User login
Scholarly Productivity and Rank in Academic Hospital Medicine
Hospital medicine has grown rapidly, with more than 50,000 hospitalists practicing nationally in 2016.1 Despite the remarkable increase in academic hospital medicine faculty (AHMF), scholarly productivity remains underdeveloped. Prior evidence suggests peer-reviewed publications remain an important aspect of promotion in academic hospital medicine.2 However, there are multiple barriers to robust scholarly productivity among AHMF, including inadequate mentorship,3 lack of protected scholarship time,4 and greater participation in nonclinical activities outside of peer-reviewed clinical research.5 Though research barriers have been described previously, the current state of scholarly productivity among AHMF has not been characterized. In this cross-sectional study, we describe the distribution of academic rank and scholarly output of a national sample of AHMF.
METHODS
Study Design and Data Source
We performed a cross-sectional study of AHMF at the top 25 internal medicine residency programs as determined by Doximity.com as of February 1, 2020 (Appendix Table 1). Between March and August 2020, two authors (NS, MT) visited each residency program’s website, identified all faculty listed as members of the hospital medicine program, and extracted demographic data, including degrees, sex, residency, medical school, year of residency graduation, completion of chief residency, completion of fellowship, and rank. We categorized all academic titles into full professor, associate professor, assistant professor, and instructor/lecturer. Missing information was supplemented by searching state licensing websites and Doximity.com. Sex was validated using Genderize.io. We queried the Scopus database for each AHMF’s name and affiliated institution to extract publications, citations, and H-index (metric of productivity and impact, derived from the number of publications and their associated citations).6 We categorized medical schools by rank (top 25, top 50, or unranked), as defined by the 2020 US News Best Medical Schools, sorted by research7 and by location (United States, international Caribbean, and international non-Caribbean). We excluded programs without hospital medicine section/division webpages and AHMF with nonpromotion titles such as “adjunct professor” or “acting professor” or those with missing data that could not be identified using these methods.
Analysis
Summary statistics were generated using means with standard deviations and medians with interquartile ranges. We evaluated postresidency years 6 to 10 and 14 to 18 as conservative time frames for promotion to associate and full professor, respectively. These windows account for time spent for additional degrees, instructor years, and alternative career pathways. Demographic differences between academic ranks were determined using chi-square and Kruskal-Wallis analyses.
Because promotion occurs sequentially, a proportional odds logistic regression model was used to evaluate the association of academic rank and H-index, number of years post residency, completion of chief residency, graduation from a top 25 medical school, and sex. Since not all programs have the instructor/lecturer rank, only assistant, associate, and full professors were included in this model. Significance was assessed with the likelihood ratio test. The proportional odds assumption was assessed using the score test. All adjusted odds ratios and their associated 95% confidence intervals were recorded. A two-tailed P value < .05 was considered significant for this study, and SAS version 9.4 (SAS Institute Inc) was used to conduct all analyses. This study was approved by the UT Southwestern Institutional Review Board.
RESULTS
Cohort Demographics
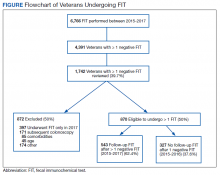
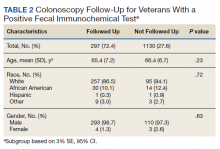
Of the top 25 internal medicine programs, 3 were excluded because they did not have websites that listed AHMF. Of the remaining 22 programs, we identified 1,829 AHMF. We excluded 166 AHMF because we could not identify title or year of residency graduation and 109 for having nonpromotion titles, leaving 1,554 AHMF (Appendix Figure). The cohort characteristics are described in Table 1. 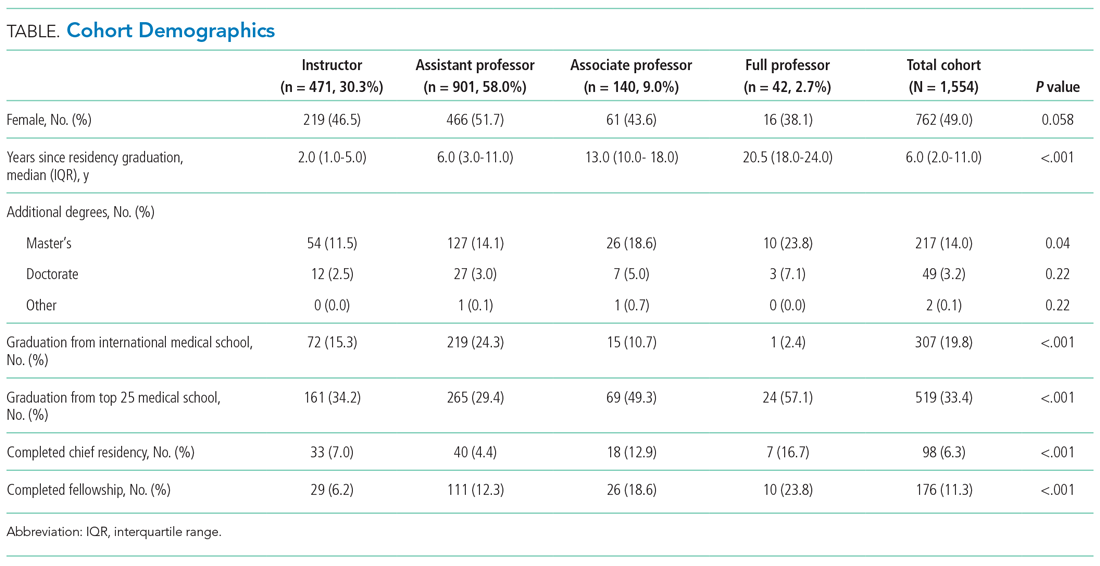
Research Productivity
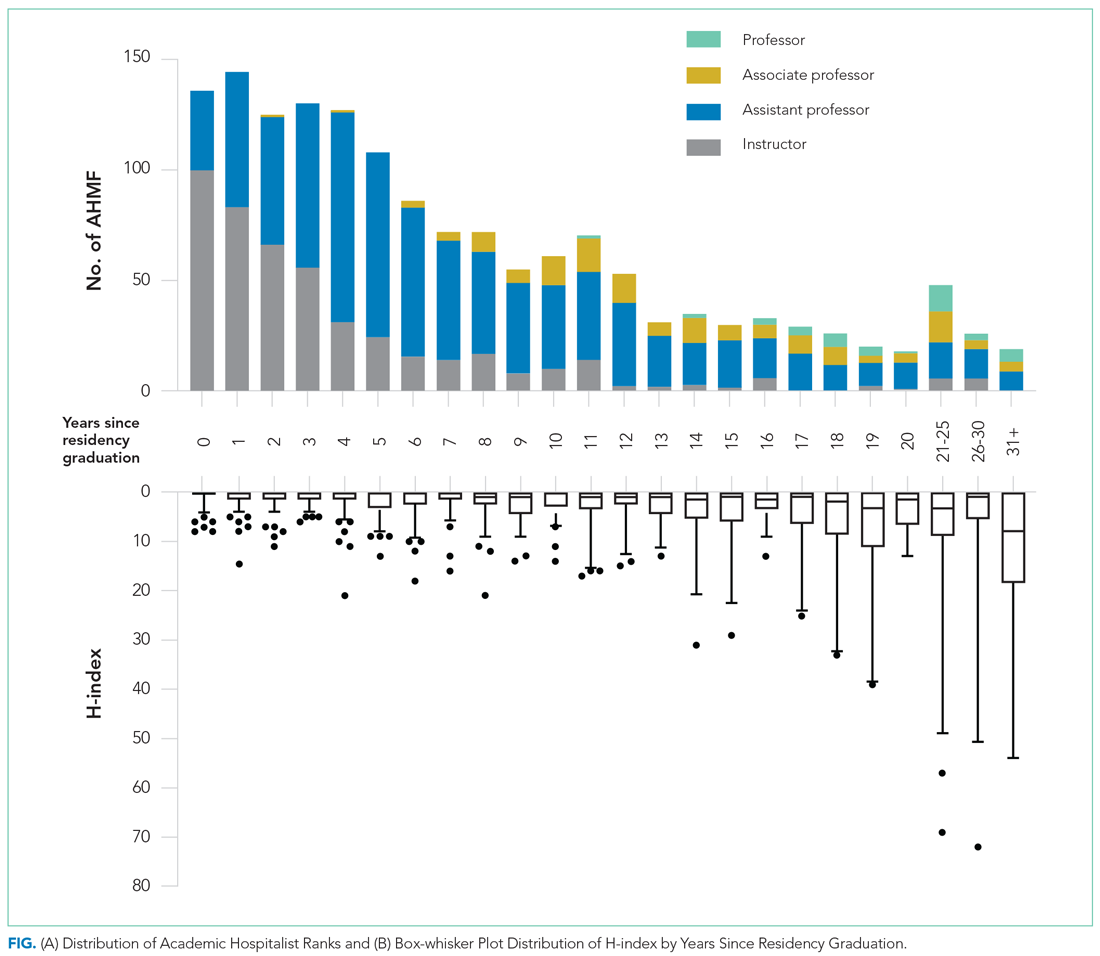
A total of 9,809 documents had been published by this cohort of academic hospitalists (Appendix Table 2). Overall mean (SD) and median (IQR) publications were 6.3 (24.3) and 0.0 (0.0-4.0), respectively. A total of 799 (51.4%) AHMF had no publications, 347 (22.3%) had one to three publications, 209 (13.4%) had 10 or more, and 39 (2.5%) had 50 or more. The median number of publications stratified by academic rank were 0.0 (IQR, 0.0-1.0) for instructors, 0.0 (IQR, 0.0-3.0) for assistant professors, 8.0 (IQR, 2.0-23.0) for associate professors, and 38.0 (IQR, 6.0-99.0) for full professors. Among men, 54.3% had published at least one manuscript, compared to 42.7% of women (P < .0001). The distribution of H-indices by years since residency graduation is shown in the Figure. The median number of documents published by faculty 6 to 10 years post residency was 1.0 (IQR, 0.0-4.0), with 46.8% of these faculty without a publication. For faculty 14 to 18 years post residency, the median number of documents was 3.0 (IQR, 0.0-11.0), with 30.1% of these faculty without a publication. Years post residency and academic rank were correlated with higher H-indices as well as more publications and citations (P < .0001).
Factors Associated With Academic Rank
Factors associated with rank are described in Appendix Table 3. In our multivariable ordinal regression model, H-index (adjusted odds ratio [aOR], 1.16 per single H-index point; 95% CI, 1.12-1.20), years post residency graduation (aOR, 1.14; 95% CI, 1.11-1.17), completion of chief residency (aOR, 2.46; 95% CI, 1.34-4.51), and graduation from a top 25 medical school (aOR, 2.10; 95% CI, 1.44-3.06) were associated with promotion.
DISCUSSION
In this cross-sectional analysis of more than 1,500 AHMF at the top 25 internal medicine residencies in the United States, 88.3% were instructors or assistant professors, while only 11.7% were associate or full professors. Furthermore, 51.4% were without a publication, and only 26.3% had published more than three manuscripts. Last, H-index, completion of a chief residency, years post residency, and graduation from a top 25 medical school were associated with higher academic rank.
Only 2.7% of the cohort were full professors, and 9.0% were associate professors. In comparison, academic cardiology faculty are 28.2% full professors and 22.9% associate professors.8 While the field of hospital medicine is relatively new, many faculty members had practiced for the expected duration of time for promotion consideration, with assistant professors or instructors constituting 89.9% of faculty at 6 to 10 years and 63.6% of faculty at 14 to 18 years post residency. We additionally observed a gender gap in publication history in hospital medicine, consistent with prior studies in hospital medicine that suggested gender disparities in scholarship.9,10 Increased focus will be needed in the future to ensure opportunities for scholarship are equitable for all faculty in hospital medicine.
Our findings suggest that scholarly productivity in academic hospital medicine remains a challenge. Prior studies have reported that less than half of academic hospitalists have ever published, and fewer than one in eight have received research funding.11,12 It is encouraging, however, that publications increase with time after residency. These data are consistent with the literature demonstrating a modest increase in hospitalists who had ever published, increasing from 43.0% in 2012 to 48.6% in 2020.12 Despite these trends, however, some early-career academic hospitalists report ambivalence toward academic productivity and promotion.13 Whether this ambivalence is the source of low scholarship output or the outcome of insufficient mentorship and limited research success is uncertain. But these factors, combined with the pressures of clinical productivity, the existing lack of mentorship, and inadequate protected research time represent barriers to successful scholarship in academic hospital medicine.3,14
Our study has several limitations. First, our inclusion criteria for the top 25 internal medicine residencies may have excluded hospital medicine divisions with substantial scholarly productivity. However, with 21 of the 25 programs listed on Doximity.com in the top 25 for internal medicine research funding, it is likely that our results overestimate scholarly productivity if compared to a complete, national cohort of AHMF.15 Second, our findings may not be generalizable to hospitalists who practice in nonacademic settings. Third, we were unable to account for differences in promotion criteria/tracks or scholarly output expectations between institutions. This limitation has been seen similarly in prior studies linking promotion and H-index.2 Furthermore, our study does not capture promotion via other pathways that may not depend on scholarly output, such as hospital leadership roles. Last, as data were abstracted from academic center websites, it is possible that not all information was accurate or updated. However, we randomly reevaluated 25% of hospital division webpages 6 months after our initial data collection and noted that all had been updated with new faculty and academic ranks, suggesting our data were accurate.
These data highlight that research productivity and academic promotion remain challenges in academic hospital medicine. Future studies may examine topics that include understanding pathways and milestones to promotion, reducing disparities in scholarship, and improving mentorship, protected time, and research funding in academic hospital medicine.
1. Wachter RM, Goldman L. Zero to 50,000—the 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958
2. Leykum LK, Parekh VI, Sharpe B, Boonyasai RT, Centor RM. Tried and true: a survey of successfully promoted academic hospitalists. J Hosp Med. 2011;6(7):411-415. https://doi.org/10.1002/jhm.894
3. Harrison R, Hunter AJ, Sharpe B, Auerbach AD. Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups. J Hosp Med. 2011;6(1):5-9. https://doi.org/10.1002/jhm.836
4. Cumbler E, Rendón P, Yirdaw E, et al. Keys to career success: resources and barriers identified by early career academic hospitalists. J Gen Intern Med. 2018;33(5):588-589. https://doi.org/10.1007/s11606-018-4336-7
5. Flanders SA, Centor B, Weber V, McGinn T, DeSalvo K, Auerbach A. Challenges and opportunities in academic hospital medicine: report from the Academic Hospital Medicine Summit. J Hosp Med. 2009;4(4):240-246. https://doi.org/10.1002/jhm.497
6. Hirsch JE. An index to quantify an individual’s scientific research output. Proc Natl Acad Sci U S A. 2005;102(46):16569-16572. https://doi.org/10.1073/pnas.0507655102
7. 2021 Best Medical Schools: Research. U.S. News & World Report. Accessed April 23, 2021. https://www.usnews.com/best-graduate-schools/top-medical-schools/research-rankings
8. Blumenthal DM, Olenski AR, Yeh RW, et al. Sex differences in faculty rank among academic cardiologists in the United States. Circulation. 2017;135(6):506-517. https://doi.org/10.1161/CIRCULATIONAHA.116.023520
9. Burden M, Frank MG, Keniston A, et al. Gender disparities in leadership and scholarly productivity of academic hospitalists. J Hosp Med. 2015;10(8):481-485. https://doi.org/10.1002/jhm.2340
10. Adler E, Hobbs A, Dhaliwal G, Babik JM. Gender differences in authorship of clinical problem-solving articles. J Hosp Med. 2020;15(8):475-478. https://doi.org/10.12788/jhm.3465
11. Chopra V, Burden M, Jones CD, et al. State of research in adult hospital medicine: results of a national survey. J Hosp Med. 2019;14(4):207-211. https://doi.org/10.12788/jhm.3136
12. Dang Do AN, Munchhof AM, Terry C, Emmett T, Kara A. Research and publication trends in hospital medicine. J Hosp Med. 2014;9(3):148-154. https://doi.org/10.1002/jhm.2148
13. Cumbler E, Yirdaw E, Kneeland P, et al. What is career success for academic hospitalists? A qualitative analysis of early-career faculty perspectives. J Hosp Med. 2018;13(6):372-377. https://doi.org/10.12788/jhm.2924
14. Reid MB, Misky GJ, Harrison RA, Sharpe B, Auerbach A, Glasheen JJ. Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27(1):23-27. https://doi.org/10.1007/s11606-011-1892-5
15. Roskoski R Jr, Parslow TG. Ranking tables of NIH funding to US medical schools in 2019. Accessed April 23, 2021. http://www.brimr.org/NIH_Awards/2019/NIH_Awards_2019.htm
Hospital medicine has grown rapidly, with more than 50,000 hospitalists practicing nationally in 2016.1 Despite the remarkable increase in academic hospital medicine faculty (AHMF), scholarly productivity remains underdeveloped. Prior evidence suggests peer-reviewed publications remain an important aspect of promotion in academic hospital medicine.2 However, there are multiple barriers to robust scholarly productivity among AHMF, including inadequate mentorship,3 lack of protected scholarship time,4 and greater participation in nonclinical activities outside of peer-reviewed clinical research.5 Though research barriers have been described previously, the current state of scholarly productivity among AHMF has not been characterized. In this cross-sectional study, we describe the distribution of academic rank and scholarly output of a national sample of AHMF.
METHODS
Study Design and Data Source
We performed a cross-sectional study of AHMF at the top 25 internal medicine residency programs as determined by Doximity.com as of February 1, 2020 (Appendix Table 1). Between March and August 2020, two authors (NS, MT) visited each residency program’s website, identified all faculty listed as members of the hospital medicine program, and extracted demographic data, including degrees, sex, residency, medical school, year of residency graduation, completion of chief residency, completion of fellowship, and rank. We categorized all academic titles into full professor, associate professor, assistant professor, and instructor/lecturer. Missing information was supplemented by searching state licensing websites and Doximity.com. Sex was validated using Genderize.io. We queried the Scopus database for each AHMF’s name and affiliated institution to extract publications, citations, and H-index (metric of productivity and impact, derived from the number of publications and their associated citations).6 We categorized medical schools by rank (top 25, top 50, or unranked), as defined by the 2020 US News Best Medical Schools, sorted by research7 and by location (United States, international Caribbean, and international non-Caribbean). We excluded programs without hospital medicine section/division webpages and AHMF with nonpromotion titles such as “adjunct professor” or “acting professor” or those with missing data that could not be identified using these methods.
Analysis
Summary statistics were generated using means with standard deviations and medians with interquartile ranges. We evaluated postresidency years 6 to 10 and 14 to 18 as conservative time frames for promotion to associate and full professor, respectively. These windows account for time spent for additional degrees, instructor years, and alternative career pathways. Demographic differences between academic ranks were determined using chi-square and Kruskal-Wallis analyses.
Because promotion occurs sequentially, a proportional odds logistic regression model was used to evaluate the association of academic rank and H-index, number of years post residency, completion of chief residency, graduation from a top 25 medical school, and sex. Since not all programs have the instructor/lecturer rank, only assistant, associate, and full professors were included in this model. Significance was assessed with the likelihood ratio test. The proportional odds assumption was assessed using the score test. All adjusted odds ratios and their associated 95% confidence intervals were recorded. A two-tailed P value < .05 was considered significant for this study, and SAS version 9.4 (SAS Institute Inc) was used to conduct all analyses. This study was approved by the UT Southwestern Institutional Review Board.
RESULTS
Cohort Demographics
Of the top 25 internal medicine programs, 3 were excluded because they did not have websites that listed AHMF. Of the remaining 22 programs, we identified 1,829 AHMF. We excluded 166 AHMF because we could not identify title or year of residency graduation and 109 for having nonpromotion titles, leaving 1,554 AHMF (Appendix Figure). The cohort characteristics are described in Table 1. 

Research Productivity
A total of 9,809 documents had been published by this cohort of academic hospitalists (Appendix Table 2). Overall mean (SD) and median (IQR) publications were 6.3 (24.3) and 0.0 (0.0-4.0), respectively. A total of 799 (51.4%) AHMF had no publications, 347 (22.3%) had one to three publications, 209 (13.4%) had 10 or more, and 39 (2.5%) had 50 or more. The median number of publications stratified by academic rank were 0.0 (IQR, 0.0-1.0) for instructors, 0.0 (IQR, 0.0-3.0) for assistant professors, 8.0 (IQR, 2.0-23.0) for associate professors, and 38.0 (IQR, 6.0-99.0) for full professors. Among men, 54.3% had published at least one manuscript, compared to 42.7% of women (P < .0001). The distribution of H-indices by years since residency graduation is shown in the Figure. The median number of documents published by faculty 6 to 10 years post residency was 1.0 (IQR, 0.0-4.0), with 46.8% of these faculty without a publication. For faculty 14 to 18 years post residency, the median number of documents was 3.0 (IQR, 0.0-11.0), with 30.1% of these faculty without a publication. Years post residency and academic rank were correlated with higher H-indices as well as more publications and citations (P < .0001).
Factors Associated With Academic Rank
Factors associated with rank are described in Appendix Table 3. In our multivariable ordinal regression model, H-index (adjusted odds ratio [aOR], 1.16 per single H-index point; 95% CI, 1.12-1.20), years post residency graduation (aOR, 1.14; 95% CI, 1.11-1.17), completion of chief residency (aOR, 2.46; 95% CI, 1.34-4.51), and graduation from a top 25 medical school (aOR, 2.10; 95% CI, 1.44-3.06) were associated with promotion.
DISCUSSION
In this cross-sectional analysis of more than 1,500 AHMF at the top 25 internal medicine residencies in the United States, 88.3% were instructors or assistant professors, while only 11.7% were associate or full professors. Furthermore, 51.4% were without a publication, and only 26.3% had published more than three manuscripts. Last, H-index, completion of a chief residency, years post residency, and graduation from a top 25 medical school were associated with higher academic rank.
Only 2.7% of the cohort were full professors, and 9.0% were associate professors. In comparison, academic cardiology faculty are 28.2% full professors and 22.9% associate professors.8 While the field of hospital medicine is relatively new, many faculty members had practiced for the expected duration of time for promotion consideration, with assistant professors or instructors constituting 89.9% of faculty at 6 to 10 years and 63.6% of faculty at 14 to 18 years post residency. We additionally observed a gender gap in publication history in hospital medicine, consistent with prior studies in hospital medicine that suggested gender disparities in scholarship.9,10 Increased focus will be needed in the future to ensure opportunities for scholarship are equitable for all faculty in hospital medicine.
Our findings suggest that scholarly productivity in academic hospital medicine remains a challenge. Prior studies have reported that less than half of academic hospitalists have ever published, and fewer than one in eight have received research funding.11,12 It is encouraging, however, that publications increase with time after residency. These data are consistent with the literature demonstrating a modest increase in hospitalists who had ever published, increasing from 43.0% in 2012 to 48.6% in 2020.12 Despite these trends, however, some early-career academic hospitalists report ambivalence toward academic productivity and promotion.13 Whether this ambivalence is the source of low scholarship output or the outcome of insufficient mentorship and limited research success is uncertain. But these factors, combined with the pressures of clinical productivity, the existing lack of mentorship, and inadequate protected research time represent barriers to successful scholarship in academic hospital medicine.3,14
Our study has several limitations. First, our inclusion criteria for the top 25 internal medicine residencies may have excluded hospital medicine divisions with substantial scholarly productivity. However, with 21 of the 25 programs listed on Doximity.com in the top 25 for internal medicine research funding, it is likely that our results overestimate scholarly productivity if compared to a complete, national cohort of AHMF.15 Second, our findings may not be generalizable to hospitalists who practice in nonacademic settings. Third, we were unable to account for differences in promotion criteria/tracks or scholarly output expectations between institutions. This limitation has been seen similarly in prior studies linking promotion and H-index.2 Furthermore, our study does not capture promotion via other pathways that may not depend on scholarly output, such as hospital leadership roles. Last, as data were abstracted from academic center websites, it is possible that not all information was accurate or updated. However, we randomly reevaluated 25% of hospital division webpages 6 months after our initial data collection and noted that all had been updated with new faculty and academic ranks, suggesting our data were accurate.
These data highlight that research productivity and academic promotion remain challenges in academic hospital medicine. Future studies may examine topics that include understanding pathways and milestones to promotion, reducing disparities in scholarship, and improving mentorship, protected time, and research funding in academic hospital medicine.
Hospital medicine has grown rapidly, with more than 50,000 hospitalists practicing nationally in 2016.1 Despite the remarkable increase in academic hospital medicine faculty (AHMF), scholarly productivity remains underdeveloped. Prior evidence suggests peer-reviewed publications remain an important aspect of promotion in academic hospital medicine.2 However, there are multiple barriers to robust scholarly productivity among AHMF, including inadequate mentorship,3 lack of protected scholarship time,4 and greater participation in nonclinical activities outside of peer-reviewed clinical research.5 Though research barriers have been described previously, the current state of scholarly productivity among AHMF has not been characterized. In this cross-sectional study, we describe the distribution of academic rank and scholarly output of a national sample of AHMF.
METHODS
Study Design and Data Source
We performed a cross-sectional study of AHMF at the top 25 internal medicine residency programs as determined by Doximity.com as of February 1, 2020 (Appendix Table 1). Between March and August 2020, two authors (NS, MT) visited each residency program’s website, identified all faculty listed as members of the hospital medicine program, and extracted demographic data, including degrees, sex, residency, medical school, year of residency graduation, completion of chief residency, completion of fellowship, and rank. We categorized all academic titles into full professor, associate professor, assistant professor, and instructor/lecturer. Missing information was supplemented by searching state licensing websites and Doximity.com. Sex was validated using Genderize.io. We queried the Scopus database for each AHMF’s name and affiliated institution to extract publications, citations, and H-index (metric of productivity and impact, derived from the number of publications and their associated citations).6 We categorized medical schools by rank (top 25, top 50, or unranked), as defined by the 2020 US News Best Medical Schools, sorted by research7 and by location (United States, international Caribbean, and international non-Caribbean). We excluded programs without hospital medicine section/division webpages and AHMF with nonpromotion titles such as “adjunct professor” or “acting professor” or those with missing data that could not be identified using these methods.
Analysis
Summary statistics were generated using means with standard deviations and medians with interquartile ranges. We evaluated postresidency years 6 to 10 and 14 to 18 as conservative time frames for promotion to associate and full professor, respectively. These windows account for time spent for additional degrees, instructor years, and alternative career pathways. Demographic differences between academic ranks were determined using chi-square and Kruskal-Wallis analyses.
Because promotion occurs sequentially, a proportional odds logistic regression model was used to evaluate the association of academic rank and H-index, number of years post residency, completion of chief residency, graduation from a top 25 medical school, and sex. Since not all programs have the instructor/lecturer rank, only assistant, associate, and full professors were included in this model. Significance was assessed with the likelihood ratio test. The proportional odds assumption was assessed using the score test. All adjusted odds ratios and their associated 95% confidence intervals were recorded. A two-tailed P value < .05 was considered significant for this study, and SAS version 9.4 (SAS Institute Inc) was used to conduct all analyses. This study was approved by the UT Southwestern Institutional Review Board.
RESULTS
Cohort Demographics
Of the top 25 internal medicine programs, 3 were excluded because they did not have websites that listed AHMF. Of the remaining 22 programs, we identified 1,829 AHMF. We excluded 166 AHMF because we could not identify title or year of residency graduation and 109 for having nonpromotion titles, leaving 1,554 AHMF (Appendix Figure). The cohort characteristics are described in Table 1. 

Research Productivity
A total of 9,809 documents had been published by this cohort of academic hospitalists (Appendix Table 2). Overall mean (SD) and median (IQR) publications were 6.3 (24.3) and 0.0 (0.0-4.0), respectively. A total of 799 (51.4%) AHMF had no publications, 347 (22.3%) had one to three publications, 209 (13.4%) had 10 or more, and 39 (2.5%) had 50 or more. The median number of publications stratified by academic rank were 0.0 (IQR, 0.0-1.0) for instructors, 0.0 (IQR, 0.0-3.0) for assistant professors, 8.0 (IQR, 2.0-23.0) for associate professors, and 38.0 (IQR, 6.0-99.0) for full professors. Among men, 54.3% had published at least one manuscript, compared to 42.7% of women (P < .0001). The distribution of H-indices by years since residency graduation is shown in the Figure. The median number of documents published by faculty 6 to 10 years post residency was 1.0 (IQR, 0.0-4.0), with 46.8% of these faculty without a publication. For faculty 14 to 18 years post residency, the median number of documents was 3.0 (IQR, 0.0-11.0), with 30.1% of these faculty without a publication. Years post residency and academic rank were correlated with higher H-indices as well as more publications and citations (P < .0001).
Factors Associated With Academic Rank
Factors associated with rank are described in Appendix Table 3. In our multivariable ordinal regression model, H-index (adjusted odds ratio [aOR], 1.16 per single H-index point; 95% CI, 1.12-1.20), years post residency graduation (aOR, 1.14; 95% CI, 1.11-1.17), completion of chief residency (aOR, 2.46; 95% CI, 1.34-4.51), and graduation from a top 25 medical school (aOR, 2.10; 95% CI, 1.44-3.06) were associated with promotion.
DISCUSSION
In this cross-sectional analysis of more than 1,500 AHMF at the top 25 internal medicine residencies in the United States, 88.3% were instructors or assistant professors, while only 11.7% were associate or full professors. Furthermore, 51.4% were without a publication, and only 26.3% had published more than three manuscripts. Last, H-index, completion of a chief residency, years post residency, and graduation from a top 25 medical school were associated with higher academic rank.
Only 2.7% of the cohort were full professors, and 9.0% were associate professors. In comparison, academic cardiology faculty are 28.2% full professors and 22.9% associate professors.8 While the field of hospital medicine is relatively new, many faculty members had practiced for the expected duration of time for promotion consideration, with assistant professors or instructors constituting 89.9% of faculty at 6 to 10 years and 63.6% of faculty at 14 to 18 years post residency. We additionally observed a gender gap in publication history in hospital medicine, consistent with prior studies in hospital medicine that suggested gender disparities in scholarship.9,10 Increased focus will be needed in the future to ensure opportunities for scholarship are equitable for all faculty in hospital medicine.
Our findings suggest that scholarly productivity in academic hospital medicine remains a challenge. Prior studies have reported that less than half of academic hospitalists have ever published, and fewer than one in eight have received research funding.11,12 It is encouraging, however, that publications increase with time after residency. These data are consistent with the literature demonstrating a modest increase in hospitalists who had ever published, increasing from 43.0% in 2012 to 48.6% in 2020.12 Despite these trends, however, some early-career academic hospitalists report ambivalence toward academic productivity and promotion.13 Whether this ambivalence is the source of low scholarship output or the outcome of insufficient mentorship and limited research success is uncertain. But these factors, combined with the pressures of clinical productivity, the existing lack of mentorship, and inadequate protected research time represent barriers to successful scholarship in academic hospital medicine.3,14
Our study has several limitations. First, our inclusion criteria for the top 25 internal medicine residencies may have excluded hospital medicine divisions with substantial scholarly productivity. However, with 21 of the 25 programs listed on Doximity.com in the top 25 for internal medicine research funding, it is likely that our results overestimate scholarly productivity if compared to a complete, national cohort of AHMF.15 Second, our findings may not be generalizable to hospitalists who practice in nonacademic settings. Third, we were unable to account for differences in promotion criteria/tracks or scholarly output expectations between institutions. This limitation has been seen similarly in prior studies linking promotion and H-index.2 Furthermore, our study does not capture promotion via other pathways that may not depend on scholarly output, such as hospital leadership roles. Last, as data were abstracted from academic center websites, it is possible that not all information was accurate or updated. However, we randomly reevaluated 25% of hospital division webpages 6 months after our initial data collection and noted that all had been updated with new faculty and academic ranks, suggesting our data were accurate.
These data highlight that research productivity and academic promotion remain challenges in academic hospital medicine. Future studies may examine topics that include understanding pathways and milestones to promotion, reducing disparities in scholarship, and improving mentorship, protected time, and research funding in academic hospital medicine.
1. Wachter RM, Goldman L. Zero to 50,000—the 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958
2. Leykum LK, Parekh VI, Sharpe B, Boonyasai RT, Centor RM. Tried and true: a survey of successfully promoted academic hospitalists. J Hosp Med. 2011;6(7):411-415. https://doi.org/10.1002/jhm.894
3. Harrison R, Hunter AJ, Sharpe B, Auerbach AD. Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups. J Hosp Med. 2011;6(1):5-9. https://doi.org/10.1002/jhm.836
4. Cumbler E, Rendón P, Yirdaw E, et al. Keys to career success: resources and barriers identified by early career academic hospitalists. J Gen Intern Med. 2018;33(5):588-589. https://doi.org/10.1007/s11606-018-4336-7
5. Flanders SA, Centor B, Weber V, McGinn T, DeSalvo K, Auerbach A. Challenges and opportunities in academic hospital medicine: report from the Academic Hospital Medicine Summit. J Hosp Med. 2009;4(4):240-246. https://doi.org/10.1002/jhm.497
6. Hirsch JE. An index to quantify an individual’s scientific research output. Proc Natl Acad Sci U S A. 2005;102(46):16569-16572. https://doi.org/10.1073/pnas.0507655102
7. 2021 Best Medical Schools: Research. U.S. News & World Report. Accessed April 23, 2021. https://www.usnews.com/best-graduate-schools/top-medical-schools/research-rankings
8. Blumenthal DM, Olenski AR, Yeh RW, et al. Sex differences in faculty rank among academic cardiologists in the United States. Circulation. 2017;135(6):506-517. https://doi.org/10.1161/CIRCULATIONAHA.116.023520
9. Burden M, Frank MG, Keniston A, et al. Gender disparities in leadership and scholarly productivity of academic hospitalists. J Hosp Med. 2015;10(8):481-485. https://doi.org/10.1002/jhm.2340
10. Adler E, Hobbs A, Dhaliwal G, Babik JM. Gender differences in authorship of clinical problem-solving articles. J Hosp Med. 2020;15(8):475-478. https://doi.org/10.12788/jhm.3465
11. Chopra V, Burden M, Jones CD, et al. State of research in adult hospital medicine: results of a national survey. J Hosp Med. 2019;14(4):207-211. https://doi.org/10.12788/jhm.3136
12. Dang Do AN, Munchhof AM, Terry C, Emmett T, Kara A. Research and publication trends in hospital medicine. J Hosp Med. 2014;9(3):148-154. https://doi.org/10.1002/jhm.2148
13. Cumbler E, Yirdaw E, Kneeland P, et al. What is career success for academic hospitalists? A qualitative analysis of early-career faculty perspectives. J Hosp Med. 2018;13(6):372-377. https://doi.org/10.12788/jhm.2924
14. Reid MB, Misky GJ, Harrison RA, Sharpe B, Auerbach A, Glasheen JJ. Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27(1):23-27. https://doi.org/10.1007/s11606-011-1892-5
15. Roskoski R Jr, Parslow TG. Ranking tables of NIH funding to US medical schools in 2019. Accessed April 23, 2021. http://www.brimr.org/NIH_Awards/2019/NIH_Awards_2019.htm
1. Wachter RM, Goldman L. Zero to 50,000—the 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958
2. Leykum LK, Parekh VI, Sharpe B, Boonyasai RT, Centor RM. Tried and true: a survey of successfully promoted academic hospitalists. J Hosp Med. 2011;6(7):411-415. https://doi.org/10.1002/jhm.894
3. Harrison R, Hunter AJ, Sharpe B, Auerbach AD. Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups. J Hosp Med. 2011;6(1):5-9. https://doi.org/10.1002/jhm.836
4. Cumbler E, Rendón P, Yirdaw E, et al. Keys to career success: resources and barriers identified by early career academic hospitalists. J Gen Intern Med. 2018;33(5):588-589. https://doi.org/10.1007/s11606-018-4336-7
5. Flanders SA, Centor B, Weber V, McGinn T, DeSalvo K, Auerbach A. Challenges and opportunities in academic hospital medicine: report from the Academic Hospital Medicine Summit. J Hosp Med. 2009;4(4):240-246. https://doi.org/10.1002/jhm.497
6. Hirsch JE. An index to quantify an individual’s scientific research output. Proc Natl Acad Sci U S A. 2005;102(46):16569-16572. https://doi.org/10.1073/pnas.0507655102
7. 2021 Best Medical Schools: Research. U.S. News & World Report. Accessed April 23, 2021. https://www.usnews.com/best-graduate-schools/top-medical-schools/research-rankings
8. Blumenthal DM, Olenski AR, Yeh RW, et al. Sex differences in faculty rank among academic cardiologists in the United States. Circulation. 2017;135(6):506-517. https://doi.org/10.1161/CIRCULATIONAHA.116.023520
9. Burden M, Frank MG, Keniston A, et al. Gender disparities in leadership and scholarly productivity of academic hospitalists. J Hosp Med. 2015;10(8):481-485. https://doi.org/10.1002/jhm.2340
10. Adler E, Hobbs A, Dhaliwal G, Babik JM. Gender differences in authorship of clinical problem-solving articles. J Hosp Med. 2020;15(8):475-478. https://doi.org/10.12788/jhm.3465
11. Chopra V, Burden M, Jones CD, et al. State of research in adult hospital medicine: results of a national survey. J Hosp Med. 2019;14(4):207-211. https://doi.org/10.12788/jhm.3136
12. Dang Do AN, Munchhof AM, Terry C, Emmett T, Kara A. Research and publication trends in hospital medicine. J Hosp Med. 2014;9(3):148-154. https://doi.org/10.1002/jhm.2148
13. Cumbler E, Yirdaw E, Kneeland P, et al. What is career success for academic hospitalists? A qualitative analysis of early-career faculty perspectives. J Hosp Med. 2018;13(6):372-377. https://doi.org/10.12788/jhm.2924
14. Reid MB, Misky GJ, Harrison RA, Sharpe B, Auerbach A, Glasheen JJ. Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27(1):23-27. https://doi.org/10.1007/s11606-011-1892-5
15. Roskoski R Jr, Parslow TG. Ranking tables of NIH funding to US medical schools in 2019. Accessed April 23, 2021. http://www.brimr.org/NIH_Awards/2019/NIH_Awards_2019.htm
© 2021 Society of Hospital Medicine
As the Story Unfolds
A 78-year-old woman presented to the ambulatory care clinic for a painful tongue mass. She noticed the mass 2 months prior to presentation, and it had not grown in the interim. She had left-sided jaw pain when opening her mouth and persistent left-sided otalgia.
In the evaluation of tongue masses, ulcerations, or other surface abnormalities, exclusion of squamous cell carcinoma is the top priority. Additional tongue surface abnormalities include benign lesions such as geographic tongue, inflammatory conditions such as lichen planus, and infections such as syphilis.
The ear and jaw pain may reflect metastatic spread, neural invasion, or referred pain from the tongue. A vasculitis with predilection for the head, such as giant cell arteritis, could present with oral and ear pain. Jaw pain with mastication could reflect jaw claudication, but pain upon mouth opening is more commonly explained by temporomandibular joint dysfunction.
The patient had hypertension, hyperlipidemia, chronic kidney disease (estimated glomerular filtration rate of 42 mL/min), and diabetes mellitus. Four months prior she was diagnosed with a chronic obstructing left renal calculus on ultrasonography to evaluate chronic kidney disease. Two months prior heart failure with preserved ejection fraction was diagnosed. Stress cardiac magnetic resonance imaging (MRI) demonstrated normal ejection fraction, asymmetric septal hypertrophy, and stress-induced subendocardial perfusion defect. Her medications were metoprolol, lisinopril, simvastatin, meloxicam, and aspirin. She never used tobacco and did not consume alcohol. She was born in the Philippines and emigrated to the United States 15 years ago. She had not experienced fever, chills, hearing loss, tinnitus, cough, dysphonia, neck swelling, or joint pain. She had lost 3 kg in the previous 4 months.
The absence of tobacco and alcohol use reduces the probability of a squamous cell carcinoma, although human papillomavirus–associated squamous cell carcinoma of the tongue remains possible. Asymmetric septal hypertrophy is characteristic of hypertrophic cardiomyopathy or an infiltrative cardiomyopathy. Sarcoidosis can affect the heart and can account for the renal calculus (via hypercalcemia). Amyloid light-chain (AL) amyloidosis could involve the heart and the tongue, although in amyloidosis the cardiac MRI typically displays late gadolinium enhancement and ventricular wall thickening. The absence of tinnitus or hearing loss suggests that the left-sided otalgia is referred pain from the tongue and oral cavity rather than a primary otologic disease (eg, infection).
On physical examination, temperature was 37.2 °C, heart rate was 88 beats per minute, blood pressure was 134/62 mm Hg, and oxygen saturation was 99% while breathing ambient air. The patient’s weight was 40.4 kg (body mass index of 19.26 kg/m2). Intraoral examination revealed induration of the bilateral tongue, an erosive 1-cm pedunculated mass of the left dorsum, rough white coating on the right dorsum, and fullness of the right lateral surface with an erosion abutting tooth #2. The right submandibular salivary gland was firm. The otoscopic examination and remainder of the head and neck examination were normal. There was no cervical, supraclavicular, or axillary adenopathy. The cranial nerve, cardiovascular, pulmonary, abdominal, and skin examinations were normal.
The left-sided lingual mass and right-sided lingual erosion likely arise from the same process. Both are compatible with an infection (eg, syphilis or tuberculosis), cancer, autoimmune disease (eg, Crohn’s disease or sarcoidosis), or an infiltrative disease such as amyloidosis. Leukoplakia could reflect a candidal infection, dysplasia, squamous cell carcinoma, oral hairy leukoplakia, or hyperkeratosis. The isolated submandibular salivary gland could reflect sialadenitis from chronic salivary duct obstruction or a primary neoplasm, but more likely is caused by the same process causing the tongue abnormalities.
The white blood cell count was 8,600/μL; hemoglobin, 11.5 g/dL; mean corpuscular volume, 102.5 fL; and platelet count, 270,000/μL3. Serum sodium was 141 mEq/L; potassium, 4.1 mEq/L; chloride, 101 mEq/L; bicarbonate, 30 mEq/L; blood urea nitrogen, 19 mg/dL; creatinine, 0.7 mg/dL; and calcium, 10.4 mg/dL (reference range, 8.5-10.3). Total serum protein was 7.3 g/dL (reference range, 6.0-8.3); albumin was 3.7 g/dL. Liver biochemistry test results were normal. Serum folate and vitamin B12 levels were normal. Serum ferritin was 423 ng/mL (reference range, 11-306); transferrin saturation, 21.4% (reference range, 15.0%-50.0%); and total iron-binding capacity, 323 µg/dL (reference range, 261-478). Parathyroid hormone (PTH) was 14 pg/mL (reference range, 15-65). HIV antibody was negative.
The calcium level is at the upper range of normal, whereas the PTH level is at the lower range of normal. The differential diagnosis for PTH-independent hypercalcemia includes hypercalcemia of malignancy and granulomatous disease such as sarcoidosis. Mild hypercalcemia could contribute to the nephrolithiasis. The iron studies exclude iron deficiency and are not suggestive of anemia of chronic disease. The triad of mild hypercalcemia, cardiomyopathy, and anemia is compatible with AL amyloidosis (perhaps with associated multiple myeloma) or sarcoidosis; both disorders can present as a mass. Imaging of the head and neck and biopsy of the tongue mass are the next steps.
The left dorsal tongue mass was excised in clinic. Histopathology revealed ulcerated squamous mucosa with inflammatory changes but no malignancy. Imaging of the head and neck was scheduled.
Neither cancer or granulomas were detected, but inadequate sampling or staining must be considered. Inflammatory changes are compatible with infection, autoimmunity, and cancer; the latter can feature reactive changes that obscure the malignant cells. The absence of granulomas lowers, but does not eliminate, the possibility of sarcoidosis, tuberculosis, fungal infection, and granulomatosis with polyangiitis. Actinomycosis is an invasive orofacial infection that disregards anatomic boundaries and is characterized by inflammatory histology; although infection of the tongue is possible, infection of the jaw and face is more typical. Immunoglobulin G4–related disease can present as an inflammatory and invasive disorder; however, the characteristic histopathologic findings (lymphoplasmacytic infiltrate, fibrosis, and phlebitis) are absent.
Culture of the tissue for mycobacteria or fungi (she is at increased risk for both given her previous residency in the Philippines) could increase the diagnostic yield. Another biopsy of the tongue or an adjacent structure—guided by imaging—may provide a more diagnostic tissue sample.
MRI of the head and neck demonstrated hyperintense signal and prominence of the right lateral pterygoid muscle (Figure 1A) and slight enlargement of a right submandibular gland (Figure 1B). No tongue abnormalities were identified. Radiograph of the chest did not reveal infiltrates, masses, or lymphadenopathy.

