User login
How to refine your approach to peripheral arterial disease
Peripheral arterial disease (PAD), the progressive disorder that results in ischemia to distal vascular territories as a result of atherosclerosis, spans a wide range of presentations, from minimally symptomatic disease to limb ischemia secondary to acute or chronic occlusion.
The prevalence of PAD is variable, due to differing diagnostic criteria used in studies, but PAD appears to affect 1 in every 22 people older than age 40.1 However, since PAD incidence increases with age, it is increasing in prevalence as the US population ages.1-3
PAD is associated with increased hospitalizations and decreased quality of life.4 Patients with PAD have an estimated 30% 5-year risk for myocardial infarction, stroke, or death from a vascular cause.3
Screening. Although PAD is underdiagnosed and appears to be undertreated,3 population-based screening for PAD in asymptomatic patients is not recommended. A Cochrane review found no studies evaluating the benefit of asymptomatic population-based screening.5 Similarly, in 2018, the USPSTF performed a comprehensive review and found no studies to support routine screening and determined there was insufficient evidence to recommend it.6,7
Risk factors and associated comorbidities
PAD risk factors, like the ones detailed below, have a potentiating effect. The presence of 2 risk factors doubles PAD risk, while 3 or more risk factors increase PAD risk by a factor of 10.1
Increasing age is the greatest single risk factor for PAD.1,2,8,9 Researchers using data from the National Health and Nutrition Examination Survey (NHANES) found that the prevalence of PAD increased from 1.4% in individuals ages 40 to 49 years to almost 17% in those age 70 or older.1
Demographic characteristics. Most studies demonstrate a higher risk for PAD in men.1-3,10 African-American patients have more than twice the risk for PAD, compared with Whites, even after adjustment for the increased prevalence of associated diseases such as hypertension and diabetes in this population.1-3,10
Continue to: Genetics...
Genetics. A study performed by the National Heart Lung and Blood Institute suggested that genetic correlations between twins were more important than environmental factors in the development of PAD.11
Smoking. Most population studies show smoking to be the greatest modifiable risk factor for PAD. An analysis of the NHANES data yielded an odds ratio (OR) of 4.1 for current smokers and of 1.8 for former smokers.1 Risk increases linearly with cumulative years of smoking.1,2,9,10
Diabetes is another significant modifiable risk factor, increasing PAD risk by 2.5 times.2 Diabetes is also associated with increases in functional limitation from claudication, risk for acute coronary syndrome, and progression to amputation.1
Hypertension nearly doubles the risk for PAD, and poor control further increases this risk.2,9,10
Chronic kidney disease (CKD). Patients with CKD have a progressively higher prevalence of PAD with worsening renal function.1 There is also an association between CKD and increased morbidity, revascularization failure, and increased mortality.1
Two additional risk factors that are less well understood are dyslipidemia and chronic inflammation. There is conflicting data regarding the role of individual components of cholesterol and their effect on PAD, although lipoprotein (a) has been shown to be an independent risk factor for both the development and progression of PAD.12 Similarly, chronic inflammation has been shown to play a role in the initiation and progression of the disease, although the role of inflammatory markers in evaluation and treatment is unclear and assessment for these purposes is not currently recommended.12,13
Continue to: Diagnosis...
Diagnosis
Clinical presentation
Lower extremity pain is the hallmark symptom of PAD, but presentation varies. The classic presentation is claudication, pain within a defined muscle group that occurs with exertion and is relieved by rest. Claudication is most common in the calf but also occurs in the buttock/thigh and the foot.
However, most patients with PAD present with pain that does not fit the definition of claudication. Patients with comorbidities, physical inactivity, and neuropathy are more likely to present with atypical pain.14 These patients may demonstrate critical or acute limb ischemia, characterized by pain at rest and most often localized to the forefoot and toes. Patients with critical limb ischemia may also present with nonhealing wounds/ulcers or gangrene.15
Physical exam findings can support the diagnosis of PAD, but none are reliable enough to rule the diagnosis in or out. Findings suggestive of PAD include cool skin, presence of a bruit (iliac, femoral, or popliteal), and palpable pulse abnormality. Multiple abnormal physical exam findings increase the likelihood of PAD, while the absence of a bruit or palpable pulse abnormality makes PAD less likely.16 In patients with PAD, an associated wound/ulcer is most often distal in the foot and usually appears dry.17
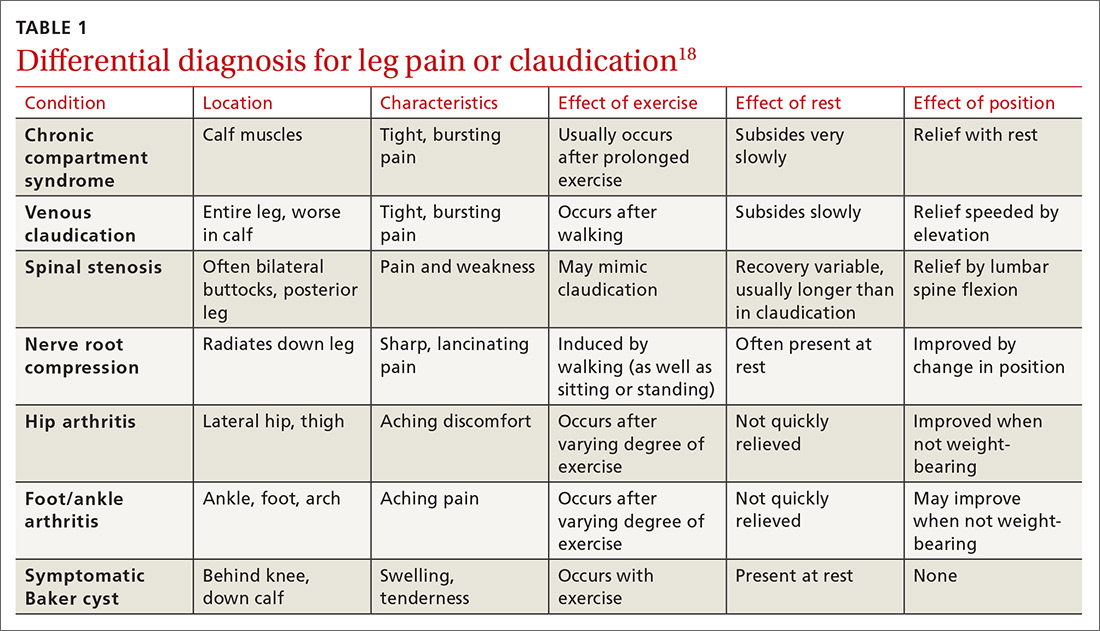
The differential diagnosis for intermittent leg pain is broad and includes neurologic, musculoskeletal, and venous etiologies. Table 118 lists some common alternate diagnoses for patients presenting with leg pain or claudication.
Continue to: Diagnostic testing...
Diagnostic testing
An ankle-brachial index (ABI) test should be performed in patients with history or physical exam findings suggestive of PAD. A resting ABI is performed with the patient in the supine position, with measurement of systolic blood pressure in both arms and ankles using a Doppler ultrasound device. Table 213 outlines ABI scoring and interpretation.
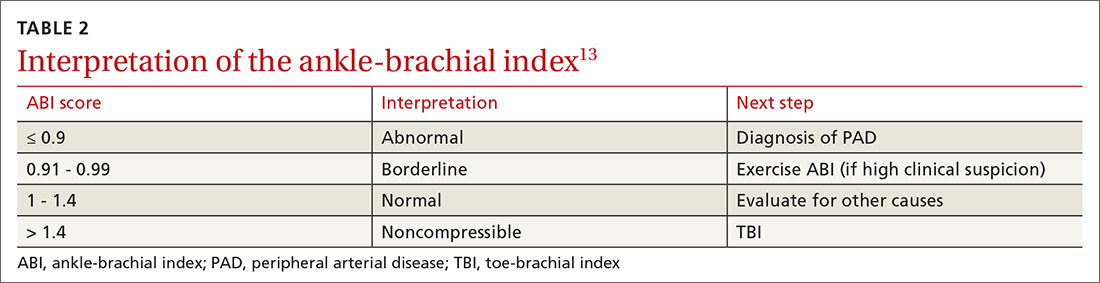
An ABI > 1.4 is an invalid measurement, indicating that the arteries are too calcified to be compressed. These highly elevated ABI measurements are common in patients with diabetes and/or advanced CKD. In these patients, a toe-brachial index (TBI) test should be performed, because the digital arteries are almost always compressible.13
Patients with symptomatic PAD who are under consideration for revascularization may benefit from radiologic imaging of the lower extremities with duplex ultrasound, computed tomography angiography, or magnetic resonance angiography to determine the anatomic location and severity of stenosis.13
Management of PAD
Lifestyle interventions
For patients with PAD, lifestyle modifications are an essential—but challenging—component of disease management.
Continue to: Smoking cessation...
Smoking cessation. As with other atherosclerotic diseases, PAD progression is strongly correlated with smoking. A trial involving 204 active smokers with PAD showed that 5-year mortality and amputation rates dropped by more than half in those who quit smoking within a year, with numbers needed to treat (NNT) of 6 for mortality and 5 for amputation.19 Because of this dramatic effect, American College of Cardiology/American Heart Association (ACC/AHA) guidelines encourage providers to address smoking at every visit and use cessation programs and medication to increase quit rates.13
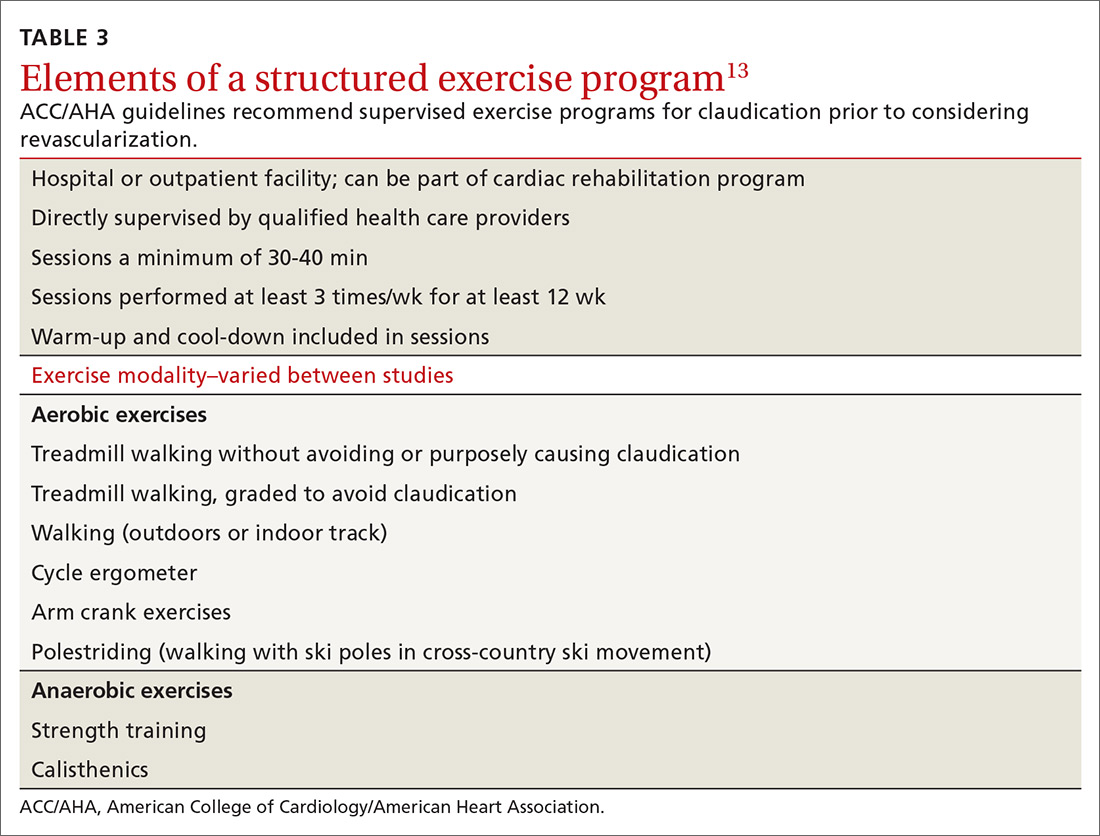
Exercise may be the most important intervention for PAD. A 2017 Cochrane review found that supervised, structured exercise programs increase pain-free and maximal walking distances by at least 20% and also improve physical and mental quality of life.20 In a trial involving 111 patients with aortoiliac PAD, supervised exercise plus medical care led to greater functional improvement than either revascularization plus medical care or medical care alone.21 In a 2018 Cochrane review, neither revascularization or revascularization added to supervised exercise were better than supervised exercise alone.22 ACC/AHA guidelines recommend supervised exercise programs for claudication prior to considering revascularization.13TABLE 313 outlines the components of a structured exercise program.
Unfortunately, the benefit of these programs has been difficult to reproduce without supervision. Another 2018 Cochrane review demonstrated significant improvement with supervised exercise and no clear improvement in patients given home exercise or advice to walk.23 A recent study examined the effect of having patients use a wearable fitness tracker for home exercise and demonstrated no benefit over usual care.24
Diet. There is some evidence that dietary interventions can prevent and possibly improve PAD. A large randomized controlled trial showed that a Mediterranean diet lowered rates of PAD over 1 year compared to a low-fat diet, with an NNT of 336 if supplemented with extra-virgin olive oil and 448 if supplemented with nuts.25 A small trial of 25 patients who consumed non-soy legumes daily for 8 weeks showed average ABI improvement of 6%, although there was no control group.26
Medical therapy to address peripheral and cardiovascular events
Standard medical therapy for coronary artery disease (CAD) is recommended for patients with PAD to reduce cardiovascular and limb events. For example, treatment of hypertension reduces cardiovascular and cerebrovascular events, and studies verify that lowering blood pressure does not worsen claudication or limb perfusion.
13TABLE 413,27-30 outlines the options for medical therapy.
Continue to: Statins...
Statins reduce cardiovascular events in PAD patients. A large study demonstrated that 40 mg of simvastatin has an NNT of 21 to prevent a coronary or cerebrovascular event in PAD, similar to the NNT of 23 seen in treatment of CAD.27 Statins also reduce adverse limb outcomes. A registry of atherosclerosis patients showed that statins have an NNT of 56 to prevent amputation in PAD and an NNT of 28 to prevent worsening claudication, critical limb ischemia, revascularization, or amputation.28
Antiplatelet therapy with low-dose aspirin or clopidogrel is recommended for symptomatic patients and for asymptomatic patients with an ABI ≤ 0.9.13 A Cochrane review demonstrated significantly reduced mortality with nonaspirin antiplatelet agents vs aspirin (NNT = 94) without increase in major bleeding.29 Only British guidelines specifically recommend clopidogrel over aspirin.31
Dual antiplatelet therapy has not shown consistent benefits over aspirin alone. ACC/AHA guidelines state that dual antiplatelet therapy is not well established for PAD but may be reasonable after revascularization.13
Voraxapar is a novel antiplatelet agent that targets the thrombin-binding receptor on platelets. However, trials show no significant coronary benefit, and slight reductions in acute limb ischemia are offset by increases in major bleeding.13
For patients receiving medical therapy, ongoing evaluation and treatment should be based on claudication symptoms and clinical assessment.
Medical therapy for claudication
Several medications have been proposed for symptomatic treatment of intermittent claudication. Cilostazol is a phosphodiesterase inhibitor with the best risk-benefit ratio. A Cochrane review showed improvements in maximal and pain-free walking distances compared to placebo and improvements in quality of life with cilostazol 100 mg taken twice daily.32 Adverse effects included headache, dizziness, palpitations, and diarrhea.29
Continue to: Pentoxifylline...
Pentoxifylline is another phosphodiesterase inhibitor with less evidence of improvement, higher adverse effect rates, and more frequent dosing. It is not recommended for treatment of intermittent claudication.13,33
Supplements. Padma 28, a Tibetan herbal formulation, appears to improve maximal walking distance with adverse effect rates similar to placebo.34 Other supplements, including vitamin E, ginkgo biloba, and omega-3 fatty acids, have no evidence of benefit.35-37
When revascularizationis needed
Patients who develop limb ischemia or lifestyle-limiting claudication despite conservative therapy are candidates for revascularization. Endovascular techniques include angioplasty, stenting, atherectomy, and precise medication delivery. Surgical approaches mainly consist of thrombectomy and bypass grafting. For intermittent claudication despite conservative care, ACC/AHA guidelines state endovascular procedures are appropriate for aortoiliac disease and reasonable for femoropopliteal disease, but unproven for infrapopliteal disease.13
Acute limb ischemia is an emergency requiring immediate intervention. Two trials revealed identical overall and amputation-free survival rates for percutaneous thrombolysis and surgical thrombectomy.38,39 ACC/AHA guidelines recommend anticoagulation with heparin followed by the revascularization technique that will most rapidly restore arterial flow.13
For chronic limb ischemia, a large trial showed angioplasty had lower initial morbidity, length of hospitalization, and cost than surgical repair. However, surgical mortality was lower after 2 years.40 ACC/AHA guidelines recommend either surgery or endovascular procedures and propose initial endovascular treatment followed by surgery if needed.13 After revascularization, the patient should be followed periodically with a clinical evaluation and ABI measurement with further consideration for routine duplex ultrasound surveillance.13
Outcomes
Patients with PAD have variable outcomes. About 70% to 80% of patients with this diagnosis will have a stable disease process with no worsening of symptoms, 10% to 20% will experience worsening symptoms over time, 5% to 10% will require revascularization within 5 years of diagnosis, and 1% to 5% will progress to critical limb ischemia, which has a 5-year amputation rate of 1% to 4%.2 Patients who require amputation have poor outcomes: Within 2 years, 30% are dead and 15% have had further amputations.18
In addition to the morbidity and mortality from its own progression, PAD is an important predictor of CAD and is associated with a significant elevation in morbidity and mortality from CAD. One small but well-designed prospective cohort study found that patients with PAD had a more than 6-fold increased risk of death from CAD than did patients without PAD.41
Acknowledgement
The authors thank Francesca Cimino, MD, FAAFP, for her help in reviewing this manuscript.
CORRESPONDENCE
Dustin K. Smith, DO, 2080 Child Street, Jacksonville, FL 32214; [email protected]
1. Eraso LH, Fukaya E, Mohler ER 3rd, et al. Peripheral arterial disease, prevalence and cumulative risk factor profile analysis. Eur J Prev Cardiol. 2014;21:704-711.
2. Pasternak RC, Criqui MH, Benjamin EJ, et al; American Heart Association. Atherosclerotic Vascular Disease Conference: Writing Group I: epidemiology. Circulation. 2004;109:2605-2612.
3. Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317-1324.
4. Olin JW, Sealove BA. Peripheral artery disease: current insight into the disease and its diagnosis and management. Mayo Clin Proc. 2010;85:678-692.
5. Andras A, Ferkert B. Screening for peripheral arterial disease. Cochrane Database Syst Rev. 2014;(4):CD010835.
6. Guirguis-Blake JM, Evans CV, Redmond N, et al. Screening for peripheral artery disease using ankle-brachial index: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;320:184-196.
7. US Preventive Services Task Force. Screening for peripheral artery disease and cardiovascular disease risk assessment with ankle-brachial index: US Preventive Services Task Force recommendation statement. JAMA. 2018;230:177-183.
8. American Heart Association Writing Group 2. Atherosclerotic Peripheral Vascular Disease Symposium II: screening for atherosclerotic vascular diseases: should nationwide programs be instituted? Circulation. 2008;118:2830-2836.
9. Berger JS, Hochman J, Lobach I, et al. Modifiable risk factor burden and the prevalence of peripheral artery disease in different vascular territories. J Vasc Surg. 2013;58:673-681.
10. Joosten MM, Pai JK, Bertoia ML, et al. Associations between conventional cardiovascular risk factors and risk of peripheral artery disease in men. JAMA. 2012;308:1660-1667.
11. Carmelli D, Fabsitz RR, Swan GE, et al. Contribution of genetic and environmental influences to ankle-brachial blood pressure index in the NHLBI Twin Study. National Heart, Lung, and Blood Institute. Am J Epidemiol. 2000;151:452-458.
12. Aboyans V, Criqui MH, Denenberg JO, et al. Risk factors for progression of peripheral arterial disease in large and small vessels. Circulation. 2006;113:2623-2629.
13. Gerald-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e726-e779.
14. McDermott MM, Greenland P, Liu K, et al. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA. 2001;286:1599-1606.
15. Cranley JJ. Ischemic rest pain. Arch Surg. 1969;98:187-188.
16. Khan NA, Rahim SA, Anand SS, et al. Does the clinical examination predict lower extremity peripheral arterial disease? JAMA. 2006;295:536-546.
17. Wennberg PW. Approach to the patient with peripheral arterial disease. Circulation. 2013;128:2241-2250.
18. Norgren L, Hiatt WR, Dormandy JA, et al. Inter-society consensus for the management of peripheral arterial disease (TASC II). Eur J Vas Endovasc Surg. 2007;33:S1-S75.
19. Armstrong EJ, Wu J, Singh GD, et al. Smoking cessation is associated with decreased mortality and improved amputation-free survival among patients with symptomatic peripheral artery disease. J Vasc Surg. 2014;60:1565-1571.
20. Lane R, Harwood A, Watson L, et al. Exercise for intermittent claudication. Cochrane Database Syst Rev. 2017;(12):CD000990.
21. Murphy TP, Cutlip DE, Regensteiner JG, et al; CLEVER Study Investigators. Supervised exercise versus primary stenting for claudication resulting from aortoiliac peripheral artery disease: six-month outcomes from the claudication: exercise versus endoluminal revascularization (CLEVER) study. Circulation. 2012;125:130-139.
22. Fakhry F, Fokkenrood HJP, Pronk S, et al. Endovascular revascularization versus conservative management for intermittent claudication. Cochrane Database Syst Rev. 2018;(3):CD010512.
23. Hageman D, Fokkenrood HJ, Gommans LN, et al. Supervised exercise therapy versus home-based exercise therapy versus walking advice for intermittent claudication. Cochrane Database Syst Rev. 2018;(4):CD005263.
24. McDermott MM, Spring B, Berger JS, et al. Effect of a home-based exercise intervention of wearable technology and telephone coaching on walking performance in peripheral artery disease: the HONOR randomized clinical trial. JAMA. 2018;319:1665-1676.
25. Ruiz-Canela M, Estruch R, Corella D, et al. Association of Mediterranean diet with peripheral artery disease: the PREDIMED randomized trial. JAMA. 2014;311:415-417.
26. Zahradka P, Wright B, Weighell W, et al. Daily non-soy legume consumption reverses vascular impairment due to peripheral artery disease. Atherosclerosis. 2013;230:310-314.
27. Heart Protection Study Collaborative Group. Randomized trial of the effects of cholesterol-lowering with simvastatin on peripheral vascular and other major vascular outcomes in 20536 people with peripheral arterial disease and other high-risk conditions. J Vasc Surg. 2007;45:645-655.
28. Kumbhani DJ, Steg G, Cannon CP, et al. Statin therapy and long-term adverse limb outcomes in patients with peripheral artery disease: insights from the REACH registry. Eur Heart J. 2014;35:2864-2872.
29. Wong PF, Chong LY, Mikhailidis DP, et al. Antiplatelet agents for intermittent claudication. Cochrane Database Syst Rev. 2011;(11):CD001272.
30. Critical Leg Ischaemia Prevention Study (CLIPS) Group, Catalano M, Born G, Peto R. Prevention of serious vascular events by aspirin amongst patients with peripheral arterial disease: randomized, double-blind trial. J Intern Med. 2007;261:276-284.
31. Morley RL, Sharma A, Horsch AD, et al. Peripheral artery disease. BMJ. 2018;360:j5842.
32. Bedenis R, Stewart M, Cleanthis M, et al. Cilostazol for intermittent claudication. Cochrane Database Syst Rev. 2014;(10):CD003748.

33. Salhiyyah K, Forster R, Senanayake E, et al. Pentoxifylline for intermittent claudication. Cochrane Database Syst Rev. 2015;(9):CD005262.
34. Stewart M, Morling JR, Maxwell H. Padma 28 for intermittent claudication. Cochrane Database Syst Rev. 2016;(3):CD007371.
35. Kleijnen J, Mackerras D. Vitamin E for intermittent claudication. Cochrane Database Syst Rev. 1998;(1):CD000987.
36. Nicolai SPA, Kruidenior LM, Bendermacher BLW, et al. Ginkgo biloba for intermittent claudication. Cochrane Database Syst Rev. 2013;(6):CD006888.
37. Campbell A, Price J, Hiatt WR. Omega-3 fatty acids for intermittent claudication. Cochrane Database Syst Rev. 2013;(7):CD003833.
38. American Surgical Association, New York Surgical Society, Philadelphia Academy of Surgery, Southern Surgical Association (US), Central Surgical Association. Results of a prospective randomized trial evaluating surgery versus thrombolysis for ischemia of the lower extremity: the STILE trial. Ann Surg. 1994;220:251-268.
39. Ouriel K, Veith FJ, Sasahara AA.
40. Bradbury AW, Ruckley CV, Fowkes FGR, et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised, controlled trial. Lancet. 2005;366:1925-1934.
41. Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381-386.
Peripheral arterial disease (PAD), the progressive disorder that results in ischemia to distal vascular territories as a result of atherosclerosis, spans a wide range of presentations, from minimally symptomatic disease to limb ischemia secondary to acute or chronic occlusion.
The prevalence of PAD is variable, due to differing diagnostic criteria used in studies, but PAD appears to affect 1 in every 22 people older than age 40.1 However, since PAD incidence increases with age, it is increasing in prevalence as the US population ages.1-3
PAD is associated with increased hospitalizations and decreased quality of life.4 Patients with PAD have an estimated 30% 5-year risk for myocardial infarction, stroke, or death from a vascular cause.3
Screening. Although PAD is underdiagnosed and appears to be undertreated,3 population-based screening for PAD in asymptomatic patients is not recommended. A Cochrane review found no studies evaluating the benefit of asymptomatic population-based screening.5 Similarly, in 2018, the USPSTF performed a comprehensive review and found no studies to support routine screening and determined there was insufficient evidence to recommend it.6,7
Risk factors and associated comorbidities
PAD risk factors, like the ones detailed below, have a potentiating effect. The presence of 2 risk factors doubles PAD risk, while 3 or more risk factors increase PAD risk by a factor of 10.1
Increasing age is the greatest single risk factor for PAD.1,2,8,9 Researchers using data from the National Health and Nutrition Examination Survey (NHANES) found that the prevalence of PAD increased from 1.4% in individuals ages 40 to 49 years to almost 17% in those age 70 or older.1

Demographic characteristics. Most studies demonstrate a higher risk for PAD in men.1-3,10 African-American patients have more than twice the risk for PAD, compared with Whites, even after adjustment for the increased prevalence of associated diseases such as hypertension and diabetes in this population.1-3,10
Continue to: Genetics...
