 1,147 trials/month between January 2020 and May 2020 (P < .001 for trend).
Once stopped (as opposed to paused), restarting a trial is a tricky prospect, said Dr. Guadino. “You can’t stop and restart a trial because it creates a lot of issues, so we should expect many of these stopped trials to never be completed.”
He said these figures likely represent an underestimate of the true impact of the pandemic because there is typically a delay in the updating of the status of a trial on ClinicalTrials.gov.
“We are likely looking only at the tip of the iceberg,” he added. “My impression is that the number of trials that will be affected and even canceled will be very high.”
As for cardiology trials, one of the report’s authors, Deepak Bhatt, MD, Brigham and Women’s Hospital, Boston, without naming specific trials, had this to say: “Several cardiovascular trials were paused, and some were permanently discontinued. It may be a while before we fully appreciate just how much information was lost and how much might be salvaged.”
He’s not worried, however, that upcoming cardiology meetings, which have moved online for the foreseeable future, might get a bit boring. “Fortunately, there is enough good work going on in the cardiovascular and cardiometabolic space that I believe there will still be ample randomized and observational data of high quality to present at the major meetings,” Dr. Bhatt said in an email.
The researchers found a weak correlation between the national population-adjusted numbers of COVID-19 cases and the proportion of non-COVID-19 trials stopped by country.
Even for trials that stopped recruiting for a period of time but are continuing, there are myriad issues involving compliance, data integrity, statistical interpretability, etc.
“Even if there is just a temporary disruption, that will most likely lead to reduced enrollment, missing follow-up visits, and protocol deviations, all things that would be red flags during normal times and impact the quality of the clinical trial,” said Dr. Guadino.
“And if your outcome of interest is mortality, well, how exactly do you measure that during a pandemic?” he added.
Stopped for lack of funding
Besides the logistical issues, another reason trials may be in jeopardy is funding. A warning early in the pandemic from the research community in Canada that funding was quickly drying up, leaving both jobs and data at risk, led to an aid package from the government to keep the lights on.
The National Institutes of Health (NIH), the Canadian Institutes of Health Research, and similar groups “have devoted large sums of money to research in COVID, which is of course very appropriate, but that clearly reduces the amount of funding that is available for other researchers,” said Dr. Guadino.
Some funding agencies around the world have canceled or put on hold all non-COVID-19 clinical trials still at the design state, Dr. Guadino said in an interview.
The NIH, he stressed, has not canceled funding and has been “extremely open and cooperative” in trying to help trialists navigate the many COVID-generated issues. They’ve even issued guidance on how to manage trials during COVID-19.
Of note, in the survey, the majority of the trials stopped (95.4%) had nongovernmental funding.
“The data are not very granular, so we’re only able to make some very simple, descriptive comments, but it does seem like the more fragile trials – those that are smaller and industry-funded – are the ones more likely to be disrupted,” said Dr. Guadino.
In some cases, he said, priorities have shifted to COVID-19. “If a small company is sponsoring a trial and they decide they want to sponsor something related to COVID, or they realize that because of the slow enrollment, the trial becomes too expensive to complete, they may opt to just abandon it,” said Dr. Guadino.
At what cost? It will take years to sort that out, he said.
This study received no funding. Dr. Guadino and Dr. Bhatt are both active trialists, participating in both industry- and government-sponsored clinical research.
A version of this article originally appeared on Medscape.com.
The COVID-19 pandemic has created unique and unprecedented challenges for the clinical research world, with potentially long-lasting consequences.
A new analysis of the extent of disruption shows that the average rate of stopped trials nearly doubled during the first 5 months of 2020, compared with the 2 previous years.
“Typically, clinical research precedes clinical practice by several years, so this disruption we’re seeing now will be felt for many years to come,” said Mario Guadino, MD, of Weill Cornell Medicine, New York.
The analysis was published online July 31 in the Journal of the American College of Cardiology.
The researchers used Python software to query meta-data from all trials reported on ClinicalTrials.gov. Of 321,218 non-COVID-19 trials queried, 28,672 (8.9%) were reported as stopped, defined as a switch in trial status from “recruiting” to “active and not recruiting,” “completed,” “suspended,” “terminated,” or “withdrawn.”
The average rate of discontinuation was 638 trials/month from January 2017 to December 2019, rising to 1,147 trials/month between January 2020 and May 2020 (P < .001 for trend).
Once stopped (as opposed to paused), restarting a trial is a tricky prospect, said Dr. Guadino. “You can’t stop and restart a trial because it creates a lot of issues, so we should expect many of these stopped trials to never be completed.”
He said these figures likely represent an underestimate of the true impact of the pandemic because there is typically a delay in the updating of the status of a trial on ClinicalTrials.gov.
“We are likely looking only at the tip of the iceberg,” he added. “My impression is that the number of trials that will be affected and even canceled will be very high.”
As for cardiology trials, one of the report’s authors, Deepak Bhatt, MD, Brigham and Women’s Hospital, Boston, without naming specific trials, had this to say: “Several cardiovascular trials were paused, and some were permanently discontinued. It may be a while before we fully appreciate just how much information was lost and how much might be salvaged.”
He’s not worried, however, that upcoming cardiology meetings, which have moved online for the foreseeable future, might get a bit boring. “Fortunately, there is enough good work going on in the cardiovascular and cardiometabolic space that I believe there will still be ample randomized and observational data of high quality to present at the major meetings,” Dr. Bhatt said in an email.
The researchers found a weak correlation between the national population-adjusted numbers of COVID-19 cases and the proportion of non-COVID-19 trials stopped by country.
Even for trials that stopped recruiting for a period of time but are continuing, there are myriad issues involving compliance, data integrity, statistical interpretability, etc.
“Even if there is just a temporary disruption, that will most likely lead to reduced enrollment, missing follow-up visits, and protocol deviations, all things that would be red flags during normal times and impact the quality of the clinical trial,” said Dr. Guadino.
“And if your outcome of interest is mortality, well, how exactly do you measure that during a pandemic?” he added.
Stopped for lack of funding
Besides the logistical issues, another reason trials may be in jeopardy is funding. A warning early in the pandemic from the research community in Canada that funding was quickly drying up, leaving both jobs and data at risk, led to an aid package from the government to keep the lights on.
The National Institutes of Health (NIH), the Canadian Institutes of Health Research, and similar groups “have devoted large sums of money to research in COVID, which is of course very appropriate, but that clearly reduces the amount of funding that is available for other researchers,” said Dr. Guadino.
Some funding agencies around the world have canceled or put on hold all non-COVID-19 clinical trials still at the design state, Dr. Guadino said in an interview.
The NIH, he stressed, has not canceled funding and has been “extremely open and cooperative” in trying to help trialists navigate the many COVID-generated issues. They’ve even issued guidance on how to manage trials during COVID-19.
Of note, in the survey, the majority of the trials stopped (95.4%) had nongovernmental funding.
“The data are not very granular, so we’re only able to make some very simple, descriptive comments, but it does seem like the more fragile trials – those that are smaller and industry-funded – are the ones more likely to be disrupted,” said Dr. Guadino.
In some cases, he said, priorities have shifted to COVID-19. “If a small company is sponsoring a trial and they decide they want to sponsor something related to COVID, or they realize that because of the slow enrollment, the trial becomes too expensive to complete, they may opt to just abandon it,” said Dr. Guadino.
At what cost? It will take years to sort that out, he said.
This study received no funding. Dr. Guadino and Dr. Bhatt are both active trialists, participating in both industry- and government-sponsored clinical research.
A version of this article originally appeared on Medscape.com.
PHM20 Virtual: Common incidental findings seen on pediatric imaging
PHM20 session title
The Incidentaloma: Common Incidental Findings Seen on Pediatric Imaging
Presenters
Jill Azok, MD; Amanda Lansell, MD; Allayne Stephans, MD; and Erin Frank, MD
Session summary
Dr. Azok, Dr. Lansell, and Dr. Frank of University Hospitals Rainbow Babies & Children’s Hospital, Cleveland, described one to three common, incidentally noted findings in central nervous system, thoracic, abdominopelvic, and musculoskeletal imaging. The presenters explained the indications for further work-up and/or intervention of these findings, and the importance of judicious use of imaging in pediatric patients.
