User login
Altered gut bacteria a biomarker of preclinical Alzheimer’s?
The findings open up the possibility of analyzing the gut microbiome to identify individuals at a higher risk for dementia and perhaps designing microbiome-altering preventive treatments to help stave off cognitive decline, researchers noted.
Study investigator Gautam Dantas, PhD, cautioned that it’s not known whether the gut is influencing the brain, or the brain is influencing the gut, “but this association is valuable to know in either case.
“It could be that the changes in the gut microbiome are just a readout of pathological changes in the brain. The other alternative is that the gut microbiome is contributing to AD, in which case, altering the gut microbiome with probiotics or fecal transfers might help change the course of the disease,” Dr. Dantas, Washington University, St. Louis, said in a news release.
The study was published online in Science Translational Medicine.
Stool test?
Multiple lines of evidence suggest a role for gut microbes in the evolution of AD pathogenesis. However, less is known about gut microbiome changes in the preclinical (presymptomatic) phase of AD.
To investigate, Dr. Dantas and colleagues studied 164 cognitively normal adults, 49 of whom had biomarker evidence of preclinical AD.
After the researchers accounted for clinical covariates and diet, those with preclinical AD had distinct gut microbial taxonomic profiles compared with their healthy controls.
The observed microbiome features correlated with amyloid and tau but not neurodegeneration biomarkers, “suggesting that the gut microbial community changes early in the disease process,” the researchers suggested.
They identified specific taxa that were associated with preclinical AD and including these microbiome features improved the accuracy, sensitivity, and specificity of machine learning classifiers for predicting preclinical AD status.
The findings suggest “markers in the stool might complement early screening measures for preclinical AD,” the researchers noted.
“The nice thing about using the gut microbiome as a screening tool is its simplicity and ease,” Beau Ances, MD, PhD, professor of neurology, at Washington University, St. Louis, said in the release.
“One day, individuals may be able to provide a stool sample and find out if they are at increased risk for developing AD. It would be much easier and less invasive and more accessible for a large proportion of the population, especially underrepresented groups, compared to brain scans or spinal taps,” Dr. Ances added.
The researchers have launched a 5-year follow-up study designed to help determine whether the differences in the gut microbiome are a cause or a result of the brain changes seen in early AD.
Caveats, cautionary notes
In a comment, Claire Sexton, DPhil, Alzheimer’s Association senior director of scientific programs and outreach, cautioned that the study design means that it’s “not possible to prove one thing causes another. What it can show is that two or more aspects are in some way related, thus setting the stage for further research.”
Dr. Sexton noted that though the authors accounted for a number of variables in their models, including age, sex, race, education, body mass index, hypertension, and diabetes, and observed no differences in intake of any major nutrient group, “it’s still not possible to rule out that additional factors beyond the variations in gut microbiome contributed to the changes in brain markers of Alzheimer’s.”
Dr. Sexton also noted that the study population is not representative of all people living with AD, with the vast majority of those with preclinical AD in the study being White.
“If these findings are replicated and confirmed in study groups that are representative of our communities, it is possible that gut microbiome signatures could be a further addition to the suite of diagnostic tools employed in certain settings,” Dr. Sexton said.
This research was supported by the Infection Disease Society of America Foundation, the National Institute on Aging, the Brennan Fund and the Paula and Rodger Riney Foundation. Dr. Dantas, Dr. Ances and Dr. Sexton have no relevant disclosures.
A version of this article first appeared on Medscape.com.
The findings open up the possibility of analyzing the gut microbiome to identify individuals at a higher risk for dementia and perhaps designing microbiome-altering preventive treatments to help stave off cognitive decline, researchers noted.
Study investigator Gautam Dantas, PhD, cautioned that it’s not known whether the gut is influencing the brain, or the brain is influencing the gut, “but this association is valuable to know in either case.
“It could be that the changes in the gut microbiome are just a readout of pathological changes in the brain. The other alternative is that the gut microbiome is contributing to AD, in which case, altering the gut microbiome with probiotics or fecal transfers might help change the course of the disease,” Dr. Dantas, Washington University, St. Louis, said in a news release.
The study was published online in Science Translational Medicine.
Stool test?
Multiple lines of evidence suggest a role for gut microbes in the evolution of AD pathogenesis. However, less is known about gut microbiome changes in the preclinical (presymptomatic) phase of AD.
To investigate, Dr. Dantas and colleagues studied 164 cognitively normal adults, 49 of whom had biomarker evidence of preclinical AD.
After the researchers accounted for clinical covariates and diet, those with preclinical AD had distinct gut microbial taxonomic profiles compared with their healthy controls.
The observed microbiome features correlated with amyloid and tau but not neurodegeneration biomarkers, “suggesting that the gut microbial community changes early in the disease process,” the researchers suggested.
They identified specific taxa that were associated with preclinical AD and including these microbiome features improved the accuracy, sensitivity, and specificity of machine learning classifiers for predicting preclinical AD status.
The findings suggest “markers in the stool might complement early screening measures for preclinical AD,” the researchers noted.
“The nice thing about using the gut microbiome as a screening tool is its simplicity and ease,” Beau Ances, MD, PhD, professor of neurology, at Washington University, St. Louis, said in the release.
“One day, individuals may be able to provide a stool sample and find out if they are at increased risk for developing AD. It would be much easier and less invasive and more accessible for a large proportion of the population, especially underrepresented groups, compared to brain scans or spinal taps,” Dr. Ances added.
The researchers have launched a 5-year follow-up study designed to help determine whether the differences in the gut microbiome are a cause or a result of the brain changes seen in early AD.
Caveats, cautionary notes
In a comment, Claire Sexton, DPhil, Alzheimer’s Association senior director of scientific programs and outreach, cautioned that the study design means that it’s “not possible to prove one thing causes another. What it can show is that two or more aspects are in some way related, thus setting the stage for further research.”
Dr. Sexton noted that though the authors accounted for a number of variables in their models, including age, sex, race, education, body mass index, hypertension, and diabetes, and observed no differences in intake of any major nutrient group, “it’s still not possible to rule out that additional factors beyond the variations in gut microbiome contributed to the changes in brain markers of Alzheimer’s.”
Dr. Sexton also noted that the study population is not representative of all people living with AD, with the vast majority of those with preclinical AD in the study being White.
“If these findings are replicated and confirmed in study groups that are representative of our communities, it is possible that gut microbiome signatures could be a further addition to the suite of diagnostic tools employed in certain settings,” Dr. Sexton said.
This research was supported by the Infection Disease Society of America Foundation, the National Institute on Aging, the Brennan Fund and the Paula and Rodger Riney Foundation. Dr. Dantas, Dr. Ances and Dr. Sexton have no relevant disclosures.
A version of this article first appeared on Medscape.com.
The findings open up the possibility of analyzing the gut microbiome to identify individuals at a higher risk for dementia and perhaps designing microbiome-altering preventive treatments to help stave off cognitive decline, researchers noted.
Study investigator Gautam Dantas, PhD, cautioned that it’s not known whether the gut is influencing the brain, or the brain is influencing the gut, “but this association is valuable to know in either case.
“It could be that the changes in the gut microbiome are just a readout of pathological changes in the brain. The other alternative is that the gut microbiome is contributing to AD, in which case, altering the gut microbiome with probiotics or fecal transfers might help change the course of the disease,” Dr. Dantas, Washington University, St. Louis, said in a news release.
The study was published online in Science Translational Medicine.
Stool test?
Multiple lines of evidence suggest a role for gut microbes in the evolution of AD pathogenesis. However, less is known about gut microbiome changes in the preclinical (presymptomatic) phase of AD.
To investigate, Dr. Dantas and colleagues studied 164 cognitively normal adults, 49 of whom had biomarker evidence of preclinical AD.
After the researchers accounted for clinical covariates and diet, those with preclinical AD had distinct gut microbial taxonomic profiles compared with their healthy controls.
The observed microbiome features correlated with amyloid and tau but not neurodegeneration biomarkers, “suggesting that the gut microbial community changes early in the disease process,” the researchers suggested.
They identified specific taxa that were associated with preclinical AD and including these microbiome features improved the accuracy, sensitivity, and specificity of machine learning classifiers for predicting preclinical AD status.
The findings suggest “markers in the stool might complement early screening measures for preclinical AD,” the researchers noted.
“The nice thing about using the gut microbiome as a screening tool is its simplicity and ease,” Beau Ances, MD, PhD, professor of neurology, at Washington University, St. Louis, said in the release.
“One day, individuals may be able to provide a stool sample and find out if they are at increased risk for developing AD. It would be much easier and less invasive and more accessible for a large proportion of the population, especially underrepresented groups, compared to brain scans or spinal taps,” Dr. Ances added.
The researchers have launched a 5-year follow-up study designed to help determine whether the differences in the gut microbiome are a cause or a result of the brain changes seen in early AD.
Caveats, cautionary notes
In a comment, Claire Sexton, DPhil, Alzheimer’s Association senior director of scientific programs and outreach, cautioned that the study design means that it’s “not possible to prove one thing causes another. What it can show is that two or more aspects are in some way related, thus setting the stage for further research.”
Dr. Sexton noted that though the authors accounted for a number of variables in their models, including age, sex, race, education, body mass index, hypertension, and diabetes, and observed no differences in intake of any major nutrient group, “it’s still not possible to rule out that additional factors beyond the variations in gut microbiome contributed to the changes in brain markers of Alzheimer’s.”
Dr. Sexton also noted that the study population is not representative of all people living with AD, with the vast majority of those with preclinical AD in the study being White.
“If these findings are replicated and confirmed in study groups that are representative of our communities, it is possible that gut microbiome signatures could be a further addition to the suite of diagnostic tools employed in certain settings,” Dr. Sexton said.
This research was supported by the Infection Disease Society of America Foundation, the National Institute on Aging, the Brennan Fund and the Paula and Rodger Riney Foundation. Dr. Dantas, Dr. Ances and Dr. Sexton have no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM SCIENCE TRANSLATIONAL MEDICINE
‘Deprescribing’: Should some older adults shed their meds?
Joanne Lynn, MD, has lost track of the number of times in her 40 years as a geriatrician she’s seen a new patient come to her office carrying a bucket full of prescription medications – many of which they don’t need.
Dr. Lynn, who is on the faculty of George Washington University,Washington, recalled one woman who unwittingly was taking two blood pressure medications with different names.
“The risks included all the side effects overdosing carries,” Dr. Lynn said, ranging from blurred vision and crankiness to organ failure and even death.
For doctors with patients who don’t know they’re taking too much of a medication, “you wonder whether the drug is causing the health problems, and it’s a symptom of the wrong medication,” rather than a symptom of an undiagnosed illness, she said.
Patients often assume their health providers check for drug interactions or assess if a medication is no longer needed, and will catch extra prescriptions. That could be a risky assumption. Some doctors may prescribe yet another prescription to manage the side effects of an unnecessary drug, instead of doing a medication review and potentially “deprescribing” or discontinuing, a treatment that’s no longer needed.
About 57% of people age 65 years or older take five or more medications regularly – a concept known as polypharmacy, a study published in 2020 in the Journal of the American Geriatrics Society shows. While doctors prescribe drugs to help patients manage various ailments, as a list of medications grows, so do potential complications.
An older adult might forget to tell their doctor what they’re taking, or maybe they don’t even know what they’re taking or why, Dr. Lynn said.
“In some cases, a doctor just added a drug to treat something, not realizing they were already taking something else for it,” she said. “Of course, the situation of whether these patients can even afford all these drugs matters a lot, too.”
Some older adults may pick and choose which medications to take based on cost, not knowing which prescriptions are necessary, Dr. Lynn said.
Finding the ‘right balance’
Indeed, if given the option, up to 80% of older adults ages 50-80 would be open to stopping one or more of their prescribed medications, according to a 2023 poll by researchers at the University of Michigan, Ann Arbor.
“A lot of drugs that people take might have been appropriate at one point, but might have outlived their usefulness for that individual,” said Michael Steinman, MD, a professor of medicine and a geriatrician at the University of California, San Francisco, and coprincipal investigator of the U.S. Deprescribing Research Network, a doctor group focused on improving medication use for older adults.
“Having fewer medications can actually be beneficial,” he said. “You can take too many medications; you can take too few. The optimal thing is finding what is the right balance for you.”
Defining how many medications is too many depends on each person, which is why caregivers and older adults can ask their doctor for a review of medications that have multiplied over time.
By reevaluating their medications, older adults can actually lower their chances of potentially harmful side effects, and avoid the spiral of being prescribed even more medications, said Sarah Vordenberg, PharmD, MPH, a clinical associate professor at the University of Michigan’s College of Pharmacy, Ann Arbor.
“It’s not really the number of medications, it’s [about] are they inappropriate or unnecessary medications for a patient,” she said.
Patients and caregivers can ask for an honest conversation with their doctor. The University of Michigan poll found that more than 90% of older adults who took prescription medications expected their health care provider to review their medicines during a regular visit.
But doctors often need prompting from patients to start a review.
“The clinical inertia, or maintaining the status quo, unfortunately is a lot of times easier than having time-intensive conversations,” Dr. Vordenberg said.
Ask questions
Sara Merwin spent many years helping manage her parents’ medical appointments and health as they transitioned from living independently in Colorado to a retirement community and finally a nursing home. Ms. Merwin, coauthor of “The Informed Patient,” said her father was taking a long list of medications, and she often asked his primary care doctor for a medication review.
“I felt that my father at his age and his frailty didn’t need as many meds as he was on,” said Ms. Merwin, who lives in Long Island, N.Y. “So we went over his meds, and I asked, ‘Does he really need to be on this?’ ‘Does he really need to be on that?’ ”
She questioned one medication in particular, a statin to lower his cholesterol and risk of a heart attack.
“I thought possibly the statin was causing some myalgia, some muscle aches in his legs, which is why I advocated for coming off it,” she said.
The primary care doctor discontinued the anticholesterol drug.
Local pharmacies can also serve as a starting point for older adults and caregivers, where a pharmacist can give them more information on whether a particular combination of the medications taken may be harmful. In states that allow for pharmacists to prescribe some medications, pharmacists may be able to consolidate some of the medications or advise that a patient stop taking one or more, Dr. Vordenberg said.
“All pharmacists have the training to do a comprehensive medication review,” she said. “All pharmacists have the ability to follow up with the patient to find out how the deprescribing is going.”
Ms. Merwin’s parents received their prescriptions from a “small mom-and-pop pharmacy, where they were on a first-name basis with the pharmacist who really looked out for them. So they had that expertise available to them,” she said.
With information in hand on potentially unnecessary medications, the work of shedding medications should be done along with health care providers, some of whom prescribed the medications in the first place.
Many older adults live in geographically isolated areas without pharmacies, or receive prescriptions from mail-order pharmacies. In this case, Medicare plans offer free medication reviews with a doctor or pharmacist – known as a medication therapy management program – and provide recommendations for taking each drug.
Ms. Merwin’s father died in early 2020. She sometimes questions whether he should have stayed on the statin for longer, or if the doctor agreed too quickly without doing more research. But overall, she doesn’t regret raising the question with his health care providers, and she advises other caregivers and older adults to pay attention to medication lists.
“It’s dangerous to be passive when it comes to one’s health care now,” Ms. Merwin said. “That’s a difficult message for older adults to hear because they have grown up with the primacy of the doctor and the authority of the doctor, as opposed to it being a collaborative relationship.”
A version of this article first appeared on WebMD.com.
Joanne Lynn, MD, has lost track of the number of times in her 40 years as a geriatrician she’s seen a new patient come to her office carrying a bucket full of prescription medications – many of which they don’t need.
Dr. Lynn, who is on the faculty of George Washington University,Washington, recalled one woman who unwittingly was taking two blood pressure medications with different names.
“The risks included all the side effects overdosing carries,” Dr. Lynn said, ranging from blurred vision and crankiness to organ failure and even death.
For doctors with patients who don’t know they’re taking too much of a medication, “you wonder whether the drug is causing the health problems, and it’s a symptom of the wrong medication,” rather than a symptom of an undiagnosed illness, she said.
Patients often assume their health providers check for drug interactions or assess if a medication is no longer needed, and will catch extra prescriptions. That could be a risky assumption. Some doctors may prescribe yet another prescription to manage the side effects of an unnecessary drug, instead of doing a medication review and potentially “deprescribing” or discontinuing, a treatment that’s no longer needed.
About 57% of people age 65 years or older take five or more medications regularly – a concept known as polypharmacy, a study published in 2020 in the Journal of the American Geriatrics Society shows. While doctors prescribe drugs to help patients manage various ailments, as a list of medications grows, so do potential complications.
An older adult might forget to tell their doctor what they’re taking, or maybe they don’t even know what they’re taking or why, Dr. Lynn said.
“In some cases, a doctor just added a drug to treat something, not realizing they were already taking something else for it,” she said. “Of course, the situation of whether these patients can even afford all these drugs matters a lot, too.”
Some older adults may pick and choose which medications to take based on cost, not knowing which prescriptions are necessary, Dr. Lynn said.
Finding the ‘right balance’
Indeed, if given the option, up to 80% of older adults ages 50-80 would be open to stopping one or more of their prescribed medications, according to a 2023 poll by researchers at the University of Michigan, Ann Arbor.
“A lot of drugs that people take might have been appropriate at one point, but might have outlived their usefulness for that individual,” said Michael Steinman, MD, a professor of medicine and a geriatrician at the University of California, San Francisco, and coprincipal investigator of the U.S. Deprescribing Research Network, a doctor group focused on improving medication use for older adults.
“Having fewer medications can actually be beneficial,” he said. “You can take too many medications; you can take too few. The optimal thing is finding what is the right balance for you.”
Defining how many medications is too many depends on each person, which is why caregivers and older adults can ask their doctor for a review of medications that have multiplied over time.
By reevaluating their medications, older adults can actually lower their chances of potentially harmful side effects, and avoid the spiral of being prescribed even more medications, said Sarah Vordenberg, PharmD, MPH, a clinical associate professor at the University of Michigan’s College of Pharmacy, Ann Arbor.
“It’s not really the number of medications, it’s [about] are they inappropriate or unnecessary medications for a patient,” she said.
Patients and caregivers can ask for an honest conversation with their doctor. The University of Michigan poll found that more than 90% of older adults who took prescription medications expected their health care provider to review their medicines during a regular visit.
But doctors often need prompting from patients to start a review.
“The clinical inertia, or maintaining the status quo, unfortunately is a lot of times easier than having time-intensive conversations,” Dr. Vordenberg said.
Ask questions
Sara Merwin spent many years helping manage her parents’ medical appointments and health as they transitioned from living independently in Colorado to a retirement community and finally a nursing home. Ms. Merwin, coauthor of “The Informed Patient,” said her father was taking a long list of medications, and she often asked his primary care doctor for a medication review.
“I felt that my father at his age and his frailty didn’t need as many meds as he was on,” said Ms. Merwin, who lives in Long Island, N.Y. “So we went over his meds, and I asked, ‘Does he really need to be on this?’ ‘Does he really need to be on that?’ ”
She questioned one medication in particular, a statin to lower his cholesterol and risk of a heart attack.
“I thought possibly the statin was causing some myalgia, some muscle aches in his legs, which is why I advocated for coming off it,” she said.
The primary care doctor discontinued the anticholesterol drug.
Local pharmacies can also serve as a starting point for older adults and caregivers, where a pharmacist can give them more information on whether a particular combination of the medications taken may be harmful. In states that allow for pharmacists to prescribe some medications, pharmacists may be able to consolidate some of the medications or advise that a patient stop taking one or more, Dr. Vordenberg said.
“All pharmacists have the training to do a comprehensive medication review,” she said. “All pharmacists have the ability to follow up with the patient to find out how the deprescribing is going.”
Ms. Merwin’s parents received their prescriptions from a “small mom-and-pop pharmacy, where they were on a first-name basis with the pharmacist who really looked out for them. So they had that expertise available to them,” she said.
With information in hand on potentially unnecessary medications, the work of shedding medications should be done along with health care providers, some of whom prescribed the medications in the first place.
Many older adults live in geographically isolated areas without pharmacies, or receive prescriptions from mail-order pharmacies. In this case, Medicare plans offer free medication reviews with a doctor or pharmacist – known as a medication therapy management program – and provide recommendations for taking each drug.
Ms. Merwin’s father died in early 2020. She sometimes questions whether he should have stayed on the statin for longer, or if the doctor agreed too quickly without doing more research. But overall, she doesn’t regret raising the question with his health care providers, and she advises other caregivers and older adults to pay attention to medication lists.
“It’s dangerous to be passive when it comes to one’s health care now,” Ms. Merwin said. “That’s a difficult message for older adults to hear because they have grown up with the primacy of the doctor and the authority of the doctor, as opposed to it being a collaborative relationship.”
A version of this article first appeared on WebMD.com.
Joanne Lynn, MD, has lost track of the number of times in her 40 years as a geriatrician she’s seen a new patient come to her office carrying a bucket full of prescription medications – many of which they don’t need.
Dr. Lynn, who is on the faculty of George Washington University,Washington, recalled one woman who unwittingly was taking two blood pressure medications with different names.
“The risks included all the side effects overdosing carries,” Dr. Lynn said, ranging from blurred vision and crankiness to organ failure and even death.
For doctors with patients who don’t know they’re taking too much of a medication, “you wonder whether the drug is causing the health problems, and it’s a symptom of the wrong medication,” rather than a symptom of an undiagnosed illness, she said.
Patients often assume their health providers check for drug interactions or assess if a medication is no longer needed, and will catch extra prescriptions. That could be a risky assumption. Some doctors may prescribe yet another prescription to manage the side effects of an unnecessary drug, instead of doing a medication review and potentially “deprescribing” or discontinuing, a treatment that’s no longer needed.
About 57% of people age 65 years or older take five or more medications regularly – a concept known as polypharmacy, a study published in 2020 in the Journal of the American Geriatrics Society shows. While doctors prescribe drugs to help patients manage various ailments, as a list of medications grows, so do potential complications.
An older adult might forget to tell their doctor what they’re taking, or maybe they don’t even know what they’re taking or why, Dr. Lynn said.
“In some cases, a doctor just added a drug to treat something, not realizing they were already taking something else for it,” she said. “Of course, the situation of whether these patients can even afford all these drugs matters a lot, too.”
Some older adults may pick and choose which medications to take based on cost, not knowing which prescriptions are necessary, Dr. Lynn said.
Finding the ‘right balance’
Indeed, if given the option, up to 80% of older adults ages 50-80 would be open to stopping one or more of their prescribed medications, according to a 2023 poll by researchers at the University of Michigan, Ann Arbor.
“A lot of drugs that people take might have been appropriate at one point, but might have outlived their usefulness for that individual,” said Michael Steinman, MD, a professor of medicine and a geriatrician at the University of California, San Francisco, and coprincipal investigator of the U.S. Deprescribing Research Network, a doctor group focused on improving medication use for older adults.
“Having fewer medications can actually be beneficial,” he said. “You can take too many medications; you can take too few. The optimal thing is finding what is the right balance for you.”
Defining how many medications is too many depends on each person, which is why caregivers and older adults can ask their doctor for a review of medications that have multiplied over time.
By reevaluating their medications, older adults can actually lower their chances of potentially harmful side effects, and avoid the spiral of being prescribed even more medications, said Sarah Vordenberg, PharmD, MPH, a clinical associate professor at the University of Michigan’s College of Pharmacy, Ann Arbor.
“It’s not really the number of medications, it’s [about] are they inappropriate or unnecessary medications for a patient,” she said.
Patients and caregivers can ask for an honest conversation with their doctor. The University of Michigan poll found that more than 90% of older adults who took prescription medications expected their health care provider to review their medicines during a regular visit.
But doctors often need prompting from patients to start a review.
“The clinical inertia, or maintaining the status quo, unfortunately is a lot of times easier than having time-intensive conversations,” Dr. Vordenberg said.
Ask questions
Sara Merwin spent many years helping manage her parents’ medical appointments and health as they transitioned from living independently in Colorado to a retirement community and finally a nursing home. Ms. Merwin, coauthor of “The Informed Patient,” said her father was taking a long list of medications, and she often asked his primary care doctor for a medication review.
