User login
Cirrhosis, Child-Pugh score predict ERCP complications
Cirrhosis may increase the risk of complications from endoscopic retrograde cholangiopancreatography (ERCP), according to a retrospective study involving almost 700 patients.
The study also showed that Child-Pugh class was a better predictor of risk than Model for End-Stage Liver Disease (MELD) score, reported lead author Michelle Bernshteyn, MD, a third-year internal medicine resident at State University of New York, Syracuse , and colleagues.
“There remains a scarcity in the literature regarding complications and adverse effects after ERCP in cirrhotic patients, particularly those incorporating Child-Pugh class and MELD score or type of intervention as predictors,” Dr. Bernshteyn said during a virtual presentation at the American College of Gastroenterology annual meeting. “Furthermore, literature review demonstrates inconsistency among results.”
To gain clarity, Dr. Bernshteyn and colleagues reviewed electronic medical records from 692 patients who underwent ERCP, of whom 174 had cirrhosis and 518 did not. For all patients, the investigators analyzed demographics, comorbidities, indications for ERCP, type of sedation, type of intervention, and complications within a 30-day period. Complications included bleeding, pancreatitis, cholangitis, perforation, mortality caused by ERCP, and mortality from other causes. Patients with cirrhosis were further analyzed based on etiology of cirrhosis, Child-Pugh class, and MELD score.
The analysis revealed that complications were significantly more common in patients with cirrhosis than in those without cirrhosis (21.30% vs. 13.51%; P = .015). No specific complications were significantly more common in patients with cirrhosis than in those without cirrhosis.
In patients with cirrhosis, 41.18% of Child-Pugh class C patients had complications, compared with 15.15% of class B patients and 19.30% of class A patients (P = .010). In contrast, MELD scores were not significantly associated with adverse events.
Further analysis showed that, in patients without cirrhosis, diagnostic-only ERCP and underlying chronic obstructive pulmonary disease were associated with high rates of complications (P = .039 and P = .003, respectively). In patients with cirrhosis, underlying chronic obstructive pulmonary disease and hypertension predicted adverse events (P = .009 and P = .003, respectively).
“The results of our study reaffirm that liver cirrhosis has an impact on the occurrence of complications during ERCP,” Dr. Bernshteyn said. “Child-Pugh class seems to be more reliable as compared to MELD score in predicting complications of ERCP in cirrhosis patients,” she added. “However, we are also aware that Child-Pugh and MELD scores are complementary to each other while evaluating outcomes of any surgery in patients with cirrhosis.”
In 2017, Udayakumar Navaneethan, MD, a gastroenterologist at AdventHealth Orlando’s Center for Interventional Endoscopy, and an assistant professor at the University of Central Florida, Orlando, and colleagues published a national database study concerning the safety of ERCP in patients with liver cirrhosis.
“[The present] study is important as it highlights the fact that ERCP is associated with significant complications in cirrhotic patients compared to those without cirrhosis,” Dr. Navaneethan said when asked to comment. “Also, Child-Pugh score appeared to be more reliable than MELD score in predicting complications of ERCP in cirrhotic patients.”
He went on to explain relevance for practicing clinicians. “The clinical implications of the study are that a detailed risk-benefit discussion needs to be done with patients with liver cirrhosis, particularly with advanced liver disease Child-Pugh class C, irrespective of the etiology,” Dr. Navaneethan said. “ERCP should be performed when there is clear evidence that the benefits outweigh the risks.” The investigators and Dr. Navaneethan reported no conflicts of interest.
SOURCE: Bernshteyn M et al. ACG 2020, Abstract S0982.
Cirrhosis may increase the risk of complications from endoscopic retrograde cholangiopancreatography (ERCP), according to a retrospective study involving almost 700 patients.
The study also showed that Child-Pugh class was a better predictor of risk than Model for End-Stage Liver Disease (MELD) score, reported lead author Michelle Bernshteyn, MD, a third-year internal medicine resident at State University of New York, Syracuse , and colleagues.
“There remains a scarcity in the literature regarding complications and adverse effects after ERCP in cirrhotic patients, particularly those incorporating Child-Pugh class and MELD score or type of intervention as predictors,” Dr. Bernshteyn said during a virtual presentation at the American College of Gastroenterology annual meeting. “Furthermore, literature review demonstrates inconsistency among results.”
To gain clarity, Dr. Bernshteyn and colleagues reviewed electronic medical records from 692 patients who underwent ERCP, of whom 174 had cirrhosis and 518 did not. For all patients, the investigators analyzed demographics, comorbidities, indications for ERCP, type of sedation, type of intervention, and complications within a 30-day period. Complications included bleeding, pancreatitis, cholangitis, perforation, mortality caused by ERCP, and mortality from other causes. Patients with cirrhosis were further analyzed based on etiology of cirrhosis, Child-Pugh class, and MELD score.
The analysis revealed that complications were significantly more common in patients with cirrhosis than in those without cirrhosis (21.30% vs. 13.51%; P = .015). No specific complications were significantly more common in patients with cirrhosis than in those without cirrhosis.
In patients with cirrhosis, 41.18% of Child-Pugh class C patients had complications, compared with 15.15% of class B patients and 19.30% of class A patients (P = .010). In contrast, MELD scores were not significantly associated with adverse events.
Further analysis showed that, in patients without cirrhosis, diagnostic-only ERCP and underlying chronic obstructive pulmonary disease were associated with high rates of complications (P = .039 and P = .003, respectively). In patients with cirrhosis, underlying chronic obstructive pulmonary disease and hypertension predicted adverse events (P = .009 and P = .003, respectively).
“The results of our study reaffirm that liver cirrhosis has an impact on the occurrence of complications during ERCP,” Dr. Bernshteyn said. “Child-Pugh class seems to be more reliable as compared to MELD score in predicting complications of ERCP in cirrhosis patients,” she added. “However, we are also aware that Child-Pugh and MELD scores are complementary to each other while evaluating outcomes of any surgery in patients with cirrhosis.”
In 2017, Udayakumar Navaneethan, MD, a gastroenterologist at AdventHealth Orlando’s Center for Interventional Endoscopy, and an assistant professor at the University of Central Florida, Orlando, and colleagues published a national database study concerning the safety of ERCP in patients with liver cirrhosis.
“[The present] study is important as it highlights the fact that ERCP is associated with significant complications in cirrhotic patients compared to those without cirrhosis,” Dr. Navaneethan said when asked to comment. “Also, Child-Pugh score appeared to be more reliable than MELD score in predicting complications of ERCP in cirrhotic patients.”
He went on to explain relevance for practicing clinicians. “The clinical implications of the study are that a detailed risk-benefit discussion needs to be done with patients with liver cirrhosis, particularly with advanced liver disease Child-Pugh class C, irrespective of the etiology,” Dr. Navaneethan said. “ERCP should be performed when there is clear evidence that the benefits outweigh the risks.” The investigators and Dr. Navaneethan reported no conflicts of interest.
SOURCE: Bernshteyn M et al. ACG 2020, Abstract S0982.
Cirrhosis may increase the risk of complications from endoscopic retrograde cholangiopancreatography (ERCP), according to a retrospective study involving almost 700 patients.
The study also showed that Child-Pugh class was a better predictor of risk than Model for End-Stage Liver Disease (MELD) score, reported lead author Michelle Bernshteyn, MD, a third-year internal medicine resident at State University of New York, Syracuse , and colleagues.
“There remains a scarcity in the literature regarding complications and adverse effects after ERCP in cirrhotic patients, particularly those incorporating Child-Pugh class and MELD score or type of intervention as predictors,” Dr. Bernshteyn said during a virtual presentation at the American College of Gastroenterology annual meeting. “Furthermore, literature review demonstrates inconsistency among results.”
To gain clarity, Dr. Bernshteyn and colleagues reviewed electronic medical records from 692 patients who underwent ERCP, of whom 174 had cirrhosis and 518 did not. For all patients, the investigators analyzed demographics, comorbidities, indications for ERCP, type of sedation, type of intervention, and complications within a 30-day period. Complications included bleeding, pancreatitis, cholangitis, perforation, mortality caused by ERCP, and mortality from other causes. Patients with cirrhosis were further analyzed based on etiology of cirrhosis, Child-Pugh class, and MELD score.
The analysis revealed that complications were significantly more common in patients with cirrhosis than in those without cirrhosis (21.30% vs. 13.51%; P = .015). No specific complications were significantly more common in patients with cirrhosis than in those without cirrhosis.
In patients with cirrhosis, 41.18% of Child-Pugh class C patients had complications, compared with 15.15% of class B patients and 19.30% of class A patients (P = .010). In contrast, MELD scores were not significantly associated with adverse events.
Further analysis showed that, in patients without cirrhosis, diagnostic-only ERCP and underlying chronic obstructive pulmonary disease were associated with high rates of complications (P = .039 and P = .003, respectively). In patients with cirrhosis, underlying chronic obstructive pulmonary disease and hypertension predicted adverse events (P = .009 and P = .003, respectively).
“The results of our study reaffirm that liver cirrhosis has an impact on the occurrence of complications during ERCP,” Dr. Bernshteyn said. “Child-Pugh class seems to be more reliable as compared to MELD score in predicting complications of ERCP in cirrhosis patients,” she added. “However, we are also aware that Child-Pugh and MELD scores are complementary to each other while evaluating outcomes of any surgery in patients with cirrhosis.”
In 2017, Udayakumar Navaneethan, MD, a gastroenterologist at AdventHealth Orlando’s Center for Interventional Endoscopy, and an assistant professor at the University of Central Florida, Orlando, and colleagues published a national database study concerning the safety of ERCP in patients with liver cirrhosis.
“[The present] study is important as it highlights the fact that ERCP is associated with significant complications in cirrhotic patients compared to those without cirrhosis,” Dr. Navaneethan said when asked to comment. “Also, Child-Pugh score appeared to be more reliable than MELD score in predicting complications of ERCP in cirrhotic patients.”
He went on to explain relevance for practicing clinicians. “The clinical implications of the study are that a detailed risk-benefit discussion needs to be done with patients with liver cirrhosis, particularly with advanced liver disease Child-Pugh class C, irrespective of the etiology,” Dr. Navaneethan said. “ERCP should be performed when there is clear evidence that the benefits outweigh the risks.” The investigators and Dr. Navaneethan reported no conflicts of interest.
SOURCE: Bernshteyn M et al. ACG 2020, Abstract S0982.
FROM ACG 2020
Cannabis may improve liver function in patients with obesity
Cannabis use is associated with a decrease in the prevalence of steatohepatitis and a slowing of its progression in patients with obesity, results from a retrospective cohort study show.
This suggests “that the anti-inflammatory effects of cannabis may be leading to reduced prevalence of steatohepatitis in cannabis users,” said Ikechukwu Achebe, MD, from the John H. Stroger, Jr. Hospital of Cook County in Chicago.
Liver injuries such as nonalcoholic steatohepatitis are characterized by hepatocellular injury and inflammation, which combine to contribute to an increase in the risk for liver failure, cirrhosis, and hepatocellular carcinoma.
“This is where cannabis comes in,” said Dr. Achebe, who presented the study results at the virtual annual meeting of the American College of Gastroenterology. “It is the most commonly used psychoactive substance worldwide and has been shown to reduce hepatic myofibroblast and stellate cell injury. Studies using mouse models have demonstrated reduced liver fibrosis and cirrhosis as a consequence of cannabis exposure.”
Given this possible connection, Dr. Achebe and colleagues set out to determine whether cannabis use affects the prevalence and progression of nonalcoholic fatty liver disease (NAFLD) in obese patients.
To do so, they analyzed the discharge records of 879,952 obese adults in the 2016 Healthcare Cost and Utilization Project National Inpatient Sample. The primary outcome was the prevalence of the four presentations of NAFLD: steatosis, steatohepatitis, cirrhosis, and hepatocellular carcinoma.
The researchers compared disease stages in cannabis users and nonusers. In the study cohort of 14,236 patients, 1.6% used cannabis. Steatohepatitis was less common among cannabis users than among nonusers (0.4% vs. 0.7%; P < .001), as was cirrhosis (1.1% vs. 1.5%; P < .001).
After propensity matching, the association between cannabis use and lower rates of steatohepatitis remained significant (0.4% vs. 0.5%; P = .035), but the association between cannabis use and the prevalence of nonalcoholic fatty liver, cirrhosis, and hepatocellular carcinoma did not.
These results might be partly explained by the protective effect of cannabis on hepatocytes regulated by the endocannabinoid system, the researchers concluded.
More studies are needed to explore this relation, said Dr. Achebe.
The challenge of self-reported use
The study is “incredibly interesting,” said Nancy S. Reau, MD, from Rush Medical College, Chicago. However, the association between cannabis and nonalcoholic fatty liver needs to be further investigated before clinicians can counsel their patients to use the agent to prevent progression.
It is difficult in a study such as this to tease out other lifestyle factors that might be linked to cannabis use, she explained. For example, “is it possible that the cannabis users exercise more, drink more coffee, or eat differently?”
And “self-reported use is challenging,” Dr. Reau said in an interview. “This cannot differentiate someone who occasionally uses from someone who is a heavy daily user. There must be some minimum level of exposure needed for it to have protective effects, if they exist.”
This study was honored at the meeting as an ACG Newsworthy Abstract and an ACG Outstanding Poster Presenter.
Dr. Achebe disclosed no relevant financial relationships. Dr. Reau reported receiving research support from Genfit and having a consultant relationship with Intercept Pharmaceuticals.
A version of this article originally appeared on Medscape.com.
Cannabis use is associated with a decrease in the prevalence of steatohepatitis and a slowing of its progression in patients with obesity, results from a retrospective cohort study show.
This suggests “that the anti-inflammatory effects of cannabis may be leading to reduced prevalence of steatohepatitis in cannabis users,” said Ikechukwu Achebe, MD, from the John H. Stroger, Jr. Hospital of Cook County in Chicago.
Liver injuries such as nonalcoholic steatohepatitis are characterized by hepatocellular injury and inflammation, which combine to contribute to an increase in the risk for liver failure, cirrhosis, and hepatocellular carcinoma.
“This is where cannabis comes in,” said Dr. Achebe, who presented the study results at the virtual annual meeting of the American College of Gastroenterology. “It is the most commonly used psychoactive substance worldwide and has been shown to reduce hepatic myofibroblast and stellate cell injury. Studies using mouse models have demonstrated reduced liver fibrosis and cirrhosis as a consequence of cannabis exposure.”
Given this possible connection, Dr. Achebe and colleagues set out to determine whether cannabis use affects the prevalence and progression of nonalcoholic fatty liver disease (NAFLD) in obese patients.
To do so, they analyzed the discharge records of 879,952 obese adults in the 2016 Healthcare Cost and Utilization Project National Inpatient Sample. The primary outcome was the prevalence of the four presentations of NAFLD: steatosis, steatohepatitis, cirrhosis, and hepatocellular carcinoma.