Genetics. A study performed by the National Heart Lung and Blood Institute suggested that genetic correlations between twins were more important than environmental factors in the development of PAD.11
Smoking. Most population studies show smoking to be the greatest modifiable risk factor for PAD. An analysis of the NHANES data yielded an odds ratio (OR) of 4.1 for current smokers and of 1.8 for former smokers.1 Risk increases linearly with cumulative years of smoking.1,2,9,10
Diabetes is another significant modifiable risk factor, increasing PAD risk by 2.5 times.2 Diabetes is also associated with increases in functional limitation from claudication, risk for acute coronary syndrome, and progression to amputation.1
Hypertension nearly doubles the risk for PAD, and poor control further increases this risk.2,9,10
Chronic kidney disease (CKD). Patients with CKD have a progressively higher prevalence of PAD with worsening renal function.1 There is also an association between CKD and increased morbidity, revascularization failure, and increased mortality.1
Two additional risk factors that are less well understood are dyslipidemia and chronic inflammation. There is conflicting data regarding the role of individual components of cholesterol and their effect on PAD, although lipoprotein (a) has been shown to be an independent risk factor for both the development and progression of PAD.12 Similarly, chronic inflammation has been shown to play a role in the initiation and progression of the disease, although the role of inflammatory markers in evaluation and treatment is unclear and assessment for these purposes is not currently recommended.12,13
Continue to: Diagnosis...
Diagnosis
Clinical presentation
Lower extremity pain is the hallmark symptom of PAD, but presentation varies. The classic presentation is claudication, pain within a defined muscle group that occurs with exertion and is relieved by rest. Claudication is most common in the calf but also occurs in the buttock/thigh and the foot.
However, most patients with PAD present with pain that does not fit the definition of claudication. Patients with comorbidities, physical inactivity, and neuropathy are more likely to present with atypical pain.14 These patients may demonstrate critical or acute limb ischemia, characterized by pain at rest and most often localized to the forefoot and toes. Patients with critical limb ischemia may also present with nonhealing wounds/ulcers or gangrene.15
Physical exam findings can support the diagnosis of PAD, but none are reliable enough to rule the diagnosis in or out. Findings suggestive of PAD include cool skin, presence of a bruit (iliac, femoral, or popliteal), and palpable pulse abnormality. Multiple abnormal physical exam findings increase the likelihood of PAD, while the absence of a bruit or palpable pulse abnormality makes PAD less likely.16 In patients with PAD, an associated wound/ulcer is most often distal in the foot and usually appears dry.17
The differential diagnosis for intermittent leg pain is broad and includes neurologic, musculoskeletal, and venous etiologies. Table 118 lists some common alternate diagnoses for patients presenting with leg pain or claudication.

Continue to: Diagnostic testing...
Diagnostic testing
An ankle-brachial index (ABI) test should be performed in patients with history or physical exam findings suggestive of PAD. A resting ABI is performed with the patient in the supine position, with measurement of systolic blood pressure in both arms and ankles using a Doppler ultrasound device. Table 213 outlines ABI scoring and interpretation.

An ABI > 1.4 is an invalid measurement, indicating that the arteries are too calcified to be compressed. These highly elevated ABI measurements are common in patients with diabetes and/or advanced CKD. In these patients, a toe-brachial index (TBI) test should be performed, because the digital arteries are almost always compressible.13
Patients with symptomatic PAD who are under consideration for revascularization may benefit from radiologic imaging of the lower extremities with duplex ultrasound, computed tomography angiography, or magnetic resonance angiography to determine the anatomic location and severity of stenosis.13
Management of PAD
Lifestyle interventions
For patients with PAD, lifestyle modifications are an essential—but challenging—component of disease management.
Continue to: Smoking cessation...
Smoking cessation. As with other atherosclerotic diseases, PAD progression is strongly correlated with smoking. A trial involving 204 active smokers with PAD showed that 5-year mortality and amputation rates dropped by more than half in those who quit smoking within a year, with numbers needed to treat (NNT) of 6 for mortality and 5 for amputation.19 Because of this dramatic effect, American College of Cardiology/American Heart Association (ACC/AHA) guidelines encourage providers to address smoking at every visit and use cessation programs and medication to increase quit rates.13
Exercise may be the most important intervention for PAD. A 2017 Cochrane review found that supervised, structured exercise programs increase pain-free and maximal walking distances by at least 20% and also improve physical and mental quality of life.20 In a trial involving 111 patients with aortoiliac PAD, supervised exercise plus medical care led to greater functional improvement than either revascularization plus medical care or medical care alone.21 In a 2018 Cochrane review, neither revascularization or revascularization added to supervised exercise were better than supervised exercise alone.22 ACC/AHA guidelines recommend supervised exercise programs for claudication prior to considering revascularization.13TABLE 313 outlines the components of a structured exercise program.

Unfortunately, the benefit of these programs has been difficult to reproduce without supervision. Another 2018 Cochrane review demonstrated significant improvement with supervised exercise and no clear improvement in patients given home exercise or advice to walk.23 A recent study examined the effect of having patients use a wearable fitness tracker for home exercise and demonstrated no benefit over usual care.24
Diet. There is some evidence that dietary interventions can prevent and possibly improve PAD. A large randomized controlled trial showed that a Mediterranean diet lowered rates of PAD over 1 year compared to a low-fat diet, with an NNT of 336 if supplemented with extra-virgin olive oil and 448 if supplemented with nuts.25 A small trial of 25 patients who consumed non-soy legumes daily for 8 weeks showed average ABI improvement of 6%, although there was no control group.26
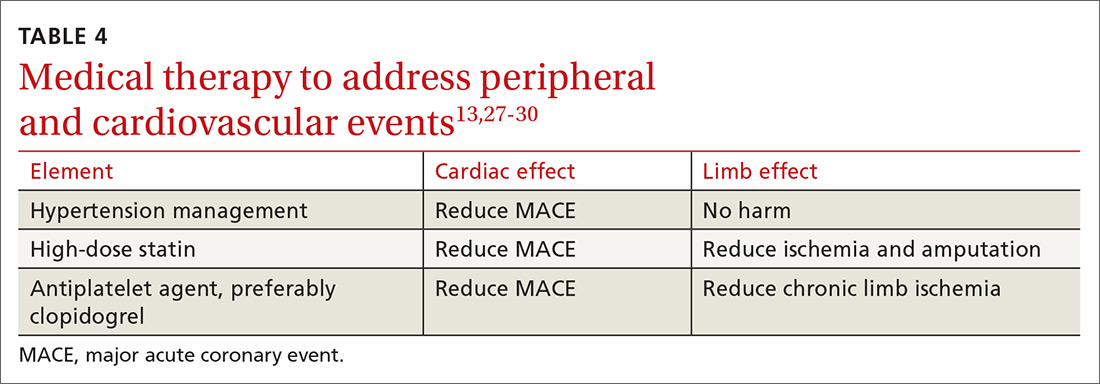
Medical therapy to address peripheral and cardiovascular events
Standard medical therapy for coronary artery disease (CAD) is recommended for patients with PAD to reduce cardiovascular and limb events. For example, treatment of hypertension reduces cardiovascular and cerebrovascular events, and studies verify that lowering blood pressure does not worsen claudication or limb perfusion.
13TABLE 413,27-30 outlines the options for medical therapy.

Continue to: Statins...
Statins reduce cardiovascular events in PAD patients. A large study demonstrated that 40 mg of simvastatin has an NNT of 21 to prevent a coronary or cerebrovascular event in PAD, similar to the NNT of 23 seen in treatment of CAD.27 Statins also reduce adverse limb outcomes. A registry of atherosclerosis patients showed that statins have an NNT of 56 to prevent amputation in PAD and an NNT of 28 to prevent worsening claudication, critical limb ischemia, revascularization, or amputation.28
Antiplatelet therapy with low-dose aspirin or clopidogrel is recommended for symptomatic patients and for asymptomatic patients with an ABI ≤ 0.9.13 A Cochrane review demonstrated significantly reduced mortality with nonaspirin antiplatelet agents vs aspirin (NNT = 94) without increase in major bleeding.29 Only British guidelines specifically recommend clopidogrel over aspirin.31
Dual antiplatelet therapy has not shown consistent benefits over aspirin alone. ACC/AHA guidelines state that dual antiplatelet therapy is not well established for PAD but may be reasonable after revascularization.13
Voraxapar is a novel antiplatelet agent that targets the thrombin-binding receptor on platelets. However, trials show no significant coronary benefit, and slight reductions in acute limb ischemia are offset by increases in major bleeding.13
For patients receiving medical therapy, ongoing evaluation and treatment should be based on claudication symptoms and clinical assessment.
Medical therapy for claudication
Several medications have been proposed for symptomatic treatment of intermittent claudication. Cilostazol is a phosphodiesterase inhibitor with the best risk-benefit ratio. A Cochrane review showed improvements in maximal and pain-free walking distances compared to placebo and improvements in quality of life with cilostazol 100 mg taken twice daily.32 Adverse effects included headache, dizziness, palpitations, and diarrhea.29
Continue to: Pentoxifylline...
Pentoxifylline is another phosphodiesterase inhibitor with less evidence of improvement, higher adverse effect rates, and more frequent dosing. It is not recommended for treatment of intermittent claudication.13,33
Supplements. Padma 28, a Tibetan herbal formulation, appears to improve maximal walking distance with adverse effect rates similar to placebo.34 Other supplements, including vitamin E, ginkgo biloba, and omega-3 fatty acids, have no evidence of benefit.35-37
When revascularizationis needed
Patients who develop limb ischemia or lifestyle-limiting claudication despite conservative therapy are candidates for revascularization. Endovascular techniques include angioplasty, stenting, atherectomy, and precise medication delivery. Surgical approaches mainly consist of thrombectomy and bypass grafting. For intermittent claudication despite conservative care, ACC/AHA guidelines state endovascular procedures are appropriate for aortoiliac disease and reasonable for femoropopliteal disease, but unproven for infrapopliteal disease.13
Acute limb ischemia is an emergency requiring immediate intervention. Two trials revealed identical overall and amputation-free survival rates for percutaneous thrombolysis and surgical thrombectomy.38,39 ACC/AHA guidelines recommend anticoagulation with heparin followed by the revascularization technique that will most rapidly restore arterial flow.13
For chronic limb ischemia, a large trial showed angioplasty had lower initial morbidity, length of hospitalization, and cost than surgical repair. However, surgical mortality was lower after 2 years.40 ACC/AHA guidelines recommend either surgery or endovascular procedures and propose initial endovascular treatment followed by surgery if needed.13 After revascularization, the patient should be followed periodically with a clinical evaluation and ABI measurement with further consideration for routine duplex ultrasound surveillance.13
Outcomes
Patients with PAD have variable outcomes. About 70% to 80% of patients with this diagnosis will have a stable disease process with no worsening of symptoms, 10% to 20% will experience worsening symptoms over time, 5% to 10% will require revascularization within 5 years of diagnosis, and 1% to 5% will progress to critical limb ischemia, which has a 5-year amputation rate of 1% to 4%.2 Patients who require amputation have poor outcomes: Within 2 years, 30% are dead and 15% have had further amputations.18
In addition to the morbidity and mortality from its own progression, PAD is an important predictor of CAD and is associated with a significant elevation in morbidity and mortality from CAD. One small but well-designed prospective cohort study found that patients with PAD had a more than 6-fold increased risk of death from CAD than did patients without PAD.41
Acknowledgement
The authors thank Francesca Cimino, MD, FAAFP, for her help in reviewing this manuscript.
CORRESPONDENCE
Dustin K. Smith, DO, 2080 Child Street, Jacksonville, FL 32214; [email protected]
Peripheral arterial disease (PAD), the progressive disorder that results in ischemia to distal vascular territories as a result of atherosclerosis, spans a wide range of presentations, from minimally symptomatic disease to limb ischemia secondary to acute or chronic occlusion.
The prevalence of PAD is variable, due to differing diagnostic criteria used in studies, but PAD appears to affect 1 in every 22 people older than age 40.1 However, since PAD incidence increases with age, it is increasing in prevalence as the US population ages.1-3
PAD is associated with increased hospitalizations and decreased quality of life.4 Patients with PAD have an estimated 30% 5-year risk for myocardial infarction, stroke, or death from a vascular cause.3
Screening. Although PAD is underdiagnosed and appears to be undertreated,3 population-based screening for PAD in asymptomatic patients is not recommended. A Cochrane review found no studies evaluating the benefit of asymptomatic population-based screening.5 Similarly, in 2018, the USPSTF performed a comprehensive review and found no studies to support routine screening and determined there was insufficient evidence to recommend it.6,7
Risk factors and associated comorbidities
PAD risk factors, like the ones detailed below, have a potentiating effect. The presence of 2 risk factors doubles PAD risk, while 3 or more risk factors increase PAD risk by a factor of 10.1
Increasing age is the greatest single risk factor for PAD.1,2,8,9 Researchers using data from the National Health and Nutrition Examination Survey (NHANES) found that the prevalence of PAD increased from 1.4% in individuals ages 40 to 49 years to almost 17% in those age 70 or older.1

Demographic characteristics. Most studies demonstrate a higher risk for PAD in men.1-3,10 African-American patients have more than twice the risk for PAD, compared with Whites, even after adjustment for the increased prevalence of associated diseases such as hypertension and diabetes in this population.1-3,10
Continue to: Genetics...
Genetics. A study performed by the National Heart Lung and Blood Institute suggested that genetic correlations between twins were more important than environmental factors in the development of PAD.11
Smoking. Most population studies show smoking to be the greatest modifiable risk factor for PAD. An analysis of the NHANES data yielded an odds ratio (OR) of 4.1 for current smokers and of 1.8 for former smokers.1 Risk increases linearly with cumulative years of smoking.1,2,9,10
Diabetes is another significant modifiable risk factor, increasing PAD risk by 2.5 times.2 Diabetes is also associated with increases in functional limitation from claudication, risk for acute coronary syndrome, and progression to amputation.1
Hypertension nearly doubles the risk for PAD, and poor control further increases this risk.2,9,10
Chronic kidney disease (CKD). Patients with CKD have a progressively higher prevalence of PAD with worsening renal function.1 There is also an association between CKD and increased morbidity, revascularization failure, and increased mortality.1
Two additional risk factors that are less well understood are dyslipidemia and chronic inflammation. There is conflicting data regarding the role of individual components of cholesterol and their effect on PAD, although lipoprotein (a) has been shown to be an independent risk factor for both the development and progression of PAD.12 Similarly, chronic inflammation has been shown to play a role in the initiation and progression of the disease, although the role of inflammatory markers in evaluation and treatment is unclear and assessment for these purposes is not currently recommended.12,13
Continue to: Diagnosis...
Diagnosis
Clinical presentation
Lower extremity pain is the hallmark symptom of PAD, but presentation varies. The classic presentation is claudication, pain within a defined muscle group that occurs with exertion and is relieved by rest. Claudication is most common in the calf but also occurs in the buttock/thigh and the foot.
However, most patients with PAD present with pain that does not fit the definition of claudication. Patients with comorbidities, physical inactivity, and neuropathy are more likely to present with atypical pain.14 These patients may demonstrate critical or acute limb ischemia, characterized by pain at rest and most often localized to the forefoot and toes. Patients with critical limb ischemia may also present with nonhealing wounds/ulcers or gangrene.15
Physical exam findings can support the diagnosis of PAD, but none are reliable enough to rule the diagnosis in or out. Findings suggestive of PAD include cool skin, presence of a bruit (iliac, femoral, or popliteal), and palpable pulse abnormality. Multiple abnormal physical exam findings increase the likelihood of PAD, while the absence of a bruit or palpable pulse abnormality makes PAD less likely.16 In patients with PAD, an associated wound/ulcer is most often distal in the foot and usually appears dry.17
The differential diagnosis for intermittent leg pain is broad and includes neurologic, musculoskeletal, and venous etiologies. Table 118 lists some common alternate diagnoses for patients presenting with leg pain or claudication.

Continue to: Diagnostic testing...
Diagnostic testing
An ankle-brachial index (ABI) test should be performed in patients with history or physical exam findings suggestive of PAD. A resting ABI is performed with the patient in the supine position, with measurement of systolic blood pressure in both arms and ankles using a Doppler ultrasound device. Table 213 outlines ABI scoring and interpretation.

An ABI > 1.4 is an invalid measurement, indicating that the arteries are too calcified to be compressed. These highly elevated ABI measurements are common in patients with diabetes and/or advanced CKD. In these patients, a toe-brachial index (TBI) test should be performed, because the digital arteries are almost always compressible.13
Patients with symptomatic PAD who are under consideration for revascularization may benefit from radiologic imaging of the lower extremities with duplex ultrasound, computed tomography angiography, or magnetic resonance angiography to determine the anatomic location and severity of stenosis.13
Management of PAD
Lifestyle interventions
For patients with PAD, lifestyle modifications are an essential—but challenging—component of disease management.
Continue to: Smoking cessation...
Smoking cessation. As with other atherosclerotic diseases, PAD progression is strongly correlated with smoking. A trial involving 204 active smokers with PAD showed that 5-year mortality and amputation rates dropped by more than half in those who quit smoking within a year, with numbers needed to treat (NNT) of 6 for mortality and 5 for amputation.19 Because of this dramatic effect, American College of Cardiology/American Heart Association (ACC/AHA) guidelines encourage providers to address smoking at every visit and use cessation programs and medication to increase quit rates.13
Exercise may be the most important intervention for PAD. A 2017 Cochrane review found that supervised, structured exercise programs increase pain-free and maximal walking distances by at least 20% and also improve physical and mental quality of life.20 In a trial involving 111 patients with aortoiliac PAD, supervised exercise plus medical care led to greater functional improvement than either revascularization plus medical care or medical care alone.21 In a 2018 Cochrane review, neither revascularization or revascularization added to supervised exercise were better than supervised exercise alone.22 ACC/AHA guidelines recommend supervised exercise programs for claudication prior to considering revascularization.13TABLE 313 outlines the components of a structured exercise program.

Unfortunately, the benefit of these programs has been difficult to reproduce without supervision. Another 2018 Cochrane review demonstrated significant improvement with supervised exercise and no clear improvement in patients given home exercise or advice to walk.23 A recent study examined the effect of having patients use a wearable fitness tracker for home exercise and demonstrated no benefit over usual care.24
Diet. There is some evidence that dietary interventions can prevent and possibly improve PAD. A large randomized controlled trial showed that a Mediterranean diet lowered rates of PAD over 1 year compared to a low-fat diet, with an NNT of 336 if supplemented with extra-virgin olive oil and 448 if supplemented with nuts.25 A small trial of 25 patients who consumed non-soy legumes daily for 8 weeks showed average ABI improvement of 6%, although there was no control group.26
Medical therapy to address peripheral and cardiovascular events
Standard medical therapy for coronary artery disease (CAD) is recommended for patients with PAD to reduce cardiovascular and limb events. For example, treatment of hypertension reduces cardiovascular and cerebrovascular events, and studies verify that lowering blood pressure does not worsen claudication or limb perfusion.
13TABLE 413,27-30 outlines the options for medical therapy.

Continue to: Statins...
Statins reduce cardiovascular events in PAD patients. A large study demonstrated that 40 mg of simvastatin has an NNT of 21 to prevent a coronary or cerebrovascular event in PAD, similar to the NNT of 23 seen in treatment of CAD.27 Statins also reduce adverse limb outcomes. A registry of atherosclerosis patients showed that statins have an NNT of 56 to prevent amputation in PAD and an NNT of 28 to prevent worsening claudication, critical limb ischemia, revascularization, or amputation.28
Antiplatelet therapy with low-dose aspirin or clopidogrel is recommended for symptomatic patients and for asymptomatic patients with an ABI ≤ 0.9.13 A Cochrane review demonstrated significantly reduced mortality with nonaspirin antiplatelet agents vs aspirin (NNT = 94) without increase in major bleeding.29 Only British guidelines specifically recommend clopidogrel over aspirin.31
Dual antiplatelet therapy has not shown consistent benefits over aspirin alone. ACC/AHA guidelines state that dual antiplatelet therapy is not well established for PAD but may be reasonable after revascularization.13
Voraxapar is a novel antiplatelet agent that targets the thrombin-binding receptor on platelets. However, trials show no significant coronary benefit, and slight reductions in acute limb ischemia are offset by increases in major bleeding.13
For patients receiving medical therapy, ongoing evaluation and treatment should be based on claudication symptoms and clinical assessment.
Medical therapy for claudication
Several medications have been proposed for symptomatic treatment of intermittent claudication. Cilostazol is a phosphodiesterase inhibitor with the best risk-benefit ratio. A Cochrane review showed improvements in maximal and pain-free walking distances compared to placebo and improvements in quality of life with cilostazol 100 mg taken twice daily.32 Adverse effects included headache, dizziness, palpitations, and diarrhea.29
Continue to: Pentoxifylline...
Pentoxifylline is another phosphodiesterase inhibitor with less evidence of improvement, higher adverse effect rates, and more frequent dosing. It is not recommended for treatment of intermittent claudication.13,33
Supplements. Padma 28, a Tibetan herbal formulation, appears to improve maximal walking distance with adverse effect rates similar to placebo.34 Other supplements, including vitamin E, ginkgo biloba, and omega-3 fatty acids, have no evidence of benefit.35-37
When revascularizationis needed
Patients who develop limb ischemia or lifestyle-limiting claudication despite conservative therapy are candidates for revascularization. Endovascular techniques include angioplasty, stenting, atherectomy, and precise medication delivery. Surgical approaches mainly consist of thrombectomy and bypass grafting. For intermittent claudication despite conservative care, ACC/AHA guidelines state endovascular procedures are appropriate for aortoiliac disease and reasonable for femoropopliteal disease, but unproven for infrapopliteal disease.13
Acute limb ischemia is an emergency requiring immediate intervention. Two trials revealed identical overall and amputation-free survival rates for percutaneous thrombolysis and surgical thrombectomy.38,39 ACC/AHA guidelines recommend anticoagulation with heparin followed by the revascularization technique that will most rapidly restore arterial flow.13
For chronic limb ischemia, a large trial showed angioplasty had lower initial morbidity, length of hospitalization, and cost than surgical repair. However, surgical mortality was lower after 2 years.40 ACC/AHA guidelines recommend either surgery or endovascular procedures and propose initial endovascular treatment followed by surgery if needed.13 After revascularization, the patient should be followed periodically with a clinical evaluation and ABI measurement with further consideration for routine duplex ultrasound surveillance.13
Outcomes
Patients with PAD have variable outcomes. About 70% to 80% of patients with this diagnosis will have a stable disease process with no worsening of symptoms, 10% to 20% will experience worsening symptoms over time, 5% to 10% will require revascularization within 5 years of diagnosis, and 1% to 5% will progress to critical limb ischemia, which has a 5-year amputation rate of 1% to 4%.2 Patients who require amputation have poor outcomes: Within 2 years, 30% are dead and 15% have had further amputations.18
In addition to the morbidity and mortality from its own progression, PAD is an important predictor of CAD and is associated with a significant elevation in morbidity and mortality from CAD. One small but well-designed prospective cohort study found that patients with PAD had a more than 6-fold increased risk of death from CAD than did patients without PAD.41
Acknowledgement
The authors thank Francesca Cimino, MD, FAAFP, for her help in reviewing this manuscript.
CORRESPONDENCE
Dustin K. Smith, DO, 2080 Child Street, Jacksonville, FL 32214; [email protected]
1. Eraso LH, Fukaya E, Mohler ER 3rd, et al. Peripheral arterial disease, prevalence and cumulative risk factor profile analysis. Eur J Prev Cardiol. 2014;21:704-711.
2. Pasternak RC, Criqui MH, Benjamin EJ, et al; American Heart Association. Atherosclerotic Vascular Disease Conference: Writing Group I: epidemiology. Circulation. 2004;109:2605-2612.
3. Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317-1324.
4. Olin JW, Sealove BA. Peripheral artery disease: current insight into the disease and its diagnosis and management. Mayo Clin Proc. 2010;85:678-692.
5. Andras A, Ferkert B. Screening for peripheral arterial disease. Cochrane Database Syst Rev. 2014;(4):CD010835.
6. Guirguis-Blake JM, Evans CV, Redmond N, et al. Screening for peripheral artery disease using ankle-brachial index: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;320:184-196.
7. US Preventive Services Task Force. Screening for peripheral artery disease and cardiovascular disease risk assessment with ankle-brachial index: US Preventive Services Task Force recommendation statement. JAMA. 2018;230:177-183.
8. American Heart Association Writing Group 2. Atherosclerotic Peripheral Vascular Disease Symposium II: screening for atherosclerotic vascular diseases: should nationwide programs be instituted? Circulation. 2008;118:2830-2836.
9. Berger JS, Hochman J, Lobach I, et al. Modifiable risk factor burden and the prevalence of peripheral artery disease in different vascular territories. J Vasc Surg. 2013;58:673-681.
10. Joosten MM, Pai JK, Bertoia ML, et al. Associations between conventional cardiovascular risk factors and risk of peripheral artery disease in men. JAMA. 2012;308:1660-1667.
11. Carmelli D, Fabsitz RR, Swan GE, et al. Contribution of genetic and environmental influences to ankle-brachial blood pressure index in the NHLBI Twin Study. National Heart, Lung, and Blood Institute. Am J Epidemiol. 2000;151:452-458.
12. Aboyans V, Criqui MH, Denenberg JO, et al. Risk factors for progression of peripheral arterial disease in large and small vessels. Circulation. 2006;113:2623-2629.
13. Gerald-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e726-e779.
14. McDermott MM, Greenland P, Liu K, et al. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA. 2001;286:1599-1606.
15. Cranley JJ. Ischemic rest pain. Arch Surg. 1969;98:187-188.
16. Khan NA, Rahim SA, Anand SS, et al. Does the clinical examination predict lower extremity peripheral arterial disease? JAMA. 2006;295:536-546.