“I felt that my father at his age and his frailty didn’t need as many meds as he was on,” said Ms. Merwin, who lives in Long Island, N.Y. “So we went over his meds, and I asked, ‘Does he really need to be on this?’ ‘Does he really need to be on that?’ ”
She questioned one medication in particular, a statin to lower his cholesterol and risk of a heart attack.
“I thought possibly the statin was causing some myalgia, some muscle aches in his legs, which is why I advocated for coming off it,” she said.
The primary care doctor discontinued the anticholesterol drug.
Local pharmacies can also serve as a starting point for older adults and caregivers, where a pharmacist can give them more information on whether a particular combination of the medications taken may be harmful. In states that allow for pharmacists to prescribe some medications, pharmacists may be able to consolidate some of the medications or advise that a patient stop taking one or more, Dr. Vordenberg said.
“All pharmacists have the training to do a comprehensive medication review,” she said. “All pharmacists have the ability to follow up with the patient to find out how the deprescribing is going.”
Ms. Merwin’s parents received their prescriptions from a “small mom-and-pop pharmacy, where they were on a first-name basis with the pharmacist who really looked out for them. So they had that expertise available to them,” she said.
With information in hand on potentially unnecessary medications, the work of shedding medications should be done along with health care providers, some of whom prescribed the medications in the first place.
Many older adults live in geographically isolated areas without pharmacies, or receive prescriptions from mail-order pharmacies. In this case, Medicare plans offer free medication reviews with a doctor or pharmacist – known as a medication therapy management program – and provide recommendations for taking each drug.
Ms. Merwin’s father died in early 2020. She sometimes questions whether he should have stayed on the statin for longer, or if the doctor agreed too quickly without doing more research. But overall, she doesn’t regret raising the question with his health care providers, and she advises other caregivers and older adults to pay attention to medication lists.
“It’s dangerous to be passive when it comes to one’s health care now,” Ms. Merwin said. “That’s a difficult message for older adults to hear because they have grown up with the primacy of the doctor and the authority of the doctor, as opposed to it being a collaborative relationship.”
A version of this article first appeared on WebMD.com.
Caring for the caregiver in dementia
THE CASE
Sam C* is a 68-year-old man who presented to his family physician in a rural health clinic due to concerns about weight loss. Since his visit 8 months prior, Mr. C unintentionally had lost 20 pounds. Upon questioning, Mr. C also reported feeling irritable and having difficulty with sleep and concentration.
A review of systems did not indicate the presence of infection or other medical conditions. In the 6 years since becoming a patient to the practice, he had reported no chronic health concerns, was taking no medications, and had only been to the clinic for his annual check-up appointments. He completed a Patient Health Questionnaire (PHQ-9) and scored 18, indicating moderately severe depression.
Mr. C had established care with his physician when he moved to the area from out of state so that he could be closer to his parents, who were in their mid-80s at the time. Mr. C’s physician also had been the family physician for his parents for the previous 20 years. Three years prior to Mr. C’s presentation for weight loss, his mother had received a diagnosis of acute leukemia; she died a year later.
Over the past year, Mr. C had needed to take a more active role in the care of his father, who was now in his early 90s. Mr. C’s father, who was previously in excellent health, had begun to develop significant health problems, including degenerative arthritis and progressive vascular dementia. He also had ataxia, leading to poor mobility, and a neurogenic bladder requiring self-catheterization, which required Mr. C’s assistance. Mr. C lived next door to his father and provided frequent assistance with activities of daily living. However, his father, who always had been the dominant figure in the family, was determined to maintain his independence and not relinquish control to others.
The strain of caregiving activities, along with managing his father’s inflexibility, was causing increasing distress for Mr. C. As he told his family physician, “I just don’t know what to do.”
●
* The patient’s name has been changed to protect his identity.
It is estimated that more than 11 million Americans provided more than 18 billion hours in unpaid support for individuals with dementia in 2022, averaging 30 hours of care per caregiver per week.1 As individuals with dementia progressively decline, they require increased assistance with activities of daily living (ADLs, such as bathing and dressing) and instrumental activities of daily living (IADLs, such as paying bills and using transportation). Most of this assistance comes from informal caregiving provided by family members and friends.
Caregiver burden can be defined as “the strain or load borne by a person who cares for a chronically ill, disabled, or elderly family member.”2 Caregiver stress has been found to be higher for dementia caregiving than other types of caregiving.3 In particular, caring for someone with greater behavioral and psychological symptoms of dementia (BPSDs) has been associated with higher caregiver burden.4-
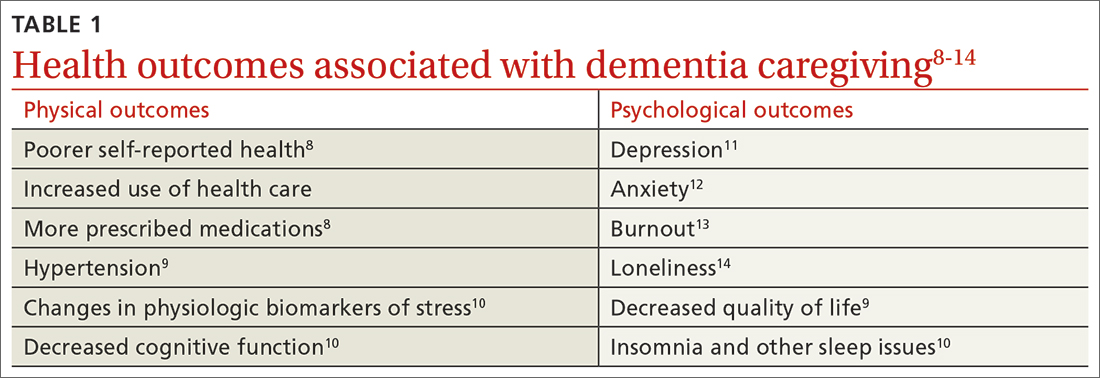
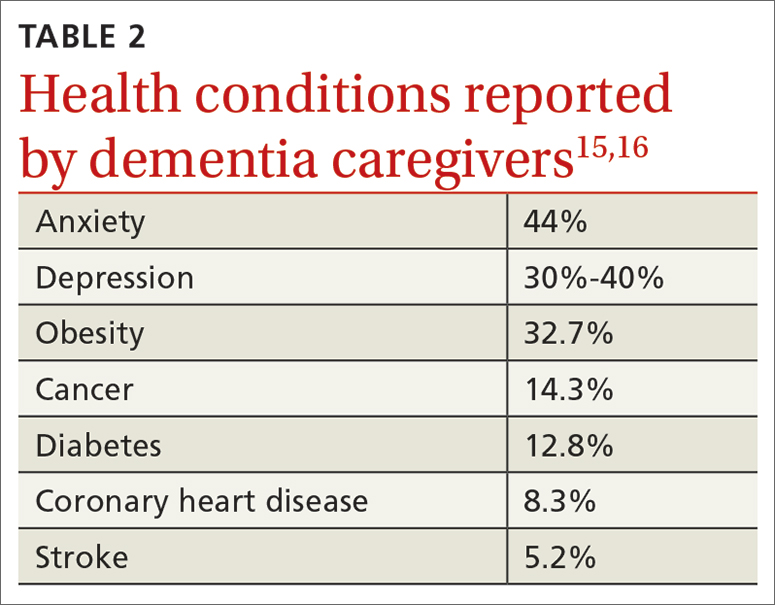
Beyond the subjective burden of caregiving, there are other potential negative consequences for dementia caregivers (see TABLE 18-14 and TABLE 215,16). In addition, caregiver distress is related to a number of care recipient outcomes, including earlier institutionalization, more hospitalizations, more BPSDs, poorer quality of life, and greater likelihood of experiencing elder abuse.17
Assessment, reassessment are key to meeting needs
Numerous factors can foster caregiver well-being, including feelings of accomplishment and contribution, a strengthening of the relationship with the care recipient, and feeling supported by friends, family, and formal care systems.18,19 Family physicians can play an important role by assessing and supporting patients with dementia and their caregivers. Ideally, the individual with dementia and the caregiver will be assessed both together and separately.
A thorough assessment includes gathering information about the context and quality of the caregiving relationship; caregiver perception of the care recipient’s health and functional status; caregiver values and preferences; caregiver well-being (including mental health assessment); caregiver skills, abilities, and knowledge about caregiving; and positive and negative consequences of caregiving.20 Caregiver needs—including informational, care support, emotional, physical, and social needs—also should be assessed.
Continue to: Tools are available...
Tools are available to facilitate caregiver assessment. For example, the Zarit Burden Interview is a 22-item self-report measure that can be given to the caregiver21; shorter versions (4 and 12 items) are also available.22 Another resource available for caregiver assessment guidance is a toolkit developed by the Family Caregiver Alliance.20
Continually assess for changing needs
As the condition of the individual with dementia progresses, it will be important to reassess the caregiver, as stressors and needs will change over the course of the caregiving relationship. Support should be adapted accordingly.
In the early stage of dementia, caregivers may need information on disease progression and dementia care planning, ways to navigate the health care system, financial planning, and useful resources. Caregivers also may need emotional support to help them adapt to the role of caregiver, deal with denial, and manage their stress.23,24
With dementia progression, caregivers may need support related to increased decision-making responsibility, managing challenging behaviors, assisting with ADLs and IADLs, and identifying opportunities to meet personal social and well-being needs. They also may need support to accept the changes they are seeing in the individual with dementia and the shifts they are experiencing in their relationship with him or her.23,25
In late-stage dementia, caregiver needs tend to shift to determining the need for long-term care placement vs staying at home, end-of-life planning, loneliness, and anticipatory grief.23,26 Support with managing changing and accumulating stress typically remains a primary need throughout the progression of dementia.27
Continue to: Specific populations have distinct needs
Specific populations have distinct needs. Some caregivers, including members of the LGBTQ+ community and different racial and ethnic groups, as well as caregivers of people with younger-onset dementia, may have additional support needs.28
For example, African American and Latino caregivers tend to have caregiving relationships of longer duration, requiring more time-intensive care, but use fewer formal support services than White caregivers.29 Caregivers from non-White racial and ethnic groups also are more likely to experience discrimination when interacting with health care services on behalf of care recipients.30
Having an awareness of potential specialized needs may help to prevent or address potential care disparities, and cultural humility may help to improve caregiver experiences with primary care physicians.
Resources to support caregivers
Family physicians are well situated to provide informational and emotional support for both patients with dementia and their informal care providers.31 Given the variability of caregiver concerns, multicomponent interventions addressing informational, self-care, social support, and financial needs often are needed.31 Supportive counseling and psychoeducation can help dementia caregivers with stress management, self-care, coping, and skills training—supporting the development of self-efficacy.32,33
Outside resources. Although significant caregiver support can be provided directly by the physician, caregivers should be connected with outside resources, including support groups, counselors, psychotherapists, financial and legal support, and formal care services
Continue to: Psychosocial and complementary interventions
Psychosocial and complementary interventions. Various psychosocial interventions (eg, psychoeducation, cognitive behavioral therapy, support groups) have been found to be beneficial in alleviating caregiver symptoms of depression, anxiety, and stress and improving well-being, perceived burden, and quality of life. However, systematic reviews have found variability in the degree of helpfulness of these interventions.35,36
Some caregivers and care recipients may benefit from complementary and integrative medicine referrals. Mind–body therapies such as mindfulness, yoga, and Tai Chi have shown some beneficial effects.37
Online resources. Caregivers also can be directed to online resources from organizations such as the Alzheimer’s Association (www.alz.org), the National Institutes of Health (www.alzheimers.gov), and the Family Caregiver Alliance (www.caregiver.org).
In rural settings, such as the one in which this case took place, online resources may decrease some barriers to supporting caregivers.38 Internet-based interventions also have been found to have some benefit for dementia caregivers.31,39
However, some rural locations continue to have limited reliable Internet services.40 In affected areas, a strong relationship with a primary care physician may be even more important to the well-being of caregivers, since other support services may be less accessible.41
Continue to: Impacts of the pandemic
Impacts of the pandemic. Although our case took place prior to the COVID-19 pandemic, it is important to acknowledge ways the pandemic has impacted informal dementia caregiving.
Caregiver stress, depression, and anxiety increased during the pandemic, and the need for greater home confinement and social distancing amplified the negative impact of social isolation, including loneliness, on caregivers.42,43 Caregivers often needed to increase their caregiving responsibilities and had more difficulty with care coordination due to limited access to in-person resources.43 The pandemic led to increased reliance on technology and telehealth in the support of dementia caregivers.43
THE CASE
The physician prescribed mirtazapine for Mr. C, titrating the dose as needed to address depressive symptoms and promote weight gain. The physician connected Mr. C’s father with home health services, including physical therapy for fall risk reduction. Mr. C also hired part-time support to provide additional assistance with ADLs and IADLs, allowing Mr. C to have time to attend to his own needs. Though provided with information about a local caregiver support group, Mr. C chose not to attend. The physician also assisted the family with advanced directives.
A particular challenge that occurred during care for the family was addressing Mr. C’s father’s driving capacity, considering his strong need for independence. To address this concern, a family meeting was held with Mr. C, his father, and his siblings from out of town. Although Mr. C’s father was not willing to relinquish his driver’s license during that meeting, he agreed to complete a functional driving assessment.
The physician continued to meet with Mr. C and his father together, as well as with Mr. C individually, to provide supportive counseling as needed. As the father’s dementia progressed and it became more difficult to complete office appointments, the physician transitioned to home visits to provide care until the father’s death.
After the death of Mr. C’s father, the physician continued to serve as Mr. C’s primary care provider.
Keeping the “family”in family medicine
Through longitudinal assessment, needs identification, and provision of relevant information, emotional support, and resources, family physicians can provide care that can improve the quality of life and well-being and help alleviate burden experienced by dementia caregivers. Family physicians also are positioned to provide treatments that can address the negative physical and psychological health outcomes associated with informal dementia caregiving. By building relationships with multiple family members across generations, family physicians can understand the context of caregiving dynamics and work together with individuals with dementia and their caregivers throughout disease progression, providing consistent support to the family unit.
CORRESPONDENCE
Kathleen M. Young, PhD, MPH, Novant Health Family Medicine Wilmington, 2523 Delaney Avenue, Wilmington, NC 28403; [email protected]
1. Alzheimer’s Association. 2023 Alzheimer’s Disease Facts and Figures. Alzheimers Dement. 202319:1598-1695. doi: 10.1002/alz.13016
2. Liu Z, Heffernan C, Tan J. Caregiver burden: a concept analysis. Int J of Nurs Sci. 2020;7:448-435. doi: 10.1016/j.ijnss.2020.07.012
3. Ory MG, Hoffman RR III, Yee JL, et al. Prevalence and impacts of caregiving: a detailed comparison between dementia and nondementia caregivers. Gerontologist. 1999;39:177-185. doi: 10.1093/geront/39.2.177
4. Baharudin AD, Din NC, Subramaniam P, et al. The associations between behavioral-psychological symptoms of dementia (BPSD) and coping strategy, burden of care and personality style among low-income caregivers of patients with dementia. BMC Public Health. 2019;19(suppl 4):447. doi: 10.1186/s12889-019-6868-0
5. Cheng S-T. Dementia caregiver burden: a research update and critical analysis. Curr Psychiatry Rep. 2017;19:64. doi: 10.1007/s11920-017-0818-2
6. Reed C, Belger M, Andrews JS, et al. Factors associated with long-term impact on informal caregivers during Alzheimer’s disease dementia progression: 36-month results from GERAS. Int Psychogeriatr. 2020;32:267-277. doi: 10.1017/S1041610219000425
7. Gilhooly KJ, Gilhooly MLM, Sullivan MP, et al. A meta-review of stress, coping and interventions in dementia and dementia caregiving. BMC Geriatr. 2016;16:106. doi: 10.1186/s12877-016-0280-8
8. Haley WE, Levine EG, Brown SL, et al. Psychological, social, and health consequences of caring for a relative with senile dementia. J Am Geriatr Soc. 1987;35:405-411.
9. Bom J, Bakx P, Schut F, et al. The impact of informal caregiving for older adults on the health of various types of caregivers: a systematic review. The Gerontologist. 2019;59:e629-e642. doi: 10.1093/geront/gny137
10. Fonareva I, Oken BS. Physiological and functional consequences of caregiving for relatives with dementia. Int Psychogeriatr. 2014;26:725-747. doi: 10.1017/S1041610214000039
11. Del-Pino-Casado R, Rodriguez Cardosa M, Lopez-Martinez C, et al. The association between subjective caregiver burden and depressive symptoms in carers of older relatives: a systematic review and meta-analysis. PLoS One. 2019;14:e0217648. doi: 10.1371/journal.pone.0217648
12. Del-Pino-Casado R, Priego-Cubero E, Lopez-Martinez C, et al. Subjective caregiver burden and anxiety in informal caregivers: a systematic review and meta-analysis. PLoS One. 2020;16:e0247143. doi: 10.1371/journal.pone.0247143
13. De Souza Alves LC, Quirino Montiero D, Ricarte Bento S, et al. Burnout syndrome in informal caregivers of older adults with dementia: a systematic review. Dement Neuropsychol. 2019;13:415-421. doi: 10.1590/1980-57642018dn13-040008
14. Victor CR, Rippon I, Quinn C, et al. The prevalence and predictors of loneliness in caregivers of people with dementia: findings from the IDEAL programme. Aging Ment Health. 2021;25:1232-1238. doi: 10.1080/13607863.2020.1753014
15. Sallim AB, Sayampanathan AA, Cuttilan A, et al. Prevalence of mental health disorders among caregivers of patients with Alzheimer disease. J Am Med Dir Assoc. 2015;16:1034-1041. doi: 10.1016/j.jamda.2015.09.007
16. Unpublished data from the 2015, 2016 2017, 2020, and 2021 Behavioral Risk Factor Surveillance System survey, analyzed by and provided to the Alzheimer’s Association by the Alzheimer’s Disease and Healthy Aging Program (AD+HP), Centers for Disease Control and Prevention (CDC).
17. Stall NM, Kim SJ, Hardacre KA, et al. Association of informal caregiver distress with health outcomes of community-dwelling dementia care recipients: a systematic review. J Am Geriatr Soc. 2018;00:1-9. doi: 10.1111/jgs.15690
18. Lindeza P, Rodrigues M, Costa J, et al. Impact of dementia on informal care: a systematic review of family caregivers’ perceptions. BMJ Support Palliat Care. 2020;bmjspcare-2020-002242. doi: 10.1136/bmjspcare-2020-002242
19. Lethin C, Guiteras AR, Zwakhalen S, et al. Psychological well-being over time among informal caregivers caring for persons with dementia living at home. Aging and Ment Health. 2017; 21:1138-1146. doi: 10.1080/13607863.2016.1211621
20. Family Caregiver Alliance. Caregivers Count Too! A Toolkit to Help Practitioners Assess the Needs of Family Caregivers. Family Caregiver Alliance; 2006. Accessed May 16, 2023. www.caregiver.org/uploads/legacy/pdfs/Assessment_Toolkit_20060802.pdf
21. Zarit SH, Zarit JM. Instructions for the Burden Interview. Pennsylvania State University; 1987.
22. University of Wisconsin. Zarit Burden Interview: assessing caregiver burden. Accessed May 19, 2023. https://wai.wisc.edu/wp-content/uploads/sites/1129/2021/11/Zarit-Caregiver-Burden-Assessment-Instruments.pdf
23. Gallagher-Thompson D, Bilbrey AC, Apesoa-Varano EC, et al. Conceptual framework to guide intervention research across the trajectory of dementia caregiving. Gerontologist. 2020;60:S29-S40. doi: 10.1093/geront/gnz157
24. Queluz FNFR, Kervin E, Wozney L, et al. Understanding the needs of caregivers of persons with dementia: a scoping review. Int Psychogeriatr. 2020;32:35-52. doi: 10.1017/S1041610219000243
25. McCabe M, You E, Tatangelo G. Hearing their voice: a systematic review of dementia family caregivers’ needs. Gerontologist. 2016;56:e70-e88. doi: 10.1093/geront/gnw07
26. Zwaanswijk M, Peeters JM, van Beek AP, et al. Informal caregivers of people with dementia: problems, needs and support in the initial stage and in subsequent stages of dementia: a questionnaire survey. Open Nurs J. 2013;7:6-13. doi: 10.2174/1874434601307010006
27. Jennings LA, Palimaru A, Corona MG, et al. Patient and caregiver goals for dementia care. Qual Life Res. 2017;26:685-693. doi: 10.1007/s11136-016-1471-7
28. Brodaty H, Donkin M. Family caregivers of people with dementia. Dialogues Clin Neurosci. 2009;11:217-228. doi: 10.31887/DCNS.2009.11.2/hbrodaty
29. Rote SM, Angel JL, Moon H, et al. Caregiving across diverse populations: new evidence from the national study of caregiving and Hispanic EPESE. Innovation in Aging. 2019;3:1-11. doi: 10.1093/geroni/igz033
30. Alzheimer’s Association. 2021 Alzheimer’s Disease facts and figures. Special report—race, ethnicity, and Alzheimer’s in America. Alzheimers Dement. 2021;17:70-104. doi: 10.1002/alz.12328
31. Swartz K, Collins LG. Caregiver care. Am Fam Physician. 2019;99:699-706.
32. Cheng ST, Au A, Losada A, et al. Psychological interventions for dementia caregivers: what we have achieved, what we have learned. Curr Psychiatry Rep. 2019;21:59. doi: 10.1007/s11920-019-1045-9
33. Jennings LA, Reuben DB, Everston LC, et al. Unmet needs of caregivers of patients referred to a dementia care program. J Am Geriatr Soc. 2015;63:282-289. doi: 10.1111/jgs.13251
34. Soong A, Au ST, Kyaw BM, et al. Information needs and information seeking behaviour of people with dementia and their non-professional caregivers: a scoping review. BMC Geriatrics. 2020;20:61. doi: 10.1186/s12877-020-1454-y
35. Cheng S-T, Zhang F. A comprehensive meta-review of systematic reviews and meta-analyses on nonpharmacological interventions for informal dementia caregivers. BMC Geriatrics. 2020;20:137. doi: 10.1186/s12877-020-01547-2
36. Wiegelmann H, Speller S, Verhaert LM, et al. Psychosocial interventions to support the mental health of informal caregivers of persons living with dementia—a systematic literature review. BMC Geriatrics. 2021;21:94. doi: 10.1186/s12877-021-02020-4
37. Nguyen SA, Oughli HA, Lavretsky H. Complementary and integrative medicine for neurocognitive disorders and caregiver health. Current Psychiatry Reports. 2022;24:469-480. doi: 10.1007/s11920-022-01355-y
38. Gibson A, Holmes SD, Fields NL, et al. Providing care for persons with dementia in rural communities: informal caregivers’ perceptions of supports and services. J Gerontol Soc Work. 2019;62:630-648. doi: 10.1080/01634372.2019.1636332
39. Leng M, Zhao Y, Xiau H, et al. Internet-based supportive interventions for family caregivers of people with dementia: systematic review and meta-analysis. J Med Internet Res. 2020;22:e19468. doi: 10.2196/19468
40. Ruggiano N, Brown EL, Li J, et al. Rural dementia caregivers and technology. What is the evidence? Res Gerontol Nurs. 2018;11:216-224. doi: 10.3928/19404921-20180628-04
41. Shuffler J, Lee K, Fields, et al. Challenges experienced by rural informal caregivers of older adults in the United States: a scoping review. J Evid Based Soc Work. Published online 24 February 24, 2023. doi:10.1080/26408066.2023.2183102
42. Hughes MC, Liu Y, Baumbach A. Impact of COVID-19 on the health and well-being of informal caregivers of people with dementia: a rapid systematic review. Gerontol Geriatric Med. 2021;7:1-8. doi: 10.1177/2333721421102164
43. Paplickar A, Rajagopalan J, Alladi S. Care for dementia patients and caregivers amid COVID-19 pandemic. Cereb Circ Cogn Behav. 2022;3:100040. doi: 10.1016/j.cccb.2022.100040
THE CASE
Sam C* is a 68-year-old man who presented to his family physician in a rural health clinic due to concerns about weight loss. Since his visit 8 months prior, Mr. C unintentionally had lost 20 pounds. Upon questioning, Mr. C also reported feeling irritable and having difficulty with sleep and concentration.
