User login
EMPEROR-Preserved findings confirmed in ‘true’ HFpEF patients
Main results from the landmark EMPEROR-Preserved trial, reported in August, established for the first time that treatment with a drug, the sodium-glucose cotransporter 2 inhibitor empagliflozin, could clearly benefit patients with heart failure with preserved ejection fraction (HFpEF).
The only caveat was that EMPEROR-Preserved enrolled patients with a left ventricular ejection fraction of at least 41%, while “true” HFpEF means patients with heart failure and an LVEF of at least 50%, according to recent definitions. About one-third of the 5,988 patients enrolled in EMPEROR-Preserved had an LVEF of 41%-49%, heart failure with mildly reduced ejection fraction.
Secondary analysis from the EMPEROR-Preserved trial has now resolved this ambiguity by showing that, among the 4,005 patients (67%) enrolled in the trial with an LVEF of at least 50%, treatment with empagliflozin (Jardiance) reduced the study’s primary endpoint – cardiovascular death or first hospitalization for heart failure – by a significant 17%, relative to patients who received placebo, dismissing any doubt about the relevance of the overall finding to the subgroup of patients with unmitigated HFpEF.
“This is the first large-scale trial to document meaningful and significant improvements associated with drug therapy in patients with ‘true’ HFpEF,” Stefan D. Anker, MD, said in presenting the results at the American Heart Association scientific sessions.
Streamlining heart failure treatment
The demonstration that empagliflozin is an effective – and safe – treatment for patients with HFpEF not only provides a new treatment for a disorder that until now had no evidence-based intervention, but also streamlines the management approach for treating patients with heart failure with an agent from empagliflozin’s class, the SGLT2 inhibitors, commented Mary Norine Walsh, MD, medical director of the heart failure and cardiac transplantation programs at Ascension St. Vincent Heart Center in Indianapolis.
That’s because empagliflozin has shown significant and consistent benefit across essentially the full range of LVEFs seen in patients with heart failure based on its performance in EMPEROR-Preserved as well as in a mirror-image trial, EMPEROR-Reduced, run in patients with heart failure with reduced ejection fraction.
“Clinicians do not need to stop and assess LVEF with echocardiography or other imaging before they decide on how to treat heart failure patients” with an SGLT2 inhibitor, noted Dr. Walsh, a designated discussant for the report. “Clinicians who are busy can now refer less to LVEF than to the patient’s phenotype.”
Treatment prevents hospitalization for heart failure
The more-detailed data reported by Dr. Anker also strengthened the case that the benefit from empagliflozin in patients with an LVEF of at least 50% mostly came from a reduction in hospitalizations for heart failure (HHF), which dropped following start of empagliflozin treatment by a relative 22%, compared with placebo for first HHF, a significant decline, and by a relative 17% for total HHF, a reduction that missed significance in this secondary analysis. The other half of the primary endpoint, cardiovascular death, declined by a nonsignificant 11% with empagliflozin treatment, compared with placebo in patients with clear-cut HFpEF.
The significant reduction in first HHF is, by itself, sufficient reason to use empagliflozin (or possibly a different SGLT2 inhibitor) in patients with HFpEF, maintained Clyde W. Yancy, MD, professor and chief of cardiology at Northwestern Medicine in Chicago.
“Attenuated HHF is a meaningful outcome,” stressed Dr. Yancy, also a discussant for the study. “This is the first time we’ve had evidence supporting that we can change the natural history of patients with HFpEF. While we still need to find interventions that save lives, we cannot overlook that this treatment can improve morbidity, and we cannot overlook that patient quality of life is better.”
Further benefits in patients with an LVEF of at least 50%
Dr. Anker, professor of cardiology and metabolism at Charité Medical University in Berlin, also reported results from several other analyses that further defined the effect of empagliflozin on clinical outcomes of patients with “true” HFpEF:
- The impact of empagliflozin, compared with placebo, for reducing both the study’s combined, primary outcome as well as total HHF was statistically consistent across all strata of LVEF, from 50% to greater than 70%. However, both outcome measures also showed a puzzling loss of benefit among patients with an LVEF of 65%-69%. In prior reports, a researcher on the EMPEROR-Preserved team, Milton Packer, MD, speculated that some patients in this LVEF stratum might not actually have had heart failure but instead had a different disorder that mimicked heart failure in clinical presentation, such as atrial fibrillation.
- Patients’ quality of life as measured by the Kansas City Cardiomyopathy Questionnaire showed a consistent benefit from empagliflozin treatment, compared with placebo, both in patients with an LVEF of at least 50% as well as in those with an LVEF of 41%-49%. In both subgroups the adjusted mean difference from placebo was significant and about 1.5 points.
- Patients showed a significant improvement in average New York Heart Association functional class while on treatment, and a strong trend toward less deterioration in functional class while on treatment.
- Deterioration of renal function on treatment slowed by an average 1.24 mL/min per 1.73 m2 per year in patients on empagliflozin, compared with placebo, in the subgroup with an LVEF of at least 50%.
Dr. Anker also reported the primary outcome and component results for the subgroup of patients with a baseline LVEF of 41%-49%. These patients had what looked like a “bigger magnitude” of effect from treatment, he noted, showing a significant 29% relative decline in the primary endpoint, compared with placebo-treated patients, and a significant 42% relative drop in first HHF and a significant 43% relative decline in total HHF, compared with placebo.
The primary analysis from EMPEROR-Preserved, which included all 5,988 randomized patients with heart failure and an LVEF of 41% or greater, showed a significant reduction in the combined, primary endpoint with empagliflozin treatment of 21%, compared with control patients during a median follow-up of about 26 months. The absolute rate reduction of the combined primary endpoint was 3.3% during 26-months’ follow-up. Statistical tests have shown no heterogeneity of this effect by diabetes status (49% of patients had diabetes), nor by renal function down to an estimated glomerular filtration rate at entry as low as 20 mL/min per 1.73 m2.
EMPEROR-Preserved was sponsored by Boehringer Ingelheim and Lilly, the two companies that market empagliflozin (Jardiance). Dr. Anker has been a consultant to Boehringer Ingelheim as well as to Abbott Vascular, Bayer, Brahms, Cardiac Dimensions, Cordio, Novartis, Servier, and Vifor. Dr. Walsh and Dr. Yancy had no disclosures.
Main results from the landmark EMPEROR-Preserved trial, reported in August, established for the first time that treatment with a drug, the sodium-glucose cotransporter 2 inhibitor empagliflozin, could clearly benefit patients with heart failure with preserved ejection fraction (HFpEF).
The only caveat was that EMPEROR-Preserved enrolled patients with a left ventricular ejection fraction of at least 41%, while “true” HFpEF means patients with heart failure and an LVEF of at least 50%, according to recent definitions. About one-third of the 5,988 patients enrolled in EMPEROR-Preserved had an LVEF of 41%-49%, heart failure with mildly reduced ejection fraction.
Secondary analysis from the EMPEROR-Preserved trial has now resolved this ambiguity by showing that, among the 4,005 patients (67%) enrolled in the trial with an LVEF of at least 50%, treatment with empagliflozin (Jardiance) reduced the study’s primary endpoint – cardiovascular death or first hospitalization for heart failure – by a significant 17%, relative to patients who received placebo, dismissing any doubt about the relevance of the overall finding to the subgroup of patients with unmitigated HFpEF.
“This is the first large-scale trial to document meaningful and significant improvements associated with drug therapy in patients with ‘true’ HFpEF,” Stefan D. Anker, MD, said in presenting the results at the American Heart Association scientific sessions.
Streamlining heart failure treatment
The demonstration that empagliflozin is an effective – and safe – treatment for patients with HFpEF not only provides a new treatment for a disorder that until now had no evidence-based intervention, but also streamlines the management approach for treating patients with heart failure with an agent from empagliflozin’s class, the SGLT2 inhibitors, commented Mary Norine Walsh, MD, medical director of the heart failure and cardiac transplantation programs at Ascension St. Vincent Heart Center in Indianapolis.
That’s because empagliflozin has shown significant and consistent benefit across essentially the full range of LVEFs seen in patients with heart failure based on its performance in EMPEROR-Preserved as well as in a mirror-image trial, EMPEROR-Reduced, run in patients with heart failure with reduced ejection fraction.
“Clinicians do not need to stop and assess LVEF with echocardiography or other imaging before they decide on how to treat heart failure patients” with an SGLT2 inhibitor, noted Dr. Walsh, a designated discussant for the report. “Clinicians who are busy can now refer less to LVEF than to the patient’s phenotype.”
Treatment prevents hospitalization for heart failure
The more-detailed data reported by Dr. Anker also strengthened the case that the benefit from empagliflozin in patients with an LVEF of at least 50% mostly came from a reduction in hospitalizations for heart failure (HHF), which dropped following start of empagliflozin treatment by a relative 22%, compared with placebo for first HHF, a significant decline, and by a relative 17% for total HHF, a reduction that missed significance in this secondary analysis. The other half of the primary endpoint, cardiovascular death, declined by a nonsignificant 11% with empagliflozin treatment, compared with placebo in patients with clear-cut HFpEF.
The significant reduction in first HHF is, by itself, sufficient reason to use empagliflozin (or possibly a different SGLT2 inhibitor) in patients with HFpEF, maintained Clyde W. Yancy, MD, professor and chief of cardiology at Northwestern Medicine in Chicago.
“Attenuated HHF is a meaningful outcome,” stressed Dr. Yancy, also a discussant for the study. “This is the first time we’ve had evidence supporting that we can change the natural history of patients with HFpEF. While we still need to find interventions that save lives, we cannot overlook that this treatment can improve morbidity, and we cannot overlook that patient quality of life is better.”
Further benefits in patients with an LVEF of at least 50%
Dr. Anker, professor of cardiology and metabolism at Charité Medical University in Berlin, also reported results from several other analyses that further defined the effect of empagliflozin on clinical outcomes of patients with “true” HFpEF:
- The impact of empagliflozin, compared with placebo, for reducing both the study’s combined, primary outcome as well as total HHF was statistically consistent across all strata of LVEF, from 50% to greater than 70%. However, both outcome measures also showed a puzzling loss of benefit among patients with an LVEF of 65%-69%. In prior reports, a researcher on the EMPEROR-Preserved team, Milton Packer, MD, speculated that some patients in this LVEF stratum might not actually have had heart failure but instead had a different disorder that mimicked heart failure in clinical presentation, such as atrial fibrillation.
- Patients’ quality of life as measured by the Kansas City Cardiomyopathy Questionnaire showed a consistent benefit from empagliflozin treatment, compared with placebo, both in patients with an LVEF of at least 50% as well as in those with an LVEF of 41%-49%. In both subgroups the adjusted mean difference from placebo was significant and about 1.5 points.
- Patients showed a significant improvement in average New York Heart Association functional class while on treatment, and a strong trend toward less deterioration in functional class while on treatment.
- Deterioration of renal function on treatment slowed by an average 1.24 mL/min per 1.73 m2 per year in patients on empagliflozin, compared with placebo, in the subgroup with an LVEF of at least 50%.
Dr. Anker also reported the primary outcome and component results for the subgroup of patients with a baseline LVEF of 41%-49%. These patients had what looked like a “bigger magnitude” of effect from treatment, he noted, showing a significant 29% relative decline in the primary endpoint, compared with placebo-treated patients, and a significant 42% relative drop in first HHF and a significant 43% relative decline in total HHF, compared with placebo.
The primary analysis from EMPEROR-Preserved, which included all 5,988 randomized patients with heart failure and an LVEF of 41% or greater, showed a significant reduction in the combined, primary endpoint with empagliflozin treatment of 21%, compared with control patients during a median follow-up of about 26 months. The absolute rate reduction of the combined primary endpoint was 3.3% during 26-months’ follow-up. Statistical tests have shown no heterogeneity of this effect by diabetes status (49% of patients had diabetes), nor by renal function down to an estimated glomerular filtration rate at entry as low as 20 mL/min per 1.73 m2.
EMPEROR-Preserved was sponsored by Boehringer Ingelheim and Lilly, the two companies that market empagliflozin (Jardiance). Dr. Anker has been a consultant to Boehringer Ingelheim as well as to Abbott Vascular, Bayer, Brahms, Cardiac Dimensions, Cordio, Novartis, Servier, and Vifor. Dr. Walsh and Dr. Yancy had no disclosures.
Main results from the landmark EMPEROR-Preserved trial, reported in August, established for the first time that treatment with a drug, the sodium-glucose cotransporter 2 inhibitor empagliflozin, could clearly benefit patients with heart failure with preserved ejection fraction (HFpEF).
The only caveat was that EMPEROR-Preserved enrolled patients with a left ventricular ejection fraction of at least 41%, while “true” HFpEF means patients with heart failure and an LVEF of at least 50%, according to recent definitions. About one-third of the 5,988 patients enrolled in EMPEROR-Preserved had an LVEF of 41%-49%, heart failure with mildly reduced ejection fraction.
Secondary analysis from the EMPEROR-Preserved trial has now resolved this ambiguity by showing that, among the 4,005 patients (67%) enrolled in the trial with an LVEF of at least 50%, treatment with empagliflozin (Jardiance) reduced the study’s primary endpoint – cardiovascular death or first hospitalization for heart failure – by a significant 17%, relative to patients who received placebo, dismissing any doubt about the relevance of the overall finding to the subgroup of patients with unmitigated HFpEF.
“This is the first large-scale trial to document meaningful and significant improvements associated with drug therapy in patients with ‘true’ HFpEF,” Stefan D. Anker, MD, said in presenting the results at the American Heart Association scientific sessions.
Streamlining heart failure treatment
The demonstration that empagliflozin is an effective – and safe – treatment for patients with HFpEF not only provides a new treatment for a disorder that until now had no evidence-based intervention, but also streamlines the management approach for treating patients with heart failure with an agent from empagliflozin’s class, the SGLT2 inhibitors, commented Mary Norine Walsh, MD, medical director of the heart failure and cardiac transplantation programs at Ascension St. Vincent Heart Center in Indianapolis.
That’s because empagliflozin has shown significant and consistent benefit across essentially the full range of LVEFs seen in patients with heart failure based on its performance in EMPEROR-Preserved as well as in a mirror-image trial, EMPEROR-Reduced, run in patients with heart failure with reduced ejection fraction.
“Clinicians do not need to stop and assess LVEF with echocardiography or other imaging before they decide on how to treat heart failure patients” with an SGLT2 inhibitor, noted Dr. Walsh, a designated discussant for the report. “Clinicians who are busy can now refer less to LVEF than to the patient’s phenotype.”
Treatment prevents hospitalization for heart failure
The more-detailed data reported by Dr. Anker also strengthened the case that the benefit from empagliflozin in patients with an LVEF of at least 50% mostly came from a reduction in hospitalizations for heart failure (HHF), which dropped following start of empagliflozin treatment by a relative 22%, compared with placebo for first HHF, a significant decline, and by a relative 17% for total HHF, a reduction that missed significance in this secondary analysis. The other half of the primary endpoint, cardiovascular death, declined by a nonsignificant 11% with empagliflozin treatment, compared with placebo in patients with clear-cut HFpEF.
The significant reduction in first HHF is, by itself, sufficient reason to use empagliflozin (or possibly a different SGLT2 inhibitor) in patients with HFpEF, maintained Clyde W. Yancy, MD, professor and chief of cardiology at Northwestern Medicine in Chicago.
“Attenuated HHF is a meaningful outcome,” stressed Dr. Yancy, also a discussant for the study. “This is the first time we’ve had evidence supporting that we can change the natural history of patients with HFpEF. While we still need to find interventions that save lives, we cannot overlook that this treatment can improve morbidity, and we cannot overlook that patient quality of life is better.”
Further benefits in patients with an LVEF of at least 50%
Dr. Anker, professor of cardiology and metabolism at Charité Medical University in Berlin, also reported results from several other analyses that further defined the effect of empagliflozin on clinical outcomes of patients with “true” HFpEF:
- The impact of empagliflozin, compared with placebo, for reducing both the study’s combined, primary outcome as well as total HHF was statistically consistent across all strata of LVEF, from 50% to greater than 70%. However, both outcome measures also showed a puzzling loss of benefit among patients with an LVEF of 65%-69%. In prior reports, a researcher on the EMPEROR-Preserved team, Milton Packer, MD, speculated that some patients in this LVEF stratum might not actually have had heart failure but instead had a different disorder that mimicked heart failure in clinical presentation, such as atrial fibrillation.
- Patients’ quality of life as measured by the Kansas City Cardiomyopathy Questionnaire showed a consistent benefit from empagliflozin treatment, compared with placebo, both in patients with an LVEF of at least 50% as well as in those with an LVEF of 41%-49%. In both subgroups the adjusted mean difference from placebo was significant and about 1.5 points.
- Patients showed a significant improvement in average New York Heart Association functional class while on treatment, and a strong trend toward less deterioration in functional class while on treatment.
- Deterioration of renal function on treatment slowed by an average 1.24 mL/min per 1.73 m2 per year in patients on empagliflozin, compared with placebo, in the subgroup with an LVEF of at least 50%.
Dr. Anker also reported the primary outcome and component results for the subgroup of patients with a baseline LVEF of 41%-49%. These patients had what looked like a “bigger magnitude” of effect from treatment, he noted, showing a significant 29% relative decline in the primary endpoint, compared with placebo-treated patients, and a significant 42% relative drop in first HHF and a significant 43% relative decline in total HHF, compared with placebo.
The primary analysis from EMPEROR-Preserved, which included all 5,988 randomized patients with heart failure and an LVEF of 41% or greater, showed a significant reduction in the combined, primary endpoint with empagliflozin treatment of 21%, compared with control patients during a median follow-up of about 26 months. The absolute rate reduction of the combined primary endpoint was 3.3% during 26-months’ follow-up. Statistical tests have shown no heterogeneity of this effect by diabetes status (49% of patients had diabetes), nor by renal function down to an estimated glomerular filtration rate at entry as low as 20 mL/min per 1.73 m2.
EMPEROR-Preserved was sponsored by Boehringer Ingelheim and Lilly, the two companies that market empagliflozin (Jardiance). Dr. Anker has been a consultant to Boehringer Ingelheim as well as to Abbott Vascular, Bayer, Brahms, Cardiac Dimensions, Cordio, Novartis, Servier, and Vifor. Dr. Walsh and Dr. Yancy had no disclosures.
FROM AHA2021
DREAM-HF: Negative stem cell trial in heart failure may still offer promise
A large, multicenter, sham-controlled trial in heart failure showed no benefit at all from stem cell delivery on the primary outcome of recurrent nonfatal decompensated HF events, but the results were still promising, according to the DREAM-HF study’s principal investigator.
When added to guideline-directed medical therapy in patients with HF, a single dose of mesenchymal progenitor cells (MPC) significantly reduced major adverse cardiovascular events (MACE) – a composite of cardiac death, nonfatal MI, and nonfatal stroke – and all-cause death in New York Heart Association (NYHA) class II (but not class III patients), reported Emerson C. Perin, MD, PhD, at the American Heart Association scientific sessions.
The problem is that none of these outcomes were included in the primary endpoint, which was recurrent events because of nonfatal decompensated heart failure. On this endpoint, the hazard ratio for events by the end of follow-up was nonsignificantly but slightly increased among those randomized to MPCs rather than sham control (HR, 1.2; P = .406).
“We learned a lot in this trial,” said Dr. Perin, who is medical director of the Texas Heart Institute in Houston, acknowledging that the expectation of benefit on the primary endpoint now appears to have been misplaced, but the positive result on other outcomes opens a new research direction.
With a negative result on the primary endpoint, a benefit on secondary endpoints is considered hypothesis generating. But Dr. Perin defended his sense of overall optimism about the results, because all of the endpoints on which benefit was demonstrated were prespecified. The positive findings “are not from a post hoc analyses,” he emphasized.
DREAM-HF
In the trial, 537 patients with chronic ischemic or nonischemic heart failure with NYHA class II or III symptoms and a left ventricular ejection fraction of 40% or lower were randomized at 51 sites in the United States and Canada. Patients were required to have elevated N-terminal of the prohormone brain natriuretic peptide levels, at least one prior hospitalization for heart failure, and have been on positive inotropic therapy for more than 1 month (but less than 9 months).
The intracardiac administration of MPCs, which are derived from adult human bone marrow, were delivered by injection guided with the NOGA left ventricular electromechanical mapping system. Multiple transendocardial injections were delivered, all in a single session.
There were no differences in baseline characteristics between those receiving MPCs and those who underwent a sham procedure. In both groups, more than half of patients had a previous MI and a coronary revascularization. Nearly 85% had an implanted defibrillator. Roughly two-thirds were in NYHA class III HF and the remaining were in class II.
Over the follow-up, the lines on a graph documenting nonfatal decompensated heart failure events were largely superimposed for the MPC-treated and sham-treated patients, with no significant differences seen over time.
However, the differences on the secondary events were sizable. For the composite outcome of nonfatal MI and nonfatal stroke over a mean follow-up of about 30 months, the rate of events was less than half as great in those randomized to MPCs (4.6% vs. 13.0%). This translated into about 65% reduction in risk (HR, 0.346; P = .001) overall, and the reduction was about the same in class II or III patients.
For a composite endpoint of MACE, events in the group treated with MPCs were about one-third lower than in the sham procedure group (20.3% vs. 30.1%), a difference that also reached significance (HR, 0.667; P = .021).
For this MACE endpoint, response was evaluated by systemic inflammation. For those with a high-sensitivity C-reactive protein (hsCRP) level of less than 2 mg/L, the risk reduction was small and not significant (HR, 0.843; P = .519). Conversely, there was a large risk reduction in those with hsCRP of at least 2 mg/L that did reach statistical significance (HR, 0.551; P = .012).
Inflammation was also found to be a discriminator for time to cardiac death among the patients with NYHA class II HF. Again, there was no benefit among those with hsCRP below 2 mg/L (HR, 1.355; P = .672), but an 80% risk reduction for those with hsCRP of at least 2 mg/L (HR, 0.204; P = .005).
In class II patients with hsCRP at least 2 mg/L, there was also a 60% reduction in all-cause death (HR, 0.401; P = .027). Neither the reduction in cardiac death nor all-cause death was observed in class III HF patients whether or not they had elevated hsCRP.
These signals of benefit provide a direction for a new set of studies, but Dr. Perin said that safety analyses from the DREAM-HF trial are reassuring for further clinical development.
In addition to the fact that “treatment-emergent adverse events and serious adverse events were similar in the MPC-treated and control patients,” Dr. Perin said that MPC administration was not associated with any clinically meaningful immune responses.
MPCs were first injected into a human 15 years ago, according to Dr. Perin. While a phase 2 trial published several years ago did show an association of MPC administration with a reduction in HF-associated events as well as a reduction in adverse ventricular remodeling, the ischemic benefits observed in this trial, particularly in those with elevated hsCRP, provide a new direction for future trials.
“This turns the page in heart failure research. We now have a new mechanism to consider,” Dr. Perin said.
