User login
Hep C, HIV coinfection tied to higher MI risk with age
, a new analysis suggests.
By contrast, the risk increases by 30% every 10 years among PWH without HCV infection.
“There is other evidence that suggests people with HIV and HCV have a greater burden of negative health outcomes,” senior author Keri N. Althoff, PhD, MPH, of the Johns Hopkins Bloomberg School of Public Health in Baltimore, said in an interview. “But the magnitude of ‘greater’ was bigger than I expected.”
“Understanding the difference HCV can make in the risk of MI with increasing age among those with – compared to without – HCV is an important step for understanding additional potential benefits of HCV treatment (among PWH),” she said.
The amplified risk with age occurred even though, overall, the association between HCV coinfection and increased risk of type 1 myocardial infarction (T1MI) was not significant, the analysis showed.
The study was published online in the Journal of the American Heart Association.
How age counts
Dr. Althoff and colleagues analyzed data from 23,361 PWH aged 40-79 who had initiated antiretroviral therapy between 2000 and 2017. The primary outcome was T1MI.
A total of 4,677 participants (20%) had HCV. Eighty-nine T1MIs occurred among PWH with HCV (1.9%) vs. 314 among PWH without HCV (1.7%). In adjusted analyses, HCV was not associated with increased T1MI risk (adjusted hazard ratio, 0.98).
However, the risk of T1MI increased with age and was augmented in those with HCV (aHR per 10-year increase in age, 1.85) vs. those without HCV (aHR, 1.30).
Specifically, compared with those without HCV, the estimated T1MI risk was 17% higher among 50- to 59-year-olds with HCV and 77% higher among those 60 and older; neither association was statistically significant, although the authors suggest this probably was because of the smaller number of participants in the older age categories.
Even without HCV, the risk of T1MI increased in participants who had traditional risk factors. The risk was significantly higher among PWH aged 40-49 with diabetes, hypertension, chronic kidney disease, protease inhibitor (PI) use, and smoking, whereas among PWH aged 50-59, the T1MI risk was significantly greater among those with hypertension, PI use, and smoking.
Among those aged 60 or older, hypertension and low CD4 counts were associated with a significantly increased T1MI risk.
“Clinicians providing health care to people with HIV should know their patients’ HCV status,” Dr. Althoff said, “and provide support regarding HCV treatment and ways to reduce their cardiovascular risk, including smoking cessation, reaching and maintaining a healthy BMI, and substance use treatment.”
Truly additive?
American Heart Association expert volunteer Nieca Goldberg, MD, a clinical associate professor of medicine at New York University and medical director of Atria NY, said the increased T1MI risk with coinfection “makes sense” because both HIV and HCV are linked to inflammation.
However, she said in an interview, “the fact that the authors didn’t control for other, more traditional heart attack risk factors is a limitation. I would like to see a study that takes other risk factors into consideration to see if HCV is truly additive.”
Meanwhile, like Dr. Althoff, she said, “Clinicians should be taking a careful history that includes chronic infections as well as traditional heart risk factors.”
Additional studies are needed, Dr. Althoff agreed. “There are two paths we are keenly interested in pursuing. The first is understanding how metabolic risk factors for MI change after HCV treatment. We are working on this.”
“Ultimately,” she said, “we want to compare MI risk in people with HIV who had successful HCV treatment to those who have not had successful HCV treatment.”
In their current study, they had nearly 2 decades of follow-up, she noted. “Although we don’t need to wait that long, we would like to have close to a decade of potential follow-up time (since 2016, when sofosbuvir/velpatasvir became available) so that we have a large enough sample size to observe a sufficient number of MIs within the first 5 years after successful HCV treatment.”
No commercial funding or relevant disclosures were reported.
A version of this article first appeared on Medscape.com.
, a new analysis suggests.
By contrast, the risk increases by 30% every 10 years among PWH without HCV infection.
“There is other evidence that suggests people with HIV and HCV have a greater burden of negative health outcomes,” senior author Keri N. Althoff, PhD, MPH, of the Johns Hopkins Bloomberg School of Public Health in Baltimore, said in an interview. “But the magnitude of ‘greater’ was bigger than I expected.”
“Understanding the difference HCV can make in the risk of MI with increasing age among those with – compared to without – HCV is an important step for understanding additional potential benefits of HCV treatment (among PWH),” she said.
The amplified risk with age occurred even though, overall, the association between HCV coinfection and increased risk of type 1 myocardial infarction (T1MI) was not significant, the analysis showed.
The study was published online in the Journal of the American Heart Association.
How age counts
Dr. Althoff and colleagues analyzed data from 23,361 PWH aged 40-79 who had initiated antiretroviral therapy between 2000 and 2017. The primary outcome was T1MI.
A total of 4,677 participants (20%) had HCV. Eighty-nine T1MIs occurred among PWH with HCV (1.9%) vs. 314 among PWH without HCV (1.7%). In adjusted analyses, HCV was not associated with increased T1MI risk (adjusted hazard ratio, 0.98).
However, the risk of T1MI increased with age and was augmented in those with HCV (aHR per 10-year increase in age, 1.85) vs. those without HCV (aHR, 1.30).
Specifically, compared with those without HCV, the estimated T1MI risk was 17% higher among 50- to 59-year-olds with HCV and 77% higher among those 60 and older; neither association was statistically significant, although the authors suggest this probably was because of the smaller number of participants in the older age categories.
Even without HCV, the risk of T1MI increased in participants who had traditional risk factors. The risk was significantly higher among PWH aged 40-49 with diabetes, hypertension, chronic kidney disease, protease inhibitor (PI) use, and smoking, whereas among PWH aged 50-59, the T1MI risk was significantly greater among those with hypertension, PI use, and smoking.
Among those aged 60 or older, hypertension and low CD4 counts were associated with a significantly increased T1MI risk.
“Clinicians providing health care to people with HIV should know their patients’ HCV status,” Dr. Althoff said, “and provide support regarding HCV treatment and ways to reduce their cardiovascular risk, including smoking cessation, reaching and maintaining a healthy BMI, and substance use treatment.”
Truly additive?
American Heart Association expert volunteer Nieca Goldberg, MD, a clinical associate professor of medicine at New York University and medical director of Atria NY, said the increased T1MI risk with coinfection “makes sense” because both HIV and HCV are linked to inflammation.
However, she said in an interview, “the fact that the authors didn’t control for other, more traditional heart attack risk factors is a limitation. I would like to see a study that takes other risk factors into consideration to see if HCV is truly additive.”
Meanwhile, like Dr. Althoff, she said, “Clinicians should be taking a careful history that includes chronic infections as well as traditional heart risk factors.”
Additional studies are needed, Dr. Althoff agreed. “There are two paths we are keenly interested in pursuing. The first is understanding how metabolic risk factors for MI change after HCV treatment. We are working on this.”
“Ultimately,” she said, “we want to compare MI risk in people with HIV who had successful HCV treatment to those who have not had successful HCV treatment.”
In their current study, they had nearly 2 decades of follow-up, she noted. “Although we don’t need to wait that long, we would like to have close to a decade of potential follow-up time (since 2016, when sofosbuvir/velpatasvir became available) so that we have a large enough sample size to observe a sufficient number of MIs within the first 5 years after successful HCV treatment.”
No commercial funding or relevant disclosures were reported.
A version of this article first appeared on Medscape.com.
, a new analysis suggests.
By contrast, the risk increases by 30% every 10 years among PWH without HCV infection.
“There is other evidence that suggests people with HIV and HCV have a greater burden of negative health outcomes,” senior author Keri N. Althoff, PhD, MPH, of the Johns Hopkins Bloomberg School of Public Health in Baltimore, said in an interview. “But the magnitude of ‘greater’ was bigger than I expected.”
“Understanding the difference HCV can make in the risk of MI with increasing age among those with – compared to without – HCV is an important step for understanding additional potential benefits of HCV treatment (among PWH),” she said.
The amplified risk with age occurred even though, overall, the association between HCV coinfection and increased risk of type 1 myocardial infarction (T1MI) was not significant, the analysis showed.
The study was published online in the Journal of the American Heart Association.
How age counts
Dr. Althoff and colleagues analyzed data from 23,361 PWH aged 40-79 who had initiated antiretroviral therapy between 2000 and 2017. The primary outcome was T1MI.
A total of 4,677 participants (20%) had HCV. Eighty-nine T1MIs occurred among PWH with HCV (1.9%) vs. 314 among PWH without HCV (1.7%). In adjusted analyses, HCV was not associated with increased T1MI risk (adjusted hazard ratio, 0.98).
However, the risk of T1MI increased with age and was augmented in those with HCV (aHR per 10-year increase in age, 1.85) vs. those without HCV (aHR, 1.30).
Specifically, compared with those without HCV, the estimated T1MI risk was 17% higher among 50- to 59-year-olds with HCV and 77% higher among those 60 and older; neither association was statistically significant, although the authors suggest this probably was because of the smaller number of participants in the older age categories.
Even without HCV, the risk of T1MI increased in participants who had traditional risk factors. The risk was significantly higher among PWH aged 40-49 with diabetes, hypertension, chronic kidney disease, protease inhibitor (PI) use, and smoking, whereas among PWH aged 50-59, the T1MI risk was significantly greater among those with hypertension, PI use, and smoking.
Among those aged 60 or older, hypertension and low CD4 counts were associated with a significantly increased T1MI risk.
“Clinicians providing health care to people with HIV should know their patients’ HCV status,” Dr. Althoff said, “and provide support regarding HCV treatment and ways to reduce their cardiovascular risk, including smoking cessation, reaching and maintaining a healthy BMI, and substance use treatment.”
Truly additive?
American Heart Association expert volunteer Nieca Goldberg, MD, a clinical associate professor of medicine at New York University and medical director of Atria NY, said the increased T1MI risk with coinfection “makes sense” because both HIV and HCV are linked to inflammation.
However, she said in an interview, “the fact that the authors didn’t control for other, more traditional heart attack risk factors is a limitation. I would like to see a study that takes other risk factors into consideration to see if HCV is truly additive.”
Meanwhile, like Dr. Althoff, she said, “Clinicians should be taking a careful history that includes chronic infections as well as traditional heart risk factors.”
Additional studies are needed, Dr. Althoff agreed. “There are two paths we are keenly interested in pursuing. The first is understanding how metabolic risk factors for MI change after HCV treatment. We are working on this.”
“Ultimately,” she said, “we want to compare MI risk in people with HIV who had successful HCV treatment to those who have not had successful HCV treatment.”
In their current study, they had nearly 2 decades of follow-up, she noted. “Although we don’t need to wait that long, we would like to have close to a decade of potential follow-up time (since 2016, when sofosbuvir/velpatasvir became available) so that we have a large enough sample size to observe a sufficient number of MIs within the first 5 years after successful HCV treatment.”
No commercial funding or relevant disclosures were reported.
A version of this article first appeared on Medscape.com.
FROM JOURNAL OF THE AMERICAN HEART ASSOCIATION
New liver stiffness thresholds refine NASH risk stratification
New liver stiffness (LS) thresholds offer accurate prediction of disease progression and clinical outcomes in patients with nonalcoholic steatohepatitis (NASH) and advanced fibrosis, according to investigators.
These new LS thresholds are more reliable because they are based on high-quality prospective data drawn from four randomized controlled trials, reported lead author Rohit Loomba, MD, of the University of California, San Diego, and colleagues.
“Retrospective studies report that increasing baseline LS by VCTE [vibration-controlled transient elastography] is associated with the risk of disease progression in patients with NAFLD [non-alcoholic fatty liver disease], but prospective data in well-characterized NASH cohorts with biopsy-confirmed advanced fibrosis are limited,” the investigators wrote in Gut. “The optimal LS thresholds for prognostication of fibrosis progression and decompensation are unknown.”
Seeking clarity, Dr. Loomba and colleagues leveraged data from two phase 3 placebo-controlled trials for selonsertib and two phase 2b placebo-controlled trials for simtuzumab.
“While the studies were discontinued prematurely due to lack of efficacy, the prospectively collected data in these well-characterized participants with serial liver biopsies provides a unique opportunity to study the association between baseline LS by VCTE and disease progression,” the investigators wrote.
Across all four studies, bridging fibrosis (F3) was present in 664 participants, while 734 individuals had cirrhosis (F4). In the selonsertib studies, fibrosis was staged at baseline and week 48. The simtuzumab studies measured liver fibrosis at baseline and week 96. Out of the 664 participants with bridging fibrosis, 103 (16%) progressed to cirrhosis. Among the 734 patients with cirrhosis, 27 (4%) experienced liver-related events. Comparing these outcomes with LS data at baseline and throughout the study revealed optimal LS thresholds.
The best threshold for predicting progression from bridging fibrosis to cirrhosis was 16.6 kPa. According to the authors, the sensitivity, specificity, positive predictive value, and negative predictive value of this threshold for progression to cirrhosis were 58%, 76%, 31%, and 91%, respectively. Among patients at or above 16.6 kPa, 31% progressed to cirrhosis, compared with 9.1% of those under that threshold. Furthermore, individuals with a baseline LS at or above 16.6 kPa had nearly four times greater risk of developing cirrhosis (adjusted hazard ratio, 3.99; 95% CI, 2.66-5.98; P < .0001).
For patients with cirrhosis at baseline, the optimal threshold for predicting liver-related events, such as ascites, hepatic encephalopathy, and portal hypertension–related GI bleeding, liver transplantation, or mortality, was 30.7 kPa. The sensitivity, specificity, PPV, and NPV of this threshold for liver-related events were 62%, 87%, 10%, and 99%, respectively, according to the authors. Patients with an LS above this mark were 10 times as likely to experience liver-related events (aHR, 10.13; 95% CI, 4.38-23.41; P < .0001).
Scott L. Friedman, MD, chief of the division of liver diseases and dean for Therapeutic Discovery at the Icahn School of Medicine at Mount Sinai, New York, called the study “an important effort” that offers valuable insights for both researchers and practitioners.
“For clinical trials, [these thresholds] really allow for greater refinement or enrichment of patients who are suitable for enrollment in the trial because they’re at a higher risk of clinical problems that might be mitigated if the drug is effective,” Dr. Friedman said in an interview. “For clinical practice, it might indicate that the patient should either be fast tracked for a clinical trial or, more importantly, maybe needs to be referred for evaluation for a liver transplant. It may also indicate – although they didn’t look at it in this study – that there’s a need to begin or accelerate screening for liver cancer, which becomes an encroaching risk as the fibrosis advances to later stages.”
The study was funded by Gilead Sciences. The investigators disclosed additional relationships with Amgen, Eli Lilly, CohBar, and others. Dr. Friedman reported no relevant conflicts of interest.
New liver stiffness (LS) thresholds offer accurate prediction of disease progression and clinical outcomes in patients with nonalcoholic steatohepatitis (NASH) and advanced fibrosis, according to investigators.
These new LS thresholds are more reliable because they are based on high-quality prospective data drawn from four randomized controlled trials, reported lead author Rohit Loomba, MD, of the University of California, San Diego, and colleagues.
“Retrospective studies report that increasing baseline LS by VCTE [vibration-controlled transient elastography] is associated with the risk of disease progression in patients with NAFLD [non-alcoholic fatty liver disease], but prospective data in well-characterized NASH cohorts with biopsy-confirmed advanced fibrosis are limited,” the investigators wrote in Gut. “The optimal LS thresholds for prognostication of fibrosis progression and decompensation are unknown.”
Seeking clarity, Dr. Loomba and colleagues leveraged data from two phase 3 placebo-controlled trials for selonsertib and two phase 2b placebo-controlled trials for simtuzumab.
“While the studies were discontinued prematurely due to lack of efficacy, the prospectively collected data in these well-characterized participants with serial liver biopsies provides a unique opportunity to study the association between baseline LS by VCTE and disease progression,” the investigators wrote.