A review of systems did not indicate the presence of infection or other medical conditions. In the 6 years since becoming a patient to the practice, he had reported no chronic health concerns, was taking no medications, and had only been to the clinic for his annual check-up appointments. He completed a Patient Health Questionnaire (PHQ-9) and scored 18, indicating moderately severe depression.
Mr. C had established care with his physician when he moved to the area from out of state so that he could be closer to his parents, who were in their mid-80s at the time. Mr. C’s physician also had been the family physician for his parents for the previous 20 years. Three years prior to Mr. C’s presentation for weight loss, his mother had received a diagnosis of acute leukemia; she died a year later.
Over the past year, Mr. C had needed to take a more active role in the care of his father, who was now in his early 90s. Mr. C’s father, who was previously in excellent health, had begun to develop significant health problems, including degenerative arthritis and progressive vascular dementia. He also had ataxia, leading to poor mobility, and a neurogenic bladder requiring self-catheterization, which required Mr. C’s assistance. Mr. C lived next door to his father and provided frequent assistance with activities of daily living. However, his father, who always had been the dominant figure in the family, was determined to maintain his independence and not relinquish control to others.
The strain of caregiving activities, along with managing his father’s inflexibility, was causing increasing distress for Mr. C. As he told his family physician, “I just don’t know what to do.”
●
* The patient’s name has been changed to protect his identity.
It is estimated that more than 11 million Americans provided more than 18 billion hours in unpaid support for individuals with dementia in 2022, averaging 30 hours of care per caregiver per week.1 As individuals with dementia progressively decline, they require increased assistance with activities of daily living (ADLs, such as bathing and dressing) and instrumental activities of daily living (IADLs, such as paying bills and using transportation). Most of this assistance comes from informal caregiving provided by family members and friends.
Caregiver burden can be defined as “the strain or load borne by a person who cares for a chronically ill, disabled, or elderly family member.”2 Caregiver stress has been found to be higher for dementia caregiving than other types of caregiving.3 In particular, caring for someone with greater behavioral and psychological symptoms of dementia (BPSDs) has been associated with higher caregiver burden.4-

Beyond the subjective burden of caregiving, there are other potential negative consequences for dementia caregivers (see TABLE 18-14 and TABLE 215,16). In addition, caregiver distress is related to a number of care recipient outcomes, including earlier institutionalization, more hospitalizations, more BPSDs, poorer quality of life, and greater likelihood of experiencing elder abuse.17

Assessment, reassessment are key to meeting needs
Numerous factors can foster caregiver well-being, including feelings of accomplishment and contribution, a strengthening of the relationship with the care recipient, and feeling supported by friends, family, and formal care systems.18,19 Family physicians can play an important role by assessing and supporting patients with dementia and their caregivers. Ideally, the individual with dementia and the caregiver will be assessed both together and separately.
A thorough assessment includes gathering information about the context and quality of the caregiving relationship; caregiver perception of the care recipient’s health and functional status; caregiver values and preferences; caregiver well-being (including mental health assessment); caregiver skills, abilities, and knowledge about caregiving; and positive and negative consequences of caregiving.20 Caregiver needs—including informational, care support, emotional, physical, and social needs—also should be assessed.
Continue to: Tools are available...
Tools are available to facilitate caregiver assessment. For example, the Zarit Burden Interview is a 22-item self-report measure that can be given to the caregiver21; shorter versions (4 and 12 items) are also available.22 Another resource available for caregiver assessment guidance is a toolkit developed by the Family Caregiver Alliance.20
Continually assess for changing needs
As the condition of the individual with dementia progresses, it will be important to reassess the caregiver, as stressors and needs will change over the course of the caregiving relationship. Support should be adapted accordingly.
In the early stage of dementia, caregivers may need information on disease progression and dementia care planning, ways to navigate the health care system, financial planning, and useful resources. Caregivers also may need emotional support to help them adapt to the role of caregiver, deal with denial, and manage their stress.23,24
With dementia progression, caregivers may need support related to increased decision-making responsibility, managing challenging behaviors, assisting with ADLs and IADLs, and identifying opportunities to meet personal social and well-being needs. They also may need support to accept the changes they are seeing in the individual with dementia and the shifts they are experiencing in their relationship with him or her.23,25
In late-stage dementia, caregiver needs tend to shift to determining the need for long-term care placement vs staying at home, end-of-life planning, loneliness, and anticipatory grief.23,26 Support with managing changing and accumulating stress typically remains a primary need throughout the progression of dementia.27
Continue to: Specific populations have distinct needs
Specific populations have distinct needs. Some caregivers, including members of the LGBTQ+ community and different racial and ethnic groups, as well as caregivers of people with younger-onset dementia, may have additional support needs.28
For example, African American and Latino caregivers tend to have caregiving relationships of longer duration, requiring more time-intensive care, but use fewer formal support services than White caregivers.29 Caregivers from non-White racial and ethnic groups also are more likely to experience discrimination when interacting with health care services on behalf of care recipients.30
Having an awareness of potential specialized needs may help to prevent or address potential care disparities, and cultural humility may help to improve caregiver experiences with primary care physicians.
Resources to support caregivers
Family physicians are well situated to provide informational and emotional support for both patients with dementia and their informal care providers.31 Given the variability of caregiver concerns, multicomponent interventions addressing informational, self-care, social support, and financial needs often are needed.31 Supportive counseling and psychoeducation can help dementia caregivers with stress management, self-care, coping, and skills training—supporting the development of self-efficacy.32,33
Outside resources. Although significant caregiver support can be provided directly by the physician, caregivers should be connected with outside resources, including support groups, counselors, psychotherapists, financial and legal support, and formal care services
Continue to: Psychosocial and complementary interventions
Psychosocial and complementary interventions. Various psychosocial interventions (eg, psychoeducation, cognitive behavioral therapy, support groups) have been found to be beneficial in alleviating caregiver symptoms of depression, anxiety, and stress and improving well-being, perceived burden, and quality of life. However, systematic reviews have found variability in the degree of helpfulness of these interventions.35,36
Some caregivers and care recipients may benefit from complementary and integrative medicine referrals. Mind–body therapies such as mindfulness, yoga, and Tai Chi have shown some beneficial effects.37
Online resources. Caregivers also can be directed to online resources from organizations such as the Alzheimer’s Association (www.alz.org), the National Institutes of Health (www.alzheimers.gov), and the Family Caregiver Alliance (www.caregiver.org).
In rural settings, such as the one in which this case took place, online resources may decrease some barriers to supporting caregivers.38 Internet-based interventions also have been found to have some benefit for dementia caregivers.31,39
However, some rural locations continue to have limited reliable Internet services.40 In affected areas, a strong relationship with a primary care physician may be even more important to the well-being of caregivers, since other support services may be less accessible.41
Continue to: Impacts of the pandemic
Impacts of the pandemic. Although our case took place prior to the COVID-19 pandemic, it is important to acknowledge ways the pandemic has impacted informal dementia caregiving.
Caregiver stress, depression, and anxiety increased during the pandemic, and the need for greater home confinement and social distancing amplified the negative impact of social isolation, including loneliness, on caregivers.42,43 Caregivers often needed to increase their caregiving responsibilities and had more difficulty with care coordination due to limited access to in-person resources.43 The pandemic led to increased reliance on technology and telehealth in the support of dementia caregivers.43
THE CASE
The physician prescribed mirtazapine for Mr. C, titrating the dose as needed to address depressive symptoms and promote weight gain. The physician connected Mr. C’s father with home health services, including physical therapy for fall risk reduction. Mr. C also hired part-time support to provide additional assistance with ADLs and IADLs, allowing Mr. C to have time to attend to his own needs. Though provided with information about a local caregiver support group, Mr. C chose not to attend. The physician also assisted the family with advanced directives.
A particular challenge that occurred during care for the family was addressing Mr. C’s father’s driving capacity, considering his strong need for independence. To address this concern, a family meeting was held with Mr. C, his father, and his siblings from out of town. Although Mr. C’s father was not willing to relinquish his driver’s license during that meeting, he agreed to complete a functional driving assessment.
The physician continued to meet with Mr. C and his father together, as well as with Mr. C individually, to provide supportive counseling as needed. As the father’s dementia progressed and it became more difficult to complete office appointments, the physician transitioned to home visits to provide care until the father’s death.
After the death of Mr. C’s father, the physician continued to serve as Mr. C’s primary care provider.
Keeping the “family”in family medicine
Through longitudinal assessment, needs identification, and provision of relevant information, emotional support, and resources, family physicians can provide care that can improve the quality of life and well-being and help alleviate burden experienced by dementia caregivers. Family physicians also are positioned to provide treatments that can address the negative physical and psychological health outcomes associated with informal dementia caregiving. By building relationships with multiple family members across generations, family physicians can understand the context of caregiving dynamics and work together with individuals with dementia and their caregivers throughout disease progression, providing consistent support to the family unit.
CORRESPONDENCE
Kathleen M. Young, PhD, MPH, Novant Health Family Medicine Wilmington, 2523 Delaney Avenue, Wilmington, NC 28403; [email protected]
THE CASE
Sam C* is a 68-year-old man who presented to his family physician in a rural health clinic due to concerns about weight loss. Since his visit 8 months prior, Mr. C unintentionally had lost 20 pounds. Upon questioning, Mr. C also reported feeling irritable and having difficulty with sleep and concentration.
A review of systems did not indicate the presence of infection or other medical conditions. In the 6 years since becoming a patient to the practice, he had reported no chronic health concerns, was taking no medications, and had only been to the clinic for his annual check-up appointments. He completed a Patient Health Questionnaire (PHQ-9) and scored 18, indicating moderately severe depression.
Mr. C had established care with his physician when he moved to the area from out of state so that he could be closer to his parents, who were in their mid-80s at the time. Mr. C’s physician also had been the family physician for his parents for the previous 20 years. Three years prior to Mr. C’s presentation for weight loss, his mother had received a diagnosis of acute leukemia; she died a year later.
Over the past year, Mr. C had needed to take a more active role in the care of his father, who was now in his early 90s. Mr. C’s father, who was previously in excellent health, had begun to develop significant health problems, including degenerative arthritis and progressive vascular dementia. He also had ataxia, leading to poor mobility, and a neurogenic bladder requiring self-catheterization, which required Mr. C’s assistance. Mr. C lived next door to his father and provided frequent assistance with activities of daily living. However, his father, who always had been the dominant figure in the family, was determined to maintain his independence and not relinquish control to others.
The strain of caregiving activities, along with managing his father’s inflexibility, was causing increasing distress for Mr. C. As he told his family physician, “I just don’t know what to do.”
●
* The patient’s name has been changed to protect his identity.
It is estimated that more than 11 million Americans provided more than 18 billion hours in unpaid support for individuals with dementia in 2022, averaging 30 hours of care per caregiver per week.1 As individuals with dementia progressively decline, they require increased assistance with activities of daily living (ADLs, such as bathing and dressing) and instrumental activities of daily living (IADLs, such as paying bills and using transportation). Most of this assistance comes from informal caregiving provided by family members and friends.
Caregiver burden can be defined as “the strain or load borne by a person who cares for a chronically ill, disabled, or elderly family member.”2 Caregiver stress has been found to be higher for dementia caregiving than other types of caregiving.3 In particular, caring for someone with greater behavioral and psychological symptoms of dementia (BPSDs) has been associated with higher caregiver burden.4-

Beyond the subjective burden of caregiving, there are other potential negative consequences for dementia caregivers (see TABLE 18-14 and TABLE 215,16). In addition, caregiver distress is related to a number of care recipient outcomes, including earlier institutionalization, more hospitalizations, more BPSDs, poorer quality of life, and greater likelihood of experiencing elder abuse.17

Assessment, reassessment are key to meeting needs
Numerous factors can foster caregiver well-being, including feelings of accomplishment and contribution, a strengthening of the relationship with the care recipient, and feeling supported by friends, family, and formal care systems.18,19 Family physicians can play an important role by assessing and supporting patients with dementia and their caregivers. Ideally, the individual with dementia and the caregiver will be assessed both together and separately.
A thorough assessment includes gathering information about the context and quality of the caregiving relationship; caregiver perception of the care recipient’s health and functional status; caregiver values and preferences; caregiver well-being (including mental health assessment); caregiver skills, abilities, and knowledge about caregiving; and positive and negative consequences of caregiving.20 Caregiver needs—including informational, care support, emotional, physical, and social needs—also should be assessed.
Continue to: Tools are available...
Tools are available to facilitate caregiver assessment. For example, the Zarit Burden Interview is a 22-item self-report measure that can be given to the caregiver21; shorter versions (4 and 12 items) are also available.22 Another resource available for caregiver assessment guidance is a toolkit developed by the Family Caregiver Alliance.20
Continually assess for changing needs
As the condition of the individual with dementia progresses, it will be important to reassess the caregiver, as stressors and needs will change over the course of the caregiving relationship. Support should be adapted accordingly.
In the early stage of dementia, caregivers may need information on disease progression and dementia care planning, ways to navigate the health care system, financial planning, and useful resources. Caregivers also may need emotional support to help them adapt to the role of caregiver, deal with denial, and manage their stress.23,24
With dementia progression, caregivers may need support related to increased decision-making responsibility, managing challenging behaviors, assisting with ADLs and IADLs, and identifying opportunities to meet personal social and well-being needs. They also may need support to accept the changes they are seeing in the individual with dementia and the shifts they are experiencing in their relationship with him or her.23,25
In late-stage dementia, caregiver needs tend to shift to determining the need for long-term care placement vs staying at home, end-of-life planning, loneliness, and anticipatory grief.23,26 Support with managing changing and accumulating stress typically remains a primary need throughout the progression of dementia.27
Continue to: Specific populations have distinct needs
Specific populations have distinct needs. Some caregivers, including members of the LGBTQ+ community and different racial and ethnic groups, as well as caregivers of people with younger-onset dementia, may have additional support needs.28
For example, African American and Latino caregivers tend to have caregiving relationships of longer duration, requiring more time-intensive care, but use fewer formal support services than White caregivers.29 Caregivers from non-White racial and ethnic groups also are more likely to experience discrimination when interacting with health care services on behalf of care recipients.30
Having an awareness of potential specialized needs may help to prevent or address potential care disparities, and cultural humility may help to improve caregiver experiences with primary care physicians.
Resources to support caregivers
Family physicians are well situated to provide informational and emotional support for both patients with dementia and their informal care providers.31 Given the variability of caregiver concerns, multicomponent interventions addressing informational, self-care, social support, and financial needs often are needed.31 Supportive counseling and psychoeducation can help dementia caregivers with stress management, self-care, coping, and skills training—supporting the development of self-efficacy.32,33
Outside resources. Although significant caregiver support can be provided directly by the physician, caregivers should be connected with outside resources, including support groups, counselors, psychotherapists, financial and legal support, and formal care services
Continue to: Psychosocial and complementary interventions
Psychosocial and complementary interventions. Various psychosocial interventions (eg, psychoeducation, cognitive behavioral therapy, support groups) have been found to be beneficial in alleviating caregiver symptoms of depression, anxiety, and stress and improving well-being, perceived burden, and quality of life. However, systematic reviews have found variability in the degree of helpfulness of these interventions.35,36
Some caregivers and care recipients may benefit from complementary and integrative medicine referrals. Mind–body therapies such as mindfulness, yoga, and Tai Chi have shown some beneficial effects.37
Online resources. Caregivers also can be directed to online resources from organizations such as the Alzheimer’s Association (www.alz.org), the National Institutes of Health (www.alzheimers.gov), and the Family Caregiver Alliance (www.caregiver.org).
In rural settings, such as the one in which this case took place, online resources may decrease some barriers to supporting caregivers.38 Internet-based interventions also have been found to have some benefit for dementia caregivers.31,39
However, some rural locations continue to have limited reliable Internet services.40 In affected areas, a strong relationship with a primary care physician may be even more important to the well-being of caregivers, since other support services may be less accessible.41
Continue to: Impacts of the pandemic
Impacts of the pandemic. Although our case took place prior to the COVID-19 pandemic, it is important to acknowledge ways the pandemic has impacted informal dementia caregiving.
Caregiver stress, depression, and anxiety increased during the pandemic, and the need for greater home confinement and social distancing amplified the negative impact of social isolation, including loneliness, on caregivers.42,43 Caregivers often needed to increase their caregiving responsibilities and had more difficulty with care coordination due to limited access to in-person resources.43 The pandemic led to increased reliance on technology and telehealth in the support of dementia caregivers.43
THE CASE
The physician prescribed mirtazapine for Mr. C, titrating the dose as needed to address depressive symptoms and promote weight gain. The physician connected Mr. C’s father with home health services, including physical therapy for fall risk reduction. Mr. C also hired part-time support to provide additional assistance with ADLs and IADLs, allowing Mr. C to have time to attend to his own needs. Though provided with information about a local caregiver support group, Mr. C chose not to attend. The physician also assisted the family with advanced directives.
A particular challenge that occurred during care for the family was addressing Mr. C’s father’s driving capacity, considering his strong need for independence. To address this concern, a family meeting was held with Mr. C, his father, and his siblings from out of town. Although Mr. C’s father was not willing to relinquish his driver’s license during that meeting, he agreed to complete a functional driving assessment.
The physician continued to meet with Mr. C and his father together, as well as with Mr. C individually, to provide supportive counseling as needed. As the father’s dementia progressed and it became more difficult to complete office appointments, the physician transitioned to home visits to provide care until the father’s death.
After the death of Mr. C’s father, the physician continued to serve as Mr. C’s primary care provider.
Keeping the “family”in family medicine
Through longitudinal assessment, needs identification, and provision of relevant information, emotional support, and resources, family physicians can provide care that can improve the quality of life and well-being and help alleviate burden experienced by dementia caregivers. Family physicians also are positioned to provide treatments that can address the negative physical and psychological health outcomes associated with informal dementia caregiving. By building relationships with multiple family members across generations, family physicians can understand the context of caregiving dynamics and work together with individuals with dementia and their caregivers throughout disease progression, providing consistent support to the family unit.
CORRESPONDENCE
Kathleen M. Young, PhD, MPH, Novant Health Family Medicine Wilmington, 2523 Delaney Avenue, Wilmington, NC 28403; [email protected]
1. Alzheimer’s Association. 2023 Alzheimer’s Disease Facts and Figures. Alzheimers Dement. 202319:1598-1695. doi: 10.1002/alz.13016
2. Liu Z, Heffernan C, Tan J. Caregiver burden: a concept analysis. Int J of Nurs Sci. 2020;7:448-435. doi: 10.1016/j.ijnss.2020.07.012
3. Ory MG, Hoffman RR III, Yee JL, et al. Prevalence and impacts of caregiving: a detailed comparison between dementia and nondementia caregivers. Gerontologist. 1999;39:177-185. doi: 10.1093/geront/39.2.177
4. Baharudin AD, Din NC, Subramaniam P, et al. The associations between behavioral-psychological symptoms of dementia (BPSD) and coping strategy, burden of care and personality style among low-income caregivers of patients with dementia. BMC Public Health. 2019;19(suppl 4):447. doi: 10.1186/s12889-019-6868-0
5. Cheng S-T. Dementia caregiver burden: a research update and critical analysis. Curr Psychiatry Rep. 2017;19:64. doi: 10.1007/s11920-017-0818-2
6. Reed C, Belger M, Andrews JS, et al. Factors associated with long-term impact on informal caregivers during Alzheimer’s disease dementia progression: 36-month results from GERAS. Int Psychogeriatr. 2020;32:267-277. doi: 10.1017/S1041610219000425
7. Gilhooly KJ, Gilhooly MLM, Sullivan MP, et al. A meta-review of stress, coping and interventions in dementia and dementia caregiving. BMC Geriatr. 2016;16:106. doi: 10.1186/s12877-016-0280-8
8. Haley WE, Levine EG, Brown SL, et al. Psychological, social, and health consequences of caring for a relative with senile dementia. J Am Geriatr Soc. 1987;35:405-411.
9. Bom J, Bakx P, Schut F, et al. The impact of informal caregiving for older adults on the health of various types of caregivers: a systematic review. The Gerontologist. 2019;59:e629-e642. doi: 10.1093/geront/gny137
10. Fonareva I, Oken BS. Physiological and functional consequences of caregiving for relatives with dementia. Int Psychogeriatr. 2014;26:725-747. doi: 10.1017/S1041610214000039
11. Del-Pino-Casado R, Rodriguez Cardosa M, Lopez-Martinez C, et al. The association between subjective caregiver burden and depressive symptoms in carers of older relatives: a systematic review and meta-analysis. PLoS One. 2019;14:e0217648. doi: 10.1371/journal.pone.0217648
12. Del-Pino-Casado R, Priego-Cubero E, Lopez-Martinez C, et al. Subjective caregiver burden and anxiety in informal caregivers: a systematic review and meta-analysis. PLoS One. 2020;16:e0247143. doi: 10.1371/journal.pone.0247143
13. De Souza Alves LC, Quirino Montiero D, Ricarte Bento S, et al. Burnout syndrome in informal caregivers of older adults with dementia: a systematic review. Dement Neuropsychol. 2019;13:415-421. doi: 10.1590/1980-57642018dn13-040008
14. Victor CR, Rippon I, Quinn C, et al. The prevalence and predictors of loneliness in caregivers of people with dementia: findings from the IDEAL programme. Aging Ment Health. 2021;25:1232-1238. doi: 10.1080/13607863.2020.1753014
15. Sallim AB, Sayampanathan AA, Cuttilan A, et al. Prevalence of mental health disorders among caregivers of patients with Alzheimer disease. J Am Med Dir Assoc. 2015;16:1034-1041. doi: 10.1016/j.jamda.2015.09.007
16. Unpublished data from the 2015, 2016 2017, 2020, and 2021 Behavioral Risk Factor Surveillance System survey, analyzed by and provided to the Alzheimer’s Association by the Alzheimer’s Disease and Healthy Aging Program (AD+HP), Centers for Disease Control and Prevention (CDC).