17. Wennberg PW. Approach to the patient with peripheral arterial disease. Circulation. 2013;128:2241-2250.
18. Norgren L, Hiatt WR, Dormandy JA, et al. Inter-society consensus for the management of peripheral arterial disease (TASC II). Eur J Vas Endovasc Surg. 2007;33:S1-S75.
19. Armstrong EJ, Wu J, Singh GD, et al. Smoking cessation is associated with decreased mortality and improved amputation-free survival among patients with symptomatic peripheral artery disease. J Vasc Surg. 2014;60:1565-1571.
20. Lane R, Harwood A, Watson L, et al. Exercise for intermittent claudication. Cochrane Database Syst Rev. 2017;(12):CD000990.
21. Murphy TP, Cutlip DE, Regensteiner JG, et al; CLEVER Study Investigators. Supervised exercise versus primary stenting for claudication resulting from aortoiliac peripheral artery disease: six-month outcomes from the claudication: exercise versus endoluminal revascularization (CLEVER) study. Circulation. 2012;125:130-139.
22. Fakhry F, Fokkenrood HJP, Pronk S, et al. Endovascular revascularization versus conservative management for intermittent claudication. Cochrane Database Syst Rev. 2018;(3):CD010512.
23. Hageman D, Fokkenrood HJ, Gommans LN, et al. Supervised exercise therapy versus home-based exercise therapy versus walking advice for intermittent claudication. Cochrane Database Syst Rev. 2018;(4):CD005263.
24. McDermott MM, Spring B, Berger JS, et al. Effect of a home-based exercise intervention of wearable technology and telephone coaching on walking performance in peripheral artery disease: the HONOR randomized clinical trial. JAMA. 2018;319:1665-1676.
25. Ruiz-Canela M, Estruch R, Corella D, et al. Association of Mediterranean diet with peripheral artery disease: the PREDIMED randomized trial. JAMA. 2014;311:415-417.
26. Zahradka P, Wright B, Weighell W, et al. Daily non-soy legume consumption reverses vascular impairment due to peripheral artery disease. Atherosclerosis. 2013;230:310-314.
27. Heart Protection Study Collaborative Group. Randomized trial of the effects of cholesterol-lowering with simvastatin on peripheral vascular and other major vascular outcomes in 20536 people with peripheral arterial disease and other high-risk conditions. J Vasc Surg. 2007;45:645-655.
28. Kumbhani DJ, Steg G, Cannon CP, et al. Statin therapy and long-term adverse limb outcomes in patients with peripheral artery disease: insights from the REACH registry. Eur Heart J. 2014;35:2864-2872.
29. Wong PF, Chong LY, Mikhailidis DP, et al. Antiplatelet agents for intermittent claudication. Cochrane Database Syst Rev. 2011;(11):CD001272.
30. Critical Leg Ischaemia Prevention Study (CLIPS) Group, Catalano M, Born G, Peto R. Prevention of serious vascular events by aspirin amongst patients with peripheral arterial disease: randomized, double-blind trial. J Intern Med. 2007;261:276-284.
31. Morley RL, Sharma A, Horsch AD, et al. Peripheral artery disease. BMJ. 2018;360:j5842.
32. Bedenis R, Stewart M, Cleanthis M, et al. Cilostazol for intermittent claudication. Cochrane Database Syst Rev. 2014;(10):CD003748.

33. Salhiyyah K, Forster R, Senanayake E, et al. Pentoxifylline for intermittent claudication. Cochrane Database Syst Rev. 2015;(9):CD005262.
34. Stewart M, Morling JR, Maxwell H. Padma 28 for intermittent claudication. Cochrane Database Syst Rev. 2016;(3):CD007371.
35. Kleijnen J, Mackerras D. Vitamin E for intermittent claudication. Cochrane Database Syst Rev. 1998;(1):CD000987.
36. Nicolai SPA, Kruidenior LM, Bendermacher BLW, et al. Ginkgo biloba for intermittent claudication. Cochrane Database Syst Rev. 2013;(6):CD006888.
37. Campbell A, Price J, Hiatt WR. Omega-3 fatty acids for intermittent claudication. Cochrane Database Syst Rev. 2013;(7):CD003833.
38. American Surgical Association, New York Surgical Society, Philadelphia Academy of Surgery, Southern Surgical Association (US), Central Surgical Association. Results of a prospective randomized trial evaluating surgery versus thrombolysis for ischemia of the lower extremity: the STILE trial. Ann Surg. 1994;220:251-268.
39. Ouriel K, Veith FJ, Sasahara AA.
40. Bradbury AW, Ruckley CV, Fowkes FGR, et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised, controlled trial. Lancet. 2005;366:1925-1934.
41. Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381-386.
1. Eraso LH, Fukaya E, Mohler ER 3rd, et al. Peripheral arterial disease, prevalence and cumulative risk factor profile analysis. Eur J Prev Cardiol. 2014;21:704-711.
2. Pasternak RC, Criqui MH, Benjamin EJ, et al; American Heart Association. Atherosclerotic Vascular Disease Conference: Writing Group I: epidemiology. Circulation. 2004;109:2605-2612.
3. Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317-1324.
4. Olin JW, Sealove BA. Peripheral artery disease: current insight into the disease and its diagnosis and management. Mayo Clin Proc. 2010;85:678-692.
5. Andras A, Ferkert B. Screening for peripheral arterial disease. Cochrane Database Syst Rev. 2014;(4):CD010835.
6. Guirguis-Blake JM, Evans CV, Redmond N, et al. Screening for peripheral artery disease using ankle-brachial index: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;320:184-196.
7. US Preventive Services Task Force. Screening for peripheral artery disease and cardiovascular disease risk assessment with ankle-brachial index: US Preventive Services Task Force recommendation statement. JAMA. 2018;230:177-183.
8. American Heart Association Writing Group 2. Atherosclerotic Peripheral Vascular Disease Symposium II: screening for atherosclerotic vascular diseases: should nationwide programs be instituted? Circulation. 2008;118:2830-2836.
9. Berger JS, Hochman J, Lobach I, et al. Modifiable risk factor burden and the prevalence of peripheral artery disease in different vascular territories. J Vasc Surg. 2013;58:673-681.
10. Joosten MM, Pai JK, Bertoia ML, et al. Associations between conventional cardiovascular risk factors and risk of peripheral artery disease in men. JAMA. 2012;308:1660-1667.
11. Carmelli D, Fabsitz RR, Swan GE, et al. Contribution of genetic and environmental influences to ankle-brachial blood pressure index in the NHLBI Twin Study. National Heart, Lung, and Blood Institute. Am J Epidemiol. 2000;151:452-458.
12. Aboyans V, Criqui MH, Denenberg JO, et al. Risk factors for progression of peripheral arterial disease in large and small vessels. Circulation. 2006;113:2623-2629.
13. Gerald-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e726-e779.
14. McDermott MM, Greenland P, Liu K, et al. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA. 2001;286:1599-1606.
15. Cranley JJ. Ischemic rest pain. Arch Surg. 1969;98:187-188.
16. Khan NA, Rahim SA, Anand SS, et al. Does the clinical examination predict lower extremity peripheral arterial disease? JAMA. 2006;295:536-546.
17. Wennberg PW. Approach to the patient with peripheral arterial disease. Circulation. 2013;128:2241-2250.
18. Norgren L, Hiatt WR, Dormandy JA, et al. Inter-society consensus for the management of peripheral arterial disease (TASC II). Eur J Vas Endovasc Surg. 2007;33:S1-S75.
19. Armstrong EJ, Wu J, Singh GD, et al. Smoking cessation is associated with decreased mortality and improved amputation-free survival among patients with symptomatic peripheral artery disease. J Vasc Surg. 2014;60:1565-1571.
20. Lane R, Harwood A, Watson L, et al. Exercise for intermittent claudication. Cochrane Database Syst Rev. 2017;(12):CD000990.
21. Murphy TP, Cutlip DE, Regensteiner JG, et al; CLEVER Study Investigators. Supervised exercise versus primary stenting for claudication resulting from aortoiliac peripheral artery disease: six-month outcomes from the claudication: exercise versus endoluminal revascularization (CLEVER) study. Circulation. 2012;125:130-139.
22. Fakhry F, Fokkenrood HJP, Pronk S, et al. Endovascular revascularization versus conservative management for intermittent claudication. Cochrane Database Syst Rev. 2018;(3):CD010512.
23. Hageman D, Fokkenrood HJ, Gommans LN, et al. Supervised exercise therapy versus home-based exercise therapy versus walking advice for intermittent claudication. Cochrane Database Syst Rev. 2018;(4):CD005263.
24. McDermott MM, Spring B, Berger JS, et al. Effect of a home-based exercise intervention of wearable technology and telephone coaching on walking performance in peripheral artery disease: the HONOR randomized clinical trial. JAMA. 2018;319:1665-1676.
25. Ruiz-Canela M, Estruch R, Corella D, et al. Association of Mediterranean diet with peripheral artery disease: the PREDIMED randomized trial. JAMA. 2014;311:415-417.
26. Zahradka P, Wright B, Weighell W, et al. Daily non-soy legume consumption reverses vascular impairment due to peripheral artery disease. Atherosclerosis. 2013;230:310-314.
27. Heart Protection Study Collaborative Group. Randomized trial of the effects of cholesterol-lowering with simvastatin on peripheral vascular and other major vascular outcomes in 20536 people with peripheral arterial disease and other high-risk conditions. J Vasc Surg. 2007;45:645-655.
28. Kumbhani DJ, Steg G, Cannon CP, et al. Statin therapy and long-term adverse limb outcomes in patients with peripheral artery disease: insights from the REACH registry. Eur Heart J. 2014;35:2864-2872.
29. Wong PF, Chong LY, Mikhailidis DP, et al. Antiplatelet agents for intermittent claudication. Cochrane Database Syst Rev. 2011;(11):CD001272.
30. Critical Leg Ischaemia Prevention Study (CLIPS) Group, Catalano M, Born G, Peto R. Prevention of serious vascular events by aspirin amongst patients with peripheral arterial disease: randomized, double-blind trial. J Intern Med. 2007;261:276-284.
31. Morley RL, Sharma A, Horsch AD, et al. Peripheral artery disease. BMJ. 2018;360:j5842.
32. Bedenis R, Stewart M, Cleanthis M, et al. Cilostazol for intermittent claudication. Cochrane Database Syst Rev. 2014;(10):CD003748.

33. Salhiyyah K, Forster R, Senanayake E, et al. Pentoxifylline for intermittent claudication. Cochrane Database Syst Rev. 2015;(9):CD005262.
34. Stewart M, Morling JR, Maxwell H. Padma 28 for intermittent claudication. Cochrane Database Syst Rev. 2016;(3):CD007371.
35. Kleijnen J, Mackerras D. Vitamin E for intermittent claudication. Cochrane Database Syst Rev. 1998;(1):CD000987.
36. Nicolai SPA, Kruidenior LM, Bendermacher BLW, et al. Ginkgo biloba for intermittent claudication. Cochrane Database Syst Rev. 2013;(6):CD006888.
37. Campbell A, Price J, Hiatt WR. Omega-3 fatty acids for intermittent claudication. Cochrane Database Syst Rev. 2013;(7):CD003833.
38. American Surgical Association, New York Surgical Society, Philadelphia Academy of Surgery, Southern Surgical Association (US), Central Surgical Association. Results of a prospective randomized trial evaluating surgery versus thrombolysis for ischemia of the lower extremity: the STILE trial. Ann Surg. 1994;220:251-268.
39. Ouriel K, Veith FJ, Sasahara AA.
40. Bradbury AW, Ruckley CV, Fowkes FGR, et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised, controlled trial. Lancet. 2005;366:1925-1934.
41. Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381-386.
PRACTICE RECOMMENDATIONS
❯ Use the ankle-brachial index for diagnosis in patients with history/physical exam findings suggestive of peripheral arterial disease (PAD). A
❯ Strongly encourage smoking cessation in patients with PAD as doing so reduces 5-year mortality and amputation rates. B
❯ Use structured exercise programs for patients with intermittent claudication prior to consideration of revascularization; doing so offers similar benefit and lower risks. A
❯ Recommend revascularization for patients who have limb ischemia or lifestyle-limiting claudication despite medical and exercise therapy. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Pediatric regimens better for adolescents/young adults with aggressive B-cell NHL
Adolescents and young adults with aggressive mature B-cell non-Hodgkin lymphomas appear to have better outcomes when they’re treated under pediatric protocols rather than adult regimens, Canadian investigators say.
Results of a study of patients from the ages of 15 to 21 years with either diffuse large B-cell lymphoma (DLBCL) or Burkitt’s lymphoma treated at regional or community cancer centers in the province of Ontario indicated that adolescents and young adult (AYA) patients treated at adult centers had a more than fourfold risk for disease relapse or progression, compared with their counterparts who were treated at pediatric centers, reported Sumit Gupta, MD, PhD, from the Hospital for Sick Children in Toronto and colleagues.
“Our data suggest that pediatric approaches are associated with improved event-free survival and overall survival, primarily due to a decrease in the risk of relapse or progression, while still using lower cumulative doses of chemotherapy,” he said in an oral abstract presented at the American Society of Hematology annual meeting, held virtually.
The findings echo those seen in the treatment of patients with acute lymphoblastic leukemia (ALL). As previously reported, a study from Nordic and Baltic countries showed that young adults with ALL who were treated with a pediatric regimen had a 4-year event-free survival rate of 73%, compared with 42% for historical controls.
Similarly, a prospective U.S. study reported in 2014 showed that AYA with ALL treated with a pediatric regimen had better overall and event-free survival rates, compared with historical controls.
As with ALL, pediatric and adult regimens for treatment of patients with aggressive mature B-cell NHL differ substantially, with pediatric patients receiving more intensive short-term therapy with lower cumulative doses.
In addition, while pediatric regimens for DLBCL and Burkitt’s lymphoma are identical, adult regimens differ substantially between the two histologies, Dr. Gupta pointed out.
Adult regimens for DLBCL most often incorporate CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone) or CHOP plus rituximab (R-CHOP), whereas Burkitt’s lymphoma in adults is generally treated with more aggressive multidrug regimens, in combination with rituximab.
Rituximab was incorporated into adults’ regimens far earlier than in pediatric regimens, with Food and Drug Administration approval of rituximab in frontline therapy of adults with DLBCL in 2006, “whereas the first pediatric large-scale randomized controlled trial of rituximab in pediatric mature B-cell lymphoma was only published earlier this year,” he noted.
Population-based study
To see how treatment patterns for AYA patients with aggressive mature B-cell non-Hodgkin lymphomas differ between pediatric and adult centers, Dr. Gupta and colleagues conducted a population-based study of all AYA in Ontario diagnosed with Burkitt’s or DLBCL from the ages of 15 to 21 years from 1992 through 2012.
AYA from the ages of 15 to 18 years who were treated at pediatric centers were identified through the Provincial Pediatric Oncology Registry, which includes data on demographics, disease treatment, and outcomes from each of Ontario’s five childhood cancer treatments centers.
Adolescents and young adults from 15 to 21 years who were treated at adult centers with adult regimens were identified through the Ontario Cancer Registry using chart abstraction by trained personnel at all treatment centers, with all data validated by clinician reviewers.
A total of 176 patients were identified, 129 with DLBCL and 47 with Burkitt’s lymphoma. In all, 62 of the 176 patients (35.2%) were treated in pediatric centers. Not surprisingly, multivariable analysis showed that AYA treated in adult centers were older, and more likely to have been treated earlier in the study period.
Comparing treatment patterns by locus of care, the investigators found that patients with DLBCL in pediatric centers received half of the cumulative anthracycline doses as those in adult centers (150 mg/m2 vs. 300 mg/m2; P < .001) and about 75% of cumulative alkylating agent doses (3,300 mg/m2 vs. 4,465 mg/m2; P = .009).
Patients with Burkitt’s lymphoma had identical exposures to anthracyclines in pediatric vs. adult centers (120 mg/m2), but those treated in pediatric centers had half the exposure to alkylators as those treated in adult centers (3,300 mg/m2 vs. 6,600 mg/m2; P = .03).
Among patients with DLBCL, none of those treated at pediatric centers received rituximab, compared with 32.3% of those treated at adult centers (P < .001), whereas only a handful of patients with Burkitt’s lymphoma received rituximab in both pediatric and adult centers (nonsignificant).
Among all patients. 5-year event-free survival was 82.3% for those treated in pediatric centers, compared with 66.7% for those treated in adult centers (P = .02). Respective 5-year overall survival rates were 85.5% and 71.1% (P = .03).
Looking at survival by histology, the investigators saw that 5-year event-free survival for patients with DLBCL was 83.3% when they were treated like children vs. 66.7% when they were treated like adults (P = .04). Respective 5-year overall survival rates were 88.9% and 72% (P = .04).
Both event-free survival (80.8% vs. 66.7%) and overall survival (80.8% vs. 66.7%) were numerically but not statistically higher among patients with Burkitt’s treated at pediatric vs. adult centers.
An analysis adjusting for disease histology, stage, and time period of diagnosis showed that treatment at an adult center was associated with higher risk for death, with a hazard ratio of 2.4 (P = .03).
Additionally, an analysis adjusted for age, disease stage, and histology showed that patients treated in adult centers had a significantly increased risk of relapse or progression, compared with a HR of 4.4 (95% confidence interval; P = .008).
There were no significant differences in the risk of treatment-related mortality between the center types, however.
“It is important to note, however, that pediatric approaches to mature B-cell NHL [non-Hodgkin lymphoma] are associated with increased inpatient needs as compared to adult approaches, and with greater supportive care requirements. Thus the safety of such approaches in adults centers need to be established,” Dr. Gupta said.
Lower doses, better outcomes
In the question and answer session following the presentation, Jennifer Teichman, MD, MSc, a fellow in hematology at the University of Toronto who was not involved in the study asked why patients treated at adult centers would have higher relapse rates despite receiving higher doses of chemotherapy, noting that the poorer outcomes in those patients were not attributable to treatment-related mortality.
“I think one of the distinctions is that higher cumulative doses versus higher intensity of treatment over a shorter period of time are two different things, perhaps, and so giving lower cumulative doses but over a short period of time, and so giving higher intensity within that short period of time, may be what explains the higher success rate in pediatric trials,” Dr. Gupta said.
R. Michael Crump, MD, from the Princess Margaret Cancer Center, also in Toronto, asked whether the study results could have been influenced by differences between the pediatric center and adult center datasets in regard to pathology review, staging information, and International Prognostic Index.
Dr. Gupta acknowledged that, while the pediatric data were captured prospectively at each center by pediatric cancer registry staff and adult data were extracted retrospectively by trained chart reviewers, “the information that we were collecting was relatively basic – basic stage, basic histology, and that is a limitation.”
He also noted that clinicians reviewed the submitted retrospective data for completeness and had the ability to request chart extractors to return to a particular record for additional information or to correct potential errors.
The study was supported by the Canadian Institutes of Health Research, the C17 Council on Children’s Cancer & Blood Disorders, and the Pediatric Oncology Group of Ontario. Dr. Gupta, Dr. Teichman, and Dr. Crump all reported no relevant conflicts of interest.
SOURCE: Gupta S et al. ASH 2020, Abstract 708.
Adolescents and young adults with aggressive mature B-cell non-Hodgkin lymphomas appear to have better outcomes when they’re treated under pediatric protocols rather than adult regimens, Canadian investigators say.
Results of a study of patients from the ages of 15 to 21 years with either diffuse large B-cell lymphoma (DLBCL) or Burkitt’s lymphoma treated at regional or community cancer centers in the province of Ontario indicated that adolescents and young adult (AYA) patients treated at adult centers had a more than fourfold risk for disease relapse or progression, compared with their counterparts who were treated at pediatric centers, reported Sumit Gupta, MD, PhD, from the Hospital for Sick Children in Toronto and colleagues.
“Our data suggest that pediatric approaches are associated with improved event-free survival and overall survival, primarily due to a decrease in the risk of relapse or progression, while still using lower cumulative doses of chemotherapy,” he said in an oral abstract presented at the American Society of Hematology annual meeting, held virtually.
The findings echo those seen in the treatment of patients with acute lymphoblastic leukemia (ALL). As previously reported, a study from Nordic and Baltic countries showed that young adults with ALL who were treated with a pediatric regimen had a 4-year event-free survival rate of 73%, compared with 42% for historical controls.
Similarly, a prospective U.S. study reported in 2014 showed that AYA with ALL treated with a pediatric regimen had better overall and event-free survival rates, compared with historical controls.
As with ALL, pediatric and adult regimens for treatment of patients with aggressive mature B-cell NHL differ substantially, with pediatric patients receiving more intensive short-term therapy with lower cumulative doses.
In addition, while pediatric regimens for DLBCL and Burkitt’s lymphoma are identical, adult regimens differ substantially between the two histologies, Dr. Gupta pointed out.
Adult regimens for DLBCL most often incorporate CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone) or CHOP plus rituximab (R-CHOP), whereas Burkitt’s lymphoma in adults is generally treated with more aggressive multidrug regimens, in combination with rituximab.
Rituximab was incorporated into adults’ regimens far earlier than in pediatric regimens, with Food and Drug Administration approval of rituximab in frontline therapy of adults with DLBCL in 2006, “whereas the first pediatric large-scale randomized controlled trial of rituximab in pediatric mature B-cell lymphoma was only published earlier this year,” he noted.
Population-based study
To see how treatment patterns for AYA patients with aggressive mature B-cell non-Hodgkin lymphomas differ between pediatric and adult centers, Dr. Gupta and colleagues conducted a population-based study of all AYA in Ontario diagnosed with Burkitt’s or DLBCL from the ages of 15 to 21 years from 1992 through 2012.
AYA from the ages of 15 to 18 years who were treated at pediatric centers were identified through the Provincial Pediatric Oncology Registry, which includes data on demographics, disease treatment, and outcomes from each of Ontario’s five childhood cancer treatments centers.
Adolescents and young adults from 15 to 21 years who were treated at adult centers with adult regimens were identified through the Ontario Cancer Registry using chart abstraction by trained personnel at all treatment centers, with all data validated by clinician reviewers.
A total of 176 patients were identified, 129 with DLBCL and 47 with Burkitt’s lymphoma. In all, 62 of the 176 patients (35.2%) were treated in pediatric centers. Not surprisingly, multivariable analysis showed that AYA treated in adult centers were older, and more likely to have been treated earlier in the study period.
Comparing treatment patterns by locus of care, the investigators found that patients with DLBCL in pediatric centers received half of the cumulative anthracycline doses as those in adult centers (150 mg/m2 vs. 300 mg/m2; P < .001) and about 75% of cumulative alkylating agent doses (3,300 mg/m2 vs. 4,465 mg/m2; P = .009).
Patients with Burkitt’s lymphoma had identical exposures to anthracyclines in pediatric vs. adult centers (120 mg/m2), but those treated in pediatric centers had half the exposure to alkylators as those treated in adult centers (3,300 mg/m2 vs. 6,600 mg/m2; P = .03).
Among patients with DLBCL, none of those treated at pediatric centers received rituximab, compared with 32.3% of those treated at adult centers (P < .001), whereas only a handful of patients with Burkitt’s lymphoma received rituximab in both pediatric and adult centers (nonsignificant).
Among all patients. 5-year event-free survival was 82.3% for those treated in pediatric centers, compared with 66.7% for those treated in adult centers (P = .02). Respective 5-year overall survival rates were 85.5% and 71.1% (P = .03).
Looking at survival by histology, the investigators saw that 5-year event-free survival for patients with DLBCL was 83.3% when they were treated like children vs. 66.7% when they were treated like adults (P = .04). Respective 5-year overall survival rates were 88.9% and 72% (P = .04).
Both event-free survival (80.8% vs. 66.7%) and overall survival (80.8% vs. 66.7%) were numerically but not statistically higher among patients with Burkitt’s treated at pediatric vs. adult centers.
An analysis adjusting for disease histology, stage, and time period of diagnosis showed that treatment at an adult center was associated with higher risk for death, with a hazard ratio of 2.4 (P = .03).
Additionally, an analysis adjusted for age, disease stage, and histology showed that patients treated in adult centers had a significantly increased risk of relapse or progression, compared with a HR of 4.4 (95% confidence interval; P = .008).
There were no significant differences in the risk of treatment-related mortality between the center types, however.
“It is important to note, however, that pediatric approaches to mature B-cell NHL [non-Hodgkin lymphoma] are associated with increased inpatient needs as compared to adult approaches, and with greater supportive care requirements. Thus the safety of such approaches in adults centers need to be established,” Dr. Gupta said.
Lower doses, better outcomes
In the question and answer session following the presentation, Jennifer Teichman, MD, MSc, a fellow in hematology at the University of Toronto who was not involved in the study asked why patients treated at adult centers would have higher relapse rates despite receiving higher doses of chemotherapy, noting that the poorer outcomes in those patients were not attributable to treatment-related mortality.
“I think one of the distinctions is that higher cumulative doses versus higher intensity of treatment over a shorter period of time are two different things, perhaps, and so giving lower cumulative doses but over a short period of time, and so giving higher intensity within that short period of time, may be what explains the higher success rate in pediatric trials,” Dr. Gupta said.
R. Michael Crump, MD, from the Princess Margaret Cancer Center, also in Toronto, asked whether the study results could have been influenced by differences between the pediatric center and adult center datasets in regard to pathology review, staging information, and International Prognostic Index.
Dr. Gupta acknowledged that, while the pediatric data were captured prospectively at each center by pediatric cancer registry staff and adult data were extracted retrospectively by trained chart reviewers, “the information that we were collecting was relatively basic – basic stage, basic histology, and that is a limitation.”
He also noted that clinicians reviewed the submitted retrospective data for completeness and had the ability to request chart extractors to return to a particular record for additional information or to correct potential errors.
The study was supported by the Canadian Institutes of Health Research, the C17 Council on Children’s Cancer & Blood Disorders, and the Pediatric Oncology Group of Ontario. Dr. Gupta, Dr. Teichman, and Dr. Crump all reported no relevant conflicts of interest.
SOURCE: Gupta S et al. ASH 2020, Abstract 708.
Adolescents and young adults with aggressive mature B-cell non-Hodgkin lymphomas appear to have better outcomes when they’re treated under pediatric protocols rather than adult regimens, Canadian investigators say.
