User login
Trends in Intravenous Magnesium Use and Outcomes for Status Asthmaticus in Children’s Hospitals from 2010 to 2017
For severe asthma exacerbations unresponsive to initial treatment, expert consensus guidelines from 2007 recommend consideration for adjunct treatments (magnesium or heliox) to decrease the likelihood of intubation.1 Over the last decade, data have emerged suggesting that intravenous (IV) magnesium may be more effective for reduction of hospital admission rates.2 Pooled meta-analyses have demonstrated improved pulmonary function and reduction of hospital admission by as much as 68% in children when IV magnesium is administered in the emergency department (ED), although the evidence is extremely limited because of a small number of studies (three) and small sample size (115 children).2-5
Though these data suggest that use of IV magnesium may reduce admission rates, a study of pediatric emergency medicine (PEM) physicians in US and Canada reported reluctance regarding use for this purpose. While PEM physicians reported awareness of the literature on admission prevention, they estimated that fewer than 5% of their patients receiving IV magnesium were discharged home.6 Their practice was generally limited to using IV magnesium in children with impending respiratory failure for the purpose of reducing intensive care unit (ICU) admission and not hospitalization.6 PEM physicians’ reluctance to use IV magnesium was related to the lack of strong available evidence supporting the impact of IV magnesium on outcomes, such as admission, and gaps in the literature about its dosing and safety profile.
The goal of this study was to assess the prevailing trends in IV magnesium use across US children’s hospitals and to assess the relationship of IV magnesium use to admission rate, length of stay (LOS), readmission rate, and ICU admission rate. We hypothesized that IV magnesium use might have increased following publication of studies demonstrating an association between IV magnesium use and fewer admissions.
METHODS
Study Design, Setting, and Participants
This is a retrospective cohort study of asthma (All Patient Refined Diagnosis Related Group 141) hospitalizations for patients less than 18 years old presenting to 35 tertiary care children’s hospitals from January 1, 2010, to December 31, 2017, included in the Pediatric Health Information System (PHIS; Children’s Hospital Association, Lenexa, Kansas) database. The PHIS database is an administrative database that contains demographics, International Classification of Diseases 9th and 10th Revision diagnoses and procedures, and daily billing records for all inpatient, observation, ED, and ambulatory surgery encounters. All data were deidentified prior to inclusion in the database and tracking of patients across ED and inpatient visits was achieved through an encrypted and unique patient identifier. Children transferred from other hospitals were excluded because we could not verify IV magnesium use prior to transfer. For hospitals to be included, they were required to provide continuous data throughout the study period.
Main Outcome Measure
The main outcome was exposure to IV magnesium as determined by billing information available in the PHIS database.
Patient Demographics
We assessed patients’ demographic characteristics, including age (younger than 5 years, 5-11 years, and 12-17 years), sex, race/ethnicity, and insurance status.
Healthcare Utilization and Hospital Characteristics
We assessed healthcare utilization using geometric mean LOS, proportion of patients admitted to the hospital and to the ICU, and the proportion of patients with a 7-day all-cause readmission. In addition, we divided hospitals into three equal groups based on their annual inpatient asthma volume (<300, 300-850, >850 cases per year).
Statistical Analysis
We compared demographic and clinical characteristics across patients receiving IV magnesium with those who did not receive it with use of chi-square tests for categorical variables and Wilcoxon rank sum test for continuous variables. We calculated annual IV magnesium use rates for each hospital and modeled the average annual rate with a general linear model in order to assess change over time. We used Pearson product moment correlation to compare the annual proportion of magnesium use and healthcare utilization measures, including geometric mean LOS, the proportion of patients using the inpatient wards or the ICU, and the proportion of cases with a 7-day all-cause readmission. Geometric mean LOS was used to normalize the compounding effect of non–normally distributed arithmetic mean LOS. A sensitivity analysis was performed stratifying IV magnesium use over time by hospital inpatient volume. Data were analyzed using SAS version 9.4 (SAS Institute, Cary, North Carolina), and P values < .05 were considered statistically significant.
RESULTS
Study Population
A total of 878,188 encounters with acute asthma exacerbation met the inclusion criteria, with 65,558 (7.5%) receiving IV magnesium (Table). Of those receiving IV magnesium, 90% were admitted to the hospital. There were statistically significant differences in IV magnesium use when compared by age, race/ethnicity, and payer type, but not gender. IV magnesium use was significantly associated with older age (more than 5 years old), non-Hispanic black race, ED visit in the year prior to admission, longer hospital LOS, and higher ICU admission rate.

Trends in Intravenous Magnesium Use
IV magnesium use among hospitalized children more than doubled from 2010 to 2017 (17% vs 36%). Low-volume hospitals had a lower frequency of IV magnesium use, compared with the moderate- and high-volume hospitals. The growth rate per year of IV magnesium use was greater in high- and moderate-volume hospitals (3.4% and 2.9% per year, respectively), compared with the low-volume hospitals (1.2% per year; P = .04).
Trends in Intravenous Magnesium Use and Hospital Outcomes
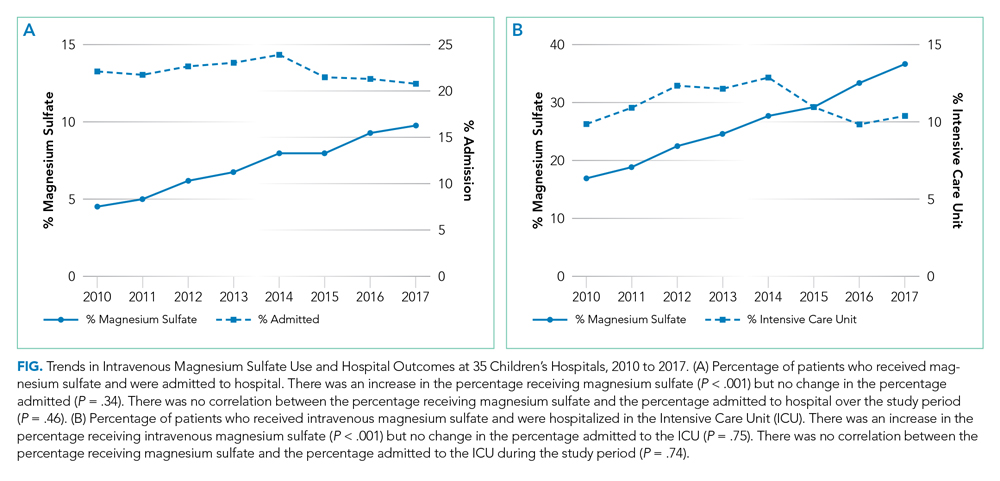
The trend in IV magnesium use was not associated with a statistically significant change in the inpatient and ICU admission rate or in the 7-day all-cause readmission rate (Figure and Appendix Figure). Although IV magnesium use increased over time, LOS decreased significantly during the same period (1.6 days in 2010 vs 1.4 days in 2017; P < .001). When analyzed by hospital volume, no significant associations were found in the inpatient admission, ICU admission, and 7-day readmission rate.
DISCUSSION
The use of IV magnesium has significantly increased in US children’s hospitals over the last 8 years, especially among those hospitalized following an ED evaluation. Over this interval, trends in inpatient and ICU admission rate, as well as 7-day all-cause readmission rate, for asthma did not change, while LOS decreased. These findings contrast with a recent Cochrane review that summarized the efficacy of IV magnesium for reducing admission rate in few small trials.2
Our study findings are more consistent with prior survey findings that IV magnesium does not reduce hospitalization and that ED physicians tend to use IV magnesium in severe asthma exacerbation for its potential therapeutic benefits because of bronchodilator and anti-inflammatory effect.6,7 Similar to PEM physicians’ estimates, only 10% of patients receiving IV magnesium were discharged home in our study.
IV magnesium use is higher in high-volume hospitals than in moderate- and low-volume ones. One potential explanation is that high- and moderate-volume hospitals may see a higher volume of children with severe or impending respiratory failure and, therefore, are more likely to use IV magnesium than the low-volume hospitals are. Alternatively, physician adoption of magnesium use for lower-acuity asthma exacerbations could vary by hospital volume.
Trend analyses of outcomes suggest that increase in IV magnesium use was not associated with an increase in inpatient and ICU admission rate or with 7-day all-cause readmission rate, although LOS reduced. LOS might be reduced because of various quality improvement initiatives (eg, discharging patients after every 3 hours albuterol treatments or respiratory therapist–driven protocols) and might not be related to IV magnesium use.8,9 To this point, a recent study of a respiratory assessment score–matched cohort did not find any therapeutic benefit of IV magnesium with severe asthma exacerbation when receiving continuous albuterol therapy on a pediatric ward.5 Perhaps future studies could explore estimating the outcome by performing comparative effectiveness studies between those with severe asthma exacerbation who did or did not receive IV magnesium. Additionally, randomized controlled trials comparing IV magnesium and standard therapy and its effects on outcomes, such as hospitalization, LOS, association with asthma chronicity, and previous oral steroid use, might provide further insight to inform clinical practice.
Certain study limitations should be noted. The study cohort included children’s hospitals only, and it is possible that care at nonchildren’s hospitals for asthma differs. PHIS dataset used in this study does not allow determination of where and when IV magnesium was given, the severity of asthma exacerbation, or the chronicity of baseline disease. Moreover, PHIS hospitals include centers in large cities, and other competing children’s hospitals may provide other tertiary care that could affect the readmission data calculation. Lastly, the temporal associations between IV magnesium use and outcomes reported in this study should not be used as evidence or lack of evidence for the effectiveness of magnesium given the limitations of the observational study design and dataset used.
In conclusion, IV magnesium use in management of asthma exacerbation in children across the United States has significantly increased. The increase occurred disproportionately in high-volume hospitals and was not associated with changes in admission rate, ICU admission rate, or 7-day all-cause readmission rate, although LOS has decreased over time.
Disclosures
The authors have no financial relationships relevant to this article or conflicts of interest to disclose.
This paper was a platform presentation at annual meetings of Pediatric Academic Societies 2019; accepted for presentation at annual meeting of Pediatric Hospital Medicine, July 2019.
Funding Source
No funding was secured for this study.
1. National Asthma Education and Prevention Program, Third Expert Panel on the Diagnosis and Management of Asthma. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, Maryland: National Heart, Lung, and Blood Institute; 2007. https://www.ncbi.nlm.nih.gov/books/NBK7232/.
2. Griffiths B, Kew KM. Intravenous magnesium sulfate for treating children with acute asthma in the emergency department. Cochrane Database Syst Rev. 2016;4(4):CD011050. https://doi.org/10.1002/14651858.CD011050.pub2.
3. Shan Z, Rong Y, Yang W, et al. Intravenous and nebulized magnesium sulfate for treating acute asthma in adults and children: a systematic review and meta-analysis. Respir Med. 2013;107(3):321-330. https://doi.org/10.1016/j.med.2012.12.001.
4. Rower J, Liu X, Yu T, Mundorff M, Sherwin C, Johnson M. Clinical pharmacokinetics of magnesium sulfate in treatment of children with severe acute asthma. Eur J Clin Pharmacol. 2017;73(3):325-331. https://doi.org/10.1007/s00228-016-2165-3.
5. Desanti R, Agasthya N, Hunter K, Hussain M. The effectiveness of magnesium sulfate for status asthmaticus outside intensive care unit. Pediatric Pulmonol. 2018;53(7):866-871. https://doi.org/10.1002/ppul.24013.Epub 2018.
6. Schuh S, Macias C, Freedman S, et al. North American practice patterns of intravenous magnesium sulfate in severe acute asthma exacerbations. Acad Emerg Med. 2010;17(11):1189-1196. https://doi.org/10.1111/j.1553-2712.2010.00913.x.
7. Cheuk DK, Chau TC, Lee SL. A meta-analysis on intravenous magnesium sulphate for treating acute asthma. Arch Dis Child. 2005;90(1):74-77. https://doi.org/10.1136/adc.2004.050005.
8. Lo HY, Messer A, Loveless J, et al. Discharging asthma patients on 3-hour β-agonist treatments: a quality improvement project. Hosp Pediatr. 2018;8(12):733-739. https://doi.org/10.1542/hpeds.2018-0072.
9. Magruder TG, Narayanan S, Walley S, et al. Improving inpatient asthma management: the implementation and evaluation of pediatric asthma clinical pathway. Pediatr Qual Saf. 2017;2(5);e041. https://doi.org/10.1097/pq9.0000000000000041.
For severe asthma exacerbations unresponsive to initial treatment, expert consensus guidelines from 2007 recommend consideration for adjunct treatments (magnesium or heliox) to decrease the likelihood of intubation.1 Over the last decade, data have emerged suggesting that intravenous (IV) magnesium may be more effective for reduction of hospital admission rates.2 Pooled meta-analyses have demonstrated improved pulmonary function and reduction of hospital admission by as much as 68% in children when IV magnesium is administered in the emergency department (ED), although the evidence is extremely limited because of a small number of studies (three) and small sample size (115 children).2-5
Though these data suggest that use of IV magnesium may reduce admission rates, a study of pediatric emergency medicine (PEM) physicians in US and Canada reported reluctance regarding use for this purpose. While PEM physicians reported awareness of the literature on admission prevention, they estimated that fewer than 5% of their patients receiving IV magnesium were discharged home.6 Their practice was generally limited to using IV magnesium in children with impending respiratory failure for the purpose of reducing intensive care unit (ICU) admission and not hospitalization.6 PEM physicians’ reluctance to use IV magnesium was related to the lack of strong available evidence supporting the impact of IV magnesium on outcomes, such as admission, and gaps in the literature about its dosing and safety profile.
The goal of this study was to assess the prevailing trends in IV magnesium use across US children’s hospitals and to assess the relationship of IV magnesium use to admission rate, length of stay (LOS), readmission rate, and ICU admission rate. We hypothesized that IV magnesium use might have increased following publication of studies demonstrating an association between IV magnesium use and fewer admissions.
METHODS
Study Design, Setting, and Participants
This is a retrospective cohort study of asthma (All Patient Refined Diagnosis Related Group 141) hospitalizations for patients less than 18 years old presenting to 35 tertiary care children’s hospitals from January 1, 2010, to December 31, 2017, included in the Pediatric Health Information System (PHIS; Children’s Hospital Association, Lenexa, Kansas) database. The PHIS database is an administrative database that contains demographics, International Classification of Diseases 9th and 10th Revision diagnoses and procedures, and daily billing records for all inpatient, observation, ED, and ambulatory surgery encounters. All data were deidentified prior to inclusion in the database and tracking of patients across ED and inpatient visits was achieved through an encrypted and unique patient identifier. Children transferred from other hospitals were excluded because we could not verify IV magnesium use prior to transfer. For hospitals to be included, they were required to provide continuous data throughout the study period.
Main Outcome Measure
The main outcome was exposure to IV magnesium as determined by billing information available in the PHIS database.
Patient Demographics
We assessed patients’ demographic characteristics, including age (younger than 5 years, 5-11 years, and 12-17 years), sex, race/ethnicity, and insurance status.
Healthcare Utilization and Hospital Characteristics
We assessed healthcare utilization using geometric mean LOS, proportion of patients admitted to the hospital and to the ICU, and the proportion of patients with a 7-day all-cause readmission. In addition, we divided hospitals into three equal groups based on their annual inpatient asthma volume (<300, 300-850, >850 cases per year).
Statistical Analysis
We compared demographic and clinical characteristics across patients receiving IV magnesium with those who did not receive it with use of chi-square tests for categorical variables and Wilcoxon rank sum test for continuous variables. We calculated annual IV magnesium use rates for each hospital and modeled the average annual rate with a general linear model in order to assess change over time. We used Pearson product moment correlation to compare the annual proportion of magnesium use and healthcare utilization measures, including geometric mean LOS, the proportion of patients using the inpatient wards or the ICU, and the proportion of cases with a 7-day all-cause readmission. Geometric mean LOS was used to normalize the compounding effect of non–normally distributed arithmetic mean LOS. A sensitivity analysis was performed stratifying IV magnesium use over time by hospital inpatient volume. Data were analyzed using SAS version 9.4 (SAS Institute, Cary, North Carolina), and P values < .05 were considered statistically significant.
RESULTS
Study Population
A total of 878,188 encounters with acute asthma exacerbation met the inclusion criteria, with 65,558 (7.5%) receiving IV magnesium (Table). Of those receiving IV magnesium, 90% were admitted to the hospital. There were statistically significant differences in IV magnesium use when compared by age, race/ethnicity, and payer type, but not gender. IV magnesium use was significantly associated with older age (more than 5 years old), non-Hispanic black race, ED visit in the year prior to admission, longer hospital LOS, and higher ICU admission rate.

Trends in Intravenous Magnesium Use
IV magnesium use among hospitalized children more than doubled from 2010 to 2017 (17% vs 36%). Low-volume hospitals had a lower frequency of IV magnesium use, compared with the moderate- and high-volume hospitals. The growth rate per year of IV magnesium use was greater in high- and moderate-volume hospitals (3.4% and 2.9% per year, respectively), compared with the low-volume hospitals (1.2% per year; P = .04).
Trends in Intravenous Magnesium Use and Hospital Outcomes
The trend in IV magnesium use was not associated with a statistically significant change in the inpatient and ICU admission rate or in the 7-day all-cause readmission rate (Figure and Appendix Figure). Although IV magnesium use increased over time, LOS decreased significantly during the same period (1.6 days in 2010 vs 1.4 days in 2017; P < .001). When analyzed by hospital volume, no significant associations were found in the inpatient admission, ICU admission, and 7-day readmission rate.

DISCUSSION
The use of IV magnesium has significantly increased in US children’s hospitals over the last 8 years, especially among those hospitalized following an ED evaluation. Over this interval, trends in inpatient and ICU admission rate, as well as 7-day all-cause readmission rate, for asthma did not change, while LOS decreased. These findings contrast with a recent Cochrane review that summarized the efficacy of IV magnesium for reducing admission rate in few small trials.2
Our study findings are more consistent with prior survey findings that IV magnesium does not reduce hospitalization and that ED physicians tend to use IV magnesium in severe asthma exacerbation for its potential therapeutic benefits because of bronchodilator and anti-inflammatory effect.6,7 Similar to PEM physicians’ estimates, only 10% of patients receiving IV magnesium were discharged home in our study.
IV magnesium use is higher in high-volume hospitals than in moderate- and low-volume ones. One potential explanation is that high- and moderate-volume hospitals may see a higher volume of children with severe or impending respiratory failure and, therefore, are more likely to use IV magnesium than the low-volume hospitals are. Alternatively, physician adoption of magnesium use for lower-acuity asthma exacerbations could vary by hospital volume.
Trend analyses of outcomes suggest that increase in IV magnesium use was not associated with an increase in inpatient and ICU admission rate or with 7-day all-cause readmission rate, although LOS reduced. LOS might be reduced because of various quality improvement initiatives (eg, discharging patients after every 3 hours albuterol treatments or respiratory therapist–driven protocols) and might not be related to IV magnesium use.8,9 To this point, a recent study of a respiratory assessment score–matched cohort did not find any therapeutic benefit of IV magnesium with severe asthma exacerbation when receiving continuous albuterol therapy on a pediatric ward.5 Perhaps future studies could explore estimating the outcome by performing comparative effectiveness studies between those with severe asthma exacerbation who did or did not receive IV magnesium. Additionally, randomized controlled trials comparing IV magnesium and standard therapy and its effects on outcomes, such as hospitalization, LOS, association with asthma chronicity, and previous oral steroid use, might provide further insight to inform clinical practice.
Certain study limitations should be noted. The study cohort included children’s hospitals only, and it is possible that care at nonchildren’s hospitals for asthma differs. PHIS dataset used in this study does not allow determination of where and when IV magnesium was given, the severity of asthma exacerbation, or the chronicity of baseline disease. Moreover, PHIS hospitals include centers in large cities, and other competing children’s hospitals may provide other tertiary care that could affect the readmission data calculation. Lastly, the temporal associations between IV magnesium use and outcomes reported in this study should not be used as evidence or lack of evidence for the effectiveness of magnesium given the limitations of the observational study design and dataset used.
In conclusion, IV magnesium use in management of asthma exacerbation in children across the United States has significantly increased. The increase occurred disproportionately in high-volume hospitals and was not associated with changes in admission rate, ICU admission rate, or 7-day all-cause readmission rate, although LOS has decreased over time.
Disclosures
The authors have no financial relationships relevant to this article or conflicts of interest to disclose.
This paper was a platform presentation at annual meetings of Pediatric Academic Societies 2019; accepted for presentation at annual meeting of Pediatric Hospital Medicine, July 2019.
Funding Source
No funding was secured for this study.
For severe asthma exacerbations unresponsive to initial treatment, expert consensus guidelines from 2007 recommend consideration for adjunct treatments (magnesium or heliox) to decrease the likelihood of intubation.1 Over the last decade, data have emerged suggesting that intravenous (IV) magnesium may be more effective for reduction of hospital admission rates.2 Pooled meta-analyses have demonstrated improved pulmonary function and reduction of hospital admission by as much as 68% in children when IV magnesium is administered in the emergency department (ED), although the evidence is extremely limited because of a small number of studies (three) and small sample size (115 children).2-5
Though these data suggest that use of IV magnesium may reduce admission rates, a study of pediatric emergency medicine (PEM) physicians in US and Canada reported reluctance regarding use for this purpose. While PEM physicians reported awareness of the literature on admission prevention, they estimated that fewer than 5% of their patients receiving IV magnesium were discharged home.6 Their practice was generally limited to using IV magnesium in children with impending respiratory failure for the purpose of reducing intensive care unit (ICU) admission and not hospitalization.6 PEM physicians’ reluctance to use IV magnesium was related to the lack of strong available evidence supporting the impact of IV magnesium on outcomes, such as admission, and gaps in the literature about its dosing and safety profile.
The goal of this study was to assess the prevailing trends in IV magnesium use across US children’s hospitals and to assess the relationship of IV magnesium use to admission rate, length of stay (LOS), readmission rate, and ICU admission rate. We hypothesized that IV magnesium use might have increased following publication of studies demonstrating an association between IV magnesium use and fewer admissions.
METHODS
Study Design, Setting, and Participants
This is a retrospective cohort study of asthma (All Patient Refined Diagnosis Related Group 141) hospitalizations for patients less than 18 years old presenting to 35 tertiary care children’s hospitals from January 1, 2010, to December 31, 2017, included in the Pediatric Health Information System (PHIS; Children’s Hospital Association, Lenexa, Kansas) database. The PHIS database is an administrative database that contains demographics, International Classification of Diseases 9th and 10th Revision diagnoses and procedures, and daily billing records for all inpatient, observation, ED, and ambulatory surgery encounters. All data were deidentified prior to inclusion in the database and tracking of patients across ED and inpatient visits was achieved through an encrypted and unique patient identifier. Children transferred from other hospitals were excluded because we could not verify IV magnesium use prior to transfer. For hospitals to be included, they were required to provide continuous data throughout the study period.
Main Outcome Measure
The main outcome was exposure to IV magnesium as determined by billing information available in the PHIS database.
Patient Demographics
We assessed patients’ demographic characteristics, including age (younger than 5 years, 5-11 years, and 12-17 years), sex, race/ethnicity, and insurance status.
Healthcare Utilization and Hospital Characteristics
We assessed healthcare utilization using geometric mean LOS, proportion of patients admitted to the hospital and to the ICU, and the proportion of patients with a 7-day all-cause readmission. In addition, we divided hospitals into three equal groups based on their annual inpatient asthma volume (<300, 300-850, >850 cases per year).
Statistical Analysis
We compared demographic and clinical characteristics across patients receiving IV magnesium with those who did not receive it with use of chi-square tests for categorical variables and Wilcoxon rank sum test for continuous variables. We calculated annual IV magnesium use rates for each hospital and modeled the average annual rate with a general linear model in order to assess change over time. We used Pearson product moment correlation to compare the annual proportion of magnesium use and healthcare utilization measures, including geometric mean LOS, the proportion of patients using the inpatient wards or the ICU, and the proportion of cases with a 7-day all-cause readmission. Geometric mean LOS was used to normalize the compounding effect of non–normally distributed arithmetic mean LOS. A sensitivity analysis was performed stratifying IV magnesium use over time by hospital inpatient volume. Data were analyzed using SAS version 9.4 (SAS Institute, Cary, North Carolina), and P values < .05 were considered statistically significant.
RESULTS
Study Population
A total of 878,188 encounters with acute asthma exacerbation met the inclusion criteria, with 65,558 (7.5%) receiving IV magnesium (Table). Of those receiving IV magnesium, 90% were admitted to the hospital. There were statistically significant differences in IV magnesium use when compared by age, race/ethnicity, and payer type, but not gender. IV magnesium use was significantly associated with older age (more than 5 years old), non-Hispanic black race, ED visit in the year prior to admission, longer hospital LOS, and higher ICU admission rate.

Trends in Intravenous Magnesium Use
IV magnesium use among hospitalized children more than doubled from 2010 to 2017 (17% vs 36%). Low-volume hospitals had a lower frequency of IV magnesium use, compared with the moderate- and high-volume hospitals. The growth rate per year of IV magnesium use was greater in high- and moderate-volume hospitals (3.4% and 2.9% per year, respectively), compared with the low-volume hospitals (1.2% per year; P = .04).
Trends in Intravenous Magnesium Use and Hospital Outcomes
The trend in IV magnesium use was not associated with a statistically significant change in the inpatient and ICU admission rate or in the 7-day all-cause readmission rate (Figure and Appendix Figure). Although IV magnesium use increased over time, LOS decreased significantly during the same period (1.6 days in 2010 vs 1.4 days in 2017; P < .001). When analyzed by hospital volume, no significant associations were found in the inpatient admission, ICU admission, and 7-day readmission rate.

DISCUSSION
The use of IV magnesium has significantly increased in US children’s hospitals over the last 8 years, especially among those hospitalized following an ED evaluation. Over this interval, trends in inpatient and ICU admission rate, as well as 7-day all-cause readmission rate, for asthma did not change, while LOS decreased. These findings contrast with a recent Cochrane review that summarized the efficacy of IV magnesium for reducing admission rate in few small trials.2
Our study findings are more consistent with prior survey findings that IV magnesium does not reduce hospitalization and that ED physicians tend to use IV magnesium in severe asthma exacerbation for its potential therapeutic benefits because of bronchodilator and anti-inflammatory effect.6,7 Similar to PEM physicians’ estimates, only 10% of patients receiving IV magnesium were discharged home in our study.
IV magnesium use is higher in high-volume hospitals than in moderate- and low-volume ones. One potential explanation is that high- and moderate-volume hospitals may see a higher volume of children with severe or impending respiratory failure and, therefore, are more likely to use IV magnesium than the low-volume hospitals are. Alternatively, physician adoption of magnesium use for lower-acuity asthma exacerbations could vary by hospital volume.
Trend analyses of outcomes suggest that increase in IV magnesium use was not associated with an increase in inpatient and ICU admission rate or with 7-day all-cause readmission rate, although LOS reduced. LOS might be reduced because of various quality improvement initiatives (eg, discharging patients after every 3 hours albuterol treatments or respiratory therapist–driven protocols) and might not be related to IV magnesium use.8,9 To this point, a recent study of a respiratory assessment score–matched cohort did not find any therapeutic benefit of IV magnesium with severe asthma exacerbation when receiving continuous albuterol therapy on a pediatric ward.5 Perhaps future studies could explore estimating the outcome by performing comparative effectiveness studies between those with severe asthma exacerbation who did or did not receive IV magnesium. Additionally, randomized controlled trials comparing IV magnesium and standard therapy and its effects on outcomes, such as hospitalization, LOS, association with asthma chronicity, and previous oral steroid use, might provide further insight to inform clinical practice.
Certain study limitations should be noted. The study cohort included children’s hospitals only, and it is possible that care at nonchildren’s hospitals for asthma differs. PHIS dataset used in this study does not allow determination of where and when IV magnesium was given, the severity of asthma exacerbation, or the chronicity of baseline disease. Moreover, PHIS hospitals include centers in large cities, and other competing children’s hospitals may provide other tertiary care that could affect the readmission data calculation. Lastly, the temporal associations between IV magnesium use and outcomes reported in this study should not be used as evidence or lack of evidence for the effectiveness of magnesium given the limitations of the observational study design and dataset used.
In conclusion, IV magnesium use in management of asthma exacerbation in children across the United States has significantly increased. The increase occurred disproportionately in high-volume hospitals and was not associated with changes in admission rate, ICU admission rate, or 7-day all-cause readmission rate, although LOS has decreased over time.
Disclosures
The authors have no financial relationships relevant to this article or conflicts of interest to disclose.
This paper was a platform presentation at annual meetings of Pediatric Academic Societies 2019; accepted for presentation at annual meeting of Pediatric Hospital Medicine, July 2019.
Funding Source
No funding was secured for this study.
1. National Asthma Education and Prevention Program, Third Expert Panel on the Diagnosis and Management of Asthma. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, Maryland: National Heart, Lung, and Blood Institute; 2007. https://www.ncbi.nlm.nih.gov/books/NBK7232/.
2. Griffiths B, Kew KM. Intravenous magnesium sulfate for treating children with acute asthma in the emergency department. Cochrane Database Syst Rev. 2016;4(4):CD011050. https://doi.org/10.1002/14651858.CD011050.pub2.
3. Shan Z, Rong Y, Yang W, et al. Intravenous and nebulized magnesium sulfate for treating acute asthma in adults and children: a systematic review and meta-analysis. Respir Med. 2013;107(3):321-330. https://doi.org/10.1016/j.med.2012.12.001.
4. Rower J, Liu X, Yu T, Mundorff M, Sherwin C, Johnson M. Clinical pharmacokinetics of magnesium sulfate in treatment of children with severe acute asthma. Eur J Clin Pharmacol. 2017;73(3):325-331. https://doi.org/10.1007/s00228-016-2165-3.
5. Desanti R, Agasthya N, Hunter K, Hussain M. The effectiveness of magnesium sulfate for status asthmaticus outside intensive care unit. Pediatric Pulmonol. 2018;53(7):866-871. https://doi.org/10.1002/ppul.24013.Epub 2018.