17. Stall NM, Kim SJ, Hardacre KA, et al. Association of informal caregiver distress with health outcomes of community-dwelling dementia care recipients: a systematic review. J Am Geriatr Soc. 2018;00:1-9. doi: 10.1111/jgs.15690
18. Lindeza P, Rodrigues M, Costa J, et al. Impact of dementia on informal care: a systematic review of family caregivers’ perceptions. BMJ Support Palliat Care. 2020;bmjspcare-2020-002242. doi: 10.1136/bmjspcare-2020-002242
19. Lethin C, Guiteras AR, Zwakhalen S, et al. Psychological well-being over time among informal caregivers caring for persons with dementia living at home. Aging and Ment Health. 2017; 21:1138-1146. doi: 10.1080/13607863.2016.1211621
20. Family Caregiver Alliance. Caregivers Count Too! A Toolkit to Help Practitioners Assess the Needs of Family Caregivers. Family Caregiver Alliance; 2006. Accessed May 16, 2023. www.caregiver.org/uploads/legacy/pdfs/Assessment_Toolkit_20060802.pdf
21. Zarit SH, Zarit JM. Instructions for the Burden Interview. Pennsylvania State University; 1987.
22. University of Wisconsin. Zarit Burden Interview: assessing caregiver burden. Accessed May 19, 2023. https://wai.wisc.edu/wp-content/uploads/sites/1129/2021/11/Zarit-Caregiver-Burden-Assessment-Instruments.pdf
23. Gallagher-Thompson D, Bilbrey AC, Apesoa-Varano EC, et al. Conceptual framework to guide intervention research across the trajectory of dementia caregiving. Gerontologist. 2020;60:S29-S40. doi: 10.1093/geront/gnz157
24. Queluz FNFR, Kervin E, Wozney L, et al. Understanding the needs of caregivers of persons with dementia: a scoping review. Int Psychogeriatr. 2020;32:35-52. doi: 10.1017/S1041610219000243
25. McCabe M, You E, Tatangelo G. Hearing their voice: a systematic review of dementia family caregivers’ needs. Gerontologist. 2016;56:e70-e88. doi: 10.1093/geront/gnw07
26. Zwaanswijk M, Peeters JM, van Beek AP, et al. Informal caregivers of people with dementia: problems, needs and support in the initial stage and in subsequent stages of dementia: a questionnaire survey. Open Nurs J. 2013;7:6-13. doi: 10.2174/1874434601307010006
27. Jennings LA, Palimaru A, Corona MG, et al. Patient and caregiver goals for dementia care. Qual Life Res. 2017;26:685-693. doi: 10.1007/s11136-016-1471-7
28. Brodaty H, Donkin M. Family caregivers of people with dementia. Dialogues Clin Neurosci. 2009;11:217-228. doi: 10.31887/DCNS.2009.11.2/hbrodaty
29. Rote SM, Angel JL, Moon H, et al. Caregiving across diverse populations: new evidence from the national study of caregiving and Hispanic EPESE. Innovation in Aging. 2019;3:1-11. doi: 10.1093/geroni/igz033
30. Alzheimer’s Association. 2021 Alzheimer’s Disease facts and figures. Special report—race, ethnicity, and Alzheimer’s in America. Alzheimers Dement. 2021;17:70-104. doi: 10.1002/alz.12328
31. Swartz K, Collins LG. Caregiver care. Am Fam Physician. 2019;99:699-706.
32. Cheng ST, Au A, Losada A, et al. Psychological interventions for dementia caregivers: what we have achieved, what we have learned. Curr Psychiatry Rep. 2019;21:59. doi: 10.1007/s11920-019-1045-9
33. Jennings LA, Reuben DB, Everston LC, et al. Unmet needs of caregivers of patients referred to a dementia care program. J Am Geriatr Soc. 2015;63:282-289. doi: 10.1111/jgs.13251
34. Soong A, Au ST, Kyaw BM, et al. Information needs and information seeking behaviour of people with dementia and their non-professional caregivers: a scoping review. BMC Geriatrics. 2020;20:61. doi: 10.1186/s12877-020-1454-y
35. Cheng S-T, Zhang F. A comprehensive meta-review of systematic reviews and meta-analyses on nonpharmacological interventions for informal dementia caregivers. BMC Geriatrics. 2020;20:137. doi: 10.1186/s12877-020-01547-2
36. Wiegelmann H, Speller S, Verhaert LM, et al. Psychosocial interventions to support the mental health of informal caregivers of persons living with dementia—a systematic literature review. BMC Geriatrics. 2021;21:94. doi: 10.1186/s12877-021-02020-4
37. Nguyen SA, Oughli HA, Lavretsky H. Complementary and integrative medicine for neurocognitive disorders and caregiver health. Current Psychiatry Reports. 2022;24:469-480. doi: 10.1007/s11920-022-01355-y
38. Gibson A, Holmes SD, Fields NL, et al. Providing care for persons with dementia in rural communities: informal caregivers’ perceptions of supports and services. J Gerontol Soc Work. 2019;62:630-648. doi: 10.1080/01634372.2019.1636332
39. Leng M, Zhao Y, Xiau H, et al. Internet-based supportive interventions for family caregivers of people with dementia: systematic review and meta-analysis. J Med Internet Res. 2020;22:e19468. doi: 10.2196/19468
40. Ruggiano N, Brown EL, Li J, et al. Rural dementia caregivers and technology. What is the evidence? Res Gerontol Nurs. 2018;11:216-224. doi: 10.3928/19404921-20180628-04
41. Shuffler J, Lee K, Fields, et al. Challenges experienced by rural informal caregivers of older adults in the United States: a scoping review. J Evid Based Soc Work. Published online 24 February 24, 2023. doi:10.1080/26408066.2023.2183102
42. Hughes MC, Liu Y, Baumbach A. Impact of COVID-19 on the health and well-being of informal caregivers of people with dementia: a rapid systematic review. Gerontol Geriatric Med. 2021;7:1-8. doi: 10.1177/2333721421102164
43. Paplickar A, Rajagopalan J, Alladi S. Care for dementia patients and caregivers amid COVID-19 pandemic. Cereb Circ Cogn Behav. 2022;3:100040. doi: 10.1016/j.cccb.2022.100040
1. Alzheimer’s Association. 2023 Alzheimer’s Disease Facts and Figures. Alzheimers Dement. 202319:1598-1695. doi: 10.1002/alz.13016
2. Liu Z, Heffernan C, Tan J. Caregiver burden: a concept analysis. Int J of Nurs Sci. 2020;7:448-435. doi: 10.1016/j.ijnss.2020.07.012
3. Ory MG, Hoffman RR III, Yee JL, et al. Prevalence and impacts of caregiving: a detailed comparison between dementia and nondementia caregivers. Gerontologist. 1999;39:177-185. doi: 10.1093/geront/39.2.177
4. Baharudin AD, Din NC, Subramaniam P, et al. The associations between behavioral-psychological symptoms of dementia (BPSD) and coping strategy, burden of care and personality style among low-income caregivers of patients with dementia. BMC Public Health. 2019;19(suppl 4):447. doi: 10.1186/s12889-019-6868-0
5. Cheng S-T. Dementia caregiver burden: a research update and critical analysis. Curr Psychiatry Rep. 2017;19:64. doi: 10.1007/s11920-017-0818-2
6. Reed C, Belger M, Andrews JS, et al. Factors associated with long-term impact on informal caregivers during Alzheimer’s disease dementia progression: 36-month results from GERAS. Int Psychogeriatr. 2020;32:267-277. doi: 10.1017/S1041610219000425
7. Gilhooly KJ, Gilhooly MLM, Sullivan MP, et al. A meta-review of stress, coping and interventions in dementia and dementia caregiving. BMC Geriatr. 2016;16:106. doi: 10.1186/s12877-016-0280-8
8. Haley WE, Levine EG, Brown SL, et al. Psychological, social, and health consequences of caring for a relative with senile dementia. J Am Geriatr Soc. 1987;35:405-411.
9. Bom J, Bakx P, Schut F, et al. The impact of informal caregiving for older adults on the health of various types of caregivers: a systematic review. The Gerontologist. 2019;59:e629-e642. doi: 10.1093/geront/gny137
10. Fonareva I, Oken BS. Physiological and functional consequences of caregiving for relatives with dementia. Int Psychogeriatr. 2014;26:725-747. doi: 10.1017/S1041610214000039
11. Del-Pino-Casado R, Rodriguez Cardosa M, Lopez-Martinez C, et al. The association between subjective caregiver burden and depressive symptoms in carers of older relatives: a systematic review and meta-analysis. PLoS One. 2019;14:e0217648. doi: 10.1371/journal.pone.0217648
12. Del-Pino-Casado R, Priego-Cubero E, Lopez-Martinez C, et al. Subjective caregiver burden and anxiety in informal caregivers: a systematic review and meta-analysis. PLoS One. 2020;16:e0247143. doi: 10.1371/journal.pone.0247143
13. De Souza Alves LC, Quirino Montiero D, Ricarte Bento S, et al. Burnout syndrome in informal caregivers of older adults with dementia: a systematic review. Dement Neuropsychol. 2019;13:415-421. doi: 10.1590/1980-57642018dn13-040008
14. Victor CR, Rippon I, Quinn C, et al. The prevalence and predictors of loneliness in caregivers of people with dementia: findings from the IDEAL programme. Aging Ment Health. 2021;25:1232-1238. doi: 10.1080/13607863.2020.1753014
15. Sallim AB, Sayampanathan AA, Cuttilan A, et al. Prevalence of mental health disorders among caregivers of patients with Alzheimer disease. J Am Med Dir Assoc. 2015;16:1034-1041. doi: 10.1016/j.jamda.2015.09.007
16. Unpublished data from the 2015, 2016 2017, 2020, and 2021 Behavioral Risk Factor Surveillance System survey, analyzed by and provided to the Alzheimer’s Association by the Alzheimer’s Disease and Healthy Aging Program (AD+HP), Centers for Disease Control and Prevention (CDC).
17. Stall NM, Kim SJ, Hardacre KA, et al. Association of informal caregiver distress with health outcomes of community-dwelling dementia care recipients: a systematic review. J Am Geriatr Soc. 2018;00:1-9. doi: 10.1111/jgs.15690
18. Lindeza P, Rodrigues M, Costa J, et al. Impact of dementia on informal care: a systematic review of family caregivers’ perceptions. BMJ Support Palliat Care. 2020;bmjspcare-2020-002242. doi: 10.1136/bmjspcare-2020-002242
19. Lethin C, Guiteras AR, Zwakhalen S, et al. Psychological well-being over time among informal caregivers caring for persons with dementia living at home. Aging and Ment Health. 2017; 21:1138-1146. doi: 10.1080/13607863.2016.1211621
20. Family Caregiver Alliance. Caregivers Count Too! A Toolkit to Help Practitioners Assess the Needs of Family Caregivers. Family Caregiver Alliance; 2006. Accessed May 16, 2023. www.caregiver.org/uploads/legacy/pdfs/Assessment_Toolkit_20060802.pdf
21. Zarit SH, Zarit JM. Instructions for the Burden Interview. Pennsylvania State University; 1987.
22. University of Wisconsin. Zarit Burden Interview: assessing caregiver burden. Accessed May 19, 2023. https://wai.wisc.edu/wp-content/uploads/sites/1129/2021/11/Zarit-Caregiver-Burden-Assessment-Instruments.pdf
23. Gallagher-Thompson D, Bilbrey AC, Apesoa-Varano EC, et al. Conceptual framework to guide intervention research across the trajectory of dementia caregiving. Gerontologist. 2020;60:S29-S40. doi: 10.1093/geront/gnz157
24. Queluz FNFR, Kervin E, Wozney L, et al. Understanding the needs of caregivers of persons with dementia: a scoping review. Int Psychogeriatr. 2020;32:35-52. doi: 10.1017/S1041610219000243
25. McCabe M, You E, Tatangelo G. Hearing their voice: a systematic review of dementia family caregivers’ needs. Gerontologist. 2016;56:e70-e88. doi: 10.1093/geront/gnw07
26. Zwaanswijk M, Peeters JM, van Beek AP, et al. Informal caregivers of people with dementia: problems, needs and support in the initial stage and in subsequent stages of dementia: a questionnaire survey. Open Nurs J. 2013;7:6-13. doi: 10.2174/1874434601307010006
27. Jennings LA, Palimaru A, Corona MG, et al. Patient and caregiver goals for dementia care. Qual Life Res. 2017;26:685-693. doi: 10.1007/s11136-016-1471-7
28. Brodaty H, Donkin M. Family caregivers of people with dementia. Dialogues Clin Neurosci. 2009;11:217-228. doi: 10.31887/DCNS.2009.11.2/hbrodaty
29. Rote SM, Angel JL, Moon H, et al. Caregiving across diverse populations: new evidence from the national study of caregiving and Hispanic EPESE. Innovation in Aging. 2019;3:1-11. doi: 10.1093/geroni/igz033
30. Alzheimer’s Association. 2021 Alzheimer’s Disease facts and figures. Special report—race, ethnicity, and Alzheimer’s in America. Alzheimers Dement. 2021;17:70-104. doi: 10.1002/alz.12328
31. Swartz K, Collins LG. Caregiver care. Am Fam Physician. 2019;99:699-706.
32. Cheng ST, Au A, Losada A, et al. Psychological interventions for dementia caregivers: what we have achieved, what we have learned. Curr Psychiatry Rep. 2019;21:59. doi: 10.1007/s11920-019-1045-9
33. Jennings LA, Reuben DB, Everston LC, et al. Unmet needs of caregivers of patients referred to a dementia care program. J Am Geriatr Soc. 2015;63:282-289. doi: 10.1111/jgs.13251
34. Soong A, Au ST, Kyaw BM, et al. Information needs and information seeking behaviour of people with dementia and their non-professional caregivers: a scoping review. BMC Geriatrics. 2020;20:61. doi: 10.1186/s12877-020-1454-y
35. Cheng S-T, Zhang F. A comprehensive meta-review of systematic reviews and meta-analyses on nonpharmacological interventions for informal dementia caregivers. BMC Geriatrics. 2020;20:137. doi: 10.1186/s12877-020-01547-2
36. Wiegelmann H, Speller S, Verhaert LM, et al. Psychosocial interventions to support the mental health of informal caregivers of persons living with dementia—a systematic literature review. BMC Geriatrics. 2021;21:94. doi: 10.1186/s12877-021-02020-4
37. Nguyen SA, Oughli HA, Lavretsky H. Complementary and integrative medicine for neurocognitive disorders and caregiver health. Current Psychiatry Reports. 2022;24:469-480. doi: 10.1007/s11920-022-01355-y
38. Gibson A, Holmes SD, Fields NL, et al. Providing care for persons with dementia in rural communities: informal caregivers’ perceptions of supports and services. J Gerontol Soc Work. 2019;62:630-648. doi: 10.1080/01634372.2019.1636332
39. Leng M, Zhao Y, Xiau H, et al. Internet-based supportive interventions for family caregivers of people with dementia: systematic review and meta-analysis. J Med Internet Res. 2020;22:e19468. doi: 10.2196/19468
40. Ruggiano N, Brown EL, Li J, et al. Rural dementia caregivers and technology. What is the evidence? Res Gerontol Nurs. 2018;11:216-224. doi: 10.3928/19404921-20180628-04
41. Shuffler J, Lee K, Fields, et al. Challenges experienced by rural informal caregivers of older adults in the United States: a scoping review. J Evid Based Soc Work. Published online 24 February 24, 2023. doi:10.1080/26408066.2023.2183102
42. Hughes MC, Liu Y, Baumbach A. Impact of COVID-19 on the health and well-being of informal caregivers of people with dementia: a rapid systematic review. Gerontol Geriatric Med. 2021;7:1-8. doi: 10.1177/2333721421102164
43. Paplickar A, Rajagopalan J, Alladi S. Care for dementia patients and caregivers amid COVID-19 pandemic. Cereb Circ Cogn Behav. 2022;3:100040. doi: 10.1016/j.cccb.2022.100040
Is the WHO’s ‘active aging’ the only healthy alternative?
MAR DEL PLATA, ARGENTINA – In the “active aging” vision promoted by the World Health Organization (WHO), older adults stay physically active, independent, and involved. This concept, though well-intentioned, is not very realistic and could easily be discouraging to individuals suffering from the psychological or physical limitations of old age. It also does not account for diversity among individuals and across cultures. These conclusions were presented by the Geriatric Psychiatry Chapter of the Argentine Psychiatric Association at its XXXVI Argentine Congress of Psychiatry.
“The WHO’s proposal of active aging is a prescriptive, standardized ideology that seems to suggest that being active is the only healthy way to age. However, that’s only part of the picture, and a biased part at that. It doesn’t account for the broad spectrum of aging processes that come in many shades,” said Mariana Pedace, psychologist with the Adult Intensive Care department at the Italian Hospital in Buenos Aires and head of the Older Adults section of the civic association Project: Unite.
“The question is whether the idea of active aging is just one more way to create mandates or rules for older adults, which make up such a heterogeneous and diverse generation,” said Ana Laura Vega, MD, psychiatrist with the Mental Health Department at the Italian Hospital of Buenos Aires.
Might it be better to speak of aging “as expected” or “aging well”? Speakers at the conference did not reach a consensus on which word would be the best to replace the adjective “active.”
“I don’t really see why there has to be an additional term when, at other stages of life, we only talk about ‘infancy,’ ‘adolescence,’ or ‘middle age,’ ” said Dr. Vega.
A thorny issue
Since the late 1990s, the WHO has defined active aging as “the process of optimizing opportunities for health, participation, and security to enhance quality of life as people age.” This concept allows older adults to “realize their potential for physical, social, and mental well-being throughout the life course and to participate in society according to their needs, desires, and capacities, while providing them with adequate protection, security, and care when they require assistance.”
The organization clarifies that the word “active” refers to continuing participation in social, economic, cultural, spiritual, and civic affairs, not just the ability to be physically active or to participate in the labor force. “However, in practice, active aging programs invariably promote physical activity and exercise as having health and social benefits,” said sociologist Elizabeth Pike, PhD, head of the Research Unit in Sport, Physical Activity, and Aging at the University of Hertfordshire in the United Kingdom.
said Dr. Pedace. Along with laying out a single prescriptive way to age healthily, which by default makes passive aging “abnormal,” it also ignores demographic, ethnographic, and cultural differences.
“Each culture has different values. The suggestion of aging well in terms of activity, autonomy, and a happy-go-lucky mindset clearly reflects Western capitalistic values. In Eastern cultures, elderly people occupy a position reflecting their experience and wisdom, while also maintaining a contemplative mindset, which is something that is held in high regard. They are at the heart of the family, and their role is to guide and counsel the younger generations,” said Dr. Pedace.
The specialist added that there are programs inspired by active aging that prioritize outward, dynamic, and observable activities to the detriment of activities that take place behind the scenes such as reflection, analysis, and contemplation. “Following this mindset, an older individual who spends their time in contemplation would be somewhat wasting their sunset years. This raises a problem, because as the years go by and death approaches, spiritual life begins to gain far more significance. And that’s not an activity that is valued or recommended in the terms of this program,” she said.
Dr. Pedace went on to say that another concern with the active-aging program is that it seems to minimize certain characteristics that are unique to old age. Resulting physical, cognitive, and emotional changes can lead to reduced activity but are merely idiosyncrasies of this stage in life and are not pathologic.
Cecilia Guerstein, psychiatrist with the Older Adults Division of Project: United in Buenos Aires, cited Julieta Oddone, PhD, a sociologist on aging who believes that the theory of activity informs the underlying supposition of most programs for older adults: that social activity in itself is beneficial and results in greater fulfillment in life. And that all older people need and desire to stay active and engaged. “The idea is that the more active they are, the happier they will be,” said Dr. Guerstein.
“But ‘doing things’ isn’t necessarily appreciated by every elderly person, nor does it automatically lead to their well-being. The fact that some find a sense of well-being from it doesn’t mean we have to always do the same activities across different contexts. There are ethnographic studies that show that there isn’t necessarily a relationship between activity and well-being, or true social integration,” said Dr. Pedace.
Not a burden
Practically speaking, few would question whether physical activity has health benefits and believe that it’s never too late to start moving. Among his more than 45 tips on how to live to a ripe old age and “ripen” slowly and nicely, George D. Lundberg, MD, who is 90 years old, gives six recommendations for exercise: walking at least 2 miles every day, trying to swim every day, learning and practicing the techniques of yoga, deliberately lifting heavy objects (resistance training), and working on balance.
“A key for health care professionals encouraging exercise among older adults is knowing what to listen for and how to identify situations that motivate the person to exercise. For example, it could be walking their granddaughter down to the ice cream parlor,” Carolina Díaz, MD, said in an interview. Dr. Díaz is a geriatrics physician and the medical director of the Hirsch nursing and rehab center for older people in San Miguel, Argentina, which is home to 180 residents with an average age of 82 years.
“Exercise shouldn’t be a burden. If someone has never gone on walks before, I wouldn’t make them walk just because they ought to. Maybe they discover well-being in meeting up with their grandchildren or reading with someone. We believe that well-being is related to mobility, but for someone to move, they need the motivation. And until they have that, there won’t be any change,” said Dr. Díaz.
She added that a physician-patient relationship must be forged and an intervention plan drafted that revolves around the person and focuses on his or her current problems such as loneliness, difficulty walking, or pain. “Based on those problems, we can draw up a plan in which physical activity may play a part; other times, it may not.”
Osvaldo Bodni, psychiatrist and psychoanalyst, former director of the Department for Older Adults within the Argentine Psychoanalytic Association and author of the book, Delegating Power in Human Aging: The Theory of Legacy and Passing the Baton) said in an interview: “Aging isn’t a disease, though it does increase vulnerability. The proposal of physical activity is not the only ‘antidote.’ In my opinion, serenity during aging provides even better protection against life’s storms.”
The physician went on to say, “Active aging programs promote physical activity because it’s easier to go on a walk with someone than it is to have a literature debate with them. However, the goal is to create a feeling of being part of a group. This isn’t bad, but it’s a replacement for family. Being part of a group has come to fill the place that was once filled by one’s children, grandchildren, and students.
“When the flood of change in modern society rushes in so quickly, there is a ‘programmed phase-out’ of knowledge, and the demand for experience drops off. It becomes less valuable, such that older adults often get more comfort from finding someone who is willing to show an interest in their stories. The best therapist is the one who listens; not necessarily the one who invites them on a walk or a bike ride,” concluded Dr. Bodni.
Dr. Vega, Dr. Guerstein, Dr. Díaz, Dr. Bodni, and Dr. Pedace have disclosed no relevant financial relationships.
This article was translated from the Medscape Spanish Edition . A version appeared on Medscape.com.
MAR DEL PLATA, ARGENTINA – In the “active aging” vision promoted by the World Health Organization (WHO), older adults stay physically active, independent, and involved. This concept, though well-intentioned, is not very realistic and could easily be discouraging to individuals suffering from the psychological or physical limitations of old age. It also does not account for diversity among individuals and across cultures. These conclusions were presented by the Geriatric Psychiatry Chapter of the Argentine Psychiatric Association at its XXXVI Argentine Congress of Psychiatry.
“The WHO’s proposal of active aging is a prescriptive, standardized ideology that seems to suggest that being active is the only healthy way to age. However, that’s only part of the picture, and a biased part at that. It doesn’t account for the broad spectrum of aging processes that come in many shades,” said Mariana Pedace, psychologist with the Adult Intensive Care department at the Italian Hospital in Buenos Aires and head of the Older Adults section of the civic association Project: Unite.
“The question is whether the idea of active aging is just one more way to create mandates or rules for older adults, which make up such a heterogeneous and diverse generation,” said Ana Laura Vega, MD, psychiatrist with the Mental Health Department at the Italian Hospital of Buenos Aires.
Might it be better to speak of aging “as expected” or “aging well”? Speakers at the conference did not reach a consensus on which word would be the best to replace the adjective “active.”
“I don’t really see why there has to be an additional term when, at other stages of life, we only talk about ‘infancy,’ ‘adolescence,’ or ‘middle age,’ ” said Dr. Vega.
A thorny issue
Since the late 1990s, the WHO has defined active aging as “the process of optimizing opportunities for health, participation, and security to enhance quality of life as people age.” This concept allows older adults to “realize their potential for physical, social, and mental well-being throughout the life course and to participate in society according to their needs, desires, and capacities, while providing them with adequate protection, security, and care when they require assistance.”
The organization clarifies that the word “active” refers to continuing participation in social, economic, cultural, spiritual, and civic affairs, not just the ability to be physically active or to participate in the labor force. “However, in practice, active aging programs invariably promote physical activity and exercise as having health and social benefits,” said sociologist Elizabeth Pike, PhD, head of the Research Unit in Sport, Physical Activity, and Aging at the University of Hertfordshire in the United Kingdom.
said Dr. Pedace. Along with laying out a single prescriptive way to age healthily, which by default makes passive aging “abnormal,” it also ignores demographic, ethnographic, and cultural differences.
“Each culture has different values. The suggestion of aging well in terms of activity, autonomy, and a happy-go-lucky mindset clearly reflects Western capitalistic values. In Eastern cultures, elderly people occupy a position reflecting their experience and wisdom, while also maintaining a contemplative mindset, which is something that is held in high regard. They are at the heart of the family, and their role is to guide and counsel the younger generations,” said Dr. Pedace.
The specialist added that there are programs inspired by active aging that prioritize outward, dynamic, and observable activities to the detriment of activities that take place behind the scenes such as reflection, analysis, and contemplation. “Following this mindset, an older individual who spends their time in contemplation would be somewhat wasting their sunset years. This raises a problem, because as the years go by and death approaches, spiritual life begins to gain far more significance. And that’s not an activity that is valued or recommended in the terms of this program,” she said.
Dr. Pedace went on to say that another concern with the active-aging program is that it seems to minimize certain characteristics that are unique to old age. Resulting physical, cognitive, and emotional changes can lead to reduced activity but are merely idiosyncrasies of this stage in life and are not pathologic.
Cecilia Guerstein, psychiatrist with the Older Adults Division of Project: United in Buenos Aires, cited Julieta Oddone, PhD, a sociologist on aging who believes that the theory of activity informs the underlying supposition of most programs for older adults: that social activity in itself is beneficial and results in greater fulfillment in life. And that all older people need and desire to stay active and engaged. “The idea is that the more active they are, the happier they will be,” said Dr. Guerstein.