Not so fast, expert says
This might be a reasonable conclusion, but the AHA-invited discussant, Larry Allen, MD, believes there is essentially no clinical message from this trial. He reiterated multiple times that this trial was neutral with no trend for benefit on the primary outcome.
“There was benefit on the secondary outcomes of nonfatal MI or stroke, but these are not the outcomes we follow in heart failure patients,” he said, noting that benefit from regenerative therapy on ischemic events has not been a major focus of the trials that preceded DREAM-HF.
Despite these intriguing results, “patients should understand that stem cells remain experimental,” he said. For the patient, it is “more important to double down on the importance of guideline directed medical therapy,” which is still being administered at levels that are suboptimal, according to Dr. Allen, medical director of advanced heart failure at the University of Colorado at Denver, Aurora.
“Keep up the investment” in the promise of stem cell therapy, he said, but he cautioned that some of the secondary benefits observed in DREAM-HF, such as the greater response in patients with elevated hsCRP, appear to be new observations that will require a great deal more study to validate.
Dr. Perin has a financial relationship with Mesoblast, which provided funding for the DREAM-HF trial. Dr. Allen reported no relevant conflicts of interest.
A large, multicenter, sham-controlled trial in heart failure showed no benefit at all from stem cell delivery on the primary outcome of recurrent nonfatal decompensated HF events, but the results were still promising, according to the DREAM-HF study’s principal investigator.
When added to guideline-directed medical therapy in patients with HF, a single dose of mesenchymal progenitor cells (MPC) significantly reduced major adverse cardiovascular events (MACE) – a composite of cardiac death, nonfatal MI, and nonfatal stroke – and all-cause death in New York Heart Association (NYHA) class II (but not class III patients), reported Emerson C. Perin, MD, PhD, at the American Heart Association scientific sessions.
The problem is that none of these outcomes were included in the primary endpoint, which was recurrent events because of nonfatal decompensated heart failure. On this endpoint, the hazard ratio for events by the end of follow-up was nonsignificantly but slightly increased among those randomized to MPCs rather than sham control (HR, 1.2; P = .406).
“We learned a lot in this trial,” said Dr. Perin, who is medical director of the Texas Heart Institute in Houston, acknowledging that the expectation of benefit on the primary endpoint now appears to have been misplaced, but the positive result on other outcomes opens a new research direction.
With a negative result on the primary endpoint, a benefit on secondary endpoints is considered hypothesis generating. But Dr. Perin defended his sense of overall optimism about the results, because all of the endpoints on which benefit was demonstrated were prespecified. The positive findings “are not from a post hoc analyses,” he emphasized.
DREAM-HF
In the trial, 537 patients with chronic ischemic or nonischemic heart failure with NYHA class II or III symptoms and a left ventricular ejection fraction of 40% or lower were randomized at 51 sites in the United States and Canada. Patients were required to have elevated N-terminal of the prohormone brain natriuretic peptide levels, at least one prior hospitalization for heart failure, and have been on positive inotropic therapy for more than 1 month (but less than 9 months).
The intracardiac administration of MPCs, which are derived from adult human bone marrow, were delivered by injection guided with the NOGA left ventricular electromechanical mapping system. Multiple transendocardial injections were delivered, all in a single session.
There were no differences in baseline characteristics between those receiving MPCs and those who underwent a sham procedure. In both groups, more than half of patients had a previous MI and a coronary revascularization. Nearly 85% had an implanted defibrillator. Roughly two-thirds were in NYHA class III HF and the remaining were in class II.
Over the follow-up, the lines on a graph documenting nonfatal decompensated heart failure events were largely superimposed for the MPC-treated and sham-treated patients, with no significant differences seen over time.
However, the differences on the secondary events were sizable. For the composite outcome of nonfatal MI and nonfatal stroke over a mean follow-up of about 30 months, the rate of events was less than half as great in those randomized to MPCs (4.6% vs. 13.0%). This translated into about 65% reduction in risk (HR, 0.346; P = .001) overall, and the reduction was about the same in class II or III patients.
For a composite endpoint of MACE, events in the group treated with MPCs were about one-third lower than in the sham procedure group (20.3% vs. 30.1%), a difference that also reached significance (HR, 0.667; P = .021).
For this MACE endpoint, response was evaluated by systemic inflammation. For those with a high-sensitivity C-reactive protein (hsCRP) level of less than 2 mg/L, the risk reduction was small and not significant (HR, 0.843; P = .519). Conversely, there was a large risk reduction in those with hsCRP of at least 2 mg/L that did reach statistical significance (HR, 0.551; P = .012).
Inflammation was also found to be a discriminator for time to cardiac death among the patients with NYHA class II HF. Again, there was no benefit among those with hsCRP below 2 mg/L (HR, 1.355; P = .672), but an 80% risk reduction for those with hsCRP of at least 2 mg/L (HR, 0.204; P = .005).
In class II patients with hsCRP at least 2 mg/L, there was also a 60% reduction in all-cause death (HR, 0.401; P = .027). Neither the reduction in cardiac death nor all-cause death was observed in class III HF patients whether or not they had elevated hsCRP.
These signals of benefit provide a direction for a new set of studies, but Dr. Perin said that safety analyses from the DREAM-HF trial are reassuring for further clinical development.
In addition to the fact that “treatment-emergent adverse events and serious adverse events were similar in the MPC-treated and control patients,” Dr. Perin said that MPC administration was not associated with any clinically meaningful immune responses.
MPCs were first injected into a human 15 years ago, according to Dr. Perin. While a phase 2 trial published several years ago did show an association of MPC administration with a reduction in HF-associated events as well as a reduction in adverse ventricular remodeling, the ischemic benefits observed in this trial, particularly in those with elevated hsCRP, provide a new direction for future trials.
“This turns the page in heart failure research. We now have a new mechanism to consider,” Dr. Perin said.
Not so fast, expert says
This might be a reasonable conclusion, but the AHA-invited discussant, Larry Allen, MD, believes there is essentially no clinical message from this trial. He reiterated multiple times that this trial was neutral with no trend for benefit on the primary outcome.
“There was benefit on the secondary outcomes of nonfatal MI or stroke, but these are not the outcomes we follow in heart failure patients,” he said, noting that benefit from regenerative therapy on ischemic events has not been a major focus of the trials that preceded DREAM-HF.
Despite these intriguing results, “patients should understand that stem cells remain experimental,” he said. For the patient, it is “more important to double down on the importance of guideline directed medical therapy,” which is still being administered at levels that are suboptimal, according to Dr. Allen, medical director of advanced heart failure at the University of Colorado at Denver, Aurora.
“Keep up the investment” in the promise of stem cell therapy, he said, but he cautioned that some of the secondary benefits observed in DREAM-HF, such as the greater response in patients with elevated hsCRP, appear to be new observations that will require a great deal more study to validate.
Dr. Perin has a financial relationship with Mesoblast, which provided funding for the DREAM-HF trial. Dr. Allen reported no relevant conflicts of interest.
A large, multicenter, sham-controlled trial in heart failure showed no benefit at all from stem cell delivery on the primary outcome of recurrent nonfatal decompensated HF events, but the results were still promising, according to the DREAM-HF study’s principal investigator.
When added to guideline-directed medical therapy in patients with HF, a single dose of mesenchymal progenitor cells (MPC) significantly reduced major adverse cardiovascular events (MACE) – a composite of cardiac death, nonfatal MI, and nonfatal stroke – and all-cause death in New York Heart Association (NYHA) class II (but not class III patients), reported Emerson C. Perin, MD, PhD, at the American Heart Association scientific sessions.
The problem is that none of these outcomes were included in the primary endpoint, which was recurrent events because of nonfatal decompensated heart failure. On this endpoint, the hazard ratio for events by the end of follow-up was nonsignificantly but slightly increased among those randomized to MPCs rather than sham control (HR, 1.2; P = .406).
“We learned a lot in this trial,” said Dr. Perin, who is medical director of the Texas Heart Institute in Houston, acknowledging that the expectation of benefit on the primary endpoint now appears to have been misplaced, but the positive result on other outcomes opens a new research direction.
With a negative result on the primary endpoint, a benefit on secondary endpoints is considered hypothesis generating. But Dr. Perin defended his sense of overall optimism about the results, because all of the endpoints on which benefit was demonstrated were prespecified. The positive findings “are not from a post hoc analyses,” he emphasized.
DREAM-HF
In the trial, 537 patients with chronic ischemic or nonischemic heart failure with NYHA class II or III symptoms and a left ventricular ejection fraction of 40% or lower were randomized at 51 sites in the United States and Canada. Patients were required to have elevated N-terminal of the prohormone brain natriuretic peptide levels, at least one prior hospitalization for heart failure, and have been on positive inotropic therapy for more than 1 month (but less than 9 months).
The intracardiac administration of MPCs, which are derived from adult human bone marrow, were delivered by injection guided with the NOGA left ventricular electromechanical mapping system. Multiple transendocardial injections were delivered, all in a single session.
There were no differences in baseline characteristics between those receiving MPCs and those who underwent a sham procedure. In both groups, more than half of patients had a previous MI and a coronary revascularization. Nearly 85% had an implanted defibrillator. Roughly two-thirds were in NYHA class III HF and the remaining were in class II.
Over the follow-up, the lines on a graph documenting nonfatal decompensated heart failure events were largely superimposed for the MPC-treated and sham-treated patients, with no significant differences seen over time.
However, the differences on the secondary events were sizable. For the composite outcome of nonfatal MI and nonfatal stroke over a mean follow-up of about 30 months, the rate of events was less than half as great in those randomized to MPCs (4.6% vs. 13.0%). This translated into about 65% reduction in risk (HR, 0.346; P = .001) overall, and the reduction was about the same in class II or III patients.
For a composite endpoint of MACE, events in the group treated with MPCs were about one-third lower than in the sham procedure group (20.3% vs. 30.1%), a difference that also reached significance (HR, 0.667; P = .021).
For this MACE endpoint, response was evaluated by systemic inflammation. For those with a high-sensitivity C-reactive protein (hsCRP) level of less than 2 mg/L, the risk reduction was small and not significant (HR, 0.843; P = .519). Conversely, there was a large risk reduction in those with hsCRP of at least 2 mg/L that did reach statistical significance (HR, 0.551; P = .012).
Inflammation was also found to be a discriminator for time to cardiac death among the patients with NYHA class II HF. Again, there was no benefit among those with hsCRP below 2 mg/L (HR, 1.355; P = .672), but an 80% risk reduction for those with hsCRP of at least 2 mg/L (HR, 0.204; P = .005).
In class II patients with hsCRP at least 2 mg/L, there was also a 60% reduction in all-cause death (HR, 0.401; P = .027). Neither the reduction in cardiac death nor all-cause death was observed in class III HF patients whether or not they had elevated hsCRP.
These signals of benefit provide a direction for a new set of studies, but Dr. Perin said that safety analyses from the DREAM-HF trial are reassuring for further clinical development.
In addition to the fact that “treatment-emergent adverse events and serious adverse events were similar in the MPC-treated and control patients,” Dr. Perin said that MPC administration was not associated with any clinically meaningful immune responses.
MPCs were first injected into a human 15 years ago, according to Dr. Perin. While a phase 2 trial published several years ago did show an association of MPC administration with a reduction in HF-associated events as well as a reduction in adverse ventricular remodeling, the ischemic benefits observed in this trial, particularly in those with elevated hsCRP, provide a new direction for future trials.
“This turns the page in heart failure research. We now have a new mechanism to consider,” Dr. Perin said.
Not so fast, expert says
This might be a reasonable conclusion, but the AHA-invited discussant, Larry Allen, MD, believes there is essentially no clinical message from this trial. He reiterated multiple times that this trial was neutral with no trend for benefit on the primary outcome.
“There was benefit on the secondary outcomes of nonfatal MI or stroke, but these are not the outcomes we follow in heart failure patients,” he said, noting that benefit from regenerative therapy on ischemic events has not been a major focus of the trials that preceded DREAM-HF.
Despite these intriguing results, “patients should understand that stem cells remain experimental,” he said. For the patient, it is “more important to double down on the importance of guideline directed medical therapy,” which is still being administered at levels that are suboptimal, according to Dr. Allen, medical director of advanced heart failure at the University of Colorado at Denver, Aurora.
“Keep up the investment” in the promise of stem cell therapy, he said, but he cautioned that some of the secondary benefits observed in DREAM-HF, such as the greater response in patients with elevated hsCRP, appear to be new observations that will require a great deal more study to validate.
Dr. Perin has a financial relationship with Mesoblast, which provided funding for the DREAM-HF trial. Dr. Allen reported no relevant conflicts of interest.
FROM AHA 2021
EHRs have no impact on inpatient heart failure clinical choices or outcomes
When provided at the bedside of patients hospitalized for heart failure, an electronic health record (EHR) alert with prognostic information did not improve outcomes or appear to have any impact on what treatments were offered.
There was no signal that the EHR prognostic alerts, which identified the 12-month risk of mortality, had any impact on any of a variety of clinical-decision-making metrics or on any of the primary or secondary outcomes, according to Tariq Ahmad, MD, who reported results of the randomized REVEAL-HF trial, presented at the American Heart Association scientific sessions.
“These results call into question the hypothesis that accurate prognostic information alone will lead to better clinical decision-making,” said Dr. Ahmad, medical director of the Heart Transplant and Mechanical Circulatory Support Program at Yale University, New Haven, Conn., and principal investigator of REVEAL-HF.
He acknowledged that the possibility that many clinicians pay little or no attention to EHR alert might have played a role in the negative results.
At four participating Yale-affiliated clinical centers, all patients hospitalized for acute heart failure were randomized as long as they were over the age of 18, had an N-terminal pro-brain natriuretic peptide (NT-proBNP) level above 500 pg/mL, and had been placed on IV diuretics within 24 hours of admission. In the experimental arm, the provider at the time of entering orders received an EHR alert with an estimate of the risk of all-cause mortality at 12 months. There was no such alert for patients managed in the control arm.
Twelve-month mortality estimates calculated
The all-cause mortality risk was calculated on a sizeable list of variables that included laboratory results, such as cell counts, and patient characteristics, such as weight and age. The risk estimate was displayed along with a five-category color-coded bar to provide context for the risk in the spectrum of very low, low, medium, high, and very high likelihood of death within 12 months.
The 1,590 patients randomized to the experimental arm and the 1,534 patients randomized to the usual care arm did not differ significantly in any baseline characteristics. The median age was about 77 years, the mean left ventricular ejection fraction (LVEF) was 55%. About 29% had an LVEF below 40%, about 40% had chronic kidney disease, and about 30% had chronic obstructive pulmonary disease (COPD).
The composite primary outcome of all-cause mortality or rehospitalization within 12 months was reached by 38.9% and 39.3% (P = 0.82) of the intervention and control arms, respectively. The components of the primary outcome were also nearly identical, as was inpatient mortality (8.4% vs. 8.8%; P = 0.72).
There were no significant differences in any of the secondary outcomes, which included rates of 30-day rehospitalizations, discharge on guideline-recommended heart failure therapies, implantation of a cardioverter defibrillator, use of a left ventricular assist device, or heart transplant.
The proportion of patients referred for palliative care was almost identical in the very low, low, and medium risk groups. In the high (23.4 vs. 15.6; P = 0.19) and very high (50% vs. 40%; P = 0.92) groups, there were numerically more referrals in the group randomized to usual care, but these rates did not reach significance.
No differences seen in discharge meds
There was essentially no difference between groups in the rates at which patients were discharged on beta-blockers, renin-angiotensin system inhibitors, sodium-glucose co-transporter type 2 (SGLT2) inhibitors, or mineralocorticoid antagonists.
When prespecified subgroups, such as those older than age 75 years relative to those younger, males relative to females, Black versus White participants, patients with reduced ejection fraction (HFrEF) relative to preserved ejection fraction (HFpEF), and intensive care unit versus non-ICU patients, were compared, there were no indications that the EHR alert improved outcomes.
Invited discussant Harriette G. C. Van Spall, MD, director of digital health and virtual care and associate professor of cardiology at McMaster University, Hamilton, Ont., did not dispute the conclusions, but she pointed out several potential explanations for the neutral result.
Not least, nearly 75% of those enrolled had low risk or very low risk for adverse outcomes within 1 year, so the opportunity to show a reduction in events, including all-cause mortality, was limited.
“This was largely a HFpEF population, for which there are no treatments for which a risk score would change therapy,” she said.
EHR alert efficacy questioned
There is considerable evidence that risk prediction tools “are common but underutilized in HF,” Dr. Van Spall added. She noted that many clinicians find alerts in the EHR more annoying than informative, and it remains unknown what proportion of clinicians pay attention to them, particularly in the absence of evidence that they lead to meaningful improvements in care over their own clinical judgment.
Dr. Ahmad agreed.
“I think that we need to study these alerts in a clinical trial format,” he said. Acknowledging that alerts have been poorly received by many clinicians, Dr. Ahmad said that trials to validate the impact of any specific alert are needed to improve their credibility. If a positive impact cannot be shown, he said the alert should be eliminated, leaving only the alerts with proven clinical value.
Dr. Ahmad reported financial relationships with Amgen, AstraZeneca, Boehringer Ingelheim, Cytokinetics, Novartis, and Relypsa. Dr. Van Spall reports no potential conflicts of interest.
When provided at the bedside of patients hospitalized for heart failure, an electronic health record (EHR) alert with prognostic information did not improve outcomes or appear to have any impact on what treatments were offered.
There was no signal that the EHR prognostic alerts, which identified the 12-month risk of mortality, had any impact on any of a variety of clinical-decision-making metrics or on any of the primary or secondary outcomes, according to Tariq Ahmad, MD, who reported results of the randomized REVEAL-HF trial, presented at the American Heart Association scientific sessions.
“These results call into question the hypothesis that accurate prognostic information alone will lead to better clinical decision-making,” said Dr. Ahmad, medical director of the Heart Transplant and Mechanical Circulatory Support Program at Yale University, New Haven, Conn., and principal investigator of REVEAL-HF.
He acknowledged that the possibility that many clinicians pay little or no attention to EHR alert might have played a role in the negative results.
At four participating Yale-affiliated clinical centers, all patients hospitalized for acute heart failure were randomized as long as they were over the age of 18, had an N-terminal pro-brain natriuretic peptide (NT-proBNP) level above 500 pg/mL, and had been placed on IV diuretics within 24 hours of admission. In the experimental arm, the provider at the time of entering orders received an EHR alert with an estimate of the risk of all-cause mortality at 12 months. There was no such alert for patients managed in the control arm.
Twelve-month mortality estimates calculated
The all-cause mortality risk was calculated on a sizeable list of variables that included laboratory results, such as cell counts, and patient characteristics, such as weight and age. The risk estimate was displayed along with a five-category color-coded bar to provide context for the risk in the spectrum of very low, low, medium, high, and very high likelihood of death within 12 months.
The 1,590 patients randomized to the experimental arm and the 1,534 patients randomized to the usual care arm did not differ significantly in any baseline characteristics. The median age was about 77 years, the mean left ventricular ejection fraction (LVEF) was 55%. About 29% had an LVEF below 40%, about 40% had chronic kidney disease, and about 30% had chronic obstructive pulmonary disease (COPD).
The composite primary outcome of all-cause mortality or rehospitalization within 12 months was reached by 38.9% and 39.3% (P = 0.82) of the intervention and control arms, respectively. The components of the primary outcome were also nearly identical, as was inpatient mortality (8.4% vs. 8.8%; P = 0.72).
There were no significant differences in any of the secondary outcomes, which included rates of 30-day rehospitalizations, discharge on guideline-recommended heart failure therapies, implantation of a cardioverter defibrillator, use of a left ventricular assist device, or heart transplant.
The proportion of patients referred for palliative care was almost identical in the very low, low, and medium risk groups. In the high (23.4 vs. 15.6; P = 0.19) and very high (50% vs. 40%; P = 0.92) groups, there were numerically more referrals in the group randomized to usual care, but these rates did not reach significance.
No differences seen in discharge meds
There was essentially no difference between groups in the rates at which patients were discharged on beta-blockers, renin-angiotensin system inhibitors, sodium-glucose co-transporter type 2 (SGLT2) inhibitors, or mineralocorticoid antagonists.
When prespecified subgroups, such as those older than age 75 years relative to those younger, males relative to females, Black versus White participants, patients with reduced ejection fraction (HFrEF) relative to preserved ejection fraction (HFpEF), and intensive care unit versus non-ICU patients, were compared, there were no indications that the EHR alert improved outcomes.
Invited discussant Harriette G. C. Van Spall, MD, director of digital health and virtual care and associate professor of cardiology at McMaster University, Hamilton, Ont., did not dispute the conclusions, but she pointed out several potential explanations for the neutral result.
Not least, nearly 75% of those enrolled had low risk or very low risk for adverse outcomes within 1 year, so the opportunity to show a reduction in events, including all-cause mortality, was limited.
“This was largely a HFpEF population, for which there are no treatments for which a risk score would change therapy,” she said.
EHR alert efficacy questioned
There is considerable evidence that risk prediction tools “are common but underutilized in HF,” Dr. Van Spall added. She noted that many clinicians find alerts in the EHR more annoying than informative, and it remains unknown what proportion of clinicians pay attention to them, particularly in the absence of evidence that they lead to meaningful improvements in care over their own clinical judgment.
Dr. Ahmad agreed.
“I think that we need to study these alerts in a clinical trial format,” he said. Acknowledging that alerts have been poorly received by many clinicians, Dr. Ahmad said that trials to validate the impact of any specific alert are needed to improve their credibility. If a positive impact cannot be shown, he said the alert should be eliminated, leaving only the alerts with proven clinical value.
Dr. Ahmad reported financial relationships with Amgen, AstraZeneca, Boehringer Ingelheim, Cytokinetics, Novartis, and Relypsa. Dr. Van Spall reports no potential conflicts of interest.
When provided at the bedside of patients hospitalized for heart failure, an electronic health record (EHR) alert with prognostic information did not improve outcomes or appear to have any impact on what treatments were offered.
There was no signal that the EHR prognostic alerts, which identified the 12-month risk of mortality, had any impact on any of a variety of clinical-decision-making metrics or on any of the primary or secondary outcomes, according to Tariq Ahmad, MD, who reported results of the randomized REVEAL-HF trial, presented at the American Heart Association scientific sessions.
“These results call into question the hypothesis that accurate prognostic information alone will lead to better clinical decision-making,” said Dr. Ahmad, medical director of the Heart Transplant and Mechanical Circulatory Support Program at Yale University, New Haven, Conn., and principal investigator of REVEAL-HF.
He acknowledged that the possibility that many clinicians pay little or no attention to EHR alert might have played a role in the negative results.
At four participating Yale-affiliated clinical centers, all patients hospitalized for acute heart failure were randomized as long as they were over the age of 18, had an N-terminal pro-brain natriuretic peptide (NT-proBNP) level above 500 pg/mL, and had been placed on IV diuretics within 24 hours of admission. In the experimental arm, the provider at the time of entering orders received an EHR alert with an estimate of the risk of all-cause mortality at 12 months. There was no such alert for patients managed in the control arm.