The absence of the tongue mass on the MRI likely reflects excision of the mass at the time of biopsy. The signal enhancement in the right lateral pterygoid muscle and submandibular gland is suggestive of an infiltrative process. Infiltration of the right lateral pterygoid muscle may also explain the patient’s pain when opening her mouth. Infiltrative processes can be neoplastic (eg, salivary gland tumor, sarcoma, lymphoma), infectious (eg, mycobacterial or fungal), cellular (eg, histiocytes, mast cells, plasma cells, eosinophils, granulomas), or related to inert substances such as amyloid or iron.
Seven weeks later, the patient presented to the hospital for scheduled percutaneous nephrolithotomy of the obstructing renal calculus. The physical examination was unchanged. The complete blood count and metabolic panel were unchanged apart from hemoglobin of 9.9 g/dL and calcium of 11.5 mg/dL. Coagulation studies were within normal limits.
A percutaneous nephroureteral stent was placed under conscious sedation. The patient then underwent rapid sequence induction of general anesthesia for the nephrolithotomy with fentanyl, propofol, and rocuronium. Within minutes of initiating mechanical ventilation, severe periorbital and perioral edema, copious oral cavity bleeding, and bilateral periorbital purpura occurred. Sugammadex (neuromuscular blockade reversal) and dexamethasone were administered. Examination of the oral cavity was limited by the brisk bleeding; the right sided tongue erosion was unchanged.
Bleeding is caused by thrombocytopenia, thrombocytopathy, coagulopathy, or disruption of vessel integrity. Oral cavity bleeding could arise from the tongue ulceration, but could also reflect pulmonary, nasal, or gastrointestinal hemorrhage. Angioedema arises from mast cell– or bradykinin-mediated pathways; mast cell degranulation may have been precipitated by the anesthetic agents, opiate, or a material in the nephroureteral stent.
The edema and bleeding are temporally related to multiple medications and mechanical ventilation. A latent bleeding diathesis may have manifested in the setting of increased tissue hydrostatic pressure or vessel permeability. Amyloidosis can lead to vessel fragility and coagulopathy, and periorbital bleeding is characteristic of AL amyloidosis.
The hypercalcemia, now more pronounced, raises concern for malignancy (including multiple myeloma) and granulomatous diseases like sarcoidosis, mycobacterial infections, and fungal infections. The declining hemoglobin could be explained by chronic blood loss, hemolysis, anemia of chronic disease, or a bone marrow process.
The cardiomyopathy, bleeding disorder, and multifocal disease in the oral cavity can be explained by AL amyloidosis; the hypercalcemia suggests concomitant multiple myeloma.
At the time of the bleeding event, the partial thromboplastin time, prothrombin time, and fibrinogen were within the reference ranges. Factor X activity level was normal. No schistocytes were observed on peripheral blood smear. Immunoglobulin G level was 1,425 mg/dL (reference range, 639-1,349); IgA and IgM levels were within the reference range. Serum lambda free light chains were 151.78 mg/dL (reference range, 0.46-2.71), and the ratio of kappa to lambda light chains was 0.01 (reference range, 0.49-2.54). Serum protein electrophoresis and immunofixation demonstrated a monoclonal paraprotein (IgG lambda) level of 1.2 g/dL. Congo red staining of the previously excised left dorsal tongue mass was negative for apple-green birefringence. Reexamination of the oral cavity revealed macroglossia and scalloping of the tongue (Figure 2).
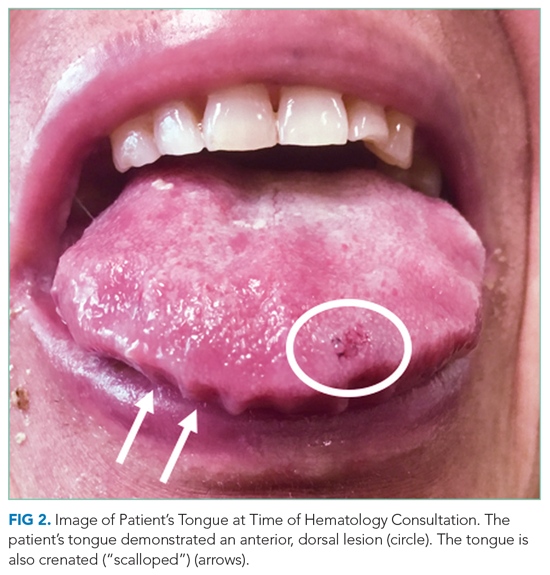
Scalloping is characteristic of an infiltrative disorder that enlarges the tongue (macroglossia) and deforms its edges, which encounter the teeth. Macroglossia is seen in AL amyloidosis, acromegaly, and hypothyroidism. A monoclonal light chain, especially a lambda light chain, is characteristic of AL amyloidosis. The Congo red stain results can support the diagnosis when positive, but it has limited sensitivity. The tongue specimen can be sent for immunohistochemistry or mass spectrometry to evaluate for light chain deposition. A bone marrow biopsy can demonstrate a clonal plasma cell population. AL amyloidosis with concomitant multiple myeloma is the most likely diagnosis.
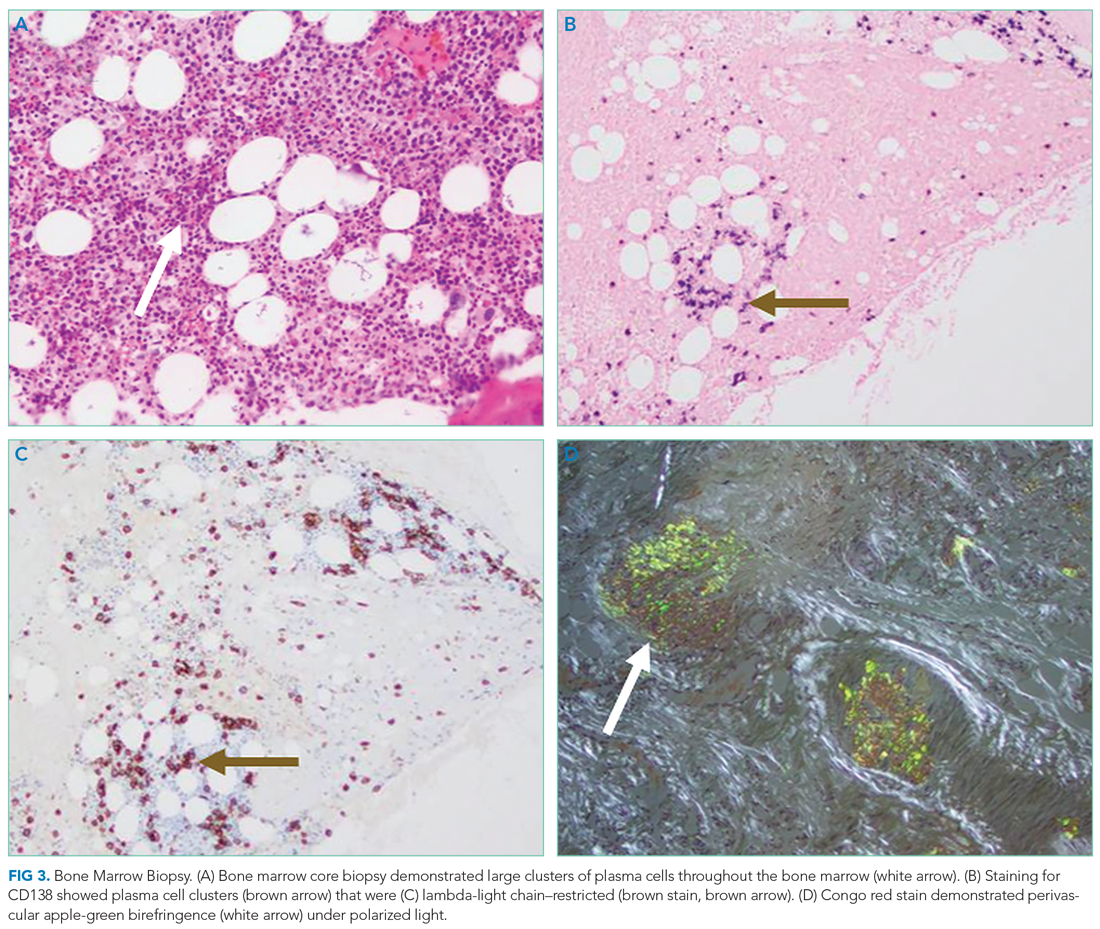
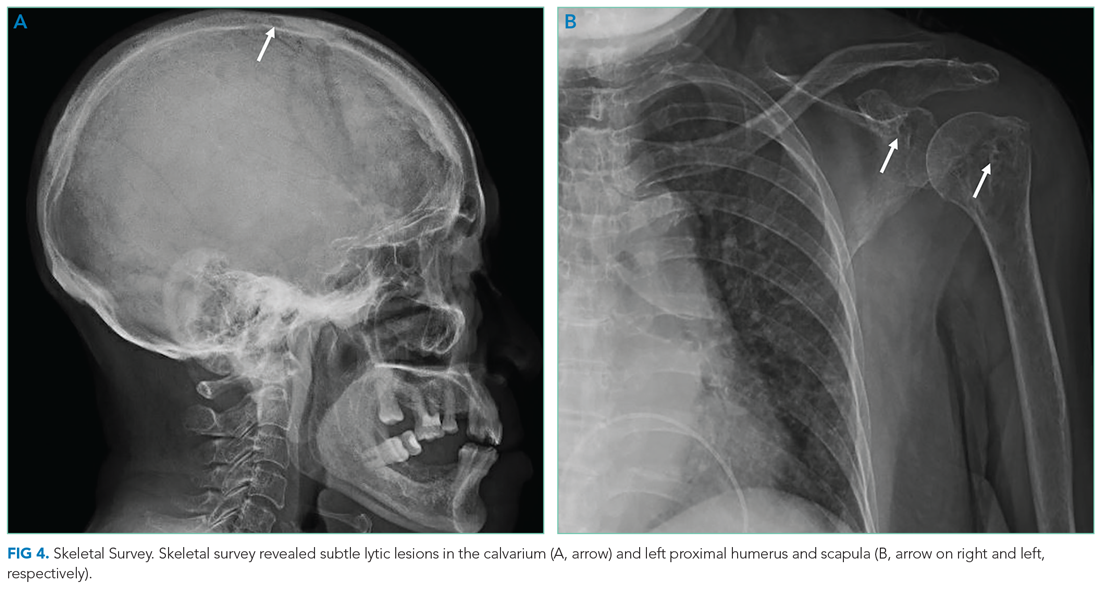
Bone marrow aspiration and core biopsy demonstrated 30% lambda-restricted plasma cells (Figure 3A-C). Congo red staining demonstrated apple-green birefringence of the bone marrow microvasculature (Figure 3D). Skeletal survey demonstrated widespread lytic bone disease involving the calvarium (Figure 4A), left humerus (Figure 4B), and left scapula (Figure 4B). Based on the monoclonal paraprotein, more than 10% monoclonal plasma cells, skeletal lesions, and hypercalcemia, she was diagnosed with IgG lambda multiple myeloma. Based on apple-green birefringence in the bone marrow and macroglossia, she was diagnosed with AL amyloidosis. The cardiac MRI findings were compatible with AL amyloidosis. 1
After three cycles of bortezomib and dexamethasone therapy to concurrently treat AL amyloidosis and multiple myeloma, the serum lambda light chain level decreased to 1.49 mg/dL and the monoclonal paraprotein level decreased to 0.3 g/dL. The calcium level was 9.8 mg/dL, and the hemoglobin level was 11.7 g/dL. The patient’s tongue pain resolved, allowing for improved oral intake and a 5.7-kg weight gain. The patient underwent nephrolithotomy 4 months after her initial presentation. She resumed an active lifestyle and recently traveled to visit relatives in the Philippines.
DISCUSSION
Oral diseases affect general health and quality of life and can be a harbinger of systemic disease. Tooth loss, caries, periodontal disease, and poorly fitting dentures commonly affect speech and nutrition.2 These common outpatient oral health issues can be the driving force for hospital admissions; for example, caries and periodontal disease can lead to suppurative odontogenic infection, endocarditis, brain abscess, and sepsis.
Tongue ulcerations, masses, and surface abnormalities often require consultation with a dentist or oral and maxillofacial surgeon to exclude squamous cell carcinoma.3 Other diagnostic considerations include benign neoplasms, trauma, inflammatory conditions (eg, sarcoidosis), infection (eg, syphilis, tuberculosis), and infiltrative processes such as amyloidosis.
Amyloidosis is a heterogeneous group of diseases caused by deposition of insoluble protein fibrils in tissues.4,5 The three most encountered forms of amyloidosis are AL, AA, and ATTR. Each form is named after the culprit protein.4 AL amyloidosis arises when a small clonal population of plasma cells in the bone marrow overproduces immunoglobulin light chain monomers.4,6 AA amyloidosis develops when the liver produces serum amyloid A protein (an acute phase reactant) in response to a chronic inflammatory condition such as rheumatoid arthritis or chronic intravenous drug injection.4 Transthyretin (TTR, also known as “prealbumin”) is a tetrameric protein that transports thyroxine and retinol; there are two forms of ATTR amyloidosis: hereditary and wild type. Hereditary ATTR amyloidosis develops from agglomeration of misfolded TTR monomers caused by mutations in the TTR gene. Wild-type ATTR amyloidosis is caused by age-related dissociation of the TTR tetramer into its constituent monomers that denature, misfold, and agglomerate into fibrils.5 Wild-type ATTR is now recognized as the most common form of amyloidosis, with 25% of myocardial autopsy specimens of patients 80 years or older demonstrating amyloid.7 The estimated incidence of AL amyloidosis is 10 cases per million person-years.8
Each amyloid protein homes in on specific anatomic sites.4 Characteristic combinations of organ dysfunction can suggest different forms of amyloidosis.9 Cardiac and peripheral nervous involvement (eg, carpal tunnel syndrome) is typical of both hereditary and wild-type ATTR amyloidosis; ATTR amyloidosis does not involve the kidney.4 AA amyloidosis most commonly manifests with proteinuria followed by declining glomerular filtration rate; heart failure is rare.4 The most common findings in AL amyloidosis are proteinuria, congestive heart failure, and sensory neuropathy.6 Gastrointestinal tract and hepatic involvement are each seen in nearly 20% of patients, and macroglossia is identified in approximately 10% of those with AL amyloidosis.6,10
Chronic deposition of amyloid can lead to acute presentations. Approximately 30% of patients with AL amyloidosis develop abnormal bleeding.11 Amyloid deposition in small blood vessels predisposes them to rupture. Bleeding events can be exacerbated by acquired coagulopathy due to plasma cell dyscrasia−associated thrombocytopenia, amyloid fibril adsorption of factor X, or hypofibrinogenemia.11,12 Periorbital purpura following minor trauma or transient venous hypertension is characteristic of AL amyloidosis.6,13 In this case, positive pressure ventilation and recumbent positioning increased hydrostatic pressure in the head and neck, causing rupture of the infiltrated small vessels around the eyes and in the oral cavity.14
Histological demonstration of tissue deposition of amyloid protein is the preferred method for amyloidosis diagnosis. Symptomatic sites or organs with dysfunction or radiologic changes are suitable for biopsy.6 If those sites are inaccessible or yield insufficient tissue quantity, abdominal fat pad aspiration or biopsy is indicated.15 Apple-green birefringence under polarized light of Congo red–stained tissue is characteristic, with sensitivity and specificity of approximately 80% and a positive predictive value of 85%.15 Immunoelectron microscopy is often performed simultaneously to confirm the diagnosis and determine the amyloid protein type.4,16 Immunoelectron microscopy’s sensitivity is approximately 80%, and it has specificity and positive predictive value both approaching 100%.15 Mass spectrometry is particularly useful in cases where the amyloid subtype is not clinically apparent (eg, a patient with an autoimmune condition or chronic infection as well as light chain abnormality).6 Cardiac MRI findings that suggest amyloidosis include a thickened left ventricle and late gadolinium enhancement.1 ATTR cardiac amyloidosis can be diagnosed using amyloid fibril–binding radiotracer technetium-99m-pyrophosphate scintigraphy; biopsy is often not necessary.1,4 Gene sequencing to differentiate between hereditary and wild-type forms of ATTR amyloidosis is beneficial.
The primary objectives of amyloidosis management are to control symptoms and inhibit amyloid protein production.6 Outcomes in AL amyloidosis have improved due to early diagnosis, new chemotherapeutic agents to eradicate the plasma cell clone, and autologous stem cell transplantation.6,17 Two new ATTR amyloidosis treatments are RNA interference therapies, which prevent TTR messenger RNA translation, and tafamidis, which stabilizes the TTR tetramer and prevents dissociation into its constituent monomers that precipitate in tissues.18 Both therapies can improve neuropathy-related quality of life.18 Tafamidis slows disease progression and decreases all-cause mortality in patients with hereditary and wild-type ATTR cardiac amyloidosis.19
Multiple myeloma and AL amyloidosis are both plasma cell dyscrasias involving the bone marrow, but they represent distinct disease processes with different clinical features.6 Multiple myeloma is characterized by marked expansion of a clonal plasma cell population within the bone marrow that aberrantly produces immunoglobulin. Conversely, the clonal plasma cell population responsible for producing the insoluble monoclonal light chain protein in AL amyloidosis typically constitutes less than 10% of the bone marrow.20 Multiple myeloma and AL amyloidosis may coexist in the same patient; nearly 15% of patients with multiple myeloma subsequently develop clinically overt AL amyloidosis, which portends a poor outcome.20
Amyloidosis is a rare group of diseases that arises when misfolded proteins aggregate in vital organs. The typical manifestations—congestive heart failure, neuropathy, chronic kidney disease, bleeding—are nearly always explained by more common conditions. Characteristic manifestations (like macroglossia) or associated diseases (like multiple myeloma) substantially increases the probability of AL amyloidosis. In a multisystem illness, the most common diseases must be excluded first, but this case reminds us that rare diseases, like amyloidosis, also warrant consideration as the story unfolds.
KEY TEACHING POINTS
- Different amyloid proteins precipitate in different anatomic sites, which leads to specific multiorgan combinations. The most common amyloidosis, ATTR, tends to manifest as heart failure and peripheral sensory neuropathy, while the constellation of AL amyloidosis includes heart failure, neuropathy, and proteinuria.
- Bleeding occurs in 30% of patients with AL amyloidosis. It is precipitated by fragile small blood vessels and exacerbated by acquired coagulopathy from adsorption of coagulation factors.
- Multiple myeloma and AL amyloidosis are both plasma cell dyscrasias involving the bone marrow, but they represent distinct disease processes with different clinical tempos and presentations. Multiple myeloma and AL amyloidosis may coexist in the same patient; nearly 15% of patients with multiple myeloma subsequently develop clinically overt AL amyloidosis.
Acknowledgment
The authors thank Benjamin A Derman, MD, of the University of Chicago, Chicago, Illinois, for critical review of the manuscript.
1. Witteles RM, Bokhari S, Damy T, et al. Screening for transthyretin amyloid cardiomyopathy in everyday practice. JACC Heart Fail. 2019;7(8):709-716. https://doi.org/10.1016/j.jchf.2019.04.010
2. Griffin SO, Jones JA, Brunson D, Griffin PM, Bailey WD. Burden of oral disease among older adults and implications for public health priorities. Am J Public Health. 2012;102(3):411-418. https://doi.org/10.2105/ajph.2011.300362
3. Ernster JA, Sciotto CG, O’Brien MM, et al. Rising incidence of oropharyngeal cancer and the role of oncogenic human papilloma virus. Laryngoscope. 2007;117(12):2115-2128. https://doi.org/10.1097/mlg.0b013e31813e5fbb
4. Wechalekar AD, Gillmore JD, Hawkins PN. Systemic amyloidosis. Lancet. 2016;387(10038):2641-2654. https://doi.org/10.1016/s0140-6736(15)01274-x
5. Riek R, Eisenberg DS. The activities of amyloids from a structural perspective. Nature. 2016;539(7628):227-235. https://doi.org/10.1038/nature20416
6. Gertz MA, Dispenzieri A. Systemic amyloidosis recognition, prognosis, and therapy: a systematic review. JAMA. 2020;324(1):79-89. https://doi.org/10.1001/jama.2020.5493
7. Ruberg FL, Berk JL. Transthyretin (TTR) cardiac amyloidosis. Circulation. 2012;126(10):1286-1300. https://doi.org/10.1161/circulationaha.111.078915
8. Quock TP, Yan T, Chang E, Guthrie S, Broder MS. Epidemiology of AL amyloidosis: a real-world study using US claims data. Blood Adv. 2018;2(10):1046-1053. https://doi.org/10.1182/bloodadvances.2018016402
9. Papoutsidakis N, Miller EJ, Rodonski A, Jacoby D. Time course of common clinical manifestations in patients with transthyretin cardiac amyloidosis: delay from symptom onset to diagnosis. J Card Fail. 2018;24(2):131-133. https://doi.org/10.1016/j.cardfail.2017.12.005
10. Shimazaki C, Hata H, Iida S, et al. Nationwide survey of 741 patients with systemic amyloid light-chain amyloidosis in Japan. Intern Med. 2018;57(2):181-187. https://doi.org/10.2169/internalmedicine.9206-17
11. Mumford AD, O’Donnell J, Gillmore JD, Manning RA, Hawkins PN, Laffan M. Bleeding symptoms and coagulation abnormalities in 337 patients with AL-amyloidosis. Br J Haematol. 2000;110(2):454-460. https://doi.org/10.1046/j.1365-2141.2000.02183.x
12. Choufani EB, Sanchorawala V, Ernst T, et al. Acquired factor X deficiency in patients with amyloid light-chain amyloidosis: incidence, bleeding manifestations, and response to high-dose chemotherapy. Blood. 2001;97(6):1885-1887. https://doi.org/10.1182/blood.v97.6.1885
13. Slagel GA, Lupton GP. Postproctoscopic periorbital purpura. Primary systemic amyloidosis. Arch Dermatol. 1986;122(4):464-465, 467-468.
14. Lupton GP. Pneomometry-induced purpura. Arch Dermatol. 1981;117(10):603. https://doi.org/10.1001/archderm.117.10.603a
15. Fernández de Larrea C, Verga L, Morbini P, et al. A practical approach to the diagnosis of systemic amyloidoses. Blood. 2015;125(14):2239-2244. https://doi.org/10.1182/blood-2014-11-609883
16. Vaxman I, Gertz M. Recent Advances in the diagnosis, risk stratification, and management of systemic light-chain amyloidosis. Acta Haematol. 2019;141(2):93-106. https://doi.org/10.1159/000495455
17. Muchtar E, Gertz MA, Kumar SK, et al. Improved outcomes for newly diagnosed AL amyloidosis between 2000 and 2014: cracking the glass ceiling of early death. Blood. 2017;129(15):2111-2119. https://doi.org/10.1182/blood-2016-11-751628
18. Quarta CC, Solomon SD. Stabilizing transthyretin to treat ATTR cardiomyopathy. N Engl J Med. 2018;379(11):1083-1084. https://doi.org/10.1056/nejme1810074
19. Maurer MS, Schwartz JH, Gundapaneni B, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379(11):1007-1016. https://doi.org/10.1056/nejmoa1805689
20. Bahlis NJ, Lazarus HM. Multiple myeloma-associated AL amyloidosis: is a distinctive therapeutic approach warranted? Bone Marrow Transplant. 2006;38(1):7-15. https://doi.org/10.1038/sj.bmt.1705395
A 78-year-old woman presented to the ambulatory care clinic for a painful tongue mass. She noticed the mass 2 months prior to presentation, and it had not grown in the interim. She had left-sided jaw pain when opening her mouth and persistent left-sided otalgia.
In the evaluation of tongue masses, ulcerations, or other surface abnormalities, exclusion of squamous cell carcinoma is the top priority. Additional tongue surface abnormalities include benign lesions such as geographic tongue, inflammatory conditions such as lichen planus, and infections such as syphilis.
The ear and jaw pain may reflect metastatic spread, neural invasion, or referred pain from the tongue. A vasculitis with predilection for the head, such as giant cell arteritis, could present with oral and ear pain. Jaw pain with mastication could reflect jaw claudication, but pain upon mouth opening is more commonly explained by temporomandibular joint dysfunction.
The patient had hypertension, hyperlipidemia, chronic kidney disease (estimated glomerular filtration rate of 42 mL/min), and diabetes mellitus. Four months prior she was diagnosed with a chronic obstructing left renal calculus on ultrasonography to evaluate chronic kidney disease. Two months prior heart failure with preserved ejection fraction was diagnosed. Stress cardiac magnetic resonance imaging (MRI) demonstrated normal ejection fraction, asymmetric septal hypertrophy, and stress-induced subendocardial perfusion defect. Her medications were metoprolol, lisinopril, simvastatin, meloxicam, and aspirin. She never used tobacco and did not consume alcohol. She was born in the Philippines and emigrated to the United States 15 years ago. She had not experienced fever, chills, hearing loss, tinnitus, cough, dysphonia, neck swelling, or joint pain. She had lost 3 kg in the previous 4 months.
The absence of tobacco and alcohol use reduces the probability of a squamous cell carcinoma, although human papillomavirus–associated squamous cell carcinoma of the tongue remains possible. Asymmetric septal hypertrophy is characteristic of hypertrophic cardiomyopathy or an infiltrative cardiomyopathy. Sarcoidosis can affect the heart and can account for the renal calculus (via hypercalcemia). Amyloid light-chain (AL) amyloidosis could involve the heart and the tongue, although in amyloidosis the cardiac MRI typically displays late gadolinium enhancement and ventricular wall thickening. The absence of tinnitus or hearing loss suggests that the left-sided otalgia is referred pain from the tongue and oral cavity rather than a primary otologic disease (eg, infection).
On physical examination, temperature was 37.2 °C, heart rate was 88 beats per minute, blood pressure was 134/62 mm Hg, and oxygen saturation was 99% while breathing ambient air. The patient’s weight was 40.4 kg (body mass index of 19.26 kg/m2). Intraoral examination revealed induration of the bilateral tongue, an erosive 1-cm pedunculated mass of the left dorsum, rough white coating on the right dorsum, and fullness of the right lateral surface with an erosion abutting tooth #2. The right submandibular salivary gland was firm. The otoscopic examination and remainder of the head and neck examination were normal. There was no cervical, supraclavicular, or axillary adenopathy. The cranial nerve, cardiovascular, pulmonary, abdominal, and skin examinations were normal.
The left-sided lingual mass and right-sided lingual erosion likely arise from the same process. Both are compatible with an infection (eg, syphilis or tuberculosis), cancer, autoimmune disease (eg, Crohn’s disease or sarcoidosis), or an infiltrative disease such as amyloidosis. Leukoplakia could reflect a candidal infection, dysplasia, squamous cell carcinoma, oral hairy leukoplakia, or hyperkeratosis. The isolated submandibular salivary gland could reflect sialadenitis from chronic salivary duct obstruction or a primary neoplasm, but more likely is caused by the same process causing the tongue abnormalities.
The white blood cell count was 8,600/μL; hemoglobin, 11.5 g/dL; mean corpuscular volume, 102.5 fL; and platelet count, 270,000/μL3. Serum sodium was 141 mEq/L; potassium, 4.1 mEq/L; chloride, 101 mEq/L; bicarbonate, 30 mEq/L; blood urea nitrogen, 19 mg/dL; creatinine, 0.7 mg/dL; and calcium, 10.4 mg/dL (reference range, 8.5-10.3). Total serum protein was 7.3 g/dL (reference range, 6.0-8.3); albumin was 3.7 g/dL. Liver biochemistry test results were normal. Serum folate and vitamin B12 levels were normal. Serum ferritin was 423 ng/mL (reference range, 11-306); transferrin saturation, 21.4% (reference range, 15.0%-50.0%); and total iron-binding capacity, 323 µg/dL (reference range, 261-478). Parathyroid hormone (PTH) was 14 pg/mL (reference range, 15-65). HIV antibody was negative.
The calcium level is at the upper range of normal, whereas the PTH level is at the lower range of normal. The differential diagnosis for PTH-independent hypercalcemia includes hypercalcemia of malignancy and granulomatous disease such as sarcoidosis. Mild hypercalcemia could contribute to the nephrolithiasis. The iron studies exclude iron deficiency and are not suggestive of anemia of chronic disease. The triad of mild hypercalcemia, cardiomyopathy, and anemia is compatible with AL amyloidosis (perhaps with associated multiple myeloma) or sarcoidosis; both disorders can present as a mass. Imaging of the head and neck and biopsy of the tongue mass are the next steps.
The left dorsal tongue mass was excised in clinic. Histopathology revealed ulcerated squamous mucosa with inflammatory changes but no malignancy. Imaging of the head and neck was scheduled.
Neither cancer or granulomas were detected, but inadequate sampling or staining must be considered. Inflammatory changes are compatible with infection, autoimmunity, and cancer; the latter can feature reactive changes that obscure the malignant cells. The absence of granulomas lowers, but does not eliminate, the possibility of sarcoidosis, tuberculosis, fungal infection, and granulomatosis with polyangiitis. Actinomycosis is an invasive orofacial infection that disregards anatomic boundaries and is characterized by inflammatory histology; although infection of the tongue is possible, infection of the jaw and face is more typical. Immunoglobulin G4–related disease can present as an inflammatory and invasive disorder; however, the characteristic histopathologic findings (lymphoplasmacytic infiltrate, fibrosis, and phlebitis) are absent.
Culture of the tissue for mycobacteria or fungi (she is at increased risk for both given her previous residency in the Philippines) could increase the diagnostic yield. Another biopsy of the tongue or an adjacent structure—guided by imaging—may provide a more diagnostic tissue sample.
MRI of the head and neck demonstrated hyperintense signal and prominence of the right lateral pterygoid muscle (Figure 1A) and slight enlargement of a right submandibular gland (Figure 1B). No tongue abnormalities were identified. Radiograph of the chest did not reveal infiltrates, masses, or lymphadenopathy.

The absence of the tongue mass on the MRI likely reflects excision of the mass at the time of biopsy. The signal enhancement in the right lateral pterygoid muscle and submandibular gland is suggestive of an infiltrative process. Infiltration of the right lateral pterygoid muscle may also explain the patient’s pain when opening her mouth. Infiltrative processes can be neoplastic (eg, salivary gland tumor, sarcoma, lymphoma), infectious (eg, mycobacterial or fungal), cellular (eg, histiocytes, mast cells, plasma cells, eosinophils, granulomas), or related to inert substances such as amyloid or iron.
Seven weeks later, the patient presented to the hospital for scheduled percutaneous nephrolithotomy of the obstructing renal calculus. The physical examination was unchanged. The complete blood count and metabolic panel were unchanged apart from hemoglobin of 9.9 g/dL and calcium of 11.5 mg/dL. Coagulation studies were within normal limits.
A percutaneous nephroureteral stent was placed under conscious sedation. The patient then underwent rapid sequence induction of general anesthesia for the nephrolithotomy with fentanyl, propofol, and rocuronium. Within minutes of initiating mechanical ventilation, severe periorbital and perioral edema, copious oral cavity bleeding, and bilateral periorbital purpura occurred. Sugammadex (neuromuscular blockade reversal) and dexamethasone were administered. Examination of the oral cavity was limited by the brisk bleeding; the right sided tongue erosion was unchanged.
Bleeding is caused by thrombocytopenia, thrombocytopathy, coagulopathy, or disruption of vessel integrity. Oral cavity bleeding could arise from the tongue ulceration, but could also reflect pulmonary, nasal, or gastrointestinal hemorrhage. Angioedema arises from mast cell– or bradykinin-mediated pathways; mast cell degranulation may have been precipitated by the anesthetic agents, opiate, or a material in the nephroureteral stent.
The edema and bleeding are temporally related to multiple medications and mechanical ventilation. A latent bleeding diathesis may have manifested in the setting of increased tissue hydrostatic pressure or vessel permeability. Amyloidosis can lead to vessel fragility and coagulopathy, and periorbital bleeding is characteristic of AL amyloidosis.
The hypercalcemia, now more pronounced, raises concern for malignancy (including multiple myeloma) and granulomatous diseases like sarcoidosis, mycobacterial infections, and fungal infections. The declining hemoglobin could be explained by chronic blood loss, hemolysis, anemia of chronic disease, or a bone marrow process.
The cardiomyopathy, bleeding disorder, and multifocal disease in the oral cavity can be explained by AL amyloidosis; the hypercalcemia suggests concomitant multiple myeloma.
At the time of the bleeding event, the partial thromboplastin time, prothrombin time, and fibrinogen were within the reference ranges. Factor X activity level was normal. No schistocytes were observed on peripheral blood smear. Immunoglobulin G level was 1,425 mg/dL (reference range, 639-1,349); IgA and IgM levels were within the reference range. Serum lambda free light chains were 151.78 mg/dL (reference range, 0.46-2.71), and the ratio of kappa to lambda light chains was 0.01 (reference range, 0.49-2.54). Serum protein electrophoresis and immunofixation demonstrated a monoclonal paraprotein (IgG lambda) level of 1.2 g/dL. Congo red staining of the previously excised left dorsal tongue mass was negative for apple-green birefringence. Reexamination of the oral cavity revealed macroglossia and scalloping of the tongue (Figure 2).

Scalloping is characteristic of an infiltrative disorder that enlarges the tongue (macroglossia) and deforms its edges, which encounter the teeth. Macroglossia is seen in AL amyloidosis, acromegaly, and hypothyroidism. A monoclonal light chain, especially a lambda light chain, is characteristic of AL amyloidosis. The Congo red stain results can support the diagnosis when positive, but it has limited sensitivity. The tongue specimen can be sent for immunohistochemistry or mass spectrometry to evaluate for light chain deposition. A bone marrow biopsy can demonstrate a clonal plasma cell population. AL amyloidosis with concomitant multiple myeloma is the most likely diagnosis.
Bone marrow aspiration and core biopsy demonstrated 30% lambda-restricted plasma cells (Figure 3A-C). Congo red staining demonstrated apple-green birefringence of the bone marrow microvasculature (Figure 3D). Skeletal survey demonstrated widespread lytic bone disease involving the calvarium (Figure 4A), left humerus (Figure 4B), and left scapula (Figure 4B). Based on the monoclonal paraprotein, more than 10% monoclonal plasma cells, skeletal lesions, and hypercalcemia, she was diagnosed with IgG lambda multiple myeloma. Based on apple-green birefringence in the bone marrow and macroglossia, she was diagnosed with AL amyloidosis. The cardiac MRI findings were compatible with AL amyloidosis. 1

After three cycles of bortezomib and dexamethasone therapy to concurrently treat AL amyloidosis and multiple myeloma, the serum lambda light chain level decreased to 1.49 mg/dL and the monoclonal paraprotein level decreased to 0.3 g/dL. The calcium level was 9.8 mg/dL, and the hemoglobin level was 11.7 g/dL. The patient’s tongue pain resolved, allowing for improved oral intake and a 5.7-kg weight gain. The patient underwent nephrolithotomy 4 months after her initial presentation. She resumed an active lifestyle and recently traveled to visit relatives in the Philippines.

DISCUSSION
Oral diseases affect general health and quality of life and can be a harbinger of systemic disease. Tooth loss, caries, periodontal disease, and poorly fitting dentures commonly affect speech and nutrition.2 These common outpatient oral health issues can be the driving force for hospital admissions; for example, caries and periodontal disease can lead to suppurative odontogenic infection, endocarditis, brain abscess, and sepsis.
Tongue ulcerations, masses, and surface abnormalities often require consultation with a dentist or oral and maxillofacial surgeon to exclude squamous cell carcinoma.3 Other diagnostic considerations include benign neoplasms, trauma, inflammatory conditions (eg, sarcoidosis), infection (eg, syphilis, tuberculosis), and infiltrative processes such as amyloidosis.
Amyloidosis is a heterogeneous group of diseases caused by deposition of insoluble protein fibrils in tissues.4,5 The three most encountered forms of amyloidosis are AL, AA, and ATTR. Each form is named after the culprit protein.4 AL amyloidosis arises when a small clonal population of plasma cells in the bone marrow overproduces immunoglobulin light chain monomers.4,6 AA amyloidosis develops when the liver produces serum amyloid A protein (an acute phase reactant) in response to a chronic inflammatory condition such as rheumatoid arthritis or chronic intravenous drug injection.4 Transthyretin (TTR, also known as “prealbumin”) is a tetrameric protein that transports thyroxine and retinol; there are two forms of ATTR amyloidosis: hereditary and wild type. Hereditary ATTR amyloidosis develops from agglomeration of misfolded TTR monomers caused by mutations in the TTR gene. Wild-type ATTR amyloidosis is caused by age-related dissociation of the TTR tetramer into its constituent monomers that denature, misfold, and agglomerate into fibrils.5 Wild-type ATTR is now recognized as the most common form of amyloidosis, with 25% of myocardial autopsy specimens of patients 80 years or older demonstrating amyloid.7 The estimated incidence of AL amyloidosis is 10 cases per million person-years.8
Each amyloid protein homes in on specific anatomic sites.4 Characteristic combinations of organ dysfunction can suggest different forms of amyloidosis.9 Cardiac and peripheral nervous involvement (eg, carpal tunnel syndrome) is typical of both hereditary and wild-type ATTR amyloidosis; ATTR amyloidosis does not involve the kidney.4 AA amyloidosis most commonly manifests with proteinuria followed by declining glomerular filtration rate; heart failure is rare.4 The most common findings in AL amyloidosis are proteinuria, congestive heart failure, and sensory neuropathy.6 Gastrointestinal tract and hepatic involvement are each seen in nearly 20% of patients, and macroglossia is identified in approximately 10% of those with AL amyloidosis.6,10
Chronic deposition of amyloid can lead to acute presentations. Approximately 30% of patients with AL amyloidosis develop abnormal bleeding.11 Amyloid deposition in small blood vessels predisposes them to rupture. Bleeding events can be exacerbated by acquired coagulopathy due to plasma cell dyscrasia−associated thrombocytopenia, amyloid fibril adsorption of factor X, or hypofibrinogenemia.11,12 Periorbital purpura following minor trauma or transient venous hypertension is characteristic of AL amyloidosis.6,13 In this case, positive pressure ventilation and recumbent positioning increased hydrostatic pressure in the head and neck, causing rupture of the infiltrated small vessels around the eyes and in the oral cavity.14
Histological demonstration of tissue deposition of amyloid protein is the preferred method for amyloidosis diagnosis. Symptomatic sites or organs with dysfunction or radiologic changes are suitable for biopsy.6 If those sites are inaccessible or yield insufficient tissue quantity, abdominal fat pad aspiration or biopsy is indicated.15 Apple-green birefringence under polarized light of Congo red–stained tissue is characteristic, with sensitivity and specificity of approximately 80% and a positive predictive value of 85%.15 Immunoelectron microscopy is often performed simultaneously to confirm the diagnosis and determine the amyloid protein type.4,16 Immunoelectron microscopy’s sensitivity is approximately 80%, and it has specificity and positive predictive value both approaching 100%.15 Mass spectrometry is particularly useful in cases where the amyloid subtype is not clinically apparent (eg, a patient with an autoimmune condition or chronic infection as well as light chain abnormality).6 Cardiac MRI findings that suggest amyloidosis include a thickened left ventricle and late gadolinium enhancement.1 ATTR cardiac amyloidosis can be diagnosed using amyloid fibril–binding radiotracer technetium-99m-pyrophosphate scintigraphy; biopsy is often not necessary.1,4 Gene sequencing to differentiate between hereditary and wild-type forms of ATTR amyloidosis is beneficial.
The primary objectives of amyloidosis management are to control symptoms and inhibit amyloid protein production.6 Outcomes in AL amyloidosis have improved due to early diagnosis, new chemotherapeutic agents to eradicate the plasma cell clone, and autologous stem cell transplantation.6,17 Two new ATTR amyloidosis treatments are RNA interference therapies, which prevent TTR messenger RNA translation, and tafamidis, which stabilizes the TTR tetramer and prevents dissociation into its constituent monomers that precipitate in tissues.18 Both therapies can improve neuropathy-related quality of life.18 Tafamidis slows disease progression and decreases all-cause mortality in patients with hereditary and wild-type ATTR cardiac amyloidosis.19
Multiple myeloma and AL amyloidosis are both plasma cell dyscrasias involving the bone marrow, but they represent distinct disease processes with different clinical features.6 Multiple myeloma is characterized by marked expansion of a clonal plasma cell population within the bone marrow that aberrantly produces immunoglobulin. Conversely, the clonal plasma cell population responsible for producing the insoluble monoclonal light chain protein in AL amyloidosis typically constitutes less than 10% of the bone marrow.20 Multiple myeloma and AL amyloidosis may coexist in the same patient; nearly 15% of patients with multiple myeloma subsequently develop clinically overt AL amyloidosis, which portends a poor outcome.20
Amyloidosis is a rare group of diseases that arises when misfolded proteins aggregate in vital organs. The typical manifestations—congestive heart failure, neuropathy, chronic kidney disease, bleeding—are nearly always explained by more common conditions. Characteristic manifestations (like macroglossia) or associated diseases (like multiple myeloma) substantially increases the probability of AL amyloidosis. In a multisystem illness, the most common diseases must be excluded first, but this case reminds us that rare diseases, like amyloidosis, also warrant consideration as the story unfolds.
KEY TEACHING POINTS
- Different amyloid proteins precipitate in different anatomic sites, which leads to specific multiorgan combinations. The most common amyloidosis, ATTR, tends to manifest as heart failure and peripheral sensory neuropathy, while the constellation of AL amyloidosis includes heart failure, neuropathy, and proteinuria.
- Bleeding occurs in 30% of patients with AL amyloidosis. It is precipitated by fragile small blood vessels and exacerbated by acquired coagulopathy from adsorption of coagulation factors.
- Multiple myeloma and AL amyloidosis are both plasma cell dyscrasias involving the bone marrow, but they represent distinct disease processes with different clinical tempos and presentations. Multiple myeloma and AL amyloidosis may coexist in the same patient; nearly 15% of patients with multiple myeloma subsequently develop clinically overt AL amyloidosis.
Acknowledgment
The authors thank Benjamin A Derman, MD, of the University of Chicago, Chicago, Illinois, for critical review of the manuscript.
A 78-year-old woman presented to the ambulatory care clinic for a painful tongue mass. She noticed the mass 2 months prior to presentation, and it had not grown in the interim. She had left-sided jaw pain when opening her mouth and persistent left-sided otalgia.
In the evaluation of tongue masses, ulcerations, or other surface abnormalities, exclusion of squamous cell carcinoma is the top priority. Additional tongue surface abnormalities include benign lesions such as geographic tongue, inflammatory conditions such as lichen planus, and infections such as syphilis.
The ear and jaw pain may reflect metastatic spread, neural invasion, or referred pain from the tongue. A vasculitis with predilection for the head, such as giant cell arteritis, could present with oral and ear pain. Jaw pain with mastication could reflect jaw claudication, but pain upon mouth opening is more commonly explained by temporomandibular joint dysfunction.
The patient had hypertension, hyperlipidemia, chronic kidney disease (estimated glomerular filtration rate of 42 mL/min), and diabetes mellitus. Four months prior she was diagnosed with a chronic obstructing left renal calculus on ultrasonography to evaluate chronic kidney disease. Two months prior heart failure with preserved ejection fraction was diagnosed. Stress cardiac magnetic resonance imaging (MRI) demonstrated normal ejection fraction, asymmetric septal hypertrophy, and stress-induced subendocardial perfusion defect. Her medications were metoprolol, lisinopril, simvastatin, meloxicam, and aspirin. She never used tobacco and did not consume alcohol. She was born in the Philippines and emigrated to the United States 15 years ago. She had not experienced fever, chills, hearing loss, tinnitus, cough, dysphonia, neck swelling, or joint pain. She had lost 3 kg in the previous 4 months.
The absence of tobacco and alcohol use reduces the probability of a squamous cell carcinoma, although human papillomavirus–associated squamous cell carcinoma of the tongue remains possible. Asymmetric septal hypertrophy is characteristic of hypertrophic cardiomyopathy or an infiltrative cardiomyopathy. Sarcoidosis can affect the heart and can account for the renal calculus (via hypercalcemia). Amyloid light-chain (AL) amyloidosis could involve the heart and the tongue, although in amyloidosis the cardiac MRI typically displays late gadolinium enhancement and ventricular wall thickening. The absence of tinnitus or hearing loss suggests that the left-sided otalgia is referred pain from the tongue and oral cavity rather than a primary otologic disease (eg, infection).
On physical examination, temperature was 37.2 °C, heart rate was 88 beats per minute, blood pressure was 134/62 mm Hg, and oxygen saturation was 99% while breathing ambient air. The patient’s weight was 40.4 kg (body mass index of 19.26 kg/m2). Intraoral examination revealed induration of the bilateral tongue, an erosive 1-cm pedunculated mass of the left dorsum, rough white coating on the right dorsum, and fullness of the right lateral surface with an erosion abutting tooth #2. The right submandibular salivary gland was firm. The otoscopic examination and remainder of the head and neck examination were normal. There was no cervical, supraclavicular, or axillary adenopathy. The cranial nerve, cardiovascular, pulmonary, abdominal, and skin examinations were normal.
The left-sided lingual mass and right-sided lingual erosion likely arise from the same process. Both are compatible with an infection (eg, syphilis or tuberculosis), cancer, autoimmune disease (eg, Crohn’s disease or sarcoidosis), or an infiltrative disease such as amyloidosis. Leukoplakia could reflect a candidal infection, dysplasia, squamous cell carcinoma, oral hairy leukoplakia, or hyperkeratosis. The isolated submandibular salivary gland could reflect sialadenitis from chronic salivary duct obstruction or a primary neoplasm, but more likely is caused by the same process causing the tongue abnormalities.
The white blood cell count was 8,600/μL; hemoglobin, 11.5 g/dL; mean corpuscular volume, 102.5 fL; and platelet count, 270,000/μL3. Serum sodium was 141 mEq/L; potassium, 4.1 mEq/L; chloride, 101 mEq/L; bicarbonate, 30 mEq/L; blood urea nitrogen, 19 mg/dL; creatinine, 0.7 mg/dL; and calcium, 10.4 mg/dL (reference range, 8.5-10.3). Total serum protein was 7.3 g/dL (reference range, 6.0-8.3); albumin was 3.7 g/dL. Liver biochemistry test results were normal. Serum folate and vitamin B12 levels were normal. Serum ferritin was 423 ng/mL (reference range, 11-306); transferrin saturation, 21.4% (reference range, 15.0%-50.0%); and total iron-binding capacity, 323 µg/dL (reference range, 261-478). Parathyroid hormone (PTH) was 14 pg/mL (reference range, 15-65). HIV antibody was negative.
The calcium level is at the upper range of normal, whereas the PTH level is at the lower range of normal. The differential diagnosis for PTH-independent hypercalcemia includes hypercalcemia of malignancy and granulomatous disease such as sarcoidosis. Mild hypercalcemia could contribute to the nephrolithiasis. The iron studies exclude iron deficiency and are not suggestive of anemia of chronic disease. The triad of mild hypercalcemia, cardiomyopathy, and anemia is compatible with AL amyloidosis (perhaps with associated multiple myeloma) or sarcoidosis; both disorders can present as a mass. Imaging of the head and neck and biopsy of the tongue mass are the next steps.
The left dorsal tongue mass was excised in clinic. Histopathology revealed ulcerated squamous mucosa with inflammatory changes but no malignancy. Imaging of the head and neck was scheduled.
Neither cancer or granulomas were detected, but inadequate sampling or staining must be considered. Inflammatory changes are compatible with infection, autoimmunity, and cancer; the latter can feature reactive changes that obscure the malignant cells. The absence of granulomas lowers, but does not eliminate, the possibility of sarcoidosis, tuberculosis, fungal infection, and granulomatosis with polyangiitis. Actinomycosis is an invasive orofacial infection that disregards anatomic boundaries and is characterized by inflammatory histology; although infection of the tongue is possible, infection of the jaw and face is more typical. Immunoglobulin G4–related disease can present as an inflammatory and invasive disorder; however, the characteristic histopathologic findings (lymphoplasmacytic infiltrate, fibrosis, and phlebitis) are absent.
Culture of the tissue for mycobacteria or fungi (she is at increased risk for both given her previous residency in the Philippines) could increase the diagnostic yield. Another biopsy of the tongue or an adjacent structure—guided by imaging—may provide a more diagnostic tissue sample.
MRI of the head and neck demonstrated hyperintense signal and prominence of the right lateral pterygoid muscle (Figure 1A) and slight enlargement of a right submandibular gland (Figure 1B). No tongue abnormalities were identified. Radiograph of the chest did not reveal infiltrates, masses, or lymphadenopathy.

The absence of the tongue mass on the MRI likely reflects excision of the mass at the time of biopsy. The signal enhancement in the right lateral pterygoid muscle and submandibular gland is suggestive of an infiltrative process. Infiltration of the right lateral pterygoid muscle may also explain the patient’s pain when opening her mouth. Infiltrative processes can be neoplastic (eg, salivary gland tumor, sarcoma, lymphoma), infectious (eg, mycobacterial or fungal), cellular (eg, histiocytes, mast cells, plasma cells, eosinophils, granulomas), or related to inert substances such as amyloid or iron.
Seven weeks later, the patient presented to the hospital for scheduled percutaneous nephrolithotomy of the obstructing renal calculus. The physical examination was unchanged. The complete blood count and metabolic panel were unchanged apart from hemoglobin of 9.9 g/dL and calcium of 11.5 mg/dL. Coagulation studies were within normal limits.
A percutaneous nephroureteral stent was placed under conscious sedation. The patient then underwent rapid sequence induction of general anesthesia for the nephrolithotomy with fentanyl, propofol, and rocuronium. Within minutes of initiating mechanical ventilation, severe periorbital and perioral edema, copious oral cavity bleeding, and bilateral periorbital purpura occurred. Sugammadex (neuromuscular blockade reversal) and dexamethasone were administered. Examination of the oral cavity was limited by the brisk bleeding; the right sided tongue erosion was unchanged.
Bleeding is caused by thrombocytopenia, thrombocytopathy, coagulopathy, or disruption of vessel integrity. Oral cavity bleeding could arise from the tongue ulceration, but could also reflect pulmonary, nasal, or gastrointestinal hemorrhage. Angioedema arises from mast cell– or bradykinin-mediated pathways; mast cell degranulation may have been precipitated by the anesthetic agents, opiate, or a material in the nephroureteral stent.
The edema and bleeding are temporally related to multiple medications and mechanical ventilation. A latent bleeding diathesis may have manifested in the setting of increased tissue hydrostatic pressure or vessel permeability. Amyloidosis can lead to vessel fragility and coagulopathy, and periorbital bleeding is characteristic of AL amyloidosis.
The hypercalcemia, now more pronounced, raises concern for malignancy (including multiple myeloma) and granulomatous diseases like sarcoidosis, mycobacterial infections, and fungal infections. The declining hemoglobin could be explained by chronic blood loss, hemolysis, anemia of chronic disease, or a bone marrow process.
The cardiomyopathy, bleeding disorder, and multifocal disease in the oral cavity can be explained by AL amyloidosis; the hypercalcemia suggests concomitant multiple myeloma.
At the time of the bleeding event, the partial thromboplastin time, prothrombin time, and fibrinogen were within the reference ranges. Factor X activity level was normal. No schistocytes were observed on peripheral blood smear. Immunoglobulin G level was 1,425 mg/dL (reference range, 639-1,349); IgA and IgM levels were within the reference range. Serum lambda free light chains were 151.78 mg/dL (reference range, 0.46-2.71), and the ratio of kappa to lambda light chains was 0.01 (reference range, 0.49-2.54). Serum protein electrophoresis and immunofixation demonstrated a monoclonal paraprotein (IgG lambda) level of 1.2 g/dL. Congo red staining of the previously excised left dorsal tongue mass was negative for apple-green birefringence. Reexamination of the oral cavity revealed macroglossia and scalloping of the tongue (Figure 2).

Scalloping is characteristic of an infiltrative disorder that enlarges the tongue (macroglossia) and deforms its edges, which encounter the teeth. Macroglossia is seen in AL amyloidosis, acromegaly, and hypothyroidism. A monoclonal light chain, especially a lambda light chain, is characteristic of AL amyloidosis. The Congo red stain results can support the diagnosis when positive, but it has limited sensitivity. The tongue specimen can be sent for immunohistochemistry or mass spectrometry to evaluate for light chain deposition. A bone marrow biopsy can demonstrate a clonal plasma cell population. AL amyloidosis with concomitant multiple myeloma is the most likely diagnosis.
Bone marrow aspiration and core biopsy demonstrated 30% lambda-restricted plasma cells (Figure 3A-C). Congo red staining demonstrated apple-green birefringence of the bone marrow microvasculature (Figure 3D). Skeletal survey demonstrated widespread lytic bone disease involving the calvarium (Figure 4A), left humerus (Figure 4B), and left scapula (Figure 4B). Based on the monoclonal paraprotein, more than 10% monoclonal plasma cells, skeletal lesions, and hypercalcemia, she was diagnosed with IgG lambda multiple myeloma. Based on apple-green birefringence in the bone marrow and macroglossia, she was diagnosed with AL amyloidosis. The cardiac MRI findings were compatible with AL amyloidosis. 1

After three cycles of bortezomib and dexamethasone therapy to concurrently treat AL amyloidosis and multiple myeloma, the serum lambda light chain level decreased to 1.49 mg/dL and the monoclonal paraprotein level decreased to 0.3 g/dL. The calcium level was 9.8 mg/dL, and the hemoglobin level was 11.7 g/dL. The patient’s tongue pain resolved, allowing for improved oral intake and a 5.7-kg weight gain. The patient underwent nephrolithotomy 4 months after her initial presentation. She resumed an active lifestyle and recently traveled to visit relatives in the Philippines.

DISCUSSION
Oral diseases affect general health and quality of life and can be a harbinger of systemic disease. Tooth loss, caries, periodontal disease, and poorly fitting dentures commonly affect speech and nutrition.2 These common outpatient oral health issues can be the driving force for hospital admissions; for example, caries and periodontal disease can lead to suppurative odontogenic infection, endocarditis, brain abscess, and sepsis.
Tongue ulcerations, masses, and surface abnormalities often require consultation with a dentist or oral and maxillofacial surgeon to exclude squamous cell carcinoma.3 Other diagnostic considerations include benign neoplasms, trauma, inflammatory conditions (eg, sarcoidosis), infection (eg, syphilis, tuberculosis), and infiltrative processes such as amyloidosis.
Amyloidosis is a heterogeneous group of diseases caused by deposition of insoluble protein fibrils in tissues.4,5 The three most encountered forms of amyloidosis are AL, AA, and ATTR. Each form is named after the culprit protein.4 AL amyloidosis arises when a small clonal population of plasma cells in the bone marrow overproduces immunoglobulin light chain monomers.4,6 AA amyloidosis develops when the liver produces serum amyloid A protein (an acute phase reactant) in response to a chronic inflammatory condition such as rheumatoid arthritis or chronic intravenous drug injection.4 Transthyretin (TTR, also known as “prealbumin”) is a tetrameric protein that transports thyroxine and retinol; there are two forms of ATTR amyloidosis: hereditary and wild type. Hereditary ATTR amyloidosis develops from agglomeration of misfolded TTR monomers caused by mutations in the TTR gene. Wild-type ATTR amyloidosis is caused by age-related dissociation of the TTR tetramer into its constituent monomers that denature, misfold, and agglomerate into fibrils.5 Wild-type ATTR is now recognized as the most common form of amyloidosis, with 25% of myocardial autopsy specimens of patients 80 years or older demonstrating amyloid.7 The estimated incidence of AL amyloidosis is 10 cases per million person-years.8
Each amyloid protein homes in on specific anatomic sites.4 Characteristic combinations of organ dysfunction can suggest different forms of amyloidosis.9 Cardiac and peripheral nervous involvement (eg, carpal tunnel syndrome) is typical of both hereditary and wild-type ATTR amyloidosis; ATTR amyloidosis does not involve the kidney.4 AA amyloidosis most commonly manifests with proteinuria followed by declining glomerular filtration rate; heart failure is rare.4 The most common findings in AL amyloidosis are proteinuria, congestive heart failure, and sensory neuropathy.6 Gastrointestinal tract and hepatic involvement are each seen in nearly 20% of patients, and macroglossia is identified in approximately 10% of those with AL amyloidosis.6,10
Chronic deposition of amyloid can lead to acute presentations. Approximately 30% of patients with AL amyloidosis develop abnormal bleeding.11 Amyloid deposition in small blood vessels predisposes them to rupture. Bleeding events can be exacerbated by acquired coagulopathy due to plasma cell dyscrasia−associated thrombocytopenia, amyloid fibril adsorption of factor X, or hypofibrinogenemia.11,12 Periorbital purpura following minor trauma or transient venous hypertension is characteristic of AL amyloidosis.6,13 In this case, positive pressure ventilation and recumbent positioning increased hydrostatic pressure in the head and neck, causing rupture of the infiltrated small vessels around the eyes and in the oral cavity.14
Histological demonstration of tissue deposition of amyloid protein is the preferred method for amyloidosis diagnosis. Symptomatic sites or organs with dysfunction or radiologic changes are suitable for biopsy.6 If those sites are inaccessible or yield insufficient tissue quantity, abdominal fat pad aspiration or biopsy is indicated.15 Apple-green birefringence under polarized light of Congo red–stained tissue is characteristic, with sensitivity and specificity of approximately 80% and a positive predictive value of 85%.15 Immunoelectron microscopy is often performed simultaneously to confirm the diagnosis and determine the amyloid protein type.4,16 Immunoelectron microscopy’s sensitivity is approximately 80%, and it has specificity and positive predictive value both approaching 100%.15 Mass spectrometry is particularly useful in cases where the amyloid subtype is not clinically apparent (eg, a patient with an autoimmune condition or chronic infection as well as light chain abnormality).6 Cardiac MRI findings that suggest amyloidosis include a thickened left ventricle and late gadolinium enhancement.1 ATTR cardiac amyloidosis can be diagnosed using amyloid fibril–binding radiotracer technetium-99m-pyrophosphate scintigraphy; biopsy is often not necessary.1,4 Gene sequencing to differentiate between hereditary and wild-type forms of ATTR amyloidosis is beneficial.
The primary objectives of amyloidosis management are to control symptoms and inhibit amyloid protein production.6 Outcomes in AL amyloidosis have improved due to early diagnosis, new chemotherapeutic agents to eradicate the plasma cell clone, and autologous stem cell transplantation.6,17 Two new ATTR amyloidosis treatments are RNA interference therapies, which prevent TTR messenger RNA translation, and tafamidis, which stabilizes the TTR tetramer and prevents dissociation into its constituent monomers that precipitate in tissues.18 Both therapies can improve neuropathy-related quality of life.18 Tafamidis slows disease progression and decreases all-cause mortality in patients with hereditary and wild-type ATTR cardiac amyloidosis.19
Multiple myeloma and AL amyloidosis are both plasma cell dyscrasias involving the bone marrow, but they represent distinct disease processes with different clinical features.6 Multiple myeloma is characterized by marked expansion of a clonal plasma cell population within the bone marrow that aberrantly produces immunoglobulin. Conversely, the clonal plasma cell population responsible for producing the insoluble monoclonal light chain protein in AL amyloidosis typically constitutes less than 10% of the bone marrow.20 Multiple myeloma and AL amyloidosis may coexist in the same patient; nearly 15% of patients with multiple myeloma subsequently develop clinically overt AL amyloidosis, which portends a poor outcome.20
Amyloidosis is a rare group of diseases that arises when misfolded proteins aggregate in vital organs. The typical manifestations—congestive heart failure, neuropathy, chronic kidney disease, bleeding—are nearly always explained by more common conditions. Characteristic manifestations (like macroglossia) or associated diseases (like multiple myeloma) substantially increases the probability of AL amyloidosis. In a multisystem illness, the most common diseases must be excluded first, but this case reminds us that rare diseases, like amyloidosis, also warrant consideration as the story unfolds.
KEY TEACHING POINTS
- Different amyloid proteins precipitate in different anatomic sites, which leads to specific multiorgan combinations. The most common amyloidosis, ATTR, tends to manifest as heart failure and peripheral sensory neuropathy, while the constellation of AL amyloidosis includes heart failure, neuropathy, and proteinuria.
- Bleeding occurs in 30% of patients with AL amyloidosis. It is precipitated by fragile small blood vessels and exacerbated by acquired coagulopathy from adsorption of coagulation factors.
- Multiple myeloma and AL amyloidosis are both plasma cell dyscrasias involving the bone marrow, but they represent distinct disease processes with different clinical tempos and presentations. Multiple myeloma and AL amyloidosis may coexist in the same patient; nearly 15% of patients with multiple myeloma subsequently develop clinically overt AL amyloidosis.
Acknowledgment
The authors thank Benjamin A Derman, MD, of the University of Chicago, Chicago, Illinois, for critical review of the manuscript.
1. Witteles RM, Bokhari S, Damy T, et al. Screening for transthyretin amyloid cardiomyopathy in everyday practice. JACC Heart Fail. 2019;7(8):709-716. https://doi.org/10.1016/j.jchf.2019.04.010
2. Griffin SO, Jones JA, Brunson D, Griffin PM, Bailey WD. Burden of oral disease among older adults and implications for public health priorities. Am J Public Health. 2012;102(3):411-418. https://doi.org/10.2105/ajph.2011.300362
3. Ernster JA, Sciotto CG, O’Brien MM, et al. Rising incidence of oropharyngeal cancer and the role of oncogenic human papilloma virus. Laryngoscope. 2007;117(12):2115-2128. https://doi.org/10.1097/mlg.0b013e31813e5fbb
4. Wechalekar AD, Gillmore JD, Hawkins PN. Systemic amyloidosis. Lancet. 2016;387(10038):2641-2654. https://doi.org/10.1016/s0140-6736(15)01274-x
5. Riek R, Eisenberg DS. The activities of amyloids from a structural perspective. Nature. 2016;539(7628):227-235. https://doi.org/10.1038/nature20416
6. Gertz MA, Dispenzieri A. Systemic amyloidosis recognition, prognosis, and therapy: a systematic review. JAMA. 2020;324(1):79-89. https://doi.org/10.1001/jama.2020.5493
7. Ruberg FL, Berk JL. Transthyretin (TTR) cardiac amyloidosis. Circulation. 2012;126(10):1286-1300. https://doi.org/10.1161/circulationaha.111.078915
8. Quock TP, Yan T, Chang E, Guthrie S, Broder MS. Epidemiology of AL amyloidosis: a real-world study using US claims data. Blood Adv. 2018;2(10):1046-1053. https://doi.org/10.1182/bloodadvances.2018016402
9. Papoutsidakis N, Miller EJ, Rodonski A, Jacoby D. Time course of common clinical manifestations in patients with transthyretin cardiac amyloidosis: delay from symptom onset to diagnosis. J Card Fail. 2018;24(2):131-133. https://doi.org/10.1016/j.cardfail.2017.12.005
10. Shimazaki C, Hata H, Iida S, et al. Nationwide survey of 741 patients with systemic amyloid light-chain amyloidosis in Japan. Intern Med. 2018;57(2):181-187. https://doi.org/10.2169/internalmedicine.9206-17
11. Mumford AD, O’Donnell J, Gillmore JD, Manning RA, Hawkins PN, Laffan M. Bleeding symptoms and coagulation abnormalities in 337 patients with AL-amyloidosis. Br J Haematol. 2000;110(2):454-460. https://doi.org/10.1046/j.1365-2141.2000.02183.x
12. Choufani EB, Sanchorawala V, Ernst T, et al. Acquired factor X deficiency in patients with amyloid light-chain amyloidosis: incidence, bleeding manifestations, and response to high-dose chemotherapy. Blood. 2001;97(6):1885-1887. https://doi.org/10.1182/blood.v97.6.1885
13. Slagel GA, Lupton GP. Postproctoscopic periorbital purpura. Primary systemic amyloidosis. Arch Dermatol. 1986;122(4):464-465, 467-468.
14. Lupton GP. Pneomometry-induced purpura. Arch Dermatol. 1981;117(10):603. https://doi.org/10.1001/archderm.117.10.603a
15. Fernández de Larrea C, Verga L, Morbini P, et al. A practical approach to the diagnosis of systemic amyloidoses. Blood. 2015;125(14):2239-2244. https://doi.org/10.1182/blood-2014-11-609883
16. Vaxman I, Gertz M. Recent Advances in the diagnosis, risk stratification, and management of systemic light-chain amyloidosis. Acta Haematol. 2019;141(2):93-106. https://doi.org/10.1159/000495455
17. Muchtar E, Gertz MA, Kumar SK, et al. Improved outcomes for newly diagnosed AL amyloidosis between 2000 and 2014: cracking the glass ceiling of early death. Blood. 2017;129(15):2111-2119. https://doi.org/10.1182/blood-2016-11-751628
18. Quarta CC, Solomon SD. Stabilizing transthyretin to treat ATTR cardiomyopathy. N Engl J Med. 2018;379(11):1083-1084. https://doi.org/10.1056/nejme1810074
19. Maurer MS, Schwartz JH, Gundapaneni B, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379(11):1007-1016. https://doi.org/10.1056/nejmoa1805689
20. Bahlis NJ, Lazarus HM. Multiple myeloma-associated AL amyloidosis: is a distinctive therapeutic approach warranted? Bone Marrow Transplant. 2006;38(1):7-15. https://doi.org/10.1038/sj.bmt.1705395
1. Witteles RM, Bokhari S, Damy T, et al. Screening for transthyretin amyloid cardiomyopathy in everyday practice. JACC Heart Fail. 2019;7(8):709-716. https://doi.org/10.1016/j.jchf.2019.04.010
2. Griffin SO, Jones JA, Brunson D, Griffin PM, Bailey WD. Burden of oral disease among older adults and implications for public health priorities. Am J Public Health. 2012;102(3):411-418. https://doi.org/10.2105/ajph.2011.300362
3. Ernster JA, Sciotto CG, O’Brien MM, et al. Rising incidence of oropharyngeal cancer and the role of oncogenic human papilloma virus. Laryngoscope. 2007;117(12):2115-2128. https://doi.org/10.1097/mlg.0b013e31813e5fbb
4. Wechalekar AD, Gillmore JD, Hawkins PN. Systemic amyloidosis. Lancet. 2016;387(10038):2641-2654. https://doi.org/10.1016/s0140-6736(15)01274-x
5. Riek R, Eisenberg DS. The activities of amyloids from a structural perspective. Nature. 2016;539(7628):227-235. https://doi.org/10.1038/nature20416
6. Gertz MA, Dispenzieri A. Systemic amyloidosis recognition, prognosis, and therapy: a systematic review. JAMA. 2020;324(1):79-89. https://doi.org/10.1001/jama.2020.5493
7. Ruberg FL, Berk JL. Transthyretin (TTR) cardiac amyloidosis. Circulation. 2012;126(10):1286-1300. https://doi.org/10.1161/circulationaha.111.078915
8. Quock TP, Yan T, Chang E, Guthrie S, Broder MS. Epidemiology of AL amyloidosis: a real-world study using US claims data. Blood Adv. 2018;2(10):1046-1053. https://doi.org/10.1182/bloodadvances.2018016402
9. Papoutsidakis N, Miller EJ, Rodonski A, Jacoby D. Time course of common clinical manifestations in patients with transthyretin cardiac amyloidosis: delay from symptom onset to diagnosis. J Card Fail. 2018;24(2):131-133. https://doi.org/10.1016/j.cardfail.2017.12.005
10. Shimazaki C, Hata H, Iida S, et al. Nationwide survey of 741 patients with systemic amyloid light-chain amyloidosis in Japan. Intern Med. 2018;57(2):181-187. https://doi.org/10.2169/internalmedicine.9206-17
11. Mumford AD, O’Donnell J, Gillmore JD, Manning RA, Hawkins PN, Laffan M. Bleeding symptoms and coagulation abnormalities in 337 patients with AL-amyloidosis. Br J Haematol. 2000;110(2):454-460. https://doi.org/10.1046/j.1365-2141.2000.02183.x
12. Choufani EB, Sanchorawala V, Ernst T, et al. Acquired factor X deficiency in patients with amyloid light-chain amyloidosis: incidence, bleeding manifestations, and response to high-dose chemotherapy. Blood. 2001;97(6):1885-1887. https://doi.org/10.1182/blood.v97.6.1885
13. Slagel GA, Lupton GP. Postproctoscopic periorbital purpura. Primary systemic amyloidosis. Arch Dermatol. 1986;122(4):464-465, 467-468.
14. Lupton GP. Pneomometry-induced purpura. Arch Dermatol. 1981;117(10):603. https://doi.org/10.1001/archderm.117.10.603a
15. Fernández de Larrea C, Verga L, Morbini P, et al. A practical approach to the diagnosis of systemic amyloidoses. Blood. 2015;125(14):2239-2244. https://doi.org/10.1182/blood-2014-11-609883
16. Vaxman I, Gertz M. Recent Advances in the diagnosis, risk stratification, and management of systemic light-chain amyloidosis. Acta Haematol. 2019;141(2):93-106. https://doi.org/10.1159/000495455
17. Muchtar E, Gertz MA, Kumar SK, et al. Improved outcomes for newly diagnosed AL amyloidosis between 2000 and 2014: cracking the glass ceiling of early death. Blood. 2017;129(15):2111-2119. https://doi.org/10.1182/blood-2016-11-751628
18. Quarta CC, Solomon SD. Stabilizing transthyretin to treat ATTR cardiomyopathy. N Engl J Med. 2018;379(11):1083-1084. https://doi.org/10.1056/nejme1810074
19. Maurer MS, Schwartz JH, Gundapaneni B, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379(11):1007-1016. https://doi.org/10.1056/nejmoa1805689
20. Bahlis NJ, Lazarus HM. Multiple myeloma-associated AL amyloidosis: is a distinctive therapeutic approach warranted? Bone Marrow Transplant. 2006;38(1):7-15. https://doi.org/10.1038/sj.bmt.1705395
© 2021 Society of Hospital Medicine
Adoption of Hospitalist Care in Asia: Experiences From Singapore, Taiwan, Korea, and Japan
Adoption of Hospitalist Care in Asia: Experiences From Singapore, Taiwan, Korea, and Japan

