User login
Potential Impact of USPS Mail Delivery Delays on Colorectal Cancer Screening Programs
Colorectal cancer (CRC) is the second leading cause of cancer deaths in the United States.1 In 2022, there were an estimated 151,030 new CRC cases and 52,580 deaths.1 Options for CRC screening of patients at average risk include stool tests (annual fecal immunochemical test [FIT], annual guaiac-based fecal occult blood test, or stool FIT-DNA test every 1 to 3 years), colonoscopies every 10 years, flexible sigmoidoscopies every 5 years (or every 10 years with annual FIT), and computed tomography (CT) colonography every 5 years.2 Many health care systems use annual FIT for patients at average risk. Compared with guaiac-based fecal occult blood testing, FIT does not require dietary or medication modifications and yields greater sensitivity and patient participation.3
The COVID-19 pandemic and staffing issues have caused a scheduling backlog for screening, diagnostic, and surveillance endoscopies at some medical centers. As a result, FIT has become the primary means of CRC screening at these institutions. FIT kits for home use are typically distributed to eligible patients at an office visit or by mail, and patients are then instructed to mail the kits back to the laboratory. For the test to be as sensitive as possible, FIT kit manufacturers advise laboratory analysis within 14 to 15 days of collection, if stored at ambient temperature, and to reject the sample if it does not meet testing criteria for stability. Delayed FIT sample analysis has been associated with higher false-negative rates because of hemoglobin degradation.4 FIT sample exposure to high ambient temperatures also has been linked to decreased sensitivity for detecting CRC.5
US Postal Service (USPS) mail delivery delays have plagued many areas of the country. A variety of factors, including the COVID-19 pandemic, understaffing, changes in USPS policies, closure of post offices, and changes in mail delivery standards, may also be contributory causes. According to the USPS website, delivery standard for first-class mail is 1 to 5 days, but this is not guaranteed.6
The Jesse Brown Veterans Affairs Medical Center (JBVAMC) laboratory in Chicago has reported receiving FIT kit envelopes in batches by the USPS, with some prepaid first-class business reply envelopes delivered up to 60 days after the time of sample collection. Polymedco, a company that assists US Department of Veterans Affairs (VA) medical centers with logistics of FIT programs for CRC screening, reports that USPS batching of FIT kits leading to delayed delivery has been a periodic problem for medical centers around the country. Polymedco staff remind USPS staff about 4 points when they encounter this issue: Mailers are first-class mail; mailers contain a human biologic specimen that has limited viability; the biological sample used for detecting cancer is time sensitive; and delays in delivery by holding/batching kits could impact morbidity and mortality. Reviewing these key points with local USPS staff usually helps, however, batching and delayed delivery of the FIT kits can sometimes recur with USPS staffing turnover.
Tracking and identifying when a patient receives the FIT kit is difficult. Patients are instructed to write the date of collection on the kit, so the receiving laboratory knows whether the sample can be reliably analyzed. When patients are notified about delayed delivery of their sample, a staff member asks if they postponed dropping the kit in the mail. Most patients report mailing the sample within 1 to 2 days of collection. Tracking and dating each step of FIT kit events is not feasible with a mass mailing campaign. In our experience, most patients write the date of collection on the kit. If a collection date is not provided, the laboratory will call the patient to confirm a date. Cheng and colleagues reviewed the causes for FIT specimen rejection in a laboratory analyzing specimens for VA patients and found that 14% of submitted samples were rejected because the specimen was received > 14 days after collection, and 6% because the patient did not record the collection date. With a series of interventions aimed at reminding patients and improving laboratory procedures, rates of rejection for these 2 causes were reduced to < 4%.7 USPS delays were not identified as a factor or tracked in this study.
It is unclear why the USPS sometimes holds FIT kits at their facilities and then delivers large bins of them at the same time. Because FIT kits should be analyzed within 14 to 15 days of sample collection to assure reliable results, mail delivery delays can result in increased sample rejection. Based on the JBVAMC experience, up to 30% of submitted samples might need to be discarded when batched delivery takes place. In these cases, patients need to be contacted, informed of the problem, and asked to submit new kits. Understandably, patients are reluctant to repeat this type of testing, and we are concerned this could lead to reduced rates of CRC screening in affected communities.
As an alternative to discarding delayed samples, laboratories could report the results of delayed FIT kits with an added comment that “negative test results may be less reliable due to delayed processing,” but this approach would raise quality and medicolegal concerns. Clinicians have reached out to local USPS supervisory personnel with mixed results. Sometimes batching and delayed deliveries stop for a few months, only to resume without warning. Dropping off the sample directly at the laboratory is not a realistic option for most patients. Some patients can be convinced to submit another sample, some elect to switch to other CRC screening strategies, while others, unfortunately, decline further screening efforts.
Laboratory staff can be overwhelmed with having to process hundreds of samples in a short time frame, especially because there is no way of knowing when USPS will make a batched delivery. Laboratory capacities can limit staff at some facilities to performing analysis of only 10 tests at a time. The FIT kits should be delivered on a rolling basis and without delay so that the samples can be reliably analyzed with a predictable workload for the laboratory personnel and without unexpected surges.
When health care facilities identify delayed mail delivery of FIT kits via USPS, laboratories should first ensure that the correct postage rates are used on the prepaid envelopes and that their USPS accounts are properly funded, so that insufficient funds are not contributing to delayed deliveries. Stakeholders should then reach out to local USPS supervisory staff and request that the practice of batching the delivery of FIT kits be stopped. Educating USPS supervisory staff about concerns related to decreased test reliability associated with delayed mail delivery can be a persuasive argument. Adding additional language to the preprinted envelopes, such as “time sensitive,” may also be helpful. Unfortunately, the JBVAMC experience has been that the problem initially gets better after contacting the USPS, only to unexpectedly resurface months later. This cycle has been repeated several times in the past 2 years at JBVAMC.
All clinicians involved in CRC screening and treatment at institutions that use FIT kits need to be aware of the impact that local USPS delays can have on the reliability of these results. Health care systems should be prepared to implement mitigation strategies if they encounter significant delays with mail delivery. If delays cannot be reliably resolved by working with the local USPS staff, consider involving national USPS oversight bodies. And if the problems persist despite an attempt to work with the USPS, some institutions might find it feasible to offer drop boxes at their clinics and instruct patients to drop off FIT kits immediately following collection, in lieu of mailing them. Switching to private carriers is not a cost-effective alternative for most health care systems, and some may exclude rural areas. Depending on the local availability and capacity of endoscopists, some clinicians might prioritize referring patients for screening colonoscopies or screening flexible sigmoidoscopies, and might deemphasize FIT kits as a preferred option for CRC screening. CT colonography is an alternative screening method that is not as widely offered, nor as widely accepted at this time.
Conclusions
CRC screening is an essential part of preventive medicine, and the percentage of eligible patients screened is a well-established quality metric in primary care settings. Health care systems, clinicians, and laboratories must be vigilant to ensure that USPS delays in delivering FIT kits do not negatively impact their CRC screening programs. Facilities should actively monitor for delays in the return of FIT kits.
Despite the widespread use of mail-order pharmacies and the use of mail to communicate notifications about test results and follow-up appointments, unreliable or delayed mail delivery traditionally has not been considered a social determinant of health.8 This article highlights the impact delayed mail delivery can have on health outcomes. Disadvantaged communities in inner cities and rural areas have been disproportionately affected by the worsening performance of the USPS over the past few years.9 This represents an underappreciated public health concern in need of a sustainable solution.
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33. doi:10.3322/caac.21708
2. Centers for Disease Control and Prevention. Colorectal cancer screening tests. Updated February 23, 2023. Accessed March 14, 2024. https://www.cdc.gov/cancer/colorectal/basic_info/screening/tests.htm
3. van Rossum LG, van Rijn AF, Laheij RJ, et al. Random comparison of guaiac and immunochemical fecal occult blood tests for colorectal cancer in a screening population. Gastroenterology. 2008;135(1):82-90. doi:10.1053/j.gastro.2008.03.040
4. van Rossum LG, van Rijn AF, van Oijen MG, et al. False negative fecal occult blood tests due to delayed sample return in colorectal cancer screening. Int J Cancer. 2009;125(4):746-750. doi:10.1002/ijc.24458
5. Doubeni CA, Jensen CD, Fedewa SA, et al. Fecal immunochemical test (FIT) for colon cancer screening: variable performance with ambient temperature. J Am Board Fam Med. 2016;29(6):672-681. doi:10.3122/jabfm.2016.06.160060
6. United States Postal Service. Shipping and mailing with USPS. Accessed March 14, 2024. https://www.usps.com/ship
7. Cheng C, Ganz DA, Chang ET, Huynh A, De Peralta S. Reducing rejected fecal immunochemical tests received in the laboratory for colorectal cancer screening. J Healthc Qual. 2019;41(2):75-82.doi:10.1097/JHQ.0000000000000181
8. Hussaini SMQ, Alexander GC. The United States Postal Service: an essential public health agency? J Gen Intern Med. 2020;35(12):3699-3701. doi:10.1007/s11606-020-06275-2
9. Hampton DJ. Colorado mountain towns are plagued by post office delays as residents wait weeks for medication and retirement checks. NBC News. February 25, 2023. Accessed March 14, 2024. https://www.nbcnews.com/news/us-news/colo-mountain-towns-are-plagued-post-office-delays-residents-wait-week-rcna72085
Colorectal cancer (CRC) is the second leading cause of cancer deaths in the United States.1 In 2022, there were an estimated 151,030 new CRC cases and 52,580 deaths.1 Options for CRC screening of patients at average risk include stool tests (annual fecal immunochemical test [FIT], annual guaiac-based fecal occult blood test, or stool FIT-DNA test every 1 to 3 years), colonoscopies every 10 years, flexible sigmoidoscopies every 5 years (or every 10 years with annual FIT), and computed tomography (CT) colonography every 5 years.2 Many health care systems use annual FIT for patients at average risk. Compared with guaiac-based fecal occult blood testing, FIT does not require dietary or medication modifications and yields greater sensitivity and patient participation.3
The COVID-19 pandemic and staffing issues have caused a scheduling backlog for screening, diagnostic, and surveillance endoscopies at some medical centers. As a result, FIT has become the primary means of CRC screening at these institutions. FIT kits for home use are typically distributed to eligible patients at an office visit or by mail, and patients are then instructed to mail the kits back to the laboratory. For the test to be as sensitive as possible, FIT kit manufacturers advise laboratory analysis within 14 to 15 days of collection, if stored at ambient temperature, and to reject the sample if it does not meet testing criteria for stability. Delayed FIT sample analysis has been associated with higher false-negative rates because of hemoglobin degradation.4 FIT sample exposure to high ambient temperatures also has been linked to decreased sensitivity for detecting CRC.5
US Postal Service (USPS) mail delivery delays have plagued many areas of the country. A variety of factors, including the COVID-19 pandemic, understaffing, changes in USPS policies, closure of post offices, and changes in mail delivery standards, may also be contributory causes. According to the USPS website, delivery standard for first-class mail is 1 to 5 days, but this is not guaranteed.6
The Jesse Brown Veterans Affairs Medical Center (JBVAMC) laboratory in Chicago has reported receiving FIT kit envelopes in batches by the USPS, with some prepaid first-class business reply envelopes delivered up to 60 days after the time of sample collection. Polymedco, a company that assists US Department of Veterans Affairs (VA) medical centers with logistics of FIT programs for CRC screening, reports that USPS batching of FIT kits leading to delayed delivery has been a periodic problem for medical centers around the country. Polymedco staff remind USPS staff about 4 points when they encounter this issue: Mailers are first-class mail; mailers contain a human biologic specimen that has limited viability; the biological sample used for detecting cancer is time sensitive; and delays in delivery by holding/batching kits could impact morbidity and mortality. Reviewing these key points with local USPS staff usually helps, however, batching and delayed delivery of the FIT kits can sometimes recur with USPS staffing turnover.
Tracking and identifying when a patient receives the FIT kit is difficult. Patients are instructed to write the date of collection on the kit, so the receiving laboratory knows whether the sample can be reliably analyzed. When patients are notified about delayed delivery of their sample, a staff member asks if they postponed dropping the kit in the mail. Most patients report mailing the sample within 1 to 2 days of collection. Tracking and dating each step of FIT kit events is not feasible with a mass mailing campaign. In our experience, most patients write the date of collection on the kit. If a collection date is not provided, the laboratory will call the patient to confirm a date. Cheng and colleagues reviewed the causes for FIT specimen rejection in a laboratory analyzing specimens for VA patients and found that 14% of submitted samples were rejected because the specimen was received > 14 days after collection, and 6% because the patient did not record the collection date. With a series of interventions aimed at reminding patients and improving laboratory procedures, rates of rejection for these 2 causes were reduced to < 4%.7 USPS delays were not identified as a factor or tracked in this study.
It is unclear why the USPS sometimes holds FIT kits at their facilities and then delivers large bins of them at the same time. Because FIT kits should be analyzed within 14 to 15 days of sample collection to assure reliable results, mail delivery delays can result in increased sample rejection. Based on the JBVAMC experience, up to 30% of submitted samples might need to be discarded when batched delivery takes place. In these cases, patients need to be contacted, informed of the problem, and asked to submit new kits. Understandably, patients are reluctant to repeat this type of testing, and we are concerned this could lead to reduced rates of CRC screening in affected communities.
As an alternative to discarding delayed samples, laboratories could report the results of delayed FIT kits with an added comment that “negative test results may be less reliable due to delayed processing,” but this approach would raise quality and medicolegal concerns. Clinicians have reached out to local USPS supervisory personnel with mixed results. Sometimes batching and delayed deliveries stop for a few months, only to resume without warning. Dropping off the sample directly at the laboratory is not a realistic option for most patients. Some patients can be convinced to submit another sample, some elect to switch to other CRC screening strategies, while others, unfortunately, decline further screening efforts.
Laboratory staff can be overwhelmed with having to process hundreds of samples in a short time frame, especially because there is no way of knowing when USPS will make a batched delivery. Laboratory capacities can limit staff at some facilities to performing analysis of only 10 tests at a time. The FIT kits should be delivered on a rolling basis and without delay so that the samples can be reliably analyzed with a predictable workload for the laboratory personnel and without unexpected surges.
When health care facilities identify delayed mail delivery of FIT kits via USPS, laboratories should first ensure that the correct postage rates are used on the prepaid envelopes and that their USPS accounts are properly funded, so that insufficient funds are not contributing to delayed deliveries. Stakeholders should then reach out to local USPS supervisory staff and request that the practice of batching the delivery of FIT kits be stopped. Educating USPS supervisory staff about concerns related to decreased test reliability associated with delayed mail delivery can be a persuasive argument. Adding additional language to the preprinted envelopes, such as “time sensitive,” may also be helpful. Unfortunately, the JBVAMC experience has been that the problem initially gets better after contacting the USPS, only to unexpectedly resurface months later. This cycle has been repeated several times in the past 2 years at JBVAMC.
All clinicians involved in CRC screening and treatment at institutions that use FIT kits need to be aware of the impact that local USPS delays can have on the reliability of these results. Health care systems should be prepared to implement mitigation strategies if they encounter significant delays with mail delivery. If delays cannot be reliably resolved by working with the local USPS staff, consider involving national USPS oversight bodies. And if the problems persist despite an attempt to work with the USPS, some institutions might find it feasible to offer drop boxes at their clinics and instruct patients to drop off FIT kits immediately following collection, in lieu of mailing them. Switching to private carriers is not a cost-effective alternative for most health care systems, and some may exclude rural areas. Depending on the local availability and capacity of endoscopists, some clinicians might prioritize referring patients for screening colonoscopies or screening flexible sigmoidoscopies, and might deemphasize FIT kits as a preferred option for CRC screening. CT colonography is an alternative screening method that is not as widely offered, nor as widely accepted at this time.
Conclusions
CRC screening is an essential part of preventive medicine, and the percentage of eligible patients screened is a well-established quality metric in primary care settings. Health care systems, clinicians, and laboratories must be vigilant to ensure that USPS delays in delivering FIT kits do not negatively impact their CRC screening programs. Facilities should actively monitor for delays in the return of FIT kits.
Despite the widespread use of mail-order pharmacies and the use of mail to communicate notifications about test results and follow-up appointments, unreliable or delayed mail delivery traditionally has not been considered a social determinant of health.8 This article highlights the impact delayed mail delivery can have on health outcomes. Disadvantaged communities in inner cities and rural areas have been disproportionately affected by the worsening performance of the USPS over the past few years.9 This represents an underappreciated public health concern in need of a sustainable solution.
Colorectal cancer (CRC) is the second leading cause of cancer deaths in the United States.1 In 2022, there were an estimated 151,030 new CRC cases and 52,580 deaths.1 Options for CRC screening of patients at average risk include stool tests (annual fecal immunochemical test [FIT], annual guaiac-based fecal occult blood test, or stool FIT-DNA test every 1 to 3 years), colonoscopies every 10 years, flexible sigmoidoscopies every 5 years (or every 10 years with annual FIT), and computed tomography (CT) colonography every 5 years.2 Many health care systems use annual FIT for patients at average risk. Compared with guaiac-based fecal occult blood testing, FIT does not require dietary or medication modifications and yields greater sensitivity and patient participation.3
The COVID-19 pandemic and staffing issues have caused a scheduling backlog for screening, diagnostic, and surveillance endoscopies at some medical centers. As a result, FIT has become the primary means of CRC screening at these institutions. FIT kits for home use are typically distributed to eligible patients at an office visit or by mail, and patients are then instructed to mail the kits back to the laboratory. For the test to be as sensitive as possible, FIT kit manufacturers advise laboratory analysis within 14 to 15 days of collection, if stored at ambient temperature, and to reject the sample if it does not meet testing criteria for stability. Delayed FIT sample analysis has been associated with higher false-negative rates because of hemoglobin degradation.4 FIT sample exposure to high ambient temperatures also has been linked to decreased sensitivity for detecting CRC.5
US Postal Service (USPS) mail delivery delays have plagued many areas of the country. A variety of factors, including the COVID-19 pandemic, understaffing, changes in USPS policies, closure of post offices, and changes in mail delivery standards, may also be contributory causes. According to the USPS website, delivery standard for first-class mail is 1 to 5 days, but this is not guaranteed.6
The Jesse Brown Veterans Affairs Medical Center (JBVAMC) laboratory in Chicago has reported receiving FIT kit envelopes in batches by the USPS, with some prepaid first-class business reply envelopes delivered up to 60 days after the time of sample collection. Polymedco, a company that assists US Department of Veterans Affairs (VA) medical centers with logistics of FIT programs for CRC screening, reports that USPS batching of FIT kits leading to delayed delivery has been a periodic problem for medical centers around the country. Polymedco staff remind USPS staff about 4 points when they encounter this issue: Mailers are first-class mail; mailers contain a human biologic specimen that has limited viability; the biological sample used for detecting cancer is time sensitive; and delays in delivery by holding/batching kits could impact morbidity and mortality. Reviewing these key points with local USPS staff usually helps, however, batching and delayed delivery of the FIT kits can sometimes recur with USPS staffing turnover.
Tracking and identifying when a patient receives the FIT kit is difficult. Patients are instructed to write the date of collection on the kit, so the receiving laboratory knows whether the sample can be reliably analyzed. When patients are notified about delayed delivery of their sample, a staff member asks if they postponed dropping the kit in the mail. Most patients report mailing the sample within 1 to 2 days of collection. Tracking and dating each step of FIT kit events is not feasible with a mass mailing campaign. In our experience, most patients write the date of collection on the kit. If a collection date is not provided, the laboratory will call the patient to confirm a date. Cheng and colleagues reviewed the causes for FIT specimen rejection in a laboratory analyzing specimens for VA patients and found that 14% of submitted samples were rejected because the specimen was received > 14 days after collection, and 6% because the patient did not record the collection date. With a series of interventions aimed at reminding patients and improving laboratory procedures, rates of rejection for these 2 causes were reduced to < 4%.7 USPS delays were not identified as a factor or tracked in this study.
It is unclear why the USPS sometimes holds FIT kits at their facilities and then delivers large bins of them at the same time. Because FIT kits should be analyzed within 14 to 15 days of sample collection to assure reliable results, mail delivery delays can result in increased sample rejection. Based on the JBVAMC experience, up to 30% of submitted samples might need to be discarded when batched delivery takes place. In these cases, patients need to be contacted, informed of the problem, and asked to submit new kits. Understandably, patients are reluctant to repeat this type of testing, and we are concerned this could lead to reduced rates of CRC screening in affected communities.
As an alternative to discarding delayed samples, laboratories could report the results of delayed FIT kits with an added comment that “negative test results may be less reliable due to delayed processing,” but this approach would raise quality and medicolegal concerns. Clinicians have reached out to local USPS supervisory personnel with mixed results. Sometimes batching and delayed deliveries stop for a few months, only to resume without warning. Dropping off the sample directly at the laboratory is not a realistic option for most patients. Some patients can be convinced to submit another sample, some elect to switch to other CRC screening strategies, while others, unfortunately, decline further screening efforts.
Laboratory staff can be overwhelmed with having to process hundreds of samples in a short time frame, especially because there is no way of knowing when USPS will make a batched delivery. Laboratory capacities can limit staff at some facilities to performing analysis of only 10 tests at a time. The FIT kits should be delivered on a rolling basis and without delay so that the samples can be reliably analyzed with a predictable workload for the laboratory personnel and without unexpected surges.
When health care facilities identify delayed mail delivery of FIT kits via USPS, laboratories should first ensure that the correct postage rates are used on the prepaid envelopes and that their USPS accounts are properly funded, so that insufficient funds are not contributing to delayed deliveries. Stakeholders should then reach out to local USPS supervisory staff and request that the practice of batching the delivery of FIT kits be stopped. Educating USPS supervisory staff about concerns related to decreased test reliability associated with delayed mail delivery can be a persuasive argument. Adding additional language to the preprinted envelopes, such as “time sensitive,” may also be helpful. Unfortunately, the JBVAMC experience has been that the problem initially gets better after contacting the USPS, only to unexpectedly resurface months later. This cycle has been repeated several times in the past 2 years at JBVAMC.
All clinicians involved in CRC screening and treatment at institutions that use FIT kits need to be aware of the impact that local USPS delays can have on the reliability of these results. Health care systems should be prepared to implement mitigation strategies if they encounter significant delays with mail delivery. If delays cannot be reliably resolved by working with the local USPS staff, consider involving national USPS oversight bodies. And if the problems persist despite an attempt to work with the USPS, some institutions might find it feasible to offer drop boxes at their clinics and instruct patients to drop off FIT kits immediately following collection, in lieu of mailing them. Switching to private carriers is not a cost-effective alternative for most health care systems, and some may exclude rural areas. Depending on the local availability and capacity of endoscopists, some clinicians might prioritize referring patients for screening colonoscopies or screening flexible sigmoidoscopies, and might deemphasize FIT kits as a preferred option for CRC screening. CT colonography is an alternative screening method that is not as widely offered, nor as widely accepted at this time.
Conclusions
CRC screening is an essential part of preventive medicine, and the percentage of eligible patients screened is a well-established quality metric in primary care settings. Health care systems, clinicians, and laboratories must be vigilant to ensure that USPS delays in delivering FIT kits do not negatively impact their CRC screening programs. Facilities should actively monitor for delays in the return of FIT kits.
Despite the widespread use of mail-order pharmacies and the use of mail to communicate notifications about test results and follow-up appointments, unreliable or delayed mail delivery traditionally has not been considered a social determinant of health.8 This article highlights the impact delayed mail delivery can have on health outcomes. Disadvantaged communities in inner cities and rural areas have been disproportionately affected by the worsening performance of the USPS over the past few years.9 This represents an underappreciated public health concern in need of a sustainable solution.
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33. doi:10.3322/caac.21708
2. Centers for Disease Control and Prevention. Colorectal cancer screening tests. Updated February 23, 2023. Accessed March 14, 2024. https://www.cdc.gov/cancer/colorectal/basic_info/screening/tests.htm
3. van Rossum LG, van Rijn AF, Laheij RJ, et al. Random comparison of guaiac and immunochemical fecal occult blood tests for colorectal cancer in a screening population. Gastroenterology. 2008;135(1):82-90. doi:10.1053/j.gastro.2008.03.040
4. van Rossum LG, van Rijn AF, van Oijen MG, et al. False negative fecal occult blood tests due to delayed sample return in colorectal cancer screening. Int J Cancer. 2009;125(4):746-750. doi:10.1002/ijc.24458
5. Doubeni CA, Jensen CD, Fedewa SA, et al. Fecal immunochemical test (FIT) for colon cancer screening: variable performance with ambient temperature. J Am Board Fam Med. 2016;29(6):672-681. doi:10.3122/jabfm.2016.06.160060
6. United States Postal Service. Shipping and mailing with USPS. Accessed March 14, 2024. https://www.usps.com/ship
7. Cheng C, Ganz DA, Chang ET, Huynh A, De Peralta S. Reducing rejected fecal immunochemical tests received in the laboratory for colorectal cancer screening. J Healthc Qual. 2019;41(2):75-82.doi:10.1097/JHQ.0000000000000181
8. Hussaini SMQ, Alexander GC. The United States Postal Service: an essential public health agency? J Gen Intern Med. 2020;35(12):3699-3701. doi:10.1007/s11606-020-06275-2
9. Hampton DJ. Colorado mountain towns are plagued by post office delays as residents wait weeks for medication and retirement checks. NBC News. February 25, 2023. Accessed March 14, 2024. https://www.nbcnews.com/news/us-news/colo-mountain-towns-are-plagued-post-office-delays-residents-wait-week-rcna72085
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33. doi:10.3322/caac.21708
2. Centers for Disease Control and Prevention. Colorectal cancer screening tests. Updated February 23, 2023. Accessed March 14, 2024. https://www.cdc.gov/cancer/colorectal/basic_info/screening/tests.htm
3. van Rossum LG, van Rijn AF, Laheij RJ, et al. Random comparison of guaiac and immunochemical fecal occult blood tests for colorectal cancer in a screening population. Gastroenterology. 2008;135(1):82-90. doi:10.1053/j.gastro.2008.03.040
4. van Rossum LG, van Rijn AF, van Oijen MG, et al. False negative fecal occult blood tests due to delayed sample return in colorectal cancer screening. Int J Cancer. 2009;125(4):746-750. doi:10.1002/ijc.24458
5. Doubeni CA, Jensen CD, Fedewa SA, et al. Fecal immunochemical test (FIT) for colon cancer screening: variable performance with ambient temperature. J Am Board Fam Med. 2016;29(6):672-681. doi:10.3122/jabfm.2016.06.160060
6. United States Postal Service. Shipping and mailing with USPS. Accessed March 14, 2024. https://www.usps.com/ship
7. Cheng C, Ganz DA, Chang ET, Huynh A, De Peralta S. Reducing rejected fecal immunochemical tests received in the laboratory for colorectal cancer screening. J Healthc Qual. 2019;41(2):75-82.doi:10.1097/JHQ.0000000000000181
8. Hussaini SMQ, Alexander GC. The United States Postal Service: an essential public health agency? J Gen Intern Med. 2020;35(12):3699-3701. doi:10.1007/s11606-020-06275-2
9. Hampton DJ. Colorado mountain towns are plagued by post office delays as residents wait weeks for medication and retirement checks. NBC News. February 25, 2023. Accessed March 14, 2024. https://www.nbcnews.com/news/us-news/colo-mountain-towns-are-plagued-post-office-delays-residents-wait-week-rcna72085
Moral Injury in Health Care: A Unified Definition and its Relationship to Burnout
Moral injury was identified by health care professionals (HCPs) as a driver of occupational distress prior to the COVID-19 pandemic, but the crisis expanded the appeal and investigation of the term.1 HCPs now consider moral injury an essential component of the framework to describe their distress, because using the term burnout alone fails to capture their full experience and has proven resistant to interventions.2 Moral injury goes beyond the transdiagnostic symptoms of exhaustion and cynicism and beyond operational, demand-resource mismatches that characterize burnout. It describes the frustration, anger, and helplessness associated with relational ruptures and the existential threats to a clinician’s professional identity as business interests erode their ability to put their patients’ needs ahead of corporate and health care system obligations.3
Proper characterization of moral injury in health care—separate from the military environments where it originated—is stymied by an ill-defined relationship between 2 definitions of the term and by an unclear relationship between moral injury and the long-standing body of scholarship in burnout. To clarify the concept, inform research agendas, and open avenues for more effective solutions to the crisis of HCP distress, we propose a unified conceptualization of moral injury and its association with burnout in health care.
CONTEXTUAL DISTINCTIONS
It is important to properly distinguish between the original use of moral injury in the military and its expanded use in civilian circumstances. Health care and the military are both professions whereupon donning the “uniform” of a physician—or soldier, sailor, airman, or marine—members must comport with strict expectations of behavior, including the refusal to engage in illegal actions or those contrary to professional ethics. Individuals in both professions acquire a highly specialized body of knowledge and enter an implied contract to provide critical services to society, specifically healing and protection, respectively. Members of both professions are trained to make complex judgments with integrity under conditions of technical and ethical uncertainty, upon which they take highly skilled action. Medical and military professionals must be free to act on their ethical principles, without confounding demands.4 However, the context of each profession’s commitment to society carries different moral implications.
The risk of moral injury is inherent in military service. The military promises protection with an implicit acknowledgment of the need to use lethal force to uphold the agreement. In contrast, HCPs promise healing and care. The military promises to protect our society, with an implicit acknowledgment of the need to use lethal force to uphold the agreement. Some military actions may inflict harm without the hope of benefitting an individual, and are therefore potentially morally injurious. The health care contract with society, promising healing and care, is devoid of inherent moral injury due to harm without potential individual benefit. Therefore, the presence of moral injury in health care settings are warning signs of a dysfunctional environment.
One complex example of the dysfunctional environments is illustrative. The military and health care are among the few industries where supply creates demand. For example, the more bad state actors there are, the more demand for the military. As we have seen since the 1950s, the more technology and therapeutics we create in health care, coupled with a larger share paid for by third parties, the greater the demand for and use of them.5 In a fee for service environment, corporate greed feeds on this reality. In most other environments, more technological and therapeutic options inevitably pit clinicians against multiple other factions: payers, who do not want to underwrite them; patients, who sometimes demand them without justification or later rail against spiraling health care costs; and administrators, especially in capitated systems, who watch their bottom lines erode. The moral injury risk in this instance demands a collective conversation among stakeholders regarding the structural determinants of health—how we choose to distribute limited resources. The intermediary of moral injury is a useful measure of the harm that results from ignoring or avoiding such challenges.
HARMONIZING DEFINITIONS
Moral injury is inherently nuanced. The 2 dominant definitions arise from work with combat veterans and create additional and perhaps unnecessary complexity. Unifying these 2 definitions eliminates inadvertent confusion, preventing the risk of unbridled interdisciplinary investigation which leads to a lack of precision in the meaning of moral injury and other related concepts, such as burnout.6
The first definition was developed by Jonathan Shay in 1994 and outlines 3 necessarycomponents, viewing the violator as a powerholder: (1) betrayal of what is right, (2) by someone who holds legitimate authority, (3) in a high stakes situation.7 Litz and colleagues describe moral injury another way: “Perpetrating, failing to prevent, bearing witness to, or learning about acts that transgress deeply held moral beliefs and expectations.”8 The violator is posited to be either the self or others.
Rather than representing “self” or “other” imposed moral injury, we propose the 2 definitions are related as exposure (ie, the perceived betrayal) and response (ie, the resulting transgression). An individual who experiences a betrayal by a legitimate authority has an opportunity to choose their response. They may acquiesce and transgress their moral beliefs (eg, their oath to provide ethical health care), or they could refuse, by speaking out, or in some way resisting the authority’s betrayal. The case of Ray Brovont is a useful illustration of reconciling the definitions (Box).9
Myriad factors—known as potentially morally injurious events—drive moral injury, such as resource-constrained decision making, witnessing the behaviors of colleagues that violate deeply held moral beliefs, questionable billing practices, and more. Each begins with a betrayal. Spotlighting the betrayal, refusing to perpetuate it, or taking actions toward change, may reduce the risk of experiencing moral injury.9 Conversely, acquiescing and transgressing one’s oath, the profession’s covenant with society, increases the risk of experiencing moral injury.8
Many HCPs believe they are not always free to resist betrayal, fearing retaliation, job loss, blacklisting, or worse. They feel constrained by debt accrued while receiving their education, being their household’s primary earner, community ties, practicing a niche specialty that requires working for a tertiary referral center, or perhaps believing the situation will be the same elsewhere. To not stand up or speak out is to choose complicity with corporate greed that uses HCPs to undermine their professional duties, which significantly increases the risk of experiencing moral injury.
MORAL INJURY AND BURNOUT
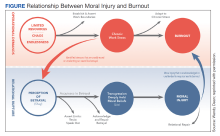
In addition to reconciling the definitions of moral injury, the relationship between moral injury and burnout are still being elucidated. We suggest that moral injury and burnout represent independent and potentially interrelated pathways to distress (Figure). Exposure to chronic, inconsonant, and transactional demands, which things like shorter work hours, better self-care, or improved health system operations might mitigate, manifests as burnout. In contrast, moral injury arises when a superior’s actions or a system’s policies and practices—such as justifiable but unnecessary testing, or referral restrictions to prevent revenue leakage—undermine one’s professional obligations to prioritize the patient’s best interest.
If concerns from HCPs about transactional demands are persistently dismissed, such inaction may be perceived as a betrayal, raising the risk of moral injury. Additionally, the resignation or helplessness of moral injury perceived as inescapable may present with emotional exhaustion, ineffectiveness, and depersonalization, all hallmarks of burnout. Both conditions can mediate and moderate the relationship between triggers for workplace distress and resulting psychological, physical, and existential harm.
CONCLUSIONS
Moral injury is increasingly recognized as a source of distress among HCPs, resulting from structural constraints on their ability to deliver optimal care and their own unwillingness to stand up for their patients, their oaths, and their professions.1 Unlike the military, where moral injury is inherent in the contract with society, moral injury in health care (and the relational rupture it connotes) is a signal of systemic dysfunction, fractured trust, and the need for relational repair.
Health care is at a crossroads, experiencing a workforce retention crisis while simultaneously predicting a significant increase in care needs by Baby Boomers over the next 3 decades.
Health care does not have the luxury of experimenting another 30 years with interventions that have limited impact. We must design a new generation of approaches, shaped by lessons learned from the pandemic while acknowledging that prepandemic standards were already failing the workforce. A unified definition of moral injury must be integrated to frame clinician distress alongside burnout, recentering ethical decision making, rather than profit, at the heart of health care. Harmonizing the definitions of moral injury and clarifying the relationship of moral injury with burnout reduces the need for further reinterpretations, allowing for more robust, easily comparable studies focused on identifying risk factors, as well as rapidly implementing effective mitigation strategies.