Dr. Frank discussed incidental findings seen on imaging of the central nervous system, using cases to focus on benign enlargement of the subarachnoid space, lipomas of the filum terminale, and pituitary abnormalities. Dr. Lansell continued by discussing possible clinical models for management of incidentally found pulmonary nodules and renal cysts. Dr. Azok completed the session with a discussion of the appearance and management of nonossifying fibromas and cortical fibrous defects. Common threads shared by all presenters were how frequent incidental findings are and the need for providers to be comfortable with a level of uncertainty.
Key takeaways
- Incidental findings are very common in pediatric imaging, occurring on up to one-third of CT scans, 25% of brain MRIs, and 21% of knee radiographs.
- An infant with personal and family history of macrocephaly, normal development, and increased extra-axial CSF on MRI likely has benign enlargement of the arachnoid space and does not need further evaluation.
- A hyperintensity of filum terminale on MRI is consistent with lipoma of the filum terminale and does not require follow-up unless symptoms of tethered cord are present.
- Pituitary abnormalities are common and call for dedicated history, physical exam, and an endocrine screening with imaging surveillance if screening is normal.
- Patient history and appearance of pulmonary nodules are important in determining appropriate follow-up.
- No single feature of renal lesions predicts future behavior, but larger lesions deserve more work-up.
- Nonossifying fibromas are well-demarcated intracortical radiolucencies of long bone metaphyses that do not require treatment or further evaluation unless they are large, painful, or occur in the proximal femur.
Dr. Miller is a second-year pediatric hospital medicine fellow at Cleveland Clinic Children’s. His academic interests include medical education, quality improvement, and high value care.
PHM20 session title
The Incidentaloma: Common Incidental Findings Seen on Pediatric Imaging
Presenters
Jill Azok, MD; Amanda Lansell, MD; Allayne Stephans, MD; and Erin Frank, MD
Session summary
Dr. Azok, Dr. Lansell, and Dr. Frank of University Hospitals Rainbow Babies & Children’s Hospital, Cleveland, described one to three common, incidentally noted findings in central nervous system, thoracic, abdominopelvic, and musculoskeletal imaging. The presenters explained the indications for further work-up and/or intervention of these findings, and the importance of judicious use of imaging in pediatric patients.
Dr. Frank discussed incidental findings seen on imaging of the central nervous system, using cases to focus on benign enlargement of the subarachnoid space, lipomas of the filum terminale, and pituitary abnormalities. Dr. Lansell continued by discussing possible clinical models for management of incidentally found pulmonary nodules and renal cysts. Dr. Azok completed the session with a discussion of the appearance and management of nonossifying fibromas and cortical fibrous defects. Common threads shared by all presenters were how frequent incidental findings are and the need for providers to be comfortable with a level of uncertainty.
Key takeaways
- Incidental findings are very common in pediatric imaging, occurring on up to one-third of CT scans, 25% of brain MRIs, and 21% of knee radiographs.
- An infant with personal and family history of macrocephaly, normal development, and increased extra-axial CSF on MRI likely has benign enlargement of the arachnoid space and does not need further evaluation.
- A hyperintensity of filum terminale on MRI is consistent with lipoma of the filum terminale and does not require follow-up unless symptoms of tethered cord are present.
- Pituitary abnormalities are common and call for dedicated history, physical exam, and an endocrine screening with imaging surveillance if screening is normal.
- Patient history and appearance of pulmonary nodules are important in determining appropriate follow-up.
- No single feature of renal lesions predicts future behavior, but larger lesions deserve more work-up.
- Nonossifying fibromas are well-demarcated intracortical radiolucencies of long bone metaphyses that do not require treatment or further evaluation unless they are large, painful, or occur in the proximal femur.
Dr. Miller is a second-year pediatric hospital medicine fellow at Cleveland Clinic Children’s. His academic interests include medical education, quality improvement, and high value care.
PHM20 session title
The Incidentaloma: Common Incidental Findings Seen on Pediatric Imaging
Presenters
Jill Azok, MD; Amanda Lansell, MD; Allayne Stephans, MD; and Erin Frank, MD
Session summary
Dr. Azok, Dr. Lansell, and Dr. Frank of University Hospitals Rainbow Babies & Children’s Hospital, Cleveland, described one to three common, incidentally noted findings in central nervous system, thoracic, abdominopelvic, and musculoskeletal imaging. The presenters explained the indications for further work-up and/or intervention of these findings, and the importance of judicious use of imaging in pediatric patients.
Dr. Frank discussed incidental findings seen on imaging of the central nervous system, using cases to focus on benign enlargement of the subarachnoid space, lipomas of the filum terminale, and pituitary abnormalities. Dr. Lansell continued by discussing possible clinical models for management of incidentally found pulmonary nodules and renal cysts. Dr. Azok completed the session with a discussion of the appearance and management of nonossifying fibromas and cortical fibrous defects. Common threads shared by all presenters were how frequent incidental findings are and the need for providers to be comfortable with a level of uncertainty.
Key takeaways
- Incidental findings are very common in pediatric imaging, occurring on up to one-third of CT scans, 25% of brain MRIs, and 21% of knee radiographs.
- An infant with personal and family history of macrocephaly, normal development, and increased extra-axial CSF on MRI likely has benign enlargement of the arachnoid space and does not need further evaluation.
- A hyperintensity of filum terminale on MRI is consistent with lipoma of the filum terminale and does not require follow-up unless symptoms of tethered cord are present.
- Pituitary abnormalities are common and call for dedicated history, physical exam, and an endocrine screening with imaging surveillance if screening is normal.
- Patient history and appearance of pulmonary nodules are important in determining appropriate follow-up.
- No single feature of renal lesions predicts future behavior, but larger lesions deserve more work-up.
- Nonossifying fibromas are well-demarcated intracortical radiolucencies of long bone metaphyses that do not require treatment or further evaluation unless they are large, painful, or occur in the proximal femur.
Dr. Miller is a second-year pediatric hospital medicine fellow at Cleveland Clinic Children’s. His academic interests include medical education, quality improvement, and high value care.
CT-FFR offers a noninvasive ‘one-stop shop’ for pre-TAVR assessment
Fractional flow reserve derived noninvasively from coronary CT angiography is a safe and accurate method for assessing the significance of coronary artery disease in patients with severe aortic stenosis who are headed for transcatheter aortic valve replacement (TAVR), according to results of the CAST-FFR prospective study.
Indeed, utilization of coronary CT angiography–derived fractional flow reserve (CT-FFR) for this purpose offers the advantage of using a single noninvasive imaging method to replace two invasive procedures: coronary angiography to assess the anatomy of coronary lesions, and conventional FFR using a pressure wire to determine the functional significance of a given coronary stenosis as a cause of ischemia, Michael Michail, MBBS, explained in reporting the results at the virtual annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
“Because up to 50% of patients with severe aortic stenosis undergoing TAVR have coexisting coronary artery disease, it remains common practice to perform prior invasive coronary angiography. However, this is associated with inherent risks, particularly in an elderly cohort with comorbidities. Additionally, coronary angiography provides no information on the functional impact of coronary stenoses, which may be important in guiding revascularization decisions prior to TAVR,” noted Dr. Michail, a cardiologist at Monash University, Melbourne.
Simulating FFR: ‘A one-stop shop cardiac CT’
Dr. Michail presented the results of the prospective CAST-FFR study, the first evaluation of CT-FFR for assessment of coronary arteries in patients with severe symptomatic aortic stenosis. This method uses computational fluid dynamics to transform data obtained noninvasively from a standard coronary CT angiography acquisition into a simulated FFR. And it offers the potential to streamline patient care.
“In current practice we see elderly patients with a long pre-TAVR assessment period, with numerous appointments and invasive procedures. Our vision is a one-stop shop cardiac CT that will provide the cardiologist with a complete assessment of the annular measurements, peripheral vasculature, and the coronary arteries ahead of their procedure,” according to Dr. Michail.