This work is licensed under a Creative Commons Attribution 4.0 International License
Since its inception in the mid-1990s, the hospitalist model of care has enjoyed robust growth in the United States, increasing to around 20,000 providers by the end of its first decade.1,2 Since then, it has far outstripped early predictions of adoption, currently standing at more than 50,000 hospitalist providers.2 Although driven by numerous factors, including system-based management needs, provision of inpatient care for unassigned patients, and demands for improved patient safety and satisfaction, this meteoric growth has been driven largely by cost pressures particular to the US healthcare system.1,2 Nonetheless, the growing complexity of healthcare systems, substantial fiscal pressures, and increasing healthcare demands from aging populations are worldwide challenges to which countries outside North America also seek solutions. Countries that have initiated hospitalist care have localized adoption, evolving the model to meet their unique fiscal and system-based needs and patients’ expectations.
While there has been keen interest in the hospitalist model in Asia, there has not yet been widespread adoption, despite numerous data demonstrating that this model is associated with lower length of stay (LOS), as well as lower costs and improved patient safety.3,4 This article explores hospitalist care adoption experiences in Singapore, Taiwan, Korea, and Japan, focusing on stakeholder demand for hospitalist care, respective adoption, outcomes, and associated challenges to date.
SINGAPORE
Stakeholder Demand for Hospitalist Care
Historically in Singapore, family physicians provided primary care and internal medicine subspecialists provided inpatient care.5 Present-day trends, including an aging population, increasing rates of chronic diseases, and multisystem health issues, have stressed the historical model, leading to care fragmentation, long LOS (>9 days), and reduced patient satisfaction.5,6 Additionally, as 80% of hospital care is government funded, public hospitals are under pressure to reduce healthcare expenditures.5
Adoption of Hospitalist Care, Outcomes, and Challenges Faced
To meet patient needs and healthcare system challenges, the hospitalist model has evolved through several iterations in Singapore. The first model, implemented at Singapore General Hospital, utilized family physicians as hospitalists to coordinate inpatient care and integrate care between hospital and community settings.3,5 This model resulted in shorter LOS and reduced costs for patients cared for by family physician hospitalists.3 Despite these benefits, the family physician hospitalist model did not spread, partly due to biases favoring subspecialist care for hospitalized patients.7
The next iteration utilized general internal medicine (GIM) specialists. Traditionally, GIM specialists cared for a small number of low-acuity hospitalized patients. Recognizing the emerging need for holistic inpatient care, the Singapore Ministry of Health supported advances in generalist care, including a financial bonus and a revamped GIM training program. This spawned hospitalist-type models nationwide. At the National University Hospital (NUH), for example, GIM physicians were recruited to care for “specialty” patients in the acute medical unit and increase their ward coverage to include complex multimorbid patients. Additionally, NUH launched the enhanced complex care program, providing integrated inpatient and outpatient care to high-utilizing, complex patients. Overall, the NUH GIM division grew by 70% (faculty) and 60% (trainees) over 5 years. Currently, fueled by government enthusiasm for generalist care, hospitalist-type models are evident at newly minted hospitals across Singapore.
Although physicians act as hospitalists, the term hospitalist is not embraced in Singapore, thus limiting its potential to develop clinical- and system-improvement competencies and establish professional identity. This may be due to the strong UK-based cultural foundations and continued systemic bias favoring subspecialists.8
TAIWAN
Stakeholder Demand for Hospitalist Care
Under its national health insurance (NHI) system, Taiwan has relatively low copayments for medical services, with acute patients paying 10% of costs for a ≤30-day hospitalization, causing demand for inpatient care to remain strong.4,9 The NHI system has also led to increased numbers of patients accessing care in emergency departments (EDs), where costs may be as low as US $16 (NT $450), causing long waits for evaluation and transfer to wards.9,10 There remains an insufficient number of hospital-based physicians to manage this high patient volume, a situation exacerbated by low reimbursements.4
Adoption of Hospitalist Care, Outcomes, and Challenges Faced
In order to address rising admissions, inefficient ED management, and physician shortages, a hospitalist care program was first introduced in Taiwan in 2002, followed by the establishment of a hospitalist-run ward in National Taiwan University Hospital in 2009.11 Subsequent studies from Taiwan have found that hospitalist-run wards had lower admission costs, shorter LOS, and more do-not-resuscitate consent, and also had similar in-hospital mortality and readmission rates compared to specialist-run wards.4,12 Reflecting these successes, the Taiwan Association of Hospital Medicine (TAHM) was established in 2018, and since January 2021, the Ministry of Health and Welfare of Taiwan has mandated hospital medicine programs as an accreditation requirement for all medical centers, with a dual role of educating residents and providing inpatient care.
Despite growing opportunities, Taiwan has seen a modest increase in the number of hospitalists, rising from three in 2009 to around 300 by January 2021. An indistinct professional identity and career path are the main barriers. Given this, TAHM is trying to strengthen hospitalist professionalism by introducing both hard and soft skills, such as utilizing point-of-care ultrasonography and implementing the concepts of Choosing Wisely® and shared decision-making.
KOREA
Stakeholder Demand for Hospitalist Care
Korea has experienced a chronic physician shortage, with just 2.4 physicians per 1,000 people (World Bank, 2017), leading to significant physician burnout. Designed to protect trainee well-being, the 2015 Improvement of Training Conditions and Status of Medical Residents Act limited resident work hours while reducing internal medicine and general surgery training periods, further exacerbating physician shortages.13 In addition, Korea’s current NHI system—including its’ healthcare insurance reimbursements scheme, established in 1989 when Korea’s per capita gross domestic product was less than US $5,000—provides low reimbursements to healthcare providers.14 Along with increased attention to patient safety and healthcare-related consumer expectations, the hospitalist system in Korea aims to maintain improvements to residents’ well-being, while increasing hospital revenue and meeting patient demand for improved services.14
Adoption of Hospitalist Care, Outcomes, and Challenges Faced
Along with the Ministry of Health and Welfare, the Korean Health Insurance Review and Assessment Service launched a hospitalist pilot program in general medicine and surgery in 2016.15 Services for hospitalist-managed inpatients are charged on a new schedule covered by the NHI system, including facility fees, which are charged per diem, and separate hospitalist fees.14 New hospital medicine programs are utilized, in part, to recruit new physicians to manage a large volume of inpatients. Previous studies found that these new hospitalist care systems also improved patient safety, quality of care, and overall patient satisfaction, while being associated with shorter LOS and fewer unnecessary intensive care unit admissions.16,17 After a successful pilot, the revamped reimbursement system for hospitalist care officially started in January 2021.
Although Korea had only 250 registered hospitalists by August 2020, this is likely a substantial underestimate, as only hospital medicine teams with more than two hospitalists were allowed formal registration during the pilot period. Wider registration is currently underway for the new official reimbursement system.
JAPAN
Stakeholder Demand for Hospitalist Care
Hospitals in Japan are organized into highly compartmentalized subspecialties. Providing quality inpatient care to senior patients, who account for more than 28% of the population, and managing smooth transitions from hospital to long-term- care facilities remain challenging. In addition, given generous caps on maximum monthly out-of-pocket payments under its NHI system, LOS for Japanese hospitals are as long as 16.1 days.18 Nonetheless, given rising financial burdens associated with long-term care, hospitals are under government pressure to further shorten LOS and transition patients to local long-term-care facilities after treatment for acute symptoms.
Adoption of Hospitalist Care, Outcomes, and Challenges Faced
To meet these challenges, an increasing number of Japanese hospitals have established departments of general medicine to triage and manage patients with multiple comorbidities and to coordinate patient care across relevant specialties. The Japanese Society of Hospital General Medicine (JSHGM) was established in 2010, and currently has 1,890 members from 896 medical institutions. In 2018, general medicine was recognized by the Japanese Board of Medical Specialties as a formal specialty for certification. Currently, JSHGM is working with the Japan Primary Care Association and other organizations to establish a specialty certification system for hospitalist physicians and raise awareness of hospital medicine. A Japanese study of elderly patients with chronic aspiration pneumonia found that care by hospitalists resulted in shorter LOS and lower costs than specialist care.19 Recently, hospitalists have played a central role in COVID-19 management, opening fever intake clinics and establishing collaborative guidelines with infectious disease experts and other specialists.
Yet, different from the prototypical hospitalist first defined by Wachter and Goldman, Japanese general medicine hospitalists continue to have substantial outpatient responsibilities, albeit in the hospital setting. Out of 81 university hospitals, 69 now have a department of hospital general medicine, though only 20 have inpatient services.20 In addition, a medical culture in which patients continue to see their surgery attendings long after surgery remains strong. Clear definitions regarding hospitalists’ roles need to be established, while promoting changes toward inpatient care for both patients and subspecialists.
DISCUSSION
The four Asian countries reviewed here have all established universal access to healthcare, with Taiwan, Korea, and Japan having strong NHI systems and Singapore providing significant healthcare subsidies for those in need. Nonetheless, they also face similar challenges, including the growing complexity of healthcare systems, substantial fiscal pressures, increased healthcare demands caused by aging populations, and increased expectations regarding stakeholder well-being. As such, these countries share common driving forces that are propelling the adoption of hospitalist care models, such as lack of a sufficient physician workforce on inpatient wards; need for extra resources to shorten ED wait times prior to inpatient admission; need for providing quality care to multimorbid senior patients across highly segmented hospital departments and coordinating medical services between hospitals and outpatient care facilities; and government pressure on cutting costs, especially by shortening inpatient LOS. Some common barriers among these Asian countries include unclear definitions of hospitalists’ roles and degree of collaboration with subspecialty departments, and social and systemic biases favoring subspecialty care for inpatients.
The four Asian countries reviewed here have chosen to adopt the hospitalist model as a supplement to already established, specialty-driven inpatient care systems; as such, further comparative outcome studies focusing on cost, care quality, and patient safety and satisfaction are warranted to bolster professional hospitalist roles, further facilitate government/policy-level support for hospital care systems, and promote future training and certification systems appropriate to each country’s unique healthcare system and medical culture. Similarly, evidence-driven educational outreach programs are warranted to facilitate patient understanding of the role of hospitalists in their care.For countries interested in establishing hospital medicine programs, the adoption experiences in Singapore, Taiwan, Korea, and Japan provide valuable insights regarding how to establish hospitalist models to meet country-specific healthcare challenges while successfully functioning in the context of their unique medical-system frameworks.
1. Wachter RM, Goldman L. The hospitalist movement 5 years later. JAMA. 2002;287:487-494. https://doi.org/10.1001/jama.287.4.487
2. Wachter RM, Goldman L. Zero to 50,000—the 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958
3. Lee KH, Yang Y, Yang KS, et al. Bringing generalists into the hospital: outcomes of a family medicine hospitalist model in Singapore. J Hosp Med. 2011;6:115-121. https://doi.org/10.1002/jhm.821
4. Shu CC, Lin JW, Lin YF, et al. Evaluating the performance of a hospitalist system in Taiwan: a pioneer study for nationwide health insurance in Asia. J Hosp Med. 2011;6(7):378-382. https://doi.org/ 10.1002/jhm.896
5. Lee KH. The hospitalist movement—a complex adaptive response to fragmentation of care in hospitals. Ann Acad Med Singap. 2008;37(2):145-150.
6. Ge L, Ya CW, Heng BH, Tan WS. Frailty and healthcare utilization across care settings among community-dwelling older adults in Singapore. BMC Geriatrics. 2020;20:389. https://doi.org/10.1186/s12877-020-01800-8
7. Lee KH. A historical perspective of the barriers to generalism. Aust Fam Physician. 2015;44(3):154-158.
8. Choo F. Alexandra Hospital provides patients with one-stop services under new care model. Updated December 14, 2018. Accessed March 26, 2021.https://www.straitstimes.com/singapore/health/alexandra-hospital-provides-patients-with-one-stop-services-under-new-care-model
9. National Health Insurance Administration, Ministry of Health and Welfare, Taiwan. Medical services. Copayments. Updated December 28, 2020. Accessed March 26, 2021. https://www.nhi.gov.tw/English/Content_List.aspx?n=E5509C8FE29950EA&topn=1D1ECC54F86E9050
10. Tsai JCH, Chen WY, Liang YW. Nonemergent emergency department visits under the National Health Insurance in Taiwan. Health Policy. 2011;100:189-195. https://doi.org/10.1016/j.healthpol.2010.10.007
11. Taiwan Society of Hospital Medicine. The birth and growth of hospital medicine in Taiwan. Accessed March 26, 2021. https://www.hospitalist.org.tw/about_25.htm
12. Hsu NC, Huang CC, Shu CC, Yang MC. Implementation of a seven-day hospitalist program to improve the outcomes of the weekend admission: a retrospective before-after study in Taiwan. PLoS One. 2018;13(3):e0194833. https://doi.org/10.1371/journal.pone.0194833
13. Ministry of Health and Welfare, Statutes of the Republic of Korea. Act on the Improvement of Training Conditions and Status of Medical Residents. Accessed March 26, 2021. https://elaw.klri.re.kr/eng_mobile/viewer.do?hseq=49563&type=sogan&key=10
14. Chae W, Park EC, Lee KY, et al. Development and evolution of hospital medicine in Korea. J Hosp Med. 2021;16(4):247-250. https://doi.org/10.12788/jhm.3573
15. Oh SJ, Jung EJ. Prospects for the Korean model of the surgical hospitalist system. J Korean Med Assoc. 2020;63(5):236-239. https://doi.org/10.5124/jkma.2020.63.5.236
16. Ohn JH, Kim NH, Kim ES, et al. An acute medical unit in a Korean tertiary care hospital reduces the length of stay and waiting time in the emergency department. J Korean Med Sci. 2017;32:1917-1920. https://doi.org/10.3346/jkms.2017.32.12.1917
17. Lee JH, Kim AJ, Kyong TY, et al. Evaluating the outcome of multi-morbid patients cared for by hospitalists: a report of integrated medical model in Korea. J Korean Med Sci. 2019;34(25):e179. https://doi.org/10.3346/jkms.2019.34.e179
18. OECD. Length of hospital stay. Accessed March 26, 2021. https://doi.org/10.1787/8dda6b7a-en
19. Hamada O, Tsutsumi T, Miki A, et al. Impact of the hospitalist system in Japan on the quality of care and healthcare economics. Intern Med. 2019;58(23):3385-3391. https://doi.org/10.2169/internalmedicine.2872-19
20. Kawashima A. Report on general medicine’s effects on specialists and other healthcare staff in the context of inclusive local medical system. Chapter in Japanese. Accessed March 26, 2021.https://soshin.pcmed-tsukuba.jp/education/report/pdf/05_004.pdf
Since its inception in the mid-1990s, the hospitalist model of care has enjoyed robust growth in the United States, increasing to around 20,000 providers by the end of its first decade.1,2 Since then, it has far outstripped early predictions of adoption, currently standing at more than 50,000 hospitalist providers.2 Although driven by numerous factors, including system-based management needs, provision of inpatient care for unassigned patients, and demands for improved patient safety and satisfaction, this meteoric growth has been driven largely by cost pressures particular to the US healthcare system.1,2 Nonetheless, the growing complexity of healthcare systems, substantial fiscal pressures, and increasing healthcare demands from aging populations are worldwide challenges to which countries outside North America also seek solutions. Countries that have initiated hospitalist care have localized adoption, evolving the model to meet their unique fiscal and system-based needs and patients’ expectations.
While there has been keen interest in the hospitalist model in Asia, there has not yet been widespread adoption, despite numerous data demonstrating that this model is associated with lower length of stay (LOS), as well as lower costs and improved patient safety.3,4 This article explores hospitalist care adoption experiences in Singapore, Taiwan, Korea, and Japan, focusing on stakeholder demand for hospitalist care, respective adoption, outcomes, and associated challenges to date.
SINGAPORE
Stakeholder Demand for Hospitalist Care
Historically in Singapore, family physicians provided primary care and internal medicine subspecialists provided inpatient care.5 Present-day trends, including an aging population, increasing rates of chronic diseases, and multisystem health issues, have stressed the historical model, leading to care fragmentation, long LOS (>9 days), and reduced patient satisfaction.5,6 Additionally, as 80% of hospital care is government funded, public hospitals are under pressure to reduce healthcare expenditures.5
Adoption of Hospitalist Care, Outcomes, and Challenges Faced
To meet patient needs and healthcare system challenges, the hospitalist model has evolved through several iterations in Singapore. The first model, implemented at Singapore General Hospital, utilized family physicians as hospitalists to coordinate inpatient care and integrate care between hospital and community settings.3,5 This model resulted in shorter LOS and reduced costs for patients cared for by family physician hospitalists.3 Despite these benefits, the family physician hospitalist model did not spread, partly due to biases favoring subspecialist care for hospitalized patients.7
The next iteration utilized general internal medicine (GIM) specialists. Traditionally, GIM specialists cared for a small number of low-acuity hospitalized patients. Recognizing the emerging need for holistic inpatient care, the Singapore Ministry of Health supported advances in generalist care, including a financial bonus and a revamped GIM training program. This spawned hospitalist-type models nationwide. At the National University Hospital (NUH), for example, GIM physicians were recruited to care for “specialty” patients in the acute medical unit and increase their ward coverage to include complex multimorbid patients. Additionally, NUH launched the enhanced complex care program, providing integrated inpatient and outpatient care to high-utilizing, complex patients. Overall, the NUH GIM division grew by 70% (faculty) and 60% (trainees) over 5 years. Currently, fueled by government enthusiasm for generalist care, hospitalist-type models are evident at newly minted hospitals across Singapore.
Although physicians act as hospitalists, the term hospitalist is not embraced in Singapore, thus limiting its potential to develop clinical- and system-improvement competencies and establish professional identity. This may be due to the strong UK-based cultural foundations and continued systemic bias favoring subspecialists.8
TAIWAN
Stakeholder Demand for Hospitalist Care
Under its national health insurance (NHI) system, Taiwan has relatively low copayments for medical services, with acute patients paying 10% of costs for a ≤30-day hospitalization, causing demand for inpatient care to remain strong.4,9 The NHI system has also led to increased numbers of patients accessing care in emergency departments (EDs), where costs may be as low as US $16 (NT $450), causing long waits for evaluation and transfer to wards.9,10 There remains an insufficient number of hospital-based physicians to manage this high patient volume, a situation exacerbated by low reimbursements.4
Adoption of Hospitalist Care, Outcomes, and Challenges Faced
In order to address rising admissions, inefficient ED management, and physician shortages, a hospitalist care program was first introduced in Taiwan in 2002, followed by the establishment of a hospitalist-run ward in National Taiwan University Hospital in 2009.11 Subsequent studies from Taiwan have found that hospitalist-run wards had lower admission costs, shorter LOS, and more do-not-resuscitate consent, and also had similar in-hospital mortality and readmission rates compared to specialist-run wards.4,12 Reflecting these successes, the Taiwan Association of Hospital Medicine (TAHM) was established in 2018, and since January 2021, the Ministry of Health and Welfare of Taiwan has mandated hospital medicine programs as an accreditation requirement for all medical centers, with a dual role of educating residents and providing inpatient care.
Despite growing opportunities, Taiwan has seen a modest increase in the number of hospitalists, rising from three in 2009 to around 300 by January 2021. An indistinct professional identity and career path are the main barriers. Given this, TAHM is trying to strengthen hospitalist professionalism by introducing both hard and soft skills, such as utilizing point-of-care ultrasonography and implementing the concepts of Choosing Wisely® and shared decision-making.
KOREA
Stakeholder Demand for Hospitalist Care
Korea has experienced a chronic physician shortage, with just 2.4 physicians per 1,000 people (World Bank, 2017), leading to significant physician burnout. Designed to protect trainee well-being, the 2015 Improvement of Training Conditions and Status of Medical Residents Act limited resident work hours while reducing internal medicine and general surgery training periods, further exacerbating physician shortages.13 In addition, Korea’s current NHI system—including its’ healthcare insurance reimbursements scheme, established in 1989 when Korea’s per capita gross domestic product was less than US $5,000—provides low reimbursements to healthcare providers.14 Along with increased attention to patient safety and healthcare-related consumer expectations, the hospitalist system in Korea aims to maintain improvements to residents’ well-being, while increasing hospital revenue and meeting patient demand for improved services.14
Adoption of Hospitalist Care, Outcomes, and Challenges Faced
Along with the Ministry of Health and Welfare, the Korean Health Insurance Review and Assessment Service launched a hospitalist pilot program in general medicine and surgery in 2016.15 Services for hospitalist-managed inpatients are charged on a new schedule covered by the NHI system, including facility fees, which are charged per diem, and separate hospitalist fees.14 New hospital medicine programs are utilized, in part, to recruit new physicians to manage a large volume of inpatients. Previous studies found that these new hospitalist care systems also improved patient safety, quality of care, and overall patient satisfaction, while being associated with shorter LOS and fewer unnecessary intensive care unit admissions.16,17 After a successful pilot, the revamped reimbursement system for hospitalist care officially started in January 2021.
Although Korea had only 250 registered hospitalists by August 2020, this is likely a substantial underestimate, as only hospital medicine teams with more than two hospitalists were allowed formal registration during the pilot period. Wider registration is currently underway for the new official reimbursement system.
JAPAN
Stakeholder Demand for Hospitalist Care
Hospitals in Japan are organized into highly compartmentalized subspecialties. Providing quality inpatient care to senior patients, who account for more than 28% of the population, and managing smooth transitions from hospital to long-term- care facilities remain challenging. In addition, given generous caps on maximum monthly out-of-pocket payments under its NHI system, LOS for Japanese hospitals are as long as 16.1 days.18 Nonetheless, given rising financial burdens associated with long-term care, hospitals are under government pressure to further shorten LOS and transition patients to local long-term-care facilities after treatment for acute symptoms.
Adoption of Hospitalist Care, Outcomes, and Challenges Faced
To meet these challenges, an increasing number of Japanese hospitals have established departments of general medicine to triage and manage patients with multiple comorbidities and to coordinate patient care across relevant specialties. The Japanese Society of Hospital General Medicine (JSHGM) was established in 2010, and currently has 1,890 members from 896 medical institutions. In 2018, general medicine was recognized by the Japanese Board of Medical Specialties as a formal specialty for certification. Currently, JSHGM is working with the Japan Primary Care Association and other organizations to establish a specialty certification system for hospitalist physicians and raise awareness of hospital medicine. A Japanese study of elderly patients with chronic aspiration pneumonia found that care by hospitalists resulted in shorter LOS and lower costs than specialist care.19 Recently, hospitalists have played a central role in COVID-19 management, opening fever intake clinics and establishing collaborative guidelines with infectious disease experts and other specialists.
Yet, different from the prototypical hospitalist first defined by Wachter and Goldman, Japanese general medicine hospitalists continue to have substantial outpatient responsibilities, albeit in the hospital setting. Out of 81 university hospitals, 69 now have a department of hospital general medicine, though only 20 have inpatient services.20 In addition, a medical culture in which patients continue to see their surgery attendings long after surgery remains strong. Clear definitions regarding hospitalists’ roles need to be established, while promoting changes toward inpatient care for both patients and subspecialists.
DISCUSSION
The four Asian countries reviewed here have all established universal access to healthcare, with Taiwan, Korea, and Japan having strong NHI systems and Singapore providing significant healthcare subsidies for those in need. Nonetheless, they also face similar challenges, including the growing complexity of healthcare systems, substantial fiscal pressures, increased healthcare demands caused by aging populations, and increased expectations regarding stakeholder well-being. As such, these countries share common driving forces that are propelling the adoption of hospitalist care models, such as lack of a sufficient physician workforce on inpatient wards; need for extra resources to shorten ED wait times prior to inpatient admission; need for providing quality care to multimorbid senior patients across highly segmented hospital departments and coordinating medical services between hospitals and outpatient care facilities; and government pressure on cutting costs, especially by shortening inpatient LOS. Some common barriers among these Asian countries include unclear definitions of hospitalists’ roles and degree of collaboration with subspecialty departments, and social and systemic biases favoring subspecialty care for inpatients.
The four Asian countries reviewed here have chosen to adopt the hospitalist model as a supplement to already established, specialty-driven inpatient care systems; as such, further comparative outcome studies focusing on cost, care quality, and patient safety and satisfaction are warranted to bolster professional hospitalist roles, further facilitate government/policy-level support for hospital care systems, and promote future training and certification systems appropriate to each country’s unique healthcare system and medical culture. Similarly, evidence-driven educational outreach programs are warranted to facilitate patient understanding of the role of hospitalists in their care.For countries interested in establishing hospital medicine programs, the adoption experiences in Singapore, Taiwan, Korea, and Japan provide valuable insights regarding how to establish hospitalist models to meet country-specific healthcare challenges while successfully functioning in the context of their unique medical-system frameworks.
Since its inception in the mid-1990s, the hospitalist model of care has enjoyed robust growth in the United States, increasing to around 20,000 providers by the end of its first decade.1,2 Since then, it has far outstripped early predictions of adoption, currently standing at more than 50,000 hospitalist providers.2 Although driven by numerous factors, including system-based management needs, provision of inpatient care for unassigned patients, and demands for improved patient safety and satisfaction, this meteoric growth has been driven largely by cost pressures particular to the US healthcare system.1,2 Nonetheless, the growing complexity of healthcare systems, substantial fiscal pressures, and increasing healthcare demands from aging populations are worldwide challenges to which countries outside North America also seek solutions. Countries that have initiated hospitalist care have localized adoption, evolving the model to meet their unique fiscal and system-based needs and patients’ expectations.
While there has been keen interest in the hospitalist model in Asia, there has not yet been widespread adoption, despite numerous data demonstrating that this model is associated with lower length of stay (LOS), as well as lower costs and improved patient safety.3,4 This article explores hospitalist care adoption experiences in Singapore, Taiwan, Korea, and Japan, focusing on stakeholder demand for hospitalist care, respective adoption, outcomes, and associated challenges to date.
SINGAPORE
Stakeholder Demand for Hospitalist Care
Historically in Singapore, family physicians provided primary care and internal medicine subspecialists provided inpatient care.5 Present-day trends, including an aging population, increasing rates of chronic diseases, and multisystem health issues, have stressed the historical model, leading to care fragmentation, long LOS (>9 days), and reduced patient satisfaction.5,6 Additionally, as 80% of hospital care is government funded, public hospitals are under pressure to reduce healthcare expenditures.5
Adoption of Hospitalist Care, Outcomes, and Challenges Faced
To meet patient needs and healthcare system challenges, the hospitalist model has evolved through several iterations in Singapore. The first model, implemented at Singapore General Hospital, utilized family physicians as hospitalists to coordinate inpatient care and integrate care between hospital and community settings.3,5 This model resulted in shorter LOS and reduced costs for patients cared for by family physician hospitalists.3 Despite these benefits, the family physician hospitalist model did not spread, partly due to biases favoring subspecialist care for hospitalized patients.7
The next iteration utilized general internal medicine (GIM) specialists. Traditionally, GIM specialists cared for a small number of low-acuity hospitalized patients. Recognizing the emerging need for holistic inpatient care, the Singapore Ministry of Health supported advances in generalist care, including a financial bonus and a revamped GIM training program. This spawned hospitalist-type models nationwide. At the National University Hospital (NUH), for example, GIM physicians were recruited to care for “specialty” patients in the acute medical unit and increase their ward coverage to include complex multimorbid patients. Additionally, NUH launched the enhanced complex care program, providing integrated inpatient and outpatient care to high-utilizing, complex patients. Overall, the NUH GIM division grew by 70% (faculty) and 60% (trainees) over 5 years. Currently, fueled by government enthusiasm for generalist care, hospitalist-type models are evident at newly minted hospitals across Singapore.
Although physicians act as hospitalists, the term hospitalist is not embraced in Singapore, thus limiting its potential to develop clinical- and system-improvement competencies and establish professional identity. This may be due to the strong UK-based cultural foundations and continued systemic bias favoring subspecialists.8
TAIWAN
Stakeholder Demand for Hospitalist Care
Under its national health insurance (NHI) system, Taiwan has relatively low copayments for medical services, with acute patients paying 10% of costs for a ≤30-day hospitalization, causing demand for inpatient care to remain strong.4,9 The NHI system has also led to increased numbers of patients accessing care in emergency departments (EDs), where costs may be as low as US $16 (NT $450), causing long waits for evaluation and transfer to wards.9,10 There remains an insufficient number of hospital-based physicians to manage this high patient volume, a situation exacerbated by low reimbursements.4
Adoption of Hospitalist Care, Outcomes, and Challenges Faced
In order to address rising admissions, inefficient ED management, and physician shortages, a hospitalist care program was first introduced in Taiwan in 2002, followed by the establishment of a hospitalist-run ward in National Taiwan University Hospital in 2009.11 Subsequent studies from Taiwan have found that hospitalist-run wards had lower admission costs, shorter LOS, and more do-not-resuscitate consent, and also had similar in-hospital mortality and readmission rates compared to specialist-run wards.4,12 Reflecting these successes, the Taiwan Association of Hospital Medicine (TAHM) was established in 2018, and since January 2021, the Ministry of Health and Welfare of Taiwan has mandated hospital medicine programs as an accreditation requirement for all medical centers, with a dual role of educating residents and providing inpatient care.
Despite growing opportunities, Taiwan has seen a modest increase in the number of hospitalists, rising from three in 2009 to around 300 by January 2021. An indistinct professional identity and career path are the main barriers. Given this, TAHM is trying to strengthen hospitalist professionalism by introducing both hard and soft skills, such as utilizing point-of-care ultrasonography and implementing the concepts of Choosing Wisely® and shared decision-making.
KOREA
Stakeholder Demand for Hospitalist Care
Korea has experienced a chronic physician shortage, with just 2.4 physicians per 1,000 people (World Bank, 2017), leading to significant physician burnout. Designed to protect trainee well-being, the 2015 Improvement of Training Conditions and Status of Medical Residents Act limited resident work hours while reducing internal medicine and general surgery training periods, further exacerbating physician shortages.13 In addition, Korea’s current NHI system—including its’ healthcare insurance reimbursements scheme, established in 1989 when Korea’s per capita gross domestic product was less than US $5,000—provides low reimbursements to healthcare providers.14 Along with increased attention to patient safety and healthcare-related consumer expectations, the hospitalist system in Korea aims to maintain improvements to residents’ well-being, while increasing hospital revenue and meeting patient demand for improved services.14
Adoption of Hospitalist Care, Outcomes, and Challenges Faced
Along with the Ministry of Health and Welfare, the Korean Health Insurance Review and Assessment Service launched a hospitalist pilot program in general medicine and surgery in 2016.15 Services for hospitalist-managed inpatients are charged on a new schedule covered by the NHI system, including facility fees, which are charged per diem, and separate hospitalist fees.14 New hospital medicine programs are utilized, in part, to recruit new physicians to manage a large volume of inpatients. Previous studies found that these new hospitalist care systems also improved patient safety, quality of care, and overall patient satisfaction, while being associated with shorter LOS and fewer unnecessary intensive care unit admissions.16,17 After a successful pilot, the revamped reimbursement system for hospitalist care officially started in January 2021.
Although Korea had only 250 registered hospitalists by August 2020, this is likely a substantial underestimate, as only hospital medicine teams with more than two hospitalists were allowed formal registration during the pilot period. Wider registration is currently underway for the new official reimbursement system.
JAPAN
Stakeholder Demand for Hospitalist Care
Hospitals in Japan are organized into highly compartmentalized subspecialties. Providing quality inpatient care to senior patients, who account for more than 28% of the population, and managing smooth transitions from hospital to long-term- care facilities remain challenging. In addition, given generous caps on maximum monthly out-of-pocket payments under its NHI system, LOS for Japanese hospitals are as long as 16.1 days.18 Nonetheless, given rising financial burdens associated with long-term care, hospitals are under government pressure to further shorten LOS and transition patients to local long-term-care facilities after treatment for acute symptoms.
Adoption of Hospitalist Care, Outcomes, and Challenges Faced
To meet these challenges, an increasing number of Japanese hospitals have established departments of general medicine to triage and manage patients with multiple comorbidities and to coordinate patient care across relevant specialties. The Japanese Society of Hospital General Medicine (JSHGM) was established in 2010, and currently has 1,890 members from 896 medical institutions. In 2018, general medicine was recognized by the Japanese Board of Medical Specialties as a formal specialty for certification. Currently, JSHGM is working with the Japan Primary Care Association and other organizations to establish a specialty certification system for hospitalist physicians and raise awareness of hospital medicine. A Japanese study of elderly patients with chronic aspiration pneumonia found that care by hospitalists resulted in shorter LOS and lower costs than specialist care.19 Recently, hospitalists have played a central role in COVID-19 management, opening fever intake clinics and establishing collaborative guidelines with infectious disease experts and other specialists.
Yet, different from the prototypical hospitalist first defined by Wachter and Goldman, Japanese general medicine hospitalists continue to have substantial outpatient responsibilities, albeit in the hospital setting. Out of 81 university hospitals, 69 now have a department of hospital general medicine, though only 20 have inpatient services.20 In addition, a medical culture in which patients continue to see their surgery attendings long after surgery remains strong. Clear definitions regarding hospitalists’ roles need to be established, while promoting changes toward inpatient care for both patients and subspecialists.
DISCUSSION
The four Asian countries reviewed here have all established universal access to healthcare, with Taiwan, Korea, and Japan having strong NHI systems and Singapore providing significant healthcare subsidies for those in need. Nonetheless, they also face similar challenges, including the growing complexity of healthcare systems, substantial fiscal pressures, increased healthcare demands caused by aging populations, and increased expectations regarding stakeholder well-being. As such, these countries share common driving forces that are propelling the adoption of hospitalist care models, such as lack of a sufficient physician workforce on inpatient wards; need for extra resources to shorten ED wait times prior to inpatient admission; need for providing quality care to multimorbid senior patients across highly segmented hospital departments and coordinating medical services between hospitals and outpatient care facilities; and government pressure on cutting costs, especially by shortening inpatient LOS. Some common barriers among these Asian countries include unclear definitions of hospitalists’ roles and degree of collaboration with subspecialty departments, and social and systemic biases favoring subspecialty care for inpatients.
The four Asian countries reviewed here have chosen to adopt the hospitalist model as a supplement to already established, specialty-driven inpatient care systems; as such, further comparative outcome studies focusing on cost, care quality, and patient safety and satisfaction are warranted to bolster professional hospitalist roles, further facilitate government/policy-level support for hospital care systems, and promote future training and certification systems appropriate to each country’s unique healthcare system and medical culture. Similarly, evidence-driven educational outreach programs are warranted to facilitate patient understanding of the role of hospitalists in their care.For countries interested in establishing hospital medicine programs, the adoption experiences in Singapore, Taiwan, Korea, and Japan provide valuable insights regarding how to establish hospitalist models to meet country-specific healthcare challenges while successfully functioning in the context of their unique medical-system frameworks.
1. Wachter RM, Goldman L. The hospitalist movement 5 years later. JAMA. 2002;287:487-494. https://doi.org/10.1001/jama.287.4.487
2. Wachter RM, Goldman L. Zero to 50,000—the 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958
3. Lee KH, Yang Y, Yang KS, et al. Bringing generalists into the hospital: outcomes of a family medicine hospitalist model in Singapore. J Hosp Med. 2011;6:115-121. https://doi.org/10.1002/jhm.821
4. Shu CC, Lin JW, Lin YF, et al. Evaluating the performance of a hospitalist system in Taiwan: a pioneer study for nationwide health insurance in Asia. J Hosp Med. 2011;6(7):378-382. https://doi.org/ 10.1002/jhm.896
5. Lee KH. The hospitalist movement—a complex adaptive response to fragmentation of care in hospitals. Ann Acad Med Singap. 2008;37(2):145-150.
6. Ge L, Ya CW, Heng BH, Tan WS. Frailty and healthcare utilization across care settings among community-dwelling older adults in Singapore. BMC Geriatrics. 2020;20:389. https://doi.org/10.1186/s12877-020-01800-8
7. Lee KH. A historical perspective of the barriers to generalism. Aust Fam Physician. 2015;44(3):154-158.
8. Choo F. Alexandra Hospital provides patients with one-stop services under new care model. Updated December 14, 2018. Accessed March 26, 2021.https://www.straitstimes.com/singapore/health/alexandra-hospital-provides-patients-with-one-stop-services-under-new-care-model
9. National Health Insurance Administration, Ministry of Health and Welfare, Taiwan. Medical services. Copayments. Updated December 28, 2020. Accessed March 26, 2021. https://www.nhi.gov.tw/English/Content_List.aspx?n=E5509C8FE29950EA&topn=1D1ECC54F86E9050
10. Tsai JCH, Chen WY, Liang YW. Nonemergent emergency department visits under the National Health Insurance in Taiwan. Health Policy. 2011;100:189-195. https://doi.org/10.1016/j.healthpol.2010.10.007
11. Taiwan Society of Hospital Medicine. The birth and growth of hospital medicine in Taiwan. Accessed March 26, 2021. https://www.hospitalist.org.tw/about_25.htm
12. Hsu NC, Huang CC, Shu CC, Yang MC. Implementation of a seven-day hospitalist program to improve the outcomes of the weekend admission: a retrospective before-after study in Taiwan. PLoS One. 2018;13(3):e0194833. https://doi.org/10.1371/journal.pone.0194833
13. Ministry of Health and Welfare, Statutes of the Republic of Korea. Act on the Improvement of Training Conditions and Status of Medical Residents. Accessed March 26, 2021. https://elaw.klri.re.kr/eng_mobile/viewer.do?hseq=49563&type=sogan&key=10
14. Chae W, Park EC, Lee KY, et al. Development and evolution of hospital medicine in Korea. J Hosp Med. 2021;16(4):247-250. https://doi.org/10.12788/jhm.3573
15. Oh SJ, Jung EJ. Prospects for the Korean model of the surgical hospitalist system. J Korean Med Assoc. 2020;63(5):236-239. https://doi.org/10.5124/jkma.2020.63.5.236
16. Ohn JH, Kim NH, Kim ES, et al. An acute medical unit in a Korean tertiary care hospital reduces the length of stay and waiting time in the emergency department. J Korean Med Sci. 2017;32:1917-1920. https://doi.org/10.3346/jkms.2017.32.12.1917
17. Lee JH, Kim AJ, Kyong TY, et al. Evaluating the outcome of multi-morbid patients cared for by hospitalists: a report of integrated medical model in Korea. J Korean Med Sci. 2019;34(25):e179. https://doi.org/10.3346/jkms.2019.34.e179
18. OECD. Length of hospital stay. Accessed March 26, 2021. https://doi.org/10.1787/8dda6b7a-en
19. Hamada O, Tsutsumi T, Miki A, et al. Impact of the hospitalist system in Japan on the quality of care and healthcare economics. Intern Med. 2019;58(23):3385-3391. https://doi.org/10.2169/internalmedicine.2872-19
20. Kawashima A. Report on general medicine’s effects on specialists and other healthcare staff in the context of inclusive local medical system. Chapter in Japanese. Accessed March 26, 2021.https://soshin.pcmed-tsukuba.jp/education/report/pdf/05_004.pdf
1. Wachter RM, Goldman L. The hospitalist movement 5 years later. JAMA. 2002;287:487-494. https://doi.org/10.1001/jama.287.4.487
2. Wachter RM, Goldman L. Zero to 50,000—the 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958
3. Lee KH, Yang Y, Yang KS, et al. Bringing generalists into the hospital: outcomes of a family medicine hospitalist model in Singapore. J Hosp Med. 2011;6:115-121. https://doi.org/10.1002/jhm.821
4. Shu CC, Lin JW, Lin YF, et al. Evaluating the performance of a hospitalist system in Taiwan: a pioneer study for nationwide health insurance in Asia. J Hosp Med. 2011;6(7):378-382. https://doi.org/ 10.1002/jhm.896
5. Lee KH. The hospitalist movement—a complex adaptive response to fragmentation of care in hospitals. Ann Acad Med Singap. 2008;37(2):145-150.
6. Ge L, Ya CW, Heng BH, Tan WS. Frailty and healthcare utilization across care settings among community-dwelling older adults in Singapore. BMC Geriatrics. 2020;20:389. https://doi.org/10.1186/s12877-020-01800-8
7. Lee KH. A historical perspective of the barriers to generalism. Aust Fam Physician. 2015;44(3):154-158.
8. Choo F. Alexandra Hospital provides patients with one-stop services under new care model. Updated December 14, 2018. Accessed March 26, 2021.https://www.straitstimes.com/singapore/health/alexandra-hospital-provides-patients-with-one-stop-services-under-new-care-model
9. National Health Insurance Administration, Ministry of Health and Welfare, Taiwan. Medical services. Copayments. Updated December 28, 2020. Accessed March 26, 2021. https://www.nhi.gov.tw/English/Content_List.aspx?n=E5509C8FE29950EA&topn=1D1ECC54F86E9050
10. Tsai JCH, Chen WY, Liang YW. Nonemergent emergency department visits under the National Health Insurance in Taiwan. Health Policy. 2011;100:189-195. https://doi.org/10.1016/j.healthpol.2010.10.007
11. Taiwan Society of Hospital Medicine. The birth and growth of hospital medicine in Taiwan. Accessed March 26, 2021. https://www.hospitalist.org.tw/about_25.htm
12. Hsu NC, Huang CC, Shu CC, Yang MC. Implementation of a seven-day hospitalist program to improve the outcomes of the weekend admission: a retrospective before-after study in Taiwan. PLoS One. 2018;13(3):e0194833. https://doi.org/10.1371/journal.pone.0194833
13. Ministry of Health and Welfare, Statutes of the Republic of Korea. Act on the Improvement of Training Conditions and Status of Medical Residents. Accessed March 26, 2021. https://elaw.klri.re.kr/eng_mobile/viewer.do?hseq=49563&type=sogan&key=10
14. Chae W, Park EC, Lee KY, et al. Development and evolution of hospital medicine in Korea. J Hosp Med. 2021;16(4):247-250. https://doi.org/10.12788/jhm.3573
15. Oh SJ, Jung EJ. Prospects for the Korean model of the surgical hospitalist system. J Korean Med Assoc. 2020;63(5):236-239. https://doi.org/10.5124/jkma.2020.63.5.236
16. Ohn JH, Kim NH, Kim ES, et al. An acute medical unit in a Korean tertiary care hospital reduces the length of stay and waiting time in the emergency department. J Korean Med Sci. 2017;32:1917-1920. https://doi.org/10.3346/jkms.2017.32.12.1917
17. Lee JH, Kim AJ, Kyong TY, et al. Evaluating the outcome of multi-morbid patients cared for by hospitalists: a report of integrated medical model in Korea. J Korean Med Sci. 2019;34(25):e179. https://doi.org/10.3346/jkms.2019.34.e179
18. OECD. Length of hospital stay. Accessed March 26, 2021. https://doi.org/10.1787/8dda6b7a-en
19. Hamada O, Tsutsumi T, Miki A, et al. Impact of the hospitalist system in Japan on the quality of care and healthcare economics. Intern Med. 2019;58(23):3385-3391. https://doi.org/10.2169/internalmedicine.2872-19
20. Kawashima A. Report on general medicine’s effects on specialists and other healthcare staff in the context of inclusive local medical system. Chapter in Japanese. Accessed March 26, 2021.https://soshin.pcmed-tsukuba.jp/education/report/pdf/05_004.pdf
Adoption of Hospitalist Care in Asia: Experiences From Singapore, Taiwan, Korea, and Japan

This work is licensed under a Creative Commons Attribution 4.0 International License
Adoption of Hospitalist Care in Asia: Experiences From Singapore, Taiwan, Korea, and Japan

