User login
Gap Analysis for the Conversion to Area Under the Curve Vancomycin Monitoring in a Small Rural Hospital
The use of weight-based dosing with trough-based monitoring of vancomycin has been in clinical practice for more than a decade. The American Society of Health-System Pharmacists (ASHP), the Infectious Diseases Society of America (IDSA), and the Society of Infectious Diseases Pharmacists (SIDP) published the first guidelines for vancomycin monitoring in 2009.1 Although it has been well established that area under the curve (AUC) over the minimal inhibitory concentration (MIC) ratio > 400 mg.h/L is the best predictor of clinical efficacy, obtaining this value in clinical practice was not pragmatic. Therefore, the 2009 guidelines recommended a goal vancomycin trough of 15 to 20 mcg/ml as a surrogate marker for AUC/MIC > 400 mg.hr/L. This has since become a common practice despite little data that support this recommendation.
The efficacy and safety of trough-based monitoring has been evaluated extensively over the past several years and more recent data suggest that there is wide patient variability in AUC with this method and higher trough levels are associated with more nephrotoxicity.2,3 ASHP, IDSA, SIDP, and the Pediatric Infectious Diseases Society (PIDS) updated the consensus guidelines in 2020.4 Trough-based monitoring is no longer recommended. Instead AUC24 monitoring should be implemented with a goal range of 400 to 600 mg.h/L for efficacy and safety. Given concerns for vancomycin penetration into the central nervous system (CNS), many facility protocols utilize higher targets (> 600 mg.h/L) for CNS infections.
Some hospitals have been utilizing AUC-based monitoring for years. There are strategies from tertiary care centers that drive this practice change in the medical literature.5,6 However, it is important to reproduce these implementation practices in small, rural facilities that may face unique challenges with limited resources and may be slower to implement consensus guidelines.7,8 As this is a major practice change, it is imperative to evaluate the extent of transition and identify areas of needed improvement.
Accurate therapeutic drug monitoring ensures both the safety and efficacy of vancomycin therapy. Unfortunately, research shows that inappropriate laboratory tests are common in medical facilities.9 Drug levels taken inappropriately can lead to delays in therapeutic decision-making, inappropriate dosage adjustments and create a need for repeated drug levels, which increases the overall cost of admission.
Given the multiple affected services needed to make successful practice transitions, it is paramount that facilities evaluate progress during the transition phase. The Agency for Healthcare Research and Quality and the Institute for Healthcare Improvement provide guidance in the Plan-Do-Study-Act Cycle for quality assessment and improvement of new initiatives.10,11 A gap analysis can be used as a simple tool for evaluating the transition of research into practice and to identify areas of needed improvement.
The Veterans Health Care System of the Ozarks (VHSO) in Fayetteville, Arkansas made the transition from trough-based monitoring to 2-level AUC-based monitoring on April 1, 2019. The purpose of this study was to evaluate the effectiveness of transition methods used to implement AUC-monitoring for vancomycin treated patients in a small, primary facility. A further goal of the study was to identify areas of needed improvement and education and whether the problems derived from deficiencies in knowledge and ordering (medical and pharmacy services) or execution (nursing and laboratory services).
Methods
VHSO is a 52-bed US Department of Veterans Affairs primary care hospital. The pharmacy and laboratory are staffed 24 hours each day. There is 1 clinical pharmacy specialist (CPS) available for therapeutic drug monitoring consults Monday through Friday between the hours of 7:30 AM and 4:00 PM. No partial full-time equivalent employees were added for this conversion. Pharmacy-driven vancomycin dosing and monitoring is conducted on a collaborative basis, with pharmacy managing the majority of vancomycin treated patients. Night and weekend pharmacy staff provide cross-coverage on vancomycin consultations. Laboratory orders and medication dosage adjustments fall within the CPS scope of practice. Nurses do not perform laboratory draws for therapeutic drug monitoring; this is done solely by phlebotomists. There is no infectious diseases specialist at the facility to champion antibiotic dosing initiatives.
The implementation strategy largely reflected those outlined from tertiary care centers.5,6 First, key personnel from the laboratory department met to discuss this practice change and to add vancomycin peaks to the ordering menu. A critical value was set at 40 mcg/ml. Vancomycin troughs and random levels already were orderable items. A comment field was added to all laboratory orders for further clarification. Verbiage was added to laboratory reports in the computerized medical record to assist clinicians in determining the appropriateness of the level. This was followed by an educational email to both the nursing and laboratory departments explaining the practice change and included a link to the Pharmacy Joe “Vancomycin Dosing by AUC:MIC Instead of Trough-level” podcast (www.pharmacyjoe.com episode 356).
The pharmacy department received an interactive 30-minute presentation, followed immediately by a group activity to discuss practice problems. This presentation was condensed, recorded, and emailed to all VHSO pharmacists. A shared folder contained pertinent material on AUC monitoring.
Finally, an interactive presentation was set up for hospitalists and a video teleconferencing was conducted for rotating medical residents. Both the podcast and recorded presentation were emailed to the entire medical staff with a brief introduction of the practice change. Additionally, the transition process was added as a standing item on the monthly antimicrobial stewardship meeting agenda.
The standardized pharmacokinetic model at the study facility consisted of a vancomycin volume of distribution of 0.7 mg/kg and elimination rate constant (Ke) by Matzke and colleagues for total daily dose calculations.12 Obese patients (BMI ≥ 30) undergo alternative clearance equations described by Crass and colleagues.13 Cockcroft-Gault methods using ideal body weight (or actual body weight if < ideal body weight) are used for determining creatinine clearance. In patients aged ≥ 65 years with a serum creatinine < 1.0 mg/dL, facility guidance was to round serum creatinine up to 1.0 mg/dL. Loading doses were determined on a case-by-case basis with a cap of 2,000 mg, maintenance doses were rounded to the nearest 250 mg.
Vancomycin levels typically are drawn at steady state and analyzed using the logarithmic trapezoidal rule.14 The pharmacy and medical staff were educated to provide details on timing and coordination in nursing and laboratory orders (Table 1). Two-level AUC monitoring typically is not performed in patients with acute renal failure, expected duration of therapy < 72 hours, urinary tract infections, skin and soft tissue infections, or in renal replacement therapy.5
This gap analysis consisted of a retrospective chart review of vancomycin levels ordered after the implementation of AUC-based monitoring to determine the effectiveness of the transition. Three months of data were collected between April 2019 and June 2019. Vancomycin levels were deemed either appropriate or inappropriate based on timing and type (peak, trough, or random) of the laboratory test in relation to the previously administered vancomycin dose. Appropriate peaks were drawn within 2 hours after the end of infusion and troughs at least 1 half-life after the dose or just prior to the next dose and within the same dosing interval as the peak. Tests drawn outside of the specified time range, trough-only laboratory tests, or those drawn after vancomycin had been discontinued were considered inappropriate. Peaks and troughs drawn from separate dosing intervals also were considered inappropriate. Random levels were considered appropriate only if they fit the clinical context in acute renal failure or renal replacement therapy. An effective transition was defined as ≥ 80% of all vancomycin treated patients monitored with AUC methods rather than trough-based methods.
