User login
Telemetry monitor watchers reduce bedside nurses’ exposure to alarms by intercepting a high number of nonactionable alarms
Cardiac telemetry, designed to monitor hospitalized patients with active cardiac conditions, is highly utilized outside the intensive care unit (ICU) and generates a large number of automated alarms. Telemetry is also costly and requires substantial time and attention commitments from nursing and technician staff, who place and maintain the recording devices and address monitoring results. 1,2 The staff address and dismiss invalid alarms caused by telemetry artifacts, 2 such as the misreporting of patient movement as ventricular tachycardia/fibrillation (VT/VF) or the mimicking of asystole by a lead disconnection.
One strategy for addressing telemetry alarms is to have dedicated staff observe telemetry monitors and notify nurses with any events or findings. Studies conducted in the 1990s showed that dedicated monitor watchers, compared with automatically generated alarms alone, did not affect most outcomes 3 but can improve accuracy of arrhythmia detection. 4 Since then, given the advances in telemetry detection software, the effect of monitor watchers has not been evaluated. Mindful of the perceived burden of nonactionable telemetry alerts, we wanted to quantify the frequency of automated telemetry alerts in the wards and analyze the proportion of alerts deemed nonactionable by monitor watchers.
METHODS
We conducted this retrospective study at a 545-bed urban academic hospital in the United States. We reviewed the cases of all non-ICU patients with telemetry monitoring ordered. The telemetry order requires providers specify the indication for monitoring and adjust alert parameters for variables such as heart rate (preset to 60 and 100 beats per minute) and baseline rhythm (preset to normal sinus). Once a telemetry order is received, 5 leads are attached to the patient, and electrocardiographic data begin transmitting to a portable wireless telemetry monitor, or telemeter (Philips Intellispace Telemetry System), which in turn transmits to a central monitoring station in the progressive care unit (PCU; cardiac/pulmonary unit). The majority of patients on telemetry are in the PCU. Telemeters are also located in the general medicine, surgical, and neurologic non-ICU units. Data from a maximum of 96 telemeters in the hospital are simultaneously displayed in the central monitoring station.
At all times, two dedicated monitor watchers oversee the central monitoring station. Watchers are certified medical assistants with extra telemetry-specific training. Each receives a salary of $17 per hour (no benefits), or about $800 per 24-hour day for two watchers. Their role is to respond to audiovisual alerts triggered by the monitoring system—they either contact the bedside nurse or intercept the alert if deemed nonactionable. Consistent with the literature, 5 nonactionable alerts and alarms were defined as either “invalid” or “nuisance.” Invalid alerts and alarms misrepresent patient status (eg, patient motion is electronically interpreted as VT/VF), and nuisance alerts and alarms do not require clinical intervention (eg, persistent sinus tachycardia has already been communicated to the nurse or provider). Monitor watchers must intercept the alert within a limited amount of time: 15 seconds for suspected lethal alerts (asystole, VT/VF), 30 seconds for extreme tachycardia/bradycardia, and 60 seconds for lead displacement or low battery.
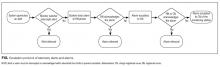
If a watcher does not intercept an alert—either intentionally or because time ran out—the alert generates an alarm, which automatically sends a text message to the patient’s nurse’s wireless phone. The nurse acknowledges the alarm and decides on further action. If the bedside nurse does not acknowledge the alarm within the same time frames as mentioned, the alarm is escalated, first to the unit charge nurse and then to the monitoring station charge nurse (Figure). All alerts are available for provider review at the central monitoring station for the duration of the telemetry order, and select telemetry strips are printed and filed in the patient’s paper chart.
For this study, we analyzed telemetry system data for all monitored non-ICU ward patients from August 1 through September 30, 2014. We focused on the rate and relevance of alerts (system-generated) and alarms (text message to nurse). As cardiac arrhythmias leading to cardiopulmonary arrest can potentially be detected by telemetry, we also reviewed all code team activations, which are recorded in a separate database that details time of code team activation, to evaluate for correlation with telemetry alerts.
RESULTS
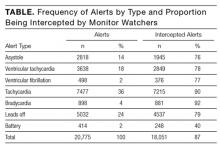
Within the 2-month study period, there were 1917 admissions to, and 1370 transfers to, non-ICU floors, for a total of 3287 unique patient-admissions and 9704 total patient-days. There were 1199 patient admissions with telemetry orders (36.5% of all admissions), 4044 total patient-days of telemetry, and an average of 66.3 patients monitored per day. In addition, the system generated 20,775 alerts, an average of 341 per day, 5.1 per patient-day, 1 every 4 minutes. Overall, 18,051 alerts (87%) were intercepted by monitor watchers, preventing nurse text-alarms. Of all alerts, 91% were from patients on medicine services, including pulmonary and cardiology; 6% were from patients on the neurology floor; and 3% were from patients on the surgery floor.
Forty percent of all alerts were for heart rates deviating outside the ranges set by the provider; of these, the overwhelming majority were intercepted as nuisance alerts (Table). In addition, 26% of all alerts were for maintenance reasons, including issues with batteries or leads. Finally, 34% (6954) were suspected lethal alerts (asystole, VT/VF); of these, 74% (5170) were intercepted by monitor watchers, suggesting they were deemed invalid. None of the suspected lethal alerts triggered a code team activation, indicating there were no telemetry-documented asystole or VT/VF episodes prompting resuscitative efforts. During the study period, there were 7 code team activations. Of the 7 patients, 2 were on telemetry, and their code team activation was for hypoxia detected by pulse oximetry; the other 5 patients, not on telemetry, were found unresponsive or apneic, and 4 of them had confirmed pulseless electrical activity.
DISCUSSION
In small studies, other investigators have directly observed nurses for hours at a time and assessed their response to telemetry-related alarms. 1,2 In the present study, we found a very large number of telemetry-detected alerts over a continuous 2-month period. The large majority (87%) of alerts were manually intercepted by monitor watchers before being communicated to a nurse or provider, indicating these alerts did not affect clinical management and likely were either false positives or nonactionable. It is possible that repeat nonactionable alerts, like continued sinus tachycardia or bradycardia, affect decision making, but this may be outside the role of continuous cardiac telemetry. In addition, it is likely that all the lethal alarms (asystole, VT/VF) forwarded to the nurses were invalid, as none resulted in code team activations.
Addressing these alerts is a major issue, as frequent telemetry alarms can lead to alarm fatigue, a widely acknowledged safety concern. 6 Furthermore, nonactionable alarms are a time sink, diverting nursing attention from other patient care needs. Finally, nonactionable alarms, especially invalid alarms, can lead to adverse patient outcomes. Although we did not specifically evaluate for harm, an earlier case series found a potential for unnecessary interventions and device implantation as a result of reporting artifactual arrhythmias. 7
Our results also highlight the role of monitor watchers in intercepting nonactionable alarms and reducing the alarm burden on nurses. Other investigators have reported on computerized paging systems that directly alert only nurses, 8 or on escalated alarm paging systems that let noncrisis alarms self-resolve. 9 In contrast, our study used a hybrid 2-step telemetry-monitoring system—an escalated paging system designed to be sensitive and less likely than human monitoring to overlook events, followed by dedicated monitor watchers who are first-responders for a large number of alarms and who increase the specificity of alarms by screening for nonactionable alarms, thereby reducing the number of alarms transmitted to nurses. We think that, for most hospitals, monitor watchers are cost-effective, as their hourly wage is lower than that of registered nurses. Furthermore, monitor watchers can screen alerts faster because they are always at the monitoring station. Their presence reduces the amount of time that nurses need to divert from other clinical tasks in order to walk to the monitoring station to evaluate alerts.
Nonetheless, there remains a large number of nonactionable alerts forwarded as alarms to nurses, likely because of monitor watchers’ inability to address the multitude of alerts, and perhaps because of alarm fatigue. Although this study showed the utility of monitor watchers in decreasing telemetry alarms to nurses, other steps can be taken to reduce telemetry alarm fatigue. A systematic review of alarm frequency interventions 5 noted that detection algorithms can be improved to decrease telemetry alert false positives. Another solution, likely easier to implement, is to encourage appropriate alterations in telemetry alarm parameters, which can decrease the alarm proportion. 10 An essential step is to decrease inappropriate telemetry use regarding the indication for and duration of monitoring, as emphasized by the Choosing Wisely campaign championing American Heart Association (AHA) guidelines for appropriate telemetry use. 11 At our institution, 20.2% of telemetry orders were for indications outside AHA guidelines, and that percentage likely is an underestimate, as this was required self-reporting on ordering. 12 Telemetry may not frequently result in changes in management in the non-ICU setting, 13 and may lead to other harms such as worsening delirium, 14 so it needs to be evaluated for harm versus benefit per patient before order.
Cardiac telemetry in the non-ICU setting produces a large number of alerts and alarms. The vast majority are not seen or addressed by nurses or physicians, leading to a negligible impact on patient care decisions. Monitor watchers reduce the nursing burden in dealing with telemetry alerts, but we emphasize the need to take additional measures to reduce telemetry-related alerts and thereby reduce alarm-related harms and alarm fatigue.
Acknowledgments
The authors thank Torberg Tonnessen, who was instrumental in providing the telemetry and clinical data used in this study, as well as the numerous Johns Hopkins Bayview Medical Center nurses, patient care technicians, and monitor watchers who answered questions about telemetry processes and allowed their work to be observed.
Disclosure
Nothing to report.
1. Gazarian PK. Nurses’ response to frequency and types of electrocardiography alarms in a non-critical care setting: a descriptive study. Int J Nurs Stud . 2014;51(2):190-197. PubMed
2. Varpio L, Kuziemsky C, MacDonald C, King WJ. The helpful or hindering effects of in-hospital patient monitor alarms on nurses. Comput Inform Nurs . 2012;30(4):210-217. PubMed
3. Funk M, Parkosewich J, Johnson C, Stukshis I. Effect of dedicated monitor watchers on patients’ outcomes. Am J Crit Care . 1997;6(4):318-323. PubMed
4. Stukshis I, Funk M, Johnson C, Parkosewich J. Accuracy of detection of clinically important dysrhythmias with and without a dedicated monitor watcher. Am J Crit Care . 1997;6(4):312-317. PubMed
5. Paine CW, Goel VV, Ely E, et al. Systematic review of physiologic monitor alarm characteristics and pragmatic interventions to reduce alarm frequency. J Hosp Med . 2016;11(2):136-144. PubMed
6. Joint Commission on Accreditation of Healthcare Organizations. The Joint Commission announces 2014 national patient safety goal. Jt Comm Perspect . 2013;33(7):1, 3-4. PubMed
7. Knight BP, Pelosi F, Michaud GF, Strickberger SA, Morady F. Clinical consequences of electrocardiographic artifact mimicking ventricular tachycardia. N Engl J Med . 1999;341(17):1270-1274. PubMed
8. Zwieg FH, Karfonta TL, Jeske LJ, et al. Arrhythmia detection and response in a monitoring technician and pocket paging system. Prog Cardiovasc Nurs . 1998;13(1):16-22, 33. PubMed
9. Cvach MM, Frank RJ, Doyle P, Stevens ZK. Use of pagers with an alarm escalation system to reduce cardiac monitor alarm signals. J Nurs Care Qual . 2013;29(1):9-18. PubMed
10. Gross B, Dahl D, Nielsen L. Physiologic monitoring alarm load on medical/surgical floors of a community hospital. Biomed Instrum Technol . 2011;Spring(suppl):29-36. PubMed
11. Drew BJ, Califf RM, Funk M, et al; American Heart Association; Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young. Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical-Care Nurses [published correction appears in Circulation . 2005;111(3):378]. Circulation . 2004;110(17):2721-2746. PubMed
12. Chen S, Palchaudhuri S, Johnson A, Trost J, Ponor I, Zakaria S. Does this patient need telemetry? An analysis of telemetry ordering practices at an academic medical center. J Eval Clin Pract . 2017 Jan 27 [Epub ahead of print] . PubMed
13. Estrada CA, Rosman HS, Prasad NK, et al. Role of telemetry monitoring in the non-intensive care unit. Am J Cardiol . 1995;76(12):960-965. PubMed
14. Chen S, Zakaria S. Behind the monitor—the trouble with telemetry: a teachable moment. JAMA Intern Med . 2015;175(6):894. PubMed
Cardiac telemetry, designed to monitor hospitalized patients with active cardiac conditions, is highly utilized outside the intensive care unit (ICU) and generates a large number of automated alarms. Telemetry is also costly and requires substantial time and attention commitments from nursing and technician staff, who place and maintain the recording devices and address monitoring results. 1,2 The staff address and dismiss invalid alarms caused by telemetry artifacts, 2 such as the misreporting of patient movement as ventricular tachycardia/fibrillation (VT/VF) or the mimicking of asystole by a lead disconnection.
One strategy for addressing telemetry alarms is to have dedicated staff observe telemetry monitors and notify nurses with any events or findings. Studies conducted in the 1990s showed that dedicated monitor watchers, compared with automatically generated alarms alone, did not affect most outcomes 3 but can improve accuracy of arrhythmia detection. 4 Since then, given the advances in telemetry detection software, the effect of monitor watchers has not been evaluated. Mindful of the perceived burden of nonactionable telemetry alerts, we wanted to quantify the frequency of automated telemetry alerts in the wards and analyze the proportion of alerts deemed nonactionable by monitor watchers.
METHODS
We conducted this retrospective study at a 545-bed urban academic hospital in the United States. We reviewed the cases of all non-ICU patients with telemetry monitoring ordered. The telemetry order requires providers specify the indication for monitoring and adjust alert parameters for variables such as heart rate (preset to 60 and 100 beats per minute) and baseline rhythm (preset to normal sinus). Once a telemetry order is received, 5 leads are attached to the patient, and electrocardiographic data begin transmitting to a portable wireless telemetry monitor, or telemeter (Philips Intellispace Telemetry System), which in turn transmits to a central monitoring station in the progressive care unit (PCU; cardiac/pulmonary unit). The majority of patients on telemetry are in the PCU. Telemeters are also located in the general medicine, surgical, and neurologic non-ICU units. Data from a maximum of 96 telemeters in the hospital are simultaneously displayed in the central monitoring station.
At all times, two dedicated monitor watchers oversee the central monitoring station. Watchers are certified medical assistants with extra telemetry-specific training. Each receives a salary of $17 per hour (no benefits), or about $800 per 24-hour day for two watchers. Their role is to respond to audiovisual alerts triggered by the monitoring system—they either contact the bedside nurse or intercept the alert if deemed nonactionable. Consistent with the literature, 5 nonactionable alerts and alarms were defined as either “invalid” or “nuisance.” Invalid alerts and alarms misrepresent patient status (eg, patient motion is electronically interpreted as VT/VF), and nuisance alerts and alarms do not require clinical intervention (eg, persistent sinus tachycardia has already been communicated to the nurse or provider). Monitor watchers must intercept the alert within a limited amount of time: 15 seconds for suspected lethal alerts (asystole, VT/VF), 30 seconds for extreme tachycardia/bradycardia, and 60 seconds for lead displacement or low battery.
If a watcher does not intercept an alert—either intentionally or because time ran out—the alert generates an alarm, which automatically sends a text message to the patient’s nurse’s wireless phone. The nurse acknowledges the alarm and decides on further action. If the bedside nurse does not acknowledge the alarm within the same time frames as mentioned, the alarm is escalated, first to the unit charge nurse and then to the monitoring station charge nurse (Figure). All alerts are available for provider review at the central monitoring station for the duration of the telemetry order, and select telemetry strips are printed and filed in the patient’s paper chart.
For this study, we analyzed telemetry system data for all monitored non-ICU ward patients from August 1 through September 30, 2014. We focused on the rate and relevance of alerts (system-generated) and alarms (text message to nurse). As cardiac arrhythmias leading to cardiopulmonary arrest can potentially be detected by telemetry, we also reviewed all code team activations, which are recorded in a separate database that details time of code team activation, to evaluate for correlation with telemetry alerts.
RESULTS
Within the 2-month study period, there were 1917 admissions to, and 1370 transfers to, non-ICU floors, for a total of 3287 unique patient-admissions and 9704 total patient-days. There were 1199 patient admissions with telemetry orders (36.5% of all admissions), 4044 total patient-days of telemetry, and an average of 66.3 patients monitored per day. In addition, the system generated 20,775 alerts, an average of 341 per day, 5.1 per patient-day, 1 every 4 minutes. Overall, 18,051 alerts (87%) were intercepted by monitor watchers, preventing nurse text-alarms. Of all alerts, 91% were from patients on medicine services, including pulmonary and cardiology; 6% were from patients on the neurology floor; and 3% were from patients on the surgery floor.
Forty percent of all alerts were for heart rates deviating outside the ranges set by the provider; of these, the overwhelming majority were intercepted as nuisance alerts (Table). In addition, 26% of all alerts were for maintenance reasons, including issues with batteries or leads. Finally, 34% (6954) were suspected lethal alerts (asystole, VT/VF); of these, 74% (5170) were intercepted by monitor watchers, suggesting they were deemed invalid. None of the suspected lethal alerts triggered a code team activation, indicating there were no telemetry-documented asystole or VT/VF episodes prompting resuscitative efforts. During the study period, there were 7 code team activations. Of the 7 patients, 2 were on telemetry, and their code team activation was for hypoxia detected by pulse oximetry; the other 5 patients, not on telemetry, were found unresponsive or apneic, and 4 of them had confirmed pulseless electrical activity.
DISCUSSION
In small studies, other investigators have directly observed nurses for hours at a time and assessed their response to telemetry-related alarms. 1,2 In the present study, we found a very large number of telemetry-detected alerts over a continuous 2-month period. The large majority (87%) of alerts were manually intercepted by monitor watchers before being communicated to a nurse or provider, indicating these alerts did not affect clinical management and likely were either false positives or nonactionable. It is possible that repeat nonactionable alerts, like continued sinus tachycardia or bradycardia, affect decision making, but this may be outside the role of continuous cardiac telemetry. In addition, it is likely that all the lethal alarms (asystole, VT/VF) forwarded to the nurses were invalid, as none resulted in code team activations.
Addressing these alerts is a major issue, as frequent telemetry alarms can lead to alarm fatigue, a widely acknowledged safety concern. 6 Furthermore, nonactionable alarms are a time sink, diverting nursing attention from other patient care needs. Finally, nonactionable alarms, especially invalid alarms, can lead to adverse patient outcomes. Although we did not specifically evaluate for harm, an earlier case series found a potential for unnecessary interventions and device implantation as a result of reporting artifactual arrhythmias. 7
Our results also highlight the role of monitor watchers in intercepting nonactionable alarms and reducing the alarm burden on nurses. Other investigators have reported on computerized paging systems that directly alert only nurses, 8 or on escalated alarm paging systems that let noncrisis alarms self-resolve. 9 In contrast, our study used a hybrid 2-step telemetry-monitoring system—an escalated paging system designed to be sensitive and less likely than human monitoring to overlook events, followed by dedicated monitor watchers who are first-responders for a large number of alarms and who increase the specificity of alarms by screening for nonactionable alarms, thereby reducing the number of alarms transmitted to nurses. We think that, for most hospitals, monitor watchers are cost-effective, as their hourly wage is lower than that of registered nurses. Furthermore, monitor watchers can screen alerts faster because they are always at the monitoring station. Their presence reduces the amount of time that nurses need to divert from other clinical tasks in order to walk to the monitoring station to evaluate alerts.
Nonetheless, there remains a large number of nonactionable alerts forwarded as alarms to nurses, likely because of monitor watchers’ inability to address the multitude of alerts, and perhaps because of alarm fatigue. Although this study showed the utility of monitor watchers in decreasing telemetry alarms to nurses, other steps can be taken to reduce telemetry alarm fatigue. A systematic review of alarm frequency interventions 5 noted that detection algorithms can be improved to decrease telemetry alert false positives. Another solution, likely easier to implement, is to encourage appropriate alterations in telemetry alarm parameters, which can decrease the alarm proportion. 10 An essential step is to decrease inappropriate telemetry use regarding the indication for and duration of monitoring, as emphasized by the Choosing Wisely campaign championing American Heart Association (AHA) guidelines for appropriate telemetry use. 11 At our institution, 20.2% of telemetry orders were for indications outside AHA guidelines, and that percentage likely is an underestimate, as this was required self-reporting on ordering. 12 Telemetry may not frequently result in changes in management in the non-ICU setting, 13 and may lead to other harms such as worsening delirium, 14 so it needs to be evaluated for harm versus benefit per patient before order.
Cardiac telemetry in the non-ICU setting produces a large number of alerts and alarms. The vast majority are not seen or addressed by nurses or physicians, leading to a negligible impact on patient care decisions. Monitor watchers reduce the nursing burden in dealing with telemetry alerts, but we emphasize the need to take additional measures to reduce telemetry-related alerts and thereby reduce alarm-related harms and alarm fatigue.
Acknowledgments
The authors thank Torberg Tonnessen, who was instrumental in providing the telemetry and clinical data used in this study, as well as the numerous Johns Hopkins Bayview Medical Center nurses, patient care technicians, and monitor watchers who answered questions about telemetry processes and allowed their work to be observed.
Disclosure
Nothing to report.
Cardiac telemetry, designed to monitor hospitalized patients with active cardiac conditions, is highly utilized outside the intensive care unit (ICU) and generates a large number of automated alarms. Telemetry is also costly and requires substantial time and attention commitments from nursing and technician staff, who place and maintain the recording devices and address monitoring results. 1,2 The staff address and dismiss invalid alarms caused by telemetry artifacts, 2 such as the misreporting of patient movement as ventricular tachycardia/fibrillation (VT/VF) or the mimicking of asystole by a lead disconnection.
One strategy for addressing telemetry alarms is to have dedicated staff observe telemetry monitors and notify nurses with any events or findings. Studies conducted in the 1990s showed that dedicated monitor watchers, compared with automatically generated alarms alone, did not affect most outcomes 3 but can improve accuracy of arrhythmia detection. 4 Since then, given the advances in telemetry detection software, the effect of monitor watchers has not been evaluated. Mindful of the perceived burden of nonactionable telemetry alerts, we wanted to quantify the frequency of automated telemetry alerts in the wards and analyze the proportion of alerts deemed nonactionable by monitor watchers.
METHODS
We conducted this retrospective study at a 545-bed urban academic hospital in the United States. We reviewed the cases of all non-ICU patients with telemetry monitoring ordered. The telemetry order requires providers specify the indication for monitoring and adjust alert parameters for variables such as heart rate (preset to 60 and 100 beats per minute) and baseline rhythm (preset to normal sinus). Once a telemetry order is received, 5 leads are attached to the patient, and electrocardiographic data begin transmitting to a portable wireless telemetry monitor, or telemeter (Philips Intellispace Telemetry System), which in turn transmits to a central monitoring station in the progressive care unit (PCU; cardiac/pulmonary unit). The majority of patients on telemetry are in the PCU. Telemeters are also located in the general medicine, surgical, and neurologic non-ICU units. Data from a maximum of 96 telemeters in the hospital are simultaneously displayed in the central monitoring station.
At all times, two dedicated monitor watchers oversee the central monitoring station. Watchers are certified medical assistants with extra telemetry-specific training. Each receives a salary of $17 per hour (no benefits), or about $800 per 24-hour day for two watchers. Their role is to respond to audiovisual alerts triggered by the monitoring system—they either contact the bedside nurse or intercept the alert if deemed nonactionable. Consistent with the literature, 5 nonactionable alerts and alarms were defined as either “invalid” or “nuisance.” Invalid alerts and alarms misrepresent patient status (eg, patient motion is electronically interpreted as VT/VF), and nuisance alerts and alarms do not require clinical intervention (eg, persistent sinus tachycardia has already been communicated to the nurse or provider). Monitor watchers must intercept the alert within a limited amount of time: 15 seconds for suspected lethal alerts (asystole, VT/VF), 30 seconds for extreme tachycardia/bradycardia, and 60 seconds for lead displacement or low battery.
If a watcher does not intercept an alert—either intentionally or because time ran out—the alert generates an alarm, which automatically sends a text message to the patient’s nurse’s wireless phone. The nurse acknowledges the alarm and decides on further action. If the bedside nurse does not acknowledge the alarm within the same time frames as mentioned, the alarm is escalated, first to the unit charge nurse and then to the monitoring station charge nurse (Figure). All alerts are available for provider review at the central monitoring station for the duration of the telemetry order, and select telemetry strips are printed and filed in the patient’s paper chart.
For this study, we analyzed telemetry system data for all monitored non-ICU ward patients from August 1 through September 30, 2014. We focused on the rate and relevance of alerts (system-generated) and alarms (text message to nurse). As cardiac arrhythmias leading to cardiopulmonary arrest can potentially be detected by telemetry, we also reviewed all code team activations, which are recorded in a separate database that details time of code team activation, to evaluate for correlation with telemetry alerts.
RESULTS
Within the 2-month study period, there were 1917 admissions to, and 1370 transfers to, non-ICU floors, for a total of 3287 unique patient-admissions and 9704 total patient-days. There were 1199 patient admissions with telemetry orders (36.5% of all admissions), 4044 total patient-days of telemetry, and an average of 66.3 patients monitored per day. In addition, the system generated 20,775 alerts, an average of 341 per day, 5.1 per patient-day, 1 every 4 minutes. Overall, 18,051 alerts (87%) were intercepted by monitor watchers, preventing nurse text-alarms. Of all alerts, 91% were from patients on medicine services, including pulmonary and cardiology; 6% were from patients on the neurology floor; and 3% were from patients on the surgery floor.
Forty percent of all alerts were for heart rates deviating outside the ranges set by the provider; of these, the overwhelming majority were intercepted as nuisance alerts (Table). In addition, 26% of all alerts were for maintenance reasons, including issues with batteries or leads. Finally, 34% (6954) were suspected lethal alerts (asystole, VT/VF); of these, 74% (5170) were intercepted by monitor watchers, suggesting they were deemed invalid. None of the suspected lethal alerts triggered a code team activation, indicating there were no telemetry-documented asystole or VT/VF episodes prompting resuscitative efforts. During the study period, there were 7 code team activations. Of the 7 patients, 2 were on telemetry, and their code team activation was for hypoxia detected by pulse oximetry; the other 5 patients, not on telemetry, were found unresponsive or apneic, and 4 of them had confirmed pulseless electrical activity.
DISCUSSION
In small studies, other investigators have directly observed nurses for hours at a time and assessed their response to telemetry-related alarms. 1,2 In the present study, we found a very large number of telemetry-detected alerts over a continuous 2-month period. The large majority (87%) of alerts were manually intercepted by monitor watchers before being communicated to a nurse or provider, indicating these alerts did not affect clinical management and likely were either false positives or nonactionable. It is possible that repeat nonactionable alerts, like continued sinus tachycardia or bradycardia, affect decision making, but this may be outside the role of continuous cardiac telemetry. In addition, it is likely that all the lethal alarms (asystole, VT/VF) forwarded to the nurses were invalid, as none resulted in code team activations.
Addressing these alerts is a major issue, as frequent telemetry alarms can lead to alarm fatigue, a widely acknowledged safety concern. 6 Furthermore, nonactionable alarms are a time sink, diverting nursing attention from other patient care needs. Finally, nonactionable alarms, especially invalid alarms, can lead to adverse patient outcomes. Although we did not specifically evaluate for harm, an earlier case series found a potential for unnecessary interventions and device implantation as a result of reporting artifactual arrhythmias. 7
Our results also highlight the role of monitor watchers in intercepting nonactionable alarms and reducing the alarm burden on nurses. Other investigators have reported on computerized paging systems that directly alert only nurses, 8 or on escalated alarm paging systems that let noncrisis alarms self-resolve. 9 In contrast, our study used a hybrid 2-step telemetry-monitoring system—an escalated paging system designed to be sensitive and less likely than human monitoring to overlook events, followed by dedicated monitor watchers who are first-responders for a large number of alarms and who increase the specificity of alarms by screening for nonactionable alarms, thereby reducing the number of alarms transmitted to nurses. We think that, for most hospitals, monitor watchers are cost-effective, as their hourly wage is lower than that of registered nurses. Furthermore, monitor watchers can screen alerts faster because they are always at the monitoring station. Their presence reduces the amount of time that nurses need to divert from other clinical tasks in order to walk to the monitoring station to evaluate alerts.
Nonetheless, there remains a large number of nonactionable alerts forwarded as alarms to nurses, likely because of monitor watchers’ inability to address the multitude of alerts, and perhaps because of alarm fatigue. Although this study showed the utility of monitor watchers in decreasing telemetry alarms to nurses, other steps can be taken to reduce telemetry alarm fatigue. A systematic review of alarm frequency interventions 5 noted that detection algorithms can be improved to decrease telemetry alert false positives. Another solution, likely easier to implement, is to encourage appropriate alterations in telemetry alarm parameters, which can decrease the alarm proportion. 10 An essential step is to decrease inappropriate telemetry use regarding the indication for and duration of monitoring, as emphasized by the Choosing Wisely campaign championing American Heart Association (AHA) guidelines for appropriate telemetry use. 11 At our institution, 20.2% of telemetry orders were for indications outside AHA guidelines, and that percentage likely is an underestimate, as this was required self-reporting on ordering. 12 Telemetry may not frequently result in changes in management in the non-ICU setting, 13 and may lead to other harms such as worsening delirium, 14 so it needs to be evaluated for harm versus benefit per patient before order.
Cardiac telemetry in the non-ICU setting produces a large number of alerts and alarms. The vast majority are not seen or addressed by nurses or physicians, leading to a negligible impact on patient care decisions. Monitor watchers reduce the nursing burden in dealing with telemetry alerts, but we emphasize the need to take additional measures to reduce telemetry-related alerts and thereby reduce alarm-related harms and alarm fatigue.
Acknowledgments
The authors thank Torberg Tonnessen, who was instrumental in providing the telemetry and clinical data used in this study, as well as the numerous Johns Hopkins Bayview Medical Center nurses, patient care technicians, and monitor watchers who answered questions about telemetry processes and allowed their work to be observed.
Disclosure
Nothing to report.
1. Gazarian PK. Nurses’ response to frequency and types of electrocardiography alarms in a non-critical care setting: a descriptive study. Int J Nurs Stud . 2014;51(2):190-197. PubMed
2. Varpio L, Kuziemsky C, MacDonald C, King WJ. The helpful or hindering effects of in-hospital patient monitor alarms on nurses. Comput Inform Nurs . 2012;30(4):210-217. PubMed
3. Funk M, Parkosewich J, Johnson C, Stukshis I. Effect of dedicated monitor watchers on patients’ outcomes. Am J Crit Care . 1997;6(4):318-323. PubMed
4. Stukshis I, Funk M, Johnson C, Parkosewich J. Accuracy of detection of clinically important dysrhythmias with and without a dedicated monitor watcher. Am J Crit Care . 1997;6(4):312-317. PubMed
5. Paine CW, Goel VV, Ely E, et al. Systematic review of physiologic monitor alarm characteristics and pragmatic interventions to reduce alarm frequency. J Hosp Med . 2016;11(2):136-144. PubMed
6. Joint Commission on Accreditation of Healthcare Organizations. The Joint Commission announces 2014 national patient safety goal. Jt Comm Perspect . 2013;33(7):1, 3-4. PubMed
7. Knight BP, Pelosi F, Michaud GF, Strickberger SA, Morady F. Clinical consequences of electrocardiographic artifact mimicking ventricular tachycardia. N Engl J Med . 1999;341(17):1270-1274. PubMed
8. Zwieg FH, Karfonta TL, Jeske LJ, et al. Arrhythmia detection and response in a monitoring technician and pocket paging system. Prog Cardiovasc Nurs . 1998;13(1):16-22, 33. PubMed
9. Cvach MM, Frank RJ, Doyle P, Stevens ZK. Use of pagers with an alarm escalation system to reduce cardiac monitor alarm signals. J Nurs Care Qual . 2013;29(1):9-18. PubMed
10. Gross B, Dahl D, Nielsen L. Physiologic monitoring alarm load on medical/surgical floors of a community hospital. Biomed Instrum Technol . 2011;Spring(suppl):29-36. PubMed
11. Drew BJ, Califf RM, Funk M, et al; American Heart Association; Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young. Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical-Care Nurses [published correction appears in Circulation . 2005;111(3):378]. Circulation . 2004;110(17):2721-2746. PubMed
12. Chen S, Palchaudhuri S, Johnson A, Trost J, Ponor I, Zakaria S. Does this patient need telemetry? An analysis of telemetry ordering practices at an academic medical center. J Eval Clin Pract . 2017 Jan 27 [Epub ahead of print] . PubMed
13. Estrada CA, Rosman HS, Prasad NK, et al. Role of telemetry monitoring in the non-intensive care unit. Am J Cardiol . 1995;76(12):960-965. PubMed
14. Chen S, Zakaria S. Behind the monitor—the trouble with telemetry: a teachable moment. JAMA Intern Med . 2015;175(6):894. PubMed
1. Gazarian PK. Nurses’ response to frequency and types of electrocardiography alarms in a non-critical care setting: a descriptive study. Int J Nurs Stud . 2014;51(2):190-197. PubMed
2. Varpio L, Kuziemsky C, MacDonald C, King WJ. The helpful or hindering effects of in-hospital patient monitor alarms on nurses. Comput Inform Nurs . 2012;30(4):210-217. PubMed
3. Funk M, Parkosewich J, Johnson C, Stukshis I. Effect of dedicated monitor watchers on patients’ outcomes. Am J Crit Care . 1997;6(4):318-323. PubMed
4. Stukshis I, Funk M, Johnson C, Parkosewich J. Accuracy of detection of clinically important dysrhythmias with and without a dedicated monitor watcher. Am J Crit Care . 1997;6(4):312-317. PubMed
5. Paine CW, Goel VV, Ely E, et al. Systematic review of physiologic monitor alarm characteristics and pragmatic interventions to reduce alarm frequency. J Hosp Med . 2016;11(2):136-144. PubMed
6. Joint Commission on Accreditation of Healthcare Organizations. The Joint Commission announces 2014 national patient safety goal. Jt Comm Perspect . 2013;33(7):1, 3-4. PubMed
7. Knight BP, Pelosi F, Michaud GF, Strickberger SA, Morady F. Clinical consequences of electrocardiographic artifact mimicking ventricular tachycardia. N Engl J Med . 1999;341(17):1270-1274. PubMed
8. Zwieg FH, Karfonta TL, Jeske LJ, et al. Arrhythmia detection and response in a monitoring technician and pocket paging system. Prog Cardiovasc Nurs . 1998;13(1):16-22, 33. PubMed
9. Cvach MM, Frank RJ, Doyle P, Stevens ZK. Use of pagers with an alarm escalation system to reduce cardiac monitor alarm signals. J Nurs Care Qual . 2013;29(1):9-18. PubMed
10. Gross B, Dahl D, Nielsen L. Physiologic monitoring alarm load on medical/surgical floors of a community hospital. Biomed Instrum Technol . 2011;Spring(suppl):29-36. PubMed
11. Drew BJ, Califf RM, Funk M, et al; American Heart Association; Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young. Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical-Care Nurses [published correction appears in Circulation . 2005;111(3):378]. Circulation . 2004;110(17):2721-2746. PubMed
12. Chen S, Palchaudhuri S, Johnson A, Trost J, Ponor I, Zakaria S. Does this patient need telemetry? An analysis of telemetry ordering practices at an academic medical center. J Eval Clin Pract . 2017 Jan 27 [Epub ahead of print] . PubMed
13. Estrada CA, Rosman HS, Prasad NK, et al. Role of telemetry monitoring in the non-intensive care unit. Am J Cardiol . 1995;76(12):960-965. PubMed
14. Chen S, Zakaria S. Behind the monitor—the trouble with telemetry: a teachable moment. JAMA Intern Med . 2015;175(6):894. PubMed
© 2017 Society of Hospital Medicine
Perceptions of hospital-dependent patients on their needs for hospitalization
In the United States, patients 65 years old or older accounted for more than one third of inpatient stays and 42% of inpatient care spending in 2012.1 Despite the identification of risk factors, the implementation of an array of interventions, and the institution of penalties on hospitals, a subset of older adults continues to spend significant time in the hospital.2,3
Hospital dependency is a concept that was only recently described. It identifies patients who improve while in the hospital but quickly deteriorate after leaving the hospital, resulting in recurring hospitalizations.4 Although little is known about hospital-dependent patients, studies have explored patients’ perspectives on readmissions.5,6 Nevertheless, it remains unclear whether there are individuals for whom frequent and prolonged hospitalizations are appropriate, and whether there are undisclosed factors that, if addressed, could decrease their hospital dependency. We conducted an exploratory study to ascertain hospital-dependent patients’ perspectives on their needs for hospitalizations.
METHODS
Study Design
This study was approved by the Yale University Institutional Review Board. From March 2015 to September 2015, Dr. Liu conducted semistructured explorative interviews with patients on the medical units of an academic medical center. Dr. Liu was not directly involved in the care of these patients. An interview guide that includes open-ended questions was created to elicit patients’ perspectives on their need for hospitalizations, health status, and outside-hospital support. This guide was pilot-tested with 6 patients, whose transcripts were not included in the final analysis, to assess for ease of understanding. After the pilot interviews, the questions were revised, and the final guide consists of 12 questions (Supplemental Table).
Recruitment
We used predetermined criteria and a purposeful sampling strategy to select potential study participants. We identified participants by periodically (~once a week) reviewing the electronic medical records of all patients admitted to the medicine service during the study period. Eligible patients were 65 years old or older and had at least 3 hospitalizations over the preceding 6 months. Patients were excluded if they met our chronic critical illness criteria: mechanical ventilation for more than 21 days, history of tracheotomy for failed weaning from mechanical ventilation,7 presence of a conservator, or admission only for comfort measures. Participants were recruited until no new themes emerged.
Data Collection
Twenty-nine patients were eligible. We obtained permission from their inpatient providers to approach them about the study. Of the 29 patients, 26 agreed to be interviewed, and 3 declined. Of the 26 participants, 6 underwent pilot interviews, and 20 underwent formal interviews with use of the finalized interview guide. The interviews, conducted in the hospital while the participants were hospitalized, lasted 17 minutes on average. The interviews were transcribed and iteratively analyzed. The themes that emerged from the initial interviews were further explored and validated in subsequent interviews. Interviews were conducted until theoretical saturation was reached and no new themes were derived from them. Demographic information, including age, sex, ethnicity, and marital status, was also collected.
Analysis
Interviews were digitally recorded and transcribed. Independently, two investigators used Atlas Ti software to analyze and code the interview transcriptions. An inductive approach was used to identify new codes from the data.8 The coders then met to discuss repeating ideas based on the codes. When a code was identified by one coder but not the other, or when there was disagreement about interpretation of a code, the coders returned to the relevant text to reach consensus and to determine whether to include or discard the code.9 We then organized and reorganized repeating ideas based on their conceptual similarities to determine the themes and subthemes.9
RESULTS
Twenty patients participated in the formal interviews. Participants’ baseline characteristics are listed in Table 1, and four dominant themes, and their subthemes and exemplary quotations, are listed in Table 2.
Perspectives on Hospital Care
Participants perceived their hospitalizations as inevitable and necessary for survival: “I think if I haven’t come to the hospital, I probably would have died.” Furthermore, participants thought only the hospital had the resources to help them (“The medications they were giving me … you can get that in the hospital but not outside the hospital”) and sustain them (“You are like an old car, and it breaks down little by little, so you have to go in periodically and get the problem fixed, so you will drive it around for a while”).
Feeling Safe in Hospital. Asked how being in the hospital makes them feel, participants attributed their feelings of safety to the constant observation, the availability of providers and nurses, and the idea that hospital care is helping. As one participant stated, “Makes me feel safer in case you go into something like cardiac arrest. You are right here where they can help you.”
Outside-Hospital Support. Despite multiple hospitalizations, most participants reported having social support (“I have the aide. I got the nurses come in. I have my daughter …”), physical support, and medical support (“I have all the doctors”) outside the hospital. A minority of participants questioned the usefulness of the services. One participant described declining the help of visiting nurses because she wanted to be independent and thought that, despite recurrent hospitalizations for physical symptoms, she still had the ability to manage her own medications.
Goals-of-Care Discussion. Some participants reported inadequate discussions about goals of care, health priorities, and health trajectories. In their reports, this inadequacy included not thinking about their goals, despite continued health decline. One participant stated, “Oh, God, I don’t know if I had any conversation like that. … I think until it is really brought to the front, you don’t make a decision really if you don’t have to.” Citing the value of a more established relationship and deeper trust, participants preferred having these serious and personal discussions with their ambulatory care clinicians: “Because I know my doctor much closer. I have been with him for a number of years. The doctors in the hospital seem to be nice and competent, but I don’t know them.”
DISCUSSION
Participants considered their hospitalizations a necessity and reported feeling safe in the hospital. Given that most already had support outside the hospital, increasing community services may be inadequate to alter participants’ perceived hospital care needs. On the other hand, a few participants reported declining services that might have prevented hospitalizations. Although there has been a study of treatment refusal among older adults with advanced illnesses,10 not much is known about refusal of services among this population. Investigators should examine the reasons for refusing services and the effect that refusal has on hospitalizations. Furthermore, although it would have been informative to ascertain clinician perspectives as well, we focused on patient perspectives because less is known on this topic.
Some participants noted their lack of discussion with their clinicians about healthcare goals and probable health trajectories. Barriers to goals-of-care discussion among this highly vulnerable population have been researched from the perspectives of clinicians and other health professionals but not patients themselves.11,12 Of particular concern in our study is the participant-noted lack of discussion about health trajectories and health priorities, given the decline that occurs in this population and even in those with good care. This inadequacy in discussion suggests continued hospital care may not always be consistent with a patient’s goals. Patients’ desire to have this discussion with their clinicians, with whom they have a relationship, supports the need to involve ambulatory care clinicians, or ensure these patients are cared for by the same clinicians, across healthcare settings.13,14 Whoever provides the care, the clinician must align treatment with the patient’s goal, whether it is to continue hospital-level care or to transition to palliative care. Such an approach also reflects the core elements of person-centered care.15
Study Limitations
Participants were recruited from the medicine service at a single large academic center, limiting the study’s generalizability to patients admitted to surgical services or community hospitals. The patients in this small sample were English-speaking and predominantly Caucasian, so our findings may not represent the perspectives of non-English-speaking or minority patients. We did not perform statistical analysis to quantify intercoder reliability. Last, as this was a qualitative study, we cannot comment on the relative importance or prevalence of the reasons cited for frequent hospitalizations, and we cannot estimate the proportion of patients who had recurrent hospitalizations and were hospital-dependent.
Implication
Although quantitative research is needed to confirm our findings, the hospital-dependent patients in this study thought their survival required hospital-level care and resources. From their perspective, increasing posthospital and community support may be insufficient to prevent some hospitalizations. The lack of goals-of-care discussion supports attempts to increase efforts to facilitate discussion about health trajectories and health priorities between patients and their preferred clinicians.
Acknowledgments
The authors thank Dr. Grace Jenq for providing feedback on the study design.
Disclosure
Nothing to report.
1. Weiss AJ, Elixhauser A. Overview of Hospital Stays in the United States, 2012: Statistical Brief 180. Rockville, MD: Agency for Healthcare Research and Quality, Healthcare Cost and Utilization Project; 2014. http://www.ncbi.nlm.nih.gov/books/NBK259100/. Published October 2014. Accessed February 17, 2016.
2. Auerbach AD, Kripalani S, Vasilevskis EE, et al. Preventability and causes of readmissions in a national cohort of general medicine patients. JAMA Intern Med. 2016;176(4):484-493. PubMed
3. Donzé JD, Williams MV, Robinson EJ, et al. International validity of the HOSPITAL score to predict 30-day potentially avoidable hospital readmissions. JAMA Intern Med. 2016;176(4):496-502. PubMed
4. Reuben DB, Tinetti ME. The hospital-dependent patient. N Engl J Med. 2014;370(8):694-697. PubMed
5. Enguidanos S, Coulourides Kogan AM, Schreibeis-Baum H, Lendon J, Lorenz K. “Because I was sick”: seriously ill veterans’ perspectives on reason for 30-day readmissions. J Am Geriatr Soc. 2015;63(3):537-542. PubMed
6. Kangovi S, Grande D, Meehan P, Mitra N, Shannon R, Long JA. Perceptions of readmitted patients on the transition from hospital to home. J Hosp Med. 2012;7(9):709-712. PubMed
7. Lamas D. Chronic critical illness. N Engl J Med. 2014;370(2):175-177. PubMed
8. Saldana J. Fundamentals of Qualitative Research. Cary, NC: Oxford University Press; 2011.
9. Auerbach CF, Silverstein LB. Qualitative Data: An Introduction to Coding and Analysis. New York, NY: New York University Press; 2003.
10. Rothman MD, Van Ness PH, O’Leary JR, Fried TR. Refusal of medical and surgical interventions by older persons with advanced chronic disease. J Gen Intern Med. 2007;22(7):982-987. PubMed
11. You JJ, Downar J, Fowler RA, et al; Canadian Researchers at the End of Life Network. Barriers to goals of care discussions with seriously ill hospitalized patients and their families: a multicenter survey of clinicians. JAMA Intern Med. 2015;175(4):549-556. PubMed
12. Schoenborn NL, Bowman TL 2nd, Cayea D, Pollack CE, Feeser S, Boyd C. Primary care practitioners’ views on incorporating long-term prognosis in the care of older adults. JAMA Intern Med. 2016;176(5):671-678. PubMed
13. Arora VM, Prochaska ML, Farnan JM, et al. Problems after discharge and understanding of communication with their primary care physicians among hospitalized seniors: a mixed methods study. J Hosp Med. 2010;5(7):385-391. PubMed
14. Jones CD, Vu MB, O’Donnell CM, et al. A failure to communicate: a qualitative exploration of care coordination between hospitalists and primary care providers around patient hospitalizations. J Gen Intern Med. 2015;30(4):417-424. PubMed
15. American Geriatrics Society Expert Panel on Person-Centered Care. Person-centered care: a definition and essential elements. J Am Geriatr Soc. 2016;64(1):15-18. PubMed
In the United States, patients 65 years old or older accounted for more than one third of inpatient stays and 42% of inpatient care spending in 2012.1 Despite the identification of risk factors, the implementation of an array of interventions, and the institution of penalties on hospitals, a subset of older adults continues to spend significant time in the hospital.2,3
Hospital dependency is a concept that was only recently described. It identifies patients who improve while in the hospital but quickly deteriorate after leaving the hospital, resulting in recurring hospitalizations.4 Although little is known about hospital-dependent patients, studies have explored patients’ perspectives on readmissions.5,6 Nevertheless, it remains unclear whether there are individuals for whom frequent and prolonged hospitalizations are appropriate, and whether there are undisclosed factors that, if addressed, could decrease their hospital dependency. We conducted an exploratory study to ascertain hospital-dependent patients’ perspectives on their needs for hospitalizations.
METHODS
Study Design
This study was approved by the Yale University Institutional Review Board. From March 2015 to September 2015, Dr. Liu conducted semistructured explorative interviews with patients on the medical units of an academic medical center. Dr. Liu was not directly involved in the care of these patients. An interview guide that includes open-ended questions was created to elicit patients’ perspectives on their need for hospitalizations, health status, and outside-hospital support. This guide was pilot-tested with 6 patients, whose transcripts were not included in the final analysis, to assess for ease of understanding. After the pilot interviews, the questions were revised, and the final guide consists of 12 questions (Supplemental Table).