This work is licensed under a Creative Commons Attribution 4.0 International License
© 2021 Society of Hospital Medicine
Rates and Characteristics of Medical Malpractice Claims Against Hospitalists
The prospect of facing a medical malpractice claim is a source of apprehension for physicians that affects physician behavior, including leading to defensive medicine.1-3 Overall, annual defensive medicine costs have been estimated at $45.6 billion,4 and surveys of hospitalists indicate that 13.0% to 37.5% of hospitalist healthcare costs involve defensive medicine.5,6 Despite the impact of malpractice concerns on hospitalist practice and the unprecedented growth of the field of hospital medicine, relatively few studies have examined the liability environment surrounding hospitalist practice.7,8 The specific issue of malpractice claims rates faced by hospitalists has received even less attention in the medical literature.8
A better understanding of the contributing factors and other attributes of malpractice claims can help guide patient safety initiatives and inform hospitalists’ level of concern regarding liability. Although most medical errors do not result in a malpractice claim,9,10 the majority of malpractice claims in which there is an indemnity payment involve medical injury due to clinician error.11 Even malpractice claims that do not result in an indemnity payment represent opportunities to identify patient safety and risk management vulnerabilities.12
We used a national malpractice claims database to analyze the characteristics of claims made against hospitalists, including claims rates. In addition to claims rates, we also analyzed the other types of providers named in hospitalist claims given the importance of interdisciplinary collaboration to hospital medicine.13,14 To provide context for understanding hospitalist liability data, we present data on other specialties. We also describe a model to predict whether hospitalist malpractice claims will close with an indemnity payment.
METHODS
Data Sources and Elements
This analysis used a repository of malpractice claims maintained by CRICO, the captive malpractice insurer of the Harvard-affiliated medical institutions. This database, the Comparative Benchmarking System, a
Injury severity was based on a widely used scale developed for malpractice claims by the National Association of Insurance Commissioners.16 Low injury severity included emotional injury and temporary insignificant injury. Medium injury severity included temporary minor, temporary major, and permanent minor injury. High injury severity included permanent significant injury through death. Because this study used a database assembled for operational and patient safety purposes and was not human subjects research, institutional review board approval was not needed.
Study Cohort
Malpractice claims included formal lawsuits or written requests for compensation for negligent medical care. Ho
Statistical Analysis
Malpractice claims rates were treated as Poisson rates and compared using a Z-test. Malpractice claims rates are expressed as claims per 100 physician-years. Each physician-year represents 1 year of coverage of one physician by the medical malpractice carrier whose data were used. Physician-years represent the duration of time physicians practiced during which they were insured by the malpractice carrier and, as such, could have been subject to a malpractice claim that would have been included in our data. Claims rates are based on the subset of the malpractice claims in the study for which the number of physician-years of coverage is available, representing 8.2% of hospitalist claims and 11.6% of all claims.
Comparisons of the percentages of cases closing with an indemnity payment, as well as the percentages of cases in different allegation type and clinical severity categories, were made using the Fisher exact test. Indemnity payment amounts were inflation-adjusted to 2018 dollars using the Consumer Price Index. Comparisons of indemnity payment amounts between physician specialties were carried out using the Wilcoxon rank sum test given that the distribution of the payment amounts appeared nonnormal; this was confirmed with the Shapiro-Wilk test. A multivariable logistic regression model was developed to predict the binary outcome of whether a hospitalist case would close with an indemnity payment (compared with no payment), based on the 1,216 hospitalist claims. The predictors used in this regression model were chosen a priori based on hypotheses about what factors drive the likelihood that a case closes with payment. Both the unadjusted and adjusted odds ratios for the predictors are presented. The adjusted model is adjusted for all the other predictors contained in the model. All reported P values are two-sided. The statistical analysis was carried out using JMP Pro version 15 (SAS Institute Inc) and Minitab version 19 (Minitab LLC).
RESULTS
We identified 1,216 hospital medicine malpractice claims from our database. Claims rates were calculated from the subset of our data for which physician-years were available—including 5,140 physician-years encompassing 100 claims, representing 8.2% of all hospitalist claims studied. An additional 18,644 malpractice claims from five other specialties—nonhospitalist general internal medicine, internal medicine subspecialists, emergency medicine, neurosurgery, and psychiatry—were analyzed to provide context for the hospitalist claims.
The malpractice claims rate for hospitalists was significantly higher than the rate for internal medicine subspecialists (1.95 vs 1.30 claims per 100 physician-years; P < .001), though they were not significantly different from the rate for nonhospitalist general internal medicine physicians (1.95 vs 1.92 claims per 100 physician-years; P = .93) (Table 1). Compared with emergency medicine physicians, with whom hospitalists are sometimes compared due to both specialties being defined by their site of practice and the absence of longitudinal patient relationships, hospitalists had a significantly lower claims rate (1.95 vs 4.07 claims per 100 physician-years; P < .001).
An assessment of the temporal trends in the claims rates, based on a comparison between the two halves of the study period (2014-2018 vs 2009-2013), showed that the claims rate for hospitalists was increasing, but at a rate that did not reach statistical significance (Table 1). In contrast, the claims rates for the five other specialties assessed decreased over time, and the decreases were significant for four of these five other specialties (internal medicine subspecialties, emergency medicine, neurosurgery, and psychiatry).
Multiple claims against a single physician were uncommon in our hospitalist malpractice claims data. Among the 100 claims that were used to calculate the claims rates, one physician was named in 2 claims, and all the other physicians were named in only a single claim. Among all of the 1,216 hospitalist malpractice claims we analyzed, there were eight physicians who were named in more than 1 claim, seven of whom were named in 2 claims, and one of whom was named in 3 claims.
The median indemnity payment for hospitalist claims was $231,454 (interquartile range [IQR], $100,000-$503,015), similar to the median indemnity payment for neurosurgery ($233,723; IQR, $85,292-$697,872), though significantly greater than the median indemnity payment for the other four specialties studied (Table 2). Among the hospitalist claims, 29.9% resulted in an indemnity payment, not significantly different from the rate for nonhospitalist general internal medicine, internal medicine subspecialties, or neurosurgery, but significantly lower than the rate for emergency medicine (33.8%; P = .011). No
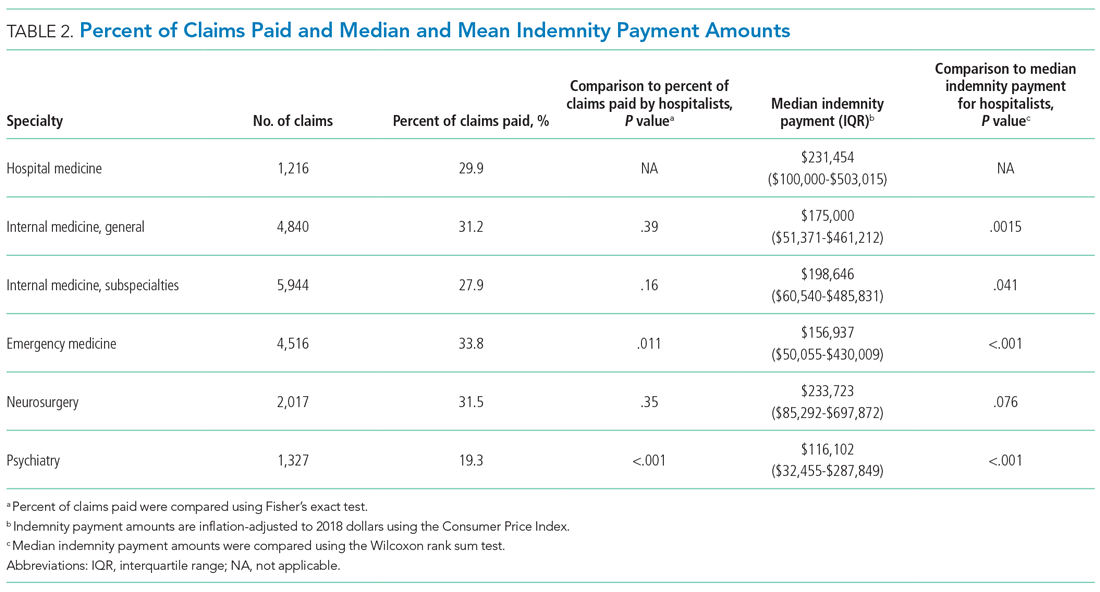
We performed a multivariable logistic regression analysis to assess the effect of different factors on the likelihood of a hospitalist case closing with an indemnity payment, compared with no payment (Table 3). In the multivariable model, the presence of an error in clinical judgment had an adjusted odds ratio (AOR) of 5.01 (95% CI, 3.37-7.45; P < .001) for a claim closing with payment, the largest effect found. The presence of problems with communication (AOR, 1.89; 95% CI, 1.42-2.51; P < .001), the clinical environment (eg, weekend/holiday or clinical busyness; AOR, 1.70; 95% CI, 1.20-2.40; P = .0026), and documentation (AOR, 1.65; 95% CI, 1.18-2.31; P = .0038) were also positive predictors of claims closing with payment. Greater patient age (per decade) was a negative predictor of the likelihood of a claim closing with payment (AOR, 0.92; 95% CI, 0.86-0.998), though it was of borderline statistical significance (P = .044).
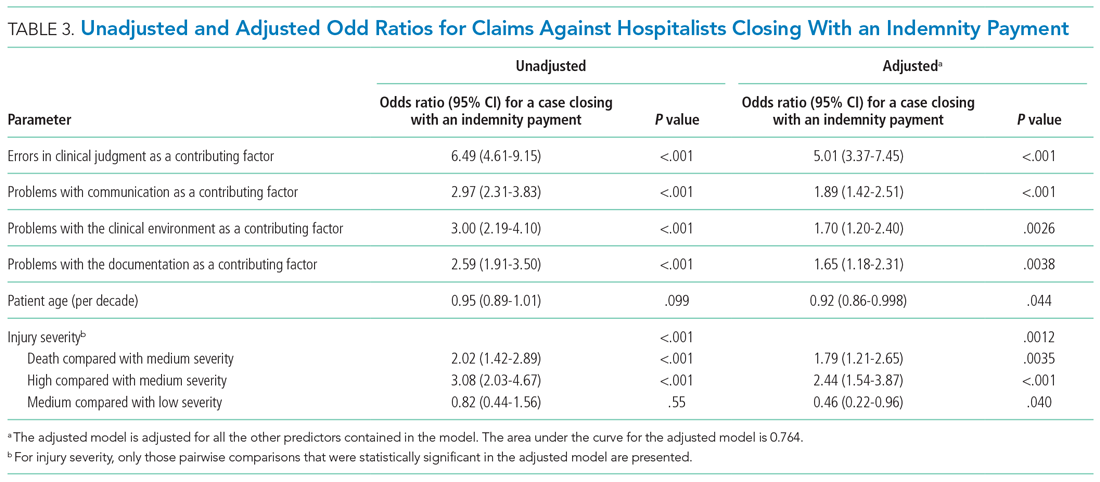
We also assessed multiple clinical attributes of hospitalist malpractice claims, including the major allegation type and injury severity (Appendix Table). Among the 1,216 hospitalist malpractice claims studied, the most common allegation types were for errors related to medical treatment (n = 482; 39.6%), diagnosis (n = 446; 36.7%), and medications (n = 157; 12.9%). Among the hospitalist claims, 888 (73.0%) involved high-severity injury, and 674 (55.4%) involved the death of the patient. The percentages of cases involving high-severity injury and death were significantly greater for hospitalists, compared with that of the other specialties studied (P < .001 for all pairwise comparisons). Of the six specialties studied, hospital medicine was the only one in which the percentage of cases involving death exceeded 50%.
Hospital medicine is typically team-based, and we evaluated which other services were named in claims with hospital medicine as the primary responsible service. The clinician groups most commonly named in hospitalist claims were nursing (n = 269; 22.1%), followed by emergency medicine (n = 91; 7.5%), general surgery (n = 51; 4.2%), cardiology (n = 49; 4.0%), and orthopedic surgery (n = 46; 3.8%) (Appendix Figure). During the first 2 years of the study period, no physician assistants (PAs) or nurse practitioners (NPs) were named in hospitalist claims. Over the study period, the proportion of hospitalist cases also naming PAs and NPs increased steadily, reaching 6.9% and 6.2% of claims, respectively, in 2018 (Figure) (P < .001 for NPs and P = .037 for PAs based on a comparison between the two halves of the study period).
DISCUSSION
We found that the average annual claims rate for hospitalists was similar to that for nonhospitalist general internists (1.95 vs 1.92 claims per 100 physician-years) but significantly greater than that for internal medicine subspecialists (1.95 vs 1.30 claims per 100 physician-years). Hospitalist claims rates showed a notable temporal trend—a nonsignificant increase—over the study period (2009-2018). This contrasts with the five other specialties studied, all of which had decreasing claims rates, four of which were significant. An analysis of a different national malpractice claims database, the NPDB, found that the rate of paid malpractice claims overall decreased 55.7% during the period 1992-2014, again contrasting with the trend we found for hospitalist claims rates.17
We posit several explanations for why the malpractice claims rate trend for hospitalists has diverged from that of other specialties. There has been a large expansion in the number of hospitalists in the United States.18 With this increasing demand, many young physicians have entered the hospital medicine field. In a survey of general internal medicine physicians conducted by the Society of General Internal Medicine, 73% of hospitalists were aged 25 to 44 years, significantly greater than the 45% in this age range among nonhospitalist general internal medicine physicians.19 Hospitalists in their first year of practice have higher mortality rates than more experienced hospitalists.20 Therefore, the relative inexperience of hospitalists, driven by this high demand, could be putting them at increased risk of medical errors and resulting malpractice claims. The higher mortality rate among hospitalists in their first year of practice could be due to a lack of familiarity with the systems of care, such as managing test results and obtaining appropriate consults.20 This possibility suggests that enhanced training and mentorship could be valuable as a strategy to both improve the quality of care and reduce medicolegal risk. The increasing demand for hospitalists could also be affecting the qualification level of physicians entering the field.
Our analysis also showed that the severity of injury in hospitalist claims was greater than that for the other specialties studied. In addition, the percentage of claims involving death was greater for hospitalists than that for the other specialties. The increased acuity of inpatients, compared with that of outpatients—and the trend, at least for some conditions, of increased inpatient acuity over time21,22— could account for the high injury severity seen among hospitalist claims. Given the positive correlation between injury severity and the size of indemnity payments made on malpractice claims,12 the high injury severity seen in hospitalist claims was very likely a driver of the high indemnity payments observed among the hospitalist claims.
The relationship between injury severity and financial outcomes is supported by the results of our multivariable regression model (Table 3). Compared with medium-severity injury claims, both death and high-severity injury cases were significantly more likely to close with an indemnity payment (compared with no payment), with AORs of 1.79 (95% CI, 1.21-2.65) and 2.44 (95% CI, 1.54-3.87), respectively.
The most striking finding in our regression model was the magnitude of the effect of an error in clinical judgement. Cases coded with this contributing factor had five times the AOR of closing with payment (compared with no payment) (AOR, 5.01; 95% CI, 3.37-7.45). A clinical judgment call may be difficult to defend when it is ultimately associated with a bad patient outcome. The importance of clinical judgment in our analysis suggests a risk management strategy: clearly and contemporaneously documenting the rationale behind one’s clinical decision-making. This may help make a claim more defensible in the event of an adverse outcome by demonstrating that the clinician was acting reasonably based on the information available at the time. The importance of specifying a rationale for a clinical decision may be especially important in the era of electronic health records (EHRs). EHRs are not structured as chronologically linear charts, which can make it challenging during a trial to retrospectively show what information was available to the physician at the time the clinical decision was made. The importance of clinical judgment also affirms the importance of effective clinical decision support as a patient safety tool.23
More broadly, it is notable that several contributing factors, including errors in clinical judgment (as discussed previously), problems with communication, and issues with the clinical environment, were significantly associated with malpractice cases closing with payment. This demonstrates that systematically examining malpractice claims to determine the underlying contributing factors can generate predictive analytics, as well as suggest risk management and patient safety strategies.
Interdisciplinary collaboration, as a component of systems-based practice, is a core principle of hospital medicine,13 and so we analyzed the involvement of other clinicians in hospitalist claims. Of the five specialties most frequently named in claims with hospitalists, two were surgical services: general surgery (n = 51; 4.2%) and orthopedic surgery (n = 46; 3.8%). With hospitalists being asked to play an increasing role in the care of surgical patients, they may be providing care to patient populations with whom they have less experience, which could put them at risk of adverse outcomes, leading to malpractice claims.24,25 Hospitalists need to be attuned to the liability risks related to the care of patients requiring surgical management and ensure areas of responsibility are clearly delineated between the hospital medicine and surgical services.26 We also found that hospitalist claims increasingly involve PAs and NPs, likely reflecting their increasing role in providing care on hospitalist services.27,28
A prior analysis of claims rates for hospitalists that covered injury dates from 1997 to 2011 found that hospitalists had a relatively low claims rate, significantly lower than that for other internal medicine physicians.8 In addition to covering an earlier time period, that analysis based its claims rates on data from academic medical centers covered by a single insurer, and physicians at academic medical centers generally have lower claims rates, likely due, at least in part, to their spending a smaller proportion of their time on patient care, compared with nonacademic physicians.29 Another analysis of hospitalist closed claims, which shared some cases with the cohort we analyzed, was performed by The Doctors Company, a commercial liability insurer.7 That analysis astutely emphasized the importance of breakdowns in diagnostic processes as a factor underlying hospitalist claims.
Our study has several limitations. First, although our database of malpractice claims includes approximately 31% of all the claims in the country and includes claims from every state, it may not be nationally representative. Another limitation relates to calculating the claims rates for physicians. Detailed information on the number of years of clinical activity, which is necessary to calculate claims rates, was available for only a subset of our data (8.2% of the hospitalist cases and 11.6% of all cases), so claims rates are based on this subset of our data (among which academic centers are overrepresented). Therefore, the claims rates should be interpreted with caution, especially regarding their application to the community hospital setting. The institutions included in the subset of our data used for determining claims rates were stable over time, so the use of a subset of our data for calculating claims rates reduces the generalizability of our claims rates but should not be a source of bias.
Potentially offsetting strengths of our claims database and study include the availability of unpaid claims (which outnumber paid claims roughly 2:1)11,12; the presence of information on contributing factors and other case characteristics obtained through structured manual review of the cases; and the availability of the specialties of the clinicians involved. These features distinguish the database we used from the NPDB, another national database of malpractice claims, which does not include unpaid claims and which does not include information on contributing factors or physician specialty.
CONCLUSION
First described in 1996, the hospitalist field is the fastest growing specialty in modern medical history.18,30 Therefore, an understanding of the malpractice risk of hospitalists is important and can shed light on the patient safety environment in hospitals. Our analysis showed that hospitalist malpractice claims rates remain roughly stable, in contrast to most other specialties, which have seen a fall in malpractice claims rates.17 In addition, unlike a previous analysis,8 we found that claims rates for hospitalists were essentially equal to those of other general internal medicine physicians (not lower, as had been previously reported), and higher than those of the internal medicine subspecialties. Hospitalist claims also have relatively high severity of injury. Potential factors driving these trends include the increasing demand for hospitalists, which results in a higher proportion of less-experienced physicians entering the field, and the expanding clinical scope of hospitalists, which may lead to their managing patients with conditions with which they may be less comfortable. Overall, our analysis suggests that the malpractice environment for hospitalists is becoming less favorable, and therefore, hospitalists should explore opportunities for mitigating liability risk and enhancing patient safety.
1. Studdert DM, Mello MM, Sage WM, et al. Defensive medicine among high-risk specialist physicians in a volatile malpractice environment. JAMA. 2005;293(21):2609-2617. https://doi.org/10.1001/jama.293.21.2609
2. Carrier ER, Reschovsky JD, Mello MM, Mayrell RC, Katz D. Physicians’ fears of malpractice lawsuits are not assuaged by tort reforms. Health Aff (Millwood). 2010;29(9):1585-1592. https://doi.org/10.1377/hlthaff.2010.0135
3. Kachalia A, Berg A, Fagerlin A, et al. Overuse of testing in preoperative evaluation and syncope: a survey of hospitalists. Ann Intern Med. 2015;162(2):100-108. https://doi.org/10.7326/m14-0694
4. Mello MM, Chandra A, Gawande AA, Studdert DM. National costs of the medical liability system. Health Aff (Millwood). 2010;29(9):1569-1577. https://doi.org/10.1377/hlthaff.2009.0807
5. Rothberg MB, Class J, Bishop TF, Friderici J, Kleppel R, Lindenauer PK. The cost of defensive medicine on 3 hospital medicine services. JAMA Intern Med. 2014;174(11):1867-1868. https://doi.org/10.1001/jamainternmed.2014.4649
6. Saint S, Vaughn VM, Chopra V, Fowler KE, Kachalia A. Perception of resources spent on defensive medicine and history of being sued among hospitalists: results from a national survey. J Hosp Med. 2018;13(1):26-29. https://doi.org/10.12788/jhm.2800
7. Ranum D, Troxel DB, Diamond R. Hospitalist Closed Claims Study: An Expert Analysis of Medical Malpractice Allegations. The Doctors Company. 2016. https://www.thedoctors.com/siteassets/pdfs/risk-management/closed-claims-studies/10392_ccs-hospitalist_academic_single-page_version_frr.pdf
8. Schaffer AC, Puopolo AL, Raman S, Kachalia A. Liability impact of the hospitalist model of care. J Hosp Med. 2014;9(12):750-755. https://doi.org/10.1002/jhm.2244
9. Localio AR, Lawthers AG, Brennan TA, et al. Relation between malpractice claims and adverse events due to negligence. results of the Harvard Medical Practice Study III. N Engl J Med. 1991;325(4):245-251. https://doi.org/10.1056/nejm199107253250405
10. Studdert DM, Thomas EJ, Burstin HR, Zbar BI, Orav EJ, Brennan TA. Negligent care and malpractice claiming behavior in Utah and Colorado. Med Care. 2000;38(3):250-260. https://doi.org/10.1097/00005650-200003000-00002
11. Studdert DM, Mello MM, Gawande AA, et al. Claims, errors, and compensation payments in medical malpractice litigation. N Engl J Med. 2006;354(19):2024-2033. https://doi.org/10.1056/nejmsa054479
12. Medical Malpractice in America: 2018 CRICO Strategies National CBS Report. CRICO Strategies; 2018.
13. Budnitz T, McKean SC. The Core Competencies in Hospital Medicine. In: McKean SC, Ross JJ, Dressler DD, Scheurer DB, eds. Principles and Practice of Hospital Medicine, 2nd ed. McGraw-Hill Education; 2017.
14. O’Leary KJ, Haviley C, Slade ME, Shah HM, Lee J, Williams MV. Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit. J Hosp Med. 2011;6(2):88-93. https://doi.org/10.1002/jhm.714
15. National Practitioner Data Bank: Public Use Data File. Division of Practitioner Data Banks, Bureau of Health Professions, Health Resources & Services Administration, U.S. Department of Health & Human Services; June 30, 2019. Updated August 2020.
16. Sowka MP, ed. NAIC Malpractice Claims, Final Compilation. National Association of Insurance Commissioners; 1980.
17. Schaffer AC, Jena AB, Seabury SA, Singh H, Chalasani V, Kachalia A. Rates and characteristics of paid malpractice claims among US physicians by specialty, 1992-2014. JAMA Intern Med. 2017;177(5):710-718. https://doi.org/10.1001/jamainternmed.2017.0311
18. Wachter RM, Goldman L. Zero to 50,000 - the 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/nejmp1607958
19. Miller CS, Fogerty RL, Gann J, Bruti CP, Klein R; The Society of General Internal Medicine Membership Committee. The growth of hospitalists and the future of the Society of General Internal Medicine: results from the 2014 membership survey. J Gen Intern Med. 2017;32(11):1179-1185. https://doi.org/10.1007/s11606-017-4126-7
20. Goodwin JS, Salameh H, Zhou J, Singh S, Kuo YF, Nattinger AB. Association of hospitalist years of experience with mortality in the hospitalized Medicare population. JAMA Intern Med. 2018;178(2):196-203. https://doi.org/10.1001/jamainternmed.2017.7049
21. Akintoye E, Briasoulis A, Egbe A, et al. National trends in admission and in-hospital mortality of patients with heart failure in the United States (2001-2014). J Am Heart Assoc. 2017;6(12):e006955. https://doi.org/10.1161/jaha.117.006955
22. Clark AV, LoPresti CM, Smith TI. Trends in inpatient admission comorbidity and electronic health data: implications for resident workload intensity. J Hosp Med. 2018;13(8):570-572. https://doi.org/10.12788/jhm.2954
23. Gilmartin HM, Liu VX, Burke RE. Annals for hospitalists inpatient notes - The role of hospitalists in the creation of learning healthcare systems. Ann Intern Med. 2020;172(2):HO2-HO3. https://doi.org/10.7326/m19-3873
24. Siegal EM. Just because you can, doesn’t mean that you should: a call for the rational application of hospitalist comanagement. J Hosp Med. 2008;3(5):398-402. https://doi.org/10.1002/jhm.361
25. Plauth WH 3rd, Pantilat SZ, Wachter RM, Fenton CL. Hospitalists’ perceptions of their residency training needs: results of a national survey. Am J Med. 2001;111(3):247-254. https://doi.org/10.1016/s0002-9343(01)00837-3
26. Thompson RE, Pfeifer K, Grant PJ, et al. Hospital medicine and perioperative care: a framework for high-quality, high-value collaborative care. J Hosp Med. 2017;12(4):277-282. https://doi.org/10.12788/jhm.2717
27. Torok H, Lackner C, Landis R, Wright S. Learning needs of physician assistants working in hospital medicine. J Hosp Med. 2012;7(3):190-194. https://doi.org/10.1002/jhm.1001
28. Kartha A, Restuccia JD, Burgess JF Jr, et al. Nurse practitioner and physician assistant scope of practice in 118 acute care hospitals. J Hosp Med. 2014;9(10):615-620. https://doi.org/10.1002/jhm.2231
29. Schaffer AC, Babayan A, Yu-Moe CW, Sato L, Einbinder JS. The effect of clinical volume on annual and per-patient encounter medical malpractice claims risk. J Patient Saf. Published online March 23, 2020. https://doi.org/10.1097/pts.0000000000000706
30. Wachter RM, Goldman L. The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335(7):514-517. https://doi.org/10.1056/nejm199608153350713
The prospect of facing a medical malpractice claim is a source of apprehension for physicians that affects physician behavior, including leading to defensive medicine.1-3 Overall, annual defensive medicine costs have been estimated at $45.6 billion,4 and surveys of hospitalists indicate that 13.0% to 37.5% of hospitalist healthcare costs involve defensive medicine.5,6 Despite the impact of malpractice concerns on hospitalist practice and the unprecedented growth of the field of hospital medicine, relatively few studies have examined the liability environment surrounding hospitalist practice.7,8 The specific issue of malpractice claims rates faced by hospitalists has received even less attention in the medical literature.8
A better understanding of the contributing factors and other attributes of malpractice claims can help guide patient safety initiatives and inform hospitalists’ level of concern regarding liability. Although most medical errors do not result in a malpractice claim,9,10 the majority of malpractice claims in which there is an indemnity payment involve medical injury due to clinician error.11 Even malpractice claims that do not result in an indemnity payment represent opportunities to identify patient safety and risk management vulnerabilities.12
We used a national malpractice claims database to analyze the characteristics of claims made against hospitalists, including claims rates. In addition to claims rates, we also analyzed the other types of providers named in hospitalist claims given the importance of interdisciplinary collaboration to hospital medicine.13,14 To provide context for understanding hospitalist liability data, we present data on other specialties. We also describe a model to predict whether hospitalist malpractice claims will close with an indemnity payment.
METHODS
Data Sources and Elements
This analysis used a repository of malpractice claims maintained by CRICO, the captive malpractice insurer of the Harvard-affiliated medical institutions. This database, the Comparative Benchmarking System, a
Injury severity was based on a widely used scale developed for malpractice claims by the National Association of Insurance Commissioners.16 Low injury severity included emotional injury and temporary insignificant injury. Medium injury severity included temporary minor, temporary major, and permanent minor injury. High injury severity included permanent significant injury through death. Because this study used a database assembled for operational and patient safety purposes and was not human subjects research, institutional review board approval was not needed.
Study Cohort
Malpractice claims included formal lawsuits or written requests for compensation for negligent medical care. Ho
Statistical Analysis
Malpractice claims rates were treated as Poisson rates and compared using a Z-test. Malpractice claims rates are expressed as claims per 100 physician-years. Each physician-year represents 1 year of coverage of one physician by the medical malpractice carrier whose data were used. Physician-years represent the duration of time physicians practiced during which they were insured by the malpractice carrier and, as such, could have been subject to a malpractice claim that would have been included in our data. Claims rates are based on the subset of the malpractice claims in the study for which the number of physician-years of coverage is available, representing 8.2% of hospitalist claims and 11.6% of all claims.
Comparisons of the percentages of cases closing with an indemnity payment, as well as the percentages of cases in different allegation type and clinical severity categories, were made using the Fisher exact test. Indemnity payment amounts were inflation-adjusted to 2018 dollars using the Consumer Price Index. Comparisons of indemnity payment amounts between physician specialties were carried out using the Wilcoxon rank sum test given that the distribution of the payment amounts appeared nonnormal; this was confirmed with the Shapiro-Wilk test. A multivariable logistic regression model was developed to predict the binary outcome of whether a hospitalist case would close with an indemnity payment (compared with no payment), based on the 1,216 hospitalist claims. The predictors used in this regression model were chosen a priori based on hypotheses about what factors drive the likelihood that a case closes with payment. Both the unadjusted and adjusted odds ratios for the predictors are presented. The adjusted model is adjusted for all the other predictors contained in the model. All reported P values are two-sided. The statistical analysis was carried out using JMP Pro version 15 (SAS Institute Inc) and Minitab version 19 (Minitab LLC).
RESULTS
We identified 1,216 hospital medicine malpractice claims from our database. Claims rates were calculated from the subset of our data for which physician-years were available—including 5,140 physician-years encompassing 100 claims, representing 8.2% of all hospitalist claims studied. An additional 18,644 malpractice claims from five other specialties—nonhospitalist general internal medicine, internal medicine subspecialists, emergency medicine, neurosurgery, and psychiatry—were analyzed to provide context for the hospitalist claims.
The malpractice claims rate for hospitalists was significantly higher than the rate for internal medicine subspecialists (1.95 vs 1.30 claims per 100 physician-years; P < .001), though they were not significantly different from the rate for nonhospitalist general internal medicine physicians (1.95 vs 1.92 claims per 100 physician-years; P = .93) (Table 1). Compared with emergency medicine physicians, with whom hospitalists are sometimes compared due to both specialties being defined by their site of practice and the absence of longitudinal patient relationships, hospitalists had a significantly lower claims rate (1.95 vs 4.07 claims per 100 physician-years; P < .001).

An assessment of the temporal trends in the claims rates, based on a comparison between the two halves of the study period (2014-2018 vs 2009-2013), showed that the claims rate for hospitalists was increasing, but at a rate that did not reach statistical significance (Table 1). In contrast, the claims rates for the five other specialties assessed decreased over time, and the decreases were significant for four of these five other specialties (internal medicine subspecialties, emergency medicine, neurosurgery, and psychiatry).
Multiple claims against a single physician were uncommon in our hospitalist malpractice claims data. Among the 100 claims that were used to calculate the claims rates, one physician was named in 2 claims, and all the other physicians were named in only a single claim. Among all of the 1,216 hospitalist malpractice claims we analyzed, there were eight physicians who were named in more than 1 claim, seven of whom were named in 2 claims, and one of whom was named in 3 claims.
The median indemnity payment for hospitalist claims was $231,454 (interquartile range [IQR], $100,000-$503,015), similar to the median indemnity payment for neurosurgery ($233,723; IQR, $85,292-$697,872), though significantly greater than the median indemnity payment for the other four specialties studied (Table 2). Among the hospitalist claims, 29.9% resulted in an indemnity payment, not significantly different from the rate for nonhospitalist general internal medicine, internal medicine subspecialties, or neurosurgery, but significantly lower than the rate for emergency medicine (33.8%; P = .011). No

We performed a multivariable logistic regression analysis to assess the effect of different factors on the likelihood of a hospitalist case closing with an indemnity payment, compared with no payment (Table 3). In the multivariable model, the presence of an error in clinical judgment had an adjusted odds ratio (AOR) of 5.01 (95% CI, 3.37-7.45; P < .001) for a claim closing with payment, the largest effect found. The presence of problems with communication (AOR, 1.89; 95% CI, 1.42-2.51; P < .001), the clinical environment (eg, weekend/holiday or clinical busyness; AOR, 1.70; 95% CI, 1.20-2.40; P = .0026), and documentation (AOR, 1.65; 95% CI, 1.18-2.31; P = .0038) were also positive predictors of claims closing with payment. Greater patient age (per decade) was a negative predictor of the likelihood of a claim closing with payment (AOR, 0.92; 95% CI, 0.86-0.998), though it was of borderline statistical significance (P = .044).

We also assessed multiple clinical attributes of hospitalist malpractice claims, including the major allegation type and injury severity (Appendix Table). Among the 1,216 hospitalist malpractice claims studied, the most common allegation types were for errors related to medical treatment (n = 482; 39.6%), diagnosis (n = 446; 36.7%), and medications (n = 157; 12.9%). Among the hospitalist claims, 888 (73.0%) involved high-severity injury, and 674 (55.4%) involved the death of the patient. The percentages of cases involving high-severity injury and death were significantly greater for hospitalists, compared with that of the other specialties studied (P < .001 for all pairwise comparisons). Of the six specialties studied, hospital medicine was the only one in which the percentage of cases involving death exceeded 50%.
Hospital medicine is typically team-based, and we evaluated which other services were named in claims with hospital medicine as the primary responsible service. The clinician groups most commonly named in hospitalist claims were nursing (n = 269; 22.1%), followed by emergency medicine (n = 91; 7.5%), general surgery (n = 51; 4.2%), cardiology (n = 49; 4.0%), and orthopedic surgery (n = 46; 3.8%) (Appendix Figure). During the first 2 years of the study period, no physician assistants (PAs) or nurse practitioners (NPs) were named in hospitalist claims. Over the study period, the proportion of hospitalist cases also naming PAs and NPs increased steadily, reaching 6.9% and 6.2% of claims, respectively, in 2018 (Figure) (P < .001 for NPs and P = .037 for PAs based on a comparison between the two halves of the study period).

DISCUSSION
We found that the average annual claims rate for hospitalists was similar to that for nonhospitalist general internists (1.95 vs 1.92 claims per 100 physician-years) but significantly greater than that for internal medicine subspecialists (1.95 vs 1.30 claims per 100 physician-years). Hospitalist claims rates showed a notable temporal trend—a nonsignificant increase—over the study period (2009-2018). This contrasts with the five other specialties studied, all of which had decreasing claims rates, four of which were significant. An analysis of a different national malpractice claims database, the NPDB, found that the rate of paid malpractice claims overall decreased 55.7% during the period 1992-2014, again contrasting with the trend we found for hospitalist claims rates.17
We posit several explanations for why the malpractice claims rate trend for hospitalists has diverged from that of other specialties. There has been a large expansion in the number of hospitalists in the United States.18 With this increasing demand, many young physicians have entered the hospital medicine field. In a survey of general internal medicine physicians conducted by the Society of General Internal Medicine, 73% of hospitalists were aged 25 to 44 years, significantly greater than the 45% in this age range among nonhospitalist general internal medicine physicians.19 Hospitalists in their first year of practice have higher mortality rates than more experienced hospitalists.20 Therefore, the relative inexperience of hospitalists, driven by this high demand, could be putting them at increased risk of medical errors and resulting malpractice claims. The higher mortality rate among hospitalists in their first year of practice could be due to a lack of familiarity with the systems of care, such as managing test results and obtaining appropriate consults.20 This possibility suggests that enhanced training and mentorship could be valuable as a strategy to both improve the quality of care and reduce medicolegal risk. The increasing demand for hospitalists could also be affecting the qualification level of physicians entering the field.
Our analysis also showed that the severity of injury in hospitalist claims was greater than that for the other specialties studied. In addition, the percentage of claims involving death was greater for hospitalists than that for the other specialties. The increased acuity of inpatients, compared with that of outpatients—and the trend, at least for some conditions, of increased inpatient acuity over time21,22— could account for the high injury severity seen among hospitalist claims. Given the positive correlation between injury severity and the size of indemnity payments made on malpractice claims,12 the high injury severity seen in hospitalist claims was very likely a driver of the high indemnity payments observed among the hospitalist claims.
The relationship between injury severity and financial outcomes is supported by the results of our multivariable regression model (Table 3). Compared with medium-severity injury claims, both death and high-severity injury cases were significantly more likely to close with an indemnity payment (compared with no payment), with AORs of 1.79 (95% CI, 1.21-2.65) and 2.44 (95% CI, 1.54-3.87), respectively.
The most striking finding in our regression model was the magnitude of the effect of an error in clinical judgement. Cases coded with this contributing factor had five times the AOR of closing with payment (compared with no payment) (AOR, 5.01; 95% CI, 3.37-7.45). A clinical judgment call may be difficult to defend when it is ultimately associated with a bad patient outcome. The importance of clinical judgment in our analysis suggests a risk management strategy: clearly and contemporaneously documenting the rationale behind one’s clinical decision-making. This may help make a claim more defensible in the event of an adverse outcome by demonstrating that the clinician was acting reasonably based on the information available at the time. The importance of specifying a rationale for a clinical decision may be especially important in the era of electronic health records (EHRs). EHRs are not structured as chronologically linear charts, which can make it challenging during a trial to retrospectively show what information was available to the physician at the time the clinical decision was made. The importance of clinical judgment also affirms the importance of effective clinical decision support as a patient safety tool.23
More broadly, it is notable that several contributing factors, including errors in clinical judgment (as discussed previously), problems with communication, and issues with the clinical environment, were significantly associated with malpractice cases closing with payment. This demonstrates that systematically examining malpractice claims to determine the underlying contributing factors can generate predictive analytics, as well as suggest risk management and patient safety strategies.
Interdisciplinary collaboration, as a component of systems-based practice, is a core principle of hospital medicine,13 and so we analyzed the involvement of other clinicians in hospitalist claims. Of the five specialties most frequently named in claims with hospitalists, two were surgical services: general surgery (n = 51; 4.2%) and orthopedic surgery (n = 46; 3.8%). With hospitalists being asked to play an increasing role in the care of surgical patients, they may be providing care to patient populations with whom they have less experience, which could put them at risk of adverse outcomes, leading to malpractice claims.24,25 Hospitalists need to be attuned to the liability risks related to the care of patients requiring surgical management and ensure areas of responsibility are clearly delineated between the hospital medicine and surgical services.26 We also found that hospitalist claims increasingly involve PAs and NPs, likely reflecting their increasing role in providing care on hospitalist services.27,28
A prior analysis of claims rates for hospitalists that covered injury dates from 1997 to 2011 found that hospitalists had a relatively low claims rate, significantly lower than that for other internal medicine physicians.8 In addition to covering an earlier time period, that analysis based its claims rates on data from academic medical centers covered by a single insurer, and physicians at academic medical centers generally have lower claims rates, likely due, at least in part, to their spending a smaller proportion of their time on patient care, compared with nonacademic physicians.29 Another analysis of hospitalist closed claims, which shared some cases with the cohort we analyzed, was performed by The Doctors Company, a commercial liability insurer.7 That analysis astutely emphasized the importance of breakdowns in diagnostic processes as a factor underlying hospitalist claims.
Our study has several limitations. First, although our database of malpractice claims includes approximately 31% of all the claims in the country and includes claims from every state, it may not be nationally representative. Another limitation relates to calculating the claims rates for physicians. Detailed information on the number of years of clinical activity, which is necessary to calculate claims rates, was available for only a subset of our data (8.2% of the hospitalist cases and 11.6% of all cases), so claims rates are based on this subset of our data (among which academic centers are overrepresented). Therefore, the claims rates should be interpreted with caution, especially regarding their application to the community hospital setting. The institutions included in the subset of our data used for determining claims rates were stable over time, so the use of a subset of our data for calculating claims rates reduces the generalizability of our claims rates but should not be a source of bias.
Potentially offsetting strengths of our claims database and study include the availability of unpaid claims (which outnumber paid claims roughly 2:1)11,12; the presence of information on contributing factors and other case characteristics obtained through structured manual review of the cases; and the availability of the specialties of the clinicians involved. These features distinguish the database we used from the NPDB, another national database of malpractice claims, which does not include unpaid claims and which does not include information on contributing factors or physician specialty.
CONCLUSION
First described in 1996, the hospitalist field is the fastest growing specialty in modern medical history.18,30 Therefore, an understanding of the malpractice risk of hospitalists is important and can shed light on the patient safety environment in hospitals. Our analysis showed that hospitalist malpractice claims rates remain roughly stable, in contrast to most other specialties, which have seen a fall in malpractice claims rates.17 In addition, unlike a previous analysis,8 we found that claims rates for hospitalists were essentially equal to those of other general internal medicine physicians (not lower, as had been previously reported), and higher than those of the internal medicine subspecialties. Hospitalist claims also have relatively high severity of injury. Potential factors driving these trends include the increasing demand for hospitalists, which results in a higher proportion of less-experienced physicians entering the field, and the expanding clinical scope of hospitalists, which may lead to their managing patients with conditions with which they may be less comfortable. Overall, our analysis suggests that the malpractice environment for hospitalists is becoming less favorable, and therefore, hospitalists should explore opportunities for mitigating liability risk and enhancing patient safety.
The prospect of facing a medical malpractice claim is a source of apprehension for physicians that affects physician behavior, including leading to defensive medicine.1-3 Overall, annual defensive medicine costs have been estimated at $45.6 billion,4 and surveys of hospitalists indicate that 13.0% to 37.5% of hospitalist healthcare costs involve defensive medicine.5,6 Despite the impact of malpractice concerns on hospitalist practice and the unprecedented growth of the field of hospital medicine, relatively few studies have examined the liability environment surrounding hospitalist practice.7,8 The specific issue of malpractice claims rates faced by hospitalists has received even less attention in the medical literature.8
A better understanding of the contributing factors and other attributes of malpractice claims can help guide patient safety initiatives and inform hospitalists’ level of concern regarding liability. Although most medical errors do not result in a malpractice claim,9,10 the majority of malpractice claims in which there is an indemnity payment involve medical injury due to clinician error.11 Even malpractice claims that do not result in an indemnity payment represent opportunities to identify patient safety and risk management vulnerabilities.12
We used a national malpractice claims database to analyze the characteristics of claims made against hospitalists, including claims rates. In addition to claims rates, we also analyzed the other types of providers named in hospitalist claims given the importance of interdisciplinary collaboration to hospital medicine.13,14 To provide context for understanding hospitalist liability data, we present data on other specialties. We also describe a model to predict whether hospitalist malpractice claims will close with an indemnity payment.
METHODS
Data Sources and Elements
This analysis used a repository of malpractice claims maintained by CRICO, the captive malpractice insurer of the Harvard-affiliated medical institutions. This database, the Comparative Benchmarking System, a
Injury severity was based on a widely used scale developed for malpractice claims by the National Association of Insurance Commissioners.16 Low injury severity included emotional injury and temporary insignificant injury. Medium injury severity included temporary minor, temporary major, and permanent minor injury. High injury severity included permanent significant injury through death. Because this study used a database assembled for operational and patient safety purposes and was not human subjects research, institutional review board approval was not needed.
Study Cohort
Malpractice claims included formal lawsuits or written requests for compensation for negligent medical care. Ho
Statistical Analysis
Malpractice claims rates were treated as Poisson rates and compared using a Z-test. Malpractice claims rates are expressed as claims per 100 physician-years. Each physician-year represents 1 year of coverage of one physician by the medical malpractice carrier whose data were used. Physician-years represent the duration of time physicians practiced during which they were insured by the malpractice carrier and, as such, could have been subject to a malpractice claim that would have been included in our data. Claims rates are based on the subset of the malpractice claims in the study for which the number of physician-years of coverage is available, representing 8.2% of hospitalist claims and 11.6% of all claims.
Comparisons of the percentages of cases closing with an indemnity payment, as well as the percentages of cases in different allegation type and clinical severity categories, were made using the Fisher exact test. Indemnity payment amounts were inflation-adjusted to 2018 dollars using the Consumer Price Index. Comparisons of indemnity payment amounts between physician specialties were carried out using the Wilcoxon rank sum test given that the distribution of the payment amounts appeared nonnormal; this was confirmed with the Shapiro-Wilk test. A multivariable logistic regression model was developed to predict the binary outcome of whether a hospitalist case would close with an indemnity payment (compared with no payment), based on the 1,216 hospitalist claims. The predictors used in this regression model were chosen a priori based on hypotheses about what factors drive the likelihood that a case closes with payment. Both the unadjusted and adjusted odds ratios for the predictors are presented. The adjusted model is adjusted for all the other predictors contained in the model. All reported P values are two-sided. The statistical analysis was carried out using JMP Pro version 15 (SAS Institute Inc) and Minitab version 19 (Minitab LLC).
RESULTS
We identified 1,216 hospital medicine malpractice claims from our database. Claims rates were calculated from the subset of our data for which physician-years were available—including 5,140 physician-years encompassing 100 claims, representing 8.2% of all hospitalist claims studied. An additional 18,644 malpractice claims from five other specialties—nonhospitalist general internal medicine, internal medicine subspecialists, emergency medicine, neurosurgery, and psychiatry—were analyzed to provide context for the hospitalist claims.
The malpractice claims rate for hospitalists was significantly higher than the rate for internal medicine subspecialists (1.95 vs 1.30 claims per 100 physician-years; P < .001), though they were not significantly different from the rate for nonhospitalist general internal medicine physicians (1.95 vs 1.92 claims per 100 physician-years; P = .93) (Table 1). Compared with emergency medicine physicians, with whom hospitalists are sometimes compared due to both specialties being defined by their site of practice and the absence of longitudinal patient relationships, hospitalists had a significantly lower claims rate (1.95 vs 4.07 claims per 100 physician-years; P < .001).

An assessment of the temporal trends in the claims rates, based on a comparison between the two halves of the study period (2014-2018 vs 2009-2013), showed that the claims rate for hospitalists was increasing, but at a rate that did not reach statistical significance (Table 1). In contrast, the claims rates for the five other specialties assessed decreased over time, and the decreases were significant for four of these five other specialties (internal medicine subspecialties, emergency medicine, neurosurgery, and psychiatry).
Multiple claims against a single physician were uncommon in our hospitalist malpractice claims data. Among the 100 claims that were used to calculate the claims rates, one physician was named in 2 claims, and all the other physicians were named in only a single claim. Among all of the 1,216 hospitalist malpractice claims we analyzed, there were eight physicians who were named in more than 1 claim, seven of whom were named in 2 claims, and one of whom was named in 3 claims.
The median indemnity payment for hospitalist claims was $231,454 (interquartile range [IQR], $100,000-$503,015), similar to the median indemnity payment for neurosurgery ($233,723; IQR, $85,292-$697,872), though significantly greater than the median indemnity payment for the other four specialties studied (Table 2). Among the hospitalist claims, 29.9% resulted in an indemnity payment, not significantly different from the rate for nonhospitalist general internal medicine, internal medicine subspecialties, or neurosurgery, but significantly lower than the rate for emergency medicine (33.8%; P = .011). No

We performed a multivariable logistic regression analysis to assess the effect of different factors on the likelihood of a hospitalist case closing with an indemnity payment, compared with no payment (Table 3). In the multivariable model, the presence of an error in clinical judgment had an adjusted odds ratio (AOR) of 5.01 (95% CI, 3.37-7.45; P < .001) for a claim closing with payment, the largest effect found. The presence of problems with communication (AOR, 1.89; 95% CI, 1.42-2.51; P < .001), the clinical environment (eg, weekend/holiday or clinical busyness; AOR, 1.70; 95% CI, 1.20-2.40; P = .0026), and documentation (AOR, 1.65; 95% CI, 1.18-2.31; P = .0038) were also positive predictors of claims closing with payment. Greater patient age (per decade) was a negative predictor of the likelihood of a claim closing with payment (AOR, 0.92; 95% CI, 0.86-0.998), though it was of borderline statistical significance (P = .044).

We also assessed multiple clinical attributes of hospitalist malpractice claims, including the major allegation type and injury severity (Appendix Table). Among the 1,216 hospitalist malpractice claims studied, the most common allegation types were for errors related to medical treatment (n = 482; 39.6%), diagnosis (n = 446; 36.7%), and medications (n = 157; 12.9%). Among the hospitalist claims, 888 (73.0%) involved high-severity injury, and 674 (55.4%) involved the death of the patient. The percentages of cases involving high-severity injury and death were significantly greater for hospitalists, compared with that of the other specialties studied (P < .001 for all pairwise comparisons). Of the six specialties studied, hospital medicine was the only one in which the percentage of cases involving death exceeded 50%.
Hospital medicine is typically team-based, and we evaluated which other services were named in claims with hospital medicine as the primary responsible service. The clinician groups most commonly named in hospitalist claims were nursing (n = 269; 22.1%), followed by emergency medicine (n = 91; 7.5%), general surgery (n = 51; 4.2%), cardiology (n = 49; 4.0%), and orthopedic surgery (n = 46; 3.8%) (Appendix Figure). During the first 2 years of the study period, no physician assistants (PAs) or nurse practitioners (NPs) were named in hospitalist claims. Over the study period, the proportion of hospitalist cases also naming PAs and NPs increased steadily, reaching 6.9% and 6.2% of claims, respectively, in 2018 (Figure) (P < .001 for NPs and P = .037 for PAs based on a comparison between the two halves of the study period).

DISCUSSION
We found that the average annual claims rate for hospitalists was similar to that for nonhospitalist general internists (1.95 vs 1.92 claims per 100 physician-years) but significantly greater than that for internal medicine subspecialists (1.95 vs 1.30 claims per 100 physician-years). Hospitalist claims rates showed a notable temporal trend—a nonsignificant increase—over the study period (2009-2018). This contrasts with the five other specialties studied, all of which had decreasing claims rates, four of which were significant. An analysis of a different national malpractice claims database, the NPDB, found that the rate of paid malpractice claims overall decreased 55.7% during the period 1992-2014, again contrasting with the trend we found for hospitalist claims rates.17
We posit several explanations for why the malpractice claims rate trend for hospitalists has diverged from that of other specialties. There has been a large expansion in the number of hospitalists in the United States.18 With this increasing demand, many young physicians have entered the hospital medicine field. In a survey of general internal medicine physicians conducted by the Society of General Internal Medicine, 73% of hospitalists were aged 25 to 44 years, significantly greater than the 45% in this age range among nonhospitalist general internal medicine physicians.19 Hospitalists in their first year of practice have higher mortality rates than more experienced hospitalists.20 Therefore, the relative inexperience of hospitalists, driven by this high demand, could be putting them at increased risk of medical errors and resulting malpractice claims. The higher mortality rate among hospitalists in their first year of practice could be due to a lack of familiarity with the systems of care, such as managing test results and obtaining appropriate consults.20 This possibility suggests that enhanced training and mentorship could be valuable as a strategy to both improve the quality of care and reduce medicolegal risk. The increasing demand for hospitalists could also be affecting the qualification level of physicians entering the field.
Our analysis also showed that the severity of injury in hospitalist claims was greater than that for the other specialties studied. In addition, the percentage of claims involving death was greater for hospitalists than that for the other specialties. The increased acuity of inpatients, compared with that of outpatients—and the trend, at least for some conditions, of increased inpatient acuity over time21,22— could account for the high injury severity seen among hospitalist claims. Given the positive correlation between injury severity and the size of indemnity payments made on malpractice claims,12 the high injury severity seen in hospitalist claims was very likely a driver of the high indemnity payments observed among the hospitalist claims.
The relationship between injury severity and financial outcomes is supported by the results of our multivariable regression model (Table 3). Compared with medium-severity injury claims, both death and high-severity injury cases were significantly more likely to close with an indemnity payment (compared with no payment), with AORs of 1.79 (95% CI, 1.21-2.65) and 2.44 (95% CI, 1.54-3.87), respectively.
The most striking finding in our regression model was the magnitude of the effect of an error in clinical judgement. Cases coded with this contributing factor had five times the AOR of closing with payment (compared with no payment) (AOR, 5.01; 95% CI, 3.37-7.45). A clinical judgment call may be difficult to defend when it is ultimately associated with a bad patient outcome. The importance of clinical judgment in our analysis suggests a risk management strategy: clearly and contemporaneously documenting the rationale behind one’s clinical decision-making. This may help make a claim more defensible in the event of an adverse outcome by demonstrating that the clinician was acting reasonably based on the information available at the time. The importance of specifying a rationale for a clinical decision may be especially important in the era of electronic health records (EHRs). EHRs are not structured as chronologically linear charts, which can make it challenging during a trial to retrospectively show what information was available to the physician at the time the clinical decision was made. The importance of clinical judgment also affirms the importance of effective clinical decision support as a patient safety tool.23
More broadly, it is notable that several contributing factors, including errors in clinical judgment (as discussed previously), problems with communication, and issues with the clinical environment, were significantly associated with malpractice cases closing with payment. This demonstrates that systematically examining malpractice claims to determine the underlying contributing factors can generate predictive analytics, as well as suggest risk management and patient safety strategies.
Interdisciplinary collaboration, as a component of systems-based practice, is a core principle of hospital medicine,13 and so we analyzed the involvement of other clinicians in hospitalist claims. Of the five specialties most frequently named in claims with hospitalists, two were surgical services: general surgery (n = 51; 4.2%) and orthopedic surgery (n = 46; 3.8%). With hospitalists being asked to play an increasing role in the care of surgical patients, they may be providing care to patient populations with whom they have less experience, which could put them at risk of adverse outcomes, leading to malpractice claims.24,25 Hospitalists need to be attuned to the liability risks related to the care of patients requiring surgical management and ensure areas of responsibility are clearly delineated between the hospital medicine and surgical services.26 We also found that hospitalist claims increasingly involve PAs and NPs, likely reflecting their increasing role in providing care on hospitalist services.27,28
A prior analysis of claims rates for hospitalists that covered injury dates from 1997 to 2011 found that hospitalists had a relatively low claims rate, significantly lower than that for other internal medicine physicians.8 In addition to covering an earlier time period, that analysis based its claims rates on data from academic medical centers covered by a single insurer, and physicians at academic medical centers generally have lower claims rates, likely due, at least in part, to their spending a smaller proportion of their time on patient care, compared with nonacademic physicians.29 Another analysis of hospitalist closed claims, which shared some cases with the cohort we analyzed, was performed by The Doctors Company, a commercial liability insurer.7 That analysis astutely emphasized the importance of breakdowns in diagnostic processes as a factor underlying hospitalist claims.
Our study has several limitations. First, although our database of malpractice claims includes approximately 31% of all the claims in the country and includes claims from every state, it may not be nationally representative. Another limitation relates to calculating the claims rates for physicians. Detailed information on the number of years of clinical activity, which is necessary to calculate claims rates, was available for only a subset of our data (8.2% of the hospitalist cases and 11.6% of all cases), so claims rates are based on this subset of our data (among which academic centers are overrepresented). Therefore, the claims rates should be interpreted with caution, especially regarding their application to the community hospital setting. The institutions included in the subset of our data used for determining claims rates were stable over time, so the use of a subset of our data for calculating claims rates reduces the generalizability of our claims rates but should not be a source of bias.
Potentially offsetting strengths of our claims database and study include the availability of unpaid claims (which outnumber paid claims roughly 2:1)11,12; the presence of information on contributing factors and other case characteristics obtained through structured manual review of the cases; and the availability of the specialties of the clinicians involved. These features distinguish the database we used from the NPDB, another national database of malpractice claims, which does not include unpaid claims and which does not include information on contributing factors or physician specialty.
CONCLUSION
First described in 1996, the hospitalist field is the fastest growing specialty in modern medical history.18,30 Therefore, an understanding of the malpractice risk of hospitalists is important and can shed light on the patient safety environment in hospitals. Our analysis showed that hospitalist malpractice claims rates remain roughly stable, in contrast to most other specialties, which have seen a fall in malpractice claims rates.17 In addition, unlike a previous analysis,8 we found that claims rates for hospitalists were essentially equal to those of other general internal medicine physicians (not lower, as had been previously reported), and higher than those of the internal medicine subspecialties. Hospitalist claims also have relatively high severity of injury. Potential factors driving these trends include the increasing demand for hospitalists, which results in a higher proportion of less-experienced physicians entering the field, and the expanding clinical scope of hospitalists, which may lead to their managing patients with conditions with which they may be less comfortable. Overall, our analysis suggests that the malpractice environment for hospitalists is becoming less favorable, and therefore, hospitalists should explore opportunities for mitigating liability risk and enhancing patient safety.
1. Studdert DM, Mello MM, Sage WM, et al. Defensive medicine among high-risk specialist physicians in a volatile malpractice environment. JAMA. 2005;293(21):2609-2617. https://doi.org/10.1001/jama.293.21.2609
2. Carrier ER, Reschovsky JD, Mello MM, Mayrell RC, Katz D. Physicians’ fears of malpractice lawsuits are not assuaged by tort reforms. Health Aff (Millwood). 2010;29(9):1585-1592. https://doi.org/10.1377/hlthaff.2010.0135
3. Kachalia A, Berg A, Fagerlin A, et al. Overuse of testing in preoperative evaluation and syncope: a survey of hospitalists. Ann Intern Med. 2015;162(2):100-108. https://doi.org/10.7326/m14-0694
4. Mello MM, Chandra A, Gawande AA, Studdert DM. National costs of the medical liability system. Health Aff (Millwood). 2010;29(9):1569-1577. https://doi.org/10.1377/hlthaff.2009.0807
5. Rothberg MB, Class J, Bishop TF, Friderici J, Kleppel R, Lindenauer PK. The cost of defensive medicine on 3 hospital medicine services. JAMA Intern Med. 2014;174(11):1867-1868. https://doi.org/10.1001/jamainternmed.2014.4649
6. Saint S, Vaughn VM, Chopra V, Fowler KE, Kachalia A. Perception of resources spent on defensive medicine and history of being sued among hospitalists: results from a national survey. J Hosp Med. 2018;13(1):26-29. https://doi.org/10.12788/jhm.2800
7. Ranum D, Troxel DB, Diamond R. Hospitalist Closed Claims Study: An Expert Analysis of Medical Malpractice Allegations. The Doctors Company. 2016. https://www.thedoctors.com/siteassets/pdfs/risk-management/closed-claims-studies/10392_ccs-hospitalist_academic_single-page_version_frr.pdf
8. Schaffer AC, Puopolo AL, Raman S, Kachalia A. Liability impact of the hospitalist model of care. J Hosp Med. 2014;9(12):750-755. https://doi.org/10.1002/jhm.2244
9. Localio AR, Lawthers AG, Brennan TA, et al. Relation between malpractice claims and adverse events due to negligence. results of the Harvard Medical Practice Study III. N Engl J Med. 1991;325(4):245-251. https://doi.org/10.1056/nejm199107253250405
10. Studdert DM, Thomas EJ, Burstin HR, Zbar BI, Orav EJ, Brennan TA. Negligent care and malpractice claiming behavior in Utah and Colorado. Med Care. 2000;38(3):250-260. https://doi.org/10.1097/00005650-200003000-00002
11. Studdert DM, Mello MM, Gawande AA, et al. Claims, errors, and compensation payments in medical malpractice litigation. N Engl J Med. 2006;354(19):2024-2033. https://doi.org/10.1056/nejmsa054479
12. Medical Malpractice in America: 2018 CRICO Strategies National CBS Report. CRICO Strategies; 2018.
13. Budnitz T, McKean SC. The Core Competencies in Hospital Medicine. In: McKean SC, Ross JJ, Dressler DD, Scheurer DB, eds. Principles and Practice of Hospital Medicine, 2nd ed. McGraw-Hill Education; 2017.
14. O’Leary KJ, Haviley C, Slade ME, Shah HM, Lee J, Williams MV. Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit. J Hosp Med. 2011;6(2):88-93. https://doi.org/10.1002/jhm.714
15. National Practitioner Data Bank: Public Use Data File. Division of Practitioner Data Banks, Bureau of Health Professions, Health Resources & Services Administration, U.S. Department of Health & Human Services; June 30, 2019. Updated August 2020.
16. Sowka MP, ed. NAIC Malpractice Claims, Final Compilation. National Association of Insurance Commissioners; 1980.
17. Schaffer AC, Jena AB, Seabury SA, Singh H, Chalasani V, Kachalia A. Rates and characteristics of paid malpractice claims among US physicians by specialty, 1992-2014. JAMA Intern Med. 2017;177(5):710-718. https://doi.org/10.1001/jamainternmed.2017.0311
18. Wachter RM, Goldman L. Zero to 50,000 - the 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/nejmp1607958
19. Miller CS, Fogerty RL, Gann J, Bruti CP, Klein R; The Society of General Internal Medicine Membership Committee. The growth of hospitalists and the future of the Society of General Internal Medicine: results from the 2014 membership survey. J Gen Intern Med. 2017;32(11):1179-1185. https://doi.org/10.1007/s11606-017-4126-7
20. Goodwin JS, Salameh H, Zhou J, Singh S, Kuo YF, Nattinger AB. Association of hospitalist years of experience with mortality in the hospitalized Medicare population. JAMA Intern Med. 2018;178(2):196-203. https://doi.org/10.1001/jamainternmed.2017.7049
21. Akintoye E, Briasoulis A, Egbe A, et al. National trends in admission and in-hospital mortality of patients with heart failure in the United States (2001-2014). J Am Heart Assoc. 2017;6(12):e006955. https://doi.org/10.1161/jaha.117.006955
22. Clark AV, LoPresti CM, Smith TI. Trends in inpatient admission comorbidity and electronic health data: implications for resident workload intensity. J Hosp Med. 2018;13(8):570-572. https://doi.org/10.12788/jhm.2954
23. Gilmartin HM, Liu VX, Burke RE. Annals for hospitalists inpatient notes - The role of hospitalists in the creation of learning healthcare systems. Ann Intern Med. 2020;172(2):HO2-HO3. https://doi.org/10.7326/m19-3873
24. Siegal EM. Just because you can, doesn’t mean that you should: a call for the rational application of hospitalist comanagement. J Hosp Med. 2008;3(5):398-402. https://doi.org/10.1002/jhm.361
25. Plauth WH 3rd, Pantilat SZ, Wachter RM, Fenton CL. Hospitalists’ perceptions of their residency training needs: results of a national survey. Am J Med. 2001;111(3):247-254. https://doi.org/10.1016/s0002-9343(01)00837-3
26. Thompson RE, Pfeifer K, Grant PJ, et al. Hospital medicine and perioperative care: a framework for high-quality, high-value collaborative care. J Hosp Med. 2017;12(4):277-282. https://doi.org/10.12788/jhm.2717
27. Torok H, Lackner C, Landis R, Wright S. Learning needs of physician assistants working in hospital medicine. J Hosp Med. 2012;7(3):190-194. https://doi.org/10.1002/jhm.1001
28. Kartha A, Restuccia JD, Burgess JF Jr, et al. Nurse practitioner and physician assistant scope of practice in 118 acute care hospitals. J Hosp Med. 2014;9(10):615-620. https://doi.org/10.1002/jhm.2231
29. Schaffer AC, Babayan A, Yu-Moe CW, Sato L, Einbinder JS. The effect of clinical volume on annual and per-patient encounter medical malpractice claims risk. J Patient Saf. Published online March 23, 2020. https://doi.org/10.1097/pts.0000000000000706
30. Wachter RM, Goldman L. The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335(7):514-517. https://doi.org/10.1056/nejm199608153350713
1. Studdert DM, Mello MM, Sage WM, et al. Defensive medicine among high-risk specialist physicians in a volatile malpractice environment. JAMA. 2005;293(21):2609-2617. https://doi.org/10.1001/jama.293.21.2609
2. Carrier ER, Reschovsky JD, Mello MM, Mayrell RC, Katz D. Physicians’ fears of malpractice lawsuits are not assuaged by tort reforms. Health Aff (Millwood). 2010;29(9):1585-1592. https://doi.org/10.1377/hlthaff.2010.0135
3. Kachalia A, Berg A, Fagerlin A, et al. Overuse of testing in preoperative evaluation and syncope: a survey of hospitalists. Ann Intern Med. 2015;162(2):100-108. https://doi.org/10.7326/m14-0694
4. Mello MM, Chandra A, Gawande AA, Studdert DM. National costs of the medical liability system. Health Aff (Millwood). 2010;29(9):1569-1577. https://doi.org/10.1377/hlthaff.2009.0807
5. Rothberg MB, Class J, Bishop TF, Friderici J, Kleppel R, Lindenauer PK. The cost of defensive medicine on 3 hospital medicine services. JAMA Intern Med. 2014;174(11):1867-1868. https://doi.org/10.1001/jamainternmed.2014.4649
6. Saint S, Vaughn VM, Chopra V, Fowler KE, Kachalia A. Perception of resources spent on defensive medicine and history of being sued among hospitalists: results from a national survey. J Hosp Med. 2018;13(1):26-29. https://doi.org/10.12788/jhm.2800
7. Ranum D, Troxel DB, Diamond R. Hospitalist Closed Claims Study: An Expert Analysis of Medical Malpractice Allegations. The Doctors Company. 2016. https://www.thedoctors.com/siteassets/pdfs/risk-management/closed-claims-studies/10392_ccs-hospitalist_academic_single-page_version_frr.pdf
8. Schaffer AC, Puopolo AL, Raman S, Kachalia A. Liability impact of the hospitalist model of care. J Hosp Med. 2014;9(12):750-755. https://doi.org/10.1002/jhm.2244
9. Localio AR, Lawthers AG, Brennan TA, et al. Relation between malpractice claims and adverse events due to negligence. results of the Harvard Medical Practice Study III. N Engl J Med. 1991;325(4):245-251. https://doi.org/10.1056/nejm199107253250405
10. Studdert DM, Thomas EJ, Burstin HR, Zbar BI, Orav EJ, Brennan TA. Negligent care and malpractice claiming behavior in Utah and Colorado. Med Care. 2000;38(3):250-260. https://doi.org/10.1097/00005650-200003000-00002
11. Studdert DM, Mello MM, Gawande AA, et al. Claims, errors, and compensation payments in medical malpractice litigation. N Engl J Med. 2006;354(19):2024-2033. https://doi.org/10.1056/nejmsa054479
12. Medical Malpractice in America: 2018 CRICO Strategies National CBS Report. CRICO Strategies; 2018.
13. Budnitz T, McKean SC. The Core Competencies in Hospital Medicine. In: McKean SC, Ross JJ, Dressler DD, Scheurer DB, eds. Principles and Practice of Hospital Medicine, 2nd ed. McGraw-Hill Education; 2017.
14. O’Leary KJ, Haviley C, Slade ME, Shah HM, Lee J, Williams MV. Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit. J Hosp Med. 2011;6(2):88-93. https://doi.org/10.1002/jhm.714
15. National Practitioner Data Bank: Public Use Data File. Division of Practitioner Data Banks, Bureau of Health Professions, Health Resources & Services Administration, U.S. Department of Health & Human Services; June 30, 2019. Updated August 2020.
16. Sowka MP, ed. NAIC Malpractice Claims, Final Compilation. National Association of Insurance Commissioners; 1980.
17. Schaffer AC, Jena AB, Seabury SA, Singh H, Chalasani V, Kachalia A. Rates and characteristics of paid malpractice claims among US physicians by specialty, 1992-2014. JAMA Intern Med. 2017;177(5):710-718. https://doi.org/10.1001/jamainternmed.2017.0311
18. Wachter RM, Goldman L. Zero to 50,000 - the 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/nejmp1607958
19. Miller CS, Fogerty RL, Gann J, Bruti CP, Klein R; The Society of General Internal Medicine Membership Committee. The growth of hospitalists and the future of the Society of General Internal Medicine: results from the 2014 membership survey. J Gen Intern Med. 2017;32(11):1179-1185. https://doi.org/10.1007/s11606-017-4126-7
20. Goodwin JS, Salameh H, Zhou J, Singh S, Kuo YF, Nattinger AB. Association of hospitalist years of experience with mortality in the hospitalized Medicare population. JAMA Intern Med. 2018;178(2):196-203. https://doi.org/10.1001/jamainternmed.2017.7049
21. Akintoye E, Briasoulis A, Egbe A, et al. National trends in admission and in-hospital mortality of patients with heart failure in the United States (2001-2014). J Am Heart Assoc. 2017;6(12):e006955. https://doi.org/10.1161/jaha.117.006955
22. Clark AV, LoPresti CM, Smith TI. Trends in inpatient admission comorbidity and electronic health data: implications for resident workload intensity. J Hosp Med. 2018;13(8):570-572. https://doi.org/10.12788/jhm.2954
23. Gilmartin HM, Liu VX, Burke RE. Annals for hospitalists inpatient notes - The role of hospitalists in the creation of learning healthcare systems. Ann Intern Med. 2020;172(2):HO2-HO3. https://doi.org/10.7326/m19-3873
24. Siegal EM. Just because you can, doesn’t mean that you should: a call for the rational application of hospitalist comanagement. J Hosp Med. 2008;3(5):398-402. https://doi.org/10.1002/jhm.361
25. Plauth WH 3rd, Pantilat SZ, Wachter RM, Fenton CL. Hospitalists’ perceptions of their residency training needs: results of a national survey. Am J Med. 2001;111(3):247-254. https://doi.org/10.1016/s0002-9343(01)00837-3
26. Thompson RE, Pfeifer K, Grant PJ, et al. Hospital medicine and perioperative care: a framework for high-quality, high-value collaborative care. J Hosp Med. 2017;12(4):277-282. https://doi.org/10.12788/jhm.2717
27. Torok H, Lackner C, Landis R, Wright S. Learning needs of physician assistants working in hospital medicine. J Hosp Med. 2012;7(3):190-194. https://doi.org/10.1002/jhm.1001
28. Kartha A, Restuccia JD, Burgess JF Jr, et al. Nurse practitioner and physician assistant scope of practice in 118 acute care hospitals. J Hosp Med. 2014;9(10):615-620. https://doi.org/10.1002/jhm.2231
29. Schaffer AC, Babayan A, Yu-Moe CW, Sato L, Einbinder JS. The effect of clinical volume on annual and per-patient encounter medical malpractice claims risk. J Patient Saf. Published online March 23, 2020. https://doi.org/10.1097/pts.0000000000000706
30. Wachter RM, Goldman L. The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335(7):514-517. https://doi.org/10.1056/nejm199608153350713
© 2021 Society of Hospital Medicine
Trauma-Informed Transformation of Evaluation and Licensure for Physicians With Mental Illness
As a physician living with bipolar disorder, I am more intimately familiar with the psychiatric ward than I would like to admit. Despite a decade of medication and weekly therapy, I live with a disease which flares, not unlike many of the patients I care for.
–Justin L Bullock, MD, MPH
Mental health conditions are common among physicians. Approximately 28% of residents experience depression or depressive symptoms during their training,1 and many will not seek mental health care due to fear of medical licensing implications.2 These fears are well-founded. Executive directors of 13 of 35 state medical boards indicated that diagnosis of mental illness alone was sufficient for sanctioning physicians.3 Another study found that two-thirds of state licensing applications pose questions about providers’ mental health that violate the American with Disabilities Act.2 What happens when a physician discloses and trusts the system to support their mental health–related needs? We address this question through the story of one trainee (JLB) recounting his experience with help-seeking and a fitness-for-duty (FFD) evaluation and conclude with recommendations to improve FFD processes.
Once again, I found myself sitting on a sheetless hospital bed with a baggy hospital gown draping my bony shoulders as I described my aborted suicide attempt to an attending psychiatrist. After a brief pause, the psychiatrist told me that they did not want to cause problems for my medical license and would drop my psychiatric hold. Even though they were the third psychiatrist to say this to me, their words caught me off guard given my recent attempt. I believe these psychiatrists factored my medical career into their clinical care because they understood that the simple act of being psychiatrically hospitalized placed my medical career in peril. After I completed a month-long outpatient treatment program, a psychiatrist and therapist cleared me to return to work.
I feel no shame about living with bipolar disorder and have always been transparent with my institution; the reason behind my absence was no secret. But before I could return to work, my case had to be reviewed by my institution’s Physician Well-being Committee. This committee was presented to me as a group that would help determine how to best support my return to work. However, before I had an opportunity to speak with the committee, I was informed that I would have to undergo a formal FFD evaluation.
FITNESS FOR DUTY
A FFD evaluation is indicated when there are credible reports of physician impairment or professional misconduct. Its purpose is to ensure that physicians can perform their essential job functions and that they are not a risk to patient safety. The Federation of State Medical Boards cautions that illness should not be conflated with impairment.4 Indeed, physicians with mental illness can function safely, thrive, and benefit patients and peers, especially when accommodations or modification reduce workplace barriers.5,6
I can best describe FFD as a 2-month-long stigmatized interrogation. I was forced to provide hair, blood, and urine samples for drug tests, complete an extensive multi-day psychiatric interview—including questions about my childhood trauma—and given a personality test. I was asked to disclose all of my private mental health records and feared I would be penalized if I refused to answer any questions. Multiple people, including my program director, confirmed that there were no performance or professionalism concerns. My suicide attempt happened outside of work; in my eyes, I had a mental illness which had been appropriately treated, not a workplace issue. I voiced my concern that the committee discriminated against me based on my mental illness. The committee told me that their decision to conduct a FFD was warranted because I had a condition that could affect my cognition, despite the lack of evidence that it actually had. My institution’s Office for Prevention of Harassment and Discrimination concurred that this was legal practice.
All of this happened despite my transparency regarding my bipolar disorder. Before I began residency, I disclosed my illness to my institution’s disability office, and I requested and received workplace accommodations. I have appropriately called-out of work whenever I felt that my bipolar could interfere with my focus on patient care. I have been openly bipolar and at the same institution for 6 years. In that time, I have received multiple teaching awards and a graduation award for exemplifying the qualities of a true physician—all while managing bipolar flares, including four hospitalizations. To destigmatize mental illness, I have discussed suicidality and getting help multiple times in front of hundreds of medical students.
The FFD evaluation found no evidence of a substance use disorder, nonadherence to treatment, or danger to my patients. Despite no adverse findings, and a record of well-managed mental illness and ongoing treatment, if I wanted to return to residency, I had to sign an agreement stipulating frequent monitoring by a “case-manager” and worksite “mentor.” I felt stigmatized and penalized for getting help. As I spoke out publicly against this process, I learned that my FFD experience is neither unique nor uncommon. Mental illness is deeply stigmatized within medicine. As a gay Black bipolar man, I hold multiple marginalized identities that inform and shape my experiences, yet the FFD committee did not have a single psychiatrist nor a single Black member. Instead, to make decisions on my case, they relied on the recommendations of the external psychiatrist, who met me for two days. In addition to the therapy and medications I had been taking for years, in order to return to work, I had to agree to a new type of therapy for the remainder of residency. I was incredulous that my employers felt it was appropriate to dictate my specific psychiatric care when I already had my own providers and my own care plan. My voice was not heard in this process, and despite my objections and the institutional mentors who spoke up for me, the FFD committee would not permit me to work without agreeing to their unmodified terms.
RE-ENVISIONING PHYSICIAN EVALUATION
Institutions face the challenging task of simultaneously navigating physician illness, patient safety, and institutional liability. In our opinion, many institutions excessively scrutinize physicians with mental illness and initiate FFD evaluations for reasons that are disproportionately skewed toward minimizing institutional liability. Moreover, in the absence of demonstrated physician workplace impairment, institutions should have systems in place to work collaboratively with the physician to ensure that they have access to professional treatment and appropriate workplace accommodations. It is possible to be simultaneously disabled and completely competent; creative and supportive accommodation processes allow physicians with disabilities to thrive, and their patients to benefit from the care of a physician with personal experience navigating disability. If a physician’s mental illness, despite accommodations, begins to impact workplace safety, a FFD evaluation may be initiated; unfortunately, the FFD evaluation itself may become a source of further harm.
FFD EVALUATIONS' POTENTIAL TO HARM PHYSICIANS WITH MENTAL ILLNESS
FFD evaluations often situate the physician as incapable of managing their own mental illness, suggesting that they must be closely monitored and restricted even when physicians come forward independently.7 These beliefs and concurrent policies can propagate harmful, inaccurate biases against physicians who live with mental illness. These biases are compounded by the structural racism endemic in healthcare and academic medicine.8 Dehumanization in medicine adds fuel to this fire, projecting the ideal physician persona as an invulnerable, infallible superhuman who can witness intense suffering and work inhumane hours without impact upon the their mental health and well-being. Altogether, these factors increase fear and discourage help-seeking among physicians with mental illness (Appendix Figure), ultimately harming physicians and patients in the process.9 Given that the FFD process involves evaluation, treatment, surveillance, and restrictions for individuals with stigmatized health conditions, these processes risk amplifying the impacts of trauma, racism, and oppression unless specifically designed to be antiracist, anti-oppressive, and trauma-informed.
TRAUMA-INFORMED APPROACH TO FFD
To encourage physician help-seeking, especially for stigmatized conditions, we must dismantle systems that traumatize physicians with mental illness (Appendix Figure) and build systems that invite and support courageous vulnerability and help-seeking.5,6 Institutions can provide evaluation and oversight of physicians while also adopting trauma-informed care principles. A trauma-informed approach to FFD would ask: How can we create systems that are informed by a genuine understanding of suffering to promote healing and avoid re-traumatization? Trauma-informed care emphasizes safety, trustworthiness, transparency, cultural humility (an antiracist, anti-oppression framework), collaboration, peer support, and patient empowerment.10 FFD evaluations differ by institution and by state, with some being performed internally and others utilizing external state physician health programs. We believe our recommendations below apply independent of context (Table).
NECESSARY CHANGES
Institutional Changes
All institutions must publish a detailed description of the FFD process, including its purpose, the definition of impairment or potential impairment, and the steps of the FFD evaluation. The FFD evaluation should be as limited in scope as possible, without invasive inquiry about the physician’s life-long history. Physicians should be invited to include a peer-support person throughout the entire process. The FFD “return to work agreement” should incorporate meaningful input from the physician as the expert in their own experience and, if already in treatment, informed by their healthcare providers. Given the stigmatization of mental health conditions and inherent power differentials in FFD processes, it is paramount that committees be diverse (including but not limited to race/ethnicity, gender, sexual orientation, and mental illness) and comprised of physicians trained in trauma-informed processes who treat the conditions affecting the individual undergoing the FFD evaluation. Finally, trustworthiness requires accountability: We recommend that all FFD systems establish an external oversight body that is equipped to effect change in real time if a physician reports a potential process violation and that collects anonymous feedback from physicians to inform required continuous quality improvement.
State and Federal Changes
It is difficult to effect meaningful change without accurate measurement of physician suicide. Therefore, we recommend mandatory reporting of physician suicide and suspected suicide to publicly available, de-identified state registries. We call for each state medical licensing board to limit licensing questions to current impairment due to mental illness, substance use disorders, or other health condition, as recommended by the Federation of State Medical Boards.4 There is a critical need for federal legislation to fund improvements in workplace safety and enhanced access to mental health treatment on demand for physicians.
Especially in these extraordinary times, as physicians are being exposed to such high burdens of stress, suffering, loss, and moral injury, we must de-stigmatize mental illness, encourage help-seeking, and provide physicians struggling with mental illness with timely and compassionate support. By creating systems that are healing and supportive for physicians, we enhance healing for all.
Bipolar is my sun and my storm. As a physician, I am not ashamed of that. For my own health and that of my patients, I must work in a system where it is safe to come forward when I am struggling.
As I fought against what I felt was a toxic and injurious process, I was fortunate to not stand alone. More than 600 residents from 18 departments at my institution signed a petition in support of reforming the FFD process informed by my experience. My institution is in the early stages of responding to this display of strength and unity with a diverse taskforce dedicated to improving the Physician Well-being Committee and FFD process.
As I accompany my patients on their healing journeys, my own experience with recovery allows me to hold the hope of healing for them. My family, friends, mentors, and providers held these rays of hope for me when I was lost in my own darkness. I now know that being cured from disease is just one form of healing. My proximity to death grounds me as some of my patients approach the end of life. Notably, some of my primary care patients have read my story online and come to their appointments to tell me that they are proud to have me as their doctor, “bipolar and all.”
1. Mata DA, Ramos MA, Bansal N, et al. Prevalence of depression and depressive symptoms among resident physicians a systematic review and meta-analysis. JAMA. 2015;314(22):2373-2383 . https://doi.org/10.1001/jama.2015.15845
2. Dyrbye LN, West CP, Sinsky CA, Goeders LE, Satele DV, Shanafelt TD. Medical Licensure questions and physician reluctance to seek care for mental health conditions. Mayo Clin Proc. 2017;92(10):1486-1493. https://doi.org/10.1016/j.mayocp.2017.06.020
3. Hendin H, Reynolds C, Fox D, et al. Licensing and physician mental health: problems and possibilities. J Med Licens Discip. 2007;93(2):6-11.
4. Federation of State Medical Boards. Physician wellness and burnout: report and recommendations of the workgroup on physician wellness and burnout. 2018. Accessed February 6, 2021. https://www.fsmb.org/siteassets/advocacy/policies/policy-on-wellness-and-burnout.pdf
5. Kirch D. Physician mental health: my personal journey and professional plea. Acad Med. 2021;96(5):618-620. https://doi.org/10.1097/ACM.0000000000003942
6. Cho HL, Huang CJ. Why mental health–related stigma matters for physician wellbeing, burnout, and patient care. J Gen Intern Med. 2020;24:1-3. https://doi.org/10.1007/s11606-019-05173-6
7. Hill AB. Breaking the stigma - a physician’s perspective on self-care and recovery. N Engl J Med. 2017;376(12):1103-1105. https://doi.org/10.1056/NEJMp1615974
8. Grubbs V. Diversity, equity, and inclusion that matter. N Engl J Med. 2020;383(4):e25. https://doi.org/10.1056/NEJMpv2022639
9. Shanafelt TD, Balch CM, Dyrbye L, et al. Special report: suicidal ideation among American surgeons. Arch Surg. 2011;146(1):54-62. https://doi.org/10.1001/archsurg.2010.292
10. Trauma-Informed Care Implementation Resource Center. Center for Healthcare Strategy. Accessed February 5, 2021. https://www.traumainformedcare.chcs.org/what-is-trauma-informed-care/
As a physician living with bipolar disorder, I am more intimately familiar with the psychiatric ward than I would like to admit. Despite a decade of medication and weekly therapy, I live with a disease which flares, not unlike many of the patients I care for.
–Justin L Bullock, MD, MPH
Mental health conditions are common among physicians. Approximately 28% of residents experience depression or depressive symptoms during their training,1 and many will not seek mental health care due to fear of medical licensing implications.2 These fears are well-founded. Executive directors of 13 of 35 state medical boards indicated that diagnosis of mental illness alone was sufficient for sanctioning physicians.3 Another study found that two-thirds of state licensing applications pose questions about providers’ mental health that violate the American with Disabilities Act.2 What happens when a physician discloses and trusts the system to support their mental health–related needs? We address this question through the story of one trainee (JLB) recounting his experience with help-seeking and a fitness-for-duty (FFD) evaluation and conclude with recommendations to improve FFD processes.
Once again, I found myself sitting on a sheetless hospital bed with a baggy hospital gown draping my bony shoulders as I described my aborted suicide attempt to an attending psychiatrist. After a brief pause, the psychiatrist told me that they did not want to cause problems for my medical license and would drop my psychiatric hold. Even though they were the third psychiatrist to say this to me, their words caught me off guard given my recent attempt. I believe these psychiatrists factored my medical career into their clinical care because they understood that the simple act of being psychiatrically hospitalized placed my medical career in peril. After I completed a month-long outpatient treatment program, a psychiatrist and therapist cleared me to return to work.
I feel no shame about living with bipolar disorder and have always been transparent with my institution; the reason behind my absence was no secret. But before I could return to work, my case had to be reviewed by my institution’s Physician Well-being Committee. This committee was presented to me as a group that would help determine how to best support my return to work. However, before I had an opportunity to speak with the committee, I was informed that I would have to undergo a formal FFD evaluation.
FITNESS FOR DUTY
A FFD evaluation is indicated when there are credible reports of physician impairment or professional misconduct. Its purpose is to ensure that physicians can perform their essential job functions and that they are not a risk to patient safety. The Federation of State Medical Boards cautions that illness should not be conflated with impairment.4 Indeed, physicians with mental illness can function safely, thrive, and benefit patients and peers, especially when accommodations or modification reduce workplace barriers.5,6
I can best describe FFD as a 2-month-long stigmatized interrogation. I was forced to provide hair, blood, and urine samples for drug tests, complete an extensive multi-day psychiatric interview—including questions about my childhood trauma—and given a personality test. I was asked to disclose all of my private mental health records and feared I would be penalized if I refused to answer any questions. Multiple people, including my program director, confirmed that there were no performance or professionalism concerns. My suicide attempt happened outside of work; in my eyes, I had a mental illness which had been appropriately treated, not a workplace issue. I voiced my concern that the committee discriminated against me based on my mental illness. The committee told me that their decision to conduct a FFD was warranted because I had a condition that could affect my cognition, despite the lack of evidence that it actually had. My institution’s Office for Prevention of Harassment and Discrimination concurred that this was legal practice.
All of this happened despite my transparency regarding my bipolar disorder. Before I began residency, I disclosed my illness to my institution’s disability office, and I requested and received workplace accommodations. I have appropriately called-out of work whenever I felt that my bipolar could interfere with my focus on patient care. I have been openly bipolar and at the same institution for 6 years. In that time, I have received multiple teaching awards and a graduation award for exemplifying the qualities of a true physician—all while managing bipolar flares, including four hospitalizations. To destigmatize mental illness, I have discussed suicidality and getting help multiple times in front of hundreds of medical students.
The FFD evaluation found no evidence of a substance use disorder, nonadherence to treatment, or danger to my patients. Despite no adverse findings, and a record of well-managed mental illness and ongoing treatment, if I wanted to return to residency, I had to sign an agreement stipulating frequent monitoring by a “case-manager” and worksite “mentor.” I felt stigmatized and penalized for getting help. As I spoke out publicly against this process, I learned that my FFD experience is neither unique nor uncommon. Mental illness is deeply stigmatized within medicine. As a gay Black bipolar man, I hold multiple marginalized identities that inform and shape my experiences, yet the FFD committee did not have a single psychiatrist nor a single Black member. Instead, to make decisions on my case, they relied on the recommendations of the external psychiatrist, who met me for two days. In addition to the therapy and medications I had been taking for years, in order to return to work, I had to agree to a new type of therapy for the remainder of residency. I was incredulous that my employers felt it was appropriate to dictate my specific psychiatric care when I already had my own providers and my own care plan. My voice was not heard in this process, and despite my objections and the institutional mentors who spoke up for me, the FFD committee would not permit me to work without agreeing to their unmodified terms.
RE-ENVISIONING PHYSICIAN EVALUATION
Institutions face the challenging task of simultaneously navigating physician illness, patient safety, and institutional liability. In our opinion, many institutions excessively scrutinize physicians with mental illness and initiate FFD evaluations for reasons that are disproportionately skewed toward minimizing institutional liability. Moreover, in the absence of demonstrated physician workplace impairment, institutions should have systems in place to work collaboratively with the physician to ensure that they have access to professional treatment and appropriate workplace accommodations. It is possible to be simultaneously disabled and completely competent; creative and supportive accommodation processes allow physicians with disabilities to thrive, and their patients to benefit from the care of a physician with personal experience navigating disability. If a physician’s mental illness, despite accommodations, begins to impact workplace safety, a FFD evaluation may be initiated; unfortunately, the FFD evaluation itself may become a source of further harm.
FFD EVALUATIONS' POTENTIAL TO HARM PHYSICIANS WITH MENTAL ILLNESS
FFD evaluations often situate the physician as incapable of managing their own mental illness, suggesting that they must be closely monitored and restricted even when physicians come forward independently.7 These beliefs and concurrent policies can propagate harmful, inaccurate biases against physicians who live with mental illness. These biases are compounded by the structural racism endemic in healthcare and academic medicine.8 Dehumanization in medicine adds fuel to this fire, projecting the ideal physician persona as an invulnerable, infallible superhuman who can witness intense suffering and work inhumane hours without impact upon the their mental health and well-being. Altogether, these factors increase fear and discourage help-seeking among physicians with mental illness (Appendix Figure), ultimately harming physicians and patients in the process.9 Given that the FFD process involves evaluation, treatment, surveillance, and restrictions for individuals with stigmatized health conditions, these processes risk amplifying the impacts of trauma, racism, and oppression unless specifically designed to be antiracist, anti-oppressive, and trauma-informed.
TRAUMA-INFORMED APPROACH TO FFD
To encourage physician help-seeking, especially for stigmatized conditions, we must dismantle systems that traumatize physicians with mental illness (Appendix Figure) and build systems that invite and support courageous vulnerability and help-seeking.5,6 Institutions can provide evaluation and oversight of physicians while also adopting trauma-informed care principles. A trauma-informed approach to FFD would ask: How can we create systems that are informed by a genuine understanding of suffering to promote healing and avoid re-traumatization? Trauma-informed care emphasizes safety, trustworthiness, transparency, cultural humility (an antiracist, anti-oppression framework), collaboration, peer support, and patient empowerment.10 FFD evaluations differ by institution and by state, with some being performed internally and others utilizing external state physician health programs. We believe our recommendations below apply independent of context (Table).