Across all four studies, bridging fibrosis (F3) was present in 664 participants, while 734 individuals had cirrhosis (F4). In the selonsertib studies, fibrosis was staged at baseline and week 48. The simtuzumab studies measured liver fibrosis at baseline and week 96. Out of the 664 participants with bridging fibrosis, 103 (16%) progressed to cirrhosis. Among the 734 patients with cirrhosis, 27 (4%) experienced liver-related events. Comparing these outcomes with LS data at baseline and throughout the study revealed optimal LS thresholds.
The best threshold for predicting progression from bridging fibrosis to cirrhosis was 16.6 kPa. According to the authors, the sensitivity, specificity, positive predictive value, and negative predictive value of this threshold for progression to cirrhosis were 58%, 76%, 31%, and 91%, respectively. Among patients at or above 16.6 kPa, 31% progressed to cirrhosis, compared with 9.1% of those under that threshold. Furthermore, individuals with a baseline LS at or above 16.6 kPa had nearly four times greater risk of developing cirrhosis (adjusted hazard ratio, 3.99; 95% CI, 2.66-5.98; P < .0001).
For patients with cirrhosis at baseline, the optimal threshold for predicting liver-related events, such as ascites, hepatic encephalopathy, and portal hypertension–related GI bleeding, liver transplantation, or mortality, was 30.7 kPa. The sensitivity, specificity, PPV, and NPV of this threshold for liver-related events were 62%, 87%, 10%, and 99%, respectively, according to the authors. Patients with an LS above this mark were 10 times as likely to experience liver-related events (aHR, 10.13; 95% CI, 4.38-23.41; P < .0001).
Scott L. Friedman, MD, chief of the division of liver diseases and dean for Therapeutic Discovery at the Icahn School of Medicine at Mount Sinai, New York, called the study “an important effort” that offers valuable insights for both researchers and practitioners.
“For clinical trials, [these thresholds] really allow for greater refinement or enrichment of patients who are suitable for enrollment in the trial because they’re at a higher risk of clinical problems that might be mitigated if the drug is effective,” Dr. Friedman said in an interview. “For clinical practice, it might indicate that the patient should either be fast tracked for a clinical trial or, more importantly, maybe needs to be referred for evaluation for a liver transplant. It may also indicate – although they didn’t look at it in this study – that there’s a need to begin or accelerate screening for liver cancer, which becomes an encroaching risk as the fibrosis advances to later stages.”
The study was funded by Gilead Sciences. The investigators disclosed additional relationships with Amgen, Eli Lilly, CohBar, and others. Dr. Friedman reported no relevant conflicts of interest.
New liver stiffness (LS) thresholds offer accurate prediction of disease progression and clinical outcomes in patients with nonalcoholic steatohepatitis (NASH) and advanced fibrosis, according to investigators.
These new LS thresholds are more reliable because they are based on high-quality prospective data drawn from four randomized controlled trials, reported lead author Rohit Loomba, MD, of the University of California, San Diego, and colleagues.
“Retrospective studies report that increasing baseline LS by VCTE [vibration-controlled transient elastography] is associated with the risk of disease progression in patients with NAFLD [non-alcoholic fatty liver disease], but prospective data in well-characterized NASH cohorts with biopsy-confirmed advanced fibrosis are limited,” the investigators wrote in Gut. “The optimal LS thresholds for prognostication of fibrosis progression and decompensation are unknown.”
Seeking clarity, Dr. Loomba and colleagues leveraged data from two phase 3 placebo-controlled trials for selonsertib and two phase 2b placebo-controlled trials for simtuzumab.
“While the studies were discontinued prematurely due to lack of efficacy, the prospectively collected data in these well-characterized participants with serial liver biopsies provides a unique opportunity to study the association between baseline LS by VCTE and disease progression,” the investigators wrote.
Across all four studies, bridging fibrosis (F3) was present in 664 participants, while 734 individuals had cirrhosis (F4). In the selonsertib studies, fibrosis was staged at baseline and week 48. The simtuzumab studies measured liver fibrosis at baseline and week 96. Out of the 664 participants with bridging fibrosis, 103 (16%) progressed to cirrhosis. Among the 734 patients with cirrhosis, 27 (4%) experienced liver-related events. Comparing these outcomes with LS data at baseline and throughout the study revealed optimal LS thresholds.
The best threshold for predicting progression from bridging fibrosis to cirrhosis was 16.6 kPa. According to the authors, the sensitivity, specificity, positive predictive value, and negative predictive value of this threshold for progression to cirrhosis were 58%, 76%, 31%, and 91%, respectively. Among patients at or above 16.6 kPa, 31% progressed to cirrhosis, compared with 9.1% of those under that threshold. Furthermore, individuals with a baseline LS at or above 16.6 kPa had nearly four times greater risk of developing cirrhosis (adjusted hazard ratio, 3.99; 95% CI, 2.66-5.98; P < .0001).
For patients with cirrhosis at baseline, the optimal threshold for predicting liver-related events, such as ascites, hepatic encephalopathy, and portal hypertension–related GI bleeding, liver transplantation, or mortality, was 30.7 kPa. The sensitivity, specificity, PPV, and NPV of this threshold for liver-related events were 62%, 87%, 10%, and 99%, respectively, according to the authors. Patients with an LS above this mark were 10 times as likely to experience liver-related events (aHR, 10.13; 95% CI, 4.38-23.41; P < .0001).
Scott L. Friedman, MD, chief of the division of liver diseases and dean for Therapeutic Discovery at the Icahn School of Medicine at Mount Sinai, New York, called the study “an important effort” that offers valuable insights for both researchers and practitioners.
“For clinical trials, [these thresholds] really allow for greater refinement or enrichment of patients who are suitable for enrollment in the trial because they’re at a higher risk of clinical problems that might be mitigated if the drug is effective,” Dr. Friedman said in an interview. “For clinical practice, it might indicate that the patient should either be fast tracked for a clinical trial or, more importantly, maybe needs to be referred for evaluation for a liver transplant. It may also indicate – although they didn’t look at it in this study – that there’s a need to begin or accelerate screening for liver cancer, which becomes an encroaching risk as the fibrosis advances to later stages.”
The study was funded by Gilead Sciences. The investigators disclosed additional relationships with Amgen, Eli Lilly, CohBar, and others. Dr. Friedman reported no relevant conflicts of interest.
FROM GUT
What barriers delay treatment in patients with hepatitis C?
EVIDENCE SUMMARY
Race, gender, and other factors are associated with lack of HCV Tx
A retrospective study (N = 894) assessed factors associated with direct-acting antiviral (DAA) initiation.1 Patients who were HCV+ with at least 1 clinical visit during the study period completed a survey of psychological, behavioral, and social life assessments. The final cohort (57% male; 64% ≥ 61 years old) was divided into patients who initiated DAA treatment (n = 690) and those who did not (n = 204).
In an adjusted multivariable analysis, factors associated with lower odds of DAA initiation included Black race (adjusted odds ratio [aOR] = 0.59 vs White race; 95% CI, 0.36-0.98); perceived difficulty accessing medical care (aOR = 0.48 vs no difficulty; 95% CI, 0.27-0.83); recent intravenous (IV) drug use (aOR = 0.11 vs no use; 95% CI, 0.02-0.54); alcohol use disorder (AUD; aOR = 0.58 vs no AUD; 95% CI, 0.38-0.90); severe depression (aOR = 0.42 vs no depression; 95% CI, 0.2-0.9); recent homelessness (aOR = 0.36 vs no homelessness; 95% CI, 0.14-0.94); and recent incarceration (aOR = 0.34 vs no incarceration; 95% CI, 0.12-0.94).1
A multicenter, observational prospective cohort study (N = 3075) evaluated receipt of HCV treatment for patients co-infected with HCV and HIV.2 The primary outcome was initiation of HCV treatment with DAAs; 1957 patients initiated therapy, while 1118 did not. Significant independent risk factors for noninitiation of treatment included age younger than 50 years, a history of IV drug use, and use of opioid substitution therapy (OST). Other factors included psychiatric comorbidity (odds ratio [OR] = 0.45; 95% CI, 0.27-0.75), incarceration (OR = 0.6; 95% CI, 0.43-0.87), and female gender (OR = 0.80; 95% CI, 0.66-0.98). In a multivariate analysis limited to those with a history of IV drug use, both use of OST (aOR = 0.55; 95% CI, 0.40-0.75) and recent IV drug use (aOR = 0.019; 95% CI, 0.004-0.087) were identified as factors with low odds of treatment implementation.2
A retrospective cohort study (N = 1024) of medical charts examined the barriers to treatment in adults with chronic HCV infection.3 Of the patient population, 208 were treated and 816 were untreated. Patients not receiving DAAs were associated with poor adherence to/loss to follow-up (n = 548; OR = 36.6; 95% CI, 19.6-68.4); significant psychiatric illness (n = 103; OR = 2.02; 95% CI, 1.13-3.71); and coinfection with HIV (n = 188; OR = 4.5; 95% CI, 2.5-8.2).3
A German multicenter retrospective case-control study (N = 793) identified factors in patient and physician decisions to initiate treatment for HCV.4 Patients were ≥ 18 years old, confirmed to be HCV+, and had visited their physician at least 1 time during the observation period. A total of 573 patients received treatment and 220 did not. Patients and clinicians of those who chose not to receive treatment completed a survey that collected reasons for not treating. The most prevalent reason for not initiating treatment was patient wish (42%). This was further delineated to reveal that 17.3% attributed their decision to fear of treatment and 13.2% to fear of adverse events. Other factors associated with nontreatment included IV drug use (aOR = 0.31; 95% CI, 0.16-0.62); HIV coinfection (aOR = 0.19; 95% CI, 0.09-0.40); and use of OST (aOR = 0.37; 95% CI, 0.21-0.68). Patient demographics associated with wish not to be treated included older age (20.2% of those ≥ 40 years old vs 6.4% of those < 40 years old; P = .03) and female gender (51.0% of females vs 35.2% of males; P = .019).4
An analysis of a French insurance database (N = 22,545) evaluated the incidence of HCV treatment with DAAs in patients who inject drugs (PWID) with a diagnosis of alcohol use disorder (AUD).5 All participants (78% male; median age, 49 years) were chronically HCV-infected and covered by national health insurance. Individuals were grouped by AUD status: untreated (n = 5176), treated (n = 3020), and no AUD (n = 14349). After multivariate adjustment, those with untreated AUD had lower uptake of DAAs than those who did not have AUD (adjusted hazard ratio [aHR] = 0.86; 95% CI, 0.78-0.94) and those with treated AUD (aHR = 0.83; 95% CI, 0.74-0.94). There were no differences between those with treated AUD and those who did not have AUD. Other factors associated with lower DAA uptake were access to care (aHR = 0.90; 95% CI, 0.83-0.98) and female gender (aHR = 0.83; 95% CI, 0.76-0.9).5
A 2017 retrospective cohort study evaluated predictors and barriers to follow-up and treatment with DAAs among veterans who were HCV+.6 Patients (94% > 50 years old; 97% male; 48% white) had established HCV care within the US Department of Veterans Affairs system. Of those who followed up with at least 1 visit to an HCV specialty clinic (n = 47,165), 29% received DAAs. Factors associated with lack of treatment included race (Black vs White: OR = 0.77; 95% CI, 0.72-0.82; Hispanic vs White: OR = 0.88; 95% CI, 0.79-0.97); IV drug use (OR = 0.84; 95% CI, 0.80-0.88); AUD (OR = 0.73; 95% CI, 0.70-0.77); medical comorbidities (OR = 0.71; 95% CI, 0.66-0.77); and hepatocellular carcinoma (OR = 0.73; 95% CI, 0.65-0.83).6
Continue to: Providers identify similar barriers to treatment of HCV
Providers identify similar barriers to treatment of HCV
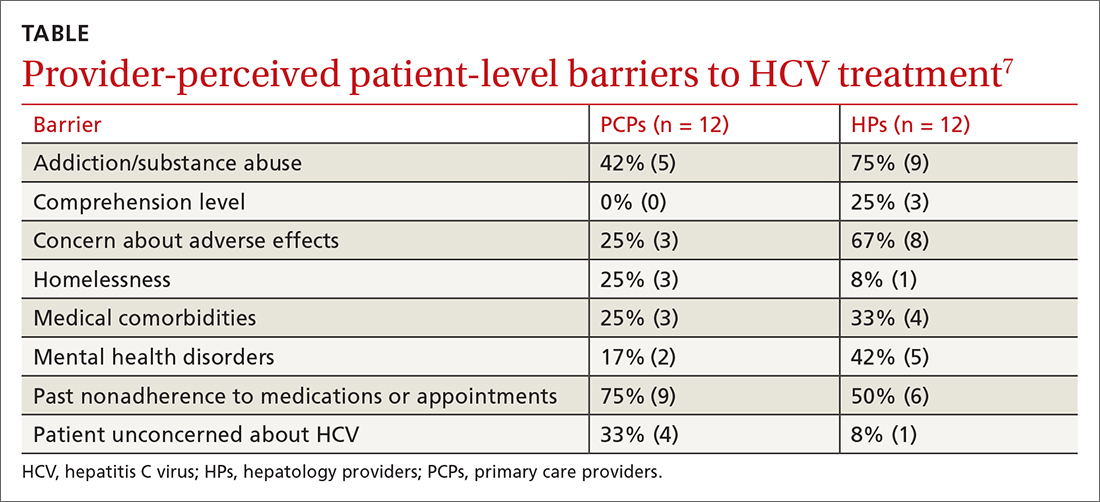
A 2017 prospective qualitative study (N = 24) from a Veterans Affairs health care system analyzed provider-perceived barriers to initiation of and adherence to HCV treatment.7 The analysis focused on differences by provider specialty. Primary care providers (PCPs; n = 12; 17% with > 40 patients with HCV) and hepatology providers (HPs; n = 12; 83% with > 40 patients with HCV) participated in a semi-structured telephone-based interview, providing their perceptions of patient-level barriers to HCV treatment. Eight patient-level barrier themes were identified; these are outlined in the TABLE7 along with data for both PCPs and HPs.
Editor’s takeaway
These 7 cohort studies show us the factors we consider and the reasons we give to not initiate HCV treatment. Some of the factors seem reasonable, but many do not. We might use this list to remind and challenge ourselves to work through barriers to provide the best possible treatment.
1. Spradling PR, Zhong Y, Moorman AC, et al. Psychosocial obstacles to hepatitis C treatment initiation among patients in care: a hitch in the cascade of cure. Hepatol Commun. 2021;5:400-411. doi: 10.1002/hep4.1632
2. Rivero-Juarez A, Tellez F, Castano-Carracedo M, et al. Parenteral drug use as the main barrier to hepatitis C treatment uptake in HIV-infected patients. HIV Medicine. 2019;20:359-367. doi: 10.1111/hiv.12715
3. Al-Khazraji A, Patel I, Saleh M, et al. Identifying barriers to the treatment of chronic hepatitis C infection. Dig Dis. 2020;38:46-52. doi: 10.1159/000501821
4. Buggisch P, Heiken H, Mauss S, et al. Barriers to initiation of hepatitis C virus therapy in Germany: a retrospective, case-controlled study. PLoS ONE. 2021;16:3p250833. doi: 10.1371/journal.pone.0250833
5. Barré T, Marcellin F, Di Beo V, et al. Untreated alcohol use disorder in people who inject drugs (PWID) in France: a major barrier to HCV treatment uptake (the ANRS-FANTASIO study). Addiction. 2019;115:573-582. doi: 10.1111/add.14820
6. Lin M, Kramer J, White D, et al. Barriers to hepatitis C treatment in the era of direct acting antiviral agents. Aliment Pharmacol Ther. 2017;46:992-1000. doi: 10.1111/apt.14328
7. Rogal SS, McCarthy R, Reid A, et al. Primary care and hepatology provider-perceived barriers to and facilitators of hepatitis C treatment candidacy and adherence. Dig Dis Sci. 2017;62:1933-1943. doi: 10.1007/s10620-017-4608-9
EVIDENCE SUMMARY
Race, gender, and other factors are associated with lack of HCV Tx
A retrospective study (N = 894) assessed factors associated with direct-acting antiviral (DAA) initiation.1 Patients who were HCV+ with at least 1 clinical visit during the study period completed a survey of psychological, behavioral, and social life assessments. The final cohort (57% male; 64% ≥ 61 years old) was divided into patients who initiated DAA treatment (n = 690) and those who did not (n = 204).