Recruitment
We used predetermined criteria and a purposeful sampling strategy to select potential study participants. We identified participants by periodically (~once a week) reviewing the electronic medical records of all patients admitted to the medicine service during the study period. Eligible patients were 65 years old or older and had at least 3 hospitalizations over the preceding 6 months. Patients were excluded if they met our chronic critical illness criteria: mechanical ventilation for more than 21 days, history of tracheotomy for failed weaning from mechanical ventilation,7 presence of a conservator, or admission only for comfort measures. Participants were recruited until no new themes emerged.
Data Collection
Twenty-nine patients were eligible. We obtained permission from their inpatient providers to approach them about the study. Of the 29 patients, 26 agreed to be interviewed, and 3 declined. Of the 26 participants, 6 underwent pilot interviews, and 20 underwent formal interviews with use of the finalized interview guide. The interviews, conducted in the hospital while the participants were hospitalized, lasted 17 minutes on average. The interviews were transcribed and iteratively analyzed. The themes that emerged from the initial interviews were further explored and validated in subsequent interviews. Interviews were conducted until theoretical saturation was reached and no new themes were derived from them. Demographic information, including age, sex, ethnicity, and marital status, was also collected.
Analysis
Interviews were digitally recorded and transcribed. Independently, two investigators used Atlas Ti software to analyze and code the interview transcriptions. An inductive approach was used to identify new codes from the data.8 The coders then met to discuss repeating ideas based on the codes. When a code was identified by one coder but not the other, or when there was disagreement about interpretation of a code, the coders returned to the relevant text to reach consensus and to determine whether to include or discard the code.9 We then organized and reorganized repeating ideas based on their conceptual similarities to determine the themes and subthemes.9
RESULTS
Twenty patients participated in the formal interviews. Participants’ baseline characteristics are listed in Table 1, and four dominant themes, and their subthemes and exemplary quotations, are listed in Table 2.
Perspectives on Hospital Care
Participants perceived their hospitalizations as inevitable and necessary for survival: “I think if I haven’t come to the hospital, I probably would have died.” Furthermore, participants thought only the hospital had the resources to help them (“The medications they were giving me … you can get that in the hospital but not outside the hospital”) and sustain them (“You are like an old car, and it breaks down little by little, so you have to go in periodically and get the problem fixed, so you will drive it around for a while”).
Feeling Safe in Hospital. Asked how being in the hospital makes them feel, participants attributed their feelings of safety to the constant observation, the availability of providers and nurses, and the idea that hospital care is helping. As one participant stated, “Makes me feel safer in case you go into something like cardiac arrest. You are right here where they can help you.”
Outside-Hospital Support. Despite multiple hospitalizations, most participants reported having social support (“I have the aide. I got the nurses come in. I have my daughter …”), physical support, and medical support (“I have all the doctors”) outside the hospital. A minority of participants questioned the usefulness of the services. One participant described declining the help of visiting nurses because she wanted to be independent and thought that, despite recurrent hospitalizations for physical symptoms, she still had the ability to manage her own medications.
Goals-of-Care Discussion. Some participants reported inadequate discussions about goals of care, health priorities, and health trajectories. In their reports, this inadequacy included not thinking about their goals, despite continued health decline. One participant stated, “Oh, God, I don’t know if I had any conversation like that. … I think until it is really brought to the front, you don’t make a decision really if you don’t have to.” Citing the value of a more established relationship and deeper trust, participants preferred having these serious and personal discussions with their ambulatory care clinicians: “Because I know my doctor much closer. I have been with him for a number of years. The doctors in the hospital seem to be nice and competent, but I don’t know them.”
DISCUSSION
Participants considered their hospitalizations a necessity and reported feeling safe in the hospital. Given that most already had support outside the hospital, increasing community services may be inadequate to alter participants’ perceived hospital care needs. On the other hand, a few participants reported declining services that might have prevented hospitalizations. Although there has been a study of treatment refusal among older adults with advanced illnesses,10 not much is known about refusal of services among this population. Investigators should examine the reasons for refusing services and the effect that refusal has on hospitalizations. Furthermore, although it would have been informative to ascertain clinician perspectives as well, we focused on patient perspectives because less is known on this topic.
Some participants noted their lack of discussion with their clinicians about healthcare goals and probable health trajectories. Barriers to goals-of-care discussion among this highly vulnerable population have been researched from the perspectives of clinicians and other health professionals but not patients themselves.11,12 Of particular concern in our study is the participant-noted lack of discussion about health trajectories and health priorities, given the decline that occurs in this population and even in those with good care. This inadequacy in discussion suggests continued hospital care may not always be consistent with a patient’s goals. Patients’ desire to have this discussion with their clinicians, with whom they have a relationship, supports the need to involve ambulatory care clinicians, or ensure these patients are cared for by the same clinicians, across healthcare settings.13,14 Whoever provides the care, the clinician must align treatment with the patient’s goal, whether it is to continue hospital-level care or to transition to palliative care. Such an approach also reflects the core elements of person-centered care.15
Study Limitations
Participants were recruited from the medicine service at a single large academic center, limiting the study’s generalizability to patients admitted to surgical services or community hospitals. The patients in this small sample were English-speaking and predominantly Caucasian, so our findings may not represent the perspectives of non-English-speaking or minority patients. We did not perform statistical analysis to quantify intercoder reliability. Last, as this was a qualitative study, we cannot comment on the relative importance or prevalence of the reasons cited for frequent hospitalizations, and we cannot estimate the proportion of patients who had recurrent hospitalizations and were hospital-dependent.
Implication
Although quantitative research is needed to confirm our findings, the hospital-dependent patients in this study thought their survival required hospital-level care and resources. From their perspective, increasing posthospital and community support may be insufficient to prevent some hospitalizations. The lack of goals-of-care discussion supports attempts to increase efforts to facilitate discussion about health trajectories and health priorities between patients and their preferred clinicians.
Acknowledgments
The authors thank Dr. Grace Jenq for providing feedback on the study design.
Disclosure
Nothing to report.
In the United States, patients 65 years old or older accounted for more than one third of inpatient stays and 42% of inpatient care spending in 2012.1 Despite the identification of risk factors, the implementation of an array of interventions, and the institution of penalties on hospitals, a subset of older adults continues to spend significant time in the hospital.2,3
Hospital dependency is a concept that was only recently described. It identifies patients who improve while in the hospital but quickly deteriorate after leaving the hospital, resulting in recurring hospitalizations.4 Although little is known about hospital-dependent patients, studies have explored patients’ perspectives on readmissions.5,6 Nevertheless, it remains unclear whether there are individuals for whom frequent and prolonged hospitalizations are appropriate, and whether there are undisclosed factors that, if addressed, could decrease their hospital dependency. We conducted an exploratory study to ascertain hospital-dependent patients’ perspectives on their needs for hospitalizations.
METHODS
Study Design
This study was approved by the Yale University Institutional Review Board. From March 2015 to September 2015, Dr. Liu conducted semistructured explorative interviews with patients on the medical units of an academic medical center. Dr. Liu was not directly involved in the care of these patients. An interview guide that includes open-ended questions was created to elicit patients’ perspectives on their need for hospitalizations, health status, and outside-hospital support. This guide was pilot-tested with 6 patients, whose transcripts were not included in the final analysis, to assess for ease of understanding. After the pilot interviews, the questions were revised, and the final guide consists of 12 questions (Supplemental Table).
Recruitment
We used predetermined criteria and a purposeful sampling strategy to select potential study participants. We identified participants by periodically (~once a week) reviewing the electronic medical records of all patients admitted to the medicine service during the study period. Eligible patients were 65 years old or older and had at least 3 hospitalizations over the preceding 6 months. Patients were excluded if they met our chronic critical illness criteria: mechanical ventilation for more than 21 days, history of tracheotomy for failed weaning from mechanical ventilation,7 presence of a conservator, or admission only for comfort measures. Participants were recruited until no new themes emerged.
Data Collection
Twenty-nine patients were eligible. We obtained permission from their inpatient providers to approach them about the study. Of the 29 patients, 26 agreed to be interviewed, and 3 declined. Of the 26 participants, 6 underwent pilot interviews, and 20 underwent formal interviews with use of the finalized interview guide. The interviews, conducted in the hospital while the participants were hospitalized, lasted 17 minutes on average. The interviews were transcribed and iteratively analyzed. The themes that emerged from the initial interviews were further explored and validated in subsequent interviews. Interviews were conducted until theoretical saturation was reached and no new themes were derived from them. Demographic information, including age, sex, ethnicity, and marital status, was also collected.
Analysis
Interviews were digitally recorded and transcribed. Independently, two investigators used Atlas Ti software to analyze and code the interview transcriptions. An inductive approach was used to identify new codes from the data.8 The coders then met to discuss repeating ideas based on the codes. When a code was identified by one coder but not the other, or when there was disagreement about interpretation of a code, the coders returned to the relevant text to reach consensus and to determine whether to include or discard the code.9 We then organized and reorganized repeating ideas based on their conceptual similarities to determine the themes and subthemes.9
RESULTS
Twenty patients participated in the formal interviews. Participants’ baseline characteristics are listed in Table 1, and four dominant themes, and their subthemes and exemplary quotations, are listed in Table 2.
Perspectives on Hospital Care
Participants perceived their hospitalizations as inevitable and necessary for survival: “I think if I haven’t come to the hospital, I probably would have died.” Furthermore, participants thought only the hospital had the resources to help them (“The medications they were giving me … you can get that in the hospital but not outside the hospital”) and sustain them (“You are like an old car, and it breaks down little by little, so you have to go in periodically and get the problem fixed, so you will drive it around for a while”).
Feeling Safe in Hospital. Asked how being in the hospital makes them feel, participants attributed their feelings of safety to the constant observation, the availability of providers and nurses, and the idea that hospital care is helping. As one participant stated, “Makes me feel safer in case you go into something like cardiac arrest. You are right here where they can help you.”
Outside-Hospital Support. Despite multiple hospitalizations, most participants reported having social support (“I have the aide. I got the nurses come in. I have my daughter …”), physical support, and medical support (“I have all the doctors”) outside the hospital. A minority of participants questioned the usefulness of the services. One participant described declining the help of visiting nurses because she wanted to be independent and thought that, despite recurrent hospitalizations for physical symptoms, she still had the ability to manage her own medications.
Goals-of-Care Discussion. Some participants reported inadequate discussions about goals of care, health priorities, and health trajectories. In their reports, this inadequacy included not thinking about their goals, despite continued health decline. One participant stated, “Oh, God, I don’t know if I had any conversation like that. … I think until it is really brought to the front, you don’t make a decision really if you don’t have to.” Citing the value of a more established relationship and deeper trust, participants preferred having these serious and personal discussions with their ambulatory care clinicians: “Because I know my doctor much closer. I have been with him for a number of years. The doctors in the hospital seem to be nice and competent, but I don’t know them.”
DISCUSSION
Participants considered their hospitalizations a necessity and reported feeling safe in the hospital. Given that most already had support outside the hospital, increasing community services may be inadequate to alter participants’ perceived hospital care needs. On the other hand, a few participants reported declining services that might have prevented hospitalizations. Although there has been a study of treatment refusal among older adults with advanced illnesses,10 not much is known about refusal of services among this population. Investigators should examine the reasons for refusing services and the effect that refusal has on hospitalizations. Furthermore, although it would have been informative to ascertain clinician perspectives as well, we focused on patient perspectives because less is known on this topic.
Some participants noted their lack of discussion with their clinicians about healthcare goals and probable health trajectories. Barriers to goals-of-care discussion among this highly vulnerable population have been researched from the perspectives of clinicians and other health professionals but not patients themselves.11,12 Of particular concern in our study is the participant-noted lack of discussion about health trajectories and health priorities, given the decline that occurs in this population and even in those with good care. This inadequacy in discussion suggests continued hospital care may not always be consistent with a patient’s goals. Patients’ desire to have this discussion with their clinicians, with whom they have a relationship, supports the need to involve ambulatory care clinicians, or ensure these patients are cared for by the same clinicians, across healthcare settings.13,14 Whoever provides the care, the clinician must align treatment with the patient’s goal, whether it is to continue hospital-level care or to transition to palliative care. Such an approach also reflects the core elements of person-centered care.15
Study Limitations
Participants were recruited from the medicine service at a single large academic center, limiting the study’s generalizability to patients admitted to surgical services or community hospitals. The patients in this small sample were English-speaking and predominantly Caucasian, so our findings may not represent the perspectives of non-English-speaking or minority patients. We did not perform statistical analysis to quantify intercoder reliability. Last, as this was a qualitative study, we cannot comment on the relative importance or prevalence of the reasons cited for frequent hospitalizations, and we cannot estimate the proportion of patients who had recurrent hospitalizations and were hospital-dependent.
Implication
Although quantitative research is needed to confirm our findings, the hospital-dependent patients in this study thought their survival required hospital-level care and resources. From their perspective, increasing posthospital and community support may be insufficient to prevent some hospitalizations. The lack of goals-of-care discussion supports attempts to increase efforts to facilitate discussion about health trajectories and health priorities between patients and their preferred clinicians.
Acknowledgments
The authors thank Dr. Grace Jenq for providing feedback on the study design.
Disclosure
Nothing to report.
1. Weiss AJ, Elixhauser A. Overview of Hospital Stays in the United States, 2012: Statistical Brief 180. Rockville, MD: Agency for Healthcare Research and Quality, Healthcare Cost and Utilization Project; 2014. http://www.ncbi.nlm.nih.gov/books/NBK259100/. Published October 2014. Accessed February 17, 2016.
2. Auerbach AD, Kripalani S, Vasilevskis EE, et al. Preventability and causes of readmissions in a national cohort of general medicine patients. JAMA Intern Med. 2016;176(4):484-493. PubMed
3. Donzé JD, Williams MV, Robinson EJ, et al. International validity of the HOSPITAL score to predict 30-day potentially avoidable hospital readmissions. JAMA Intern Med. 2016;176(4):496-502. PubMed
4. Reuben DB, Tinetti ME. The hospital-dependent patient. N Engl J Med. 2014;370(8):694-697. PubMed
5. Enguidanos S, Coulourides Kogan AM, Schreibeis-Baum H, Lendon J, Lorenz K. “Because I was sick”: seriously ill veterans’ perspectives on reason for 30-day readmissions. J Am Geriatr Soc. 2015;63(3):537-542. PubMed
6. Kangovi S, Grande D, Meehan P, Mitra N, Shannon R, Long JA. Perceptions of readmitted patients on the transition from hospital to home. J Hosp Med. 2012;7(9):709-712. PubMed
7. Lamas D. Chronic critical illness. N Engl J Med. 2014;370(2):175-177. PubMed
8. Saldana J. Fundamentals of Qualitative Research. Cary, NC: Oxford University Press; 2011.
9. Auerbach CF, Silverstein LB. Qualitative Data: An Introduction to Coding and Analysis. New York, NY: New York University Press; 2003.
10. Rothman MD, Van Ness PH, O’Leary JR, Fried TR. Refusal of medical and surgical interventions by older persons with advanced chronic disease. J Gen Intern Med. 2007;22(7):982-987. PubMed
11. You JJ, Downar J, Fowler RA, et al; Canadian Researchers at the End of Life Network. Barriers to goals of care discussions with seriously ill hospitalized patients and their families: a multicenter survey of clinicians. JAMA Intern Med. 2015;175(4):549-556. PubMed
12. Schoenborn NL, Bowman TL 2nd, Cayea D, Pollack CE, Feeser S, Boyd C. Primary care practitioners’ views on incorporating long-term prognosis in the care of older adults. JAMA Intern Med. 2016;176(5):671-678. PubMed
13. Arora VM, Prochaska ML, Farnan JM, et al. Problems after discharge and understanding of communication with their primary care physicians among hospitalized seniors: a mixed methods study. J Hosp Med. 2010;5(7):385-391. PubMed
14. Jones CD, Vu MB, O’Donnell CM, et al. A failure to communicate: a qualitative exploration of care coordination between hospitalists and primary care providers around patient hospitalizations. J Gen Intern Med. 2015;30(4):417-424. PubMed
15. American Geriatrics Society Expert Panel on Person-Centered Care. Person-centered care: a definition and essential elements. J Am Geriatr Soc. 2016;64(1):15-18. PubMed
1. Weiss AJ, Elixhauser A. Overview of Hospital Stays in the United States, 2012: Statistical Brief 180. Rockville, MD: Agency for Healthcare Research and Quality, Healthcare Cost and Utilization Project; 2014. http://www.ncbi.nlm.nih.gov/books/NBK259100/. Published October 2014. Accessed February 17, 2016.
2. Auerbach AD, Kripalani S, Vasilevskis EE, et al. Preventability and causes of readmissions in a national cohort of general medicine patients. JAMA Intern Med. 2016;176(4):484-493. PubMed
3. Donzé JD, Williams MV, Robinson EJ, et al. International validity of the HOSPITAL score to predict 30-day potentially avoidable hospital readmissions. JAMA Intern Med. 2016;176(4):496-502. PubMed
4. Reuben DB, Tinetti ME. The hospital-dependent patient. N Engl J Med. 2014;370(8):694-697. PubMed
5. Enguidanos S, Coulourides Kogan AM, Schreibeis-Baum H, Lendon J, Lorenz K. “Because I was sick”: seriously ill veterans’ perspectives on reason for 30-day readmissions. J Am Geriatr Soc. 2015;63(3):537-542. PubMed
6. Kangovi S, Grande D, Meehan P, Mitra N, Shannon R, Long JA. Perceptions of readmitted patients on the transition from hospital to home. J Hosp Med. 2012;7(9):709-712. PubMed
7. Lamas D. Chronic critical illness. N Engl J Med. 2014;370(2):175-177. PubMed
8. Saldana J. Fundamentals of Qualitative Research. Cary, NC: Oxford University Press; 2011.
9. Auerbach CF, Silverstein LB. Qualitative Data: An Introduction to Coding and Analysis. New York, NY: New York University Press; 2003.
10. Rothman MD, Van Ness PH, O’Leary JR, Fried TR. Refusal of medical and surgical interventions by older persons with advanced chronic disease. J Gen Intern Med. 2007;22(7):982-987. PubMed
11. You JJ, Downar J, Fowler RA, et al; Canadian Researchers at the End of Life Network. Barriers to goals of care discussions with seriously ill hospitalized patients and their families: a multicenter survey of clinicians. JAMA Intern Med. 2015;175(4):549-556. PubMed
12. Schoenborn NL, Bowman TL 2nd, Cayea D, Pollack CE, Feeser S, Boyd C. Primary care practitioners’ views on incorporating long-term prognosis in the care of older adults. JAMA Intern Med. 2016;176(5):671-678. PubMed
13. Arora VM, Prochaska ML, Farnan JM, et al. Problems after discharge and understanding of communication with their primary care physicians among hospitalized seniors: a mixed methods study. J Hosp Med. 2010;5(7):385-391. PubMed
14. Jones CD, Vu MB, O’Donnell CM, et al. A failure to communicate: a qualitative exploration of care coordination between hospitalists and primary care providers around patient hospitalizations. J Gen Intern Med. 2015;30(4):417-424. PubMed
15. American Geriatrics Society Expert Panel on Person-Centered Care. Person-centered care: a definition and essential elements. J Am Geriatr Soc. 2016;64(1):15-18. PubMed
© 2017 Society of Hospital Medicine
Incidental pulmonary nodules reported on CT abdominal imaging: Frequency and factors affecting inclusion in the hospital discharge summary
Incidental findings create both medical and logistical challenges regarding communication.1,2 Pulmonary nodules are among the most frequent and medically relevant incidental findings, being noted in up to 8.4% of abdominal computed tomography (CT) scans.3 There are guidelines regarding proper follow-up and management of such incidental pulmonary nodules, but appropriate evidence-based surveillance imaging is often not performed, and many patients remain uninformed. Collins et al.4 reported that, before initiation of a standardized protocol, only 17.7% of incidental findings were communicated to patients admitted to the trauma service; after protocol initiation, the rate increased to 32.4%. The hospital discharge summary provides an opportunity to communicate incidental findings to patients and their medical care providers, but Kripalani et al.5 raised questions regarding the current completeness and accuracy of discharge summaries, reporting that 65% of discharge summaries omitted relevant diagnostic testing, and 30% omitted a follow-up plan.
We conducted a study to determine how often incidental pulmonary nodules found on abdominal CT are documented in the discharge summary, and to identify factors associated with pulmonary nodule inclusion.
METHODS
This was a retrospective cohort study of hospitalized patients ≥35 years of age who underwent in-patient abdominal CT between January 1, 2012 and December 31, 2014. Patients were identified by cross-referencing hospital admissions with Current Procedural Terminology (CPT) codes indicating abdominal CT (74176, 74177, 74178, 74160, 74150, 74170). Patients with chest CT (CPT codes 71260, 71250, 71270) during that hospitalization or within 30 days before admission were excluded to ensure that pulmonary nodules were incidental and asymptomatic. The index hospitalization was defined as the first hospitalization during which the patient was diagnosed with an incidental pulmonary nodule on abdominal CT, or the first hospitalization during the study period for patients without pulmonary nodules. All patient charts were manually reviewed, and baseline age, sex, and smoking status data collected.
Radiology reports were electronically screened for the words nodule and nodules and then confirmed through manual review of the full text reports. Nodules described as tiny (without other size description) were assumed to be <4 mm in size, per manual review of a small sample. Nodules were deemed as falling outside the Fleischner Society criteria guidelines (designed for indeterminate pulmonary nodules), and were therefore excluded, if any of seven criteria were met: The nodule was (1) cavitary, (2) associated with a known metastatic disease, (3) associated with a known granulomatous disease, (4) associated with a known inflammatory process, (5) reported likely to represent atelectasis, (6) reported likely to be a lymph node, or (7) previously biopsied.4
For each patient with pulmonary nodules, a personal history of cancer was obtained. Nodule size, characteristics, and stability compared with available prior imaging were recorded. Radiology reports were reviewed to determine if pulmonary nodules were mentioned in the summary headings of the reports or in the body of the reports and whether specific follow-up recommendations were provided. Hospital discharge summaries were reviewed for documentation of pulmonary nodule(s) and follow-up recommendations. Discharging service (medical/medical subspecialty, surgical/surgical subspecialty) was noted, along with the patients’ condition at discharge (alive, alive on hospice, deceased).
The frequency of incidental pulmonary nodules on abdominal CT during hospitalization and the frequency of nodules requiring follow-up were reported using a point estimate and corresponding 95% confidence interval (CI). The χ2 test was used to compare the frequency of pulmonary nodules across patient groups. In addition, for patients found to have incidental nodules requiring follow-up, the χ2 test was used to compare across groups the percentage of patients with discharge documentation of the incidental nodule. In all cases, 2-tailed Ps are reported, with P ≤ 0.05 considered statistically significant.
RESULTS
Between January 1, 2012 and December 31, 2014, 7173 patients ≥35 years old underwent in-patient abdominal CT without concurrent chest CT. Of these patients, 62.2% were ≥60 years old, 50.6% were men, and 45.5% were current or former smokers. Incidental pulmonary nodules were noted in 402 patients (5.6%; 95% CI, 5.1%-6.2%), of whom 68.7% were ≥60 years old, 56.5% were men, and 46.3% were current or former smokers. Increasing age (P = 0.004) and male sex (P = 0.015) were associated with increased frequency of incidental pulmonary nodules, but smoking status (P = 0.586) was not. Of patients with incidental nodules, 71.6% had solitary nodules, and 58.5% had a maximum nodule size of ≤4 mm (Table 1). Based on smoking status, nodule size, and reported size stability, 208 patients (2.9%; 95% CI, 2.5%-3.3%) required follow-up surveillance as per 2005 Fleischner Society guidelines. Among solitary pulmonary nodules requiring further surveillance (n = 147), the mean risk of malignancy based on the Mayo Clinic solitary pulmonary nodule risk calculator was 7.9% (interquartile range, 3.0%-10.5%), with 28% having a malignancy risk of ≥10%.6
Of the 208 patients with nodules requiring further surveillance, only 48 (23%) received discharge summaries documenting the nodule; 34 of these summaries included a recommendation for nodule follow-up, with 19 of the recommendations including a time frame for repeat CT. Three factors were positively associated with documentation of the pulmonary nodule in the discharge summary: mention of the pulmonary nodule in the summary headings of the radiology report (P < 0.001), radiologist recommendation for further surveillance (P < 0.001), and medical discharging service (P = 0.016) (Table 2). The highest rate of pulmonary nodule inclusion in the discharge summary (42%) was noted among patients for whom the radiology report included specific recommendations.
DISCUSSION
The frequency of incidental pulmonary nodules reported on abdominal CT in our study (5.6%) is consistent with frequencies reported in similar studies. Wu et al.7 (reviewing 141,406 abdominal CT scans) and Alpert et al.8 (reviewing 12,287 abdominal CT scans) reported frequencies of 2.5% and 3%, respectively, while Rinaldi et al.3 (reviewing 243 abdominal CT scans) reported a higher frequency, 8.4%. Variation likely results from patient factors and the individual radiologist’s attention to incidental pulmonary findings. Rinaldi et al. suggested that up to 39% of abdominal CT scans include pulmonary nodules on independent review, raising the possibility of significant underreporting. In our study, we focused on pulmonary nodules included in the radiology report to tailor the relevance of our study to the hospital medicine community. We also included only those incidental nodules falling within the purview of the Fleischner Society criteria in order to analyze only findings with established follow-up guidelines.
The rate of pulmonary nodule documentation in our study was low overall (23%) but consistent with the literature. Collins et al.,4 for example, reported that only 17.7% of patients with trauma were notified of incidental CT findings by either the discharge summary or an appropriate specialist consultation. Various contributing factors can be hypothesized. First, incidental pulmonary nodules are discovered largely in the context of evaluation for other symptomatic conditions, which can overshadow their importance. Second, the lack of clear patient-friendly education materials regarding incidental pulmonary nodules can complicate discussions with patients. Third, many electronic health record (EHR) systems cannot automatically pull incidental findings into the discharge summary and instead rely on provider vigilance.
As our study does, the literature highlights the importance of the radiology report in communicating incidental findings. In a review of >1000 pulmonary angiographic CT studies, Blagev et al.9 reported an overall follow-up rate of 29% (28/96) among patients with incidental pulmonary nodules, but none of the 12 patients with pulmonary nodules mentioned in the body of the report (rather than in the summary headings) received adequate follow-up. Similarly, in Shuaib et al.,10 radiology reports that included follow-up recommendations were more likely to change patient treatment than reports without follow-up recommendations (70% vs 2%). However, our data also show that radiologist recommendations alone are insufficient to ensure adequate communication of incidental findings.
The literature regarding the most cost-effective means of addressing this quality gap is limited. Some institutions have integrated their EHR systems to allow radiologists to flag incidental findings for auto-population in a dedicated section of the discharge summary. Although these efforts can be helpful, documentation alone does not save lives without appropriate follow-up and intervention. Some institutions have hired dedicated nursing staff as incidental finding coordinators. For high-risk incidental findings, Sperry et al.11 reported that hiring an incidental findings coordinator helped their level I trauma center achieve nearly complete documentation, patient notification, and confirmation of posthospital follow-up appointments. Such solutions, however, are labor-intensive and still rely on appropriate primary care follow-up.
Strengths of our study include its relatively large size and particular focus on the issues and decisions facing hospital medicine providers. By focusing on incidental pulmonary nodules reported on abdominal CT, and excluding patients with concurrent chest CT, we avoided including patients with symptomatic or previously identified pulmonary findings. Study limitations include the cross-sectional, retrospective design, which did not include follow-up data regarding such outcomes as rates of appropriate follow-up surveillance and subsequent lung cancer diagnoses. Our single-center study findings may not apply to all hospital practice settings, though they are consistent with the literature with comparison data.
Our study results highlight the need for a multidisciplinary systems-based approach to incidental pulmonary nodule documentation, communication, and follow-up surveillance.
Disclosure
Nothing to report.
1. Armao D, Smith JK. Overuse of computed tomography and the onslaught of incidental findings. N C Med J. 2014;75(2):127. PubMed
2. Gould MK, Tang T, Liu IL, et al. Recent trends in the identification of incidental pulmonary nodules. Am J Respir Crit Care Med. 2015;192(10):1208-1214. PubMed
3. Rinaldi MF, Bartalena T, Giannelli G, et al. Incidental lung nodules on CT examinations of the abdomen: prevalence and reporting rates in the PACS era. Eur J Radiol. 2010;74(3):e84-e88. PubMed
4. Collins CE, Cherng N, McDade T, et al. Improving patient notification of solid abdominal viscera incidental findings with a standardized protocol. J Trauma Manag Outcomes. 2015;9(1):1. PubMed
5. Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital-based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831-841. PubMed
6. Swensen SJ, Silverstein MD, Ilstrup DM, Schleck CD, Edell ES. The probability of malignancy in solitary pulmonary nodules. Application to small radiologically indeterminate nodules. Arch Intern Med. 1997;157(8):849-855. PubMed
7. Wu CC, Cronin CG, Chu JT, et al. Incidental pulmonary nodules detected on abdominal computed tomography. J Comput Assist Tomogr. 2012;36(6):641-645. PubMed
8. Alpert JB, Fantauzzi JP, Melamud K, Greenwood H, Naidich DP, Ko JP. Clinical significance of lung nodules reported on abdominal CT. AJR Am J Roentgenol. 2012;198(4):793-799. PubMed
9. Blagev DP, Lloyd JF, Conner K, et al. Follow-up of incidental pulmonary nodules and the radiology report. J Am Coll Radiol. 2014;11(4):378-383. PubMed
10. Shuaib W, Johnson JO, Salastekar N, Maddu KK, Khosa F. Incidental findings detected on abdomino-pelvic multidetector computed tomography performed in the acute setting [published correction appears in Am J Emerg Med. 2014;32(7):811. Waqas, Shuaib (corrected to Shuaib, Waqas)]. Am J Emerg Med. 2014;32(1):36-39. PubMed
11. Sperry JL, Massaro MS, Collage RD, et al. Incidental radiographic findings after injury: dedicated attention results in improved capture, documentation, and management. Surgery. 2010;148(4):618-624. PubMed
Incidental findings create both medical and logistical challenges regarding communication.1,2 Pulmonary nodules are among the most frequent and medically relevant incidental findings, being noted in up to 8.4% of abdominal computed tomography (CT) scans.3 There are guidelines regarding proper follow-up and management of such incidental pulmonary nodules, but appropriate evidence-based surveillance imaging is often not performed, and many patients remain uninformed. Collins et al.4 reported that, before initiation of a standardized protocol, only 17.7% of incidental findings were communicated to patients admitted to the trauma service; after protocol initiation, the rate increased to 32.4%. The hospital discharge summary provides an opportunity to communicate incidental findings to patients and their medical care providers, but Kripalani et al.5 raised questions regarding the current completeness and accuracy of discharge summaries, reporting that 65% of discharge summaries omitted relevant diagnostic testing, and 30% omitted a follow-up plan.
We conducted a study to determine how often incidental pulmonary nodules found on abdominal CT are documented in the discharge summary, and to identify factors associated with pulmonary nodule inclusion.
METHODS
This was a retrospective cohort study of hospitalized patients ≥35 years of age who underwent in-patient abdominal CT between January 1, 2012 and December 31, 2014. Patients were identified by cross-referencing hospital admissions with Current Procedural Terminology (CPT) codes indicating abdominal CT (74176, 74177, 74178, 74160, 74150, 74170). Patients with chest CT (CPT codes 71260, 71250, 71270) during that hospitalization or within 30 days before admission were excluded to ensure that pulmonary nodules were incidental and asymptomatic. The index hospitalization was defined as the first hospitalization during which the patient was diagnosed with an incidental pulmonary nodule on abdominal CT, or the first hospitalization during the study period for patients without pulmonary nodules. All patient charts were manually reviewed, and baseline age, sex, and smoking status data collected.
Radiology reports were electronically screened for the words nodule and nodules and then confirmed through manual review of the full text reports. Nodules described as tiny (without other size description) were assumed to be <4 mm in size, per manual review of a small sample. Nodules were deemed as falling outside the Fleischner Society criteria guidelines (designed for indeterminate pulmonary nodules), and were therefore excluded, if any of seven criteria were met: The nodule was (1) cavitary, (2) associated with a known metastatic disease, (3) associated with a known granulomatous disease, (4) associated with a known inflammatory process, (5) reported likely to represent atelectasis, (6) reported likely to be a lymph node, or (7) previously biopsied.4
For each patient with pulmonary nodules, a personal history of cancer was obtained. Nodule size, characteristics, and stability compared with available prior imaging were recorded. Radiology reports were reviewed to determine if pulmonary nodules were mentioned in the summary headings of the reports or in the body of the reports and whether specific follow-up recommendations were provided. Hospital discharge summaries were reviewed for documentation of pulmonary nodule(s) and follow-up recommendations. Discharging service (medical/medical subspecialty, surgical/surgical subspecialty) was noted, along with the patients’ condition at discharge (alive, alive on hospice, deceased).
The frequency of incidental pulmonary nodules on abdominal CT during hospitalization and the frequency of nodules requiring follow-up were reported using a point estimate and corresponding 95% confidence interval (CI). The χ2 test was used to compare the frequency of pulmonary nodules across patient groups. In addition, for patients found to have incidental nodules requiring follow-up, the χ2 test was used to compare across groups the percentage of patients with discharge documentation of the incidental nodule. In all cases, 2-tailed Ps are reported, with P ≤ 0.05 considered statistically significant.
RESULTS
Between January 1, 2012 and December 31, 2014, 7173 patients ≥35 years old underwent in-patient abdominal CT without concurrent chest CT. Of these patients, 62.2% were ≥60 years old, 50.6% were men, and 45.5% were current or former smokers. Incidental pulmonary nodules were noted in 402 patients (5.6%; 95% CI, 5.1%-6.2%), of whom 68.7% were ≥60 years old, 56.5% were men, and 46.3% were current or former smokers. Increasing age (P = 0.004) and male sex (P = 0.015) were associated with increased frequency of incidental pulmonary nodules, but smoking status (P = 0.586) was not. Of patients with incidental nodules, 71.6% had solitary nodules, and 58.5% had a maximum nodule size of ≤4 mm (Table 1). Based on smoking status, nodule size, and reported size stability, 208 patients (2.9%; 95% CI, 2.5%-3.3%) required follow-up surveillance as per 2005 Fleischner Society guidelines. Among solitary pulmonary nodules requiring further surveillance (n = 147), the mean risk of malignancy based on the Mayo Clinic solitary pulmonary nodule risk calculator was 7.9% (interquartile range, 3.0%-10.5%), with 28% having a malignancy risk of ≥10%.6
Of the 208 patients with nodules requiring further surveillance, only 48 (23%) received discharge summaries documenting the nodule; 34 of these summaries included a recommendation for nodule follow-up, with 19 of the recommendations including a time frame for repeat CT. Three factors were positively associated with documentation of the pulmonary nodule in the discharge summary: mention of the pulmonary nodule in the summary headings of the radiology report (P < 0.001), radiologist recommendation for further surveillance (P < 0.001), and medical discharging service (P = 0.016) (Table 2). The highest rate of pulmonary nodule inclusion in the discharge summary (42%) was noted among patients for whom the radiology report included specific recommendations.
DISCUSSION
The frequency of incidental pulmonary nodules reported on abdominal CT in our study (5.6%) is consistent with frequencies reported in similar studies. Wu et al.7 (reviewing 141,406 abdominal CT scans) and Alpert et al.8 (reviewing 12,287 abdominal CT scans) reported frequencies of 2.5% and 3%, respectively, while Rinaldi et al.3 (reviewing 243 abdominal CT scans) reported a higher frequency, 8.4%. Variation likely results from patient factors and the individual radiologist’s attention to incidental pulmonary findings. Rinaldi et al. suggested that up to 39% of abdominal CT scans include pulmonary nodules on independent review, raising the possibility of significant underreporting. In our study, we focused on pulmonary nodules included in the radiology report to tailor the relevance of our study to the hospital medicine community. We also included only those incidental nodules falling within the purview of the Fleischner Society criteria in order to analyze only findings with established follow-up guidelines.
The rate of pulmonary nodule documentation in our study was low overall (23%) but consistent with the literature. Collins et al.,4 for example, reported that only 17.7% of patients with trauma were notified of incidental CT findings by either the discharge summary or an appropriate specialist consultation. Various contributing factors can be hypothesized. First, incidental pulmonary nodules are discovered largely in the context of evaluation for other symptomatic conditions, which can overshadow their importance. Second, the lack of clear patient-friendly education materials regarding incidental pulmonary nodules can complicate discussions with patients. Third, many electronic health record (EHR) systems cannot automatically pull incidental findings into the discharge summary and instead rely on provider vigilance.
As our study does, the literature highlights the importance of the radiology report in communicating incidental findings. In a review of >1000 pulmonary angiographic CT studies, Blagev et al.9 reported an overall follow-up rate of 29% (28/96) among patients with incidental pulmonary nodules, but none of the 12 patients with pulmonary nodules mentioned in the body of the report (rather than in the summary headings) received adequate follow-up. Similarly, in Shuaib et al.,10 radiology reports that included follow-up recommendations were more likely to change patient treatment than reports without follow-up recommendations (70% vs 2%). However, our data also show that radiologist recommendations alone are insufficient to ensure adequate communication of incidental findings.
The literature regarding the most cost-effective means of addressing this quality gap is limited. Some institutions have integrated their EHR systems to allow radiologists to flag incidental findings for auto-population in a dedicated section of the discharge summary. Although these efforts can be helpful, documentation alone does not save lives without appropriate follow-up and intervention. Some institutions have hired dedicated nursing staff as incidental finding coordinators. For high-risk incidental findings, Sperry et al.11 reported that hiring an incidental findings coordinator helped their level I trauma center achieve nearly complete documentation, patient notification, and confirmation of posthospital follow-up appointments. Such solutions, however, are labor-intensive and still rely on appropriate primary care follow-up.
Strengths of our study include its relatively large size and particular focus on the issues and decisions facing hospital medicine providers. By focusing on incidental pulmonary nodules reported on abdominal CT, and excluding patients with concurrent chest CT, we avoided including patients with symptomatic or previously identified pulmonary findings. Study limitations include the cross-sectional, retrospective design, which did not include follow-up data regarding such outcomes as rates of appropriate follow-up surveillance and subsequent lung cancer diagnoses. Our single-center study findings may not apply to all hospital practice settings, though they are consistent with the literature with comparison data.
Our study results highlight the need for a multidisciplinary systems-based approach to incidental pulmonary nodule documentation, communication, and follow-up surveillance.
Disclosure
Nothing to report.
Incidental findings create both medical and logistical challenges regarding communication.1,2 Pulmonary nodules are among the most frequent and medically relevant incidental findings, being noted in up to 8.4% of abdominal computed tomography (CT) scans.3 There are guidelines regarding proper follow-up and management of such incidental pulmonary nodules, but appropriate evidence-based surveillance imaging is often not performed, and many patients remain uninformed. Collins et al.4 reported that, before initiation of a standardized protocol, only 17.7% of incidental findings were communicated to patients admitted to the trauma service; after protocol initiation, the rate increased to 32.4%. The hospital discharge summary provides an opportunity to communicate incidental findings to patients and their medical care providers, but Kripalani et al.5 raised questions regarding the current completeness and accuracy of discharge summaries, reporting that 65% of discharge summaries omitted relevant diagnostic testing, and 30% omitted a follow-up plan.
We conducted a study to determine how often incidental pulmonary nodules found on abdominal CT are documented in the discharge summary, and to identify factors associated with pulmonary nodule inclusion.
METHODS
This was a retrospective cohort study of hospitalized patients ≥35 years of age who underwent in-patient abdominal CT between January 1, 2012 and December 31, 2014. Patients were identified by cross-referencing hospital admissions with Current Procedural Terminology (CPT) codes indicating abdominal CT (74176, 74177, 74178, 74160, 74150, 74170). Patients with chest CT (CPT codes 71260, 71250, 71270) during that hospitalization or within 30 days before admission were excluded to ensure that pulmonary nodules were incidental and asymptomatic. The index hospitalization was defined as the first hospitalization during which the patient was diagnosed with an incidental pulmonary nodule on abdominal CT, or the first hospitalization during the study period for patients without pulmonary nodules. All patient charts were manually reviewed, and baseline age, sex, and smoking status data collected.
Radiology reports were electronically screened for the words nodule and nodules and then confirmed through manual review of the full text reports. Nodules described as tiny (without other size description) were assumed to be <4 mm in size, per manual review of a small sample. Nodules were deemed as falling outside the Fleischner Society criteria guidelines (designed for indeterminate pulmonary nodules), and were therefore excluded, if any of seven criteria were met: The nodule was (1) cavitary, (2) associated with a known metastatic disease, (3) associated with a known granulomatous disease, (4) associated with a known inflammatory process, (5) reported likely to represent atelectasis, (6) reported likely to be a lymph node, or (7) previously biopsied.4
For each patient with pulmonary nodules, a personal history of cancer was obtained. Nodule size, characteristics, and stability compared with available prior imaging were recorded. Radiology reports were reviewed to determine if pulmonary nodules were mentioned in the summary headings of the reports or in the body of the reports and whether specific follow-up recommendations were provided. Hospital discharge summaries were reviewed for documentation of pulmonary nodule(s) and follow-up recommendations. Discharging service (medical/medical subspecialty, surgical/surgical subspecialty) was noted, along with the patients’ condition at discharge (alive, alive on hospice, deceased).
The frequency of incidental pulmonary nodules on abdominal CT during hospitalization and the frequency of nodules requiring follow-up were reported using a point estimate and corresponding 95% confidence interval (CI). The χ2 test was used to compare the frequency of pulmonary nodules across patient groups. In addition, for patients found to have incidental nodules requiring follow-up, the χ2 test was used to compare across groups the percentage of patients with discharge documentation of the incidental nodule. In all cases, 2-tailed Ps are reported, with P ≤ 0.05 considered statistically significant.
RESULTS
Between January 1, 2012 and December 31, 2014, 7173 patients ≥35 years old underwent in-patient abdominal CT without concurrent chest CT. Of these patients, 62.2% were ≥60 years old, 50.6% were men, and 45.5% were current or former smokers. Incidental pulmonary nodules were noted in 402 patients (5.6%; 95% CI, 5.1%-6.2%), of whom 68.7% were ≥60 years old, 56.5% were men, and 46.3% were current or former smokers. Increasing age (P = 0.004) and male sex (P = 0.015) were associated with increased frequency of incidental pulmonary nodules, but smoking status (P = 0.586) was not. Of patients with incidental nodules, 71.6% had solitary nodules, and 58.5% had a maximum nodule size of ≤4 mm (Table 1). Based on smoking status, nodule size, and reported size stability, 208 patients (2.9%; 95% CI, 2.5%-3.3%) required follow-up surveillance as per 2005 Fleischner Society guidelines. Among solitary pulmonary nodules requiring further surveillance (n = 147), the mean risk of malignancy based on the Mayo Clinic solitary pulmonary nodule risk calculator was 7.9% (interquartile range, 3.0%-10.5%), with 28% having a malignancy risk of ≥10%.6
Of the 208 patients with nodules requiring further surveillance, only 48 (23%) received discharge summaries documenting the nodule; 34 of these summaries included a recommendation for nodule follow-up, with 19 of the recommendations including a time frame for repeat CT. Three factors were positively associated with documentation of the pulmonary nodule in the discharge summary: mention of the pulmonary nodule in the summary headings of the radiology report (P < 0.001), radiologist recommendation for further surveillance (P < 0.001), and medical discharging service (P = 0.016) (Table 2). The highest rate of pulmonary nodule inclusion in the discharge summary (42%) was noted among patients for whom the radiology report included specific recommendations.
DISCUSSION
The frequency of incidental pulmonary nodules reported on abdominal CT in our study (5.6%) is consistent with frequencies reported in similar studies. Wu et al.7 (reviewing 141,406 abdominal CT scans) and Alpert et al.8 (reviewing 12,287 abdominal CT scans) reported frequencies of 2.5% and 3%, respectively, while Rinaldi et al.3 (reviewing 243 abdominal CT scans) reported a higher frequency, 8.4%. Variation likely results from patient factors and the individual radiologist’s attention to incidental pulmonary findings. Rinaldi et al. suggested that up to 39% of abdominal CT scans include pulmonary nodules on independent review, raising the possibility of significant underreporting. In our study, we focused on pulmonary nodules included in the radiology report to tailor the relevance of our study to the hospital medicine community. We also included only those incidental nodules falling within the purview of the Fleischner Society criteria in order to analyze only findings with established follow-up guidelines.
The rate of pulmonary nodule documentation in our study was low overall (23%) but consistent with the literature. Collins et al.,4 for example, reported that only 17.7% of patients with trauma were notified of incidental CT findings by either the discharge summary or an appropriate specialist consultation. Various contributing factors can be hypothesized. First, incidental pulmonary nodules are discovered largely in the context of evaluation for other symptomatic conditions, which can overshadow their importance. Second, the lack of clear patient-friendly education materials regarding incidental pulmonary nodules can complicate discussions with patients. Third, many electronic health record (EHR) systems cannot automatically pull incidental findings into the discharge summary and instead rely on provider vigilance.
As our study does, the literature highlights the importance of the radiology report in communicating incidental findings. In a review of >1000 pulmonary angiographic CT studies, Blagev et al.9 reported an overall follow-up rate of 29% (28/96) among patients with incidental pulmonary nodules, but none of the 12 patients with pulmonary nodules mentioned in the body of the report (rather than in the summary headings) received adequate follow-up. Similarly, in Shuaib et al.,10 radiology reports that included follow-up recommendations were more likely to change patient treatment than reports without follow-up recommendations (70% vs 2%). However, our data also show that radiologist recommendations alone are insufficient to ensure adequate communication of incidental findings.
The literature regarding the most cost-effective means of addressing this quality gap is limited. Some institutions have integrated their EHR systems to allow radiologists to flag incidental findings for auto-population in a dedicated section of the discharge summary. Although these efforts can be helpful, documentation alone does not save lives without appropriate follow-up and intervention. Some institutions have hired dedicated nursing staff as incidental finding coordinators. For high-risk incidental findings, Sperry et al.11 reported that hiring an incidental findings coordinator helped their level I trauma center achieve nearly complete documentation, patient notification, and confirmation of posthospital follow-up appointments. Such solutions, however, are labor-intensive and still rely on appropriate primary care follow-up.
Strengths of our study include its relatively large size and particular focus on the issues and decisions facing hospital medicine providers. By focusing on incidental pulmonary nodules reported on abdominal CT, and excluding patients with concurrent chest CT, we avoided including patients with symptomatic or previously identified pulmonary findings. Study limitations include the cross-sectional, retrospective design, which did not include follow-up data regarding such outcomes as rates of appropriate follow-up surveillance and subsequent lung cancer diagnoses. Our single-center study findings may not apply to all hospital practice settings, though they are consistent with the literature with comparison data.
Our study results highlight the need for a multidisciplinary systems-based approach to incidental pulmonary nodule documentation, communication, and follow-up surveillance.
Disclosure
Nothing to report.