Results of a study of patients from the ages of 15 to 21 years with either diffuse large B-cell lymphoma (DLBCL) or Burkitt’s lymphoma treated at regional or community cancer centers in the province of Ontario indicated that adolescents and young adult (AYA) patients treated at adult centers had a more than fourfold risk for disease relapse or progression, compared with their counterparts who were treated at pediatric centers, reported Sumit Gupta, MD, PhD, from the Hospital for Sick Children in Toronto and colleagues.
“Our data suggest that pediatric approaches are associated with improved event-free survival and overall survival, primarily due to a decrease in the risk of relapse or progression, while still using lower cumulative doses of chemotherapy,” he said in an oral abstract presented at the American Society of Hematology annual meeting, held virtually.
The findings echo those seen in the treatment of patients with acute lymphoblastic leukemia (ALL). As previously reported, a study from Nordic and Baltic countries showed that young adults with ALL who were treated with a pediatric regimen had a 4-year event-free survival rate of 73%, compared with 42% for historical controls.
Similarly, a prospective U.S. study reported in 2014 showed that AYA with ALL treated with a pediatric regimen had better overall and event-free survival rates, compared with historical controls.
As with ALL, pediatric and adult regimens for treatment of patients with aggressive mature B-cell NHL differ substantially, with pediatric patients receiving more intensive short-term therapy with lower cumulative doses.
In addition, while pediatric regimens for DLBCL and Burkitt’s lymphoma are identical, adult regimens differ substantially between the two histologies, Dr. Gupta pointed out.
Adult regimens for DLBCL most often incorporate CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone) or CHOP plus rituximab (R-CHOP), whereas Burkitt’s lymphoma in adults is generally treated with more aggressive multidrug regimens, in combination with rituximab.
Rituximab was incorporated into adults’ regimens far earlier than in pediatric regimens, with Food and Drug Administration approval of rituximab in frontline therapy of adults with DLBCL in 2006, “whereas the first pediatric large-scale randomized controlled trial of rituximab in pediatric mature B-cell lymphoma was only published earlier this year,” he noted.
Population-based study
To see how treatment patterns for AYA patients with aggressive mature B-cell non-Hodgkin lymphomas differ between pediatric and adult centers, Dr. Gupta and colleagues conducted a population-based study of all AYA in Ontario diagnosed with Burkitt’s or DLBCL from the ages of 15 to 21 years from 1992 through 2012.
AYA from the ages of 15 to 18 years who were treated at pediatric centers were identified through the Provincial Pediatric Oncology Registry, which includes data on demographics, disease treatment, and outcomes from each of Ontario’s five childhood cancer treatments centers.
Adolescents and young adults from 15 to 21 years who were treated at adult centers with adult regimens were identified through the Ontario Cancer Registry using chart abstraction by trained personnel at all treatment centers, with all data validated by clinician reviewers.
A total of 176 patients were identified, 129 with DLBCL and 47 with Burkitt’s lymphoma. In all, 62 of the 176 patients (35.2%) were treated in pediatric centers. Not surprisingly, multivariable analysis showed that AYA treated in adult centers were older, and more likely to have been treated earlier in the study period.
Comparing treatment patterns by locus of care, the investigators found that patients with DLBCL in pediatric centers received half of the cumulative anthracycline doses as those in adult centers (150 mg/m2 vs. 300 mg/m2; P < .001) and about 75% of cumulative alkylating agent doses (3,300 mg/m2 vs. 4,465 mg/m2; P = .009).
Patients with Burkitt’s lymphoma had identical exposures to anthracyclines in pediatric vs. adult centers (120 mg/m2), but those treated in pediatric centers had half the exposure to alkylators as those treated in adult centers (3,300 mg/m2 vs. 6,600 mg/m2; P = .03).
Among patients with DLBCL, none of those treated at pediatric centers received rituximab, compared with 32.3% of those treated at adult centers (P < .001), whereas only a handful of patients with Burkitt’s lymphoma received rituximab in both pediatric and adult centers (nonsignificant).
Among all patients. 5-year event-free survival was 82.3% for those treated in pediatric centers, compared with 66.7% for those treated in adult centers (P = .02). Respective 5-year overall survival rates were 85.5% and 71.1% (P = .03).
Looking at survival by histology, the investigators saw that 5-year event-free survival for patients with DLBCL was 83.3% when they were treated like children vs. 66.7% when they were treated like adults (P = .04). Respective 5-year overall survival rates were 88.9% and 72% (P = .04).
Both event-free survival (80.8% vs. 66.7%) and overall survival (80.8% vs. 66.7%) were numerically but not statistically higher among patients with Burkitt’s treated at pediatric vs. adult centers.
An analysis adjusting for disease histology, stage, and time period of diagnosis showed that treatment at an adult center was associated with higher risk for death, with a hazard ratio of 2.4 (P = .03).
Additionally, an analysis adjusted for age, disease stage, and histology showed that patients treated in adult centers had a significantly increased risk of relapse or progression, compared with a HR of 4.4 (95% confidence interval; P = .008).
There were no significant differences in the risk of treatment-related mortality between the center types, however.
“It is important to note, however, that pediatric approaches to mature B-cell NHL [non-Hodgkin lymphoma] are associated with increased inpatient needs as compared to adult approaches, and with greater supportive care requirements. Thus the safety of such approaches in adults centers need to be established,” Dr. Gupta said.
Lower doses, better outcomes
In the question and answer session following the presentation, Jennifer Teichman, MD, MSc, a fellow in hematology at the University of Toronto who was not involved in the study asked why patients treated at adult centers would have higher relapse rates despite receiving higher doses of chemotherapy, noting that the poorer outcomes in those patients were not attributable to treatment-related mortality.
“I think one of the distinctions is that higher cumulative doses versus higher intensity of treatment over a shorter period of time are two different things, perhaps, and so giving lower cumulative doses but over a short period of time, and so giving higher intensity within that short period of time, may be what explains the higher success rate in pediatric trials,” Dr. Gupta said.
R. Michael Crump, MD, from the Princess Margaret Cancer Center, also in Toronto, asked whether the study results could have been influenced by differences between the pediatric center and adult center datasets in regard to pathology review, staging information, and International Prognostic Index.
Dr. Gupta acknowledged that, while the pediatric data were captured prospectively at each center by pediatric cancer registry staff and adult data were extracted retrospectively by trained chart reviewers, “the information that we were collecting was relatively basic – basic stage, basic histology, and that is a limitation.”
He also noted that clinicians reviewed the submitted retrospective data for completeness and had the ability to request chart extractors to return to a particular record for additional information or to correct potential errors.
The study was supported by the Canadian Institutes of Health Research, the C17 Council on Children’s Cancer & Blood Disorders, and the Pediatric Oncology Group of Ontario. Dr. Gupta, Dr. Teichman, and Dr. Crump all reported no relevant conflicts of interest.
SOURCE: Gupta S et al. ASH 2020, Abstract 708.
FROM ASH 2020
Key clinical point: Pediatric cancer regimens may offer better outcomes for adolescents/young adults with aggressive mature B-cell lymphomas.
Major finding: The hazard ratio for relapse or progression for patients treated in adults centers was 4.4 (P = .008)
Study details: Retrospective study of 176 adolescents/young adults with diffuse large B-cell lymphoma or Burkitt’s lymphoma.
Disclosures: The study was supported the Canadian Institutes of Health Research, the C17 Council on Children’s Cancer & Blood Disorders, and the Pediatric Oncology Group of Ontario. Dr. Gupta, Dr. Teichman, and Dr. Crump all reported no relevant conflicts of interest.
Source: Gupta S. et al. ASH 2020, Abstract 708.
Emotions, worse attention linked to pain-related health care use in SCD
The cognitive and emotional status of children with sickle cell disease (SCD) appears to have a significant effect on how they cope with pain and use health care resources, investigators have found.
Results of a retrospective study of 112 children and adolescents with SCD, the majority of whom had sickle cell anemia, showed that ED visits and hospitalizations were significantly lower among children with SCD who performed better on an attention task, as well as those who were better able to cope emotionally with having SCD and pain, reported Zaria Williams, a second-year medical student at Howard University, Washington, and colleagues.
“Since I started learning more about sickle cell disease, I’ve been very concerned about the great disease burden that this condition can place on pediatric patients, particularly those who suffer from pain,” Ms. Williams said in an oral abstract presented at the annual meeting of the American Society of Hematology.
Although many children and adolescents with SCD can have their pain effectively managed at home with opioids and other medications, some require ED visits and potentially hospitalizations for pain management.
“There is great variability in health care utilization among patients with sickle cell disease, with some having to come to the ED and be admit to the hospital more than others. In searching for reasons why this might be the case, we thought about cognitive function and emotional differences between children with sickle cell disease as potentially affecting disease management,” she said.
Anxiety and catastrophizing
Children with SCD are known to be susceptible to affective comorbidities such as anxiety and catastrophizing, and to conditions that have the potential for deleterious effects on executive function, attention, and working memory. To determine whether cognitive and emotional factors affect the disease self-management in children and adolescents with SCD, Ms. Williams and coinvestigators looked at a cohort of 112 SCD patients aged 7-16 years treated at Children’s National Hospital in Washington, D.C.
The patients had participated in a previous pilot study of computerized working memory training. The authors reviewed charts for data on health care utilization, focusing on ED visits and hospitalization for pain 1 and 3 years after enrollment in the study.
They collected data on SCD genotype, disease-related variables, psychosocial information, and measures of cognition and emotion from the dataset. The information included socioeconomic status, parent education level, household income, and number of adults in the household.
Cognitive measures included the Weschler Intelligence Scale for Children full scale IQ, and the Cogstate computerized cognitive assessment system, which measures attention, executive function, and working memory.
Emotional measures were captured from the Pediatric Quality of Life Inventory Sickle Cell Disease module, including questions about worrying and emotions such as anger regarding SCD and pain.
The mean age of participants was 10.61 years. Of the 112 children/adolescents in the study, 65 (58%) were female, and 83 (74%) had sickle cell anemia (either HbSS or HbSβ0 thalassemia).
The participants had a median number of ED visits for pain of one within a year of enrollment, and a median of three within 3 years of enrollment,
The median number of hospital admissions for pain was zero and one, respectively.
Attention, emotions linked to higher use
Factors significantly associated with ED visits for pain within the first year were higher (worse) scores for attention (P = .001) and self-reported emotion (P = .049). ED visits within 3 years of enrollment were associated with attention (P = .003) and working memory (P = .039).
Similarly, hospitalizations for pain within the first year were significantly associated with worse attention scores (P = .009) and child-reported emotion (P = .013). Hospitalizations for pain within 3 years of enrollment were also significantly associated with attention deficits (P = .006) and with worse emotional function as reported by a parent (P = .020).
There was no significant effect of SCD genotype or socioeconomic status on either pain-related ED visits or hospitalizations, however.
The investigators theorized that poor attention may make it difficult to distract children from focusing on their pain, and could also hamper disease self-management strategies such as medication adherence and avoiding pain triggers.
Age-related differences?
In the question-and-answer session following her presentation, comoderator Susanna A Curtis, MD, from Yale New Haven (Conn.) Hospital, commented that “some previous work has shown that adolescents and young adults with sickle cell disease have higher utilization as compared to their younger counterparts,” and asked whether the investigators found differences between cognition and utilization among different age groups within the cohort.
“We didn’t find a significant association with age, but I’m also very interested in that as well, especially considering that maybe there is more or less parent involvement, considering how old the child is,” Ms. Williams said.
Dr. Curtis noted that many of the comorbidities of sickle cell disease such as stroke or degree of anemia can affect cognitive function, but can also have an effect on health care utilization as well, asked whether the investigators were able to look at the potential confounding effects of comorbidities.
Ms. Williams said that, although they have not looked at potential confounders as yet, they hope to do so in future research.
Asked by another audience member whether the authors had considered using the Pain Catastrophizing Scale for children and/or their parents, in addition to other markers, Ms. Williams replied that “I definitely have considered it. Under recommendations from my mentors, we just focused on the quality-of-life scale first, but catastrophizing is something I’m very interested in. Especially, I would love to have the parent factors as well, so along the journey I hope to include that.”
The study was sponsored in part by a grant from the Doris Duke Charitable Foundation. Ms Williams is the recipient of an ASH Minority Medical Student Award. Dr. Curtis and Ms. Williams both reported no relevant conflicts of interest to disclose.
SOURCE: Williams Z et al. ASH 2020, Abstract 366
The cognitive and emotional status of children with sickle cell disease (SCD) appears to have a significant effect on how they cope with pain and use health care resources, investigators have found.
Results of a retrospective study of 112 children and adolescents with SCD, the majority of whom had sickle cell anemia, showed that ED visits and hospitalizations were significantly lower among children with SCD who performed better on an attention task, as well as those who were better able to cope emotionally with having SCD and pain, reported Zaria Williams, a second-year medical student at Howard University, Washington, and colleagues.
“Since I started learning more about sickle cell disease, I’ve been very concerned about the great disease burden that this condition can place on pediatric patients, particularly those who suffer from pain,” Ms. Williams said in an oral abstract presented at the annual meeting of the American Society of Hematology.
Although many children and adolescents with SCD can have their pain effectively managed at home with opioids and other medications, some require ED visits and potentially hospitalizations for pain management.
“There is great variability in health care utilization among patients with sickle cell disease, with some having to come to the ED and be admit to the hospital more than others. In searching for reasons why this might be the case, we thought about cognitive function and emotional differences between children with sickle cell disease as potentially affecting disease management,” she said.
Anxiety and catastrophizing
Children with SCD are known to be susceptible to affective comorbidities such as anxiety and catastrophizing, and to conditions that have the potential for deleterious effects on executive function, attention, and working memory. To determine whether cognitive and emotional factors affect the disease self-management in children and adolescents with SCD, Ms. Williams and coinvestigators looked at a cohort of 112 SCD patients aged 7-16 years treated at Children’s National Hospital in Washington, D.C.
The patients had participated in a previous pilot study of computerized working memory training. The authors reviewed charts for data on health care utilization, focusing on ED visits and hospitalization for pain 1 and 3 years after enrollment in the study.
They collected data on SCD genotype, disease-related variables, psychosocial information, and measures of cognition and emotion from the dataset. The information included socioeconomic status, parent education level, household income, and number of adults in the household.
Cognitive measures included the Weschler Intelligence Scale for Children full scale IQ, and the Cogstate computerized cognitive assessment system, which measures attention, executive function, and working memory.
Emotional measures were captured from the Pediatric Quality of Life Inventory Sickle Cell Disease module, including questions about worrying and emotions such as anger regarding SCD and pain.
The mean age of participants was 10.61 years. Of the 112 children/adolescents in the study, 65 (58%) were female, and 83 (74%) had sickle cell anemia (either HbSS or HbSβ0 thalassemia).
The participants had a median number of ED visits for pain of one within a year of enrollment, and a median of three within 3 years of enrollment,
The median number of hospital admissions for pain was zero and one, respectively.
Attention, emotions linked to higher use
Factors significantly associated with ED visits for pain within the first year were higher (worse) scores for attention (P = .001) and self-reported emotion (P = .049). ED visits within 3 years of enrollment were associated with attention (P = .003) and working memory (P = .039).
Similarly, hospitalizations for pain within the first year were significantly associated with worse attention scores (P = .009) and child-reported emotion (P = .013). Hospitalizations for pain within 3 years of enrollment were also significantly associated with attention deficits (P = .006) and with worse emotional function as reported by a parent (P = .020).
There was no significant effect of SCD genotype or socioeconomic status on either pain-related ED visits or hospitalizations, however.
The investigators theorized that poor attention may make it difficult to distract children from focusing on their pain, and could also hamper disease self-management strategies such as medication adherence and avoiding pain triggers.
Age-related differences?
In the question-and-answer session following her presentation, comoderator Susanna A Curtis, MD, from Yale New Haven (Conn.) Hospital, commented that “some previous work has shown that adolescents and young adults with sickle cell disease have higher utilization as compared to their younger counterparts,” and asked whether the investigators found differences between cognition and utilization among different age groups within the cohort.
“We didn’t find a significant association with age, but I’m also very interested in that as well, especially considering that maybe there is more or less parent involvement, considering how old the child is,” Ms. Williams said.
Dr. Curtis noted that many of the comorbidities of sickle cell disease such as stroke or degree of anemia can affect cognitive function, but can also have an effect on health care utilization as well, asked whether the investigators were able to look at the potential confounding effects of comorbidities.
Ms. Williams said that, although they have not looked at potential confounders as yet, they hope to do so in future research.
Asked by another audience member whether the authors had considered using the Pain Catastrophizing Scale for children and/or their parents, in addition to other markers, Ms. Williams replied that “I definitely have considered it. Under recommendations from my mentors, we just focused on the quality-of-life scale first, but catastrophizing is something I’m very interested in. Especially, I would love to have the parent factors as well, so along the journey I hope to include that.”
The study was sponsored in part by a grant from the Doris Duke Charitable Foundation. Ms Williams is the recipient of an ASH Minority Medical Student Award. Dr. Curtis and Ms. Williams both reported no relevant conflicts of interest to disclose.
SOURCE: Williams Z et al. ASH 2020, Abstract 366
The cognitive and emotional status of children with sickle cell disease (SCD) appears to have a significant effect on how they cope with pain and use health care resources, investigators have found.
Results of a retrospective study of 112 children and adolescents with SCD, the majority of whom had sickle cell anemia, showed that ED visits and hospitalizations were significantly lower among children with SCD who performed better on an attention task, as well as those who were better able to cope emotionally with having SCD and pain, reported Zaria Williams, a second-year medical student at Howard University, Washington, and colleagues.
“Since I started learning more about sickle cell disease, I’ve been very concerned about the great disease burden that this condition can place on pediatric patients, particularly those who suffer from pain,” Ms. Williams said in an oral abstract presented at the annual meeting of the American Society of Hematology.
Although many children and adolescents with SCD can have their pain effectively managed at home with opioids and other medications, some require ED visits and potentially hospitalizations for pain management.
“There is great variability in health care utilization among patients with sickle cell disease, with some having to come to the ED and be admit to the hospital more than others. In searching for reasons why this might be the case, we thought about cognitive function and emotional differences between children with sickle cell disease as potentially affecting disease management,” she said.
Anxiety and catastrophizing
Children with SCD are known to be susceptible to affective comorbidities such as anxiety and catastrophizing, and to conditions that have the potential for deleterious effects on executive function, attention, and working memory. To determine whether cognitive and emotional factors affect the disease self-management in children and adolescents with SCD, Ms. Williams and coinvestigators looked at a cohort of 112 SCD patients aged 7-16 years treated at Children’s National Hospital in Washington, D.C.
The patients had participated in a previous pilot study of computerized working memory training. The authors reviewed charts for data on health care utilization, focusing on ED visits and hospitalization for pain 1 and 3 years after enrollment in the study.
They collected data on SCD genotype, disease-related variables, psychosocial information, and measures of cognition and emotion from the dataset. The information included socioeconomic status, parent education level, household income, and number of adults in the household.
Cognitive measures included the Weschler Intelligence Scale for Children full scale IQ, and the Cogstate computerized cognitive assessment system, which measures attention, executive function, and working memory.
Emotional measures were captured from the Pediatric Quality of Life Inventory Sickle Cell Disease module, including questions about worrying and emotions such as anger regarding SCD and pain.
The mean age of participants was 10.61 years. Of the 112 children/adolescents in the study, 65 (58%) were female, and 83 (74%) had sickle cell anemia (either HbSS or HbSβ0 thalassemia).
The participants had a median number of ED visits for pain of one within a year of enrollment, and a median of three within 3 years of enrollment,
The median number of hospital admissions for pain was zero and one, respectively.
Attention, emotions linked to higher use
Factors significantly associated with ED visits for pain within the first year were higher (worse) scores for attention (P = .001) and self-reported emotion (P = .049). ED visits within 3 years of enrollment were associated with attention (P = .003) and working memory (P = .039).
Similarly, hospitalizations for pain within the first year were significantly associated with worse attention scores (P = .009) and child-reported emotion (P = .013). Hospitalizations for pain within 3 years of enrollment were also significantly associated with attention deficits (P = .006) and with worse emotional function as reported by a parent (P = .020).
There was no significant effect of SCD genotype or socioeconomic status on either pain-related ED visits or hospitalizations, however.
The investigators theorized that poor attention may make it difficult to distract children from focusing on their pain, and could also hamper disease self-management strategies such as medication adherence and avoiding pain triggers.
Age-related differences?
In the question-and-answer session following her presentation, comoderator Susanna A Curtis, MD, from Yale New Haven (Conn.) Hospital, commented that “some previous work has shown that adolescents and young adults with sickle cell disease have higher utilization as compared to their younger counterparts,” and asked whether the investigators found differences between cognition and utilization among different age groups within the cohort.
“We didn’t find a significant association with age, but I’m also very interested in that as well, especially considering that maybe there is more or less parent involvement, considering how old the child is,” Ms. Williams said.
Dr. Curtis noted that many of the comorbidities of sickle cell disease such as stroke or degree of anemia can affect cognitive function, but can also have an effect on health care utilization as well, asked whether the investigators were able to look at the potential confounding effects of comorbidities.
Ms. Williams said that, although they have not looked at potential confounders as yet, they hope to do so in future research.
Asked by another audience member whether the authors had considered using the Pain Catastrophizing Scale for children and/or their parents, in addition to other markers, Ms. Williams replied that “I definitely have considered it. Under recommendations from my mentors, we just focused on the quality-of-life scale first, but catastrophizing is something I’m very interested in. Especially, I would love to have the parent factors as well, so along the journey I hope to include that.”
The study was sponsored in part by a grant from the Doris Duke Charitable Foundation. Ms Williams is the recipient of an ASH Minority Medical Student Award. Dr. Curtis and Ms. Williams both reported no relevant conflicts of interest to disclose.
SOURCE: Williams Z et al. ASH 2020, Abstract 366
FROM ASH 2020
Cost is the main hurdle to broad use of caplacizumab for TTP
As hematologists debated the role of the anti–von Willebrand factor agent caplacizumab for acquired thrombotic thrombocytopenic purpura (TTP), an investigator on the phase 3 trial that led to its approval had a message.
,” said hematologist Spero Cataland, MD, of the department of internal medicine at Ohio State University in Columbus.
If cost is going to be a factor, and it “has to be in our world these days, it’s more of a discussion,” he said during his presentation at the 2020 Update in Nonneoplastic Hematology virtual conference.
The HERCULES trial Dr. Cataland helped conduct found a median time to platelet count normalization of 2.69 days when caplacizumab was started during plasma exchange versus 2.88 days for placebo; 12% of patients had a TTP recurrence while they continued caplacizumab for 30 days past their last exchange and were followed for an additional 28 days versus 38% randomized to placebo. Caplacizumab subjects needed an average of 5.8 days of plasma exchange versus 9.4 days in the placebo arm (N Engl J Med. 2019 Jan 24;380(4):335-46).
Based on the results, the Food and Drug Administration approved the agent for acquired TTP in combination with plasma exchange and immunosuppressives in Feb. 2019 for 30 days beyond the last plasma exchange, with up to 28 additional days if ADAMTS13 activity remains suppressed. Labeling notes a risk of severe bleeding.
“The data on refractory disease and mortality aren’t quite there yet, but there’s a suggestion [caplacizumab] might impact that as well,” Dr. Cataland said. In its recent TTP guidelines, the International Society on Thrombosis and Haemostasis gave the agent only a conditional recommendation, in part because it’s backed up only by HERCULES and a phase 2 trial.
Also, the group noted that in the phase 2 study caplacizumab patients had a clinically and statistically significant increase in the number of relapses at 12 months: 31% versus 8% placebo. “Caplacizumab may leave patients prone to experience a later recurrence owing to the unresolved ADAMTS13 deficiency and inhibitors,” Dr. Cataland said.
“We do see some early recurrence” when caplacizumab is stopped, suggesting that when the agent’s “protective effect is removed, the risk is still there,” said Dr. Cataland, who was also an author on the ISTH guidelines, as well as the phase 2 trial.
It raises the question of how long patients should be kept on caplacizumab. There are few data on the issue, “but the consensus has been to stop caplacizumab when two consecutive ADAMTS13 measurements show 20% or greater activity,” or perhaps with one reading above 20% in a patient trending in the right direction. “With a bleeding complication, you might stop it sooner,” he said.
Dr. Cataland anticipates TTP management will eventually move away from plasma exchange to more directed therapies, including caplacizumab and perhaps recombinant ADAMTS13, which is in development.
There have been a few reports of TTP patients who refuse plasma exchange on religious grounds being successfully treated with caplacizumab. Dr. Cataland also noted a patient of his with relapsing TTP who didn’t want to be admitted yet again for plasma exchange and steroids at the start of a new episode.
“We managed her with caplacizumab and rituximab, and in a couple weeks she had recovered her ADAMTS13 activity and was able to stop the caplacizumab.” She was a motivated, knowledgeable person, “someone I trusted, so I was comfortable with the approach. I think that may be where we are headed in the future, hopefully,” he said.
Dr. Cataland disclosed research funding and consulting fees from Alexion, caplacizumab’s maker, Sanofi Genzyme, and Takeda,. The conference was sponsored by MedscapeLive. MedscapeLive and this news organization are owned by the same parent company.
As hematologists debated the role of the anti–von Willebrand factor agent caplacizumab for acquired thrombotic thrombocytopenic purpura (TTP), an investigator on the phase 3 trial that led to its approval had a message.
,” said hematologist Spero Cataland, MD, of the department of internal medicine at Ohio State University in Columbus.
If cost is going to be a factor, and it “has to be in our world these days, it’s more of a discussion,” he said during his presentation at the 2020 Update in Nonneoplastic Hematology virtual conference.
The HERCULES trial Dr. Cataland helped conduct found a median time to platelet count normalization of 2.69 days when caplacizumab was started during plasma exchange versus 2.88 days for placebo; 12% of patients had a TTP recurrence while they continued caplacizumab for 30 days past their last exchange and were followed for an additional 28 days versus 38% randomized to placebo. Caplacizumab subjects needed an average of 5.8 days of plasma exchange versus 9.4 days in the placebo arm (N Engl J Med. 2019 Jan 24;380(4):335-46).
Based on the results, the Food and Drug Administration approved the agent for acquired TTP in combination with plasma exchange and immunosuppressives in Feb. 2019 for 30 days beyond the last plasma exchange, with up to 28 additional days if ADAMTS13 activity remains suppressed. Labeling notes a risk of severe bleeding.