NECESSARY CHANGES
Institutional Changes
All institutions must publish a detailed description of the FFD process, including its purpose, the definition of impairment or potential impairment, and the steps of the FFD evaluation. The FFD evaluation should be as limited in scope as possible, without invasive inquiry about the physician’s life-long history. Physicians should be invited to include a peer-support person throughout the entire process. The FFD “return to work agreement” should incorporate meaningful input from the physician as the expert in their own experience and, if already in treatment, informed by their healthcare providers. Given the stigmatization of mental health conditions and inherent power differentials in FFD processes, it is paramount that committees be diverse (including but not limited to race/ethnicity, gender, sexual orientation, and mental illness) and comprised of physicians trained in trauma-informed processes who treat the conditions affecting the individual undergoing the FFD evaluation. Finally, trustworthiness requires accountability: We recommend that all FFD systems establish an external oversight body that is equipped to effect change in real time if a physician reports a potential process violation and that collects anonymous feedback from physicians to inform required continuous quality improvement.
State and Federal Changes
It is difficult to effect meaningful change without accurate measurement of physician suicide. Therefore, we recommend mandatory reporting of physician suicide and suspected suicide to publicly available, de-identified state registries. We call for each state medical licensing board to limit licensing questions to current impairment due to mental illness, substance use disorders, or other health condition, as recommended by the Federation of State Medical Boards.4 There is a critical need for federal legislation to fund improvements in workplace safety and enhanced access to mental health treatment on demand for physicians.
Especially in these extraordinary times, as physicians are being exposed to such high burdens of stress, suffering, loss, and moral injury, we must de-stigmatize mental illness, encourage help-seeking, and provide physicians struggling with mental illness with timely and compassionate support. By creating systems that are healing and supportive for physicians, we enhance healing for all.
Bipolar is my sun and my storm. As a physician, I am not ashamed of that. For my own health and that of my patients, I must work in a system where it is safe to come forward when I am struggling.
As I fought against what I felt was a toxic and injurious process, I was fortunate to not stand alone. More than 600 residents from 18 departments at my institution signed a petition in support of reforming the FFD process informed by my experience. My institution is in the early stages of responding to this display of strength and unity with a diverse taskforce dedicated to improving the Physician Well-being Committee and FFD process.
As I accompany my patients on their healing journeys, my own experience with recovery allows me to hold the hope of healing for them. My family, friends, mentors, and providers held these rays of hope for me when I was lost in my own darkness. I now know that being cured from disease is just one form of healing. My proximity to death grounds me as some of my patients approach the end of life. Notably, some of my primary care patients have read my story online and come to their appointments to tell me that they are proud to have me as their doctor, “bipolar and all.”
As a physician living with bipolar disorder, I am more intimately familiar with the psychiatric ward than I would like to admit. Despite a decade of medication and weekly therapy, I live with a disease which flares, not unlike many of the patients I care for.
–Justin L Bullock, MD, MPH
Mental health conditions are common among physicians. Approximately 28% of residents experience depression or depressive symptoms during their training,1 and many will not seek mental health care due to fear of medical licensing implications.2 These fears are well-founded. Executive directors of 13 of 35 state medical boards indicated that diagnosis of mental illness alone was sufficient for sanctioning physicians.3 Another study found that two-thirds of state licensing applications pose questions about providers’ mental health that violate the American with Disabilities Act.2 What happens when a physician discloses and trusts the system to support their mental health–related needs? We address this question through the story of one trainee (JLB) recounting his experience with help-seeking and a fitness-for-duty (FFD) evaluation and conclude with recommendations to improve FFD processes.
Once again, I found myself sitting on a sheetless hospital bed with a baggy hospital gown draping my bony shoulders as I described my aborted suicide attempt to an attending psychiatrist. After a brief pause, the psychiatrist told me that they did not want to cause problems for my medical license and would drop my psychiatric hold. Even though they were the third psychiatrist to say this to me, their words caught me off guard given my recent attempt. I believe these psychiatrists factored my medical career into their clinical care because they understood that the simple act of being psychiatrically hospitalized placed my medical career in peril. After I completed a month-long outpatient treatment program, a psychiatrist and therapist cleared me to return to work.
I feel no shame about living with bipolar disorder and have always been transparent with my institution; the reason behind my absence was no secret. But before I could return to work, my case had to be reviewed by my institution’s Physician Well-being Committee. This committee was presented to me as a group that would help determine how to best support my return to work. However, before I had an opportunity to speak with the committee, I was informed that I would have to undergo a formal FFD evaluation.
FITNESS FOR DUTY
A FFD evaluation is indicated when there are credible reports of physician impairment or professional misconduct. Its purpose is to ensure that physicians can perform their essential job functions and that they are not a risk to patient safety. The Federation of State Medical Boards cautions that illness should not be conflated with impairment.4 Indeed, physicians with mental illness can function safely, thrive, and benefit patients and peers, especially when accommodations or modification reduce workplace barriers.5,6
I can best describe FFD as a 2-month-long stigmatized interrogation. I was forced to provide hair, blood, and urine samples for drug tests, complete an extensive multi-day psychiatric interview—including questions about my childhood trauma—and given a personality test. I was asked to disclose all of my private mental health records and feared I would be penalized if I refused to answer any questions. Multiple people, including my program director, confirmed that there were no performance or professionalism concerns. My suicide attempt happened outside of work; in my eyes, I had a mental illness which had been appropriately treated, not a workplace issue. I voiced my concern that the committee discriminated against me based on my mental illness. The committee told me that their decision to conduct a FFD was warranted because I had a condition that could affect my cognition, despite the lack of evidence that it actually had. My institution’s Office for Prevention of Harassment and Discrimination concurred that this was legal practice.
All of this happened despite my transparency regarding my bipolar disorder. Before I began residency, I disclosed my illness to my institution’s disability office, and I requested and received workplace accommodations. I have appropriately called-out of work whenever I felt that my bipolar could interfere with my focus on patient care. I have been openly bipolar and at the same institution for 6 years. In that time, I have received multiple teaching awards and a graduation award for exemplifying the qualities of a true physician—all while managing bipolar flares, including four hospitalizations. To destigmatize mental illness, I have discussed suicidality and getting help multiple times in front of hundreds of medical students.
The FFD evaluation found no evidence of a substance use disorder, nonadherence to treatment, or danger to my patients. Despite no adverse findings, and a record of well-managed mental illness and ongoing treatment, if I wanted to return to residency, I had to sign an agreement stipulating frequent monitoring by a “case-manager” and worksite “mentor.” I felt stigmatized and penalized for getting help. As I spoke out publicly against this process, I learned that my FFD experience is neither unique nor uncommon. Mental illness is deeply stigmatized within medicine. As a gay Black bipolar man, I hold multiple marginalized identities that inform and shape my experiences, yet the FFD committee did not have a single psychiatrist nor a single Black member. Instead, to make decisions on my case, they relied on the recommendations of the external psychiatrist, who met me for two days. In addition to the therapy and medications I had been taking for years, in order to return to work, I had to agree to a new type of therapy for the remainder of residency. I was incredulous that my employers felt it was appropriate to dictate my specific psychiatric care when I already had my own providers and my own care plan. My voice was not heard in this process, and despite my objections and the institutional mentors who spoke up for me, the FFD committee would not permit me to work without agreeing to their unmodified terms.
RE-ENVISIONING PHYSICIAN EVALUATION
Institutions face the challenging task of simultaneously navigating physician illness, patient safety, and institutional liability. In our opinion, many institutions excessively scrutinize physicians with mental illness and initiate FFD evaluations for reasons that are disproportionately skewed toward minimizing institutional liability. Moreover, in the absence of demonstrated physician workplace impairment, institutions should have systems in place to work collaboratively with the physician to ensure that they have access to professional treatment and appropriate workplace accommodations. It is possible to be simultaneously disabled and completely competent; creative and supportive accommodation processes allow physicians with disabilities to thrive, and their patients to benefit from the care of a physician with personal experience navigating disability. If a physician’s mental illness, despite accommodations, begins to impact workplace safety, a FFD evaluation may be initiated; unfortunately, the FFD evaluation itself may become a source of further harm.
FFD EVALUATIONS' POTENTIAL TO HARM PHYSICIANS WITH MENTAL ILLNESS
FFD evaluations often situate the physician as incapable of managing their own mental illness, suggesting that they must be closely monitored and restricted even when physicians come forward independently.7 These beliefs and concurrent policies can propagate harmful, inaccurate biases against physicians who live with mental illness. These biases are compounded by the structural racism endemic in healthcare and academic medicine.8 Dehumanization in medicine adds fuel to this fire, projecting the ideal physician persona as an invulnerable, infallible superhuman who can witness intense suffering and work inhumane hours without impact upon the their mental health and well-being. Altogether, these factors increase fear and discourage help-seeking among physicians with mental illness (Appendix Figure), ultimately harming physicians and patients in the process.9 Given that the FFD process involves evaluation, treatment, surveillance, and restrictions for individuals with stigmatized health conditions, these processes risk amplifying the impacts of trauma, racism, and oppression unless specifically designed to be antiracist, anti-oppressive, and trauma-informed.
TRAUMA-INFORMED APPROACH TO FFD
To encourage physician help-seeking, especially for stigmatized conditions, we must dismantle systems that traumatize physicians with mental illness (Appendix Figure) and build systems that invite and support courageous vulnerability and help-seeking.5,6 Institutions can provide evaluation and oversight of physicians while also adopting trauma-informed care principles. A trauma-informed approach to FFD would ask: How can we create systems that are informed by a genuine understanding of suffering to promote healing and avoid re-traumatization? Trauma-informed care emphasizes safety, trustworthiness, transparency, cultural humility (an antiracist, anti-oppression framework), collaboration, peer support, and patient empowerment.10 FFD evaluations differ by institution and by state, with some being performed internally and others utilizing external state physician health programs. We believe our recommendations below apply independent of context (Table).