6. Schuh S, Macias C, Freedman S, et al. North American practice patterns of intravenous magnesium sulfate in severe acute asthma exacerbations. Acad Emerg Med. 2010;17(11):1189-1196. https://doi.org/10.1111/j.1553-2712.2010.00913.x.
7. Cheuk DK, Chau TC, Lee SL. A meta-analysis on intravenous magnesium sulphate for treating acute asthma. Arch Dis Child. 2005;90(1):74-77. https://doi.org/10.1136/adc.2004.050005.
8. Lo HY, Messer A, Loveless J, et al. Discharging asthma patients on 3-hour β-agonist treatments: a quality improvement project. Hosp Pediatr. 2018;8(12):733-739. https://doi.org/10.1542/hpeds.2018-0072.
9. Magruder TG, Narayanan S, Walley S, et al. Improving inpatient asthma management: the implementation and evaluation of pediatric asthma clinical pathway. Pediatr Qual Saf. 2017;2(5);e041. https://doi.org/10.1097/pq9.0000000000000041.
1. National Asthma Education and Prevention Program, Third Expert Panel on the Diagnosis and Management of Asthma. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, Maryland: National Heart, Lung, and Blood Institute; 2007. https://www.ncbi.nlm.nih.gov/books/NBK7232/.
2. Griffiths B, Kew KM. Intravenous magnesium sulfate for treating children with acute asthma in the emergency department. Cochrane Database Syst Rev. 2016;4(4):CD011050. https://doi.org/10.1002/14651858.CD011050.pub2.
3. Shan Z, Rong Y, Yang W, et al. Intravenous and nebulized magnesium sulfate for treating acute asthma in adults and children: a systematic review and meta-analysis. Respir Med. 2013;107(3):321-330. https://doi.org/10.1016/j.med.2012.12.001.
4. Rower J, Liu X, Yu T, Mundorff M, Sherwin C, Johnson M. Clinical pharmacokinetics of magnesium sulfate in treatment of children with severe acute asthma. Eur J Clin Pharmacol. 2017;73(3):325-331. https://doi.org/10.1007/s00228-016-2165-3.
5. Desanti R, Agasthya N, Hunter K, Hussain M. The effectiveness of magnesium sulfate for status asthmaticus outside intensive care unit. Pediatric Pulmonol. 2018;53(7):866-871. https://doi.org/10.1002/ppul.24013.Epub 2018.
6. Schuh S, Macias C, Freedman S, et al. North American practice patterns of intravenous magnesium sulfate in severe acute asthma exacerbations. Acad Emerg Med. 2010;17(11):1189-1196. https://doi.org/10.1111/j.1553-2712.2010.00913.x.
7. Cheuk DK, Chau TC, Lee SL. A meta-analysis on intravenous magnesium sulphate for treating acute asthma. Arch Dis Child. 2005;90(1):74-77. https://doi.org/10.1136/adc.2004.050005.
8. Lo HY, Messer A, Loveless J, et al. Discharging asthma patients on 3-hour β-agonist treatments: a quality improvement project. Hosp Pediatr. 2018;8(12):733-739. https://doi.org/10.1542/hpeds.2018-0072.
9. Magruder TG, Narayanan S, Walley S, et al. Improving inpatient asthma management: the implementation and evaluation of pediatric asthma clinical pathway. Pediatr Qual Saf. 2017;2(5);e041. https://doi.org/10.1097/pq9.0000000000000041.
©2020 Society of Hospital Medicine
Communicating Effectively With Hospitalized Patients and Families During the COVID-19 Pandemic
For parents of children with medical complexity (CMC), bringing a child to the hospital for needed expertise, equipment, and support is necessarily accompanied by a loss of power, freedom, and control. Two of our authors (K.L., P.M.) are parents of CMC—patients affectionately known as “frequent flyers” at their local hospitals. When health needs present, these experienced parents quickly identify what can be managed at home and what needs a higher level of care. The autonomy and security that accompany this parental expertise have been mitigated by, and in some cases even lost in, the COVID-19 pandemic. In particular, one of the most obvious changes to patients’ and families’ roles in inpatient care has been in communication practices, including changes to patient- and family-centered rounding that result from current isolation procedures and visitation policies. Over the past few months, we’ve learned a tremendous amount from providers and caregivers of hospitalized patients; in this article, we share some of what they’ve taught us.
Before we continue, we take a humble pause. The process of writing this piece spanned weeks during which certain areas of the world were overwhelmed. Our perspective has been informed by others who shared their experiences, and as a result, our health systems are more prepared. We offer this perspective recognizing the importance of learning from others and feeling a sense of gratitude to the providers and patients on the front lines.
CHANGING CIRCUMSTANCES OF CARE
As a group of parents, nurses, physicians, educators, and researchers who have spent the last 10 years studying how to communicate more effectively in the healthcare setting,1,2 we find ourselves in uncharted territory. Even now, we are engaged in an ongoing mentored implementation program examining the effects of a communication bundle on patient- and family- centered rounds (PFCRs) at 21 teaching hospitals across North America (the SHM I-PASS SCORE Study).3 COVID-19 has put that study on hold, and we have taken a step back to reassess the most basic communication needs of patients and families under any circumstance.
Even among our study group, our family advisors have also been on the front lines as patients and caregivers. One author (P.M.), shared a recent experience that she and her son, John Michael had:
“My son [who has autoimmune hepatitis and associated conditions] began coughing and had an intense sinus headache. As his symptoms continued, our concern steadily grew: Could we push through at home or would we have to go in [to the hospital] to seek care? My mind raced. We faced this decision many times, but never with the overwhelming threat of COVID-19 in the equation. My son, who is able to recognize troublesome symptoms, was afraid his sinuses were infected and decided that we should go in. My heart sank.”
Now, amid the COVID-19 pandemic, we have heard that patients like John Michael, who are accustomed to the healthcare setting, are “terrified with this additional concern of just being safe in the hospital,” reported a member of our Family Advisory Council. One of our members added, “We recognize this extends to the providers as well, who maintain great care despite their own family and personal safety concerns.” Although families affirmed the necessity of the enhanced isolation procedures and strict visitation policies, they also highlighted the effects of these changes on usual communication practices, including PFCRs.
CORE VALUES DURING COVID-19
In response to these sentiments, we reached out to all of our family advisors, as well as other team members, for suggestions on how healthcare teams could help patients and families best manage their hospital experiences in the setting of COVID-19. Additionally, we asked our physician and nursing colleagues across health systems about current inpatient unit adaptations. Their suggestions and adaptations reinforced and directly aligned with some of the core values of family engagement and patient- and family-centered care,4 namely, (1) prioritizing communication, (2) maintaining active engagement with patients and families, and (3) enhancing communication with technology.
Prioritizing Communication
Timely and clear communication can help providers manage the expectations of patients and families, build patient and family feelings of confidence, and reduce their feelings of anxiety and vulnerability. Almost universally, families acknowledged the importance of infection control and physical distancing measures while fearing that decreased entry into rooms would lead to decreased communication. “Since COVID-19 is contagious, families will want to see every precaution taken … but in a way that doesn’t cut off communication and leave an already sick and scared child and their family feeling emotionally isolated in a scary situation,” an Advisory Council member recounted. Importantly, one parent shared that hearing about personal protective equipment conservation could amplify stress because of fear their child wouldn’t be protected. These perspectives remind us that families may be experiencing heightened sensitivity and vulnerability during this pandemic.
Maintaining Active Engagement With Patients and Families
PFCRs continue to be an ideal setting for providers, patients, and families to communicate and build shared understanding, as well as build rapport and connection through human interactions. Maintaining rounding structures, when possible, reinforces familiarity with roles and expectations, among both patients who have been hospitalized in the past and those hospitalized for the first time. Adapting rounds may be as simple as opening the door during walk-rounds to invite caregiver participation while being aware of distancing. With large rounding teams, more substantial workflow changes may be necessary.
Beyond PFCRs, patients and family members can be further engaged through tasks/responsibilities for the time in between rounding communication. Examples include recording patient symptoms (eg, work of breathing) or actions (eg, how much water their child drinks). By doing this, patients and caregivers who feel helpless and anxious may be given a greater sense of control while at the same time making helpful contributions to medical care.
Parents also expressed value in reinforcing the message that patients and families are experts about themselves/their loved ones. Healthcare teams can invite their insights, questions, and concerns to show respect for their expertise and value. This builds trust and leads to a feeling of togetherness and teamwork. Across the board, families stressed the value of family engagement and communication in ideal conditions, and even more so in this time of upheaval.
Enhancing Communication With Technology
Many hospitals are leveraging technology to promote communication by integrating workstations on wheels & tablets with video-conferencing software (eg, Zoom, Skype) and even by adding communication via email and phone. While fewer team members are entering rooms, rounding teams are still including the voices of pharmacists, nutritionists, social workers, primary care physicians, and caregivers who are unable to be at the bedside.
These alternative communication methods may actually provide patients with more comfortable avenues for participating in their own care even beyond the pandemic. Children, in particular, may have strong opinions about their care but may not be comfortable speaking up in front of providers whom they don’t know very well. Telehealth, whiteboards, email, and limiting the number of providers in the room might actually create a more approachable environment for these patients even under routine conditions.
CONCLUSION
Patients, families, nurses, physicians, and other team members all feel the current stress on our healthcare system. As we continue to change workflows, alignment with principles of family engagement and patient- and family-centered care4 remain a priority for all involved. Prioritizing effective communication, maintaining engagement with patients and families, and using technology in new ways will all help us maintain high standards of care in both typical and completely atypical settings, such as during this pandemic. Nothing captures the benefits of effective communication better than P.M.’s description of John Michael’s experience during his hospitalization:
“Although usually an expedited triage patient, we spent hours in the ER among other ill and anxious patients. Ultimately, John Michael tested positive for influenza A. We spent 5 days in the hospital on droplet protection.
“The staff was amazing! The doctors and nurses communicated with us every step of the way. They made us aware of extra precautions and explained limitations, like not being able to go in the nutrition room or only having the doctors come in once midday. Whenever they did use [personal protective equipment] and come in, the nurses and team kept a safe distance but made sure to connect with John Michael, talking about what was on TV, what his favorite teams are, asking about his sisters, and always asking if we needed anything or if there was anything they could do. I am grateful for the kind, compassionate, and professional people who continue to care for our children under the intense danger and overwhelming magnitude of COVID-19.”
Disclosures
Dr Landrigan has served as a paid consultant to the Midwest Lighting Institute to help study the effect of blue light on health care provider performance and safety. He has consulted with and holds equity in the I-PASS Institute, which seeks to train institutions in best handoff practices and aid in their implementation. Dr Landrigan has received consulting fees from the Missouri Hospital Association/Executive Speakers Bureau for consulting on I-PASS. In addition, he has received monetary awards, honoraria, and travel reimbursement from multiple academic and professional organizations for teaching and consulting on sleep deprivation, physician performance, handoffs, and safety and has served as an expert witness in cases regarding patient safety and sleep deprivation. Drs Spector and Baird have also consulted with and hold equity in the I-PASS Institute. Dr Baird has consulted with the I-PASS Patient Safety Institute. Dr Patel holds equity/stock options in and has consulted for the I-PASS Patient Safety Institute. Dr Rosenbluth previously consulted with the I-PASS Patient Safety Institute, but not within the past 36 months. The other authors have no conflicts of interest or external support other than the existing PCORI funding for the Society of Hospital Medicine I-PASS SCORE study.
Disclaimer
The I-PASS Patient Safety Institute did not provide support to any authors for this work.
1. Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803-1812. https://doi.org/10.1056/nejmsa1405556.
2. Khan A, Spector ND, Baird JD, et al. Patient safety after implementation of a coproduced family centered communication programme: multicenter before and after intervention study. BMJ. 2018;363:k4764. https://doi.org/10.1136/bmj.k4764.
3. Patient-Centered Outcomes Research Institute. Helping Children’s Hospitals Use a Program to Improve Communication with Families. December 27, 2019. https://www.pcori.org/research-results/2018/helping-childrens-hospitals-use-program-improve-communication-families. Accessed March 26, 2020.
4. Institute for Patient- and Family-Centered Care (IPFCC). PFCC and COVID-19. https://www.ipfcc.org/bestpractices/covid-19/index.html. Accessed April 10, 2020.
For parents of children with medical complexity (CMC), bringing a child to the hospital for needed expertise, equipment, and support is necessarily accompanied by a loss of power, freedom, and control. Two of our authors (K.L., P.M.) are parents of CMC—patients affectionately known as “frequent flyers” at their local hospitals. When health needs present, these experienced parents quickly identify what can be managed at home and what needs a higher level of care. The autonomy and security that accompany this parental expertise have been mitigated by, and in some cases even lost in, the COVID-19 pandemic. In particular, one of the most obvious changes to patients’ and families’ roles in inpatient care has been in communication practices, including changes to patient- and family-centered rounding that result from current isolation procedures and visitation policies. Over the past few months, we’ve learned a tremendous amount from providers and caregivers of hospitalized patients; in this article, we share some of what they’ve taught us.
Before we continue, we take a humble pause. The process of writing this piece spanned weeks during which certain areas of the world were overwhelmed. Our perspective has been informed by others who shared their experiences, and as a result, our health systems are more prepared. We offer this perspective recognizing the importance of learning from others and feeling a sense of gratitude to the providers and patients on the front lines.
CHANGING CIRCUMSTANCES OF CARE
As a group of parents, nurses, physicians, educators, and researchers who have spent the last 10 years studying how to communicate more effectively in the healthcare setting,1,2 we find ourselves in uncharted territory. Even now, we are engaged in an ongoing mentored implementation program examining the effects of a communication bundle on patient- and family- centered rounds (PFCRs) at 21 teaching hospitals across North America (the SHM I-PASS SCORE Study).3 COVID-19 has put that study on hold, and we have taken a step back to reassess the most basic communication needs of patients and families under any circumstance.
Even among our study group, our family advisors have also been on the front lines as patients and caregivers. One author (P.M.), shared a recent experience that she and her son, John Michael had:
“My son [who has autoimmune hepatitis and associated conditions] began coughing and had an intense sinus headache. As his symptoms continued, our concern steadily grew: Could we push through at home or would we have to go in [to the hospital] to seek care? My mind raced. We faced this decision many times, but never with the overwhelming threat of COVID-19 in the equation. My son, who is able to recognize troublesome symptoms, was afraid his sinuses were infected and decided that we should go in. My heart sank.”
Now, amid the COVID-19 pandemic, we have heard that patients like John Michael, who are accustomed to the healthcare setting, are “terrified with this additional concern of just being safe in the hospital,” reported a member of our Family Advisory Council. One of our members added, “We recognize this extends to the providers as well, who maintain great care despite their own family and personal safety concerns.” Although families affirmed the necessity of the enhanced isolation procedures and strict visitation policies, they also highlighted the effects of these changes on usual communication practices, including PFCRs.
CORE VALUES DURING COVID-19
In response to these sentiments, we reached out to all of our family advisors, as well as other team members, for suggestions on how healthcare teams could help patients and families best manage their hospital experiences in the setting of COVID-19. Additionally, we asked our physician and nursing colleagues across health systems about current inpatient unit adaptations. Their suggestions and adaptations reinforced and directly aligned with some of the core values of family engagement and patient- and family-centered care,4 namely, (1) prioritizing communication, (2) maintaining active engagement with patients and families, and (3) enhancing communication with technology.
Prioritizing Communication
Timely and clear communication can help providers manage the expectations of patients and families, build patient and family feelings of confidence, and reduce their feelings of anxiety and vulnerability. Almost universally, families acknowledged the importance of infection control and physical distancing measures while fearing that decreased entry into rooms would lead to decreased communication. “Since COVID-19 is contagious, families will want to see every precaution taken … but in a way that doesn’t cut off communication and leave an already sick and scared child and their family feeling emotionally isolated in a scary situation,” an Advisory Council member recounted. Importantly, one parent shared that hearing about personal protective equipment conservation could amplify stress because of fear their child wouldn’t be protected. These perspectives remind us that families may be experiencing heightened sensitivity and vulnerability during this pandemic.
Maintaining Active Engagement With Patients and Families
PFCRs continue to be an ideal setting for providers, patients, and families to communicate and build shared understanding, as well as build rapport and connection through human interactions. Maintaining rounding structures, when possible, reinforces familiarity with roles and expectations, among both patients who have been hospitalized in the past and those hospitalized for the first time. Adapting rounds may be as simple as opening the door during walk-rounds to invite caregiver participation while being aware of distancing. With large rounding teams, more substantial workflow changes may be necessary.
Beyond PFCRs, patients and family members can be further engaged through tasks/responsibilities for the time in between rounding communication. Examples include recording patient symptoms (eg, work of breathing) or actions (eg, how much water their child drinks). By doing this, patients and caregivers who feel helpless and anxious may be given a greater sense of control while at the same time making helpful contributions to medical care.
Parents also expressed value in reinforcing the message that patients and families are experts about themselves/their loved ones. Healthcare teams can invite their insights, questions, and concerns to show respect for their expertise and value. This builds trust and leads to a feeling of togetherness and teamwork. Across the board, families stressed the value of family engagement and communication in ideal conditions, and even more so in this time of upheaval.
Enhancing Communication With Technology
Many hospitals are leveraging technology to promote communication by integrating workstations on wheels & tablets with video-conferencing software (eg, Zoom, Skype) and even by adding communication via email and phone. While fewer team members are entering rooms, rounding teams are still including the voices of pharmacists, nutritionists, social workers, primary care physicians, and caregivers who are unable to be at the bedside.
These alternative communication methods may actually provide patients with more comfortable avenues for participating in their own care even beyond the pandemic. Children, in particular, may have strong opinions about their care but may not be comfortable speaking up in front of providers whom they don’t know very well. Telehealth, whiteboards, email, and limiting the number of providers in the room might actually create a more approachable environment for these patients even under routine conditions.
CONCLUSION
Patients, families, nurses, physicians, and other team members all feel the current stress on our healthcare system. As we continue to change workflows, alignment with principles of family engagement and patient- and family-centered care4 remain a priority for all involved. Prioritizing effective communication, maintaining engagement with patients and families, and using technology in new ways will all help us maintain high standards of care in both typical and completely atypical settings, such as during this pandemic. Nothing captures the benefits of effective communication better than P.M.’s description of John Michael’s experience during his hospitalization:
“Although usually an expedited triage patient, we spent hours in the ER among other ill and anxious patients. Ultimately, John Michael tested positive for influenza A. We spent 5 days in the hospital on droplet protection.
“The staff was amazing! The doctors and nurses communicated with us every step of the way. They made us aware of extra precautions and explained limitations, like not being able to go in the nutrition room or only having the doctors come in once midday. Whenever they did use [personal protective equipment] and come in, the nurses and team kept a safe distance but made sure to connect with John Michael, talking about what was on TV, what his favorite teams are, asking about his sisters, and always asking if we needed anything or if there was anything they could do. I am grateful for the kind, compassionate, and professional people who continue to care for our children under the intense danger and overwhelming magnitude of COVID-19.”
Disclosures
Dr Landrigan has served as a paid consultant to the Midwest Lighting Institute to help study the effect of blue light on health care provider performance and safety. He has consulted with and holds equity in the I-PASS Institute, which seeks to train institutions in best handoff practices and aid in their implementation. Dr Landrigan has received consulting fees from the Missouri Hospital Association/Executive Speakers Bureau for consulting on I-PASS. In addition, he has received monetary awards, honoraria, and travel reimbursement from multiple academic and professional organizations for teaching and consulting on sleep deprivation, physician performance, handoffs, and safety and has served as an expert witness in cases regarding patient safety and sleep deprivation. Drs Spector and Baird have also consulted with and hold equity in the I-PASS Institute. Dr Baird has consulted with the I-PASS Patient Safety Institute. Dr Patel holds equity/stock options in and has consulted for the I-PASS Patient Safety Institute. Dr Rosenbluth previously consulted with the I-PASS Patient Safety Institute, but not within the past 36 months. The other authors have no conflicts of interest or external support other than the existing PCORI funding for the Society of Hospital Medicine I-PASS SCORE study.
Disclaimer
The I-PASS Patient Safety Institute did not provide support to any authors for this work.
For parents of children with medical complexity (CMC), bringing a child to the hospital for needed expertise, equipment, and support is necessarily accompanied by a loss of power, freedom, and control. Two of our authors (K.L., P.M.) are parents of CMC—patients affectionately known as “frequent flyers” at their local hospitals. When health needs present, these experienced parents quickly identify what can be managed at home and what needs a higher level of care. The autonomy and security that accompany this parental expertise have been mitigated by, and in some cases even lost in, the COVID-19 pandemic. In particular, one of the most obvious changes to patients’ and families’ roles in inpatient care has been in communication practices, including changes to patient- and family-centered rounding that result from current isolation procedures and visitation policies. Over the past few months, we’ve learned a tremendous amount from providers and caregivers of hospitalized patients; in this article, we share some of what they’ve taught us.
Before we continue, we take a humble pause. The process of writing this piece spanned weeks during which certain areas of the world were overwhelmed. Our perspective has been informed by others who shared their experiences, and as a result, our health systems are more prepared. We offer this perspective recognizing the importance of learning from others and feeling a sense of gratitude to the providers and patients on the front lines.
CHANGING CIRCUMSTANCES OF CARE
As a group of parents, nurses, physicians, educators, and researchers who have spent the last 10 years studying how to communicate more effectively in the healthcare setting,1,2 we find ourselves in uncharted territory. Even now, we are engaged in an ongoing mentored implementation program examining the effects of a communication bundle on patient- and family- centered rounds (PFCRs) at 21 teaching hospitals across North America (the SHM I-PASS SCORE Study).3 COVID-19 has put that study on hold, and we have taken a step back to reassess the most basic communication needs of patients and families under any circumstance.
Even among our study group, our family advisors have also been on the front lines as patients and caregivers. One author (P.M.), shared a recent experience that she and her son, John Michael had:
“My son [who has autoimmune hepatitis and associated conditions] began coughing and had an intense sinus headache. As his symptoms continued, our concern steadily grew: Could we push through at home or would we have to go in [to the hospital] to seek care? My mind raced. We faced this decision many times, but never with the overwhelming threat of COVID-19 in the equation. My son, who is able to recognize troublesome symptoms, was afraid his sinuses were infected and decided that we should go in. My heart sank.”
Now, amid the COVID-19 pandemic, we have heard that patients like John Michael, who are accustomed to the healthcare setting, are “terrified with this additional concern of just being safe in the hospital,” reported a member of our Family Advisory Council. One of our members added, “We recognize this extends to the providers as well, who maintain great care despite their own family and personal safety concerns.” Although families affirmed the necessity of the enhanced isolation procedures and strict visitation policies, they also highlighted the effects of these changes on usual communication practices, including PFCRs.
CORE VALUES DURING COVID-19
In response to these sentiments, we reached out to all of our family advisors, as well as other team members, for suggestions on how healthcare teams could help patients and families best manage their hospital experiences in the setting of COVID-19. Additionally, we asked our physician and nursing colleagues across health systems about current inpatient unit adaptations. Their suggestions and adaptations reinforced and directly aligned with some of the core values of family engagement and patient- and family-centered care,4 namely, (1) prioritizing communication, (2) maintaining active engagement with patients and families, and (3) enhancing communication with technology.
Prioritizing Communication
Timely and clear communication can help providers manage the expectations of patients and families, build patient and family feelings of confidence, and reduce their feelings of anxiety and vulnerability. Almost universally, families acknowledged the importance of infection control and physical distancing measures while fearing that decreased entry into rooms would lead to decreased communication. “Since COVID-19 is contagious, families will want to see every precaution taken … but in a way that doesn’t cut off communication and leave an already sick and scared child and their family feeling emotionally isolated in a scary situation,” an Advisory Council member recounted. Importantly, one parent shared that hearing about personal protective equipment conservation could amplify stress because of fear their child wouldn’t be protected. These perspectives remind us that families may be experiencing heightened sensitivity and vulnerability during this pandemic.
Maintaining Active Engagement With Patients and Families
PFCRs continue to be an ideal setting for providers, patients, and families to communicate and build shared understanding, as well as build rapport and connection through human interactions. Maintaining rounding structures, when possible, reinforces familiarity with roles and expectations, among both patients who have been hospitalized in the past and those hospitalized for the first time. Adapting rounds may be as simple as opening the door during walk-rounds to invite caregiver participation while being aware of distancing. With large rounding teams, more substantial workflow changes may be necessary.
Beyond PFCRs, patients and family members can be further engaged through tasks/responsibilities for the time in between rounding communication. Examples include recording patient symptoms (eg, work of breathing) or actions (eg, how much water their child drinks). By doing this, patients and caregivers who feel helpless and anxious may be given a greater sense of control while at the same time making helpful contributions to medical care.
Parents also expressed value in reinforcing the message that patients and families are experts about themselves/their loved ones. Healthcare teams can invite their insights, questions, and concerns to show respect for their expertise and value. This builds trust and leads to a feeling of togetherness and teamwork. Across the board, families stressed the value of family engagement and communication in ideal conditions, and even more so in this time of upheaval.
Enhancing Communication With Technology
Many hospitals are leveraging technology to promote communication by integrating workstations on wheels & tablets with video-conferencing software (eg, Zoom, Skype) and even by adding communication via email and phone. While fewer team members are entering rooms, rounding teams are still including the voices of pharmacists, nutritionists, social workers, primary care physicians, and caregivers who are unable to be at the bedside.
These alternative communication methods may actually provide patients with more comfortable avenues for participating in their own care even beyond the pandemic. Children, in particular, may have strong opinions about their care but may not be comfortable speaking up in front of providers whom they don’t know very well. Telehealth, whiteboards, email, and limiting the number of providers in the room might actually create a more approachable environment for these patients even under routine conditions.
CONCLUSION
Patients, families, nurses, physicians, and other team members all feel the current stress on our healthcare system. As we continue to change workflows, alignment with principles of family engagement and patient- and family-centered care4 remain a priority for all involved. Prioritizing effective communication, maintaining engagement with patients and families, and using technology in new ways will all help us maintain high standards of care in both typical and completely atypical settings, such as during this pandemic. Nothing captures the benefits of effective communication better than P.M.’s description of John Michael’s experience during his hospitalization:
“Although usually an expedited triage patient, we spent hours in the ER among other ill and anxious patients. Ultimately, John Michael tested positive for influenza A. We spent 5 days in the hospital on droplet protection.
“The staff was amazing! The doctors and nurses communicated with us every step of the way. They made us aware of extra precautions and explained limitations, like not being able to go in the nutrition room or only having the doctors come in once midday. Whenever they did use [personal protective equipment] and come in, the nurses and team kept a safe distance but made sure to connect with John Michael, talking about what was on TV, what his favorite teams are, asking about his sisters, and always asking if we needed anything or if there was anything they could do. I am grateful for the kind, compassionate, and professional people who continue to care for our children under the intense danger and overwhelming magnitude of COVID-19.”
Disclosures
Dr Landrigan has served as a paid consultant to the Midwest Lighting Institute to help study the effect of blue light on health care provider performance and safety. He has consulted with and holds equity in the I-PASS Institute, which seeks to train institutions in best handoff practices and aid in their implementation. Dr Landrigan has received consulting fees from the Missouri Hospital Association/Executive Speakers Bureau for consulting on I-PASS. In addition, he has received monetary awards, honoraria, and travel reimbursement from multiple academic and professional organizations for teaching and consulting on sleep deprivation, physician performance, handoffs, and safety and has served as an expert witness in cases regarding patient safety and sleep deprivation. Drs Spector and Baird have also consulted with and hold equity in the I-PASS Institute. Dr Baird has consulted with the I-PASS Patient Safety Institute. Dr Patel holds equity/stock options in and has consulted for the I-PASS Patient Safety Institute. Dr Rosenbluth previously consulted with the I-PASS Patient Safety Institute, but not within the past 36 months. The other authors have no conflicts of interest or external support other than the existing PCORI funding for the Society of Hospital Medicine I-PASS SCORE study.
Disclaimer
The I-PASS Patient Safety Institute did not provide support to any authors for this work.
1. Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803-1812. https://doi.org/10.1056/nejmsa1405556.
2. Khan A, Spector ND, Baird JD, et al. Patient safety after implementation of a coproduced family centered communication programme: multicenter before and after intervention study. BMJ. 2018;363:k4764. https://doi.org/10.1136/bmj.k4764.
3. Patient-Centered Outcomes Research Institute. Helping Children’s Hospitals Use a Program to Improve Communication with Families. December 27, 2019. https://www.pcori.org/research-results/2018/helping-childrens-hospitals-use-program-improve-communication-families. Accessed March 26, 2020.
4. Institute for Patient- and Family-Centered Care (IPFCC). PFCC and COVID-19. https://www.ipfcc.org/bestpractices/covid-19/index.html. Accessed April 10, 2020.
1. Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803-1812. https://doi.org/10.1056/nejmsa1405556.
2. Khan A, Spector ND, Baird JD, et al. Patient safety after implementation of a coproduced family centered communication programme: multicenter before and after intervention study. BMJ. 2018;363:k4764. https://doi.org/10.1136/bmj.k4764.
3. Patient-Centered Outcomes Research Institute. Helping Children’s Hospitals Use a Program to Improve Communication with Families. December 27, 2019. https://www.pcori.org/research-results/2018/helping-childrens-hospitals-use-program-improve-communication-families. Accessed March 26, 2020.
4. Institute for Patient- and Family-Centered Care (IPFCC). PFCC and COVID-19. https://www.ipfcc.org/bestpractices/covid-19/index.html. Accessed April 10, 2020.
© 2020 Society of Hospital Medicine
Trust in Public Health Is Essential Amid the COVID-19 Pandemic
The visibility of public health—both as a science and a government responsibility—has increased dramatically with the COVID-19 pandemic. Public health science, surveillance, and emergency interventions are saving lives across the globe. Public health leaders are advising local, state, national, and international policymakers and have a consistent and strong voice in the media. We describe here the trust challenges facing public health in this moment of crisis, as well as the strategies necessary to maintain and increase that trust.