“But ‘doing things’ isn’t necessarily appreciated by every elderly person, nor does it automatically lead to their well-being. The fact that some find a sense of well-being from it doesn’t mean we have to always do the same activities across different contexts. There are ethnographic studies that show that there isn’t necessarily a relationship between activity and well-being, or true social integration,” said Dr. Pedace.
Not a burden
Practically speaking, few would question whether physical activity has health benefits and believe that it’s never too late to start moving. Among his more than 45 tips on how to live to a ripe old age and “ripen” slowly and nicely, George D. Lundberg, MD, who is 90 years old, gives six recommendations for exercise: walking at least 2 miles every day, trying to swim every day, learning and practicing the techniques of yoga, deliberately lifting heavy objects (resistance training), and working on balance.
“A key for health care professionals encouraging exercise among older adults is knowing what to listen for and how to identify situations that motivate the person to exercise. For example, it could be walking their granddaughter down to the ice cream parlor,” Carolina Díaz, MD, said in an interview. Dr. Díaz is a geriatrics physician and the medical director of the Hirsch nursing and rehab center for older people in San Miguel, Argentina, which is home to 180 residents with an average age of 82 years.
“Exercise shouldn’t be a burden. If someone has never gone on walks before, I wouldn’t make them walk just because they ought to. Maybe they discover well-being in meeting up with their grandchildren or reading with someone. We believe that well-being is related to mobility, but for someone to move, they need the motivation. And until they have that, there won’t be any change,” said Dr. Díaz.
She added that a physician-patient relationship must be forged and an intervention plan drafted that revolves around the person and focuses on his or her current problems such as loneliness, difficulty walking, or pain. “Based on those problems, we can draw up a plan in which physical activity may play a part; other times, it may not.”
Osvaldo Bodni, psychiatrist and psychoanalyst, former director of the Department for Older Adults within the Argentine Psychoanalytic Association and author of the book, Delegating Power in Human Aging: The Theory of Legacy and Passing the Baton) said in an interview: “Aging isn’t a disease, though it does increase vulnerability. The proposal of physical activity is not the only ‘antidote.’ In my opinion, serenity during aging provides even better protection against life’s storms.”
The physician went on to say, “Active aging programs promote physical activity because it’s easier to go on a walk with someone than it is to have a literature debate with them. However, the goal is to create a feeling of being part of a group. This isn’t bad, but it’s a replacement for family. Being part of a group has come to fill the place that was once filled by one’s children, grandchildren, and students.
“When the flood of change in modern society rushes in so quickly, there is a ‘programmed phase-out’ of knowledge, and the demand for experience drops off. It becomes less valuable, such that older adults often get more comfort from finding someone who is willing to show an interest in their stories. The best therapist is the one who listens; not necessarily the one who invites them on a walk or a bike ride,” concluded Dr. Bodni.
Dr. Vega, Dr. Guerstein, Dr. Díaz, Dr. Bodni, and Dr. Pedace have disclosed no relevant financial relationships.
This article was translated from the Medscape Spanish Edition . A version appeared on Medscape.com.
MAR DEL PLATA, ARGENTINA – In the “active aging” vision promoted by the World Health Organization (WHO), older adults stay physically active, independent, and involved. This concept, though well-intentioned, is not very realistic and could easily be discouraging to individuals suffering from the psychological or physical limitations of old age. It also does not account for diversity among individuals and across cultures. These conclusions were presented by the Geriatric Psychiatry Chapter of the Argentine Psychiatric Association at its XXXVI Argentine Congress of Psychiatry.
“The WHO’s proposal of active aging is a prescriptive, standardized ideology that seems to suggest that being active is the only healthy way to age. However, that’s only part of the picture, and a biased part at that. It doesn’t account for the broad spectrum of aging processes that come in many shades,” said Mariana Pedace, psychologist with the Adult Intensive Care department at the Italian Hospital in Buenos Aires and head of the Older Adults section of the civic association Project: Unite.
“The question is whether the idea of active aging is just one more way to create mandates or rules for older adults, which make up such a heterogeneous and diverse generation,” said Ana Laura Vega, MD, psychiatrist with the Mental Health Department at the Italian Hospital of Buenos Aires.
Might it be better to speak of aging “as expected” or “aging well”? Speakers at the conference did not reach a consensus on which word would be the best to replace the adjective “active.”
“I don’t really see why there has to be an additional term when, at other stages of life, we only talk about ‘infancy,’ ‘adolescence,’ or ‘middle age,’ ” said Dr. Vega.
A thorny issue
Since the late 1990s, the WHO has defined active aging as “the process of optimizing opportunities for health, participation, and security to enhance quality of life as people age.” This concept allows older adults to “realize their potential for physical, social, and mental well-being throughout the life course and to participate in society according to their needs, desires, and capacities, while providing them with adequate protection, security, and care when they require assistance.”
The organization clarifies that the word “active” refers to continuing participation in social, economic, cultural, spiritual, and civic affairs, not just the ability to be physically active or to participate in the labor force. “However, in practice, active aging programs invariably promote physical activity and exercise as having health and social benefits,” said sociologist Elizabeth Pike, PhD, head of the Research Unit in Sport, Physical Activity, and Aging at the University of Hertfordshire in the United Kingdom.
said Dr. Pedace. Along with laying out a single prescriptive way to age healthily, which by default makes passive aging “abnormal,” it also ignores demographic, ethnographic, and cultural differences.
“Each culture has different values. The suggestion of aging well in terms of activity, autonomy, and a happy-go-lucky mindset clearly reflects Western capitalistic values. In Eastern cultures, elderly people occupy a position reflecting their experience and wisdom, while also maintaining a contemplative mindset, which is something that is held in high regard. They are at the heart of the family, and their role is to guide and counsel the younger generations,” said Dr. Pedace.
The specialist added that there are programs inspired by active aging that prioritize outward, dynamic, and observable activities to the detriment of activities that take place behind the scenes such as reflection, analysis, and contemplation. “Following this mindset, an older individual who spends their time in contemplation would be somewhat wasting their sunset years. This raises a problem, because as the years go by and death approaches, spiritual life begins to gain far more significance. And that’s not an activity that is valued or recommended in the terms of this program,” she said.
Dr. Pedace went on to say that another concern with the active-aging program is that it seems to minimize certain characteristics that are unique to old age. Resulting physical, cognitive, and emotional changes can lead to reduced activity but are merely idiosyncrasies of this stage in life and are not pathologic.
Cecilia Guerstein, psychiatrist with the Older Adults Division of Project: United in Buenos Aires, cited Julieta Oddone, PhD, a sociologist on aging who believes that the theory of activity informs the underlying supposition of most programs for older adults: that social activity in itself is beneficial and results in greater fulfillment in life. And that all older people need and desire to stay active and engaged. “The idea is that the more active they are, the happier they will be,” said Dr. Guerstein.
“But ‘doing things’ isn’t necessarily appreciated by every elderly person, nor does it automatically lead to their well-being. The fact that some find a sense of well-being from it doesn’t mean we have to always do the same activities across different contexts. There are ethnographic studies that show that there isn’t necessarily a relationship between activity and well-being, or true social integration,” said Dr. Pedace.
Not a burden
Practically speaking, few would question whether physical activity has health benefits and believe that it’s never too late to start moving. Among his more than 45 tips on how to live to a ripe old age and “ripen” slowly and nicely, George D. Lundberg, MD, who is 90 years old, gives six recommendations for exercise: walking at least 2 miles every day, trying to swim every day, learning and practicing the techniques of yoga, deliberately lifting heavy objects (resistance training), and working on balance.
“A key for health care professionals encouraging exercise among older adults is knowing what to listen for and how to identify situations that motivate the person to exercise. For example, it could be walking their granddaughter down to the ice cream parlor,” Carolina Díaz, MD, said in an interview. Dr. Díaz is a geriatrics physician and the medical director of the Hirsch nursing and rehab center for older people in San Miguel, Argentina, which is home to 180 residents with an average age of 82 years.
“Exercise shouldn’t be a burden. If someone has never gone on walks before, I wouldn’t make them walk just because they ought to. Maybe they discover well-being in meeting up with their grandchildren or reading with someone. We believe that well-being is related to mobility, but for someone to move, they need the motivation. And until they have that, there won’t be any change,” said Dr. Díaz.
She added that a physician-patient relationship must be forged and an intervention plan drafted that revolves around the person and focuses on his or her current problems such as loneliness, difficulty walking, or pain. “Based on those problems, we can draw up a plan in which physical activity may play a part; other times, it may not.”
Osvaldo Bodni, psychiatrist and psychoanalyst, former director of the Department for Older Adults within the Argentine Psychoanalytic Association and author of the book, Delegating Power in Human Aging: The Theory of Legacy and Passing the Baton) said in an interview: “Aging isn’t a disease, though it does increase vulnerability. The proposal of physical activity is not the only ‘antidote.’ In my opinion, serenity during aging provides even better protection against life’s storms.”
The physician went on to say, “Active aging programs promote physical activity because it’s easier to go on a walk with someone than it is to have a literature debate with them. However, the goal is to create a feeling of being part of a group. This isn’t bad, but it’s a replacement for family. Being part of a group has come to fill the place that was once filled by one’s children, grandchildren, and students.
“When the flood of change in modern society rushes in so quickly, there is a ‘programmed phase-out’ of knowledge, and the demand for experience drops off. It becomes less valuable, such that older adults often get more comfort from finding someone who is willing to show an interest in their stories. The best therapist is the one who listens; not necessarily the one who invites them on a walk or a bike ride,” concluded Dr. Bodni.
Dr. Vega, Dr. Guerstein, Dr. Díaz, Dr. Bodni, and Dr. Pedace have disclosed no relevant financial relationships.
This article was translated from the Medscape Spanish Edition . A version appeared on Medscape.com.
Muscle fat: A new risk factor for cognitive decline?
Investigators assessed muscle fat in more than 1,600 adults in their 70s and evaluated their cognitive function over a 10-year period. They found that increases in muscle adiposity from year 1 to year 6 were associated with greater cognitive decline over time, independent of total weight, other fat deposits, muscle characteristics, and traditional dementia risk factors.
The findings were similar between Black and White people and between men and women.
“Increasing adiposity – or fat deposition – in skeletal muscles predicted faster cognitive decline, irrespective of demographics or other disease, and this effect was distinct from that of other types of fat or other muscle characteristics, such as strength or mass,” study investigator Caterina Rosano MD, MPH, professor of epidemiology at the University of Pittsburgh, said in an interview.
The study was published in the Journal of the American Geriatrics Society.
Biologically plausible
“There has been a growing recognition that overall adiposity and muscle measures, such as strength and mass, are individual indicators of future dementia risk and both strengthen the algorithms to predict cognitive decline,” said Dr. Rosano, associate director for clinical translation at the University of Pittsburgh’s Aging Institute. “However, adiposity in the muscle has not been examined.”
Some evidence supports a “biologically plausible link” between muscle adiposity and dementia risk. For example, muscle adiposity increases the risk for type 2 diabetes and hypertension, both of which are dementia risk factors.
Skeletal muscle adiposity increases with older age, even in older adults who lose weight, and is “highly prevalent” among older adults of African ancestry.
The researchers examined a large, biracial sample of older adults participating in the Health, Aging and Body Composition study, which enrolled men and women aged between 70 and 79 years. Participants were followed for an average of 9.0 ± 1.8 years.
During years 1 and 6, participants’ body composition was analyzed, including intermuscular adipose tissue (IMAT), visceral and subcutaneous adiposity, total fat mass, and muscle area.
In years 1, 3, 5, 8, and 10, participants’ cognition was measured using the modified Mini-Mental State (3MS) exam.
The main independent variable was 5-year change in thigh IMAT (year 6 minus year 1), and the main dependent variable was 3MS decline (from year 5 to year 10).
The researchers adjusted all the models for traditional dementia risk factors at baseline including 3MS, education, apo E4 allele, diabetes, hypertension, and physical activity and also calculated interactions between IMAT change by race or sex.
These models also accounted for change in muscle strength, muscle area, body weight, abdominal subcutaneous and visceral adiposity, and total body fat mass as well as cytokines related to adiposity.
‘Rich and engaging crosstalk’
The final sample included 1634 participants (mean age, 73.38 years at baseline; 48% female; 35% Black; mean baseline 3MS score, 91.6).
Thigh IMAT increased by 39.0% in all participants from year 1 to year 6, which corresponded to an increase of 4.85 cm2 or 0.97 cm2/year. During the same time period, muscle strength decreased by 14.0% (P < .05), although thigh muscle area remained stable, decreasing less than 0.5%.
There were decreases in both abdominal subcutaneous and visceral adiposity of 3.92% and 6.43%, respectively (P < .05). There was a decrease of 3.3% in 3MS from year 5 to year 10.
Several variables were associated with 3MS decline, independent of any change in thigh IMAT: older age, less education, and having at least one copy of the APOe4 allele. These variables were included in the model of IMAT change predicting 3MS change.
A statistically significant association of IMAT increase with 3MS decline was found. The IMAT increase of 4.85 cm2 corresponded to a 3MS decline of an additional 3.6 points (P < .0001) from year 5 to year 10, “indicating a clinically important change.”
The association between increasing thigh IMAT with declining 3MS “remained statistically significant” after adjusting for race, age, education, and apo E4 (P < .0001) and was independent of changes in thigh muscle area, muscle strength, and other adiposity measures.
In participants with increased IMAT in years 1-6, the mean 3MS score fell to approximately 87 points at year 10, compared with those without increased IMAT, with a 3MS score that dropped to approximately 89 points.
Interactions by race and sex were not statistically significant (P > .08).
“Our results suggest that adiposity in muscles can predict cognitive decline, in addition to (not instead of) other traditional dementia risk factors,” said Dr. Rosano.
There is “a rich and engaging crosstalk between muscle, adipose tissue, and the brain all throughout our lives, happening through factors released in the bloodstream that can reach the brain, however, the specific identity of the factors responsible for the crosstalk of muscle adiposity and brain in older adults has not yet been discovered,” she noted.
Although muscle adiposity is “not yet routinely measured in clinical settings, it is being measured opportunistically on clinical CT scans obtained as part of routine patient care,” she added. “These CT measurements have already been validated in many studies of older adults; thus, clinicians could have access to this novel information without additional cost, time, or radiation exposure.”
Causality not proven
In a comment, Bruce Albala, PhD, professor, department of environmental and occupational health, University of California, Irvine, noted that the 3MS assessment is scored on a 100-point scale, with a score less than 78 “generally regarded as indicating cognitive impairment or approaching a dementia condition.” In the current study, the mean 3MS score of participants with increased IMAT was still “well above the dementia cut-off.”
Moreover, “even if there is a relationship or correlation between IMAT and cognition, this does not prove or even suggest causality, especially from a biological mechanistic approach,” said Dr. Albaba, an adjunct professor of neurology, who was not involved in the study. “Clearly, more research is needed even to understand the relationship between these two factors.”
The study was supported by the National Institute on Aging. Dr. Rosano and coauthors and Dr. Albala declared no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Investigators assessed muscle fat in more than 1,600 adults in their 70s and evaluated their cognitive function over a 10-year period. They found that increases in muscle adiposity from year 1 to year 6 were associated with greater cognitive decline over time, independent of total weight, other fat deposits, muscle characteristics, and traditional dementia risk factors.
The findings were similar between Black and White people and between men and women.
“Increasing adiposity – or fat deposition – in skeletal muscles predicted faster cognitive decline, irrespective of demographics or other disease, and this effect was distinct from that of other types of fat or other muscle characteristics, such as strength or mass,” study investigator Caterina Rosano MD, MPH, professor of epidemiology at the University of Pittsburgh, said in an interview.
The study was published in the Journal of the American Geriatrics Society.
Biologically plausible
“There has been a growing recognition that overall adiposity and muscle measures, such as strength and mass, are individual indicators of future dementia risk and both strengthen the algorithms to predict cognitive decline,” said Dr. Rosano, associate director for clinical translation at the University of Pittsburgh’s Aging Institute. “However, adiposity in the muscle has not been examined.”
Some evidence supports a “biologically plausible link” between muscle adiposity and dementia risk. For example, muscle adiposity increases the risk for type 2 diabetes and hypertension, both of which are dementia risk factors.
Skeletal muscle adiposity increases with older age, even in older adults who lose weight, and is “highly prevalent” among older adults of African ancestry.
The researchers examined a large, biracial sample of older adults participating in the Health, Aging and Body Composition study, which enrolled men and women aged between 70 and 79 years. Participants were followed for an average of 9.0 ± 1.8 years.
During years 1 and 6, participants’ body composition was analyzed, including intermuscular adipose tissue (IMAT), visceral and subcutaneous adiposity, total fat mass, and muscle area.
In years 1, 3, 5, 8, and 10, participants’ cognition was measured using the modified Mini-Mental State (3MS) exam.
The main independent variable was 5-year change in thigh IMAT (year 6 minus year 1), and the main dependent variable was 3MS decline (from year 5 to year 10).
The researchers adjusted all the models for traditional dementia risk factors at baseline including 3MS, education, apo E4 allele, diabetes, hypertension, and physical activity and also calculated interactions between IMAT change by race or sex.
These models also accounted for change in muscle strength, muscle area, body weight, abdominal subcutaneous and visceral adiposity, and total body fat mass as well as cytokines related to adiposity.
‘Rich and engaging crosstalk’
The final sample included 1634 participants (mean age, 73.38 years at baseline; 48% female; 35% Black; mean baseline 3MS score, 91.6).
Thigh IMAT increased by 39.0% in all participants from year 1 to year 6, which corresponded to an increase of 4.85 cm2 or 0.97 cm2/year. During the same time period, muscle strength decreased by 14.0% (P < .05), although thigh muscle area remained stable, decreasing less than 0.5%.
There were decreases in both abdominal subcutaneous and visceral adiposity of 3.92% and 6.43%, respectively (P < .05). There was a decrease of 3.3% in 3MS from year 5 to year 10.
Several variables were associated with 3MS decline, independent of any change in thigh IMAT: older age, less education, and having at least one copy of the APOe4 allele. These variables were included in the model of IMAT change predicting 3MS change.
A statistically significant association of IMAT increase with 3MS decline was found. The IMAT increase of 4.85 cm2 corresponded to a 3MS decline of an additional 3.6 points (P < .0001) from year 5 to year 10, “indicating a clinically important change.”
The association between increasing thigh IMAT with declining 3MS “remained statistically significant” after adjusting for race, age, education, and apo E4 (P < .0001) and was independent of changes in thigh muscle area, muscle strength, and other adiposity measures.
In participants with increased IMAT in years 1-6, the mean 3MS score fell to approximately 87 points at year 10, compared with those without increased IMAT, with a 3MS score that dropped to approximately 89 points.
Interactions by race and sex were not statistically significant (P > .08).
“Our results suggest that adiposity in muscles can predict cognitive decline, in addition to (not instead of) other traditional dementia risk factors,” said Dr. Rosano.
There is “a rich and engaging crosstalk between muscle, adipose tissue, and the brain all throughout our lives, happening through factors released in the bloodstream that can reach the brain, however, the specific identity of the factors responsible for the crosstalk of muscle adiposity and brain in older adults has not yet been discovered,” she noted.
Although muscle adiposity is “not yet routinely measured in clinical settings, it is being measured opportunistically on clinical CT scans obtained as part of routine patient care,” she added. “These CT measurements have already been validated in many studies of older adults; thus, clinicians could have access to this novel information without additional cost, time, or radiation exposure.”
Causality not proven
In a comment, Bruce Albala, PhD, professor, department of environmental and occupational health, University of California, Irvine, noted that the 3MS assessment is scored on a 100-point scale, with a score less than 78 “generally regarded as indicating cognitive impairment or approaching a dementia condition.” In the current study, the mean 3MS score of participants with increased IMAT was still “well above the dementia cut-off.”
Moreover, “even if there is a relationship or correlation between IMAT and cognition, this does not prove or even suggest causality, especially from a biological mechanistic approach,” said Dr. Albaba, an adjunct professor of neurology, who was not involved in the study. “Clearly, more research is needed even to understand the relationship between these two factors.”
The study was supported by the National Institute on Aging. Dr. Rosano and coauthors and Dr. Albala declared no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Investigators assessed muscle fat in more than 1,600 adults in their 70s and evaluated their cognitive function over a 10-year period. They found that increases in muscle adiposity from year 1 to year 6 were associated with greater cognitive decline over time, independent of total weight, other fat deposits, muscle characteristics, and traditional dementia risk factors.
The findings were similar between Black and White people and between men and women.
“Increasing adiposity – or fat deposition – in skeletal muscles predicted faster cognitive decline, irrespective of demographics or other disease, and this effect was distinct from that of other types of fat or other muscle characteristics, such as strength or mass,” study investigator Caterina Rosano MD, MPH, professor of epidemiology at the University of Pittsburgh, said in an interview.
The study was published in the Journal of the American Geriatrics Society.
Biologically plausible
“There has been a growing recognition that overall adiposity and muscle measures, such as strength and mass, are individual indicators of future dementia risk and both strengthen the algorithms to predict cognitive decline,” said Dr. Rosano, associate director for clinical translation at the University of Pittsburgh’s Aging Institute. “However, adiposity in the muscle has not been examined.”
Some evidence supports a “biologically plausible link” between muscle adiposity and dementia risk. For example, muscle adiposity increases the risk for type 2 diabetes and hypertension, both of which are dementia risk factors.
Skeletal muscle adiposity increases with older age, even in older adults who lose weight, and is “highly prevalent” among older adults of African ancestry.
The researchers examined a large, biracial sample of older adults participating in the Health, Aging and Body Composition study, which enrolled men and women aged between 70 and 79 years. Participants were followed for an average of 9.0 ± 1.8 years.
During years 1 and 6, participants’ body composition was analyzed, including intermuscular adipose tissue (IMAT), visceral and subcutaneous adiposity, total fat mass, and muscle area.
In years 1, 3, 5, 8, and 10, participants’ cognition was measured using the modified Mini-Mental State (3MS) exam.
The main independent variable was 5-year change in thigh IMAT (year 6 minus year 1), and the main dependent variable was 3MS decline (from year 5 to year 10).
The researchers adjusted all the models for traditional dementia risk factors at baseline including 3MS, education, apo E4 allele, diabetes, hypertension, and physical activity and also calculated interactions between IMAT change by race or sex.
These models also accounted for change in muscle strength, muscle area, body weight, abdominal subcutaneous and visceral adiposity, and total body fat mass as well as cytokines related to adiposity.
‘Rich and engaging crosstalk’
The final sample included 1634 participants (mean age, 73.38 years at baseline; 48% female; 35% Black; mean baseline 3MS score, 91.6).
Thigh IMAT increased by 39.0% in all participants from year 1 to year 6, which corresponded to an increase of 4.85 cm2 or 0.97 cm2/year. During the same time period, muscle strength decreased by 14.0% (P < .05), although thigh muscle area remained stable, decreasing less than 0.5%.
There were decreases in both abdominal subcutaneous and visceral adiposity of 3.92% and 6.43%, respectively (P < .05). There was a decrease of 3.3% in 3MS from year 5 to year 10.
Several variables were associated with 3MS decline, independent of any change in thigh IMAT: older age, less education, and having at least one copy of the APOe4 allele. These variables were included in the model of IMAT change predicting 3MS change.
A statistically significant association of IMAT increase with 3MS decline was found. The IMAT increase of 4.85 cm2 corresponded to a 3MS decline of an additional 3.6 points (P < .0001) from year 5 to year 10, “indicating a clinically important change.”
The association between increasing thigh IMAT with declining 3MS “remained statistically significant” after adjusting for race, age, education, and apo E4 (P < .0001) and was independent of changes in thigh muscle area, muscle strength, and other adiposity measures.
In participants with increased IMAT in years 1-6, the mean 3MS score fell to approximately 87 points at year 10, compared with those without increased IMAT, with a 3MS score that dropped to approximately 89 points.
Interactions by race and sex were not statistically significant (P > .08).
“Our results suggest that adiposity in muscles can predict cognitive decline, in addition to (not instead of) other traditional dementia risk factors,” said Dr. Rosano.