NECESSARY CHANGES
Institutional Changes
All institutions must publish a detailed description of the FFD process, including its purpose, the definition of impairment or potential impairment, and the steps of the FFD evaluation. The FFD evaluation should be as limited in scope as possible, without invasive inquiry about the physician’s life-long history. Physicians should be invited to include a peer-support person throughout the entire process. The FFD “return to work agreement” should incorporate meaningful input from the physician as the expert in their own experience and, if already in treatment, informed by their healthcare providers. Given the stigmatization of mental health conditions and inherent power differentials in FFD processes, it is paramount that committees be diverse (including but not limited to race/ethnicity, gender, sexual orientation, and mental illness) and comprised of physicians trained in trauma-informed processes who treat the conditions affecting the individual undergoing the FFD evaluation. Finally, trustworthiness requires accountability: We recommend that all FFD systems establish an external oversight body that is equipped to effect change in real time if a physician reports a potential process violation and that collects anonymous feedback from physicians to inform required continuous quality improvement.
State and Federal Changes
It is difficult to effect meaningful change without accurate measurement of physician suicide. Therefore, we recommend mandatory reporting of physician suicide and suspected suicide to publicly available, de-identified state registries. We call for each state medical licensing board to limit licensing questions to current impairment due to mental illness, substance use disorders, or other health condition, as recommended by the Federation of State Medical Boards.4 There is a critical need for federal legislation to fund improvements in workplace safety and enhanced access to mental health treatment on demand for physicians.
Especially in these extraordinary times, as physicians are being exposed to such high burdens of stress, suffering, loss, and moral injury, we must de-stigmatize mental illness, encourage help-seeking, and provide physicians struggling with mental illness with timely and compassionate support. By creating systems that are healing and supportive for physicians, we enhance healing for all.
Bipolar is my sun and my storm. As a physician, I am not ashamed of that. For my own health and that of my patients, I must work in a system where it is safe to come forward when I am struggling.
As I fought against what I felt was a toxic and injurious process, I was fortunate to not stand alone. More than 600 residents from 18 departments at my institution signed a petition in support of reforming the FFD process informed by my experience. My institution is in the early stages of responding to this display of strength and unity with a diverse taskforce dedicated to improving the Physician Well-being Committee and FFD process.
As I accompany my patients on their healing journeys, my own experience with recovery allows me to hold the hope of healing for them. My family, friends, mentors, and providers held these rays of hope for me when I was lost in my own darkness. I now know that being cured from disease is just one form of healing. My proximity to death grounds me as some of my patients approach the end of life. Notably, some of my primary care patients have read my story online and come to their appointments to tell me that they are proud to have me as their doctor, “bipolar and all.”
1. Mata DA, Ramos MA, Bansal N, et al. Prevalence of depression and depressive symptoms among resident physicians a systematic review and meta-analysis. JAMA. 2015;314(22):2373-2383 . https://doi.org/10.1001/jama.2015.15845
2. Dyrbye LN, West CP, Sinsky CA, Goeders LE, Satele DV, Shanafelt TD. Medical Licensure questions and physician reluctance to seek care for mental health conditions. Mayo Clin Proc. 2017;92(10):1486-1493. https://doi.org/10.1016/j.mayocp.2017.06.020
3. Hendin H, Reynolds C, Fox D, et al. Licensing and physician mental health: problems and possibilities. J Med Licens Discip. 2007;93(2):6-11.
4. Federation of State Medical Boards. Physician wellness and burnout: report and recommendations of the workgroup on physician wellness and burnout. 2018. Accessed February 6, 2021. https://www.fsmb.org/siteassets/advocacy/policies/policy-on-wellness-and-burnout.pdf
5. Kirch D. Physician mental health: my personal journey and professional plea. Acad Med. 2021;96(5):618-620. https://doi.org/10.1097/ACM.0000000000003942
6. Cho HL, Huang CJ. Why mental health–related stigma matters for physician wellbeing, burnout, and patient care. J Gen Intern Med. 2020;24:1-3. https://doi.org/10.1007/s11606-019-05173-6
7. Hill AB. Breaking the stigma - a physician’s perspective on self-care and recovery. N Engl J Med. 2017;376(12):1103-1105. https://doi.org/10.1056/NEJMp1615974
8. Grubbs V. Diversity, equity, and inclusion that matter. N Engl J Med. 2020;383(4):e25. https://doi.org/10.1056/NEJMpv2022639
9. Shanafelt TD, Balch CM, Dyrbye L, et al. Special report: suicidal ideation among American surgeons. Arch Surg. 2011;146(1):54-62. https://doi.org/10.1001/archsurg.2010.292
10. Trauma-Informed Care Implementation Resource Center. Center for Healthcare Strategy. Accessed February 5, 2021. https://www.traumainformedcare.chcs.org/what-is-trauma-informed-care/
1. Mata DA, Ramos MA, Bansal N, et al. Prevalence of depression and depressive symptoms among resident physicians a systematic review and meta-analysis. JAMA. 2015;314(22):2373-2383 . https://doi.org/10.1001/jama.2015.15845
2. Dyrbye LN, West CP, Sinsky CA, Goeders LE, Satele DV, Shanafelt TD. Medical Licensure questions and physician reluctance to seek care for mental health conditions. Mayo Clin Proc. 2017;92(10):1486-1493. https://doi.org/10.1016/j.mayocp.2017.06.020
3. Hendin H, Reynolds C, Fox D, et al. Licensing and physician mental health: problems and possibilities. J Med Licens Discip. 2007;93(2):6-11.
4. Federation of State Medical Boards. Physician wellness and burnout: report and recommendations of the workgroup on physician wellness and burnout. 2018. Accessed February 6, 2021. https://www.fsmb.org/siteassets/advocacy/policies/policy-on-wellness-and-burnout.pdf
5. Kirch D. Physician mental health: my personal journey and professional plea. Acad Med. 2021;96(5):618-620. https://doi.org/10.1097/ACM.0000000000003942
6. Cho HL, Huang CJ. Why mental health–related stigma matters for physician wellbeing, burnout, and patient care. J Gen Intern Med. 2020;24:1-3. https://doi.org/10.1007/s11606-019-05173-6
7. Hill AB. Breaking the stigma - a physician’s perspective on self-care and recovery. N Engl J Med. 2017;376(12):1103-1105. https://doi.org/10.1056/NEJMp1615974
8. Grubbs V. Diversity, equity, and inclusion that matter. N Engl J Med. 2020;383(4):e25. https://doi.org/10.1056/NEJMpv2022639
9. Shanafelt TD, Balch CM, Dyrbye L, et al. Special report: suicidal ideation among American surgeons. Arch Surg. 2011;146(1):54-62. https://doi.org/10.1001/archsurg.2010.292
10. Trauma-Informed Care Implementation Resource Center. Center for Healthcare Strategy. Accessed February 5, 2021. https://www.traumainformedcare.chcs.org/what-is-trauma-informed-care/
© 2021 Society of Hospital Medicine
Role of 3D Printing and Modeling to Aid in Neuroradiology Education for Medical Trainees
Applications of 3-dimensional (3D) printing in medical imaging and health care are expanding. 3D printing may serve a variety of roles and is used increasingly in the context of presurgical planning, as specific medical models may be created using individual patient imaging data.1 These patient-specific models may assist in medical trainee education, decrease operating room time, improve patient education for potential planned surgery, and guide clinicians for optimizing therapy.1,2 This article discusses the utility of 3D printing at a single institution to serve in enhancing specifically neuroradiology education.
Background
As digital imaging and 3D printing have increased in popularity, the potential application of using imaging data to guide patient therapy has shown significant promise. Computed tomography (CT) is a commonly used modality that can be used to create 3D anatomical models, as it is frequently used in the medical setting, demonstrates excellent resolution to the millimeter scale, and can readily pinpoint pathology on imaging.
Image Acquisition
CT scans can be rapidly obtained, which adds significant value, particularly in the context of point-of-care 3D printing. Another modality commonly used for 3D printing is magnetic resonance imaging (MRI), which unlike CT, does not expose the patient to ionizing radiation. The 3D printing process is initiated with patient-specific CT or MRI data stored in the digital imaging and communications in medicine (DICOM) format, which is the international standard for communication and management of medical imaging information and related data. DICOM allows for faster and robust collaboration among imaging professionals.3
Image Processing
To print 3D anatomical models, patient-specific data must be converted from DICOM into standard tessellation language (STL) format, which can be created and edited with a variety of softwares.3 At James A. Haley Veterans’ Hospital in Tampa, Florida, we use an image processing package that includes the Materialise 3-matic and interactive medical image control system. Image quality is essential; therefore, careful attention to details such as pixel dimensions, slice thickness, and slice increments must be considered.3,4
An STL file creates a 3D image from triangle approximations. The entire 3D shape will be made of numerous large or small triangles, depending on the slice thickness, therefore, quality of the original radiologic image. The size and position of the triangles used to make the model can be varied to approximate the object’s shape. The smaller the triangles, the better the image quality and vice versa. This concept is analogous to approximating a circle using straight lines of equal length—more, smaller lines will result in better approximation of a circle (Figure 1).5,6 Similarly, using smaller triangles allows for better approximation of the image. As the human body is a complex structure, mimicking the body requires a system able to create nongeometrical shapes, which is made possible via these triangle approximations in a 3D STL file.
The creation of an STL file from DICOM data starts with a threshold-based segmentation process followed by additional fine-tuning and edits, and ends in the creation of a 3D part. The initial segmentation can be created with the threshold tool, using a Hounsfield unit range based on the area of interest desired (eg, bone, blood, fat). This is used to create an initial mask, which can be further optimized. The region grow tool allows the user to focus the segmentation by discarding areas that are not directly connected to the region of interest. In contrast, the split mask tool divides areas that are connected. Next, fine-tuning the segmentation using tools such as multiple slice edit helps to optimize the model. After all edits are made, the calculate part tool converts the mask into a 3D component that can be used in downstream applications. For the purposes of demonstration and proof of concept, the models provided in this article were created via open-source hardware designs under free or open licenses.7-9
3D Printing in Neuroradiology Education
Neuroradiologists focus on diagnosing pathology related to the brain, head and neck, and spine. CT and MRI scans are the primary modalities used to diagnose these conditions. 3D printing is a useful tool for the trainee who wishes to fully understand neuroanatomy and obtain further appreciation of imaging pathology as it relates to 3D anatomy. Head and neck imaging are a complex subdiscipline of neuroradiology that often require further training beyond radiology residency. A neuroradiology fellowship that focuses on head and neck imaging extends the training.
3D printing has the potential to improve the understanding of various imaging pathologies by providing the trainee with a more in-depth appreciation of the anterior, middle, and posterior cranial fossa, the skull base foramina (ie, foramen ovale, spinosum, rotundum), and complex 3D areas, such as the pterygopalatine fossa, which are all critical areas to investigate on imaging. Figure 2 highlights how a complex anatomical structure, such as the sphenoid bone when printed in 3D, can be correlated with CT cross-sectional images to supplement the educational experience.
Furthermore, the various lobes, sulci, and gyri of the brain and cerebellum and how they interrelate to nearby vasculature and bony structures can be difficult to conceptualize for early trainees. A 3D-printed cerebellum and its relation to the brainstem is illustrated in Figure 3A. Additional complex head and neck structures of the middle ear membranous and bony labyrinth and ossicles and multiple views of the mandible are shown in Figures 3B through 3E.
3D printing in the context of neurovascular pathology holds great promise, particularly as these models may provide the trainee, patient, and proceduralist essential details such as appearance and morphology of an intracranial aneurysm, relationship and size of the neck of aneurysm, incorporation of vessels emanating from the aneurysmal sac, and details of the dome of the aneurysm. For example, the normal circle of Willis in Figure 4A is juxtaposed with an example of a saccular internal carotid artery aneurysm (Figure 4B).
A variety of conditions can affect the bony spine from degenerative, trauma, neoplastic, and inflammatory etiologies. A CT scan of the spine is readily used to detect these different conditions and often is used in the initial evaluation of trauma as indicated in the American College of Radiology appropriateness criteria.10 In addition, MRI is used to evaluate the spinal cord and to further define spinal stenosis as well as evaluate radiculopathy. An appreciation of the bony and soft tissue structures within the spine can be garnered with the use of 3D models (Figure 5).
Trainees can further their understanding of approaches in spinal procedures, including lumbar puncture, myelography, and facet injections. A variety of approaches to access the spinal canal have been documented, such as interspinous, paraspinous, and interlaminar oblique; 3D-printed models can aid in practicing these procedures.11 For example, a water-filled tube can be inserted into the vertebral canal to provide realistic tactile feedback for simulation of a lumbar puncture. An appreciation of the 3D anatomy can guide the clinician on the optimal approach, which can help limit time and potentially improve outcomes.
Future Directions
Artificial Intelligence (AI) offers the ability to teach computers to perform tasks that ordinarily require human intelligence. In the context of 3D printing, the ability to use AI to readily convert and process DICOM data into printable STL models holds significant promise. Currently, the manual conversion of a DICOM file into a segmented 3D model may take several days, necessitating a number of productive hours even from the imaging and engineering champion. If machines could aid in this process, the ability to readily scale clinical 3D printing and promote widespread adoption would be feasible. Several studies already are looking into this concept to determine how deep learning networks may automatically recognize lesions on medical imaging to assist a human operator, potentially cutting hours from the clinical 3D printing workflow.12,13
Furthermore, there are several applications for AI in the context of 3D printing upstream or before the creation of a 3D model. A number of AI tools are already in use at the CT and MRI scanner. Current strategies leverage deep learning and advances in neural networks to improve image quality and create thin section DICOM data, which can be converted into printable 3D files. Additionally, the ability to automate tasks using AI can improve production capacity by assessing material costs and ensuring cost efficiency, which will be critical as point-of-care 3D printing develops widespread adoption. AI also can reduce printing errors by using automated adaptive feedback, using machine learning to search for possible print errors, and sending feedback to the computer to ensure appropriate settings (eg, temperature settings/environmental conditions).
Conclusions
Based on this single-institution experience, 3D-printed complex neuroanatomical structures seems feasible and may enhance resident education and patient safety. Interested trainees may have the opportunity to learn and be involved in the printing process of new and innovative ideas. Further studies may involve printing various pathologic processes and applying these same steps and principles to other subspecialties of radiology. Finally, AI has the potential to advance the 3D printing process in the future.
1. Rengier F, Mehndiratta A, von Tengg-Kobligk H, et al. 3D printing based on imaging data: review of medical applications. Int J Comput Assist Radiol Surg. 2010;5(4):335-341. doi:10.1007/s11548-010-0476-x
2. Perica E, Sun Z. Patient-specific three-dimensional printing for pre-surgical planning in hepatocellular carcinoma treatment. Quant Imaging Med Surg. 2017;7(6):668-677. doi:10.21037/qims.2017.11.02
3. Hwang JJ, Jung Y-H, Cho B-H. The need for DICOM encapsulation of 3D scanning STL data. Imaging Sci Dent. 2018;48(4):301-302. doi:10.5624/isd.2018.48.4.301
4. Whyms BJ, Vorperian HK, Gentry LR, Schimek EM, Bersu ET, Chung MK. The effect of computed tomographic scanner parameters and 3-dimensional volume rendering techniques on the accuracy of linear, angular, and volumetric measurements of the mandible. Oral Surg Oral Med, Oral Pathol Oral Radiol. 2013;115(5):682-691. doi:10.1016/j.oooo.2013.02.008
5. Materialise Cloud. Triangle reduction. Accessed May 20, 2021. https://cloud.materialise.com/tools/triangle-reduction
6. Comaneanu RM, Tarcolea M, Vlasceanu D, Cotrut MC. Virtual 3D reconstruction, diagnosis and surgical planning with Mimics software. Int J Nano Biomaterials. 2012;4(1);69-77.
7. Thingiverse: Digital designs for physical objects. Accessed May 20, 2021. https://www.thingiverse.com
8. Cults. Download for free 3D models for 3D printers. Accessed May 20, 2021. https://cults3d.com/en
9. yeggi. Search engine for 3D printer models. Accessed May 20, 2021. https://www.yeggi.com
10. Expert Panel on Neurological Imaging and Musculoskeletal Imaging; Beckmann NM, West OC, Nunez D, et al. ACR appropriateness criteria suspected spine trauma. J Am Coll Radiol. 2919;16(5):S264-285. doi:10.1016/j.jacr.2019.02.002
11. McKinney AM. Normal variants of the lumbar and sacral spine. In: Atlas of Head/Neck and Spine Normal Imaging Variants. Springer; 2018:263-321.
12. Sollini M, Bartoli F, Marciano A, et al. Artificial intelligence and hybrid imaging: the best match for personalized medicine in oncology. Eur J Hybrid Imaging. 2020;4(1):24. doi:10.1186/s41824-020-00094-8
13. Küstner T, Hepp T, Fischer M, et al. Fully automated and standardized segmentation of adipose tissue compartments via deep learning in 3D whole-body MRI of epidemiologic cohort studies. Radiol Artif Intell.2020;2(6):e200010. doi:10.1148/ryai.2020200010
Applications of 3-dimensional (3D) printing in medical imaging and health care are expanding. 3D printing may serve a variety of roles and is used increasingly in the context of presurgical planning, as specific medical models may be created using individual patient imaging data.1 These patient-specific models may assist in medical trainee education, decrease operating room time, improve patient education for potential planned surgery, and guide clinicians for optimizing therapy.1,2 This article discusses the utility of 3D printing at a single institution to serve in enhancing specifically neuroradiology education.
Background
As digital imaging and 3D printing have increased in popularity, the potential application of using imaging data to guide patient therapy has shown significant promise. Computed tomography (CT) is a commonly used modality that can be used to create 3D anatomical models, as it is frequently used in the medical setting, demonstrates excellent resolution to the millimeter scale, and can readily pinpoint pathology on imaging.
Image Acquisition
CT scans can be rapidly obtained, which adds significant value, particularly in the context of point-of-care 3D printing. Another modality commonly used for 3D printing is magnetic resonance imaging (MRI), which unlike CT, does not expose the patient to ionizing radiation. The 3D printing process is initiated with patient-specific CT or MRI data stored in the digital imaging and communications in medicine (DICOM) format, which is the international standard for communication and management of medical imaging information and related data. DICOM allows for faster and robust collaboration among imaging professionals.3
Image Processing
To print 3D anatomical models, patient-specific data must be converted from DICOM into standard tessellation language (STL) format, which can be created and edited with a variety of softwares.3 At James A. Haley Veterans’ Hospital in Tampa, Florida, we use an image processing package that includes the Materialise 3-matic and interactive medical image control system. Image quality is essential; therefore, careful attention to details such as pixel dimensions, slice thickness, and slice increments must be considered.3,4
An STL file creates a 3D image from triangle approximations. The entire 3D shape will be made of numerous large or small triangles, depending on the slice thickness, therefore, quality of the original radiologic image. The size and position of the triangles used to make the model can be varied to approximate the object’s shape. The smaller the triangles, the better the image quality and vice versa. This concept is analogous to approximating a circle using straight lines of equal length—more, smaller lines will result in better approximation of a circle (Figure 1).5,6 Similarly, using smaller triangles allows for better approximation of the image. As the human body is a complex structure, mimicking the body requires a system able to create nongeometrical shapes, which is made possible via these triangle approximations in a 3D STL file.
The creation of an STL file from DICOM data starts with a threshold-based segmentation process followed by additional fine-tuning and edits, and ends in the creation of a 3D part. The initial segmentation can be created with the threshold tool, using a Hounsfield unit range based on the area of interest desired (eg, bone, blood, fat). This is used to create an initial mask, which can be further optimized. The region grow tool allows the user to focus the segmentation by discarding areas that are not directly connected to the region of interest. In contrast, the split mask tool divides areas that are connected. Next, fine-tuning the segmentation using tools such as multiple slice edit helps to optimize the model. After all edits are made, the calculate part tool converts the mask into a 3D component that can be used in downstream applications. For the purposes of demonstration and proof of concept, the models provided in this article were created via open-source hardware designs under free or open licenses.7-9
3D Printing in Neuroradiology Education
Neuroradiologists focus on diagnosing pathology related to the brain, head and neck, and spine. CT and MRI scans are the primary modalities used to diagnose these conditions. 3D printing is a useful tool for the trainee who wishes to fully understand neuroanatomy and obtain further appreciation of imaging pathology as it relates to 3D anatomy. Head and neck imaging are a complex subdiscipline of neuroradiology that often require further training beyond radiology residency. A neuroradiology fellowship that focuses on head and neck imaging extends the training.
3D printing has the potential to improve the understanding of various imaging pathologies by providing the trainee with a more in-depth appreciation of the anterior, middle, and posterior cranial fossa, the skull base foramina (ie, foramen ovale, spinosum, rotundum), and complex 3D areas, such as the pterygopalatine fossa, which are all critical areas to investigate on imaging. Figure 2 highlights how a complex anatomical structure, such as the sphenoid bone when printed in 3D, can be correlated with CT cross-sectional images to supplement the educational experience.
Furthermore, the various lobes, sulci, and gyri of the brain and cerebellum and how they interrelate to nearby vasculature and bony structures can be difficult to conceptualize for early trainees. A 3D-printed cerebellum and its relation to the brainstem is illustrated in Figure 3A. Additional complex head and neck structures of the middle ear membranous and bony labyrinth and ossicles and multiple views of the mandible are shown in Figures 3B through 3E.
3D printing in the context of neurovascular pathology holds great promise, particularly as these models may provide the trainee, patient, and proceduralist essential details such as appearance and morphology of an intracranial aneurysm, relationship and size of the neck of aneurysm, incorporation of vessels emanating from the aneurysmal sac, and details of the dome of the aneurysm. For example, the normal circle of Willis in Figure 4A is juxtaposed with an example of a saccular internal carotid artery aneurysm (Figure 4B).
A variety of conditions can affect the bony spine from degenerative, trauma, neoplastic, and inflammatory etiologies. A CT scan of the spine is readily used to detect these different conditions and often is used in the initial evaluation of trauma as indicated in the American College of Radiology appropriateness criteria.10 In addition, MRI is used to evaluate the spinal cord and to further define spinal stenosis as well as evaluate radiculopathy. An appreciation of the bony and soft tissue structures within the spine can be garnered with the use of 3D models (Figure 5).
Trainees can further their understanding of approaches in spinal procedures, including lumbar puncture, myelography, and facet injections. A variety of approaches to access the spinal canal have been documented, such as interspinous, paraspinous, and interlaminar oblique; 3D-printed models can aid in practicing these procedures.11 For example, a water-filled tube can be inserted into the vertebral canal to provide realistic tactile feedback for simulation of a lumbar puncture. An appreciation of the 3D anatomy can guide the clinician on the optimal approach, which can help limit time and potentially improve outcomes.
Future Directions
Artificial Intelligence (AI) offers the ability to teach computers to perform tasks that ordinarily require human intelligence. In the context of 3D printing, the ability to use AI to readily convert and process DICOM data into printable STL models holds significant promise. Currently, the manual conversion of a DICOM file into a segmented 3D model may take several days, necessitating a number of productive hours even from the imaging and engineering champion. If machines could aid in this process, the ability to readily scale clinical 3D printing and promote widespread adoption would be feasible. Several studies already are looking into this concept to determine how deep learning networks may automatically recognize lesions on medical imaging to assist a human operator, potentially cutting hours from the clinical 3D printing workflow.12,13
Furthermore, there are several applications for AI in the context of 3D printing upstream or before the creation of a 3D model. A number of AI tools are already in use at the CT and MRI scanner. Current strategies leverage deep learning and advances in neural networks to improve image quality and create thin section DICOM data, which can be converted into printable 3D files. Additionally, the ability to automate tasks using AI can improve production capacity by assessing material costs and ensuring cost efficiency, which will be critical as point-of-care 3D printing develops widespread adoption. AI also can reduce printing errors by using automated adaptive feedback, using machine learning to search for possible print errors, and sending feedback to the computer to ensure appropriate settings (eg, temperature settings/environmental conditions).
Conclusions
Based on this single-institution experience, 3D-printed complex neuroanatomical structures seems feasible and may enhance resident education and patient safety. Interested trainees may have the opportunity to learn and be involved in the printing process of new and innovative ideas. Further studies may involve printing various pathologic processes and applying these same steps and principles to other subspecialties of radiology. Finally, AI has the potential to advance the 3D printing process in the future.
Applications of 3-dimensional (3D) printing in medical imaging and health care are expanding. 3D printing may serve a variety of roles and is used increasingly in the context of presurgical planning, as specific medical models may be created using individual patient imaging data.1 These patient-specific models may assist in medical trainee education, decrease operating room time, improve patient education for potential planned surgery, and guide clinicians for optimizing therapy.1,2 This article discusses the utility of 3D printing at a single institution to serve in enhancing specifically neuroradiology education.
Background
As digital imaging and 3D printing have increased in popularity, the potential application of using imaging data to guide patient therapy has shown significant promise. Computed tomography (CT) is a commonly used modality that can be used to create 3D anatomical models, as it is frequently used in the medical setting, demonstrates excellent resolution to the millimeter scale, and can readily pinpoint pathology on imaging.
Image Acquisition
CT scans can be rapidly obtained, which adds significant value, particularly in the context of point-of-care 3D printing. Another modality commonly used for 3D printing is magnetic resonance imaging (MRI), which unlike CT, does not expose the patient to ionizing radiation. The 3D printing process is initiated with patient-specific CT or MRI data stored in the digital imaging and communications in medicine (DICOM) format, which is the international standard for communication and management of medical imaging information and related data. DICOM allows for faster and robust collaboration among imaging professionals.3
Image Processing
To print 3D anatomical models, patient-specific data must be converted from DICOM into standard tessellation language (STL) format, which can be created and edited with a variety of softwares.3 At James A. Haley Veterans’ Hospital in Tampa, Florida, we use an image processing package that includes the Materialise 3-matic and interactive medical image control system. Image quality is essential; therefore, careful attention to details such as pixel dimensions, slice thickness, and slice increments must be considered.3,4
An STL file creates a 3D image from triangle approximations. The entire 3D shape will be made of numerous large or small triangles, depending on the slice thickness, therefore, quality of the original radiologic image. The size and position of the triangles used to make the model can be varied to approximate the object’s shape. The smaller the triangles, the better the image quality and vice versa. This concept is analogous to approximating a circle using straight lines of equal length—more, smaller lines will result in better approximation of a circle (Figure 1).5,6 Similarly, using smaller triangles allows for better approximation of the image. As the human body is a complex structure, mimicking the body requires a system able to create nongeometrical shapes, which is made possible via these triangle approximations in a 3D STL file.
The creation of an STL file from DICOM data starts with a threshold-based segmentation process followed by additional fine-tuning and edits, and ends in the creation of a 3D part. The initial segmentation can be created with the threshold tool, using a Hounsfield unit range based on the area of interest desired (eg, bone, blood, fat). This is used to create an initial mask, which can be further optimized. The region grow tool allows the user to focus the segmentation by discarding areas that are not directly connected to the region of interest. In contrast, the split mask tool divides areas that are connected. Next, fine-tuning the segmentation using tools such as multiple slice edit helps to optimize the model. After all edits are made, the calculate part tool converts the mask into a 3D component that can be used in downstream applications. For the purposes of demonstration and proof of concept, the models provided in this article were created via open-source hardware designs under free or open licenses.7-9
3D Printing in Neuroradiology Education
Neuroradiologists focus on diagnosing pathology related to the brain, head and neck, and spine. CT and MRI scans are the primary modalities used to diagnose these conditions. 3D printing is a useful tool for the trainee who wishes to fully understand neuroanatomy and obtain further appreciation of imaging pathology as it relates to 3D anatomy. Head and neck imaging are a complex subdiscipline of neuroradiology that often require further training beyond radiology residency. A neuroradiology fellowship that focuses on head and neck imaging extends the training.
3D printing has the potential to improve the understanding of various imaging pathologies by providing the trainee with a more in-depth appreciation of the anterior, middle, and posterior cranial fossa, the skull base foramina (ie, foramen ovale, spinosum, rotundum), and complex 3D areas, such as the pterygopalatine fossa, which are all critical areas to investigate on imaging. Figure 2 highlights how a complex anatomical structure, such as the sphenoid bone when printed in 3D, can be correlated with CT cross-sectional images to supplement the educational experience.
Furthermore, the various lobes, sulci, and gyri of the brain and cerebellum and how they interrelate to nearby vasculature and bony structures can be difficult to conceptualize for early trainees. A 3D-printed cerebellum and its relation to the brainstem is illustrated in Figure 3A. Additional complex head and neck structures of the middle ear membranous and bony labyrinth and ossicles and multiple views of the mandible are shown in Figures 3B through 3E.
3D printing in the context of neurovascular pathology holds great promise, particularly as these models may provide the trainee, patient, and proceduralist essential details such as appearance and morphology of an intracranial aneurysm, relationship and size of the neck of aneurysm, incorporation of vessels emanating from the aneurysmal sac, and details of the dome of the aneurysm. For example, the normal circle of Willis in Figure 4A is juxtaposed with an example of a saccular internal carotid artery aneurysm (Figure 4B).
A variety of conditions can affect the bony spine from degenerative, trauma, neoplastic, and inflammatory etiologies. A CT scan of the spine is readily used to detect these different conditions and often is used in the initial evaluation of trauma as indicated in the American College of Radiology appropriateness criteria.10 In addition, MRI is used to evaluate the spinal cord and to further define spinal stenosis as well as evaluate radiculopathy. An appreciation of the bony and soft tissue structures within the spine can be garnered with the use of 3D models (Figure 5).
Trainees can further their understanding of approaches in spinal procedures, including lumbar puncture, myelography, and facet injections. A variety of approaches to access the spinal canal have been documented, such as interspinous, paraspinous, and interlaminar oblique; 3D-printed models can aid in practicing these procedures.11 For example, a water-filled tube can be inserted into the vertebral canal to provide realistic tactile feedback for simulation of a lumbar puncture. An appreciation of the 3D anatomy can guide the clinician on the optimal approach, which can help limit time and potentially improve outcomes.
Future Directions
Artificial Intelligence (AI) offers the ability to teach computers to perform tasks that ordinarily require human intelligence. In the context of 3D printing, the ability to use AI to readily convert and process DICOM data into printable STL models holds significant promise. Currently, the manual conversion of a DICOM file into a segmented 3D model may take several days, necessitating a number of productive hours even from the imaging and engineering champion. If machines could aid in this process, the ability to readily scale clinical 3D printing and promote widespread adoption would be feasible. Several studies already are looking into this concept to determine how deep learning networks may automatically recognize lesions on medical imaging to assist a human operator, potentially cutting hours from the clinical 3D printing workflow.12,13
Furthermore, there are several applications for AI in the context of 3D printing upstream or before the creation of a 3D model. A number of AI tools are already in use at the CT and MRI scanner. Current strategies leverage deep learning and advances in neural networks to improve image quality and create thin section DICOM data, which can be converted into printable 3D files. Additionally, the ability to automate tasks using AI can improve production capacity by assessing material costs and ensuring cost efficiency, which will be critical as point-of-care 3D printing develops widespread adoption. AI also can reduce printing errors by using automated adaptive feedback, using machine learning to search for possible print errors, and sending feedback to the computer to ensure appropriate settings (eg, temperature settings/environmental conditions).
Conclusions
Based on this single-institution experience, 3D-printed complex neuroanatomical structures seems feasible and may enhance resident education and patient safety. Interested trainees may have the opportunity to learn and be involved in the printing process of new and innovative ideas. Further studies may involve printing various pathologic processes and applying these same steps and principles to other subspecialties of radiology. Finally, AI has the potential to advance the 3D printing process in the future.
1. Rengier F, Mehndiratta A, von Tengg-Kobligk H, et al. 3D printing based on imaging data: review of medical applications. Int J Comput Assist Radiol Surg. 2010;5(4):335-341. doi:10.1007/s11548-010-0476-x
2. Perica E, Sun Z. Patient-specific three-dimensional printing for pre-surgical planning in hepatocellular carcinoma treatment. Quant Imaging Med Surg. 2017;7(6):668-677. doi:10.21037/qims.2017.11.02
3. Hwang JJ, Jung Y-H, Cho B-H. The need for DICOM encapsulation of 3D scanning STL data. Imaging Sci Dent. 2018;48(4):301-302. doi:10.5624/isd.2018.48.4.301
4. Whyms BJ, Vorperian HK, Gentry LR, Schimek EM, Bersu ET, Chung MK. The effect of computed tomographic scanner parameters and 3-dimensional volume rendering techniques on the accuracy of linear, angular, and volumetric measurements of the mandible. Oral Surg Oral Med, Oral Pathol Oral Radiol. 2013;115(5):682-691. doi:10.1016/j.oooo.2013.02.008
5. Materialise Cloud. Triangle reduction. Accessed May 20, 2021. https://cloud.materialise.com/tools/triangle-reduction
6. Comaneanu RM, Tarcolea M, Vlasceanu D, Cotrut MC. Virtual 3D reconstruction, diagnosis and surgical planning with Mimics software. Int J Nano Biomaterials. 2012;4(1);69-77.
7. Thingiverse: Digital designs for physical objects. Accessed May 20, 2021. https://www.thingiverse.com
8. Cults. Download for free 3D models for 3D printers. Accessed May 20, 2021. https://cults3d.com/en
9. yeggi. Search engine for 3D printer models. Accessed May 20, 2021. https://www.yeggi.com
10. Expert Panel on Neurological Imaging and Musculoskeletal Imaging; Beckmann NM, West OC, Nunez D, et al. ACR appropriateness criteria suspected spine trauma. J Am Coll Radiol. 2919;16(5):S264-285. doi:10.1016/j.jacr.2019.02.002
11. McKinney AM. Normal variants of the lumbar and sacral spine. In: Atlas of Head/Neck and Spine Normal Imaging Variants. Springer; 2018:263-321.
12. Sollini M, Bartoli F, Marciano A, et al. Artificial intelligence and hybrid imaging: the best match for personalized medicine in oncology. Eur J Hybrid Imaging. 2020;4(1):24. doi:10.1186/s41824-020-00094-8
13. Küstner T, Hepp T, Fischer M, et al. Fully automated and standardized segmentation of adipose tissue compartments via deep learning in 3D whole-body MRI of epidemiologic cohort studies. Radiol Artif Intell.2020;2(6):e200010. doi:10.1148/ryai.2020200010
1. Rengier F, Mehndiratta A, von Tengg-Kobligk H, et al. 3D printing based on imaging data: review of medical applications. Int J Comput Assist Radiol Surg. 2010;5(4):335-341. doi:10.1007/s11548-010-0476-x
2. Perica E, Sun Z. Patient-specific three-dimensional printing for pre-surgical planning in hepatocellular carcinoma treatment. Quant Imaging Med Surg. 2017;7(6):668-677. doi:10.21037/qims.2017.11.02
3. Hwang JJ, Jung Y-H, Cho B-H. The need for DICOM encapsulation of 3D scanning STL data. Imaging Sci Dent. 2018;48(4):301-302. doi:10.5624/isd.2018.48.4.301
4. Whyms BJ, Vorperian HK, Gentry LR, Schimek EM, Bersu ET, Chung MK. The effect of computed tomographic scanner parameters and 3-dimensional volume rendering techniques on the accuracy of linear, angular, and volumetric measurements of the mandible. Oral Surg Oral Med, Oral Pathol Oral Radiol. 2013;115(5):682-691. doi:10.1016/j.oooo.2013.02.008
5. Materialise Cloud. Triangle reduction. Accessed May 20, 2021. https://cloud.materialise.com/tools/triangle-reduction
6. Comaneanu RM, Tarcolea M, Vlasceanu D, Cotrut MC. Virtual 3D reconstruction, diagnosis and surgical planning with Mimics software. Int J Nano Biomaterials. 2012;4(1);69-77.
7. Thingiverse: Digital designs for physical objects. Accessed May 20, 2021. https://www.thingiverse.com
8. Cults. Download for free 3D models for 3D printers. Accessed May 20, 2021. https://cults3d.com/en
9. yeggi. Search engine for 3D printer models. Accessed May 20, 2021. https://www.yeggi.com
10. Expert Panel on Neurological Imaging and Musculoskeletal Imaging; Beckmann NM, West OC, Nunez D, et al. ACR appropriateness criteria suspected spine trauma. J Am Coll Radiol. 2919;16(5):S264-285. doi:10.1016/j.jacr.2019.02.002
11. McKinney AM. Normal variants of the lumbar and sacral spine. In: Atlas of Head/Neck and Spine Normal Imaging Variants. Springer; 2018:263-321.
12. Sollini M, Bartoli F, Marciano A, et al. Artificial intelligence and hybrid imaging: the best match for personalized medicine in oncology. Eur J Hybrid Imaging. 2020;4(1):24. doi:10.1186/s41824-020-00094-8
13. Küstner T, Hepp T, Fischer M, et al. Fully automated and standardized segmentation of adipose tissue compartments via deep learning in 3D whole-body MRI of epidemiologic cohort studies. Radiol Artif Intell.2020;2(6):e200010. doi:10.1148/ryai.2020200010
Audit and Feedback: A Quality Improvement Study to Improve Antimicrobial Stewardship
Antibiotics are commonly overused for several viral respiratory conditions where antibiotic treatment is not clinically indicated. For example, a 2016 study by Fleming-Dutra and colleagues showed that at least 30% of all antibiotics prescribed in an outpatient setting were inappropriate and for acute bronchitis, antibiotic prescriptions were inappropriate in 50% of cases.1 Acute bronchitis is predominantly a viral illness where antibiotics should be rarely used.2-8 The Healthcare Effectiveness Data and Information Set has measured the avoidance of antibiotic treatment in adults with acute bronchitis since 2006. The National Committee for Quality Assurance reported in 2018 that about 75% of adults received antibiotics for acute bronchitis.9 Inappropriate antibiotic use contributes to antimicrobial resistance, resulting in the increase of morbidity and mortality of treatable infections.10 Reducing inappropriate antibiotic use in outpatient settings is a high-priority public health issue and is a Healthy People 2030 objective.11
Antimicrobial Stewardship
Antimicrobial stewardship programs measure and track how antibiotics are prescribed by health care providers (HCPs) and used by patients. The Centers for Disease Control and Prevention (CDC) created a framework for outpatient antimicrobial stewardship programs by outlining 4 core elements: (1) commitment from every person involved in patient care to act as an antibiotic steward; (2) policies and interventions to promote appropriate antibiotic prescribing practices; (3) antibiotic prescription tracking and reporting; and (4) appropriate antibiotic use education.12
Audit and feedback (A&F) is a form of antibiotic prescription tracking and reporting that involves measuring and comparing a HCP’s performance (ie, antibiotic prescribing) with a standard, and the results of this audit are shared with the HCP. This strategy is based on the belief that a HCP is motivated to modify practice habits when given feedback showing that his or her performance is inconsistent with targeted expectations. A&F is most effective when feedback is provided by a supervisor or respected peer, presented more than once, individualized, delivered in both verbal and written formats, and includes explicit targets and an action plan.13,14
This study focuses on an antimicrobial stewardship program implemented in an outpatient Indian Health Service ambulatory care clinic in the Pacific Northwest. The clinic was staffed by 9 HCPs serving about 12,000 American Indian and Alaskan Native patients. The clinic includes a full-service pharmacy where nearly all prescriptions issued by in-house HCPs are filled. The clinic’s antibiotic prescribing rate for adult patients with acute bronchitis was similar to the national mean in 2018 (75%).9 The study objective was to reduce the rate of potentially inappropriate (not guideline-concordant) antibiotic prescribing in patients with acute bronchitis without underlying chronic lung disease or evidence of bacterial infection through A&F.
Methods
The antimicrobial stewardship program was implemented by 3 pharmacists, including a pharmacy resident. HCPs received education by pharmacy staff on evidence-based prescribing for adult acute bronchitis and quarterly feedback on antibiotic prescribing rates. All prescribing and dispensing records necessary for the program were available in the clinic electronic health record. The rate of potentially inappropriate antibiotic prescribing was calculated as the proportion of eligible bronchitis cases who received antibiotics.
In October 2018, a 60-minute educational session was provided by 2 pharmacists to HCPs. The material covered an overview of acute bronchitis presentation, diagnosis, treatment (Table 1), and a comparison of national and local prescribing data (baseline audit).2-4 The educational session concluded with prescription strategies to reduce inappropriate antibiotic prescribing, including but not limited to: delayed prescriptions, patient and caregiver education, use of nonantibiotic medications to control symptoms, and use of A&F reports.5-8 At the conclusion of the session, HCPs committed to engage in the antimicrobial stewardship program.
Audit
To determine the total number of eligible bronchitis cases (denominator), a visit report was generated by a pharmacist for a primary diagnosis of acute bronchitis using International Statistical Classification of Diseases, Tenth Revision (ICD 10) codes (J20.3 - J20.9) for the review period. Only adults aged ≥ 18 years were included. Patients with a chronic lung disease (eg, chronic obstructive pulmonary disease, asthma) and those who had a concomitant bacterial infection (eg, urinary tract infection, cellulitis) were excluded. A visit for acute bronchitis that included additional ICD 10 codes indicating the patient had a chronic lung disease or concomitant bacterial infection were used to determine exclusion. The remaining patients who received a potentially inappropriate antibiotic prescription (numerator) were those who were prescribed or dispensed antibiotics on the date of service.
Feedback
Baseline data were presented to HCPs during the educational session in October 2018. Prospective audits were performed quarterly thereafter (January, April, and July) by the pharmacy resident using the criteria described above. Audit data were compiled into personalized reports and provided to HCPs by the pharmacy resident with written and verbal individual feedback. Written feedback was sent by email to each HCP containing the HCP’s rate, the clinic rate in aggregate, rates from the prior year and quarter(s) for comparison, and clinical pearls from the guidelines (Figure). Verbal feedback included a review of the written feedback and answering any questions concerning the report.
Implementation
Study periods were chosen to coincide with the pharmacy residency training year, which starts in July and ends in June. The start date of October 2018 differed from the start of the residency year (July 2018) owing to delays in obtaining permissions. A&F and analysis of prescribing rates continued through the end of the residency year, for total duration of 9 months (October 1, 2018 to June 30, 2019). For ease of reporting, quarterly reports followed the federal government’s fiscal year (FY) which runs from October 1 of the prior calendar year through September 30 of the year being described. HCPs received 4 feedback reports: baseline (October 1, 2018 - June 30, 2018) in October 2018, quarter 1 (October 1, 2018 - December 31, 2018) in January 2019, quarter 2 (January 1, 2019 - March 31, 2019) in April 2019, and quarter 3 (April 1, 2019 - June 30, 2019) in July 2019.
Statistical Analysis
Prescribing rates were compared between identical 9 -month periods. A 2-sample binomial test for proportions was used to derive an approximate CI of prescribing rates at the patient level. However, to account for clustering of patients within HCP panels and dependence of observations over study periods stemming from examining the same HCPs within each of the periods, the Wilcoxon signed rank test for paired data was used to evaluate prescribing rates at the HCP level. Statistical analysis was performed using R statistical software version 4.0.3. Differences were considered significant at P < .05 set a priori.
This study was approved by the Portland Area Indian Health Service Institutional Review Board (Study ID: 1316730).
Results
All 9 HCPs who see adult patients at the clinic agreed to participate and were all fully present in each study period. Among HCPs, there were 5 physicians and 4 physician assistants or nurse practitioners. There was a total of 213 visits that met study criteria during the baseline period (October 1, 2017 to June 30, 2018) and 177 visits in the posteducation period (October 1, 2018 to June 30, 2019). The total number of acute bronchitis encounters varied by HCP (Ranges, 5-63 [baseline] and 2-57 [posteducation]); however, the relative number of encounters each HCP contributed was similar in each study period (Table 2). The pharmacy resident spent about 2 hours each quarter to generate 9 feedback reports, 1 for each HCP.
Antibiotic Prescribing
Antibiotic prescribing rates decreased from 75% at baseline to 60% at posteducation month 9 (absolute difference, -15% [95% CI, 5 - 24%]; P ≤ .01) (Table 3). The clinic rate was lower for each quarter in FY 2019 (posteducation) compared with the same quarter of FY 2018 (baseline), with the lowest rate observed in the final quarter of the study. Comparing pre- and post- A&F, the rates for HCPs prescribing antibiotics were lower for 7 HCPs, unchanged for 1 HCP, and slightly increased for 1 HCP(P = .02).
Discussion
Acute bronchitis remains a common diagnosis where antibiotics are prescribed despite being a predominately viral illness. Guidelines and evidence-based practices advise against antibiotics for this diagnosis. According to the American Academy of Family Physicians, antibiotics are reserved for cases where chronic lung disease is present as these patients are at a high risk of developing pneumonia.3 The decision to prescribe antibiotics is complex and driven by several interdependent factors, such as patient expectations, health system limitations, clinician training, and specialty.15 HCPs may more aggressively treat acute bronchitis among American Indian/Alaskan Native (AI/AN) people due to a high risk of developing serious complications from respiratory illnesses.16 A clinician’s background, usual patient cohort (ie, mostly pediatric or geriatric), and time spent in urgent care or in activities outside of patient care (administration) may account for the difference in patient encounters by HCP for acute bronchitis.
Following the CDC framework, this antimicrobial stewardship program helped empower people involved in patient care (eg, pharmacists, HCPs), educate staff on proper use of antibiotics for acute bronchitis, and track and report antibiotic prescribing through the A&F process. Educational interventions coupled with ongoing A&F are reproducible by other health care facilities and are not usually time consuming. This study showcases a successful example of implementing A&F in an antimicrobial stewardship quality improvement project that could be translated toward other conditions (eg, sinusitis, urinary tract infection, community-acquired pneumonia).
In a similar study, Meeker and colleagues used a variation of an A&F intervention using a monthly email showing peer comparisons to notify clinicians who were prescribing too many unnecessary antibiotics for common respiratory illnesses that did not require antibiotics, such as the common cold.17 The peer comparison intervention arm emailed a rank order that listed prescribers by the number of prescriptions for common respiratory illnesses. This intervention demonstrated a reduction of 5.2% in inappropriate antibiotic prescribing.
Limitations
This quality improvement study had several limitations. The study did not account for the duration of symptoms as a factor to judge appropriateness. Although this was identified early in the study, it was unavoidable since there was no report that could extract the duration of symptoms in the electronic health record. Future studies should consider a manual review of each encounter to overcome this limitation. Another limitation was that only three-quarters of the year and not the entire year were reviewed. Future studies should include longer time frames to measure the durability of changes to antibiotic prescriptions. Lastly, the study did not assess diagnosis shifting (the practice of changing the proportion of antibiotic-appropriate acute respiratory tract infection diagnosis over time), effects of patient demographics (patient age and sex were not recorded), or any sustained effect on prescribing rates after the study ended.
Conclusions
Clinician education coupled with A&F are components of the CDC’s framework for an effective antimicrobial stewardship program. The intervention seem to be an effective means toward reducing inappropriate antibiotic prescribing for acute bronchitis and has the potential for application to other antimicrobial stewardship initiatives. The present study adds to the growing body of evidence on the importance and impact an antimicrobial stewardship program has on a clinic or health system.
Acknowledgment
The results of this study have been reported at the 2019 IHS Southwest Regional Pharmacy Continuing Education Seminar, April 12-14, 2019.
1. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315(17):1864-1873. doi:10.1001/jama.2016.4151
2. Barnett ML, Linder JA. Antibiotic prescribing for adults with acute bronchitis in the United States, 1996-2010. JAMA. 2014;311(19):2020-2022. doi:10.1001/jama.2013.286141
3. Kinkade S, Long NA. Acute bronchitis. Am Fam Physician. 2016;94(7):560-565.
4. Harris AM, Hicks LA, Qaseem A; High Value Care Task Force of the American College of Physicians and for the Centers for Disease Control and Prevention. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016;164(6):425-434. doi:10.7326/M15-1840
5. Gonzales R, Bartlett JG, Besser RE, et al. Principles of appropriate antibiotic use for treatment of uncomplicated acute bronchitis: background. Ann Intern Med. 2001;134(6):521-529. doi:10.7326/0003-4819-134-6-200103200-00021
6. Centers for Disease Control and Prevention. Adult outpatient treatment recommendations. Updated October 3, 2017. Accessed May 19, 2021. www.cdc.gov/antibiotic-use/community/for-hcp/outpatient-hcp/adult-treatment-rec.html
7. Braman SS. Chronic cough due to chronic bronchitis: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 suppl):104S-115S. doi:10.1378/chest.129.1_suppl.104S
8. Petersen I, Johnson AM, Islam A, Duckworth G, Livermore DM, Hayward AC. Protective effect of antibiotics against serious complications of common respiratory tract infections: retrospective cohort study with the UK General Practice Research Database. BMJ. 2007;335(7627):982. doi:10.1136/bmj.39345.405243.BE
9. National Committee for Quality Assurance. Avoidance of antibiotic treatment in adults with acute bronchitis (AAB). Accessed May 19, 2021. https://www.ncqa.org/hedis/measures/avoidance-of-antibiotic-treatment-in-adults-with-acute-bronchitis
10. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Published April 23, 2013. Accessed May 19, 2021. https://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf
11. US Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Healthy People 2030: reduce inappropriate antibiotic use in outpatient settings — HAI‑D01. Accessed May 19, 2021. https://health.gov/healthypeople/objectives-and-data/browse-objectives/healthcare-associated-infections/reduce-inappropriate-antibiotic-use-outpatient-settings-hai-d01
12. Sanchez GV, Fleming-Dutra KE, Roberts RM, Hicks LA. Core elements of outpatient antibiotic stewardship. MMWR Recomm Rep. 2016;65(6):1-12. Published 2016 Nov 11. doi:10.15585/mmwr.rr6506a1
13. Ivers N, Jamtvedt G, Flottorp S, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2012;(6):CD000259. Published 2012 Jun 13. doi:10.1002/14651858.CD000259.pub3
14. Ivers NM, Grimshaw JM, Jamtvedt G, et al. Growing literature, stagnant science? Systematic review, meta-regression and cumulative analysis of audit and feedback interventions in health care. J Gen Intern Med. 2014;29(11):1534-1541. doi:10.1007/s11606-014-2913-y
15. Ranji SR, Steinman MA, Shojania KG, et al. Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies. Vol. 4: Antibiotic Prescribing Behavior. Agency for Healthcare Research and Quality (US); 2006. Accessed May 20, 2021. https://www.ncbi.nlm.nih.gov/books/NBK43956/
16. Groom AV, Hennessy TW, Singleton RJ, Butler JC, Holve S, Cheek JE. Pneumonia and influenza mortality among American Indian and Alaska Native people, 1990-2009. Am J Public Health. 2014;104 Suppl 3(suppl 3):S460-S469. doi:10.2105/AJPH.2013.301740
17. Meeker D, Linder JA, Fox CR, et al. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. JAMA. 2016;315(6):562-570. doi:10.1001/jama.2016.0275
Antibiotics are commonly overused for several viral respiratory conditions where antibiotic treatment is not clinically indicated. For example, a 2016 study by Fleming-Dutra and colleagues showed that at least 30% of all antibiotics prescribed in an outpatient setting were inappropriate and for acute bronchitis, antibiotic prescriptions were inappropriate in 50% of cases.1 Acute bronchitis is predominantly a viral illness where antibiotics should be rarely used.2-8 The Healthcare Effectiveness Data and Information Set has measured the avoidance of antibiotic treatment in adults with acute bronchitis since 2006. The National Committee for Quality Assurance reported in 2018 that about 75% of adults received antibiotics for acute bronchitis.9 Inappropriate antibiotic use contributes to antimicrobial resistance, resulting in the increase of morbidity and mortality of treatable infections.10 Reducing inappropriate antibiotic use in outpatient settings is a high-priority public health issue and is a Healthy People 2030 objective.11
Antimicrobial Stewardship
Antimicrobial stewardship programs measure and track how antibiotics are prescribed by health care providers (HCPs) and used by patients. The Centers for Disease Control and Prevention (CDC) created a framework for outpatient antimicrobial stewardship programs by outlining 4 core elements: (1) commitment from every person involved in patient care to act as an antibiotic steward; (2) policies and interventions to promote appropriate antibiotic prescribing practices; (3) antibiotic prescription tracking and reporting; and (4) appropriate antibiotic use education.12
Audit and feedback (A&F) is a form of antibiotic prescription tracking and reporting that involves measuring and comparing a HCP’s performance (ie, antibiotic prescribing) with a standard, and the results of this audit are shared with the HCP. This strategy is based on the belief that a HCP is motivated to modify practice habits when given feedback showing that his or her performance is inconsistent with targeted expectations. A&F is most effective when feedback is provided by a supervisor or respected peer, presented more than once, individualized, delivered in both verbal and written formats, and includes explicit targets and an action plan.13,14
This study focuses on an antimicrobial stewardship program implemented in an outpatient Indian Health Service ambulatory care clinic in the Pacific Northwest. The clinic was staffed by 9 HCPs serving about 12,000 American Indian and Alaskan Native patients. The clinic includes a full-service pharmacy where nearly all prescriptions issued by in-house HCPs are filled. The clinic’s antibiotic prescribing rate for adult patients with acute bronchitis was similar to the national mean in 2018 (75%).9 The study objective was to reduce the rate of potentially inappropriate (not guideline-concordant) antibiotic prescribing in patients with acute bronchitis without underlying chronic lung disease or evidence of bacterial infection through A&F.
Methods
The antimicrobial stewardship program was implemented by 3 pharmacists, including a pharmacy resident. HCPs received education by pharmacy staff on evidence-based prescribing for adult acute bronchitis and quarterly feedback on antibiotic prescribing rates. All prescribing and dispensing records necessary for the program were available in the clinic electronic health record. The rate of potentially inappropriate antibiotic prescribing was calculated as the proportion of eligible bronchitis cases who received antibiotics.
In October 2018, a 60-minute educational session was provided by 2 pharmacists to HCPs. The material covered an overview of acute bronchitis presentation, diagnosis, treatment (Table 1), and a comparison of national and local prescribing data (baseline audit).2-4 The educational session concluded with prescription strategies to reduce inappropriate antibiotic prescribing, including but not limited to: delayed prescriptions, patient and caregiver education, use of nonantibiotic medications to control symptoms, and use of A&F reports.5-8 At the conclusion of the session, HCPs committed to engage in the antimicrobial stewardship program.
Audit
To determine the total number of eligible bronchitis cases (denominator), a visit report was generated by a pharmacist for a primary diagnosis of acute bronchitis using International Statistical Classification of Diseases, Tenth Revision (ICD 10) codes (J20.3 - J20.9) for the review period. Only adults aged ≥ 18 years were included. Patients with a chronic lung disease (eg, chronic obstructive pulmonary disease, asthma) and those who had a concomitant bacterial infection (eg, urinary tract infection, cellulitis) were excluded. A visit for acute bronchitis that included additional ICD 10 codes indicating the patient had a chronic lung disease or concomitant bacterial infection were used to determine exclusion. The remaining patients who received a potentially inappropriate antibiotic prescription (numerator) were those who were prescribed or dispensed antibiotics on the date of service.
Feedback
Baseline data were presented to HCPs during the educational session in October 2018. Prospective audits were performed quarterly thereafter (January, April, and July) by the pharmacy resident using the criteria described above. Audit data were compiled into personalized reports and provided to HCPs by the pharmacy resident with written and verbal individual feedback. Written feedback was sent by email to each HCP containing the HCP’s rate, the clinic rate in aggregate, rates from the prior year and quarter(s) for comparison, and clinical pearls from the guidelines (Figure). Verbal feedback included a review of the written feedback and answering any questions concerning the report.
Implementation
Study periods were chosen to coincide with the pharmacy residency training year, which starts in July and ends in June. The start date of October 2018 differed from the start of the residency year (July 2018) owing to delays in obtaining permissions. A&F and analysis of prescribing rates continued through the end of the residency year, for total duration of 9 months (October 1, 2018 to June 30, 2019). For ease of reporting, quarterly reports followed the federal government’s fiscal year (FY) which runs from October 1 of the prior calendar year through September 30 of the year being described. HCPs received 4 feedback reports: baseline (October 1, 2018 - June 30, 2018) in October 2018, quarter 1 (October 1, 2018 - December 31, 2018) in January 2019, quarter 2 (January 1, 2019 - March 31, 2019) in April 2019, and quarter 3 (April 1, 2019 - June 30, 2019) in July 2019.
Statistical Analysis
Prescribing rates were compared between identical 9 -month periods. A 2-sample binomial test for proportions was used to derive an approximate CI of prescribing rates at the patient level. However, to account for clustering of patients within HCP panels and dependence of observations over study periods stemming from examining the same HCPs within each of the periods, the Wilcoxon signed rank test for paired data was used to evaluate prescribing rates at the HCP level. Statistical analysis was performed using R statistical software version 4.0.3. Differences were considered significant at P < .05 set a priori.
This study was approved by the Portland Area Indian Health Service Institutional Review Board (Study ID: 1316730).
Results
All 9 HCPs who see adult patients at the clinic agreed to participate and were all fully present in each study period. Among HCPs, there were 5 physicians and 4 physician assistants or nurse practitioners. There was a total of 213 visits that met study criteria during the baseline period (October 1, 2017 to June 30, 2018) and 177 visits in the posteducation period (October 1, 2018 to June 30, 2019). The total number of acute bronchitis encounters varied by HCP (Ranges, 5-63 [baseline] and 2-57 [posteducation]); however, the relative number of encounters each HCP contributed was similar in each study period (Table 2). The pharmacy resident spent about 2 hours each quarter to generate 9 feedback reports, 1 for each HCP.
Antibiotic Prescribing
Antibiotic prescribing rates decreased from 75% at baseline to 60% at posteducation month 9 (absolute difference, -15% [95% CI, 5 - 24%]; P ≤ .01) (Table 3). The clinic rate was lower for each quarter in FY 2019 (posteducation) compared with the same quarter of FY 2018 (baseline), with the lowest rate observed in the final quarter of the study. Comparing pre- and post- A&F, the rates for HCPs prescribing antibiotics were lower for 7 HCPs, unchanged for 1 HCP, and slightly increased for 1 HCP(P = .02).
Discussion
Acute bronchitis remains a common diagnosis where antibiotics are prescribed despite being a predominately viral illness. Guidelines and evidence-based practices advise against antibiotics for this diagnosis. According to the American Academy of Family Physicians, antibiotics are reserved for cases where chronic lung disease is present as these patients are at a high risk of developing pneumonia.3 The decision to prescribe antibiotics is complex and driven by several interdependent factors, such as patient expectations, health system limitations, clinician training, and specialty.15 HCPs may more aggressively treat acute bronchitis among American Indian/Alaskan Native (AI/AN) people due to a high risk of developing serious complications from respiratory illnesses.16 A clinician’s background, usual patient cohort (ie, mostly pediatric or geriatric), and time spent in urgent care or in activities outside of patient care (administration) may account for the difference in patient encounters by HCP for acute bronchitis.
Following the CDC framework, this antimicrobial stewardship program helped empower people involved in patient care (eg, pharmacists, HCPs), educate staff on proper use of antibiotics for acute bronchitis, and track and report antibiotic prescribing through the A&F process. Educational interventions coupled with ongoing A&F are reproducible by other health care facilities and are not usually time consuming. This study showcases a successful example of implementing A&F in an antimicrobial stewardship quality improvement project that could be translated toward other conditions (eg, sinusitis, urinary tract infection, community-acquired pneumonia).
In a similar study, Meeker and colleagues used a variation of an A&F intervention using a monthly email showing peer comparisons to notify clinicians who were prescribing too many unnecessary antibiotics for common respiratory illnesses that did not require antibiotics, such as the common cold.17 The peer comparison intervention arm emailed a rank order that listed prescribers by the number of prescriptions for common respiratory illnesses. This intervention demonstrated a reduction of 5.2% in inappropriate antibiotic prescribing.
Limitations
This quality improvement study had several limitations. The study did not account for the duration of symptoms as a factor to judge appropriateness. Although this was identified early in the study, it was unavoidable since there was no report that could extract the duration of symptoms in the electronic health record. Future studies should consider a manual review of each encounter to overcome this limitation. Another limitation was that only three-quarters of the year and not the entire year were reviewed. Future studies should include longer time frames to measure the durability of changes to antibiotic prescriptions. Lastly, the study did not assess diagnosis shifting (the practice of changing the proportion of antibiotic-appropriate acute respiratory tract infection diagnosis over time), effects of patient demographics (patient age and sex were not recorded), or any sustained effect on prescribing rates after the study ended.
Conclusions
Clinician education coupled with A&F are components of the CDC’s framework for an effective antimicrobial stewardship program. The intervention seem to be an effective means toward reducing inappropriate antibiotic prescribing for acute bronchitis and has the potential for application to other antimicrobial stewardship initiatives. The present study adds to the growing body of evidence on the importance and impact an antimicrobial stewardship program has on a clinic or health system.
Acknowledgment
The results of this study have been reported at the 2019 IHS Southwest Regional Pharmacy Continuing Education Seminar, April 12-14, 2019.
Antibiotics are commonly overused for several viral respiratory conditions where antibiotic treatment is not clinically indicated. For example, a 2016 study by Fleming-Dutra and colleagues showed that at least 30% of all antibiotics prescribed in an outpatient setting were inappropriate and for acute bronchitis, antibiotic prescriptions were inappropriate in 50% of cases.1 Acute bronchitis is predominantly a viral illness where antibiotics should be rarely used.2-8 The Healthcare Effectiveness Data and Information Set has measured the avoidance of antibiotic treatment in adults with acute bronchitis since 2006. The National Committee for Quality Assurance reported in 2018 that about 75% of adults received antibiotics for acute bronchitis.9 Inappropriate antibiotic use contributes to antimicrobial resistance, resulting in the increase of morbidity and mortality of treatable infections.10 Reducing inappropriate antibiotic use in outpatient settings is a high-priority public health issue and is a Healthy People 2030 objective.11
Antimicrobial Stewardship
Antimicrobial stewardship programs measure and track how antibiotics are prescribed by health care providers (HCPs) and used by patients. The Centers for Disease Control and Prevention (CDC) created a framework for outpatient antimicrobial stewardship programs by outlining 4 core elements: (1) commitment from every person involved in patient care to act as an antibiotic steward; (2) policies and interventions to promote appropriate antibiotic prescribing practices; (3) antibiotic prescription tracking and reporting; and (4) appropriate antibiotic use education.12
Audit and feedback (A&F) is a form of antibiotic prescription tracking and reporting that involves measuring and comparing a HCP’s performance (ie, antibiotic prescribing) with a standard, and the results of this audit are shared with the HCP. This strategy is based on the belief that a HCP is motivated to modify practice habits when given feedback showing that his or her performance is inconsistent with targeted expectations. A&F is most effective when feedback is provided by a supervisor or respected peer, presented more than once, individualized, delivered in both verbal and written formats, and includes explicit targets and an action plan.13,14
This study focuses on an antimicrobial stewardship program implemented in an outpatient Indian Health Service ambulatory care clinic in the Pacific Northwest. The clinic was staffed by 9 HCPs serving about 12,000 American Indian and Alaskan Native patients. The clinic includes a full-service pharmacy where nearly all prescriptions issued by in-house HCPs are filled. The clinic’s antibiotic prescribing rate for adult patients with acute bronchitis was similar to the national mean in 2018 (75%).9 The study objective was to reduce the rate of potentially inappropriate (not guideline-concordant) antibiotic prescribing in patients with acute bronchitis without underlying chronic lung disease or evidence of bacterial infection through A&F.
Methods
The antimicrobial stewardship program was implemented by 3 pharmacists, including a pharmacy resident. HCPs received education by pharmacy staff on evidence-based prescribing for adult acute bronchitis and quarterly feedback on antibiotic prescribing rates. All prescribing and dispensing records necessary for the program were available in the clinic electronic health record. The rate of potentially inappropriate antibiotic prescribing was calculated as the proportion of eligible bronchitis cases who received antibiotics.
In October 2018, a 60-minute educational session was provided by 2 pharmacists to HCPs. The material covered an overview of acute bronchitis presentation, diagnosis, treatment (Table 1), and a comparison of national and local prescribing data (baseline audit).2-4 The educational session concluded with prescription strategies to reduce inappropriate antibiotic prescribing, including but not limited to: delayed prescriptions, patient and caregiver education, use of nonantibiotic medications to control symptoms, and use of A&F reports.5-8 At the conclusion of the session, HCPs committed to engage in the antimicrobial stewardship program.
Audit
To determine the total number of eligible bronchitis cases (denominator), a visit report was generated by a pharmacist for a primary diagnosis of acute bronchitis using International Statistical Classification of Diseases, Tenth Revision (ICD 10) codes (J20.3 - J20.9) for the review period. Only adults aged ≥ 18 years were included. Patients with a chronic lung disease (eg, chronic obstructive pulmonary disease, asthma) and those who had a concomitant bacterial infection (eg, urinary tract infection, cellulitis) were excluded. A visit for acute bronchitis that included additional ICD 10 codes indicating the patient had a chronic lung disease or concomitant bacterial infection were used to determine exclusion. The remaining patients who received a potentially inappropriate antibiotic prescription (numerator) were those who were prescribed or dispensed antibiotics on the date of service.
Feedback
Baseline data were presented to HCPs during the educational session in October 2018. Prospective audits were performed quarterly thereafter (January, April, and July) by the pharmacy resident using the criteria described above. Audit data were compiled into personalized reports and provided to HCPs by the pharmacy resident with written and verbal individual feedback. Written feedback was sent by email to each HCP containing the HCP’s rate, the clinic rate in aggregate, rates from the prior year and quarter(s) for comparison, and clinical pearls from the guidelines (Figure). Verbal feedback included a review of the written feedback and answering any questions concerning the report.
Implementation
Study periods were chosen to coincide with the pharmacy residency training year, which starts in July and ends in June. The start date of October 2018 differed from the start of the residency year (July 2018) owing to delays in obtaining permissions. A&F and analysis of prescribing rates continued through the end of the residency year, for total duration of 9 months (October 1, 2018 to June 30, 2019). For ease of reporting, quarterly reports followed the federal government’s fiscal year (FY) which runs from October 1 of the prior calendar year through September 30 of the year being described. HCPs received 4 feedback reports: baseline (October 1, 2018 - June 30, 2018) in October 2018, quarter 1 (October 1, 2018 - December 31, 2018) in January 2019, quarter 2 (January 1, 2019 - March 31, 2019) in April 2019, and quarter 3 (April 1, 2019 - June 30, 2019) in July 2019.
Statistical Analysis
Prescribing rates were compared between identical 9 -month periods. A 2-sample binomial test for proportions was used to derive an approximate CI of prescribing rates at the patient level. However, to account for clustering of patients within HCP panels and dependence of observations over study periods stemming from examining the same HCPs within each of the periods, the Wilcoxon signed rank test for paired data was used to evaluate prescribing rates at the HCP level. Statistical analysis was performed using R statistical software version 4.0.3. Differences were considered significant at P < .05 set a priori.
This study was approved by the Portland Area Indian Health Service Institutional Review Board (Study ID: 1316730).
Results
All 9 HCPs who see adult patients at the clinic agreed to participate and were all fully present in each study period. Among HCPs, there were 5 physicians and 4 physician assistants or nurse practitioners. There was a total of 213 visits that met study criteria during the baseline period (October 1, 2017 to June 30, 2018) and 177 visits in the posteducation period (October 1, 2018 to June 30, 2019). The total number of acute bronchitis encounters varied by HCP (Ranges, 5-63 [baseline] and 2-57 [posteducation]); however, the relative number of encounters each HCP contributed was similar in each study period (Table 2). The pharmacy resident spent about 2 hours each quarter to generate 9 feedback reports, 1 for each HCP.
Antibiotic Prescribing
Antibiotic prescribing rates decreased from 75% at baseline to 60% at posteducation month 9 (absolute difference, -15% [95% CI, 5 - 24%]; P ≤ .01) (Table 3). The clinic rate was lower for each quarter in FY 2019 (posteducation) compared with the same quarter of FY 2018 (baseline), with the lowest rate observed in the final quarter of the study. Comparing pre- and post- A&F, the rates for HCPs prescribing antibiotics were lower for 7 HCPs, unchanged for 1 HCP, and slightly increased for 1 HCP(P = .02).
Discussion
Acute bronchitis remains a common diagnosis where antibiotics are prescribed despite being a predominately viral illness. Guidelines and evidence-based practices advise against antibiotics for this diagnosis. According to the American Academy of Family Physicians, antibiotics are reserved for cases where chronic lung disease is present as these patients are at a high risk of developing pneumonia.3 The decision to prescribe antibiotics is complex and driven by several interdependent factors, such as patient expectations, health system limitations, clinician training, and specialty.15 HCPs may more aggressively treat acute bronchitis among American Indian/Alaskan Native (AI/AN) people due to a high risk of developing serious complications from respiratory illnesses.16 A clinician’s background, usual patient cohort (ie, mostly pediatric or geriatric), and time spent in urgent care or in activities outside of patient care (administration) may account for the difference in patient encounters by HCP for acute bronchitis.
Following the CDC framework, this antimicrobial stewardship program helped empower people involved in patient care (eg, pharmacists, HCPs), educate staff on proper use of antibiotics for acute bronchitis, and track and report antibiotic prescribing through the A&F process. Educational interventions coupled with ongoing A&F are reproducible by other health care facilities and are not usually time consuming. This study showcases a successful example of implementing A&F in an antimicrobial stewardship quality improvement project that could be translated toward other conditions (eg, sinusitis, urinary tract infection, community-acquired pneumonia).
In a similar study, Meeker and colleagues used a variation of an A&F intervention using a monthly email showing peer comparisons to notify clinicians who were prescribing too many unnecessary antibiotics for common respiratory illnesses that did not require antibiotics, such as the common cold.17 The peer comparison intervention arm emailed a rank order that listed prescribers by the number of prescriptions for common respiratory illnesses. This intervention demonstrated a reduction of 5.2% in inappropriate antibiotic prescribing.
Limitations
This quality improvement study had several limitations. The study did not account for the duration of symptoms as a factor to judge appropriateness. Although this was identified early in the study, it was unavoidable since there was no report that could extract the duration of symptoms in the electronic health record. Future studies should consider a manual review of each encounter to overcome this limitation. Another limitation was that only three-quarters of the year and not the entire year were reviewed. Future studies should include longer time frames to measure the durability of changes to antibiotic prescriptions. Lastly, the study did not assess diagnosis shifting (the practice of changing the proportion of antibiotic-appropriate acute respiratory tract infection diagnosis over time), effects of patient demographics (patient age and sex were not recorded), or any sustained effect on prescribing rates after the study ended.
Conclusions
Clinician education coupled with A&F are components of the CDC’s framework for an effective antimicrobial stewardship program. The intervention seem to be an effective means toward reducing inappropriate antibiotic prescribing for acute bronchitis and has the potential for application to other antimicrobial stewardship initiatives. The present study adds to the growing body of evidence on the importance and impact an antimicrobial stewardship program has on a clinic or health system.
Acknowledgment
The results of this study have been reported at the 2019 IHS Southwest Regional Pharmacy Continuing Education Seminar, April 12-14, 2019.
1. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315(17):1864-1873. doi:10.1001/jama.2016.4151
2. Barnett ML, Linder JA. Antibiotic prescribing for adults with acute bronchitis in the United States, 1996-2010. JAMA. 2014;311(19):2020-2022. doi:10.1001/jama.2013.286141
3. Kinkade S, Long NA. Acute bronchitis. Am Fam Physician. 2016;94(7):560-565.
4. Harris AM, Hicks LA, Qaseem A; High Value Care Task Force of the American College of Physicians and for the Centers for Disease Control and Prevention. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016;164(6):425-434. doi:10.7326/M15-1840
5. Gonzales R, Bartlett JG, Besser RE, et al. Principles of appropriate antibiotic use for treatment of uncomplicated acute bronchitis: background. Ann Intern Med. 2001;134(6):521-529. doi:10.7326/0003-4819-134-6-200103200-00021
6. Centers for Disease Control and Prevention. Adult outpatient treatment recommendations. Updated October 3, 2017. Accessed May 19, 2021. www.cdc.gov/antibiotic-use/community/for-hcp/outpatient-hcp/adult-treatment-rec.html
7. Braman SS. Chronic cough due to chronic bronchitis: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 suppl):104S-115S. doi:10.1378/chest.129.1_suppl.104S
8. Petersen I, Johnson AM, Islam A, Duckworth G, Livermore DM, Hayward AC. Protective effect of antibiotics against serious complications of common respiratory tract infections: retrospective cohort study with the UK General Practice Research Database. BMJ. 2007;335(7627):982. doi:10.1136/bmj.39345.405243.BE
9. National Committee for Quality Assurance. Avoidance of antibiotic treatment in adults with acute bronchitis (AAB). Accessed May 19, 2021. https://www.ncqa.org/hedis/measures/avoidance-of-antibiotic-treatment-in-adults-with-acute-bronchitis
10. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Published April 23, 2013. Accessed May 19, 2021. https://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf
11. US Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Healthy People 2030: reduce inappropriate antibiotic use in outpatient settings — HAI‑D01. Accessed May 19, 2021. https://health.gov/healthypeople/objectives-and-data/browse-objectives/healthcare-associated-infections/reduce-inappropriate-antibiotic-use-outpatient-settings-hai-d01
12. Sanchez GV, Fleming-Dutra KE, Roberts RM, Hicks LA. Core elements of outpatient antibiotic stewardship. MMWR Recomm Rep. 2016;65(6):1-12. Published 2016 Nov 11. doi:10.15585/mmwr.rr6506a1
13. Ivers N, Jamtvedt G, Flottorp S, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2012;(6):CD000259. Published 2012 Jun 13. doi:10.1002/14651858.CD000259.pub3
14. Ivers NM, Grimshaw JM, Jamtvedt G, et al. Growing literature, stagnant science? Systematic review, meta-regression and cumulative analysis of audit and feedback interventions in health care. J Gen Intern Med. 2014;29(11):1534-1541. doi:10.1007/s11606-014-2913-y
15. Ranji SR, Steinman MA, Shojania KG, et al. Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies. Vol. 4: Antibiotic Prescribing Behavior. Agency for Healthcare Research and Quality (US); 2006. Accessed May 20, 2021. https://www.ncbi.nlm.nih.gov/books/NBK43956/
16. Groom AV, Hennessy TW, Singleton RJ, Butler JC, Holve S, Cheek JE. Pneumonia and influenza mortality among American Indian and Alaska Native people, 1990-2009. Am J Public Health. 2014;104 Suppl 3(suppl 3):S460-S469. doi:10.2105/AJPH.2013.301740
17. Meeker D, Linder JA, Fox CR, et al. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. JAMA. 2016;315(6):562-570. doi:10.1001/jama.2016.0275
1. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315(17):1864-1873. doi:10.1001/jama.2016.4151
2. Barnett ML, Linder JA. Antibiotic prescribing for adults with acute bronchitis in the United States, 1996-2010. JAMA. 2014;311(19):2020-2022. doi:10.1001/jama.2013.286141
3. Kinkade S, Long NA. Acute bronchitis. Am Fam Physician. 2016;94(7):560-565.
4. Harris AM, Hicks LA, Qaseem A; High Value Care Task Force of the American College of Physicians and for the Centers for Disease Control and Prevention. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016;164(6):425-434. doi:10.7326/M15-1840
5. Gonzales R, Bartlett JG, Besser RE, et al. Principles of appropriate antibiotic use for treatment of uncomplicated acute bronchitis: background. Ann Intern Med. 2001;134(6):521-529. doi:10.7326/0003-4819-134-6-200103200-00021
6. Centers for Disease Control and Prevention. Adult outpatient treatment recommendations. Updated October 3, 2017. Accessed May 19, 2021. www.cdc.gov/antibiotic-use/community/for-hcp/outpatient-hcp/adult-treatment-rec.html
7. Braman SS. Chronic cough due to chronic bronchitis: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 suppl):104S-115S. doi:10.1378/chest.129.1_suppl.104S
8. Petersen I, Johnson AM, Islam A, Duckworth G, Livermore DM, Hayward AC. Protective effect of antibiotics against serious complications of common respiratory tract infections: retrospective cohort study with the UK General Practice Research Database. BMJ. 2007;335(7627):982. doi:10.1136/bmj.39345.405243.BE
9. National Committee for Quality Assurance. Avoidance of antibiotic treatment in adults with acute bronchitis (AAB). Accessed May 19, 2021. https://www.ncqa.org/hedis/measures/avoidance-of-antibiotic-treatment-in-adults-with-acute-bronchitis
10. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Published April 23, 2013. Accessed May 19, 2021. https://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf
11. US Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Healthy People 2030: reduce inappropriate antibiotic use in outpatient settings — HAI‑D01. Accessed May 19, 2021. https://health.gov/healthypeople/objectives-and-data/browse-objectives/healthcare-associated-infections/reduce-inappropriate-antibiotic-use-outpatient-settings-hai-d01
12. Sanchez GV, Fleming-Dutra KE, Roberts RM, Hicks LA. Core elements of outpatient antibiotic stewardship. MMWR Recomm Rep. 2016;65(6):1-12. Published 2016 Nov 11. doi:10.15585/mmwr.rr6506a1
13. Ivers N, Jamtvedt G, Flottorp S, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2012;(6):CD000259. Published 2012 Jun 13. doi:10.1002/14651858.CD000259.pub3
14. Ivers NM, Grimshaw JM, Jamtvedt G, et al. Growing literature, stagnant science? Systematic review, meta-regression and cumulative analysis of audit and feedback interventions in health care. J Gen Intern Med. 2014;29(11):1534-1541. doi:10.1007/s11606-014-2913-y
15. Ranji SR, Steinman MA, Shojania KG, et al. Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies. Vol. 4: Antibiotic Prescribing Behavior. Agency for Healthcare Research and Quality (US); 2006. Accessed May 20, 2021. https://www.ncbi.nlm.nih.gov/books/NBK43956/
16. Groom AV, Hennessy TW, Singleton RJ, Butler JC, Holve S, Cheek JE. Pneumonia and influenza mortality among American Indian and Alaska Native people, 1990-2009. Am J Public Health. 2014;104 Suppl 3(suppl 3):S460-S469. doi:10.2105/AJPH.2013.301740
17. Meeker D, Linder JA, Fox CR, et al. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. JAMA. 2016;315(6):562-570. doi:10.1001/jama.2016.0275
High Rate of Inappropriate Fecal Immunochemical Testing at a Large Veterans Affairs Health Care System
Colonoscopies and annual fecal immunochemical tests (FITs), are 2 of the preferred modalities for colorectal cancer (CRC) screening endorsed by the US Preventive Services Task Forces as well as the US Multi-Society Task Force of Colorectal Cancer, which represents the American Gastroenterological Association, American College of Gastroenterology, and the American Society of Gastrointestinal Endoscopy.1,2 The recommendations include proper patient selection (patients aged 50 - 75 years with a life expectancy of at least 10 years), and a discussion with the patient regarding both options.
Background
It is known that patients with a positive FIT are at an increased risk for CRC. Lee and colleagues found that patients who do not undergo subsequent colonoscopy after a positive FIT have a 1.64 relative risk of death from colon cancer compared with those who undergo follow-up colonoscopy.3 Studies also have shown that longer wait times (10 months vs 1 month) between a positive FIT and colonoscopy also are associated with a higher risk of CRC.4 FIT utilize antibodies specific for the globin moiety of human hemoglobin and measure the development of antibody-globin complexes using immunoassay techniques. FIT has largely replaced the fecal occult blood test (FOBT), which depends on the detection of heme in feces through oxidation.
A US Department of Veterans Affairs (VA) study found that a longer time to colonoscopy was associated with a higher risk of neoplasia in veterans with a positive FOBT (odds ratio [OR], 1.10).5 It is thus crucial that a positive FOBT or FIT be investigated with follow-up colonoscopy. However, a retrospective study at a single safety-net hospital in San Francisco found that only 55.6% of patients with a positive FIT completed colonoscopy within 1 year.6 Importantly, almost half the patients examined in this study lacked documentation of the result of the FIT or counseling regarding the significance of the positive FIT by the patient’s primary care provider who ordered the test. A VA study looked at veterans aged > 70 years at 4 VA medical centers who did not receive a follow-up colonoscopy within 1 year and reported that 26% of patients studied had a documented refusal to undergo colonoscopy.7
It also is clear that FOBT is used inappropriately for colon cancer screening in some patients. A 2005 single-center VA study looked at inappropriate fecal occult blood tests and found that 18% of veterans for whom FOBTs were ordered had a severe comorbid illness, 13% had signs or symptoms of gastrointestinal (GI) blood loss, and 7% had a history of colorectal neoplasia or inflammatory bowel disease.8 An additional national VA study looked at all veterans aged ≥ 50 years who underwent FOBT or screening colonoscopy between 2009 and 2011 and found 26% to be inappropriate (13.9% of veterans not due for screening, 7.8% with limited life expectancy, and 11% receiving a FOBT when colonoscopy was indicated).9
An often-misunderstood additional requirement in utilizing FIT for CRC screening is that negative tests should be repeated annually.2 A study from Kaiser Permanente in California found that 75.3 to 86.1% of eligible patients underwent yearly FIT.10 In this study, programmatic FIT detected 80.4% of all patients with CRC detected within 1 year of testing.
Since most of the VA-specific studies are based on inappropriate or inadequate use of FOBT, we feel it is essential that further data be gained on appropriate and inappropriate testing. The aim of this study is to determine the frequency at which improper FIT occurs because of failure to obtain serial FIT over time with a negative result, failure to follow-up a positive FIT result with a diagnostic colonoscopy, or performance of FIT in veterans undergoing a recent colonoscopy with adequate bowel preparation. This quality assurance study received an institutional review board exemption from the VA Pittsburgh Healthcare System (VAPHS) in Pennsylvania.
Methods
VAPHS has a data repository of all veterans served within the health care system, which was queried for all veterans who underwent a FIT in the system from January 1, 2015 through December 31, 2017 as well as the number and results of FITs during the interval. In addition, the data repository was also queried specifically for veterans who had at least 1 colonoscopy as well as FIT between 2015 and 2017. The ordering location for each FIT also was queried.
We made 3 calculations for this study. First, we measured the rate of a negative initial FIT in 2015 and/or 2016 followed by a second FIT in 2016 and/or 2017 in a random selection of veterans (3% SE, 95% CI). Demographics were compared in an equal random number of veterans who did and did not have a follow up FIT (5% SE, 95% CI of all negative FIT). Second, we measured the rate of completing colonoscopy following a positive FIT in a random selection of veterans (3% SE, 95% SI). Finally, we calculated FITs following a colonoscopy for all veterans.
Using a power analysis with a 3% SE and 95% CI for sample size calculation and accounting for the approximate 50% exclusion rate from the final eligible population of veterans with at least 1 negative FIT, a random sample of 1,742 patient charts with a negative FIT in the interval were then reviewed to determine the frequency with which they underwent multiple FITs in the interval as well as for the presence of exclusionary factors. Because of the large number of veterans involved in this category, a more detailed demographics review was performed of a subset of these patients using a 95% CI and 5% SE. Using a 95% CI and 3% SE, 445 veterans with a positive FIT in the interval were reviewed to determine the frequency at which they underwent a follow-up diagnostic colonoscopy.
Because of a relatively small sample size, all 108 veterans who underwent a colonoscopy followed by a FIT were reviewed to determine the reason for follow-up FIT. In addition, in veterans who then went on to have a subsequent repeat colonoscopy, the examination findings were recorded.
Results
From January 1, 2015 to December 31, 2017, 6,766 FIT, were ordered at VAPHS. Of these, 4,391 unique veterans had at least 1 negative FIT during the period and 709 unique veterans had a positive FIT. There were 832 veterans who had both a FIT and colonoscopy during the study period. Of these, 108 had a colonoscopy with a subsequent FIT (Figure).
Of 1,742 randomly selected veterans with at least 1 negative FIT in the study interval, 870 were eligible for multiple FITs during this period as they were in the appropriate screening age (50-75 years or 85 years based on an assessment of life expectancy by the ordering health care provider [HCP]), did not have exclusionary comorbidities to multiple FIT, were not lost to follow-up, and had at least 1 negative FIT collected from 2015 to 2016 (veterans who only had a FIT in 2017 were excluded from this aim to avoid confounding). Of these 870 veterans, 543 (62.4%) underwent at least 2 FITs during the study period. In a demographic comparison of 110 veterans with 1 FIT and 110 veterans with > 1 FIT, there were no statistically significant differences in demographics (Table 1).
In a random chart review of 410 veterans with a positive FIT, 113 (27.5%) veterans did not undergo a subsequent colonoscopy within 1 year due to patient refusal, failure to schedule, or failure to keep colonoscopy appointment. There were no differences in demographics between those that underwent a diagnostic colonoscopy and those that did not (Table 2).
Of the 108 patients with a FIT following colonoscopy in the study interval, 97 FITs were negative. Ninety-five of the 108 FITs (88%) were judged to be inappropriate, having been performed for indications, including 38 for colon cancer screening, 23 for anemia, 32 for GI symptoms (eg, diarrhea, rectal bleeding, possible GI bleeding), and 2 for unclear indications. Thirteen FITs were deemed appropriate, as they were performed on veterans who refused to have a repeat colonoscopy following an examination with inadequate bowel preparation (Table 3). There was no difference in age or race between these 2 groups, although there was a statistically significant difference in gender (Table 4).
There were 19 patients who had a colonoscopy following a prior colonoscopy and subsequent positive FIT in the interval. Eight patients had no significant findings, 10 had nonadvanced adenomas, and 1 had an advanced adenoma (this patient had inadequate preparation with recommendation to repeat colonoscopy in 1 year).
While not a specific aim of the study we were able to identify certain HCPs by clinic location who systematically performed inappropriate or appropriate FIT. There were 47 separate ordering locations for the 95 inappropriate FIT following recent colonoscopy. Of these, 1 location was responsible for ordering 20 (21%) inappropriate FIT. Eight locations accounted for 51% of all the inappropriately ordered FIT. Two clinics seemed to be high performers in regard to overall appropriate vs inappropriate FIT use. The appropriate FIT rate for these locations was 30 of 33 (90.9%) and 26 of 28 (92.8%), respectively.
Discussion
In this retrospective study, we found that a large percentage of veterans eligible for colon cancer screening utilizing FIT did not undergo appropriate screening. Almost 40% of veterans in a 3-year interval received only 1 FIT. This seemed to occur due to a combination of patient refusal and inadequate education by HCPs regarding how to screen appropriately for CRC using FIT. This occurred despite a reminder in the VA Computerized Patient Record System regarding CRC screening.
There did not seem to be significant differences in demographics between those who were screened appropriately vs inappropriately. While there was a statistically significant difference in gender between those who had an appropriate FIT following recent colonoscopy (2 of 13 were female) and those who had an inappropriate FIT after recent colonoscopy (1 of 95 was a female), we are uncertain of the significance of this finding given the small number of female veterans in the analysis.
We do believe that the ratio of veterans in our study with a single FIT likely underestimates the true prevalence. To avoid confounding from factors such as inadequate prior follow-up in the study interval, we excluded veterans who underwent FIT only in 2017 for this analysis. As such, a significant percentage of these veterans were actually eligible to be screened throughout the study interval.
In spite of recommendations regarding the need for diagnostic colonoscopy following a positive FIT, we found that more than one-quarter of patients did not undergo colonoscopy. Although this number is an improvement over previously published literature that found almost half of patients at a safety-net hospital did not undergo diagnostic colonoscopy following a positive FIT, this is still clearly suboptimal.6
VAPHS has a mandate that all patients with a positive FIT be scheduled for colonoscopy within 30 days, either at VAPHS or in the community. An alert is sent to both ordering HCP regarding the positive FIT as well as to the GI department. In addition to contact from the ordering HCP, all veterans also are contacted by either a physician or nurse practitioner GI provider to provide test results and an explanation of its clinical significance and to facilitate colonoscopy scheduling. If a patient cannot be reached by telephone, the patient is sent a certified letter from the GI department regarding the significance of a positive FIT and instructions for scheduling a colonoscopy.
Despite this outreach, 27.5% of veterans did not have a diagnostic colonoscopy following a positive FIT. This suggests that there may be inadequate education and counseling of veterans at the time of the FIT order about the subsequent series of events and need for diagnostic colonoscopy following a positive FIT. If a patient refuses to undergo a colonoscopy under any circumstances (including after a positive FIT), the utility of placing a FIT order is questionable.
There is also a need for more education of ordering HCPs on appropriate indications for FITs. We found that 35% of FIT ordered after a recent colonoscopy were done for the purpose of CRC screening, despite clear guidelines recommending against this. In addition, another 50% of FIT ordered after recent colonoscopy was done either for evaluation of GI symptoms like diarrhea and rectal bleeding or in the evaluation of anemia, both of which are inappropriate uses for FIT. Since FIT is an antibody test against globin, the protein component of hemoglobin that degrades during passage through the small bowel, it is not a useful test for the evaluation of upper GI or small bowel bleeding. A relatively recent database study in the Netherlands looking at the diagnosis of upper GI malignancies within 3 years of a positive FIT found a < 1% rate.11
In our study, albeit limited by the small number of veterans undergoing a repeat colonoscopy following a prior colonoscopy and subsequent positive FIT, there were few significant findings. Only 1 veteran had an advanced adenoma detected, and this veteran had already been recommended a repeat colonoscopy in 1 year due to an inadequate bowel preparation on the last examination.
Lastly, we found that certain HCPs (based on ordering clinic location) systematically performed improper FIT compared with other HCPs. This presumably is due to a lack of education on appropriate FIT usage and suggests opportunity for educational and/or systems interventions.
Limitations
While our study strengths include a relatively large number of veterans and detailed review of individual patient data, it has multiple limitations. As a retrospective chart review-based study, incomplete or inaccurate data are a possibility. It is possible that patients underwent repeat FIT or underwent colonoscopy outside of the VA system and never recorded into the VA records. In addition, there is likely a sampling bias in this study as only veterans who underwent at least 1 FIT in the interval were included. These patients may be different from those who choose to undergo colonoscopy for CRC screening or from those who do not undergo screening at all.
Conclusions
A large percentage of patients underwent improper FIT at a tertiary referral academic VA medical center. Additional education and systems interventions are necessary to improve both provider and patient adherence to appropriate CRC screening. For example, one measure may include providing HCPs with a list of their patients not up-to-date with CRC screening that was shown to increase patient participation in FIT screening compared with patients who received usual care in a 2017 study.12 In addition, a 2018 study showed that a digital health intervention that allows patients to self-order tests (eg, on an iPad) can increase CRC screening rates.13
Author Contributions
Adam Gluskin: Study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript. Jeffrey Dueker: Study concept and design; analysis and interpretation of data; statistical analysis; critical revision of the manuscript for important intellectual content. Asif Khalid: Study concept and design; analysis and interpretation of data; drafting of the manuscripts; critical revision of the manuscript for important intellectual content; study supervision.
1. US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for Colorectal Cancer: US Preventive Services Task Force recommendation statement [published correction appears in JAMA. 2016 Aug 2;316(5):545] [published correction appears in JAMA. 2017 Jun 6;317(21):2239]. JAMA. 2016;315(23):2564-2575. doi:10.1001/jama.2016.5989
2. Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;153(1):307-323. doi:10.1053/j.gastro.2017.05.013
3. Lee YC, Li-Sheng Chen S, Ming-Fang Yen A, et al. Association between colorectal cancer mortality and gradient fecal hemoglobin concentration in colonoscopy noncompliers. J Natl Cancer Inst. 2017;109(5):djw269. doi:10.1093/jnci/djw269
4. Corley DA, Jensen CD, Quinn VP, et al. Association between time to colonoscopy after a positive fecal test result and risk of colorectal cancer and cancer stage at diagnosis. JAMA. 2017;317(16):1631-1641. doi:10.1001/jama.2017.3634
5. Gellad ZF, Almirall D, Provenzale D, Fisher DA. Time from positive screening fecal occult blood test to colonoscopy and risk of neoplasia. Dig Dis Sci. 2009;54(11):2497-2502. doi:10.1007/s10620-008-0653-8
6. Issaka RB, Singh MH, Oshima SM, et al. Inadequate utilization of diagnostic colonoscopy following abnormal FIT results in an integrated safety-net System. Am J Gastroenterol. 2017;112(2):375-382. doi:10.1038/ajg.2016.555
7. Carlson CM, Kirby KA, Casadei MA, Partin MR, Kistler CE, Walter LC. Lack of follow-up after fecal occult blood testing in older adults: inappropriate screening or failure to follow up?. Arch Intern Med. 2011;171(3):249-256. doi:10.1001/archinternmed.2010.372
8. Fisher DA, Judd L, Sanford NS. Inappropriate colorectal cancer screening: findings and implications. Am J Gastroenterol. 2005;100(11):2526-2530. doi:10.1111/j.1572-0241.2005.00322.x
9. Powell AA, Saini SD, Breitenstein MK, Noorbaloochi S, Cutting A, Fisher DA, Bloomfield HE, Halek K, Partin MR. Rates and correlates of potentially inappropriate colorectal cancer screening in the Veterans Health Administration. J Gen Intern Med. 2015 Jun;30(6):732-41. doi: 10.1007/s11606-014-3163-8
10. Jensen CD, Corley DA, Quinn VP, et al. Fecal immunochemical test program performance over 4 rounds of annual screening: a retrospective cohort study. Ann Intern Med. 2016;164(7):456-463. doi:10.7326/M15-0983
11. van der Vlugt M, Grobbee EJ, Bossuyt PM, et al. Risk of oral and upper gastrointestinal cancers in persons with positive results from a fecal immunochemical test in a colorectal cancer screening program. Clin Gastroenterol Hepatol. 2018;16(8):1237-1243.e2. doi:10.1016/j.cgh.2018.01.037
12. Rat C, Pogu C, Le Donné D, et al. Effect of physician notification regarding nonadherence to colorectal cancer screening on patient participation in fecal immunochemical test cancer screening: a randomized clinical trial. JAMA. 2017;318(9):816-824. doi:10.1001/jama.2017.11387
13. Miller DP Jr, Denizard-Thompson N, Weaver KE, et al. Effect of a digital health intervention on receipt of colorectal cancer screening in vulnerable patients: a randomized controlled trial. Ann Intern Med. 2018;168(8):550-557. doi:10.7326/M17-2315
Colonoscopies and annual fecal immunochemical tests (FITs), are 2 of the preferred modalities for colorectal cancer (CRC) screening endorsed by the US Preventive Services Task Forces as well as the US Multi-Society Task Force of Colorectal Cancer, which represents the American Gastroenterological Association, American College of Gastroenterology, and the American Society of Gastrointestinal Endoscopy.1,2 The recommendations include proper patient selection (patients aged 50 - 75 years with a life expectancy of at least 10 years), and a discussion with the patient regarding both options.
Background
It is known that patients with a positive FIT are at an increased risk for CRC. Lee and colleagues found that patients who do not undergo subsequent colonoscopy after a positive FIT have a 1.64 relative risk of death from colon cancer compared with those who undergo follow-up colonoscopy.3 Studies also have shown that longer wait times (10 months vs 1 month) between a positive FIT and colonoscopy also are associated with a higher risk of CRC.4 FIT utilize antibodies specific for the globin moiety of human hemoglobin and measure the development of antibody-globin complexes using immunoassay techniques. FIT has largely replaced the fecal occult blood test (FOBT), which depends on the detection of heme in feces through oxidation.
A US Department of Veterans Affairs (VA) study found that a longer time to colonoscopy was associated with a higher risk of neoplasia in veterans with a positive FOBT (odds ratio [OR], 1.10).5 It is thus crucial that a positive FOBT or FIT be investigated with follow-up colonoscopy. However, a retrospective study at a single safety-net hospital in San Francisco found that only 55.6% of patients with a positive FIT completed colonoscopy within 1 year.6 Importantly, almost half the patients examined in this study lacked documentation of the result of the FIT or counseling regarding the significance of the positive FIT by the patient’s primary care provider who ordered the test. A VA study looked at veterans aged > 70 years at 4 VA medical centers who did not receive a follow-up colonoscopy within 1 year and reported that 26% of patients studied had a documented refusal to undergo colonoscopy.7
It also is clear that FOBT is used inappropriately for colon cancer screening in some patients. A 2005 single-center VA study looked at inappropriate fecal occult blood tests and found that 18% of veterans for whom FOBTs were ordered had a severe comorbid illness, 13% had signs or symptoms of gastrointestinal (GI) blood loss, and 7% had a history of colorectal neoplasia or inflammatory bowel disease.8 An additional national VA study looked at all veterans aged ≥ 50 years who underwent FOBT or screening colonoscopy between 2009 and 2011 and found 26% to be inappropriate (13.9% of veterans not due for screening, 7.8% with limited life expectancy, and 11% receiving a FOBT when colonoscopy was indicated).9
An often-misunderstood additional requirement in utilizing FIT for CRC screening is that negative tests should be repeated annually.2 A study from Kaiser Permanente in California found that 75.3 to 86.1% of eligible patients underwent yearly FIT.10 In this study, programmatic FIT detected 80.4% of all patients with CRC detected within 1 year of testing.
Since most of the VA-specific studies are based on inappropriate or inadequate use of FOBT, we feel it is essential that further data be gained on appropriate and inappropriate testing. The aim of this study is to determine the frequency at which improper FIT occurs because of failure to obtain serial FIT over time with a negative result, failure to follow-up a positive FIT result with a diagnostic colonoscopy, or performance of FIT in veterans undergoing a recent colonoscopy with adequate bowel preparation. This quality assurance study received an institutional review board exemption from the VA Pittsburgh Healthcare System (VAPHS) in Pennsylvania.
Methods
VAPHS has a data repository of all veterans served within the health care system, which was queried for all veterans who underwent a FIT in the system from January 1, 2015 through December 31, 2017 as well as the number and results of FITs during the interval. In addition, the data repository was also queried specifically for veterans who had at least 1 colonoscopy as well as FIT between 2015 and 2017. The ordering location for each FIT also was queried.
We made 3 calculations for this study. First, we measured the rate of a negative initial FIT in 2015 and/or 2016 followed by a second FIT in 2016 and/or 2017 in a random selection of veterans (3% SE, 95% CI). Demographics were compared in an equal random number of veterans who did and did not have a follow up FIT (5% SE, 95% CI of all negative FIT). Second, we measured the rate of completing colonoscopy following a positive FIT in a random selection of veterans (3% SE, 95% SI). Finally, we calculated FITs following a colonoscopy for all veterans.
Using a power analysis with a 3% SE and 95% CI for sample size calculation and accounting for the approximate 50% exclusion rate from the final eligible population of veterans with at least 1 negative FIT, a random sample of 1,742 patient charts with a negative FIT in the interval were then reviewed to determine the frequency with which they underwent multiple FITs in the interval as well as for the presence of exclusionary factors. Because of the large number of veterans involved in this category, a more detailed demographics review was performed of a subset of these patients using a 95% CI and 5% SE. Using a 95% CI and 3% SE, 445 veterans with a positive FIT in the interval were reviewed to determine the frequency at which they underwent a follow-up diagnostic colonoscopy.
Because of a relatively small sample size, all 108 veterans who underwent a colonoscopy followed by a FIT were reviewed to determine the reason for follow-up FIT. In addition, in veterans who then went on to have a subsequent repeat colonoscopy, the examination findings were recorded.
Results
From January 1, 2015 to December 31, 2017, 6,766 FIT, were ordered at VAPHS. Of these, 4,391 unique veterans had at least 1 negative FIT during the period and 709 unique veterans had a positive FIT. There were 832 veterans who had both a FIT and colonoscopy during the study period. Of these, 108 had a colonoscopy with a subsequent FIT (Figure).
Of 1,742 randomly selected veterans with at least 1 negative FIT in the study interval, 870 were eligible for multiple FITs during this period as they were in the appropriate screening age (50-75 years or 85 years based on an assessment of life expectancy by the ordering health care provider [HCP]), did not have exclusionary comorbidities to multiple FIT, were not lost to follow-up, and had at least 1 negative FIT collected from 2015 to 2016 (veterans who only had a FIT in 2017 were excluded from this aim to avoid confounding). Of these 870 veterans, 543 (62.4%) underwent at least 2 FITs during the study period. In a demographic comparison of 110 veterans with 1 FIT and 110 veterans with > 1 FIT, there were no statistically significant differences in demographics (Table 1).
In a random chart review of 410 veterans with a positive FIT, 113 (27.5%) veterans did not undergo a subsequent colonoscopy within 1 year due to patient refusal, failure to schedule, or failure to keep colonoscopy appointment. There were no differences in demographics between those that underwent a diagnostic colonoscopy and those that did not (Table 2).
Of the 108 patients with a FIT following colonoscopy in the study interval, 97 FITs were negative. Ninety-five of the 108 FITs (88%) were judged to be inappropriate, having been performed for indications, including 38 for colon cancer screening, 23 for anemia, 32 for GI symptoms (eg, diarrhea, rectal bleeding, possible GI bleeding), and 2 for unclear indications. Thirteen FITs were deemed appropriate, as they were performed on veterans who refused to have a repeat colonoscopy following an examination with inadequate bowel preparation (Table 3). There was no difference in age or race between these 2 groups, although there was a statistically significant difference in gender (Table 4).
There were 19 patients who had a colonoscopy following a prior colonoscopy and subsequent positive FIT in the interval. Eight patients had no significant findings, 10 had nonadvanced adenomas, and 1 had an advanced adenoma (this patient had inadequate preparation with recommendation to repeat colonoscopy in 1 year).
While not a specific aim of the study we were able to identify certain HCPs by clinic location who systematically performed inappropriate or appropriate FIT. There were 47 separate ordering locations for the 95 inappropriate FIT following recent colonoscopy. Of these, 1 location was responsible for ordering 20 (21%) inappropriate FIT. Eight locations accounted for 51% of all the inappropriately ordered FIT. Two clinics seemed to be high performers in regard to overall appropriate vs inappropriate FIT use. The appropriate FIT rate for these locations was 30 of 33 (90.9%) and 26 of 28 (92.8%), respectively.
Discussion
In this retrospective study, we found that a large percentage of veterans eligible for colon cancer screening utilizing FIT did not undergo appropriate screening. Almost 40% of veterans in a 3-year interval received only 1 FIT. This seemed to occur due to a combination of patient refusal and inadequate education by HCPs regarding how to screen appropriately for CRC using FIT. This occurred despite a reminder in the VA Computerized Patient Record System regarding CRC screening.
There did not seem to be significant differences in demographics between those who were screened appropriately vs inappropriately. While there was a statistically significant difference in gender between those who had an appropriate FIT following recent colonoscopy (2 of 13 were female) and those who had an inappropriate FIT after recent colonoscopy (1 of 95 was a female), we are uncertain of the significance of this finding given the small number of female veterans in the analysis.
We do believe that the ratio of veterans in our study with a single FIT likely underestimates the true prevalence. To avoid confounding from factors such as inadequate prior follow-up in the study interval, we excluded veterans who underwent FIT only in 2017 for this analysis. As such, a significant percentage of these veterans were actually eligible to be screened throughout the study interval.
In spite of recommendations regarding the need for diagnostic colonoscopy following a positive FIT, we found that more than one-quarter of patients did not undergo colonoscopy. Although this number is an improvement over previously published literature that found almost half of patients at a safety-net hospital did not undergo diagnostic colonoscopy following a positive FIT, this is still clearly suboptimal.6
VAPHS has a mandate that all patients with a positive FIT be scheduled for colonoscopy within 30 days, either at VAPHS or in the community. An alert is sent to both ordering HCP regarding the positive FIT as well as to the GI department. In addition to contact from the ordering HCP, all veterans also are contacted by either a physician or nurse practitioner GI provider to provide test results and an explanation of its clinical significance and to facilitate colonoscopy scheduling. If a patient cannot be reached by telephone, the patient is sent a certified letter from the GI department regarding the significance of a positive FIT and instructions for scheduling a colonoscopy.
Despite this outreach, 27.5% of veterans did not have a diagnostic colonoscopy following a positive FIT. This suggests that there may be inadequate education and counseling of veterans at the time of the FIT order about the subsequent series of events and need for diagnostic colonoscopy following a positive FIT. If a patient refuses to undergo a colonoscopy under any circumstances (including after a positive FIT), the utility of placing a FIT order is questionable.
There is also a need for more education of ordering HCPs on appropriate indications for FITs. We found that 35% of FIT ordered after a recent colonoscopy were done for the purpose of CRC screening, despite clear guidelines recommending against this. In addition, another 50% of FIT ordered after recent colonoscopy was done either for evaluation of GI symptoms like diarrhea and rectal bleeding or in the evaluation of anemia, both of which are inappropriate uses for FIT. Since FIT is an antibody test against globin, the protein component of hemoglobin that degrades during passage through the small bowel, it is not a useful test for the evaluation of upper GI or small bowel bleeding. A relatively recent database study in the Netherlands looking at the diagnosis of upper GI malignancies within 3 years of a positive FIT found a < 1% rate.11
In our study, albeit limited by the small number of veterans undergoing a repeat colonoscopy following a prior colonoscopy and subsequent positive FIT, there were few significant findings. Only 1 veteran had an advanced adenoma detected, and this veteran had already been recommended a repeat colonoscopy in 1 year due to an inadequate bowel preparation on the last examination.
Lastly, we found that certain HCPs (based on ordering clinic location) systematically performed improper FIT compared with other HCPs. This presumably is due to a lack of education on appropriate FIT usage and suggests opportunity for educational and/or systems interventions.
Limitations
While our study strengths include a relatively large number of veterans and detailed review of individual patient data, it has multiple limitations. As a retrospective chart review-based study, incomplete or inaccurate data are a possibility. It is possible that patients underwent repeat FIT or underwent colonoscopy outside of the VA system and never recorded into the VA records. In addition, there is likely a sampling bias in this study as only veterans who underwent at least 1 FIT in the interval were included. These patients may be different from those who choose to undergo colonoscopy for CRC screening or from those who do not undergo screening at all.
Conclusions
A large percentage of patients underwent improper FIT at a tertiary referral academic VA medical center. Additional education and systems interventions are necessary to improve both provider and patient adherence to appropriate CRC screening. For example, one measure may include providing HCPs with a list of their patients not up-to-date with CRC screening that was shown to increase patient participation in FIT screening compared with patients who received usual care in a 2017 study.12 In addition, a 2018 study showed that a digital health intervention that allows patients to self-order tests (eg, on an iPad) can increase CRC screening rates.13
Author Contributions
Adam Gluskin: Study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript. Jeffrey Dueker: Study concept and design; analysis and interpretation of data; statistical analysis; critical revision of the manuscript for important intellectual content. Asif Khalid: Study concept and design; analysis and interpretation of data; drafting of the manuscripts; critical revision of the manuscript for important intellectual content; study supervision.
Colonoscopies and annual fecal immunochemical tests (FITs), are 2 of the preferred modalities for colorectal cancer (CRC) screening endorsed by the US Preventive Services Task Forces as well as the US Multi-Society Task Force of Colorectal Cancer, which represents the American Gastroenterological Association, American College of Gastroenterology, and the American Society of Gastrointestinal Endoscopy.1,2 The recommendations include proper patient selection (patients aged 50 - 75 years with a life expectancy of at least 10 years), and a discussion with the patient regarding both options.
Background
It is known that patients with a positive FIT are at an increased risk for CRC. Lee and colleagues found that patients who do not undergo subsequent colonoscopy after a positive FIT have a 1.64 relative risk of death from colon cancer compared with those who undergo follow-up colonoscopy.3 Studies also have shown that longer wait times (10 months vs 1 month) between a positive FIT and colonoscopy also are associated with a higher risk of CRC.4 FIT utilize antibodies specific for the globin moiety of human hemoglobin and measure the development of antibody-globin complexes using immunoassay techniques. FIT has largely replaced the fecal occult blood test (FOBT), which depends on the detection of heme in feces through oxidation.
A US Department of Veterans Affairs (VA) study found that a longer time to colonoscopy was associated with a higher risk of neoplasia in veterans with a positive FOBT (odds ratio [OR], 1.10).5 It is thus crucial that a positive FOBT or FIT be investigated with follow-up colonoscopy. However, a retrospective study at a single safety-net hospital in San Francisco found that only 55.6% of patients with a positive FIT completed colonoscopy within 1 year.6 Importantly, almost half the patients examined in this study lacked documentation of the result of the FIT or counseling regarding the significance of the positive FIT by the patient’s primary care provider who ordered the test. A VA study looked at veterans aged > 70 years at 4 VA medical centers who did not receive a follow-up colonoscopy within 1 year and reported that 26% of patients studied had a documented refusal to undergo colonoscopy.7
It also is clear that FOBT is used inappropriately for colon cancer screening in some patients. A 2005 single-center VA study looked at inappropriate fecal occult blood tests and found that 18% of veterans for whom FOBTs were ordered had a severe comorbid illness, 13% had signs or symptoms of gastrointestinal (GI) blood loss, and 7% had a history of colorectal neoplasia or inflammatory bowel disease.8 An additional national VA study looked at all veterans aged ≥ 50 years who underwent FOBT or screening colonoscopy between 2009 and 2011 and found 26% to be inappropriate (13.9% of veterans not due for screening, 7.8% with limited life expectancy, and 11% receiving a FOBT when colonoscopy was indicated).9
An often-misunderstood additional requirement in utilizing FIT for CRC screening is that negative tests should be repeated annually.2 A study from Kaiser Permanente in California found that 75.3 to 86.1% of eligible patients underwent yearly FIT.10 In this study, programmatic FIT detected 80.4% of all patients with CRC detected within 1 year of testing.
Since most of the VA-specific studies are based on inappropriate or inadequate use of FOBT, we feel it is essential that further data be gained on appropriate and inappropriate testing. The aim of this study is to determine the frequency at which improper FIT occurs because of failure to obtain serial FIT over time with a negative result, failure to follow-up a positive FIT result with a diagnostic colonoscopy, or performance of FIT in veterans undergoing a recent colonoscopy with adequate bowel preparation. This quality assurance study received an institutional review board exemption from the VA Pittsburgh Healthcare System (VAPHS) in Pennsylvania.
Methods
VAPHS has a data repository of all veterans served within the health care system, which was queried for all veterans who underwent a FIT in the system from January 1, 2015 through December 31, 2017 as well as the number and results of FITs during the interval. In addition, the data repository was also queried specifically for veterans who had at least 1 colonoscopy as well as FIT between 2015 and 2017. The ordering location for each FIT also was queried.
We made 3 calculations for this study. First, we measured the rate of a negative initial FIT in 2015 and/or 2016 followed by a second FIT in 2016 and/or 2017 in a random selection of veterans (3% SE, 95% CI). Demographics were compared in an equal random number of veterans who did and did not have a follow up FIT (5% SE, 95% CI of all negative FIT). Second, we measured the rate of completing colonoscopy following a positive FIT in a random selection of veterans (3% SE, 95% SI). Finally, we calculated FITs following a colonoscopy for all veterans.
Using a power analysis with a 3% SE and 95% CI for sample size calculation and accounting for the approximate 50% exclusion rate from the final eligible population of veterans with at least 1 negative FIT, a random sample of 1,742 patient charts with a negative FIT in the interval were then reviewed to determine the frequency with which they underwent multiple FITs in the interval as well as for the presence of exclusionary factors. Because of the large number of veterans involved in this category, a more detailed demographics review was performed of a subset of these patients using a 95% CI and 5% SE. Using a 95% CI and 3% SE, 445 veterans with a positive FIT in the interval were reviewed to determine the frequency at which they underwent a follow-up diagnostic colonoscopy.
Because of a relatively small sample size, all 108 veterans who underwent a colonoscopy followed by a FIT were reviewed to determine the reason for follow-up FIT. In addition, in veterans who then went on to have a subsequent repeat colonoscopy, the examination findings were recorded.
Results
From January 1, 2015 to December 31, 2017, 6,766 FIT, were ordered at VAPHS. Of these, 4,391 unique veterans had at least 1 negative FIT during the period and 709 unique veterans had a positive FIT. There were 832 veterans who had both a FIT and colonoscopy during the study period. Of these, 108 had a colonoscopy with a subsequent FIT (Figure).
Of 1,742 randomly selected veterans with at least 1 negative FIT in the study interval, 870 were eligible for multiple FITs during this period as they were in the appropriate screening age (50-75 years or 85 years based on an assessment of life expectancy by the ordering health care provider [HCP]), did not have exclusionary comorbidities to multiple FIT, were not lost to follow-up, and had at least 1 negative FIT collected from 2015 to 2016 (veterans who only had a FIT in 2017 were excluded from this aim to avoid confounding). Of these 870 veterans, 543 (62.4%) underwent at least 2 FITs during the study period. In a demographic comparison of 110 veterans with 1 FIT and 110 veterans with > 1 FIT, there were no statistically significant differences in demographics (Table 1).
In a random chart review of 410 veterans with a positive FIT, 113 (27.5%) veterans did not undergo a subsequent colonoscopy within 1 year due to patient refusal, failure to schedule, or failure to keep colonoscopy appointment. There were no differences in demographics between those that underwent a diagnostic colonoscopy and those that did not (Table 2).
Of the 108 patients with a FIT following colonoscopy in the study interval, 97 FITs were negative. Ninety-five of the 108 FITs (88%) were judged to be inappropriate, having been performed for indications, including 38 for colon cancer screening, 23 for anemia, 32 for GI symptoms (eg, diarrhea, rectal bleeding, possible GI bleeding), and 2 for unclear indications. Thirteen FITs were deemed appropriate, as they were performed on veterans who refused to have a repeat colonoscopy following an examination with inadequate bowel preparation (Table 3). There was no difference in age or race between these 2 groups, although there was a statistically significant difference in gender (Table 4).
There were 19 patients who had a colonoscopy following a prior colonoscopy and subsequent positive FIT in the interval. Eight patients had no significant findings, 10 had nonadvanced adenomas, and 1 had an advanced adenoma (this patient had inadequate preparation with recommendation to repeat colonoscopy in 1 year).
While not a specific aim of the study we were able to identify certain HCPs by clinic location who systematically performed inappropriate or appropriate FIT. There were 47 separate ordering locations for the 95 inappropriate FIT following recent colonoscopy. Of these, 1 location was responsible for ordering 20 (21%) inappropriate FIT. Eight locations accounted for 51% of all the inappropriately ordered FIT. Two clinics seemed to be high performers in regard to overall appropriate vs inappropriate FIT use. The appropriate FIT rate for these locations was 30 of 33 (90.9%) and 26 of 28 (92.8%), respectively.
Discussion
In this retrospective study, we found that a large percentage of veterans eligible for colon cancer screening utilizing FIT did not undergo appropriate screening. Almost 40% of veterans in a 3-year interval received only 1 FIT. This seemed to occur due to a combination of patient refusal and inadequate education by HCPs regarding how to screen appropriately for CRC using FIT. This occurred despite a reminder in the VA Computerized Patient Record System regarding CRC screening.
There did not seem to be significant differences in demographics between those who were screened appropriately vs inappropriately. While there was a statistically significant difference in gender between those who had an appropriate FIT following recent colonoscopy (2 of 13 were female) and those who had an inappropriate FIT after recent colonoscopy (1 of 95 was a female), we are uncertain of the significance of this finding given the small number of female veterans in the analysis.
We do believe that the ratio of veterans in our study with a single FIT likely underestimates the true prevalence. To avoid confounding from factors such as inadequate prior follow-up in the study interval, we excluded veterans who underwent FIT only in 2017 for this analysis. As such, a significant percentage of these veterans were actually eligible to be screened throughout the study interval.
In spite of recommendations regarding the need for diagnostic colonoscopy following a positive FIT, we found that more than one-quarter of patients did not undergo colonoscopy. Although this number is an improvement over previously published literature that found almost half of patients at a safety-net hospital did not undergo diagnostic colonoscopy following a positive FIT, this is still clearly suboptimal.6
VAPHS has a mandate that all patients with a positive FIT be scheduled for colonoscopy within 30 days, either at VAPHS or in the community. An alert is sent to both ordering HCP regarding the positive FIT as well as to the GI department. In addition to contact from the ordering HCP, all veterans also are contacted by either a physician or nurse practitioner GI provider to provide test results and an explanation of its clinical significance and to facilitate colonoscopy scheduling. If a patient cannot be reached by telephone, the patient is sent a certified letter from the GI department regarding the significance of a positive FIT and instructions for scheduling a colonoscopy.
Despite this outreach, 27.5% of veterans did not have a diagnostic colonoscopy following a positive FIT. This suggests that there may be inadequate education and counseling of veterans at the time of the FIT order about the subsequent series of events and need for diagnostic colonoscopy following a positive FIT. If a patient refuses to undergo a colonoscopy under any circumstances (including after a positive FIT), the utility of placing a FIT order is questionable.
There is also a need for more education of ordering HCPs on appropriate indications for FITs. We found that 35% of FIT ordered after a recent colonoscopy were done for the purpose of CRC screening, despite clear guidelines recommending against this. In addition, another 50% of FIT ordered after recent colonoscopy was done either for evaluation of GI symptoms like diarrhea and rectal bleeding or in the evaluation of anemia, both of which are inappropriate uses for FIT. Since FIT is an antibody test against globin, the protein component of hemoglobin that degrades during passage through the small bowel, it is not a useful test for the evaluation of upper GI or small bowel bleeding. A relatively recent database study in the Netherlands looking at the diagnosis of upper GI malignancies within 3 years of a positive FIT found a < 1% rate.11
In our study, albeit limited by the small number of veterans undergoing a repeat colonoscopy following a prior colonoscopy and subsequent positive FIT, there were few significant findings. Only 1 veteran had an advanced adenoma detected, and this veteran had already been recommended a repeat colonoscopy in 1 year due to an inadequate bowel preparation on the last examination.
Lastly, we found that certain HCPs (based on ordering clinic location) systematically performed improper FIT compared with other HCPs. This presumably is due to a lack of education on appropriate FIT usage and suggests opportunity for educational and/or systems interventions.
Limitations
While our study strengths include a relatively large number of veterans and detailed review of individual patient data, it has multiple limitations. As a retrospective chart review-based study, incomplete or inaccurate data are a possibility. It is possible that patients underwent repeat FIT or underwent colonoscopy outside of the VA system and never recorded into the VA records. In addition, there is likely a sampling bias in this study as only veterans who underwent at least 1 FIT in the interval were included. These patients may be different from those who choose to undergo colonoscopy for CRC screening or from those who do not undergo screening at all.
Conclusions
A large percentage of patients underwent improper FIT at a tertiary referral academic VA medical center. Additional education and systems interventions are necessary to improve both provider and patient adherence to appropriate CRC screening. For example, one measure may include providing HCPs with a list of their patients not up-to-date with CRC screening that was shown to increase patient participation in FIT screening compared with patients who received usual care in a 2017 study.12 In addition, a 2018 study showed that a digital health intervention that allows patients to self-order tests (eg, on an iPad) can increase CRC screening rates.13
Author Contributions
Adam Gluskin: Study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript. Jeffrey Dueker: Study concept and design; analysis and interpretation of data; statistical analysis; critical revision of the manuscript for important intellectual content. Asif Khalid: Study concept and design; analysis and interpretation of data; drafting of the manuscripts; critical revision of the manuscript for important intellectual content; study supervision.
1. US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for Colorectal Cancer: US Preventive Services Task Force recommendation statement [published correction appears in JAMA. 2016 Aug 2;316(5):545] [published correction appears in JAMA. 2017 Jun 6;317(21):2239]. JAMA. 2016;315(23):2564-2575. doi:10.1001/jama.2016.5989
2. Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;153(1):307-323. doi:10.1053/j.gastro.2017.05.013
3. Lee YC, Li-Sheng Chen S, Ming-Fang Yen A, et al. Association between colorectal cancer mortality and gradient fecal hemoglobin concentration in colonoscopy noncompliers. J Natl Cancer Inst. 2017;109(5):djw269. doi:10.1093/jnci/djw269
4. Corley DA, Jensen CD, Quinn VP, et al. Association between time to colonoscopy after a positive fecal test result and risk of colorectal cancer and cancer stage at diagnosis. JAMA. 2017;317(16):1631-1641. doi:10.1001/jama.2017.3634
5. Gellad ZF, Almirall D, Provenzale D, Fisher DA. Time from positive screening fecal occult blood test to colonoscopy and risk of neoplasia. Dig Dis Sci. 2009;54(11):2497-2502. doi:10.1007/s10620-008-0653-8
6. Issaka RB, Singh MH, Oshima SM, et al. Inadequate utilization of diagnostic colonoscopy following abnormal FIT results in an integrated safety-net System. Am J Gastroenterol. 2017;112(2):375-382. doi:10.1038/ajg.2016.555
7. Carlson CM, Kirby KA, Casadei MA, Partin MR, Kistler CE, Walter LC. Lack of follow-up after fecal occult blood testing in older adults: inappropriate screening or failure to follow up?. Arch Intern Med. 2011;171(3):249-256. doi:10.1001/archinternmed.2010.372
8. Fisher DA, Judd L, Sanford NS. Inappropriate colorectal cancer screening: findings and implications. Am J Gastroenterol. 2005;100(11):2526-2530. doi:10.1111/j.1572-0241.2005.00322.x
9. Powell AA, Saini SD, Breitenstein MK, Noorbaloochi S, Cutting A, Fisher DA, Bloomfield HE, Halek K, Partin MR. Rates and correlates of potentially inappropriate colorectal cancer screening in the Veterans Health Administration. J Gen Intern Med. 2015 Jun;30(6):732-41. doi: 10.1007/s11606-014-3163-8
10. Jensen CD, Corley DA, Quinn VP, et al. Fecal immunochemical test program performance over 4 rounds of annual screening: a retrospective cohort study. Ann Intern Med. 2016;164(7):456-463. doi:10.7326/M15-0983
11. van der Vlugt M, Grobbee EJ, Bossuyt PM, et al. Risk of oral and upper gastrointestinal cancers in persons with positive results from a fecal immunochemical test in a colorectal cancer screening program. Clin Gastroenterol Hepatol. 2018;16(8):1237-1243.e2. doi:10.1016/j.cgh.2018.01.037
12. Rat C, Pogu C, Le Donné D, et al. Effect of physician notification regarding nonadherence to colorectal cancer screening on patient participation in fecal immunochemical test cancer screening: a randomized clinical trial. JAMA. 2017;318(9):816-824. doi:10.1001/jama.2017.11387
13. Miller DP Jr, Denizard-Thompson N, Weaver KE, et al. Effect of a digital health intervention on receipt of colorectal cancer screening in vulnerable patients: a randomized controlled trial. Ann Intern Med. 2018;168(8):550-557. doi:10.7326/M17-2315
1. US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for Colorectal Cancer: US Preventive Services Task Force recommendation statement [published correction appears in JAMA. 2016 Aug 2;316(5):545] [published correction appears in JAMA. 2017 Jun 6;317(21):2239]. JAMA. 2016;315(23):2564-2575. doi:10.1001/jama.2016.5989
2. Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;153(1):307-323. doi:10.1053/j.gastro.2017.05.013
3. Lee YC, Li-Sheng Chen S, Ming-Fang Yen A, et al. Association between colorectal cancer mortality and gradient fecal hemoglobin concentration in colonoscopy noncompliers. J Natl Cancer Inst. 2017;109(5):djw269. doi:10.1093/jnci/djw269
4. Corley DA, Jensen CD, Quinn VP, et al. Association between time to colonoscopy after a positive fecal test result and risk of colorectal cancer and cancer stage at diagnosis. JAMA. 2017;317(16):1631-1641. doi:10.1001/jama.2017.3634
5. Gellad ZF, Almirall D, Provenzale D, Fisher DA. Time from positive screening fecal occult blood test to colonoscopy and risk of neoplasia. Dig Dis Sci. 2009;54(11):2497-2502. doi:10.1007/s10620-008-0653-8
6. Issaka RB, Singh MH, Oshima SM, et al. Inadequate utilization of diagnostic colonoscopy following abnormal FIT results in an integrated safety-net System. Am J Gastroenterol. 2017;112(2):375-382. doi:10.1038/ajg.2016.555
7. Carlson CM, Kirby KA, Casadei MA, Partin MR, Kistler CE, Walter LC. Lack of follow-up after fecal occult blood testing in older adults: inappropriate screening or failure to follow up?. Arch Intern Med. 2011;171(3):249-256. doi:10.1001/archinternmed.2010.372
8. Fisher DA, Judd L, Sanford NS. Inappropriate colorectal cancer screening: findings and implications. Am J Gastroenterol. 2005;100(11):2526-2530. doi:10.1111/j.1572-0241.2005.00322.x
9. Powell AA, Saini SD, Breitenstein MK, Noorbaloochi S, Cutting A, Fisher DA, Bloomfield HE, Halek K, Partin MR. Rates and correlates of potentially inappropriate colorectal cancer screening in the Veterans Health Administration. J Gen Intern Med. 2015 Jun;30(6):732-41. doi: 10.1007/s11606-014-3163-8
10. Jensen CD, Corley DA, Quinn VP, et al. Fecal immunochemical test program performance over 4 rounds of annual screening: a retrospective cohort study. Ann Intern Med. 2016;164(7):456-463. doi:10.7326/M15-0983
11. van der Vlugt M, Grobbee EJ, Bossuyt PM, et al. Risk of oral and upper gastrointestinal cancers in persons with positive results from a fecal immunochemical test in a colorectal cancer screening program. Clin Gastroenterol Hepatol. 2018;16(8):1237-1243.e2. doi:10.1016/j.cgh.2018.01.037
12. Rat C, Pogu C, Le Donné D, et al. Effect of physician notification regarding nonadherence to colorectal cancer screening on patient participation in fecal immunochemical test cancer screening: a randomized clinical trial. JAMA. 2017;318(9):816-824. doi:10.1001/jama.2017.11387
13. Miller DP Jr, Denizard-Thompson N, Weaver KE, et al. Effect of a digital health intervention on receipt of colorectal cancer screening in vulnerable patients: a randomized controlled trial. Ann Intern Med. 2018;168(8):550-557. doi:10.7326/M17-2315
Outcomes Following Implementation of a Hospital-Wide, Multicomponent Delirium Care Pathway
Delirium is an acute disturbance in mental status characterized by fluctuations in cognition and attention that affects more than 2.6 million hospitalized older adults in the United States annually, a rate that is expected to increase as the population ages.1-4 Hospital-acquired delirium is associated with poor outcomes, including prolonged hospital length of stay (LOS), loss of independence, cognitive impairment, and even death.5-10 Individuals who develop delirium do poorly after hospital discharge and are more likely to be readmitted within 30 days.11 Approximately 30% to 40% of hospital-acquired delirium cases are preventable.10,12 However, programs designed to prevent delirium and associated complications, such as increased LOS, have demonstrated variable success.12-14 Many studies are limited by small sample sizes, lack of generalizability to different hospitalized patient populations, poor adherence, or reliance on outside funding.12,13,15-18
Delirium prevention programs face several challenges because delirium could be caused by a variety of risk factors and precipitants.19,20 Some risk factors that occur frequently among hospitalized patients can be mitigated, such as sensory impairment, immobility from physical restraints or urinary catheters, and polypharmacy.20,21 Effective delirium care pathways targeting these risk factors must be multifaceted, interdisciplinary, and interprofessional. Accurate risk assessment is critical to allocate resources to high-risk patients. Delirium affects patients in all medical and surgical disciplines, and often is underdiagnosed.19,22 Comprehensive screening is necessary to identify cases early and track outcomes, and educational efforts must reach all providers in the hospital. These challenges require a systematic, pragmatic approach to change.
The purpose of this study was to evaluate the association between a delirium care pathway and clinical outcomes for hospitalized patients. We hypothesized that this program would be associated with reduced hospital LOS, with secondary benefits to hospitalization costs, odds of 30-day readmission, and delirium rates.
METHODS
Study Design
In this retrospective cohort study, we compared clinical outcomes the year before and after implementation of a delirium care pathway across seven hospital units. The study period spanned October 1, 2015, through February 28, 2019. The study was approved by the University of California, San Francisco Institutional Review Board (#13-12500).
Multicomponent Delirium Care Pathway
The delirium care pathway was developed collaboratively among geriatrics, hospital medicine, neurology, anesthesiology, surgery, and psychiatry services, with an interprofessional team of physicians, nurses, pharmacists, and physical and occupational therapists. This pathway was implemented in units consecutively, approximately every 4 months in the following order: neurosciences, medicine, cardiology, general surgery, specialty surgery, hematology-oncology, and transplant. The same implementation education protocols were performed in each unit. The pathway consisted of several components targeting delirium prevention and management (Appendix Figure 1 and Appendix Figure 2). Systematic screening for delirium was introduced as part of the multicomponent intervention. Nursing staff assessed each patient’s risk of developing delirium at admission using the AWOL score, a validated delirium prediction tool.23 AWOL consists of: patient Age, spelling “World” backwards correctly, Orientation, and assessment of iLlness severity by the nurse. For patients who spoke a language other than English, spelling of “world” backwards was translated to his or her primary language, or if this was not possible, the task was modified to serial 7s (subtracting 7 from 100 in a serial fashion). This modification has been validated for use in other languages.24 Patients at high risk for delirium based on an AWOL score ≥2 received a multidisciplinary intervention with four components: (1) notifying the primary team by pager and electronic medical record (EMR), (2) a nurse-led, evidence-based, nonpharmacologic multicomponent intervention,25 (3) placement of a delirium order set by the physician, and (4) review of medications by the unit pharmacist who adjusted administration timing to occur during waking hours and placed a note in the EMR notifying the primary team of potentially deliriogenic medications. The delirium order set reinforced the nonpharmacologic multicomponent intervention through a nursing order, placed an automatic consult to occupational therapy, and included options to order physical therapy, order speech/language therapy, obtain vital signs three times daily with minimal night interruptions, remove an indwelling bladder catheter, and prescribe melatonin as a sleep aid.
The bedside nurse screened all patients for active delirium every 12-hour shift using the Nursing Delirium Screening Scale (NuDESC) and entered the results into the EMR.23,26 Capturing NuDESC results in the EMR allowed communication across medical providers as well as monitoring of screening adherence. Each nurse received two in-person trainings in staff meetings and one-to-one instruction during the first week of implementation. All nurses were required to complete a 15-minute training module and had the option of completing an additional 1-hour continuing medical education module. If a patient was transferred to the intensive care unit (ICU), delirium was identified through use of the ICU-specific Confusion Assessment Method (CAM-ICU) assessments, which the bedside nurse performed each shift throughout the intervention period.27 Nurses were instructed to call the primary team physician after every positive screen. Before each unit’s implementation start date, physicians with patients on that unit received education through a combination of grand rounds, resident lectures and seminars, and a pocket card on delirium evaluation and management.
Participants and Eligibility Criteria
We included all patients aged ≥50 years hospitalized for >1 day on each hospital unit (Figure). We included adults aged ≥50 years to maximize the number of participants for this study while also capturing a population at risk for delirium. Because the delirium care pathway was unit-based and the pathway was rolled out sequentially across units, only patients who were admitted to and discharged from the same unit were included to better isolate the effect of the pathway. Patients who were transferred to the ICU were only included if they were discharged from the original unit of admission. Only the first hospitalization was included for patients with multiple hospitalizations during the study period.
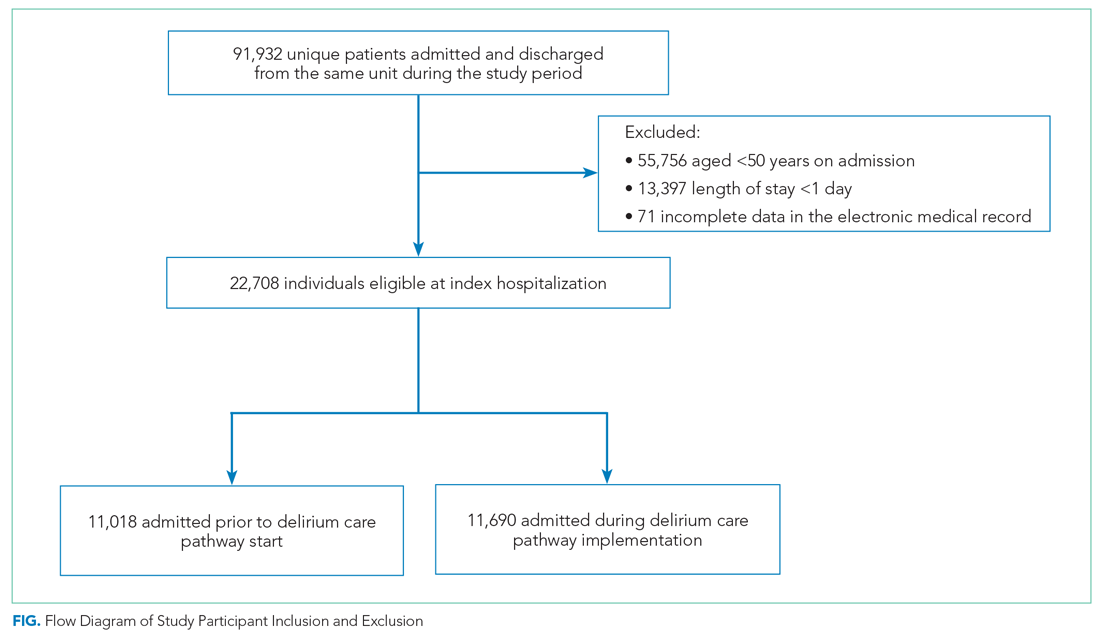
Patient Characteristics
Patient demographics and clinical data were collected after discharge through Clarity and Vizient electronic databases (Table 1 and Table 2). All Elixhauser comorbidities were included except for the following International Classification of Disease, Tenth Revision, Clinical Modification (ICD-10) codes that overlapped with a delirium diagnosis: G31.2, G93.89, G93.9, G94, R41.0, and R41.82 (Appendix Table 1). Severity of illness was obtained from Vizient, which calculates illness severity based on clinical and claims data (Appendix Table 1).
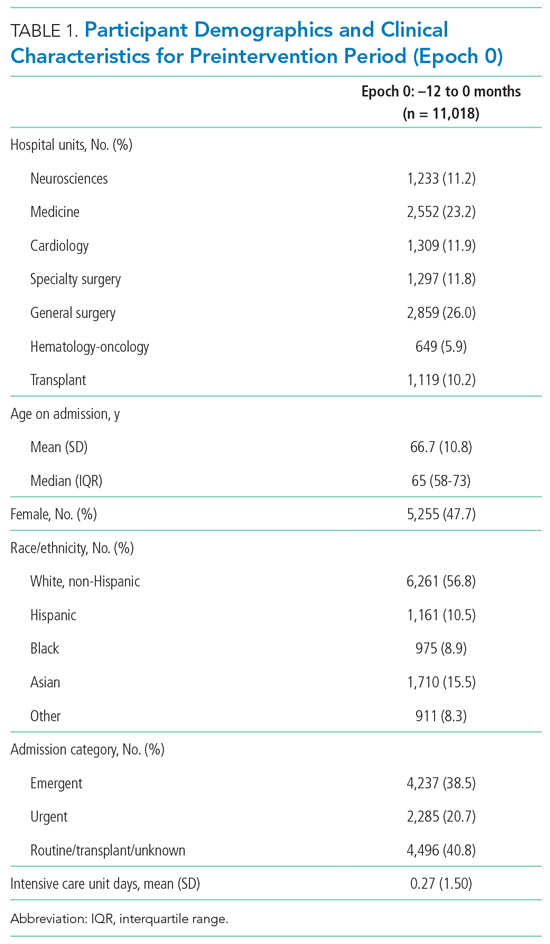
Delirium Metrics
Delirium screening was introduced as part of the multicomponent intervention, and therefore delirium rates before the intervention could not be determined. Trends in delirium prevalence and incidence after the intervention are reported. Prevalent delirium was defined as a single score of ≥2 on the nurse-administered NuDESC or a positive CAM-ICU at any point during the hospital stay. Incident delirium was identified if the first NuDESC score was negative and any subsequent NuDESC or CAM-ICU score was positive.