1. Griffin BJ, Weber MC, Hinkson KD, et al. Toward a dimensional contextual model of moral injury: a scoping review on healthcare workers. Curr Treat Options Psych. 2023;10:199-216. doi:10.1007/s40501-023-00296-4
2. National Academies of Sciences, Engineering, and Medicine; National Academy of Medicine; Committee on Systems Approaches to Improve Patient Care by Supporting Clinician Well-Being. Taking Action Against Clinician Burnout: A Systems Approach to Professional Well-Being. The National Academies Press; 2019. doi:10.17226/25521
3. Dean W, Talbot S, Dean A. Reframing clinician distress: moral injury not burnout. Fed Pract. 2019;36(9):400-402.
4. Gardner HE, Schulman LS. The professions in America today: crucial but fragile. Daedalus. 2005;134(3):13-18. doi:10.1162/0011526054622132
5. Fuchs VR. Major trends in the U.S. health economy since 1950. N Engl J Med. 2012;366(11):973-977. doi:10.1056/NEJMp1200478
6. Molendijk T. Warnings against romanticising moral injury. Br J Psychiatry. 2022;220(1):1-3. doi:10.1192/bjp.2021.114
7. Shay J. Moral injury. Psychoanalytic Psychol. 2014;31(2):182-191. doi:10.1037/a0036090
8. Litz BT, Stein N, Delaney E, et al. Moral injury and moral repair in war veterans: a preliminary model and intervention strategy. Clin Psychol Rev. 2009;29(8):695-706. doi:10.1016/j.cpr.2009.07.003
9. Brovont v KS-I Med. Servs., P.A., 622 SW3d 671 (Mo Ct App 2020).
Moral injury was identified by health care professionals (HCPs) as a driver of occupational distress prior to the COVID-19 pandemic, but the crisis expanded the appeal and investigation of the term.1 HCPs now consider moral injury an essential component of the framework to describe their distress, because using the term burnout alone fails to capture their full experience and has proven resistant to interventions.2 Moral injury goes beyond the transdiagnostic symptoms of exhaustion and cynicism and beyond operational, demand-resource mismatches that characterize burnout. It describes the frustration, anger, and helplessness associated with relational ruptures and the existential threats to a clinician’s professional identity as business interests erode their ability to put their patients’ needs ahead of corporate and health care system obligations.3
Proper characterization of moral injury in health care—separate from the military environments where it originated—is stymied by an ill-defined relationship between 2 definitions of the term and by an unclear relationship between moral injury and the long-standing body of scholarship in burnout. To clarify the concept, inform research agendas, and open avenues for more effective solutions to the crisis of HCP distress, we propose a unified conceptualization of moral injury and its association with burnout in health care.
CONTEXTUAL DISTINCTIONS
It is important to properly distinguish between the original use of moral injury in the military and its expanded use in civilian circumstances. Health care and the military are both professions whereupon donning the “uniform” of a physician—or soldier, sailor, airman, or marine—members must comport with strict expectations of behavior, including the refusal to engage in illegal actions or those contrary to professional ethics. Individuals in both professions acquire a highly specialized body of knowledge and enter an implied contract to provide critical services to society, specifically healing and protection, respectively. Members of both professions are trained to make complex judgments with integrity under conditions of technical and ethical uncertainty, upon which they take highly skilled action. Medical and military professionals must be free to act on their ethical principles, without confounding demands.4 However, the context of each profession’s commitment to society carries different moral implications.
The risk of moral injury is inherent in military service. The military promises protection with an implicit acknowledgment of the need to use lethal force to uphold the agreement. In contrast, HCPs promise healing and care. The military promises to protect our society, with an implicit acknowledgment of the need to use lethal force to uphold the agreement. Some military actions may inflict harm without the hope of benefitting an individual, and are therefore potentially morally injurious. The health care contract with society, promising healing and care, is devoid of inherent moral injury due to harm without potential individual benefit. Therefore, the presence of moral injury in health care settings are warning signs of a dysfunctional environment.
One complex example of the dysfunctional environments is illustrative. The military and health care are among the few industries where supply creates demand. For example, the more bad state actors there are, the more demand for the military. As we have seen since the 1950s, the more technology and therapeutics we create in health care, coupled with a larger share paid for by third parties, the greater the demand for and use of them.5 In a fee for service environment, corporate greed feeds on this reality. In most other environments, more technological and therapeutic options inevitably pit clinicians against multiple other factions: payers, who do not want to underwrite them; patients, who sometimes demand them without justification or later rail against spiraling health care costs; and administrators, especially in capitated systems, who watch their bottom lines erode. The moral injury risk in this instance demands a collective conversation among stakeholders regarding the structural determinants of health—how we choose to distribute limited resources. The intermediary of moral injury is a useful measure of the harm that results from ignoring or avoiding such challenges.
HARMONIZING DEFINITIONS
Moral injury is inherently nuanced. The 2 dominant definitions arise from work with combat veterans and create additional and perhaps unnecessary complexity. Unifying these 2 definitions eliminates inadvertent confusion, preventing the risk of unbridled interdisciplinary investigation which leads to a lack of precision in the meaning of moral injury and other related concepts, such as burnout.6
The first definition was developed by Jonathan Shay in 1994 and outlines 3 necessarycomponents, viewing the violator as a powerholder: (1) betrayal of what is right, (2) by someone who holds legitimate authority, (3) in a high stakes situation.7 Litz and colleagues describe moral injury another way: “Perpetrating, failing to prevent, bearing witness to, or learning about acts that transgress deeply held moral beliefs and expectations.”8 The violator is posited to be either the self or others.
Rather than representing “self” or “other” imposed moral injury, we propose the 2 definitions are related as exposure (ie, the perceived betrayal) and response (ie, the resulting transgression). An individual who experiences a betrayal by a legitimate authority has an opportunity to choose their response. They may acquiesce and transgress their moral beliefs (eg, their oath to provide ethical health care), or they could refuse, by speaking out, or in some way resisting the authority’s betrayal. The case of Ray Brovont is a useful illustration of reconciling the definitions (Box).9
Myriad factors—known as potentially morally injurious events—drive moral injury, such as resource-constrained decision making, witnessing the behaviors of colleagues that violate deeply held moral beliefs, questionable billing practices, and more. Each begins with a betrayal. Spotlighting the betrayal, refusing to perpetuate it, or taking actions toward change, may reduce the risk of experiencing moral injury.9 Conversely, acquiescing and transgressing one’s oath, the profession’s covenant with society, increases the risk of experiencing moral injury.8
Many HCPs believe they are not always free to resist betrayal, fearing retaliation, job loss, blacklisting, or worse. They feel constrained by debt accrued while receiving their education, being their household’s primary earner, community ties, practicing a niche specialty that requires working for a tertiary referral center, or perhaps believing the situation will be the same elsewhere. To not stand up or speak out is to choose complicity with corporate greed that uses HCPs to undermine their professional duties, which significantly increases the risk of experiencing moral injury.
MORAL INJURY AND BURNOUT
In addition to reconciling the definitions of moral injury, the relationship between moral injury and burnout are still being elucidated. We suggest that moral injury and burnout represent independent and potentially interrelated pathways to distress (Figure). Exposure to chronic, inconsonant, and transactional demands, which things like shorter work hours, better self-care, or improved health system operations might mitigate, manifests as burnout. In contrast, moral injury arises when a superior’s actions or a system’s policies and practices—such as justifiable but unnecessary testing, or referral restrictions to prevent revenue leakage—undermine one’s professional obligations to prioritize the patient’s best interest.
If concerns from HCPs about transactional demands are persistently dismissed, such inaction may be perceived as a betrayal, raising the risk of moral injury. Additionally, the resignation or helplessness of moral injury perceived as inescapable may present with emotional exhaustion, ineffectiveness, and depersonalization, all hallmarks of burnout. Both conditions can mediate and moderate the relationship between triggers for workplace distress and resulting psychological, physical, and existential harm.
CONCLUSIONS
Moral injury is increasingly recognized as a source of distress among HCPs, resulting from structural constraints on their ability to deliver optimal care and their own unwillingness to stand up for their patients, their oaths, and their professions.1 Unlike the military, where moral injury is inherent in the contract with society, moral injury in health care (and the relational rupture it connotes) is a signal of systemic dysfunction, fractured trust, and the need for relational repair.
Health care is at a crossroads, experiencing a workforce retention crisis while simultaneously predicting a significant increase in care needs by Baby Boomers over the next 3 decades.
Health care does not have the luxury of experimenting another 30 years with interventions that have limited impact. We must design a new generation of approaches, shaped by lessons learned from the pandemic while acknowledging that prepandemic standards were already failing the workforce. A unified definition of moral injury must be integrated to frame clinician distress alongside burnout, recentering ethical decision making, rather than profit, at the heart of health care. Harmonizing the definitions of moral injury and clarifying the relationship of moral injury with burnout reduces the need for further reinterpretations, allowing for more robust, easily comparable studies focused on identifying risk factors, as well as rapidly implementing effective mitigation strategies.
Moral injury was identified by health care professionals (HCPs) as a driver of occupational distress prior to the COVID-19 pandemic, but the crisis expanded the appeal and investigation of the term.1 HCPs now consider moral injury an essential component of the framework to describe their distress, because using the term burnout alone fails to capture their full experience and has proven resistant to interventions.2 Moral injury goes beyond the transdiagnostic symptoms of exhaustion and cynicism and beyond operational, demand-resource mismatches that characterize burnout. It describes the frustration, anger, and helplessness associated with relational ruptures and the existential threats to a clinician’s professional identity as business interests erode their ability to put their patients’ needs ahead of corporate and health care system obligations.3
Proper characterization of moral injury in health care—separate from the military environments where it originated—is stymied by an ill-defined relationship between 2 definitions of the term and by an unclear relationship between moral injury and the long-standing body of scholarship in burnout. To clarify the concept, inform research agendas, and open avenues for more effective solutions to the crisis of HCP distress, we propose a unified conceptualization of moral injury and its association with burnout in health care.
CONTEXTUAL DISTINCTIONS
It is important to properly distinguish between the original use of moral injury in the military and its expanded use in civilian circumstances. Health care and the military are both professions whereupon donning the “uniform” of a physician—or soldier, sailor, airman, or marine—members must comport with strict expectations of behavior, including the refusal to engage in illegal actions or those contrary to professional ethics. Individuals in both professions acquire a highly specialized body of knowledge and enter an implied contract to provide critical services to society, specifically healing and protection, respectively. Members of both professions are trained to make complex judgments with integrity under conditions of technical and ethical uncertainty, upon which they take highly skilled action. Medical and military professionals must be free to act on their ethical principles, without confounding demands.4 However, the context of each profession’s commitment to society carries different moral implications.
The risk of moral injury is inherent in military service. The military promises protection with an implicit acknowledgment of the need to use lethal force to uphold the agreement. In contrast, HCPs promise healing and care. The military promises to protect our society, with an implicit acknowledgment of the need to use lethal force to uphold the agreement. Some military actions may inflict harm without the hope of benefitting an individual, and are therefore potentially morally injurious. The health care contract with society, promising healing and care, is devoid of inherent moral injury due to harm without potential individual benefit. Therefore, the presence of moral injury in health care settings are warning signs of a dysfunctional environment.
One complex example of the dysfunctional environments is illustrative. The military and health care are among the few industries where supply creates demand. For example, the more bad state actors there are, the more demand for the military. As we have seen since the 1950s, the more technology and therapeutics we create in health care, coupled with a larger share paid for by third parties, the greater the demand for and use of them.5 In a fee for service environment, corporate greed feeds on this reality. In most other environments, more technological and therapeutic options inevitably pit clinicians against multiple other factions: payers, who do not want to underwrite them; patients, who sometimes demand them without justification or later rail against spiraling health care costs; and administrators, especially in capitated systems, who watch their bottom lines erode. The moral injury risk in this instance demands a collective conversation among stakeholders regarding the structural determinants of health—how we choose to distribute limited resources. The intermediary of moral injury is a useful measure of the harm that results from ignoring or avoiding such challenges.
HARMONIZING DEFINITIONS
Moral injury is inherently nuanced. The 2 dominant definitions arise from work with combat veterans and create additional and perhaps unnecessary complexity. Unifying these 2 definitions eliminates inadvertent confusion, preventing the risk of unbridled interdisciplinary investigation which leads to a lack of precision in the meaning of moral injury and other related concepts, such as burnout.6
The first definition was developed by Jonathan Shay in 1994 and outlines 3 necessarycomponents, viewing the violator as a powerholder: (1) betrayal of what is right, (2) by someone who holds legitimate authority, (3) in a high stakes situation.7 Litz and colleagues describe moral injury another way: “Perpetrating, failing to prevent, bearing witness to, or learning about acts that transgress deeply held moral beliefs and expectations.”8 The violator is posited to be either the self or others.
Rather than representing “self” or “other” imposed moral injury, we propose the 2 definitions are related as exposure (ie, the perceived betrayal) and response (ie, the resulting transgression). An individual who experiences a betrayal by a legitimate authority has an opportunity to choose their response. They may acquiesce and transgress their moral beliefs (eg, their oath to provide ethical health care), or they could refuse, by speaking out, or in some way resisting the authority’s betrayal. The case of Ray Brovont is a useful illustration of reconciling the definitions (Box).9
Myriad factors—known as potentially morally injurious events—drive moral injury, such as resource-constrained decision making, witnessing the behaviors of colleagues that violate deeply held moral beliefs, questionable billing practices, and more. Each begins with a betrayal. Spotlighting the betrayal, refusing to perpetuate it, or taking actions toward change, may reduce the risk of experiencing moral injury.9 Conversely, acquiescing and transgressing one’s oath, the profession’s covenant with society, increases the risk of experiencing moral injury.8
Many HCPs believe they are not always free to resist betrayal, fearing retaliation, job loss, blacklisting, or worse. They feel constrained by debt accrued while receiving their education, being their household’s primary earner, community ties, practicing a niche specialty that requires working for a tertiary referral center, or perhaps believing the situation will be the same elsewhere. To not stand up or speak out is to choose complicity with corporate greed that uses HCPs to undermine their professional duties, which significantly increases the risk of experiencing moral injury.
MORAL INJURY AND BURNOUT
In addition to reconciling the definitions of moral injury, the relationship between moral injury and burnout are still being elucidated. We suggest that moral injury and burnout represent independent and potentially interrelated pathways to distress (Figure). Exposure to chronic, inconsonant, and transactional demands, which things like shorter work hours, better self-care, or improved health system operations might mitigate, manifests as burnout. In contrast, moral injury arises when a superior’s actions or a system’s policies and practices—such as justifiable but unnecessary testing, or referral restrictions to prevent revenue leakage—undermine one’s professional obligations to prioritize the patient’s best interest.
If concerns from HCPs about transactional demands are persistently dismissed, such inaction may be perceived as a betrayal, raising the risk of moral injury. Additionally, the resignation or helplessness of moral injury perceived as inescapable may present with emotional exhaustion, ineffectiveness, and depersonalization, all hallmarks of burnout. Both conditions can mediate and moderate the relationship between triggers for workplace distress and resulting psychological, physical, and existential harm.
CONCLUSIONS
Moral injury is increasingly recognized as a source of distress among HCPs, resulting from structural constraints on their ability to deliver optimal care and their own unwillingness to stand up for their patients, their oaths, and their professions.1 Unlike the military, where moral injury is inherent in the contract with society, moral injury in health care (and the relational rupture it connotes) is a signal of systemic dysfunction, fractured trust, and the need for relational repair.
Health care is at a crossroads, experiencing a workforce retention crisis while simultaneously predicting a significant increase in care needs by Baby Boomers over the next 3 decades.
Health care does not have the luxury of experimenting another 30 years with interventions that have limited impact. We must design a new generation of approaches, shaped by lessons learned from the pandemic while acknowledging that prepandemic standards were already failing the workforce. A unified definition of moral injury must be integrated to frame clinician distress alongside burnout, recentering ethical decision making, rather than profit, at the heart of health care. Harmonizing the definitions of moral injury and clarifying the relationship of moral injury with burnout reduces the need for further reinterpretations, allowing for more robust, easily comparable studies focused on identifying risk factors, as well as rapidly implementing effective mitigation strategies.
1. Griffin BJ, Weber MC, Hinkson KD, et al. Toward a dimensional contextual model of moral injury: a scoping review on healthcare workers. Curr Treat Options Psych. 2023;10:199-216. doi:10.1007/s40501-023-00296-4
2. National Academies of Sciences, Engineering, and Medicine; National Academy of Medicine; Committee on Systems Approaches to Improve Patient Care by Supporting Clinician Well-Being. Taking Action Against Clinician Burnout: A Systems Approach to Professional Well-Being. The National Academies Press; 2019. doi:10.17226/25521
3. Dean W, Talbot S, Dean A. Reframing clinician distress: moral injury not burnout. Fed Pract. 2019;36(9):400-402.
4. Gardner HE, Schulman LS. The professions in America today: crucial but fragile. Daedalus. 2005;134(3):13-18. doi:10.1162/0011526054622132
5. Fuchs VR. Major trends in the U.S. health economy since 1950. N Engl J Med. 2012;366(11):973-977. doi:10.1056/NEJMp1200478
6. Molendijk T. Warnings against romanticising moral injury. Br J Psychiatry. 2022;220(1):1-3. doi:10.1192/bjp.2021.114
7. Shay J. Moral injury. Psychoanalytic Psychol. 2014;31(2):182-191. doi:10.1037/a0036090
8. Litz BT, Stein N, Delaney E, et al. Moral injury and moral repair in war veterans: a preliminary model and intervention strategy. Clin Psychol Rev. 2009;29(8):695-706. doi:10.1016/j.cpr.2009.07.003
9. Brovont v KS-I Med. Servs., P.A., 622 SW3d 671 (Mo Ct App 2020).
1. Griffin BJ, Weber MC, Hinkson KD, et al. Toward a dimensional contextual model of moral injury: a scoping review on healthcare workers. Curr Treat Options Psych. 2023;10:199-216. doi:10.1007/s40501-023-00296-4
2. National Academies of Sciences, Engineering, and Medicine; National Academy of Medicine; Committee on Systems Approaches to Improve Patient Care by Supporting Clinician Well-Being. Taking Action Against Clinician Burnout: A Systems Approach to Professional Well-Being. The National Academies Press; 2019. doi:10.17226/25521
3. Dean W, Talbot S, Dean A. Reframing clinician distress: moral injury not burnout. Fed Pract. 2019;36(9):400-402.
4. Gardner HE, Schulman LS. The professions in America today: crucial but fragile. Daedalus. 2005;134(3):13-18. doi:10.1162/0011526054622132
5. Fuchs VR. Major trends in the U.S. health economy since 1950. N Engl J Med. 2012;366(11):973-977. doi:10.1056/NEJMp1200478
6. Molendijk T. Warnings against romanticising moral injury. Br J Psychiatry. 2022;220(1):1-3. doi:10.1192/bjp.2021.114
7. Shay J. Moral injury. Psychoanalytic Psychol. 2014;31(2):182-191. doi:10.1037/a0036090
8. Litz BT, Stein N, Delaney E, et al. Moral injury and moral repair in war veterans: a preliminary model and intervention strategy. Clin Psychol Rev. 2009;29(8):695-706. doi:10.1016/j.cpr.2009.07.003
9. Brovont v KS-I Med. Servs., P.A., 622 SW3d 671 (Mo Ct App 2020).
Graduate Medical Education Financing in the US Department of Veterans Affairs
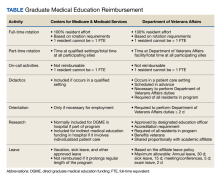
The US Department of Veterans Affairs (VA) has partnered with academic medical centers and programs since 1946 to provide clinical training for physician residents. Ranking second in federal graduate medical education (GME) funding to the Centers for Medicare and Medicaid Services (CMS), the $850 million VA GME budget annually reimburses > 250 GME-sponsoring institutions (affiliates) of 8000 GME programs for the clinical training of 49,000 individual residents rotating through > 11,000 full-time equivalent (FTE) positions.1 The VA also distributes $1.6 billion to VA facilities to offset the costs of conducting health professions education (HPE) (eg, facility infrastructure, salary support for VA instructors and preceptors, education office administration, and instructional equipment).2 The VA financial and educational contributions account for payment of 11% of resident positions nationally and allow academic medical centers to be less reliant on CMS GME funding.3,4 The VA contributions also provide opportunities for GME expansion,1,5,6 educational innovations,5,7 interprofessional and team-based care,8,9 and quality and safety training.10,11 The Table provides a comparison of CMS and VA GME reimbursability based on activity.
GME financing is complex, particularly the formulaic approach used by CMS, the details of which are often obscured in federal regulations. Due to this complexity and the $16 billion CMS GME budget, academic publications have focused on CMS GME financing while not fully explaining the VA GME policies and processes.4,12-14 By comparison, the VA GME financing model is relatively straightforward and governed by different statues and VA regulations, yet sharing some of the same principles as CMS regulations. Given the challenges in CMS reimbursement to fully support the cost of resident education, as well as the educational opportunities at the VA, the VA designs its reimbursement model to assure that affiliates receive appropriate payments.4,12,15 To ensure the continued success of VA GME partnerships, knowledge of VA GME financing has become increasingly important for designated institutional officers (DIOs) and residency program directors, particularly in light of recent investigations into oversight of the VA’s reimbursement to academic affiliates.
VA AUTHORITY
While the VA’s primary mission is “to provide a complete hospital medical service for the medical care and treatment of veterans,” early VA leaders recognized the importance of affiliating with the nation’s academic institutions.19 In 1946, the VA Policy Memorandum Number 2 established a partnership between the VA and the academic medical community.20 Additional legislation authorized specific agreements with academic affiliates for the central administration of salary and benefits for residents rotating at VA facilities. This process, known as disbursement, is an alternative payroll mechanism whereby the VA reimburses the academic affiliate for resident salary and benefits and the affiliate acts as the disbursing agent, issuing paychecks to residents.21,22
Resident FUNDING
By policy, with rare exceptions, the VA does not sponsor residency programs due to the challenges of providing an appropriate patient mix of age, sex, and medical conditions to meet accreditation standards.4 Nearly all VA reimbursements are for residents in affiliate-sponsored programs, while just 1% pays for residents in legacy, VA-sponsored residency programs at 2 VA facilities. The VA budget for resident (including fellows) salary and benefits is managed by the VA Office of Academic Affiliations (OAA), the national VA office responsible for oversight, policy, and funding of VA HPE programs.
Resident Salaries and Benefits
VA funding of resident salary and benefits are analogous with CMS direct GME (DGME), which is designed to cover resident salary and benefits costs.4,14,23 CMS DGME payments depend on a hospital’s volume of CMS inpatients and are based on a statutory formula, which uses the hospital’s resident FTE positions, the per-resident amount, and Medicare’s share of inpatient beds (Medicare patient load) to determine payments.12 The per-resident amount is set by statute, varies geographically, and is calculated by dividing the hospital’s allowable costs of GME (percentage of CMS inpatient days) divided by the number of residents.12,24
By comparison, the VA GME payment reimburses for each FTE based on the salary and benefits rate set by the academic affiliate. Reimbursement is calculated based on resident time spent at the VA multiplied by a daily salary rate. The daily salary rate is determined by dividing the resident’s total compensation (salary and benefits) by the number of calendar days in an academic year. Resident time spent at the VA facility is determined by obtaining rotation schedules provided by the academic affiliate and verifying resident clinical and educational activity during scheduled rotations.
Indirect Medical Education Funding
In addition to resident salary and benefits, funds to offset the cost of conducting HPE are provided to VA facilities. These funds are intended to improve and maintain necessary infrastructure for all HPE programs not just GME, including education office administration needs, teaching costs (ie, a portion of VA preceptors salary), and instructional equipment.
The Veterans Equitable Resource Allocation (VERA) is a national budgeting process for VA medical facilities that funds facility operational needs such as staff salary and benefits, infrastructure, and equipment.2 The education portion of the VERA, the VERA Education Support Component (VESC), is not managed by the OAA, but rather is distributed through the VERA model to the general budget of VA facilities hosting HPE (Figure). VESC funding in the VA budget is based on labor mapping of physician time spent in education; other labor mapping categories include clinical care, research, and administration. VA facility VESC funding is calculated based on the number of paid health profession trainees (HPTs) from all professions, apportioned according to the number of FTEs for physician residents and VA-paid HPTs in other disciplines. In fiscal year 2024, VA facilities received $115,812 for each physician resident FTE position and $84,906 for each VA-paid, non-GME FTE position.
The VESC is like CMS's indirect GME funding, termed Indirect Medical Education (IME), an additional payment for each Medicare patient discharged reflecting teaching hospitals’ higher patient care costs relative to nonteaching hospitals. Described elsewhere, IME is calculated using a resident-to-bed ratio and a multiplier, which is set by statute.4,25 While IME can be used for reimbursement for some resident clinical and educational activities(eg, research), VA VESC funds cannot be used for such activities and are part of the general facility budget and appropriated per the discretion of the medical facility director.
ESTABLISHING GME PARTNERSHIPS
An affiliation agreement establishes the administrative and legal requirements for educational relationships with academic affiliates and includes standards for conducting HPE, responsibilities for accreditation standards, program leadership, faculty, resources, supervision, academic policies, and procedures. The VA uses standardized affiliation agreement templates that have been vetted with accrediting bodies and the VA Office of General Counsel.
A disbursement agreement authorizes the VA to reimburse affiliates for resident salary and benefits for VA clinical and educational activities. The disbursement agreement details the fiscal arrangements (eg, payment in advance vs arrears, salary, and benefit rates, leave) for the reimbursement payments. Veterans Health Administration (VHA) Directive 1400.05 provides the policy and procedures for calculating reimbursement for HPT educational activities.26
The VA facility designated education officer (DEO) oversees all HPE programs and coordinates the affiliation and disbursement agreement processes.27 The DEO, affiliate DIO, residency program director, and VA residency site director determine the physician resident FTE positions assigned to a VA facility based on educational objectives and availability of educational resources at the VA facility, such as patient care opportunities, faculty supervisors, space, and equipment. The VA facility requests for resident FTE positions are submitted to the OAA by the facility DEO.
Once GME FTE positions are approved by the OAA, VA facilities work with their academic affiliate to submit the physician resident salary and benefit rate. Affiliate DIOs attest to the accuracy of the salary rate schedule and the local DEO submits the budget request to the OAA. Upon approval, the funds are transferred to the VA facility each fiscal year, which begins October 1. DEOs report quarterly to the OAA both budget needs and excesses based on variations in the approved FTEs due to additional VA rotations, physician resident attrition, or reassignment.
Resident Position Allocation
VA GME financing provides flexibility through periodic needs assessments and expansion initiatives. In August and December, DEOs collaborate with an academic affiliate to submit reports to the OAA confirming their projected GME needs for the next academic year. Additional positions requests are reviewed by the OAA; funding depends on budget and the educational justification. The OAA periodically issues GME expansion requests for proposal, which typically arise from legislation to address specific VA workforce needs. The VA facility DEO and affiliate GME leaders collaborate to apply for additional positions. For example, a VA GME expansion under the Veterans Access, Choice, and Accountability Act of 2014 added 1500 GME positions in 8 years for critically needed specialties and in rural and underserved areas.5 The Maintaining Internal Systems and Strengthening Outside Networks (MISSION) Act of 2018 authorized a pilot program for VA to fund residents at non-VA facilities with priority for Indian Health Services, Tribes and Tribal Organizations, Federally Qualified Health Centers, and US Department of Defense facilities to provide access to veterans in underserved areas.6
The VA GME financing system has flexibility to meet local needs for additional resident positions and to address broader VA workforce gaps through targeted expansion. Generally, CMS does not fund positions to address workforce needs, place residents in specific geographic areas, or require the training of certain types of residents.4 However, the Consolidated Appropriations Act of 2021 has provided the opportunity to address rural workforce needs.28
Reimbursement
The VA provides reimbursement for clinical and educational activities performed in VA facilities for the benefit of veterans as well as research, didactics, meetings and conferences, annual and sick leave, and orientation. The VA also may provide reimbursement for educational activities that occur off VA grounds (eg, the VA proportional share of a residency program’s didactic sessions). The VA does not reimburse for affiliate clinical duties or administrative costs, although a national policy allows VA facilities to reimburse affiliates for some GME overhead costs.29
CMS similarly reimburses for residency training time spent in patient care activities as well as orientation activities, didactics, leave, and, in some cases, research.4,30,31 CMS makes payments to hospitals, which may include sponsoring institutions and Medicare-eligible participating training sites.4,30,31 For both the VA and CMS, residents may not be counted twice for reimbursement by 2 federal agencies; in other words, a resident may not count for > 1 FTE.4,30-32
GME Oversight
VA GME funding came under significant scrutiny. At a 2016 House Veterans Affairs Committee hearing, Representative Phil Roe, MD (R-Tennessee), noted that no process existed at many VA facilities for “determining trainee presence” and that many VA medical centers had “difficulty tracking resident rotations”16 A VA Office of the Inspector General investigation recommended that the VA implement policies and procedures to improve oversight to “ensure residents are fully participating in educational activities” and that the VA is “paying the correct amount” to the affiliate.17 A 2020 General Accountability Office report outlined unclear policy guidance, incomplete tracking of resident activities, and improper fiscal processes for reimbursement and reconciliation of affiliate invoices.18
In response, the OAA created an oversight and compliance unit, revised VHA Directive 1400.05 (the policy for disbursement), and improved resident tracking procedures.26 The standard operating procedure that accompanied VHA Directive 1400.05 provides detailed information for the DEO and VA facility staff for tracking resident clinical and educational activities. FTE counts are essential to both VA and CMS for accurate reimbursement. The eAppendix and the Table provide a guide to reimbursable activities in the VA for the calculation of reimbursement, with a comparison to CMS.33,34 The OAA in cooperation with other VA staff and officers periodically conducts audits to assess compliance with disbursement policy and affiliate reimbursement accuracy.
In the VA, resident activities are captured on the VA Educational Activity Record, a standardized spreadsheet to track activities and calculate reimbursement. Each VA facility hosting resident physicians manually records resident activity by the half-day. This process is labor intensive, involving both VA and affiliate staff to accurately reconcile payments. To address the workload demands, the OAA is developing an online tool that will automate aspects of the tracking process. Also, to ensure adequate staffing, the OAA is in the process of implementing an office optimization project, providing standardized position descriptions, an organizational chart, and staffing levels for DEO offices in VA facilities.
Conclusions
This report describes the key policies and principles of VA GME financing, highlighting the essential similarities and differences between VA and CMS. Neither the VA nor CMS regulations allow for reimbursement for > 1 FTE position per resident, a principle that underpins the assignment of resident rotations and federal funding for GME and are similar with respect to reimbursement for patient care activities, didactics, research, orientation, and scholarly activity. While reimbursable activities in the VA require physical presence and care of veteran patients, CMS also limits reimbursement to resident activities in the hospital and approved other settings if the hospital is paying for resident salary and benefits in these settings. The VA provides some flexibility for offsite activities including didactics and, in specific circumstances, remote care of veteran patients (eg, teleradiology).
The VA and CMS use different GME financing models. For example, the CMS calculations for resident FTEs are complex, whereas VA calculations reimburse the salary and benefits as set by the academic affiliate. The VA process accounts for local variation in salary rates, whereas the per-resident amount set by CMS varies regionally and does not fully account for differences in the cost of living.24 Because all patients in VA facilities are veterans, VA calculations for reimbursement do not involve ratios of beds like the CMS calculations to determine a proportional share of reimbursement. The VA GME expansion tends to be more directed to VA health workforce needs than CMS, specifying the types of programs and geographic locations to address these needs.
The VA regularly reevaluates how affiliates are reimbursed for VA resident activity, balancing compliance with VA policies and the workload for VA and its affiliates. The VA obtains input from key stakeholders including DEOs, DIOs, and professional organizations such as the Association of American Medical Colleges and the Accreditation Council for Graduate Medical Education.35,36
Looking ahead, the VA is developing an online tool to improve the accuracy of affiliate reimbursement. The VA will also implement a standardized staffing model, organizational structure, and position descriptions for DEO offices. These initiatives will help reduce the burden of tracking and verifying resident activity and continue to support the 77-year partnership between VA and its affiliated institutions.
1. Klink KA, Albanese AP, Bope ET, Sanders KM. Veterans Affairs graduate medical education expansion addresses US physician workforce needs. Acad Med. 2022;97(8):1144-1150. doi:10.1097/ACM.0000000000004545
2. Andrus CH, Johnson K, Pierce E, Romito PJ, Hartel P, Berrios‐Guccione S, Best W. Finance modeling in the delivery of medical care in tertiary‐care hospitals in the Department of Veterans Affairs. J Surg Res. 2001;96(2):152-157. doi:10.1006/jsre.1999.5728
3. Petrakis IL, Kozal M. Academic medical centers and the U.S. Department of Veterans Affairs: a 75-year partnership influences medical education, scientific discovery, and clinical care. Acad Med. 2022;97(8):1110-1113. doi:10.1097/ACM.0000000000004734
4. Heisler EJ, Mendez BH, Mitchell A, Panangala SV, Villagrana MA. Federal support for graduate medical education: an overview (R44376). Congressional Research Service report R44376; version 11. Updated December 27, 2018. Accessed March 2, 2024. https://crsreports.congress.gov/product/pdf/R/R44376/11
5. Chang BK, Brannen JL. The Veterans Access, Choice, and Accountability Act of 2014: examining graduate medical education enhancement in the Department of Veterans Affairs. Acad Med. 2015;90(9):1196-1198. doi:10.1097/ACM.0000000000000795
6. Albanese AP, Bope ET, Sanders KM, Bowman M. The VA MISSION Act of 2018: a potential game changer for rural GME expansion and veteran health care. J Rural Health. 2020;36(1):133-136. doi:10.1111/jrh.12360
7. Lypson ML, Roberts LW. Valuing the partnership between the Veterans Health Administration and academic medicine. Acad Med. 2022;97(8):1091-1093. doi:10.1097/ACM.0000000000004748
8. Harada ND, Traylor L, Rugen KW, et al. Interprofessional transformation of clinical education: the first six years of the Veterans Affairs Centers of Excellence in Primary Care Education. J Interprof Care. 2023;37(suppl 1):S86-S94. doi:10.1080/13561820.2018.1433642

9. Harada ND, Rajashekara S, Sansgiry S, et al. Developing interprofessional primary care teams: alumni evaluation of the Department of Veterans Affairs Centers of Excellence in Primary Care Education Program. J Med Educ Curric Dev. 2019;6:2382120519875455. doi:10.1177/2382120519875455
10. Splaine ME, Ogrinc G, Gilman SC, et al. The Department of Veterans Affairs National Quality Scholars Fellowship Program: experience from 10 years of training quality scholars. Acad Med. 2009;84(12):1741-1748. doi:10.1097/ACM.0b013e3181bfdcef
11. Watts BV, Paull DE, Williams LC, Neily J, Hemphill RR, Brannen JL. Department of Veterans Affairs chief resident in quality and patient safety program: a model to spread change. Am J Med Qual. 2016;31(6):598-600. doi:10.1177/1062860616643403
12. He K, Whang E, Kristo G. Graduate medical education funding mechanisms, challenges, and solutions: a narrative review. Am J Surg. 2021;221(1):65-71. doi:10.1016/j.amjsurg.2020.06.007
13. Villagrana M. Medicare graduate medical education payments: an overview. Congressional Research Service report IF10960. Updated September 29, 2022. Accessed March 2, 2024. https://crsreports.congress.gov/product/pdf/IF/IF10960
14. Committee on the Governance and Financing of Graduate Medical Education; Board on Health Care Services; Institute of Medicine. Graduate Medical Education That Meets the Nation’s Health Needs. Eden J, Berwick DM, Wilensky GR, eds. Washington, DC: National Academies Press; 2014. doi:10.17226/18754
15. Physician workforce: caps on Medicare-funded graduate medical education at teaching hospitals. Report to congressional requesters. GAO-21-391. May 21, 2021. Accessed March 1, 2024. https://www.gao.gov/assets/gao-21-391.pdf
16. VA and Academic Affiliates: Who Benefits? Hearing Before the Subcommittee on Oversight and Investigations of the Committee on Veterans’ Affairs, 114th Cong, 2nd Sess (2016). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/CHRG-115hhrg29685/html/CHRG-115hhrg29685.htm
17. US Department of Veterans Affairs, Office of Inspector General (OIG). Veterans Health Administration. Review of resident and part-time physician time and attendance at the Oklahoma City VA Health Care System. OIG report 17-00253-93. March 28, 2018. Accessed March 1, 2024. https://www.oversight.gov/sites/default/files/oig-reports/VAOIG-17-00253-93.pdf
18. VA health care: actions needed to improve oversight of graduate medical education reimbursement. Report to the ranking member, Committee on Veterans’ Affairs, House of Representatives. GAO-20-553. July 2020. Accessed March 1, 2024. https://www.gao.gov/assets/710/708275.pdf
19. Functions of Veterans Health Administration: in general, 38 USC §7301 (2022). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/USCODE-2022-title38/pdf/USCODE-2022-title38-partV-chap73-subchapI-sec7301.pdf
20. US Department of Veterans Affairs. Policy memorandum no. 2, policy in association of veterans’ hospitals with medical schools. January 30, 1946.
