User login
A Concise Tool for Measuring Care Coordination from the Provider’s Perspective in the Hospital Setting
Care Coordination has been defined as “…the deliberate organization of patient care activities between two or more participants (including the patient) involved in a patient’s care to facilitate the appropriate delivery of healthcare services.”1 The Institute of Medicine identified care coordination as a key strategy to improve the American healthcare system,2 and evidence has been building that well-coordinated care improves patient outcomes and reduces healthcare costs associated with chronic conditions.3-5 In 2012, Johns Hopkins Medicine was awarded a Healthcare Innovation Award by the Centers for Medicare & Medicaid Services to improve coordination of care across the continuum of care for adult patients admitted to Johns Hopkins Hospital (JHH) and Johns Hopkins Bayview Medical Center (JHBMC), and for high-risk low-income Medicare and Medicaid beneficiaries receiving ambulatory care in targeted zip codes. The purpose of this project, known as the Johns Hopkins Community Health Partnership (J-CHiP), was to improve health and healthcare and to reduce healthcare costs. The acute care component of the program consisted of a bundle of interventions focused on improving coordination of care for all patients, including a “bridge to home” discharge process, as they transitioned back to the community from inpatient admission. The bundle included the following: early screening for discharge planning to predict needed postdischarge services; discussion in daily multidisciplinary rounds about goals and priorities of the hospitalization and potential postdischarge needs; patient and family self-care management; education enhanced medication management, including the option of “medications in hand” at the time of discharge; postdischarge telephone follow-up by nurses; and, for patients identified as high-risk, a “transition guide” (a nurse who works with the patient via home visits and by phone to optimize compliance with care for 30 days postdischarge).6 While the primary endpoints of the J-CHiP program were to improve clinical outcomes and reduce healthcare costs, we were also interested in the impact of the program on care coordination processes in the acute care setting. This created the need for an instrument to measure healthcare professionals’ views of care coordination in their immediate work environments.
We began our search for existing measures by reviewing the Coordination Measures Atlas published in 2014.7 Although this report evaluates over 80 different measures of care coordination, most of them focus on the perspective of the patient and/or family members, on specific conditions, and on primary care or outpatient settings.7,8 We were unable to identify an existing measure from the provider perspective, designed for the inpatient setting, that was both brief but comprehensive enough to cover a range of care coordination domains.8
Consequently, our first aim was to develop a brief, comprehensive tool to measure care coordination from the perspective of hospital inpatient staff that could be used to compare different units or types of providers, or to conduct longitudinal assessment. The second aim was to conduct a preliminary evaluation of the tool in our healthcare setting, including to assess its psychometric properties, to describe provider perceptions of care coordination after the implementation of J-CHiP, and to explore potential differences among departments, types of professionals, and between the 2 hospitals.
METHODS
Development of the Care Coordination Questionnaire
The survey was developed in collaboration with leaders of the J-CHiP Acute Care Team. We met at the outset and on multiple subsequent occasions to align survey domains with the main components of the J-CHiP acute care intervention and to assure that the survey would be relevant and understandable to a variety of multidisciplinary professionals, including physicians, nurses, social workers, physical therapists, and other health professionals. Care was taken to avoid redundancy with existing evaluation efforts and to minimize respondent burden. This process helped to ensure the content validity of the items, the usefulness of the results, and the future usability of the tool.
We modeled the Care Coordination Questionnaire (CCQ) after the Safety Attitudes Questionnaire (SAQ),9 a widely used survey that is deployed approximately annually at JHH and JHBMC. While the SAQ focuses on healthcare provider attitudes about issues relevant to patient safety (often referred to as safety climate or safety culture), this new tool was designed to focus on healthcare professionals’ attitudes about care coordination. Similar to the way that the SAQ “elicits a snapshot of the safety climate through surveys of frontline worker perceptions,” we sought to elicit a picture of our care coordination climate through a survey of frontline hospital staff.
The CCQ was built upon the domains and approaches to care coordination described in the Agency for Healthcare Research and Quality Care Coordination Atlas.3 This report identifies 9 mechanisms for achieving care coordination, including the following: Establish Accountability or Negotiate Responsibility; Communicate; Facilitate Transitions; Assess Needs and Goals; Create a Proactive Plan of Care; Monitor, Follow Up, and Respond to Change; Support Self-Management Goals; Link to Community Resources; and Align Resources with Patient and Population Needs; as well as 5 broad approaches commonly used to improve the delivery of healthcare, including Teamwork Focused on Coordination, Healthcare Home, Care Management, Medication Management, and Health IT-Enabled Coordination.7 We generated at least 1 item to represent 8 of the 9 domains, as well as the broad approach described as Teamwork Focused on Coordination. After developing an initial set of items, we sought input from 3 senior leaders of the J-CHiP Acute Care Team to determine if the items covered the care coordination domains of interest, and to provide feedback on content validity. To test the interpretability of survey items and consistency across professional groups, we sent an initial version of the survey questions to at least 1 person from each of the following professional groups: hospitalist, social worker, case manager, clinical pharmacist, and nurse. We asked them to review all of our survey questions and to provide us with feedback on all aspects of the questions, such as whether they believed the questions were relevant and understandable to the members of their professional discipline, the appropriateness of the wording of the questions, and other comments. Modifications were made to the content and wording of the questions based on the feedback received. The final draft of the questionnaire was reviewed by the leadership team of the J-CHiP Acute Care Team to ensure its usefulness in providing actionable information.
The resulting 12-item questionnaire used a 5-point Likert response scale ranging from 1 = “disagree strongly” to 5 = “agree strongly,” and an additional option of “not applicable (N/A).” To help assess construct validity, a global question was added at the end of the questionnaire asking, “Overall, how would you rate the care coordination at the hospital of your primary work setting?” The response was measured on a 10-point Likert-type scale ranging from 1 = “totally uncoordinated care” to 10 = “perfectly coordinated care” (see Appendix). In addition, the questionnaire requested information about the respondents’ gender, position, and their primary unit, department, and hospital affiliation.
Data Collection Procedures
An invitation to complete an anonymous questionnaire was sent to the following inpatient care professionals: all nursing staff working on care coordination units in the departments of medicine, surgery, and neurology/neurosurgery, as well as physicians, pharmacists, acute care therapists (eg, occupational and physical therapists), and other frontline staff. All healthcare staff fitting these criteria was sent an e-mail with a request to fill out the survey online using QualtricsTM (Qualtrics Labs Inc., Provo, UT), as well as multiple follow-up reminders. The participants worked either at the JHH (a 1194-bed tertiary academic medical center in Baltimore, MD) or the JHBMC (a 440-bed academic community hospital located nearby). Data were collected from October 2015 through January 2016.
Analysis
Means and standard deviations were calculated by treating the responses as continuous variables. We tried 3 different methods to handle missing data: (1) without imputation, (2) imputing the mean value of each item, and (3) substituting a neutral score. Because all 3 methods produced very similar results, we treated the N/A responses as missing values without imputation for simplicity of analysis. We used STATA 13.1 (Stata Corporation, College Station, Texas) to analyze the data.
To identify subscales, we performed exploratory factor analysis on responses to the 12 specific items. Promax rotation was selected based on the simple structure. Subscale scores for each respondent were generated by computing the mean of responses to the items in the subscale. Internal consistency reliability of the subscales was estimated using Cronbach’s alpha. We calculated Pearson correlation coefficients for the items in each subscale, and examined Cronbach’s alpha deleting each item in turn. For each of the subscales identified and the global scale, we calculated the mean, standard deviation, median and interquartile range. Although distributions of scores tended to be non-normal, this was done to increase interpretability. We also calculated percent scoring at the ceiling (highest possible score).
We analyzed the data with 3 research questions in mind: Was there a difference in perceptions of care coordination between (1) staff affiliated with the 2 different hospitals, (2) staff affiliated with different clinical departments, or (3) staff with different professional roles? For comparisons based on hospital and department, and type of professional, nonparametric tests (Wilcoxon rank-sum and Kruskal-Wallis test) were used with a level of statistical significance set at 0.05. The comparison between hospitals and departments was made only among nurses to minimize the confounding effect of different distribution of professionals. We tested the distribution of “years in specialty” between hospitals and departments for this comparison using Pearson’s χ2 test. The difference was not statistically significant (P = 0.167 for hospitals, and P = 0.518 for departments), so we assumed that the potential confounding effect of this variable was negligible in this analysis. The comparison of scores within each professional group used the Friedman test. Pearson’s χ2 test was used to compare the baseline characteristics between 2 hospitals.
RESULTS
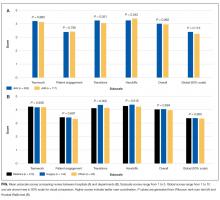
Among the 1486 acute care professionals asked to participate in the survey, 841 completed the questionnaire (response rate 56.6%). Table 1 shows the characteristics of the participants from each hospital. Table 2 summarizes the item response rates, proportion scoring at the ceiling, and weighting from the factor analysis. All items had completion rates of 99.2% or higher, with N/A responses ranging from 0% (item 2) to 3.1% (item 7). The percent scoring at the ceiling was 1.7% for the global item and ranged from 18.3% up to 63.3% for other individual items.
We also examined differences in perceptions of care coordination among nursing units to illustrate the tool’s ability to detect variation in Patient Engagement subscale scores for JHH nurses (see Appendix).
DISCUSSION
This study resulted in one of the first measurement tools to succinctly measure multiple aspects of care coordination in the hospital from the perspective of healthcare professionals. Given the hectic work environment of healthcare professionals, and the increasing emphasis on collecting data for evaluation and improvement, it is important to minimize respondent burden. This effort was catalyzed by a multifaceted initiative to redesign acute care delivery and promote seamless transitions of care, supported by the Center for Medicare & Medicaid Innovation. In initial testing, this questionnaire has evidence for reliability and validity. It was encouraging to find that the preliminary psychometric performance of the measure was very similar in 2 different settings of a tertiary academic hospital and a community hospital.
Our analysis of the survey data explored potential differences between the 2 hospitals, among different types of healthcare professionals and across different departments. Although we expected differences, we had no specific hypotheses about what those differences might be, and, in fact, did not observe any substantial differences. This could be taken to indicate that the intervention was uniformly and successfully implemented in both hospitals, and engaged various professionals in different departments. The ability to detect differences in care coordination at the nursing unit level could also prove to be beneficial for more precisely targeting where process improvement is needed. Further data collection and analyses should be conducted to more systematically compare units and to help identify those where practice is most advanced and those where improvements may be needed. It would also be informative to link differences in care coordination scores with patient outcomes. In addition, differences identified on specific domains between professional groups could be helpful to identify where greater efforts are needed to improve interdisciplinary practice. Sampling strategies stratified by provider type would need to be targeted to make this kind of analysis informative.
The consistently lower scores observed for patient engagement, from the perspective of care professionals in all groups, suggest that this is an area where improvement is needed. These findings are consistent with published reports on the common failure by hospitals to include patients as a member of their own care team. In addition to measuring care processes from the perspective of frontline healthcare workers, future evaluations within the healthcare system would also benefit from including data collected from the perspective of the patient and family.
This study had some limitations. First, there may be more than 4 domains of care coordination that are important and can be measured in the acute care setting from provider perspective. However, the addition of more domains should be balanced against practicality and respondent burden. It may be possible to further clarify priority domains in hospital settings as opposed to the primary care setting. Future research should be directed to find these areas and to develop a more comprehensive, yet still concise measurement instrument. Second, the tool was developed to measure the impact of a large-scale intervention, and to fit into the specific context of 2 hospitals. Therefore, it should be tested in different settings of hospital care to see how it performs. However, virtually all hospitals in the United States today are adapting to changes in both financing and healthcare delivery. A tool such as the one described in this paper could be helpful to many organizations. Third, the scoring system for the overall scale score is not weighted and therefore reflects teamwork more than other components of care coordination, which are represented by fewer items. In general, we believe that use of the subscale scores may be more informative. Alternative scoring systems might also be proposed, including item weighting based on factor scores.
For the purposes of evaluation in this specific instance, we only collected data at a single point in time, after the intervention had been deployed. Thus, we were not able to evaluate the effectiveness of the J-CHiP intervention. We also did not intend to focus too much on the differences between units, given the limited number of respondents from individual units. It would be useful to collect more data at future time points, both to test the responsiveness of the scales and to evaluate the impact of future interventions at both the hospital and unit level.
The preliminary data from this study have generated insights about gaps in current practice, such as in engaging patients in the inpatient care process. It has also increased awareness by hospital leaders about the need to achieve high reliability in the adoption of new procedures and interdisciplinary practice. This tool might be used to find areas in need of improvement, to evaluate the effect of initiatives to improve care coordination, to monitor the change over time in the perception of care coordination among healthcare professionals, and to develop better intervention strategies for coordination activities in acute care settings. Additional research is needed to provide further evidence for the reliability and validity of this measure in diverse settings.
Disclosure
The project described was supported by Grant Number 1C1CMS331053-01-00 from the US Department of Health and Human Services, Centers for Medicare & Medicaid Services. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the US Department of Health and Human Services or any of its agencies. The research presented was conducted by the awardee. Results may or may not be consistent with or confirmed by the findings of the independent evaluation contractor.
The authors have no other disclosures.
1. McDonald KM, Sundaram V, Bravata DM, et al. Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies (Vol. 7: Care Coordination). Technical Reviews, No. 9.7. Rockville (MD): Agency for Healthcare Research and Quality (US); 2007. PubMed
2. Adams K, Corrigan J. Priority areas for national action: transforming health care quality. Washington, DC: National Academies Press; 2003. PubMed
3. Renders CM, Valk GD, Griffin S, Wagner EH, Eijk JT, Assendelft WJ. Interventions to improve the management of diabetes mellitus in primary care, outpatient and community settings. Cochrane Database Syst Rev. 2001(1):CD001481. PubMed
4. McAlister FA, Lawson FM, Teo KK, Armstrong PW. A systematic review of randomized trials of disease management programs in heart failure. Am J Med. 2001;110(5):378-384. PubMed
5. Bruce ML, Raue PJ, Reilly CF, et al. Clinical effectiveness of integrating depression care management into medicare home health: the Depression CAREPATH Randomized trial. JAMA Intern Med. 2015;175(1):55-64. PubMed
6. Berkowitz SA, Brown P, Brotman DJ, et al. Case Study: Johns Hopkins Community Health Partnership: A model for transformation. Healthc (Amst). 2016;4(4):264-270. PubMed
7. McDonald. KM, Schultz. E, Albin. L, et al. Care Coordination Measures Atlas Version 4. Rockville, MD: Agency for Healthcare Research and Quality; 2014.
8 Schultz EM, Pineda N, Lonhart J, Davies SM, McDonald KM. A systematic review of the care coordination measurement landscape. BMC Health Serv Res. 2013;13:119. PubMed
9. Sexton JB, Helmreich RL, Neilands TB, et al. The Safety Attitudes Questionnaire: psychometric properties, benchmarking data, and emerging research. BMC Health Serv Res. 2006;6:44. PubMed
Care Coordination has been defined as “…the deliberate organization of patient care activities between two or more participants (including the patient) involved in a patient’s care to facilitate the appropriate delivery of healthcare services.”1 The Institute of Medicine identified care coordination as a key strategy to improve the American healthcare system,2 and evidence has been building that well-coordinated care improves patient outcomes and reduces healthcare costs associated with chronic conditions.3-5 In 2012, Johns Hopkins Medicine was awarded a Healthcare Innovation Award by the Centers for Medicare & Medicaid Services to improve coordination of care across the continuum of care for adult patients admitted to Johns Hopkins Hospital (JHH) and Johns Hopkins Bayview Medical Center (JHBMC), and for high-risk low-income Medicare and Medicaid beneficiaries receiving ambulatory care in targeted zip codes. The purpose of this project, known as the Johns Hopkins Community Health Partnership (J-CHiP), was to improve health and healthcare and to reduce healthcare costs. The acute care component of the program consisted of a bundle of interventions focused on improving coordination of care for all patients, including a “bridge to home” discharge process, as they transitioned back to the community from inpatient admission. The bundle included the following: early screening for discharge planning to predict needed postdischarge services; discussion in daily multidisciplinary rounds about goals and priorities of the hospitalization and potential postdischarge needs; patient and family self-care management; education enhanced medication management, including the option of “medications in hand” at the time of discharge; postdischarge telephone follow-up by nurses; and, for patients identified as high-risk, a “transition guide” (a nurse who works with the patient via home visits and by phone to optimize compliance with care for 30 days postdischarge).6 While the primary endpoints of the J-CHiP program were to improve clinical outcomes and reduce healthcare costs, we were also interested in the impact of the program on care coordination processes in the acute care setting. This created the need for an instrument to measure healthcare professionals’ views of care coordination in their immediate work environments.
We began our search for existing measures by reviewing the Coordination Measures Atlas published in 2014.7 Although this report evaluates over 80 different measures of care coordination, most of them focus on the perspective of the patient and/or family members, on specific conditions, and on primary care or outpatient settings.7,8 We were unable to identify an existing measure from the provider perspective, designed for the inpatient setting, that was both brief but comprehensive enough to cover a range of care coordination domains.8
Consequently, our first aim was to develop a brief, comprehensive tool to measure care coordination from the perspective of hospital inpatient staff that could be used to compare different units or types of providers, or to conduct longitudinal assessment. The second aim was to conduct a preliminary evaluation of the tool in our healthcare setting, including to assess its psychometric properties, to describe provider perceptions of care coordination after the implementation of J-CHiP, and to explore potential differences among departments, types of professionals, and between the 2 hospitals.
METHODS
Development of the Care Coordination Questionnaire
The survey was developed in collaboration with leaders of the J-CHiP Acute Care Team. We met at the outset and on multiple subsequent occasions to align survey domains with the main components of the J-CHiP acute care intervention and to assure that the survey would be relevant and understandable to a variety of multidisciplinary professionals, including physicians, nurses, social workers, physical therapists, and other health professionals. Care was taken to avoid redundancy with existing evaluation efforts and to minimize respondent burden. This process helped to ensure the content validity of the items, the usefulness of the results, and the future usability of the tool.
We modeled the Care Coordination Questionnaire (CCQ) after the Safety Attitudes Questionnaire (SAQ),9 a widely used survey that is deployed approximately annually at JHH and JHBMC. While the SAQ focuses on healthcare provider attitudes about issues relevant to patient safety (often referred to as safety climate or safety culture), this new tool was designed to focus on healthcare professionals’ attitudes about care coordination. Similar to the way that the SAQ “elicits a snapshot of the safety climate through surveys of frontline worker perceptions,” we sought to elicit a picture of our care coordination climate through a survey of frontline hospital staff.
The CCQ was built upon the domains and approaches to care coordination described in the Agency for Healthcare Research and Quality Care Coordination Atlas.3 This report identifies 9 mechanisms for achieving care coordination, including the following: Establish Accountability or Negotiate Responsibility; Communicate; Facilitate Transitions; Assess Needs and Goals; Create a Proactive Plan of Care; Monitor, Follow Up, and Respond to Change; Support Self-Management Goals; Link to Community Resources; and Align Resources with Patient and Population Needs; as well as 5 broad approaches commonly used to improve the delivery of healthcare, including Teamwork Focused on Coordination, Healthcare Home, Care Management, Medication Management, and Health IT-Enabled Coordination.7 We generated at least 1 item to represent 8 of the 9 domains, as well as the broad approach described as Teamwork Focused on Coordination. After developing an initial set of items, we sought input from 3 senior leaders of the J-CHiP Acute Care Team to determine if the items covered the care coordination domains of interest, and to provide feedback on content validity. To test the interpretability of survey items and consistency across professional groups, we sent an initial version of the survey questions to at least 1 person from each of the following professional groups: hospitalist, social worker, case manager, clinical pharmacist, and nurse. We asked them to review all of our survey questions and to provide us with feedback on all aspects of the questions, such as whether they believed the questions were relevant and understandable to the members of their professional discipline, the appropriateness of the wording of the questions, and other comments. Modifications were made to the content and wording of the questions based on the feedback received. The final draft of the questionnaire was reviewed by the leadership team of the J-CHiP Acute Care Team to ensure its usefulness in providing actionable information.
The resulting 12-item questionnaire used a 5-point Likert response scale ranging from 1 = “disagree strongly” to 5 = “agree strongly,” and an additional option of “not applicable (N/A).” To help assess construct validity, a global question was added at the end of the questionnaire asking, “Overall, how would you rate the care coordination at the hospital of your primary work setting?” The response was measured on a 10-point Likert-type scale ranging from 1 = “totally uncoordinated care” to 10 = “perfectly coordinated care” (see Appendix). In addition, the questionnaire requested information about the respondents’ gender, position, and their primary unit, department, and hospital affiliation.
Data Collection Procedures
An invitation to complete an anonymous questionnaire was sent to the following inpatient care professionals: all nursing staff working on care coordination units in the departments of medicine, surgery, and neurology/neurosurgery, as well as physicians, pharmacists, acute care therapists (eg, occupational and physical therapists), and other frontline staff. All healthcare staff fitting these criteria was sent an e-mail with a request to fill out the survey online using QualtricsTM (Qualtrics Labs Inc., Provo, UT), as well as multiple follow-up reminders. The participants worked either at the JHH (a 1194-bed tertiary academic medical center in Baltimore, MD) or the JHBMC (a 440-bed academic community hospital located nearby). Data were collected from October 2015 through January 2016.
Analysis
Means and standard deviations were calculated by treating the responses as continuous variables. We tried 3 different methods to handle missing data: (1) without imputation, (2) imputing the mean value of each item, and (3) substituting a neutral score. Because all 3 methods produced very similar results, we treated the N/A responses as missing values without imputation for simplicity of analysis. We used STATA 13.1 (Stata Corporation, College Station, Texas) to analyze the data.
To identify subscales, we performed exploratory factor analysis on responses to the 12 specific items. Promax rotation was selected based on the simple structure. Subscale scores for each respondent were generated by computing the mean of responses to the items in the subscale. Internal consistency reliability of the subscales was estimated using Cronbach’s alpha. We calculated Pearson correlation coefficients for the items in each subscale, and examined Cronbach’s alpha deleting each item in turn. For each of the subscales identified and the global scale, we calculated the mean, standard deviation, median and interquartile range. Although distributions of scores tended to be non-normal, this was done to increase interpretability. We also calculated percent scoring at the ceiling (highest possible score).
We analyzed the data with 3 research questions in mind: Was there a difference in perceptions of care coordination between (1) staff affiliated with the 2 different hospitals, (2) staff affiliated with different clinical departments, or (3) staff with different professional roles? For comparisons based on hospital and department, and type of professional, nonparametric tests (Wilcoxon rank-sum and Kruskal-Wallis test) were used with a level of statistical significance set at 0.05. The comparison between hospitals and departments was made only among nurses to minimize the confounding effect of different distribution of professionals. We tested the distribution of “years in specialty” between hospitals and departments for this comparison using Pearson’s χ2 test. The difference was not statistically significant (P = 0.167 for hospitals, and P = 0.518 for departments), so we assumed that the potential confounding effect of this variable was negligible in this analysis. The comparison of scores within each professional group used the Friedman test. Pearson’s χ2 test was used to compare the baseline characteristics between 2 hospitals.
RESULTS
Among the 1486 acute care professionals asked to participate in the survey, 841 completed the questionnaire (response rate 56.6%). Table 1 shows the characteristics of the participants from each hospital. Table 2 summarizes the item response rates, proportion scoring at the ceiling, and weighting from the factor analysis. All items had completion rates of 99.2% or higher, with N/A responses ranging from 0% (item 2) to 3.1% (item 7). The percent scoring at the ceiling was 1.7% for the global item and ranged from 18.3% up to 63.3% for other individual items.
We also examined differences in perceptions of care coordination among nursing units to illustrate the tool’s ability to detect variation in Patient Engagement subscale scores for JHH nurses (see Appendix).
DISCUSSION
This study resulted in one of the first measurement tools to succinctly measure multiple aspects of care coordination in the hospital from the perspective of healthcare professionals. Given the hectic work environment of healthcare professionals, and the increasing emphasis on collecting data for evaluation and improvement, it is important to minimize respondent burden. This effort was catalyzed by a multifaceted initiative to redesign acute care delivery and promote seamless transitions of care, supported by the Center for Medicare & Medicaid Innovation. In initial testing, this questionnaire has evidence for reliability and validity. It was encouraging to find that the preliminary psychometric performance of the measure was very similar in 2 different settings of a tertiary academic hospital and a community hospital.
Our analysis of the survey data explored potential differences between the 2 hospitals, among different types of healthcare professionals and across different departments. Although we expected differences, we had no specific hypotheses about what those differences might be, and, in fact, did not observe any substantial differences. This could be taken to indicate that the intervention was uniformly and successfully implemented in both hospitals, and engaged various professionals in different departments. The ability to detect differences in care coordination at the nursing unit level could also prove to be beneficial for more precisely targeting where process improvement is needed. Further data collection and analyses should be conducted to more systematically compare units and to help identify those where practice is most advanced and those where improvements may be needed. It would also be informative to link differences in care coordination scores with patient outcomes. In addition, differences identified on specific domains between professional groups could be helpful to identify where greater efforts are needed to improve interdisciplinary practice. Sampling strategies stratified by provider type would need to be targeted to make this kind of analysis informative.
The consistently lower scores observed for patient engagement, from the perspective of care professionals in all groups, suggest that this is an area where improvement is needed. These findings are consistent with published reports on the common failure by hospitals to include patients as a member of their own care team. In addition to measuring care processes from the perspective of frontline healthcare workers, future evaluations within the healthcare system would also benefit from including data collected from the perspective of the patient and family.
This study had some limitations. First, there may be more than 4 domains of care coordination that are important and can be measured in the acute care setting from provider perspective. However, the addition of more domains should be balanced against practicality and respondent burden. It may be possible to further clarify priority domains in hospital settings as opposed to the primary care setting. Future research should be directed to find these areas and to develop a more comprehensive, yet still concise measurement instrument. Second, the tool was developed to measure the impact of a large-scale intervention, and to fit into the specific context of 2 hospitals. Therefore, it should be tested in different settings of hospital care to see how it performs. However, virtually all hospitals in the United States today are adapting to changes in both financing and healthcare delivery. A tool such as the one described in this paper could be helpful to many organizations. Third, the scoring system for the overall scale score is not weighted and therefore reflects teamwork more than other components of care coordination, which are represented by fewer items. In general, we believe that use of the subscale scores may be more informative. Alternative scoring systems might also be proposed, including item weighting based on factor scores.
For the purposes of evaluation in this specific instance, we only collected data at a single point in time, after the intervention had been deployed. Thus, we were not able to evaluate the effectiveness of the J-CHiP intervention. We also did not intend to focus too much on the differences between units, given the limited number of respondents from individual units. It would be useful to collect more data at future time points, both to test the responsiveness of the scales and to evaluate the impact of future interventions at both the hospital and unit level.
The preliminary data from this study have generated insights about gaps in current practice, such as in engaging patients in the inpatient care process. It has also increased awareness by hospital leaders about the need to achieve high reliability in the adoption of new procedures and interdisciplinary practice. This tool might be used to find areas in need of improvement, to evaluate the effect of initiatives to improve care coordination, to monitor the change over time in the perception of care coordination among healthcare professionals, and to develop better intervention strategies for coordination activities in acute care settings. Additional research is needed to provide further evidence for the reliability and validity of this measure in diverse settings.
Disclosure
The project described was supported by Grant Number 1C1CMS331053-01-00 from the US Department of Health and Human Services, Centers for Medicare & Medicaid Services. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the US Department of Health and Human Services or any of its agencies. The research presented was conducted by the awardee. Results may or may not be consistent with or confirmed by the findings of the independent evaluation contractor.
The authors have no other disclosures.
Care Coordination has been defined as “…the deliberate organization of patient care activities between two or more participants (including the patient) involved in a patient’s care to facilitate the appropriate delivery of healthcare services.”1 The Institute of Medicine identified care coordination as a key strategy to improve the American healthcare system,2 and evidence has been building that well-coordinated care improves patient outcomes and reduces healthcare costs associated with chronic conditions.3-5 In 2012, Johns Hopkins Medicine was awarded a Healthcare Innovation Award by the Centers for Medicare & Medicaid Services to improve coordination of care across the continuum of care for adult patients admitted to Johns Hopkins Hospital (JHH) and Johns Hopkins Bayview Medical Center (JHBMC), and for high-risk low-income Medicare and Medicaid beneficiaries receiving ambulatory care in targeted zip codes. The purpose of this project, known as the Johns Hopkins Community Health Partnership (J-CHiP), was to improve health and healthcare and to reduce healthcare costs. The acute care component of the program consisted of a bundle of interventions focused on improving coordination of care for all patients, including a “bridge to home” discharge process, as they transitioned back to the community from inpatient admission. The bundle included the following: early screening for discharge planning to predict needed postdischarge services; discussion in daily multidisciplinary rounds about goals and priorities of the hospitalization and potential postdischarge needs; patient and family self-care management; education enhanced medication management, including the option of “medications in hand” at the time of discharge; postdischarge telephone follow-up by nurses; and, for patients identified as high-risk, a “transition guide” (a nurse who works with the patient via home visits and by phone to optimize compliance with care for 30 days postdischarge).6 While the primary endpoints of the J-CHiP program were to improve clinical outcomes and reduce healthcare costs, we were also interested in the impact of the program on care coordination processes in the acute care setting. This created the need for an instrument to measure healthcare professionals’ views of care coordination in their immediate work environments.
We began our search for existing measures by reviewing the Coordination Measures Atlas published in 2014.7 Although this report evaluates over 80 different measures of care coordination, most of them focus on the perspective of the patient and/or family members, on specific conditions, and on primary care or outpatient settings.7,8 We were unable to identify an existing measure from the provider perspective, designed for the inpatient setting, that was both brief but comprehensive enough to cover a range of care coordination domains.8
Consequently, our first aim was to develop a brief, comprehensive tool to measure care coordination from the perspective of hospital inpatient staff that could be used to compare different units or types of providers, or to conduct longitudinal assessment. The second aim was to conduct a preliminary evaluation of the tool in our healthcare setting, including to assess its psychometric properties, to describe provider perceptions of care coordination after the implementation of J-CHiP, and to explore potential differences among departments, types of professionals, and between the 2 hospitals.
METHODS
Development of the Care Coordination Questionnaire
The survey was developed in collaboration with leaders of the J-CHiP Acute Care Team. We met at the outset and on multiple subsequent occasions to align survey domains with the main components of the J-CHiP acute care intervention and to assure that the survey would be relevant and understandable to a variety of multidisciplinary professionals, including physicians, nurses, social workers, physical therapists, and other health professionals. Care was taken to avoid redundancy with existing evaluation efforts and to minimize respondent burden. This process helped to ensure the content validity of the items, the usefulness of the results, and the future usability of the tool.
We modeled the Care Coordination Questionnaire (CCQ) after the Safety Attitudes Questionnaire (SAQ),9 a widely used survey that is deployed approximately annually at JHH and JHBMC. While the SAQ focuses on healthcare provider attitudes about issues relevant to patient safety (often referred to as safety climate or safety culture), this new tool was designed to focus on healthcare professionals’ attitudes about care coordination. Similar to the way that the SAQ “elicits a snapshot of the safety climate through surveys of frontline worker perceptions,” we sought to elicit a picture of our care coordination climate through a survey of frontline hospital staff.
The CCQ was built upon the domains and approaches to care coordination described in the Agency for Healthcare Research and Quality Care Coordination Atlas.3 This report identifies 9 mechanisms for achieving care coordination, including the following: Establish Accountability or Negotiate Responsibility; Communicate; Facilitate Transitions; Assess Needs and Goals; Create a Proactive Plan of Care; Monitor, Follow Up, and Respond to Change; Support Self-Management Goals; Link to Community Resources; and Align Resources with Patient and Population Needs; as well as 5 broad approaches commonly used to improve the delivery of healthcare, including Teamwork Focused on Coordination, Healthcare Home, Care Management, Medication Management, and Health IT-Enabled Coordination.7 We generated at least 1 item to represent 8 of the 9 domains, as well as the broad approach described as Teamwork Focused on Coordination. After developing an initial set of items, we sought input from 3 senior leaders of the J-CHiP Acute Care Team to determine if the items covered the care coordination domains of interest, and to provide feedback on content validity. To test the interpretability of survey items and consistency across professional groups, we sent an initial version of the survey questions to at least 1 person from each of the following professional groups: hospitalist, social worker, case manager, clinical pharmacist, and nurse. We asked them to review all of our survey questions and to provide us with feedback on all aspects of the questions, such as whether they believed the questions were relevant and understandable to the members of their professional discipline, the appropriateness of the wording of the questions, and other comments. Modifications were made to the content and wording of the questions based on the feedback received. The final draft of the questionnaire was reviewed by the leadership team of the J-CHiP Acute Care Team to ensure its usefulness in providing actionable information.
The resulting 12-item questionnaire used a 5-point Likert response scale ranging from 1 = “disagree strongly” to 5 = “agree strongly,” and an additional option of “not applicable (N/A).” To help assess construct validity, a global question was added at the end of the questionnaire asking, “Overall, how would you rate the care coordination at the hospital of your primary work setting?” The response was measured on a 10-point Likert-type scale ranging from 1 = “totally uncoordinated care” to 10 = “perfectly coordinated care” (see Appendix). In addition, the questionnaire requested information about the respondents’ gender, position, and their primary unit, department, and hospital affiliation.
Data Collection Procedures
An invitation to complete an anonymous questionnaire was sent to the following inpatient care professionals: all nursing staff working on care coordination units in the departments of medicine, surgery, and neurology/neurosurgery, as well as physicians, pharmacists, acute care therapists (eg, occupational and physical therapists), and other frontline staff. All healthcare staff fitting these criteria was sent an e-mail with a request to fill out the survey online using QualtricsTM (Qualtrics Labs Inc., Provo, UT), as well as multiple follow-up reminders. The participants worked either at the JHH (a 1194-bed tertiary academic medical center in Baltimore, MD) or the JHBMC (a 440-bed academic community hospital located nearby). Data were collected from October 2015 through January 2016.
Analysis
Means and standard deviations were calculated by treating the responses as continuous variables. We tried 3 different methods to handle missing data: (1) without imputation, (2) imputing the mean value of each item, and (3) substituting a neutral score. Because all 3 methods produced very similar results, we treated the N/A responses as missing values without imputation for simplicity of analysis. We used STATA 13.1 (Stata Corporation, College Station, Texas) to analyze the data.
To identify subscales, we performed exploratory factor analysis on responses to the 12 specific items. Promax rotation was selected based on the simple structure. Subscale scores for each respondent were generated by computing the mean of responses to the items in the subscale. Internal consistency reliability of the subscales was estimated using Cronbach’s alpha. We calculated Pearson correlation coefficients for the items in each subscale, and examined Cronbach’s alpha deleting each item in turn. For each of the subscales identified and the global scale, we calculated the mean, standard deviation, median and interquartile range. Although distributions of scores tended to be non-normal, this was done to increase interpretability. We also calculated percent scoring at the ceiling (highest possible score).
We analyzed the data with 3 research questions in mind: Was there a difference in perceptions of care coordination between (1) staff affiliated with the 2 different hospitals, (2) staff affiliated with different clinical departments, or (3) staff with different professional roles? For comparisons based on hospital and department, and type of professional, nonparametric tests (Wilcoxon rank-sum and Kruskal-Wallis test) were used with a level of statistical significance set at 0.05. The comparison between hospitals and departments was made only among nurses to minimize the confounding effect of different distribution of professionals. We tested the distribution of “years in specialty” between hospitals and departments for this comparison using Pearson’s χ2 test. The difference was not statistically significant (P = 0.167 for hospitals, and P = 0.518 for departments), so we assumed that the potential confounding effect of this variable was negligible in this analysis. The comparison of scores within each professional group used the Friedman test. Pearson’s χ2 test was used to compare the baseline characteristics between 2 hospitals.
RESULTS
Among the 1486 acute care professionals asked to participate in the survey, 841 completed the questionnaire (response rate 56.6%). Table 1 shows the characteristics of the participants from each hospital. Table 2 summarizes the item response rates, proportion scoring at the ceiling, and weighting from the factor analysis. All items had completion rates of 99.2% or higher, with N/A responses ranging from 0% (item 2) to 3.1% (item 7). The percent scoring at the ceiling was 1.7% for the global item and ranged from 18.3% up to 63.3% for other individual items.
We also examined differences in perceptions of care coordination among nursing units to illustrate the tool’s ability to detect variation in Patient Engagement subscale scores for JHH nurses (see Appendix).
DISCUSSION
This study resulted in one of the first measurement tools to succinctly measure multiple aspects of care coordination in the hospital from the perspective of healthcare professionals. Given the hectic work environment of healthcare professionals, and the increasing emphasis on collecting data for evaluation and improvement, it is important to minimize respondent burden. This effort was catalyzed by a multifaceted initiative to redesign acute care delivery and promote seamless transitions of care, supported by the Center for Medicare & Medicaid Innovation. In initial testing, this questionnaire has evidence for reliability and validity. It was encouraging to find that the preliminary psychometric performance of the measure was very similar in 2 different settings of a tertiary academic hospital and a community hospital.
Our analysis of the survey data explored potential differences between the 2 hospitals, among different types of healthcare professionals and across different departments. Although we expected differences, we had no specific hypotheses about what those differences might be, and, in fact, did not observe any substantial differences. This could be taken to indicate that the intervention was uniformly and successfully implemented in both hospitals, and engaged various professionals in different departments. The ability to detect differences in care coordination at the nursing unit level could also prove to be beneficial for more precisely targeting where process improvement is needed. Further data collection and analyses should be conducted to more systematically compare units and to help identify those where practice is most advanced and those where improvements may be needed. It would also be informative to link differences in care coordination scores with patient outcomes. In addition, differences identified on specific domains between professional groups could be helpful to identify where greater efforts are needed to improve interdisciplinary practice. Sampling strategies stratified by provider type would need to be targeted to make this kind of analysis informative.
The consistently lower scores observed for patient engagement, from the perspective of care professionals in all groups, suggest that this is an area where improvement is needed. These findings are consistent with published reports on the common failure by hospitals to include patients as a member of their own care team. In addition to measuring care processes from the perspective of frontline healthcare workers, future evaluations within the healthcare system would also benefit from including data collected from the perspective of the patient and family.
This study had some limitations. First, there may be more than 4 domains of care coordination that are important and can be measured in the acute care setting from provider perspective. However, the addition of more domains should be balanced against practicality and respondent burden. It may be possible to further clarify priority domains in hospital settings as opposed to the primary care setting. Future research should be directed to find these areas and to develop a more comprehensive, yet still concise measurement instrument. Second, the tool was developed to measure the impact of a large-scale intervention, and to fit into the specific context of 2 hospitals. Therefore, it should be tested in different settings of hospital care to see how it performs. However, virtually all hospitals in the United States today are adapting to changes in both financing and healthcare delivery. A tool such as the one described in this paper could be helpful to many organizations. Third, the scoring system for the overall scale score is not weighted and therefore reflects teamwork more than other components of care coordination, which are represented by fewer items. In general, we believe that use of the subscale scores may be more informative. Alternative scoring systems might also be proposed, including item weighting based on factor scores.
For the purposes of evaluation in this specific instance, we only collected data at a single point in time, after the intervention had been deployed. Thus, we were not able to evaluate the effectiveness of the J-CHiP intervention. We also did not intend to focus too much on the differences between units, given the limited number of respondents from individual units. It would be useful to collect more data at future time points, both to test the responsiveness of the scales and to evaluate the impact of future interventions at both the hospital and unit level.
The preliminary data from this study have generated insights about gaps in current practice, such as in engaging patients in the inpatient care process. It has also increased awareness by hospital leaders about the need to achieve high reliability in the adoption of new procedures and interdisciplinary practice. This tool might be used to find areas in need of improvement, to evaluate the effect of initiatives to improve care coordination, to monitor the change over time in the perception of care coordination among healthcare professionals, and to develop better intervention strategies for coordination activities in acute care settings. Additional research is needed to provide further evidence for the reliability and validity of this measure in diverse settings.
Disclosure
The project described was supported by Grant Number 1C1CMS331053-01-00 from the US Department of Health and Human Services, Centers for Medicare & Medicaid Services. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the US Department of Health and Human Services or any of its agencies. The research presented was conducted by the awardee. Results may or may not be consistent with or confirmed by the findings of the independent evaluation contractor.
The authors have no other disclosures.
1. McDonald KM, Sundaram V, Bravata DM, et al. Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies (Vol. 7: Care Coordination). Technical Reviews, No. 9.7. Rockville (MD): Agency for Healthcare Research and Quality (US); 2007. PubMed
2. Adams K, Corrigan J. Priority areas for national action: transforming health care quality. Washington, DC: National Academies Press; 2003. PubMed
3. Renders CM, Valk GD, Griffin S, Wagner EH, Eijk JT, Assendelft WJ. Interventions to improve the management of diabetes mellitus in primary care, outpatient and community settings. Cochrane Database Syst Rev. 2001(1):CD001481. PubMed
4. McAlister FA, Lawson FM, Teo KK, Armstrong PW. A systematic review of randomized trials of disease management programs in heart failure. Am J Med. 2001;110(5):378-384. PubMed
5. Bruce ML, Raue PJ, Reilly CF, et al. Clinical effectiveness of integrating depression care management into medicare home health: the Depression CAREPATH Randomized trial. JAMA Intern Med. 2015;175(1):55-64. PubMed
6. Berkowitz SA, Brown P, Brotman DJ, et al. Case Study: Johns Hopkins Community Health Partnership: A model for transformation. Healthc (Amst). 2016;4(4):264-270. PubMed
7. McDonald. KM, Schultz. E, Albin. L, et al. Care Coordination Measures Atlas Version 4. Rockville, MD: Agency for Healthcare Research and Quality; 2014.
8 Schultz EM, Pineda N, Lonhart J, Davies SM, McDonald KM. A systematic review of the care coordination measurement landscape. BMC Health Serv Res. 2013;13:119. PubMed
9. Sexton JB, Helmreich RL, Neilands TB, et al. The Safety Attitudes Questionnaire: psychometric properties, benchmarking data, and emerging research. BMC Health Serv Res. 2006;6:44. PubMed
1. McDonald KM, Sundaram V, Bravata DM, et al. Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies (Vol. 7: Care Coordination). Technical Reviews, No. 9.7. Rockville (MD): Agency for Healthcare Research and Quality (US); 2007. PubMed
2. Adams K, Corrigan J. Priority areas for national action: transforming health care quality. Washington, DC: National Academies Press; 2003. PubMed
3. Renders CM, Valk GD, Griffin S, Wagner EH, Eijk JT, Assendelft WJ. Interventions to improve the management of diabetes mellitus in primary care, outpatient and community settings. Cochrane Database Syst Rev. 2001(1):CD001481. PubMed
4. McAlister FA, Lawson FM, Teo KK, Armstrong PW. A systematic review of randomized trials of disease management programs in heart failure. Am J Med. 2001;110(5):378-384. PubMed
5. Bruce ML, Raue PJ, Reilly CF, et al. Clinical effectiveness of integrating depression care management into medicare home health: the Depression CAREPATH Randomized trial. JAMA Intern Med. 2015;175(1):55-64. PubMed
6. Berkowitz SA, Brown P, Brotman DJ, et al. Case Study: Johns Hopkins Community Health Partnership: A model for transformation. Healthc (Amst). 2016;4(4):264-270. PubMed
7. McDonald. KM, Schultz. E, Albin. L, et al. Care Coordination Measures Atlas Version 4. Rockville, MD: Agency for Healthcare Research and Quality; 2014.
8 Schultz EM, Pineda N, Lonhart J, Davies SM, McDonald KM. A systematic review of the care coordination measurement landscape. BMC Health Serv Res. 2013;13:119. PubMed
9. Sexton JB, Helmreich RL, Neilands TB, et al. The Safety Attitudes Questionnaire: psychometric properties, benchmarking data, and emerging research. BMC Health Serv Res. 2006;6:44. PubMed
© 2017 Society of Hospital Medicine
Associations of Physician Empathy with Patient Anxiety and Ratings of Communication in Hospital Admission Encounters
Admission to a hospital can be a stressful event,1,2 and patients report having many concerns at the time of hospital admission.3 Over the last 20 years, the United States has widely adopted the hospitalist model of inpatient care. Although this model has clear benefits, it also has the potential to contribute to patient stress, as hospitalized patients generally lack preexisting relationships with their inpatient physicians.4,5 In this changing hospital environment, defining and promoting effective medical communication has become an essential goal of both individual practitioners and medical centers.
Successful communication and strong therapeutic relationships with physicians support patients’ coping with illness-associated stress6,7 as well as promote adherence to medical treatment plans.8 Empathy serves as an important building block of patient-centered communication and encourages a strong therapeutic alliance.9 Studies from primary care, oncology, and intensive care unit (ICU) settings indicate that physician empathy is associated with decreased emotional distress,10,11 improved ratings of communication,12 and even better medical outcomes.13
Prior work has shown that hospitalists, like other clinicians, underutilize empathy as a tool in their daily interactions with patients.14-16 Our prior qualitative analysis of audio-recorded hospitalist-patient admission encounters indicated that how hospitalists respond to patient expressions of negative emotion influences relationships with patients and alignment around care plans.17 To determine whether empathic communication is associated with patient-reported outcomes in the hospitalist model, we quantitatively analyzed coded admission encounters and survey data to examine the association between hospitalists’ responses to patient expressions of negative emotion (anxiety, sadness, and anger) and patient anxiety and ratings of communication. Given the often-limited time hospitalists have to complete admission encounters, we also examined the association between response to emotion and encounter length.
METHODS
We analyzed data collected as part of an observational study of hospitalist-patient communication during hospital admission encounters14 to assess the association between the way physicians responded to patient expressions of negative emotion and patient anxiety, ratings of communication in the encounter, and encounter length. We collected data between August 2008 and March 2009 on the general medical service at 2 urban hospitals that are part of an academic medical center. Participants were attending hospitalists (not physician trainees), and patients admitted under participating hospitalists’ care who were able to communicate verbally in English and provide informed consent for the study. The institutional review board at the University of California, San Francisco approved the study; physician and patient participants provided written informed consent.
Enrollment and data collection has been described previously.17 Our cohort for this analysis included 76 patients of 27 physicians who completed encounter audio recordings and pre- and postencounter surveys. Following enrollment, patients completed a preencounter survey to collect demographic information and to measure their baseline anxiety via the State Anxiety Scale (STAI-S), which assesses transient anxious mood using 20 items answered on a 4-point scale for a final score range of 20 to 80.10,18,19 We timed and audio-recorded admission encounters. Encounter recordings were obtained solely from patient interactions with attending hospitalists and did not take into account the time patients may have spent with other physicians, including trainees. After the encounter, patients completed postencounter surveys, which included the STAI-S and patients’ ratings of communication during the encounter. To rate communication, patients responded to 7 items on a 0- to 10-point scale that were derived from previous work (Table 1)12,20,21; the anchors were “not at all” and “completely.” To identify patients with serious illness, which we used as a covariate in regression models, we asked physicians on a postencounter survey whether or not they “would be surprised by this patient’s death or admission to the ICU in the next year.”22
We considered physician as a clustering variable in the calculation of robust standard errors for all models. In addition, we included in each model covariates that were associated with the outcome at P ≤ 0.10, including patient gender, patient age, serious illness,22 preencounter anxiety, encounter length, and hospital. We considered P values < 0.05 to be statistically significant. We used Stata SE 13 (StataCorp LLC, College Station, TX) for all statistical analyses.
RESULTS
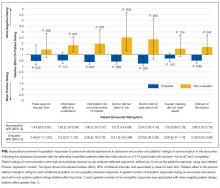
We analyzed data from admission encounters with 76 patients (consent rate 63%) and 27 hospitalists (consent rate 91%). Their characteristics are shown in Table 3. Median encounter length was 19 minutes (mean 21 minutes, range 3-68). Patients expressed negative emotion in 190 instances across all encounters; median number of expressions per encounter was 1 (range 0-14). Hospitalists responded empathically to 32% (n = 61) of the patient expressions, neutrally to 43% (n = 81), and nonempathically to 25% (n = 48).
The STAI-S was normally distributed. The mean preencounter STAI-S score was 39 (standard deviation [SD] 8.9). Mean postencounter STAI-S score was 38 (SD 10.7). Mean change in anxiety over the course of the encounter, calculated as the postencounter minus preencounter mean was −1.2 (SD 7.6). Table 1 shows summary statistics for the patient ratings of communication items. All items were rated highly. Across the items, between 51% and 78% of patients rated the highest score of 10.
Across the range of frequencies of emotional expressions per encounter in our data set (0-14 expressions), each additional empathic hospitalist response was associated with a 1.65-point decrease in the STAI-S (95% confidence interval [CI], 0.48-2.82). We did not find significant associations between changes in the STAI-S and the number of neutral hospitalist responses (−0.65 per response; 95% CI, −1.67-0.37) or nonempathic hospitalist responses (0.61 per response; 95% CI, −0.88-2.10).
In addition, nonempathic responses were associated with more negative ratings of communication for 5 of the 7 items: ease of understanding information, covering points of interest, the doctor listening, the doctor caring, and trusting the doctor. For example, for the item “I felt this doctor cared about me,” each nonempathic hospitalist response was associated with a more than doubling of negative patient ratings (aRE: 2.3; 95% CI, 1.32-4.16). Neutral physician responses to patient expressions of negative emotion were associated with less negative patient ratings for 2 of the items: covering points of interest (aRE 0.68; 95% CI, 0.51-0.90) and trusting the doctor (aRE: 0.86; 95% CI, 0.75-0.99).
We did not find a statistical association between encounter length and the number of empathic hospitalist responses in the encounter (percent change in encounter length per response [PC]: 1%; 95% CI, −8%-10%) or the number of nonempathic responses (PC: 18%; 95% CI, −2%-42%). We did find a statistically significant association between the number of neutral responses and encounter length (PC: 13%; 95% CI, 3%-24%), corresponding to 2.5 minutes of additional encounter time per neutral response for the median encounter length of 19 minutes.
DISCUSSION
Our study set out to measure how hospitalists responded to expressions of negative emotion during admission encounters with patients and how those responses correlated with patient anxiety, ratings of communication, and encounter length. We found that empathic responses were associated with diminishing patient anxiety after the visit, as well as with better ratings of several domains of hospitalist communication. Moreover, nonempathic responses to negative emotion were associated with more strongly negative ratings of hospitalist communication. Finally, while clinicians may worry that encouraging patients to speak further about emotion will result in excessive visit lengths, we did not find a statistical association between empathic responses and encounter duration. To our knowledge, this is the first study to indicate an association between empathy and patient anxiety and communication ratings within the hospitalist model, which is rapidly becoming the predominant model for providing inpatient care in the United States.4,5
As in oncologic care, anxiety is an emotion commonly confronted by clinicians meeting admitted medical patients for the first time. Studies show that not only do patient anxiety levels remain high throughout a hospital course, patients who experience higher levels of anxiety tend to stay longer in the hospital.1,2,27-30 But unlike oncologic care or other therapy provided in an outpatient setting, the hospitalist model does not facilitate “continuity” of care, or the ability to care for the same patients over a long period of time. This reality of inpatient care makes rapid, effective rapport-building critical to establishing strong physician-patient relationships. In this setting, a simple communication tool that is potentially able to reduce inpatients’ anxiety could have a meaningful impact on hospitalist-provided care and patient outcomes.
In terms of the magnitude of the effect of empathic responses, the clinical significance of a 1.65-point decrease in the STAI-S anxiety score is not precisely clear. A prior study that examined the effect of music therapy on anxiety levels in patients with cancer found an average anxiety reduction of approximately 9.5 units on the STAIS-S scale after sensitivity analysis, suggesting a rather large meaningful effect size.31 Given we found a reduction of 1.65 points for each empathic response, however, with a range of 0-14 negative emotions expressed over a median 19-minute encounter, there is opportunity for hospitalists to achieve a clinically significant decrease in patient anxiety during an admission encounter. The potential to reduce anxiety is extended further when we consider that the impact of an empathic response may apply not just to the admission encounter alone but also to numerous other patient-clinician interactions over the course of a hospitalization.
A healthy body of communication research supports the associations we found in our study between empathy and patient ratings of communication and physicians. Families in ICU conferences rate communication more positively when physicians express empathy,12 and a number of studies indicate an association between empathy and patient satisfaction in outpatient settings.8 Given the associations we found with negative ratings on the items in our study, promoting empathic responses to expressions of emotion and, more importantly, stressing avoidance of nonempathic responses may be relevant efforts in working to improve patient satisfaction scores on surveys reporting “top box” percentages, such as Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS). More notably, evidence indicates that empathy has positive impacts beyond satisfaction surveys, such as adherence, better diagnostic and clinical outcomes, and strengthening of patient enablement.8Not all hospitalist responses to emotion were associated with patient ratings across the 7 communication items we assessed. For example, we did not find an association between how physicians responded to patient expressions of negative emotion and patient perception that enough time was spent in the visit or the degree to which talking with the doctor met a patient’s overall needs. It follows logically, and other research supports, that empathy would influence patient ratings of physician caring and trust,32 whereas other communication factors we were unable to measure (eg, physician body language, tone, and use of jargon and patient health literacy and primary language) may have a more significant association with patient ratings of the other items we assessed.
In considering the clinical application of our results, it is important to note that communication skills, including responding empathically to patient expressions of negative emotion, can be imparted through training in the same way as abdominal examination or electrocardiogram interpretation skills.33-35 However, training of hospitalists in communication skills requires time and some financial investment on the part of the physician, their hospital or group, or, ideally, both. Effective training methods, like those for other skill acquisition, involve learner-centered teaching and practicing skills with role-play and feedback.36 Given the importance of a learner-centered approach, learning would likely be better received and more effective if it was tailored to the specific needs and patient scenarios commonly encountered by hospitalist physicians. As these programs are developed, it will be important to assess the impact of any training on the patient-reported outcomes we assessed in this observational study, along with clinical outcomes.
Our study has several limitations. First, we were only able to evaluate whether hospitalists verbally responded to patient emotion and were thus not able to account for nonverbal empathy such as facial expressions, body language, or voice tone. Second, given our patient consent rate of 63%, patients who agreed to participate in the study may have had different opinions than those who declined to participate. Also, hospitalists and patients may have behaved differently as a result of being audio recorded. We only included patients who spoke English, and our patient population was predominately non-Hispanic white. Patients who spoke other languages or came from other cultural backgrounds may have had different responses. Third, we did not use a single validated scale for patient ratings of communication, and multiple analyses increase our risk of finding statistically significant associations by chance. The skewing of the communication rating items toward high scores may also have led to our results being driven by outliers, although the model we chose for analysis does penalize for this. Furthermore, our sample size was small, leading to wide CIs and potential for lack of statistical associations due to insufficient power. Our findings warrant replication in larger studies. Fourth, the setting of our study in an academic center may affect generalizability. Finally, the age of our data (collected between 2008 and 2009) is also a limitation. Given a recent focus on communication and patient experience since the initiation of HCAHPS feedback, a similar analysis of empathy and communication methods now may result in different outcomes.
In conclusion, our results suggest that enhancing hospitalists’ empathic responses to patient expressions of negative emotion could decrease patient anxiety and improve patients’ perceptions of (and thus possibly their relationships with) hospitalists, without sacrificing efficiency. Future work should focus on tailoring and implementing communication skills training programs for hospitalists and evaluating the impact of training on patient outcomes.
Acknowledgments
The authors extend their sincere thanks to the patients and physicians who participated in this study. Dr. Anderson was funded by the National Palliative Care Research Center and the University of California, San Francisco Clinical and Translational Science Institute Career Development Program, National Institutes of Health (NIH) grant number 5 KL2 RR024130-04. Project costs were funded by a grant from the University of California, San Francisco Academic Senate.
Disclosure
All coauthors have seen and agree with the contents of this manuscript. This submission is not under review by any other publication. Wendy Anderson received funding for this project from the National Palliative Care Research Center, University of California San Francisco Clinical and Translational Science Institute (NIH grant number 5KL2RR024130-04), and the University of San Francisco Academic Senate [From Section 2 of Author Disclosure Form]. Andy Auerbach has a Patient-Centered Outcomes Research Institute research grant in development [From Section 3 of the Author Disclosure Form].
1. Walker FB, Novack DH, Kaiser DL, Knight A, Oblinger P. Anxiety and depression among medical and surgical patients nearing hospital discharge. J Gen Intern Med. 1987;2(2):99-101. PubMed
2. Castillo MI, Cooke M, Macfarlane B, Aitken LM. Factors associated with anxiety in critically ill patients: A prospective observational cohort study. Int J Nurs Stud. 2016;60:225-233. PubMed
3. Anderson WG, Winters K, Auerbach AD. Patient concerns at hospital admission. Arch Intern Med. 2011;171(15):1399-1400. PubMed
4. Kuo Y-F, Sharma G, Freeman JL, Goodwin JS. Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102-1112. PubMed
5. Wachter RM, Goldman L. Zero to 50,000 - The 20th Anniversary of the Hospitalist. N Engl J Med. 2016;375(11):1009-1011. PubMed
6. Mack JW, Block SD, Nilsson M, et al. Measuring therapeutic alliance between oncologists and patients with advanced cancer: the Human Connection Scale. Cancer. 2009;115(14):3302-3311. PubMed
7. Huff NG, Nadig N, Ford DW, Cox CE. Therapeutic Alliance between the Caregivers of Critical Illness Survivors and Intensive Care Unit Clinicians. [published correction appears in Ann Am Thorac Soc. 2016;13(4):576]. Ann Am Thorac Soc. 2015;12(11):1646-1653. PubMed
8. Derksen F, Bensing J, Lagro-Janssen A. Effectiveness of empathy in general practice: a systematic review. Br J Gen Pract. 2013;63(606):e76-e84. PubMed
9. Dwamena F, Holmes-Rovner M, Gaulden CM, et al. Interventions for providers to promote a patient-centred approach in clinical consultations. Cochrane Database Syst Rev. 2012;12:CD003267. PubMed
10. Fogarty LA, Curbow BA, Wingard JR, McDonnell K, Somerfield MR. Can 40 seconds of compassion reduce patient anxiety? J Clin Oncol. 1999;17(1):371-379. PubMed
11. Roter DL, Hall JA, Kern DE, Barker LR, Cole KA, Roca RP. Improving physicians’ interviewing skills and reducing patients’ emotional distress. A randomized clinical trial. Arch Intern Med. 1995;155(17):1877-1884. PubMed
12. Stapleton RD, Engelberg RA, Wenrich MD, Goss CH, Curtis JR. Clinician statements and family satisfaction with family conferences in the intensive care unit. Crit Care Med. 2006;34(6):1679-1685. PubMed
13. Hojat M, Louis DZ, Markham FW, Wender R, Rabinowitz C, Gonnella JS. Physicians’ empathy and clinical outcomes for diabetic patients. Acad Med. 2011;86(3):359-364. PubMed
14. Anderson WG, Winters K, Arnold RM, Puntillo KA, White DB, Auerbach AD. Studying physician-patient communication in the acute care setting: the hospitalist rapport study. Patient Educ Couns. 2011;82(2):275-279. PubMed
15. Pollak KI, Arnold RM, Jeffreys AS, et al. Oncologist communication about emotion during visits with patients with advanced cancer. J Clin Oncol. 2007;25(36):5748-5752. PubMed
16. Suchman AL, Markakis K, Beckman HB, Frankel R. A model of empathic communication in the medical interview. JAMA. 1997;277(8):678-682. PubMed
17. Adams K, Cimino JEW, Arnold RM, Anderson WG. Why should I talk about emotion? Communication patterns associated with physician discussion of patient expressions of negative emotion in hospital admission encounters. Patient Educ Couns. 2012;89(1):44-50. PubMed
18. Julian LJ. Measures of anxiety: State-Trait Anxiety Inventory (STAI), Beck Anxiety Inventory (BAI), and Hospital Anxiety and Depression Scale-Anxiety (HADS-A). Arthritis Care Res (Hoboken). 2011;63 Suppl 11:S467-S472. PubMed
19. Speilberger C, Ritterband L, Sydeman S, Reheiser E, Unger K. Assessment of emotional states and personality traits: measuring psychological vital signs. In: Butcher J, editor. Clinical personality assessment: practical approaches. New York: Oxford University Press; 1995.