In the United States, public opinion data suggest that, while trust in science and government is relatively low and has been declining in recent years, trust in public health is high.1,2 In a survey released in April, 2020, the most trusted groups “to do the right thing” on COVID-19 were doctors, hospitals, scientists, researchers, and the Centers for Disease Control and Prevention (CDC).3 Trust in state government was the next highest. Some governors have been particularly strong in supporting public health messages. For example, Governor Gretchen Whitmer in Michigan has repeatedly stated that her decisions are based on science and public health4; Michiganders reported trust in state government at 79%, compared with trust in the White House at 54%.3 In Ohio, where Governor Mike DeWine has stood with his director of public health, Amy Acton, MD, MPH, in his pandemic response, trust in state government was 80%, compared with trust in the White House at 62%.3
Until there is an effective vaccine with high levels of uptake, COVID-19 prevention and control efforts are going to primarily rely on intrusive and challenging public health interventions such as school/business closures, stay-at-home orders, crowd limits, and travel restrictions. Maintaining trust in and support for both public health interventions and leaders requires intentional strategies that are sophisticated and deploy effective social marketing and risk communication strategies.
CHALLENGES TO MAINTAINING TRUST IN PUBLIC HEALTH
Early in the trajectory of COVID-19, Americans were almost uniform in their support for stay-at-home orders.5 Later, as the economic and social impact of self-quarantine, business, and school closures deepened, backlash began to increase.6 As recent protests against stay-at-home orders and other COVID-19-interventions reveal, many people do not understand the breadth of government’s duty to protect the public’s health and welfare. In fact, the US Constitution gives states a significant amount of power to protect the health, safety, and welfare of their populations, including “police powers” that generally fall into three categories: (a) protecting people who cannot protect themselves, (b) protecting people from others, and (c) protecting people from themselves.7,8 Current executive orders and other government actions designed to combat COVID-19 represent the use of police powers in all three of these areas.
It is exceedingly difficult for governments to design effective pandemic interventions—including executive orders and laws based on “police power”—that protect the public’s health without negatively affecting the economy, healthcare system, schools, and the financial and psychosocial welfare of citizens.
To compound this challenge, while local, state, and federal governments have the authority to act strongly and swiftly in a public crisis, American’s passionate political and philosophical attachments to freedom and self-determination and their skepticism about government interference cannot be dismissed. “Life, liberty, and the pursuit of happiness” is more than a line in the Declaration of Independence—it reflects a strong set of American values that make the case for action that is collectively based while honoring individual interests. Although Americans have a deep-seated belief in individual freedoms, public health relies on collective action for success. Public health leaders must understand this tension and effectively articulate why and when collective action is necessary while also articulating a path to move from a uniform, state-imposed emergency response to one that relies on responsible individual actions.
The federal government’s conflicting messages on science and the public health are also an enormous threat to public health. When the White House’s top trade adviser publicly criticizes the response of the CDC, the CDC guidance appears politicized, which erodes public trust.
Unfortunately, public health in the United States has generally struggled to make a clear and compelling case for prevention and nonmedical approaches to health and well-being. As the saying goes, “Public health is invisible when it is most effective.” Public health leaders are trained in epidemiology and other sciences, in community-based partnerships, and sometimes medicine. However, few public health leaders have been trained in advocacy communication.
STRATEGIES TO STRENGTHEN TRUST IN PUBLIC HEALTH
Government leaders and their partners can better balance the health, economic, and other needs of the population if they effectively communicate the rationale and need for population-based public health interventions in ways that are based on communication science and are politically savvy. A civics lesson from public health officials about constitutional law and the role of police power in combating COVID-19 is not likely to be effective. However, sophisticated messaging tailored to different audiences about the government’s role in protecting the health of everyone could be.
While much is still unknown regarding COVID-19, the evidence is clear that nonpharmaceutical interventions like self-quarantine and isolation, physical distancing, business and school closures, and other core public health strategies are effective in reducing community spread and can flatten the infectious-disease epidemiologic curve.9,10 Countries such as South Korea, New Zealand, Australia, and Germany—countries that have taken strong public-health approaches on social distancing and stay-at-home orders along with extensive testing and contact tracing—have demonstrated reduced rates of severe morbidity and mortality from COVID-19. Vietnam, a developing country of 96 million people that borders China, has reported zero deaths from COVID-19 to date because of both swift public health actions and strong communication strategies.11
Public health communication efforts regarding COVID-19 should be based on risk and crisis communication science and on best practices for social marketing that rallies people around shared values.12,13 For example, communications from Dr Acton have attempted to “inspire” rather than “order” people to physically isolate by appealing to widely shared core values.14 This includes acknowledging the hardships people are experiencing, emphasizing the important historic role that everyone is playing in their sacrifices, promoting determination rather than fear, and declaring that “not all heroes wear capes.” Best practices in communication also include segmenting audiences for the design and testing of different communication approaches.12
Public health leaders can also learn from the extensive research from other fields in how to build trust. Consumer product research emphasizes the importance of transparency in sharing known and unknown risks and admitting error when errors are made.15
Engagement of the public in policy decision-making is also essential in situations of uncertainty. Since much is unknown about COVID-19, policy guidance about mitigation and prevention strategies has changed in real time. Changing messages on the importance of face masks is an example of the trust challenge for public health. In the initial stages of the pandemic, the CDC discouraged the use of face masks. As more data became available, the CDC changed its guidance. Such changed guidance can undermine the entire public health message on protective factors. Acknowledging uncertainty and engaging the public in decision-making through a process of reflexive learning can build public trust in a time of uncertainty.16
COVID-19 has also reaffirmed and illuminated that the public health and healthcare delivery systems are intertwined. Failure to “flatten the curve” results in an overrun healthcare system, enormous costs, and significant mortality. However, public health efforts that successfully slow and limit community spread also produce significant financial losses for healthcare systems because the use of all types of nonemergent care greatly decreases. Public health and healthcare system leaders must partner in the strategic design and reinforcement of messages to build strong and lasting trust in the ongoing public health interventions and mandates that are going to be with us for the unforeseen future.
Finally, maintaining trust in the face of political attacks on our agencies of public health requires the healthcare community speak out in unity—endorsing science-based recommendations and supporting the CDC, the World Health Organization, and local public health.
CONCLUSION
Public health is at an unprecedented and crucial moment in this global pandemic, with growing societal understanding of the role that public health plays in our lives. Public health leaders have a unique opportunity to build on that understanding, strengthen trust, and increase funding and support for core public health services.
Balancing risks and benefits in the face of great uncertainty is never easy. With COVID-19, the horrific number of deaths and speed of community spread has led to a strong and essential public health emergency response throughout most of the country. Keeping the public committed to the important and ongoing measures necessary to ensure that prevention/control efforts are effective and that as few lives as possible are lost will require strengthening the widespread and deep trust in the science and practice of public health.
Disclosures
The authors have nothing to disclose.
1. Pew Research Center. Trust and Distrust in America. July 2019. https://www.people-press.org/wp-content/uploads/sites/4/2019/07/pew-research-center_trust-distrust-in-america-report_2019-07-22-1.pdf. Accessed May 24, 2020.
2. Kirzinger A, Kearney A, Hamel L, Brodie M. KFF Health Tracking Poll – Early April 2020: The Impact of Coronavirus on Life in America. Kaiser Family Foundation. April 2, 2020. https://www.kff.org/health-reform/report/kff-health-tracking-poll-early-april-2020/. Accessed May 24, 2020.
3. Lazer D, Baum MA, Ognyanova K, Della Volpe J. The State of the Nation: A 50-State COVID-19 Survey. April 30, 2020. http://www.kateto.net/COVID19%20CONSORTIUM%20REPORT%20April%202020.pdf. Accessed May 24, 2020
4. Whitmer G. I have made gut-wrenching choices to keep people safe. New York Times. April 21, 2020. https://www.nytimes.com/2020/04/21/opinion/gretchen-whitmer-coronavirus-michigan.html. Accessed May 24, 2020.
5. Kluch S. The compliance curve: Will people stay home much longer? Gallup Blog. April 29, 2020. https://news.gallup.com/opinion/gallup/309491/compliance-curve-americans-stay-home-covid.aspx. Accessed May 24, 2020.
6. Deutsch J, Wheaton S. Public health experts are now the bad guys. Politico. April 21, 2020. https://www.politico.com/news/2020/04/21/public-health-experts-are-now-the-bad-guys-198174. Accessed May 24, 2020.
7. Galva JE, Atchinson C, Levey S. Public health strategy and the police powers of the state. Public Health Rep. 2005;120(Suppl 1):20-27. https://doi.org/10.1177/00333549051200s106.
8. Gostin LO. Public health law in a new century: part III: public health regulation: a systematic evaluation. JAMA. 2000;283(23):3118-3122. https://doi.org/10.1001/jama.283.23.3118.
9. Smith SMS, Sonego S, Wallen G, et al. Use of non-pharmaceutical interventions to reduce the transmission of influenza in adults: a systematic review. Respirology. 2015;20(6):896-903. https://doi.org/10.1111/resp.12541.
10. Harris JE. The coronavirus epidemic curve is already flattening in New York City. National Bureau of Economic Research. April 2020. https://www.nber.org/papers/w26917. Accessed May 24, 2020.
11. La VP, Pham TH, Ho MT, et al. Policy response, social media and scientific journals for the sustainability of the public health system amid the COVID-19 outbreak: the Vietnam lessons. Sustainability. 2020;12(7):2931. https://doi.org/10.3390/su12072931.
12. Glik DC. Risk communication for public health emergencies. Annu Rev Public Health. 2007;28:33-54. https://doi.org/10.1146/annurev.publhealth.28.021406.144123.
13. MacDonald L, Cairns G, Angus K, Stead M. Evidence Review: Social Marketing for the Prevention and Control of Communicable Disease. Stockholm: ECDC; 2012. https://www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/Social-marketing-prevention-control-of-communicable-disease.pdf. Accessed May 8, 2020.
14. Dosani S and Westbrook A. The leader we wish we all had: A look at the style of Dr Amy Acton, who has earned praise for her daily briefings on the pandemic. New York Times. May 5, 2020. https://www.nytimes.com/2020/05/05/opinion/coronavirus-ohio-amy-acton.html.
15. Snyder L. An anniversary review and critique: the Tylenol crisis. Public Relations Rev. 1983;9(3):24-34. https://doi.org/10.1016/S0363-8111(83)80182-9.
16. Millar H, Davidson A, White LA. Puzzling publics: the role of reflexive learning in universal pre-kindergarten (UPK) policy formulation in Canada and the US. Public Policy Adm. 2020;35(3):312-336. https://doi.org/10.1177/0952076719889100.
The visibility of public health—both as a science and a government responsibility—has increased dramatically with the COVID-19 pandemic. Public health science, surveillance, and emergency interventions are saving lives across the globe. Public health leaders are advising local, state, national, and international policymakers and have a consistent and strong voice in the media. We describe here the trust challenges facing public health in this moment of crisis, as well as the strategies necessary to maintain and increase that trust.
In the United States, public opinion data suggest that, while trust in science and government is relatively low and has been declining in recent years, trust in public health is high.1,2 In a survey released in April, 2020, the most trusted groups “to do the right thing” on COVID-19 were doctors, hospitals, scientists, researchers, and the Centers for Disease Control and Prevention (CDC).3 Trust in state government was the next highest. Some governors have been particularly strong in supporting public health messages. For example, Governor Gretchen Whitmer in Michigan has repeatedly stated that her decisions are based on science and public health4; Michiganders reported trust in state government at 79%, compared with trust in the White House at 54%.3 In Ohio, where Governor Mike DeWine has stood with his director of public health, Amy Acton, MD, MPH, in his pandemic response, trust in state government was 80%, compared with trust in the White House at 62%.3
Until there is an effective vaccine with high levels of uptake, COVID-19 prevention and control efforts are going to primarily rely on intrusive and challenging public health interventions such as school/business closures, stay-at-home orders, crowd limits, and travel restrictions. Maintaining trust in and support for both public health interventions and leaders requires intentional strategies that are sophisticated and deploy effective social marketing and risk communication strategies.
CHALLENGES TO MAINTAINING TRUST IN PUBLIC HEALTH
Early in the trajectory of COVID-19, Americans were almost uniform in their support for stay-at-home orders.5 Later, as the economic and social impact of self-quarantine, business, and school closures deepened, backlash began to increase.6 As recent protests against stay-at-home orders and other COVID-19-interventions reveal, many people do not understand the breadth of government’s duty to protect the public’s health and welfare. In fact, the US Constitution gives states a significant amount of power to protect the health, safety, and welfare of their populations, including “police powers” that generally fall into three categories: (a) protecting people who cannot protect themselves, (b) protecting people from others, and (c) protecting people from themselves.7,8 Current executive orders and other government actions designed to combat COVID-19 represent the use of police powers in all three of these areas.
It is exceedingly difficult for governments to design effective pandemic interventions—including executive orders and laws based on “police power”—that protect the public’s health without negatively affecting the economy, healthcare system, schools, and the financial and psychosocial welfare of citizens.
To compound this challenge, while local, state, and federal governments have the authority to act strongly and swiftly in a public crisis, American’s passionate political and philosophical attachments to freedom and self-determination and their skepticism about government interference cannot be dismissed. “Life, liberty, and the pursuit of happiness” is more than a line in the Declaration of Independence—it reflects a strong set of American values that make the case for action that is collectively based while honoring individual interests. Although Americans have a deep-seated belief in individual freedoms, public health relies on collective action for success. Public health leaders must understand this tension and effectively articulate why and when collective action is necessary while also articulating a path to move from a uniform, state-imposed emergency response to one that relies on responsible individual actions.
The federal government’s conflicting messages on science and the public health are also an enormous threat to public health. When the White House’s top trade adviser publicly criticizes the response of the CDC, the CDC guidance appears politicized, which erodes public trust.
Unfortunately, public health in the United States has generally struggled to make a clear and compelling case for prevention and nonmedical approaches to health and well-being. As the saying goes, “Public health is invisible when it is most effective.” Public health leaders are trained in epidemiology and other sciences, in community-based partnerships, and sometimes medicine. However, few public health leaders have been trained in advocacy communication.
STRATEGIES TO STRENGTHEN TRUST IN PUBLIC HEALTH
Government leaders and their partners can better balance the health, economic, and other needs of the population if they effectively communicate the rationale and need for population-based public health interventions in ways that are based on communication science and are politically savvy. A civics lesson from public health officials about constitutional law and the role of police power in combating COVID-19 is not likely to be effective. However, sophisticated messaging tailored to different audiences about the government’s role in protecting the health of everyone could be.
While much is still unknown regarding COVID-19, the evidence is clear that nonpharmaceutical interventions like self-quarantine and isolation, physical distancing, business and school closures, and other core public health strategies are effective in reducing community spread and can flatten the infectious-disease epidemiologic curve.9,10 Countries such as South Korea, New Zealand, Australia, and Germany—countries that have taken strong public-health approaches on social distancing and stay-at-home orders along with extensive testing and contact tracing—have demonstrated reduced rates of severe morbidity and mortality from COVID-19. Vietnam, a developing country of 96 million people that borders China, has reported zero deaths from COVID-19 to date because of both swift public health actions and strong communication strategies.11
Public health communication efforts regarding COVID-19 should be based on risk and crisis communication science and on best practices for social marketing that rallies people around shared values.12,13 For example, communications from Dr Acton have attempted to “inspire” rather than “order” people to physically isolate by appealing to widely shared core values.14 This includes acknowledging the hardships people are experiencing, emphasizing the important historic role that everyone is playing in their sacrifices, promoting determination rather than fear, and declaring that “not all heroes wear capes.” Best practices in communication also include segmenting audiences for the design and testing of different communication approaches.12
Public health leaders can also learn from the extensive research from other fields in how to build trust. Consumer product research emphasizes the importance of transparency in sharing known and unknown risks and admitting error when errors are made.15
Engagement of the public in policy decision-making is also essential in situations of uncertainty. Since much is unknown about COVID-19, policy guidance about mitigation and prevention strategies has changed in real time. Changing messages on the importance of face masks is an example of the trust challenge for public health. In the initial stages of the pandemic, the CDC discouraged the use of face masks. As more data became available, the CDC changed its guidance. Such changed guidance can undermine the entire public health message on protective factors. Acknowledging uncertainty and engaging the public in decision-making through a process of reflexive learning can build public trust in a time of uncertainty.16
COVID-19 has also reaffirmed and illuminated that the public health and healthcare delivery systems are intertwined. Failure to “flatten the curve” results in an overrun healthcare system, enormous costs, and significant mortality. However, public health efforts that successfully slow and limit community spread also produce significant financial losses for healthcare systems because the use of all types of nonemergent care greatly decreases. Public health and healthcare system leaders must partner in the strategic design and reinforcement of messages to build strong and lasting trust in the ongoing public health interventions and mandates that are going to be with us for the unforeseen future.
Finally, maintaining trust in the face of political attacks on our agencies of public health requires the healthcare community speak out in unity—endorsing science-based recommendations and supporting the CDC, the World Health Organization, and local public health.
CONCLUSION
Public health is at an unprecedented and crucial moment in this global pandemic, with growing societal understanding of the role that public health plays in our lives. Public health leaders have a unique opportunity to build on that understanding, strengthen trust, and increase funding and support for core public health services.
Balancing risks and benefits in the face of great uncertainty is never easy. With COVID-19, the horrific number of deaths and speed of community spread has led to a strong and essential public health emergency response throughout most of the country. Keeping the public committed to the important and ongoing measures necessary to ensure that prevention/control efforts are effective and that as few lives as possible are lost will require strengthening the widespread and deep trust in the science and practice of public health.
Disclosures
The authors have nothing to disclose.
The visibility of public health—both as a science and a government responsibility—has increased dramatically with the COVID-19 pandemic. Public health science, surveillance, and emergency interventions are saving lives across the globe. Public health leaders are advising local, state, national, and international policymakers and have a consistent and strong voice in the media. We describe here the trust challenges facing public health in this moment of crisis, as well as the strategies necessary to maintain and increase that trust.
In the United States, public opinion data suggest that, while trust in science and government is relatively low and has been declining in recent years, trust in public health is high.1,2 In a survey released in April, 2020, the most trusted groups “to do the right thing” on COVID-19 were doctors, hospitals, scientists, researchers, and the Centers for Disease Control and Prevention (CDC).3 Trust in state government was the next highest. Some governors have been particularly strong in supporting public health messages. For example, Governor Gretchen Whitmer in Michigan has repeatedly stated that her decisions are based on science and public health4; Michiganders reported trust in state government at 79%, compared with trust in the White House at 54%.3 In Ohio, where Governor Mike DeWine has stood with his director of public health, Amy Acton, MD, MPH, in his pandemic response, trust in state government was 80%, compared with trust in the White House at 62%.3
Until there is an effective vaccine with high levels of uptake, COVID-19 prevention and control efforts are going to primarily rely on intrusive and challenging public health interventions such as school/business closures, stay-at-home orders, crowd limits, and travel restrictions. Maintaining trust in and support for both public health interventions and leaders requires intentional strategies that are sophisticated and deploy effective social marketing and risk communication strategies.
CHALLENGES TO MAINTAINING TRUST IN PUBLIC HEALTH
Early in the trajectory of COVID-19, Americans were almost uniform in their support for stay-at-home orders.5 Later, as the economic and social impact of self-quarantine, business, and school closures deepened, backlash began to increase.6 As recent protests against stay-at-home orders and other COVID-19-interventions reveal, many people do not understand the breadth of government’s duty to protect the public’s health and welfare. In fact, the US Constitution gives states a significant amount of power to protect the health, safety, and welfare of their populations, including “police powers” that generally fall into three categories: (a) protecting people who cannot protect themselves, (b) protecting people from others, and (c) protecting people from themselves.7,8 Current executive orders and other government actions designed to combat COVID-19 represent the use of police powers in all three of these areas.
It is exceedingly difficult for governments to design effective pandemic interventions—including executive orders and laws based on “police power”—that protect the public’s health without negatively affecting the economy, healthcare system, schools, and the financial and psychosocial welfare of citizens.
To compound this challenge, while local, state, and federal governments have the authority to act strongly and swiftly in a public crisis, American’s passionate political and philosophical attachments to freedom and self-determination and their skepticism about government interference cannot be dismissed. “Life, liberty, and the pursuit of happiness” is more than a line in the Declaration of Independence—it reflects a strong set of American values that make the case for action that is collectively based while honoring individual interests. Although Americans have a deep-seated belief in individual freedoms, public health relies on collective action for success. Public health leaders must understand this tension and effectively articulate why and when collective action is necessary while also articulating a path to move from a uniform, state-imposed emergency response to one that relies on responsible individual actions.
The federal government’s conflicting messages on science and the public health are also an enormous threat to public health. When the White House’s top trade adviser publicly criticizes the response of the CDC, the CDC guidance appears politicized, which erodes public trust.
Unfortunately, public health in the United States has generally struggled to make a clear and compelling case for prevention and nonmedical approaches to health and well-being. As the saying goes, “Public health is invisible when it is most effective.” Public health leaders are trained in epidemiology and other sciences, in community-based partnerships, and sometimes medicine. However, few public health leaders have been trained in advocacy communication.
STRATEGIES TO STRENGTHEN TRUST IN PUBLIC HEALTH
Government leaders and their partners can better balance the health, economic, and other needs of the population if they effectively communicate the rationale and need for population-based public health interventions in ways that are based on communication science and are politically savvy. A civics lesson from public health officials about constitutional law and the role of police power in combating COVID-19 is not likely to be effective. However, sophisticated messaging tailored to different audiences about the government’s role in protecting the health of everyone could be.
While much is still unknown regarding COVID-19, the evidence is clear that nonpharmaceutical interventions like self-quarantine and isolation, physical distancing, business and school closures, and other core public health strategies are effective in reducing community spread and can flatten the infectious-disease epidemiologic curve.9,10 Countries such as South Korea, New Zealand, Australia, and Germany—countries that have taken strong public-health approaches on social distancing and stay-at-home orders along with extensive testing and contact tracing—have demonstrated reduced rates of severe morbidity and mortality from COVID-19. Vietnam, a developing country of 96 million people that borders China, has reported zero deaths from COVID-19 to date because of both swift public health actions and strong communication strategies.11
Public health communication efforts regarding COVID-19 should be based on risk and crisis communication science and on best practices for social marketing that rallies people around shared values.12,13 For example, communications from Dr Acton have attempted to “inspire” rather than “order” people to physically isolate by appealing to widely shared core values.14 This includes acknowledging the hardships people are experiencing, emphasizing the important historic role that everyone is playing in their sacrifices, promoting determination rather than fear, and declaring that “not all heroes wear capes.” Best practices in communication also include segmenting audiences for the design and testing of different communication approaches.12
Public health leaders can also learn from the extensive research from other fields in how to build trust. Consumer product research emphasizes the importance of transparency in sharing known and unknown risks and admitting error when errors are made.15
Engagement of the public in policy decision-making is also essential in situations of uncertainty. Since much is unknown about COVID-19, policy guidance about mitigation and prevention strategies has changed in real time. Changing messages on the importance of face masks is an example of the trust challenge for public health. In the initial stages of the pandemic, the CDC discouraged the use of face masks. As more data became available, the CDC changed its guidance. Such changed guidance can undermine the entire public health message on protective factors. Acknowledging uncertainty and engaging the public in decision-making through a process of reflexive learning can build public trust in a time of uncertainty.16
COVID-19 has also reaffirmed and illuminated that the public health and healthcare delivery systems are intertwined. Failure to “flatten the curve” results in an overrun healthcare system, enormous costs, and significant mortality. However, public health efforts that successfully slow and limit community spread also produce significant financial losses for healthcare systems because the use of all types of nonemergent care greatly decreases. Public health and healthcare system leaders must partner in the strategic design and reinforcement of messages to build strong and lasting trust in the ongoing public health interventions and mandates that are going to be with us for the unforeseen future.
Finally, maintaining trust in the face of political attacks on our agencies of public health requires the healthcare community speak out in unity—endorsing science-based recommendations and supporting the CDC, the World Health Organization, and local public health.
CONCLUSION
Public health is at an unprecedented and crucial moment in this global pandemic, with growing societal understanding of the role that public health plays in our lives. Public health leaders have a unique opportunity to build on that understanding, strengthen trust, and increase funding and support for core public health services.
Balancing risks and benefits in the face of great uncertainty is never easy. With COVID-19, the horrific number of deaths and speed of community spread has led to a strong and essential public health emergency response throughout most of the country. Keeping the public committed to the important and ongoing measures necessary to ensure that prevention/control efforts are effective and that as few lives as possible are lost will require strengthening the widespread and deep trust in the science and practice of public health.
Disclosures
The authors have nothing to disclose.
1. Pew Research Center. Trust and Distrust in America. July 2019. https://www.people-press.org/wp-content/uploads/sites/4/2019/07/pew-research-center_trust-distrust-in-america-report_2019-07-22-1.pdf. Accessed May 24, 2020.
2. Kirzinger A, Kearney A, Hamel L, Brodie M. KFF Health Tracking Poll – Early April 2020: The Impact of Coronavirus on Life in America. Kaiser Family Foundation. April 2, 2020. https://www.kff.org/health-reform/report/kff-health-tracking-poll-early-april-2020/. Accessed May 24, 2020.
3. Lazer D, Baum MA, Ognyanova K, Della Volpe J. The State of the Nation: A 50-State COVID-19 Survey. April 30, 2020. http://www.kateto.net/COVID19%20CONSORTIUM%20REPORT%20April%202020.pdf. Accessed May 24, 2020
4. Whitmer G. I have made gut-wrenching choices to keep people safe. New York Times. April 21, 2020. https://www.nytimes.com/2020/04/21/opinion/gretchen-whitmer-coronavirus-michigan.html. Accessed May 24, 2020.
5. Kluch S. The compliance curve: Will people stay home much longer? Gallup Blog. April 29, 2020. https://news.gallup.com/opinion/gallup/309491/compliance-curve-americans-stay-home-covid.aspx. Accessed May 24, 2020.
6. Deutsch J, Wheaton S. Public health experts are now the bad guys. Politico. April 21, 2020. https://www.politico.com/news/2020/04/21/public-health-experts-are-now-the-bad-guys-198174. Accessed May 24, 2020.
7. Galva JE, Atchinson C, Levey S. Public health strategy and the police powers of the state. Public Health Rep. 2005;120(Suppl 1):20-27. https://doi.org/10.1177/00333549051200s106.
8. Gostin LO. Public health law in a new century: part III: public health regulation: a systematic evaluation. JAMA. 2000;283(23):3118-3122. https://doi.org/10.1001/jama.283.23.3118.
9. Smith SMS, Sonego S, Wallen G, et al. Use of non-pharmaceutical interventions to reduce the transmission of influenza in adults: a systematic review. Respirology. 2015;20(6):896-903. https://doi.org/10.1111/resp.12541.
10. Harris JE. The coronavirus epidemic curve is already flattening in New York City. National Bureau of Economic Research. April 2020. https://www.nber.org/papers/w26917. Accessed May 24, 2020.
11. La VP, Pham TH, Ho MT, et al. Policy response, social media and scientific journals for the sustainability of the public health system amid the COVID-19 outbreak: the Vietnam lessons. Sustainability. 2020;12(7):2931. https://doi.org/10.3390/su12072931.
12. Glik DC. Risk communication for public health emergencies. Annu Rev Public Health. 2007;28:33-54. https://doi.org/10.1146/annurev.publhealth.28.021406.144123.
13. MacDonald L, Cairns G, Angus K, Stead M. Evidence Review: Social Marketing for the Prevention and Control of Communicable Disease. Stockholm: ECDC; 2012. https://www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/Social-marketing-prevention-control-of-communicable-disease.pdf. Accessed May 8, 2020.
14. Dosani S and Westbrook A. The leader we wish we all had: A look at the style of Dr Amy Acton, who has earned praise for her daily briefings on the pandemic. New York Times. May 5, 2020. https://www.nytimes.com/2020/05/05/opinion/coronavirus-ohio-amy-acton.html.
15. Snyder L. An anniversary review and critique: the Tylenol crisis. Public Relations Rev. 1983;9(3):24-34. https://doi.org/10.1016/S0363-8111(83)80182-9.
16. Millar H, Davidson A, White LA. Puzzling publics: the role of reflexive learning in universal pre-kindergarten (UPK) policy formulation in Canada and the US. Public Policy Adm. 2020;35(3):312-336. https://doi.org/10.1177/0952076719889100.
1. Pew Research Center. Trust and Distrust in America. July 2019. https://www.people-press.org/wp-content/uploads/sites/4/2019/07/pew-research-center_trust-distrust-in-america-report_2019-07-22-1.pdf. Accessed May 24, 2020.
2. Kirzinger A, Kearney A, Hamel L, Brodie M. KFF Health Tracking Poll – Early April 2020: The Impact of Coronavirus on Life in America. Kaiser Family Foundation. April 2, 2020. https://www.kff.org/health-reform/report/kff-health-tracking-poll-early-april-2020/. Accessed May 24, 2020.
3. Lazer D, Baum MA, Ognyanova K, Della Volpe J. The State of the Nation: A 50-State COVID-19 Survey. April 30, 2020. http://www.kateto.net/COVID19%20CONSORTIUM%20REPORT%20April%202020.pdf. Accessed May 24, 2020
4. Whitmer G. I have made gut-wrenching choices to keep people safe. New York Times. April 21, 2020. https://www.nytimes.com/2020/04/21/opinion/gretchen-whitmer-coronavirus-michigan.html. Accessed May 24, 2020.
5. Kluch S. The compliance curve: Will people stay home much longer? Gallup Blog. April 29, 2020. https://news.gallup.com/opinion/gallup/309491/compliance-curve-americans-stay-home-covid.aspx. Accessed May 24, 2020.
6. Deutsch J, Wheaton S. Public health experts are now the bad guys. Politico. April 21, 2020. https://www.politico.com/news/2020/04/21/public-health-experts-are-now-the-bad-guys-198174. Accessed May 24, 2020.
7. Galva JE, Atchinson C, Levey S. Public health strategy and the police powers of the state. Public Health Rep. 2005;120(Suppl 1):20-27. https://doi.org/10.1177/00333549051200s106.