21. Veterans Health Care Expansion Act of 1973, Public Law 93-82. August 2, 1973. Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/STATUTE-87/pdf/STATUTE-87-Pg179.pdf
22. Residencies and internships, 38 USC § 7406 (2022). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/USCODE-2022-title38/pdf/USCODE-2022-title38-partV-chap74-subchapI-sec7406.pdf
23. Direct graduate medical education (DGME). Centers for Medicaid and Medicare Services. Updated December 5, 2023. Accessed March 1, 2024. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/DGME
24. Drezdzon MK, Cowley NJ, Sweeney DP, et al. Going for broke: the impact of cost of living on surgery resident stipend value. Ann Surg. 2023;278(6):1053-1059. doi:10.1097/SLA.0000000000005923
25. Special treatment: hospitals that incur indirect costs for graduate medical education programs, 42 CFR § 412.105 (2023). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/CFR-2023-title42-vol2/pdf/CFR-2023-title42-vol2-sec412-105.pdf
26. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1400.05, Disbursement agreements for health professions trainees appointed under 38 U.S.C. § 7406. June 2, 2021. Accessed March 1, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=9293
27. Harada ND, Sanders KM, Bowman MA. Health systems education leadership: learning from the VA designated education officer role. Fed Pract. 2022;39(6):266-273. doi:10.12788/fp.0278
28. Schleiter Hitchell K, Johnson L. CMS finalizes rules for distribution of 1000 new Medicare-funded residency positions and changes to rural training track programs. J Grad Med Educ. 2022;14(2):245-249. doi:10.4300/JGME-D-22-00193.1

29. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1400.10, Educational cost contracts for health professions education. September 25, 2023. Accessed March 1, 2024. https://www.va.gov/VHAPUBLICATIONS/ViewPublication.asp?pub_ID=11480
30. Direct GME payments: general requirements, 42 CFR § 413.75 (2023). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/CFR-2023-title42-vol2/pdf/CFR-2023-title42-vol2-sec413-75.pdf
31. Direct GME payments: determination of the total number of FTE residents, 42 CFR § 413.78 (2023). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/CFR-2023-title42-vol2/pdf/CFR-2023-title42-vol2-sec413-78.pdf
32. US Department of Health and Human Services, Centers for Medicare and Medicaid Services. Medicare financial management manual, chapter 8. Contractor procedures for provider audits. Accessed March 1, 2024. https://www.cms.gov/regulations-and-guidance/guidance/manuals/downloads/fin106c08.pdf
33. US Department of Health and Human Services, Office of Inspector General. CMS did not always ensure hospitals complied with Medicare reimbursement requirements for graduate medical education. OIG report A-02-17-01017. November 2018. Accessed March 1, 2024. https://oig.hhs.gov/oas/reports/region2/21701017.pdf
34. US Department of Health and Human Services, Centers for Medicare and Medicaid Services. Interns and Residents Information System (IRIS) XML format. Publication 100-20. Transmittal 11418. Change request 12724. May 19, 2022. Accessed March 1, 2024. https://www.hhs.gov/guidance/sites/default/files/hhs-guidance-documents/R11418OTN.pdf
35. Birnbaum AD, Byrne J, on behalf of the VA Office of Academic Affiliations. VHA Updates: Disbursement Policy and Education Cost Contracts. Presented at: American Association of Medical Colleges Webinar; June 2021. Accessed March 1, 2024. https://vimeo.com/644415670
36. Byrne JM, on behalf of the VA Office of Academic Affiliations. Disbursement procedures update for AY 23-24. Accessed March 1, 2024. https://www.va.gov/oaa/Videos/AffiliatePresentationDisbursementandEARsAY23-24.pptx
The US Department of Veterans Affairs (VA) has partnered with academic medical centers and programs since 1946 to provide clinical training for physician residents. Ranking second in federal graduate medical education (GME) funding to the Centers for Medicare and Medicaid Services (CMS), the $850 million VA GME budget annually reimburses > 250 GME-sponsoring institutions (affiliates) of 8000 GME programs for the clinical training of 49,000 individual residents rotating through > 11,000 full-time equivalent (FTE) positions.1 The VA also distributes $1.6 billion to VA facilities to offset the costs of conducting health professions education (HPE) (eg, facility infrastructure, salary support for VA instructors and preceptors, education office administration, and instructional equipment).2 The VA financial and educational contributions account for payment of 11% of resident positions nationally and allow academic medical centers to be less reliant on CMS GME funding.3,4 The VA contributions also provide opportunities for GME expansion,1,5,6 educational innovations,5,7 interprofessional and team-based care,8,9 and quality and safety training.10,11 The Table provides a comparison of CMS and VA GME reimbursability based on activity.
GME financing is complex, particularly the formulaic approach used by CMS, the details of which are often obscured in federal regulations. Due to this complexity and the $16 billion CMS GME budget, academic publications have focused on CMS GME financing while not fully explaining the VA GME policies and processes.4,12-14 By comparison, the VA GME financing model is relatively straightforward and governed by different statues and VA regulations, yet sharing some of the same principles as CMS regulations. Given the challenges in CMS reimbursement to fully support the cost of resident education, as well as the educational opportunities at the VA, the VA designs its reimbursement model to assure that affiliates receive appropriate payments.4,12,15 To ensure the continued success of VA GME partnerships, knowledge of VA GME financing has become increasingly important for designated institutional officers (DIOs) and residency program directors, particularly in light of recent investigations into oversight of the VA’s reimbursement to academic affiliates.
VA AUTHORITY
While the VA’s primary mission is “to provide a complete hospital medical service for the medical care and treatment of veterans,” early VA leaders recognized the importance of affiliating with the nation’s academic institutions.19 In 1946, the VA Policy Memorandum Number 2 established a partnership between the VA and the academic medical community.20 Additional legislation authorized specific agreements with academic affiliates for the central administration of salary and benefits for residents rotating at VA facilities. This process, known as disbursement, is an alternative payroll mechanism whereby the VA reimburses the academic affiliate for resident salary and benefits and the affiliate acts as the disbursing agent, issuing paychecks to residents.21,22
Resident FUNDING
By policy, with rare exceptions, the VA does not sponsor residency programs due to the challenges of providing an appropriate patient mix of age, sex, and medical conditions to meet accreditation standards.4 Nearly all VA reimbursements are for residents in affiliate-sponsored programs, while just 1% pays for residents in legacy, VA-sponsored residency programs at 2 VA facilities. The VA budget for resident (including fellows) salary and benefits is managed by the VA Office of Academic Affiliations (OAA), the national VA office responsible for oversight, policy, and funding of VA HPE programs.
Resident Salaries and Benefits
VA funding of resident salary and benefits are analogous with CMS direct GME (DGME), which is designed to cover resident salary and benefits costs.4,14,23 CMS DGME payments depend on a hospital’s volume of CMS inpatients and are based on a statutory formula, which uses the hospital’s resident FTE positions, the per-resident amount, and Medicare’s share of inpatient beds (Medicare patient load) to determine payments.12 The per-resident amount is set by statute, varies geographically, and is calculated by dividing the hospital’s allowable costs of GME (percentage of CMS inpatient days) divided by the number of residents.12,24
By comparison, the VA GME payment reimburses for each FTE based on the salary and benefits rate set by the academic affiliate. Reimbursement is calculated based on resident time spent at the VA multiplied by a daily salary rate. The daily salary rate is determined by dividing the resident’s total compensation (salary and benefits) by the number of calendar days in an academic year. Resident time spent at the VA facility is determined by obtaining rotation schedules provided by the academic affiliate and verifying resident clinical and educational activity during scheduled rotations.
Indirect Medical Education Funding
In addition to resident salary and benefits, funds to offset the cost of conducting HPE are provided to VA facilities. These funds are intended to improve and maintain necessary infrastructure for all HPE programs not just GME, including education office administration needs, teaching costs (ie, a portion of VA preceptors salary), and instructional equipment.
The Veterans Equitable Resource Allocation (VERA) is a national budgeting process for VA medical facilities that funds facility operational needs such as staff salary and benefits, infrastructure, and equipment.2 The education portion of the VERA, the VERA Education Support Component (VESC), is not managed by the OAA, but rather is distributed through the VERA model to the general budget of VA facilities hosting HPE (Figure). VESC funding in the VA budget is based on labor mapping of physician time spent in education; other labor mapping categories include clinical care, research, and administration. VA facility VESC funding is calculated based on the number of paid health profession trainees (HPTs) from all professions, apportioned according to the number of FTEs for physician residents and VA-paid HPTs in other disciplines. In fiscal year 2024, VA facilities received $115,812 for each physician resident FTE position and $84,906 for each VA-paid, non-GME FTE position.
The VESC is like CMS's indirect GME funding, termed Indirect Medical Education (IME), an additional payment for each Medicare patient discharged reflecting teaching hospitals’ higher patient care costs relative to nonteaching hospitals. Described elsewhere, IME is calculated using a resident-to-bed ratio and a multiplier, which is set by statute.4,25 While IME can be used for reimbursement for some resident clinical and educational activities(eg, research), VA VESC funds cannot be used for such activities and are part of the general facility budget and appropriated per the discretion of the medical facility director.
ESTABLISHING GME PARTNERSHIPS
An affiliation agreement establishes the administrative and legal requirements for educational relationships with academic affiliates and includes standards for conducting HPE, responsibilities for accreditation standards, program leadership, faculty, resources, supervision, academic policies, and procedures. The VA uses standardized affiliation agreement templates that have been vetted with accrediting bodies and the VA Office of General Counsel.
A disbursement agreement authorizes the VA to reimburse affiliates for resident salary and benefits for VA clinical and educational activities. The disbursement agreement details the fiscal arrangements (eg, payment in advance vs arrears, salary, and benefit rates, leave) for the reimbursement payments. Veterans Health Administration (VHA) Directive 1400.05 provides the policy and procedures for calculating reimbursement for HPT educational activities.26
The VA facility designated education officer (DEO) oversees all HPE programs and coordinates the affiliation and disbursement agreement processes.27 The DEO, affiliate DIO, residency program director, and VA residency site director determine the physician resident FTE positions assigned to a VA facility based on educational objectives and availability of educational resources at the VA facility, such as patient care opportunities, faculty supervisors, space, and equipment. The VA facility requests for resident FTE positions are submitted to the OAA by the facility DEO.
Once GME FTE positions are approved by the OAA, VA facilities work with their academic affiliate to submit the physician resident salary and benefit rate. Affiliate DIOs attest to the accuracy of the salary rate schedule and the local DEO submits the budget request to the OAA. Upon approval, the funds are transferred to the VA facility each fiscal year, which begins October 1. DEOs report quarterly to the OAA both budget needs and excesses based on variations in the approved FTEs due to additional VA rotations, physician resident attrition, or reassignment.
Resident Position Allocation
VA GME financing provides flexibility through periodic needs assessments and expansion initiatives. In August and December, DEOs collaborate with an academic affiliate to submit reports to the OAA confirming their projected GME needs for the next academic year. Additional positions requests are reviewed by the OAA; funding depends on budget and the educational justification. The OAA periodically issues GME expansion requests for proposal, which typically arise from legislation to address specific VA workforce needs. The VA facility DEO and affiliate GME leaders collaborate to apply for additional positions. For example, a VA GME expansion under the Veterans Access, Choice, and Accountability Act of 2014 added 1500 GME positions in 8 years for critically needed specialties and in rural and underserved areas.5 The Maintaining Internal Systems and Strengthening Outside Networks (MISSION) Act of 2018 authorized a pilot program for VA to fund residents at non-VA facilities with priority for Indian Health Services, Tribes and Tribal Organizations, Federally Qualified Health Centers, and US Department of Defense facilities to provide access to veterans in underserved areas.6
The VA GME financing system has flexibility to meet local needs for additional resident positions and to address broader VA workforce gaps through targeted expansion. Generally, CMS does not fund positions to address workforce needs, place residents in specific geographic areas, or require the training of certain types of residents.4 However, the Consolidated Appropriations Act of 2021 has provided the opportunity to address rural workforce needs.28
Reimbursement
The VA provides reimbursement for clinical and educational activities performed in VA facilities for the benefit of veterans as well as research, didactics, meetings and conferences, annual and sick leave, and orientation. The VA also may provide reimbursement for educational activities that occur off VA grounds (eg, the VA proportional share of a residency program’s didactic sessions). The VA does not reimburse for affiliate clinical duties or administrative costs, although a national policy allows VA facilities to reimburse affiliates for some GME overhead costs.29
CMS similarly reimburses for residency training time spent in patient care activities as well as orientation activities, didactics, leave, and, in some cases, research.4,30,31 CMS makes payments to hospitals, which may include sponsoring institutions and Medicare-eligible participating training sites.4,30,31 For both the VA and CMS, residents may not be counted twice for reimbursement by 2 federal agencies; in other words, a resident may not count for > 1 FTE.4,30-32
GME Oversight
VA GME funding came under significant scrutiny. At a 2016 House Veterans Affairs Committee hearing, Representative Phil Roe, MD (R-Tennessee), noted that no process existed at many VA facilities for “determining trainee presence” and that many VA medical centers had “difficulty tracking resident rotations”16 A VA Office of the Inspector General investigation recommended that the VA implement policies and procedures to improve oversight to “ensure residents are fully participating in educational activities” and that the VA is “paying the correct amount” to the affiliate.17 A 2020 General Accountability Office report outlined unclear policy guidance, incomplete tracking of resident activities, and improper fiscal processes for reimbursement and reconciliation of affiliate invoices.18
In response, the OAA created an oversight and compliance unit, revised VHA Directive 1400.05 (the policy for disbursement), and improved resident tracking procedures.26 The standard operating procedure that accompanied VHA Directive 1400.05 provides detailed information for the DEO and VA facility staff for tracking resident clinical and educational activities. FTE counts are essential to both VA and CMS for accurate reimbursement. The eAppendix and the Table provide a guide to reimbursable activities in the VA for the calculation of reimbursement, with a comparison to CMS.33,34 The OAA in cooperation with other VA staff and officers periodically conducts audits to assess compliance with disbursement policy and affiliate reimbursement accuracy.
In the VA, resident activities are captured on the VA Educational Activity Record, a standardized spreadsheet to track activities and calculate reimbursement. Each VA facility hosting resident physicians manually records resident activity by the half-day. This process is labor intensive, involving both VA and affiliate staff to accurately reconcile payments. To address the workload demands, the OAA is developing an online tool that will automate aspects of the tracking process. Also, to ensure adequate staffing, the OAA is in the process of implementing an office optimization project, providing standardized position descriptions, an organizational chart, and staffing levels for DEO offices in VA facilities.
Conclusions
This report describes the key policies and principles of VA GME financing, highlighting the essential similarities and differences between VA and CMS. Neither the VA nor CMS regulations allow for reimbursement for > 1 FTE position per resident, a principle that underpins the assignment of resident rotations and federal funding for GME and are similar with respect to reimbursement for patient care activities, didactics, research, orientation, and scholarly activity. While reimbursable activities in the VA require physical presence and care of veteran patients, CMS also limits reimbursement to resident activities in the hospital and approved other settings if the hospital is paying for resident salary and benefits in these settings. The VA provides some flexibility for offsite activities including didactics and, in specific circumstances, remote care of veteran patients (eg, teleradiology).
The VA and CMS use different GME financing models. For example, the CMS calculations for resident FTEs are complex, whereas VA calculations reimburse the salary and benefits as set by the academic affiliate. The VA process accounts for local variation in salary rates, whereas the per-resident amount set by CMS varies regionally and does not fully account for differences in the cost of living.24 Because all patients in VA facilities are veterans, VA calculations for reimbursement do not involve ratios of beds like the CMS calculations to determine a proportional share of reimbursement. The VA GME expansion tends to be more directed to VA health workforce needs than CMS, specifying the types of programs and geographic locations to address these needs.
The VA regularly reevaluates how affiliates are reimbursed for VA resident activity, balancing compliance with VA policies and the workload for VA and its affiliates. The VA obtains input from key stakeholders including DEOs, DIOs, and professional organizations such as the Association of American Medical Colleges and the Accreditation Council for Graduate Medical Education.35,36
Looking ahead, the VA is developing an online tool to improve the accuracy of affiliate reimbursement. The VA will also implement a standardized staffing model, organizational structure, and position descriptions for DEO offices. These initiatives will help reduce the burden of tracking and verifying resident activity and continue to support the 77-year partnership between VA and its affiliated institutions.
The US Department of Veterans Affairs (VA) has partnered with academic medical centers and programs since 1946 to provide clinical training for physician residents. Ranking second in federal graduate medical education (GME) funding to the Centers for Medicare and Medicaid Services (CMS), the $850 million VA GME budget annually reimburses > 250 GME-sponsoring institutions (affiliates) of 8000 GME programs for the clinical training of 49,000 individual residents rotating through > 11,000 full-time equivalent (FTE) positions.1 The VA also distributes $1.6 billion to VA facilities to offset the costs of conducting health professions education (HPE) (eg, facility infrastructure, salary support for VA instructors and preceptors, education office administration, and instructional equipment).2 The VA financial and educational contributions account for payment of 11% of resident positions nationally and allow academic medical centers to be less reliant on CMS GME funding.3,4 The VA contributions also provide opportunities for GME expansion,1,5,6 educational innovations,5,7 interprofessional and team-based care,8,9 and quality and safety training.10,11 The Table provides a comparison of CMS and VA GME reimbursability based on activity.
GME financing is complex, particularly the formulaic approach used by CMS, the details of which are often obscured in federal regulations. Due to this complexity and the $16 billion CMS GME budget, academic publications have focused on CMS GME financing while not fully explaining the VA GME policies and processes.4,12-14 By comparison, the VA GME financing model is relatively straightforward and governed by different statues and VA regulations, yet sharing some of the same principles as CMS regulations. Given the challenges in CMS reimbursement to fully support the cost of resident education, as well as the educational opportunities at the VA, the VA designs its reimbursement model to assure that affiliates receive appropriate payments.4,12,15 To ensure the continued success of VA GME partnerships, knowledge of VA GME financing has become increasingly important for designated institutional officers (DIOs) and residency program directors, particularly in light of recent investigations into oversight of the VA’s reimbursement to academic affiliates.
VA AUTHORITY
While the VA’s primary mission is “to provide a complete hospital medical service for the medical care and treatment of veterans,” early VA leaders recognized the importance of affiliating with the nation’s academic institutions.19 In 1946, the VA Policy Memorandum Number 2 established a partnership between the VA and the academic medical community.20 Additional legislation authorized specific agreements with academic affiliates for the central administration of salary and benefits for residents rotating at VA facilities. This process, known as disbursement, is an alternative payroll mechanism whereby the VA reimburses the academic affiliate for resident salary and benefits and the affiliate acts as the disbursing agent, issuing paychecks to residents.21,22
Resident FUNDING
By policy, with rare exceptions, the VA does not sponsor residency programs due to the challenges of providing an appropriate patient mix of age, sex, and medical conditions to meet accreditation standards.4 Nearly all VA reimbursements are for residents in affiliate-sponsored programs, while just 1% pays for residents in legacy, VA-sponsored residency programs at 2 VA facilities. The VA budget for resident (including fellows) salary and benefits is managed by the VA Office of Academic Affiliations (OAA), the national VA office responsible for oversight, policy, and funding of VA HPE programs.
Resident Salaries and Benefits
VA funding of resident salary and benefits are analogous with CMS direct GME (DGME), which is designed to cover resident salary and benefits costs.4,14,23 CMS DGME payments depend on a hospital’s volume of CMS inpatients and are based on a statutory formula, which uses the hospital’s resident FTE positions, the per-resident amount, and Medicare’s share of inpatient beds (Medicare patient load) to determine payments.12 The per-resident amount is set by statute, varies geographically, and is calculated by dividing the hospital’s allowable costs of GME (percentage of CMS inpatient days) divided by the number of residents.12,24
By comparison, the VA GME payment reimburses for each FTE based on the salary and benefits rate set by the academic affiliate. Reimbursement is calculated based on resident time spent at the VA multiplied by a daily salary rate. The daily salary rate is determined by dividing the resident’s total compensation (salary and benefits) by the number of calendar days in an academic year. Resident time spent at the VA facility is determined by obtaining rotation schedules provided by the academic affiliate and verifying resident clinical and educational activity during scheduled rotations.
Indirect Medical Education Funding
In addition to resident salary and benefits, funds to offset the cost of conducting HPE are provided to VA facilities. These funds are intended to improve and maintain necessary infrastructure for all HPE programs not just GME, including education office administration needs, teaching costs (ie, a portion of VA preceptors salary), and instructional equipment.
The Veterans Equitable Resource Allocation (VERA) is a national budgeting process for VA medical facilities that funds facility operational needs such as staff salary and benefits, infrastructure, and equipment.2 The education portion of the VERA, the VERA Education Support Component (VESC), is not managed by the OAA, but rather is distributed through the VERA model to the general budget of VA facilities hosting HPE (Figure). VESC funding in the VA budget is based on labor mapping of physician time spent in education; other labor mapping categories include clinical care, research, and administration. VA facility VESC funding is calculated based on the number of paid health profession trainees (HPTs) from all professions, apportioned according to the number of FTEs for physician residents and VA-paid HPTs in other disciplines. In fiscal year 2024, VA facilities received $115,812 for each physician resident FTE position and $84,906 for each VA-paid, non-GME FTE position.
The VESC is like CMS's indirect GME funding, termed Indirect Medical Education (IME), an additional payment for each Medicare patient discharged reflecting teaching hospitals’ higher patient care costs relative to nonteaching hospitals. Described elsewhere, IME is calculated using a resident-to-bed ratio and a multiplier, which is set by statute.4,25 While IME can be used for reimbursement for some resident clinical and educational activities(eg, research), VA VESC funds cannot be used for such activities and are part of the general facility budget and appropriated per the discretion of the medical facility director.
ESTABLISHING GME PARTNERSHIPS
An affiliation agreement establishes the administrative and legal requirements for educational relationships with academic affiliates and includes standards for conducting HPE, responsibilities for accreditation standards, program leadership, faculty, resources, supervision, academic policies, and procedures. The VA uses standardized affiliation agreement templates that have been vetted with accrediting bodies and the VA Office of General Counsel.
A disbursement agreement authorizes the VA to reimburse affiliates for resident salary and benefits for VA clinical and educational activities. The disbursement agreement details the fiscal arrangements (eg, payment in advance vs arrears, salary, and benefit rates, leave) for the reimbursement payments. Veterans Health Administration (VHA) Directive 1400.05 provides the policy and procedures for calculating reimbursement for HPT educational activities.26
The VA facility designated education officer (DEO) oversees all HPE programs and coordinates the affiliation and disbursement agreement processes.27 The DEO, affiliate DIO, residency program director, and VA residency site director determine the physician resident FTE positions assigned to a VA facility based on educational objectives and availability of educational resources at the VA facility, such as patient care opportunities, faculty supervisors, space, and equipment. The VA facility requests for resident FTE positions are submitted to the OAA by the facility DEO.
Once GME FTE positions are approved by the OAA, VA facilities work with their academic affiliate to submit the physician resident salary and benefit rate. Affiliate DIOs attest to the accuracy of the salary rate schedule and the local DEO submits the budget request to the OAA. Upon approval, the funds are transferred to the VA facility each fiscal year, which begins October 1. DEOs report quarterly to the OAA both budget needs and excesses based on variations in the approved FTEs due to additional VA rotations, physician resident attrition, or reassignment.
Resident Position Allocation
VA GME financing provides flexibility through periodic needs assessments and expansion initiatives. In August and December, DEOs collaborate with an academic affiliate to submit reports to the OAA confirming their projected GME needs for the next academic year. Additional positions requests are reviewed by the OAA; funding depends on budget and the educational justification. The OAA periodically issues GME expansion requests for proposal, which typically arise from legislation to address specific VA workforce needs. The VA facility DEO and affiliate GME leaders collaborate to apply for additional positions. For example, a VA GME expansion under the Veterans Access, Choice, and Accountability Act of 2014 added 1500 GME positions in 8 years for critically needed specialties and in rural and underserved areas.5 The Maintaining Internal Systems and Strengthening Outside Networks (MISSION) Act of 2018 authorized a pilot program for VA to fund residents at non-VA facilities with priority for Indian Health Services, Tribes and Tribal Organizations, Federally Qualified Health Centers, and US Department of Defense facilities to provide access to veterans in underserved areas.6
The VA GME financing system has flexibility to meet local needs for additional resident positions and to address broader VA workforce gaps through targeted expansion. Generally, CMS does not fund positions to address workforce needs, place residents in specific geographic areas, or require the training of certain types of residents.4 However, the Consolidated Appropriations Act of 2021 has provided the opportunity to address rural workforce needs.28
Reimbursement
The VA provides reimbursement for clinical and educational activities performed in VA facilities for the benefit of veterans as well as research, didactics, meetings and conferences, annual and sick leave, and orientation. The VA also may provide reimbursement for educational activities that occur off VA grounds (eg, the VA proportional share of a residency program’s didactic sessions). The VA does not reimburse for affiliate clinical duties or administrative costs, although a national policy allows VA facilities to reimburse affiliates for some GME overhead costs.29
CMS similarly reimburses for residency training time spent in patient care activities as well as orientation activities, didactics, leave, and, in some cases, research.4,30,31 CMS makes payments to hospitals, which may include sponsoring institutions and Medicare-eligible participating training sites.4,30,31 For both the VA and CMS, residents may not be counted twice for reimbursement by 2 federal agencies; in other words, a resident may not count for > 1 FTE.4,30-32
GME Oversight
VA GME funding came under significant scrutiny. At a 2016 House Veterans Affairs Committee hearing, Representative Phil Roe, MD (R-Tennessee), noted that no process existed at many VA facilities for “determining trainee presence” and that many VA medical centers had “difficulty tracking resident rotations”16 A VA Office of the Inspector General investigation recommended that the VA implement policies and procedures to improve oversight to “ensure residents are fully participating in educational activities” and that the VA is “paying the correct amount” to the affiliate.17 A 2020 General Accountability Office report outlined unclear policy guidance, incomplete tracking of resident activities, and improper fiscal processes for reimbursement and reconciliation of affiliate invoices.18
In response, the OAA created an oversight and compliance unit, revised VHA Directive 1400.05 (the policy for disbursement), and improved resident tracking procedures.26 The standard operating procedure that accompanied VHA Directive 1400.05 provides detailed information for the DEO and VA facility staff for tracking resident clinical and educational activities. FTE counts are essential to both VA and CMS for accurate reimbursement. The eAppendix and the Table provide a guide to reimbursable activities in the VA for the calculation of reimbursement, with a comparison to CMS.33,34 The OAA in cooperation with other VA staff and officers periodically conducts audits to assess compliance with disbursement policy and affiliate reimbursement accuracy.
In the VA, resident activities are captured on the VA Educational Activity Record, a standardized spreadsheet to track activities and calculate reimbursement. Each VA facility hosting resident physicians manually records resident activity by the half-day. This process is labor intensive, involving both VA and affiliate staff to accurately reconcile payments. To address the workload demands, the OAA is developing an online tool that will automate aspects of the tracking process. Also, to ensure adequate staffing, the OAA is in the process of implementing an office optimization project, providing standardized position descriptions, an organizational chart, and staffing levels for DEO offices in VA facilities.
Conclusions
This report describes the key policies and principles of VA GME financing, highlighting the essential similarities and differences between VA and CMS. Neither the VA nor CMS regulations allow for reimbursement for > 1 FTE position per resident, a principle that underpins the assignment of resident rotations and federal funding for GME and are similar with respect to reimbursement for patient care activities, didactics, research, orientation, and scholarly activity. While reimbursable activities in the VA require physical presence and care of veteran patients, CMS also limits reimbursement to resident activities in the hospital and approved other settings if the hospital is paying for resident salary and benefits in these settings. The VA provides some flexibility for offsite activities including didactics and, in specific circumstances, remote care of veteran patients (eg, teleradiology).
The VA and CMS use different GME financing models. For example, the CMS calculations for resident FTEs are complex, whereas VA calculations reimburse the salary and benefits as set by the academic affiliate. The VA process accounts for local variation in salary rates, whereas the per-resident amount set by CMS varies regionally and does not fully account for differences in the cost of living.24 Because all patients in VA facilities are veterans, VA calculations for reimbursement do not involve ratios of beds like the CMS calculations to determine a proportional share of reimbursement. The VA GME expansion tends to be more directed to VA health workforce needs than CMS, specifying the types of programs and geographic locations to address these needs.
The VA regularly reevaluates how affiliates are reimbursed for VA resident activity, balancing compliance with VA policies and the workload for VA and its affiliates. The VA obtains input from key stakeholders including DEOs, DIOs, and professional organizations such as the Association of American Medical Colleges and the Accreditation Council for Graduate Medical Education.35,36
Looking ahead, the VA is developing an online tool to improve the accuracy of affiliate reimbursement. The VA will also implement a standardized staffing model, organizational structure, and position descriptions for DEO offices. These initiatives will help reduce the burden of tracking and verifying resident activity and continue to support the 77-year partnership between VA and its affiliated institutions.
1. Klink KA, Albanese AP, Bope ET, Sanders KM. Veterans Affairs graduate medical education expansion addresses US physician workforce needs. Acad Med. 2022;97(8):1144-1150. doi:10.1097/ACM.0000000000004545
2. Andrus CH, Johnson K, Pierce E, Romito PJ, Hartel P, Berrios‐Guccione S, Best W. Finance modeling in the delivery of medical care in tertiary‐care hospitals in the Department of Veterans Affairs. J Surg Res. 2001;96(2):152-157. doi:10.1006/jsre.1999.5728
3. Petrakis IL, Kozal M. Academic medical centers and the U.S. Department of Veterans Affairs: a 75-year partnership influences medical education, scientific discovery, and clinical care. Acad Med. 2022;97(8):1110-1113. doi:10.1097/ACM.0000000000004734
4. Heisler EJ, Mendez BH, Mitchell A, Panangala SV, Villagrana MA. Federal support for graduate medical education: an overview (R44376). Congressional Research Service report R44376; version 11. Updated December 27, 2018. Accessed March 2, 2024. https://crsreports.congress.gov/product/pdf/R/R44376/11
5. Chang BK, Brannen JL. The Veterans Access, Choice, and Accountability Act of 2014: examining graduate medical education enhancement in the Department of Veterans Affairs. Acad Med. 2015;90(9):1196-1198. doi:10.1097/ACM.0000000000000795
6. Albanese AP, Bope ET, Sanders KM, Bowman M. The VA MISSION Act of 2018: a potential game changer for rural GME expansion and veteran health care. J Rural Health. 2020;36(1):133-136. doi:10.1111/jrh.12360
7. Lypson ML, Roberts LW. Valuing the partnership between the Veterans Health Administration and academic medicine. Acad Med. 2022;97(8):1091-1093. doi:10.1097/ACM.0000000000004748
8. Harada ND, Traylor L, Rugen KW, et al. Interprofessional transformation of clinical education: the first six years of the Veterans Affairs Centers of Excellence in Primary Care Education. J Interprof Care. 2023;37(suppl 1):S86-S94. doi:10.1080/13561820.2018.1433642

9. Harada ND, Rajashekara S, Sansgiry S, et al. Developing interprofessional primary care teams: alumni evaluation of the Department of Veterans Affairs Centers of Excellence in Primary Care Education Program. J Med Educ Curric Dev. 2019;6:2382120519875455. doi:10.1177/2382120519875455
10. Splaine ME, Ogrinc G, Gilman SC, et al. The Department of Veterans Affairs National Quality Scholars Fellowship Program: experience from 10 years of training quality scholars. Acad Med. 2009;84(12):1741-1748. doi:10.1097/ACM.0b013e3181bfdcef
11. Watts BV, Paull DE, Williams LC, Neily J, Hemphill RR, Brannen JL. Department of Veterans Affairs chief resident in quality and patient safety program: a model to spread change. Am J Med Qual. 2016;31(6):598-600. doi:10.1177/1062860616643403
12. He K, Whang E, Kristo G. Graduate medical education funding mechanisms, challenges, and solutions: a narrative review. Am J Surg. 2021;221(1):65-71. doi:10.1016/j.amjsurg.2020.06.007
13. Villagrana M. Medicare graduate medical education payments: an overview. Congressional Research Service report IF10960. Updated September 29, 2022. Accessed March 2, 2024. https://crsreports.congress.gov/product/pdf/IF/IF10960
14. Committee on the Governance and Financing of Graduate Medical Education; Board on Health Care Services; Institute of Medicine. Graduate Medical Education That Meets the Nation’s Health Needs. Eden J, Berwick DM, Wilensky GR, eds. Washington, DC: National Academies Press; 2014. doi:10.17226/18754
15. Physician workforce: caps on Medicare-funded graduate medical education at teaching hospitals. Report to congressional requesters. GAO-21-391. May 21, 2021. Accessed March 1, 2024. https://www.gao.gov/assets/gao-21-391.pdf
16. VA and Academic Affiliates: Who Benefits? Hearing Before the Subcommittee on Oversight and Investigations of the Committee on Veterans’ Affairs, 114th Cong, 2nd Sess (2016). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/CHRG-115hhrg29685/html/CHRG-115hhrg29685.htm
17. US Department of Veterans Affairs, Office of Inspector General (OIG). Veterans Health Administration. Review of resident and part-time physician time and attendance at the Oklahoma City VA Health Care System. OIG report 17-00253-93. March 28, 2018. Accessed March 1, 2024. https://www.oversight.gov/sites/default/files/oig-reports/VAOIG-17-00253-93.pdf
18. VA health care: actions needed to improve oversight of graduate medical education reimbursement. Report to the ranking member, Committee on Veterans’ Affairs, House of Representatives. GAO-20-553. July 2020. Accessed March 1, 2024. https://www.gao.gov/assets/710/708275.pdf
19. Functions of Veterans Health Administration: in general, 38 USC §7301 (2022). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/USCODE-2022-title38/pdf/USCODE-2022-title38-partV-chap73-subchapI-sec7301.pdf
20. US Department of Veterans Affairs. Policy memorandum no. 2, policy in association of veterans’ hospitals with medical schools. January 30, 1946.