Twelve-month mortality estimates calculated
The all-cause mortality risk was calculated on a sizeable list of variables that included laboratory results, such as cell counts, and patient characteristics, such as weight and age. The risk estimate was displayed along with a five-category color-coded bar to provide context for the risk in the spectrum of very low, low, medium, high, and very high likelihood of death within 12 months.
The 1,590 patients randomized to the experimental arm and the 1,534 patients randomized to the usual care arm did not differ significantly in any baseline characteristics. The median age was about 77 years, the mean left ventricular ejection fraction (LVEF) was 55%. About 29% had an LVEF below 40%, about 40% had chronic kidney disease, and about 30% had chronic obstructive pulmonary disease (COPD).
The composite primary outcome of all-cause mortality or rehospitalization within 12 months was reached by 38.9% and 39.3% (P = 0.82) of the intervention and control arms, respectively. The components of the primary outcome were also nearly identical, as was inpatient mortality (8.4% vs. 8.8%; P = 0.72).
There were no significant differences in any of the secondary outcomes, which included rates of 30-day rehospitalizations, discharge on guideline-recommended heart failure therapies, implantation of a cardioverter defibrillator, use of a left ventricular assist device, or heart transplant.
The proportion of patients referred for palliative care was almost identical in the very low, low, and medium risk groups. In the high (23.4 vs. 15.6; P = 0.19) and very high (50% vs. 40%; P = 0.92) groups, there were numerically more referrals in the group randomized to usual care, but these rates did not reach significance.
No differences seen in discharge meds
There was essentially no difference between groups in the rates at which patients were discharged on beta-blockers, renin-angiotensin system inhibitors, sodium-glucose co-transporter type 2 (SGLT2) inhibitors, or mineralocorticoid antagonists.
When prespecified subgroups, such as those older than age 75 years relative to those younger, males relative to females, Black versus White participants, patients with reduced ejection fraction (HFrEF) relative to preserved ejection fraction (HFpEF), and intensive care unit versus non-ICU patients, were compared, there were no indications that the EHR alert improved outcomes.
Invited discussant Harriette G. C. Van Spall, MD, director of digital health and virtual care and associate professor of cardiology at McMaster University, Hamilton, Ont., did not dispute the conclusions, but she pointed out several potential explanations for the neutral result.
Not least, nearly 75% of those enrolled had low risk or very low risk for adverse outcomes within 1 year, so the opportunity to show a reduction in events, including all-cause mortality, was limited.
“This was largely a HFpEF population, for which there are no treatments for which a risk score would change therapy,” she said.
EHR alert efficacy questioned
There is considerable evidence that risk prediction tools “are common but underutilized in HF,” Dr. Van Spall added. She noted that many clinicians find alerts in the EHR more annoying than informative, and it remains unknown what proportion of clinicians pay attention to them, particularly in the absence of evidence that they lead to meaningful improvements in care over their own clinical judgment.
Dr. Ahmad agreed.
“I think that we need to study these alerts in a clinical trial format,” he said. Acknowledging that alerts have been poorly received by many clinicians, Dr. Ahmad said that trials to validate the impact of any specific alert are needed to improve their credibility. If a positive impact cannot be shown, he said the alert should be eliminated, leaving only the alerts with proven clinical value.
Dr. Ahmad reported financial relationships with Amgen, AstraZeneca, Boehringer Ingelheim, Cytokinetics, Novartis, and Relypsa. Dr. Van Spall reports no potential conflicts of interest.
FROM AHA 2021
Finerenone, sotagliflozin exert heart failure benefits despite renal dysfunction
New analyses of trial results for the cardiorenal agents finerenone and sotagliflozin continued the pattern showing that they exert consistent heart failure benefits in patients who span a broad spectrum of renal function, further disproving the notion that more severe stages of chronic kidney disease preclude aggressive medical management.
Analysis of combined data from two pivotal trials of the nonsteroidal mineralocorticoid receptor antagonist (MRA) finerenone (Kerendia), which together enrolled more than 13,000 patients with type 2 diabetes and chronic kidney disease, showed in greater detail that .
That spectrum included patients with estimated glomerular filtration rates (eGFR) as low as 25 mL/min per 1.73m2 and patients with micro- or macroalbuminuria, as well as those with normal urinary albumin levels, Gerasimos Filippatos, MD, reported at the American Heart Association scientific sessions.
And in a separate, unrelated report, combined data from the two pivotal trials, with a total of nearly 12,000 patients with type 2 diabetes, for sotagliflozin (Zynquista), a novel and still unapproved agent that inhibits both the sodium-glucose cotransporter (SGLT) 1 and 2 enzymes, showed a consistent effect significantly reducing cardiovascular death, hospitalization for heart failure, or urgent heart failure outpatient events in patients with eGFR rates as low as 25 mL/min per 1.73m2, Deepak L. Bhatt, MD, reported at the meeting.
These two reports follow a third, presented just a week earlier during Kidney Week, that showed the benefit from the SGLT2 inhibitor empagliflozin (Jardiance) for preventing heart failure hospitalizations or cardiovascular death in patients with heart failure with preserved ejection fraction remained consistent even in patients with an eGFR as low as 20 mL/min/1.73m2 in results from the EMPEROR-Preserved trial. Similar findings for empagliflozin in patients with heart failure with reduced ejection fraction in the EMPEROR-Reduced trial came out nearly a year ago.
A message to clinicians from these reports is, “don’t wait for patients to develop heart failure” to start these drugs, according to Dipti Itchhaporia, MD, director of disease management for the Hoag Heart and Vascular Institute in Newport Beach, Calif. “It’s time to start using these drugs upstream to have fewer patients with heart failure downstream,” she said in an interview.
Finerenone works differently than spironolactone
The new finerenone analysis included 5,734 patients enrolled in the FIDELIO-DKD trial, and 7,437 in the FIGARO-DKD trial, two very similar trials that differed by transposing the primary endpoint of one to the secondary endpoint of the other, and vice versa. The combined analysis is known as FIDELITY.
Expanding on a report that he first gave at the European Society of Cardiology annual congress in August 2021, Dr. Filippatos provided a few additional details on the analysis that showed a consistent effect of finerenone on preventing hospitalizations for heart failure, and on preventing a combined endpoint of hospitalizations for heart failure and cardiovascular death regardless of the severity of chronic kidney disease down to 25 mL/min per 1.73 m2. Statistical analysis showed no hint of an interaction between finerenone’s effect on these outcomes in patients with an eGFR of 60 mL/min per 1.73 m2 or greater and those with reduced renal function. Analyses also showed no interaction based on urinary albumin-to-creatinine ratio, be it more or less than 300 mg/g, reported Dr. Filippatos, professor and director of the heart failure unit at Attikon University Hospital in Athens.
“We use MRAs [such as spironolactone] in heart failure patients, but it’s difficult to use because of the risk of patients developing hyperkalemia,” noted Dr. Itchhaporia, who added that reluctance to use spironolactone is especially high for patients with depressed renal function, which could exacerbate a hyperkalemic effect. Evidence shows that finerenone poses a substantially reduced risk for raising serum potassium levels, making finerenone a more attractive agent to use in patients with CKD who have an elevated risk for heart failure events as well as an increased risk for hyperkalemia, like those enrolled in the two finerenone trials, she said.
Sotagliflozin uniquely inhibits SGLT1 and SGLT2
The new sotagliflozin analyses reported by Dr. Bhatt combined data for more than 11,800 patients randomized into either of two trials, SCORED, which randomized more than 10,000 patients with type 2 diabetes and chronic kidney disease, and SOLOIST, which randomized more than 1,000 patients with type 2 diabetes who were recently hospitalized for worsening heart failure.
A prespecified analysis for the combined data from both studies looked at the impact of sotagliflozin treatment on the combined outcome of cardiovascular death, hospitalization for heart failure, or an urgent outpatient visit because of heart failure based on kidney function at baseline. The analysis showed that sotagliflozin was at least as effective in the 8% of study patients who at baseline had an eGFR of 25-29 mL/min per 1.73 m2 as it was in patients with more preserved renal function.
Benefit from sotagliflozin treatment “was consistent across the full range of eGFR,” said Dr. Bhatt, professor at Harvard Medical School in Boston and executive director of interventional cardiovascular programs at Brigham and Women’s Hospital in Boston.
Results from a second analysis that he reported also showed a consistent effect of sotagliflozin on reducing hemoglobin A1c levels in the enrolled patients, even those with the lowest levels of renal function, an effect not previously seen with the related class of SGLT2 inhibitors (which includes empagliflozin, canagliflozin [Invokana], and dapagliflozin [Farxiga]). Dr. Bhatt suggested that, while SGLT2 inhibitors act entirely in the kidneys and hence their effect on glycemic control is blunted by renal dysfunction, sotagliflozin also inhibits the SGLT1 enzyme, which functions in the gut to transport glucose out of the digestive tract and into the blood, a glycemic control pathway that’s independent of renal function.
FIDELIO-DKD, FIGARO-DKD, and FIDELITY were sponsored by Bayer, the company that markets finerenone (Kerendia). SCORED and SOLOIST were sponsored by Sanofi, and later by Lexicon, the companies developing sotagliflozin (Zynquista). EMPEROR-Preserved and EMPEROR-Reduced were sponsored by Boehringer-Ingelheim and Lilly, the companies that market empagliflozin (Jardiance). Dr. Filippatos has had financial relationships with Bayer and Boehringer-Ingelheim, as well as with Amgen, Medtronic, Novartis, Servier, and Vifor. Dr. Bhatt has received research funding from Sanofi, Lexicon, Bayer, and Boehringer-Ingelheim, Lilly, and numerous other companies, and he has been an adviser to Boehringer-Ingelheim and several other companies.
New analyses of trial results for the cardiorenal agents finerenone and sotagliflozin continued the pattern showing that they exert consistent heart failure benefits in patients who span a broad spectrum of renal function, further disproving the notion that more severe stages of chronic kidney disease preclude aggressive medical management.
Analysis of combined data from two pivotal trials of the nonsteroidal mineralocorticoid receptor antagonist (MRA) finerenone (Kerendia), which together enrolled more than 13,000 patients with type 2 diabetes and chronic kidney disease, showed in greater detail that .
That spectrum included patients with estimated glomerular filtration rates (eGFR) as low as 25 mL/min per 1.73m2 and patients with micro- or macroalbuminuria, as well as those with normal urinary albumin levels, Gerasimos Filippatos, MD, reported at the American Heart Association scientific sessions.
And in a separate, unrelated report, combined data from the two pivotal trials, with a total of nearly 12,000 patients with type 2 diabetes, for sotagliflozin (Zynquista), a novel and still unapproved agent that inhibits both the sodium-glucose cotransporter (SGLT) 1 and 2 enzymes, showed a consistent effect significantly reducing cardiovascular death, hospitalization for heart failure, or urgent heart failure outpatient events in patients with eGFR rates as low as 25 mL/min per 1.73m2, Deepak L. Bhatt, MD, reported at the meeting.
These two reports follow a third, presented just a week earlier during Kidney Week, that showed the benefit from the SGLT2 inhibitor empagliflozin (Jardiance) for preventing heart failure hospitalizations or cardiovascular death in patients with heart failure with preserved ejection fraction remained consistent even in patients with an eGFR as low as 20 mL/min/1.73m2 in results from the EMPEROR-Preserved trial. Similar findings for empagliflozin in patients with heart failure with reduced ejection fraction in the EMPEROR-Reduced trial came out nearly a year ago.
A message to clinicians from these reports is, “don’t wait for patients to develop heart failure” to start these drugs, according to Dipti Itchhaporia, MD, director of disease management for the Hoag Heart and Vascular Institute in Newport Beach, Calif. “It’s time to start using these drugs upstream to have fewer patients with heart failure downstream,” she said in an interview.
Finerenone works differently than spironolactone
The new finerenone analysis included 5,734 patients enrolled in the FIDELIO-DKD trial, and 7,437 in the FIGARO-DKD trial, two very similar trials that differed by transposing the primary endpoint of one to the secondary endpoint of the other, and vice versa. The combined analysis is known as FIDELITY.
Expanding on a report that he first gave at the European Society of Cardiology annual congress in August 2021, Dr. Filippatos provided a few additional details on the analysis that showed a consistent effect of finerenone on preventing hospitalizations for heart failure, and on preventing a combined endpoint of hospitalizations for heart failure and cardiovascular death regardless of the severity of chronic kidney disease down to 25 mL/min per 1.73 m2. Statistical analysis showed no hint of an interaction between finerenone’s effect on these outcomes in patients with an eGFR of 60 mL/min per 1.73 m2 or greater and those with reduced renal function. Analyses also showed no interaction based on urinary albumin-to-creatinine ratio, be it more or less than 300 mg/g, reported Dr. Filippatos, professor and director of the heart failure unit at Attikon University Hospital in Athens.
“We use MRAs [such as spironolactone] in heart failure patients, but it’s difficult to use because of the risk of patients developing hyperkalemia,” noted Dr. Itchhaporia, who added that reluctance to use spironolactone is especially high for patients with depressed renal function, which could exacerbate a hyperkalemic effect. Evidence shows that finerenone poses a substantially reduced risk for raising serum potassium levels, making finerenone a more attractive agent to use in patients with CKD who have an elevated risk for heart failure events as well as an increased risk for hyperkalemia, like those enrolled in the two finerenone trials, she said.
Sotagliflozin uniquely inhibits SGLT1 and SGLT2
The new sotagliflozin analyses reported by Dr. Bhatt combined data for more than 11,800 patients randomized into either of two trials, SCORED, which randomized more than 10,000 patients with type 2 diabetes and chronic kidney disease, and SOLOIST, which randomized more than 1,000 patients with type 2 diabetes who were recently hospitalized for worsening heart failure.
A prespecified analysis for the combined data from both studies looked at the impact of sotagliflozin treatment on the combined outcome of cardiovascular death, hospitalization for heart failure, or an urgent outpatient visit because of heart failure based on kidney function at baseline. The analysis showed that sotagliflozin was at least as effective in the 8% of study patients who at baseline had an eGFR of 25-29 mL/min per 1.73 m2 as it was in patients with more preserved renal function.
Benefit from sotagliflozin treatment “was consistent across the full range of eGFR,” said Dr. Bhatt, professor at Harvard Medical School in Boston and executive director of interventional cardiovascular programs at Brigham and Women’s Hospital in Boston.
Results from a second analysis that he reported also showed a consistent effect of sotagliflozin on reducing hemoglobin A1c levels in the enrolled patients, even those with the lowest levels of renal function, an effect not previously seen with the related class of SGLT2 inhibitors (which includes empagliflozin, canagliflozin [Invokana], and dapagliflozin [Farxiga]). Dr. Bhatt suggested that, while SGLT2 inhibitors act entirely in the kidneys and hence their effect on glycemic control is blunted by renal dysfunction, sotagliflozin also inhibits the SGLT1 enzyme, which functions in the gut to transport glucose out of the digestive tract and into the blood, a glycemic control pathway that’s independent of renal function.
FIDELIO-DKD, FIGARO-DKD, and FIDELITY were sponsored by Bayer, the company that markets finerenone (Kerendia). SCORED and SOLOIST were sponsored by Sanofi, and later by Lexicon, the companies developing sotagliflozin (Zynquista). EMPEROR-Preserved and EMPEROR-Reduced were sponsored by Boehringer-Ingelheim and Lilly, the companies that market empagliflozin (Jardiance). Dr. Filippatos has had financial relationships with Bayer and Boehringer-Ingelheim, as well as with Amgen, Medtronic, Novartis, Servier, and Vifor. Dr. Bhatt has received research funding from Sanofi, Lexicon, Bayer, and Boehringer-Ingelheim, Lilly, and numerous other companies, and he has been an adviser to Boehringer-Ingelheim and several other companies.
New analyses of trial results for the cardiorenal agents finerenone and sotagliflozin continued the pattern showing that they exert consistent heart failure benefits in patients who span a broad spectrum of renal function, further disproving the notion that more severe stages of chronic kidney disease preclude aggressive medical management.
Analysis of combined data from two pivotal trials of the nonsteroidal mineralocorticoid receptor antagonist (MRA) finerenone (Kerendia), which together enrolled more than 13,000 patients with type 2 diabetes and chronic kidney disease, showed in greater detail that .
That spectrum included patients with estimated glomerular filtration rates (eGFR) as low as 25 mL/min per 1.73m2 and patients with micro- or macroalbuminuria, as well as those with normal urinary albumin levels, Gerasimos Filippatos, MD, reported at the American Heart Association scientific sessions.
And in a separate, unrelated report, combined data from the two pivotal trials, with a total of nearly 12,000 patients with type 2 diabetes, for sotagliflozin (Zynquista), a novel and still unapproved agent that inhibits both the sodium-glucose cotransporter (SGLT) 1 and 2 enzymes, showed a consistent effect significantly reducing cardiovascular death, hospitalization for heart failure, or urgent heart failure outpatient events in patients with eGFR rates as low as 25 mL/min per 1.73m2, Deepak L. Bhatt, MD, reported at the meeting.
These two reports follow a third, presented just a week earlier during Kidney Week, that showed the benefit from the SGLT2 inhibitor empagliflozin (Jardiance) for preventing heart failure hospitalizations or cardiovascular death in patients with heart failure with preserved ejection fraction remained consistent even in patients with an eGFR as low as 20 mL/min/1.73m2 in results from the EMPEROR-Preserved trial. Similar findings for empagliflozin in patients with heart failure with reduced ejection fraction in the EMPEROR-Reduced trial came out nearly a year ago.
A message to clinicians from these reports is, “don’t wait for patients to develop heart failure” to start these drugs, according to Dipti Itchhaporia, MD, director of disease management for the Hoag Heart and Vascular Institute in Newport Beach, Calif. “It’s time to start using these drugs upstream to have fewer patients with heart failure downstream,” she said in an interview.
Finerenone works differently than spironolactone
The new finerenone analysis included 5,734 patients enrolled in the FIDELIO-DKD trial, and 7,437 in the FIGARO-DKD trial, two very similar trials that differed by transposing the primary endpoint of one to the secondary endpoint of the other, and vice versa. The combined analysis is known as FIDELITY.
Expanding on a report that he first gave at the European Society of Cardiology annual congress in August 2021, Dr. Filippatos provided a few additional details on the analysis that showed a consistent effect of finerenone on preventing hospitalizations for heart failure, and on preventing a combined endpoint of hospitalizations for heart failure and cardiovascular death regardless of the severity of chronic kidney disease down to 25 mL/min per 1.73 m2. Statistical analysis showed no hint of an interaction between finerenone’s effect on these outcomes in patients with an eGFR of 60 mL/min per 1.73 m2 or greater and those with reduced renal function. Analyses also showed no interaction based on urinary albumin-to-creatinine ratio, be it more or less than 300 mg/g, reported Dr. Filippatos, professor and director of the heart failure unit at Attikon University Hospital in Athens.
“We use MRAs [such as spironolactone] in heart failure patients, but it’s difficult to use because of the risk of patients developing hyperkalemia,” noted Dr. Itchhaporia, who added that reluctance to use spironolactone is especially high for patients with depressed renal function, which could exacerbate a hyperkalemic effect. Evidence shows that finerenone poses a substantially reduced risk for raising serum potassium levels, making finerenone a more attractive agent to use in patients with CKD who have an elevated risk for heart failure events as well as an increased risk for hyperkalemia, like those enrolled in the two finerenone trials, she said.
Sotagliflozin uniquely inhibits SGLT1 and SGLT2
The new sotagliflozin analyses reported by Dr. Bhatt combined data for more than 11,800 patients randomized into either of two trials, SCORED, which randomized more than 10,000 patients with type 2 diabetes and chronic kidney disease, and SOLOIST, which randomized more than 1,000 patients with type 2 diabetes who were recently hospitalized for worsening heart failure.
A prespecified analysis for the combined data from both studies looked at the impact of sotagliflozin treatment on the combined outcome of cardiovascular death, hospitalization for heart failure, or an urgent outpatient visit because of heart failure based on kidney function at baseline. The analysis showed that sotagliflozin was at least as effective in the 8% of study patients who at baseline had an eGFR of 25-29 mL/min per 1.73 m2 as it was in patients with more preserved renal function.
Benefit from sotagliflozin treatment “was consistent across the full range of eGFR,” said Dr. Bhatt, professor at Harvard Medical School in Boston and executive director of interventional cardiovascular programs at Brigham and Women’s Hospital in Boston.
Results from a second analysis that he reported also showed a consistent effect of sotagliflozin on reducing hemoglobin A1c levels in the enrolled patients, even those with the lowest levels of renal function, an effect not previously seen with the related class of SGLT2 inhibitors (which includes empagliflozin, canagliflozin [Invokana], and dapagliflozin [Farxiga]). Dr. Bhatt suggested that, while SGLT2 inhibitors act entirely in the kidneys and hence their effect on glycemic control is blunted by renal dysfunction, sotagliflozin also inhibits the SGLT1 enzyme, which functions in the gut to transport glucose out of the digestive tract and into the blood, a glycemic control pathway that’s independent of renal function.
FIDELIO-DKD, FIGARO-DKD, and FIDELITY were sponsored by Bayer, the company that markets finerenone (Kerendia). SCORED and SOLOIST were sponsored by Sanofi, and later by Lexicon, the companies developing sotagliflozin (Zynquista). EMPEROR-Preserved and EMPEROR-Reduced were sponsored by Boehringer-Ingelheim and Lilly, the companies that market empagliflozin (Jardiance). Dr. Filippatos has had financial relationships with Bayer and Boehringer-Ingelheim, as well as with Amgen, Medtronic, Novartis, Servier, and Vifor. Dr. Bhatt has received research funding from Sanofi, Lexicon, Bayer, and Boehringer-Ingelheim, Lilly, and numerous other companies, and he has been an adviser to Boehringer-Ingelheim and several other companies.
FROM AHA 2021
BP Track: Blood pressure control rates dropped during pandemic
Wave of CV events possible
, if the data from 24 health systems is representative of national trends.
The decline in blood pressure control corresponded with – and might be explained by – a parallel decline in follow-up visits for uncontrolled hypertension from the same data source, according to Alanna M. Chamberlain, PhD, associate professor of epidemiology in the division of quantitative health sciences, Mayo Clinic, Rochester, Minn.
If the data are representative, a wave of cardiovascular (CV) events might be coming.
The study, called BP Track, collated electronic medical data on almost 1.8 million patients with hypertension from 2017 through 2020. Up until the end of 2019 and prior to the pandemic, slightly less than 60% of these patients had blood pressure control, defined as less than 140/90 mm Hg.
While the pre-COVID control rates were already “suboptimal,” a decline began almost immediately when the full force of the COVID-19 pandemic began in March of 2020, said Dr. Chamberlain in reporting the BP Track results at the American Heart Association scientific sessions.