20. Safran DG, Kosinski M, Tarlov AR, et al. The Primary Care Assessment Survey: tests of data quality and measurement performance. Med Care. 1998;36(5):728-739. PubMed
21. Azoulay E, Pochard F, Kentish-Barnes N, et al. Risk of post-traumatic stress symptoms in family members of intensive care unit patients. Am J Respir Crit Care Med. 2005;171(9):987-994. PubMed
22. Lynn J. Perspectives on care at the close of life. Serving patients who may die soon and their families: the role of hospice and other services. JAMA. 2001;285(7):925-932. PubMed
23. Kennifer SL, Alexander SC, Pollak KI, et al. Negative emotions in cancer care: do oncologists’ responses depend on severity and type of emotion? Patient Educ Couns. 2009;76(1):51-56. PubMed
24. Butow PN, Brown RF, Cogar S, Tattersall MHN, Dunn SM. Oncologists’ reactions to cancer patients’ verbal cues. Psychooncology. 2002;11(1):47-58. PubMed
25. Levinson W, Gorawara-Bhat R, Lamb J. A study of patient clues and physician responses in primary care and surgical settings. JAMA. 2000;284(8):1021-1027. PubMed
26. Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20(1):37-46.
27. Fulop G. Anxiety disorders in the general hospital setting. Psychiatr Med. 1990;8(3):187-195. PubMed
28. Gerson S, Mistry R, Bastani R, et al. Symptoms of depression and anxiety (MHI) following acute medical/surgical hospitalization and post-discharge psychiatric diagnoses (DSM) in 839 geriatric US veterans. Int J Geriatr Psychiatry. 2004;19(12):1155-1167. PubMed
29. Kathol RG, Wenzel RP. Natural history of symptoms of depression and anxiety during inpatient treatment on general medicine wards. J Gen Intern Med. 1992;7(3):287-293. PubMed
30. Unsal A, Unaldi C, Baytemir C. Anxiety and depression levels of inpatients in the city centre of Kirşehir in Turkey. Int J Nurs Pract. 2011;17(4):411-418. PubMed
31. Bradt J, Dileo C, Grocke D, Magill L. Music interventions for improving psychological and physical outcomes in cancer patients. [Update appears in Cochrane Database Syst Rev. 2016;(8):CD006911] Cochrane Database Syst Rev. 2011;(8):CD006911. PubMed
32. Kim SS, Kaplowitz S, Johnston MV. The effects of physician empathy on patient satisfaction and compliance. Eval Health Prof. 2004;27(3):237-251. PubMed
33. Tulsky JA, Arnold RM, Alexander SC, et al. Enhancing communication between oncologists and patients with a computer-based training program: a randomized trial. Ann Intern Med. 2011;155(9):593-601. PubMed
34. Bays AM, Engelberg RA, Back AL, et al. Interprofessional communication skills training for serious illness: evaluation of a small-group, simulated patient intervention. J Palliat Med. 2014;17(2):159-166. PubMed
35. Epstein RM, Duberstein PR, Fenton JJ, et al. Effect of a Patient-Centered Communication Intervention on Oncologist-Patient Communication, Quality of Life, and Health Care Utilization in Advanced Cancer: The VOICE Randomized Clinical Trial. JAMA Oncol. 2017;3(1):92-100. PubMed
36. Berkhof M, van Rijssen HJ, Schellart AJM, Anema JR, van der Beek AJ. Effective training strategies for teaching communication skills to physicians: an overview of systematic reviews. Patient Educ Couns. 2011;84(2):152-162. PubMed
Admission to a hospital can be a stressful event,1,2 and patients report having many concerns at the time of hospital admission.3 Over the last 20 years, the United States has widely adopted the hospitalist model of inpatient care. Although this model has clear benefits, it also has the potential to contribute to patient stress, as hospitalized patients generally lack preexisting relationships with their inpatient physicians.4,5 In this changing hospital environment, defining and promoting effective medical communication has become an essential goal of both individual practitioners and medical centers.
Successful communication and strong therapeutic relationships with physicians support patients’ coping with illness-associated stress6,7 as well as promote adherence to medical treatment plans.8 Empathy serves as an important building block of patient-centered communication and encourages a strong therapeutic alliance.9 Studies from primary care, oncology, and intensive care unit (ICU) settings indicate that physician empathy is associated with decreased emotional distress,10,11 improved ratings of communication,12 and even better medical outcomes.13
Prior work has shown that hospitalists, like other clinicians, underutilize empathy as a tool in their daily interactions with patients.14-16 Our prior qualitative analysis of audio-recorded hospitalist-patient admission encounters indicated that how hospitalists respond to patient expressions of negative emotion influences relationships with patients and alignment around care plans.17 To determine whether empathic communication is associated with patient-reported outcomes in the hospitalist model, we quantitatively analyzed coded admission encounters and survey data to examine the association between hospitalists’ responses to patient expressions of negative emotion (anxiety, sadness, and anger) and patient anxiety and ratings of communication. Given the often-limited time hospitalists have to complete admission encounters, we also examined the association between response to emotion and encounter length.
METHODS
We analyzed data collected as part of an observational study of hospitalist-patient communication during hospital admission encounters14 to assess the association between the way physicians responded to patient expressions of negative emotion and patient anxiety, ratings of communication in the encounter, and encounter length. We collected data between August 2008 and March 2009 on the general medical service at 2 urban hospitals that are part of an academic medical center. Participants were attending hospitalists (not physician trainees), and patients admitted under participating hospitalists’ care who were able to communicate verbally in English and provide informed consent for the study. The institutional review board at the University of California, San Francisco approved the study; physician and patient participants provided written informed consent.
Enrollment and data collection has been described previously.17 Our cohort for this analysis included 76 patients of 27 physicians who completed encounter audio recordings and pre- and postencounter surveys. Following enrollment, patients completed a preencounter survey to collect demographic information and to measure their baseline anxiety via the State Anxiety Scale (STAI-S), which assesses transient anxious mood using 20 items answered on a 4-point scale for a final score range of 20 to 80.10,18,19 We timed and audio-recorded admission encounters. Encounter recordings were obtained solely from patient interactions with attending hospitalists and did not take into account the time patients may have spent with other physicians, including trainees. After the encounter, patients completed postencounter surveys, which included the STAI-S and patients’ ratings of communication during the encounter. To rate communication, patients responded to 7 items on a 0- to 10-point scale that were derived from previous work (Table 1)12,20,21; the anchors were “not at all” and “completely.” To identify patients with serious illness, which we used as a covariate in regression models, we asked physicians on a postencounter survey whether or not they “would be surprised by this patient’s death or admission to the ICU in the next year.”22
We considered physician as a clustering variable in the calculation of robust standard errors for all models. In addition, we included in each model covariates that were associated with the outcome at P ≤ 0.10, including patient gender, patient age, serious illness,22 preencounter anxiety, encounter length, and hospital. We considered P values < 0.05 to be statistically significant. We used Stata SE 13 (StataCorp LLC, College Station, TX) for all statistical analyses.
RESULTS
We analyzed data from admission encounters with 76 patients (consent rate 63%) and 27 hospitalists (consent rate 91%). Their characteristics are shown in Table 3. Median encounter length was 19 minutes (mean 21 minutes, range 3-68). Patients expressed negative emotion in 190 instances across all encounters; median number of expressions per encounter was 1 (range 0-14). Hospitalists responded empathically to 32% (n = 61) of the patient expressions, neutrally to 43% (n = 81), and nonempathically to 25% (n = 48).
The STAI-S was normally distributed. The mean preencounter STAI-S score was 39 (standard deviation [SD] 8.9). Mean postencounter STAI-S score was 38 (SD 10.7). Mean change in anxiety over the course of the encounter, calculated as the postencounter minus preencounter mean was −1.2 (SD 7.6). Table 1 shows summary statistics for the patient ratings of communication items. All items were rated highly. Across the items, between 51% and 78% of patients rated the highest score of 10.
Across the range of frequencies of emotional expressions per encounter in our data set (0-14 expressions), each additional empathic hospitalist response was associated with a 1.65-point decrease in the STAI-S (95% confidence interval [CI], 0.48-2.82). We did not find significant associations between changes in the STAI-S and the number of neutral hospitalist responses (−0.65 per response; 95% CI, −1.67-0.37) or nonempathic hospitalist responses (0.61 per response; 95% CI, −0.88-2.10).
In addition, nonempathic responses were associated with more negative ratings of communication for 5 of the 7 items: ease of understanding information, covering points of interest, the doctor listening, the doctor caring, and trusting the doctor. For example, for the item “I felt this doctor cared about me,” each nonempathic hospitalist response was associated with a more than doubling of negative patient ratings (aRE: 2.3; 95% CI, 1.32-4.16). Neutral physician responses to patient expressions of negative emotion were associated with less negative patient ratings for 2 of the items: covering points of interest (aRE 0.68; 95% CI, 0.51-0.90) and trusting the doctor (aRE: 0.86; 95% CI, 0.75-0.99).
We did not find a statistical association between encounter length and the number of empathic hospitalist responses in the encounter (percent change in encounter length per response [PC]: 1%; 95% CI, −8%-10%) or the number of nonempathic responses (PC: 18%; 95% CI, −2%-42%). We did find a statistically significant association between the number of neutral responses and encounter length (PC: 13%; 95% CI, 3%-24%), corresponding to 2.5 minutes of additional encounter time per neutral response for the median encounter length of 19 minutes.
DISCUSSION
Our study set out to measure how hospitalists responded to expressions of negative emotion during admission encounters with patients and how those responses correlated with patient anxiety, ratings of communication, and encounter length. We found that empathic responses were associated with diminishing patient anxiety after the visit, as well as with better ratings of several domains of hospitalist communication. Moreover, nonempathic responses to negative emotion were associated with more strongly negative ratings of hospitalist communication. Finally, while clinicians may worry that encouraging patients to speak further about emotion will result in excessive visit lengths, we did not find a statistical association between empathic responses and encounter duration. To our knowledge, this is the first study to indicate an association between empathy and patient anxiety and communication ratings within the hospitalist model, which is rapidly becoming the predominant model for providing inpatient care in the United States.4,5
As in oncologic care, anxiety is an emotion commonly confronted by clinicians meeting admitted medical patients for the first time. Studies show that not only do patient anxiety levels remain high throughout a hospital course, patients who experience higher levels of anxiety tend to stay longer in the hospital.1,2,27-30 But unlike oncologic care or other therapy provided in an outpatient setting, the hospitalist model does not facilitate “continuity” of care, or the ability to care for the same patients over a long period of time. This reality of inpatient care makes rapid, effective rapport-building critical to establishing strong physician-patient relationships. In this setting, a simple communication tool that is potentially able to reduce inpatients’ anxiety could have a meaningful impact on hospitalist-provided care and patient outcomes.
In terms of the magnitude of the effect of empathic responses, the clinical significance of a 1.65-point decrease in the STAI-S anxiety score is not precisely clear. A prior study that examined the effect of music therapy on anxiety levels in patients with cancer found an average anxiety reduction of approximately 9.5 units on the STAIS-S scale after sensitivity analysis, suggesting a rather large meaningful effect size.31 Given we found a reduction of 1.65 points for each empathic response, however, with a range of 0-14 negative emotions expressed over a median 19-minute encounter, there is opportunity for hospitalists to achieve a clinically significant decrease in patient anxiety during an admission encounter. The potential to reduce anxiety is extended further when we consider that the impact of an empathic response may apply not just to the admission encounter alone but also to numerous other patient-clinician interactions over the course of a hospitalization.
A healthy body of communication research supports the associations we found in our study between empathy and patient ratings of communication and physicians. Families in ICU conferences rate communication more positively when physicians express empathy,12 and a number of studies indicate an association between empathy and patient satisfaction in outpatient settings.8 Given the associations we found with negative ratings on the items in our study, promoting empathic responses to expressions of emotion and, more importantly, stressing avoidance of nonempathic responses may be relevant efforts in working to improve patient satisfaction scores on surveys reporting “top box” percentages, such as Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS). More notably, evidence indicates that empathy has positive impacts beyond satisfaction surveys, such as adherence, better diagnostic and clinical outcomes, and strengthening of patient enablement.8Not all hospitalist responses to emotion were associated with patient ratings across the 7 communication items we assessed. For example, we did not find an association between how physicians responded to patient expressions of negative emotion and patient perception that enough time was spent in the visit or the degree to which talking with the doctor met a patient’s overall needs. It follows logically, and other research supports, that empathy would influence patient ratings of physician caring and trust,32 whereas other communication factors we were unable to measure (eg, physician body language, tone, and use of jargon and patient health literacy and primary language) may have a more significant association with patient ratings of the other items we assessed.
In considering the clinical application of our results, it is important to note that communication skills, including responding empathically to patient expressions of negative emotion, can be imparted through training in the same way as abdominal examination or electrocardiogram interpretation skills.33-35 However, training of hospitalists in communication skills requires time and some financial investment on the part of the physician, their hospital or group, or, ideally, both. Effective training methods, like those for other skill acquisition, involve learner-centered teaching and practicing skills with role-play and feedback.36 Given the importance of a learner-centered approach, learning would likely be better received and more effective if it was tailored to the specific needs and patient scenarios commonly encountered by hospitalist physicians. As these programs are developed, it will be important to assess the impact of any training on the patient-reported outcomes we assessed in this observational study, along with clinical outcomes.
Our study has several limitations. First, we were only able to evaluate whether hospitalists verbally responded to patient emotion and were thus not able to account for nonverbal empathy such as facial expressions, body language, or voice tone. Second, given our patient consent rate of 63%, patients who agreed to participate in the study may have had different opinions than those who declined to participate. Also, hospitalists and patients may have behaved differently as a result of being audio recorded. We only included patients who spoke English, and our patient population was predominately non-Hispanic white. Patients who spoke other languages or came from other cultural backgrounds may have had different responses. Third, we did not use a single validated scale for patient ratings of communication, and multiple analyses increase our risk of finding statistically significant associations by chance. The skewing of the communication rating items toward high scores may also have led to our results being driven by outliers, although the model we chose for analysis does penalize for this. Furthermore, our sample size was small, leading to wide CIs and potential for lack of statistical associations due to insufficient power. Our findings warrant replication in larger studies. Fourth, the setting of our study in an academic center may affect generalizability. Finally, the age of our data (collected between 2008 and 2009) is also a limitation. Given a recent focus on communication and patient experience since the initiation of HCAHPS feedback, a similar analysis of empathy and communication methods now may result in different outcomes.
In conclusion, our results suggest that enhancing hospitalists’ empathic responses to patient expressions of negative emotion could decrease patient anxiety and improve patients’ perceptions of (and thus possibly their relationships with) hospitalists, without sacrificing efficiency. Future work should focus on tailoring and implementing communication skills training programs for hospitalists and evaluating the impact of training on patient outcomes.
Acknowledgments
The authors extend their sincere thanks to the patients and physicians who participated in this study. Dr. Anderson was funded by the National Palliative Care Research Center and the University of California, San Francisco Clinical and Translational Science Institute Career Development Program, National Institutes of Health (NIH) grant number 5 KL2 RR024130-04. Project costs were funded by a grant from the University of California, San Francisco Academic Senate.
Disclosure
All coauthors have seen and agree with the contents of this manuscript. This submission is not under review by any other publication. Wendy Anderson received funding for this project from the National Palliative Care Research Center, University of California San Francisco Clinical and Translational Science Institute (NIH grant number 5KL2RR024130-04), and the University of San Francisco Academic Senate [From Section 2 of Author Disclosure Form]. Andy Auerbach has a Patient-Centered Outcomes Research Institute research grant in development [From Section 3 of the Author Disclosure Form].
Admission to a hospital can be a stressful event,1,2 and patients report having many concerns at the time of hospital admission.3 Over the last 20 years, the United States has widely adopted the hospitalist model of inpatient care. Although this model has clear benefits, it also has the potential to contribute to patient stress, as hospitalized patients generally lack preexisting relationships with their inpatient physicians.4,5 In this changing hospital environment, defining and promoting effective medical communication has become an essential goal of both individual practitioners and medical centers.
Successful communication and strong therapeutic relationships with physicians support patients’ coping with illness-associated stress6,7 as well as promote adherence to medical treatment plans.8 Empathy serves as an important building block of patient-centered communication and encourages a strong therapeutic alliance.9 Studies from primary care, oncology, and intensive care unit (ICU) settings indicate that physician empathy is associated with decreased emotional distress,10,11 improved ratings of communication,12 and even better medical outcomes.13
Prior work has shown that hospitalists, like other clinicians, underutilize empathy as a tool in their daily interactions with patients.14-16 Our prior qualitative analysis of audio-recorded hospitalist-patient admission encounters indicated that how hospitalists respond to patient expressions of negative emotion influences relationships with patients and alignment around care plans.17 To determine whether empathic communication is associated with patient-reported outcomes in the hospitalist model, we quantitatively analyzed coded admission encounters and survey data to examine the association between hospitalists’ responses to patient expressions of negative emotion (anxiety, sadness, and anger) and patient anxiety and ratings of communication. Given the often-limited time hospitalists have to complete admission encounters, we also examined the association between response to emotion and encounter length.
METHODS
We analyzed data collected as part of an observational study of hospitalist-patient communication during hospital admission encounters14 to assess the association between the way physicians responded to patient expressions of negative emotion and patient anxiety, ratings of communication in the encounter, and encounter length. We collected data between August 2008 and March 2009 on the general medical service at 2 urban hospitals that are part of an academic medical center. Participants were attending hospitalists (not physician trainees), and patients admitted under participating hospitalists’ care who were able to communicate verbally in English and provide informed consent for the study. The institutional review board at the University of California, San Francisco approved the study; physician and patient participants provided written informed consent.
Enrollment and data collection has been described previously.17 Our cohort for this analysis included 76 patients of 27 physicians who completed encounter audio recordings and pre- and postencounter surveys. Following enrollment, patients completed a preencounter survey to collect demographic information and to measure their baseline anxiety via the State Anxiety Scale (STAI-S), which assesses transient anxious mood using 20 items answered on a 4-point scale for a final score range of 20 to 80.10,18,19 We timed and audio-recorded admission encounters. Encounter recordings were obtained solely from patient interactions with attending hospitalists and did not take into account the time patients may have spent with other physicians, including trainees. After the encounter, patients completed postencounter surveys, which included the STAI-S and patients’ ratings of communication during the encounter. To rate communication, patients responded to 7 items on a 0- to 10-point scale that were derived from previous work (Table 1)12,20,21; the anchors were “not at all” and “completely.” To identify patients with serious illness, which we used as a covariate in regression models, we asked physicians on a postencounter survey whether or not they “would be surprised by this patient’s death or admission to the ICU in the next year.”22
We considered physician as a clustering variable in the calculation of robust standard errors for all models. In addition, we included in each model covariates that were associated with the outcome at P ≤ 0.10, including patient gender, patient age, serious illness,22 preencounter anxiety, encounter length, and hospital. We considered P values < 0.05 to be statistically significant. We used Stata SE 13 (StataCorp LLC, College Station, TX) for all statistical analyses.
RESULTS
We analyzed data from admission encounters with 76 patients (consent rate 63%) and 27 hospitalists (consent rate 91%). Their characteristics are shown in Table 3. Median encounter length was 19 minutes (mean 21 minutes, range 3-68). Patients expressed negative emotion in 190 instances across all encounters; median number of expressions per encounter was 1 (range 0-14). Hospitalists responded empathically to 32% (n = 61) of the patient expressions, neutrally to 43% (n = 81), and nonempathically to 25% (n = 48).
The STAI-S was normally distributed. The mean preencounter STAI-S score was 39 (standard deviation [SD] 8.9). Mean postencounter STAI-S score was 38 (SD 10.7). Mean change in anxiety over the course of the encounter, calculated as the postencounter minus preencounter mean was −1.2 (SD 7.6). Table 1 shows summary statistics for the patient ratings of communication items. All items were rated highly. Across the items, between 51% and 78% of patients rated the highest score of 10.
Across the range of frequencies of emotional expressions per encounter in our data set (0-14 expressions), each additional empathic hospitalist response was associated with a 1.65-point decrease in the STAI-S (95% confidence interval [CI], 0.48-2.82). We did not find significant associations between changes in the STAI-S and the number of neutral hospitalist responses (−0.65 per response; 95% CI, −1.67-0.37) or nonempathic hospitalist responses (0.61 per response; 95% CI, −0.88-2.10).
In addition, nonempathic responses were associated with more negative ratings of communication for 5 of the 7 items: ease of understanding information, covering points of interest, the doctor listening, the doctor caring, and trusting the doctor. For example, for the item “I felt this doctor cared about me,” each nonempathic hospitalist response was associated with a more than doubling of negative patient ratings (aRE: 2.3; 95% CI, 1.32-4.16). Neutral physician responses to patient expressions of negative emotion were associated with less negative patient ratings for 2 of the items: covering points of interest (aRE 0.68; 95% CI, 0.51-0.90) and trusting the doctor (aRE: 0.86; 95% CI, 0.75-0.99).
We did not find a statistical association between encounter length and the number of empathic hospitalist responses in the encounter (percent change in encounter length per response [PC]: 1%; 95% CI, −8%-10%) or the number of nonempathic responses (PC: 18%; 95% CI, −2%-42%). We did find a statistically significant association between the number of neutral responses and encounter length (PC: 13%; 95% CI, 3%-24%), corresponding to 2.5 minutes of additional encounter time per neutral response for the median encounter length of 19 minutes.
DISCUSSION
Our study set out to measure how hospitalists responded to expressions of negative emotion during admission encounters with patients and how those responses correlated with patient anxiety, ratings of communication, and encounter length. We found that empathic responses were associated with diminishing patient anxiety after the visit, as well as with better ratings of several domains of hospitalist communication. Moreover, nonempathic responses to negative emotion were associated with more strongly negative ratings of hospitalist communication. Finally, while clinicians may worry that encouraging patients to speak further about emotion will result in excessive visit lengths, we did not find a statistical association between empathic responses and encounter duration. To our knowledge, this is the first study to indicate an association between empathy and patient anxiety and communication ratings within the hospitalist model, which is rapidly becoming the predominant model for providing inpatient care in the United States.4,5
As in oncologic care, anxiety is an emotion commonly confronted by clinicians meeting admitted medical patients for the first time. Studies show that not only do patient anxiety levels remain high throughout a hospital course, patients who experience higher levels of anxiety tend to stay longer in the hospital.1,2,27-30 But unlike oncologic care or other therapy provided in an outpatient setting, the hospitalist model does not facilitate “continuity” of care, or the ability to care for the same patients over a long period of time. This reality of inpatient care makes rapid, effective rapport-building critical to establishing strong physician-patient relationships. In this setting, a simple communication tool that is potentially able to reduce inpatients’ anxiety could have a meaningful impact on hospitalist-provided care and patient outcomes.
In terms of the magnitude of the effect of empathic responses, the clinical significance of a 1.65-point decrease in the STAI-S anxiety score is not precisely clear. A prior study that examined the effect of music therapy on anxiety levels in patients with cancer found an average anxiety reduction of approximately 9.5 units on the STAIS-S scale after sensitivity analysis, suggesting a rather large meaningful effect size.31 Given we found a reduction of 1.65 points for each empathic response, however, with a range of 0-14 negative emotions expressed over a median 19-minute encounter, there is opportunity for hospitalists to achieve a clinically significant decrease in patient anxiety during an admission encounter. The potential to reduce anxiety is extended further when we consider that the impact of an empathic response may apply not just to the admission encounter alone but also to numerous other patient-clinician interactions over the course of a hospitalization.
A healthy body of communication research supports the associations we found in our study between empathy and patient ratings of communication and physicians. Families in ICU conferences rate communication more positively when physicians express empathy,12 and a number of studies indicate an association between empathy and patient satisfaction in outpatient settings.8 Given the associations we found with negative ratings on the items in our study, promoting empathic responses to expressions of emotion and, more importantly, stressing avoidance of nonempathic responses may be relevant efforts in working to improve patient satisfaction scores on surveys reporting “top box” percentages, such as Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS). More notably, evidence indicates that empathy has positive impacts beyond satisfaction surveys, such as adherence, better diagnostic and clinical outcomes, and strengthening of patient enablement.8Not all hospitalist responses to emotion were associated with patient ratings across the 7 communication items we assessed. For example, we did not find an association between how physicians responded to patient expressions of negative emotion and patient perception that enough time was spent in the visit or the degree to which talking with the doctor met a patient’s overall needs. It follows logically, and other research supports, that empathy would influence patient ratings of physician caring and trust,32 whereas other communication factors we were unable to measure (eg, physician body language, tone, and use of jargon and patient health literacy and primary language) may have a more significant association with patient ratings of the other items we assessed.
In considering the clinical application of our results, it is important to note that communication skills, including responding empathically to patient expressions of negative emotion, can be imparted through training in the same way as abdominal examination or electrocardiogram interpretation skills.33-35 However, training of hospitalists in communication skills requires time and some financial investment on the part of the physician, their hospital or group, or, ideally, both. Effective training methods, like those for other skill acquisition, involve learner-centered teaching and practicing skills with role-play and feedback.36 Given the importance of a learner-centered approach, learning would likely be better received and more effective if it was tailored to the specific needs and patient scenarios commonly encountered by hospitalist physicians. As these programs are developed, it will be important to assess the impact of any training on the patient-reported outcomes we assessed in this observational study, along with clinical outcomes.
Our study has several limitations. First, we were only able to evaluate whether hospitalists verbally responded to patient emotion and were thus not able to account for nonverbal empathy such as facial expressions, body language, or voice tone. Second, given our patient consent rate of 63%, patients who agreed to participate in the study may have had different opinions than those who declined to participate. Also, hospitalists and patients may have behaved differently as a result of being audio recorded. We only included patients who spoke English, and our patient population was predominately non-Hispanic white. Patients who spoke other languages or came from other cultural backgrounds may have had different responses. Third, we did not use a single validated scale for patient ratings of communication, and multiple analyses increase our risk of finding statistically significant associations by chance. The skewing of the communication rating items toward high scores may also have led to our results being driven by outliers, although the model we chose for analysis does penalize for this. Furthermore, our sample size was small, leading to wide CIs and potential for lack of statistical associations due to insufficient power. Our findings warrant replication in larger studies. Fourth, the setting of our study in an academic center may affect generalizability. Finally, the age of our data (collected between 2008 and 2009) is also a limitation. Given a recent focus on communication and patient experience since the initiation of HCAHPS feedback, a similar analysis of empathy and communication methods now may result in different outcomes.
In conclusion, our results suggest that enhancing hospitalists’ empathic responses to patient expressions of negative emotion could decrease patient anxiety and improve patients’ perceptions of (and thus possibly their relationships with) hospitalists, without sacrificing efficiency. Future work should focus on tailoring and implementing communication skills training programs for hospitalists and evaluating the impact of training on patient outcomes.
Acknowledgments
The authors extend their sincere thanks to the patients and physicians who participated in this study. Dr. Anderson was funded by the National Palliative Care Research Center and the University of California, San Francisco Clinical and Translational Science Institute Career Development Program, National Institutes of Health (NIH) grant number 5 KL2 RR024130-04. Project costs were funded by a grant from the University of California, San Francisco Academic Senate.
Disclosure
All coauthors have seen and agree with the contents of this manuscript. This submission is not under review by any other publication. Wendy Anderson received funding for this project from the National Palliative Care Research Center, University of California San Francisco Clinical and Translational Science Institute (NIH grant number 5KL2RR024130-04), and the University of San Francisco Academic Senate [From Section 2 of Author Disclosure Form]. Andy Auerbach has a Patient-Centered Outcomes Research Institute research grant in development [From Section 3 of the Author Disclosure Form].
1. Walker FB, Novack DH, Kaiser DL, Knight A, Oblinger P. Anxiety and depression among medical and surgical patients nearing hospital discharge. J Gen Intern Med. 1987;2(2):99-101. PubMed
2. Castillo MI, Cooke M, Macfarlane B, Aitken LM. Factors associated with anxiety in critically ill patients: A prospective observational cohort study. Int J Nurs Stud. 2016;60:225-233. PubMed
3. Anderson WG, Winters K, Auerbach AD. Patient concerns at hospital admission. Arch Intern Med. 2011;171(15):1399-1400. PubMed
4. Kuo Y-F, Sharma G, Freeman JL, Goodwin JS. Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102-1112. PubMed
5. Wachter RM, Goldman L. Zero to 50,000 - The 20th Anniversary of the Hospitalist. N Engl J Med. 2016;375(11):1009-1011. PubMed
6. Mack JW, Block SD, Nilsson M, et al. Measuring therapeutic alliance between oncologists and patients with advanced cancer: the Human Connection Scale. Cancer. 2009;115(14):3302-3311. PubMed
7. Huff NG, Nadig N, Ford DW, Cox CE. Therapeutic Alliance between the Caregivers of Critical Illness Survivors and Intensive Care Unit Clinicians. [published correction appears in Ann Am Thorac Soc. 2016;13(4):576]. Ann Am Thorac Soc. 2015;12(11):1646-1653. PubMed
8. Derksen F, Bensing J, Lagro-Janssen A. Effectiveness of empathy in general practice: a systematic review. Br J Gen Pract. 2013;63(606):e76-e84. PubMed
9. Dwamena F, Holmes-Rovner M, Gaulden CM, et al. Interventions for providers to promote a patient-centred approach in clinical consultations. Cochrane Database Syst Rev. 2012;12:CD003267. PubMed
10. Fogarty LA, Curbow BA, Wingard JR, McDonnell K, Somerfield MR. Can 40 seconds of compassion reduce patient anxiety? J Clin Oncol. 1999;17(1):371-379. PubMed
11. Roter DL, Hall JA, Kern DE, Barker LR, Cole KA, Roca RP. Improving physicians’ interviewing skills and reducing patients’ emotional distress. A randomized clinical trial. Arch Intern Med. 1995;155(17):1877-1884. PubMed
12. Stapleton RD, Engelberg RA, Wenrich MD, Goss CH, Curtis JR. Clinician statements and family satisfaction with family conferences in the intensive care unit. Crit Care Med. 2006;34(6):1679-1685. PubMed
13. Hojat M, Louis DZ, Markham FW, Wender R, Rabinowitz C, Gonnella JS. Physicians’ empathy and clinical outcomes for diabetic patients. Acad Med. 2011;86(3):359-364. PubMed
14. Anderson WG, Winters K, Arnold RM, Puntillo KA, White DB, Auerbach AD. Studying physician-patient communication in the acute care setting: the hospitalist rapport study. Patient Educ Couns. 2011;82(2):275-279. PubMed
15. Pollak KI, Arnold RM, Jeffreys AS, et al. Oncologist communication about emotion during visits with patients with advanced cancer. J Clin Oncol. 2007;25(36):5748-5752. PubMed
16. Suchman AL, Markakis K, Beckman HB, Frankel R. A model of empathic communication in the medical interview. JAMA. 1997;277(8):678-682. PubMed
17. Adams K, Cimino JEW, Arnold RM, Anderson WG. Why should I talk about emotion? Communication patterns associated with physician discussion of patient expressions of negative emotion in hospital admission encounters. Patient Educ Couns. 2012;89(1):44-50. PubMed
18. Julian LJ. Measures of anxiety: State-Trait Anxiety Inventory (STAI), Beck Anxiety Inventory (BAI), and Hospital Anxiety and Depression Scale-Anxiety (HADS-A). Arthritis Care Res (Hoboken). 2011;63 Suppl 11:S467-S472. PubMed
19. Speilberger C, Ritterband L, Sydeman S, Reheiser E, Unger K. Assessment of emotional states and personality traits: measuring psychological vital signs. In: Butcher J, editor. Clinical personality assessment: practical approaches. New York: Oxford University Press; 1995.
20. Safran DG, Kosinski M, Tarlov AR, et al. The Primary Care Assessment Survey: tests of data quality and measurement performance. Med Care. 1998;36(5):728-739. PubMed
21. Azoulay E, Pochard F, Kentish-Barnes N, et al. Risk of post-traumatic stress symptoms in family members of intensive care unit patients. Am J Respir Crit Care Med. 2005;171(9):987-994. PubMed
22. Lynn J. Perspectives on care at the close of life. Serving patients who may die soon and their families: the role of hospice and other services. JAMA. 2001;285(7):925-932. PubMed
23. Kennifer SL, Alexander SC, Pollak KI, et al. Negative emotions in cancer care: do oncologists’ responses depend on severity and type of emotion? Patient Educ Couns. 2009;76(1):51-56. PubMed
24. Butow PN, Brown RF, Cogar S, Tattersall MHN, Dunn SM. Oncologists’ reactions to cancer patients’ verbal cues. Psychooncology. 2002;11(1):47-58. PubMed
25. Levinson W, Gorawara-Bhat R, Lamb J. A study of patient clues and physician responses in primary care and surgical settings. JAMA. 2000;284(8):1021-1027. PubMed
26. Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20(1):37-46.
27. Fulop G. Anxiety disorders in the general hospital setting. Psychiatr Med. 1990;8(3):187-195. PubMed
28. Gerson S, Mistry R, Bastani R, et al. Symptoms of depression and anxiety (MHI) following acute medical/surgical hospitalization and post-discharge psychiatric diagnoses (DSM) in 839 geriatric US veterans. Int J Geriatr Psychiatry. 2004;19(12):1155-1167. PubMed
29. Kathol RG, Wenzel RP. Natural history of symptoms of depression and anxiety during inpatient treatment on general medicine wards. J Gen Intern Med. 1992;7(3):287-293. PubMed
30. Unsal A, Unaldi C, Baytemir C. Anxiety and depression levels of inpatients in the city centre of Kirşehir in Turkey. Int J Nurs Pract. 2011;17(4):411-418. PubMed
31. Bradt J, Dileo C, Grocke D, Magill L. Music interventions for improving psychological and physical outcomes in cancer patients. [Update appears in Cochrane Database Syst Rev. 2016;(8):CD006911] Cochrane Database Syst Rev. 2011;(8):CD006911. PubMed
32. Kim SS, Kaplowitz S, Johnston MV. The effects of physician empathy on patient satisfaction and compliance. Eval Health Prof. 2004;27(3):237-251. PubMed
33. Tulsky JA, Arnold RM, Alexander SC, et al. Enhancing communication between oncologists and patients with a computer-based training program: a randomized trial. Ann Intern Med. 2011;155(9):593-601. PubMed
34. Bays AM, Engelberg RA, Back AL, et al. Interprofessional communication skills training for serious illness: evaluation of a small-group, simulated patient intervention. J Palliat Med. 2014;17(2):159-166. PubMed
35. Epstein RM, Duberstein PR, Fenton JJ, et al. Effect of a Patient-Centered Communication Intervention on Oncologist-Patient Communication, Quality of Life, and Health Care Utilization in Advanced Cancer: The VOICE Randomized Clinical Trial. JAMA Oncol. 2017;3(1):92-100. PubMed
36. Berkhof M, van Rijssen HJ, Schellart AJM, Anema JR, van der Beek AJ. Effective training strategies for teaching communication skills to physicians: an overview of systematic reviews. Patient Educ Couns. 2011;84(2):152-162. PubMed
1. Walker FB, Novack DH, Kaiser DL, Knight A, Oblinger P. Anxiety and depression among medical and surgical patients nearing hospital discharge. J Gen Intern Med. 1987;2(2):99-101. PubMed
2. Castillo MI, Cooke M, Macfarlane B, Aitken LM. Factors associated with anxiety in critically ill patients: A prospective observational cohort study. Int J Nurs Stud. 2016;60:225-233. PubMed
3. Anderson WG, Winters K, Auerbach AD. Patient concerns at hospital admission. Arch Intern Med. 2011;171(15):1399-1400. PubMed
4. Kuo Y-F, Sharma G, Freeman JL, Goodwin JS. Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102-1112. PubMed
5. Wachter RM, Goldman L. Zero to 50,000 - The 20th Anniversary of the Hospitalist. N Engl J Med. 2016;375(11):1009-1011. PubMed
6. Mack JW, Block SD, Nilsson M, et al. Measuring therapeutic alliance between oncologists and patients with advanced cancer: the Human Connection Scale. Cancer. 2009;115(14):3302-3311. PubMed
7. Huff NG, Nadig N, Ford DW, Cox CE. Therapeutic Alliance between the Caregivers of Critical Illness Survivors and Intensive Care Unit Clinicians. [published correction appears in Ann Am Thorac Soc. 2016;13(4):576]. Ann Am Thorac Soc. 2015;12(11):1646-1653. PubMed
8. Derksen F, Bensing J, Lagro-Janssen A. Effectiveness of empathy in general practice: a systematic review. Br J Gen Pract. 2013;63(606):e76-e84. PubMed
9. Dwamena F, Holmes-Rovner M, Gaulden CM, et al. Interventions for providers to promote a patient-centred approach in clinical consultations. Cochrane Database Syst Rev. 2012;12:CD003267. PubMed
10. Fogarty LA, Curbow BA, Wingard JR, McDonnell K, Somerfield MR. Can 40 seconds of compassion reduce patient anxiety? J Clin Oncol. 1999;17(1):371-379. PubMed
11. Roter DL, Hall JA, Kern DE, Barker LR, Cole KA, Roca RP. Improving physicians’ interviewing skills and reducing patients’ emotional distress. A randomized clinical trial. Arch Intern Med. 1995;155(17):1877-1884. PubMed
12. Stapleton RD, Engelberg RA, Wenrich MD, Goss CH, Curtis JR. Clinician statements and family satisfaction with family conferences in the intensive care unit. Crit Care Med. 2006;34(6):1679-1685. PubMed
13. Hojat M, Louis DZ, Markham FW, Wender R, Rabinowitz C, Gonnella JS. Physicians’ empathy and clinical outcomes for diabetic patients. Acad Med. 2011;86(3):359-364. PubMed
14. Anderson WG, Winters K, Arnold RM, Puntillo KA, White DB, Auerbach AD. Studying physician-patient communication in the acute care setting: the hospitalist rapport study. Patient Educ Couns. 2011;82(2):275-279. PubMed
15. Pollak KI, Arnold RM, Jeffreys AS, et al. Oncologist communication about emotion during visits with patients with advanced cancer. J Clin Oncol. 2007;25(36):5748-5752. PubMed
16. Suchman AL, Markakis K, Beckman HB, Frankel R. A model of empathic communication in the medical interview. JAMA. 1997;277(8):678-682. PubMed
17. Adams K, Cimino JEW, Arnold RM, Anderson WG. Why should I talk about emotion? Communication patterns associated with physician discussion of patient expressions of negative emotion in hospital admission encounters. Patient Educ Couns. 2012;89(1):44-50. PubMed
18. Julian LJ. Measures of anxiety: State-Trait Anxiety Inventory (STAI), Beck Anxiety Inventory (BAI), and Hospital Anxiety and Depression Scale-Anxiety (HADS-A). Arthritis Care Res (Hoboken). 2011;63 Suppl 11:S467-S472. PubMed
19. Speilberger C, Ritterband L, Sydeman S, Reheiser E, Unger K. Assessment of emotional states and personality traits: measuring psychological vital signs. In: Butcher J, editor. Clinical personality assessment: practical approaches. New York: Oxford University Press; 1995.
20. Safran DG, Kosinski M, Tarlov AR, et al. The Primary Care Assessment Survey: tests of data quality and measurement performance. Med Care. 1998;36(5):728-739. PubMed
21. Azoulay E, Pochard F, Kentish-Barnes N, et al. Risk of post-traumatic stress symptoms in family members of intensive care unit patients. Am J Respir Crit Care Med. 2005;171(9):987-994. PubMed
22. Lynn J. Perspectives on care at the close of life. Serving patients who may die soon and their families: the role of hospice and other services. JAMA. 2001;285(7):925-932. PubMed
23. Kennifer SL, Alexander SC, Pollak KI, et al. Negative emotions in cancer care: do oncologists’ responses depend on severity and type of emotion? Patient Educ Couns. 2009;76(1):51-56. PubMed
24. Butow PN, Brown RF, Cogar S, Tattersall MHN, Dunn SM. Oncologists’ reactions to cancer patients’ verbal cues. Psychooncology. 2002;11(1):47-58. PubMed
25. Levinson W, Gorawara-Bhat R, Lamb J. A study of patient clues and physician responses in primary care and surgical settings. JAMA. 2000;284(8):1021-1027. PubMed
26. Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20(1):37-46.
27. Fulop G. Anxiety disorders in the general hospital setting. Psychiatr Med. 1990;8(3):187-195. PubMed
28. Gerson S, Mistry R, Bastani R, et al. Symptoms of depression and anxiety (MHI) following acute medical/surgical hospitalization and post-discharge psychiatric diagnoses (DSM) in 839 geriatric US veterans. Int J Geriatr Psychiatry. 2004;19(12):1155-1167. PubMed
29. Kathol RG, Wenzel RP. Natural history of symptoms of depression and anxiety during inpatient treatment on general medicine wards. J Gen Intern Med. 1992;7(3):287-293. PubMed
30. Unsal A, Unaldi C, Baytemir C. Anxiety and depression levels of inpatients in the city centre of Kirşehir in Turkey. Int J Nurs Pract. 2011;17(4):411-418. PubMed
31. Bradt J, Dileo C, Grocke D, Magill L. Music interventions for improving psychological and physical outcomes in cancer patients. [Update appears in Cochrane Database Syst Rev. 2016;(8):CD006911] Cochrane Database Syst Rev. 2011;(8):CD006911. PubMed
32. Kim SS, Kaplowitz S, Johnston MV. The effects of physician empathy on patient satisfaction and compliance. Eval Health Prof. 2004;27(3):237-251. PubMed
33. Tulsky JA, Arnold RM, Alexander SC, et al. Enhancing communication between oncologists and patients with a computer-based training program: a randomized trial. Ann Intern Med. 2011;155(9):593-601. PubMed
34. Bays AM, Engelberg RA, Back AL, et al. Interprofessional communication skills training for serious illness: evaluation of a small-group, simulated patient intervention. J Palliat Med. 2014;17(2):159-166. PubMed
35. Epstein RM, Duberstein PR, Fenton JJ, et al. Effect of a Patient-Centered Communication Intervention on Oncologist-Patient Communication, Quality of Life, and Health Care Utilization in Advanced Cancer: The VOICE Randomized Clinical Trial. JAMA Oncol. 2017;3(1):92-100. PubMed
36. Berkhof M, van Rijssen HJ, Schellart AJM, Anema JR, van der Beek AJ. Effective training strategies for teaching communication skills to physicians: an overview of systematic reviews. Patient Educ Couns. 2011;84(2):152-162. PubMed
© 2017 Society of Hospital Medicine
Sound and Light Levels Are Similarly Disruptive in ICU and non-ICU Wards
The hospital environment fails to promote adequate sleep for acutely or critically ill patients. Intensive care units (ICUs) have received the most scrutiny, because critically ill patients suffer from severely fragmented sleep as well as a lack of deeper, more restorative sleep.1-4 ICU survivors frequently cite sleep deprivation, contributed to by ambient noise, as a major stressor while receiving care.5,6 Importantly, efforts to modify the ICU environment to promote sleep have been associated with reductions in delirium.7,8 However, sleep deprivation and delirium in the hospital are not limited to ICU patients.
Sleep in the non-ICU setting is also notoriously poor, with 50%-80% of patients reporting sleep as “unsound” or otherwise subjectively poor.9-11 Additionally, patients frequently ask for and/or receive pharmacological sleeping aids12 despite little evidence of efficacy13 and increasing evidence of harm.14 Here too, efforts to improve sleep seems to attenuate risk of delirium,15 which remains a substantial problem on general wards, with incidence reported as high as 20%-30%. The reasons for poor sleep in the hospital are multifactorial, but data suggest that the inpatient environment, including noise and light levels, which are measurable and modifiable entities, contribute significantly to the problem.16
The World Health Organization (WHO) recommends that nighttime baseline noise levels do not exceed 30 decibels (dB) and that nighttime noise peaks (ie, loud noises) do not exceed 40 dB17; most studies suggest that ICU and general ward rooms are above this range on average.10,18 Others have also demonstrated an association between loud noises and patients’ subjective perception of poor sleep.10,19 However, when considering clinically important noise, peak and average noise levels may not be the key factor in causing arousals from sleep. Buxton and colleagues20 found that noise quality affects arousal probability; for example, electronic alarms and conversational noise are more likely to cause awakenings compared with the opening or closing of doors and ice machines. Importantly, peak and average noise levels may also matter less for sleep than do sound level changes (SLCs), which are defined as the difference between background/baseline noise and peak noise. Using healthy subjects exposed to simulated ICU noise, Stanchina et al.21 found that SLCs >17.5 dB were more likely to cause polysomnographic arousals from sleep regardless of peak noise level. This sound pressure change of approximately 20 dB would be perceived as 4 times louder, or, as an example, would be the difference between normal conversation between 2 people (~40 dB) that is then interrupted by the start of a vacuum cleaner (~60 dB). To our knowledge, no other studies have closely examined SLCs in different hospital environments.
Ambient light also likely affects sleep quality in the hospital. The circadian rhythm system, which controls the human sleep–wake cycle as well as multiple other physiologic functions, depends on ambient light as the primary external factor for regulating the internal clock.22,23 Insufficient and inappropriately timed light exposure can desynchronize the biological clock, thereby negatively affecting sleep quality.24,25 Conversely, patients exposed to early-morning bright light may sleep better while in the hospital.16 In addition to sleep patterns, ambient light affects other aspects of patient care; for example, lower light levels in the hospital have recently been associated with higher levels of fatigue and mood disturbance.26A growing body of data has investigated the ambient environment in the ICU, but fewer studies have focused on sound and light analysis in other inpatient areas such as the general ward and telemetry floors. We examined sound and light levels in the ICU and non-ICU environment, hypothesizing that average sound levels would be higher in the ICU than on non-ICU floors but that the number of SLCs >17.5 dB would be similar. Additionally, we expected that average light levels would be higher in the ICU than on non-ICU floors.
METHODS
This was an observational study of the sound and light environment in the inpatient setting. Per our Institutional Review Board, no consent was required. Battery-operated sound-level (SDL600, Extech Instruments, Nashua, NH) and light-level (SDL400, Extech Instruments, Nashua, NH) meters were placed in 24 patient rooms in our tertiary-care adult hospital in La Jolla, CA. Recordings were obtained in randomly selected, single-patient occupied rooms that were from 3 different hospital units and included 8 general ward rooms, 8 telemetry floor rooms, and 8 ICU rooms. We recorded for approximately 24-72 hours. Depending on the geographic layout of the room, meters were placed as close to the head of the patient’s bed as possible and were generally not placed farther than 2 meters away from the patient’s head of bed; all rooms contained a window.
Sound Measurements
Sound meters measured ambient noise in dB every 2 seconds and were set for A-weighted frequency measurements. We averaged individual data points to obtain hourly averages for ICU and non-ICU rooms. For hourly sound averages, we further separated the data to compare the general ward telemetry floors (both non-ICU), the latter of which has more patient monitoring and a lower nurse-to-patient ratio compared with the general ward floor.
Data from ICU versus non-ICU rooms were analyzed for the number of sound peaks throughout the 24-hour day and for sound peak over the nighttime, defined as the number of times sound levels exceeded 65 dB, 70 dB, or 80 dB, which were averaged over 24 hours and over the nighttime (10 PM to 6 AM). We also calculated the number of average SLCs ≥17.5 dB observed over 24 hours and over the nighttime.
Light Measurements
Light meters measured luminescence in lux at a frequency of 120 seconds. We averaged individual data points to obtain hourly averages for ICU and non-ICU rooms. In addition to hourly averages, light-level data were analyzed for maximum levels throughout the day and night.
Statistical Analysis
Hourly sound-level averages between the 3 floors were evaluated using a 1-way analysis of variance (ANOVA); sound averages from the general ward and telemetry floor were also compared at each hour using a Student t test. Light-level data, sound-level peak data, as well as SLC data were also evaluated using a Student t test.
RESULTS
Sound Measurements
Examples of the raw data distribution for individual sound recordings in an ICU and non-ICU room are shown in Figure 1A and 1B. Sound-level analysis with specific average values and significance levels between ICU and non-ICU rooms (with non-ICU rooms further divided between telemetry and general ward floors for purposes of hourly averages) are shown in Table 1. The average hourly values in all 3 locations were always above the 30-35 dB level (nighttime and daytime, respectively) recommended by the WHO (Figure 1C). A 1-way ANOVA analysis revealed significant differences between the 3 floors at all time points except for 10 AM. An analysis of the means at each time point between the telemetry floor and the general ward floor showed that the telemetry floor had significantly higher sound averages compared with the general ward floor at 10 PM, 11 PM, and 12 AM. Sound levels dropped during the nighttime on both non-ICU wards but remained fairly constant throughout the day and night in the ICU.
Importantly, despite average and peak sound levels showing that the ICU environment is louder overall, there were an equivalent number of SLCs ≥ 17.5 dB in the ICU and on non-ICU floors. The number of SLCs ≥ 17.5 dB is not statistically different when comparing ICU and non-ICU rooms either averaged over 24 hours or averaged over the nighttime (Figure 1E).
Light Measurements
Examples of light levels over a 24-hour period in an ICU and non-ICU room are shown in Figure 2A and 2B, respectively. Maximum average light levels (reported here as average value ± standard deviation to demonstrate variability within the data) in the ICU were 169.7 ± 127.1 lux and occurred at 1 PM, while maximum average light levels in the non-ICU rooms were 213.5 ± 341.6 lux and occurred at 5 PM (Figure 2C). Average light levels in the morning hours remained low and ranged from 15.9 ± 12.7 lux to 38.9 ± 43.4 lux in the ICU and from 22.3 ± 17.5 lux to 100.7 ± 92.0 lux on the non-ICU floors. The maximum measured level from any of the recordings was 2530 lux and occurred in a general ward room in the 5 PM hour. Overall, light averages remained low, but this particular room had light levels that were significantly higher than the others. A t test analysis of the hourly averages revealed only 1 time point of significant difference between the 2 floors; at 7 AM, the general ward floor had a higher lux level of 49.9 ± 27.5 versus 19.2 ± 10.7 in the ICU (P = 0.038). Otherwise, there were no differences between light levels in ICU rooms versus non-ICU rooms. Evaluation of the data revealed a substantial amount of variability in light levels throughout the daytime hours. Light levels during the nighttime remained low and were not significantly different between the 2 groups.
DISCUSSION
To our knowledge, this is the first study to directly compare the ICU and non-ICU environment for its potential impact on sleep and circadian alignment. Our study adds to the literature with several novel findings. First, average sound levels on non-ICU wards are lower than in the ICU. Second, although quieter on average, SLCs >17.5 dB occurred an equivalent number of times for both the ICU and non-ICU wards. Third, average daytime light levels in both the ICU and non-ICU environment are low. Lastly, peak light levels for both ICU and non-ICU wards occur later in the day instead of in the morning. All of the above have potential impact for optimizing the ward environment to better aid in sleep for patients.
Sound-Level Findings
Data on sound levels for non-ICU floors are limited but mostly consistent with our finding
Average and peak sound levels contribute to the ambient noise experienced by patients but may not be the source of sleep disruptions. Using polysomnography in healthy subjects exposed to recordings of ICU noise, Stanchina et al.21 showed that SLCs from baseline and not peak sound levels determined whether a subject was aroused from sleep by sound. Accordingly, they also found that increasing baseline sound levels by using white noise reduced the number of arousals that subjects experienced. To our knowledge, other studies have not quantified and compared SLCs in the ICU and non-ICU environments. Our data show that patients on non-ICU floors experience at least the same number of SLCs, and thereby the same potential for arousals from sleep, when compared with ICU patients. The higher baseline level of noise in the ICU likely explains the relatively lower number of SLCs when compared with the non-ICU floors. Although decreasing overall noise to promote sleep in the hospital seems like the obvious solution, the treatment for noise pollution in the hospital may actually be more background noise, not less.
Recent studies support the clinical implications of our findings. First, decreasing overall noise levels is difficult to accomplish.29 Second, recent studies utilized white noise in different hospital settings with some success in improving patients’ subjective sleep quality, although more studies using objective data measurements are needed to further understand the impact of white noise on sleep in hospitalized patients.30,31 Third, efforts at reducing interruptions—which likely will decrease the number of SLCs—such as clustering nursing care or reducing intermittent alarms may be more beneficial in improving sleep than efforts at decreasing average sound levels. For example, Bartick et al. reduced the number of patient interruptions at night by eliminating routine vital signs and clustering medication administration. Although they included other interventions as well, we note that this approach likely reduced SLCs and was associated with a reduction in the use of sedative medications.32 Ultimately, our data show that a focus on reducing SLCs will be one necessary component of a multipronged solution to improving inpatient sleep.33
Light-Level Findings
Because of its effect on circadian rhythms, the daily light-dark cycle has a powerful impact on human physiology and behavior, which includes sleep.34 Little is understood about how light affects sleep and other circadian-related functions in general ward patients, as it is not commonly measured. Our findings suggest that patients admitted to the hospital are exposed to light levels and patterns that may not optimally promote wake and sleep. Encouragingly, we did not find excessive average light levels during the nighttime in either ICU or non-ICU environment of our hospital, although others have described intrusive nighttime light in the hospital setting.35,36 Even short bursts of low or moderate light during the nighttime can cause circadian phase delay,37 and efforts to maintain darkness in patient rooms at night should continue.
Our measurements show that average daytime light levels did not exceed 250 lux, which corresponds to low, office-level lighting, while the brightest average light levels occurred in the afternoon for both environments. These levels are consistent with other reports26,35,36 as is the light-level variability noted throughout the day (which is not unexpected given room positioning, patient preference, curtains, etc). The level and amount of daytime light needed to maintain circadian rhythms in humans is still unknown.38 Brighter light is generally more effective at influencing the circadian pacemaker in a dose-dependent manner.39 Although entrainment (synchronization of the body’s biological rhythm with environmental cues such as ambient light) of the human circadian rhythm has been shown with low light levels (eg, <100 lux), these studies included healthy volunteers in a carefully controlled, constant, routine environment.23 How these data apply to acutely ill subjects in the hospital environment is not clear. We note that low to moderate levels of light (50-1000 lux) are less effective for entrainment of the circadian rhythm in older people (age >65 years, the majority of our admissions) compared with younger people. Thus, older, hospitalized patients may require greater light levels for regulation of the sleep-wake cycle.40 These data are important when designing interventions to improve light for and maintain circadian rhythms in hospitalized patients. For example, Simons et al. found that dynamic light-application therapy, which achieved a maximum average lux level of <800 lux, did not reduce rates of delirium in critically ill patients (mean age ~65). One interpretation of these results, though there are many others, is that the light levels achieved were not high enough to influence circadian timing in hospitalized, mostly elderly patients. The physiological impact of light on the circadian rhythm in hospitalized patients still remains to be measured.
LIMITATIONS
Our study does have a few limitations. We did not assess sound quality, which is another determinant of arousal potential.20 Also, a shorter measurement interval might be useful in determining sharper sound increases. It may also be important to consider A- versus C-weighted measurements of sound levels, as A-weighted measurements usually reflect higher-frequency sound while C-weighted measurements usually reflect low-frequency noise18; we obtained only A-weighted measurements in our study. However, A-weighted measurements are generally considered more reflective of what the human ear considers noise and are used more standardly than C-weighted measurements.
Regarding light measurements, we recorded from rooms facing different cardinal directions and during different times of the year, which likely contributed to some of the variability in the daytime light levels on both floors. Additionally, light levels were not measured directly at the patient’s eye level. However, given that overhead fluorescent lighting was the primary source of lighting, it is doubtful that we substantially underestimated optic-nerve light levels. In the future, it may also be important to measure the different wavelengths of lights, as blue light may have a greater impact on sleep than other wavelengths.41 Although our findings align with others’, we note that this was a single-center study, which could limit the generalizability of our findings given inter-hospital variations in patient volume, interior layout and structure, and geographic location.
CONCLUSIONS
Overall, our study suggests that the light and sound environment for sleep in the inpatient setting, including both the ICU and non-ICU wards, has multiple areas for improvement. Our data also suggest specific directions for future clinical efforts at improvement. For example, efforts to decrease average sound levels may worsen sleep fragmentation. Similarly, more light during the day may be more helpful than further attempts to limit light during the night.
Disclosure
This research was funded in part by a NIH/NCATS flagship Clinical and Translational Science Award Grant (5KL2TR001112). None of the authors report any conflict of interest, financial or otherwise, in the preparation of this article.