The researchers compared disease stages in cannabis users and nonusers. In the study cohort of 14,236 patients, 1.6% used cannabis. Steatohepatitis was less common among cannabis users than among nonusers (0.4% vs. 0.7%; P < .001), as was cirrhosis (1.1% vs. 1.5%; P < .001).
After propensity matching, the association between cannabis use and lower rates of steatohepatitis remained significant (0.4% vs. 0.5%; P = .035), but the association between cannabis use and the prevalence of nonalcoholic fatty liver, cirrhosis, and hepatocellular carcinoma did not.
These results might be partly explained by the protective effect of cannabis on hepatocytes regulated by the endocannabinoid system, the researchers concluded.
More studies are needed to explore this relation, said Dr. Achebe.
The challenge of self-reported use
The study is “incredibly interesting,” said Nancy S. Reau, MD, from Rush Medical College, Chicago. However, the association between cannabis and nonalcoholic fatty liver needs to be further investigated before clinicians can counsel their patients to use the agent to prevent progression.
It is difficult in a study such as this to tease out other lifestyle factors that might be linked to cannabis use, she explained. For example, “is it possible that the cannabis users exercise more, drink more coffee, or eat differently?”
And “self-reported use is challenging,” Dr. Reau said in an interview. “This cannot differentiate someone who occasionally uses from someone who is a heavy daily user. There must be some minimum level of exposure needed for it to have protective effects, if they exist.”
This study was honored at the meeting as an ACG Newsworthy Abstract and an ACG Outstanding Poster Presenter.
Dr. Achebe disclosed no relevant financial relationships. Dr. Reau reported receiving research support from Genfit and having a consultant relationship with Intercept Pharmaceuticals.
A version of this article originally appeared on Medscape.com.
Cannabis use is associated with a decrease in the prevalence of steatohepatitis and a slowing of its progression in patients with obesity, results from a retrospective cohort study show.
This suggests “that the anti-inflammatory effects of cannabis may be leading to reduced prevalence of steatohepatitis in cannabis users,” said Ikechukwu Achebe, MD, from the John H. Stroger, Jr. Hospital of Cook County in Chicago.
Liver injuries such as nonalcoholic steatohepatitis are characterized by hepatocellular injury and inflammation, which combine to contribute to an increase in the risk for liver failure, cirrhosis, and hepatocellular carcinoma.
“This is where cannabis comes in,” said Dr. Achebe, who presented the study results at the virtual annual meeting of the American College of Gastroenterology. “It is the most commonly used psychoactive substance worldwide and has been shown to reduce hepatic myofibroblast and stellate cell injury. Studies using mouse models have demonstrated reduced liver fibrosis and cirrhosis as a consequence of cannabis exposure.”
Given this possible connection, Dr. Achebe and colleagues set out to determine whether cannabis use affects the prevalence and progression of nonalcoholic fatty liver disease (NAFLD) in obese patients.
To do so, they analyzed the discharge records of 879,952 obese adults in the 2016 Healthcare Cost and Utilization Project National Inpatient Sample. The primary outcome was the prevalence of the four presentations of NAFLD: steatosis, steatohepatitis, cirrhosis, and hepatocellular carcinoma.
The researchers compared disease stages in cannabis users and nonusers. In the study cohort of 14,236 patients, 1.6% used cannabis. Steatohepatitis was less common among cannabis users than among nonusers (0.4% vs. 0.7%; P < .001), as was cirrhosis (1.1% vs. 1.5%; P < .001).
After propensity matching, the association between cannabis use and lower rates of steatohepatitis remained significant (0.4% vs. 0.5%; P = .035), but the association between cannabis use and the prevalence of nonalcoholic fatty liver, cirrhosis, and hepatocellular carcinoma did not.
These results might be partly explained by the protective effect of cannabis on hepatocytes regulated by the endocannabinoid system, the researchers concluded.
More studies are needed to explore this relation, said Dr. Achebe.
The challenge of self-reported use
The study is “incredibly interesting,” said Nancy S. Reau, MD, from Rush Medical College, Chicago. However, the association between cannabis and nonalcoholic fatty liver needs to be further investigated before clinicians can counsel their patients to use the agent to prevent progression.
It is difficult in a study such as this to tease out other lifestyle factors that might be linked to cannabis use, she explained. For example, “is it possible that the cannabis users exercise more, drink more coffee, or eat differently?”
And “self-reported use is challenging,” Dr. Reau said in an interview. “This cannot differentiate someone who occasionally uses from someone who is a heavy daily user. There must be some minimum level of exposure needed for it to have protective effects, if they exist.”
This study was honored at the meeting as an ACG Newsworthy Abstract and an ACG Outstanding Poster Presenter.
Dr. Achebe disclosed no relevant financial relationships. Dr. Reau reported receiving research support from Genfit and having a consultant relationship with Intercept Pharmaceuticals.
A version of this article originally appeared on Medscape.com.
FROM ACG 2020
INR fails to predict bleeding in patients with cirrhosis
International normalized ratio (INR) does not predict periprocedural bleeding in patients with cirrhosis, according to a meta-analysis of 29 studies.
This finding should deter the common practice of delivering blood products to cirrhotic patients with an elevated INR, reported lead author Alexander J. Kovalic, MD, of Novant Forsyth Medical Center in Winston Salem, N.C., and colleagues.
“INR measurement among cirrhotic patients is important in MELD [Model for End-Stage Liver Disease] prognostication and assessment of underlying hepatic synthetic function, however the INR alone does not capture the complicated interplay of anticoagulant and procoagulant deficiencies present in cirrhotic coagulopathy,” Dr. Kovalic and colleagues wrote in Alimentary Pharmacology & Therapeutics. “Yet, the ‘correction’ of these aberrancies among peripheral coagulation tests remains common ... even in modern practice, and not uncommonly occurs in the periprocedural setting.”
According to investigators, addressing INR with blood transfusion can have a litany of negative effects. Beyond the risks faced by all patient populations, increasing blood volume in those with cirrhosis can increase portal venous pressure, thereby raising risks of portal gastropathy or variceal hemorrhage. In addition, giving plasma products to patients with cirrhotic coagulopathy may further disrupt the balance between anticoagulants and procoagulants, potentially triggering disseminated intravascular coagulation.
Dr. Kovalic and colleagues noted that the lack of correlation between peripheral coagulation tests and bleeding risk has been a longstanding subject of investigation, citing studies from as early as 1981.
To add further weight to this body of evidence, the investigators conducted a systematic review and meta-analysis involving 13,276 patients with cirrhosis who underwent various procedures between 1999 and 2019. Primary outcomes included periprocedural bleeding events and the association between preprocedural INR and periprocedural bleeding events. Secondary outcomes included mortality, quantity of blood and/or plasma products used, and relationship between preprocedural platelet count and periprocedural bleeding events.
The analysis showed that preprocedural INR was not significantly associated with periprocedural bleeding events (pooled odds ratio, 1.52; 95% confidence interval, 0.99-2.33; P = .06), a finding that held across INR threshold subgroups. Similarly, no significant difference was found between mean INR of patients who had bleeding events versus that of those who did not (pooled mean difference, 0.05; 95% CI, 0.03-0.13; P = .23).
Preprocedural platelet count was also a poor predictor of periprocedural bleeding, with a pooled odds ratio of 1.24 (95% CI, 0.55-2.77; P = .60), although the investigators noted that platelet count thresholds varied widely across studies, from 30 to 150 × 109/L. When studies were stratified by procedural bleeding risk or procedure type, subgroup effects were no longer significant. Other secondary endpoints were incalculable because of insufficient data.
“Hopefully, these findings will spark initiation of more large-scale, higher-quality studies ... to reinforce minimizing administration of fresh frozen plasma for inappropriate correction of INR, which carries a multitude of adverse effects among cirrhotic [patients],” the investigators concluded.
According to Stephen H. Caldwell, MD, of the University of Virginia in Charlottesville, “The present paper augments accumulating literature over the past 15 years that INR should be discarded as a measure of procedure-related bleeding risk.”
Dr. Caldwell pointed out that “bleeding in cirrhosis is usually related to portal hypertension not with impaired hemostasis, with the occasional exception of hyperfibrinolysis, which is very different from a prolonged INR.”
He went on to suggest that the present findings should dissuade clinicians from a practice that, for some, is reflexive rather than evidence based.
“It’s remarkable how many medical practices become entrenched based on hand-me-down teaching during our early training years, and remain so for many years beyond as we disperse into various medical and surgical fields,” Dr. Caldwell said. “These learned approaches to common problems can clearly persist for generations despite overwhelming evidence to the contrary that usually evolve slowly and well-insulated within subspecialties or sub-subspecialties, and hence take several generations of training to diffuse into the wider practice of medical care for common problems. These may become matters of expedience in decision-making, much like the old antibiotic conundrum of ‘no-think-a-cillin,’ as critics referred to over-use of broad spectrum antibiotics. And so it has been with the INR.”The investigators disclosed relationships with AbbVie, Eisai, Gilead, and others. Dr. Caldwell disclosed research support from Daiichi concerning the potential role of anticoagulation therapy in preventing cirrhosis progression.
SOURCE: Kovalic AJ et al. Aliment Pharmacol Ther. 2020 Sep 10. doi: 10.1111/apt.16078.
International normalized ratio (INR) does not predict periprocedural bleeding in patients with cirrhosis, according to a meta-analysis of 29 studies.
This finding should deter the common practice of delivering blood products to cirrhotic patients with an elevated INR, reported lead author Alexander J. Kovalic, MD, of Novant Forsyth Medical Center in Winston Salem, N.C., and colleagues.
“INR measurement among cirrhotic patients is important in MELD [Model for End-Stage Liver Disease] prognostication and assessment of underlying hepatic synthetic function, however the INR alone does not capture the complicated interplay of anticoagulant and procoagulant deficiencies present in cirrhotic coagulopathy,” Dr. Kovalic and colleagues wrote in Alimentary Pharmacology & Therapeutics. “Yet, the ‘correction’ of these aberrancies among peripheral coagulation tests remains common ... even in modern practice, and not uncommonly occurs in the periprocedural setting.”
According to investigators, addressing INR with blood transfusion can have a litany of negative effects. Beyond the risks faced by all patient populations, increasing blood volume in those with cirrhosis can increase portal venous pressure, thereby raising risks of portal gastropathy or variceal hemorrhage. In addition, giving plasma products to patients with cirrhotic coagulopathy may further disrupt the balance between anticoagulants and procoagulants, potentially triggering disseminated intravascular coagulation.
Dr. Kovalic and colleagues noted that the lack of correlation between peripheral coagulation tests and bleeding risk has been a longstanding subject of investigation, citing studies from as early as 1981.
To add further weight to this body of evidence, the investigators conducted a systematic review and meta-analysis involving 13,276 patients with cirrhosis who underwent various procedures between 1999 and 2019. Primary outcomes included periprocedural bleeding events and the association between preprocedural INR and periprocedural bleeding events. Secondary outcomes included mortality, quantity of blood and/or plasma products used, and relationship between preprocedural platelet count and periprocedural bleeding events.
The analysis showed that preprocedural INR was not significantly associated with periprocedural bleeding events (pooled odds ratio, 1.52; 95% confidence interval, 0.99-2.33; P = .06), a finding that held across INR threshold subgroups. Similarly, no significant difference was found between mean INR of patients who had bleeding events versus that of those who did not (pooled mean difference, 0.05; 95% CI, 0.03-0.13; P = .23).
Preprocedural platelet count was also a poor predictor of periprocedural bleeding, with a pooled odds ratio of 1.24 (95% CI, 0.55-2.77; P = .60), although the investigators noted that platelet count thresholds varied widely across studies, from 30 to 150 × 109/L. When studies were stratified by procedural bleeding risk or procedure type, subgroup effects were no longer significant. Other secondary endpoints were incalculable because of insufficient data.
“Hopefully, these findings will spark initiation of more large-scale, higher-quality studies ... to reinforce minimizing administration of fresh frozen plasma for inappropriate correction of INR, which carries a multitude of adverse effects among cirrhotic [patients],” the investigators concluded.
According to Stephen H. Caldwell, MD, of the University of Virginia in Charlottesville, “The present paper augments accumulating literature over the past 15 years that INR should be discarded as a measure of procedure-related bleeding risk.”
Dr. Caldwell pointed out that “bleeding in cirrhosis is usually related to portal hypertension not with impaired hemostasis, with the occasional exception of hyperfibrinolysis, which is very different from a prolonged INR.”
He went on to suggest that the present findings should dissuade clinicians from a practice that, for some, is reflexive rather than evidence based.
“It’s remarkable how many medical practices become entrenched based on hand-me-down teaching during our early training years, and remain so for many years beyond as we disperse into various medical and surgical fields,” Dr. Caldwell said. “These learned approaches to common problems can clearly persist for generations despite overwhelming evidence to the contrary that usually evolve slowly and well-insulated within subspecialties or sub-subspecialties, and hence take several generations of training to diffuse into the wider practice of medical care for common problems. These may become matters of expedience in decision-making, much like the old antibiotic conundrum of ‘no-think-a-cillin,’ as critics referred to over-use of broad spectrum antibiotics. And so it has been with the INR.”The investigators disclosed relationships with AbbVie, Eisai, Gilead, and others. Dr. Caldwell disclosed research support from Daiichi concerning the potential role of anticoagulation therapy in preventing cirrhosis progression.
SOURCE: Kovalic AJ et al. Aliment Pharmacol Ther. 2020 Sep 10. doi: 10.1111/apt.16078.
International normalized ratio (INR) does not predict periprocedural bleeding in patients with cirrhosis, according to a meta-analysis of 29 studies.
This finding should deter the common practice of delivering blood products to cirrhotic patients with an elevated INR, reported lead author Alexander J. Kovalic, MD, of Novant Forsyth Medical Center in Winston Salem, N.C., and colleagues.
“INR measurement among cirrhotic patients is important in MELD [Model for End-Stage Liver Disease] prognostication and assessment of underlying hepatic synthetic function, however the INR alone does not capture the complicated interplay of anticoagulant and procoagulant deficiencies present in cirrhotic coagulopathy,” Dr. Kovalic and colleagues wrote in Alimentary Pharmacology & Therapeutics. “Yet, the ‘correction’ of these aberrancies among peripheral coagulation tests remains common ... even in modern practice, and not uncommonly occurs in the periprocedural setting.”
According to investigators, addressing INR with blood transfusion can have a litany of negative effects. Beyond the risks faced by all patient populations, increasing blood volume in those with cirrhosis can increase portal venous pressure, thereby raising risks of portal gastropathy or variceal hemorrhage. In addition, giving plasma products to patients with cirrhotic coagulopathy may further disrupt the balance between anticoagulants and procoagulants, potentially triggering disseminated intravascular coagulation.
Dr. Kovalic and colleagues noted that the lack of correlation between peripheral coagulation tests and bleeding risk has been a longstanding subject of investigation, citing studies from as early as 1981.
To add further weight to this body of evidence, the investigators conducted a systematic review and meta-analysis involving 13,276 patients with cirrhosis who underwent various procedures between 1999 and 2019. Primary outcomes included periprocedural bleeding events and the association between preprocedural INR and periprocedural bleeding events. Secondary outcomes included mortality, quantity of blood and/or plasma products used, and relationship between preprocedural platelet count and periprocedural bleeding events.