Inclusion criteria included all vancomycin levels ordered during the study period with no exclusions. The primary endpoint was the proportion of vancomycin levels drawn appropriately. Secondary endpoints were the proportion of AUC24 calculations within therapeutic range and a stratification of reasons for inappropriate levels. Descriptive statistics were collected to describe the scope of the project. Levels drawn from various shifts were compared (ie, day, night, or weekend). Calculated AUC24 levels between 400 and 600 mg.h/L were considered therapeutic unless treating CNS infection (600-700 mg.h/L). Given the operational outcomes (rather than clinical outcomes) and no comparator group, patient specific data were not collected.
Descriptive statistics without further analysis were used to describe proportions. The goal level for compliance was set at 100%. These methods were reviewed by the VHSO Institutional Review Board and granted nonresearch status, waiving the requirement for informed consent.
Results
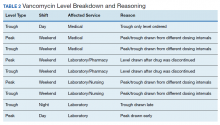
The transition was effective with 97% of all cases utilizing AUC-based methods for monitoring. A total of 65 vancomycin levels were drawn in the study period; 32 peaks, 32 troughs, and 1 random level (drawn appropriately during acute renal failure 24 hours after starting therapy). All shifts were affected proportionately; days (n = 26, 40%), nights (n = 18, 27.7%), and weekends (n = 21, 32.3%). Based on time of dosage administration and laboratory test, there were 9 levels (13.8%) deemed inappropriate, 56 levels (86.1%) were appropriate. Reasons for inappropriate levels gleaned from chart review are presented in Table 2. Four levels had to be repeated for accurate calculations.
From the peak/trough couplets drawn appropriately, calculated AUC24 fell with the desired range in 61% (n = 17) of cases. Of the 11 that fell outside of range, 8 were subtherapeutic (< 400 mg.h/L) and 3 were supratherapeutic (> 600 mg.h/L). All levels were drawn at steady state. Indications for vancomycin monitoring were osteomyelitis (n = 13, 43%), sepsis (n = 10, 33%), pneumonia (n = 6, 20%), and 1 case of meningitis (3%).
Discussion
To the author’s knowledge, this is the first report of a vancomycin AUC24 monitoring conversion in a rural facility. This study adds to the existing medical literature in that it demonstrates that: (1) implementation methods described in large, tertiary centers can be effectively utilized in primary care, rural facilities; (2) the gap analysis used can be duplicated with minimal personnel and resources to ensure effective implementation (Table 3); and (3) the reported improvement needs can serve as a model for preventative measures at other facilities. The incidence of appropriate vancomycin levels was notably better than those reported in other single center studies.15-17 However, given variations in study design and facility operating procedures, it would be difficult to compare incidence among medical facilities. As such, there are no consensus benchmarks for comparison. The majority of inappropriate levels occurred early in the study period and on weekends. Appropriateness of drug levels may have improved with continued feedback and familiarity.
The calculated AUC24 fell within predicted range in 61% of cases. For comparison, a recent study from a large academic medical center reported that 73.5% of 2-level AUC24 cases had initial values within the therapeutic range.18 Of note, the target range used was much wider (400 - 800 mg.h/L) than the present study. Another study reported dose adjustments for subtherapeutic AUC levels in 25% of cases and dose reductions for supratherapeutic levels in 33.3% of cases.19
Of the AUC24 calculations that fell outside of therapeutic range, the majority (n = 8, 73%) were subtherapeutic (< 400 mg.h/L), half of these were for patients who were obese. It was unclear in the medical record which equation was used for initial dosing (Matzke vs Crass), or whether more conservative AUCs were used for calculating the total daily dose. The VHSO policy limiting loading doses also may have played a role; indeed the updated guidelines recommend a maximum loading dose of 3,000 mg depending on the severity of infection.4 Two of the 3 supratherapeutic levels were thought to be due to accumulation with long-term therapy.
Given such a large change from long-standing practices, there was surprisingly little resistance from the various clinical services. A recent survey of academic medical centers reported that the majority (88%) of all respondents who did not currently utilize AUC24 monitoring did not plan on making this immediate transition, largely citing unfamiliarity and training requirements.20 It is conceivable that the transition to AUC monitoring in smaller facilities may have fewer barriers than those seen in tertiary care centers. There are fewer health care providers and pharmacists to educate with the primary responsibilities falling on relatively few clinicians. There is little question as to who will be conducting follow up or whom to contact for questions. A smaller patient load and lesser patient acuity may translate to fewer vancomycin cases that require monitoring.
The interactive meetings were an important element for facility implementation. Research shows that emails alone are not effective for health care provider education, and interactive methods are recommended over passive methods.21,22 Assessing and avoiding barriers up front such as unclear laboratory orders, or communication failures is paramount to successful implementation strategies.23 Additionally, the detailed written ordering communication may have contributed to a smoother transition. The educational recording proved to be helpful in educating new staff and residents. An identified logistical error was that laboratory orders entered while patients were enrolled in sham clinics for electronic workload capture (eg, Pharmacy Inpatient Clinic) created confusion on the physical location of the patient for the phlebotomists, potentially causing delays in specimen collection.
A major development that stemmed from this intervention was that the Medical Service asked that policy changes be made so that the Pharmacy Service take over all vancomycin dosing at the facility. Previously, this had been done on a collaborative basis. Similar facilities with a collaborative practice model may need to anticipate such a request as this may present a new set of challenges. Accordingly, the pharmacy department is in the process of establishing standing operating procedures, pharmacist competencies, and a facility memorandum. Future research should evaluate the safety and efficacy of vancomycin therapy after the switch to AUC-based monitoring.
Limitations
There are several limitations to consider with this study. Operating procedures and implementation processes may vary between facilities, which could limit the generalizability of these results. Given the small facility size, the overall number of laboratory tests drawn was much smaller than those seen in larger facilities. The time needed for AUC calculations is notably longer than older methods of monitoring; however, this was not objectively assessed. It is important to note that clinical outcomes were beyond the scope of this gap analysis and this is an area of future research at the study facility. Vancomycin laboratory tests that were missed due to procedures and subsequently rescheduled were occasionally observed but not accounted for in this analysis. Additionally, vancomycin courses without monitoring (appropriate or otherwise) when indicated were not assessed. However, anecdotally speaking, this would be a very unlikely occurrence.
Conclusion
Conversion to AUC-based vancomycin monitoring is feasible in primary, rural medical centers. Implementation strategies from tertiary facilities can be successfully utilized in smaller hospitals. Quality assessment strategies such as a gap analysis can be utilized with minimal resources for facility uptake of new clinical practices.
1. Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists [published correction appears in Am J Health Syst Pharm. 2009;66(10):887]. Am J Health Syst Pharm. 2009;66(1):82‐98. doi:10.2146/ajhp080434
2. van Hal SJ, Paterson DL, Lodise TP. Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob Agents Chemother. 2013;57(2):734‐744. doi:10.1128/AAC.01568-12
3. Pai MP, Neely M, Rodvold KA, Lodise TP. Innovative approaches to optimizing the delivery of vancomycin in individual patients. Adv Drug Deliv Rev. 2014;77:50‐57. doi:10.1016/j.addr.2014.05.016
4. Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists [published online ahead of print, 2020 Mar 19]. Am J Health Syst Pharm. 2020;zxaa036. doi:10.1093/ajhp/zxaa036
5. Heil EL, Claeys KC, Mynatt RP, et al. Making the change to area under the curve-based vancomycin dosing. Am J Health Syst Pharm. 2018;75(24):1986‐1995. doi:10.2146/ajhp180034
6. Gregory ER, Burgess DR, Cotner SE, et al. Vancomycin area under the curve dosing and monitoring at an academic medical center: transition strategies and lessons learned [published online ahead of print, 2019 Mar 10]. J Pharm Pract. 2019;897190019834369. doi:10.1177/0897190019834369
7. Septimus EJ, Owens RC Jr. Need and potential of antimicrobial stewardship in community hospitals. Clin Infect Dis. 2011;53 Suppl 1:S8‐S14. doi:10.1093/cid/cir363
8. Goldman LE, Dudley RA. United States rural hospital quality in the Hospital Compare database-accounting for hospital characteristics. Health Policy. 2008;87(1):112‐127. doi:10.1016/j.healthpol.2008.02.002
9. Zhi M, Ding EL, Theisen-Toupal J, Whelan J, Arnaout R. The landscape of inappropriate laboratory testing: a 15-year meta-analysis. PLoS One. 2013;8(11):e78962. doi:10.1371/journal.pone.0078962
10. Institute for Healthcare Improvement. Plan-do-study-act (PDSA) worksheet. http://www.ihi.org/resources/Pages/Tools/PlanDoStudyActWorksheet.aspx. Accessed May 13, 2020.
11. Agency for Healthcare Research and Quality. Plan-do-study-act (PDSA) cycle. https://innovations.ahrq.gov/qualitytools/plan-do-study-act-pdsa-cycle. Updated April 10, 2013. Accessed May 13, 2020.
12. Matzke GR, McGory RW, Halstenson CE, Keane WF. Pharmacokinetics of vancomycin in patients with various degrees of renal function. Antimicrob Agents Chemother. 1984;25(4):433‐437. doi:10.1128/aac.25.4.433
13. Crass RL, Dunn R, Hong J, Krop LC, Pai MP. Dosing vancomycin in the super obese: less is more. J Antimicrob Chemother. 2018;73(11):3081‐3086. doi:10.1093/jac/dky310
14. Pai MP, Russo A, Novelli A, Venditti M, Falcone M. Simplified equations using two concentrations to calculate area under the curve for antimicrobials with concentration-dependent pharmacodynamics: daptomycin as a motivating example. Antimicrob Agents Chemother. 2014;58(6):3162‐3167. doi:10.1128/AAC.02355-14
15. Suryadevara M, Steidl KE, Probst LA, Shaw J. Inappropriate vancomycin therapeutic drug monitoring in hospitalized pediatric patients increases pediatric trauma and hospital costs. J Pediatr Pharmacol Ther. 2012;17(2):159‐165. doi:10.5863/1551-6776-17.2.159
16. Morrison AP, Melanson SE, Carty MG, Bates DW, Szumita PM, Tanasijevic MJ. What proportion of vancomycin trough levels are drawn too early?: frequency and impact on clinical actions. Am J Clin Pathol. 2012;137(3):472‐478. doi:10.1309/AJCPDSYS0DVLKFOH
17. Melanson SE, Mijailovic AS, Wright AP, Szumita PM, Bates DW, Tanasijevic MJ. An intervention to improve the timing of vancomycin levels. Am J Clin Pathol. 2013;140(6):801‐806. doi:10.1309/AJCPKQ6EAH7OYQLB
18. Meng L, Wong T, Huang S, et al. Conversion from vancomycin trough concentration-guided dosing to area under the curve-guided dosing using two sample measurements in adults: implementation at an academic medical center. Pharmacotherapy. 2019;39(4):433‐442. doi:10.1002/phar.2234
19. Stoessel AM, Hale CM, Seabury RW, Miller CD, Steele JM. The impact of AUC-based monitoring on pharmacist-directed vancomycin dose adjustments in complicated methicillin-resistant staphylococcus aureus Infection. J Pharm Pract. 2019;32(4):442‐446. doi:10.1177/0897190018764564
20. Kufel WD, Seabury RW, Mogle BT, Beccari MV, Probst LA, Steele JM. Readiness to implement vancomycin monitoring based on area under the concentration-time curve: a cross-sectional survey of a national health consortium. Am J Health Syst Pharm. 2019;76(12):889‐894. doi:10.1093/ajhp/zxz070
21. Bluestone J, Johnson P, Fullerton J, Carr C, Alderman J, BonTempo J. Effective in-service training design and delivery: evidence from an integrative literature review. Hum Resour Health. 2013;11:51. doi:10.1186/1478-4491-11-51
22. Ebben RHA, Siqeca F, Madsen UR, Vloet LCM, van Achterberg T. Effectiveness of implementation strategies for the improvement of guideline and protocol adherence in emergency care: a systematic review. BMJ Open. 2018;8(11):e017572. doi:10.1136/bmjopen-2017-017572
23. Fischer F, Lange K, Klose K, Greiner W, Kraemer A. Barriers and Strategies in Guideline Implementation-A Scoping Review. Healthcare (Basel). 2016;4(3):36. doi:10.3390/healthcare4030036
The use of weight-based dosing with trough-based monitoring of vancomycin has been in clinical practice for more than a decade. The American Society of Health-System Pharmacists (ASHP), the Infectious Diseases Society of America (IDSA), and the Society of Infectious Diseases Pharmacists (SIDP) published the first guidelines for vancomycin monitoring in 2009.1 Although it has been well established that area under the curve (AUC) over the minimal inhibitory concentration (MIC) ratio > 400 mg.h/L is the best predictor of clinical efficacy, obtaining this value in clinical practice was not pragmatic. Therefore, the 2009 guidelines recommended a goal vancomycin trough of 15 to 20 mcg/ml as a surrogate marker for AUC/MIC > 400 mg.hr/L. This has since become a common practice despite little data that support this recommendation.
The efficacy and safety of trough-based monitoring has been evaluated extensively over the past several years and more recent data suggest that there is wide patient variability in AUC with this method and higher trough levels are associated with more nephrotoxicity.2,3 ASHP, IDSA, SIDP, and the Pediatric Infectious Diseases Society (PIDS) updated the consensus guidelines in 2020.4 Trough-based monitoring is no longer recommended. Instead AUC24 monitoring should be implemented with a goal range of 400 to 600 mg.h/L for efficacy and safety. Given concerns for vancomycin penetration into the central nervous system (CNS), many facility protocols utilize higher targets (> 600 mg.h/L) for CNS infections.
Some hospitals have been utilizing AUC-based monitoring for years. There are strategies from tertiary care centers that drive this practice change in the medical literature.5,6 However, it is important to reproduce these implementation practices in small, rural facilities that may face unique challenges with limited resources and may be slower to implement consensus guidelines.7,8 As this is a major practice change, it is imperative to evaluate the extent of transition and identify areas of needed improvement.
Accurate therapeutic drug monitoring ensures both the safety and efficacy of vancomycin therapy. Unfortunately, research shows that inappropriate laboratory tests are common in medical facilities.9 Drug levels taken inappropriately can lead to delays in therapeutic decision-making, inappropriate dosage adjustments and create a need for repeated drug levels, which increases the overall cost of admission.