1. Freedman NS, Gazendam J, Levan L, Pack AI, Schwab RJ. Abnormal sleep/wake
cycles and the effect of environmental noise on sleep disruption in the intensive
care unit. Am J Respir Crit Care Med. 2001;163(2):451-457. PubMed
2. Watson PL, Pandharipande P, Gehlbach BK, et al. Atypical sleep in ventilated
patients: empirical electroencephalography findings and the path toward revised ICU sleep scoring criteria. Crit Care Med. 2013;41(8):1958-1967. PubMed
3. Gehlbach BK, Chapotot F, Leproult R, et al. Temporal disorganization of circadian rhythmicity and sleep-wake regulation in mechanically ventilated patients receiving continuous intravenous sedation. Sleep. 2012;35(8):1105-1114. PubMed
4. Elliott R, McKinley S, Cistulli P, Fien M. Characterisation of sleep in intensive care using 24-hour polysomnography: an observational study. Crit Care. 2013;17(2):R46. PubMed
5. Novaes MA, Aronovich A, Ferraz MB, Knobel E. Stressors in ICU: patients’ evaluation. Intensive Care Med. 1997;23(12):1282-1285. PubMed
6. Tembo AC, Parker V, Higgins I. The experience of sleep deprivation in intensive care patients: findings from a larger hermeneutic phenomenological study. Intensive Crit Care Nurs. 2013;29(6):310-316. PubMed
7. Kamdar BB, Yang J, King LM, et al. Developing, implementing, and evaluating a multifaceted quality improvement intervention to promote sleep in an ICU. Am J Med Qual. 2014;29(6):546-554. PubMed
8. Patel J, Baldwin J, Bunting P, Laha S. The effect of a multicomponent multidisciplinary bundle of interventions on sleep and delirium in medical and surgical intensive care patients. Anaesthesia. 2014;69(6):540-549. PubMed
9. Manian FA, Manian CJ. Sleep quality in adult hospitalized patients with infection: an observational study. Am J Med Sci. 2015;349(1):56-60. PubMed
10. Park MJ, Yoo JH, Cho BW, Kim KT, Jeong WC, Ha M. Noise in hospital rooms and sleep disturbance in hospitalized medical patients. Environ Health Toxicol. 2014;29:e2014006. PubMed
11. Dobing S, Frolova N, McAlister F, Ringrose J. Sleep quality and factors influencing self-reported sleep duration and quality in the general internal medicine inpatient population. PLoS One. 2016;11(6):e0156735. PubMed
12. Gillis CM, Poyant JO, Degrado JR, Ye L, Anger KE, Owens RL. Inpatient pharmacological
sleep aid utilization is common at a tertiary medical center. J Hosp Med. 2014;9(10):652-657. PubMed
13. Krenk L, Jennum P, Kehlet H. Postoperative sleep disturbances after zolpidem treatment in fast-track hip and knee replacement. J Clin Sleep Med. 2014;10(3):321-326. PubMed
14. Kolla BP, Lovely JK, Mansukhani MP, Morgenthaler TI. Zolpidem is independently
associated with increased risk of inpatient falls. J Hosp Med. 2013;8(1):1-6. PubMed
15. Inouye SK, Bogardus ST Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669-676. PubMed
16. Bano M, Chiaromanni F, Corrias M, et al. The influence of environmental factors on sleep quality in hospitalized medical patients. Front Neurol. 2014;5:267. PubMed
17. Berglund BLTSD. Guidelines for Community Noise. World Health Organization. 1999.
18. Knauert M, Jeon S, Murphy TE, Yaggi HK, Pisani MA, Redeker NS. Comparing average levels and peak occurrence of overnight sound in the medical intensive care unit on A-weighted and C-weighted decibel scales. J Crit Care. 2016;36:1-7. PubMed
19. Yoder JC, Staisiunas PG, Meltzer DO, Knutson KL, Arora VM. Noise and sleep among adult medical inpatients: far from a quiet night. Arch Intern Med. 2012;172(1):68-70. PubMed
20. Buxton OM, Ellenbogen JM, Wang W, et al. Sleep disruption due to hospital noises: a prospective evaluation. Ann Intern Med. 2012;157(3):170-179. PubMed
21. Stanchina ML, Abu-Hijleh M, Chaudhry BK, Carlisle CC, Millman RP. The influence of white noise on sleep in subjects exposed to ICU noise. Sleep Med. 2005;6(5):423-428. PubMed
22. Czeisler CA, Allan JS, Strogatz SH, et al. Bright light resets the human circadian pacemaker independent of the timing of the sleep-wake cycle. Science. 1986;233(4764):667-671. PubMed
23. Duffy JF, Czeisler CA. Effect of light on human circadian physiology. Sleep Med Clin. 2009;4(2):165-177. PubMed
24. Lewy AJ, Wehr TA, Goodwin FK, Newsome DA, Markey SP. Light suppresses melatonin secretion in humans. Science. 1980;210(4475):1267-1269. PubMed
25. Zeitzer JM, Dijk DJ, Kronauer R, Brown E, Czeisler C. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol. 2000;526:695-702. PubMed
26. Bernhofer EI, Higgins PA, Daly BJ, Burant CJ, Hornick TR. Hospital lighting and its association with sleep, mood and pain in medical inpatients. J Adv Nurs. 2014;70(5):1164-1173. PubMed
27. Darbyshire JL, Young JD. An investigation of sound levels on intensive care units with reference to the WHO guidelines. Crit Care. 2013;17(5):R187. PubMed
28. Gillis S. Pharmacologic treatment of depression during pregnancy. J Midwifery Womens Health. 2000;45(4):357-359. PubMed
29. Tainter CR, Levine AR, Quraishi SA, et al. Noise levels in surgical ICUs are consistently above recommended standards. Crit Care Med. 2016;44(1):147-152. PubMed
30. Farrehi PM, Clore KR, Scott JR, Vanini G, Clauw DJ. Efficacy of Sleep Tool Education During Hospitalization: A Randomized Controlled Trial. Am J Med. 2016;129(12):1329.e9-1329.e17. PubMed
31. Farokhnezhad Afshar P, Bahramnezhad F, Asgari P, Shiri M. Effect of white noise on sleep in patients admitted to a coronary care. J Caring Sci. 2016;5(2):103-109. PubMed
32. Bartick MC, Thai X, Schmidt T, Altaye A, Solet JM. Decrease in as-needed sedative use by limiting nighttime sleep disruptions from hospital staff. J Hosp Med. 2010;5(3):E20-E24. PubMed
33. Tamrat R, Huynh-Le MP, Goyal M. Non-pharmacologic interventions to improve the sleep of hospitalized patients: a systematic review. J Gen Intern Med. 2014;29(5):788-795. PubMed
34. Dijk DJ, Archer SN. Light, sleep, and circadian rhythms: together again. PLoS Biol. 2009;7(6):e1000145. PubMed
35. Verceles AC, Liu X, Terrin ML, et al. Ambient light levels and critical care outcomes. J Crit Care. 2013;28(1):110.e1-110.e8. PubMed
36. Hu RF, Hegadoren KM, Wang XY, Jiang XY. An investigation of light and sound levels on intensive care units in China. Aust Crit Care. 2016;29(2):62-67. PubMed
37. Zeitzer JM, Ruby NF, Fisicaro RA, Heller HC. Response of the human circadian system to millisecond flashes of light. PLoS One. 2011;6(7):e22078. PubMed
38. Duffy JF, Wright KP, Jr. Entrainment of the human circadian system by light. J Biol Rhythms. 2005;20(4):326-338. PubMed
39. Wright KP Jr, Gronfier C, Duffy JF, Czeisler CA. Intrinsic period and light intensity determine the phase relationship between melatonin and sleep in humans. J Biol Rhythms. 2005;20(2):168-177. PubMed
40. Duffy JF, Zeitzer JM, Czeisler CA. Decreased sensitivity to phase-delaying effects of moderate intensity light in older subjects. Neurobiol Aging. 2007;28(5):799-807. PubMed
41. Figueiro MG, Plitnick BA, Lok A, et al. Tailored lighting intervention improves measures of sleep, depression, and agitation in persons with Alzheimer’s disease and related dementia living in long-term care facilities. Clin Interv Aging. 2014;9:1527-1537. PubMed
The hospital environment fails to promote adequate sleep for acutely or critically ill patients. Intensive care units (ICUs) have received the most scrutiny, because critically ill patients suffer from severely fragmented sleep as well as a lack of deeper, more restorative sleep.1-4 ICU survivors frequently cite sleep deprivation, contributed to by ambient noise, as a major stressor while receiving care.5,6 Importantly, efforts to modify the ICU environment to promote sleep have been associated with reductions in delirium.7,8 However, sleep deprivation and delirium in the hospital are not limited to ICU patients.
Sleep in the non-ICU setting is also notoriously poor, with 50%-80% of patients reporting sleep as “unsound” or otherwise subjectively poor.9-11 Additionally, patients frequently ask for and/or receive pharmacological sleeping aids12 despite little evidence of efficacy13 and increasing evidence of harm.14 Here too, efforts to improve sleep seems to attenuate risk of delirium,15 which remains a substantial problem on general wards, with incidence reported as high as 20%-30%. The reasons for poor sleep in the hospital are multifactorial, but data suggest that the inpatient environment, including noise and light levels, which are measurable and modifiable entities, contribute significantly to the problem.16
The World Health Organization (WHO) recommends that nighttime baseline noise levels do not exceed 30 decibels (dB) and that nighttime noise peaks (ie, loud noises) do not exceed 40 dB17; most studies suggest that ICU and general ward rooms are above this range on average.10,18 Others have also demonstrated an association between loud noises and patients’ subjective perception of poor sleep.10,19 However, when considering clinically important noise, peak and average noise levels may not be the key factor in causing arousals from sleep. Buxton and colleagues20 found that noise quality affects arousal probability; for example, electronic alarms and conversational noise are more likely to cause awakenings compared with the opening or closing of doors and ice machines. Importantly, peak and average noise levels may also matter less for sleep than do sound level changes (SLCs), which are defined as the difference between background/baseline noise and peak noise. Using healthy subjects exposed to simulated ICU noise, Stanchina et al.21 found that SLCs >17.5 dB were more likely to cause polysomnographic arousals from sleep regardless of peak noise level. This sound pressure change of approximately 20 dB would be perceived as 4 times louder, or, as an example, would be the difference between normal conversation between 2 people (~40 dB) that is then interrupted by the start of a vacuum cleaner (~60 dB). To our knowledge, no other studies have closely examined SLCs in different hospital environments.
Ambient light also likely affects sleep quality in the hospital. The circadian rhythm system, which controls the human sleep–wake cycle as well as multiple other physiologic functions, depends on ambient light as the primary external factor for regulating the internal clock.22,23 Insufficient and inappropriately timed light exposure can desynchronize the biological clock, thereby negatively affecting sleep quality.24,25 Conversely, patients exposed to early-morning bright light may sleep better while in the hospital.16 In addition to sleep patterns, ambient light affects other aspects of patient care; for example, lower light levels in the hospital have recently been associated with higher levels of fatigue and mood disturbance.26A growing body of data has investigated the ambient environment in the ICU, but fewer studies have focused on sound and light analysis in other inpatient areas such as the general ward and telemetry floors. We examined sound and light levels in the ICU and non-ICU environment, hypothesizing that average sound levels would be higher in the ICU than on non-ICU floors but that the number of SLCs >17.5 dB would be similar. Additionally, we expected that average light levels would be higher in the ICU than on non-ICU floors.
METHODS
This was an observational study of the sound and light environment in the inpatient setting. Per our Institutional Review Board, no consent was required. Battery-operated sound-level (SDL600, Extech Instruments, Nashua, NH) and light-level (SDL400, Extech Instruments, Nashua, NH) meters were placed in 24 patient rooms in our tertiary-care adult hospital in La Jolla, CA. Recordings were obtained in randomly selected, single-patient occupied rooms that were from 3 different hospital units and included 8 general ward rooms, 8 telemetry floor rooms, and 8 ICU rooms. We recorded for approximately 24-72 hours. Depending on the geographic layout of the room, meters were placed as close to the head of the patient’s bed as possible and were generally not placed farther than 2 meters away from the patient’s head of bed; all rooms contained a window.
Sound Measurements
Sound meters measured ambient noise in dB every 2 seconds and were set for A-weighted frequency measurements. We averaged individual data points to obtain hourly averages for ICU and non-ICU rooms. For hourly sound averages, we further separated the data to compare the general ward telemetry floors (both non-ICU), the latter of which has more patient monitoring and a lower nurse-to-patient ratio compared with the general ward floor.
Data from ICU versus non-ICU rooms were analyzed for the number of sound peaks throughout the 24-hour day and for sound peak over the nighttime, defined as the number of times sound levels exceeded 65 dB, 70 dB, or 80 dB, which were averaged over 24 hours and over the nighttime (10 PM to 6 AM). We also calculated the number of average SLCs ≥17.5 dB observed over 24 hours and over the nighttime.
Light Measurements
Light meters measured luminescence in lux at a frequency of 120 seconds. We averaged individual data points to obtain hourly averages for ICU and non-ICU rooms. In addition to hourly averages, light-level data were analyzed for maximum levels throughout the day and night.
Statistical Analysis
Hourly sound-level averages between the 3 floors were evaluated using a 1-way analysis of variance (ANOVA); sound averages from the general ward and telemetry floor were also compared at each hour using a Student t test. Light-level data, sound-level peak data, as well as SLC data were also evaluated using a Student t test.
RESULTS
Sound Measurements
Examples of the raw data distribution for individual sound recordings in an ICU and non-ICU room are shown in Figure 1A and 1B. Sound-level analysis with specific average values and significance levels between ICU and non-ICU rooms (with non-ICU rooms further divided between telemetry and general ward floors for purposes of hourly averages) are shown in Table 1. The average hourly values in all 3 locations were always above the 30-35 dB level (nighttime and daytime, respectively) recommended by the WHO (Figure 1C). A 1-way ANOVA analysis revealed significant differences between the 3 floors at all time points except for 10 AM. An analysis of the means at each time point between the telemetry floor and the general ward floor showed that the telemetry floor had significantly higher sound averages compared with the general ward floor at 10 PM, 11 PM, and 12 AM. Sound levels dropped during the nighttime on both non-ICU wards but remained fairly constant throughout the day and night in the ICU.
Importantly, despite average and peak sound levels showing that the ICU environment is louder overall, there were an equivalent number of SLCs ≥ 17.5 dB in the ICU and on non-ICU floors. The number of SLCs ≥ 17.5 dB is not statistically different when comparing ICU and non-ICU rooms either averaged over 24 hours or averaged over the nighttime (Figure 1E).
Light Measurements
Examples of light levels over a 24-hour period in an ICU and non-ICU room are shown in Figure 2A and 2B, respectively. Maximum average light levels (reported here as average value ± standard deviation to demonstrate variability within the data) in the ICU were 169.7 ± 127.1 lux and occurred at 1 PM, while maximum average light levels in the non-ICU rooms were 213.5 ± 341.6 lux and occurred at 5 PM (Figure 2C). Average light levels in the morning hours remained low and ranged from 15.9 ± 12.7 lux to 38.9 ± 43.4 lux in the ICU and from 22.3 ± 17.5 lux to 100.7 ± 92.0 lux on the non-ICU floors. The maximum measured level from any of the recordings was 2530 lux and occurred in a general ward room in the 5 PM hour. Overall, light averages remained low, but this particular room had light levels that were significantly higher than the others. A t test analysis of the hourly averages revealed only 1 time point of significant difference between the 2 floors; at 7 AM, the general ward floor had a higher lux level of 49.9 ± 27.5 versus 19.2 ± 10.7 in the ICU (P = 0.038). Otherwise, there were no differences between light levels in ICU rooms versus non-ICU rooms. Evaluation of the data revealed a substantial amount of variability in light levels throughout the daytime hours. Light levels during the nighttime remained low and were not significantly different between the 2 groups.
DISCUSSION
To our knowledge, this is the first study to directly compare the ICU and non-ICU environment for its potential impact on sleep and circadian alignment. Our study adds to the literature with several novel findings. First, average sound levels on non-ICU wards are lower than in the ICU. Second, although quieter on average, SLCs >17.5 dB occurred an equivalent number of times for both the ICU and non-ICU wards. Third, average daytime light levels in both the ICU and non-ICU environment are low. Lastly, peak light levels for both ICU and non-ICU wards occur later in the day instead of in the morning. All of the above have potential impact for optimizing the ward environment to better aid in sleep for patients.
Sound-Level Findings
Data on sound levels for non-ICU floors are limited but mostly consistent with our finding
Average and peak sound levels contribute to the ambient noise experienced by patients but may not be the source of sleep disruptions. Using polysomnography in healthy subjects exposed to recordings of ICU noise, Stanchina et al.21 showed that SLCs from baseline and not peak sound levels determined whether a subject was aroused from sleep by sound. Accordingly, they also found that increasing baseline sound levels by using white noise reduced the number of arousals that subjects experienced. To our knowledge, other studies have not quantified and compared SLCs in the ICU and non-ICU environments. Our data show that patients on non-ICU floors experience at least the same number of SLCs, and thereby the same potential for arousals from sleep, when compared with ICU patients. The higher baseline level of noise in the ICU likely explains the relatively lower number of SLCs when compared with the non-ICU floors. Although decreasing overall noise to promote sleep in the hospital seems like the obvious solution, the treatment for noise pollution in the hospital may actually be more background noise, not less.
Recent studies support the clinical implications of our findings. First, decreasing overall noise levels is difficult to accomplish.29 Second, recent studies utilized white noise in different hospital settings with some success in improving patients’ subjective sleep quality, although more studies using objective data measurements are needed to further understand the impact of white noise on sleep in hospitalized patients.30,31 Third, efforts at reducing interruptions—which likely will decrease the number of SLCs—such as clustering nursing care or reducing intermittent alarms may be more beneficial in improving sleep than efforts at decreasing average sound levels. For example, Bartick et al. reduced the number of patient interruptions at night by eliminating routine vital signs and clustering medication administration. Although they included other interventions as well, we note that this approach likely reduced SLCs and was associated with a reduction in the use of sedative medications.32 Ultimately, our data show that a focus on reducing SLCs will be one necessary component of a multipronged solution to improving inpatient sleep.33
Light-Level Findings
Because of its effect on circadian rhythms, the daily light-dark cycle has a powerful impact on human physiology and behavior, which includes sleep.34 Little is understood about how light affects sleep and other circadian-related functions in general ward patients, as it is not commonly measured. Our findings suggest that patients admitted to the hospital are exposed to light levels and patterns that may not optimally promote wake and sleep. Encouragingly, we did not find excessive average light levels during the nighttime in either ICU or non-ICU environment of our hospital, although others have described intrusive nighttime light in the hospital setting.35,36 Even short bursts of low or moderate light during the nighttime can cause circadian phase delay,37 and efforts to maintain darkness in patient rooms at night should continue.
Our measurements show that average daytime light levels did not exceed 250 lux, which corresponds to low, office-level lighting, while the brightest average light levels occurred in the afternoon for both environments. These levels are consistent with other reports26,35,36 as is the light-level variability noted throughout the day (which is not unexpected given room positioning, patient preference, curtains, etc). The level and amount of daytime light needed to maintain circadian rhythms in humans is still unknown.38 Brighter light is generally more effective at influencing the circadian pacemaker in a dose-dependent manner.39 Although entrainment (synchronization of the body’s biological rhythm with environmental cues such as ambient light) of the human circadian rhythm has been shown with low light levels (eg, <100 lux), these studies included healthy volunteers in a carefully controlled, constant, routine environment.23 How these data apply to acutely ill subjects in the hospital environment is not clear. We note that low to moderate levels of light (50-1000 lux) are less effective for entrainment of the circadian rhythm in older people (age >65 years, the majority of our admissions) compared with younger people. Thus, older, hospitalized patients may require greater light levels for regulation of the sleep-wake cycle.40 These data are important when designing interventions to improve light for and maintain circadian rhythms in hospitalized patients. For example, Simons et al. found that dynamic light-application therapy, which achieved a maximum average lux level of <800 lux, did not reduce rates of delirium in critically ill patients (mean age ~65). One interpretation of these results, though there are many others, is that the light levels achieved were not high enough to influence circadian timing in hospitalized, mostly elderly patients. The physiological impact of light on the circadian rhythm in hospitalized patients still remains to be measured.
LIMITATIONS
Our study does have a few limitations. We did not assess sound quality, which is another determinant of arousal potential.20 Also, a shorter measurement interval might be useful in determining sharper sound increases. It may also be important to consider A- versus C-weighted measurements of sound levels, as A-weighted measurements usually reflect higher-frequency sound while C-weighted measurements usually reflect low-frequency noise18; we obtained only A-weighted measurements in our study. However, A-weighted measurements are generally considered more reflective of what the human ear considers noise and are used more standardly than C-weighted measurements.
Regarding light measurements, we recorded from rooms facing different cardinal directions and during different times of the year, which likely contributed to some of the variability in the daytime light levels on both floors. Additionally, light levels were not measured directly at the patient’s eye level. However, given that overhead fluorescent lighting was the primary source of lighting, it is doubtful that we substantially underestimated optic-nerve light levels. In the future, it may also be important to measure the different wavelengths of lights, as blue light may have a greater impact on sleep than other wavelengths.41 Although our findings align with others’, we note that this was a single-center study, which could limit the generalizability of our findings given inter-hospital variations in patient volume, interior layout and structure, and geographic location.
CONCLUSIONS
Overall, our study suggests that the light and sound environment for sleep in the inpatient setting, including both the ICU and non-ICU wards, has multiple areas for improvement. Our data also suggest specific directions for future clinical efforts at improvement. For example, efforts to decrease average sound levels may worsen sleep fragmentation. Similarly, more light during the day may be more helpful than further attempts to limit light during the night.
Disclosure
This research was funded in part by a NIH/NCATS flagship Clinical and Translational Science Award Grant (5KL2TR001112). None of the authors report any conflict of interest, financial or otherwise, in the preparation of this article.
The hospital environment fails to promote adequate sleep for acutely or critically ill patients. Intensive care units (ICUs) have received the most scrutiny, because critically ill patients suffer from severely fragmented sleep as well as a lack of deeper, more restorative sleep.1-4 ICU survivors frequently cite sleep deprivation, contributed to by ambient noise, as a major stressor while receiving care.5,6 Importantly, efforts to modify the ICU environment to promote sleep have been associated with reductions in delirium.7,8 However, sleep deprivation and delirium in the hospital are not limited to ICU patients.
Sleep in the non-ICU setting is also notoriously poor, with 50%-80% of patients reporting sleep as “unsound” or otherwise subjectively poor.9-11 Additionally, patients frequently ask for and/or receive pharmacological sleeping aids12 despite little evidence of efficacy13 and increasing evidence of harm.14 Here too, efforts to improve sleep seems to attenuate risk of delirium,15 which remains a substantial problem on general wards, with incidence reported as high as 20%-30%. The reasons for poor sleep in the hospital are multifactorial, but data suggest that the inpatient environment, including noise and light levels, which are measurable and modifiable entities, contribute significantly to the problem.16
The World Health Organization (WHO) recommends that nighttime baseline noise levels do not exceed 30 decibels (dB) and that nighttime noise peaks (ie, loud noises) do not exceed 40 dB17; most studies suggest that ICU and general ward rooms are above this range on average.10,18 Others have also demonstrated an association between loud noises and patients’ subjective perception of poor sleep.10,19 However, when considering clinically important noise, peak and average noise levels may not be the key factor in causing arousals from sleep. Buxton and colleagues20 found that noise quality affects arousal probability; for example, electronic alarms and conversational noise are more likely to cause awakenings compared with the opening or closing of doors and ice machines. Importantly, peak and average noise levels may also matter less for sleep than do sound level changes (SLCs), which are defined as the difference between background/baseline noise and peak noise. Using healthy subjects exposed to simulated ICU noise, Stanchina et al.21 found that SLCs >17.5 dB were more likely to cause polysomnographic arousals from sleep regardless of peak noise level. This sound pressure change of approximately 20 dB would be perceived as 4 times louder, or, as an example, would be the difference between normal conversation between 2 people (~40 dB) that is then interrupted by the start of a vacuum cleaner (~60 dB). To our knowledge, no other studies have closely examined SLCs in different hospital environments.
Ambient light also likely affects sleep quality in the hospital. The circadian rhythm system, which controls the human sleep–wake cycle as well as multiple other physiologic functions, depends on ambient light as the primary external factor for regulating the internal clock.22,23 Insufficient and inappropriately timed light exposure can desynchronize the biological clock, thereby negatively affecting sleep quality.24,25 Conversely, patients exposed to early-morning bright light may sleep better while in the hospital.16 In addition to sleep patterns, ambient light affects other aspects of patient care; for example, lower light levels in the hospital have recently been associated with higher levels of fatigue and mood disturbance.26A growing body of data has investigated the ambient environment in the ICU, but fewer studies have focused on sound and light analysis in other inpatient areas such as the general ward and telemetry floors. We examined sound and light levels in the ICU and non-ICU environment, hypothesizing that average sound levels would be higher in the ICU than on non-ICU floors but that the number of SLCs >17.5 dB would be similar. Additionally, we expected that average light levels would be higher in the ICU than on non-ICU floors.
METHODS
This was an observational study of the sound and light environment in the inpatient setting. Per our Institutional Review Board, no consent was required. Battery-operated sound-level (SDL600, Extech Instruments, Nashua, NH) and light-level (SDL400, Extech Instruments, Nashua, NH) meters were placed in 24 patient rooms in our tertiary-care adult hospital in La Jolla, CA. Recordings were obtained in randomly selected, single-patient occupied rooms that were from 3 different hospital units and included 8 general ward rooms, 8 telemetry floor rooms, and 8 ICU rooms. We recorded for approximately 24-72 hours. Depending on the geographic layout of the room, meters were placed as close to the head of the patient’s bed as possible and were generally not placed farther than 2 meters away from the patient’s head of bed; all rooms contained a window.
Sound Measurements
Sound meters measured ambient noise in dB every 2 seconds and were set for A-weighted frequency measurements. We averaged individual data points to obtain hourly averages for ICU and non-ICU rooms. For hourly sound averages, we further separated the data to compare the general ward telemetry floors (both non-ICU), the latter of which has more patient monitoring and a lower nurse-to-patient ratio compared with the general ward floor.
Data from ICU versus non-ICU rooms were analyzed for the number of sound peaks throughout the 24-hour day and for sound peak over the nighttime, defined as the number of times sound levels exceeded 65 dB, 70 dB, or 80 dB, which were averaged over 24 hours and over the nighttime (10 PM to 6 AM). We also calculated the number of average SLCs ≥17.5 dB observed over 24 hours and over the nighttime.
Light Measurements
Light meters measured luminescence in lux at a frequency of 120 seconds. We averaged individual data points to obtain hourly averages for ICU and non-ICU rooms. In addition to hourly averages, light-level data were analyzed for maximum levels throughout the day and night.
Statistical Analysis
Hourly sound-level averages between the 3 floors were evaluated using a 1-way analysis of variance (ANOVA); sound averages from the general ward and telemetry floor were also compared at each hour using a Student t test. Light-level data, sound-level peak data, as well as SLC data were also evaluated using a Student t test.
RESULTS
Sound Measurements
Examples of the raw data distribution for individual sound recordings in an ICU and non-ICU room are shown in Figure 1A and 1B. Sound-level analysis with specific average values and significance levels between ICU and non-ICU rooms (with non-ICU rooms further divided between telemetry and general ward floors for purposes of hourly averages) are shown in Table 1. The average hourly values in all 3 locations were always above the 30-35 dB level (nighttime and daytime, respectively) recommended by the WHO (Figure 1C). A 1-way ANOVA analysis revealed significant differences between the 3 floors at all time points except for 10 AM. An analysis of the means at each time point between the telemetry floor and the general ward floor showed that the telemetry floor had significantly higher sound averages compared with the general ward floor at 10 PM, 11 PM, and 12 AM. Sound levels dropped during the nighttime on both non-ICU wards but remained fairly constant throughout the day and night in the ICU.
Importantly, despite average and peak sound levels showing that the ICU environment is louder overall, there were an equivalent number of SLCs ≥ 17.5 dB in the ICU and on non-ICU floors. The number of SLCs ≥ 17.5 dB is not statistically different when comparing ICU and non-ICU rooms either averaged over 24 hours or averaged over the nighttime (Figure 1E).
Light Measurements
Examples of light levels over a 24-hour period in an ICU and non-ICU room are shown in Figure 2A and 2B, respectively. Maximum average light levels (reported here as average value ± standard deviation to demonstrate variability within the data) in the ICU were 169.7 ± 127.1 lux and occurred at 1 PM, while maximum average light levels in the non-ICU rooms were 213.5 ± 341.6 lux and occurred at 5 PM (Figure 2C). Average light levels in the morning hours remained low and ranged from 15.9 ± 12.7 lux to 38.9 ± 43.4 lux in the ICU and from 22.3 ± 17.5 lux to 100.7 ± 92.0 lux on the non-ICU floors. The maximum measured level from any of the recordings was 2530 lux and occurred in a general ward room in the 5 PM hour. Overall, light averages remained low, but this particular room had light levels that were significantly higher than the others. A t test analysis of the hourly averages revealed only 1 time point of significant difference between the 2 floors; at 7 AM, the general ward floor had a higher lux level of 49.9 ± 27.5 versus 19.2 ± 10.7 in the ICU (P = 0.038). Otherwise, there were no differences between light levels in ICU rooms versus non-ICU rooms. Evaluation of the data revealed a substantial amount of variability in light levels throughout the daytime hours. Light levels during the nighttime remained low and were not significantly different between the 2 groups.
DISCUSSION
To our knowledge, this is the first study to directly compare the ICU and non-ICU environment for its potential impact on sleep and circadian alignment. Our study adds to the literature with several novel findings. First, average sound levels on non-ICU wards are lower than in the ICU. Second, although quieter on average, SLCs >17.5 dB occurred an equivalent number of times for both the ICU and non-ICU wards. Third, average daytime light levels in both the ICU and non-ICU environment are low. Lastly, peak light levels for both ICU and non-ICU wards occur later in the day instead of in the morning. All of the above have potential impact for optimizing the ward environment to better aid in sleep for patients.
Sound-Level Findings
Data on sound levels for non-ICU floors are limited but mostly consistent with our finding
Average and peak sound levels contribute to the ambient noise experienced by patients but may not be the source of sleep disruptions. Using polysomnography in healthy subjects exposed to recordings of ICU noise, Stanchina et al.21 showed that SLCs from baseline and not peak sound levels determined whether a subject was aroused from sleep by sound. Accordingly, they also found that increasing baseline sound levels by using white noise reduced the number of arousals that subjects experienced. To our knowledge, other studies have not quantified and compared SLCs in the ICU and non-ICU environments. Our data show that patients on non-ICU floors experience at least the same number of SLCs, and thereby the same potential for arousals from sleep, when compared with ICU patients. The higher baseline level of noise in the ICU likely explains the relatively lower number of SLCs when compared with the non-ICU floors. Although decreasing overall noise to promote sleep in the hospital seems like the obvious solution, the treatment for noise pollution in the hospital may actually be more background noise, not less.
Recent studies support the clinical implications of our findings. First, decreasing overall noise levels is difficult to accomplish.29 Second, recent studies utilized white noise in different hospital settings with some success in improving patients’ subjective sleep quality, although more studies using objective data measurements are needed to further understand the impact of white noise on sleep in hospitalized patients.30,31 Third, efforts at reducing interruptions—which likely will decrease the number of SLCs—such as clustering nursing care or reducing intermittent alarms may be more beneficial in improving sleep than efforts at decreasing average sound levels. For example, Bartick et al. reduced the number of patient interruptions at night by eliminating routine vital signs and clustering medication administration. Although they included other interventions as well, we note that this approach likely reduced SLCs and was associated with a reduction in the use of sedative medications.32 Ultimately, our data show that a focus on reducing SLCs will be one necessary component of a multipronged solution to improving inpatient sleep.33
Light-Level Findings
Because of its effect on circadian rhythms, the daily light-dark cycle has a powerful impact on human physiology and behavior, which includes sleep.34 Little is understood about how light affects sleep and other circadian-related functions in general ward patients, as it is not commonly measured. Our findings suggest that patients admitted to the hospital are exposed to light levels and patterns that may not optimally promote wake and sleep. Encouragingly, we did not find excessive average light levels during the nighttime in either ICU or non-ICU environment of our hospital, although others have described intrusive nighttime light in the hospital setting.35,36 Even short bursts of low or moderate light during the nighttime can cause circadian phase delay,37 and efforts to maintain darkness in patient rooms at night should continue.
Our measurements show that average daytime light levels did not exceed 250 lux, which corresponds to low, office-level lighting, while the brightest average light levels occurred in the afternoon for both environments. These levels are consistent with other reports26,35,36 as is the light-level variability noted throughout the day (which is not unexpected given room positioning, patient preference, curtains, etc). The level and amount of daytime light needed to maintain circadian rhythms in humans is still unknown.38 Brighter light is generally more effective at influencing the circadian pacemaker in a dose-dependent manner.39 Although entrainment (synchronization of the body’s biological rhythm with environmental cues such as ambient light) of the human circadian rhythm has been shown with low light levels (eg, <100 lux), these studies included healthy volunteers in a carefully controlled, constant, routine environment.23 How these data apply to acutely ill subjects in the hospital environment is not clear. We note that low to moderate levels of light (50-1000 lux) are less effective for entrainment of the circadian rhythm in older people (age >65 years, the majority of our admissions) compared with younger people. Thus, older, hospitalized patients may require greater light levels for regulation of the sleep-wake cycle.40 These data are important when designing interventions to improve light for and maintain circadian rhythms in hospitalized patients. For example, Simons et al. found that dynamic light-application therapy, which achieved a maximum average lux level of <800 lux, did not reduce rates of delirium in critically ill patients (mean age ~65). One interpretation of these results, though there are many others, is that the light levels achieved were not high enough to influence circadian timing in hospitalized, mostly elderly patients. The physiological impact of light on the circadian rhythm in hospitalized patients still remains to be measured.
LIMITATIONS
Our study does have a few limitations. We did not assess sound quality, which is another determinant of arousal potential.20 Also, a shorter measurement interval might be useful in determining sharper sound increases. It may also be important to consider A- versus C-weighted measurements of sound levels, as A-weighted measurements usually reflect higher-frequency sound while C-weighted measurements usually reflect low-frequency noise18; we obtained only A-weighted measurements in our study. However, A-weighted measurements are generally considered more reflective of what the human ear considers noise and are used more standardly than C-weighted measurements.
Regarding light measurements, we recorded from rooms facing different cardinal directions and during different times of the year, which likely contributed to some of the variability in the daytime light levels on both floors. Additionally, light levels were not measured directly at the patient’s eye level. However, given that overhead fluorescent lighting was the primary source of lighting, it is doubtful that we substantially underestimated optic-nerve light levels. In the future, it may also be important to measure the different wavelengths of lights, as blue light may have a greater impact on sleep than other wavelengths.41 Although our findings align with others’, we note that this was a single-center study, which could limit the generalizability of our findings given inter-hospital variations in patient volume, interior layout and structure, and geographic location.
CONCLUSIONS
Overall, our study suggests that the light and sound environment for sleep in the inpatient setting, including both the ICU and non-ICU wards, has multiple areas for improvement. Our data also suggest specific directions for future clinical efforts at improvement. For example, efforts to decrease average sound levels may worsen sleep fragmentation. Similarly, more light during the day may be more helpful than further attempts to limit light during the night.
Disclosure
This research was funded in part by a NIH/NCATS flagship Clinical and Translational Science Award Grant (5KL2TR001112). None of the authors report any conflict of interest, financial or otherwise, in the preparation of this article.
1. Freedman NS, Gazendam J, Levan L, Pack AI, Schwab RJ. Abnormal sleep/wake
cycles and the effect of environmental noise on sleep disruption in the intensive
care unit. Am J Respir Crit Care Med. 2001;163(2):451-457. PubMed
2. Watson PL, Pandharipande P, Gehlbach BK, et al. Atypical sleep in ventilated
patients: empirical electroencephalography findings and the path toward revised ICU sleep scoring criteria. Crit Care Med. 2013;41(8):1958-1967. PubMed
3. Gehlbach BK, Chapotot F, Leproult R, et al. Temporal disorganization of circadian rhythmicity and sleep-wake regulation in mechanically ventilated patients receiving continuous intravenous sedation. Sleep. 2012;35(8):1105-1114. PubMed
4. Elliott R, McKinley S, Cistulli P, Fien M. Characterisation of sleep in intensive care using 24-hour polysomnography: an observational study. Crit Care. 2013;17(2):R46. PubMed
5. Novaes MA, Aronovich A, Ferraz MB, Knobel E. Stressors in ICU: patients’ evaluation. Intensive Care Med. 1997;23(12):1282-1285. PubMed
6. Tembo AC, Parker V, Higgins I. The experience of sleep deprivation in intensive care patients: findings from a larger hermeneutic phenomenological study. Intensive Crit Care Nurs. 2013;29(6):310-316. PubMed
7. Kamdar BB, Yang J, King LM, et al. Developing, implementing, and evaluating a multifaceted quality improvement intervention to promote sleep in an ICU. Am J Med Qual. 2014;29(6):546-554. PubMed
8. Patel J, Baldwin J, Bunting P, Laha S. The effect of a multicomponent multidisciplinary bundle of interventions on sleep and delirium in medical and surgical intensive care patients. Anaesthesia. 2014;69(6):540-549. PubMed
9. Manian FA, Manian CJ. Sleep quality in adult hospitalized patients with infection: an observational study. Am J Med Sci. 2015;349(1):56-60. PubMed
10. Park MJ, Yoo JH, Cho BW, Kim KT, Jeong WC, Ha M. Noise in hospital rooms and sleep disturbance in hospitalized medical patients. Environ Health Toxicol. 2014;29:e2014006. PubMed
11. Dobing S, Frolova N, McAlister F, Ringrose J. Sleep quality and factors influencing self-reported sleep duration and quality in the general internal medicine inpatient population. PLoS One. 2016;11(6):e0156735. PubMed
12. Gillis CM, Poyant JO, Degrado JR, Ye L, Anger KE, Owens RL. Inpatient pharmacological
sleep aid utilization is common at a tertiary medical center. J Hosp Med. 2014;9(10):652-657. PubMed
13. Krenk L, Jennum P, Kehlet H. Postoperative sleep disturbances after zolpidem treatment in fast-track hip and knee replacement. J Clin Sleep Med. 2014;10(3):321-326. PubMed
14. Kolla BP, Lovely JK, Mansukhani MP, Morgenthaler TI. Zolpidem is independently
associated with increased risk of inpatient falls. J Hosp Med. 2013;8(1):1-6. PubMed
15. Inouye SK, Bogardus ST Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669-676. PubMed
16. Bano M, Chiaromanni F, Corrias M, et al. The influence of environmental factors on sleep quality in hospitalized medical patients. Front Neurol. 2014;5:267. PubMed
17. Berglund BLTSD. Guidelines for Community Noise. World Health Organization. 1999.
18. Knauert M, Jeon S, Murphy TE, Yaggi HK, Pisani MA, Redeker NS. Comparing average levels and peak occurrence of overnight sound in the medical intensive care unit on A-weighted and C-weighted decibel scales. J Crit Care. 2016;36:1-7. PubMed
19. Yoder JC, Staisiunas PG, Meltzer DO, Knutson KL, Arora VM. Noise and sleep among adult medical inpatients: far from a quiet night. Arch Intern Med. 2012;172(1):68-70. PubMed
20. Buxton OM, Ellenbogen JM, Wang W, et al. Sleep disruption due to hospital noises: a prospective evaluation. Ann Intern Med. 2012;157(3):170-179. PubMed
21. Stanchina ML, Abu-Hijleh M, Chaudhry BK, Carlisle CC, Millman RP. The influence of white noise on sleep in subjects exposed to ICU noise. Sleep Med. 2005;6(5):423-428. PubMed
22. Czeisler CA, Allan JS, Strogatz SH, et al. Bright light resets the human circadian pacemaker independent of the timing of the sleep-wake cycle. Science. 1986;233(4764):667-671. PubMed
23. Duffy JF, Czeisler CA. Effect of light on human circadian physiology. Sleep Med Clin. 2009;4(2):165-177. PubMed
24. Lewy AJ, Wehr TA, Goodwin FK, Newsome DA, Markey SP. Light suppresses melatonin secretion in humans. Science. 1980;210(4475):1267-1269. PubMed
25. Zeitzer JM, Dijk DJ, Kronauer R, Brown E, Czeisler C. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol. 2000;526:695-702. PubMed
26. Bernhofer EI, Higgins PA, Daly BJ, Burant CJ, Hornick TR. Hospital lighting and its association with sleep, mood and pain in medical inpatients. J Adv Nurs. 2014;70(5):1164-1173. PubMed
27. Darbyshire JL, Young JD. An investigation of sound levels on intensive care units with reference to the WHO guidelines. Crit Care. 2013;17(5):R187. PubMed
28. Gillis S. Pharmacologic treatment of depression during pregnancy. J Midwifery Womens Health. 2000;45(4):357-359. PubMed
29. Tainter CR, Levine AR, Quraishi SA, et al. Noise levels in surgical ICUs are consistently above recommended standards. Crit Care Med. 2016;44(1):147-152. PubMed
30. Farrehi PM, Clore KR, Scott JR, Vanini G, Clauw DJ. Efficacy of Sleep Tool Education During Hospitalization: A Randomized Controlled Trial. Am J Med. 2016;129(12):1329.e9-1329.e17. PubMed
31. Farokhnezhad Afshar P, Bahramnezhad F, Asgari P, Shiri M. Effect of white noise on sleep in patients admitted to a coronary care. J Caring Sci. 2016;5(2):103-109. PubMed
32. Bartick MC, Thai X, Schmidt T, Altaye A, Solet JM. Decrease in as-needed sedative use by limiting nighttime sleep disruptions from hospital staff. J Hosp Med. 2010;5(3):E20-E24. PubMed
33. Tamrat R, Huynh-Le MP, Goyal M. Non-pharmacologic interventions to improve the sleep of hospitalized patients: a systematic review. J Gen Intern Med. 2014;29(5):788-795. PubMed
34. Dijk DJ, Archer SN. Light, sleep, and circadian rhythms: together again. PLoS Biol. 2009;7(6):e1000145. PubMed
35. Verceles AC, Liu X, Terrin ML, et al. Ambient light levels and critical care outcomes. J Crit Care. 2013;28(1):110.e1-110.e8. PubMed
36. Hu RF, Hegadoren KM, Wang XY, Jiang XY. An investigation of light and sound levels on intensive care units in China. Aust Crit Care. 2016;29(2):62-67. PubMed
37. Zeitzer JM, Ruby NF, Fisicaro RA, Heller HC. Response of the human circadian system to millisecond flashes of light. PLoS One. 2011;6(7):e22078. PubMed
38. Duffy JF, Wright KP, Jr. Entrainment of the human circadian system by light. J Biol Rhythms. 2005;20(4):326-338. PubMed
39. Wright KP Jr, Gronfier C, Duffy JF, Czeisler CA. Intrinsic period and light intensity determine the phase relationship between melatonin and sleep in humans. J Biol Rhythms. 2005;20(2):168-177. PubMed
40. Duffy JF, Zeitzer JM, Czeisler CA. Decreased sensitivity to phase-delaying effects of moderate intensity light in older subjects. Neurobiol Aging. 2007;28(5):799-807. PubMed
41. Figueiro MG, Plitnick BA, Lok A, et al. Tailored lighting intervention improves measures of sleep, depression, and agitation in persons with Alzheimer’s disease and related dementia living in long-term care facilities. Clin Interv Aging. 2014;9:1527-1537. PubMed
1. Freedman NS, Gazendam J, Levan L, Pack AI, Schwab RJ. Abnormal sleep/wake
cycles and the effect of environmental noise on sleep disruption in the intensive
care unit. Am J Respir Crit Care Med. 2001;163(2):451-457. PubMed
2. Watson PL, Pandharipande P, Gehlbach BK, et al. Atypical sleep in ventilated
patients: empirical electroencephalography findings and the path toward revised ICU sleep scoring criteria. Crit Care Med. 2013;41(8):1958-1967. PubMed
3. Gehlbach BK, Chapotot F, Leproult R, et al. Temporal disorganization of circadian rhythmicity and sleep-wake regulation in mechanically ventilated patients receiving continuous intravenous sedation. Sleep. 2012;35(8):1105-1114. PubMed
4. Elliott R, McKinley S, Cistulli P, Fien M. Characterisation of sleep in intensive care using 24-hour polysomnography: an observational study. Crit Care. 2013;17(2):R46. PubMed
5. Novaes MA, Aronovich A, Ferraz MB, Knobel E. Stressors in ICU: patients’ evaluation. Intensive Care Med. 1997;23(12):1282-1285. PubMed
6. Tembo AC, Parker V, Higgins I. The experience of sleep deprivation in intensive care patients: findings from a larger hermeneutic phenomenological study. Intensive Crit Care Nurs. 2013;29(6):310-316. PubMed
7. Kamdar BB, Yang J, King LM, et al. Developing, implementing, and evaluating a multifaceted quality improvement intervention to promote sleep in an ICU. Am J Med Qual. 2014;29(6):546-554. PubMed
8. Patel J, Baldwin J, Bunting P, Laha S. The effect of a multicomponent multidisciplinary bundle of interventions on sleep and delirium in medical and surgical intensive care patients. Anaesthesia. 2014;69(6):540-549. PubMed
9. Manian FA, Manian CJ. Sleep quality in adult hospitalized patients with infection: an observational study. Am J Med Sci. 2015;349(1):56-60. PubMed
10. Park MJ, Yoo JH, Cho BW, Kim KT, Jeong WC, Ha M. Noise in hospital rooms and sleep disturbance in hospitalized medical patients. Environ Health Toxicol. 2014;29:e2014006. PubMed
11. Dobing S, Frolova N, McAlister F, Ringrose J. Sleep quality and factors influencing self-reported sleep duration and quality in the general internal medicine inpatient population. PLoS One. 2016;11(6):e0156735. PubMed
12. Gillis CM, Poyant JO, Degrado JR, Ye L, Anger KE, Owens RL. Inpatient pharmacological
sleep aid utilization is common at a tertiary medical center. J Hosp Med. 2014;9(10):652-657. PubMed
13. Krenk L, Jennum P, Kehlet H. Postoperative sleep disturbances after zolpidem treatment in fast-track hip and knee replacement. J Clin Sleep Med. 2014;10(3):321-326. PubMed
14. Kolla BP, Lovely JK, Mansukhani MP, Morgenthaler TI. Zolpidem is independently
associated with increased risk of inpatient falls. J Hosp Med. 2013;8(1):1-6. PubMed
15. Inouye SK, Bogardus ST Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669-676. PubMed
16. Bano M, Chiaromanni F, Corrias M, et al. The influence of environmental factors on sleep quality in hospitalized medical patients. Front Neurol. 2014;5:267. PubMed
17. Berglund BLTSD. Guidelines for Community Noise. World Health Organization. 1999.
18. Knauert M, Jeon S, Murphy TE, Yaggi HK, Pisani MA, Redeker NS. Comparing average levels and peak occurrence of overnight sound in the medical intensive care unit on A-weighted and C-weighted decibel scales. J Crit Care. 2016;36:1-7. PubMed
19. Yoder JC, Staisiunas PG, Meltzer DO, Knutson KL, Arora VM. Noise and sleep among adult medical inpatients: far from a quiet night. Arch Intern Med. 2012;172(1):68-70. PubMed
20. Buxton OM, Ellenbogen JM, Wang W, et al. Sleep disruption due to hospital noises: a prospective evaluation. Ann Intern Med. 2012;157(3):170-179. PubMed
21. Stanchina ML, Abu-Hijleh M, Chaudhry BK, Carlisle CC, Millman RP. The influence of white noise on sleep in subjects exposed to ICU noise. Sleep Med. 2005;6(5):423-428. PubMed
22. Czeisler CA, Allan JS, Strogatz SH, et al. Bright light resets the human circadian pacemaker independent of the timing of the sleep-wake cycle. Science. 1986;233(4764):667-671. PubMed
23. Duffy JF, Czeisler CA. Effect of light on human circadian physiology. Sleep Med Clin. 2009;4(2):165-177. PubMed
24. Lewy AJ, Wehr TA, Goodwin FK, Newsome DA, Markey SP. Light suppresses melatonin secretion in humans. Science. 1980;210(4475):1267-1269. PubMed
25. Zeitzer JM, Dijk DJ, Kronauer R, Brown E, Czeisler C. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol. 2000;526:695-702. PubMed
26. Bernhofer EI, Higgins PA, Daly BJ, Burant CJ, Hornick TR. Hospital lighting and its association with sleep, mood and pain in medical inpatients. J Adv Nurs. 2014;70(5):1164-1173. PubMed
27. Darbyshire JL, Young JD. An investigation of sound levels on intensive care units with reference to the WHO guidelines. Crit Care. 2013;17(5):R187. PubMed
28. Gillis S. Pharmacologic treatment of depression during pregnancy. J Midwifery Womens Health. 2000;45(4):357-359. PubMed
29. Tainter CR, Levine AR, Quraishi SA, et al. Noise levels in surgical ICUs are consistently above recommended standards. Crit Care Med. 2016;44(1):147-152. PubMed
30. Farrehi PM, Clore KR, Scott JR, Vanini G, Clauw DJ. Efficacy of Sleep Tool Education During Hospitalization: A Randomized Controlled Trial. Am J Med. 2016;129(12):1329.e9-1329.e17. PubMed
31. Farokhnezhad Afshar P, Bahramnezhad F, Asgari P, Shiri M. Effect of white noise on sleep in patients admitted to a coronary care. J Caring Sci. 2016;5(2):103-109. PubMed
32. Bartick MC, Thai X, Schmidt T, Altaye A, Solet JM. Decrease in as-needed sedative use by limiting nighttime sleep disruptions from hospital staff. J Hosp Med. 2010;5(3):E20-E24. PubMed
33. Tamrat R, Huynh-Le MP, Goyal M. Non-pharmacologic interventions to improve the sleep of hospitalized patients: a systematic review. J Gen Intern Med. 2014;29(5):788-795. PubMed
34. Dijk DJ, Archer SN. Light, sleep, and circadian rhythms: together again. PLoS Biol. 2009;7(6):e1000145. PubMed
35. Verceles AC, Liu X, Terrin ML, et al. Ambient light levels and critical care outcomes. J Crit Care. 2013;28(1):110.e1-110.e8. PubMed
36. Hu RF, Hegadoren KM, Wang XY, Jiang XY. An investigation of light and sound levels on intensive care units in China. Aust Crit Care. 2016;29(2):62-67. PubMed
37. Zeitzer JM, Ruby NF, Fisicaro RA, Heller HC. Response of the human circadian system to millisecond flashes of light. PLoS One. 2011;6(7):e22078. PubMed
38. Duffy JF, Wright KP, Jr. Entrainment of the human circadian system by light. J Biol Rhythms. 2005;20(4):326-338. PubMed
39. Wright KP Jr, Gronfier C, Duffy JF, Czeisler CA. Intrinsic period and light intensity determine the phase relationship between melatonin and sleep in humans. J Biol Rhythms. 2005;20(2):168-177. PubMed
40. Duffy JF, Zeitzer JM, Czeisler CA. Decreased sensitivity to phase-delaying effects of moderate intensity light in older subjects. Neurobiol Aging. 2007;28(5):799-807. PubMed
41. Figueiro MG, Plitnick BA, Lok A, et al. Tailored lighting intervention improves measures of sleep, depression, and agitation in persons with Alzheimer’s disease and related dementia living in long-term care facilities. Clin Interv Aging. 2014;9:1527-1537. PubMed
© 2017 Society of Hospital Medicine
Florence A. Blanchfield: A Lifetime of Nursing Leadership
The U.S. Army hospital at Fort Campbell, Kentucky, was named for army nurse, Colonel Florence A. Blanchfield—making it the only current army hospital named for a nurse.
Florence Aby Blanchfield was born into a large family in Shepherdstown, West Virginia, in 1882. Her mother was a nurse, and her father was a mason and stonecutter. She grew up in Oranda, Virginia, and attended both public and private schools. Following in her mother’s footsteps to become a nurse, she attended Southside Hospital Training School in Pittsburgh, Pennsylvania, and graduated in 1906. She moved to Baltimore after graduation and worked with Howard Atwood Kelly, one of the “Big Four” along with William Osler, William Henry Welch, and William Stewart Halsted who were known as the founding physicians of the Johns Hopkins Hospital.
After what must have been a remarkable experience with the innovative Kelly (inventor of many groundbreaking medical instruments and procedures, including the Kelly clamp), Blanchfield returned to Pittsburgh. She held positions of increasing responsibility over several years, including operating room supervisor at Southside Hospital and Montefiore Hospital and superintendent of the training school at Suburban General Hospital. Looking for adventure as well as service, she gave up her positions of leadership and headed to Panama in 1913 to become an operating room nurse and an anesthetist at Ancon Hospital in the U.S. Canal Zone.
As America prepared for its probable entry into World War I, Blanchfield joined the Army Nurse Corps (ANC) at age 35 to serve with the Medical School Unit of the University of Pittsburgh’s Base Hospital 27. She arrived in France in October 1917 and became acting chief nurse of Base Hospital 27 in Angers, Maine et Loire department. She also served as acting chief nurse of Camp Hospital 15 at Coëtquidan, Ille et Vil department.
Blanchfield returned to civilian life following World War I for a short period but returned to active duty in 1920. Over the next 15 years, she had several assignments within the continental U.S. and overseas in the Philippines and in Tianjin, China (formally known in English as Tientsin). In 1935, Blanchfield joined the Office of the Army Surgeon General in Washington, DC, where she was assigned to work on personnel matters in the office of the superintendent of the ANC. She became assistant superintendent in 1939, acting superintendent in 1942, and served as superintendent from June 1943 until September 1947. During World War II, she presided over the growth of the ANC from about 7,000 nurses on the day Pearl Harbor was attacked to more than 50,000 by the end of the war. She was awarded the Distinguished Service Medal for her contributions and accomplishments during World War II.
Blanchfield, a long-time senior leader in the ANC, was instrumental in many of the significant changes that took place during and after World War II, including nurses gaining full rank and benefits. This was an incremental process that culminated with passage of the Army and Navy Nurse Corps Act of April 1947, with nurses being granted full commissioned status. As a result of this act, she became a lieutenant colonel and the first woman to receive a commission in the regular army.
Blanchfield remained active in national nursing affairs after her retirement from the U.S. Army. At a time when many believed that nurses did not need specialty training, she promoted the establishment of specialized courses of study. In 1951, she received the Florence Nightingale Medal of the International Red Cross.
Blanchfield died on May 12, 1971, and was buried in the nurse’s section of Arlington National Cemetery with full military honors. In 1978, ANC leadership began a drive to memorialize Blanchfield by naming the new hospital at Fort Campbell, Kentucky, in her honor. A successful letter writing campaign by army nurses inundated the senior commander at Fort Campbell. The Colonel Florence A. Blanchfield Army Community Hospital, which was dedicated in her memory on September 17, 1982.
About this column
This column provides biographical sketches of the namesakes of military and VA health care facilities. To learn more about the individual your facility was named for or to offer a topic suggestion, contact us at [email protected] or on Facebook.
The U.S. Army hospital at Fort Campbell, Kentucky, was named for army nurse, Colonel Florence A. Blanchfield—making it the only current army hospital named for a nurse.
Florence Aby Blanchfield was born into a large family in Shepherdstown, West Virginia, in 1882. Her mother was a nurse, and her father was a mason and stonecutter. She grew up in Oranda, Virginia, and attended both public and private schools. Following in her mother’s footsteps to become a nurse, she attended Southside Hospital Training School in Pittsburgh, Pennsylvania, and graduated in 1906. She moved to Baltimore after graduation and worked with Howard Atwood Kelly, one of the “Big Four” along with William Osler, William Henry Welch, and William Stewart Halsted who were known as the founding physicians of the Johns Hopkins Hospital.
After what must have been a remarkable experience with the innovative Kelly (inventor of many groundbreaking medical instruments and procedures, including the Kelly clamp), Blanchfield returned to Pittsburgh. She held positions of increasing responsibility over several years, including operating room supervisor at Southside Hospital and Montefiore Hospital and superintendent of the training school at Suburban General Hospital. Looking for adventure as well as service, she gave up her positions of leadership and headed to Panama in 1913 to become an operating room nurse and an anesthetist at Ancon Hospital in the U.S. Canal Zone.
As America prepared for its probable entry into World War I, Blanchfield joined the Army Nurse Corps (ANC) at age 35 to serve with the Medical School Unit of the University of Pittsburgh’s Base Hospital 27. She arrived in France in October 1917 and became acting chief nurse of Base Hospital 27 in Angers, Maine et Loire department. She also served as acting chief nurse of Camp Hospital 15 at Coëtquidan, Ille et Vil department.
Blanchfield returned to civilian life following World War I for a short period but returned to active duty in 1920. Over the next 15 years, she had several assignments within the continental U.S. and overseas in the Philippines and in Tianjin, China (formally known in English as Tientsin). In 1935, Blanchfield joined the Office of the Army Surgeon General in Washington, DC, where she was assigned to work on personnel matters in the office of the superintendent of the ANC. She became assistant superintendent in 1939, acting superintendent in 1942, and served as superintendent from June 1943 until September 1947. During World War II, she presided over the growth of the ANC from about 7,000 nurses on the day Pearl Harbor was attacked to more than 50,000 by the end of the war. She was awarded the Distinguished Service Medal for her contributions and accomplishments during World War II.
Blanchfield, a long-time senior leader in the ANC, was instrumental in many of the significant changes that took place during and after World War II, including nurses gaining full rank and benefits. This was an incremental process that culminated with passage of the Army and Navy Nurse Corps Act of April 1947, with nurses being granted full commissioned status. As a result of this act, she became a lieutenant colonel and the first woman to receive a commission in the regular army.
Blanchfield remained active in national nursing affairs after her retirement from the U.S. Army. At a time when many believed that nurses did not need specialty training, she promoted the establishment of specialized courses of study. In 1951, she received the Florence Nightingale Medal of the International Red Cross.
Blanchfield died on May 12, 1971, and was buried in the nurse’s section of Arlington National Cemetery with full military honors. In 1978, ANC leadership began a drive to memorialize Blanchfield by naming the new hospital at Fort Campbell, Kentucky, in her honor. A successful letter writing campaign by army nurses inundated the senior commander at Fort Campbell. The Colonel Florence A. Blanchfield Army Community Hospital, which was dedicated in her memory on September 17, 1982.
About this column
This column provides biographical sketches of the namesakes of military and VA health care facilities. To learn more about the individual your facility was named for or to offer a topic suggestion, contact us at [email protected] or on Facebook.
The U.S. Army hospital at Fort Campbell, Kentucky, was named for army nurse, Colonel Florence A. Blanchfield—making it the only current army hospital named for a nurse.