“We believe the ability to perform the requisite coronary assessment using CT-FFR will translate to improved patient care in several ways,” he continued. “Firstly, this will shorten the number of tests and overall diagnostic journey for patients. It will reduce the risk from unnecessary invasive procedures, and this will also reduce discomfort for the patient. Based on emerging evidence on the adverse prognostic impact of functionally significant coronary disease in aortic stenosis, this data has the potential to improve procedural risk stratification. And finally, contingent on further data, this may improve lesion selection for upfront revascularization.”
The CAST-FFR study was a small, single-center, proof-of-concept study in which 42 patients with severe aortic stenosis underwent both coronary CT angiography and conventional FFR with a pressure wire. The CT data was sent to a core laboratory for conversion into CT-FFR by evaluators blinded to the conventional FFR values.
Of the 42 participants, 39 (93%) had usable CT-FFR data on 60 coronary vessels. Dr. Michail and coinvestigators found a strong correlation between the conventional pressure wire FFR and CT-FFR findings, with a receiver operating characteristic area under the curve of 0.83 per vessel. CT-FFR had a diagnostic sensitivity and specificity of 73.9% and 78.4%, respectively, with a positive predictive value of 68%, a negative predictive value of 82.9%, and a diagnostic accuracy of 76.7%.
He cited as study limitations the small size, the fact that patients with previous revascularization or significant left ventricular impairment were excluded, and the study cohort’s relative youth.
“With a mean age of 76.2 years, it’s unclear whether these results can be extrapolated to very elderly patients with more calcified arteries undergoing TAVR. Encouragingly, though, a subgroup analysis based on calcium score showed no effect on accuracy,” according to the cardiologist.
CT-FFR may ‘shorten the diagnostic journey’ for fragile patients
Discussant Daniele Andreini, MD, PhD, praised the investigators’ concept of integrating the functional assessment provided by CT-FFR into a one-stop shop examination by cardiac CT angiography for TAVR planning.
“I would like to underline one of Dr. Michail’s messages: It’s really important to shorten the diagnostic journey for these fragile, older patients with aortic stenosis in order to improve safety, use less contrast, and avoid complications,” said Dr. Andreini, a cardiologist at the University of Milan and director of the cardiovascular CT and radiology unit at Monzino Cardiology Center, also in Milan.
Both Dr. Michail and Dr. Andreini reported having no financial conflicts of interest.
Fractional flow reserve derived noninvasively from coronary CT angiography is a safe and accurate method for assessing the significance of coronary artery disease in patients with severe aortic stenosis who are headed for transcatheter aortic valve replacement (TAVR), according to results of the CAST-FFR prospective study.
Indeed, utilization of coronary CT angiography–derived fractional flow reserve (CT-FFR) for this purpose offers the advantage of using a single noninvasive imaging method to replace two invasive procedures: coronary angiography to assess the anatomy of coronary lesions, and conventional FFR using a pressure wire to determine the functional significance of a given coronary stenosis as a cause of ischemia, Michael Michail, MBBS, explained in reporting the results at the virtual annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
“Because up to 50% of patients with severe aortic stenosis undergoing TAVR have coexisting coronary artery disease, it remains common practice to perform prior invasive coronary angiography. However, this is associated with inherent risks, particularly in an elderly cohort with comorbidities. Additionally, coronary angiography provides no information on the functional impact of coronary stenoses, which may be important in guiding revascularization decisions prior to TAVR,” noted Dr. Michail, a cardiologist at Monash University, Melbourne.
Simulating FFR: ‘A one-stop shop cardiac CT’
Dr. Michail presented the results of the prospective CAST-FFR study, the first evaluation of CT-FFR for assessment of coronary arteries in patients with severe symptomatic aortic stenosis. This method uses computational fluid dynamics to transform data obtained noninvasively from a standard coronary CT angiography acquisition into a simulated FFR. And it offers the potential to streamline patient care.
“In current practice we see elderly patients with a long pre-TAVR assessment period, with numerous appointments and invasive procedures. Our vision is a one-stop shop cardiac CT that will provide the cardiologist with a complete assessment of the annular measurements, peripheral vasculature, and the coronary arteries ahead of their procedure,” according to Dr. Michail.
“We believe the ability to perform the requisite coronary assessment using CT-FFR will translate to improved patient care in several ways,” he continued. “Firstly, this will shorten the number of tests and overall diagnostic journey for patients. It will reduce the risk from unnecessary invasive procedures, and this will also reduce discomfort for the patient. Based on emerging evidence on the adverse prognostic impact of functionally significant coronary disease in aortic stenosis, this data has the potential to improve procedural risk stratification. And finally, contingent on further data, this may improve lesion selection for upfront revascularization.”
The CAST-FFR study was a small, single-center, proof-of-concept study in which 42 patients with severe aortic stenosis underwent both coronary CT angiography and conventional FFR with a pressure wire. The CT data was sent to a core laboratory for conversion into CT-FFR by evaluators blinded to the conventional FFR values.
Of the 42 participants, 39 (93%) had usable CT-FFR data on 60 coronary vessels. Dr. Michail and coinvestigators found a strong correlation between the conventional pressure wire FFR and CT-FFR findings, with a receiver operating characteristic area under the curve of 0.83 per vessel. CT-FFR had a diagnostic sensitivity and specificity of 73.9% and 78.4%, respectively, with a positive predictive value of 68%, a negative predictive value of 82.9%, and a diagnostic accuracy of 76.7%.
He cited as study limitations the small size, the fact that patients with previous revascularization or significant left ventricular impairment were excluded, and the study cohort’s relative youth.
“With a mean age of 76.2 years, it’s unclear whether these results can be extrapolated to very elderly patients with more calcified arteries undergoing TAVR. Encouragingly, though, a subgroup analysis based on calcium score showed no effect on accuracy,” according to the cardiologist.
CT-FFR may ‘shorten the diagnostic journey’ for fragile patients
Discussant Daniele Andreini, MD, PhD, praised the investigators’ concept of integrating the functional assessment provided by CT-FFR into a one-stop shop examination by cardiac CT angiography for TAVR planning.
“I would like to underline one of Dr. Michail’s messages: It’s really important to shorten the diagnostic journey for these fragile, older patients with aortic stenosis in order to improve safety, use less contrast, and avoid complications,” said Dr. Andreini, a cardiologist at the University of Milan and director of the cardiovascular CT and radiology unit at Monzino Cardiology Center, also in Milan.
Both Dr. Michail and Dr. Andreini reported having no financial conflicts of interest.
Fractional flow reserve derived noninvasively from coronary CT angiography is a safe and accurate method for assessing the significance of coronary artery disease in patients with severe aortic stenosis who are headed for transcatheter aortic valve replacement (TAVR), according to results of the CAST-FFR prospective study.
Indeed, utilization of coronary CT angiography–derived fractional flow reserve (CT-FFR) for this purpose offers the advantage of using a single noninvasive imaging method to replace two invasive procedures: coronary angiography to assess the anatomy of coronary lesions, and conventional FFR using a pressure wire to determine the functional significance of a given coronary stenosis as a cause of ischemia, Michael Michail, MBBS, explained in reporting the results at the virtual annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
“Because up to 50% of patients with severe aortic stenosis undergoing TAVR have coexisting coronary artery disease, it remains common practice to perform prior invasive coronary angiography. However, this is associated with inherent risks, particularly in an elderly cohort with comorbidities. Additionally, coronary angiography provides no information on the functional impact of coronary stenoses, which may be important in guiding revascularization decisions prior to TAVR,” noted Dr. Michail, a cardiologist at Monash University, Melbourne.
Simulating FFR: ‘A one-stop shop cardiac CT’
Dr. Michail presented the results of the prospective CAST-FFR study, the first evaluation of CT-FFR for assessment of coronary arteries in patients with severe symptomatic aortic stenosis. This method uses computational fluid dynamics to transform data obtained noninvasively from a standard coronary CT angiography acquisition into a simulated FFR. And it offers the potential to streamline patient care.
“In current practice we see elderly patients with a long pre-TAVR assessment period, with numerous appointments and invasive procedures. Our vision is a one-stop shop cardiac CT that will provide the cardiologist with a complete assessment of the annular measurements, peripheral vasculature, and the coronary arteries ahead of their procedure,” according to Dr. Michail.