The analysis showed that preprocedural INR was not significantly associated with periprocedural bleeding events (pooled odds ratio, 1.52; 95% confidence interval, 0.99-2.33; P = .06), a finding that held across INR threshold subgroups. Similarly, no significant difference was found between mean INR of patients who had bleeding events versus that of those who did not (pooled mean difference, 0.05; 95% CI, 0.03-0.13; P = .23).
Preprocedural platelet count was also a poor predictor of periprocedural bleeding, with a pooled odds ratio of 1.24 (95% CI, 0.55-2.77; P = .60), although the investigators noted that platelet count thresholds varied widely across studies, from 30 to 150 × 109/L. When studies were stratified by procedural bleeding risk or procedure type, subgroup effects were no longer significant. Other secondary endpoints were incalculable because of insufficient data.
“Hopefully, these findings will spark initiation of more large-scale, higher-quality studies ... to reinforce minimizing administration of fresh frozen plasma for inappropriate correction of INR, which carries a multitude of adverse effects among cirrhotic [patients],” the investigators concluded.
According to Stephen H. Caldwell, MD, of the University of Virginia in Charlottesville, “The present paper augments accumulating literature over the past 15 years that INR should be discarded as a measure of procedure-related bleeding risk.”
Dr. Caldwell pointed out that “bleeding in cirrhosis is usually related to portal hypertension not with impaired hemostasis, with the occasional exception of hyperfibrinolysis, which is very different from a prolonged INR.”
He went on to suggest that the present findings should dissuade clinicians from a practice that, for some, is reflexive rather than evidence based.
“It’s remarkable how many medical practices become entrenched based on hand-me-down teaching during our early training years, and remain so for many years beyond as we disperse into various medical and surgical fields,” Dr. Caldwell said. “These learned approaches to common problems can clearly persist for generations despite overwhelming evidence to the contrary that usually evolve slowly and well-insulated within subspecialties or sub-subspecialties, and hence take several generations of training to diffuse into the wider practice of medical care for common problems. These may become matters of expedience in decision-making, much like the old antibiotic conundrum of ‘no-think-a-cillin,’ as critics referred to over-use of broad spectrum antibiotics. And so it has been with the INR.”The investigators disclosed relationships with AbbVie, Eisai, Gilead, and others. Dr. Caldwell disclosed research support from Daiichi concerning the potential role of anticoagulation therapy in preventing cirrhosis progression.
SOURCE: Kovalic AJ et al. Aliment Pharmacol Ther. 2020 Sep 10. doi: 10.1111/apt.16078.
FROM ALIMENTARY PHARMACOLOGY & THERAPEUTICS
Hepatocellular carcinoma shows risk factor shift
Rates of hepatocellular carcinoma (HCC) continue to rise in the United States, but unevenly so given how the incidence has become highest in the Hispanic population, which is reflected in increased rates in the southern and western states, Hashem B. El-Serag, MD, of Baylor College of Medicine, Houston, said in a virtual presentation at the annual Digestive Diseases: New Advances, which is jointly provided by Rutgers and Global Academy for Medical Education.
In addition to this demographic shift, the risk factors for HCC are shifting, he said. Hepatitis C virus (HCV) has been the dominant risk factor for HCC; for patients with active HCV, the factors historically associated with increased HCC risk have included alcohol consumption, obesity, diabetes, coinfection, and genetics, he said.
This pattern is starting to change. In fact, for patients with active HCV, antiviral treatment with a sustained virologic response has surfaced as the most significant risk factor in the development of HCC, said Dr. El-Serag: Among these patients, sustained virologic response from direct-acting antivirals is associated with a significant reduction in HCC risk. However, it is important to recognize that a residual risk of HCC remains that doesn’t go away for several years, he noted.
“Who are those people who got treated, got cured, and still developed HCC? Those with cirrhosis at the time of treatment,” he said. Those with cirrhosis have cumulative incidence of 1.8% per year, but those without cirrhosis had very low risk, he said.
Some good news in HCC is that rates appear to be declining among young men, and this is thought to be one of the groups who are achieving a cure of HCV, he said.
“One would hope, if goals for HCV elimination are met, that will translate into massive reduction of HCC,” he said.
“The issue now for hepatitis is finding infected patients and curing them,” he noted.
Dr. El-Serag touched on hepatitis B (HBV), which continues to be the driving force of hepatitis infections globally. However, in patients who receive and respond to antiviral treatment “there is a significant and considerable reduction in HCC in the context of hepatitis B” similar to that seen with hepatitis C. Vaccination programs for HBV have started to make the desired impact of reducing HCC in HBV-endemic areas, he noted.
However, current risk factors for HCC are related less to HCV and HBV and more to metabolic syndrome because more people are treated for HCV and HBV, Dr. El-Serag said. He went on to address the new dominant global risk factor for HCC: obesity. Based on data from multiple studies, those who are obese, defined as a body mass index greater than 30 kg/m2, carry a twofold increased risk of developing HCC, he said.
To reduce this risk, treatment targets might address intermediate factors such as abdominal obesity, said Dr. El-Serag. He cited a study published in Hepatology in which individuals in the highest tertile for waist-hip ratio had a threefold higher risk of HCC, compared with those in the lowest tertile.
In addition, consideration of obesity must include type 2 diabetes, which is often linked to obesity and occurs in approximately one-third of adults in the United States, Dr. El-Serag said.
Treatment of type 2 diabetes may make a difference in HCC risk reduction, Dr. El-Serag noted. “The impact of treatment of diabetes on HCC risk is an area of intense interest,” he said. Based on the latest research, “the bottom line is that those treated with metformin experience a 50% reduction in the risk of HCC,” he said
Dr. El-Serag also acknowledged the impact of other risk factors for HCC: the use of statins and the presence of nonalcoholic fatty liver disease (NAFLD).
Dr. El-Serag noted that, among NAFLD patients, subgroups at even greater risk for HCC include those with diabetes, those older than 65 years, Hispanic race, and those with cirrhosis. These patients should be candidates for surveillance. Metabolic dysfunction traits such as obesity and diabetes are very common conditions, so it’s important to look at other, more specific factors, he added. “I hope that there will be tools to help clinicians classify or risk-stratify patients into different buckets,” he said.
Areas for further research on HCC continue to include risk stratification, mechanisms of action, and HCC prevention related to treatment of metabolic syndrome, he emphasized.
Dr. El-Serag had no financial conflicts to disclose. Global Academy for Medical Education and this news organization are owned by the same parent company.
*This story was updated on Oct. 28, 2020.
SOURCE: El-Serag HB. Digestive Diseases: New Advances 2020.
Rates of hepatocellular carcinoma (HCC) continue to rise in the United States, but unevenly so given how the incidence has become highest in the Hispanic population, which is reflected in increased rates in the southern and western states, Hashem B. El-Serag, MD, of Baylor College of Medicine, Houston, said in a virtual presentation at the annual Digestive Diseases: New Advances, which is jointly provided by Rutgers and Global Academy for Medical Education.
In addition to this demographic shift, the risk factors for HCC are shifting, he said. Hepatitis C virus (HCV) has been the dominant risk factor for HCC; for patients with active HCV, the factors historically associated with increased HCC risk have included alcohol consumption, obesity, diabetes, coinfection, and genetics, he said.
This pattern is starting to change. In fact, for patients with active HCV, antiviral treatment with a sustained virologic response has surfaced as the most significant risk factor in the development of HCC, said Dr. El-Serag: Among these patients, sustained virologic response from direct-acting antivirals is associated with a significant reduction in HCC risk. However, it is important to recognize that a residual risk of HCC remains that doesn’t go away for several years, he noted.
“Who are those people who got treated, got cured, and still developed HCC? Those with cirrhosis at the time of treatment,” he said. Those with cirrhosis have cumulative incidence of 1.8% per year, but those without cirrhosis had very low risk, he said.
Some good news in HCC is that rates appear to be declining among young men, and this is thought to be one of the groups who are achieving a cure of HCV, he said.
“One would hope, if goals for HCV elimination are met, that will translate into massive reduction of HCC,” he said.
“The issue now for hepatitis is finding infected patients and curing them,” he noted.
Dr. El-Serag touched on hepatitis B (HBV), which continues to be the driving force of hepatitis infections globally. However, in patients who receive and respond to antiviral treatment “there is a significant and considerable reduction in HCC in the context of hepatitis B” similar to that seen with hepatitis C. Vaccination programs for HBV have started to make the desired impact of reducing HCC in HBV-endemic areas, he noted.
However, current risk factors for HCC are related less to HCV and HBV and more to metabolic syndrome because more people are treated for HCV and HBV, Dr. El-Serag said. He went on to address the new dominant global risk factor for HCC: obesity. Based on data from multiple studies, those who are obese, defined as a body mass index greater than 30 kg/m2, carry a twofold increased risk of developing HCC, he said.
To reduce this risk, treatment targets might address intermediate factors such as abdominal obesity, said Dr. El-Serag. He cited a study published in Hepatology in which individuals in the highest tertile for waist-hip ratio had a threefold higher risk of HCC, compared with those in the lowest tertile.
In addition, consideration of obesity must include type 2 diabetes, which is often linked to obesity and occurs in approximately one-third of adults in the United States, Dr. El-Serag said.
Treatment of type 2 diabetes may make a difference in HCC risk reduction, Dr. El-Serag noted. “The impact of treatment of diabetes on HCC risk is an area of intense interest,” he said. Based on the latest research, “the bottom line is that those treated with metformin experience a 50% reduction in the risk of HCC,” he said
Dr. El-Serag also acknowledged the impact of other risk factors for HCC: the use of statins and the presence of nonalcoholic fatty liver disease (NAFLD).
Dr. El-Serag noted that, among NAFLD patients, subgroups at even greater risk for HCC include those with diabetes, those older than 65 years, Hispanic race, and those with cirrhosis. These patients should be candidates for surveillance. Metabolic dysfunction traits such as obesity and diabetes are very common conditions, so it’s important to look at other, more specific factors, he added. “I hope that there will be tools to help clinicians classify or risk-stratify patients into different buckets,” he said.
Areas for further research on HCC continue to include risk stratification, mechanisms of action, and HCC prevention related to treatment of metabolic syndrome, he emphasized.
Dr. El-Serag had no financial conflicts to disclose. Global Academy for Medical Education and this news organization are owned by the same parent company.
*This story was updated on Oct. 28, 2020.
SOURCE: El-Serag HB. Digestive Diseases: New Advances 2020.
Rates of hepatocellular carcinoma (HCC) continue to rise in the United States, but unevenly so given how the incidence has become highest in the Hispanic population, which is reflected in increased rates in the southern and western states, Hashem B. El-Serag, MD, of Baylor College of Medicine, Houston, said in a virtual presentation at the annual Digestive Diseases: New Advances, which is jointly provided by Rutgers and Global Academy for Medical Education.
In addition to this demographic shift, the risk factors for HCC are shifting, he said. Hepatitis C virus (HCV) has been the dominant risk factor for HCC; for patients with active HCV, the factors historically associated with increased HCC risk have included alcohol consumption, obesity, diabetes, coinfection, and genetics, he said.
This pattern is starting to change. In fact, for patients with active HCV, antiviral treatment with a sustained virologic response has surfaced as the most significant risk factor in the development of HCC, said Dr. El-Serag: Among these patients, sustained virologic response from direct-acting antivirals is associated with a significant reduction in HCC risk. However, it is important to recognize that a residual risk of HCC remains that doesn’t go away for several years, he noted.
“Who are those people who got treated, got cured, and still developed HCC? Those with cirrhosis at the time of treatment,” he said. Those with cirrhosis have cumulative incidence of 1.8% per year, but those without cirrhosis had very low risk, he said.
Some good news in HCC is that rates appear to be declining among young men, and this is thought to be one of the groups who are achieving a cure of HCV, he said.
“One would hope, if goals for HCV elimination are met, that will translate into massive reduction of HCC,” he said.
“The issue now for hepatitis is finding infected patients and curing them,” he noted.
Dr. El-Serag touched on hepatitis B (HBV), which continues to be the driving force of hepatitis infections globally. However, in patients who receive and respond to antiviral treatment “there is a significant and considerable reduction in HCC in the context of hepatitis B” similar to that seen with hepatitis C. Vaccination programs for HBV have started to make the desired impact of reducing HCC in HBV-endemic areas, he noted.
However, current risk factors for HCC are related less to HCV and HBV and more to metabolic syndrome because more people are treated for HCV and HBV, Dr. El-Serag said. He went on to address the new dominant global risk factor for HCC: obesity. Based on data from multiple studies, those who are obese, defined as a body mass index greater than 30 kg/m2, carry a twofold increased risk of developing HCC, he said.
To reduce this risk, treatment targets might address intermediate factors such as abdominal obesity, said Dr. El-Serag. He cited a study published in Hepatology in which individuals in the highest tertile for waist-hip ratio had a threefold higher risk of HCC, compared with those in the lowest tertile.
In addition, consideration of obesity must include type 2 diabetes, which is often linked to obesity and occurs in approximately one-third of adults in the United States, Dr. El-Serag said.
Treatment of type 2 diabetes may make a difference in HCC risk reduction, Dr. El-Serag noted. “The impact of treatment of diabetes on HCC risk is an area of intense interest,” he said. Based on the latest research, “the bottom line is that those treated with metformin experience a 50% reduction in the risk of HCC,” he said
Dr. El-Serag also acknowledged the impact of other risk factors for HCC: the use of statins and the presence of nonalcoholic fatty liver disease (NAFLD).
Dr. El-Serag noted that, among NAFLD patients, subgroups at even greater risk for HCC include those with diabetes, those older than 65 years, Hispanic race, and those with cirrhosis. These patients should be candidates for surveillance. Metabolic dysfunction traits such as obesity and diabetes are very common conditions, so it’s important to look at other, more specific factors, he added. “I hope that there will be tools to help clinicians classify or risk-stratify patients into different buckets,” he said.
Areas for further research on HCC continue to include risk stratification, mechanisms of action, and HCC prevention related to treatment of metabolic syndrome, he emphasized.
Dr. El-Serag had no financial conflicts to disclose. Global Academy for Medical Education and this news organization are owned by the same parent company.
*This story was updated on Oct. 28, 2020.
SOURCE: El-Serag HB. Digestive Diseases: New Advances 2020.
FROM DIGESTIVE DISEASES: NEW ADVANCES
Address root causes to manage NASH
Not only the prevalence, but the impact of nonalcoholic fatty liver disease (NAFLD) is increasing in much of the world, Arun J. Sanyal, MD, said in a virtual presentation at the meeting jointly provided by Rutgers and Global Academy for Medical Education. “It is currently estimated that the number of people living with cirrhosis or with decompensated cirrhosis will increase two- to threefold from 2015 to 2030,” which underlines the public health impact and the need for improved treatment paradigms, he emphasized.