When graphed from the start of the pandemic until the end of 2020, the proportion under control fell 7.2% to a level just above 50%. For the more rigorous target of less than 130/80 mm Hg, the proportion fell 4.6% over the same period of time, leaving only about 25% at that level of control.
Repeat visits for BP control rebounded
The proportion of patients with a repeat office visit within 4 weeks of a diagnosis of uncontrolled hypertension fell even more steeply, reaching a nadir at about the middle of 2020, but it was followed by a partial recovery. The rate was 5% lower by the end of 2020, relative to the prepandemic rate (31.7% vs. 36.7%), but that was 5% higher than the nadir.
A similar phenomenon was observed with several other metrics. For example, there was a steep, immediate fall correlating with the onset of the pandemic in the proportion of patients who achieved at least a 10–mm Hg reduction or a BP under 140/90 mm Hg when treated for hypertension. Again, the nadir in this proportion was reached in about mid-2020 followed by a partial recovery. By the end of 2020, 5.9% fewer patients were achieving 10–mm Hg or better improvement in BP control when treated relative to the prepandemic level (23.8% vs. 29.7%), but this level was almost 10% higher than the nadir.
Data based on electronic medical records
The nearly 1.8 million patient records evaluated in the BP Track study were drawn from the 24 centers participating in the PCORnet Blood Pressure Control Laboratory Surveillance System. Nationally distributed, 18 of the 24 systems were academically affiliated.
When stratified by race, the proportion of Asians meeting the definition of BP control prior to the pandemic was about 5% higher than the overall average, and the proportion in Blacks was more than 5% lower. Whites had rates of blood pressure control very near the average. The relative declines in BP and the proportion of patients with uncontrolled blood pressure who had a repeat visit within 4 weeks during the pandemic were generally parallel across racial groups.
The implications of these data and the role of the COVID-19 pandemic on blood pressure control are “concerning,” according to Adam Bress, PharmD, department of population health sciences, University of Utah, Salt Lake City.
Citing a study published in 2020 that suggested blood pressure control rates in the United States were already declining before the COVID-19 pandemic, he said the COVID-19 epidemic appears to be exacerbating an existing problem. He expressed particular concern for populations who already have low rates of control, such as African Americans.
“The impact of COVID-19 is likely to be disproportionately greater for underserved and minoritized patients,” said Dr. Bress, who was the lead author of a recent article on this specific topic.
The implication of BP Track is that a wave of cardiovascular events will be coming if the data are nationally representative.
“A recent meta-analysis shows that each 5–mm Hg reduction in blood pressure is associated with age-related reductions in CV events,” Dr. Bress said. For those 55 years of age or older, he said the risk reduction is about 10%. Given that the inverse is almost certainly true, he expects diminishing blood pressure control, whether COVID-19-related or not, to translate into increased CV events.
However, there is no guarantee that the BP Track data are representative of the U.S. population, cautioned Eugene Yang, MD, professor in the division of cardiology, University of Washington, Seattle. Even though a large group of patients was included, they were largely drawn from academic centers.
Nevertheless, Dr. Yang, who chairs the Hypertension Working Group of the American College of Cardiology’s Prevention of Cardiovascular Disease Council, acknowledged that the implications are “scary.”
If the data are representative, “this type of reduction in blood pressure control would be expected to have a significant impact on morbidity and mortality, but we also have to think of all the variables that were not tracked and might add to risk,” he said. He named such risk factors as weight gain, diminished exercise, and increased alcohol consumption, which have been cited by others as being exacerbated by the pandemic.
If these lead to more cardiovascular events on a population basis, the timing of these events would be expected to be age dependent.
“If you look at the patients included in this study, about 50% were 65 years of age or older. In a population like this you would expect to see an increase in events sooner rather than later,” said Dr. Wang.
In other words, if the trial is representative, a wave of cardiovascular events might be seen in the most vulnerable patients “within the next few years,” Dr. Yang speculated.
Dr. Chamberlain reports a research grant from EpidStrategies. Dr. Bress and Dr. Yang report no potential financial conflicts of interest.
Wave of CV events possible
Wave of CV events possible
, if the data from 24 health systems is representative of national trends.
The decline in blood pressure control corresponded with – and might be explained by – a parallel decline in follow-up visits for uncontrolled hypertension from the same data source, according to Alanna M. Chamberlain, PhD, associate professor of epidemiology in the division of quantitative health sciences, Mayo Clinic, Rochester, Minn.
If the data are representative, a wave of cardiovascular (CV) events might be coming.
The study, called BP Track, collated electronic medical data on almost 1.8 million patients with hypertension from 2017 through 2020. Up until the end of 2019 and prior to the pandemic, slightly less than 60% of these patients had blood pressure control, defined as less than 140/90 mm Hg.
While the pre-COVID control rates were already “suboptimal,” a decline began almost immediately when the full force of the COVID-19 pandemic began in March of 2020, said Dr. Chamberlain in reporting the BP Track results at the American Heart Association scientific sessions.
When graphed from the start of the pandemic until the end of 2020, the proportion under control fell 7.2% to a level just above 50%. For the more rigorous target of less than 130/80 mm Hg, the proportion fell 4.6% over the same period of time, leaving only about 25% at that level of control.
Repeat visits for BP control rebounded
The proportion of patients with a repeat office visit within 4 weeks of a diagnosis of uncontrolled hypertension fell even more steeply, reaching a nadir at about the middle of 2020, but it was followed by a partial recovery. The rate was 5% lower by the end of 2020, relative to the prepandemic rate (31.7% vs. 36.7%), but that was 5% higher than the nadir.
A similar phenomenon was observed with several other metrics. For example, there was a steep, immediate fall correlating with the onset of the pandemic in the proportion of patients who achieved at least a 10–mm Hg reduction or a BP under 140/90 mm Hg when treated for hypertension. Again, the nadir in this proportion was reached in about mid-2020 followed by a partial recovery. By the end of 2020, 5.9% fewer patients were achieving 10–mm Hg or better improvement in BP control when treated relative to the prepandemic level (23.8% vs. 29.7%), but this level was almost 10% higher than the nadir.
Data based on electronic medical records
The nearly 1.8 million patient records evaluated in the BP Track study were drawn from the 24 centers participating in the PCORnet Blood Pressure Control Laboratory Surveillance System. Nationally distributed, 18 of the 24 systems were academically affiliated.
When stratified by race, the proportion of Asians meeting the definition of BP control prior to the pandemic was about 5% higher than the overall average, and the proportion in Blacks was more than 5% lower. Whites had rates of blood pressure control very near the average. The relative declines in BP and the proportion of patients with uncontrolled blood pressure who had a repeat visit within 4 weeks during the pandemic were generally parallel across racial groups.
The implications of these data and the role of the COVID-19 pandemic on blood pressure control are “concerning,” according to Adam Bress, PharmD, department of population health sciences, University of Utah, Salt Lake City.
Citing a study published in 2020 that suggested blood pressure control rates in the United States were already declining before the COVID-19 pandemic, he said the COVID-19 epidemic appears to be exacerbating an existing problem. He expressed particular concern for populations who already have low rates of control, such as African Americans.
“The impact of COVID-19 is likely to be disproportionately greater for underserved and minoritized patients,” said Dr. Bress, who was the lead author of a recent article on this specific topic.
The implication of BP Track is that a wave of cardiovascular events will be coming if the data are nationally representative.
“A recent meta-analysis shows that each 5–mm Hg reduction in blood pressure is associated with age-related reductions in CV events,” Dr. Bress said. For those 55 years of age or older, he said the risk reduction is about 10%. Given that the inverse is almost certainly true, he expects diminishing blood pressure control, whether COVID-19-related or not, to translate into increased CV events.
However, there is no guarantee that the BP Track data are representative of the U.S. population, cautioned Eugene Yang, MD, professor in the division of cardiology, University of Washington, Seattle. Even though a large group of patients was included, they were largely drawn from academic centers.
Nevertheless, Dr. Yang, who chairs the Hypertension Working Group of the American College of Cardiology’s Prevention of Cardiovascular Disease Council, acknowledged that the implications are “scary.”
If the data are representative, “this type of reduction in blood pressure control would be expected to have a significant impact on morbidity and mortality, but we also have to think of all the variables that were not tracked and might add to risk,” he said. He named such risk factors as weight gain, diminished exercise, and increased alcohol consumption, which have been cited by others as being exacerbated by the pandemic.
If these lead to more cardiovascular events on a population basis, the timing of these events would be expected to be age dependent.
“If you look at the patients included in this study, about 50% were 65 years of age or older. In a population like this you would expect to see an increase in events sooner rather than later,” said Dr. Wang.
In other words, if the trial is representative, a wave of cardiovascular events might be seen in the most vulnerable patients “within the next few years,” Dr. Yang speculated.
Dr. Chamberlain reports a research grant from EpidStrategies. Dr. Bress and Dr. Yang report no potential financial conflicts of interest.
, if the data from 24 health systems is representative of national trends.
The decline in blood pressure control corresponded with – and might be explained by – a parallel decline in follow-up visits for uncontrolled hypertension from the same data source, according to Alanna M. Chamberlain, PhD, associate professor of epidemiology in the division of quantitative health sciences, Mayo Clinic, Rochester, Minn.
If the data are representative, a wave of cardiovascular (CV) events might be coming.
The study, called BP Track, collated electronic medical data on almost 1.8 million patients with hypertension from 2017 through 2020. Up until the end of 2019 and prior to the pandemic, slightly less than 60% of these patients had blood pressure control, defined as less than 140/90 mm Hg.
While the pre-COVID control rates were already “suboptimal,” a decline began almost immediately when the full force of the COVID-19 pandemic began in March of 2020, said Dr. Chamberlain in reporting the BP Track results at the American Heart Association scientific sessions.
When graphed from the start of the pandemic until the end of 2020, the proportion under control fell 7.2% to a level just above 50%. For the more rigorous target of less than 130/80 mm Hg, the proportion fell 4.6% over the same period of time, leaving only about 25% at that level of control.
Repeat visits for BP control rebounded
The proportion of patients with a repeat office visit within 4 weeks of a diagnosis of uncontrolled hypertension fell even more steeply, reaching a nadir at about the middle of 2020, but it was followed by a partial recovery. The rate was 5% lower by the end of 2020, relative to the prepandemic rate (31.7% vs. 36.7%), but that was 5% higher than the nadir.
A similar phenomenon was observed with several other metrics. For example, there was a steep, immediate fall correlating with the onset of the pandemic in the proportion of patients who achieved at least a 10–mm Hg reduction or a BP under 140/90 mm Hg when treated for hypertension. Again, the nadir in this proportion was reached in about mid-2020 followed by a partial recovery. By the end of 2020, 5.9% fewer patients were achieving 10–mm Hg or better improvement in BP control when treated relative to the prepandemic level (23.8% vs. 29.7%), but this level was almost 10% higher than the nadir.
Data based on electronic medical records
The nearly 1.8 million patient records evaluated in the BP Track study were drawn from the 24 centers participating in the PCORnet Blood Pressure Control Laboratory Surveillance System. Nationally distributed, 18 of the 24 systems were academically affiliated.
When stratified by race, the proportion of Asians meeting the definition of BP control prior to the pandemic was about 5% higher than the overall average, and the proportion in Blacks was more than 5% lower. Whites had rates of blood pressure control very near the average. The relative declines in BP and the proportion of patients with uncontrolled blood pressure who had a repeat visit within 4 weeks during the pandemic were generally parallel across racial groups.
The implications of these data and the role of the COVID-19 pandemic on blood pressure control are “concerning,” according to Adam Bress, PharmD, department of population health sciences, University of Utah, Salt Lake City.
Citing a study published in 2020 that suggested blood pressure control rates in the United States were already declining before the COVID-19 pandemic, he said the COVID-19 epidemic appears to be exacerbating an existing problem. He expressed particular concern for populations who already have low rates of control, such as African Americans.
“The impact of COVID-19 is likely to be disproportionately greater for underserved and minoritized patients,” said Dr. Bress, who was the lead author of a recent article on this specific topic.
The implication of BP Track is that a wave of cardiovascular events will be coming if the data are nationally representative.
“A recent meta-analysis shows that each 5–mm Hg reduction in blood pressure is associated with age-related reductions in CV events,” Dr. Bress said. For those 55 years of age or older, he said the risk reduction is about 10%. Given that the inverse is almost certainly true, he expects diminishing blood pressure control, whether COVID-19-related or not, to translate into increased CV events.
However, there is no guarantee that the BP Track data are representative of the U.S. population, cautioned Eugene Yang, MD, professor in the division of cardiology, University of Washington, Seattle. Even though a large group of patients was included, they were largely drawn from academic centers.
Nevertheless, Dr. Yang, who chairs the Hypertension Working Group of the American College of Cardiology’s Prevention of Cardiovascular Disease Council, acknowledged that the implications are “scary.”
If the data are representative, “this type of reduction in blood pressure control would be expected to have a significant impact on morbidity and mortality, but we also have to think of all the variables that were not tracked and might add to risk,” he said. He named such risk factors as weight gain, diminished exercise, and increased alcohol consumption, which have been cited by others as being exacerbated by the pandemic.
If these lead to more cardiovascular events on a population basis, the timing of these events would be expected to be age dependent.
“If you look at the patients included in this study, about 50% were 65 years of age or older. In a population like this you would expect to see an increase in events sooner rather than later,” said Dr. Wang.
In other words, if the trial is representative, a wave of cardiovascular events might be seen in the most vulnerable patients “within the next few years,” Dr. Yang speculated.
Dr. Chamberlain reports a research grant from EpidStrategies. Dr. Bress and Dr. Yang report no potential financial conflicts of interest.
FROM AHA 2021
Early SAVR tops watchful waiting in severe, asymptomatic aortic stenosis: AVATAR
Better to intervene early with a new valve in patients with severe aortic stenosis (AS) who are asymptomatic, even during exercise, than to wait for the disease to progress and symptoms to emerge before operating, suggests a small, randomized trial that challenges the guidelines.
Of the trial’s 157 patients, all with negative results on stress tests and normal left ventricular (LV) function despite severe AS, those assigned to early surgical aortic valve replacement (SAVR), compared with standard watchful waiting, showed a better-than-50% drop in risk for death or major adverse cardiac events (MACE) over 2-3 years. The benefit appeared driven by fewer hospitalizations for heart failure (HF) and deaths in the early-surgery group.
The findings “advocate for early surgery once aortic stenosis becomes significant and regardless of symptom status,” Marko Banovic, MD, PhD, said during his presentation at the American Heart Association scientific sessions.
Dr. Banovic, from the University of Belgrade Medical School in Serbia, is coprincipal investigator on the trial, called AVATAR (Aortic Valve Replacement vs. Conservative Treatment in Asymptomatic Severe Aortic Stenosis). He is also lead author on the study’s publication in Circulation, timed to coincide with his AHA presentation.
“The AVATAR findings provide additional evidence to help clinicians in guiding their decision when seeing a patient with significant aortic stenosis, normal left ventricular function, overall low surgical risk, and without significant comorbidities,” Dr. Banovic told this news organization.
European and North American Guidelines favor watchful waiting for asymptomatic patients with severe aortic stenosis, with surgery upon development of symptoms or LV dysfunction, observed Victoria Delgado, MD, PhD, Leiden (the Netherlands) University Medical Center, an invited discussant for the AVATAR presentation.
AVATAR does suggest that “early surgery in truly asymptomatic patients with severe aortic stenosis and preserved ejection fraction seems to provide better outcomes as compared to the conservative treatment,” she said. “But I think that the long-term follow-up for potential events, such as valve durability or endocarditis, is still needed.”
The trial has strengths, compared with the recent RECOVERY trial, which also concluded in favor of early SAVR over watchful waiting in patients described as asymptomatic with severe aortic stenosis. Dr. Delgado and other observers, however, have pointed out limitations of that trial, including questions about whether the patients were truly asymptomatic – stress testing wasn›t routinely performed.
In AVATAR, all patients were negative at stress testing, which required them to reach their estimated maximum heart rate, Dr. Banovic noted. As he and his colleagues write, the trial expands on RECOVERY “by providing evidence of the benefit of early surgery in a setting representative of a dilemma in decision making, in truly asymptomatic patients with severe but not critical aortic stenosis and normal LV function.”
A role for TAVR?
Guidelines in general “can be very conservative and lag behind evidence a bit,” Patricia A. Pellikka, MD, Mayo Clinic, Rochester, Minn., who is not associated with AVATAR, said in an interview.
“I think when we see patients clinically, we can advise them that if they don’t have symptoms and they do have severe aortic stenosis,” she said, “they’re likely going to get symptoms within a reasonably short period of time, according to our retrospective databases, and that doing the intervention early may yield better long-term outcomes.”
The results of AVATAR, in which valve replacement consisted only of SAVR, “probably could be extrapolated” to transcatheter aortic valve replacement (TAVR), Dr. Pellikka observed. “Certainly, TAVR is the procedure that patients come asking for. It’s attractive to avoid a major surgery, and it seems very plausible that TAVR would have yielded similar results if that had been a therapy in this trial.”
In practice, patient age and functional status would figure heavily in deciding whether early valve replacement, and which procedure, is appropriate, Dr. Banovic said in an interview. Importantly, the trial’s patients were at low surgical risk and free of major chronic diseases or other important health concerns.
“Frailty and older age are known risk factors for suboptimal recovery” after SAVR, Dr. Banovic said when interviewed. Therefore, frail patients, who were not many in AVATAR, might be “more suitable for TAVR than SAVR, based on the TAVR-vs.-SAVR results in symptomatic AS patients,” he said.
“One might extrapolate experience from AVATAR trial to TAVR, which may lower the bar for TAVR indications,” but that would require more supporting evidence, Dr. Banovic said.
Confirmed asymptomatic
AVATAR, conducted at nine centers in seven countries in the European Union, randomly assigned 157 adults with severe AS by echocardiography and a LV ejection fraction (LVEF) greater than 50% to early SAVR or conservative management. They averaged 67 years in age, and 43% were women.
The trial excluded anyone with dyspnea, syncope, presyncope, angina, or LV dysfunction and anyone with a history of atrial fibrillation or significant cardiac, renal, or lung disease. The cohort’s average Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM) score was 1.7%.
The 78 patients in the early-surgery group “were expected” to have the procedure within 8 weeks of randomization, the published report states; the median time was 55 days. Six of them ultimately did not have the surgery. There was only one periprocedural death, for an operative mortality of 1.4%.
The 79 patients assigned to conservative care were later referred for surgery if they developed symptoms, their LVEF dropped below 50%, or they showed a 0.3-m/sec jump in peak aortic jet velocity at follow-up echocardiography. That occurred with 25 patients a median of 400 days after randomization.
The rate of the primary endpoint – death from any cause, acute myocardial infarction, stroke, or unplanned HF hospitalization – was 16.6% in the early-surgery group and 32.9% for those managed conservatively over a median of 32 months. The hazard ratio by intention-to-treat analysis was 0.46 (95% confidence interval, 0.23-0.90; P = .02). The HR for death from any cause or HF hospitalization was 0.40 (95% CI, 0.19-0.84; P = .013). Any differences in the individual endpoints of death, first HF hospitalizations, thromboembolic complications, or major bleeding were not significant.
If early aortic valve replacement is better for patients like those in AVATAR, some sort of screening for previously unknown severe aortic stenosis may seem attractive for selected populations. “Echocardiography would be the screening test for aortic stenosis, but it’s fairly expensive and therefore has never been advocated as a test to screen everyone,” Dr. Pellikka observed.
“But things are changing,” given innovations such as point-of-care ultrasonography and machine learning, she noted. “Artificial intelligence is progressing in its application to echocardiography, and it’s conceivable that in the future, there might be some abbreviated or screening type of test. But I don’t think we’re quite there yet.”
Dr. Banovic had no conflicts; disclosures for the other authors are in the report. Dr. Delgado disclosed speaker fees from Edwards Lifesciences, Abbott Vascular, Medtronic, Merck, Novartis, and GE Healthcare and unrestricted research grants to her institution from Abbott Vascular, Bayer, Biotronik, Bioventrix, Boston Scientific, Edwards Lifesciences, GE Healthcare, Ionis, and Medtronic. Dr. Pellikka disclosed receiving a research grant from Ultromics and having unspecified modest relationships with GE Healthcare, Lantheus, and OxThera.
A version of this article first appeared on Medscape.com.
Better to intervene early with a new valve in patients with severe aortic stenosis (AS) who are asymptomatic, even during exercise, than to wait for the disease to progress and symptoms to emerge before operating, suggests a small, randomized trial that challenges the guidelines.
Of the trial’s 157 patients, all with negative results on stress tests and normal left ventricular (LV) function despite severe AS, those assigned to early surgical aortic valve replacement (SAVR), compared with standard watchful waiting, showed a better-than-50% drop in risk for death or major adverse cardiac events (MACE) over 2-3 years. The benefit appeared driven by fewer hospitalizations for heart failure (HF) and deaths in the early-surgery group.
The findings “advocate for early surgery once aortic stenosis becomes significant and regardless of symptom status,” Marko Banovic, MD, PhD, said during his presentation at the American Heart Association scientific sessions.
Dr. Banovic, from the University of Belgrade Medical School in Serbia, is coprincipal investigator on the trial, called AVATAR (Aortic Valve Replacement vs. Conservative Treatment in Asymptomatic Severe Aortic Stenosis). He is also lead author on the study’s publication in Circulation, timed to coincide with his AHA presentation.
“The AVATAR findings provide additional evidence to help clinicians in guiding their decision when seeing a patient with significant aortic stenosis, normal left ventricular function, overall low surgical risk, and without significant comorbidities,” Dr. Banovic told this news organization.
European and North American Guidelines favor watchful waiting for asymptomatic patients with severe aortic stenosis, with surgery upon development of symptoms or LV dysfunction, observed Victoria Delgado, MD, PhD, Leiden (the Netherlands) University Medical Center, an invited discussant for the AVATAR presentation.
AVATAR does suggest that “early surgery in truly asymptomatic patients with severe aortic stenosis and preserved ejection fraction seems to provide better outcomes as compared to the conservative treatment,” she said. “But I think that the long-term follow-up for potential events, such as valve durability or endocarditis, is still needed.”
The trial has strengths, compared with the recent RECOVERY trial, which also concluded in favor of early SAVR over watchful waiting in patients described as asymptomatic with severe aortic stenosis. Dr. Delgado and other observers, however, have pointed out limitations of that trial, including questions about whether the patients were truly asymptomatic – stress testing wasn›t routinely performed.
In AVATAR, all patients were negative at stress testing, which required them to reach their estimated maximum heart rate, Dr. Banovic noted. As he and his colleagues write, the trial expands on RECOVERY “by providing evidence of the benefit of early surgery in a setting representative of a dilemma in decision making, in truly asymptomatic patients with severe but not critical aortic stenosis and normal LV function.”
A role for TAVR?
Guidelines in general “can be very conservative and lag behind evidence a bit,” Patricia A. Pellikka, MD, Mayo Clinic, Rochester, Minn., who is not associated with AVATAR, said in an interview.