8. Gostin LO. Public health law in a new century: part III: public health regulation: a systematic evaluation. JAMA. 2000;283(23):3118-3122. https://doi.org/10.1001/jama.283.23.3118.
9. Smith SMS, Sonego S, Wallen G, et al. Use of non-pharmaceutical interventions to reduce the transmission of influenza in adults: a systematic review. Respirology. 2015;20(6):896-903. https://doi.org/10.1111/resp.12541.
10. Harris JE. The coronavirus epidemic curve is already flattening in New York City. National Bureau of Economic Research. April 2020. https://www.nber.org/papers/w26917. Accessed May 24, 2020.
11. La VP, Pham TH, Ho MT, et al. Policy response, social media and scientific journals for the sustainability of the public health system amid the COVID-19 outbreak: the Vietnam lessons. Sustainability. 2020;12(7):2931. https://doi.org/10.3390/su12072931.
12. Glik DC. Risk communication for public health emergencies. Annu Rev Public Health. 2007;28:33-54. https://doi.org/10.1146/annurev.publhealth.28.021406.144123.
13. MacDonald L, Cairns G, Angus K, Stead M. Evidence Review: Social Marketing for the Prevention and Control of Communicable Disease. Stockholm: ECDC; 2012. https://www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/Social-marketing-prevention-control-of-communicable-disease.pdf. Accessed May 8, 2020.
14. Dosani S and Westbrook A. The leader we wish we all had: A look at the style of Dr Amy Acton, who has earned praise for her daily briefings on the pandemic. New York Times. May 5, 2020. https://www.nytimes.com/2020/05/05/opinion/coronavirus-ohio-amy-acton.html.
15. Snyder L. An anniversary review and critique: the Tylenol crisis. Public Relations Rev. 1983;9(3):24-34. https://doi.org/10.1016/S0363-8111(83)80182-9.
16. Millar H, Davidson A, White LA. Puzzling publics: the role of reflexive learning in universal pre-kindergarten (UPK) policy formulation in Canada and the US. Public Policy Adm. 2020;35(3):312-336. https://doi.org/10.1177/0952076719889100.
© 2020 Society of Hospital Medicine
Empiric Therapies for COVID-19: Destined to Fail by Ignoring the Lessons of History
Manifestations of disease, as perceived by physicians, can shape conceptual views and favor specific therapeutic actions. Historically, these factors appear to have an outsized influence on medical thinking in general. Disease concepts derived from empirical observations during pandemics impose a trade-off. We obtain unparalleled insight into medical thought and practice, but risk incurring the cost of unfortunate mistakes. The psychologist and Nobel Prize winner in economics Daniel Kahneman describes two mental systems that shape our judgments and decision-making in his book, Thinking, Fast and Slow: System One is intuitive, emotional, and fast, whereas System Two is deliberative and logical and has slower onset.1 If we extrapolate these observations to clinical medicine, we often rely on either System One or System Two depending on particular situations. Errors can emerge when we default to fast and emotional responses in situations that instead require more deliberate and logical assessments. These include instances in which the desire to help—our humanitarian role as physicians, associated with an “adrenaline rush”—results from attempts to relieve human suffering. As mercenaries of misfortune, it is inevitable we engage medical interventions based on an incomplete understanding of the pathophysiology—in other words, without understanding the full risks and benefits.
During the ongoing COVID-19 pandemic, members of the medical community continue to provide care with the utmost nobility, empathy, and desire for action amid uncertainty. However, as the number of cases continues to increase worldwide, we urge caution in evaluating the current state of scientific understanding, our approaches to treatment, and the safety of empiric medical interventions targeting COVID-19. We are concerned that the extensive history of unintended adverse consequences of therapies for emerging infectious diseases in the past is being ignored in the development of approaches to COVID-19 treatment. It is likely harms will emerge from current empiric therapies for COVID-19 given what can be learned from history.
HISTORICAL EXAMPLES OF UNINTENDED ADVERSE CONSEQUENCES
Whereas influenza can be treated with neuraminidase inhibitors,2 there are currently no established effective antiviral therapies for COVID-19, which is similar to two other coronavirus diseases from the 21st century, SARS (Severe Acute Respiratory Syndrome) in 2003 and MERS (Middle-Eastern Respiratory Syndrome) in 2012.3 Even in times of global pandemic, we need to consider potential harms and adverse consequences of novel treatments and show justifiable ratio of risk versus benefit. With the absence of proven COVID-19 therapy and the desire to fulfill our oath of primum non nocere (first, do no harm) in mind, we review selected unintended adverse events of developing therapies for infectious diseases.
Two types of error in our decision-making strategies are errors of omission and errors of commission.4 Errors of omission, defined as instances in which a medical intervention was not carried out when there was a clear indication to do so, are less conspicuous in the history of infectious disease therapeutics. Errors of commission, in contrast, have become a more concerning component of our approach to COVID-19 therapy, perhaps prompted by our desire to act. Errors of commission are defined as instances in which a specific medical intervention that should have been avoided was instead performed. We will discuss historical examples of errors of commission to highlight parallels with the current pandemic (Appendix Figure).
During influenza epidemics in the 18th century, some physicians advocated the use of therapeutic lancet phlebotomies, while others recommended indiscriminate use of opium, which led to high rates of addiction.5 Neither intervention was supported by a reassuring body of evidence. Many recommended mercury-based preparations during major outbreaks of syphilis in medieval protestant Europe. Because of accumulated mercurial toxicity, many persons suffered long-term sequelae including chronic kidney injury and peripheral neuropathy.6 After the discovery of the tuberculous bacillus, Robert Koch attempted the inoculation of tuberculin as a curative intervention for tuberculosis.7 Under pressure from the king of Prussia to present his findings at the International Medical Meeting in Berlin, Germany, in 1890, Koch conducted a poorly executed clinical trial. Rudolf Virchow then demonstrated endobronchial spread of the infection with resultant clinical worsening in those who received Koch’s tuberculin. In 1905, Harold Wolfersan Thomas at the Liverpool School of Tropical Medicine treated cases of African trypanosomiasis with the arsenical drug Atoxyl (arsanilic acid), which demonstrated some efficacy but also caused optic nerve atrophy leading to blindness.8
There have also been errors of commission in the development of vaccines. One such event, known as the Cutter incident, followed from an incompletely inactivated batch of polio vaccine that caused 40,000 cases of abortive poliomyelitis and many cases of paralysis and death.9 In the early phases of the development of the yellow fever vaccine, Hideyo Noguchi tried to develop a vaccine based on the erroneous assumption that yellow fever was caused by Leptospira icteroides.10 In 1976, an error of commission occurred in response to an outbreak of a few dozen cases of Influenza A/H1N1 in Fort Dix, New Jersey: The accelerated implementation of a swine influenza–vaccination program led to many cases of Guillian-Barré Syndrome among recipients.11 Immunization experts defended this decision to vaccinate by arguing that “when lives are at risk, it’s better to err on the side of overreaction over underreaction.”11 However, this is a risk-perception versus risk-management concept that drives potential errors of commission.
A more recent error of commission involved the use of drotrecogin alfa (activated protein C) in the treatment of sepsis. This drug became the first and only Food and Drug Administration–approved drug for sepsis treatment. The approval process of this medication relied on one clinical trial, which was terminated early because of perceived overwhelming evidence of efficacy. Despite the initial high medical and financial expectations, Eli Lilly (Indianapolis) withdrew the drug when a larger, international clinical trial (PROWESS-SHOCK) did not show a similar benefit.12
THE COVID-19 ERA
The gravity of the COVID-19 pandemic has motivated the repurposing of previously available therapies. This explains the use of medications like hydroxychloroquine, interleukin-6 (IL-6) receptor antagonists, and remdesivir.13-15
Despite early authorization of emergency use for hydroxychloroquine by the FDA based on limited and poor-quality evidence,16 this drug has yet to demonstrate treatment efficacy for COVID-19. On the contrary, other, controlled, retrospective studies have shown that hydroxychloroquine might actually increase mortality, possibly through prolongation of the QT-interval.16,17 Also, diversion of this drug to treat COVID-19 raises the concern of hydroxychloroquine shortages for treatment of patients with autoimmune disease, in whom the drug has proven benefit. We question the hasty FDA authorization for emergency use of hydroxychloroquine for COVID-19.
There is also great enthusiasm among the medical community to administer IL-6 receptor antagonists as a COVID-19 treatment. The rationale for this approach includes observations in case series in which IL-6 levels correlated with adverse clinical outcomes.13 IL-6 antagonists have a proven track record of improving the outcome in autoimmune diseases. However, we must avoid the logical trap of post hoc, ergo propter hoc (after this, therefore because of this) dictum from which one would assume that, based on those observations of high IL-6 levels and adverse outcomes, lowering IL-6 levels will necessarily improve outcomes in COVID-19. The supposed role of IL-6 in causing COVID-19 is based on scant preliminary observations and on the yet unproven assumption that IL-6 association with disease severity is a cause-effect relationship and not an association separate from pathogenesis. Moreover, there is sufficient scientific evidence that, in the case of severe influenza infections, IL-6 limits inflammation and protects against severe and potentially life-threatening lung injury. The road ahead for IL-6 inhibition to treat COVID-19 is perilous and should be entered cautiously. One immediate concern of administering IL-6 receptor antagonists in this patient population is the potential reactivation of latent tuberculosis infection and hepatitis B, colonic perforation, and increased rate of infections in general.
The greatest hope at this early stage of the COVID-19 pandemic may be remdesivir, which is a direct-acting antiviral. Here again, initial case series in prestigious medical journals signaled the possibility of a morbidity and mortality benefit.14 Despite these encouraging signs, a recent clinical trial from China that was limited by incomplete patient enrollment demonstrated a lack of efficacy of remdesivir in accelerating clinical improvement or limiting mortality.18 In spite of these negative results, preliminary data from the Adaptive COVID-19 Treatment Trial (ACTT) has revealed a nonsignificant signal of reduced mortality and shorter time to recovery in the remdesivir group. In response to these reports, the FDA has now issued emergency use authorization of remdesivir for treating COVID-19. Given the precedence of conflicting study data in therapeutic development for infectious diseases, we urge caution in drawing interpretations of benefit based on these early reports. Early termination of clinical studies is often associated with a 30% overestimation of clinical benefit.19 Furthermore, the availability of remdesivir is limited, which raises substantial ethical concerns on the preferential allocation of the drug to selected populations in high-income countries. At the time of this report, uncertainty regarding the risk-benefit balance of remdesivir and other COVID-19 treatments should be emphasized among decision makers.
CONCLUSION
Errors of commission present particular concerns for risk in treating COVID-19 patients and suggest that sometimes inaction is preferable to action. With many pandemics, there is a history of repeating mistakes, and we believe this can be curtailed by heeding the lessons of history. In the end, we may learn that avoiding therapeutic interventions that are poorly supported may prove to be one of the most important legacies of the COVID-19 pandemic.
Disclosures
The authors reported having nothing to disclose.
Funding
Dr Shapiro is supported by The Emily Foundation, Boston, Massachusetts. For all other authors, no financial support was declared.
1. Kahneman D. Thinking, Fast and Slow. New York: Farrar, Straus and Giroux; 2011.
2. Boikos C, Caya C, Doll MK, et al. Safety and effectiveness of neuraminidase inhibitors in situations of pandemic and/or novel/variant influenza: a systematic review of the literature, 2009-15. J Antimicrob Chemother. 2017;72(6):1556-1573. https://doi.org/10.1093/jac/dkx013.
3. de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14(8):523-534. https://doi.org/10.1038/nrmicro.2016.81.
4. Grober ED, Bohnen JM. Defining medical error. Can J Surg. 2005;48(1):39-44.
5. Schofield AT. Opium in influenza. Lancet. 1894;143(3676):372. https://doi.org/10.1016/S0140-6736(01)66349-9.
6. Abraham JJ. Some account of the history of the treatment of syphilis. Br J Vener Dis. 1948;24(4):153-161. https://doi.org/10.1136/sti.24.4.153.
7. Gradmann C. Laboratory Disease: Robert Koch’s Medical Bacteriology. Baltimore, MD: Johns Hopkins University Press; 2009. .
8. Steverding D. The history of African trypanosomiasis. Parasit Vectors. 2008;1(1):3. https://doi.org/10.1186/1756-3305-1-3
9. Offit PA. The Cutter incident, 50 years later. N Engl J Med. 2005;352(14):1411-1412. https://doi.org/10.1056/nejmp048180.
10. Frierson JG. The yellow fever vaccine: a history. Yale J Biol Med. 2010;83(2):77-85.
11. Sencer DJ, Millar JD. Reflections on the 1976 swine flu vaccination program. Emerg Infect Dis. 2006;12(1):29-33. https://doi.org/10.3201/eid1201.051007.
12. Ranieri VM, Thompson BT, Barie PS, et al. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366(22):2055-2064. https://doi.org/10.1056/nejmoa1202290.
13. Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab [online first]. ChinaXiv. 2020. https://doi.org/10.1073/pnas.2005615117.
14. Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe COVID-19 [online first]. N Engl J Med. 2020. https://doi.org/10.1056/nejmoa2007016.
15. Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial [online first]. Int J Antimicrob Agents. 2020. https://doi.org/10.1016/j.ijantimicag.2020.105949.
16. Magagnoli J, Narendran S, Pereira F, et al. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19 [preprint]. medRxiv. 2020. https://doi.org/10.1101/2020.04.16.20065920.
17. Bessière F, Roccia H, Delinière A, et al. Assessment of QT intervals in a case series of patients with coronavirus disease 2019 (COVID-19) infection treated with hydroxychloroquine alone or in combination with azithromycin in an intensive care unit [online first]. JAMA Cardiology. 2020. https://doi.org/10.1001/jamacardio.2020.1787.
18. Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569-1578. https://doi.org/10.1016/S0140-6736(20)31022-9.
19. Bassler D, Briel M, Montori VM, et al; STOPIT-2 Study Group. Stopping randomized trials early for benefit and estimation of treatment effects, systematic review and meta-regression analysis. JAMA. 2010 Mar 24;303(12):1180-1187. https://doi.org/jama.2010.310.
Manifestations of disease, as perceived by physicians, can shape conceptual views and favor specific therapeutic actions. Historically, these factors appear to have an outsized influence on medical thinking in general. Disease concepts derived from empirical observations during pandemics impose a trade-off. We obtain unparalleled insight into medical thought and practice, but risk incurring the cost of unfortunate mistakes. The psychologist and Nobel Prize winner in economics Daniel Kahneman describes two mental systems that shape our judgments and decision-making in his book, Thinking, Fast and Slow: System One is intuitive, emotional, and fast, whereas System Two is deliberative and logical and has slower onset.1 If we extrapolate these observations to clinical medicine, we often rely on either System One or System Two depending on particular situations. Errors can emerge when we default to fast and emotional responses in situations that instead require more deliberate and logical assessments. These include instances in which the desire to help—our humanitarian role as physicians, associated with an “adrenaline rush”—results from attempts to relieve human suffering. As mercenaries of misfortune, it is inevitable we engage medical interventions based on an incomplete understanding of the pathophysiology—in other words, without understanding the full risks and benefits.
During the ongoing COVID-19 pandemic, members of the medical community continue to provide care with the utmost nobility, empathy, and desire for action amid uncertainty. However, as the number of cases continues to increase worldwide, we urge caution in evaluating the current state of scientific understanding, our approaches to treatment, and the safety of empiric medical interventions targeting COVID-19. We are concerned that the extensive history of unintended adverse consequences of therapies for emerging infectious diseases in the past is being ignored in the development of approaches to COVID-19 treatment. It is likely harms will emerge from current empiric therapies for COVID-19 given what can be learned from history.
HISTORICAL EXAMPLES OF UNINTENDED ADVERSE CONSEQUENCES
Whereas influenza can be treated with neuraminidase inhibitors,2 there are currently no established effective antiviral therapies for COVID-19, which is similar to two other coronavirus diseases from the 21st century, SARS (Severe Acute Respiratory Syndrome) in 2003 and MERS (Middle-Eastern Respiratory Syndrome) in 2012.3 Even in times of global pandemic, we need to consider potential harms and adverse consequences of novel treatments and show justifiable ratio of risk versus benefit. With the absence of proven COVID-19 therapy and the desire to fulfill our oath of primum non nocere (first, do no harm) in mind, we review selected unintended adverse events of developing therapies for infectious diseases.
Two types of error in our decision-making strategies are errors of omission and errors of commission.4 Errors of omission, defined as instances in which a medical intervention was not carried out when there was a clear indication to do so, are less conspicuous in the history of infectious disease therapeutics. Errors of commission, in contrast, have become a more concerning component of our approach to COVID-19 therapy, perhaps prompted by our desire to act. Errors of commission are defined as instances in which a specific medical intervention that should have been avoided was instead performed. We will discuss historical examples of errors of commission to highlight parallels with the current pandemic (Appendix Figure).
During influenza epidemics in the 18th century, some physicians advocated the use of therapeutic lancet phlebotomies, while others recommended indiscriminate use of opium, which led to high rates of addiction.5 Neither intervention was supported by a reassuring body of evidence. Many recommended mercury-based preparations during major outbreaks of syphilis in medieval protestant Europe. Because of accumulated mercurial toxicity, many persons suffered long-term sequelae including chronic kidney injury and peripheral neuropathy.6 After the discovery of the tuberculous bacillus, Robert Koch attempted the inoculation of tuberculin as a curative intervention for tuberculosis.7 Under pressure from the king of Prussia to present his findings at the International Medical Meeting in Berlin, Germany, in 1890, Koch conducted a poorly executed clinical trial. Rudolf Virchow then demonstrated endobronchial spread of the infection with resultant clinical worsening in those who received Koch’s tuberculin. In 1905, Harold Wolfersan Thomas at the Liverpool School of Tropical Medicine treated cases of African trypanosomiasis with the arsenical drug Atoxyl (arsanilic acid), which demonstrated some efficacy but also caused optic nerve atrophy leading to blindness.8
There have also been errors of commission in the development of vaccines. One such event, known as the Cutter incident, followed from an incompletely inactivated batch of polio vaccine that caused 40,000 cases of abortive poliomyelitis and many cases of paralysis and death.9 In the early phases of the development of the yellow fever vaccine, Hideyo Noguchi tried to develop a vaccine based on the erroneous assumption that yellow fever was caused by Leptospira icteroides.10 In 1976, an error of commission occurred in response to an outbreak of a few dozen cases of Influenza A/H1N1 in Fort Dix, New Jersey: The accelerated implementation of a swine influenza–vaccination program led to many cases of Guillian-Barré Syndrome among recipients.11 Immunization experts defended this decision to vaccinate by arguing that “when lives are at risk, it’s better to err on the side of overreaction over underreaction.”11 However, this is a risk-perception versus risk-management concept that drives potential errors of commission.
A more recent error of commission involved the use of drotrecogin alfa (activated protein C) in the treatment of sepsis. This drug became the first and only Food and Drug Administration–approved drug for sepsis treatment. The approval process of this medication relied on one clinical trial, which was terminated early because of perceived overwhelming evidence of efficacy. Despite the initial high medical and financial expectations, Eli Lilly (Indianapolis) withdrew the drug when a larger, international clinical trial (PROWESS-SHOCK) did not show a similar benefit.12
THE COVID-19 ERA
The gravity of the COVID-19 pandemic has motivated the repurposing of previously available therapies. This explains the use of medications like hydroxychloroquine, interleukin-6 (IL-6) receptor antagonists, and remdesivir.13-15
Despite early authorization of emergency use for hydroxychloroquine by the FDA based on limited and poor-quality evidence,16 this drug has yet to demonstrate treatment efficacy for COVID-19. On the contrary, other, controlled, retrospective studies have shown that hydroxychloroquine might actually increase mortality, possibly through prolongation of the QT-interval.16,17 Also, diversion of this drug to treat COVID-19 raises the concern of hydroxychloroquine shortages for treatment of patients with autoimmune disease, in whom the drug has proven benefit. We question the hasty FDA authorization for emergency use of hydroxychloroquine for COVID-19.
There is also great enthusiasm among the medical community to administer IL-6 receptor antagonists as a COVID-19 treatment. The rationale for this approach includes observations in case series in which IL-6 levels correlated with adverse clinical outcomes.13 IL-6 antagonists have a proven track record of improving the outcome in autoimmune diseases. However, we must avoid the logical trap of post hoc, ergo propter hoc (after this, therefore because of this) dictum from which one would assume that, based on those observations of high IL-6 levels and adverse outcomes, lowering IL-6 levels will necessarily improve outcomes in COVID-19. The supposed role of IL-6 in causing COVID-19 is based on scant preliminary observations and on the yet unproven assumption that IL-6 association with disease severity is a cause-effect relationship and not an association separate from pathogenesis. Moreover, there is sufficient scientific evidence that, in the case of severe influenza infections, IL-6 limits inflammation and protects against severe and potentially life-threatening lung injury. The road ahead for IL-6 inhibition to treat COVID-19 is perilous and should be entered cautiously. One immediate concern of administering IL-6 receptor antagonists in this patient population is the potential reactivation of latent tuberculosis infection and hepatitis B, colonic perforation, and increased rate of infections in general.
The greatest hope at this early stage of the COVID-19 pandemic may be remdesivir, which is a direct-acting antiviral. Here again, initial case series in prestigious medical journals signaled the possibility of a morbidity and mortality benefit.14 Despite these encouraging signs, a recent clinical trial from China that was limited by incomplete patient enrollment demonstrated a lack of efficacy of remdesivir in accelerating clinical improvement or limiting mortality.18 In spite of these negative results, preliminary data from the Adaptive COVID-19 Treatment Trial (ACTT) has revealed a nonsignificant signal of reduced mortality and shorter time to recovery in the remdesivir group. In response to these reports, the FDA has now issued emergency use authorization of remdesivir for treating COVID-19. Given the precedence of conflicting study data in therapeutic development for infectious diseases, we urge caution in drawing interpretations of benefit based on these early reports. Early termination of clinical studies is often associated with a 30% overestimation of clinical benefit.19 Furthermore, the availability of remdesivir is limited, which raises substantial ethical concerns on the preferential allocation of the drug to selected populations in high-income countries. At the time of this report, uncertainty regarding the risk-benefit balance of remdesivir and other COVID-19 treatments should be emphasized among decision makers.
CONCLUSION
Errors of commission present particular concerns for risk in treating COVID-19 patients and suggest that sometimes inaction is preferable to action. With many pandemics, there is a history of repeating mistakes, and we believe this can be curtailed by heeding the lessons of history. In the end, we may learn that avoiding therapeutic interventions that are poorly supported may prove to be one of the most important legacies of the COVID-19 pandemic.
Disclosures
The authors reported having nothing to disclose.
Funding
Dr Shapiro is supported by The Emily Foundation, Boston, Massachusetts. For all other authors, no financial support was declared.
Manifestations of disease, as perceived by physicians, can shape conceptual views and favor specific therapeutic actions. Historically, these factors appear to have an outsized influence on medical thinking in general. Disease concepts derived from empirical observations during pandemics impose a trade-off. We obtain unparalleled insight into medical thought and practice, but risk incurring the cost of unfortunate mistakes. The psychologist and Nobel Prize winner in economics Daniel Kahneman describes two mental systems that shape our judgments and decision-making in his book, Thinking, Fast and Slow: System One is intuitive, emotional, and fast, whereas System Two is deliberative and logical and has slower onset.1 If we extrapolate these observations to clinical medicine, we often rely on either System One or System Two depending on particular situations. Errors can emerge when we default to fast and emotional responses in situations that instead require more deliberate and logical assessments. These include instances in which the desire to help—our humanitarian role as physicians, associated with an “adrenaline rush”—results from attempts to relieve human suffering. As mercenaries of misfortune, it is inevitable we engage medical interventions based on an incomplete understanding of the pathophysiology—in other words, without understanding the full risks and benefits.
During the ongoing COVID-19 pandemic, members of the medical community continue to provide care with the utmost nobility, empathy, and desire for action amid uncertainty. However, as the number of cases continues to increase worldwide, we urge caution in evaluating the current state of scientific understanding, our approaches to treatment, and the safety of empiric medical interventions targeting COVID-19. We are concerned that the extensive history of unintended adverse consequences of therapies for emerging infectious diseases in the past is being ignored in the development of approaches to COVID-19 treatment. It is likely harms will emerge from current empiric therapies for COVID-19 given what can be learned from history.
HISTORICAL EXAMPLES OF UNINTENDED ADVERSE CONSEQUENCES
Whereas influenza can be treated with neuraminidase inhibitors,2 there are currently no established effective antiviral therapies for COVID-19, which is similar to two other coronavirus diseases from the 21st century, SARS (Severe Acute Respiratory Syndrome) in 2003 and MERS (Middle-Eastern Respiratory Syndrome) in 2012.3 Even in times of global pandemic, we need to consider potential harms and adverse consequences of novel treatments and show justifiable ratio of risk versus benefit. With the absence of proven COVID-19 therapy and the desire to fulfill our oath of primum non nocere (first, do no harm) in mind, we review selected unintended adverse events of developing therapies for infectious diseases.
Two types of error in our decision-making strategies are errors of omission and errors of commission.4 Errors of omission, defined as instances in which a medical intervention was not carried out when there was a clear indication to do so, are less conspicuous in the history of infectious disease therapeutics. Errors of commission, in contrast, have become a more concerning component of our approach to COVID-19 therapy, perhaps prompted by our desire to act. Errors of commission are defined as instances in which a specific medical intervention that should have been avoided was instead performed. We will discuss historical examples of errors of commission to highlight parallels with the current pandemic (Appendix Figure).
During influenza epidemics in the 18th century, some physicians advocated the use of therapeutic lancet phlebotomies, while others recommended indiscriminate use of opium, which led to high rates of addiction.5 Neither intervention was supported by a reassuring body of evidence. Many recommended mercury-based preparations during major outbreaks of syphilis in medieval protestant Europe. Because of accumulated mercurial toxicity, many persons suffered long-term sequelae including chronic kidney injury and peripheral neuropathy.6 After the discovery of the tuberculous bacillus, Robert Koch attempted the inoculation of tuberculin as a curative intervention for tuberculosis.7 Under pressure from the king of Prussia to present his findings at the International Medical Meeting in Berlin, Germany, in 1890, Koch conducted a poorly executed clinical trial. Rudolf Virchow then demonstrated endobronchial spread of the infection with resultant clinical worsening in those who received Koch’s tuberculin. In 1905, Harold Wolfersan Thomas at the Liverpool School of Tropical Medicine treated cases of African trypanosomiasis with the arsenical drug Atoxyl (arsanilic acid), which demonstrated some efficacy but also caused optic nerve atrophy leading to blindness.8
There have also been errors of commission in the development of vaccines. One such event, known as the Cutter incident, followed from an incompletely inactivated batch of polio vaccine that caused 40,000 cases of abortive poliomyelitis and many cases of paralysis and death.9 In the early phases of the development of the yellow fever vaccine, Hideyo Noguchi tried to develop a vaccine based on the erroneous assumption that yellow fever was caused by Leptospira icteroides.10 In 1976, an error of commission occurred in response to an outbreak of a few dozen cases of Influenza A/H1N1 in Fort Dix, New Jersey: The accelerated implementation of a swine influenza–vaccination program led to many cases of Guillian-Barré Syndrome among recipients.11 Immunization experts defended this decision to vaccinate by arguing that “when lives are at risk, it’s better to err on the side of overreaction over underreaction.”11 However, this is a risk-perception versus risk-management concept that drives potential errors of commission.
A more recent error of commission involved the use of drotrecogin alfa (activated protein C) in the treatment of sepsis. This drug became the first and only Food and Drug Administration–approved drug for sepsis treatment. The approval process of this medication relied on one clinical trial, which was terminated early because of perceived overwhelming evidence of efficacy. Despite the initial high medical and financial expectations, Eli Lilly (Indianapolis) withdrew the drug when a larger, international clinical trial (PROWESS-SHOCK) did not show a similar benefit.12
THE COVID-19 ERA
The gravity of the COVID-19 pandemic has motivated the repurposing of previously available therapies. This explains the use of medications like hydroxychloroquine, interleukin-6 (IL-6) receptor antagonists, and remdesivir.13-15
Despite early authorization of emergency use for hydroxychloroquine by the FDA based on limited and poor-quality evidence,16 this drug has yet to demonstrate treatment efficacy for COVID-19. On the contrary, other, controlled, retrospective studies have shown that hydroxychloroquine might actually increase mortality, possibly through prolongation of the QT-interval.16,17 Also, diversion of this drug to treat COVID-19 raises the concern of hydroxychloroquine shortages for treatment of patients with autoimmune disease, in whom the drug has proven benefit. We question the hasty FDA authorization for emergency use of hydroxychloroquine for COVID-19.
There is also great enthusiasm among the medical community to administer IL-6 receptor antagonists as a COVID-19 treatment. The rationale for this approach includes observations in case series in which IL-6 levels correlated with adverse clinical outcomes.13 IL-6 antagonists have a proven track record of improving the outcome in autoimmune diseases. However, we must avoid the logical trap of post hoc, ergo propter hoc (after this, therefore because of this) dictum from which one would assume that, based on those observations of high IL-6 levels and adverse outcomes, lowering IL-6 levels will necessarily improve outcomes in COVID-19. The supposed role of IL-6 in causing COVID-19 is based on scant preliminary observations and on the yet unproven assumption that IL-6 association with disease severity is a cause-effect relationship and not an association separate from pathogenesis. Moreover, there is sufficient scientific evidence that, in the case of severe influenza infections, IL-6 limits inflammation and protects against severe and potentially life-threatening lung injury. The road ahead for IL-6 inhibition to treat COVID-19 is perilous and should be entered cautiously. One immediate concern of administering IL-6 receptor antagonists in this patient population is the potential reactivation of latent tuberculosis infection and hepatitis B, colonic perforation, and increased rate of infections in general.