“We believe the ability to perform the requisite coronary assessment using CT-FFR will translate to improved patient care in several ways,” he continued. “Firstly, this will shorten the number of tests and overall diagnostic journey for patients. It will reduce the risk from unnecessary invasive procedures, and this will also reduce discomfort for the patient. Based on emerging evidence on the adverse prognostic impact of functionally significant coronary disease in aortic stenosis, this data has the potential to improve procedural risk stratification. And finally, contingent on further data, this may improve lesion selection for upfront revascularization.”
The CAST-FFR study was a small, single-center, proof-of-concept study in which 42 patients with severe aortic stenosis underwent both coronary CT angiography and conventional FFR with a pressure wire. The CT data was sent to a core laboratory for conversion into CT-FFR by evaluators blinded to the conventional FFR values.
Of the 42 participants, 39 (93%) had usable CT-FFR data on 60 coronary vessels. Dr. Michail and coinvestigators found a strong correlation between the conventional pressure wire FFR and CT-FFR findings, with a receiver operating characteristic area under the curve of 0.83 per vessel. CT-FFR had a diagnostic sensitivity and specificity of 73.9% and 78.4%, respectively, with a positive predictive value of 68%, a negative predictive value of 82.9%, and a diagnostic accuracy of 76.7%.
He cited as study limitations the small size, the fact that patients with previous revascularization or significant left ventricular impairment were excluded, and the study cohort’s relative youth.
“With a mean age of 76.2 years, it’s unclear whether these results can be extrapolated to very elderly patients with more calcified arteries undergoing TAVR. Encouragingly, though, a subgroup analysis based on calcium score showed no effect on accuracy,” according to the cardiologist.
CT-FFR may ‘shorten the diagnostic journey’ for fragile patients
Discussant Daniele Andreini, MD, PhD, praised the investigators’ concept of integrating the functional assessment provided by CT-FFR into a one-stop shop examination by cardiac CT angiography for TAVR planning.
“I would like to underline one of Dr. Michail’s messages: It’s really important to shorten the diagnostic journey for these fragile, older patients with aortic stenosis in order to improve safety, use less contrast, and avoid complications,” said Dr. Andreini, a cardiologist at the University of Milan and director of the cardiovascular CT and radiology unit at Monzino Cardiology Center, also in Milan.
Both Dr. Michail and Dr. Andreini reported having no financial conflicts of interest.
REPORTING FROM EUROPCR 2020
Stress-induced brain activity linked to chest pain in CAD patients
The brain’s reaction to stress may be an important contributor to chest pain in patients with coronary artery disease (CAD), according to results of a cohort study.
“Although more research is needed, these results may potentially shift the paradigm by which angina is evaluated by refocusing clinical evaluation and management of psychological stress as adjunct to traditional cardiac evaluations,” wrote Kasra Moazzami, MD, MPH, of Emory University in Atlanta, and his coauthors in Circulation: Cardiovascular Imaging.
To determine if an association exists between stress-induced frontal lobe activity and angina, the researchers launched a study of 148 patients with stable CAD. Their mean age was 62, 69% were male, and roughly 36% were Black. Angina symptoms were assessed at baseline and also after 2 years through the Seattle Angina Questionnaire’s angina frequency subscale.
As the patients underwent stress testing that included both speech and arithmetic stressors, they also received eight brain scans via high-resolution positron emission tomography (HR-PET) brain imaging. Two scans occurred during each of the two control and two stress conditions. Subsequent analysis of these images evaluated regional blood flow relative to total brain flow. Each patient also underwent myocardial perfusion imaging (MPI) at rest, under stress conditions, and during conventional stress testing.
At baseline, patients who reported experiencing angina monthly (35) or daily/weekly (19) had higher rates of mental stress–induced ischemia, more common symptoms of depression and anxiety, and more use of antidepressants and nitrates. Patients reporting angina during stress testing with MPI had higher inferior frontal lobe activation (1.43), compared with patients without active chest pain (1.19; P = 0.03). Patients reporting angina during stress testing also had fewer years of education, higher Beck Depression Inventory scores, and higher posttraumatic stress disorder (PTSD) checklist scores.
More angina correlates with more mental stress
At 2-year-follow-up, 28 (24%) of the 112 returning patients reported an increase in angina episodes. Those patients had a higher mean inferior frontal lobe activation with mental stress at baseline, compared with returning patients who reported a decrease in chest pain frequency (1.82 versus 0.92; P = .01).
After adjustment for sociodemographic and lifestyle variables, any doubling in inferior frontal lobe activation led to an increase in angina frequency by 13.7 units at baseline (95% confidence interval, 6.3-21.7; P = .008) and 11.6 units during follow-up (95% CI, 4.1-19.2; P = .01). After relative importance analysis, the most important correlate of angina was found to be inferior frontal lobe activation at 36.5%, followed by Beck Depression Inventory score and PTSD checklist score.
‘It shows that the heart and brain are connected’
“Previous studies have linked mental stress with ischemia using nuclear stress testing. This study is unique in that it looked at brain activity associated with mental stress and was able to correlate that activity with angina,” said cardiologist Nieca Goldberg, MD, of NYU Langone in New York City in an interview. “It shows that the heart and brain are connected.”
The authors acknowledged their study’s limitations, including using standard stress-inducing protocols that did not account for or reflect any real-life stressors. In addition, although their methods are still considered clinically relevant, retrospectively collecting angina symptoms via questionnaire rather than a prospective diary could have led to incomplete responses.
Dr. Goldberg noted that additional research should include a more diverse population – women in particular were underrepresented in this study – while focusing on how interventions for stress can play a role in angina symptoms and brain activity.
That said, she added, “until there are more studies, it is important to consider mental stress in assessing angina symptoms in patients.”
The study was supported by grants from the National Institutes of Health. The authors reported no potential conflicts of interest.
SOURCE: Moazzami K et al. Circ Cardiovasc Imaging. 2020 Aug 10. doi: 10.1161/circimaging.120.010710.
The brain’s reaction to stress may be an important contributor to chest pain in patients with coronary artery disease (CAD), according to results of a cohort study.
“Although more research is needed, these results may potentially shift the paradigm by which angina is evaluated by refocusing clinical evaluation and management of psychological stress as adjunct to traditional cardiac evaluations,” wrote Kasra Moazzami, MD, MPH, of Emory University in Atlanta, and his coauthors in Circulation: Cardiovascular Imaging.
To determine if an association exists between stress-induced frontal lobe activity and angina, the researchers launched a study of 148 patients with stable CAD. Their mean age was 62, 69% were male, and roughly 36% were Black. Angina symptoms were assessed at baseline and also after 2 years through the Seattle Angina Questionnaire’s angina frequency subscale.
As the patients underwent stress testing that included both speech and arithmetic stressors, they also received eight brain scans via high-resolution positron emission tomography (HR-PET) brain imaging. Two scans occurred during each of the two control and two stress conditions. Subsequent analysis of these images evaluated regional blood flow relative to total brain flow. Each patient also underwent myocardial perfusion imaging (MPI) at rest, under stress conditions, and during conventional stress testing.
At baseline, patients who reported experiencing angina monthly (35) or daily/weekly (19) had higher rates of mental stress–induced ischemia, more common symptoms of depression and anxiety, and more use of antidepressants and nitrates. Patients reporting angina during stress testing with MPI had higher inferior frontal lobe activation (1.43), compared with patients without active chest pain (1.19; P = 0.03). Patients reporting angina during stress testing also had fewer years of education, higher Beck Depression Inventory scores, and higher posttraumatic stress disorder (PTSD) checklist scores.
More angina correlates with more mental stress
At 2-year-follow-up, 28 (24%) of the 112 returning patients reported an increase in angina episodes. Those patients had a higher mean inferior frontal lobe activation with mental stress at baseline, compared with returning patients who reported a decrease in chest pain frequency (1.82 versus 0.92; P = .01).