Given the multiple affected services needed to make successful practice transitions, it is paramount that facilities evaluate progress during the transition phase. The Agency for Healthcare Research and Quality and the Institute for Healthcare Improvement provide guidance in the Plan-Do-Study-Act Cycle for quality assessment and improvement of new initiatives.10,11 A gap analysis can be used as a simple tool for evaluating the transition of research into practice and to identify areas of needed improvement.
The Veterans Health Care System of the Ozarks (VHSO) in Fayetteville, Arkansas made the transition from trough-based monitoring to 2-level AUC-based monitoring on April 1, 2019. The purpose of this study was to evaluate the effectiveness of transition methods used to implement AUC-monitoring for vancomycin treated patients in a small, primary facility. A further goal of the study was to identify areas of needed improvement and education and whether the problems derived from deficiencies in knowledge and ordering (medical and pharmacy services) or execution (nursing and laboratory services).
Methods
VHSO is a 52-bed US Department of Veterans Affairs primary care hospital. The pharmacy and laboratory are staffed 24 hours each day. There is 1 clinical pharmacy specialist (CPS) available for therapeutic drug monitoring consults Monday through Friday between the hours of 7:30 AM and 4:00 PM. No partial full-time equivalent employees were added for this conversion. Pharmacy-driven vancomycin dosing and monitoring is conducted on a collaborative basis, with pharmacy managing the majority of vancomycin treated patients. Night and weekend pharmacy staff provide cross-coverage on vancomycin consultations. Laboratory orders and medication dosage adjustments fall within the CPS scope of practice. Nurses do not perform laboratory draws for therapeutic drug monitoring; this is done solely by phlebotomists. There is no infectious diseases specialist at the facility to champion antibiotic dosing initiatives.
The implementation strategy largely reflected those outlined from tertiary care centers.5,6 First, key personnel from the laboratory department met to discuss this practice change and to add vancomycin peaks to the ordering menu. A critical value was set at 40 mcg/ml. Vancomycin troughs and random levels already were orderable items. A comment field was added to all laboratory orders for further clarification. Verbiage was added to laboratory reports in the computerized medical record to assist clinicians in determining the appropriateness of the level. This was followed by an educational email to both the nursing and laboratory departments explaining the practice change and included a link to the Pharmacy Joe “Vancomycin Dosing by AUC:MIC Instead of Trough-level” podcast (www.pharmacyjoe.com episode 356).
The pharmacy department received an interactive 30-minute presentation, followed immediately by a group activity to discuss practice problems. This presentation was condensed, recorded, and emailed to all VHSO pharmacists. A shared folder contained pertinent material on AUC monitoring.
Finally, an interactive presentation was set up for hospitalists and a video teleconferencing was conducted for rotating medical residents. Both the podcast and recorded presentation were emailed to the entire medical staff with a brief introduction of the practice change. Additionally, the transition process was added as a standing item on the monthly antimicrobial stewardship meeting agenda.
The standardized pharmacokinetic model at the study facility consisted of a vancomycin volume of distribution of 0.7 mg/kg and elimination rate constant (Ke) by Matzke and colleagues for total daily dose calculations.12 Obese patients (BMI ≥ 30) undergo alternative clearance equations described by Crass and colleagues.13 Cockcroft-Gault methods using ideal body weight (or actual body weight if < ideal body weight) are used for determining creatinine clearance. In patients aged ≥ 65 years with a serum creatinine < 1.0 mg/dL, facility guidance was to round serum creatinine up to 1.0 mg/dL. Loading doses were determined on a case-by-case basis with a cap of 2,000 mg, maintenance doses were rounded to the nearest 250 mg.
Vancomycin levels typically are drawn at steady state and analyzed using the logarithmic trapezoidal rule.14 The pharmacy and medical staff were educated to provide details on timing and coordination in nursing and laboratory orders (Table 1). Two-level AUC monitoring typically is not performed in patients with acute renal failure, expected duration of therapy < 72 hours, urinary tract infections, skin and soft tissue infections, or in renal replacement therapy.5
This gap analysis consisted of a retrospective chart review of vancomycin levels ordered after the implementation of AUC-based monitoring to determine the effectiveness of the transition. Three months of data were collected between April 2019 and June 2019. Vancomycin levels were deemed either appropriate or inappropriate based on timing and type (peak, trough, or random) of the laboratory test in relation to the previously administered vancomycin dose. Appropriate peaks were drawn within 2 hours after the end of infusion and troughs at least 1 half-life after the dose or just prior to the next dose and within the same dosing interval as the peak. Tests drawn outside of the specified time range, trough-only laboratory tests, or those drawn after vancomycin had been discontinued were considered inappropriate. Peaks and troughs drawn from separate dosing intervals also were considered inappropriate. Random levels were considered appropriate only if they fit the clinical context in acute renal failure or renal replacement therapy. An effective transition was defined as ≥ 80% of all vancomycin treated patients monitored with AUC methods rather than trough-based methods.
Inclusion criteria included all vancomycin levels ordered during the study period with no exclusions. The primary endpoint was the proportion of vancomycin levels drawn appropriately. Secondary endpoints were the proportion of AUC24 calculations within therapeutic range and a stratification of reasons for inappropriate levels. Descriptive statistics were collected to describe the scope of the project. Levels drawn from various shifts were compared (ie, day, night, or weekend). Calculated AUC24 levels between 400 and 600 mg.h/L were considered therapeutic unless treating CNS infection (600-700 mg.h/L). Given the operational outcomes (rather than clinical outcomes) and no comparator group, patient specific data were not collected.
Descriptive statistics without further analysis were used to describe proportions. The goal level for compliance was set at 100%. These methods were reviewed by the VHSO Institutional Review Board and granted nonresearch status, waiving the requirement for informed consent.
Results
The transition was effective with 97% of all cases utilizing AUC-based methods for monitoring. A total of 65 vancomycin levels were drawn in the study period; 32 peaks, 32 troughs, and 1 random level (drawn appropriately during acute renal failure 24 hours after starting therapy). All shifts were affected proportionately; days (n = 26, 40%), nights (n = 18, 27.7%), and weekends (n = 21, 32.3%). Based on time of dosage administration and laboratory test, there were 9 levels (13.8%) deemed inappropriate, 56 levels (86.1%) were appropriate. Reasons for inappropriate levels gleaned from chart review are presented in Table 2. Four levels had to be repeated for accurate calculations.
From the peak/trough couplets drawn appropriately, calculated AUC24 fell with the desired range in 61% (n = 17) of cases. Of the 11 that fell outside of range, 8 were subtherapeutic (< 400 mg.h/L) and 3 were supratherapeutic (> 600 mg.h/L). All levels were drawn at steady state. Indications for vancomycin monitoring were osteomyelitis (n = 13, 43%), sepsis (n = 10, 33%), pneumonia (n = 6, 20%), and 1 case of meningitis (3%).
Discussion
To the author’s knowledge, this is the first report of a vancomycin AUC24 monitoring conversion in a rural facility. This study adds to the existing medical literature in that it demonstrates that: (1) implementation methods described in large, tertiary centers can be effectively utilized in primary care, rural facilities; (2) the gap analysis used can be duplicated with minimal personnel and resources to ensure effective implementation (Table 3); and (3) the reported improvement needs can serve as a model for preventative measures at other facilities. The incidence of appropriate vancomycin levels was notably better than those reported in other single center studies.15-17 However, given variations in study design and facility operating procedures, it would be difficult to compare incidence among medical facilities. As such, there are no consensus benchmarks for comparison. The majority of inappropriate levels occurred early in the study period and on weekends. Appropriateness of drug levels may have improved with continued feedback and familiarity.