21. Veterans Health Care Expansion Act of 1973, Public Law 93-82. August 2, 1973. Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/STATUTE-87/pdf/STATUTE-87-Pg179.pdf
22. Residencies and internships, 38 USC § 7406 (2022). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/USCODE-2022-title38/pdf/USCODE-2022-title38-partV-chap74-subchapI-sec7406.pdf
23. Direct graduate medical education (DGME). Centers for Medicaid and Medicare Services. Updated December 5, 2023. Accessed March 1, 2024. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/DGME
24. Drezdzon MK, Cowley NJ, Sweeney DP, et al. Going for broke: the impact of cost of living on surgery resident stipend value. Ann Surg. 2023;278(6):1053-1059. doi:10.1097/SLA.0000000000005923
25. Special treatment: hospitals that incur indirect costs for graduate medical education programs, 42 CFR § 412.105 (2023). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/CFR-2023-title42-vol2/pdf/CFR-2023-title42-vol2-sec412-105.pdf
26. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1400.05, Disbursement agreements for health professions trainees appointed under 38 U.S.C. § 7406. June 2, 2021. Accessed March 1, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=9293
27. Harada ND, Sanders KM, Bowman MA. Health systems education leadership: learning from the VA designated education officer role. Fed Pract. 2022;39(6):266-273. doi:10.12788/fp.0278
28. Schleiter Hitchell K, Johnson L. CMS finalizes rules for distribution of 1000 new Medicare-funded residency positions and changes to rural training track programs. J Grad Med Educ. 2022;14(2):245-249. doi:10.4300/JGME-D-22-00193.1

29. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1400.10, Educational cost contracts for health professions education. September 25, 2023. Accessed March 1, 2024. https://www.va.gov/VHAPUBLICATIONS/ViewPublication.asp?pub_ID=11480
30. Direct GME payments: general requirements, 42 CFR § 413.75 (2023). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/CFR-2023-title42-vol2/pdf/CFR-2023-title42-vol2-sec413-75.pdf
31. Direct GME payments: determination of the total number of FTE residents, 42 CFR § 413.78 (2023). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/CFR-2023-title42-vol2/pdf/CFR-2023-title42-vol2-sec413-78.pdf
32. US Department of Health and Human Services, Centers for Medicare and Medicaid Services. Medicare financial management manual, chapter 8. Contractor procedures for provider audits. Accessed March 1, 2024. https://www.cms.gov/regulations-and-guidance/guidance/manuals/downloads/fin106c08.pdf
33. US Department of Health and Human Services, Office of Inspector General. CMS did not always ensure hospitals complied with Medicare reimbursement requirements for graduate medical education. OIG report A-02-17-01017. November 2018. Accessed March 1, 2024. https://oig.hhs.gov/oas/reports/region2/21701017.pdf
34. US Department of Health and Human Services, Centers for Medicare and Medicaid Services. Interns and Residents Information System (IRIS) XML format. Publication 100-20. Transmittal 11418. Change request 12724. May 19, 2022. Accessed March 1, 2024. https://www.hhs.gov/guidance/sites/default/files/hhs-guidance-documents/R11418OTN.pdf
35. Birnbaum AD, Byrne J, on behalf of the VA Office of Academic Affiliations. VHA Updates: Disbursement Policy and Education Cost Contracts. Presented at: American Association of Medical Colleges Webinar; June 2021. Accessed March 1, 2024. https://vimeo.com/644415670
36. Byrne JM, on behalf of the VA Office of Academic Affiliations. Disbursement procedures update for AY 23-24. Accessed March 1, 2024. https://www.va.gov/oaa/Videos/AffiliatePresentationDisbursementandEARsAY23-24.pptx
1. Klink KA, Albanese AP, Bope ET, Sanders KM. Veterans Affairs graduate medical education expansion addresses US physician workforce needs. Acad Med. 2022;97(8):1144-1150. doi:10.1097/ACM.0000000000004545
2. Andrus CH, Johnson K, Pierce E, Romito PJ, Hartel P, Berrios‐Guccione S, Best W. Finance modeling in the delivery of medical care in tertiary‐care hospitals in the Department of Veterans Affairs. J Surg Res. 2001;96(2):152-157. doi:10.1006/jsre.1999.5728
3. Petrakis IL, Kozal M. Academic medical centers and the U.S. Department of Veterans Affairs: a 75-year partnership influences medical education, scientific discovery, and clinical care. Acad Med. 2022;97(8):1110-1113. doi:10.1097/ACM.0000000000004734
4. Heisler EJ, Mendez BH, Mitchell A, Panangala SV, Villagrana MA. Federal support for graduate medical education: an overview (R44376). Congressional Research Service report R44376; version 11. Updated December 27, 2018. Accessed March 2, 2024. https://crsreports.congress.gov/product/pdf/R/R44376/11
5. Chang BK, Brannen JL. The Veterans Access, Choice, and Accountability Act of 2014: examining graduate medical education enhancement in the Department of Veterans Affairs. Acad Med. 2015;90(9):1196-1198. doi:10.1097/ACM.0000000000000795
6. Albanese AP, Bope ET, Sanders KM, Bowman M. The VA MISSION Act of 2018: a potential game changer for rural GME expansion and veteran health care. J Rural Health. 2020;36(1):133-136. doi:10.1111/jrh.12360
7. Lypson ML, Roberts LW. Valuing the partnership between the Veterans Health Administration and academic medicine. Acad Med. 2022;97(8):1091-1093. doi:10.1097/ACM.0000000000004748
8. Harada ND, Traylor L, Rugen KW, et al. Interprofessional transformation of clinical education: the first six years of the Veterans Affairs Centers of Excellence in Primary Care Education. J Interprof Care. 2023;37(suppl 1):S86-S94. doi:10.1080/13561820.2018.1433642

9. Harada ND, Rajashekara S, Sansgiry S, et al. Developing interprofessional primary care teams: alumni evaluation of the Department of Veterans Affairs Centers of Excellence in Primary Care Education Program. J Med Educ Curric Dev. 2019;6:2382120519875455. doi:10.1177/2382120519875455
10. Splaine ME, Ogrinc G, Gilman SC, et al. The Department of Veterans Affairs National Quality Scholars Fellowship Program: experience from 10 years of training quality scholars. Acad Med. 2009;84(12):1741-1748. doi:10.1097/ACM.0b013e3181bfdcef
11. Watts BV, Paull DE, Williams LC, Neily J, Hemphill RR, Brannen JL. Department of Veterans Affairs chief resident in quality and patient safety program: a model to spread change. Am J Med Qual. 2016;31(6):598-600. doi:10.1177/1062860616643403
12. He K, Whang E, Kristo G. Graduate medical education funding mechanisms, challenges, and solutions: a narrative review. Am J Surg. 2021;221(1):65-71. doi:10.1016/j.amjsurg.2020.06.007
13. Villagrana M. Medicare graduate medical education payments: an overview. Congressional Research Service report IF10960. Updated September 29, 2022. Accessed March 2, 2024. https://crsreports.congress.gov/product/pdf/IF/IF10960
14. Committee on the Governance and Financing of Graduate Medical Education; Board on Health Care Services; Institute of Medicine. Graduate Medical Education That Meets the Nation’s Health Needs. Eden J, Berwick DM, Wilensky GR, eds. Washington, DC: National Academies Press; 2014. doi:10.17226/18754
15. Physician workforce: caps on Medicare-funded graduate medical education at teaching hospitals. Report to congressional requesters. GAO-21-391. May 21, 2021. Accessed March 1, 2024. https://www.gao.gov/assets/gao-21-391.pdf
16. VA and Academic Affiliates: Who Benefits? Hearing Before the Subcommittee on Oversight and Investigations of the Committee on Veterans’ Affairs, 114th Cong, 2nd Sess (2016). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/CHRG-115hhrg29685/html/CHRG-115hhrg29685.htm
17. US Department of Veterans Affairs, Office of Inspector General (OIG). Veterans Health Administration. Review of resident and part-time physician time and attendance at the Oklahoma City VA Health Care System. OIG report 17-00253-93. March 28, 2018. Accessed March 1, 2024. https://www.oversight.gov/sites/default/files/oig-reports/VAOIG-17-00253-93.pdf
18. VA health care: actions needed to improve oversight of graduate medical education reimbursement. Report to the ranking member, Committee on Veterans’ Affairs, House of Representatives. GAO-20-553. July 2020. Accessed March 1, 2024. https://www.gao.gov/assets/710/708275.pdf
19. Functions of Veterans Health Administration: in general, 38 USC §7301 (2022). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/USCODE-2022-title38/pdf/USCODE-2022-title38-partV-chap73-subchapI-sec7301.pdf
20. US Department of Veterans Affairs. Policy memorandum no. 2, policy in association of veterans’ hospitals with medical schools. January 30, 1946.
21. Veterans Health Care Expansion Act of 1973, Public Law 93-82. August 2, 1973. Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/STATUTE-87/pdf/STATUTE-87-Pg179.pdf
22. Residencies and internships, 38 USC § 7406 (2022). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/USCODE-2022-title38/pdf/USCODE-2022-title38-partV-chap74-subchapI-sec7406.pdf
23. Direct graduate medical education (DGME). Centers for Medicaid and Medicare Services. Updated December 5, 2023. Accessed March 1, 2024. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/DGME
24. Drezdzon MK, Cowley NJ, Sweeney DP, et al. Going for broke: the impact of cost of living on surgery resident stipend value. Ann Surg. 2023;278(6):1053-1059. doi:10.1097/SLA.0000000000005923
25. Special treatment: hospitals that incur indirect costs for graduate medical education programs, 42 CFR § 412.105 (2023). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/CFR-2023-title42-vol2/pdf/CFR-2023-title42-vol2-sec412-105.pdf
26. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1400.05, Disbursement agreements for health professions trainees appointed under 38 U.S.C. § 7406. June 2, 2021. Accessed March 1, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=9293
27. Harada ND, Sanders KM, Bowman MA. Health systems education leadership: learning from the VA designated education officer role. Fed Pract. 2022;39(6):266-273. doi:10.12788/fp.0278
28. Schleiter Hitchell K, Johnson L. CMS finalizes rules for distribution of 1000 new Medicare-funded residency positions and changes to rural training track programs. J Grad Med Educ. 2022;14(2):245-249. doi:10.4300/JGME-D-22-00193.1

29. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1400.10, Educational cost contracts for health professions education. September 25, 2023. Accessed March 1, 2024. https://www.va.gov/VHAPUBLICATIONS/ViewPublication.asp?pub_ID=11480
30. Direct GME payments: general requirements, 42 CFR § 413.75 (2023). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/CFR-2023-title42-vol2/pdf/CFR-2023-title42-vol2-sec413-75.pdf
31. Direct GME payments: determination of the total number of FTE residents, 42 CFR § 413.78 (2023). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/CFR-2023-title42-vol2/pdf/CFR-2023-title42-vol2-sec413-78.pdf
32. US Department of Health and Human Services, Centers for Medicare and Medicaid Services. Medicare financial management manual, chapter 8. Contractor procedures for provider audits. Accessed March 1, 2024. https://www.cms.gov/regulations-and-guidance/guidance/manuals/downloads/fin106c08.pdf
33. US Department of Health and Human Services, Office of Inspector General. CMS did not always ensure hospitals complied with Medicare reimbursement requirements for graduate medical education. OIG report A-02-17-01017. November 2018. Accessed March 1, 2024. https://oig.hhs.gov/oas/reports/region2/21701017.pdf
34. US Department of Health and Human Services, Centers for Medicare and Medicaid Services. Interns and Residents Information System (IRIS) XML format. Publication 100-20. Transmittal 11418. Change request 12724. May 19, 2022. Accessed March 1, 2024. https://www.hhs.gov/guidance/sites/default/files/hhs-guidance-documents/R11418OTN.pdf
35. Birnbaum AD, Byrne J, on behalf of the VA Office of Academic Affiliations. VHA Updates: Disbursement Policy and Education Cost Contracts. Presented at: American Association of Medical Colleges Webinar; June 2021. Accessed March 1, 2024. https://vimeo.com/644415670
36. Byrne JM, on behalf of the VA Office of Academic Affiliations. Disbursement procedures update for AY 23-24. Accessed March 1, 2024. https://www.va.gov/oaa/Videos/AffiliatePresentationDisbursementandEARsAY23-24.pptx
Could EHR Pharmacy Errors Put Veterans at Risk?
Will the new US Department of Veterans Affairs (VA) pharmacy software be safe and effective? That was the topic when David Case, the VA Deputy Inspector General, spoke in the US House of Representatives Veterans Affairs Committee technology modernization subcommittee hearing on February 15.
Questions like that have dogged the project since 2018, when the VA began rolling out the Oracle Cerner electronic health record (EHR) system as the successor to ViSTA.
The Oracle system has been beset by one glitch after another since its arrival. And in that time, Case said, the VA Office of Inspector General (OIG) has been engaging with VA employees at sites in Washington, Oregon, Ohio, Illinois, and other locations where the modernization program has been piloted.
The most recent OIG investigation of pharmacy-related patient safety issues began with a review of an allegation of a prescription backlog at Columbus, Ohio, where the system went live on April 30, 2022. The OIG found that facility leaders took “timely and sustainable steps” to manage that issue. However, other unresolved patient safety issues came to light, such as medication inaccuracies, inaccurate medication data, and insufficient staffing. The OIG also found staff were creating “numerous work arounds” to provide patient care, and that the volume of staff educational materials for pharmacy-related functions was “overwhelming.”
Those problems were just the latest in a long queue. In May 2021, after the first VA deployment of the new EHR at the Mann-Grandstaff VA Medical Center in Spokane, Washington, a pharmacy patient safety team under the VA National Center for Patient Safety (NCPS) also had identified patient safety issues and “multiple” concerns regarding the system’s usability. For example, updates to a patient’s active medication list were not routinely reflected at the patient’s next appointment. Despite knowing about such challenges, Case noted in his report, VA leaders deployed the new EHR at 4 more VA medical centers.
Cerner/ViSTA Communication
One major cause of the current problems is the way the systems “talk” to each other. EHR information is communicated between VHA facilities through channels that include the Joint Longitudinal Viewer (JLV) and the Health Data Repository, which stores patient-specific clinical information from both the legacy and the new EHR systems. The JLV application allows clinicians to access a read only version of a patient’s EHR from both systems.
Every medication used in VHA has a VA Unique Identifier (VUID). When a patient is prescribed a medication at a new EHR site, that medication’s VUID is sent to the Health Data Repository. If that patient seeks care from a legacy health care practitioner (HCP), and that HCP enters a medication order, a software interface accesses the VUID from the Health Data Repository to verify that the medication being prescribed is safe and compatible with the medications and allergies previously documented in the patient’s record.
However, on March 31, 2023, staff from a ViSTA site found an incorrect medication order when prescribing a new medication to a patient who had received care and medications at a new EHR site. This in turn led to the discovery that an error in Oracle software coding had resulted in the “widespread transmission” of incorrect VUIDs from new EHR sites to legacy EHR sites, the OIG found. VA leaders and HCPs were notified of the potential clinical impact and were given specific instructions on how to mitigate the issue. They were asked to “please share widely.”
On top of that, days later, patient safety managers across the Veterans Health Administration (VHA) were told that drug-to-drug interactions, duplicate medication orders, and allergy checks were not functioning as expected, and they too were provided with remedial actions.
Oracle applied a successful software patch on in April 2023, to ensure accurate VUIDs were attached to all mail order pharmacy–processed prescriptions from that date forward. However, the OIG learned the incorrect VUIDs sent from new EHR sites and stored in the Health Data Repository from as far back as October 2020 had not been corrected. Case told the subcommittee that on November 29, 2023, the VHA Pharmacy Council reported withdrawing a request for Oracle to send corrected medication VUID data to the Health Data Repository, on the presumption that remaining inaccurate VUIDs would expire in early April 2024, and the data would be corrected at that time.
The OIG is concerned, Case said, that patient medication data remains inaccurate almost a year after VA learned of the issue. The mail order pharmacy-related data generated from approximately 120,000 patients served by new EHR sites are still incorrect. These patients face an ongoing risk of an adverse medication-related event if they receive care and medications from a VA medical center using the legacy EHR system.
The OIG also learned of other problems associated with transmission of medication and allergy information, which could have consequences such as:
- Patient medications being discontinued or stopped by new HCPs using Cerner that appear in ViSTA as active and current prescriptions;
- Allergy-warning messages not appearing when intended or inappropriately appearing for the wrong medication;
- Duplicate medication order checks not appearing when intended or inappropriately appearing for the wrong drug;
- Patient active medication lists having incomplete or inaccurate information, such as missing prescriptions, duplicate prescriptions, or incorrect medication order statuses.
The OIG warned VHA employees about the risks, although it wasn’t possible to determine who might actually be at risk. A VHA leader told the OIG that all patients who have been prescribed any medications or have medication allergies documented at a at a Cerner site are at risk. That could mean as many as 250,000 patients: As of September 2023, approximately 190,000 patients had a medication prescribed and 126,000 had an allergy documented at a new EHR site.
Case Example
Not surprisingly, “the OIG is not confident in [EHRM-Integration Office] leaders’ oversight and control of the new systems’ Health Data Repository interface programming,” Case said. He cited the case of a patient with posttraumatic stress disorder and traumatic brain injury with adrenal insufficiency. Four days prior to admission, a ViSTA site pharmacist used the EHR to perform a medication reconciliation for the patient. The data available did not include the patient’s most recent prednisone prescription, which had been ordered by an HCP at a facility using Cerner.
A nurse practitioner performed another reconciliation when the patient was admitted to the residential program, but the patient was unsure of all their medications. Because the most recent prednisone prescription was not visible in ViSTA, the prednisone appeared to have been completed at least 3 months prior to admission and was therefore not prescribed in the admission medication orders.
Five days into the residential program, the patient began exhibiting unusual behaviors associated with the lack of prednisone. The patient realized they needed more prednisone, but the nurse explained there was no prednisone on the patient’s medication list. Eventually, the patient found the active prednisone order on their personal cell phone and was transferred to a local emergency department for care.
Work Arounds
The VHA’s efforts to forestall or mitigate system errors have in some cases had a cascade effect. For example, HCPs must essentially back up what the automated software is intended to do, with “complex, time-consuming” multistep manual safety checks when prescribing new medications for patients previously cared for at a Cerner site. The OIG is concerned that this increased vigilance is “unsustainable” by pharmacists and frontline staff and could lead to burnout and medication-related patient safety events. After the new EHR launched, the OIG found, burnout symptoms for pharmacy staff increased. Nonetheless, Case told the committee, OIG staff “have observed [employees’] unwavering commitment to prioritizing the care of patients while mitigating implementation challenges.”
EHR-related workload burdens have necessitated other adjustments. Columbus, for instance, hired 9 full-time clinical pharmacists—a 62% staffing increase—to help reduce the backlog. Pharmacy leaders created approximately 29 additional work-arounds to support pharmacy staff and prevent delays. Facility pharmacy leaders also developed approximately 25 educational materials, such as tip sheets, reference guides, and job aids. The OIG’s concern—apart from the overwhelming amount of information for staff to implement—is that such prophylactic measures may in fact give rise to inconsistent practices, which increase risks to patient safety.
Committed to Working With the VA
Mike Sicilia, executive vice president of Oracle Corporation, told lawmakers in the hearing, “After the initial deployments, it became clear that the pharmacy system needed to be enhanced to better meet VA’s needs. To that end, in August 2022, shortly after Oracle completed its acquisition of Cerner, VA contracted with us for seven enhancements that overall would adapt the pharmacy system to a more bidirectional system between VA providers placing prescription orders and VA pharmacists fulfilling and dispensing them.” Those enhancements are all live for VA providers and pharmacists to use now, he said, except for one that is undergoing additional testing.
He added, “As with any healthcare technology system, there is a need for continuous improvements but that does not mean the system is not safe and effective in its current state. Oracle is committed to working with VA … throughout the reset period to identify workflows and other items that can be simplified or streamlined to improve the overall user and pharmacy experience.”
Standardizing workflows and ensuring training and communications to pharmacists about the latest updates will discourage use of work-arounds, Sicilia said, and “help with improving morale and satisfaction with the system.” During a visit in early February by VA and the Oracle team to the Lovell Federal Health Care Center in North Chicago, “feedback from pharmacists was positive about the training and readiness for using the new pharmacy system.”
The backlog, at least, may be resolved. Sicilia said on average more than 215,000 outpatient prescriptions are being filled each month. “The current live sites do not have a backlog in filling prescriptions. Recent data from this month show that three of the five live sites have zero prescriptions waiting to be processed that are older than seven days. The two other live sites have an average of two prescriptions older than seven days,” he said.
Although Oracle Health has since resolved some of the identified issues, the OIG is concerned that the new EHR will continue to be deployed at medical facilities despite “myriad” as-yet unresolved issues related to inaccurate medication ordering, reconciliation, and dispensing. The VHA has paused Cerner deployments multiple times.
“It is unclear whether identified problems are being adequately resolved before additional deployments,” Case said. “There is also the question of whether there is sufficient transparency and communication among EHRM-IO, VHA and facility leaders, VA leaders, and Oracle Health needed for quality control and critical coordination. Trust in VA is also dependent on patients being fully and quickly advised when issues affecting them are identified and addressed. As VA moves toward its deployment next month at a complex facility jointly operated with the Department of Defense, transparency, communication, and program management will be essential to getting it right. Failures in these areas risk cascading problems.”
Will the new US Department of Veterans Affairs (VA) pharmacy software be safe and effective? That was the topic when David Case, the VA Deputy Inspector General, spoke in the US House of Representatives Veterans Affairs Committee technology modernization subcommittee hearing on February 15.
Questions like that have dogged the project since 2018, when the VA began rolling out the Oracle Cerner electronic health record (EHR) system as the successor to ViSTA.
The Oracle system has been beset by one glitch after another since its arrival. And in that time, Case said, the VA Office of Inspector General (OIG) has been engaging with VA employees at sites in Washington, Oregon, Ohio, Illinois, and other locations where the modernization program has been piloted.
The most recent OIG investigation of pharmacy-related patient safety issues began with a review of an allegation of a prescription backlog at Columbus, Ohio, where the system went live on April 30, 2022. The OIG found that facility leaders took “timely and sustainable steps” to manage that issue. However, other unresolved patient safety issues came to light, such as medication inaccuracies, inaccurate medication data, and insufficient staffing. The OIG also found staff were creating “numerous work arounds” to provide patient care, and that the volume of staff educational materials for pharmacy-related functions was “overwhelming.”
Those problems were just the latest in a long queue. In May 2021, after the first VA deployment of the new EHR at the Mann-Grandstaff VA Medical Center in Spokane, Washington, a pharmacy patient safety team under the VA National Center for Patient Safety (NCPS) also had identified patient safety issues and “multiple” concerns regarding the system’s usability. For example, updates to a patient’s active medication list were not routinely reflected at the patient’s next appointment. Despite knowing about such challenges, Case noted in his report, VA leaders deployed the new EHR at 4 more VA medical centers.
Cerner/ViSTA Communication
One major cause of the current problems is the way the systems “talk” to each other. EHR information is communicated between VHA facilities through channels that include the Joint Longitudinal Viewer (JLV) and the Health Data Repository, which stores patient-specific clinical information from both the legacy and the new EHR systems. The JLV application allows clinicians to access a read only version of a patient’s EHR from both systems.
Every medication used in VHA has a VA Unique Identifier (VUID). When a patient is prescribed a medication at a new EHR site, that medication’s VUID is sent to the Health Data Repository. If that patient seeks care from a legacy health care practitioner (HCP), and that HCP enters a medication order, a software interface accesses the VUID from the Health Data Repository to verify that the medication being prescribed is safe and compatible with the medications and allergies previously documented in the patient’s record.
However, on March 31, 2023, staff from a ViSTA site found an incorrect medication order when prescribing a new medication to a patient who had received care and medications at a new EHR site. This in turn led to the discovery that an error in Oracle software coding had resulted in the “widespread transmission” of incorrect VUIDs from new EHR sites to legacy EHR sites, the OIG found. VA leaders and HCPs were notified of the potential clinical impact and were given specific instructions on how to mitigate the issue. They were asked to “please share widely.”
On top of that, days later, patient safety managers across the Veterans Health Administration (VHA) were told that drug-to-drug interactions, duplicate medication orders, and allergy checks were not functioning as expected, and they too were provided with remedial actions.
Oracle applied a successful software patch on in April 2023, to ensure accurate VUIDs were attached to all mail order pharmacy–processed prescriptions from that date forward. However, the OIG learned the incorrect VUIDs sent from new EHR sites and stored in the Health Data Repository from as far back as October 2020 had not been corrected. Case told the subcommittee that on November 29, 2023, the VHA Pharmacy Council reported withdrawing a request for Oracle to send corrected medication VUID data to the Health Data Repository, on the presumption that remaining inaccurate VUIDs would expire in early April 2024, and the data would be corrected at that time.
The OIG is concerned, Case said, that patient medication data remains inaccurate almost a year after VA learned of the issue. The mail order pharmacy-related data generated from approximately 120,000 patients served by new EHR sites are still incorrect. These patients face an ongoing risk of an adverse medication-related event if they receive care and medications from a VA medical center using the legacy EHR system.
The OIG also learned of other problems associated with transmission of medication and allergy information, which could have consequences such as:
- Patient medications being discontinued or stopped by new HCPs using Cerner that appear in ViSTA as active and current prescriptions;
- Allergy-warning messages not appearing when intended or inappropriately appearing for the wrong medication;
- Duplicate medication order checks not appearing when intended or inappropriately appearing for the wrong drug;
- Patient active medication lists having incomplete or inaccurate information, such as missing prescriptions, duplicate prescriptions, or incorrect medication order statuses.
The OIG warned VHA employees about the risks, although it wasn’t possible to determine who might actually be at risk. A VHA leader told the OIG that all patients who have been prescribed any medications or have medication allergies documented at a at a Cerner site are at risk. That could mean as many as 250,000 patients: As of September 2023, approximately 190,000 patients had a medication prescribed and 126,000 had an allergy documented at a new EHR site.
Case Example
Not surprisingly, “the OIG is not confident in [EHRM-Integration Office] leaders’ oversight and control of the new systems’ Health Data Repository interface programming,” Case said. He cited the case of a patient with posttraumatic stress disorder and traumatic brain injury with adrenal insufficiency. Four days prior to admission, a ViSTA site pharmacist used the EHR to perform a medication reconciliation for the patient. The data available did not include the patient’s most recent prednisone prescription, which had been ordered by an HCP at a facility using Cerner.
A nurse practitioner performed another reconciliation when the patient was admitted to the residential program, but the patient was unsure of all their medications. Because the most recent prednisone prescription was not visible in ViSTA, the prednisone appeared to have been completed at least 3 months prior to admission and was therefore not prescribed in the admission medication orders.
Five days into the residential program, the patient began exhibiting unusual behaviors associated with the lack of prednisone. The patient realized they needed more prednisone, but the nurse explained there was no prednisone on the patient’s medication list. Eventually, the patient found the active prednisone order on their personal cell phone and was transferred to a local emergency department for care.
Work Arounds
The VHA’s efforts to forestall or mitigate system errors have in some cases had a cascade effect. For example, HCPs must essentially back up what the automated software is intended to do, with “complex, time-consuming” multistep manual safety checks when prescribing new medications for patients previously cared for at a Cerner site. The OIG is concerned that this increased vigilance is “unsustainable” by pharmacists and frontline staff and could lead to burnout and medication-related patient safety events. After the new EHR launched, the OIG found, burnout symptoms for pharmacy staff increased. Nonetheless, Case told the committee, OIG staff “have observed [employees’] unwavering commitment to prioritizing the care of patients while mitigating implementation challenges.”
EHR-related workload burdens have necessitated other adjustments. Columbus, for instance, hired 9 full-time clinical pharmacists—a 62% staffing increase—to help reduce the backlog. Pharmacy leaders created approximately 29 additional work-arounds to support pharmacy staff and prevent delays. Facility pharmacy leaders also developed approximately 25 educational materials, such as tip sheets, reference guides, and job aids. The OIG’s concern—apart from the overwhelming amount of information for staff to implement—is that such prophylactic measures may in fact give rise to inconsistent practices, which increase risks to patient safety.
Committed to Working With the VA
Mike Sicilia, executive vice president of Oracle Corporation, told lawmakers in the hearing, “After the initial deployments, it became clear that the pharmacy system needed to be enhanced to better meet VA’s needs. To that end, in August 2022, shortly after Oracle completed its acquisition of Cerner, VA contracted with us for seven enhancements that overall would adapt the pharmacy system to a more bidirectional system between VA providers placing prescription orders and VA pharmacists fulfilling and dispensing them.” Those enhancements are all live for VA providers and pharmacists to use now, he said, except for one that is undergoing additional testing.
He added, “As with any healthcare technology system, there is a need for continuous improvements but that does not mean the system is not safe and effective in its current state. Oracle is committed to working with VA … throughout the reset period to identify workflows and other items that can be simplified or streamlined to improve the overall user and pharmacy experience.”
Standardizing workflows and ensuring training and communications to pharmacists about the latest updates will discourage use of work-arounds, Sicilia said, and “help with improving morale and satisfaction with the system.” During a visit in early February by VA and the Oracle team to the Lovell Federal Health Care Center in North Chicago, “feedback from pharmacists was positive about the training and readiness for using the new pharmacy system.”
The backlog, at least, may be resolved. Sicilia said on average more than 215,000 outpatient prescriptions are being filled each month. “The current live sites do not have a backlog in filling prescriptions. Recent data from this month show that three of the five live sites have zero prescriptions waiting to be processed that are older than seven days. The two other live sites have an average of two prescriptions older than seven days,” he said.
Although Oracle Health has since resolved some of the identified issues, the OIG is concerned that the new EHR will continue to be deployed at medical facilities despite “myriad” as-yet unresolved issues related to inaccurate medication ordering, reconciliation, and dispensing. The VHA has paused Cerner deployments multiple times.
“It is unclear whether identified problems are being adequately resolved before additional deployments,” Case said. “There is also the question of whether there is sufficient transparency and communication among EHRM-IO, VHA and facility leaders, VA leaders, and Oracle Health needed for quality control and critical coordination. Trust in VA is also dependent on patients being fully and quickly advised when issues affecting them are identified and addressed. As VA moves toward its deployment next month at a complex facility jointly operated with the Department of Defense, transparency, communication, and program management will be essential to getting it right. Failures in these areas risk cascading problems.”
Will the new US Department of Veterans Affairs (VA) pharmacy software be safe and effective? That was the topic when David Case, the VA Deputy Inspector General, spoke in the US House of Representatives Veterans Affairs Committee technology modernization subcommittee hearing on February 15.
Questions like that have dogged the project since 2018, when the VA began rolling out the Oracle Cerner electronic health record (EHR) system as the successor to ViSTA.
The Oracle system has been beset by one glitch after another since its arrival. And in that time, Case said, the VA Office of Inspector General (OIG) has been engaging with VA employees at sites in Washington, Oregon, Ohio, Illinois, and other locations where the modernization program has been piloted.
The most recent OIG investigation of pharmacy-related patient safety issues began with a review of an allegation of a prescription backlog at Columbus, Ohio, where the system went live on April 30, 2022. The OIG found that facility leaders took “timely and sustainable steps” to manage that issue. However, other unresolved patient safety issues came to light, such as medication inaccuracies, inaccurate medication data, and insufficient staffing. The OIG also found staff were creating “numerous work arounds” to provide patient care, and that the volume of staff educational materials for pharmacy-related functions was “overwhelming.”
Those problems were just the latest in a long queue. In May 2021, after the first VA deployment of the new EHR at the Mann-Grandstaff VA Medical Center in Spokane, Washington, a pharmacy patient safety team under the VA National Center for Patient Safety (NCPS) also had identified patient safety issues and “multiple” concerns regarding the system’s usability. For example, updates to a patient’s active medication list were not routinely reflected at the patient’s next appointment. Despite knowing about such challenges, Case noted in his report, VA leaders deployed the new EHR at 4 more VA medical centers.
Cerner/ViSTA Communication
One major cause of the current problems is the way the systems “talk” to each other. EHR information is communicated between VHA facilities through channels that include the Joint Longitudinal Viewer (JLV) and the Health Data Repository, which stores patient-specific clinical information from both the legacy and the new EHR systems. The JLV application allows clinicians to access a read only version of a patient’s EHR from both systems.
Every medication used in VHA has a VA Unique Identifier (VUID). When a patient is prescribed a medication at a new EHR site, that medication’s VUID is sent to the Health Data Repository. If that patient seeks care from a legacy health care practitioner (HCP), and that HCP enters a medication order, a software interface accesses the VUID from the Health Data Repository to verify that the medication being prescribed is safe and compatible with the medications and allergies previously documented in the patient’s record.
However, on March 31, 2023, staff from a ViSTA site found an incorrect medication order when prescribing a new medication to a patient who had received care and medications at a new EHR site. This in turn led to the discovery that an error in Oracle software coding had resulted in the “widespread transmission” of incorrect VUIDs from new EHR sites to legacy EHR sites, the OIG found. VA leaders and HCPs were notified of the potential clinical impact and were given specific instructions on how to mitigate the issue. They were asked to “please share widely.”