There is “a rich and engaging crosstalk between muscle, adipose tissue, and the brain all throughout our lives, happening through factors released in the bloodstream that can reach the brain, however, the specific identity of the factors responsible for the crosstalk of muscle adiposity and brain in older adults has not yet been discovered,” she noted.
Although muscle adiposity is “not yet routinely measured in clinical settings, it is being measured opportunistically on clinical CT scans obtained as part of routine patient care,” she added. “These CT measurements have already been validated in many studies of older adults; thus, clinicians could have access to this novel information without additional cost, time, or radiation exposure.”
Causality not proven
In a comment, Bruce Albala, PhD, professor, department of environmental and occupational health, University of California, Irvine, noted that the 3MS assessment is scored on a 100-point scale, with a score less than 78 “generally regarded as indicating cognitive impairment or approaching a dementia condition.” In the current study, the mean 3MS score of participants with increased IMAT was still “well above the dementia cut-off.”
Moreover, “even if there is a relationship or correlation between IMAT and cognition, this does not prove or even suggest causality, especially from a biological mechanistic approach,” said Dr. Albaba, an adjunct professor of neurology, who was not involved in the study. “Clearly, more research is needed even to understand the relationship between these two factors.”
The study was supported by the National Institute on Aging. Dr. Rosano and coauthors and Dr. Albala declared no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN GERIATRICS SOCIETY
Blood biomarker may help predict who will develop Alzheimer’s
A blood biomarker that measures astrocyte reactivity may help determine who, among cognitively unimpaired older adults with amyloid-beta, will go on to develop Alzheimer’s disease (AD), new research suggests.
Investigators tested the blood of 1,000 cognitively healthy individuals with and without amyloid-beta pathology and found that only those with a combination of amyloid-beta burden and abnormal astrocyte activation subsequently progressed to AD.
“Our study argues that testing for the presence of brain amyloid along with blood biomarkers of astrocyte reactivity is the optimal screening to identify patients who are most at risk for progressing to Alzheimer’s disease,” senior investigator Tharick A. Pascoal, MD, PhD, associate professor of psychiatry and neurology, University of Pittsburgh, said in a release.
At this point, the biomarker is a research tool, but its application in clinical practice “is not very far away,” Dr. Pascoal told this news organization.
The study was published online in Nature Medicine.
Multicenter study
In AD, accumulation of amyloid-beta in the brain precedes tau pathology, but not everyone with amyloid-beta develops tau, and, consequently, clinical symptoms. Approximately 30% of older adults have brain amyloid but many never progress to AD, said Dr. Pascoal.
This suggests other biological processes may trigger the deleterious effects of amyloid-beta in the early stages of AD.
Finding predictive markers of early amyloid-beta–related tau pathology would help identify cognitively normal individuals who are more likely to develop AD.
Post-mortem studies show astrocyte reactivity – changes in glial cells in the brain and spinal cord because of an insult in the brain – is an early AD abnormality. Other research suggests a close link between amyloid-beta, astrocyte reactivity, and tau.
In addition, evidence suggests plasma measures of glial fibrillary acidic protein (GFAP) could be a strong proxy of astrocyte reactivity in the brain. Dr. Pascoal explained that when astrocytes are changed or become bigger, more GFAP is released.
The study included 1,016 cognitively normal individuals from three centers; some had amyloid pathology, some did not. Participants’ mean age was 69.6 years, and all were deemed negative or positive for astrocyte reactivity based on plasma GFAP levels.
Results showed amyloid-beta is associated with increased plasma phosphorylated tau only in individuals positive for astrocyte reactivity. In addition, analyses using PET scans showed an AD-like pattern of tau tangle accumulation as a function of amyloid-beta exclusively in those same individuals.
Early upstream event
The findings suggest abnormalities in astrocyte reactivity is an early upstream event that likely occurs prior to tau pathology, which is closely related to the development of neurodegeneration and cognitive decline.
It’s likely many types of insults or processes can lead to astrocyte reactivity, possibly including COVID, but more research in this area is needed, said Dr. Pascoal.
“Our study only looked at the consequence of having both amyloid and astrocyte reactivity; it did not elucidate what is causing either of them,” he said.
Although “we were able to have very good results” in the current study, additional studies are needed to better establish the cut-off for GFAP levels that signal progression, said Dr. Pascoal.
The effect of astrocyte reactivity on the association between amyloid-beta and tau phosphorylation was greater in men than women. Dr. Pascoal noted anti-amyloid therapies, which might be modifying the amyloid-beta-astrocyte-tau pathway, tend to have a much larger effect in men than women.
Further studies that measure amyloid-beta, tau, and GFAP biomarkers at multiple timepoints, and with long follow-up, are needed, the investigators note.
The results may have implications for clinical trials, which have increasingly focused on individuals in the earliest preclinical phases of AD. Future studies should include cognitively normal patients who are positive for both amyloid pathology and astrocyte reactivity but have no overt p-tau abnormality, said Dr. Pascoal.
This may provide a time window for interventions very early in the disease process in those at increased risk for AD-related progression.
The study did not determine whether participants with both amyloid and astrocyte reactivity will inevitably develop AD, and to do so would require a longer follow up. “Our outcome was correlation to tau in the brain, which is something we know will lead to AD.”
Although the cohort represents significant socioeconomic diversity, a main limitation of the study was that subjects were mainly White, which limits the generalizability of the findings to a more diverse population.
The study received support from the National Institute of Aging; National Heart Lung and Blood Institute; Alzheimer’s Association; Fonds de Recherche du Québec-Santé; Canadian Consortium of Neurodegeneration in Aging; Weston Brain Institute; Colin Adair Charitable Foundation; Swedish Research Council; Wallenberg Scholar; BrightFocus Foundation; Swedish Alzheimer Foundation; Swedish Brain Foundation; Agneta Prytz-Folkes & Gösta Folkes Foundation; European Union; Swedish State Support for Clinical Research; Alzheimer Drug Discovery Foundation; Bluefield Project, the Olav Thon Foundation, the Erling-Persson Family Foundation, Stiftelsen för Gamla Tjänarinnor, Hjärnfonden, Sweden; the UK Dementia Research Institute at UCL; National Academy of Neuropsychology; Fundação de Amparo a pesquisa do Rio Grande do Sul; Instituto Serrapilheira; and Hjärnfonden.
Dr. Pascoal reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A blood biomarker that measures astrocyte reactivity may help determine who, among cognitively unimpaired older adults with amyloid-beta, will go on to develop Alzheimer’s disease (AD), new research suggests.
Investigators tested the blood of 1,000 cognitively healthy individuals with and without amyloid-beta pathology and found that only those with a combination of amyloid-beta burden and abnormal astrocyte activation subsequently progressed to AD.
“Our study argues that testing for the presence of brain amyloid along with blood biomarkers of astrocyte reactivity is the optimal screening to identify patients who are most at risk for progressing to Alzheimer’s disease,” senior investigator Tharick A. Pascoal, MD, PhD, associate professor of psychiatry and neurology, University of Pittsburgh, said in a release.
At this point, the biomarker is a research tool, but its application in clinical practice “is not very far away,” Dr. Pascoal told this news organization.
The study was published online in Nature Medicine.
Multicenter study
In AD, accumulation of amyloid-beta in the brain precedes tau pathology, but not everyone with amyloid-beta develops tau, and, consequently, clinical symptoms. Approximately 30% of older adults have brain amyloid but many never progress to AD, said Dr. Pascoal.
This suggests other biological processes may trigger the deleterious effects of amyloid-beta in the early stages of AD.
Finding predictive markers of early amyloid-beta–related tau pathology would help identify cognitively normal individuals who are more likely to develop AD.
Post-mortem studies show astrocyte reactivity – changes in glial cells in the brain and spinal cord because of an insult in the brain – is an early AD abnormality. Other research suggests a close link between amyloid-beta, astrocyte reactivity, and tau.
In addition, evidence suggests plasma measures of glial fibrillary acidic protein (GFAP) could be a strong proxy of astrocyte reactivity in the brain. Dr. Pascoal explained that when astrocytes are changed or become bigger, more GFAP is released.
The study included 1,016 cognitively normal individuals from three centers; some had amyloid pathology, some did not. Participants’ mean age was 69.6 years, and all were deemed negative or positive for astrocyte reactivity based on plasma GFAP levels.
Results showed amyloid-beta is associated with increased plasma phosphorylated tau only in individuals positive for astrocyte reactivity. In addition, analyses using PET scans showed an AD-like pattern of tau tangle accumulation as a function of amyloid-beta exclusively in those same individuals.
Early upstream event
The findings suggest abnormalities in astrocyte reactivity is an early upstream event that likely occurs prior to tau pathology, which is closely related to the development of neurodegeneration and cognitive decline.
It’s likely many types of insults or processes can lead to astrocyte reactivity, possibly including COVID, but more research in this area is needed, said Dr. Pascoal.
“Our study only looked at the consequence of having both amyloid and astrocyte reactivity; it did not elucidate what is causing either of them,” he said.
Although “we were able to have very good results” in the current study, additional studies are needed to better establish the cut-off for GFAP levels that signal progression, said Dr. Pascoal.
The effect of astrocyte reactivity on the association between amyloid-beta and tau phosphorylation was greater in men than women. Dr. Pascoal noted anti-amyloid therapies, which might be modifying the amyloid-beta-astrocyte-tau pathway, tend to have a much larger effect in men than women.
Further studies that measure amyloid-beta, tau, and GFAP biomarkers at multiple timepoints, and with long follow-up, are needed, the investigators note.
The results may have implications for clinical trials, which have increasingly focused on individuals in the earliest preclinical phases of AD. Future studies should include cognitively normal patients who are positive for both amyloid pathology and astrocyte reactivity but have no overt p-tau abnormality, said Dr. Pascoal.
This may provide a time window for interventions very early in the disease process in those at increased risk for AD-related progression.
The study did not determine whether participants with both amyloid and astrocyte reactivity will inevitably develop AD, and to do so would require a longer follow up. “Our outcome was correlation to tau in the brain, which is something we know will lead to AD.”
Although the cohort represents significant socioeconomic diversity, a main limitation of the study was that subjects were mainly White, which limits the generalizability of the findings to a more diverse population.
The study received support from the National Institute of Aging; National Heart Lung and Blood Institute; Alzheimer’s Association; Fonds de Recherche du Québec-Santé; Canadian Consortium of Neurodegeneration in Aging; Weston Brain Institute; Colin Adair Charitable Foundation; Swedish Research Council; Wallenberg Scholar; BrightFocus Foundation; Swedish Alzheimer Foundation; Swedish Brain Foundation; Agneta Prytz-Folkes & Gösta Folkes Foundation; European Union; Swedish State Support for Clinical Research; Alzheimer Drug Discovery Foundation; Bluefield Project, the Olav Thon Foundation, the Erling-Persson Family Foundation, Stiftelsen för Gamla Tjänarinnor, Hjärnfonden, Sweden; the UK Dementia Research Institute at UCL; National Academy of Neuropsychology; Fundação de Amparo a pesquisa do Rio Grande do Sul; Instituto Serrapilheira; and Hjärnfonden.
Dr. Pascoal reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A blood biomarker that measures astrocyte reactivity may help determine who, among cognitively unimpaired older adults with amyloid-beta, will go on to develop Alzheimer’s disease (AD), new research suggests.
Investigators tested the blood of 1,000 cognitively healthy individuals with and without amyloid-beta pathology and found that only those with a combination of amyloid-beta burden and abnormal astrocyte activation subsequently progressed to AD.
“Our study argues that testing for the presence of brain amyloid along with blood biomarkers of astrocyte reactivity is the optimal screening to identify patients who are most at risk for progressing to Alzheimer’s disease,” senior investigator Tharick A. Pascoal, MD, PhD, associate professor of psychiatry and neurology, University of Pittsburgh, said in a release.
At this point, the biomarker is a research tool, but its application in clinical practice “is not very far away,” Dr. Pascoal told this news organization.
The study was published online in Nature Medicine.
Multicenter study
In AD, accumulation of amyloid-beta in the brain precedes tau pathology, but not everyone with amyloid-beta develops tau, and, consequently, clinical symptoms. Approximately 30% of older adults have brain amyloid but many never progress to AD, said Dr. Pascoal.
This suggests other biological processes may trigger the deleterious effects of amyloid-beta in the early stages of AD.
Finding predictive markers of early amyloid-beta–related tau pathology would help identify cognitively normal individuals who are more likely to develop AD.
Post-mortem studies show astrocyte reactivity – changes in glial cells in the brain and spinal cord because of an insult in the brain – is an early AD abnormality. Other research suggests a close link between amyloid-beta, astrocyte reactivity, and tau.
In addition, evidence suggests plasma measures of glial fibrillary acidic protein (GFAP) could be a strong proxy of astrocyte reactivity in the brain. Dr. Pascoal explained that when astrocytes are changed or become bigger, more GFAP is released.
The study included 1,016 cognitively normal individuals from three centers; some had amyloid pathology, some did not. Participants’ mean age was 69.6 years, and all were deemed negative or positive for astrocyte reactivity based on plasma GFAP levels.
Results showed amyloid-beta is associated with increased plasma phosphorylated tau only in individuals positive for astrocyte reactivity. In addition, analyses using PET scans showed an AD-like pattern of tau tangle accumulation as a function of amyloid-beta exclusively in those same individuals.
Early upstream event
The findings suggest abnormalities in astrocyte reactivity is an early upstream event that likely occurs prior to tau pathology, which is closely related to the development of neurodegeneration and cognitive decline.
It’s likely many types of insults or processes can lead to astrocyte reactivity, possibly including COVID, but more research in this area is needed, said Dr. Pascoal.
“Our study only looked at the consequence of having both amyloid and astrocyte reactivity; it did not elucidate what is causing either of them,” he said.
Although “we were able to have very good results” in the current study, additional studies are needed to better establish the cut-off for GFAP levels that signal progression, said Dr. Pascoal.
The effect of astrocyte reactivity on the association between amyloid-beta and tau phosphorylation was greater in men than women. Dr. Pascoal noted anti-amyloid therapies, which might be modifying the amyloid-beta-astrocyte-tau pathway, tend to have a much larger effect in men than women.
Further studies that measure amyloid-beta, tau, and GFAP biomarkers at multiple timepoints, and with long follow-up, are needed, the investigators note.
The results may have implications for clinical trials, which have increasingly focused on individuals in the earliest preclinical phases of AD. Future studies should include cognitively normal patients who are positive for both amyloid pathology and astrocyte reactivity but have no overt p-tau abnormality, said Dr. Pascoal.
This may provide a time window for interventions very early in the disease process in those at increased risk for AD-related progression.
The study did not determine whether participants with both amyloid and astrocyte reactivity will inevitably develop AD, and to do so would require a longer follow up. “Our outcome was correlation to tau in the brain, which is something we know will lead to AD.”
Although the cohort represents significant socioeconomic diversity, a main limitation of the study was that subjects were mainly White, which limits the generalizability of the findings to a more diverse population.
The study received support from the National Institute of Aging; National Heart Lung and Blood Institute; Alzheimer’s Association; Fonds de Recherche du Québec-Santé; Canadian Consortium of Neurodegeneration in Aging; Weston Brain Institute; Colin Adair Charitable Foundation; Swedish Research Council; Wallenberg Scholar; BrightFocus Foundation; Swedish Alzheimer Foundation; Swedish Brain Foundation; Agneta Prytz-Folkes & Gösta Folkes Foundation; European Union; Swedish State Support for Clinical Research; Alzheimer Drug Discovery Foundation; Bluefield Project, the Olav Thon Foundation, the Erling-Persson Family Foundation, Stiftelsen för Gamla Tjänarinnor, Hjärnfonden, Sweden; the UK Dementia Research Institute at UCL; National Academy of Neuropsychology; Fundação de Amparo a pesquisa do Rio Grande do Sul; Instituto Serrapilheira; and Hjärnfonden.
Dr. Pascoal reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Medicaid patients with heart failure get poor follow-up after hospital discharge
Nearly 60% of Medicaid-covered adults with concurrent diabetes and heart failure did not receive guideline-concordant postdischarge care within 7-10 days of leaving the hospital, according to a large Alabama study. Moreover, affected Black and Hispanic/other Alabamians were less likely than were their White counterparts to receive recommended postdischarge care.
In comparison with White participants, Black and Hispanic adults were less likely to have any postdischarge ambulatory care visits after HF hospitalization or had a delayed visit, according to researchers led by Yulia Khodneva, MD, PhD, an internist at the University of Alabama at Birmingham. “This is likely a reflection of a structural racism and implicit bias against racial and ethnic minorities that persists in the U.S. health care system,” she and her colleagues wrote.
The findings point to the need for strategies to improve access to postdischarge care for lower-income HF patients.
Among U.S. states, Alabama is the sixth-poorest, the third in diabetes prevalence (14%), and has the highest rates of heart failure hospitalizations and cardiovascular mortality, the authors noted.
Study details
The cohort included 9,857 adults with diabetes and first hospitalizations for heart failure who were covered by Alabama Medicaid during 2010-2019. The investigators analyzed patients’ claims for ambulatory care (any, primary, cardiology, or endocrinology) within 60 days of discharge.
The mean age of participants was 53.7 years; 47.3% were Black; 41.8% non-Hispanic White; and 10.9% Hispanic/other, with other including those identifying as non-White Hispanic, American Indian, Pacific Islander, and Asian. About two-thirds (65.4%) of participants were women.
Analysis revealed low rates of follow-up care after hospital discharge; 26.7% had an ambulatory visit within 0-7 days, 15.2% within 8-14 days, 31.3% within 15-60 days, and 26.8% had no follow-up visit at all. Of those having a follow-up visit, 71% saw a primary care physician and 12% saw a cardiologist.
In contrast, a much higher proportion of heart failure patients in a Swedish registry – 63% – received ambulatory follow-up in cardiology.
Ethnic/gender/age disparities
Black and Hispanic/other adults were less likely to have any postdischarge ambulatory visit (P <.0001) or had the visit delayed by 1.8 days (P = .0006) and 2.8 days (P = .0016), respectively. They were less likely to see a primary care physician than were non-Hispanic White adults: adjusted incidence rate ratio, 0.96 (95% confidence interval [CI], 0.91-1.00) and 0.91 (95% CI, 0.89-0.98), respectively.
Men and those with longer-standing heart failure were less likely to be seen in primary care, while the presence of multiple comorbidities was associated with a higher likelihood of a postdischarge primary care visit. Men were more likely to be seen by a cardiologist, while older discharged patients were less likely to be seen by an endocrinologist within 60 days. There was a U-shaped relationship between the timing of the first postdischarge ambulatory visit and all-cause mortality among adults with diabetes and heart failure. Higher rates of 60-day all-cause mortality were observed both in those who had seen a provider within 0-7 days after discharge and in those who had not seen any provider during the 60-day study period compared with those having an ambulatory care visit within 7-14 or 15-60 days. “The group with early follow-up (0-7 days) likely represents a sicker population of patients with heart failure with more comorbidity burden and higher overall health care use, including readmissions, as was demonstrated in our analysis,” Dr. Khodneva and associates wrote. “Interventions that improve access to postdischarge ambulatory care for low-income patients with diabetes and heart failure and eliminate racial and ethnic disparities may be warranted,” they added.
This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases and the University of Alabama at Birmingham Diabetes Research Center. Dr. Khodneva reported funding from the University of Alabama at Birmingham and the Forge Ahead Center as well as from the NIDDK, the National Institutes of Health, the Agency for Healthcare Research and Quality, and the Alabama Medicaid Agency. Coauthor Emily Levitan, ScD, reported research funding from Amgen and has served on Amgen advisory boards. She has also served as a scientific consultant for a research project funded by Novartis.
Nearly 60% of Medicaid-covered adults with concurrent diabetes and heart failure did not receive guideline-concordant postdischarge care within 7-10 days of leaving the hospital, according to a large Alabama study. Moreover, affected Black and Hispanic/other Alabamians were less likely than were their White counterparts to receive recommended postdischarge care.
In comparison with White participants, Black and Hispanic adults were less likely to have any postdischarge ambulatory care visits after HF hospitalization or had a delayed visit, according to researchers led by Yulia Khodneva, MD, PhD, an internist at the University of Alabama at Birmingham. “This is likely a reflection of a structural racism and implicit bias against racial and ethnic minorities that persists in the U.S. health care system,” she and her colleagues wrote.
The findings point to the need for strategies to improve access to postdischarge care for lower-income HF patients.
Among U.S. states, Alabama is the sixth-poorest, the third in diabetes prevalence (14%), and has the highest rates of heart failure hospitalizations and cardiovascular mortality, the authors noted.
Study details
The cohort included 9,857 adults with diabetes and first hospitalizations for heart failure who were covered by Alabama Medicaid during 2010-2019. The investigators analyzed patients’ claims for ambulatory care (any, primary, cardiology, or endocrinology) within 60 days of discharge.
The mean age of participants was 53.7 years; 47.3% were Black; 41.8% non-Hispanic White; and 10.9% Hispanic/other, with other including those identifying as non-White Hispanic, American Indian, Pacific Islander, and Asian. About two-thirds (65.4%) of participants were women.
Analysis revealed low rates of follow-up care after hospital discharge; 26.7% had an ambulatory visit within 0-7 days, 15.2% within 8-14 days, 31.3% within 15-60 days, and 26.8% had no follow-up visit at all. Of those having a follow-up visit, 71% saw a primary care physician and 12% saw a cardiologist.
In contrast, a much higher proportion of heart failure patients in a Swedish registry – 63% – received ambulatory follow-up in cardiology.
Ethnic/gender/age disparities
Black and Hispanic/other adults were less likely to have any postdischarge ambulatory visit (P <.0001) or had the visit delayed by 1.8 days (P = .0006) and 2.8 days (P = .0016), respectively. They were less likely to see a primary care physician than were non-Hispanic White adults: adjusted incidence rate ratio, 0.96 (95% confidence interval [CI], 0.91-1.00) and 0.91 (95% CI, 0.89-0.98), respectively.
Men and those with longer-standing heart failure were less likely to be seen in primary care, while the presence of multiple comorbidities was associated with a higher likelihood of a postdischarge primary care visit. Men were more likely to be seen by a cardiologist, while older discharged patients were less likely to be seen by an endocrinologist within 60 days. There was a U-shaped relationship between the timing of the first postdischarge ambulatory visit and all-cause mortality among adults with diabetes and heart failure. Higher rates of 60-day all-cause mortality were observed both in those who had seen a provider within 0-7 days after discharge and in those who had not seen any provider during the 60-day study period compared with those having an ambulatory care visit within 7-14 or 15-60 days. “The group with early follow-up (0-7 days) likely represents a sicker population of patients with heart failure with more comorbidity burden and higher overall health care use, including readmissions, as was demonstrated in our analysis,” Dr. Khodneva and associates wrote. “Interventions that improve access to postdischarge ambulatory care for low-income patients with diabetes and heart failure and eliminate racial and ethnic disparities may be warranted,” they added.
This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases and the University of Alabama at Birmingham Diabetes Research Center. Dr. Khodneva reported funding from the University of Alabama at Birmingham and the Forge Ahead Center as well as from the NIDDK, the National Institutes of Health, the Agency for Healthcare Research and Quality, and the Alabama Medicaid Agency. Coauthor Emily Levitan, ScD, reported research funding from Amgen and has served on Amgen advisory boards. She has also served as a scientific consultant for a research project funded by Novartis.
Nearly 60% of Medicaid-covered adults with concurrent diabetes and heart failure did not receive guideline-concordant postdischarge care within 7-10 days of leaving the hospital, according to a large Alabama study. Moreover, affected Black and Hispanic/other Alabamians were less likely than were their White counterparts to receive recommended postdischarge care.
In comparison with White participants, Black and Hispanic adults were less likely to have any postdischarge ambulatory care visits after HF hospitalization or had a delayed visit, according to researchers led by Yulia Khodneva, MD, PhD, an internist at the University of Alabama at Birmingham. “This is likely a reflection of a structural racism and implicit bias against racial and ethnic minorities that persists in the U.S. health care system,” she and her colleagues wrote.
The findings point to the need for strategies to improve access to postdischarge care for lower-income HF patients.
Among U.S. states, Alabama is the sixth-poorest, the third in diabetes prevalence (14%), and has the highest rates of heart failure hospitalizations and cardiovascular mortality, the authors noted.