The calculated AUC24 fell within predicted range in 61% of cases. For comparison, a recent study from a large academic medical center reported that 73.5% of 2-level AUC24 cases had initial values within the therapeutic range.18 Of note, the target range used was much wider (400 - 800 mg.h/L) than the present study. Another study reported dose adjustments for subtherapeutic AUC levels in 25% of cases and dose reductions for supratherapeutic levels in 33.3% of cases.19
Of the AUC24 calculations that fell outside of therapeutic range, the majority (n = 8, 73%) were subtherapeutic (< 400 mg.h/L), half of these were for patients who were obese. It was unclear in the medical record which equation was used for initial dosing (Matzke vs Crass), or whether more conservative AUCs were used for calculating the total daily dose. The VHSO policy limiting loading doses also may have played a role; indeed the updated guidelines recommend a maximum loading dose of 3,000 mg depending on the severity of infection.4 Two of the 3 supratherapeutic levels were thought to be due to accumulation with long-term therapy.
Given such a large change from long-standing practices, there was surprisingly little resistance from the various clinical services. A recent survey of academic medical centers reported that the majority (88%) of all respondents who did not currently utilize AUC24 monitoring did not plan on making this immediate transition, largely citing unfamiliarity and training requirements.20 It is conceivable that the transition to AUC monitoring in smaller facilities may have fewer barriers than those seen in tertiary care centers. There are fewer health care providers and pharmacists to educate with the primary responsibilities falling on relatively few clinicians. There is little question as to who will be conducting follow up or whom to contact for questions. A smaller patient load and lesser patient acuity may translate to fewer vancomycin cases that require monitoring.
The interactive meetings were an important element for facility implementation. Research shows that emails alone are not effective for health care provider education, and interactive methods are recommended over passive methods.21,22 Assessing and avoiding barriers up front such as unclear laboratory orders, or communication failures is paramount to successful implementation strategies.23 Additionally, the detailed written ordering communication may have contributed to a smoother transition. The educational recording proved to be helpful in educating new staff and residents. An identified logistical error was that laboratory orders entered while patients were enrolled in sham clinics for electronic workload capture (eg, Pharmacy Inpatient Clinic) created confusion on the physical location of the patient for the phlebotomists, potentially causing delays in specimen collection.
A major development that stemmed from this intervention was that the Medical Service asked that policy changes be made so that the Pharmacy Service take over all vancomycin dosing at the facility. Previously, this had been done on a collaborative basis. Similar facilities with a collaborative practice model may need to anticipate such a request as this may present a new set of challenges. Accordingly, the pharmacy department is in the process of establishing standing operating procedures, pharmacist competencies, and a facility memorandum. Future research should evaluate the safety and efficacy of vancomycin therapy after the switch to AUC-based monitoring.
Limitations
There are several limitations to consider with this study. Operating procedures and implementation processes may vary between facilities, which could limit the generalizability of these results. Given the small facility size, the overall number of laboratory tests drawn was much smaller than those seen in larger facilities. The time needed for AUC calculations is notably longer than older methods of monitoring; however, this was not objectively assessed. It is important to note that clinical outcomes were beyond the scope of this gap analysis and this is an area of future research at the study facility. Vancomycin laboratory tests that were missed due to procedures and subsequently rescheduled were occasionally observed but not accounted for in this analysis. Additionally, vancomycin courses without monitoring (appropriate or otherwise) when indicated were not assessed. However, anecdotally speaking, this would be a very unlikely occurrence.
Conclusion
Conversion to AUC-based vancomycin monitoring is feasible in primary, rural medical centers. Implementation strategies from tertiary facilities can be successfully utilized in smaller hospitals. Quality assessment strategies such as a gap analysis can be utilized with minimal resources for facility uptake of new clinical practices.
The use of weight-based dosing with trough-based monitoring of vancomycin has been in clinical practice for more than a decade. The American Society of Health-System Pharmacists (ASHP), the Infectious Diseases Society of America (IDSA), and the Society of Infectious Diseases Pharmacists (SIDP) published the first guidelines for vancomycin monitoring in 2009.1 Although it has been well established that area under the curve (AUC) over the minimal inhibitory concentration (MIC) ratio > 400 mg.h/L is the best predictor of clinical efficacy, obtaining this value in clinical practice was not pragmatic. Therefore, the 2009 guidelines recommended a goal vancomycin trough of 15 to 20 mcg/ml as a surrogate marker for AUC/MIC > 400 mg.hr/L. This has since become a common practice despite little data that support this recommendation.
The efficacy and safety of trough-based monitoring has been evaluated extensively over the past several years and more recent data suggest that there is wide patient variability in AUC with this method and higher trough levels are associated with more nephrotoxicity.2,3 ASHP, IDSA, SIDP, and the Pediatric Infectious Diseases Society (PIDS) updated the consensus guidelines in 2020.4 Trough-based monitoring is no longer recommended. Instead AUC24 monitoring should be implemented with a goal range of 400 to 600 mg.h/L for efficacy and safety. Given concerns for vancomycin penetration into the central nervous system (CNS), many facility protocols utilize higher targets (> 600 mg.h/L) for CNS infections.
Some hospitals have been utilizing AUC-based monitoring for years. There are strategies from tertiary care centers that drive this practice change in the medical literature.5,6 However, it is important to reproduce these implementation practices in small, rural facilities that may face unique challenges with limited resources and may be slower to implement consensus guidelines.7,8 As this is a major practice change, it is imperative to evaluate the extent of transition and identify areas of needed improvement.
Accurate therapeutic drug monitoring ensures both the safety and efficacy of vancomycin therapy. Unfortunately, research shows that inappropriate laboratory tests are common in medical facilities.9 Drug levels taken inappropriately can lead to delays in therapeutic decision-making, inappropriate dosage adjustments and create a need for repeated drug levels, which increases the overall cost of admission.
Given the multiple affected services needed to make successful practice transitions, it is paramount that facilities evaluate progress during the transition phase. The Agency for Healthcare Research and Quality and the Institute for Healthcare Improvement provide guidance in the Plan-Do-Study-Act Cycle for quality assessment and improvement of new initiatives.10,11 A gap analysis can be used as a simple tool for evaluating the transition of research into practice and to identify areas of needed improvement.
The Veterans Health Care System of the Ozarks (VHSO) in Fayetteville, Arkansas made the transition from trough-based monitoring to 2-level AUC-based monitoring on April 1, 2019. The purpose of this study was to evaluate the effectiveness of transition methods used to implement AUC-monitoring for vancomycin treated patients in a small, primary facility. A further goal of the study was to identify areas of needed improvement and education and whether the problems derived from deficiencies in knowledge and ordering (medical and pharmacy services) or execution (nursing and laboratory services).