“I think when we see patients clinically, we can advise them that if they don’t have symptoms and they do have severe aortic stenosis,” she said, “they’re likely going to get symptoms within a reasonably short period of time, according to our retrospective databases, and that doing the intervention early may yield better long-term outcomes.”
The results of AVATAR, in which valve replacement consisted only of SAVR, “probably could be extrapolated” to transcatheter aortic valve replacement (TAVR), Dr. Pellikka observed. “Certainly, TAVR is the procedure that patients come asking for. It’s attractive to avoid a major surgery, and it seems very plausible that TAVR would have yielded similar results if that had been a therapy in this trial.”
In practice, patient age and functional status would figure heavily in deciding whether early valve replacement, and which procedure, is appropriate, Dr. Banovic said in an interview. Importantly, the trial’s patients were at low surgical risk and free of major chronic diseases or other important health concerns.
“Frailty and older age are known risk factors for suboptimal recovery” after SAVR, Dr. Banovic said when interviewed. Therefore, frail patients, who were not many in AVATAR, might be “more suitable for TAVR than SAVR, based on the TAVR-vs.-SAVR results in symptomatic AS patients,” he said.
“One might extrapolate experience from AVATAR trial to TAVR, which may lower the bar for TAVR indications,” but that would require more supporting evidence, Dr. Banovic said.
Confirmed asymptomatic
AVATAR, conducted at nine centers in seven countries in the European Union, randomly assigned 157 adults with severe AS by echocardiography and a LV ejection fraction (LVEF) greater than 50% to early SAVR or conservative management. They averaged 67 years in age, and 43% were women.
The trial excluded anyone with dyspnea, syncope, presyncope, angina, or LV dysfunction and anyone with a history of atrial fibrillation or significant cardiac, renal, or lung disease. The cohort’s average Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM) score was 1.7%.
The 78 patients in the early-surgery group “were expected” to have the procedure within 8 weeks of randomization, the published report states; the median time was 55 days. Six of them ultimately did not have the surgery. There was only one periprocedural death, for an operative mortality of 1.4%.
The 79 patients assigned to conservative care were later referred for surgery if they developed symptoms, their LVEF dropped below 50%, or they showed a 0.3-m/sec jump in peak aortic jet velocity at follow-up echocardiography. That occurred with 25 patients a median of 400 days after randomization.
The rate of the primary endpoint – death from any cause, acute myocardial infarction, stroke, or unplanned HF hospitalization – was 16.6% in the early-surgery group and 32.9% for those managed conservatively over a median of 32 months. The hazard ratio by intention-to-treat analysis was 0.46 (95% confidence interval, 0.23-0.90; P = .02). The HR for death from any cause or HF hospitalization was 0.40 (95% CI, 0.19-0.84; P = .013). Any differences in the individual endpoints of death, first HF hospitalizations, thromboembolic complications, or major bleeding were not significant.
If early aortic valve replacement is better for patients like those in AVATAR, some sort of screening for previously unknown severe aortic stenosis may seem attractive for selected populations. “Echocardiography would be the screening test for aortic stenosis, but it’s fairly expensive and therefore has never been advocated as a test to screen everyone,” Dr. Pellikka observed.
“But things are changing,” given innovations such as point-of-care ultrasonography and machine learning, she noted. “Artificial intelligence is progressing in its application to echocardiography, and it’s conceivable that in the future, there might be some abbreviated or screening type of test. But I don’t think we’re quite there yet.”
Dr. Banovic had no conflicts; disclosures for the other authors are in the report. Dr. Delgado disclosed speaker fees from Edwards Lifesciences, Abbott Vascular, Medtronic, Merck, Novartis, and GE Healthcare and unrestricted research grants to her institution from Abbott Vascular, Bayer, Biotronik, Bioventrix, Boston Scientific, Edwards Lifesciences, GE Healthcare, Ionis, and Medtronic. Dr. Pellikka disclosed receiving a research grant from Ultromics and having unspecified modest relationships with GE Healthcare, Lantheus, and OxThera.
A version of this article first appeared on Medscape.com.
Better to intervene early with a new valve in patients with severe aortic stenosis (AS) who are asymptomatic, even during exercise, than to wait for the disease to progress and symptoms to emerge before operating, suggests a small, randomized trial that challenges the guidelines.
Of the trial’s 157 patients, all with negative results on stress tests and normal left ventricular (LV) function despite severe AS, those assigned to early surgical aortic valve replacement (SAVR), compared with standard watchful waiting, showed a better-than-50% drop in risk for death or major adverse cardiac events (MACE) over 2-3 years. The benefit appeared driven by fewer hospitalizations for heart failure (HF) and deaths in the early-surgery group.
The findings “advocate for early surgery once aortic stenosis becomes significant and regardless of symptom status,” Marko Banovic, MD, PhD, said during his presentation at the American Heart Association scientific sessions.
Dr. Banovic, from the University of Belgrade Medical School in Serbia, is coprincipal investigator on the trial, called AVATAR (Aortic Valve Replacement vs. Conservative Treatment in Asymptomatic Severe Aortic Stenosis). He is also lead author on the study’s publication in Circulation, timed to coincide with his AHA presentation.
“The AVATAR findings provide additional evidence to help clinicians in guiding their decision when seeing a patient with significant aortic stenosis, normal left ventricular function, overall low surgical risk, and without significant comorbidities,” Dr. Banovic told this news organization.
European and North American Guidelines favor watchful waiting for asymptomatic patients with severe aortic stenosis, with surgery upon development of symptoms or LV dysfunction, observed Victoria Delgado, MD, PhD, Leiden (the Netherlands) University Medical Center, an invited discussant for the AVATAR presentation.
AVATAR does suggest that “early surgery in truly asymptomatic patients with severe aortic stenosis and preserved ejection fraction seems to provide better outcomes as compared to the conservative treatment,” she said. “But I think that the long-term follow-up for potential events, such as valve durability or endocarditis, is still needed.”
The trial has strengths, compared with the recent RECOVERY trial, which also concluded in favor of early SAVR over watchful waiting in patients described as asymptomatic with severe aortic stenosis. Dr. Delgado and other observers, however, have pointed out limitations of that trial, including questions about whether the patients were truly asymptomatic – stress testing wasn›t routinely performed.
In AVATAR, all patients were negative at stress testing, which required them to reach their estimated maximum heart rate, Dr. Banovic noted. As he and his colleagues write, the trial expands on RECOVERY “by providing evidence of the benefit of early surgery in a setting representative of a dilemma in decision making, in truly asymptomatic patients with severe but not critical aortic stenosis and normal LV function.”
A role for TAVR?
Guidelines in general “can be very conservative and lag behind evidence a bit,” Patricia A. Pellikka, MD, Mayo Clinic, Rochester, Minn., who is not associated with AVATAR, said in an interview.
“I think when we see patients clinically, we can advise them that if they don’t have symptoms and they do have severe aortic stenosis,” she said, “they’re likely going to get symptoms within a reasonably short period of time, according to our retrospective databases, and that doing the intervention early may yield better long-term outcomes.”
The results of AVATAR, in which valve replacement consisted only of SAVR, “probably could be extrapolated” to transcatheter aortic valve replacement (TAVR), Dr. Pellikka observed. “Certainly, TAVR is the procedure that patients come asking for. It’s attractive to avoid a major surgery, and it seems very plausible that TAVR would have yielded similar results if that had been a therapy in this trial.”
In practice, patient age and functional status would figure heavily in deciding whether early valve replacement, and which procedure, is appropriate, Dr. Banovic said in an interview. Importantly, the trial’s patients were at low surgical risk and free of major chronic diseases or other important health concerns.
“Frailty and older age are known risk factors for suboptimal recovery” after SAVR, Dr. Banovic said when interviewed. Therefore, frail patients, who were not many in AVATAR, might be “more suitable for TAVR than SAVR, based on the TAVR-vs.-SAVR results in symptomatic AS patients,” he said.
“One might extrapolate experience from AVATAR trial to TAVR, which may lower the bar for TAVR indications,” but that would require more supporting evidence, Dr. Banovic said.
Confirmed asymptomatic
AVATAR, conducted at nine centers in seven countries in the European Union, randomly assigned 157 adults with severe AS by echocardiography and a LV ejection fraction (LVEF) greater than 50% to early SAVR or conservative management. They averaged 67 years in age, and 43% were women.
The trial excluded anyone with dyspnea, syncope, presyncope, angina, or LV dysfunction and anyone with a history of atrial fibrillation or significant cardiac, renal, or lung disease. The cohort’s average Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM) score was 1.7%.
The 78 patients in the early-surgery group “were expected” to have the procedure within 8 weeks of randomization, the published report states; the median time was 55 days. Six of them ultimately did not have the surgery. There was only one periprocedural death, for an operative mortality of 1.4%.
The 79 patients assigned to conservative care were later referred for surgery if they developed symptoms, their LVEF dropped below 50%, or they showed a 0.3-m/sec jump in peak aortic jet velocity at follow-up echocardiography. That occurred with 25 patients a median of 400 days after randomization.
The rate of the primary endpoint – death from any cause, acute myocardial infarction, stroke, or unplanned HF hospitalization – was 16.6% in the early-surgery group and 32.9% for those managed conservatively over a median of 32 months. The hazard ratio by intention-to-treat analysis was 0.46 (95% confidence interval, 0.23-0.90; P = .02). The HR for death from any cause or HF hospitalization was 0.40 (95% CI, 0.19-0.84; P = .013). Any differences in the individual endpoints of death, first HF hospitalizations, thromboembolic complications, or major bleeding were not significant.
If early aortic valve replacement is better for patients like those in AVATAR, some sort of screening for previously unknown severe aortic stenosis may seem attractive for selected populations. “Echocardiography would be the screening test for aortic stenosis, but it’s fairly expensive and therefore has never been advocated as a test to screen everyone,” Dr. Pellikka observed.
“But things are changing,” given innovations such as point-of-care ultrasonography and machine learning, she noted. “Artificial intelligence is progressing in its application to echocardiography, and it’s conceivable that in the future, there might be some abbreviated or screening type of test. But I don’t think we’re quite there yet.”
Dr. Banovic had no conflicts; disclosures for the other authors are in the report. Dr. Delgado disclosed speaker fees from Edwards Lifesciences, Abbott Vascular, Medtronic, Merck, Novartis, and GE Healthcare and unrestricted research grants to her institution from Abbott Vascular, Bayer, Biotronik, Bioventrix, Boston Scientific, Edwards Lifesciences, GE Healthcare, Ionis, and Medtronic. Dr. Pellikka disclosed receiving a research grant from Ultromics and having unspecified modest relationships with GE Healthcare, Lantheus, and OxThera.
A version of this article first appeared on Medscape.com.
FROM AHA 2021
Concomitant tricuspid-mitral surgery beneficial but with a trade-off
Tricuspid valve repair at the time of mitral valve surgery reduces tricuspid regurgitation progression, but at the cost of more than a fivefold increase in permanent pacemakers, results of a new Cardiothoracic Surgical Trials Network study show.
The results were presented during the opening late-breaking science session at the American Heart Association scientific sessions and published simultaneously in the New England Journal of Medicine.
Tricuspid regurgitation (TR) is common among patients undergoing mitral valve surgery, and there’s broad agreement to intervene when a patient has severe TR. There’s uncertainty, however, about the management of moderate or less TR during mitral valve surgery, which is reflected in current guidelines on the basis of observational data, explained coprimary investigator James Gammie, MD, codirector and surgical director of the Johns Hopkins Heart and Vascular Institute, Baltimore. As a result, rates of concomitant tricuspid-mitral surgery range from 5% to 75% at various centers.
To help fill the gap, Dr. Gammie and colleagues screened 5,208 patients at 29 centers in the United States, Canada, and Germany undergoing surgery for degenerative mitral regurgitation, and randomly assigned 401 patients (75% male) to mitral valve surgery alone or with tricuspid annuloplasty.
Patients had either moderate TR (37%) or less than moderate TR with a dilated tricuspid annulus of at least 40 mm or at least 21 mm/m2 indexed for body surface area. Importantly, there was a uniform surgical approach using undersized (26-30 mm) rigid nonplanar annuloplasty rings to repair the tricuspid valve, he said.
The study’s primary outcome of treatment failure at 2 years was defined as the composite of death, reoperation for TR, or progression of TR from baseline by 2 grades or severe TR.
The primary endpoint occurred in 10.2% of patients who underwent mitral valve surgery alone and 3.9% who underwent concomitant tricuspid annuloplasty (relative risk, 0.37; 95% confidence interval, 0.16-0.86; P = .02).
The endpoint was driven exclusively by less TR progression in the annuloplasty group, with no TR reoperations in either group, observed Dr. Gammie. At 2 years, just 0.6% of the annuloplasty group had severe TR, compared with 5.6% of the surgery-alone group.
The rate of permanent pacemaker implantations, however, jumped from 2.5% with surgery alone to 14.1% with concomitant tricuspid annuloplasty (rate ratio, 5.75; 95% CI, 2.27-14.60). More than half of pacemakers were placed during the first 2 days after surgery.
There was no between-group difference in 2-year rates of all-cause mortality, major adverse cardiac and cerebrovascular events, readmission, quality of life, or functional status.
Less than moderate TR
In a post hoc analysis stratified by baseline TR severity, treatment failure was significantly less common with surgery plus tricuspid annuloplasty among patients with moderate TR (4.5% vs. 18.1%) but not among those with less than moderate TR and tricuspid annular dilation (3.4% vs. 6.1%).
Although the trial was not powered for the subgroup analysis, “these results call into question the idea that less than moderate TR with annular dilation should be an indication for tricuspid valve repair,” Dr. Gammie told this news organization.
“I did not repair the tricuspid valve in the setting of less than moderate TR before the trial, and my practice won’t change; but it will be based on much better evidence,” he added. “Of course, long-term data from our trial will be of great interest.”
Discussant Joseph Woo, MD, chair of surgery at Stanford (Calif.) University, congratulated the authors on a “landmark trial” that addresses a highly relevant problem without a clear-cut indication.
In the 2020 AHA/American College of Cardiology heart valve disease guideline, tricuspid valve surgery is a class I recommendation when there’s severe TR (stages C and D) and left-sided valve surgery but a class IIa recommendation in patients with progressive TR (stage B) with an annular dilation of at least 40 mm.
“The interesting findings in this study include that moderate TR was only 37% of the enrolled patients, and only 97% of the patients with degenerative MR received a mitral valve repair,” Dr. Woo said. “This level of mitral valve repair is perhaps lower than what we might expect at these centers and lower, certainly, than what the AHA/ACC guidelines recommend for surgery on asymptomatic severe mitral regurgitation.”
Panelist Roxanna Mehran, MD, of Icahn School of Medicine at Mount Sinai in New York said, “What I was struck by is that we, as clinicians, believe that if you fix the mitral valve, maybe the tricuspid regurgitation will improve. And it seems like that is not what’s happening, and I think that’s a big takeaway.”
Session comoderator Joanna Chikwe, MD, head of cardiac surgery at Cedars-Sinai Medical Center, Los Angeles, said, “I think we can all agree that severe tricuspid regurgitation is a disaster for patients, and I think the fact the trial is designed for an additional 5 years’ follow-up will hopefully give us some insights into the clinical impact of severe tricuspid regurgitation.”
For now, “a back of the envelope calculation suggests that, for every 20 patients with moderate tricuspid regurgitation who we repair the tricuspid valve in, we would prevent severe tricuspid valve regurgitation in 1 at the price of pacemakers in 2,” she said.
Dr. Chikwe said in an interview that “transcatheter tricuspid repair is increasingly helping these patients, but if you could avoid it with a technique that doesn’t cause incremental harm beyond, perhaps, the need for pacemakers, then this is helpful data that supports that approach.”
The pacemaker burden is not negligible, she said, but also not surprising to surgeons. “If you look at national practice of mitral-tricuspid surgery, it’s about 15% after that, and it’s simply because the conduction tissue is so close to the tricuspid annulus.”
Pacemaker implantation rates, like those for concomitant tricuspid-mitral surgery, are also highly variable, and in some single-center series only around 2%, Dr. Chikwe said. “So that suggests there are technical approaches that can minimize the pacemaker rate [such as] being extremely careful to avoid suture placement around the area of the conduction tissues.”
For some the trade-off between reduced TR progression and the risk of a permanent pacemaker is worth it. “But the fact that the trial didn’t show a difference in survival, a difference in symptoms or quality of life, might suggest that patients you anticipated were high risk for surgery or didn’t have a longer projected survival aren’t going to benefit from what is quite an aggressive surgical approach,” Dr. Chikwe said.
In an accompanying editorial, Dr. Chikwe and Mario Gaudino, MD, of Weill Cornell Medicine, New York, also point out that the “very dynamic nature of tricuspid regurgitation and wide variability in assessing tricuspid annular dilatation are additional compelling reasons to leave lesser regurgitation alone.”
Julia Grapsa, MD, PhD, Kings College and tricuspid service lead at Guys and St. Thomas NHS, London, also pointed to the need for longer-term follow-up but said increased use of imaging markers is also needed to help pinpoint TR progression in these patients. “For the moment, the results should remind imagers and clinicians to refer patients earlier.”
“As a valvular heart physician, I see more and more patients coming in with significant severe tricuspid regurgitation post–mitral valve surgery and because of the time that’s passed, there’s dysfunction of the right heart, the left heart, and it’s very hard to suggest an operation because they’re at high risk,” she said. “So we’re discussing with these patients whether to do an intervention or medical management.”
“Now, with this study, and the pending longer follow-up by the authors, I’m optimistic that the class II recommendation will be class I in order to help our patients treat tricuspid regurgitation earlier than late,” said Dr. Grapsa, who is also editor-in-chief of JACC: Case Reports.
The study was funded by the National Heart, Lung, and Blood Institute and the German Center for Cardiovascular Research. Dr. Gammie reports a consultant/stockholder relationship with Edwards Lifesciences. Dr. Grapsa reports no conflicts of interest. Dr. Chikwe reports that as coprincipal investigator/study director of NCT 05051033 (an NHLBI-sponsored Cardiothoracic Surgical Trials Network trial), she collaborates with several of the study authors.
A version of this article first appeared on Medscape.com.
Tricuspid valve repair at the time of mitral valve surgery reduces tricuspid regurgitation progression, but at the cost of more than a fivefold increase in permanent pacemakers, results of a new Cardiothoracic Surgical Trials Network study show.
The results were presented during the opening late-breaking science session at the American Heart Association scientific sessions and published simultaneously in the New England Journal of Medicine.
Tricuspid regurgitation (TR) is common among patients undergoing mitral valve surgery, and there’s broad agreement to intervene when a patient has severe TR. There’s uncertainty, however, about the management of moderate or less TR during mitral valve surgery, which is reflected in current guidelines on the basis of observational data, explained coprimary investigator James Gammie, MD, codirector and surgical director of the Johns Hopkins Heart and Vascular Institute, Baltimore. As a result, rates of concomitant tricuspid-mitral surgery range from 5% to 75% at various centers.
To help fill the gap, Dr. Gammie and colleagues screened 5,208 patients at 29 centers in the United States, Canada, and Germany undergoing surgery for degenerative mitral regurgitation, and randomly assigned 401 patients (75% male) to mitral valve surgery alone or with tricuspid annuloplasty.
Patients had either moderate TR (37%) or less than moderate TR with a dilated tricuspid annulus of at least 40 mm or at least 21 mm/m2 indexed for body surface area. Importantly, there was a uniform surgical approach using undersized (26-30 mm) rigid nonplanar annuloplasty rings to repair the tricuspid valve, he said.
The study’s primary outcome of treatment failure at 2 years was defined as the composite of death, reoperation for TR, or progression of TR from baseline by 2 grades or severe TR.
The primary endpoint occurred in 10.2% of patients who underwent mitral valve surgery alone and 3.9% who underwent concomitant tricuspid annuloplasty (relative risk, 0.37; 95% confidence interval, 0.16-0.86; P = .02).
The endpoint was driven exclusively by less TR progression in the annuloplasty group, with no TR reoperations in either group, observed Dr. Gammie. At 2 years, just 0.6% of the annuloplasty group had severe TR, compared with 5.6% of the surgery-alone group.
The rate of permanent pacemaker implantations, however, jumped from 2.5% with surgery alone to 14.1% with concomitant tricuspid annuloplasty (rate ratio, 5.75; 95% CI, 2.27-14.60). More than half of pacemakers were placed during the first 2 days after surgery.
There was no between-group difference in 2-year rates of all-cause mortality, major adverse cardiac and cerebrovascular events, readmission, quality of life, or functional status.
Less than moderate TR
In a post hoc analysis stratified by baseline TR severity, treatment failure was significantly less common with surgery plus tricuspid annuloplasty among patients with moderate TR (4.5% vs. 18.1%) but not among those with less than moderate TR and tricuspid annular dilation (3.4% vs. 6.1%).
Although the trial was not powered for the subgroup analysis, “these results call into question the idea that less than moderate TR with annular dilation should be an indication for tricuspid valve repair,” Dr. Gammie told this news organization.
“I did not repair the tricuspid valve in the setting of less than moderate TR before the trial, and my practice won’t change; but it will be based on much better evidence,” he added. “Of course, long-term data from our trial will be of great interest.”
Discussant Joseph Woo, MD, chair of surgery at Stanford (Calif.) University, congratulated the authors on a “landmark trial” that addresses a highly relevant problem without a clear-cut indication.
In the 2020 AHA/American College of Cardiology heart valve disease guideline, tricuspid valve surgery is a class I recommendation when there’s severe TR (stages C and D) and left-sided valve surgery but a class IIa recommendation in patients with progressive TR (stage B) with an annular dilation of at least 40 mm.
“The interesting findings in this study include that moderate TR was only 37% of the enrolled patients, and only 97% of the patients with degenerative MR received a mitral valve repair,” Dr. Woo said. “This level of mitral valve repair is perhaps lower than what we might expect at these centers and lower, certainly, than what the AHA/ACC guidelines recommend for surgery on asymptomatic severe mitral regurgitation.”
Panelist Roxanna Mehran, MD, of Icahn School of Medicine at Mount Sinai in New York said, “What I was struck by is that we, as clinicians, believe that if you fix the mitral valve, maybe the tricuspid regurgitation will improve. And it seems like that is not what’s happening, and I think that’s a big takeaway.”
Session comoderator Joanna Chikwe, MD, head of cardiac surgery at Cedars-Sinai Medical Center, Los Angeles, said, “I think we can all agree that severe tricuspid regurgitation is a disaster for patients, and I think the fact the trial is designed for an additional 5 years’ follow-up will hopefully give us some insights into the clinical impact of severe tricuspid regurgitation.”
For now, “a back of the envelope calculation suggests that, for every 20 patients with moderate tricuspid regurgitation who we repair the tricuspid valve in, we would prevent severe tricuspid valve regurgitation in 1 at the price of pacemakers in 2,” she said.