Study details
The cohort included 9,857 adults with diabetes and first hospitalizations for heart failure who were covered by Alabama Medicaid during 2010-2019. The investigators analyzed patients’ claims for ambulatory care (any, primary, cardiology, or endocrinology) within 60 days of discharge.
The mean age of participants was 53.7 years; 47.3% were Black; 41.8% non-Hispanic White; and 10.9% Hispanic/other, with other including those identifying as non-White Hispanic, American Indian, Pacific Islander, and Asian. About two-thirds (65.4%) of participants were women.
Analysis revealed low rates of follow-up care after hospital discharge; 26.7% had an ambulatory visit within 0-7 days, 15.2% within 8-14 days, 31.3% within 15-60 days, and 26.8% had no follow-up visit at all. Of those having a follow-up visit, 71% saw a primary care physician and 12% saw a cardiologist.
In contrast, a much higher proportion of heart failure patients in a Swedish registry – 63% – received ambulatory follow-up in cardiology.
Ethnic/gender/age disparities
Black and Hispanic/other adults were less likely to have any postdischarge ambulatory visit (P <.0001) or had the visit delayed by 1.8 days (P = .0006) and 2.8 days (P = .0016), respectively. They were less likely to see a primary care physician than were non-Hispanic White adults: adjusted incidence rate ratio, 0.96 (95% confidence interval [CI], 0.91-1.00) and 0.91 (95% CI, 0.89-0.98), respectively.
Men and those with longer-standing heart failure were less likely to be seen in primary care, while the presence of multiple comorbidities was associated with a higher likelihood of a postdischarge primary care visit. Men were more likely to be seen by a cardiologist, while older discharged patients were less likely to be seen by an endocrinologist within 60 days. There was a U-shaped relationship between the timing of the first postdischarge ambulatory visit and all-cause mortality among adults with diabetes and heart failure. Higher rates of 60-day all-cause mortality were observed both in those who had seen a provider within 0-7 days after discharge and in those who had not seen any provider during the 60-day study period compared with those having an ambulatory care visit within 7-14 or 15-60 days. “The group with early follow-up (0-7 days) likely represents a sicker population of patients with heart failure with more comorbidity burden and higher overall health care use, including readmissions, as was demonstrated in our analysis,” Dr. Khodneva and associates wrote. “Interventions that improve access to postdischarge ambulatory care for low-income patients with diabetes and heart failure and eliminate racial and ethnic disparities may be warranted,” they added.
This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases and the University of Alabama at Birmingham Diabetes Research Center. Dr. Khodneva reported funding from the University of Alabama at Birmingham and the Forge Ahead Center as well as from the NIDDK, the National Institutes of Health, the Agency for Healthcare Research and Quality, and the Alabama Medicaid Agency. Coauthor Emily Levitan, ScD, reported research funding from Amgen and has served on Amgen advisory boards. She has also served as a scientific consultant for a research project funded by Novartis.
FROM JOURNAL OF THE AMERICAN HEART ASSOCIATION
Game-changing Alzheimer’s research: The latest on biomarkers
The field of neurodegenerative dementias, particularly Alzheimer’s disease (AD), has been revolutionized by the development of imaging and cerebrospinal fluid biomarkers and is on the brink of a new development: emerging plasma biomarkers. Research now recognizes the relationship between the cognitive-behavioral syndromic diagnosis (that is, the illness) and the etiologic diagnosis (the disease) – and the need to consider each separately when developing a diagnostic formulation. The National Institute on Aging and Alzheimer’s Association Research Framework uses the amyloid, tau, and neurodegeneration system to define AD biologically in living patients. Here is an overview of the framework, which requires biomarker evidence of amyloid plaques (amyloid positivity) and neurofibrillary tangles (tau positivity), with evidence of neurodegeneration (neurodegeneration positivity) to support the diagnosis.
The diagnostic approach for symptomatic patients
The differential diagnosis in symptomatic patients with mild cognitive impairment (MCI), mild behavioral impairment, or dementia is broad and includes multiple neurodegenerative diseases (for example, AD, frontotemporal lobar degeneration, dementia with Lewy bodies, argyrophilic grain disease, hippocampal sclerosis); vascular ischemic brain injury (for example, stroke); tumors; infectious, inflammatory, paraneoplastic, or demyelinating diseases; trauma; hydrocephalus; toxic/metabolic insults; and other rare diseases. The patient’s clinical syndrome narrows the differential diagnosis.
Once the clinician has a prioritized differential diagnosis of the brain disease or condition that is probably causing or contributing to the patient’s signs and symptoms, they can then select appropriate assessments and tests, typically starting with a laboratory panel and brain MRI. Strong evidence backed by practice recommendations also supports the use of fluorodeoxyglucose PET as a marker of functional brain abnormalities associated with dementia. Although molecular biomarkers are typically considered at the later stage of the clinical workup, the anticipated future availability of plasma biomarkers will probably change the timing of molecular biomarker assessment in patients with suspected cognitive impairment owing to AD.
Molecular PET biomarkers
Three PET tracers approved by the U.S. Food and Drug Administration for the detection of cerebral amyloid plaques have high sensitivity (89%-98%) and specificity (88%-100%), compared with autopsy, the gold standard diagnostic tool. However, these scans are costly and are not reimbursed by Medicare and Medicaid. Because all amyloid PET scans are covered by the Veterans Administration, this test is more readily accessible for patients receiving VA benefits.
The appropriate-use criteria developed by the Amyloid Imaging Task Force recommends amyloid PET for patients with persistent or progressive MCI or dementia. In such patients, a negative amyloid PET scan would strongly weigh against AD, supporting a differential diagnosis of other etiologies. Although a positive amyloid PET scan in patients with MCI or dementia indicates the presence of amyloid plaques, it does not necessarily confirm AD as the cause. Cerebral amyloid plaques may coexist with other pathologies and increase with age, even in cognitively normal individuals.
The IDEAS study looked at the clinical utility of amyloid PET in a real-world dementia specialist setting. In the study, dementia subspecialists documented their presumed etiologic diagnosis (and level of confidence) before and after amyloid PET. Of the 11,409 patients who completed the study, the etiologic diagnosis changed from AD to non-AD in just over 25% of cases and from non-AD to AD in 10.5%. Clinical management changed in about 60% of patients with MCI and 63.5% of patients with dementia.
In May 2020, the FDA approved flortaucipir F-18, the first diagnostic tau radiotracer for use with PET to estimate the density and distribution of aggregated tau neurofibrillary tangles in adults with cognitive impairment undergoing evaluation for AD. Regulatory approval of flortaucipir F-18 was based on findings from two clinical trials of terminally ill patients who were followed to autopsy. The studies included patients with a spectrum of clinically diagnosed dementias and those with normal cognition. The primary outcome of the studies was accurate visual interpretation of the images in detecting advanced AD tau neurofibrillary tangle pathology (Braak stage V or VI tau pathology). Sensitivity of five trained readers ranged from 68% to 86%, and specificity ranged from 63% to 100%; interrater agreement was 0.87. Tau PET is not yet reimbursed and is therefore not yet readily available in the clinical setting. Moreover, appropriate use criteria have not yet been published.
Molecular fluid biomarkers
Cerebrospinal fluid (CSF) analysis is currently the most readily available and reimbursed test to aid in diagnosing AD, with appropriate-use criteria for patients with suspected AD. CSF biomarkers for AD are useful in cognitively impaired patients when the etiologic diagnosis is equivocal, there is only an intermediate level of diagnostic confidence, or there is very high confidence in the etiologic diagnosis. Testing for CSF biomarkers is also recommended for patients at very early clinical stages (for example, early MCI) or with atypical clinical presentations.
A decreased concentration of amyloid-beta 42 in CSF is a marker of amyloid neuritic plaques in the brain. An increased concentration of total tau in CSF reflects injury to neurons, and an increased concentration of specific isoforms of hyperphosphorylated tau reflects neurofibrillary tangles. Presently, the ratios of t-tau to amyloid-beta 42, amyloid-beta 42 to amyloid-beta 40, and phosphorylated-tau 181 to amyloid-beta 42 are the best-performing markers of AD neuropathologic changes and are more accurate than assessing individual biomarkers. These CSF biomarkers of AD have been validated against autopsy, and ratio values of CSF amyloid-beta 42 have been further validated against amyloid PET, with overall sensitivity and specificity of approximately 90% and 84%, respectively.
Some of the most exciting recent advances in AD center around the measurement of these proteins and others in plasma. Appropriate-use criteria for plasma biomarkers in the evaluation of patients with cognitive impairment were published in 2022. In addition to their use in clinical trials, these criteria cautiously recommend using these biomarkers in specialized memory clinics in the diagnostic workup of patients with cognitive symptoms, along with confirmatory CSF markers or PET. Additional data are needed before plasma biomarkers of AD are used as standalone diagnostic markers or considered in the primary care setting.
We have made remarkable progress toward more precise molecular diagnosis of brain diseases underlying cognitive impairment and dementia. Ongoing efforts to evaluate the utility of these measures in clinical practice include the need to increase diversity of patients and providers. Ultimately, the tremendous progress in molecular biomarkers for the diseases causing dementia will help the field work toward our common goal of early and accurate diagnosis, better management, and hope for people living with these diseases.
Bradford C. Dickerson, MD, MMSc, is a professor, department of neurology, Harvard Medical School, and director, Frontotemporal Disorders Unit, department of neurology, at Massachusetts General Hospital, both in Boston.
A version of this article first appeared on Medscape.com.
The field of neurodegenerative dementias, particularly Alzheimer’s disease (AD), has been revolutionized by the development of imaging and cerebrospinal fluid biomarkers and is on the brink of a new development: emerging plasma biomarkers. Research now recognizes the relationship between the cognitive-behavioral syndromic diagnosis (that is, the illness) and the etiologic diagnosis (the disease) – and the need to consider each separately when developing a diagnostic formulation. The National Institute on Aging and Alzheimer’s Association Research Framework uses the amyloid, tau, and neurodegeneration system to define AD biologically in living patients. Here is an overview of the framework, which requires biomarker evidence of amyloid plaques (amyloid positivity) and neurofibrillary tangles (tau positivity), with evidence of neurodegeneration (neurodegeneration positivity) to support the diagnosis.
The diagnostic approach for symptomatic patients
The differential diagnosis in symptomatic patients with mild cognitive impairment (MCI), mild behavioral impairment, or dementia is broad and includes multiple neurodegenerative diseases (for example, AD, frontotemporal lobar degeneration, dementia with Lewy bodies, argyrophilic grain disease, hippocampal sclerosis); vascular ischemic brain injury (for example, stroke); tumors; infectious, inflammatory, paraneoplastic, or demyelinating diseases; trauma; hydrocephalus; toxic/metabolic insults; and other rare diseases. The patient’s clinical syndrome narrows the differential diagnosis.
Once the clinician has a prioritized differential diagnosis of the brain disease or condition that is probably causing or contributing to the patient’s signs and symptoms, they can then select appropriate assessments and tests, typically starting with a laboratory panel and brain MRI. Strong evidence backed by practice recommendations also supports the use of fluorodeoxyglucose PET as a marker of functional brain abnormalities associated with dementia. Although molecular biomarkers are typically considered at the later stage of the clinical workup, the anticipated future availability of plasma biomarkers will probably change the timing of molecular biomarker assessment in patients with suspected cognitive impairment owing to AD.
Molecular PET biomarkers
Three PET tracers approved by the U.S. Food and Drug Administration for the detection of cerebral amyloid plaques have high sensitivity (89%-98%) and specificity (88%-100%), compared with autopsy, the gold standard diagnostic tool. However, these scans are costly and are not reimbursed by Medicare and Medicaid. Because all amyloid PET scans are covered by the Veterans Administration, this test is more readily accessible for patients receiving VA benefits.
The appropriate-use criteria developed by the Amyloid Imaging Task Force recommends amyloid PET for patients with persistent or progressive MCI or dementia. In such patients, a negative amyloid PET scan would strongly weigh against AD, supporting a differential diagnosis of other etiologies. Although a positive amyloid PET scan in patients with MCI or dementia indicates the presence of amyloid plaques, it does not necessarily confirm AD as the cause. Cerebral amyloid plaques may coexist with other pathologies and increase with age, even in cognitively normal individuals.
The IDEAS study looked at the clinical utility of amyloid PET in a real-world dementia specialist setting. In the study, dementia subspecialists documented their presumed etiologic diagnosis (and level of confidence) before and after amyloid PET. Of the 11,409 patients who completed the study, the etiologic diagnosis changed from AD to non-AD in just over 25% of cases and from non-AD to AD in 10.5%. Clinical management changed in about 60% of patients with MCI and 63.5% of patients with dementia.
In May 2020, the FDA approved flortaucipir F-18, the first diagnostic tau radiotracer for use with PET to estimate the density and distribution of aggregated tau neurofibrillary tangles in adults with cognitive impairment undergoing evaluation for AD. Regulatory approval of flortaucipir F-18 was based on findings from two clinical trials of terminally ill patients who were followed to autopsy. The studies included patients with a spectrum of clinically diagnosed dementias and those with normal cognition. The primary outcome of the studies was accurate visual interpretation of the images in detecting advanced AD tau neurofibrillary tangle pathology (Braak stage V or VI tau pathology). Sensitivity of five trained readers ranged from 68% to 86%, and specificity ranged from 63% to 100%; interrater agreement was 0.87. Tau PET is not yet reimbursed and is therefore not yet readily available in the clinical setting. Moreover, appropriate use criteria have not yet been published.
Molecular fluid biomarkers
Cerebrospinal fluid (CSF) analysis is currently the most readily available and reimbursed test to aid in diagnosing AD, with appropriate-use criteria for patients with suspected AD. CSF biomarkers for AD are useful in cognitively impaired patients when the etiologic diagnosis is equivocal, there is only an intermediate level of diagnostic confidence, or there is very high confidence in the etiologic diagnosis. Testing for CSF biomarkers is also recommended for patients at very early clinical stages (for example, early MCI) or with atypical clinical presentations.
A decreased concentration of amyloid-beta 42 in CSF is a marker of amyloid neuritic plaques in the brain. An increased concentration of total tau in CSF reflects injury to neurons, and an increased concentration of specific isoforms of hyperphosphorylated tau reflects neurofibrillary tangles. Presently, the ratios of t-tau to amyloid-beta 42, amyloid-beta 42 to amyloid-beta 40, and phosphorylated-tau 181 to amyloid-beta 42 are the best-performing markers of AD neuropathologic changes and are more accurate than assessing individual biomarkers. These CSF biomarkers of AD have been validated against autopsy, and ratio values of CSF amyloid-beta 42 have been further validated against amyloid PET, with overall sensitivity and specificity of approximately 90% and 84%, respectively.
Some of the most exciting recent advances in AD center around the measurement of these proteins and others in plasma. Appropriate-use criteria for plasma biomarkers in the evaluation of patients with cognitive impairment were published in 2022. In addition to their use in clinical trials, these criteria cautiously recommend using these biomarkers in specialized memory clinics in the diagnostic workup of patients with cognitive symptoms, along with confirmatory CSF markers or PET. Additional data are needed before plasma biomarkers of AD are used as standalone diagnostic markers or considered in the primary care setting.
We have made remarkable progress toward more precise molecular diagnosis of brain diseases underlying cognitive impairment and dementia. Ongoing efforts to evaluate the utility of these measures in clinical practice include the need to increase diversity of patients and providers. Ultimately, the tremendous progress in molecular biomarkers for the diseases causing dementia will help the field work toward our common goal of early and accurate diagnosis, better management, and hope for people living with these diseases.
Bradford C. Dickerson, MD, MMSc, is a professor, department of neurology, Harvard Medical School, and director, Frontotemporal Disorders Unit, department of neurology, at Massachusetts General Hospital, both in Boston.
A version of this article first appeared on Medscape.com.
The field of neurodegenerative dementias, particularly Alzheimer’s disease (AD), has been revolutionized by the development of imaging and cerebrospinal fluid biomarkers and is on the brink of a new development: emerging plasma biomarkers. Research now recognizes the relationship between the cognitive-behavioral syndromic diagnosis (that is, the illness) and the etiologic diagnosis (the disease) – and the need to consider each separately when developing a diagnostic formulation. The National Institute on Aging and Alzheimer’s Association Research Framework uses the amyloid, tau, and neurodegeneration system to define AD biologically in living patients. Here is an overview of the framework, which requires biomarker evidence of amyloid plaques (amyloid positivity) and neurofibrillary tangles (tau positivity), with evidence of neurodegeneration (neurodegeneration positivity) to support the diagnosis.
The diagnostic approach for symptomatic patients
The differential diagnosis in symptomatic patients with mild cognitive impairment (MCI), mild behavioral impairment, or dementia is broad and includes multiple neurodegenerative diseases (for example, AD, frontotemporal lobar degeneration, dementia with Lewy bodies, argyrophilic grain disease, hippocampal sclerosis); vascular ischemic brain injury (for example, stroke); tumors; infectious, inflammatory, paraneoplastic, or demyelinating diseases; trauma; hydrocephalus; toxic/metabolic insults; and other rare diseases. The patient’s clinical syndrome narrows the differential diagnosis.
Once the clinician has a prioritized differential diagnosis of the brain disease or condition that is probably causing or contributing to the patient’s signs and symptoms, they can then select appropriate assessments and tests, typically starting with a laboratory panel and brain MRI. Strong evidence backed by practice recommendations also supports the use of fluorodeoxyglucose PET as a marker of functional brain abnormalities associated with dementia. Although molecular biomarkers are typically considered at the later stage of the clinical workup, the anticipated future availability of plasma biomarkers will probably change the timing of molecular biomarker assessment in patients with suspected cognitive impairment owing to AD.
Molecular PET biomarkers
Three PET tracers approved by the U.S. Food and Drug Administration for the detection of cerebral amyloid plaques have high sensitivity (89%-98%) and specificity (88%-100%), compared with autopsy, the gold standard diagnostic tool. However, these scans are costly and are not reimbursed by Medicare and Medicaid. Because all amyloid PET scans are covered by the Veterans Administration, this test is more readily accessible for patients receiving VA benefits.
The appropriate-use criteria developed by the Amyloid Imaging Task Force recommends amyloid PET for patients with persistent or progressive MCI or dementia. In such patients, a negative amyloid PET scan would strongly weigh against AD, supporting a differential diagnosis of other etiologies. Although a positive amyloid PET scan in patients with MCI or dementia indicates the presence of amyloid plaques, it does not necessarily confirm AD as the cause. Cerebral amyloid plaques may coexist with other pathologies and increase with age, even in cognitively normal individuals.
The IDEAS study looked at the clinical utility of amyloid PET in a real-world dementia specialist setting. In the study, dementia subspecialists documented their presumed etiologic diagnosis (and level of confidence) before and after amyloid PET. Of the 11,409 patients who completed the study, the etiologic diagnosis changed from AD to non-AD in just over 25% of cases and from non-AD to AD in 10.5%. Clinical management changed in about 60% of patients with MCI and 63.5% of patients with dementia.
In May 2020, the FDA approved flortaucipir F-18, the first diagnostic tau radiotracer for use with PET to estimate the density and distribution of aggregated tau neurofibrillary tangles in adults with cognitive impairment undergoing evaluation for AD. Regulatory approval of flortaucipir F-18 was based on findings from two clinical trials of terminally ill patients who were followed to autopsy. The studies included patients with a spectrum of clinically diagnosed dementias and those with normal cognition. The primary outcome of the studies was accurate visual interpretation of the images in detecting advanced AD tau neurofibrillary tangle pathology (Braak stage V or VI tau pathology). Sensitivity of five trained readers ranged from 68% to 86%, and specificity ranged from 63% to 100%; interrater agreement was 0.87. Tau PET is not yet reimbursed and is therefore not yet readily available in the clinical setting. Moreover, appropriate use criteria have not yet been published.
Molecular fluid biomarkers
Cerebrospinal fluid (CSF) analysis is currently the most readily available and reimbursed test to aid in diagnosing AD, with appropriate-use criteria for patients with suspected AD. CSF biomarkers for AD are useful in cognitively impaired patients when the etiologic diagnosis is equivocal, there is only an intermediate level of diagnostic confidence, or there is very high confidence in the etiologic diagnosis. Testing for CSF biomarkers is also recommended for patients at very early clinical stages (for example, early MCI) or with atypical clinical presentations.
A decreased concentration of amyloid-beta 42 in CSF is a marker of amyloid neuritic plaques in the brain. An increased concentration of total tau in CSF reflects injury to neurons, and an increased concentration of specific isoforms of hyperphosphorylated tau reflects neurofibrillary tangles. Presently, the ratios of t-tau to amyloid-beta 42, amyloid-beta 42 to amyloid-beta 40, and phosphorylated-tau 181 to amyloid-beta 42 are the best-performing markers of AD neuropathologic changes and are more accurate than assessing individual biomarkers. These CSF biomarkers of AD have been validated against autopsy, and ratio values of CSF amyloid-beta 42 have been further validated against amyloid PET, with overall sensitivity and specificity of approximately 90% and 84%, respectively.
Some of the most exciting recent advances in AD center around the measurement of these proteins and others in plasma. Appropriate-use criteria for plasma biomarkers in the evaluation of patients with cognitive impairment were published in 2022. In addition to their use in clinical trials, these criteria cautiously recommend using these biomarkers in specialized memory clinics in the diagnostic workup of patients with cognitive symptoms, along with confirmatory CSF markers or PET. Additional data are needed before plasma biomarkers of AD are used as standalone diagnostic markers or considered in the primary care setting.
We have made remarkable progress toward more precise molecular diagnosis of brain diseases underlying cognitive impairment and dementia. Ongoing efforts to evaluate the utility of these measures in clinical practice include the need to increase diversity of patients and providers. Ultimately, the tremendous progress in molecular biomarkers for the diseases causing dementia will help the field work toward our common goal of early and accurate diagnosis, better management, and hope for people living with these diseases.
Bradford C. Dickerson, MD, MMSc, is a professor, department of neurology, Harvard Medical School, and director, Frontotemporal Disorders Unit, department of neurology, at Massachusetts General Hospital, both in Boston.
A version of this article first appeared on Medscape.com.
The new vaccine your patients may not want
Compared with the complicated and ever-changing recommended vaccine schedule for infants and children, vaccines for adults have been straightforward. Adults without compromised immunity who received all their childhood vaccinations are eligible for a tetanus and diphtheria (Td) or tetanus, diphtheria, and pertussis (Tdap) booster every 10 years, recombinant herpes zoster vaccine at age 50, and pneumococcal vaccines at age 65, along with annual influenza and (likely) COVID-19 vaccines. Last year, due to rising rates of acute hepatitis B, the Centers for Disease Control and Prevention first recommended universal hepatitis B vaccination for adults aged 19-59 years without a record of previous hepatitis B infection or vaccination.
An additional routine vaccine for adults is now on the horizon. The U.S. Food and Drug Administration recently approved Arexvy, a vaccine against respiratory syncytial virus (RSV) for adults aged 60 years or older. Two more RSV vaccines are in the final stages of development. Why should family physicians prioritize vaccinating older adults against RSV, and how can we incorporate this new vaccine into our practices and overcome patient hesitancy to receive yet another vaccine?
Clinicians tend to think of RSV as a serious disease in young children – which it is – but data suggest that in 2019, RSV infection led to more than 100,000 hospitalizations and 7,700 deaths in older adults in the United States. In a randomized controlled trial of 25,000 adults aged 60 years or older with a median of 6.7 months of follow-up, Arexvy reduced severe RSV disease by 94% and RSV-related acute respiratory infections by 71%, with similar effectiveness in adults with underlying health conditions. That’s considerably better protection than current influenza vaccines and comparable to COVID-19 mRNA vaccines before variants became widespread. Pain and fatigue were the most common side effects and usually resolved within 1-2 days.
Although the seasonal pattern of RSV shifted during the COVID-19 pandemic, RSV season historically begins in October, peaks in December, and ends in April. If the vaccine is recommended by the CDC and is widely available by fall, as the manufacturer, GSK, expects, it could be administered around the same time as influenza and COVID-19 vaccines.