The greatest hope at this early stage of the COVID-19 pandemic may be remdesivir, which is a direct-acting antiviral. Here again, initial case series in prestigious medical journals signaled the possibility of a morbidity and mortality benefit.14 Despite these encouraging signs, a recent clinical trial from China that was limited by incomplete patient enrollment demonstrated a lack of efficacy of remdesivir in accelerating clinical improvement or limiting mortality.18 In spite of these negative results, preliminary data from the Adaptive COVID-19 Treatment Trial (ACTT) has revealed a nonsignificant signal of reduced mortality and shorter time to recovery in the remdesivir group. In response to these reports, the FDA has now issued emergency use authorization of remdesivir for treating COVID-19. Given the precedence of conflicting study data in therapeutic development for infectious diseases, we urge caution in drawing interpretations of benefit based on these early reports. Early termination of clinical studies is often associated with a 30% overestimation of clinical benefit.19 Furthermore, the availability of remdesivir is limited, which raises substantial ethical concerns on the preferential allocation of the drug to selected populations in high-income countries. At the time of this report, uncertainty regarding the risk-benefit balance of remdesivir and other COVID-19 treatments should be emphasized among decision makers.
CONCLUSION
Errors of commission present particular concerns for risk in treating COVID-19 patients and suggest that sometimes inaction is preferable to action. With many pandemics, there is a history of repeating mistakes, and we believe this can be curtailed by heeding the lessons of history. In the end, we may learn that avoiding therapeutic interventions that are poorly supported may prove to be one of the most important legacies of the COVID-19 pandemic.
Disclosures
The authors reported having nothing to disclose.
Funding
Dr Shapiro is supported by The Emily Foundation, Boston, Massachusetts. For all other authors, no financial support was declared.
1. Kahneman D. Thinking, Fast and Slow. New York: Farrar, Straus and Giroux; 2011.
2. Boikos C, Caya C, Doll MK, et al. Safety and effectiveness of neuraminidase inhibitors in situations of pandemic and/or novel/variant influenza: a systematic review of the literature, 2009-15. J Antimicrob Chemother. 2017;72(6):1556-1573. https://doi.org/10.1093/jac/dkx013.
3. de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14(8):523-534. https://doi.org/10.1038/nrmicro.2016.81.
4. Grober ED, Bohnen JM. Defining medical error. Can J Surg. 2005;48(1):39-44.
5. Schofield AT. Opium in influenza. Lancet. 1894;143(3676):372. https://doi.org/10.1016/S0140-6736(01)66349-9.
6. Abraham JJ. Some account of the history of the treatment of syphilis. Br J Vener Dis. 1948;24(4):153-161. https://doi.org/10.1136/sti.24.4.153.
7. Gradmann C. Laboratory Disease: Robert Koch’s Medical Bacteriology. Baltimore, MD: Johns Hopkins University Press; 2009. .
8. Steverding D. The history of African trypanosomiasis. Parasit Vectors. 2008;1(1):3. https://doi.org/10.1186/1756-3305-1-3
9. Offit PA. The Cutter incident, 50 years later. N Engl J Med. 2005;352(14):1411-1412. https://doi.org/10.1056/nejmp048180.
10. Frierson JG. The yellow fever vaccine: a history. Yale J Biol Med. 2010;83(2):77-85.
11. Sencer DJ, Millar JD. Reflections on the 1976 swine flu vaccination program. Emerg Infect Dis. 2006;12(1):29-33. https://doi.org/10.3201/eid1201.051007.
12. Ranieri VM, Thompson BT, Barie PS, et al. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366(22):2055-2064. https://doi.org/10.1056/nejmoa1202290.
13. Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab [online first]. ChinaXiv. 2020. https://doi.org/10.1073/pnas.2005615117.
14. Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe COVID-19 [online first]. N Engl J Med. 2020. https://doi.org/10.1056/nejmoa2007016.
15. Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial [online first]. Int J Antimicrob Agents. 2020. https://doi.org/10.1016/j.ijantimicag.2020.105949.
16. Magagnoli J, Narendran S, Pereira F, et al. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19 [preprint]. medRxiv. 2020. https://doi.org/10.1101/2020.04.16.20065920.
17. Bessière F, Roccia H, Delinière A, et al. Assessment of QT intervals in a case series of patients with coronavirus disease 2019 (COVID-19) infection treated with hydroxychloroquine alone or in combination with azithromycin in an intensive care unit [online first]. JAMA Cardiology. 2020. https://doi.org/10.1001/jamacardio.2020.1787.
18. Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569-1578. https://doi.org/10.1016/S0140-6736(20)31022-9.
19. Bassler D, Briel M, Montori VM, et al; STOPIT-2 Study Group. Stopping randomized trials early for benefit and estimation of treatment effects, systematic review and meta-regression analysis. JAMA. 2010 Mar 24;303(12):1180-1187. https://doi.org/jama.2010.310.
1. Kahneman D. Thinking, Fast and Slow. New York: Farrar, Straus and Giroux; 2011.
2. Boikos C, Caya C, Doll MK, et al. Safety and effectiveness of neuraminidase inhibitors in situations of pandemic and/or novel/variant influenza: a systematic review of the literature, 2009-15. J Antimicrob Chemother. 2017;72(6):1556-1573. https://doi.org/10.1093/jac/dkx013.
3. de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14(8):523-534. https://doi.org/10.1038/nrmicro.2016.81.
4. Grober ED, Bohnen JM. Defining medical error. Can J Surg. 2005;48(1):39-44.
5. Schofield AT. Opium in influenza. Lancet. 1894;143(3676):372. https://doi.org/10.1016/S0140-6736(01)66349-9.
6. Abraham JJ. Some account of the history of the treatment of syphilis. Br J Vener Dis. 1948;24(4):153-161. https://doi.org/10.1136/sti.24.4.153.
7. Gradmann C. Laboratory Disease: Robert Koch’s Medical Bacteriology. Baltimore, MD: Johns Hopkins University Press; 2009. .
8. Steverding D. The history of African trypanosomiasis. Parasit Vectors. 2008;1(1):3. https://doi.org/10.1186/1756-3305-1-3
9. Offit PA. The Cutter incident, 50 years later. N Engl J Med. 2005;352(14):1411-1412. https://doi.org/10.1056/nejmp048180.
10. Frierson JG. The yellow fever vaccine: a history. Yale J Biol Med. 2010;83(2):77-85.
11. Sencer DJ, Millar JD. Reflections on the 1976 swine flu vaccination program. Emerg Infect Dis. 2006;12(1):29-33. https://doi.org/10.3201/eid1201.051007.
12. Ranieri VM, Thompson BT, Barie PS, et al. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366(22):2055-2064. https://doi.org/10.1056/nejmoa1202290.
13. Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab [online first]. ChinaXiv. 2020. https://doi.org/10.1073/pnas.2005615117.
14. Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe COVID-19 [online first]. N Engl J Med. 2020. https://doi.org/10.1056/nejmoa2007016.
15. Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial [online first]. Int J Antimicrob Agents. 2020. https://doi.org/10.1016/j.ijantimicag.2020.105949.
16. Magagnoli J, Narendran S, Pereira F, et al. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19 [preprint]. medRxiv. 2020. https://doi.org/10.1101/2020.04.16.20065920.
17. Bessière F, Roccia H, Delinière A, et al. Assessment of QT intervals in a case series of patients with coronavirus disease 2019 (COVID-19) infection treated with hydroxychloroquine alone or in combination with azithromycin in an intensive care unit [online first]. JAMA Cardiology. 2020. https://doi.org/10.1001/jamacardio.2020.1787.
18. Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569-1578. https://doi.org/10.1016/S0140-6736(20)31022-9.
19. Bassler D, Briel M, Montori VM, et al; STOPIT-2 Study Group. Stopping randomized trials early for benefit and estimation of treatment effects, systematic review and meta-regression analysis. JAMA. 2010 Mar 24;303(12):1180-1187. https://doi.org/jama.2010.310.
© 2020 Society of Hospital Medicine
Compassionate Communication Amid the COVID-19 Pandemic
The coronavirus disease of 2019 (COVID-19) pandemic is the health crisis of our generation and will inevitably leave a lasting mark on how we practice medicine.1,2 It has already rapidly changed the way we communicate with patients, families, and colleagues. From the explosion of virtual care—which has been accelerated by need and new reimbursement policies3—to the physical barriers created by personal protective equipment (PPE) and no-visitor policies, the landscape of caring for hospitalized patients has seismically shifted in a few short months. At its core, the practice of medicine is about human connection—a connection between healers and the sick—and should remain as such to provide compassionate care to patients and their loved ones.4,5 In this perspective, we discuss challenges arising from communication barriers in the time of COVID-19 and opportunities to overcome them by preserving human connection to deliver high-quality care (Table).
COMMUNICATION WITH PATIENTS
While critically important to prevent transmission of the COVID-19 pathogen (ie, SARS-CoV-2), physical distancing and PPE create myriad challenges to achieving effective communication between healthcare providers and patients. Telemedicine has been leveraged to allow distanced communication between patients with COVID-19 and their providers from separate rooms. For face-to-face conversations, physical barriers, including distance between individuals and the wearing of face masks, impose new types of hindrances to nonverbal and verbal communication.
Challenges
Nonverbal communication helps build the therapeutic alliance and influences patient adherence to care plans, satisfaction, trust, and clinical outcomes.6,7 Expressions of emotion and reciprocity of nonverbal communication serve as important foundations for physician-patient encounters.6 Face masks, a necessity to reduce transmission of SARS-CoV-2, lead to fewer facial cues and may impede the ability to express and recognize emotional cues for patients and providers. A study of over 1,000 patients randomized to mask-wearing and non–mask-wearing physicians revealed a significant and negative effect on patient perception of physician empathy in consultations performed by mask-wearing physicians.8 Additionally, simple handshakes that convey respect and appreciation are no longer practiced.
Verbal communication is also affected by measures designed to reduce infection. The face mask and face shield worn by clinicians caring for patients with respiratory illnesses like COVID-19 diminish the volume and clarity of the spoken word. This is particularly problematic for patients who have sensory disturbances like hearing impairment. Additionally, these patients may rely on lipreading to effectively understand others, a strategy lost once the face mask is donned.
Opportunities
Healthcare providers may respond to nonverbal communication impediments by explicitly shifting nonverbal to verbal communication. For instance, when delivering serious news, a physician might previously have “mirrored” the patient’s sadness through a light touch on the hand and facial expressions congruent with that emotion. With physical distancing and PPE, the physician may instead express empathy through verbal statements such as acknowledging, validating, and respecting the patient’s emotions; making supportive statements; or exploring the patient’s feelings. The physician may also thank the patient for providing their input for the conversation.
Physicians should introduce themselves at the start of every daily encounter with a patient since there may be few distinct features above the face mask to distinguish the numerous individuals on a healthcare team. Some medical teams have provided “facesheets” with photographs and information about each member in an effort to humanize the team and connect more genuinely with the patient. In some cases, this may be the only way for a patient to see their healthcare providers’ faces.
To address obstacles to effective verbal communication, physicians should inquire about patients’ possible sensory disturbances on admission and, if necessary, arrange for hearing aids or other assistive devices. When communicating, physicians should articulate, enunciate, and increase volume to overcome the physical barrier created by the face mask. They should speak slowly, use plain language without jargon, and intentionally pause to check for understanding using the teach-back method.9
COMMUNICATION WITH FAMILIES AND CAREGIVERS
Challenges
With the aim of mitigating SARS-CoV-2 transmission, most healthcare systems have implemented no-visitor policies for hospitalized patients. This often leads to feelings of isolation among patients and their families. Goals-of-care discussions for COVID-19 and other serious diagnoses such as cancer can become even more difficult because family members often cannot witness how ill patients have become and clinicians cannot easily communicate virtually with multiple family members simultaneously.
Lack of family at the bedside also makes critical activities, such as discharge planning and education, more vulnerable to poor coordination and medical errors.10 Patients who are continuing to recover from acute illness may be expected to learn the details of home infusion for intravenous antibiotics, tracheostomy care, or specialized nutritional feeds. Without caregiver support, the patient may be at risk for readmission or other untoward safety events.
Opportunities
Several strategies may be used to improve virtual communication with families. The healthcare team should identify one family point of contact (ideally with the durable power of attorney for healthcare) who will receive and disseminate to others information about the patient’s status. This reduces the potential for multiple telephone conversations. We have witnessed some remarkable family points of contact call many family members to relay medical updates and moderate discussion. Care teams may decide to call the family contact during rounds so that they may listen in on the conversation with the patient or call after rounds to provide succinct updates. Family meetings may benefit greatly if conducted through a video platform, when possible, particularly if significant interval events have occurred. Connection through video allows eye contact and recognition of other nonverbal cues, as well as allowing findings like diagnostic images to be shared.
Because of increased anxiety associated with isolation, we recommend that one member of the primary healthcare team conduct telephone updates to the family point of contact on at least a daily basis. This simple act reduces potential for disjointed or discrepant messages from the healthcare team.11 It also demonstrates the value of keeping those individuals most important to the patient informed and has been shown to increase satisfaction with care and perceived effectiveness of meeting informational needs.12
Regarding discharge planning, physicians should engage the patient and family/caregivers in developing a patient-centered plan as early in the hospital stay as possible. The adage “discharge planning starts at admission” has never been more relevant. The team should avoid assumptions about patient/family sophistication for understanding complex healthcare concepts. Rather, physicians should assess patients’ and caregivers’ health literacy at the beginning of a hospital stay by asking simple, validated questions in a nonjudgmental way.13,14 This valuable information then allows the team to tailor medical information and discharge education appropriately for both patients and caregivers.
COMMUNICATION WITHIN THE HEALTHCARE TEAM
Challenges
As a result of the COVID-19 pandemic, various members of the healthcare team may be working remotely, and therefore, team members may feel less connected with each other. This could lead to a loss of camaraderie and fellowship within the team, as well as depersonalization, one of the main facets of burnout.15 Even if colocalized in the same area, those wearing face masks may experience disconnection and depersonalization. In an anecdote at our medical center, one clinician did not know what her team members’ faces looked like until they removed their masks for a moment to have a snack just before the end of the rotation.
In addition, healthcare systems have witnessed an increase in the volume of electronic consultations in which faculty and house staff review the patient’s medical record and render medical decision-making and recommendations without physically examining or interviewing the patient at the bedside. The purpose of this is twofold: to reduce the risk of transmitting SARS-CoV-2 and to conserve PPE. Electronic consultations could threaten to reduce collaborative communication and teaching among primary and consulting teams, which may lead to greater misunderstanding, less-effective patient care, and decreased satisfaction within the healthcare team.
Opportunities
Now more than ever, physicians should purposefully engage in regular communication with the multidisciplinary healthcare team that includes nurses, pharmacists, social workers, and other critical members. Because many of these individuals may now be working remotely or not joining in-person rounds, several strategies are needed to ensure care coordination within the primary healthcare team. For example, all members should “huddle” at least once daily to review each patient’s care and progress in meeting discharge goals. Team members who are working remotely should be dialed into these huddles and included in coordinating the plan for the day. While in-person multidisciplinary rounds may be temporarily halted to allow for physical distancing of staff, physician leaders can still encourage regular check-ins and updates throughout the day with multidisciplinary team members by other means, such as discussions by phone or a secure instant messenger, if available.
Another strategy to improve care coordination is to engage consulting teams in direct patient/family communication at critical junctures. For example, when a patient’s renal failure has gotten severe enough that dialysis is a consideration, the primary team may ask the nephrology consult service to participate in a joint telephone discussion with the family about risks, benefits, and alternatives to renal replacement therapy. Additionally, our palliative care consult service volunteered to be automatically consulted for all COVID-19 patients in the intensive care unit and high-risk COVID-19 patients on the acute care wards because of the disease’s high potential morbidity and mortality. Their roles included proactively confirming the patient’s surrogate decision maker, reviewing the patient’s decision-making capacity, eliciting specific goals of care and life-sustaining treatment preferences, and establishing relationships with the family. They also conducted daily huddles with the respective teams, another approach that fostered high-quality, collaborative care.
CONCLUSION
The COVID-19 pandemic has forced us to change the approaches we usually employ to interact with patients and their loved ones, as well as healthcare team members, but it has not changed the heart of medicine, which is to heal. Here we provide tangible and discrete strategies to achieve this goal through clear and compassionate communication, including shifting nonverbal to verbal communication with patients, speaking at least daily to one family point of contact, ensuring early and tailored discharge planning, emphasizing continued close care coordination among the multidisciplinary team, and thoughtfully engaging consultants in patient/family communication. We hope this guidance will assist us in striving to cultivate connection with our patients, their loved ones, and each other, just as we have always sought to do. With these strategies in mind, coupled with a continued focus on patient- and family-centered care for hospitalized patients, no amount of distance or PPE will diminish the power of human connection.
Acknowledgments
The authors wish to thank their colleagues—the physicians, nurses, respiratory therapists, clerks, custodial staff, security, and administrative professionals, to name a few—of the VA Ann Arbor Healthcare System for their collaboration, dedication, and grace in this time of crisis. The authors are indebted to the patients and their loved ones for putting their trust in their team, for teaching team members, and for providing the privilege of being a part of their lives.
Disclosures
The authors reported having nothing to disclose.
1. Ross JE. Resident response during pandemic: this is our time [online first]. Ann Intern Med. 2020. https://doi.org/10.7326/M20-1240
2. Berwick DM. Choices for the “new normal” [online first]. JAMA. 2020. https://doi.org/10.1001/jama.2020.6949.
3. Centers for Medicare & Medicaid Services. President Trump expands telehealth benefits for Medicare beneficiaries during COVID-19 outbreak. CMS.gov. Mar 17, 2020. https://www.cms.gov/newsroom/press-releases/president-trump-expands-telehealth-benefits-medicare-beneficiaries-during-covid-19-outbreak. Accessed May 09, 2020.
4. Zulman DM, Haverfield MC, Shaw JG, et al. Practices to foster physician presence and connection with patients in the clinical encounter. JAMA. 2020;323(1):70‐81. https://doi.org/10.1001/jama.2019.19003.
5. Haverfield MC, Tierney A, Schwartz R, et al. Can patient-provider interpersonal interventions achieve the quadruple aim of healthcare? a systematic review [online first]. J Gen Intern Med. 2020. https://doi.org/10.1007/s11606-019-05525-2.
6. Roter DL, Frankel RM, Hall JA, Sluyter D. The expression of emotion through nonverbal behavior in medical visits: mechanisms and outcomes. J Gen Intern Med. 2006;21(Suppl 1):S28-S34. https://doi.org/10.1111/j.1525-1497.2006.00306.x.
7. Mast MS. On the importance of nonverbal communication in the physician-patient interaction. Patient Educ Couns. 2007;67(3):315-318. https://doi.org/10.1016/j.pec.2007.03.005.
8. Wong CK, Yip BH, Mercer S, et al. Effect of facemasks on empathy and relational continuity: a randomised controlled trial in primary care. BMC Fam Pract. 2013;14:200. https://doi.org/10.1186/1471-2296-14-200.
9. Talevski J, Wong Shee A, Rasmussen B, Kemp G, Beauchamp A. Teach-back: a systematic review of implementation and impacts. PLoS One. 2020;15(4):e0231350. https://doi.org/10.1371/journal.pone.0231350.
10. Kripalani S, Jackson AT, Schnipper JL, Coleman EA. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314-323. https://doi.org/10.1002/jhm.228.
11. Ahrens T, Yancey V, Kollef M. Improving family communications at the end of life: implications for length of stay in the intensive care unit and resource use. Am J Crit Care. 2003;12(4):317-324.
12. Medland JJ, Ferrans CE. Effectiveness of a structured communication program for family members of patients in an ICU. Am J Crit Care. 1998;7(1):24-29.
13. Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Fam Med. 2004;36(8):588-594.
14. Wallace LS, Rogers ES, Roskos SE, Holiday DB, Weiss BD. Brief report: screening items to identify patients with limited health literacy skills. J Gen Intern Med. 2006;21:874-877. https://doi.org/10.1111/j.1525-1497.2006.00532.x.
15. West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283(6):516‐529. https://doi.org/10.1111/joim.12752.
The coronavirus disease of 2019 (COVID-19) pandemic is the health crisis of our generation and will inevitably leave a lasting mark on how we practice medicine.1,2 It has already rapidly changed the way we communicate with patients, families, and colleagues. From the explosion of virtual care—which has been accelerated by need and new reimbursement policies3—to the physical barriers created by personal protective equipment (PPE) and no-visitor policies, the landscape of caring for hospitalized patients has seismically shifted in a few short months. At its core, the practice of medicine is about human connection—a connection between healers and the sick—and should remain as such to provide compassionate care to patients and their loved ones.4,5 In this perspective, we discuss challenges arising from communication barriers in the time of COVID-19 and opportunities to overcome them by preserving human connection to deliver high-quality care (Table).
COMMUNICATION WITH PATIENTS
While critically important to prevent transmission of the COVID-19 pathogen (ie, SARS-CoV-2), physical distancing and PPE create myriad challenges to achieving effective communication between healthcare providers and patients. Telemedicine has been leveraged to allow distanced communication between patients with COVID-19 and their providers from separate rooms. For face-to-face conversations, physical barriers, including distance between individuals and the wearing of face masks, impose new types of hindrances to nonverbal and verbal communication.
Challenges
Nonverbal communication helps build the therapeutic alliance and influences patient adherence to care plans, satisfaction, trust, and clinical outcomes.6,7 Expressions of emotion and reciprocity of nonverbal communication serve as important foundations for physician-patient encounters.6 Face masks, a necessity to reduce transmission of SARS-CoV-2, lead to fewer facial cues and may impede the ability to express and recognize emotional cues for patients and providers. A study of over 1,000 patients randomized to mask-wearing and non–mask-wearing physicians revealed a significant and negative effect on patient perception of physician empathy in consultations performed by mask-wearing physicians.8 Additionally, simple handshakes that convey respect and appreciation are no longer practiced.
Verbal communication is also affected by measures designed to reduce infection. The face mask and face shield worn by clinicians caring for patients with respiratory illnesses like COVID-19 diminish the volume and clarity of the spoken word. This is particularly problematic for patients who have sensory disturbances like hearing impairment. Additionally, these patients may rely on lipreading to effectively understand others, a strategy lost once the face mask is donned.
Opportunities
Healthcare providers may respond to nonverbal communication impediments by explicitly shifting nonverbal to verbal communication. For instance, when delivering serious news, a physician might previously have “mirrored” the patient’s sadness through a light touch on the hand and facial expressions congruent with that emotion. With physical distancing and PPE, the physician may instead express empathy through verbal statements such as acknowledging, validating, and respecting the patient’s emotions; making supportive statements; or exploring the patient’s feelings. The physician may also thank the patient for providing their input for the conversation.
Physicians should introduce themselves at the start of every daily encounter with a patient since there may be few distinct features above the face mask to distinguish the numerous individuals on a healthcare team. Some medical teams have provided “facesheets” with photographs and information about each member in an effort to humanize the team and connect more genuinely with the patient. In some cases, this may be the only way for a patient to see their healthcare providers’ faces.
To address obstacles to effective verbal communication, physicians should inquire about patients’ possible sensory disturbances on admission and, if necessary, arrange for hearing aids or other assistive devices. When communicating, physicians should articulate, enunciate, and increase volume to overcome the physical barrier created by the face mask. They should speak slowly, use plain language without jargon, and intentionally pause to check for understanding using the teach-back method.9
COMMUNICATION WITH FAMILIES AND CAREGIVERS
Challenges
With the aim of mitigating SARS-CoV-2 transmission, most healthcare systems have implemented no-visitor policies for hospitalized patients. This often leads to feelings of isolation among patients and their families. Goals-of-care discussions for COVID-19 and other serious diagnoses such as cancer can become even more difficult because family members often cannot witness how ill patients have become and clinicians cannot easily communicate virtually with multiple family members simultaneously.
Lack of family at the bedside also makes critical activities, such as discharge planning and education, more vulnerable to poor coordination and medical errors.10 Patients who are continuing to recover from acute illness may be expected to learn the details of home infusion for intravenous antibiotics, tracheostomy care, or specialized nutritional feeds. Without caregiver support, the patient may be at risk for readmission or other untoward safety events.
Opportunities
Several strategies may be used to improve virtual communication with families. The healthcare team should identify one family point of contact (ideally with the durable power of attorney for healthcare) who will receive and disseminate to others information about the patient’s status. This reduces the potential for multiple telephone conversations. We have witnessed some remarkable family points of contact call many family members to relay medical updates and moderate discussion. Care teams may decide to call the family contact during rounds so that they may listen in on the conversation with the patient or call after rounds to provide succinct updates. Family meetings may benefit greatly if conducted through a video platform, when possible, particularly if significant interval events have occurred. Connection through video allows eye contact and recognition of other nonverbal cues, as well as allowing findings like diagnostic images to be shared.
Because of increased anxiety associated with isolation, we recommend that one member of the primary healthcare team conduct telephone updates to the family point of contact on at least a daily basis. This simple act reduces potential for disjointed or discrepant messages from the healthcare team.11 It also demonstrates the value of keeping those individuals most important to the patient informed and has been shown to increase satisfaction with care and perceived effectiveness of meeting informational needs.12
Regarding discharge planning, physicians should engage the patient and family/caregivers in developing a patient-centered plan as early in the hospital stay as possible. The adage “discharge planning starts at admission” has never been more relevant. The team should avoid assumptions about patient/family sophistication for understanding complex healthcare concepts. Rather, physicians should assess patients’ and caregivers’ health literacy at the beginning of a hospital stay by asking simple, validated questions in a nonjudgmental way.13,14 This valuable information then allows the team to tailor medical information and discharge education appropriately for both patients and caregivers.
COMMUNICATION WITHIN THE HEALTHCARE TEAM
Challenges
As a result of the COVID-19 pandemic, various members of the healthcare team may be working remotely, and therefore, team members may feel less connected with each other. This could lead to a loss of camaraderie and fellowship within the team, as well as depersonalization, one of the main facets of burnout.15 Even if colocalized in the same area, those wearing face masks may experience disconnection and depersonalization. In an anecdote at our medical center, one clinician did not know what her team members’ faces looked like until they removed their masks for a moment to have a snack just before the end of the rotation.
In addition, healthcare systems have witnessed an increase in the volume of electronic consultations in which faculty and house staff review the patient’s medical record and render medical decision-making and recommendations without physically examining or interviewing the patient at the bedside. The purpose of this is twofold: to reduce the risk of transmitting SARS-CoV-2 and to conserve PPE. Electronic consultations could threaten to reduce collaborative communication and teaching among primary and consulting teams, which may lead to greater misunderstanding, less-effective patient care, and decreased satisfaction within the healthcare team.
Opportunities
Now more than ever, physicians should purposefully engage in regular communication with the multidisciplinary healthcare team that includes nurses, pharmacists, social workers, and other critical members. Because many of these individuals may now be working remotely or not joining in-person rounds, several strategies are needed to ensure care coordination within the primary healthcare team. For example, all members should “huddle” at least once daily to review each patient’s care and progress in meeting discharge goals. Team members who are working remotely should be dialed into these huddles and included in coordinating the plan for the day. While in-person multidisciplinary rounds may be temporarily halted to allow for physical distancing of staff, physician leaders can still encourage regular check-ins and updates throughout the day with multidisciplinary team members by other means, such as discussions by phone or a secure instant messenger, if available.
Another strategy to improve care coordination is to engage consulting teams in direct patient/family communication at critical junctures. For example, when a patient’s renal failure has gotten severe enough that dialysis is a consideration, the primary team may ask the nephrology consult service to participate in a joint telephone discussion with the family about risks, benefits, and alternatives to renal replacement therapy. Additionally, our palliative care consult service volunteered to be automatically consulted for all COVID-19 patients in the intensive care unit and high-risk COVID-19 patients on the acute care wards because of the disease’s high potential morbidity and mortality. Their roles included proactively confirming the patient’s surrogate decision maker, reviewing the patient’s decision-making capacity, eliciting specific goals of care and life-sustaining treatment preferences, and establishing relationships with the family. They also conducted daily huddles with the respective teams, another approach that fostered high-quality, collaborative care.
CONCLUSION
The COVID-19 pandemic has forced us to change the approaches we usually employ to interact with patients and their loved ones, as well as healthcare team members, but it has not changed the heart of medicine, which is to heal. Here we provide tangible and discrete strategies to achieve this goal through clear and compassionate communication, including shifting nonverbal to verbal communication with patients, speaking at least daily to one family point of contact, ensuring early and tailored discharge planning, emphasizing continued close care coordination among the multidisciplinary team, and thoughtfully engaging consultants in patient/family communication. We hope this guidance will assist us in striving to cultivate connection with our patients, their loved ones, and each other, just as we have always sought to do. With these strategies in mind, coupled with a continued focus on patient- and family-centered care for hospitalized patients, no amount of distance or PPE will diminish the power of human connection.
Acknowledgments
The authors wish to thank their colleagues—the physicians, nurses, respiratory therapists, clerks, custodial staff, security, and administrative professionals, to name a few—of the VA Ann Arbor Healthcare System for their collaboration, dedication, and grace in this time of crisis. The authors are indebted to the patients and their loved ones for putting their trust in their team, for teaching team members, and for providing the privilege of being a part of their lives.
Disclosures
The authors reported having nothing to disclose.
The coronavirus disease of 2019 (COVID-19) pandemic is the health crisis of our generation and will inevitably leave a lasting mark on how we practice medicine.1,2 It has already rapidly changed the way we communicate with patients, families, and colleagues. From the explosion of virtual care—which has been accelerated by need and new reimbursement policies3—to the physical barriers created by personal protective equipment (PPE) and no-visitor policies, the landscape of caring for hospitalized patients has seismically shifted in a few short months. At its core, the practice of medicine is about human connection—a connection between healers and the sick—and should remain as such to provide compassionate care to patients and their loved ones.4,5 In this perspective, we discuss challenges arising from communication barriers in the time of COVID-19 and opportunities to overcome them by preserving human connection to deliver high-quality care (Table).
COMMUNICATION WITH PATIENTS
While critically important to prevent transmission of the COVID-19 pathogen (ie, SARS-CoV-2), physical distancing and PPE create myriad challenges to achieving effective communication between healthcare providers and patients. Telemedicine has been leveraged to allow distanced communication between patients with COVID-19 and their providers from separate rooms. For face-to-face conversations, physical barriers, including distance between individuals and the wearing of face masks, impose new types of hindrances to nonverbal and verbal communication.