“The thing to remember about NAFLD is that it does not exist in a vacuum,” Dr. Sanyal said. NAFLD is a multisystem disorder. Most patients have concomitant cardiovascular disease, but others may have type 2 diabetes, hypertension, and dyslipidemia, all of which are now accepted as risk factors for nonalcoholic steatohepatitis (NASH), he said.
“What ties these conditions together is metabolic stress leading to systemic inflammation and fibrosis. This is primarily due to diet-induced obesity. If you think about treating all of these competing risks to the patient’s life, the optimal way is to treat the root cause,” he said.
Various options exist to manage the conditions that can lead to NASH, but several of these also appear promising as a treatment of NASH, Dr. Sanyal said. Glucagonlike peptide–1 agonists and sodium-glucose transporter 2 inhibitors have been shown to improve multiple outcomes of interest in type 2 diabetes. However, insulin can cause weight gain at the expense of controlling HbA1C levels, he said.
Bariatric surgery can improve histology, but many patients with advanced fibrosis do not demonstrate improvement in fibrosis. Also, bariatric surgery has its own associated morbidity, including an increased suicide rate across multiple studies, Dr. Sanyal noted.
A new and interesting option is duodenal mucosal resurfacing (DMR) “a novel, minimally invasive outpatient upper-endoscopic procedure,” said Dr. Sanyal. DMR involves use of a catheter to perform a submucosal lift and hydrothermal mucosal ablation, prompting healthy epithelial regrowth, he explained. “The mucosa sloughs off, fresh epithelium grows in, and the hormonal signal from the gut to the rest of the body is restored to a more normal pattern,” he noted.
In the REVITA-2 study of patients with diabetes and NAFLD, the average fat loss was 5.4% in those randomized to DMR vs. 2.4% in sham-procedure patients and represented “quite significant defatting of the liver,” Dr. Sanyal said.
Dr. Sanyal then focused on fatty liver disease. “The first step when you see a patient with fatty liver disease is to see how scarred is the liver, and whether the patient has silent cirrhosis. The more scarred the liver, the greater risk of liver-related outcomes,” he said. The goal of therapy for these patients is to reduce the risk of progression to cirrhosis, he added. Dr. Sanyal recommended evaluating fibrosis using the Fibrosis 4 score (Fib4). “If the Fib4 is less than 1.3, the likelihood of significant scarring in the liver is less than 10%,” he said. On the other hand, a Fib4 greater than 2.67 suggests advanced fibrosis, he noted.
Overall, the goals of treatment for NASH patients are to prevent cirrhosis, reduce decompensation, and prevent hepatocellular carcinoma, said Dr. Sanyal.
“The ideal drug for NASH should also help other end organs, or at least be neutral,” said Dr. Sanyal.
Current frontline therapies for precirrhotic NASH include thiazolidinediones (TZD), farnesoid X receptor (FXR)/fibroblast growth factor 19 (FGF-19), FGF21, thyroxine B-R, and glucagonlike peptide-1. Clinical evidence varies based on different populations, endpoints, assessment methods, and treatment duration, he said.
Looking ahead to the next decade, a NASH management paradigm will likely play out that can be applied in the clinic today, Dr. Sanyal said. First, make an initial assessment of the status of the end organs. Start with a weight-loss regimen; use statins and GLP-1 and SGLT2 inhibitors as needed. Follow and reassess, and if the patient still has disease, progress to targeted therapy for active NASH while continuing to encourage weight loss and healthy living, he said.
“The ultimate proof that what we are doing is working is that we are improving mortality, reducing health care costs, and improving patients’ function and quality of life,” he concluded.
Dr. Sanyal is president of Sanyal Biotechnologies. He also disclosed stock options for Durect, Exhalenz, Galmed, Genfit, Immuton, Indalo, and Tiziana, as well as various relationships with Allergan, AMRA, Astra Zeneca-Medimmune, Birdrock, Boehringer Ingelheim, Bristol Myers, Echosense, GE, Genentech, Gilead, Hemoshear, IFMO, Innovate, Intercept, Lilly, Lipocine, Merck, Novartis, Novo Nordisk, OWL, Pfizer, RedX, Sundise, Tern, and Zydus.
Global Academy for Medical Education and this news organization are owned by the same parent company.
Not only the prevalence, but the impact of nonalcoholic fatty liver disease (NAFLD) is increasing in much of the world, Arun J. Sanyal, MD, said in a virtual presentation at the meeting jointly provided by Rutgers and Global Academy for Medical Education. “It is currently estimated that the number of people living with cirrhosis or with decompensated cirrhosis will increase two- to threefold from 2015 to 2030,” which underlines the public health impact and the need for improved treatment paradigms, he emphasized.
“The thing to remember about NAFLD is that it does not exist in a vacuum,” Dr. Sanyal said. NAFLD is a multisystem disorder. Most patients have concomitant cardiovascular disease, but others may have type 2 diabetes, hypertension, and dyslipidemia, all of which are now accepted as risk factors for nonalcoholic steatohepatitis (NASH), he said.
“What ties these conditions together is metabolic stress leading to systemic inflammation and fibrosis. This is primarily due to diet-induced obesity. If you think about treating all of these competing risks to the patient’s life, the optimal way is to treat the root cause,” he said.
Various options exist to manage the conditions that can lead to NASH, but several of these also appear promising as a treatment of NASH, Dr. Sanyal said. Glucagonlike peptide–1 agonists and sodium-glucose transporter 2 inhibitors have been shown to improve multiple outcomes of interest in type 2 diabetes. However, insulin can cause weight gain at the expense of controlling HbA1C levels, he said.
Bariatric surgery can improve histology, but many patients with advanced fibrosis do not demonstrate improvement in fibrosis. Also, bariatric surgery has its own associated morbidity, including an increased suicide rate across multiple studies, Dr. Sanyal noted.
A new and interesting option is duodenal mucosal resurfacing (DMR) “a novel, minimally invasive outpatient upper-endoscopic procedure,” said Dr. Sanyal. DMR involves use of a catheter to perform a submucosal lift and hydrothermal mucosal ablation, prompting healthy epithelial regrowth, he explained. “The mucosa sloughs off, fresh epithelium grows in, and the hormonal signal from the gut to the rest of the body is restored to a more normal pattern,” he noted.
In the REVITA-2 study of patients with diabetes and NAFLD, the average fat loss was 5.4% in those randomized to DMR vs. 2.4% in sham-procedure patients and represented “quite significant defatting of the liver,” Dr. Sanyal said.
Dr. Sanyal then focused on fatty liver disease. “The first step when you see a patient with fatty liver disease is to see how scarred is the liver, and whether the patient has silent cirrhosis. The more scarred the liver, the greater risk of liver-related outcomes,” he said. The goal of therapy for these patients is to reduce the risk of progression to cirrhosis, he added. Dr. Sanyal recommended evaluating fibrosis using the Fibrosis 4 score (Fib4). “If the Fib4 is less than 1.3, the likelihood of significant scarring in the liver is less than 10%,” he said. On the other hand, a Fib4 greater than 2.67 suggests advanced fibrosis, he noted.
Overall, the goals of treatment for NASH patients are to prevent cirrhosis, reduce decompensation, and prevent hepatocellular carcinoma, said Dr. Sanyal.
“The ideal drug for NASH should also help other end organs, or at least be neutral,” said Dr. Sanyal.
Current frontline therapies for precirrhotic NASH include thiazolidinediones (TZD), farnesoid X receptor (FXR)/fibroblast growth factor 19 (FGF-19), FGF21, thyroxine B-R, and glucagonlike peptide-1. Clinical evidence varies based on different populations, endpoints, assessment methods, and treatment duration, he said.
Looking ahead to the next decade, a NASH management paradigm will likely play out that can be applied in the clinic today, Dr. Sanyal said. First, make an initial assessment of the status of the end organs. Start with a weight-loss regimen; use statins and GLP-1 and SGLT2 inhibitors as needed. Follow and reassess, and if the patient still has disease, progress to targeted therapy for active NASH while continuing to encourage weight loss and healthy living, he said.
“The ultimate proof that what we are doing is working is that we are improving mortality, reducing health care costs, and improving patients’ function and quality of life,” he concluded.
Dr. Sanyal is president of Sanyal Biotechnologies. He also disclosed stock options for Durect, Exhalenz, Galmed, Genfit, Immuton, Indalo, and Tiziana, as well as various relationships with Allergan, AMRA, Astra Zeneca-Medimmune, Birdrock, Boehringer Ingelheim, Bristol Myers, Echosense, GE, Genentech, Gilead, Hemoshear, IFMO, Innovate, Intercept, Lilly, Lipocine, Merck, Novartis, Novo Nordisk, OWL, Pfizer, RedX, Sundise, Tern, and Zydus.
Global Academy for Medical Education and this news organization are owned by the same parent company.
Not only the prevalence, but the impact of nonalcoholic fatty liver disease (NAFLD) is increasing in much of the world, Arun J. Sanyal, MD, said in a virtual presentation at the meeting jointly provided by Rutgers and Global Academy for Medical Education. “It is currently estimated that the number of people living with cirrhosis or with decompensated cirrhosis will increase two- to threefold from 2015 to 2030,” which underlines the public health impact and the need for improved treatment paradigms, he emphasized.
“The thing to remember about NAFLD is that it does not exist in a vacuum,” Dr. Sanyal said. NAFLD is a multisystem disorder. Most patients have concomitant cardiovascular disease, but others may have type 2 diabetes, hypertension, and dyslipidemia, all of which are now accepted as risk factors for nonalcoholic steatohepatitis (NASH), he said.
“What ties these conditions together is metabolic stress leading to systemic inflammation and fibrosis. This is primarily due to diet-induced obesity. If you think about treating all of these competing risks to the patient’s life, the optimal way is to treat the root cause,” he said.
Various options exist to manage the conditions that can lead to NASH, but several of these also appear promising as a treatment of NASH, Dr. Sanyal said. Glucagonlike peptide–1 agonists and sodium-glucose transporter 2 inhibitors have been shown to improve multiple outcomes of interest in type 2 diabetes. However, insulin can cause weight gain at the expense of controlling HbA1C levels, he said.
Bariatric surgery can improve histology, but many patients with advanced fibrosis do not demonstrate improvement in fibrosis. Also, bariatric surgery has its own associated morbidity, including an increased suicide rate across multiple studies, Dr. Sanyal noted.
A new and interesting option is duodenal mucosal resurfacing (DMR) “a novel, minimally invasive outpatient upper-endoscopic procedure,” said Dr. Sanyal. DMR involves use of a catheter to perform a submucosal lift and hydrothermal mucosal ablation, prompting healthy epithelial regrowth, he explained. “The mucosa sloughs off, fresh epithelium grows in, and the hormonal signal from the gut to the rest of the body is restored to a more normal pattern,” he noted.
In the REVITA-2 study of patients with diabetes and NAFLD, the average fat loss was 5.4% in those randomized to DMR vs. 2.4% in sham-procedure patients and represented “quite significant defatting of the liver,” Dr. Sanyal said.
Dr. Sanyal then focused on fatty liver disease. “The first step when you see a patient with fatty liver disease is to see how scarred is the liver, and whether the patient has silent cirrhosis. The more scarred the liver, the greater risk of liver-related outcomes,” he said. The goal of therapy for these patients is to reduce the risk of progression to cirrhosis, he added. Dr. Sanyal recommended evaluating fibrosis using the Fibrosis 4 score (Fib4). “If the Fib4 is less than 1.3, the likelihood of significant scarring in the liver is less than 10%,” he said. On the other hand, a Fib4 greater than 2.67 suggests advanced fibrosis, he noted.
Overall, the goals of treatment for NASH patients are to prevent cirrhosis, reduce decompensation, and prevent hepatocellular carcinoma, said Dr. Sanyal.
“The ideal drug for NASH should also help other end organs, or at least be neutral,” said Dr. Sanyal.
Current frontline therapies for precirrhotic NASH include thiazolidinediones (TZD), farnesoid X receptor (FXR)/fibroblast growth factor 19 (FGF-19), FGF21, thyroxine B-R, and glucagonlike peptide-1. Clinical evidence varies based on different populations, endpoints, assessment methods, and treatment duration, he said.
Looking ahead to the next decade, a NASH management paradigm will likely play out that can be applied in the clinic today, Dr. Sanyal said. First, make an initial assessment of the status of the end organs. Start with a weight-loss regimen; use statins and GLP-1 and SGLT2 inhibitors as needed. Follow and reassess, and if the patient still has disease, progress to targeted therapy for active NASH while continuing to encourage weight loss and healthy living, he said.
“The ultimate proof that what we are doing is working is that we are improving mortality, reducing health care costs, and improving patients’ function and quality of life,” he concluded.
Dr. Sanyal is president of Sanyal Biotechnologies. He also disclosed stock options for Durect, Exhalenz, Galmed, Genfit, Immuton, Indalo, and Tiziana, as well as various relationships with Allergan, AMRA, Astra Zeneca-Medimmune, Birdrock, Boehringer Ingelheim, Bristol Myers, Echosense, GE, Genentech, Gilead, Hemoshear, IFMO, Innovate, Intercept, Lilly, Lipocine, Merck, Novartis, Novo Nordisk, OWL, Pfizer, RedX, Sundise, Tern, and Zydus.
Global Academy for Medical Education and this news organization are owned by the same parent company.
FROM DIGESTIVE DISEASES: NEW ADVANCES
Lusutrombopag found safe, effective for severe thrombocytopenia in patients with hepatocellular carcinoma
For patients with severe thrombocytopenia and chronic liver diseases, including hepatocellular carcinoma, treatment with lusutrombopag prior to invasive procedures significantly decreased the need for platelet transfusions without increasing the need for rescue treatment for bleeding or the rate of thromboembolic events.
In a post hoc analysis of data from 270 patients in two manufacturer-sponsored, multicenter, randomized, double-blind, placebo-controlled, phase 3 trials, significantly more lusutrombopag recipients met the primary efficacy endpoint, including patients with hepatocellular carcinoma (68.0% vs. 8.9% in the placebo group; P < .0001) and those without it (77.0% vs. 21.6%; P < .0001). Rates of treatment-emergent adverse events were similar between the lusutrombopag and placebo groups, and patients with hepatocellular carcinoma were not at increased risk for thrombosis, Naim Alkhouri, MD, of Texas Liver Institute in San Antonio, and associates wrote in Clinical Gastroenterology and Hepatology.
Platelet transfusion is the treatment mainstay for patients with thrombocytopenia related to cirrhosis who are undergoing invasive procedures, but its effects are short-lived, and at least one in five transfusions fails. Thrombopoietin agonists such as lusutrombopag are efficacious and approved in this setting, but they can be prothrombotic, particularly in patients with hepatocellular carcinoma, who already are at heightened risk for portal vein thrombosis.