Florence Aby Blanchfield was born into a large family in Shepherdstown, West Virginia, in 1882. Her mother was a nurse, and her father was a mason and stonecutter. She grew up in Oranda, Virginia, and attended both public and private schools. Following in her mother’s footsteps to become a nurse, she attended Southside Hospital Training School in Pittsburgh, Pennsylvania, and graduated in 1906. She moved to Baltimore after graduation and worked with Howard Atwood Kelly, one of the “Big Four” along with William Osler, William Henry Welch, and William Stewart Halsted who were known as the founding physicians of the Johns Hopkins Hospital.
After what must have been a remarkable experience with the innovative Kelly (inventor of many groundbreaking medical instruments and procedures, including the Kelly clamp), Blanchfield returned to Pittsburgh. She held positions of increasing responsibility over several years, including operating room supervisor at Southside Hospital and Montefiore Hospital and superintendent of the training school at Suburban General Hospital. Looking for adventure as well as service, she gave up her positions of leadership and headed to Panama in 1913 to become an operating room nurse and an anesthetist at Ancon Hospital in the U.S. Canal Zone.
As America prepared for its probable entry into World War I, Blanchfield joined the Army Nurse Corps (ANC) at age 35 to serve with the Medical School Unit of the University of Pittsburgh’s Base Hospital 27. She arrived in France in October 1917 and became acting chief nurse of Base Hospital 27 in Angers, Maine et Loire department. She also served as acting chief nurse of Camp Hospital 15 at Coëtquidan, Ille et Vil department.
Blanchfield returned to civilian life following World War I for a short period but returned to active duty in 1920. Over the next 15 years, she had several assignments within the continental U.S. and overseas in the Philippines and in Tianjin, China (formally known in English as Tientsin). In 1935, Blanchfield joined the Office of the Army Surgeon General in Washington, DC, where she was assigned to work on personnel matters in the office of the superintendent of the ANC. She became assistant superintendent in 1939, acting superintendent in 1942, and served as superintendent from June 1943 until September 1947. During World War II, she presided over the growth of the ANC from about 7,000 nurses on the day Pearl Harbor was attacked to more than 50,000 by the end of the war. She was awarded the Distinguished Service Medal for her contributions and accomplishments during World War II.
Blanchfield, a long-time senior leader in the ANC, was instrumental in many of the significant changes that took place during and after World War II, including nurses gaining full rank and benefits. This was an incremental process that culminated with passage of the Army and Navy Nurse Corps Act of April 1947, with nurses being granted full commissioned status. As a result of this act, she became a lieutenant colonel and the first woman to receive a commission in the regular army.
Blanchfield remained active in national nursing affairs after her retirement from the U.S. Army. At a time when many believed that nurses did not need specialty training, she promoted the establishment of specialized courses of study. In 1951, she received the Florence Nightingale Medal of the International Red Cross.
Blanchfield died on May 12, 1971, and was buried in the nurse’s section of Arlington National Cemetery with full military honors. In 1978, ANC leadership began a drive to memorialize Blanchfield by naming the new hospital at Fort Campbell, Kentucky, in her honor. A successful letter writing campaign by army nurses inundated the senior commander at Fort Campbell. The Colonel Florence A. Blanchfield Army Community Hospital, which was dedicated in her memory on September 17, 1982.
About this column
This column provides biographical sketches of the namesakes of military and VA health care facilities. To learn more about the individual your facility was named for or to offer a topic suggestion, contact us at [email protected] or on Facebook.
Keeping Up-to-Date on Health Disparity Data
The DATA2020 HealthyPeople.gov data search function just got more user friendly. The Office of Disease Prevention and Health Promotion, National Center for Health Statistics, and Office of Minority Health, partners in DATA2020, have released a shareable widget that gives users easy access to regularly updated information.
The health disparities tool at DATA2020 lets users sort and view health disparities by demographic groups, including race/ethnicity, age, disability status, and geographic location. Users also can easily navigate and visualize data and changes in disparities over time; compare data points for each population group; and see all rates, rate ratios, confidence intervals and other technical details about data collection.
The new widget is an easy way (by simply copying the provided code) to share health disparities information on individual websites. Users can browse by disparity type or by Leading Health Indicator. The widget needs no maintenance; content updates automatically.
The DATA2020 HealthyPeople.gov data search function just got more user friendly. The Office of Disease Prevention and Health Promotion, National Center for Health Statistics, and Office of Minority Health, partners in DATA2020, have released a shareable widget that gives users easy access to regularly updated information.
The health disparities tool at DATA2020 lets users sort and view health disparities by demographic groups, including race/ethnicity, age, disability status, and geographic location. Users also can easily navigate and visualize data and changes in disparities over time; compare data points for each population group; and see all rates, rate ratios, confidence intervals and other technical details about data collection.
The new widget is an easy way (by simply copying the provided code) to share health disparities information on individual websites. Users can browse by disparity type or by Leading Health Indicator. The widget needs no maintenance; content updates automatically.
The DATA2020 HealthyPeople.gov data search function just got more user friendly. The Office of Disease Prevention and Health Promotion, National Center for Health Statistics, and Office of Minority Health, partners in DATA2020, have released a shareable widget that gives users easy access to regularly updated information.
The health disparities tool at DATA2020 lets users sort and view health disparities by demographic groups, including race/ethnicity, age, disability status, and geographic location. Users also can easily navigate and visualize data and changes in disparities over time; compare data points for each population group; and see all rates, rate ratios, confidence intervals and other technical details about data collection.
The new widget is an easy way (by simply copying the provided code) to share health disparities information on individual websites. Users can browse by disparity type or by Leading Health Indicator. The widget needs no maintenance; content updates automatically.
Association Between Anemia and Fatigue in Hospitalized Patients: Does the Measure of Anemia Matter?
Fatigue is the most common clinical symptom of anemia and is a significant concern to patients.1,2 In ambulatory patients, lower hemoglobin (Hb) concentration is associated with increased fatigue.2,3 Accordingly, therapies that treat anemia by increasing Hb concentration, such as erythropoiesis stimulating agents,4-7 often use fatigue as an outcome measure.
In hospitalized patients, transfusion of red blood cell increases Hb concentration and is the primary treatment for anemia. However, the extent to which transfusion and changes in Hb concentration affect hospitalized patients’ fatigue levels is not well established. Guidelines support transfusing patients with symptoms of anemia, such as fatigue, on the assumption that the increased oxygen delivery will improve the symptoms of anemia. While transfusion studies in hospitalized patients have consistently reported that transfusion at lower or “restrictive” Hb concentrations is safe compared with transfusion at higher Hb concentrations,8-10 these studies have mainly used cardiac events and mortality as outcomes rather than patient symptoms, such as fatigue. Nevertheless, they have resulted in hospitals increasingly adopting restrictive transfusion policies that discourage transfusion at higher Hb levels.11,12 Consequently, the rate of transfusion in hospitalized patients has decreased,13 raising questions of whether some patients with lower Hb concentrations may experience increased fatigue as a result of restrictive transfusion policies. Fatigue among hospitalized patients is important not only because it is an adverse symptom but because it may result in decreased activity levels, deconditioning, and losses in functional status.14,15While the effect of alternative transfusion policies on fatigue in hospitalized patients could be answered by a randomized clinical trial using fatigue and functional status as outcomes, an important first step is to assess whether the Hb concentration of hospitalized patients is associated with their fatigue level during hospitalization. Because hospitalized patients often have acute illnesses that can cause fatigue in and of themselves, it is possible that anemia is not associated with fatigue in hospitalized patients despite anemia’s association with fatigue in ambulatory patients. Additionally, Hb concentration varies during hospitalization,16 raising the question of what measures of Hb during hospitalization might be most associated with anemia-related fatigue.
The objective of this study is to explore multiple Hb measures in hospitalized medical patients with anemia and test whether any of these Hb measures are associated with patients’ fatigue level.
METHODS
Study Design
We performed a prospective, observational study of hospitalized patients with anemia on the general medicine services at The University of Chicago Medical Center (UCMC). The institutional review board approved the study procedures, and all study subjects provided informed consent.
Study Eligibility
Between April 2014 and June 2015, all general medicine inpatients were approached for written consent for The University of Chicago Hospitalist Project,17 a research infrastructure at UCMC. Among patients consenting to participate in the Hospitalist Project, patients were eligible if they had Hb <9 g/dL at any point during their hospitalization and were age ≥50 years. Hb concentration of <9 g/dL was chosen to include the range of Hb values covered by most restrictive transfusion policies.8-10,18 Age ≥50 years was an inclusion criteria because anemia is more strongly associated with poor outcomes, including functional impairment, among older patients compared with younger patients.14,19-21 If patients were not eligible for inclusion at the time of consent for the Hospitalist Project, their Hb values were reviewed twice daily until hospital discharge to assess if their Hb was <9 g/dL. Proxies were sought to answer questions for patients who failed the Short Portable Mental Status Questionnaire.22
Patient Demographic Data Collection
Research assistants abstracted patient age and sex from the electronic health record (EHR), and asked patients to self-identify their race. The individual comorbidities included as part of the Charlson Comorbidity Index were identified using International Classification of Diseases, 9th Revision codes from hospital administrative data for each encounter and specifically included the following: myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, rheumatic disease, peptic ulcer disease, liver disease, diabetes, hemiplegia and/or paraplegia, renal disease, cancer, and human immunodeficiency virus/acquired immunodeficiency syndrome.23 We also used Healthcare Cost and Utilization Project (www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp) diagnosis categories to identify whether patients had sickle cell (SC) anemia, gastrointestinal bleeding (GIB), or a depressive disorder (DD) because these conditions are associated with anemia (SC and GIB) and fatigue (DD).24
Measuring Anemia
Hb measures were available only when hospital providers ordered them as part of routine practice. The first Hb concentration <9 g/dL during a patient’s hospitalization, which made them eligible for study participation, was obtained through manual review of the EHR. All additional Hb values during the patient’s hospitalization were obtained from the hospital’s administrative data mart. All Hb values collected for each patient during the hospitalization were used to calculate summary measures of Hb during the hospitalization, including the mean Hb, median Hb, minimum Hb, maximum Hb, admission (first recorded) Hb, and discharge (last recorded) Hb. Hb measures were analyzed both as a continuous variable and as a categorical variable created by dividing the continuous Hb measures into integer ranges of 3 groups of approximately the same size.
Measuring Fatigue
Our primary outcome was patients’ level of fatigue reported during hospitalization, measured using the Functional Assessment of Chronic Illness Therapy (FACIT)-Anemia questionnaire. Fatigue was measured using a 13-question fatigue subscale,1,2,25 which measures fatigue within the past 7 days. Scores on the fatigue subscale range from 0 to 52, with lower scores reflecting greater levels of fatigue. As soon as patients met the eligibility criteria for study participation during their hospitalization (age ≥50 years and Hb <9 g/dL), they were approached to answer the FACIT questions. Values for missing data in the fatigue subscale for individual subjects were filled in using a prorated score from their answered questions as long as >50% of the items in the fatigue subscale were answered, in accordance with recommendations for addressing missing data in the FACIT.26 Fatigue was analyzed as a continuous variable and as a dichotomous variable created by dividing the sample into high (FACIT <27) and low (FACIT ≥27) levels of fatigue based on the median FACIT score of the population. Previous literature has shown a FACIT fatigue subscale score between 23 and 26 to be associated with an Eastern Cooperative Oncology Group (ECOG)27 C Performance Status rating of 2 to 33 compared to scores ≥27.
Statistical Analysis
Statistical analysis was performed using Stata statistical software (StataCorp, College Station, TX). Descriptive statistics were used to characterize patient demographics. Analysis of variance was used to test for differences in the mean fatigue levels across Hb measures. χ2 tests were performed to test for associations between high fatigue levels and the Hb measures. Multivariable analysis, including both linear and logistic regression models, were used to test the association of Hb concentration and fatigue. P values <0.05 using a 2-tailed test were deemed statistically significant.
RESULTS
Patient Characteristics
During the study period, 8559 patients were admitted to the general medicine service. Of those, 5073 (59%) consented for participation in the Hospitalist Project, and 3670 (72%) completed the Hospitalist Project inpatient interview. Of these patients, 1292 (35%) had Hb <9 g/dL, and 784 (61%) were 50 years or older and completed the FACIT questionnaire.
Table 1 reports the demographic characteristics and comorbidities for the sample, the mean (standard deviation [SD]) for the 6 Hb measures, and mean (SD) and median FACIT scores.
Bivariate Association of Fatigue and Hb
Categorizing patients into low, middle, or high Hb for each of the 6 Hb measures, minimum Hb was strongly associated with fatigue, with a weaker association for mean Hb and no statistically significant association for the other measures.
Minimum Hb. Patients with a minimum Hb <7 g/dL and patients with Hb 7-8 g/dL had higher fatigue levels (FACIT = 25 for each) than patients with a minimum Hb ≥8 g/dL (FACIT = 29; P < 0.001; Table 2). When excluding patients with SC and/or GIB because their average minimum Hb differed from the average minimum Hb of the full population (P < 0.001), patients with a minimum Hb <7 g/dL or 7-8 g/dL had even higher fatigue levels (FACIT = 23 and FACIT = 24, respectively), with no change in the fatigue level of patients with a minimum Hb ≥8 g/dL (FACIT = 29; P < 0.001; Table 2). Lower minimum Hb continued to be associated with higher fatigue levels when analyzed in 0.5 g/dL increments (Figure).
Mean Hb and Other Measures. Fatigue levels were high for 47% of patients with a mean Hb <8g /dL and 53% of patients with a mean Hb 8-9 g/dL compared with 43% of patients with a mean Hb ≥9 g/dL (P = 0.05). However, the association between high fatigue and mean Hb was not statistically significant when patients with SC and/or GIB were excluded (Table 2). None of the other 4 Hb measures was significantly associated with fatigue.
Linear Regression of Fatigue on Hb
In linear regression models, minimum Hb consistently predicted patient fatigue, mean Hb had a less robust association with fatigue, and the other Hb measures were not associated with patient fatigue. Increases in minimum Hb (analyzed as a continuous variable) were associated with reduced fatigue (higher FACIT score; β = 1.4; P = 0.005). In models in which minimum Hb was a categorical variable, patients with a minimum Hb of <7 g/dL or 7-8 g/dL had greater fatigue (lower FACIT score) than patients whose minimum Hb was ≥8 g/dL (Hb <7 g/dL: β = −4.2; P ≤ 0.001; Hb 7-8 g/dL: β = −4.1; P < 0.001). These results control for patients’ age, sex, individual comorbidities, and whether their minimum Hb occurred before or after the measurement of fatigue during hospitalization (Model 1), and the results are unchanged when also controlling for the number of Hb laboratory draws patients had during their hospitalization (Model 2; Table 3). In a stratified analysis excluding patients with either SC and/or GIB, changes in minimum Hb were associated with larger changes in patient fatigue levels (Supplemental Table 1). We also stratified our analysis to include only patients whose minimum Hb occurred before the measurement of their fatigue level during hospitalization to avoid a spurious association of fatigue with minimum Hb occurring after fatigue was measured. In both Models 1 and 2, minimum Hb remained a predictor of patients’ fatigue levels with similar effect sizes, although in Model 2, the results did not quite reach a statistically significant level, in part due to larger confidence intervals from the smaller sample size of this stratified analysis (Supplemental Table 2a). We further stratified this analysis to include only patients whose transfusion, if they received one, occurred after their minimum Hb and the measurement of their fatigue level to account for the possibility that a transfusion could affect the fatigue level patients report. In this analysis, most of the estimates of the effect of minimum Hb on fatigue were larger than those seen when only analyzing patients whose minimum Hb occurred before the measurement of their fatigue level, although again, the smaller sample size of this additional stratified analysis does produce larger confidence intervals for these estimates (Supplemental Table 2b).
No Hb measure other than minimum or mean had significant association with patient fatigue levels in linear regression models.
Logistic Regression of High Fatigue Level on Hb
Using logistic regression, minimum Hb analyzed as a categorical variable predicted increased odds of a high fatigue level. Patients with a minimum Hb <7 g/dL were 50% (odds ratio [OR] = 1.5; P = 0.03) more likely to have high fatigue and patients with a minimum Hb 7-8 g/dL were 90% (OR = 1.9; P < 0.001) more likely to have high fatigue compared with patients with a minimum Hb ≥8 g/dL in Model 1. These results were similar in Model 2, although the effect was only statistically significant in the 7-8 g/dL Hb group (Table 3). When excluding SC and/or GIB patients, the odds of having high fatigue as minimum Hb decreased were the same or higher for both models compared to the full population of patients. However, again, in Model 2, the effect was only statistically significant in the 7-8 g/dL Hb group (Supplemental Table 1).
Patients with a mean Hb <8 g/dL were 20% to 30% more likely to have high fatigue and patients with mean Hb 8-9 g/dL were 50% more likely to have high fatigue compared with patients with a mean Hb ≥9 g/dL, but the effects were only statistically significant for patients with a mean Hb 8-9 g/dL in both Models 1 and 2 (Table 3). These results were similar when excluding patients with SC and/or GIB, but they were only significant for patients with a mean Hb 8-9 g/dL in Model 1 and patients with a mean Hb <8 g/dL in the Model 2 (Supplemental Table 3).
DISCUSSION
These results demonstrate that minimum Hb during hospitalization is associated with fatigue in hospitalized patients age ≥50 years, and the association is stronger among patients without SC and/or GIB as comorbidities. The analysis of Hb as a continuous and categorical variable and the use of both linear and logistic regression models support the robustness of these associations and illuminate their clinical significance. For example, in linear regression with minimum Hb a continuous variable, the coefficient of 1.4 suggests that an increase of 2 g/dL in Hb, as might be expected from transfusion of 2 units of red blood cells, would be associated with about a 3-point improvement in fatigue. Additionally, as a categorical variable, a minimum Hb ≥8 g/dL compared with a minimum Hb <7 g/dL or 7-8 g/dL is associated with a 3- to 4-point improvement in fatigue. Previous literature suggests that a difference of 3 in the FACIT score is the minimum clinically important difference in fatigue,3 and changes in minimum Hb in either model predict changes in fatigue that are in the range of potential clinical significance.
The clinical significance of the findings is also reflected in the results of the logistic regressions, which may be mapped to potential effects on functional status. Specifically, the odds of having a high fatigue level (FACIT <27) increase 90% for persons with a minimum Hb 7–8 g/dL compared with persons with a minimum Hb ≥8 g/dL. For persons with a minimum Hb <7 g/dL, point estimates suggest a smaller (50%) increase in the odds of high fatigue, but the 95% confidence interval overlaps heavily with the estimate of patients whose minimum Hb is 7-8 g/dL. While it might be expected that patients with a minimum Hb <7 g/dL have greater levels of fatigue compared with patients with a minimum Hb 7-8 g/dL, we did not observe such a pattern. One reason may be that the confidence intervals of our estimated effects are wide enough that we cannot exclude such a pattern. Another possible explanation is that in both groups, the fatigue levels are sufficiently severe, such that the difference in their fatigue levels may not be clinically meaningful. For example, a FACIT score of 23 to 26 has been shown to be associated with an ECOG performance status of 2 to 3, requiring bed rest for at least part of the day.3 Therefore, patients with a minimum Hb 7-8 g/dL (mean FACIT score = 24; Table 2) or a minimum Hb of <7 g/dL (mean FACIT score = 23; Table 2) are already functionally limited to the point of being partially bed bound, such that further decreases in their Hb may not produce additional fatigue in part because they reduce their activity sufficiently to prevent an increase in fatigue. In such cases, the potential benefits of increased Hb may be better assessed by measuring fatigue in response to a specific and provoked activity level, a concept known as fatigability.20
That minimum Hb is more strongly associated with fatigue than any other measure of Hb during hospitalization may not be surprising. Mean, median, maximum, and discharge Hb may all be affected by transfusion during hospitalization that could affect fatigue. Admission Hb may not reflect true oxygen-carrying capacity because of hemoconcentration.
The association between Hb and fatigue in hospitalized patients is important because increased fatigue could contribute to slower clinical recovery in hospitalized patients. Additionally, increased fatigue during hospitalization and at hospital discharge could exacerbate the known deleterious consequences of fatigue on patients and their health outcomes14,15 after hospital discharge. Although one previous study, the Functional Outcomes in Cardiovascular Patients Undergoing Surgical Hip Fracture Repair (FOCUS)8 trial, did not report differences in patients’ fatigue levels at 30 and 60 days postdischarge when transfused at restrictive (8 g/dL) compared with liberal (10 g/dL) Hb thresholds, confidence in the validity of this finding is reduced by the fact that more than half of the patients were lost to follow-up at the 30- and 60-day time points. Further, patients in the restrictive transfusion arm of FOCUS were transfused to maintain Hb levels at or above 8 g/dL. This transfusion threshold of 8 g/dL may have mitigated the high levels of fatigue that are seen in our study when patients’ Hb drops below 8 g/dL, and maintaining a Hb level of 7 g/dL is now the standard of care in stable hospitalized patients. Lastly, FOCUS was limited to postoperative hip fracture patients, and the generalizability of FOCUS to hospitalized medicine patients with anemia is limited.
Therefore, our results support guideline suggestions that practitioners incorporate the presence of patient symptoms such as fatigue into transfusion decisions, particularly if patients’ Hb is <8 g/dL.18 Though reasonable, the suggestion to incorporate symptoms such as fatigue into transfusion decisions has not been strongly supported by evidence so far, and it may often be neglected in practice. Definitive evidence to support such recommendations would benefit from study through an optimal trial18 that incorporates symptoms into decision making. Our findings add support for a study of transfusion strategies that incorporates patients’ fatigue level in addition to Hb concentration.
This study has several limitations. Although our sample size is large and includes patients with a range of comorbidities that we believe are representative of hospitalized general medicine patients, as a single-center, observational study, our results may not be generalizable to other centers. Additionally, although these data support a reliable association between hospitalized patients’ minimum Hb and fatigue level, the observational design of this study cannot prove that this relationship is causal. Also, patients’ Hb values were measured at the discretion of their clinician, and therefore, the measures of Hb were not uniformly measured for participating patients. In addition, fatigue was only measured at one time point during a patient’s hospitalization, and it is possible that patients’ fatigue levels change during hospitalization in relation to variables we did not consider. Finally, our study was not designed to assess the association of Hb with longer-term functional outcomes, which may be of greater concern than fatigue.
CONCLUSION
In hospitalized patients ≥50 years old, minimum Hb is reliably associated with patients’ fatigue level. Patients whose minimum Hb is <8 g/dL experience higher fatigue levels compared to patients whose minimum Hb is ≥8 g/dL. Additional studies are warranted to understand if patients may benefit from improved fatigue levels by correcting their anemia through transfusion.
1. Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13(2):63-74.
2. Cella D, Lai JS, Chang CH, Peterman A, Slavin M. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer. 2002;94(2):528-538. doi:10.1002/cncr.10245.
3. Cella D, Eton DT, Lai J-S, Peterman AH, Merkel DE. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. J Pain Symptom Manage. 2002;24(6):547-561.
4. Tonelli M, Hemmelgarn B, Reiman T, et al. Benefits and harms of erythropoiesis-stimulating agents for anemia related to cancer: a meta-analysis. CMAJ Can Med Assoc J J Assoc Medicale Can. 2009;180(11):E62-E71. doi:10.1503/cmaj.090470.
5. Foley RN, Curtis BM, Parfrey PS. Erythropoietin Therapy, Hemoglobin Targets, and Quality of Life in Healthy Hemodialysis Patients: A Randomized Trial. Clin J Am Soc Nephrol. 2009;4(4):726-733. doi:10.2215/CJN.04950908.
6. Keown PA, Churchill DN, Poulin-Costello M, et al. Dialysis patients treated with Epoetin alfa show improved anemia symptoms: A new analysis of the Canadian Erythropoietin Study Group trial. Hemodial Int Int Symp Home Hemodial. 2010;14(2):168-173. doi:10.1111/j.1542-4758.2009.00422.x.
7. Palmer SC, Saglimbene V, Mavridis D, et al. Erythropoiesis-stimulating agents for anaemia in adults with chronic kidney disease: a network meta-analysis. Cochrane Database Syst Rev. 2014:CD010590.
8. Carson JL, Terrin ML, Noveck H, et al. Liberal or Restrictive Transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365(26):2453-2462. doi:10.1056/NEJMoa1012452.
9. Holst LB, Haase N, Wetterslev J, et al. Transfusion requirements in septic shock (TRISS) trial – comparing the effects and safety of liberal versus restrictive red blood cell transfusion in septic shock patients in the ICU: protocol for a randomised controlled trial. Trials. 2013;14:150. doi:10.1186/1745-6215-14-150.
10. Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med. 1999;340(6):409-417. doi:10.1056/NEJM199902113400601.
11. Corwin HL, Theus JW, Cargile CS, Lang NP. Red blood cell transfusion: Impact of an education program and a clinical guideline on transfusion practice. J Hosp Med. 2014;9(12):745-749. doi:10.1002/jhm.2237.
12. Saxena, S, editor. The Transfusion Committee: Putting Patient Safety First, 2nd Edition. Bethesda (MD): American Association of Blood Banks; 2013.
13. The 2011 National Blood Collection and Utilization Report. http://www.hhs.gov/ash/bloodsafety/2011-nbcus.pdf. Accessed August 16, 2017.
14. Vestergaard S, Nayfield SG, Patel KV, et al. Fatigue in a Representative Population of Older Persons and Its Association With Functional Impairment, Functional Limitation, and Disability. J Gerontol A Biol Sci Med Sci. 2009;64A(1):76-82. doi:10.1093/gerona/gln017.
15. Gill TM, Desai MM, Gahbauer EA, Holford TR, Williams CS. Restricted activity among community-living older persons: incidence, precipitants, and health care utilization. Ann Intern Med. 2001;135(5):313-321.
16. Koch CG, Li L, Sun Z, et al. Hospital-acquired anemia: Prevalence, outcomes, and healthcare implications. J Hosp Med. 2013;8(9):506-512. doi:10.1002/jhm.2061.
17. Meltzer D, Manning WG, Morrison J, et al. Effects of Physician Experience on Costs and Outcomes on an Academic General Medicine Service: Results of a Trial of Hospitalists. Ann Intern Med. 2002;137(11):866-874. doi:10.7326/0003-4819-137-11-200212030-00007.
18. Carson JL, Grossman BJ, Kleinman S, et al. Red Blood Cell Transfusion: A Clinical Practice Guideline From the AABB*. Ann Intern Med. 2012;157(1):49-58. doi:10.7326/0003-4819-157-1-201206190-00429.
19. Moreh E, Jacobs JM, Stessman J. Fatigue, function, and mortality in older adults. J Gerontol A Biol Sci Med Sci. 2010;65(8):887-895. doi:10.1093/gerona/glq064.
20. Eldadah BA. Fatigue and Fatigability in Older Adults. PM&R. 2010;2(5):406-413. doi:10.1016/j.pmrj.2010.03.022.
21. Hardy SE, Studenski SA. Fatigue Predicts Mortality among Older Adults. J Am Geriatr Soc. 2008;56(10):1910-1914. doi:10.1111/j.1532-5415.2008.01957.x.
22. Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23(10):433-441.
23. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139.
24. HCUP Clinical Classifications Software (CCS) for ICD-9-CM. Healthcare Cost and Utilization Project (HCUP). 2006-2009. Agency for Healthcare Research and Quality, Rockville, MD. https://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed November 22, 2016.
25. Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol Off J Am Soc Clin Oncol. 1993;11(3):570-579.
26. Webster K, Cella D, Yost K. The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: properties, applications, and interpretation. Health Qual Life Outcomes. 2003;1:79. doi:10.1186/1477-7525-1-79.
27. Oken MMMD a, Creech RHMD b, Tormey DCMD, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. J Clin Oncol. 1982;5(6):649-656.
Fatigue is the most common clinical symptom of anemia and is a significant concern to patients.1,2 In ambulatory patients, lower hemoglobin (Hb) concentration is associated with increased fatigue.2,3 Accordingly, therapies that treat anemia by increasing Hb concentration, such as erythropoiesis stimulating agents,4-7 often use fatigue as an outcome measure.
In hospitalized patients, transfusion of red blood cell increases Hb concentration and is the primary treatment for anemia. However, the extent to which transfusion and changes in Hb concentration affect hospitalized patients’ fatigue levels is not well established. Guidelines support transfusing patients with symptoms of anemia, such as fatigue, on the assumption that the increased oxygen delivery will improve the symptoms of anemia. While transfusion studies in hospitalized patients have consistently reported that transfusion at lower or “restrictive” Hb concentrations is safe compared with transfusion at higher Hb concentrations,8-10 these studies have mainly used cardiac events and mortality as outcomes rather than patient symptoms, such as fatigue. Nevertheless, they have resulted in hospitals increasingly adopting restrictive transfusion policies that discourage transfusion at higher Hb levels.11,12 Consequently, the rate of transfusion in hospitalized patients has decreased,13 raising questions of whether some patients with lower Hb concentrations may experience increased fatigue as a result of restrictive transfusion policies. Fatigue among hospitalized patients is important not only because it is an adverse symptom but because it may result in decreased activity levels, deconditioning, and losses in functional status.14,15While the effect of alternative transfusion policies on fatigue in hospitalized patients could be answered by a randomized clinical trial using fatigue and functional status as outcomes, an important first step is to assess whether the Hb concentration of hospitalized patients is associated with their fatigue level during hospitalization. Because hospitalized patients often have acute illnesses that can cause fatigue in and of themselves, it is possible that anemia is not associated with fatigue in hospitalized patients despite anemia’s association with fatigue in ambulatory patients. Additionally, Hb concentration varies during hospitalization,16 raising the question of what measures of Hb during hospitalization might be most associated with anemia-related fatigue.
The objective of this study is to explore multiple Hb measures in hospitalized medical patients with anemia and test whether any of these Hb measures are associated with patients’ fatigue level.
METHODS
Study Design
We performed a prospective, observational study of hospitalized patients with anemia on the general medicine services at The University of Chicago Medical Center (UCMC). The institutional review board approved the study procedures, and all study subjects provided informed consent.
Study Eligibility
Between April 2014 and June 2015, all general medicine inpatients were approached for written consent for The University of Chicago Hospitalist Project,17 a research infrastructure at UCMC. Among patients consenting to participate in the Hospitalist Project, patients were eligible if they had Hb <9 g/dL at any point during their hospitalization and were age ≥50 years. Hb concentration of <9 g/dL was chosen to include the range of Hb values covered by most restrictive transfusion policies.8-10,18 Age ≥50 years was an inclusion criteria because anemia is more strongly associated with poor outcomes, including functional impairment, among older patients compared with younger patients.14,19-21 If patients were not eligible for inclusion at the time of consent for the Hospitalist Project, their Hb values were reviewed twice daily until hospital discharge to assess if their Hb was <9 g/dL. Proxies were sought to answer questions for patients who failed the Short Portable Mental Status Questionnaire.22
Patient Demographic Data Collection
Research assistants abstracted patient age and sex from the electronic health record (EHR), and asked patients to self-identify their race. The individual comorbidities included as part of the Charlson Comorbidity Index were identified using International Classification of Diseases, 9th Revision codes from hospital administrative data for each encounter and specifically included the following: myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, rheumatic disease, peptic ulcer disease, liver disease, diabetes, hemiplegia and/or paraplegia, renal disease, cancer, and human immunodeficiency virus/acquired immunodeficiency syndrome.23 We also used Healthcare Cost and Utilization Project (www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp) diagnosis categories to identify whether patients had sickle cell (SC) anemia, gastrointestinal bleeding (GIB), or a depressive disorder (DD) because these conditions are associated with anemia (SC and GIB) and fatigue (DD).24
Measuring Anemia
Hb measures were available only when hospital providers ordered them as part of routine practice. The first Hb concentration <9 g/dL during a patient’s hospitalization, which made them eligible for study participation, was obtained through manual review of the EHR. All additional Hb values during the patient’s hospitalization were obtained from the hospital’s administrative data mart. All Hb values collected for each patient during the hospitalization were used to calculate summary measures of Hb during the hospitalization, including the mean Hb, median Hb, minimum Hb, maximum Hb, admission (first recorded) Hb, and discharge (last recorded) Hb. Hb measures were analyzed both as a continuous variable and as a categorical variable created by dividing the continuous Hb measures into integer ranges of 3 groups of approximately the same size.
Measuring Fatigue
Our primary outcome was patients’ level of fatigue reported during hospitalization, measured using the Functional Assessment of Chronic Illness Therapy (FACIT)-Anemia questionnaire. Fatigue was measured using a 13-question fatigue subscale,1,2,25 which measures fatigue within the past 7 days. Scores on the fatigue subscale range from 0 to 52, with lower scores reflecting greater levels of fatigue. As soon as patients met the eligibility criteria for study participation during their hospitalization (age ≥50 years and Hb <9 g/dL), they were approached to answer the FACIT questions. Values for missing data in the fatigue subscale for individual subjects were filled in using a prorated score from their answered questions as long as >50% of the items in the fatigue subscale were answered, in accordance with recommendations for addressing missing data in the FACIT.26 Fatigue was analyzed as a continuous variable and as a dichotomous variable created by dividing the sample into high (FACIT <27) and low (FACIT ≥27) levels of fatigue based on the median FACIT score of the population. Previous literature has shown a FACIT fatigue subscale score between 23 and 26 to be associated with an Eastern Cooperative Oncology Group (ECOG)27 C Performance Status rating of 2 to 33 compared to scores ≥27.
Statistical Analysis
Statistical analysis was performed using Stata statistical software (StataCorp, College Station, TX). Descriptive statistics were used to characterize patient demographics. Analysis of variance was used to test for differences in the mean fatigue levels across Hb measures. χ2 tests were performed to test for associations between high fatigue levels and the Hb measures. Multivariable analysis, including both linear and logistic regression models, were used to test the association of Hb concentration and fatigue. P values <0.05 using a 2-tailed test were deemed statistically significant.
RESULTS
Patient Characteristics
During the study period, 8559 patients were admitted to the general medicine service. Of those, 5073 (59%) consented for participation in the Hospitalist Project, and 3670 (72%) completed the Hospitalist Project inpatient interview. Of these patients, 1292 (35%) had Hb <9 g/dL, and 784 (61%) were 50 years or older and completed the FACIT questionnaire.
Table 1 reports the demographic characteristics and comorbidities for the sample, the mean (standard deviation [SD]) for the 6 Hb measures, and mean (SD) and median FACIT scores.
Bivariate Association of Fatigue and Hb
Categorizing patients into low, middle, or high Hb for each of the 6 Hb measures, minimum Hb was strongly associated with fatigue, with a weaker association for mean Hb and no statistically significant association for the other measures.
Minimum Hb. Patients with a minimum Hb <7 g/dL and patients with Hb 7-8 g/dL had higher fatigue levels (FACIT = 25 for each) than patients with a minimum Hb ≥8 g/dL (FACIT = 29; P < 0.001; Table 2). When excluding patients with SC and/or GIB because their average minimum Hb differed from the average minimum Hb of the full population (P < 0.001), patients with a minimum Hb <7 g/dL or 7-8 g/dL had even higher fatigue levels (FACIT = 23 and FACIT = 24, respectively), with no change in the fatigue level of patients with a minimum Hb ≥8 g/dL (FACIT = 29; P < 0.001; Table 2). Lower minimum Hb continued to be associated with higher fatigue levels when analyzed in 0.5 g/dL increments (Figure).
Mean Hb and Other Measures. Fatigue levels were high for 47% of patients with a mean Hb <8g /dL and 53% of patients with a mean Hb 8-9 g/dL compared with 43% of patients with a mean Hb ≥9 g/dL (P = 0.05). However, the association between high fatigue and mean Hb was not statistically significant when patients with SC and/or GIB were excluded (Table 2). None of the other 4 Hb measures was significantly associated with fatigue.
Linear Regression of Fatigue on Hb
In linear regression models, minimum Hb consistently predicted patient fatigue, mean Hb had a less robust association with fatigue, and the other Hb measures were not associated with patient fatigue. Increases in minimum Hb (analyzed as a continuous variable) were associated with reduced fatigue (higher FACIT score; β = 1.4; P = 0.005). In models in which minimum Hb was a categorical variable, patients with a minimum Hb of <7 g/dL or 7-8 g/dL had greater fatigue (lower FACIT score) than patients whose minimum Hb was ≥8 g/dL (Hb <7 g/dL: β = −4.2; P ≤ 0.001; Hb 7-8 g/dL: β = −4.1; P < 0.001). These results control for patients’ age, sex, individual comorbidities, and whether their minimum Hb occurred before or after the measurement of fatigue during hospitalization (Model 1), and the results are unchanged when also controlling for the number of Hb laboratory draws patients had during their hospitalization (Model 2; Table 3). In a stratified analysis excluding patients with either SC and/or GIB, changes in minimum Hb were associated with larger changes in patient fatigue levels (Supplemental Table 1). We also stratified our analysis to include only patients whose minimum Hb occurred before the measurement of their fatigue level during hospitalization to avoid a spurious association of fatigue with minimum Hb occurring after fatigue was measured. In both Models 1 and 2, minimum Hb remained a predictor of patients’ fatigue levels with similar effect sizes, although in Model 2, the results did not quite reach a statistically significant level, in part due to larger confidence intervals from the smaller sample size of this stratified analysis (Supplemental Table 2a). We further stratified this analysis to include only patients whose transfusion, if they received one, occurred after their minimum Hb and the measurement of their fatigue level to account for the possibility that a transfusion could affect the fatigue level patients report. In this analysis, most of the estimates of the effect of minimum Hb on fatigue were larger than those seen when only analyzing patients whose minimum Hb occurred before the measurement of their fatigue level, although again, the smaller sample size of this additional stratified analysis does produce larger confidence intervals for these estimates (Supplemental Table 2b).
No Hb measure other than minimum or mean had significant association with patient fatigue levels in linear regression models.
Logistic Regression of High Fatigue Level on Hb
Using logistic regression, minimum Hb analyzed as a categorical variable predicted increased odds of a high fatigue level. Patients with a minimum Hb <7 g/dL were 50% (odds ratio [OR] = 1.5; P = 0.03) more likely to have high fatigue and patients with a minimum Hb 7-8 g/dL were 90% (OR = 1.9; P < 0.001) more likely to have high fatigue compared with patients with a minimum Hb ≥8 g/dL in Model 1. These results were similar in Model 2, although the effect was only statistically significant in the 7-8 g/dL Hb group (Table 3). When excluding SC and/or GIB patients, the odds of having high fatigue as minimum Hb decreased were the same or higher for both models compared to the full population of patients. However, again, in Model 2, the effect was only statistically significant in the 7-8 g/dL Hb group (Supplemental Table 1).
Patients with a mean Hb <8 g/dL were 20% to 30% more likely to have high fatigue and patients with mean Hb 8-9 g/dL were 50% more likely to have high fatigue compared with patients with a mean Hb ≥9 g/dL, but the effects were only statistically significant for patients with a mean Hb 8-9 g/dL in both Models 1 and 2 (Table 3). These results were similar when excluding patients with SC and/or GIB, but they were only significant for patients with a mean Hb 8-9 g/dL in Model 1 and patients with a mean Hb <8 g/dL in the Model 2 (Supplemental Table 3).
DISCUSSION
These results demonstrate that minimum Hb during hospitalization is associated with fatigue in hospitalized patients age ≥50 years, and the association is stronger among patients without SC and/or GIB as comorbidities. The analysis of Hb as a continuous and categorical variable and the use of both linear and logistic regression models support the robustness of these associations and illuminate their clinical significance. For example, in linear regression with minimum Hb a continuous variable, the coefficient of 1.4 suggests that an increase of 2 g/dL in Hb, as might be expected from transfusion of 2 units of red blood cells, would be associated with about a 3-point improvement in fatigue. Additionally, as a categorical variable, a minimum Hb ≥8 g/dL compared with a minimum Hb <7 g/dL or 7-8 g/dL is associated with a 3- to 4-point improvement in fatigue. Previous literature suggests that a difference of 3 in the FACIT score is the minimum clinically important difference in fatigue,3 and changes in minimum Hb in either model predict changes in fatigue that are in the range of potential clinical significance.
The clinical significance of the findings is also reflected in the results of the logistic regressions, which may be mapped to potential effects on functional status. Specifically, the odds of having a high fatigue level (FACIT <27) increase 90% for persons with a minimum Hb 7–8 g/dL compared with persons with a minimum Hb ≥8 g/dL. For persons with a minimum Hb <7 g/dL, point estimates suggest a smaller (50%) increase in the odds of high fatigue, but the 95% confidence interval overlaps heavily with the estimate of patients whose minimum Hb is 7-8 g/dL. While it might be expected that patients with a minimum Hb <7 g/dL have greater levels of fatigue compared with patients with a minimum Hb 7-8 g/dL, we did not observe such a pattern. One reason may be that the confidence intervals of our estimated effects are wide enough that we cannot exclude such a pattern. Another possible explanation is that in both groups, the fatigue levels are sufficiently severe, such that the difference in their fatigue levels may not be clinically meaningful. For example, a FACIT score of 23 to 26 has been shown to be associated with an ECOG performance status of 2 to 3, requiring bed rest for at least part of the day.3 Therefore, patients with a minimum Hb 7-8 g/dL (mean FACIT score = 24; Table 2) or a minimum Hb of <7 g/dL (mean FACIT score = 23; Table 2) are already functionally limited to the point of being partially bed bound, such that further decreases in their Hb may not produce additional fatigue in part because they reduce their activity sufficiently to prevent an increase in fatigue. In such cases, the potential benefits of increased Hb may be better assessed by measuring fatigue in response to a specific and provoked activity level, a concept known as fatigability.20
That minimum Hb is more strongly associated with fatigue than any other measure of Hb during hospitalization may not be surprising. Mean, median, maximum, and discharge Hb may all be affected by transfusion during hospitalization that could affect fatigue. Admission Hb may not reflect true oxygen-carrying capacity because of hemoconcentration.
The association between Hb and fatigue in hospitalized patients is important because increased fatigue could contribute to slower clinical recovery in hospitalized patients. Additionally, increased fatigue during hospitalization and at hospital discharge could exacerbate the known deleterious consequences of fatigue on patients and their health outcomes14,15 after hospital discharge. Although one previous study, the Functional Outcomes in Cardiovascular Patients Undergoing Surgical Hip Fracture Repair (FOCUS)8 trial, did not report differences in patients’ fatigue levels at 30 and 60 days postdischarge when transfused at restrictive (8 g/dL) compared with liberal (10 g/dL) Hb thresholds, confidence in the validity of this finding is reduced by the fact that more than half of the patients were lost to follow-up at the 30- and 60-day time points. Further, patients in the restrictive transfusion arm of FOCUS were transfused to maintain Hb levels at or above 8 g/dL. This transfusion threshold of 8 g/dL may have mitigated the high levels of fatigue that are seen in our study when patients’ Hb drops below 8 g/dL, and maintaining a Hb level of 7 g/dL is now the standard of care in stable hospitalized patients. Lastly, FOCUS was limited to postoperative hip fracture patients, and the generalizability of FOCUS to hospitalized medicine patients with anemia is limited.
Therefore, our results support guideline suggestions that practitioners incorporate the presence of patient symptoms such as fatigue into transfusion decisions, particularly if patients’ Hb is <8 g/dL.18 Though reasonable, the suggestion to incorporate symptoms such as fatigue into transfusion decisions has not been strongly supported by evidence so far, and it may often be neglected in practice. Definitive evidence to support such recommendations would benefit from study through an optimal trial18 that incorporates symptoms into decision making. Our findings add support for a study of transfusion strategies that incorporates patients’ fatigue level in addition to Hb concentration.
This study has several limitations. Although our sample size is large and includes patients with a range of comorbidities that we believe are representative of hospitalized general medicine patients, as a single-center, observational study, our results may not be generalizable to other centers. Additionally, although these data support a reliable association between hospitalized patients’ minimum Hb and fatigue level, the observational design of this study cannot prove that this relationship is causal. Also, patients’ Hb values were measured at the discretion of their clinician, and therefore, the measures of Hb were not uniformly measured for participating patients. In addition, fatigue was only measured at one time point during a patient’s hospitalization, and it is possible that patients’ fatigue levels change during hospitalization in relation to variables we did not consider. Finally, our study was not designed to assess the association of Hb with longer-term functional outcomes, which may be of greater concern than fatigue.
CONCLUSION
In hospitalized patients ≥50 years old, minimum Hb is reliably associated with patients’ fatigue level. Patients whose minimum Hb is <8 g/dL experience higher fatigue levels compared to patients whose minimum Hb is ≥8 g/dL. Additional studies are warranted to understand if patients may benefit from improved fatigue levels by correcting their anemia through transfusion.
Fatigue is the most common clinical symptom of anemia and is a significant concern to patients.1,2 In ambulatory patients, lower hemoglobin (Hb) concentration is associated with increased fatigue.2,3 Accordingly, therapies that treat anemia by increasing Hb concentration, such as erythropoiesis stimulating agents,4-7 often use fatigue as an outcome measure.
In hospitalized patients, transfusion of red blood cell increases Hb concentration and is the primary treatment for anemia. However, the extent to which transfusion and changes in Hb concentration affect hospitalized patients’ fatigue levels is not well established. Guidelines support transfusing patients with symptoms of anemia, such as fatigue, on the assumption that the increased oxygen delivery will improve the symptoms of anemia. While transfusion studies in hospitalized patients have consistently reported that transfusion at lower or “restrictive” Hb concentrations is safe compared with transfusion at higher Hb concentrations,8-10 these studies have mainly used cardiac events and mortality as outcomes rather than patient symptoms, such as fatigue. Nevertheless, they have resulted in hospitals increasingly adopting restrictive transfusion policies that discourage transfusion at higher Hb levels.11,12 Consequently, the rate of transfusion in hospitalized patients has decreased,13 raising questions of whether some patients with lower Hb concentrations may experience increased fatigue as a result of restrictive transfusion policies. Fatigue among hospitalized patients is important not only because it is an adverse symptom but because it may result in decreased activity levels, deconditioning, and losses in functional status.14,15While the effect of alternative transfusion policies on fatigue in hospitalized patients could be answered by a randomized clinical trial using fatigue and functional status as outcomes, an important first step is to assess whether the Hb concentration of hospitalized patients is associated with their fatigue level during hospitalization. Because hospitalized patients often have acute illnesses that can cause fatigue in and of themselves, it is possible that anemia is not associated with fatigue in hospitalized patients despite anemia’s association with fatigue in ambulatory patients. Additionally, Hb concentration varies during hospitalization,16 raising the question of what measures of Hb during hospitalization might be most associated with anemia-related fatigue.
The objective of this study is to explore multiple Hb measures in hospitalized medical patients with anemia and test whether any of these Hb measures are associated with patients’ fatigue level.
METHODS
Study Design
We performed a prospective, observational study of hospitalized patients with anemia on the general medicine services at The University of Chicago Medical Center (UCMC). The institutional review board approved the study procedures, and all study subjects provided informed consent.
Study Eligibility
Between April 2014 and June 2015, all general medicine inpatients were approached for written consent for The University of Chicago Hospitalist Project,17 a research infrastructure at UCMC. Among patients consenting to participate in the Hospitalist Project, patients were eligible if they had Hb <9 g/dL at any point during their hospitalization and were age ≥50 years. Hb concentration of <9 g/dL was chosen to include the range of Hb values covered by most restrictive transfusion policies.8-10,18 Age ≥50 years was an inclusion criteria because anemia is more strongly associated with poor outcomes, including functional impairment, among older patients compared with younger patients.14,19-21 If patients were not eligible for inclusion at the time of consent for the Hospitalist Project, their Hb values were reviewed twice daily until hospital discharge to assess if their Hb was <9 g/dL. Proxies were sought to answer questions for patients who failed the Short Portable Mental Status Questionnaire.22
Patient Demographic Data Collection
Research assistants abstracted patient age and sex from the electronic health record (EHR), and asked patients to self-identify their race. The individual comorbidities included as part of the Charlson Comorbidity Index were identified using International Classification of Diseases, 9th Revision codes from hospital administrative data for each encounter and specifically included the following: myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, rheumatic disease, peptic ulcer disease, liver disease, diabetes, hemiplegia and/or paraplegia, renal disease, cancer, and human immunodeficiency virus/acquired immunodeficiency syndrome.23 We also used Healthcare Cost and Utilization Project (www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp) diagnosis categories to identify whether patients had sickle cell (SC) anemia, gastrointestinal bleeding (GIB), or a depressive disorder (DD) because these conditions are associated with anemia (SC and GIB) and fatigue (DD).24
Measuring Anemia
Hb measures were available only when hospital providers ordered them as part of routine practice. The first Hb concentration <9 g/dL during a patient’s hospitalization, which made them eligible for study participation, was obtained through manual review of the EHR. All additional Hb values during the patient’s hospitalization were obtained from the hospital’s administrative data mart. All Hb values collected for each patient during the hospitalization were used to calculate summary measures of Hb during the hospitalization, including the mean Hb, median Hb, minimum Hb, maximum Hb, admission (first recorded) Hb, and discharge (last recorded) Hb. Hb measures were analyzed both as a continuous variable and as a categorical variable created by dividing the continuous Hb measures into integer ranges of 3 groups of approximately the same size.
Measuring Fatigue
Our primary outcome was patients’ level of fatigue reported during hospitalization, measured using the Functional Assessment of Chronic Illness Therapy (FACIT)-Anemia questionnaire. Fatigue was measured using a 13-question fatigue subscale,1,2,25 which measures fatigue within the past 7 days. Scores on the fatigue subscale range from 0 to 52, with lower scores reflecting greater levels of fatigue. As soon as patients met the eligibility criteria for study participation during their hospitalization (age ≥50 years and Hb <9 g/dL), they were approached to answer the FACIT questions. Values for missing data in the fatigue subscale for individual subjects were filled in using a prorated score from their answered questions as long as >50% of the items in the fatigue subscale were answered, in accordance with recommendations for addressing missing data in the FACIT.26 Fatigue was analyzed as a continuous variable and as a dichotomous variable created by dividing the sample into high (FACIT <27) and low (FACIT ≥27) levels of fatigue based on the median FACIT score of the population. Previous literature has shown a FACIT fatigue subscale score between 23 and 26 to be associated with an Eastern Cooperative Oncology Group (ECOG)27 C Performance Status rating of 2 to 33 compared to scores ≥27.
Statistical Analysis
Statistical analysis was performed using Stata statistical software (StataCorp, College Station, TX). Descriptive statistics were used to characterize patient demographics. Analysis of variance was used to test for differences in the mean fatigue levels across Hb measures. χ2 tests were performed to test for associations between high fatigue levels and the Hb measures. Multivariable analysis, including both linear and logistic regression models, were used to test the association of Hb concentration and fatigue. P values <0.05 using a 2-tailed test were deemed statistically significant.
RESULTS
Patient Characteristics
During the study period, 8559 patients were admitted to the general medicine service. Of those, 5073 (59%) consented for participation in the Hospitalist Project, and 3670 (72%) completed the Hospitalist Project inpatient interview. Of these patients, 1292 (35%) had Hb <9 g/dL, and 784 (61%) were 50 years or older and completed the FACIT questionnaire.
Table 1 reports the demographic characteristics and comorbidities for the sample, the mean (standard deviation [SD]) for the 6 Hb measures, and mean (SD) and median FACIT scores.
Bivariate Association of Fatigue and Hb
Categorizing patients into low, middle, or high Hb for each of the 6 Hb measures, minimum Hb was strongly associated with fatigue, with a weaker association for mean Hb and no statistically significant association for the other measures.
Minimum Hb. Patients with a minimum Hb <7 g/dL and patients with Hb 7-8 g/dL had higher fatigue levels (FACIT = 25 for each) than patients with a minimum Hb ≥8 g/dL (FACIT = 29; P < 0.001; Table 2). When excluding patients with SC and/or GIB because their average minimum Hb differed from the average minimum Hb of the full population (P < 0.001), patients with a minimum Hb <7 g/dL or 7-8 g/dL had even higher fatigue levels (FACIT = 23 and FACIT = 24, respectively), with no change in the fatigue level of patients with a minimum Hb ≥8 g/dL (FACIT = 29; P < 0.001; Table 2). Lower minimum Hb continued to be associated with higher fatigue levels when analyzed in 0.5 g/dL increments (Figure).
Mean Hb and Other Measures. Fatigue levels were high for 47% of patients with a mean Hb <8g /dL and 53% of patients with a mean Hb 8-9 g/dL compared with 43% of patients with a mean Hb ≥9 g/dL (P = 0.05). However, the association between high fatigue and mean Hb was not statistically significant when patients with SC and/or GIB were excluded (Table 2). None of the other 4 Hb measures was significantly associated with fatigue.
Linear Regression of Fatigue on Hb
In linear regression models, minimum Hb consistently predicted patient fatigue, mean Hb had a less robust association with fatigue, and the other Hb measures were not associated with patient fatigue. Increases in minimum Hb (analyzed as a continuous variable) were associated with reduced fatigue (higher FACIT score; β = 1.4; P = 0.005). In models in which minimum Hb was a categorical variable, patients with a minimum Hb of <7 g/dL or 7-8 g/dL had greater fatigue (lower FACIT score) than patients whose minimum Hb was ≥8 g/dL (Hb <7 g/dL: β = −4.2; P ≤ 0.001; Hb 7-8 g/dL: β = −4.1; P < 0.001). These results control for patients’ age, sex, individual comorbidities, and whether their minimum Hb occurred before or after the measurement of fatigue during hospitalization (Model 1), and the results are unchanged when also controlling for the number of Hb laboratory draws patients had during their hospitalization (Model 2; Table 3). In a stratified analysis excluding patients with either SC and/or GIB, changes in minimum Hb were associated with larger changes in patient fatigue levels (Supplemental Table 1). We also stratified our analysis to include only patients whose minimum Hb occurred before the measurement of their fatigue level during hospitalization to avoid a spurious association of fatigue with minimum Hb occurring after fatigue was measured. In both Models 1 and 2, minimum Hb remained a predictor of patients’ fatigue levels with similar effect sizes, although in Model 2, the results did not quite reach a statistically significant level, in part due to larger confidence intervals from the smaller sample size of this stratified analysis (Supplemental Table 2a). We further stratified this analysis to include only patients whose transfusion, if they received one, occurred after their minimum Hb and the measurement of their fatigue level to account for the possibility that a transfusion could affect the fatigue level patients report. In this analysis, most of the estimates of the effect of minimum Hb on fatigue were larger than those seen when only analyzing patients whose minimum Hb occurred before the measurement of their fatigue level, although again, the smaller sample size of this additional stratified analysis does produce larger confidence intervals for these estimates (Supplemental Table 2b).
No Hb measure other than minimum or mean had significant association with patient fatigue levels in linear regression models.
Logistic Regression of High Fatigue Level on Hb
Using logistic regression, minimum Hb analyzed as a categorical variable predicted increased odds of a high fatigue level. Patients with a minimum Hb <7 g/dL were 50% (odds ratio [OR] = 1.5; P = 0.03) more likely to have high fatigue and patients with a minimum Hb 7-8 g/dL were 90% (OR = 1.9; P < 0.001) more likely to have high fatigue compared with patients with a minimum Hb ≥8 g/dL in Model 1. These results were similar in Model 2, although the effect was only statistically significant in the 7-8 g/dL Hb group (Table 3). When excluding SC and/or GIB patients, the odds of having high fatigue as minimum Hb decreased were the same or higher for both models compared to the full population of patients. However, again, in Model 2, the effect was only statistically significant in the 7-8 g/dL Hb group (Supplemental Table 1).
Patients with a mean Hb <8 g/dL were 20% to 30% more likely to have high fatigue and patients with mean Hb 8-9 g/dL were 50% more likely to have high fatigue compared with patients with a mean Hb ≥9 g/dL, but the effects were only statistically significant for patients with a mean Hb 8-9 g/dL in both Models 1 and 2 (Table 3). These results were similar when excluding patients with SC and/or GIB, but they were only significant for patients with a mean Hb 8-9 g/dL in Model 1 and patients with a mean Hb <8 g/dL in the Model 2 (Supplemental Table 3).
DISCUSSION
These results demonstrate that minimum Hb during hospitalization is associated with fatigue in hospitalized patients age ≥50 years, and the association is stronger among patients without SC and/or GIB as comorbidities. The analysis of Hb as a continuous and categorical variable and the use of both linear and logistic regression models support the robustness of these associations and illuminate their clinical significance. For example, in linear regression with minimum Hb a continuous variable, the coefficient of 1.4 suggests that an increase of 2 g/dL in Hb, as might be expected from transfusion of 2 units of red blood cells, would be associated with about a 3-point improvement in fatigue. Additionally, as a categorical variable, a minimum Hb ≥8 g/dL compared with a minimum Hb <7 g/dL or 7-8 g/dL is associated with a 3- to 4-point improvement in fatigue. Previous literature suggests that a difference of 3 in the FACIT score is the minimum clinically important difference in fatigue,3 and changes in minimum Hb in either model predict changes in fatigue that are in the range of potential clinical significance.
The clinical significance of the findings is also reflected in the results of the logistic regressions, which may be mapped to potential effects on functional status. Specifically, the odds of having a high fatigue level (FACIT <27) increase 90% for persons with a minimum Hb 7–8 g/dL compared with persons with a minimum Hb ≥8 g/dL. For persons with a minimum Hb <7 g/dL, point estimates suggest a smaller (50%) increase in the odds of high fatigue, but the 95% confidence interval overlaps heavily with the estimate of patients whose minimum Hb is 7-8 g/dL. While it might be expected that patients with a minimum Hb <7 g/dL have greater levels of fatigue compared with patients with a minimum Hb 7-8 g/dL, we did not observe such a pattern. One reason may be that the confidence intervals of our estimated effects are wide enough that we cannot exclude such a pattern. Another possible explanation is that in both groups, the fatigue levels are sufficiently severe, such that the difference in their fatigue levels may not be clinically meaningful. For example, a FACIT score of 23 to 26 has been shown to be associated with an ECOG performance status of 2 to 3, requiring bed rest for at least part of the day.3 Therefore, patients with a minimum Hb 7-8 g/dL (mean FACIT score = 24; Table 2) or a minimum Hb of <7 g/dL (mean FACIT score = 23; Table 2) are already functionally limited to the point of being partially bed bound, such that further decreases in their Hb may not produce additional fatigue in part because they reduce their activity sufficiently to prevent an increase in fatigue. In such cases, the potential benefits of increased Hb may be better assessed by measuring fatigue in response to a specific and provoked activity level, a concept known as fatigability.20
That minimum Hb is more strongly associated with fatigue than any other measure of Hb during hospitalization may not be surprising. Mean, median, maximum, and discharge Hb may all be affected by transfusion during hospitalization that could affect fatigue. Admission Hb may not reflect true oxygen-carrying capacity because of hemoconcentration.