Challenges
Nonverbal communication helps build the therapeutic alliance and influences patient adherence to care plans, satisfaction, trust, and clinical outcomes.6,7 Expressions of emotion and reciprocity of nonverbal communication serve as important foundations for physician-patient encounters.6 Face masks, a necessity to reduce transmission of SARS-CoV-2, lead to fewer facial cues and may impede the ability to express and recognize emotional cues for patients and providers. A study of over 1,000 patients randomized to mask-wearing and non–mask-wearing physicians revealed a significant and negative effect on patient perception of physician empathy in consultations performed by mask-wearing physicians.8 Additionally, simple handshakes that convey respect and appreciation are no longer practiced.
Verbal communication is also affected by measures designed to reduce infection. The face mask and face shield worn by clinicians caring for patients with respiratory illnesses like COVID-19 diminish the volume and clarity of the spoken word. This is particularly problematic for patients who have sensory disturbances like hearing impairment. Additionally, these patients may rely on lipreading to effectively understand others, a strategy lost once the face mask is donned.
Opportunities
Healthcare providers may respond to nonverbal communication impediments by explicitly shifting nonverbal to verbal communication. For instance, when delivering serious news, a physician might previously have “mirrored” the patient’s sadness through a light touch on the hand and facial expressions congruent with that emotion. With physical distancing and PPE, the physician may instead express empathy through verbal statements such as acknowledging, validating, and respecting the patient’s emotions; making supportive statements; or exploring the patient’s feelings. The physician may also thank the patient for providing their input for the conversation.
Physicians should introduce themselves at the start of every daily encounter with a patient since there may be few distinct features above the face mask to distinguish the numerous individuals on a healthcare team. Some medical teams have provided “facesheets” with photographs and information about each member in an effort to humanize the team and connect more genuinely with the patient. In some cases, this may be the only way for a patient to see their healthcare providers’ faces.
To address obstacles to effective verbal communication, physicians should inquire about patients’ possible sensory disturbances on admission and, if necessary, arrange for hearing aids or other assistive devices. When communicating, physicians should articulate, enunciate, and increase volume to overcome the physical barrier created by the face mask. They should speak slowly, use plain language without jargon, and intentionally pause to check for understanding using the teach-back method.9
COMMUNICATION WITH FAMILIES AND CAREGIVERS
Challenges
With the aim of mitigating SARS-CoV-2 transmission, most healthcare systems have implemented no-visitor policies for hospitalized patients. This often leads to feelings of isolation among patients and their families. Goals-of-care discussions for COVID-19 and other serious diagnoses such as cancer can become even more difficult because family members often cannot witness how ill patients have become and clinicians cannot easily communicate virtually with multiple family members simultaneously.
Lack of family at the bedside also makes critical activities, such as discharge planning and education, more vulnerable to poor coordination and medical errors.10 Patients who are continuing to recover from acute illness may be expected to learn the details of home infusion for intravenous antibiotics, tracheostomy care, or specialized nutritional feeds. Without caregiver support, the patient may be at risk for readmission or other untoward safety events.
Opportunities
Several strategies may be used to improve virtual communication with families. The healthcare team should identify one family point of contact (ideally with the durable power of attorney for healthcare) who will receive and disseminate to others information about the patient’s status. This reduces the potential for multiple telephone conversations. We have witnessed some remarkable family points of contact call many family members to relay medical updates and moderate discussion. Care teams may decide to call the family contact during rounds so that they may listen in on the conversation with the patient or call after rounds to provide succinct updates. Family meetings may benefit greatly if conducted through a video platform, when possible, particularly if significant interval events have occurred. Connection through video allows eye contact and recognition of other nonverbal cues, as well as allowing findings like diagnostic images to be shared.
Because of increased anxiety associated with isolation, we recommend that one member of the primary healthcare team conduct telephone updates to the family point of contact on at least a daily basis. This simple act reduces potential for disjointed or discrepant messages from the healthcare team.11 It also demonstrates the value of keeping those individuals most important to the patient informed and has been shown to increase satisfaction with care and perceived effectiveness of meeting informational needs.12
Regarding discharge planning, physicians should engage the patient and family/caregivers in developing a patient-centered plan as early in the hospital stay as possible. The adage “discharge planning starts at admission” has never been more relevant. The team should avoid assumptions about patient/family sophistication for understanding complex healthcare concepts. Rather, physicians should assess patients’ and caregivers’ health literacy at the beginning of a hospital stay by asking simple, validated questions in a nonjudgmental way.13,14 This valuable information then allows the team to tailor medical information and discharge education appropriately for both patients and caregivers.
COMMUNICATION WITHIN THE HEALTHCARE TEAM
Challenges
As a result of the COVID-19 pandemic, various members of the healthcare team may be working remotely, and therefore, team members may feel less connected with each other. This could lead to a loss of camaraderie and fellowship within the team, as well as depersonalization, one of the main facets of burnout.15 Even if colocalized in the same area, those wearing face masks may experience disconnection and depersonalization. In an anecdote at our medical center, one clinician did not know what her team members’ faces looked like until they removed their masks for a moment to have a snack just before the end of the rotation.
In addition, healthcare systems have witnessed an increase in the volume of electronic consultations in which faculty and house staff review the patient’s medical record and render medical decision-making and recommendations without physically examining or interviewing the patient at the bedside. The purpose of this is twofold: to reduce the risk of transmitting SARS-CoV-2 and to conserve PPE. Electronic consultations could threaten to reduce collaborative communication and teaching among primary and consulting teams, which may lead to greater misunderstanding, less-effective patient care, and decreased satisfaction within the healthcare team.
Opportunities
Now more than ever, physicians should purposefully engage in regular communication with the multidisciplinary healthcare team that includes nurses, pharmacists, social workers, and other critical members. Because many of these individuals may now be working remotely or not joining in-person rounds, several strategies are needed to ensure care coordination within the primary healthcare team. For example, all members should “huddle” at least once daily to review each patient’s care and progress in meeting discharge goals. Team members who are working remotely should be dialed into these huddles and included in coordinating the plan for the day. While in-person multidisciplinary rounds may be temporarily halted to allow for physical distancing of staff, physician leaders can still encourage regular check-ins and updates throughout the day with multidisciplinary team members by other means, such as discussions by phone or a secure instant messenger, if available.
Another strategy to improve care coordination is to engage consulting teams in direct patient/family communication at critical junctures. For example, when a patient’s renal failure has gotten severe enough that dialysis is a consideration, the primary team may ask the nephrology consult service to participate in a joint telephone discussion with the family about risks, benefits, and alternatives to renal replacement therapy. Additionally, our palliative care consult service volunteered to be automatically consulted for all COVID-19 patients in the intensive care unit and high-risk COVID-19 patients on the acute care wards because of the disease’s high potential morbidity and mortality. Their roles included proactively confirming the patient’s surrogate decision maker, reviewing the patient’s decision-making capacity, eliciting specific goals of care and life-sustaining treatment preferences, and establishing relationships with the family. They also conducted daily huddles with the respective teams, another approach that fostered high-quality, collaborative care.
CONCLUSION
The COVID-19 pandemic has forced us to change the approaches we usually employ to interact with patients and their loved ones, as well as healthcare team members, but it has not changed the heart of medicine, which is to heal. Here we provide tangible and discrete strategies to achieve this goal through clear and compassionate communication, including shifting nonverbal to verbal communication with patients, speaking at least daily to one family point of contact, ensuring early and tailored discharge planning, emphasizing continued close care coordination among the multidisciplinary team, and thoughtfully engaging consultants in patient/family communication. We hope this guidance will assist us in striving to cultivate connection with our patients, their loved ones, and each other, just as we have always sought to do. With these strategies in mind, coupled with a continued focus on patient- and family-centered care for hospitalized patients, no amount of distance or PPE will diminish the power of human connection.
Acknowledgments
The authors wish to thank their colleagues—the physicians, nurses, respiratory therapists, clerks, custodial staff, security, and administrative professionals, to name a few—of the VA Ann Arbor Healthcare System for their collaboration, dedication, and grace in this time of crisis. The authors are indebted to the patients and their loved ones for putting their trust in their team, for teaching team members, and for providing the privilege of being a part of their lives.
Disclosures
The authors reported having nothing to disclose.
1. Ross JE. Resident response during pandemic: this is our time [online first]. Ann Intern Med. 2020. https://doi.org/10.7326/M20-1240
2. Berwick DM. Choices for the “new normal” [online first]. JAMA. 2020. https://doi.org/10.1001/jama.2020.6949.
3. Centers for Medicare & Medicaid Services. President Trump expands telehealth benefits for Medicare beneficiaries during COVID-19 outbreak. CMS.gov. Mar 17, 2020. https://www.cms.gov/newsroom/press-releases/president-trump-expands-telehealth-benefits-medicare-beneficiaries-during-covid-19-outbreak. Accessed May 09, 2020.
4. Zulman DM, Haverfield MC, Shaw JG, et al. Practices to foster physician presence and connection with patients in the clinical encounter. JAMA. 2020;323(1):70‐81. https://doi.org/10.1001/jama.2019.19003.
5. Haverfield MC, Tierney A, Schwartz R, et al. Can patient-provider interpersonal interventions achieve the quadruple aim of healthcare? a systematic review [online first]. J Gen Intern Med. 2020. https://doi.org/10.1007/s11606-019-05525-2.
6. Roter DL, Frankel RM, Hall JA, Sluyter D. The expression of emotion through nonverbal behavior in medical visits: mechanisms and outcomes. J Gen Intern Med. 2006;21(Suppl 1):S28-S34. https://doi.org/10.1111/j.1525-1497.2006.00306.x.
7. Mast MS. On the importance of nonverbal communication in the physician-patient interaction. Patient Educ Couns. 2007;67(3):315-318. https://doi.org/10.1016/j.pec.2007.03.005.
8. Wong CK, Yip BH, Mercer S, et al. Effect of facemasks on empathy and relational continuity: a randomised controlled trial in primary care. BMC Fam Pract. 2013;14:200. https://doi.org/10.1186/1471-2296-14-200.
9. Talevski J, Wong Shee A, Rasmussen B, Kemp G, Beauchamp A. Teach-back: a systematic review of implementation and impacts. PLoS One. 2020;15(4):e0231350. https://doi.org/10.1371/journal.pone.0231350.
10. Kripalani S, Jackson AT, Schnipper JL, Coleman EA. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314-323. https://doi.org/10.1002/jhm.228.
11. Ahrens T, Yancey V, Kollef M. Improving family communications at the end of life: implications for length of stay in the intensive care unit and resource use. Am J Crit Care. 2003;12(4):317-324.
12. Medland JJ, Ferrans CE. Effectiveness of a structured communication program for family members of patients in an ICU. Am J Crit Care. 1998;7(1):24-29.
13. Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Fam Med. 2004;36(8):588-594.
14. Wallace LS, Rogers ES, Roskos SE, Holiday DB, Weiss BD. Brief report: screening items to identify patients with limited health literacy skills. J Gen Intern Med. 2006;21:874-877. https://doi.org/10.1111/j.1525-1497.2006.00532.x.
15. West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283(6):516‐529. https://doi.org/10.1111/joim.12752.
1. Ross JE. Resident response during pandemic: this is our time [online first]. Ann Intern Med. 2020. https://doi.org/10.7326/M20-1240
2. Berwick DM. Choices for the “new normal” [online first]. JAMA. 2020. https://doi.org/10.1001/jama.2020.6949.
3. Centers for Medicare & Medicaid Services. President Trump expands telehealth benefits for Medicare beneficiaries during COVID-19 outbreak. CMS.gov. Mar 17, 2020. https://www.cms.gov/newsroom/press-releases/president-trump-expands-telehealth-benefits-medicare-beneficiaries-during-covid-19-outbreak. Accessed May 09, 2020.
4. Zulman DM, Haverfield MC, Shaw JG, et al. Practices to foster physician presence and connection with patients in the clinical encounter. JAMA. 2020;323(1):70‐81. https://doi.org/10.1001/jama.2019.19003.
5. Haverfield MC, Tierney A, Schwartz R, et al. Can patient-provider interpersonal interventions achieve the quadruple aim of healthcare? a systematic review [online first]. J Gen Intern Med. 2020. https://doi.org/10.1007/s11606-019-05525-2.
6. Roter DL, Frankel RM, Hall JA, Sluyter D. The expression of emotion through nonverbal behavior in medical visits: mechanisms and outcomes. J Gen Intern Med. 2006;21(Suppl 1):S28-S34. https://doi.org/10.1111/j.1525-1497.2006.00306.x.
7. Mast MS. On the importance of nonverbal communication in the physician-patient interaction. Patient Educ Couns. 2007;67(3):315-318. https://doi.org/10.1016/j.pec.2007.03.005.
8. Wong CK, Yip BH, Mercer S, et al. Effect of facemasks on empathy and relational continuity: a randomised controlled trial in primary care. BMC Fam Pract. 2013;14:200. https://doi.org/10.1186/1471-2296-14-200.
9. Talevski J, Wong Shee A, Rasmussen B, Kemp G, Beauchamp A. Teach-back: a systematic review of implementation and impacts. PLoS One. 2020;15(4):e0231350. https://doi.org/10.1371/journal.pone.0231350.
10. Kripalani S, Jackson AT, Schnipper JL, Coleman EA. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314-323. https://doi.org/10.1002/jhm.228.
11. Ahrens T, Yancey V, Kollef M. Improving family communications at the end of life: implications for length of stay in the intensive care unit and resource use. Am J Crit Care. 2003;12(4):317-324.
12. Medland JJ, Ferrans CE. Effectiveness of a structured communication program for family members of patients in an ICU. Am J Crit Care. 1998;7(1):24-29.
13. Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Fam Med. 2004;36(8):588-594.
14. Wallace LS, Rogers ES, Roskos SE, Holiday DB, Weiss BD. Brief report: screening items to identify patients with limited health literacy skills. J Gen Intern Med. 2006;21:874-877. https://doi.org/10.1111/j.1525-1497.2006.00532.x.
15. West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283(6):516‐529. https://doi.org/10.1111/joim.12752.
© 2020 Society of Hospital Medicine
SECTION 4: HEALTHCARE SYSTEMS: SUPPORTING AND ADVANCING CHILD HEALTH
Alvarez F, Alverson B, Balighian E, Beauchamp-Walters J, Biondi E, Blankenberg R, Bridgeman C, Brown J, Buchanan AO, Carlson D, Chang P, Coon E, Daud YN, Denniston S, DeWolfe CC, Deutsch SA, Doshi A, Fisher E, Gage S, Gallagher MP, Gill A, Goel Jones V, Grill J, Gupta A, Herbst BF Jr, Hershey D, Hoang K, Holmes AV, Hopkins A, Jones Y, Khan A, Lee V, Li ST, Lye, PS, Maginot T, Maloney C, Maniscalco J, Mannino Avila E, Markowsky A, Marks M, Matheny Antommaria AH, Maul E, McCulloh R, Melwani A, Miller C, Mittal V, Natt B, O'Toole J, Ottolini M, Percelay J, Phillips S, Pressel D, Quinonez R, Ralston S, Rappaport DI, Rauch D, Rhee K, Riese J, Roberts K, Rogers A, Rosenberg RE, Ruhlen M, Russell CJ, Russo C, Schwenk KM, Sekaran A, Shadman KA, Shah SS, Shen M, Simon T, Singh A, Smith K, Srinivas N Srivastava R, Sterni L, Thompson ED Jr, Thomson J, Tieder J, Tremoulet A, Wang ME, Williams R, Wu S. Name of Chapter. In: Pediatric Hospital Medicine Core Competencies: 2020 Revision. Section 4: Healthcare Systems: Supporting and Advancing Child Health. J Hosp Med. 2020;15(S1):xxx-xxx (insert page numbers). https://doi.org/jhm.3400
Alvarez F, Alverson B, Balighian E, Beauchamp-Walters J, Biondi E, Blankenberg R, Bridgeman C, Brown J, Buchanan AO, Carlson D, Chang P, Coon E, Daud YN, Denniston S, DeWolfe CC, Deutsch SA, Doshi A, Fisher E, Gage S, Gallagher MP, Gill A, Goel Jones V, Grill J, Gupta A, Herbst BF Jr, Hershey D, Hoang K, Holmes AV, Hopkins A, Jones Y, Khan A, Lee V, Li ST, Lye, PS, Maginot T, Maloney C, Maniscalco J, Mannino Avila E, Markowsky A, Marks M, Matheny Antommaria AH, Maul E, McCulloh R, Melwani A, Miller C, Mittal V, Natt B, O'Toole J, Ottolini M, Percelay J, Phillips S, Pressel D, Quinonez R, Ralston S, Rappaport DI, Rauch D, Rhee K, Riese J, Roberts K, Rogers A, Rosenberg RE, Ruhlen M, Russell CJ, Russo C, Schwenk KM, Sekaran A, Shadman KA, Shah SS, Shen M, Simon T, Singh A, Smith K, Srinivas N Srivastava R, Sterni L, Thompson ED Jr, Thomson J, Tieder J, Tremoulet A, Wang ME, Williams R, Wu S. Name of Chapter. In: Pediatric Hospital Medicine Core Competencies: 2020 Revision. Section 4: Healthcare Systems: Supporting and Advancing Child Health. J Hosp Med. 2020;15(S1):xxx-xxx (insert page numbers). https://doi.org/jhm.3400
Alvarez F, Alverson B, Balighian E, Beauchamp-Walters J, Biondi E, Blankenberg R, Bridgeman C, Brown J, Buchanan AO, Carlson D, Chang P, Coon E, Daud YN, Denniston S, DeWolfe CC, Deutsch SA, Doshi A, Fisher E, Gage S, Gallagher MP, Gill A, Goel Jones V, Grill J, Gupta A, Herbst BF Jr, Hershey D, Hoang K, Holmes AV, Hopkins A, Jones Y, Khan A, Lee V, Li ST, Lye, PS, Maginot T, Maloney C, Maniscalco J, Mannino Avila E, Markowsky A, Marks M, Matheny Antommaria AH, Maul E, McCulloh R, Melwani A, Miller C, Mittal V, Natt B, O'Toole J, Ottolini M, Percelay J, Phillips S, Pressel D, Quinonez R, Ralston S, Rappaport DI, Rauch D, Rhee K, Riese J, Roberts K, Rogers A, Rosenberg RE, Ruhlen M, Russell CJ, Russo C, Schwenk KM, Sekaran A, Shadman KA, Shah SS, Shen M, Simon T, Singh A, Smith K, Srinivas N Srivastava R, Sterni L, Thompson ED Jr, Thomson J, Tieder J, Tremoulet A, Wang ME, Williams R, Wu S. Name of Chapter. In: Pediatric Hospital Medicine Core Competencies: 2020 Revision. Section 4: Healthcare Systems: Supporting and Advancing Child Health. J Hosp Med. 2020;15(S1):xxx-xxx (insert page numbers). https://doi.org/jhm.3400
SECTION 3: SPECIALIZED SERVICES
How to cite articles within Section 2
Alvarez F, Alverson B, Balighian E, Beauchamp-Walters J, Biondi E, Blankenberg R, Bridgeman C, Brown J, Buchanan AO, Carlson D, Chang P, Coon E, Daud YN, Denniston S, DeWolfe CC, Deutsch SA, Doshi A, Fisher E, Gage S, Gallagher MP, Gill A, Goel Jones V, Grill J, Gupta A, Herbst BF Jr, Hershey D, Hoang K, Holmes AV, Hopkins A, Jones Y, Khan A, Lee V, Li ST, Lye, PS, Maginot T, Maloney C, Maniscalco J, Mannino Avila E, Markowsky A, Marks M, Matheny Antommaria AH, Maul E, McCulloh R, Melwani A, Miller C, Mittal V, Natt B, O'Toole J, Ottolini M, Percelay J, Phillips S, Pressel D, Quinonez R, Ralston S, Rappaport DI, Rauch D, Rhee K, Riese J, Roberts K, Rogers A, Rosenberg RE, Ruhlen M, Russell CJ, Russo C, Schwenk KM, Sekaran A, Shadman KA, Shah SS, Shen M, Simon T, Singh A, Smith K, Srinivas N Srivastava R, Sterni L, Thompson ED Jr, Thomson J, Tieder J, Tremoulet A, Wang ME, Williams R, Wu S. Name of Chapter. In: Pediatric Hospital Medicine Core Competencies: 2020 Revision. Section 3: Specialized Services. J Hosp Med. 2020;15(S1):xx-xxx (insert page numbers). https://doi.org/10.12788/jhm.3399
How to cite articles within Section 2
Alvarez F, Alverson B, Balighian E, Beauchamp-Walters J, Biondi E, Blankenberg R, Bridgeman C, Brown J, Buchanan AO, Carlson D, Chang P, Coon E, Daud YN, Denniston S, DeWolfe CC, Deutsch SA, Doshi A, Fisher E, Gage S, Gallagher MP, Gill A, Goel Jones V, Grill J, Gupta A, Herbst BF Jr, Hershey D, Hoang K, Holmes AV, Hopkins A, Jones Y, Khan A, Lee V, Li ST, Lye, PS, Maginot T, Maloney C, Maniscalco J, Mannino Avila E, Markowsky A, Marks M, Matheny Antommaria AH, Maul E, McCulloh R, Melwani A, Miller C, Mittal V, Natt B, O'Toole J, Ottolini M, Percelay J, Phillips S, Pressel D, Quinonez R, Ralston S, Rappaport DI, Rauch D, Rhee K, Riese J, Roberts K, Rogers A, Rosenberg RE, Ruhlen M, Russell CJ, Russo C, Schwenk KM, Sekaran A, Shadman KA, Shah SS, Shen M, Simon T, Singh A, Smith K, Srinivas N Srivastava R, Sterni L, Thompson ED Jr, Thomson J, Tieder J, Tremoulet A, Wang ME, Williams R, Wu S. Name of Chapter. In: Pediatric Hospital Medicine Core Competencies: 2020 Revision. Section 3: Specialized Services. J Hosp Med. 2020;15(S1):xx-xxx (insert page numbers). https://doi.org/10.12788/jhm.3399
How to cite articles within Section 2
Alvarez F, Alverson B, Balighian E, Beauchamp-Walters J, Biondi E, Blankenberg R, Bridgeman C, Brown J, Buchanan AO, Carlson D, Chang P, Coon E, Daud YN, Denniston S, DeWolfe CC, Deutsch SA, Doshi A, Fisher E, Gage S, Gallagher MP, Gill A, Goel Jones V, Grill J, Gupta A, Herbst BF Jr, Hershey D, Hoang K, Holmes AV, Hopkins A, Jones Y, Khan A, Lee V, Li ST, Lye, PS, Maginot T, Maloney C, Maniscalco J, Mannino Avila E, Markowsky A, Marks M, Matheny Antommaria AH, Maul E, McCulloh R, Melwani A, Miller C, Mittal V, Natt B, O'Toole J, Ottolini M, Percelay J, Phillips S, Pressel D, Quinonez R, Ralston S, Rappaport DI, Rauch D, Rhee K, Riese J, Roberts K, Rogers A, Rosenberg RE, Ruhlen M, Russell CJ, Russo C, Schwenk KM, Sekaran A, Shadman KA, Shah SS, Shen M, Simon T, Singh A, Smith K, Srinivas N Srivastava R, Sterni L, Thompson ED Jr, Thomson J, Tieder J, Tremoulet A, Wang ME, Williams R, Wu S. Name of Chapter. In: Pediatric Hospital Medicine Core Competencies: 2020 Revision. Section 3: Specialized Services. J Hosp Med. 2020;15(S1):xx-xxx (insert page numbers). https://doi.org/10.12788/jhm.3399
SECTION 2: CORE SKILLS
How to cite articles within Section 2
Alvarez F, Alverson B, Balighian E, Beauchamp-Walters J, Biondi E, Blankenberg R, Bridgeman C, Brown J, Buchanan AO, Carlson D, Chang P, Coon E, Daud YN, Denniston S, DeWolfe CC, Deutsch SA, Doshi A, Fisher E, Gage S, Gallagher MP, Gill A, Goel Jones V, Grill J, Gupta A, Herbst BF Jr, Hershey D, Hoang K, Holmes AV, Hopkins A, Jones Y, Khan A, Lee V, Li ST, Lye, PS, Maginot T, Maloney C, Maniscalco J, Mannino Avila E, Markowsky A, Marks M, Matheny Antommaria AH, Maul E, McCulloh R, Melwani A, Miller C, Mittal V, Natt B, O'Toole J, Ottolini M, Percelay J, Phillips S, Pressel D, Quinonez R, Ralston S, Rappaport DI, Rauch D, Rhee K, Riese J, Roberts K, Rogers A, Rosenberg RE, Ruhlen M, Russell CJ, Russo C, Schwenk KM, Sekaran A, Shadman KA, Shah SS, Shen M, Simon T, Singh A, Smith K, Srinivas N Srivastava R, Sterni L, Thompson ED Jr, Thomson J, Tieder J, Tremoulet A, Wang ME, Williams R, Wu S. Name of Chapter. In: Pediatric Hospital Medicine Core Competencies: 2020 Revision. Section 2: Core Skills. J Hosp Med. 2020;15(S1):XX-XX (insert page numbers). https://doi.org/10.12788/jhm.3398
How to cite articles within Section 2
Alvarez F, Alverson B, Balighian E, Beauchamp-Walters J, Biondi E, Blankenberg R, Bridgeman C, Brown J, Buchanan AO, Carlson D, Chang P, Coon E, Daud YN, Denniston S, DeWolfe CC, Deutsch SA, Doshi A, Fisher E, Gage S, Gallagher MP, Gill A, Goel Jones V, Grill J, Gupta A, Herbst BF Jr, Hershey D, Hoang K, Holmes AV, Hopkins A, Jones Y, Khan A, Lee V, Li ST, Lye, PS, Maginot T, Maloney C, Maniscalco J, Mannino Avila E, Markowsky A, Marks M, Matheny Antommaria AH, Maul E, McCulloh R, Melwani A, Miller C, Mittal V, Natt B, O'Toole J, Ottolini M, Percelay J, Phillips S, Pressel D, Quinonez R, Ralston S, Rappaport DI, Rauch D, Rhee K, Riese J, Roberts K, Rogers A, Rosenberg RE, Ruhlen M, Russell CJ, Russo C, Schwenk KM, Sekaran A, Shadman KA, Shah SS, Shen M, Simon T, Singh A, Smith K, Srinivas N Srivastava R, Sterni L, Thompson ED Jr, Thomson J, Tieder J, Tremoulet A, Wang ME, Williams R, Wu S. Name of Chapter. In: Pediatric Hospital Medicine Core Competencies: 2020 Revision. Section 2: Core Skills. J Hosp Med. 2020;15(S1):XX-XX (insert page numbers). https://doi.org/10.12788/jhm.3398
How to cite articles within Section 2
Alvarez F, Alverson B, Balighian E, Beauchamp-Walters J, Biondi E, Blankenberg R, Bridgeman C, Brown J, Buchanan AO, Carlson D, Chang P, Coon E, Daud YN, Denniston S, DeWolfe CC, Deutsch SA, Doshi A, Fisher E, Gage S, Gallagher MP, Gill A, Goel Jones V, Grill J, Gupta A, Herbst BF Jr, Hershey D, Hoang K, Holmes AV, Hopkins A, Jones Y, Khan A, Lee V, Li ST, Lye, PS, Maginot T, Maloney C, Maniscalco J, Mannino Avila E, Markowsky A, Marks M, Matheny Antommaria AH, Maul E, McCulloh R, Melwani A, Miller C, Mittal V, Natt B, O'Toole J, Ottolini M, Percelay J, Phillips S, Pressel D, Quinonez R, Ralston S, Rappaport DI, Rauch D, Rhee K, Riese J, Roberts K, Rogers A, Rosenberg RE, Ruhlen M, Russell CJ, Russo C, Schwenk KM, Sekaran A, Shadman KA, Shah SS, Shen M, Simon T, Singh A, Smith K, Srinivas N Srivastava R, Sterni L, Thompson ED Jr, Thomson J, Tieder J, Tremoulet A, Wang ME, Williams R, Wu S. Name of Chapter. In: Pediatric Hospital Medicine Core Competencies: 2020 Revision. Section 2: Core Skills. J Hosp Med. 2020;15(S1):XX-XX (insert page numbers). https://doi.org/10.12788/jhm.3398
SECTION 1: COMMON CLINICAL DIAGNOSES AND CONDITIONS
How to cite articles within Section 1
Alvarez F, Alverson B, Balighian E, Beauchamp-Walters J, Biondi E, Blankenberg R, Bridgeman C, Brown J, Buchanan AO, Carlson D, Chang P, Coon E, Daud YN, Denniston S, DeWolfe CC, Deutsch SA, Doshi A, Fisher E, Gage S, Gallagher MP, Gill A, Goel Jones V, Grill J, Gupta A, Herbst BF Jr, Hershey D, Hoang K, Holmes AV, Hopkins A, Jones Y, Khan A, Lee V, Li ST, Lye, PS, Maginot T, Maloney C, Maniscalco J, Mannino Avila E, Markowsky A, Marks M, Matheny Antommaria AH, Maul E, McCulloh R, Melwani A, Miller C, Mittal V, Natt B, O'Toole J, Ottolini M, Percelay J, Phillips S, Pressel D, Quinonez R, Ralston S, Rappaport DI, Rauch D, Rhee K, Riese J, Roberts K, Rogers A, Rosenberg RE, Ruhlen M, Russell CJ, Russo C, Schwenk KM, Sekaran A, Shadman KA, Shah SS, Shen M, Simon T, Singh A, Smith K, Srinivas N Srivastava R, Sterni L, Thompson ED Jr, Thomson J, Tieder J, Tremoulet A, Wang ME, Williams R, Wu S. Name of Chapter. In: Pediatric Hospital Medicine Core Competencies: 2020 Revision. Section 1: Common Clinical Diagnoses and Conditions. J Hosp Med. 2020;15(S1):xx-xx (insert page numbers). https://doi.org/10.12788/jhm.3397
How to cite articles within Section 1
Alvarez F, Alverson B, Balighian E, Beauchamp-Walters J, Biondi E, Blankenberg R, Bridgeman C, Brown J, Buchanan AO, Carlson D, Chang P, Coon E, Daud YN, Denniston S, DeWolfe CC, Deutsch SA, Doshi A, Fisher E, Gage S, Gallagher MP, Gill A, Goel Jones V, Grill J, Gupta A, Herbst BF Jr, Hershey D, Hoang K, Holmes AV, Hopkins A, Jones Y, Khan A, Lee V, Li ST, Lye, PS, Maginot T, Maloney C, Maniscalco J, Mannino Avila E, Markowsky A, Marks M, Matheny Antommaria AH, Maul E, McCulloh R, Melwani A, Miller C, Mittal V, Natt B, O'Toole J, Ottolini M, Percelay J, Phillips S, Pressel D, Quinonez R, Ralston S, Rappaport DI, Rauch D, Rhee K, Riese J, Roberts K, Rogers A, Rosenberg RE, Ruhlen M, Russell CJ, Russo C, Schwenk KM, Sekaran A, Shadman KA, Shah SS, Shen M, Simon T, Singh A, Smith K, Srinivas N Srivastava R, Sterni L, Thompson ED Jr, Thomson J, Tieder J, Tremoulet A, Wang ME, Williams R, Wu S. Name of Chapter. In: Pediatric Hospital Medicine Core Competencies: 2020 Revision. Section 1: Common Clinical Diagnoses and Conditions. J Hosp Med. 2020;15(S1):xx-xx (insert page numbers). https://doi.org/10.12788/jhm.3397
How to cite articles within Section 1
Alvarez F, Alverson B, Balighian E, Beauchamp-Walters J, Biondi E, Blankenberg R, Bridgeman C, Brown J, Buchanan AO, Carlson D, Chang P, Coon E, Daud YN, Denniston S, DeWolfe CC, Deutsch SA, Doshi A, Fisher E, Gage S, Gallagher MP, Gill A, Goel Jones V, Grill J, Gupta A, Herbst BF Jr, Hershey D, Hoang K, Holmes AV, Hopkins A, Jones Y, Khan A, Lee V, Li ST, Lye, PS, Maginot T, Maloney C, Maniscalco J, Mannino Avila E, Markowsky A, Marks M, Matheny Antommaria AH, Maul E, McCulloh R, Melwani A, Miller C, Mittal V, Natt B, O'Toole J, Ottolini M, Percelay J, Phillips S, Pressel D, Quinonez R, Ralston S, Rappaport DI, Rauch D, Rhee K, Riese J, Roberts K, Rogers A, Rosenberg RE, Ruhlen M, Russell CJ, Russo C, Schwenk KM, Sekaran A, Shadman KA, Shah SS, Shen M, Simon T, Singh A, Smith K, Srinivas N Srivastava R, Sterni L, Thompson ED Jr, Thomson J, Tieder J, Tremoulet A, Wang ME, Williams R, Wu S. Name of Chapter. In: Pediatric Hospital Medicine Core Competencies: 2020 Revision. Section 1: Common Clinical Diagnoses and Conditions. J Hosp Med. 2020;15(S1):xx-xx (insert page numbers). https://doi.org/10.12788/jhm.3397
Gap Analysis for the Conversion to Area Under the Curve Vancomycin Monitoring in a Small Rural Hospital
The use of weight-based dosing with trough-based monitoring of vancomycin has been in clinical practice for more than a decade. The American Society of Health-System Pharmacists (ASHP), the Infectious Diseases Society of America (IDSA), and the Society of Infectious Diseases Pharmacists (SIDP) published the first guidelines for vancomycin monitoring in 2009.1 Although it has been well established that area under the curve (AUC) over the minimal inhibitory concentration (MIC) ratio > 400 mg.h/L is the best predictor of clinical efficacy, obtaining this value in clinical practice was not pragmatic. Therefore, the 2009 guidelines recommended a goal vancomycin trough of 15 to 20 mcg/ml as a surrogate marker for AUC/MIC > 400 mg.hr/L. This has since become a common practice despite little data that support this recommendation.