“The data on refractory disease and mortality aren’t quite there yet, but there’s a suggestion [caplacizumab] might impact that as well,” Dr. Cataland said. In its recent TTP guidelines, the International Society on Thrombosis and Haemostasis gave the agent only a conditional recommendation, in part because it’s backed up only by HERCULES and a phase 2 trial.
Also, the group noted that in the phase 2 study caplacizumab patients had a clinically and statistically significant increase in the number of relapses at 12 months: 31% versus 8% placebo. “Caplacizumab may leave patients prone to experience a later recurrence owing to the unresolved ADAMTS13 deficiency and inhibitors,” Dr. Cataland said.
“We do see some early recurrence” when caplacizumab is stopped, suggesting that when the agent’s “protective effect is removed, the risk is still there,” said Dr. Cataland, who was also an author on the ISTH guidelines, as well as the phase 2 trial.
It raises the question of how long patients should be kept on caplacizumab. There are few data on the issue, “but the consensus has been to stop caplacizumab when two consecutive ADAMTS13 measurements show 20% or greater activity,” or perhaps with one reading above 20% in a patient trending in the right direction. “With a bleeding complication, you might stop it sooner,” he said.
Dr. Cataland anticipates TTP management will eventually move away from plasma exchange to more directed therapies, including caplacizumab and perhaps recombinant ADAMTS13, which is in development.
There have been a few reports of TTP patients who refuse plasma exchange on religious grounds being successfully treated with caplacizumab. Dr. Cataland also noted a patient of his with relapsing TTP who didn’t want to be admitted yet again for plasma exchange and steroids at the start of a new episode.
“We managed her with caplacizumab and rituximab, and in a couple weeks she had recovered her ADAMTS13 activity and was able to stop the caplacizumab.” She was a motivated, knowledgeable person, “someone I trusted, so I was comfortable with the approach. I think that may be where we are headed in the future, hopefully,” he said.
Dr. Cataland disclosed research funding and consulting fees from Alexion, caplacizumab’s maker, Sanofi Genzyme, and Takeda,. The conference was sponsored by MedscapeLive. MedscapeLive and this news organization are owned by the same parent company.
As hematologists debated the role of the anti–von Willebrand factor agent caplacizumab for acquired thrombotic thrombocytopenic purpura (TTP), an investigator on the phase 3 trial that led to its approval had a message.
,” said hematologist Spero Cataland, MD, of the department of internal medicine at Ohio State University in Columbus.
If cost is going to be a factor, and it “has to be in our world these days, it’s more of a discussion,” he said during his presentation at the 2020 Update in Nonneoplastic Hematology virtual conference.
The HERCULES trial Dr. Cataland helped conduct found a median time to platelet count normalization of 2.69 days when caplacizumab was started during plasma exchange versus 2.88 days for placebo; 12% of patients had a TTP recurrence while they continued caplacizumab for 30 days past their last exchange and were followed for an additional 28 days versus 38% randomized to placebo. Caplacizumab subjects needed an average of 5.8 days of plasma exchange versus 9.4 days in the placebo arm (N Engl J Med. 2019 Jan 24;380(4):335-46).
Based on the results, the Food and Drug Administration approved the agent for acquired TTP in combination with plasma exchange and immunosuppressives in Feb. 2019 for 30 days beyond the last plasma exchange, with up to 28 additional days if ADAMTS13 activity remains suppressed. Labeling notes a risk of severe bleeding.
“The data on refractory disease and mortality aren’t quite there yet, but there’s a suggestion [caplacizumab] might impact that as well,” Dr. Cataland said. In its recent TTP guidelines, the International Society on Thrombosis and Haemostasis gave the agent only a conditional recommendation, in part because it’s backed up only by HERCULES and a phase 2 trial.
Also, the group noted that in the phase 2 study caplacizumab patients had a clinically and statistically significant increase in the number of relapses at 12 months: 31% versus 8% placebo. “Caplacizumab may leave patients prone to experience a later recurrence owing to the unresolved ADAMTS13 deficiency and inhibitors,” Dr. Cataland said.
“We do see some early recurrence” when caplacizumab is stopped, suggesting that when the agent’s “protective effect is removed, the risk is still there,” said Dr. Cataland, who was also an author on the ISTH guidelines, as well as the phase 2 trial.
It raises the question of how long patients should be kept on caplacizumab. There are few data on the issue, “but the consensus has been to stop caplacizumab when two consecutive ADAMTS13 measurements show 20% or greater activity,” or perhaps with one reading above 20% in a patient trending in the right direction. “With a bleeding complication, you might stop it sooner,” he said.
Dr. Cataland anticipates TTP management will eventually move away from plasma exchange to more directed therapies, including caplacizumab and perhaps recombinant ADAMTS13, which is in development.
There have been a few reports of TTP patients who refuse plasma exchange on religious grounds being successfully treated with caplacizumab. Dr. Cataland also noted a patient of his with relapsing TTP who didn’t want to be admitted yet again for plasma exchange and steroids at the start of a new episode.
“We managed her with caplacizumab and rituximab, and in a couple weeks she had recovered her ADAMTS13 activity and was able to stop the caplacizumab.” She was a motivated, knowledgeable person, “someone I trusted, so I was comfortable with the approach. I think that may be where we are headed in the future, hopefully,” he said.
Dr. Cataland disclosed research funding and consulting fees from Alexion, caplacizumab’s maker, Sanofi Genzyme, and Takeda,. The conference was sponsored by MedscapeLive. MedscapeLive and this news organization are owned by the same parent company.
FROM 2020 UNNH
Fixed duration ibrutinib/venetoclax appears feasible for some CLL/SLL patients
Among chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) patients in the minimal residual disease (MRD) cohort of the phase 2 CAPTIVATE trial, a 1-year disease-free survival (DFS) rate of 95% in those randomized to placebo after 12 cycles of combined ibrutinib plus venetoclax supports a fixed-duration treatment approach, according to William G. Wierda, MD, PhD, University of Texas, MD Anderson Cancer Center, Houston.
Ibrutinib, a once-daily Bruton kinase inhibitor, is the only targeted therapy for first-line treatment of CLL that has demonstrated significant overall survival benefit in randomized phase 3 studies, Dr. Wierda said at the American Society of Hematology annual meeting, held virtually.
Ibrutinib and venetoclax have synergistic and complementary antitumor activity, he noted, through mobilizing and clearing CLL cells from protective niches and disease compartments beyond blood and bone marrow.
Fixed-duration study
CAPTIVATE (PCYC-1142), an international phase 2 study, evaluated first-line treatment with 12 cycles of the ibrutinib/venetoclax combination in MRD and fixed-duration cohorts. The current primary analysis of 1-year DFS from the MRD cohort tested whether the regimen allows for treatment-free remission in the setting of confirmed undetectable MRD (uMRD).
Patients (n = 164, median age 58 years) in the CAPTIVATE study MRD cohort had previously untreated active CLL/SLL requiring treatment per International Workshop on Chronic Lymphocytic Leukemia criteria.
They received 3 cycles of lead-in ibrutinib (420 mg once daily) followed by 12 cycles of ibrutinib (420 mg once daily plus venetoclax ramp-up to 400 mg once daily). Thereafter, in an MRD-guided 1:1 randomization stratified by immunoglobulin heavy chain (IGHV) mutational status, those with confirmed uMRD received either placebo or ibrutinib, and those with uMRD not confirmed received either ibrutinib or ibrutinib plus venetoclax (both open-label).
Among high-risk features in CAPTIVATE subjects, 60% of patients had unmutated IGHV, with del(17p)/TP53 mutation in 20%, del(11Q) in 17%, complex karyotype in 19%, cytopenias in 36%, bulky lymph nodes in 32%, and absolute neutrophil count ≥25x109/L in 76%.
Response findings
The ibrutinib lead-in, Dr. Wierda said, reduced tumor lysis syndrome (TLS) risk, shifting 90% of patients with high baseline TLS risk to medium or low-risk categories (from 77 to 51 patients), precluding need for hospitalization with venetoclax initiation.
The rate for best response of uMRD (defined as uMRD over at least 3 cycles in both peripheral blood and bone marrow) in evaluable patients was 75% in peripheral blood (n = 163) and 72% in bone marrow (n = 155).
Confirmed uMRD was achieved in 86/149 (58%), with uMRD not confirmed in 63/149 (uMRD 32% in bone marrow and 48% in peripheral blood). One-year DFS after the further randomization to placebo or ibrutinib in the confirmed uMRD group was 95.3% in the placebo group and 100% in the ibrutinib group (P = .1475). In the uMRD not confirmed group, 30-month progression-free survival (PFS) was 95.2% and 96.7% in the ibrutinib and ibrutinib plus venetoclax groups, respectively. Thirty-month PFS rates in the confirmed uMRD placebo and ibrutinib arms were 95.3% and 100%. “Thirty-month PFS rates were greater than 95% across all randomized arms,” Dr. Wierda stated.
In patients without confirmed uMRD after 12 cycles of combined ibrutinib plus venetoclax, additional randomized treatment led to greater increases in uMRD in the ibrutinib plus venetoclax group than in the ibrutinib alone group (bone marrow additional 10% ibrutinib alone, 34% ibrutinib plus venetoclax; peripheral blood 0% ibrutinib, 19% ibrutinib plus venetoclax).
Adverse events generally decreased after the first 6 months of ibrutinib plus venetoclax treatment, with no new safety signals emerging over time. “There were no safety concerns with this highly active combination of first-line ibrutinib plus venetoclax. It’s an oral, once-daily fixed duration regimen that achieves undetectable MRD in blood or bone marrow in three-fourths of patients after 12 cycles of combined treatment.”
When asked, in a question-and-answer session after his presentation, if the findings were “practice changing,” Dr. Wierda responded: “We need additional data from ongoing studies looking at various combinations of targeted therapy. But this study does clearly show efficacy in terms of depth of remission, and it supports the concept of fixed duration treatment, particularly for those patients who achieved undetectable MRD status.”
SOURCE: William G. Wierda, MD, PhD. ASH 2020, Abstract 123.
Among chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) patients in the minimal residual disease (MRD) cohort of the phase 2 CAPTIVATE trial, a 1-year disease-free survival (DFS) rate of 95% in those randomized to placebo after 12 cycles of combined ibrutinib plus venetoclax supports a fixed-duration treatment approach, according to William G. Wierda, MD, PhD, University of Texas, MD Anderson Cancer Center, Houston.
Ibrutinib, a once-daily Bruton kinase inhibitor, is the only targeted therapy for first-line treatment of CLL that has demonstrated significant overall survival benefit in randomized phase 3 studies, Dr. Wierda said at the American Society of Hematology annual meeting, held virtually.
Ibrutinib and venetoclax have synergistic and complementary antitumor activity, he noted, through mobilizing and clearing CLL cells from protective niches and disease compartments beyond blood and bone marrow.
Fixed-duration study
CAPTIVATE (PCYC-1142), an international phase 2 study, evaluated first-line treatment with 12 cycles of the ibrutinib/venetoclax combination in MRD and fixed-duration cohorts. The current primary analysis of 1-year DFS from the MRD cohort tested whether the regimen allows for treatment-free remission in the setting of confirmed undetectable MRD (uMRD).
Patients (n = 164, median age 58 years) in the CAPTIVATE study MRD cohort had previously untreated active CLL/SLL requiring treatment per International Workshop on Chronic Lymphocytic Leukemia criteria.
They received 3 cycles of lead-in ibrutinib (420 mg once daily) followed by 12 cycles of ibrutinib (420 mg once daily plus venetoclax ramp-up to 400 mg once daily). Thereafter, in an MRD-guided 1:1 randomization stratified by immunoglobulin heavy chain (IGHV) mutational status, those with confirmed uMRD received either placebo or ibrutinib, and those with uMRD not confirmed received either ibrutinib or ibrutinib plus venetoclax (both open-label).
Among high-risk features in CAPTIVATE subjects, 60% of patients had unmutated IGHV, with del(17p)/TP53 mutation in 20%, del(11Q) in 17%, complex karyotype in 19%, cytopenias in 36%, bulky lymph nodes in 32%, and absolute neutrophil count ≥25x109/L in 76%.
Response findings
The ibrutinib lead-in, Dr. Wierda said, reduced tumor lysis syndrome (TLS) risk, shifting 90% of patients with high baseline TLS risk to medium or low-risk categories (from 77 to 51 patients), precluding need for hospitalization with venetoclax initiation.
The rate for best response of uMRD (defined as uMRD over at least 3 cycles in both peripheral blood and bone marrow) in evaluable patients was 75% in peripheral blood (n = 163) and 72% in bone marrow (n = 155).
Confirmed uMRD was achieved in 86/149 (58%), with uMRD not confirmed in 63/149 (uMRD 32% in bone marrow and 48% in peripheral blood). One-year DFS after the further randomization to placebo or ibrutinib in the confirmed uMRD group was 95.3% in the placebo group and 100% in the ibrutinib group (P = .1475). In the uMRD not confirmed group, 30-month progression-free survival (PFS) was 95.2% and 96.7% in the ibrutinib and ibrutinib plus venetoclax groups, respectively. Thirty-month PFS rates in the confirmed uMRD placebo and ibrutinib arms were 95.3% and 100%. “Thirty-month PFS rates were greater than 95% across all randomized arms,” Dr. Wierda stated.
In patients without confirmed uMRD after 12 cycles of combined ibrutinib plus venetoclax, additional randomized treatment led to greater increases in uMRD in the ibrutinib plus venetoclax group than in the ibrutinib alone group (bone marrow additional 10% ibrutinib alone, 34% ibrutinib plus venetoclax; peripheral blood 0% ibrutinib, 19% ibrutinib plus venetoclax).
Adverse events generally decreased after the first 6 months of ibrutinib plus venetoclax treatment, with no new safety signals emerging over time. “There were no safety concerns with this highly active combination of first-line ibrutinib plus venetoclax. It’s an oral, once-daily fixed duration regimen that achieves undetectable MRD in blood or bone marrow in three-fourths of patients after 12 cycles of combined treatment.”
When asked, in a question-and-answer session after his presentation, if the findings were “practice changing,” Dr. Wierda responded: “We need additional data from ongoing studies looking at various combinations of targeted therapy. But this study does clearly show efficacy in terms of depth of remission, and it supports the concept of fixed duration treatment, particularly for those patients who achieved undetectable MRD status.”
SOURCE: William G. Wierda, MD, PhD. ASH 2020, Abstract 123.
Among chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) patients in the minimal residual disease (MRD) cohort of the phase 2 CAPTIVATE trial, a 1-year disease-free survival (DFS) rate of 95% in those randomized to placebo after 12 cycles of combined ibrutinib plus venetoclax supports a fixed-duration treatment approach, according to William G. Wierda, MD, PhD, University of Texas, MD Anderson Cancer Center, Houston.
Ibrutinib, a once-daily Bruton kinase inhibitor, is the only targeted therapy for first-line treatment of CLL that has demonstrated significant overall survival benefit in randomized phase 3 studies, Dr. Wierda said at the American Society of Hematology annual meeting, held virtually.
Ibrutinib and venetoclax have synergistic and complementary antitumor activity, he noted, through mobilizing and clearing CLL cells from protective niches and disease compartments beyond blood and bone marrow.
Fixed-duration study
CAPTIVATE (PCYC-1142), an international phase 2 study, evaluated first-line treatment with 12 cycles of the ibrutinib/venetoclax combination in MRD and fixed-duration cohorts. The current primary analysis of 1-year DFS from the MRD cohort tested whether the regimen allows for treatment-free remission in the setting of confirmed undetectable MRD (uMRD).
Patients (n = 164, median age 58 years) in the CAPTIVATE study MRD cohort had previously untreated active CLL/SLL requiring treatment per International Workshop on Chronic Lymphocytic Leukemia criteria.
They received 3 cycles of lead-in ibrutinib (420 mg once daily) followed by 12 cycles of ibrutinib (420 mg once daily plus venetoclax ramp-up to 400 mg once daily). Thereafter, in an MRD-guided 1:1 randomization stratified by immunoglobulin heavy chain (IGHV) mutational status, those with confirmed uMRD received either placebo or ibrutinib, and those with uMRD not confirmed received either ibrutinib or ibrutinib plus venetoclax (both open-label).
Among high-risk features in CAPTIVATE subjects, 60% of patients had unmutated IGHV, with del(17p)/TP53 mutation in 20%, del(11Q) in 17%, complex karyotype in 19%, cytopenias in 36%, bulky lymph nodes in 32%, and absolute neutrophil count ≥25x109/L in 76%.
Response findings
The ibrutinib lead-in, Dr. Wierda said, reduced tumor lysis syndrome (TLS) risk, shifting 90% of patients with high baseline TLS risk to medium or low-risk categories (from 77 to 51 patients), precluding need for hospitalization with venetoclax initiation.
The rate for best response of uMRD (defined as uMRD over at least 3 cycles in both peripheral blood and bone marrow) in evaluable patients was 75% in peripheral blood (n = 163) and 72% in bone marrow (n = 155).
Confirmed uMRD was achieved in 86/149 (58%), with uMRD not confirmed in 63/149 (uMRD 32% in bone marrow and 48% in peripheral blood). One-year DFS after the further randomization to placebo or ibrutinib in the confirmed uMRD group was 95.3% in the placebo group and 100% in the ibrutinib group (P = .1475). In the uMRD not confirmed group, 30-month progression-free survival (PFS) was 95.2% and 96.7% in the ibrutinib and ibrutinib plus venetoclax groups, respectively. Thirty-month PFS rates in the confirmed uMRD placebo and ibrutinib arms were 95.3% and 100%. “Thirty-month PFS rates were greater than 95% across all randomized arms,” Dr. Wierda stated.
In patients without confirmed uMRD after 12 cycles of combined ibrutinib plus venetoclax, additional randomized treatment led to greater increases in uMRD in the ibrutinib plus venetoclax group than in the ibrutinib alone group (bone marrow additional 10% ibrutinib alone, 34% ibrutinib plus venetoclax; peripheral blood 0% ibrutinib, 19% ibrutinib plus venetoclax).
Adverse events generally decreased after the first 6 months of ibrutinib plus venetoclax treatment, with no new safety signals emerging over time. “There were no safety concerns with this highly active combination of first-line ibrutinib plus venetoclax. It’s an oral, once-daily fixed duration regimen that achieves undetectable MRD in blood or bone marrow in three-fourths of patients after 12 cycles of combined treatment.”
When asked, in a question-and-answer session after his presentation, if the findings were “practice changing,” Dr. Wierda responded: “We need additional data from ongoing studies looking at various combinations of targeted therapy. But this study does clearly show efficacy in terms of depth of remission, and it supports the concept of fixed duration treatment, particularly for those patients who achieved undetectable MRD status.”
SOURCE: William G. Wierda, MD, PhD. ASH 2020, Abstract 123.
FROM ASH 2020
Key clinical point: A favorable 1-year DFS in patients after 12 cycles of ibrutinib plus venetoclax in the MRD cohort of the phase 2 CAPTIVATE trial supports fixed-duration treatment for chronic lymphocytic leukemia/small lymphocytic lymphoma.
Major finding: One-year DFS after randomization to placebo or ibrutinib in the confirmed undetectable MRD group was 95.3% in the placebo group and 100.0 percent in the ibrutinib group (P = .1475).
Study details: The phase 2 CAPTIVATE study included 164 patients with previously untreated active chronic lymphocytic leukemia/small lymphocytic lymphoma requiring treatment per International Workshop on Chronic Lymphocytic Leukemia criteria.
Disclosures: Dr. Wierda disclosed consultancy and research funding with multiple pharmaceutical companies.
Source: William G. Wierda, MD, PhD. ASH 2020 Abstract 123.
Durable responses with anti-BCMA CAR T-cell for multiple myeloma
For patients with heavily-pretreated multiple myeloma, the early and deep responses seen with the novel chimeric antigen receptor T-cell (CAR T-cell) construct ciltacabtagene autoleucel (cilta-cel) have also been durable, according to investigators in the CARTITUDE-1 trial.
Among 97 patients with multiple myeloma that had progressed on three or more prior lines of therapy or following treatment with at least two lines of therapy with a proteasome inhibitor and immunomodulating agent, the overall response rate (ORR) was 96.9%, with a median duration of response not reached after a median of 12.4 months of follow-up, reported Deepu Madduri, MD of Mount Sinai Medical Center in New York, and colleagues.
“We saw how heavily pretreated these patients were, and to see a one-time treatment get these kind of response rates is quite exceptional. What’s even more impressive is that 72% of these patients were still maintaining their response at the time of data cutoff,“ she said in an oral abstract presented during the virtual American Society of Hematology annual meeting.
Cilta-cel is a second-generation CAR T containing two single-domain antibodies targeted against B-cell maturation protein (BCMA). BCMA was first described in myeloma in 2004 as a mechanism for the growth and survival of malignant plasma cells.
As previously reported, the same CAR T-cell construct showed a high overall response with manageable toxicities in 74 patients with relapsed/refractory multiple myeloma.
Ciltacabtagene autoleucel was granted a breakthrough therapy designation for relapsed/refractory multiple myeloma by the Food and Drug Administration in December 2019, a priority medicines (PRIME) designation by the European Medicines Agency in April 2019, and breakthrough designation in China in September 2020.
At the 2019 ASH annual meeting, Dr. Madduri reported phase 1b results from the trial, which showed that for 29 patients with heavily pretreated, relapsed/refractory multiple myeloma, the ORR at 6 months median follow-up was 100%, including 69% complete responses, with 27 patients remaining free of disease progression.
Combined data
For the 2020 ASH annual meeting, Dr. Madduri reported combined results from phases 1b and 2 of the CARTITUDE-1 study.
The investigators enrolled patients with multiple myeloma with measurable diseases as assessed by M-protein or serum free light chain levels who had experienced disease progression on at least three prior lines of therapy, or whose disease was refractory to at least two lines of therapy with a proteasome inhibitor, immunomodulatory drug, and an anti-CD38 antibody.
Patients underwent apheresis for T-cell collection, with bridging therapy allowed until the expanded T cells could be delivered.
Following T-cell depletion with cyclophosphamide 300 mg/m2 and fludarabine 30 mg/m2 over 3 days, patients received a single weight-based infusion (compared with fixed-dose infusions used with other CAR T-cell constructs).
The dose was targeted at 0.75x106 CAR-positive cells/kg, with a target range of 0.5–1.0x106, administered 5-7 days after the start of the conditioning regimen.
Of the 101 patients who underwent lymphodepletion, 97 (29 in phase 1b and 68 in phase 2) were treated with cilta-cel. Five of the patients in phase 1b and nine in phase 2 died on study, five of whom succumbed to progressive disease, and three due to adverse events unrelated to treatment. The remaining six patients died from treatment-related causes, including two patients from sepsis or septic shock, and one each from the cytokine release syndrome (CRS)/hemophagocytic lymphohistiocytosis (HLH), lung abscess, respiratory failure, and neurotoxicity.
At the time of data cutoff, 83 patients remained on study.
High ORR
The ORR was 96.9% (94 of 97 patients), comprising 67% stringent complete responses (sCR), 25.8% very good partial responses (VGPR), and 4.1% partial responses (PR).
Among 57 patients evaluable for minimal residual disease (MRD), 53 (93%) were MRD negative. Of this group, 49 had a VGPR or better.
The median time to first response was 1 month (range 0.9 to 8.5 months). At the time of data cutoff 70 patients had an ongoing response.
Among patients followed for a minimum of 6 months, most had cilta-cel CAR T-cells below the level of quantification (2 cells per microliter) in peripheral blood.
At a median follow-up of 12.4 months, 12-month overall progression-free survival rate was 76%, with the median PFS not reached. The 12-month overall survival rate was 88.5%, with the median OS not reached.
Safety data
All patients had at least one hematologic adverse event, 96 of which were grade 3 or 4 in severity. The events include neutropenia, anemia, thrombocytopenia, leukopenia, and lymphopenia. The median time to recovery was 2 weeks for grade 3 or 4 neutropenia and 4 weeks for thrombocytopenia.
Infections of any grade occurred in 57.7% of patients, including grade 3/4 pneumonia in 8.2% and grade 3/4 sepsis in 4.1%.
Grade 3 or 4 nonhematologic toxicities were uncommon, Dr. Madduri noted.
CRS of any grade occurred in 92 patients, but only 4 had grade 3 or 4 CRS.
Neurotoxicities occurred in 20 patients, of whom 10 had grade 3 or 4 neurotoxicity.
Immune effector cell-associated neurotoxicity syndrome (ICANS) occurred in 16 patients, with 2 having grade 3 or greater ICANS. Other neurotoxicities of any grade, many which overlapped with ICANS, occurred in 12 patients, with 9 having grade 3 or 4 neurotoxicity.
The median time to ICANS onset was 8 days, with a median time to recovery of 4 days. Other neurotoxicities took longer to manifest and disappear, however, with a median time to onset of 27 days, and median time to recovery of 75 days.
Neurotoxicity mechanism questioned
In the question-and-answer session following her presentation, an audience member asked whether the investigators had any insights into the mechanism underlying the non-ICANS neurotoxicities they saw.
“We saw no clear etiology in the other neurotoxicities, but we saw that maybe there could be some mild associations with high tumor burden, prior CRS, ICANS, or even the higher expansion and persistence of these cells,” Dr. Madduri replied.
She noted that subsequent to these findings, the investigators have implemented mitigation strategies including allowing patients to have more bridging chemotherapy, more aggressive steroid use for early ICANS, and extensive monitoring.
Eric Smith, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York, said that the non-ICANS neurotoxicity profile of cilta-cel was different from that seen in other CAR T-cell trials, and asked how it compared to that of bi-specific BCMA/CD3 CAR T constructs.
“We did see some nerve palsies and peripheral motor neuropathy, but it wasn’t that many patients, and it’s really hard to compare what happened here with the bi-specifics, as every product is very different,” she said.
The study was sponsored by Janssen Research & Development and Legend Biotech. Dr. Madduri disclosed honoraria, consultancy, and speakers bureau activities for those companies and others.
SOURCE: Madduri D et al. ASH 2020. Abstract 177.
For patients with heavily-pretreated multiple myeloma, the early and deep responses seen with the novel chimeric antigen receptor T-cell (CAR T-cell) construct ciltacabtagene autoleucel (cilta-cel) have also been durable, according to investigators in the CARTITUDE-1 trial.