The association between Hb and fatigue in hospitalized patients is important because increased fatigue could contribute to slower clinical recovery in hospitalized patients. Additionally, increased fatigue during hospitalization and at hospital discharge could exacerbate the known deleterious consequences of fatigue on patients and their health outcomes14,15 after hospital discharge. Although one previous study, the Functional Outcomes in Cardiovascular Patients Undergoing Surgical Hip Fracture Repair (FOCUS)8 trial, did not report differences in patients’ fatigue levels at 30 and 60 days postdischarge when transfused at restrictive (8 g/dL) compared with liberal (10 g/dL) Hb thresholds, confidence in the validity of this finding is reduced by the fact that more than half of the patients were lost to follow-up at the 30- and 60-day time points. Further, patients in the restrictive transfusion arm of FOCUS were transfused to maintain Hb levels at or above 8 g/dL. This transfusion threshold of 8 g/dL may have mitigated the high levels of fatigue that are seen in our study when patients’ Hb drops below 8 g/dL, and maintaining a Hb level of 7 g/dL is now the standard of care in stable hospitalized patients. Lastly, FOCUS was limited to postoperative hip fracture patients, and the generalizability of FOCUS to hospitalized medicine patients with anemia is limited.
Therefore, our results support guideline suggestions that practitioners incorporate the presence of patient symptoms such as fatigue into transfusion decisions, particularly if patients’ Hb is <8 g/dL.18 Though reasonable, the suggestion to incorporate symptoms such as fatigue into transfusion decisions has not been strongly supported by evidence so far, and it may often be neglected in practice. Definitive evidence to support such recommendations would benefit from study through an optimal trial18 that incorporates symptoms into decision making. Our findings add support for a study of transfusion strategies that incorporates patients’ fatigue level in addition to Hb concentration.
This study has several limitations. Although our sample size is large and includes patients with a range of comorbidities that we believe are representative of hospitalized general medicine patients, as a single-center, observational study, our results may not be generalizable to other centers. Additionally, although these data support a reliable association between hospitalized patients’ minimum Hb and fatigue level, the observational design of this study cannot prove that this relationship is causal. Also, patients’ Hb values were measured at the discretion of their clinician, and therefore, the measures of Hb were not uniformly measured for participating patients. In addition, fatigue was only measured at one time point during a patient’s hospitalization, and it is possible that patients’ fatigue levels change during hospitalization in relation to variables we did not consider. Finally, our study was not designed to assess the association of Hb with longer-term functional outcomes, which may be of greater concern than fatigue.
CONCLUSION
In hospitalized patients ≥50 years old, minimum Hb is reliably associated with patients’ fatigue level. Patients whose minimum Hb is <8 g/dL experience higher fatigue levels compared to patients whose minimum Hb is ≥8 g/dL. Additional studies are warranted to understand if patients may benefit from improved fatigue levels by correcting their anemia through transfusion.
1. Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13(2):63-74.
2. Cella D, Lai JS, Chang CH, Peterman A, Slavin M. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer. 2002;94(2):528-538. doi:10.1002/cncr.10245.
3. Cella D, Eton DT, Lai J-S, Peterman AH, Merkel DE. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. J Pain Symptom Manage. 2002;24(6):547-561.
4. Tonelli M, Hemmelgarn B, Reiman T, et al. Benefits and harms of erythropoiesis-stimulating agents for anemia related to cancer: a meta-analysis. CMAJ Can Med Assoc J J Assoc Medicale Can. 2009;180(11):E62-E71. doi:10.1503/cmaj.090470.
5. Foley RN, Curtis BM, Parfrey PS. Erythropoietin Therapy, Hemoglobin Targets, and Quality of Life in Healthy Hemodialysis Patients: A Randomized Trial. Clin J Am Soc Nephrol. 2009;4(4):726-733. doi:10.2215/CJN.04950908.
6. Keown PA, Churchill DN, Poulin-Costello M, et al. Dialysis patients treated with Epoetin alfa show improved anemia symptoms: A new analysis of the Canadian Erythropoietin Study Group trial. Hemodial Int Int Symp Home Hemodial. 2010;14(2):168-173. doi:10.1111/j.1542-4758.2009.00422.x.
7. Palmer SC, Saglimbene V, Mavridis D, et al. Erythropoiesis-stimulating agents for anaemia in adults with chronic kidney disease: a network meta-analysis. Cochrane Database Syst Rev. 2014:CD010590.
8. Carson JL, Terrin ML, Noveck H, et al. Liberal or Restrictive Transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365(26):2453-2462. doi:10.1056/NEJMoa1012452.
9. Holst LB, Haase N, Wetterslev J, et al. Transfusion requirements in septic shock (TRISS) trial – comparing the effects and safety of liberal versus restrictive red blood cell transfusion in septic shock patients in the ICU: protocol for a randomised controlled trial. Trials. 2013;14:150. doi:10.1186/1745-6215-14-150.
10. Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med. 1999;340(6):409-417. doi:10.1056/NEJM199902113400601.
11. Corwin HL, Theus JW, Cargile CS, Lang NP. Red blood cell transfusion: Impact of an education program and a clinical guideline on transfusion practice. J Hosp Med. 2014;9(12):745-749. doi:10.1002/jhm.2237.
12. Saxena, S, editor. The Transfusion Committee: Putting Patient Safety First, 2nd Edition. Bethesda (MD): American Association of Blood Banks; 2013.
13. The 2011 National Blood Collection and Utilization Report. http://www.hhs.gov/ash/bloodsafety/2011-nbcus.pdf. Accessed August 16, 2017.
14. Vestergaard S, Nayfield SG, Patel KV, et al. Fatigue in a Representative Population of Older Persons and Its Association With Functional Impairment, Functional Limitation, and Disability. J Gerontol A Biol Sci Med Sci. 2009;64A(1):76-82. doi:10.1093/gerona/gln017.
15. Gill TM, Desai MM, Gahbauer EA, Holford TR, Williams CS. Restricted activity among community-living older persons: incidence, precipitants, and health care utilization. Ann Intern Med. 2001;135(5):313-321.
16. Koch CG, Li L, Sun Z, et al. Hospital-acquired anemia: Prevalence, outcomes, and healthcare implications. J Hosp Med. 2013;8(9):506-512. doi:10.1002/jhm.2061.
17. Meltzer D, Manning WG, Morrison J, et al. Effects of Physician Experience on Costs and Outcomes on an Academic General Medicine Service: Results of a Trial of Hospitalists. Ann Intern Med. 2002;137(11):866-874. doi:10.7326/0003-4819-137-11-200212030-00007.
18. Carson JL, Grossman BJ, Kleinman S, et al. Red Blood Cell Transfusion: A Clinical Practice Guideline From the AABB*. Ann Intern Med. 2012;157(1):49-58. doi:10.7326/0003-4819-157-1-201206190-00429.
19. Moreh E, Jacobs JM, Stessman J. Fatigue, function, and mortality in older adults. J Gerontol A Biol Sci Med Sci. 2010;65(8):887-895. doi:10.1093/gerona/glq064.
20. Eldadah BA. Fatigue and Fatigability in Older Adults. PM&R. 2010;2(5):406-413. doi:10.1016/j.pmrj.2010.03.022.
21. Hardy SE, Studenski SA. Fatigue Predicts Mortality among Older Adults. J Am Geriatr Soc. 2008;56(10):1910-1914. doi:10.1111/j.1532-5415.2008.01957.x.
22. Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23(10):433-441.
23. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139.
24. HCUP Clinical Classifications Software (CCS) for ICD-9-CM. Healthcare Cost and Utilization Project (HCUP). 2006-2009. Agency for Healthcare Research and Quality, Rockville, MD. https://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed November 22, 2016.
25. Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol Off J Am Soc Clin Oncol. 1993;11(3):570-579.
26. Webster K, Cella D, Yost K. The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: properties, applications, and interpretation. Health Qual Life Outcomes. 2003;1:79. doi:10.1186/1477-7525-1-79.
27. Oken MMMD a, Creech RHMD b, Tormey DCMD, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. J Clin Oncol. 1982;5(6):649-656.
1. Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13(2):63-74.
2. Cella D, Lai JS, Chang CH, Peterman A, Slavin M. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer. 2002;94(2):528-538. doi:10.1002/cncr.10245.
3. Cella D, Eton DT, Lai J-S, Peterman AH, Merkel DE. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. J Pain Symptom Manage. 2002;24(6):547-561.
4. Tonelli M, Hemmelgarn B, Reiman T, et al. Benefits and harms of erythropoiesis-stimulating agents for anemia related to cancer: a meta-analysis. CMAJ Can Med Assoc J J Assoc Medicale Can. 2009;180(11):E62-E71. doi:10.1503/cmaj.090470.
5. Foley RN, Curtis BM, Parfrey PS. Erythropoietin Therapy, Hemoglobin Targets, and Quality of Life in Healthy Hemodialysis Patients: A Randomized Trial. Clin J Am Soc Nephrol. 2009;4(4):726-733. doi:10.2215/CJN.04950908.
6. Keown PA, Churchill DN, Poulin-Costello M, et al. Dialysis patients treated with Epoetin alfa show improved anemia symptoms: A new analysis of the Canadian Erythropoietin Study Group trial. Hemodial Int Int Symp Home Hemodial. 2010;14(2):168-173. doi:10.1111/j.1542-4758.2009.00422.x.
7. Palmer SC, Saglimbene V, Mavridis D, et al. Erythropoiesis-stimulating agents for anaemia in adults with chronic kidney disease: a network meta-analysis. Cochrane Database Syst Rev. 2014:CD010590.
8. Carson JL, Terrin ML, Noveck H, et al. Liberal or Restrictive Transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365(26):2453-2462. doi:10.1056/NEJMoa1012452.
9. Holst LB, Haase N, Wetterslev J, et al. Transfusion requirements in septic shock (TRISS) trial – comparing the effects and safety of liberal versus restrictive red blood cell transfusion in septic shock patients in the ICU: protocol for a randomised controlled trial. Trials. 2013;14:150. doi:10.1186/1745-6215-14-150.
10. Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med. 1999;340(6):409-417. doi:10.1056/NEJM199902113400601.
11. Corwin HL, Theus JW, Cargile CS, Lang NP. Red blood cell transfusion: Impact of an education program and a clinical guideline on transfusion practice. J Hosp Med. 2014;9(12):745-749. doi:10.1002/jhm.2237.
12. Saxena, S, editor. The Transfusion Committee: Putting Patient Safety First, 2nd Edition. Bethesda (MD): American Association of Blood Banks; 2013.
13. The 2011 National Blood Collection and Utilization Report. http://www.hhs.gov/ash/bloodsafety/2011-nbcus.pdf. Accessed August 16, 2017.
14. Vestergaard S, Nayfield SG, Patel KV, et al. Fatigue in a Representative Population of Older Persons and Its Association With Functional Impairment, Functional Limitation, and Disability. J Gerontol A Biol Sci Med Sci. 2009;64A(1):76-82. doi:10.1093/gerona/gln017.
15. Gill TM, Desai MM, Gahbauer EA, Holford TR, Williams CS. Restricted activity among community-living older persons: incidence, precipitants, and health care utilization. Ann Intern Med. 2001;135(5):313-321.
16. Koch CG, Li L, Sun Z, et al. Hospital-acquired anemia: Prevalence, outcomes, and healthcare implications. J Hosp Med. 2013;8(9):506-512. doi:10.1002/jhm.2061.
17. Meltzer D, Manning WG, Morrison J, et al. Effects of Physician Experience on Costs and Outcomes on an Academic General Medicine Service: Results of a Trial of Hospitalists. Ann Intern Med. 2002;137(11):866-874. doi:10.7326/0003-4819-137-11-200212030-00007.
18. Carson JL, Grossman BJ, Kleinman S, et al. Red Blood Cell Transfusion: A Clinical Practice Guideline From the AABB*. Ann Intern Med. 2012;157(1):49-58. doi:10.7326/0003-4819-157-1-201206190-00429.
19. Moreh E, Jacobs JM, Stessman J. Fatigue, function, and mortality in older adults. J Gerontol A Biol Sci Med Sci. 2010;65(8):887-895. doi:10.1093/gerona/glq064.
20. Eldadah BA. Fatigue and Fatigability in Older Adults. PM&R. 2010;2(5):406-413. doi:10.1016/j.pmrj.2010.03.022.
21. Hardy SE, Studenski SA. Fatigue Predicts Mortality among Older Adults. J Am Geriatr Soc. 2008;56(10):1910-1914. doi:10.1111/j.1532-5415.2008.01957.x.
22. Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23(10):433-441.
23. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139.
24. HCUP Clinical Classifications Software (CCS) for ICD-9-CM. Healthcare Cost and Utilization Project (HCUP). 2006-2009. Agency for Healthcare Research and Quality, Rockville, MD. https://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed November 22, 2016.
25. Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol Off J Am Soc Clin Oncol. 1993;11(3):570-579.
26. Webster K, Cella D, Yost K. The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: properties, applications, and interpretation. Health Qual Life Outcomes. 2003;1:79. doi:10.1186/1477-7525-1-79.
27. Oken MMMD a, Creech RHMD b, Tormey DCMD, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. J Clin Oncol. 1982;5(6):649-656.
© 2017 Society of Hospital Medicine
Impact of an Educational Training Program on Restorative Care Practice of Nursing Assistants Working with Hospitalized Older Patients
Abstract
- Background: Acute and prolonged exposure to hospital medical care can cause hospital-associated deconditioning with deleterious effects on patient care provision and quality of life. Physical rehabilitation provided by allied healthcare professionals can enable reacquisition of function via professional input into attainment of set goals. Separate to rehabilitative efforts, restorative care optimizes independence by motivating individuals to maintain and restore function. Nursing assistants (NAs) provide a significant amount of direct patient care and are well placed to deliver restorative care.
- Objective: To increase proportional restorative care interactions with hospitalized older adults by training NAs.
- Methods: A prospective cohort quality improvement (QI) project was undertaken at 3 acute hospital wards (patient minimum age 65 years) and 2 community subacute care wards in the UK. NAs working within the target settings received a 2-part restorative care training package. The primary evaluation tool was 51 hours in total of observation measuring the proportional change in restorative care events delivered by NAs.
- Results: NA-led restorative care events increased from 40 (pre-intervention) to 94 (post-intervention), representing a statistically significant proportional increase from 74% to 92% (χ2(1) 9.53, P = 0.002). NAs on occasions inadvertently emphasized restriction of function to manage risk and oblige with rest periods.
- Conclusion: Investing in NAs can influence the amount of restorative care delivered to hospitalized older adults at risk of hospital-associated deconditioning. Continued investment in NAs is indicated to influence top-down, mandated restorative care practice in this patient group.
Key words: older people; restorative care; hospital associated deconditioning; nursing assistants; rehabilitation; training.
Hospital-associated deconditioning is defined as a significant decline in functional abilities developed through acute and prolonged exposure to a medical care facility environment, and is independent of that attributed to primary pathologies resulting in acute admission [1]. Considerable research on iatrogenic complications in older hospitalized populations [1–5] has shown the impacts of hospital-associated deconditioning and associated dysfunctions on quality of life for patients and the resultant burden on health and social care provision [6].
Physical rehabilitation has been shown to restore function through high-dose repetition of task-specific activity [7], and the benefits attributed to extra physical therapy include improved mobility, activity, and participation [8]. Simply defined, physical rehabilitation is the reacquisition of function through multidisciplinary assessment and professional therapeutic input in attainment of set goals. A more recent nomenclature in health settings is “restorative care,” defined as a philosophy of care that encourages, enables, and motivates individuals to maintain and restore function, thereby optimizing independence [9]. It has been clearly defined as a philosophy separate from that of rehabilitation [9] and remote from task-related or “custodial care,” which is designed to assist in meeting patients’ daily activity needs without any therapeutic value.
In UK rehabilitation wards, nursing staff provide 4.5 times as much direct patient care time compared with allied health professionals, with paraprofessional nursing assistants (NAs, equivalent to certified nurse assistants [CNAs] in the United States) responsible for half of this direct nursing care [10]. Kessler’s group examined the evolving role of NAs in UK hospitals [11]. From a national survey of 700 NAs and 600 trained nurses, the authors upheld the view that NAs act as direct caregivers including through routine tasks traditionally delivered by nurses. They identified that NAs exhibit distinct qualities, which are valued by qualified nurses, including routine task fulfilment and abilities relating to patients, which enable NAs to enhance care quality. Indeed, the national findings of Kessler’s group were generalizable to our own clinical setting where a NA cohort was a well-placed, available, and motivated resource to deliver therapeutically focused care for our hospitalized older population.
The theoretical relationship between care approaches is complex and represents a challenge for service users and policy makers. For instance, comprehensive rehabilitation delivery during an acute care episode may lead to users not seeking custodial care at home. Conversely, day-to-day activities realized by custodial care at home may lead to users not seeking acute rehabilitative care [12]. With stable resources being assigned to more dependent users in higher numbers, reactive care regardless of environment has often been the model of choice.
However, an economic rationale has developed more recently where investment in maintenance and preventative models results in healthcare savings with models including the 4Rs; reablement, reactivation, rehabilitation, and restorative care [13]. In North America, restorative care approaches have resulted in favorable results in nursing home facilities [14] and at home [15], and restorative care education and motivation training for nursing assistants was effective in supporting a change in beliefs and practice behaviors [16]. While results show restorative care practices in the non-acute care sector are advantageous, it is unknown whether these approaches if adopted in hospital settings affect subsequent healthcare utilization in the non-acute facilities, or even if they are feasible to implement in acute facilities by a staff group able to do so. Therefore, the purpose of this QI project was to deploy a restorative care educational intervention for NA staff working with hospitalized older adults with the aim of increasing the proportion of restorative care delivered.
Methods
Context
This project was conducted at a UK National Health Service university teaching hospital trust at 3 acute hospital wards (patient minimum age 65 years) and 2 community subacute care wards for older patients. Participants consisted of all permanent or long-term temporary (> 3 months continuous employment) NAs working in the target settings (n = 36). The QI project design is summarized in Figure 1. The project applied the 4Es translational approach to regulate the QI intervention: Engage, Educate, Execute, and Evaluate [17]. The reporting of this study follows SQUIRE guidance [18].
Intervention
The QI activity was a holistic educational process for all NA participants.
Didactic Study Day
Each NA attended a study day led by a physical therapist (up to 10 NAs per group). A student-centered training approach was adopted, recognizing variations in adult learning styles [19], and included seminar style theory, video case scenarios, group work, practical skills, open discussion, and reflection. The training package outline was compiled following consensus among the multi-disciplinary team working in the target settings and the steering group. Topics covered were theory on the risk of hospitalization and benefits of early mobilization; case scenarios and examples of restorative care; identifying and overcoming barriers to restorative care; identifying appro-priate patients for a restorative care approach; practical skills, including assisting patients out of bed, ambulation, and eating/drinking; and challenging, problem-solving scenarios. All participants received a course handbook to facilitate learning.
Ward-Based Practice
Measures
Type of Care Event
The quantity and nature of all NA-patient functional task-related care events was established by independent systematic observation pre- and post-intervention. Observers rated the type of care for observed patients as either custodial or restorative events using a tool described below. In addition, the numbers of patients receiving no restorative care events at all during observation was calculated to capture changes in rates between patients observed. The observational tool used was adapted from that utilized in a North American study of a long-term care facility [20], which demonstrated favorable intra-rater reliability (person separation reliability of 0.77), inter-rater reliability (80% to 100% agreement on each of the care behaviors), and validity (evidence of unidimensionality and good fit of the items). Adaptations accommodated for data collection in a hospital environment and alteration to UK nomenclature.
Three blinded volunteer assessors undertook observations. The observers monitored for activity in any 1 of 8 functional domains: bed mobility, transfers, mobility, washing and dressing, exercise, hygiene (mouth care/shaving/hair/nail care), toileting, and eating. Patient activity observed within these domains was identified as either a restorative or custodial care event. For example: “asks or encourages patient to walk/independently propel wheelchair to bathroom/toilet/day room/activities and gives them time to perform activity” was identified as a restorative care event, while “utilizes wheelchair instead of encourages ambulation and does not encourage patient to self-propel” was considered a custodial care event. All observations were carried out by student physical therapists in training or physical therapy assistants, all of whom were familiar in working in the acute facility with hospitalized older people. In an attempt to optimize internal consistency, observer skill was quality-controlled by ensuring observers were trained and their competency assessed in the use of the evaluation measurement tool.
Bays of 3 to 6 beds comprised each observation space. Three 90-minute time epochs were selected for observation—awakening (early morning), lunchtime (middle of the day), and afternoon (before evening meal)—with the aim that each time frame be observed on a minimum of 1 occasion on each of the 5 wards to generate a minimum of 15 observation sessions. Resources dictated observational periods to be 90-minutes maximum, per epoch, on weekdays only. The mean (range) time between the didactic study day and the ward-based practice day was 4 (1–8) weeks, and between the ward-based practice day and the second observational period was 6 (1–14) weeks.
Patient Characteristics
Differences in the acuity of patients between pre- and post-QI activity in the observational environments could influence care demands. Therefore, patient characteristics before and after the QI activity were measured to assess for stability. Prior to each session, observers recorded patient demographic details and current STRATIFY score, a predictive tool used at the time to segment fall risk [21], from patients’ clinical records. Two measures were used to offer contemporaneous representation of the observed population in the observation environment: a modified Barthel index [22], which provides a measure of activities of daily living [23], and the Abbreviated Mental Test Score [24], a simple diagnostic screen for cognitive impairment. All patients were considered as recuperating and thus eligible for observation except those with a “Patient-At-Risk” score ≥ 4, indicating physiological factors associated with established or impending critical illness [25], or if an end of life care plan was clearly detailed in the clinical record.
Data Analysis
Patient demographics are reported descriptively. Ordinal data are summarized using median and inter-quartile ranges (IQR), interval/ratio data using mean and standard deviation (SD) unless otherwise stated. Categorical data are reported as percentages. Comparison of observed patient samples before and after the QI period were compared with the Mann-Whitney U-test for ordinal data, 2 sample t tests for interval/ratio data, and chi squared tests of proportions for other variables.
Analyses were carried out using STATA 11 ME (StataCorp, College Station, TX) and SPSS v17 (SPSS, Chicago). Statistical significance was set at P ≤ 0.05.
Ethical Issues
This study was approved by the local UK NHS Trust clinical audit committee (Quality Improvement project 2038).
Results
Care Events by NAs
Observations were undertaken across the 5 wards on 14 workdays (Monday–Friday) over 6 weeks in the pre-QI period, and on 16 workdays over 4 weeks in the post-QI period, yielding a total of 51 hours of observation.
Overall, across all care environments, there was a statistically significant proportional increase in restorative care from 74% to 92% [χ2(1) 9.53, P = 0.002] (Figure 2). This represents an increase in restorative care events from 40 to 94. Observed custodial care events decreased from 14 to 8, a 43% reduction in custodial care events overall, a difference which remained irrespective of the environment (acute or subacute care), pre- and post-QI activity (P = 0.538 and P = 0.695, respectively).
There was a marked decrease in the number of patients receiving no NA-led restorative care events from 59 (74%) to 32 (48%) before and after QI activity respectively, [χ2 (1) 10.63, P = 0.001].
Patients Observed
Patient population characteristics remained stable during the course of the QI activity; there were no significant differences in the observed patient characteristics pre- and post-QI activity (Table). In 51 hours of observation undertaken by 3 independent observers there were 80 and 71 occupied beds before and after QI activity, respectively, representing a stable bed occupancy rate of 94% and 83% (P = 0.074). Of the occupied beds, 98.7% and 98.6% of patients (pre- and post-QI activity, respectively) were considered recuperating and therefore appropriate for a restorative care approach.
Discussion
although significantly decreased from pre-QI proportions (74%). We therefore conclude that a meaningful decrease across patients receiving no restorative care and a meaningful increase in within-patient restorative care events post-QI intervention occurred.
Our study furthers research in methods of increasing restorative care events delivered by NAs. In a randomized controlled trial by Resnick et al [16], a structured 6-week restorative care program incorporating teaching NAs
restorative care philosophies (tier 1) and facilitating NAs to motivate residents to engage in functional activities (tier 2) was compared to placebo (a single 30-minute educational session in managing residents’ behavioral symptoms) [16]. Results showed the 6-week program led to more restorative care, with NAs demonstrating enhanced knowledge and expectations of restorative care outcomes and better job satisfaction. Our educational package (1 day) and ward-based-learning session (3–4 hours) was much shorter than Resnick et al’s 6-week intervention [16], and the optimal dose of educational packages for NAs is yet to be determined and needs to be addressed in future studies. Furthermore, while we found education increased restorative care across multiple environments, it is yet to be determined whether more restorative care has a positive impact on patient function downstream of an acute inpatient stay. In fact, determination of restorative care’s influence on augmenting rehabilitation outcomes is a neglected aspect of nursing-AHP practice that we aim to define and investigate in ongoing studies.
The patient population characteristics within the target wards were stable over the course of the QI project. Observed patients’ median Barthel (11) and Abbreviated Mental Test (6) scores remained stable and are indicative of high levels of day-to-day activity dependence [24,26–28]. Over the QI activity period it was therefore unsurprising that modest proportions of patients direc-ted their own care (28% and 33% pre and post-QI, respectively). Subsequently, demands on staff to lead patient care were substantial, leading to high risks of social or clinical iatrogenesis and hospital-associated deconditioning.
In a previous observational study, substantial patient inactivity was found in a highly dependent cohort of patients [29]. Fear of falling and insignificant emphasis on ambulation were cited as patient and organizational-centric reasons, respectively. Furthermore, in a selective observational study, patients receiving function-focused care (FFC; synonymous to restorative care) in an acute hospital environment developed less physical functional decline compared to those receiving custodial care [30]. However, patients who had fallen during their hospital stay received less FFC. The authors suggest limited FFC in fallers was deployed to minimize further risk but concluded there is need for nursing and therapy interventions that manage fall risks through endorsing functional activities instead of mobility restriction [30].
Limitations
While observational studies are more robust for measuring behavioral activities compared to self or proxy reporting [34], they are exposed to observer judgment and drift. An attempt was made to minimize this with the binary measurement of restorative versus custodial care and by random sampling of wards and time frames to capture an entire healthcare environment.
The observational study tool was based on one previously developed where acceptable reliability and validity was established and where observations were based on what individual care staff were practicing regardless of their operational environment [20]. In contrast, our observations were based in predetermined environmental spaces regardless of what care practice occurred within it. We consider our approach justifiable in minimizing observer influence on an individual’s practice by emphasizing to them that observers were interested in what happened in an environment [35,36]. However, we acknowledge the risk of under representation of care by observers not following the care delivery, and that local validity and reliability of our methods was not undertaken. Lastly, whilst training for observers was undertaken in this study to standardize the observations undertaken, validation of this method would be a feature required of any future experimental work.
Conclusions
Our findings support the current understanding of restorative care [14–16] and provides proof of concept that dedicating resources in a previously under-invested part of the workforce is feasible, well-accepted, and meaningful. The results are in keeping with the concept that the NA staff group is ready and able to fulfil their roles as direct caregivers, supporting and relieving other trained staff [11].
Corresponding author: Gareth D. Jones, MSc, Physiotherapy Dept, 3rd Fl Lambeth Wing, St Thomas’ Hospital, Westminster Bridge Rd, London SE1 7EH, UK, [email protected].
Funding/support: This work was supported by a small grants application to the Guy's and St Thomas' Charity, project code S100414.
Financial disclosures: No conflicts of interest to declare.
Acknowledgment: The authors acknowledge members of the steering group for their input: Rebekah Schiff, Carrie-Ann Wood, Judith Centofanti, Judith Hall, and Richard Page; Anne Bisset-Smith and Claudia Jacob for their initial pilot work; Amanda Buttery, Lottie Prowse, and Ryan Mackie for practical assistance; Siobhan Crichton for her statistical help; and Jacky Jones, Michael Thacker, Tisha Pryor, and Sarah Ritchie for helping review the manuscript.
1. Kortebein P. Rehabilitation for hospital-associated deconditioning. Am J Phys Med Rehabil 2009;88:66–77.
2. Creditor MC. Hazards of hospitalization of the elderly. Ann Intern Med 1993;118:219–23.
3. Davydow DS, Hough CL, Levine DA, et al. Functional disability, cognitive impairment, and depression after hospitalization for pneumonia. Am J Med 2013;126:615–24.e5.
4. Sager MA, Franke T, Inouye SK, et al. Functional outcomes of acute medical illness and hospitalization in older persons. Arch Intern Med 1996;156:645–52.
5. Warshaw GA, Moore JT, Friedman SW, et al. Functional disability in the hospitalized elderly. JAMA 1982;248:847–50.
6. Covinsky KE, Pierluissi E, Johnston CB. Hospitalization-associated disability: "She was probably able to ambulate, but I'm not sure". JAMA 2011;306:1782–93.
7. Kwakkel G, Kollen B, Lindeman E. Understanding the pattern of functional recovery after stroke: facts and theories. Restor Neurol Neurosci 2004;22:281–99.
8. Peiris CL, Taylor NF, Shields N. Extra physical therapy reduces patient length of stay and improves functional outcomes and quality of life in people with acute or subacute conditions: a systematic review. Arch Phys Med
Rehabil 2011;92:1490–500.
9. Resnick B, Boltz M, Galik E, Pretzer-Aboff I. Restorative care nursing for older adults: a guide for all care settings. 2nd ed. New York: Springer; 2012.
10. Rudd AG, Jenkinson D, Grant RL, Hoffman A. Staffing levels and patient dependence in English stroke units. Clin Med (Lond). 2009;9:110–5.
11. Kessler I, Heron P, Dopson S, et al. The nature and consequences of support workers in a hospital setting, Final Report. London: National Institute for Health Research, Service Delivery and Organization Programme; 2010.
12. Kashner TM, Krompholz B, McDonnell C, et al. Acute and custodial care among impaired aged. J Aging Health 1990;2:28–41.
13. Sims-Gould J, Tong CE, Wallis-Mayer L, Ashe MC. Reablement, reactivation, rehabilitation and restorative interventions with older adults in receipt of home care: a systematic review. J Am Med Dir Assoc 2017;18:653–63.
14. Shanti C, Johnson J, Meyers AM, et al. Evaluation of the restorative care education and training program for nursing homes. Can J Aging 2005;24:115–26.
15. Tinetti ME, Baker D, Gallo WT, et al. Evaluation of restorative care vs usual care for older adults receiving an acute episode of home care. JAMA 2002;287:2098–105.
16. Resnick B, Gruber-Baldini AL, Galik E, et al. Changing the philosophy of care in long-term care: testing of the restorative care intervention. Gerontologist 2009;49:175–84.
17. Pronovost PJ, Berenholtz SM, Needham DM. Translating evidence into practice: a model for large scale knowledge translation. BMJ 2008;337:a1714.
18. Davidoff F, Batalden P, Stevens D, et al; SQUIRE development group. Publication guidelines for quality improvement studies in health care: evolution of the SQUIRE project. BMJ 2009;338:a3152.
19. Sweeney JF. Nurse education: learner-centred or teacher-centred? Nurse Educ Today 1986;6:257–62.
20. Resnick B, Rogers V, Galik E, Gruber-Baldini AL. Measuring restorative care provided by nursing assistants: reliability and validity of the Restorative Care Behavior Checklist. Nurs Res 2007;56:387–98.
21. Oliver D, Britton M, Seed P, et al. Development and evaluation of evidence based risk assessment tool (STRATIFY) to predict which elderly inpatients will fall: case-control and cohort studies. BMJ 1997;315:1049–53.
22. Colin C, Wade DT, Davies S, Horne V. The Barthel ADL Index: a reliability study. Int Disabil Stud 1988;10:61–3.
23. Richards SH, Peters TJ, Coast J, et al. Inter-rater reliability of the Barthel ADL index: how does a researcher compare to a nurse? Clin Rehabil 2000;14:72–8.
24. Hodkinson HM. Evaluation of a mental test score for assessment of mental impairment in the elderly. Age Ageing 1972;1:233–8.
25. Morgan CD, Baade LE. Neuropsychological testing and assessment scales for dementia of the Alzheimer's type. Psychiatr Clin North Am 1997;20:25–43.
26. Granger CV, Hamilton BB, Gresham GE, Kramer AA. The stroke rehabilitation outcome study: Part II. Relative merits of the total Barthel index score and a four-item subscore in predicting patient outcomes. Arch Phys Med Rehabil
1989;70:100–3.
27. MacKenzie DM, Copp P, Shaw RJ, Goodwin GM. Brief cognitive screening of the elderly: a comparison of the Mini-Mental State Examination (MMSE), Abbreviated Mental Test (AMT) and Mental Status Questionnaire (MSQ). Psychol Med
1996;26:427–30.
28. Uyttenboogaart M, Stewart RE, Vroomen PC, et al. Optimizing cutoff scores for the Barthel index and the modified Rankin scale for defining outcome in acute stroke trials. Stroke 2005;36:1984–7.
29. Callen BL, Mahoney JE, Grieves CB, et al. Frequency of hallway ambulation by hospitalized older adults on medical units of an academic hospital. Geriatr Nurs 2004;25:212–7.
30. Boltz M, Resnick B, Capezuti E, Shuluk J. Activity restriction vs. self-direction: hospitalised older adults' response to fear of falling. Int J Older People Nurs 2014;9:44–53.
31. Moyle W, Borbasi S, Wallis M, et al. Acute care management of older people with dementia: a qualitative perspective. J Clin Nurs 2011;20:420–8.
32. Olson DM, Borel CO, Laskowitz DT, et al. Quiet time: a nursing intervention to promote sleep in neurocritical care units. Am J Crit Care 2001;10:74–8.
33. Gardner C, Collins C, Osborne S, et al. Creating a therapeutic environment: a non-randomised controlled trial of a quiet time intervention for patients in acute care. Int J Nurs Stud 2009;46:778–86.
34. Kupek E. Bias and heteroscedastic memory error in self-reported health behavior: an investigation using covariance structure analysis. BMC Med Res Methodol 2002;2:14.
35. Fromme HB, Karani R, Downing SM. Direct observation in medical education: a review of the literature and evidence for validity. Mt Sinai J Med 2009;76:365–71.
36. Williams RG, Klamen DA, McGaghie WC. Cognitive, social and environmental sources of bias in clinical performance ratings. Teach Learn Med 2003;15:270–92.
Abstract
- Background: Acute and prolonged exposure to hospital medical care can cause hospital-associated deconditioning with deleterious effects on patient care provision and quality of life. Physical rehabilitation provided by allied healthcare professionals can enable reacquisition of function via professional input into attainment of set goals. Separate to rehabilitative efforts, restorative care optimizes independence by motivating individuals to maintain and restore function. Nursing assistants (NAs) provide a significant amount of direct patient care and are well placed to deliver restorative care.
- Objective: To increase proportional restorative care interactions with hospitalized older adults by training NAs.
- Methods: A prospective cohort quality improvement (QI) project was undertaken at 3 acute hospital wards (patient minimum age 65 years) and 2 community subacute care wards in the UK. NAs working within the target settings received a 2-part restorative care training package. The primary evaluation tool was 51 hours in total of observation measuring the proportional change in restorative care events delivered by NAs.
- Results: NA-led restorative care events increased from 40 (pre-intervention) to 94 (post-intervention), representing a statistically significant proportional increase from 74% to 92% (χ2(1) 9.53, P = 0.002). NAs on occasions inadvertently emphasized restriction of function to manage risk and oblige with rest periods.
- Conclusion: Investing in NAs can influence the amount of restorative care delivered to hospitalized older adults at risk of hospital-associated deconditioning. Continued investment in NAs is indicated to influence top-down, mandated restorative care practice in this patient group.
Key words: older people; restorative care; hospital associated deconditioning; nursing assistants; rehabilitation; training.
Hospital-associated deconditioning is defined as a significant decline in functional abilities developed through acute and prolonged exposure to a medical care facility environment, and is independent of that attributed to primary pathologies resulting in acute admission [1]. Considerable research on iatrogenic complications in older hospitalized populations [1–5] has shown the impacts of hospital-associated deconditioning and associated dysfunctions on quality of life for patients and the resultant burden on health and social care provision [6].
Physical rehabilitation has been shown to restore function through high-dose repetition of task-specific activity [7], and the benefits attributed to extra physical therapy include improved mobility, activity, and participation [8]. Simply defined, physical rehabilitation is the reacquisition of function through multidisciplinary assessment and professional therapeutic input in attainment of set goals. A more recent nomenclature in health settings is “restorative care,” defined as a philosophy of care that encourages, enables, and motivates individuals to maintain and restore function, thereby optimizing independence [9]. It has been clearly defined as a philosophy separate from that of rehabilitation [9] and remote from task-related or “custodial care,” which is designed to assist in meeting patients’ daily activity needs without any therapeutic value.
In UK rehabilitation wards, nursing staff provide 4.5 times as much direct patient care time compared with allied health professionals, with paraprofessional nursing assistants (NAs, equivalent to certified nurse assistants [CNAs] in the United States) responsible for half of this direct nursing care [10]. Kessler’s group examined the evolving role of NAs in UK hospitals [11]. From a national survey of 700 NAs and 600 trained nurses, the authors upheld the view that NAs act as direct caregivers including through routine tasks traditionally delivered by nurses. They identified that NAs exhibit distinct qualities, which are valued by qualified nurses, including routine task fulfilment and abilities relating to patients, which enable NAs to enhance care quality. Indeed, the national findings of Kessler’s group were generalizable to our own clinical setting where a NA cohort was a well-placed, available, and motivated resource to deliver therapeutically focused care for our hospitalized older population.
The theoretical relationship between care approaches is complex and represents a challenge for service users and policy makers. For instance, comprehensive rehabilitation delivery during an acute care episode may lead to users not seeking custodial care at home. Conversely, day-to-day activities realized by custodial care at home may lead to users not seeking acute rehabilitative care [12]. With stable resources being assigned to more dependent users in higher numbers, reactive care regardless of environment has often been the model of choice.
However, an economic rationale has developed more recently where investment in maintenance and preventative models results in healthcare savings with models including the 4Rs; reablement, reactivation, rehabilitation, and restorative care [13]. In North America, restorative care approaches have resulted in favorable results in nursing home facilities [14] and at home [15], and restorative care education and motivation training for nursing assistants was effective in supporting a change in beliefs and practice behaviors [16]. While results show restorative care practices in the non-acute care sector are advantageous, it is unknown whether these approaches if adopted in hospital settings affect subsequent healthcare utilization in the non-acute facilities, or even if they are feasible to implement in acute facilities by a staff group able to do so. Therefore, the purpose of this QI project was to deploy a restorative care educational intervention for NA staff working with hospitalized older adults with the aim of increasing the proportion of restorative care delivered.
Methods
Context
This project was conducted at a UK National Health Service university teaching hospital trust at 3 acute hospital wards (patient minimum age 65 years) and 2 community subacute care wards for older patients. Participants consisted of all permanent or long-term temporary (> 3 months continuous employment) NAs working in the target settings (n = 36). The QI project design is summarized in Figure 1. The project applied the 4Es translational approach to regulate the QI intervention: Engage, Educate, Execute, and Evaluate [17]. The reporting of this study follows SQUIRE guidance [18].
Intervention
The QI activity was a holistic educational process for all NA participants.
Didactic Study Day
Each NA attended a study day led by a physical therapist (up to 10 NAs per group). A student-centered training approach was adopted, recognizing variations in adult learning styles [19], and included seminar style theory, video case scenarios, group work, practical skills, open discussion, and reflection. The training package outline was compiled following consensus among the multi-disciplinary team working in the target settings and the steering group. Topics covered were theory on the risk of hospitalization and benefits of early mobilization; case scenarios and examples of restorative care; identifying and overcoming barriers to restorative care; identifying appro-priate patients for a restorative care approach; practical skills, including assisting patients out of bed, ambulation, and eating/drinking; and challenging, problem-solving scenarios. All participants received a course handbook to facilitate learning.
Ward-Based Practice
Measures
Type of Care Event
The quantity and nature of all NA-patient functional task-related care events was established by independent systematic observation pre- and post-intervention. Observers rated the type of care for observed patients as either custodial or restorative events using a tool described below. In addition, the numbers of patients receiving no restorative care events at all during observation was calculated to capture changes in rates between patients observed. The observational tool used was adapted from that utilized in a North American study of a long-term care facility [20], which demonstrated favorable intra-rater reliability (person separation reliability of 0.77), inter-rater reliability (80% to 100% agreement on each of the care behaviors), and validity (evidence of unidimensionality and good fit of the items). Adaptations accommodated for data collection in a hospital environment and alteration to UK nomenclature.
Three blinded volunteer assessors undertook observations. The observers monitored for activity in any 1 of 8 functional domains: bed mobility, transfers, mobility, washing and dressing, exercise, hygiene (mouth care/shaving/hair/nail care), toileting, and eating. Patient activity observed within these domains was identified as either a restorative or custodial care event. For example: “asks or encourages patient to walk/independently propel wheelchair to bathroom/toilet/day room/activities and gives them time to perform activity” was identified as a restorative care event, while “utilizes wheelchair instead of encourages ambulation and does not encourage patient to self-propel” was considered a custodial care event. All observations were carried out by student physical therapists in training or physical therapy assistants, all of whom were familiar in working in the acute facility with hospitalized older people. In an attempt to optimize internal consistency, observer skill was quality-controlled by ensuring observers were trained and their competency assessed in the use of the evaluation measurement tool.
Bays of 3 to 6 beds comprised each observation space. Three 90-minute time epochs were selected for observation—awakening (early morning), lunchtime (middle of the day), and afternoon (before evening meal)—with the aim that each time frame be observed on a minimum of 1 occasion on each of the 5 wards to generate a minimum of 15 observation sessions. Resources dictated observational periods to be 90-minutes maximum, per epoch, on weekdays only. The mean (range) time between the didactic study day and the ward-based practice day was 4 (1–8) weeks, and between the ward-based practice day and the second observational period was 6 (1–14) weeks.
Patient Characteristics
Differences in the acuity of patients between pre- and post-QI activity in the observational environments could influence care demands. Therefore, patient characteristics before and after the QI activity were measured to assess for stability. Prior to each session, observers recorded patient demographic details and current STRATIFY score, a predictive tool used at the time to segment fall risk [21], from patients’ clinical records. Two measures were used to offer contemporaneous representation of the observed population in the observation environment: a modified Barthel index [22], which provides a measure of activities of daily living [23], and the Abbreviated Mental Test Score [24], a simple diagnostic screen for cognitive impairment. All patients were considered as recuperating and thus eligible for observation except those with a “Patient-At-Risk” score ≥ 4, indicating physiological factors associated with established or impending critical illness [25], or if an end of life care plan was clearly detailed in the clinical record.
Data Analysis
Patient demographics are reported descriptively. Ordinal data are summarized using median and inter-quartile ranges (IQR), interval/ratio data using mean and standard deviation (SD) unless otherwise stated. Categorical data are reported as percentages. Comparison of observed patient samples before and after the QI period were compared with the Mann-Whitney U-test for ordinal data, 2 sample t tests for interval/ratio data, and chi squared tests of proportions for other variables.
Analyses were carried out using STATA 11 ME (StataCorp, College Station, TX) and SPSS v17 (SPSS, Chicago). Statistical significance was set at P ≤ 0.05.
Ethical Issues
This study was approved by the local UK NHS Trust clinical audit committee (Quality Improvement project 2038).
Results
Care Events by NAs
Observations were undertaken across the 5 wards on 14 workdays (Monday–Friday) over 6 weeks in the pre-QI period, and on 16 workdays over 4 weeks in the post-QI period, yielding a total of 51 hours of observation.
Overall, across all care environments, there was a statistically significant proportional increase in restorative care from 74% to 92% [χ2(1) 9.53, P = 0.002] (Figure 2). This represents an increase in restorative care events from 40 to 94. Observed custodial care events decreased from 14 to 8, a 43% reduction in custodial care events overall, a difference which remained irrespective of the environment (acute or subacute care), pre- and post-QI activity (P = 0.538 and P = 0.695, respectively).
There was a marked decrease in the number of patients receiving no NA-led restorative care events from 59 (74%) to 32 (48%) before and after QI activity respectively, [χ2 (1) 10.63, P = 0.001].
Patients Observed
Patient population characteristics remained stable during the course of the QI activity; there were no significant differences in the observed patient characteristics pre- and post-QI activity (Table). In 51 hours of observation undertaken by 3 independent observers there were 80 and 71 occupied beds before and after QI activity, respectively, representing a stable bed occupancy rate of 94% and 83% (P = 0.074). Of the occupied beds, 98.7% and 98.6% of patients (pre- and post-QI activity, respectively) were considered recuperating and therefore appropriate for a restorative care approach.
Discussion
although significantly decreased from pre-QI proportions (74%). We therefore conclude that a meaningful decrease across patients receiving no restorative care and a meaningful increase in within-patient restorative care events post-QI intervention occurred.
Our study furthers research in methods of increasing restorative care events delivered by NAs. In a randomized controlled trial by Resnick et al [16], a structured 6-week restorative care program incorporating teaching NAs
restorative care philosophies (tier 1) and facilitating NAs to motivate residents to engage in functional activities (tier 2) was compared to placebo (a single 30-minute educational session in managing residents’ behavioral symptoms) [16]. Results showed the 6-week program led to more restorative care, with NAs demonstrating enhanced knowledge and expectations of restorative care outcomes and better job satisfaction. Our educational package (1 day) and ward-based-learning session (3–4 hours) was much shorter than Resnick et al’s 6-week intervention [16], and the optimal dose of educational packages for NAs is yet to be determined and needs to be addressed in future studies. Furthermore, while we found education increased restorative care across multiple environments, it is yet to be determined whether more restorative care has a positive impact on patient function downstream of an acute inpatient stay. In fact, determination of restorative care’s influence on augmenting rehabilitation outcomes is a neglected aspect of nursing-AHP practice that we aim to define and investigate in ongoing studies.
The patient population characteristics within the target wards were stable over the course of the QI project. Observed patients’ median Barthel (11) and Abbreviated Mental Test (6) scores remained stable and are indicative of high levels of day-to-day activity dependence [24,26–28]. Over the QI activity period it was therefore unsurprising that modest proportions of patients direc-ted their own care (28% and 33% pre and post-QI, respectively). Subsequently, demands on staff to lead patient care were substantial, leading to high risks of social or clinical iatrogenesis and hospital-associated deconditioning.
In a previous observational study, substantial patient inactivity was found in a highly dependent cohort of patients [29]. Fear of falling and insignificant emphasis on ambulation were cited as patient and organizational-centric reasons, respectively. Furthermore, in a selective observational study, patients receiving function-focused care (FFC; synonymous to restorative care) in an acute hospital environment developed less physical functional decline compared to those receiving custodial care [30]. However, patients who had fallen during their hospital stay received less FFC. The authors suggest limited FFC in fallers was deployed to minimize further risk but concluded there is need for nursing and therapy interventions that manage fall risks through endorsing functional activities instead of mobility restriction [30].
Limitations
While observational studies are more robust for measuring behavioral activities compared to self or proxy reporting [34], they are exposed to observer judgment and drift. An attempt was made to minimize this with the binary measurement of restorative versus custodial care and by random sampling of wards and time frames to capture an entire healthcare environment.
The observational study tool was based on one previously developed where acceptable reliability and validity was established and where observations were based on what individual care staff were practicing regardless of their operational environment [20]. In contrast, our observations were based in predetermined environmental spaces regardless of what care practice occurred within it. We consider our approach justifiable in minimizing observer influence on an individual’s practice by emphasizing to them that observers were interested in what happened in an environment [35,36]. However, we acknowledge the risk of under representation of care by observers not following the care delivery, and that local validity and reliability of our methods was not undertaken. Lastly, whilst training for observers was undertaken in this study to standardize the observations undertaken, validation of this method would be a feature required of any future experimental work.
Conclusions
Our findings support the current understanding of restorative care [14–16] and provides proof of concept that dedicating resources in a previously under-invested part of the workforce is feasible, well-accepted, and meaningful. The results are in keeping with the concept that the NA staff group is ready and able to fulfil their roles as direct caregivers, supporting and relieving other trained staff [11].
Corresponding author: Gareth D. Jones, MSc, Physiotherapy Dept, 3rd Fl Lambeth Wing, St Thomas’ Hospital, Westminster Bridge Rd, London SE1 7EH, UK, [email protected].
Funding/support: This work was supported by a small grants application to the Guy's and St Thomas' Charity, project code S100414.
Financial disclosures: No conflicts of interest to declare.
Acknowledgment: The authors acknowledge members of the steering group for their input: Rebekah Schiff, Carrie-Ann Wood, Judith Centofanti, Judith Hall, and Richard Page; Anne Bisset-Smith and Claudia Jacob for their initial pilot work; Amanda Buttery, Lottie Prowse, and Ryan Mackie for practical assistance; Siobhan Crichton for her statistical help; and Jacky Jones, Michael Thacker, Tisha Pryor, and Sarah Ritchie for helping review the manuscript.
Abstract
- Background: Acute and prolonged exposure to hospital medical care can cause hospital-associated deconditioning with deleterious effects on patient care provision and quality of life. Physical rehabilitation provided by allied healthcare professionals can enable reacquisition of function via professional input into attainment of set goals. Separate to rehabilitative efforts, restorative care optimizes independence by motivating individuals to maintain and restore function. Nursing assistants (NAs) provide a significant amount of direct patient care and are well placed to deliver restorative care.
- Objective: To increase proportional restorative care interactions with hospitalized older adults by training NAs.
- Methods: A prospective cohort quality improvement (QI) project was undertaken at 3 acute hospital wards (patient minimum age 65 years) and 2 community subacute care wards in the UK. NAs working within the target settings received a 2-part restorative care training package. The primary evaluation tool was 51 hours in total of observation measuring the proportional change in restorative care events delivered by NAs.
- Results: NA-led restorative care events increased from 40 (pre-intervention) to 94 (post-intervention), representing a statistically significant proportional increase from 74% to 92% (χ2(1) 9.53, P = 0.002). NAs on occasions inadvertently emphasized restriction of function to manage risk and oblige with rest periods.
- Conclusion: Investing in NAs can influence the amount of restorative care delivered to hospitalized older adults at risk of hospital-associated deconditioning. Continued investment in NAs is indicated to influence top-down, mandated restorative care practice in this patient group.
Key words: older people; restorative care; hospital associated deconditioning; nursing assistants; rehabilitation; training.
Hospital-associated deconditioning is defined as a significant decline in functional abilities developed through acute and prolonged exposure to a medical care facility environment, and is independent of that attributed to primary pathologies resulting in acute admission [1]. Considerable research on iatrogenic complications in older hospitalized populations [1–5] has shown the impacts of hospital-associated deconditioning and associated dysfunctions on quality of life for patients and the resultant burden on health and social care provision [6].
Physical rehabilitation has been shown to restore function through high-dose repetition of task-specific activity [7], and the benefits attributed to extra physical therapy include improved mobility, activity, and participation [8]. Simply defined, physical rehabilitation is the reacquisition of function through multidisciplinary assessment and professional therapeutic input in attainment of set goals. A more recent nomenclature in health settings is “restorative care,” defined as a philosophy of care that encourages, enables, and motivates individuals to maintain and restore function, thereby optimizing independence [9]. It has been clearly defined as a philosophy separate from that of rehabilitation [9] and remote from task-related or “custodial care,” which is designed to assist in meeting patients’ daily activity needs without any therapeutic value.
In UK rehabilitation wards, nursing staff provide 4.5 times as much direct patient care time compared with allied health professionals, with paraprofessional nursing assistants (NAs, equivalent to certified nurse assistants [CNAs] in the United States) responsible for half of this direct nursing care [10]. Kessler’s group examined the evolving role of NAs in UK hospitals [11]. From a national survey of 700 NAs and 600 trained nurses, the authors upheld the view that NAs act as direct caregivers including through routine tasks traditionally delivered by nurses. They identified that NAs exhibit distinct qualities, which are valued by qualified nurses, including routine task fulfilment and abilities relating to patients, which enable NAs to enhance care quality. Indeed, the national findings of Kessler’s group were generalizable to our own clinical setting where a NA cohort was a well-placed, available, and motivated resource to deliver therapeutically focused care for our hospitalized older population.
The theoretical relationship between care approaches is complex and represents a challenge for service users and policy makers. For instance, comprehensive rehabilitation delivery during an acute care episode may lead to users not seeking custodial care at home. Conversely, day-to-day activities realized by custodial care at home may lead to users not seeking acute rehabilitative care [12]. With stable resources being assigned to more dependent users in higher numbers, reactive care regardless of environment has often been the model of choice.
However, an economic rationale has developed more recently where investment in maintenance and preventative models results in healthcare savings with models including the 4Rs; reablement, reactivation, rehabilitation, and restorative care [13]. In North America, restorative care approaches have resulted in favorable results in nursing home facilities [14] and at home [15], and restorative care education and motivation training for nursing assistants was effective in supporting a change in beliefs and practice behaviors [16]. While results show restorative care practices in the non-acute care sector are advantageous, it is unknown whether these approaches if adopted in hospital settings affect subsequent healthcare utilization in the non-acute facilities, or even if they are feasible to implement in acute facilities by a staff group able to do so. Therefore, the purpose of this QI project was to deploy a restorative care educational intervention for NA staff working with hospitalized older adults with the aim of increasing the proportion of restorative care delivered.
Methods
Context
This project was conducted at a UK National Health Service university teaching hospital trust at 3 acute hospital wards (patient minimum age 65 years) and 2 community subacute care wards for older patients. Participants consisted of all permanent or long-term temporary (> 3 months continuous employment) NAs working in the target settings (n = 36). The QI project design is summarized in Figure 1. The project applied the 4Es translational approach to regulate the QI intervention: Engage, Educate, Execute, and Evaluate [17]. The reporting of this study follows SQUIRE guidance [18].
Intervention
The QI activity was a holistic educational process for all NA participants.
Didactic Study Day
Each NA attended a study day led by a physical therapist (up to 10 NAs per group). A student-centered training approach was adopted, recognizing variations in adult learning styles [19], and included seminar style theory, video case scenarios, group work, practical skills, open discussion, and reflection. The training package outline was compiled following consensus among the multi-disciplinary team working in the target settings and the steering group. Topics covered were theory on the risk of hospitalization and benefits of early mobilization; case scenarios and examples of restorative care; identifying and overcoming barriers to restorative care; identifying appro-priate patients for a restorative care approach; practical skills, including assisting patients out of bed, ambulation, and eating/drinking; and challenging, problem-solving scenarios. All participants received a course handbook to facilitate learning.
Ward-Based Practice
Measures
Type of Care Event
The quantity and nature of all NA-patient functional task-related care events was established by independent systematic observation pre- and post-intervention. Observers rated the type of care for observed patients as either custodial or restorative events using a tool described below. In addition, the numbers of patients receiving no restorative care events at all during observation was calculated to capture changes in rates between patients observed. The observational tool used was adapted from that utilized in a North American study of a long-term care facility [20], which demonstrated favorable intra-rater reliability (person separation reliability of 0.77), inter-rater reliability (80% to 100% agreement on each of the care behaviors), and validity (evidence of unidimensionality and good fit of the items). Adaptations accommodated for data collection in a hospital environment and alteration to UK nomenclature.
Three blinded volunteer assessors undertook observations. The observers monitored for activity in any 1 of 8 functional domains: bed mobility, transfers, mobility, washing and dressing, exercise, hygiene (mouth care/shaving/hair/nail care), toileting, and eating. Patient activity observed within these domains was identified as either a restorative or custodial care event. For example: “asks or encourages patient to walk/independently propel wheelchair to bathroom/toilet/day room/activities and gives them time to perform activity” was identified as a restorative care event, while “utilizes wheelchair instead of encourages ambulation and does not encourage patient to self-propel” was considered a custodial care event. All observations were carried out by student physical therapists in training or physical therapy assistants, all of whom were familiar in working in the acute facility with hospitalized older people. In an attempt to optimize internal consistency, observer skill was quality-controlled by ensuring observers were trained and their competency assessed in the use of the evaluation measurement tool.
Bays of 3 to 6 beds comprised each observation space. Three 90-minute time epochs were selected for observation—awakening (early morning), lunchtime (middle of the day), and afternoon (before evening meal)—with the aim that each time frame be observed on a minimum of 1 occasion on each of the 5 wards to generate a minimum of 15 observation sessions. Resources dictated observational periods to be 90-minutes maximum, per epoch, on weekdays only. The mean (range) time between the didactic study day and the ward-based practice day was 4 (1–8) weeks, and between the ward-based practice day and the second observational period was 6 (1–14) weeks.
Patient Characteristics
Differences in the acuity of patients between pre- and post-QI activity in the observational environments could influence care demands. Therefore, patient characteristics before and after the QI activity were measured to assess for stability. Prior to each session, observers recorded patient demographic details and current STRATIFY score, a predictive tool used at the time to segment fall risk [21], from patients’ clinical records. Two measures were used to offer contemporaneous representation of the observed population in the observation environment: a modified Barthel index [22], which provides a measure of activities of daily living [23], and the Abbreviated Mental Test Score [24], a simple diagnostic screen for cognitive impairment. All patients were considered as recuperating and thus eligible for observation except those with a “Patient-At-Risk” score ≥ 4, indicating physiological factors associated with established or impending critical illness [25], or if an end of life care plan was clearly detailed in the clinical record.
Data Analysis
Patient demographics are reported descriptively. Ordinal data are summarized using median and inter-quartile ranges (IQR), interval/ratio data using mean and standard deviation (SD) unless otherwise stated. Categorical data are reported as percentages. Comparison of observed patient samples before and after the QI period were compared with the Mann-Whitney U-test for ordinal data, 2 sample t tests for interval/ratio data, and chi squared tests of proportions for other variables.
Analyses were carried out using STATA 11 ME (StataCorp, College Station, TX) and SPSS v17 (SPSS, Chicago). Statistical significance was set at P ≤ 0.05.
Ethical Issues
This study was approved by the local UK NHS Trust clinical audit committee (Quality Improvement project 2038).
Results
Care Events by NAs
Observations were undertaken across the 5 wards on 14 workdays (Monday–Friday) over 6 weeks in the pre-QI period, and on 16 workdays over 4 weeks in the post-QI period, yielding a total of 51 hours of observation.