The efficacy and safety of trough-based monitoring has been evaluated extensively over the past several years and more recent data suggest that there is wide patient variability in AUC with this method and higher trough levels are associated with more nephrotoxicity.2,3 ASHP, IDSA, SIDP, and the Pediatric Infectious Diseases Society (PIDS) updated the consensus guidelines in 2020.4 Trough-based monitoring is no longer recommended. Instead AUC24 monitoring should be implemented with a goal range of 400 to 600 mg.h/L for efficacy and safety. Given concerns for vancomycin penetration into the central nervous system (CNS), many facility protocols utilize higher targets (> 600 mg.h/L) for CNS infections.
Some hospitals have been utilizing AUC-based monitoring for years. There are strategies from tertiary care centers that drive this practice change in the medical literature.5,6 However, it is important to reproduce these implementation practices in small, rural facilities that may face unique challenges with limited resources and may be slower to implement consensus guidelines.7,8 As this is a major practice change, it is imperative to evaluate the extent of transition and identify areas of needed improvement.
Accurate therapeutic drug monitoring ensures both the safety and efficacy of vancomycin therapy. Unfortunately, research shows that inappropriate laboratory tests are common in medical facilities.9 Drug levels taken inappropriately can lead to delays in therapeutic decision-making, inappropriate dosage adjustments and create a need for repeated drug levels, which increases the overall cost of admission.
Given the multiple affected services needed to make successful practice transitions, it is paramount that facilities evaluate progress during the transition phase. The Agency for Healthcare Research and Quality and the Institute for Healthcare Improvement provide guidance in the Plan-Do-Study-Act Cycle for quality assessment and improvement of new initiatives.10,11 A gap analysis can be used as a simple tool for evaluating the transition of research into practice and to identify areas of needed improvement.
The Veterans Health Care System of the Ozarks (VHSO) in Fayetteville, Arkansas made the transition from trough-based monitoring to 2-level AUC-based monitoring on April 1, 2019. The purpose of this study was to evaluate the effectiveness of transition methods used to implement AUC-monitoring for vancomycin treated patients in a small, primary facility. A further goal of the study was to identify areas of needed improvement and education and whether the problems derived from deficiencies in knowledge and ordering (medical and pharmacy services) or execution (nursing and laboratory services).
Methods
VHSO is a 52-bed US Department of Veterans Affairs primary care hospital. The pharmacy and laboratory are staffed 24 hours each day. There is 1 clinical pharmacy specialist (CPS) available for therapeutic drug monitoring consults Monday through Friday between the hours of 7:30 AM and 4:00 PM. No partial full-time equivalent employees were added for this conversion. Pharmacy-driven vancomycin dosing and monitoring is conducted on a collaborative basis, with pharmacy managing the majority of vancomycin treated patients. Night and weekend pharmacy staff provide cross-coverage on vancomycin consultations. Laboratory orders and medication dosage adjustments fall within the CPS scope of practice. Nurses do not perform laboratory draws for therapeutic drug monitoring; this is done solely by phlebotomists. There is no infectious diseases specialist at the facility to champion antibiotic dosing initiatives.
The implementation strategy largely reflected those outlined from tertiary care centers.5,6 First, key personnel from the laboratory department met to discuss this practice change and to add vancomycin peaks to the ordering menu. A critical value was set at 40 mcg/ml. Vancomycin troughs and random levels already were orderable items. A comment field was added to all laboratory orders for further clarification. Verbiage was added to laboratory reports in the computerized medical record to assist clinicians in determining the appropriateness of the level. This was followed by an educational email to both the nursing and laboratory departments explaining the practice change and included a link to the Pharmacy Joe “Vancomycin Dosing by AUC:MIC Instead of Trough-level” podcast (www.pharmacyjoe.com episode 356).
The pharmacy department received an interactive 30-minute presentation, followed immediately by a group activity to discuss practice problems. This presentation was condensed, recorded, and emailed to all VHSO pharmacists. A shared folder contained pertinent material on AUC monitoring.
Finally, an interactive presentation was set up for hospitalists and a video teleconferencing was conducted for rotating medical residents. Both the podcast and recorded presentation were emailed to the entire medical staff with a brief introduction of the practice change. Additionally, the transition process was added as a standing item on the monthly antimicrobial stewardship meeting agenda.
The standardized pharmacokinetic model at the study facility consisted of a vancomycin volume of distribution of 0.7 mg/kg and elimination rate constant (Ke) by Matzke and colleagues for total daily dose calculations.12 Obese patients (BMI ≥ 30) undergo alternative clearance equations described by Crass and colleagues.13 Cockcroft-Gault methods using ideal body weight (or actual body weight if < ideal body weight) are used for determining creatinine clearance. In patients aged ≥ 65 years with a serum creatinine < 1.0 mg/dL, facility guidance was to round serum creatinine up to 1.0 mg/dL. Loading doses were determined on a case-by-case basis with a cap of 2,000 mg, maintenance doses were rounded to the nearest 250 mg.
Vancomycin levels typically are drawn at steady state and analyzed using the logarithmic trapezoidal rule.14 The pharmacy and medical staff were educated to provide details on timing and coordination in nursing and laboratory orders (Table 1). Two-level AUC monitoring typically is not performed in patients with acute renal failure, expected duration of therapy < 72 hours, urinary tract infections, skin and soft tissue infections, or in renal replacement therapy.5
This gap analysis consisted of a retrospective chart review of vancomycin levels ordered after the implementation of AUC-based monitoring to determine the effectiveness of the transition. Three months of data were collected between April 2019 and June 2019. Vancomycin levels were deemed either appropriate or inappropriate based on timing and type (peak, trough, or random) of the laboratory test in relation to the previously administered vancomycin dose. Appropriate peaks were drawn within 2 hours after the end of infusion and troughs at least 1 half-life after the dose or just prior to the next dose and within the same dosing interval as the peak. Tests drawn outside of the specified time range, trough-only laboratory tests, or those drawn after vancomycin had been discontinued were considered inappropriate. Peaks and troughs drawn from separate dosing intervals also were considered inappropriate. Random levels were considered appropriate only if they fit the clinical context in acute renal failure or renal replacement therapy. An effective transition was defined as ≥ 80% of all vancomycin treated patients monitored with AUC methods rather than trough-based methods.
Inclusion criteria included all vancomycin levels ordered during the study period with no exclusions. The primary endpoint was the proportion of vancomycin levels drawn appropriately. Secondary endpoints were the proportion of AUC24 calculations within therapeutic range and a stratification of reasons for inappropriate levels. Descriptive statistics were collected to describe the scope of the project. Levels drawn from various shifts were compared (ie, day, night, or weekend). Calculated AUC24 levels between 400 and 600 mg.h/L were considered therapeutic unless treating CNS infection (600-700 mg.h/L). Given the operational outcomes (rather than clinical outcomes) and no comparator group, patient specific data were not collected.
Descriptive statistics without further analysis were used to describe proportions. The goal level for compliance was set at 100%. These methods were reviewed by the VHSO Institutional Review Board and granted nonresearch status, waiving the requirement for informed consent.
Results
The transition was effective with 97% of all cases utilizing AUC-based methods for monitoring. A total of 65 vancomycin levels were drawn in the study period; 32 peaks, 32 troughs, and 1 random level (drawn appropriately during acute renal failure 24 hours after starting therapy). All shifts were affected proportionately; days (n = 26, 40%), nights (n = 18, 27.7%), and weekends (n = 21, 32.3%). Based on time of dosage administration and laboratory test, there were 9 levels (13.8%) deemed inappropriate, 56 levels (86.1%) were appropriate. Reasons for inappropriate levels gleaned from chart review are presented in Table 2. Four levels had to be repeated for accurate calculations.
From the peak/trough couplets drawn appropriately, calculated AUC24 fell with the desired range in 61% (n = 17) of cases. Of the 11 that fell outside of range, 8 were subtherapeutic (< 400 mg.h/L) and 3 were supratherapeutic (> 600 mg.h/L). All levels were drawn at steady state. Indications for vancomycin monitoring were osteomyelitis (n = 13, 43%), sepsis (n = 10, 33%), pneumonia (n = 6, 20%), and 1 case of meningitis (3%).
Discussion
To the author’s knowledge, this is the first report of a vancomycin AUC24 monitoring conversion in a rural facility. This study adds to the existing medical literature in that it demonstrates that: (1) implementation methods described in large, tertiary centers can be effectively utilized in primary care, rural facilities; (2) the gap analysis used can be duplicated with minimal personnel and resources to ensure effective implementation (Table 3); and (3) the reported improvement needs can serve as a model for preventative measures at other facilities. The incidence of appropriate vancomycin levels was notably better than those reported in other single center studies.15-17 However, given variations in study design and facility operating procedures, it would be difficult to compare incidence among medical facilities. As such, there are no consensus benchmarks for comparison. The majority of inappropriate levels occurred early in the study period and on weekends. Appropriateness of drug levels may have improved with continued feedback and familiarity.
The calculated AUC24 fell within predicted range in 61% of cases. For comparison, a recent study from a large academic medical center reported that 73.5% of 2-level AUC24 cases had initial values within the therapeutic range.18 Of note, the target range used was much wider (400 - 800 mg.h/L) than the present study. Another study reported dose adjustments for subtherapeutic AUC levels in 25% of cases and dose reductions for supratherapeutic levels in 33.3% of cases.19
Of the AUC24 calculations that fell outside of therapeutic range, the majority (n = 8, 73%) were subtherapeutic (< 400 mg.h/L), half of these were for patients who were obese. It was unclear in the medical record which equation was used for initial dosing (Matzke vs Crass), or whether more conservative AUCs were used for calculating the total daily dose. The VHSO policy limiting loading doses also may have played a role; indeed the updated guidelines recommend a maximum loading dose of 3,000 mg depending on the severity of infection.4 Two of the 3 supratherapeutic levels were thought to be due to accumulation with long-term therapy.
Given such a large change from long-standing practices, there was surprisingly little resistance from the various clinical services. A recent survey of academic medical centers reported that the majority (88%) of all respondents who did not currently utilize AUC24 monitoring did not plan on making this immediate transition, largely citing unfamiliarity and training requirements.20 It is conceivable that the transition to AUC monitoring in smaller facilities may have fewer barriers than those seen in tertiary care centers. There are fewer health care providers and pharmacists to educate with the primary responsibilities falling on relatively few clinicians. There is little question as to who will be conducting follow up or whom to contact for questions. A smaller patient load and lesser patient acuity may translate to fewer vancomycin cases that require monitoring.
The interactive meetings were an important element for facility implementation. Research shows that emails alone are not effective for health care provider education, and interactive methods are recommended over passive methods.21,22 Assessing and avoiding barriers up front such as unclear laboratory orders, or communication failures is paramount to successful implementation strategies.23 Additionally, the detailed written ordering communication may have contributed to a smoother transition. The educational recording proved to be helpful in educating new staff and residents. An identified logistical error was that laboratory orders entered while patients were enrolled in sham clinics for electronic workload capture (eg, Pharmacy Inpatient Clinic) created confusion on the physical location of the patient for the phlebotomists, potentially causing delays in specimen collection.
A major development that stemmed from this intervention was that the Medical Service asked that policy changes be made so that the Pharmacy Service take over all vancomycin dosing at the facility. Previously, this had been done on a collaborative basis. Similar facilities with a collaborative practice model may need to anticipate such a request as this may present a new set of challenges. Accordingly, the pharmacy department is in the process of establishing standing operating procedures, pharmacist competencies, and a facility memorandum. Future research should evaluate the safety and efficacy of vancomycin therapy after the switch to AUC-based monitoring.
Limitations
There are several limitations to consider with this study. Operating procedures and implementation processes may vary between facilities, which could limit the generalizability of these results. Given the small facility size, the overall number of laboratory tests drawn was much smaller than those seen in larger facilities. The time needed for AUC calculations is notably longer than older methods of monitoring; however, this was not objectively assessed. It is important to note that clinical outcomes were beyond the scope of this gap analysis and this is an area of future research at the study facility. Vancomycin laboratory tests that were missed due to procedures and subsequently rescheduled were occasionally observed but not accounted for in this analysis. Additionally, vancomycin courses without monitoring (appropriate or otherwise) when indicated were not assessed. However, anecdotally speaking, this would be a very unlikely occurrence.
Conclusion
Conversion to AUC-based vancomycin monitoring is feasible in primary, rural medical centers. Implementation strategies from tertiary facilities can be successfully utilized in smaller hospitals. Quality assessment strategies such as a gap analysis can be utilized with minimal resources for facility uptake of new clinical practices.
1. Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists [published correction appears in Am J Health Syst Pharm. 2009;66(10):887]. Am J Health Syst Pharm. 2009;66(1):82‐98. doi:10.2146/ajhp080434
2. van Hal SJ, Paterson DL, Lodise TP. Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob Agents Chemother. 2013;57(2):734‐744. doi:10.1128/AAC.01568-12
3. Pai MP, Neely M, Rodvold KA, Lodise TP. Innovative approaches to optimizing the delivery of vancomycin in individual patients. Adv Drug Deliv Rev. 2014;77:50‐57. doi:10.1016/j.addr.2014.05.016
4. Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists [published online ahead of print, 2020 Mar 19]. Am J Health Syst Pharm. 2020;zxaa036. doi:10.1093/ajhp/zxaa036
5. Heil EL, Claeys KC, Mynatt RP, et al. Making the change to area under the curve-based vancomycin dosing. Am J Health Syst Pharm. 2018;75(24):1986‐1995. doi:10.2146/ajhp180034
6. Gregory ER, Burgess DR, Cotner SE, et al. Vancomycin area under the curve dosing and monitoring at an academic medical center: transition strategies and lessons learned [published online ahead of print, 2019 Mar 10]. J Pharm Pract. 2019;897190019834369. doi:10.1177/0897190019834369
7. Septimus EJ, Owens RC Jr. Need and potential of antimicrobial stewardship in community hospitals. Clin Infect Dis. 2011;53 Suppl 1:S8‐S14. doi:10.1093/cid/cir363
8. Goldman LE, Dudley RA. United States rural hospital quality in the Hospital Compare database-accounting for hospital characteristics. Health Policy. 2008;87(1):112‐127. doi:10.1016/j.healthpol.2008.02.002
9. Zhi M, Ding EL, Theisen-Toupal J, Whelan J, Arnaout R. The landscape of inappropriate laboratory testing: a 15-year meta-analysis. PLoS One. 2013;8(11):e78962. doi:10.1371/journal.pone.0078962
10. Institute for Healthcare Improvement. Plan-do-study-act (PDSA) worksheet. http://www.ihi.org/resources/Pages/Tools/PlanDoStudyActWorksheet.aspx. Accessed May 13, 2020.
11. Agency for Healthcare Research and Quality. Plan-do-study-act (PDSA) cycle. https://innovations.ahrq.gov/qualitytools/plan-do-study-act-pdsa-cycle. Updated April 10, 2013. Accessed May 13, 2020.
12. Matzke GR, McGory RW, Halstenson CE, Keane WF. Pharmacokinetics of vancomycin in patients with various degrees of renal function. Antimicrob Agents Chemother. 1984;25(4):433‐437. doi:10.1128/aac.25.4.433
13. Crass RL, Dunn R, Hong J, Krop LC, Pai MP. Dosing vancomycin in the super obese: less is more. J Antimicrob Chemother. 2018;73(11):3081‐3086. doi:10.1093/jac/dky310
14. Pai MP, Russo A, Novelli A, Venditti M, Falcone M. Simplified equations using two concentrations to calculate area under the curve for antimicrobials with concentration-dependent pharmacodynamics: daptomycin as a motivating example. Antimicrob Agents Chemother. 2014;58(6):3162‐3167. doi:10.1128/AAC.02355-14
15. Suryadevara M, Steidl KE, Probst LA, Shaw J. Inappropriate vancomycin therapeutic drug monitoring in hospitalized pediatric patients increases pediatric trauma and hospital costs. J Pediatr Pharmacol Ther. 2012;17(2):159‐165. doi:10.5863/1551-6776-17.2.159
16. Morrison AP, Melanson SE, Carty MG, Bates DW, Szumita PM, Tanasijevic MJ. What proportion of vancomycin trough levels are drawn too early?: frequency and impact on clinical actions. Am J Clin Pathol. 2012;137(3):472‐478. doi:10.1309/AJCPDSYS0DVLKFOH
17. Melanson SE, Mijailovic AS, Wright AP, Szumita PM, Bates DW, Tanasijevic MJ. An intervention to improve the timing of vancomycin levels. Am J Clin Pathol. 2013;140(6):801‐806. doi:10.1309/AJCPKQ6EAH7OYQLB
18. Meng L, Wong T, Huang S, et al. Conversion from vancomycin trough concentration-guided dosing to area under the curve-guided dosing using two sample measurements in adults: implementation at an academic medical center. Pharmacotherapy. 2019;39(4):433‐442. doi:10.1002/phar.2234
19. Stoessel AM, Hale CM, Seabury RW, Miller CD, Steele JM. The impact of AUC-based monitoring on pharmacist-directed vancomycin dose adjustments in complicated methicillin-resistant staphylococcus aureus Infection. J Pharm Pract. 2019;32(4):442‐446. doi:10.1177/0897190018764564
20. Kufel WD, Seabury RW, Mogle BT, Beccari MV, Probst LA, Steele JM. Readiness to implement vancomycin monitoring based on area under the concentration-time curve: a cross-sectional survey of a national health consortium. Am J Health Syst Pharm. 2019;76(12):889‐894. doi:10.1093/ajhp/zxz070
21. Bluestone J, Johnson P, Fullerton J, Carr C, Alderman J, BonTempo J. Effective in-service training design and delivery: evidence from an integrative literature review. Hum Resour Health. 2013;11:51. doi:10.1186/1478-4491-11-51
22. Ebben RHA, Siqeca F, Madsen UR, Vloet LCM, van Achterberg T. Effectiveness of implementation strategies for the improvement of guideline and protocol adherence in emergency care: a systematic review. BMJ Open. 2018;8(11):e017572. doi:10.1136/bmjopen-2017-017572
23. Fischer F, Lange K, Klose K, Greiner W, Kraemer A. Barriers and Strategies in Guideline Implementation-A Scoping Review. Healthcare (Basel). 2016;4(3):36. doi:10.3390/healthcare4030036
The use of weight-based dosing with trough-based monitoring of vancomycin has been in clinical practice for more than a decade. The American Society of Health-System Pharmacists (ASHP), the Infectious Diseases Society of America (IDSA), and the Society of Infectious Diseases Pharmacists (SIDP) published the first guidelines for vancomycin monitoring in 2009.1 Although it has been well established that area under the curve (AUC) over the minimal inhibitory concentration (MIC) ratio > 400 mg.h/L is the best predictor of clinical efficacy, obtaining this value in clinical practice was not pragmatic. Therefore, the 2009 guidelines recommended a goal vancomycin trough of 15 to 20 mcg/ml as a surrogate marker for AUC/MIC > 400 mg.hr/L. This has since become a common practice despite little data that support this recommendation.
The efficacy and safety of trough-based monitoring has been evaluated extensively over the past several years and more recent data suggest that there is wide patient variability in AUC with this method and higher trough levels are associated with more nephrotoxicity.2,3 ASHP, IDSA, SIDP, and the Pediatric Infectious Diseases Society (PIDS) updated the consensus guidelines in 2020.4 Trough-based monitoring is no longer recommended. Instead AUC24 monitoring should be implemented with a goal range of 400 to 600 mg.h/L for efficacy and safety. Given concerns for vancomycin penetration into the central nervous system (CNS), many facility protocols utilize higher targets (> 600 mg.h/L) for CNS infections.
Some hospitals have been utilizing AUC-based monitoring for years. There are strategies from tertiary care centers that drive this practice change in the medical literature.5,6 However, it is important to reproduce these implementation practices in small, rural facilities that may face unique challenges with limited resources and may be slower to implement consensus guidelines.7,8 As this is a major practice change, it is imperative to evaluate the extent of transition and identify areas of needed improvement.
Accurate therapeutic drug monitoring ensures both the safety and efficacy of vancomycin therapy. Unfortunately, research shows that inappropriate laboratory tests are common in medical facilities.9 Drug levels taken inappropriately can lead to delays in therapeutic decision-making, inappropriate dosage adjustments and create a need for repeated drug levels, which increases the overall cost of admission.
Given the multiple affected services needed to make successful practice transitions, it is paramount that facilities evaluate progress during the transition phase. The Agency for Healthcare Research and Quality and the Institute for Healthcare Improvement provide guidance in the Plan-Do-Study-Act Cycle for quality assessment and improvement of new initiatives.10,11 A gap analysis can be used as a simple tool for evaluating the transition of research into practice and to identify areas of needed improvement.
The Veterans Health Care System of the Ozarks (VHSO) in Fayetteville, Arkansas made the transition from trough-based monitoring to 2-level AUC-based monitoring on April 1, 2019. The purpose of this study was to evaluate the effectiveness of transition methods used to implement AUC-monitoring for vancomycin treated patients in a small, primary facility. A further goal of the study was to identify areas of needed improvement and education and whether the problems derived from deficiencies in knowledge and ordering (medical and pharmacy services) or execution (nursing and laboratory services).
Methods
VHSO is a 52-bed US Department of Veterans Affairs primary care hospital. The pharmacy and laboratory are staffed 24 hours each day. There is 1 clinical pharmacy specialist (CPS) available for therapeutic drug monitoring consults Monday through Friday between the hours of 7:30 AM and 4:00 PM. No partial full-time equivalent employees were added for this conversion. Pharmacy-driven vancomycin dosing and monitoring is conducted on a collaborative basis, with pharmacy managing the majority of vancomycin treated patients. Night and weekend pharmacy staff provide cross-coverage on vancomycin consultations. Laboratory orders and medication dosage adjustments fall within the CPS scope of practice. Nurses do not perform laboratory draws for therapeutic drug monitoring; this is done solely by phlebotomists. There is no infectious diseases specialist at the facility to champion antibiotic dosing initiatives.
The implementation strategy largely reflected those outlined from tertiary care centers.5,6 First, key personnel from the laboratory department met to discuss this practice change and to add vancomycin peaks to the ordering menu. A critical value was set at 40 mcg/ml. Vancomycin troughs and random levels already were orderable items. A comment field was added to all laboratory orders for further clarification. Verbiage was added to laboratory reports in the computerized medical record to assist clinicians in determining the appropriateness of the level. This was followed by an educational email to both the nursing and laboratory departments explaining the practice change and included a link to the Pharmacy Joe “Vancomycin Dosing by AUC:MIC Instead of Trough-level” podcast (www.pharmacyjoe.com episode 356).
The pharmacy department received an interactive 30-minute presentation, followed immediately by a group activity to discuss practice problems. This presentation was condensed, recorded, and emailed to all VHSO pharmacists. A shared folder contained pertinent material on AUC monitoring.
Finally, an interactive presentation was set up for hospitalists and a video teleconferencing was conducted for rotating medical residents. Both the podcast and recorded presentation were emailed to the entire medical staff with a brief introduction of the practice change. Additionally, the transition process was added as a standing item on the monthly antimicrobial stewardship meeting agenda.
The standardized pharmacokinetic model at the study facility consisted of a vancomycin volume of distribution of 0.7 mg/kg and elimination rate constant (Ke) by Matzke and colleagues for total daily dose calculations.12 Obese patients (BMI ≥ 30) undergo alternative clearance equations described by Crass and colleagues.13 Cockcroft-Gault methods using ideal body weight (or actual body weight if < ideal body weight) are used for determining creatinine clearance. In patients aged ≥ 65 years with a serum creatinine < 1.0 mg/dL, facility guidance was to round serum creatinine up to 1.0 mg/dL. Loading doses were determined on a case-by-case basis with a cap of 2,000 mg, maintenance doses were rounded to the nearest 250 mg.
Vancomycin levels typically are drawn at steady state and analyzed using the logarithmic trapezoidal rule.14 The pharmacy and medical staff were educated to provide details on timing and coordination in nursing and laboratory orders (Table 1). Two-level AUC monitoring typically is not performed in patients with acute renal failure, expected duration of therapy < 72 hours, urinary tract infections, skin and soft tissue infections, or in renal replacement therapy.5
This gap analysis consisted of a retrospective chart review of vancomycin levels ordered after the implementation of AUC-based monitoring to determine the effectiveness of the transition. Three months of data were collected between April 2019 and June 2019. Vancomycin levels were deemed either appropriate or inappropriate based on timing and type (peak, trough, or random) of the laboratory test in relation to the previously administered vancomycin dose. Appropriate peaks were drawn within 2 hours after the end of infusion and troughs at least 1 half-life after the dose or just prior to the next dose and within the same dosing interval as the peak. Tests drawn outside of the specified time range, trough-only laboratory tests, or those drawn after vancomycin had been discontinued were considered inappropriate. Peaks and troughs drawn from separate dosing intervals also were considered inappropriate. Random levels were considered appropriate only if they fit the clinical context in acute renal failure or renal replacement therapy. An effective transition was defined as ≥ 80% of all vancomycin treated patients monitored with AUC methods rather than trough-based methods.
Inclusion criteria included all vancomycin levels ordered during the study period with no exclusions. The primary endpoint was the proportion of vancomycin levels drawn appropriately. Secondary endpoints were the proportion of AUC24 calculations within therapeutic range and a stratification of reasons for inappropriate levels. Descriptive statistics were collected to describe the scope of the project. Levels drawn from various shifts were compared (ie, day, night, or weekend). Calculated AUC24 levels between 400 and 600 mg.h/L were considered therapeutic unless treating CNS infection (600-700 mg.h/L). Given the operational outcomes (rather than clinical outcomes) and no comparator group, patient specific data were not collected.
Descriptive statistics without further analysis were used to describe proportions. The goal level for compliance was set at 100%. These methods were reviewed by the VHSO Institutional Review Board and granted nonresearch status, waiving the requirement for informed consent.