Among 97 patients with multiple myeloma that had progressed on three or more prior lines of therapy or following treatment with at least two lines of therapy with a proteasome inhibitor and immunomodulating agent, the overall response rate (ORR) was 96.9%, with a median duration of response not reached after a median of 12.4 months of follow-up, reported Deepu Madduri, MD of Mount Sinai Medical Center in New York, and colleagues.
“We saw how heavily pretreated these patients were, and to see a one-time treatment get these kind of response rates is quite exceptional. What’s even more impressive is that 72% of these patients were still maintaining their response at the time of data cutoff,“ she said in an oral abstract presented during the virtual American Society of Hematology annual meeting.
Cilta-cel is a second-generation CAR T containing two single-domain antibodies targeted against B-cell maturation protein (BCMA). BCMA was first described in myeloma in 2004 as a mechanism for the growth and survival of malignant plasma cells.
As previously reported, the same CAR T-cell construct showed a high overall response with manageable toxicities in 74 patients with relapsed/refractory multiple myeloma.
Ciltacabtagene autoleucel was granted a breakthrough therapy designation for relapsed/refractory multiple myeloma by the Food and Drug Administration in December 2019, a priority medicines (PRIME) designation by the European Medicines Agency in April 2019, and breakthrough designation in China in September 2020.
At the 2019 ASH annual meeting, Dr. Madduri reported phase 1b results from the trial, which showed that for 29 patients with heavily pretreated, relapsed/refractory multiple myeloma, the ORR at 6 months median follow-up was 100%, including 69% complete responses, with 27 patients remaining free of disease progression.
Combined data
For the 2020 ASH annual meeting, Dr. Madduri reported combined results from phases 1b and 2 of the CARTITUDE-1 study.
The investigators enrolled patients with multiple myeloma with measurable diseases as assessed by M-protein or serum free light chain levels who had experienced disease progression on at least three prior lines of therapy, or whose disease was refractory to at least two lines of therapy with a proteasome inhibitor, immunomodulatory drug, and an anti-CD38 antibody.
Patients underwent apheresis for T-cell collection, with bridging therapy allowed until the expanded T cells could be delivered.
Following T-cell depletion with cyclophosphamide 300 mg/m2 and fludarabine 30 mg/m2 over 3 days, patients received a single weight-based infusion (compared with fixed-dose infusions used with other CAR T-cell constructs).
The dose was targeted at 0.75x106 CAR-positive cells/kg, with a target range of 0.5–1.0x106, administered 5-7 days after the start of the conditioning regimen.
Of the 101 patients who underwent lymphodepletion, 97 (29 in phase 1b and 68 in phase 2) were treated with cilta-cel. Five of the patients in phase 1b and nine in phase 2 died on study, five of whom succumbed to progressive disease, and three due to adverse events unrelated to treatment. The remaining six patients died from treatment-related causes, including two patients from sepsis or septic shock, and one each from the cytokine release syndrome (CRS)/hemophagocytic lymphohistiocytosis (HLH), lung abscess, respiratory failure, and neurotoxicity.
At the time of data cutoff, 83 patients remained on study.
High ORR
The ORR was 96.9% (94 of 97 patients), comprising 67% stringent complete responses (sCR), 25.8% very good partial responses (VGPR), and 4.1% partial responses (PR).
Among 57 patients evaluable for minimal residual disease (MRD), 53 (93%) were MRD negative. Of this group, 49 had a VGPR or better.
The median time to first response was 1 month (range 0.9 to 8.5 months). At the time of data cutoff 70 patients had an ongoing response.
Among patients followed for a minimum of 6 months, most had cilta-cel CAR T-cells below the level of quantification (2 cells per microliter) in peripheral blood.
At a median follow-up of 12.4 months, 12-month overall progression-free survival rate was 76%, with the median PFS not reached. The 12-month overall survival rate was 88.5%, with the median OS not reached.
Safety data
All patients had at least one hematologic adverse event, 96 of which were grade 3 or 4 in severity. The events include neutropenia, anemia, thrombocytopenia, leukopenia, and lymphopenia. The median time to recovery was 2 weeks for grade 3 or 4 neutropenia and 4 weeks for thrombocytopenia.
Infections of any grade occurred in 57.7% of patients, including grade 3/4 pneumonia in 8.2% and grade 3/4 sepsis in 4.1%.
Grade 3 or 4 nonhematologic toxicities were uncommon, Dr. Madduri noted.
CRS of any grade occurred in 92 patients, but only 4 had grade 3 or 4 CRS.
Neurotoxicities occurred in 20 patients, of whom 10 had grade 3 or 4 neurotoxicity.
Immune effector cell-associated neurotoxicity syndrome (ICANS) occurred in 16 patients, with 2 having grade 3 or greater ICANS. Other neurotoxicities of any grade, many which overlapped with ICANS, occurred in 12 patients, with 9 having grade 3 or 4 neurotoxicity.
The median time to ICANS onset was 8 days, with a median time to recovery of 4 days. Other neurotoxicities took longer to manifest and disappear, however, with a median time to onset of 27 days, and median time to recovery of 75 days.
Neurotoxicity mechanism questioned
In the question-and-answer session following her presentation, an audience member asked whether the investigators had any insights into the mechanism underlying the non-ICANS neurotoxicities they saw.
“We saw no clear etiology in the other neurotoxicities, but we saw that maybe there could be some mild associations with high tumor burden, prior CRS, ICANS, or even the higher expansion and persistence of these cells,” Dr. Madduri replied.
She noted that subsequent to these findings, the investigators have implemented mitigation strategies including allowing patients to have more bridging chemotherapy, more aggressive steroid use for early ICANS, and extensive monitoring.
Eric Smith, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York, said that the non-ICANS neurotoxicity profile of cilta-cel was different from that seen in other CAR T-cell trials, and asked how it compared to that of bi-specific BCMA/CD3 CAR T constructs.
“We did see some nerve palsies and peripheral motor neuropathy, but it wasn’t that many patients, and it’s really hard to compare what happened here with the bi-specifics, as every product is very different,” she said.
The study was sponsored by Janssen Research & Development and Legend Biotech. Dr. Madduri disclosed honoraria, consultancy, and speakers bureau activities for those companies and others.
SOURCE: Madduri D et al. ASH 2020. Abstract 177.
For patients with heavily-pretreated multiple myeloma, the early and deep responses seen with the novel chimeric antigen receptor T-cell (CAR T-cell) construct ciltacabtagene autoleucel (cilta-cel) have also been durable, according to investigators in the CARTITUDE-1 trial.
Among 97 patients with multiple myeloma that had progressed on three or more prior lines of therapy or following treatment with at least two lines of therapy with a proteasome inhibitor and immunomodulating agent, the overall response rate (ORR) was 96.9%, with a median duration of response not reached after a median of 12.4 months of follow-up, reported Deepu Madduri, MD of Mount Sinai Medical Center in New York, and colleagues.
“We saw how heavily pretreated these patients were, and to see a one-time treatment get these kind of response rates is quite exceptional. What’s even more impressive is that 72% of these patients were still maintaining their response at the time of data cutoff,“ she said in an oral abstract presented during the virtual American Society of Hematology annual meeting.
Cilta-cel is a second-generation CAR T containing two single-domain antibodies targeted against B-cell maturation protein (BCMA). BCMA was first described in myeloma in 2004 as a mechanism for the growth and survival of malignant plasma cells.
As previously reported, the same CAR T-cell construct showed a high overall response with manageable toxicities in 74 patients with relapsed/refractory multiple myeloma.
Ciltacabtagene autoleucel was granted a breakthrough therapy designation for relapsed/refractory multiple myeloma by the Food and Drug Administration in December 2019, a priority medicines (PRIME) designation by the European Medicines Agency in April 2019, and breakthrough designation in China in September 2020.
At the 2019 ASH annual meeting, Dr. Madduri reported phase 1b results from the trial, which showed that for 29 patients with heavily pretreated, relapsed/refractory multiple myeloma, the ORR at 6 months median follow-up was 100%, including 69% complete responses, with 27 patients remaining free of disease progression.
Combined data
For the 2020 ASH annual meeting, Dr. Madduri reported combined results from phases 1b and 2 of the CARTITUDE-1 study.
The investigators enrolled patients with multiple myeloma with measurable diseases as assessed by M-protein or serum free light chain levels who had experienced disease progression on at least three prior lines of therapy, or whose disease was refractory to at least two lines of therapy with a proteasome inhibitor, immunomodulatory drug, and an anti-CD38 antibody.
Patients underwent apheresis for T-cell collection, with bridging therapy allowed until the expanded T cells could be delivered.
Following T-cell depletion with cyclophosphamide 300 mg/m2 and fludarabine 30 mg/m2 over 3 days, patients received a single weight-based infusion (compared with fixed-dose infusions used with other CAR T-cell constructs).
The dose was targeted at 0.75x106 CAR-positive cells/kg, with a target range of 0.5–1.0x106, administered 5-7 days after the start of the conditioning regimen.
Of the 101 patients who underwent lymphodepletion, 97 (29 in phase 1b and 68 in phase 2) were treated with cilta-cel. Five of the patients in phase 1b and nine in phase 2 died on study, five of whom succumbed to progressive disease, and three due to adverse events unrelated to treatment. The remaining six patients died from treatment-related causes, including two patients from sepsis or septic shock, and one each from the cytokine release syndrome (CRS)/hemophagocytic lymphohistiocytosis (HLH), lung abscess, respiratory failure, and neurotoxicity.
At the time of data cutoff, 83 patients remained on study.
High ORR
The ORR was 96.9% (94 of 97 patients), comprising 67% stringent complete responses (sCR), 25.8% very good partial responses (VGPR), and 4.1% partial responses (PR).
Among 57 patients evaluable for minimal residual disease (MRD), 53 (93%) were MRD negative. Of this group, 49 had a VGPR or better.
The median time to first response was 1 month (range 0.9 to 8.5 months). At the time of data cutoff 70 patients had an ongoing response.
Among patients followed for a minimum of 6 months, most had cilta-cel CAR T-cells below the level of quantification (2 cells per microliter) in peripheral blood.
At a median follow-up of 12.4 months, 12-month overall progression-free survival rate was 76%, with the median PFS not reached. The 12-month overall survival rate was 88.5%, with the median OS not reached.
Safety data
All patients had at least one hematologic adverse event, 96 of which were grade 3 or 4 in severity. The events include neutropenia, anemia, thrombocytopenia, leukopenia, and lymphopenia. The median time to recovery was 2 weeks for grade 3 or 4 neutropenia and 4 weeks for thrombocytopenia.
Infections of any grade occurred in 57.7% of patients, including grade 3/4 pneumonia in 8.2% and grade 3/4 sepsis in 4.1%.
Grade 3 or 4 nonhematologic toxicities were uncommon, Dr. Madduri noted.
CRS of any grade occurred in 92 patients, but only 4 had grade 3 or 4 CRS.
Neurotoxicities occurred in 20 patients, of whom 10 had grade 3 or 4 neurotoxicity.
Immune effector cell-associated neurotoxicity syndrome (ICANS) occurred in 16 patients, with 2 having grade 3 or greater ICANS. Other neurotoxicities of any grade, many which overlapped with ICANS, occurred in 12 patients, with 9 having grade 3 or 4 neurotoxicity.
The median time to ICANS onset was 8 days, with a median time to recovery of 4 days. Other neurotoxicities took longer to manifest and disappear, however, with a median time to onset of 27 days, and median time to recovery of 75 days.
Neurotoxicity mechanism questioned
In the question-and-answer session following her presentation, an audience member asked whether the investigators had any insights into the mechanism underlying the non-ICANS neurotoxicities they saw.
“We saw no clear etiology in the other neurotoxicities, but we saw that maybe there could be some mild associations with high tumor burden, prior CRS, ICANS, or even the higher expansion and persistence of these cells,” Dr. Madduri replied.
She noted that subsequent to these findings, the investigators have implemented mitigation strategies including allowing patients to have more bridging chemotherapy, more aggressive steroid use for early ICANS, and extensive monitoring.
Eric Smith, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York, said that the non-ICANS neurotoxicity profile of cilta-cel was different from that seen in other CAR T-cell trials, and asked how it compared to that of bi-specific BCMA/CD3 CAR T constructs.
“We did see some nerve palsies and peripheral motor neuropathy, but it wasn’t that many patients, and it’s really hard to compare what happened here with the bi-specifics, as every product is very different,” she said.
The study was sponsored by Janssen Research & Development and Legend Biotech. Dr. Madduri disclosed honoraria, consultancy, and speakers bureau activities for those companies and others.
SOURCE: Madduri D et al. ASH 2020. Abstract 177.
FROM ASH 2020
Allogeneic transplant leads to durable remissions in T-cell lymphomas
, results of a large retrospective observational study suggest.
Five-year progression-free survival (PFS) approached 40% and 5-year overall survival (OS) was over 50% in the study, which according to an investigator is the largest-ever reported patient series of allogeneic stem cell transplantation in T-cell lymphomas.
“We believe that eligible patients with relapsed/refractory T-cell lymphomas should be considered for consultation for allogeneic transplant by an expert clinician,” said investigator Neha Mehta-Shah, MD, of Washington University in St. Louis.
“These decisions should occur on a patient by patient level – but it’s important to consider this,” Dr. Mehta-Shah said at the annual meeting of the American Society of Hematology, held virtually this year.
Notably, patients with cutaneous T-cell lymphoma (CTCL) had a higher rate of relapse yet similar overall survival (OS) compared to patients with common peripheral T-cell lymphoma (PTCL) subtypes, according to Dr. Mehta-Shah.
Among PTCL subtypes, there was a trend toward improved PFS and OS for angioimmunoblastic T-cell lymphoma (AITL), compared with PTCL not otherwise specified (PTCL-NOS) and anaplastic large-cell lymphoma (ALCL), she added.
Catherine M. Diefenbach, MD, director of the clinical lymphoma program at NYU Langone’s Perlmutter Cancer Center, said the results of this retrospective study need to considered in light of the treatment-related risks associated with allogeneic transplantation.
Treatment-related mortality in the study ranged from about 8% to 24%, depending on the donor type, while acute and chronic graft-versus-host-disease (GvHD) was seen in more than 40% of patients, the reported data show.
“If I have a relapsed patient with AITL, I would look to this data and say that patients with AITL appear in a retrospective study to have a strong benefit,” Dr. Diefenbach said in an interview.
“For the other patients, you would describe both potential benefits and also discuss the treatment-associated risks – both the chronic GvHD and transplant-related mortality – and you’d have to balance the risk with the benefits for each individual case,” Dr. Diefenbach added.
The retrospective analysis by Dr. Mehta-Shah and colleagues included 508 consecutive T-cell lymphoma patients receiving allogeneic transplants at 12 academic centers between 2000 and 2019. The most common subtypes were PTCL-NOS in 26%, AITL in 16%, CTCL in 13%, and hepatosplenic T-cell lymphoma (HSTCL) in 7%. About 40% had a matched related donor (MRD) and 39% had a matched unrelated donor (MUD). The conditioning regimen was myeloablative in about a third of patients and nonmyeloablative in two-thirds.
At 5 years, PFS was 39.4% and OS was 50.8% for the overall study cohort, Dr. Mehta-Shah reported, noting that the median time from relapse to death post allogeneic transplant was 10.2 months.
Patients in complete remission at the time of transplant fared better than others, with a median PFS of 44.6 months vs. 8.5 months for those in partial remission, 21.0 months in those with stable disease, and 3.5 months for those with progressive disease at time of transplant, data show.
Patients with common PTCL subtypes had better PFS compared to patients with CTCL, yet OS was similar, according to the investigator. At 5 years, PFS was 43.7% and 18.6%, respectively, for PTCL and CTCL, while OS was 53.1% and 44.0%, respectively.
There was a trend toward improved outcomes for AITL relative to PTCL-NOS and ALCL, with a median PFS of 51.4 months for AITL versus 18.3 months those other subtypes. Similarly, median OS was not reached for AITL versus 73.1 months in the other subtypes.
Treatment-related mortality was lowest for patients with MRDs, or 8.2% at 12 months, Dr. Mehta-Shah reported, while patients with MUDs, mismatched donors, or haploidentical donors had treatment-related mortality of 13% to 16% at 12 months, and those with cord blood donors had treatment-related mortality of nearly 24% at 12 months.
Acute GvHD was observed in 46% of patients and chronic GvHD was seen in nearly 41%, the investigator added.
While these findings are important to consider in individual patient consultations, the study is nevertheless subject to limitations including patient selection and referral bias, according to Dr. Mehta-Shah.
“This was a retrospective analysis of patients who underwent transplant,” she said in a question-and-answer period. “Of course, that is heavily biased by who got to a transplant center, who was well enough to achieve transplant, and who had a donor or donor options, as well as their overall health and depth of remission,” the researcher said.
“I think this just represents what we could tell patients about what may happen to them once they embark on a transplant,” she added, “but really, there would be more prospective work needed to be done for what happens to patients overarching, and how many of them even get to a transplant consultation.”
Further studies should be done to develop predictive tools or biomarkers to determine who benefits from an allogeneic transplant, if there are predictors of relapse following allogeneic transplant, and what are the mechanisms of relapse following allogeneic transplant, according to Dr. Mehta-Shah.
Dr. Mehta-Shah reported research funding from Bristol Myers-Squibb, Celgene, Verastem, Corvus, Innate Pharmaceuticals, and Genentech/Roche. She reported consultancy with Kyowa Hakko Kirin, C4 Therapeutics, and Karyopharm Therapeutics.
SOURCE: Mehta-Shah N et al. ASH 2020, Abstract 41.
, results of a large retrospective observational study suggest.
Five-year progression-free survival (PFS) approached 40% and 5-year overall survival (OS) was over 50% in the study, which according to an investigator is the largest-ever reported patient series of allogeneic stem cell transplantation in T-cell lymphomas.
“We believe that eligible patients with relapsed/refractory T-cell lymphomas should be considered for consultation for allogeneic transplant by an expert clinician,” said investigator Neha Mehta-Shah, MD, of Washington University in St. Louis.
“These decisions should occur on a patient by patient level – but it’s important to consider this,” Dr. Mehta-Shah said at the annual meeting of the American Society of Hematology, held virtually this year.
Notably, patients with cutaneous T-cell lymphoma (CTCL) had a higher rate of relapse yet similar overall survival (OS) compared to patients with common peripheral T-cell lymphoma (PTCL) subtypes, according to Dr. Mehta-Shah.
Among PTCL subtypes, there was a trend toward improved PFS and OS for angioimmunoblastic T-cell lymphoma (AITL), compared with PTCL not otherwise specified (PTCL-NOS) and anaplastic large-cell lymphoma (ALCL), she added.
Catherine M. Diefenbach, MD, director of the clinical lymphoma program at NYU Langone’s Perlmutter Cancer Center, said the results of this retrospective study need to considered in light of the treatment-related risks associated with allogeneic transplantation.
Treatment-related mortality in the study ranged from about 8% to 24%, depending on the donor type, while acute and chronic graft-versus-host-disease (GvHD) was seen in more than 40% of patients, the reported data show.
“If I have a relapsed patient with AITL, I would look to this data and say that patients with AITL appear in a retrospective study to have a strong benefit,” Dr. Diefenbach said in an interview.
“For the other patients, you would describe both potential benefits and also discuss the treatment-associated risks – both the chronic GvHD and transplant-related mortality – and you’d have to balance the risk with the benefits for each individual case,” Dr. Diefenbach added.
The retrospective analysis by Dr. Mehta-Shah and colleagues included 508 consecutive T-cell lymphoma patients receiving allogeneic transplants at 12 academic centers between 2000 and 2019. The most common subtypes were PTCL-NOS in 26%, AITL in 16%, CTCL in 13%, and hepatosplenic T-cell lymphoma (HSTCL) in 7%. About 40% had a matched related donor (MRD) and 39% had a matched unrelated donor (MUD). The conditioning regimen was myeloablative in about a third of patients and nonmyeloablative in two-thirds.
At 5 years, PFS was 39.4% and OS was 50.8% for the overall study cohort, Dr. Mehta-Shah reported, noting that the median time from relapse to death post allogeneic transplant was 10.2 months.
Patients in complete remission at the time of transplant fared better than others, with a median PFS of 44.6 months vs. 8.5 months for those in partial remission, 21.0 months in those with stable disease, and 3.5 months for those with progressive disease at time of transplant, data show.
Patients with common PTCL subtypes had better PFS compared to patients with CTCL, yet OS was similar, according to the investigator. At 5 years, PFS was 43.7% and 18.6%, respectively, for PTCL and CTCL, while OS was 53.1% and 44.0%, respectively.
There was a trend toward improved outcomes for AITL relative to PTCL-NOS and ALCL, with a median PFS of 51.4 months for AITL versus 18.3 months those other subtypes. Similarly, median OS was not reached for AITL versus 73.1 months in the other subtypes.
Treatment-related mortality was lowest for patients with MRDs, or 8.2% at 12 months, Dr. Mehta-Shah reported, while patients with MUDs, mismatched donors, or haploidentical donors had treatment-related mortality of 13% to 16% at 12 months, and those with cord blood donors had treatment-related mortality of nearly 24% at 12 months.
Acute GvHD was observed in 46% of patients and chronic GvHD was seen in nearly 41%, the investigator added.
While these findings are important to consider in individual patient consultations, the study is nevertheless subject to limitations including patient selection and referral bias, according to Dr. Mehta-Shah.
“This was a retrospective analysis of patients who underwent transplant,” she said in a question-and-answer period. “Of course, that is heavily biased by who got to a transplant center, who was well enough to achieve transplant, and who had a donor or donor options, as well as their overall health and depth of remission,” the researcher said.
“I think this just represents what we could tell patients about what may happen to them once they embark on a transplant,” she added, “but really, there would be more prospective work needed to be done for what happens to patients overarching, and how many of them even get to a transplant consultation.”
Further studies should be done to develop predictive tools or biomarkers to determine who benefits from an allogeneic transplant, if there are predictors of relapse following allogeneic transplant, and what are the mechanisms of relapse following allogeneic transplant, according to Dr. Mehta-Shah.
Dr. Mehta-Shah reported research funding from Bristol Myers-Squibb, Celgene, Verastem, Corvus, Innate Pharmaceuticals, and Genentech/Roche. She reported consultancy with Kyowa Hakko Kirin, C4 Therapeutics, and Karyopharm Therapeutics.
SOURCE: Mehta-Shah N et al. ASH 2020, Abstract 41.
, results of a large retrospective observational study suggest.
Five-year progression-free survival (PFS) approached 40% and 5-year overall survival (OS) was over 50% in the study, which according to an investigator is the largest-ever reported patient series of allogeneic stem cell transplantation in T-cell lymphomas.
“We believe that eligible patients with relapsed/refractory T-cell lymphomas should be considered for consultation for allogeneic transplant by an expert clinician,” said investigator Neha Mehta-Shah, MD, of Washington University in St. Louis.
“These decisions should occur on a patient by patient level – but it’s important to consider this,” Dr. Mehta-Shah said at the annual meeting of the American Society of Hematology, held virtually this year.
Notably, patients with cutaneous T-cell lymphoma (CTCL) had a higher rate of relapse yet similar overall survival (OS) compared to patients with common peripheral T-cell lymphoma (PTCL) subtypes, according to Dr. Mehta-Shah.
Among PTCL subtypes, there was a trend toward improved PFS and OS for angioimmunoblastic T-cell lymphoma (AITL), compared with PTCL not otherwise specified (PTCL-NOS) and anaplastic large-cell lymphoma (ALCL), she added.
Catherine M. Diefenbach, MD, director of the clinical lymphoma program at NYU Langone’s Perlmutter Cancer Center, said the results of this retrospective study need to considered in light of the treatment-related risks associated with allogeneic transplantation.
Treatment-related mortality in the study ranged from about 8% to 24%, depending on the donor type, while acute and chronic graft-versus-host-disease (GvHD) was seen in more than 40% of patients, the reported data show.
“If I have a relapsed patient with AITL, I would look to this data and say that patients with AITL appear in a retrospective study to have a strong benefit,” Dr. Diefenbach said in an interview.
“For the other patients, you would describe both potential benefits and also discuss the treatment-associated risks – both the chronic GvHD and transplant-related mortality – and you’d have to balance the risk with the benefits for each individual case,” Dr. Diefenbach added.
The retrospective analysis by Dr. Mehta-Shah and colleagues included 508 consecutive T-cell lymphoma patients receiving allogeneic transplants at 12 academic centers between 2000 and 2019. The most common subtypes were PTCL-NOS in 26%, AITL in 16%, CTCL in 13%, and hepatosplenic T-cell lymphoma (HSTCL) in 7%. About 40% had a matched related donor (MRD) and 39% had a matched unrelated donor (MUD). The conditioning regimen was myeloablative in about a third of patients and nonmyeloablative in two-thirds.
At 5 years, PFS was 39.4% and OS was 50.8% for the overall study cohort, Dr. Mehta-Shah reported, noting that the median time from relapse to death post allogeneic transplant was 10.2 months.
Patients in complete remission at the time of transplant fared better than others, with a median PFS of 44.6 months vs. 8.5 months for those in partial remission, 21.0 months in those with stable disease, and 3.5 months for those with progressive disease at time of transplant, data show.
Patients with common PTCL subtypes had better PFS compared to patients with CTCL, yet OS was similar, according to the investigator. At 5 years, PFS was 43.7% and 18.6%, respectively, for PTCL and CTCL, while OS was 53.1% and 44.0%, respectively.
There was a trend toward improved outcomes for AITL relative to PTCL-NOS and ALCL, with a median PFS of 51.4 months for AITL versus 18.3 months those other subtypes. Similarly, median OS was not reached for AITL versus 73.1 months in the other subtypes.
Treatment-related mortality was lowest for patients with MRDs, or 8.2% at 12 months, Dr. Mehta-Shah reported, while patients with MUDs, mismatched donors, or haploidentical donors had treatment-related mortality of 13% to 16% at 12 months, and those with cord blood donors had treatment-related mortality of nearly 24% at 12 months.
Acute GvHD was observed in 46% of patients and chronic GvHD was seen in nearly 41%, the investigator added.
While these findings are important to consider in individual patient consultations, the study is nevertheless subject to limitations including patient selection and referral bias, according to Dr. Mehta-Shah.
“This was a retrospective analysis of patients who underwent transplant,” she said in a question-and-answer period. “Of course, that is heavily biased by who got to a transplant center, who was well enough to achieve transplant, and who had a donor or donor options, as well as their overall health and depth of remission,” the researcher said.