Overall, across all care environments, there was a statistically significant proportional increase in restorative care from 74% to 92% [χ2(1) 9.53, P = 0.002] (Figure 2). This represents an increase in restorative care events from 40 to 94. Observed custodial care events decreased from 14 to 8, a 43% reduction in custodial care events overall, a difference which remained irrespective of the environment (acute or subacute care), pre- and post-QI activity (P = 0.538 and P = 0.695, respectively).
There was a marked decrease in the number of patients receiving no NA-led restorative care events from 59 (74%) to 32 (48%) before and after QI activity respectively, [χ2 (1) 10.63, P = 0.001].
Patients Observed
Patient population characteristics remained stable during the course of the QI activity; there were no significant differences in the observed patient characteristics pre- and post-QI activity (Table). In 51 hours of observation undertaken by 3 independent observers there were 80 and 71 occupied beds before and after QI activity, respectively, representing a stable bed occupancy rate of 94% and 83% (P = 0.074). Of the occupied beds, 98.7% and 98.6% of patients (pre- and post-QI activity, respectively) were considered recuperating and therefore appropriate for a restorative care approach.
Discussion
although significantly decreased from pre-QI proportions (74%). We therefore conclude that a meaningful decrease across patients receiving no restorative care and a meaningful increase in within-patient restorative care events post-QI intervention occurred.
Our study furthers research in methods of increasing restorative care events delivered by NAs. In a randomized controlled trial by Resnick et al [16], a structured 6-week restorative care program incorporating teaching NAs
restorative care philosophies (tier 1) and facilitating NAs to motivate residents to engage in functional activities (tier 2) was compared to placebo (a single 30-minute educational session in managing residents’ behavioral symptoms) [16]. Results showed the 6-week program led to more restorative care, with NAs demonstrating enhanced knowledge and expectations of restorative care outcomes and better job satisfaction. Our educational package (1 day) and ward-based-learning session (3–4 hours) was much shorter than Resnick et al’s 6-week intervention [16], and the optimal dose of educational packages for NAs is yet to be determined and needs to be addressed in future studies. Furthermore, while we found education increased restorative care across multiple environments, it is yet to be determined whether more restorative care has a positive impact on patient function downstream of an acute inpatient stay. In fact, determination of restorative care’s influence on augmenting rehabilitation outcomes is a neglected aspect of nursing-AHP practice that we aim to define and investigate in ongoing studies.
The patient population characteristics within the target wards were stable over the course of the QI project. Observed patients’ median Barthel (11) and Abbreviated Mental Test (6) scores remained stable and are indicative of high levels of day-to-day activity dependence [24,26–28]. Over the QI activity period it was therefore unsurprising that modest proportions of patients direc-ted their own care (28% and 33% pre and post-QI, respectively). Subsequently, demands on staff to lead patient care were substantial, leading to high risks of social or clinical iatrogenesis and hospital-associated deconditioning.
In a previous observational study, substantial patient inactivity was found in a highly dependent cohort of patients [29]. Fear of falling and insignificant emphasis on ambulation were cited as patient and organizational-centric reasons, respectively. Furthermore, in a selective observational study, patients receiving function-focused care (FFC; synonymous to restorative care) in an acute hospital environment developed less physical functional decline compared to those receiving custodial care [30]. However, patients who had fallen during their hospital stay received less FFC. The authors suggest limited FFC in fallers was deployed to minimize further risk but concluded there is need for nursing and therapy interventions that manage fall risks through endorsing functional activities instead of mobility restriction [30].
Limitations
While observational studies are more robust for measuring behavioral activities compared to self or proxy reporting [34], they are exposed to observer judgment and drift. An attempt was made to minimize this with the binary measurement of restorative versus custodial care and by random sampling of wards and time frames to capture an entire healthcare environment.
The observational study tool was based on one previously developed where acceptable reliability and validity was established and where observations were based on what individual care staff were practicing regardless of their operational environment [20]. In contrast, our observations were based in predetermined environmental spaces regardless of what care practice occurred within it. We consider our approach justifiable in minimizing observer influence on an individual’s practice by emphasizing to them that observers were interested in what happened in an environment [35,36]. However, we acknowledge the risk of under representation of care by observers not following the care delivery, and that local validity and reliability of our methods was not undertaken. Lastly, whilst training for observers was undertaken in this study to standardize the observations undertaken, validation of this method would be a feature required of any future experimental work.
Conclusions
Our findings support the current understanding of restorative care [14–16] and provides proof of concept that dedicating resources in a previously under-invested part of the workforce is feasible, well-accepted, and meaningful. The results are in keeping with the concept that the NA staff group is ready and able to fulfil their roles as direct caregivers, supporting and relieving other trained staff [11].
Corresponding author: Gareth D. Jones, MSc, Physiotherapy Dept, 3rd Fl Lambeth Wing, St Thomas’ Hospital, Westminster Bridge Rd, London SE1 7EH, UK, [email protected].
Funding/support: This work was supported by a small grants application to the Guy's and St Thomas' Charity, project code S100414.
Financial disclosures: No conflicts of interest to declare.
Acknowledgment: The authors acknowledge members of the steering group for their input: Rebekah Schiff, Carrie-Ann Wood, Judith Centofanti, Judith Hall, and Richard Page; Anne Bisset-Smith and Claudia Jacob for their initial pilot work; Amanda Buttery, Lottie Prowse, and Ryan Mackie for practical assistance; Siobhan Crichton for her statistical help; and Jacky Jones, Michael Thacker, Tisha Pryor, and Sarah Ritchie for helping review the manuscript.
1. Kortebein P. Rehabilitation for hospital-associated deconditioning. Am J Phys Med Rehabil 2009;88:66–77.
2. Creditor MC. Hazards of hospitalization of the elderly. Ann Intern Med 1993;118:219–23.
3. Davydow DS, Hough CL, Levine DA, et al. Functional disability, cognitive impairment, and depression after hospitalization for pneumonia. Am J Med 2013;126:615–24.e5.
4. Sager MA, Franke T, Inouye SK, et al. Functional outcomes of acute medical illness and hospitalization in older persons. Arch Intern Med 1996;156:645–52.
5. Warshaw GA, Moore JT, Friedman SW, et al. Functional disability in the hospitalized elderly. JAMA 1982;248:847–50.
6. Covinsky KE, Pierluissi E, Johnston CB. Hospitalization-associated disability: "She was probably able to ambulate, but I'm not sure". JAMA 2011;306:1782–93.
7. Kwakkel G, Kollen B, Lindeman E. Understanding the pattern of functional recovery after stroke: facts and theories. Restor Neurol Neurosci 2004;22:281–99.
8. Peiris CL, Taylor NF, Shields N. Extra physical therapy reduces patient length of stay and improves functional outcomes and quality of life in people with acute or subacute conditions: a systematic review. Arch Phys Med
Rehabil 2011;92:1490–500.
9. Resnick B, Boltz M, Galik E, Pretzer-Aboff I. Restorative care nursing for older adults: a guide for all care settings. 2nd ed. New York: Springer; 2012.
10. Rudd AG, Jenkinson D, Grant RL, Hoffman A. Staffing levels and patient dependence in English stroke units. Clin Med (Lond). 2009;9:110–5.
11. Kessler I, Heron P, Dopson S, et al. The nature and consequences of support workers in a hospital setting, Final Report. London: National Institute for Health Research, Service Delivery and Organization Programme; 2010.
12. Kashner TM, Krompholz B, McDonnell C, et al. Acute and custodial care among impaired aged. J Aging Health 1990;2:28–41.
13. Sims-Gould J, Tong CE, Wallis-Mayer L, Ashe MC. Reablement, reactivation, rehabilitation and restorative interventions with older adults in receipt of home care: a systematic review. J Am Med Dir Assoc 2017;18:653–63.
14. Shanti C, Johnson J, Meyers AM, et al. Evaluation of the restorative care education and training program for nursing homes. Can J Aging 2005;24:115–26.
15. Tinetti ME, Baker D, Gallo WT, et al. Evaluation of restorative care vs usual care for older adults receiving an acute episode of home care. JAMA 2002;287:2098–105.
16. Resnick B, Gruber-Baldini AL, Galik E, et al. Changing the philosophy of care in long-term care: testing of the restorative care intervention. Gerontologist 2009;49:175–84.
17. Pronovost PJ, Berenholtz SM, Needham DM. Translating evidence into practice: a model for large scale knowledge translation. BMJ 2008;337:a1714.
18. Davidoff F, Batalden P, Stevens D, et al; SQUIRE development group. Publication guidelines for quality improvement studies in health care: evolution of the SQUIRE project. BMJ 2009;338:a3152.
19. Sweeney JF. Nurse education: learner-centred or teacher-centred? Nurse Educ Today 1986;6:257–62.
20. Resnick B, Rogers V, Galik E, Gruber-Baldini AL. Measuring restorative care provided by nursing assistants: reliability and validity of the Restorative Care Behavior Checklist. Nurs Res 2007;56:387–98.
21. Oliver D, Britton M, Seed P, et al. Development and evaluation of evidence based risk assessment tool (STRATIFY) to predict which elderly inpatients will fall: case-control and cohort studies. BMJ 1997;315:1049–53.
22. Colin C, Wade DT, Davies S, Horne V. The Barthel ADL Index: a reliability study. Int Disabil Stud 1988;10:61–3.
23. Richards SH, Peters TJ, Coast J, et al. Inter-rater reliability of the Barthel ADL index: how does a researcher compare to a nurse? Clin Rehabil 2000;14:72–8.
24. Hodkinson HM. Evaluation of a mental test score for assessment of mental impairment in the elderly. Age Ageing 1972;1:233–8.
25. Morgan CD, Baade LE. Neuropsychological testing and assessment scales for dementia of the Alzheimer's type. Psychiatr Clin North Am 1997;20:25–43.
26. Granger CV, Hamilton BB, Gresham GE, Kramer AA. The stroke rehabilitation outcome study: Part II. Relative merits of the total Barthel index score and a four-item subscore in predicting patient outcomes. Arch Phys Med Rehabil
1989;70:100–3.
27. MacKenzie DM, Copp P, Shaw RJ, Goodwin GM. Brief cognitive screening of the elderly: a comparison of the Mini-Mental State Examination (MMSE), Abbreviated Mental Test (AMT) and Mental Status Questionnaire (MSQ). Psychol Med
1996;26:427–30.
28. Uyttenboogaart M, Stewart RE, Vroomen PC, et al. Optimizing cutoff scores for the Barthel index and the modified Rankin scale for defining outcome in acute stroke trials. Stroke 2005;36:1984–7.
29. Callen BL, Mahoney JE, Grieves CB, et al. Frequency of hallway ambulation by hospitalized older adults on medical units of an academic hospital. Geriatr Nurs 2004;25:212–7.
30. Boltz M, Resnick B, Capezuti E, Shuluk J. Activity restriction vs. self-direction: hospitalised older adults' response to fear of falling. Int J Older People Nurs 2014;9:44–53.
31. Moyle W, Borbasi S, Wallis M, et al. Acute care management of older people with dementia: a qualitative perspective. J Clin Nurs 2011;20:420–8.
32. Olson DM, Borel CO, Laskowitz DT, et al. Quiet time: a nursing intervention to promote sleep in neurocritical care units. Am J Crit Care 2001;10:74–8.
33. Gardner C, Collins C, Osborne S, et al. Creating a therapeutic environment: a non-randomised controlled trial of a quiet time intervention for patients in acute care. Int J Nurs Stud 2009;46:778–86.
34. Kupek E. Bias and heteroscedastic memory error in self-reported health behavior: an investigation using covariance structure analysis. BMC Med Res Methodol 2002;2:14.
35. Fromme HB, Karani R, Downing SM. Direct observation in medical education: a review of the literature and evidence for validity. Mt Sinai J Med 2009;76:365–71.
36. Williams RG, Klamen DA, McGaghie WC. Cognitive, social and environmental sources of bias in clinical performance ratings. Teach Learn Med 2003;15:270–92.
1. Kortebein P. Rehabilitation for hospital-associated deconditioning. Am J Phys Med Rehabil 2009;88:66–77.
2. Creditor MC. Hazards of hospitalization of the elderly. Ann Intern Med 1993;118:219–23.
3. Davydow DS, Hough CL, Levine DA, et al. Functional disability, cognitive impairment, and depression after hospitalization for pneumonia. Am J Med 2013;126:615–24.e5.
4. Sager MA, Franke T, Inouye SK, et al. Functional outcomes of acute medical illness and hospitalization in older persons. Arch Intern Med 1996;156:645–52.
5. Warshaw GA, Moore JT, Friedman SW, et al. Functional disability in the hospitalized elderly. JAMA 1982;248:847–50.
6. Covinsky KE, Pierluissi E, Johnston CB. Hospitalization-associated disability: "She was probably able to ambulate, but I'm not sure". JAMA 2011;306:1782–93.
7. Kwakkel G, Kollen B, Lindeman E. Understanding the pattern of functional recovery after stroke: facts and theories. Restor Neurol Neurosci 2004;22:281–99.
8. Peiris CL, Taylor NF, Shields N. Extra physical therapy reduces patient length of stay and improves functional outcomes and quality of life in people with acute or subacute conditions: a systematic review. Arch Phys Med
Rehabil 2011;92:1490–500.
9. Resnick B, Boltz M, Galik E, Pretzer-Aboff I. Restorative care nursing for older adults: a guide for all care settings. 2nd ed. New York: Springer; 2012.
10. Rudd AG, Jenkinson D, Grant RL, Hoffman A. Staffing levels and patient dependence in English stroke units. Clin Med (Lond). 2009;9:110–5.
11. Kessler I, Heron P, Dopson S, et al. The nature and consequences of support workers in a hospital setting, Final Report. London: National Institute for Health Research, Service Delivery and Organization Programme; 2010.
12. Kashner TM, Krompholz B, McDonnell C, et al. Acute and custodial care among impaired aged. J Aging Health 1990;2:28–41.
13. Sims-Gould J, Tong CE, Wallis-Mayer L, Ashe MC. Reablement, reactivation, rehabilitation and restorative interventions with older adults in receipt of home care: a systematic review. J Am Med Dir Assoc 2017;18:653–63.
14. Shanti C, Johnson J, Meyers AM, et al. Evaluation of the restorative care education and training program for nursing homes. Can J Aging 2005;24:115–26.
15. Tinetti ME, Baker D, Gallo WT, et al. Evaluation of restorative care vs usual care for older adults receiving an acute episode of home care. JAMA 2002;287:2098–105.
16. Resnick B, Gruber-Baldini AL, Galik E, et al. Changing the philosophy of care in long-term care: testing of the restorative care intervention. Gerontologist 2009;49:175–84.
17. Pronovost PJ, Berenholtz SM, Needham DM. Translating evidence into practice: a model for large scale knowledge translation. BMJ 2008;337:a1714.
18. Davidoff F, Batalden P, Stevens D, et al; SQUIRE development group. Publication guidelines for quality improvement studies in health care: evolution of the SQUIRE project. BMJ 2009;338:a3152.
19. Sweeney JF. Nurse education: learner-centred or teacher-centred? Nurse Educ Today 1986;6:257–62.
20. Resnick B, Rogers V, Galik E, Gruber-Baldini AL. Measuring restorative care provided by nursing assistants: reliability and validity of the Restorative Care Behavior Checklist. Nurs Res 2007;56:387–98.
21. Oliver D, Britton M, Seed P, et al. Development and evaluation of evidence based risk assessment tool (STRATIFY) to predict which elderly inpatients will fall: case-control and cohort studies. BMJ 1997;315:1049–53.
22. Colin C, Wade DT, Davies S, Horne V. The Barthel ADL Index: a reliability study. Int Disabil Stud 1988;10:61–3.
23. Richards SH, Peters TJ, Coast J, et al. Inter-rater reliability of the Barthel ADL index: how does a researcher compare to a nurse? Clin Rehabil 2000;14:72–8.
24. Hodkinson HM. Evaluation of a mental test score for assessment of mental impairment in the elderly. Age Ageing 1972;1:233–8.
25. Morgan CD, Baade LE. Neuropsychological testing and assessment scales for dementia of the Alzheimer's type. Psychiatr Clin North Am 1997;20:25–43.
26. Granger CV, Hamilton BB, Gresham GE, Kramer AA. The stroke rehabilitation outcome study: Part II. Relative merits of the total Barthel index score and a four-item subscore in predicting patient outcomes. Arch Phys Med Rehabil
1989;70:100–3.
27. MacKenzie DM, Copp P, Shaw RJ, Goodwin GM. Brief cognitive screening of the elderly: a comparison of the Mini-Mental State Examination (MMSE), Abbreviated Mental Test (AMT) and Mental Status Questionnaire (MSQ). Psychol Med
1996;26:427–30.
28. Uyttenboogaart M, Stewart RE, Vroomen PC, et al. Optimizing cutoff scores for the Barthel index and the modified Rankin scale for defining outcome in acute stroke trials. Stroke 2005;36:1984–7.
29. Callen BL, Mahoney JE, Grieves CB, et al. Frequency of hallway ambulation by hospitalized older adults on medical units of an academic hospital. Geriatr Nurs 2004;25:212–7.
30. Boltz M, Resnick B, Capezuti E, Shuluk J. Activity restriction vs. self-direction: hospitalised older adults' response to fear of falling. Int J Older People Nurs 2014;9:44–53.
31. Moyle W, Borbasi S, Wallis M, et al. Acute care management of older people with dementia: a qualitative perspective. J Clin Nurs 2011;20:420–8.
32. Olson DM, Borel CO, Laskowitz DT, et al. Quiet time: a nursing intervention to promote sleep in neurocritical care units. Am J Crit Care 2001;10:74–8.
33. Gardner C, Collins C, Osborne S, et al. Creating a therapeutic environment: a non-randomised controlled trial of a quiet time intervention for patients in acute care. Int J Nurs Stud 2009;46:778–86.
34. Kupek E. Bias and heteroscedastic memory error in self-reported health behavior: an investigation using covariance structure analysis. BMC Med Res Methodol 2002;2:14.
35. Fromme HB, Karani R, Downing SM. Direct observation in medical education: a review of the literature and evidence for validity. Mt Sinai J Med 2009;76:365–71.
36. Williams RG, Klamen DA, McGaghie WC. Cognitive, social and environmental sources of bias in clinical performance ratings. Teach Learn Med 2003;15:270–92.
A Longitudinal Study of Transfusion Utilization in Hospitalized Veterans
Abstract
- Background: Although transfusion guidelines have changed considerably over the past 2 decades, the adoption of patient blood management programs has not been fully realized across hospitals in the United States.
- Objective: To evaluate trends in red blood cell (RBC), platelet, and plasma transfusion at 3 Veterans Health Administration (VHA) hospitals from 2000 through 2010.
- Methods: Data from all hospitalizations were collected from January 2000 through December 2010. Blood bank data (including the type and volume of products administered) were available electronically from each hospital. These files were linked to inpatient data, which included ICD-9-CM diagnoses (principal and secondary) and procedures during hospitalization. Statistical analyses were conducted using generalized linear models to evaluate trends over time. The unit of observation was hospitalization, with categorization by type.
- Results: There were 176,521 hospitalizations in 69,621 patients; of these, 13.6% of hospitalizations involved transfusion of blood products (12.7% RBCs, 1.4% platelets, 3.0% plasma). Transfusion occurred in 25.2% of surgical and 5.3% of medical hospitalizations. Transfusion use peaked in 2002 for surgical hospitalizations and declined afterwards (P < 0.001). There was no significant change in transfusion use over time (P = 0.126) for medical hospitalizations. In hospitalizations that involved transfusions, there was a 20.3% reduction in the proportion of hospitalizations in which ≥ 3 units of RBCs were given (from 51.7% to 41.1%; P < 0.001) and a 73.6% increase when 1 RBC unit was given (from 8.0% to 13.8%; P < 0.001) from 2000-2010. Of the hospitalizations with RBC transfusion, 9.6% involved the use of 1 unit over the entire study period. The most common principal diagnoses for medical patients receiving transfusion were anemia, malignancy, heart failure, pneumonia and renal failure. Over time, transfusion utilization increased in patients who were admitted for infection (P = 0.009).
- Conclusion: Blood transfusions in 3 VHA hospitals have decreased over time for surgical patients but remained the same for medical patients. Further study examining appropriateness of blood products in medical patients appears necessary.
Key words: Transfusion; red blood cells; plasma; platelets; veterans.
Transfusion practices during hospitalization have changed considerably over the past 2 decades. Guided by evidence from randomized controlled trials, patient blood management programs have been expanded [1]. Such programs include recommendations regarding minimization of blood loss during surgery, prevention and treatment of anemia, strategies for reducing transfusions in both medical and surgical patients, improved blood utilization, education of health professionals, and standardization of blood management-related metrics [2]. Some of the guidelines have been incorporated into the Choosing Wisely initiative of the American Board of Internal Medicine Foundation, including: (a) don’t transfuse more units of blood than absolutely necessary, (b) don’t transfuse red blood cells for iron deficiency without hemodynamic instability, (c) don’t routinely use blood products to reverse warfarin, and (d) don’t perform serial blood counts on clinically stable patients [3]. Although there has been growing interest in blood management, only 37.8% of the 607 AABB (formerly, American Association of Blood Banks) facilities in the United States reported having a patient blood management program in 2013 [2].
While the importance of blood safety is recognized, data regarding the overall trends in practices are conflicting. A study using the Nationwide Inpatient Sample indicated that there was a 5.6% annual mean increase in the transfusion of blood products from 2002 to 2011 in the United States [4]. This contrasts with the experience of Kaiser Permanente in Northern California, in which the incidence of RBC transfusion decreased by 3.2% from 2009 to 2013 [5]. A decline in rates of intraoperative transfusion was also reported among elderly veterans in the United States from 1997 to 2009 [6].
We conducted a study in hospitalized veterans with 2 main objectives: (a) to evaluate trends in utilization of red blood cells (RBCs), platelets, and plasma over time, and (b) to identify those groups of veterans who received specific blood products. We were particularly interested in transfusion use in medical patients.
Methods
Participants were hospitalized veterans at 3 Department of Veterans Affairs (VA) medical centers. Data from all hospitalizations were collected from January 2000 through December 2010. Blood bank data (including the type and volume of products administered) were available electronically from each hospital. These files were linked to inpatient data, which included ICD-9-CM diagnoses (principal and secondary) and procedures during hospitalization.
Statistical analyses were conducted using generalized linear models to evaluate trends over time. The unit of observation was hospitalization, with categorization by type. Surgical hospitalizations were defined as admissions in which any surgical procedure occurred, whereas medical hospitalizations were defined as admissions without any surgery. Alpha was set at 0.05, 2-tailed. All analyses were conducted in Stata/MP 14.1 (StataCorp, College Station, TX). The study received institutional review board approval from the VA Ann Arbor Healthcare System.
Results
From 2000 through 2010, there were 176,521 hospitalizations in 69,621 patients. Within this cohort, 6% were < 40 years of age, 66% were 40 to 69 years of age, and 28% were 70 years or older at the time of admission. In this cohort, 96% of patients were male. Overall, 13.6% of all hospitalizations involved transfusion of a blood product (12.7% RBCs, 1.4% platelets, 3.0% plasma).
Transfusion occurred in 25.2% of surgical hospitalizations and 5.3% of medical hospitalizations. For surgical hospitalizations, transfusion use peaked in 2002 (when 30.9% of the surgical hospitalizations involved a trans-fusion) and significantly declined afterwards (P < 0.001). By 2010, 22.5% of the surgical hospitalizations involved a transfusion. Most of the surgeries where blood products were transfused involved cardiovascular procedures. For medical hospitalizations only, there was no significant change in transfusion use over time, either from 2000 to 2010 (P = 0.126) or from 2002 to 2010 (P = 0.072). In 2010, 5.2% of the medical hospitalizations involved a transfusion.
Rates of transfusion varied by principal diagnosis (Figure 1). For patients admitted with a principal diagnosis of infection (n = 20,981 hospitalizations), there was an increase in the percentage of hospitalizations in which transfusions (RBCs, platelet, plasma) were administered over time (P = 0.009) (Figure 1). For patients admitted with a principal diagnosis of malignancy (n = 12,904 hospitalizations), cardiovascular disease (n = 40,324 hospitalizations), and other diagnoses (n = 102,312 hospitalizations), there were no significant linear trends over the entire study period (P = 0.191, P = 0.052, P = 0.314, respectively). Rather, blood utilization peaked in year 2002 and significantly declined afterwards for patients admitted for malignancy (P < 0.001) and for cardiovascular disease (P < 0.001).
The most common principal diagnoses for medical patients receiving any transfusion (RBCs, platelet, plasma) are listed in Table 1. For medical patients with a principal diagnosis of anemia, 88% of hospitalizations involved a transfusion (Table 1). Transfusion occurred in 6% to 11% of medical hospitalizations with malignancies, heart failure, pneumonia or renal failure (Table 1). A considerable proportion (43%) of medical patients with gastrointestinal hemorrhage received a transfusion.
9.6% (2154/22,344) involved the use of only 1 unit, 43.8% (9791/22,344) involved 2 units, and 46.5% (10,399/22,344) involved 3 or more units during the hospitalization. From 2000 through 2010, there was a 20.3% reduction in the proportion of hospitalizations in which 3 or more units of RBCs were given (from 51.7% to 41.1%; P < 0.001). That is, among those hospitalizations in which a RBC transfusion occurred, a smaller proportion of hospitalizations involved the administration of 3 or more units of RBCs from 2000 through 2010 (Figure 2). There was an 11.5% increase in the proportion of hospitalizations in which 2 units of RBCs were used (from 40.4% to 45.0%; P < 0.001). In addition, there was a 73.6% increase in the proportion of hospitalizations in which 1 RBC unit was given (from 8.0% to 13.8%;
P = 0.001).
16.8 mL/hospitalization in 2010. For plasma, the mean mL/hospitalization was 28.9 in year 2000, increased to 50.1 mL/hospitalization in year 2008, and declined, thereafter, to 35.1 mL/hospitalization in year 2010.
Discussion
We also observed secular trends in the volume of RBCs administered. There was an increase in the percentage of hospitalizations in which 1 or 2 RBC units were used and a decline in transfusion of 3 or more units. The reduction in the use of 3 or more RBC units may reflect the adoption and integration of recommendations in patient blood management by clinicians,
which encourage assessment of the patients’ symptoms in determining whether additional units are necessary [7]. Such guidelines also endorse the avoidance of routine
administration of 2 units of RBCs if 1 unit is sufficient [8]. We have previously shown that, after coronary artery bypass grafting, 2 RBC units doubled the risk of pneumonia [9]; additional analyses indicated that 1 or 2 units of RBCs were associated with increased postoperative morbidity [10]. In addition, our previous research indicated that the probability of infection increased considerably between 1 and 2 RBC units, with a more gradual increase beyond 2 units [11]. With this evidence in mind, some studies at single sites have reported that there was a dramatic decline from 2 RBC units before initiation of patient blood management programs to 1 unit after the programs were implemented [12,13].
Medical patients who received a transfusion were often admitted for reason of anemia, cancer, organ failure, or pneumonia. Some researchers are now reporting that blood use, at certain sites, is becoming more common in medical rather than surgical patients, which may be due to an expansion of patient blood management procedures in surgery [16]. There are a substantial number of patient blood management programs among surgical specialties and their adoption has expanded [17]. Although there are fewer patient blood management programs in the nonsurgical setting, some have been targeted to internal medicine physicians and specifically, to hospitalists [1,18]. For example, a toolkit from the Society of Hospital Medicine centers on anemia management and includes anemia assessment, treatment, evaluation of RBC transfusion risk, blood conservation, optimization of coagulation, and patient-centered decision-making [19]. Additionally, bundling of patient blood management strategies has been launched to help encourage a wider adoption of such programs [20].
While guidelines regarding use of RBCs are becoming increasingly recognized, recommendations for the use of platelets and plasma are hampered by the paucity of evidence from randomized controlled trials [21,22]. There is moderate-quality evidence for the use of platelets with therapy-induced hypoproliferative thrombocytopenia in hospitalized patients [21], but low quality evidence for other uses. Moreover, a recent review of plasma transfusion in bleeding patients found no randomized controlled trials on plasma use in hospitalized patients, although several trials were currently underway [22].
Our findings need to be considered in the context of the following limitations. The data were from 3 VA hospitals, so the results may not reflect patterns of usage at other hospitals. However, AABB reports that there has been a general decrease in transfusion of allogeneic whole blood and RBC units since 2008 at the AABB-affiliated sites in the United States [2]; this is similar to the pattern that we observed in surgical patients. In addition, we report an overall view of trends without having details regarding which specific factors influenced changes in transfusion during this 11-year period. It is possible that the severity of hospitalized patients may have changed with time which could have influenced decisions regarding the need for transfusion.
In conclusion, the use of blood products decreased in surgical patients since 2002 but remained the same in medical patients in this VA population. Transfusions increased over time for patients who were admitted to the hospital for reason of infection, but decreased since 2002 for those admitted for cardiovascular disease or cancer. The number of RBC units per hospitalization decreased over time. Additional surveillance is needed to determine whether recent evidence regarding blood management has been incorporated into clinical practice for medical patients, as we strive to deliver optimal care to our veterans.
Corresponding author: Mary A.M. Rogers, PhD, MS, Dept. of Internal Medicine, Univ. of Michigan, 016-422W NCRC, Ann Arbor, MI 48109-2800, [email protected].
Funding/support: Department of Veterans Affairs, Clinical Sciences Research & Development Service Merit Review Award (EPID-011-11S). The contents do not represent the views of the U.S. Department of Veterans Affairs or the U.S. Government.
Financial disclosures: None.
Author contributions: conception and design, MAMR, SS; analysis and interpretation of data, MAMR, JDB, DR, LK, SS; drafting of article, MAMR; critical revision of the article, MAMR, MTG, DR, LK, SS, VC; statistical expertise, MAMR, DR; obtaining of funding, MTG, SS, VC; administrative or technical support, MTG, LK, SS, VC; collection and assembly of data, JDB, LK.
1. Hohmuth B, Ozawa S, Ashton M, Melseth RL. Patient-centered blood management. J Hosp Med 2014;9:60–5.
2. Whitaker B, Rajbhandary S, Harris A. The 2013 AABB blood collection, utilization, and patient blood management survey report. United States Department of Health and Human Services, AABB; 2015.
3. Cassel CK, Guest JA. Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA 2012;307:1801–2.
4. Pathak R, Bhatt VR, Karmacharya P, et al. Trends in blood-product transfusion among inpatients in the United States from 2002 to 2011: data from the nationwide inpatient sample. J Hosp Med 2014;9:800–1.
5. Roubinian NH, Escobar GJ, Liu V, et al. Trends in red blood cell transfusion and 30-day mortality among hospitalized patients. Transfusion 2014;54:2678–86.
6. Chen A, Trivedi AN, Jiang L, et al. Hospital blood transfusion patterns during major noncardiac surgery and surgical mortality. Medicine (Baltimore) 2015;94:e1342.
7. Carson JL, Guyatt G, Heddle NM, et al. Clinical practice guidelines from the AABB: Red blood cell transfusion thresholds and storage. JAMA 2016;316:2025–35.
8. Hicks LK, Bering H, Carson KR, et al. The ASH choosing wisely® campaign: five hematologic tests and treatments to question. Blood 2013;122:3879–83.
9. Likosky DS, Paone G, Zhang M, et al. Red blood cell transfusions impact pneumonia rates after coronary artery bypass grafting. Ann Thorac Surg 2015;100:794–801.
10. Paone G, Likosky DS, Brewer R, et al. Transfusion of 1 and 2 units of red blood cells is associated with increased morbidity and mortality. Ann Thorac Surg 2014;97:87–93; discussion 93–4.
11. Rogers MAM, Blumberg N, Heal JM, et al. Role of transfusion in the development of urinary tract–related bloodstream infection. Arch Intern Med 2011;171:1587–9.
12. Oliver JC, Griffin RL, Hannon T, Marques MB. The success of our patient blood management program depended on an institution-wide change in transfusion practices. Transfusion 2014;54:2617–24.
13. Yerrabothala S, Desrosiers KP, Szczepiorkowski ZM, Dunbar NM. Significant reduction in red blood cell transfusions in a general hospital after successful implementation of a restrictive transfusion policy supported by prospective computerized order auditing. Transfusion 2014;54:2640–5.
14. Rehm JP, Otto PS, West WW, et al. Hospital-wide educational program decreases red blood cell transfusions. J Surg Res 1998;75:183–6.
15. Lawler EV, Bradbury BD, Fonda JR, et al. Transfusion burden among patients with chronic kidney disease and anemia. Clin J Am Soc Nephrol 2010;5:667–72.
16. Tinegate H, Pendry K, Murphy M, et al. Where do all the red blood cells (RBCs) go? Results of a survey of RBC use in England and North Wales in 2014. Transfusion 2016;56:139–45.
17. Meybohm P, Herrmann E, Steinbicker AU, et al. Patient blood management is associated with a substantial reduction of red blood cell utilization and safe for patient’s outcome: a prospective, multicenter cohort study with a noninferiority design. Ann Surg 2016;264:203–11.
18. Corwin HL, Theus JW, Cargile CS, Lang NP. Red blood cell transfusion: impact of an education program and a clinical guideline on transfusion practice. J Hosp Med 2014;9:745–9.
19. Society of Hospital Medicine. Anemia prevention and management program implementation toolkit. Accessed at www.hospitalmedicine.org/Web/Quality___Innovation/Implementation_Toolkit/Anemia/anemia_overview.aspx on 9 June 2017.
20. Meybohm P, Richards T, Isbister J, et al. Patient blood management bundles to facilitate implementation. Transfus Med Rev 2017;31:62–71.
21. Kaufman RM, Djulbegovic B, Gernsheimer T, et al. Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med 2015;162:205–13.
22. Levy JH, Grottke O, Fries D, Kozek-Langenecker S. Therapeutic plasma transfusion in bleeding patients: A systematic review. Anesth Analg 2017;124:1268–76.
Abstract
- Background: Although transfusion guidelines have changed considerably over the past 2 decades, the adoption of patient blood management programs has not been fully realized across hospitals in the United States.
- Objective: To evaluate trends in red blood cell (RBC), platelet, and plasma transfusion at 3 Veterans Health Administration (VHA) hospitals from 2000 through 2010.
- Methods: Data from all hospitalizations were collected from January 2000 through December 2010. Blood bank data (including the type and volume of products administered) were available electronically from each hospital. These files were linked to inpatient data, which included ICD-9-CM diagnoses (principal and secondary) and procedures during hospitalization. Statistical analyses were conducted using generalized linear models to evaluate trends over time. The unit of observation was hospitalization, with categorization by type.
- Results: There were 176,521 hospitalizations in 69,621 patients; of these, 13.6% of hospitalizations involved transfusion of blood products (12.7% RBCs, 1.4% platelets, 3.0% plasma). Transfusion occurred in 25.2% of surgical and 5.3% of medical hospitalizations. Transfusion use peaked in 2002 for surgical hospitalizations and declined afterwards (P < 0.001). There was no significant change in transfusion use over time (P = 0.126) for medical hospitalizations. In hospitalizations that involved transfusions, there was a 20.3% reduction in the proportion of hospitalizations in which ≥ 3 units of RBCs were given (from 51.7% to 41.1%; P < 0.001) and a 73.6% increase when 1 RBC unit was given (from 8.0% to 13.8%; P < 0.001) from 2000-2010. Of the hospitalizations with RBC transfusion, 9.6% involved the use of 1 unit over the entire study period. The most common principal diagnoses for medical patients receiving transfusion were anemia, malignancy, heart failure, pneumonia and renal failure. Over time, transfusion utilization increased in patients who were admitted for infection (P = 0.009).
- Conclusion: Blood transfusions in 3 VHA hospitals have decreased over time for surgical patients but remained the same for medical patients. Further study examining appropriateness of blood products in medical patients appears necessary.
Key words: Transfusion; red blood cells; plasma; platelets; veterans.
Transfusion practices during hospitalization have changed considerably over the past 2 decades. Guided by evidence from randomized controlled trials, patient blood management programs have been expanded [1]. Such programs include recommendations regarding minimization of blood loss during surgery, prevention and treatment of anemia, strategies for reducing transfusions in both medical and surgical patients, improved blood utilization, education of health professionals, and standardization of blood management-related metrics [2]. Some of the guidelines have been incorporated into the Choosing Wisely initiative of the American Board of Internal Medicine Foundation, including: (a) don’t transfuse more units of blood than absolutely necessary, (b) don’t transfuse red blood cells for iron deficiency without hemodynamic instability, (c) don’t routinely use blood products to reverse warfarin, and (d) don’t perform serial blood counts on clinically stable patients [3]. Although there has been growing interest in blood management, only 37.8% of the 607 AABB (formerly, American Association of Blood Banks) facilities in the United States reported having a patient blood management program in 2013 [2].
While the importance of blood safety is recognized, data regarding the overall trends in practices are conflicting. A study using the Nationwide Inpatient Sample indicated that there was a 5.6% annual mean increase in the transfusion of blood products from 2002 to 2011 in the United States [4]. This contrasts with the experience of Kaiser Permanente in Northern California, in which the incidence of RBC transfusion decreased by 3.2% from 2009 to 2013 [5]. A decline in rates of intraoperative transfusion was also reported among elderly veterans in the United States from 1997 to 2009 [6].
We conducted a study in hospitalized veterans with 2 main objectives: (a) to evaluate trends in utilization of red blood cells (RBCs), platelets, and plasma over time, and (b) to identify those groups of veterans who received specific blood products. We were particularly interested in transfusion use in medical patients.
Methods
Participants were hospitalized veterans at 3 Department of Veterans Affairs (VA) medical centers. Data from all hospitalizations were collected from January 2000 through December 2010. Blood bank data (including the type and volume of products administered) were available electronically from each hospital. These files were linked to inpatient data, which included ICD-9-CM diagnoses (principal and secondary) and procedures during hospitalization.
Statistical analyses were conducted using generalized linear models to evaluate trends over time. The unit of observation was hospitalization, with categorization by type. Surgical hospitalizations were defined as admissions in which any surgical procedure occurred, whereas medical hospitalizations were defined as admissions without any surgery. Alpha was set at 0.05, 2-tailed. All analyses were conducted in Stata/MP 14.1 (StataCorp, College Station, TX). The study received institutional review board approval from the VA Ann Arbor Healthcare System.
Results
From 2000 through 2010, there were 176,521 hospitalizations in 69,621 patients. Within this cohort, 6% were < 40 years of age, 66% were 40 to 69 years of age, and 28% were 70 years or older at the time of admission. In this cohort, 96% of patients were male. Overall, 13.6% of all hospitalizations involved transfusion of a blood product (12.7% RBCs, 1.4% platelets, 3.0% plasma).
Transfusion occurred in 25.2% of surgical hospitalizations and 5.3% of medical hospitalizations. For surgical hospitalizations, transfusion use peaked in 2002 (when 30.9% of the surgical hospitalizations involved a trans-fusion) and significantly declined afterwards (P < 0.001). By 2010, 22.5% of the surgical hospitalizations involved a transfusion. Most of the surgeries where blood products were transfused involved cardiovascular procedures. For medical hospitalizations only, there was no significant change in transfusion use over time, either from 2000 to 2010 (P = 0.126) or from 2002 to 2010 (P = 0.072). In 2010, 5.2% of the medical hospitalizations involved a transfusion.
Rates of transfusion varied by principal diagnosis (Figure 1). For patients admitted with a principal diagnosis of infection (n = 20,981 hospitalizations), there was an increase in the percentage of hospitalizations in which transfusions (RBCs, platelet, plasma) were administered over time (P = 0.009) (Figure 1). For patients admitted with a principal diagnosis of malignancy (n = 12,904 hospitalizations), cardiovascular disease (n = 40,324 hospitalizations), and other diagnoses (n = 102,312 hospitalizations), there were no significant linear trends over the entire study period (P = 0.191, P = 0.052, P = 0.314, respectively). Rather, blood utilization peaked in year 2002 and significantly declined afterwards for patients admitted for malignancy (P < 0.001) and for cardiovascular disease (P < 0.001).
The most common principal diagnoses for medical patients receiving any transfusion (RBCs, platelet, plasma) are listed in Table 1. For medical patients with a principal diagnosis of anemia, 88% of hospitalizations involved a transfusion (Table 1). Transfusion occurred in 6% to 11% of medical hospitalizations with malignancies, heart failure, pneumonia or renal failure (Table 1). A considerable proportion (43%) of medical patients with gastrointestinal hemorrhage received a transfusion.
9.6% (2154/22,344) involved the use of only 1 unit, 43.8% (9791/22,344) involved 2 units, and 46.5% (10,399/22,344) involved 3 or more units during the hospitalization. From 2000 through 2010, there was a 20.3% reduction in the proportion of hospitalizations in which 3 or more units of RBCs were given (from 51.7% to 41.1%; P < 0.001). That is, among those hospitalizations in which a RBC transfusion occurred, a smaller proportion of hospitalizations involved the administration of 3 or more units of RBCs from 2000 through 2010 (Figure 2). There was an 11.5% increase in the proportion of hospitalizations in which 2 units of RBCs were used (from 40.4% to 45.0%; P < 0.001). In addition, there was a 73.6% increase in the proportion of hospitalizations in which 1 RBC unit was given (from 8.0% to 13.8%;
P = 0.001).
16.8 mL/hospitalization in 2010. For plasma, the mean mL/hospitalization was 28.9 in year 2000, increased to 50.1 mL/hospitalization in year 2008, and declined, thereafter, to 35.1 mL/hospitalization in year 2010.
Discussion
We also observed secular trends in the volume of RBCs administered. There was an increase in the percentage of hospitalizations in which 1 or 2 RBC units were used and a decline in transfusion of 3 or more units. The reduction in the use of 3 or more RBC units may reflect the adoption and integration of recommendations in patient blood management by clinicians,
which encourage assessment of the patients’ symptoms in determining whether additional units are necessary [7]. Such guidelines also endorse the avoidance of routine
administration of 2 units of RBCs if 1 unit is sufficient [8]. We have previously shown that, after coronary artery bypass grafting, 2 RBC units doubled the risk of pneumonia [9]; additional analyses indicated that 1 or 2 units of RBCs were associated with increased postoperative morbidity [10]. In addition, our previous research indicated that the probability of infection increased considerably between 1 and 2 RBC units, with a more gradual increase beyond 2 units [11]. With this evidence in mind, some studies at single sites have reported that there was a dramatic decline from 2 RBC units before initiation of patient blood management programs to 1 unit after the programs were implemented [12,13].
Medical patients who received a transfusion were often admitted for reason of anemia, cancer, organ failure, or pneumonia. Some researchers are now reporting that blood use, at certain sites, is becoming more common in medical rather than surgical patients, which may be due to an expansion of patient blood management procedures in surgery [16]. There are a substantial number of patient blood management programs among surgical specialties and their adoption has expanded [17]. Although there are fewer patient blood management programs in the nonsurgical setting, some have been targeted to internal medicine physicians and specifically, to hospitalists [1,18]. For example, a toolkit from the Society of Hospital Medicine centers on anemia management and includes anemia assessment, treatment, evaluation of RBC transfusion risk, blood conservation, optimization of coagulation, and patient-centered decision-making [19]. Additionally, bundling of patient blood management strategies has been launched to help encourage a wider adoption of such programs [20].
While guidelines regarding use of RBCs are becoming increasingly recognized, recommendations for the use of platelets and plasma are hampered by the paucity of evidence from randomized controlled trials [21,22]. There is moderate-quality evidence for the use of platelets with therapy-induced hypoproliferative thrombocytopenia in hospitalized patients [21], but low quality evidence for other uses. Moreover, a recent review of plasma transfusion in bleeding patients found no randomized controlled trials on plasma use in hospitalized patients, although several trials were currently underway [22].
Our findings need to be considered in the context of the following limitations. The data were from 3 VA hospitals, so the results may not reflect patterns of usage at other hospitals. However, AABB reports that there has been a general decrease in transfusion of allogeneic whole blood and RBC units since 2008 at the AABB-affiliated sites in the United States [2]; this is similar to the pattern that we observed in surgical patients. In addition, we report an overall view of trends without having details regarding which specific factors influenced changes in transfusion during this 11-year period. It is possible that the severity of hospitalized patients may have changed with time which could have influenced decisions regarding the need for transfusion.
In conclusion, the use of blood products decreased in surgical patients since 2002 but remained the same in medical patients in this VA population. Transfusions increased over time for patients who were admitted to the hospital for reason of infection, but decreased since 2002 for those admitted for cardiovascular disease or cancer. The number of RBC units per hospitalization decreased over time. Additional surveillance is needed to determine whether recent evidence regarding blood management has been incorporated into clinical practice for medical patients, as we strive to deliver optimal care to our veterans.
Corresponding author: Mary A.M. Rogers, PhD, MS, Dept. of Internal Medicine, Univ. of Michigan, 016-422W NCRC, Ann Arbor, MI 48109-2800, [email protected].
Funding/support: Department of Veterans Affairs, Clinical Sciences Research & Development Service Merit Review Award (EPID-011-11S). The contents do not represent the views of the U.S. Department of Veterans Affairs or the U.S. Government.
Financial disclosures: None.
Author contributions: conception and design, MAMR, SS; analysis and interpretation of data, MAMR, JDB, DR, LK, SS; drafting of article, MAMR; critical revision of the article, MAMR, MTG, DR, LK, SS, VC; statistical expertise, MAMR, DR; obtaining of funding, MTG, SS, VC; administrative or technical support, MTG, LK, SS, VC; collection and assembly of data, JDB, LK.
Abstract
- Background: Although transfusion guidelines have changed considerably over the past 2 decades, the adoption of patient blood management programs has not been fully realized across hospitals in the United States.
- Objective: To evaluate trends in red blood cell (RBC), platelet, and plasma transfusion at 3 Veterans Health Administration (VHA) hospitals from 2000 through 2010.
- Methods: Data from all hospitalizations were collected from January 2000 through December 2010. Blood bank data (including the type and volume of products administered) were available electronically from each hospital. These files were linked to inpatient data, which included ICD-9-CM diagnoses (principal and secondary) and procedures during hospitalization. Statistical analyses were conducted using generalized linear models to evaluate trends over time. The unit of observation was hospitalization, with categorization by type.
- Results: There were 176,521 hospitalizations in 69,621 patients; of these, 13.6% of hospitalizations involved transfusion of blood products (12.7% RBCs, 1.4% platelets, 3.0% plasma). Transfusion occurred in 25.2% of surgical and 5.3% of medical hospitalizations. Transfusion use peaked in 2002 for surgical hospitalizations and declined afterwards (P < 0.001). There was no significant change in transfusion use over time (P = 0.126) for medical hospitalizations. In hospitalizations that involved transfusions, there was a 20.3% reduction in the proportion of hospitalizations in which ≥ 3 units of RBCs were given (from 51.7% to 41.1%; P < 0.001) and a 73.6% increase when 1 RBC unit was given (from 8.0% to 13.8%; P < 0.001) from 2000-2010. Of the hospitalizations with RBC transfusion, 9.6% involved the use of 1 unit over the entire study period. The most common principal diagnoses for medical patients receiving transfusion were anemia, malignancy, heart failure, pneumonia and renal failure. Over time, transfusion utilization increased in patients who were admitted for infection (P = 0.009).
- Conclusion: Blood transfusions in 3 VHA hospitals have decreased over time for surgical patients but remained the same for medical patients. Further study examining appropriateness of blood products in medical patients appears necessary.
Key words: Transfusion; red blood cells; plasma; platelets; veterans.
Transfusion practices during hospitalization have changed considerably over the past 2 decades. Guided by evidence from randomized controlled trials, patient blood management programs have been expanded [1]. Such programs include recommendations regarding minimization of blood loss during surgery, prevention and treatment of anemia, strategies for reducing transfusions in both medical and surgical patients, improved blood utilization, education of health professionals, and standardization of blood management-related metrics [2]. Some of the guidelines have been incorporated into the Choosing Wisely initiative of the American Board of Internal Medicine Foundation, including: (a) don’t transfuse more units of blood than absolutely necessary, (b) don’t transfuse red blood cells for iron deficiency without hemodynamic instability, (c) don’t routinely use blood products to reverse warfarin, and (d) don’t perform serial blood counts on clinically stable patients [3]. Although there has been growing interest in blood management, only 37.8% of the 607 AABB (formerly, American Association of Blood Banks) facilities in the United States reported having a patient blood management program in 2013 [2].
While the importance of blood safety is recognized, data regarding the overall trends in practices are conflicting. A study using the Nationwide Inpatient Sample indicated that there was a 5.6% annual mean increase in the transfusion of blood products from 2002 to 2011 in the United States [4]. This contrasts with the experience of Kaiser Permanente in Northern California, in which the incidence of RBC transfusion decreased by 3.2% from 2009 to 2013 [5]. A decline in rates of intraoperative transfusion was also reported among elderly veterans in the United States from 1997 to 2009 [6].
We conducted a study in hospitalized veterans with 2 main objectives: (a) to evaluate trends in utilization of red blood cells (RBCs), platelets, and plasma over time, and (b) to identify those groups of veterans who received specific blood products. We were particularly interested in transfusion use in medical patients.
Methods
Participants were hospitalized veterans at 3 Department of Veterans Affairs (VA) medical centers. Data from all hospitalizations were collected from January 2000 through December 2010. Blood bank data (including the type and volume of products administered) were available electronically from each hospital. These files were linked to inpatient data, which included ICD-9-CM diagnoses (principal and secondary) and procedures during hospitalization.
Statistical analyses were conducted using generalized linear models to evaluate trends over time. The unit of observation was hospitalization, with categorization by type. Surgical hospitalizations were defined as admissions in which any surgical procedure occurred, whereas medical hospitalizations were defined as admissions without any surgery. Alpha was set at 0.05, 2-tailed. All analyses were conducted in Stata/MP 14.1 (StataCorp, College Station, TX). The study received institutional review board approval from the VA Ann Arbor Healthcare System.
Results
From 2000 through 2010, there were 176,521 hospitalizations in 69,621 patients. Within this cohort, 6% were < 40 years of age, 66% were 40 to 69 years of age, and 28% were 70 years or older at the time of admission. In this cohort, 96% of patients were male. Overall, 13.6% of all hospitalizations involved transfusion of a blood product (12.7% RBCs, 1.4% platelets, 3.0% plasma).
Transfusion occurred in 25.2% of surgical hospitalizations and 5.3% of medical hospitalizations. For surgical hospitalizations, transfusion use peaked in 2002 (when 30.9% of the surgical hospitalizations involved a trans-fusion) and significantly declined afterwards (P < 0.001). By 2010, 22.5% of the surgical hospitalizations involved a transfusion. Most of the surgeries where blood products were transfused involved cardiovascular procedures. For medical hospitalizations only, there was no significant change in transfusion use over time, either from 2000 to 2010 (P = 0.126) or from 2002 to 2010 (P = 0.072). In 2010, 5.2% of the medical hospitalizations involved a transfusion.
Rates of transfusion varied by principal diagnosis (Figure 1). For patients admitted with a principal diagnosis of infection (n = 20,981 hospitalizations), there was an increase in the percentage of hospitalizations in which transfusions (RBCs, platelet, plasma) were administered over time (P = 0.009) (Figure 1). For patients admitted with a principal diagnosis of malignancy (n = 12,904 hospitalizations), cardiovascular disease (n = 40,324 hospitalizations), and other diagnoses (n = 102,312 hospitalizations), there were no significant linear trends over the entire study period (P = 0.191, P = 0.052, P = 0.314, respectively). Rather, blood utilization peaked in year 2002 and significantly declined afterwards for patients admitted for malignancy (P < 0.001) and for cardiovascular disease (P < 0.001).
The most common principal diagnoses for medical patients receiving any transfusion (RBCs, platelet, plasma) are listed in Table 1. For medical patients with a principal diagnosis of anemia, 88% of hospitalizations involved a transfusion (Table 1). Transfusion occurred in 6% to 11% of medical hospitalizations with malignancies, heart failure, pneumonia or renal failure (Table 1). A considerable proportion (43%) of medical patients with gastrointestinal hemorrhage received a transfusion.
9.6% (2154/22,344) involved the use of only 1 unit, 43.8% (9791/22,344) involved 2 units, and 46.5% (10,399/22,344) involved 3 or more units during the hospitalization. From 2000 through 2010, there was a 20.3% reduction in the proportion of hospitalizations in which 3 or more units of RBCs were given (from 51.7% to 41.1%; P < 0.001). That is, among those hospitalizations in which a RBC transfusion occurred, a smaller proportion of hospitalizations involved the administration of 3 or more units of RBCs from 2000 through 2010 (Figure 2). There was an 11.5% increase in the proportion of hospitalizations in which 2 units of RBCs were used (from 40.4% to 45.0%; P < 0.001). In addition, there was a 73.6% increase in the proportion of hospitalizations in which 1 RBC unit was given (from 8.0% to 13.8%;
P = 0.001).
16.8 mL/hospitalization in 2010. For plasma, the mean mL/hospitalization was 28.9 in year 2000, increased to 50.1 mL/hospitalization in year 2008, and declined, thereafter, to 35.1 mL/hospitalization in year 2010.
Discussion
We also observed secular trends in the volume of RBCs administered. There was an increase in the percentage of hospitalizations in which 1 or 2 RBC units were used and a decline in transfusion of 3 or more units. The reduction in the use of 3 or more RBC units may reflect the adoption and integration of recommendations in patient blood management by clinicians,
which encourage assessment of the patients’ symptoms in determining whether additional units are necessary [7]. Such guidelines also endorse the avoidance of routine
administration of 2 units of RBCs if 1 unit is sufficient [8]. We have previously shown that, after coronary artery bypass grafting, 2 RBC units doubled the risk of pneumonia [9]; additional analyses indicated that 1 or 2 units of RBCs were associated with increased postoperative morbidity [10]. In addition, our previous research indicated that the probability of infection increased considerably between 1 and 2 RBC units, with a more gradual increase beyond 2 units [11]. With this evidence in mind, some studies at single sites have reported that there was a dramatic decline from 2 RBC units before initiation of patient blood management programs to 1 unit after the programs were implemented [12,13].
Medical patients who received a transfusion were often admitted for reason of anemia, cancer, organ failure, or pneumonia. Some researchers are now reporting that blood use, at certain sites, is becoming more common in medical rather than surgical patients, which may be due to an expansion of patient blood management procedures in surgery [16]. There are a substantial number of patient blood management programs among surgical specialties and their adoption has expanded [17]. Although there are fewer patient blood management programs in the nonsurgical setting, some have been targeted to internal medicine physicians and specifically, to hospitalists [1,18]. For example, a toolkit from the Society of Hospital Medicine centers on anemia management and includes anemia assessment, treatment, evaluation of RBC transfusion risk, blood conservation, optimization of coagulation, and patient-centered decision-making [19]. Additionally, bundling of patient blood management strategies has been launched to help encourage a wider adoption of such programs [20].
While guidelines regarding use of RBCs are becoming increasingly recognized, recommendations for the use of platelets and plasma are hampered by the paucity of evidence from randomized controlled trials [21,22]. There is moderate-quality evidence for the use of platelets with therapy-induced hypoproliferative thrombocytopenia in hospitalized patients [21], but low quality evidence for other uses. Moreover, a recent review of plasma transfusion in bleeding patients found no randomized controlled trials on plasma use in hospitalized patients, although several trials were currently underway [22].
Our findings need to be considered in the context of the following limitations. The data were from 3 VA hospitals, so the results may not reflect patterns of usage at other hospitals. However, AABB reports that there has been a general decrease in transfusion of allogeneic whole blood and RBC units since 2008 at the AABB-affiliated sites in the United States [2]; this is similar to the pattern that we observed in surgical patients. In addition, we report an overall view of trends without having details regarding which specific factors influenced changes in transfusion during this 11-year period. It is possible that the severity of hospitalized patients may have changed with time which could have influenced decisions regarding the need for transfusion.
In conclusion, the use of blood products decreased in surgical patients since 2002 but remained the same in medical patients in this VA population. Transfusions increased over time for patients who were admitted to the hospital for reason of infection, but decreased since 2002 for those admitted for cardiovascular disease or cancer. The number of RBC units per hospitalization decreased over time. Additional surveillance is needed to determine whether recent evidence regarding blood management has been incorporated into clinical practice for medical patients, as we strive to deliver optimal care to our veterans.
Corresponding author: Mary A.M. Rogers, PhD, MS, Dept. of Internal Medicine, Univ. of Michigan, 016-422W NCRC, Ann Arbor, MI 48109-2800, [email protected].
Funding/support: Department of Veterans Affairs, Clinical Sciences Research & Development Service Merit Review Award (EPID-011-11S). The contents do not represent the views of the U.S. Department of Veterans Affairs or the U.S. Government.
Financial disclosures: None.
Author contributions: conception and design, MAMR, SS; analysis and interpretation of data, MAMR, JDB, DR, LK, SS; drafting of article, MAMR; critical revision of the article, MAMR, MTG, DR, LK, SS, VC; statistical expertise, MAMR, DR; obtaining of funding, MTG, SS, VC; administrative or technical support, MTG, LK, SS, VC; collection and assembly of data, JDB, LK.
1. Hohmuth B, Ozawa S, Ashton M, Melseth RL. Patient-centered blood management. J Hosp Med 2014;9:60–5.
2. Whitaker B, Rajbhandary S, Harris A. The 2013 AABB blood collection, utilization, and patient blood management survey report. United States Department of Health and Human Services, AABB; 2015.
3. Cassel CK, Guest JA. Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA 2012;307:1801–2.
4. Pathak R, Bhatt VR, Karmacharya P, et al. Trends in blood-product transfusion among inpatients in the United States from 2002 to 2011: data from the nationwide inpatient sample. J Hosp Med 2014;9:800–1.
5. Roubinian NH, Escobar GJ, Liu V, et al. Trends in red blood cell transfusion and 30-day mortality among hospitalized patients. Transfusion 2014;54:2678–86.
6. Chen A, Trivedi AN, Jiang L, et al. Hospital blood transfusion patterns during major noncardiac surgery and surgical mortality. Medicine (Baltimore) 2015;94:e1342.
7. Carson JL, Guyatt G, Heddle NM, et al. Clinical practice guidelines from the AABB: Red blood cell transfusion thresholds and storage. JAMA 2016;316:2025–35.
8. Hicks LK, Bering H, Carson KR, et al. The ASH choosing wisely® campaign: five hematologic tests and treatments to question. Blood 2013;122:3879–83.