In an adjusted multivariable analysis, factors associated with lower odds of DAA initiation included Black race (adjusted odds ratio [aOR] = 0.59 vs White race; 95% CI, 0.36-0.98); perceived difficulty accessing medical care (aOR = 0.48 vs no difficulty; 95% CI, 0.27-0.83); recent intravenous (IV) drug use (aOR = 0.11 vs no use; 95% CI, 0.02-0.54); alcohol use disorder (AUD; aOR = 0.58 vs no AUD; 95% CI, 0.38-0.90); severe depression (aOR = 0.42 vs no depression; 95% CI, 0.2-0.9); recent homelessness (aOR = 0.36 vs no homelessness; 95% CI, 0.14-0.94); and recent incarceration (aOR = 0.34 vs no incarceration; 95% CI, 0.12-0.94).1
A multicenter, observational prospective cohort study (N = 3075) evaluated receipt of HCV treatment for patients co-infected with HCV and HIV.2 The primary outcome was initiation of HCV treatment with DAAs; 1957 patients initiated therapy, while 1118 did not. Significant independent risk factors for noninitiation of treatment included age younger than 50 years, a history of IV drug use, and use of opioid substitution therapy (OST). Other factors included psychiatric comorbidity (odds ratio [OR] = 0.45; 95% CI, 0.27-0.75), incarceration (OR = 0.6; 95% CI, 0.43-0.87), and female gender (OR = 0.80; 95% CI, 0.66-0.98). In a multivariate analysis limited to those with a history of IV drug use, both use of OST (aOR = 0.55; 95% CI, 0.40-0.75) and recent IV drug use (aOR = 0.019; 95% CI, 0.004-0.087) were identified as factors with low odds of treatment implementation.2
A retrospective cohort study (N = 1024) of medical charts examined the barriers to treatment in adults with chronic HCV infection.3 Of the patient population, 208 were treated and 816 were untreated. Patients not receiving DAAs were associated with poor adherence to/loss to follow-up (n = 548; OR = 36.6; 95% CI, 19.6-68.4); significant psychiatric illness (n = 103; OR = 2.02; 95% CI, 1.13-3.71); and coinfection with HIV (n = 188; OR = 4.5; 95% CI, 2.5-8.2).3
A German multicenter retrospective case-control study (N = 793) identified factors in patient and physician decisions to initiate treatment for HCV.4 Patients were ≥ 18 years old, confirmed to be HCV+, and had visited their physician at least 1 time during the observation period. A total of 573 patients received treatment and 220 did not. Patients and clinicians of those who chose not to receive treatment completed a survey that collected reasons for not treating. The most prevalent reason for not initiating treatment was patient wish (42%). This was further delineated to reveal that 17.3% attributed their decision to fear of treatment and 13.2% to fear of adverse events. Other factors associated with nontreatment included IV drug use (aOR = 0.31; 95% CI, 0.16-0.62); HIV coinfection (aOR = 0.19; 95% CI, 0.09-0.40); and use of OST (aOR = 0.37; 95% CI, 0.21-0.68). Patient demographics associated with wish not to be treated included older age (20.2% of those ≥ 40 years old vs 6.4% of those < 40 years old; P = .03) and female gender (51.0% of females vs 35.2% of males; P = .019).4
An analysis of a French insurance database (N = 22,545) evaluated the incidence of HCV treatment with DAAs in patients who inject drugs (PWID) with a diagnosis of alcohol use disorder (AUD).5 All participants (78% male; median age, 49 years) were chronically HCV-infected and covered by national health insurance. Individuals were grouped by AUD status: untreated (n = 5176), treated (n = 3020), and no AUD (n = 14349). After multivariate adjustment, those with untreated AUD had lower uptake of DAAs than those who did not have AUD (adjusted hazard ratio [aHR] = 0.86; 95% CI, 0.78-0.94) and those with treated AUD (aHR = 0.83; 95% CI, 0.74-0.94). There were no differences between those with treated AUD and those who did not have AUD. Other factors associated with lower DAA uptake were access to care (aHR = 0.90; 95% CI, 0.83-0.98) and female gender (aHR = 0.83; 95% CI, 0.76-0.9).5
A 2017 retrospective cohort study evaluated predictors and barriers to follow-up and treatment with DAAs among veterans who were HCV+.6 Patients (94% > 50 years old; 97% male; 48% white) had established HCV care within the US Department of Veterans Affairs system. Of those who followed up with at least 1 visit to an HCV specialty clinic (n = 47,165), 29% received DAAs. Factors associated with lack of treatment included race (Black vs White: OR = 0.77; 95% CI, 0.72-0.82; Hispanic vs White: OR = 0.88; 95% CI, 0.79-0.97); IV drug use (OR = 0.84; 95% CI, 0.80-0.88); AUD (OR = 0.73; 95% CI, 0.70-0.77); medical comorbidities (OR = 0.71; 95% CI, 0.66-0.77); and hepatocellular carcinoma (OR = 0.73; 95% CI, 0.65-0.83).6
Continue to: Providers identify similar barriers to treatment of HCV
Providers identify similar barriers to treatment of HCV
A 2017 prospective qualitative study (N = 24) from a Veterans Affairs health care system analyzed provider-perceived barriers to initiation of and adherence to HCV treatment.7 The analysis focused on differences by provider specialty. Primary care providers (PCPs; n = 12; 17% with > 40 patients with HCV) and hepatology providers (HPs; n = 12; 83% with > 40 patients with HCV) participated in a semi-structured telephone-based interview, providing their perceptions of patient-level barriers to HCV treatment. Eight patient-level barrier themes were identified; these are outlined in the TABLE7 along with data for both PCPs and HPs.

Editor’s takeaway
These 7 cohort studies show us the factors we consider and the reasons we give to not initiate HCV treatment. Some of the factors seem reasonable, but many do not. We might use this list to remind and challenge ourselves to work through barriers to provide the best possible treatment.
EVIDENCE SUMMARY
Race, gender, and other factors are associated with lack of HCV Tx
A retrospective study (N = 894) assessed factors associated with direct-acting antiviral (DAA) initiation.1 Patients who were HCV+ with at least 1 clinical visit during the study period completed a survey of psychological, behavioral, and social life assessments. The final cohort (57% male; 64% ≥ 61 years old) was divided into patients who initiated DAA treatment (n = 690) and those who did not (n = 204).
In an adjusted multivariable analysis, factors associated with lower odds of DAA initiation included Black race (adjusted odds ratio [aOR] = 0.59 vs White race; 95% CI, 0.36-0.98); perceived difficulty accessing medical care (aOR = 0.48 vs no difficulty; 95% CI, 0.27-0.83); recent intravenous (IV) drug use (aOR = 0.11 vs no use; 95% CI, 0.02-0.54); alcohol use disorder (AUD; aOR = 0.58 vs no AUD; 95% CI, 0.38-0.90); severe depression (aOR = 0.42 vs no depression; 95% CI, 0.2-0.9); recent homelessness (aOR = 0.36 vs no homelessness; 95% CI, 0.14-0.94); and recent incarceration (aOR = 0.34 vs no incarceration; 95% CI, 0.12-0.94).1
A multicenter, observational prospective cohort study (N = 3075) evaluated receipt of HCV treatment for patients co-infected with HCV and HIV.2 The primary outcome was initiation of HCV treatment with DAAs; 1957 patients initiated therapy, while 1118 did not. Significant independent risk factors for noninitiation of treatment included age younger than 50 years, a history of IV drug use, and use of opioid substitution therapy (OST). Other factors included psychiatric comorbidity (odds ratio [OR] = 0.45; 95% CI, 0.27-0.75), incarceration (OR = 0.6; 95% CI, 0.43-0.87), and female gender (OR = 0.80; 95% CI, 0.66-0.98). In a multivariate analysis limited to those with a history of IV drug use, both use of OST (aOR = 0.55; 95% CI, 0.40-0.75) and recent IV drug use (aOR = 0.019; 95% CI, 0.004-0.087) were identified as factors with low odds of treatment implementation.2
A retrospective cohort study (N = 1024) of medical charts examined the barriers to treatment in adults with chronic HCV infection.3 Of the patient population, 208 were treated and 816 were untreated. Patients not receiving DAAs were associated with poor adherence to/loss to follow-up (n = 548; OR = 36.6; 95% CI, 19.6-68.4); significant psychiatric illness (n = 103; OR = 2.02; 95% CI, 1.13-3.71); and coinfection with HIV (n = 188; OR = 4.5; 95% CI, 2.5-8.2).3
A German multicenter retrospective case-control study (N = 793) identified factors in patient and physician decisions to initiate treatment for HCV.4 Patients were ≥ 18 years old, confirmed to be HCV+, and had visited their physician at least 1 time during the observation period. A total of 573 patients received treatment and 220 did not. Patients and clinicians of those who chose not to receive treatment completed a survey that collected reasons for not treating. The most prevalent reason for not initiating treatment was patient wish (42%). This was further delineated to reveal that 17.3% attributed their decision to fear of treatment and 13.2% to fear of adverse events. Other factors associated with nontreatment included IV drug use (aOR = 0.31; 95% CI, 0.16-0.62); HIV coinfection (aOR = 0.19; 95% CI, 0.09-0.40); and use of OST (aOR = 0.37; 95% CI, 0.21-0.68). Patient demographics associated with wish not to be treated included older age (20.2% of those ≥ 40 years old vs 6.4% of those < 40 years old; P = .03) and female gender (51.0% of females vs 35.2% of males; P = .019).4
An analysis of a French insurance database (N = 22,545) evaluated the incidence of HCV treatment with DAAs in patients who inject drugs (PWID) with a diagnosis of alcohol use disorder (AUD).5 All participants (78% male; median age, 49 years) were chronically HCV-infected and covered by national health insurance. Individuals were grouped by AUD status: untreated (n = 5176), treated (n = 3020), and no AUD (n = 14349). After multivariate adjustment, those with untreated AUD had lower uptake of DAAs than those who did not have AUD (adjusted hazard ratio [aHR] = 0.86; 95% CI, 0.78-0.94) and those with treated AUD (aHR = 0.83; 95% CI, 0.74-0.94). There were no differences between those with treated AUD and those who did not have AUD. Other factors associated with lower DAA uptake were access to care (aHR = 0.90; 95% CI, 0.83-0.98) and female gender (aHR = 0.83; 95% CI, 0.76-0.9).5
A 2017 retrospective cohort study evaluated predictors and barriers to follow-up and treatment with DAAs among veterans who were HCV+.6 Patients (94% > 50 years old; 97% male; 48% white) had established HCV care within the US Department of Veterans Affairs system. Of those who followed up with at least 1 visit to an HCV specialty clinic (n = 47,165), 29% received DAAs. Factors associated with lack of treatment included race (Black vs White: OR = 0.77; 95% CI, 0.72-0.82; Hispanic vs White: OR = 0.88; 95% CI, 0.79-0.97); IV drug use (OR = 0.84; 95% CI, 0.80-0.88); AUD (OR = 0.73; 95% CI, 0.70-0.77); medical comorbidities (OR = 0.71; 95% CI, 0.66-0.77); and hepatocellular carcinoma (OR = 0.73; 95% CI, 0.65-0.83).6
Continue to: Providers identify similar barriers to treatment of HCV
Providers identify similar barriers to treatment of HCV
A 2017 prospective qualitative study (N = 24) from a Veterans Affairs health care system analyzed provider-perceived barriers to initiation of and adherence to HCV treatment.7 The analysis focused on differences by provider specialty. Primary care providers (PCPs; n = 12; 17% with > 40 patients with HCV) and hepatology providers (HPs; n = 12; 83% with > 40 patients with HCV) participated in a semi-structured telephone-based interview, providing their perceptions of patient-level barriers to HCV treatment. Eight patient-level barrier themes were identified; these are outlined in the TABLE7 along with data for both PCPs and HPs.

Editor’s takeaway
These 7 cohort studies show us the factors we consider and the reasons we give to not initiate HCV treatment. Some of the factors seem reasonable, but many do not. We might use this list to remind and challenge ourselves to work through barriers to provide the best possible treatment.
1. Spradling PR, Zhong Y, Moorman AC, et al. Psychosocial obstacles to hepatitis C treatment initiation among patients in care: a hitch in the cascade of cure. Hepatol Commun. 2021;5:400-411. doi: 10.1002/hep4.1632
2. Rivero-Juarez A, Tellez F, Castano-Carracedo M, et al. Parenteral drug use as the main barrier to hepatitis C treatment uptake in HIV-infected patients. HIV Medicine. 2019;20:359-367. doi: 10.1111/hiv.12715
3. Al-Khazraji A, Patel I, Saleh M, et al. Identifying barriers to the treatment of chronic hepatitis C infection. Dig Dis. 2020;38:46-52. doi: 10.1159/000501821
4. Buggisch P, Heiken H, Mauss S, et al. Barriers to initiation of hepatitis C virus therapy in Germany: a retrospective, case-controlled study. PLoS ONE. 2021;16:3p250833. doi: 10.1371/journal.pone.0250833
5. Barré T, Marcellin F, Di Beo V, et al. Untreated alcohol use disorder in people who inject drugs (PWID) in France: a major barrier to HCV treatment uptake (the ANRS-FANTASIO study). Addiction. 2019;115:573-582. doi: 10.1111/add.14820
6. Lin M, Kramer J, White D, et al. Barriers to hepatitis C treatment in the era of direct acting antiviral agents. Aliment Pharmacol Ther. 2017;46:992-1000. doi: 10.1111/apt.14328
7. Rogal SS, McCarthy R, Reid A, et al. Primary care and hepatology provider-perceived barriers to and facilitators of hepatitis C treatment candidacy and adherence. Dig Dis Sci. 2017;62:1933-1943. doi: 10.1007/s10620-017-4608-9
1. Spradling PR, Zhong Y, Moorman AC, et al. Psychosocial obstacles to hepatitis C treatment initiation among patients in care: a hitch in the cascade of cure. Hepatol Commun. 2021;5:400-411. doi: 10.1002/hep4.1632
2. Rivero-Juarez A, Tellez F, Castano-Carracedo M, et al. Parenteral drug use as the main barrier to hepatitis C treatment uptake in HIV-infected patients. HIV Medicine. 2019;20:359-367. doi: 10.1111/hiv.12715
3. Al-Khazraji A, Patel I, Saleh M, et al. Identifying barriers to the treatment of chronic hepatitis C infection. Dig Dis. 2020;38:46-52. doi: 10.1159/000501821
4. Buggisch P, Heiken H, Mauss S, et al. Barriers to initiation of hepatitis C virus therapy in Germany: a retrospective, case-controlled study. PLoS ONE. 2021;16:3p250833. doi: 10.1371/journal.pone.0250833
5. Barré T, Marcellin F, Di Beo V, et al. Untreated alcohol use disorder in people who inject drugs (PWID) in France: a major barrier to HCV treatment uptake (the ANRS-FANTASIO study). Addiction. 2019;115:573-582. doi: 10.1111/add.14820
6. Lin M, Kramer J, White D, et al. Barriers to hepatitis C treatment in the era of direct acting antiviral agents. Aliment Pharmacol Ther. 2017;46:992-1000. doi: 10.1111/apt.14328
7. Rogal SS, McCarthy R, Reid A, et al. Primary care and hepatology provider-perceived barriers to and facilitators of hepatitis C treatment candidacy and adherence. Dig Dis Sci. 2017;62:1933-1943. doi: 10.1007/s10620-017-4608-9
EVIDENCE-BASED ANSWER:
Multiple patient-specific and provider-perceived factors delay initiation of treatment in patients with hepatitis C. Patient-specific barriers to initiation of treatment for hepatitis C virus (HCV) include age, race, gender, economic status, insurance status, and comorbidities such as HIV coinfection, psychiatric illness, and other psychosocial factors.