Dr. Alkhouri and associates performed an integrated analysis of the PLUS 1 trial (Japan, October 2013–May 2014) and the L-PLUS 2 (global, June 2015–April 2017). Participants were adults with Child-Pugh Class A or B chronic liver disease and baseline platelet counts under 50 x 109 per L who were scheduled for invasive procedures. Of the 270 patients, 95 had hepatocellular carcinoma. Patients were randomly assigned on a one-to-one basis to receive either lusutrombopag (3 mg) or placebo daily for up to 7 days before procedures. The primary endpoint was the percentage of patients in the per-protocol population who did not need a platelet transfusion before the invasive procedure or rescue therapy within 7 days afterward.
The treatment and placebo arms were similar except that patients with hepatocellular carcinoma were about 10 years older on average. In patients with hepatocellular carcinoma, 60.5% more lusutrombopag recipients than placebo recipients met the primary endpoint, and rates of bleeding-related adverse events were 9.1% and 15.7%, respectively. In patients with other chronic liver diseases, 52.6% more lusutrombopag recipients met the primary endpoint. Rates of bleeding-related adverse events were 5% and 10.6%.
“Approximately 88% of patients with hepatocellular carcinoma underwent a liver-related procedure, compared with approximately 10% of patients without hepatocellular carcinoma,” the investigators wrote. “This is significant because ablations or transcatheter arterial chemoembolizations can be associated with serious bleeding complications. It is clinically important that, given the greater number of liver-related procedures, the incidence of bleeding-related adverse events was lower in patients treated with lusutrombopag than placebo.”
Imaging after the procedures confirmed low rates of thromboses in both groups and subgroups. Four patients developed portal vein thromboses, including two lusutrombopag recipients (one of whom had hepatocellular carcinoma) and two placebo recipients without hepatocellular carcinoma.
These trials excluded patients undergoing major surgical procedures and those with decompensated cirrhosis; portal vein thrombosis; hematopoietic tumors; aplastic anemia; myelodysplastic syndrome; myelofibrosis; liver transplantation; splenectomy; and thrombocytopenia that was congenital, autoimmune, or drug induced. “A limitation of this study was the high rate of protocol violations related to platelet transfusions,” the researchers noted. “A number of patients [42 in all] were excluded from the per-protocol population owing to receipt of unnecessary platelet transfusions, or because they did not receive a needed platelet transfusion.”Shionogi makes lusutrombopag and sponsored the study. Dr. Alkhouri reported an advisory relationship with Shionogi and Dova Pharma. Two coinvestigators reported being employed by Shionogi. Three coinvestigators also disclosed ties to Shionogi and to several other pharmaceutical companies.
SOURCE: Alkhouri N et al. Clin Gastroenterol Hepatol. 2020 Mar 20. doi: 10.1016/j.cgh.2020.03.032.
Thrombocytopenia is of clinical concern in patients with cirrhosis, as it complicates routine patient care and results in delayed or canceled procedures due to concern for risk of bleeding. In the last few years, availability of thrombopoietin (TPO) receptor agonists have facilitated the performance of elective invasive procedures in cirrhotic patients with severe thrombocytopenia.
In this integrated analysis of data from two phase 3 studies, Alkhouri et al. demonstrated the efficacy of a novel TPO receptor agonist, lusutrombopag, in reducing bleeding events and need for platelet transfusion in cirrhotic patients undergoing invasive procedures. The risk for thrombosis-related adverse events was not increased in lusutrombopag recipients with or without HCC. Previous studies with another TPO, eltrombopag, resulted in high rate of symptomatic portal vein thrombosis. Avatrombopag, a recently approved TPO receptor agonist reported few thrombotic symptomatic events but no prospective imaging for evaluation of thrombotic events was included in the protocol. A unique strength of this study was inclusion of prospective imaging for evaluation of portal vein thrombosis. Strategic scheduling is required with use of TPO agonists. Lusutrombopag can be given orally in convenient daily doses and provides a 7-10-day procedural window for scheduling and performing elective invasive procedures. However, because of several days of lag period for platelet production, these agents cannot be used for emergent cases.
Gagan K. Sood, MD, AGAF, FAASLD, is an associate professor of medicine and surgery, division of gastroenterology and hepatology and division of abdominal transplantation, Baylor College of Medicine, Houston. He has no conflicts of interest.
Thrombocytopenia is of clinical concern in patients with cirrhosis, as it complicates routine patient care and results in delayed or canceled procedures due to concern for risk of bleeding. In the last few years, availability of thrombopoietin (TPO) receptor agonists have facilitated the performance of elective invasive procedures in cirrhotic patients with severe thrombocytopenia.
In this integrated analysis of data from two phase 3 studies, Alkhouri et al. demonstrated the efficacy of a novel TPO receptor agonist, lusutrombopag, in reducing bleeding events and need for platelet transfusion in cirrhotic patients undergoing invasive procedures. The risk for thrombosis-related adverse events was not increased in lusutrombopag recipients with or without HCC. Previous studies with another TPO, eltrombopag, resulted in high rate of symptomatic portal vein thrombosis. Avatrombopag, a recently approved TPO receptor agonist reported few thrombotic symptomatic events but no prospective imaging for evaluation of thrombotic events was included in the protocol. A unique strength of this study was inclusion of prospective imaging for evaluation of portal vein thrombosis. Strategic scheduling is required with use of TPO agonists. Lusutrombopag can be given orally in convenient daily doses and provides a 7-10-day procedural window for scheduling and performing elective invasive procedures. However, because of several days of lag period for platelet production, these agents cannot be used for emergent cases.
Gagan K. Sood, MD, AGAF, FAASLD, is an associate professor of medicine and surgery, division of gastroenterology and hepatology and division of abdominal transplantation, Baylor College of Medicine, Houston. He has no conflicts of interest.
Thrombocytopenia is of clinical concern in patients with cirrhosis, as it complicates routine patient care and results in delayed or canceled procedures due to concern for risk of bleeding. In the last few years, availability of thrombopoietin (TPO) receptor agonists have facilitated the performance of elective invasive procedures in cirrhotic patients with severe thrombocytopenia.
In this integrated analysis of data from two phase 3 studies, Alkhouri et al. demonstrated the efficacy of a novel TPO receptor agonist, lusutrombopag, in reducing bleeding events and need for platelet transfusion in cirrhotic patients undergoing invasive procedures. The risk for thrombosis-related adverse events was not increased in lusutrombopag recipients with or without HCC. Previous studies with another TPO, eltrombopag, resulted in high rate of symptomatic portal vein thrombosis. Avatrombopag, a recently approved TPO receptor agonist reported few thrombotic symptomatic events but no prospective imaging for evaluation of thrombotic events was included in the protocol. A unique strength of this study was inclusion of prospective imaging for evaluation of portal vein thrombosis. Strategic scheduling is required with use of TPO agonists. Lusutrombopag can be given orally in convenient daily doses and provides a 7-10-day procedural window for scheduling and performing elective invasive procedures. However, because of several days of lag period for platelet production, these agents cannot be used for emergent cases.
Gagan K. Sood, MD, AGAF, FAASLD, is an associate professor of medicine and surgery, division of gastroenterology and hepatology and division of abdominal transplantation, Baylor College of Medicine, Houston. He has no conflicts of interest.
For patients with severe thrombocytopenia and chronic liver diseases, including hepatocellular carcinoma, treatment with lusutrombopag prior to invasive procedures significantly decreased the need for platelet transfusions without increasing the need for rescue treatment for bleeding or the rate of thromboembolic events.
In a post hoc analysis of data from 270 patients in two manufacturer-sponsored, multicenter, randomized, double-blind, placebo-controlled, phase 3 trials, significantly more lusutrombopag recipients met the primary efficacy endpoint, including patients with hepatocellular carcinoma (68.0% vs. 8.9% in the placebo group; P < .0001) and those without it (77.0% vs. 21.6%; P < .0001). Rates of treatment-emergent adverse events were similar between the lusutrombopag and placebo groups, and patients with hepatocellular carcinoma were not at increased risk for thrombosis, Naim Alkhouri, MD, of Texas Liver Institute in San Antonio, and associates wrote in Clinical Gastroenterology and Hepatology.
Platelet transfusion is the treatment mainstay for patients with thrombocytopenia related to cirrhosis who are undergoing invasive procedures, but its effects are short-lived, and at least one in five transfusions fails. Thrombopoietin agonists such as lusutrombopag are efficacious and approved in this setting, but they can be prothrombotic, particularly in patients with hepatocellular carcinoma, who already are at heightened risk for portal vein thrombosis.
Dr. Alkhouri and associates performed an integrated analysis of the PLUS 1 trial (Japan, October 2013–May 2014) and the L-PLUS 2 (global, June 2015–April 2017). Participants were adults with Child-Pugh Class A or B chronic liver disease and baseline platelet counts under 50 x 109 per L who were scheduled for invasive procedures. Of the 270 patients, 95 had hepatocellular carcinoma. Patients were randomly assigned on a one-to-one basis to receive either lusutrombopag (3 mg) or placebo daily for up to 7 days before procedures. The primary endpoint was the percentage of patients in the per-protocol population who did not need a platelet transfusion before the invasive procedure or rescue therapy within 7 days afterward.
The treatment and placebo arms were similar except that patients with hepatocellular carcinoma were about 10 years older on average. In patients with hepatocellular carcinoma, 60.5% more lusutrombopag recipients than placebo recipients met the primary endpoint, and rates of bleeding-related adverse events were 9.1% and 15.7%, respectively. In patients with other chronic liver diseases, 52.6% more lusutrombopag recipients met the primary endpoint. Rates of bleeding-related adverse events were 5% and 10.6%.
“Approximately 88% of patients with hepatocellular carcinoma underwent a liver-related procedure, compared with approximately 10% of patients without hepatocellular carcinoma,” the investigators wrote. “This is significant because ablations or transcatheter arterial chemoembolizations can be associated with serious bleeding complications. It is clinically important that, given the greater number of liver-related procedures, the incidence of bleeding-related adverse events was lower in patients treated with lusutrombopag than placebo.”
Imaging after the procedures confirmed low rates of thromboses in both groups and subgroups. Four patients developed portal vein thromboses, including two lusutrombopag recipients (one of whom had hepatocellular carcinoma) and two placebo recipients without hepatocellular carcinoma.
These trials excluded patients undergoing major surgical procedures and those with decompensated cirrhosis; portal vein thrombosis; hematopoietic tumors; aplastic anemia; myelodysplastic syndrome; myelofibrosis; liver transplantation; splenectomy; and thrombocytopenia that was congenital, autoimmune, or drug induced. “A limitation of this study was the high rate of protocol violations related to platelet transfusions,” the researchers noted. “A number of patients [42 in all] were excluded from the per-protocol population owing to receipt of unnecessary platelet transfusions, or because they did not receive a needed platelet transfusion.”Shionogi makes lusutrombopag and sponsored the study. Dr. Alkhouri reported an advisory relationship with Shionogi and Dova Pharma. Two coinvestigators reported being employed by Shionogi. Three coinvestigators also disclosed ties to Shionogi and to several other pharmaceutical companies.
SOURCE: Alkhouri N et al. Clin Gastroenterol Hepatol. 2020 Mar 20. doi: 10.1016/j.cgh.2020.03.032.
For patients with severe thrombocytopenia and chronic liver diseases, including hepatocellular carcinoma, treatment with lusutrombopag prior to invasive procedures significantly decreased the need for platelet transfusions without increasing the need for rescue treatment for bleeding or the rate of thromboembolic events.
In a post hoc analysis of data from 270 patients in two manufacturer-sponsored, multicenter, randomized, double-blind, placebo-controlled, phase 3 trials, significantly more lusutrombopag recipients met the primary efficacy endpoint, including patients with hepatocellular carcinoma (68.0% vs. 8.9% in the placebo group; P < .0001) and those without it (77.0% vs. 21.6%; P < .0001). Rates of treatment-emergent adverse events were similar between the lusutrombopag and placebo groups, and patients with hepatocellular carcinoma were not at increased risk for thrombosis, Naim Alkhouri, MD, of Texas Liver Institute in San Antonio, and associates wrote in Clinical Gastroenterology and Hepatology.
Platelet transfusion is the treatment mainstay for patients with thrombocytopenia related to cirrhosis who are undergoing invasive procedures, but its effects are short-lived, and at least one in five transfusions fails. Thrombopoietin agonists such as lusutrombopag are efficacious and approved in this setting, but they can be prothrombotic, particularly in patients with hepatocellular carcinoma, who already are at heightened risk for portal vein thrombosis.
Dr. Alkhouri and associates performed an integrated analysis of the PLUS 1 trial (Japan, October 2013–May 2014) and the L-PLUS 2 (global, June 2015–April 2017). Participants were adults with Child-Pugh Class A or B chronic liver disease and baseline platelet counts under 50 x 109 per L who were scheduled for invasive procedures. Of the 270 patients, 95 had hepatocellular carcinoma. Patients were randomly assigned on a one-to-one basis to receive either lusutrombopag (3 mg) or placebo daily for up to 7 days before procedures. The primary endpoint was the percentage of patients in the per-protocol population who did not need a platelet transfusion before the invasive procedure or rescue therapy within 7 days afterward.
The treatment and placebo arms were similar except that patients with hepatocellular carcinoma were about 10 years older on average. In patients with hepatocellular carcinoma, 60.5% more lusutrombopag recipients than placebo recipients met the primary endpoint, and rates of bleeding-related adverse events were 9.1% and 15.7%, respectively. In patients with other chronic liver diseases, 52.6% more lusutrombopag recipients met the primary endpoint. Rates of bleeding-related adverse events were 5% and 10.6%.
“Approximately 88% of patients with hepatocellular carcinoma underwent a liver-related procedure, compared with approximately 10% of patients without hepatocellular carcinoma,” the investigators wrote. “This is significant because ablations or transcatheter arterial chemoembolizations can be associated with serious bleeding complications. It is clinically important that, given the greater number of liver-related procedures, the incidence of bleeding-related adverse events was lower in patients treated with lusutrombopag than placebo.”
Imaging after the procedures confirmed low rates of thromboses in both groups and subgroups. Four patients developed portal vein thromboses, including two lusutrombopag recipients (one of whom had hepatocellular carcinoma) and two placebo recipients without hepatocellular carcinoma.
These trials excluded patients undergoing major surgical procedures and those with decompensated cirrhosis; portal vein thrombosis; hematopoietic tumors; aplastic anemia; myelodysplastic syndrome; myelofibrosis; liver transplantation; splenectomy; and thrombocytopenia that was congenital, autoimmune, or drug induced. “A limitation of this study was the high rate of protocol violations related to platelet transfusions,” the researchers noted. “A number of patients [42 in all] were excluded from the per-protocol population owing to receipt of unnecessary platelet transfusions, or because they did not receive a needed platelet transfusion.”Shionogi makes lusutrombopag and sponsored the study. Dr. Alkhouri reported an advisory relationship with Shionogi and Dova Pharma. Two coinvestigators reported being employed by Shionogi. Three coinvestigators also disclosed ties to Shionogi and to several other pharmaceutical companies.
SOURCE: Alkhouri N et al. Clin Gastroenterol Hepatol. 2020 Mar 20. doi: 10.1016/j.cgh.2020.03.032.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Bariatric surgery achieved long-term resolution of NASH without worsening fibrosis
Bariatric surgery resolved nonalcoholic steatohepatitis (NASH) without worsening fibrosis in 84% of patients with evaluable biopsies, according to the findings of a prospective study.