Dr. Chikwe said in an interview that “transcatheter tricuspid repair is increasingly helping these patients, but if you could avoid it with a technique that doesn’t cause incremental harm beyond, perhaps, the need for pacemakers, then this is helpful data that supports that approach.”
The pacemaker burden is not negligible, she said, but also not surprising to surgeons. “If you look at national practice of mitral-tricuspid surgery, it’s about 15% after that, and it’s simply because the conduction tissue is so close to the tricuspid annulus.”
Pacemaker implantation rates, like those for concomitant tricuspid-mitral surgery, are also highly variable, and in some single-center series only around 2%, Dr. Chikwe said. “So that suggests there are technical approaches that can minimize the pacemaker rate [such as] being extremely careful to avoid suture placement around the area of the conduction tissues.”
For some the trade-off between reduced TR progression and the risk of a permanent pacemaker is worth it. “But the fact that the trial didn’t show a difference in survival, a difference in symptoms or quality of life, might suggest that patients you anticipated were high risk for surgery or didn’t have a longer projected survival aren’t going to benefit from what is quite an aggressive surgical approach,” Dr. Chikwe said.
In an accompanying editorial, Dr. Chikwe and Mario Gaudino, MD, of Weill Cornell Medicine, New York, also point out that the “very dynamic nature of tricuspid regurgitation and wide variability in assessing tricuspid annular dilatation are additional compelling reasons to leave lesser regurgitation alone.”
Julia Grapsa, MD, PhD, Kings College and tricuspid service lead at Guys and St. Thomas NHS, London, also pointed to the need for longer-term follow-up but said increased use of imaging markers is also needed to help pinpoint TR progression in these patients. “For the moment, the results should remind imagers and clinicians to refer patients earlier.”
“As a valvular heart physician, I see more and more patients coming in with significant severe tricuspid regurgitation post–mitral valve surgery and because of the time that’s passed, there’s dysfunction of the right heart, the left heart, and it’s very hard to suggest an operation because they’re at high risk,” she said. “So we’re discussing with these patients whether to do an intervention or medical management.”
“Now, with this study, and the pending longer follow-up by the authors, I’m optimistic that the class II recommendation will be class I in order to help our patients treat tricuspid regurgitation earlier than late,” said Dr. Grapsa, who is also editor-in-chief of JACC: Case Reports.
The study was funded by the National Heart, Lung, and Blood Institute and the German Center for Cardiovascular Research. Dr. Gammie reports a consultant/stockholder relationship with Edwards Lifesciences. Dr. Grapsa reports no conflicts of interest. Dr. Chikwe reports that as coprincipal investigator/study director of NCT 05051033 (an NHLBI-sponsored Cardiothoracic Surgical Trials Network trial), she collaborates with several of the study authors.
A version of this article first appeared on Medscape.com.
Tricuspid valve repair at the time of mitral valve surgery reduces tricuspid regurgitation progression, but at the cost of more than a fivefold increase in permanent pacemakers, results of a new Cardiothoracic Surgical Trials Network study show.
The results were presented during the opening late-breaking science session at the American Heart Association scientific sessions and published simultaneously in the New England Journal of Medicine.
Tricuspid regurgitation (TR) is common among patients undergoing mitral valve surgery, and there’s broad agreement to intervene when a patient has severe TR. There’s uncertainty, however, about the management of moderate or less TR during mitral valve surgery, which is reflected in current guidelines on the basis of observational data, explained coprimary investigator James Gammie, MD, codirector and surgical director of the Johns Hopkins Heart and Vascular Institute, Baltimore. As a result, rates of concomitant tricuspid-mitral surgery range from 5% to 75% at various centers.
To help fill the gap, Dr. Gammie and colleagues screened 5,208 patients at 29 centers in the United States, Canada, and Germany undergoing surgery for degenerative mitral regurgitation, and randomly assigned 401 patients (75% male) to mitral valve surgery alone or with tricuspid annuloplasty.
Patients had either moderate TR (37%) or less than moderate TR with a dilated tricuspid annulus of at least 40 mm or at least 21 mm/m2 indexed for body surface area. Importantly, there was a uniform surgical approach using undersized (26-30 mm) rigid nonplanar annuloplasty rings to repair the tricuspid valve, he said.
The study’s primary outcome of treatment failure at 2 years was defined as the composite of death, reoperation for TR, or progression of TR from baseline by 2 grades or severe TR.
The primary endpoint occurred in 10.2% of patients who underwent mitral valve surgery alone and 3.9% who underwent concomitant tricuspid annuloplasty (relative risk, 0.37; 95% confidence interval, 0.16-0.86; P = .02).
The endpoint was driven exclusively by less TR progression in the annuloplasty group, with no TR reoperations in either group, observed Dr. Gammie. At 2 years, just 0.6% of the annuloplasty group had severe TR, compared with 5.6% of the surgery-alone group.
The rate of permanent pacemaker implantations, however, jumped from 2.5% with surgery alone to 14.1% with concomitant tricuspid annuloplasty (rate ratio, 5.75; 95% CI, 2.27-14.60). More than half of pacemakers were placed during the first 2 days after surgery.
There was no between-group difference in 2-year rates of all-cause mortality, major adverse cardiac and cerebrovascular events, readmission, quality of life, or functional status.
Less than moderate TR
In a post hoc analysis stratified by baseline TR severity, treatment failure was significantly less common with surgery plus tricuspid annuloplasty among patients with moderate TR (4.5% vs. 18.1%) but not among those with less than moderate TR and tricuspid annular dilation (3.4% vs. 6.1%).
Although the trial was not powered for the subgroup analysis, “these results call into question the idea that less than moderate TR with annular dilation should be an indication for tricuspid valve repair,” Dr. Gammie told this news organization.
“I did not repair the tricuspid valve in the setting of less than moderate TR before the trial, and my practice won’t change; but it will be based on much better evidence,” he added. “Of course, long-term data from our trial will be of great interest.”
Discussant Joseph Woo, MD, chair of surgery at Stanford (Calif.) University, congratulated the authors on a “landmark trial” that addresses a highly relevant problem without a clear-cut indication.
In the 2020 AHA/American College of Cardiology heart valve disease guideline, tricuspid valve surgery is a class I recommendation when there’s severe TR (stages C and D) and left-sided valve surgery but a class IIa recommendation in patients with progressive TR (stage B) with an annular dilation of at least 40 mm.
“The interesting findings in this study include that moderate TR was only 37% of the enrolled patients, and only 97% of the patients with degenerative MR received a mitral valve repair,” Dr. Woo said. “This level of mitral valve repair is perhaps lower than what we might expect at these centers and lower, certainly, than what the AHA/ACC guidelines recommend for surgery on asymptomatic severe mitral regurgitation.”
Panelist Roxanna Mehran, MD, of Icahn School of Medicine at Mount Sinai in New York said, “What I was struck by is that we, as clinicians, believe that if you fix the mitral valve, maybe the tricuspid regurgitation will improve. And it seems like that is not what’s happening, and I think that’s a big takeaway.”
Session comoderator Joanna Chikwe, MD, head of cardiac surgery at Cedars-Sinai Medical Center, Los Angeles, said, “I think we can all agree that severe tricuspid regurgitation is a disaster for patients, and I think the fact the trial is designed for an additional 5 years’ follow-up will hopefully give us some insights into the clinical impact of severe tricuspid regurgitation.”
For now, “a back of the envelope calculation suggests that, for every 20 patients with moderate tricuspid regurgitation who we repair the tricuspid valve in, we would prevent severe tricuspid valve regurgitation in 1 at the price of pacemakers in 2,” she said.
Dr. Chikwe said in an interview that “transcatheter tricuspid repair is increasingly helping these patients, but if you could avoid it with a technique that doesn’t cause incremental harm beyond, perhaps, the need for pacemakers, then this is helpful data that supports that approach.”
The pacemaker burden is not negligible, she said, but also not surprising to surgeons. “If you look at national practice of mitral-tricuspid surgery, it’s about 15% after that, and it’s simply because the conduction tissue is so close to the tricuspid annulus.”
Pacemaker implantation rates, like those for concomitant tricuspid-mitral surgery, are also highly variable, and in some single-center series only around 2%, Dr. Chikwe said. “So that suggests there are technical approaches that can minimize the pacemaker rate [such as] being extremely careful to avoid suture placement around the area of the conduction tissues.”
For some the trade-off between reduced TR progression and the risk of a permanent pacemaker is worth it. “But the fact that the trial didn’t show a difference in survival, a difference in symptoms or quality of life, might suggest that patients you anticipated were high risk for surgery or didn’t have a longer projected survival aren’t going to benefit from what is quite an aggressive surgical approach,” Dr. Chikwe said.
In an accompanying editorial, Dr. Chikwe and Mario Gaudino, MD, of Weill Cornell Medicine, New York, also point out that the “very dynamic nature of tricuspid regurgitation and wide variability in assessing tricuspid annular dilatation are additional compelling reasons to leave lesser regurgitation alone.”
Julia Grapsa, MD, PhD, Kings College and tricuspid service lead at Guys and St. Thomas NHS, London, also pointed to the need for longer-term follow-up but said increased use of imaging markers is also needed to help pinpoint TR progression in these patients. “For the moment, the results should remind imagers and clinicians to refer patients earlier.”
“As a valvular heart physician, I see more and more patients coming in with significant severe tricuspid regurgitation post–mitral valve surgery and because of the time that’s passed, there’s dysfunction of the right heart, the left heart, and it’s very hard to suggest an operation because they’re at high risk,” she said. “So we’re discussing with these patients whether to do an intervention or medical management.”
“Now, with this study, and the pending longer follow-up by the authors, I’m optimistic that the class II recommendation will be class I in order to help our patients treat tricuspid regurgitation earlier than late,” said Dr. Grapsa, who is also editor-in-chief of JACC: Case Reports.
The study was funded by the National Heart, Lung, and Blood Institute and the German Center for Cardiovascular Research. Dr. Gammie reports a consultant/stockholder relationship with Edwards Lifesciences. Dr. Grapsa reports no conflicts of interest. Dr. Chikwe reports that as coprincipal investigator/study director of NCT 05051033 (an NHLBI-sponsored Cardiothoracic Surgical Trials Network trial), she collaborates with several of the study authors.
A version of this article first appeared on Medscape.com.
FROM AHA 2021
Direct comparison shows differing strengths for left atrial closure devices
On the basis of outcomes, there was no clear winner from a trial that directly compared two modern devices used in patients undergoing percutaneous left atrial appendage (LAA) closure.
But the devices were not interchangeable for rates of complications or leaks, according to results of the open-label SWISS APERO trial, which compared the Amplatzer Amulet to the Watchman FLX device at eight participating centers in Europe.
At 45 days, the overall rates of leaks and the clinical outcomes in the two randomized groups were not significantly different, but there were differences in secondary endpoints, such as rates of peridevice leak (PDL), which were lower in the Amulet device group, and procedural complications, which were higher, Roberto Galea, MD, reported at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando.
LAA closure devices were developed as an alternative to oral anticoagulation in patients with nonvalvular atrial fibrillation. Although a similar comparison of LAA closure devices, called Amulet IDE, was recently published, that trial compared Amulet to Watchman 2.5, an earlier generation device.
Started later, SWISS APERO was also a planned comparison of Amulet and the Watchman 2.5, but the comparison switched to the Watchman FLX, when it was released in March of 2019.
First randomized comparison with Watchman FLX
“This is the first multicenter randomized controlled trial to include the Watchman FLX,” said Dr. Galea, a clinical investigator in the department of cardiology, Bern (Switzerland) University Hospital. He noted that Watchman FLX included some adjustments in design with the potential to reduce leak rates.
After preprocedural transesophageal echocardiography confirmed that patients had suitable anatomy to receive either device, the 221 patients who qualified for SWISS APERO were randomized. The primary endpoint was a composite of a justified crossover to a device other than the one to which they were assigned or residual patency detected by coronary computed tomography angiography (CCTA) at 45 days.
The primary endpoint was reached by 67.6% of patients randomized to the Amulet device and 70% of those randomized to Watchman Flex, a statistically nonsignificant difference (P = .71).
Because only one patient in the Amulet group and none in the Watchman group had a justified crossover to a nonrandomized device, most of the differences in the 45-day CCTA involved patency, defined as LAA density of at least 100 Hounsfield units. While the proportion of patients with leaks was similar, the types of leaks, which were stratified by underlying leak mechanism into PDL, mixed leaks (including incomplete side sealing), intradevice leaks, and leaks of unclear origin, were different.
Peridevice leaks twofold greater with Watchman
Those randomized to the Watchman device were more than twice as likely to have PDL (27.5% vs. 13.7%; P = .02), although no visible leak exceeded 5 mm in size. They were also more likely to have mixed leaks (14% vs. 3.8%; P = .01) and patency with no visible leak (21.0% vs. 9.5%; P = .02). There were also more device-related thrombi in the Watchman group even though the difference did not reach statistical significance (9.9% vs. 3.7%; P = .08).
Intradevice leaks (44.8% vs. 23.0%; P = .001) were the only type of patency significantly more common among patients randomized to Amulet, but the difference was relatively large. In addition, procedural complications of any type (32.4% vs. 19.1%; P = .023) were higher in the Amulet group. Most of these involved non–clinically relevant pericardial effusions, Dr. Galea said at the meeting, sponsored by the Cardiovascular Research Foundation.
The proportion of patients with adverse outcomes by 45 days was similar, but the types of complications differed. Of the six deaths, two occurred in the Amulet group as a result of periprocedural complications (one stemming from an air embolism and the other from a series of events following pericardial effusion). Three of the four deaths in the Watchman group were due to fatal bleeding. The fourth was a sudden death that occurred 30 days after the procedure.
Amulet IDE trial generates similar data
The much larger Amulet IDE trial, which compared Amulet to the Watchman 2.5 device, produced generally similar results. Again, the proportion of patients reaching the composite primary endpoints was similar.
The primary safety endpoint, which included death and major bleeding within 12 months of randomization, occurred in 14.5% and 14.7% of the Amulet and Watchman patients, respectively (P < .001 for noninferiority). The primary efficacy endpoint, which included stroke or systemic embolism within 18 months of randomization, occurred in 2.8% of patients in both groups.
As in SWISS APERO, the 1,878-patient Amulet IDE trial showed that the devices are similarly effective and safe but not necessarily interchangeable. Ultimately, the rate of LAA occlusion was higher for Amulet than the older generation Watchman (98.9% vs. 96.8%; P = .003) but procedural complication occurred more frequently among those randomized to the Amulet device (4.5% vs. 2.5%).
“The closure mechanisms are not the same, which might explain why we see differences in some secondary outcomes even when they perform similarly on the primary outcomes,” said Dhanunjaya R. Lakkireddy, MD, executive medical director, Kansas City (Kansas) Heart Rhythm Institute.
The lead investigator of the Amulet IDE trial, Dr. Lakkireddy was referring to both the AMULET IDE and the SWISS APERO study when he said that the currently available data do not allow one device to be considered superior to the other. He did suggest that differences between devices might still be considered meaningful in specific clinical situations or to specific clinicians.
Without studies to show objective differences, Dr. Lakkireddy suggested that training and experience is probably the most important variable in achieving treatment goals. “Operator comfort is certainly important,” he said.
Dr. Galea reports no significant financial relationships. The investigator-initiated study received funding from Abbott, the manufacturer of the Amulet device. Dr. Lakkireddy has financial relationships with Abbott, AltaThera, Medtronic, Biotronik, and Boston Scientific, which makes the Watchman device.
On the basis of outcomes, there was no clear winner from a trial that directly compared two modern devices used in patients undergoing percutaneous left atrial appendage (LAA) closure.
But the devices were not interchangeable for rates of complications or leaks, according to results of the open-label SWISS APERO trial, which compared the Amplatzer Amulet to the Watchman FLX device at eight participating centers in Europe.
At 45 days, the overall rates of leaks and the clinical outcomes in the two randomized groups were not significantly different, but there were differences in secondary endpoints, such as rates of peridevice leak (PDL), which were lower in the Amulet device group, and procedural complications, which were higher, Roberto Galea, MD, reported at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando.
LAA closure devices were developed as an alternative to oral anticoagulation in patients with nonvalvular atrial fibrillation. Although a similar comparison of LAA closure devices, called Amulet IDE, was recently published, that trial compared Amulet to Watchman 2.5, an earlier generation device.
Started later, SWISS APERO was also a planned comparison of Amulet and the Watchman 2.5, but the comparison switched to the Watchman FLX, when it was released in March of 2019.
First randomized comparison with Watchman FLX
“This is the first multicenter randomized controlled trial to include the Watchman FLX,” said Dr. Galea, a clinical investigator in the department of cardiology, Bern (Switzerland) University Hospital. He noted that Watchman FLX included some adjustments in design with the potential to reduce leak rates.
After preprocedural transesophageal echocardiography confirmed that patients had suitable anatomy to receive either device, the 221 patients who qualified for SWISS APERO were randomized. The primary endpoint was a composite of a justified crossover to a device other than the one to which they were assigned or residual patency detected by coronary computed tomography angiography (CCTA) at 45 days.
The primary endpoint was reached by 67.6% of patients randomized to the Amulet device and 70% of those randomized to Watchman Flex, a statistically nonsignificant difference (P = .71).
Because only one patient in the Amulet group and none in the Watchman group had a justified crossover to a nonrandomized device, most of the differences in the 45-day CCTA involved patency, defined as LAA density of at least 100 Hounsfield units. While the proportion of patients with leaks was similar, the types of leaks, which were stratified by underlying leak mechanism into PDL, mixed leaks (including incomplete side sealing), intradevice leaks, and leaks of unclear origin, were different.
Peridevice leaks twofold greater with Watchman
Those randomized to the Watchman device were more than twice as likely to have PDL (27.5% vs. 13.7%; P = .02), although no visible leak exceeded 5 mm in size. They were also more likely to have mixed leaks (14% vs. 3.8%; P = .01) and patency with no visible leak (21.0% vs. 9.5%; P = .02). There were also more device-related thrombi in the Watchman group even though the difference did not reach statistical significance (9.9% vs. 3.7%; P = .08).
Intradevice leaks (44.8% vs. 23.0%; P = .001) were the only type of patency significantly more common among patients randomized to Amulet, but the difference was relatively large. In addition, procedural complications of any type (32.4% vs. 19.1%; P = .023) were higher in the Amulet group. Most of these involved non–clinically relevant pericardial effusions, Dr. Galea said at the meeting, sponsored by the Cardiovascular Research Foundation.
The proportion of patients with adverse outcomes by 45 days was similar, but the types of complications differed. Of the six deaths, two occurred in the Amulet group as a result of periprocedural complications (one stemming from an air embolism and the other from a series of events following pericardial effusion). Three of the four deaths in the Watchman group were due to fatal bleeding. The fourth was a sudden death that occurred 30 days after the procedure.
Amulet IDE trial generates similar data
The much larger Amulet IDE trial, which compared Amulet to the Watchman 2.5 device, produced generally similar results. Again, the proportion of patients reaching the composite primary endpoints was similar.
The primary safety endpoint, which included death and major bleeding within 12 months of randomization, occurred in 14.5% and 14.7% of the Amulet and Watchman patients, respectively (P < .001 for noninferiority). The primary efficacy endpoint, which included stroke or systemic embolism within 18 months of randomization, occurred in 2.8% of patients in both groups.
As in SWISS APERO, the 1,878-patient Amulet IDE trial showed that the devices are similarly effective and safe but not necessarily interchangeable. Ultimately, the rate of LAA occlusion was higher for Amulet than the older generation Watchman (98.9% vs. 96.8%; P = .003) but procedural complication occurred more frequently among those randomized to the Amulet device (4.5% vs. 2.5%).
“The closure mechanisms are not the same, which might explain why we see differences in some secondary outcomes even when they perform similarly on the primary outcomes,” said Dhanunjaya R. Lakkireddy, MD, executive medical director, Kansas City (Kansas) Heart Rhythm Institute.
The lead investigator of the Amulet IDE trial, Dr. Lakkireddy was referring to both the AMULET IDE and the SWISS APERO study when he said that the currently available data do not allow one device to be considered superior to the other. He did suggest that differences between devices might still be considered meaningful in specific clinical situations or to specific clinicians.
Without studies to show objective differences, Dr. Lakkireddy suggested that training and experience is probably the most important variable in achieving treatment goals. “Operator comfort is certainly important,” he said.
Dr. Galea reports no significant financial relationships. The investigator-initiated study received funding from Abbott, the manufacturer of the Amulet device. Dr. Lakkireddy has financial relationships with Abbott, AltaThera, Medtronic, Biotronik, and Boston Scientific, which makes the Watchman device.
On the basis of outcomes, there was no clear winner from a trial that directly compared two modern devices used in patients undergoing percutaneous left atrial appendage (LAA) closure.
But the devices were not interchangeable for rates of complications or leaks, according to results of the open-label SWISS APERO trial, which compared the Amplatzer Amulet to the Watchman FLX device at eight participating centers in Europe.
At 45 days, the overall rates of leaks and the clinical outcomes in the two randomized groups were not significantly different, but there were differences in secondary endpoints, such as rates of peridevice leak (PDL), which were lower in the Amulet device group, and procedural complications, which were higher, Roberto Galea, MD, reported at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando.
LAA closure devices were developed as an alternative to oral anticoagulation in patients with nonvalvular atrial fibrillation. Although a similar comparison of LAA closure devices, called Amulet IDE, was recently published, that trial compared Amulet to Watchman 2.5, an earlier generation device.
Started later, SWISS APERO was also a planned comparison of Amulet and the Watchman 2.5, but the comparison switched to the Watchman FLX, when it was released in March of 2019.
First randomized comparison with Watchman FLX
“This is the first multicenter randomized controlled trial to include the Watchman FLX,” said Dr. Galea, a clinical investigator in the department of cardiology, Bern (Switzerland) University Hospital. He noted that Watchman FLX included some adjustments in design with the potential to reduce leak rates.
After preprocedural transesophageal echocardiography confirmed that patients had suitable anatomy to receive either device, the 221 patients who qualified for SWISS APERO were randomized. The primary endpoint was a composite of a justified crossover to a device other than the one to which they were assigned or residual patency detected by coronary computed tomography angiography (CCTA) at 45 days.
The primary endpoint was reached by 67.6% of patients randomized to the Amulet device and 70% of those randomized to Watchman Flex, a statistically nonsignificant difference (P = .71).
Because only one patient in the Amulet group and none in the Watchman group had a justified crossover to a nonrandomized device, most of the differences in the 45-day CCTA involved patency, defined as LAA density of at least 100 Hounsfield units. While the proportion of patients with leaks was similar, the types of leaks, which were stratified by underlying leak mechanism into PDL, mixed leaks (including incomplete side sealing), intradevice leaks, and leaks of unclear origin, were different.