1. Armao D, Smith JK. Overuse of computed tomography and the onslaught of incidental findings. N C Med J. 2014;75(2):127. PubMed
2. Gould MK, Tang T, Liu IL, et al. Recent trends in the identification of incidental pulmonary nodules. Am J Respir Crit Care Med. 2015;192(10):1208-1214. PubMed
3. Rinaldi MF, Bartalena T, Giannelli G, et al. Incidental lung nodules on CT examinations of the abdomen: prevalence and reporting rates in the PACS era. Eur J Radiol. 2010;74(3):e84-e88. PubMed
4. Collins CE, Cherng N, McDade T, et al. Improving patient notification of solid abdominal viscera incidental findings with a standardized protocol. J Trauma Manag Outcomes. 2015;9(1):1. PubMed
5. Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital-based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831-841. PubMed
6. Swensen SJ, Silverstein MD, Ilstrup DM, Schleck CD, Edell ES. The probability of malignancy in solitary pulmonary nodules. Application to small radiologically indeterminate nodules. Arch Intern Med. 1997;157(8):849-855. PubMed
7. Wu CC, Cronin CG, Chu JT, et al. Incidental pulmonary nodules detected on abdominal computed tomography. J Comput Assist Tomogr. 2012;36(6):641-645. PubMed
8. Alpert JB, Fantauzzi JP, Melamud K, Greenwood H, Naidich DP, Ko JP. Clinical significance of lung nodules reported on abdominal CT. AJR Am J Roentgenol. 2012;198(4):793-799. PubMed
9. Blagev DP, Lloyd JF, Conner K, et al. Follow-up of incidental pulmonary nodules and the radiology report. J Am Coll Radiol. 2014;11(4):378-383. PubMed
10. Shuaib W, Johnson JO, Salastekar N, Maddu KK, Khosa F. Incidental findings detected on abdomino-pelvic multidetector computed tomography performed in the acute setting [published correction appears in Am J Emerg Med. 2014;32(7):811. Waqas, Shuaib (corrected to Shuaib, Waqas)]. Am J Emerg Med. 2014;32(1):36-39. PubMed
11. Sperry JL, Massaro MS, Collage RD, et al. Incidental radiographic findings after injury: dedicated attention results in improved capture, documentation, and management. Surgery. 2010;148(4):618-624. PubMed
1. Armao D, Smith JK. Overuse of computed tomography and the onslaught of incidental findings. N C Med J. 2014;75(2):127. PubMed
2. Gould MK, Tang T, Liu IL, et al. Recent trends in the identification of incidental pulmonary nodules. Am J Respir Crit Care Med. 2015;192(10):1208-1214. PubMed
3. Rinaldi MF, Bartalena T, Giannelli G, et al. Incidental lung nodules on CT examinations of the abdomen: prevalence and reporting rates in the PACS era. Eur J Radiol. 2010;74(3):e84-e88. PubMed
4. Collins CE, Cherng N, McDade T, et al. Improving patient notification of solid abdominal viscera incidental findings with a standardized protocol. J Trauma Manag Outcomes. 2015;9(1):1. PubMed
5. Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital-based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831-841. PubMed
6. Swensen SJ, Silverstein MD, Ilstrup DM, Schleck CD, Edell ES. The probability of malignancy in solitary pulmonary nodules. Application to small radiologically indeterminate nodules. Arch Intern Med. 1997;157(8):849-855. PubMed
7. Wu CC, Cronin CG, Chu JT, et al. Incidental pulmonary nodules detected on abdominal computed tomography. J Comput Assist Tomogr. 2012;36(6):641-645. PubMed
8. Alpert JB, Fantauzzi JP, Melamud K, Greenwood H, Naidich DP, Ko JP. Clinical significance of lung nodules reported on abdominal CT. AJR Am J Roentgenol. 2012;198(4):793-799. PubMed
9. Blagev DP, Lloyd JF, Conner K, et al. Follow-up of incidental pulmonary nodules and the radiology report. J Am Coll Radiol. 2014;11(4):378-383. PubMed
10. Shuaib W, Johnson JO, Salastekar N, Maddu KK, Khosa F. Incidental findings detected on abdomino-pelvic multidetector computed tomography performed in the acute setting [published correction appears in Am J Emerg Med. 2014;32(7):811. Waqas, Shuaib (corrected to Shuaib, Waqas)]. Am J Emerg Med. 2014;32(1):36-39. PubMed
11. Sperry JL, Massaro MS, Collage RD, et al. Incidental radiographic findings after injury: dedicated attention results in improved capture, documentation, and management. Surgery. 2010;148(4):618-624. PubMed
© 2017 Society of Hospital Medicine
Empiric <i>Listeria monocytogenes</i> antibiotic coverage for febrile infants (age, 0-90 days)
The “Things We Do for No Reason” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

Evaluation and treatment of the febrile infant 0 to 90 days of age are common clinical issues in pediatrics, family medicine, emergency medicine, and pediatric hospital medicine. Traditional teaching has been that Listeria monocytogenes is 1 of the 3 most common pathogens causing neonatal sepsis. Many practitioners routinely use antibiotic regimens, including ampicillin, to specifically target Listeria. However, a large body of evidence, including a meta-analysis and several multicenter studies, has shown that listeriosis is extremely rare in the United States. The practice of empiric ampicillin thus exposes the patient to harms and costs with little if any potential benefit, while increasing pressure on the bacterial flora in the community to generate antibiotic resistance. Empiric ampicillin for all infants admitted for sepsis evaluation is a tradition-based practice no longer founded on the best available evidence.
CASE REPORT
A 32-day-old, full-term, previously healthy girl presented with fever of 1 day’s duration. Her parents reported she had appeared well until the evening before admission, when she became a bit less active and spent less time breastfeeding. The morning of admission, she was fussier than usual. Rectal temperature, taken by her parents, was 101°F. There were no other symptoms and no sick contacts.
On examination, the patient’s rectal temperature was 101.5°F. Her other vitals and the physical examination findings were unremarkable. Laboratory test results included a normal urinalysis and a peripheral white blood cell (WBC) count of 21,300 cells/µL. Cerebrospinal fluid (CSF) analysis revealed normal protein and glucose levels with 3 WBCs/µL and a negative gram stain. Due to stratifying at higher risk for serious bacterial infection (SBI), the child was admitted and started on ampicillin and cefotaxime while awaiting culture results.
BACKGROUND
Evaluation and treatment of febrile infants are common clinical issues in pediatrics, emergency medicine, and general practice. Practice guidelines for evaluation of febrile infants recommend hospitalization and parenteral antibiotics for children younger than 28 days and children 29 to 90 days old if stratified at high risk for SBI.1,2 Recommendations for empiric antibiotic regimens include ampicillin in addition to either gentamicin or cefotaxime.1,2
WHY YOU MIGHT THINK AMPICILLIN IS HELPFUL
Generations of pediatrics students have been taught that the 3 pathogens most likely to cause bacterial sepsis in infants are group B Streptococcus (GBS), Escherichia coli, and Listeria monocytogenes. This teaching is still espoused in the latest editions of pediatrics textbooks.3 Ampicillin is specifically recommended for covering Listeria, and studies have found that 62% to 78% of practitioners choose empiric ampicillin-containing antibiotic regimens for the treatment of febrile infants.4-6
WHY EMPIRIC AMPICILLIN IS UNNECESSARY
In the past, Listeria was a potential though still uncommon infant pathogen. Over the past few decades, however, the epidemiology of infant sepsis has changed significantly. Estimates of the rate of infection with Listeria now range from extremely rare to nonexistent across multiple studies4,7-15 (Table). In a 4-year retrospective case series at a single urban academic center in Washington, DC, Sadow et al.4 reported no instances of Listeria among 121 positive bacterial cultures in infants younger than 60 days seen in the emergency department (ED). Byington et al.7 examined all positive cultures for infants 0 to 90 days old at a large academic referral center in Utah over a 3-year period and reported no cases of Listeria (1298 patients, 105 SBI cases). A study at a North Carolina academic center found 1 case of Listeria meningitis among 72 SBIs (668 febrile infants) without a localizing source.8 At a large group-practice in northern California, Greenhow et al.9 examined all blood cultures (N = 4255) performed over 4 years for otherwise healthy infants 1 week to 3 months old and found no cases of Listeria. In a follow-up study, the same authors examined all blood (n = 5396), urine (n = 4599), and CSF (n = 1796) cultures in the same population and found no Listeria cases.10 Hassoun et al.11 studied SBI rates among infants younger than 28 days with any blood, urine, or CSF culture performed over 4 years at two Michigan EDs. One (0.08%) of the 1192 infants evaluated had bacteremia caused by Listeria.
Multicenter studies have reported similar results. In a study of 6 hospital systems in geographically diverse areas of the United States, Biondi et al.12 examined all positive blood cultures (N = 181) for febrile infants younger than 90 days admitted to a general pediatric ward, and found no listeriosis. Mischler et al.13 examined all positive blood cultures (N = 392) for otherwise healthy febrile infants 0 to 90 days old admitted to a hospital in 1 of 17 geographically diverse healthcare systems and found no cases of Listeria. A recent meta-analysis of studies that reported SBI rates for febrile infants 0 to 90 days old found the weighted prevalence of Listeria bacteremia to be 0.03% (2/20,703) and that of meningitis to be 0.02% (3/13,375).14 Veesenmeyer and Edmonson15 used a national inpatient database to identify all Listeria cases among infants over a 6-year period and estimated listeriosis rates for the US population. Over the 6 years, there were 212 total cases, which were extrapolated to 344 in the United States during that period, yielding a pooled annual incidence rate of 1.41 in 100,000 births.
Ampicillin offers no significant improvement in coverage for GBS or E coli beyond other β-lactam antibiotics, such as cefotaxime. Therefore, though the cost and potential harms of 24 to 48 hours of intravenous ampicillin are low for the individual patient, there is almost no potential benefit. Using the weighted prevalence of 0.03% for Listeria bacteremia reported in the recent meta-analysis,14 the number needed to treat to cover 1 case of Listeria bacteremia would be 3333. In addition, the increasing incidence of ampicillin resistance, particularly among gram-negative organisms,4,7,9 argues strongly for better antibiotic stewardship on a national level. A number of expert authors have advocated dropping empiric Listeria coverage as part of the treatment of febrile infants, particularly infants 29 to 90 days old.16,17 Some authors continue to advocate empiric Listeria coverage.6 It is interesting to note, however, that the incidence of Staph aureus bacteremia in recent case series is much higher than that reported for Listeria, accounting for 6-9% of bacteremia cases.9,11,13 Yet few if any authors advocate for empiric S. aureus coverage.
WHEN EMPIRIC AMPICILLIN COVERAGE MAY BE REASONABLE
The rate of listeriosis remains low across age groups in recent studies, but the rate is slightly higher in very young infants. In the recent national database study of listeriosis cases over a 6-year period, almost half involved infants younger than 7 days, and most of these infants showed no evidence of meningitis.15 Therefore, it may be reasonable to include empiric Listeria coverage in febrile infants younger than 7 days, though the study authors estimated 22.5 annual cases of Listeria in this age range in the United States. Eighty percent of the Listeria cases were in infants younger than 28 days, but more than 85% of infants 7 to 28 days old had meningitis. Therefore, broad antimicrobial coverage for infants with CSF pleocytosis and/or a high bacterial meningitis score is reasonable, especially for infants younger than 28 days.
Other potential indications for ampicillin are enterococcal infections. Though enteroccocal SBI rates in febrile infants are also quite low,7-9,11,12 if Enterococcus were highly suspected, such as in an infant with pyuria and gram positive organisms on gram stain, ampicillin offers good additional coverage. In the case of a local outbreak of listeriosis, or a specific exposure to Listeria-contaminated products on a patient history, antibiotics with efficacy against Listeria should be used. Last, in cases in which gentamicin is used as empiric coverage for gram-negative organisms, ampicillin offers important additional coverage for GBS.
Some practitioners advocate ampicillin and gentamicin over cefotaxime regimens on the basis of an often cited study that found a survival benefit for febrile neonates in the intensive care setting.18 There are a number of reasons that this study should not influence care for typical infants admitted with possible sepsis. First, the study was retrospective and limited by its use of administrative data. The authors acknowledged that a potential explanation for their results is unmeasured confounding. Second, the patients included in the study were dramatically different from the group of well infants admitted with possible sepsis; the study included neonatal critical care unit patients treated with antibiotics within the first 3 days of life. Third, the study’s results have not been replicated in otherwise healthy febrile infants.
WHAT YOU SHOULD USE INSTEAD OF AMPICILLIN FOR EMPIRIC LISTERIA COVERAGE
For febrile children 0 to 90 days old, empiric antibiotic coverage should be aimed at covering the current predominant pathogens, which include E coli and GBS. Therefore, for most children and US regions, a third-generation cephalosporin (eg, cefotaxime) is sufficient.
RECOMMENDATIONS
- Empiric antibiotics for treatment of febrile children 0-90 days should target E. coli and GBS; a third generation cephalosporin, (e.g. cefotaxime) alone is a reasonable choice for most patients.
- Prescribing ampicillin to specifically cover Listeria is unnecessary for the vast majority of febrile infants
- Prescribing ampicillin is reasonable in certain subgroups of febrile infants: those less than seven days of age, those with evidence of bacterial meningitis (especially if also <28 days of age), those in whom enterococcal infection is strongly suspected, and those with specific Listeria exposures related to local outbreaks.
CONCLUSION
The 32-day-old infant described in the clinical scenario was at extremely low risk for listeriosis. Antibiotic coverage with a third-generation cephalosporin is sufficient for the most likely pathogens. The common practice of empirically covering Listeria in otherwise healthy febrile infants considered to be at higher risk for SBI is no longer based on best available evidence and represents overtreatment with at least theoretical harms. Avoidance of the risks associated with the side effects of antibiotics, costs saved by forgoing multiple antibiotics, a decrease in medication dosing frequency, and improved antibiotic stewardship for the general population all argue forcefully for making empiric Listeria coverage a thing of the past.
Disclosure
Nothing to report.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and Liking It on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
1. Baraff LJ, Bass JW, Fleisher GR, et al. Practice guideline for the management of infants and children 0 to 36 months of age with fever without source. Agency for Health Care Policy and Research. Ann Emerg Med. 1993;22(7):1198-1210. PubMed
2. American College of Emergency Physicians Clinical Policies Committee; American College of Emergency Physicians Clinical Policies Subcommittee on Pediatric Fever. Clinical policy for children younger than three years presenting to the emergency department with fever. Ann Emerg Med. 2003;42(4):530-545. PubMed
3. Nield L, Kamat D. Fever without a focus. In: Kliegman R, Stanton B, eds. Nelson’s Textbook of Pediatrics. 20th ed. Philadelphia, PA: Elsevier; 2016.
4. Sadow KB, Derr R, Teach SJ. Bacterial infections in infants 60 days and younger: epidemiology, resistance, and implications for treatment. Arch Pediatr Adolesc Med. 1999;153(6):611-614. PubMed
5. Aronson PL, Thurm C, Alpern ER, et al. Variation in care of the febrile young infant <90 days in US pediatric emergency departments. Pediatrics. 2014;134(4):667-677. PubMed
6. Cantey JB, Lopez-Medina E, Nguyen S, Doern C, Garcia C. Empiric antibiotics for serious bacterial infection in young infants: opportunities for stewardship. Pediatr Emerg Care. 2015;31(8):568-571. PubMed
7. Byington CL, Rittichier KK, Bassett KE, et al. Serious bacterial infections in febrile infants younger than 90 days of age: the importance of ampicillin-resistant pathogens. Pediatrics. 2003;111(5 pt 1):964-968. PubMed
8. Watt K, Waddle E, Jhaveri R. Changing epidemiology of serious bacterial infections in febrile infants without localizing signs. PLoS One. 2010;5(8):e12448. PubMed
9. Greenhow TL, Hung YY, Herz AM. Changing epidemiology of bacteremia in infants aged 1 week to 3 months. Pediatrics. 2012;129(3):e590-e596. PubMed
10. Greenhow TL, Hung YY, Herz AM, Losada E, Pantell RH. The changing epidemiology of serious bacterial infections in young infants. Pediatr Infect Dis J. 2014;33(6):595-599. PubMed
11. Hassoun A, Stankovic C, Rogers A, et al. Listeria and enterococcal infections in neonates 28 days of age and younger: is empiric parenteral ampicillin still indicated? Pediatr Emerg Care. 2014;30(4):240-243. PubMed
12. Biondi E, Evans R, Mischler M, et al. Epidemiology of bacteremia in febrile infants in the United States. Pediatrics. 2013;132(6):990-996. PubMed
13. Mischler M, Ryan MS, Leyenaar JK, et al. Epidemiology of bacteremia in previously healthy febrile infants: a follow-up study. Hosp Pediatr. 2015;5(6):293-300. PubMed
14. Leazer R, Perkins AM, Shomaker K, Fine B. A meta-analysis of the rates of Listeria monocytogenes and Enterococcus in febrile infants. Hosp Pediatr. 2016;6(4):187-195. PubMed
15. Veesenmeyer AF, Edmonson MB. Trends in US hospital stays for listeriosis in infants. Hosp Pediatr. 2016;6(4):196-203. PubMed
16. Schroeder AR, Roberts KB. Is tradition trumping evidence in the treatment of young, febrile infants? Hosp Pediatr. 2016;6(4):252-253. PubMed
17. Cioffredi LA, Jhaveri R. Evaluation and management of febrile children: a review. JAMA Pediatr. 2016;170(8):794-800. PubMed
18. Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Empiric use of ampicillin and cefotaxime, compared with ampicillin and gentamicin, for neonates at risk for sepsis is associated with an increased risk of neonatal death. Pediatrics. 2006;117(1):67-74. PubMed
The “Things We Do for No Reason” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

Evaluation and treatment of the febrile infant 0 to 90 days of age are common clinical issues in pediatrics, family medicine, emergency medicine, and pediatric hospital medicine. Traditional teaching has been that Listeria monocytogenes is 1 of the 3 most common pathogens causing neonatal sepsis. Many practitioners routinely use antibiotic regimens, including ampicillin, to specifically target Listeria. However, a large body of evidence, including a meta-analysis and several multicenter studies, has shown that listeriosis is extremely rare in the United States. The practice of empiric ampicillin thus exposes the patient to harms and costs with little if any potential benefit, while increasing pressure on the bacterial flora in the community to generate antibiotic resistance. Empiric ampicillin for all infants admitted for sepsis evaluation is a tradition-based practice no longer founded on the best available evidence.
CASE REPORT
A 32-day-old, full-term, previously healthy girl presented with fever of 1 day’s duration. Her parents reported she had appeared well until the evening before admission, when she became a bit less active and spent less time breastfeeding. The morning of admission, she was fussier than usual. Rectal temperature, taken by her parents, was 101°F. There were no other symptoms and no sick contacts.
On examination, the patient’s rectal temperature was 101.5°F. Her other vitals and the physical examination findings were unremarkable. Laboratory test results included a normal urinalysis and a peripheral white blood cell (WBC) count of 21,300 cells/µL. Cerebrospinal fluid (CSF) analysis revealed normal protein and glucose levels with 3 WBCs/µL and a negative gram stain. Due to stratifying at higher risk for serious bacterial infection (SBI), the child was admitted and started on ampicillin and cefotaxime while awaiting culture results.
BACKGROUND
Evaluation and treatment of febrile infants are common clinical issues in pediatrics, emergency medicine, and general practice. Practice guidelines for evaluation of febrile infants recommend hospitalization and parenteral antibiotics for children younger than 28 days and children 29 to 90 days old if stratified at high risk for SBI.1,2 Recommendations for empiric antibiotic regimens include ampicillin in addition to either gentamicin or cefotaxime.1,2
WHY YOU MIGHT THINK AMPICILLIN IS HELPFUL
Generations of pediatrics students have been taught that the 3 pathogens most likely to cause bacterial sepsis in infants are group B Streptococcus (GBS), Escherichia coli, and Listeria monocytogenes. This teaching is still espoused in the latest editions of pediatrics textbooks.3 Ampicillin is specifically recommended for covering Listeria, and studies have found that 62% to 78% of practitioners choose empiric ampicillin-containing antibiotic regimens for the treatment of febrile infants.4-6
WHY EMPIRIC AMPICILLIN IS UNNECESSARY
In the past, Listeria was a potential though still uncommon infant pathogen. Over the past few decades, however, the epidemiology of infant sepsis has changed significantly. Estimates of the rate of infection with Listeria now range from extremely rare to nonexistent across multiple studies4,7-15 (Table). In a 4-year retrospective case series at a single urban academic center in Washington, DC, Sadow et al.4 reported no instances of Listeria among 121 positive bacterial cultures in infants younger than 60 days seen in the emergency department (ED). Byington et al.7 examined all positive cultures for infants 0 to 90 days old at a large academic referral center in Utah over a 3-year period and reported no cases of Listeria (1298 patients, 105 SBI cases). A study at a North Carolina academic center found 1 case of Listeria meningitis among 72 SBIs (668 febrile infants) without a localizing source.8 At a large group-practice in northern California, Greenhow et al.9 examined all blood cultures (N = 4255) performed over 4 years for otherwise healthy infants 1 week to 3 months old and found no cases of Listeria. In a follow-up study, the same authors examined all blood (n = 5396), urine (n = 4599), and CSF (n = 1796) cultures in the same population and found no Listeria cases.10 Hassoun et al.11 studied SBI rates among infants younger than 28 days with any blood, urine, or CSF culture performed over 4 years at two Michigan EDs. One (0.08%) of the 1192 infants evaluated had bacteremia caused by Listeria.
Multicenter studies have reported similar results. In a study of 6 hospital systems in geographically diverse areas of the United States, Biondi et al.12 examined all positive blood cultures (N = 181) for febrile infants younger than 90 days admitted to a general pediatric ward, and found no listeriosis. Mischler et al.13 examined all positive blood cultures (N = 392) for otherwise healthy febrile infants 0 to 90 days old admitted to a hospital in 1 of 17 geographically diverse healthcare systems and found no cases of Listeria. A recent meta-analysis of studies that reported SBI rates for febrile infants 0 to 90 days old found the weighted prevalence of Listeria bacteremia to be 0.03% (2/20,703) and that of meningitis to be 0.02% (3/13,375).14 Veesenmeyer and Edmonson15 used a national inpatient database to identify all Listeria cases among infants over a 6-year period and estimated listeriosis rates for the US population. Over the 6 years, there were 212 total cases, which were extrapolated to 344 in the United States during that period, yielding a pooled annual incidence rate of 1.41 in 100,000 births.
Ampicillin offers no significant improvement in coverage for GBS or E coli beyond other β-lactam antibiotics, such as cefotaxime. Therefore, though the cost and potential harms of 24 to 48 hours of intravenous ampicillin are low for the individual patient, there is almost no potential benefit. Using the weighted prevalence of 0.03% for Listeria bacteremia reported in the recent meta-analysis,14 the number needed to treat to cover 1 case of Listeria bacteremia would be 3333. In addition, the increasing incidence of ampicillin resistance, particularly among gram-negative organisms,4,7,9 argues strongly for better antibiotic stewardship on a national level. A number of expert authors have advocated dropping empiric Listeria coverage as part of the treatment of febrile infants, particularly infants 29 to 90 days old.16,17 Some authors continue to advocate empiric Listeria coverage.6 It is interesting to note, however, that the incidence of Staph aureus bacteremia in recent case series is much higher than that reported for Listeria, accounting for 6-9% of bacteremia cases.9,11,13 Yet few if any authors advocate for empiric S. aureus coverage.
WHEN EMPIRIC AMPICILLIN COVERAGE MAY BE REASONABLE
The rate of listeriosis remains low across age groups in recent studies, but the rate is slightly higher in very young infants. In the recent national database study of listeriosis cases over a 6-year period, almost half involved infants younger than 7 days, and most of these infants showed no evidence of meningitis.15 Therefore, it may be reasonable to include empiric Listeria coverage in febrile infants younger than 7 days, though the study authors estimated 22.5 annual cases of Listeria in this age range in the United States. Eighty percent of the Listeria cases were in infants younger than 28 days, but more than 85% of infants 7 to 28 days old had meningitis. Therefore, broad antimicrobial coverage for infants with CSF pleocytosis and/or a high bacterial meningitis score is reasonable, especially for infants younger than 28 days.
Other potential indications for ampicillin are enterococcal infections. Though enteroccocal SBI rates in febrile infants are also quite low,7-9,11,12 if Enterococcus were highly suspected, such as in an infant with pyuria and gram positive organisms on gram stain, ampicillin offers good additional coverage. In the case of a local outbreak of listeriosis, or a specific exposure to Listeria-contaminated products on a patient history, antibiotics with efficacy against Listeria should be used. Last, in cases in which gentamicin is used as empiric coverage for gram-negative organisms, ampicillin offers important additional coverage for GBS.
Some practitioners advocate ampicillin and gentamicin over cefotaxime regimens on the basis of an often cited study that found a survival benefit for febrile neonates in the intensive care setting.18 There are a number of reasons that this study should not influence care for typical infants admitted with possible sepsis. First, the study was retrospective and limited by its use of administrative data. The authors acknowledged that a potential explanation for their results is unmeasured confounding. Second, the patients included in the study were dramatically different from the group of well infants admitted with possible sepsis; the study included neonatal critical care unit patients treated with antibiotics within the first 3 days of life. Third, the study’s results have not been replicated in otherwise healthy febrile infants.
WHAT YOU SHOULD USE INSTEAD OF AMPICILLIN FOR EMPIRIC LISTERIA COVERAGE
For febrile children 0 to 90 days old, empiric antibiotic coverage should be aimed at covering the current predominant pathogens, which include E coli and GBS. Therefore, for most children and US regions, a third-generation cephalosporin (eg, cefotaxime) is sufficient.
RECOMMENDATIONS
- Empiric antibiotics for treatment of febrile children 0-90 days should target E. coli and GBS; a third generation cephalosporin, (e.g. cefotaxime) alone is a reasonable choice for most patients.
- Prescribing ampicillin to specifically cover Listeria is unnecessary for the vast majority of febrile infants
- Prescribing ampicillin is reasonable in certain subgroups of febrile infants: those less than seven days of age, those with evidence of bacterial meningitis (especially if also <28 days of age), those in whom enterococcal infection is strongly suspected, and those with specific Listeria exposures related to local outbreaks.
CONCLUSION
The 32-day-old infant described in the clinical scenario was at extremely low risk for listeriosis. Antibiotic coverage with a third-generation cephalosporin is sufficient for the most likely pathogens. The common practice of empirically covering Listeria in otherwise healthy febrile infants considered to be at higher risk for SBI is no longer based on best available evidence and represents overtreatment with at least theoretical harms. Avoidance of the risks associated with the side effects of antibiotics, costs saved by forgoing multiple antibiotics, a decrease in medication dosing frequency, and improved antibiotic stewardship for the general population all argue forcefully for making empiric Listeria coverage a thing of the past.
Disclosure
Nothing to report.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and Liking It on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
The “Things We Do for No Reason” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

Evaluation and treatment of the febrile infant 0 to 90 days of age are common clinical issues in pediatrics, family medicine, emergency medicine, and pediatric hospital medicine. Traditional teaching has been that Listeria monocytogenes is 1 of the 3 most common pathogens causing neonatal sepsis. Many practitioners routinely use antibiotic regimens, including ampicillin, to specifically target Listeria. However, a large body of evidence, including a meta-analysis and several multicenter studies, has shown that listeriosis is extremely rare in the United States. The practice of empiric ampicillin thus exposes the patient to harms and costs with little if any potential benefit, while increasing pressure on the bacterial flora in the community to generate antibiotic resistance. Empiric ampicillin for all infants admitted for sepsis evaluation is a tradition-based practice no longer founded on the best available evidence.
CASE REPORT
A 32-day-old, full-term, previously healthy girl presented with fever of 1 day’s duration. Her parents reported she had appeared well until the evening before admission, when she became a bit less active and spent less time breastfeeding. The morning of admission, she was fussier than usual. Rectal temperature, taken by her parents, was 101°F. There were no other symptoms and no sick contacts.
On examination, the patient’s rectal temperature was 101.5°F. Her other vitals and the physical examination findings were unremarkable. Laboratory test results included a normal urinalysis and a peripheral white blood cell (WBC) count of 21,300 cells/µL. Cerebrospinal fluid (CSF) analysis revealed normal protein and glucose levels with 3 WBCs/µL and a negative gram stain. Due to stratifying at higher risk for serious bacterial infection (SBI), the child was admitted and started on ampicillin and cefotaxime while awaiting culture results.
BACKGROUND
Evaluation and treatment of febrile infants are common clinical issues in pediatrics, emergency medicine, and general practice. Practice guidelines for evaluation of febrile infants recommend hospitalization and parenteral antibiotics for children younger than 28 days and children 29 to 90 days old if stratified at high risk for SBI.1,2 Recommendations for empiric antibiotic regimens include ampicillin in addition to either gentamicin or cefotaxime.1,2
WHY YOU MIGHT THINK AMPICILLIN IS HELPFUL
Generations of pediatrics students have been taught that the 3 pathogens most likely to cause bacterial sepsis in infants are group B Streptococcus (GBS), Escherichia coli, and Listeria monocytogenes. This teaching is still espoused in the latest editions of pediatrics textbooks.3 Ampicillin is specifically recommended for covering Listeria, and studies have found that 62% to 78% of practitioners choose empiric ampicillin-containing antibiotic regimens for the treatment of febrile infants.4-6
WHY EMPIRIC AMPICILLIN IS UNNECESSARY
In the past, Listeria was a potential though still uncommon infant pathogen. Over the past few decades, however, the epidemiology of infant sepsis has changed significantly. Estimates of the rate of infection with Listeria now range from extremely rare to nonexistent across multiple studies4,7-15 (Table). In a 4-year retrospective case series at a single urban academic center in Washington, DC, Sadow et al.4 reported no instances of Listeria among 121 positive bacterial cultures in infants younger than 60 days seen in the emergency department (ED). Byington et al.7 examined all positive cultures for infants 0 to 90 days old at a large academic referral center in Utah over a 3-year period and reported no cases of Listeria (1298 patients, 105 SBI cases). A study at a North Carolina academic center found 1 case of Listeria meningitis among 72 SBIs (668 febrile infants) without a localizing source.8 At a large group-practice in northern California, Greenhow et al.9 examined all blood cultures (N = 4255) performed over 4 years for otherwise healthy infants 1 week to 3 months old and found no cases of Listeria. In a follow-up study, the same authors examined all blood (n = 5396), urine (n = 4599), and CSF (n = 1796) cultures in the same population and found no Listeria cases.10 Hassoun et al.11 studied SBI rates among infants younger than 28 days with any blood, urine, or CSF culture performed over 4 years at two Michigan EDs. One (0.08%) of the 1192 infants evaluated had bacteremia caused by Listeria.
Multicenter studies have reported similar results. In a study of 6 hospital systems in geographically diverse areas of the United States, Biondi et al.12 examined all positive blood cultures (N = 181) for febrile infants younger than 90 days admitted to a general pediatric ward, and found no listeriosis. Mischler et al.13 examined all positive blood cultures (N = 392) for otherwise healthy febrile infants 0 to 90 days old admitted to a hospital in 1 of 17 geographically diverse healthcare systems and found no cases of Listeria. A recent meta-analysis of studies that reported SBI rates for febrile infants 0 to 90 days old found the weighted prevalence of Listeria bacteremia to be 0.03% (2/20,703) and that of meningitis to be 0.02% (3/13,375).14 Veesenmeyer and Edmonson15 used a national inpatient database to identify all Listeria cases among infants over a 6-year period and estimated listeriosis rates for the US population. Over the 6 years, there were 212 total cases, which were extrapolated to 344 in the United States during that period, yielding a pooled annual incidence rate of 1.41 in 100,000 births.
Ampicillin offers no significant improvement in coverage for GBS or E coli beyond other β-lactam antibiotics, such as cefotaxime. Therefore, though the cost and potential harms of 24 to 48 hours of intravenous ampicillin are low for the individual patient, there is almost no potential benefit. Using the weighted prevalence of 0.03% for Listeria bacteremia reported in the recent meta-analysis,14 the number needed to treat to cover 1 case of Listeria bacteremia would be 3333. In addition, the increasing incidence of ampicillin resistance, particularly among gram-negative organisms,4,7,9 argues strongly for better antibiotic stewardship on a national level. A number of expert authors have advocated dropping empiric Listeria coverage as part of the treatment of febrile infants, particularly infants 29 to 90 days old.16,17 Some authors continue to advocate empiric Listeria coverage.6 It is interesting to note, however, that the incidence of Staph aureus bacteremia in recent case series is much higher than that reported for Listeria, accounting for 6-9% of bacteremia cases.9,11,13 Yet few if any authors advocate for empiric S. aureus coverage.
WHEN EMPIRIC AMPICILLIN COVERAGE MAY BE REASONABLE
The rate of listeriosis remains low across age groups in recent studies, but the rate is slightly higher in very young infants. In the recent national database study of listeriosis cases over a 6-year period, almost half involved infants younger than 7 days, and most of these infants showed no evidence of meningitis.15 Therefore, it may be reasonable to include empiric Listeria coverage in febrile infants younger than 7 days, though the study authors estimated 22.5 annual cases of Listeria in this age range in the United States. Eighty percent of the Listeria cases were in infants younger than 28 days, but more than 85% of infants 7 to 28 days old had meningitis. Therefore, broad antimicrobial coverage for infants with CSF pleocytosis and/or a high bacterial meningitis score is reasonable, especially for infants younger than 28 days.
Other potential indications for ampicillin are enterococcal infections. Though enteroccocal SBI rates in febrile infants are also quite low,7-9,11,12 if Enterococcus were highly suspected, such as in an infant with pyuria and gram positive organisms on gram stain, ampicillin offers good additional coverage. In the case of a local outbreak of listeriosis, or a specific exposure to Listeria-contaminated products on a patient history, antibiotics with efficacy against Listeria should be used. Last, in cases in which gentamicin is used as empiric coverage for gram-negative organisms, ampicillin offers important additional coverage for GBS.
Some practitioners advocate ampicillin and gentamicin over cefotaxime regimens on the basis of an often cited study that found a survival benefit for febrile neonates in the intensive care setting.18 There are a number of reasons that this study should not influence care for typical infants admitted with possible sepsis. First, the study was retrospective and limited by its use of administrative data. The authors acknowledged that a potential explanation for their results is unmeasured confounding. Second, the patients included in the study were dramatically different from the group of well infants admitted with possible sepsis; the study included neonatal critical care unit patients treated with antibiotics within the first 3 days of life. Third, the study’s results have not been replicated in otherwise healthy febrile infants.
WHAT YOU SHOULD USE INSTEAD OF AMPICILLIN FOR EMPIRIC LISTERIA COVERAGE
For febrile children 0 to 90 days old, empiric antibiotic coverage should be aimed at covering the current predominant pathogens, which include E coli and GBS. Therefore, for most children and US regions, a third-generation cephalosporin (eg, cefotaxime) is sufficient.
RECOMMENDATIONS
- Empiric antibiotics for treatment of febrile children 0-90 days should target E. coli and GBS; a third generation cephalosporin, (e.g. cefotaxime) alone is a reasonable choice for most patients.
- Prescribing ampicillin to specifically cover Listeria is unnecessary for the vast majority of febrile infants
- Prescribing ampicillin is reasonable in certain subgroups of febrile infants: those less than seven days of age, those with evidence of bacterial meningitis (especially if also <28 days of age), those in whom enterococcal infection is strongly suspected, and those with specific Listeria exposures related to local outbreaks.
CONCLUSION
The 32-day-old infant described in the clinical scenario was at extremely low risk for listeriosis. Antibiotic coverage with a third-generation cephalosporin is sufficient for the most likely pathogens. The common practice of empirically covering Listeria in otherwise healthy febrile infants considered to be at higher risk for SBI is no longer based on best available evidence and represents overtreatment with at least theoretical harms. Avoidance of the risks associated with the side effects of antibiotics, costs saved by forgoing multiple antibiotics, a decrease in medication dosing frequency, and improved antibiotic stewardship for the general population all argue forcefully for making empiric Listeria coverage a thing of the past.
Disclosure
Nothing to report.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and Liking It on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
1. Baraff LJ, Bass JW, Fleisher GR, et al. Practice guideline for the management of infants and children 0 to 36 months of age with fever without source. Agency for Health Care Policy and Research. Ann Emerg Med. 1993;22(7):1198-1210. PubMed
2. American College of Emergency Physicians Clinical Policies Committee; American College of Emergency Physicians Clinical Policies Subcommittee on Pediatric Fever. Clinical policy for children younger than three years presenting to the emergency department with fever. Ann Emerg Med. 2003;42(4):530-545. PubMed
3. Nield L, Kamat D. Fever without a focus. In: Kliegman R, Stanton B, eds. Nelson’s Textbook of Pediatrics. 20th ed. Philadelphia, PA: Elsevier; 2016.
4. Sadow KB, Derr R, Teach SJ. Bacterial infections in infants 60 days and younger: epidemiology, resistance, and implications for treatment. Arch Pediatr Adolesc Med. 1999;153(6):611-614. PubMed
5. Aronson PL, Thurm C, Alpern ER, et al. Variation in care of the febrile young infant <90 days in US pediatric emergency departments. Pediatrics. 2014;134(4):667-677. PubMed
6. Cantey JB, Lopez-Medina E, Nguyen S, Doern C, Garcia C. Empiric antibiotics for serious bacterial infection in young infants: opportunities for stewardship. Pediatr Emerg Care. 2015;31(8):568-571. PubMed
7. Byington CL, Rittichier KK, Bassett KE, et al. Serious bacterial infections in febrile infants younger than 90 days of age: the importance of ampicillin-resistant pathogens. Pediatrics. 2003;111(5 pt 1):964-968. PubMed
8. Watt K, Waddle E, Jhaveri R. Changing epidemiology of serious bacterial infections in febrile infants without localizing signs. PLoS One. 2010;5(8):e12448. PubMed
9. Greenhow TL, Hung YY, Herz AM. Changing epidemiology of bacteremia in infants aged 1 week to 3 months. Pediatrics. 2012;129(3):e590-e596. PubMed
10. Greenhow TL, Hung YY, Herz AM, Losada E, Pantell RH. The changing epidemiology of serious bacterial infections in young infants. Pediatr Infect Dis J. 2014;33(6):595-599. PubMed
11. Hassoun A, Stankovic C, Rogers A, et al. Listeria and enterococcal infections in neonates 28 days of age and younger: is empiric parenteral ampicillin still indicated? Pediatr Emerg Care. 2014;30(4):240-243. PubMed
12. Biondi E, Evans R, Mischler M, et al. Epidemiology of bacteremia in febrile infants in the United States. Pediatrics. 2013;132(6):990-996. PubMed
13. Mischler M, Ryan MS, Leyenaar JK, et al. Epidemiology of bacteremia in previously healthy febrile infants: a follow-up study. Hosp Pediatr. 2015;5(6):293-300. PubMed
14. Leazer R, Perkins AM, Shomaker K, Fine B. A meta-analysis of the rates of Listeria monocytogenes and Enterococcus in febrile infants. Hosp Pediatr. 2016;6(4):187-195. PubMed
15. Veesenmeyer AF, Edmonson MB. Trends in US hospital stays for listeriosis in infants. Hosp Pediatr. 2016;6(4):196-203. PubMed
16. Schroeder AR, Roberts KB. Is tradition trumping evidence in the treatment of young, febrile infants? Hosp Pediatr. 2016;6(4):252-253. PubMed
17. Cioffredi LA, Jhaveri R. Evaluation and management of febrile children: a review. JAMA Pediatr. 2016;170(8):794-800. PubMed
18. Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Empiric use of ampicillin and cefotaxime, compared with ampicillin and gentamicin, for neonates at risk for sepsis is associated with an increased risk of neonatal death. Pediatrics. 2006;117(1):67-74. PubMed
1. Baraff LJ, Bass JW, Fleisher GR, et al. Practice guideline for the management of infants and children 0 to 36 months of age with fever without source. Agency for Health Care Policy and Research. Ann Emerg Med. 1993;22(7):1198-1210. PubMed
2. American College of Emergency Physicians Clinical Policies Committee; American College of Emergency Physicians Clinical Policies Subcommittee on Pediatric Fever. Clinical policy for children younger than three years presenting to the emergency department with fever. Ann Emerg Med. 2003;42(4):530-545. PubMed
3. Nield L, Kamat D. Fever without a focus. In: Kliegman R, Stanton B, eds. Nelson’s Textbook of Pediatrics. 20th ed. Philadelphia, PA: Elsevier; 2016.
4. Sadow KB, Derr R, Teach SJ. Bacterial infections in infants 60 days and younger: epidemiology, resistance, and implications for treatment. Arch Pediatr Adolesc Med. 1999;153(6):611-614. PubMed
5. Aronson PL, Thurm C, Alpern ER, et al. Variation in care of the febrile young infant <90 days in US pediatric emergency departments. Pediatrics. 2014;134(4):667-677. PubMed
6. Cantey JB, Lopez-Medina E, Nguyen S, Doern C, Garcia C. Empiric antibiotics for serious bacterial infection in young infants: opportunities for stewardship. Pediatr Emerg Care. 2015;31(8):568-571. PubMed
7. Byington CL, Rittichier KK, Bassett KE, et al. Serious bacterial infections in febrile infants younger than 90 days of age: the importance of ampicillin-resistant pathogens. Pediatrics. 2003;111(5 pt 1):964-968. PubMed
8. Watt K, Waddle E, Jhaveri R. Changing epidemiology of serious bacterial infections in febrile infants without localizing signs. PLoS One. 2010;5(8):e12448. PubMed
9. Greenhow TL, Hung YY, Herz AM. Changing epidemiology of bacteremia in infants aged 1 week to 3 months. Pediatrics. 2012;129(3):e590-e596. PubMed
10. Greenhow TL, Hung YY, Herz AM, Losada E, Pantell RH. The changing epidemiology of serious bacterial infections in young infants. Pediatr Infect Dis J. 2014;33(6):595-599. PubMed
11. Hassoun A, Stankovic C, Rogers A, et al. Listeria and enterococcal infections in neonates 28 days of age and younger: is empiric parenteral ampicillin still indicated? Pediatr Emerg Care. 2014;30(4):240-243. PubMed
12. Biondi E, Evans R, Mischler M, et al. Epidemiology of bacteremia in febrile infants in the United States. Pediatrics. 2013;132(6):990-996. PubMed
13. Mischler M, Ryan MS, Leyenaar JK, et al. Epidemiology of bacteremia in previously healthy febrile infants: a follow-up study. Hosp Pediatr. 2015;5(6):293-300. PubMed
14. Leazer R, Perkins AM, Shomaker K, Fine B. A meta-analysis of the rates of Listeria monocytogenes and Enterococcus in febrile infants. Hosp Pediatr. 2016;6(4):187-195. PubMed
15. Veesenmeyer AF, Edmonson MB. Trends in US hospital stays for listeriosis in infants. Hosp Pediatr. 2016;6(4):196-203. PubMed
16. Schroeder AR, Roberts KB. Is tradition trumping evidence in the treatment of young, febrile infants? Hosp Pediatr. 2016;6(4):252-253. PubMed
17. Cioffredi LA, Jhaveri R. Evaluation and management of febrile children: a review. JAMA Pediatr. 2016;170(8):794-800. PubMed
18. Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Empiric use of ampicillin and cefotaxime, compared with ampicillin and gentamicin, for neonates at risk for sepsis is associated with an increased risk of neonatal death. Pediatrics. 2006;117(1):67-74. PubMed
© 2017 Society of Hospital Medicine
Hot in the tropics
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient’s case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant. The bolded text represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 42-year-old Malaysian construction worker with subjective fevers of 4 days’ duration presented to an emergency department in Singapore. He reported nonproductive cough, chills without rigors, sore throat, and body aches. He denied sick contacts. Past medical history included chronic hepatitis B virus (HBV) infection. The patient was not taking any medications.
For this patient presenting acutely with subjective fevers, nonproductive cough, chills, aches, and lethargy, initial considerations include infection with a common virus (influenza virus, adenovirus, Epstein-Barr virus [EBV]), acute human immunodeficiency virus (HIV) infection, emerging infection (severe acute respiratory syndrome [SARS], Middle Eastern respiratory syndrome coronavirus [MERS-CoV] infection, avian influenza), and tropical infection (dengue, chikungunya). Also possible are bacterial infections (eg, with Salmonella typhi or Rickettsia or Mycoplasma species), parasitic infections (eg, malaria), and noninfectious illnesses (eg, autoimmune diseases, thyroiditis, acute leukemia, environmental exposures).
The patient’s temperature was 38.5°C; blood pressure, 133/73 mm Hg; heart rate, 95 beats per minute; respiratory rate, 18 breaths per minute; and oxygen saturation, 100% on ambient air. On physical examination, he appeared comfortable, and heart, lung, abdomen, skin, and extremities were normal. Laboratory test results included white blood cell (WBC) count, 4400/μL (with normal differential); hemoglobin, 16.1 g/dL; and platelet count, 207,000/μL. Serum chemistries were normal. C-reactive protein (CRP) level was 44.6 mg/L (reference range, 0.2-9.1 mg/L), and procalcitonin level was 0.13 ng/mL (reference range, <0.50 ng/mL). Chest radiograph was normal. Dengue antibodies (immunoglobulin M, immunoglobulin G [IgG]) and dengue NS1 antigen were negative. The patient was discharged with a presumptive diagnosis of viral upper respiratory tract infection.
There is no left shift characteristic of bacterial infection or lymphopenia characteristic of rickettsial disease or acute HIV infection. The serologic testing and the patient’s overall appearance make dengue unlikely. The low procalcitonin supports a nonbacterial cause of illness. CRP elevation may indicate an inflammatory process and is relatively nonspecific.
Myalgias, pharyngitis, and cough improved over several days, but fevers persisted, and a rash developed over the lower abdomen. The patient returned to the emergency department and was admitted. He denied weight loss and night sweats. He had multiple female sexual partners, including commercial sex workers, within the previous 6 months. Temperature was 38.5°C. The posterior oropharynx was slightly erythematous. There was no lymphadenopathy. Firm, mildly erythematous macules were present on the anterior abdominal wall (Figure 1). The rest of the physical examination was normal.
Laboratory testing revealed WBC count, 5800/μL (75% neutrophils, 19% lymphocytes, 3% monocytes, 2% atypical mononuclear cells); hemoglobin, 16.3 g/dL; platelet count, 185,000/μL; sodium, 131 mmol/L; potassium, 3.4 mmol/L; creatinine, 0.9 mg/dL; albumin, 3.2 g/dL; alanine aminotransferase (ALT), 99 U/L; aspartate aminotransferase (AST), 137 U/L; alkaline phosphatase (ALP), 63 U/L; and total bilirubin, 1.9 mg/dL. Prothrombin time was 11.1 seconds; partial thromboplastin time, 36.1 seconds; erythrocyte sedimentation rate, 14 mm/h; and CRP, 62.2 mg/L.
EBV, acute HIV, and cytomegalovirus infections often present with adenopathy, which is absent here. Disseminated gonococcal infection can manifest with fever, body aches, and rash, but his rash and the absence of penile discharge, migratory arthritis, and enthesitis are not characteristic. Mycoplasma infection can present with macules, urticaria, or erythema multiforme. Rickettsia illnesses typically cause vasculitis with progression to petechiae or purpura resulting from endothelial damage. Patients with secondary syphilis may have widespread macular lesions, and the accompanying syphilitic hepatitis often manifests with elevations in ALP instead of ALT and AST. The mild elevation in ALT and AST can occur with many systemic viral infections. Sweet syndrome may manifest with febrile illness and rash, but the acuity of this patient’s illness and the rapid evolution favor infection.
The patient’s fevers (35°-40°C) continued without pattern over the next 3 days. Blood and urine cultures were negative. Polymerase chain reaction (PCR) test of the nasal mucosa was negative for respiratory viruses. PCR blood tests for EBV, HIV-1, and cytomegalovirus were also negative. Antistreptolysin O (ASO) titer was 400 IU/mm (reference range, <200 IU/mm). Antinuclear antibodies were negative, and rheumatoid factor was 12.4 U/mL (reference range, <10.3 U/mL). Computed tomography (CT) of the thorax, abdomen, and pelvis was normal. Results of a biopsy of an anterior abdominal wall skin lesion showed perivascular and periadnexal lymphocytic inflammation. Amoxicillin was started for the treatment of possible group A streptococcal infection.