The study included 180 severely or morbidly obese adults (body mass index >35 kg/m2) with NASH who underwent bariatric surgery at a center in France. Among 94 patients evaluated 5 years later, 68% had follow-up liver biopsies, of whom 84% (95% confidence interval, 73.1%-92.2%) met the primary endpoint of resolution of NASH without worsening of fibrosis. All histologic aspects of NASH had improved, median nonalcoholic fatty liver disease scores (NAS) fell from 5 (interquartile range, 4 to 5) to 1 (IQR, 0-2; P < .001), and 90% of patients achieved at least a 2-point NAS improvement. Hepatocellular ballooning also improved in 87.5% of patients. Baseline severity of NASH did not affect the chances of it resolving at 5 years. “The reduction of fibrosis [was] progressive, beginning during the first year and continuing through 5 years,” Guillaume Lassailly, MD, and associates wrote in Gastroenterology.
NASH is a priority for clinical research because of the substantial risk for subsequent cirrhosis, added Dr. Lassailly of CHU Lille (France). For NASH to resolve, most patients need to lose at least 7%-10% of their body weight, but “only 10% of patients reach this objective with lifestyle therapy at 1 year, and less than half maintain the weight loss 5 years later.” Despite ongoing drug development efforts, no medications have been approved for treating NASH. Although weight loss after bariatric surgery has been reported to resolve NASH in approximately 80% of patients at 1 year, longer-term data have been unavailable, and it has remained unclear whether bariatric surgery can slow or halt fibrosis progression.
All patients in this study had biopsy-confirmed NASH and at least a 5-year history of severe or morbid obesity as well as at least one comorbidity, such as diabetes mellitus or arterial hypertension. Patients were not heavy drinkers, and none had detectable markers of chronic liver disease.
Bariatric surgery produced a median 12-kg/m2 drop in body mass index. At 5-year follow-up, 93% of patients meeting or exceeding this threshold who had biopsies performed showed resolution of NASH without worsening of fibrosis. Furthermore, 56% of patients (95% CI, 42.4%-69.3%) had no histologic evidence of fibrosis, including 45.5% of patients who had bridging fibrosis at baseline.
Participants in this study received intensive preoperative support, including evaluations by numerous specialists, a nutrition plan, and a 6- to 12-month therapeutic education program. Bariatric surgery techniques included Roux-en-Y gastric bypass, gastric banding, and sleeve gastrectomy. A subgroup analysis linked gastric bypass to a significantly higher probability of meeting the primary endpoint, compared with gastric banding. Refusal was the most common reason for not having a follow-up biopsy, the researchers said. “Patients without liver biopsy after bariatric surgery were not significantly different from those with a histological follow-up except for a lower BMI at 1 year. Baseline fibrosis did not influence the probability of undergoing histological reevaluation at 5 years.”
Two study participants died from surgical complications within 1 month after surgery, and one patient died from cardiac dysfunction 4 years later. No fatality was deemed liver related.
The study was funded by the French Ministry of Health, Conseil Régional Nord-Pas de Calais, National de la Recherche, and the European commission (FEDER). The researchers reported having no conflicts of interest.
SOURCE: Lassailly G et al. Gastroenterology. 2020 Jun 15. doi: 10.1053/j.gastro.2020.06.006.
As obesity prevalence increases at an alarming pace, nonalcoholic steatohepatitis (NASH) has become the most common indication for liver transplantation in women and the second most common in men in the United States. Impeding the inflammation and reversing the resultant fibrosis prior to the development of end-stage liver disease and needing liver transplantation are essential goals in NASH management. The lack of Food and Drug Administration–approved pharmacotherapy triggered interest in the effect of weight loss on NASH and short-term benefits were noted.
In this article, Lassailly et al. demonstrated long-term benefits of bariatric surgery in patients with NASH. They prospectively enrolled 180 patients and histologically followed 64 patients at 1 year and 5 years postoperatively. NASH resolved in 84% of patients and fibrosis regressed in >70%. Importantly, advanced fibrosis (F3) regressed in 15/19 patients. Cirrhosis regressed to F3 in two-thirds of patients. No liver-related mortality or decompensation was observed.
These favorable outcomes embolden the practice of referring NASH patients with morbid obesity to bariatric surgery before liver disease severity becomes prohibitive of this approach. NASH pharmacotherapy may become available in the future. However, we must not forget that cardiovascular disease remains a common cause of morbidity and mortality in NASH patients.
With these study findings and previously established benefits of bariatric surgery on mitigating cardiovascular risk and treating relevant metabolic derangements (e.g., diabetes mellitus), pursuing bariatric surgery in NASH patients may be the seed that, if planted early on, can later flourish with resolution of NASH, prevention of cardiovascular disease, metabolic optimization, and potentially longer and healthier life.
Manhal J. Izzy, MD, is assistant professor of medicine, Vanderbilt Digestive Disease Center, Vanderbilt University, Nashville, Tenn.
As obesity prevalence increases at an alarming pace, nonalcoholic steatohepatitis (NASH) has become the most common indication for liver transplantation in women and the second most common in men in the United States. Impeding the inflammation and reversing the resultant fibrosis prior to the development of end-stage liver disease and needing liver transplantation are essential goals in NASH management. The lack of Food and Drug Administration–approved pharmacotherapy triggered interest in the effect of weight loss on NASH and short-term benefits were noted.
In this article, Lassailly et al. demonstrated long-term benefits of bariatric surgery in patients with NASH. They prospectively enrolled 180 patients and histologically followed 64 patients at 1 year and 5 years postoperatively. NASH resolved in 84% of patients and fibrosis regressed in >70%. Importantly, advanced fibrosis (F3) regressed in 15/19 patients. Cirrhosis regressed to F3 in two-thirds of patients. No liver-related mortality or decompensation was observed.
These favorable outcomes embolden the practice of referring NASH patients with morbid obesity to bariatric surgery before liver disease severity becomes prohibitive of this approach. NASH pharmacotherapy may become available in the future. However, we must not forget that cardiovascular disease remains a common cause of morbidity and mortality in NASH patients.
With these study findings and previously established benefits of bariatric surgery on mitigating cardiovascular risk and treating relevant metabolic derangements (e.g., diabetes mellitus), pursuing bariatric surgery in NASH patients may be the seed that, if planted early on, can later flourish with resolution of NASH, prevention of cardiovascular disease, metabolic optimization, and potentially longer and healthier life.
Manhal J. Izzy, MD, is assistant professor of medicine, Vanderbilt Digestive Disease Center, Vanderbilt University, Nashville, Tenn.
As obesity prevalence increases at an alarming pace, nonalcoholic steatohepatitis (NASH) has become the most common indication for liver transplantation in women and the second most common in men in the United States. Impeding the inflammation and reversing the resultant fibrosis prior to the development of end-stage liver disease and needing liver transplantation are essential goals in NASH management. The lack of Food and Drug Administration–approved pharmacotherapy triggered interest in the effect of weight loss on NASH and short-term benefits were noted.
In this article, Lassailly et al. demonstrated long-term benefits of bariatric surgery in patients with NASH. They prospectively enrolled 180 patients and histologically followed 64 patients at 1 year and 5 years postoperatively. NASH resolved in 84% of patients and fibrosis regressed in >70%. Importantly, advanced fibrosis (F3) regressed in 15/19 patients. Cirrhosis regressed to F3 in two-thirds of patients. No liver-related mortality or decompensation was observed.
These favorable outcomes embolden the practice of referring NASH patients with morbid obesity to bariatric surgery before liver disease severity becomes prohibitive of this approach. NASH pharmacotherapy may become available in the future. However, we must not forget that cardiovascular disease remains a common cause of morbidity and mortality in NASH patients.
With these study findings and previously established benefits of bariatric surgery on mitigating cardiovascular risk and treating relevant metabolic derangements (e.g., diabetes mellitus), pursuing bariatric surgery in NASH patients may be the seed that, if planted early on, can later flourish with resolution of NASH, prevention of cardiovascular disease, metabolic optimization, and potentially longer and healthier life.
Manhal J. Izzy, MD, is assistant professor of medicine, Vanderbilt Digestive Disease Center, Vanderbilt University, Nashville, Tenn.
Bariatric surgery resolved nonalcoholic steatohepatitis (NASH) without worsening fibrosis in 84% of patients with evaluable biopsies, according to the findings of a prospective study.
The study included 180 severely or morbidly obese adults (body mass index >35 kg/m2) with NASH who underwent bariatric surgery at a center in France. Among 94 patients evaluated 5 years later, 68% had follow-up liver biopsies, of whom 84% (95% confidence interval, 73.1%-92.2%) met the primary endpoint of resolution of NASH without worsening of fibrosis. All histologic aspects of NASH had improved, median nonalcoholic fatty liver disease scores (NAS) fell from 5 (interquartile range, 4 to 5) to 1 (IQR, 0-2; P < .001), and 90% of patients achieved at least a 2-point NAS improvement. Hepatocellular ballooning also improved in 87.5% of patients. Baseline severity of NASH did not affect the chances of it resolving at 5 years. “The reduction of fibrosis [was] progressive, beginning during the first year and continuing through 5 years,” Guillaume Lassailly, MD, and associates wrote in Gastroenterology.
NASH is a priority for clinical research because of the substantial risk for subsequent cirrhosis, added Dr. Lassailly of CHU Lille (France). For NASH to resolve, most patients need to lose at least 7%-10% of their body weight, but “only 10% of patients reach this objective with lifestyle therapy at 1 year, and less than half maintain the weight loss 5 years later.” Despite ongoing drug development efforts, no medications have been approved for treating NASH. Although weight loss after bariatric surgery has been reported to resolve NASH in approximately 80% of patients at 1 year, longer-term data have been unavailable, and it has remained unclear whether bariatric surgery can slow or halt fibrosis progression.
All patients in this study had biopsy-confirmed NASH and at least a 5-year history of severe or morbid obesity as well as at least one comorbidity, such as diabetes mellitus or arterial hypertension. Patients were not heavy drinkers, and none had detectable markers of chronic liver disease.
Bariatric surgery produced a median 12-kg/m2 drop in body mass index. At 5-year follow-up, 93% of patients meeting or exceeding this threshold who had biopsies performed showed resolution of NASH without worsening of fibrosis. Furthermore, 56% of patients (95% CI, 42.4%-69.3%) had no histologic evidence of fibrosis, including 45.5% of patients who had bridging fibrosis at baseline.
Participants in this study received intensive preoperative support, including evaluations by numerous specialists, a nutrition plan, and a 6- to 12-month therapeutic education program. Bariatric surgery techniques included Roux-en-Y gastric bypass, gastric banding, and sleeve gastrectomy. A subgroup analysis linked gastric bypass to a significantly higher probability of meeting the primary endpoint, compared with gastric banding. Refusal was the most common reason for not having a follow-up biopsy, the researchers said. “Patients without liver biopsy after bariatric surgery were not significantly different from those with a histological follow-up except for a lower BMI at 1 year. Baseline fibrosis did not influence the probability of undergoing histological reevaluation at 5 years.”
Two study participants died from surgical complications within 1 month after surgery, and one patient died from cardiac dysfunction 4 years later. No fatality was deemed liver related.
The study was funded by the French Ministry of Health, Conseil Régional Nord-Pas de Calais, National de la Recherche, and the European commission (FEDER). The researchers reported having no conflicts of interest.
SOURCE: Lassailly G et al. Gastroenterology. 2020 Jun 15. doi: 10.1053/j.gastro.2020.06.006.
Bariatric surgery resolved nonalcoholic steatohepatitis (NASH) without worsening fibrosis in 84% of patients with evaluable biopsies, according to the findings of a prospective study.
The study included 180 severely or morbidly obese adults (body mass index >35 kg/m2) with NASH who underwent bariatric surgery at a center in France. Among 94 patients evaluated 5 years later, 68% had follow-up liver biopsies, of whom 84% (95% confidence interval, 73.1%-92.2%) met the primary endpoint of resolution of NASH without worsening of fibrosis. All histologic aspects of NASH had improved, median nonalcoholic fatty liver disease scores (NAS) fell from 5 (interquartile range, 4 to 5) to 1 (IQR, 0-2; P < .001), and 90% of patients achieved at least a 2-point NAS improvement. Hepatocellular ballooning also improved in 87.5% of patients. Baseline severity of NASH did not affect the chances of it resolving at 5 years. “The reduction of fibrosis [was] progressive, beginning during the first year and continuing through 5 years,” Guillaume Lassailly, MD, and associates wrote in Gastroenterology.
NASH is a priority for clinical research because of the substantial risk for subsequent cirrhosis, added Dr. Lassailly of CHU Lille (France). For NASH to resolve, most patients need to lose at least 7%-10% of their body weight, but “only 10% of patients reach this objective with lifestyle therapy at 1 year, and less than half maintain the weight loss 5 years later.” Despite ongoing drug development efforts, no medications have been approved for treating NASH. Although weight loss after bariatric surgery has been reported to resolve NASH in approximately 80% of patients at 1 year, longer-term data have been unavailable, and it has remained unclear whether bariatric surgery can slow or halt fibrosis progression.
All patients in this study had biopsy-confirmed NASH and at least a 5-year history of severe or morbid obesity as well as at least one comorbidity, such as diabetes mellitus or arterial hypertension. Patients were not heavy drinkers, and none had detectable markers of chronic liver disease.
Bariatric surgery produced a median 12-kg/m2 drop in body mass index. At 5-year follow-up, 93% of patients meeting or exceeding this threshold who had biopsies performed showed resolution of NASH without worsening of fibrosis. Furthermore, 56% of patients (95% CI, 42.4%-69.3%) had no histologic evidence of fibrosis, including 45.5% of patients who had bridging fibrosis at baseline.
Participants in this study received intensive preoperative support, including evaluations by numerous specialists, a nutrition plan, and a 6- to 12-month therapeutic education program. Bariatric surgery techniques included Roux-en-Y gastric bypass, gastric banding, and sleeve gastrectomy. A subgroup analysis linked gastric bypass to a significantly higher probability of meeting the primary endpoint, compared with gastric banding. Refusal was the most common reason for not having a follow-up biopsy, the researchers said. “Patients without liver biopsy after bariatric surgery were not significantly different from those with a histological follow-up except for a lower BMI at 1 year. Baseline fibrosis did not influence the probability of undergoing histological reevaluation at 5 years.”
Two study participants died from surgical complications within 1 month after surgery, and one patient died from cardiac dysfunction 4 years later. No fatality was deemed liver related.
The study was funded by the French Ministry of Health, Conseil Régional Nord-Pas de Calais, National de la Recherche, and the European commission (FEDER). The researchers reported having no conflicts of interest.
SOURCE: Lassailly G et al. Gastroenterology. 2020 Jun 15. doi: 10.1053/j.gastro.2020.06.006.