Methods
VHSO is a 52-bed US Department of Veterans Affairs primary care hospital. The pharmacy and laboratory are staffed 24 hours each day. There is 1 clinical pharmacy specialist (CPS) available for therapeutic drug monitoring consults Monday through Friday between the hours of 7:30 AM and 4:00 PM. No partial full-time equivalent employees were added for this conversion. Pharmacy-driven vancomycin dosing and monitoring is conducted on a collaborative basis, with pharmacy managing the majority of vancomycin treated patients. Night and weekend pharmacy staff provide cross-coverage on vancomycin consultations. Laboratory orders and medication dosage adjustments fall within the CPS scope of practice. Nurses do not perform laboratory draws for therapeutic drug monitoring; this is done solely by phlebotomists. There is no infectious diseases specialist at the facility to champion antibiotic dosing initiatives.
The implementation strategy largely reflected those outlined from tertiary care centers.5,6 First, key personnel from the laboratory department met to discuss this practice change and to add vancomycin peaks to the ordering menu. A critical value was set at 40 mcg/ml. Vancomycin troughs and random levels already were orderable items. A comment field was added to all laboratory orders for further clarification. Verbiage was added to laboratory reports in the computerized medical record to assist clinicians in determining the appropriateness of the level. This was followed by an educational email to both the nursing and laboratory departments explaining the practice change and included a link to the Pharmacy Joe “Vancomycin Dosing by AUC:MIC Instead of Trough-level” podcast (www.pharmacyjoe.com episode 356).
The pharmacy department received an interactive 30-minute presentation, followed immediately by a group activity to discuss practice problems. This presentation was condensed, recorded, and emailed to all VHSO pharmacists. A shared folder contained pertinent material on AUC monitoring.
Finally, an interactive presentation was set up for hospitalists and a video teleconferencing was conducted for rotating medical residents. Both the podcast and recorded presentation were emailed to the entire medical staff with a brief introduction of the practice change. Additionally, the transition process was added as a standing item on the monthly antimicrobial stewardship meeting agenda.
The standardized pharmacokinetic model at the study facility consisted of a vancomycin volume of distribution of 0.7 mg/kg and elimination rate constant (Ke) by Matzke and colleagues for total daily dose calculations.12 Obese patients (BMI ≥ 30) undergo alternative clearance equations described by Crass and colleagues.13 Cockcroft-Gault methods using ideal body weight (or actual body weight if < ideal body weight) are used for determining creatinine clearance. In patients aged ≥ 65 years with a serum creatinine < 1.0 mg/dL, facility guidance was to round serum creatinine up to 1.0 mg/dL. Loading doses were determined on a case-by-case basis with a cap of 2,000 mg, maintenance doses were rounded to the nearest 250 mg.
Vancomycin levels typically are drawn at steady state and analyzed using the logarithmic trapezoidal rule.14 The pharmacy and medical staff were educated to provide details on timing and coordination in nursing and laboratory orders (Table 1). Two-level AUC monitoring typically is not performed in patients with acute renal failure, expected duration of therapy < 72 hours, urinary tract infections, skin and soft tissue infections, or in renal replacement therapy.5
This gap analysis consisted of a retrospective chart review of vancomycin levels ordered after the implementation of AUC-based monitoring to determine the effectiveness of the transition. Three months of data were collected between April 2019 and June 2019. Vancomycin levels were deemed either appropriate or inappropriate based on timing and type (peak, trough, or random) of the laboratory test in relation to the previously administered vancomycin dose. Appropriate peaks were drawn within 2 hours after the end of infusion and troughs at least 1 half-life after the dose or just prior to the next dose and within the same dosing interval as the peak. Tests drawn outside of the specified time range, trough-only laboratory tests, or those drawn after vancomycin had been discontinued were considered inappropriate. Peaks and troughs drawn from separate dosing intervals also were considered inappropriate. Random levels were considered appropriate only if they fit the clinical context in acute renal failure or renal replacement therapy. An effective transition was defined as ≥ 80% of all vancomycin treated patients monitored with AUC methods rather than trough-based methods.
Inclusion criteria included all vancomycin levels ordered during the study period with no exclusions. The primary endpoint was the proportion of vancomycin levels drawn appropriately. Secondary endpoints were the proportion of AUC24 calculations within therapeutic range and a stratification of reasons for inappropriate levels. Descriptive statistics were collected to describe the scope of the project. Levels drawn from various shifts were compared (ie, day, night, or weekend). Calculated AUC24 levels between 400 and 600 mg.h/L were considered therapeutic unless treating CNS infection (600-700 mg.h/L). Given the operational outcomes (rather than clinical outcomes) and no comparator group, patient specific data were not collected.
Descriptive statistics without further analysis were used to describe proportions. The goal level for compliance was set at 100%. These methods were reviewed by the VHSO Institutional Review Board and granted nonresearch status, waiving the requirement for informed consent.
Results
The transition was effective with 97% of all cases utilizing AUC-based methods for monitoring. A total of 65 vancomycin levels were drawn in the study period; 32 peaks, 32 troughs, and 1 random level (drawn appropriately during acute renal failure 24 hours after starting therapy). All shifts were affected proportionately; days (n = 26, 40%), nights (n = 18, 27.7%), and weekends (n = 21, 32.3%). Based on time of dosage administration and laboratory test, there were 9 levels (13.8%) deemed inappropriate, 56 levels (86.1%) were appropriate. Reasons for inappropriate levels gleaned from chart review are presented in Table 2. Four levels had to be repeated for accurate calculations.
From the peak/trough couplets drawn appropriately, calculated AUC24 fell with the desired range in 61% (n = 17) of cases. Of the 11 that fell outside of range, 8 were subtherapeutic (< 400 mg.h/L) and 3 were supratherapeutic (> 600 mg.h/L). All levels were drawn at steady state. Indications for vancomycin monitoring were osteomyelitis (n = 13, 43%), sepsis (n = 10, 33%), pneumonia (n = 6, 20%), and 1 case of meningitis (3%).
Discussion
To the author’s knowledge, this is the first report of a vancomycin AUC24 monitoring conversion in a rural facility. This study adds to the existing medical literature in that it demonstrates that: (1) implementation methods described in large, tertiary centers can be effectively utilized in primary care, rural facilities; (2) the gap analysis used can be duplicated with minimal personnel and resources to ensure effective implementation (Table 3); and (3) the reported improvement needs can serve as a model for preventative measures at other facilities. The incidence of appropriate vancomycin levels was notably better than those reported in other single center studies.15-17 However, given variations in study design and facility operating procedures, it would be difficult to compare incidence among medical facilities. As such, there are no consensus benchmarks for comparison. The majority of inappropriate levels occurred early in the study period and on weekends. Appropriateness of drug levels may have improved with continued feedback and familiarity.
The calculated AUC24 fell within predicted range in 61% of cases. For comparison, a recent study from a large academic medical center reported that 73.5% of 2-level AUC24 cases had initial values within the therapeutic range.18 Of note, the target range used was much wider (400 - 800 mg.h/L) than the present study. Another study reported dose adjustments for subtherapeutic AUC levels in 25% of cases and dose reductions for supratherapeutic levels in 33.3% of cases.19
Of the AUC24 calculations that fell outside of therapeutic range, the majority (n = 8, 73%) were subtherapeutic (< 400 mg.h/L), half of these were for patients who were obese. It was unclear in the medical record which equation was used for initial dosing (Matzke vs Crass), or whether more conservative AUCs were used for calculating the total daily dose. The VHSO policy limiting loading doses also may have played a role; indeed the updated guidelines recommend a maximum loading dose of 3,000 mg depending on the severity of infection.4 Two of the 3 supratherapeutic levels were thought to be due to accumulation with long-term therapy.