Provider-perceived patient factors include substance abuse history, older age, psychiatric illness, medical comorbidities, treatment adverse effect risks, and factors that might limit adherence (eg, comprehension level).
Study limitations included problems with generalizability of the populations studied and variability in reporting or interpreting data associated with substance or alcohol use disorders
Living-donor liver transplants linked with substantial survival benefit
Living-donor liver transplant recipients gained an additional 13-17 years of life, compared with patients who remained on the wait list, according to a retrospective case-control study.
The data suggest that the life-years gained are comparable to or greater than those conferred by either other lifesaving procedures or liver transplant from a deceased donor, wrote the researchers, led by Whitney Jackson, MD, assistant professor of gastroenterology and medical director of living-donor liver transplantation at the University of Colorado Anschutz Medical Campus.
“Despite the acceptance of living-donor liver transplant as a lifesaving procedure for end-stage liver disease, it remains underused in the United States,” the authors wrote in JAMA Surgery. “This study’s findings challenge current perceptions regarding when the survival benefit of a living-donor transplant occurs.”
Dr. Jackson and colleagues conducted a retrospective, secondary analysis of the Scientific Registry of Transplant Recipients database for 119,275 U.S. liver transplant candidates and recipients from January 2012 to September 2021. They assessed the survival benefit, life-years saved, and the Model for End-Stage Liver Disease incorporating sodium levels (MELD-Na) score at which the survival benefit was obtained, compared with those who remained on the wait list.
The research team included 116,455 liver transplant candidates who were 18 and older and assigned to the wait list, as well as 2,820 patients who received a living-donor liver transplant. Patients listed for retransplant or multiorgan transplant were excluded, as were those with prior kidney or liver transplants.
The mean age of the study participants was 55 years, and 63% were men. Overall, 70.2% were White, 15.8% were Hispanic or Latinx, 8.2% were Black or African American, 4.3% were Asian, 0.9% were American Indian or Alaska Native, and 0.2% were Native Hawaiian or Pacific Islander. The most common etiologies were alcoholic cirrhosis (23.8%) and nonalcoholic steatohepatitis (15.9%).
Compared with patients on the wait list, recipients of a living-donor liver transplant were younger, more often women, more educated, and more often White. A greater proportion of transplant recipients had a primary etiology of nonalcoholic steatohepatitis (19.8%) and cholestatic liver disease (24.1%). At wait list placement, one-third of candidates had a MELD-Na score of 14 or higher.
The research team found a significant survival benefit for patients receiving a living-donor liver transplant based on mortality risk and survival scores. The survival benefit was significant at a MELD-Na score as low as 11, with a 34% decrease(95% confidence interval [CI], 17.4%-52.0%) in mortality compared with the wait list. In addition, mortality risk models confirmed a survival benefit for patients with a MELD-Na score of 11 or higher at 1 year after transplant (adjusted hazard ratio, 0.64; 95% CI, 0.47-0.88; P = .006). At a MELD-Na score of 14-16, mortality decreased by about 50% (aHR, 0.47; 95% CI, 0.34-0.66; P < .001).
The probability of death from a living-donor liver transplant for patients with very low MELD-Na scores (between 6 and 10) was greater than that for patients on the wait list for the first 259 days, at which point the risk of death for both groups was equal. At 471 days, the probability of survival in both groups was equal. As the MELD-Na score increased, both the time to equal risk of death and the time to equal survival decreased, demonstrating that the survival benefit occurs much earlier for patients with a higher MELD-Na score.
Analysis of life-years from transplant showed living-donor transplant recipients gained 13-17 life-years compared to those who didn’t receive one.
“Living-donor liver transplantation is a valuable yet underutilized strategy to address the significant organ shortage and long waiting times on the transplant list in the U.S.,” said Renu Dhanasekaran, MD, PhD, assistant professor of gastroenterology and hepatology at Stanford (Calif.) University.
Dr. Dhanasekaran, who wasn’t involved with this study, also welcomed the finding that living-donor liver transplantation can benefit patients with low MELD-Na scores, even below the expected cutoff at 15. According to the study authors, previous research had suggested benefit would be seen only at MELD-Na 15 and above.
“In my practice, I have several patients whose symptoms are out of proportion to their MELD score, and data like this will convince them and their potential donors to avail a transplant at an earlier stage,” she said.
The findings challenge the current paradigm around the timing of referral for a liver transplant and may have ramifications for allocation policies for deceased donors, the study authors wrote. The data can also help to contextualize risk-benefit discussions for donors and recipients.
“Donating a part of one’s liver to save a patient suffering from end-stage liver disease is an incredible act of selfless love,” Dr. Dhanasekaran said. “I hope strong positive data from studies like this one encourage more donors, patients, and transplant centers to expand the use of [living-donor liver transplant].”
The authors reported no grant support or funding sources for this study. One author disclosed being married to the current chair of the United Network for Organ Sharing’s Liver and Intestinal Organ Transplantation Committee. No other conflicts of interest were reported. Dr. Dhanasekaran reported no relevant disclosures.
Living-donor liver transplant recipients gained an additional 13-17 years of life, compared with patients who remained on the wait list, according to a retrospective case-control study.
The data suggest that the life-years gained are comparable to or greater than those conferred by either other lifesaving procedures or liver transplant from a deceased donor, wrote the researchers, led by Whitney Jackson, MD, assistant professor of gastroenterology and medical director of living-donor liver transplantation at the University of Colorado Anschutz Medical Campus.
“Despite the acceptance of living-donor liver transplant as a lifesaving procedure for end-stage liver disease, it remains underused in the United States,” the authors wrote in JAMA Surgery. “This study’s findings challenge current perceptions regarding when the survival benefit of a living-donor transplant occurs.”
Dr. Jackson and colleagues conducted a retrospective, secondary analysis of the Scientific Registry of Transplant Recipients database for 119,275 U.S. liver transplant candidates and recipients from January 2012 to September 2021. They assessed the survival benefit, life-years saved, and the Model for End-Stage Liver Disease incorporating sodium levels (MELD-Na) score at which the survival benefit was obtained, compared with those who remained on the wait list.
The research team included 116,455 liver transplant candidates who were 18 and older and assigned to the wait list, as well as 2,820 patients who received a living-donor liver transplant. Patients listed for retransplant or multiorgan transplant were excluded, as were those with prior kidney or liver transplants.
The mean age of the study participants was 55 years, and 63% were men. Overall, 70.2% were White, 15.8% were Hispanic or Latinx, 8.2% were Black or African American, 4.3% were Asian, 0.9% were American Indian or Alaska Native, and 0.2% were Native Hawaiian or Pacific Islander. The most common etiologies were alcoholic cirrhosis (23.8%) and nonalcoholic steatohepatitis (15.9%).
Compared with patients on the wait list, recipients of a living-donor liver transplant were younger, more often women, more educated, and more often White. A greater proportion of transplant recipients had a primary etiology of nonalcoholic steatohepatitis (19.8%) and cholestatic liver disease (24.1%). At wait list placement, one-third of candidates had a MELD-Na score of 14 or higher.
The research team found a significant survival benefit for patients receiving a living-donor liver transplant based on mortality risk and survival scores. The survival benefit was significant at a MELD-Na score as low as 11, with a 34% decrease(95% confidence interval [CI], 17.4%-52.0%) in mortality compared with the wait list. In addition, mortality risk models confirmed a survival benefit for patients with a MELD-Na score of 11 or higher at 1 year after transplant (adjusted hazard ratio, 0.64; 95% CI, 0.47-0.88; P = .006). At a MELD-Na score of 14-16, mortality decreased by about 50% (aHR, 0.47; 95% CI, 0.34-0.66; P < .001).
The probability of death from a living-donor liver transplant for patients with very low MELD-Na scores (between 6 and 10) was greater than that for patients on the wait list for the first 259 days, at which point the risk of death for both groups was equal. At 471 days, the probability of survival in both groups was equal. As the MELD-Na score increased, both the time to equal risk of death and the time to equal survival decreased, demonstrating that the survival benefit occurs much earlier for patients with a higher MELD-Na score.
Analysis of life-years from transplant showed living-donor transplant recipients gained 13-17 life-years compared to those who didn’t receive one.
“Living-donor liver transplantation is a valuable yet underutilized strategy to address the significant organ shortage and long waiting times on the transplant list in the U.S.,” said Renu Dhanasekaran, MD, PhD, assistant professor of gastroenterology and hepatology at Stanford (Calif.) University.
Dr. Dhanasekaran, who wasn’t involved with this study, also welcomed the finding that living-donor liver transplantation can benefit patients with low MELD-Na scores, even below the expected cutoff at 15. According to the study authors, previous research had suggested benefit would be seen only at MELD-Na 15 and above.
“In my practice, I have several patients whose symptoms are out of proportion to their MELD score, and data like this will convince them and their potential donors to avail a transplant at an earlier stage,” she said.
The findings challenge the current paradigm around the timing of referral for a liver transplant and may have ramifications for allocation policies for deceased donors, the study authors wrote. The data can also help to contextualize risk-benefit discussions for donors and recipients.
“Donating a part of one’s liver to save a patient suffering from end-stage liver disease is an incredible act of selfless love,” Dr. Dhanasekaran said. “I hope strong positive data from studies like this one encourage more donors, patients, and transplant centers to expand the use of [living-donor liver transplant].”
The authors reported no grant support or funding sources for this study. One author disclosed being married to the current chair of the United Network for Organ Sharing’s Liver and Intestinal Organ Transplantation Committee. No other conflicts of interest were reported. Dr. Dhanasekaran reported no relevant disclosures.
Living-donor liver transplant recipients gained an additional 13-17 years of life, compared with patients who remained on the wait list, according to a retrospective case-control study.
The data suggest that the life-years gained are comparable to or greater than those conferred by either other lifesaving procedures or liver transplant from a deceased donor, wrote the researchers, led by Whitney Jackson, MD, assistant professor of gastroenterology and medical director of living-donor liver transplantation at the University of Colorado Anschutz Medical Campus.
“Despite the acceptance of living-donor liver transplant as a lifesaving procedure for end-stage liver disease, it remains underused in the United States,” the authors wrote in JAMA Surgery. “This study’s findings challenge current perceptions regarding when the survival benefit of a living-donor transplant occurs.”
Dr. Jackson and colleagues conducted a retrospective, secondary analysis of the Scientific Registry of Transplant Recipients database for 119,275 U.S. liver transplant candidates and recipients from January 2012 to September 2021. They assessed the survival benefit, life-years saved, and the Model for End-Stage Liver Disease incorporating sodium levels (MELD-Na) score at which the survival benefit was obtained, compared with those who remained on the wait list.
The research team included 116,455 liver transplant candidates who were 18 and older and assigned to the wait list, as well as 2,820 patients who received a living-donor liver transplant. Patients listed for retransplant or multiorgan transplant were excluded, as were those with prior kidney or liver transplants.
The mean age of the study participants was 55 years, and 63% were men. Overall, 70.2% were White, 15.8% were Hispanic or Latinx, 8.2% were Black or African American, 4.3% were Asian, 0.9% were American Indian or Alaska Native, and 0.2% were Native Hawaiian or Pacific Islander. The most common etiologies were alcoholic cirrhosis (23.8%) and nonalcoholic steatohepatitis (15.9%).
Compared with patients on the wait list, recipients of a living-donor liver transplant were younger, more often women, more educated, and more often White. A greater proportion of transplant recipients had a primary etiology of nonalcoholic steatohepatitis (19.8%) and cholestatic liver disease (24.1%). At wait list placement, one-third of candidates had a MELD-Na score of 14 or higher.
The research team found a significant survival benefit for patients receiving a living-donor liver transplant based on mortality risk and survival scores. The survival benefit was significant at a MELD-Na score as low as 11, with a 34% decrease(95% confidence interval [CI], 17.4%-52.0%) in mortality compared with the wait list. In addition, mortality risk models confirmed a survival benefit for patients with a MELD-Na score of 11 or higher at 1 year after transplant (adjusted hazard ratio, 0.64; 95% CI, 0.47-0.88; P = .006). At a MELD-Na score of 14-16, mortality decreased by about 50% (aHR, 0.47; 95% CI, 0.34-0.66; P < .001).
The probability of death from a living-donor liver transplant for patients with very low MELD-Na scores (between 6 and 10) was greater than that for patients on the wait list for the first 259 days, at which point the risk of death for both groups was equal. At 471 days, the probability of survival in both groups was equal. As the MELD-Na score increased, both the time to equal risk of death and the time to equal survival decreased, demonstrating that the survival benefit occurs much earlier for patients with a higher MELD-Na score.
Analysis of life-years from transplant showed living-donor transplant recipients gained 13-17 life-years compared to those who didn’t receive one.
“Living-donor liver transplantation is a valuable yet underutilized strategy to address the significant organ shortage and long waiting times on the transplant list in the U.S.,” said Renu Dhanasekaran, MD, PhD, assistant professor of gastroenterology and hepatology at Stanford (Calif.) University.
Dr. Dhanasekaran, who wasn’t involved with this study, also welcomed the finding that living-donor liver transplantation can benefit patients with low MELD-Na scores, even below the expected cutoff at 15. According to the study authors, previous research had suggested benefit would be seen only at MELD-Na 15 and above.
“In my practice, I have several patients whose symptoms are out of proportion to their MELD score, and data like this will convince them and their potential donors to avail a transplant at an earlier stage,” she said.
The findings challenge the current paradigm around the timing of referral for a liver transplant and may have ramifications for allocation policies for deceased donors, the study authors wrote. The data can also help to contextualize risk-benefit discussions for donors and recipients.
“Donating a part of one’s liver to save a patient suffering from end-stage liver disease is an incredible act of selfless love,” Dr. Dhanasekaran said. “I hope strong positive data from studies like this one encourage more donors, patients, and transplant centers to expand the use of [living-donor liver transplant].”
The authors reported no grant support or funding sources for this study. One author disclosed being married to the current chair of the United Network for Organ Sharing’s Liver and Intestinal Organ Transplantation Committee. No other conflicts of interest were reported. Dr. Dhanasekaran reported no relevant disclosures.
FROM JAMA SURGERY
Pervasive ‘forever chemical’ linked to liver cancer
The correlation does not prove that PFOS causes this cancer, and more research is needed, but in the meantime, people should limit their exposure to it and others in its class, said Jesse Goodrich, PhD, a postdoctoral scholar in environmental medicine at the University of Southern California, Los Angeles.
“If you’re at risk for liver cancer because you have other risk factors, then these chemicals have the potential to kind of send you over the edge,” he told this news organization.
Dr. Goodrich and colleagues published their research online in JHEP Reports.
Dubbed “forever chemicals” because they can take thousands of years to break down, polyfluoroalkyl substances (PFAS) figure in makeup, food packaging, waterproof clothing, nonstick cookware, firefighting foams, and groundwater. They have spread through the atmosphere into rain and can be found in the blood of most Americans. PFOS is one of the most widely used PFAS.
“You can’t really escape them,” Dr. Goodrich said.
Previous research has linked PFAS to infertility, pregnancy complications, learning and behavioral problems in children, immune system issues, and higher cholesterol, as well as other cancers. Some experiments in animals suggested PFAS could cause liver cancer, and others showed a correlation between PFAS serum levels and biomarkers associated with liver cancer. But many of these health effects take a long time to develop.
“It wasn’t until we started to get really highly exposed groups of people that we started, as scientists, to be able to figure out what was going on,” said Dr. Goodrich.