PCR for HIV would be positive at a high level in acute HIV. The skin biopsy is not characteristic of Sweet syndrome, which typically shows neutrophilic infiltrate without leukocytoclastic vasculitis, or of syphilis, which typically shows a plasma cell infiltrate.
The patient’s erythematous oropharynx may indicate recent streptococcal pharyngitis. The fevers, elevated ASO titer, and CRP level are consistent with acute rheumatic fever, but arthritis, carditis, and neurologic manifestations are lacking. Erythema marginatum manifests on the trunk and limbs as macules or papules with central clearing as the lesions spread outward—and differs from the patient’s rash, which is firm and restricted to the abdominal wall.
Fevers persisted through hospital day 7. The WBC count was 1100/μL (75.7% neutrophils, 22.5% lymphocytes), hemoglobin was 10.3 g/dL, and platelet count was 52,000/μL. Additional laboratory test results included ALP, 234 U/L; ALT, 250 U/L; AST, 459 U/L; lactate dehydrogenase, 2303 U/L (reference range, 222-454 U/L); and ferritin, 14,964 ng/mL (reference range, 47-452 ng/mL).
The duration of illness and negative diagnostic tests for infections increases suspicion for a noninfectious illness. Conditions commonly associated with marked hyperferritinemia include adult-onset Still disease (AOSD) and hemophagocytic lymphohistiocytosis (HLH). Of the 9 AOSD diagnostic (Yamaguchi) criteria, 5 are met in this case: fever, rash, sore throat, abnormal liver function tests, and negative rheumatologic tests. However, the patient lacks arthritis, leukocytosis, lymphadenopathy, and hepatosplenomegaly. Except for the elevated ferritin, the AOSD criteria overlap substantially with the criteria for acute rheumatic fever, and still require that infections be adequately excluded. HLH, a state of abnormal immune activation with resultant organ dysfunction, can be a primary disorder, but in adults more often is secondary to underlying infectious, autoimmune, or malignant (often lymphoma) conditions. Elevated ferritin, cytopenias, elevated ALT and AST, elevated CRP and erythrocyte sedimentation rate, and elevated lactate dehydrogenase are consistent with HLH. The HLH diagnosis can be more firmly established with the more specific findings of hypertriglyceridemia, hypofibrinogenemia, and elevated soluble CD25 level. The histopathologic finding of hemophagocytosis in the bone marrow, lymph nodes, or liver may further support the diagnosis of HLH.
Rash and fevers persisted. Hepatitis A, hepatitis C, Rickettsia IgG, Burkholderia pseudomallei (the causative organism of melioidosis), and Leptospira serologies, as well as PCR for herpes simplex virus and parvovirus, were all negative. Hepatitis B viral load was 962 IU/mL (2.98 log), hepatitis B envelope antigen was negative, and hepatitis B envelope antibody was positive. Orientia tsutsugamushi (organism responsible for scrub typhus) IgG titer was elevated at 1:128. Antiliver kidney microsomal antibodies and antineutrophil cytoplasmic antibodies were negative. Fibrinogen level was 0.69 g/L (reference range, 1.8-4.8 g/L), and beta-2 microglobulin level was 5078 ng/mL (reference range, 878-2000 ng/mL). Bone marrow biopsy results showed hypocellular marrow with suppressed myelopoiesis, few atypical lymphoid cells, and few hemophagocytes. Flow cytometry was negative for clonal B lymphocytes and aberrant expression of T lymphocytes. Bone marrow myobacterial PCR and fungal cultures were negative.
The patient’s chronic HBV infection is unlikely to be related to his presentation given his low viral load and absence of signs of hepatic dysfunction. Excluding rickettsial disease requires paired acute and convalescent serologies. O tsutsugamushi, the causative agent of the rickettsial disease scrub typhus, is endemic in Malaysia; thus, his positive O tsutsugamushi IgG may indicate past exposure. His fevers, myalgias, truncal rash, and hepatitis are consistent with scrub typhus, but he lacks the characteristic severe headache and generalized lymphadenopathy. Although eschar formation with evolution of a papular rash is common in scrub typhus, it is often absent in the variant found in Southeast Asia. Although elevated β2 microglobulin level is used as a prognostic marker in multiple myeloma and Waldenström macroglobulinemia, it can be elevated in many immune-active states. The patient likely has HLH, which is supported by the hemophagocytosis seen on bone marrow biopsy, and the hypofibrinogenemia. Potential HLH triggers include O tsutsugamushi infection or recent streptococcal pharyngitis.
A deep-punch skin biopsy of the anterior abdominal wall skin lesion was performed because of the absence of subcutaneous fat in the first biopsy specimen. The latest biopsy results showed irregular interstitial expansion of medium-size lymphocytes in a lobular panniculated pattern. The lymphocytes contained enlarged, irregularly contoured nucleoli and were positive for T-cell markers CD2 and CD3 with reduction in CD5 expression. The lymphomatous cells were of CD8+ with uniform expression of activated cytotoxic granule protein granzyme B and were positive for T-cell hemireceptor β.
Positron emission tomography (PET) CT, obtained for staging purposes, showed multiple hypermetabolic subcutaneous and cutaneous lesions over the torso and upper and lower limbs—compatible with lymphomatous infiltrates (Figure 2). Examination, pathology, and imaging findings suggested a rare neoplasm: subcutaneous panniculitis-like T-cell lymphoma (SPTCL). SPTCL was confirmed by T-cell receptor gene rearrangements studies.
HLH was diagnosed on the basis of the fevers, cytopenias, hypofibrinogenemia, elevated ferritin level, and evidence of hemophagocytosis. SPTCL was suspected as the HLH trigger.
The patient was treated with cyclophosphamide, hydroxydoxorubicin, vincristine, and prednisone. While on this regimen, he developed new skin lesions, and his ferritin level was persistently elevated. He was switched to romidepsin, a histone deacetylase inhibitor that specifically targets cutaneous T-cell lymphoma, but the lesions continued to progress. The patient then was treated with gemcitabine, dexamethasone, and cisplatin, and the rashes resolved. The most recent PET-CT showed nearly complete resolution of the subcutaneous lesions.
DISCUSSION
When residents or visitors to tropical or sub-tropical regions, those located near or between the Tropics of Cancer and Capricorn, present with fever, physicians usually first think of infectious diseases. This patient’s case is a reminder that these important first considerations should not be the last.
Generating a differential diagnosis for tropical illnesses begins with the patient’s history. Factors to be considered include location (regional disease prevalence), exposures (food/water ingestion, outdoor work/recreation, sexual contact, animal contact), and timing (temporal relationship of symptom development to possible exposure). Common tropical infections are malaria, dengue, typhoid, and emerging infections such as chikungunya, avian influenza, and Zika virus infection.1This case underscores the need to analyze diagnostic tests critically. Interpreting tests as simply positive or negative, irrespective of disease features, epidemiology, and test characteristics, can contribute to diagnostic error. For example, the patient’s positive ASO titer requires an understanding of disease features and a nuanced interpretation based on the clinical presentation. The erythematous posterior oropharynx prompted concern for postinfectious sequelae of streptococcal pharyngitis, but his illness was more severe and more prolonged than is typical of that condition. The isolated elevated O tsutsugamushi IgG titer provides an example of the role of epidemiology in test interpretation. Although a single positive value might indicate a new exposure for a visitor to an endemic region, IgG seropositivity in Singapore, where scrub typhus is endemic, likely reflects prior exposure to the organism. Diagnosing an acute scrub typhus infection in a patient in an endemic region requires PCR testing. The skin biopsy results highlight the importance of understanding test characteristics. A skin biopsy specimen must be adequate in order to draw valid and accurate conclusions. In this case, the initial skin biopsy was superficial, and the specimen inadequate, but the test was not “negative.” In the diagnostic skin biopsy, deeper tissue was sampled, and panniculitis (inflammation of subcutaneous fat), which arises in inflammatory, infectious, traumatic, enzymatic, and malignant conditions, was identified. An adequate biopsy specimen that contains subcutaneous fat is essential in making this diagnosis.2This patient eventually manifested several elements of hemophagocytic lymphohistiocytosis (HLH), a syndrome of excessive inflammation and resultant organ injury relating to abnormal immune activation and excessive inflammation. HLH results from deficient down-regulation of activated macrophages and lymphocytes.3 It was initially described in pediatric patients but is now recognized in adults, and associated with mortality as high as 50%.3 A high ferritin level (>2000 ng/mL) has 70% sensitivity and 68% specificity for pediatric HLH and should trigger consideration of HLH in any age group.4 The diagnostic criteria for HLH initially proposed in 2004 by the Histiocyte Society to identify patients for recruitment into a clinical trial included molecular testing consistent with HLH and/or 5 of 8 clinical, laboratory, or histopathologic features (Table 1).5 HScore is a more recent validated scoring system that predicts the probability of HLH (Table 2). A score above 169 signifies diagnostic sensitivity of 93% and specificity of 86%.6
The diagnosis of HLH warrants a search for its underlying cause. Common triggers are viral infections (eg, EBV), autoimmune diseases (eg, systemic lupus erythematosus), and hematologic malignancies. These triggers typically stimulate or suppress the immune system. Initial management involves treatment of the underlying trigger and, potentially, immunosuppression with high
In this case, SPTCL triggered HLH. SPTCL is a rare non-Hodgkin lymphoma characterized by painless subcutaneous nodules or indurated plaques (panniculitis-like) on the trunk or extremities, constitutional symptoms, and, in some cases, HLH.7-10 SPTCL is diagnosed by deep skin biopsy, with immunohistochemistry showing CD8-positive pathologic T cells expressing cytotoxic proteins (eg, granzyme B).9,11 SPTCL can either have an alpha/beta T-cell phenotype (SPTCL-AB) or gamma/delta T-cell phenotype (SPTCL-GD). Seventeen percent of patients with SPTCL-AB and 45% of patients with SPTCL-GD have HLH on diagnosis. Concomitant HLH is associated with decreased 5-year survival.12This patient presented with fevers and was ultimately diagnosed with HLH secondary to SPLTCL. His case is a reminder that not all diseases in the tropics are tropical diseases. In the diagnosis of a febrile illness, a broad evaluative framework and rigorous test results evaluation are essential—no matter where a patient lives or visits.
KEY TEACHING POINTS
- A febrile illness acquired in the tropics is not always attributable to a tropical infection.
- To avoid diagnostic error, weigh positive or negative test results against disease features, patient epidemiology, and test characteristics.
- HLH is characterized by fevers, cytopenias, hepatosplenomegaly, hyperferritinemia, hypertriglyceridemia, and hypofibrinogenemia. In tissue specimens, hemophagocytosis may help differentiate HLH from competing conditions.
- After HLH is diagnosed, try to determine its underlying cause, which may be an infection, autoimmunity, or a malignancy (commonly, a lymphoma).
Disclosure
Nothing to report.
1. Centers for Disease Control and Prevention. Destinations [list]. http://wwwnc.cdc.gov/travel/destinations/list/. Accessed April 22, 2016.
2. Diaz Cascajo C, Borghi S, Weyers W. Panniculitis: definition of terms and diagnostic strategy. Am J Dermatopathol. 2000;22(6):530-549. PubMed
3. Ramos-Casals M, Brito-Zerón P, López-Guillermo A, Khamashta MA, Bosch X. Adult haemophagocytic syndrome. Lancet. 2014;383(9927):1503-1516. PubMed
4. Lehmberg K, McClain KL, Janka GE, Allen CE. Determination of an appropriate cut-off value for ferritin in the diagnosis of hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2014;61(11):2101-2103. PubMed
5. Henter JI, Horne A, Aricó M, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48(2):124-131. PubMed
6. Fardet L, Galicier L, Lambotte O, et al. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014;66(9):2613-2620. PubMed
7. Aronson IK, Worobed CM. Cytophagic histiocytic panniculitis and hemophagocytic lymphohistiocytosis: an overview. Dermatol Ther. 2010;23(4):389-402. PubMed
8. Willemze R, Jansen PM, Cerroni L, et al; EORTC Cutaneous Lymphoma Group. Subcutaneous panniculitis-like T-cell lymphoma: definition, classification, and prognostic factors: an EORTC Cutaneous Lymphoma Group study of 83 cases. Blood. 2008;111(2):838-845. PubMed
9. Kumar S, Krenacs L, Medeiros J, et al. Subcutaneous panniculitic T-cell lymphoma is a tumor of cytotoxic T lymphocytes. Hum Pathol. 1998;29(4):397-403. PubMed
10. Salhany KE, Macon WR, Choi JK, et al. Subcutaneous panniculitis-like T-cell lymphoma: clinicopathologic, immunophenotypic, and genotypic analysis of alpha/beta and gamma/delta subtypes. Am J Surg Pathol. 1998;22(7):881-893. PubMed
11. Jaffe ES, Nicolae A, Pittaluga S. Peripheral T-cell and NK-cell lymphomas in the WHO classification: pearls and pitfalls. Mod Pathol. 2013;26(suppl 1):S71-S87. PubMed
12. Willemze R, Hodak E, Zinzani PL, Specht L, Ladetto M; ESMO Guidelines Working Group. Primary cutaneous lymphomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(suppl 6):vi149-vi154. PubMed
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient’s case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant. The bolded text represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 42-year-old Malaysian construction worker with subjective fevers of 4 days’ duration presented to an emergency department in Singapore. He reported nonproductive cough, chills without rigors, sore throat, and body aches. He denied sick contacts. Past medical history included chronic hepatitis B virus (HBV) infection. The patient was not taking any medications.
For this patient presenting acutely with subjective fevers, nonproductive cough, chills, aches, and lethargy, initial considerations include infection with a common virus (influenza virus, adenovirus, Epstein-Barr virus [EBV]), acute human immunodeficiency virus (HIV) infection, emerging infection (severe acute respiratory syndrome [SARS], Middle Eastern respiratory syndrome coronavirus [MERS-CoV] infection, avian influenza), and tropical infection (dengue, chikungunya). Also possible are bacterial infections (eg, with Salmonella typhi or Rickettsia or Mycoplasma species), parasitic infections (eg, malaria), and noninfectious illnesses (eg, autoimmune diseases, thyroiditis, acute leukemia, environmental exposures).
The patient’s temperature was 38.5°C; blood pressure, 133/73 mm Hg; heart rate, 95 beats per minute; respiratory rate, 18 breaths per minute; and oxygen saturation, 100% on ambient air. On physical examination, he appeared comfortable, and heart, lung, abdomen, skin, and extremities were normal. Laboratory test results included white blood cell (WBC) count, 4400/μL (with normal differential); hemoglobin, 16.1 g/dL; and platelet count, 207,000/μL. Serum chemistries were normal. C-reactive protein (CRP) level was 44.6 mg/L (reference range, 0.2-9.1 mg/L), and procalcitonin level was 0.13 ng/mL (reference range, <0.50 ng/mL). Chest radiograph was normal. Dengue antibodies (immunoglobulin M, immunoglobulin G [IgG]) and dengue NS1 antigen were negative. The patient was discharged with a presumptive diagnosis of viral upper respiratory tract infection.
There is no left shift characteristic of bacterial infection or lymphopenia characteristic of rickettsial disease or acute HIV infection. The serologic testing and the patient’s overall appearance make dengue unlikely. The low procalcitonin supports a nonbacterial cause of illness. CRP elevation may indicate an inflammatory process and is relatively nonspecific.
Myalgias, pharyngitis, and cough improved over several days, but fevers persisted, and a rash developed over the lower abdomen. The patient returned to the emergency department and was admitted. He denied weight loss and night sweats. He had multiple female sexual partners, including commercial sex workers, within the previous 6 months. Temperature was 38.5°C. The posterior oropharynx was slightly erythematous. There was no lymphadenopathy. Firm, mildly erythematous macules were present on the anterior abdominal wall (Figure 1). The rest of the physical examination was normal.
Laboratory testing revealed WBC count, 5800/μL (75% neutrophils, 19% lymphocytes, 3% monocytes, 2% atypical mononuclear cells); hemoglobin, 16.3 g/dL; platelet count, 185,000/μL; sodium, 131 mmol/L; potassium, 3.4 mmol/L; creatinine, 0.9 mg/dL; albumin, 3.2 g/dL; alanine aminotransferase (ALT), 99 U/L; aspartate aminotransferase (AST), 137 U/L; alkaline phosphatase (ALP), 63 U/L; and total bilirubin, 1.9 mg/dL. Prothrombin time was 11.1 seconds; partial thromboplastin time, 36.1 seconds; erythrocyte sedimentation rate, 14 mm/h; and CRP, 62.2 mg/L.
EBV, acute HIV, and cytomegalovirus infections often present with adenopathy, which is absent here. Disseminated gonococcal infection can manifest with fever, body aches, and rash, but his rash and the absence of penile discharge, migratory arthritis, and enthesitis are not characteristic. Mycoplasma infection can present with macules, urticaria, or erythema multiforme. Rickettsia illnesses typically cause vasculitis with progression to petechiae or purpura resulting from endothelial damage. Patients with secondary syphilis may have widespread macular lesions, and the accompanying syphilitic hepatitis often manifests with elevations in ALP instead of ALT and AST. The mild elevation in ALT and AST can occur with many systemic viral infections. Sweet syndrome may manifest with febrile illness and rash, but the acuity of this patient’s illness and the rapid evolution favor infection.
The patient’s fevers (35°-40°C) continued without pattern over the next 3 days. Blood and urine cultures were negative. Polymerase chain reaction (PCR) test of the nasal mucosa was negative for respiratory viruses. PCR blood tests for EBV, HIV-1, and cytomegalovirus were also negative. Antistreptolysin O (ASO) titer was 400 IU/mm (reference range, <200 IU/mm). Antinuclear antibodies were negative, and rheumatoid factor was 12.4 U/mL (reference range, <10.3 U/mL). Computed tomography (CT) of the thorax, abdomen, and pelvis was normal. Results of a biopsy of an anterior abdominal wall skin lesion showed perivascular and periadnexal lymphocytic inflammation. Amoxicillin was started for the treatment of possible group A streptococcal infection.
PCR for HIV would be positive at a high level in acute HIV. The skin biopsy is not characteristic of Sweet syndrome, which typically shows neutrophilic infiltrate without leukocytoclastic vasculitis, or of syphilis, which typically shows a plasma cell infiltrate.
The patient’s erythematous oropharynx may indicate recent streptococcal pharyngitis. The fevers, elevated ASO titer, and CRP level are consistent with acute rheumatic fever, but arthritis, carditis, and neurologic manifestations are lacking. Erythema marginatum manifests on the trunk and limbs as macules or papules with central clearing as the lesions spread outward—and differs from the patient’s rash, which is firm and restricted to the abdominal wall.
Fevers persisted through hospital day 7. The WBC count was 1100/μL (75.7% neutrophils, 22.5% lymphocytes), hemoglobin was 10.3 g/dL, and platelet count was 52,000/μL. Additional laboratory test results included ALP, 234 U/L; ALT, 250 U/L; AST, 459 U/L; lactate dehydrogenase, 2303 U/L (reference range, 222-454 U/L); and ferritin, 14,964 ng/mL (reference range, 47-452 ng/mL).
The duration of illness and negative diagnostic tests for infections increases suspicion for a noninfectious illness. Conditions commonly associated with marked hyperferritinemia include adult-onset Still disease (AOSD) and hemophagocytic lymphohistiocytosis (HLH). Of the 9 AOSD diagnostic (Yamaguchi) criteria, 5 are met in this case: fever, rash, sore throat, abnormal liver function tests, and negative rheumatologic tests. However, the patient lacks arthritis, leukocytosis, lymphadenopathy, and hepatosplenomegaly. Except for the elevated ferritin, the AOSD criteria overlap substantially with the criteria for acute rheumatic fever, and still require that infections be adequately excluded. HLH, a state of abnormal immune activation with resultant organ dysfunction, can be a primary disorder, but in adults more often is secondary to underlying infectious, autoimmune, or malignant (often lymphoma) conditions. Elevated ferritin, cytopenias, elevated ALT and AST, elevated CRP and erythrocyte sedimentation rate, and elevated lactate dehydrogenase are consistent with HLH. The HLH diagnosis can be more firmly established with the more specific findings of hypertriglyceridemia, hypofibrinogenemia, and elevated soluble CD25 level. The histopathologic finding of hemophagocytosis in the bone marrow, lymph nodes, or liver may further support the diagnosis of HLH.
Rash and fevers persisted. Hepatitis A, hepatitis C, Rickettsia IgG, Burkholderia pseudomallei (the causative organism of melioidosis), and Leptospira serologies, as well as PCR for herpes simplex virus and parvovirus, were all negative. Hepatitis B viral load was 962 IU/mL (2.98 log), hepatitis B envelope antigen was negative, and hepatitis B envelope antibody was positive. Orientia tsutsugamushi (organism responsible for scrub typhus) IgG titer was elevated at 1:128. Antiliver kidney microsomal antibodies and antineutrophil cytoplasmic antibodies were negative. Fibrinogen level was 0.69 g/L (reference range, 1.8-4.8 g/L), and beta-2 microglobulin level was 5078 ng/mL (reference range, 878-2000 ng/mL). Bone marrow biopsy results showed hypocellular marrow with suppressed myelopoiesis, few atypical lymphoid cells, and few hemophagocytes. Flow cytometry was negative for clonal B lymphocytes and aberrant expression of T lymphocytes. Bone marrow myobacterial PCR and fungal cultures were negative.
The patient’s chronic HBV infection is unlikely to be related to his presentation given his low viral load and absence of signs of hepatic dysfunction. Excluding rickettsial disease requires paired acute and convalescent serologies. O tsutsugamushi, the causative agent of the rickettsial disease scrub typhus, is endemic in Malaysia; thus, his positive O tsutsugamushi IgG may indicate past exposure. His fevers, myalgias, truncal rash, and hepatitis are consistent with scrub typhus, but he lacks the characteristic severe headache and generalized lymphadenopathy. Although eschar formation with evolution of a papular rash is common in scrub typhus, it is often absent in the variant found in Southeast Asia. Although elevated β2 microglobulin level is used as a prognostic marker in multiple myeloma and Waldenström macroglobulinemia, it can be elevated in many immune-active states. The patient likely has HLH, which is supported by the hemophagocytosis seen on bone marrow biopsy, and the hypofibrinogenemia. Potential HLH triggers include O tsutsugamushi infection or recent streptococcal pharyngitis.
A deep-punch skin biopsy of the anterior abdominal wall skin lesion was performed because of the absence of subcutaneous fat in the first biopsy specimen. The latest biopsy results showed irregular interstitial expansion of medium-size lymphocytes in a lobular panniculated pattern. The lymphocytes contained enlarged, irregularly contoured nucleoli and were positive for T-cell markers CD2 and CD3 with reduction in CD5 expression. The lymphomatous cells were of CD8+ with uniform expression of activated cytotoxic granule protein granzyme B and were positive for T-cell hemireceptor β.
Positron emission tomography (PET) CT, obtained for staging purposes, showed multiple hypermetabolic subcutaneous and cutaneous lesions over the torso and upper and lower limbs—compatible with lymphomatous infiltrates (Figure 2). Examination, pathology, and imaging findings suggested a rare neoplasm: subcutaneous panniculitis-like T-cell lymphoma (SPTCL). SPTCL was confirmed by T-cell receptor gene rearrangements studies.
HLH was diagnosed on the basis of the fevers, cytopenias, hypofibrinogenemia, elevated ferritin level, and evidence of hemophagocytosis. SPTCL was suspected as the HLH trigger.
The patient was treated with cyclophosphamide, hydroxydoxorubicin, vincristine, and prednisone. While on this regimen, he developed new skin lesions, and his ferritin level was persistently elevated. He was switched to romidepsin, a histone deacetylase inhibitor that specifically targets cutaneous T-cell lymphoma, but the lesions continued to progress. The patient then was treated with gemcitabine, dexamethasone, and cisplatin, and the rashes resolved. The most recent PET-CT showed nearly complete resolution of the subcutaneous lesions.
DISCUSSION
When residents or visitors to tropical or sub-tropical regions, those located near or between the Tropics of Cancer and Capricorn, present with fever, physicians usually first think of infectious diseases. This patient’s case is a reminder that these important first considerations should not be the last.
Generating a differential diagnosis for tropical illnesses begins with the patient’s history. Factors to be considered include location (regional disease prevalence), exposures (food/water ingestion, outdoor work/recreation, sexual contact, animal contact), and timing (temporal relationship of symptom development to possible exposure). Common tropical infections are malaria, dengue, typhoid, and emerging infections such as chikungunya, avian influenza, and Zika virus infection.1This case underscores the need to analyze diagnostic tests critically. Interpreting tests as simply positive or negative, irrespective of disease features, epidemiology, and test characteristics, can contribute to diagnostic error. For example, the patient’s positive ASO titer requires an understanding of disease features and a nuanced interpretation based on the clinical presentation. The erythematous posterior oropharynx prompted concern for postinfectious sequelae of streptococcal pharyngitis, but his illness was more severe and more prolonged than is typical of that condition. The isolated elevated O tsutsugamushi IgG titer provides an example of the role of epidemiology in test interpretation. Although a single positive value might indicate a new exposure for a visitor to an endemic region, IgG seropositivity in Singapore, where scrub typhus is endemic, likely reflects prior exposure to the organism. Diagnosing an acute scrub typhus infection in a patient in an endemic region requires PCR testing. The skin biopsy results highlight the importance of understanding test characteristics. A skin biopsy specimen must be adequate in order to draw valid and accurate conclusions. In this case, the initial skin biopsy was superficial, and the specimen inadequate, but the test was not “negative.” In the diagnostic skin biopsy, deeper tissue was sampled, and panniculitis (inflammation of subcutaneous fat), which arises in inflammatory, infectious, traumatic, enzymatic, and malignant conditions, was identified. An adequate biopsy specimen that contains subcutaneous fat is essential in making this diagnosis.2This patient eventually manifested several elements of hemophagocytic lymphohistiocytosis (HLH), a syndrome of excessive inflammation and resultant organ injury relating to abnormal immune activation and excessive inflammation. HLH results from deficient down-regulation of activated macrophages and lymphocytes.3 It was initially described in pediatric patients but is now recognized in adults, and associated with mortality as high as 50%.3 A high ferritin level (>2000 ng/mL) has 70% sensitivity and 68% specificity for pediatric HLH and should trigger consideration of HLH in any age group.4 The diagnostic criteria for HLH initially proposed in 2004 by the Histiocyte Society to identify patients for recruitment into a clinical trial included molecular testing consistent with HLH and/or 5 of 8 clinical, laboratory, or histopathologic features (Table 1).5 HScore is a more recent validated scoring system that predicts the probability of HLH (Table 2). A score above 169 signifies diagnostic sensitivity of 93% and specificity of 86%.6
The diagnosis of HLH warrants a search for its underlying cause. Common triggers are viral infections (eg, EBV), autoimmune diseases (eg, systemic lupus erythematosus), and hematologic malignancies. These triggers typically stimulate or suppress the immune system. Initial management involves treatment of the underlying trigger and, potentially, immunosuppression with high
In this case, SPTCL triggered HLH. SPTCL is a rare non-Hodgkin lymphoma characterized by painless subcutaneous nodules or indurated plaques (panniculitis-like) on the trunk or extremities, constitutional symptoms, and, in some cases, HLH.7-10 SPTCL is diagnosed by deep skin biopsy, with immunohistochemistry showing CD8-positive pathologic T cells expressing cytotoxic proteins (eg, granzyme B).9,11 SPTCL can either have an alpha/beta T-cell phenotype (SPTCL-AB) or gamma/delta T-cell phenotype (SPTCL-GD). Seventeen percent of patients with SPTCL-AB and 45% of patients with SPTCL-GD have HLH on diagnosis. Concomitant HLH is associated with decreased 5-year survival.12This patient presented with fevers and was ultimately diagnosed with HLH secondary to SPLTCL. His case is a reminder that not all diseases in the tropics are tropical diseases. In the diagnosis of a febrile illness, a broad evaluative framework and rigorous test results evaluation are essential—no matter where a patient lives or visits.
KEY TEACHING POINTS
- A febrile illness acquired in the tropics is not always attributable to a tropical infection.
- To avoid diagnostic error, weigh positive or negative test results against disease features, patient epidemiology, and test characteristics.
- HLH is characterized by fevers, cytopenias, hepatosplenomegaly, hyperferritinemia, hypertriglyceridemia, and hypofibrinogenemia. In tissue specimens, hemophagocytosis may help differentiate HLH from competing conditions.
- After HLH is diagnosed, try to determine its underlying cause, which may be an infection, autoimmunity, or a malignancy (commonly, a lymphoma).
Disclosure
Nothing to report.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient’s case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant. The bolded text represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 42-year-old Malaysian construction worker with subjective fevers of 4 days’ duration presented to an emergency department in Singapore. He reported nonproductive cough, chills without rigors, sore throat, and body aches. He denied sick contacts. Past medical history included chronic hepatitis B virus (HBV) infection. The patient was not taking any medications.
For this patient presenting acutely with subjective fevers, nonproductive cough, chills, aches, and lethargy, initial considerations include infection with a common virus (influenza virus, adenovirus, Epstein-Barr virus [EBV]), acute human immunodeficiency virus (HIV) infection, emerging infection (severe acute respiratory syndrome [SARS], Middle Eastern respiratory syndrome coronavirus [MERS-CoV] infection, avian influenza), and tropical infection (dengue, chikungunya). Also possible are bacterial infections (eg, with Salmonella typhi or Rickettsia or Mycoplasma species), parasitic infections (eg, malaria), and noninfectious illnesses (eg, autoimmune diseases, thyroiditis, acute leukemia, environmental exposures).
The patient’s temperature was 38.5°C; blood pressure, 133/73 mm Hg; heart rate, 95 beats per minute; respiratory rate, 18 breaths per minute; and oxygen saturation, 100% on ambient air. On physical examination, he appeared comfortable, and heart, lung, abdomen, skin, and extremities were normal. Laboratory test results included white blood cell (WBC) count, 4400/μL (with normal differential); hemoglobin, 16.1 g/dL; and platelet count, 207,000/μL. Serum chemistries were normal. C-reactive protein (CRP) level was 44.6 mg/L (reference range, 0.2-9.1 mg/L), and procalcitonin level was 0.13 ng/mL (reference range, <0.50 ng/mL). Chest radiograph was normal. Dengue antibodies (immunoglobulin M, immunoglobulin G [IgG]) and dengue NS1 antigen were negative. The patient was discharged with a presumptive diagnosis of viral upper respiratory tract infection.
There is no left shift characteristic of bacterial infection or lymphopenia characteristic of rickettsial disease or acute HIV infection. The serologic testing and the patient’s overall appearance make dengue unlikely. The low procalcitonin supports a nonbacterial cause of illness. CRP elevation may indicate an inflammatory process and is relatively nonspecific.
Myalgias, pharyngitis, and cough improved over several days, but fevers persisted, and a rash developed over the lower abdomen. The patient returned to the emergency department and was admitted. He denied weight loss and night sweats. He had multiple female sexual partners, including commercial sex workers, within the previous 6 months. Temperature was 38.5°C. The posterior oropharynx was slightly erythematous. There was no lymphadenopathy. Firm, mildly erythematous macules were present on the anterior abdominal wall (Figure 1). The rest of the physical examination was normal.
Laboratory testing revealed WBC count, 5800/μL (75% neutrophils, 19% lymphocytes, 3% monocytes, 2% atypical mononuclear cells); hemoglobin, 16.3 g/dL; platelet count, 185,000/μL; sodium, 131 mmol/L; potassium, 3.4 mmol/L; creatinine, 0.9 mg/dL; albumin, 3.2 g/dL; alanine aminotransferase (ALT), 99 U/L; aspartate aminotransferase (AST), 137 U/L; alkaline phosphatase (ALP), 63 U/L; and total bilirubin, 1.9 mg/dL. Prothrombin time was 11.1 seconds; partial thromboplastin time, 36.1 seconds; erythrocyte sedimentation rate, 14 mm/h; and CRP, 62.2 mg/L.
EBV, acute HIV, and cytomegalovirus infections often present with adenopathy, which is absent here. Disseminated gonococcal infection can manifest with fever, body aches, and rash, but his rash and the absence of penile discharge, migratory arthritis, and enthesitis are not characteristic. Mycoplasma infection can present with macules, urticaria, or erythema multiforme. Rickettsia illnesses typically cause vasculitis with progression to petechiae or purpura resulting from endothelial damage. Patients with secondary syphilis may have widespread macular lesions, and the accompanying syphilitic hepatitis often manifests with elevations in ALP instead of ALT and AST. The mild elevation in ALT and AST can occur with many systemic viral infections. Sweet syndrome may manifest with febrile illness and rash, but the acuity of this patient’s illness and the rapid evolution favor infection.
The patient’s fevers (35°-40°C) continued without pattern over the next 3 days. Blood and urine cultures were negative. Polymerase chain reaction (PCR) test of the nasal mucosa was negative for respiratory viruses. PCR blood tests for EBV, HIV-1, and cytomegalovirus were also negative. Antistreptolysin O (ASO) titer was 400 IU/mm (reference range, <200 IU/mm). Antinuclear antibodies were negative, and rheumatoid factor was 12.4 U/mL (reference range, <10.3 U/mL). Computed tomography (CT) of the thorax, abdomen, and pelvis was normal. Results of a biopsy of an anterior abdominal wall skin lesion showed perivascular and periadnexal lymphocytic inflammation. Amoxicillin was started for the treatment of possible group A streptococcal infection.
PCR for HIV would be positive at a high level in acute HIV. The skin biopsy is not characteristic of Sweet syndrome, which typically shows neutrophilic infiltrate without leukocytoclastic vasculitis, or of syphilis, which typically shows a plasma cell infiltrate.
The patient’s erythematous oropharynx may indicate recent streptococcal pharyngitis. The fevers, elevated ASO titer, and CRP level are consistent with acute rheumatic fever, but arthritis, carditis, and neurologic manifestations are lacking. Erythema marginatum manifests on the trunk and limbs as macules or papules with central clearing as the lesions spread outward—and differs from the patient’s rash, which is firm and restricted to the abdominal wall.
Fevers persisted through hospital day 7. The WBC count was 1100/μL (75.7% neutrophils, 22.5% lymphocytes), hemoglobin was 10.3 g/dL, and platelet count was 52,000/μL. Additional laboratory test results included ALP, 234 U/L; ALT, 250 U/L; AST, 459 U/L; lactate dehydrogenase, 2303 U/L (reference range, 222-454 U/L); and ferritin, 14,964 ng/mL (reference range, 47-452 ng/mL).
The duration of illness and negative diagnostic tests for infections increases suspicion for a noninfectious illness. Conditions commonly associated with marked hyperferritinemia include adult-onset Still disease (AOSD) and hemophagocytic lymphohistiocytosis (HLH). Of the 9 AOSD diagnostic (Yamaguchi) criteria, 5 are met in this case: fever, rash, sore throat, abnormal liver function tests, and negative rheumatologic tests. However, the patient lacks arthritis, leukocytosis, lymphadenopathy, and hepatosplenomegaly. Except for the elevated ferritin, the AOSD criteria overlap substantially with the criteria for acute rheumatic fever, and still require that infections be adequately excluded. HLH, a state of abnormal immune activation with resultant organ dysfunction, can be a primary disorder, but in adults more often is secondary to underlying infectious, autoimmune, or malignant (often lymphoma) conditions. Elevated ferritin, cytopenias, elevated ALT and AST, elevated CRP and erythrocyte sedimentation rate, and elevated lactate dehydrogenase are consistent with HLH. The HLH diagnosis can be more firmly established with the more specific findings of hypertriglyceridemia, hypofibrinogenemia, and elevated soluble CD25 level. The histopathologic finding of hemophagocytosis in the bone marrow, lymph nodes, or liver may further support the diagnosis of HLH.
Rash and fevers persisted. Hepatitis A, hepatitis C, Rickettsia IgG, Burkholderia pseudomallei (the causative organism of melioidosis), and Leptospira serologies, as well as PCR for herpes simplex virus and parvovirus, were all negative. Hepatitis B viral load was 962 IU/mL (2.98 log), hepatitis B envelope antigen was negative, and hepatitis B envelope antibody was positive. Orientia tsutsugamushi (organism responsible for scrub typhus) IgG titer was elevated at 1:128. Antiliver kidney microsomal antibodies and antineutrophil cytoplasmic antibodies were negative. Fibrinogen level was 0.69 g/L (reference range, 1.8-4.8 g/L), and beta-2 microglobulin level was 5078 ng/mL (reference range, 878-2000 ng/mL). Bone marrow biopsy results showed hypocellular marrow with suppressed myelopoiesis, few atypical lymphoid cells, and few hemophagocytes. Flow cytometry was negative for clonal B lymphocytes and aberrant expression of T lymphocytes. Bone marrow myobacterial PCR and fungal cultures were negative.
The patient’s chronic HBV infection is unlikely to be related to his presentation given his low viral load and absence of signs of hepatic dysfunction. Excluding rickettsial disease requires paired acute and convalescent serologies. O tsutsugamushi, the causative agent of the rickettsial disease scrub typhus, is endemic in Malaysia; thus, his positive O tsutsugamushi IgG may indicate past exposure. His fevers, myalgias, truncal rash, and hepatitis are consistent with scrub typhus, but he lacks the characteristic severe headache and generalized lymphadenopathy. Although eschar formation with evolution of a papular rash is common in scrub typhus, it is often absent in the variant found in Southeast Asia. Although elevated β2 microglobulin level is used as a prognostic marker in multiple myeloma and Waldenström macroglobulinemia, it can be elevated in many immune-active states. The patient likely has HLH, which is supported by the hemophagocytosis seen on bone marrow biopsy, and the hypofibrinogenemia. Potential HLH triggers include O tsutsugamushi infection or recent streptococcal pharyngitis.
A deep-punch skin biopsy of the anterior abdominal wall skin lesion was performed because of the absence of subcutaneous fat in the first biopsy specimen. The latest biopsy results showed irregular interstitial expansion of medium-size lymphocytes in a lobular panniculated pattern. The lymphocytes contained enlarged, irregularly contoured nucleoli and were positive for T-cell markers CD2 and CD3 with reduction in CD5 expression. The lymphomatous cells were of CD8+ with uniform expression of activated cytotoxic granule protein granzyme B and were positive for T-cell hemireceptor β.
Positron emission tomography (PET) CT, obtained for staging purposes, showed multiple hypermetabolic subcutaneous and cutaneous lesions over the torso and upper and lower limbs—compatible with lymphomatous infiltrates (Figure 2). Examination, pathology, and imaging findings suggested a rare neoplasm: subcutaneous panniculitis-like T-cell lymphoma (SPTCL). SPTCL was confirmed by T-cell receptor gene rearrangements studies.
HLH was diagnosed on the basis of the fevers, cytopenias, hypofibrinogenemia, elevated ferritin level, and evidence of hemophagocytosis. SPTCL was suspected as the HLH trigger.
The patient was treated with cyclophosphamide, hydroxydoxorubicin, vincristine, and prednisone. While on this regimen, he developed new skin lesions, and his ferritin level was persistently elevated. He was switched to romidepsin, a histone deacetylase inhibitor that specifically targets cutaneous T-cell lymphoma, but the lesions continued to progress. The patient then was treated with gemcitabine, dexamethasone, and cisplatin, and the rashes resolved. The most recent PET-CT showed nearly complete resolution of the subcutaneous lesions.
DISCUSSION
When residents or visitors to tropical or sub-tropical regions, those located near or between the Tropics of Cancer and Capricorn, present with fever, physicians usually first think of infectious diseases. This patient’s case is a reminder that these important first considerations should not be the last.
Generating a differential diagnosis for tropical illnesses begins with the patient’s history. Factors to be considered include location (regional disease prevalence), exposures (food/water ingestion, outdoor work/recreation, sexual contact, animal contact), and timing (temporal relationship of symptom development to possible exposure). Common tropical infections are malaria, dengue, typhoid, and emerging infections such as chikungunya, avian influenza, and Zika virus infection.1This case underscores the need to analyze diagnostic tests critically. Interpreting tests as simply positive or negative, irrespective of disease features, epidemiology, and test characteristics, can contribute to diagnostic error. For example, the patient’s positive ASO titer requires an understanding of disease features and a nuanced interpretation based on the clinical presentation. The erythematous posterior oropharynx prompted concern for postinfectious sequelae of streptococcal pharyngitis, but his illness was more severe and more prolonged than is typical of that condition. The isolated elevated O tsutsugamushi IgG titer provides an example of the role of epidemiology in test interpretation. Although a single positive value might indicate a new exposure for a visitor to an endemic region, IgG seropositivity in Singapore, where scrub typhus is endemic, likely reflects prior exposure to the organism. Diagnosing an acute scrub typhus infection in a patient in an endemic region requires PCR testing. The skin biopsy results highlight the importance of understanding test characteristics. A skin biopsy specimen must be adequate in order to draw valid and accurate conclusions. In this case, the initial skin biopsy was superficial, and the specimen inadequate, but the test was not “negative.” In the diagnostic skin biopsy, deeper tissue was sampled, and panniculitis (inflammation of subcutaneous fat), which arises in inflammatory, infectious, traumatic, enzymatic, and malignant conditions, was identified. An adequate biopsy specimen that contains subcutaneous fat is essential in making this diagnosis.2This patient eventually manifested several elements of hemophagocytic lymphohistiocytosis (HLH), a syndrome of excessive inflammation and resultant organ injury relating to abnormal immune activation and excessive inflammation. HLH results from deficient down-regulation of activated macrophages and lymphocytes.3 It was initially described in pediatric patients but is now recognized in adults, and associated with mortality as high as 50%.3 A high ferritin level (>2000 ng/mL) has 70% sensitivity and 68% specificity for pediatric HLH and should trigger consideration of HLH in any age group.4 The diagnostic criteria for HLH initially proposed in 2004 by the Histiocyte Society to identify patients for recruitment into a clinical trial included molecular testing consistent with HLH and/or 5 of 8 clinical, laboratory, or histopathologic features (Table 1).5 HScore is a more recent validated scoring system that predicts the probability of HLH (Table 2). A score above 169 signifies diagnostic sensitivity of 93% and specificity of 86%.6
The diagnosis of HLH warrants a search for its underlying cause. Common triggers are viral infections (eg, EBV), autoimmune diseases (eg, systemic lupus erythematosus), and hematologic malignancies. These triggers typically stimulate or suppress the immune system. Initial management involves treatment of the underlying trigger and, potentially, immunosuppression with high
In this case, SPTCL triggered HLH. SPTCL is a rare non-Hodgkin lymphoma characterized by painless subcutaneous nodules or indurated plaques (panniculitis-like) on the trunk or extremities, constitutional symptoms, and, in some cases, HLH.7-10 SPTCL is diagnosed by deep skin biopsy, with immunohistochemistry showing CD8-positive pathologic T cells expressing cytotoxic proteins (eg, granzyme B).9,11 SPTCL can either have an alpha/beta T-cell phenotype (SPTCL-AB) or gamma/delta T-cell phenotype (SPTCL-GD). Seventeen percent of patients with SPTCL-AB and 45% of patients with SPTCL-GD have HLH on diagnosis. Concomitant HLH is associated with decreased 5-year survival.12This patient presented with fevers and was ultimately diagnosed with HLH secondary to SPLTCL. His case is a reminder that not all diseases in the tropics are tropical diseases. In the diagnosis of a febrile illness, a broad evaluative framework and rigorous test results evaluation are essential—no matter where a patient lives or visits.
KEY TEACHING POINTS
- A febrile illness acquired in the tropics is not always attributable to a tropical infection.
- To avoid diagnostic error, weigh positive or negative test results against disease features, patient epidemiology, and test characteristics.
- HLH is characterized by fevers, cytopenias, hepatosplenomegaly, hyperferritinemia, hypertriglyceridemia, and hypofibrinogenemia. In tissue specimens, hemophagocytosis may help differentiate HLH from competing conditions.
- After HLH is diagnosed, try to determine its underlying cause, which may be an infection, autoimmunity, or a malignancy (commonly, a lymphoma).
Disclosure
Nothing to report.
1. Centers for Disease Control and Prevention. Destinations [list]. http://wwwnc.cdc.gov/travel/destinations/list/. Accessed April 22, 2016.
2. Diaz Cascajo C, Borghi S, Weyers W. Panniculitis: definition of terms and diagnostic strategy. Am J Dermatopathol. 2000;22(6):530-549. PubMed
3. Ramos-Casals M, Brito-Zerón P, López-Guillermo A, Khamashta MA, Bosch X. Adult haemophagocytic syndrome. Lancet. 2014;383(9927):1503-1516. PubMed
4. Lehmberg K, McClain KL, Janka GE, Allen CE. Determination of an appropriate cut-off value for ferritin in the diagnosis of hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2014;61(11):2101-2103. PubMed
5. Henter JI, Horne A, Aricó M, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48(2):124-131. PubMed
6. Fardet L, Galicier L, Lambotte O, et al. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014;66(9):2613-2620. PubMed
7. Aronson IK, Worobed CM. Cytophagic histiocytic panniculitis and hemophagocytic lymphohistiocytosis: an overview. Dermatol Ther. 2010;23(4):389-402. PubMed
8. Willemze R, Jansen PM, Cerroni L, et al; EORTC Cutaneous Lymphoma Group. Subcutaneous panniculitis-like T-cell lymphoma: definition, classification, and prognostic factors: an EORTC Cutaneous Lymphoma Group study of 83 cases. Blood. 2008;111(2):838-845. PubMed
9. Kumar S, Krenacs L, Medeiros J, et al. Subcutaneous panniculitic T-cell lymphoma is a tumor of cytotoxic T lymphocytes. Hum Pathol. 1998;29(4):397-403. PubMed
10. Salhany KE, Macon WR, Choi JK, et al. Subcutaneous panniculitis-like T-cell lymphoma: clinicopathologic, immunophenotypic, and genotypic analysis of alpha/beta and gamma/delta subtypes. Am J Surg Pathol. 1998;22(7):881-893. PubMed
11. Jaffe ES, Nicolae A, Pittaluga S. Peripheral T-cell and NK-cell lymphomas in the WHO classification: pearls and pitfalls. Mod Pathol. 2013;26(suppl 1):S71-S87. PubMed
12. Willemze R, Hodak E, Zinzani PL, Specht L, Ladetto M; ESMO Guidelines Working Group. Primary cutaneous lymphomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(suppl 6):vi149-vi154. PubMed
1. Centers for Disease Control and Prevention. Destinations [list]. http://wwwnc.cdc.gov/travel/destinations/list/. Accessed April 22, 2016.