Peridevice leaks twofold greater with Watchman
Those randomized to the Watchman device were more than twice as likely to have PDL (27.5% vs. 13.7%; P = .02), although no visible leak exceeded 5 mm in size. They were also more likely to have mixed leaks (14% vs. 3.8%; P = .01) and patency with no visible leak (21.0% vs. 9.5%; P = .02). There were also more device-related thrombi in the Watchman group even though the difference did not reach statistical significance (9.9% vs. 3.7%; P = .08).
Intradevice leaks (44.8% vs. 23.0%; P = .001) were the only type of patency significantly more common among patients randomized to Amulet, but the difference was relatively large. In addition, procedural complications of any type (32.4% vs. 19.1%; P = .023) were higher in the Amulet group. Most of these involved non–clinically relevant pericardial effusions, Dr. Galea said at the meeting, sponsored by the Cardiovascular Research Foundation.
The proportion of patients with adverse outcomes by 45 days was similar, but the types of complications differed. Of the six deaths, two occurred in the Amulet group as a result of periprocedural complications (one stemming from an air embolism and the other from a series of events following pericardial effusion). Three of the four deaths in the Watchman group were due to fatal bleeding. The fourth was a sudden death that occurred 30 days after the procedure.
Amulet IDE trial generates similar data
The much larger Amulet IDE trial, which compared Amulet to the Watchman 2.5 device, produced generally similar results. Again, the proportion of patients reaching the composite primary endpoints was similar.
The primary safety endpoint, which included death and major bleeding within 12 months of randomization, occurred in 14.5% and 14.7% of the Amulet and Watchman patients, respectively (P < .001 for noninferiority). The primary efficacy endpoint, which included stroke or systemic embolism within 18 months of randomization, occurred in 2.8% of patients in both groups.
As in SWISS APERO, the 1,878-patient Amulet IDE trial showed that the devices are similarly effective and safe but not necessarily interchangeable. Ultimately, the rate of LAA occlusion was higher for Amulet than the older generation Watchman (98.9% vs. 96.8%; P = .003) but procedural complication occurred more frequently among those randomized to the Amulet device (4.5% vs. 2.5%).
“The closure mechanisms are not the same, which might explain why we see differences in some secondary outcomes even when they perform similarly on the primary outcomes,” said Dhanunjaya R. Lakkireddy, MD, executive medical director, Kansas City (Kansas) Heart Rhythm Institute.
The lead investigator of the Amulet IDE trial, Dr. Lakkireddy was referring to both the AMULET IDE and the SWISS APERO study when he said that the currently available data do not allow one device to be considered superior to the other. He did suggest that differences between devices might still be considered meaningful in specific clinical situations or to specific clinicians.
Without studies to show objective differences, Dr. Lakkireddy suggested that training and experience is probably the most important variable in achieving treatment goals. “Operator comfort is certainly important,” he said.
Dr. Galea reports no significant financial relationships. The investigator-initiated study received funding from Abbott, the manufacturer of the Amulet device. Dr. Lakkireddy has financial relationships with Abbott, AltaThera, Medtronic, Biotronik, and Boston Scientific, which makes the Watchman device.
FROM TCT 2021
Fully endovascular mitral valve replacement a limited success in feasibility study
It remains early days for transcatheter mitral-valve replacement (TMVR) as a minimally invasive way to treat severe, mitral regurgitation (MR), but it’s even earlier days for TMVR as an endovascular procedure. Most of the technique’s limited experience with a dedicated mitral prosthesis has involved transapical delivery.
But now a 15-patient study of transfemoral, transeptal TMVR – with a prosthesis designed for the mitral position and previously tested only transapically – has shown good 30-day results in that MR was essentially abolished with virtually no paravalvular leakage.
Nor were there adverse clinical events such as death, stroke, reintervention, or new need for a pacemaker in any of the high-surgical-risk patients with MR in this feasibility study of the transfemoral Intrepid TMVR System (Medtronic). Implantation failed, however, in one patient who then received a surgical valve via sternotomy.
The current cohort is part of a larger ongoing trial that will track whether patients implanted transfemorally with the Intrepid also show reverse remodeling and good clinical outcomes over at least a year. That study, called APOLLO, is one of several exploring dedicated TMVR valves from different companies, with names like SUMMIT, MISCEND, and TIARA-2.
Currently, TMVR is approved in the United States only using one device designed for the aortic position and only for treating failed surgical mitral bioprostheses in high-risk patients.
If the Intrepid transfemoral system has an Achilles’ heel, at least in the current iteration, it might be its 35 F catheter delivery system that requires surgical access to the femoral vein. Seven of the patients in the small series experienced major bleeding events, including six at the femoral access site, listed as major vascular complications.
Overall, the study’s patients “were extremely sick with a lot of comorbidity. A lot of them had atrial fibrillation, a lot of them were on anticoagulation to start with,” observed Firas Zahr, MD, Oregon Health & Science University, Portland, as part of his presentation of the study at Transcatheter Cardiovascular Therapeutics (TCT) 2021, held virtually as well as onsite in Orlando, Florida.
All had moderate-to-severe, usually primary MR; two thirds of the cohort had been in NYHA class III or IV at baseline, and 40% had been hospitalized for heart failure within the past year. Eight had a history of cardiovascular surgery, and eight had diabetes. Their mean Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM) score was 4.7, Dr. Zahr reported.
“At 30 days, there was a significant improvement in their heart failure classification; the vast majority of the patients were [NYHA] class I and class II,” said Dr. Zahr, who is also lead author on the study’s Nov. 6 publication in JACC: Cardiovascular Interventions.
Observers of the study at TCT 2021 seemed enthusiastic about the study’s results but recognized that TMVR in its current form still has formidable limitations.
“This is clearly an exciting look into the future and very reassuring to a degree, aside from the complications, which are somewhat expected as we go with 30-plus French devices,” Rajiv Tayal, MD, MPH, said at a press conference on the Intrepid study held before Dr. Zahr’s formal presentation. Dr. Tayal is an interventional cardiologist with Valley Health System, Ridgewood, New Jersey, and New York Medical College, Valhalla.
“I think we’ve all learned that transapical [access] is just not a viable procedure for a lot of these patients, and so we’ve got to get to transfemoral,” Susheel K. Kodali, MD, interventional cardiologist at New York-Presbyterian/Columbia University Irving Medical Center, said at the same forum.
A 35 F device “is going to be too big,” he said. However, “it is the first step to iterate to a smaller device.” Dr. Kodali said his center contributed a patient to the study, and he is listed as a coauthor on the publication.
The delivery system’s large profile is only part of the vascular complication issue. Not only did the procedure require surgical cutdown for venous access, but “we were fairly aggressive in anticoagulating these patients with the fear of thrombus formation,” Dr. Zahr said in the discussion following his presentation.
“A postprocedure anticoagulation regimen is recommended within the protocol, but ultimate therapy was left to the discretion of the treating site physician,” the published report states, noting that all 14 patients with successful TMVR were discharged on warfarin. They included 12 who were also put on a single antiplatelet and one given dual antiplatelet therapy on top of the oral anticoagulant.
“One thing that we learned is that we probably should standardize our approach to perioperative anticoagulation,” Dr. Zahr observed. Also, a 29 F sheath for the system is in the works, “and we’re hoping that with smaller sheath size, and hopefully going even to percutaneous, might have an impact on lowering the vascular complications.”
Explanations for the “higher-than-expected vascular complication rate” remains somewhat unclear, agreed an editorial accompanying the study’s publication, “but may include a learning curve with the system, the large introducer sheath, the need for surgical cutdown, and postprocedural anticoagulation.”
For trans-septal TMVR to become a default approach, “venous access will need to be achieved percutaneously and vascular complications need to be infrequent,” contends the editorial, with lead author Mohamad Alkhouli, MD, Mayo Clinic, Rochester, Minn.
“These data provide a glimpse into the future of TMVR. The excellent short-term safety and effectiveness of this still very early-stage procedure represent a major step forward in the field,” they write.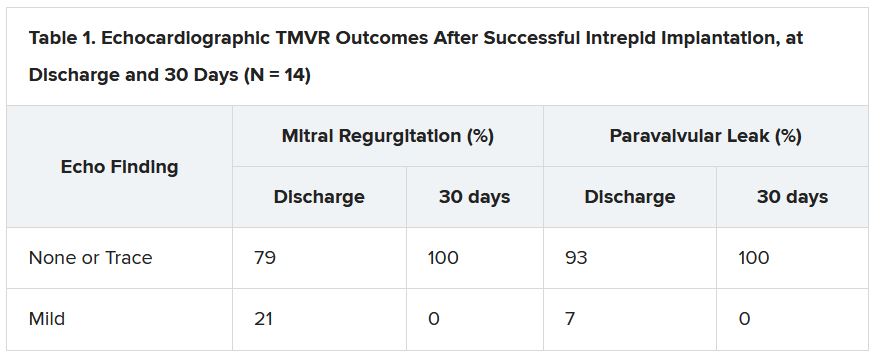
“The main question that the Intrepid early feasibility data raise is whether transfemoral, trans-septal TMVR will evolve to become the preferred strategy over transapical TMVR,” as occurred with transcatheter aortic-valve replacement (TAVR), the editorial states. “The answer is likely yes, but a few matters specific to trans-septal route will need be addressed first.”
Among those matters: The 35 F catheter leaves behind a considerable atrial septal defect (ASD). At operator discretion in this series, 11 patients received an ASD closure device.
None of the remaining four patients “developed significant heart failure or right ventricular dysfunction,” Dr. Zahr observed. “So, it seems like those patients who had their ASD left open tolerated it fairly well, at least until 30 days.”
But “we still need to learn what to do with those ASDs,” he said. “What is an acceptable residual shunt and what is an acceptable ASD size is to be determined.”
In general, the editorial notes, “the TMVR population has a high prevalence of cardiomyopathy, and a large residual iatrogenic ASD may lead to worsening volume overload and heart failure decompensation in some patients.”
Insertion of a closure device has its own issues, it continues. “Closure of the ASD might impede future access to the left atrium, which could impact life-long management of this high-risk population. A large septal occluder may hinder potentially needed procedures such as paravalvular leak closure, left atrial appendage closure, or pulmonary vein isolation.”
Patients like those in the current series, Dr. Kodali observed, will face “a lifetime of management challenges, and you want to make sure you don’t take away other options.”
The study was funded by Medtronic. Dr. Zahr reported institutional grant support from Edwards Lifesciences and Medtronic. Dr. Kodali disclosed consultant fees from Admedus and Dura Biotech; equity in Dura Biotech, Microinterventional Devices, Thubrika Aortic Valve, Supira, Admedus, TriFlo, and Anona; and institutional grant support from Edwards Lifesciences, Medtronic, Abbott Vascular, Boston Scientific, and JenaValve. The editorial writers have disclosed no relevant financial relationships. Dr. Tayal disclosed consultant fees or honoraria from or serving on a speakers bureau for Abiomed, Edwards Lifesciences, Abbott Vascular, and Shockwave Medical.
A version of this article first appeared on Medscape.com.
It remains early days for transcatheter mitral-valve replacement (TMVR) as a minimally invasive way to treat severe, mitral regurgitation (MR), but it’s even earlier days for TMVR as an endovascular procedure. Most of the technique’s limited experience with a dedicated mitral prosthesis has involved transapical delivery.
But now a 15-patient study of transfemoral, transeptal TMVR – with a prosthesis designed for the mitral position and previously tested only transapically – has shown good 30-day results in that MR was essentially abolished with virtually no paravalvular leakage.
Nor were there adverse clinical events such as death, stroke, reintervention, or new need for a pacemaker in any of the high-surgical-risk patients with MR in this feasibility study of the transfemoral Intrepid TMVR System (Medtronic). Implantation failed, however, in one patient who then received a surgical valve via sternotomy.
The current cohort is part of a larger ongoing trial that will track whether patients implanted transfemorally with the Intrepid also show reverse remodeling and good clinical outcomes over at least a year. That study, called APOLLO, is one of several exploring dedicated TMVR valves from different companies, with names like SUMMIT, MISCEND, and TIARA-2.
Currently, TMVR is approved in the United States only using one device designed for the aortic position and only for treating failed surgical mitral bioprostheses in high-risk patients.
If the Intrepid transfemoral system has an Achilles’ heel, at least in the current iteration, it might be its 35 F catheter delivery system that requires surgical access to the femoral vein. Seven of the patients in the small series experienced major bleeding events, including six at the femoral access site, listed as major vascular complications.
Overall, the study’s patients “were extremely sick with a lot of comorbidity. A lot of them had atrial fibrillation, a lot of them were on anticoagulation to start with,” observed Firas Zahr, MD, Oregon Health & Science University, Portland, as part of his presentation of the study at Transcatheter Cardiovascular Therapeutics (TCT) 2021, held virtually as well as onsite in Orlando, Florida.
All had moderate-to-severe, usually primary MR; two thirds of the cohort had been in NYHA class III or IV at baseline, and 40% had been hospitalized for heart failure within the past year. Eight had a history of cardiovascular surgery, and eight had diabetes. Their mean Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM) score was 4.7, Dr. Zahr reported.
“At 30 days, there was a significant improvement in their heart failure classification; the vast majority of the patients were [NYHA] class I and class II,” said Dr. Zahr, who is also lead author on the study’s Nov. 6 publication in JACC: Cardiovascular Interventions.
Observers of the study at TCT 2021 seemed enthusiastic about the study’s results but recognized that TMVR in its current form still has formidable limitations.
“This is clearly an exciting look into the future and very reassuring to a degree, aside from the complications, which are somewhat expected as we go with 30-plus French devices,” Rajiv Tayal, MD, MPH, said at a press conference on the Intrepid study held before Dr. Zahr’s formal presentation. Dr. Tayal is an interventional cardiologist with Valley Health System, Ridgewood, New Jersey, and New York Medical College, Valhalla.
“I think we’ve all learned that transapical [access] is just not a viable procedure for a lot of these patients, and so we’ve got to get to transfemoral,” Susheel K. Kodali, MD, interventional cardiologist at New York-Presbyterian/Columbia University Irving Medical Center, said at the same forum.
A 35 F device “is going to be too big,” he said. However, “it is the first step to iterate to a smaller device.” Dr. Kodali said his center contributed a patient to the study, and he is listed as a coauthor on the publication.
The delivery system’s large profile is only part of the vascular complication issue. Not only did the procedure require surgical cutdown for venous access, but “we were fairly aggressive in anticoagulating these patients with the fear of thrombus formation,” Dr. Zahr said in the discussion following his presentation.
“A postprocedure anticoagulation regimen is recommended within the protocol, but ultimate therapy was left to the discretion of the treating site physician,” the published report states, noting that all 14 patients with successful TMVR were discharged on warfarin. They included 12 who were also put on a single antiplatelet and one given dual antiplatelet therapy on top of the oral anticoagulant.
“One thing that we learned is that we probably should standardize our approach to perioperative anticoagulation,” Dr. Zahr observed. Also, a 29 F sheath for the system is in the works, “and we’re hoping that with smaller sheath size, and hopefully going even to percutaneous, might have an impact on lowering the vascular complications.”
Explanations for the “higher-than-expected vascular complication rate” remains somewhat unclear, agreed an editorial accompanying the study’s publication, “but may include a learning curve with the system, the large introducer sheath, the need for surgical cutdown, and postprocedural anticoagulation.”
For trans-septal TMVR to become a default approach, “venous access will need to be achieved percutaneously and vascular complications need to be infrequent,” contends the editorial, with lead author Mohamad Alkhouli, MD, Mayo Clinic, Rochester, Minn.
“These data provide a glimpse into the future of TMVR. The excellent short-term safety and effectiveness of this still very early-stage procedure represent a major step forward in the field,” they write.
“The main question that the Intrepid early feasibility data raise is whether transfemoral, trans-septal TMVR will evolve to become the preferred strategy over transapical TMVR,” as occurred with transcatheter aortic-valve replacement (TAVR), the editorial states. “The answer is likely yes, but a few matters specific to trans-septal route will need be addressed first.”
Among those matters: The 35 F catheter leaves behind a considerable atrial septal defect (ASD). At operator discretion in this series, 11 patients received an ASD closure device.
None of the remaining four patients “developed significant heart failure or right ventricular dysfunction,” Dr. Zahr observed. “So, it seems like those patients who had their ASD left open tolerated it fairly well, at least until 30 days.”
But “we still need to learn what to do with those ASDs,” he said. “What is an acceptable residual shunt and what is an acceptable ASD size is to be determined.”
In general, the editorial notes, “the TMVR population has a high prevalence of cardiomyopathy, and a large residual iatrogenic ASD may lead to worsening volume overload and heart failure decompensation in some patients.”
Insertion of a closure device has its own issues, it continues. “Closure of the ASD might impede future access to the left atrium, which could impact life-long management of this high-risk population. A large septal occluder may hinder potentially needed procedures such as paravalvular leak closure, left atrial appendage closure, or pulmonary vein isolation.”
Patients like those in the current series, Dr. Kodali observed, will face “a lifetime of management challenges, and you want to make sure you don’t take away other options.”
The study was funded by Medtronic. Dr. Zahr reported institutional grant support from Edwards Lifesciences and Medtronic. Dr. Kodali disclosed consultant fees from Admedus and Dura Biotech; equity in Dura Biotech, Microinterventional Devices, Thubrika Aortic Valve, Supira, Admedus, TriFlo, and Anona; and institutional grant support from Edwards Lifesciences, Medtronic, Abbott Vascular, Boston Scientific, and JenaValve. The editorial writers have disclosed no relevant financial relationships. Dr. Tayal disclosed consultant fees or honoraria from or serving on a speakers bureau for Abiomed, Edwards Lifesciences, Abbott Vascular, and Shockwave Medical.
A version of this article first appeared on Medscape.com.
It remains early days for transcatheter mitral-valve replacement (TMVR) as a minimally invasive way to treat severe, mitral regurgitation (MR), but it’s even earlier days for TMVR as an endovascular procedure. Most of the technique’s limited experience with a dedicated mitral prosthesis has involved transapical delivery.
But now a 15-patient study of transfemoral, transeptal TMVR – with a prosthesis designed for the mitral position and previously tested only transapically – has shown good 30-day results in that MR was essentially abolished with virtually no paravalvular leakage.
Nor were there adverse clinical events such as death, stroke, reintervention, or new need for a pacemaker in any of the high-surgical-risk patients with MR in this feasibility study of the transfemoral Intrepid TMVR System (Medtronic). Implantation failed, however, in one patient who then received a surgical valve via sternotomy.
The current cohort is part of a larger ongoing trial that will track whether patients implanted transfemorally with the Intrepid also show reverse remodeling and good clinical outcomes over at least a year. That study, called APOLLO, is one of several exploring dedicated TMVR valves from different companies, with names like SUMMIT, MISCEND, and TIARA-2.
Currently, TMVR is approved in the United States only using one device designed for the aortic position and only for treating failed surgical mitral bioprostheses in high-risk patients.
If the Intrepid transfemoral system has an Achilles’ heel, at least in the current iteration, it might be its 35 F catheter delivery system that requires surgical access to the femoral vein. Seven of the patients in the small series experienced major bleeding events, including six at the femoral access site, listed as major vascular complications.
Overall, the study’s patients “were extremely sick with a lot of comorbidity. A lot of them had atrial fibrillation, a lot of them were on anticoagulation to start with,” observed Firas Zahr, MD, Oregon Health & Science University, Portland, as part of his presentation of the study at Transcatheter Cardiovascular Therapeutics (TCT) 2021, held virtually as well as onsite in Orlando, Florida.
All had moderate-to-severe, usually primary MR; two thirds of the cohort had been in NYHA class III or IV at baseline, and 40% had been hospitalized for heart failure within the past year. Eight had a history of cardiovascular surgery, and eight had diabetes. Their mean Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM) score was 4.7, Dr. Zahr reported.
“At 30 days, there was a significant improvement in their heart failure classification; the vast majority of the patients were [NYHA] class I and class II,” said Dr. Zahr, who is also lead author on the study’s Nov. 6 publication in JACC: Cardiovascular Interventions.
Observers of the study at TCT 2021 seemed enthusiastic about the study’s results but recognized that TMVR in its current form still has formidable limitations.
“This is clearly an exciting look into the future and very reassuring to a degree, aside from the complications, which are somewhat expected as we go with 30-plus French devices,” Rajiv Tayal, MD, MPH, said at a press conference on the Intrepid study held before Dr. Zahr’s formal presentation. Dr. Tayal is an interventional cardiologist with Valley Health System, Ridgewood, New Jersey, and New York Medical College, Valhalla.
“I think we’ve all learned that transapical [access] is just not a viable procedure for a lot of these patients, and so we’ve got to get to transfemoral,” Susheel K. Kodali, MD, interventional cardiologist at New York-Presbyterian/Columbia University Irving Medical Center, said at the same forum.
A 35 F device “is going to be too big,” he said. However, “it is the first step to iterate to a smaller device.” Dr. Kodali said his center contributed a patient to the study, and he is listed as a coauthor on the publication.
The delivery system’s large profile is only part of the vascular complication issue. Not only did the procedure require surgical cutdown for venous access, but “we were fairly aggressive in anticoagulating these patients with the fear of thrombus formation,” Dr. Zahr said in the discussion following his presentation.
“A postprocedure anticoagulation regimen is recommended within the protocol, but ultimate therapy was left to the discretion of the treating site physician,” the published report states, noting that all 14 patients with successful TMVR were discharged on warfarin. They included 12 who were also put on a single antiplatelet and one given dual antiplatelet therapy on top of the oral anticoagulant.
“One thing that we learned is that we probably should standardize our approach to perioperative anticoagulation,” Dr. Zahr observed. Also, a 29 F sheath for the system is in the works, “and we’re hoping that with smaller sheath size, and hopefully going even to percutaneous, might have an impact on lowering the vascular complications.”
Explanations for the “higher-than-expected vascular complication rate” remains somewhat unclear, agreed an editorial accompanying the study’s publication, “but may include a learning curve with the system, the large introducer sheath, the need for surgical cutdown, and postprocedural anticoagulation.”
For trans-septal TMVR to become a default approach, “venous access will need to be achieved percutaneously and vascular complications need to be infrequent,” contends the editorial, with lead author Mohamad Alkhouli, MD, Mayo Clinic, Rochester, Minn.
“These data provide a glimpse into the future of TMVR. The excellent short-term safety and effectiveness of this still very early-stage procedure represent a major step forward in the field,” they write.
“The main question that the Intrepid early feasibility data raise is whether transfemoral, trans-septal TMVR will evolve to become the preferred strategy over transapical TMVR,” as occurred with transcatheter aortic-valve replacement (TAVR), the editorial states. “The answer is likely yes, but a few matters specific to trans-septal route will need be addressed first.”
Among those matters: The 35 F catheter leaves behind a considerable atrial septal defect (ASD). At operator discretion in this series, 11 patients received an ASD closure device.
None of the remaining four patients “developed significant heart failure or right ventricular dysfunction,” Dr. Zahr observed. “So, it seems like those patients who had their ASD left open tolerated it fairly well, at least until 30 days.”