Outcomes
The primary study outcome was hospital LOS across all participants. Secondary outcomes included total direct cost and odds of 30-day hospital readmission. Readmissions tracked as part of hospital quality reporting were obtained from Vizient and were not captured if they occurred at another hospital. We also examined rates of safety attendant and restraint use during the study period, defined as the number of safety attendant days or restraint days per 1,000 patient days.
Because previous studies have demonstrated the effectiveness of multicomponent delirium interventions among elderly general medical patients,12 we also investigated these same outcomes in the medicine unit alone.
Statistical Analysis
The date of intervention implementation was determined for each hospital unit, which was defined as time(0) [t(0)]. The 12-month postintervention period was divided into four 3-month epochs to assess for trends. Data were aggregated across the seven units using t(0) as the start date, agnostic to the calendar month. Demographic and clinical characteristics were collected for the 12-months before t(0) and the four 3-month epochs after t(0). Univariate analysis of outcome variables comparing trends across the same epochs were conducted in the same manner, except for the rate of delirium, which was measured after t(0) and therefore could not be compared with the preintervention period.
Multivariable models were adjusted for age, sex, race/ethnicity, admission category, Elixhauser comorbidities, severity of illness quartile, and number days spent in the ICU. Admission category referred to whether the admission was emergent, urgent, or elective/unknown. Because it took 3 months after t(0) for each unit to reach a delirium screening compliance rate of 90%, the intervention was only considered fully implemented after this period. A ramp-up variable was set to 0 for admissions occurring prior to the intervention to t(0), 1/3 for admissions occurring 1 month post intervention, 2/3 for 2 months post intervention, and 1 for admissions occurring 3 to 12 months post intervention. In this way, the coefficient for the ramp-up variable estimated the postintervention versus preintervention effect. Numerical outcomes (LOS, cost) were log transformed to reduce skewness and analyzed using linear models. Coefficients were back-transformed to provide interpretations as proportional change in the median outcomes.
For LOS and readmission, we assessed secular trends by including admission date and admission date squared, in case the trend was nonlinear, as possible predictors; admission date was the specific date—not time from t(0)—to account for secular trends and allow contemporaneous controls in the analysis. To be conservative, we retained secular terms (first considering the quadratic and then the linear) if P <.10. The categorical outcome (30-day readmission) was analyzed using a logistic model. Count variables (delirium, safety attendants, restraints) were analyzed using Poisson regression models with a log link, and coefficients were back-transformed to provide rate ratio interpretations. Because delirium was not measured before t(0), and because the intervention was considered to take 3 months to become fully effective, baseline delirium rates were defined as those in the first 3 months adjusted by the ramp-up variable. For each outcome we included hospital unit, a ramp-up variable (measuring the pre- vs postintervention effect), and their interaction. If there was no statistically significant interaction, we presented the outcome for all units combined. If the interaction was statistically significant, we looked for consistency across units and reported results for all units combined when consistent, along with site-specific results. If the results were not consistent across the units, we provided site-specific results only. All statistical analyses were performed using SAS software, version 9.4 (SAS Institute Inc).
RESULTS
Participant Demographics and Clinical Characteristics
A total of 22,708 individuals were included in this study, with 11,018 in the preintervention period (Table 1 and Table 2). Most patients were cared for on the general surgery unit (n = 5,899), followed by the medicine unit (n = 4,923). The smallest number of patients were cared for on the hematology-oncology unit (n = 1,709). Across the five epochs, patients were of similar age and sex, and spent a similar number of days in the ICU. The population was diverse with regard to race and ethnicity; there were minor differences in admission category. There were also minor differences in severity of illness and some comorbidities between timepoints (Appendix Table 1).
Delirium Metrics
Delirium prevalence was 13.0% during the first epoch post intervention, followed by 12.0%, 11.7%, and 13.0% in the subsequent epochs (P = .91). Incident delirium occurred in 6.1% of patients during the first epoch post intervention, followed by 5.3%, 5.3%, and 5.8% in the subsequent epochs (P = .63).
Primary Outcome
Epoch-level data for LOS before and after the intervention is shown in Appendix Table 2. The mean unadjusted LOS for all units combined did not decrease after the intervention, but in the adjusted model, the mean LOS decreased by 2% after the intervention (P = .0087; Table 3).
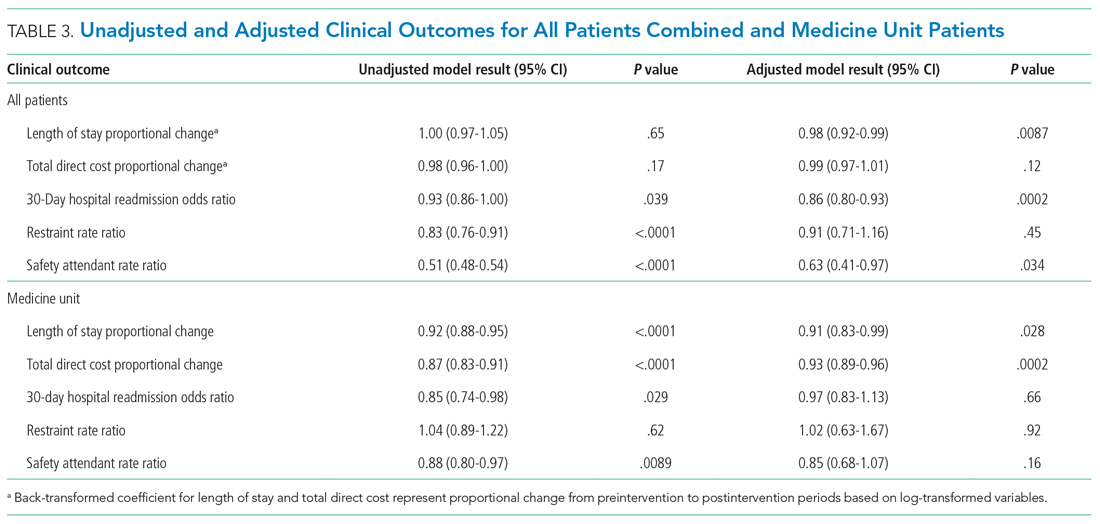
Secondary Outcomes
The odds of 30-day readmission decreased by 14% (P = .0002) in the adjusted models for all units combined (Table 3). There was no statistically significant reduction in adjusted total direct hospitalization cost or rate of restraint use. The safety attendant results showed strong effect modification across sites; the site-specific estimates are provided in Appendix Table 3. However, the estimated values all showed reductions, and a number were large and statistically significant.
Medicine Unit Outcomes
On the medicine unit alone, we observed a statistically significant reduction in LOS of 9% after implementation of the delirium care pathway (P = .028) in the adjusted model (Table 3). There was an associated 7% proportional decrease in total direct cost (P = .0002). Reductions in 30-day readmission and safety attendant use did not remain statistically significant in the adjusted models.
DISCUSSION
Implementation of a hospital-wide multicomponent delirium care pathway was associated with reduced hospital LOS and 30-day hospital readmission in a study of 22,708 hospitalized adults at a tertiary care, university hospital in Northern California, encompassing both medical and surgical acute care patients. When evaluating general medicine patients alone, pathway implementation was associated with reductions in LOS and total direct cost. The cost savings of 7% among medical patients translates to median savings of $1,237 per hospitalization. This study—one of the largest to date examining implementation of a hospital-wide delirium care pathway—supports use of a multicomponent delirium care pathway for older adults hospitalized for a range of conditions.
Multicomponent pathways for delirium prevention and management are increasingly being used in hospital settings. The United Kingdom National Institute for Health and Care Excellence guidelines recommend delirium assessment and intervention by a multidisciplinary team within 24 hours of hospital admission for those at risk.25 These guidelines are based on evidence accumulated in clinical studies over the past 30 years suggesting that multicomponent interventions reduce incident delirium by 30% to 40% among medical and surgical patients.12,13,25,28
Although multicomponent delirium care pathways are associated with improved patient outcomes, the specific clinical benefits might vary across patient populations. Here, we found larger reductions in LOS and total direct cost among medicine patients. Medical patients might respond more robustly to nonpharmacologic multicomponent delirium interventions because of differing delirium etiologies (eg, constipation and sleep deprivation in a medical patient vs seizures or encephalitis in a neurosciences patient). Another explanation for the difference observed in total direct cost might be the inclusion of surgical units in the total study population. For example, not all hospital days are equivalent in cost for patients on a surgical unit.29 For patients requiring surgical care, most of the hospitalization cost might be incurred during the initial days of hospitalization, when there are perioperative costs; therefore, reduced LOS might have a lower economic impact.29 Multicomponent, nonpharmacologic delirium interventions encourage discontinuing restraints. As a result, one might expect a need for more frequent safety attendant use and an associated cost increase. However, we found that the estimated unit-specific values for safety attendant use showed reductions, which were large and highly statistically significant. For all units combined and the medicine unit alone, we found that the rate of restraint use decreased, although the change was not statistically significant. It is possible that some of the interventions taught to nurses and physicians as part of care pathway implementation, such as the use of family support for at-risk and delirious patients, led to a reduction in both safety attendants and restraints.
Our study had several strengths. This is one of the largest hospital-based delirium interventions studied, both in terms of its scope across seven diverse medical and surgical hospital units and the number of hospitalized patients studied. This intervention did not require additional staff or creating a specialized ward. Adherence to the pathway, as measured by risk assessment and delirium screening, was high (>90%) 3 months after implementation. This allowed for robust outcome ascertainment. The patient population’s characteristics and rates of delirium were stable over time. Because different hospital units incorporated the multicomponent delirium care pathway at different times, limiting enrollment to patients admitted and discharged from the same unit isolated the analysis to patients exposed to the pathway on each unit. This design also limited potential influence of other hospital quality improvement projects that might have occurred at the same time.
The primary limitation of this study is that screening for delirium was introduced as part of the multicomponent intervention. This decision was made to maximize buy-in from bedside nurses performing delirium screening because this addition to their workflow was explicitly linked to delirium prevention and management measures. Delirium could not be ascertained preintervention from the EMR because it is a clinical diagnosis and is coded inadequately.30 We could only measure the change in delirium metrics after implementation of the delirium care pathway. Because baseline delirium rates before the intervention were not measured systematically, conclusions about the intervention’s association with delirium metrics are limited. All other outcomes were measured before and after the intervention.
Although the comprehensive delirium screening program and high rate of adherence are a methodologic strength of this study, a second limitation is the use of the NuDESC. Our previous research demonstrated that the NuDESC has low sensitivity but high specificity and positive predictive value,26 which might underestimate delirium rates in this study. However, any underestimation should be stable over time and temporal trends should remain meaningful. This could allow more widespread study of delirium among hospitalized individuals. Because this care pathway was hospital-wide, it was important to ensure both consistency of screening and longevity of the initiative, and it was necessary to select a delirium assessment tool that was efficient and validated for nursing implementation. For these reasons, the NuDESC was an appropriate choice.
It is possible that our results could be influenced by unmeasured confounders. For example, although we incorporated Elixhauser medical comorbidities and illness severity into our model, we were unable to adjust for baseline functional status or frailty. Baseline functional status and frailty were not reliably recorded in the EMR, although these are potential confounders when investigating clinical outcomes including hospital readmission.
CONCLUSION
Implementation of a systematic, hospital-wide multicomponent delirium care pathway is associated with reductions in hospital LOS and 30-day readmission. In general medicine units, the reduction in LOS and associated cost savings were robust. These results demonstrate the feasibility and effectiveness of implementing an interprofessional, multidisciplinary multicomponent delirium care pathway through medical center funding to benefit patients and the hospital system.
Acknowledgments
The authors thank the many hospital staff members, especially the nurses, pharmacists, therapists, and patient care assistants, who helped implement the multicomponent delirium care pathway. All persons who have contributed significantly to this work are listed as authors of this work.
1. Bidwell J. Interventions for preventing delirium in hospitalized non-ICU patients: A Cochrane review summary. Int J Nurs Stud. 2017;70:142-143. https://doi.org/ 10.1016/j.ijnurstu.2016.11.010
2. Maldonado JR. Delirium in the acute care setting: characteristics, diagnosis and treatment. Crit Care Clin. 2008;24(4):657-722, vii. https://doi.org/10.1016/j.ccc.2008.05.008
3. Field RR, Wall MH. Delirium: past, present, and future. Semin Cardiothorac Vasc Anesth. 2013;17(3):170-179. https://doi.org/10.1177/1089253213476957
4. Oh ST, Park JY. Postoperative delirium. Korean J Anesthesiol. 2019;72(1):4-12. https://doi.org/10.4097/kja.d.18.00073.1
5. Francis J, Martin D, Kapoor WN. A prospective study of delirium in hospitalized elderly. JAMA. 1990;263(8):1097-1101.
6. Salluh JI, Soares M, Teles JM, et al. Delirium epidemiology in critical care (DECCA): an international study. Crit Care. 2010;14(6):R210. https://doi.org/10.1186/cc9333
7. Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753-1762. https://doi.org/
8. McCusker J, Cole MG, Dendukuri N, Belzile E. Does delirium increase hospital stay? J Am Geriatr Soc. 2003;51(11):1539-1546. https://doi.org/10.1001/jama.291.14.1753
9. Inouye SK, Rushing JT, Foreman MD, Palmer RM, Pompei P. Does delirium contribute to poor hospital outcomes? A three-site epidemiologic study. J Gen Intern Med. 1998;13(4):234-242. https://doi.org/10.1046/j.1525-1497.1998.00073.x
10. Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in-patients: a systematic literature review. Age Ageing. 2006;35(4):350-364. https://doi.org/10.1093/ageing/afl005
11. LaHue SC, Douglas VC, Kuo T, et al. Association between inpatient delirium and hospital readmission in patients >/= 65 years of age: a retrospective cohort study. J Hosp Med. 2019;14(4):201-206. https://doi.org/10.12788/jhm.3130
12. Hshieh TT, Yue J, Oh E, et al. Effectiveness of multicomponent nonpharmacological delirium interventions: a meta-analysis. JAMA Intern Med. 2015;175(4):512-520. https://doi.org/10.1001/jamainternmed.2014.7779
13. Inouye SK, Bogardus ST, Jr., Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669-676. https://doi.org/10.1056/NEJM199903043400901
14. Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49(5):516-522. https://doi.org/
15. Alhaidari AA, Allen-Narker RA. An evolving approach to delirium: A mixed-methods process evaluation of a hospital-wide delirium program in New Zealand. Australas J Ageing. 2017. https://doi.org/10.1046/j.1532-5415.2001.49108.x
16. Holroyd-Leduc JM, Khandwala F, Sink KM. How can delirium best be prevented and managed in older patients in hospital? CMAJ. 2010;182(5):465-470. https://doi.org/10.1503/cmaj.080519
17. Siddiqi N, Stockdale R, Britton AM, Holmes J. Interventions for preventing delirium in hospitalised patients. Cochrane Database Syst Rev. 2007(2):CD005563. https://doi.org/ 10.1002/14651858.CD005563.pub2
18. Siddiqi N, Harrison JK, Clegg A, et al. Interventions for preventing delirium in hospitalised non-ICU patients. Cochrane Database Syst Rev. 2016;3:CD005563. https://doi.org/10.1002/14651858.CD005563.pub3
19. Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922. https://doi.org/10.1016/S0140-6736(13)60688-1
20. Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275(11):852-857.
21. LaHue SC, Liu VX. Loud and clear: sensory impairment, delirium, and functional recovery in critical illness. Am J Respir Crit Care Med. 2016;194(3):252-253. https://doi.org/10.1164/rccm.201602-0372ED
22. Ritter SRF, Cardoso AF, Lins MMP, Zoccoli TLV, Freitas MPD, Camargos EF. Underdiagnosis of delirium in the elderly in acute care hospital settings: lessons not learned. Psychogeriatrics. 2018;18(4):268-275. https://doi.org/10.1111/psyg.12324
23. Douglas VC, Hessler CS, Dhaliwal G, et al. The AWOL tool: derivation and validation of a delirium prediction rule. J Hosp Med. 2013;8(9):493-499. https://doi.org/10.1002/jhm.2062
24. Tombaugh TN, McDowell I, Kristjansson B, Hubley AM. Mini-Mental State Examination (MMSE) and the modified MMSE (3MS): A psychometric comparison and normative data. Psychol Assessment. 1996;8(1):48-59. https://doi.org/10.1037/1040-3590.8.1.48
25. Young J, Murthy L, Westby M, Akunne A, O’Mahony R, Guideline Development Group. Diagnosis, prevention, and management of delirium: summary of NICE guidance. BMJ. 2010;341:c3704. https://doi.org/10.1136/bmj.c3704
26. Hargrave A, Bastiaens J, Bourgeois JA, et al. Validation of a nurse-based delirium-screening tool for hospitalized patients. Psychosomatics. 2017;58(6):594-603. https://doi.org/10.1016/j.psym.2017.05.005
27. Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA. 2001;286(21):2703-2710. https://doi.org/10.1001/jama.286.21.2703
28. Strijbos MJ, Steunenberg B, van der Mast RC, Inouye SK, Schuurmans MJ. Design and methods of the Hospital Elder Life Program (HELP), a multicomponent targeted intervention to prevent delirium in hospitalized older patients: efficacy and cost-effectiveness in Dutch health care. BMC Geriatr. 2013;13:78. https://doi.org/10.1186/1471-2318-13-78
29. Taheri PA, Butz DA, Greenfield LJ. Length of stay has minimal impact on the cost of hospital admission. J Am Coll Surg. 2000;191(2):123-130. https://doi.org/10.1016/s1072-7515(00)00352-5
30. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5(4):210-220. https://doi.org/10.1038/nrneurol.2009.24
Delirium is an acute disturbance in mental status characterized by fluctuations in cognition and attention that affects more than 2.6 million hospitalized older adults in the United States annually, a rate that is expected to increase as the population ages.1-4 Hospital-acquired delirium is associated with poor outcomes, including prolonged hospital length of stay (LOS), loss of independence, cognitive impairment, and even death.5-10 Individuals who develop delirium do poorly after hospital discharge and are more likely to be readmitted within 30 days.11 Approximately 30% to 40% of hospital-acquired delirium cases are preventable.10,12 However, programs designed to prevent delirium and associated complications, such as increased LOS, have demonstrated variable success.12-14 Many studies are limited by small sample sizes, lack of generalizability to different hospitalized patient populations, poor adherence, or reliance on outside funding.12,13,15-18
Delirium prevention programs face several challenges because delirium could be caused by a variety of risk factors and precipitants.19,20 Some risk factors that occur frequently among hospitalized patients can be mitigated, such as sensory impairment, immobility from physical restraints or urinary catheters, and polypharmacy.20,21 Effective delirium care pathways targeting these risk factors must be multifaceted, interdisciplinary, and interprofessional. Accurate risk assessment is critical to allocate resources to high-risk patients. Delirium affects patients in all medical and surgical disciplines, and often is underdiagnosed.19,22 Comprehensive screening is necessary to identify cases early and track outcomes, and educational efforts must reach all providers in the hospital. These challenges require a systematic, pragmatic approach to change.
The purpose of this study was to evaluate the association between a delirium care pathway and clinical outcomes for hospitalized patients. We hypothesized that this program would be associated with reduced hospital LOS, with secondary benefits to hospitalization costs, odds of 30-day readmission, and delirium rates.
METHODS
Study Design
In this retrospective cohort study, we compared clinical outcomes the year before and after implementation of a delirium care pathway across seven hospital units. The study period spanned October 1, 2015, through February 28, 2019. The study was approved by the University of California, San Francisco Institutional Review Board (#13-12500).
Multicomponent Delirium Care Pathway
The delirium care pathway was developed collaboratively among geriatrics, hospital medicine, neurology, anesthesiology, surgery, and psychiatry services, with an interprofessional team of physicians, nurses, pharmacists, and physical and occupational therapists. This pathway was implemented in units consecutively, approximately every 4 months in the following order: neurosciences, medicine, cardiology, general surgery, specialty surgery, hematology-oncology, and transplant. The same implementation education protocols were performed in each unit. The pathway consisted of several components targeting delirium prevention and management (Appendix Figure 1 and Appendix Figure 2). Systematic screening for delirium was introduced as part of the multicomponent intervention. Nursing staff assessed each patient’s risk of developing delirium at admission using the AWOL score, a validated delirium prediction tool.23 AWOL consists of: patient Age, spelling “World” backwards correctly, Orientation, and assessment of iLlness severity by the nurse. For patients who spoke a language other than English, spelling of “world” backwards was translated to his or her primary language, or if this was not possible, the task was modified to serial 7s (subtracting 7 from 100 in a serial fashion). This modification has been validated for use in other languages.24 Patients at high risk for delirium based on an AWOL score ≥2 received a multidisciplinary intervention with four components: (1) notifying the primary team by pager and electronic medical record (EMR), (2) a nurse-led, evidence-based, nonpharmacologic multicomponent intervention,25 (3) placement of a delirium order set by the physician, and (4) review of medications by the unit pharmacist who adjusted administration timing to occur during waking hours and placed a note in the EMR notifying the primary team of potentially deliriogenic medications. The delirium order set reinforced the nonpharmacologic multicomponent intervention through a nursing order, placed an automatic consult to occupational therapy, and included options to order physical therapy, order speech/language therapy, obtain vital signs three times daily with minimal night interruptions, remove an indwelling bladder catheter, and prescribe melatonin as a sleep aid.
The bedside nurse screened all patients for active delirium every 12-hour shift using the Nursing Delirium Screening Scale (NuDESC) and entered the results into the EMR.23,26 Capturing NuDESC results in the EMR allowed communication across medical providers as well as monitoring of screening adherence. Each nurse received two in-person trainings in staff meetings and one-to-one instruction during the first week of implementation. All nurses were required to complete a 15-minute training module and had the option of completing an additional 1-hour continuing medical education module. If a patient was transferred to the intensive care unit (ICU), delirium was identified through use of the ICU-specific Confusion Assessment Method (CAM-ICU) assessments, which the bedside nurse performed each shift throughout the intervention period.27 Nurses were instructed to call the primary team physician after every positive screen. Before each unit’s implementation start date, physicians with patients on that unit received education through a combination of grand rounds, resident lectures and seminars, and a pocket card on delirium evaluation and management.
Participants and Eligibility Criteria
We included all patients aged ≥50 years hospitalized for >1 day on each hospital unit (Figure). We included adults aged ≥50 years to maximize the number of participants for this study while also capturing a population at risk for delirium. Because the delirium care pathway was unit-based and the pathway was rolled out sequentially across units, only patients who were admitted to and discharged from the same unit were included to better isolate the effect of the pathway. Patients who were transferred to the ICU were only included if they were discharged from the original unit of admission. Only the first hospitalization was included for patients with multiple hospitalizations during the study period.