“I think this just represents what we could tell patients about what may happen to them once they embark on a transplant,” she added, “but really, there would be more prospective work needed to be done for what happens to patients overarching, and how many of them even get to a transplant consultation.”
Further studies should be done to develop predictive tools or biomarkers to determine who benefits from an allogeneic transplant, if there are predictors of relapse following allogeneic transplant, and what are the mechanisms of relapse following allogeneic transplant, according to Dr. Mehta-Shah.
Dr. Mehta-Shah reported research funding from Bristol Myers-Squibb, Celgene, Verastem, Corvus, Innate Pharmaceuticals, and Genentech/Roche. She reported consultancy with Kyowa Hakko Kirin, C4 Therapeutics, and Karyopharm Therapeutics.
SOURCE: Mehta-Shah N et al. ASH 2020, Abstract 41.
FROM ASH 2020
ZUMA-5: Axi-cel yields high response rate in indolent NHL
, according to phase 2 study results presented at the annual meeting of the American Society of Hematology, held virtually this year.
The overall response rate exceeded 90% in the ZUMA-5 study, which included patients with multiply relapsed follicular lymphoma (FL) or marginal zone lymphoma (MZL) who were treated with this anti-CD19 chimeric antigen receptor (CAR) T cell therapy.
“Although longer follow-up is needed, these responses appear to be durable,” said investigator Caron Jacobson, MD, of Dana-Farber Cancer Institute in Boston.
Complete responses (CRs) after axi-cel treatment were seen in about three-quarters of patients, and most of those patients were still in response with a median follow-up that approached 1.5 years as of this report at the ASH meeting.
In her presentation, Dr. Jacobson said the safety profile of axi-cel in ZUMA-5 was manageable and “at least similar” to what was previously seen in aggressive relapsed lymphomas, referring to the ZUMA-1 study that led to 2017 approval by the Food and Drug Administration of the treatment for relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy.
The FL patient cohort in ZUMA-5 appeared to have lower rates of cytokine release syndrome (CRS) and high-grade neurotoxicity, compared with the MZL cohort in the study, she added.
Catherine Bollard, MD, of Children’s National Research Institute in Washington, said these results suggest axi-cel may be a “viable treatment option” for some patients with indolent lymphomas who have not responded to other therapies.
“What the field does need is long-term follow-up in the real-world setting to see what the true progression-free and disease-free survival is for these patients,” said Dr. Bollard, who moderated a media briefing that included the ZUMA-5 study.
“It’s really exciting to see this data in the [indolent] lymphoma setting, and I actually would like to see it moved further up in the treatment of patients, earlier in their disease process, if that’s going to be possible,” she added.
Promising results
The report on ZUMA-5, presented by Dr. Jacobson, involved 146 patients with relapsed/refractory indolent NHL: 124 patients with FL and an exploratory cohort of 22 patients with MZL. All patients had received at least two prior lines of therapy.
Following a fludarabine/cyclophosphamide conditioning regimen, patients received axi-cel at the FDA-approved dose of 2 x 106 CAR-positive T cells per kg of body weight. The primary endpoint of the study was overall response rate (ORR).
For 104 patients evaluable for efficacy, the ORR was 92% (96 patients), including CR in 76% (79 patients), data show. Among 84 FL patients evaluable for efficacy, ORR and CR were 94% (79 patients) and 80% (67 patients), respectively, while among 20 evaluable patients in the exploratory MZL cohort, ORR and CR were 60% (12 patients) and 25% (5 patients), respectively.
Sixty-four percent of patients with FL had an ongoing response at a median follow-up of 17.5 months, according to Dr. Jacobson, who added that median duration of response (DOR) had not been reached, while the 12-month DOR rate approached 72%.
The 12-month progression-free survival and overall survival rates were 73.7% and 92.9%, respectively, with medians not yet reached for either survival outcome, according to reported data.
Adverse effects
The incidence of grade 3 or greater neurologic events was lower in FL patients (15%), compared with MZL patients (41%), according to Dr. Jacobson.
While CRS occurred in 82% of patients, rates of grade 3 or greater CRS occurred in just 6% of FL patients and 9% of MZL patients, the investigator said.
There were no grade 5 neurologic events, and one grade 5 CRS was observed, she noted in her presentation.
The median time to onset of CRS was 4 days, compared with 2 days in the ZUMA-1 trial. “This may have implications for the possibility of outpatient therapy,” she said.
A study is planned to look at outpatient administration of axi-cel in patients with indolent NHL, she added.
Dr. Jacobson said she had no conflicts of interest to declare. Coauthors reported disclosures related to Kite, a Gilead Company; Genentech; Epizyme; Verastem; Novartis; and Pfizer, among others.
Correction, 12/7/20: An earlier version of this article misattributed some aspects of the ZUMA-5 trial to ZUMA-1.
SOURCE: Jacobson CA et al. ASH 2020, Abstract 700.
, according to phase 2 study results presented at the annual meeting of the American Society of Hematology, held virtually this year.
The overall response rate exceeded 90% in the ZUMA-5 study, which included patients with multiply relapsed follicular lymphoma (FL) or marginal zone lymphoma (MZL) who were treated with this anti-CD19 chimeric antigen receptor (CAR) T cell therapy.
“Although longer follow-up is needed, these responses appear to be durable,” said investigator Caron Jacobson, MD, of Dana-Farber Cancer Institute in Boston.
Complete responses (CRs) after axi-cel treatment were seen in about three-quarters of patients, and most of those patients were still in response with a median follow-up that approached 1.5 years as of this report at the ASH meeting.
In her presentation, Dr. Jacobson said the safety profile of axi-cel in ZUMA-5 was manageable and “at least similar” to what was previously seen in aggressive relapsed lymphomas, referring to the ZUMA-1 study that led to 2017 approval by the Food and Drug Administration of the treatment for relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy.
The FL patient cohort in ZUMA-5 appeared to have lower rates of cytokine release syndrome (CRS) and high-grade neurotoxicity, compared with the MZL cohort in the study, she added.
Catherine Bollard, MD, of Children’s National Research Institute in Washington, said these results suggest axi-cel may be a “viable treatment option” for some patients with indolent lymphomas who have not responded to other therapies.
“What the field does need is long-term follow-up in the real-world setting to see what the true progression-free and disease-free survival is for these patients,” said Dr. Bollard, who moderated a media briefing that included the ZUMA-5 study.
“It’s really exciting to see this data in the [indolent] lymphoma setting, and I actually would like to see it moved further up in the treatment of patients, earlier in their disease process, if that’s going to be possible,” she added.
Promising results
The report on ZUMA-5, presented by Dr. Jacobson, involved 146 patients with relapsed/refractory indolent NHL: 124 patients with FL and an exploratory cohort of 22 patients with MZL. All patients had received at least two prior lines of therapy.
Following a fludarabine/cyclophosphamide conditioning regimen, patients received axi-cel at the FDA-approved dose of 2 x 106 CAR-positive T cells per kg of body weight. The primary endpoint of the study was overall response rate (ORR).
For 104 patients evaluable for efficacy, the ORR was 92% (96 patients), including CR in 76% (79 patients), data show. Among 84 FL patients evaluable for efficacy, ORR and CR were 94% (79 patients) and 80% (67 patients), respectively, while among 20 evaluable patients in the exploratory MZL cohort, ORR and CR were 60% (12 patients) and 25% (5 patients), respectively.
Sixty-four percent of patients with FL had an ongoing response at a median follow-up of 17.5 months, according to Dr. Jacobson, who added that median duration of response (DOR) had not been reached, while the 12-month DOR rate approached 72%.
The 12-month progression-free survival and overall survival rates were 73.7% and 92.9%, respectively, with medians not yet reached for either survival outcome, according to reported data.
Adverse effects
The incidence of grade 3 or greater neurologic events was lower in FL patients (15%), compared with MZL patients (41%), according to Dr. Jacobson.
While CRS occurred in 82% of patients, rates of grade 3 or greater CRS occurred in just 6% of FL patients and 9% of MZL patients, the investigator said.
There were no grade 5 neurologic events, and one grade 5 CRS was observed, she noted in her presentation.
The median time to onset of CRS was 4 days, compared with 2 days in the ZUMA-1 trial. “This may have implications for the possibility of outpatient therapy,” she said.
A study is planned to look at outpatient administration of axi-cel in patients with indolent NHL, she added.
Dr. Jacobson said she had no conflicts of interest to declare. Coauthors reported disclosures related to Kite, a Gilead Company; Genentech; Epizyme; Verastem; Novartis; and Pfizer, among others.
Correction, 12/7/20: An earlier version of this article misattributed some aspects of the ZUMA-5 trial to ZUMA-1.
SOURCE: Jacobson CA et al. ASH 2020, Abstract 700.
, according to phase 2 study results presented at the annual meeting of the American Society of Hematology, held virtually this year.
The overall response rate exceeded 90% in the ZUMA-5 study, which included patients with multiply relapsed follicular lymphoma (FL) or marginal zone lymphoma (MZL) who were treated with this anti-CD19 chimeric antigen receptor (CAR) T cell therapy.
“Although longer follow-up is needed, these responses appear to be durable,” said investigator Caron Jacobson, MD, of Dana-Farber Cancer Institute in Boston.
Complete responses (CRs) after axi-cel treatment were seen in about three-quarters of patients, and most of those patients were still in response with a median follow-up that approached 1.5 years as of this report at the ASH meeting.
In her presentation, Dr. Jacobson said the safety profile of axi-cel in ZUMA-5 was manageable and “at least similar” to what was previously seen in aggressive relapsed lymphomas, referring to the ZUMA-1 study that led to 2017 approval by the Food and Drug Administration of the treatment for relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy.
The FL patient cohort in ZUMA-5 appeared to have lower rates of cytokine release syndrome (CRS) and high-grade neurotoxicity, compared with the MZL cohort in the study, she added.
Catherine Bollard, MD, of Children’s National Research Institute in Washington, said these results suggest axi-cel may be a “viable treatment option” for some patients with indolent lymphomas who have not responded to other therapies.
“What the field does need is long-term follow-up in the real-world setting to see what the true progression-free and disease-free survival is for these patients,” said Dr. Bollard, who moderated a media briefing that included the ZUMA-5 study.
“It’s really exciting to see this data in the [indolent] lymphoma setting, and I actually would like to see it moved further up in the treatment of patients, earlier in their disease process, if that’s going to be possible,” she added.
Promising results
The report on ZUMA-5, presented by Dr. Jacobson, involved 146 patients with relapsed/refractory indolent NHL: 124 patients with FL and an exploratory cohort of 22 patients with MZL. All patients had received at least two prior lines of therapy.
Following a fludarabine/cyclophosphamide conditioning regimen, patients received axi-cel at the FDA-approved dose of 2 x 106 CAR-positive T cells per kg of body weight. The primary endpoint of the study was overall response rate (ORR).
For 104 patients evaluable for efficacy, the ORR was 92% (96 patients), including CR in 76% (79 patients), data show. Among 84 FL patients evaluable for efficacy, ORR and CR were 94% (79 patients) and 80% (67 patients), respectively, while among 20 evaluable patients in the exploratory MZL cohort, ORR and CR were 60% (12 patients) and 25% (5 patients), respectively.
Sixty-four percent of patients with FL had an ongoing response at a median follow-up of 17.5 months, according to Dr. Jacobson, who added that median duration of response (DOR) had not been reached, while the 12-month DOR rate approached 72%.
The 12-month progression-free survival and overall survival rates were 73.7% and 92.9%, respectively, with medians not yet reached for either survival outcome, according to reported data.
Adverse effects
The incidence of grade 3 or greater neurologic events was lower in FL patients (15%), compared with MZL patients (41%), according to Dr. Jacobson.
While CRS occurred in 82% of patients, rates of grade 3 or greater CRS occurred in just 6% of FL patients and 9% of MZL patients, the investigator said.
There were no grade 5 neurologic events, and one grade 5 CRS was observed, she noted in her presentation.
The median time to onset of CRS was 4 days, compared with 2 days in the ZUMA-1 trial. “This may have implications for the possibility of outpatient therapy,” she said.
A study is planned to look at outpatient administration of axi-cel in patients with indolent NHL, she added.
Dr. Jacobson said she had no conflicts of interest to declare. Coauthors reported disclosures related to Kite, a Gilead Company; Genentech; Epizyme; Verastem; Novartis; and Pfizer, among others.
Correction, 12/7/20: An earlier version of this article misattributed some aspects of the ZUMA-5 trial to ZUMA-1.
SOURCE: Jacobson CA et al. ASH 2020, Abstract 700.
FROM ASH 2020
COVID-19–related outcomes poor for patients with hematologic disease in ASH registry
Patients with hematologic disease who develop COVID-19 may experience substantial morbidity and mortality related to SARS-CoV-2 infection, according to recent registry data reported at the all-virtual annual meeting of the American Society of Hematology.
Overall mortality was 28% for the first 250 patients entered into the ASH Research Collaborative COVID-19 Registry for Hematology, researchers reported in an abstract of their study findings.
However, the burden of death and moderate-to-severe COVID-19 outcomes was highest in patients with poorer prognosis and those with relapsed/refractory hematological disease, they added.
The most commonly represented malignancies were acute leukemia, non-Hodgkin lymphoma, and myeloma or amyloidosis, according to the report.
Taken together, the findings do support an “emerging consensus” that COVID-19 related morbidity and mortality is significant in these patients, authors said – however, the current findings may not be reason enough to support a change in treatment course for the underlying disease.
“We see no reason, based on our data, to withhold intensive therapies from patients with underlying hematologic malignancies and favorable prognoses, if aggressive supportive care is consistent with patient preferences,” wrote the researchers.
ASH President Stephanie Lee, MD, MPH, said these registry findings are important to better understand how SARS-CoV-2 is affecting not only patients with hematologic diseases, but also individuals who experience COVID-19-related hematologic complications.
However, the findings are limited due to the heterogeneity of diseases, symptoms, and treatments represented in the registry, said Dr. Lee, associate director of the clinical research division at Fred Hutchinson Cancer Center in Seattle.
“More data will be coming in, but I think this is an example of trying to harness real-world information to try to learn things until we get more controlled studies,” Dr. Lee said in a media briefing held in advance of the ASH meeting.
Comorbidities and more
Patients with blood cancers are often older and may have comorbidities such as diabetes or hypertension that have been linked to poor COVID-19 outcomes, according to the authors of the report, led by William A. Wood, MD, MPH, associate professor of medicine with the UNC Lineberger Comprehensive Cancer Center in Chapel Hill, N.C.
Moreover, these patients may have underlying immune dysfunction and may receive chemotherapy or immunotherapy that is “profoundly immunosuppressive,” Dr. Wood and coauthors said in their report.
To date, however, risks of morbidity and mortality related to SARS-CoV-2 infection have not been well defined in this patient population, authors said.
More data is emerging now from the ASH Research Collaborative COVID-19 Registry for Hematology, which includes data on patients positive for COVID-19 who have a past or present hematologic condition or have experienced a hematologic complication related to COVID-19.
All data from the registry is being made available through a dashboard on the ASH Research Collaborative website, which as of Dec. 1, 2020, included 693 complete cases.
The data cut in the ASH abstract includes the first 250 patients enrolled at 74 sites around the world, the authors said. The most common malignancies included acute leukemia in 33%, non-Hodgkin lymphoma in 27%, and myeloma or amyloidosis in 16%.
The most frequently reported symptoms included fever in 73%, cough in 67%, dyspnea in 50%, and fatigue in 40%, according to that report.
At the time of this data snapshot, treatment with COVID-19-directed therapies including hydroxychloroquine or azithromycin were common, reported in 76 and 59 patients, respectively, in the cohort.
Batch submissions from sites with high incidence of COVID-19 infection are ongoing. The registry has been expanded to include nonmalignant hematologic diseases, and the registry will continue to accumulate data as a resource for the hematology community.
Overall mortality was 28% at the time, according to the abstract, with nearly all of the deaths occurring in patients classified as having COVID-19 that was moderate (i.e., requiring hospitalization) or severe (i.e., requiring ICU admission).
“In some instances, death occurred after a decision was made to forgo ICU admission in favor of a palliative approach,” said Dr. Wood and coauthors in their report.
Dr. Wood reported research funding from Pfizer, consultancy with Teladoc/Best Doctors, and honoraria from the ASH Research Collaborative. Coauthors provided disclosures related to Celgene, Madrigal Pharmaceuticals, Pharmacyclics, and Amgen, among others.
SOURCE: Wood WA et al. ASH 2020, Abstract 215.
Patients with hematologic disease who develop COVID-19 may experience substantial morbidity and mortality related to SARS-CoV-2 infection, according to recent registry data reported at the all-virtual annual meeting of the American Society of Hematology.
Overall mortality was 28% for the first 250 patients entered into the ASH Research Collaborative COVID-19 Registry for Hematology, researchers reported in an abstract of their study findings.
However, the burden of death and moderate-to-severe COVID-19 outcomes was highest in patients with poorer prognosis and those with relapsed/refractory hematological disease, they added.
The most commonly represented malignancies were acute leukemia, non-Hodgkin lymphoma, and myeloma or amyloidosis, according to the report.
Taken together, the findings do support an “emerging consensus” that COVID-19 related morbidity and mortality is significant in these patients, authors said – however, the current findings may not be reason enough to support a change in treatment course for the underlying disease.
“We see no reason, based on our data, to withhold intensive therapies from patients with underlying hematologic malignancies and favorable prognoses, if aggressive supportive care is consistent with patient preferences,” wrote the researchers.
ASH President Stephanie Lee, MD, MPH, said these registry findings are important to better understand how SARS-CoV-2 is affecting not only patients with hematologic diseases, but also individuals who experience COVID-19-related hematologic complications.
However, the findings are limited due to the heterogeneity of diseases, symptoms, and treatments represented in the registry, said Dr. Lee, associate director of the clinical research division at Fred Hutchinson Cancer Center in Seattle.
“More data will be coming in, but I think this is an example of trying to harness real-world information to try to learn things until we get more controlled studies,” Dr. Lee said in a media briefing held in advance of the ASH meeting.
Comorbidities and more
Patients with blood cancers are often older and may have comorbidities such as diabetes or hypertension that have been linked to poor COVID-19 outcomes, according to the authors of the report, led by William A. Wood, MD, MPH, associate professor of medicine with the UNC Lineberger Comprehensive Cancer Center in Chapel Hill, N.C.
Moreover, these patients may have underlying immune dysfunction and may receive chemotherapy or immunotherapy that is “profoundly immunosuppressive,” Dr. Wood and coauthors said in their report.
To date, however, risks of morbidity and mortality related to SARS-CoV-2 infection have not been well defined in this patient population, authors said.
More data is emerging now from the ASH Research Collaborative COVID-19 Registry for Hematology, which includes data on patients positive for COVID-19 who have a past or present hematologic condition or have experienced a hematologic complication related to COVID-19.
All data from the registry is being made available through a dashboard on the ASH Research Collaborative website, which as of Dec. 1, 2020, included 693 complete cases.
The data cut in the ASH abstract includes the first 250 patients enrolled at 74 sites around the world, the authors said. The most common malignancies included acute leukemia in 33%, non-Hodgkin lymphoma in 27%, and myeloma or amyloidosis in 16%.
The most frequently reported symptoms included fever in 73%, cough in 67%, dyspnea in 50%, and fatigue in 40%, according to that report.
At the time of this data snapshot, treatment with COVID-19-directed therapies including hydroxychloroquine or azithromycin were common, reported in 76 and 59 patients, respectively, in the cohort.
Batch submissions from sites with high incidence of COVID-19 infection are ongoing. The registry has been expanded to include nonmalignant hematologic diseases, and the registry will continue to accumulate data as a resource for the hematology community.
Overall mortality was 28% at the time, according to the abstract, with nearly all of the deaths occurring in patients classified as having COVID-19 that was moderate (i.e., requiring hospitalization) or severe (i.e., requiring ICU admission).
“In some instances, death occurred after a decision was made to forgo ICU admission in favor of a palliative approach,” said Dr. Wood and coauthors in their report.
Dr. Wood reported research funding from Pfizer, consultancy with Teladoc/Best Doctors, and honoraria from the ASH Research Collaborative. Coauthors provided disclosures related to Celgene, Madrigal Pharmaceuticals, Pharmacyclics, and Amgen, among others.
SOURCE: Wood WA et al. ASH 2020, Abstract 215.
Patients with hematologic disease who develop COVID-19 may experience substantial morbidity and mortality related to SARS-CoV-2 infection, according to recent registry data reported at the all-virtual annual meeting of the American Society of Hematology.
Overall mortality was 28% for the first 250 patients entered into the ASH Research Collaborative COVID-19 Registry for Hematology, researchers reported in an abstract of their study findings.
However, the burden of death and moderate-to-severe COVID-19 outcomes was highest in patients with poorer prognosis and those with relapsed/refractory hematological disease, they added.
The most commonly represented malignancies were acute leukemia, non-Hodgkin lymphoma, and myeloma or amyloidosis, according to the report.
Taken together, the findings do support an “emerging consensus” that COVID-19 related morbidity and mortality is significant in these patients, authors said – however, the current findings may not be reason enough to support a change in treatment course for the underlying disease.
“We see no reason, based on our data, to withhold intensive therapies from patients with underlying hematologic malignancies and favorable prognoses, if aggressive supportive care is consistent with patient preferences,” wrote the researchers.
ASH President Stephanie Lee, MD, MPH, said these registry findings are important to better understand how SARS-CoV-2 is affecting not only patients with hematologic diseases, but also individuals who experience COVID-19-related hematologic complications.
However, the findings are limited due to the heterogeneity of diseases, symptoms, and treatments represented in the registry, said Dr. Lee, associate director of the clinical research division at Fred Hutchinson Cancer Center in Seattle.
“More data will be coming in, but I think this is an example of trying to harness real-world information to try to learn things until we get more controlled studies,” Dr. Lee said in a media briefing held in advance of the ASH meeting.
Comorbidities and more
Patients with blood cancers are often older and may have comorbidities such as diabetes or hypertension that have been linked to poor COVID-19 outcomes, according to the authors of the report, led by William A. Wood, MD, MPH, associate professor of medicine with the UNC Lineberger Comprehensive Cancer Center in Chapel Hill, N.C.
Moreover, these patients may have underlying immune dysfunction and may receive chemotherapy or immunotherapy that is “profoundly immunosuppressive,” Dr. Wood and coauthors said in their report.
To date, however, risks of morbidity and mortality related to SARS-CoV-2 infection have not been well defined in this patient population, authors said.
More data is emerging now from the ASH Research Collaborative COVID-19 Registry for Hematology, which includes data on patients positive for COVID-19 who have a past or present hematologic condition or have experienced a hematologic complication related to COVID-19.
All data from the registry is being made available through a dashboard on the ASH Research Collaborative website, which as of Dec. 1, 2020, included 693 complete cases.
The data cut in the ASH abstract includes the first 250 patients enrolled at 74 sites around the world, the authors said. The most common malignancies included acute leukemia in 33%, non-Hodgkin lymphoma in 27%, and myeloma or amyloidosis in 16%.
The most frequently reported symptoms included fever in 73%, cough in 67%, dyspnea in 50%, and fatigue in 40%, according to that report.
At the time of this data snapshot, treatment with COVID-19-directed therapies including hydroxychloroquine or azithromycin were common, reported in 76 and 59 patients, respectively, in the cohort.
Batch submissions from sites with high incidence of COVID-19 infection are ongoing. The registry has been expanded to include nonmalignant hematologic diseases, and the registry will continue to accumulate data as a resource for the hematology community.
Overall mortality was 28% at the time, according to the abstract, with nearly all of the deaths occurring in patients classified as having COVID-19 that was moderate (i.e., requiring hospitalization) or severe (i.e., requiring ICU admission).
“In some instances, death occurred after a decision was made to forgo ICU admission in favor of a palliative approach,” said Dr. Wood and coauthors in their report.
Dr. Wood reported research funding from Pfizer, consultancy with Teladoc/Best Doctors, and honoraria from the ASH Research Collaborative. Coauthors provided disclosures related to Celgene, Madrigal Pharmaceuticals, Pharmacyclics, and Amgen, among others.
SOURCE: Wood WA et al. ASH 2020, Abstract 215.
FROM ASH 2020
In MDS, transplant ups survival in elderly and may be reimbursed
New results suggest that allogeneic hematopoietic cell transplantation (HCT), which is typically reserved for younger patients, may well be offered to older patients with advanced myelodysplastic syndrome (MDS).
In patients with a median age of 66 years who had received a donor transplant, the overall survival (OS) at 3 years was almost double compared with patients who did not receive a transplant – 47.9% vs. 26.6% for the “no-donor” group.
The finding comes from the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) Study 1102 (NCT02016781) presented at the American Society of Hematology (ASH) 2020 virtual meeting.
“This study conclusively solidifies the role of transplantation in older individuals with MDS,” presenter Corey Cutler, MD, MPH, of the Dana-Farber Cancer Center, Boston, said in an interview.
Coauthor Ryotaro Nakamura, MD, of City of Hope, Duarte, Calif., said in an interview that this was the largest and first trial in the United States to determine in a prospective fashion that allogeneic stem cell transplantation offers a significant survival in older patients. “There was more than a 20% benefit in OS in this age group,” he said.
“This is an incredibly important study,” said Andrew Brunner, MD, medical oncologist at the Mass General Cancer Center in Boston, who was approached for comment. He explained that for years early transplant was recommended as important for patients who have higher-risk MDS. “This study validates this in a prospective, pseudo-randomized (donor/no donor) fashion,” he said in an interview.
“[This study] is really a seminal advance in the care of patients with MDS. Transplant should be integrated into the care algorithm, if not already, and we as a community need to build upon this study further,” Dr. Brunner added.
Several experts in addition to the authors hailed the study as practice changing.
Robert A. Brodsky, MD, ASH, director of the division of hematology at Johns Hopkins University, Baltimore, noted that in younger patients bone marrow transplant is the standard of care for aggressive MDS, but a lot of practices do not refer older patients or those with comorbidities for transplant and prefer to give these patients palliative care with hypomethylating agents for fear that the transplant process would be too toxic.
“There has been an institutional bias to do transplant in older patients, but until now there was no randomized clinical trial to show that this is the right choice. Now we have the data,” Dr Brodsky said, predicting that “this study will change the standard of care.”
Henry Fung, MD, chair of the department of bone marrow transplant and cellular therapies at Fox Chase Cancer Center, Philadelphia, agreed. “We should congratulate all the investigators and our patients who participated in this study. Reduced intensity allogeneic stem cell transplantation improved disease control and overall survival with similar quality of life.