2. Diaz Cascajo C, Borghi S, Weyers W. Panniculitis: definition of terms and diagnostic strategy. Am J Dermatopathol. 2000;22(6):530-549. PubMed
3. Ramos-Casals M, Brito-Zerón P, López-Guillermo A, Khamashta MA, Bosch X. Adult haemophagocytic syndrome. Lancet. 2014;383(9927):1503-1516. PubMed
4. Lehmberg K, McClain KL, Janka GE, Allen CE. Determination of an appropriate cut-off value for ferritin in the diagnosis of hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2014;61(11):2101-2103. PubMed
5. Henter JI, Horne A, Aricó M, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48(2):124-131. PubMed
6. Fardet L, Galicier L, Lambotte O, et al. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014;66(9):2613-2620. PubMed
7. Aronson IK, Worobed CM. Cytophagic histiocytic panniculitis and hemophagocytic lymphohistiocytosis: an overview. Dermatol Ther. 2010;23(4):389-402. PubMed
8. Willemze R, Jansen PM, Cerroni L, et al; EORTC Cutaneous Lymphoma Group. Subcutaneous panniculitis-like T-cell lymphoma: definition, classification, and prognostic factors: an EORTC Cutaneous Lymphoma Group study of 83 cases. Blood. 2008;111(2):838-845. PubMed
9. Kumar S, Krenacs L, Medeiros J, et al. Subcutaneous panniculitic T-cell lymphoma is a tumor of cytotoxic T lymphocytes. Hum Pathol. 1998;29(4):397-403. PubMed
10. Salhany KE, Macon WR, Choi JK, et al. Subcutaneous panniculitis-like T-cell lymphoma: clinicopathologic, immunophenotypic, and genotypic analysis of alpha/beta and gamma/delta subtypes. Am J Surg Pathol. 1998;22(7):881-893. PubMed
11. Jaffe ES, Nicolae A, Pittaluga S. Peripheral T-cell and NK-cell lymphomas in the WHO classification: pearls and pitfalls. Mod Pathol. 2013;26(suppl 1):S71-S87. PubMed
12. Willemze R, Hodak E, Zinzani PL, Specht L, Ladetto M; ESMO Guidelines Working Group. Primary cutaneous lymphomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(suppl 6):vi149-vi154. PubMed
© 2017 Society of Hospital Medicine
Postdischarge clinics and hospitalists: A review of the evidence and existing models
Readmission prevention is paramount for hospitals and, by extension, hospitalist programs. Hospitalists see early and reliable outpatient follow-up as a safe landing for their most complicated patient cases. The option of a postdischarge clinic arises from the challenge to arrange adequate postdischarge care for patients who lack easy access because of insurance or provider availability. Guaranteeing postdischarge access by opening a dedicated, hospitalist-led postdischarge clinic appears to be an easy solution, but it is a solution that requires significant investment (including investment in physician and staff training and administrative support) and careful navigation of existing primary care relationships. In addition, a clinic staffed only with physicians may not be well equipped to address the complex social factors in healthcare utilization and readmission. Better understanding of the evidence supporting post discharge physician visits, several models of clinics, and the key operational questions are essential to address before crossing the inpatient-outpatient divide.
POSTDISCHARGE PHYSICIAN VISITS AND READMISSIONS
A postdischarge outpatient provider visit is often seen as a key factor in reducing readmissions. In 2013, Medicare added strength to this association by establishing transitional care management codes, which provide enhanced reimbursement to providers for a visit within 7 or 14 days of discharge, with focused attention on transitional issues.1 However, whether a postdischarge visit reduces readmissions remains unclear. Given evidence that higher primary care density is associated with lower healthcare utilization,2 CMS’s financial investment in incentivizing post discharge physician visits may be a good bet. On the other hand, simply having a primary care physician (PCP) may be a risk factor for readmission. This association suggests that postdischarge vigilance leads to identification of medical problems that lead to rehospitalization.3 This uncertainty is not resolved in systematic reviews of readmission reduction initiatives, which were not focused solely on the impact of a physician visit.4,5
The earliest study of postdischarge visits in a general medical population found an association between intensive outpatient follow-up by new providers in a Veterans Affairs population and an increase in hospital readmissions.6 This model is similar to some hospitalist models for postdischarge clinics, as the visit was with a noncontinuity provider. The largest recent study, of patients hospitalized with acute myocardial infarction, community-acquired pneumonia, or congestive heart failure (CHF) between 2009 and 2012, found increased frequency of postdischarge follow-up but no concomitant reduction in readmissions.7 Although small observational studies8 have found a postdischarge primary care visit may reduce the risk for readmission in general medical patients, the bulk of the recent data is negative.
In high-risk patients, however, there may be a clear benefit to postdischarge follow-up. In a North Carolina Medicaid population, a physician visit after discharge was associated with fewer readmissions among high-risk patients, but not among lower risk patients, whose readmission rates were low to start.9 The results of that study support the idea that risk stratification may identify patients who can benefit from more intensive outpatient follow-up. In general medical populations, existing studies may suffer from an absence of adequate risk assessment.
The evidence in specific disease states may show a clearer association between a postdischarge physician visit and reduced risk for readmission. One quarter of patients with CHF are rehospitalized within 30 days of discharge.10 In this disease with frequent exacerbations, a clinic visit to monitor volume status, weight, and medication adherence might reduce the frequency of readmissions or prolong the interval between rehospitalizations. A large observational study observed that earlier post discharge follow up by a cardiologist or a PCP was associated with lower risk of readmission, but only in the quintile with the closest follow-up. In addition, fewer than 40% of patients in this group had a visit within 7 days.11 In another heart failure population, follow-up with either a PCP or cardiologist within 7 days of discharge was again associated with lower risk for readmission.12 Thus, data suggest a protective effect of postdischarge visits in CHF patients, in contrast to a general medical population. Patients with end-stage renal disease may also fit in this group protected by a postdischarge physician visit, as 1 additional visit within the month after discharge was estimated to reduce rehospitalizations and produce significant cost savings.13
With other specific discharge diagnoses, results are varied. Two small observational studies in chronic obstructive pulmonary disease had conflicting results—one found a modest reduction in readmission and emergency department (ED) visits for patients seen by a PCP or pulmonologist within 30 days of discharge,14 and the other found no effect on readmissions but an associated reduction in mortality.15 More data are needed to clarify further the interaction of postdischarge visits with mortality, but the association between postdischarge physician visits and readmission reduction is controversial for patients with chronic obstructive pulmonary disease.
Finally, the evidence for dedicated postdischarge clinics is even more limited. A study of a hospitalist-led postdischarge clinic in a Veterans Affairs hospital found reduced length of stay and earlier postdischarge follow-up in a postdischarge clinic, but no effect on readmissions.16 Other studies have found earlier postdischarge follow-up with dedicated discharge clinics but have not evaluated readmission rates specifically.17In summary, the effect of postdischarge visits on risk for readmission is an area of active research, but remains unclear. The data reviewed suggest a benefit for the highest risk patients, specifically those with severe chronic illness, or those deemed high-risk with a readmission tool.9 At present, because physicians cannot accurately predict which patients will be readmitted,18 discharging physicians often take a broad approach and schedule outpatient visits for all patients. As readmission tools are further refined, the group of patients who will benefit from postdischarge care will be easier to identify, and a benefit to postdischarge visits may be seen
It is also important to note that this review emphasizes the physician visit and its potential impact on readmissions. Socioeconomic causes are increasingly being recognized as driving readmissions and other utilization.19 Whether an isolated physician visit is sufficient to prevent readmissions for patients with nonmedical drivers of healthcare utilization is unclear. For those patients, a discharge visit likely is a necessary component of a readmission reduction strategy for high-risk patients, but may be insufficient for patients who require not just an isolated visit but rather a more integrated and comprehensive care program.8,20,21
POSTDISCHARGE CLINIC MODELS
Despite the unclear relationship between postdischarge physician care and readmissions, dedicated postdischarge clinics, some staffed by hospitalists, have been adopted over the past 10 years. The three primary types of clinics arise in safety net environments, in academic medical centers, and as comprehensive high-risk patient solutions. Reviewing several types of clinics further clarifies the nature of this structural innovation.
Safety Net Hospital Models
Safety net hospitals and their hospitalists struggle with securing adequate postdischarge access for their population, which has inadequate insurance and poor access to primary care. Patient characteristics also play a role in the complex postdischarge care for this population, given its high rate of ED use (owing to perceived convenience and capabilities) for ambulatory-sensitive conditions.22 In addition, immigrants, particularly those with low English-language proficiency, underuse and have poor access to primary care.23,24 Postdischarge clinics in this environment focus first on providing a reliable postdischarge plan and then on linking to primary care. Examples of two clinics are at Harborview Medical Center in Seattle, Washington25 and Texas Health in Fort Worth.
Harborview is a 400-bed hospital affiliated with the University of Washington. More than 50% of its patients are considered indigent. The clinic was established in 2007 to provide a postdischarge option for uninsured patients, and a link to primary care in federally qualified health centers. The clinic was staffed 5 days a week with one or two hospitalists or advanced practice nurses. Visit duration was 20 minutes, 270 visits occurred per month, and the no-show rate was 30%. A small subgroup of the hospitalist group staffed the clinic. Particular clinical foci included CHF patients, patients with wound-care needs, and homeless, immigrant, and recently incarcerated patients. A key goal was connecting to longitudinal primary care, and the clinic successfully connected more than 70% of patients to primary care in community health centers. This clinic ultimately transitioned from a hospitalist practice to a primary care practice with a primary focus on post-ED follow-up for unaffiliated patients.26
In 2010, Texas Health faced a similar challenge with unaffiliated patients, and established a nurse practitioner–based clinic with hospitalist oversight to provide care primarily for patients without insurance or without an existing primary care relationship.
Academic Medical Center Models
Another clinical model is designed for patients who receive primary care at practices affiliated with academic medical centers. Although many of these patients have insurance and a PCP, there is often no availability with their continuity provider, because of the resident’s inpatient schedule or the faculty member’s conflicting priorities.27,28 Academic medical centers, including the University of California at San Francisco, the University of New Mexico, and the Beth Israel Deaconess Medical Center, have established discharge clinics within their faculty primary care practices. A model of this type of clinic was set up at Beth Israel Deaconess in 2010. Staffed by four hospitalists and using 40-minute appointments, this clinic was physically based in the primary care practice. As such, it took advantage of the existing clinic’s administrative and clinical functions, including triage, billing, and scheduling. A visit was scheduled in that clinic by the discharging physician team if a primary care appointment was not available with the patient’s continuity provider. Visits were standardized and focused on outstanding issues at discharge, medication reconciliation, and symptom trajectory. The hospitalists used the clinic’s clinical resources, including nurses, social workers, and pharmacists, but had no other dedicated staff. That there were only four hospitalists meant they were able to gain sufficient exposure to the outpatient setting, provide consistent high-quality care, and gain credibility with the PCPs. As the patients who were seen had PCPs of their own, during the visit significant attention was focused first on the postdischarge concerns, and then on promptly returning the patients to routine primary care. Significant patient outreach was used to address the clinic’s no-show rate, which was almost 50% in the early months. Within a year, the rate was down, closer to 20%. This clinic closed in 2015 after the primary care practice, in which it was based, transitioned to a patient-centered medical home. Since that time, this type of initiative has spread further, with neurohospitalist discharge clinics established, and postdischarge neurology follow-up becoming faster and more reliable.29
Academic medical centers and safety net hospitals substitute for routine primary care to address the basic challenge of primary care access, often without significant enhancements or additional resources, such as dedicated care management and pharmacy, social work, and nursing support. Commonalities of these clinics include dedicated physician staff, appointments generally longer than average outpatient appointments, and visit content concentrated on the key issues at transition (medication reconciliation, outstanding tests, symptom trajectory). As possible, clinics adopted a multidisciplinary approach, with social workers, community health workers, and nurses, to respond to the breadth of patients’ postdischarge needs, which often extend beyond pure medical need. The most frequent barriers encountered included the knowledge gap for hospitalist providers in the outpatient setting (a gap mitigated by using dedicated providers) and the patients’ high no-show rate (not surprising given that the providers are generally new to them). Few clinics have attempted to create continuity across inpatient and outpatient providers, though continuity might reduce no-shows as well as eliminate at least 1 transition.
Comprehensive High-Risk Patient Solutions
At the other end of the clinic spectrum are more integrated postdischarge approaches, which also evolved from the hospitalist model with hospitalist staffing. However, these approaches were introduced in response to the clinical needs of the highest risk patients (who are most vulnerable to frequent provider transitions), not to a systemic inability to provide routine postdischarge care.30
The most long-standing model for this type of clinic is represented by CareMore Health System, a subsidiary of Anthem.30-32 The extensivist, an expanded-scope hospitalist, acts as primary care coordinator, coordinating a multidisciplinary team for a panel of about 100 patients, representing the sickest 5% of the Medicare Advantage–insured population. Unlike the traditional hospitalist, the extensivist follows patients across all care sites, including hospital, rehabilitation sites, and outpatient clinic. For the most part, this relationship is not designed to evolve into a longitudinal relationship, but rather is an intervention only for the several-months period of acute need. Internal data have shown effects on hospital readmissions as well as length of stay.30
Another integrated clinic was established in 2013, at the University of Chicago. This was an effort to redesign care for patients at highest risk for hospitalization.33 Similar to the CareMore process, a high-risk population is identified by prior hospitalization and expected high Medicare costs. A comprehensive care physician cares for these patients across care settings. The clinic takes a team-based approach to patient care, with team members selected on the basis of patient need. Physicians have panels limited to only 200 patients, and generally spend part of the day in clinic, and part in seeing their hospitalized patients. Although reminiscent of a traditional primary care setting, this clinic is designed specifically for a high-risk, frequently hospitalized population, and therefore requires physicians with both a skill set akin to that of hospitalists, and an approach of palliative care and holistic patient care. Outcomes from this trial clinic are expected in 2017 or 2018.
LOGISTICAL CONSIDERATIONS FOR DISCHARGE CLINICS
Considering some key operational questions (Table) can help guide hospitals, hospitalists, and healthcare systems as they venture into the postdischarge clinic space. Return on investment and sustainability are two key questions for postdischarge clinics.
Return on investment varies by payment structure. In capitated environments with a strong emphasis on readmissions and total medical expenditure, a successful postdischarge clinic would recoup the investment through readmission reduction. However, maintaining adequate patient volume against high no-show rates may strain the group financially. In addition, although a hospitalist group may reap few measurable benefits from this clinical exposure, the unique view of the outpatient world afforded to hospitalists working in this environment could enrich the group as a whole by providing a more well-rounded vantage point.
Another key question surrounds sustainability. The clinic at the Beth Israel Deaconess Medical Center in Boston temporarily closed due to high inpatient volume and corresponding need for those hospitalists in the inpatient setting, early in its inception. It subsequently closed due to evolution in the clinic where it was based, rendering it unnecessary. Clinics that are contingent on other clinics will be vulnerable to external forces. Finally, staffing these clinics may be a stretch for a hospitalist group, as a partly different skill set is required for patient care in the outpatient setting. Hospitalists interested in care transitions are well suited for this role. In addition, hospitalists interested in more clinical variety, or in more schedule variety than that provided in a traditional hospitalist schedule, often enjoy the work. A vast majority of hospitalists think PCPs are responsible for postdischarge problems, and would not be interested in working in the postdischarge world.34 A poor fit for providers may lead to clinic failure.
As evident from this review, gaps in understanding the benefits of postdischarge care have persisted for 10 years. Discharge clinics have been scantly described in the literature. The primary unanswered question remains the effect on readmissions, but this has been the sole research focus to date. Other key research areas are the impact on other patient-centered clinical and system outcomes (eg, patient satisfaction, particularly for patients seeing new providers), postdischarge mortality, the effect on other adverse events, and total medical expenditure.
The healthcare system is evolving in the context of a focus on readmissions, primary care access challenges, and high-risk patients’ specific needs. These forces are spurring innovation in the realm of postdischarge physician clinics, as even the basic need for an appointment may not be met by the existing outpatient primary care system. In this context, multiple new outpatient care structures have arisen, many staffed by hospitalists. Some, such as clinics based in safety net hospitals and academic medical centers, address the simple requirement that patients who lack easy access, because of insurance status or provider availability, can see a doctor after discharge. This type of clinic may be an essential step in alleviating a strained system but may not represent a sustainable long-term solution. More comprehensive solutions for improving patient care and clinical outcomes may be offered by integrated systems, such as CareMore, which also emerged from the hospitalist model. A lasting question is whether these clinics, both the narrowly focused and the comprehensive, will have longevity in the evolving healthcare market. Inevitably, though, hospitalist directors will continue to raise such questions, and should stand to benefit from the experiences of others described in this review.
Disclosure
Nothing to report.
1. US Department of Health and Human Services, Centers for Medicare & Medicaid Services. Transitional Care Management Services. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/Downloads/Transitional-Care-Management-Services-Fact-Sheet-ICN908628.pdf. Fact sheet ICN 908628.. Accessed June 29, 2016.
2. Kravet SJ, Shore AD, Miller R, Green GB, Kolodner K, Wright SM. Health care utilization and the proportion of primary care physicians. Am J Med. 2008;121(2):142-148. PubMed
3. Hasan O, Meltzer DO, Shaykevich SA, et al. Hospital readmission in general medicine patients: a prediction model. J Gen Intern Med. 2010;25(3):211-219. PubMed
4. Hansen LO, Young RS, Hinami K, Leung A, Williams MV. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520-528. PubMed
5. Leppin AL, Gionfriddo MR, Kessler M, et al. Preventing 30-day hospital readmissions: a systematic review and meta-analysis of randomized trials. JAMA Intern Med. 2014;174(7):1095-1107. PubMed
6. Weinberger M, Oddone EZ, Henderson WG. Does increased access to primary care reduce hospital readmissions? Veterans Affairs Cooperative Study Group on Primary Care and Hospital Readmission. N Engl J Med. 1996;334(22):1441-1447. PubMed
7. DeLia D, Tong J, Gaboda D, Casalino LP. Post-discharge follow-up visits and hospital utilization by Medicare patients, 2007-2010. Medicare Medicaid Res Rev. 2014;4(2). PubMed
8. Dedhia P, Kravet S, Bulger J, et al. A quality improvement intervention to facilitate the transition of older adults from three hospitals back to their homes. J Am Geriatr Soc. 2009;57(9):1540-1546. PubMed
9. Jackson C, Shahsahebi M, Wedlake T, DuBard CA. Timeliness of outpatient follow-up: an evidence-based approach for planning after hospital discharge. Ann Fam Med. 2015;13(2):115-122. PubMed
10. Dharmarajan K, Hsieh AF, Lin Z, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309(4):355-363. PubMed
11. Hernandez AF, Greiner MA, Fonarow GC, et al. Relationship between early physician follow-up and 30-day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303(17):1716-1722. PubMed
12. Lee KK, Yang J, Hernandez AF, Steimle AE, Go AS. Post-discharge follow-up characteristics associated with 30-day readmission after heart failure hospitalization. Med Care. 2016;54(4):365-372. PubMed
13. Erickson KF, Winkelmayer WC, Chertow GM, Bhattacharya J. Physician visits and 30-day hospital readmissions in patients receiving hemodialysis. J Am Soc Nephrol. 2014;25(9):2079-2087. PubMed
14. Sharma G, Kuo YF, Freeman JL, Zhang DD, Goodwin JS. Outpatient follow-up visit and 30-day emergency department visit and readmission in patients hospitalized for chronic obstructive pulmonary disease. Arch Intern Med. 2010;170(18):1664-1670. PubMed
15. Fidahussein SS, Croghan IT, Cha SS, Klocke DL. Posthospital follow-up visits and 30-day readmission rates in chronic obstructive pulmonary disease. Risk Manag Healthc Policy. 2014;7:105-112. PubMed
16. Burke RE, Whitfield E, Prochazka AV. Effect of a hospitalist-run postdischarge clinic on outcomes. J Hosp Med. 2014;9(1):7-12. PubMed
17. Doctoroff L, Nijhawan A, McNally D, Vanka A, Yu R, Mukamal KJ. The characteristics and impact of a hospitalist-staffed post-discharge clinic. Am J Med. 2013;126(11):1016.e9-e15. PubMed
18. Allaudeen N, Schnipper JL, Orav EJ, Wachter RM, Vidyarthi AR. Inability of providers to predict unplanned readmissions. J Gen Intern Med. 2011;26(7):771-776. PubMed
19. Barnett ML, Hsu J, McWilliams J. Patient characteristics and differences in hospital readmission rates. JAMA Intern Med. 2015;175(11):1803-1812. PubMed
20. Jack BW, Chetty VK, Anthony D, et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178-187. PubMed
21. Naylor M, Brooten D, Jones R, Lavizzo-Mourey R, Mezey M, Pauly M. Comprehensive discharge planning for the hospitalized elderly. A randomized clinical trial. Ann Intern Med. 1994;120(12):999-1006. PubMed
22. Capp R, Camp-Binford M, Sobolewski S, Bulmer S, Kelley L. Do adult Medicaid enrollees prefer going to their primary care provider’s clinic rather than emergency department (ED) for low acuity conditions? Med Care. 2015;53(6):530-533. PubMed
23. Vargas Bustamante A, Fang H, Garza J, et al. Variations in healthcare access and utilization among Mexican immigrants: the role of documentation status. J Immigr Minor Health. 2012;14(1):146-155. PubMed
24. Chi JT, Handcock MS. Identifying sources of health care underutilization among California’s immigrants. J Racial Ethn Health Disparities. 2014;1(3):207-218. PubMed
25. Martinez S. Bridging the Gap: Discharge Clinics Providing Safe Transitions for High Risk Patients. Workshop presented at: Northwest Patient Safety Conference; May 15, 2012; Seattle, WA. http://www.wapatientsafety.org/downloads/Martinez.pdf. Published 2011. Accessed April 26, 2017.
26. Elliott K, W Klein J, Basu A, Sabbatini AK. Transitional care clinics for follow-up and primary care linkage for patients discharged from the ED. Am J Emerg Med. 2016;34(7):1230-1235. PubMed
27. Baxley EG, Weir S. Advanced access in academic settings: definitional challenges. Ann Fam Med. 2009;7(1):90-91. PubMed
28. Doctoroff L, McNally D, Vanka A, Nall R, Mukamal KJ. Inpatient–outpatient transitions for patients with resident primary care physicians: access and readmission. Am J Med. 2014;127(9):886.e15-e20. PubMed
29. Shah M, Douglas V, Scott B, Josephson SA. A neurohospitalist discharge clinic shortens the transition from inpatient to outpatient care. Neurohospitalist. 2016;6(2):64-69. PubMed
30. Powers BW, Milstein A, Jain SH. Delivery models for high-risk older patients: back to the future? JAMA. 2016;315(1):23-24. PubMed
31. Milstein A, Gilbertson E. American medical home runs. Health Aff (Millwood). 2009;28(5):1317-1326. PubMed
32. Reuben DB. Physicians in supporting roles in chronic disease care: the CareMore model. J Am Geriatr Soc. 2011;59(1):158-160. PubMed
33. Meltzer DO, Ruhnke GW. Redesigning care for patients at increased hospitalization risk: the comprehensive care physician model. Health Aff (Millwood). 2014;33(5):770-777. PubMed
34. Burke RE, Ryan P. Postdischarge clinics: hospitalist attitudes and experiences. J Hosp Med. 2013;8(10):578-581. PubMed
Readmission prevention is paramount for hospitals and, by extension, hospitalist programs. Hospitalists see early and reliable outpatient follow-up as a safe landing for their most complicated patient cases. The option of a postdischarge clinic arises from the challenge to arrange adequate postdischarge care for patients who lack easy access because of insurance or provider availability. Guaranteeing postdischarge access by opening a dedicated, hospitalist-led postdischarge clinic appears to be an easy solution, but it is a solution that requires significant investment (including investment in physician and staff training and administrative support) and careful navigation of existing primary care relationships. In addition, a clinic staffed only with physicians may not be well equipped to address the complex social factors in healthcare utilization and readmission. Better understanding of the evidence supporting post discharge physician visits, several models of clinics, and the key operational questions are essential to address before crossing the inpatient-outpatient divide.
POSTDISCHARGE PHYSICIAN VISITS AND READMISSIONS
A postdischarge outpatient provider visit is often seen as a key factor in reducing readmissions. In 2013, Medicare added strength to this association by establishing transitional care management codes, which provide enhanced reimbursement to providers for a visit within 7 or 14 days of discharge, with focused attention on transitional issues.1 However, whether a postdischarge visit reduces readmissions remains unclear. Given evidence that higher primary care density is associated with lower healthcare utilization,2 CMS’s financial investment in incentivizing post discharge physician visits may be a good bet. On the other hand, simply having a primary care physician (PCP) may be a risk factor for readmission. This association suggests that postdischarge vigilance leads to identification of medical problems that lead to rehospitalization.3 This uncertainty is not resolved in systematic reviews of readmission reduction initiatives, which were not focused solely on the impact of a physician visit.4,5
The earliest study of postdischarge visits in a general medical population found an association between intensive outpatient follow-up by new providers in a Veterans Affairs population and an increase in hospital readmissions.6 This model is similar to some hospitalist models for postdischarge clinics, as the visit was with a noncontinuity provider. The largest recent study, of patients hospitalized with acute myocardial infarction, community-acquired pneumonia, or congestive heart failure (CHF) between 2009 and 2012, found increased frequency of postdischarge follow-up but no concomitant reduction in readmissions.7 Although small observational studies8 have found a postdischarge primary care visit may reduce the risk for readmission in general medical patients, the bulk of the recent data is negative.
In high-risk patients, however, there may be a clear benefit to postdischarge follow-up. In a North Carolina Medicaid population, a physician visit after discharge was associated with fewer readmissions among high-risk patients, but not among lower risk patients, whose readmission rates were low to start.9 The results of that study support the idea that risk stratification may identify patients who can benefit from more intensive outpatient follow-up. In general medical populations, existing studies may suffer from an absence of adequate risk assessment.
The evidence in specific disease states may show a clearer association between a postdischarge physician visit and reduced risk for readmission. One quarter of patients with CHF are rehospitalized within 30 days of discharge.10 In this disease with frequent exacerbations, a clinic visit to monitor volume status, weight, and medication adherence might reduce the frequency of readmissions or prolong the interval between rehospitalizations. A large observational study observed that earlier post discharge follow up by a cardiologist or a PCP was associated with lower risk of readmission, but only in the quintile with the closest follow-up. In addition, fewer than 40% of patients in this group had a visit within 7 days.11 In another heart failure population, follow-up with either a PCP or cardiologist within 7 days of discharge was again associated with lower risk for readmission.12 Thus, data suggest a protective effect of postdischarge visits in CHF patients, in contrast to a general medical population. Patients with end-stage renal disease may also fit in this group protected by a postdischarge physician visit, as 1 additional visit within the month after discharge was estimated to reduce rehospitalizations and produce significant cost savings.13
With other specific discharge diagnoses, results are varied. Two small observational studies in chronic obstructive pulmonary disease had conflicting results—one found a modest reduction in readmission and emergency department (ED) visits for patients seen by a PCP or pulmonologist within 30 days of discharge,14 and the other found no effect on readmissions but an associated reduction in mortality.15 More data are needed to clarify further the interaction of postdischarge visits with mortality, but the association between postdischarge physician visits and readmission reduction is controversial for patients with chronic obstructive pulmonary disease.
Finally, the evidence for dedicated postdischarge clinics is even more limited. A study of a hospitalist-led postdischarge clinic in a Veterans Affairs hospital found reduced length of stay and earlier postdischarge follow-up in a postdischarge clinic, but no effect on readmissions.16 Other studies have found earlier postdischarge follow-up with dedicated discharge clinics but have not evaluated readmission rates specifically.17In summary, the effect of postdischarge visits on risk for readmission is an area of active research, but remains unclear. The data reviewed suggest a benefit for the highest risk patients, specifically those with severe chronic illness, or those deemed high-risk with a readmission tool.9 At present, because physicians cannot accurately predict which patients will be readmitted,18 discharging physicians often take a broad approach and schedule outpatient visits for all patients. As readmission tools are further refined, the group of patients who will benefit from postdischarge care will be easier to identify, and a benefit to postdischarge visits may be seen
It is also important to note that this review emphasizes the physician visit and its potential impact on readmissions. Socioeconomic causes are increasingly being recognized as driving readmissions and other utilization.19 Whether an isolated physician visit is sufficient to prevent readmissions for patients with nonmedical drivers of healthcare utilization is unclear. For those patients, a discharge visit likely is a necessary component of a readmission reduction strategy for high-risk patients, but may be insufficient for patients who require not just an isolated visit but rather a more integrated and comprehensive care program.8,20,21
POSTDISCHARGE CLINIC MODELS
Despite the unclear relationship between postdischarge physician care and readmissions, dedicated postdischarge clinics, some staffed by hospitalists, have been adopted over the past 10 years. The three primary types of clinics arise in safety net environments, in academic medical centers, and as comprehensive high-risk patient solutions. Reviewing several types of clinics further clarifies the nature of this structural innovation.
Safety Net Hospital Models
Safety net hospitals and their hospitalists struggle with securing adequate postdischarge access for their population, which has inadequate insurance and poor access to primary care. Patient characteristics also play a role in the complex postdischarge care for this population, given its high rate of ED use (owing to perceived convenience and capabilities) for ambulatory-sensitive conditions.22 In addition, immigrants, particularly those with low English-language proficiency, underuse and have poor access to primary care.23,24 Postdischarge clinics in this environment focus first on providing a reliable postdischarge plan and then on linking to primary care. Examples of two clinics are at Harborview Medical Center in Seattle, Washington25 and Texas Health in Fort Worth.
Harborview is a 400-bed hospital affiliated with the University of Washington. More than 50% of its patients are considered indigent. The clinic was established in 2007 to provide a postdischarge option for uninsured patients, and a link to primary care in federally qualified health centers. The clinic was staffed 5 days a week with one or two hospitalists or advanced practice nurses. Visit duration was 20 minutes, 270 visits occurred per month, and the no-show rate was 30%. A small subgroup of the hospitalist group staffed the clinic. Particular clinical foci included CHF patients, patients with wound-care needs, and homeless, immigrant, and recently incarcerated patients. A key goal was connecting to longitudinal primary care, and the clinic successfully connected more than 70% of patients to primary care in community health centers. This clinic ultimately transitioned from a hospitalist practice to a primary care practice with a primary focus on post-ED follow-up for unaffiliated patients.26
In 2010, Texas Health faced a similar challenge with unaffiliated patients, and established a nurse practitioner–based clinic with hospitalist oversight to provide care primarily for patients without insurance or without an existing primary care relationship.
Academic Medical Center Models
Another clinical model is designed for patients who receive primary care at practices affiliated with academic medical centers. Although many of these patients have insurance and a PCP, there is often no availability with their continuity provider, because of the resident’s inpatient schedule or the faculty member’s conflicting priorities.27,28 Academic medical centers, including the University of California at San Francisco, the University of New Mexico, and the Beth Israel Deaconess Medical Center, have established discharge clinics within their faculty primary care practices. A model of this type of clinic was set up at Beth Israel Deaconess in 2010. Staffed by four hospitalists and using 40-minute appointments, this clinic was physically based in the primary care practice. As such, it took advantage of the existing clinic’s administrative and clinical functions, including triage, billing, and scheduling. A visit was scheduled in that clinic by the discharging physician team if a primary care appointment was not available with the patient’s continuity provider. Visits were standardized and focused on outstanding issues at discharge, medication reconciliation, and symptom trajectory. The hospitalists used the clinic’s clinical resources, including nurses, social workers, and pharmacists, but had no other dedicated staff. That there were only four hospitalists meant they were able to gain sufficient exposure to the outpatient setting, provide consistent high-quality care, and gain credibility with the PCPs. As the patients who were seen had PCPs of their own, during the visit significant attention was focused first on the postdischarge concerns, and then on promptly returning the patients to routine primary care. Significant patient outreach was used to address the clinic’s no-show rate, which was almost 50% in the early months. Within a year, the rate was down, closer to 20%. This clinic closed in 2015 after the primary care practice, in which it was based, transitioned to a patient-centered medical home. Since that time, this type of initiative has spread further, with neurohospitalist discharge clinics established, and postdischarge neurology follow-up becoming faster and more reliable.29
Academic medical centers and safety net hospitals substitute for routine primary care to address the basic challenge of primary care access, often without significant enhancements or additional resources, such as dedicated care management and pharmacy, social work, and nursing support. Commonalities of these clinics include dedicated physician staff, appointments generally longer than average outpatient appointments, and visit content concentrated on the key issues at transition (medication reconciliation, outstanding tests, symptom trajectory). As possible, clinics adopted a multidisciplinary approach, with social workers, community health workers, and nurses, to respond to the breadth of patients’ postdischarge needs, which often extend beyond pure medical need. The most frequent barriers encountered included the knowledge gap for hospitalist providers in the outpatient setting (a gap mitigated by using dedicated providers) and the patients’ high no-show rate (not surprising given that the providers are generally new to them). Few clinics have attempted to create continuity across inpatient and outpatient providers, though continuity might reduce no-shows as well as eliminate at least 1 transition.
Comprehensive High-Risk Patient Solutions
At the other end of the clinic spectrum are more integrated postdischarge approaches, which also evolved from the hospitalist model with hospitalist staffing. However, these approaches were introduced in response to the clinical needs of the highest risk patients (who are most vulnerable to frequent provider transitions), not to a systemic inability to provide routine postdischarge care.30
The most long-standing model for this type of clinic is represented by CareMore Health System, a subsidiary of Anthem.30-32 The extensivist, an expanded-scope hospitalist, acts as primary care coordinator, coordinating a multidisciplinary team for a panel of about 100 patients, representing the sickest 5% of the Medicare Advantage–insured population. Unlike the traditional hospitalist, the extensivist follows patients across all care sites, including hospital, rehabilitation sites, and outpatient clinic. For the most part, this relationship is not designed to evolve into a longitudinal relationship, but rather is an intervention only for the several-months period of acute need. Internal data have shown effects on hospital readmissions as well as length of stay.30
Another integrated clinic was established in 2013, at the University of Chicago. This was an effort to redesign care for patients at highest risk for hospitalization.33 Similar to the CareMore process, a high-risk population is identified by prior hospitalization and expected high Medicare costs. A comprehensive care physician cares for these patients across care settings. The clinic takes a team-based approach to patient care, with team members selected on the basis of patient need. Physicians have panels limited to only 200 patients, and generally spend part of the day in clinic, and part in seeing their hospitalized patients. Although reminiscent of a traditional primary care setting, this clinic is designed specifically for a high-risk, frequently hospitalized population, and therefore requires physicians with both a skill set akin to that of hospitalists, and an approach of palliative care and holistic patient care. Outcomes from this trial clinic are expected in 2017 or 2018.
LOGISTICAL CONSIDERATIONS FOR DISCHARGE CLINICS
Considering some key operational questions (Table) can help guide hospitals, hospitalists, and healthcare systems as they venture into the postdischarge clinic space. Return on investment and sustainability are two key questions for postdischarge clinics.
Return on investment varies by payment structure. In capitated environments with a strong emphasis on readmissions and total medical expenditure, a successful postdischarge clinic would recoup the investment through readmission reduction. However, maintaining adequate patient volume against high no-show rates may strain the group financially. In addition, although a hospitalist group may reap few measurable benefits from this clinical exposure, the unique view of the outpatient world afforded to hospitalists working in this environment could enrich the group as a whole by providing a more well-rounded vantage point.
Another key question surrounds sustainability. The clinic at the Beth Israel Deaconess Medical Center in Boston temporarily closed due to high inpatient volume and corresponding need for those hospitalists in the inpatient setting, early in its inception. It subsequently closed due to evolution in the clinic where it was based, rendering it unnecessary. Clinics that are contingent on other clinics will be vulnerable to external forces. Finally, staffing these clinics may be a stretch for a hospitalist group, as a partly different skill set is required for patient care in the outpatient setting. Hospitalists interested in care transitions are well suited for this role. In addition, hospitalists interested in more clinical variety, or in more schedule variety than that provided in a traditional hospitalist schedule, often enjoy the work. A vast majority of hospitalists think PCPs are responsible for postdischarge problems, and would not be interested in working in the postdischarge world.34 A poor fit for providers may lead to clinic failure.
As evident from this review, gaps in understanding the benefits of postdischarge care have persisted for 10 years. Discharge clinics have been scantly described in the literature. The primary unanswered question remains the effect on readmissions, but this has been the sole research focus to date. Other key research areas are the impact on other patient-centered clinical and system outcomes (eg, patient satisfaction, particularly for patients seeing new providers), postdischarge mortality, the effect on other adverse events, and total medical expenditure.
The healthcare system is evolving in the context of a focus on readmissions, primary care access challenges, and high-risk patients’ specific needs. These forces are spurring innovation in the realm of postdischarge physician clinics, as even the basic need for an appointment may not be met by the existing outpatient primary care system. In this context, multiple new outpatient care structures have arisen, many staffed by hospitalists. Some, such as clinics based in safety net hospitals and academic medical centers, address the simple requirement that patients who lack easy access, because of insurance status or provider availability, can see a doctor after discharge. This type of clinic may be an essential step in alleviating a strained system but may not represent a sustainable long-term solution. More comprehensive solutions for improving patient care and clinical outcomes may be offered by integrated systems, such as CareMore, which also emerged from the hospitalist model. A lasting question is whether these clinics, both the narrowly focused and the comprehensive, will have longevity in the evolving healthcare market. Inevitably, though, hospitalist directors will continue to raise such questions, and should stand to benefit from the experiences of others described in this review.
Disclosure
Nothing to report.
Readmission prevention is paramount for hospitals and, by extension, hospitalist programs. Hospitalists see early and reliable outpatient follow-up as a safe landing for their most complicated patient cases. The option of a postdischarge clinic arises from the challenge to arrange adequate postdischarge care for patients who lack easy access because of insurance or provider availability. Guaranteeing postdischarge access by opening a dedicated, hospitalist-led postdischarge clinic appears to be an easy solution, but it is a solution that requires significant investment (including investment in physician and staff training and administrative support) and careful navigation of existing primary care relationships. In addition, a clinic staffed only with physicians may not be well equipped to address the complex social factors in healthcare utilization and readmission. Better understanding of the evidence supporting post discharge physician visits, several models of clinics, and the key operational questions are essential to address before crossing the inpatient-outpatient divide.
POSTDISCHARGE PHYSICIAN VISITS AND READMISSIONS
A postdischarge outpatient provider visit is often seen as a key factor in reducing readmissions. In 2013, Medicare added strength to this association by establishing transitional care management codes, which provide enhanced reimbursement to providers for a visit within 7 or 14 days of discharge, with focused attention on transitional issues.1 However, whether a postdischarge visit reduces readmissions remains unclear. Given evidence that higher primary care density is associated with lower healthcare utilization,2 CMS’s financial investment in incentivizing post discharge physician visits may be a good bet. On the other hand, simply having a primary care physician (PCP) may be a risk factor for readmission. This association suggests that postdischarge vigilance leads to identification of medical problems that lead to rehospitalization.3 This uncertainty is not resolved in systematic reviews of readmission reduction initiatives, which were not focused solely on the impact of a physician visit.4,5
The earliest study of postdischarge visits in a general medical population found an association between intensive outpatient follow-up by new providers in a Veterans Affairs population and an increase in hospital readmissions.6 This model is similar to some hospitalist models for postdischarge clinics, as the visit was with a noncontinuity provider. The largest recent study, of patients hospitalized with acute myocardial infarction, community-acquired pneumonia, or congestive heart failure (CHF) between 2009 and 2012, found increased frequency of postdischarge follow-up but no concomitant reduction in readmissions.7 Although small observational studies8 have found a postdischarge primary care visit may reduce the risk for readmission in general medical patients, the bulk of the recent data is negative.
In high-risk patients, however, there may be a clear benefit to postdischarge follow-up. In a North Carolina Medicaid population, a physician visit after discharge was associated with fewer readmissions among high-risk patients, but not among lower risk patients, whose readmission rates were low to start.9 The results of that study support the idea that risk stratification may identify patients who can benefit from more intensive outpatient follow-up. In general medical populations, existing studies may suffer from an absence of adequate risk assessment.
The evidence in specific disease states may show a clearer association between a postdischarge physician visit and reduced risk for readmission. One quarter of patients with CHF are rehospitalized within 30 days of discharge.10 In this disease with frequent exacerbations, a clinic visit to monitor volume status, weight, and medication adherence might reduce the frequency of readmissions or prolong the interval between rehospitalizations. A large observational study observed that earlier post discharge follow up by a cardiologist or a PCP was associated with lower risk of readmission, but only in the quintile with the closest follow-up. In addition, fewer than 40% of patients in this group had a visit within 7 days.11 In another heart failure population, follow-up with either a PCP or cardiologist within 7 days of discharge was again associated with lower risk for readmission.12 Thus, data suggest a protective effect of postdischarge visits in CHF patients, in contrast to a general medical population. Patients with end-stage renal disease may also fit in this group protected by a postdischarge physician visit, as 1 additional visit within the month after discharge was estimated to reduce rehospitalizations and produce significant cost savings.13
With other specific discharge diagnoses, results are varied. Two small observational studies in chronic obstructive pulmonary disease had conflicting results—one found a modest reduction in readmission and emergency department (ED) visits for patients seen by a PCP or pulmonologist within 30 days of discharge,14 and the other found no effect on readmissions but an associated reduction in mortality.15 More data are needed to clarify further the interaction of postdischarge visits with mortality, but the association between postdischarge physician visits and readmission reduction is controversial for patients with chronic obstructive pulmonary disease.
Finally, the evidence for dedicated postdischarge clinics is even more limited. A study of a hospitalist-led postdischarge clinic in a Veterans Affairs hospital found reduced length of stay and earlier postdischarge follow-up in a postdischarge clinic, but no effect on readmissions.16 Other studies have found earlier postdischarge follow-up with dedicated discharge clinics but have not evaluated readmission rates specifically.17In summary, the effect of postdischarge visits on risk for readmission is an area of active research, but remains unclear. The data reviewed suggest a benefit for the highest risk patients, specifically those with severe chronic illness, or those deemed high-risk with a readmission tool.9 At present, because physicians cannot accurately predict which patients will be readmitted,18 discharging physicians often take a broad approach and schedule outpatient visits for all patients. As readmission tools are further refined, the group of patients who will benefit from postdischarge care will be easier to identify, and a benefit to postdischarge visits may be seen
It is also important to note that this review emphasizes the physician visit and its potential impact on readmissions. Socioeconomic causes are increasingly being recognized as driving readmissions and other utilization.19 Whether an isolated physician visit is sufficient to prevent readmissions for patients with nonmedical drivers of healthcare utilization is unclear. For those patients, a discharge visit likely is a necessary component of a readmission reduction strategy for high-risk patients, but may be insufficient for patients who require not just an isolated visit but rather a more integrated and comprehensive care program.8,20,21
POSTDISCHARGE CLINIC MODELS
Despite the unclear relationship between postdischarge physician care and readmissions, dedicated postdischarge clinics, some staffed by hospitalists, have been adopted over the past 10 years. The three primary types of clinics arise in safety net environments, in academic medical centers, and as comprehensive high-risk patient solutions. Reviewing several types of clinics further clarifies the nature of this structural innovation.
Safety Net Hospital Models
Safety net hospitals and their hospitalists struggle with securing adequate postdischarge access for their population, which has inadequate insurance and poor access to primary care. Patient characteristics also play a role in the complex postdischarge care for this population, given its high rate of ED use (owing to perceived convenience and capabilities) for ambulatory-sensitive conditions.22 In addition, immigrants, particularly those with low English-language proficiency, underuse and have poor access to primary care.23,24 Postdischarge clinics in this environment focus first on providing a reliable postdischarge plan and then on linking to primary care. Examples of two clinics are at Harborview Medical Center in Seattle, Washington25 and Texas Health in Fort Worth.
Harborview is a 400-bed hospital affiliated with the University of Washington. More than 50% of its patients are considered indigent. The clinic was established in 2007 to provide a postdischarge option for uninsured patients, and a link to primary care in federally qualified health centers. The clinic was staffed 5 days a week with one or two hospitalists or advanced practice nurses. Visit duration was 20 minutes, 270 visits occurred per month, and the no-show rate was 30%. A small subgroup of the hospitalist group staffed the clinic. Particular clinical foci included CHF patients, patients with wound-care needs, and homeless, immigrant, and recently incarcerated patients. A key goal was connecting to longitudinal primary care, and the clinic successfully connected more than 70% of patients to primary care in community health centers. This clinic ultimately transitioned from a hospitalist practice to a primary care practice with a primary focus on post-ED follow-up for unaffiliated patients.26
In 2010, Texas Health faced a similar challenge with unaffiliated patients, and established a nurse practitioner–based clinic with hospitalist oversight to provide care primarily for patients without insurance or without an existing primary care relationship.
Academic Medical Center Models
Another clinical model is designed for patients who receive primary care at practices affiliated with academic medical centers. Although many of these patients have insurance and a PCP, there is often no availability with their continuity provider, because of the resident’s inpatient schedule or the faculty member’s conflicting priorities.27,28 Academic medical centers, including the University of California at San Francisco, the University of New Mexico, and the Beth Israel Deaconess Medical Center, have established discharge clinics within their faculty primary care practices. A model of this type of clinic was set up at Beth Israel Deaconess in 2010. Staffed by four hospitalists and using 40-minute appointments, this clinic was physically based in the primary care practice. As such, it took advantage of the existing clinic’s administrative and clinical functions, including triage, billing, and scheduling. A visit was scheduled in that clinic by the discharging physician team if a primary care appointment was not available with the patient’s continuity provider. Visits were standardized and focused on outstanding issues at discharge, medication reconciliation, and symptom trajectory. The hospitalists used the clinic’s clinical resources, including nurses, social workers, and pharmacists, but had no other dedicated staff. That there were only four hospitalists meant they were able to gain sufficient exposure to the outpatient setting, provide consistent high-quality care, and gain credibility with the PCPs. As the patients who were seen had PCPs of their own, during the visit significant attention was focused first on the postdischarge concerns, and then on promptly returning the patients to routine primary care. Significant patient outreach was used to address the clinic’s no-show rate, which was almost 50% in the early months. Within a year, the rate was down, closer to 20%. This clinic closed in 2015 after the primary care practice, in which it was based, transitioned to a patient-centered medical home. Since that time, this type of initiative has spread further, with neurohospitalist discharge clinics established, and postdischarge neurology follow-up becoming faster and more reliable.29
Academic medical centers and safety net hospitals substitute for routine primary care to address the basic challenge of primary care access, often without significant enhancements or additional resources, such as dedicated care management and pharmacy, social work, and nursing support. Commonalities of these clinics include dedicated physician staff, appointments generally longer than average outpatient appointments, and visit content concentrated on the key issues at transition (medication reconciliation, outstanding tests, symptom trajectory). As possible, clinics adopted a multidisciplinary approach, with social workers, community health workers, and nurses, to respond to the breadth of patients’ postdischarge needs, which often extend beyond pure medical need. The most frequent barriers encountered included the knowledge gap for hospitalist providers in the outpatient setting (a gap mitigated by using dedicated providers) and the patients’ high no-show rate (not surprising given that the providers are generally new to them). Few clinics have attempted to create continuity across inpatient and outpatient providers, though continuity might reduce no-shows as well as eliminate at least 1 transition.
Comprehensive High-Risk Patient Solutions
At the other end of the clinic spectrum are more integrated postdischarge approaches, which also evolved from the hospitalist model with hospitalist staffing. However, these approaches were introduced in response to the clinical needs of the highest risk patients (who are most vulnerable to frequent provider transitions), not to a systemic inability to provide routine postdischarge care.30
The most long-standing model for this type of clinic is represented by CareMore Health System, a subsidiary of Anthem.30-32 The extensivist, an expanded-scope hospitalist, acts as primary care coordinator, coordinating a multidisciplinary team for a panel of about 100 patients, representing the sickest 5% of the Medicare Advantage–insured population. Unlike the traditional hospitalist, the extensivist follows patients across all care sites, including hospital, rehabilitation sites, and outpatient clinic. For the most part, this relationship is not designed to evolve into a longitudinal relationship, but rather is an intervention only for the several-months period of acute need. Internal data have shown effects on hospital readmissions as well as length of stay.30
Another integrated clinic was established in 2013, at the University of Chicago. This was an effort to redesign care for patients at highest risk for hospitalization.33 Similar to the CareMore process, a high-risk population is identified by prior hospitalization and expected high Medicare costs. A comprehensive care physician cares for these patients across care settings. The clinic takes a team-based approach to patient care, with team members selected on the basis of patient need. Physicians have panels limited to only 200 patients, and generally spend part of the day in clinic, and part in seeing their hospitalized patients. Although reminiscent of a traditional primary care setting, this clinic is designed specifically for a high-risk, frequently hospitalized population, and therefore requires physicians with both a skill set akin to that of hospitalists, and an approach of palliative care and holistic patient care. Outcomes from this trial clinic are expected in 2017 or 2018.