Given such a large change from long-standing practices, there was surprisingly little resistance from the various clinical services. A recent survey of academic medical centers reported that the majority (88%) of all respondents who did not currently utilize AUC24 monitoring did not plan on making this immediate transition, largely citing unfamiliarity and training requirements.20 It is conceivable that the transition to AUC monitoring in smaller facilities may have fewer barriers than those seen in tertiary care centers. There are fewer health care providers and pharmacists to educate with the primary responsibilities falling on relatively few clinicians. There is little question as to who will be conducting follow up or whom to contact for questions. A smaller patient load and lesser patient acuity may translate to fewer vancomycin cases that require monitoring.
The interactive meetings were an important element for facility implementation. Research shows that emails alone are not effective for health care provider education, and interactive methods are recommended over passive methods.21,22 Assessing and avoiding barriers up front such as unclear laboratory orders, or communication failures is paramount to successful implementation strategies.23 Additionally, the detailed written ordering communication may have contributed to a smoother transition. The educational recording proved to be helpful in educating new staff and residents. An identified logistical error was that laboratory orders entered while patients were enrolled in sham clinics for electronic workload capture (eg, Pharmacy Inpatient Clinic) created confusion on the physical location of the patient for the phlebotomists, potentially causing delays in specimen collection.
A major development that stemmed from this intervention was that the Medical Service asked that policy changes be made so that the Pharmacy Service take over all vancomycin dosing at the facility. Previously, this had been done on a collaborative basis. Similar facilities with a collaborative practice model may need to anticipate such a request as this may present a new set of challenges. Accordingly, the pharmacy department is in the process of establishing standing operating procedures, pharmacist competencies, and a facility memorandum. Future research should evaluate the safety and efficacy of vancomycin therapy after the switch to AUC-based monitoring.
Limitations
There are several limitations to consider with this study. Operating procedures and implementation processes may vary between facilities, which could limit the generalizability of these results. Given the small facility size, the overall number of laboratory tests drawn was much smaller than those seen in larger facilities. The time needed for AUC calculations is notably longer than older methods of monitoring; however, this was not objectively assessed. It is important to note that clinical outcomes were beyond the scope of this gap analysis and this is an area of future research at the study facility. Vancomycin laboratory tests that were missed due to procedures and subsequently rescheduled were occasionally observed but not accounted for in this analysis. Additionally, vancomycin courses without monitoring (appropriate or otherwise) when indicated were not assessed. However, anecdotally speaking, this would be a very unlikely occurrence.
Conclusion
Conversion to AUC-based vancomycin monitoring is feasible in primary, rural medical centers. Implementation strategies from tertiary facilities can be successfully utilized in smaller hospitals. Quality assessment strategies such as a gap analysis can be utilized with minimal resources for facility uptake of new clinical practices.
1. Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists [published correction appears in Am J Health Syst Pharm. 2009;66(10):887]. Am J Health Syst Pharm. 2009;66(1):82‐98. doi:10.2146/ajhp080434
2. van Hal SJ, Paterson DL, Lodise TP. Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob Agents Chemother. 2013;57(2):734‐744. doi:10.1128/AAC.01568-12
3. Pai MP, Neely M, Rodvold KA, Lodise TP. Innovative approaches to optimizing the delivery of vancomycin in individual patients. Adv Drug Deliv Rev. 2014;77:50‐57. doi:10.1016/j.addr.2014.05.016
4. Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists [published online ahead of print, 2020 Mar 19]. Am J Health Syst Pharm. 2020;zxaa036. doi:10.1093/ajhp/zxaa036
5. Heil EL, Claeys KC, Mynatt RP, et al. Making the change to area under the curve-based vancomycin dosing. Am J Health Syst Pharm. 2018;75(24):1986‐1995. doi:10.2146/ajhp180034
6. Gregory ER, Burgess DR, Cotner SE, et al. Vancomycin area under the curve dosing and monitoring at an academic medical center: transition strategies and lessons learned [published online ahead of print, 2019 Mar 10]. J Pharm Pract. 2019;897190019834369. doi:10.1177/0897190019834369
7. Septimus EJ, Owens RC Jr. Need and potential of antimicrobial stewardship in community hospitals. Clin Infect Dis. 2011;53 Suppl 1:S8‐S14. doi:10.1093/cid/cir363
8. Goldman LE, Dudley RA. United States rural hospital quality in the Hospital Compare database-accounting for hospital characteristics. Health Policy. 2008;87(1):112‐127. doi:10.1016/j.healthpol.2008.02.002
9. Zhi M, Ding EL, Theisen-Toupal J, Whelan J, Arnaout R. The landscape of inappropriate laboratory testing: a 15-year meta-analysis. PLoS One. 2013;8(11):e78962. doi:10.1371/journal.pone.0078962
10. Institute for Healthcare Improvement. Plan-do-study-act (PDSA) worksheet. http://www.ihi.org/resources/Pages/Tools/PlanDoStudyActWorksheet.aspx. Accessed May 13, 2020.
11. Agency for Healthcare Research and Quality. Plan-do-study-act (PDSA) cycle. https://innovations.ahrq.gov/qualitytools/plan-do-study-act-pdsa-cycle. Updated April 10, 2013. Accessed May 13, 2020.