FROM GASTROENTEROLOGY
Increasing hepatitis C treatment may curb hepatocellular carcinoma
Widespread treatment of hepatitis C virus significantly reduced the risk of hepatocellular carcinoma, based on 18 years of data from patients in Veterans Health Administration hospitals.
Although eradication of hepatitis C virus (HCV) infections has been shown to reduce the risk of hepatocellular carcinoma (HCC), effective direct-acting antiviral therapies available since 2013 appear underused in the United States, with a 14% cure rate for HCV patients as of 2016, wrote Lauren A. Beste, MD, of Veterans Affairs Puget Sound Health Care System, Seattle, and colleagues.
However, “the Veterans Health Administration, the largest integrated health care system in the U.S., provides unrestricted access to HCV treatments and approximately 85% of its case load has achieved cure,” the researchers said.
In a letter published in JAMA, the researchers identified all patients in the VHA diagnosed with HCC based on electronic health records for each year between 2002 and 2018. HCV infection was based on any history of detectable viral load, and HCV cure was defined as a negative viral load at least 12 weeks following completion of antiviral treatment, the researchers said.
“We categorized patients into 3 groups as of the time of HCC diagnosis: HCC/HCV viremic (latest HCV RNA before HCC diagnosis was positive), HCC/HCV cured (HCV eradicated before HCC diagnosis), and HCC/non-HCV (no positive lifetime HCV RNA),” they explained.
The sum of HCC/HCV viremic plus HCC/HCV cured made up the HCC/HCV total. Overall, the incidence of HCC/HCV total increased from 2000 to 2015, peaked at 31.0 per 100,000 patients in 2015, and decreased to 21.8 per 100,000 in 2018 after the introduction of viral eradication efforts from 2014 to 2016.
HCV treatment shows value despite lack of causality
Although the study could not prove causality, “the timing of HCV eradication and declining HCC incidence, lack of decline in non–HCV-related HCC, and prior studies demonstrating that HCV eradication reduces HCC risk, provide indirect evidence that this decline may be related to widespread HCV treatment,” the researchers said.
The findings were limited by several factors including the observational study design, use of the International Classification of Diseases to define HCC, use of a VA population that might limit generalizability, and lack of data on the severity of disease prior to treatment, the researchers noted. However, “HCC incidence trends should continue to be monitored closely because patients cured of HCV may have yet to experience the full potential of risk reduction,” and the study results support large-scale campaigns to eliminate HCV as well as monitoring for HCC and HCV patients who achieve disease eradication, they concluded.
The study was supported in part by grants from the National Institutes of Health/National Cancer Institute and the Veterans Affairs Clinical Science Research and Development. The researchers had no financial conflicts to disclose.
SOURCE: Beste LA et al. JAMA. 2020 Sep 8;324(10):1003-5.
Widespread treatment of hepatitis C virus significantly reduced the risk of hepatocellular carcinoma, based on 18 years of data from patients in Veterans Health Administration hospitals.
Although eradication of hepatitis C virus (HCV) infections has been shown to reduce the risk of hepatocellular carcinoma (HCC), effective direct-acting antiviral therapies available since 2013 appear underused in the United States, with a 14% cure rate for HCV patients as of 2016, wrote Lauren A. Beste, MD, of Veterans Affairs Puget Sound Health Care System, Seattle, and colleagues.
However, “the Veterans Health Administration, the largest integrated health care system in the U.S., provides unrestricted access to HCV treatments and approximately 85% of its case load has achieved cure,” the researchers said.
In a letter published in JAMA, the researchers identified all patients in the VHA diagnosed with HCC based on electronic health records for each year between 2002 and 2018. HCV infection was based on any history of detectable viral load, and HCV cure was defined as a negative viral load at least 12 weeks following completion of antiviral treatment, the researchers said.
“We categorized patients into 3 groups as of the time of HCC diagnosis: HCC/HCV viremic (latest HCV RNA before HCC diagnosis was positive), HCC/HCV cured (HCV eradicated before HCC diagnosis), and HCC/non-HCV (no positive lifetime HCV RNA),” they explained.
The sum of HCC/HCV viremic plus HCC/HCV cured made up the HCC/HCV total. Overall, the incidence of HCC/HCV total increased from 2000 to 2015, peaked at 31.0 per 100,000 patients in 2015, and decreased to 21.8 per 100,000 in 2018 after the introduction of viral eradication efforts from 2014 to 2016.
HCV treatment shows value despite lack of causality
Although the study could not prove causality, “the timing of HCV eradication and declining HCC incidence, lack of decline in non–HCV-related HCC, and prior studies demonstrating that HCV eradication reduces HCC risk, provide indirect evidence that this decline may be related to widespread HCV treatment,” the researchers said.
The findings were limited by several factors including the observational study design, use of the International Classification of Diseases to define HCC, use of a VA population that might limit generalizability, and lack of data on the severity of disease prior to treatment, the researchers noted. However, “HCC incidence trends should continue to be monitored closely because patients cured of HCV may have yet to experience the full potential of risk reduction,” and the study results support large-scale campaigns to eliminate HCV as well as monitoring for HCC and HCV patients who achieve disease eradication, they concluded.
The study was supported in part by grants from the National Institutes of Health/National Cancer Institute and the Veterans Affairs Clinical Science Research and Development. The researchers had no financial conflicts to disclose.
SOURCE: Beste LA et al. JAMA. 2020 Sep 8;324(10):1003-5.
Widespread treatment of hepatitis C virus significantly reduced the risk of hepatocellular carcinoma, based on 18 years of data from patients in Veterans Health Administration hospitals.
Although eradication of hepatitis C virus (HCV) infections has been shown to reduce the risk of hepatocellular carcinoma (HCC), effective direct-acting antiviral therapies available since 2013 appear underused in the United States, with a 14% cure rate for HCV patients as of 2016, wrote Lauren A. Beste, MD, of Veterans Affairs Puget Sound Health Care System, Seattle, and colleagues.
However, “the Veterans Health Administration, the largest integrated health care system in the U.S., provides unrestricted access to HCV treatments and approximately 85% of its case load has achieved cure,” the researchers said.
In a letter published in JAMA, the researchers identified all patients in the VHA diagnosed with HCC based on electronic health records for each year between 2002 and 2018. HCV infection was based on any history of detectable viral load, and HCV cure was defined as a negative viral load at least 12 weeks following completion of antiviral treatment, the researchers said.
“We categorized patients into 3 groups as of the time of HCC diagnosis: HCC/HCV viremic (latest HCV RNA before HCC diagnosis was positive), HCC/HCV cured (HCV eradicated before HCC diagnosis), and HCC/non-HCV (no positive lifetime HCV RNA),” they explained.
The sum of HCC/HCV viremic plus HCC/HCV cured made up the HCC/HCV total. Overall, the incidence of HCC/HCV total increased from 2000 to 2015, peaked at 31.0 per 100,000 patients in 2015, and decreased to 21.8 per 100,000 in 2018 after the introduction of viral eradication efforts from 2014 to 2016.
HCV treatment shows value despite lack of causality
Although the study could not prove causality, “the timing of HCV eradication and declining HCC incidence, lack of decline in non–HCV-related HCC, and prior studies demonstrating that HCV eradication reduces HCC risk, provide indirect evidence that this decline may be related to widespread HCV treatment,” the researchers said.
The findings were limited by several factors including the observational study design, use of the International Classification of Diseases to define HCC, use of a VA population that might limit generalizability, and lack of data on the severity of disease prior to treatment, the researchers noted. However, “HCC incidence trends should continue to be monitored closely because patients cured of HCV may have yet to experience the full potential of risk reduction,” and the study results support large-scale campaigns to eliminate HCV as well as monitoring for HCC and HCV patients who achieve disease eradication, they concluded.
The study was supported in part by grants from the National Institutes of Health/National Cancer Institute and the Veterans Affairs Clinical Science Research and Development. The researchers had no financial conflicts to disclose.
SOURCE: Beste LA et al. JAMA. 2020 Sep 8;324(10):1003-5.
FROM JAMA
Tools emerging to predict liver failure in cirrhosis
Systemic inflammation and portal hypertension are key predictors of acute-on-chronic liver failure (ACLF) in the 3 months after a hospital stay for acute decompensated cirrhosis and also of death after 12 months, a preliminary analysis of data from the PREDICT study shows.
“Before this, we never had any patient signatures to identify ACLF,” said Jonel Trebicka, MD, PhD, from the JW Goethe University Hospital in Frankfurt, Germany.
Now, Dr. Trebicka’s team has “characterized the phenotypes in pre-ACLF that will progress within 3 months,” he said in an interview. “Those with high levels of inflammatory proteins, white blood cell count, are more likely to develop ACLF.”
ACLF is a highly complex disorder that can lead liver, cardiovascular, renal, cerebral, pulmonary, intestinal, adrenal, and immune systems to fail, Dr. Trebicka explained when he discussed the analysis – published online in the Journal of Hepatology – during the virtual International Liver Congress (ILC) 2020.
The chance of survival after the onset of ACLF is low – the 28-day survival rate is 30% – and “the only treatment we have is liver transplant,” he said.
For their prospective observational study, Dr. Trebicka and his colleagues assessed 1071 participants from 48 European hospitals in 14 countries who were admitted for an episode of acute decompensation, defined as the development of ascites, hepatic encephalopathy, gastrointestinal hemorrhage, infection, or a combination thereof.
The researchers identified three distinct clinical courses for a patient hospitalized with acute decompensated cirrhosis that will help clinicians predict the development of ACLF.
At study enrollment, more than half of the patients at highest risk for ACLF had pre-ACLF and high-grade systemic inflammation. The patients at intermediate risk had unstable decompensated cirrhosis with low-grade systemic inflammation and complications related to severe portal hypertension. And those at lowest risk for ACLF had stable decompensated cirrhosis and no severe systemic inflammation or portal hypertension complications, and did not develop ACLF or another episode of acute decompensation in the subsequent 3 months.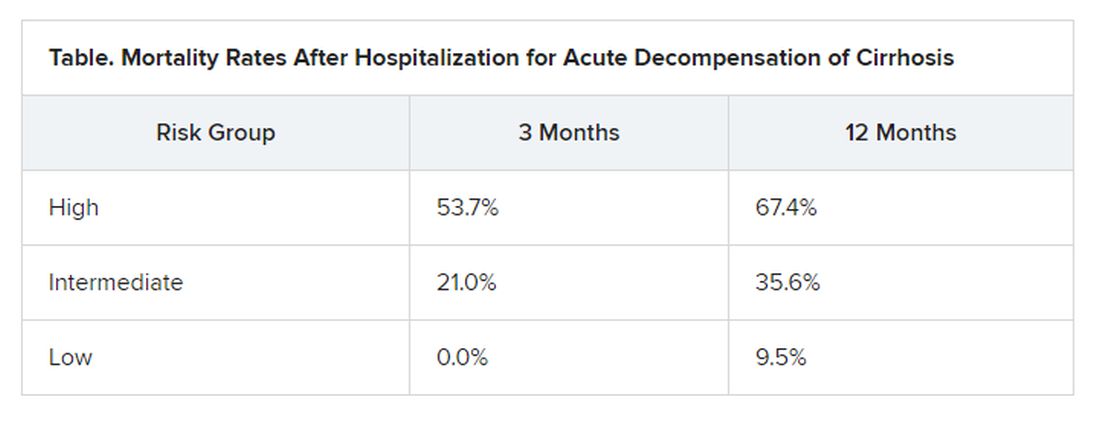
“There have been hints of possible phenotypes before – for stable and unstable ACLF – but we never had anything specific to diagnose,” Trebicka reported.
“We found that there are two main mechanisms in the development of ACLF that are most important,” he said. The first is systemic inflammation with high levels of proteins, which “leads to organ failure. This is the most striking acute mechanism.”
The second is the development of portal hypertension. “This is slower, but also very important, causing increased pressure in the portal vein, and leading to bleeding if the pressure is too great,” he said.
More tools emerging to help predict ACLF
The Albumin-functionality-test (AFT), which uses serum albumin levels to evaluate liver and kidney function, might also be useful in the prediction of ACLF and 12-month survival, according to a separate study an Italian group presented at the virtual ILC.
“Our main results are that parameters from albumin predict the development of ACLF in acute decompensated patients with the same diagnostic performance as the CLIF-AD score,” said Katja Waterstradt, PhD, from the University of Bologna in Italy.
And when the two tests are combined, diagnostic performance is increased, she added.
Dr. Trebicka has disclosed no relevant financial relationships. Dr. Waterstrand is a researcher for MedInnovation GmbH.
This article first appeared on Medscape.com.
Systemic inflammation and portal hypertension are key predictors of acute-on-chronic liver failure (ACLF) in the 3 months after a hospital stay for acute decompensated cirrhosis and also of death after 12 months, a preliminary analysis of data from the PREDICT study shows.
“Before this, we never had any patient signatures to identify ACLF,” said Jonel Trebicka, MD, PhD, from the JW Goethe University Hospital in Frankfurt, Germany.
Now, Dr. Trebicka’s team has “characterized the phenotypes in pre-ACLF that will progress within 3 months,” he said in an interview. “Those with high levels of inflammatory proteins, white blood cell count, are more likely to develop ACLF.”
ACLF is a highly complex disorder that can lead liver, cardiovascular, renal, cerebral, pulmonary, intestinal, adrenal, and immune systems to fail, Dr. Trebicka explained when he discussed the analysis – published online in the Journal of Hepatology – during the virtual International Liver Congress (ILC) 2020.
The chance of survival after the onset of ACLF is low – the 28-day survival rate is 30% – and “the only treatment we have is liver transplant,” he said.
For their prospective observational study, Dr. Trebicka and his colleagues assessed 1071 participants from 48 European hospitals in 14 countries who were admitted for an episode of acute decompensation, defined as the development of ascites, hepatic encephalopathy, gastrointestinal hemorrhage, infection, or a combination thereof.
The researchers identified three distinct clinical courses for a patient hospitalized with acute decompensated cirrhosis that will help clinicians predict the development of ACLF.
At study enrollment, more than half of the patients at highest risk for ACLF had pre-ACLF and high-grade systemic inflammation. The patients at intermediate risk had unstable decompensated cirrhosis with low-grade systemic inflammation and complications related to severe portal hypertension. And those at lowest risk for ACLF had stable decompensated cirrhosis and no severe systemic inflammation or portal hypertension complications, and did not develop ACLF or another episode of acute decompensation in the subsequent 3 months.
“There have been hints of possible phenotypes before – for stable and unstable ACLF – but we never had anything specific to diagnose,” Trebicka reported.
“We found that there are two main mechanisms in the development of ACLF that are most important,” he said. The first is systemic inflammation with high levels of proteins, which “leads to organ failure. This is the most striking acute mechanism.”
The second is the development of portal hypertension. “This is slower, but also very important, causing increased pressure in the portal vein, and leading to bleeding if the pressure is too great,” he said.
More tools emerging to help predict ACLF
The Albumin-functionality-test (AFT), which uses serum albumin levels to evaluate liver and kidney function, might also be useful in the prediction of ACLF and 12-month survival, according to a separate study an Italian group presented at the virtual ILC.