LOGISTICAL CONSIDERATIONS FOR DISCHARGE CLINICS
Considering some key operational questions (Table) can help guide hospitals, hospitalists, and healthcare systems as they venture into the postdischarge clinic space. Return on investment and sustainability are two key questions for postdischarge clinics.
Return on investment varies by payment structure. In capitated environments with a strong emphasis on readmissions and total medical expenditure, a successful postdischarge clinic would recoup the investment through readmission reduction. However, maintaining adequate patient volume against high no-show rates may strain the group financially. In addition, although a hospitalist group may reap few measurable benefits from this clinical exposure, the unique view of the outpatient world afforded to hospitalists working in this environment could enrich the group as a whole by providing a more well-rounded vantage point.
Another key question surrounds sustainability. The clinic at the Beth Israel Deaconess Medical Center in Boston temporarily closed due to high inpatient volume and corresponding need for those hospitalists in the inpatient setting, early in its inception. It subsequently closed due to evolution in the clinic where it was based, rendering it unnecessary. Clinics that are contingent on other clinics will be vulnerable to external forces. Finally, staffing these clinics may be a stretch for a hospitalist group, as a partly different skill set is required for patient care in the outpatient setting. Hospitalists interested in care transitions are well suited for this role. In addition, hospitalists interested in more clinical variety, or in more schedule variety than that provided in a traditional hospitalist schedule, often enjoy the work. A vast majority of hospitalists think PCPs are responsible for postdischarge problems, and would not be interested in working in the postdischarge world.34 A poor fit for providers may lead to clinic failure.
As evident from this review, gaps in understanding the benefits of postdischarge care have persisted for 10 years. Discharge clinics have been scantly described in the literature. The primary unanswered question remains the effect on readmissions, but this has been the sole research focus to date. Other key research areas are the impact on other patient-centered clinical and system outcomes (eg, patient satisfaction, particularly for patients seeing new providers), postdischarge mortality, the effect on other adverse events, and total medical expenditure.
The healthcare system is evolving in the context of a focus on readmissions, primary care access challenges, and high-risk patients’ specific needs. These forces are spurring innovation in the realm of postdischarge physician clinics, as even the basic need for an appointment may not be met by the existing outpatient primary care system. In this context, multiple new outpatient care structures have arisen, many staffed by hospitalists. Some, such as clinics based in safety net hospitals and academic medical centers, address the simple requirement that patients who lack easy access, because of insurance status or provider availability, can see a doctor after discharge. This type of clinic may be an essential step in alleviating a strained system but may not represent a sustainable long-term solution. More comprehensive solutions for improving patient care and clinical outcomes may be offered by integrated systems, such as CareMore, which also emerged from the hospitalist model. A lasting question is whether these clinics, both the narrowly focused and the comprehensive, will have longevity in the evolving healthcare market. Inevitably, though, hospitalist directors will continue to raise such questions, and should stand to benefit from the experiences of others described in this review.
Disclosure
Nothing to report.
1. US Department of Health and Human Services, Centers for Medicare & Medicaid Services. Transitional Care Management Services. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/Downloads/Transitional-Care-Management-Services-Fact-Sheet-ICN908628.pdf. Fact sheet ICN 908628.. Accessed June 29, 2016.
2. Kravet SJ, Shore AD, Miller R, Green GB, Kolodner K, Wright SM. Health care utilization and the proportion of primary care physicians. Am J Med. 2008;121(2):142-148. PubMed
3. Hasan O, Meltzer DO, Shaykevich SA, et al. Hospital readmission in general medicine patients: a prediction model. J Gen Intern Med. 2010;25(3):211-219. PubMed
4. Hansen LO, Young RS, Hinami K, Leung A, Williams MV. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520-528. PubMed
5. Leppin AL, Gionfriddo MR, Kessler M, et al. Preventing 30-day hospital readmissions: a systematic review and meta-analysis of randomized trials. JAMA Intern Med. 2014;174(7):1095-1107. PubMed
6. Weinberger M, Oddone EZ, Henderson WG. Does increased access to primary care reduce hospital readmissions? Veterans Affairs Cooperative Study Group on Primary Care and Hospital Readmission. N Engl J Med. 1996;334(22):1441-1447. PubMed
7. DeLia D, Tong J, Gaboda D, Casalino LP. Post-discharge follow-up visits and hospital utilization by Medicare patients, 2007-2010. Medicare Medicaid Res Rev. 2014;4(2). PubMed
8. Dedhia P, Kravet S, Bulger J, et al. A quality improvement intervention to facilitate the transition of older adults from three hospitals back to their homes. J Am Geriatr Soc. 2009;57(9):1540-1546. PubMed
9. Jackson C, Shahsahebi M, Wedlake T, DuBard CA. Timeliness of outpatient follow-up: an evidence-based approach for planning after hospital discharge. Ann Fam Med. 2015;13(2):115-122. PubMed
10. Dharmarajan K, Hsieh AF, Lin Z, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309(4):355-363. PubMed
11. Hernandez AF, Greiner MA, Fonarow GC, et al. Relationship between early physician follow-up and 30-day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303(17):1716-1722. PubMed
12. Lee KK, Yang J, Hernandez AF, Steimle AE, Go AS. Post-discharge follow-up characteristics associated with 30-day readmission after heart failure hospitalization. Med Care. 2016;54(4):365-372. PubMed
13. Erickson KF, Winkelmayer WC, Chertow GM, Bhattacharya J. Physician visits and 30-day hospital readmissions in patients receiving hemodialysis. J Am Soc Nephrol. 2014;25(9):2079-2087. PubMed
14. Sharma G, Kuo YF, Freeman JL, Zhang DD, Goodwin JS. Outpatient follow-up visit and 30-day emergency department visit and readmission in patients hospitalized for chronic obstructive pulmonary disease. Arch Intern Med. 2010;170(18):1664-1670. PubMed
15. Fidahussein SS, Croghan IT, Cha SS, Klocke DL. Posthospital follow-up visits and 30-day readmission rates in chronic obstructive pulmonary disease. Risk Manag Healthc Policy. 2014;7:105-112. PubMed
16. Burke RE, Whitfield E, Prochazka AV. Effect of a hospitalist-run postdischarge clinic on outcomes. J Hosp Med. 2014;9(1):7-12. PubMed
17. Doctoroff L, Nijhawan A, McNally D, Vanka A, Yu R, Mukamal KJ. The characteristics and impact of a hospitalist-staffed post-discharge clinic. Am J Med. 2013;126(11):1016.e9-e15. PubMed
18. Allaudeen N, Schnipper JL, Orav EJ, Wachter RM, Vidyarthi AR. Inability of providers to predict unplanned readmissions. J Gen Intern Med. 2011;26(7):771-776. PubMed
19. Barnett ML, Hsu J, McWilliams J. Patient characteristics and differences in hospital readmission rates. JAMA Intern Med. 2015;175(11):1803-1812. PubMed
20. Jack BW, Chetty VK, Anthony D, et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178-187. PubMed
21. Naylor M, Brooten D, Jones R, Lavizzo-Mourey R, Mezey M, Pauly M. Comprehensive discharge planning for the hospitalized elderly. A randomized clinical trial. Ann Intern Med. 1994;120(12):999-1006. PubMed
22. Capp R, Camp-Binford M, Sobolewski S, Bulmer S, Kelley L. Do adult Medicaid enrollees prefer going to their primary care provider’s clinic rather than emergency department (ED) for low acuity conditions? Med Care. 2015;53(6):530-533. PubMed
23. Vargas Bustamante A, Fang H, Garza J, et al. Variations in healthcare access and utilization among Mexican immigrants: the role of documentation status. J Immigr Minor Health. 2012;14(1):146-155. PubMed
24. Chi JT, Handcock MS. Identifying sources of health care underutilization among California’s immigrants. J Racial Ethn Health Disparities. 2014;1(3):207-218. PubMed
25. Martinez S. Bridging the Gap: Discharge Clinics Providing Safe Transitions for High Risk Patients. Workshop presented at: Northwest Patient Safety Conference; May 15, 2012; Seattle, WA. http://www.wapatientsafety.org/downloads/Martinez.pdf. Published 2011. Accessed April 26, 2017.
26. Elliott K, W Klein J, Basu A, Sabbatini AK. Transitional care clinics for follow-up and primary care linkage for patients discharged from the ED. Am J Emerg Med. 2016;34(7):1230-1235. PubMed
27. Baxley EG, Weir S. Advanced access in academic settings: definitional challenges. Ann Fam Med. 2009;7(1):90-91. PubMed
28. Doctoroff L, McNally D, Vanka A, Nall R, Mukamal KJ. Inpatient–outpatient transitions for patients with resident primary care physicians: access and readmission. Am J Med. 2014;127(9):886.e15-e20. PubMed
29. Shah M, Douglas V, Scott B, Josephson SA. A neurohospitalist discharge clinic shortens the transition from inpatient to outpatient care. Neurohospitalist. 2016;6(2):64-69. PubMed
30. Powers BW, Milstein A, Jain SH. Delivery models for high-risk older patients: back to the future? JAMA. 2016;315(1):23-24. PubMed
31. Milstein A, Gilbertson E. American medical home runs. Health Aff (Millwood). 2009;28(5):1317-1326. PubMed
32. Reuben DB. Physicians in supporting roles in chronic disease care: the CareMore model. J Am Geriatr Soc. 2011;59(1):158-160. PubMed
33. Meltzer DO, Ruhnke GW. Redesigning care for patients at increased hospitalization risk: the comprehensive care physician model. Health Aff (Millwood). 2014;33(5):770-777. PubMed
34. Burke RE, Ryan P. Postdischarge clinics: hospitalist attitudes and experiences. J Hosp Med. 2013;8(10):578-581. PubMed
1. US Department of Health and Human Services, Centers for Medicare & Medicaid Services. Transitional Care Management Services. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/Downloads/Transitional-Care-Management-Services-Fact-Sheet-ICN908628.pdf. Fact sheet ICN 908628.. Accessed June 29, 2016.
2. Kravet SJ, Shore AD, Miller R, Green GB, Kolodner K, Wright SM. Health care utilization and the proportion of primary care physicians. Am J Med. 2008;121(2):142-148. PubMed
3. Hasan O, Meltzer DO, Shaykevich SA, et al. Hospital readmission in general medicine patients: a prediction model. J Gen Intern Med. 2010;25(3):211-219. PubMed
4. Hansen LO, Young RS, Hinami K, Leung A, Williams MV. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520-528. PubMed
5. Leppin AL, Gionfriddo MR, Kessler M, et al. Preventing 30-day hospital readmissions: a systematic review and meta-analysis of randomized trials. JAMA Intern Med. 2014;174(7):1095-1107. PubMed
6. Weinberger M, Oddone EZ, Henderson WG. Does increased access to primary care reduce hospital readmissions? Veterans Affairs Cooperative Study Group on Primary Care and Hospital Readmission. N Engl J Med. 1996;334(22):1441-1447. PubMed
7. DeLia D, Tong J, Gaboda D, Casalino LP. Post-discharge follow-up visits and hospital utilization by Medicare patients, 2007-2010. Medicare Medicaid Res Rev. 2014;4(2). PubMed
8. Dedhia P, Kravet S, Bulger J, et al. A quality improvement intervention to facilitate the transition of older adults from three hospitals back to their homes. J Am Geriatr Soc. 2009;57(9):1540-1546. PubMed
9. Jackson C, Shahsahebi M, Wedlake T, DuBard CA. Timeliness of outpatient follow-up: an evidence-based approach for planning after hospital discharge. Ann Fam Med. 2015;13(2):115-122. PubMed
10. Dharmarajan K, Hsieh AF, Lin Z, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309(4):355-363. PubMed
11. Hernandez AF, Greiner MA, Fonarow GC, et al. Relationship between early physician follow-up and 30-day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303(17):1716-1722. PubMed
12. Lee KK, Yang J, Hernandez AF, Steimle AE, Go AS. Post-discharge follow-up characteristics associated with 30-day readmission after heart failure hospitalization. Med Care. 2016;54(4):365-372. PubMed
13. Erickson KF, Winkelmayer WC, Chertow GM, Bhattacharya J. Physician visits and 30-day hospital readmissions in patients receiving hemodialysis. J Am Soc Nephrol. 2014;25(9):2079-2087. PubMed
14. Sharma G, Kuo YF, Freeman JL, Zhang DD, Goodwin JS. Outpatient follow-up visit and 30-day emergency department visit and readmission in patients hospitalized for chronic obstructive pulmonary disease. Arch Intern Med. 2010;170(18):1664-1670. PubMed
15. Fidahussein SS, Croghan IT, Cha SS, Klocke DL. Posthospital follow-up visits and 30-day readmission rates in chronic obstructive pulmonary disease. Risk Manag Healthc Policy. 2014;7:105-112. PubMed
16. Burke RE, Whitfield E, Prochazka AV. Effect of a hospitalist-run postdischarge clinic on outcomes. J Hosp Med. 2014;9(1):7-12. PubMed
17. Doctoroff L, Nijhawan A, McNally D, Vanka A, Yu R, Mukamal KJ. The characteristics and impact of a hospitalist-staffed post-discharge clinic. Am J Med. 2013;126(11):1016.e9-e15. PubMed
18. Allaudeen N, Schnipper JL, Orav EJ, Wachter RM, Vidyarthi AR. Inability of providers to predict unplanned readmissions. J Gen Intern Med. 2011;26(7):771-776. PubMed
19. Barnett ML, Hsu J, McWilliams J. Patient characteristics and differences in hospital readmission rates. JAMA Intern Med. 2015;175(11):1803-1812. PubMed
20. Jack BW, Chetty VK, Anthony D, et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178-187. PubMed
21. Naylor M, Brooten D, Jones R, Lavizzo-Mourey R, Mezey M, Pauly M. Comprehensive discharge planning for the hospitalized elderly. A randomized clinical trial. Ann Intern Med. 1994;120(12):999-1006. PubMed
22. Capp R, Camp-Binford M, Sobolewski S, Bulmer S, Kelley L. Do adult Medicaid enrollees prefer going to their primary care provider’s clinic rather than emergency department (ED) for low acuity conditions? Med Care. 2015;53(6):530-533. PubMed
23. Vargas Bustamante A, Fang H, Garza J, et al. Variations in healthcare access and utilization among Mexican immigrants: the role of documentation status. J Immigr Minor Health. 2012;14(1):146-155. PubMed
24. Chi JT, Handcock MS. Identifying sources of health care underutilization among California’s immigrants. J Racial Ethn Health Disparities. 2014;1(3):207-218. PubMed
25. Martinez S. Bridging the Gap: Discharge Clinics Providing Safe Transitions for High Risk Patients. Workshop presented at: Northwest Patient Safety Conference; May 15, 2012; Seattle, WA. http://www.wapatientsafety.org/downloads/Martinez.pdf. Published 2011. Accessed April 26, 2017.
26. Elliott K, W Klein J, Basu A, Sabbatini AK. Transitional care clinics for follow-up and primary care linkage for patients discharged from the ED. Am J Emerg Med. 2016;34(7):1230-1235. PubMed
27. Baxley EG, Weir S. Advanced access in academic settings: definitional challenges. Ann Fam Med. 2009;7(1):90-91. PubMed
28. Doctoroff L, McNally D, Vanka A, Nall R, Mukamal KJ. Inpatient–outpatient transitions for patients with resident primary care physicians: access and readmission. Am J Med. 2014;127(9):886.e15-e20. PubMed
29. Shah M, Douglas V, Scott B, Josephson SA. A neurohospitalist discharge clinic shortens the transition from inpatient to outpatient care. Neurohospitalist. 2016;6(2):64-69. PubMed
30. Powers BW, Milstein A, Jain SH. Delivery models for high-risk older patients: back to the future? JAMA. 2016;315(1):23-24. PubMed
31. Milstein A, Gilbertson E. American medical home runs. Health Aff (Millwood). 2009;28(5):1317-1326. PubMed
32. Reuben DB. Physicians in supporting roles in chronic disease care: the CareMore model. J Am Geriatr Soc. 2011;59(1):158-160. PubMed
33. Meltzer DO, Ruhnke GW. Redesigning care for patients at increased hospitalization risk: the comprehensive care physician model. Health Aff (Millwood). 2014;33(5):770-777. PubMed
34. Burke RE, Ryan P. Postdischarge clinics: hospitalist attitudes and experiences. J Hosp Med. 2013;8(10):578-581. PubMed
© 2017 Society of Hospital Medicine
Forgotten but not gone: Update on measles infection for hospitalists
Measles is a highly contagious acute respiratory illness that includes a characteristic rash. After exposure, up to 90% of susceptible persons develop measles.1 Even though it is considered a childhood illness, measles can affect people of all age groups. Measles continues to be major health problem around the world, despite the availability of a safe and effective vaccine, and it remains one of the leading causes of childhood mortality, with nearly 115,000 deaths reported by the World Health Organization2 in 2014. In 2000, measles was declared eliminated from the United States, but outbreaks still occasionally occur.3-6
The disease is self-limited, but some patients develop complications that may require hospitalization for treatment. People at highest risk for complications are children younger than 5 years, adults older than 20 years, pregnant women, and immunocompromised individuals.7
HISTORY AND EPIDEMIOLOGY
During the licensure of live measles vaccine in 1963, an average of 549,000 measles cases and 495 measles deaths, as well as 48,000 hospitalizations and 4000 encephalitis cases, were reported annually in the United States. Almost all Americans were affected by measles by adolescence.
Implementation of the 1-dose vaccine program substantially reduced reported incidence in the United States by 1988, and led to a dramatic decline in measles-related hospitalizations and deaths.3-6 The 2-dose MMR (measles, mumps, rubella) vaccination was introduced in 1989, and measles was declared eliminated in the United States in 2000.3-6
National–level one-dose MMR coverage among children 19-35 months has remained above 90% during the last two decades.8 NIS-Teen vaccination coverage data for 13- to 17-year-olds since 2008 has been near or above 90%,9 and 94% of children enrolled in kindergarten had evidence of 2 MMR doses in the 2014-2015 school year.10
A large multistate measles outbreak was reported in the United States in 2014-2015.4,11 One hundred fifty-nine cases were reported in the United States between January 4 and April 5, 2015. The majority of patients either were unvaccinated (45%) or had an unknown vaccination status (38%). Age ranged from 6 weeks to 70 years, and 22 patients (14%) were hospitalized.4
CLINICAL PRESENTATION AND PATHOPHYSIOLOGY
Measles is caused by an RNA-containing paramyxovirus that is spread by the respiratory route. Average incubation period from exposure to rash onset is 14 days (range, 7-21 days).12,13 Peak infectivity occurs during the prodromal phase, before rash onset (Figure 1), but patients are infectious from 4 days before rash onset through 4 days after rash onset.7,12,13
The disease prodrome consists of a high fever (39°C-40.5°C), coryza, cough, and conjunctivitis followed by Koplik spots (Figure 2A). Koplik spots are pathognomonic for measles but rarely discovered. They appear before the skin rash alongside second molars on the buccal surface of the cheeks. The spots usually disappear when the characteristic maculopapular, nonpruritic rash erupts initially at the hairline and behind the ears, and within four days progresses toward the trunk and limbs, including the palms and soles (Figures 2B, 2C).
The patient remains febrile while the rash spreads.12,13 Usually the fever resolves while the rash fades in the same order in which it appeared. Fever that persists for more than 5 days usually indicates complications.13
Cellular immunity plays an important role in host defense; the virus invades T lymphocytes and triggers suppressive cytokine (interleukin 4) production. Leukopenia, expansion of mainly measles-specific T and B lymphocytes, and replacement of lymphocyte memory cell population results in further depression of cellular immunity, and predisposes patients to secondary bacterial infections for up to 2 years after measles infection.14,15
Patients immunocompromised by congenital cellular immunity deficiency, cancer, human immunodeficiency virus (HIV) infection without effective antiretroviral therapy, or immunosuppression treatment are at higher risk for developing severe complications or dying from measles. As the rash may fail to develop in these patients, diagnosis can be challenging.16
Modified measles is milder and may occur in patients with preexisting partial immunity: those with an immunization history (2-dose vaccine effectiveness is ∼97%), and infants with minimal immunity from their mothers.1,7 Patients may have mild respiratory symptoms with rash but little or no fever.7
Atypical measles is now extremely rare. It was described only among people who were vaccinated with the killed vaccine in the United States between 1963 and 1968 and subsequently exposed to measles. The disease is characterized by high fever, edema of extremities, and a rash that develops on the palms and soles and spreads centerward. It is considered noncommunicable.17
Measles infection during pregnancy is associated with increased maternal and fetal morbidity. The virus can induce neonatal low birth weight, spontaneous abortion, intrauterine fetal death, and maternal death. Pregnant women with measles are more likely to be hospitalized.18,19
DIFFERENTIAL DIAGNOSIS
The presenting symptoms of primary measles infection are nonspecific, particularly if Koplik spots are not identified. The differential diagnosis for a patient who presents with high fever and rash include Kawasaki disease, dengue, parvovirus B19, serum sickness, syphilis, systemic lupus erythematous, toxic shock syndrome, enterovirus infection, human herpes virus 6 (roseola), viral hemorrhagic fever, drug eruption, infectious mononucleosis, Rocky Mountain spotted fever, rubella, scarlet fever, chikungunya, and Zika virus infection.
COMPLICATIONS
Measles complications can affect nearly every organ system (Table). Rates of complications from measles infection depend on age and underlying condition. Coexisting vitamin A deficiency increases complication rates.20
Bacterial infections in the setting of measles infection are more common in adults than in children, and are more severe among people who are malnourished or have an immunodeficiency disorder. The most common infectious complications, which involve the respiratory tract, include pneumonia, laryngotracheitis (“measles croup”), bronchitis, otitis media (most common complication among children in the United States), and sinusitis.7,13,21
Indications for hospitalizing children include respiratory distress, laryngeal obstruction, dehydration that requires intravenous fluids, diarrhea with more than 10 stools a day or bloody stool, severe anemia, altered mental status, convulsion, severe rash with developing hemorrhagic areas, extensive mouth ulcers, corneal clouding or ulcers, visual disturbance, and mastoiditis.22
Pneumonia is a common indication for hospitalizing adults.23,24 Measles-associated interstitial giant cell (Hecht) pneumonia is most often recognized among immunocompromised and malnourished patients.13 Primary pneumonia is caused by the measles virus, but bacterial superinfection can occur. The most common bacterial pathogens include Streptococcus, Pneumococcus, and Staphylococcus,13,24 and less commonly isolated organisms include gram-negative bacteria, such as Haemophilus influenzae, Pseudomonas aeruginosa, Neisseria meningitides, and Enterobacter cloacae.23
Uncommon complications of measles are myocarditis, glomerulonephritis, acute renal failure, and thrombocytopenic purpura.25,26
Neurologic complications in measles are an important concern. Measles-associated central nervous system complications are considered a result of an immune-mediated reaction to myelin protein and not from direct viral insult.26-28 Immunocompromised patients are at risk for developing fatal encephalitis, and those who survive often experience cognitive decline or seizures.
Measles is associated with four different encephalitic diseases: primary measles encephalitis, acute post-measles encephalomyelitis, measles inclusion body encephalitis, and subacute sclerosing panencephalitis.
Primary measles encephalitis is characterized by fever, headache, stiff neck, and meningeal signs. Onset occurs between 1 and 15 days after rash onset, and the disease affects 1/1000 patients. Seizure, altered mental status, and coma can also develop. Viral RNA detection in the cerebrospinal fluid (CSF) confirms the diagnosis.29Acute post-measles encephalomyelitis is more common in adults than in children.12 It typically develops after the rash fades and the other symptoms subside. Patients suddenly experience a recurrence of fevers or seizures. Deafness, intellectual decline, epilepsy, postencephalitic hyperkinesia, hemiplegia, and/or paraplegia also can develop.27-29
Measles inclusion body encephalitis is described only in immunocompromised patients, and onset occurs within 1 year of infection. Seizures are an initial and common symptom, and some patients also experience hemiplegia, stupor, hypertonia, and dysarthria.29 Diagnostic findings include seroconversion during the disease course, improvement after withholding of the immunosuppressive regimen, and normal CSF. Brain biopsy confirms the diagnosis.
Subacute sclerosing panencephalitis (SSPE) is a slowly progressing and untreatable degenerative neurologic disorder characterized by demyelination of multiple brain areas. SSPE develops 7 to 10 years after natural measles infection, and usually affects children or adolescents. Clinical presentation includes intellectual decline, frequent rhythmic myoclonic jerks, seizure, and dementia. As the disease progresses, coma, quadriplegia, vegetative state, and autonomic instability develop. Death usually occurs within 2 years of onset.30,31 In children, the risk for SSPE after measles infection is estimated to be 4 to 11 per 100,000 infections. After the 1989-1991 resurgence of measles in the United States, however, the risk for SSPE was estimated to be 22 per 100,000 infections.30-32 The pathogenesis of SSPE is not fully understood but is thought to result from persistent aberrant measles virus infection.32
The SSPE diagnosis is based on clinical presentation, presence of anti-measles antibodies in CSF, typical electroencephalography pattern (periodic paroxysmal bursts) with accompanying myoclonus, tissue analysis, and magnetic resonance imaging.30
LABORATORY DIAGNOSIS
Suspicion for measles should prompt immediate consultation with local or state public health officials. Laboratory testing can be carefully considered after consultation, and care is needed in interpreting serologic studies.
The mainstays of measles infection diagnosis are detection of viral RNA by reverse transcriptase–polymerase chain reaction, or isolation of the virus in the clinical specimen, and detection of measles-specific IgM (immunoglobulin M) antibodies. A detailed protocol for collecting specimens for viral isolation appears on the Centers for Disease Control and Prevention website (http://www.cdc.gov/measles/lab-tools/rt-pcr.html).
IgM antibodies are detectable over the 15 weeks after rash onset, but the recommendation is to collect serum between 72 hours and 4 weeks after rash onset.33 Clinicians should be aware that false-positive IgM results may occur with rheumatologic diseases, parvovirus B19 infection, rubella, and infectious mononucleosis.
IgG (immunoglobulin G) antibodies are usually detectable a week after rash onset. The laboratory can confirm measles by detecting more than a 4-fold increase in IgG titers between the acute phase and the convalescent phase. After measles infection, most adults develop lifelong immunity with positive IgG serology.34
Additional tests, such as IgG avidity and plaque reduction neutralization assay, can be used to confirm suspected cases in previously vaccinated individuals.34
MANAGEMENT
General Principles
Uncomplicated measles treatment is supportive and includes oral fluids and antipyretics.7,22 Severe bacterial infections, encephalitis, or dehydration may require hospitalization, and in these cases infectious disease consultation is recommended. Patients with pneumonia, purulent otitis media, or tonsillitis should be treated with antibiotics.35 Observational data suggest antibiotics may reduce the occurrence of bacterial infection in children, but there are no usage guidelines.35 Vitamin A supplementation has been associated with a 50% decrease in morbidity and mortality and with blindness prevention.22 This supplementation should be considered in severe measles cases (all hospitalized patients), especially for children, regardless of country of residence, and for adult patients who exhibit clinical signs of vitamin A deficiency.22,24
Antiviral Treatment
No specific treatment is available.36 Ribavirin demonstrates in vitro activity against the virus, but the Food and Drug Administration has not approved the drug for treatment of measles. Ribavirin has been used for cases of severe measles, and for patients with SSPE along with intrathecal interferon alpha. This antiviral treatment is considered experimental.37
All patients hospitalized with measles infection should be cautioned about the potential downstream complications of the disease and should follow up with their primary care physician for surveillance after discharge.38
If measles symptoms develop, patients should self-quarantine and contact their primary care physician or public health department as soon as possible. Regardless of immune status, family members and other exposed persons should be educated about the measles symptoms that may occur during the 21 days after exposure.38
Both suspected and confirmed cases of measles should be reported immediately to local public health authorities.
Infection Control and Prophylaxis
Current guidelines recommend 2 doses of measles-containing vaccine to all adults at higher risk for contracting measles: international travelers, healthcare personnel, and high school and college students. Infants 6 or 11 months old should receive 1 MMR dose before international travel.1,38
Strict airborne isolation—use of N95 respirator or respirator with similar effectiveness in preventing airborne transmission—is mandatory from 3 to 5 days before rash onset to 4 days after rash onset (immunocompetent patients) or for the duration of the disease (immunocompromised patients).38
Healthcare workers should have documented presumptive evidence of immunity to measles.39 Healthcare providers without evidence of immunity should be excused from work from day 5 to day 21 of exposure, even if they have received postexposure vaccine or intramuscular immunoglobulin. They should be offered the first MMR dose within 72 hours of measles exposure to prevent or modify the disease. Susceptible family members or visitors should not be allowed in the patient’s room.1
Postexposure Prophylaxis
Standard MMR vaccination within 72 hours after exposure may protect against disease in people without a contraindication to measles vaccine. The public health department usually identifies these individuals and provides postexposure prophylaxis recommendations.38,39
People with HIV, patients receiving immunosuppressive therapy, and pregnant women and infants who have been exposed to measles and who are at risk for developing morbid disease can be treated with immunoglobulin (IG). If administered within 6 days of exposure, IG can prevent or modify disease in people who are unvaccinated or severely immunocompromised (ie, not immune). The recommended dose of IG administered intramuscularly is 0.5 mL/kg of body weight (maximum, 15 mL), and the recommended dose of IG given intravenously is 400 mg/kg. Anyone heavier than 30 kg would require intravenous IG to achieve adequate antibody levels.
Physicians should not vaccinate pregnant women, patients with severe immunosuppression from disease or therapy, patients with moderate or severe illness, and people with a history of severe allergic reaction to the vaccine.1,40 The measles vaccine should be deferred for 6 months after IG administration.36 More details are available in the recommendations made by the Advisory Committee on Immunization Practices.1
CONCLUSION
Although rare in the United States, measles remains a common and potentially devastating infection among patients who have not been vaccinated. Diagnosis requires clinical suspicion, engagement of public health authorities, and judicious use of laboratory testing. Hospitalists may encounter infectious and neurologic complications of measles long after the initial infection and should be aware of these associations.
Disclosure
Nothing to report.
1. McLean HQ, Fiebelkorn AP, Temte JL, Wallace, GS; Centers for Disease Control and Prevention. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2013;62(RR-04):1-34.
2. World Health Organization. Measles [fact sheet]. http://www.who.int/mediacentre/factsheets/fs286/en/. Accessed April 27, 2017.
3. Kutty P, Rota J, Bellini W, Redd SB, Barskey A, Wallace G. Chapter 7: measles. In: Manual for the Surveillance of Vaccine-Preventable Disease. 6th ed. https://www.cdc.gov/vaccines/pubs/surv-manual/chpt07-measles.html. Published 2013. Accessed April 27, 2017.
4. Clemmons NS, Gastanaduy PA, Fiebelkorn AP, Redd SB, Wallace GS; Centers for Disease Control and Prevention (CDC). Measles—United States, January 4-April 2, 2015. MMWR Morb Mortal Wkly Rep. 2015;64(14):373-376.
5. Fiebelkorn AP, Redd SB, Gallagher K, et al. Measles in the United States during the postelimination era. J Infect Dis. 2010;202(10):1520-1528.
6. Fiebelkorn AP, Redd SB, Gastañaduy PA, et al. A comparison of postelimination measles epidemiology in the United States, 2009-2014 versus 2001-2008. J Pediatric Infect Dis Soc. 2017;6(1):40-48.
7. Gershon A. Measles (rubeola). In: Braunwald E, Fauci AS, Kasper DL, Hauser SL, Longo DL, Jameson JL, eds. Harrison’s Principles of Internal Medicine. 15th ed. New York, NY: McGraw-Hill; 2001:1143-1145.
8. Hill HA, Elam-Evans LD, Yankey D, Singleton JA, Kolasa M. National, state, and selected local area vaccination coverage among children aged 19-35 months—United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64(33):889-896.
9. Reagan-Steiner S, Yankey D, Jayarajah J, et al. National, state and selected local area vaccination coverage among children aged 13-17 years—United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64(29):784-792.
10. Seither R, Calhoun K, Knighton CL, et al. Vaccination coverage among children in kindergarten—United States, 2014-15 school year. MMWR Morb Mortal Wkly Rep. 2015;64(33):897-904.
11. Zipprich J, Winter K, Hacker J, Xia D, Watt J, Harriman K; Centers for Disease Control and Prevention (CDC). Measles outbreak—California, December 2014-February 2015. MMWR Morb Mortal Wkly Rep. 2015;64(6):153-154.
12. Perry RT, Halsey NA. The clinical significance of measles: a review. J Infect Dis. 2004;189(suppl 1):S4-S6.
13. Bernstein DI, Schiff GM. Measles. In: Gorbach SL, Bartlett JG, Blacklow NR, eds. Infectious Diseases. Philadelphia, PA: Saunders; 1998:1296.
14. Scheider-Schaulies S, Schneider-Schaulies J. Measles virus induced immunosuppression. Curr Top Microbiol Immunol. 2009;330:243-69
15. Mina MJ, Metcalf JE, de Swart RL, Osterhaus AD, Grenfell BT. Vaccines. Long-term measles-induced immunomodulation increases overall childhood infectious disease mortality. Science. 2015;348(6235):694-699.
16. Kaplan LJ, Daum RS, Smaron M, McCarthy CA. Severe measles may occur in immunocompromised patients. JAMA. 1992;267(9):1237-1241.
17. Melenotte C, Cassir N, Tessonnier L, Brouqui P. Atypical measles syndrome in adults: still around [published online September 23, 2015]. BMJ Case Rep. doi:10.1136/bcr-2015-211054.
18. Ogbuano IU, Zeko S, Chu SY, et al. Maternal, fetal and neonatal outcomes associated with measles during pregnancy: Namibia, 2009-2010. Clin Infect Dis. 2014;58(8):1086-1092.
19. Rasmussen SA, Jameson DJ. What obstetric healthcare providers need to know about measles and pregnancy. Obstet Gynecol. 2015;126(1):163-170.
20. Davis AT. Exanthematous diseases. In: Shulman ST, Phair JP, Peterson LR, Warren JR, eds. The Biologic and Clinical Basis of Infectious Diseases. 5th ed. Philadelphia, PA: Saunders; 1997:467-469.
21. Fortenberry JD, Mariscalco MM, Louis PT, Stein F, Jones JK, Jefferson LS. Severe laryngotracheobronchitis complicating measles. Am J Dis Child. 1992;146(9):1040-1043.
22. World Health Organization, Department of Immunization, Vaccines and Biologicals. Treating Measles in Children. http://www.who.int/immunization/programmes_systems/interventions/TreatingMeaslesENG300.pdf. Published 1997. Updated 2004. Accessed April 27, 2017.
23. Rafat C, Klouche K, Ricard JD, et al. Severe measles infection: the spectrum of disease in 36 critically ill adult patients. Medicine (Baltimore). 2013;92(5):257-272.
24. Ortac Ersoy E, Tanriover MD, Ocal S, Ozisik L, Inkaya C, Topeli A. Severe measles pneumonia in adults with respiratory failure: role of ribavirin and high-dose vitamin A. Clin Respir J. 2016;10(5):673-675.
25. Chassort A, Coutherut J, Moreau-Klein A, et al. Renal dysfunction in adults during measles. Med Mal Infect. 2015;45(5):165-168.
26. Sunnetcioglu M, Baran A, Sunnetcioglu A, Mentes O, Karadas S, Aypak A. Clinical and laboratory features of adult measles cases detected in Van, Turkey. J Pak Med Assoc. 2015;65(3):273-276.
27. Honarmand S, Glaser CA, Chow E, et al. Subacute sclerosing panencephalitis in the differential diagnosis of encephalitis. Neurology. 2004;63(8):1489-1493.
28. Liko J, Guzman-Cottrill JA, Cieslak PR. Notes from the field: subacute sclerosing panencephalitis death—Oregon, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(1):10-11.
29. Fisher DL, Defres S, Solomon T. Measles-induced encephalitis. QJM. 2015;108(3):177-182.
30. Rodriguez D, Fishman D. Measles and subacute sclerosing panencephalitis. In: Samuels MA, Feske SK, eds. Office Practice of Neurology. Philadelphia, PA: Churchill Livingstone; 2003:419-420.
31. Gutierrez J, Issacson RS, Koppel BS. Subacute sclerosing panencephalitis: an update. Dev Med Child Neurol. 2010;52(10):901-907.
32. Bellini WJ, Rota JS, Lowe LE, et al. Subacute sclerosing panencephalitis: more cases
of this fatal disease are prevented by measles immunization than was previously
recognized. J Infect Dis. 2005;192(10);1686-1693.
33. Helfand RF, Heath JL, Anderson LJ, Maes EF, Guris D, Bellini WJ. Diagnosis of
measles with an IgM capture EIA: the optimal timing of specimen collection after
rash onset. J Infect Dis. 1997;175(1):195-199.
34. Hickman CJ, Hyde TB, Sowers SB, et al. Laboratory characterization of measles
virus infection in previously vaccinated and unvaccinated individuals. J Infect Dis.
2011;204(suppl 1):S549-S558.
35. Kabra SK, Lodha R. Antibiotics for preventing complications in children with
measles. Cochrane Database Syst Rev. 2013;(8):CD001477.
36. Sabella C. Measles: not just a childhood rash. Cleve Clin J Med. 2010;77(3):
207-213.
37. Hosoya M, Shigeta S, Mori S, et al. High-dose intravenous ribavirin therapy
for subacute sclerosing panencephalitis. Antimicrob Agents Chemother.
2001;45(3):943-945.
38. Siegel JD, Rhinehart E, Jackson M, Chiarello L; Healthcare Infection Control
Practices Advisory Committee. 2007 Guideline for Isolation Precautions: Preventing
Transmission of Infectious Agents in Healthcare Settings. Centers for Disease Control
and Prevention website. https://www.cdc.gov/hicpac/pdf/isolation/isolation2007.
pdf. Accessed April 27, 2017.
39. Houck P, Scott-Johnson G, Krebs L. Measles immunity among community hospital
employees. Infect Control Hosp Epidemiol. 1991;12(11):663-668.
40. Kumar D, Sabella C. Measles: back again. Cleve Clin J Med. 2016;83(5):340-344.
Measles is a highly contagious acute respiratory illness that includes a characteristic rash. After exposure, up to 90% of susceptible persons develop measles.1 Even though it is considered a childhood illness, measles can affect people of all age groups. Measles continues to be major health problem around the world, despite the availability of a safe and effective vaccine, and it remains one of the leading causes of childhood mortality, with nearly 115,000 deaths reported by the World Health Organization2 in 2014. In 2000, measles was declared eliminated from the United States, but outbreaks still occasionally occur.3-6
The disease is self-limited, but some patients develop complications that may require hospitalization for treatment. People at highest risk for complications are children younger than 5 years, adults older than 20 years, pregnant women, and immunocompromised individuals.7
HISTORY AND EPIDEMIOLOGY
During the licensure of live measles vaccine in 1963, an average of 549,000 measles cases and 495 measles deaths, as well as 48,000 hospitalizations and 4000 encephalitis cases, were reported annually in the United States. Almost all Americans were affected by measles by adolescence.
Implementation of the 1-dose vaccine program substantially reduced reported incidence in the United States by 1988, and led to a dramatic decline in measles-related hospitalizations and deaths.3-6 The 2-dose MMR (measles, mumps, rubella) vaccination was introduced in 1989, and measles was declared eliminated in the United States in 2000.3-6
National–level one-dose MMR coverage among children 19-35 months has remained above 90% during the last two decades.8 NIS-Teen vaccination coverage data for 13- to 17-year-olds since 2008 has been near or above 90%,9 and 94% of children enrolled in kindergarten had evidence of 2 MMR doses in the 2014-2015 school year.10
A large multistate measles outbreak was reported in the United States in 2014-2015.4,11 One hundred fifty-nine cases were reported in the United States between January 4 and April 5, 2015. The majority of patients either were unvaccinated (45%) or had an unknown vaccination status (38%). Age ranged from 6 weeks to 70 years, and 22 patients (14%) were hospitalized.4
CLINICAL PRESENTATION AND PATHOPHYSIOLOGY
Measles is caused by an RNA-containing paramyxovirus that is spread by the respiratory route. Average incubation period from exposure to rash onset is 14 days (range, 7-21 days).12,13 Peak infectivity occurs during the prodromal phase, before rash onset (Figure 1), but patients are infectious from 4 days before rash onset through 4 days after rash onset.7,12,13
The disease prodrome consists of a high fever (39°C-40.5°C), coryza, cough, and conjunctivitis followed by Koplik spots (Figure 2A). Koplik spots are pathognomonic for measles but rarely discovered. They appear before the skin rash alongside second molars on the buccal surface of the cheeks. The spots usually disappear when the characteristic maculopapular, nonpruritic rash erupts initially at the hairline and behind the ears, and within four days progresses toward the trunk and limbs, including the palms and soles (Figures 2B, 2C).
The patient remains febrile while the rash spreads.12,13 Usually the fever resolves while the rash fades in the same order in which it appeared. Fever that persists for more than 5 days usually indicates complications.13
Cellular immunity plays an important role in host defense; the virus invades T lymphocytes and triggers suppressive cytokine (interleukin 4) production. Leukopenia, expansion of mainly measles-specific T and B lymphocytes, and replacement of lymphocyte memory cell population results in further depression of cellular immunity, and predisposes patients to secondary bacterial infections for up to 2 years after measles infection.14,15
Patients immunocompromised by congenital cellular immunity deficiency, cancer, human immunodeficiency virus (HIV) infection without effective antiretroviral therapy, or immunosuppression treatment are at higher risk for developing severe complications or dying from measles. As the rash may fail to develop in these patients, diagnosis can be challenging.16
Modified measles is milder and may occur in patients with preexisting partial immunity: those with an immunization history (2-dose vaccine effectiveness is ∼97%), and infants with minimal immunity from their mothers.1,7 Patients may have mild respiratory symptoms with rash but little or no fever.7
Atypical measles is now extremely rare. It was described only among people who were vaccinated with the killed vaccine in the United States between 1963 and 1968 and subsequently exposed to measles. The disease is characterized by high fever, edema of extremities, and a rash that develops on the palms and soles and spreads centerward. It is considered noncommunicable.17
Measles infection during pregnancy is associated with increased maternal and fetal morbidity. The virus can induce neonatal low birth weight, spontaneous abortion, intrauterine fetal death, and maternal death. Pregnant women with measles are more likely to be hospitalized.18,19
DIFFERENTIAL DIAGNOSIS
The presenting symptoms of primary measles infection are nonspecific, particularly if Koplik spots are not identified. The differential diagnosis for a patient who presents with high fever and rash include Kawasaki disease, dengue, parvovirus B19, serum sickness, syphilis, systemic lupus erythematous, toxic shock syndrome, enterovirus infection, human herpes virus 6 (roseola), viral hemorrhagic fever, drug eruption, infectious mononucleosis, Rocky Mountain spotted fever, rubella, scarlet fever, chikungunya, and Zika virus infection.
COMPLICATIONS
Measles complications can affect nearly every organ system (Table). Rates of complications from measles infection depend on age and underlying condition. Coexisting vitamin A deficiency increases complication rates.20
Bacterial infections in the setting of measles infection are more common in adults than in children, and are more severe among people who are malnourished or have an immunodeficiency disorder. The most common infectious complications, which involve the respiratory tract, include pneumonia, laryngotracheitis (“measles croup”), bronchitis, otitis media (most common complication among children in the United States), and sinusitis.7,13,21
Indications for hospitalizing children include respiratory distress, laryngeal obstruction, dehydration that requires intravenous fluids, diarrhea with more than 10 stools a day or bloody stool, severe anemia, altered mental status, convulsion, severe rash with developing hemorrhagic areas, extensive mouth ulcers, corneal clouding or ulcers, visual disturbance, and mastoiditis.22
Pneumonia is a common indication for hospitalizing adults.23,24 Measles-associated interstitial giant cell (Hecht) pneumonia is most often recognized among immunocompromised and malnourished patients.13 Primary pneumonia is caused by the measles virus, but bacterial superinfection can occur. The most common bacterial pathogens include Streptococcus, Pneumococcus, and Staphylococcus,13,24 and less commonly isolated organisms include gram-negative bacteria, such as Haemophilus influenzae, Pseudomonas aeruginosa, Neisseria meningitides, and Enterobacter cloacae.23
Uncommon complications of measles are myocarditis, glomerulonephritis, acute renal failure, and thrombocytopenic purpura.25,26
Neurologic complications in measles are an important concern. Measles-associated central nervous system complications are considered a result of an immune-mediated reaction to myelin protein and not from direct viral insult.26-28 Immunocompromised patients are at risk for developing fatal encephalitis, and those who survive often experience cognitive decline or seizures.
Measles is associated with four different encephalitic diseases: primary measles encephalitis, acute post-measles encephalomyelitis, measles inclusion body encephalitis, and subacute sclerosing panencephalitis.
Primary measles encephalitis is characterized by fever, headache, stiff neck, and meningeal signs. Onset occurs between 1 and 15 days after rash onset, and the disease affects 1/1000 patients. Seizure, altered mental status, and coma can also develop. Viral RNA detection in the cerebrospinal fluid (CSF) confirms the diagnosis.29Acute post-measles encephalomyelitis is more common in adults than in children.12 It typically develops after the rash fades and the other symptoms subside. Patients suddenly experience a recurrence of fevers or seizures. Deafness, intellectual decline, epilepsy, postencephalitic hyperkinesia, hemiplegia, and/or paraplegia also can develop.27-29
Measles inclusion body encephalitis is described only in immunocompromised patients, and onset occurs within 1 year of infection. Seizures are an initial and common symptom, and some patients also experience hemiplegia, stupor, hypertonia, and dysarthria.29 Diagnostic findings include seroconversion during the disease course, improvement after withholding of the immunosuppressive regimen, and normal CSF. Brain biopsy confirms the diagnosis.
Subacute sclerosing panencephalitis (SSPE) is a slowly progressing and untreatable degenerative neurologic disorder characterized by demyelination of multiple brain areas. SSPE develops 7 to 10 years after natural measles infection, and usually affects children or adolescents. Clinical presentation includes intellectual decline, frequent rhythmic myoclonic jerks, seizure, and dementia. As the disease progresses, coma, quadriplegia, vegetative state, and autonomic instability develop. Death usually occurs within 2 years of onset.30,31 In children, the risk for SSPE after measles infection is estimated to be 4 to 11 per 100,000 infections. After the 1989-1991 resurgence of measles in the United States, however, the risk for SSPE was estimated to be 22 per 100,000 infections.30-32 The pathogenesis of SSPE is not fully understood but is thought to result from persistent aberrant measles virus infection.32
The SSPE diagnosis is based on clinical presentation, presence of anti-measles antibodies in CSF, typical electroencephalography pattern (periodic paroxysmal bursts) with accompanying myoclonus, tissue analysis, and magnetic resonance imaging.30
LABORATORY DIAGNOSIS
Suspicion for measles should prompt immediate consultation with local or state public health officials. Laboratory testing can be carefully considered after consultation, and care is needed in interpreting serologic studies.