12. Matzke GR, McGory RW, Halstenson CE, Keane WF. Pharmacokinetics of vancomycin in patients with various degrees of renal function. Antimicrob Agents Chemother. 1984;25(4):433‐437. doi:10.1128/aac.25.4.433
13. Crass RL, Dunn R, Hong J, Krop LC, Pai MP. Dosing vancomycin in the super obese: less is more. J Antimicrob Chemother. 2018;73(11):3081‐3086. doi:10.1093/jac/dky310
14. Pai MP, Russo A, Novelli A, Venditti M, Falcone M. Simplified equations using two concentrations to calculate area under the curve for antimicrobials with concentration-dependent pharmacodynamics: daptomycin as a motivating example. Antimicrob Agents Chemother. 2014;58(6):3162‐3167. doi:10.1128/AAC.02355-14
15. Suryadevara M, Steidl KE, Probst LA, Shaw J. Inappropriate vancomycin therapeutic drug monitoring in hospitalized pediatric patients increases pediatric trauma and hospital costs. J Pediatr Pharmacol Ther. 2012;17(2):159‐165. doi:10.5863/1551-6776-17.2.159
16. Morrison AP, Melanson SE, Carty MG, Bates DW, Szumita PM, Tanasijevic MJ. What proportion of vancomycin trough levels are drawn too early?: frequency and impact on clinical actions. Am J Clin Pathol. 2012;137(3):472‐478. doi:10.1309/AJCPDSYS0DVLKFOH
17. Melanson SE, Mijailovic AS, Wright AP, Szumita PM, Bates DW, Tanasijevic MJ. An intervention to improve the timing of vancomycin levels. Am J Clin Pathol. 2013;140(6):801‐806. doi:10.1309/AJCPKQ6EAH7OYQLB
18. Meng L, Wong T, Huang S, et al. Conversion from vancomycin trough concentration-guided dosing to area under the curve-guided dosing using two sample measurements in adults: implementation at an academic medical center. Pharmacotherapy. 2019;39(4):433‐442. doi:10.1002/phar.2234
19. Stoessel AM, Hale CM, Seabury RW, Miller CD, Steele JM. The impact of AUC-based monitoring on pharmacist-directed vancomycin dose adjustments in complicated methicillin-resistant staphylococcus aureus Infection. J Pharm Pract. 2019;32(4):442‐446. doi:10.1177/0897190018764564
20. Kufel WD, Seabury RW, Mogle BT, Beccari MV, Probst LA, Steele JM. Readiness to implement vancomycin monitoring based on area under the concentration-time curve: a cross-sectional survey of a national health consortium. Am J Health Syst Pharm. 2019;76(12):889‐894. doi:10.1093/ajhp/zxz070
21. Bluestone J, Johnson P, Fullerton J, Carr C, Alderman J, BonTempo J. Effective in-service training design and delivery: evidence from an integrative literature review. Hum Resour Health. 2013;11:51. doi:10.1186/1478-4491-11-51
22. Ebben RHA, Siqeca F, Madsen UR, Vloet LCM, van Achterberg T. Effectiveness of implementation strategies for the improvement of guideline and protocol adherence in emergency care: a systematic review. BMJ Open. 2018;8(11):e017572. doi:10.1136/bmjopen-2017-017572
23. Fischer F, Lange K, Klose K, Greiner W, Kraemer A. Barriers and Strategies in Guideline Implementation-A Scoping Review. Healthcare (Basel). 2016;4(3):36. doi:10.3390/healthcare4030036
1. Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists [published correction appears in Am J Health Syst Pharm. 2009;66(10):887]. Am J Health Syst Pharm. 2009;66(1):82‐98. doi:10.2146/ajhp080434
2. van Hal SJ, Paterson DL, Lodise TP. Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob Agents Chemother. 2013;57(2):734‐744. doi:10.1128/AAC.01568-12
3. Pai MP, Neely M, Rodvold KA, Lodise TP. Innovative approaches to optimizing the delivery of vancomycin in individual patients. Adv Drug Deliv Rev. 2014;77:50‐57. doi:10.1016/j.addr.2014.05.016
4. Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists [published online ahead of print, 2020 Mar 19]. Am J Health Syst Pharm. 2020;zxaa036. doi:10.1093/ajhp/zxaa036
5. Heil EL, Claeys KC, Mynatt RP, et al. Making the change to area under the curve-based vancomycin dosing. Am J Health Syst Pharm. 2018;75(24):1986‐1995. doi:10.2146/ajhp180034
6. Gregory ER, Burgess DR, Cotner SE, et al. Vancomycin area under the curve dosing and monitoring at an academic medical center: transition strategies and lessons learned [published online ahead of print, 2019 Mar 10]. J Pharm Pract. 2019;897190019834369. doi:10.1177/0897190019834369
7. Septimus EJ, Owens RC Jr. Need and potential of antimicrobial stewardship in community hospitals. Clin Infect Dis. 2011;53 Suppl 1:S8‐S14. doi:10.1093/cid/cir363
8. Goldman LE, Dudley RA. United States rural hospital quality in the Hospital Compare database-accounting for hospital characteristics. Health Policy. 2008;87(1):112‐127. doi:10.1016/j.healthpol.2008.02.002
9. Zhi M, Ding EL, Theisen-Toupal J, Whelan J, Arnaout R. The landscape of inappropriate laboratory testing: a 15-year meta-analysis. PLoS One. 2013;8(11):e78962. doi:10.1371/journal.pone.0078962
10. Institute for Healthcare Improvement. Plan-do-study-act (PDSA) worksheet. http://www.ihi.org/resources/Pages/Tools/PlanDoStudyActWorksheet.aspx. Accessed May 13, 2020.
11. Agency for Healthcare Research and Quality. Plan-do-study-act (PDSA) cycle. https://innovations.ahrq.gov/qualitytools/plan-do-study-act-pdsa-cycle. Updated April 10, 2013. Accessed May 13, 2020.
12. Matzke GR, McGory RW, Halstenson CE, Keane WF. Pharmacokinetics of vancomycin in patients with various degrees of renal function. Antimicrob Agents Chemother. 1984;25(4):433‐437. doi:10.1128/aac.25.4.433
13. Crass RL, Dunn R, Hong J, Krop LC, Pai MP. Dosing vancomycin in the super obese: less is more. J Antimicrob Chemother. 2018;73(11):3081‐3086. doi:10.1093/jac/dky310
14. Pai MP, Russo A, Novelli A, Venditti M, Falcone M. Simplified equations using two concentrations to calculate area under the curve for antimicrobials with concentration-dependent pharmacodynamics: daptomycin as a motivating example. Antimicrob Agents Chemother. 2014;58(6):3162‐3167. doi:10.1128/AAC.02355-14
15. Suryadevara M, Steidl KE, Probst LA, Shaw J. Inappropriate vancomycin therapeutic drug monitoring in hospitalized pediatric patients increases pediatric trauma and hospital costs. J Pediatr Pharmacol Ther. 2012;17(2):159‐165. doi:10.5863/1551-6776-17.2.159
16. Morrison AP, Melanson SE, Carty MG, Bates DW, Szumita PM, Tanasijevic MJ. What proportion of vancomycin trough levels are drawn too early?: frequency and impact on clinical actions. Am J Clin Pathol. 2012;137(3):472‐478. doi:10.1309/AJCPDSYS0DVLKFOH
17. Melanson SE, Mijailovic AS, Wright AP, Szumita PM, Bates DW, Tanasijevic MJ. An intervention to improve the timing of vancomycin levels. Am J Clin Pathol. 2013;140(6):801‐806. doi:10.1309/AJCPKQ6EAH7OYQLB
18. Meng L, Wong T, Huang S, et al. Conversion from vancomycin trough concentration-guided dosing to area under the curve-guided dosing using two sample measurements in adults: implementation at an academic medical center. Pharmacotherapy. 2019;39(4):433‐442. doi:10.1002/phar.2234
19. Stoessel AM, Hale CM, Seabury RW, Miller CD, Steele JM. The impact of AUC-based monitoring on pharmacist-directed vancomycin dose adjustments in complicated methicillin-resistant staphylococcus aureus Infection. J Pharm Pract. 2019;32(4):442‐446. doi:10.1177/0897190018764564
20. Kufel WD, Seabury RW, Mogle BT, Beccari MV, Probst LA, Steele JM. Readiness to implement vancomycin monitoring based on area under the concentration-time curve: a cross-sectional survey of a national health consortium. Am J Health Syst Pharm. 2019;76(12):889‐894. doi:10.1093/ajhp/zxz070
21. Bluestone J, Johnson P, Fullerton J, Carr C, Alderman J, BonTempo J. Effective in-service training design and delivery: evidence from an integrative literature review. Hum Resour Health. 2013;11:51. doi:10.1186/1478-4491-11-51
22. Ebben RHA, Siqeca F, Madsen UR, Vloet LCM, van Achterberg T. Effectiveness of implementation strategies for the improvement of guideline and protocol adherence in emergency care: a systematic review. BMJ Open. 2018;8(11):e017572. doi:10.1136/bmjopen-2017-017572
23. Fischer F, Lange K, Klose K, Greiner W, Kraemer A. Barriers and Strategies in Guideline Implementation-A Scoping Review. Healthcare (Basel). 2016;4(3):36. doi:10.3390/healthcare4030036