9. Likosky DS, Paone G, Zhang M, et al. Red blood cell transfusions impact pneumonia rates after coronary artery bypass grafting. Ann Thorac Surg 2015;100:794–801.
10. Paone G, Likosky DS, Brewer R, et al. Transfusion of 1 and 2 units of red blood cells is associated with increased morbidity and mortality. Ann Thorac Surg 2014;97:87–93; discussion 93–4.
11. Rogers MAM, Blumberg N, Heal JM, et al. Role of transfusion in the development of urinary tract–related bloodstream infection. Arch Intern Med 2011;171:1587–9.
12. Oliver JC, Griffin RL, Hannon T, Marques MB. The success of our patient blood management program depended on an institution-wide change in transfusion practices. Transfusion 2014;54:2617–24.
13. Yerrabothala S, Desrosiers KP, Szczepiorkowski ZM, Dunbar NM. Significant reduction in red blood cell transfusions in a general hospital after successful implementation of a restrictive transfusion policy supported by prospective computerized order auditing. Transfusion 2014;54:2640–5.
14. Rehm JP, Otto PS, West WW, et al. Hospital-wide educational program decreases red blood cell transfusions. J Surg Res 1998;75:183–6.
15. Lawler EV, Bradbury BD, Fonda JR, et al. Transfusion burden among patients with chronic kidney disease and anemia. Clin J Am Soc Nephrol 2010;5:667–72.
16. Tinegate H, Pendry K, Murphy M, et al. Where do all the red blood cells (RBCs) go? Results of a survey of RBC use in England and North Wales in 2014. Transfusion 2016;56:139–45.
17. Meybohm P, Herrmann E, Steinbicker AU, et al. Patient blood management is associated with a substantial reduction of red blood cell utilization and safe for patient’s outcome: a prospective, multicenter cohort study with a noninferiority design. Ann Surg 2016;264:203–11.
18. Corwin HL, Theus JW, Cargile CS, Lang NP. Red blood cell transfusion: impact of an education program and a clinical guideline on transfusion practice. J Hosp Med 2014;9:745–9.
19. Society of Hospital Medicine. Anemia prevention and management program implementation toolkit. Accessed at www.hospitalmedicine.org/Web/Quality___Innovation/Implementation_Toolkit/Anemia/anemia_overview.aspx on 9 June 2017.
20. Meybohm P, Richards T, Isbister J, et al. Patient blood management bundles to facilitate implementation. Transfus Med Rev 2017;31:62–71.
21. Kaufman RM, Djulbegovic B, Gernsheimer T, et al. Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med 2015;162:205–13.
22. Levy JH, Grottke O, Fries D, Kozek-Langenecker S. Therapeutic plasma transfusion in bleeding patients: A systematic review. Anesth Analg 2017;124:1268–76.
1. Hohmuth B, Ozawa S, Ashton M, Melseth RL. Patient-centered blood management. J Hosp Med 2014;9:60–5.
2. Whitaker B, Rajbhandary S, Harris A. The 2013 AABB blood collection, utilization, and patient blood management survey report. United States Department of Health and Human Services, AABB; 2015.
3. Cassel CK, Guest JA. Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA 2012;307:1801–2.
4. Pathak R, Bhatt VR, Karmacharya P, et al. Trends in blood-product transfusion among inpatients in the United States from 2002 to 2011: data from the nationwide inpatient sample. J Hosp Med 2014;9:800–1.
5. Roubinian NH, Escobar GJ, Liu V, et al. Trends in red blood cell transfusion and 30-day mortality among hospitalized patients. Transfusion 2014;54:2678–86.
6. Chen A, Trivedi AN, Jiang L, et al. Hospital blood transfusion patterns during major noncardiac surgery and surgical mortality. Medicine (Baltimore) 2015;94:e1342.
7. Carson JL, Guyatt G, Heddle NM, et al. Clinical practice guidelines from the AABB: Red blood cell transfusion thresholds and storage. JAMA 2016;316:2025–35.
8. Hicks LK, Bering H, Carson KR, et al. The ASH choosing wisely® campaign: five hematologic tests and treatments to question. Blood 2013;122:3879–83.
9. Likosky DS, Paone G, Zhang M, et al. Red blood cell transfusions impact pneumonia rates after coronary artery bypass grafting. Ann Thorac Surg 2015;100:794–801.
10. Paone G, Likosky DS, Brewer R, et al. Transfusion of 1 and 2 units of red blood cells is associated with increased morbidity and mortality. Ann Thorac Surg 2014;97:87–93; discussion 93–4.
11. Rogers MAM, Blumberg N, Heal JM, et al. Role of transfusion in the development of urinary tract–related bloodstream infection. Arch Intern Med 2011;171:1587–9.
12. Oliver JC, Griffin RL, Hannon T, Marques MB. The success of our patient blood management program depended on an institution-wide change in transfusion practices. Transfusion 2014;54:2617–24.
13. Yerrabothala S, Desrosiers KP, Szczepiorkowski ZM, Dunbar NM. Significant reduction in red blood cell transfusions in a general hospital after successful implementation of a restrictive transfusion policy supported by prospective computerized order auditing. Transfusion 2014;54:2640–5.
14. Rehm JP, Otto PS, West WW, et al. Hospital-wide educational program decreases red blood cell transfusions. J Surg Res 1998;75:183–6.
15. Lawler EV, Bradbury BD, Fonda JR, et al. Transfusion burden among patients with chronic kidney disease and anemia. Clin J Am Soc Nephrol 2010;5:667–72.
16. Tinegate H, Pendry K, Murphy M, et al. Where do all the red blood cells (RBCs) go? Results of a survey of RBC use in England and North Wales in 2014. Transfusion 2016;56:139–45.
17. Meybohm P, Herrmann E, Steinbicker AU, et al. Patient blood management is associated with a substantial reduction of red blood cell utilization and safe for patient’s outcome: a prospective, multicenter cohort study with a noninferiority design. Ann Surg 2016;264:203–11.
18. Corwin HL, Theus JW, Cargile CS, Lang NP. Red blood cell transfusion: impact of an education program and a clinical guideline on transfusion practice. J Hosp Med 2014;9:745–9.
19. Society of Hospital Medicine. Anemia prevention and management program implementation toolkit. Accessed at www.hospitalmedicine.org/Web/Quality___Innovation/Implementation_Toolkit/Anemia/anemia_overview.aspx on 9 June 2017.
20. Meybohm P, Richards T, Isbister J, et al. Patient blood management bundles to facilitate implementation. Transfus Med Rev 2017;31:62–71.
21. Kaufman RM, Djulbegovic B, Gernsheimer T, et al. Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med 2015;162:205–13.
22. Levy JH, Grottke O, Fries D, Kozek-Langenecker S. Therapeutic plasma transfusion in bleeding patients: A systematic review. Anesth Analg 2017;124:1268–76.
Measuring both serum amylase and lipase for acute pancreatitis lowers quality and raises cost
A 43-year-old, previously healthy woman was admitted to the hospital after 1 day of severe epigastric abdominal pain, nausea, and vomiting. She denied alcohol or tobacco use.
Her physical examination revealed normal vital signs and epigastric tenderness without rebound tenderness.
Notable laboratory results:
- Aspartate aminotransferase 149 U/L (reference range 10–35)
- Alanine aminotransferase 140 U/L (10–35)
- Alkaline phosphatase 178 IU/L (35–104)
- Total bilirubin 1.8 mg/dL (0.2–1.3)
- Amylase 1,244 U/L (28–100)
- Lipase 14,628 U/L (7–59).
Abdominal ultrasonography showed a dilated bile duct and gallstones.
The patient was diagnosed with biliary pancreatitis and was treated by placing her on nothing-by-mouth (NPO) status and giving intravenous fluids and analgesics. All symptoms had resolved by hospital day 3. She underwent laparoscopic cholecystectomy and was discharged the following day.
This is a typical case of biliary pancreatitis that was diagnosed and treated appropriately with a positive outcome. But was it necessary or beneficial to measure both the serum amylase and serum lipase to make the correct diagnosis and treat the patient appropriately?
IS MEASURING SERUM AMYLASE NECESSARY?
The American College of Gastroenterology practice guidelines suggest that measuring both serum amylase and serum lipase is not necessary.1 Serum lipase alone is the preferred test for diagnosing acute pancreatitis, since it is more sensitive than serum amylase, just as specific, rises more quickly, and remains elevated longer.
In a retrospective study of 151 patients with acute pancreatitis,2 the sensitivity of lipase was 96.6% and the specificity was 99.4%.2 In contrast, the sensitivity of amylase was 78.6% and the specificity was 99.1%.
In another study,3 in 476 patients with acute pancreatitis, lipase had a sensitivity of 91% vs 62% for amylase. Again, specificity was similar between the two tests (92% for lipase and 93% for amylase). The authors concluded that lipase should replace amylase as the first-line laboratory investigation for suspected acute pancreatitis.
Smith et al4 reviewed 1,825 patients with acute pancreatitis and similarly concluded that pancreatic lipase is a more accurate biomarker of acute pancreatitis than serum amylase.
PRACTICE AT OUR HOSPITAL
Despite this guideline and evidence, concurrent ordering of serum amylase and lipase is common at many institutions.
We evaluated the practice of ordering both serum amylase and lipase for diagnosis of acute pancreatitis at our 300-bed academic medical hospital. From January 2011 through August 2014, our institution completed 26,254 orders for serum amylase and lipase measurement in 13,198 patients. In 9,938 (75%) of the patients, amylase and lipase were ordered concurrently. Of these, 482 patients (4.8%) had either amylase or lipase elevated above the diagnostic threshold, ie, 3 times the upper limit of normal, and 63 of the 482 patients had an elevation in serum amylase greater than 3 times the upper limit of normal without an elevation in serum lipase.
None of the patients had acute pancreatitis clinically (eg, typical abdominal pain, nausea, vomiting) or on imaging (pancreatic edema). The definitive cause of nonpancreatic hyperamylasemia could not be determined in these patients; they did not have evidence of salivary disorder, malignancy, or tubo-ovarian disease, and the hyperamylasemia was believed to be related to renal disease, diabetic ketoacidosis, infection, or medications, or to be idiopathic.
In 12 patients, the discrepancy between an elevated amylase and normal lipase resulted in additional imaging with computed tomography. Four patients were also unnecessarily kept NPO for 1 to 3 days, depriving them of nutrition and prolonging their hospital stay.
To minimize concurrent ordering of serum amylase and lipase, we introduced a best-practice alert in the computerized physician order entry systems. The alert mentioned that “ordering both serum amylase and lipase in cases of suspected pancreatitis is unnecessary. Serum lipase alone is sufficient.” However, ordering providers could still order both tests if they wanted to.
In the 3 months after the alert was implemented, serum lipase was ordered 1,780 times with 532 (30%) concurrent orders of amylase. Before the alert was instituted, amylase testing was ordered a mean of 450 times per month; afterward, this decreased by about 60%.
We are now considering eliminating serum amylase testing, as suggested by prior studies5 and the American Society of Clinical Pathology.6
ELIMINATING NEEDLESS EXPENSES
The relentless and unsustainable rise in healthcare costs has prompted physician-led groups such as the American Board of Internal Medicine Foundation and the American College of Physicians to focus on ways to cut waste and incorporate high-value, cost-conscious care into clinical practice.
In 2009 alone, waste in total healthcare expenditures was estimated at $765 billion. More than half of this astronomical figure was attributed to unnecessary and inefficiently delivered services, expenditures that physicians can directly avoid with changes to their practice.7,8 Unnecessary laboratory tests such as serum amylase are just one of many wasteful practices.
Hospitals have much to lose when unnecessary tests are ordered. For inpatient hospital admissions in the United States, payment is based on the diagnosis-related group system, in which hospitals are paid a fixed amount per diagnosis. There is no additional reimbursement for laboratory tests. An unnecessary test such as serum amylase in suspected cases of acute pancreatitis thus becomes an expense with no corresponding benefit.
The cost of performing a serum amylase test for a typical laboratory is around $4 to $6. Serum amylase testing at our hospital resulted in unnecessary expense of about $35,000 annually. If we add the costs of additional imaging and prolonged hospitalization, the expenses are substantially more.
Despite this, most hospitals have been unwilling or unable to tackle the problem. This may be due to respect for physician autonomy, seemingly small financial loss, or organizational inertia. For the entire healthcare system, these seemingly minor costs add up. For example, from 2011 to 2014, Medicare Part B alone spent $19.4 million on serum amylase testing.
Ordering unnecessary laboratory tests is not a problem specific to our hospital, but rather a common problem encountered at many hospitals. Recognizing the widespread practice of ordering amylase, the Choosing Wisely initiative shared new recommendations from the American Society for Clinical Pathology supporting the use of lipase instead of amylase in suspected acute pancreatitis.7
Physicians who continue to order these tests show a disregard for evidence-based medicine, patient care, and healthcare costs.
CLINICAL BOTTOM LINE
Concurrent use of amylase and lipase testing to diagnose acute pancreatitis is an unnecessary expense for the hospital and can negatively affect patient care as it can lead to additional tests and prolonged hospitalization. Steps should be taken to minimize ordering of amylase by educating physicians and instituting best-practice alerts, or by eliminating the test altogether.
- Banks PA, Freeman ML, Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. Am J Gastroenterol 2006; 101:2379–2400.
- Gomez D, Addison A, De Rosa A, Brooks A, Cameron IC. Retrospective study of patients with acute pancreatitis: is serum amylase still required? BMJ Open 2012; 2. pii:e001471.
- Hofmeyr S, Meyer C, Warren BL. Serum lipase should be the laboratory test of choice for suspected acute pancreatitis. S Afr J Surg 2014; 52:72–75.
- Smith RC, Southwell-Keely J, Chesher D. Should serum pancreatic lipase replace serum amylase as a biomarker of acute pancreatitis? ANZ J Surg 2005; 75:399–404.
- Volz KA, McGillicuddy DC, Horowitz GL, Wolfe RE, Joyce N, Sanchez LD. Eliminating amylase testing from the evaluation of pancreatitis in the emergency department. West J Emerg Med 2010; 11:344–347.
- American Society for Clinical Pathology. Do not test for amylase in cases of suspected pancreatitis. Instead, test for lipase. Choosing Wisely; 2016. www.choosingwisely.org/clinician-lists/american-society-clinical-pathology-testing-for-amylase. Accessed August 3, 2017.
- Smith M, Saunders R, Stuckhardt L, McGinnis JM; Committee on the Learning Health Care System in America, eds. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington, DC: The National Academies Press; 2013. www.hep.fsu.edu/~wahl/artic/NAP/HealthCare13444.pdf. Accessed August 3, 2017.
- American College of Physicians. Eliminating healthcare waste and overordering of tests. www.acponline.org/clinical-information/high-value-care/medical-educators-resources/curriculum-for-educators-and-residents/curriculum-version-3. Accessed August 3, 2017.
A 43-year-old, previously healthy woman was admitted to the hospital after 1 day of severe epigastric abdominal pain, nausea, and vomiting. She denied alcohol or tobacco use.
Her physical examination revealed normal vital signs and epigastric tenderness without rebound tenderness.
Notable laboratory results:
- Aspartate aminotransferase 149 U/L (reference range 10–35)
- Alanine aminotransferase 140 U/L (10–35)
- Alkaline phosphatase 178 IU/L (35–104)
- Total bilirubin 1.8 mg/dL (0.2–1.3)
- Amylase 1,244 U/L (28–100)
- Lipase 14,628 U/L (7–59).
Abdominal ultrasonography showed a dilated bile duct and gallstones.
The patient was diagnosed with biliary pancreatitis and was treated by placing her on nothing-by-mouth (NPO) status and giving intravenous fluids and analgesics. All symptoms had resolved by hospital day 3. She underwent laparoscopic cholecystectomy and was discharged the following day.
This is a typical case of biliary pancreatitis that was diagnosed and treated appropriately with a positive outcome. But was it necessary or beneficial to measure both the serum amylase and serum lipase to make the correct diagnosis and treat the patient appropriately?
IS MEASURING SERUM AMYLASE NECESSARY?
The American College of Gastroenterology practice guidelines suggest that measuring both serum amylase and serum lipase is not necessary.1 Serum lipase alone is the preferred test for diagnosing acute pancreatitis, since it is more sensitive than serum amylase, just as specific, rises more quickly, and remains elevated longer.
In a retrospective study of 151 patients with acute pancreatitis,2 the sensitivity of lipase was 96.6% and the specificity was 99.4%.2 In contrast, the sensitivity of amylase was 78.6% and the specificity was 99.1%.
In another study,3 in 476 patients with acute pancreatitis, lipase had a sensitivity of 91% vs 62% for amylase. Again, specificity was similar between the two tests (92% for lipase and 93% for amylase). The authors concluded that lipase should replace amylase as the first-line laboratory investigation for suspected acute pancreatitis.
Smith et al4 reviewed 1,825 patients with acute pancreatitis and similarly concluded that pancreatic lipase is a more accurate biomarker of acute pancreatitis than serum amylase.
PRACTICE AT OUR HOSPITAL
Despite this guideline and evidence, concurrent ordering of serum amylase and lipase is common at many institutions.
We evaluated the practice of ordering both serum amylase and lipase for diagnosis of acute pancreatitis at our 300-bed academic medical hospital. From January 2011 through August 2014, our institution completed 26,254 orders for serum amylase and lipase measurement in 13,198 patients. In 9,938 (75%) of the patients, amylase and lipase were ordered concurrently. Of these, 482 patients (4.8%) had either amylase or lipase elevated above the diagnostic threshold, ie, 3 times the upper limit of normal, and 63 of the 482 patients had an elevation in serum amylase greater than 3 times the upper limit of normal without an elevation in serum lipase.
None of the patients had acute pancreatitis clinically (eg, typical abdominal pain, nausea, vomiting) or on imaging (pancreatic edema). The definitive cause of nonpancreatic hyperamylasemia could not be determined in these patients; they did not have evidence of salivary disorder, malignancy, or tubo-ovarian disease, and the hyperamylasemia was believed to be related to renal disease, diabetic ketoacidosis, infection, or medications, or to be idiopathic.
In 12 patients, the discrepancy between an elevated amylase and normal lipase resulted in additional imaging with computed tomography. Four patients were also unnecessarily kept NPO for 1 to 3 days, depriving them of nutrition and prolonging their hospital stay.
To minimize concurrent ordering of serum amylase and lipase, we introduced a best-practice alert in the computerized physician order entry systems. The alert mentioned that “ordering both serum amylase and lipase in cases of suspected pancreatitis is unnecessary. Serum lipase alone is sufficient.” However, ordering providers could still order both tests if they wanted to.
In the 3 months after the alert was implemented, serum lipase was ordered 1,780 times with 532 (30%) concurrent orders of amylase. Before the alert was instituted, amylase testing was ordered a mean of 450 times per month; afterward, this decreased by about 60%.
We are now considering eliminating serum amylase testing, as suggested by prior studies5 and the American Society of Clinical Pathology.6
ELIMINATING NEEDLESS EXPENSES
The relentless and unsustainable rise in healthcare costs has prompted physician-led groups such as the American Board of Internal Medicine Foundation and the American College of Physicians to focus on ways to cut waste and incorporate high-value, cost-conscious care into clinical practice.
In 2009 alone, waste in total healthcare expenditures was estimated at $765 billion. More than half of this astronomical figure was attributed to unnecessary and inefficiently delivered services, expenditures that physicians can directly avoid with changes to their practice.7,8 Unnecessary laboratory tests such as serum amylase are just one of many wasteful practices.
Hospitals have much to lose when unnecessary tests are ordered. For inpatient hospital admissions in the United States, payment is based on the diagnosis-related group system, in which hospitals are paid a fixed amount per diagnosis. There is no additional reimbursement for laboratory tests. An unnecessary test such as serum amylase in suspected cases of acute pancreatitis thus becomes an expense with no corresponding benefit.
The cost of performing a serum amylase test for a typical laboratory is around $4 to $6. Serum amylase testing at our hospital resulted in unnecessary expense of about $35,000 annually. If we add the costs of additional imaging and prolonged hospitalization, the expenses are substantially more.
Despite this, most hospitals have been unwilling or unable to tackle the problem. This may be due to respect for physician autonomy, seemingly small financial loss, or organizational inertia. For the entire healthcare system, these seemingly minor costs add up. For example, from 2011 to 2014, Medicare Part B alone spent $19.4 million on serum amylase testing.
Ordering unnecessary laboratory tests is not a problem specific to our hospital, but rather a common problem encountered at many hospitals. Recognizing the widespread practice of ordering amylase, the Choosing Wisely initiative shared new recommendations from the American Society for Clinical Pathology supporting the use of lipase instead of amylase in suspected acute pancreatitis.7
Physicians who continue to order these tests show a disregard for evidence-based medicine, patient care, and healthcare costs.
CLINICAL BOTTOM LINE
Concurrent use of amylase and lipase testing to diagnose acute pancreatitis is an unnecessary expense for the hospital and can negatively affect patient care as it can lead to additional tests and prolonged hospitalization. Steps should be taken to minimize ordering of amylase by educating physicians and instituting best-practice alerts, or by eliminating the test altogether.
A 43-year-old, previously healthy woman was admitted to the hospital after 1 day of severe epigastric abdominal pain, nausea, and vomiting. She denied alcohol or tobacco use.
Her physical examination revealed normal vital signs and epigastric tenderness without rebound tenderness.
Notable laboratory results:
- Aspartate aminotransferase 149 U/L (reference range 10–35)
- Alanine aminotransferase 140 U/L (10–35)
- Alkaline phosphatase 178 IU/L (35–104)
- Total bilirubin 1.8 mg/dL (0.2–1.3)
- Amylase 1,244 U/L (28–100)
- Lipase 14,628 U/L (7–59).
Abdominal ultrasonography showed a dilated bile duct and gallstones.
The patient was diagnosed with biliary pancreatitis and was treated by placing her on nothing-by-mouth (NPO) status and giving intravenous fluids and analgesics. All symptoms had resolved by hospital day 3. She underwent laparoscopic cholecystectomy and was discharged the following day.
This is a typical case of biliary pancreatitis that was diagnosed and treated appropriately with a positive outcome. But was it necessary or beneficial to measure both the serum amylase and serum lipase to make the correct diagnosis and treat the patient appropriately?
IS MEASURING SERUM AMYLASE NECESSARY?
The American College of Gastroenterology practice guidelines suggest that measuring both serum amylase and serum lipase is not necessary.1 Serum lipase alone is the preferred test for diagnosing acute pancreatitis, since it is more sensitive than serum amylase, just as specific, rises more quickly, and remains elevated longer.
In a retrospective study of 151 patients with acute pancreatitis,2 the sensitivity of lipase was 96.6% and the specificity was 99.4%.2 In contrast, the sensitivity of amylase was 78.6% and the specificity was 99.1%.
In another study,3 in 476 patients with acute pancreatitis, lipase had a sensitivity of 91% vs 62% for amylase. Again, specificity was similar between the two tests (92% for lipase and 93% for amylase). The authors concluded that lipase should replace amylase as the first-line laboratory investigation for suspected acute pancreatitis.
Smith et al4 reviewed 1,825 patients with acute pancreatitis and similarly concluded that pancreatic lipase is a more accurate biomarker of acute pancreatitis than serum amylase.
PRACTICE AT OUR HOSPITAL
Despite this guideline and evidence, concurrent ordering of serum amylase and lipase is common at many institutions.
We evaluated the practice of ordering both serum amylase and lipase for diagnosis of acute pancreatitis at our 300-bed academic medical hospital. From January 2011 through August 2014, our institution completed 26,254 orders for serum amylase and lipase measurement in 13,198 patients. In 9,938 (75%) of the patients, amylase and lipase were ordered concurrently. Of these, 482 patients (4.8%) had either amylase or lipase elevated above the diagnostic threshold, ie, 3 times the upper limit of normal, and 63 of the 482 patients had an elevation in serum amylase greater than 3 times the upper limit of normal without an elevation in serum lipase.
None of the patients had acute pancreatitis clinically (eg, typical abdominal pain, nausea, vomiting) or on imaging (pancreatic edema). The definitive cause of nonpancreatic hyperamylasemia could not be determined in these patients; they did not have evidence of salivary disorder, malignancy, or tubo-ovarian disease, and the hyperamylasemia was believed to be related to renal disease, diabetic ketoacidosis, infection, or medications, or to be idiopathic.
In 12 patients, the discrepancy between an elevated amylase and normal lipase resulted in additional imaging with computed tomography. Four patients were also unnecessarily kept NPO for 1 to 3 days, depriving them of nutrition and prolonging their hospital stay.
To minimize concurrent ordering of serum amylase and lipase, we introduced a best-practice alert in the computerized physician order entry systems. The alert mentioned that “ordering both serum amylase and lipase in cases of suspected pancreatitis is unnecessary. Serum lipase alone is sufficient.” However, ordering providers could still order both tests if they wanted to.
In the 3 months after the alert was implemented, serum lipase was ordered 1,780 times with 532 (30%) concurrent orders of amylase. Before the alert was instituted, amylase testing was ordered a mean of 450 times per month; afterward, this decreased by about 60%.
We are now considering eliminating serum amylase testing, as suggested by prior studies5 and the American Society of Clinical Pathology.6
ELIMINATING NEEDLESS EXPENSES
The relentless and unsustainable rise in healthcare costs has prompted physician-led groups such as the American Board of Internal Medicine Foundation and the American College of Physicians to focus on ways to cut waste and incorporate high-value, cost-conscious care into clinical practice.
In 2009 alone, waste in total healthcare expenditures was estimated at $765 billion. More than half of this astronomical figure was attributed to unnecessary and inefficiently delivered services, expenditures that physicians can directly avoid with changes to their practice.7,8 Unnecessary laboratory tests such as serum amylase are just one of many wasteful practices.
Hospitals have much to lose when unnecessary tests are ordered. For inpatient hospital admissions in the United States, payment is based on the diagnosis-related group system, in which hospitals are paid a fixed amount per diagnosis. There is no additional reimbursement for laboratory tests. An unnecessary test such as serum amylase in suspected cases of acute pancreatitis thus becomes an expense with no corresponding benefit.
The cost of performing a serum amylase test for a typical laboratory is around $4 to $6. Serum amylase testing at our hospital resulted in unnecessary expense of about $35,000 annually. If we add the costs of additional imaging and prolonged hospitalization, the expenses are substantially more.
Despite this, most hospitals have been unwilling or unable to tackle the problem. This may be due to respect for physician autonomy, seemingly small financial loss, or organizational inertia. For the entire healthcare system, these seemingly minor costs add up. For example, from 2011 to 2014, Medicare Part B alone spent $19.4 million on serum amylase testing.
Ordering unnecessary laboratory tests is not a problem specific to our hospital, but rather a common problem encountered at many hospitals. Recognizing the widespread practice of ordering amylase, the Choosing Wisely initiative shared new recommendations from the American Society for Clinical Pathology supporting the use of lipase instead of amylase in suspected acute pancreatitis.7
Physicians who continue to order these tests show a disregard for evidence-based medicine, patient care, and healthcare costs.
CLINICAL BOTTOM LINE
Concurrent use of amylase and lipase testing to diagnose acute pancreatitis is an unnecessary expense for the hospital and can negatively affect patient care as it can lead to additional tests and prolonged hospitalization. Steps should be taken to minimize ordering of amylase by educating physicians and instituting best-practice alerts, or by eliminating the test altogether.
- Banks PA, Freeman ML, Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. Am J Gastroenterol 2006; 101:2379–2400.
- Gomez D, Addison A, De Rosa A, Brooks A, Cameron IC. Retrospective study of patients with acute pancreatitis: is serum amylase still required? BMJ Open 2012; 2. pii:e001471.
- Hofmeyr S, Meyer C, Warren BL. Serum lipase should be the laboratory test of choice for suspected acute pancreatitis. S Afr J Surg 2014; 52:72–75.
- Smith RC, Southwell-Keely J, Chesher D. Should serum pancreatic lipase replace serum amylase as a biomarker of acute pancreatitis? ANZ J Surg 2005; 75:399–404.
- Volz KA, McGillicuddy DC, Horowitz GL, Wolfe RE, Joyce N, Sanchez LD. Eliminating amylase testing from the evaluation of pancreatitis in the emergency department. West J Emerg Med 2010; 11:344–347.
- American Society for Clinical Pathology. Do not test for amylase in cases of suspected pancreatitis. Instead, test for lipase. Choosing Wisely; 2016. www.choosingwisely.org/clinician-lists/american-society-clinical-pathology-testing-for-amylase. Accessed August 3, 2017.
- Smith M, Saunders R, Stuckhardt L, McGinnis JM; Committee on the Learning Health Care System in America, eds. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington, DC: The National Academies Press; 2013. www.hep.fsu.edu/~wahl/artic/NAP/HealthCare13444.pdf. Accessed August 3, 2017.
- American College of Physicians. Eliminating healthcare waste and overordering of tests. www.acponline.org/clinical-information/high-value-care/medical-educators-resources/curriculum-for-educators-and-residents/curriculum-version-3. Accessed August 3, 2017.
- Banks PA, Freeman ML, Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. Am J Gastroenterol 2006; 101:2379–2400.
- Gomez D, Addison A, De Rosa A, Brooks A, Cameron IC. Retrospective study of patients with acute pancreatitis: is serum amylase still required? BMJ Open 2012; 2. pii:e001471.
- Hofmeyr S, Meyer C, Warren BL. Serum lipase should be the laboratory test of choice for suspected acute pancreatitis. S Afr J Surg 2014; 52:72–75.
- Smith RC, Southwell-Keely J, Chesher D. Should serum pancreatic lipase replace serum amylase as a biomarker of acute pancreatitis? ANZ J Surg 2005; 75:399–404.
- Volz KA, McGillicuddy DC, Horowitz GL, Wolfe RE, Joyce N, Sanchez LD. Eliminating amylase testing from the evaluation of pancreatitis in the emergency department. West J Emerg Med 2010; 11:344–347.
- American Society for Clinical Pathology. Do not test for amylase in cases of suspected pancreatitis. Instead, test for lipase. Choosing Wisely; 2016. www.choosingwisely.org/clinician-lists/american-society-clinical-pathology-testing-for-amylase. Accessed August 3, 2017.
- Smith M, Saunders R, Stuckhardt L, McGinnis JM; Committee on the Learning Health Care System in America, eds. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington, DC: The National Academies Press; 2013. www.hep.fsu.edu/~wahl/artic/NAP/HealthCare13444.pdf. Accessed August 3, 2017.
- American College of Physicians. Eliminating healthcare waste and overordering of tests. www.acponline.org/clinical-information/high-value-care/medical-educators-resources/curriculum-for-educators-and-residents/curriculum-version-3. Accessed August 3, 2017.
Postoperative delirium in a 64-year-old woman
A 64-year-old woman undergoes elective T10-S1 nerve decompression with fusion for chronic idiopathic scoliosis. Soon afterward, she develops acute urinary retention attributed to an Escherichia coli urinary tract infection and narcotic medications. She is treated with antibiotics, an indwelling catheter is inserted, and her symptoms resolve. She is transferred to the inpatient physical rehabilitation unit.
On postoperative day 9, she develops an acute change in mental status, suddenly becoming extremely anxious and falsely believing she has a “terminal illness.” A psychiatrist suggests that these symptoms are a manifestation of delirium, given the patient’s recent surgery and exposure to benzodiazepine and narcotic medications. On postoperative day 10, she is awake but is now mute and uncooperative. An internist is consulted for an evaluation for encephalopathy and delirium.
MEDICAL HISTORY
Her medical history, obtained by chart review and interviewing her husband, includes well-controlled bipolar disorder over the last 4 years, with no episodes of frank psychosis or mania. She had a “bout of delirium” 4 years earlier attributed to a catastrophic life event, but the symptoms resolved after adjustment of her anxiolytic and mood-stabilizing drugs. She also has well-controlled hypertension, hypothyroidism, and gastroesophageal reflux. Her only surgery was her recent elective procedure.
She has a family history of dementia (Pick disease in her mother).
She is married, lives with her husband, and has an adult son. She is employed as a media specialist and also teaches English as a second language. Before this hospital admission, she was described as happy and content, though her primary psychiatrist had noted intermittent anxiety. Her husband does not suspect illicit drug use and denies significant alcohol or tobacco abuse.
A thorough review of systems is not possible, given her encephalopathy. But before her acute decline, she had complained of “choking on blood” and a subjective inability to swallow.
Her home medications include dextroamphetamine extended-release, alprazolam as needed for sleep, venlafaxine extended-release, lamotrigine, lisinopril, propranolol, amlodipine, atorvastatin, levothyroxine, omeprazole, iron, and vitamin B12. At the time of the evaluation, she is on her home medications with the addition of olanzapine, vitamin D, polyethylene glycol, and an intravenous infusion of dextrose 5% with 0.45% saline at a rate of 100 mL/hour. She has allergies to latex, penicillin, peanuts, and shellfish.
PHYSICAL EXAMINATION
On physical examination, the patient seems healthy and appears normal for her stated age. She is wearing a spinal brace and is in no apparent distress. She is afebrile, pulse 104 beats per minute, respirations 16 breaths per minute and unlabored, and oxygen saturation good on room air. The skin is normal. No thyromegaly, bruits, or lymphadenopathy is noted. Cardiovascular, respiratory, and abdominal examinations, though limited by the spinal brace, are unremarkable. She has no evidence of peripheral edema or vascular insufficiency. Muscle bulk and tone are adequate and symmetric.
She is awake and alert and able to follow simple commands with some prompting. She does not initiate movements spontaneously. She makes some eye contact but does not track or acknowledge the interviewer consistently and does not respond verbally to questions. Her sclera are nonicteric, the pupils are equally round and reactive to light, and the external ocular muscles are intact. There is no facial asymmetry, and the tongue protrudes at midline. She blinks appropriately to threat bilaterally. Strength is at least 3/5 in the upper extremities and 2/5 in the lower extremities, though the examination is limited by lack of patient cooperation. She shows minimal grimace on noxious stimulation but does not withdraw extremities. Reflexes are present and mildly depressed symmetrically. Plantar reflexes are downgoing bilaterally.
INITIAL LABORATORY EVALUATION
On initial laboratory testing, the serum sodium is 132 mmol/L (reference range 136–144), stable since admission. Point-of-care glucose is 98 mg/dL. Aspartate aminotransferase and alanine aminotransferase levels are mildly elevated at 59 U/L (13–35) and 51 U/L (7–38), respectively, but serum ammonia is undetectable. Vitamin B12, folate, thyroid-stimulating hormone, and free thyroxine are within the normal ranges. Leukocytosis is noted, with 14 × 109 cells/L (3.7–11.0), 86% neutrophils, and a mild left shift. Urinalysis is negative for leukocyte esterase, nitrites, and white blood cells.
APPROACH TO ALTERED MENTAL STATUS
1. Which of the following risk factors predisposes this patient to postoperative delirium?
- Hyponatremia
- Polypharmacy
- Family history of dementia
- Depression
Altered mental status, or encephalopathy, is one of the most common yet challenging conditions in medicine. When a consult is placed for altered mental status, it is important to determine the affected domain that has changed from the patient’s normal state. Changes can include alterations in consciousness, attention, behavior, cognition, language, speech, and praxis and can reflect varying degrees of cerebral dysfunction.
Electrolyte abnormalities
Disorders of sodium homeostasis are common in hospitalized patients and may contribute to the onset of delirium. Hyponatremia is especially frequent and often iatrogenic, with a prevalence significantly higher in women (2.1% vs 1.3%, P = .0044) and in the elderly.2
Neurologic manifestations are often the result of cerebral edema due to osmolar volume shifts.3–6 Acute hyponatremic encephalopathy is most likely to occur when sodium shifts are rapid, usually within 24 hours, and is often seen in postoperative patients requiring significant volume resuscitation with hypotonic fluids.6 Young premenopausal women appear to be at especially high risk of permanent brain damage secondary to hyponatremic encephalopathy,7 a finding that may reflect the limited compliance within the intracranial vault and lack of significant involutional parenchymal changes that occur with aging.8–11
Aging also has important effects on fluid balance, as restoration of body fluid homeostasis is slower in older patients.12
Hormonal effects of estrogen appear to play a synergistic role in the expression of arginine vasopressin in postmenopausal women, further contributing to hyponatremia.
Although our patient has mild hyponatremia, there has been no acute change in her sodium balance since admission to the hospital, and so it is unlikely to be the cause of her acute delirium. Her mild hyponatremia may in part be from hypo-osmolar maintenance fluids with dextrose 5% and 0.45% normal saline.
Mild chronic hyponatremia may affect balance and has been associated with increased mortality risk in certain chronic disease states, but this is unlikely to be the main cause of acute delirium.
Polypharmacy
Patients admitted to the hospital with polypharmacy are at high risk of drug-induced delirium. In approaching delirium, a patient’s medications should be evaluated for interactions, as well as for possible effects of newly prescribed drugs. New medications that affect cytochrome P450 enzymes warrant investigation, as do drugs with narrow therapeutic windows that the patient has been using long-term.
Consultation with a clinical pharmacist is often helpful. Macrolides, protease inhibitors, and nondihydropyridine calcium channel blockers are common P450 inhibitors, while many anticonvulsants are known inducers of the P450 system. Selective serotonin reuptake inhibitors and diuretics can lead to electrolyte imbalances such as hyponatremia, which may further predispose to bouts of delirium, as described above.
The patient’s extensive list of psychoactive medications makes polypharmacy a significant risk factor for delirium. Quetiapine and venlafaxine both cause sedation and increase the risk of serotonin syndrome. However, in this case, the patient does not have marked fever, rigidity, or hyperreflexia to corroborate that diagnosis.
Dementia
The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), defines dementia as a disorder involving cognitive impairment in at least 1 cognitive domain, with a significant decline from a previous level of functioning.1 These impairments need not necessarily occur separately from bouts of delirium, but the time course for most forms of dementia tends to be progressive over a subacute to chronic duration.
Dementia increases the risk for acute confusion and delirium in hospitalized patients.13 This is partly reflected by pathophysiologic changes that leave elderly patients susceptible to the effects of anticholinergic drugs.14 Structural changes due to small-vessel ischemia may also predispose patients to seizures in the setting of metabolic derangement or critical illness. Diagnosing dementia thus remains a challenge, as dementia must be clearly distinguished from other disorders such as delirium and depression.
The acute change in this patient’s case makes the isolated diagnosis of dementia much less likely than other causes of altered mental status. Also, her previous level of function does not suggest a clinically significant personal history of impairment.
Mental illness
Several studies have examined the link between preoperative mental health disorders and postoperative delirium.15–17 Depression appears to be a risk factor for postoperative delirium in patients undergoing elective orthopedic surgery,15 and this includes elderly patients.16 While a clear etiologic link has yet to be determined, disruption of circadian rhythm and abnormal cerebral response to stress may play a role. Studies have also suggested an association between schizophrenia and delirium, though this may be related to perioperative suspension of medications.17
Bipolar disorder has not been well studied with regard to postoperative complications. However, this patient has had a previous episode of decompensated mania, therefore making bipolar disorder a plausible condition in the differential diagnosis.
CASE CONTINUED: ACUTE DETERIORATION
Without a clearly identifiable cause for our patient’s acute confusional state, neurology and medical consultants recommend neuroimaging.
Computed tomography (CT) and magnetic resonance imaging (MRI) without contrast are ordered and performed on postoperative day 11 and demonstrate chronic small-vessel ischemic disease, consistent with our patient’s age, as well as frontotemporal atrophy. There is no evidence of mass effect, bleeding, or acute ischemia.
Overnight, she becomes obtunded, and the rapid response team is called. Her vital signs appear stable, and she is afebrile. Basic laboratory studies, imaging, and electrocardiography are repeated, and the results are unchanged from recent tests. She is transferred to the intensive care unit (ICU) for closer monitoring.
2. What is most likely cause of the patient’s declining mental status, and what is the next appropriate step?
- Acute stroke: repeat MRI with contrast
- Urinary tract infection: order blood and urine cultures, and start empiric antibiotics
- Neuroleptic malignant syndrome: start dantrolene
- Seizures: order electroencephalography (EEG)
Acute stroke
Acute stroke can affect mental status and consciousness through several pathways. Stroke syndromes can vary in presentation depending on the level of cortical and subcortical involvement, with clinical manifestations including confusion, aphasia, neglect, and inattention. Wakefulness and the ability to maintain consciousness is impaired, with disruption of the ascending reticular activating system, often seen in injuries to the brainstem. Large territorial or hemispheric infarcts, with subsequent cerebral edema, can also disrupt this system and lead to cerebral herniation and coma.
MRI without contrast is extremely sensitive for ischemia and can typically detect ischemia in acute stroke within 3 to 30 minutes.18–20 Repeating the study with contrast is unlikely to provide additional benefit.
In our patient’s case, the lack of localizing neurologic symptoms, in addition to her recent negative neuroimaging workup, makes the diagnosis of acute stroke unlikely.
Infection
The role of severe infection in patients with altered mental status is well documented and likely relates to diffuse cerebral dysfunction caused by an inflammatory cascade. Less well understood is the role of occult infection, especially urinary tract infection, in otherwise immunocompetent patients. Urinary tract infection has long been thought to cause delirium in otherwise asymptomatic elderly patients, but few studies have examined this relationship, and those studies have been shown to have significant methodologic errors.21 In the absence of better data, urinary tract infection as the cause of frank delirium in an otherwise well patient should be viewed with skepticism, and alternative causes should be sought.
Although the patient has a nonspecific leukocytosis, her benign urinalysis and lack of corroborating evidence makes urinary tract infection an unlikely cause of her frank delirium.
Neuroleptic malignant syndrome
Neuroleptic malignant syndrome is defined as fever, rigidity, mental status changes, and autonomic instability after exposure to antidopaminergic drugs. It is classically seen after administration of typical antipsychotics, though atypical antipsychotics and antiemetic drugs may be implicated as well.
Patients often exhibit agitation and confusion, which when severe may progress to mutism and catatonia. Likewise, psychotropic drugs such as quetiapine and venlafaxine, used in combination, have the additional risk of serotonin syndrome.
Additional symptoms include hyperreflexia, ataxia, and myoclonus. Withdrawal of the causative agent and supportive care are the mainstays of therapy. Targeted therapies with agents such as dantrolene, bromocriptine, and amantadine have also been reported anecdotally, but their efficacy is unclear, with variable results.22
As noted earlier, the addition of quetiapine to the patient’s already lengthy medication list could conceivably cause neuroleptic malignant syndrome or serotonin syndrome and should be considered. However, additional neurologic findings to confirm this diagnosis are lacking.
Seizures
Nonconvulsive seizure, particularly nonconvulsive status epilepticus (NCSE), is not well recognized and is particularly challenging to diagnose without EEG. In several case series of patients presenting to the emergency room with altered mental status, NCSE was found in 16% to 28% of patients in whom EEG was performed after an initial evaluation failed to show an obvious cause for the delirium.23,24 Historical features are unreliable for ruling out NCSE as a cause of delirium, as up to 41% of patients in whom the condition is ultimately diagnosed have only confusion as the presenting clinical symptom.25
Likewise, alternating ictal and postictal periods may mimic the typical waxing and waning course classically associated with delirium of other causes. Physical findings such as nystagmus, anisocoria, and hippus may be helpful but are often overlooked or absent. EEG is thus an essential requirement for the diagnosis.26
Given the lack of a clear diagnosis, a workup with EEG should be considered in this patient.
CASE CONTINUED: ADDITIONAL SIGNS
In the ICU, our patient is evaluated by the intensivist team. Her vital signs are stable, and while she is now awakening, she is unable to follow commands and remains mute. She does not initiate movement spontaneously but offers slight resistance to passive movements, holding and maintaining postures her extremities are placed in. She keeps her eyes closed, but when opened by the examining physician, dysconjugate gaze and anisocoria are noted.
3. What clinical entity is most consistent with these physical findings, and what is the next step in management?
- Catatonia secondary to bipolar disorder type I: challenge with intravenous lorazepam 2 mg
- Oculomotor nerve palsy due to enlarging intracranial aneurysm: aggressive blood pressure lowering, elevation of the head of the bed
- Toxic leukoencephalopathy: supportive care and withdrawal of the causative agent
- NCSE: challenge with intravenous lorazepam 2 mg and order EEG
Catatonia
The DSM-5 defines catatonia as a behavioral syndrome complicating an underlying psychiatric or medical condition, as opposed to a distinct diagnosis. It is most commonly encountered in psychiatric illnesses including bipolar disorder, major depression, and schizophrenia. Akinesis, stupor, mutism, and “waxy” flexibility often dominate the clinical picture.
The pathophysiology is poorly defined, but likely involves neurotransmitter imbalances particularly with an increase in N-methyl-d-aspartate (NMDA) activity and suppression of gamma-aminobutyric acid (GABA) activity. This hypothesis is supported by the finding that benzodiazepines, electroconvulsive therapy, and NMDA antagonists such as amantadine are all effective in treating catatonia.27,28 Findings of focal neurologic abnormalities warrant further investigation. EEG may be necessary to differentiate catatonia from NCSE, as both may respond to a benzodiazepine challenge.
As pure catatonia is a diagnosis of exclusion, further workup, including EEG, is necessary to confirm the diagnosis.
Oculomotor nerve palsy
Anisocoria together with dysconjugate gaze should prompt consideration of a lesion involving the oculomotor nerve. Loss of tonic muscle activity from the lateral rectus and superior oblique cause a downward and outward gaze. Furthermore, loss of parasympathetic tone occurs with compressive palsies of the oculomotor nerve, clinically manifesting as a mydriatic and unreactive pupil with ptosis. Given its anatomic course and proximity to other vascular and parenchymal structures, the oculomotor nerve is vulnerable to compression from many sources, including aneurysmal dilation (especially of the posterior cerebral artery), uncal herniation, and inflammation of the cavernous sinus.
Noncontrast CT and lumbar puncture are very sensitive for making the diagnosis of sentinel bleeding within the first 24 hours,29 whereas computed tomographic angiography and magnetic resonance angiography can reliably detect unruptured aneurysms as small as 3 mm.30
Conditions that can lead to oculomotor palsy are unlikely to cause an acute gain in appendicular muscle tone, as noted by the catatonia this patient is demonstrating. Also, mass lesions or bleeding associated with oculomotor palsy is likely to cause acute loss of tone. Chronic upper-motor neuron lesions lead to spasticity rather than the waxy flexibility seen in this patient. In our patient, the findings of isolated anisocoria without further clinical evidence of oculomotor nerve compression make this diagnosis unlikely.
Toxic leukoencephalopathy
Toxic leukoencephalopathy—widespread destruction of myelin, particularly in the white matter tracts that support higher cortical functions—can be caused by antineoplastic agents, immunosuppressant agents, and industrial solvents, as well as by abuse of vaporized drugs such as heroin (“chasing the dragon”). In its mild forms it may cause behavioral disturbances or inattention. In severe forms, a neurobehavioral syndrome of akinetic mutism may be present and can mimic catatonia.31
The diagnosis is often based on the clinical history and neuroimaging, particularly MRI, which demonstrates hyperintensity of the white matter tracts in T2-weighted images.32
This patient does not have a clear history of exposure to an agent typically associated with toxic leukoencephalopathy and does not have the corroborating MRI findings to support this diagnosis.
CASE CONTINUED
Because recent neuroimaging revealed no structural brain lesions and no cause for brain herniation, the patient receives a challenge of 2 mg of intravenous lorazepam to treat potential NCSE. Subsequent improvement is noted in her anisocoria, gaze deviation, and encephalopathy. EEG reveals frequent focal seizures arising from mesial frontal regions with bilateral hemisphere propagation, consistent with bifrontal focal NCSE.
As our patient is being transferred to a room for continuous EEG monitoring, her condition begins to deteriorate, and she again becomes more encephalopathic, with anisocoria and dysconjugate gaze. Additional doses of lorazepam are given (to complete a 0.1-mg/kg load), and additional therapy with intravenous fosphenytoin (20-mg/kg load) is given. Intubation is done for airway protection.
Continuous EEG monitoring reveals multiple frequent electrographic seizures arising from the bifrontal territories, concerning for persistent focal NCSE. A midazolam drip is initiated for EEG burst suppression of cerebral activity. Over 24 hours, EEG shows resolution of seizure activity. As the patient is weaned from sedation, she awakens and follows commands consistently, tolerating extubation without complications. Her neurologic status remains stable over the next 48 hours, having returned to her neurologic baseline level of functioning. She is able to be transferred out of the ICU in stable condition while continuing on scheduled antiepileptic therapy with phenytoin.
ALTERED MENTAL STATUS IN INPATIENTS
Altered mental status is one of the most frequently encountered reasons for medical consultation from nonmedical services. The workup and management of metabolic, toxic, psychiatric, and neurologic causes requires a deep appreciation for the broad differential diagnosis and a multidisciplinary approach. Physicians caring for these patients should avoid prematurely drawing conclusions when the patient’s clinical condition fails to respond to typical measures.
Delirium is a challenging adverse event in older patients during hospitalization, with a significant national financial burden of $164 billion per year.33 The prevalence of delirium in adults on hospital admission is estimated as 14% to 24%, with an inpatient hospitalization incidence ranging from 6% to 56% in general hospital patients.34 In addition, postoperative delirium has been reported in 15% to 53% of older patients.35
While delirium is preventable in 30% to 40% of cases,36,37 it remains an important independent prognostic determinant of hospital outcomes.38–40
Delirium in hospitalized patients requires a thorough, individualized workup. In our patient’s case, the clinical findings of hypoactive delirium were found to be manifestations of NCSE, a rare life-threatening and potentially reversible neurologic disease.
While establishing seizures as a diagnosis, careful attention must first be directed towards investigating environmental or metabolic triggers that may be inciting the disease. This often involves a similar workup for metabolic derangements, as seen in the approach to delirium.
The diagnosis of NCSE, while made in this patient’s case, remains challenging. Careful physical examination should assess for automatisms, “negative” symptoms (staring, aphasia, weakness), and “positive” symptoms (hallucinations, psychosis). Cataplexy, mutism, and other acute psychiatric features have been associated with NCSE,44 highlighting the importance of EEG. A trial of a benzodiazepine in conjunction with clinical and EEG monitoring may help guide clinical decision- making.
As there is no current universally accepted definition for NCSE nor an accepted agreement on required EEG diagnostic features at this time,41 accurate diagnosis is most likely to be obtained in facilities with both subspecialty neurologic consultation and EEG capabilities.
Our patient’s family history of Pick disease is interesting, as this is a progressive form of frontotemporal dementia with both sporadic and genetically linked cases. Recent studies have shown evidence that patients with neurodegenerative disease have increased seizure frequency early in the disease course,31 and efforts are under way to establish the incidence of first unprovoked seizure in patients with frontotemporal dementia. In our patient’s case, resolution of seizure activity yielded a return to her baseline level of neurologic function.
Early use of selective serotonin reuptake inhibitors has been shown to help with the behavioral symptoms of frontotemporal dementia,45 but increasing requirements over time may indicate progression of neurodegeneration and should warrant further appropriate investigation.
In our patient’s case, escalating dose requirements may have reflected worsening frontotemporal atrophy. However, the diagnosis of a neurodegenerative disease such as frontotemporal dementia in a patient such as ours is not definitively established at this time and is being investigated on an outpatient basis.
Given the frequency of delirium and its many risk factors in the inpatient setting, verifying a causative diagnosis can be difficult. Detailed consideration of the patient’s individual clinical circumstances, often in concert with appropriate subspecialty consultations, is essential to the evaluation. Although it is time-intensive, multidisciplinary intervention can lead to safer outcomes and shorter hospital stays.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association Publishing; 2013. http://psychiatryonline.org/doi/book/10.1176/appi.books.9780890425596. Accessed July 7, 2017.
- Mohan S, Gu S, Parikh A, Radhakrishnan J. Prevalence of hyponatremia and association with mortality: results from NHANES. Am J Med 2013; 126:1127–1137.e1.
- Sterns RH. Disorders of plasma sodium—causes, consequences, and correction. N Engl J Med 2015; 372:55–65.
- Rose B, Post T. Clinical physiology of acid-base and electrolyte disorders. 5th ed. New York, NY: McGraw-Hill; 2001.
- McManus ML, Churchwell KB, Strange K. Regulation of cell volume in health and disease. N Engl J Med 1995; 333:1260–1266.
- Strange K. Regulation of solute and water balance and cell volume in the central nervous system. J Am Soc Nephrol 1992; 3:12–27.
- Ayus JC, Wheeler JM, Arieff AI. Postoperative hyponatremic encephalopathy in menstruant women. Ann Intern Med 1992; 117:891–897.
- Gur RC, Mozley PD, Resnick SM, et al. Gender differences in age effect on brain atrophy measured by magnetic resonance imaging. Proc Natl Acad Sci USA 1991; 88:2845–2849.
- Rosomoff HL, Zugibe FT. Distribution of intracranial contents in experimental edema. Arch Neurol 1963; 9:26–34.
- Melton JE, Nattie EE. Brain and CSF water and ions during dilutional and isosmotic hyponatremia in the rat. Am J Physiol 1983; 244:R724–R732.
- Nattie EE, Edwards WH. Brain and CSF water and ions in newborn puppies during acute hypo- and hypernatremia. J Appl Physiol Respir Environ Exerc Physiol 1981; 51:1086–1091.
- Stachenfeld NS, DiPietro L, Palter SF, Nadel ER. Estrogen influences osmotic secretion of AVP and body water balance in postmenopausal women. Am J Physiol 1998; 274:R187–R195.
- Fick DM, Agostini JV, Inouye SK. Delirium superimposed on dementia: a systematic review. J Am Geriatr Soc 2002; 50:1723–1732.
- de Smet Y, Ruberg M, Serdaru M, Dubois B, Lhermitte F, Agid Y. Confusion, dementia and anticholinergics in Parkinson’s disease. J Neurol Neurosurg Psychiatry 1982; 45:1161–1164.
- Mollon B, Mahure SA, Ding DY, Zuckerman JD, Kwon YW. The influence of a history of clinical depression on peri-operative outcomes in elective total shoulder arthroplasty: a ten-year national analysis. Bone Joint J 2016; 98-B:818–824.
- Kosar CM, Tabloski PA, Travison TG, et al. Effect of preoperative pain and depressive symptoms on the development of postoperative delirium. Lancet Psychiatry 2014; 1:431–436.
- Copeland LA, Zeber JE, Pugh MJ, Mortensen EM, Restrepo MI, Lawrence VA. Postoperative complications in the seriously mentally ill: a systematic review of the literature. Ann Surg 2008; 248:31–38.
- Warach S, Gaa J, Siewert B, Wielopolski P, Edelman RR. Acute human stroke studied by whole brain echo planar diffusion-weighted magnetic resonance imaging. Ann Neurol 1995; 37:231–241.
- Sorensen AG, Buonanno FS, Gonzalez RG, et al. Hyperacute stroke: evaluation with combined multisection diffusion-weighted and hemodynamically weighted echo-planar MR imaging. Radiology 1996; 199:391–401.
- Li F, Han S, Tatlisumak T, et al. A new method to improve in-bore middle cerebral artery occlusion in rats: demonstration with diffusion—and perfusion—weighted imaging. Stroke 1998; 29:1715–1720.
- Balogun SA, Philbrick JT. Delirium, a symptom of UTI in the elderly: fact or fable? A systematic review. Can Geriatr J 2013; 17:22–26.
- Reulbach U, Dütsch C, Biermann T, et al. Managing an effective treatment for neuroleptic malignant syndrome. Crit Care 2007; 11:R4.
- Naeije G, Depondt C, Meeus C, Korpak K, Pepersack T, Legros B. EEG patterns compatible with nonconvulsive status epilepticus are common in elderly patients with delirium: a prospective study with continuous EEG monitoring. Epilepsy Behav 2014; 36:18–21.
- Veran O, Kahane P, Thomas P, Hamelin S, Sabourdy C, Vercueil L. De novo epileptic confusion in the elderly: a 1-year prospective study. Epilepsia 2010; 51:1030–1035.
- Sutter R, Rüegg S, Kaplan PW. Epidemiology, diagnosis, and management of nonconvulsive status epilepticus. Opening Pandora’s box. Neurol Clin Pract 2012; 2:275–286.
- Husain AM, Horn GJ, Jacobson MP. Non-convulsive status epilepticus: usefulness of clinical features in selecting patients for urgent EEG. J Neurol Neurosurg Psychiatry 2003; 74:189–191.
- Ungvari GS, Chiu HF, Chow LY, Lau BS, Tang WK. Lorazepam for chronic catatonia: a randomized, double-blind, placebo-controlled cross-over study. Psychopharmacology (Berl) 1999; 142:393–398.
- Carroll BT, Goforth HW, Thomas C, et al. Review of adjunctive glutamate antagonist therapy in the treatment of catatonic syndromes. J Neuropsychiatry Clin Neurosci 2007; 19:406– 412.
- Perry JJ, Spacek A, Forbes M, et al. Is the combination of negative computed tomography result and negative lumbar puncture result sufficient to rule out subarachnoid hemorrhage? Ann Emerg Med 2008; 51:707–713.
- Li MH, Cheng YS, Li YD, et al. Large-cohort comparison between three-dimensional time-of-flight magnetic resonance and rotational digital subtraction angiographies in intracranial aneurysm detection. Stroke 2009; 40:3127–3129.
- Filley CM, Kleinschmidt-DeMasters BK. Toxic leukoencephalopathy. N Engl J Med 2001; 345:425–432.
- Magnetic resonance imaging of the central nervous system. Council on Scientific Affairs. Report of the Panel on Magnetic Resonance Imaging. JAMA 1988; 259:1211–1222.
- Leslie DL, Marcantonio ER, Zhang Y, Leo-Summers L, Inouye SK. One-year health care costs associated with delirium in the elderly population. Arch Intern Med 2008; 168:27–32.
- Inouye SK. Delirium in hospitalized older patients. Clin Geriatr Med 1998; 14:745–764.
- Agostini JV, Inouye SK, Hazzard W, Blass J. Delirium. In: Principles of Geriatric Medicine and Gerontology. 5th ed. New York, NY: McGraw-Hill; 2003:1503–1515.
- Inouye SK, Bogardus ST Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med 1999; 340:669–676.
- Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc 2001; 49:516–522.
- Inouye SK, Rushing JT, Foreman MD, Palmer RM, Pompei P. Does delirium contribute to poor hospital outcomes? A three-site epidemiologic study. J Gen Intern Med 1998; 13:234–242.
- Rothschild JM, Bates DW, Leape LL. Preventable medical injuries in older patients. Arch Intern Med 2000; 160:2717–2728.
- Gillick MR, Serrell NA, Gillick LS. Adverse consequences of hospitalization in the elderly. Soc Sci Med 1982; 16:1033–1038.