After adjustment for sociodemographic and lifestyle variables, any doubling in inferior frontal lobe activation led to an increase in angina frequency by 13.7 units at baseline (95% confidence interval, 6.3-21.7; P = .008) and 11.6 units during follow-up (95% CI, 4.1-19.2; P = .01). After relative importance analysis, the most important correlate of angina was found to be inferior frontal lobe activation at 36.5%, followed by Beck Depression Inventory score and PTSD checklist score.
‘It shows that the heart and brain are connected’
“Previous studies have linked mental stress with ischemia using nuclear stress testing. This study is unique in that it looked at brain activity associated with mental stress and was able to correlate that activity with angina,” said cardiologist Nieca Goldberg, MD, of NYU Langone in New York City in an interview. “It shows that the heart and brain are connected.”
The authors acknowledged their study’s limitations, including using standard stress-inducing protocols that did not account for or reflect any real-life stressors. In addition, although their methods are still considered clinically relevant, retrospectively collecting angina symptoms via questionnaire rather than a prospective diary could have led to incomplete responses.
Dr. Goldberg noted that additional research should include a more diverse population – women in particular were underrepresented in this study – while focusing on how interventions for stress can play a role in angina symptoms and brain activity.
That said, she added, “until there are more studies, it is important to consider mental stress in assessing angina symptoms in patients.”
The study was supported by grants from the National Institutes of Health. The authors reported no potential conflicts of interest.
SOURCE: Moazzami K et al. Circ Cardiovasc Imaging. 2020 Aug 10. doi: 10.1161/circimaging.120.010710.
The brain’s reaction to stress may be an important contributor to chest pain in patients with coronary artery disease (CAD), according to results of a cohort study.
“Although more research is needed, these results may potentially shift the paradigm by which angina is evaluated by refocusing clinical evaluation and management of psychological stress as adjunct to traditional cardiac evaluations,” wrote Kasra Moazzami, MD, MPH, of Emory University in Atlanta, and his coauthors in Circulation: Cardiovascular Imaging.
To determine if an association exists between stress-induced frontal lobe activity and angina, the researchers launched a study of 148 patients with stable CAD. Their mean age was 62, 69% were male, and roughly 36% were Black. Angina symptoms were assessed at baseline and also after 2 years through the Seattle Angina Questionnaire’s angina frequency subscale.
As the patients underwent stress testing that included both speech and arithmetic stressors, they also received eight brain scans via high-resolution positron emission tomography (HR-PET) brain imaging. Two scans occurred during each of the two control and two stress conditions. Subsequent analysis of these images evaluated regional blood flow relative to total brain flow. Each patient also underwent myocardial perfusion imaging (MPI) at rest, under stress conditions, and during conventional stress testing.
At baseline, patients who reported experiencing angina monthly (35) or daily/weekly (19) had higher rates of mental stress–induced ischemia, more common symptoms of depression and anxiety, and more use of antidepressants and nitrates. Patients reporting angina during stress testing with MPI had higher inferior frontal lobe activation (1.43), compared with patients without active chest pain (1.19; P = 0.03). Patients reporting angina during stress testing also had fewer years of education, higher Beck Depression Inventory scores, and higher posttraumatic stress disorder (PTSD) checklist scores.
More angina correlates with more mental stress
At 2-year-follow-up, 28 (24%) of the 112 returning patients reported an increase in angina episodes. Those patients had a higher mean inferior frontal lobe activation with mental stress at baseline, compared with returning patients who reported a decrease in chest pain frequency (1.82 versus 0.92; P = .01).
After adjustment for sociodemographic and lifestyle variables, any doubling in inferior frontal lobe activation led to an increase in angina frequency by 13.7 units at baseline (95% confidence interval, 6.3-21.7; P = .008) and 11.6 units during follow-up (95% CI, 4.1-19.2; P = .01). After relative importance analysis, the most important correlate of angina was found to be inferior frontal lobe activation at 36.5%, followed by Beck Depression Inventory score and PTSD checklist score.
‘It shows that the heart and brain are connected’
“Previous studies have linked mental stress with ischemia using nuclear stress testing. This study is unique in that it looked at brain activity associated with mental stress and was able to correlate that activity with angina,” said cardiologist Nieca Goldberg, MD, of NYU Langone in New York City in an interview. “It shows that the heart and brain are connected.”
The authors acknowledged their study’s limitations, including using standard stress-inducing protocols that did not account for or reflect any real-life stressors. In addition, although their methods are still considered clinically relevant, retrospectively collecting angina symptoms via questionnaire rather than a prospective diary could have led to incomplete responses.
Dr. Goldberg noted that additional research should include a more diverse population – women in particular were underrepresented in this study – while focusing on how interventions for stress can play a role in angina symptoms and brain activity.
That said, she added, “until there are more studies, it is important to consider mental stress in assessing angina symptoms in patients.”
The study was supported by grants from the National Institutes of Health. The authors reported no potential conflicts of interest.
SOURCE: Moazzami K et al. Circ Cardiovasc Imaging. 2020 Aug 10. doi: 10.1161/circimaging.120.010710.
FROM CIRCULATION: CARDIOVASCULAR IMAGING
Ultrasound, cardiac CT valuable in COVID-19 assessment
As if the management of patients with severe COVID-19 infections is not complicated enough, an estimated 50%-60% of patients admitted to an ICU with the disease will have some form of cardiovascular involvement, which further increases their already high risk for morbidity and mortality.
Multimodality cardiovascular imaging, chosen wisely, can both help to direct management of cardiovascular complications associated with COVID-19 and lessen risk of exposure of health care workers to SARS-CoV-2, said members of an expert panel from the American College of Cardiology Cardiovascular Imaging Leadership Council.
“When we face a patient with known or suspected COVID-19, it’s not like any other disease because we face potential exposure risk to personnel doing imaging studies and also to other patients,” corresponding author Marcelo F. Di Carli, MD, of Brigham and Women’s Hospital Boston said in an interview.
“Any imaging study that is being considered should be performed only if we think it will help us make a change in the way that we’re going to treat that particular patient. This is true for imaging in any disease – why would you do an imaging study that will make no difference in treatment? – but the stakes are even higher in COVID-19,” he said.
The panel’s recommendations for cardiovascular imaging in patients with COVID-19 are outlined in a guidance document published online in the Journal of the American College of Cardiology.
Testing and biomarkers
The guidance begins by highlighting the importance of diagnostic testing for COVID-19 infection and the use of universal precautions for health care personnel performing imaging studies, as well as disinfection of imaging equipment and rooms after each use.
Circulating biomarkers that measure end-organ stress or injury, inflammation, hypoperfusion, and activation of thrombosis/hemostasis pathways may be prognostically useful, but “almost none of the widely measured biomarkers represent a specific trigger for imaging outside of that supported by clinical judgment,” the guidance states.
In contrast, low to moderate, nonrising concentrations of markers for myocardial stress, such as B-type natriuretic peptide (BNP) and N-terminal pro-BNP (NT-proBNP), or of myocardial injury, such as cardiac troponins (cTn), may be helpful for excluding the need for imaging.
“Importantly, clinicians should be aware that most patients with abnormal BNP/NT-proBNP or cTn do not have acute heart failure or myocardial infarction; and rise in concentration of either class of biomarker presumably reflects complex processes including direct myocardial stress/injury related to systemic illness,” the panel members wrote.
Oldies but goodies
“One thing that we found out in our review of the literature and in our experiences in our own work settings is that cardiac ultrasound plays a huge role in this disease – like in any disease – but this one in particular,” Dr. Di Carli said. “One of the most feared complications in COVID-19 leads to inflammation of the heart muscle, which then leads to heart dysfunction. And of course cardiac ultrasound, because of its portability, can be performed at bedside to help clinicians ascertain an abnormality in the heart.”
Cardiac CT is also extremely helpful for determining whether patients with ECG findings suggestive of infarction have suffered an actual thrombotic event.
“These patients may best be served by a noninvasive study as compared to an invasive coronary angiogram,” he said.
Clinical scenarios
Cardiologists may be called in to consult on the evaluation of possible cardiogenic components of pulmonary abnormalities in patients who present with dyspnea and chest x-rays showing airspace or interstitial infiltrates suggestive of pneumonia, the authors noted.