On top of that, days later, patient safety managers across the Veterans Health Administration (VHA) were told that drug-to-drug interactions, duplicate medication orders, and allergy checks were not functioning as expected, and they too were provided with remedial actions.
Oracle applied a successful software patch on in April 2023, to ensure accurate VUIDs were attached to all mail order pharmacy–processed prescriptions from that date forward. However, the OIG learned the incorrect VUIDs sent from new EHR sites and stored in the Health Data Repository from as far back as October 2020 had not been corrected. Case told the subcommittee that on November 29, 2023, the VHA Pharmacy Council reported withdrawing a request for Oracle to send corrected medication VUID data to the Health Data Repository, on the presumption that remaining inaccurate VUIDs would expire in early April 2024, and the data would be corrected at that time.
The OIG is concerned, Case said, that patient medication data remains inaccurate almost a year after VA learned of the issue. The mail order pharmacy-related data generated from approximately 120,000 patients served by new EHR sites are still incorrect. These patients face an ongoing risk of an adverse medication-related event if they receive care and medications from a VA medical center using the legacy EHR system.
The OIG also learned of other problems associated with transmission of medication and allergy information, which could have consequences such as:
- Patient medications being discontinued or stopped by new HCPs using Cerner that appear in ViSTA as active and current prescriptions;
- Allergy-warning messages not appearing when intended or inappropriately appearing for the wrong medication;
- Duplicate medication order checks not appearing when intended or inappropriately appearing for the wrong drug;
- Patient active medication lists having incomplete or inaccurate information, such as missing prescriptions, duplicate prescriptions, or incorrect medication order statuses.
The OIG warned VHA employees about the risks, although it wasn’t possible to determine who might actually be at risk. A VHA leader told the OIG that all patients who have been prescribed any medications or have medication allergies documented at a at a Cerner site are at risk. That could mean as many as 250,000 patients: As of September 2023, approximately 190,000 patients had a medication prescribed and 126,000 had an allergy documented at a new EHR site.
Case Example
Not surprisingly, “the OIG is not confident in [EHRM-Integration Office] leaders’ oversight and control of the new systems’ Health Data Repository interface programming,” Case said. He cited the case of a patient with posttraumatic stress disorder and traumatic brain injury with adrenal insufficiency. Four days prior to admission, a ViSTA site pharmacist used the EHR to perform a medication reconciliation for the patient. The data available did not include the patient’s most recent prednisone prescription, which had been ordered by an HCP at a facility using Cerner.
A nurse practitioner performed another reconciliation when the patient was admitted to the residential program, but the patient was unsure of all their medications. Because the most recent prednisone prescription was not visible in ViSTA, the prednisone appeared to have been completed at least 3 months prior to admission and was therefore not prescribed in the admission medication orders.
Five days into the residential program, the patient began exhibiting unusual behaviors associated with the lack of prednisone. The patient realized they needed more prednisone, but the nurse explained there was no prednisone on the patient’s medication list. Eventually, the patient found the active prednisone order on their personal cell phone and was transferred to a local emergency department for care.
Work Arounds
The VHA’s efforts to forestall or mitigate system errors have in some cases had a cascade effect. For example, HCPs must essentially back up what the automated software is intended to do, with “complex, time-consuming” multistep manual safety checks when prescribing new medications for patients previously cared for at a Cerner site. The OIG is concerned that this increased vigilance is “unsustainable” by pharmacists and frontline staff and could lead to burnout and medication-related patient safety events. After the new EHR launched, the OIG found, burnout symptoms for pharmacy staff increased. Nonetheless, Case told the committee, OIG staff “have observed [employees’] unwavering commitment to prioritizing the care of patients while mitigating implementation challenges.”
EHR-related workload burdens have necessitated other adjustments. Columbus, for instance, hired 9 full-time clinical pharmacists—a 62% staffing increase—to help reduce the backlog. Pharmacy leaders created approximately 29 additional work-arounds to support pharmacy staff and prevent delays. Facility pharmacy leaders also developed approximately 25 educational materials, such as tip sheets, reference guides, and job aids. The OIG’s concern—apart from the overwhelming amount of information for staff to implement—is that such prophylactic measures may in fact give rise to inconsistent practices, which increase risks to patient safety.
Committed to Working With the VA
Mike Sicilia, executive vice president of Oracle Corporation, told lawmakers in the hearing, “After the initial deployments, it became clear that the pharmacy system needed to be enhanced to better meet VA’s needs. To that end, in August 2022, shortly after Oracle completed its acquisition of Cerner, VA contracted with us for seven enhancements that overall would adapt the pharmacy system to a more bidirectional system between VA providers placing prescription orders and VA pharmacists fulfilling and dispensing them.” Those enhancements are all live for VA providers and pharmacists to use now, he said, except for one that is undergoing additional testing.
He added, “As with any healthcare technology system, there is a need for continuous improvements but that does not mean the system is not safe and effective in its current state. Oracle is committed to working with VA … throughout the reset period to identify workflows and other items that can be simplified or streamlined to improve the overall user and pharmacy experience.”
Standardizing workflows and ensuring training and communications to pharmacists about the latest updates will discourage use of work-arounds, Sicilia said, and “help with improving morale and satisfaction with the system.” During a visit in early February by VA and the Oracle team to the Lovell Federal Health Care Center in North Chicago, “feedback from pharmacists was positive about the training and readiness for using the new pharmacy system.”
The backlog, at least, may be resolved. Sicilia said on average more than 215,000 outpatient prescriptions are being filled each month. “The current live sites do not have a backlog in filling prescriptions. Recent data from this month show that three of the five live sites have zero prescriptions waiting to be processed that are older than seven days. The two other live sites have an average of two prescriptions older than seven days,” he said.
Although Oracle Health has since resolved some of the identified issues, the OIG is concerned that the new EHR will continue to be deployed at medical facilities despite “myriad” as-yet unresolved issues related to inaccurate medication ordering, reconciliation, and dispensing. The VHA has paused Cerner deployments multiple times.
“It is unclear whether identified problems are being adequately resolved before additional deployments,” Case said. “There is also the question of whether there is sufficient transparency and communication among EHRM-IO, VHA and facility leaders, VA leaders, and Oracle Health needed for quality control and critical coordination. Trust in VA is also dependent on patients being fully and quickly advised when issues affecting them are identified and addressed. As VA moves toward its deployment next month at a complex facility jointly operated with the Department of Defense, transparency, communication, and program management will be essential to getting it right. Failures in these areas risk cascading problems.”
Implementing Trustworthy AI in VA High Reliability Health Care Organizations
Artificial intelligence (AI) has lagged in health care but has considerable potential to improve quality, safety, clinician experience, and access to care. It is being tested in areas like billing, hospital operations, and preventing adverse events (eg, sepsis mortality) with some early success. However, there are still many barriers preventing the widespread use of AI, such as data problems, mismatched rewards, and workplace obstacles. Innovative projects, partnerships, better rewards, and more investment could remove barriers. Implemented reliably and safely, AI can add to what clinicians know, help them work faster, cut costs, and, most importantly, improve patient care.1
AI can potentially bring several clinical benefits, such as reducing the administrative strain on clinicians and granting them more time for direct patient care. It can also improve diagnostic accuracy by analyzing patient data and diagnostic images, providing differential diagnoses, and increasing access to care by providing medical information and essential online services to patients.2
High Reliability Organizations
High reliability health care organizations have considerable experience safely launching new programs. For example, the Patient Safety Adoption Framework gives practical tips for smoothly rolling out safety initiatives (Table 1). Developed with experts and diverse views, this framework has 5 key areas: leadership, culture and context, process, measurement, and person-centeredness. These address adoption problems, guide leaders step-by-step, and focus on leadership buy-in, safety culture, cooperation, and local customization. Checklists and tools make it systematic to go from ideas to action on patient safety.3
Leadership involves establishing organizational commitment behind new safety programs. This visible commitment signals importance and priorities to others. Leaders model desired behaviors and language around safety, allocate resources, remove obstacles, and keep initiatives energized over time through consistent messaging.4 Culture and context recognizes that safety culture differs across units and facilities. Local input tailors programs to fit and examines strengths to build on, like psychological safety. Surveys gauge the existing culture and its need for change. Process details how to plan, design, test, implement, and improve new safety practices and provides a phased roadmap from idea to results. Measurement collects data to drive improvement and show impact. Metrics track progress and allow benchmarking. Person-centeredness puts patients first in safety efforts through participation, education, and transparency.
The Veterans Health Administration piloted a comprehensive high reliability hospital (HRH) model. Over 3 years, the Veterans Health Administration focused on leadership, culture, and process improvement at a hospital. After initiating the model, the pilot hospital improved its safety culture, reported more minor safety issues, and reduced deaths and complications better than other hospitals. The high-reliability approach successfully instilled principles and improved culture and outcomes. The HRH model is set to be expanded to 18 more US Department of Veterans Affairs (VA) sites for further evaluation across diverse settings.5
Trustworthy AI Framework
AI systems are growing more powerful and widespread, including in health care. Unfortunately, irresponsible AI can introduce new harm. ChatGPT and other large language models, for example, sometimes are known to provide erroneous information in a compelling way. Clinicians and patients who use such programs can act on such information, which would lead to unforeseen negative consequences. Several frameworks on ethical AI have come from governmental groups.6-9 In 2023, the VA National AI Institute suggested a Trustworthy AI Framework based on core principles tailored for federal health care. The framework has 6 key principles: purposeful, effective and safe, secure and private, fair and equitable, transparent and explainable, and accountable and monitored (Table 2).10
First, AI must clearly help veterans while minimizing risks. To ensure purpose, the VA will assess patient and clinician needs and design AI that targets meaningful problems to avoid scope creep or feature bloat. For example, adding new features to the AI software after release can clutter and complicate the interface, making it difficult to use. Rigorous testing will confirm that AI meets intent prior to deployment. Second, AI is designed and checked for effectiveness, safety, and reliability. The VA pledges to monitor AI’s impact to ensure it performs as expected without unintended consequences. Algorithms will be stress tested across representative datasets and approval processes will screen for safety issues. Third, AI models are secured from vulnerabilities and misuse. Technical controls will prevent unauthorized access or changes to AI systems. Audits will check for appropriate internal usage per policies. Continual patches and upgrades will maintain security. Fourth, the VA manages AI for fairness, avoiding bias. They will proactively assess datasets and algorithms for potential biases based on protected attributes like race, gender, or age. Biased outputs will be addressed through techniques such as data augmentation, reweighting, and algorithm tweaks. Fifth, transparency explains AI’s role in care. Documentation will detail an AI system’s data sources, methodology, testing, limitations, and integration with clinical workflows. Clinicians and patients will receive education on interpreting AI outputs. Finally, the VA pledges to closely monitor AI systems to sustain trust. The VA will establish oversight processes to quickly identify any declines in reliability or unfair impacts on subgroups. AI models will be retrained as needed based on incoming data patterns.
Each Trustworthy AI Framework principle connects to others in existing frameworks. The purpose principle aligns with human-centric AI focused on benefits. Effectiveness and safety link to technical robustness and risk management principles. Security maps to privacy protection principles. Fairness connects to principles of avoiding bias and discrimination. Transparency corresponds with accountable and explainable AI. Monitoring and accountability tie back to governance principles. Overall, the VA framework aims to guide ethical AI based on context. It offers a model for managing risks and building trust in health care AI.
Combining VA principles with high-reliability safety principles can ensure that AI benefits veterans. The leadership and culture aspects will drive commitment to trustworthy AI practices. Leaders will communicate the importance of responsible AI through words and actions. Culture surveys can assess baseline awareness of AI ethics issues to target education. AI security and fairness will be emphasized as safety critical. The process aspect will institute policies and procedures to uphold AI principles through the project lifecycle. For example, structured testing processes will validate safety. Measurement will collect data on principles like transparency and fairness. Dashboards can track metrics like explainability and biases. A patient-centered approach will incorporate veteran perspectives on AI through participatory design and advisory councils. They can give input on AI explainability and potential biases based on their diverse backgrounds.
Conclusions
Joint principles will lead to successful AI that improves care while proactively managing risks. Involve leaders to stress the necessity of eliminating biases. Build security into the AI development process. Co-design AI transparency features with end users. Closely monitor the impact of AI across safety, fairness, and other principles. Adhering to both Trustworthy AI and high reliability organizations principles will earn veterans’ confidence. Health care organizations like the VA can integrate ethical AI safely via established frameworks. With responsible design and implementation, AI’s potential to enhance care quality, safety, and access can be realized.
Acknowledgments
We would like to acknowledge Joshua Mueller, Theo Tiffney, John Zachary, and Gil Alterovitz for their excellent work creating the VA Trustworthy Principles. This material is the result of work supported by resources and the use of facilities at the James A. Haley Veterans’ Hospital.
1. Sahni NR, Carrus B. Artificial intelligence in U.S. health care delivery. N Engl J Med. 2023;389(4):348-358. doi:10.1056/NEJMra2204673
2. Borkowski AA, Jakey CE, Mastorides SM, et al. Applications of ChatGPT and large language models in medicine and health care: benefits and pitfalls. Fed Pract. 2023;40(6):170-173. doi:10.12788/fp.0386
3. Moyal-Smith R, Margo J, Maloney FL, et al. The patient safety adoption framework: a practical framework to bridge the know-do gap. J Patient Saf. 2023;19(4):243-248. doi:10.1097/PTS.0000000000001118
4. Isaacks DB, Anderson TM, Moore SC, Patterson W, Govindan S. High reliability organization principles improve VA workplace burnout: the Truman THRIVE2 model. Am J Med Qual. 2021;36(6):422-428. doi:10.1097/01.JMQ.0000735516.35323.97
5. Sculli GL, Pendley-Louis R, Neily J, et al. A high-reliability organization framework for health care: a multiyear implementation strategy and associated outcomes. J Patient Saf. 2022;18(1):64-70. doi:10.1097/PTS.0000000000000788
6. National Institute of Standards and Technology. AI risk management framework. Accessed January 2, 2024. https://www.nist.gov/itl/ai-risk-management-framework
7. Executive Office of the President, Office of Science and Technology Policy. Blueprint for an AI Bill of Rights. Accessed January 11, 2024. https://www.whitehouse.gov/ostp/ai-bill-of-rights
8. Executive Office of the President. Executive Order 13960: promoting the use of trustworthy artificial intelligence in the federal government. Fed Regist. 2020;89(236):78939-78943.
9. Biden JR. Executive Order on the safe, secure, and trustworthy development and use of artificial intelligence. Published October 30, 2023. Accessed January 11, 2024. https://www.whitehouse.gov/briefing-room/presidential-actions/2023/10/30/executive-order-on-the-safe-secure-and-trustworthy-development-and-use-of-artificial-intelligence/
10. US Department of Veterans Affairs. Trustworthy AI. Accessed January 11, 2024. https://department.va.gov/ai/trustworthy/
Artificial intelligence (AI) has lagged in health care but has considerable potential to improve quality, safety, clinician experience, and access to care. It is being tested in areas like billing, hospital operations, and preventing adverse events (eg, sepsis mortality) with some early success. However, there are still many barriers preventing the widespread use of AI, such as data problems, mismatched rewards, and workplace obstacles. Innovative projects, partnerships, better rewards, and more investment could remove barriers. Implemented reliably and safely, AI can add to what clinicians know, help them work faster, cut costs, and, most importantly, improve patient care.1
AI can potentially bring several clinical benefits, such as reducing the administrative strain on clinicians and granting them more time for direct patient care. It can also improve diagnostic accuracy by analyzing patient data and diagnostic images, providing differential diagnoses, and increasing access to care by providing medical information and essential online services to patients.2
High Reliability Organizations
High reliability health care organizations have considerable experience safely launching new programs. For example, the Patient Safety Adoption Framework gives practical tips for smoothly rolling out safety initiatives (Table 1). Developed with experts and diverse views, this framework has 5 key areas: leadership, culture and context, process, measurement, and person-centeredness. These address adoption problems, guide leaders step-by-step, and focus on leadership buy-in, safety culture, cooperation, and local customization. Checklists and tools make it systematic to go from ideas to action on patient safety.3
Leadership involves establishing organizational commitment behind new safety programs. This visible commitment signals importance and priorities to others. Leaders model desired behaviors and language around safety, allocate resources, remove obstacles, and keep initiatives energized over time through consistent messaging.4 Culture and context recognizes that safety culture differs across units and facilities. Local input tailors programs to fit and examines strengths to build on, like psychological safety. Surveys gauge the existing culture and its need for change. Process details how to plan, design, test, implement, and improve new safety practices and provides a phased roadmap from idea to results. Measurement collects data to drive improvement and show impact. Metrics track progress and allow benchmarking. Person-centeredness puts patients first in safety efforts through participation, education, and transparency.
The Veterans Health Administration piloted a comprehensive high reliability hospital (HRH) model. Over 3 years, the Veterans Health Administration focused on leadership, culture, and process improvement at a hospital. After initiating the model, the pilot hospital improved its safety culture, reported more minor safety issues, and reduced deaths and complications better than other hospitals. The high-reliability approach successfully instilled principles and improved culture and outcomes. The HRH model is set to be expanded to 18 more US Department of Veterans Affairs (VA) sites for further evaluation across diverse settings.5
Trustworthy AI Framework
AI systems are growing more powerful and widespread, including in health care. Unfortunately, irresponsible AI can introduce new harm. ChatGPT and other large language models, for example, sometimes are known to provide erroneous information in a compelling way. Clinicians and patients who use such programs can act on such information, which would lead to unforeseen negative consequences. Several frameworks on ethical AI have come from governmental groups.6-9 In 2023, the VA National AI Institute suggested a Trustworthy AI Framework based on core principles tailored for federal health care. The framework has 6 key principles: purposeful, effective and safe, secure and private, fair and equitable, transparent and explainable, and accountable and monitored (Table 2).10
First, AI must clearly help veterans while minimizing risks. To ensure purpose, the VA will assess patient and clinician needs and design AI that targets meaningful problems to avoid scope creep or feature bloat. For example, adding new features to the AI software after release can clutter and complicate the interface, making it difficult to use. Rigorous testing will confirm that AI meets intent prior to deployment. Second, AI is designed and checked for effectiveness, safety, and reliability. The VA pledges to monitor AI’s impact to ensure it performs as expected without unintended consequences. Algorithms will be stress tested across representative datasets and approval processes will screen for safety issues. Third, AI models are secured from vulnerabilities and misuse. Technical controls will prevent unauthorized access or changes to AI systems. Audits will check for appropriate internal usage per policies. Continual patches and upgrades will maintain security. Fourth, the VA manages AI for fairness, avoiding bias. They will proactively assess datasets and algorithms for potential biases based on protected attributes like race, gender, or age. Biased outputs will be addressed through techniques such as data augmentation, reweighting, and algorithm tweaks. Fifth, transparency explains AI’s role in care. Documentation will detail an AI system’s data sources, methodology, testing, limitations, and integration with clinical workflows. Clinicians and patients will receive education on interpreting AI outputs. Finally, the VA pledges to closely monitor AI systems to sustain trust. The VA will establish oversight processes to quickly identify any declines in reliability or unfair impacts on subgroups. AI models will be retrained as needed based on incoming data patterns.
Each Trustworthy AI Framework principle connects to others in existing frameworks. The purpose principle aligns with human-centric AI focused on benefits. Effectiveness and safety link to technical robustness and risk management principles. Security maps to privacy protection principles. Fairness connects to principles of avoiding bias and discrimination. Transparency corresponds with accountable and explainable AI. Monitoring and accountability tie back to governance principles. Overall, the VA framework aims to guide ethical AI based on context. It offers a model for managing risks and building trust in health care AI.
Combining VA principles with high-reliability safety principles can ensure that AI benefits veterans. The leadership and culture aspects will drive commitment to trustworthy AI practices. Leaders will communicate the importance of responsible AI through words and actions. Culture surveys can assess baseline awareness of AI ethics issues to target education. AI security and fairness will be emphasized as safety critical. The process aspect will institute policies and procedures to uphold AI principles through the project lifecycle. For example, structured testing processes will validate safety. Measurement will collect data on principles like transparency and fairness. Dashboards can track metrics like explainability and biases. A patient-centered approach will incorporate veteran perspectives on AI through participatory design and advisory councils. They can give input on AI explainability and potential biases based on their diverse backgrounds.
Conclusions
Joint principles will lead to successful AI that improves care while proactively managing risks. Involve leaders to stress the necessity of eliminating biases. Build security into the AI development process. Co-design AI transparency features with end users. Closely monitor the impact of AI across safety, fairness, and other principles. Adhering to both Trustworthy AI and high reliability organizations principles will earn veterans’ confidence. Health care organizations like the VA can integrate ethical AI safely via established frameworks. With responsible design and implementation, AI’s potential to enhance care quality, safety, and access can be realized.
Acknowledgments
We would like to acknowledge Joshua Mueller, Theo Tiffney, John Zachary, and Gil Alterovitz for their excellent work creating the VA Trustworthy Principles. This material is the result of work supported by resources and the use of facilities at the James A. Haley Veterans’ Hospital.
Artificial intelligence (AI) has lagged in health care but has considerable potential to improve quality, safety, clinician experience, and access to care. It is being tested in areas like billing, hospital operations, and preventing adverse events (eg, sepsis mortality) with some early success. However, there are still many barriers preventing the widespread use of AI, such as data problems, mismatched rewards, and workplace obstacles. Innovative projects, partnerships, better rewards, and more investment could remove barriers. Implemented reliably and safely, AI can add to what clinicians know, help them work faster, cut costs, and, most importantly, improve patient care.1
AI can potentially bring several clinical benefits, such as reducing the administrative strain on clinicians and granting them more time for direct patient care. It can also improve diagnostic accuracy by analyzing patient data and diagnostic images, providing differential diagnoses, and increasing access to care by providing medical information and essential online services to patients.2
High Reliability Organizations
High reliability health care organizations have considerable experience safely launching new programs. For example, the Patient Safety Adoption Framework gives practical tips for smoothly rolling out safety initiatives (Table 1). Developed with experts and diverse views, this framework has 5 key areas: leadership, culture and context, process, measurement, and person-centeredness. These address adoption problems, guide leaders step-by-step, and focus on leadership buy-in, safety culture, cooperation, and local customization. Checklists and tools make it systematic to go from ideas to action on patient safety.3
Leadership involves establishing organizational commitment behind new safety programs. This visible commitment signals importance and priorities to others. Leaders model desired behaviors and language around safety, allocate resources, remove obstacles, and keep initiatives energized over time through consistent messaging.4 Culture and context recognizes that safety culture differs across units and facilities. Local input tailors programs to fit and examines strengths to build on, like psychological safety. Surveys gauge the existing culture and its need for change. Process details how to plan, design, test, implement, and improve new safety practices and provides a phased roadmap from idea to results. Measurement collects data to drive improvement and show impact. Metrics track progress and allow benchmarking. Person-centeredness puts patients first in safety efforts through participation, education, and transparency.
The Veterans Health Administration piloted a comprehensive high reliability hospital (HRH) model. Over 3 years, the Veterans Health Administration focused on leadership, culture, and process improvement at a hospital. After initiating the model, the pilot hospital improved its safety culture, reported more minor safety issues, and reduced deaths and complications better than other hospitals. The high-reliability approach successfully instilled principles and improved culture and outcomes. The HRH model is set to be expanded to 18 more US Department of Veterans Affairs (VA) sites for further evaluation across diverse settings.5
Trustworthy AI Framework
AI systems are growing more powerful and widespread, including in health care. Unfortunately, irresponsible AI can introduce new harm. ChatGPT and other large language models, for example, sometimes are known to provide erroneous information in a compelling way. Clinicians and patients who use such programs can act on such information, which would lead to unforeseen negative consequences. Several frameworks on ethical AI have come from governmental groups.6-9 In 2023, the VA National AI Institute suggested a Trustworthy AI Framework based on core principles tailored for federal health care. The framework has 6 key principles: purposeful, effective and safe, secure and private, fair and equitable, transparent and explainable, and accountable and monitored (Table 2).10
First, AI must clearly help veterans while minimizing risks. To ensure purpose, the VA will assess patient and clinician needs and design AI that targets meaningful problems to avoid scope creep or feature bloat. For example, adding new features to the AI software after release can clutter and complicate the interface, making it difficult to use. Rigorous testing will confirm that AI meets intent prior to deployment. Second, AI is designed and checked for effectiveness, safety, and reliability. The VA pledges to monitor AI’s impact to ensure it performs as expected without unintended consequences. Algorithms will be stress tested across representative datasets and approval processes will screen for safety issues. Third, AI models are secured from vulnerabilities and misuse. Technical controls will prevent unauthorized access or changes to AI systems. Audits will check for appropriate internal usage per policies. Continual patches and upgrades will maintain security. Fourth, the VA manages AI for fairness, avoiding bias. They will proactively assess datasets and algorithms for potential biases based on protected attributes like race, gender, or age. Biased outputs will be addressed through techniques such as data augmentation, reweighting, and algorithm tweaks. Fifth, transparency explains AI’s role in care. Documentation will detail an AI system’s data sources, methodology, testing, limitations, and integration with clinical workflows. Clinicians and patients will receive education on interpreting AI outputs. Finally, the VA pledges to closely monitor AI systems to sustain trust. The VA will establish oversight processes to quickly identify any declines in reliability or unfair impacts on subgroups. AI models will be retrained as needed based on incoming data patterns.
Each Trustworthy AI Framework principle connects to others in existing frameworks. The purpose principle aligns with human-centric AI focused on benefits. Effectiveness and safety link to technical robustness and risk management principles. Security maps to privacy protection principles. Fairness connects to principles of avoiding bias and discrimination. Transparency corresponds with accountable and explainable AI. Monitoring and accountability tie back to governance principles. Overall, the VA framework aims to guide ethical AI based on context. It offers a model for managing risks and building trust in health care AI.
Combining VA principles with high-reliability safety principles can ensure that AI benefits veterans. The leadership and culture aspects will drive commitment to trustworthy AI practices. Leaders will communicate the importance of responsible AI through words and actions. Culture surveys can assess baseline awareness of AI ethics issues to target education. AI security and fairness will be emphasized as safety critical. The process aspect will institute policies and procedures to uphold AI principles through the project lifecycle. For example, structured testing processes will validate safety. Measurement will collect data on principles like transparency and fairness. Dashboards can track metrics like explainability and biases. A patient-centered approach will incorporate veteran perspectives on AI through participatory design and advisory councils. They can give input on AI explainability and potential biases based on their diverse backgrounds.
Conclusions
Joint principles will lead to successful AI that improves care while proactively managing risks. Involve leaders to stress the necessity of eliminating biases. Build security into the AI development process. Co-design AI transparency features with end users. Closely monitor the impact of AI across safety, fairness, and other principles. Adhering to both Trustworthy AI and high reliability organizations principles will earn veterans’ confidence. Health care organizations like the VA can integrate ethical AI safely via established frameworks. With responsible design and implementation, AI’s potential to enhance care quality, safety, and access can be realized.
Acknowledgments
We would like to acknowledge Joshua Mueller, Theo Tiffney, John Zachary, and Gil Alterovitz for their excellent work creating the VA Trustworthy Principles. This material is the result of work supported by resources and the use of facilities at the James A. Haley Veterans’ Hospital.
1. Sahni NR, Carrus B. Artificial intelligence in U.S. health care delivery. N Engl J Med. 2023;389(4):348-358. doi:10.1056/NEJMra2204673
2. Borkowski AA, Jakey CE, Mastorides SM, et al. Applications of ChatGPT and large language models in medicine and health care: benefits and pitfalls. Fed Pract. 2023;40(6):170-173. doi:10.12788/fp.0386
3. Moyal-Smith R, Margo J, Maloney FL, et al. The patient safety adoption framework: a practical framework to bridge the know-do gap. J Patient Saf. 2023;19(4):243-248. doi:10.1097/PTS.0000000000001118
4. Isaacks DB, Anderson TM, Moore SC, Patterson W, Govindan S. High reliability organization principles improve VA workplace burnout: the Truman THRIVE2 model. Am J Med Qual. 2021;36(6):422-428. doi:10.1097/01.JMQ.0000735516.35323.97
5. Sculli GL, Pendley-Louis R, Neily J, et al. A high-reliability organization framework for health care: a multiyear implementation strategy and associated outcomes. J Patient Saf. 2022;18(1):64-70. doi:10.1097/PTS.0000000000000788
6. National Institute of Standards and Technology. AI risk management framework. Accessed January 2, 2024. https://www.nist.gov/itl/ai-risk-management-framework
7. Executive Office of the President, Office of Science and Technology Policy. Blueprint for an AI Bill of Rights. Accessed January 11, 2024. https://www.whitehouse.gov/ostp/ai-bill-of-rights
8. Executive Office of the President. Executive Order 13960: promoting the use of trustworthy artificial intelligence in the federal government. Fed Regist. 2020;89(236):78939-78943.
9. Biden JR. Executive Order on the safe, secure, and trustworthy development and use of artificial intelligence. Published October 30, 2023. Accessed January 11, 2024. https://www.whitehouse.gov/briefing-room/presidential-actions/2023/10/30/executive-order-on-the-safe-secure-and-trustworthy-development-and-use-of-artificial-intelligence/
10. US Department of Veterans Affairs. Trustworthy AI. Accessed January 11, 2024. https://department.va.gov/ai/trustworthy/
1. Sahni NR, Carrus B. Artificial intelligence in U.S. health care delivery. N Engl J Med. 2023;389(4):348-358. doi:10.1056/NEJMra2204673
2. Borkowski AA, Jakey CE, Mastorides SM, et al. Applications of ChatGPT and large language models in medicine and health care: benefits and pitfalls. Fed Pract. 2023;40(6):170-173. doi:10.12788/fp.0386
3. Moyal-Smith R, Margo J, Maloney FL, et al. The patient safety adoption framework: a practical framework to bridge the know-do gap. J Patient Saf. 2023;19(4):243-248. doi:10.1097/PTS.0000000000001118
4. Isaacks DB, Anderson TM, Moore SC, Patterson W, Govindan S. High reliability organization principles improve VA workplace burnout: the Truman THRIVE2 model. Am J Med Qual. 2021;36(6):422-428. doi:10.1097/01.JMQ.0000735516.35323.97
5. Sculli GL, Pendley-Louis R, Neily J, et al. A high-reliability organization framework for health care: a multiyear implementation strategy and associated outcomes. J Patient Saf. 2022;18(1):64-70. doi:10.1097/PTS.0000000000000788
6. National Institute of Standards and Technology. AI risk management framework. Accessed January 2, 2024. https://www.nist.gov/itl/ai-risk-management-framework
7. Executive Office of the President, Office of Science and Technology Policy. Blueprint for an AI Bill of Rights. Accessed January 11, 2024. https://www.whitehouse.gov/ostp/ai-bill-of-rights
8. Executive Office of the President. Executive Order 13960: promoting the use of trustworthy artificial intelligence in the federal government. Fed Regist. 2020;89(236):78939-78943.
9. Biden JR. Executive Order on the safe, secure, and trustworthy development and use of artificial intelligence. Published October 30, 2023. Accessed January 11, 2024. https://www.whitehouse.gov/briefing-room/presidential-actions/2023/10/30/executive-order-on-the-safe-secure-and-trustworthy-development-and-use-of-artificial-intelligence/
10. US Department of Veterans Affairs. Trustworthy AI. Accessed January 11, 2024. https://department.va.gov/ai/trustworthy/
Age-Friendly Health Systems and Meeting the Principles of High Reliability Organizations in the VHA
The Veterans Health Administration (VHA) is the largest integrated health care system in the US, providing care to more than 9 million enrolled veterans at 1298 facilities.1 In February 2019, the VHA identified key action steps to become a high reliability organization (HRO), transforming how employees think about patient safety and care quality.2 The VHA is also working toward becoming the largest age-friendly health system in the US to be recognized by the Institute for Healthcare Improvement (IHI) for its commitment to providing care guided by the 4Ms (what matters, medication, mentation, and mobility), causing no harm, and aligning care with what matters to older veterans.3 In this article, we describe how the Age-Friendly Health Systems (AFHS) movement supports the culture shift observed in HROs.
Age-Friendly Veteran Care
By 2060, the US population of adults aged ≥ 65 years is projected to increase to about 95 million.3 In the VHA, nearly half of veteran enrollees are aged ≥ 65 years, necessitating evidence-based models of care, such as the 4Ms, to meet their complex care needs.3 Historically, the VHA has been a leader in caring for older adults, recognizing the value of age-friendly care for veterans.4 In 1975, the VHA established the Geriatric Research, Education, and Clinical Centers (GRECCs) to serve as catalysts for developing, implementing, and refining enduring models of geriatric care.4 For 5 decades, GRECCs have driven innovations related to the 4Ms.
The VHA is well positioned to be a leader in the AFHS movement, building on decades of GRECC innovations and geriatric programs that align with the 4Ms and providing specialized geriatric training for health care professionals to expand age-friendly care to new settings and health systems.4 The AFHS movement organizes the 4Ms into a simple framework for frontline staff, and the VHA has recently begun tracking 4Ms care in the electronic health record (EHR) to facilitate evaluation and continuous improvement.
AFHS use the 4Ms as a framework to be implemented in every care setting, from the emergency department to inpatient units, outpatient settings, and postacute and long-term care. By assessing and acting on each M and practicing the 4Ms collectively, all members of the care team work to improve health outcomes and prevent avoidable harm.5
The 4Ms
What matters, is the driver of this person-centered approach. Any member of the care team may initiate a what matters conversation with the older adult to understand their personal values, health goals, and care preferences. When compared with usual care, care aligned with the older adult’s health priorities has been shown to decrease the use of high-risk medications and reduce treatment burden.6 The VHA has adopted Whole Health principles of care and the Patient Priorities Care approach to identify and support what matters to veterans.7,8
Addressing polypharmacy and identifying and deprescribing potentially inappropriate medications are essential in preventing adverse drug events, drug-drug interactions, and medication nonadherence.9 In the VHA, VIONE (Vital, Important, Optional, Not indicated, Every medication has an indication) is a rapidly expanding medication deprescribing program that exemplifies HRO principles.9 VIONE provides medication management that supports shared decision making, reducing risk and improving patient safety and quality of life.9 As of June 2023, > 600,000 unique veterans have benefited from VIONE, with an average of 2.2 medications deprescribed per patient with an annual cost avoidance of > $100 million.10
Assessing and acting on mentation includes preventing, identifying, and managing depression and dementia in outpatient settings and delirium in hospital and long-term care settings.5 There are many tools and clinical reminders available in the EHR so that interdisciplinary teams can document changes to mentation and identify opportunities for continuous improvement.