High exposure, increased incidence
To measure the relationship between PFAS exposure and the incidence of hepatocellular carcinoma more definitively, Dr. Goodrich and colleagues analyzed data from the Multiethnic Cohort Study, a cohort of more than 200,000 people of African, Latin, Native Hawaiian, Japanese, and European ancestry tracked since the early 1990s in California and Hawaii. About 67,000 participants provided blood samples from 2001 to 2007.
From this cohort, the researchers found 50 people who later developed hepatocellular carcinoma. The researchers matched these patients with 50 controls of similar age at blood collection, sex, race, ethnicity, and study area who did not develop the cancer.
They found that people with more than 54.9 mcg/L of PFOS in their blood before any diagnosis of hepatocellular carcinoma were almost five times more likely to get the cancer (odds ratio 4.5; 95% confidence interval, 1.2-16.0), which was statistically significant (P = .02).
This level of PFOS corresponds to the 90th percentile found in the U.S. National Health and Nutrition Examination Survey (NHANES).
To get some idea of the mechanism by which PFOS might do its damage, the researchers also looked for linkage to levels of metabolites.
They found an overlap among high PFOS levels, hepatocellular carcinoma, and high levels of glucose, butyric acid (a short chain fatty acid), alpha-Ketoisovaleric acid (alpha branched-chain alpha-keto acid), and 7alpha-Hydroxy-3-oxo-4-cholestenoate (a bile acid). These metabolites have been associated in previous studies with metabolic disorders and liver disease.
Similarly, the researchers identified an association among the cancer, PFOS, and alterations in amino acid and glycan biosynthesis pathways.
Risk mitigation
The half-life of PFAS in the human body is about 3-7 years, said Dr. Goodrich.
“There’s not much you can do once they’re in there,” he said. “So, the focus needs to be on preventing the exposure in the first place.”
People can limit exposure by avoiding water contaminated with PFAS or filtering it out, Dr. Goodrich said. He recommended avoiding fish from contaminated waterways and nonstick cookware. The Environmental Protection Agency has more detailed recommendations.
But giving patients individualized recommendations is difficult, said Vincent Chen, MD, MS, a clinical instructor in gastroenterology at the University of Michigan, Ann Arbor, who was not involved in the study. Most clinicians don’t know their patients’ PFOS levels.
“It’s not that easy to get a test,” Dr. Chen told this news organization.
People can also mitigate their risk factors for hepatocellular carcinoma, such as a poor diet, a lack of exercise, and smoking, said Dr. Goodrich.
The researchers found that patients with hepatocellular carcinoma were more likely to be overweight and have diabetes, and PFOS was associated with higher fasting glucose levels. This raises the possibility that PFOS increases the risk for hepatocellular carcinoma by causing diabetes and obesity.
Dr. Goodrich and his colleagues tried to address this question by adjusting for baseline body mass index (BMI) and diabetes diagnosis in their statistical analysis.
After adjusting for BMI, they found that the association between PFOS and hepatocellular carcinoma diminished to a threefold risk (OR, 2.90; 95% CI, 0.78-10.00) and was no longer statistically significant (P = .11).
On the other hand, adjusting for diabetes did not change the significance of the relationship between PFOS and the cancer (OR, 5.7; 95% CI, 1.10-30.00; P = .04).
The sample size was probably too small to adequately tease out this relationship, Dr. Chen said. Still, he said, “I thought it was a very, very important study.”
The levels of PFOS found in the blood of Americans has been declining since the 1999-2000 NHANES, Dr. Chen pointed out. But that’s not as reassuring as it sounds.
“The problem is that if you put a regulation limiting the use of one PFAS, what people can do is just substitute with another PFAS or another molecule, which for all we know could be equally harmful,” Dr. Chen said.
Funding was provided by the Southern California Environmental Health Science Center supported by the National Institutes of Health. Dr. Goodrich and Dr. Chen report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The correlation does not prove that PFOS causes this cancer, and more research is needed, but in the meantime, people should limit their exposure to it and others in its class, said Jesse Goodrich, PhD, a postdoctoral scholar in environmental medicine at the University of Southern California, Los Angeles.
“If you’re at risk for liver cancer because you have other risk factors, then these chemicals have the potential to kind of send you over the edge,” he told this news organization.
Dr. Goodrich and colleagues published their research online in JHEP Reports.
Dubbed “forever chemicals” because they can take thousands of years to break down, polyfluoroalkyl substances (PFAS) figure in makeup, food packaging, waterproof clothing, nonstick cookware, firefighting foams, and groundwater. They have spread through the atmosphere into rain and can be found in the blood of most Americans. PFOS is one of the most widely used PFAS.
“You can’t really escape them,” Dr. Goodrich said.
Previous research has linked PFAS to infertility, pregnancy complications, learning and behavioral problems in children, immune system issues, and higher cholesterol, as well as other cancers. Some experiments in animals suggested PFAS could cause liver cancer, and others showed a correlation between PFAS serum levels and biomarkers associated with liver cancer. But many of these health effects take a long time to develop.
“It wasn’t until we started to get really highly exposed groups of people that we started, as scientists, to be able to figure out what was going on,” said Dr. Goodrich.
High exposure, increased incidence
To measure the relationship between PFAS exposure and the incidence of hepatocellular carcinoma more definitively, Dr. Goodrich and colleagues analyzed data from the Multiethnic Cohort Study, a cohort of more than 200,000 people of African, Latin, Native Hawaiian, Japanese, and European ancestry tracked since the early 1990s in California and Hawaii. About 67,000 participants provided blood samples from 2001 to 2007.
From this cohort, the researchers found 50 people who later developed hepatocellular carcinoma. The researchers matched these patients with 50 controls of similar age at blood collection, sex, race, ethnicity, and study area who did not develop the cancer.
They found that people with more than 54.9 mcg/L of PFOS in their blood before any diagnosis of hepatocellular carcinoma were almost five times more likely to get the cancer (odds ratio 4.5; 95% confidence interval, 1.2-16.0), which was statistically significant (P = .02).
This level of PFOS corresponds to the 90th percentile found in the U.S. National Health and Nutrition Examination Survey (NHANES).
To get some idea of the mechanism by which PFOS might do its damage, the researchers also looked for linkage to levels of metabolites.
They found an overlap among high PFOS levels, hepatocellular carcinoma, and high levels of glucose, butyric acid (a short chain fatty acid), alpha-Ketoisovaleric acid (alpha branched-chain alpha-keto acid), and 7alpha-Hydroxy-3-oxo-4-cholestenoate (a bile acid). These metabolites have been associated in previous studies with metabolic disorders and liver disease.
Similarly, the researchers identified an association among the cancer, PFOS, and alterations in amino acid and glycan biosynthesis pathways.
Risk mitigation
The half-life of PFAS in the human body is about 3-7 years, said Dr. Goodrich.
“There’s not much you can do once they’re in there,” he said. “So, the focus needs to be on preventing the exposure in the first place.”
People can limit exposure by avoiding water contaminated with PFAS or filtering it out, Dr. Goodrich said. He recommended avoiding fish from contaminated waterways and nonstick cookware. The Environmental Protection Agency has more detailed recommendations.
But giving patients individualized recommendations is difficult, said Vincent Chen, MD, MS, a clinical instructor in gastroenterology at the University of Michigan, Ann Arbor, who was not involved in the study. Most clinicians don’t know their patients’ PFOS levels.
“It’s not that easy to get a test,” Dr. Chen told this news organization.
People can also mitigate their risk factors for hepatocellular carcinoma, such as a poor diet, a lack of exercise, and smoking, said Dr. Goodrich.
The researchers found that patients with hepatocellular carcinoma were more likely to be overweight and have diabetes, and PFOS was associated with higher fasting glucose levels. This raises the possibility that PFOS increases the risk for hepatocellular carcinoma by causing diabetes and obesity.
Dr. Goodrich and his colleagues tried to address this question by adjusting for baseline body mass index (BMI) and diabetes diagnosis in their statistical analysis.
After adjusting for BMI, they found that the association between PFOS and hepatocellular carcinoma diminished to a threefold risk (OR, 2.90; 95% CI, 0.78-10.00) and was no longer statistically significant (P = .11).
On the other hand, adjusting for diabetes did not change the significance of the relationship between PFOS and the cancer (OR, 5.7; 95% CI, 1.10-30.00; P = .04).
The sample size was probably too small to adequately tease out this relationship, Dr. Chen said. Still, he said, “I thought it was a very, very important study.”
The levels of PFOS found in the blood of Americans has been declining since the 1999-2000 NHANES, Dr. Chen pointed out. But that’s not as reassuring as it sounds.
“The problem is that if you put a regulation limiting the use of one PFAS, what people can do is just substitute with another PFAS or another molecule, which for all we know could be equally harmful,” Dr. Chen said.
Funding was provided by the Southern California Environmental Health Science Center supported by the National Institutes of Health. Dr. Goodrich and Dr. Chen report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The correlation does not prove that PFOS causes this cancer, and more research is needed, but in the meantime, people should limit their exposure to it and others in its class, said Jesse Goodrich, PhD, a postdoctoral scholar in environmental medicine at the University of Southern California, Los Angeles.
“If you’re at risk for liver cancer because you have other risk factors, then these chemicals have the potential to kind of send you over the edge,” he told this news organization.
Dr. Goodrich and colleagues published their research online in JHEP Reports.
Dubbed “forever chemicals” because they can take thousands of years to break down, polyfluoroalkyl substances (PFAS) figure in makeup, food packaging, waterproof clothing, nonstick cookware, firefighting foams, and groundwater. They have spread through the atmosphere into rain and can be found in the blood of most Americans. PFOS is one of the most widely used PFAS.
“You can’t really escape them,” Dr. Goodrich said.
Previous research has linked PFAS to infertility, pregnancy complications, learning and behavioral problems in children, immune system issues, and higher cholesterol, as well as other cancers. Some experiments in animals suggested PFAS could cause liver cancer, and others showed a correlation between PFAS serum levels and biomarkers associated with liver cancer. But many of these health effects take a long time to develop.
“It wasn’t until we started to get really highly exposed groups of people that we started, as scientists, to be able to figure out what was going on,” said Dr. Goodrich.
High exposure, increased incidence
To measure the relationship between PFAS exposure and the incidence of hepatocellular carcinoma more definitively, Dr. Goodrich and colleagues analyzed data from the Multiethnic Cohort Study, a cohort of more than 200,000 people of African, Latin, Native Hawaiian, Japanese, and European ancestry tracked since the early 1990s in California and Hawaii. About 67,000 participants provided blood samples from 2001 to 2007.
From this cohort, the researchers found 50 people who later developed hepatocellular carcinoma. The researchers matched these patients with 50 controls of similar age at blood collection, sex, race, ethnicity, and study area who did not develop the cancer.
They found that people with more than 54.9 mcg/L of PFOS in their blood before any diagnosis of hepatocellular carcinoma were almost five times more likely to get the cancer (odds ratio 4.5; 95% confidence interval, 1.2-16.0), which was statistically significant (P = .02).
This level of PFOS corresponds to the 90th percentile found in the U.S. National Health and Nutrition Examination Survey (NHANES).
To get some idea of the mechanism by which PFOS might do its damage, the researchers also looked for linkage to levels of metabolites.
They found an overlap among high PFOS levels, hepatocellular carcinoma, and high levels of glucose, butyric acid (a short chain fatty acid), alpha-Ketoisovaleric acid (alpha branched-chain alpha-keto acid), and 7alpha-Hydroxy-3-oxo-4-cholestenoate (a bile acid). These metabolites have been associated in previous studies with metabolic disorders and liver disease.
Similarly, the researchers identified an association among the cancer, PFOS, and alterations in amino acid and glycan biosynthesis pathways.
Risk mitigation
The half-life of PFAS in the human body is about 3-7 years, said Dr. Goodrich.
“There’s not much you can do once they’re in there,” he said. “So, the focus needs to be on preventing the exposure in the first place.”
People can limit exposure by avoiding water contaminated with PFAS or filtering it out, Dr. Goodrich said. He recommended avoiding fish from contaminated waterways and nonstick cookware. The Environmental Protection Agency has more detailed recommendations.
But giving patients individualized recommendations is difficult, said Vincent Chen, MD, MS, a clinical instructor in gastroenterology at the University of Michigan, Ann Arbor, who was not involved in the study. Most clinicians don’t know their patients’ PFOS levels.
“It’s not that easy to get a test,” Dr. Chen told this news organization.
People can also mitigate their risk factors for hepatocellular carcinoma, such as a poor diet, a lack of exercise, and smoking, said Dr. Goodrich.
The researchers found that patients with hepatocellular carcinoma were more likely to be overweight and have diabetes, and PFOS was associated with higher fasting glucose levels. This raises the possibility that PFOS increases the risk for hepatocellular carcinoma by causing diabetes and obesity.
Dr. Goodrich and his colleagues tried to address this question by adjusting for baseline body mass index (BMI) and diabetes diagnosis in their statistical analysis.
After adjusting for BMI, they found that the association between PFOS and hepatocellular carcinoma diminished to a threefold risk (OR, 2.90; 95% CI, 0.78-10.00) and was no longer statistically significant (P = .11).
On the other hand, adjusting for diabetes did not change the significance of the relationship between PFOS and the cancer (OR, 5.7; 95% CI, 1.10-30.00; P = .04).
The sample size was probably too small to adequately tease out this relationship, Dr. Chen said. Still, he said, “I thought it was a very, very important study.”
The levels of PFOS found in the blood of Americans has been declining since the 1999-2000 NHANES, Dr. Chen pointed out. But that’s not as reassuring as it sounds.
“The problem is that if you put a regulation limiting the use of one PFAS, what people can do is just substitute with another PFAS or another molecule, which for all we know could be equally harmful,” Dr. Chen said.
Funding was provided by the Southern California Environmental Health Science Center supported by the National Institutes of Health. Dr. Goodrich and Dr. Chen report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JHEP REPORTS
Fewer GI docs, more alcohol-associated liver disease deaths
People are more likely to die of alcohol-associated liver disease (ALD) when there are fewer gastroenterologists in their state, researchers say.
The finding raises questions about steps that policymakers could take to increase the number of gastroenterologists and spread them more evenly around the United States.
“We found that there’s a fivefold difference in density of gastroenterologists through different states,” said Brian P. Lee, MD, MAS, an assistant professor of clinical medicine at the University of Southern California, Los Angeles.
Dr. Lee and colleagues published their findings in Clinical Gastroenterology and Hepatology.
ALD is becoming more common, and it is killing more people. Research among veterans has linked visits to gastroenterologists to a lower risk for death from liver disease.
To see whether that correlation applies more broadly, Dr. Lee and colleagues compared multiple datasets. One from the U.S. Health Resources & Service Administration provided the number of gastroenterologists per 100,000 population. The other from the U.S. Centers for Disease Control and Prevention provided ALD-related deaths per 1,000,000 adults for each state and the District of Columbia.
The researchers adjusted for many variables that could affect the relationship between the availability of gastroenterologists and deaths related to ALD, including the age distribution of the population in each state, the gender balance, race and ethnicity, binge drinking, household income, obesity, and the proportion of rural residents.
They found that for every additional gastroenterologist, there is almost one fewer ALD-related death each year per 100,000 population (9.0 [95% confidence interval, 1.3-16.7] fewer ALD-related deaths per 1,000,000 population for each additional gastroenterologist per 100,000 population).
The strength of the association appeared to plateau when there were at least 7.5 gastroenterologists per 100,000 people.
From these findings, the researchers calculated that as many as 40% of deaths from ALD nationwide could be prevented by providing more gastroenterologists in the places where they are lacking.
The mean number of gastroenterologists per 100,000 people in the United States was 4.6, and the annual ALD-related death rate was 85.6 per 1,000,000 people.
The Atlantic states had the greatest concentration of gastroenterologists and the lowest ALD-related mortality, whereas the Mountain states had the lowest concentration of gastroenterologists and the highest ALD-related mortality.