The mainstays of measles infection diagnosis are detection of viral RNA by reverse transcriptase–polymerase chain reaction, or isolation of the virus in the clinical specimen, and detection of measles-specific IgM (immunoglobulin M) antibodies. A detailed protocol for collecting specimens for viral isolation appears on the Centers for Disease Control and Prevention website (http://www.cdc.gov/measles/lab-tools/rt-pcr.html).
IgM antibodies are detectable over the 15 weeks after rash onset, but the recommendation is to collect serum between 72 hours and 4 weeks after rash onset.33 Clinicians should be aware that false-positive IgM results may occur with rheumatologic diseases, parvovirus B19 infection, rubella, and infectious mononucleosis.
IgG (immunoglobulin G) antibodies are usually detectable a week after rash onset. The laboratory can confirm measles by detecting more than a 4-fold increase in IgG titers between the acute phase and the convalescent phase. After measles infection, most adults develop lifelong immunity with positive IgG serology.34
Additional tests, such as IgG avidity and plaque reduction neutralization assay, can be used to confirm suspected cases in previously vaccinated individuals.34
MANAGEMENT
General Principles
Uncomplicated measles treatment is supportive and includes oral fluids and antipyretics.7,22 Severe bacterial infections, encephalitis, or dehydration may require hospitalization, and in these cases infectious disease consultation is recommended. Patients with pneumonia, purulent otitis media, or tonsillitis should be treated with antibiotics.35 Observational data suggest antibiotics may reduce the occurrence of bacterial infection in children, but there are no usage guidelines.35 Vitamin A supplementation has been associated with a 50% decrease in morbidity and mortality and with blindness prevention.22 This supplementation should be considered in severe measles cases (all hospitalized patients), especially for children, regardless of country of residence, and for adult patients who exhibit clinical signs of vitamin A deficiency.22,24
Antiviral Treatment
No specific treatment is available.36 Ribavirin demonstrates in vitro activity against the virus, but the Food and Drug Administration has not approved the drug for treatment of measles. Ribavirin has been used for cases of severe measles, and for patients with SSPE along with intrathecal interferon alpha. This antiviral treatment is considered experimental.37
All patients hospitalized with measles infection should be cautioned about the potential downstream complications of the disease and should follow up with their primary care physician for surveillance after discharge.38
If measles symptoms develop, patients should self-quarantine and contact their primary care physician or public health department as soon as possible. Regardless of immune status, family members and other exposed persons should be educated about the measles symptoms that may occur during the 21 days after exposure.38
Both suspected and confirmed cases of measles should be reported immediately to local public health authorities.
Infection Control and Prophylaxis
Current guidelines recommend 2 doses of measles-containing vaccine to all adults at higher risk for contracting measles: international travelers, healthcare personnel, and high school and college students. Infants 6 or 11 months old should receive 1 MMR dose before international travel.1,38
Strict airborne isolation—use of N95 respirator or respirator with similar effectiveness in preventing airborne transmission—is mandatory from 3 to 5 days before rash onset to 4 days after rash onset (immunocompetent patients) or for the duration of the disease (immunocompromised patients).38
Healthcare workers should have documented presumptive evidence of immunity to measles.39 Healthcare providers without evidence of immunity should be excused from work from day 5 to day 21 of exposure, even if they have received postexposure vaccine or intramuscular immunoglobulin. They should be offered the first MMR dose within 72 hours of measles exposure to prevent or modify the disease. Susceptible family members or visitors should not be allowed in the patient’s room.1
Postexposure Prophylaxis
Standard MMR vaccination within 72 hours after exposure may protect against disease in people without a contraindication to measles vaccine. The public health department usually identifies these individuals and provides postexposure prophylaxis recommendations.38,39
People with HIV, patients receiving immunosuppressive therapy, and pregnant women and infants who have been exposed to measles and who are at risk for developing morbid disease can be treated with immunoglobulin (IG). If administered within 6 days of exposure, IG can prevent or modify disease in people who are unvaccinated or severely immunocompromised (ie, not immune). The recommended dose of IG administered intramuscularly is 0.5 mL/kg of body weight (maximum, 15 mL), and the recommended dose of IG given intravenously is 400 mg/kg. Anyone heavier than 30 kg would require intravenous IG to achieve adequate antibody levels.
Physicians should not vaccinate pregnant women, patients with severe immunosuppression from disease or therapy, patients with moderate or severe illness, and people with a history of severe allergic reaction to the vaccine.1,40 The measles vaccine should be deferred for 6 months after IG administration.36 More details are available in the recommendations made by the Advisory Committee on Immunization Practices.1
CONCLUSION
Although rare in the United States, measles remains a common and potentially devastating infection among patients who have not been vaccinated. Diagnosis requires clinical suspicion, engagement of public health authorities, and judicious use of laboratory testing. Hospitalists may encounter infectious and neurologic complications of measles long after the initial infection and should be aware of these associations.
Disclosure
Nothing to report.
Measles is a highly contagious acute respiratory illness that includes a characteristic rash. After exposure, up to 90% of susceptible persons develop measles.1 Even though it is considered a childhood illness, measles can affect people of all age groups. Measles continues to be major health problem around the world, despite the availability of a safe and effective vaccine, and it remains one of the leading causes of childhood mortality, with nearly 115,000 deaths reported by the World Health Organization2 in 2014. In 2000, measles was declared eliminated from the United States, but outbreaks still occasionally occur.3-6
The disease is self-limited, but some patients develop complications that may require hospitalization for treatment. People at highest risk for complications are children younger than 5 years, adults older than 20 years, pregnant women, and immunocompromised individuals.7
HISTORY AND EPIDEMIOLOGY
During the licensure of live measles vaccine in 1963, an average of 549,000 measles cases and 495 measles deaths, as well as 48,000 hospitalizations and 4000 encephalitis cases, were reported annually in the United States. Almost all Americans were affected by measles by adolescence.
Implementation of the 1-dose vaccine program substantially reduced reported incidence in the United States by 1988, and led to a dramatic decline in measles-related hospitalizations and deaths.3-6 The 2-dose MMR (measles, mumps, rubella) vaccination was introduced in 1989, and measles was declared eliminated in the United States in 2000.3-6
National–level one-dose MMR coverage among children 19-35 months has remained above 90% during the last two decades.8 NIS-Teen vaccination coverage data for 13- to 17-year-olds since 2008 has been near or above 90%,9 and 94% of children enrolled in kindergarten had evidence of 2 MMR doses in the 2014-2015 school year.10
A large multistate measles outbreak was reported in the United States in 2014-2015.4,11 One hundred fifty-nine cases were reported in the United States between January 4 and April 5, 2015. The majority of patients either were unvaccinated (45%) or had an unknown vaccination status (38%). Age ranged from 6 weeks to 70 years, and 22 patients (14%) were hospitalized.4
CLINICAL PRESENTATION AND PATHOPHYSIOLOGY
Measles is caused by an RNA-containing paramyxovirus that is spread by the respiratory route. Average incubation period from exposure to rash onset is 14 days (range, 7-21 days).12,13 Peak infectivity occurs during the prodromal phase, before rash onset (Figure 1), but patients are infectious from 4 days before rash onset through 4 days after rash onset.7,12,13
The disease prodrome consists of a high fever (39°C-40.5°C), coryza, cough, and conjunctivitis followed by Koplik spots (Figure 2A). Koplik spots are pathognomonic for measles but rarely discovered. They appear before the skin rash alongside second molars on the buccal surface of the cheeks. The spots usually disappear when the characteristic maculopapular, nonpruritic rash erupts initially at the hairline and behind the ears, and within four days progresses toward the trunk and limbs, including the palms and soles (Figures 2B, 2C).
The patient remains febrile while the rash spreads.12,13 Usually the fever resolves while the rash fades in the same order in which it appeared. Fever that persists for more than 5 days usually indicates complications.13
Cellular immunity plays an important role in host defense; the virus invades T lymphocytes and triggers suppressive cytokine (interleukin 4) production. Leukopenia, expansion of mainly measles-specific T and B lymphocytes, and replacement of lymphocyte memory cell population results in further depression of cellular immunity, and predisposes patients to secondary bacterial infections for up to 2 years after measles infection.14,15
Patients immunocompromised by congenital cellular immunity deficiency, cancer, human immunodeficiency virus (HIV) infection without effective antiretroviral therapy, or immunosuppression treatment are at higher risk for developing severe complications or dying from measles. As the rash may fail to develop in these patients, diagnosis can be challenging.16
Modified measles is milder and may occur in patients with preexisting partial immunity: those with an immunization history (2-dose vaccine effectiveness is ∼97%), and infants with minimal immunity from their mothers.1,7 Patients may have mild respiratory symptoms with rash but little or no fever.7
Atypical measles is now extremely rare. It was described only among people who were vaccinated with the killed vaccine in the United States between 1963 and 1968 and subsequently exposed to measles. The disease is characterized by high fever, edema of extremities, and a rash that develops on the palms and soles and spreads centerward. It is considered noncommunicable.17
Measles infection during pregnancy is associated with increased maternal and fetal morbidity. The virus can induce neonatal low birth weight, spontaneous abortion, intrauterine fetal death, and maternal death. Pregnant women with measles are more likely to be hospitalized.18,19
DIFFERENTIAL DIAGNOSIS
The presenting symptoms of primary measles infection are nonspecific, particularly if Koplik spots are not identified. The differential diagnosis for a patient who presents with high fever and rash include Kawasaki disease, dengue, parvovirus B19, serum sickness, syphilis, systemic lupus erythematous, toxic shock syndrome, enterovirus infection, human herpes virus 6 (roseola), viral hemorrhagic fever, drug eruption, infectious mononucleosis, Rocky Mountain spotted fever, rubella, scarlet fever, chikungunya, and Zika virus infection.
COMPLICATIONS
Measles complications can affect nearly every organ system (Table). Rates of complications from measles infection depend on age and underlying condition. Coexisting vitamin A deficiency increases complication rates.20
Bacterial infections in the setting of measles infection are more common in adults than in children, and are more severe among people who are malnourished or have an immunodeficiency disorder. The most common infectious complications, which involve the respiratory tract, include pneumonia, laryngotracheitis (“measles croup”), bronchitis, otitis media (most common complication among children in the United States), and sinusitis.7,13,21
Indications for hospitalizing children include respiratory distress, laryngeal obstruction, dehydration that requires intravenous fluids, diarrhea with more than 10 stools a day or bloody stool, severe anemia, altered mental status, convulsion, severe rash with developing hemorrhagic areas, extensive mouth ulcers, corneal clouding or ulcers, visual disturbance, and mastoiditis.22
Pneumonia is a common indication for hospitalizing adults.23,24 Measles-associated interstitial giant cell (Hecht) pneumonia is most often recognized among immunocompromised and malnourished patients.13 Primary pneumonia is caused by the measles virus, but bacterial superinfection can occur. The most common bacterial pathogens include Streptococcus, Pneumococcus, and Staphylococcus,13,24 and less commonly isolated organisms include gram-negative bacteria, such as Haemophilus influenzae, Pseudomonas aeruginosa, Neisseria meningitides, and Enterobacter cloacae.23
Uncommon complications of measles are myocarditis, glomerulonephritis, acute renal failure, and thrombocytopenic purpura.25,26
Neurologic complications in measles are an important concern. Measles-associated central nervous system complications are considered a result of an immune-mediated reaction to myelin protein and not from direct viral insult.26-28 Immunocompromised patients are at risk for developing fatal encephalitis, and those who survive often experience cognitive decline or seizures.
Measles is associated with four different encephalitic diseases: primary measles encephalitis, acute post-measles encephalomyelitis, measles inclusion body encephalitis, and subacute sclerosing panencephalitis.
Primary measles encephalitis is characterized by fever, headache, stiff neck, and meningeal signs. Onset occurs between 1 and 15 days after rash onset, and the disease affects 1/1000 patients. Seizure, altered mental status, and coma can also develop. Viral RNA detection in the cerebrospinal fluid (CSF) confirms the diagnosis.29Acute post-measles encephalomyelitis is more common in adults than in children.12 It typically develops after the rash fades and the other symptoms subside. Patients suddenly experience a recurrence of fevers or seizures. Deafness, intellectual decline, epilepsy, postencephalitic hyperkinesia, hemiplegia, and/or paraplegia also can develop.27-29
Measles inclusion body encephalitis is described only in immunocompromised patients, and onset occurs within 1 year of infection. Seizures are an initial and common symptom, and some patients also experience hemiplegia, stupor, hypertonia, and dysarthria.29 Diagnostic findings include seroconversion during the disease course, improvement after withholding of the immunosuppressive regimen, and normal CSF. Brain biopsy confirms the diagnosis.
Subacute sclerosing panencephalitis (SSPE) is a slowly progressing and untreatable degenerative neurologic disorder characterized by demyelination of multiple brain areas. SSPE develops 7 to 10 years after natural measles infection, and usually affects children or adolescents. Clinical presentation includes intellectual decline, frequent rhythmic myoclonic jerks, seizure, and dementia. As the disease progresses, coma, quadriplegia, vegetative state, and autonomic instability develop. Death usually occurs within 2 years of onset.30,31 In children, the risk for SSPE after measles infection is estimated to be 4 to 11 per 100,000 infections. After the 1989-1991 resurgence of measles in the United States, however, the risk for SSPE was estimated to be 22 per 100,000 infections.30-32 The pathogenesis of SSPE is not fully understood but is thought to result from persistent aberrant measles virus infection.32
The SSPE diagnosis is based on clinical presentation, presence of anti-measles antibodies in CSF, typical electroencephalography pattern (periodic paroxysmal bursts) with accompanying myoclonus, tissue analysis, and magnetic resonance imaging.30
LABORATORY DIAGNOSIS
Suspicion for measles should prompt immediate consultation with local or state public health officials. Laboratory testing can be carefully considered after consultation, and care is needed in interpreting serologic studies.
The mainstays of measles infection diagnosis are detection of viral RNA by reverse transcriptase–polymerase chain reaction, or isolation of the virus in the clinical specimen, and detection of measles-specific IgM (immunoglobulin M) antibodies. A detailed protocol for collecting specimens for viral isolation appears on the Centers for Disease Control and Prevention website (http://www.cdc.gov/measles/lab-tools/rt-pcr.html).
IgM antibodies are detectable over the 15 weeks after rash onset, but the recommendation is to collect serum between 72 hours and 4 weeks after rash onset.33 Clinicians should be aware that false-positive IgM results may occur with rheumatologic diseases, parvovirus B19 infection, rubella, and infectious mononucleosis.
IgG (immunoglobulin G) antibodies are usually detectable a week after rash onset. The laboratory can confirm measles by detecting more than a 4-fold increase in IgG titers between the acute phase and the convalescent phase. After measles infection, most adults develop lifelong immunity with positive IgG serology.34
Additional tests, such as IgG avidity and plaque reduction neutralization assay, can be used to confirm suspected cases in previously vaccinated individuals.34
MANAGEMENT
General Principles
Uncomplicated measles treatment is supportive and includes oral fluids and antipyretics.7,22 Severe bacterial infections, encephalitis, or dehydration may require hospitalization, and in these cases infectious disease consultation is recommended. Patients with pneumonia, purulent otitis media, or tonsillitis should be treated with antibiotics.35 Observational data suggest antibiotics may reduce the occurrence of bacterial infection in children, but there are no usage guidelines.35 Vitamin A supplementation has been associated with a 50% decrease in morbidity and mortality and with blindness prevention.22 This supplementation should be considered in severe measles cases (all hospitalized patients), especially for children, regardless of country of residence, and for adult patients who exhibit clinical signs of vitamin A deficiency.22,24
Antiviral Treatment
No specific treatment is available.36 Ribavirin demonstrates in vitro activity against the virus, but the Food and Drug Administration has not approved the drug for treatment of measles. Ribavirin has been used for cases of severe measles, and for patients with SSPE along with intrathecal interferon alpha. This antiviral treatment is considered experimental.37
All patients hospitalized with measles infection should be cautioned about the potential downstream complications of the disease and should follow up with their primary care physician for surveillance after discharge.38
If measles symptoms develop, patients should self-quarantine and contact their primary care physician or public health department as soon as possible. Regardless of immune status, family members and other exposed persons should be educated about the measles symptoms that may occur during the 21 days after exposure.38
Both suspected and confirmed cases of measles should be reported immediately to local public health authorities.
Infection Control and Prophylaxis
Current guidelines recommend 2 doses of measles-containing vaccine to all adults at higher risk for contracting measles: international travelers, healthcare personnel, and high school and college students. Infants 6 or 11 months old should receive 1 MMR dose before international travel.1,38
Strict airborne isolation—use of N95 respirator or respirator with similar effectiveness in preventing airborne transmission—is mandatory from 3 to 5 days before rash onset to 4 days after rash onset (immunocompetent patients) or for the duration of the disease (immunocompromised patients).38
Healthcare workers should have documented presumptive evidence of immunity to measles.39 Healthcare providers without evidence of immunity should be excused from work from day 5 to day 21 of exposure, even if they have received postexposure vaccine or intramuscular immunoglobulin. They should be offered the first MMR dose within 72 hours of measles exposure to prevent or modify the disease. Susceptible family members or visitors should not be allowed in the patient’s room.1
Postexposure Prophylaxis
Standard MMR vaccination within 72 hours after exposure may protect against disease in people without a contraindication to measles vaccine. The public health department usually identifies these individuals and provides postexposure prophylaxis recommendations.38,39
People with HIV, patients receiving immunosuppressive therapy, and pregnant women and infants who have been exposed to measles and who are at risk for developing morbid disease can be treated with immunoglobulin (IG). If administered within 6 days of exposure, IG can prevent or modify disease in people who are unvaccinated or severely immunocompromised (ie, not immune). The recommended dose of IG administered intramuscularly is 0.5 mL/kg of body weight (maximum, 15 mL), and the recommended dose of IG given intravenously is 400 mg/kg. Anyone heavier than 30 kg would require intravenous IG to achieve adequate antibody levels.
Physicians should not vaccinate pregnant women, patients with severe immunosuppression from disease or therapy, patients with moderate or severe illness, and people with a history of severe allergic reaction to the vaccine.1,40 The measles vaccine should be deferred for 6 months after IG administration.36 More details are available in the recommendations made by the Advisory Committee on Immunization Practices.1
CONCLUSION
Although rare in the United States, measles remains a common and potentially devastating infection among patients who have not been vaccinated. Diagnosis requires clinical suspicion, engagement of public health authorities, and judicious use of laboratory testing. Hospitalists may encounter infectious and neurologic complications of measles long after the initial infection and should be aware of these associations.
Disclosure
Nothing to report.
1. McLean HQ, Fiebelkorn AP, Temte JL, Wallace, GS; Centers for Disease Control and Prevention. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2013;62(RR-04):1-34.
2. World Health Organization. Measles [fact sheet]. http://www.who.int/mediacentre/factsheets/fs286/en/. Accessed April 27, 2017.
3. Kutty P, Rota J, Bellini W, Redd SB, Barskey A, Wallace G. Chapter 7: measles. In: Manual for the Surveillance of Vaccine-Preventable Disease. 6th ed. https://www.cdc.gov/vaccines/pubs/surv-manual/chpt07-measles.html. Published 2013. Accessed April 27, 2017.
4. Clemmons NS, Gastanaduy PA, Fiebelkorn AP, Redd SB, Wallace GS; Centers for Disease Control and Prevention (CDC). Measles—United States, January 4-April 2, 2015. MMWR Morb Mortal Wkly Rep. 2015;64(14):373-376.
5. Fiebelkorn AP, Redd SB, Gallagher K, et al. Measles in the United States during the postelimination era. J Infect Dis. 2010;202(10):1520-1528.
6. Fiebelkorn AP, Redd SB, Gastañaduy PA, et al. A comparison of postelimination measles epidemiology in the United States, 2009-2014 versus 2001-2008. J Pediatric Infect Dis Soc. 2017;6(1):40-48.
7. Gershon A. Measles (rubeola). In: Braunwald E, Fauci AS, Kasper DL, Hauser SL, Longo DL, Jameson JL, eds. Harrison’s Principles of Internal Medicine. 15th ed. New York, NY: McGraw-Hill; 2001:1143-1145.
8. Hill HA, Elam-Evans LD, Yankey D, Singleton JA, Kolasa M. National, state, and selected local area vaccination coverage among children aged 19-35 months—United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64(33):889-896.
9. Reagan-Steiner S, Yankey D, Jayarajah J, et al. National, state and selected local area vaccination coverage among children aged 13-17 years—United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64(29):784-792.
10. Seither R, Calhoun K, Knighton CL, et al. Vaccination coverage among children in kindergarten—United States, 2014-15 school year. MMWR Morb Mortal Wkly Rep. 2015;64(33):897-904.
11. Zipprich J, Winter K, Hacker J, Xia D, Watt J, Harriman K; Centers for Disease Control and Prevention (CDC). Measles outbreak—California, December 2014-February 2015. MMWR Morb Mortal Wkly Rep. 2015;64(6):153-154.
12. Perry RT, Halsey NA. The clinical significance of measles: a review. J Infect Dis. 2004;189(suppl 1):S4-S6.
13. Bernstein DI, Schiff GM. Measles. In: Gorbach SL, Bartlett JG, Blacklow NR, eds. Infectious Diseases. Philadelphia, PA: Saunders; 1998:1296.
14. Scheider-Schaulies S, Schneider-Schaulies J. Measles virus induced immunosuppression. Curr Top Microbiol Immunol. 2009;330:243-69
15. Mina MJ, Metcalf JE, de Swart RL, Osterhaus AD, Grenfell BT. Vaccines. Long-term measles-induced immunomodulation increases overall childhood infectious disease mortality. Science. 2015;348(6235):694-699.
16. Kaplan LJ, Daum RS, Smaron M, McCarthy CA. Severe measles may occur in immunocompromised patients. JAMA. 1992;267(9):1237-1241.
17. Melenotte C, Cassir N, Tessonnier L, Brouqui P. Atypical measles syndrome in adults: still around [published online September 23, 2015]. BMJ Case Rep. doi:10.1136/bcr-2015-211054.
18. Ogbuano IU, Zeko S, Chu SY, et al. Maternal, fetal and neonatal outcomes associated with measles during pregnancy: Namibia, 2009-2010. Clin Infect Dis. 2014;58(8):1086-1092.
19. Rasmussen SA, Jameson DJ. What obstetric healthcare providers need to know about measles and pregnancy. Obstet Gynecol. 2015;126(1):163-170.
20. Davis AT. Exanthematous diseases. In: Shulman ST, Phair JP, Peterson LR, Warren JR, eds. The Biologic and Clinical Basis of Infectious Diseases. 5th ed. Philadelphia, PA: Saunders; 1997:467-469.
21. Fortenberry JD, Mariscalco MM, Louis PT, Stein F, Jones JK, Jefferson LS. Severe laryngotracheobronchitis complicating measles. Am J Dis Child. 1992;146(9):1040-1043.
22. World Health Organization, Department of Immunization, Vaccines and Biologicals. Treating Measles in Children. http://www.who.int/immunization/programmes_systems/interventions/TreatingMeaslesENG300.pdf. Published 1997. Updated 2004. Accessed April 27, 2017.
23. Rafat C, Klouche K, Ricard JD, et al. Severe measles infection: the spectrum of disease in 36 critically ill adult patients. Medicine (Baltimore). 2013;92(5):257-272.
24. Ortac Ersoy E, Tanriover MD, Ocal S, Ozisik L, Inkaya C, Topeli A. Severe measles pneumonia in adults with respiratory failure: role of ribavirin and high-dose vitamin A. Clin Respir J. 2016;10(5):673-675.
25. Chassort A, Coutherut J, Moreau-Klein A, et al. Renal dysfunction in adults during measles. Med Mal Infect. 2015;45(5):165-168.
26. Sunnetcioglu M, Baran A, Sunnetcioglu A, Mentes O, Karadas S, Aypak A. Clinical and laboratory features of adult measles cases detected in Van, Turkey. J Pak Med Assoc. 2015;65(3):273-276.
27. Honarmand S, Glaser CA, Chow E, et al. Subacute sclerosing panencephalitis in the differential diagnosis of encephalitis. Neurology. 2004;63(8):1489-1493.
28. Liko J, Guzman-Cottrill JA, Cieslak PR. Notes from the field: subacute sclerosing panencephalitis death—Oregon, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(1):10-11.
29. Fisher DL, Defres S, Solomon T. Measles-induced encephalitis. QJM. 2015;108(3):177-182.
30. Rodriguez D, Fishman D. Measles and subacute sclerosing panencephalitis. In: Samuels MA, Feske SK, eds. Office Practice of Neurology. Philadelphia, PA: Churchill Livingstone; 2003:419-420.
31. Gutierrez J, Issacson RS, Koppel BS. Subacute sclerosing panencephalitis: an update. Dev Med Child Neurol. 2010;52(10):901-907.
32. Bellini WJ, Rota JS, Lowe LE, et al. Subacute sclerosing panencephalitis: more cases
of this fatal disease are prevented by measles immunization than was previously
recognized. J Infect Dis. 2005;192(10);1686-1693.
33. Helfand RF, Heath JL, Anderson LJ, Maes EF, Guris D, Bellini WJ. Diagnosis of
measles with an IgM capture EIA: the optimal timing of specimen collection after
rash onset. J Infect Dis. 1997;175(1):195-199.
34. Hickman CJ, Hyde TB, Sowers SB, et al. Laboratory characterization of measles
virus infection in previously vaccinated and unvaccinated individuals. J Infect Dis.
2011;204(suppl 1):S549-S558.
35. Kabra SK, Lodha R. Antibiotics for preventing complications in children with
measles. Cochrane Database Syst Rev. 2013;(8):CD001477.
36. Sabella C. Measles: not just a childhood rash. Cleve Clin J Med. 2010;77(3):
207-213.
37. Hosoya M, Shigeta S, Mori S, et al. High-dose intravenous ribavirin therapy
for subacute sclerosing panencephalitis. Antimicrob Agents Chemother.
2001;45(3):943-945.
38. Siegel JD, Rhinehart E, Jackson M, Chiarello L; Healthcare Infection Control
Practices Advisory Committee. 2007 Guideline for Isolation Precautions: Preventing
Transmission of Infectious Agents in Healthcare Settings. Centers for Disease Control
and Prevention website. https://www.cdc.gov/hicpac/pdf/isolation/isolation2007.
pdf. Accessed April 27, 2017.
39. Houck P, Scott-Johnson G, Krebs L. Measles immunity among community hospital
employees. Infect Control Hosp Epidemiol. 1991;12(11):663-668.
40. Kumar D, Sabella C. Measles: back again. Cleve Clin J Med. 2016;83(5):340-344.
1. McLean HQ, Fiebelkorn AP, Temte JL, Wallace, GS; Centers for Disease Control and Prevention. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2013;62(RR-04):1-34.
2. World Health Organization. Measles [fact sheet]. http://www.who.int/mediacentre/factsheets/fs286/en/. Accessed April 27, 2017.
3. Kutty P, Rota J, Bellini W, Redd SB, Barskey A, Wallace G. Chapter 7: measles. In: Manual for the Surveillance of Vaccine-Preventable Disease. 6th ed. https://www.cdc.gov/vaccines/pubs/surv-manual/chpt07-measles.html. Published 2013. Accessed April 27, 2017.
4. Clemmons NS, Gastanaduy PA, Fiebelkorn AP, Redd SB, Wallace GS; Centers for Disease Control and Prevention (CDC). Measles—United States, January 4-April 2, 2015. MMWR Morb Mortal Wkly Rep. 2015;64(14):373-376.
5. Fiebelkorn AP, Redd SB, Gallagher K, et al. Measles in the United States during the postelimination era. J Infect Dis. 2010;202(10):1520-1528.
6. Fiebelkorn AP, Redd SB, Gastañaduy PA, et al. A comparison of postelimination measles epidemiology in the United States, 2009-2014 versus 2001-2008. J Pediatric Infect Dis Soc. 2017;6(1):40-48.
7. Gershon A. Measles (rubeola). In: Braunwald E, Fauci AS, Kasper DL, Hauser SL, Longo DL, Jameson JL, eds. Harrison’s Principles of Internal Medicine. 15th ed. New York, NY: McGraw-Hill; 2001:1143-1145.
8. Hill HA, Elam-Evans LD, Yankey D, Singleton JA, Kolasa M. National, state, and selected local area vaccination coverage among children aged 19-35 months—United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64(33):889-896.
9. Reagan-Steiner S, Yankey D, Jayarajah J, et al. National, state and selected local area vaccination coverage among children aged 13-17 years—United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64(29):784-792.
10. Seither R, Calhoun K, Knighton CL, et al. Vaccination coverage among children in kindergarten—United States, 2014-15 school year. MMWR Morb Mortal Wkly Rep. 2015;64(33):897-904.
11. Zipprich J, Winter K, Hacker J, Xia D, Watt J, Harriman K; Centers for Disease Control and Prevention (CDC). Measles outbreak—California, December 2014-February 2015. MMWR Morb Mortal Wkly Rep. 2015;64(6):153-154.
12. Perry RT, Halsey NA. The clinical significance of measles: a review. J Infect Dis. 2004;189(suppl 1):S4-S6.
13. Bernstein DI, Schiff GM. Measles. In: Gorbach SL, Bartlett JG, Blacklow NR, eds. Infectious Diseases. Philadelphia, PA: Saunders; 1998:1296.
14. Scheider-Schaulies S, Schneider-Schaulies J. Measles virus induced immunosuppression. Curr Top Microbiol Immunol. 2009;330:243-69
15. Mina MJ, Metcalf JE, de Swart RL, Osterhaus AD, Grenfell BT. Vaccines. Long-term measles-induced immunomodulation increases overall childhood infectious disease mortality. Science. 2015;348(6235):694-699.
16. Kaplan LJ, Daum RS, Smaron M, McCarthy CA. Severe measles may occur in immunocompromised patients. JAMA. 1992;267(9):1237-1241.
17. Melenotte C, Cassir N, Tessonnier L, Brouqui P. Atypical measles syndrome in adults: still around [published online September 23, 2015]. BMJ Case Rep. doi:10.1136/bcr-2015-211054.
18. Ogbuano IU, Zeko S, Chu SY, et al. Maternal, fetal and neonatal outcomes associated with measles during pregnancy: Namibia, 2009-2010. Clin Infect Dis. 2014;58(8):1086-1092.
19. Rasmussen SA, Jameson DJ. What obstetric healthcare providers need to know about measles and pregnancy. Obstet Gynecol. 2015;126(1):163-170.
20. Davis AT. Exanthematous diseases. In: Shulman ST, Phair JP, Peterson LR, Warren JR, eds. The Biologic and Clinical Basis of Infectious Diseases. 5th ed. Philadelphia, PA: Saunders; 1997:467-469.
21. Fortenberry JD, Mariscalco MM, Louis PT, Stein F, Jones JK, Jefferson LS. Severe laryngotracheobronchitis complicating measles. Am J Dis Child. 1992;146(9):1040-1043.
22. World Health Organization, Department of Immunization, Vaccines and Biologicals. Treating Measles in Children. http://www.who.int/immunization/programmes_systems/interventions/TreatingMeaslesENG300.pdf. Published 1997. Updated 2004. Accessed April 27, 2017.
23. Rafat C, Klouche K, Ricard JD, et al. Severe measles infection: the spectrum of disease in 36 critically ill adult patients. Medicine (Baltimore). 2013;92(5):257-272.
24. Ortac Ersoy E, Tanriover MD, Ocal S, Ozisik L, Inkaya C, Topeli A. Severe measles pneumonia in adults with respiratory failure: role of ribavirin and high-dose vitamin A. Clin Respir J. 2016;10(5):673-675.
25. Chassort A, Coutherut J, Moreau-Klein A, et al. Renal dysfunction in adults during measles. Med Mal Infect. 2015;45(5):165-168.
26. Sunnetcioglu M, Baran A, Sunnetcioglu A, Mentes O, Karadas S, Aypak A. Clinical and laboratory features of adult measles cases detected in Van, Turkey. J Pak Med Assoc. 2015;65(3):273-276.
27. Honarmand S, Glaser CA, Chow E, et al. Subacute sclerosing panencephalitis in the differential diagnosis of encephalitis. Neurology. 2004;63(8):1489-1493.
28. Liko J, Guzman-Cottrill JA, Cieslak PR. Notes from the field: subacute sclerosing panencephalitis death—Oregon, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(1):10-11.
29. Fisher DL, Defres S, Solomon T. Measles-induced encephalitis. QJM. 2015;108(3):177-182.
30. Rodriguez D, Fishman D. Measles and subacute sclerosing panencephalitis. In: Samuels MA, Feske SK, eds. Office Practice of Neurology. Philadelphia, PA: Churchill Livingstone; 2003:419-420.
31. Gutierrez J, Issacson RS, Koppel BS. Subacute sclerosing panencephalitis: an update. Dev Med Child Neurol. 2010;52(10):901-907.
32. Bellini WJ, Rota JS, Lowe LE, et al. Subacute sclerosing panencephalitis: more cases
of this fatal disease are prevented by measles immunization than was previously
recognized. J Infect Dis. 2005;192(10);1686-1693.
33. Helfand RF, Heath JL, Anderson LJ, Maes EF, Guris D, Bellini WJ. Diagnosis of
measles with an IgM capture EIA: the optimal timing of specimen collection after
rash onset. J Infect Dis. 1997;175(1):195-199.
34. Hickman CJ, Hyde TB, Sowers SB, et al. Laboratory characterization of measles
virus infection in previously vaccinated and unvaccinated individuals. J Infect Dis.
2011;204(suppl 1):S549-S558.
35. Kabra SK, Lodha R. Antibiotics for preventing complications in children with
measles. Cochrane Database Syst Rev. 2013;(8):CD001477.
36. Sabella C. Measles: not just a childhood rash. Cleve Clin J Med. 2010;77(3):
207-213.
37. Hosoya M, Shigeta S, Mori S, et al. High-dose intravenous ribavirin therapy
for subacute sclerosing panencephalitis. Antimicrob Agents Chemother.
2001;45(3):943-945.
38. Siegel JD, Rhinehart E, Jackson M, Chiarello L; Healthcare Infection Control
Practices Advisory Committee. 2007 Guideline for Isolation Precautions: Preventing
Transmission of Infectious Agents in Healthcare Settings. Centers for Disease Control
and Prevention website. https://www.cdc.gov/hicpac/pdf/isolation/isolation2007.
pdf. Accessed April 27, 2017.
39. Houck P, Scott-Johnson G, Krebs L. Measles immunity among community hospital
employees. Infect Control Hosp Epidemiol. 1991;12(11):663-668.
40. Kumar D, Sabella C. Measles: back again. Cleve Clin J Med. 2016;83(5):340-344.
© 2017 Society of Hospital Medicine
Mobility assessment in the hospital: What are the “next steps”?
Mobility impairment (reduced ability to change body position or ambulate) is common among older adults during hospitalization1 and is correlated with higher rates of readmission,2 long-term care placement,3 and even death.4 Although some may perceive mobility impairment during hospitalization as a temporary inconvenience, recent research suggests disruptions of basic activities of daily life such as mobility may be “traumatic” 5 or “toxic”6 to older adults with long-term post-hospital effects.7 While these studies highlight the underestimated effects of low mobility during hospitalization, they are based on data collected for research purposes using mobility measurement tools not typically utilized in routine hospital care.
The absence of a standardized mobility measurement tool used as part of routine hospital care poses a barrier to estimating the effects of low hospital mobility and programs seeking to improve mobility levels in hospitalized patients. In this issue of the Journal of Hospital Medicine, Valiani et al.8 found a novel approach to measure mobility using a universally disseminated clinical scale (Braden). Using the activity subscale of the Braden scale, the authors found that mobility level changes during hospitalization can have a striking impact on post-discharge mortality. Their results indicate that older adults who develop mobility impairment during hospitalization had higher odds of death, specifically 1.23 times greater risk, within 6 months after discharge (23% decreased chance of survival). Most of the risk applies in the first 30 days and remains to a lesser extent for up to 5 years post-hospitalization. An equally interesting finding was that those who enter the hospital with low mobility but improve have a 46% higher survival rate. Again, most of the benefit is seen during hospitalization or immediately afterward, but the benefit persists for up to 5 years. A schematic of the results are presented in the Figure. Notably, Valiani et al.8 did not find regression to the mean Braden score of 3.
This novel use of the Braden activity subscale raises a question: Should we be using the Braden activity component to measure mobility in the hospital? Put another way, what scale should we be using in the hospital? Using the Braden activity subscale is convenient, since it capitalizes on data already being gathered. However, this subscale focuses solely on ambulation frequency; it doesn’t capture other mobility domains, such as ability to change body position. Ambulation is only half of the mobility story. It is interesting that although the Braden scale does have a mobility subscale that captures body position changes, the authors chose not to use it. This begs the question of whether an ideal mobility scale should encompass both components.
Previous studies of hospital mobility have deployed tools such as Katz Activities of Daily Living (ADLs)9 and the Short Physical Performance Battery (SPPB),10 and there is a recent trend toward using the Activity Measure for Post-Acute Care (AM-PAC).11 However, none of these tools, including the one discussed in this review, were designed to capture mobility levels in hospitalized patients. The Katz ADLs and the SPPB were designed for community living adults, and the AM-PAC was designed for a more mobile post-acute-care patient population. Although these tools do have limitations for use with hospitalized patients, they have shown promising results.10,12
What does all this mean for implementation? Do we have enough data on the existing scales to say we should be implementing them—or in the case of Braden, continuing to use them—to measure function and mobility in hospitalized patients? Implementing an ideal mobility assessment tool into the routinized care of the hospital patient may be necessary but insufficient. Complementing the use of these tools with more objective and precise mobility measures (eg, activity counts or steps from wearable sensors) would greatly increase the ability to accurately assess mobility and potentially enable providers to recommend specific mobility goals for patients in the form of steps or minutes of activity per day. In conclusion, the provocative results by Valiani et al.8 underscore the importance of mobility for hospitalized patients but also suggest many opportunities for future research and implementation to improve hospital care, especially for older adults.
Disclosure
Nothing to report.
1. Covinsky KE, Pierluissi E, Johnston CB. Hospitalization-associated disability: “She was probably able to ambulate, but I’m not sure.” JAMA. 2011;306(16):1782-1793. PubMed
2. Greysen SR, Stijacic Cenzer I, Auerbach AD, Covinsky KE. Functional impairment and hospital readmission in Medicare seniors. JAMA Intern Med. 2015;175(4):559-565. PubMed
3. Covinsky KE, Palmer RM, Fortinsky RH, et al. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. J Amer Geriatr Soc. 2003;51(4):451-458. PubMed
4. Barnes DE, Mehta KM, Boscardin WJ, et al. Prediction of recovery, dependence or death in elders who become disabled during hospitalization. J Gen Intern Med. 2013;28(2):261-268. PubMed
5. Detsky AS, Krumholz HM. Reducing the trauma of hospitalization. JAMA. 2014;311(21):2169-2170. PubMed
6. Creditor MC. Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118(3):219-223. PubMed
7. Krumholz HM. Post-hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100-102. PubMed
8. Valiani V, Chen Z, Lipori G, Pahor M, Sabbá C, Manini TM. Prognostic value of Braden activity subscale for mobility status in hospitalized older adults. J Hosp Med. 2017;12(6):396-401. PubMed
9. Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914-919. PubMed
10. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol A Bio Sci Med Sci. 1994;49(2):M85-M94. PubMed
11. Haley SM, Andres PL, Coster WJ, Kosinski M, Ni P, Jette AM. Short-form activity measure for post-acute care. Arch Phys Med Rehabil. 2004;85(4):649-660. PubMed
12. Wallace M, Shelkey M. Monitoring functional status in hospitalized older adults. Am J Nurs. 2008;108(4):64-71. PubMed
Mobility impairment (reduced ability to change body position or ambulate) is common among older adults during hospitalization1 and is correlated with higher rates of readmission,2 long-term care placement,3 and even death.4 Although some may perceive mobility impairment during hospitalization as a temporary inconvenience, recent research suggests disruptions of basic activities of daily life such as mobility may be “traumatic” 5 or “toxic”6 to older adults with long-term post-hospital effects.7 While these studies highlight the underestimated effects of low mobility during hospitalization, they are based on data collected for research purposes using mobility measurement tools not typically utilized in routine hospital care.
The absence of a standardized mobility measurement tool used as part of routine hospital care poses a barrier to estimating the effects of low hospital mobility and programs seeking to improve mobility levels in hospitalized patients. In this issue of the Journal of Hospital Medicine, Valiani et al.8 found a novel approach to measure mobility using a universally disseminated clinical scale (Braden). Using the activity subscale of the Braden scale, the authors found that mobility level changes during hospitalization can have a striking impact on post-discharge mortality. Their results indicate that older adults who develop mobility impairment during hospitalization had higher odds of death, specifically 1.23 times greater risk, within 6 months after discharge (23% decreased chance of survival). Most of the risk applies in the first 30 days and remains to a lesser extent for up to 5 years post-hospitalization. An equally interesting finding was that those who enter the hospital with low mobility but improve have a 46% higher survival rate. Again, most of the benefit is seen during hospitalization or immediately afterward, but the benefit persists for up to 5 years. A schematic of the results are presented in the Figure. Notably, Valiani et al.8 did not find regression to the mean Braden score of 3.
This novel use of the Braden activity subscale raises a question: Should we be using the Braden activity component to measure mobility in the hospital? Put another way, what scale should we be using in the hospital? Using the Braden activity subscale is convenient, since it capitalizes on data already being gathered. However, this subscale focuses solely on ambulation frequency; it doesn’t capture other mobility domains, such as ability to change body position. Ambulation is only half of the mobility story. It is interesting that although the Braden scale does have a mobility subscale that captures body position changes, the authors chose not to use it. This begs the question of whether an ideal mobility scale should encompass both components.
Previous studies of hospital mobility have deployed tools such as Katz Activities of Daily Living (ADLs)9 and the Short Physical Performance Battery (SPPB),10 and there is a recent trend toward using the Activity Measure for Post-Acute Care (AM-PAC).11 However, none of these tools, including the one discussed in this review, were designed to capture mobility levels in hospitalized patients. The Katz ADLs and the SPPB were designed for community living adults, and the AM-PAC was designed for a more mobile post-acute-care patient population. Although these tools do have limitations for use with hospitalized patients, they have shown promising results.10,12
What does all this mean for implementation? Do we have enough data on the existing scales to say we should be implementing them—or in the case of Braden, continuing to use them—to measure function and mobility in hospitalized patients? Implementing an ideal mobility assessment tool into the routinized care of the hospital patient may be necessary but insufficient. Complementing the use of these tools with more objective and precise mobility measures (eg, activity counts or steps from wearable sensors) would greatly increase the ability to accurately assess mobility and potentially enable providers to recommend specific mobility goals for patients in the form of steps or minutes of activity per day. In conclusion, the provocative results by Valiani et al.8 underscore the importance of mobility for hospitalized patients but also suggest many opportunities for future research and implementation to improve hospital care, especially for older adults.
Disclosure
Nothing to report.
Mobility impairment (reduced ability to change body position or ambulate) is common among older adults during hospitalization1 and is correlated with higher rates of readmission,2 long-term care placement,3 and even death.4 Although some may perceive mobility impairment during hospitalization as a temporary inconvenience, recent research suggests disruptions of basic activities of daily life such as mobility may be “traumatic” 5 or “toxic”6 to older adults with long-term post-hospital effects.7 While these studies highlight the underestimated effects of low mobility during hospitalization, they are based on data collected for research purposes using mobility measurement tools not typically utilized in routine hospital care.
The absence of a standardized mobility measurement tool used as part of routine hospital care poses a barrier to estimating the effects of low hospital mobility and programs seeking to improve mobility levels in hospitalized patients. In this issue of the Journal of Hospital Medicine, Valiani et al.8 found a novel approach to measure mobility using a universally disseminated clinical scale (Braden). Using the activity subscale of the Braden scale, the authors found that mobility level changes during hospitalization can have a striking impact on post-discharge mortality. Their results indicate that older adults who develop mobility impairment during hospitalization had higher odds of death, specifically 1.23 times greater risk, within 6 months after discharge (23% decreased chance of survival). Most of the risk applies in the first 30 days and remains to a lesser extent for up to 5 years post-hospitalization. An equally interesting finding was that those who enter the hospital with low mobility but improve have a 46% higher survival rate. Again, most of the benefit is seen during hospitalization or immediately afterward, but the benefit persists for up to 5 years. A schematic of the results are presented in the Figure. Notably, Valiani et al.8 did not find regression to the mean Braden score of 3.
This novel use of the Braden activity subscale raises a question: Should we be using the Braden activity component to measure mobility in the hospital? Put another way, what scale should we be using in the hospital? Using the Braden activity subscale is convenient, since it capitalizes on data already being gathered. However, this subscale focuses solely on ambulation frequency; it doesn’t capture other mobility domains, such as ability to change body position. Ambulation is only half of the mobility story. It is interesting that although the Braden scale does have a mobility subscale that captures body position changes, the authors chose not to use it. This begs the question of whether an ideal mobility scale should encompass both components.
Previous studies of hospital mobility have deployed tools such as Katz Activities of Daily Living (ADLs)9 and the Short Physical Performance Battery (SPPB),10 and there is a recent trend toward using the Activity Measure for Post-Acute Care (AM-PAC).11 However, none of these tools, including the one discussed in this review, were designed to capture mobility levels in hospitalized patients. The Katz ADLs and the SPPB were designed for community living adults, and the AM-PAC was designed for a more mobile post-acute-care patient population. Although these tools do have limitations for use with hospitalized patients, they have shown promising results.10,12
What does all this mean for implementation? Do we have enough data on the existing scales to say we should be implementing them—or in the case of Braden, continuing to use them—to measure function and mobility in hospitalized patients? Implementing an ideal mobility assessment tool into the routinized care of the hospital patient may be necessary but insufficient. Complementing the use of these tools with more objective and precise mobility measures (eg, activity counts or steps from wearable sensors) would greatly increase the ability to accurately assess mobility and potentially enable providers to recommend specific mobility goals for patients in the form of steps or minutes of activity per day. In conclusion, the provocative results by Valiani et al.8 underscore the importance of mobility for hospitalized patients but also suggest many opportunities for future research and implementation to improve hospital care, especially for older adults.
Disclosure
Nothing to report.