Closely aligned with mentation is mobility, with evidence suggesting that regular physical activity reduces the risk of falls (preventing associated complications), maintains physical functioning, and lowers the risk of cognitive impairment and depression.5 Ensuring early, frequent, and safe mobility helps patients achieve better health outcomes and prevent injury.5 Mobility programs within the VHA include the STRIDE program for the inpatient setting and Gerofit for outpatient settings.11,12
HRO Principles
An HRO is a complex environment of care that experiences fewer than anticipated accidents or adverse events by (1) establishing trust among leaders and staff by balancing individual accountability with systems thinking; (2) empowering staff to lead continuous process improvements; and (3) creating an environment where employees feel safe to report harm or near misses, focusing on the reasons errors occur.13 The work of AFHS incorporates HRO principles with an emphasis on 3 elements. First, it involves interactive systems and processes needed to support 4Ms care across care settings. Second, AFHS acknowledge the complexity of age-friendly work and deference to the expertise of interdisciplinary team members. Finally, AFHS are committed to resilience by overcoming failures and challenges to implementation and long-term sustainment as a standard of practice.
Case study
The names and details in this case have been modified to protect patient privacy. It is representative of many Community Living Centers (CLCs) involved in AFHS that work to create a safe, person-centered environment for veterans.
In a CLC team workroom, 2 nurses were discussing a long-term care resident. The nurses approached the attending physician and explained that they were worried about Sgt Johnson, who seemed depressed and sometimes combative. They had noticed a change in his behavior when they helped him clean up after an episode of incontinence and were concerned that he would try to get out of bed on his own and fall. The attending physician thanked them for sharing their concerns. Sgt Johnson was a retired Army veteran who had a long, decorated military career. His chronic health conditions had led to muscle weakness, and he fell and broke a hip before this admission. He had an uneventful hip replacement but was showing signs of depression due to his limited mobility, loss of independence, and inability to live at home without additional support.
The attending physician knocked on the door of his room, sat down next to the bed, and asked, “How are you feeling today?” Sgt Johnson tersely replied, “About the same.” The physician asked, “Sgt Johnson, what matters most to you related to your recovery? What is important to you?” Sgt Johnson responded, “Feeling like a man!” The doctor replied, “So what makes you feel ‘not like a man’?” The Sgt replied, “Having to be cleaned up by the nurses and not being able to use the toilet on my own.” The physician surmised that his decline in physical functioning had a connection to his worsening depression and combativeness and said to the Sgt, “Let’s get the team together and work out a plan to get you strong enough to use a bedside commode by yourself. Let’s make that the first goal in our plan to get you back to using the toilet independently. Can you work with us on that?” He smiled and said, “Sir, yes Sir!”
At the weekly interdisciplinary team meeting, the team discussed Sgt Johnson’s wishes and the nurses’ safety concerns. The physician reported to the team what mattered to the veteran. The nurses arranged for a bedside commode and supplies to be placed in his room, encouraged and assisted him, and provided a privacy screen. The physical therapist continued to support his mobility needs, concentrating on transfers, small steps like standing and turning with a walker to get in position to use the bedside commode, and later the bathroom toilet. The psychologist addressed what matters to Sgt Johnson and his mentation, health goals, and coping strategies. The social worker provided support and counseling for the veteran and his family. The pharmacist checked his medications to be sure that none were affecting his gastrointestinal tract and his ability to move safely and do what matters to him. Knowing what mattered to Sgt Johnson was the driver of the interdisciplinary care plan to provide 4Ms care.
The team worked collaboratively with the veteran to develop and set attainable goals around toileting and regaining his dignity. This improved his overall recovery. As Sgt Johnson became more independent, his mood gradually improved and he began to participate in other activities and interact with other residents on the unit, and he did not experience any falls. By addressing the 4Ms, the interdisciplinary team coordinated efforts to provide high-quality, person-centered care. They built trust with the veteran, shared accountability, and followed HRO principles to keep the veteran safe.
Becoming an Age-Friendly HRO
Becoming an HRO is a dynamic, ever-changing process to maintain high standards, improve care quality, and cause no harm. There are 3 pillars and 5 principles that guide an HRO. The pillars are critical areas of focus and include leadership commitment, culture of safety, and continuous process improvement.14 The first of 5 HRO principles is sensitivity to operations. This is defined as an awareness of how processes and systems impact the entire organization, the downstream impact.15 Focusing on the 4Ms helps develop the capability of frontline staff to provide high-quality care for older adults while ensuring that processes are in place to support the work. The 4Ms provide an efficient way to organize interdisciplinary team meetings, provide warm handoffs using Situation-Background-Assessment-Recommendation, and standardize documentation. Involvement in the AFHS movement improves communication, care quality, and patient and staff satisfaction to meet this HRO principle.15
The second HRO principle, reluctance to simplify, ensures that direct care staff and leaders delve further into issues to find solutions.15 AFHS use the Plan-Do-Study-Act cycle to put the 4Ms into practice; this cycle helps teams test small increments of change, study their performance, and act to ensure that all 4Ms are being practiced as a set. AFHS teams are encouraged to review at least 3 months of data after implementation of the 4Ms, working to find solutions if there are gaps or issues identified.
The third principle, preoccupation with failure, refers to shared attentiveness—being prepared for the unexpected and learning from mistakes.15 The entire AFHS team shares responsibility for providing 4Ms care, where staff are empowered to report any safety concerns or close calls. The fourth principle of deference to expertise includes listening to staff who have the most knowledge for the task at hand, which aligns with the collaborative interdisciplinary teamwork of age-friendly teams.15
The final HRO principle, commitment to resilience, includes continuous learning, interdisciplinary team training, and sharing of lessons learned.15 Although IHI offers 2 levels of AFHS recognition, teams are continuously learning to improve and sustain care beyond level 2, Committed to Care Excellence recognition.16
The Table shows the VHA’s AFHS implementation strategies and the HRO principles adapted from the Joint Commission’s High Reliability Health Care Maturity Model and the IHI’s Framework for Safe, Reliable, and Effective Care. The VHA is developing a national dashboard to capture age-friendly processes and health outcome measures that address patient safety and care quality.
Conclusions
AFHS empowers VHA teams to honor veterans’ care preferences and values, supporting their independence, dignity, and quality of life across care settings. The adoption of AFHS brings evidence-based practices to the point of care by addressing common pitfalls in the care of older adults, drawing attention to, and calling for action on inappropriate medication use, physical inactivity, and assessment of the vulnerable brain. The 4Ms also serve as a framework to continuously improve care and cause zero harm, reinforcing HRO pillars and principles across the VHA, and ensuring that older adults reliably receive the evidence-based, high-quality care they deserve.
1. Veterans Health Administration. Providing healthcare for veterans. Updated June 20, 2023. Accessed June 26, 2023. https://www.va.gov/health
2. Veazie S, Peterson K, Bourne D. Evidence brief: implementation of high reliability organization principles. Washington, DC: Evidence Synthesis Program, Health Services Research and Development Service, Office of Research and Development, Department of Veterans Affairs. VA ESP Project #09-199; 2019. Accessed November 30, 2023. https://www.hsrd.research.va.gov/publications/esp/high-reliability-org.cfm
3. Church K, Munro S, Shaughnessy M, Clancy C. Age-Friendly Health Systems: improving care for older adults in the Veterans Health Administration. Health Serv Res. 2023;58(suppl 1):5-8. doi:10.1111/1475-6773.14110
4. Farrell TW, Volden TA, Butler JM, et al. Age-friendly care in the Veterans Health Administration: past, present, and future. J Am Geriatr Soc. 2023;71(1):18-25. doi:10.1111/jgs.18070
5. Mate K, Fulmer T, Pelton L, et al. Evidence for the 4Ms: interactions and outcomes across the care continuum. J Aging Health. 2021;33(7-8):469-481. doi:10.1177/0898264321991658
6. Tinetti ME, Naik AD, Dindo L, et al. Association of patient priorities-aligned decision-making with patient outcomes and ambulatory health care burden among older adults with multiple chronic conditions: A nonrandomized clinical trial. JAMA Intern Med. 2019;179(12):1688-1697. doi:10.1001/jamainternmed.2019.4235
7. US Department of Veterans Affairs. What is whole health? Updated: October 31, 2023. November 30, 2023. https://www.va.gov/wholehealth
8. Patient Priorities Care. Updated 2019. Accessed November 30, 2023. https://patientprioritiescare.org
9. Battar S, Watson Dickerson KR, Sedgwick C, Cmelik T. Understanding principles of high reliability organizations through the eyes of VIONE: a clinical program to improve patient safety by deprescribing potentially inappropriate medications and reducing polypharmacy. Fed Pract. 2019;36(12):564-568.
10. VA Diffusion Marketplace. VIONE- medication optimization and polypharmacy reduction initiative. Accessed November 30, 2023. https://marketplace.va.gov/innovations/vione
11. US Department of Veterans Affairs, Office of Research and Development. STRIDE program to keep hospitalized veterans mobile. Updated November 6, 2018. Accessed November 30, 2023. https://www.research.va.gov/research_in_action/STRIDE-program-to-keep-hospitalized-Veterans-mobile.cfm
12. US Department of Veterans Affairs, VA Geriatrics and Extended Care. Gerofit: a program promoting exercise and health for older veterans. Updated August 2, 2023. Accessed November 30, 2023. https://www.va.gov/GERIATRICS/pages/gerofit_Home.asp
13. US Department of Veterans Affairs, Health Services Research and Development. VHA’s vision for a high reliability organization. Updated August 14, 2020. Accessed November 30, 2023. https://www.hsrd.research.va.gov/publications/forum/summer20/default.cfm?ForumMenu=summer20-1
14. US Department of Veterans Affairs, Health Services Research and Development. Three HRO evaluation priorities. Updated August 14, 2020. Accessed November 30, 2023. https://www.hsrd.research.va.gov/publications/forum/summer20/default.cfm?ForumMenu=summer20-2
15. Oster CA, Deakins S. Practical application of high-reliability principles in healthcare to optimize quality and safety outcomes. J Nurs Adm. 2018;48(1):50-55. doi:10.1097/NNA.0000000000000570
16. Institute for Healthcare Improvement. Age-Friendly Health Systems recognitions. Accessed November 30, 2023. https://www.ihi.org/Engage/Initiatives/Age-Friendly-Health-Systems/Pages/Recognition.aspx
The Veterans Health Administration (VHA) is the largest integrated health care system in the US, providing care to more than 9 million enrolled veterans at 1298 facilities.1 In February 2019, the VHA identified key action steps to become a high reliability organization (HRO), transforming how employees think about patient safety and care quality.2 The VHA is also working toward becoming the largest age-friendly health system in the US to be recognized by the Institute for Healthcare Improvement (IHI) for its commitment to providing care guided by the 4Ms (what matters, medication, mentation, and mobility), causing no harm, and aligning care with what matters to older veterans.3 In this article, we describe how the Age-Friendly Health Systems (AFHS) movement supports the culture shift observed in HROs.
Age-Friendly Veteran Care
By 2060, the US population of adults aged ≥ 65 years is projected to increase to about 95 million.3 In the VHA, nearly half of veteran enrollees are aged ≥ 65 years, necessitating evidence-based models of care, such as the 4Ms, to meet their complex care needs.3 Historically, the VHA has been a leader in caring for older adults, recognizing the value of age-friendly care for veterans.4 In 1975, the VHA established the Geriatric Research, Education, and Clinical Centers (GRECCs) to serve as catalysts for developing, implementing, and refining enduring models of geriatric care.4 For 5 decades, GRECCs have driven innovations related to the 4Ms.
The VHA is well positioned to be a leader in the AFHS movement, building on decades of GRECC innovations and geriatric programs that align with the 4Ms and providing specialized geriatric training for health care professionals to expand age-friendly care to new settings and health systems.4 The AFHS movement organizes the 4Ms into a simple framework for frontline staff, and the VHA has recently begun tracking 4Ms care in the electronic health record (EHR) to facilitate evaluation and continuous improvement.
AFHS use the 4Ms as a framework to be implemented in every care setting, from the emergency department to inpatient units, outpatient settings, and postacute and long-term care. By assessing and acting on each M and practicing the 4Ms collectively, all members of the care team work to improve health outcomes and prevent avoidable harm.5
The 4Ms
What matters, is the driver of this person-centered approach. Any member of the care team may initiate a what matters conversation with the older adult to understand their personal values, health goals, and care preferences. When compared with usual care, care aligned with the older adult’s health priorities has been shown to decrease the use of high-risk medications and reduce treatment burden.6 The VHA has adopted Whole Health principles of care and the Patient Priorities Care approach to identify and support what matters to veterans.7,8
Addressing polypharmacy and identifying and deprescribing potentially inappropriate medications are essential in preventing adverse drug events, drug-drug interactions, and medication nonadherence.9 In the VHA, VIONE (Vital, Important, Optional, Not indicated, Every medication has an indication) is a rapidly expanding medication deprescribing program that exemplifies HRO principles.9 VIONE provides medication management that supports shared decision making, reducing risk and improving patient safety and quality of life.9 As of June 2023, > 600,000 unique veterans have benefited from VIONE, with an average of 2.2 medications deprescribed per patient with an annual cost avoidance of > $100 million.10
Assessing and acting on mentation includes preventing, identifying, and managing depression and dementia in outpatient settings and delirium in hospital and long-term care settings.5 There are many tools and clinical reminders available in the EHR so that interdisciplinary teams can document changes to mentation and identify opportunities for continuous improvement.
Closely aligned with mentation is mobility, with evidence suggesting that regular physical activity reduces the risk of falls (preventing associated complications), maintains physical functioning, and lowers the risk of cognitive impairment and depression.5 Ensuring early, frequent, and safe mobility helps patients achieve better health outcomes and prevent injury.5 Mobility programs within the VHA include the STRIDE program for the inpatient setting and Gerofit for outpatient settings.11,12
HRO Principles
An HRO is a complex environment of care that experiences fewer than anticipated accidents or adverse events by (1) establishing trust among leaders and staff by balancing individual accountability with systems thinking; (2) empowering staff to lead continuous process improvements; and (3) creating an environment where employees feel safe to report harm or near misses, focusing on the reasons errors occur.13 The work of AFHS incorporates HRO principles with an emphasis on 3 elements. First, it involves interactive systems and processes needed to support 4Ms care across care settings. Second, AFHS acknowledge the complexity of age-friendly work and deference to the expertise of interdisciplinary team members. Finally, AFHS are committed to resilience by overcoming failures and challenges to implementation and long-term sustainment as a standard of practice.
Case study
The names and details in this case have been modified to protect patient privacy. It is representative of many Community Living Centers (CLCs) involved in AFHS that work to create a safe, person-centered environment for veterans.
In a CLC team workroom, 2 nurses were discussing a long-term care resident. The nurses approached the attending physician and explained that they were worried about Sgt Johnson, who seemed depressed and sometimes combative. They had noticed a change in his behavior when they helped him clean up after an episode of incontinence and were concerned that he would try to get out of bed on his own and fall. The attending physician thanked them for sharing their concerns. Sgt Johnson was a retired Army veteran who had a long, decorated military career. His chronic health conditions had led to muscle weakness, and he fell and broke a hip before this admission. He had an uneventful hip replacement but was showing signs of depression due to his limited mobility, loss of independence, and inability to live at home without additional support.
The attending physician knocked on the door of his room, sat down next to the bed, and asked, “How are you feeling today?” Sgt Johnson tersely replied, “About the same.” The physician asked, “Sgt Johnson, what matters most to you related to your recovery? What is important to you?” Sgt Johnson responded, “Feeling like a man!” The doctor replied, “So what makes you feel ‘not like a man’?” The Sgt replied, “Having to be cleaned up by the nurses and not being able to use the toilet on my own.” The physician surmised that his decline in physical functioning had a connection to his worsening depression and combativeness and said to the Sgt, “Let’s get the team together and work out a plan to get you strong enough to use a bedside commode by yourself. Let’s make that the first goal in our plan to get you back to using the toilet independently. Can you work with us on that?” He smiled and said, “Sir, yes Sir!”
At the weekly interdisciplinary team meeting, the team discussed Sgt Johnson’s wishes and the nurses’ safety concerns. The physician reported to the team what mattered to the veteran. The nurses arranged for a bedside commode and supplies to be placed in his room, encouraged and assisted him, and provided a privacy screen. The physical therapist continued to support his mobility needs, concentrating on transfers, small steps like standing and turning with a walker to get in position to use the bedside commode, and later the bathroom toilet. The psychologist addressed what matters to Sgt Johnson and his mentation, health goals, and coping strategies. The social worker provided support and counseling for the veteran and his family. The pharmacist checked his medications to be sure that none were affecting his gastrointestinal tract and his ability to move safely and do what matters to him. Knowing what mattered to Sgt Johnson was the driver of the interdisciplinary care plan to provide 4Ms care.
The team worked collaboratively with the veteran to develop and set attainable goals around toileting and regaining his dignity. This improved his overall recovery. As Sgt Johnson became more independent, his mood gradually improved and he began to participate in other activities and interact with other residents on the unit, and he did not experience any falls. By addressing the 4Ms, the interdisciplinary team coordinated efforts to provide high-quality, person-centered care. They built trust with the veteran, shared accountability, and followed HRO principles to keep the veteran safe.
Becoming an Age-Friendly HRO
Becoming an HRO is a dynamic, ever-changing process to maintain high standards, improve care quality, and cause no harm. There are 3 pillars and 5 principles that guide an HRO. The pillars are critical areas of focus and include leadership commitment, culture of safety, and continuous process improvement.14 The first of 5 HRO principles is sensitivity to operations. This is defined as an awareness of how processes and systems impact the entire organization, the downstream impact.15 Focusing on the 4Ms helps develop the capability of frontline staff to provide high-quality care for older adults while ensuring that processes are in place to support the work. The 4Ms provide an efficient way to organize interdisciplinary team meetings, provide warm handoffs using Situation-Background-Assessment-Recommendation, and standardize documentation. Involvement in the AFHS movement improves communication, care quality, and patient and staff satisfaction to meet this HRO principle.15
The second HRO principle, reluctance to simplify, ensures that direct care staff and leaders delve further into issues to find solutions.15 AFHS use the Plan-Do-Study-Act cycle to put the 4Ms into practice; this cycle helps teams test small increments of change, study their performance, and act to ensure that all 4Ms are being practiced as a set. AFHS teams are encouraged to review at least 3 months of data after implementation of the 4Ms, working to find solutions if there are gaps or issues identified.
The third principle, preoccupation with failure, refers to shared attentiveness—being prepared for the unexpected and learning from mistakes.15 The entire AFHS team shares responsibility for providing 4Ms care, where staff are empowered to report any safety concerns or close calls. The fourth principle of deference to expertise includes listening to staff who have the most knowledge for the task at hand, which aligns with the collaborative interdisciplinary teamwork of age-friendly teams.15
The final HRO principle, commitment to resilience, includes continuous learning, interdisciplinary team training, and sharing of lessons learned.15 Although IHI offers 2 levels of AFHS recognition, teams are continuously learning to improve and sustain care beyond level 2, Committed to Care Excellence recognition.16
The Table shows the VHA’s AFHS implementation strategies and the HRO principles adapted from the Joint Commission’s High Reliability Health Care Maturity Model and the IHI’s Framework for Safe, Reliable, and Effective Care. The VHA is developing a national dashboard to capture age-friendly processes and health outcome measures that address patient safety and care quality.
Conclusions
AFHS empowers VHA teams to honor veterans’ care preferences and values, supporting their independence, dignity, and quality of life across care settings. The adoption of AFHS brings evidence-based practices to the point of care by addressing common pitfalls in the care of older adults, drawing attention to, and calling for action on inappropriate medication use, physical inactivity, and assessment of the vulnerable brain. The 4Ms also serve as a framework to continuously improve care and cause zero harm, reinforcing HRO pillars and principles across the VHA, and ensuring that older adults reliably receive the evidence-based, high-quality care they deserve.
The Veterans Health Administration (VHA) is the largest integrated health care system in the US, providing care to more than 9 million enrolled veterans at 1298 facilities.1 In February 2019, the VHA identified key action steps to become a high reliability organization (HRO), transforming how employees think about patient safety and care quality.2 The VHA is also working toward becoming the largest age-friendly health system in the US to be recognized by the Institute for Healthcare Improvement (IHI) for its commitment to providing care guided by the 4Ms (what matters, medication, mentation, and mobility), causing no harm, and aligning care with what matters to older veterans.3 In this article, we describe how the Age-Friendly Health Systems (AFHS) movement supports the culture shift observed in HROs.
Age-Friendly Veteran Care
By 2060, the US population of adults aged ≥ 65 years is projected to increase to about 95 million.3 In the VHA, nearly half of veteran enrollees are aged ≥ 65 years, necessitating evidence-based models of care, such as the 4Ms, to meet their complex care needs.3 Historically, the VHA has been a leader in caring for older adults, recognizing the value of age-friendly care for veterans.4 In 1975, the VHA established the Geriatric Research, Education, and Clinical Centers (GRECCs) to serve as catalysts for developing, implementing, and refining enduring models of geriatric care.4 For 5 decades, GRECCs have driven innovations related to the 4Ms.
The VHA is well positioned to be a leader in the AFHS movement, building on decades of GRECC innovations and geriatric programs that align with the 4Ms and providing specialized geriatric training for health care professionals to expand age-friendly care to new settings and health systems.4 The AFHS movement organizes the 4Ms into a simple framework for frontline staff, and the VHA has recently begun tracking 4Ms care in the electronic health record (EHR) to facilitate evaluation and continuous improvement.
AFHS use the 4Ms as a framework to be implemented in every care setting, from the emergency department to inpatient units, outpatient settings, and postacute and long-term care. By assessing and acting on each M and practicing the 4Ms collectively, all members of the care team work to improve health outcomes and prevent avoidable harm.5
The 4Ms
What matters, is the driver of this person-centered approach. Any member of the care team may initiate a what matters conversation with the older adult to understand their personal values, health goals, and care preferences. When compared with usual care, care aligned with the older adult’s health priorities has been shown to decrease the use of high-risk medications and reduce treatment burden.6 The VHA has adopted Whole Health principles of care and the Patient Priorities Care approach to identify and support what matters to veterans.7,8
Addressing polypharmacy and identifying and deprescribing potentially inappropriate medications are essential in preventing adverse drug events, drug-drug interactions, and medication nonadherence.9 In the VHA, VIONE (Vital, Important, Optional, Not indicated, Every medication has an indication) is a rapidly expanding medication deprescribing program that exemplifies HRO principles.9 VIONE provides medication management that supports shared decision making, reducing risk and improving patient safety and quality of life.9 As of June 2023, > 600,000 unique veterans have benefited from VIONE, with an average of 2.2 medications deprescribed per patient with an annual cost avoidance of > $100 million.10
Assessing and acting on mentation includes preventing, identifying, and managing depression and dementia in outpatient settings and delirium in hospital and long-term care settings.5 There are many tools and clinical reminders available in the EHR so that interdisciplinary teams can document changes to mentation and identify opportunities for continuous improvement.
Closely aligned with mentation is mobility, with evidence suggesting that regular physical activity reduces the risk of falls (preventing associated complications), maintains physical functioning, and lowers the risk of cognitive impairment and depression.5 Ensuring early, frequent, and safe mobility helps patients achieve better health outcomes and prevent injury.5 Mobility programs within the VHA include the STRIDE program for the inpatient setting and Gerofit for outpatient settings.11,12
HRO Principles
An HRO is a complex environment of care that experiences fewer than anticipated accidents or adverse events by (1) establishing trust among leaders and staff by balancing individual accountability with systems thinking; (2) empowering staff to lead continuous process improvements; and (3) creating an environment where employees feel safe to report harm or near misses, focusing on the reasons errors occur.13 The work of AFHS incorporates HRO principles with an emphasis on 3 elements. First, it involves interactive systems and processes needed to support 4Ms care across care settings. Second, AFHS acknowledge the complexity of age-friendly work and deference to the expertise of interdisciplinary team members. Finally, AFHS are committed to resilience by overcoming failures and challenges to implementation and long-term sustainment as a standard of practice.
Case study
The names and details in this case have been modified to protect patient privacy. It is representative of many Community Living Centers (CLCs) involved in AFHS that work to create a safe, person-centered environment for veterans.
In a CLC team workroom, 2 nurses were discussing a long-term care resident. The nurses approached the attending physician and explained that they were worried about Sgt Johnson, who seemed depressed and sometimes combative. They had noticed a change in his behavior when they helped him clean up after an episode of incontinence and were concerned that he would try to get out of bed on his own and fall. The attending physician thanked them for sharing their concerns. Sgt Johnson was a retired Army veteran who had a long, decorated military career. His chronic health conditions had led to muscle weakness, and he fell and broke a hip before this admission. He had an uneventful hip replacement but was showing signs of depression due to his limited mobility, loss of independence, and inability to live at home without additional support.
The attending physician knocked on the door of his room, sat down next to the bed, and asked, “How are you feeling today?” Sgt Johnson tersely replied, “About the same.” The physician asked, “Sgt Johnson, what matters most to you related to your recovery? What is important to you?” Sgt Johnson responded, “Feeling like a man!” The doctor replied, “So what makes you feel ‘not like a man’?” The Sgt replied, “Having to be cleaned up by the nurses and not being able to use the toilet on my own.” The physician surmised that his decline in physical functioning had a connection to his worsening depression and combativeness and said to the Sgt, “Let’s get the team together and work out a plan to get you strong enough to use a bedside commode by yourself. Let’s make that the first goal in our plan to get you back to using the toilet independently. Can you work with us on that?” He smiled and said, “Sir, yes Sir!”
At the weekly interdisciplinary team meeting, the team discussed Sgt Johnson’s wishes and the nurses’ safety concerns. The physician reported to the team what mattered to the veteran. The nurses arranged for a bedside commode and supplies to be placed in his room, encouraged and assisted him, and provided a privacy screen. The physical therapist continued to support his mobility needs, concentrating on transfers, small steps like standing and turning with a walker to get in position to use the bedside commode, and later the bathroom toilet. The psychologist addressed what matters to Sgt Johnson and his mentation, health goals, and coping strategies. The social worker provided support and counseling for the veteran and his family. The pharmacist checked his medications to be sure that none were affecting his gastrointestinal tract and his ability to move safely and do what matters to him. Knowing what mattered to Sgt Johnson was the driver of the interdisciplinary care plan to provide 4Ms care.
The team worked collaboratively with the veteran to develop and set attainable goals around toileting and regaining his dignity. This improved his overall recovery. As Sgt Johnson became more independent, his mood gradually improved and he began to participate in other activities and interact with other residents on the unit, and he did not experience any falls. By addressing the 4Ms, the interdisciplinary team coordinated efforts to provide high-quality, person-centered care. They built trust with the veteran, shared accountability, and followed HRO principles to keep the veteran safe.
Becoming an Age-Friendly HRO
Becoming an HRO is a dynamic, ever-changing process to maintain high standards, improve care quality, and cause no harm. There are 3 pillars and 5 principles that guide an HRO. The pillars are critical areas of focus and include leadership commitment, culture of safety, and continuous process improvement.14 The first of 5 HRO principles is sensitivity to operations. This is defined as an awareness of how processes and systems impact the entire organization, the downstream impact.15 Focusing on the 4Ms helps develop the capability of frontline staff to provide high-quality care for older adults while ensuring that processes are in place to support the work. The 4Ms provide an efficient way to organize interdisciplinary team meetings, provide warm handoffs using Situation-Background-Assessment-Recommendation, and standardize documentation. Involvement in the AFHS movement improves communication, care quality, and patient and staff satisfaction to meet this HRO principle.15
The second HRO principle, reluctance to simplify, ensures that direct care staff and leaders delve further into issues to find solutions.15 AFHS use the Plan-Do-Study-Act cycle to put the 4Ms into practice; this cycle helps teams test small increments of change, study their performance, and act to ensure that all 4Ms are being practiced as a set. AFHS teams are encouraged to review at least 3 months of data after implementation of the 4Ms, working to find solutions if there are gaps or issues identified.
The third principle, preoccupation with failure, refers to shared attentiveness—being prepared for the unexpected and learning from mistakes.15 The entire AFHS team shares responsibility for providing 4Ms care, where staff are empowered to report any safety concerns or close calls. The fourth principle of deference to expertise includes listening to staff who have the most knowledge for the task at hand, which aligns with the collaborative interdisciplinary teamwork of age-friendly teams.15
The final HRO principle, commitment to resilience, includes continuous learning, interdisciplinary team training, and sharing of lessons learned.15 Although IHI offers 2 levels of AFHS recognition, teams are continuously learning to improve and sustain care beyond level 2, Committed to Care Excellence recognition.16
The Table shows the VHA’s AFHS implementation strategies and the HRO principles adapted from the Joint Commission’s High Reliability Health Care Maturity Model and the IHI’s Framework for Safe, Reliable, and Effective Care. The VHA is developing a national dashboard to capture age-friendly processes and health outcome measures that address patient safety and care quality.
Conclusions
AFHS empowers VHA teams to honor veterans’ care preferences and values, supporting their independence, dignity, and quality of life across care settings. The adoption of AFHS brings evidence-based practices to the point of care by addressing common pitfalls in the care of older adults, drawing attention to, and calling for action on inappropriate medication use, physical inactivity, and assessment of the vulnerable brain. The 4Ms also serve as a framework to continuously improve care and cause zero harm, reinforcing HRO pillars and principles across the VHA, and ensuring that older adults reliably receive the evidence-based, high-quality care they deserve.
1. Veterans Health Administration. Providing healthcare for veterans. Updated June 20, 2023. Accessed June 26, 2023. https://www.va.gov/health
2. Veazie S, Peterson K, Bourne D. Evidence brief: implementation of high reliability organization principles. Washington, DC: Evidence Synthesis Program, Health Services Research and Development Service, Office of Research and Development, Department of Veterans Affairs. VA ESP Project #09-199; 2019. Accessed November 30, 2023. https://www.hsrd.research.va.gov/publications/esp/high-reliability-org.cfm
3. Church K, Munro S, Shaughnessy M, Clancy C. Age-Friendly Health Systems: improving care for older adults in the Veterans Health Administration. Health Serv Res. 2023;58(suppl 1):5-8. doi:10.1111/1475-6773.14110
4. Farrell TW, Volden TA, Butler JM, et al. Age-friendly care in the Veterans Health Administration: past, present, and future. J Am Geriatr Soc. 2023;71(1):18-25. doi:10.1111/jgs.18070
5. Mate K, Fulmer T, Pelton L, et al. Evidence for the 4Ms: interactions and outcomes across the care continuum. J Aging Health. 2021;33(7-8):469-481. doi:10.1177/0898264321991658
6. Tinetti ME, Naik AD, Dindo L, et al. Association of patient priorities-aligned decision-making with patient outcomes and ambulatory health care burden among older adults with multiple chronic conditions: A nonrandomized clinical trial. JAMA Intern Med. 2019;179(12):1688-1697. doi:10.1001/jamainternmed.2019.4235
7. US Department of Veterans Affairs. What is whole health? Updated: October 31, 2023. November 30, 2023. https://www.va.gov/wholehealth
8. Patient Priorities Care. Updated 2019. Accessed November 30, 2023. https://patientprioritiescare.org
9. Battar S, Watson Dickerson KR, Sedgwick C, Cmelik T. Understanding principles of high reliability organizations through the eyes of VIONE: a clinical program to improve patient safety by deprescribing potentially inappropriate medications and reducing polypharmacy. Fed Pract. 2019;36(12):564-568.
10. VA Diffusion Marketplace. VIONE- medication optimization and polypharmacy reduction initiative. Accessed November 30, 2023. https://marketplace.va.gov/innovations/vione
11. US Department of Veterans Affairs, Office of Research and Development. STRIDE program to keep hospitalized veterans mobile. Updated November 6, 2018. Accessed November 30, 2023. https://www.research.va.gov/research_in_action/STRIDE-program-to-keep-hospitalized-Veterans-mobile.cfm
12. US Department of Veterans Affairs, VA Geriatrics and Extended Care. Gerofit: a program promoting exercise and health for older veterans. Updated August 2, 2023. Accessed November 30, 2023. https://www.va.gov/GERIATRICS/pages/gerofit_Home.asp
13. US Department of Veterans Affairs, Health Services Research and Development. VHA’s vision for a high reliability organization. Updated August 14, 2020. Accessed November 30, 2023. https://www.hsrd.research.va.gov/publications/forum/summer20/default.cfm?ForumMenu=summer20-1
14. US Department of Veterans Affairs, Health Services Research and Development. Three HRO evaluation priorities. Updated August 14, 2020. Accessed November 30, 2023. https://www.hsrd.research.va.gov/publications/forum/summer20/default.cfm?ForumMenu=summer20-2
15. Oster CA, Deakins S. Practical application of high-reliability principles in healthcare to optimize quality and safety outcomes. J Nurs Adm. 2018;48(1):50-55. doi:10.1097/NNA.0000000000000570
16. Institute for Healthcare Improvement. Age-Friendly Health Systems recognitions. Accessed November 30, 2023. https://www.ihi.org/Engage/Initiatives/Age-Friendly-Health-Systems/Pages/Recognition.aspx
1. Veterans Health Administration. Providing healthcare for veterans. Updated June 20, 2023. Accessed June 26, 2023. https://www.va.gov/health
2. Veazie S, Peterson K, Bourne D. Evidence brief: implementation of high reliability organization principles. Washington, DC: Evidence Synthesis Program, Health Services Research and Development Service, Office of Research and Development, Department of Veterans Affairs. VA ESP Project #09-199; 2019. Accessed November 30, 2023. https://www.hsrd.research.va.gov/publications/esp/high-reliability-org.cfm
3. Church K, Munro S, Shaughnessy M, Clancy C. Age-Friendly Health Systems: improving care for older adults in the Veterans Health Administration. Health Serv Res. 2023;58(suppl 1):5-8. doi:10.1111/1475-6773.14110
4. Farrell TW, Volden TA, Butler JM, et al. Age-friendly care in the Veterans Health Administration: past, present, and future. J Am Geriatr Soc. 2023;71(1):18-25. doi:10.1111/jgs.18070
5. Mate K, Fulmer T, Pelton L, et al. Evidence for the 4Ms: interactions and outcomes across the care continuum. J Aging Health. 2021;33(7-8):469-481. doi:10.1177/0898264321991658
6. Tinetti ME, Naik AD, Dindo L, et al. Association of patient priorities-aligned decision-making with patient outcomes and ambulatory health care burden among older adults with multiple chronic conditions: A nonrandomized clinical trial. JAMA Intern Med. 2019;179(12):1688-1697. doi:10.1001/jamainternmed.2019.4235
7. US Department of Veterans Affairs. What is whole health? Updated: October 31, 2023. November 30, 2023. https://www.va.gov/wholehealth
8. Patient Priorities Care. Updated 2019. Accessed November 30, 2023. https://patientprioritiescare.org
9. Battar S, Watson Dickerson KR, Sedgwick C, Cmelik T. Understanding principles of high reliability organizations through the eyes of VIONE: a clinical program to improve patient safety by deprescribing potentially inappropriate medications and reducing polypharmacy. Fed Pract. 2019;36(12):564-568.