The lowest mortality related to ALD was in New Jersey, Maryland, and Hawaii, with 52 per 1,000,000 people, and the highest was in Wyoming, with 289.
Study shines spotlight on general GI care
Access to liver transplants did not make a statistically significant difference in mortality from ALD.
“It makes you realize that transplant will only be accessible for really just a small fraction of the population who needs it,” Dr. Lee told this news organization.
General gastroenterologic care appears to make a bigger difference in saving patients’ lives. “Are they getting endoscopy for bleeding from varices?” Dr. Lee asked. “Are they getting appropriate antibiotics prescribed to prevent bacterial infection of ascites?”
The concentration of primary care physicians did not reduce mortality from ALD, and neither did the concentration of substance use, behavioral disorder, and mental health counselors.
Previous research has shown that substance abuse therapy is effective. But many people do not want to undertake it, or they face barriers of transportation, language, or insurance, said Dr. Lee.
“I have many patients whose insurance will provide them access to medical visits to me but will not to substance-use rehab, for example,” he said.
To see whether the effect was more generally due to the concentration of medical specialists, the researchers examined the state-level density of ophthalmologists and dermatologists. They found no significant difference in ALD-related mortality.
The finding builds on reports by the American Gastroenterological Association and the American Association for the Study of Liver Diseases that the number of gastroenterologists has not kept up with the U.S. population nor the burden of digestive diseases, and that predicts a critical shortage in the future.
Overcoming barriers to care for liver disease
The overall supply of gastroenterologists could be increased by reducing the educational requirements and increasing the funding for fellowships, said Dr. Lee.
“We have to have a better understanding as to the barriers to gastroenterology practice in certain areas, then interventions to address those barriers and also incentives to attract gastroenterologists to those areas,” Dr. Lee said.
The study underscores the importance of access to gastroenterological care, said George Cholankeril, MD, assistant professor of medicine at Baylor College of Medicine, Houston, who was not involved in the study. That urgency has only grown as ALD has spiraled up with the COVID-19 pandemic, he said.
“Anyone in clinical practice right now will be able to say that there’s been a clear rising tide of patients with alcohol-related liver disease,” he told this news organization. “There’s an urgent need to address this and provide the necessary resources.”
Prevention remains essential, Dr. Cholankeril said.
Gastroenterologists and primary care physicians can help stem the tide of ALD by screening their patients for the disease through a tool like AUDIT (Alcohol Use Disorders Identification Test), he said. They can then refer patients to substance abuse treatment centers or to psychologists and psychiatrists.
Dr. Lee and Dr. Cholankeril report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
People are more likely to die of alcohol-associated liver disease (ALD) when there are fewer gastroenterologists in their state, researchers say.
The finding raises questions about steps that policymakers could take to increase the number of gastroenterologists and spread them more evenly around the United States.
“We found that there’s a fivefold difference in density of gastroenterologists through different states,” said Brian P. Lee, MD, MAS, an assistant professor of clinical medicine at the University of Southern California, Los Angeles.
Dr. Lee and colleagues published their findings in Clinical Gastroenterology and Hepatology.
ALD is becoming more common, and it is killing more people. Research among veterans has linked visits to gastroenterologists to a lower risk for death from liver disease.
To see whether that correlation applies more broadly, Dr. Lee and colleagues compared multiple datasets. One from the U.S. Health Resources & Service Administration provided the number of gastroenterologists per 100,000 population. The other from the U.S. Centers for Disease Control and Prevention provided ALD-related deaths per 1,000,000 adults for each state and the District of Columbia.
The researchers adjusted for many variables that could affect the relationship between the availability of gastroenterologists and deaths related to ALD, including the age distribution of the population in each state, the gender balance, race and ethnicity, binge drinking, household income, obesity, and the proportion of rural residents.
They found that for every additional gastroenterologist, there is almost one fewer ALD-related death each year per 100,000 population (9.0 [95% confidence interval, 1.3-16.7] fewer ALD-related deaths per 1,000,000 population for each additional gastroenterologist per 100,000 population).
The strength of the association appeared to plateau when there were at least 7.5 gastroenterologists per 100,000 people.
From these findings, the researchers calculated that as many as 40% of deaths from ALD nationwide could be prevented by providing more gastroenterologists in the places where they are lacking.
The mean number of gastroenterologists per 100,000 people in the United States was 4.6, and the annual ALD-related death rate was 85.6 per 1,000,000 people.
The Atlantic states had the greatest concentration of gastroenterologists and the lowest ALD-related mortality, whereas the Mountain states had the lowest concentration of gastroenterologists and the highest ALD-related mortality.
The lowest mortality related to ALD was in New Jersey, Maryland, and Hawaii, with 52 per 1,000,000 people, and the highest was in Wyoming, with 289.
Study shines spotlight on general GI care
Access to liver transplants did not make a statistically significant difference in mortality from ALD.
“It makes you realize that transplant will only be accessible for really just a small fraction of the population who needs it,” Dr. Lee told this news organization.
General gastroenterologic care appears to make a bigger difference in saving patients’ lives. “Are they getting endoscopy for bleeding from varices?” Dr. Lee asked. “Are they getting appropriate antibiotics prescribed to prevent bacterial infection of ascites?”
The concentration of primary care physicians did not reduce mortality from ALD, and neither did the concentration of substance use, behavioral disorder, and mental health counselors.
Previous research has shown that substance abuse therapy is effective. But many people do not want to undertake it, or they face barriers of transportation, language, or insurance, said Dr. Lee.
“I have many patients whose insurance will provide them access to medical visits to me but will not to substance-use rehab, for example,” he said.
To see whether the effect was more generally due to the concentration of medical specialists, the researchers examined the state-level density of ophthalmologists and dermatologists. They found no significant difference in ALD-related mortality.
The finding builds on reports by the American Gastroenterological Association and the American Association for the Study of Liver Diseases that the number of gastroenterologists has not kept up with the U.S. population nor the burden of digestive diseases, and that predicts a critical shortage in the future.
Overcoming barriers to care for liver disease
The overall supply of gastroenterologists could be increased by reducing the educational requirements and increasing the funding for fellowships, said Dr. Lee.
“We have to have a better understanding as to the barriers to gastroenterology practice in certain areas, then interventions to address those barriers and also incentives to attract gastroenterologists to those areas,” Dr. Lee said.
The study underscores the importance of access to gastroenterological care, said George Cholankeril, MD, assistant professor of medicine at Baylor College of Medicine, Houston, who was not involved in the study. That urgency has only grown as ALD has spiraled up with the COVID-19 pandemic, he said.
“Anyone in clinical practice right now will be able to say that there’s been a clear rising tide of patients with alcohol-related liver disease,” he told this news organization. “There’s an urgent need to address this and provide the necessary resources.”
Prevention remains essential, Dr. Cholankeril said.
Gastroenterologists and primary care physicians can help stem the tide of ALD by screening their patients for the disease through a tool like AUDIT (Alcohol Use Disorders Identification Test), he said. They can then refer patients to substance abuse treatment centers or to psychologists and psychiatrists.
Dr. Lee and Dr. Cholankeril report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
People are more likely to die of alcohol-associated liver disease (ALD) when there are fewer gastroenterologists in their state, researchers say.
The finding raises questions about steps that policymakers could take to increase the number of gastroenterologists and spread them more evenly around the United States.
“We found that there’s a fivefold difference in density of gastroenterologists through different states,” said Brian P. Lee, MD, MAS, an assistant professor of clinical medicine at the University of Southern California, Los Angeles.
Dr. Lee and colleagues published their findings in Clinical Gastroenterology and Hepatology.
ALD is becoming more common, and it is killing more people. Research among veterans has linked visits to gastroenterologists to a lower risk for death from liver disease.
To see whether that correlation applies more broadly, Dr. Lee and colleagues compared multiple datasets. One from the U.S. Health Resources & Service Administration provided the number of gastroenterologists per 100,000 population. The other from the U.S. Centers for Disease Control and Prevention provided ALD-related deaths per 1,000,000 adults for each state and the District of Columbia.
The researchers adjusted for many variables that could affect the relationship between the availability of gastroenterologists and deaths related to ALD, including the age distribution of the population in each state, the gender balance, race and ethnicity, binge drinking, household income, obesity, and the proportion of rural residents.
They found that for every additional gastroenterologist, there is almost one fewer ALD-related death each year per 100,000 population (9.0 [95% confidence interval, 1.3-16.7] fewer ALD-related deaths per 1,000,000 population for each additional gastroenterologist per 100,000 population).
The strength of the association appeared to plateau when there were at least 7.5 gastroenterologists per 100,000 people.
From these findings, the researchers calculated that as many as 40% of deaths from ALD nationwide could be prevented by providing more gastroenterologists in the places where they are lacking.
The mean number of gastroenterologists per 100,000 people in the United States was 4.6, and the annual ALD-related death rate was 85.6 per 1,000,000 people.
The Atlantic states had the greatest concentration of gastroenterologists and the lowest ALD-related mortality, whereas the Mountain states had the lowest concentration of gastroenterologists and the highest ALD-related mortality.
The lowest mortality related to ALD was in New Jersey, Maryland, and Hawaii, with 52 per 1,000,000 people, and the highest was in Wyoming, with 289.
Study shines spotlight on general GI care
Access to liver transplants did not make a statistically significant difference in mortality from ALD.
“It makes you realize that transplant will only be accessible for really just a small fraction of the population who needs it,” Dr. Lee told this news organization.
General gastroenterologic care appears to make a bigger difference in saving patients’ lives. “Are they getting endoscopy for bleeding from varices?” Dr. Lee asked. “Are they getting appropriate antibiotics prescribed to prevent bacterial infection of ascites?”
The concentration of primary care physicians did not reduce mortality from ALD, and neither did the concentration of substance use, behavioral disorder, and mental health counselors.
Previous research has shown that substance abuse therapy is effective. But many people do not want to undertake it, or they face barriers of transportation, language, or insurance, said Dr. Lee.
“I have many patients whose insurance will provide them access to medical visits to me but will not to substance-use rehab, for example,” he said.
To see whether the effect was more generally due to the concentration of medical specialists, the researchers examined the state-level density of ophthalmologists and dermatologists. They found no significant difference in ALD-related mortality.
The finding builds on reports by the American Gastroenterological Association and the American Association for the Study of Liver Diseases that the number of gastroenterologists has not kept up with the U.S. population nor the burden of digestive diseases, and that predicts a critical shortage in the future.
Overcoming barriers to care for liver disease
The overall supply of gastroenterologists could be increased by reducing the educational requirements and increasing the funding for fellowships, said Dr. Lee.
“We have to have a better understanding as to the barriers to gastroenterology practice in certain areas, then interventions to address those barriers and also incentives to attract gastroenterologists to those areas,” Dr. Lee said.
The study underscores the importance of access to gastroenterological care, said George Cholankeril, MD, assistant professor of medicine at Baylor College of Medicine, Houston, who was not involved in the study. That urgency has only grown as ALD has spiraled up with the COVID-19 pandemic, he said.
“Anyone in clinical practice right now will be able to say that there’s been a clear rising tide of patients with alcohol-related liver disease,” he told this news organization. “There’s an urgent need to address this and provide the necessary resources.”
Prevention remains essential, Dr. Cholankeril said.
Gastroenterologists and primary care physicians can help stem the tide of ALD by screening their patients for the disease through a tool like AUDIT (Alcohol Use Disorders Identification Test), he said. They can then refer patients to substance abuse treatment centers or to psychologists and psychiatrists.
Dr. Lee and Dr. Cholankeril report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Gene variants found to protect against liver disease
Rare gene variants are associated with a reduced risk for multiple types of liver disease, including cirrhosis, researchers say.
People with certain variants in the gene CIDEB are one-third less likely to develop any sort of liver disease, according to Aris Baras, MD, a senior vice president at Regeneron, and colleagues.
“The unprecedented protective effect that these CIDEB genetic variants have against liver disease provides us with one of our most exciting targets and potential therapeutic approaches for a notoriously hard-to-treat disease where there are currently no approved treatments,” said Dr. Baras in a press release.
Dr. Baras and colleagues published the finding in The New England Journal of Medicine.
The finding follows on a similar discovery about a common variant in the gene HSD17B13. Treatments targeting this gene are being tested in clinical trials.
To search for more such genes, the researchers analyzed human exomes – the part of the genome that codes for proteins – to look for associations between gene variants and liver function.
The researchers used exome sequencing on 542,904 people from the UK Biobank, the Geisinger Health System MyCode cohort, and other datasets.
They found that coding variants in APOB, ABCB4, SLC30A10, and TM6SF2 were associated with increased aminotransferase levels and an increased risk for liver disease.
But variants in CIDEB were associated with decreased levels of alanine aminotransferase, a biomarker of hepatocellular injury. And they were associated with a decreased risk for liver disease of any cause (odds ratio per allele, 0.67; 95% confidence interval, 0.57-0.79).
The CIDEB variants were present in only 0.7% of the persons in the study.
Zeroing in on various kinds of liver disease, the researchers found that the CIDEB variants were associated with a reduced risk for alcoholic liver disease, nonalcoholic liver disease, any liver cirrhosis, alcoholic liver cirrhosis, nonalcoholic liver cirrhosis, and viral hepatitis.
In 3,599 patients who had undergone bariatric surgery, variants in CIDEB were associated with a reduced nonalcoholic fatty liver disease activity score of –0.98 beta per allele in score units, where scores range from 0-8, with a higher score indicating more severe disease.
In patients for whom MRI data were available, those with rare coding variants in CIDEB had lower proportions of liver fat. However, percentage of liver fat did not fully explain the reduced risk for liver disease.
Pursuing another line of investigation, the researchers found that they could prevent the buildup of large lipid droplets in oleic acid-treated human hepatoma cell lines by silencing the CIDEB gene using small interfering RNA.
The association was particularly strong among people with higher body mass indices and type 2 diabetes.
The associations with the rare protective CIDEB variants were consistent across ancestries, but people of non-European ancestry, who might be disproportionately affected by liver disease, were underrepresented in the database, the researchers note.
The study was supported by Regeneron Pharmaceuticals, which also employed several of the researchers.
A version of this article first appeared on Medscape.com.
Rare gene variants are associated with a reduced risk for multiple types of liver disease, including cirrhosis, researchers say.
People with certain variants in the gene CIDEB are one-third less likely to develop any sort of liver disease, according to Aris Baras, MD, a senior vice president at Regeneron, and colleagues.
“The unprecedented protective effect that these CIDEB genetic variants have against liver disease provides us with one of our most exciting targets and potential therapeutic approaches for a notoriously hard-to-treat disease where there are currently no approved treatments,” said Dr. Baras in a press release.
Dr. Baras and colleagues published the finding in The New England Journal of Medicine.
The finding follows on a similar discovery about a common variant in the gene HSD17B13. Treatments targeting this gene are being tested in clinical trials.
To search for more such genes, the researchers analyzed human exomes – the part of the genome that codes for proteins – to look for associations between gene variants and liver function.
The researchers used exome sequencing on 542,904 people from the UK Biobank, the Geisinger Health System MyCode cohort, and other datasets.
They found that coding variants in APOB, ABCB4, SLC30A10, and TM6SF2 were associated with increased aminotransferase levels and an increased risk for liver disease.
But variants in CIDEB were associated with decreased levels of alanine aminotransferase, a biomarker of hepatocellular injury. And they were associated with a decreased risk for liver disease of any cause (odds ratio per allele, 0.67; 95% confidence interval, 0.57-0.79).
The CIDEB variants were present in only 0.7% of the persons in the study.
Zeroing in on various kinds of liver disease, the researchers found that the CIDEB variants were associated with a reduced risk for alcoholic liver disease, nonalcoholic liver disease, any liver cirrhosis, alcoholic liver cirrhosis, nonalcoholic liver cirrhosis, and viral hepatitis.