“Clinicians will rely on history, physical exam, ECG [electrocardiogram] and biomarkers, and recent cardiac imaging tests if available. Underlying cardiac history including [coronary artery disease], cardiomyopathy, heart failure, and arrhythmia should be sought, and frequent contributors to decompensation should be eliminated,” they wrote.
For patients with suspected cardiac injury, either point-of-care ultrasound or limited echocardiography can be used for the initial evaluation, with additional, more advanced technologies called into play for specific clinical scenarios outlined in the guidance.
For example, the guidance recommends that patients with chest pain and abnormal ECG readings with clinical concern for ST-elevation acute coronary syndrome or high clinical risk for in-hospital mortality from conditions such as cardiogenic shock, dynamic ST-segment changes, or left ventricular ejection fraction less than 40% thought to be caused by non–ST-elevation myocardial infarction be referred for emergent coronary angiography and reperfusion.
In contrast, in patients with chest pain and abnormal ECG but equivocal symptoms, atypical or equivocal ECG abnormalities, or late presentations, point-of-care ultrasound or limited echocardiogram could be used to look for regional wall motion abnormalities and left ventricular ejection fraction, whereas in patients with chest pain and ST-elevation without clear evidence of ST-elevation myocardial infarction, coronary CT angiography can help to rule out ACS and point to alternate diagnoses, the authors said.
The guidance also offers recommendations for imaging in patients with hemodynamic instability (shock or hypotension), patients with new left ventricular dysfunction in the absence of shock or hypotension, and patients with subacute and chronic-phase disease.
Development of the guidance document was supported by the ACC. Dr. Di Carli disclosed institutional grant support from Gilead Sciences and Spectrum Dynamics, and consulting income from Janssen and Bayer.
SOURCE: Rudski L et al. J Am Coll Cardiol. 2020 Jul 22. doi: 10.1016/j.jacc.2020.06.080.
As if the management of patients with severe COVID-19 infections is not complicated enough, an estimated 50%-60% of patients admitted to an ICU with the disease will have some form of cardiovascular involvement, which further increases their already high risk for morbidity and mortality.
Multimodality cardiovascular imaging, chosen wisely, can both help to direct management of cardiovascular complications associated with COVID-19 and lessen risk of exposure of health care workers to SARS-CoV-2, said members of an expert panel from the American College of Cardiology Cardiovascular Imaging Leadership Council.
“When we face a patient with known or suspected COVID-19, it’s not like any other disease because we face potential exposure risk to personnel doing imaging studies and also to other patients,” corresponding author Marcelo F. Di Carli, MD, of Brigham and Women’s Hospital Boston said in an interview.
“Any imaging study that is being considered should be performed only if we think it will help us make a change in the way that we’re going to treat that particular patient. This is true for imaging in any disease – why would you do an imaging study that will make no difference in treatment? – but the stakes are even higher in COVID-19,” he said.
The panel’s recommendations for cardiovascular imaging in patients with COVID-19 are outlined in a guidance document published online in the Journal of the American College of Cardiology.
Testing and biomarkers
The guidance begins by highlighting the importance of diagnostic testing for COVID-19 infection and the use of universal precautions for health care personnel performing imaging studies, as well as disinfection of imaging equipment and rooms after each use.
Circulating biomarkers that measure end-organ stress or injury, inflammation, hypoperfusion, and activation of thrombosis/hemostasis pathways may be prognostically useful, but “almost none of the widely measured biomarkers represent a specific trigger for imaging outside of that supported by clinical judgment,” the guidance states.
In contrast, low to moderate, nonrising concentrations of markers for myocardial stress, such as B-type natriuretic peptide (BNP) and N-terminal pro-BNP (NT-proBNP), or of myocardial injury, such as cardiac troponins (cTn), may be helpful for excluding the need for imaging.
“Importantly, clinicians should be aware that most patients with abnormal BNP/NT-proBNP or cTn do not have acute heart failure or myocardial infarction; and rise in concentration of either class of biomarker presumably reflects complex processes including direct myocardial stress/injury related to systemic illness,” the panel members wrote.
Oldies but goodies
“One thing that we found out in our review of the literature and in our experiences in our own work settings is that cardiac ultrasound plays a huge role in this disease – like in any disease – but this one in particular,” Dr. Di Carli said. “One of the most feared complications in COVID-19 leads to inflammation of the heart muscle, which then leads to heart dysfunction. And of course cardiac ultrasound, because of its portability, can be performed at bedside to help clinicians ascertain an abnormality in the heart.”
Cardiac CT is also extremely helpful for determining whether patients with ECG findings suggestive of infarction have suffered an actual thrombotic event.
“These patients may best be served by a noninvasive study as compared to an invasive coronary angiogram,” he said.
Clinical scenarios
Cardiologists may be called in to consult on the evaluation of possible cardiogenic components of pulmonary abnormalities in patients who present with dyspnea and chest x-rays showing airspace or interstitial infiltrates suggestive of pneumonia, the authors noted.
“Clinicians will rely on history, physical exam, ECG [electrocardiogram] and biomarkers, and recent cardiac imaging tests if available. Underlying cardiac history including [coronary artery disease], cardiomyopathy, heart failure, and arrhythmia should be sought, and frequent contributors to decompensation should be eliminated,” they wrote.
For patients with suspected cardiac injury, either point-of-care ultrasound or limited echocardiography can be used for the initial evaluation, with additional, more advanced technologies called into play for specific clinical scenarios outlined in the guidance.
For example, the guidance recommends that patients with chest pain and abnormal ECG readings with clinical concern for ST-elevation acute coronary syndrome or high clinical risk for in-hospital mortality from conditions such as cardiogenic shock, dynamic ST-segment changes, or left ventricular ejection fraction less than 40% thought to be caused by non–ST-elevation myocardial infarction be referred for emergent coronary angiography and reperfusion.
In contrast, in patients with chest pain and abnormal ECG but equivocal symptoms, atypical or equivocal ECG abnormalities, or late presentations, point-of-care ultrasound or limited echocardiogram could be used to look for regional wall motion abnormalities and left ventricular ejection fraction, whereas in patients with chest pain and ST-elevation without clear evidence of ST-elevation myocardial infarction, coronary CT angiography can help to rule out ACS and point to alternate diagnoses, the authors said.
The guidance also offers recommendations for imaging in patients with hemodynamic instability (shock or hypotension), patients with new left ventricular dysfunction in the absence of shock or hypotension, and patients with subacute and chronic-phase disease.
Development of the guidance document was supported by the ACC. Dr. Di Carli disclosed institutional grant support from Gilead Sciences and Spectrum Dynamics, and consulting income from Janssen and Bayer.
SOURCE: Rudski L et al. J Am Coll Cardiol. 2020 Jul 22. doi: 10.1016/j.jacc.2020.06.080.
As if the management of patients with severe COVID-19 infections is not complicated enough, an estimated 50%-60% of patients admitted to an ICU with the disease will have some form of cardiovascular involvement, which further increases their already high risk for morbidity and mortality.
Multimodality cardiovascular imaging, chosen wisely, can both help to direct management of cardiovascular complications associated with COVID-19 and lessen risk of exposure of health care workers to SARS-CoV-2, said members of an expert panel from the American College of Cardiology Cardiovascular Imaging Leadership Council.
“When we face a patient with known or suspected COVID-19, it’s not like any other disease because we face potential exposure risk to personnel doing imaging studies and also to other patients,” corresponding author Marcelo F. Di Carli, MD, of Brigham and Women’s Hospital Boston said in an interview.
“Any imaging study that is being considered should be performed only if we think it will help us make a change in the way that we’re going to treat that particular patient. This is true for imaging in any disease – why would you do an imaging study that will make no difference in treatment? – but the stakes are even higher in COVID-19,” he said.
The panel’s recommendations for cardiovascular imaging in patients with COVID-19 are outlined in a guidance document published online in the Journal of the American College of Cardiology.
Testing and biomarkers
The guidance begins by highlighting the importance of diagnostic testing for COVID-19 infection and the use of universal precautions for health care personnel performing imaging studies, as well as disinfection of imaging equipment and rooms after each use.