Results
The transition was effective with 97% of all cases utilizing AUC-based methods for monitoring. A total of 65 vancomycin levels were drawn in the study period; 32 peaks, 32 troughs, and 1 random level (drawn appropriately during acute renal failure 24 hours after starting therapy). All shifts were affected proportionately; days (n = 26, 40%), nights (n = 18, 27.7%), and weekends (n = 21, 32.3%). Based on time of dosage administration and laboratory test, there were 9 levels (13.8%) deemed inappropriate, 56 levels (86.1%) were appropriate. Reasons for inappropriate levels gleaned from chart review are presented in Table 2. Four levels had to be repeated for accurate calculations.
From the peak/trough couplets drawn appropriately, calculated AUC24 fell with the desired range in 61% (n = 17) of cases. Of the 11 that fell outside of range, 8 were subtherapeutic (< 400 mg.h/L) and 3 were supratherapeutic (> 600 mg.h/L). All levels were drawn at steady state. Indications for vancomycin monitoring were osteomyelitis (n = 13, 43%), sepsis (n = 10, 33%), pneumonia (n = 6, 20%), and 1 case of meningitis (3%).
Discussion
To the author’s knowledge, this is the first report of a vancomycin AUC24 monitoring conversion in a rural facility. This study adds to the existing medical literature in that it demonstrates that: (1) implementation methods described in large, tertiary centers can be effectively utilized in primary care, rural facilities; (2) the gap analysis used can be duplicated with minimal personnel and resources to ensure effective implementation (Table 3); and (3) the reported improvement needs can serve as a model for preventative measures at other facilities. The incidence of appropriate vancomycin levels was notably better than those reported in other single center studies.15-17 However, given variations in study design and facility operating procedures, it would be difficult to compare incidence among medical facilities. As such, there are no consensus benchmarks for comparison. The majority of inappropriate levels occurred early in the study period and on weekends. Appropriateness of drug levels may have improved with continued feedback and familiarity.
The calculated AUC24 fell within predicted range in 61% of cases. For comparison, a recent study from a large academic medical center reported that 73.5% of 2-level AUC24 cases had initial values within the therapeutic range.18 Of note, the target range used was much wider (400 - 800 mg.h/L) than the present study. Another study reported dose adjustments for subtherapeutic AUC levels in 25% of cases and dose reductions for supratherapeutic levels in 33.3% of cases.19
Of the AUC24 calculations that fell outside of therapeutic range, the majority (n = 8, 73%) were subtherapeutic (< 400 mg.h/L), half of these were for patients who were obese. It was unclear in the medical record which equation was used for initial dosing (Matzke vs Crass), or whether more conservative AUCs were used for calculating the total daily dose. The VHSO policy limiting loading doses also may have played a role; indeed the updated guidelines recommend a maximum loading dose of 3,000 mg depending on the severity of infection.4 Two of the 3 supratherapeutic levels were thought to be due to accumulation with long-term therapy.
Given such a large change from long-standing practices, there was surprisingly little resistance from the various clinical services. A recent survey of academic medical centers reported that the majority (88%) of all respondents who did not currently utilize AUC24 monitoring did not plan on making this immediate transition, largely citing unfamiliarity and training requirements.20 It is conceivable that the transition to AUC monitoring in smaller facilities may have fewer barriers than those seen in tertiary care centers. There are fewer health care providers and pharmacists to educate with the primary responsibilities falling on relatively few clinicians. There is little question as to who will be conducting follow up or whom to contact for questions. A smaller patient load and lesser patient acuity may translate to fewer vancomycin cases that require monitoring.
The interactive meetings were an important element for facility implementation. Research shows that emails alone are not effective for health care provider education, and interactive methods are recommended over passive methods.21,22 Assessing and avoiding barriers up front such as unclear laboratory orders, or communication failures is paramount to successful implementation strategies.23 Additionally, the detailed written ordering communication may have contributed to a smoother transition. The educational recording proved to be helpful in educating new staff and residents. An identified logistical error was that laboratory orders entered while patients were enrolled in sham clinics for electronic workload capture (eg, Pharmacy Inpatient Clinic) created confusion on the physical location of the patient for the phlebotomists, potentially causing delays in specimen collection.
A major development that stemmed from this intervention was that the Medical Service asked that policy changes be made so that the Pharmacy Service take over all vancomycin dosing at the facility. Previously, this had been done on a collaborative basis. Similar facilities with a collaborative practice model may need to anticipate such a request as this may present a new set of challenges. Accordingly, the pharmacy department is in the process of establishing standing operating procedures, pharmacist competencies, and a facility memorandum. Future research should evaluate the safety and efficacy of vancomycin therapy after the switch to AUC-based monitoring.
Limitations
There are several limitations to consider with this study. Operating procedures and implementation processes may vary between facilities, which could limit the generalizability of these results. Given the small facility size, the overall number of laboratory tests drawn was much smaller than those seen in larger facilities. The time needed for AUC calculations is notably longer than older methods of monitoring; however, this was not objectively assessed. It is important to note that clinical outcomes were beyond the scope of this gap analysis and this is an area of future research at the study facility. Vancomycin laboratory tests that were missed due to procedures and subsequently rescheduled were occasionally observed but not accounted for in this analysis. Additionally, vancomycin courses without monitoring (appropriate or otherwise) when indicated were not assessed. However, anecdotally speaking, this would be a very unlikely occurrence.
Conclusion
Conversion to AUC-based vancomycin monitoring is feasible in primary, rural medical centers. Implementation strategies from tertiary facilities can be successfully utilized in smaller hospitals. Quality assessment strategies such as a gap analysis can be utilized with minimal resources for facility uptake of new clinical practices.
The use of weight-based dosing with trough-based monitoring of vancomycin has been in clinical practice for more than a decade. The American Society of Health-System Pharmacists (ASHP), the Infectious Diseases Society of America (IDSA), and the Society of Infectious Diseases Pharmacists (SIDP) published the first guidelines for vancomycin monitoring in 2009.1 Although it has been well established that area under the curve (AUC) over the minimal inhibitory concentration (MIC) ratio > 400 mg.h/L is the best predictor of clinical efficacy, obtaining this value in clinical practice was not pragmatic. Therefore, the 2009 guidelines recommended a goal vancomycin trough of 15 to 20 mcg/ml as a surrogate marker for AUC/MIC > 400 mg.hr/L. This has since become a common practice despite little data that support this recommendation.
The efficacy and safety of trough-based monitoring has been evaluated extensively over the past several years and more recent data suggest that there is wide patient variability in AUC with this method and higher trough levels are associated with more nephrotoxicity.2,3 ASHP, IDSA, SIDP, and the Pediatric Infectious Diseases Society (PIDS) updated the consensus guidelines in 2020.4 Trough-based monitoring is no longer recommended. Instead AUC24 monitoring should be implemented with a goal range of 400 to 600 mg.h/L for efficacy and safety. Given concerns for vancomycin penetration into the central nervous system (CNS), many facility protocols utilize higher targets (> 600 mg.h/L) for CNS infections.
Some hospitals have been utilizing AUC-based monitoring for years. There are strategies from tertiary care centers that drive this practice change in the medical literature.5,6 However, it is important to reproduce these implementation practices in small, rural facilities that may face unique challenges with limited resources and may be slower to implement consensus guidelines.7,8 As this is a major practice change, it is imperative to evaluate the extent of transition and identify areas of needed improvement.
Accurate therapeutic drug monitoring ensures both the safety and efficacy of vancomycin therapy. Unfortunately, research shows that inappropriate laboratory tests are common in medical facilities.9 Drug levels taken inappropriately can lead to delays in therapeutic decision-making, inappropriate dosage adjustments and create a need for repeated drug levels, which increases the overall cost of admission.
Given the multiple affected services needed to make successful practice transitions, it is paramount that facilities evaluate progress during the transition phase. The Agency for Healthcare Research and Quality and the Institute for Healthcare Improvement provide guidance in the Plan-Do-Study-Act Cycle for quality assessment and improvement of new initiatives.10,11 A gap analysis can be used as a simple tool for evaluating the transition of research into practice and to identify areas of needed improvement.
The Veterans Health Care System of the Ozarks (VHSO) in Fayetteville, Arkansas made the transition from trough-based monitoring to 2-level AUC-based monitoring on April 1, 2019. The purpose of this study was to evaluate the effectiveness of transition methods used to implement AUC-monitoring for vancomycin treated patients in a small, primary facility. A further goal of the study was to identify areas of needed improvement and education and whether the problems derived from deficiencies in knowledge and ordering (medical and pharmacy services) or execution (nursing and laboratory services).
Methods
VHSO is a 52-bed US Department of Veterans Affairs primary care hospital. The pharmacy and laboratory are staffed 24 hours each day. There is 1 clinical pharmacy specialist (CPS) available for therapeutic drug monitoring consults Monday through Friday between the hours of 7:30 AM and 4:00 PM. No partial full-time equivalent employees were added for this conversion. Pharmacy-driven vancomycin dosing and monitoring is conducted on a collaborative basis, with pharmacy managing the majority of vancomycin treated patients. Night and weekend pharmacy staff provide cross-coverage on vancomycin consultations. Laboratory orders and medication dosage adjustments fall within the CPS scope of practice. Nurses do not perform laboratory draws for therapeutic drug monitoring; this is done solely by phlebotomists. There is no infectious diseases specialist at the facility to champion antibiotic dosing initiatives.
The implementation strategy largely reflected those outlined from tertiary care centers.5,6 First, key personnel from the laboratory department met to discuss this practice change and to add vancomycin peaks to the ordering menu. A critical value was set at 40 mcg/ml. Vancomycin troughs and random levels already were orderable items. A comment field was added to all laboratory orders for further clarification. Verbiage was added to laboratory reports in the computerized medical record to assist clinicians in determining the appropriateness of the level. This was followed by an educational email to both the nursing and laboratory departments explaining the practice change and included a link to the Pharmacy Joe “Vancomycin Dosing by AUC:MIC Instead of Trough-level” podcast (www.pharmacyjoe.com episode 356).
The pharmacy department received an interactive 30-minute presentation, followed immediately by a group activity to discuss practice problems. This presentation was condensed, recorded, and emailed to all VHSO pharmacists. A shared folder contained pertinent material on AUC monitoring.
Finally, an interactive presentation was set up for hospitalists and a video teleconferencing was conducted for rotating medical residents. Both the podcast and recorded presentation were emailed to the entire medical staff with a brief introduction of the practice change. Additionally, the transition process was added as a standing item on the monthly antimicrobial stewardship meeting agenda.
The standardized pharmacokinetic model at the study facility consisted of a vancomycin volume of distribution of 0.7 mg/kg and elimination rate constant (Ke) by Matzke and colleagues for total daily dose calculations.12 Obese patients (BMI ≥ 30) undergo alternative clearance equations described by Crass and colleagues.13 Cockcroft-Gault methods using ideal body weight (or actual body weight if < ideal body weight) are used for determining creatinine clearance. In patients aged ≥ 65 years with a serum creatinine < 1.0 mg/dL, facility guidance was to round serum creatinine up to 1.0 mg/dL. Loading doses were determined on a case-by-case basis with a cap of 2,000 mg, maintenance doses were rounded to the nearest 250 mg.
Vancomycin levels typically are drawn at steady state and analyzed using the logarithmic trapezoidal rule.14 The pharmacy and medical staff were educated to provide details on timing and coordination in nursing and laboratory orders (Table 1). Two-level AUC monitoring typically is not performed in patients with acute renal failure, expected duration of therapy < 72 hours, urinary tract infections, skin and soft tissue infections, or in renal replacement therapy.5
This gap analysis consisted of a retrospective chart review of vancomycin levels ordered after the implementation of AUC-based monitoring to determine the effectiveness of the transition. Three months of data were collected between April 2019 and June 2019. Vancomycin levels were deemed either appropriate or inappropriate based on timing and type (peak, trough, or random) of the laboratory test in relation to the previously administered vancomycin dose. Appropriate peaks were drawn within 2 hours after the end of infusion and troughs at least 1 half-life after the dose or just prior to the next dose and within the same dosing interval as the peak. Tests drawn outside of the specified time range, trough-only laboratory tests, or those drawn after vancomycin had been discontinued were considered inappropriate. Peaks and troughs drawn from separate dosing intervals also were considered inappropriate. Random levels were considered appropriate only if they fit the clinical context in acute renal failure or renal replacement therapy. An effective transition was defined as ≥ 80% of all vancomycin treated patients monitored with AUC methods rather than trough-based methods.
Inclusion criteria included all vancomycin levels ordered during the study period with no exclusions. The primary endpoint was the proportion of vancomycin levels drawn appropriately. Secondary endpoints were the proportion of AUC24 calculations within therapeutic range and a stratification of reasons for inappropriate levels. Descriptive statistics were collected to describe the scope of the project. Levels drawn from various shifts were compared (ie, day, night, or weekend). Calculated AUC24 levels between 400 and 600 mg.h/L were considered therapeutic unless treating CNS infection (600-700 mg.h/L). Given the operational outcomes (rather than clinical outcomes) and no comparator group, patient specific data were not collected.
Descriptive statistics without further analysis were used to describe proportions. The goal level for compliance was set at 100%. These methods were reviewed by the VHSO Institutional Review Board and granted nonresearch status, waiving the requirement for informed consent.
Results
The transition was effective with 97% of all cases utilizing AUC-based methods for monitoring. A total of 65 vancomycin levels were drawn in the study period; 32 peaks, 32 troughs, and 1 random level (drawn appropriately during acute renal failure 24 hours after starting therapy). All shifts were affected proportionately; days (n = 26, 40%), nights (n = 18, 27.7%), and weekends (n = 21, 32.3%). Based on time of dosage administration and laboratory test, there were 9 levels (13.8%) deemed inappropriate, 56 levels (86.1%) were appropriate. Reasons for inappropriate levels gleaned from chart review are presented in Table 2. Four levels had to be repeated for accurate calculations.
From the peak/trough couplets drawn appropriately, calculated AUC24 fell with the desired range in 61% (n = 17) of cases. Of the 11 that fell outside of range, 8 were subtherapeutic (< 400 mg.h/L) and 3 were supratherapeutic (> 600 mg.h/L). All levels were drawn at steady state. Indications for vancomycin monitoring were osteomyelitis (n = 13, 43%), sepsis (n = 10, 33%), pneumonia (n = 6, 20%), and 1 case of meningitis (3%).
Discussion
To the author’s knowledge, this is the first report of a vancomycin AUC24 monitoring conversion in a rural facility. This study adds to the existing medical literature in that it demonstrates that: (1) implementation methods described in large, tertiary centers can be effectively utilized in primary care, rural facilities; (2) the gap analysis used can be duplicated with minimal personnel and resources to ensure effective implementation (Table 3); and (3) the reported improvement needs can serve as a model for preventative measures at other facilities. The incidence of appropriate vancomycin levels was notably better than those reported in other single center studies.15-17 However, given variations in study design and facility operating procedures, it would be difficult to compare incidence among medical facilities. As such, there are no consensus benchmarks for comparison. The majority of inappropriate levels occurred early in the study period and on weekends. Appropriateness of drug levels may have improved with continued feedback and familiarity.
The calculated AUC24 fell within predicted range in 61% of cases. For comparison, a recent study from a large academic medical center reported that 73.5% of 2-level AUC24 cases had initial values within the therapeutic range.18 Of note, the target range used was much wider (400 - 800 mg.h/L) than the present study. Another study reported dose adjustments for subtherapeutic AUC levels in 25% of cases and dose reductions for supratherapeutic levels in 33.3% of cases.19
Of the AUC24 calculations that fell outside of therapeutic range, the majority (n = 8, 73%) were subtherapeutic (< 400 mg.h/L), half of these were for patients who were obese. It was unclear in the medical record which equation was used for initial dosing (Matzke vs Crass), or whether more conservative AUCs were used for calculating the total daily dose. The VHSO policy limiting loading doses also may have played a role; indeed the updated guidelines recommend a maximum loading dose of 3,000 mg depending on the severity of infection.4 Two of the 3 supratherapeutic levels were thought to be due to accumulation with long-term therapy.
Given such a large change from long-standing practices, there was surprisingly little resistance from the various clinical services. A recent survey of academic medical centers reported that the majority (88%) of all respondents who did not currently utilize AUC24 monitoring did not plan on making this immediate transition, largely citing unfamiliarity and training requirements.20 It is conceivable that the transition to AUC monitoring in smaller facilities may have fewer barriers than those seen in tertiary care centers. There are fewer health care providers and pharmacists to educate with the primary responsibilities falling on relatively few clinicians. There is little question as to who will be conducting follow up or whom to contact for questions. A smaller patient load and lesser patient acuity may translate to fewer vancomycin cases that require monitoring.
The interactive meetings were an important element for facility implementation. Research shows that emails alone are not effective for health care provider education, and interactive methods are recommended over passive methods.21,22 Assessing and avoiding barriers up front such as unclear laboratory orders, or communication failures is paramount to successful implementation strategies.23 Additionally, the detailed written ordering communication may have contributed to a smoother transition. The educational recording proved to be helpful in educating new staff and residents. An identified logistical error was that laboratory orders entered while patients were enrolled in sham clinics for electronic workload capture (eg, Pharmacy Inpatient Clinic) created confusion on the physical location of the patient for the phlebotomists, potentially causing delays in specimen collection.
A major development that stemmed from this intervention was that the Medical Service asked that policy changes be made so that the Pharmacy Service take over all vancomycin dosing at the facility. Previously, this had been done on a collaborative basis. Similar facilities with a collaborative practice model may need to anticipate such a request as this may present a new set of challenges. Accordingly, the pharmacy department is in the process of establishing standing operating procedures, pharmacist competencies, and a facility memorandum. Future research should evaluate the safety and efficacy of vancomycin therapy after the switch to AUC-based monitoring.
Limitations
There are several limitations to consider with this study. Operating procedures and implementation processes may vary between facilities, which could limit the generalizability of these results. Given the small facility size, the overall number of laboratory tests drawn was much smaller than those seen in larger facilities. The time needed for AUC calculations is notably longer than older methods of monitoring; however, this was not objectively assessed. It is important to note that clinical outcomes were beyond the scope of this gap analysis and this is an area of future research at the study facility. Vancomycin laboratory tests that were missed due to procedures and subsequently rescheduled were occasionally observed but not accounted for in this analysis. Additionally, vancomycin courses without monitoring (appropriate or otherwise) when indicated were not assessed. However, anecdotally speaking, this would be a very unlikely occurrence.
Conclusion
Conversion to AUC-based vancomycin monitoring is feasible in primary, rural medical centers. Implementation strategies from tertiary facilities can be successfully utilized in smaller hospitals. Quality assessment strategies such as a gap analysis can be utilized with minimal resources for facility uptake of new clinical practices.
1. Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists [published correction appears in Am J Health Syst Pharm. 2009;66(10):887]. Am J Health Syst Pharm. 2009;66(1):82‐98. doi:10.2146/ajhp080434
2. van Hal SJ, Paterson DL, Lodise TP. Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob Agents Chemother. 2013;57(2):734‐744. doi:10.1128/AAC.01568-12
3. Pai MP, Neely M, Rodvold KA, Lodise TP. Innovative approaches to optimizing the delivery of vancomycin in individual patients. Adv Drug Deliv Rev. 2014;77:50‐57. doi:10.1016/j.addr.2014.05.016
4. Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists [published online ahead of print, 2020 Mar 19]. Am J Health Syst Pharm. 2020;zxaa036. doi:10.1093/ajhp/zxaa036
5. Heil EL, Claeys KC, Mynatt RP, et al. Making the change to area under the curve-based vancomycin dosing. Am J Health Syst Pharm. 2018;75(24):1986‐1995. doi:10.2146/ajhp180034
6. Gregory ER, Burgess DR, Cotner SE, et al. Vancomycin area under the curve dosing and monitoring at an academic medical center: transition strategies and lessons learned [published online ahead of print, 2019 Mar 10]. J Pharm Pract. 2019;897190019834369. doi:10.1177/0897190019834369
7. Septimus EJ, Owens RC Jr. Need and potential of antimicrobial stewardship in community hospitals. Clin Infect Dis. 2011;53 Suppl 1:S8‐S14. doi:10.1093/cid/cir363
8. Goldman LE, Dudley RA. United States rural hospital quality in the Hospital Compare database-accounting for hospital characteristics. Health Policy. 2008;87(1):112‐127. doi:10.1016/j.healthpol.2008.02.002
9. Zhi M, Ding EL, Theisen-Toupal J, Whelan J, Arnaout R. The landscape of inappropriate laboratory testing: a 15-year meta-analysis. PLoS One. 2013;8(11):e78962. doi:10.1371/journal.pone.0078962
10. Institute for Healthcare Improvement. Plan-do-study-act (PDSA) worksheet. http://www.ihi.org/resources/Pages/Tools/PlanDoStudyActWorksheet.aspx. Accessed May 13, 2020.
11. Agency for Healthcare Research and Quality. Plan-do-study-act (PDSA) cycle. https://innovations.ahrq.gov/qualitytools/plan-do-study-act-pdsa-cycle. Updated April 10, 2013. Accessed May 13, 2020.
12. Matzke GR, McGory RW, Halstenson CE, Keane WF. Pharmacokinetics of vancomycin in patients with various degrees of renal function. Antimicrob Agents Chemother. 1984;25(4):433‐437. doi:10.1128/aac.25.4.433
13. Crass RL, Dunn R, Hong J, Krop LC, Pai MP. Dosing vancomycin in the super obese: less is more. J Antimicrob Chemother. 2018;73(11):3081‐3086. doi:10.1093/jac/dky310
14. Pai MP, Russo A, Novelli A, Venditti M, Falcone M. Simplified equations using two concentrations to calculate area under the curve for antimicrobials with concentration-dependent pharmacodynamics: daptomycin as a motivating example. Antimicrob Agents Chemother. 2014;58(6):3162‐3167. doi:10.1128/AAC.02355-14
15. Suryadevara M, Steidl KE, Probst LA, Shaw J. Inappropriate vancomycin therapeutic drug monitoring in hospitalized pediatric patients increases pediatric trauma and hospital costs. J Pediatr Pharmacol Ther. 2012;17(2):159‐165. doi:10.5863/1551-6776-17.2.159
16. Morrison AP, Melanson SE, Carty MG, Bates DW, Szumita PM, Tanasijevic MJ. What proportion of vancomycin trough levels are drawn too early?: frequency and impact on clinical actions. Am J Clin Pathol. 2012;137(3):472‐478. doi:10.1309/AJCPDSYS0DVLKFOH
17. Melanson SE, Mijailovic AS, Wright AP, Szumita PM, Bates DW, Tanasijevic MJ. An intervention to improve the timing of vancomycin levels. Am J Clin Pathol. 2013;140(6):801‐806. doi:10.1309/AJCPKQ6EAH7OYQLB
18. Meng L, Wong T, Huang S, et al. Conversion from vancomycin trough concentration-guided dosing to area under the curve-guided dosing using two sample measurements in adults: implementation at an academic medical center. Pharmacotherapy. 2019;39(4):433‐442. doi:10.1002/phar.2234
19. Stoessel AM, Hale CM, Seabury RW, Miller CD, Steele JM. The impact of AUC-based monitoring on pharmacist-directed vancomycin dose adjustments in complicated methicillin-resistant staphylococcus aureus Infection. J Pharm Pract. 2019;32(4):442‐446. doi:10.1177/0897190018764564
20. Kufel WD, Seabury RW, Mogle BT, Beccari MV, Probst LA, Steele JM. Readiness to implement vancomycin monitoring based on area under the concentration-time curve: a cross-sectional survey of a national health consortium. Am J Health Syst Pharm. 2019;76(12):889‐894. doi:10.1093/ajhp/zxz070
21. Bluestone J, Johnson P, Fullerton J, Carr C, Alderman J, BonTempo J. Effective in-service training design and delivery: evidence from an integrative literature review. Hum Resour Health. 2013;11:51. doi:10.1186/1478-4491-11-51
22. Ebben RHA, Siqeca F, Madsen UR, Vloet LCM, van Achterberg T. Effectiveness of implementation strategies for the improvement of guideline and protocol adherence in emergency care: a systematic review. BMJ Open. 2018;8(11):e017572. doi:10.1136/bmjopen-2017-017572
23. Fischer F, Lange K, Klose K, Greiner W, Kraemer A. Barriers and Strategies in Guideline Implementation-A Scoping Review. Healthcare (Basel). 2016;4(3):36. doi:10.3390/healthcare4030036
1. Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists [published correction appears in Am J Health Syst Pharm. 2009;66(10):887]. Am J Health Syst Pharm. 2009;66(1):82‐98. doi:10.2146/ajhp080434
2. van Hal SJ, Paterson DL, Lodise TP. Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob Agents Chemother. 2013;57(2):734‐744. doi:10.1128/AAC.01568-12
3. Pai MP, Neely M, Rodvold KA, Lodise TP. Innovative approaches to optimizing the delivery of vancomycin in individual patients. Adv Drug Deliv Rev. 2014;77:50‐57. doi:10.1016/j.addr.2014.05.016
4. Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists [published online ahead of print, 2020 Mar 19]. Am J Health Syst Pharm. 2020;zxaa036. doi:10.1093/ajhp/zxaa036
5. Heil EL, Claeys KC, Mynatt RP, et al. Making the change to area under the curve-based vancomycin dosing. Am J Health Syst Pharm. 2018;75(24):1986‐1995. doi:10.2146/ajhp180034
6. Gregory ER, Burgess DR, Cotner SE, et al. Vancomycin area under the curve dosing and monitoring at an academic medical center: transition strategies and lessons learned [published online ahead of print, 2019 Mar 10]. J Pharm Pract. 2019;897190019834369. doi:10.1177/0897190019834369
7. Septimus EJ, Owens RC Jr. Need and potential of antimicrobial stewardship in community hospitals. Clin Infect Dis. 2011;53 Suppl 1:S8‐S14. doi:10.1093/cid/cir363
8. Goldman LE, Dudley RA. United States rural hospital quality in the Hospital Compare database-accounting for hospital characteristics. Health Policy. 2008;87(1):112‐127. doi:10.1016/j.healthpol.2008.02.002
9. Zhi M, Ding EL, Theisen-Toupal J, Whelan J, Arnaout R. The landscape of inappropriate laboratory testing: a 15-year meta-analysis. PLoS One. 2013;8(11):e78962. doi:10.1371/journal.pone.0078962
10. Institute for Healthcare Improvement. Plan-do-study-act (PDSA) worksheet. http://www.ihi.org/resources/Pages/Tools/PlanDoStudyActWorksheet.aspx. Accessed May 13, 2020.
11. Agency for Healthcare Research and Quality. Plan-do-study-act (PDSA) cycle. https://innovations.ahrq.gov/qualitytools/plan-do-study-act-pdsa-cycle. Updated April 10, 2013. Accessed May 13, 2020.
12. Matzke GR, McGory RW, Halstenson CE, Keane WF. Pharmacokinetics of vancomycin in patients with various degrees of renal function. Antimicrob Agents Chemother. 1984;25(4):433‐437. doi:10.1128/aac.25.4.433
13. Crass RL, Dunn R, Hong J, Krop LC, Pai MP. Dosing vancomycin in the super obese: less is more. J Antimicrob Chemother. 2018;73(11):3081‐3086. doi:10.1093/jac/dky310
14. Pai MP, Russo A, Novelli A, Venditti M, Falcone M. Simplified equations using two concentrations to calculate area under the curve for antimicrobials with concentration-dependent pharmacodynamics: daptomycin as a motivating example. Antimicrob Agents Chemother. 2014;58(6):3162‐3167. doi:10.1128/AAC.02355-14
15. Suryadevara M, Steidl KE, Probst LA, Shaw J. Inappropriate vancomycin therapeutic drug monitoring in hospitalized pediatric patients increases pediatric trauma and hospital costs. J Pediatr Pharmacol Ther. 2012;17(2):159‐165. doi:10.5863/1551-6776-17.2.159
16. Morrison AP, Melanson SE, Carty MG, Bates DW, Szumita PM, Tanasijevic MJ. What proportion of vancomycin trough levels are drawn too early?: frequency and impact on clinical actions. Am J Clin Pathol. 2012;137(3):472‐478. doi:10.1309/AJCPDSYS0DVLKFOH
17. Melanson SE, Mijailovic AS, Wright AP, Szumita PM, Bates DW, Tanasijevic MJ. An intervention to improve the timing of vancomycin levels. Am J Clin Pathol. 2013;140(6):801‐806. doi:10.1309/AJCPKQ6EAH7OYQLB
18. Meng L, Wong T, Huang S, et al. Conversion from vancomycin trough concentration-guided dosing to area under the curve-guided dosing using two sample measurements in adults: implementation at an academic medical center. Pharmacotherapy. 2019;39(4):433‐442. doi:10.1002/phar.2234
19. Stoessel AM, Hale CM, Seabury RW, Miller CD, Steele JM. The impact of AUC-based monitoring on pharmacist-directed vancomycin dose adjustments in complicated methicillin-resistant staphylococcus aureus Infection. J Pharm Pract. 2019;32(4):442‐446. doi:10.1177/0897190018764564
20. Kufel WD, Seabury RW, Mogle BT, Beccari MV, Probst LA, Steele JM. Readiness to implement vancomycin monitoring based on area under the concentration-time curve: a cross-sectional survey of a national health consortium. Am J Health Syst Pharm. 2019;76(12):889‐894. doi:10.1093/ajhp/zxz070
21. Bluestone J, Johnson P, Fullerton J, Carr C, Alderman J, BonTempo J. Effective in-service training design and delivery: evidence from an integrative literature review. Hum Resour Health. 2013;11:51. doi:10.1186/1478-4491-11-51
22. Ebben RHA, Siqeca F, Madsen UR, Vloet LCM, van Achterberg T. Effectiveness of implementation strategies for the improvement of guideline and protocol adherence in emergency care: a systematic review. BMJ Open. 2018;8(11):e017572. doi:10.1136/bmjopen-2017-017572
23. Fischer F, Lange K, Klose K, Greiner W, Kraemer A. Barriers and Strategies in Guideline Implementation-A Scoping Review. Healthcare (Basel). 2016;4(3):36. doi:10.3390/healthcare4030036