1. Covinsky KE, Pierluissi E, Johnston CB. Hospitalization-associated disability: “She was probably able to ambulate, but I’m not sure.” JAMA. 2011;306(16):1782-1793. PubMed
2. Greysen SR, Stijacic Cenzer I, Auerbach AD, Covinsky KE. Functional impairment and hospital readmission in Medicare seniors. JAMA Intern Med. 2015;175(4):559-565. PubMed
3. Covinsky KE, Palmer RM, Fortinsky RH, et al. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. J Amer Geriatr Soc. 2003;51(4):451-458. PubMed
4. Barnes DE, Mehta KM, Boscardin WJ, et al. Prediction of recovery, dependence or death in elders who become disabled during hospitalization. J Gen Intern Med. 2013;28(2):261-268. PubMed
5. Detsky AS, Krumholz HM. Reducing the trauma of hospitalization. JAMA. 2014;311(21):2169-2170. PubMed
6. Creditor MC. Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118(3):219-223. PubMed
7. Krumholz HM. Post-hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100-102. PubMed
8. Valiani V, Chen Z, Lipori G, Pahor M, Sabbá C, Manini TM. Prognostic value of Braden activity subscale for mobility status in hospitalized older adults. J Hosp Med. 2017;12(6):396-401. PubMed
9. Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914-919. PubMed
10. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol A Bio Sci Med Sci. 1994;49(2):M85-M94. PubMed
11. Haley SM, Andres PL, Coster WJ, Kosinski M, Ni P, Jette AM. Short-form activity measure for post-acute care. Arch Phys Med Rehabil. 2004;85(4):649-660. PubMed
12. Wallace M, Shelkey M. Monitoring functional status in hospitalized older adults. Am J Nurs. 2008;108(4):64-71. PubMed
1. Covinsky KE, Pierluissi E, Johnston CB. Hospitalization-associated disability: “She was probably able to ambulate, but I’m not sure.” JAMA. 2011;306(16):1782-1793. PubMed
2. Greysen SR, Stijacic Cenzer I, Auerbach AD, Covinsky KE. Functional impairment and hospital readmission in Medicare seniors. JAMA Intern Med. 2015;175(4):559-565. PubMed
3. Covinsky KE, Palmer RM, Fortinsky RH, et al. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. J Amer Geriatr Soc. 2003;51(4):451-458. PubMed
4. Barnes DE, Mehta KM, Boscardin WJ, et al. Prediction of recovery, dependence or death in elders who become disabled during hospitalization. J Gen Intern Med. 2013;28(2):261-268. PubMed
5. Detsky AS, Krumholz HM. Reducing the trauma of hospitalization. JAMA. 2014;311(21):2169-2170. PubMed
6. Creditor MC. Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118(3):219-223. PubMed
7. Krumholz HM. Post-hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100-102. PubMed
8. Valiani V, Chen Z, Lipori G, Pahor M, Sabbá C, Manini TM. Prognostic value of Braden activity subscale for mobility status in hospitalized older adults. J Hosp Med. 2017;12(6):396-401. PubMed
9. Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914-919. PubMed
10. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol A Bio Sci Med Sci. 1994;49(2):M85-M94. PubMed
11. Haley SM, Andres PL, Coster WJ, Kosinski M, Ni P, Jette AM. Short-form activity measure for post-acute care. Arch Phys Med Rehabil. 2004;85(4):649-660. PubMed
12. Wallace M, Shelkey M. Monitoring functional status in hospitalized older adults. Am J Nurs. 2008;108(4):64-71. PubMed
© 2017 Society of Hospital Medicine
It’s time for a strategic approach to observation care
After patients have experienced an illness requiring a hospital stay, they are increasingly finding that despite having received treatment in a hospital bed, they were never actually admitted—at least not from the perspective of their insurers. Instead, these patients were kept under observation, an outpatient designation that allows a hospital to bill for observation services without formally admitting a patient.
Recent studies have recorded significant increases in hospitals’ use of observation stays among the Medicare population,1-3 raising concerns about the financial ramifications for patients. Under observation, patients are potentially responsible for a greater share of the cost and bear the financial consequences of inappropriate observation stays. Currently, around 6% of Medicare patients hospitalized as outpatients spend more than 48 hours (or two midnights) in observation, sometimes much longer, exposing them to significant out-of-pocket costs.3 In addition, liberal use of observation can lead to increased hospital stays, for example among lower-severity emergency department (ED) patients who could have been safely discharged but were instead kept for a costly observation stay.4 At the same time, hospitals do not necessarily benefit from this cost shifting; in fact, hospital margin is worse for patients under Medicare observation care.5 Yet hospitals are obligated to be compliant with CMS observation regulations and may try to avoid the consequences (eg, audits, non-payment) for inpatient stays that are deemed inappropriate by CMS.
While the nuances of how CMS finances observation stays have made the practice controversial, the use of observation care in other payer groups that may not have the same reimbursement policies, and its impact on patients, have not been well studied. In this issue of the Journal of Hospital Medicine, Nuckols et al.6 begins to address this gap by carefully exploring trends in observation stays in a multipayer data set.
The authors use data for four states (Georgia, Nebraska, South Carolina, and Tennessee) from the Healthcare Cost and Utilization Project (Agency for Healthcare Quality and Research) and the American Community Survey (US Census Bureau) to calculate population based rates of ED visits, observation stays, and inpatient admissions. To date, this is the first study to examine and compare the use of observation stays in an all-payer data set. Similar to prior work that examined the Medicare population, the authors find increased rates of treat-and-release ED visits and observation stays over time with a corresponding decline in inpatient admissions. As this study clearly shows, observation stays are comprising a greater fraction of the total hospital care delivered to patients with acute illnesses.
In many ways, the findings of Nuckols et al.6 raise more questions than they answer. For example, does the rise in observation stays represent a fundamental shift in how hospitals deliver care, an alternative to costly inpatient admissions? Are changing payer incentives driving hospitals to be more prudent in their inpatient admission practices, or are similar services simply being delivered under a new billing designation? And, most important, does this shift have any repercussions for the quality and safety of patient care?
Ultimately, the answer to these questions is, “It depends.” As the authors mention, most US hospitals admit observation patients to general medical wards, where they receive care at the admitting provider’s discretion instead of utilizing specific care pathways or observation protocols.7 In some of these hospitals, there may be little to no difference in how the observation patient is treated compared with a similar patient who is hospitalized as an inpatient.
However, a minority of hospitals has been more strategic in their delivery of observation care and have developed observation units. While observation units vary in design, common features include a dedicated location in the hospital with dedicated staff, reliance on clear inclusion-exclusion criteria for admission to the unit, and the use of rapid diagnostic or treatment protocols for a limited number of conditions. About half of these observation units are ED-based, reducing transitions of care between services. Protocol-driven observation units have the potential to prevent unnecessary inpatient admissions, standardize evidence-based practice, and reduce practice variation and resource use, apparently without increasing adverse events.8 In addition, they may also lead to better experiences of care for many patients compared with inpatient admissions.
Medicare’s own policy on observation hospital care succinctly describes ED observation units: “Observation services are commonly ordered for patients who present to the emergency department and who then require a significant period of treatment in order to make a decision concerning their admission or discharge…usually in less than 24 hours.” Due to regulatory changes and auditing pressure, observation care has expanded beyond this definition in length of stay, scope, and practice such that much of observation care now occurs on general hospital wards. Ideally, observation policy must be realigned with its original intent and investment made in ED observation units.
The shifting landscape of hospital-based care as described by Nuckols et al.6 highlights the need for a more strategic approach to the delivery of acute care. Unfortunately, to date, there has been a lack of attention among policymakers towards promoting a system of emergent and urgent care that is coordinated and efficient. Observation stays are one major area for which innovations in the acute care delivery system may result in meaningful improvement in patient outcomes and greater value for the healthcare system. Incentivizing a system of high-value observation care, such as promoting the use of observation units that employ evidence-based practices, should be a key priority when considering approaches to reducing the cost of hospital-based and other acute care.
One strategy is to better define and possibly expand the cohort of patients likely to benefit from care in an observation unit. Hospitals with significant experience using observation units treat not only common observation conditions like chest pain, asthma, or cellulitis, but also higher-risk inpatient conditions like syncope and diabetic ketoacidosis using rapid diagnostic and treatment protocols.
Identifying high-value observation care also will require developing patient outcome measures specific for observation stays. Observation-specific quality measures will allow a comparison of hospitals that use different care pathways for observation patients or treat certain populations of patients in observation units. This necessitates looking beyond resource use (costs and length of stay), which most studies on observation units have focused on, and examining a broader range of patient outcomes like time to symptomatic resolution, quality of life, or return to productivity after an acute illness.
Finally, observation care is also a good target for payment redesign. For example, incentive payments could be provided to hospitals that choose to develop observation units, employ observation units that utilize best known practices for observation care (such as protocols and clearly defined patient cohorts), or deliver particularly good acute care outcomes for patients with observation-amenable conditions. On the consumer side, value-based contracting could be used to shunt patients with acute conditions that require evaluation in an urgent care center or ED to hospitals that use observation units.
While the declines in inpatient admission and increases in treat-and-release ED patients have been well-documented over time, perhaps the biggest contribution of this study from Nuckols et al.6 lies in its identification of the changes in observation care, which have been increasing in all payer groups. Our opportunity now is to shape whether these shifts toward observation care deliver greater value for patients.
Acknowledgment
The authors thank Joanna Guo, BA, for her editorial and technical assistance.
Disclosure
Nothing to report.
1. Feng Z, Wright B, Mor V. Sharp rise in Medicare enrollees being held in hospitals for observation raises concerns about causes and consequences. Health Aff (Millwood). 2012;31(6):1251-1259. PubMed
2. Zuckerman RB, Sheingold SH, Orav EJ, Ruhter J, Epstein AM. Readmissions, observation, and the Hospital Readmissions Reduction Program. N Engl J Med. 2016;374(16):1543-1551. PubMed
3. Office of Inspector General. Vulnerabilities Remain Under Medicare’s 2-Midnight Hospital Policy. US Department of Health & Human Services. Published 2016. https://oig.hhs.gov/oei/reports/oei-02-15-00020.pdf. Accessed April 25, 2017.
4. Blecker S, Gavin NP, Park H, Ladapo JA, Katz SD. Observation units as substitutes for hospitalization or home discharge. Ann Emerg Med. 2016;67(6):706-713.e702. PubMed
5. Medicare Payment Advisory Commission. Report to the Congress: Medicare Payment Policy. Published 2015. http://medpac.gov/docs/default-source/reports/mar2015_entirereport_revised.pdf?sfvrsn=0). Accessed April 25, 2017.
6. Nuckols TN, Fingar KR, Barrett M, Steiner C, Stocks C, Owens PL. The shifting landscape in utilization of inpatient, observation, and emergency department services across payers. J Hosp Med. 2017;12(6):444-446. PubMed
7. Ross MA, Hockenberry JM, Mutter R, Barrett M, Wheatley M, Pitts SR. Protocol-driven emergency department observation units offer savings, shorter stays, and reduced admissions. Health Aff (Millwood). 2013;32(12):2149-2156. PubMed
8. Ross MA, Aurora T, Graff L, et al. State of the art: emergency department observation units. Crit Pathw Cardiol. 2012;11(3):128-138. PubMed
After patients have experienced an illness requiring a hospital stay, they are increasingly finding that despite having received treatment in a hospital bed, they were never actually admitted—at least not from the perspective of their insurers. Instead, these patients were kept under observation, an outpatient designation that allows a hospital to bill for observation services without formally admitting a patient.
Recent studies have recorded significant increases in hospitals’ use of observation stays among the Medicare population,1-3 raising concerns about the financial ramifications for patients. Under observation, patients are potentially responsible for a greater share of the cost and bear the financial consequences of inappropriate observation stays. Currently, around 6% of Medicare patients hospitalized as outpatients spend more than 48 hours (or two midnights) in observation, sometimes much longer, exposing them to significant out-of-pocket costs.3 In addition, liberal use of observation can lead to increased hospital stays, for example among lower-severity emergency department (ED) patients who could have been safely discharged but were instead kept for a costly observation stay.4 At the same time, hospitals do not necessarily benefit from this cost shifting; in fact, hospital margin is worse for patients under Medicare observation care.5 Yet hospitals are obligated to be compliant with CMS observation regulations and may try to avoid the consequences (eg, audits, non-payment) for inpatient stays that are deemed inappropriate by CMS.
While the nuances of how CMS finances observation stays have made the practice controversial, the use of observation care in other payer groups that may not have the same reimbursement policies, and its impact on patients, have not been well studied. In this issue of the Journal of Hospital Medicine, Nuckols et al.6 begins to address this gap by carefully exploring trends in observation stays in a multipayer data set.
The authors use data for four states (Georgia, Nebraska, South Carolina, and Tennessee) from the Healthcare Cost and Utilization Project (Agency for Healthcare Quality and Research) and the American Community Survey (US Census Bureau) to calculate population based rates of ED visits, observation stays, and inpatient admissions. To date, this is the first study to examine and compare the use of observation stays in an all-payer data set. Similar to prior work that examined the Medicare population, the authors find increased rates of treat-and-release ED visits and observation stays over time with a corresponding decline in inpatient admissions. As this study clearly shows, observation stays are comprising a greater fraction of the total hospital care delivered to patients with acute illnesses.
In many ways, the findings of Nuckols et al.6 raise more questions than they answer. For example, does the rise in observation stays represent a fundamental shift in how hospitals deliver care, an alternative to costly inpatient admissions? Are changing payer incentives driving hospitals to be more prudent in their inpatient admission practices, or are similar services simply being delivered under a new billing designation? And, most important, does this shift have any repercussions for the quality and safety of patient care?
Ultimately, the answer to these questions is, “It depends.” As the authors mention, most US hospitals admit observation patients to general medical wards, where they receive care at the admitting provider’s discretion instead of utilizing specific care pathways or observation protocols.7 In some of these hospitals, there may be little to no difference in how the observation patient is treated compared with a similar patient who is hospitalized as an inpatient.
However, a minority of hospitals has been more strategic in their delivery of observation care and have developed observation units. While observation units vary in design, common features include a dedicated location in the hospital with dedicated staff, reliance on clear inclusion-exclusion criteria for admission to the unit, and the use of rapid diagnostic or treatment protocols for a limited number of conditions. About half of these observation units are ED-based, reducing transitions of care between services. Protocol-driven observation units have the potential to prevent unnecessary inpatient admissions, standardize evidence-based practice, and reduce practice variation and resource use, apparently without increasing adverse events.8 In addition, they may also lead to better experiences of care for many patients compared with inpatient admissions.
Medicare’s own policy on observation hospital care succinctly describes ED observation units: “Observation services are commonly ordered for patients who present to the emergency department and who then require a significant period of treatment in order to make a decision concerning their admission or discharge…usually in less than 24 hours.” Due to regulatory changes and auditing pressure, observation care has expanded beyond this definition in length of stay, scope, and practice such that much of observation care now occurs on general hospital wards. Ideally, observation policy must be realigned with its original intent and investment made in ED observation units.
The shifting landscape of hospital-based care as described by Nuckols et al.6 highlights the need for a more strategic approach to the delivery of acute care. Unfortunately, to date, there has been a lack of attention among policymakers towards promoting a system of emergent and urgent care that is coordinated and efficient. Observation stays are one major area for which innovations in the acute care delivery system may result in meaningful improvement in patient outcomes and greater value for the healthcare system. Incentivizing a system of high-value observation care, such as promoting the use of observation units that employ evidence-based practices, should be a key priority when considering approaches to reducing the cost of hospital-based and other acute care.
One strategy is to better define and possibly expand the cohort of patients likely to benefit from care in an observation unit. Hospitals with significant experience using observation units treat not only common observation conditions like chest pain, asthma, or cellulitis, but also higher-risk inpatient conditions like syncope and diabetic ketoacidosis using rapid diagnostic and treatment protocols.
Identifying high-value observation care also will require developing patient outcome measures specific for observation stays. Observation-specific quality measures will allow a comparison of hospitals that use different care pathways for observation patients or treat certain populations of patients in observation units. This necessitates looking beyond resource use (costs and length of stay), which most studies on observation units have focused on, and examining a broader range of patient outcomes like time to symptomatic resolution, quality of life, or return to productivity after an acute illness.
Finally, observation care is also a good target for payment redesign. For example, incentive payments could be provided to hospitals that choose to develop observation units, employ observation units that utilize best known practices for observation care (such as protocols and clearly defined patient cohorts), or deliver particularly good acute care outcomes for patients with observation-amenable conditions. On the consumer side, value-based contracting could be used to shunt patients with acute conditions that require evaluation in an urgent care center or ED to hospitals that use observation units.
While the declines in inpatient admission and increases in treat-and-release ED patients have been well-documented over time, perhaps the biggest contribution of this study from Nuckols et al.6 lies in its identification of the changes in observation care, which have been increasing in all payer groups. Our opportunity now is to shape whether these shifts toward observation care deliver greater value for patients.
Acknowledgment
The authors thank Joanna Guo, BA, for her editorial and technical assistance.
Disclosure
Nothing to report.
After patients have experienced an illness requiring a hospital stay, they are increasingly finding that despite having received treatment in a hospital bed, they were never actually admitted—at least not from the perspective of their insurers. Instead, these patients were kept under observation, an outpatient designation that allows a hospital to bill for observation services without formally admitting a patient.
Recent studies have recorded significant increases in hospitals’ use of observation stays among the Medicare population,1-3 raising concerns about the financial ramifications for patients. Under observation, patients are potentially responsible for a greater share of the cost and bear the financial consequences of inappropriate observation stays. Currently, around 6% of Medicare patients hospitalized as outpatients spend more than 48 hours (or two midnights) in observation, sometimes much longer, exposing them to significant out-of-pocket costs.3 In addition, liberal use of observation can lead to increased hospital stays, for example among lower-severity emergency department (ED) patients who could have been safely discharged but were instead kept for a costly observation stay.4 At the same time, hospitals do not necessarily benefit from this cost shifting; in fact, hospital margin is worse for patients under Medicare observation care.5 Yet hospitals are obligated to be compliant with CMS observation regulations and may try to avoid the consequences (eg, audits, non-payment) for inpatient stays that are deemed inappropriate by CMS.
While the nuances of how CMS finances observation stays have made the practice controversial, the use of observation care in other payer groups that may not have the same reimbursement policies, and its impact on patients, have not been well studied. In this issue of the Journal of Hospital Medicine, Nuckols et al.6 begins to address this gap by carefully exploring trends in observation stays in a multipayer data set.
The authors use data for four states (Georgia, Nebraska, South Carolina, and Tennessee) from the Healthcare Cost and Utilization Project (Agency for Healthcare Quality and Research) and the American Community Survey (US Census Bureau) to calculate population based rates of ED visits, observation stays, and inpatient admissions. To date, this is the first study to examine and compare the use of observation stays in an all-payer data set. Similar to prior work that examined the Medicare population, the authors find increased rates of treat-and-release ED visits and observation stays over time with a corresponding decline in inpatient admissions. As this study clearly shows, observation stays are comprising a greater fraction of the total hospital care delivered to patients with acute illnesses.
In many ways, the findings of Nuckols et al.6 raise more questions than they answer. For example, does the rise in observation stays represent a fundamental shift in how hospitals deliver care, an alternative to costly inpatient admissions? Are changing payer incentives driving hospitals to be more prudent in their inpatient admission practices, or are similar services simply being delivered under a new billing designation? And, most important, does this shift have any repercussions for the quality and safety of patient care?
Ultimately, the answer to these questions is, “It depends.” As the authors mention, most US hospitals admit observation patients to general medical wards, where they receive care at the admitting provider’s discretion instead of utilizing specific care pathways or observation protocols.7 In some of these hospitals, there may be little to no difference in how the observation patient is treated compared with a similar patient who is hospitalized as an inpatient.
However, a minority of hospitals has been more strategic in their delivery of observation care and have developed observation units. While observation units vary in design, common features include a dedicated location in the hospital with dedicated staff, reliance on clear inclusion-exclusion criteria for admission to the unit, and the use of rapid diagnostic or treatment protocols for a limited number of conditions. About half of these observation units are ED-based, reducing transitions of care between services. Protocol-driven observation units have the potential to prevent unnecessary inpatient admissions, standardize evidence-based practice, and reduce practice variation and resource use, apparently without increasing adverse events.8 In addition, they may also lead to better experiences of care for many patients compared with inpatient admissions.
Medicare’s own policy on observation hospital care succinctly describes ED observation units: “Observation services are commonly ordered for patients who present to the emergency department and who then require a significant period of treatment in order to make a decision concerning their admission or discharge…usually in less than 24 hours.” Due to regulatory changes and auditing pressure, observation care has expanded beyond this definition in length of stay, scope, and practice such that much of observation care now occurs on general hospital wards. Ideally, observation policy must be realigned with its original intent and investment made in ED observation units.
The shifting landscape of hospital-based care as described by Nuckols et al.6 highlights the need for a more strategic approach to the delivery of acute care. Unfortunately, to date, there has been a lack of attention among policymakers towards promoting a system of emergent and urgent care that is coordinated and efficient. Observation stays are one major area for which innovations in the acute care delivery system may result in meaningful improvement in patient outcomes and greater value for the healthcare system. Incentivizing a system of high-value observation care, such as promoting the use of observation units that employ evidence-based practices, should be a key priority when considering approaches to reducing the cost of hospital-based and other acute care.
One strategy is to better define and possibly expand the cohort of patients likely to benefit from care in an observation unit. Hospitals with significant experience using observation units treat not only common observation conditions like chest pain, asthma, or cellulitis, but also higher-risk inpatient conditions like syncope and diabetic ketoacidosis using rapid diagnostic and treatment protocols.
Identifying high-value observation care also will require developing patient outcome measures specific for observation stays. Observation-specific quality measures will allow a comparison of hospitals that use different care pathways for observation patients or treat certain populations of patients in observation units. This necessitates looking beyond resource use (costs and length of stay), which most studies on observation units have focused on, and examining a broader range of patient outcomes like time to symptomatic resolution, quality of life, or return to productivity after an acute illness.
Finally, observation care is also a good target for payment redesign. For example, incentive payments could be provided to hospitals that choose to develop observation units, employ observation units that utilize best known practices for observation care (such as protocols and clearly defined patient cohorts), or deliver particularly good acute care outcomes for patients with observation-amenable conditions. On the consumer side, value-based contracting could be used to shunt patients with acute conditions that require evaluation in an urgent care center or ED to hospitals that use observation units.
While the declines in inpatient admission and increases in treat-and-release ED patients have been well-documented over time, perhaps the biggest contribution of this study from Nuckols et al.6 lies in its identification of the changes in observation care, which have been increasing in all payer groups. Our opportunity now is to shape whether these shifts toward observation care deliver greater value for patients.
Acknowledgment
The authors thank Joanna Guo, BA, for her editorial and technical assistance.
Disclosure
Nothing to report.
1. Feng Z, Wright B, Mor V. Sharp rise in Medicare enrollees being held in hospitals for observation raises concerns about causes and consequences. Health Aff (Millwood). 2012;31(6):1251-1259. PubMed
2. Zuckerman RB, Sheingold SH, Orav EJ, Ruhter J, Epstein AM. Readmissions, observation, and the Hospital Readmissions Reduction Program. N Engl J Med. 2016;374(16):1543-1551. PubMed
3. Office of Inspector General. Vulnerabilities Remain Under Medicare’s 2-Midnight Hospital Policy. US Department of Health & Human Services. Published 2016. https://oig.hhs.gov/oei/reports/oei-02-15-00020.pdf. Accessed April 25, 2017.
4. Blecker S, Gavin NP, Park H, Ladapo JA, Katz SD. Observation units as substitutes for hospitalization or home discharge. Ann Emerg Med. 2016;67(6):706-713.e702. PubMed
5. Medicare Payment Advisory Commission. Report to the Congress: Medicare Payment Policy. Published 2015. http://medpac.gov/docs/default-source/reports/mar2015_entirereport_revised.pdf?sfvrsn=0). Accessed April 25, 2017.
6. Nuckols TN, Fingar KR, Barrett M, Steiner C, Stocks C, Owens PL. The shifting landscape in utilization of inpatient, observation, and emergency department services across payers. J Hosp Med. 2017;12(6):444-446. PubMed
7. Ross MA, Hockenberry JM, Mutter R, Barrett M, Wheatley M, Pitts SR. Protocol-driven emergency department observation units offer savings, shorter stays, and reduced admissions. Health Aff (Millwood). 2013;32(12):2149-2156. PubMed
8. Ross MA, Aurora T, Graff L, et al. State of the art: emergency department observation units. Crit Pathw Cardiol. 2012;11(3):128-138. PubMed
1. Feng Z, Wright B, Mor V. Sharp rise in Medicare enrollees being held in hospitals for observation raises concerns about causes and consequences. Health Aff (Millwood). 2012;31(6):1251-1259. PubMed
2. Zuckerman RB, Sheingold SH, Orav EJ, Ruhter J, Epstein AM. Readmissions, observation, and the Hospital Readmissions Reduction Program. N Engl J Med. 2016;374(16):1543-1551. PubMed
3. Office of Inspector General. Vulnerabilities Remain Under Medicare’s 2-Midnight Hospital Policy. US Department of Health & Human Services. Published 2016. https://oig.hhs.gov/oei/reports/oei-02-15-00020.pdf. Accessed April 25, 2017.
4. Blecker S, Gavin NP, Park H, Ladapo JA, Katz SD. Observation units as substitutes for hospitalization or home discharge. Ann Emerg Med. 2016;67(6):706-713.e702. PubMed
5. Medicare Payment Advisory Commission. Report to the Congress: Medicare Payment Policy. Published 2015. http://medpac.gov/docs/default-source/reports/mar2015_entirereport_revised.pdf?sfvrsn=0). Accessed April 25, 2017.
6. Nuckols TN, Fingar KR, Barrett M, Steiner C, Stocks C, Owens PL. The shifting landscape in utilization of inpatient, observation, and emergency department services across payers. J Hosp Med. 2017;12(6):444-446. PubMed
7. Ross MA, Hockenberry JM, Mutter R, Barrett M, Wheatley M, Pitts SR. Protocol-driven emergency department observation units offer savings, shorter stays, and reduced admissions. Health Aff (Millwood). 2013;32(12):2149-2156. PubMed
8. Ross MA, Aurora T, Graff L, et al. State of the art: emergency department observation units. Crit Pathw Cardiol. 2012;11(3):128-138. PubMed
© 2017 Society of Hospital Medicine
Monitor watchers and alarm fatigue: Cautious optimism
Monitor watcher personnel are frequently used to assist nurses with identifying meaningful events on telemetry monitors. Although effectiveness of monitor watchers on patient outcomes has not been demonstrated conclusively,1 as many as 60% of United States hospitals may be using monitor watchers in some capacity.2 Presumed benefits of monitor watchers include prompt recognition of changes in patients’ conditions and the potential to reduce alarm fatigue among hospital staff. Alarm fatigue is desensitization resulting from overexposure to alarm signals that are either invalid or clinically irrelevant. Alarm fatigue has resulted in missed patient events and preventable deaths.3 In this issue of the Journal of Hospital Medicine, Palchaudhuri et al.4 report findings from their observational study of telemetry monitor alarms intercepted by monitor watchers as a mechanism for reducing both nurses’ exposure to alarm signals and subsequent alarm fatigue.
To our knowledge, the study by Palchaudhuri et al.4 is the first to report the effect of monitor watchers on nurses’ exposure to alarm signals. In this study, over a 2-month period monitor watchers intercepted 87% of alarms before they were sent to the nurse’s telephone. Monitor watchers intercepted over 90% of bradycardia and tachycardia alarms, indicating that they believed these alarms to be clinically irrelevant. Monitor watchers also intercepted about 75% of alarms for lethal arrhythmias, indicating that they believed these alarms to be invalid.
In this study, decisions about alarm validity and relevance were made through close communication between monitor watchers and nursing staff. If an alarm was sounding and the monitor watcher had already spoken with the nurse about it and established that the nurse was addressing the problem, the monitor watcher would intercept subsequent alarms for that issue or event (according to personal communication with S. Palchaudhuri). The results of the study not only indicate that monitor watchers can reduce the number of alarms to which a nurse is exposed, but also support previous findings that few alarms are valid or clinically relevant.5-7 The results of this study also suggest that “nuisance” alarms should include not only clinically irrelevant alarms, but also relevant alarms for which the nurse is actively seeking a solution. Monitor watchers may have an important role in addressing these alarms.
The study raises important considerations regarding monitor watcher practice and alarm fatigue. If monitor watchers are to be effective in reducing nurses’ exposure to alarms, they must use good judgment to determine when to intercept an alarm, call the nurse, or both. In the absence of proper judgment, monitor watchers may inadvertently increase nurses’ fatigue through redundant calls or inappropriately suppress valid relevant alarms. In free-text responses to our national monitor watcher survey, nurses expressed frustration over redundant calls from monitor watchers for invalid and irrelevant alarms.2 Research suggests that monitor watchers may not identify potentially dangerous alarms with complete accuracy. In a recent study reported in The Journal of the American Medical Society (JAMA), monitor watchers missed about 18% of patients with detectable rhythm or rate changes on telemetry in the hour before an emergency response team was activated.8
Several factors and conditions may affect monitor watchers’ judgment: 1) education and training, 2) location and access to contextual patient information, and 3) fatigue. First, across the US, the level of education required for monitor watcher positions ranges from a high school diploma to licensure as a registered nurse. The content and frequency of in-service training required also varies.2 These differing requirements may influence monitor watchers’ ability to interpret alarms.
Second, most monitor watchers are located off the patient care unit,2 which influences their access to information. Even in remote locations, monitor watchers can assess alarm validity by reviewing parameter waveforms for artifact. However, determining the relevance of an alarm to a particular patient is a more complex task requiring contextual information about the patient.9 Monitor watchers must work closely with clinicians at the bedside to determine the relevance of alarms, and repeated contact between monitor watchers and nurses over alarm conditions may itself increase nurses’ alarm fatigue.
Finally, fatigue may affect monitor watchers themselves and reduce their effectiveness. This issue was raised by Palchaudhuri et al. Both the number of monitors watched and the length of the monitor watcher’s shift likely influence alertness and effectiveness. In a simulation study, Segall et al.10 found that monitor watchers’ recognition of serious arrhythmias was significantly delayed when they were responsible for more than 40 patient monitors. Monitor watchers often work 12-hour shifts,2 and although no research has been reported on their shift-related alertness, this is a long time to remain attentive.
Given these potential challenges, future research should specifically address adverse patient outcomes and missed clinically relevant alarms. Only two of the seven patients who arrested during the study by Palchaudhuri et al.4 were on telemetry, and neither arrested due to lethal arrhythmias. While this is an important indication that no alarms for lethal arrhythmias were inadvertently suppressed, it is difficult to achieve adequate statistical power to assess rare outcomes like cardiac arrests. In a future study, alarms intercepted by monitor watchers could be assessed for accuracy and relevance to patient care to determine whether important alarms were inadvertently suppressed.
In summary, the study by Palchaudhuri et al.4 represents a preliminary step in considering the potential utility of monitor watchers for reducing invalid and clinically irrelevant alarms as well as subsequent alarm fatigue. As the authors note, dedicated monitor watchers can screen alarms much more quickly than nurses who may be engaged in other activities when an alarm signals. The study raises interesting questions about how monitor watchers should be incorporated into workflow. Should their only responsibility be to call regarding potentially critical events, or should they be able to prevent alarms from reaching the nurse? Could monitor watchers provide guidance to reduce alarm fatigue, such as suggesting parameter changes when they see trends in irrelevant alarms? Future research is warranted to understand how monitor watchers can be used most effectively to reduce alarm fatigue, and which characteristics of monitor watchers and their practice result in the best patient outcomes.
Disclosure
Nothing to report.
1. Funk M, Parkosewich JA, Johnson CR, Stukshis I. Effect of dedicated monitor watchers on patients’ outcomes. Am J Crit Care. 1997;6(4):318-323. PubMed
2. Funk M, Ruppel H, Blake N, Phillips J. Research: Use of monitor watchers in hospitals: characteristics, training, and practices. Biomed Instrum Technol. 2016;50(6):428-438. PubMed
3. Joint Commission. Medical device alarm safety in hospitals. Sentinel Event Alert. 2013;(50):1-3. PubMed
4. Palchaudhuri S, Chen S, Clayton E, Accurso A, Zakaria S. Telemetry monitor watchers reduce bedside nurses’ exposure to alarms by intercepting a high number of nonactionable alarms. J Hosp Med. 2017;12(6):447-449. PubMed
5. Bonafide CP, Lin R, Zander M, et al. Association between exposure to nonactionable physiologic monitor alarms and response time in a children’s hospital. J Hosp Med. 2015;10(6):345-351. PubMed
6. Drew BJ, Harris P, Zègre-Hemsey JK, et al. Insights into the problem of alarm fatigue with physiologic monitor devices: a comprehensive observational study of consecutive intensive care unit patients. PLoS One. 2014;9(10):e110274. PubMed
7. Siebig S, Kuhls S, Imhoff M, Gather U, Schölmerich J, Wrede CE. Intensive care unit alarms—how many do we need? Crit Care Med. 2010;38(2):451-456. PubMed
8. Cantillon DJ, Loy M, Burkle A, et al. Association between off-site central monitoring using standardized cardiac telemetry and clinical outcomes among non-critically ill patients. JAMA. 2016;316(5):519-524. PubMed
9. Rayo MF, Moffatt-Bruce SD. Alarm system management: evidence-based guidance encouraging direct measurement of informativeness to improve alarm response. BMJ Qual Saf. 2015;24(4):282-286. PubMed
10. Segall N, Hobbs G, Granger CB, et al. Patient load effects on response time to critical arrhythmias in cardiac telemetry: a randomized trial. Crit Care Med. 2015;43(5):1036-1042. PubMed
Monitor watcher personnel are frequently used to assist nurses with identifying meaningful events on telemetry monitors. Although effectiveness of monitor watchers on patient outcomes has not been demonstrated conclusively,1 as many as 60% of United States hospitals may be using monitor watchers in some capacity.2 Presumed benefits of monitor watchers include prompt recognition of changes in patients’ conditions and the potential to reduce alarm fatigue among hospital staff. Alarm fatigue is desensitization resulting from overexposure to alarm signals that are either invalid or clinically irrelevant. Alarm fatigue has resulted in missed patient events and preventable deaths.3 In this issue of the Journal of Hospital Medicine, Palchaudhuri et al.4 report findings from their observational study of telemetry monitor alarms intercepted by monitor watchers as a mechanism for reducing both nurses’ exposure to alarm signals and subsequent alarm fatigue.
To our knowledge, the study by Palchaudhuri et al.4 is the first to report the effect of monitor watchers on nurses’ exposure to alarm signals. In this study, over a 2-month period monitor watchers intercepted 87% of alarms before they were sent to the nurse’s telephone. Monitor watchers intercepted over 90% of bradycardia and tachycardia alarms, indicating that they believed these alarms to be clinically irrelevant. Monitor watchers also intercepted about 75% of alarms for lethal arrhythmias, indicating that they believed these alarms to be invalid.
In this study, decisions about alarm validity and relevance were made through close communication between monitor watchers and nursing staff. If an alarm was sounding and the monitor watcher had already spoken with the nurse about it and established that the nurse was addressing the problem, the monitor watcher would intercept subsequent alarms for that issue or event (according to personal communication with S. Palchaudhuri). The results of the study not only indicate that monitor watchers can reduce the number of alarms to which a nurse is exposed, but also support previous findings that few alarms are valid or clinically relevant.5-7 The results of this study also suggest that “nuisance” alarms should include not only clinically irrelevant alarms, but also relevant alarms for which the nurse is actively seeking a solution. Monitor watchers may have an important role in addressing these alarms.
The study raises important considerations regarding monitor watcher practice and alarm fatigue. If monitor watchers are to be effective in reducing nurses’ exposure to alarms, they must use good judgment to determine when to intercept an alarm, call the nurse, or both. In the absence of proper judgment, monitor watchers may inadvertently increase nurses’ fatigue through redundant calls or inappropriately suppress valid relevant alarms. In free-text responses to our national monitor watcher survey, nurses expressed frustration over redundant calls from monitor watchers for invalid and irrelevant alarms.2 Research suggests that monitor watchers may not identify potentially dangerous alarms with complete accuracy. In a recent study reported in The Journal of the American Medical Society (JAMA), monitor watchers missed about 18% of patients with detectable rhythm or rate changes on telemetry in the hour before an emergency response team was activated.8
Several factors and conditions may affect monitor watchers’ judgment: 1) education and training, 2) location and access to contextual patient information, and 3) fatigue. First, across the US, the level of education required for monitor watcher positions ranges from a high school diploma to licensure as a registered nurse. The content and frequency of in-service training required also varies.2 These differing requirements may influence monitor watchers’ ability to interpret alarms.
Second, most monitor watchers are located off the patient care unit,2 which influences their access to information. Even in remote locations, monitor watchers can assess alarm validity by reviewing parameter waveforms for artifact. However, determining the relevance of an alarm to a particular patient is a more complex task requiring contextual information about the patient.9 Monitor watchers must work closely with clinicians at the bedside to determine the relevance of alarms, and repeated contact between monitor watchers and nurses over alarm conditions may itself increase nurses’ alarm fatigue.
Finally, fatigue may affect monitor watchers themselves and reduce their effectiveness. This issue was raised by Palchaudhuri et al. Both the number of monitors watched and the length of the monitor watcher’s shift likely influence alertness and effectiveness. In a simulation study, Segall et al.10 found that monitor watchers’ recognition of serious arrhythmias was significantly delayed when they were responsible for more than 40 patient monitors. Monitor watchers often work 12-hour shifts,2 and although no research has been reported on their shift-related alertness, this is a long time to remain attentive.
Given these potential challenges, future research should specifically address adverse patient outcomes and missed clinically relevant alarms. Only two of the seven patients who arrested during the study by Palchaudhuri et al.4 were on telemetry, and neither arrested due to lethal arrhythmias. While this is an important indication that no alarms for lethal arrhythmias were inadvertently suppressed, it is difficult to achieve adequate statistical power to assess rare outcomes like cardiac arrests. In a future study, alarms intercepted by monitor watchers could be assessed for accuracy and relevance to patient care to determine whether important alarms were inadvertently suppressed.
In summary, the study by Palchaudhuri et al.4 represents a preliminary step in considering the potential utility of monitor watchers for reducing invalid and clinically irrelevant alarms as well as subsequent alarm fatigue. As the authors note, dedicated monitor watchers can screen alarms much more quickly than nurses who may be engaged in other activities when an alarm signals. The study raises interesting questions about how monitor watchers should be incorporated into workflow. Should their only responsibility be to call regarding potentially critical events, or should they be able to prevent alarms from reaching the nurse? Could monitor watchers provide guidance to reduce alarm fatigue, such as suggesting parameter changes when they see trends in irrelevant alarms? Future research is warranted to understand how monitor watchers can be used most effectively to reduce alarm fatigue, and which characteristics of monitor watchers and their practice result in the best patient outcomes.
Disclosure
Nothing to report.
Monitor watcher personnel are frequently used to assist nurses with identifying meaningful events on telemetry monitors. Although effectiveness of monitor watchers on patient outcomes has not been demonstrated conclusively,1 as many as 60% of United States hospitals may be using monitor watchers in some capacity.2 Presumed benefits of monitor watchers include prompt recognition of changes in patients’ conditions and the potential to reduce alarm fatigue among hospital staff. Alarm fatigue is desensitization resulting from overexposure to alarm signals that are either invalid or clinically irrelevant. Alarm fatigue has resulted in missed patient events and preventable deaths.3 In this issue of the Journal of Hospital Medicine, Palchaudhuri et al.4 report findings from their observational study of telemetry monitor alarms intercepted by monitor watchers as a mechanism for reducing both nurses’ exposure to alarm signals and subsequent alarm fatigue.
To our knowledge, the study by Palchaudhuri et al.4 is the first to report the effect of monitor watchers on nurses’ exposure to alarm signals. In this study, over a 2-month period monitor watchers intercepted 87% of alarms before they were sent to the nurse’s telephone. Monitor watchers intercepted over 90% of bradycardia and tachycardia alarms, indicating that they believed these alarms to be clinically irrelevant. Monitor watchers also intercepted about 75% of alarms for lethal arrhythmias, indicating that they believed these alarms to be invalid.
In this study, decisions about alarm validity and relevance were made through close communication between monitor watchers and nursing staff. If an alarm was sounding and the monitor watcher had already spoken with the nurse about it and established that the nurse was addressing the problem, the monitor watcher would intercept subsequent alarms for that issue or event (according to personal communication with S. Palchaudhuri). The results of the study not only indicate that monitor watchers can reduce the number of alarms to which a nurse is exposed, but also support previous findings that few alarms are valid or clinically relevant.5-7 The results of this study also suggest that “nuisance” alarms should include not only clinically irrelevant alarms, but also relevant alarms for which the nurse is actively seeking a solution. Monitor watchers may have an important role in addressing these alarms.
The study raises important considerations regarding monitor watcher practice and alarm fatigue. If monitor watchers are to be effective in reducing nurses’ exposure to alarms, they must use good judgment to determine when to intercept an alarm, call the nurse, or both. In the absence of proper judgment, monitor watchers may inadvertently increase nurses’ fatigue through redundant calls or inappropriately suppress valid relevant alarms. In free-text responses to our national monitor watcher survey, nurses expressed frustration over redundant calls from monitor watchers for invalid and irrelevant alarms.2 Research suggests that monitor watchers may not identify potentially dangerous alarms with complete accuracy. In a recent study reported in The Journal of the American Medical Society (JAMA), monitor watchers missed about 18% of patients with detectable rhythm or rate changes on telemetry in the hour before an emergency response team was activated.8
Several factors and conditions may affect monitor watchers’ judgment: 1) education and training, 2) location and access to contextual patient information, and 3) fatigue. First, across the US, the level of education required for monitor watcher positions ranges from a high school diploma to licensure as a registered nurse. The content and frequency of in-service training required also varies.2 These differing requirements may influence monitor watchers’ ability to interpret alarms.
Second, most monitor watchers are located off the patient care unit,2 which influences their access to information. Even in remote locations, monitor watchers can assess alarm validity by reviewing parameter waveforms for artifact. However, determining the relevance of an alarm to a particular patient is a more complex task requiring contextual information about the patient.9 Monitor watchers must work closely with clinicians at the bedside to determine the relevance of alarms, and repeated contact between monitor watchers and nurses over alarm conditions may itself increase nurses’ alarm fatigue.
Finally, fatigue may affect monitor watchers themselves and reduce their effectiveness. This issue was raised by Palchaudhuri et al. Both the number of monitors watched and the length of the monitor watcher’s shift likely influence alertness and effectiveness. In a simulation study, Segall et al.10 found that monitor watchers’ recognition of serious arrhythmias was significantly delayed when they were responsible for more than 40 patient monitors. Monitor watchers often work 12-hour shifts,2 and although no research has been reported on their shift-related alertness, this is a long time to remain attentive.
Given these potential challenges, future research should specifically address adverse patient outcomes and missed clinically relevant alarms. Only two of the seven patients who arrested during the study by Palchaudhuri et al.4 were on telemetry, and neither arrested due to lethal arrhythmias. While this is an important indication that no alarms for lethal arrhythmias were inadvertently suppressed, it is difficult to achieve adequate statistical power to assess rare outcomes like cardiac arrests. In a future study, alarms intercepted by monitor watchers could be assessed for accuracy and relevance to patient care to determine whether important alarms were inadvertently suppressed.
In summary, the study by Palchaudhuri et al.4 represents a preliminary step in considering the potential utility of monitor watchers for reducing invalid and clinically irrelevant alarms as well as subsequent alarm fatigue. As the authors note, dedicated monitor watchers can screen alarms much more quickly than nurses who may be engaged in other activities when an alarm signals. The study raises interesting questions about how monitor watchers should be incorporated into workflow. Should their only responsibility be to call regarding potentially critical events, or should they be able to prevent alarms from reaching the nurse? Could monitor watchers provide guidance to reduce alarm fatigue, such as suggesting parameter changes when they see trends in irrelevant alarms? Future research is warranted to understand how monitor watchers can be used most effectively to reduce alarm fatigue, and which characteristics of monitor watchers and their practice result in the best patient outcomes.
Disclosure
Nothing to report.
1. Funk M, Parkosewich JA, Johnson CR, Stukshis I. Effect of dedicated monitor watchers on patients’ outcomes. Am J Crit Care. 1997;6(4):318-323. PubMed
2. Funk M, Ruppel H, Blake N, Phillips J. Research: Use of monitor watchers in hospitals: characteristics, training, and practices. Biomed Instrum Technol. 2016;50(6):428-438. PubMed
3. Joint Commission. Medical device alarm safety in hospitals. Sentinel Event Alert. 2013;(50):1-3. PubMed
4. Palchaudhuri S, Chen S, Clayton E, Accurso A, Zakaria S. Telemetry monitor watchers reduce bedside nurses’ exposure to alarms by intercepting a high number of nonactionable alarms. J Hosp Med. 2017;12(6):447-449. PubMed
5. Bonafide CP, Lin R, Zander M, et al. Association between exposure to nonactionable physiologic monitor alarms and response time in a children’s hospital. J Hosp Med. 2015;10(6):345-351. PubMed
6. Drew BJ, Harris P, Zègre-Hemsey JK, et al. Insights into the problem of alarm fatigue with physiologic monitor devices: a comprehensive observational study of consecutive intensive care unit patients. PLoS One. 2014;9(10):e110274. PubMed
7. Siebig S, Kuhls S, Imhoff M, Gather U, Schölmerich J, Wrede CE. Intensive care unit alarms—how many do we need? Crit Care Med. 2010;38(2):451-456. PubMed
8. Cantillon DJ, Loy M, Burkle A, et al. Association between off-site central monitoring using standardized cardiac telemetry and clinical outcomes among non-critically ill patients. JAMA. 2016;316(5):519-524. PubMed
9. Rayo MF, Moffatt-Bruce SD. Alarm system management: evidence-based guidance encouraging direct measurement of informativeness to improve alarm response. BMJ Qual Saf. 2015;24(4):282-286. PubMed
10. Segall N, Hobbs G, Granger CB, et al. Patient load effects on response time to critical arrhythmias in cardiac telemetry: a randomized trial. Crit Care Med. 2015;43(5):1036-1042. PubMed
1. Funk M, Parkosewich JA, Johnson CR, Stukshis I. Effect of dedicated monitor watchers on patients’ outcomes. Am J Crit Care. 1997;6(4):318-323. PubMed
2. Funk M, Ruppel H, Blake N, Phillips J. Research: Use of monitor watchers in hospitals: characteristics, training, and practices. Biomed Instrum Technol. 2016;50(6):428-438. PubMed
3. Joint Commission. Medical device alarm safety in hospitals. Sentinel Event Alert. 2013;(50):1-3. PubMed
4. Palchaudhuri S, Chen S, Clayton E, Accurso A, Zakaria S. Telemetry monitor watchers reduce bedside nurses’ exposure to alarms by intercepting a high number of nonactionable alarms. J Hosp Med. 2017;12(6):447-449. PubMed
5. Bonafide CP, Lin R, Zander M, et al. Association between exposure to nonactionable physiologic monitor alarms and response time in a children’s hospital. J Hosp Med. 2015;10(6):345-351. PubMed
6. Drew BJ, Harris P, Zègre-Hemsey JK, et al. Insights into the problem of alarm fatigue with physiologic monitor devices: a comprehensive observational study of consecutive intensive care unit patients. PLoS One. 2014;9(10):e110274. PubMed
7. Siebig S, Kuhls S, Imhoff M, Gather U, Schölmerich J, Wrede CE. Intensive care unit alarms—how many do we need? Crit Care Med. 2010;38(2):451-456. PubMed
8. Cantillon DJ, Loy M, Burkle A, et al. Association between off-site central monitoring using standardized cardiac telemetry and clinical outcomes among non-critically ill patients. JAMA. 2016;316(5):519-524. PubMed
9. Rayo MF, Moffatt-Bruce SD. Alarm system management: evidence-based guidance encouraging direct measurement of informativeness to improve alarm response. BMJ Qual Saf. 2015;24(4):282-286. PubMed
10. Segall N, Hobbs G, Granger CB, et al. Patient load effects on response time to critical arrhythmias in cardiac telemetry: a randomized trial. Crit Care Med. 2015;43(5):1036-1042. PubMed
© 2017 Society of Hospital Medicine