Circulating biomarkers that measure end-organ stress or injury, inflammation, hypoperfusion, and activation of thrombosis/hemostasis pathways may be prognostically useful, but “almost none of the widely measured biomarkers represent a specific trigger for imaging outside of that supported by clinical judgment,” the guidance states.
In contrast, low to moderate, nonrising concentrations of markers for myocardial stress, such as B-type natriuretic peptide (BNP) and N-terminal pro-BNP (NT-proBNP), or of myocardial injury, such as cardiac troponins (cTn), may be helpful for excluding the need for imaging.
“Importantly, clinicians should be aware that most patients with abnormal BNP/NT-proBNP or cTn do not have acute heart failure or myocardial infarction; and rise in concentration of either class of biomarker presumably reflects complex processes including direct myocardial stress/injury related to systemic illness,” the panel members wrote.
Oldies but goodies
“One thing that we found out in our review of the literature and in our experiences in our own work settings is that cardiac ultrasound plays a huge role in this disease – like in any disease – but this one in particular,” Dr. Di Carli said. “One of the most feared complications in COVID-19 leads to inflammation of the heart muscle, which then leads to heart dysfunction. And of course cardiac ultrasound, because of its portability, can be performed at bedside to help clinicians ascertain an abnormality in the heart.”
Cardiac CT is also extremely helpful for determining whether patients with ECG findings suggestive of infarction have suffered an actual thrombotic event.
“These patients may best be served by a noninvasive study as compared to an invasive coronary angiogram,” he said.
Clinical scenarios
Cardiologists may be called in to consult on the evaluation of possible cardiogenic components of pulmonary abnormalities in patients who present with dyspnea and chest x-rays showing airspace or interstitial infiltrates suggestive of pneumonia, the authors noted.
“Clinicians will rely on history, physical exam, ECG [electrocardiogram] and biomarkers, and recent cardiac imaging tests if available. Underlying cardiac history including [coronary artery disease], cardiomyopathy, heart failure, and arrhythmia should be sought, and frequent contributors to decompensation should be eliminated,” they wrote.
For patients with suspected cardiac injury, either point-of-care ultrasound or limited echocardiography can be used for the initial evaluation, with additional, more advanced technologies called into play for specific clinical scenarios outlined in the guidance.
For example, the guidance recommends that patients with chest pain and abnormal ECG readings with clinical concern for ST-elevation acute coronary syndrome or high clinical risk for in-hospital mortality from conditions such as cardiogenic shock, dynamic ST-segment changes, or left ventricular ejection fraction less than 40% thought to be caused by non–ST-elevation myocardial infarction be referred for emergent coronary angiography and reperfusion.
In contrast, in patients with chest pain and abnormal ECG but equivocal symptoms, atypical or equivocal ECG abnormalities, or late presentations, point-of-care ultrasound or limited echocardiogram could be used to look for regional wall motion abnormalities and left ventricular ejection fraction, whereas in patients with chest pain and ST-elevation without clear evidence of ST-elevation myocardial infarction, coronary CT angiography can help to rule out ACS and point to alternate diagnoses, the authors said.
The guidance also offers recommendations for imaging in patients with hemodynamic instability (shock or hypotension), patients with new left ventricular dysfunction in the absence of shock or hypotension, and patients with subacute and chronic-phase disease.
Development of the guidance document was supported by the ACC. Dr. Di Carli disclosed institutional grant support from Gilead Sciences and Spectrum Dynamics, and consulting income from Janssen and Bayer.
SOURCE: Rudski L et al. J Am Coll Cardiol. 2020 Jul 22. doi: 10.1016/j.jacc.2020.06.080.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Internists’ use of ultrasound can reduce radiology referrals
researchers say.
“It’s a safe and very useful tool,” Marco Barchiesi, MD, an internal medicine resident at Luigi Sacco Hospital in Milan, said in an interview. “We had a great reduction in chest x-rays because of the use of ultrasound.”
The finding addresses concerns that ultrasound used in primary care could consume more health care resources or put patients at risk.
Dr. Barchiesi and colleagues published their findings July 20 in the European Journal of Internal Medicine.
Point-of-care ultrasound has become increasingly common as miniaturization of devices has made them more portable. The approach has caught on particularly in emergency departments where quick decisions are of the essence.
Its use in internal medicine has been more controversial, with concerns raised that improperly trained practitioners may miss diagnoses or refer patients for unnecessary tests as a result of uncertainty about their findings.
To measure the effect of point-of-care ultrasound in an internal medicine hospital ward, Dr. Barchiesi and colleagues alternated months when point-of-care ultrasound was allowed with months when it was not allowed, for a total of 4 months each, on an internal medicine unit. They allowed the ultrasound to be used for invasive procedures and excluded patients whose critical condition made point-of-care ultrasound crucial.
The researchers analyzed data on 263 patients in the “on” months when point-of-care ultrasound was used, and 255 in the “off” months when it wasn’t used. The two groups were well balanced in age, sex, comorbidity, and clinical impairment.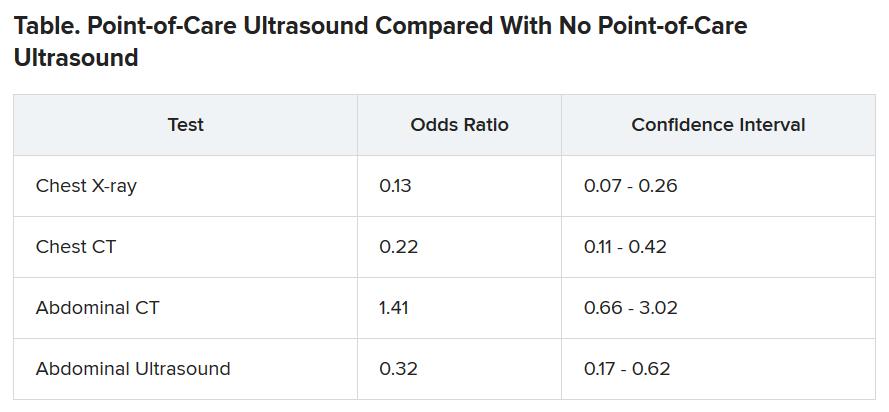
During the on months, the internists ordered 113 diagnostic tests (0.43 per patient). During the off months they ordered 329 tests (1.29 per patient).
The odds of being referred for a chest x-ray were 87% less in the “on” months, compared with the off months, a statistically significant finding (P < .001). The risk for a chest CT scan and abdominal ultrasound were also reduced during the on months, but the risk for an abdominal CT was increased.
Nineteen patients died during the o” months and 10 during the off months, a difference that was not statistically significant (P = .15). The median length of stay in the hospital was almost the same for the two groups: 9 days for the on months and 9 days for the off months. The difference was also not statistically significant (P = .094).
Point-of-care ultrasound is particularly accurate in identifying cardiac abnormalities and pleural fluid and pneumonia, and it can be used effectively for monitoring heart conditions, the researchers wrote. This could explain the reduction in chest x-rays and CT scans.
On the other hand, ultrasound cannot address such questions as staging in an abdominal malignancy, and unexpected findings are more common with abdominal than chest ultrasound. This could explain why the point-of-care ultrasound did not reduce the use of abdominal CT, the researchers speculated.
They acknowledged that the patients in their sample had an average age of 81 years, raising questions about how well their data could be applied to a younger population. And they noted that they used point-of-care ultrasound frequently, so they were particularly adept with it. “We use it almost every day in our clinical practice,” said Dr. Barchiesi.
Those factors may have played a key role in the success of point-of-care ultrasound in this study, said Michael Wagner, MD, an assistant professor of medicine at the University of South Carolina, Greenville, who has helped colleagues incorporate ultrasound into their practices.
Elderly patients often present with multiple comorbidities and atypical signs and symptoms, he said. “Sometimes they can be very confusing as to the underlying clinical picture. Ultrasound is being used frequently to better assess these complicated patients.”
Dr. Wagner said extensive training is required to use point-of-care ultrasound accurately.
Dr. Barchiesi also acknowledged that the devices used in this study were large portable machines, not the simpler and less expensive hand-held versions that are also available for similar purposes.