10. VA Diffusion Marketplace. VIONE- medication optimization and polypharmacy reduction initiative. Accessed November 30, 2023. https://marketplace.va.gov/innovations/vione
11. US Department of Veterans Affairs, Office of Research and Development. STRIDE program to keep hospitalized veterans mobile. Updated November 6, 2018. Accessed November 30, 2023. https://www.research.va.gov/research_in_action/STRIDE-program-to-keep-hospitalized-Veterans-mobile.cfm
12. US Department of Veterans Affairs, VA Geriatrics and Extended Care. Gerofit: a program promoting exercise and health for older veterans. Updated August 2, 2023. Accessed November 30, 2023. https://www.va.gov/GERIATRICS/pages/gerofit_Home.asp
13. US Department of Veterans Affairs, Health Services Research and Development. VHA’s vision for a high reliability organization. Updated August 14, 2020. Accessed November 30, 2023. https://www.hsrd.research.va.gov/publications/forum/summer20/default.cfm?ForumMenu=summer20-1
14. US Department of Veterans Affairs, Health Services Research and Development. Three HRO evaluation priorities. Updated August 14, 2020. Accessed November 30, 2023. https://www.hsrd.research.va.gov/publications/forum/summer20/default.cfm?ForumMenu=summer20-2
15. Oster CA, Deakins S. Practical application of high-reliability principles in healthcare to optimize quality and safety outcomes. J Nurs Adm. 2018;48(1):50-55. doi:10.1097/NNA.0000000000000570
16. Institute for Healthcare Improvement. Age-Friendly Health Systems recognitions. Accessed November 30, 2023. https://www.ihi.org/Engage/Initiatives/Age-Friendly-Health-Systems/Pages/Recognition.aspx
Fellowships in Complex Medical Dermatology
Complex medical dermatology has become an emerging field in dermatology. Although a rather protean and broad term, complex medical dermatology encompasses patients with autoimmune conditions, bullous disease, connective tissue disease, vasculitis, severe dermatoses requiring immunomodulation, and inpatient consultations. Importantly, dermatology inpatient consultations aid in lowering health care costs due to accurate diagnoses, correct treatment, and decreased hospital stays.1 A fellowship is not required for holding an inpatient role in the hospital system as a dermatologist but can be beneficial. There are combined internal medicine–dermatology programs available for medical students applying to dermatology residency, but a complex medical dermatology fellowship is an option after residency for those who are interested. I believe that a focused complex medical dermatology fellowship differs from the training offered in combined internal medicine–dermatology residency. My fellow colleagues in combined internal medicine–dermatology programs are exposed to systemic manifestations of cutaneous disease and are experts in the interplay between the skin and other organ systems. However, the focus of their programs is with the intention of becoming double boarded in internal medicine and dermatology with comprehensive exposure to both fields. In my fellowship, I am able to tailor my schedule to focus on any dermatologic disease such as connective tissue disease, pruritus, graft vs host disease, and Merkel cell carcinoma. I ultimately can determine a niche in dermatology and hone my skills for a year under supervision.
Available Fellowships
Fellowship Locations—Importantly, the complex medical dermatology fellowship is not accredited by the Accreditation Council for Graduate Medical Education, which can make it difficult to identify and apply to programs. The complex medical dermatology fellowship is different than a rheumatology-dermatology fellowship, cutaneous oncology fellowship, pediatric dermatology fellowship, or other subspecialty fellowships such as those in itch or autoimmune blistering diseases. The fellowship often encompasses gaining clinical expertise in many of these conditions. I performed a thorough search online and spoke with complex medical dermatologists to compile a list of programs that offer a complex medical dermatology fellowship: Brigham and Women’s Hospital (Boston, Massachusetts); University of California San Francisco (San Francisco, California); University of Pennsylvania (Philadelphia, Pennsylvania); Cleveland Clinic (Cleveland, Ohio); and New York University (New York, New York)(Table). Only 1 spot is offered at each of these programs.
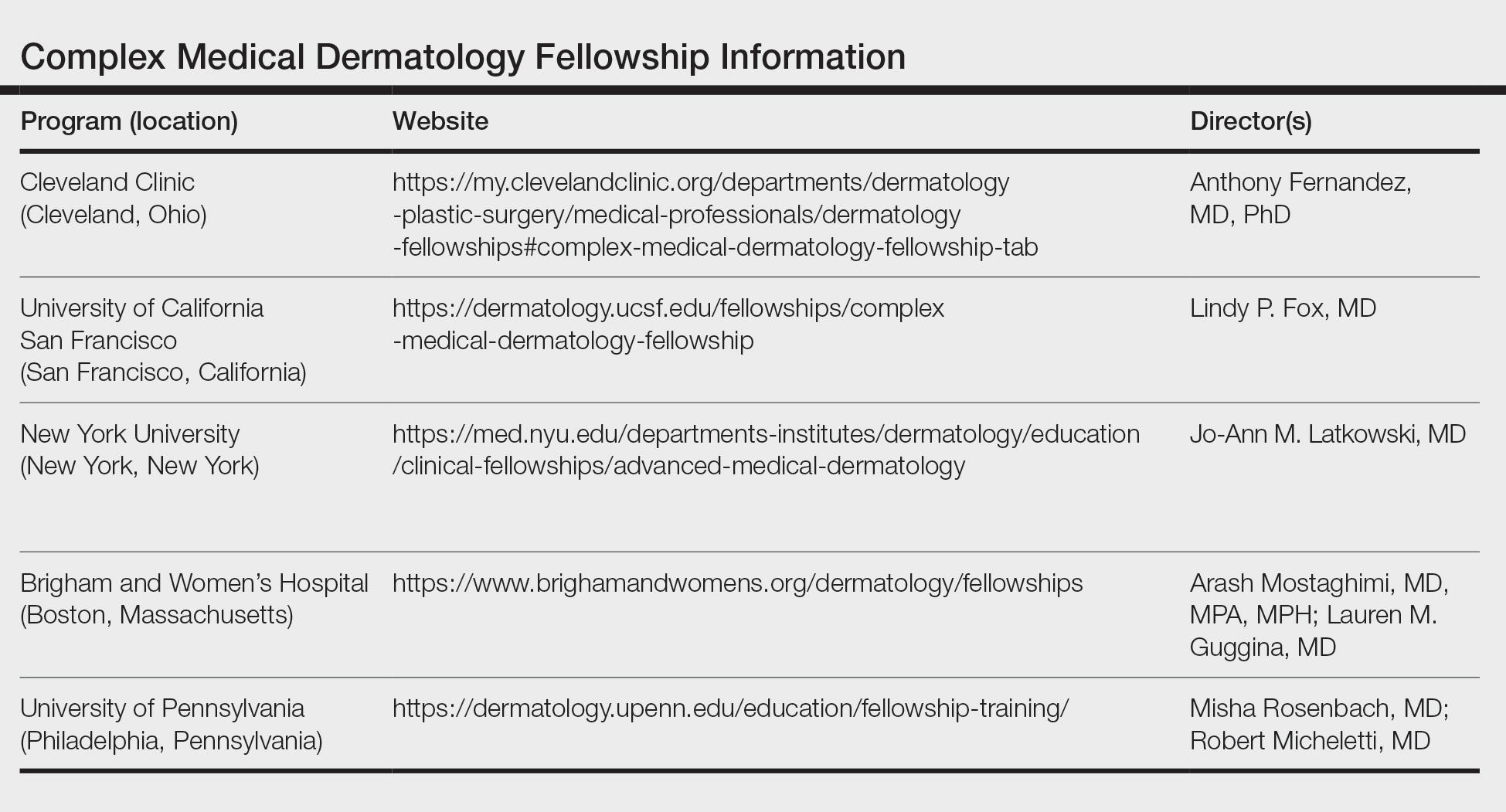
Reason to Pursue the Fellowship—There are many reasons to pursue a fellowship in complex medical dermatology such as a desire to enhance exposure to the field, to practice in an academic center and develop a niche within dermatology, to practice dermatology in an inpatient setting, to improve delivery of health care to medically challenging populations in a community setting, and to become an expert on cutaneous manifestations of internal and systemic disease.
Application—There is no standardized application or deadline for this fellowship; however, there is a concerted attempt from some of the programs to offer interviews and decisions at a similar time. Deadlines and contact information are listed on the program websites, along with more details (Table).
Recommendations—I would recommend reaching out at the beginning of postgraduate year (PGY) 4 to these programs and voicing your interest in the fellowship. It is possible to set up an away rotation at some of the programs, and if your program offers elective time, pursuing an away rotation during PGY-3 or early in PGY-4 can prove to be advantageous. Furthermore, during my application cycle I toured the University of California San Francisco, University of Pennsylvania, and Brigham and Women’s Hospital to gain further insight into each program.
Brigham and Women’s Complex Medical Dermatology Fellowship
I am currently the complex medical dermatology fellow at Brigham and Women’s Hospital, and it has been an outstanding experience thus far. The program offers numerous subspecialty clinics focusing solely on cutaneous-oncodermatology, psoriasis, rheumatology-dermatology, skin of color, mole mapping backed by artificial intelligence, cosmetics, high-risk skin cancer, neutrophilic dermatoses, patch testing, phototherapy, psychodermatology, and transplant dermatology. In addition to a wide variety of subspecialty clinics, fellows have the opportunity to participate in inpatient dermatology rounds and act as a junior attending. I appreciate the flexibility of this program combined with the ability to work alongside worldwide experts. There are numerous teaching opportunities, and all of the faculty are amiable and intelligent and emphasize wellness, education, and autonomy. Overall, my experience and decision to pursue a complex medical dermatology fellowship has been extremely rewarding and invaluable. I am gaining additional skills to aid medically challenging patients while pursuing my true passion in dermatology.
1. Sahni DR. Inpatient dermatology consultation services in hospital institutions. Cutis. 2023;111:E11-E12. doi:10.12788/cutis.0776.
Complex medical dermatology has become an emerging field in dermatology. Although a rather protean and broad term, complex medical dermatology encompasses patients with autoimmune conditions, bullous disease, connective tissue disease, vasculitis, severe dermatoses requiring immunomodulation, and inpatient consultations. Importantly, dermatology inpatient consultations aid in lowering health care costs due to accurate diagnoses, correct treatment, and decreased hospital stays.1 A fellowship is not required for holding an inpatient role in the hospital system as a dermatologist but can be beneficial. There are combined internal medicine–dermatology programs available for medical students applying to dermatology residency, but a complex medical dermatology fellowship is an option after residency for those who are interested. I believe that a focused complex medical dermatology fellowship differs from the training offered in combined internal medicine–dermatology residency. My fellow colleagues in combined internal medicine–dermatology programs are exposed to systemic manifestations of cutaneous disease and are experts in the interplay between the skin and other organ systems. However, the focus of their programs is with the intention of becoming double boarded in internal medicine and dermatology with comprehensive exposure to both fields. In my fellowship, I am able to tailor my schedule to focus on any dermatologic disease such as connective tissue disease, pruritus, graft vs host disease, and Merkel cell carcinoma. I ultimately can determine a niche in dermatology and hone my skills for a year under supervision.
Available Fellowships
Fellowship Locations—Importantly, the complex medical dermatology fellowship is not accredited by the Accreditation Council for Graduate Medical Education, which can make it difficult to identify and apply to programs. The complex medical dermatology fellowship is different than a rheumatology-dermatology fellowship, cutaneous oncology fellowship, pediatric dermatology fellowship, or other subspecialty fellowships such as those in itch or autoimmune blistering diseases. The fellowship often encompasses gaining clinical expertise in many of these conditions. I performed a thorough search online and spoke with complex medical dermatologists to compile a list of programs that offer a complex medical dermatology fellowship: Brigham and Women’s Hospital (Boston, Massachusetts); University of California San Francisco (San Francisco, California); University of Pennsylvania (Philadelphia, Pennsylvania); Cleveland Clinic (Cleveland, Ohio); and New York University (New York, New York)(Table). Only 1 spot is offered at each of these programs.

Reason to Pursue the Fellowship—There are many reasons to pursue a fellowship in complex medical dermatology such as a desire to enhance exposure to the field, to practice in an academic center and develop a niche within dermatology, to practice dermatology in an inpatient setting, to improve delivery of health care to medically challenging populations in a community setting, and to become an expert on cutaneous manifestations of internal and systemic disease.
Application—There is no standardized application or deadline for this fellowship; however, there is a concerted attempt from some of the programs to offer interviews and decisions at a similar time. Deadlines and contact information are listed on the program websites, along with more details (Table).
Recommendations—I would recommend reaching out at the beginning of postgraduate year (PGY) 4 to these programs and voicing your interest in the fellowship. It is possible to set up an away rotation at some of the programs, and if your program offers elective time, pursuing an away rotation during PGY-3 or early in PGY-4 can prove to be advantageous. Furthermore, during my application cycle I toured the University of California San Francisco, University of Pennsylvania, and Brigham and Women’s Hospital to gain further insight into each program.
Brigham and Women’s Complex Medical Dermatology Fellowship
I am currently the complex medical dermatology fellow at Brigham and Women’s Hospital, and it has been an outstanding experience thus far. The program offers numerous subspecialty clinics focusing solely on cutaneous-oncodermatology, psoriasis, rheumatology-dermatology, skin of color, mole mapping backed by artificial intelligence, cosmetics, high-risk skin cancer, neutrophilic dermatoses, patch testing, phototherapy, psychodermatology, and transplant dermatology. In addition to a wide variety of subspecialty clinics, fellows have the opportunity to participate in inpatient dermatology rounds and act as a junior attending. I appreciate the flexibility of this program combined with the ability to work alongside worldwide experts. There are numerous teaching opportunities, and all of the faculty are amiable and intelligent and emphasize wellness, education, and autonomy. Overall, my experience and decision to pursue a complex medical dermatology fellowship has been extremely rewarding and invaluable. I am gaining additional skills to aid medically challenging patients while pursuing my true passion in dermatology.
Complex medical dermatology has become an emerging field in dermatology. Although a rather protean and broad term, complex medical dermatology encompasses patients with autoimmune conditions, bullous disease, connective tissue disease, vasculitis, severe dermatoses requiring immunomodulation, and inpatient consultations. Importantly, dermatology inpatient consultations aid in lowering health care costs due to accurate diagnoses, correct treatment, and decreased hospital stays.1 A fellowship is not required for holding an inpatient role in the hospital system as a dermatologist but can be beneficial. There are combined internal medicine–dermatology programs available for medical students applying to dermatology residency, but a complex medical dermatology fellowship is an option after residency for those who are interested. I believe that a focused complex medical dermatology fellowship differs from the training offered in combined internal medicine–dermatology residency. My fellow colleagues in combined internal medicine–dermatology programs are exposed to systemic manifestations of cutaneous disease and are experts in the interplay between the skin and other organ systems. However, the focus of their programs is with the intention of becoming double boarded in internal medicine and dermatology with comprehensive exposure to both fields. In my fellowship, I am able to tailor my schedule to focus on any dermatologic disease such as connective tissue disease, pruritus, graft vs host disease, and Merkel cell carcinoma. I ultimately can determine a niche in dermatology and hone my skills for a year under supervision.
Available Fellowships
Fellowship Locations—Importantly, the complex medical dermatology fellowship is not accredited by the Accreditation Council for Graduate Medical Education, which can make it difficult to identify and apply to programs. The complex medical dermatology fellowship is different than a rheumatology-dermatology fellowship, cutaneous oncology fellowship, pediatric dermatology fellowship, or other subspecialty fellowships such as those in itch or autoimmune blistering diseases. The fellowship often encompasses gaining clinical expertise in many of these conditions. I performed a thorough search online and spoke with complex medical dermatologists to compile a list of programs that offer a complex medical dermatology fellowship: Brigham and Women’s Hospital (Boston, Massachusetts); University of California San Francisco (San Francisco, California); University of Pennsylvania (Philadelphia, Pennsylvania); Cleveland Clinic (Cleveland, Ohio); and New York University (New York, New York)(Table). Only 1 spot is offered at each of these programs.

Reason to Pursue the Fellowship—There are many reasons to pursue a fellowship in complex medical dermatology such as a desire to enhance exposure to the field, to practice in an academic center and develop a niche within dermatology, to practice dermatology in an inpatient setting, to improve delivery of health care to medically challenging populations in a community setting, and to become an expert on cutaneous manifestations of internal and systemic disease.
Application—There is no standardized application or deadline for this fellowship; however, there is a concerted attempt from some of the programs to offer interviews and decisions at a similar time. Deadlines and contact information are listed on the program websites, along with more details (Table).
Recommendations—I would recommend reaching out at the beginning of postgraduate year (PGY) 4 to these programs and voicing your interest in the fellowship. It is possible to set up an away rotation at some of the programs, and if your program offers elective time, pursuing an away rotation during PGY-3 or early in PGY-4 can prove to be advantageous. Furthermore, during my application cycle I toured the University of California San Francisco, University of Pennsylvania, and Brigham and Women’s Hospital to gain further insight into each program.
Brigham and Women’s Complex Medical Dermatology Fellowship
I am currently the complex medical dermatology fellow at Brigham and Women’s Hospital, and it has been an outstanding experience thus far. The program offers numerous subspecialty clinics focusing solely on cutaneous-oncodermatology, psoriasis, rheumatology-dermatology, skin of color, mole mapping backed by artificial intelligence, cosmetics, high-risk skin cancer, neutrophilic dermatoses, patch testing, phototherapy, psychodermatology, and transplant dermatology. In addition to a wide variety of subspecialty clinics, fellows have the opportunity to participate in inpatient dermatology rounds and act as a junior attending. I appreciate the flexibility of this program combined with the ability to work alongside worldwide experts. There are numerous teaching opportunities, and all of the faculty are amiable and intelligent and emphasize wellness, education, and autonomy. Overall, my experience and decision to pursue a complex medical dermatology fellowship has been extremely rewarding and invaluable. I am gaining additional skills to aid medically challenging patients while pursuing my true passion in dermatology.
1. Sahni DR. Inpatient dermatology consultation services in hospital institutions. Cutis. 2023;111:E11-E12. doi:10.12788/cutis.0776.
1. Sahni DR. Inpatient dermatology consultation services in hospital institutions. Cutis. 2023;111:E11-E12. doi:10.12788/cutis.0776.
RESIDENT PEARL
- Complex medical dermatology is a rewarding and fascinating subspecialty of dermatology, and additional training can be accomplished through a fellowship at a variety of prestigious institutions.
Camp Lejeune Family Members Now Eligible for Health Care Reimbursement Related to Parkinson Disease
Family members of veterans exposed to contaminated drinking water at Marine Corps Base Camp Lejeune, Jacksonville, North Carolina, from August 1, 1953, to December 31, 1987, are now eligible for reimbursement of health care costs associated with Parkinson disease (PD) under the Camp Lejeune Family Member Program, the US Department of Veterans Affairs (VA) has announced.
That brings the number of illnesses or conditions those family members can be reimbursed for to 16: esophageal, lung, breast, bladder, and kidney cancer, leukemia, multiple myeloma, renal toxicity, miscarriage, hepatic steatosis, female infertility, myelodysplastic syndromes, scleroderma, neurobehavioral effects, non-Hodgkin lymphoma, and Parkinson disease.
A recent JAMA study of 340,489 service members found that the risk of PD is 70% higher for veterans stationed at Camp Lejeune (n = 279) compared with veterans stationed at Camp Pendleton, California (n = 151).
The researchers say water supplies at Camp Lejeune were contaminated with several volatile organic compounds. They suggest that the risk of PD may be related to trichloroethylene exposure (TCE), a volatile organic compound widely used as a cleaning agent, in the manufacturing of some refrigerants, and found in paints and other products. In January, the US Environmental Protection Agency issued a revised risk determination saying that TCE presents an unreasonable risk to the health of workers, occupational nonusers (workers nearby but not in direct contact with this chemical), consumers, and bystanders.
Levels at Camp Lejeune were highest for TCE, with monthly median values greater than 70-fold the permissible amount.
Camp Lejeune veterans also had a significantly increased risk of prodromal PD diagnoses, including tremor, anxiety, and erectile dysfunction, and higher cumulative prodromal risk scores. No excess risk was found for other forms of neurodegenerative parkinsonism.
The PACT Act allows veterans and their families to file lawsuits for harm caused by exposure to contaminated water at Camp Lejeune. “Veterans and their families deserve no-cost health care for the conditions they developed due to the contaminated water at Camp Lejeune,” said VA’s Under Secretary for Health, Dr. Shereef Elnahal, MD. “We’re proud to add Parkinson disease to the list of conditions that are covered for veteran family members, and we implore anyone who may be living with this disease—or any of the other conditions covered by VA’s Camp Lejeune Family Member Program—to apply for assistance today.”
Family members of veterans exposed to contaminated drinking water at Marine Corps Base Camp Lejeune, Jacksonville, North Carolina, from August 1, 1953, to December 31, 1987, are now eligible for reimbursement of health care costs associated with Parkinson disease (PD) under the Camp Lejeune Family Member Program, the US Department of Veterans Affairs (VA) has announced.
That brings the number of illnesses or conditions those family members can be reimbursed for to 16: esophageal, lung, breast, bladder, and kidney cancer, leukemia, multiple myeloma, renal toxicity, miscarriage, hepatic steatosis, female infertility, myelodysplastic syndromes, scleroderma, neurobehavioral effects, non-Hodgkin lymphoma, and Parkinson disease.
A recent JAMA study of 340,489 service members found that the risk of PD is 70% higher for veterans stationed at Camp Lejeune (n = 279) compared with veterans stationed at Camp Pendleton, California (n = 151).
The researchers say water supplies at Camp Lejeune were contaminated with several volatile organic compounds. They suggest that the risk of PD may be related to trichloroethylene exposure (TCE), a volatile organic compound widely used as a cleaning agent, in the manufacturing of some refrigerants, and found in paints and other products. In January, the US Environmental Protection Agency issued a revised risk determination saying that TCE presents an unreasonable risk to the health of workers, occupational nonusers (workers nearby but not in direct contact with this chemical), consumers, and bystanders.
Levels at Camp Lejeune were highest for TCE, with monthly median values greater than 70-fold the permissible amount.
Camp Lejeune veterans also had a significantly increased risk of prodromal PD diagnoses, including tremor, anxiety, and erectile dysfunction, and higher cumulative prodromal risk scores. No excess risk was found for other forms of neurodegenerative parkinsonism.
The PACT Act allows veterans and their families to file lawsuits for harm caused by exposure to contaminated water at Camp Lejeune. “Veterans and their families deserve no-cost health care for the conditions they developed due to the contaminated water at Camp Lejeune,” said VA’s Under Secretary for Health, Dr. Shereef Elnahal, MD. “We’re proud to add Parkinson disease to the list of conditions that are covered for veteran family members, and we implore anyone who may be living with this disease—or any of the other conditions covered by VA’s Camp Lejeune Family Member Program—to apply for assistance today.”
Family members of veterans exposed to contaminated drinking water at Marine Corps Base Camp Lejeune, Jacksonville, North Carolina, from August 1, 1953, to December 31, 1987, are now eligible for reimbursement of health care costs associated with Parkinson disease (PD) under the Camp Lejeune Family Member Program, the US Department of Veterans Affairs (VA) has announced.
That brings the number of illnesses or conditions those family members can be reimbursed for to 16: esophageal, lung, breast, bladder, and kidney cancer, leukemia, multiple myeloma, renal toxicity, miscarriage, hepatic steatosis, female infertility, myelodysplastic syndromes, scleroderma, neurobehavioral effects, non-Hodgkin lymphoma, and Parkinson disease.
A recent JAMA study of 340,489 service members found that the risk of PD is 70% higher for veterans stationed at Camp Lejeune (n = 279) compared with veterans stationed at Camp Pendleton, California (n = 151).
The researchers say water supplies at Camp Lejeune were contaminated with several volatile organic compounds. They suggest that the risk of PD may be related to trichloroethylene exposure (TCE), a volatile organic compound widely used as a cleaning agent, in the manufacturing of some refrigerants, and found in paints and other products. In January, the US Environmental Protection Agency issued a revised risk determination saying that TCE presents an unreasonable risk to the health of workers, occupational nonusers (workers nearby but not in direct contact with this chemical), consumers, and bystanders.
Levels at Camp Lejeune were highest for TCE, with monthly median values greater than 70-fold the permissible amount.
Camp Lejeune veterans also had a significantly increased risk of prodromal PD diagnoses, including tremor, anxiety, and erectile dysfunction, and higher cumulative prodromal risk scores. No excess risk was found for other forms of neurodegenerative parkinsonism.
The PACT Act allows veterans and their families to file lawsuits for harm caused by exposure to contaminated water at Camp Lejeune. “Veterans and their families deserve no-cost health care for the conditions they developed due to the contaminated water at Camp Lejeune,” said VA’s Under Secretary for Health, Dr. Shereef Elnahal, MD. “We’re proud to add Parkinson disease to the list of conditions that are covered for veteran family members, and we implore anyone who may be living with this disease—or any of the other conditions covered by VA’s Camp Lejeune Family Member Program—to apply for assistance today.”
VA Partners to Open Clinics, Build Facilities that Increase Veteran Access to Health Care
The US Department of Veterans Affairs (VA) has been establishing partnerships right, left, and center to improve and expand care for veterans. Instead of going it alone, VA is partnering with academic affiliates, Native American tribes, and the military to take advantage of state and federal funds.
In California, the VA Palo Alto Health Care System and Stanford Medicine announced a deal to plan, build, and operate a National Cancer Institute–designated joint cancer care and research center on the VA Palo Alto campus. The partnership is another offshoot of the PACT Act, in part because of the number of veterans who need cancer treatment related to, for instance, airborne toxins. The influx of veterans via the PACT Act could represent “the largest expansion of veterans’ benefits in history,” VA Under Secretary for Health Shereef Elnahal, MD, MBA, said at a press event about the collaboration. “This will allow us to partner with every powerhouse academic center in the country if we do this right. For research, training, and care delivery, it’s all one bucket of cancer care that veterans deserve.”
A separate partnership between the Cherokee Nation and Eastern Oklahoma VA Healthcare System will establish a VA clinic inside the Cherokee Nation’s Vinita Health Center, an hour northeast of Tulsa. The clinic, expected to open early next year, will serve any veteran. “Cherokees and other Native Americans serve in the US military at a higher rate than any other group, and veterans hold a special place in our hearts,” Cherokee Nation Principal Chief Chuck Hoskin Jr. said in a statement. “I am honored to do my part in covering veterans’ long-term health needs.”
The VA serves about 53,000 veterans living in eastern Oklahoma. Officials predict that partnership could serve as a roadmap for how rural America can work with tribes to increase care for veterans. “As we look ahead, this partnership with the VA can be a model for other tribes and communities across the nation,” Hoskin said.
Another collaborative plan, this one by the VA and US Department of Defense (DoD), will give about 37,000 Gulf Coast–area veterans access to care at a new Naval Hospital Pensacola clinic. Local veterans who previously received care from community clinicians or traveled to the Biloxi VA Medical Center in Mississippi will now be able to receive same-day, outpatient surgical care. “This partnership will help VA provide more care, more quickly, to more Gulf Coast veterans—as close to their homes as possible,” said Elnahal.
An agreement with the University of Pennsylvania Health System (UPHS) will improve infrastructure at the Coatesville VA Medical Center by repurposing a recently closed hospital nearby for outpatient, acute mental health, and long-term care services. “The PACT Act allows for great synergy between Penn Medicine and the VA, and we hope to leverage this new model to set the standard for how our nation approaches military medicine,” UPHS CEO Kevin B. Mahoney said.
An Eastern Oklahoma VA Health Care System hospital scheduled to open in 2025 in Tulsa was partially funded through the Communities Helping Invest through Property and Improvements Needed (CHIP-IN) program, the state of Oklahoma, the city of Tulsa, and the nonprofit team of Oklahoma State University Medical and the Anne and Henry Zarrow Foundation. When completed, the 58-bed hospital will serve approximately 38,000 veterans.
The US Department of Veterans Affairs (VA) has been establishing partnerships right, left, and center to improve and expand care for veterans. Instead of going it alone, VA is partnering with academic affiliates, Native American tribes, and the military to take advantage of state and federal funds.
In California, the VA Palo Alto Health Care System and Stanford Medicine announced a deal to plan, build, and operate a National Cancer Institute–designated joint cancer care and research center on the VA Palo Alto campus. The partnership is another offshoot of the PACT Act, in part because of the number of veterans who need cancer treatment related to, for instance, airborne toxins. The influx of veterans via the PACT Act could represent “the largest expansion of veterans’ benefits in history,” VA Under Secretary for Health Shereef Elnahal, MD, MBA, said at a press event about the collaboration. “This will allow us to partner with every powerhouse academic center in the country if we do this right. For research, training, and care delivery, it’s all one bucket of cancer care that veterans deserve.”
A separate partnership between the Cherokee Nation and Eastern Oklahoma VA Healthcare System will establish a VA clinic inside the Cherokee Nation’s Vinita Health Center, an hour northeast of Tulsa. The clinic, expected to open early next year, will serve any veteran. “Cherokees and other Native Americans serve in the US military at a higher rate than any other group, and veterans hold a special place in our hearts,” Cherokee Nation Principal Chief Chuck Hoskin Jr. said in a statement. “I am honored to do my part in covering veterans’ long-term health needs.”
The VA serves about 53,000 veterans living in eastern Oklahoma. Officials predict that partnership could serve as a roadmap for how rural America can work with tribes to increase care for veterans. “As we look ahead, this partnership with the VA can be a model for other tribes and communities across the nation,” Hoskin said.
Another collaborative plan, this one by the VA and US Department of Defense (DoD), will give about 37,000 Gulf Coast–area veterans access to care at a new Naval Hospital Pensacola clinic. Local veterans who previously received care from community clinicians or traveled to the Biloxi VA Medical Center in Mississippi will now be able to receive same-day, outpatient surgical care. “This partnership will help VA provide more care, more quickly, to more Gulf Coast veterans—as close to their homes as possible,” said Elnahal.
An agreement with the University of Pennsylvania Health System (UPHS) will improve infrastructure at the Coatesville VA Medical Center by repurposing a recently closed hospital nearby for outpatient, acute mental health, and long-term care services. “The PACT Act allows for great synergy between Penn Medicine and the VA, and we hope to leverage this new model to set the standard for how our nation approaches military medicine,” UPHS CEO Kevin B. Mahoney said.
An Eastern Oklahoma VA Health Care System hospital scheduled to open in 2025 in Tulsa was partially funded through the Communities Helping Invest through Property and Improvements Needed (CHIP-IN) program, the state of Oklahoma, the city of Tulsa, and the nonprofit team of Oklahoma State University Medical and the Anne and Henry Zarrow Foundation. When completed, the 58-bed hospital will serve approximately 38,000 veterans.
The US Department of Veterans Affairs (VA) has been establishing partnerships right, left, and center to improve and expand care for veterans. Instead of going it alone, VA is partnering with academic affiliates, Native American tribes, and the military to take advantage of state and federal funds.
In California, the VA Palo Alto Health Care System and Stanford Medicine announced a deal to plan, build, and operate a National Cancer Institute–designated joint cancer care and research center on the VA Palo Alto campus. The partnership is another offshoot of the PACT Act, in part because of the number of veterans who need cancer treatment related to, for instance, airborne toxins. The influx of veterans via the PACT Act could represent “the largest expansion of veterans’ benefits in history,” VA Under Secretary for Health Shereef Elnahal, MD, MBA, said at a press event about the collaboration. “This will allow us to partner with every powerhouse academic center in the country if we do this right. For research, training, and care delivery, it’s all one bucket of cancer care that veterans deserve.”
A separate partnership between the Cherokee Nation and Eastern Oklahoma VA Healthcare System will establish a VA clinic inside the Cherokee Nation’s Vinita Health Center, an hour northeast of Tulsa. The clinic, expected to open early next year, will serve any veteran. “Cherokees and other Native Americans serve in the US military at a higher rate than any other group, and veterans hold a special place in our hearts,” Cherokee Nation Principal Chief Chuck Hoskin Jr. said in a statement. “I am honored to do my part in covering veterans’ long-term health needs.”
The VA serves about 53,000 veterans living in eastern Oklahoma. Officials predict that partnership could serve as a roadmap for how rural America can work with tribes to increase care for veterans. “As we look ahead, this partnership with the VA can be a model for other tribes and communities across the nation,” Hoskin said.
Another collaborative plan, this one by the VA and US Department of Defense (DoD), will give about 37,000 Gulf Coast–area veterans access to care at a new Naval Hospital Pensacola clinic. Local veterans who previously received care from community clinicians or traveled to the Biloxi VA Medical Center in Mississippi will now be able to receive same-day, outpatient surgical care. “This partnership will help VA provide more care, more quickly, to more Gulf Coast veterans—as close to their homes as possible,” said Elnahal.
An agreement with the University of Pennsylvania Health System (UPHS) will improve infrastructure at the Coatesville VA Medical Center by repurposing a recently closed hospital nearby for outpatient, acute mental health, and long-term care services. “The PACT Act allows for great synergy between Penn Medicine and the VA, and we hope to leverage this new model to set the standard for how our nation approaches military medicine,” UPHS CEO Kevin B. Mahoney said.
An Eastern Oklahoma VA Health Care System hospital scheduled to open in 2025 in Tulsa was partially funded through the Communities Helping Invest through Property and Improvements Needed (CHIP-IN) program, the state of Oklahoma, the city of Tulsa, and the nonprofit team of Oklahoma State University Medical and the Anne and Henry Zarrow Foundation. When completed, the 58-bed hospital will serve approximately 38,000 veterans.
Who Gets to Determine Whether Home Is “Unsafe” at the End of Life?
Sometimes a patient at the end of life (EOL) just wants to go home. We recently treated such a patient, “Joe,” a 66-year-old veteran with end-stage chronic obstructive pulmonary disorder (COPD), severe hearing loss, and heavy alcohol use. A neighbor brought Joe to the hospital when he developed a urinary tract infection. Before hospitalization, Joe spent his days in bed. His neighbor was his designated health care agent (HCA) and caregiver, dropping off meals and bringing Joe to medical appointments. Joe had no other social support. In the hospital, Joe could not participate in physical therapy (PT) evaluations due to severe dyspnea on exertion. He was recommended for home PT, a home health aide, and home nursing, but Joe declined these services out of concern for encroachment on his independence. Given his heavy alcohol use, limited support, and functional limitations, the hospitalist team felt that Joe would be best served in a skilled nursing facility. As the palliative care team, we were consulted and felt that he was eligible for hospice. Joe simply wanted to go home.