“I will recommend all patients with intermediate-2 or higher-risk MDS to be evaluated by the transplant team at diagnosis and eligible patients should be considered for a transplant,” Dr. Fung said in an interview.
Immediate impact on clinical practice
Lead author Dr. Cutler suggested that the study results had an immediate impact for changing clinical practice. “Individuals between the ages of 50 and 75 years with intermediate-2 or high-risk MDS who are eligible to undergo reduced-intensity transplantation had superior outcomes if they had a suitable donor for transplantation in comparison with those who did not have a donor,” he said.
Dr. Cutler further explained that many community-based hematologists do not refer their patients for transplantation. In addition, there is a lack of a uniform payer position for transplantation for MDS, he noted. Also, there is a lack of understanding of the cost-effectiveness of transplantation in comparison to nontransplant strategies, he suggested.
“Transplant is curative for MDS,” he emphasized. Most transplant recipients will eventually become transfusion-independent within weeks to months from transplant.
“We do transplants in this age group all the time,” Dr. Cutler noted. He said that academic centers will continue to offer transplants, and suggested that community oncologists encourage referral to transplant centers early in a patient’s disease course to maximize search time and provide patients all potential options for therapy.
Dr. Brunner agreed and noted that there is a need to build capacity for higher transplant volume, and in general physicians should seek ways to expand this treatment option to more patients. “At this time, allogeneic transplant still requires close collaboration with referral centers; that said, more and more we are able to work closely with colleagues in the community to share management, including earlier after the actual transplant,” he said.
He noted that one silver lining of the pandemic in 2020 has been increased use of telemedicine to collaborate. “Ongoing advances may be able to further encourage these virtual connections to enhance the entire patient care experience,” Dr. Brunner said.
Reimbursement by CMS for Medicare recipients
Despite the data showing benefit, allogeneic stem cell transplantation is not offered to older individuals with high-risk MDS and is not covered by Medicare in the United States, Dr. Cutler noted in his presentation.
“This study was spurred by the CMS [Centers for Medicare & Medicaid Services] ruling for transplantation in MDS and the story has come full circle,” Aaron T. Gerds, MD, MS, noted at a preconference press briefing. Dr. Gerds is chair of the ASH Committee on Communications and assistant professor at the Cleveland Clinic Taussig Cancer Institute, Cleveland.
Dr. Nakamura explained that in 2010 a CMS decision memo noted that the evidence of a benefit for transplantation in MDS was lacking and Medicare would not cover transplant unless patients were enrolled in a clinical study. That memo outlined criteria that a clinical trial would have to address before it could consider reimbursement for Medicare beneficiaries.
“The BMT CTN Study 1102 was one of two studies that met the criteria set by CMS,” Dr. Nakamura said, noting that the data are being prepared for CMS review.
“This study will likely be the deciding factor for CMS to begin to cover payment for transplantation for MDS,” said Dr. Cutler.
The other study, published earlier this year in JAMA Oncology, showed that outcomes for patients older than ager 65 were similar to those of patients aged 55-65.
BMT CTN 1102 study details
Dr. Cutler noted that the study was designed to address the issue of whether transplantation was beneficial to Medicare-aged individuals with high-risk MDS, and the trial had been approved by Medicare.
The multicenter study enrolled patients who were between ages 50 and 75 years and had newly diagnosed MDS of higher risk (International Prognostic Scoring System [IPSS] intermediate-2 or higher) and were candidates for reduced intensity conditioning (RIC) allogeneic HCT.
Patients were enrolled prior to a formal donor search and were initially assigned to the “no donor” group and reassigned to the donor group when a suitable donor (matched sibling or unrelated donor) was identified. Patients underwent RIC HCT according to institution protocol.
Of 384 patients, 260 received RIC HCT and 124 received hypomethylating therapy. Median follow-up was 34.2 months for the donor group and 26.9 months for the no-donor group.
The two arms were well balanced with respect to age (median 66 years), gender, disease risk [two-thirds of the patients had an intermediate-2 and one third had a high-risk MDS], and response to hypomethylating therapy. The majority of subjects in the donor arm had unrelated donors and more than one-third had a high comorbidity score, Dr. Cutler indicated.
At 3 years, absolute improvement in OS was 21.3% in favor of donor-arm subjects. Leukemia-free survival was also higher in the donor group: 35.8% vs. 20.6% for the no-donor group.
Improvement in OS for patients receiving transplants was seen across all patient subtypes, regardless of age, response to hypomethylating therapy, and IPSS score. “Treatment effects were seen in any subgroup, but particularly in subjects above age 65,” Dr. Cutler stressed.
In an as-treated analysis that excluded subjects who died, the treatment effects were even more pronounced, with an absolute improvement in OS of 31.4% (47.4% vs. 16% for the no-donor arm) and improvement in leukemia-free survival of 28.4% (39.3% vs. 10.9% for the no-donor arm).
In 25 patients in the no-donor arm who subsequently went on to receive alternate donor transplant, the 3-year OS and leukemia-free survival was 58.5%, underscoring the potential value of alternate donor transplant, Dr. Cutler noted.
Dr. Nakamura emphasized that the gains in survival benefits were not seen at the expense of quality of life, as preliminary results showed no difference in quality-of-life measures across those who received donor transplants and those who did not.
Dr. Brunner noted that physicians often highlight the toxicities of transplant as a consideration for whether to proceed, and while there are toxicities specific to transplant that should be considered, in this study it is seen that, even early on, survival is improved in those patients who move toward early transplant. “It also underscores the limitations of current nontransplant treatments for MDS – there is much room to improve,” he said.
Role for alternate donors
Dr. Cutler noted that the majority of patients in the no-donor group died without transplantation. “We need to establish the role of alternative donor transplantation in this population,” he said. Dr. Nakamura indicated that mismatched donors and haploidentical donors such as family donors and umbilical cord blood may be alternate donor sources; outcomes from published studies show similar results, he said.
However, Dr. Brunner noted that the study looked only at traditional fully matched donors, leaving open some questions about alternative donor options such as haploidentical donors and umbilical cord blood donation.
“Our experience in other areas of transplant would suggest that these donor sources may be as good as traditional fully matched options, when using newer conditioning and prophylaxis regimens,” Dr. Brunner said.
Dr. Cutler added, “With the increased acceptance of alternate transplant modalities, we need to determine the outcomes associated with these in prospective trials.”
“I think a significant consideration here as well is health equity,” Dr. Brunner said. “Donor options vary according to race and ethnicity and we need to be proactive as a community to ensure that all MDS patients have access to a potentially curative option early in their diagnosis.”
Dr. Cutler reports consultancy for Mesoblast, Generon, Medsenic, Jazz, Kadmon, and Incyte. Dr. Nakamura reports relationships with Magenta Therapeutics, Kyowa-Kirin, Alexion, Merck, NapaJen Pharma, Kadmon Corporation, Celgene, and Viracor. Dr. Fung has disclosed no relevant financial relationships. Dr. Brodsky reports receiving funding from and being on the board/advisory committee for Achillion Pharmaceuticals, consults with Alexion Pharmaceuticals, and receives honoraria from UpToDate. Dr. Brunner reports relationships with Biogen, Acceleron Pharma Inc, Celgene/BMS, Forty Seven Inc, Jazz Pharma, Novartis, Takeda, Xcenda, GSK, Janssen, and AstraZeneca.
A version of this article originally appeared on Medscape.com.
New results suggest that allogeneic hematopoietic cell transplantation (HCT), which is typically reserved for younger patients, may well be offered to older patients with advanced myelodysplastic syndrome (MDS).
In patients with a median age of 66 years who had received a donor transplant, the overall survival (OS) at 3 years was almost double compared with patients who did not receive a transplant – 47.9% vs. 26.6% for the “no-donor” group.
The finding comes from the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) Study 1102 (NCT02016781) presented at the American Society of Hematology (ASH) 2020 virtual meeting.
“This study conclusively solidifies the role of transplantation in older individuals with MDS,” presenter Corey Cutler, MD, MPH, of the Dana-Farber Cancer Center, Boston, said in an interview.
Coauthor Ryotaro Nakamura, MD, of City of Hope, Duarte, Calif., said in an interview that this was the largest and first trial in the United States to determine in a prospective fashion that allogeneic stem cell transplantation offers a significant survival in older patients. “There was more than a 20% benefit in OS in this age group,” he said.
“This is an incredibly important study,” said Andrew Brunner, MD, medical oncologist at the Mass General Cancer Center in Boston, who was approached for comment. He explained that for years early transplant was recommended as important for patients who have higher-risk MDS. “This study validates this in a prospective, pseudo-randomized (donor/no donor) fashion,” he said in an interview.
“[This study] is really a seminal advance in the care of patients with MDS. Transplant should be integrated into the care algorithm, if not already, and we as a community need to build upon this study further,” Dr. Brunner added.
Several experts in addition to the authors hailed the study as practice changing.
Robert A. Brodsky, MD, ASH, director of the division of hematology at Johns Hopkins University, Baltimore, noted that in younger patients bone marrow transplant is the standard of care for aggressive MDS, but a lot of practices do not refer older patients or those with comorbidities for transplant and prefer to give these patients palliative care with hypomethylating agents for fear that the transplant process would be too toxic.
“There has been an institutional bias to do transplant in older patients, but until now there was no randomized clinical trial to show that this is the right choice. Now we have the data,” Dr Brodsky said, predicting that “this study will change the standard of care.”
Henry Fung, MD, chair of the department of bone marrow transplant and cellular therapies at Fox Chase Cancer Center, Philadelphia, agreed. “We should congratulate all the investigators and our patients who participated in this study. Reduced intensity allogeneic stem cell transplantation improved disease control and overall survival with similar quality of life.
“I will recommend all patients with intermediate-2 or higher-risk MDS to be evaluated by the transplant team at diagnosis and eligible patients should be considered for a transplant,” Dr. Fung said in an interview.
Immediate impact on clinical practice
Lead author Dr. Cutler suggested that the study results had an immediate impact for changing clinical practice. “Individuals between the ages of 50 and 75 years with intermediate-2 or high-risk MDS who are eligible to undergo reduced-intensity transplantation had superior outcomes if they had a suitable donor for transplantation in comparison with those who did not have a donor,” he said.
Dr. Cutler further explained that many community-based hematologists do not refer their patients for transplantation. In addition, there is a lack of a uniform payer position for transplantation for MDS, he noted. Also, there is a lack of understanding of the cost-effectiveness of transplantation in comparison to nontransplant strategies, he suggested.
“Transplant is curative for MDS,” he emphasized. Most transplant recipients will eventually become transfusion-independent within weeks to months from transplant.
“We do transplants in this age group all the time,” Dr. Cutler noted. He said that academic centers will continue to offer transplants, and suggested that community oncologists encourage referral to transplant centers early in a patient’s disease course to maximize search time and provide patients all potential options for therapy.
Dr. Brunner agreed and noted that there is a need to build capacity for higher transplant volume, and in general physicians should seek ways to expand this treatment option to more patients. “At this time, allogeneic transplant still requires close collaboration with referral centers; that said, more and more we are able to work closely with colleagues in the community to share management, including earlier after the actual transplant,” he said.
He noted that one silver lining of the pandemic in 2020 has been increased use of telemedicine to collaborate. “Ongoing advances may be able to further encourage these virtual connections to enhance the entire patient care experience,” Dr. Brunner said.
Reimbursement by CMS for Medicare recipients
Despite the data showing benefit, allogeneic stem cell transplantation is not offered to older individuals with high-risk MDS and is not covered by Medicare in the United States, Dr. Cutler noted in his presentation.
“This study was spurred by the CMS [Centers for Medicare & Medicaid Services] ruling for transplantation in MDS and the story has come full circle,” Aaron T. Gerds, MD, MS, noted at a preconference press briefing. Dr. Gerds is chair of the ASH Committee on Communications and assistant professor at the Cleveland Clinic Taussig Cancer Institute, Cleveland.
Dr. Nakamura explained that in 2010 a CMS decision memo noted that the evidence of a benefit for transplantation in MDS was lacking and Medicare would not cover transplant unless patients were enrolled in a clinical study. That memo outlined criteria that a clinical trial would have to address before it could consider reimbursement for Medicare beneficiaries.
“The BMT CTN Study 1102 was one of two studies that met the criteria set by CMS,” Dr. Nakamura said, noting that the data are being prepared for CMS review.
“This study will likely be the deciding factor for CMS to begin to cover payment for transplantation for MDS,” said Dr. Cutler.
The other study, published earlier this year in JAMA Oncology, showed that outcomes for patients older than ager 65 were similar to those of patients aged 55-65.
BMT CTN 1102 study details
Dr. Cutler noted that the study was designed to address the issue of whether transplantation was beneficial to Medicare-aged individuals with high-risk MDS, and the trial had been approved by Medicare.
The multicenter study enrolled patients who were between ages 50 and 75 years and had newly diagnosed MDS of higher risk (International Prognostic Scoring System [IPSS] intermediate-2 or higher) and were candidates for reduced intensity conditioning (RIC) allogeneic HCT.
Patients were enrolled prior to a formal donor search and were initially assigned to the “no donor” group and reassigned to the donor group when a suitable donor (matched sibling or unrelated donor) was identified. Patients underwent RIC HCT according to institution protocol.
Of 384 patients, 260 received RIC HCT and 124 received hypomethylating therapy. Median follow-up was 34.2 months for the donor group and 26.9 months for the no-donor group.
The two arms were well balanced with respect to age (median 66 years), gender, disease risk [two-thirds of the patients had an intermediate-2 and one third had a high-risk MDS], and response to hypomethylating therapy. The majority of subjects in the donor arm had unrelated donors and more than one-third had a high comorbidity score, Dr. Cutler indicated.
At 3 years, absolute improvement in OS was 21.3% in favor of donor-arm subjects. Leukemia-free survival was also higher in the donor group: 35.8% vs. 20.6% for the no-donor group.
Improvement in OS for patients receiving transplants was seen across all patient subtypes, regardless of age, response to hypomethylating therapy, and IPSS score. “Treatment effects were seen in any subgroup, but particularly in subjects above age 65,” Dr. Cutler stressed.
In an as-treated analysis that excluded subjects who died, the treatment effects were even more pronounced, with an absolute improvement in OS of 31.4% (47.4% vs. 16% for the no-donor arm) and improvement in leukemia-free survival of 28.4% (39.3% vs. 10.9% for the no-donor arm).
In 25 patients in the no-donor arm who subsequently went on to receive alternate donor transplant, the 3-year OS and leukemia-free survival was 58.5%, underscoring the potential value of alternate donor transplant, Dr. Cutler noted.
Dr. Nakamura emphasized that the gains in survival benefits were not seen at the expense of quality of life, as preliminary results showed no difference in quality-of-life measures across those who received donor transplants and those who did not.
Dr. Brunner noted that physicians often highlight the toxicities of transplant as a consideration for whether to proceed, and while there are toxicities specific to transplant that should be considered, in this study it is seen that, even early on, survival is improved in those patients who move toward early transplant. “It also underscores the limitations of current nontransplant treatments for MDS – there is much room to improve,” he said.
Role for alternate donors
Dr. Cutler noted that the majority of patients in the no-donor group died without transplantation. “We need to establish the role of alternative donor transplantation in this population,” he said. Dr. Nakamura indicated that mismatched donors and haploidentical donors such as family donors and umbilical cord blood may be alternate donor sources; outcomes from published studies show similar results, he said.
However, Dr. Brunner noted that the study looked only at traditional fully matched donors, leaving open some questions about alternative donor options such as haploidentical donors and umbilical cord blood donation.
“Our experience in other areas of transplant would suggest that these donor sources may be as good as traditional fully matched options, when using newer conditioning and prophylaxis regimens,” Dr. Brunner said.
Dr. Cutler added, “With the increased acceptance of alternate transplant modalities, we need to determine the outcomes associated with these in prospective trials.”
“I think a significant consideration here as well is health equity,” Dr. Brunner said. “Donor options vary according to race and ethnicity and we need to be proactive as a community to ensure that all MDS patients have access to a potentially curative option early in their diagnosis.”
Dr. Cutler reports consultancy for Mesoblast, Generon, Medsenic, Jazz, Kadmon, and Incyte. Dr. Nakamura reports relationships with Magenta Therapeutics, Kyowa-Kirin, Alexion, Merck, NapaJen Pharma, Kadmon Corporation, Celgene, and Viracor. Dr. Fung has disclosed no relevant financial relationships. Dr. Brodsky reports receiving funding from and being on the board/advisory committee for Achillion Pharmaceuticals, consults with Alexion Pharmaceuticals, and receives honoraria from UpToDate. Dr. Brunner reports relationships with Biogen, Acceleron Pharma Inc, Celgene/BMS, Forty Seven Inc, Jazz Pharma, Novartis, Takeda, Xcenda, GSK, Janssen, and AstraZeneca.
A version of this article originally appeared on Medscape.com.
New results suggest that allogeneic hematopoietic cell transplantation (HCT), which is typically reserved for younger patients, may well be offered to older patients with advanced myelodysplastic syndrome (MDS).
In patients with a median age of 66 years who had received a donor transplant, the overall survival (OS) at 3 years was almost double compared with patients who did not receive a transplant – 47.9% vs. 26.6% for the “no-donor” group.
The finding comes from the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) Study 1102 (NCT02016781) presented at the American Society of Hematology (ASH) 2020 virtual meeting.
“This study conclusively solidifies the role of transplantation in older individuals with MDS,” presenter Corey Cutler, MD, MPH, of the Dana-Farber Cancer Center, Boston, said in an interview.
Coauthor Ryotaro Nakamura, MD, of City of Hope, Duarte, Calif., said in an interview that this was the largest and first trial in the United States to determine in a prospective fashion that allogeneic stem cell transplantation offers a significant survival in older patients. “There was more than a 20% benefit in OS in this age group,” he said.
“This is an incredibly important study,” said Andrew Brunner, MD, medical oncologist at the Mass General Cancer Center in Boston, who was approached for comment. He explained that for years early transplant was recommended as important for patients who have higher-risk MDS. “This study validates this in a prospective, pseudo-randomized (donor/no donor) fashion,” he said in an interview.
“[This study] is really a seminal advance in the care of patients with MDS. Transplant should be integrated into the care algorithm, if not already, and we as a community need to build upon this study further,” Dr. Brunner added.
Several experts in addition to the authors hailed the study as practice changing.
Robert A. Brodsky, MD, ASH, director of the division of hematology at Johns Hopkins University, Baltimore, noted that in younger patients bone marrow transplant is the standard of care for aggressive MDS, but a lot of practices do not refer older patients or those with comorbidities for transplant and prefer to give these patients palliative care with hypomethylating agents for fear that the transplant process would be too toxic.
“There has been an institutional bias to do transplant in older patients, but until now there was no randomized clinical trial to show that this is the right choice. Now we have the data,” Dr Brodsky said, predicting that “this study will change the standard of care.”
Henry Fung, MD, chair of the department of bone marrow transplant and cellular therapies at Fox Chase Cancer Center, Philadelphia, agreed. “We should congratulate all the investigators and our patients who participated in this study. Reduced intensity allogeneic stem cell transplantation improved disease control and overall survival with similar quality of life.
“I will recommend all patients with intermediate-2 or higher-risk MDS to be evaluated by the transplant team at diagnosis and eligible patients should be considered for a transplant,” Dr. Fung said in an interview.
Immediate impact on clinical practice
Lead author Dr. Cutler suggested that the study results had an immediate impact for changing clinical practice. “Individuals between the ages of 50 and 75 years with intermediate-2 or high-risk MDS who are eligible to undergo reduced-intensity transplantation had superior outcomes if they had a suitable donor for transplantation in comparison with those who did not have a donor,” he said.
Dr. Cutler further explained that many community-based hematologists do not refer their patients for transplantation. In addition, there is a lack of a uniform payer position for transplantation for MDS, he noted. Also, there is a lack of understanding of the cost-effectiveness of transplantation in comparison to nontransplant strategies, he suggested.
“Transplant is curative for MDS,” he emphasized. Most transplant recipients will eventually become transfusion-independent within weeks to months from transplant.
“We do transplants in this age group all the time,” Dr. Cutler noted. He said that academic centers will continue to offer transplants, and suggested that community oncologists encourage referral to transplant centers early in a patient’s disease course to maximize search time and provide patients all potential options for therapy.
Dr. Brunner agreed and noted that there is a need to build capacity for higher transplant volume, and in general physicians should seek ways to expand this treatment option to more patients. “At this time, allogeneic transplant still requires close collaboration with referral centers; that said, more and more we are able to work closely with colleagues in the community to share management, including earlier after the actual transplant,” he said.
He noted that one silver lining of the pandemic in 2020 has been increased use of telemedicine to collaborate. “Ongoing advances may be able to further encourage these virtual connections to enhance the entire patient care experience,” Dr. Brunner said.
Reimbursement by CMS for Medicare recipients
Despite the data showing benefit, allogeneic stem cell transplantation is not offered to older individuals with high-risk MDS and is not covered by Medicare in the United States, Dr. Cutler noted in his presentation.
“This study was spurred by the CMS [Centers for Medicare & Medicaid Services] ruling for transplantation in MDS and the story has come full circle,” Aaron T. Gerds, MD, MS, noted at a preconference press briefing. Dr. Gerds is chair of the ASH Committee on Communications and assistant professor at the Cleveland Clinic Taussig Cancer Institute, Cleveland.
Dr. Nakamura explained that in 2010 a CMS decision memo noted that the evidence of a benefit for transplantation in MDS was lacking and Medicare would not cover transplant unless patients were enrolled in a clinical study. That memo outlined criteria that a clinical trial would have to address before it could consider reimbursement for Medicare beneficiaries.
“The BMT CTN Study 1102 was one of two studies that met the criteria set by CMS,” Dr. Nakamura said, noting that the data are being prepared for CMS review.
“This study will likely be the deciding factor for CMS to begin to cover payment for transplantation for MDS,” said Dr. Cutler.
The other study, published earlier this year in JAMA Oncology, showed that outcomes for patients older than ager 65 were similar to those of patients aged 55-65.
BMT CTN 1102 study details
Dr. Cutler noted that the study was designed to address the issue of whether transplantation was beneficial to Medicare-aged individuals with high-risk MDS, and the trial had been approved by Medicare.
The multicenter study enrolled patients who were between ages 50 and 75 years and had newly diagnosed MDS of higher risk (International Prognostic Scoring System [IPSS] intermediate-2 or higher) and were candidates for reduced intensity conditioning (RIC) allogeneic HCT.
Patients were enrolled prior to a formal donor search and were initially assigned to the “no donor” group and reassigned to the donor group when a suitable donor (matched sibling or unrelated donor) was identified. Patients underwent RIC HCT according to institution protocol.
Of 384 patients, 260 received RIC HCT and 124 received hypomethylating therapy. Median follow-up was 34.2 months for the donor group and 26.9 months for the no-donor group.
The two arms were well balanced with respect to age (median 66 years), gender, disease risk [two-thirds of the patients had an intermediate-2 and one third had a high-risk MDS], and response to hypomethylating therapy. The majority of subjects in the donor arm had unrelated donors and more than one-third had a high comorbidity score, Dr. Cutler indicated.
At 3 years, absolute improvement in OS was 21.3% in favor of donor-arm subjects. Leukemia-free survival was also higher in the donor group: 35.8% vs. 20.6% for the no-donor group.
Improvement in OS for patients receiving transplants was seen across all patient subtypes, regardless of age, response to hypomethylating therapy, and IPSS score. “Treatment effects were seen in any subgroup, but particularly in subjects above age 65,” Dr. Cutler stressed.
In an as-treated analysis that excluded subjects who died, the treatment effects were even more pronounced, with an absolute improvement in OS of 31.4% (47.4% vs. 16% for the no-donor arm) and improvement in leukemia-free survival of 28.4% (39.3% vs. 10.9% for the no-donor arm).
In 25 patients in the no-donor arm who subsequently went on to receive alternate donor transplant, the 3-year OS and leukemia-free survival was 58.5%, underscoring the potential value of alternate donor transplant, Dr. Cutler noted.
Dr. Nakamura emphasized that the gains in survival benefits were not seen at the expense of quality of life, as preliminary results showed no difference in quality-of-life measures across those who received donor transplants and those who did not.
Dr. Brunner noted that physicians often highlight the toxicities of transplant as a consideration for whether to proceed, and while there are toxicities specific to transplant that should be considered, in this study it is seen that, even early on, survival is improved in those patients who move toward early transplant. “It also underscores the limitations of current nontransplant treatments for MDS – there is much room to improve,” he said.
Role for alternate donors
Dr. Cutler noted that the majority of patients in the no-donor group died without transplantation. “We need to establish the role of alternative donor transplantation in this population,” he said. Dr. Nakamura indicated that mismatched donors and haploidentical donors such as family donors and umbilical cord blood may be alternate donor sources; outcomes from published studies show similar results, he said.
However, Dr. Brunner noted that the study looked only at traditional fully matched donors, leaving open some questions about alternative donor options such as haploidentical donors and umbilical cord blood donation.
“Our experience in other areas of transplant would suggest that these donor sources may be as good as traditional fully matched options, when using newer conditioning and prophylaxis regimens,” Dr. Brunner said.
Dr. Cutler added, “With the increased acceptance of alternate transplant modalities, we need to determine the outcomes associated with these in prospective trials.”
“I think a significant consideration here as well is health equity,” Dr. Brunner said. “Donor options vary according to race and ethnicity and we need to be proactive as a community to ensure that all MDS patients have access to a potentially curative option early in their diagnosis.”
Dr. Cutler reports consultancy for Mesoblast, Generon, Medsenic, Jazz, Kadmon, and Incyte. Dr. Nakamura reports relationships with Magenta Therapeutics, Kyowa-Kirin, Alexion, Merck, NapaJen Pharma, Kadmon Corporation, Celgene, and Viracor. Dr. Fung has disclosed no relevant financial relationships. Dr. Brodsky reports receiving funding from and being on the board/advisory committee for Achillion Pharmaceuticals, consults with Alexion Pharmaceuticals, and receives honoraria from UpToDate. Dr. Brunner reports relationships with Biogen, Acceleron Pharma Inc, Celgene/BMS, Forty Seven Inc, Jazz Pharma, Novartis, Takeda, Xcenda, GSK, Janssen, and AstraZeneca.
A version of this article originally appeared on Medscape.com.