Patient Characteristics
Patient demographics and clinical data were collected after discharge through Clarity and Vizient electronic databases (Table 1 and Table 2). All Elixhauser comorbidities were included except for the following International Classification of Disease, Tenth Revision, Clinical Modification (ICD-10) codes that overlapped with a delirium diagnosis: G31.2, G93.89, G93.9, G94, R41.0, and R41.82 (Appendix Table 1). Severity of illness was obtained from Vizient, which calculates illness severity based on clinical and claims data (Appendix Table 1).

Delirium Metrics
Delirium screening was introduced as part of the multicomponent intervention, and therefore delirium rates before the intervention could not be determined. Trends in delirium prevalence and incidence after the intervention are reported. Prevalent delirium was defined as a single score of ≥2 on the nurse-administered NuDESC or a positive CAM-ICU at any point during the hospital stay. Incident delirium was identified if the first NuDESC score was negative and any subsequent NuDESC or CAM-ICU score was positive.

Outcomes
The primary study outcome was hospital LOS across all participants. Secondary outcomes included total direct cost and odds of 30-day hospital readmission. Readmissions tracked as part of hospital quality reporting were obtained from Vizient and were not captured if they occurred at another hospital. We also examined rates of safety attendant and restraint use during the study period, defined as the number of safety attendant days or restraint days per 1,000 patient days.
Because previous studies have demonstrated the effectiveness of multicomponent delirium interventions among elderly general medical patients,12 we also investigated these same outcomes in the medicine unit alone.
Statistical Analysis
The date of intervention implementation was determined for each hospital unit, which was defined as time(0) [t(0)]. The 12-month postintervention period was divided into four 3-month epochs to assess for trends. Data were aggregated across the seven units using t(0) as the start date, agnostic to the calendar month. Demographic and clinical characteristics were collected for the 12-months before t(0) and the four 3-month epochs after t(0). Univariate analysis of outcome variables comparing trends across the same epochs were conducted in the same manner, except for the rate of delirium, which was measured after t(0) and therefore could not be compared with the preintervention period.
Multivariable models were adjusted for age, sex, race/ethnicity, admission category, Elixhauser comorbidities, severity of illness quartile, and number days spent in the ICU. Admission category referred to whether the admission was emergent, urgent, or elective/unknown. Because it took 3 months after t(0) for each unit to reach a delirium screening compliance rate of 90%, the intervention was only considered fully implemented after this period. A ramp-up variable was set to 0 for admissions occurring prior to the intervention to t(0), 1/3 for admissions occurring 1 month post intervention, 2/3 for 2 months post intervention, and 1 for admissions occurring 3 to 12 months post intervention. In this way, the coefficient for the ramp-up variable estimated the postintervention versus preintervention effect. Numerical outcomes (LOS, cost) were log transformed to reduce skewness and analyzed using linear models. Coefficients were back-transformed to provide interpretations as proportional change in the median outcomes.
For LOS and readmission, we assessed secular trends by including admission date and admission date squared, in case the trend was nonlinear, as possible predictors; admission date was the specific date—not time from t(0)—to account for secular trends and allow contemporaneous controls in the analysis. To be conservative, we retained secular terms (first considering the quadratic and then the linear) if P <.10. The categorical outcome (30-day readmission) was analyzed using a logistic model. Count variables (delirium, safety attendants, restraints) were analyzed using Poisson regression models with a log link, and coefficients were back-transformed to provide rate ratio interpretations. Because delirium was not measured before t(0), and because the intervention was considered to take 3 months to become fully effective, baseline delirium rates were defined as those in the first 3 months adjusted by the ramp-up variable. For each outcome we included hospital unit, a ramp-up variable (measuring the pre- vs postintervention effect), and their interaction. If there was no statistically significant interaction, we presented the outcome for all units combined. If the interaction was statistically significant, we looked for consistency across units and reported results for all units combined when consistent, along with site-specific results. If the results were not consistent across the units, we provided site-specific results only. All statistical analyses were performed using SAS software, version 9.4 (SAS Institute Inc).
RESULTS
Participant Demographics and Clinical Characteristics
A total of 22,708 individuals were included in this study, with 11,018 in the preintervention period (Table 1 and Table 2). Most patients were cared for on the general surgery unit (n = 5,899), followed by the medicine unit (n = 4,923). The smallest number of patients were cared for on the hematology-oncology unit (n = 1,709). Across the five epochs, patients were of similar age and sex, and spent a similar number of days in the ICU. The population was diverse with regard to race and ethnicity; there were minor differences in admission category. There were also minor differences in severity of illness and some comorbidities between timepoints (Appendix Table 1).
Delirium Metrics
Delirium prevalence was 13.0% during the first epoch post intervention, followed by 12.0%, 11.7%, and 13.0% in the subsequent epochs (P = .91). Incident delirium occurred in 6.1% of patients during the first epoch post intervention, followed by 5.3%, 5.3%, and 5.8% in the subsequent epochs (P = .63).
Primary Outcome
Epoch-level data for LOS before and after the intervention is shown in Appendix Table 2. The mean unadjusted LOS for all units combined did not decrease after the intervention, but in the adjusted model, the mean LOS decreased by 2% after the intervention (P = .0087; Table 3).

Secondary Outcomes
The odds of 30-day readmission decreased by 14% (P = .0002) in the adjusted models for all units combined (Table 3). There was no statistically significant reduction in adjusted total direct hospitalization cost or rate of restraint use. The safety attendant results showed strong effect modification across sites; the site-specific estimates are provided in Appendix Table 3. However, the estimated values all showed reductions, and a number were large and statistically significant.
Medicine Unit Outcomes
On the medicine unit alone, we observed a statistically significant reduction in LOS of 9% after implementation of the delirium care pathway (P = .028) in the adjusted model (Table 3). There was an associated 7% proportional decrease in total direct cost (P = .0002). Reductions in 30-day readmission and safety attendant use did not remain statistically significant in the adjusted models.
DISCUSSION
Implementation of a hospital-wide multicomponent delirium care pathway was associated with reduced hospital LOS and 30-day hospital readmission in a study of 22,708 hospitalized adults at a tertiary care, university hospital in Northern California, encompassing both medical and surgical acute care patients. When evaluating general medicine patients alone, pathway implementation was associated with reductions in LOS and total direct cost. The cost savings of 7% among medical patients translates to median savings of $1,237 per hospitalization. This study—one of the largest to date examining implementation of a hospital-wide delirium care pathway—supports use of a multicomponent delirium care pathway for older adults hospitalized for a range of conditions.
Multicomponent pathways for delirium prevention and management are increasingly being used in hospital settings. The United Kingdom National Institute for Health and Care Excellence guidelines recommend delirium assessment and intervention by a multidisciplinary team within 24 hours of hospital admission for those at risk.25 These guidelines are based on evidence accumulated in clinical studies over the past 30 years suggesting that multicomponent interventions reduce incident delirium by 30% to 40% among medical and surgical patients.12,13,25,28
Although multicomponent delirium care pathways are associated with improved patient outcomes, the specific clinical benefits might vary across patient populations. Here, we found larger reductions in LOS and total direct cost among medicine patients. Medical patients might respond more robustly to nonpharmacologic multicomponent delirium interventions because of differing delirium etiologies (eg, constipation and sleep deprivation in a medical patient vs seizures or encephalitis in a neurosciences patient). Another explanation for the difference observed in total direct cost might be the inclusion of surgical units in the total study population. For example, not all hospital days are equivalent in cost for patients on a surgical unit.29 For patients requiring surgical care, most of the hospitalization cost might be incurred during the initial days of hospitalization, when there are perioperative costs; therefore, reduced LOS might have a lower economic impact.29 Multicomponent, nonpharmacologic delirium interventions encourage discontinuing restraints. As a result, one might expect a need for more frequent safety attendant use and an associated cost increase. However, we found that the estimated unit-specific values for safety attendant use showed reductions, which were large and highly statistically significant. For all units combined and the medicine unit alone, we found that the rate of restraint use decreased, although the change was not statistically significant. It is possible that some of the interventions taught to nurses and physicians as part of care pathway implementation, such as the use of family support for at-risk and delirious patients, led to a reduction in both safety attendants and restraints.
Our study had several strengths. This is one of the largest hospital-based delirium interventions studied, both in terms of its scope across seven diverse medical and surgical hospital units and the number of hospitalized patients studied. This intervention did not require additional staff or creating a specialized ward. Adherence to the pathway, as measured by risk assessment and delirium screening, was high (>90%) 3 months after implementation. This allowed for robust outcome ascertainment. The patient population’s characteristics and rates of delirium were stable over time. Because different hospital units incorporated the multicomponent delirium care pathway at different times, limiting enrollment to patients admitted and discharged from the same unit isolated the analysis to patients exposed to the pathway on each unit. This design also limited potential influence of other hospital quality improvement projects that might have occurred at the same time.
The primary limitation of this study is that screening for delirium was introduced as part of the multicomponent intervention. This decision was made to maximize buy-in from bedside nurses performing delirium screening because this addition to their workflow was explicitly linked to delirium prevention and management measures. Delirium could not be ascertained preintervention from the EMR because it is a clinical diagnosis and is coded inadequately.30 We could only measure the change in delirium metrics after implementation of the delirium care pathway. Because baseline delirium rates before the intervention were not measured systematically, conclusions about the intervention’s association with delirium metrics are limited. All other outcomes were measured before and after the intervention.
Although the comprehensive delirium screening program and high rate of adherence are a methodologic strength of this study, a second limitation is the use of the NuDESC. Our previous research demonstrated that the NuDESC has low sensitivity but high specificity and positive predictive value,26 which might underestimate delirium rates in this study. However, any underestimation should be stable over time and temporal trends should remain meaningful. This could allow more widespread study of delirium among hospitalized individuals. Because this care pathway was hospital-wide, it was important to ensure both consistency of screening and longevity of the initiative, and it was necessary to select a delirium assessment tool that was efficient and validated for nursing implementation. For these reasons, the NuDESC was an appropriate choice.
It is possible that our results could be influenced by unmeasured confounders. For example, although we incorporated Elixhauser medical comorbidities and illness severity into our model, we were unable to adjust for baseline functional status or frailty. Baseline functional status and frailty were not reliably recorded in the EMR, although these are potential confounders when investigating clinical outcomes including hospital readmission.
CONCLUSION
Implementation of a systematic, hospital-wide multicomponent delirium care pathway is associated with reductions in hospital LOS and 30-day readmission. In general medicine units, the reduction in LOS and associated cost savings were robust. These results demonstrate the feasibility and effectiveness of implementing an interprofessional, multidisciplinary multicomponent delirium care pathway through medical center funding to benefit patients and the hospital system.
Acknowledgments
The authors thank the many hospital staff members, especially the nurses, pharmacists, therapists, and patient care assistants, who helped implement the multicomponent delirium care pathway. All persons who have contributed significantly to this work are listed as authors of this work.
Delirium is an acute disturbance in mental status characterized by fluctuations in cognition and attention that affects more than 2.6 million hospitalized older adults in the United States annually, a rate that is expected to increase as the population ages.1-4 Hospital-acquired delirium is associated with poor outcomes, including prolonged hospital length of stay (LOS), loss of independence, cognitive impairment, and even death.5-10 Individuals who develop delirium do poorly after hospital discharge and are more likely to be readmitted within 30 days.11 Approximately 30% to 40% of hospital-acquired delirium cases are preventable.10,12 However, programs designed to prevent delirium and associated complications, such as increased LOS, have demonstrated variable success.12-14 Many studies are limited by small sample sizes, lack of generalizability to different hospitalized patient populations, poor adherence, or reliance on outside funding.12,13,15-18
Delirium prevention programs face several challenges because delirium could be caused by a variety of risk factors and precipitants.19,20 Some risk factors that occur frequently among hospitalized patients can be mitigated, such as sensory impairment, immobility from physical restraints or urinary catheters, and polypharmacy.20,21 Effective delirium care pathways targeting these risk factors must be multifaceted, interdisciplinary, and interprofessional. Accurate risk assessment is critical to allocate resources to high-risk patients. Delirium affects patients in all medical and surgical disciplines, and often is underdiagnosed.19,22 Comprehensive screening is necessary to identify cases early and track outcomes, and educational efforts must reach all providers in the hospital. These challenges require a systematic, pragmatic approach to change.
The purpose of this study was to evaluate the association between a delirium care pathway and clinical outcomes for hospitalized patients. We hypothesized that this program would be associated with reduced hospital LOS, with secondary benefits to hospitalization costs, odds of 30-day readmission, and delirium rates.
METHODS
Study Design
In this retrospective cohort study, we compared clinical outcomes the year before and after implementation of a delirium care pathway across seven hospital units. The study period spanned October 1, 2015, through February 28, 2019. The study was approved by the University of California, San Francisco Institutional Review Board (#13-12500).
Multicomponent Delirium Care Pathway
The delirium care pathway was developed collaboratively among geriatrics, hospital medicine, neurology, anesthesiology, surgery, and psychiatry services, with an interprofessional team of physicians, nurses, pharmacists, and physical and occupational therapists. This pathway was implemented in units consecutively, approximately every 4 months in the following order: neurosciences, medicine, cardiology, general surgery, specialty surgery, hematology-oncology, and transplant. The same implementation education protocols were performed in each unit. The pathway consisted of several components targeting delirium prevention and management (Appendix Figure 1 and Appendix Figure 2). Systematic screening for delirium was introduced as part of the multicomponent intervention. Nursing staff assessed each patient’s risk of developing delirium at admission using the AWOL score, a validated delirium prediction tool.23 AWOL consists of: patient Age, spelling “World” backwards correctly, Orientation, and assessment of iLlness severity by the nurse. For patients who spoke a language other than English, spelling of “world” backwards was translated to his or her primary language, or if this was not possible, the task was modified to serial 7s (subtracting 7 from 100 in a serial fashion). This modification has been validated for use in other languages.24 Patients at high risk for delirium based on an AWOL score ≥2 received a multidisciplinary intervention with four components: (1) notifying the primary team by pager and electronic medical record (EMR), (2) a nurse-led, evidence-based, nonpharmacologic multicomponent intervention,25 (3) placement of a delirium order set by the physician, and (4) review of medications by the unit pharmacist who adjusted administration timing to occur during waking hours and placed a note in the EMR notifying the primary team of potentially deliriogenic medications. The delirium order set reinforced the nonpharmacologic multicomponent intervention through a nursing order, placed an automatic consult to occupational therapy, and included options to order physical therapy, order speech/language therapy, obtain vital signs three times daily with minimal night interruptions, remove an indwelling bladder catheter, and prescribe melatonin as a sleep aid.
The bedside nurse screened all patients for active delirium every 12-hour shift using the Nursing Delirium Screening Scale (NuDESC) and entered the results into the EMR.23,26 Capturing NuDESC results in the EMR allowed communication across medical providers as well as monitoring of screening adherence. Each nurse received two in-person trainings in staff meetings and one-to-one instruction during the first week of implementation. All nurses were required to complete a 15-minute training module and had the option of completing an additional 1-hour continuing medical education module. If a patient was transferred to the intensive care unit (ICU), delirium was identified through use of the ICU-specific Confusion Assessment Method (CAM-ICU) assessments, which the bedside nurse performed each shift throughout the intervention period.27 Nurses were instructed to call the primary team physician after every positive screen. Before each unit’s implementation start date, physicians with patients on that unit received education through a combination of grand rounds, resident lectures and seminars, and a pocket card on delirium evaluation and management.
Participants and Eligibility Criteria
We included all patients aged ≥50 years hospitalized for >1 day on each hospital unit (Figure). We included adults aged ≥50 years to maximize the number of participants for this study while also capturing a population at risk for delirium. Because the delirium care pathway was unit-based and the pathway was rolled out sequentially across units, only patients who were admitted to and discharged from the same unit were included to better isolate the effect of the pathway. Patients who were transferred to the ICU were only included if they were discharged from the original unit of admission. Only the first hospitalization was included for patients with multiple hospitalizations during the study period.

Patient Characteristics
Patient demographics and clinical data were collected after discharge through Clarity and Vizient electronic databases (Table 1 and Table 2). All Elixhauser comorbidities were included except for the following International Classification of Disease, Tenth Revision, Clinical Modification (ICD-10) codes that overlapped with a delirium diagnosis: G31.2, G93.89, G93.9, G94, R41.0, and R41.82 (Appendix Table 1). Severity of illness was obtained from Vizient, which calculates illness severity based on clinical and claims data (Appendix Table 1).

Delirium Metrics
Delirium screening was introduced as part of the multicomponent intervention, and therefore delirium rates before the intervention could not be determined. Trends in delirium prevalence and incidence after the intervention are reported. Prevalent delirium was defined as a single score of ≥2 on the nurse-administered NuDESC or a positive CAM-ICU at any point during the hospital stay. Incident delirium was identified if the first NuDESC score was negative and any subsequent NuDESC or CAM-ICU score was positive.

Outcomes
The primary study outcome was hospital LOS across all participants. Secondary outcomes included total direct cost and odds of 30-day hospital readmission. Readmissions tracked as part of hospital quality reporting were obtained from Vizient and were not captured if they occurred at another hospital. We also examined rates of safety attendant and restraint use during the study period, defined as the number of safety attendant days or restraint days per 1,000 patient days.
Because previous studies have demonstrated the effectiveness of multicomponent delirium interventions among elderly general medical patients,12 we also investigated these same outcomes in the medicine unit alone.
Statistical Analysis
The date of intervention implementation was determined for each hospital unit, which was defined as time(0) [t(0)]. The 12-month postintervention period was divided into four 3-month epochs to assess for trends. Data were aggregated across the seven units using t(0) as the start date, agnostic to the calendar month. Demographic and clinical characteristics were collected for the 12-months before t(0) and the four 3-month epochs after t(0). Univariate analysis of outcome variables comparing trends across the same epochs were conducted in the same manner, except for the rate of delirium, which was measured after t(0) and therefore could not be compared with the preintervention period.
Multivariable models were adjusted for age, sex, race/ethnicity, admission category, Elixhauser comorbidities, severity of illness quartile, and number days spent in the ICU. Admission category referred to whether the admission was emergent, urgent, or elective/unknown. Because it took 3 months after t(0) for each unit to reach a delirium screening compliance rate of 90%, the intervention was only considered fully implemented after this period. A ramp-up variable was set to 0 for admissions occurring prior to the intervention to t(0), 1/3 for admissions occurring 1 month post intervention, 2/3 for 2 months post intervention, and 1 for admissions occurring 3 to 12 months post intervention. In this way, the coefficient for the ramp-up variable estimated the postintervention versus preintervention effect. Numerical outcomes (LOS, cost) were log transformed to reduce skewness and analyzed using linear models. Coefficients were back-transformed to provide interpretations as proportional change in the median outcomes.
For LOS and readmission, we assessed secular trends by including admission date and admission date squared, in case the trend was nonlinear, as possible predictors; admission date was the specific date—not time from t(0)—to account for secular trends and allow contemporaneous controls in the analysis. To be conservative, we retained secular terms (first considering the quadratic and then the linear) if P <.10. The categorical outcome (30-day readmission) was analyzed using a logistic model. Count variables (delirium, safety attendants, restraints) were analyzed using Poisson regression models with a log link, and coefficients were back-transformed to provide rate ratio interpretations. Because delirium was not measured before t(0), and because the intervention was considered to take 3 months to become fully effective, baseline delirium rates were defined as those in the first 3 months adjusted by the ramp-up variable. For each outcome we included hospital unit, a ramp-up variable (measuring the pre- vs postintervention effect), and their interaction. If there was no statistically significant interaction, we presented the outcome for all units combined. If the interaction was statistically significant, we looked for consistency across units and reported results for all units combined when consistent, along with site-specific results. If the results were not consistent across the units, we provided site-specific results only. All statistical analyses were performed using SAS software, version 9.4 (SAS Institute Inc).
RESULTS
Participant Demographics and Clinical Characteristics
A total of 22,708 individuals were included in this study, with 11,018 in the preintervention period (Table 1 and Table 2). Most patients were cared for on the general surgery unit (n = 5,899), followed by the medicine unit (n = 4,923). The smallest number of patients were cared for on the hematology-oncology unit (n = 1,709). Across the five epochs, patients were of similar age and sex, and spent a similar number of days in the ICU. The population was diverse with regard to race and ethnicity; there were minor differences in admission category. There were also minor differences in severity of illness and some comorbidities between timepoints (Appendix Table 1).
Delirium Metrics
Delirium prevalence was 13.0% during the first epoch post intervention, followed by 12.0%, 11.7%, and 13.0% in the subsequent epochs (P = .91). Incident delirium occurred in 6.1% of patients during the first epoch post intervention, followed by 5.3%, 5.3%, and 5.8% in the subsequent epochs (P = .63).
Primary Outcome
Epoch-level data for LOS before and after the intervention is shown in Appendix Table 2. The mean unadjusted LOS for all units combined did not decrease after the intervention, but in the adjusted model, the mean LOS decreased by 2% after the intervention (P = .0087; Table 3).

Secondary Outcomes
The odds of 30-day readmission decreased by 14% (P = .0002) in the adjusted models for all units combined (Table 3). There was no statistically significant reduction in adjusted total direct hospitalization cost or rate of restraint use. The safety attendant results showed strong effect modification across sites; the site-specific estimates are provided in Appendix Table 3. However, the estimated values all showed reductions, and a number were large and statistically significant.
Medicine Unit Outcomes
On the medicine unit alone, we observed a statistically significant reduction in LOS of 9% after implementation of the delirium care pathway (P = .028) in the adjusted model (Table 3). There was an associated 7% proportional decrease in total direct cost (P = .0002). Reductions in 30-day readmission and safety attendant use did not remain statistically significant in the adjusted models.
DISCUSSION
Implementation of a hospital-wide multicomponent delirium care pathway was associated with reduced hospital LOS and 30-day hospital readmission in a study of 22,708 hospitalized adults at a tertiary care, university hospital in Northern California, encompassing both medical and surgical acute care patients. When evaluating general medicine patients alone, pathway implementation was associated with reductions in LOS and total direct cost. The cost savings of 7% among medical patients translates to median savings of $1,237 per hospitalization. This study—one of the largest to date examining implementation of a hospital-wide delirium care pathway—supports use of a multicomponent delirium care pathway for older adults hospitalized for a range of conditions.
Multicomponent pathways for delirium prevention and management are increasingly being used in hospital settings. The United Kingdom National Institute for Health and Care Excellence guidelines recommend delirium assessment and intervention by a multidisciplinary team within 24 hours of hospital admission for those at risk.25 These guidelines are based on evidence accumulated in clinical studies over the past 30 years suggesting that multicomponent interventions reduce incident delirium by 30% to 40% among medical and surgical patients.12,13,25,28
Although multicomponent delirium care pathways are associated with improved patient outcomes, the specific clinical benefits might vary across patient populations. Here, we found larger reductions in LOS and total direct cost among medicine patients. Medical patients might respond more robustly to nonpharmacologic multicomponent delirium interventions because of differing delirium etiologies (eg, constipation and sleep deprivation in a medical patient vs seizures or encephalitis in a neurosciences patient). Another explanation for the difference observed in total direct cost might be the inclusion of surgical units in the total study population. For example, not all hospital days are equivalent in cost for patients on a surgical unit.29 For patients requiring surgical care, most of the hospitalization cost might be incurred during the initial days of hospitalization, when there are perioperative costs; therefore, reduced LOS might have a lower economic impact.29 Multicomponent, nonpharmacologic delirium interventions encourage discontinuing restraints. As a result, one might expect a need for more frequent safety attendant use and an associated cost increase. However, we found that the estimated unit-specific values for safety attendant use showed reductions, which were large and highly statistically significant. For all units combined and the medicine unit alone, we found that the rate of restraint use decreased, although the change was not statistically significant. It is possible that some of the interventions taught to nurses and physicians as part of care pathway implementation, such as the use of family support for at-risk and delirious patients, led to a reduction in both safety attendants and restraints.
Our study had several strengths. This is one of the largest hospital-based delirium interventions studied, both in terms of its scope across seven diverse medical and surgical hospital units and the number of hospitalized patients studied. This intervention did not require additional staff or creating a specialized ward. Adherence to the pathway, as measured by risk assessment and delirium screening, was high (>90%) 3 months after implementation. This allowed for robust outcome ascertainment. The patient population’s characteristics and rates of delirium were stable over time. Because different hospital units incorporated the multicomponent delirium care pathway at different times, limiting enrollment to patients admitted and discharged from the same unit isolated the analysis to patients exposed to the pathway on each unit. This design also limited potential influence of other hospital quality improvement projects that might have occurred at the same time.
The primary limitation of this study is that screening for delirium was introduced as part of the multicomponent intervention. This decision was made to maximize buy-in from bedside nurses performing delirium screening because this addition to their workflow was explicitly linked to delirium prevention and management measures. Delirium could not be ascertained preintervention from the EMR because it is a clinical diagnosis and is coded inadequately.30 We could only measure the change in delirium metrics after implementation of the delirium care pathway. Because baseline delirium rates before the intervention were not measured systematically, conclusions about the intervention’s association with delirium metrics are limited. All other outcomes were measured before and after the intervention.
Although the comprehensive delirium screening program and high rate of adherence are a methodologic strength of this study, a second limitation is the use of the NuDESC. Our previous research demonstrated that the NuDESC has low sensitivity but high specificity and positive predictive value,26 which might underestimate delirium rates in this study. However, any underestimation should be stable over time and temporal trends should remain meaningful. This could allow more widespread study of delirium among hospitalized individuals. Because this care pathway was hospital-wide, it was important to ensure both consistency of screening and longevity of the initiative, and it was necessary to select a delirium assessment tool that was efficient and validated for nursing implementation. For these reasons, the NuDESC was an appropriate choice.
It is possible that our results could be influenced by unmeasured confounders. For example, although we incorporated Elixhauser medical comorbidities and illness severity into our model, we were unable to adjust for baseline functional status or frailty. Baseline functional status and frailty were not reliably recorded in the EMR, although these are potential confounders when investigating clinical outcomes including hospital readmission.
CONCLUSION
Implementation of a systematic, hospital-wide multicomponent delirium care pathway is associated with reductions in hospital LOS and 30-day readmission. In general medicine units, the reduction in LOS and associated cost savings were robust. These results demonstrate the feasibility and effectiveness of implementing an interprofessional, multidisciplinary multicomponent delirium care pathway through medical center funding to benefit patients and the hospital system.
Acknowledgments
The authors thank the many hospital staff members, especially the nurses, pharmacists, therapists, and patient care assistants, who helped implement the multicomponent delirium care pathway. All persons who have contributed significantly to this work are listed as authors of this work.
1. Bidwell J. Interventions for preventing delirium in hospitalized non-ICU patients: A Cochrane review summary. Int J Nurs Stud. 2017;70:142-143. https://doi.org/ 10.1016/j.ijnurstu.2016.11.010
2. Maldonado JR. Delirium in the acute care setting: characteristics, diagnosis and treatment. Crit Care Clin. 2008;24(4):657-722, vii. https://doi.org/10.1016/j.ccc.2008.05.008
3. Field RR, Wall MH. Delirium: past, present, and future. Semin Cardiothorac Vasc Anesth. 2013;17(3):170-179. https://doi.org/10.1177/1089253213476957
4. Oh ST, Park JY. Postoperative delirium. Korean J Anesthesiol. 2019;72(1):4-12. https://doi.org/10.4097/kja.d.18.00073.1
5. Francis J, Martin D, Kapoor WN. A prospective study of delirium in hospitalized elderly. JAMA. 1990;263(8):1097-1101.
6. Salluh JI, Soares M, Teles JM, et al. Delirium epidemiology in critical care (DECCA): an international study. Crit Care. 2010;14(6):R210. https://doi.org/10.1186/cc9333
7. Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753-1762. https://doi.org/
8. McCusker J, Cole MG, Dendukuri N, Belzile E. Does delirium increase hospital stay? J Am Geriatr Soc. 2003;51(11):1539-1546. https://doi.org/10.1001/jama.291.14.1753
9. Inouye SK, Rushing JT, Foreman MD, Palmer RM, Pompei P. Does delirium contribute to poor hospital outcomes? A three-site epidemiologic study. J Gen Intern Med. 1998;13(4):234-242. https://doi.org/10.1046/j.1525-1497.1998.00073.x
10. Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in-patients: a systematic literature review. Age Ageing. 2006;35(4):350-364. https://doi.org/10.1093/ageing/afl005
11. LaHue SC, Douglas VC, Kuo T, et al. Association between inpatient delirium and hospital readmission in patients >/= 65 years of age: a retrospective cohort study. J Hosp Med. 2019;14(4):201-206. https://doi.org/10.12788/jhm.3130
12. Hshieh TT, Yue J, Oh E, et al. Effectiveness of multicomponent nonpharmacological delirium interventions: a meta-analysis. JAMA Intern Med. 2015;175(4):512-520. https://doi.org/10.1001/jamainternmed.2014.7779
13. Inouye SK, Bogardus ST, Jr., Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669-676. https://doi.org/10.1056/NEJM199903043400901
14. Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49(5):516-522. https://doi.org/
15. Alhaidari AA, Allen-Narker RA. An evolving approach to delirium: A mixed-methods process evaluation of a hospital-wide delirium program in New Zealand. Australas J Ageing. 2017. https://doi.org/10.1046/j.1532-5415.2001.49108.x
16. Holroyd-Leduc JM, Khandwala F, Sink KM. How can delirium best be prevented and managed in older patients in hospital? CMAJ. 2010;182(5):465-470. https://doi.org/10.1503/cmaj.080519
17. Siddiqi N, Stockdale R, Britton AM, Holmes J. Interventions for preventing delirium in hospitalised patients. Cochrane Database Syst Rev. 2007(2):CD005563. https://doi.org/ 10.1002/14651858.CD005563.pub2
18. Siddiqi N, Harrison JK, Clegg A, et al. Interventions for preventing delirium in hospitalised non-ICU patients. Cochrane Database Syst Rev. 2016;3:CD005563. https://doi.org/10.1002/14651858.CD005563.pub3
19. Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922. https://doi.org/10.1016/S0140-6736(13)60688-1
20. Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275(11):852-857.
21. LaHue SC, Liu VX. Loud and clear: sensory impairment, delirium, and functional recovery in critical illness. Am J Respir Crit Care Med. 2016;194(3):252-253. https://doi.org/10.1164/rccm.201602-0372ED
22. Ritter SRF, Cardoso AF, Lins MMP, Zoccoli TLV, Freitas MPD, Camargos EF. Underdiagnosis of delirium in the elderly in acute care hospital settings: lessons not learned. Psychogeriatrics. 2018;18(4):268-275. https://doi.org/10.1111/psyg.12324
23. Douglas VC, Hessler CS, Dhaliwal G, et al. The AWOL tool: derivation and validation of a delirium prediction rule. J Hosp Med. 2013;8(9):493-499. https://doi.org/10.1002/jhm.2062
24. Tombaugh TN, McDowell I, Kristjansson B, Hubley AM. Mini-Mental State Examination (MMSE) and the modified MMSE (3MS): A psychometric comparison and normative data. Psychol Assessment. 1996;8(1):48-59. https://doi.org/10.1037/1040-3590.8.1.48
25. Young J, Murthy L, Westby M, Akunne A, O’Mahony R, Guideline Development Group. Diagnosis, prevention, and management of delirium: summary of NICE guidance. BMJ. 2010;341:c3704. https://doi.org/10.1136/bmj.c3704
26. Hargrave A, Bastiaens J, Bourgeois JA, et al. Validation of a nurse-based delirium-screening tool for hospitalized patients. Psychosomatics. 2017;58(6):594-603. https://doi.org/10.1016/j.psym.2017.05.005
27. Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA. 2001;286(21):2703-2710. https://doi.org/10.1001/jama.286.21.2703
28. Strijbos MJ, Steunenberg B, van der Mast RC, Inouye SK, Schuurmans MJ. Design and methods of the Hospital Elder Life Program (HELP), a multicomponent targeted intervention to prevent delirium in hospitalized older patients: efficacy and cost-effectiveness in Dutch health care. BMC Geriatr. 2013;13:78. https://doi.org/10.1186/1471-2318-13-78
29. Taheri PA, Butz DA, Greenfield LJ. Length of stay has minimal impact on the cost of hospital admission. J Am Coll Surg. 2000;191(2):123-130. https://doi.org/10.1016/s1072-7515(00)00352-5
30. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5(4):210-220. https://doi.org/10.1038/nrneurol.2009.24
1. Bidwell J. Interventions for preventing delirium in hospitalized non-ICU patients: A Cochrane review summary. Int J Nurs Stud. 2017;70:142-143. https://doi.org/ 10.1016/j.ijnurstu.2016.11.010
2. Maldonado JR. Delirium in the acute care setting: characteristics, diagnosis and treatment. Crit Care Clin. 2008;24(4):657-722, vii. https://doi.org/10.1016/j.ccc.2008.05.008
3. Field RR, Wall MH. Delirium: past, present, and future. Semin Cardiothorac Vasc Anesth. 2013;17(3):170-179. https://doi.org/10.1177/1089253213476957
4. Oh ST, Park JY. Postoperative delirium. Korean J Anesthesiol. 2019;72(1):4-12. https://doi.org/10.4097/kja.d.18.00073.1
5. Francis J, Martin D, Kapoor WN. A prospective study of delirium in hospitalized elderly. JAMA. 1990;263(8):1097-1101.
6. Salluh JI, Soares M, Teles JM, et al. Delirium epidemiology in critical care (DECCA): an international study. Crit Care. 2010;14(6):R210. https://doi.org/10.1186/cc9333
7. Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753-1762. https://doi.org/
8. McCusker J, Cole MG, Dendukuri N, Belzile E. Does delirium increase hospital stay? J Am Geriatr Soc. 2003;51(11):1539-1546. https://doi.org/10.1001/jama.291.14.1753
9. Inouye SK, Rushing JT, Foreman MD, Palmer RM, Pompei P. Does delirium contribute to poor hospital outcomes? A three-site epidemiologic study. J Gen Intern Med. 1998;13(4):234-242. https://doi.org/10.1046/j.1525-1497.1998.00073.x
10. Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in-patients: a systematic literature review. Age Ageing. 2006;35(4):350-364. https://doi.org/10.1093/ageing/afl005
11. LaHue SC, Douglas VC, Kuo T, et al. Association between inpatient delirium and hospital readmission in patients >/= 65 years of age: a retrospective cohort study. J Hosp Med. 2019;14(4):201-206. https://doi.org/10.12788/jhm.3130
12. Hshieh TT, Yue J, Oh E, et al. Effectiveness of multicomponent nonpharmacological delirium interventions: a meta-analysis. JAMA Intern Med. 2015;175(4):512-520. https://doi.org/10.1001/jamainternmed.2014.7779
13. Inouye SK, Bogardus ST, Jr., Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669-676. https://doi.org/10.1056/NEJM199903043400901
14. Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49(5):516-522. https://doi.org/
15. Alhaidari AA, Allen-Narker RA. An evolving approach to delirium: A mixed-methods process evaluation of a hospital-wide delirium program in New Zealand. Australas J Ageing. 2017. https://doi.org/10.1046/j.1532-5415.2001.49108.x
16. Holroyd-Leduc JM, Khandwala F, Sink KM. How can delirium best be prevented and managed in older patients in hospital? CMAJ. 2010;182(5):465-470. https://doi.org/10.1503/cmaj.080519
17. Siddiqi N, Stockdale R, Britton AM, Holmes J. Interventions for preventing delirium in hospitalised patients. Cochrane Database Syst Rev. 2007(2):CD005563. https://doi.org/ 10.1002/14651858.CD005563.pub2
18. Siddiqi N, Harrison JK, Clegg A, et al. Interventions for preventing delirium in hospitalised non-ICU patients. Cochrane Database Syst Rev. 2016;3:CD005563. https://doi.org/10.1002/14651858.CD005563.pub3
19. Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922. https://doi.org/10.1016/S0140-6736(13)60688-1
20. Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275(11):852-857.
21. LaHue SC, Liu VX. Loud and clear: sensory impairment, delirium, and functional recovery in critical illness. Am J Respir Crit Care Med. 2016;194(3):252-253. https://doi.org/10.1164/rccm.201602-0372ED
22. Ritter SRF, Cardoso AF, Lins MMP, Zoccoli TLV, Freitas MPD, Camargos EF. Underdiagnosis of delirium in the elderly in acute care hospital settings: lessons not learned. Psychogeriatrics. 2018;18(4):268-275. https://doi.org/10.1111/psyg.12324
23. Douglas VC, Hessler CS, Dhaliwal G, et al. The AWOL tool: derivation and validation of a delirium prediction rule. J Hosp Med. 2013;8(9):493-499. https://doi.org/10.1002/jhm.2062
24. Tombaugh TN, McDowell I, Kristjansson B, Hubley AM. Mini-Mental State Examination (MMSE) and the modified MMSE (3MS): A psychometric comparison and normative data. Psychol Assessment. 1996;8(1):48-59. https://doi.org/10.1037/1040-3590.8.1.48
25. Young J, Murthy L, Westby M, Akunne A, O’Mahony R, Guideline Development Group. Diagnosis, prevention, and management of delirium: summary of NICE guidance. BMJ. 2010;341:c3704. https://doi.org/10.1136/bmj.c3704
26. Hargrave A, Bastiaens J, Bourgeois JA, et al. Validation of a nurse-based delirium-screening tool for hospitalized patients. Psychosomatics. 2017;58(6):594-603. https://doi.org/10.1016/j.psym.2017.05.005
27. Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA. 2001;286(21):2703-2710. https://doi.org/10.1001/jama.286.21.2703
28. Strijbos MJ, Steunenberg B, van der Mast RC, Inouye SK, Schuurmans MJ. Design and methods of the Hospital Elder Life Program (HELP), a multicomponent targeted intervention to prevent delirium in hospitalized older patients: efficacy and cost-effectiveness in Dutch health care. BMC Geriatr. 2013;13:78. https://doi.org/10.1186/1471-2318-13-78
29. Taheri PA, Butz DA, Greenfield LJ. Length of stay has minimal impact on the cost of hospital admission. J Am Coll Surg. 2000;191(2):123-130. https://doi.org/10.1016/s1072-7515(00)00352-5
30. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5(4):210-220. https://doi.org/10.1038/nrneurol.2009.24
© 2021 Society of Hospital Medicine
New Editor in Chief: Ebrahim Barkoudah, MD, MPH
With another long winter officially in the rearview mirror and spring sunshine displaying new signs of life outdoors, I am excited to share some of the changes happening inside the offices of the Journal of Clinical Outcomes Management (JCOM). It is my pleasure to introduce Ebrahim Barkoudah, MD, MPH, as the journal’s new physician Editor in Chief. Dr. Barkoudah’s extensive experience in education and his work to improve patient outcomes will be assets to JCOM.
Specializing in both internal medicine and hospital medicine, Dr. Barkoudah is the Associate Director of the Hospital Medicine Unit and a Medical Director in the Department of Medicine at Brigham and Women’s Hospital in Boston. He is also Assistant Professor of Medicine at Harvard Medical School, where he led the school’s international education efforts.
Dr. Barkoudah serves patients with a range of complex clinical disorders, managing their care and seeking innovative treatment options. His research interest is in health care outcomes as well as clinical trials of therapeutic interventions. Dr. Barkoudah also serves on numerous clinical innovation committees at Brigham Health and national task forces.
Dr. Barkoudah is an active member of several professional societies including the American College of Physicians, Society of Hospital Medicine, American Heart Association, Massachusetts Medical Society, among others. He was the Institutional Administration Fellow of the Safety and Quality Fellowship Program at the Institution for Healthcare Improvement.
On behalf of the JCOM Editorial Review Board, I want to extend a special thank you to outgoing editor Lori Tishler, MD, MPH. Dr. Tishler’s impact on the journal cannot be overstated, and we are indebted to the time and expertise she shared with the journal during her tenure.
—Eric Seger
With another long winter officially in the rearview mirror and spring sunshine displaying new signs of life outdoors, I am excited to share some of the changes happening inside the offices of the Journal of Clinical Outcomes Management (JCOM). It is my pleasure to introduce Ebrahim Barkoudah, MD, MPH, as the journal’s new physician Editor in Chief. Dr. Barkoudah’s extensive experience in education and his work to improve patient outcomes will be assets to JCOM.
Specializing in both internal medicine and hospital medicine, Dr. Barkoudah is the Associate Director of the Hospital Medicine Unit and a Medical Director in the Department of Medicine at Brigham and Women’s Hospital in Boston. He is also Assistant Professor of Medicine at Harvard Medical School, where he led the school’s international education efforts.
Dr. Barkoudah serves patients with a range of complex clinical disorders, managing their care and seeking innovative treatment options. His research interest is in health care outcomes as well as clinical trials of therapeutic interventions. Dr. Barkoudah also serves on numerous clinical innovation committees at Brigham Health and national task forces.
Dr. Barkoudah is an active member of several professional societies including the American College of Physicians, Society of Hospital Medicine, American Heart Association, Massachusetts Medical Society, among others. He was the Institutional Administration Fellow of the Safety and Quality Fellowship Program at the Institution for Healthcare Improvement.
On behalf of the JCOM Editorial Review Board, I want to extend a special thank you to outgoing editor Lori Tishler, MD, MPH. Dr. Tishler’s impact on the journal cannot be overstated, and we are indebted to the time and expertise she shared with the journal during her tenure.
—Eric Seger
With another long winter officially in the rearview mirror and spring sunshine displaying new signs of life outdoors, I am excited to share some of the changes happening inside the offices of the Journal of Clinical Outcomes Management (JCOM). It is my pleasure to introduce Ebrahim Barkoudah, MD, MPH, as the journal’s new physician Editor in Chief. Dr. Barkoudah’s extensive experience in education and his work to improve patient outcomes will be assets to JCOM.
Specializing in both internal medicine and hospital medicine, Dr. Barkoudah is the Associate Director of the Hospital Medicine Unit and a Medical Director in the Department of Medicine at Brigham and Women’s Hospital in Boston. He is also Assistant Professor of Medicine at Harvard Medical School, where he led the school’s international education efforts.
Dr. Barkoudah serves patients with a range of complex clinical disorders, managing their care and seeking innovative treatment options. His research interest is in health care outcomes as well as clinical trials of therapeutic interventions. Dr. Barkoudah also serves on numerous clinical innovation committees at Brigham Health and national task forces.
Dr. Barkoudah is an active member of several professional societies including the American College of Physicians, Society of Hospital Medicine, American Heart Association, Massachusetts Medical Society, among others. He was the Institutional Administration Fellow of the Safety and Quality Fellowship Program at the Institution for Healthcare Improvement.
On behalf of the JCOM Editorial Review Board, I want to extend a special thank you to outgoing editor Lori Tishler, MD, MPH. Dr. Tishler’s impact on the journal cannot be overstated, and we are indebted to the time and expertise she shared with the journal during her tenure.
—Eric Seger