Point-of-care ultrasound is a promising innovation, said Thomas Melgar, MD, a professor of medicine at Western Michigan University, Kalamazoo. “The advantage is that the exam is being done by someone who knows the patient and specifically what they’re looking for. It’s done at the bedside so you don’t have to move the patient.”
The study could help address opposition to internal medicine residents being trained in the technique, he said, adding that “I think it’s very exciting.”
The study was partially supported by Philips, which provided the ultrasound devices. Dr. Barchiesi, Dr. Melgar, and Dr. Wagner disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
researchers say.
“It’s a safe and very useful tool,” Marco Barchiesi, MD, an internal medicine resident at Luigi Sacco Hospital in Milan, said in an interview. “We had a great reduction in chest x-rays because of the use of ultrasound.”
The finding addresses concerns that ultrasound used in primary care could consume more health care resources or put patients at risk.
Dr. Barchiesi and colleagues published their findings July 20 in the European Journal of Internal Medicine.
Point-of-care ultrasound has become increasingly common as miniaturization of devices has made them more portable. The approach has caught on particularly in emergency departments where quick decisions are of the essence.
Its use in internal medicine has been more controversial, with concerns raised that improperly trained practitioners may miss diagnoses or refer patients for unnecessary tests as a result of uncertainty about their findings.
To measure the effect of point-of-care ultrasound in an internal medicine hospital ward, Dr. Barchiesi and colleagues alternated months when point-of-care ultrasound was allowed with months when it was not allowed, for a total of 4 months each, on an internal medicine unit. They allowed the ultrasound to be used for invasive procedures and excluded patients whose critical condition made point-of-care ultrasound crucial.
The researchers analyzed data on 263 patients in the “on” months when point-of-care ultrasound was used, and 255 in the “off” months when it wasn’t used. The two groups were well balanced in age, sex, comorbidity, and clinical impairment.
During the on months, the internists ordered 113 diagnostic tests (0.43 per patient). During the off months they ordered 329 tests (1.29 per patient).
The odds of being referred for a chest x-ray were 87% less in the “on” months, compared with the off months, a statistically significant finding (P < .001). The risk for a chest CT scan and abdominal ultrasound were also reduced during the on months, but the risk for an abdominal CT was increased.
Nineteen patients died during the o” months and 10 during the off months, a difference that was not statistically significant (P = .15). The median length of stay in the hospital was almost the same for the two groups: 9 days for the on months and 9 days for the off months. The difference was also not statistically significant (P = .094).
Point-of-care ultrasound is particularly accurate in identifying cardiac abnormalities and pleural fluid and pneumonia, and it can be used effectively for monitoring heart conditions, the researchers wrote. This could explain the reduction in chest x-rays and CT scans.
On the other hand, ultrasound cannot address such questions as staging in an abdominal malignancy, and unexpected findings are more common with abdominal than chest ultrasound. This could explain why the point-of-care ultrasound did not reduce the use of abdominal CT, the researchers speculated.
They acknowledged that the patients in their sample had an average age of 81 years, raising questions about how well their data could be applied to a younger population. And they noted that they used point-of-care ultrasound frequently, so they were particularly adept with it. “We use it almost every day in our clinical practice,” said Dr. Barchiesi.
Those factors may have played a key role in the success of point-of-care ultrasound in this study, said Michael Wagner, MD, an assistant professor of medicine at the University of South Carolina, Greenville, who has helped colleagues incorporate ultrasound into their practices.
Elderly patients often present with multiple comorbidities and atypical signs and symptoms, he said. “Sometimes they can be very confusing as to the underlying clinical picture. Ultrasound is being used frequently to better assess these complicated patients.”
Dr. Wagner said extensive training is required to use point-of-care ultrasound accurately.
Dr. Barchiesi also acknowledged that the devices used in this study were large portable machines, not the simpler and less expensive hand-held versions that are also available for similar purposes.
Point-of-care ultrasound is a promising innovation, said Thomas Melgar, MD, a professor of medicine at Western Michigan University, Kalamazoo. “The advantage is that the exam is being done by someone who knows the patient and specifically what they’re looking for. It’s done at the bedside so you don’t have to move the patient.”
The study could help address opposition to internal medicine residents being trained in the technique, he said, adding that “I think it’s very exciting.”
The study was partially supported by Philips, which provided the ultrasound devices. Dr. Barchiesi, Dr. Melgar, and Dr. Wagner disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
researchers say.
“It’s a safe and very useful tool,” Marco Barchiesi, MD, an internal medicine resident at Luigi Sacco Hospital in Milan, said in an interview. “We had a great reduction in chest x-rays because of the use of ultrasound.”
The finding addresses concerns that ultrasound used in primary care could consume more health care resources or put patients at risk.
Dr. Barchiesi and colleagues published their findings July 20 in the European Journal of Internal Medicine.
Point-of-care ultrasound has become increasingly common as miniaturization of devices has made them more portable. The approach has caught on particularly in emergency departments where quick decisions are of the essence.
Its use in internal medicine has been more controversial, with concerns raised that improperly trained practitioners may miss diagnoses or refer patients for unnecessary tests as a result of uncertainty about their findings.
To measure the effect of point-of-care ultrasound in an internal medicine hospital ward, Dr. Barchiesi and colleagues alternated months when point-of-care ultrasound was allowed with months when it was not allowed, for a total of 4 months each, on an internal medicine unit. They allowed the ultrasound to be used for invasive procedures and excluded patients whose critical condition made point-of-care ultrasound crucial.
The researchers analyzed data on 263 patients in the “on” months when point-of-care ultrasound was used, and 255 in the “off” months when it wasn’t used. The two groups were well balanced in age, sex, comorbidity, and clinical impairment.
During the on months, the internists ordered 113 diagnostic tests (0.43 per patient). During the off months they ordered 329 tests (1.29 per patient).
The odds of being referred for a chest x-ray were 87% less in the “on” months, compared with the off months, a statistically significant finding (P < .001). The risk for a chest CT scan and abdominal ultrasound were also reduced during the on months, but the risk for an abdominal CT was increased.
Nineteen patients died during the o” months and 10 during the off months, a difference that was not statistically significant (P = .15). The median length of stay in the hospital was almost the same for the two groups: 9 days for the on months and 9 days for the off months. The difference was also not statistically significant (P = .094).
Point-of-care ultrasound is particularly accurate in identifying cardiac abnormalities and pleural fluid and pneumonia, and it can be used effectively for monitoring heart conditions, the researchers wrote. This could explain the reduction in chest x-rays and CT scans.
On the other hand, ultrasound cannot address such questions as staging in an abdominal malignancy, and unexpected findings are more common with abdominal than chest ultrasound. This could explain why the point-of-care ultrasound did not reduce the use of abdominal CT, the researchers speculated.
They acknowledged that the patients in their sample had an average age of 81 years, raising questions about how well their data could be applied to a younger population. And they noted that they used point-of-care ultrasound frequently, so they were particularly adept with it. “We use it almost every day in our clinical practice,” said Dr. Barchiesi.
Those factors may have played a key role in the success of point-of-care ultrasound in this study, said Michael Wagner, MD, an assistant professor of medicine at the University of South Carolina, Greenville, who has helped colleagues incorporate ultrasound into their practices.
Elderly patients often present with multiple comorbidities and atypical signs and symptoms, he said. “Sometimes they can be very confusing as to the underlying clinical picture. Ultrasound is being used frequently to better assess these complicated patients.”
Dr. Wagner said extensive training is required to use point-of-care ultrasound accurately.
Dr. Barchiesi also acknowledged that the devices used in this study were large portable machines, not the simpler and less expensive hand-held versions that are also available for similar purposes.
Point-of-care ultrasound is a promising innovation, said Thomas Melgar, MD, a professor of medicine at Western Michigan University, Kalamazoo. “The advantage is that the exam is being done by someone who knows the patient and specifically what they’re looking for. It’s done at the bedside so you don’t have to move the patient.”
The study could help address opposition to internal medicine residents being trained in the technique, he said, adding that “I think it’s very exciting.”
The study was partially supported by Philips, which provided the ultrasound devices. Dr. Barchiesi, Dr. Melgar, and Dr. Wagner disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.