In 3,599 patients who had undergone bariatric surgery, variants in CIDEB were associated with a reduced nonalcoholic fatty liver disease activity score of –0.98 beta per allele in score units, where scores range from 0-8, with a higher score indicating more severe disease.
In patients for whom MRI data were available, those with rare coding variants in CIDEB had lower proportions of liver fat. However, percentage of liver fat did not fully explain the reduced risk for liver disease.
Pursuing another line of investigation, the researchers found that they could prevent the buildup of large lipid droplets in oleic acid-treated human hepatoma cell lines by silencing the CIDEB gene using small interfering RNA.
The association was particularly strong among people with higher body mass indices and type 2 diabetes.
The associations with the rare protective CIDEB variants were consistent across ancestries, but people of non-European ancestry, who might be disproportionately affected by liver disease, were underrepresented in the database, the researchers note.
The study was supported by Regeneron Pharmaceuticals, which also employed several of the researchers.
A version of this article first appeared on Medscape.com.
Rare gene variants are associated with a reduced risk for multiple types of liver disease, including cirrhosis, researchers say.
People with certain variants in the gene CIDEB are one-third less likely to develop any sort of liver disease, according to Aris Baras, MD, a senior vice president at Regeneron, and colleagues.
“The unprecedented protective effect that these CIDEB genetic variants have against liver disease provides us with one of our most exciting targets and potential therapeutic approaches for a notoriously hard-to-treat disease where there are currently no approved treatments,” said Dr. Baras in a press release.
Dr. Baras and colleagues published the finding in The New England Journal of Medicine.
The finding follows on a similar discovery about a common variant in the gene HSD17B13. Treatments targeting this gene are being tested in clinical trials.
To search for more such genes, the researchers analyzed human exomes – the part of the genome that codes for proteins – to look for associations between gene variants and liver function.
The researchers used exome sequencing on 542,904 people from the UK Biobank, the Geisinger Health System MyCode cohort, and other datasets.
They found that coding variants in APOB, ABCB4, SLC30A10, and TM6SF2 were associated with increased aminotransferase levels and an increased risk for liver disease.
But variants in CIDEB were associated with decreased levels of alanine aminotransferase, a biomarker of hepatocellular injury. And they were associated with a decreased risk for liver disease of any cause (odds ratio per allele, 0.67; 95% confidence interval, 0.57-0.79).
The CIDEB variants were present in only 0.7% of the persons in the study.
Zeroing in on various kinds of liver disease, the researchers found that the CIDEB variants were associated with a reduced risk for alcoholic liver disease, nonalcoholic liver disease, any liver cirrhosis, alcoholic liver cirrhosis, nonalcoholic liver cirrhosis, and viral hepatitis.
In 3,599 patients who had undergone bariatric surgery, variants in CIDEB were associated with a reduced nonalcoholic fatty liver disease activity score of –0.98 beta per allele in score units, where scores range from 0-8, with a higher score indicating more severe disease.
In patients for whom MRI data were available, those with rare coding variants in CIDEB had lower proportions of liver fat. However, percentage of liver fat did not fully explain the reduced risk for liver disease.
Pursuing another line of investigation, the researchers found that they could prevent the buildup of large lipid droplets in oleic acid-treated human hepatoma cell lines by silencing the CIDEB gene using small interfering RNA.
The association was particularly strong among people with higher body mass indices and type 2 diabetes.
The associations with the rare protective CIDEB variants were consistent across ancestries, but people of non-European ancestry, who might be disproportionately affected by liver disease, were underrepresented in the database, the researchers note.
The study was supported by Regeneron Pharmaceuticals, which also employed several of the researchers.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Neighborhood factors contribute to liver cancer disparities in Texas
Factors operating at the community level may help explain disparities in rates of hepatocellular carcinoma (HCC) across Texas.
Researchers found that the risk for HCC is higher in neighborhoods characterized by minority populations, socioeconomic disadvantage, and blue-collar workers from specific industries.
However, these relationships are not uniform across the state, report researchers from Baylor College of Medicine, Houston.
“HCC is a serious health concern in Texas, and our foundational work is a step forward to better prevent this deadly disease,” study investigator Hashem El-Serag, MD, PhD, said in a news release.
The study was published online in Clinical Gastroenterology and Hepatology.
HCC is the most common type of liver cancer in the United States, and Texas has the highest rate of HCC. Yet, within Texas, incidence rates vary by race, ethnicity, and geographic location.
The Baylor team examined these disparities at the neighborhood level, with a focus on measures of social determinants of health and the industries where most neighborhood residents work.
They identified 11,547 Texas residents diagnosed with HCC between 2011 and 2015, at a mean age of 63 years. Roughly three-quarters were men, and 44% were non-Hispanic White, 14% non-Hispanic Black, 37% Hispanic, and 5% other.
The researchers used demographics, socioeconomic status, and employment provided by the U.S. Census Bureau to characterize the neighborhoods where these people lived when they were diagnosed with HCC.
Among their key findings, the risk for HCC among African American and Hispanic residents was highest in West Texas, South Texas, and the panhandle. However, some factors, like age and socioeconomic status, were not affected by location.
Across the entire state, however, people older than 60 years and those of low socioeconomic status had a higher relative risk for HCC.
Two areas of employment – construction and service occupations – also stood out as being associated with a higher risk for HCC, whereas employment in agriculture was associated with lower risk.
The authors caution that the ecological nature of the study precludes any firm conclusions regarding a causal link between working in these industries and HCC.
“Further research, including longitudinal studies, [is] needed to clarify the roles of specific occupations in HCC risk,” corresponding author Abiodun Oluyomi, PhD, said in the news release.
“Our findings validate factors previously associated with HCC, and our geographic analysis shows areas of Texas where specific intervention strategies may be most relevant,” Dr. Oluyomi added.
This research was supported by the Cancer Prevention & Research Institute of Texas. The authors have no relevant disclosures.
A version of this article first appeared on Medscape.com.
Factors operating at the community level may help explain disparities in rates of hepatocellular carcinoma (HCC) across Texas.
Researchers found that the risk for HCC is higher in neighborhoods characterized by minority populations, socioeconomic disadvantage, and blue-collar workers from specific industries.
However, these relationships are not uniform across the state, report researchers from Baylor College of Medicine, Houston.
“HCC is a serious health concern in Texas, and our foundational work is a step forward to better prevent this deadly disease,” study investigator Hashem El-Serag, MD, PhD, said in a news release.
The study was published online in Clinical Gastroenterology and Hepatology.
HCC is the most common type of liver cancer in the United States, and Texas has the highest rate of HCC. Yet, within Texas, incidence rates vary by race, ethnicity, and geographic location.
The Baylor team examined these disparities at the neighborhood level, with a focus on measures of social determinants of health and the industries where most neighborhood residents work.
They identified 11,547 Texas residents diagnosed with HCC between 2011 and 2015, at a mean age of 63 years. Roughly three-quarters were men, and 44% were non-Hispanic White, 14% non-Hispanic Black, 37% Hispanic, and 5% other.
The researchers used demographics, socioeconomic status, and employment provided by the U.S. Census Bureau to characterize the neighborhoods where these people lived when they were diagnosed with HCC.
Among their key findings, the risk for HCC among African American and Hispanic residents was highest in West Texas, South Texas, and the panhandle. However, some factors, like age and socioeconomic status, were not affected by location.
Across the entire state, however, people older than 60 years and those of low socioeconomic status had a higher relative risk for HCC.
Two areas of employment – construction and service occupations – also stood out as being associated with a higher risk for HCC, whereas employment in agriculture was associated with lower risk.
The authors caution that the ecological nature of the study precludes any firm conclusions regarding a causal link between working in these industries and HCC.
“Further research, including longitudinal studies, [is] needed to clarify the roles of specific occupations in HCC risk,” corresponding author Abiodun Oluyomi, PhD, said in the news release.
“Our findings validate factors previously associated with HCC, and our geographic analysis shows areas of Texas where specific intervention strategies may be most relevant,” Dr. Oluyomi added.
This research was supported by the Cancer Prevention & Research Institute of Texas. The authors have no relevant disclosures.
A version of this article first appeared on Medscape.com.
Factors operating at the community level may help explain disparities in rates of hepatocellular carcinoma (HCC) across Texas.
Researchers found that the risk for HCC is higher in neighborhoods characterized by minority populations, socioeconomic disadvantage, and blue-collar workers from specific industries.
However, these relationships are not uniform across the state, report researchers from Baylor College of Medicine, Houston.
“HCC is a serious health concern in Texas, and our foundational work is a step forward to better prevent this deadly disease,” study investigator Hashem El-Serag, MD, PhD, said in a news release.
The study was published online in Clinical Gastroenterology and Hepatology.
HCC is the most common type of liver cancer in the United States, and Texas has the highest rate of HCC. Yet, within Texas, incidence rates vary by race, ethnicity, and geographic location.
The Baylor team examined these disparities at the neighborhood level, with a focus on measures of social determinants of health and the industries where most neighborhood residents work.
They identified 11,547 Texas residents diagnosed with HCC between 2011 and 2015, at a mean age of 63 years. Roughly three-quarters were men, and 44% were non-Hispanic White, 14% non-Hispanic Black, 37% Hispanic, and 5% other.
The researchers used demographics, socioeconomic status, and employment provided by the U.S. Census Bureau to characterize the neighborhoods where these people lived when they were diagnosed with HCC.
Among their key findings, the risk for HCC among African American and Hispanic residents was highest in West Texas, South Texas, and the panhandle. However, some factors, like age and socioeconomic status, were not affected by location.
Across the entire state, however, people older than 60 years and those of low socioeconomic status had a higher relative risk for HCC.
Two areas of employment – construction and service occupations – also stood out as being associated with a higher risk for HCC, whereas employment in agriculture was associated with lower risk.
The authors caution that the ecological nature of the study precludes any firm conclusions regarding a causal link between working in these industries and HCC.
“Further research, including longitudinal studies, [is] needed to clarify the roles of specific occupations in HCC risk,” corresponding author Abiodun Oluyomi, PhD, said in the news release.
“Our findings validate factors previously associated with HCC, and our geographic analysis shows areas of Texas where specific intervention strategies may be most relevant,” Dr. Oluyomi added.
This research was supported by the Cancer Prevention & Research Institute of Texas. The authors have no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Degree of PPG reduction linked with ascites control after TIPS
A reduction in portal hepatic pressure gradient (PPG) soon after implantation of a transjugular intrahepatic portosystemic shunt (TIPS) greater than 60% was associated with improved ascites control at 6 weeks in a study published in Hepatology.
“The probability of ascites resolution is much higher if PPG reduction exceeded 60% of PPG before TIPS,” wrote the researchers, led by co–first authors Alexander Queck, MD, a postdoctoral researcher in the department of internal medicine at University Hospital Frankfurt (Germany) and Goethe University Frankfurt, and Louise Schwierz, MD, of the department of internal medicine in the University Hospital Bonn (Germany). “This study suggests that, even in patients with uncomplicated TIPS insertion, a short-term follow-up 6 weeks after TIPS should be scheduled to be able to predict their course of disease.”
The authors investigated the decrease of PPG in a single-center, retrospective analysis of 341 patients with liver cirrhosis undergoing TIPS insertion for recurrent or refractory ascites between March 1994 and July 2015. During each procedure, portal and inferior vena cava pressures were invasively measured and correlated with patients’ outcomes and ascites progression over time. In 241 patients, or 71%, chronic alcohol consumption was the reason for cirrhosis development, followed by 13% with chronic viral hepatitis (n = 43). Median survival after TIPS insertion was 102 weeks, and 19 patients received liver transplants over time.
Median portal pressure before TIPS placement was 28 mm Hg, which decreased to a median of 21 mm Hg after TIPS. Median PPG levels were 19 mm Hg before TIPS and 8 mm Hg immediately after TIPS placement.
At the time of TIPS placement, 65 patients, or 19%, had hepatic encephalopathy, and nine had severe hepatic encephalopathy. Six weeks after TIPS, two had episodes of hepatic encephalopathy.
After 6 weeks, ascites significantly improved through TIPS insertion. About 47% had a complete resolution of ascites at 6 weeks, whereas 29% had ascites detectable only by ultrasound and 24% of patients still needed large-volume paracentesis. There was an association between extent of PPG reduction and ascites resolution: Median PPG reduction was 55% of initial PPG in patients with persistence of severe ascites, 58% in patients with ascites detected by ultrasound, and 65% in patients with complete resolution of ascites at 6 weeks after TIPS.
Ascites resolved in 54% of patients with higher PPG reduction (60% or above), compared with 39% of patients with lower PPG reduction (below 60%). Ascites that was detected by ultrasound in another 27% of patients with higher PPG reduction, compared with 31% of patients with lower PPG reduction. In addition, persistent severe ascites was seen in 19% of patients with higher PPG reduction, compared with 30% of patients with lower PPG reduction.
The authors also noted the importance of timing follow-up evaluation: They noted that post-TIPS follow-up is a frequent question and not yet standardized; in this study, they found that, with follow-up at 6 weeks, they could “clearly stratify the course post TIPS” and this could “detect patients at high risk of unstable course of disease.”
PPG reduction of more than 60% after TIPS correlated with resolution of severe ascites 6 weeks after TIPS, the study authors concluded.
“This is one of the first studies that highlights the optimal goal for a portal pressure gradient in the setting of refractory ascites post TIPS procedure,” said Neeral Shah, MD, an associate professor of gastroenterology and hepatology and transplant hepatology specialist at the University of Virginia, Charlottesville.
“It is exciting to see some data from patients examining a question we have always thought to be true but have never quantified,” he said. “As a clinician who refers patients for TIPS, one of my biggest concerns is that significant shunting of blood past liver tissue through a TIPS can lead to the development of confusion.”
Dr. Shah, who wasn’t involved with the study, pointed to ongoing questions about hepatic encephalopathy around TIPS. The study authors didn’t find an issue with this among their study population, and some patients had improvements in their mental status after TIPS.
“This has not been my experience in those patients with hepatic encephalopathy at baseline pre-TIPS,” Dr. Shah said. “This point will need to be clarified further, especially if we are aiming for portal pressure gradients of 10 mm Hg or less in all patients with refractory ascites.”
The study authors declared that the research was conducted without commercial or financial relationships that could be construed as a potential conflict of interest. The authors were supported by the German Research Foundation, the German Federal Ministry of Education and Research, the European Union’s Horizon 2020 research program, and Goethe University Frankfurt. Dr. Shah reported no relevant disclosures.
A reduction in portal hepatic pressure gradient (PPG) soon after implantation of a transjugular intrahepatic portosystemic shunt (TIPS) greater than 60% was associated with improved ascites control at 6 weeks in a study published in Hepatology.
“The probability of ascites resolution is much higher if PPG reduction exceeded 60% of PPG before TIPS,” wrote the researchers, led by co–first authors Alexander Queck, MD, a postdoctoral researcher in the department of internal medicine at University Hospital Frankfurt (Germany) and Goethe University Frankfurt, and Louise Schwierz, MD, of the department of internal medicine in the University Hospital Bonn (Germany). “This study suggests that, even in patients with uncomplicated TIPS insertion, a short-term follow-up 6 weeks after TIPS should be scheduled to be able to predict their course of disease.”
The authors investigated the decrease of PPG in a single-center, retrospective analysis of 341 patients with liver cirrhosis undergoing TIPS insertion for recurrent or refractory ascites between March 1994 and July 2015. During each procedure, portal and inferior vena cava pressures were invasively measured and correlated with patients’ outcomes and ascites progression over time. In 241 patients, or 71%, chronic alcohol consumption was the reason for cirrhosis development, followed by 13% with chronic viral hepatitis (n = 43). Median survival after TIPS insertion was 102 weeks, and 19 patients received liver transplants over time.
Median portal pressure before TIPS placement was 28 mm Hg, which decreased to a median of 21 mm Hg after TIPS. Median PPG levels were 19 mm Hg before TIPS and 8 mm Hg immediately after TIPS placement.