But “we still need to learn what to do with those ASDs,” he said. “What is an acceptable residual shunt and what is an acceptable ASD size is to be determined.”
In general, the editorial notes, “the TMVR population has a high prevalence of cardiomyopathy, and a large residual iatrogenic ASD may lead to worsening volume overload and heart failure decompensation in some patients.”
Insertion of a closure device has its own issues, it continues. “Closure of the ASD might impede future access to the left atrium, which could impact life-long management of this high-risk population. A large septal occluder may hinder potentially needed procedures such as paravalvular leak closure, left atrial appendage closure, or pulmonary vein isolation.”
Patients like those in the current series, Dr. Kodali observed, will face “a lifetime of management challenges, and you want to make sure you don’t take away other options.”
The study was funded by Medtronic. Dr. Zahr reported institutional grant support from Edwards Lifesciences and Medtronic. Dr. Kodali disclosed consultant fees from Admedus and Dura Biotech; equity in Dura Biotech, Microinterventional Devices, Thubrika Aortic Valve, Supira, Admedus, TriFlo, and Anona; and institutional grant support from Edwards Lifesciences, Medtronic, Abbott Vascular, Boston Scientific, and JenaValve. The editorial writers have disclosed no relevant financial relationships. Dr. Tayal disclosed consultant fees or honoraria from or serving on a speakers bureau for Abiomed, Edwards Lifesciences, Abbott Vascular, and Shockwave Medical.
A version of this article first appeared on Medscape.com.
AHA 2021 puts scientific dialogue, health equity center stage
Virtual platforms democratized scientific meetings during the COVID-19 pandemic but, as any meeting-goer will tell you, it’s the questions from the floor and the back-and-forth of an expert panel that often reveal the importance of and/or problems with a presentation. It’s the scrutiny that makes the science resonate, especially in this postfactual era.
The all-virtual American Heart Association Scientific Sessions 2021 is looking to recreate the engagement of an in-person meeting by offering more live interactive events. They range from seven late-breaking science (LBS) sessions to Saturday’s fireside chat on the Pfizer and Moderna COVID-19 vaccines and Monday’s dive into the controversial new AHA/American College of Cardiology Chest Pain guidelines.
To help digest the latest science, attendees will be able to have their questions answered in real-time via Slido, meet with the trialists, and hear live commentary from key opinion leaders after the live events. A networking function will also allow attendees and exhibitors to chat or meet virtually.
“In this day and age, many people pretty quickly can get access to the science but it’s what I call the IC sort of phenomenon – the presentation of the information, the context of the information, putting it into how I’m going to use it in my practice, and then the critical appraisal – that’s what most people want at the Scientific Sessions,” program committee chair Manesh R. Patel, MD, of Duke University School of Medicine, said in an interview. “We’re all craving ways in which we can interact with one another to put things in context.”
Plans for a hybrid in-person meeting in Boston were scuttled in September because of the Delta variant surge, but the theme of the meeting remained: “One World. Together for Science.” Attendees will be able to access more than 500 live and on-demand sessions including 117 oral abstracts, 286 poster sessions, 59 moderated digital posters, and over a dozen sessions focused on strategies to promote health equity.
“Last year there was a Presidential Session and a statement on structural racism, so we wanted to take the next step and say, What are the ways in which people are starting to interact and do things to make a difference?” explained Dr. Patel. “So, this year, you’ll see different versions of that from the Main Event session, which has some case vignettes and a panel discussion, to other health equity sessions that describe not just COVID care, but blood pressure care, maternal-fetal medicine, and congenital kids. Wherever we can, we’ve tried to infuse it throughout the sessions and will continue to.”
Late-breaking science
The LBS sessions kick off at 9:30 a.m. ET Saturday with AVATAR, a randomized trial of aortic valve replacement vs. watchful waiting in severe aortic stenosis proved asymptomatic through exercise testing.
“The findings of that trial, depending on what they are, could certainly impact clinical practice because it’s a very common scenario in which we have elderly patients with aortic valve stenosis that might be severe but they may not be symptomatic,” he said.
It’s followed by a randomized trial from the Cardiothoracic Surgical Trials Network, examining whether tricuspid repair at the time of mitral valve surgery leads to beneficial outcomes. “I think it’s a pretty important study,” Dr. Patel said, “because it’ll again affect how we think about our clinical practice.”
Rounding out the LBS.01 session is RAPID CABG, comparing early vs. delayed coronary bypass graft surgery (CABG) in patients with acute coronary syndromes on ticagrelor, and the pivotal U.S. VEST trial of an external support device already approved in Europe for saphenous vein grafts during CABG.
Saturday’s LBS.02 at 3:00 p.m. ET is devoted to hypertension and looks at how the COVID-19 pandemic affected blood pressure control. There’s also a study of remotely delivered hypertension and lipid management in 10,000 patients across the Partners Healthcare System and a cluster randomized trial of a village doctor–led blood pressure intervention in rural China.
Sunday’s LBS.03 at 8:00 a.m. ET is focused on atrial arrhythmias, starting with the CRAVE trial examining the effect of caffeine consumption on cardiac ectopy burden in 108 patients using an N-of-1 design and 2-day blocks on and off caffeine. “There’s an ability to identify a dose response that you get arrhythmias when you increase the amount of coffee you drink vs. not in an individual, so I think that will be likely discussed a lot and worth paying attention to,” Dr. Patel said.
The session also includes GIRAF, a comparison of cognitive outcomes with dabigatran (Pradaxa) vs. warfarin (Coumadin) in nonvalvular atrial fibrillation (AF); PALACS, a randomized trial examining whether left-sided pericardiotomy prevents AF after cardiac surgery; and AMAZE, which study sponsor AtriCure revealed missed its primary efficacy endpoint of freedom from AF with the LARIAT suture delivery device for left atrial appendage closure plus pulmonary vein isolation.
LBS.04 at 3:30 p.m. ET Sunday takes on digital health, with results from the nonrandomized Fitbit Heart Study on AF notifications from 450,000 participants wearing a single-lead ECG patch. “A lot of technologies claim that they can detect things, and we should ask that people go through the rigorous evaluation to see if they in fact do. So, in that respect, I think it›s an important step,” observed Dr. Patel.
Also on tap is I-STOP-AFib, another N-of-1 study using mobile apps and the AliveCor device to identify individual AF triggers; and REVeAL-HF, a 4,000-patient study examining whether electronic alerts that provide clinicians with prognostic information on their heart failure (HF) patients will reduce mortality and 30-day HF hospitalizations.
LBS.05 at 5:00 p.m. ET provides new information from EMPEROR-Preserved in HF with preserved ejection fraction and main results from EMPULSE, also using the sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin (Jardiance) in 530 patients hospitalized for acute HF.
The session also features CHIEF-HF, a randomized trial leveraging mobile technologies to test whether 12 weeks of another SGLT2 inhibitor, canagliflozin (Invokana), is superior to placebo for improving HF symptoms; and DREAM-HF, a comparison of transendocardial delivery of allogeneic mesenchymal precursor cells vs. a sham comparator in chronic HF as a result of left ventricular systolic dysfunction.
Monday’s LBS.06 at 8:00 a.m. ET details the safety and cholesterol-lowering efficacy of MK-0616, an investigational oral PCSK9 inhibitor. “It’s just a phase 2 [trial], but there’s interest in an oral PCSK9 inhibitor, given that the current ones are subcutaneous,” Dr. Patel said.
Results will also be presented from PREPARE-IT 2, which tested icosapent ethyl vs. placebo in outpatients with COVID-19. In the recently reported PREPARE-IT 1, a loading dose of icosapent ethyl failed to reduce the risk of hospitalization with SARS-CoV-2 infection among at-risk individuals.
LBS.07 at 11:00 a.m. Monday completes the late-breakers with new results from ASCEND, this time examining the effect of aspirin on dementia and cognitive impairment in patients with diabetes.
Next up is a look at the effectiveness of P2Y12 inhibitors in hospitalized patients with COVID-19 in the adaptive ACTIV-4a trial, followed by results of the pivotal phase 3 REVERSE-IT trial of bentracimab, a recombinant human monoclonal antibody antigen fragment designed to reverse the antiplatelet activity of ticagrelor in the event of major bleeding or when urgent surgery is needed.
Closing out the session is AXIOMATIC-TKR, a double-blind comparison of the safety and efficacy of the investigational oral factor XI anticoagulant JNJ-70033093 vs. subcutaneous enoxaparin (Lovenox) in elective total knee replacement.
For those searching for more AHA-related science online, the Resuscitation Science Symposium (ReSS) will run from this Friday through Sunday and the Quality of Care and Outcomes Research (QCOR) Scientific Sessions will take the stage next Monday, Nov. 15.
A version of this article first appeared on Medscape.com.
Virtual platforms democratized scientific meetings during the COVID-19 pandemic but, as any meeting-goer will tell you, it’s the questions from the floor and the back-and-forth of an expert panel that often reveal the importance of and/or problems with a presentation. It’s the scrutiny that makes the science resonate, especially in this postfactual era.
The all-virtual American Heart Association Scientific Sessions 2021 is looking to recreate the engagement of an in-person meeting by offering more live interactive events. They range from seven late-breaking science (LBS) sessions to Saturday’s fireside chat on the Pfizer and Moderna COVID-19 vaccines and Monday’s dive into the controversial new AHA/American College of Cardiology Chest Pain guidelines.
To help digest the latest science, attendees will be able to have their questions answered in real-time via Slido, meet with the trialists, and hear live commentary from key opinion leaders after the live events. A networking function will also allow attendees and exhibitors to chat or meet virtually.
“In this day and age, many people pretty quickly can get access to the science but it’s what I call the IC sort of phenomenon – the presentation of the information, the context of the information, putting it into how I’m going to use it in my practice, and then the critical appraisal – that’s what most people want at the Scientific Sessions,” program committee chair Manesh R. Patel, MD, of Duke University School of Medicine, said in an interview. “We’re all craving ways in which we can interact with one another to put things in context.”
Plans for a hybrid in-person meeting in Boston were scuttled in September because of the Delta variant surge, but the theme of the meeting remained: “One World. Together for Science.” Attendees will be able to access more than 500 live and on-demand sessions including 117 oral abstracts, 286 poster sessions, 59 moderated digital posters, and over a dozen sessions focused on strategies to promote health equity.
“Last year there was a Presidential Session and a statement on structural racism, so we wanted to take the next step and say, What are the ways in which people are starting to interact and do things to make a difference?” explained Dr. Patel. “So, this year, you’ll see different versions of that from the Main Event session, which has some case vignettes and a panel discussion, to other health equity sessions that describe not just COVID care, but blood pressure care, maternal-fetal medicine, and congenital kids. Wherever we can, we’ve tried to infuse it throughout the sessions and will continue to.”
Late-breaking science
The LBS sessions kick off at 9:30 a.m. ET Saturday with AVATAR, a randomized trial of aortic valve replacement vs. watchful waiting in severe aortic stenosis proved asymptomatic through exercise testing.
“The findings of that trial, depending on what they are, could certainly impact clinical practice because it’s a very common scenario in which we have elderly patients with aortic valve stenosis that might be severe but they may not be symptomatic,” he said.
It’s followed by a randomized trial from the Cardiothoracic Surgical Trials Network, examining whether tricuspid repair at the time of mitral valve surgery leads to beneficial outcomes. “I think it’s a pretty important study,” Dr. Patel said, “because it’ll again affect how we think about our clinical practice.”
Rounding out the LBS.01 session is RAPID CABG, comparing early vs. delayed coronary bypass graft surgery (CABG) in patients with acute coronary syndromes on ticagrelor, and the pivotal U.S. VEST trial of an external support device already approved in Europe for saphenous vein grafts during CABG.
Saturday’s LBS.02 at 3:00 p.m. ET is devoted to hypertension and looks at how the COVID-19 pandemic affected blood pressure control. There’s also a study of remotely delivered hypertension and lipid management in 10,000 patients across the Partners Healthcare System and a cluster randomized trial of a village doctor–led blood pressure intervention in rural China.
Sunday’s LBS.03 at 8:00 a.m. ET is focused on atrial arrhythmias, starting with the CRAVE trial examining the effect of caffeine consumption on cardiac ectopy burden in 108 patients using an N-of-1 design and 2-day blocks on and off caffeine. “There’s an ability to identify a dose response that you get arrhythmias when you increase the amount of coffee you drink vs. not in an individual, so I think that will be likely discussed a lot and worth paying attention to,” Dr. Patel said.
The session also includes GIRAF, a comparison of cognitive outcomes with dabigatran (Pradaxa) vs. warfarin (Coumadin) in nonvalvular atrial fibrillation (AF); PALACS, a randomized trial examining whether left-sided pericardiotomy prevents AF after cardiac surgery; and AMAZE, which study sponsor AtriCure revealed missed its primary efficacy endpoint of freedom from AF with the LARIAT suture delivery device for left atrial appendage closure plus pulmonary vein isolation.
LBS.04 at 3:30 p.m. ET Sunday takes on digital health, with results from the nonrandomized Fitbit Heart Study on AF notifications from 450,000 participants wearing a single-lead ECG patch. “A lot of technologies claim that they can detect things, and we should ask that people go through the rigorous evaluation to see if they in fact do. So, in that respect, I think it›s an important step,” observed Dr. Patel.
Also on tap is I-STOP-AFib, another N-of-1 study using mobile apps and the AliveCor device to identify individual AF triggers; and REVeAL-HF, a 4,000-patient study examining whether electronic alerts that provide clinicians with prognostic information on their heart failure (HF) patients will reduce mortality and 30-day HF hospitalizations.
LBS.05 at 5:00 p.m. ET provides new information from EMPEROR-Preserved in HF with preserved ejection fraction and main results from EMPULSE, also using the sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin (Jardiance) in 530 patients hospitalized for acute HF.
The session also features CHIEF-HF, a randomized trial leveraging mobile technologies to test whether 12 weeks of another SGLT2 inhibitor, canagliflozin (Invokana), is superior to placebo for improving HF symptoms; and DREAM-HF, a comparison of transendocardial delivery of allogeneic mesenchymal precursor cells vs. a sham comparator in chronic HF as a result of left ventricular systolic dysfunction.
Monday’s LBS.06 at 8:00 a.m. ET details the safety and cholesterol-lowering efficacy of MK-0616, an investigational oral PCSK9 inhibitor. “It’s just a phase 2 [trial], but there’s interest in an oral PCSK9 inhibitor, given that the current ones are subcutaneous,” Dr. Patel said.
Results will also be presented from PREPARE-IT 2, which tested icosapent ethyl vs. placebo in outpatients with COVID-19. In the recently reported PREPARE-IT 1, a loading dose of icosapent ethyl failed to reduce the risk of hospitalization with SARS-CoV-2 infection among at-risk individuals.
LBS.07 at 11:00 a.m. Monday completes the late-breakers with new results from ASCEND, this time examining the effect of aspirin on dementia and cognitive impairment in patients with diabetes.
Next up is a look at the effectiveness of P2Y12 inhibitors in hospitalized patients with COVID-19 in the adaptive ACTIV-4a trial, followed by results of the pivotal phase 3 REVERSE-IT trial of bentracimab, a recombinant human monoclonal antibody antigen fragment designed to reverse the antiplatelet activity of ticagrelor in the event of major bleeding or when urgent surgery is needed.
Closing out the session is AXIOMATIC-TKR, a double-blind comparison of the safety and efficacy of the investigational oral factor XI anticoagulant JNJ-70033093 vs. subcutaneous enoxaparin (Lovenox) in elective total knee replacement.
For those searching for more AHA-related science online, the Resuscitation Science Symposium (ReSS) will run from this Friday through Sunday and the Quality of Care and Outcomes Research (QCOR) Scientific Sessions will take the stage next Monday, Nov. 15.
A version of this article first appeared on Medscape.com.
Virtual platforms democratized scientific meetings during the COVID-19 pandemic but, as any meeting-goer will tell you, it’s the questions from the floor and the back-and-forth of an expert panel that often reveal the importance of and/or problems with a presentation. It’s the scrutiny that makes the science resonate, especially in this postfactual era.
The all-virtual American Heart Association Scientific Sessions 2021 is looking to recreate the engagement of an in-person meeting by offering more live interactive events. They range from seven late-breaking science (LBS) sessions to Saturday’s fireside chat on the Pfizer and Moderna COVID-19 vaccines and Monday’s dive into the controversial new AHA/American College of Cardiology Chest Pain guidelines.
To help digest the latest science, attendees will be able to have their questions answered in real-time via Slido, meet with the trialists, and hear live commentary from key opinion leaders after the live events. A networking function will also allow attendees and exhibitors to chat or meet virtually.
“In this day and age, many people pretty quickly can get access to the science but it’s what I call the IC sort of phenomenon – the presentation of the information, the context of the information, putting it into how I’m going to use it in my practice, and then the critical appraisal – that’s what most people want at the Scientific Sessions,” program committee chair Manesh R. Patel, MD, of Duke University School of Medicine, said in an interview. “We’re all craving ways in which we can interact with one another to put things in context.”
Plans for a hybrid in-person meeting in Boston were scuttled in September because of the Delta variant surge, but the theme of the meeting remained: “One World. Together for Science.” Attendees will be able to access more than 500 live and on-demand sessions including 117 oral abstracts, 286 poster sessions, 59 moderated digital posters, and over a dozen sessions focused on strategies to promote health equity.
“Last year there was a Presidential Session and a statement on structural racism, so we wanted to take the next step and say, What are the ways in which people are starting to interact and do things to make a difference?” explained Dr. Patel. “So, this year, you’ll see different versions of that from the Main Event session, which has some case vignettes and a panel discussion, to other health equity sessions that describe not just COVID care, but blood pressure care, maternal-fetal medicine, and congenital kids. Wherever we can, we’ve tried to infuse it throughout the sessions and will continue to.”
Late-breaking science
The LBS sessions kick off at 9:30 a.m. ET Saturday with AVATAR, a randomized trial of aortic valve replacement vs. watchful waiting in severe aortic stenosis proved asymptomatic through exercise testing.
“The findings of that trial, depending on what they are, could certainly impact clinical practice because it’s a very common scenario in which we have elderly patients with aortic valve stenosis that might be severe but they may not be symptomatic,” he said.
It’s followed by a randomized trial from the Cardiothoracic Surgical Trials Network, examining whether tricuspid repair at the time of mitral valve surgery leads to beneficial outcomes. “I think it’s a pretty important study,” Dr. Patel said, “because it’ll again affect how we think about our clinical practice.”
Rounding out the LBS.01 session is RAPID CABG, comparing early vs. delayed coronary bypass graft surgery (CABG) in patients with acute coronary syndromes on ticagrelor, and the pivotal U.S. VEST trial of an external support device already approved in Europe for saphenous vein grafts during CABG.
Saturday’s LBS.02 at 3:00 p.m. ET is devoted to hypertension and looks at how the COVID-19 pandemic affected blood pressure control. There’s also a study of remotely delivered hypertension and lipid management in 10,000 patients across the Partners Healthcare System and a cluster randomized trial of a village doctor–led blood pressure intervention in rural China.
Sunday’s LBS.03 at 8:00 a.m. ET is focused on atrial arrhythmias, starting with the CRAVE trial examining the effect of caffeine consumption on cardiac ectopy burden in 108 patients using an N-of-1 design and 2-day blocks on and off caffeine. “There’s an ability to identify a dose response that you get arrhythmias when you increase the amount of coffee you drink vs. not in an individual, so I think that will be likely discussed a lot and worth paying attention to,” Dr. Patel said.
The session also includes GIRAF, a comparison of cognitive outcomes with dabigatran (Pradaxa) vs. warfarin (Coumadin) in nonvalvular atrial fibrillation (AF); PALACS, a randomized trial examining whether left-sided pericardiotomy prevents AF after cardiac surgery; and AMAZE, which study sponsor AtriCure revealed missed its primary efficacy endpoint of freedom from AF with the LARIAT suture delivery device for left atrial appendage closure plus pulmonary vein isolation.
LBS.04 at 3:30 p.m. ET Sunday takes on digital health, with results from the nonrandomized Fitbit Heart Study on AF notifications from 450,000 participants wearing a single-lead ECG patch. “A lot of technologies claim that they can detect things, and we should ask that people go through the rigorous evaluation to see if they in fact do. So, in that respect, I think it›s an important step,” observed Dr. Patel.
Also on tap is I-STOP-AFib, another N-of-1 study using mobile apps and the AliveCor device to identify individual AF triggers; and REVeAL-HF, a 4,000-patient study examining whether electronic alerts that provide clinicians with prognostic information on their heart failure (HF) patients will reduce mortality and 30-day HF hospitalizations.
LBS.05 at 5:00 p.m. ET provides new information from EMPEROR-Preserved in HF with preserved ejection fraction and main results from EMPULSE, also using the sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin (Jardiance) in 530 patients hospitalized for acute HF.
The session also features CHIEF-HF, a randomized trial leveraging mobile technologies to test whether 12 weeks of another SGLT2 inhibitor, canagliflozin (Invokana), is superior to placebo for improving HF symptoms; and DREAM-HF, a comparison of transendocardial delivery of allogeneic mesenchymal precursor cells vs. a sham comparator in chronic HF as a result of left ventricular systolic dysfunction.
Monday’s LBS.06 at 8:00 a.m. ET details the safety and cholesterol-lowering efficacy of MK-0616, an investigational oral PCSK9 inhibitor. “It’s just a phase 2 [trial], but there’s interest in an oral PCSK9 inhibitor, given that the current ones are subcutaneous,” Dr. Patel said.
Results will also be presented from PREPARE-IT 2, which tested icosapent ethyl vs. placebo in outpatients with COVID-19. In the recently reported PREPARE-IT 1, a loading dose of icosapent ethyl failed to reduce the risk of hospitalization with SARS-CoV-2 infection among at-risk individuals.
LBS.07 at 11:00 a.m. Monday completes the late-breakers with new results from ASCEND, this time examining the effect of aspirin on dementia and cognitive impairment in patients with diabetes.
Next up is a look at the effectiveness of P2Y12 inhibitors in hospitalized patients with COVID-19 in the adaptive ACTIV-4a trial, followed by results of the pivotal phase 3 REVERSE-IT trial of bentracimab, a recombinant human monoclonal antibody antigen fragment designed to reverse the antiplatelet activity of ticagrelor in the event of major bleeding or when urgent surgery is needed.
Closing out the session is AXIOMATIC-TKR, a double-blind comparison of the safety and efficacy of the investigational oral factor XI anticoagulant JNJ-70033093 vs. subcutaneous enoxaparin (Lovenox) in elective total knee replacement.
For those searching for more AHA-related science online, the Resuscitation Science Symposium (ReSS) will run from this Friday through Sunday and the Quality of Care and Outcomes Research (QCOR) Scientific Sessions will take the stage next Monday, Nov. 15.
A version of this article first appeared on Medscape.com.
FROM AHA 2021





