“Our main results are that parameters from albumin predict the development of ACLF in acute decompensated patients with the same diagnostic performance as the CLIF-AD score,” said Katja Waterstradt, PhD, from the University of Bologna in Italy.
And when the two tests are combined, diagnostic performance is increased, she added.
Dr. Trebicka has disclosed no relevant financial relationships. Dr. Waterstrand is a researcher for MedInnovation GmbH.
This article first appeared on Medscape.com.
Systemic inflammation and portal hypertension are key predictors of acute-on-chronic liver failure (ACLF) in the 3 months after a hospital stay for acute decompensated cirrhosis and also of death after 12 months, a preliminary analysis of data from the PREDICT study shows.
“Before this, we never had any patient signatures to identify ACLF,” said Jonel Trebicka, MD, PhD, from the JW Goethe University Hospital in Frankfurt, Germany.
Now, Dr. Trebicka’s team has “characterized the phenotypes in pre-ACLF that will progress within 3 months,” he said in an interview. “Those with high levels of inflammatory proteins, white blood cell count, are more likely to develop ACLF.”
ACLF is a highly complex disorder that can lead liver, cardiovascular, renal, cerebral, pulmonary, intestinal, adrenal, and immune systems to fail, Dr. Trebicka explained when he discussed the analysis – published online in the Journal of Hepatology – during the virtual International Liver Congress (ILC) 2020.
The chance of survival after the onset of ACLF is low – the 28-day survival rate is 30% – and “the only treatment we have is liver transplant,” he said.
For their prospective observational study, Dr. Trebicka and his colleagues assessed 1071 participants from 48 European hospitals in 14 countries who were admitted for an episode of acute decompensation, defined as the development of ascites, hepatic encephalopathy, gastrointestinal hemorrhage, infection, or a combination thereof.
The researchers identified three distinct clinical courses for a patient hospitalized with acute decompensated cirrhosis that will help clinicians predict the development of ACLF.
At study enrollment, more than half of the patients at highest risk for ACLF had pre-ACLF and high-grade systemic inflammation. The patients at intermediate risk had unstable decompensated cirrhosis with low-grade systemic inflammation and complications related to severe portal hypertension. And those at lowest risk for ACLF had stable decompensated cirrhosis and no severe systemic inflammation or portal hypertension complications, and did not develop ACLF or another episode of acute decompensation in the subsequent 3 months.
“There have been hints of possible phenotypes before – for stable and unstable ACLF – but we never had anything specific to diagnose,” Trebicka reported.
“We found that there are two main mechanisms in the development of ACLF that are most important,” he said. The first is systemic inflammation with high levels of proteins, which “leads to organ failure. This is the most striking acute mechanism.”
The second is the development of portal hypertension. “This is slower, but also very important, causing increased pressure in the portal vein, and leading to bleeding if the pressure is too great,” he said.
More tools emerging to help predict ACLF
The Albumin-functionality-test (AFT), which uses serum albumin levels to evaluate liver and kidney function, might also be useful in the prediction of ACLF and 12-month survival, according to a separate study an Italian group presented at the virtual ILC.
“Our main results are that parameters from albumin predict the development of ACLF in acute decompensated patients with the same diagnostic performance as the CLIF-AD score,” said Katja Waterstradt, PhD, from the University of Bologna in Italy.
And when the two tests are combined, diagnostic performance is increased, she added.
Dr. Trebicka has disclosed no relevant financial relationships. Dr. Waterstrand is a researcher for MedInnovation GmbH.
This article first appeared on Medscape.com.
Liver transplant doesn’t raise COVID death risk
A history of liver transplant conveyed no increased risk of death from COVID-19 infections, according to data from a multicenter cohort study of 151 transplant recipients who became infected.
Although current data suggest a possible increased risk of adverse outcomes if liver transplant patients develop COVID-19 infections, the effects remain unclear, wrote Gwilym J. Webb, PhD, of the University of Oxford (England) and colleagues.
In a study published in the Lancet Gastroenterology & Hepatology, the researchers identified adults from 18 countries who had laboratory-confirmed COVID-19 infections between March 25, 2020, and June 26, 2020. The average age of the patients was 60 years, and 68% were men. A contemporaneous group of 627 consecutive adults with confirmed COVID-19 infections who had not undergone liver transplants served as controls.
Overall, 28 of the liver transplant patients and 167 of the controls died (19% vs. 27%; P = .046).
In addition, no differences appeared between infected transplant patients and infected controls in terms of hospitalization (82% vs. 76%) and the need for intensive care (31% vs. 30%), although the transplant patients were significantly more likely to require invasive ventilation (20% vs. 5%).
However, in a multivariate analysis, older age, serum creatinine concentration, and the presence of nonliver cancers were independently associated with increased risk of death in the liver transplant patients, with odds ratios of 1.06 for each year increase in age, 1.57 for each mg/dL increase in serum creatinine concentration, and 18.30 with the presence of a nonliver cancer.
The study findings were limited by several factors including the potential overreporting of severe COVID-19 cases because of reporting bias in the transplant registry, as well as the inability of the sample size to rule out mortality differences, the differences in comorbidities between the transplant patients and controls, and the impact of unmeasured confounding variables such as diet, physical activity, or fibrosis or cirrhosis in recipient grafts, the researchers noted. However, the results suggest that a history of liver transplantation does not increase the risk of death following COVID-19 infection, they wrote.
“Thus, traditional risk factors for adverse outcomes from COVID-19 should be preferentially considered when considering the risks and benefits of hospital attendance, immunosuppression, and social-distancing requirements for liver transplant recipients during the ongoing COVID-19 pandemic,” they concluded.
Focus on comorbidities and combined transplants
“Given that age and presence of comorbidities were significantly associated with risk of death in the cohorts of patients who had and had not undergone liver transplantation, greater emphasis should be placed on other coexisting comorbidities, rather than transplantation status per se, when risk-stratifying liver transplant recipients,” the researchers noted. “Indirectly, these findings suggest that liver transplantation, where indicated, should not be delayed during the COVID-19 pandemic, and that supportive care should not be limited for patients with existing liver transplants with COVID-19,” they suggested.
Given the high prevalence of COVID-19 in many countries, “it is inevitable that liver transplant patients will become infected,” said Wajahat Mehal, MD, of Yale University, New Haven, Conn., in an interview.
Going forward, “it is important to know the natural history of COVID in the immunocompromised population,” he emphasized.
One of the study limitations was the lack of data on how patients’ immunosuppression regimens were changed, if at all, while they were infected. “Since some other immunocompromised patients have had a higher rate of complications [in the wake of COVID-19 infections], I was pleasantly surprised that liver transplant recipients did so well,” Dr. Mehal said.
Dr. Mehal noted that additional research is needed to promote safety in patients with liver disease in the context of COVID-19. “It would be important to evaluate combined transplants, particularly combined liver/kidney transplants,” he said.
The study was supported by the European Association for the Study of the Liver, the National Institutes of Health, and the United Kingdom National Institute for Health Research. Lead author Dr. Webb had no financial conflicts to disclose. One coauthor disclosed unrelated fees from AbbVie and grants from the Fondation du Centre Hospitalier de l’Université de Montréal. Dr. Mehal had no financial conflicts to disclose.
SOURCE: Webb GJ et al. Lancet Gastroenterol Hepatol. 2020 Aug 28. doi: 10.1016/ S2468-1253(20)30271-5.
A history of liver transplant conveyed no increased risk of death from COVID-19 infections, according to data from a multicenter cohort study of 151 transplant recipients who became infected.
Although current data suggest a possible increased risk of adverse outcomes if liver transplant patients develop COVID-19 infections, the effects remain unclear, wrote Gwilym J. Webb, PhD, of the University of Oxford (England) and colleagues.
In a study published in the Lancet Gastroenterology & Hepatology, the researchers identified adults from 18 countries who had laboratory-confirmed COVID-19 infections between March 25, 2020, and June 26, 2020. The average age of the patients was 60 years, and 68% were men. A contemporaneous group of 627 consecutive adults with confirmed COVID-19 infections who had not undergone liver transplants served as controls.
Overall, 28 of the liver transplant patients and 167 of the controls died (19% vs. 27%; P = .046).
In addition, no differences appeared between infected transplant patients and infected controls in terms of hospitalization (82% vs. 76%) and the need for intensive care (31% vs. 30%), although the transplant patients were significantly more likely to require invasive ventilation (20% vs. 5%).
However, in a multivariate analysis, older age, serum creatinine concentration, and the presence of nonliver cancers were independently associated with increased risk of death in the liver transplant patients, with odds ratios of 1.06 for each year increase in age, 1.57 for each mg/dL increase in serum creatinine concentration, and 18.30 with the presence of a nonliver cancer.
The study findings were limited by several factors including the potential overreporting of severe COVID-19 cases because of reporting bias in the transplant registry, as well as the inability of the sample size to rule out mortality differences, the differences in comorbidities between the transplant patients and controls, and the impact of unmeasured confounding variables such as diet, physical activity, or fibrosis or cirrhosis in recipient grafts, the researchers noted. However, the results suggest that a history of liver transplantation does not increase the risk of death following COVID-19 infection, they wrote.
“Thus, traditional risk factors for adverse outcomes from COVID-19 should be preferentially considered when considering the risks and benefits of hospital attendance, immunosuppression, and social-distancing requirements for liver transplant recipients during the ongoing COVID-19 pandemic,” they concluded.
Focus on comorbidities and combined transplants
“Given that age and presence of comorbidities were significantly associated with risk of death in the cohorts of patients who had and had not undergone liver transplantation, greater emphasis should be placed on other coexisting comorbidities, rather than transplantation status per se, when risk-stratifying liver transplant recipients,” the researchers noted. “Indirectly, these findings suggest that liver transplantation, where indicated, should not be delayed during the COVID-19 pandemic, and that supportive care should not be limited for patients with existing liver transplants with COVID-19,” they suggested.
Given the high prevalence of COVID-19 in many countries, “it is inevitable that liver transplant patients will become infected,” said Wajahat Mehal, MD, of Yale University, New Haven, Conn., in an interview.
Going forward, “it is important to know the natural history of COVID in the immunocompromised population,” he emphasized.
One of the study limitations was the lack of data on how patients’ immunosuppression regimens were changed, if at all, while they were infected. “Since some other immunocompromised patients have had a higher rate of complications [in the wake of COVID-19 infections], I was pleasantly surprised that liver transplant recipients did so well,” Dr. Mehal said.
Dr. Mehal noted that additional research is needed to promote safety in patients with liver disease in the context of COVID-19. “It would be important to evaluate combined transplants, particularly combined liver/kidney transplants,” he said.
The study was supported by the European Association for the Study of the Liver, the National Institutes of Health, and the United Kingdom National Institute for Health Research. Lead author Dr. Webb had no financial conflicts to disclose. One coauthor disclosed unrelated fees from AbbVie and grants from the Fondation du Centre Hospitalier de l’Université de Montréal. Dr. Mehal had no financial conflicts to disclose.
SOURCE: Webb GJ et al. Lancet Gastroenterol Hepatol. 2020 Aug 28. doi: 10.1016/ S2468-1253(20)30271-5.
A history of liver transplant conveyed no increased risk of death from COVID-19 infections, according to data from a multicenter cohort study of 151 transplant recipients who became infected.
Although current data suggest a possible increased risk of adverse outcomes if liver transplant patients develop COVID-19 infections, the effects remain unclear, wrote Gwilym J. Webb, PhD, of the University of Oxford (England) and colleagues.
In a study published in the Lancet Gastroenterology & Hepatology, the researchers identified adults from 18 countries who had laboratory-confirmed COVID-19 infections between March 25, 2020, and June 26, 2020. The average age of the patients was 60 years, and 68% were men. A contemporaneous group of 627 consecutive adults with confirmed COVID-19 infections who had not undergone liver transplants served as controls.
Overall, 28 of the liver transplant patients and 167 of the controls died (19% vs. 27%; P = .046).
In addition, no differences appeared between infected transplant patients and infected controls in terms of hospitalization (82% vs. 76%) and the need for intensive care (31% vs. 30%), although the transplant patients were significantly more likely to require invasive ventilation (20% vs. 5%).
However, in a multivariate analysis, older age, serum creatinine concentration, and the presence of nonliver cancers were independently associated with increased risk of death in the liver transplant patients, with odds ratios of 1.06 for each year increase in age, 1.57 for each mg/dL increase in serum creatinine concentration, and 18.30 with the presence of a nonliver cancer.
The study findings were limited by several factors including the potential overreporting of severe COVID-19 cases because of reporting bias in the transplant registry, as well as the inability of the sample size to rule out mortality differences, the differences in comorbidities between the transplant patients and controls, and the impact of unmeasured confounding variables such as diet, physical activity, or fibrosis or cirrhosis in recipient grafts, the researchers noted. However, the results suggest that a history of liver transplantation does not increase the risk of death following COVID-19 infection, they wrote.
“Thus, traditional risk factors for adverse outcomes from COVID-19 should be preferentially considered when considering the risks and benefits of hospital attendance, immunosuppression, and social-distancing requirements for liver transplant recipients during the ongoing COVID-19 pandemic,” they concluded.
Focus on comorbidities and combined transplants
“Given that age and presence of comorbidities were significantly associated with risk of death in the cohorts of patients who had and had not undergone liver transplantation, greater emphasis should be placed on other coexisting comorbidities, rather than transplantation status per se, when risk-stratifying liver transplant recipients,” the researchers noted. “Indirectly, these findings suggest that liver transplantation, where indicated, should not be delayed during the COVID-19 pandemic, and that supportive care should not be limited for patients with existing liver transplants with COVID-19,” they suggested.
Given the high prevalence of COVID-19 in many countries, “it is inevitable that liver transplant patients will become infected,” said Wajahat Mehal, MD, of Yale University, New Haven, Conn., in an interview.
Going forward, “it is important to know the natural history of COVID in the immunocompromised population,” he emphasized.
One of the study limitations was the lack of data on how patients’ immunosuppression regimens were changed, if at all, while they were infected. “Since some other immunocompromised patients have had a higher rate of complications [in the wake of COVID-19 infections], I was pleasantly surprised that liver transplant recipients did so well,” Dr. Mehal said.
Dr. Mehal noted that additional research is needed to promote safety in patients with liver disease in the context of COVID-19. “It would be important to evaluate combined transplants, particularly combined liver/kidney transplants,” he said.
The study was supported by the European Association for the Study of the Liver, the National Institutes of Health, and the United Kingdom National Institute for Health Research. Lead author Dr. Webb had no financial conflicts to disclose. One coauthor disclosed unrelated fees from AbbVie and grants from the Fondation du Centre Hospitalier de l’Université de Montréal. Dr. Mehal had no financial conflicts to disclose.
SOURCE: Webb GJ et al. Lancet Gastroenterol Hepatol. 2020 Aug 28. doi: 10.1016/ S2468-1253(20)30271-5.
FROM THE LANCET GASTROENTEROLOGY & HEPATOLOGY