The challenges of incorporating this new vaccine into practice will feel familiar: Many of our patients won’t have heard about it, may feel that they don’t need it, or may decline it because of concerns about side effects, real or imagined. (Of note, the FDA is requiring GSK to perform a postmarketing study to rule out associations with rare cases of Guillain-Barré syndrome and acute disseminated encephalomyelitis, and the company also plans to monitor the incidence of atrial fibrillation, which was slightly more common in the vaccine group than the placebo group.)
While a strong recommendation from a family physician is often enough to convince patients to accept vaccination, rampant misinformation during the pandemic may have worsened vaccine hesitancy for some. It may feel like a fruitless exercise to try to convince adults who have refused COVID-19 and influenza vaccines to accept a newer vaccine against a respiratory virus that causes less serious illness overall. But with other RSV vaccines and monoclonal antibodies for older adults and infants likely to be approved soon, it’s important for us to start laying the groundwork now by educating colleagues, staff, and patients about preventing serious illness caused by RSV.
Dr. Lin is an associate professor in the Department of Family Medicine at Georgetown University and a staff physician atMedStar Health Center, both in Washington. He has received income from UpToDate, Wiley-Blackwell, and the American Academy of Family Physicians.
A version of this article first appeared on Medscape.com.
Compared with the complicated and ever-changing recommended vaccine schedule for infants and children, vaccines for adults have been straightforward. Adults without compromised immunity who received all their childhood vaccinations are eligible for a tetanus and diphtheria (Td) or tetanus, diphtheria, and pertussis (Tdap) booster every 10 years, recombinant herpes zoster vaccine at age 50, and pneumococcal vaccines at age 65, along with annual influenza and (likely) COVID-19 vaccines. Last year, due to rising rates of acute hepatitis B, the Centers for Disease Control and Prevention first recommended universal hepatitis B vaccination for adults aged 19-59 years without a record of previous hepatitis B infection or vaccination.
An additional routine vaccine for adults is now on the horizon. The U.S. Food and Drug Administration recently approved Arexvy, a vaccine against respiratory syncytial virus (RSV) for adults aged 60 years or older. Two more RSV vaccines are in the final stages of development. Why should family physicians prioritize vaccinating older adults against RSV, and how can we incorporate this new vaccine into our practices and overcome patient hesitancy to receive yet another vaccine?
Clinicians tend to think of RSV as a serious disease in young children – which it is – but data suggest that in 2019, RSV infection led to more than 100,000 hospitalizations and 7,700 deaths in older adults in the United States. In a randomized controlled trial of 25,000 adults aged 60 years or older with a median of 6.7 months of follow-up, Arexvy reduced severe RSV disease by 94% and RSV-related acute respiratory infections by 71%, with similar effectiveness in adults with underlying health conditions. That’s considerably better protection than current influenza vaccines and comparable to COVID-19 mRNA vaccines before variants became widespread. Pain and fatigue were the most common side effects and usually resolved within 1-2 days.
Although the seasonal pattern of RSV shifted during the COVID-19 pandemic, RSV season historically begins in October, peaks in December, and ends in April. If the vaccine is recommended by the CDC and is widely available by fall, as the manufacturer, GSK, expects, it could be administered around the same time as influenza and COVID-19 vaccines.
The challenges of incorporating this new vaccine into practice will feel familiar: Many of our patients won’t have heard about it, may feel that they don’t need it, or may decline it because of concerns about side effects, real or imagined. (Of note, the FDA is requiring GSK to perform a postmarketing study to rule out associations with rare cases of Guillain-Barré syndrome and acute disseminated encephalomyelitis, and the company also plans to monitor the incidence of atrial fibrillation, which was slightly more common in the vaccine group than the placebo group.)
While a strong recommendation from a family physician is often enough to convince patients to accept vaccination, rampant misinformation during the pandemic may have worsened vaccine hesitancy for some. It may feel like a fruitless exercise to try to convince adults who have refused COVID-19 and influenza vaccines to accept a newer vaccine against a respiratory virus that causes less serious illness overall. But with other RSV vaccines and monoclonal antibodies for older adults and infants likely to be approved soon, it’s important for us to start laying the groundwork now by educating colleagues, staff, and patients about preventing serious illness caused by RSV.
Dr. Lin is an associate professor in the Department of Family Medicine at Georgetown University and a staff physician atMedStar Health Center, both in Washington. He has received income from UpToDate, Wiley-Blackwell, and the American Academy of Family Physicians.
A version of this article first appeared on Medscape.com.
Compared with the complicated and ever-changing recommended vaccine schedule for infants and children, vaccines for adults have been straightforward. Adults without compromised immunity who received all their childhood vaccinations are eligible for a tetanus and diphtheria (Td) or tetanus, diphtheria, and pertussis (Tdap) booster every 10 years, recombinant herpes zoster vaccine at age 50, and pneumococcal vaccines at age 65, along with annual influenza and (likely) COVID-19 vaccines. Last year, due to rising rates of acute hepatitis B, the Centers for Disease Control and Prevention first recommended universal hepatitis B vaccination for adults aged 19-59 years without a record of previous hepatitis B infection or vaccination.
An additional routine vaccine for adults is now on the horizon. The U.S. Food and Drug Administration recently approved Arexvy, a vaccine against respiratory syncytial virus (RSV) for adults aged 60 years or older. Two more RSV vaccines are in the final stages of development. Why should family physicians prioritize vaccinating older adults against RSV, and how can we incorporate this new vaccine into our practices and overcome patient hesitancy to receive yet another vaccine?
Clinicians tend to think of RSV as a serious disease in young children – which it is – but data suggest that in 2019, RSV infection led to more than 100,000 hospitalizations and 7,700 deaths in older adults in the United States. In a randomized controlled trial of 25,000 adults aged 60 years or older with a median of 6.7 months of follow-up, Arexvy reduced severe RSV disease by 94% and RSV-related acute respiratory infections by 71%, with similar effectiveness in adults with underlying health conditions. That’s considerably better protection than current influenza vaccines and comparable to COVID-19 mRNA vaccines before variants became widespread. Pain and fatigue were the most common side effects and usually resolved within 1-2 days.
Although the seasonal pattern of RSV shifted during the COVID-19 pandemic, RSV season historically begins in October, peaks in December, and ends in April. If the vaccine is recommended by the CDC and is widely available by fall, as the manufacturer, GSK, expects, it could be administered around the same time as influenza and COVID-19 vaccines.
The challenges of incorporating this new vaccine into practice will feel familiar: Many of our patients won’t have heard about it, may feel that they don’t need it, or may decline it because of concerns about side effects, real or imagined. (Of note, the FDA is requiring GSK to perform a postmarketing study to rule out associations with rare cases of Guillain-Barré syndrome and acute disseminated encephalomyelitis, and the company also plans to monitor the incidence of atrial fibrillation, which was slightly more common in the vaccine group than the placebo group.)
While a strong recommendation from a family physician is often enough to convince patients to accept vaccination, rampant misinformation during the pandemic may have worsened vaccine hesitancy for some. It may feel like a fruitless exercise to try to convince adults who have refused COVID-19 and influenza vaccines to accept a newer vaccine against a respiratory virus that causes less serious illness overall. But with other RSV vaccines and monoclonal antibodies for older adults and infants likely to be approved soon, it’s important for us to start laying the groundwork now by educating colleagues, staff, and patients about preventing serious illness caused by RSV.
Dr. Lin is an associate professor in the Department of Family Medicine at Georgetown University and a staff physician atMedStar Health Center, both in Washington. He has received income from UpToDate, Wiley-Blackwell, and the American Academy of Family Physicians.
A version of this article first appeared on Medscape.com.
Flavanol supplement improves memory in adults with poor diets
Taking a daily flavanol supplement improves hippocampal-dependent memory in older adults who have a relatively poor diet, results of a large new study suggest.
There’s increasing evidence that certain nutrients are important for the aging body and brain, study investigator Scott Small, MD, the Boris and Rose Katz Professor of Neurology, Columbia University Vagelos College of Physicians and Surgeons, New York, told this news organization.
“With this new study, I think we can begin to say flavanols might be the first one that really is a nutrient for the aging brain.”
These findings, said Dr. Small, represent “the beginning of a new era” that will eventually lead to formal recommendations” related to ideal intake of flavanols to reduce cognitive aging.
The findings were published online in the Proceedings of the National Academy of Science.
Better cognitive aging
Cognitive aging refers to the decline in cognitive abilities that are not thought to be caused by neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease. Cognitive aging targets two areas of the brain: the hippocampus, which is related to memory function, and the prefrontal cortex, which is related to attention and executive function.
Previous research has linked flavanols, which are found in foods like apples, pears, berries, and cocoa beans, to improved cognitive aging. The evidence shows that consuming these nutrients might be associated with the hippocampal-dependent memory component of cognitive aging.
The new study, known as COcoa Supplement and Multivitamin Outcomes Study-Web (COSMOS-Web), included 3,562 generally healthy men and women, mean age 71 years, who were mostly well-educated and non-Hispanic/non-Latinx White individuals.
Participants were randomly assigned to receive oral flavanol-containing cocoa extract (500 mg of cocoa flavanols, including 80 mg of epicatechin) or a placebo daily.
The primary endpoint was hippocampal-dependent memory at year 1 as assessed with the ModRey, a neuropsychological test designed to measure hippocampal function.
Results showed participants in both groups had a typical learning (practice) effect, with similar improvements (d = 0.025; P = .42).
Researchers used other tests to measure cognition: the Color/Directional Flanker Task, a measure of prefrontal cortex function, and the ModBent, a measure that’s sensitive to dentate gyrus function. The flavanol intervention did not affect ModBent results or performance on the Flanker test after 1 year.
However, it was a different story for those with a poor diet at baseline. Researchers stratified participants into tertiles on the basis of diet quality as measured by the Healthy Eating Index (HEI) scores. Those in the lowest tertile had poorer baseline hippocampal-dependent memory performance but not memory related to the prefrontal cortex.
The flavanol intervention improved performance on the ModRey test, compared with placebo in participants in the low HEI tertile (overall effect: d = 0.086; P = .011) but not among those with a medium or high HEI at baseline.
“We confirmed that the flavanol intervention only benefits people who are relatively deficient at baseline,” said Dr. Small.
The correlation with hippocampal-dependent memory was confirmed in a subset of 1,361 study participants who provided a urine sample. Researchers measured urinary 5-(3′,4′-dihydroxyphenyl)-gamma-valerolactone metabolite (gVLM) concentrations, a validated biomarker of flavanol consumption.
After stratifying these results into tertiles, researchers found performance on the ModRey was significantly improved with the dietary flavanol intervention (overall effect: d = 0.141; P = .006) in the lowest gVLM tertile.
Memory restored
When participants in the lowest tertile consumed the supplement, “their flavanol levels went back to normal, and when that happened, their memory was restored,” said Dr. Small.
It appears that there is a sort of ceiling effect to the flavanol benefits. “It seems what you need to do is normalize your flavanol levels; if you go above normal, there was no evidence that your memory keeps on getting better,” said Dr. Small.
The study included only older adults, so it’s unclear what the impact of flavanol supplementation is in younger adults. But cognitive aging “begins its slippery side” in the 40s, said Dr. Small. “If this is truly a nutrient that is taken to prevent that slide from happening, it might be beneficial to start in our 40s.”
He recognized that the effect size is not large but said this is “very dependent” on baseline factors and most study participants had a rather healthy diet. “None of our participants were really highly deficient” in flavanols, he said.
“To see a stronger effect size, we need to do another study where we recruit people who are very low, truly deficient, in flavanols, and then see what happens.”
Showing that flavanols are linked to the hippocampal and not to the prefrontal component of cognitive aging “speaks to the mechanism,” said Dr. Small.
Though the exact mechanism linking flavanols with enhanced memory isn’t clear, there are some clues; for example, research suggests cognitive aging affects the dentate gyrus, a subregion of the hippocampus.
The flavanol supplements were well tolerated. “I can say with close to certainty that this is very safe,” said Dr. Small, adding the flavanols have now been used in numerous studies.
The findings suggest flavanol consumption might be part of future dietary guidelines. “I suspect that once there is sufficient evidence, flavanols will be part of the dietary recommendations for healthy aging,” said Dr. Small.
A word of caution
Heather M. Snyder, PhD, vice president of medical and scientific relations, Alzheimer’s Association, said that though science suggests a balanced diet is good for overall brain health, no single food, beverage, ingredient, vitamin, or supplement has yet been proven to prevent dementia, treat or cure Alzheimer’s, or benefit cognitive function or brain health.
Experts agree the best source of vitamins and other nutrients is from whole foods as part of a balanced diet. “We recognize that, for a variety of reasons, this may not always be possible,” said Dr. Snyder.
However, she noted, dietary supplements are not subject to the same rigorous review and regulation process as medications.
“The Alzheimer’s Association strongly encourages individuals to have conversations with their physicians about all medications and dietary supplements they are currently taking or interested in starting.”
COSMOS is supported by an investigator-initiated grant from Mars Edge, a segment of Mars, company engaged in flavanol research and flavanol-related commercial activities, which included infrastructure support and the donation of study pills and packaging. Small reports receiving an unrestricted research grant from Mars.
A version of this article first appeared on Medscape.com.
Taking a daily flavanol supplement improves hippocampal-dependent memory in older adults who have a relatively poor diet, results of a large new study suggest.
There’s increasing evidence that certain nutrients are important for the aging body and brain, study investigator Scott Small, MD, the Boris and Rose Katz Professor of Neurology, Columbia University Vagelos College of Physicians and Surgeons, New York, told this news organization.
“With this new study, I think we can begin to say flavanols might be the first one that really is a nutrient for the aging brain.”
These findings, said Dr. Small, represent “the beginning of a new era” that will eventually lead to formal recommendations” related to ideal intake of flavanols to reduce cognitive aging.
The findings were published online in the Proceedings of the National Academy of Science.
Better cognitive aging
Cognitive aging refers to the decline in cognitive abilities that are not thought to be caused by neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease. Cognitive aging targets two areas of the brain: the hippocampus, which is related to memory function, and the prefrontal cortex, which is related to attention and executive function.
Previous research has linked flavanols, which are found in foods like apples, pears, berries, and cocoa beans, to improved cognitive aging. The evidence shows that consuming these nutrients might be associated with the hippocampal-dependent memory component of cognitive aging.
The new study, known as COcoa Supplement and Multivitamin Outcomes Study-Web (COSMOS-Web), included 3,562 generally healthy men and women, mean age 71 years, who were mostly well-educated and non-Hispanic/non-Latinx White individuals.
Participants were randomly assigned to receive oral flavanol-containing cocoa extract (500 mg of cocoa flavanols, including 80 mg of epicatechin) or a placebo daily.
The primary endpoint was hippocampal-dependent memory at year 1 as assessed with the ModRey, a neuropsychological test designed to measure hippocampal function.
Results showed participants in both groups had a typical learning (practice) effect, with similar improvements (d = 0.025; P = .42).
Researchers used other tests to measure cognition: the Color/Directional Flanker Task, a measure of prefrontal cortex function, and the ModBent, a measure that’s sensitive to dentate gyrus function. The flavanol intervention did not affect ModBent results or performance on the Flanker test after 1 year.
However, it was a different story for those with a poor diet at baseline. Researchers stratified participants into tertiles on the basis of diet quality as measured by the Healthy Eating Index (HEI) scores. Those in the lowest tertile had poorer baseline hippocampal-dependent memory performance but not memory related to the prefrontal cortex.
The flavanol intervention improved performance on the ModRey test, compared with placebo in participants in the low HEI tertile (overall effect: d = 0.086; P = .011) but not among those with a medium or high HEI at baseline.
“We confirmed that the flavanol intervention only benefits people who are relatively deficient at baseline,” said Dr. Small.
The correlation with hippocampal-dependent memory was confirmed in a subset of 1,361 study participants who provided a urine sample. Researchers measured urinary 5-(3′,4′-dihydroxyphenyl)-gamma-valerolactone metabolite (gVLM) concentrations, a validated biomarker of flavanol consumption.
After stratifying these results into tertiles, researchers found performance on the ModRey was significantly improved with the dietary flavanol intervention (overall effect: d = 0.141; P = .006) in the lowest gVLM tertile.
Memory restored
When participants in the lowest tertile consumed the supplement, “their flavanol levels went back to normal, and when that happened, their memory was restored,” said Dr. Small.
It appears that there is a sort of ceiling effect to the flavanol benefits. “It seems what you need to do is normalize your flavanol levels; if you go above normal, there was no evidence that your memory keeps on getting better,” said Dr. Small.
The study included only older adults, so it’s unclear what the impact of flavanol supplementation is in younger adults. But cognitive aging “begins its slippery side” in the 40s, said Dr. Small. “If this is truly a nutrient that is taken to prevent that slide from happening, it might be beneficial to start in our 40s.”
He recognized that the effect size is not large but said this is “very dependent” on baseline factors and most study participants had a rather healthy diet. “None of our participants were really highly deficient” in flavanols, he said.
“To see a stronger effect size, we need to do another study where we recruit people who are very low, truly deficient, in flavanols, and then see what happens.”
Showing that flavanols are linked to the hippocampal and not to the prefrontal component of cognitive aging “speaks to the mechanism,” said Dr. Small.
Though the exact mechanism linking flavanols with enhanced memory isn’t clear, there are some clues; for example, research suggests cognitive aging affects the dentate gyrus, a subregion of the hippocampus.
The flavanol supplements were well tolerated. “I can say with close to certainty that this is very safe,” said Dr. Small, adding the flavanols have now been used in numerous studies.
The findings suggest flavanol consumption might be part of future dietary guidelines. “I suspect that once there is sufficient evidence, flavanols will be part of the dietary recommendations for healthy aging,” said Dr. Small.
A word of caution
Heather M. Snyder, PhD, vice president of medical and scientific relations, Alzheimer’s Association, said that though science suggests a balanced diet is good for overall brain health, no single food, beverage, ingredient, vitamin, or supplement has yet been proven to prevent dementia, treat or cure Alzheimer’s, or benefit cognitive function or brain health.
Experts agree the best source of vitamins and other nutrients is from whole foods as part of a balanced diet. “We recognize that, for a variety of reasons, this may not always be possible,” said Dr. Snyder.
However, she noted, dietary supplements are not subject to the same rigorous review and regulation process as medications.
“The Alzheimer’s Association strongly encourages individuals to have conversations with their physicians about all medications and dietary supplements they are currently taking or interested in starting.”
COSMOS is supported by an investigator-initiated grant from Mars Edge, a segment of Mars, company engaged in flavanol research and flavanol-related commercial activities, which included infrastructure support and the donation of study pills and packaging. Small reports receiving an unrestricted research grant from Mars.
A version of this article first appeared on Medscape.com.
Taking a daily flavanol supplement improves hippocampal-dependent memory in older adults who have a relatively poor diet, results of a large new study suggest.
There’s increasing evidence that certain nutrients are important for the aging body and brain, study investigator Scott Small, MD, the Boris and Rose Katz Professor of Neurology, Columbia University Vagelos College of Physicians and Surgeons, New York, told this news organization.
“With this new study, I think we can begin to say flavanols might be the first one that really is a nutrient for the aging brain.”
These findings, said Dr. Small, represent “the beginning of a new era” that will eventually lead to formal recommendations” related to ideal intake of flavanols to reduce cognitive aging.
The findings were published online in the Proceedings of the National Academy of Science.
Better cognitive aging
Cognitive aging refers to the decline in cognitive abilities that are not thought to be caused by neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease. Cognitive aging targets two areas of the brain: the hippocampus, which is related to memory function, and the prefrontal cortex, which is related to attention and executive function.
Previous research has linked flavanols, which are found in foods like apples, pears, berries, and cocoa beans, to improved cognitive aging. The evidence shows that consuming these nutrients might be associated with the hippocampal-dependent memory component of cognitive aging.
The new study, known as COcoa Supplement and Multivitamin Outcomes Study-Web (COSMOS-Web), included 3,562 generally healthy men and women, mean age 71 years, who were mostly well-educated and non-Hispanic/non-Latinx White individuals.
Participants were randomly assigned to receive oral flavanol-containing cocoa extract (500 mg of cocoa flavanols, including 80 mg of epicatechin) or a placebo daily.
The primary endpoint was hippocampal-dependent memory at year 1 as assessed with the ModRey, a neuropsychological test designed to measure hippocampal function.
Results showed participants in both groups had a typical learning (practice) effect, with similar improvements (d = 0.025; P = .42).
Researchers used other tests to measure cognition: the Color/Directional Flanker Task, a measure of prefrontal cortex function, and the ModBent, a measure that’s sensitive to dentate gyrus function. The flavanol intervention did not affect ModBent results or performance on the Flanker test after 1 year.
However, it was a different story for those with a poor diet at baseline. Researchers stratified participants into tertiles on the basis of diet quality as measured by the Healthy Eating Index (HEI) scores. Those in the lowest tertile had poorer baseline hippocampal-dependent memory performance but not memory related to the prefrontal cortex.
The flavanol intervention improved performance on the ModRey test, compared with placebo in participants in the low HEI tertile (overall effect: d = 0.086; P = .011) but not among those with a medium or high HEI at baseline.
“We confirmed that the flavanol intervention only benefits people who are relatively deficient at baseline,” said Dr. Small.
The correlation with hippocampal-dependent memory was confirmed in a subset of 1,361 study participants who provided a urine sample. Researchers measured urinary 5-(3′,4′-dihydroxyphenyl)-gamma-valerolactone metabolite (gVLM) concentrations, a validated biomarker of flavanol consumption.
After stratifying these results into tertiles, researchers found performance on the ModRey was significantly improved with the dietary flavanol intervention (overall effect: d = 0.141; P = .006) in the lowest gVLM tertile.
Memory restored
When participants in the lowest tertile consumed the supplement, “their flavanol levels went back to normal, and when that happened, their memory was restored,” said Dr. Small.
It appears that there is a sort of ceiling effect to the flavanol benefits. “It seems what you need to do is normalize your flavanol levels; if you go above normal, there was no evidence that your memory keeps on getting better,” said Dr. Small.
The study included only older adults, so it’s unclear what the impact of flavanol supplementation is in younger adults. But cognitive aging “begins its slippery side” in the 40s, said Dr. Small. “If this is truly a nutrient that is taken to prevent that slide from happening, it might be beneficial to start in our 40s.”
He recognized that the effect size is not large but said this is “very dependent” on baseline factors and most study participants had a rather healthy diet. “None of our participants were really highly deficient” in flavanols, he said.
“To see a stronger effect size, we need to do another study where we recruit people who are very low, truly deficient, in flavanols, and then see what happens.”
Showing that flavanols are linked to the hippocampal and not to the prefrontal component of cognitive aging “speaks to the mechanism,” said Dr. Small.
Though the exact mechanism linking flavanols with enhanced memory isn’t clear, there are some clues; for example, research suggests cognitive aging affects the dentate gyrus, a subregion of the hippocampus.
The flavanol supplements were well tolerated. “I can say with close to certainty that this is very safe,” said Dr. Small, adding the flavanols have now been used in numerous studies.
The findings suggest flavanol consumption might be part of future dietary guidelines. “I suspect that once there is sufficient evidence, flavanols will be part of the dietary recommendations for healthy aging,” said Dr. Small.
A word of caution
Heather M. Snyder, PhD, vice president of medical and scientific relations, Alzheimer’s Association, said that though science suggests a balanced diet is good for overall brain health, no single food, beverage, ingredient, vitamin, or supplement has yet been proven to prevent dementia, treat or cure Alzheimer’s, or benefit cognitive function or brain health.
Experts agree the best source of vitamins and other nutrients is from whole foods as part of a balanced diet. “We recognize that, for a variety of reasons, this may not always be possible,” said Dr. Snyder.
However, she noted, dietary supplements are not subject to the same rigorous review and regulation process as medications.
“The Alzheimer’s Association strongly encourages individuals to have conversations with their physicians about all medications and dietary supplements they are currently taking or interested in starting.”
COSMOS is supported by an investigator-initiated grant from Mars Edge, a segment of Mars, company engaged in flavanol research and flavanol-related commercial activities, which included infrastructure support and the donation of study pills and packaging. Small reports receiving an unrestricted research grant from Mars.
A version of this article first appeared on Medscape.com.