Many patients like Joe experience functional decline at EOL, leading to increased care needs and transitions between sites of care.1 Some hospitalized patients at EOL want to transition directly to home, but due to their limited functioning and social support, discharge home may be deemed unsafe by health care professionals (HCPs). Clinicians then face the difficult balancing act of honoring patient wishes and avoiding a bad outcome. For patients at EOL, issues of capacity and risk become particularly salient. Furthermore, the unique structure of the US Department of Veterans Affairs (VA) health system and the psychosocial needs of some veterans add additional considerations for complex EOL discharges.2
End-of-life Decision Making
While patients may express strong preferences regarding their health care, their decision-making ability may worsen as they approach EOL. Contributing factors include older age, effects of hospitalization, treatment adverse effects, and comorbidities, including cognitive impairment. Studies of terminally ill patients show high rates of impaired decisional capacity.3,4 It is critical to assess capacity as part of discharge planning. Even when patients have the capacity, families and caregivers have an important voice, since they are often instrumental in maintaining patients at home.
Defining Risk
Determining whether a discharge is risky or unsafe is highly subjective, with differing opinions among clinicians and between patients and clinicians.5-7 In a qualitative study by Coombs and colleagues, HCPs tended toward a risk-averse approach to discharge decisions, sometimes favoring discharge to care facilities despite patient preferences.6 This approach also reflects pressures from the health care system to decrease the length of stay and reduce readmissions, important metrics for patient care and cost containment. However, keeping patients hospitalized or in nursing facilities does not completely mitigate risks (eg, falls) and carries other hazards (eg, nosocomial infections), as highlighted during the COVID-19 pandemic.7,8 The prospect of malpractice lawsuits and HCP moral distress about perceived risky home situations can also understandably affect decision making.
At the same time, risk calculation changes depending on the patient’s clinical status and priorities. Coombs and colleagues found that in contrast to clinicians, patients nearing EOL are willing to accept increasing risks and suboptimal living conditions to remain at home.6 What may be intolerable for a younger, healthier patient with a long life expectancy may be acceptable for someone who is approaching EOL. In our framework, a risky home discharge at EOL is considered one in which other adverse events, such as falls or inadequate symptom management, are likely.
Ethical Considerations
Unsafe discharges are challenging in part because some of the pillars of medical ethics can conflict. Prior articles have analyzed the ethical concerns of unsafe discharges in detail.9-11 Briefly, when patients wish to return home against initial medical recommendations, treatment teams may focus on the principles of beneficence and nonmaleficence, as exemplified by the desire to minimize harm, and justice, in which clinicians consider resource allocation and risks that a home discharge poses to family members, caregivers, and home health professionals. However, autonomy is important to consider as well. The concept of dignity of risk highlights the imperative to respect others’ decisions even when they increase the chance of harm, particularly given the overall shift in medicine from paternalism to shared decision making.12 Accommodating patient choice in how and where health care is received allows patients to regain some control over their lives, thereby enhancing their quality of life and promoting patient dignity, especially in their remaining days.13
Discharge Risk Framework
Our risk assessment framework helps clinicians more objectively identify factors that increase or decrease risk, inform discharge planning, partner with patients and families, give patients a prominent role in EOL decisions, and mitigate the risk of a bad outcome. This concept has been used in psychiatry, in which formal suicide assessment includes identifying risk factors and protective factors to estimate suicide risk and determine interventions.14 Similar to suicide risk estimation, this framework is based on clinical judgment rather than a specific calculation.
While this framework serves as a guide for determining and mitigating risk, we encourage teams to consider legal or ethical consultations in challenging cases, such as those in which patients lack both capacity and an involved HCA.
Step 1: Determine the patient’s capacity regarding disposition planning. Patients at EOL are at a higher risk of impaired decision-making capabilities; therefore, capacity evaluation is a critical step.
Step 2: Identify risk factors and protective factors for discharge home. Risk factors are intrinsic and extrinsic factors that increase risk such as functional or sensory impairments. Protective factors are intrinsic and extrinsic factors that decrease risk, including a good understanding of illness and consistent connection with the health care system (Table 1).
Step 3: Determine discharge to home risk level based on identified risk factors and protective factors. Patients may be at low, moderate, or high risk of having an adverse event, such as a fall or inadequate symptom control (Table 2).
Step 4: Identify risk mitigation strategies. These should be tailored to the patient based on the factors identified in Step 2. Examples include home nursing and therapy, mental health treatment, a medical alert system, and frequent contact between the patient and health care team.
Step 5: Meet with inpatient and outpatient HCP teams. Meetings should include the primary care professional (PCP) or relevant subspecialist, such as an oncologist for patients with cancer. For veterans receiving care solely at a local VA medical center, this can be easier to facilitate, but for veterans who receive care through both VA and non-VA systems, this step may require additional coordination. We also recommend including interdisciplinary team members, such as social workers, case managers, and the relevant home care or hospice agency. Certain agencies may decline admission if they perceive increased risk, such as no 24-hour care, perceived self-neglect, and limited instrumental support. During this meeting, HCPs discuss risk mitigation strategies identified in Step 4 and create a plan to propose to patients and families.
Step 6: Meet with patient, HCA, and family members. In addition to sharing information about prognosis, assessing caregiver capabilities and burden can guide conversations about discharge. The discharge plan should be determined through shared decision making.11 If the patient lacks capacity regarding disposition planning, this should be shared with the HCA. However, even when patients lack capacity, it is important to continue to engage them to understand their goals and preferences.
Step 7: Maximize risk mitigation strategies. If a moderate- or high-risk discharge is requested, the health care team should maximize risk mitigation strategies. For low-risk discharges, risk mitigation strategies can still promote safety, especially since risk increases as patients progress toward EOL. In some instances, patients, their HCAs, or caregivers may decline all risk mitigation strategies despite best efforts to communicate and negotiate options. In such circumstances, we recommend discussing the case with the outpatient team for a warm handoff. HCPs should also document all efforts (helpful from a legal standpoint as well as for the patient’s future treatment teams) and respect the decision to discharge home.
Applying the Framework
Our patient Joe provides a good illustration of how to implement this EOL framework. He was deemed to have the capacity to make decisions regarding discharge (Step 1). We determined his risk factors and protective factors for discharge (Step 2). His poor functional status, limited instrumental support, heavy alcohol use, rejection of home services, and communication barriers due to severe hearing impairment all increased his risk. Protective factors included an appreciation of functional limitations, intact cognition, and an involved HCA. Based on his limited instrumental support and poor function but good insight into limitations, discharge home was deemed to be of moderate risk (Step 3). Although risk factors such as alcohol use and severe hearing impairment could have raised his level to high risk, we felt that his involved HCA maintained him in the moderate-risk category.
We worked with the hospitalist team, PT, and audiology to identify multiple risk mitigation strategies: frequent phone calls between the HCA and outpatient palliative care team, home PT to improve transfers from bed to bedside commode, home nursing services either through a routine agency or hospice, and hearing aids for better communication (Steps 4 and 5). We then proposed these strategies to Joe and his HCA (Step 6). Due to concerns about infringement on his independence, Joe declined all home services but agreed to twice-daily check-ins by his HCA, frequent communication between his HCA and our team, and new hearing aids.
Joe returned home with the agreed-upon risk mitigation strategies in place (Step 7). Despite clinicians’ original reservations about sending Joe home without formal services, his HCA maintained close contact with our team, noting that Joe remained stable and happy to be at home in the months following discharge.
Conclusions
Fortunately, VA HCPs operate in an integrated health care system with access to psychological, social, and at-home medical support that can help mitigate risks. Still, we have benefitted from having a tool to help us evaluate risk systematically. Even if patients, families, and HCPs disagree on ideal discharge plans, this tool helps clinicians approach discharges methodically while maintaining open communication and partnership with patients. In doing so, our framework reflects the shift in medical culture from a patriarchal approach to shared decision-making practices regarding all aspects of medical care. Furthermore, we hope that this can help reduce clinician moral distress stemming from these challenging cases.
Future research on best practices for discharge risk assessment and optimizing home safety are needed. We also hope to evaluate the impact and effectiveness of our framework through interviews with key stakeholders. For Joe and other veterans like him, where to spend their final days may be the last important decision they make in life, and our framework allows for their voices to be better heard throughout the decision-making process.
Acknowledgments
We thank Brooke Lifland, MD, for her theoretical contributions to the concept behind this paper.
1. Committee on Approaching Death: Addressing Key End of Life Issues; Institute of Medicine. Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Washington (DC): National Academies Press (US); March 19, 2015.
2. Casarett D, Pickard A, Amos Bailey F, et al. Important aspects of end-of-life care among veterans: implications for measurement and quality improvement. J Pain Symptom Manage. 2008;35(2):115-125. doi:10.1016/j.jpainsymman.2007.03.008
3. Kolva E, Rosenfeld B, Brescia R, Comfort C. Assessing decision-making capacity at end of life. Gen Hosp Psychiatry. 2014;36(4):392-397. doi:10.1016/j.genhosppsych.2014.02.013
4. Kolva E, Rosenfeld B, Saracino R. Assessing the decision-making capacity of terminally ill patients with cancer. Am J Geriatr Psychiatry. 2018;26(5):523-531. doi:10.1016/j.jagp.2017.11.012
5. Macmillan MS. Hospital staff’s perceptions of risk associated with the discharge of elderly people from acute hospital care. J Adv Nurs. 1994;19(2):249-256. doi:10.1111/j.1365-2648.1994.tb01078.x
6. Coombs MA, Parker R, de Vries K. Managing risk during care transitions when approaching end of life: A qualitative study of patients’ and health care professionals’ decision making. Palliat Med. 2017;31(7):617-624. doi:10.1177/0269216316673476
7. Hyslop B. ‘Not safe for discharge’? Words, values, and person-centred care. Age Ageing. 2020;49(3):334-336. doi:10.1093/ageing/afz170
8. Goodacre S. Safe discharge: an irrational, unhelpful and unachievable concept. Emerg Med J. 2006;23(10):753-755. doi:10.1136/emj.2006.037903
9. Swidler RN, Seastrum T, Shelton W. Difficult hospital inpatient discharge decisions: ethical, legal and clinical practice issues. Am J Bioeth. 2007;7(3):23-28. doi:10.1080/15265160601171739
10. Hill J, Filer W. Safety and ethical considerations in discharging patients to suboptimal living situations. AMA J Ethics. 2015;17(6):506-510. Published 2015 Jun 1. doi:10.1001/journalofethics.2015.17.6.ecas2-1506
11. West JC. What is an ethically informed approach to managing patient safety risk during discharge planning?. AMA J Ethics. 2020;22(11):E919-E923. Published 2020 Nov 1. doi:10.1001/amajethics.2020.919
12. Mukherjee D. Discharge decisions and the dignity of risk. Hastings Cent Rep. 2015;45(3):7-8. doi:10.1002/hast.441
13. Wheatley VJ, Baker JI. “Please, I want to go home”: ethical issues raised when considering choice of place of care in palliative care. Postgrad Med J. 2007;83(984):643-648. doi:10.1136/pgmj.2007.058487
14. Work Group on Suicidal Behaviors. Practice guideline for the assessment and treatment of patients with suicidal behaviors. Am J Psychiatry. 2003;160(suppl 11):1-60.
Sometimes a patient at the end of life (EOL) just wants to go home. We recently treated such a patient, “Joe,” a 66-year-old veteran with end-stage chronic obstructive pulmonary disorder (COPD), severe hearing loss, and heavy alcohol use. A neighbor brought Joe to the hospital when he developed a urinary tract infection. Before hospitalization, Joe spent his days in bed. His neighbor was his designated health care agent (HCA) and caregiver, dropping off meals and bringing Joe to medical appointments. Joe had no other social support. In the hospital, Joe could not participate in physical therapy (PT) evaluations due to severe dyspnea on exertion. He was recommended for home PT, a home health aide, and home nursing, but Joe declined these services out of concern for encroachment on his independence. Given his heavy alcohol use, limited support, and functional limitations, the hospitalist team felt that Joe would be best served in a skilled nursing facility. As the palliative care team, we were consulted and felt that he was eligible for hospice. Joe simply wanted to go home.
Many patients like Joe experience functional decline at EOL, leading to increased care needs and transitions between sites of care.1 Some hospitalized patients at EOL want to transition directly to home, but due to their limited functioning and social support, discharge home may be deemed unsafe by health care professionals (HCPs). Clinicians then face the difficult balancing act of honoring patient wishes and avoiding a bad outcome. For patients at EOL, issues of capacity and risk become particularly salient. Furthermore, the unique structure of the US Department of Veterans Affairs (VA) health system and the psychosocial needs of some veterans add additional considerations for complex EOL discharges.2
End-of-life Decision Making
While patients may express strong preferences regarding their health care, their decision-making ability may worsen as they approach EOL. Contributing factors include older age, effects of hospitalization, treatment adverse effects, and comorbidities, including cognitive impairment. Studies of terminally ill patients show high rates of impaired decisional capacity.3,4 It is critical to assess capacity as part of discharge planning. Even when patients have the capacity, families and caregivers have an important voice, since they are often instrumental in maintaining patients at home.
Defining Risk
Determining whether a discharge is risky or unsafe is highly subjective, with differing opinions among clinicians and between patients and clinicians.5-7 In a qualitative study by Coombs and colleagues, HCPs tended toward a risk-averse approach to discharge decisions, sometimes favoring discharge to care facilities despite patient preferences.6 This approach also reflects pressures from the health care system to decrease the length of stay and reduce readmissions, important metrics for patient care and cost containment. However, keeping patients hospitalized or in nursing facilities does not completely mitigate risks (eg, falls) and carries other hazards (eg, nosocomial infections), as highlighted during the COVID-19 pandemic.7,8 The prospect of malpractice lawsuits and HCP moral distress about perceived risky home situations can also understandably affect decision making.
At the same time, risk calculation changes depending on the patient’s clinical status and priorities. Coombs and colleagues found that in contrast to clinicians, patients nearing EOL are willing to accept increasing risks and suboptimal living conditions to remain at home.6 What may be intolerable for a younger, healthier patient with a long life expectancy may be acceptable for someone who is approaching EOL. In our framework, a risky home discharge at EOL is considered one in which other adverse events, such as falls or inadequate symptom management, are likely.
Ethical Considerations
Unsafe discharges are challenging in part because some of the pillars of medical ethics can conflict. Prior articles have analyzed the ethical concerns of unsafe discharges in detail.9-11 Briefly, when patients wish to return home against initial medical recommendations, treatment teams may focus on the principles of beneficence and nonmaleficence, as exemplified by the desire to minimize harm, and justice, in which clinicians consider resource allocation and risks that a home discharge poses to family members, caregivers, and home health professionals. However, autonomy is important to consider as well. The concept of dignity of risk highlights the imperative to respect others’ decisions even when they increase the chance of harm, particularly given the overall shift in medicine from paternalism to shared decision making.12 Accommodating patient choice in how and where health care is received allows patients to regain some control over their lives, thereby enhancing their quality of life and promoting patient dignity, especially in their remaining days.13
Discharge Risk Framework
Our risk assessment framework helps clinicians more objectively identify factors that increase or decrease risk, inform discharge planning, partner with patients and families, give patients a prominent role in EOL decisions, and mitigate the risk of a bad outcome. This concept has been used in psychiatry, in which formal suicide assessment includes identifying risk factors and protective factors to estimate suicide risk and determine interventions.14 Similar to suicide risk estimation, this framework is based on clinical judgment rather than a specific calculation.
While this framework serves as a guide for determining and mitigating risk, we encourage teams to consider legal or ethical consultations in challenging cases, such as those in which patients lack both capacity and an involved HCA.
Step 1: Determine the patient’s capacity regarding disposition planning. Patients at EOL are at a higher risk of impaired decision-making capabilities; therefore, capacity evaluation is a critical step.
Step 2: Identify risk factors and protective factors for discharge home. Risk factors are intrinsic and extrinsic factors that increase risk such as functional or sensory impairments. Protective factors are intrinsic and extrinsic factors that decrease risk, including a good understanding of illness and consistent connection with the health care system (Table 1).
Step 3: Determine discharge to home risk level based on identified risk factors and protective factors. Patients may be at low, moderate, or high risk of having an adverse event, such as a fall or inadequate symptom control (Table 2).
Step 4: Identify risk mitigation strategies. These should be tailored to the patient based on the factors identified in Step 2. Examples include home nursing and therapy, mental health treatment, a medical alert system, and frequent contact between the patient and health care team.
Step 5: Meet with inpatient and outpatient HCP teams. Meetings should include the primary care professional (PCP) or relevant subspecialist, such as an oncologist for patients with cancer. For veterans receiving care solely at a local VA medical center, this can be easier to facilitate, but for veterans who receive care through both VA and non-VA systems, this step may require additional coordination. We also recommend including interdisciplinary team members, such as social workers, case managers, and the relevant home care or hospice agency. Certain agencies may decline admission if they perceive increased risk, such as no 24-hour care, perceived self-neglect, and limited instrumental support. During this meeting, HCPs discuss risk mitigation strategies identified in Step 4 and create a plan to propose to patients and families.
Step 6: Meet with patient, HCA, and family members. In addition to sharing information about prognosis, assessing caregiver capabilities and burden can guide conversations about discharge. The discharge plan should be determined through shared decision making.11 If the patient lacks capacity regarding disposition planning, this should be shared with the HCA. However, even when patients lack capacity, it is important to continue to engage them to understand their goals and preferences.
Step 7: Maximize risk mitigation strategies. If a moderate- or high-risk discharge is requested, the health care team should maximize risk mitigation strategies. For low-risk discharges, risk mitigation strategies can still promote safety, especially since risk increases as patients progress toward EOL. In some instances, patients, their HCAs, or caregivers may decline all risk mitigation strategies despite best efforts to communicate and negotiate options. In such circumstances, we recommend discussing the case with the outpatient team for a warm handoff. HCPs should also document all efforts (helpful from a legal standpoint as well as for the patient’s future treatment teams) and respect the decision to discharge home.
Applying the Framework
Our patient Joe provides a good illustration of how to implement this EOL framework. He was deemed to have the capacity to make decisions regarding discharge (Step 1). We determined his risk factors and protective factors for discharge (Step 2). His poor functional status, limited instrumental support, heavy alcohol use, rejection of home services, and communication barriers due to severe hearing impairment all increased his risk. Protective factors included an appreciation of functional limitations, intact cognition, and an involved HCA. Based on his limited instrumental support and poor function but good insight into limitations, discharge home was deemed to be of moderate risk (Step 3). Although risk factors such as alcohol use and severe hearing impairment could have raised his level to high risk, we felt that his involved HCA maintained him in the moderate-risk category.
We worked with the hospitalist team, PT, and audiology to identify multiple risk mitigation strategies: frequent phone calls between the HCA and outpatient palliative care team, home PT to improve transfers from bed to bedside commode, home nursing services either through a routine agency or hospice, and hearing aids for better communication (Steps 4 and 5). We then proposed these strategies to Joe and his HCA (Step 6). Due to concerns about infringement on his independence, Joe declined all home services but agreed to twice-daily check-ins by his HCA, frequent communication between his HCA and our team, and new hearing aids.
Joe returned home with the agreed-upon risk mitigation strategies in place (Step 7). Despite clinicians’ original reservations about sending Joe home without formal services, his HCA maintained close contact with our team, noting that Joe remained stable and happy to be at home in the months following discharge.
Conclusions
Fortunately, VA HCPs operate in an integrated health care system with access to psychological, social, and at-home medical support that can help mitigate risks. Still, we have benefitted from having a tool to help us evaluate risk systematically. Even if patients, families, and HCPs disagree on ideal discharge plans, this tool helps clinicians approach discharges methodically while maintaining open communication and partnership with patients. In doing so, our framework reflects the shift in medical culture from a patriarchal approach to shared decision-making practices regarding all aspects of medical care. Furthermore, we hope that this can help reduce clinician moral distress stemming from these challenging cases.
Future research on best practices for discharge risk assessment and optimizing home safety are needed. We also hope to evaluate the impact and effectiveness of our framework through interviews with key stakeholders. For Joe and other veterans like him, where to spend their final days may be the last important decision they make in life, and our framework allows for their voices to be better heard throughout the decision-making process.
Acknowledgments
We thank Brooke Lifland, MD, for her theoretical contributions to the concept behind this paper.
Sometimes a patient at the end of life (EOL) just wants to go home. We recently treated such a patient, “Joe,” a 66-year-old veteran with end-stage chronic obstructive pulmonary disorder (COPD), severe hearing loss, and heavy alcohol use. A neighbor brought Joe to the hospital when he developed a urinary tract infection. Before hospitalization, Joe spent his days in bed. His neighbor was his designated health care agent (HCA) and caregiver, dropping off meals and bringing Joe to medical appointments. Joe had no other social support. In the hospital, Joe could not participate in physical therapy (PT) evaluations due to severe dyspnea on exertion. He was recommended for home PT, a home health aide, and home nursing, but Joe declined these services out of concern for encroachment on his independence. Given his heavy alcohol use, limited support, and functional limitations, the hospitalist team felt that Joe would be best served in a skilled nursing facility. As the palliative care team, we were consulted and felt that he was eligible for hospice. Joe simply wanted to go home.
Many patients like Joe experience functional decline at EOL, leading to increased care needs and transitions between sites of care.1 Some hospitalized patients at EOL want to transition directly to home, but due to their limited functioning and social support, discharge home may be deemed unsafe by health care professionals (HCPs). Clinicians then face the difficult balancing act of honoring patient wishes and avoiding a bad outcome. For patients at EOL, issues of capacity and risk become particularly salient. Furthermore, the unique structure of the US Department of Veterans Affairs (VA) health system and the psychosocial needs of some veterans add additional considerations for complex EOL discharges.2
End-of-life Decision Making
While patients may express strong preferences regarding their health care, their decision-making ability may worsen as they approach EOL. Contributing factors include older age, effects of hospitalization, treatment adverse effects, and comorbidities, including cognitive impairment. Studies of terminally ill patients show high rates of impaired decisional capacity.3,4 It is critical to assess capacity as part of discharge planning. Even when patients have the capacity, families and caregivers have an important voice, since they are often instrumental in maintaining patients at home.
Defining Risk
Determining whether a discharge is risky or unsafe is highly subjective, with differing opinions among clinicians and between patients and clinicians.5-7 In a qualitative study by Coombs and colleagues, HCPs tended toward a risk-averse approach to discharge decisions, sometimes favoring discharge to care facilities despite patient preferences.6 This approach also reflects pressures from the health care system to decrease the length of stay and reduce readmissions, important metrics for patient care and cost containment. However, keeping patients hospitalized or in nursing facilities does not completely mitigate risks (eg, falls) and carries other hazards (eg, nosocomial infections), as highlighted during the COVID-19 pandemic.7,8 The prospect of malpractice lawsuits and HCP moral distress about perceived risky home situations can also understandably affect decision making.
At the same time, risk calculation changes depending on the patient’s clinical status and priorities. Coombs and colleagues found that in contrast to clinicians, patients nearing EOL are willing to accept increasing risks and suboptimal living conditions to remain at home.6 What may be intolerable for a younger, healthier patient with a long life expectancy may be acceptable for someone who is approaching EOL. In our framework, a risky home discharge at EOL is considered one in which other adverse events, such as falls or inadequate symptom management, are likely.
Ethical Considerations
Unsafe discharges are challenging in part because some of the pillars of medical ethics can conflict. Prior articles have analyzed the ethical concerns of unsafe discharges in detail.9-11 Briefly, when patients wish to return home against initial medical recommendations, treatment teams may focus on the principles of beneficence and nonmaleficence, as exemplified by the desire to minimize harm, and justice, in which clinicians consider resource allocation and risks that a home discharge poses to family members, caregivers, and home health professionals. However, autonomy is important to consider as well. The concept of dignity of risk highlights the imperative to respect others’ decisions even when they increase the chance of harm, particularly given the overall shift in medicine from paternalism to shared decision making.12 Accommodating patient choice in how and where health care is received allows patients to regain some control over their lives, thereby enhancing their quality of life and promoting patient dignity, especially in their remaining days.13
Discharge Risk Framework
Our risk assessment framework helps clinicians more objectively identify factors that increase or decrease risk, inform discharge planning, partner with patients and families, give patients a prominent role in EOL decisions, and mitigate the risk of a bad outcome. This concept has been used in psychiatry, in which formal suicide assessment includes identifying risk factors and protective factors to estimate suicide risk and determine interventions.14 Similar to suicide risk estimation, this framework is based on clinical judgment rather than a specific calculation.
While this framework serves as a guide for determining and mitigating risk, we encourage teams to consider legal or ethical consultations in challenging cases, such as those in which patients lack both capacity and an involved HCA.
Step 1: Determine the patient’s capacity regarding disposition planning. Patients at EOL are at a higher risk of impaired decision-making capabilities; therefore, capacity evaluation is a critical step.
Step 2: Identify risk factors and protective factors for discharge home. Risk factors are intrinsic and extrinsic factors that increase risk such as functional or sensory impairments. Protective factors are intrinsic and extrinsic factors that decrease risk, including a good understanding of illness and consistent connection with the health care system (Table 1).
Step 3: Determine discharge to home risk level based on identified risk factors and protective factors. Patients may be at low, moderate, or high risk of having an adverse event, such as a fall or inadequate symptom control (Table 2).
Step 4: Identify risk mitigation strategies. These should be tailored to the patient based on the factors identified in Step 2. Examples include home nursing and therapy, mental health treatment, a medical alert system, and frequent contact between the patient and health care team.
Step 5: Meet with inpatient and outpatient HCP teams. Meetings should include the primary care professional (PCP) or relevant subspecialist, such as an oncologist for patients with cancer. For veterans receiving care solely at a local VA medical center, this can be easier to facilitate, but for veterans who receive care through both VA and non-VA systems, this step may require additional coordination. We also recommend including interdisciplinary team members, such as social workers, case managers, and the relevant home care or hospice agency. Certain agencies may decline admission if they perceive increased risk, such as no 24-hour care, perceived self-neglect, and limited instrumental support. During this meeting, HCPs discuss risk mitigation strategies identified in Step 4 and create a plan to propose to patients and families.
Step 6: Meet with patient, HCA, and family members. In addition to sharing information about prognosis, assessing caregiver capabilities and burden can guide conversations about discharge. The discharge plan should be determined through shared decision making.11 If the patient lacks capacity regarding disposition planning, this should be shared with the HCA. However, even when patients lack capacity, it is important to continue to engage them to understand their goals and preferences.
Step 7: Maximize risk mitigation strategies. If a moderate- or high-risk discharge is requested, the health care team should maximize risk mitigation strategies. For low-risk discharges, risk mitigation strategies can still promote safety, especially since risk increases as patients progress toward EOL. In some instances, patients, their HCAs, or caregivers may decline all risk mitigation strategies despite best efforts to communicate and negotiate options. In such circumstances, we recommend discussing the case with the outpatient team for a warm handoff. HCPs should also document all efforts (helpful from a legal standpoint as well as for the patient’s future treatment teams) and respect the decision to discharge home.
Applying the Framework
Our patient Joe provides a good illustration of how to implement this EOL framework. He was deemed to have the capacity to make decisions regarding discharge (Step 1). We determined his risk factors and protective factors for discharge (Step 2). His poor functional status, limited instrumental support, heavy alcohol use, rejection of home services, and communication barriers due to severe hearing impairment all increased his risk. Protective factors included an appreciation of functional limitations, intact cognition, and an involved HCA. Based on his limited instrumental support and poor function but good insight into limitations, discharge home was deemed to be of moderate risk (Step 3). Although risk factors such as alcohol use and severe hearing impairment could have raised his level to high risk, we felt that his involved HCA maintained him in the moderate-risk category.
We worked with the hospitalist team, PT, and audiology to identify multiple risk mitigation strategies: frequent phone calls between the HCA and outpatient palliative care team, home PT to improve transfers from bed to bedside commode, home nursing services either through a routine agency or hospice, and hearing aids for better communication (Steps 4 and 5). We then proposed these strategies to Joe and his HCA (Step 6). Due to concerns about infringement on his independence, Joe declined all home services but agreed to twice-daily check-ins by his HCA, frequent communication between his HCA and our team, and new hearing aids.
Joe returned home with the agreed-upon risk mitigation strategies in place (Step 7). Despite clinicians’ original reservations about sending Joe home without formal services, his HCA maintained close contact with our team, noting that Joe remained stable and happy to be at home in the months following discharge.
Conclusions
Fortunately, VA HCPs operate in an integrated health care system with access to psychological, social, and at-home medical support that can help mitigate risks. Still, we have benefitted from having a tool to help us evaluate risk systematically. Even if patients, families, and HCPs disagree on ideal discharge plans, this tool helps clinicians approach discharges methodically while maintaining open communication and partnership with patients. In doing so, our framework reflects the shift in medical culture from a patriarchal approach to shared decision-making practices regarding all aspects of medical care. Furthermore, we hope that this can help reduce clinician moral distress stemming from these challenging cases.
Future research on best practices for discharge risk assessment and optimizing home safety are needed. We also hope to evaluate the impact and effectiveness of our framework through interviews with key stakeholders. For Joe and other veterans like him, where to spend their final days may be the last important decision they make in life, and our framework allows for their voices to be better heard throughout the decision-making process.
Acknowledgments
We thank Brooke Lifland, MD, for her theoretical contributions to the concept behind this paper.
1. Committee on Approaching Death: Addressing Key End of Life Issues; Institute of Medicine. Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Washington (DC): National Academies Press (US); March 19, 2015.
2. Casarett D, Pickard A, Amos Bailey F, et al. Important aspects of end-of-life care among veterans: implications for measurement and quality improvement. J Pain Symptom Manage. 2008;35(2):115-125. doi:10.1016/j.jpainsymman.2007.03.008
3. Kolva E, Rosenfeld B, Brescia R, Comfort C. Assessing decision-making capacity at end of life. Gen Hosp Psychiatry. 2014;36(4):392-397. doi:10.1016/j.genhosppsych.2014.02.013
4. Kolva E, Rosenfeld B, Saracino R. Assessing the decision-making capacity of terminally ill patients with cancer. Am J Geriatr Psychiatry. 2018;26(5):523-531. doi:10.1016/j.jagp.2017.11.012
5. Macmillan MS. Hospital staff’s perceptions of risk associated with the discharge of elderly people from acute hospital care. J Adv Nurs. 1994;19(2):249-256. doi:10.1111/j.1365-2648.1994.tb01078.x
6. Coombs MA, Parker R, de Vries K. Managing risk during care transitions when approaching end of life: A qualitative study of patients’ and health care professionals’ decision making. Palliat Med. 2017;31(7):617-624. doi:10.1177/0269216316673476
7. Hyslop B. ‘Not safe for discharge’? Words, values, and person-centred care. Age Ageing. 2020;49(3):334-336. doi:10.1093/ageing/afz170
8. Goodacre S. Safe discharge: an irrational, unhelpful and unachievable concept. Emerg Med J. 2006;23(10):753-755. doi:10.1136/emj.2006.037903
9. Swidler RN, Seastrum T, Shelton W. Difficult hospital inpatient discharge decisions: ethical, legal and clinical practice issues. Am J Bioeth. 2007;7(3):23-28. doi:10.1080/15265160601171739
10. Hill J, Filer W. Safety and ethical considerations in discharging patients to suboptimal living situations. AMA J Ethics. 2015;17(6):506-510. Published 2015 Jun 1. doi:10.1001/journalofethics.2015.17.6.ecas2-1506
11. West JC. What is an ethically informed approach to managing patient safety risk during discharge planning?. AMA J Ethics. 2020;22(11):E919-E923. Published 2020 Nov 1. doi:10.1001/amajethics.2020.919
12. Mukherjee D. Discharge decisions and the dignity of risk. Hastings Cent Rep. 2015;45(3):7-8. doi:10.1002/hast.441
13. Wheatley VJ, Baker JI. “Please, I want to go home”: ethical issues raised when considering choice of place of care in palliative care. Postgrad Med J. 2007;83(984):643-648. doi:10.1136/pgmj.2007.058487
14. Work Group on Suicidal Behaviors. Practice guideline for the assessment and treatment of patients with suicidal behaviors. Am J Psychiatry. 2003;160(suppl 11):1-60.
1. Committee on Approaching Death: Addressing Key End of Life Issues; Institute of Medicine. Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Washington (DC): National Academies Press (US); March 19, 2015.
2. Casarett D, Pickard A, Amos Bailey F, et al. Important aspects of end-of-life care among veterans: implications for measurement and quality improvement. J Pain Symptom Manage. 2008;35(2):115-125. doi:10.1016/j.jpainsymman.2007.03.008
3. Kolva E, Rosenfeld B, Brescia R, Comfort C. Assessing decision-making capacity at end of life. Gen Hosp Psychiatry. 2014;36(4):392-397. doi:10.1016/j.genhosppsych.2014.02.013
4. Kolva E, Rosenfeld B, Saracino R. Assessing the decision-making capacity of terminally ill patients with cancer. Am J Geriatr Psychiatry. 2018;26(5):523-531. doi:10.1016/j.jagp.2017.11.012
5. Macmillan MS. Hospital staff’s perceptions of risk associated with the discharge of elderly people from acute hospital care. J Adv Nurs. 1994;19(2):249-256. doi:10.1111/j.1365-2648.1994.tb01078.x
6. Coombs MA, Parker R, de Vries K. Managing risk during care transitions when approaching end of life: A qualitative study of patients’ and health care professionals’ decision making. Palliat Med. 2017;31(7):617-624. doi:10.1177/0269216316673476
7. Hyslop B. ‘Not safe for discharge’? Words, values, and person-centred care. Age Ageing. 2020;49(3):334-336. doi:10.1093/ageing/afz170
8. Goodacre S. Safe discharge: an irrational, unhelpful and unachievable concept. Emerg Med J. 2006;23(10):753-755. doi:10.1136/emj.2006.037903
9. Swidler RN, Seastrum T, Shelton W. Difficult hospital inpatient discharge decisions: ethical, legal and clinical practice issues. Am J Bioeth. 2007;7(3):23-28. doi:10.1080/15265160601171739
10. Hill J, Filer W. Safety and ethical considerations in discharging patients to suboptimal living situations. AMA J Ethics. 2015;17(6):506-510. Published 2015 Jun 1. doi:10.1001/journalofethics.2015.17.6.ecas2-1506
11. West JC. What is an ethically informed approach to managing patient safety risk during discharge planning?. AMA J Ethics. 2020;22(11):E919-E923. Published 2020 Nov 1. doi:10.1001/amajethics.2020.919
12. Mukherjee D. Discharge decisions and the dignity of risk. Hastings Cent Rep. 2015;45(3):7-8. doi:10.1002/hast.441
13. Wheatley VJ, Baker JI. “Please, I want to go home”: ethical issues raised when considering choice of place of care in palliative care. Postgrad Med J. 2007;83(984):643-648. doi:10.1136/pgmj.2007.058487
14. Work Group on Suicidal Behaviors. Practice guideline for the assessment and treatment of patients with suicidal behaviors. Am J Psychiatry. 2003;160(suppl 11):1-60.