- Drislane FW. Presentation, evaluation, and treatment of nonconvulsive status epilepticus. Epilepsy Behav 2000; 1:301-314.
- Rosenow F, Hamer HM, Knake S. The epidemiology of convulsive and nonconvulsive status epilepticus. Epilepsia 2007; 48(suppl 8):82–84.
- Woodford HJ, George J, Jackson M. Non-convulsive status epilepticus: a practical approach to diagnosis in confused older people. Postgrad Med J 2015; 91:655–661.
- Kaplan PW. Nonconvulsive status epilepticus in the emergency room. Epilepsia 1996; 37:643–650.
- Swartz JR, Miller BL, Lesser IM, Darby AL. Frontotemporal dementia: treatment response to serotonin selective reuptake inhibitors. J Clin Psychiatry 1997; 58:212–216.
A 64-year-old woman undergoes elective T10-S1 nerve decompression with fusion for chronic idiopathic scoliosis. Soon afterward, she develops acute urinary retention attributed to an Escherichia coli urinary tract infection and narcotic medications. She is treated with antibiotics, an indwelling catheter is inserted, and her symptoms resolve. She is transferred to the inpatient physical rehabilitation unit.
On postoperative day 9, she develops an acute change in mental status, suddenly becoming extremely anxious and falsely believing she has a “terminal illness.” A psychiatrist suggests that these symptoms are a manifestation of delirium, given the patient’s recent surgery and exposure to benzodiazepine and narcotic medications. On postoperative day 10, she is awake but is now mute and uncooperative. An internist is consulted for an evaluation for encephalopathy and delirium.
MEDICAL HISTORY
Her medical history, obtained by chart review and interviewing her husband, includes well-controlled bipolar disorder over the last 4 years, with no episodes of frank psychosis or mania. She had a “bout of delirium” 4 years earlier attributed to a catastrophic life event, but the symptoms resolved after adjustment of her anxiolytic and mood-stabilizing drugs. She also has well-controlled hypertension, hypothyroidism, and gastroesophageal reflux. Her only surgery was her recent elective procedure.
She has a family history of dementia (Pick disease in her mother).
She is married, lives with her husband, and has an adult son. She is employed as a media specialist and also teaches English as a second language. Before this hospital admission, she was described as happy and content, though her primary psychiatrist had noted intermittent anxiety. Her husband does not suspect illicit drug use and denies significant alcohol or tobacco abuse.
A thorough review of systems is not possible, given her encephalopathy. But before her acute decline, she had complained of “choking on blood” and a subjective inability to swallow.
Her home medications include dextroamphetamine extended-release, alprazolam as needed for sleep, venlafaxine extended-release, lamotrigine, lisinopril, propranolol, amlodipine, atorvastatin, levothyroxine, omeprazole, iron, and vitamin B12. At the time of the evaluation, she is on her home medications with the addition of olanzapine, vitamin D, polyethylene glycol, and an intravenous infusion of dextrose 5% with 0.45% saline at a rate of 100 mL/hour. She has allergies to latex, penicillin, peanuts, and shellfish.
PHYSICAL EXAMINATION
On physical examination, the patient seems healthy and appears normal for her stated age. She is wearing a spinal brace and is in no apparent distress. She is afebrile, pulse 104 beats per minute, respirations 16 breaths per minute and unlabored, and oxygen saturation good on room air. The skin is normal. No thyromegaly, bruits, or lymphadenopathy is noted. Cardiovascular, respiratory, and abdominal examinations, though limited by the spinal brace, are unremarkable. She has no evidence of peripheral edema or vascular insufficiency. Muscle bulk and tone are adequate and symmetric.
She is awake and alert and able to follow simple commands with some prompting. She does not initiate movements spontaneously. She makes some eye contact but does not track or acknowledge the interviewer consistently and does not respond verbally to questions. Her sclera are nonicteric, the pupils are equally round and reactive to light, and the external ocular muscles are intact. There is no facial asymmetry, and the tongue protrudes at midline. She blinks appropriately to threat bilaterally. Strength is at least 3/5 in the upper extremities and 2/5 in the lower extremities, though the examination is limited by lack of patient cooperation. She shows minimal grimace on noxious stimulation but does not withdraw extremities. Reflexes are present and mildly depressed symmetrically. Plantar reflexes are downgoing bilaterally.
INITIAL LABORATORY EVALUATION
On initial laboratory testing, the serum sodium is 132 mmol/L (reference range 136–144), stable since admission. Point-of-care glucose is 98 mg/dL. Aspartate aminotransferase and alanine aminotransferase levels are mildly elevated at 59 U/L (13–35) and 51 U/L (7–38), respectively, but serum ammonia is undetectable. Vitamin B12, folate, thyroid-stimulating hormone, and free thyroxine are within the normal ranges. Leukocytosis is noted, with 14 × 109 cells/L (3.7–11.0), 86% neutrophils, and a mild left shift. Urinalysis is negative for leukocyte esterase, nitrites, and white blood cells.
APPROACH TO ALTERED MENTAL STATUS
1. Which of the following risk factors predisposes this patient to postoperative delirium?
- Hyponatremia
- Polypharmacy
- Family history of dementia
- Depression
Altered mental status, or encephalopathy, is one of the most common yet challenging conditions in medicine. When a consult is placed for altered mental status, it is important to determine the affected domain that has changed from the patient’s normal state. Changes can include alterations in consciousness, attention, behavior, cognition, language, speech, and praxis and can reflect varying degrees of cerebral dysfunction.
Electrolyte abnormalities
Disorders of sodium homeostasis are common in hospitalized patients and may contribute to the onset of delirium. Hyponatremia is especially frequent and often iatrogenic, with a prevalence significantly higher in women (2.1% vs 1.3%, P = .0044) and in the elderly.2
Neurologic manifestations are often the result of cerebral edema due to osmolar volume shifts.3–6 Acute hyponatremic encephalopathy is most likely to occur when sodium shifts are rapid, usually within 24 hours, and is often seen in postoperative patients requiring significant volume resuscitation with hypotonic fluids.6 Young premenopausal women appear to be at especially high risk of permanent brain damage secondary to hyponatremic encephalopathy,7 a finding that may reflect the limited compliance within the intracranial vault and lack of significant involutional parenchymal changes that occur with aging.8–11
Aging also has important effects on fluid balance, as restoration of body fluid homeostasis is slower in older patients.12
Hormonal effects of estrogen appear to play a synergistic role in the expression of arginine vasopressin in postmenopausal women, further contributing to hyponatremia.
Although our patient has mild hyponatremia, there has been no acute change in her sodium balance since admission to the hospital, and so it is unlikely to be the cause of her acute delirium. Her mild hyponatremia may in part be from hypo-osmolar maintenance fluids with dextrose 5% and 0.45% normal saline.
Mild chronic hyponatremia may affect balance and has been associated with increased mortality risk in certain chronic disease states, but this is unlikely to be the main cause of acute delirium.
Polypharmacy
Patients admitted to the hospital with polypharmacy are at high risk of drug-induced delirium. In approaching delirium, a patient’s medications should be evaluated for interactions, as well as for possible effects of newly prescribed drugs. New medications that affect cytochrome P450 enzymes warrant investigation, as do drugs with narrow therapeutic windows that the patient has been using long-term.
Consultation with a clinical pharmacist is often helpful. Macrolides, protease inhibitors, and nondihydropyridine calcium channel blockers are common P450 inhibitors, while many anticonvulsants are known inducers of the P450 system. Selective serotonin reuptake inhibitors and diuretics can lead to electrolyte imbalances such as hyponatremia, which may further predispose to bouts of delirium, as described above.
The patient’s extensive list of psychoactive medications makes polypharmacy a significant risk factor for delirium. Quetiapine and venlafaxine both cause sedation and increase the risk of serotonin syndrome. However, in this case, the patient does not have marked fever, rigidity, or hyperreflexia to corroborate that diagnosis.
Dementia
The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), defines dementia as a disorder involving cognitive impairment in at least 1 cognitive domain, with a significant decline from a previous level of functioning.1 These impairments need not necessarily occur separately from bouts of delirium, but the time course for most forms of dementia tends to be progressive over a subacute to chronic duration.
Dementia increases the risk for acute confusion and delirium in hospitalized patients.13 This is partly reflected by pathophysiologic changes that leave elderly patients susceptible to the effects of anticholinergic drugs.14 Structural changes due to small-vessel ischemia may also predispose patients to seizures in the setting of metabolic derangement or critical illness. Diagnosing dementia thus remains a challenge, as dementia must be clearly distinguished from other disorders such as delirium and depression.
The acute change in this patient’s case makes the isolated diagnosis of dementia much less likely than other causes of altered mental status. Also, her previous level of function does not suggest a clinically significant personal history of impairment.
Mental illness
Several studies have examined the link between preoperative mental health disorders and postoperative delirium.15–17 Depression appears to be a risk factor for postoperative delirium in patients undergoing elective orthopedic surgery,15 and this includes elderly patients.16 While a clear etiologic link has yet to be determined, disruption of circadian rhythm and abnormal cerebral response to stress may play a role. Studies have also suggested an association between schizophrenia and delirium, though this may be related to perioperative suspension of medications.17
Bipolar disorder has not been well studied with regard to postoperative complications. However, this patient has had a previous episode of decompensated mania, therefore making bipolar disorder a plausible condition in the differential diagnosis.
CASE CONTINUED: ACUTE DETERIORATION
Without a clearly identifiable cause for our patient’s acute confusional state, neurology and medical consultants recommend neuroimaging.
Computed tomography (CT) and magnetic resonance imaging (MRI) without contrast are ordered and performed on postoperative day 11 and demonstrate chronic small-vessel ischemic disease, consistent with our patient’s age, as well as frontotemporal atrophy. There is no evidence of mass effect, bleeding, or acute ischemia.
Overnight, she becomes obtunded, and the rapid response team is called. Her vital signs appear stable, and she is afebrile. Basic laboratory studies, imaging, and electrocardiography are repeated, and the results are unchanged from recent tests. She is transferred to the intensive care unit (ICU) for closer monitoring.
2. What is most likely cause of the patient’s declining mental status, and what is the next appropriate step?
- Acute stroke: repeat MRI with contrast
- Urinary tract infection: order blood and urine cultures, and start empiric antibiotics
- Neuroleptic malignant syndrome: start dantrolene
- Seizures: order electroencephalography (EEG)
Acute stroke
Acute stroke can affect mental status and consciousness through several pathways. Stroke syndromes can vary in presentation depending on the level of cortical and subcortical involvement, with clinical manifestations including confusion, aphasia, neglect, and inattention. Wakefulness and the ability to maintain consciousness is impaired, with disruption of the ascending reticular activating system, often seen in injuries to the brainstem. Large territorial or hemispheric infarcts, with subsequent cerebral edema, can also disrupt this system and lead to cerebral herniation and coma.
MRI without contrast is extremely sensitive for ischemia and can typically detect ischemia in acute stroke within 3 to 30 minutes.18–20 Repeating the study with contrast is unlikely to provide additional benefit.
In our patient’s case, the lack of localizing neurologic symptoms, in addition to her recent negative neuroimaging workup, makes the diagnosis of acute stroke unlikely.
Infection
The role of severe infection in patients with altered mental status is well documented and likely relates to diffuse cerebral dysfunction caused by an inflammatory cascade. Less well understood is the role of occult infection, especially urinary tract infection, in otherwise immunocompetent patients. Urinary tract infection has long been thought to cause delirium in otherwise asymptomatic elderly patients, but few studies have examined this relationship, and those studies have been shown to have significant methodologic errors.21 In the absence of better data, urinary tract infection as the cause of frank delirium in an otherwise well patient should be viewed with skepticism, and alternative causes should be sought.
Although the patient has a nonspecific leukocytosis, her benign urinalysis and lack of corroborating evidence makes urinary tract infection an unlikely cause of her frank delirium.
Neuroleptic malignant syndrome
Neuroleptic malignant syndrome is defined as fever, rigidity, mental status changes, and autonomic instability after exposure to antidopaminergic drugs. It is classically seen after administration of typical antipsychotics, though atypical antipsychotics and antiemetic drugs may be implicated as well.
Patients often exhibit agitation and confusion, which when severe may progress to mutism and catatonia. Likewise, psychotropic drugs such as quetiapine and venlafaxine, used in combination, have the additional risk of serotonin syndrome.
Additional symptoms include hyperreflexia, ataxia, and myoclonus. Withdrawal of the causative agent and supportive care are the mainstays of therapy. Targeted therapies with agents such as dantrolene, bromocriptine, and amantadine have also been reported anecdotally, but their efficacy is unclear, with variable results.22
As noted earlier, the addition of quetiapine to the patient’s already lengthy medication list could conceivably cause neuroleptic malignant syndrome or serotonin syndrome and should be considered. However, additional neurologic findings to confirm this diagnosis are lacking.
Seizures
Nonconvulsive seizure, particularly nonconvulsive status epilepticus (NCSE), is not well recognized and is particularly challenging to diagnose without EEG. In several case series of patients presenting to the emergency room with altered mental status, NCSE was found in 16% to 28% of patients in whom EEG was performed after an initial evaluation failed to show an obvious cause for the delirium.23,24 Historical features are unreliable for ruling out NCSE as a cause of delirium, as up to 41% of patients in whom the condition is ultimately diagnosed have only confusion as the presenting clinical symptom.25
Likewise, alternating ictal and postictal periods may mimic the typical waxing and waning course classically associated with delirium of other causes. Physical findings such as nystagmus, anisocoria, and hippus may be helpful but are often overlooked or absent. EEG is thus an essential requirement for the diagnosis.26
Given the lack of a clear diagnosis, a workup with EEG should be considered in this patient.
CASE CONTINUED: ADDITIONAL SIGNS
In the ICU, our patient is evaluated by the intensivist team. Her vital signs are stable, and while she is now awakening, she is unable to follow commands and remains mute. She does not initiate movement spontaneously but offers slight resistance to passive movements, holding and maintaining postures her extremities are placed in. She keeps her eyes closed, but when opened by the examining physician, dysconjugate gaze and anisocoria are noted.
3. What clinical entity is most consistent with these physical findings, and what is the next step in management?
- Catatonia secondary to bipolar disorder type I: challenge with intravenous lorazepam 2 mg
- Oculomotor nerve palsy due to enlarging intracranial aneurysm: aggressive blood pressure lowering, elevation of the head of the bed
- Toxic leukoencephalopathy: supportive care and withdrawal of the causative agent
- NCSE: challenge with intravenous lorazepam 2 mg and order EEG
Catatonia
The DSM-5 defines catatonia as a behavioral syndrome complicating an underlying psychiatric or medical condition, as opposed to a distinct diagnosis. It is most commonly encountered in psychiatric illnesses including bipolar disorder, major depression, and schizophrenia. Akinesis, stupor, mutism, and “waxy” flexibility often dominate the clinical picture.
The pathophysiology is poorly defined, but likely involves neurotransmitter imbalances particularly with an increase in N-methyl-d-aspartate (NMDA) activity and suppression of gamma-aminobutyric acid (GABA) activity. This hypothesis is supported by the finding that benzodiazepines, electroconvulsive therapy, and NMDA antagonists such as amantadine are all effective in treating catatonia.27,28 Findings of focal neurologic abnormalities warrant further investigation. EEG may be necessary to differentiate catatonia from NCSE, as both may respond to a benzodiazepine challenge.
As pure catatonia is a diagnosis of exclusion, further workup, including EEG, is necessary to confirm the diagnosis.
Oculomotor nerve palsy
Anisocoria together with dysconjugate gaze should prompt consideration of a lesion involving the oculomotor nerve. Loss of tonic muscle activity from the lateral rectus and superior oblique cause a downward and outward gaze. Furthermore, loss of parasympathetic tone occurs with compressive palsies of the oculomotor nerve, clinically manifesting as a mydriatic and unreactive pupil with ptosis. Given its anatomic course and proximity to other vascular and parenchymal structures, the oculomotor nerve is vulnerable to compression from many sources, including aneurysmal dilation (especially of the posterior cerebral artery), uncal herniation, and inflammation of the cavernous sinus.
Noncontrast CT and lumbar puncture are very sensitive for making the diagnosis of sentinel bleeding within the first 24 hours,29 whereas computed tomographic angiography and magnetic resonance angiography can reliably detect unruptured aneurysms as small as 3 mm.30
Conditions that can lead to oculomotor palsy are unlikely to cause an acute gain in appendicular muscle tone, as noted by the catatonia this patient is demonstrating. Also, mass lesions or bleeding associated with oculomotor palsy is likely to cause acute loss of tone. Chronic upper-motor neuron lesions lead to spasticity rather than the waxy flexibility seen in this patient. In our patient, the findings of isolated anisocoria without further clinical evidence of oculomotor nerve compression make this diagnosis unlikely.
Toxic leukoencephalopathy
Toxic leukoencephalopathy—widespread destruction of myelin, particularly in the white matter tracts that support higher cortical functions—can be caused by antineoplastic agents, immunosuppressant agents, and industrial solvents, as well as by abuse of vaporized drugs such as heroin (“chasing the dragon”). In its mild forms it may cause behavioral disturbances or inattention. In severe forms, a neurobehavioral syndrome of akinetic mutism may be present and can mimic catatonia.31
The diagnosis is often based on the clinical history and neuroimaging, particularly MRI, which demonstrates hyperintensity of the white matter tracts in T2-weighted images.32
This patient does not have a clear history of exposure to an agent typically associated with toxic leukoencephalopathy and does not have the corroborating MRI findings to support this diagnosis.
CASE CONTINUED
Because recent neuroimaging revealed no structural brain lesions and no cause for brain herniation, the patient receives a challenge of 2 mg of intravenous lorazepam to treat potential NCSE. Subsequent improvement is noted in her anisocoria, gaze deviation, and encephalopathy. EEG reveals frequent focal seizures arising from mesial frontal regions with bilateral hemisphere propagation, consistent with bifrontal focal NCSE.
As our patient is being transferred to a room for continuous EEG monitoring, her condition begins to deteriorate, and she again becomes more encephalopathic, with anisocoria and dysconjugate gaze. Additional doses of lorazepam are given (to complete a 0.1-mg/kg load), and additional therapy with intravenous fosphenytoin (20-mg/kg load) is given. Intubation is done for airway protection.
Continuous EEG monitoring reveals multiple frequent electrographic seizures arising from the bifrontal territories, concerning for persistent focal NCSE. A midazolam drip is initiated for EEG burst suppression of cerebral activity. Over 24 hours, EEG shows resolution of seizure activity. As the patient is weaned from sedation, she awakens and follows commands consistently, tolerating extubation without complications. Her neurologic status remains stable over the next 48 hours, having returned to her neurologic baseline level of functioning. She is able to be transferred out of the ICU in stable condition while continuing on scheduled antiepileptic therapy with phenytoin.
ALTERED MENTAL STATUS IN INPATIENTS
Altered mental status is one of the most frequently encountered reasons for medical consultation from nonmedical services. The workup and management of metabolic, toxic, psychiatric, and neurologic causes requires a deep appreciation for the broad differential diagnosis and a multidisciplinary approach. Physicians caring for these patients should avoid prematurely drawing conclusions when the patient’s clinical condition fails to respond to typical measures.
Delirium is a challenging adverse event in older patients during hospitalization, with a significant national financial burden of $164 billion per year.33 The prevalence of delirium in adults on hospital admission is estimated as 14% to 24%, with an inpatient hospitalization incidence ranging from 6% to 56% in general hospital patients.34 In addition, postoperative delirium has been reported in 15% to 53% of older patients.35
While delirium is preventable in 30% to 40% of cases,36,37 it remains an important independent prognostic determinant of hospital outcomes.38–40
Delirium in hospitalized patients requires a thorough, individualized workup. In our patient’s case, the clinical findings of hypoactive delirium were found to be manifestations of NCSE, a rare life-threatening and potentially reversible neurologic disease.
While establishing seizures as a diagnosis, careful attention must first be directed towards investigating environmental or metabolic triggers that may be inciting the disease. This often involves a similar workup for metabolic derangements, as seen in the approach to delirium.
The diagnosis of NCSE, while made in this patient’s case, remains challenging. Careful physical examination should assess for automatisms, “negative” symptoms (staring, aphasia, weakness), and “positive” symptoms (hallucinations, psychosis). Cataplexy, mutism, and other acute psychiatric features have been associated with NCSE,44 highlighting the importance of EEG. A trial of a benzodiazepine in conjunction with clinical and EEG monitoring may help guide clinical decision- making.
As there is no current universally accepted definition for NCSE nor an accepted agreement on required EEG diagnostic features at this time,41 accurate diagnosis is most likely to be obtained in facilities with both subspecialty neurologic consultation and EEG capabilities.
Our patient’s family history of Pick disease is interesting, as this is a progressive form of frontotemporal dementia with both sporadic and genetically linked cases. Recent studies have shown evidence that patients with neurodegenerative disease have increased seizure frequency early in the disease course,31 and efforts are under way to establish the incidence of first unprovoked seizure in patients with frontotemporal dementia. In our patient’s case, resolution of seizure activity yielded a return to her baseline level of neurologic function.
Early use of selective serotonin reuptake inhibitors has been shown to help with the behavioral symptoms of frontotemporal dementia,45 but increasing requirements over time may indicate progression of neurodegeneration and should warrant further appropriate investigation.
In our patient’s case, escalating dose requirements may have reflected worsening frontotemporal atrophy. However, the diagnosis of a neurodegenerative disease such as frontotemporal dementia in a patient such as ours is not definitively established at this time and is being investigated on an outpatient basis.
Given the frequency of delirium and its many risk factors in the inpatient setting, verifying a causative diagnosis can be difficult. Detailed consideration of the patient’s individual clinical circumstances, often in concert with appropriate subspecialty consultations, is essential to the evaluation. Although it is time-intensive, multidisciplinary intervention can lead to safer outcomes and shorter hospital stays.
A 64-year-old woman undergoes elective T10-S1 nerve decompression with fusion for chronic idiopathic scoliosis. Soon afterward, she develops acute urinary retention attributed to an Escherichia coli urinary tract infection and narcotic medications. She is treated with antibiotics, an indwelling catheter is inserted, and her symptoms resolve. She is transferred to the inpatient physical rehabilitation unit.
On postoperative day 9, she develops an acute change in mental status, suddenly becoming extremely anxious and falsely believing she has a “terminal illness.” A psychiatrist suggests that these symptoms are a manifestation of delirium, given the patient’s recent surgery and exposure to benzodiazepine and narcotic medications. On postoperative day 10, she is awake but is now mute and uncooperative. An internist is consulted for an evaluation for encephalopathy and delirium.
MEDICAL HISTORY
Her medical history, obtained by chart review and interviewing her husband, includes well-controlled bipolar disorder over the last 4 years, with no episodes of frank psychosis or mania. She had a “bout of delirium” 4 years earlier attributed to a catastrophic life event, but the symptoms resolved after adjustment of her anxiolytic and mood-stabilizing drugs. She also has well-controlled hypertension, hypothyroidism, and gastroesophageal reflux. Her only surgery was her recent elective procedure.
She has a family history of dementia (Pick disease in her mother).
She is married, lives with her husband, and has an adult son. She is employed as a media specialist and also teaches English as a second language. Before this hospital admission, she was described as happy and content, though her primary psychiatrist had noted intermittent anxiety. Her husband does not suspect illicit drug use and denies significant alcohol or tobacco abuse.
A thorough review of systems is not possible, given her encephalopathy. But before her acute decline, she had complained of “choking on blood” and a subjective inability to swallow.
Her home medications include dextroamphetamine extended-release, alprazolam as needed for sleep, venlafaxine extended-release, lamotrigine, lisinopril, propranolol, amlodipine, atorvastatin, levothyroxine, omeprazole, iron, and vitamin B12. At the time of the evaluation, she is on her home medications with the addition of olanzapine, vitamin D, polyethylene glycol, and an intravenous infusion of dextrose 5% with 0.45% saline at a rate of 100 mL/hour. She has allergies to latex, penicillin, peanuts, and shellfish.
PHYSICAL EXAMINATION
On physical examination, the patient seems healthy and appears normal for her stated age. She is wearing a spinal brace and is in no apparent distress. She is afebrile, pulse 104 beats per minute, respirations 16 breaths per minute and unlabored, and oxygen saturation good on room air. The skin is normal. No thyromegaly, bruits, or lymphadenopathy is noted. Cardiovascular, respiratory, and abdominal examinations, though limited by the spinal brace, are unremarkable. She has no evidence of peripheral edema or vascular insufficiency. Muscle bulk and tone are adequate and symmetric.
She is awake and alert and able to follow simple commands with some prompting. She does not initiate movements spontaneously. She makes some eye contact but does not track or acknowledge the interviewer consistently and does not respond verbally to questions. Her sclera are nonicteric, the pupils are equally round and reactive to light, and the external ocular muscles are intact. There is no facial asymmetry, and the tongue protrudes at midline. She blinks appropriately to threat bilaterally. Strength is at least 3/5 in the upper extremities and 2/5 in the lower extremities, though the examination is limited by lack of patient cooperation. She shows minimal grimace on noxious stimulation but does not withdraw extremities. Reflexes are present and mildly depressed symmetrically. Plantar reflexes are downgoing bilaterally.
INITIAL LABORATORY EVALUATION
On initial laboratory testing, the serum sodium is 132 mmol/L (reference range 136–144), stable since admission. Point-of-care glucose is 98 mg/dL. Aspartate aminotransferase and alanine aminotransferase levels are mildly elevated at 59 U/L (13–35) and 51 U/L (7–38), respectively, but serum ammonia is undetectable. Vitamin B12, folate, thyroid-stimulating hormone, and free thyroxine are within the normal ranges. Leukocytosis is noted, with 14 × 109 cells/L (3.7–11.0), 86% neutrophils, and a mild left shift. Urinalysis is negative for leukocyte esterase, nitrites, and white blood cells.
APPROACH TO ALTERED MENTAL STATUS
1. Which of the following risk factors predisposes this patient to postoperative delirium?
- Hyponatremia
- Polypharmacy
- Family history of dementia
- Depression
Altered mental status, or encephalopathy, is one of the most common yet challenging conditions in medicine. When a consult is placed for altered mental status, it is important to determine the affected domain that has changed from the patient’s normal state. Changes can include alterations in consciousness, attention, behavior, cognition, language, speech, and praxis and can reflect varying degrees of cerebral dysfunction.
Electrolyte abnormalities
Disorders of sodium homeostasis are common in hospitalized patients and may contribute to the onset of delirium. Hyponatremia is especially frequent and often iatrogenic, with a prevalence significantly higher in women (2.1% vs 1.3%, P = .0044) and in the elderly.2
Neurologic manifestations are often the result of cerebral edema due to osmolar volume shifts.3–6 Acute hyponatremic encephalopathy is most likely to occur when sodium shifts are rapid, usually within 24 hours, and is often seen in postoperative patients requiring significant volume resuscitation with hypotonic fluids.6 Young premenopausal women appear to be at especially high risk of permanent brain damage secondary to hyponatremic encephalopathy,7 a finding that may reflect the limited compliance within the intracranial vault and lack of significant involutional parenchymal changes that occur with aging.8–11
Aging also has important effects on fluid balance, as restoration of body fluid homeostasis is slower in older patients.12
Hormonal effects of estrogen appear to play a synergistic role in the expression of arginine vasopressin in postmenopausal women, further contributing to hyponatremia.
Although our patient has mild hyponatremia, there has been no acute change in her sodium balance since admission to the hospital, and so it is unlikely to be the cause of her acute delirium. Her mild hyponatremia may in part be from hypo-osmolar maintenance fluids with dextrose 5% and 0.45% normal saline.
Mild chronic hyponatremia may affect balance and has been associated with increased mortality risk in certain chronic disease states, but this is unlikely to be the main cause of acute delirium.
Polypharmacy
Patients admitted to the hospital with polypharmacy are at high risk of drug-induced delirium. In approaching delirium, a patient’s medications should be evaluated for interactions, as well as for possible effects of newly prescribed drugs. New medications that affect cytochrome P450 enzymes warrant investigation, as do drugs with narrow therapeutic windows that the patient has been using long-term.
Consultation with a clinical pharmacist is often helpful. Macrolides, protease inhibitors, and nondihydropyridine calcium channel blockers are common P450 inhibitors, while many anticonvulsants are known inducers of the P450 system. Selective serotonin reuptake inhibitors and diuretics can lead to electrolyte imbalances such as hyponatremia, which may further predispose to bouts of delirium, as described above.
The patient’s extensive list of psychoactive medications makes polypharmacy a significant risk factor for delirium. Quetiapine and venlafaxine both cause sedation and increase the risk of serotonin syndrome. However, in this case, the patient does not have marked fever, rigidity, or hyperreflexia to corroborate that diagnosis.
Dementia
The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), defines dementia as a disorder involving cognitive impairment in at least 1 cognitive domain, with a significant decline from a previous level of functioning.1 These impairments need not necessarily occur separately from bouts of delirium, but the time course for most forms of dementia tends to be progressive over a subacute to chronic duration.
Dementia increases the risk for acute confusion and delirium in hospitalized patients.13 This is partly reflected by pathophysiologic changes that leave elderly patients susceptible to the effects of anticholinergic drugs.14 Structural changes due to small-vessel ischemia may also predispose patients to seizures in the setting of metabolic derangement or critical illness. Diagnosing dementia thus remains a challenge, as dementia must be clearly distinguished from other disorders such as delirium and depression.
The acute change in this patient’s case makes the isolated diagnosis of dementia much less likely than other causes of altered mental status. Also, her previous level of function does not suggest a clinically significant personal history of impairment.
Mental illness
Several studies have examined the link between preoperative mental health disorders and postoperative delirium.15–17 Depression appears to be a risk factor for postoperative delirium in patients undergoing elective orthopedic surgery,15 and this includes elderly patients.16 While a clear etiologic link has yet to be determined, disruption of circadian rhythm and abnormal cerebral response to stress may play a role. Studies have also suggested an association between schizophrenia and delirium, though this may be related to perioperative suspension of medications.17
Bipolar disorder has not been well studied with regard to postoperative complications. However, this patient has had a previous episode of decompensated mania, therefore making bipolar disorder a plausible condition in the differential diagnosis.
CASE CONTINUED: ACUTE DETERIORATION
Without a clearly identifiable cause for our patient’s acute confusional state, neurology and medical consultants recommend neuroimaging.
Computed tomography (CT) and magnetic resonance imaging (MRI) without contrast are ordered and performed on postoperative day 11 and demonstrate chronic small-vessel ischemic disease, consistent with our patient’s age, as well as frontotemporal atrophy. There is no evidence of mass effect, bleeding, or acute ischemia.
Overnight, she becomes obtunded, and the rapid response team is called. Her vital signs appear stable, and she is afebrile. Basic laboratory studies, imaging, and electrocardiography are repeated, and the results are unchanged from recent tests. She is transferred to the intensive care unit (ICU) for closer monitoring.
2. What is most likely cause of the patient’s declining mental status, and what is the next appropriate step?
- Acute stroke: repeat MRI with contrast
- Urinary tract infection: order blood and urine cultures, and start empiric antibiotics
- Neuroleptic malignant syndrome: start dantrolene
- Seizures: order electroencephalography (EEG)
Acute stroke
Acute stroke can affect mental status and consciousness through several pathways. Stroke syndromes can vary in presentation depending on the level of cortical and subcortical involvement, with clinical manifestations including confusion, aphasia, neglect, and inattention. Wakefulness and the ability to maintain consciousness is impaired, with disruption of the ascending reticular activating system, often seen in injuries to the brainstem. Large territorial or hemispheric infarcts, with subsequent cerebral edema, can also disrupt this system and lead to cerebral herniation and coma.
MRI without contrast is extremely sensitive for ischemia and can typically detect ischemia in acute stroke within 3 to 30 minutes.18–20 Repeating the study with contrast is unlikely to provide additional benefit.
In our patient’s case, the lack of localizing neurologic symptoms, in addition to her recent negative neuroimaging workup, makes the diagnosis of acute stroke unlikely.
Infection
The role of severe infection in patients with altered mental status is well documented and likely relates to diffuse cerebral dysfunction caused by an inflammatory cascade. Less well understood is the role of occult infection, especially urinary tract infection, in otherwise immunocompetent patients. Urinary tract infection has long been thought to cause delirium in otherwise asymptomatic elderly patients, but few studies have examined this relationship, and those studies have been shown to have significant methodologic errors.21 In the absence of better data, urinary tract infection as the cause of frank delirium in an otherwise well patient should be viewed with skepticism, and alternative causes should be sought.
Although the patient has a nonspecific leukocytosis, her benign urinalysis and lack of corroborating evidence makes urinary tract infection an unlikely cause of her frank delirium.
Neuroleptic malignant syndrome
Neuroleptic malignant syndrome is defined as fever, rigidity, mental status changes, and autonomic instability after exposure to antidopaminergic drugs. It is classically seen after administration of typical antipsychotics, though atypical antipsychotics and antiemetic drugs may be implicated as well.
Patients often exhibit agitation and confusion, which when severe may progress to mutism and catatonia. Likewise, psychotropic drugs such as quetiapine and venlafaxine, used in combination, have the additional risk of serotonin syndrome.
Additional symptoms include hyperreflexia, ataxia, and myoclonus. Withdrawal of the causative agent and supportive care are the mainstays of therapy. Targeted therapies with agents such as dantrolene, bromocriptine, and amantadine have also been reported anecdotally, but their efficacy is unclear, with variable results.22
As noted earlier, the addition of quetiapine to the patient’s already lengthy medication list could conceivably cause neuroleptic malignant syndrome or serotonin syndrome and should be considered. However, additional neurologic findings to confirm this diagnosis are lacking.
Seizures
Nonconvulsive seizure, particularly nonconvulsive status epilepticus (NCSE), is not well recognized and is particularly challenging to diagnose without EEG. In several case series of patients presenting to the emergency room with altered mental status, NCSE was found in 16% to 28% of patients in whom EEG was performed after an initial evaluation failed to show an obvious cause for the delirium.23,24 Historical features are unreliable for ruling out NCSE as a cause of delirium, as up to 41% of patients in whom the condition is ultimately diagnosed have only confusion as the presenting clinical symptom.25
Likewise, alternating ictal and postictal periods may mimic the typical waxing and waning course classically associated with delirium of other causes. Physical findings such as nystagmus, anisocoria, and hippus may be helpful but are often overlooked or absent. EEG is thus an essential requirement for the diagnosis.26
Given the lack of a clear diagnosis, a workup with EEG should be considered in this patient.
CASE CONTINUED: ADDITIONAL SIGNS
In the ICU, our patient is evaluated by the intensivist team. Her vital signs are stable, and while she is now awakening, she is unable to follow commands and remains mute. She does not initiate movement spontaneously but offers slight resistance to passive movements, holding and maintaining postures her extremities are placed in. She keeps her eyes closed, but when opened by the examining physician, dysconjugate gaze and anisocoria are noted.
3. What clinical entity is most consistent with these physical findings, and what is the next step in management?
- Catatonia secondary to bipolar disorder type I: challenge with intravenous lorazepam 2 mg
- Oculomotor nerve palsy due to enlarging intracranial aneurysm: aggressive blood pressure lowering, elevation of the head of the bed
- Toxic leukoencephalopathy: supportive care and withdrawal of the causative agent
- NCSE: challenge with intravenous lorazepam 2 mg and order EEG
Catatonia
The DSM-5 defines catatonia as a behavioral syndrome complicating an underlying psychiatric or medical condition, as opposed to a distinct diagnosis. It is most commonly encountered in psychiatric illnesses including bipolar disorder, major depression, and schizophrenia. Akinesis, stupor, mutism, and “waxy” flexibility often dominate the clinical picture.
The pathophysiology is poorly defined, but likely involves neurotransmitter imbalances particularly with an increase in N-methyl-d-aspartate (NMDA) activity and suppression of gamma-aminobutyric acid (GABA) activity. This hypothesis is supported by the finding that benzodiazepines, electroconvulsive therapy, and NMDA antagonists such as amantadine are all effective in treating catatonia.27,28 Findings of focal neurologic abnormalities warrant further investigation. EEG may be necessary to differentiate catatonia from NCSE, as both may respond to a benzodiazepine challenge.
As pure catatonia is a diagnosis of exclusion, further workup, including EEG, is necessary to confirm the diagnosis.
Oculomotor nerve palsy
Anisocoria together with dysconjugate gaze should prompt consideration of a lesion involving the oculomotor nerve. Loss of tonic muscle activity from the lateral rectus and superior oblique cause a downward and outward gaze. Furthermore, loss of parasympathetic tone occurs with compressive palsies of the oculomotor nerve, clinically manifesting as a mydriatic and unreactive pupil with ptosis. Given its anatomic course and proximity to other vascular and parenchymal structures, the oculomotor nerve is vulnerable to compression from many sources, including aneurysmal dilation (especially of the posterior cerebral artery), uncal herniation, and inflammation of the cavernous sinus.
Noncontrast CT and lumbar puncture are very sensitive for making the diagnosis of sentinel bleeding within the first 24 hours,29 whereas computed tomographic angiography and magnetic resonance angiography can reliably detect unruptured aneurysms as small as 3 mm.30
Conditions that can lead to oculomotor palsy are unlikely to cause an acute gain in appendicular muscle tone, as noted by the catatonia this patient is demonstrating. Also, mass lesions or bleeding associated with oculomotor palsy is likely to cause acute loss of tone. Chronic upper-motor neuron lesions lead to spasticity rather than the waxy flexibility seen in this patient. In our patient, the findings of isolated anisocoria without further clinical evidence of oculomotor nerve compression make this diagnosis unlikely.
Toxic leukoencephalopathy
Toxic leukoencephalopathy—widespread destruction of myelin, particularly in the white matter tracts that support higher cortical functions—can be caused by antineoplastic agents, immunosuppressant agents, and industrial solvents, as well as by abuse of vaporized drugs such as heroin (“chasing the dragon”). In its mild forms it may cause behavioral disturbances or inattention. In severe forms, a neurobehavioral syndrome of akinetic mutism may be present and can mimic catatonia.31
The diagnosis is often based on the clinical history and neuroimaging, particularly MRI, which demonstrates hyperintensity of the white matter tracts in T2-weighted images.32
This patient does not have a clear history of exposure to an agent typically associated with toxic leukoencephalopathy and does not have the corroborating MRI findings to support this diagnosis.
CASE CONTINUED
Because recent neuroimaging revealed no structural brain lesions and no cause for brain herniation, the patient receives a challenge of 2 mg of intravenous lorazepam to treat potential NCSE. Subsequent improvement is noted in her anisocoria, gaze deviation, and encephalopathy. EEG reveals frequent focal seizures arising from mesial frontal regions with bilateral hemisphere propagation, consistent with bifrontal focal NCSE.
As our patient is being transferred to a room for continuous EEG monitoring, her condition begins to deteriorate, and she again becomes more encephalopathic, with anisocoria and dysconjugate gaze. Additional doses of lorazepam are given (to complete a 0.1-mg/kg load), and additional therapy with intravenous fosphenytoin (20-mg/kg load) is given. Intubation is done for airway protection.
Continuous EEG monitoring reveals multiple frequent electrographic seizures arising from the bifrontal territories, concerning for persistent focal NCSE. A midazolam drip is initiated for EEG burst suppression of cerebral activity. Over 24 hours, EEG shows resolution of seizure activity. As the patient is weaned from sedation, she awakens and follows commands consistently, tolerating extubation without complications. Her neurologic status remains stable over the next 48 hours, having returned to her neurologic baseline level of functioning. She is able to be transferred out of the ICU in stable condition while continuing on scheduled antiepileptic therapy with phenytoin.
ALTERED MENTAL STATUS IN INPATIENTS
Altered mental status is one of the most frequently encountered reasons for medical consultation from nonmedical services. The workup and management of metabolic, toxic, psychiatric, and neurologic causes requires a deep appreciation for the broad differential diagnosis and a multidisciplinary approach. Physicians caring for these patients should avoid prematurely drawing conclusions when the patient’s clinical condition fails to respond to typical measures.
Delirium is a challenging adverse event in older patients during hospitalization, with a significant national financial burden of $164 billion per year.33 The prevalence of delirium in adults on hospital admission is estimated as 14% to 24%, with an inpatient hospitalization incidence ranging from 6% to 56% in general hospital patients.34 In addition, postoperative delirium has been reported in 15% to 53% of older patients.35
While delirium is preventable in 30% to 40% of cases,36,37 it remains an important independent prognostic determinant of hospital outcomes.38–40
Delirium in hospitalized patients requires a thorough, individualized workup. In our patient’s case, the clinical findings of hypoactive delirium were found to be manifestations of NCSE, a rare life-threatening and potentially reversible neurologic disease.
While establishing seizures as a diagnosis, careful attention must first be directed towards investigating environmental or metabolic triggers that may be inciting the disease. This often involves a similar workup for metabolic derangements, as seen in the approach to delirium.
The diagnosis of NCSE, while made in this patient’s case, remains challenging. Careful physical examination should assess for automatisms, “negative” symptoms (staring, aphasia, weakness), and “positive” symptoms (hallucinations, psychosis). Cataplexy, mutism, and other acute psychiatric features have been associated with NCSE,44 highlighting the importance of EEG. A trial of a benzodiazepine in conjunction with clinical and EEG monitoring may help guide clinical decision- making.
As there is no current universally accepted definition for NCSE nor an accepted agreement on required EEG diagnostic features at this time,41 accurate diagnosis is most likely to be obtained in facilities with both subspecialty neurologic consultation and EEG capabilities.
Our patient’s family history of Pick disease is interesting, as this is a progressive form of frontotemporal dementia with both sporadic and genetically linked cases. Recent studies have shown evidence that patients with neurodegenerative disease have increased seizure frequency early in the disease course,31 and efforts are under way to establish the incidence of first unprovoked seizure in patients with frontotemporal dementia. In our patient’s case, resolution of seizure activity yielded a return to her baseline level of neurologic function.
Early use of selective serotonin reuptake inhibitors has been shown to help with the behavioral symptoms of frontotemporal dementia,45 but increasing requirements over time may indicate progression of neurodegeneration and should warrant further appropriate investigation.
In our patient’s case, escalating dose requirements may have reflected worsening frontotemporal atrophy. However, the diagnosis of a neurodegenerative disease such as frontotemporal dementia in a patient such as ours is not definitively established at this time and is being investigated on an outpatient basis.
Given the frequency of delirium and its many risk factors in the inpatient setting, verifying a causative diagnosis can be difficult. Detailed consideration of the patient’s individual clinical circumstances, often in concert with appropriate subspecialty consultations, is essential to the evaluation. Although it is time-intensive, multidisciplinary intervention can lead to safer outcomes and shorter hospital stays.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association Publishing; 2013. http://psychiatryonline.org/doi/book/10.1176/appi.books.9780890425596. Accessed July 7, 2017.
- Mohan S, Gu S, Parikh A, Radhakrishnan J. Prevalence of hyponatremia and association with mortality: results from NHANES. Am J Med 2013; 126:1127–1137.e1.
- Sterns RH. Disorders of plasma sodium—causes, consequences, and correction. N Engl J Med 2015; 372:55–65.
- Rose B, Post T. Clinical physiology of acid-base and electrolyte disorders. 5th ed. New York, NY: McGraw-Hill; 2001.
- McManus ML, Churchwell KB, Strange K. Regulation of cell volume in health and disease. N Engl J Med 1995; 333:1260–1266.
- Strange K. Regulation of solute and water balance and cell volume in the central nervous system. J Am Soc Nephrol 1992; 3:12–27.
- Ayus JC, Wheeler JM, Arieff AI. Postoperative hyponatremic encephalopathy in menstruant women. Ann Intern Med 1992; 117:891–897.
- Gur RC, Mozley PD, Resnick SM, et al. Gender differences in age effect on brain atrophy measured by magnetic resonance imaging. Proc Natl Acad Sci USA 1991; 88:2845–2849.
- Rosomoff HL, Zugibe FT. Distribution of intracranial contents in experimental edema. Arch Neurol 1963; 9:26–34.
- Melton JE, Nattie EE. Brain and CSF water and ions during dilutional and isosmotic hyponatremia in the rat. Am J Physiol 1983; 244:R724–R732.
- Nattie EE, Edwards WH. Brain and CSF water and ions in newborn puppies during acute hypo- and hypernatremia. J Appl Physiol Respir Environ Exerc Physiol 1981; 51:1086–1091.
- Stachenfeld NS, DiPietro L, Palter SF, Nadel ER. Estrogen influences osmotic secretion of AVP and body water balance in postmenopausal women. Am J Physiol 1998; 274:R187–R195.
- Fick DM, Agostini JV, Inouye SK. Delirium superimposed on dementia: a systematic review. J Am Geriatr Soc 2002; 50:1723–1732.
- de Smet Y, Ruberg M, Serdaru M, Dubois B, Lhermitte F, Agid Y. Confusion, dementia and anticholinergics in Parkinson’s disease. J Neurol Neurosurg Psychiatry 1982; 45:1161–1164.
- Mollon B, Mahure SA, Ding DY, Zuckerman JD, Kwon YW. The influence of a history of clinical depression on peri-operative outcomes in elective total shoulder arthroplasty: a ten-year national analysis. Bone Joint J 2016; 98-B:818–824.
- Kosar CM, Tabloski PA, Travison TG, et al. Effect of preoperative pain and depressive symptoms on the development of postoperative delirium. Lancet Psychiatry 2014; 1:431–436.
- Copeland LA, Zeber JE, Pugh MJ, Mortensen EM, Restrepo MI, Lawrence VA. Postoperative complications in the seriously mentally ill: a systematic review of the literature. Ann Surg 2008; 248:31–38.
- Warach S, Gaa J, Siewert B, Wielopolski P, Edelman RR. Acute human stroke studied by whole brain echo planar diffusion-weighted magnetic resonance imaging. Ann Neurol 1995; 37:231–241.
- Sorensen AG, Buonanno FS, Gonzalez RG, et al. Hyperacute stroke: evaluation with combined multisection diffusion-weighted and hemodynamically weighted echo-planar MR imaging. Radiology 1996; 199:391–401.
- Li F, Han S, Tatlisumak T, et al. A new method to improve in-bore middle cerebral artery occlusion in rats: demonstration with diffusion—and perfusion—weighted imaging. Stroke 1998; 29:1715–1720.
- Balogun SA, Philbrick JT. Delirium, a symptom of UTI in the elderly: fact or fable? A systematic review. Can Geriatr J 2013; 17:22–26.
- Reulbach U, Dütsch C, Biermann T, et al. Managing an effective treatment for neuroleptic malignant syndrome. Crit Care 2007; 11:R4.
- Naeije G, Depondt C, Meeus C, Korpak K, Pepersack T, Legros B. EEG patterns compatible with nonconvulsive status epilepticus are common in elderly patients with delirium: a prospective study with continuous EEG monitoring. Epilepsy Behav 2014; 36:18–21.
- Veran O, Kahane P, Thomas P, Hamelin S, Sabourdy C, Vercueil L. De novo epileptic confusion in the elderly: a 1-year prospective study. Epilepsia 2010; 51:1030–1035.
- Sutter R, Rüegg S, Kaplan PW. Epidemiology, diagnosis, and management of nonconvulsive status epilepticus. Opening Pandora’s box. Neurol Clin Pract 2012; 2:275–286.
- Husain AM, Horn GJ, Jacobson MP. Non-convulsive status epilepticus: usefulness of clinical features in selecting patients for urgent EEG. J Neurol Neurosurg Psychiatry 2003; 74:189–191.
- Ungvari GS, Chiu HF, Chow LY, Lau BS, Tang WK. Lorazepam for chronic catatonia: a randomized, double-blind, placebo-controlled cross-over study. Psychopharmacology (Berl) 1999; 142:393–398.
- Carroll BT, Goforth HW, Thomas C, et al. Review of adjunctive glutamate antagonist therapy in the treatment of catatonic syndromes. J Neuropsychiatry Clin Neurosci 2007; 19:406– 412.
- Perry JJ, Spacek A, Forbes M, et al. Is the combination of negative computed tomography result and negative lumbar puncture result sufficient to rule out subarachnoid hemorrhage? Ann Emerg Med 2008; 51:707–713.
- Li MH, Cheng YS, Li YD, et al. Large-cohort comparison between three-dimensional time-of-flight magnetic resonance and rotational digital subtraction angiographies in intracranial aneurysm detection. Stroke 2009; 40:3127–3129.
- Filley CM, Kleinschmidt-DeMasters BK. Toxic leukoencephalopathy. N Engl J Med 2001; 345:425–432.
- Magnetic resonance imaging of the central nervous system. Council on Scientific Affairs. Report of the Panel on Magnetic Resonance Imaging. JAMA 1988; 259:1211–1222.
- Leslie DL, Marcantonio ER, Zhang Y, Leo-Summers L, Inouye SK. One-year health care costs associated with delirium in the elderly population. Arch Intern Med 2008; 168:27–32.
- Inouye SK. Delirium in hospitalized older patients. Clin Geriatr Med 1998; 14:745–764.
- Agostini JV, Inouye SK, Hazzard W, Blass J. Delirium. In: Principles of Geriatric Medicine and Gerontology. 5th ed. New York, NY: McGraw-Hill; 2003:1503–1515.
- Inouye SK, Bogardus ST Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med 1999; 340:669–676.
- Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc 2001; 49:516–522.
- Inouye SK, Rushing JT, Foreman MD, Palmer RM, Pompei P. Does delirium contribute to poor hospital outcomes? A three-site epidemiologic study. J Gen Intern Med 1998; 13:234–242.
- Rothschild JM, Bates DW, Leape LL. Preventable medical injuries in older patients. Arch Intern Med 2000; 160:2717–2728.
- Gillick MR, Serrell NA, Gillick LS. Adverse consequences of hospitalization in the elderly. Soc Sci Med 1982; 16:1033–1038.
- Drislane FW. Presentation, evaluation, and treatment of nonconvulsive status epilepticus. Epilepsy Behav 2000; 1:301-314.
- Rosenow F, Hamer HM, Knake S. The epidemiology of convulsive and nonconvulsive status epilepticus. Epilepsia 2007; 48(suppl 8):82–84.
- Woodford HJ, George J, Jackson M. Non-convulsive status epilepticus: a practical approach to diagnosis in confused older people. Postgrad Med J 2015; 91:655–661.
- Kaplan PW. Nonconvulsive status epilepticus in the emergency room. Epilepsia 1996; 37:643–650.
- Swartz JR, Miller BL, Lesser IM, Darby AL. Frontotemporal dementia: treatment response to serotonin selective reuptake inhibitors. J Clin Psychiatry 1997; 58:212–216.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association Publishing; 2013. http://psychiatryonline.org/doi/book/10.1176/appi.books.9780890425596. Accessed July 7, 2017.
- Mohan S, Gu S, Parikh A, Radhakrishnan J. Prevalence of hyponatremia and association with mortality: results from NHANES. Am J Med 2013; 126:1127–1137.e1.
- Sterns RH. Disorders of plasma sodium—causes, consequences, and correction. N Engl J Med 2015; 372:55–65.
- Rose B, Post T. Clinical physiology of acid-base and electrolyte disorders. 5th ed. New York, NY: McGraw-Hill; 2001.
- McManus ML, Churchwell KB, Strange K. Regulation of cell volume in health and disease. N Engl J Med 1995; 333:1260–1266.
- Strange K. Regulation of solute and water balance and cell volume in the central nervous system. J Am Soc Nephrol 1992; 3:12–27.
- Ayus JC, Wheeler JM, Arieff AI. Postoperative hyponatremic encephalopathy in menstruant women. Ann Intern Med 1992; 117:891–897.
- Gur RC, Mozley PD, Resnick SM, et al. Gender differences in age effect on brain atrophy measured by magnetic resonance imaging. Proc Natl Acad Sci USA 1991; 88:2845–2849.
- Rosomoff HL, Zugibe FT. Distribution of intracranial contents in experimental edema. Arch Neurol 1963; 9:26–34.
- Melton JE, Nattie EE. Brain and CSF water and ions during dilutional and isosmotic hyponatremia in the rat. Am J Physiol 1983; 244:R724–R732.
- Nattie EE, Edwards WH. Brain and CSF water and ions in newborn puppies during acute hypo- and hypernatremia. J Appl Physiol Respir Environ Exerc Physiol 1981; 51:1086–1091.
- Stachenfeld NS, DiPietro L, Palter SF, Nadel ER. Estrogen influences osmotic secretion of AVP and body water balance in postmenopausal women. Am J Physiol 1998; 274:R187–R195.
- Fick DM, Agostini JV, Inouye SK. Delirium superimposed on dementia: a systematic review. J Am Geriatr Soc 2002; 50:1723–1732.
- de Smet Y, Ruberg M, Serdaru M, Dubois B, Lhermitte F, Agid Y. Confusion, dementia and anticholinergics in Parkinson’s disease. J Neurol Neurosurg Psychiatry 1982; 45:1161–1164.
- Mollon B, Mahure SA, Ding DY, Zuckerman JD, Kwon YW. The influence of a history of clinical depression on peri-operative outcomes in elective total shoulder arthroplasty: a ten-year national analysis. Bone Joint J 2016; 98-B:818–824.
- Kosar CM, Tabloski PA, Travison TG, et al. Effect of preoperative pain and depressive symptoms on the development of postoperative delirium. Lancet Psychiatry 2014; 1:431–436.
- Copeland LA, Zeber JE, Pugh MJ, Mortensen EM, Restrepo MI, Lawrence VA. Postoperative complications in the seriously mentally ill: a systematic review of the literature. Ann Surg 2008; 248:31–38.
- Warach S, Gaa J, Siewert B, Wielopolski P, Edelman RR. Acute human stroke studied by whole brain echo planar diffusion-weighted magnetic resonance imaging. Ann Neurol 1995; 37:231–241.
- Sorensen AG, Buonanno FS, Gonzalez RG, et al. Hyperacute stroke: evaluation with combined multisection diffusion-weighted and hemodynamically weighted echo-planar MR imaging. Radiology 1996; 199:391–401.
- Li F, Han S, Tatlisumak T, et al. A new method to improve in-bore middle cerebral artery occlusion in rats: demonstration with diffusion—and perfusion—weighted imaging. Stroke 1998; 29:1715–1720.
- Balogun SA, Philbrick JT. Delirium, a symptom of UTI in the elderly: fact or fable? A systematic review. Can Geriatr J 2013; 17:22–26.
- Reulbach U, Dütsch C, Biermann T, et al. Managing an effective treatment for neuroleptic malignant syndrome. Crit Care 2007; 11:R4.
- Naeije G, Depondt C, Meeus C, Korpak K, Pepersack T, Legros B. EEG patterns compatible with nonconvulsive status epilepticus are common in elderly patients with delirium: a prospective study with continuous EEG monitoring. Epilepsy Behav 2014; 36:18–21.
- Veran O, Kahane P, Thomas P, Hamelin S, Sabourdy C, Vercueil L. De novo epileptic confusion in the elderly: a 1-year prospective study. Epilepsia 2010; 51:1030–1035.
- Sutter R, Rüegg S, Kaplan PW. Epidemiology, diagnosis, and management of nonconvulsive status epilepticus. Opening Pandora’s box. Neurol Clin Pract 2012; 2:275–286.
- Husain AM, Horn GJ, Jacobson MP. Non-convulsive status epilepticus: usefulness of clinical features in selecting patients for urgent EEG. J Neurol Neurosurg Psychiatry 2003; 74:189–191.
- Ungvari GS, Chiu HF, Chow LY, Lau BS, Tang WK. Lorazepam for chronic catatonia: a randomized, double-blind, placebo-controlled cross-over study. Psychopharmacology (Berl) 1999; 142:393–398.
- Carroll BT, Goforth HW, Thomas C, et al. Review of adjunctive glutamate antagonist therapy in the treatment of catatonic syndromes. J Neuropsychiatry Clin Neurosci 2007; 19:406– 412.
- Perry JJ, Spacek A, Forbes M, et al. Is the combination of negative computed tomography result and negative lumbar puncture result sufficient to rule out subarachnoid hemorrhage? Ann Emerg Med 2008; 51:707–713.
- Li MH, Cheng YS, Li YD, et al. Large-cohort comparison between three-dimensional time-of-flight magnetic resonance and rotational digital subtraction angiographies in intracranial aneurysm detection. Stroke 2009; 40:3127–3129.
- Filley CM, Kleinschmidt-DeMasters BK. Toxic leukoencephalopathy. N Engl J Med 2001; 345:425–432.
- Magnetic resonance imaging of the central nervous system. Council on Scientific Affairs. Report of the Panel on Magnetic Resonance Imaging. JAMA 1988; 259:1211–1222.
- Leslie DL, Marcantonio ER, Zhang Y, Leo-Summers L, Inouye SK. One-year health care costs associated with delirium in the elderly population. Arch Intern Med 2008; 168:27–32.
- Inouye SK. Delirium in hospitalized older patients. Clin Geriatr Med 1998; 14:745–764.
- Agostini JV, Inouye SK, Hazzard W, Blass J. Delirium. In: Principles of Geriatric Medicine and Gerontology. 5th ed. New York, NY: McGraw-Hill; 2003:1503–1515.
- Inouye SK, Bogardus ST Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med 1999; 340:669–676.
- Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc 2001; 49:516–522.
- Inouye SK, Rushing JT, Foreman MD, Palmer RM, Pompei P. Does delirium contribute to poor hospital outcomes? A three-site epidemiologic study. J Gen Intern Med 1998; 13:234–242.
- Rothschild JM, Bates DW, Leape LL. Preventable medical injuries in older patients. Arch Intern Med 2000; 160:2717–2728.
- Gillick MR, Serrell NA, Gillick LS. Adverse consequences of hospitalization in the elderly. Soc Sci Med 1982; 16:1033–1038.
- Drislane FW. Presentation, evaluation, and treatment of nonconvulsive status epilepticus. Epilepsy Behav 2000; 1:301-314.
- Rosenow F, Hamer HM, Knake S. The epidemiology of convulsive and nonconvulsive status epilepticus. Epilepsia 2007; 48(suppl 8):82–84.
- Woodford HJ, George J, Jackson M. Non-convulsive status epilepticus: a practical approach to diagnosis in confused older people. Postgrad Med J 2015; 91:655–661.
- Kaplan PW. Nonconvulsive status epilepticus in the emergency room. Epilepsia 1996; 37:643–650.
- Swartz JR, Miller BL, Lesser IM, Darby AL. Frontotemporal dementia: treatment response to serotonin selective reuptake inhibitors. J Clin Psychiatry 1997; 58:212–216.