At the time of TIPS placement, 65 patients, or 19%, had hepatic encephalopathy, and nine had severe hepatic encephalopathy. Six weeks after TIPS, two had episodes of hepatic encephalopathy.
After 6 weeks, ascites significantly improved through TIPS insertion. About 47% had a complete resolution of ascites at 6 weeks, whereas 29% had ascites detectable only by ultrasound and 24% of patients still needed large-volume paracentesis. There was an association between extent of PPG reduction and ascites resolution: Median PPG reduction was 55% of initial PPG in patients with persistence of severe ascites, 58% in patients with ascites detected by ultrasound, and 65% in patients with complete resolution of ascites at 6 weeks after TIPS.
Ascites resolved in 54% of patients with higher PPG reduction (60% or above), compared with 39% of patients with lower PPG reduction (below 60%). Ascites that was detected by ultrasound in another 27% of patients with higher PPG reduction, compared with 31% of patients with lower PPG reduction. In addition, persistent severe ascites was seen in 19% of patients with higher PPG reduction, compared with 30% of patients with lower PPG reduction.
The authors also noted the importance of timing follow-up evaluation: They noted that post-TIPS follow-up is a frequent question and not yet standardized; in this study, they found that, with follow-up at 6 weeks, they could “clearly stratify the course post TIPS” and this could “detect patients at high risk of unstable course of disease.”
PPG reduction of more than 60% after TIPS correlated with resolution of severe ascites 6 weeks after TIPS, the study authors concluded.
“This is one of the first studies that highlights the optimal goal for a portal pressure gradient in the setting of refractory ascites post TIPS procedure,” said Neeral Shah, MD, an associate professor of gastroenterology and hepatology and transplant hepatology specialist at the University of Virginia, Charlottesville.
“It is exciting to see some data from patients examining a question we have always thought to be true but have never quantified,” he said. “As a clinician who refers patients for TIPS, one of my biggest concerns is that significant shunting of blood past liver tissue through a TIPS can lead to the development of confusion.”
Dr. Shah, who wasn’t involved with the study, pointed to ongoing questions about hepatic encephalopathy around TIPS. The study authors didn’t find an issue with this among their study population, and some patients had improvements in their mental status after TIPS.
“This has not been my experience in those patients with hepatic encephalopathy at baseline pre-TIPS,” Dr. Shah said. “This point will need to be clarified further, especially if we are aiming for portal pressure gradients of 10 mm Hg or less in all patients with refractory ascites.”
The study authors declared that the research was conducted without commercial or financial relationships that could be construed as a potential conflict of interest. The authors were supported by the German Research Foundation, the German Federal Ministry of Education and Research, the European Union’s Horizon 2020 research program, and Goethe University Frankfurt. Dr. Shah reported no relevant disclosures.
A reduction in portal hepatic pressure gradient (PPG) soon after implantation of a transjugular intrahepatic portosystemic shunt (TIPS) greater than 60% was associated with improved ascites control at 6 weeks in a study published in Hepatology.
“The probability of ascites resolution is much higher if PPG reduction exceeded 60% of PPG before TIPS,” wrote the researchers, led by co–first authors Alexander Queck, MD, a postdoctoral researcher in the department of internal medicine at University Hospital Frankfurt (Germany) and Goethe University Frankfurt, and Louise Schwierz, MD, of the department of internal medicine in the University Hospital Bonn (Germany). “This study suggests that, even in patients with uncomplicated TIPS insertion, a short-term follow-up 6 weeks after TIPS should be scheduled to be able to predict their course of disease.”
The authors investigated the decrease of PPG in a single-center, retrospective analysis of 341 patients with liver cirrhosis undergoing TIPS insertion for recurrent or refractory ascites between March 1994 and July 2015. During each procedure, portal and inferior vena cava pressures were invasively measured and correlated with patients’ outcomes and ascites progression over time. In 241 patients, or 71%, chronic alcohol consumption was the reason for cirrhosis development, followed by 13% with chronic viral hepatitis (n = 43). Median survival after TIPS insertion was 102 weeks, and 19 patients received liver transplants over time.
Median portal pressure before TIPS placement was 28 mm Hg, which decreased to a median of 21 mm Hg after TIPS. Median PPG levels were 19 mm Hg before TIPS and 8 mm Hg immediately after TIPS placement.
At the time of TIPS placement, 65 patients, or 19%, had hepatic encephalopathy, and nine had severe hepatic encephalopathy. Six weeks after TIPS, two had episodes of hepatic encephalopathy.
After 6 weeks, ascites significantly improved through TIPS insertion. About 47% had a complete resolution of ascites at 6 weeks, whereas 29% had ascites detectable only by ultrasound and 24% of patients still needed large-volume paracentesis. There was an association between extent of PPG reduction and ascites resolution: Median PPG reduction was 55% of initial PPG in patients with persistence of severe ascites, 58% in patients with ascites detected by ultrasound, and 65% in patients with complete resolution of ascites at 6 weeks after TIPS.
Ascites resolved in 54% of patients with higher PPG reduction (60% or above), compared with 39% of patients with lower PPG reduction (below 60%). Ascites that was detected by ultrasound in another 27% of patients with higher PPG reduction, compared with 31% of patients with lower PPG reduction. In addition, persistent severe ascites was seen in 19% of patients with higher PPG reduction, compared with 30% of patients with lower PPG reduction.
The authors also noted the importance of timing follow-up evaluation: They noted that post-TIPS follow-up is a frequent question and not yet standardized; in this study, they found that, with follow-up at 6 weeks, they could “clearly stratify the course post TIPS” and this could “detect patients at high risk of unstable course of disease.”
PPG reduction of more than 60% after TIPS correlated with resolution of severe ascites 6 weeks after TIPS, the study authors concluded.
“This is one of the first studies that highlights the optimal goal for a portal pressure gradient in the setting of refractory ascites post TIPS procedure,” said Neeral Shah, MD, an associate professor of gastroenterology and hepatology and transplant hepatology specialist at the University of Virginia, Charlottesville.
“It is exciting to see some data from patients examining a question we have always thought to be true but have never quantified,” he said. “As a clinician who refers patients for TIPS, one of my biggest concerns is that significant shunting of blood past liver tissue through a TIPS can lead to the development of confusion.”
Dr. Shah, who wasn’t involved with the study, pointed to ongoing questions about hepatic encephalopathy around TIPS. The study authors didn’t find an issue with this among their study population, and some patients had improvements in their mental status after TIPS.
“This has not been my experience in those patients with hepatic encephalopathy at baseline pre-TIPS,” Dr. Shah said. “This point will need to be clarified further, especially if we are aiming for portal pressure gradients of 10 mm Hg or less in all patients with refractory ascites.”
The study authors declared that the research was conducted without commercial or financial relationships that could be construed as a potential conflict of interest. The authors were supported by the German Research Foundation, the German Federal Ministry of Education and Research, the European Union’s Horizon 2020 research program, and Goethe University Frankfurt. Dr. Shah reported no relevant disclosures.
FROM HEPATOLOGY
Ultrasound on par with CT for evaluating sarcopenia in patients with cirrhosis
Using ultrasound (US) to evaluate sarcopenic obesity in patients with cirrhosis may offer accuracy on par with computed tomography (CT), according to investigators.
US-based assessment presents a more affordable point-of-care strategy that limits radiation exposure, which enables sequential monitoring, reported lead author Sukhpal Dhariwal, MBBS, MD, of the Postgraduate Institute of Medical Education and Research, Chandigarh, India.
“Preliminary data in patients with liver disease ... suggest that US muscle assessment–derived indices, especially thigh muscle thickness, identify sarcopenia CT-skeletal muscle index (SMI) and also predict hospitalization and mortality,” the investigators wrote in Journal of Clinical Gastroenterology. “However, the applicability of US-based techniques to measure muscle mass in the high-risk group of patients with cirrhosis and sarcopenic obesity has not been evaluated.”
To address this knowledge gap, the investigators performed both US- and CT-based muscle assessments in 52 patients with obesity and evidence of cirrhosis; 40 patients were male and the mean age was 50.9 years. In all, 20 (38.5%) were diagnosed with sarcopenia based on CT-determined SMI scores of less than 39 cm2/m2 for women and 50 cm2/m2 for men.
US showed that it was similarly capable of categorizing patients. The modality significantly differentiated individuals with or without sarcopenia based on high area under the curve values in four muscle indices: quadriceps muscle thickness (0.98), quadriceps muscle feather index (0.95), forearm muscle thickness (0.85), and forearm feather index (0.80).
Direct comparison of US-based assessment against CT-based SMI revealed positive correlations, with significant r values ranging from 0.40 to 0.58. These correlations were stronger in a male-only subgroup analysis, in which r values ranged from 0.52 to 0.70. R values were not calculated in the female subgroup because of the small sample size (n = 12).
The investigators adjusted indices for height, which may pose bias for overestimating muscle mass. Another limitation is the small sample size.
“US-based assessment of sarcopenia has excellent diagnostic accuracy and correlates highly with cross-sectional imaging-based SMI in cirrhosis patients with sarcopenic obesity,” the investigators concluded. “US may serve as an easy-to-use, point-of-care tool for assessing sarcopenia in sarcopenic obesity with the advantage of repeated sequential assessment.”
According to Jamile Wakim-Fleming, MD, of the Cleveland Clinic, “US-based muscle mass assessment seems to be reliable, reproducible, and simple to perform and should be encouraged along with nutrition assessments in all patients with cirrhosis and obesity.”
In a written comment, Dr. Wakim-Fleming noted the importance of timely monitoring and intervention in this patient population.
“Considering the morbidity and the poor outcomes associated with sarcopenic obesity and its frequency in cirrhosis, it is important to make early diagnosis and institute a management plan to improve muscle mass and function,” she said.
The study was supported the Patient-Centered Outcomes Research Institute, the American Medical Association, and the American Heart Association. The investigators disclosed additional relationships with Pfizer, Bristol Myers Squibb, and Novartis. Dr. Wakim-Fleming reported no relevant conflicts of interest.
Using ultrasound (US) to evaluate sarcopenic obesity in patients with cirrhosis may offer accuracy on par with computed tomography (CT), according to investigators.
US-based assessment presents a more affordable point-of-care strategy that limits radiation exposure, which enables sequential monitoring, reported lead author Sukhpal Dhariwal, MBBS, MD, of the Postgraduate Institute of Medical Education and Research, Chandigarh, India.
“Preliminary data in patients with liver disease ... suggest that US muscle assessment–derived indices, especially thigh muscle thickness, identify sarcopenia CT-skeletal muscle index (SMI) and also predict hospitalization and mortality,” the investigators wrote in Journal of Clinical Gastroenterology. “However, the applicability of US-based techniques to measure muscle mass in the high-risk group of patients with cirrhosis and sarcopenic obesity has not been evaluated.”
To address this knowledge gap, the investigators performed both US- and CT-based muscle assessments in 52 patients with obesity and evidence of cirrhosis; 40 patients were male and the mean age was 50.9 years. In all, 20 (38.5%) were diagnosed with sarcopenia based on CT-determined SMI scores of less than 39 cm2/m2 for women and 50 cm2/m2 for men.
US showed that it was similarly capable of categorizing patients. The modality significantly differentiated individuals with or without sarcopenia based on high area under the curve values in four muscle indices: quadriceps muscle thickness (0.98), quadriceps muscle feather index (0.95), forearm muscle thickness (0.85), and forearm feather index (0.80).
Direct comparison of US-based assessment against CT-based SMI revealed positive correlations, with significant r values ranging from 0.40 to 0.58. These correlations were stronger in a male-only subgroup analysis, in which r values ranged from 0.52 to 0.70. R values were not calculated in the female subgroup because of the small sample size (n = 12).
The investigators adjusted indices for height, which may pose bias for overestimating muscle mass. Another limitation is the small sample size.
“US-based assessment of sarcopenia has excellent diagnostic accuracy and correlates highly with cross-sectional imaging-based SMI in cirrhosis patients with sarcopenic obesity,” the investigators concluded. “US may serve as an easy-to-use, point-of-care tool for assessing sarcopenia in sarcopenic obesity with the advantage of repeated sequential assessment.”
According to Jamile Wakim-Fleming, MD, of the Cleveland Clinic, “US-based muscle mass assessment seems to be reliable, reproducible, and simple to perform and should be encouraged along with nutrition assessments in all patients with cirrhosis and obesity.”
In a written comment, Dr. Wakim-Fleming noted the importance of timely monitoring and intervention in this patient population.
“Considering the morbidity and the poor outcomes associated with sarcopenic obesity and its frequency in cirrhosis, it is important to make early diagnosis and institute a management plan to improve muscle mass and function,” she said.
The study was supported the Patient-Centered Outcomes Research Institute, the American Medical Association, and the American Heart Association. The investigators disclosed additional relationships with Pfizer, Bristol Myers Squibb, and Novartis. Dr. Wakim-Fleming reported no relevant conflicts of interest.
Using ultrasound (US) to evaluate sarcopenic obesity in patients with cirrhosis may offer accuracy on par with computed tomography (CT), according to investigators.
US-based assessment presents a more affordable point-of-care strategy that limits radiation exposure, which enables sequential monitoring, reported lead author Sukhpal Dhariwal, MBBS, MD, of the Postgraduate Institute of Medical Education and Research, Chandigarh, India.
“Preliminary data in patients with liver disease ... suggest that US muscle assessment–derived indices, especially thigh muscle thickness, identify sarcopenia CT-skeletal muscle index (SMI) and also predict hospitalization and mortality,” the investigators wrote in Journal of Clinical Gastroenterology. “However, the applicability of US-based techniques to measure muscle mass in the high-risk group of patients with cirrhosis and sarcopenic obesity has not been evaluated.”
To address this knowledge gap, the investigators performed both US- and CT-based muscle assessments in 52 patients with obesity and evidence of cirrhosis; 40 patients were male and the mean age was 50.9 years. In all, 20 (38.5%) were diagnosed with sarcopenia based on CT-determined SMI scores of less than 39 cm2/m2 for women and 50 cm2/m2 for men.
US showed that it was similarly capable of categorizing patients. The modality significantly differentiated individuals with or without sarcopenia based on high area under the curve values in four muscle indices: quadriceps muscle thickness (0.98), quadriceps muscle feather index (0.95), forearm muscle thickness (0.85), and forearm feather index (0.80).
Direct comparison of US-based assessment against CT-based SMI revealed positive correlations, with significant r values ranging from 0.40 to 0.58. These correlations were stronger in a male-only subgroup analysis, in which r values ranged from 0.52 to 0.70. R values were not calculated in the female subgroup because of the small sample size (n = 12).
The investigators adjusted indices for height, which may pose bias for overestimating muscle mass. Another limitation is the small sample size.
“US-based assessment of sarcopenia has excellent diagnostic accuracy and correlates highly with cross-sectional imaging-based SMI in cirrhosis patients with sarcopenic obesity,” the investigators concluded. “US may serve as an easy-to-use, point-of-care tool for assessing sarcopenia in sarcopenic obesity with the advantage of repeated sequential assessment.”
According to Jamile Wakim-Fleming, MD, of the Cleveland Clinic, “US-based muscle mass assessment seems to be reliable, reproducible, and simple to perform and should be encouraged along with nutrition assessments in all patients with cirrhosis and obesity.”
In a written comment, Dr. Wakim-Fleming noted the importance of timely monitoring and intervention in this patient population.
“Considering the morbidity and the poor outcomes associated with sarcopenic obesity and its frequency in cirrhosis, it is important to make early diagnosis and institute a management plan to improve muscle mass and function,” she said.
The study was supported the Patient-Centered Outcomes Research Institute, the American Medical Association, and the American Heart Association. The investigators disclosed additional relationships with Pfizer, Bristol Myers Squibb, and Novartis. Dr. Wakim-Fleming reported no relevant conflicts of interest.
FROM JAMA INTERNAL MEDICINE